Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 05/513/02. The contractual start date was in October 2006. The draft report began editorial review in July 2010 and was accepted for publication in January 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The 50% salicylic acid (Verrugon) plasters and felt pads were provided to the University of York, free of charge, by the manufacturer William Ransom & Son Plc. BOC Cryospeed provided liquid nitrogen storage equipment at reduced cost. Neither company has had any input into the design, analysis and reporting of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Cockayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
What are verrucae?
Verrucae (or plantar warts) are caused by the human papillomavirus. They are extremely common, being experienced by most people at some time during their lives. Verrucae are infectious and can be painful, especially when affecting the soles of the feet or the nails. Although most verrucae will spontaneously disappear without treatment, many patients seek treatment because they are painful or because they are being prevented from doing sports and other activities of daily living.
Various studies have examined the prevalence of warts/verrucae and have produced a wide range of estimates. Three population-based studies reported point prevalence rates ranging from 0.84% (USA)1 to 3.3% (UK)2 and up to 12.9% (Russian Federation). 3 Studies of school-age populations have reported prevalence of 12% in 4 to 6-year-olds, 3.9% to 4.7% in 11 to 16-year-olds4 and 24% in 16 to 18-year-olds. 5 A recent cross-sectional study, including 1465 children in four primary schools in the Netherlands, reported prevalence rates in children aged 4–12 years of 33% (9% had hand warts, 20% had plantar warts and 4% had both). 6
Estimates of the rate of natural resolution of warts vary widely. Massing7 found that two-thirds resolved within 2 years, but the resolution rates reported in the placebo arms of trials suggest that warts may resolve more rapidly. In a Cochrane systematic review8 of wart treatment, 21 trials with placebo groups were reviewed. The average proportion that were clear of warts in the placebo groups in these trials was 27% (range 0 to 73%), after an average period of 15 weeks (range 4 to 24 weeks). This has led some to suggest that warts should not be treated at all. 9,10 However, some viral warts may persist for many years and there is no reliable means of predicting which ones will resolve spontaneously.
Verrucae are spread by direct skin-to-skin contact or indirectly via contact with contaminated surfaces (e.g. swimming pools or communal showers),11 although having a family member with a wart and having a high incidence of warts within a child’s class have been shown to be stronger risk factors than the use of swimming pools and shared bathing areas. 6 If a verruca is scratched or knocked it can bleed, making it easier for the virus to infect another part of the body through a breach in the skin. 12
What treatments are available?
Many treatments are available for the treatment of verrucae, including cryotherapy, topically applied treatments, surgical curettage, and complementary and alternative therapies. The most commonly prescribed treatments are cryotherapy with liquid nitrogen and topical salicylic acid. 13
Side-effects are common with all verrucae treatments, and include pain, burning, blistering, bleeding and scarring. Pain and blistering are more commonly reported for cryotherapy treatments,14 and, for this reason, cryotherapy is not recommended for young children. 10
What evidence is there for the most commonly used treatments?
A Cochrane systematic review8 that assessed the effects of different local treatments of cutaneous, non-genital warts was updated in 2006 (search date March 2005). This review highlighted considerable uncertainty around the optimal treatment of verrucae.
The best available evidence was for topical treatments containing salicylic acid (of varied strengths). These preparations were significantly better than placebo. Data pooled from five placebo-controlled trials showed a cure rate of 117/160 (73%) compared with 78/162 (48%) in control subjects. 8
Evidence for the effectiveness of cryotherapy was limited. The review found two trials comparing cryotherapy with salicylic acid and one comparing duct tape with cryotherapy. These trials showed no significant difference in efficacy for the compared treatments. More recently, a head-to-head trial of salicylic acid compared with cryotherapy has been reported in a primary care setting in the Netherlands. This trial found that cryotherapy was significantly better than salicylic acid for the treatment of hand warts, but that there was no significant benefit of cryotherapy compared with salicylic acid in plantar warts. 15 Cure rates for plantar warts were 29% for cryotherapy, 33% for salicylic acid and 23% for a no-treatment control group.
Why did we do the trial?
The treatment of warts and verrucae represents a considerable cost burden to both patients and the NHS. An economic decision model assessing the effectiveness and cost-effectiveness of salicylic acid and cryotherapy estimated that almost 2 million people in England and Wales see their general practitioner (GP) for the treatment of cutaneous warts each year, at a cost of at least £40M per annum. 14
Despite this, the evidence base on which to inform clinical decision-making is poor. Of the 60 trials identified in the 2006 Cochrane systematic review,8 46 (77%) were classified as low quality; in addition, heterogeneity between the trials was high and analyses were often inappropriate or misleading. A major conclusion from the Cochrane review8 was that a trial comparing topical salicylic acid with cryotherapy was urgently needed.
In response to an open call for trial proposals looking at medicines for children, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme agreed to fund the EVerT (Effective Verruca Treatments) trial, with the aim of establishing the efficacy and cost-effectiveness of these two treatments.
Specific objectives of the trial
-
To assess the clinical effectiveness and acceptability of cryotherapy compared with salicylic acid for the treatment of verrucae.
-
To assess the cost-effectiveness of the compared treatments.
Chapter 2 Methods
Trial design
The EVerT trial was an open, pragmatic, multicentred, two-armed, randomised controlled trial (RCT) with equal randomisation. Participants with verrucae were randomised (1 : 1) to receive either:
-
cryotherapy using liquid nitrogen, delivered by a health-care professional (a podiatrist, practice nurse or GP) or
-
once-daily self-treatment with 50% salicylic acid (Verrugon, William Ransom & Son Plc, Hitchin, UK).
Approvals obtained
The Trent Multicentre Research Ethics Committee (MREC) approved the study and substantial amendment to address the NIHR HTA programme reviewers’ comments on 26 October 2004 and 16 August 2006, respectively. Galway Research Ethics Committee approved the study on 20 March 2009.
Salicylic acid was classified as a medicinal product, therefore, clinical trial authorisations (CTAs) were obtained from the competent authorities in the UK and Ireland: the Medicines and Healthcare products Regulatory Agency (MHRA) (CTA number 22803/0001/001-0001) on 8 February 2005 and the Irish Medicines Board (clinical trial number CT 1552/1/1 Salicylic Acid/Liquid Nitrogen) on 30 January 2009.
The details of MREC, local research ethics committees (LRECs), competent authorities and research and development department approvals are provided in Appendix 1.
The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) of ISRCTN18994246; EudraCT number 2004-000905-24; and National Research Register number N0484189151.
Trial sites
The study was conducted in 16 study sites: 15 in the UK and 1 in Ireland. Sites were recruited throughout the duration of the trial. The sites were podiatry schools, outpatient podiatry clinics, GP practices or, in one case, a primary care trust (PCT) podiatry service outpatient clinic. Details of the study sites are provided in Appendix 2.
Participant eligibility
People with one or more verrucae were recruited into this study.
Inclusion criteria
Potential participants were eligible for inclusion in the trial if they met the following criteria:
-
They had a verruca which, in the opinion of the health-care professional, was suitable for treatment with both salicylic acid and cryotherapy.
-
They were aged 12 years and over.
The study was funded via the NIHR HTA’s medicines for children call. Consequently, the initial inclusion criteria focused on children and young people between the ages of 12 and 24 years, inclusive. However, because of poor recruitment the upper age restriction was lifted.
Exclusion criteria
Potential participants were excluded if they met one or more of the following criteria:
-
They were currently in a trial evaluating other treatments for their verruca.
-
They had impaired healing, for example owing to diabetes, peripheral vascular disease or any other condition.
-
They were immunosuppressed, for example had agammaglobulinaemia or were taking immunosuppressant drugs such as oral corticosteroids.
-
They had neuropathy.
-
They were currently on renal dialysis.
-
They had cold intolerance, for example Raynaud syndrome or cold urticaria.
-
They had any of the following conditions: blood dyscrasias of unknown origin, cryoglobulinaemia, cryofibrinogenaemia or collagen or autoimmune disease.
-
They were unable to give informed consent.
Recruitment into the trial
Members of the research team participating in the study received ‘Good Clinical Practice’ training, as well as training in all aspects of the trial, including participant recruitment, eligibility criteria, trial protocol, adverse event reporting procedures and trial documentation. In order to standardise the study prior to commencement, each study site also received a trial handbook.
Potential participants for the trial were identified by a health-care professional at the study site from GP referrals, or self-referrals received by the podiatry clinic or GP practice for the treatment of verrucae. Participants were provided with an appointment for assessment/treatment and sent an invitation letter, information sheet about the trial, baseline questionnaire and consent form for the study (see Appendices 3 and 4). The flow of participants through the trial is presented in a CONSORT (Consolidated Standards of Reporting Trials) diagram (see Figure 2).
In order to aid recruitment, one or more of the following strategies were adopted at some sites to increase the number of people with verrucae presenting to the clinics:
-
GPs in the recruiting area were approached by either the York Trials Unit (YTU: University of York, UK) or the local Primary Care Research Network. They were requested to refer patients presenting with a verruca and who expressed an interest in taking part in the trial to the recruiting site.
-
The trial was promoted by means of a recruitment poster that was displayed in 41 libraries, 15 pharmacies, 19 swimming pools, 8 supermarkets, 2 universities and 2 hospitals.
-
Secondary schools were approached and asked to send out study information to their students. Fifteen schools in three different recruiting areas agreed to send out study information to 7410 students and displayed recruitment posters.
-
The trial was publicised in two local newspapers, in three university press releases, on three university websites and on two local radio stations. Potential participants were directed to the local recruiting site.
The documentation used to aid recruitment to the study is included in Appendix 5.
For individuals responding to an advert for trial participants, telephone screening by the study sites was recommended to ensure that the potential participants fulfilled the inclusion criteria.
Participants were given a minimum of 24 hours to read the information sheet and consider participation. In Ireland, where possible, there was a minimum of 6 days between the patient signing the consent form and the start of treatment in order to comply with local regulations. Participants who wished to take part in the study and who returned their baseline questionnaire were screened by the health-care professional using a randomisation form that listed the eligibility criteria (see Appendix 4). Eligible patients, and their parent/guardian for those under 16 years of age, were able to discuss the study in more detail prior to providing written informed consent. Baseline data were then recorded and a digital photograph taken of the verruca(e). Participants’ GPs were notified of their involvement in the EVerT trial after recruitment.
Baseline assessment
After written informed consent had been obtained, baseline data were collected using the podiatrist treatment assessment form and the baseline questionnaire (see Appendix 4). The following data were collected.
Type and number of verruca(e)
The number and type of verruca(e) (mosaic or non-mosaic) were collected on the podiatrist treatment assessment form in order to examine whether or not mosaic verrucae respond less well to treatment than simple verrucae.
Duration and previous treatment of current verruca(e)
The duration of the current verruca(e) and type of any previous treatment received were recorded on the participant baseline questionnaire.
Reason for seeking treatment
The reasons for seeking treatment for the verrucae were recorded on the participant baseline questionnaire.
Level of pain
Participants were asked to rate how painful their current verruca was at baseline on a five-point Likert scale of 0–4, where 0 was not at all painful and 4 was extremely painful.
Number of previous verrucae and age at which they occurred
The number of previous verrucae and age at which they occurred were recorded on the participant baseline questionnaire.
Patient’s treatment preference
The patient’s treatment preference was recorded on the podiatrist treatment assessment form to allow us to explore the influence of the patient’s treatment preference on treatment outcomes.
Date of birth
Date of birth was recorded on the participant baseline questionnaire, allowing age at recruitment to be calculated and to allow us to explore the influence of the participant’s age on treatment outcomes.
Gender
The gender of participants was recorded on the participant baseline questionnaire.
Ineligible patients
The health-care professionals were asked to complete an ineligible patient form (see Appendix 4) for those participants who wished to take part in the trial, but were ineligible to do so. Data collected on this form were reasons why the patient was not eligible, date of birth, gender, type of wart and date of consideration for trial entry. Where the patient was willing, a completed baseline questionnaire was also collected.
Randomisation
Patients were randomised equally between the two treatment arms: cryotherapy using liquid nitrogen delivered by the health-care professional (podiatrist, practice nurse and GP) or daily self-treatment by the patient with 50% salicylic acid. The health-care professional at the recruiting site randomised the patient using the secure, remote, independent YTU telephone or web-based randomisation service. Randomisation was simple, i.e. it was not restricted in any way. Stratified randomisation was not used in order to reduce the risk of subversion, which can occur using forms of restricted randomisation. The allocation sequence was computer generated, with the treatment allocation being concealed from both the health-care professional and YTU until the moment of randomisation.
Sample size
The Cochrane systematic review8 found only one small trial directly comparing the effectiveness of a chemical treatment, salicylic acid, with cryotherapy in patients with warts on their feet alone. This poor-quality study found a 58% cure rate among the patients allocated to cryotherapy compared with 41% among those treated with salicylic acid. This difference of 17% was not statistically significant. The overall cure rates from this study are smaller than those observed in two placebo-controlled trials of salicylic acid, both of which reported cure rates of 85% for active treatment, possibly because more resistant verrucae were included in the study comparing cryotherapy with salicylic acid. The EVerT trial was powered to show a 15% difference in effectiveness. To give us 80% power (5% two-sided significance) to show a difference in cure rates of 70% versus 85% at 12 weeks, we required a sample size of 120 patients in each treatment group or 133 patients in each group after allowing for 10% attrition (i.e. 266 in total).
Trial interventions
Participants were randomised to receive either cryotherapy using liquid nitrogen delivered by a health-care professional or daily self-treatment with 50% salicylic acid (Verrugon).
Cryotherapy using liquid nitrogen delivered by the health-care professional
Patients randomised to cryotherapy using liquid nitrogen received up to a maximum of four treatments 14–21 days apart. Treatment was delivered by the health-care professional according to the usual practice of each trial site. Most of the health-care professionals delivering the cryotherapy had several years’ experience in delivering cryotherapy using liquid nitrogen. If a patient presented with more than one verruca, the health-care professional was instructed to treat the verrucae as they would in normal practice.
Prior to treatment, if it was the site’s normal practice, the callus surrounding the verruca(e) was debrided (e.g. with a scalpel or file) with any haemorrhages stopped by digital pressure only. The tissue surrounding the verruca was either masked (e.g. with petroleum jelly) or left unmasked, as per usual practice. Liquid nitrogen was applied using a spray (method of choice if available) or probe until the health-care professional was satisfied that the tissue had been frozen adequately. On the advice from the Trial Steering Committee (TSC), clinicians were advised that the first treatment should be a gentle freeze (approximately 10 seconds’ duration) in order to ensure that the patient could tolerate the treatment. Silver nitrate was not applied to the verruca. If necessary, the health-care professional could pad the area surrounding the verruca after treatment, for example with 7 mm of felt-cavity padding. Patients were given a cryotherapy patient’s advice sheet (see Appendix 3). Patients were also advised to keep the area dry for 24 hours and that the area may blister and be uncomfortable. If required, patients were recommended to use painkillers, as they would for a headache, if the area was very painful.
Daily self-treatment by the patient with 50% salicylic acid
Patients randomised to self-treatment with 50% salicylic acid were instructed how to use the salicylic acid by the health-care professional and were provided with a salicylic acid patient’s advice sheet (see Appendix 3) at the first trial appointment. Thereafter, the salicylic acid was applied once daily by the patient (or parent/guardian if appropriate) for a maximum of 8 weeks as per the manufacturer’s instructions as follows:
-
The self-adhesive ring should be fixed with the hole over the verruca.
-
Squeeze a little Verrugon ointment into the hole and directly onto the verruca.
-
Remove backing paper from plaster.
-
Cover ring completely with plaster. Seal into position.
-
Repeat treatment daily after gently pumicing or filing off the dead part of the verruca.
All patients were given a follow-up appointment at 2 weeks as a safety check. Further supplies of felt pads, plasters and salicylic acid were provided to the patient when required. Patients were asked to return all of the tubes of salicylic acid they had received during the trial to the treating health-care professional at their 12-week appointment. The health-care professional weighed the tube(s) to determine how much salicylic acid had been used over the 8-week period.
Participant follow-up
Appendix 6 shows a summary of participant follow-up for the EVerT trial. Participants were given the option to complete participant questionnaires in either paper or web-based format according to their preference. In order to increase the response rate to the week-12 questionnaire, participants received an unconditional £5 (€5 for the site in Ireland) with their week-12 questionnaire. The week-12 questionnaire was preceded by a letter notifying the participant that their week-12 questionnaire would arrive shortly and that it would be accompanied by a five pound (or five euro) note as an acknowledgement for their taking part in the trial and completing the questionnaires.
In order to minimise the difference in attendance between participants in the two groups, participants were reimbursed £20 for attending their week-12 outcome assessment appointment with the health-care professional. Information about this reimbursement was included in the patient information sheet.
Trial completion
Participants were deemed to have exited the trial when:
-
the participant had been in the trial for 6 months
-
the participant wished to exit the trial fully
-
the participant’s health-care professional withdrew him/her from the trial
-
the participant was lost to follow-up
-
the participant died.
Instead of withdrawing fully from the trial, participants had the option of:
-
withdrawing only from receiving the trial treatment
-
withdrawing only from postal or web-based questionnaires
-
withdrawing from the collection of data by the health-care professional
-
any combination of the above.
