Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/65/02. The contractual start date was in April 2009. The draft report began editorial review in April 2010 and was accepted for publication in November 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Brush et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Epidemiology
Worldwide, large bowel (colorectal) cancer (CRC) accounts for > 1 million cancers per year or 9% of all new cancer cases. 1 In the UK, CRC is the third most common malignancy (behind lung and breast cancer), with 37,514 new cases registered in 2006, of which around two-thirds (23,384) were in the colon and one-third (14,130) in the rectum. The proportions by gender are similar for colon cancer (M : F = 12,005 : 11,379) but for rectal cancer the number of cases is higher in men (M : F = 8425 : 5705). 2
Data from the EUROCARE (EUROpean CAncer REgistry-based study on survival and CARE of cancer patients)] analyses (2000–2) show that the age-adjusted 5-year relative survival for England, Northern Ireland, Scotland and Wales ranges between 51.8% and 54.5%, rates that lag behind those of other European countries (e.g. Switzerland 63.8%) and the USA (SEER-13 registries 65.5%). 3 The reasons for these variations are multifactorial, but in part reflect variability in standards of care between regions and countries. In turn, these differences point to the continued need to improve the quality of diagnosis and treatment in patients with CRC – a theme central to this report.
Clinical presentation
For the purposes of clinical presentation and treatment, new cancer cases arising from the colon are distinct from those arising from the rectum (defined as the distal large bowel up to 15 cm from the anal verge). 4 In turn, clinical presentation may be considered as (1) elective and (2) emergency.
Symptomatic presentations of colon cancer in the non-emergency setting range from findings on surveillance for polyps or inflammatory bowel disease through abdominal discomfort, change in bowel habit and anaemia, to more advanced disease with weight loss, palpable tumour or metastatic disease. By contrast, rectal cancer typically presents with symptoms localised to the pelvis, including bleeding, diarrhoea and, in advanced cases, pain, fistulation to other adjacent viscera and/or tenesmus (a symptom characterised by the persistent sensation to defecate due to the presence of a rectal mass). Importantly, in general there is a poor correlation between symptoms and tumour stage.
A national CRC screening programme has been rolled out across the UK since 2007 using the faecal occult blood test as the primary screening modality. 5 Asymptomatic presentations of cancer of the colon or rectum will become increasingly more common as a result. Importantly, there is emerging evidence that screening substantially reduces the proportion of emergency cases at a population level – for example from 29% in 1999 to 16% in 2004 in the Coventry and North Warwickshire pilot studies. 6
Diagnosis and staging
Cancers of the colon and rectum are usually diagnosed either by direct endoscopic visualisation – for example colonoscopy for investigation of symptoms or flexible sigmoidoscopy, which is now widely used throughout the UK in ‘one-stop’ rectal bleeding clinics – or by a radiological investigation [barium enema has been largely replaced by computerised tomography (CT), plain imaging or CT colonography]. For the majority of cases, histological confirmation is obtained through endoscopic biopsy. Some 85% of CRCs are adenocarcinomas (not otherwise specified), 10% are mucinous adenocarcinomas and the remainder are rare histological types such as papillary carcinoma, adenosquamous carcinoma and signet ring cell carcinoma.
In the peri-operative period there should be comprehensive clinical and pathological assessment of the tumour, its invasion characteristics and its spread. This forms the basis of tumour ‘staging’. Traditionally in the UK, pathological staging of cancer of the colon and rectum comprised the Dukes’ staging system;7 this has increasingly been replaced by the internationally accepted tumour, node, metastasis (TNM) staging system. The TNM system classifies the extent of the tumour (T), the extent of spread to the lymph nodes (N) and the presence of metastases (M). 8
Treatment of primary tumour
For cancers of both the colon and the rectum, surgical resection is the mainstay of definitive treatment. However, the pathways before surgery differ for colon and rectal cancers. For most colon cancers, surgical resection is the primary treatment, followed by histopathological staging. Adjuvant chemotherapy is then standard care for patients with node-positive disease and for select cases of node-negative disease when there are adverse prognostic histological features, for example extravascular invasion.
For rectal cancer, the algorithms for treatment are more complex. For some T1 rectal tumours, a local surgical procedure (e.g. transanal endoscopic microsurgery) may be considered,9 but these tumours represent a small proportion of all cases. The majority of rectal cancers are larger yet confined within the anatomical boundaries of the mesorectal fascial plane and require major surgery, either as anterior resection [with total mesorectal excision (TME) for middle lower and third sections] or by total abdominoperineal resection (APR). These procedures are associated with considerable short- and long-term morbidity, including permanent colostomy if APR is required. Pre-operative radiotherapy has been shown to be superior to postoperative (salvage) radiotherapy, but there is no evidence that radiotherapy confers an overall survival advantage. Risk of local recurrence is a key issue for rectal cancer, and for this reason many patients in the UK receive pre-operative radiotherapy or chemoradiotherapy, where radiotherapy and chemotherapy are delivered as a combination (CRT). 10 Pre-operative therapies are conventionally administered as either (1) short-course pre-operative radiotherapy (SCPRT) over 4–5 days followed by surgery within 10 days; or (2) long-course radiotherapy, usually in combination with chemotherapy [chemoradiotherapy (CRT)], and then a period to allow ‘tumour downstaging’ ranging from 6 to 12 weeks before surgery. The effectiveness of SCPRT11 and CRT12 in reducing local recurrence has been shown in randomised clinical trials (RCTs), but it is increasingly recognised that these adjuvant therapies are associated with long-term morbidities, for example sexual and bowel dysfunction. 13 However, the selection criteria for which pre-operative therapy is used varied between studies, but, in general, CRT is indicated for locally advanced rectal cancer where there is a high risk of local recurrence – for example a threatened resection margin (known as the ‘circumference’ margin) or several positive lymph nodes. 14 In modern clinical practice, these criteria are generally predicted from magnetic resonance imaging (MRI). 15
In a small proportion of rectal cancers there is advancement of neoplastic disease locally beyond the mesorectal fascial plane to involve adjacent viscera. These cases require major complex surgery, often in the form of multivisceral exenteration and formation of colostomy and urostomy. These patients are generally treated within a centralised setting by the multidisciplinary surgical team (colorectal surgeon, urologist, gynaecologist and/or plastic surgeon). Cure rates are low to modest and morbidity is high; thus, pre-operative selection through staging is a key clinical process.
Treatment of recurrent disease
Locoregional recurrence is well recognised following initial treatment for colon and rectal cancers. The problem is best described for local pelvic recurrence following rectal cancer treatment. These cases require major complex ‘salvage’ surgery, often in the form of multivisceral exenteration with formation of colostomy and urostomy. Cure rates are low to modest and morbidity is high. 16
Treatment of metastatic disease
Cancer of the colon and rectum spreads via lymphatics and the blood system to the liver and lungs, the most common sites for ‘distant’ metastatic disease. A small proportion also spreads principally via the peritoneal lining and omentum (known as transcoelomic spread) to manifest as peritoneal deposits or ovarian masses in women (known as Krukenberg tumours).
Resections of metastatic tumour (referred to as ‘metastatectomy’), with or without pre-operative chemotherapy, are commonly performed in the UK for metastases of colorectal origin, with long-term cure rates greater than one-third. 17 Aggressive approaches to peritoneal deposits of colorectal origin are increasing recognised and involve major surgery known as cytoreductive surgery, and the administration of hyperthermic intraoperative intraperitoneal chemotherapy.
Rationale for pre-operative staging
With the wide range of clinical scenarios, treatment options and their timings outlined above for colon and rectal cancer, the rationale for pre-operative staging becomes clearly apparent. Over the past two decades, a number of diagnostic tools have entered clinical practice and now facilitate the process of pre-operative staging. A number of imaging modalities are used in the pre-operative staging of CRCs including CT, MRI, ultrasound imaging and positron emission tomography (PET).
For this report, the focus is on imaging; the use of prognostic indicators, for instance blood-borne tumour markers, alone or in combination with imaging, is beyond the scope of the report.
Specifically, this report examines the role of CT in combination with PET scanning (‘hybrid’ scan) in pre-operative staging for CRC. The literature contains reports of the use of PET scanning alone compared with other imaging modalities for staging CRC, but as stand-alone PET technology is no longer commercially available, the present report is limited to the role of hybrid PET/CT scanning.
Pre-operative staging modalities
Computerised tomography
Computerised tomography is a cross-sectional technique using ionising radiation (X-rays) to produce images of sections of the body. Modern scanners are capable of producing high spatial resolution images with the ability to define anatomy and different tissues in great detail. However, one major drawback of CT is that it does not provide functional information and hence cannot reliably discriminate between active cancer cells and scar tissue following previous successful treatment. CT relies predominantly on morphology and size for diagnosis and it may be impossible to confidently detect cancer in small (< 1 cm) lymph nodes or, alternatively, to tell the difference between enlarged cancerous nodes and enlarged benign reactive nodes. 18
Modern CT scanners are capable of fast scanning and are the workhorse of modern imaging departments. Most protocols to stage CRC would involve injection of iodine-containing intravenous contrast, which also gives information on perfusion and the relationship of tumours to blood vessels and often makes metastases easier to discriminate from background tissues. Iodine-containing oral contrast is often used to highlight loops of bowel, allowing a higher degree of diagnostic certainty when evaluating adjacent soft tissue masses.
Computerised tomography scans can therefore be performed quickly [a total in-department time of approximately 30 minutes would be standard (not including administration of initial oral contrast), although actual scanning time is usually < 2 minutes].
Staging of CRCs uses CT as the first test and this test is capable of identifying the primary tumour, local and distant lymph nodes and the presence of liver and lung metastases.
Magnetic resonance imaging
Magnetic resonance imaging is a specialised investigation that is capable of producing very high spatial resolution images of targeted body parts. Unlike CT, MRI uses a powerful magnetic field and the properties of atoms within this field. A radiofrequency pulse allows a signal that can be measured and applied to an image. There is no radiation dose with MRI as X-rays are not used.
Magnetic resonance imaging is therefore capable of assessing the primary tumour and its relationship to the bowel wall, thus guiding surgeon and oncologist towards either curative surgery or radiotherapy, both to downstage the primary tumour and as palliation.
Magnetic resonance imaging scans take considerably longer than CT scans and require the patient to be still within the confined space of the magnet for prolonged periods. Claustrophobia can often result in incomplete imaging or the inability to even start the scan. MRI images are therefore best performed on parts of the body that are static, as motion can considerably degrade image quality. In CRC, imaging of the rectum is ideal as this segment of bowel is relatively fixed – peristaltic waves, although they obviously do affect the rectum, do not cause as much of a problem because the rectum is partly fixed by adjacent musculature forming part of the sphincter mechanism. For this reason, use of MRI to stage rectal cancers to assess the primary tumour and its relationship to the bowel wall is standard, whereas MRI is not used to assess primary colonic tumours elsewhere in the large bowel because of image quality degradation by peristalsis. In addition, the rectum, because of its relatively fixed position, can be targeted with radiotherapy with minimal risks to adjacent tissues – this is not the case with colonic tumours elsewhere. The use of MRI to pre-operatively stage rectal cancer is part of routine clinical practice in the UK and is recommended in UK clinical guidelines. 4,19
Magnetic resonance imaging can also be used to obtain high-quality, high spatial resolution images of the liver in the assessment of metastatic disease to the liver, and is capable of both identifying metastases that have not been seen by standard CT and providing a roadmap for surgery in the case of metastatic disease to liver being worked up for surgical resection. MRI to assess the liver is also excellent as a problem-solving tool in cases in which there is some doubt as to the nature of the liver lesion(s) present.
Ultrasound imaging
Standard transabdominal ultrasound has no role in assessment of primary CRCs, although it is possible to diagnose CRC with this modality. Transabdominal ultrasound is capable of identifying liver metastases and is a useful test to gain information on a liver lesion identified by cross-sectional imaging.
Transrectal ultrasound is a specialised test that is used to assess rectal cancers and is capable of producing high-resolution images of the tumour and its relationship to the bowel wall. This procedure is relatively invasive but can guide the surgeon and oncologist as to the presence or absence of extension through the wall. This may change the operative approach and may act as a guide in the use of pre-operative radiotherapy.
Positron emission tomography
Positron emission tomography has been in use for > 25 years as a research tool and during the last 15 years in a clinical role. The major clinical applications of PET are in the areas of oncology, cardiology and neurology, with by far the greatest use in oncology.
Positron emission tomography is a functional imaging technique that uses short-lived radioisotopes (with half-lives ranging from 2 to 110 minutes currently) attached to tracers to examine abnormal biochemical processes associated with disease. The most commonly used radiopharmaceutical in PET is fluorine-18-labelled deoxyglucose (FDG) – this acts as an analogue of glucose and can be used to identify tissues showing increased glucose transport and metabolism, such as cancer cells. There are potentially many more radiopharmaceuticals that can be used to investigate aspects of metabolism in the body, but currently FDG is the only readily available tracer.
The rationale behind PET is that biochemical changes caused by disease usually precede changes in size or structure of a particular organ or tissue. Hence, PET is capable of identifying cancer earlier than anatomical imaging techniques (such as CT and MRI).
A drawback of PET, however, is that the functional images obtained often lack fine anatomical definition, and it can be difficult, because of the inherently low spatial resolution of the technique, to accurately localise the abnormality.
Depending on the tumour type, PET can be highly effective as a prognostic indicator or as a technique for primary tumour staging, for assessing treatment response or for detecting disease recurrence. However, there are specific areas in which PET is not helpful, such as for specific subtypes of CRC with histological features consistent with mucinous carcinoma – PET scans can be falsely negative because of the inherent low metabolic rate of these tumours.
Positron emission tomography is also known to produce false-positive (FP) results when inflammation is present and can produce both FP and false-negative (FN) findings in people who have high plasma glucose levels as a result of poorly controlled diabetes. 20,21 Providing that the standard recommendations for PET are followed, the use of PET has already been shown to alter patient management in approximately one-third of cancer cases. 22
With regards to CRC, the main indications currently for PET are:
-
assessment of a residual mass following treatment
-
assessment of apparently isolated metastatic disease.
Combined positron emission tomography and computerised tomography imaging
Positron emission tomography and CT are complementary imaging techniques that, when combined, can maximise their individual advantages and minimise their disadvantages. The functional imaging provided by PET can be accurately superimposed on high spatial resolution CT images using combined FDG PET/CT imaging. Thus, when reporting a modern FDG PET/CT study, it is possible to analyse the functional PET study, the anatomical CT study and a combined fused image. A further advantage of this combination is that the CT component can be used to correct PET images for attenuation errors, thus further improving PET imaging quality and increasing scan speed.
The recommendation from the Royal College of Radiologists is that every new PET scanner should be a PET/CT scanner and that every cancer network should have access to FDG PET/CT services. 23
Combined PET/CT scanners using standard FDG allow scans to be acquired within approximately 30–40 minutes.
Several studies have shown FDG PET/CT to be more accurate than diagnostic CT and stand-alone PET for cancer staging, including staging of CRC. 24,25
It is also recognised that technology is rapidly changing and, although the first generation of combined PET/CT scanners used lower-specification CT scanners, modern scanners now use fast, multiple-slice CT components with technology as advanced as that of modern stand-alone CT scanners. This could obviously affect FDG PET/CT interpretation as higher-quality, more accurate scanners replace older technology.
Although the CT component of most FDG PET/CT scans is currently performed without oral and intravenous contrast agents (non-contrast-enhanced CT), the role of contrast-enhanced FDG PET/CT has been evaluated in more recent studies. 21 Currently, almost all patients with CRC who are undergoing FDG PET/CT studies will have had a diagnostic CT scan prior to referral for FDG PET/CT and there is, therefore, little to be gained by use of oral and intravenous contrast. It would be possible to perform an FDG PET/CT scan with oral and intravenous contrast to optimise the CT component if a role for use of FDG PET/CT in primary staging of CRC was identified. There is evidence that it may have a role in staging of primary rectal cancer. 21,26,27
Replacing diagnostic CT with FDG PET/CT as an imaging investigation has considerable resource and cost implications. However, the potential improvement in correctly diagnosing the extent of the disease could lead to a beneficial therapeutic impact in which unnecessary surgery and the prescribing of expensive chemotherapies are avoided. Cost and resource considerations currently limit FDG PET/CT use to an add-on test in most centres where the technology is available. 28
Information about safety is essential to guide clinicians’, patients’ and policy-makers’ decisions, and there are operational and pharmacological factors that need to be considered when FDG PET/CT scans are performed in hospital premises. These apply to all indications for FDG PET/CT scans, including CRC. A systematic review of potential harms from FDG PET/CT is outwith the scope of this research; however, we briefly outline below what is currently known about the safety of FDG PET/CT.
FDG is a radiopharmaceutical substance that contains a small amount of radioactivity. It has a short half-life of 110 minutes, after which it is rendered ‘safe’, and the Medicines and Healthcare Products Regulatory Agency supports its use in clinical practice. 29 However, there are some groups of patients for whom FDG is not advised: pregnant or lactating women should avoid FDG unless the benefits outweigh the risks. Nursing mothers are advised that breastfeeding should be stopped for 12 hours, and close contact between mother and infant is discouraged within 12 hours of the injection.
Although the whole-body approach to FDG PET/CT imaging allows broad coverage of multiple organs, the radiation dose to the patient is potentially much higher than in conventional CT. 30 Staff working closely with patients undergoing an FDG PET/CT procedure may inadvertently receive a dose of radiation, and care in planning the FDG PET/CT procedure can reduce this. Staff radiation exposure can be maintained below regulatory limits by appropriate design, particularly shielding, and workflow in FDG PET/CT facilities. 30 The physical space in which FDG PET/CT scans are performed needs to shield equipment (syringes and vials) as well as rooms (walls, floors and ceiling), and to incorporate a ‘hot’ waiting area in which patients who have had an FDG injection can rest until the scan is performed. 30 In a study by Carson et al. ,31 a two-stage process involving a ‘cold’ pre-injection set-up session reduced the radiation received by radiotherapy radiographers working with small-cell lung cancer patients by a factor of three. 31 The staffing levels in radiography departments with FDG PET/CT equipment may necessarily be higher to reduce the exposure of individual staff to FDG. 31
Although the patient remains radioactive after the scan, this reduces quickly over time (the half-life of fluorine-18 is approximately 110 minutes); however, people who come into contact with the patient once he or she leaves the hospital will receive a small dose of radiation. The associated risk of harm from such low doses is thought to be low, although the risk to repeated contacts such as hospital transport drivers is unknown. NHS Scotland provides advice for patients’ friends and family members, and ambulance and taxi drivers who might accompany patients from the hospital after a scan. It recommends that there should be a space between the patients and other passengers whilst in a car or ambulance and suggests that drivers who may routinely transport patients from an FDG PET/CT facility may be at risk of higher than background doses of radiation (Dr Jim Hannan, Royal Infirmary Edinburgh, 2009, personal communication).
Chapter 2 Research objectives
The main objectives of the review are to compare the accuracy of FDG PET/CT scans with that of other staging modalities in the pre-operative staging of CRC, and to model the cost-effectiveness of these different diagnostic tests.
This study focuses on the use of FDG PET/CT for staging CRC in three different groups of patients:
-
those with primary CRC
-
those with recurrent CRC
-
those with metastatic CRC.
Research questions
The following questions were considered to reflect UK staging practice.
Primary colorectal cancer
How accurate is FDG PET/CT combined with pelvic MRI or routinely used imaging modalities compared with routinely used imaging modalities (CT chest/abdomen/pelvis combined with pelvic MRI) for pre-operative staging of primary rectal cancer?
How accurate is FDG PET/CT in addition to routinely used imaging modalities compared with routinely used imaging modalities for pre-operative staging of primary colon cancer?
Recurrent colorectal cancer
How accurate is FDG PET/CT combined with pelvic MRI compared with routinely used imaging modalities (CT chest/abdomen/pelvis combined with pelvic MRI) for pre-operative staging of patients with pelvic recurrence of rectal cancer?
How accurate is FDG PET/CT ± MRI compared with routinely used imaging modalities (CT chest/abdomen/pelvis ± MRI) for pre-operative staging of patients with recurrent colon cancer?
Metastatic colorectal cancers
How accurate is FDG PET/CT imaging compared with routinely used imaging modalities for pre-operative staging in patients with metastatic CRC?
Secondary objective
The secondary objective was to determine the impact of diagnostic information provided by FDG PET/CT over conventional imaging techniques on decisions about patient management. We also extracted data pertaining to adverse effects when these were reported in the included studies.
Economic evaluation
An economic evaluation of FDG PET/CT as an add-on test compared with routinely used imaging modalities for pre-operative staging in patients with primary, recurrent or metastatic CRC.
Chapter 3 Review methods
A steering committee was convened that included all the authors of this report plus three public partners from the South East Scotland Cancer Network (see Acknowledgements). The committee considered the current use of FDG PET/CT for the pre-operative staging of CRC in radiological and surgical practice in the UK, and the following methodological design was agreed following discussion.
Search methods for the systematic review(s)
A database was assembled from a comprehensive search for published and unpublished studies, which included database searches, reference list searches and contact with experts. The software used was Reference Manager version 10 (Thomson Reuters, CA, USA).
The search of online databases was performed combining the concepts ‘colorectal’, ‘neoplasm’ and ‘FDG-PET’ using a sensitive variety of free-text and subject heading terms. No limits, such as language or publication year, were used. The search histories were based on scoping searches and refined by adapting searches used in previous Cochrane reviews32,33 and optimal search strategies. 34,35 Available guidance on search methods for diagnostic accuracy tests was followed. 36 Intermediate search results were tested against known relevant publications and revised to ensure enough sensitivity to include those known publications with the intention that additional, unknown publications would not be missed.
The following online databases were searched for relevant studies from their inception until May 2009: BIOSIS Previews; Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus; The Cochrane Library; Compendex; ProQuest Dissertations and Theses; EMBASE; Global Health; Global Health Library regional indexes (comprising LILACS, AFRO, EMRO, PAHO, WHOLIS); Index to Theses; Inspec; MEDLINE; metaRegister of Current Controlled Trials; National Technical Information Services; OpenSIGLE (System for Information on Grey Literature in Europe); UK Clinical Research Network; and Web of Science, including Conference Proceedings Citation Index.
Reference lists in review publications identified by the database searches were obtained and had the review inclusion criteria applied. All conference abstracts reporting studies that met the inclusion criteria were followed up to obtain published full reports.
In addition to the main systematic review and quality of life searches, which were structured to include data on adverse effects, a supplementary search for reports of safety and adverse events of PET in CRC was performed in EMBASE. The sensitive floating subheading for adverse effects was used in combination with subject headings and keyword terms for CRC, and the principal subject heading for PET.
The main search strategy can be found in Appendix 1.
Study selection
One reviewer screened titles and abstracts for relevance, and a second screened a 25% sample of the titles identified by the search activities as resources permitted. It was intended that disagreements were resolved by discussion, but there was a high level of agreement (> 80%). Full papers of potentially relevant studies were obtained and assessed for inclusion by one reviewer, and the whole sample was double-checked by a second. Two different sets of criteria were used for studies evaluating the diagnostic test accuracy of FDG PET/CT and those evaluating its therapeutic impact. No language restrictions were applied in either type of review, and non-English-language studies were read by individuals with language-specific reading skills. These individuals are identified in the acknowledgements section of this report.
Studies evaluating the accuracy of FDG PET/CT in the pre-operative staging of primary, recurrent and metastatic colorectal cancer
Eligibility criteria
Types of studies
Prospective and retrospective patient series (diagnostic cohort), cross-sectional, before and after studies and RCTs were eligible for inclusion. Both consecutive series and series not explicitly reported as consecutive were included. Diagnostic case–control studies (two-gate design) were excluded because clinically relevant estimates of specificity and sensitivity can be derived only from an unselected sample of the clinical population.
Participants
Adults with known or suspected primary cancer of the colon or rectum undergoing pre-operative staging prior to curative surgery in a secondary care setting were eligible for inclusion. Patients with any stage of disease were included. Studies solely in patients with anal cancer were excluded because this rare cancer differs from CRCs both biologically and in terms of the treatment pathway. Studies that included colorectal and anal cancer patients in which data were not reported separately for the colorectal and anal cancer groups were included in the review only if < 20% of patients had anal cancer. It was intended that the effect of including these studies would be explored using sensitivity analysis where possible.
Index tests
Studies using only integrated FDG PET/CT equipment with both contrast-enhanced and non-contrast-enhanced CT were considered eligible for inclusion.
Comparator tests
Standard imaging tests including ultrasound, diagnostic CT, MRI and PET, alone or in combination.
Target conditions
Known or suspected primary, recurrent or metastatic CRC.
Reference standards
Histopathology of surgical resected specimens is the gold standard for tests used in CRC pre-operative staging; however, patients who do not undergo surgical resection (because tests show they have incurable disease) have their results verified by an alternative standard. These include histopathology based on biopsy and follow-up, which can include both clinical examination and imaging tests, but wide variations in practice for CRC follow-up are well recognised. Although it was intended to restrict the eligibility criteria to surgical histopathology and follow-up, so few studies met these criteria that we deviated from the original protocol to include studies using any reference standard either singly or mixed. Any duration of follow-up and frequency of follow-up were permitted.
Data extraction and management
Data were extracted by two reviewers (HMc and FCr) independently using a standard form, which included the quality assessment criteria, and disagreements were resolved by discussion. Data to populate 2 × 2 contingency tables consisting of the numbers of true positives (TP), true negatives (TN), FPs and FNs using the studies’ own definitions were extracted as reported, including both patient- and lesion-level data, and qualitative and quantitative definitions of diagnostic thresholds. Numbers of uninterpretable test results were also extracted when these were reported.
Data that described the clinical characteristics of FP and FN FDG PET/CT findings, and additional cases detected and cases re-staged by the use of FDG PET/CT, actual changes in planned management directed by FDG PET/CT findings and the clinical consequences of the changes were also collected. Data on mortality, adverse events (including how these were monitored and recorded) and technical failures for both index and comparator tests were also extracted.
To facilitate our interpretation of the results, we extracted data on comorbidities (e.g. diabetes) and previous treatment in the study population; the FDG PET/CT system; fasting duration; FDG dose and time between administration of FDG and performance of the scan; comparator imaging test system(s), patient preparation and test interpretation; interval between index and comparator tests (more or less than 3 months); assessors (number, expertise, experience, consensus procedures and learning effect data); and, in regard to the reference standard, whether histopathology was by surgery or biopsy, the duration, frequency, type and interpretation of clinical and imaging follow-up tests, and the numbers of patients whose results were confirmed by each type of reference standard.
Non-English-language studies had data extracted by one reviewer (FCr) during face-to face discussions with fluent individuals who translated all available data outlined above.
The assessment of methodological quality of studies evaluating the accuracy of FDG PET/CT in the pre-operative staging of colorectal cancer
Fourteen items from the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist were used to assess the methodological quality of the included studies. Two reviewers (HMc and FCr) applied the criteria independently and resolved disagreements by discussion. We intended to use the results of the quality assessment for descriptive purposes to provide an evaluation of the overall quality of the included studies and to investigate potential sources of heterogeneity. 37
The classification of responses to each of the QUADAS items is summarised in Appendix 2.
Statistical analysis and data synthesis of studies evaluating the accuracy of FDG PET/CT in the pre-operative staging of colorectal cancer
We intended to analyse data pertaining to the accuracy of FDG PET/CT in the following way. Because of methodological problems, particularly those caused by the difficulty of estimating within-study variance when patients contribute more than one data point, and when the individual patient data are not available, it was our intention that the 2 × 2 tables should report the lesion-level data, but that the analyses be restricted to patient-level data.
The 2 × 2 tables for the patient-level data were used to calculate sensitivity and specificity with confidence intervals (CIs). Depending on the degree of heterogeneity, data were plotted graphically in forest plots to give an indication of the extent of heterogeneity between studies. 38
A random effects meta-analysis was planned to fit the bivariate summary receiver operating characteristic (SROC) curve model38 with the within-study variances fitted as binomial. The DiagMeta package in R was used for all meta-analyses and forest plots (www.r-project.org). In the event that the data were not amenable to bivariate random effects meta-analyses, separate meta-analyses for sensitivity and specificity were presented instead, using fixed or random effects as appropriate, depending on the degree of heterogeneity. Estimates include the average sensitivity and specificity for each test.
Investigation of sources of heterogeneity
Several potential sources of heterogeneity were identified in other systematic reviews and meta-analyses of diagnostic imaging techniques in CRC. 39–44 These were considered by the clinical authors of this report, who identified the factors most likely to affect diagnostic accuracy in studies of FDG PET/CT from these previously published systematic reviews and also from their own clinical and surgical experience.
We planned to investigate the following potential sources of heterogeneity, using subgroup analysis where possible: academic (e.g. university hospital) versus non-academic setting; indication known or suspected; study conducted up to 2005 and post 2005 (reflecting differences in FDG PET/CT technology); blinding of index and reference standard test interpretation. It was also our intention that heterogeneity in the statistical analysis would be initially assessed graphically where possible using meta-regression (see Investigations of heterogeneity).
Investigations of heterogeneity
We intended to explore heterogeneity due to individual diagnostic studies using different diagnostic thresholds as a standard part of fitting the bivariate SROC curve model. Investigations of heterogeneity due to other study characteristics were planned using meta-regression, but only if the data were adequate for such analyses. In either case, we planned graphical displays of estimates from individual studies grouped according to the pre-specified sources of heterogeneity.
Sensitivity analyses
We planned to conduct sensitivity analysis where possible by including only prospective studies (excluding retrospective) with explicitly consecutive samples (excluding non-consecutive or unclear), studies with a histopathology (surgical specimen or biopsy) reference standard for all participants (excluding studies in which some or all participants received only clinical or imaging follow-up as the reference standard) and studies that included only rectal and colon cancer patients (excluding anal cancer).
Studies evaluating the therapeutic impact of pre-operative staging of FDG PET/CT for primary, recurrent and metastatic colorectal cancer
For studies evaluating the therapeutic impact (changes in management) of FDG PET/CT, we adapted our methodological approach detailed above. The main differences were the eligibility criteria and the quality assessment processes; it was no longer necessary for the studies to include 2 × 2 data and this was dropped as an eligibility criterion, and the items used in the quality assessment process were assembled from three different sources in recognition of the different study designs required to evaluate changes in management arising from the use of diagnostic tests. 37,45,46
Eligibility criteria
Types of studies
We included case series, cross-sectional studies, before and after designs and RCTs.
Participants
Adults with known or suspected primary cancer of the colon or rectum undergoing pre-operative staging prior to curative surgery in a secondary care setting were eligible for inclusion. Patients with any stage of disease were included. Studies solely in patients with anal cancer were excluded. Studies that included colorectal and anal cancer patients in which data were not reported separately for the colorectal and anal cancer group, were included in the review only if < 20% had anal cancer. It was intended that the effect of including these studies would be explored using sensitivity analysis where possible.