If the participant elected to withdraw from all three (trial treatment, questionnaires and data collection) then he or she was deemed as a full withdrawal (trial exit). Health-care professionals were able to indicate any change in the patient’s level of participation by completing the change of circumstances form (see Appendix 4). This ensured appropriate follow-up from the YTU.
Measurement of primary outcome
The primary outcome was complete clearance of all verrucae at 12 weeks after randomisation. Clearance of verrucae was defined as the restoration of normal skin on close inspection.
At the 12-week appointment the treating health-care professional or other member of the research team took a digital photograph of the participant’s foot. Participants who did not attend their 12-week outcome assessment appointment were asked to take a digital photograph of their foot and send it to the YTU. Two blinded assessors independently assessed the photographs for each participant from all the sites to determine whether or not the verrucae had cleared, and whether or not they could tell which treatment the patient had received. The assessors discussed any discrepancies with referral to a third assessor for a final decision if required.
Previous studies co-ordinated by the YTU had found that using cameras to obtain blinded outcome assessments was not without its challenges. We therefore undertook an additional blinded outcome assessment at the recruiting site at the participant’s 12-week appointment. This assessment would then be used in cases in which assessment of the digital photograph was not possible, for example when the photograph was not interpretable or was missing. The blinded outcome assessment at the site was undertaken by another member of the research team who was unaware of the treatment the participant had received. The health-care professional recorded whether or not the verruca(e) had completely cleared on the podiatrist outcome assessment form (see Appendix 4). Participants were reminded not to tell the person undertaking the blinded assessment which treatment they received and participants allocated to the salicylic acid group were asked not to return any used or unused Verrugon tubes to them. If the outcome assessment was not blinded, this was recorded on the podiatrist outcome assessment form.
The primary outcome was then calculated using whether or not the verrucae had cleared, as decided by the blinded assessors from the photographs. However, if no photographs were available for a participant, or if the photograph was not interpretable, then the outcome from the blinded assessment at 12 weeks was taken. If neither of these were available for a participant then the patient’s self-reported outcome recorded in the week-12 questionnaire or on the ‘verrucae gone’ form (see Appendix 4) were used.
Measurement of secondary outcomes
Self-reported time to clearance of verrucae
Participants were asked to report if their verruca(e) had cleared on their week-3, week-12 and 6-month questionnaires (see Appendix 4) and, if it had cleared, on what date it cleared. In addition to this, participants were asked to return their ‘verruca gone form’ if their verrucae cleared at any other time points. If there was any discrepancy between the dates reported by the participant then the longest date to clearance was used.
Clearance of verrucae at 6 months
Clearance of verrucae at 6 months was recorded on the participant’s 6-month questionnaire. If the participant had verrucae at 6 months then the position of the verrucae (either in the original or in a new position) was recorded.
Number of verrucae remaining at the 12-week appointment
The number of verrucae remaining at 12 weeks was recorded on the podiatrist outcome assessment form to summarise the effects of the two regimens.
Additional data collected
Recurrence of verrucae at 6 months
Participants were asked whether or not they had a verruca at 6 months on the participant’s 6-month questionnaire. If a verruca was present they were asked to record whether or not it was in the original or in a different place.
Patient satisfaction with treatment
Patient satisfaction with treatment (on a five-point scale, from ‘very unhappy’ to ‘very happy’) was reported on the participant week-1, week-3 and week-12 questionnaires.
Pain associated with first treatment
Pain associated with the first treatment (on a scale of 0–10, where 0 is no pain and 10 is the worst pain imaginable) was recorded on the patient pain questionnaire (see Appendix 4). This questionnaire was designed for participants to complete after their first treatment and return to the YTU using a reply-paid envelope.
Pain associated with verrucae and use of painkillers
Participants were asked to rate how painful their verrucae were on a five-point Likert scale of 0–4, where 0 was not at all painful and 4 was extremely painful. They were also asked to record if they needed to take a painkiller because of their verruca treatment during the first 3 weeks following entry into the study and, if yes, the number of days they took painkillers. Data were collected on the week-1 and week-3 questionnaires.
Treatment details
The number of appointments attended by each participant, excluding the week-12 outcome assessment appointment, were recorded by the health-care professional on the podiatrist treatment assessment form. Details of the cryotherapy delivered at each appointment were recorded by the health-care professional on the same form, including the number of freezes performed, the duration of the first freeze on that visit, whether or not the health-care professional considered that sufficient freezing took place and whether or not the patient asked for the freeze(s) to be stopped, and, if so, why. As a means of assessing adherence, the weight of salicylic acid ointment used over the treatment period was recorded by the health-care professional on the podiatrist treatment assessment form by weighing the tubes of salicylic acid at the start and end of treatment. In addition, the number of times salicylic acid was applied within the past 7 days was reported on the participants’ week-1 and week-3 questionnaires.
Adverse events
An adverse event was defined as ‘any untoward medical occurrence in a subject to whom a medicinal product has been administered, including occurrences which are not necessarily caused by or related to that product’.
Health-care professionals were asked to report any adverse events occurring in participants in both groups to the trial office using either the ‘serious adverse event form’ or the ‘non-serious adverse event form’ (see Appendix 4). The reporting health-care professional was asked to indicate whether or not, in his or her opinion, the event was related to the treatment. Serious adverse events were defined as an event that resulted in death, was life-threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in a persistent or significant disability or incapacity, or resulted in a congenital anomaly or birth defect. When appropriate an assessment of intensity and expectedness was also undertaken.
A list of possible treatment-related adverse events was established, a priori, based on reports in the literature. These were pain, blistering, irritation to the skin, infection, burning sensation, bleeding, scarring and allergic contact reaction.
Health-care professionals were asked to report any serious adverse events within 24 hours of becoming aware of the event and provide a follow-up report if necessary.
Reasons for stopping treatment and any new treatments
Whether or not the participant found it necessary to stop the treatment to which they had been allocated and, if so, the reasons for this were recorded on the participant’s week-12 questionnaires. Whether or not they started another treatment, and, if so, what was the new treatment, was also recorded on the week-12 questionnaire.
Statistical analysis
All analyses were conducted on an intention-to-treat basis, including all randomised patients in the groups to which they were randomised. All of the analyses were conducted using Stata statistic and data analysis software version 10.1 (StataCorp LP, College Station, TX, USA), except the logistic regression model accounting for centre clustering effects, which was undertaken using Sas version 9.2 (SAS Institute Inc., Cary, NC, USA) and two-sided significance tests at the 5% significance level for the primary outcome measure and 1% significance level for secondary outcome measures. Multiple imputation methods were used to handle missing data. The statistician conducting the analysis remained blind to treatment group and data were unblinded only once all data summaries and analyses were completed.
Trial completion
The flow of participants through the trial is presented in a CONSORT diagram. The numbers of participants withdrawing from treatment and/or the trial were summarised together with the reasons where available.
Baseline data
All baseline data were summarised by treatment group and described descriptively. No formal statistical comparisons were undertaken. Continuous measures were reported as means and standard deviations (SDs), whereas categorical data were reported as counts and percentages.
Primary analysis
The primary outcome was complete clearance of all verrucae at 12 weeks. This was a dichotomous outcome (presence or absence of verruca). We compared the proportions of participants with complete clearance of all verrucae using a chi-squared test.
The Cohen’s kappa measure of inter-rater agreement was used to assess the agreement between the two assessors of the blinded photographs whether or not the verrucae had cleared.
Secondary analysis
Clearance of verrucae at 12 weeks
A logistic regression model was used to adjust the primary analysis for important prognostic variables (age, whether or not the verrucae have been previously treated and type of verruca). Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were obtained from this model.
Time to clearance of verrucae
Time to clearance was derived as the number of days from randomisation until the date of clearance as detailed from the participant’s self-reported questionnaire. Participants’ verrucae that had not cleared were treated as censored and their date of trial exit, or date of last available assessment, or 183 days/trial cessation, as appropriate, was used to calculate their duration in the trial.
A Cox proportional hazards model was used to compare the time to clearance of the verrucae between the two groups, adjusting for the same covariates as for the primary outcome.
Clearance of verrucae at 6 months
The complete clearance of all verrucae at 6 months was analysed in the same way as the primary outcome, with adjustments for the same covariates.
Number of verrucae at 12 weeks
Negative binomial regression was used to compare the number of verrucae at 12 weeks between the two treatment groups, with adjustment for the number of verrucae at baseline. These models are used to estimate the number of occurrences of an event when the event has Poisson variation with overdispersion.
Patient’s treatment preference
As patients and health-care professionals were not blinded to treatments, we carried out an analysis to assess the influence of participant’s treatment preference on treatment outcomes. A logistic regression model was developed using the primary outcome and included patient preference and an interaction term between randomised treatment and preferred treatment in the model.
Missing data
We investigated the sensitivity of the results to missing data with multiple imputation analysis. Five imputations were created using a set of appropriate imputation models constructed using variables that were predictive of the missing data. Multiple imputation analysis was performed using the multiple imputation procedure in Sas.
Additional data collected
The following additional data were collected:
-
recurrence of verrucae at 6 months
-
patient satisfaction with treatment
-
pain associated with the first treatment
-
pain associated with verrucae and use of painkillers
-
treatment details for the cryotherapy delivered and adherence data for the salicylic acid arm
-
adverse events
-
reasons for stopping treatment and any new treatments
-
if patients had verrucae at 6 months were they in the original or a different place?
All additional data were summarised by treatment group (where appropriate), but no statistical analyses were performed.
Economic analysis
Aim of the economic analysis
Economic evaluation of health interventions is a tool used to assist decision-makers in prioritising and allocating resources in the health-care sector, by assessing the value for money (cost-effectiveness) of alternative interventions.
The aim of the economic analysis was to assess the relative costs and effectiveness of cryotherapy and salicylic acid for the treatment of verrucae. Data on both costs and effectiveness of the two comparators were synthesised to assess the additional cost required for an additional unit of outcome. For this analysis, a cost-effectiveness approach was taken, where the outcome was defined as complete clearance of verrucae at 12 weeks.
The analysis was conducted on an ‘intention-to-treat’ basis. Hence, the analysis compared the treatment groups based on their original random allocation, regardless of protocol deviations and participants’ compliance or withdrawal. The NHS perspective was taken for the analysis where only costs directly linked to the NHS budget (GP or nurse visits, podiatrist time and cost of equipment and medications) were included.
Data
Resource use data
During the participant’s treatment period within the study, data on the resource use component of the economic analysis were collected from both participants’ self-completed questionnaires and the relevant form (podiatrist treatment assessment form) completed by the health-care professionals.
The number of visits to the podiatrist, nurse or GP for treatment was recorded by the health-care professional who treated the trial participant. In particular, details on the number of cryotherapy sessions administered and the number of tubes of salicylic acid provided to the patients were collected.
In addition, data on other resource usage were collected at 12 weeks after randomisation on a patient self-reported questionnaire. The questionnaire was designed for participant completion and was returned to the trial office using a reply-paid envelope. Participants were asked to complete the questionnaire about the number of visits to the clinic for treatment of their verruca and health service use (e.g. if they had seen a GP, practice nurse or attended an emergency visit with a GP because of their verruca).
Outcome data
The outcome data used for the economic analysis were the complete clearance of verrucae at 12 weeks. The data on outcome were extracted primarily by two independent assessors from digital photographs taken at 12 weeks. In cases where the digital photograph was not interpretable, the data were extracted from the podiatrist outcome assessment form and, finally, the patient self-reported questionnaire at 12 weeks. This has been described above (see Measurement of primary outcome).
Methods for calculation of costs
Cost of the cryotherapy treatment
The cost of cryotherapy treatment comprised two components: the cost of the equipment and the opportunity cost of the health-care professional’s time for attending the patients.
The list of equipment required for cryotherapy was compiled by a combination of interviewing podiatrists who run podiatric clinics and the equipment that was bought as part of setting up a trial centre. The equipment list included:
-
cryogenic gloves
-
safety glasses
-
aluminium Dewar
-
tipping trolley for Dewar
-
withdrawal device
-
cryosurgery applicator
-
slim probe
-
apron.
In the economic analysis, annuitisation of the equipment cost was performed (see Equation 1). For this procedure, the cost of the equipment (K), which was incurred on its purchase, is spread over the lifetime of the equipment to obtain an equivalent annual cost (E). 16 An interest rate (r) of 3.5% and a lifespan (n) for the cryogenic equipment of approximately 5 years were used in the calculations of the annuity factor.
To assign an equipment cost per treatment, the annual cost (E) was divided by the maximum number of the treatments that can be provided by a GP or podiatrist. The maximum number of treatments was calculated based on an average appointment time of 20 minutes and assuming full capacity of the clinics for the total number of working days per year (i.e. 253 excluding bank holidays in the UK). The average appointment time of 20 minutes was based on the experience of podiatrists and practice nurses.
In addition to the equipment cost, the cost of liquid nitrogen, which was the freezing agent for the cryotherapy, was calculated. Liquid nitrogen is nitrogen in a liquid state at a very low temperature. 17 Hence, the Dewars are refilled frequently, approximately every 4–6 weeks, even though the liquid nitrogen is not being used fully for patient treatments. It is, therefore, difficult to assess the quantity of liquid nitrogen that is required for a single treatment. However, from the trial data, it was noticed that in one trial centre (Galway, Ireland) that exclusively treated trial participants, four refills of a 25-litre Dewar were ordered in a time frame of 3 months. The cost of liquid nitrogen per treatment was calculated by dividing the cost of four refills of a 25-l Dewar by the total number of treatments performed in that centre.
The clinician’s time was calculated based on an average appointment time of 20 minutes. The treatments were administered to the trial participants by either a GP, nurse or podiatrist. The unit costs for these health-care professionals were retrieved from the Unit costs of health and social care 2009. 18
Cost of the salicylic acid treatment
The cost of the salicylic acid treatment comprised two components: the cost of the medication and the health professional’s time spent for each treatment assessment visit.
The cost of the medication included the:
-
Verrugon ointment tubes
-
felt pads
-
plasters.
The cost of Verrugon tubes was calculated based on the number of tubes used by the patients, irrespective of whether or not the patient had used up the entire content of the last tube received. For example, if the patient did not finish the second tube, the total of the two tubes was used for cost calculations. It should be noted that the maximum number of tubes used per participant in the trial was two.
The total numbers of felt pads and plasters were calculated based on the total number of applications, which, in turn, was based on the number of Verrugon tubes that a patient used. Hence, if the patient used only one tube of Verrugon then he or she needed pads and plasters sufficient for 28 applications. This is half of the total number of applications possible during the 8-week treatment period of the trial. Similarly, if the patients used two tubes then pads and plasters were required for 56 applications.
The total number of felt pads boxes used per patient was calculated by dividing the number of applications by 36 (this is the total number of felt pads in a box: www.nu-careproducts.co.uk/chiropody.htm#feltpads, product PPD12619) and rounded up to a whole number. Similarly, for the plasters, a box of 10 fabric strips sold by a national pharmacy chain,20 equivalent to 20 applications, was used as a reference. The total number of boxes needed for the treatment was calculated by dividing the number of applications by 20 and rounding up to a whole number.
The cost of the health-care professional’s time for the administration of treatment was calculated based on an average appointment time of 20 minutes.