Index tests
Studies using only integrated FDG PET/CT equipment with either contrast-enhanced or non-contrast-enhanced CT were considered eligible for inclusion.
Comparator tests
Standard imaging tests including ultrasound, diagnostic CT, MRI and PET, alone or in combination.
Target conditions
Known or suspected primary, recurrent or metastatic CRC.
Reference standards
Surgically resected specimens, histology and clinical and imaging follow-up were considered for inclusion, and studies using these reference standards singly or mixed were eligible for inclusion. Any duration of follow-up and frequency of follow-up testing were included.
Data extraction and management
Data were extracted by two reviewers (HMc and FCr) independently using a standard form, which included the quality assessment criteria, and disagreements were resolved by discussion.
Additional cases detected and cases restaged by the use of FDG PET/CT were collected, as were the actual changes in planned management directed by FDG PET/CT findings and the clinical consequences of the changes. Data on mortality, adverse events (including how these were monitored and recorded) and technical failures for both index and comparator tests were also extracted.
To facilitate interpretation of the findings, we extracted data on comorbidities (e.g. diabetes) and previous treatment in the study population; the FDG PET/CT system; fasting duration; FDG dose and time between administration of FDG and performance of the scan; comparator imaging test system(s), patient preparation and test interpretation; interval between index and comparator tests (more or less than 3 months); assessors (number, expertise, experience, consensus procedures and learning effect data); and, in regard to the reference standard, whether histopathology was by surgery or biopsy and the duration, frequency, type and interpretation of clinical and imaging follow-up tests, and the numbers of patients whose results were confirmed by each type of reference standard. Diagnostic findings, changes in management or treatment intent, appropriateness of management decisions or patient outcomes, adverse events, numbers of patients with each type of event, and consequences, for example withdrawal from the study, were all sought from each report. Non-English-language studies had data extracted by one reviewer (FCr) during face-to-face discussions with fluent individuals who translated all available data outlined above.
Contact with authors
When necessary, authors of studies identified by our search activities were contacted to request points of clarification in assessing a study’s eligibility for inclusion in the review or to request specific data.
Search methods for FDG PET/CT economics, decision-making and quality of life
Additional database searches for studies with data on economics, decision-making and quality of life were undertaken. The searches used a sensitive combination of subject headings and free-text search terms, constructed by drawing on key terms identified by the expert health economists in combination with adaptations of the Scottish Intercollegiate Guideline Network’s published search filters (www.sign.ac.uk/methodology/filters.htmlwww.sign.ac.uk/methodology/filters.html).
The following databases were also searched: Cost-effectiveness Analysis Registry; CINAHL; The Cochrane Library; EMBASE; Health Management Information Consortium; MEDLINE; and Web of Science. The search strategies used can be found in Appendix 3.
FDG PET/CT as an add-on imaging test versus routinely used imaging modalities for pre-operative staging in patients with primary, recurrent or metastatic colorectal cancer
Handsearch study
Systematic reviews adopt an approach of searching extensively for studies to ensure as much available evidence as possible can inform the review and to minimise bias, including publication bias. Handsearching has formed part of the extensive search approach for systematic reviews of effects.
Guidance for conducting Cochrane systematic reviews of effects evidence and more recently diagnostic test accuracy studies recommends that handsearching should be considered to enhance the retrieval of relevant studies, but there is sparse research evidence for the value of this approach. We therefore designed a complementary but distinct research project to exploit the opportunity to explore the value of handsearching to inform an imaging systematic review and to contribute to our understanding of the role of handsearching in the identification of reports of diagnostic test accuracy studies. A full report of the method employed and the results obtained can be found in Appendix 4.
A copy of the protocol for the systematic review can be found in Appendix 5.
Chapter 4 Included and excluded studies identified by the search strategy
FIGURE 1.
Flow chart of study selection process for studies of diagnostic test accuracy and therapeutic impact. CRC, colorectal cancer; DTA, diagnostic test accuracy.
A table of excluded studies with reasons for exclusion can be found in Appendix 6.
Chapter 5 FDG PET/CT for the pre-operative staging of primary colorectal cancer
Recent statistics on the incidence of CRC report that 37,514 new cases of large bowel cancer were registered in the UK in 2006. Primary colonic tumours (any part of the colon other than the rectum) outnumber primary rectal tumours by approximately 2 : 1. 47
After the first diagnosis of a new CRC with colonoscopy and biopsy, and occasionally following an incidental finding on radiological imaging performed for another reason, more sophisticated medical imaging tests are required to accurately stage the tumour to determine the extent to which other tissues might be involved. The aim of radiological imaging is to obtain information regarding the primary tumour (T stage), local and distant nodal involvement (N stage) and distant metastatic disease (M stage).
The containment of the tumour to the mucosa or the muscularis propria is associated with improved survival. 48 However, because of delayed presentation before development of symptoms, it is not uncommon for CRCs to have either spread locally through the colonic wall, with involvement of local and distant nodal groups, or metastasised. Metastases tend to occur to the liver and lung.
For staging primary cancer of the colon, CT with spiral acquisition through the chest, abdomen and pelvis following intravenous contrast administration is the conventional imaging technique and is readily available and reproducible. MRI is not currently used for assessment of primary colonic tumours because of image degradation by peristalsis, and does not add any value to standard CT imaging in this area.
Magnetic resonance imaging is used as standard for staging of rectal cancers, with the benefits of imaging a part of the bowel that is not degraded by peristaltic motion resulting in more accurate assessment of the primary tumour and relationships to bowel wall. MRI is also capable of identifying involved local nodal groups, although, currently, standard practice is to rely on size criteria to suggest involvement. This results in a large inherent inaccuracy, as many studies have shown that size alone is a poor indicator of disease status within nodal groups. Spiral CT is also used to give information on local and distant nodal involvement and the presence or absence of metastatic disease, again usually to the liver and lungs.
FDG PET/CT is currently recommended only for the assessment of suspected recurrence of CRC and in pre-operative staging prior to metastectomy, and clinical opinion on the role of FDG PET/CT in the routine management of primary colon cancer varies. Some investigators suggest that in certain clinical circumstances it should be considered as part of the standard pre-operative assessment and acknowledge it may have an up-and-coming role in the initial staging of primary rectal cancer. 21,26–28 More recently, small studies have suggested that FDG PET/CT may offer a clinically useful addition for the routine staging of rectal cancers. 27 In one study, FDG PET/CT was able to identify involved nodes outwith the mesorectal fascia not seen with standard imaging, particularly with low rectal tumours in which iliac and inguinofemoral nodal involvement was a common finding. 49 This resulted in a change in planned management in 27% of patients, improving the accuracy of pre-treatment staging.
As 11% of the CRC population present with metastases at the time of first diagnosis,50 the early identification of those with advanced disease might lead to improved survival. Replacing diagnostic CT with FDG PET/CT as the initial imaging investigation has considerable resource and cost implications; in a cost-effectiveness study in 2005, a single PET scan (using non-integrated equipment) was estimated to cost €1038 compared with €313 for a single CT scan. 51 These cost considerations currently limit FDG PET/CT use to an add-on test in most centres where the technology is available.
The rationale for using FDG PET/CT (or PET/contrast-enhanced CT) as a replacement test, at the outset of the CRC diagnostic pathway, is the avoidance of unnecessary and expensive surgery in individuals who have advanced incurable disease at the time of the first diagnosis. 50
Replacing the diagnostic CT scan with FDG PET/CT implies that the CT component of both investigations should be of a similar standard. Modern FDG PET/CT systems tend to use low-dose spiral CT to localise a functional abnormality – this is because the patient has already undergone a high-quality, higher-dose spiral CT scan with intravenous contrast in almost all cases. Using FDG PET/CT as a replacement should involve performance of the CT component to the same standards as the diagnostic scan – this increases the complexity of the FDG PET/CT study but may have a role in the future.
It is anticipated that there will be a knock-on effect from the UK CRC screening programmes on NHS CRC radiology services, and this also deserves consideration. 50,52 Results from the second round of the UK CRC screening programme show that the majority of detected cancers are early tumours, classified as Dukes A or B. FDG PET/CT is more commonly used for the detection of recurrence and liver metastases, and the value of its use in the detection of small, contained primary CRCs remains under-researched. The limitation of the spatial resolution of modern scanners (5–8 mm) is a potential issue here and, although modern FDG PET/CT systems are capable of identifying primary CRCs, the standard roles for this imaging modality are concerned with disease spread, rather than assessment of the primary tumour.
Aim
The aim of this systematic review is to compare FDG PET/CT imaging with routinely used imaging modalities for pre-operative staging in patients with a primary diagnosis of CRC.
Objectives
The primary objective is to determine the diagnostic accuracy of integrated FDG PET/CT over (in addition to) conventional imaging for the pre-operative staging of primary CRC. The comparisons of interest are:
-
PET/CT combined with pelvic MRI or routinely used imaging modalities versus routinely used imaging modalities (CT chest/abdomen/pelvis combined with pelvic MRI) for pre-operative staging of primary rectal cancer.
-
PET/CT in addition to routinely used imaging modalities versus routinely used imaging modalities for pre-operative staging of primary colon cancer.
Results
Our search did not identify any systematic reviews to evaluate the diagnostic test accuracy of integrated FDG PET/CT in the pre-operative staging of primary CRC.
We found no studies that met the stated objectives, but have presented all studies that included patients with primary CRC who received FDG PET/CT for pre-operative staging.
Number of included studies
The search identified two studies53,54 that evaluated the diagnostic test accuracy of integrated FDG PET/CT for the detection of primary CRC.
Study characteristics and study designs
The study characteristics are shown in Table 1 and accuracy data are shown in Table 2.
| Study | Population | Index test | Comparator(s) | Reference standard |
|---|---|---|---|---|
|
Tsunoda 200853 Country: Japan Year: 2004–5 Study design: prospective/retrospective: unclear; consecutive sample: unclear Setting: national cancer centre Aim: to assess the value of FDG PET/CT for detection of LN metastases |
88 patients (52 men, 36 women), mean age 60.6 years (range 23–89 years) Indication: pre-operative nodal diagnosis Exclusion criteria: NR Disease: primary CRC; colon (n = 37), rectum (n = 51) Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery LS8® (GE Healthcare, Fairfield, CT); fasting duration at least 6 hours; FDG 370 MBq, scan 60 minutes later; non-contrast-enhanced CT Image interpretation Assessors: two observers experienced in interpreting FDG PET/CT, blind to clinical information, disagreement resolved by discussion to reach consensus Qualitative: LN metastases diagnosed by abnormal uptake regardless of node shape or size (visual diagnosis) Quantitative: maximal nodal diameter (size diagnosis) cut-off value 10 mm; SUVmax of LNs with greater uptake than normal organs or surrounding tissue (SUV diagnosis): the optimal cut-off value was where accuracy was greatest; when more than one LN in the proximal or distant region was malignant, the one with the highest SUV was used in the analysis |
None | Histopathology; all patients had surgical resection and regional LN dissection |
|
Tateishi 200754 Country: Japan Year: NR Study design: retrospective; consecutive sample: unclear Setting: cancer centre hospital Aim: to compare contrast-enhanced FDG PET/CT and non-contrast-enhanced FDG PET/CT for nodal staging of rectal cancer |
53 patients (32 men, 21 women), mean age 61 years (range 27–79 years) Indication: pre-operative staging of rectal cancer Exclusion criteria: evidence of distant metastases, diabetes, pregnancy, lactation; performance status other than 0 (fully active) or 1 (restricted in physically strenuous activity but ambulatory) Disease: histologically proven rectal cancer; rectosigmoid (n = 6), upper rectal (n = 26), lower rectal (n = 21); Stage I (n=12), Stage IIA (n = 5), Stage IIB (n = 2), Stage IIIA (n = 6), Stage IIIB (n = 24), Stage IIIC (n = 4); mucinous tumour (n = 6) Comorbidities: NR Previous treatment: NR |
FDG PET/CT Biograph® (Siemens Health Care Diagnostics, Surrey, UK); fasting duration at least 6 hours; FDG 370–555 MBq, time to scan NR; non-contrast-enhanced CT Image interpretation Assessors: a certified radiologist and a nuclear medicine specialist, blind to clinical information and results of other studies, in consensus Qualitative: Abnormal LN: focal increased uptake at a location corresponding to LN chains on CT scans; abnormal uptake: focal increased activity higher than that of the background soft tissue Quantitative: SUVmax; no further details |
CE FDG PET/CT CT as index test with IV contrast agent Image interpretation As index test Time between index and comparator tests: sequential; time between assessment of the two data sets 3 months |
Histopathology, all patients had total mesorectal resection and lymphadenectomy performed within 2 weeks of the radiological tests; 14 nodal stations were examined in each patient |
| Study | Test and outcome level | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| TP/TP + FN | TN/TN + FP | TP/TP + FP | TN/TN + FN | ||||||
| Tsunoda 200853 | FDG PET/CT, nodal staging, LN group level (proximal, distal), visual diagnosis | 14 | 9 | 118 | 35 | 0.286 | 0.929 | 0.609 | 0.771 |
| FDG PET/CT, nodal staging, LN group level, size diagnosis | 15 | 6 | 121 | 34 | 0.306 | 0.953 | 0.714 | 0.781 | |
| FDG PET/CT, nodal staging, LN group level, SUV diagnosis 1.5 | 26 | 12 | 115 | 23 | 0.531 | 0.906 | 0.684 | 0.833 | |
| FDG PET/CT, nodal staging, LN group level, SUV diagnosis 2.5 | 19 | 7 | 120 | 30 | 0.388 | 0.945 | 0.731 | 0.800 | |
| FDG PET/CT, nodal staging, LN group level, SUV diagnosis 3.5 | 12 | 0 | 127 | 37 | 0.245 | 1.000 | 1.000 | 0.774 | |
| FDG PET/CT, proximal nodal group staging, patient level, SUV diagnosis 1.5 (optimal cut-off value) | 21 | 7 | 40 | 20 | 0.512 | 0.851 | 0.750 | 0.667 | |
| FDG PET/CT, distant nodal group staging, SUV diagnosis 1.5 (optimal cut-off value) | 5 | 6 | 74 | 3 | 0.625 | 0.925 | 0.455 | 0.961 | |
| Tateishi 200754 | FDG PET/CT, nodal staging, patient level | 29 | 11 | 8 | 5 | 0.853 | 0.421 | 0.725 | 0.615 |
| Contrast-enhanced FDG PET/CT, nodal staging, patient level | 29 | 6 | 13 | 5 | 0.853 | 0.684 | 0.829 | 0.722 | |
| FDG PET/CT, pararectal nodal staging, patient level | 24 | 10 | 11 | 8 | 0.750 | 0.524 | 0.706 | 0.579 | |
| Contrast-enhanced FDG PET/CT, pararectal nodal staging, patient level | 29 | 5 | 16 | 3 | 0.906 | 0.762 | 0.853 | 0.842 | |
| FDG PET/CT, internal iliac nodal staging, patient level | 5 | 7 | 30 | 11 | 0.313 | 0.811 | 0.417 | 0.732 | |
| Contrast-enhanced FDG PET/CT, internal iliac nodal staging, patient level | 12 | 5 | 32 | 4 | 0.750 | 0.865 | 0.706 | 0.889 | |
| FDG PET/CT, obturator nodal staging, patient level | 8 | 16 | 25 | 4 | 0.667 | 0.610 | 0.333 | 0.862 | |
| Contrast-enhanced FDG PET/CT, obturator nodal staging, patient level | 8 | 2 | 39 | 4 | 0.667 | 0.951 | 0.800 | 0.907 |
One study54 reported using a retrospective design. In the second study53 the study design was unclear.
Study setting and country in which the research was conducted
Both studies were conducted in cancer centres in Japan.
Patient populations
The studies reported outcomes for a total of 141 patients, who were mostly men. The mean age of the patients in the two studies was 6053 and 6154 years, respectively, with a range of 23–89 years. Rectal cancer affected 104 patients and cancer of the colon affected 37.
Indication for FDG PET/CT
FDG PET/CT was undertaken in order to pre-operatively stage the primary CRC, and was reported to be specifically for the diagnosis of nodal disease in one study. 53
FDG PET/CT equipment and patient preparation
Full details of all the equipment used in the studies can be found in Table 1. FDG PET/CT equipment was manufactured by GE Healthcare (Fairfield, CT) and Siemens Medical Solutions (Surrey, UK). The fasting duration prior to the scan was at least 6 hours in both studies.
A range of injected FDG doses was reported with units ranging from 370 to 555 MBq. Where the information was reported, patients were scanned 60 minutes after the administration of the radioactive tracer. 53 Neither study used contrast-enhanced CT.
Image interpretation
The index and comparator tests, when one was performed,54 were assessed by at least two individuals blind to clinical information. The qualitative and quantitative image interpretations were conducted in similar ways in each of the studies.
In one study,53 lymph node metastases were qualitatively diagnosed by abnormal uptake regardless of node shape or size (visual diagnosis). A maximal nodal diameter (size diagnosis) cut-off value of 10 mm was used; maximum standardised uptake value (SUVmax) of lymph nodes with greater uptake than normal organs or surrounding tissue (SUV diagnosis): the optimal cut-off value was where accuracy was greatest; when more than one lymph node in the proximal or distant region was malignant, the one with the highest SUV was used in the analysis.
In the second study54 the qualitative assessment judged lesions positive if there was an abnormal focal uptake at a location corresponding to lymph node chains on CT scans. Abnormal uptake was defined as a focal increased activity higher than that of the background soft tissue. The authors also reported using SUVmax in the quantitative diagnosis but did not report any other details.
Reference standard
Both studies reported histopathology from surgically resected specimens and lymph node resection as the reference standard in all patients.
Data synthesis – diagnostic performance
FDG PET/CT versus none
In proximal nodal staging based on patient-level analysis with an SUV threshold of 1.5, FDG PET/CT demonstrated a sensitivity of 51% (95% CI 36% to 66%) and a specificity of 85% (95% CI 72% to 92%). For distal nodal staging using an SUV threshold of 1.5, the sensitivity of FDG PET/CT was 62% (95% CI 30% to 86%) and specificity was 92% (95% CI 84% to 96%).
In analysis based on group-level data and compared with findings from surgical excisions and region lymph node dissection,FDG PET/CT was found to have a sensitivity of 28% (95% CI 18% to 42%) and specificity of 92% (95% CI 87% to 96%) when used for nodal staging of proximal and distal lymph nodes (n = 176).
In the detection of lymph nodes based on size (nodal maximum axial diameter) and a threshold of ≥ 10 mm, the authors found FDG PET/CT to have a sensitivity of 30% (95% CI 19% to 44%) and a specificity of 95% (95% CI 90% to 97%).
For SUV diagnosis using the optimal cut-off value of 1.5, the sensitivity of FDG PET/CT was 53% (95% CI 39% to 66%) and the specificity was 90% (95% CI 84% to 94%). At a threshold of 2.5, the sensitivity of FDG PET/CT was 38% (95% CI 26% to 53%) and the specificity was 94% (95% CI 89% to 97%), and, at a threshold of 3.5, the sensitivity was 24% (95% CI 15% to 38%) and the specificity was 100% (95% CI not calculable).
FDG PET/CT versus contrast-enhanced FDG PET/CT
Accuracy in diagnosing nodal staging appeared to be improved by adding contrast enhancement to the FDG PET/CT in 53 patients. FDG PET/CT had a sensitivity of 85% (95% CI 69% to 93%) and a specificity of 42% (95% CI 23% to 67%), and the respective accuracy of contrast-enhanced FDG PET/CT was 85% (95% CI 69% to 93%) and 68% (95% CI 46% to 84%).
FDG PET/CT produced poorer estimates of accuracy than contrast-enhanced FDG PET/CT in imaging all lymph nodes: a sensitivity of 75% (95% CI 57% to 87%) and a specificity of 52% (95% CI 32% to 71%) for FDG PET/CT compared with 90% sensitivity (95% CI 76% to 97%) and 76% specificity (95% CI 55% to 89%) for contrast-enhanced FDG PET/CT for pararectal nodes; a sensitivity of 31% (95% CI 14% to 55%) and specificity of 81% (95% CI 66% to 90%) for FDG PET/CT but 75% sensitivity (CI 95% 50% to 90%) and 86% specificity (95% CI 72% to 94%) for contrast-enhanced FDG PET/CT for internal iliac nodes; and a sensitivity of 67% (95% CI 39% to 86%) and a specificity of 61% (95% CI 46% to 74%) for FDG PET/CT compared with a sensitivity of 67% (95% CI 39% to 86%) and a specificity of 95% (95% CI 84% to 99%) for contrast-enhanced FDG PET/CT for obturator nodes.
Quality assessment
Fourteen items from the QUADAS checklist were used to assess the methodological quality of the results and the findings from this process are shown in Table 3.
| Study | Spectrum of patients’ representative? | Selection criteria clearly described? | Reference standard likely to classify the target condition? | Time between the reference standard and index test short enough? | Whole or a random sample receives verification using a reference standard? | Patients received the same reference standard regardless of the index test result? | Reference standard independent of the index test? | Execution of the test described in sufficient detail to permit replication? | Execution of the reference standard described in sufficient detail? | Index test results interpreted without knowledge of the reference standard results? | Reference standard results interpreted without knowledge of the index test results? | Same clinical data available when tests results were interpreted as in clinical practice? | Uninterpretable intermediate test results reported? | Withdrawals from the study explained? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tateishi 200754 | N | Y | Y | Y | Y | Y | Y | Y | UC | UC | UC | N | UC | Y |
| Tsunoda 200853 | Y | N | Y | UC | Y | Y | Y | Y | UC | UC | UC | N | UC | Y |
Only one study reported including a consecutive series of patients nor a random sample of adults undergoing pre-operative staging of primary CRC. 53 In both studies the assessors were blind to the clinical information and results of other studies. The reference standard test of histopathology was applied to all patients and is therefore likely to be 100% sensitive and specific, but neither study gave details of the execution of the reference standard. The reviewers considered 6 weeks to be the time limit after which disease progression might occur, and the time between the reference standard and the index test was reported to be ≤ 6 weeks in one study. 53 The withdrawals from the study were explained for both studies.
In addition to selection bias, the validity of the findings from these studies is also potentially compromised by review bias arising from the lack of information about the ‘blinding’ of individuals reviewing the scans.
Summary
-
The accuracy data to support the use of FDG PET/CT or contrast-enhanced FDG PET/CT in the pre-operative staging for primary CRC are very limited and both studies53,54 include small samples of patients. Furthermore, cross-tabulation of results of different tests for patients contributing to the same study was not available. This meant that significance testing for differences between the sensitivity and specificity of different tests was not carried out.
-
Both studies53,54 produced estimates of accuracy for the detection of lymph node disease associated with primary CRC, but the lack of comparisons with other tests makes it difficult to place a value on the use of this test in clinical practice.
-
An analysis based on lymph node size showed FDG PET/CT to have a sensitivity of 30% and a specificity of 85% for tumours > 10 mm.
-
The patient population was not well described in terms of disease, and the accuracy estimates from both studies may be compromised by reviewer bias.
-
In one study,53 patients had a primary diagnosis of both rectal and colon cancer, which makes the clinical interpretation of this study difficult because colon cancer and rectal cancer are regarded as two distinct pathologies, and are investigated and treated differently.
-
There is a suggestion that contrast-enhanced FDG PET/CT is more accurate for pre-operative staging of primary CRC than non-contrast-enhanced FDG PET/CT, but the poor quality of the data makes a reasonable interpretation difficult.
-
Both studies53,54 suggest that FDG PET/CT is able to identify nodal disease remote from the primary site, and this may suggest a future role for FDG PET/CT in the pre-operative staging of primary rectal cancer.
-
There is a lack of data to support the use of FDG PET/CT in the routine staging of all patients diagnosed with primary CRC.
Chapter 6 FDG PET/CT in the pre-operative staging of recurrent colorectal cancer
The rate of recurrence of CRCs is high, and long-term postoperative surveillance of patients is regarded by many as essential to identify relapsing malignancy early. 55,56 Estimates suggest that 40% of patients who have undergone a surgical resection of primary cancer in the colon or rectum will have recurrent disease confirmed during a follow-up period of 2–3 years. 56,57 Recurrent lesions may occur locally at the site of the previous tumour or in distant tissues, typically the liver or lungs. Two main classification systems are used to stage CRCs – the Dukes’ and TNM systems – and survival diminishes as numeric values increase. 21,25 This chapter reports the evidence that FDG PET/CT accurately stages local recurrence of CRC pre-operatively.
Local recurrence of colon cancer is less common than recurrent rectal cancer because the surgical removal of primary tumours of the colon involves extensive resection and the removal of lymph nodes. 58 Most primary rectal tumours are confined to the pelvis, and the prognosis is improved in this group of patients compared with those with worse TNM stages. 59 TME is the procedure used to remove both the tumour and the surrounding mesorectal fat, and this is currently the standard surgical treatment for all patients with primary rectal cancer. This technique is associated with a reduction in rates of recurrence and mortality. 60
Although successful surgical outcomes can increase life expectancy for some patients, secondary surgical resections often result in considerable morbidity and greatly diminished quality of life for survivors. 56 Accurate pre-operative re-staging is essential to distinguish those most likely to benefit from additional, sometimes drastic, surgery. 60
The procedures used in the routine follow-up of patients with a history of CRC vary but generally include blood testing to detect rising levels of carcinoembryonic antigen (CEA), imaging tests (predominantly CT) and clinical examination. 55,56 When recurrence is suspected, combinations of MRI, CT, chest radiography and ultrasound are used to confirm the presence or absence of disease and allow pre-operative staging to reclassify the disease status. MRI is regarded by many as the best method to image the rectum. 56 In hospitals with access to FDG PET/CT, this imaging test is now used alongside conventional imaging techniques and is widely believed to have an important role in the detection and the delineation of the extent of recurrent colorectal tumours. 21,55
The accuracy of all radiological imaging tests used for pre-operative staging of recurrent CRC is hampered by the gross disruption to the pelvic anatomy arising from the first surgery and associated chemotherapy. 56 The ability of individual imaging tests to differentiate between fibrous scar tissue and tumour is considered to be especially valuable in staging recurrent CRC.
A systematic review and meta-analysis of studies evaluating the diagnostic test accuracy of PET without a CT integrated component42 reported a sensitivity of 94% and a specificity of 98% in 366 patients with locally recurrent rectal cancer. When data pertaining to changes in the management of these patients were pooled, 29% (95% CI 25% to 34%) of management decisions were changed, the majority of which were the avoidance of surgery as a result of the upstaging of the patients’ disease classification.
Aim
The aim of this systematic review is to compare FDG PET/CT imaging with routinely used imaging modalities for pre-operative staging in patients with a local recurrence of CRC.
Objectives
The primary objective is to determine the diagnostic accuracy of integrated FDG PET/CT over (in addition to) conventional imaging for the pre-operative staging of recurrent CRC. The comparisons of interest are:
-
DG PET/CT combined with pelvic MRI versus routinely used imaging modalities (CT chest/abdomen/pelvis combined with pelvic MRI) for staging of patients with pelvic recurrence of rectal cancer.
-
PET/CT ± MRI versus routinely used imaging modalities (CT chest/abdomen/pelvis ± MRI) for staging of patients with recurrent colon cancer.
Results
Our search did not identify any systematic reviews evaluating the diagnostic test accuracy of integrated FDG PET/CT in the pre-operative staging of recurrent CRC.
We found no studies that met the stated objectives but have presented all studies that included patients with suspected recurrent CRC who received FDG PET/CT for pre-operative staging.
Number of included studies
The search identified eight studies61–68 that evaluated the diagnostic test accuracy of integrated FDG PET/CT for the detection of colorectal recurrence.
Study characteristics and study designs
The study characteristics are shown in Table 4 and accuracy data are shown in Table 5. All eight studies used a retrospective design. Three62,65,67 studies reported recruiting patients consecutively.