Unit costs of the treatments
The unit costs for the cryotherapy equipment were retrieved either from the supplier’s website or from a catalogue that was sent to the different trial centres. When more than one type of the same item was available, the average unit cost was calculated. The unit costs for the cryotherapy equipment are presented in Table 1.
| Item | Source | Size/type | Pricea,b | Average price | Price (£)c | Price including VAT (£)d |
|---|---|---|---|---|---|---|
| Cryogenic gloves | Catalogue sent to Galway | €35.00 | €35.00 | 28.88 | 33.94 | |
| Safety glasses | BOC Products21 | £4.42 | £4.42 | 4.42 | 5.19 | |
| Dewar | Catalogue sent to Galway | 25 l aluminium | €833.00 | €868.00 | 716.30 | 841.65 |
| 25 l stainless steel | €903.00 | |||||
| Tipping trolley for Dewar | Catalogue sent to Galway | €433.00 | €433.00 | 357.33 | 419.86 | |
| Withdrawal device | Catalogue sent to Galway | €708.00 | €708.00 | 584.26 | 686.51 | |
| Cryosurgery applicator | Catalogue sent to Galway | 330 ml capacity applicator | €630.40 | €642.10 | 529.88 | 622.61 |
| 450 ml capacity applicator | €653.80 | |||||
| Slim probe | Catalogue sent to Galway | 1 mm | €99.40 | €99.40 | 82.03 | 96.38 |
| 2 mm | €99.40 | |||||
| 3 mm | €99.40 | |||||
| Cryogenic apron | BOC Products21 | Small | £137.28 | £163.02 | 163.02 | 191.55 |
| Medium | £154.44 | |||||
| Large | £171.60 | |||||
| Extra large | £188.76 |
The cost data that were used for calculating the cost of liquid nitrogen per treatment were retrieved from the purchases of liquid nitrogen of a single centre. The costs included the cost of the liquid nitrogen and the cost of delivery. The average cost over four purchases was calculated. Details are provided in Table 2.
| Item | Source | Price (€)a | Average (€) | Price (£)b | Price including VAT (£)c | |
|---|---|---|---|---|---|---|
| Liquid nitrogen (calculated for 25-l Dewar) | Galway invoice | Invoice 1 | 2.79/l | 65.88 | ||
| Invoice 2 | 2.79/l | |||||
| Invoice 3 | 2.48/l | |||||
| Invoice 4 | 2.48/l | |||||
| Delivery charges | Galway invoice | Invoice 1 | 38/delivery | 27.43 | ||
| Invoice 2 | 38/delivery | |||||
| Invoice 3 | 16.86/delivery | |||||
| Invoice 4 | 16.86/delivery | |||||
| Total for liquid nitrogen and delivery | 93.31 | 77.00 | 90.47 | |||
For the salicylic acid treatment, the unit costs for the medication, pads and plasters are presented in Table 3.
| Item | Source | Price including VAT (£)a |
|---|---|---|
| Verrugon 6 g | BNF 5922 | 3.00 |
| Fabric plasters | Boots the Chemist | 1.49 |
| Pads | www.nu-careproducts.co.uk/chiropody.htm#feltpads, product PPD126 | 2.30 |
Unit costs of the health-care professionals’ time
The unit costs of the health-care professional’s time were retrieved from the Unit costs of health and social care 200918 document published by the Personal Social Services Research Unit (PSSRU) of Kent University. Unit costs for health-care professionals with the lowest qualifications were chosen. These are presented in Table 4.
| Health-care professional | Source | Unit of measurement | Unit cost (£) | Used for |
|---|---|---|---|---|
| Nurse (GP practice) | PSSRU | Per hour (minute) in clinic | 28.00 (0.47) | Administration of cryotherapy/salicylic acid |
| Nurse (GP practice) | PSSRU | Per surgery consultation | 10 | Additional nurse visits |
| GP | PSSRU | Per surgery/clinic minute | 2.70 | Administration of cryotherapy/salicylic acid |
| GP | PSSRU | Per surgery consultation lasting 11.7 minutes | 31 | Additional GP visits |
| Community chiropodist/podiatrist | PSSRU | Per clinic visit | 11.00 | Administration of cryotherapy/salicylic acid |
Data analysis
The analysis of data was mainly dictated by the level of missing data for the primary outcome. The base-case analysis was conducted as a ‘complete case analysis’, where only patients with available primary outcome data were included. Where resource use data were missing, mean values were imputed based on the response group of the patients.
An additional analysis was conducted by including all the patients and performing multiple imputations on both the primary outcome and the missing total costs.
For both analyses, the mean differences in costs and effects and the 95% CIs around those were calculated by using bias-corrected and -accelerated (BCA) bootstrap methods. For the mean difference in costs, a linear regression was used, whereas logistic regression was used for the difference in primary outcome, given the binary nature of the data.
All the analyses were conducted using Stata statistic and data analysis software, version 10.1.
Cost-effectiveness analysis
The cost-effectiveness of cryotherapy versus salicylic acid was assessed by comparing the incremental costs between the two arms of the trial with the incremental benefit, which is expressed as the difference in the proportion of patients with completely cleared verrucae at 12 weeks.
When two options are compared, one is said to ‘dominate’ the other, and thereby is considered to be the more cost-effective option, if it is associated with a mean cost saving (a negative incremental cost) and positive mean incremental effect. Where one intervention does not dominate the other it is usual practice to calculate the incremental cost-effectiveness ratio (ICER) associated with each intervention group, relative to the next best alternative.
The ICER was calculated by dividing the mean incremental cost (ΔC) by the mean incremental effect (ΔE) (ICER = ΔC/ΔE), where E is the difference in effectiveness and C is the cost. Subsequently, the decision-makers can assess whether or not the additional benefit is worth the additional cost. Hence, a treatment strategy can be considered cost-effective only if the decision-maker’s willingness to pay for an additional unit of outcome, i.e. the cost per additional patient cured at 12 weeks, is greater than (or equal to) the ICER. Cost-effectiveness acceptances curves (CEACs) were plotted. CEACs express the probability that a treatment is more cost-effective than its comparator for different thresholds the decision-makers may be willing to pay for an additional unit of outcome.
Chapter 3 Protocol changes
Inclusion and exclusion criteria
This study was funded via the NIHR HTA’s ‘medicines for children’ call, so the initial inclusion criteria focused on participants aged between 12 and 24 years of age. However, owing to poor recruitment, the possibility of opening up the inclusion criteria to participants over the age of 24 years was considered. The study investigators, TSC and Data Monitoring and Ethics Committee (DMEC) could see no reason why participants over the age of 24 years should not be included in the study. It was felt that including these patients would improve the generalisability of the study’s findings, making the results of the study of greater interest to health-care practitioners. Therefore, it was decided to include patients over the age of 24 years, and although there was no known reason why results from participants from older patients should not be applicable to younger patients, it was decided to undertake an analysis looking for an interaction with age.
Following advice from the TSC (20 September 2006 and 19 July 2007), it was decided to exclude the following patients from the study in order to enhance patient safety:
-
patients who were currently on renal dialysis
-
patients who had cold intolerance, for example Raynaud syndrome or cold urticaria
-
patients who had any of the following conditions: blood dyscrasias of unknown origin; cryoglobulinaemia; cryofibrinogenaemia; collagen and autoimmune disease
-
patients who were immunosuppressed, for example had agammaglobulinaemia or were currently taking immunosuppressant drugs such as oral corticosteroids
-
patients with neuropathy.
Treatment regimens
In order to increase the generalisability of the study’s results it was decided that debridement prior to treatment with cryotherapy was no longer a requirement, but could be performed if it was the site’s usual practice. Following advice from the TSC (20 September 2006), further clarifications to the cryotherapy regimen and the treatment of patients with more than one verruca were made (see Chapter 2, Cyrotherapy using liquid nitrogen delivered by the health-care professional).
Clarification of secondary outcomes and analysis
Following advice from the TSC (20 September 2006) it was decided to clarify the secondary outcomes, the adverse event reporting procedure and the economic analysis plan, and it was decided that the influence of prognostic variables on the primary outcome should be investigated.
Questionnaire response rates
The response rate to the 12-week questionnaire was initially lower than anticipated. Results of a systematic review23 identified the use of financial incentives as a means of increasing response rates to postal questionnaires. The YTU had also identified that participant questionnaire return rates in previous NIHR HTA trials24 could be improved if participants were sent an unconditional £5 as a token ‘thank you’ reimbursement at the end of the trial.
We therefore applied to the regulatory authorities for permission to send participants £5 or €5 with their week-12 questionnaire, i.e. the primary outcome data point. This was not mentioned in the patient information sheet, so that any possibility that it would be interpreted as a financial incentive to taking part in the trial was minimised. The week-12 questionnaire was preceded by a letter notifying the participant that their week-12 questionnaire was due to arrive shortly. This letter also stated that the questionnaire would be accompanied by a £5 (or €5) note as a thank you for their taking part in the trial and completing the questionnaires.
Recruitment
The original proposal contained five recruiting sites that planned to recruit three participants per month over an 18-month period. As the trial progressed, recruitment fell below expected levels despite the recruitment of extra study sites. Details regarding the recruitment of each site can be found in Appendix 1. An extension in time and funding was obtained from the funder and the recruitment period was extended to 39 months (November 2006 to January 2010).
In order to increase the number of eligible patients presenting to the recruiting sites, a variety of recruitment strategies were introduced. Details regarding the recruitment strategies can be found in Appendix 5.
Chapter 4 Clinical results section
Trial recruitment
Over the course of the trial there was a total of 16 participating sites: 15 in the UK and one in Ireland. These were the podiatry schools at the University of Northampton, the University of Huddersfield, the University of Brighton (at Leaf Hospital, Eastbourne), Glasgow Caledonian University (at Southern General Hospital) and the National University of Ireland, Galway (NUI Galway); Brownlow Group Practice, Liverpool; Springfield Surgery, Bingley; Sheffield PCT podiatry clinic; Sacriston Surgery, Sacriston; The Haven Surgery, Burnhope; Peaseway Medical Centre, Newton Aycliffe; Arlington Road Medical Practice, Eastbourne; Claughton Medical Centre, Birkenhead; Harbinson House Surgery, Sedgefield; Annfield Plain Surgery, Stanley; and Islington PCT podiatry service.
Recruitment of at least one trial participant took place in 14 out of the 16 sites. Recruitment was staggered, with sites joining and leaving the trial over its course. The two sites that did not recruit any patients were Annfield Plain Surgery, because of the short time period between the site initiation visit and the end of the recruitment period, and Islington PCT podiatry services, which withdrew from the study before it had recruited any patients.
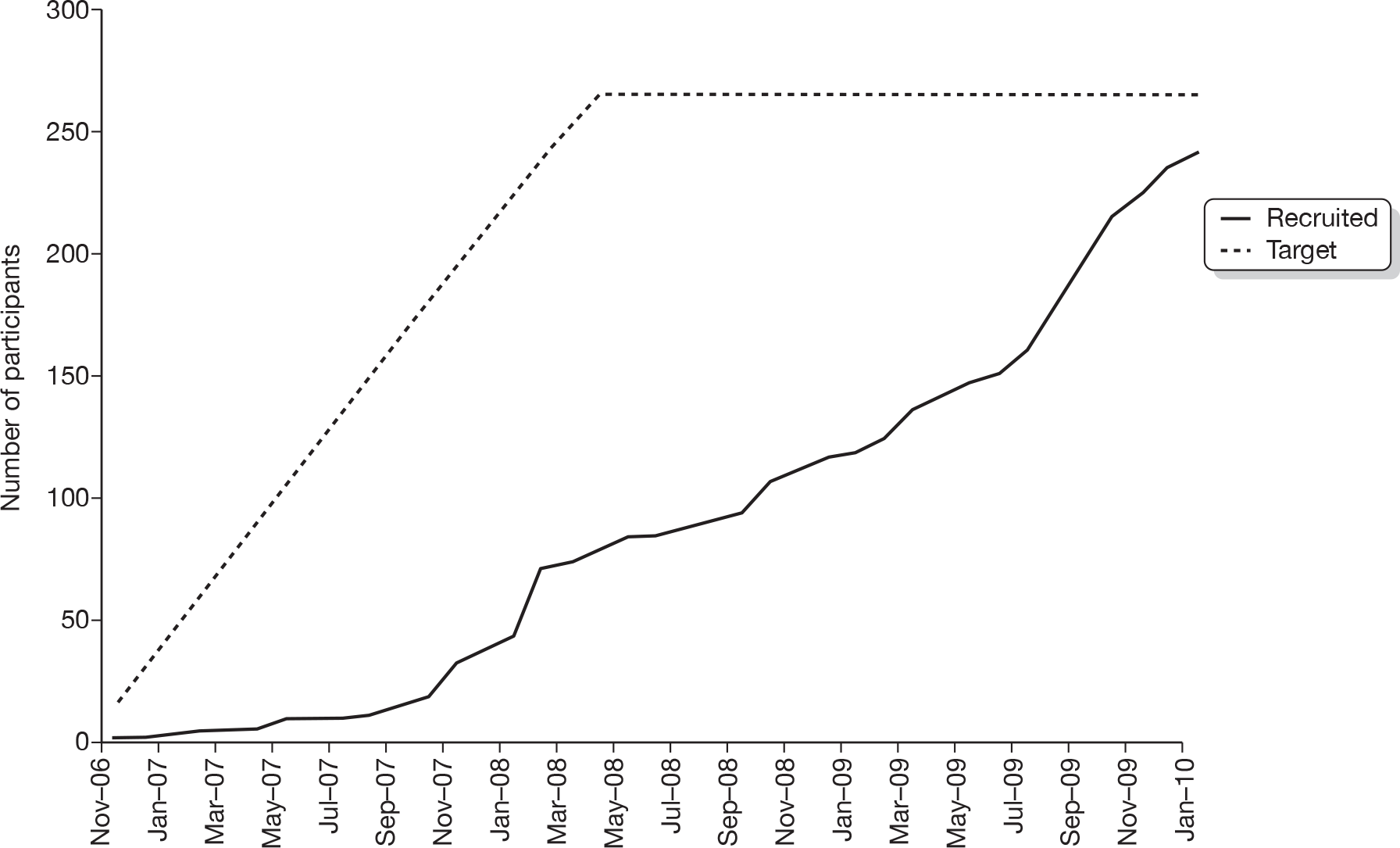
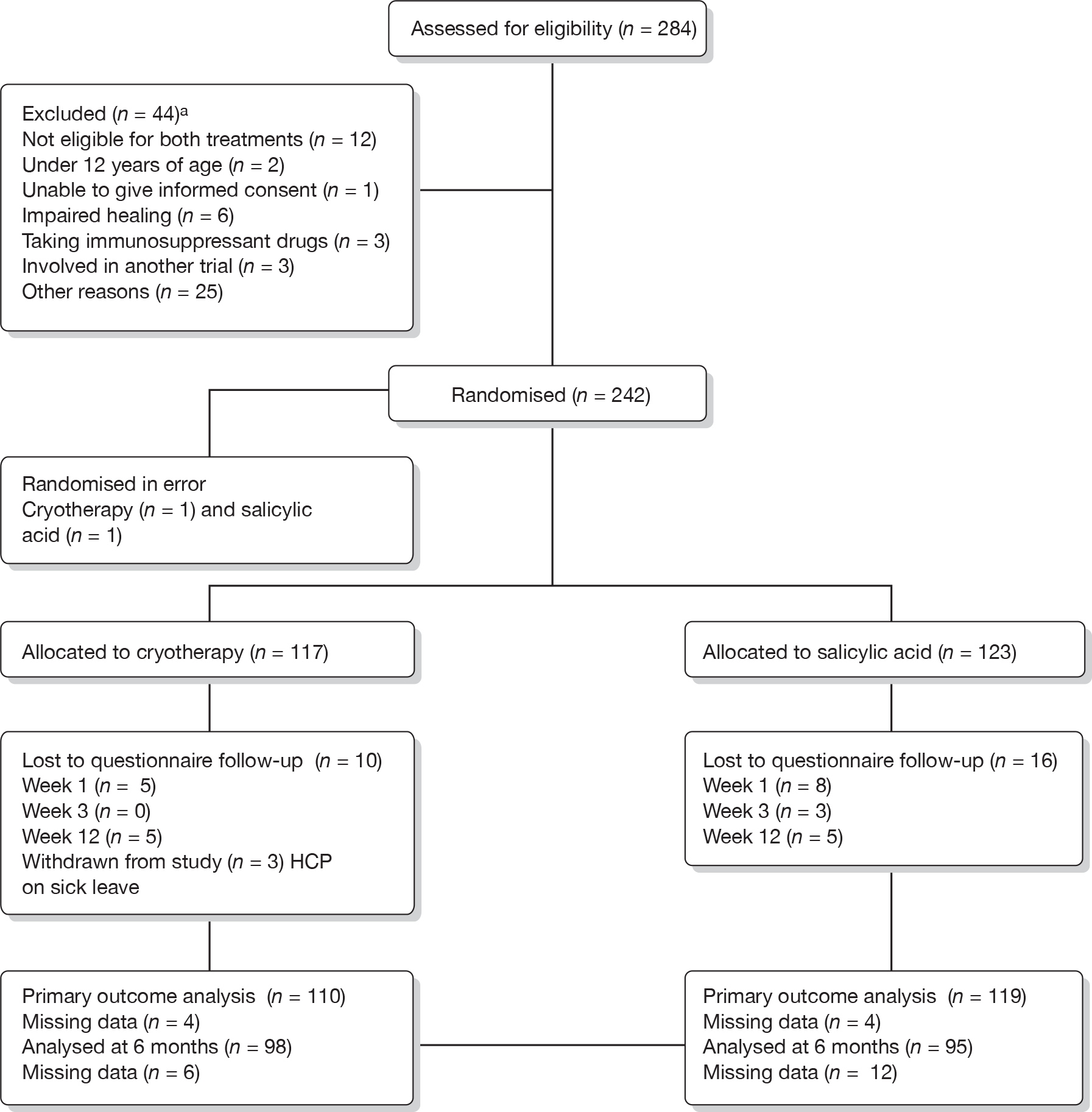
Recruitment began in November 2006 and ceased in January 2010. In total, 284 individuals were screened as potential participants and, of these, 242 (85.2%) were randomised. The overall rate of recruitment is shown in Figure 1. The number of participants recruited per site ranged from 2 to 58 (Table 5). Figure 2 shows the CONSORT flow chart of participants through the trial. Two ineligible participants with hand warts rather than verrucae on their feet were randomised in error, one to each treatment group. These two patients have been excluded from all tables, figures, summaries and analyses (for exceptions, see Table 5 and Figures 1 and 2).
FIGURE 1.