| Study | Population | Index test | Comparator(s) | Reference standard |
|---|---|---|---|---|
|
Sarikaya 200761 Country: USA Year: 2005–6 Study design: retrospective; consecutive sample: unclear Setting: university hospital Aim: to assess the value of FDG-PET in patients with suspicion of CRC recurrence but with normal CEA |
39 patients who had PET (n = 27) or FDG PET/CT (n = 12); mean age 55 years (range 33–91 years) Indication: clinical and/or radiological suspicion of recurrence but normal CEA (0–5 ng/ml); suspicion based on history and physical exam (n = 20), equivocal lesions on CT (n = 17), barium study (n = 2) Exclusion criteria: no histopathological evaluation following PET scan Disease: NR Comorbidities: NR Previous treatment: surgical resection alone, or chemotherapy and/or RT before or after resection (numbers NR) |
FDG PET/CT Biograph 16® (Siemens Health Care Diagnostics, Surrey, UK); fasting duration approximately 6 hours; FDG 370–555 MBq, scan approximately 60 minutes later; CT: non-contrast-enhanced CT Image interpretation Assessors: two certified nuclear medicine physicians, no further details Qualitative: positive or suspicious: abnormal or non-physiological activity; focal hypermetabolic activity in the liver greater than adjacent normal liver; isometabolic liver lesions identified with the help of the CT component; diffuse mild activity in bowel considered normal physiologic uptake Quantitative: mean SUVmax compared between PET TPs and FPs but not used as a threshold |
None | Histopathology, surgery within 2 months of FDG PET/CT scan |
|
Bellomi 200762 Country: Italy Year: NR Study design: retrospective; consecutive sample: yes Setting: university hospital Aim: to compare MDCT and FDG PET/CT for detection of local and distant recurrence of rectal cancer |
67 patients (gender NR), age NR Indication: suspicion of local or distant recurrence on routine follow-up (CEA, abdominal and pelvic MDCT, chest radiography or colonoscopy) following radical surgery Exclusion criteria: diabetes Disease: local recurrence (n = 15), distant recurrence (n = 27); hepatic metastases (n = 17); local recurrence and hepatic metastases (n=7); lung metastases (n = 8); local recurrence and lung metastases (n = 2) Comorbidities: NR Previous treatment: all underwent radical surgery; pre-surgical RT (n = 20); post-surgical RT (n = 5); pre-surgical chemotherapy (n = 5); post-surgical chemotherapy (n = 18); post-surgical RT and chemotherapy (n = 19) |
FDG PET/CT Discovery LS® (GE Medical Systems, Fairfield, CT); fasting duration 6 hours; FDG 5 MBq/kg, scan approximately 60 minutes later; non- contrast-enhanced CT Image interpretation Assessors: experts in nuclear medicine, aware of other clinical, laboratory and diagnostic investigations results, who had just started interpreting FDG PET/CT images at the time of the study Qualitative: Local recurrence: any new tissue at or close to anastomosis with asymmetrical, irregular, inhomogeneous contrast enhancement or changes compared with previous findings; hepatic or lung metastases: lesions not present at pre-operative CT Quantitative: SUV not used |
MDCT Lightspeed scanner® (GE Medical Systems, Fairfield, CT); intravenous contrast agent Image interpretation Assessors: expert radiologists with several years’ experience of reading MDCT scans Qualitative: as index test Quantitative: NR Time between index and comparator tests: within 30 days, mean 22 days |
Histology (biopsy or surgical) or follow-up of at least 2 years; independently compared with FDG PET/CT and MDCT findings |
|
Votrubova 200663 Country: Czech Republic Year: NR Study design: retrospective; consecutive sample: unclear Setting: hospital PET centre Aim: to compare PET and FDG PET/CT for detection of CRC recurrence |
84 patients (54 men, 30 women), mean age 64 years (range 41–78 years) Indication: suspected recurrence of CRC Exclusion criteria: NR Disease: NR Comorbidities: NR Previous treatment: colonic resection or rectal amputation |
FDG PET/CT Biograph Duo LSO® (Siemens Health Care Diagnostics, Surrey, UK); fasting duration at least 6 hours; FDG 370 MBq, scan 60–90 minutes later; CT: 30 patients received intravenous contrast agent; patients who previously underwent contrast-enhanced CT received oral contrast agent only Image interpretation Assessors: a skilled radiologist and a nuclear physician, in consensus; assessors first analysed CT and PET corrected and uncorrected images blind but aware of results of patients’ other investigations, then CT and FDG PET/CT images read with knowledge of previous PET reading Qualitative: positive: lesions with increased uptake and CT abnormality; pulmonary/liver nodules < 1 cm, even in absence of FDG uptake; clearly increased focal uptake in normal structures; pathological structures with slightly higher uptake than in the liver Quantitative: NA |
PET component of FDG PET/CT: using attenuation-corrected and -uncorrected images Image interpretation As index test Time between index and comparator tests: sequential |
Histopathology within 4 weeks (no further details) and/or follow-up, mean duration 6.5 months (range 5–8 months) |
|
Kim 200568 Country: USA Year: 2002–3 Study design: retrospective; consecutive sample: unclear Setting: university hospital Aim: to compare PET and FDG PET/CT for restaging recurrent CRC |
51 patients (30 men, 21 women), mean age 65 years (range 54–76 years) Indication: staging of biopsy proven (n = 12) or suspected (n = 39) recurrent CRC; suspicion based on clinical symptoms, tumour markers or other imaging tests Exclusion criteria: chemotherapy or RT within 4 weeks prior to FDG PET/CT; < 6-month follow-up Disease: colon (n = 35), rectum (n = 16); primary tumour non-mucinous adenocarcinoma (n = 41), mucinous adenocarcinoma (n = 6), unknown (n = 5) Comorbidities: NR Previous treatment: NR |
FDG PET/CT Reveal RT® (CPS Innovations, Knoxville, TN); fasting duration NR; FDG 7.77 MBq/kg, scan 60 minutes later; CT with oral contrast agent Image interpretation Assessors: three experienced nuclear medicine physicians, independently and unaware of clinical data; each assessor evaluated either PET or FDG PET/CT (not both) for each patient, and interpreted approximately one out of three PET and FDG PET/CT images Qualitative: abnormalities: multiple lung nodules on CT < 1 cm even in absence of increased uptake, and sclerotic bone lesions that did not demonstrate features of degenerative changes considered malignant; abnormalities on CT without corresponding increased uptake generally considered benign; characterisation: 1 = definitely benign, 2 = probably benign, 3 = equivocal, 4 = probably malignant, 5 = definitely malignant; location: 1 = uncertain, 2 = probable, 3 = definite Quantitative: NA |
PET component of FDG PET/CT: CT used for attenuation correction Image interpretation As index test Time between index and comparator tests: sequential |
Histology (33/143 regions) or clinical and imaging follow-up including physical examination, laboratory tests, CT, FDG PET/CT and MRI, for at least 6 months; data collected by one physician unaware of the interpretation of the PET or CT scans |
|
Even-Sapir 200464 Country: Israel Year: NR Study design: retrospective; consecutive sample: unclear Setting: university hospital/cancer centre Aim: to assess the role of FDG PET/CT for detection of pelvic recurrence of rectal cancer |
62 patients (37 men, 25 women), mean age 62 years (range 34–86 years) Indication: suspected recurrence of rectal cancer; increase in CEA (n = 16), suspected pelvic recurrence at CT (n = 19) or colonoscopy (n = 3), suspected extrapelvic recurrence or restaging prior to surgical removal of presumed respectable metastases (n = 17), monitoring treatment response (n = 5), suspected second primary in lung (n = 1), unexplained anal pain (n = 1) Exclusion criteria: NR Disease: free of disease (n = 19), extrapelvic metastases with no evidence of pelvic recurrence (n = 19), extrapelvic metastases and pelvic recurrence (n = 8), pelvic recurrence only (n = 16) Comorbidities: diabetes (number NR) Previous treatment: abdominoperineal (n = 17) or anterior (n = 45) resection; neoadjuvant chemoradiation (n = 7); adjuvant chemo (n = 16); post-surgical RT (n = 3) |
FDG PET/CT Discovery LS; fasting duration at least 4 hours; FDG 370–666 MBq, time to scan NR; CT: first 20 patients with oral contrast agent and 42 without Image interpretation Assessors: two experienced readers, in consensus; patients’ names were removed from reports; PET and FDG PET/CT images interpreted on separate days at least 1 week apart, presented in a different order Qualitative: uptake sites defined as malignant, benign or indeterminate on basis of shape, location and intensity; characterisation: 1 = benign, 2 = probably benign, 3 = equivocal, 4 = probably malignant, 5 = malignant Quantitative: SUVmax; no further details |
PET component of FDG PET/CT: CT used for attenuation correction Image interpretation As index test Time between index and comparator tests: sequential |
Histology (30/81 lesions) or clinical and imaging follow-up for at least 6 months (mean 8 ± 2.6 months); follow-up imaging included contrast-enhanced CT (n = 38), ultrasound (n = 4), MRI (n = 5), TRUS (n = 5), colonoscopy (n = 11), repeat FDG PET/CT (n = 9); two physicians, who did not participate in FDG PET/CT, PET interpretation, reviewed patient records together |
|
Schmidt 200965 Country: Germany Year: NR Study design: retrospective; consecutive sample: yes Setting: University hospital Aim: to compare the accuracy of FDG PET/CT and MRI for detection of recurrent CRC |
24 patients (gender NR), mean age 62 years (range 47–80 years) Indication: suspected recurrence (n = 10), conspicuous finding on another imaging modality (n = 14) Exclusion criteria: chemotherapy or RT immediately before or between imaging tests; lesions found with MRI outside the overlapping field of view of both tests excluded from analysis of DTA Disease: NR Comorbidities: NR Previous treatment: primarily curative; no further details |
FDG PET/CT Gemini® (Philips Medical Systems, Andover, MA); fasting duration at least 6 hours; FDG 197–390 MBq, time to scan 60 minutes; CT with intravenous contrast agent, except one patient who refused it Image interpretation Assessors: a radiologist and a nuclear medicine physician with 3 and 6 years’ experience, blind to the other investigation (MRI) and information on previous or current diagnostic imaging results, in consensus Qualitative: malignant: signs of aggressive expansion of lesion (e.g. ill-defined borders, erosion or infiltration of neighbouring structures, haemorrhage, necrosis); abnormal contrast uptake Quantitative: malignant: focally increased uptake with SUVmax > 2.5 |
Whole body MRI 1.5-T system® (Magnetom Avento, Siemens Health Care Diagnostics, Surrey, UK) (n = 14); 3-T system® (Magnetom Tim Trio, Siemens Health Care Diagnostics, Surrey, UK) (n = 10) Image interpretation Assessors: two certified radiologists with > 6 years’ experience, in consensus Qualitative: malignant: signs of aggressive expansion of lesion; established sequence-specific signal changes; abnormal contrast uptake Quantitative: NR Time between index and comparator tests: mean 3 days (maximum 12 days) |
Radiological or nuclear medicine follow-up, FDG PET/CT (n = 12), CT (n = 10), whole-body MRI (n = 6), MRI (n = 6), radiographs (n = 5), abdominal ultrasound (n = 1), average duration 11 months (range 5–30 months) |
|
Strunk 200567 Country: Germany Year: NR Study design: retrospective; consecutive sample: yes Setting: university hospital |
29 patients (12 men, 17 women) age range 51–76 years Indication: unexplained raise in CEA, suspected recurrence, pelvic metastases or response to therapy Exclusion criteria: > 130 mg/dl glucose blood level Disease: CRC |
PET/CT Biograph oral contrast was used; FDG 370 MBq FDG, scan approximately 90 minutes later Image interpretation Assessors: blindly and independently scored by two nuclear medicine physicists Qualitative: five-point scale was used; consensus reading during which a virtual (meaning mental) fusion of PET and CT images and afterwards real fusion (meaning co-registered) |
CT Image interpretation Scored blindly and independently by two radiologists; consensus reading during which a virtual (meaning mental) fusion of PET and CT images and afterwards real fusion (meaning co-registered) Time between index and comparator tests: < 3 months |
Histopathology 86 malignant lesion PET/CT detected 68, CT detected 65; seven patients had histology and the rest had disease progression as the final confirmation Follow-up and the course of the disease |
|
Kula 200466 Country: Poland Year: 2003–4 Study design: retrospective; consecutive sample: unclear Setting: hospital oncology centre Aim: to evaluate the usefulness of PET/CT in the recurrence of colorectal cancer |
120 patients (44 men, 76 women), mean age 58.8 years women, 61.2 years men (range 24–80 years) Indication: suspected recurrence based on examination, routine imaging tests and raised CEA Exclusion criteria: > 8.4 mm/l glucose Disease: rectal cancer (n = 62), colon cancer (n = 28), hemicolorectomy left side (n = 12), hemicolorectomy right side (n = 10), not specified (n = 8) Comorbidities: NR Previous treatment: all had radical surgery; 62 rectal resection (51.7%) |
PET/CT Biograph; fasting duration 1 hour; FDG 370 MBq FDG, scan approximately 60–90 minutes later Image interpretation Assessors: NR Qualitative: NR Quantitative: NR |
CEA and compared with all other tests Image interpretation 5 ng/ml threshold Time between index and comparator tests: 6 weeks before the PET/CT scan |
Histopathology and surgery; (n = 56) recurrence confirmed by surgical resection, (n = 24) recurrence was confirmed by histopathology Interpretation; (n = 10) liver, (n = 6) lungs, (n = 5) pelvis, (n = 3) post-operative scars Follow-up conducted within 12 months (average 6.3 months) |
| Study | Test and outcome level | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| TP/TP + FN | TN/TN + FP | TP/TP + FP | TN/TN + FN | ||||||
| Sarikaya 200761 | FDG PET/CT, recurrence, patient level | 6 | 4 | 0 | 2 | 0.750 | 0.000 | 0.600 | 0.000 |
| Bellomi 200762 | FDG PET/CT, local recurrence, patient level | 14 | 1 | 51 | 1 | 0.933 | 0.981 | 0.933 | 0.981 |
| MDCT, local recurrence, patient level | 15 | 1 | 50 | 0 | 1.000 | 0.980 | 0.938 | 1.000 | |
| FDG PET/CT, hepatic metastases, patient level | 17 | 0 | 50 | 0 | 1.000 | 1.000 | 1.000 | 1.000 | |
| MDCT, hepatic metastases, patient level | 17 | 0 | 50 | 0 | 1.000 | 1.000 | 1.000 | 1.000 | |
| FDG PET/CT, lung metastases, patient level | 6 | 0 | 0 | 2 | 0.750 | 0.000 | 1.000 | 0.000 | |
| MDCT, lung metastases, patient level | 8 | 0 | 0 | 0 | 1.000 | 0.000 | 1.000 | 0.000 | |
| Votrubova 200663 | FDG PET/CT, recurrence, patient level | 40 | 3 | 36 | 5 | 0.889 | 0.923 | 0.930 | 0.878 |
| PET component of FDG PET/CT, recurrence, patient level | 36 | 12 | 27 | 9 | 0.800 | 0.692 | 0.750 | 0.750 | |
| FDG PET/CT, intra-abdominal extrahepatic recurrence, patient level | 29 | 3 | 48 | 4 | 0.879 | 0.941 | 0.906 | 0.923 | |
| PET component of FDG PET/CT, intra-abdominal extrahepatic recurrence, patient level | 27 | 6 | 45 | 6 | 0.818 | 0.882 | 0.818 | 0.882 | |
| FDG PET/CT, extra-abdominal and/or -hepatic recurrence, patient level | 18 | 0 | 65 | 1 | 0.947 | 1.000 | 1.000 | 0.985 | |
| PET component of FDG PET/CT, extra-abdominal and/or -hepatic recurrence, patient level | 14 | 8 | 57 | 5 | 0.737 | 0.877 | 0.636 | 0.919 | |
| Even-Sapir 200464 | FDG PET/CT, pelvic recurrence, patient level | 23 | 4 | 34 | 1 | 0.958 | 0.895 | 0.852 | 0.971 |
| PET component of FDG PET/CT, pelvic recurrence, patient level | 21 | 10 | 28 | 3 | 0.875 | 0.737 | 0.677 | 0.903 | |
| FDG PET/CT, pelvic recurrence, lesion level (1) | 43 | 1 | 32 | 1 | 0.977 | 0.970 | 0.977 | 0.970 | |
| PET component of FDG PET/CT, pelvic recurrence, lesion level (2) | 36 | 10 | 24 | 5 | 0.878 | 0.706 | 0.783 | 0.828 | |
| Schmidt 200965 | FDG PET/CT, recurrence, patient level | 17 | 0 | 6 | 1 | 0.944 | 1.000 | 1.000 | 0.857 |
| Whole-body MRI, recurrence, patient level | 17 | 0 | 6 | 1 | 0.944 | 1.000 | 1.000 | 0.857 | |
| FDG PET/CT, local recurrence, lesion level | 2 | 1 | 0 | 0 | 1.000 | 0.000 | 0.667 | #DIV/0! | |
| Whole-body MRI, local recurrence, lesion level | 2 | 0 | 1 | 0 | 1.000 | 1.000 | 1.000 | 1.000 | |
| FDG PET/CT, nodal recurrence, lesion level | 27 | 0 | 47 | 2 | 0.931 | 1.000 | 1.000 | 0.959 | |
| Whole-body MRI, nodal recurrence, lesion level | 18 | 4 | 43 | 11 | 0.621 | 0.915 | 0.818 | 0.796 | |
| FDG PET/CT, distant recurrence, lesion level | 37 | 2 | 35 | 9 | 0.804 | 0.946 | 0.949 | 0.795 | |
| Whole-body MRI, distant recurrence, lesion level | 36 | 2 | 35 | 10 | 0.783 | 0.946 | 0.947 | 0.778 | |
| FDG PET/CT, overall recurrence, lesion level | 66 | 3 | 82 | 11 | 0.857 | 0.965 | 0.957 | 0.882 | |
| Whole-body MRI, overall recurrence, lesion level | 56 | 6 | 79 | 21 | 0.727 | 0.929 | 0.903 | 0.790 | |
| Kula 200466 | FDG PET/CT, recurrence, patient level | 54 | 2 | 35 | 1 | 0.982 | 0.946 | 0.964 | 0.972 |
| CEA, recurrence, patient level | 38 | 5 | 23 | 18 | 0.679 | 0.821 | 0.884 | 0.561 | |
| Strunk 200567 | FDG PET/CT, recurrence, lesion level | 68 | 3 | 15 | 18 | 0.791 | 0.833 | 0.958 | 0.455 |
| CT, recurrence, lesion level | 65 | 5 | 16 | 11 | 0.855 | 0.762 | 0.929 | 0.593 | |
| Kim 200568 | FDG PET/CT, staging accuracy, patient level | 20 | 2 | 25 | 4 | 0.833 | 0.926 | 0.909 | 0.862 |
| PET component of FDG PET/CT, staging accuracy, patient level | 16 | 7 | 20 | 8 | 0.667 | 0.741 | 0.696 | 0.714 | |
| FDG PET/CT, hepatic recurrence, region level | 13 | 0 | 33 | 2 | 0.867 | 1.000 | 1.000 | 0.943 | |
| PET component of FDG PET/CT, hepatic recurrence, region level | 13 | 1 | 32 | 2 | 0.867 | 0.970 | 0.929 | 0.941 | |
| FDG PET/CT, extra-abdominal recurrence, region level | 9 | 1 | 36 | 0 | 1.000 | 0.973 | 0.900 | 1.000 | |
| PET component of FDG PET/CT, extra-abdominal recurrence, region level | 7 | 5 | 32 | 2 | 0.778 | 0.865 | 0.583 | 0.941 | |
| FDG PET/CT, recurrence overall, region level (hepatic, extrahepatic abdominal, extra-abdominal) | 31 | 2 | 106 | 4 | 0.886 | 0.981 | 0.939 | 0.964 | |
| PET component of FDG PET/CT, recurrence overall, region level | 26 | 8 | 100 | 9 | 0.743 | 0.926 | 0.765 | 0.917 |
Study setting and country in which the research was conducted
All studies were conducted in university hospitals and specialist cancer centres in Europe,62,63,65–67 Israel64 and the USA. 61,68
Patient populations
The eight included studies reported outcomes for 476 patients. When gender proportions were reported, men were usually in the majority, with the exception of two studies66,67 that reported mostly women and two studies62,65 that did not report gender. The mean ages of patients when reported were 55–65 years, with a range of 33–91 years.
The distinction between cancer of the colon and rectum was not reported in four studies,61,63,65,67 in two the population was mixed (colon and rectal disease)66,68 and two studies reported outcomes in people with a diagnosis of rectal cancer. 62,64
Indication for FDG PET/CT
In all eight studies61–68 the indication for FDG PET/CT was a suspicion of CRC from blood markers, a conspicuous finding on other imaging tests or suspicion based on history and a clinical examination.
FDG PET/CT equipment and patient preparation
Full details of all of the equipment used in the primary studies can be found in Table 4. FDG PET/CT equipment was manufactured by one of four companies: Philips Medical Systems, GE Healthcare, CPS Innovations or Siemens Medical Solutions. The fasting duration prior to the scan was 4 or 6 hours in the majority of studies that reported these data, but in one study66 the fasting duration was 1 hour.
A wide range of injected FDG doses were reported, with units ranging from 197 to 740 MBq, or 5 to 7.77 MBq/kg, and patients were scanned between 60 and 90 minutes after the administration of the radioactive tracer.
Patients received oral67,68 or intravenous65 contrast agents with the CT component of the FDG PET/CT test in three studies. One study64 reported a mixture of contrast-enhanced CT and non-contrast-enhanced CT images obtained from study patients.
Image interpretation
Where reported, FDG PET/CT and comparison tests were assessed by at least two individuals, usually experts in nuclear medicine, blind to the results of the other investigations.
Image interpretation was reported to be based on the assessors’ qualitative judgement using various approaches including a five-point scoring system of lesions: 1 = benign, 2 = probably benign, 3 = equivocal, 4 = probably malignant and 5 = definitely malignant. 64,67,68 Positive lesions from the PET component were defined as those associated with a focal increased uptake of FDG which was greater than that of the background tissues,61,63 any new tissue close to an anastomosis with asymmetrical, or irregular, contrast enhancement or changes compared with previous findings,62 signs of aggressive expansion of a lesion and ill-defined borders or erosion/infiltration of neighbouring tissues. 65
No information regarding the methods of assessment was given in one study. 66
Reference standard
The studies reported the use of various methods as reference standard: histopathology, surgically resected specimens, other imaging modalities, laboratory tests and clinical follow-up. None of the studies attributed a specific reference standard test to individual patients’ index tests. Five studies62–65,68 reported a period of follow-up, and these ranged from 6 months to 2 years. Patient mortality was not reported.
Data synthesis – diagnostic performance
The accuracy data of FDG PET/CT in identifying recurrent disease in 276 patients are presented in a forest plot in Figure 2. 61–65 Patients were excluded from these studies if they lacked histopathology based on surgical resection or biopsy,61 had a diagnosis of diabetes62 or had received chemotherapy or radiotherapy immediately before or between imaging tests. 65
FIGURE 2.
Accuracy data of FDG PET/CT in the detection of recurrent CRC based on patient-level data.
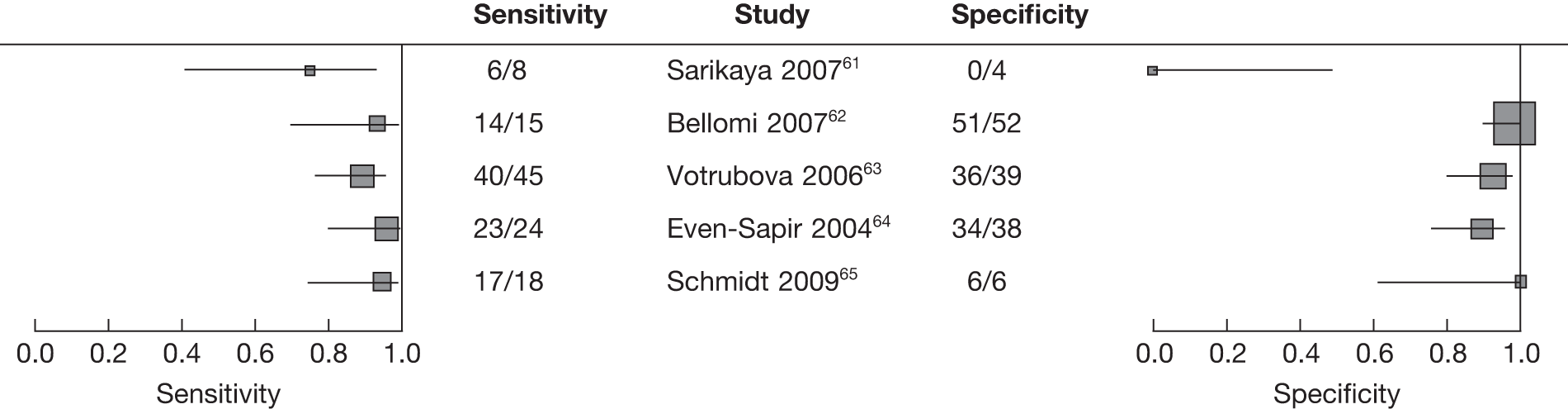
The overall estimate of sensitivity is 91% (95% CI 87% to 95%). There was little evidence of heterogeneity in sensitivity estimates, hence a fixed-effects meta-analysis was used. The overall estimate of specificity is 91% (95% CI 85% to 95%). There was some evidence of heterogeneity in specificity estimates, so a random effects bivariate/hierarchical summary receiver operating characteristic (HSROC) method was used. Two separate univariate meta-analyses were used as the data were not adequate to fit the bivariate/HSROC model.
FDG PET/CT versus no comparator
A study61 with no comparator reported FDG PET/CT to have a sensitivity of 75% (the specificity was incalculable as a result of no TNs) in 39 patients undergoing postoperative follow-up.
FDG PET/CT versus multi-detector computerised tomography or computerised tomography
One study62 compared FDG PET/CT with multidetector CT in the detection of local recurrence of CRC in 67 patients. The accuracy of FDG PET/CT was less than that of multidetector CT: the sensitivity of FDG PET/CT was 93% (95% CI 70% to 99%) and the specificity 98% (95% CI 89% to 100%), whereas for multidetector CT the sensitivity was 100% (95% CI not calculable) and the specificity was 98% (95% CI 90% to 100%).
A second study67 comparing FDG PET/CT with CT in patients with an unexplained rise in CEA levels and suspected recurrent CRC found that FDG PET/CT had a sensitivity of 79% (95% CI 69% to 86%) and a specificity of 83% (95% CI 61% to 94%). The accuracy estimates for CT for the detection of recurrence of CRC were a sensitivity of 85% (95% CI 76% to 92%) and a specificity of 76% (95% CI 55% to 89%) based on lesion-level data.
FDG PET/CT versus positron emission tomography alone
Three studies63,64,68 compared the accuracy of FDG PET/CT with FDG PET alone for the pre-operative staging of 197 patients with suspected recurrence and produced broadly similar data.
FDG PET/CT was reported to be more accurate in pre-operative staging than FDG PET alone in all three studies,63,64,68 regardless of the unit of analysis (patient or lesion). For patient-level analyses,FDG PET/CT sensitivities were 89% (95% CI 76% to 95%),63 83% (95% CI 64% to 93%)68 and 96% (95% CI 80% to 99%),64 while the reported corresponding specificities were 92% (95% CI 80% to 97%),63 93% (95% CI 76% to 97%)68 and 89% (95% CI 76% to 96%). 64 For FDG PET alone, the sensitivities were 80% (95% CI 66% to 89%),63 67% (95% CI 47% to 82%)68 and 87% (95% CI 69% to 96%),64 with reported corresponding specificities of 69% (95% CI 53% to 81%),63 74% (95% CI 60% to 86%)68 and 74% (95% CI 58% to 80%). 64
In the analysis of lesion-level data relating to recurrence, FDG PET/CT had a sensitivity of 88% (95% CI 73% to 95%) and a specificity of 94% (95% CI 84% to 97%), and FDG PET alone had a sensitivity of 81% (95% CI 66% to 91%) and a specificity of 88% (95% CI 77% to 94%) in the detection of intra-abdominal extrahepatic lesions. 63
Lesion-level data for the accuracy of FDG PET/CT in the detection of extra-abdominal recurrence showed a sensitivity of 100% (95% CI 70% to 100%) and a specificity of 97% (95% CI 86% to 99%), while FDG PET alone had a sensitivity of 78% (95% CI 45% to 93%) and a specificity of 86% (95% CI 72% to 94%). 68
Lesion-level data for the accuracy of staging pelvic recurrence demonstrated a sensitivity of 98% (95% CI 88% to 99%) and a specificity of 97% (95% CI 85% to 99%) for FDG PET/CT, while PET alone had a sensitivity of 88% (95% CI 69% to 95%) and a specificity of 70% (95% CI 57% to 85%). 64
FDG PET/CT versus whole-body magnetic resonance imaging
One study65 compared FDG PET/CT with whole-body MRI and found both tests to be equally good in the detection of all recurrent lesions, with a sensitivity of 94% (95% CI 74% to 99%) and a specificity of 100% (95% CI 60% to 100%) in analyses conducted at the patient level (n = 24). Lesion-level analyses for the detection of nodal recurrence suggest that FDG PET/CT has greater accuracy, with a sensitivity of 93% (95% CI 78% to 98%) and a specificity of 100% (92% to 100%) whole-body MRI, with a sensitivity and specificity of 62% (95% CI 44% to 77%) and 91% (95% CI 80% to 96%), respectively.
FDG PET/CT versus carcinoembryonic antigen
Finally, one study66 compared FDG PET/CT with levels of CEA in 120 patients. Recurrence data with patients as the unit of analysis revealed FDG PET/CT to be more accurate than the blood test [FDG PET/CT: sensitivity 98% (95% CI 90% to 100%), specificity 95% (95% CI 82% to 99%); CEA: sensitivity 68% (95% CI 55% to 79%), specificity 82% (95% CI 64% to 92%)].
Metastatic disease
In 20–40% of patients, hepatic metastases are the first presentation of recurrence,58 and three of the studies62,63,68 detected metastases in patients suspected of recurrence. These are included in the chapter relating to FDG PET/CT in the pre-operative staging of metastatic disease.
Quality assessment of included studies
Fourteen items from the QUADAS checklist were used to assess the methodological quality of the results and the findings from this process are shown in Table 6. 37
| Study | Spectrum of patients’ representative? | Selection criteria clearly described? | Reference standard likely to classify the target condition? | Time between the reference standard and index test short enough? | Whole or a random sample receive verification using a reference standard? | Patients received the same reference standard regardless of the index test result? | Reference standard independent of the index test? | Execution of the test described in sufficient detail to permit replication? | Execution of the reference standard described in sufficient detail? | Index test results interpreted without knowledge of the reference standard results? | Reference standard results interpreted without knowledge of the index test results? | Same clinical data available when tests results were interpreted as in clinical practice? | Uninterpretable intermediate test results reported? | Withdrawals from the study explained? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarikaya 200761 | N | Y | Y | Y | N | N | Y | Y | UC | Y | UC | UC | UC | N |
| Bellomi 200762 | Y | Y | Y | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Votrubova 200663 | UC | N | Y | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Even-Sapir 200464 | UC | N | UC | N | Y | N | Y | Y | UC | UC | UC | UC | Y | Y |
| Schmidt 200965 | Y | N | N | N | Y | Y | Y | Y | UC | UC | UC | N | UC | Y |
| Kula 200466 | Y | Y | UC | Y | N | UC | Y | Y | UC | UC | UC | UC | N | UC |
| Strunk 200467 | UC | N | UC | UC | UC | UC | Y | Y | N | Y | UC | UC | UC | Y |
| Kim 200568 | N | Y | Y | N | Y | N | Y | Y | UC | UC | Y | N | UC | Y |
In the majority of studies the spectrum of patients was not representative of those who would receive the test in clinical practice according to our criteria:61,63,64,67,68 a consecutive series or random sample of patients was reported in only three studies. 61,65,66 A reference standard of surgical resection, biopsy or clinical imaging follow-up of at least 6 months was reported in four studies. 61–63,68 FDG PET/CT results were verified using a variety of reference standards that were independent of the index test in all studies. Six weeks was considered by us to be the time limit after which disease progression might occur, but the time between the reference standard and the index test was reported to be ≤ 6 weeks in only two studies. 61,66 Two studies did not report whether the whole or a random selection of the sample received verification using a reference standard of diagnosis, and in another this information was unclear. 67 Only one study reported that patients had received the same reference standard regardless of the index test results. 65 It was unclear in most studies whether the index test was interpreted without knowledge of the reference standard test results and vice versa. 62–65 Withdrawals were explained in the majority of studies. 62–65,67,68
The validity of the conclusions from these studies was found to be compromised by spectrum, disease progression, differential verification and review bias.
Summary
-
The published evidence regarding the accuracy of FDG PET/CT in the pre-operative staging for recurrent CRC cancer is of poor quality.
-
All studies were retrospective.
-
Patient populations were not well described in terms of disease classification systems or the primary diagnosis and all included small numbers of patients.
-
The largest study66 compared FDG PET/CT with CEA, arguably a clinically irrelevant comparison, and the study authors did not report information about how the tests were interpreted or by whom, a potentially important source of bias.
-
PET/CT has been reported to be more accurate than FDG PET alone in staging recurrent CRC in three studies including 197 patients,63,64,68 but cross-tabulation of results of different tests for patients contributing to the same study was not available. This meant that significance testing for differences between estimates of sensitivity and specificity was not carried out.