Trial recruitment rate.
| Site | Cryotherapy (N = 118) | Salicylic acid (N = 124) | Total (N = 242) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| University of Northampton | 25 (21.2) | 33 (26.6) | 58 (24.0) |
| University of Huddersfield | 24 (20.3) | 21 (16.9) | 45 (18.6) |
| Glasgow Caledonian University | 15 (12.7) | 21 (16.9) | 36 (14.9) |
| Arlington Road Medical Practice | 14 (11.9) | 5 (4.0) | 19 (7.9) |
| Brownlow Group Practice | 8 (6.8) | 9 (7.3) | 17 (7.0) |
| NUI Galway | 5 (4.2) | 8 (6.5) | 13 (5.4) |
| Sacriston Surgery | 6 (5.1) | 7 (5.6) | 13 (5.4) |
| University of Brighton | 6 (5.1) | 7 (5.6) | 13 (5.4) |
| Sheffield PCT | 4 (3.4) | 5 (4.0) | 9 (3.7) |
| Claughton Medical Centre | 3 (2.5) | 3 (2.4) | 6 (2.5) |
| Peaseway Medical Centre | 4 (3.4) | 1 (0.8) | 5 (2.1) |
| Harbinson House Surgery | 2 (1.7) | 2 (1.6) | 4 (1.7) |
| Springfield Surgery | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| The Haven Surgery | 1 (0.8) | 1 (0.8) | 2 (0.8) |
FIGURE 2.
EVerT CONSORT diagram. a, More than one category could be checked for each patient. HCP, health-care professional.
Baseline participant characteristics
In total, 240 eligible participants were recruited to the study: 117 in the cryotherapy group and 123 in the salicylic acid group. We received a completed baseline questionnaire for 237 participants (114 and 123 individuals in the cryotherapy group and salicylic acid groups, respectively). Three patients did not return their baseline questionnaires. The baseline characteristics are summarised by treatment group in Tables 6 and 7. Data collected on participants’ previous verrucae are summarised by treatment group in Table 8.
| Characteristics | Cryotherapy (N = 114) | Salicylic acid (N = 123) |
|---|---|---|
| Gender | ||
| Female n (%) | 84 (73.7) | 73 (59.3) |
| Male n (%) | 30 (26.3) | 50 (40.7) |
| Age (years) | ||
| Mean (SD) | 30.1 (15.7) | 30.2 (16.4) |
| Median (minimum, maximum) | 24.3 (12.2, 75.3) | 23.2 (12.0, 70.6) |
| Characteristics | Cryotherapy | Salicylic acid |
|---|---|---|
| No. of verrucae at baselinea | ||
| N | 106 | 119 |
| Mean (SD) | 4.0 (6.6) | 3.4 (3.6) |
| Median (minimum, maximum) | 2.0 (1.0, 55.0) | 2.0 (1.0, 20.0) |
| Duration of verrucae in months | ||
| N | 108 | 119 |
| Mean (SD) | 25.0 (24.9) | 26.9 (24.5) |
| Median (minimum, maximum) | 14.5 (0.9, 144.0) | 20 (1.5, 130.0) |
| Type of verrucae | ||
| Mosaic, n (%) | 29 (26.9) | 21 (17.5) |
| Non-mosaic, n (%) | 79 (73.1) | 99 (82.5) |
| Previous treatment | ||
| Yes, n (%) | 89 (78.1) | 96 (78.0) |
| No, n (%) | 25 (21.9) | 27 (22.0) |
| Type of previous treatmenta | ||
| N | 89 | 96 |
| Self-treatment, n (%) | 81 (91.0) | 82 (85.4) |
| Podiatrist/chiropodist, n (%) | 24 (27.0) | 29 (30.2) |
| GP, n (%) | 30 (33.7) | 43 (44.8) |
| Trial investigating verruca treatments, n (%) | 0 (0.0) | 2 (2.1) |
| Other, n (%) | 6 (6.7) | 8 (8.3) |
| Reasons for seeking verruca treatmentb | ||
| N | 114 | 123 |
| Painful, n (%) | 68 (59.6) | 71 (57.7) |
| Unable to go swimming, n (%) | 29 (25.4) | 41 (33.3) |
| Unable to participate in other sports, n (%) | 18 (15.8) | 28 (22.8) |
| Other, n (%) | 46 (40.4) | 59 (48.0) |
| Pain intensity | ||
| Not at all, n (%) | 44 (38.9) | 44 (36.1) |
| A little bit, n (%) | 37 (32.7) | 34 (27.9) |
| Moderately, n (%) | 20 (17.7) | 22 (18.0) |
| Quite a lot, n (%) | 10 (8.8) | 18 (14.8) |
| Extremely, n (%) | 2 (1.8) | 4 (3.3) |
| Characteristics | Cryotherapy | Salicylic acid |
|---|---|---|
| Previous verrucae | ||
| Yes, n (%) | 72 (63.7) | 81 (65.9) |
| No, n (%) | 34 (30.1) | 36 (29.3) |
| Don’t know, n (%) | 7 (6.2) | 6 (4.9) |
| No. of previous verrucae | ||
| N | 69 | 76 |
| Mean (SD) | 3.7 (3.5) | 4.0 (4.01) |
| Median (minimum, maximum) | 2.0 (1, 15) | 2.0 (1, 25) |
| Age at which previous verrucae occurred (years) | ||
| N | 72 | 77 |
| Mean (SD) | 18.9 (14.9) | 17.9 (12.8) |
| Median (minimum, maximum) | 13.5 (7.0, 74.0) | 13.0 (5.0, 60.0) |
The majority of patients in the study were female (n = 157, 66%) and the median age of patients was 24 years, with the youngest person in the study being 12.0 and the oldest person being 75.3 years. The majority (n = 185, 78%) of participants had received previous treatment for their verrucae. In most cases this included self-treatment using an over-the-counter (OTC) preparation. Preparations previously used included salicylic acid preparations (Bazuka Gel, Bazuka Extra-Strength Gel, Verrugon, Salactol and Boots own-brand gel) and cryotherapy self-treatments (Wartner, Scholl Freeze and Bazuka Sub-Zero). In both groups, a small number of individuals (six in the cryotherapy arm and eight in the salicylic acid arm) reported they had tried other treatments, including tea tree oil (four participants).
Just under 60% (n = 139) of participants reported that they were seeking treatment for their verrucae because it was painful; however, when patients were asked how painful their verrucae were, only 34 (14%) reported that they were in quite a lot or extreme pain. A large number of individuals reported ‘other’ reasons for seeking treatment as a free-text comment. The most frequently reported reasons included the unfavourable appearance of the verrucae, risk of infecting other individuals, the verrucae were annoying or embarrassing, the verrucae had been present for a long time and that the participants just wished to get rid of them. A large number of participants previously had verrucae and the median number of previous verrucae was two, occurring at 5–74 years of age.
In general, the two groups were well balanced at baseline; however, there were slight imbalances in gender and the type of verrucae. The proportion of women was greater in the cryotherapy group than in the salicylic acid group. However, as there is no evidence that gender is a prognostic factor for verrucae clearance, this imbalance is unlikely to affect clearance outcomes and gender was not included in any analyses. The proportion of participants with a mosaic verruca was greater in the cryotherapy group than in the salicylic acid group.
Primary outcome: complete clearance of verrucae at 12 weeks
In total, 229 participants had a response for whether or not there was complete clearance of all verrucae at 12 weeks after randomisation, with 206 (90.0%) having a blinded outcome assessment: 159 (69.4%) had a blinded outcome assessment from a digital photograph with 31 photographs deemed to be of insufficient quality to allow an assessment to be undertaken (The two assessors agreed that they were unable to assess 28 photographs. However, they disagreed on a further 51 photographs. When these 51 photographs were sent to the third assessor she was unable to assess three of these photographs, making a total of 31 photographs which could not be assessed.) Forty-seven (20.5%) had a blinded outcome assessment from a health-care professional assessment; four (1.7%) had an unblinded outcome assessment from a health-care professional assessment; and 19 (8.3%) had patient self-reported data. Overall, 32 of the 229 (14.0%) had complete clearance of all verrucae at 12 weeks: 17 out of the 119 (14.3%) patients in salicylic acid group and 15 of the 110 (13.6%) patients in the cryotherapy group. We compared the proportions of participants with complete clearance of all verrucae and there was no evidence of a difference between the salicylic acid and the cryotherapy groups (14.3% vs 13.6%, difference = 0.6%, 95% CI –9.6% to 8.3%; p = 0.89).
Determination of primary outcome
Table 9 shows the data comparing the outcomes from two independent assessors for photographs from 190 patients. Of these, 106 patients were deemed by both assessors to have verrucae, although both agreed that five patients’ verrucae had all cleared. The assessors disagreed in 51 cases: Assessor 1 classified that two patients had no verrucae, whereas Assessor 2 classified these patients as having verrucae. Similarly, Assessor 2 classified that one patient had no verrucae, whereas Assessor 1 classified them as still present. There were five cases in which Assessor 1 classified the verrucae as cleared, but Assessor 2 was unable to assess whether or not the verrucae had cleared. In the remaining 43 cases, the disagreement was between ‘not cleared’ and ‘unable to assess’ classifications. To quantify the strength of this association the kappa measure of agreement was estimated as 0.45 [standard error (SE) 0.05, 95% CI 0.35 to 0.55]. This indicates a moderate level of agreement.
| Assessor 1 | Assessor 2 | Total | ||
|---|---|---|---|---|
| Cleared | Not cleared | Unable to assess | ||
| Cleared | 5 | 1 | 5 | 11 |
| Not cleared | 2 | 106 | 41 | 149 |
| Unable to assess | 0 | 2 | 28 | 30 |
| Total | 7 | 109 | 74 | 190 |
Secondary outcomes
Complete clearance of verrucae at 12 weeks adjusted analysis
The primary analysis was repeated but controlled for age, whether or not the verrucae had been previously treated (yes/no) and the type of verrucae (mosaic/non-mosaic). The results from the logistic regression highlighted that there was no evidence of a difference between the salicylic acid and the cryotherapy groups (OR 0.96, 95% CI 0.44 to 2.11; p = 0.92).
Age was categorised into three groups (< 18 years, > 18 but < 25 years, and over 25 years). There was a non-significant effect of age (< 18 years vs > 25 years, OR 0.66, 95% CI 0.22 to 1.98; and > 18 years but < 25 years vs > 25 years, OR 0.80, 95% CI 0.35 to 1.82).
After adjusting for clustering of healing rates within a centre there was still no evidence of a difference between the salicylic acid and the cryotherapy groups (OR 1.04, 95% CI 0.43 to 2.50; p = 0.92). The resultant intraclass correlation was almost zero (2.74 × 10–10; p = 1.00).
Self-reported time to clearance of verrucae
We compared the time to clearance of the verrucae between the two groups, adjusting for the same covariates as above (age, previous treatment and type of verrucae). There was no evidence of a difference in the time to clearance between the two groups when compared in the Cox proportional hazards model [hazard ratio (HR) 0.80, 95% CI 0.51 to 1.25; p = 0.33].
Clearance of verrucae at 6 months
We received data on presence/absence of verrucae at 6 months from 193 participants. Overall, 62 of the 193 (32.1%) had complete clearance of all verrucae at 6 months: 29 of the 95 (30.5%) patients in the salicylic acid group and 33 out of the 98 (33.7%) patients in the cryotherapy group. There was no evidence of a difference between the salicylic acid and the cryotherapy groups (30.5% vs 33.7%, difference = –3.1%, 95% CI –10.0% to 16.3%; p = 0.64). The findings from the adjusted analysis were similar to the unadjusted analysis (OR 1.17, 95% CI 0.62 to 2.21; p = 0.62).
Number of verrucae remaining at 12 weeks
The median number of verrucae at 12 weeks in the salicylic acid group was 2 (minimum to maximum = 0–20) and in the cryotherapy group was 1 (minimum to maximum = 0–40). There was no evidence of a difference in the number of verrucae at 12 weeks between the two groups [incidence rate ratio (IRR) 1.08, 95% CI 0.8 to 1.43; p = 0.62].
Patient’s treatment preference
Twenty-eight (11.7%) and 86 (35.8%) participants expressed a preference at baseline for salicylic acid and cryotherapy, respectively, whereas 104 (43.3%) did not have a preference and 22 people did not respond to this question. When we extended the primary analysis to include an interaction term between randomised treatment and preferred treatment we found no evidence to suggest that patients’ preferences at baseline influenced the outcome.
Missing data
We investigated the sensitivity of the results to missing data with multiple imputation analysis. There was little difference in the estimates obtained from the complete case analysis and the multiple imputation analysis. The summary of the sensitivity of results to missing data is presented in Table 10.
| Outcome | Complete cases estimate (95% CI) | Multiple imputation estimate (95% CI) |
|---|---|---|
| Clearance at 12 weeks (unadjusted) | 0.95 (0.45 to 2.00) | 1.01 (0.49 to 2.08) |
| Clearance at 12 weeks (adjusted) | 0.96 (0.44 to 2.11) | 0.99 (0.47 to 2.07) |
| Clearance at 6 months | 1.17 (0.62 to 2.21) | 1.18 (0.68 to 2.08) |
| No. of verrucae | 1.08 (0.81 to 1.43) | 1.06 (0.82 to 1.38) |
Patients’ willingness to have the same treatment allocation
There was an association between willingness to have the same allocation and treatment randomised {chi-squared test statistic 17.90 [2 degrees of freedom (df)]; p = 0.0001}. More patients were willing to have cryotherapy again and fewer patients were willing to have salicylic acid again than expected under independent association.
Additional data collected
Recurrence of verrucae at 6 months
Thirty-two patients had clearance of verrucae at 12 weeks. At 6 months, 22 of these 32 patients had reported their verrucae as gone, six had missing data and four (two patients in each group) reported that their verrucae had returned in its original place.
Patient satisfaction with treatment
Table 11 summarises patient satisfaction with treatment, reported on a five-point scale (from ‘very unhappy’ to ‘very happy’) on the participant week-1, week-3 and week-12 questionnaires.
| Questionnaire | Satisfaction with treatment | ||||
|---|---|---|---|---|---|
| Very unhappy | Unhappy | Indifferent | Happy | Very happy | |
| Week 1 | |||||
| Cryotherapy, n (%) | 6 (5.9) | 1 (1.0) | 26 (25.7) | 43 (42.6) | 25 (24.8) |
| Salicylic acid, n (%) | 1 (0.9) | 5 (4.7) | 32 (29.9) | 54 (50.5) | 15 (14.0) |
| Week 3 | |||||
| Cryotherapy, n (%) | 10 (9.6) | 0 (0.0) | 18 (17.3) | 48 (46.2) | 28 (26.9) |
| Salicylic acid, n (%) | 3.9 (4.0) | 8 (7.7) | 29 (27.9) | 46 (44.2) | 17 (16.4) |
| Week 12 | |||||
| Cryotherapy, n (%) | 7 (7.9) | 5 (5.5) | 23 (25.3) | 30 (33.0) | 26 (28.6) |
| Salicylic acid, n (%) | 10 (10.2) | 21 (21.4) | 27 (27.6) | 25 (25.5) | 15 (15.3) |
At week 1, the majority of participants in both groups were happy with their treatment, with 68 individuals (67%) and 69 individuals (65%) in the cryotherapy and salicylic acid treatment groups, respectively, answering that they were either ‘happy’ or ‘very happy’. Only a small proportion of individuals (7% in the cryotherapy group and 6% in the salicylic acid group) were unhappy (answered ‘unhappy’ or ‘very unhappy’) with their treatment. At week 3, participants in both groups reported a similar level of satisfaction with their treatment to week 1. The majority (73% in the cryotherapy group and 61% in the salicylic acid group) reported that they were happy, and only 10% and 12% in the cryotherapy and salicylic acid treatment groups, respectively, reported that they were unhappy. At week 12, once again the majority (62%) of participants in the cryotherapy group reported that they were happy with their treatment and only 13% reported that they were unhappy. However, in the salicylic acid group individuals were less happy with their treatment than they were at previous time points and compared with the cryotherapy group at the week-12 time point. Forty individuals (41%) were happy, whereas 31 individuals (32%) were unhappy.
Whether or not the participants would be willing to receive the same treatment again is summarised in Table 12.
| Response | Cryotherapy (N = 91) | Salicylic acid (N = 98) |
|---|---|---|
| Yes, n (%) | 65 (71.4) | 42 (42.9) |
| No, n (%) | 9 (9.9) | 30 (30.6) |
| Not sure, n (%) | 17 (18.7) | 26 (26.5) |
In total, 146 (77%) participants indicated at 12 weeks whether they would or would not have the same treatment again. The majority (n = 65, 71%) of cryotherapy patients reported that they would be willing to receive the same treatment again, whereas only 42 (43%) of salicylic acid patients were willing to repeat their treatment.
Pain associated with first treatment
Participants were asked to record the level of pain associated with their first treatment. This was reported on a 0–10 pain scale, where 0 represents no pain and 10 is the worst pain imaginable. The mean pain intensity associated with the first cryotherapy treatment was 3.05 (with a minimum score of 0 and a maximum score of 8), whereas the mean pain intensity associated with the first salicylic acid treatment was lower at 0.75 (with a minimum score of 0 and a maximum score of 7).
Pain associated with verrucae and use of painkillers
Pain associated with participants’ verrucae is summarised in Table 13. At weeks 1 and 3, the majority of participants in both treatment groups reported that their verrucae were not painful or a little painful. A minority reported a lot of pain (answering ‘quite a lot of pain’ or ‘extremely painful’).
| Verruca painful today? | Week 1 | Week 3 | ||
|---|---|---|---|---|
| Cryotherapy | Salicylic acid | Cryotherapy | Salicylic acid | |
| Not at all, n (%) | 43 (41.7) | 42 (39.3) | 62 (59.6) | 46 (44.7) |
| A little bit, n (%) | 35 (34.0) | 34 (31.8) | 25 (24.0) | 34 (33.0) |
| Moderately, n (%) | 16 (15.5) | 19 (17.8) | 13 (12.5) | 14 (13.6) |
| Quite a lot, n (%) | 9 (8.7) | 10 (9.3) | 3 (2.9) | 8 (7.8) |
| Extremely, n (%) | 0 (0.0) | 2 (1.9) | 1 (1.0) | 1 (1.0) |
A minority of participants found it necessary to take painkillers during the first 3 weeks of the study (n = 9), with more individuals taking painkillers in the cryotherapy group (n = 8) than in the salicylic acid group (n = 1). Those individuals who had used painkillers took them for between 1 and 4 days.
Treatment details
Table 14 summarises the cryotherapy treatment details reported by the treating health-care professional. Out of the 117 patients randomised to cryotherapy, treatment details were returned on the podiatrist treatment assessment form for 109 (93.2%) individuals and are summarised here. The mean number of visits to the clinic or GP practice for cryotherapy treatment was 3.6, with a minimum of one and a maximum of five visits. The mean duration between visits for treatment was 18.3 days, with a minimum of 9.7 days and a maximum of 52.5 days. At each treatment visit, participants received a mean of 1.6 applications of liquid nitrogen, with each application lasting a mean of 10.9 seconds. In the vast majority of cases (94.2%), the health-care professional considered that a sufficient freeze had been achieved and for only 9% of the freezes did the patient request that the freeze was stopped. The main reason for stopping the freeze was that it was painful.
| Treatment details | Cryotherapy (N = 109) |
|---|---|
| No. of visits | |
| Mean (SD) | 3.6 (0.70) |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) |
| Duration between visits (days)a | |
| Mean (SD) | 18.3 (6.8) |
| Median (minimum, maximum) | 15.5 (9.7, 52.5) |
| No. of times applied | |
| Mean (SD) | 1.6 (0.7) |
| Median (minimum, maximum) | 1.5 (0.3, 4.3) |
| Duration of each application (seconds) | |
| Mean (SD) | 9.5 (8.6) |
| Median (minimum, maximum) | 9.5 (2.0, 60.0) |
| Sufficient freezing took place (%) | 94.2 |
| Patients asked to stop the freeze (%) | 9.0 |
Table 15 summarises the data collected on adherence for the salicylic acid treatment group. The majority (76%) of individuals received one tube of salicylic acid during the trial, and a mean of 2.8 g (SD 2.2 g) of ointment from each tube was used during the treatment period. Self-reported adherence was reasonably high, with participants applying salicylic acid on a mean of 6.3 days and 5.4 days out of 7 days at weeks 1 and 3, respectively.
| Treatment details | Salicylic acid |
|---|---|
| No. of tubes dispensed | |
| N | 108 |
| One tube, n (%) | 82 (75.9) |
| Two tubes, n (%) | 26 (24.1) |
| Weight of salicylic acid used (g) | |
| N | 58 |
| Mean (SD) | 2.8 (2.2) |
| Median (minimum, maximum) | 2.4 (0.06, 9.3) |
| No. of times salicylic acid applied in the last 7 days | |
| Week 1 | |
| N | 106 |
| Mean (SD) | 6.3 (1.5) |
| Median (minimum, maximum) | 7.0 (0.0, 7.0) |
| Week 3 | |
| N | 103 |
| Mean (SD) | 5.4 (2.8) |
| Median (minimum, maximum) | 6.0 (0.0, 22.0) |
Adverse events
In total, 19 participants reported 28 adverse events. Of these 28 events, one was classed as serious and unrelated to the treatment (salicylic acid). Of the remaining 27 events, 13 were in the salicylic acid group and 14 were in the cryotherapy group. The relationship between the non-serious adverse events and treatment group is reported in Table 16. There were two treatment-related non-serious adverse events, both of which were in the cryotherapy group. Both patients developed a blister that was larger in size than expected in routine practice.
| Relationship | Salicylic acid | Cryotherapy | Total (%) |
|---|---|---|---|
| Unrelated | 9 | 7 | 16 |
| Unlikely to be related | 4 | 5 | 9 |
| Possibly related | 0 | 0 | 0 |
| Probably related | 0 | 0 | 0 |
| Definitely related | 0 | 2 | 2 |
| Unable to assess if related | 0 | 0 | 0 |
| Total | 13 | 14 | 27 |
Reasons for stopping treatment and any new treatments
Table 17 summarises the number of participants who found it necessary to stop their allocated treatment.
| Cryotherapy | Salicylic acid | |
|---|---|---|
| Necessary to stop the original treatment? | ||
| Yes (%) | 5 (5.6) | 16 (17.0) |
| No (%) | 84 (94.3) | 78 (83.0) |
| If yes, was another treatment started? | ||
| Yes (%) | 1 (25.0) | 2 (12.5) |
| No (%) | 3 (75.0) | 14 (87.5) |
There was a low incidence of participants stopping their original treatment. Twenty-one participants (11.5%) reported stopping their original treatment. Of these, 16 participants were in the salicylic acid group and five in the cryotherapy group. The reasons for stopping treatment are summarised in Table 18.
| Reason | Cryotherapy group | Salicylic acid group |
|---|---|---|
| Pain | 2 | 7 |
| Poor condition of the participant’s skin | 0 | 5 |
| Blistering | 2 | 0 |
| Ran out of plasters | 0 | 1 |
| Participant believed verruca had gone | 0 | 4a |
Of the participants who reported stopping their original treatment, three (15%) reported starting another treatment. The cryotherapy patient started salicylic acid treatment and one of the salicylic acid patients continued their treatment with salicylic acid after a temporary stop. The other salicylic acid patient did not state which treatment they started.
Chapter 5 Economic analysis
This chapter presents the results of the economic analysis of the EVerT trial. We have undertaken several analyses to assess whether or not costing assumptions and missing data could have affected the results.
Summary of the resource usage
Data on resource usage were collected for the treatment visits, any additional contact with GPs or nurses, and emergency visits to the GP, as well as items related to the medication for both groups. The average resource usage on the EVerT study is reported in Table 19.
| Item | Cryotherapy | Salicylic acid |
|---|---|---|
| Average no. of treatment visits | ||
| n | 109 | 120 |
| Mean (SE) | 3.59 (0.072) | 1.94 (0.38) |
| SD | 0.75 | 0.42 |
| Median (minimum, maximum) | 4 (1, 5) | 2 (1, 4) |
| Missing (%) | 8 (7%) | 3 (2%) |
| Average no. of additional GP visits | ||
| n | 82 | 89 |
| Mean (SE) | 0.04 (0.03) | 0.01 (0.01) |
| SD | 0.25 | 0.11 |
| Median (minimum, maximum) | 0 (0, 2) | 0 (0, 1) |
| Missing (%) | 35 (30%) | 34 (28%) |
| Average no. of additional nurse visits | ||
| n | 82 | 89 |
| Mean (SE) | 0.05 (0.03) | 0.08 (0.04) |
| SD | 0.27 | 0.34 |
| Median (minimum, maximum) | 0 (0, 2) | 0 (0, 2) |
| Missing (%) | 35 (30%) | 34 (28%) |
| Average no. of tubes of Verrugon | ||
| n | NA | 116 |
| Mean (SE) | NA | 1.25 (0.04) |
| SD | NA | 0.44 |
| Median (minimum, maximum) | NA | 1 (1, 2) |
| Missing (%) | NA | 7 (6%) |
| Average no. of cryotherapy treatments given to patients | ||
| n | 109 | NA |
| Mean (SE) | 3.49 (0.08) | NA |
| SD | 0.80 | NA |
| Median (minimum, maximum) | 4 (1, 5) | NA |
| Missing (%) | 8 (7%) | NA |
During the trial, participants in the cryotherapy arm had a mean of 3.59 visits to the GP, nurse or podiatrist for treatment. The salicylic acid arm participants had a mean of 1.94 visits. Only a small number of patients (three in each group) had extra visits to the GP, in addition to the planned treatment visits. Participants in the cryotherapy arm had a mean of 0.04 additional visits to the GP, whereas those in the salicylic acid arm had a mean of 0.01 additional visits. Eight patients from both groups had additional visits to a nurse. This resulted in a mean number of additional nurse visits of 0.05 for the patients undergoing cryotherapy and 0.08 for the salicylic acid group. Salicylic acid patients received a mean of 1.25 tubes of Verrugon, whereas cryotherapy patients received a mean of 3.49 treatments.
Two emergency visits were reported, one in each group. For the salicylic acid patient, the comments referred to an event that happened before the randomisation date. The cryotherapy patient did see the GP, but, after reviewing the trial co-ordinator’s notes for this patient, it was concluded that this visit was already reported as an additional visit to the GP.
Missing data on resource use and outcome
There was a significant level of missing data on resource usage relating to additional GP or nurse visits: 30% and 28% for the cryotherapy and salicylic acid groups, respectively. The level of missing data for treatment visits, number of tubes of Verrugon and cryotherapy applications was much lower, ranging from 2% to 7%.
The missing items were a result of either the trial participants not returning the questionnaire or not completing the relevant questions on the questionnaire. Missing data on number of treatment visits was because of missing podiatrist treatment assessment forms. The level of missing data was not related to the treatment allocation as demonstrated by a chi-squared test.
Table 20 presents details on the missing data for various resource usage items.
| Resource use or outcome data item | Missing response, n (%) | Treatment arm impact on level of missing data (Pearson chi-squared, p-value) | |
|---|---|---|---|
| Cryotherapy (n = 117) | Salicylic acid (n = 123) | ||
| Additional visits to GP or nurse | 35 (30) | 34 (28) | (0.1511, p = 0.697) |
| Treatment visits | 8 (7) | 3 (2) | (2.6528, p = 0.103) |
| No. of tubes of Verrugon | NA | 7 (6) | NA |
| No. of cryotherapy treatments | 8 (7) | NA | NA |
| Primary outcome | 7 (6) | 4 (3) | (0.1345, p = 0.714) |
Table 20 supports the notion that the amount of missing data is not related to group allocation, which reduces the risk of bias. In the following analyses we adjusted for missing data through multiple imputation methods.
Data analysis
Given the level of missing data for both the primary outcome and the resource use items, the analysis of data was based on two scenarios as will be described below.
Scenario 1: complete case analysis based on the primary outcome data
For the first scenario, only the patients who had primary outcome data were considered. Mean values were imputed for the missing resource usage items (i.e. treatments visits, additional visits to the GP or nurse, number of cryotherapy treatments, number of tubes of Verrugon). For the visits, the mean imputation was performed based on the outcome group (i.e. verrucae gone or not gone) and the treatment allocation. For the cryotherapy treatments and the number of Verrugon tubes, the means were imputed based on the outcome group only.
The total costs were calculated by summing up the cost of treatment visits, additional GP or nurse visits and the medication costs, i.e. the cost of cryotherapy equipment and liquid nitrogen per patient treatment for the cryotherapy group and the cost of Verrugon, pads and plasters for the salicylic acid group. Table 21 presents the costs by items of resource usage. The majority of costs for both groups was owing to the cost of treatment visits, with the average cost per patient being larger in the cryotherapy group than in the salicylic acid group (£88.69 vs £39.59). The second largest cost for the cryotherapy group was the cost of treatment, which included the cost of equipment and liquid nitrogen. The average cost of the cryotherapy treatment per patient was £60.05.
| Item | Treatment group | Mean cost (£) | 95% CI |
|---|---|---|---|
| Treatment visits (health-care professional’s time) | Salicylic acid | 39.59 | 33.26 to 45.92 |
| Cryotherapy | 88.69 | 74.70 to 102.68 | |
| Verrugon (including pads and plasters) | Salicylic acid | 8.50 | 7.97 to 9.03 |
| Cryotherapy cost (liquid nitrogen and equipment cost) | Cryotherapy | 60.05 | 57.57 to 62.53 |
| Additional visit to GP | Salicylic acid | 0.35 | –0.17 to 0.86 |
| Cryotherapy | 1.15 | –0.09 to 2.38 | |
| Additional visit to nurse | Salicylic acid | 0.78 | 0.24 to 1.31 |
| Cryotherapy | 0.49 | 0.06 to 0.93 | |
| Total costs | Salicylic acid | 49.22 | 42.39 to 56.04 |
| Cryotherapy | 150.39 | 135.65 to 165.13 |
To avoid any distributional assumptions on the cost and outcome data, the BCA 95% CIs around the mean difference in costs and outcomes were calculated by the bootstrapping method. The mean differences in costs and the proportion of patients with cleared verrucae were calculated based on linear regression for the former and logistic regression for the latter. Two types of analyses were conducted: first, based on unadjusted costs and outcomes and, second, by adjusting them based on the age of the participants, whether or not they had received previous treatment and the type of verrucae.
Unadjusted costs and outcomes
The results of the base case unadjusted analysis demonstrate that there is a significant difference in the total cost per patient between the two arms of the study, with cryotherapy costing on average £101.17 more per patient. The treatment effect for cryotherapy is smaller than that for salicylic acid, although statistically non-significant. The mean total costs and outcomes based on data after imputation are presented in Table 22, whereas the mean difference in costs and outcomes and the corresponding 95% CI are presented in Table 23.
| Variable | Treatment | n | Mean cost/outcome | SE |
|---|---|---|---|---|
| Costs (£) | Salicylic acid | 119 | 49.22 | 3.46 |
| Cryotherapy | 110 | 150.39 | 7.48 | |
| Difference = 101.17; p < 0.001 | ||||
| Outcomes | Salicylic acid | 119 | 0.143 (17 patients) | 0.032 |
| Cryotherapy | 110 | 0.136 (15 patients) | 0.033 | |
| Difference = –0.006; p = 0.89 | ||||
| Variable | Mean difference | 95% CI | ICER |
|---|---|---|---|
| Costs (£) | 101.17 | 85.09 to 117.26 | Cryotherapy is dominated |
| Outcomes | –0.0065 | –0.10 to 0.08 |
Adjusted costs and outcomes
The adjusted results lead to the same conclusion as the unadjusted results, i.e. cryotherapy is more costly and less effective (not statistically significant) than salicylic acid treatment. Table 24 presents the mean difference in adjusted costs and outcomes.
| Variable | Mean difference | 95% CI | ICER |
|---|---|---|---|
| Costs (£) | 101.21 | 84.18 to 118.25 | Cryotherapy is dominated |
| Outcomes | –0.00336 | –0.09 to 0.08 |
Cost-effectiveness and uncertainty
As demonstrated in Tables 23 and 24, cryotherapy is more costly and non-significantly less effective. The bootstrapped pairs of difference in costs and outcomes for both adjusted and unadjusted results are presented on a cost-effectiveness plane (CE plane) (see Figure 3) to visually demonstrate the above conclusions. As evident from the CE plane, almost 50% of the bootstrapped replicates falls either side of the line that goes through the zero difference in outcomes point (x-axis). This is indicative of high uncertainty around the difference in effectiveness of the two treatments. In contrast, all of the cost replicates are above the zero line of the y-axis, i.e. no difference in costs. Figure 3 presents the CE plane for both unadjusted and adjusted results.
FIGURE 3.
Cost-effectiveness plane for unadjusted and adjusted costs and outcomes.
Figure 4 presents the CEACs. This demonstrates the probability of the cryotherapy being cost-effective given a specific willingness-to-pay value per ‘cured’ patient. The adjusted and unadjusted data give the same results. In both cases, the probability that cryotherapy is cost-effective is around 40% at a threshold value of £15,000 per cured patient.
FIGURE 4.
Cost-effectiveness acceptability curves (adjusted and unadjusted data).