-
PET/CT was less accurate than multidetector CT, of equivalent accuracy to MRI and more accurate than CEA in the detection of recurrent CRC, but these estimates are based on small numbers of patients and differences may have arisen by chance.
-
Pooled estimates of accuracy from five studies61–65 found FDG PET/CT to have a sensitivity of 91% (95% CI 87% to 95%) and a specificity of 91% (95% CI 85% to 95%).
Chapter 7 FDG PET/CT for the pre-operative staging of metastatic colorectal cancer
Approximately 37,000 CRC cases are diagnosed each year and 17,000 deaths are attributed to the disease,2 mainly due to metastatic disease. At presentation, approximately 20–25% of patients have clinically detectable metastases and a further 40–50% of patients subsequently develop metastases after resection of the primary tumour, most commonly within the first 3 years of follow-up.
The liver is often the first site of metastatic disease. It has been postulated that the principal mode of tumour dissemination to the liver is via the portal system and therefore that surgical resection of isolated hepatic metastases from CRC may be curative. However, the natural history of metastatic CRC is variable. Median survival without treatment is < 8 months from presentation, but the prognosis is better for those patients with isolated hepatic metastases. Patients with a limited number of metastases or those with disease confined to one lobe of the liver have a longer duration of survival than those with more advanced disease.
Thus, some 20–30% of patients with metastatic CRC have disease that is confined to the liver and is potentially resectable. For the UK, this equates to approximately 3500 patients per year. 72 Following the seminal series reported by Scheele and colleagues in 1995,73 several large series on resection for colorectal liver metastases have reported 5-year survival rates ranging from 25% to 44%, with operative mortality of approximately 2.5%. 74
The key issue after hepatic resection for metastatic disease is the high relapse rate and/or metastatic disease elsewhere. There are a number of predictors of relapse including the number of intrahepatic metastases and extrahepatic disease. 75 To address this, pre-operative chemotherapy is increasingly used and shown to reduce the risk of progression in resected patients. 76 Furthermore, modern combinational chemotherapy regimens may downstage unresectable liver disease to resectable in approximately 15% of cases. 77
The lung is the second most common site for metastases. In modern oncological practice it is not uncommon to consider patients with isolated lung metastases for metastatectomy, including patients undergoing resection for hepatic metastases, with moderate success rates. 78 However, as the overall number of cases of patients undergoing pulmonary metastatectomy is small, the lung is not the primary site of interest in this section of the report.
Given the high morbidity and potential mortality associated with hepatic resection, and the need to predict cases most likely to benefit from such major surgery, the rationale for accurate pre-operative staging becomes clear. 25
Systematic reviews to evaluate FDG PET against standard imaging techniques used in staging metastatic CRCs have been conducted and FDG PET was shown to compare favourably with helical CT, non-helical CT and MRI in a meta-analysis of data from 1058 patients, with a sensitivity of 94.6% (95% CI 92.5% to 96.1%). The authors also found PET to be most accurate in a meta-analysis of overall diagnostic performance based on lesion-level data, with a sensitivity of 75% (95% CI 61.1% to 86.3%). 40
In a second systematic review that compared FDG PET with CT scanning for the detection of hepatic and extrahepatic liver lesions, PET was shown to be superior to CT in 1843 patients, with a sensitivity of 88.0% (95% CI 88.0% to 98.0%) and a specificity of 96.1% (95% CI 70.4% to 104.3%). 44 Integrated FDG PET/CT equipment has not been evaluated in a systematic review, and the benefits or harms associated with this new technology are unclear.
This systematic review considers diagnostic test accuracy of integrated or hybrid FDG PET/CT for the staging of metastatic CRC.
Aim
The aim of this systematic review is to compare the performance of combined functional and anatomical imaging with integrated FDG PET/CT scanning with standard imaging modalities in the investigation of patients with suspected metastatic disease.
Objectives
To evaluate the diagnostic accuracy of FDG PET/CT for the detection of extrahepatic and intrahepatic lesions.
Results
Our search did not identify any systematic reviews to evaluate the diagnostic test accuracy of integrated FDG PET/CT in the pre-operative staging of metastatic CRC.
Number of included studies
Sixteen studies24,62,63,68–71,79–87 evaluating the diagnostic test accuracy of combined functional and anatomical imaging with FDG PET/CT for the detection of colorectal metastases were identified. The majority of the studies identified investigated metastatic disease to the liver. However, some studies also evaluated extrahepatic disease including local recurrence. 62,63,68
Study characteristics and study designs
The study characteristics are shown in Table 7 and accuracy data are shown in Table 8.
| Study | Population | Index test | Comparator(s) | Reference standard |
|---|---|---|---|---|
|
D’Souza 200970 Country: India Year: NR Study design: prospective; consecutive sample: unclear Setting: university-affiliated nuclear medicine institute Aim: to compare FDG PET/CT and contrast-enhanced CT for detection of hepatic metastases |
Mixed cancer population, 8/45 CRC patients, age and gender not reported separately for CRC Indication: suspected hepatic metastases based on clinical or ultrasound findings Exclusion criteria: NR Disease: primary site colorectal (n = 1), colon (n = 5), rectum (n = 2) Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery STE 16® (GE Healthcare, Fairfield, CT); fasting duration 6–8 hours; FDG 370 MBq, scan 60 minutes later; non-contrast-enhanced CT Image interpretation Assessors: an experienced nuclear medicine physician and a radiologist, independently, blind (no further details) Qualitative: NR Quantitative: SUVmax more than three criterion for metastases |
Contrast-enhanced CT Triphasic contrast-enhanced abdominal CT, Discovery STE 16, intravenous contrast agent Image interpretation As index test Time between index and comparator tests: 24–72 hours |
Histology (no further details) and/or follow-up, duration 6–12 months |
|
Chua 200779 Country: UK Year: NR Study design: retrospective; consecutive sample: unclear Setting: university hospital (University College London) Aim: to compare FDG PET/CT and dedicated contrast-enhanced CT for detection of hepatic metastases |
Mixed cancer population, 75/131 CRC patients, age and gender not reported separately for CRC Indication: pre-operative staging (n = 2), assess suitability for liver resection (n = 21), assess suitability for radiofrequency therapy (n = 5), recurrence (n = 12), indeterminate CT findings (n = 12), suspected extrahepatic lesions on CT (n = 12), asymptomatic rise in tumour markers (n = 7), reassessment after chemo/radiotherapy (n = 4) Exclusion criteria: NR Disease: primary colorectal cancer Comorbidities: NR Previous treatment: some had undergone chemotherapy at least 6 months prior to imaging; at least four had chemo/radiotherapy |
FDG PET/CT Discovery LS; fasting duration NR; FDG 350–370 MBq, scan 60 minutes later; non-contrast-enhanced CT Image interpretation Assessors: two experienced nuclear medicine physicians/radiologists, aware of clinical history, blinding not enforced Qualitative: visual assessment and maximum intensity tomographic data; negative: lesions not associated with focal increased uptake greater than background level Quantitative: NR |
Contrast-enhanced CT Siemens MDCT, oral and intravenous contrast agent Image interpretation Assessors: images reported under supervision of an experienced radiologist Qualitative: NR Quantitative: NR Time between index and comparator tests: within 6 weeks |
Histopathology where available; clinical and radiological (both contrast-enhanced CT and FDG PET/CT) follow-up in discordant cases, duration at least 6 months |
|
Lubezky 200769 Country: Israel Year: 2002–5 Study design: unclear; consecutive sample: unclear Setting: teaching and research medical centre Aim: to examine the effect on FDG PET/CT and CT findings of neoadjuvant chemotherapy for colorectal hepatic metastases |
75 patients (18 men, 57 women), mean age 61 (SD 10.9) years neoadjuvant treatment group, 66 (SD 9.8) years no neoadjuvant treatment group Indication: pre-operative staging of hepatic metastases (with or without neoadjuvant chemotherapy) Exclusion criteria: NR Disease: no neoadjuvant treatment group: lymph node metastases 81.5%, mean number of liver tumours 1.19 (SD 0.4), extrahepatic disease n = 7; neoadjuvant treatment group: lymph node metastases 82%, mean number of liver tumours 2.52 (SD 1.9), extrahepatic disease n = 9 Comorbidities: NR Previous treatment: prior liver resection (n = 10); neoadjuvant chemotherapy: 5FU, leucovorin (folinic acid) and either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) (n = 31); 17 patients also had bevacizumab; at least 2 weeks between last course and FDG PET/CT |
FDG PET/CT Discovery LS; fasting duration at least4 hours; FDG 370–666 MBq, scan 60–120 minutes later; CT with oral contrast agent Image interpretation Assessors: two experts, in consensus, no further details Qualitative: sites of metastatic disease showing increased uptake were recorded, no further details; hepatic segmental classification recorded Quantitative: NR |
Contrast-enhanced CT Triphasic contrast-enhanced abdominal CT, no further details Image interpretation Assessors: as index test Qualitative: NR Quantitative: NR Time between index and comparator tests: unclear |
Histopathology, surgical; surgical exploration within 1 month following FDG PET/CT in most cases; two patients had explorative laparotomy only (no resection) |
|
Kong 200880 Country: UK Year: 2004–6 Study design: retrospective; consecutive sample: unclear Setting: specialist cancer centre (Royal Marsden) Aim: to compare FDG PET/CT with CT to identify extrahepatic disease, and with liver MRI to identify liver metastases |
65 patients (42 men, 23 women), median age 65 years (range NR) Indication: known or suspected potentially operable liver metastases Exclusion criteria: chemotherapy < 3 months before FDG PET/CT Disease: NR Comorbidities: NR Previous treatment: NR |
FDG PET/CT Gemini; fasting duration at least 4 hours; FDG 400 MBq, scan 60 minutes later; CT unenhanced Image interpretation Assessors: imaging results discussed at MDT meeting only, not directly reviewed Qualitative: NR Quantitative: NR |
Contrast-enhanced CT Lightspeed scanner; intravenous contrast agent Liver MRI Magafodipir trisodium contrast enhanced (Mn-DPDP MRI); 1.5-T MRI system® (Gyroscan Intera Master, Philips Medical Systems, Andover, MA) Image interpretation As index test Time between index and comparator tests: median < 1 month (range 0–49 days); 40/65 received liver MRI before FDG PET/CT |
Histopathology (surgery or biopsy unclear) (n = 23); clinical/imaging follow-up (n = 42), median duration 13 months (range 1.5–30.3 months) |
|
Ramos 200881 Country: Spain Year: 2006–7 Study design: prospective; consecutive sample: unclear Setting: university hospital Aim: to assess the additional value of FDG PET/CT over conventional imaging for pre-surgical staging of liver metastases |
63 patients (41 men, 22 women), median age 61.8 years (range 38–78 years) Indication: referred for FDG PET/CT with a diagnosis of CRC liver metastasis on CT or MRI Exclusion criteria: intact primary tumour; previous treatment for liver metastases by surgery or RT Disease: primary tumour T4 (n = 13), T3 (n = 42), T2 (n = 7), T1 (n = 1); N0 (n = 21), one to three affected nodes (n = 24), more than three affected nodes (n = 18); synchronous metastasis (n = 31), metachronous metastasis (n = 32) Comorbidities: NR Previous treatment: chemotherapy in last 3 months but discontinued at least 1 month before FDG PET/CT(n = 17) |
FDG PET/CT Discovery ST® (GE Healthcare, Fairfield, CT); fasting duration over 6 hours; FDG 3.7–7.4 MBq/kg (0.25 mg/kg intravenous furosemide 30 minutes after FDG), scan 50–90 minutes later; CT non- contrast-enhanced, no further details Image interpretation Assessors: tumour staging done systematically, no further details Qualitative: NR Quantitative: NR |
Contrast-enhanced CT 16-slice multidetector; intravenous contrast agent Liver MRI 1.5-T system Image interpretation As index test Time between index and comparator tests: between CT and FDG PET/CT median 16.8 days (range 1–62) |
An extension study including thoraco–abdominal CT, FDG PET/CT and a colon study if none was available that was < 1year old (protocol included MRI in patients with contrast agent allergy or steatosis), and manual abdominal exam of areas noted on intra-operative liver ultrasound; 1-year follow-up of all patients was planned to definitively confirm the results of the extension study – this report was based only on data available on completion of the extension study and the data from surgery only |
|
Rappeport 200783 Country: Denmark Year: 2004–5 Study design: prospective; consecutive sample Setting: university hospital Aim: to compare the performance of the three modalities for detection of liver lesions |
35 patients (16 men, 19 women), median age 62 years (range 33–74 years) Indication: patients referred for surgery for known or suspected CRC liver metastases Exclusion criteria: diabetes, MRI contraindications, imaging could not be performed before scheduled surgery Disease: NR Comorbidities: NR Previous treatment: chemotherapy within 1 month prior to FDG PET/CT (n = 4) |
FDG PET/CT Discovery LS; fasting duration NR; FDG 400 MBq, scan 50–90 minutes later; CT with intravenous contrast agent Image interpretation Assessors: a radiologist and a nuclear medicine physician with > 10 years’ experience, blind to other imaging results, in consensus; the radiologist had access to the complete CT data set Qualitative: assessors told to rely upon criteria from daily practice to decide whether a lesion was benign or malignant; characterisation: 1 = definitely benign, 2 = possibly malignant, 3 = definitely malignant; maximum diameter and segment location also recorded Quantitative: NR |
PET component of FDG PET/CT: CT used for attenuation correction CT component of FDG PET/CT MRI SPIO-enhanced 1.5-T Horizon Sigma LX scanner (GE Medical Systems, Fairfield, CT) Image interpretation Assessors: single observers, CT by a radiologist and MRI by a different single observer; no further details Qualitative: suspicious: hypovascular liver lesion with low TI signal and high T2 signal not classified as haemangioma on contrast enhancement pattern Quantitative: NR Time between index and comparator tests: 1–2 days |
28/31 surgical patients had liver metastases verified at surgery (23/28 histological confirmation, 5/28 verified intraoperatively), 2/31 had follow up imaging; median time from scan to surgery was 7 days for FDG PET/CT, 12 days for MRI; data reviewed by three assessors, in consensus |
|
Coenegrachts 200984 Country: Belgium Year: 2005–8 Study design: prospective; consecutive sample Setting: academic hospital Aim: to compare FDG PET/CT and MRI for detection of liver metastases |