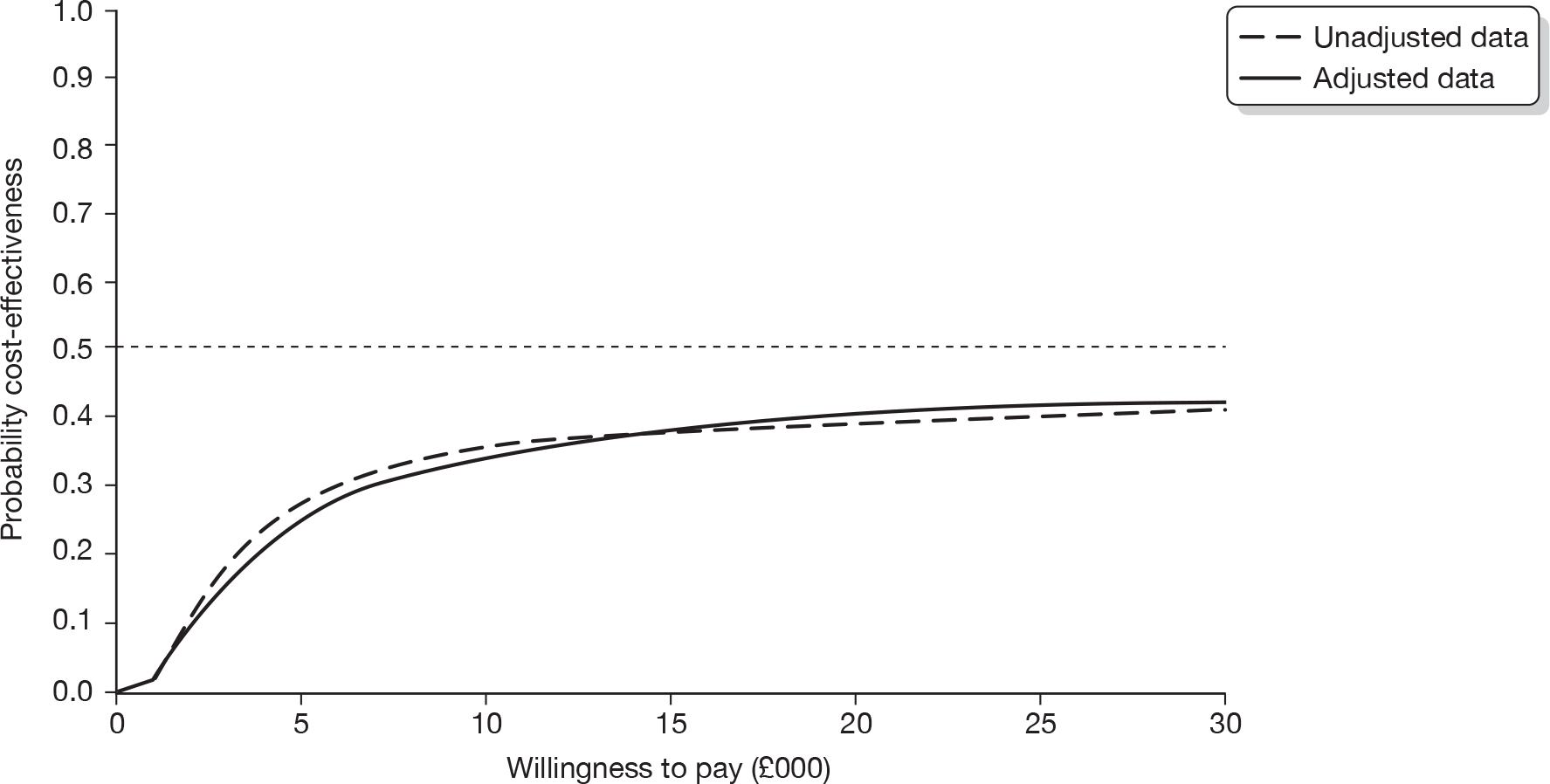
Sensitivity analysis based on the data analysis of scenario 1 (complete case for primary outcome data)
The majority of costs for the cryotherapy group are owing to treatment visits (i.e. health-care professional’s time) and the cost of the treatment itself, i.e. the cost of the equipment and liquid nitrogen. A sensitivity analysis was carried out by adopting an extreme approach whereby the administration of the treatment was assumed to be carried out by a nurse (rather than a GP) in those study sites that were set up in GP practices and by excluding completely the cost of cryotherapy equipment and liquid nitrogen. In effect, this analysis would result in comparing both treatments based on the treatment visits only rather than including the cost of medication as well. Table 25 presents the results of the sensitivity analysis. Cryotherapy is again more costly than salicylic acid treatment. This is because of the greater number of treatment visits that the patients made, even though the cost of the health-care professional who administered the cryotherapy was lower (nurse vs GP).
| Variable | Mean difference | 95% CI | ICER |
|---|---|---|---|
| Unadjusted | |||
| Costs (£) | 9.18 | 7.09 to 11.26 | Cryotherapy is dominated |
| Outcome | –0.0065 | –0.10 to 0.08 | |
| Adjusted | |||
| Costs (£) | 9.17 | 7.00 to 11.33 | Cryotherapy is dominated |
| Outcomes | –0.00336 | –0.09 to 0.08 | |
Figures 5 and 6 present the CE planes and CEACs for both unadjusted and adjusted results of the sensitivity analysis. Cryotherapy remains more costly and all of the bootstrapped replicates of difference in costs are above the zero line, although outcome results do not change. The smaller difference in costs between the two treatments (compared with the base-case analysis) results in cryotherapy having an approximately 40% probability of being cost-effective at a lower (than the base-case analysis) threshold.
FIGURE 5.
Cost-effectiveness planes for unadjusted and adjusted costs and outcomes.
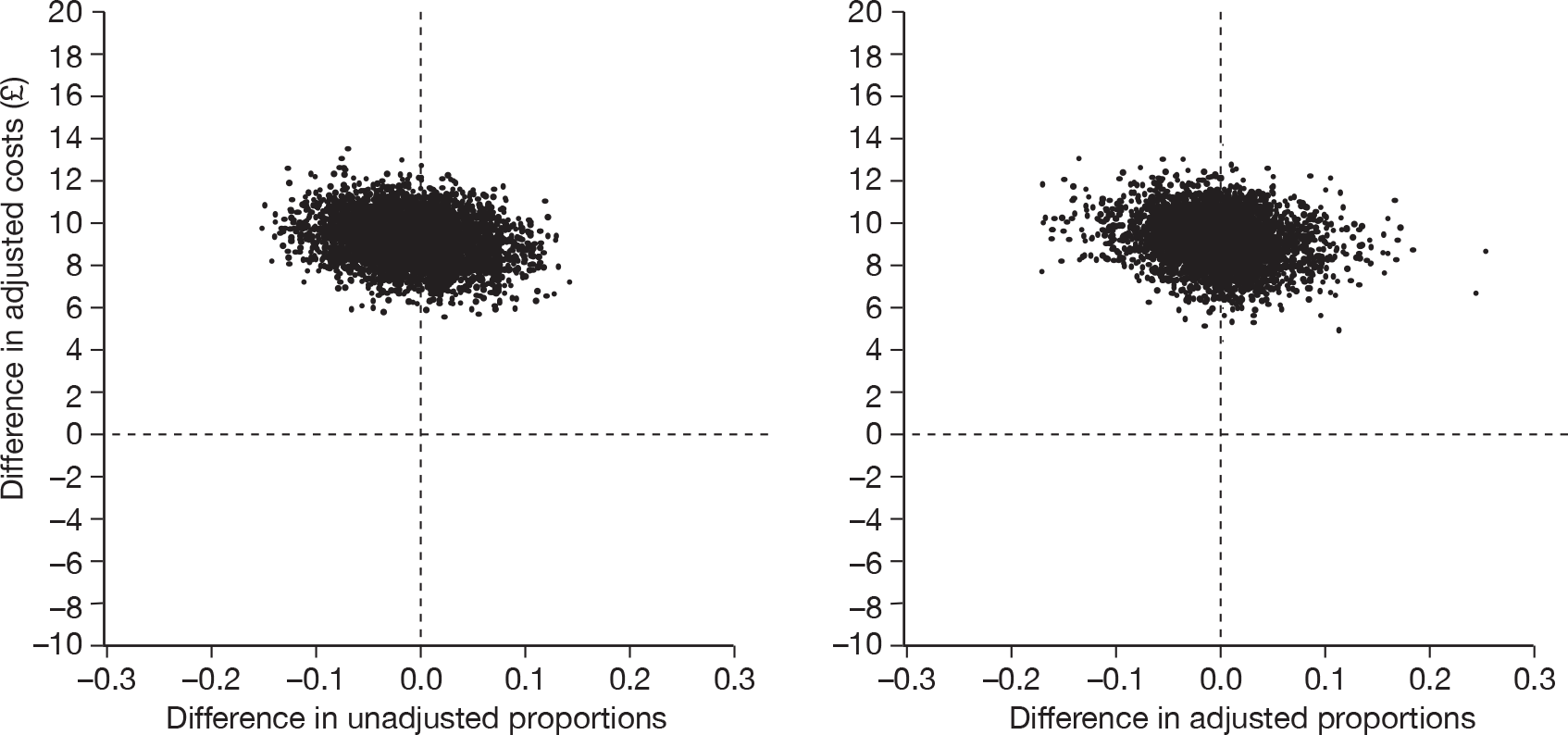
FIGURE 6.
Cost-effectiveness acceptability curve for the sensitivity analysis.
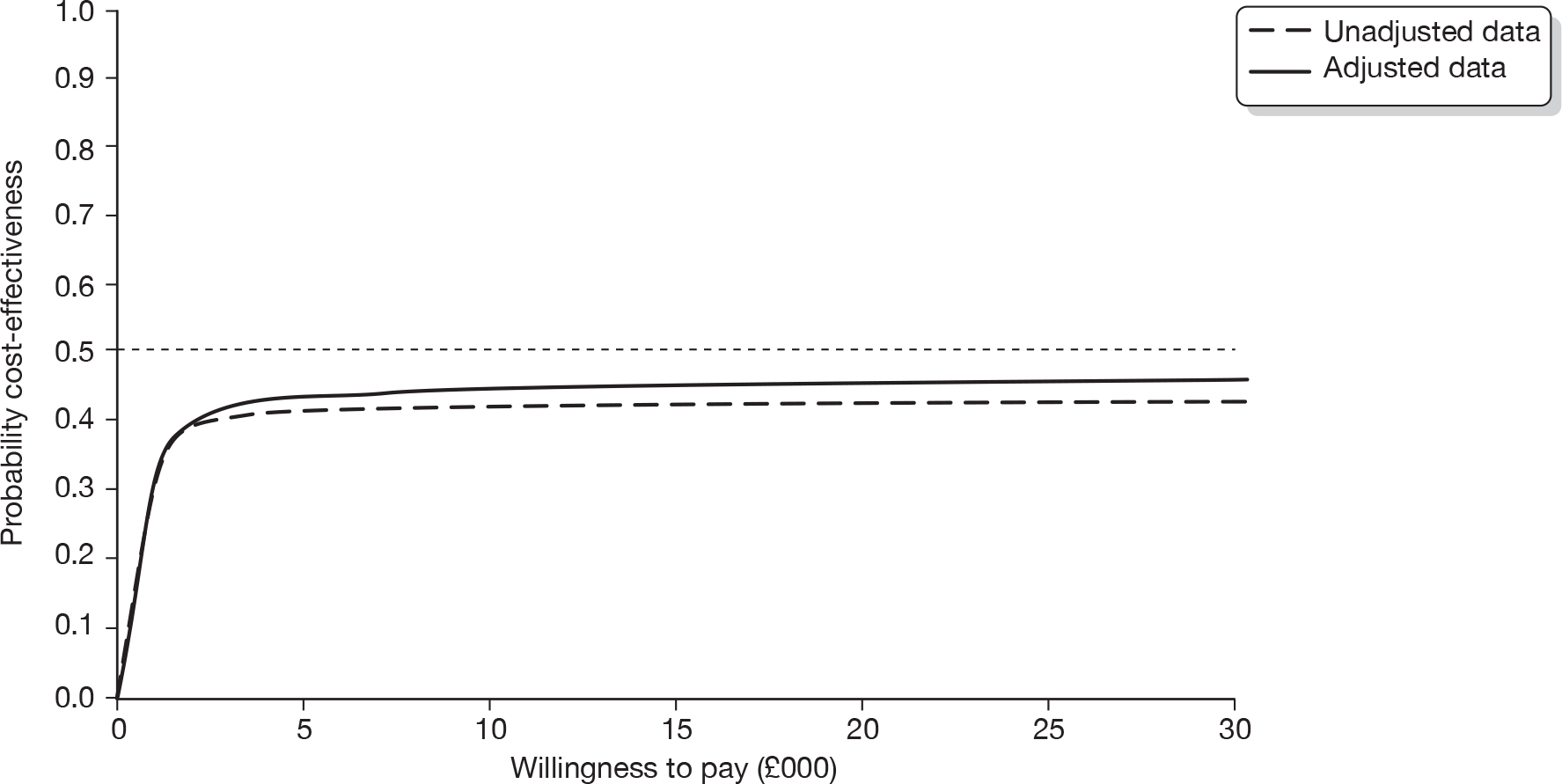
Scenario 2: multiple imputation on the primary outcome data and on the missing total costs
Data were imputed by using multiple imputation methods for the 11 patients who had missing primary outcome data. The multiple imputations were performed by using age, previous treatment and type of verrucae as covariates. Data were imputed also for the missing total costs of these 11 patients.
The mean differences in costs and outcomes after multiple imputation are presented in Table 26. The CE plane and CEAC are presented in Figures 7 and 8. The results of the multiple imputation do not alter the overall conclusion of the study that cryotherapy is more costly than salicylic acid and that there is no evidence of it being more effective.
| Variable | Mean difference | 95% CI | ICER |
|---|---|---|---|
| Costs (£) | 101.39 | 86.29 to 117.29 | Cryotherapy is dominated |
| Outcomes | –0.0083 | –0.10 to 0.08 |
FIGURE 7.
Cost-effectiveness plane (multiply imputed data).
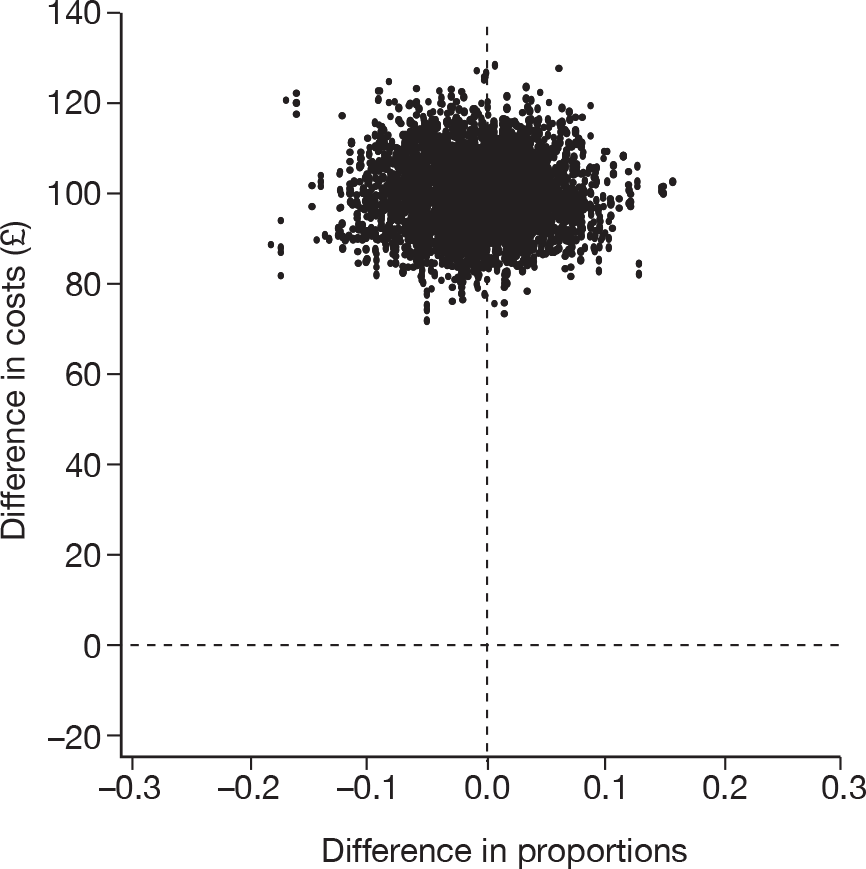
FIGURE 8.
Cost-effectiveness acceptability curve (multiply imputed data).
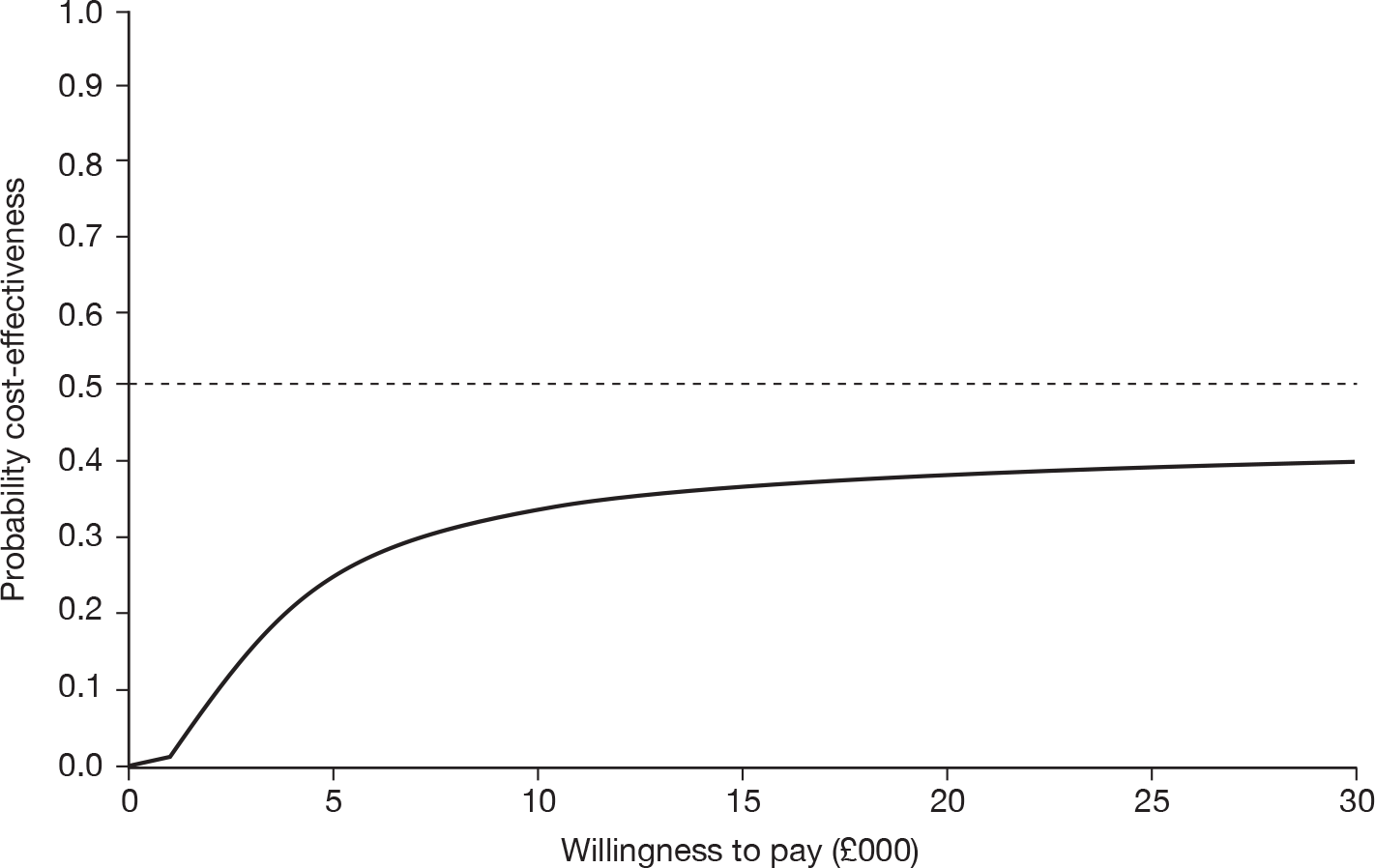
Summary of findings
The EVerT trial has demonstrated that there is no evidence of a difference in effectiveness between cryotherapy and salicylic acid at 12 weeks. In fact, cryotherapy appears to be marginally worse than salicylic acid, without reaching statistical significance. Cryotherapy is also more expensive than salicylic acid, at an average incremental cost of approximately £101 per patient. This evidence results in cryotherapy being dominated (i.e. more costly, less effective) by salicylic acid.
Two scenarios for analysing the data were developed. One was based on complete case analysis for patients who had primary outcome data and mean imputation of cost data for the patients with missing information on different cost items. The second analysis was based on multiple imputation of the primary outcome and missing total cost data. Both analyses resulted in the same conclusions, i.e. cryotherapy is more costly and less effective than salicylic acid, and, hence, dominated by salicylic acid.
An extreme case sensitivity analysis was conducted by replacing the provision of treatment from a GP with nurses, and excluding the cost of cryotherapy equipment and liquid nitrogen, the implicit assumption being that the equipment has a dual use. However, this analysis still resulted in cryotherapy being more expensive than salicylic acid. By excluding the cryotherapy treatment costs completely and reducing the cost of the health-care professional who administers the treatment it is made evident that the results are strongly driven by the lack of effectiveness as well as the larger number of treatment visits that cryotherapy patients have. When the costs of the cryotherapy equipment and liquid nitrogen are included, it is found that cryotherapy is even less cost-effective.
In conclusion, self-treatment with salicylic acid is more cost-effective for the treatment of verrucae than cryotherapy administered by a health-care professional.
Chapter 6 Discussion
Here we report the results of a large RCT assessing the clinical effectiveness and cost-effectiveness of cryotherapy and salicylic acid for the treatment of verrucae. We were motivated to conduct this trial when the Cochrane systematic review into the treatment of cutaneous warts highlighted the lack of good-quality evidence to support the use of cryotherapy over simple topical treatments. This discussion summarises the key findings, compares these results with published studies, considers the strengths and limitations of the present study and summarises the clinical and research implications of the work.
Key findings
We compared the clinical effectiveness and cost-effectiveness of cryotherapy using liquid nitrogen and 50% salicylic acid for the treatment of verrucae and found no evidence to suggest that cryotherapy was more effective than salicylic acid in clearing verrucae. Overall, 32 of 229 (14.0%) patients had complete clearance of all verrucae at 12 weeks; 17 out of 119 (14.3%) were in the salicylic acid group and 15 of 110 (13.6%) in the cryotherapy group, p = 0.89. When the analysis was repeated, adjusting for potentially important prognostic variables (age, whether or not the verrucae had previously been treated and type of verruca), there was no difference in the overall findings (OR 0.96, 95% CI 0.44 to 2.11; p = 0.92). In addition, cryotherapy is associated with higher costs per cured patient. The combination of lack of difference in effectiveness between cryotherapy and salicylic acid, and higher costs leads to cryotherapy being dominated by salicylic acid. Sensitivity analysis conducted by completely excluding the cost of liquid nitrogen and the cost of cryotherapy equipment, and assuming that the provision of the treatment is undertaken by a nurse instead of a GP, did not alter the conclusions of the study, i.e. there is very small probability of cryotherapy being cost-effective for a wide range of willingness-to-pay values. The sensitivity analysis clearly demonstrates, primarily, the lack of treatment benefit of cryotherapy over salicylic acid and, secondly, that the larger number of treatment visits required for the cryotherapy drives the cost-effectiveness results.
Comparison with other studies/reviews
Our results confirm the findings of the two published studies25,26 and the results from a more recent Dutch primary care study,15 which compared cryotherapy with a salicylic acid or a combination of salicylic and lactic acid for the treatment of plantar and hand warts. These previous studies, like ours, showed no evidence for the effectiveness of cryotherapy compared with salicylic acid alone or in combination with lactic acid for the treatment of plantar warts. In Figure 9 we put the two studies reporting clearance rates for plantar warts15,26 (including our own) in a meta-analysis (the results of the Bunney et al. trial25 have not been included, as this trial included only participants with hand warts), which shows that the OR for cure is 1.07 (95% CI 0.63 to 1.79). This result is not statistically significant and indicates that the odds of clearance of verrucae was similar in both groups.
FIGURE 9.
Forest plot of RCTs comparing salicylic acid and cryotherapy for the treatment of verrucae.
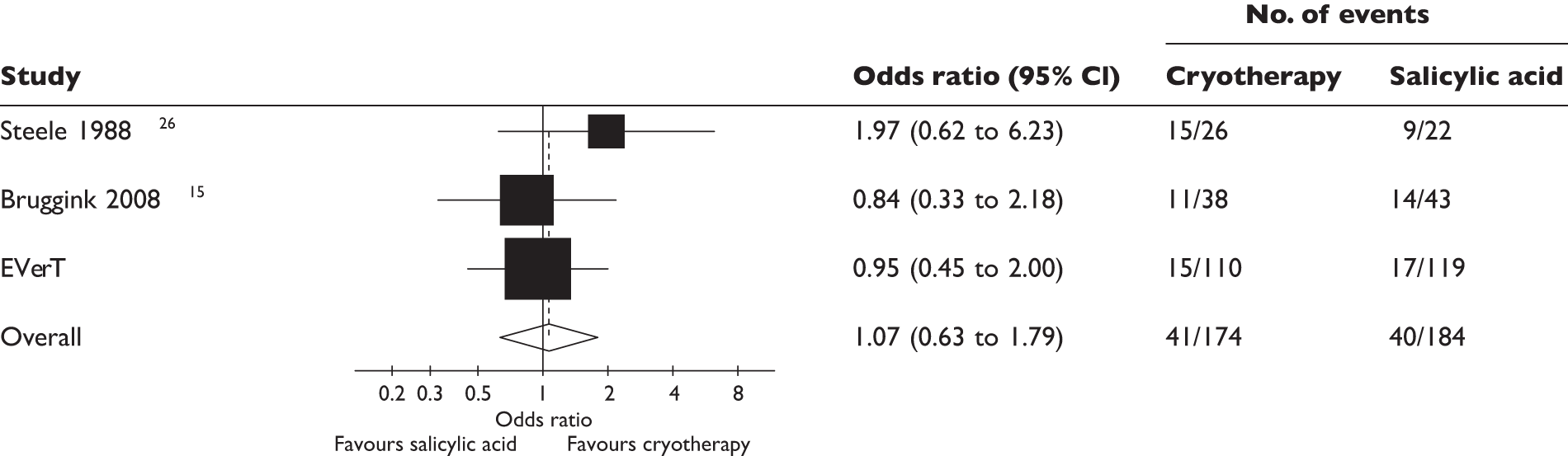
Our trial, however, does differ from the previous studies with respect to the cure rate. The cure rate in previous studies ranged from about 30%15 to 68%,26 which is at least twice the cure rate we observed.
This difference in cure rate could be attributed to different populations recruited to the study. The type of wart being treated was different between the studies. For example, Bunney and colleagues25 included only patients with hand warts, whereas Steele and Irwin26 excluded what are generally regarded as harder-to-treat mosaic warts. They also excluded patients with five or more lesions, lesions outside an average diameter of 3–9 mm and patients who had self-treated within the past month. In our study, 22% of participants had a mosaic wart, 17% had more than five verrucae and patients were not excluded if they had tried previous treatment. There was a difference in the age of the populations. Patients were younger in the Steele and Irwin25 and Bruggink et al. 15 studies, with 59% of participants under the age of 16 in the Steele and Irwin study25 compared with 17.3% in our study. The median age of patients in the Bruggink et al. study15 was 15 [interquartile range (IQR) 7–39] for cryotherapy patients and 13 (IQR 7–31) years for salicylic acid patients compared with median ages of 24 years and 23 years, respectively, in our study.
Treatment regimen
We anticipated that a large percentage of potential participants would have received some form of treatment of their verruca prior to entry into the trial. In the UK the first line of treatment is generally an OTC salicylic acid preparation with a strength of 15–26% salicylic acid, with cryotherapy treatment using liquid nitrogen and higher concentrations of salicylic acid viewed as second-line treatments. This appeared to be the case, as 78% of our participants reported receiving some form of treatment and, of this 78%, 88% reported that they had self-treated and 29% reported receiving treatment from a GP or a podiatrist. We considered it unlikely that patients would be willing to be randomised to a treatment that they had previously tried and found to be ineffective, so in order to maximise recruitment to the study we decided to use a 50% salicylic acid preparation for that arm of the trial.
The 50% salicylic acid preparation chosen was an OTC medication, available as a ‘pharmacy-only’ medication, and was used within its marketing authorisation. Although we wished to replicate how this OTC treatment would be delivered in normal practice as far as possible, it was felt that in order to enhance patient safety, participants allocated to salicylic acid should be seen for a safety check at 2 weeks post randomisation. Using a 50% salicylic acid preparation had the additional benefit that this was a similar concentration to that often used by podiatrists to treat verrucae. If the results of the study demonstrated daily patient self-treatment to be more effective than cryotherapy then it would seem likely that podiatrists using a similar concentration of salicylic acid would be able to achieve similar cure rates.
A strength of this study is that it was a pragmatic trial. As far as possible we allowed clinicians to follow their normal practice in terms in delivering of the cryotherapy treatment. Consequently, we asked the clinicians to treat the verrucae as they would in normal practice, with the recommendation that the first freeze should be relatively gentle, in order to assess how well the patient could tolerate the treatment. Subsequent treatments could be more aggressive if it were appropriate and the patient was able to tolerate the treatment. Pooled data from Gibbs et al. ’s systematic review8 demonstrated that higher cure rates could be achieved if a more aggressive cryotherapy regimen was used. However, these trials were in different populations and on different types of warts. They also used different definitions of ‘aggressive treatment’ ranging from one 10-second freeze to 2 minutes with a cryoprobe. We did originally propose that verrucae should be treated by applying three 10-second applications of liquid nitrogen; however, most health-care professionals reported that this did not reflect their normal practice and that they were unwilling to follow what they considered to be such an aggressive regimen.
In terms of frequency of freezing, evidence from Gibbs et al. ’s systematic review8 showed no significant difference in long-term cure rates between applying cryotherapy treatments at 2-, 3- or 4-weekly intervals and no significant benefit to prolonging 3-weekly cryotherapy treatments beyond 3 months (approximately four freezes). It was decided that cryotherapy patients should therefore receive a maximum of four treatments, 2–3 weeks apart. Treating at 2- to 3-week intervals allowed the treatment to be delivered followed by a minimum of 3 weeks before the outcome assessment at 12 weeks to allow the participant to heal. This would minimise the possibility of unblinding the outcome assessor to the treatment group.
From the limited data available on adherence to treatment, the salicylic acid patients were applying their salicylic acid for the first 3 weeks. However, the overall amount applied (mean amount applied 2.8 g) could suggest that either patients stopped self-treating after the third week or the amount applied during the course of the 8 weeks was relatively small. For some deep-seated verrucae, this might not have not been sufficient to clear the verruca.
Patient satisfaction with treatment
The majority of participants in both groups were happy with their treatment at week 1 and week 3. However, there was a difference in patient satisfaction between the two groups at week 12. The majority of the cryotherapy group reported that they were happy with their treatment, and only 13% reported being unhappy. However, the salicylic acid group individuals were less happy with their treatment at week 12 than at previous time points. In addition to this, a larger proportion of salicylic acid patients (31%) were unwilling to have the same treatment again compared with only 10% of cryotherapy patients.
Strengths and limitations of the study
This is a large pragmatic study that recruited patients with longstanding verrucae, the majority of which had been previously treated either by the patient themselves or by a health-care professional. This is typical of the characteristics of patients presenting to health-care professionals for treatment.
We were able to undertake a blinded outcome assessment for the primary outcome by a clinician present with the patient at the 12-week visit for the majority of participants and we had to rely on blinded assessment of photographs. However, our experience of using cameras to obtain outcome assessments was not without challenges. First, some sites found it difficult to find the additional time required to take digital photographs during busy clinics and, second, the quality of several photographs was such that an assessment of clearance could not be made. It was anticipated that as the cameras given to sites were a similar make and model to that used successfully on another NIHR HTA-funded trial,24 and because members of the EVerT research team owned their own digital cameras and used them to take photographs outside work, the quality of the photographs would not be too great an issue. However, members of the research team encountered difficulties, as they rarely took photographs of such a relatively small scale out of work, and overall a total of 31 out of 190 (16%) photographs were uninterpretable. In an effort to improve the quality of the photographs taken, researchers took several photographs at the same time point and reviewed them on an ongoing basis, taking additional photographs if necessary. However, on several occasions it was noted that although the photographs appeared to be of an acceptable quality on the camera’s LCD screen, once uploaded/sent to the YTU the quality of the photograph meant that an assessment was not possible. Further issues were raised in the amount of time it took to send photographs to the YTU. At the time of undertaking the trial it was not possible to upload the photographs directly to the YTU so the photographs had to be sent via e-mail or copied on to a disk and put in the post, both of which were time-consuming. The delay in sending photographs to the YTU, which in some cases was several weeks, meant that it was not possible to always monitor the activity at the site, for example adherence to treatment regimen, as closely as we had first envisaged. Despite these problems, we were still able to achieve blinded outcome data from digital photographs for a total of 159 (69.4%) participants and overall for 206 (85.8%) of the 240 trial participants when we combined the blinded clinician assessment at 12 weeks with the blinded photographic assessment.
One limitation of our study is the lack of a no-treatment group, so we were unable to determine the spontaneous clearance rate of verrucae in this population. We did consider having a no-treatment arm to the study, but decided against this for several reasons. First, Gibbs and Harvey’s systematic review8 showed that salicylic acid was more effective than no treatment, while failing to find any evidence for the effectiveness of cryotherapy. Therefore, the important clinical question was whether or not the use of cryotherapy was superior to that of the salicylic acid treatment. Second, overall recruitment to the study could have been jeopardised, as patients might have been unwilling to be randomised to a no-treatment arm. Finally, a no-treatment arm might have led to bias owing to resentful demoralisation, particularly in those patients in whom the verrucae were painful, longstanding and resistant to previous treatment.
Generalisability of the results
The EVerT study was a pragmatic trial that recruited from 14 centres across England, Scotland and Ireland. The inclusion of patients recruited from podiatry clinics, from GP practices and from the community means that we can be confident that these results are broadly generalisable and that the study has external validity across the UK and Ireland. However, although the 50% salicylic acid preparation used in this study was an OTC treatment, it is not the most commonly used concentration and may be viewed as a second-line treatment. Typically, weaker preparations of 15–26% salicylic acid are used as the first form of treatment and so the results of this study may not mimic the usual clinical situation. Therefore, it is possible that cryotherapy using liquid nitrogen is superior to using these lower concentrations of salicylic acid. However, some GPs are no longer offering cryotherapy using liquid nitrogen as a form of treatment. This is because of the additional treatment cost incurred in order to comply with changes to the health and safety rules regarding the storage of liquid nitrogen. Therefore, the availability of the treatment may be lower than that reported in 2002 and, in some cases, may be considered a third-line treatment.
Implications for health care
There is no evidence from this trial to suggest that cryotherapy used for the treatment of verrucae is more effective than patient self-treatment with 50% salicylic acid and our economic evaluation concludes that self-treatment with salicylic acid is the most cost-effective option. Our results are applicable only to verrucae or plantar warts. Warts at other sites, such as the hands, may respond differently to cryotherapy.
We evaluated only patient self-treatment with salicylic acid and, therefore, the results cannot be extrapolated to the effectiveness of salicylic acid if it had been delivered by a health-care professional. The freezing agent used for the cryotherapy was liquid nitrogen, so the results from this study cannot be extrapolated to include other freezing agents, such as nitrous oxide, frozen carbon dioxide (dry ice) or OTC freezing treatments, which freeze lesions at higher temperatures.