24 patients (14 men, 10 women), mean age 65.3 (SD 10.8) years Indication: suspected CRC liver metastases based on ultrasound and/or lab results Exclusion criteria: contraindications for MRI (pacemakers, electronic implants); < 3 weeks between FDG PET/CT and MRI Disease: NR Comorbidities: NR Previous treatment: chemotherapy (14/24) within 1 month of FDG PET/CT, ongoing (n = 7) |
FDG PET/CT Discovery ST; fasting duration 6 hours; FDG at least 222 MBq, scan 60 minutes later; CT with oral and intravenous contrast agent (intravenous contraindicated n = 2) Image interpretation Assessors: two nuclear medicine physicians with 15 and 8 years’ experience, independently, blind to MRI result but aware of patients’ history, consensus reading performed if they disagreed; a radiologist helped interpret the CT images Qualitative: malignant: focal increased uptake in liver, with or without corresponding hypodense enhancing CT lesion; latter not considered as metastases in the absence of increased uptake; hepatic segmental classification Quantitative: NR |
MRI SPIO-enhanced and unenhanced single-shot spin-echo planar imaging: 1.5-T whole-body MRI system® (Intera, Philips Medical Systems, Andover, MA) Image interpretation Assessors: two radiologists with 15 and 17 years’ experience in abdominal MRI, independently, blind (unclear to what), aware of patients’ history and liver ultrasound findings, consensus reading performed if they disagreed Qualitative: phase-related signal intensity, ring enhancement and lesion conspicuity Quantitative: NR Time between index and comparator tests: mean 10.2 (SD 5.2) days |
Histopathology, postsurgery with IOUS (n = 18); follow-up imaging (n = 6), duration NR |
|
Wildi 200885 Country: Switzerland Year: NR Study design: retrospective; consecutive sample: unclear Setting: tertiary referral centre Aim: to compare FDG PET/CT with FDG PET/CT in combination with IOUS to determine the additional value of IOUS in pre-operative evaluation of hepatic metastases |
31 patients (16 men, 15 women), mean age 63.5 years (range 53–82 years) Indication: potentially respectable metastatic disease by other imaging modalities (ultrasound, CT and/or MRI) Exclusion criteria: second primary tumour Disease: primary tumour sigmoid colon (n = 10), transverse colon (n = 2), ascending colon (n = 5), caecum (n = 3), rectum (n = 11); T2 (n = 29), T3 (n = 21), T4 (n = 28), N0 (n = 5), node positive (n = 26) Comorbidities: NR Previous treatment: adjuvant therapy for primary tumour (n = 25); pre-operative chemotherapy within 6 months of surgery for metastases (n = 15); surgery for recurrent metastatic disease (n = 7) |
FDG PET/CT Discovery LS; fasting duration NR; FDG dose NR, time to scan NR; CT with intravenous contrast agent (n = 8) or non-contrast-enhanced (n = 23) Image interpretation Assessors: a certified radiologist, no further details Qualitative: based on identification of regions with increased uptake on PET and anatomic delineation of FDG-avid lesions on the co-registered CT; CT images also viewed separately to identify lesions without FDG uptake Quantitative: NR |
FDG PET/CT: as index test, plus IOUS (Nemio 30 scanner, Toshiba Medical Systems) Image interpretation Assessors: examinations performed by a certified gastroenterologist who had performed > 50 IOUS in patients with liver metastases, not blind to other liver imaging results including FDG PET/CT Qualitative: NR Quantitative: NR Time between index and comparator tests: mean time between FDG PET/CT and IOUS (i.e. resection of the metastases) 22.6 days (range 1–56 days) |
Histopathology, intra-operative frozen sections and pathologic specimen, and/or clinical follow up, no further details |
|
Bellomi 200762 Country: Italy Year: NR Study design: retrospective; consecutive sample: yes Setting: university hospital Aim: to compare MDCT and FDG PET/CT for detection of local and distant recurrence of rectal cancer |
67 patients (gender NR), age NR Indication: suspicion of local or distant recurrence on routine follow-up (CEA, abdominal and pelvic MDCT, chest radiograph or colonoscopy) following radical surgery Exclusion criteria: diabetes Disease: local recurrence (n = 15), distant recurrence (n = 27); hepatic metastases (n = 17); local recurrence and hepatic metastases (n = 7); lung metastases (n = 8); local recurrence and lung metastases (n = 2) Comorbidities: NR Previous treatment: all underwent radical surgery; pre-surgical RT (n = 20); post-surgical RT (n = 5); pre-surgical chemotherapy (n = 5); post-surgical chemotherapy (n = 18); post-surgical RT and chemo (n = 19) |
FDG PET/CT Discovery LS; fasting duration 6 hours; FDG 5 MBq/kg, scan approximately 60 minutes later; non-contrast-enhanced CT Image interpretation Assessors: experts in nuclear medicine, aware of other clinical, laboratory and diagnostic investigations results, who had just started interpreting FDG PET/CT images at the time of the study Qualitative: local recurrence: any new tissue at or close to anastomosis with asymmetrical, irregular, inhomogeneous contrast enhancement or changes compared with previous findings; hepatic or lung metastases: lesions not present at pre-operative CT Quantitative: SUV not used |
MDCT Lightspeed scanner; intravenous contrast agent Image interpretation Assessors: expert radiologists with several years’ experience of reading MDCT Qualitative: as index test Quantitative: NR Time between index and comparator tests: within 30 days (mean 22 days) |
Histology (biopsy or surgical) or follow-up of at least 2 years; independently compared with FDG PET/CT and MDCT findings |
|
Votrubova 200663 Country: Czech Republic Year: NR Study design: retrospective; consecutive sample: unclear Setting: hospital PET centre Aim: to compare PET and FDG PET/CT for detection of CRC recurrence |
84 patients (54 men, 30 women), mean age 64 years (range 41–78 years) Indication: suspected recurrence of CRC Exclusion criteria: NR Disease: NR Comorbidities: NR Previous treatment: colonic resection or rectal amputation |
FDG PET/CT Biograph Duo LSO; fasting duration at least 6 hours; FDG 370 MBq, scan 60–90 minutes later; CT: 30 patients received intravenous contrast agent; patients who previously underwent contrast-enhanced CT received oral contrast agent only Image interpretation Assessors: a skilled radiologist and a nuclear physician, in consensus; assessors first analysed CT and PET corrected and uncorrected images blind but aware of results of patients’ other investigations, then CT and FDG PET/CT images read with knowledge of previous PET reading Qualitative: positive: lesions with increased uptake and CT abnormality; pulmonary/liver nodules < 1 cm even in absence of FDG uptake; clearly increased focal uptake in normal structures; pathological structures with slightly higher uptake than the liver Quantitative: NA |
PET component of FDG PET/CT: using attenuation-corrected and uncorrected images Image interpretation As index test Time between index and comparator tests: sequential |
Histopathology within 4 weeks (no further details) and/or follow-up, mean duration 6.5 months (range 5–8 months) |
|
Kim 200568 Country: USA Year: 2002–3 Study design: retrospective; consecutive sample: unclear Setting: university hospital Aim: to compare PET and FDG PET/CT for re-staging recurrent CRC |
51 patients (30 men, 21 women), mean age 65 years (range 54–76 years) Indication: staging of biopsy proven (n = 12) or suspected (n = 39) recurrent CRC; suspicion based on clinical symptoms, tumour markers or other imaging tests Exclusion criteria: chemotherapy or RT within 4 weeks prior to FDG PET/CT; < 6 months’ follow-up Disease: colon (n = 35), rectum (n = 16); primary tumour non-mucinous adenocarcinoma (n = 41), mucinous adenocarcinoma (n = 6), unknown (n = 4) Comorbidities: NR Previous treatment: NR |
FDG PET/CT Reveal RT; fasting duration NR; FDG 7.77 MBq/kg, scan 60 minutes later; CT with oral contrast agent Image interpretation Assessors: three experienced nuclear medicine physicians, independently and unaware of clinical data; each assessor evaluated either PET or FDG PET/CT (not both) for each patient, and interpreted approximately one-third of PET and FDG PET/CT images Qualitative: abnormalities: multiple lung nodules on CT < 1 cm even in absence of increased uptake, and sclerotic bone lesions that did not demonstrate features of degenerative changes considered malignant; abnormalities on CT without corresponding increased uptake generally considered benign; characterisation: 1 = definitely benign, 2 = probably benign, 3 = equivocal, 4 = probably malignant, 5 = definitely malignant; location: 1 = uncertain, 2 = probable, 3 = definite Quantitative: NA |
PET component of FDG PET/CT: CT used for attenuation correction Image interpretation As index test Time between index and comparator tests: sequential |
Histology (33/143 regions) and clinical follow-up |
|
Cantwell 200882 Country: USA Year: 2004–5 Study design: retrospective; non-consecutive sample Setting: university hospital Aim: to compare the performance of the three modalities for detection of liver lesions |
33 patients (22 men, 11 women), mean age 63 years (range NR) Indication: liver metastases, included primary staging, restaging and treatment assessment; patients had to have at least one lesion to be included Exclusion criteria: > 10 liver lesions Disease: adenocarcinoma of colon (n = 24) or rectum (n = 9), none mucinous; low grade (n = 30), high (n = 2), high and low n = 1; T1 N1 M1 (n = 2), T2 N0 M1 (n = 5), T2 N1 M1 (n = 2), T3 N0 M1 (n = 7), T3 N1 M1 (n = 13), T4 N0 M1 (n = 1), T4 N1 M1 (n = 4); more than five hepatic lesions (n = 23), five to nine hepatic lesions (n = 10); extrahepatic metastatic disease (n = 11) Comorbidities: NR Previous treatment: prior hepatic resection (n = 11); chemotherapy (mean 1.5 courses, range 1–2) (n = 24); mean time from last dose to imaging 124 days (range 5–720 days) |
PET/CT LSO Biograph-16® (Siemens Health Care Diagnostics, Surrey, UK); fasting duration 6 hours; FDG 555–740 MBq, time to scan NR; low-dose CT Image interpretation Assessors: two radiologists with 2 and 5 years’ experience in reporting PET/CT and MRI, blind to patient demographics and clinical data, in consensus Qualitative: criteria NR; lesion characterisation: 0 = no lesion or normal, 1 = definitely benign, 2 = probably benign, 3 = possibly benign, 4 = possibly malignant, 5 = probably malignant, 6 = definitely malignant; malignant = 4–6, benign = 1–3 Quantitative: NR |
CE PET/CT: as index test with intravenous contrast agent, 170–220 mA Liver MRI: 1.5-T system® (Signa Advantage, GE Medical Systems, Fairfield, CT) or Siemens Magnetom (Siemens Medical Solutions) Image interpretation As index test Time between index and comparator tests: CE CT sequential; MRI mean 12 days (range 0–42 days) |
Histopathology, percutaneous biopsy (n = 3/100 malignant lesions, 0 benign lesions); serial follow-up imaging (CT, MRI) (n = 97/100 malignant lesions, 10/10 benign lesions), duration at least 6 months; data reviewed by one assessor |
|
Selzner 200487 Country: Switzerland and the USA Year: 2002–3 Study design: prospective; consecutive sample: yes Setting: university hospital Aim: to assess how PET/CT would change the indications for surgery and the diagnostic accuracy |
76 patients (52 men and 24 women) mean age 63 years (range 35–78 years) Indication; consideration for liver resection Exclusion criteria: cases with synchronous metastases were not present in this series Disease: metastatic CRC Comorbidities: NR Previous treatment: 62 patients received chemotherapy after the colorectal surgery with a median interval of 3 months between last chemotherapy and PET/CT |
PET/CT Discovery LS; fasting duration 4 hours; FDG 10 mCi (370 MBq), time to scan 45 minutes. No contrast enhancement used Image interpretation Assessors: single board registered radiologist and nuclear medicine physician viewed all images as co-registered on a using eNTEGRA software as well as separately Qualitative: based on the identification of regions with increased FDG uptake on PET images and the anatomical delineation of all FDG avid lesions on the co-registered images Quantitative: liver lesions categorised into (a) unilobar disease up to three lesions, (b) unilobar disease more than four lesions, (c) bilobar disease |
Contrast-enhanced CT: CT MD row scanner (Somatom Volume Zoom Siemens Medical Solutions, Erlangen, Germany). Intravenous contrast agent used | Biopsy, surgical histopathology, clinical and imaging follow-up, ultrasound and follow-up contrast-enhanced CT in all patients including those who did not undergo surgery |
| Study | Test and outcome level | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| TP/(TP + FN) | TN/(TN + FP) | TP/(TP + FP) | TN/(TN + FN) | ||||||
| D’Souza 200970 | FDG PET/CT, hepatic metastases, patient level | 7 | 0 | 0 | 1 | 0.875 | 0.000 | 1.000 | 0.000 |
| Contrast-enhanced CT, hepatic metastases, patient level | 6 | 0 | 0 | 2 | 0.750 | 0.000 | 1.000 | 0.000 | |
| Chua 20779 | FDG PET/CT, hepatic metastases, patient level | 63 | 2 | 6 | 4 | 0.940 | 0.750 | 0.969 | 0.600 |
| Contrast-enhanced CT, hepatic metastases, patient level | 61 | 6 | 2 | 6 | 0.910 | 0.250 | 0.910 | 0.250 | |
| Lubezky 200769 | FDG PET/CT, hepatic metastases, lesion level | 77 | 6 | 20 | 52 | 0.597 | 0.769 | 0.928 | 0.278 |
| Contrast-enhanced CT, hepatic metastases, lesion level | 92 | 7 | 18 | 38 | 0.708 | 0.720 | 0.929 | 0.321 | |
| FDG PET/CT, hepatic metastases, without neoadjuvant chemotherapy, lesion level | 29 | 2 | 0 | 2 | 0.935 | 0.000 | 0.935 | 0.000 | |
| Contrast-enhanced CT, hepatic metastases, without neoadjuvant chemotherapy, lesion level | 28 | 1 | 0 | 4 | 0.875 | 0.000 | 0.966 | 0.000 | |
| FDG PET/CT, hepatic metastases, with neoadjuvant chemotherapy, lesion level | 48 | 4 | 20 | 50 | 0.490 | 0.833 | 0.923 | 0.286 | |
| Contrast-enhanced CT, hepatic metastases, with neoadjuvant chemotherapy, lesion level | 64 | 6 | 18 | 34 | 0.653 | 0.750 | 0.914 | 0.346 | |
| Kong 200880 |
FDG PET/CT, hepatic metastases, patient level |
60 | 0 | 4 | 1 | 0.984 | 1.000 | 1.000 | 0.800 |
| Contrast-enhanced CT, hepatic metastases, patient level | 60 | 0 | 4 | 1 | 0.984 | 1.000 | 1.000 | 0.800 | |
| FDG PET/CT, hepatic metastases, lesion level | 155 | 0 | 6 | 10 | 0.939 | 1.000 | 1.000 | 0.375 | |
| Contrast-enhanced CT, hepatic metastases, lesion level | 163 | 0 | 6 | 2 | 0.988 | 1.000 | 1.000 | 0.750 | |
| Ramos 200881 | FDG PET/CT, local recurrence, patient level | 2 | 2 | 59 | 0 | 1.000 | 0.967 | 0.500 | 1.000 |
| Contrast-enhanced CT, local recurrence, patient level | 0 | 0 | 61 | 2 | 0.000 | 1.000 | 0.000 | 0.968 | |
| FDG PET/CT, hepatic recurrence, lesion level | 69 | 0 | 9 | 56 | 0.552 | 1.000 | 1.000 | 0.138 | |
| Contrast-enhanced CT, hepatic recurrence, lesion level | 98 | 4 | 5 | 27 | 0.784 | 0.556 | 0.961 | 0.156 | |
| Selzner 200487 | FDG PET/CT, extrahepatic metastases, patient level | 32 | 2 | 4 | 38 | 0.457 | 0.667 | 0.941 | 0.095 |
| Contrast-enhanced CT, extrahepatic metastases, patient level | 23 | 1 | 13 | 39 | 0.371 | 0.929 | 0.958 | 0.250 | |
| Cantwell 200882 | FDG PET/CT, hepatic metastases, lesion level | 67 | 4 | 6 | 33 | 0.670 | 0.600 | 0.944 | 0.154 |
| Contrast-enhanced FDG PET/CT, hepatic metastases, lesion level | 85 | 0 | 10 | 15 | 0.850 | 1.000 | 1.000 | 0.400 | |
| Liver MRI, hepatic metastases, lesion level | 98 | 0 | 10 | 2 | 0.980 | 1.000 | 1.000 | 0.833