Implications for research
Health-care professionals will need to write patient information sheets in such a way to give patients realistic expectations in relation to the effectiveness of cryotherapy treatment.
There are other treatments available for cutaneous warts, but very little good-quality evidence assessing their effectiveness. Further research assessing the effectiveness of these treatments is required in order to inform future practice.
Acknowledgements
We would like to thank the participants for taking part in the trial, the podiatrists, GPs and practice nurses for recruiting participants to the study and completing the trial documentation, the principal investigators at each site for co-ordinating participant recruitment, and the TSC and DMEC members for overseeing the study.
We would specifically like to thank:
Peter Arthur, Lucy Bellas, Beverly Brown, Lynne Bryan, Amanda Clark, Andrew Clarke, Michael Concannon, Beryl Cooling, Dawn Curruthers, Chris Davies, Gary Denby, Sean Dinneen, Diane Exley, Lisa Farndon, Simon Gazeley, Elizabeth Green, Stephanie Haughy, Julia Haswell, Christine Hearmon, Christine Howell, Jamil Karolia, Susan Kitchener, Phillip LeDune, Susan Lightfoot, Maria Madigan, Julie Mandehzadeh, Rina Miah, Caroline McIntosh, Christine Northern, Frances Price, Julie Poland, Ilan Rajap, Jayne Robinson, Julie Robinson, Raymond Skinner, Deborah Turner, Susan Walton, Linda White and Catriona Williams, who were members of the research team at each site and recruited participants to the study, as well as Fiona Aitken and Helen Hill from the Medicines for Children Local Research Network, and Kate Biscomb and Kate Wyer from the Primary Care Research Network.
Jill Hall (JH), Farina Hashmi and Jude Watson, who undertook the blinded outcome assessment of clinical verrucae photographs.
Collaborations and contributions of the authors
The EVerT collaborators (current and past) are:
Sally Baker, Diane Bilton, Stephen Brealey, Ling-Hsiang Chuang, Sarah Cockayne (SC), Sue Collins, Ben Cross, Mike Curran (MC), Sarah Gardner, Farina Hashmi (FH), Catherine Hewitt (CH) Kathryn Hicks (KH), Shalmini Jayakody (SJ), Arthur Kang’ombe, Nichola McLarnon (NM), Veronica Morton, Jo Orchard, Eugena Stamuli (ES), Kim Thomas (KT), David Torgerson (DT), Gwen Turner (GT), Val Wadsworth, Ian Watt and Gill Worthy (GW).
William Ransom & Son Plc supplied the Verrugon, plasters and felt pads at no cost, and BOC provided one site with liquid nitrogen storage equipment at a reduced cost.
Statement of independence of researchers
Neither the Verrugon manufacturers nor BOC had any role in the design of EVerT, or in the collection, analysis and interpretation of data.
DT and JH wrote the original protocol. SC, MC, FH, NM, DT and KT were co-applicants on the HTA application and refined the protocol. IW was the chief investigator and oversaw the study. SC and KH were the trial co-ordinators and GT the trial support officer. GW, SJ and CH designed the clinical analysis. CH oversaw the conduct of the analysis. SJ conducted the clinical analysis and AK undertook additional analysis requested by the reviewers. ES designed and undertook the economic analysis. The writing team consisted of SC, KH, CH, ES and KT, who drafted the report. GD, CM, FH, SJ, DT and IW commented on the report.
Trial Steering Committee members
Dr Sam Gibbs, (Independent Chairperson) Consultant Dermatologist, The Great Western Hospital, Swindon.
Dr Jill Mollison (member of the TSC October 2006 to December 2008), Senior Medical Statistician, Centre for Statistics in Medicine, Wolfson College Annexe, University of Oxford, Oxford.
Dr Elaine Thomas (member of the TSC from December 2008 to end), Senior Lecturer in Biostatistics, Arthritis Research Campaign National Primary Care Centre, Keele University, Keele.
Professor Wesley Vernon, Head of Podiatry Service and Research Lead, Sheffield Primary Care Trust, Sheffield.
Data Monitoring and Ethics Committee members
Dr Anne-Maree Keenan (Independent Chairperson), Assistant Director, NIHR Leeds Musculoskeletal Biomedical Research Unit, University of Leeds, Leeds.
Matthew Hankins, Senior Research Fellow, Division of Public Health & Primary Care, Brighton & Sussex Medical School, University of Brighton, Brighton.
Katharine Speaks, Clinical Lead Podiatrist – Diabetes, Centre for Diabetes and Endocrinology, York Hospital, York.
Publications
Cockayne E. The EVerT (Effective Verruca Treatments) trial protocol: a pragmatic randomised controlled trial to evaluate cryotherapy versus salicyclic acid for the treatment of verrucae. Trials 2010;11:1-5.
Cockayne S. Verrucae trial: call for participants. Podiatry Now 9:July 2006.
Cockayne S, et al. , on behalf of the EVerT team. Cryotherapy versus salicylic acid for the treatment of plantar warts (verrucae): a randomised controlled trial. BMJ 2011;342:d3271.
Turner G. Cryotherapy versus salicylic acid for the treatment of verrucae. Society of Chiropodists and Podiatrists. Newsletter, January 2009.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Johnson ML, Roberts J. Skin conditions and related need for medical care among persons 1–74 years. Vital Health Stat 11 1978;1660:1-26.
- Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med 1976;30:107-14.
- Beliaeva TL. The population incidence of warts. Vestn Dermatol Vener 1990;2:55-8.
- Williams HC, Pottier A, Strachan D. The descriptive epidemiology of warts in British schoolchildren. Br J Dermatol 1993;128:504-11.
- Kilkenny M, Merlin K, Young R, Marks R. The prevalence of common skin conditions in Australian school students. 1. Common plane and plantar viral warts. Br J Dermatol 1998;138:840-5.
- van Haalen FM, Bruggink SC, Gussekloo J, Assendelft WJ, Eekhof JA. Warts in primary schoolchildren: prevalence and relation with environmental factors. Br J Dermatol 2009;161:148-52.
- Massing AM. Natural history of warts. Arch Dermatol 1963;87:306-10.
- Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev 2006;3.
- Sterling JC, Handfield-Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol 2001;144:4-11.
- Clinical Knowledge Summaries . Warts and Verrucae 2009. www.cks.nhs.uk/warts_and_verrucae# (accessed May 2010).
- Johnson LW. Communal showers and the risk of plantar warts. J Fam Pract 1995;40:136-8.
- Health Protection Agency (HPA) . School Health Matters: A Guide to Communicable Diseases and Infection Control 2008. www.hpa.org.uk (accessed May 2010).
- Schofield J, Grindlay D, Williams HC. Skin conditions in the UK: a health care needs assessment. Nottingham: Centre of Evidence Based Dermatology; 2009.
- Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al. Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model. Health Technol Assess 2006;10.
- Bruggink SC, Gussekloo J, Zaaijer K, Assendelft WJJ, Berger MY, Koes BW, et al. Warts: cryotherapy, salicylic acid or expectantly awaiting? a randomised controlled trial. J Invest Dermatol 2008;128.
- Drummond MF, O’Brien B, Stoddart GL, Torrance. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- BOC n.d. www.boconline.co.uk/ (accessed June 2010).
- Personal Social Service Research Unit (PSSRU) . Unit Costs of Health and Social Care 2009 2009.
- Nu-Care Products n.d. www.nu-careproducts.co.uk/chiropody.htm#feltpads (accessed June 2010).
- Boots n.d. www.boots.com/en/Boots-Fabric-Strip-10-pack_5466/ (accessed June 2010).
- BOC Products n.d. www.bocsafetyproducts.co.uk/main/home/index.aspx (accessed June 2010).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2010.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev 2007;2.
- Dumville J, Worthy G, Soares M, Bland JM, Cullum N, Dowson C, et al. VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers. Health Technol Assess 2009;13.
- Bunney M, Nolan M, Williams D. An assessment of methods of treating viral warts by comparative treatment trials based on a standard design. Br J Dermatol 1976;94:667-79.
- Steele K, Irwin W. Liquid nitrogen and salicylic/lactic acid paint in the treatment of cutaneous warts in general practice. J Roy Coll Gen Pract 1988;38.
Appendix 1 Regulatory approvals
| Research site | Ethical site assessor | Date of favourable ethical opinion | R&D approval | Competent authority approval |
|---|---|---|---|---|
| School of Podiatry, Centre for Healthcare Education School of Podiatry, University of Northamptona | Leicestershire, Northamptonshire & Rutland REC 2 | 26 October 2004 | N/A | 8 February 2005 |
| With approval to contact GPs with the PCT Leicestershire Primary Care Alliance | 6 November 2006 | 4 January 2007 | ||
| University of Brighton (Leaf Hospital) and Eastbourne PCT Sussex NHS Research Consortium | East Sussex LREC | 23 September 2006 | 24 October 2006 | 10 August 2006 |
| Southern General Hospital, South University Division, Greater Glasgow and Clyde Health Board and Glasgow Caledonian University | South Glasgow and Clyde REC | 29 March 2007 | 21 December 2007 | 13 November 2007 |
| Huddersfield University, Division of Podiatry, Department of Clinical and Health Sciences, Huddersfielda | Airedale REC |
5 May 2007b 3 December 2008b |
15 June 2007 | 13 November 2006 |
| Brownlow Group Practice, Liverpool | Liverpool Paediatric REC | 13 May 2007 | 19 September 2007 | 26 June 2007 |
| Springfield Surgery, Bingley | Bradford REC | 5 December 2007 | 21 November 2007 | 12 October 2007 |
| Sheffield PCT | North Sheffield LREC | 3 April 2008 | 28 August 2008 | 23 April 2008 |
| Claughton Medical Centre, Birkenhead | Cheshire REC | 3 November 2008 | 10 March 2009 | 9 July 2008 |
| Arlington Road GP Surgery, Eastbourne | Brighton East REC | 23 December 2008 | 23 January 2009 | 4 December 2008 |
| Harbinson House, Sedgefield | Research Management & Governance Unit of County Durham & Tees Valley PCTs | 6 October 2009 | 6 October 2009 | 16 October 2009 |
| Sacriston Surgery, Sacriston | Research Management & Governance Unit of County Durham & Tees Valley PCTs | 7 August 2009 | 28 July 2009 | 29 July 2009 |
| Peaseway Medical Centre, Newton Aycliffe | Research Management & Governance Unit of County Durham & Tees Valley PCTs | 7 August 2009 | 28 July 2009 | 29 July 2009 |
| The Haven Surgery, Burnhope | Research Management & Governance Unit of County Durham & Tees Valley PCTs | 7 August 2009 | 28 July 2009 | 29 July 2009 |
| The National University of Ireland,a Galway | Galway REC | 20 March 2009 | N/A | 30 January 2009 |
Appendix 2 Details of the study sites
The following sites recruited at least one patient:
-
The University of Northampton Podiatry School Clinic, Northampton
-
The University of Huddersfield Podiatry School Clinic, Huddersfield
-
The University of Brighton Podiatry School Clinic at the Leaf Hospital, Eastbourne
-
Southern General Hospital, Glasgow/Glasgow Caledonian University Podiatry School, Glasgow
-
The National University of Ireland, Galway (NUI Galway) Podiatry School Clinic, Galway
-
Brownlow Group Practice, Liverpool
-
Springfield Surgery, Bingley
-
Sheffield Primary Care Trust Podiatry Clinic, Sheffield
-
Sacriston Surgery, Sacriston
-
The Haven Surgery, Burnhope
-
Peaseway Medical Centre, Newton Aycliffe
-
Arlington Road Medical Practice, Eastbourne
-
Claughton Medical Centre, Birkenhead
-
Harbinson House Surgery, Sedgefield.
Approval was gained and an initiation visit was performed at the following two sites, but neither recruited any patients:
-
Annfield Plain Surgery, Stanley, Co. Durham
-
Islington Primary Care Trust Services, London.
Appendix 3 Patient information sheets and consent form
Patient information sheets
Patient information sheet for children
Consent form
Cryotherapy patient’s advice sheet
Acid therapy patient’s advice sheet
Appendix 4 Data collection forms
Baseline patient questionnaire
Randomisation form
Patient ineligible form
Podiatrist’s treatment assessment form
Patient pain questionnaire
Week-1 patient questionnaire
Week-3 patient questionnaire
Week-12 patient questionnaire
Verruca(e) has gone form
Podiatrist outcome assessment form
Six-month patient questionnaire
Change of circumstances form
Non-serious adverse event form
Review of non-serious adverse event form
Serious adverse event form
Review of serious adverse events
Appendix 5 Advertising material
Poster – version 1
Poster – version 2
Press releases
Letters to pharmacists/GPs/parents of school students
Letter to GPs (version 1 21 November 2006)
Letter to parent of school children
Appendix 6 Flow chart for EVerT trial
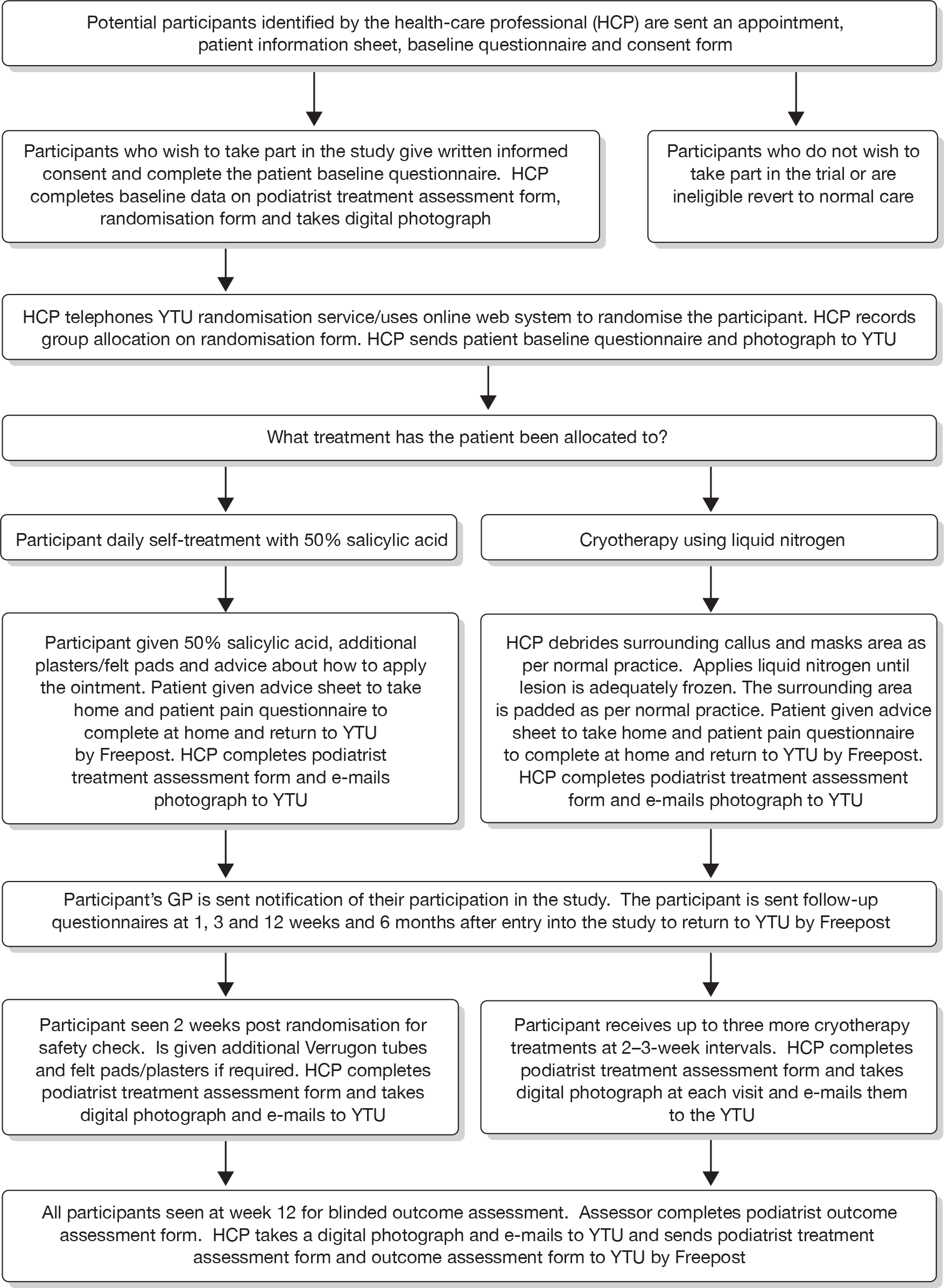
Appendix 7 Trial protocol
List of abbreviations
- BCA
- bias-corrected and accelerated
- CE plane
- cost-effectiveness plane
- CEAC
- cost-effectiveness acceptance curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTA
- clinical trial authorisation
- df
- degrees of freedom
- DMEC
- Data Monitoring and Ethics Committee
- EVerT
- Effective Verruca Treatments
- GP
- general practitioner
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LREC
- Local Research Ethics Committee
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MREC
- Multicentre Research Ethics Committee
- NIHR
- National Institute for Health Research
- NUI
- National University of Ireland
- OR
- odds ratio
- OTC
- over-the-counter
- PCT
- primary care trust
- PSSRU
- Personal Social Services Research Unit
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee
- YTU
- York Trials Unit
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington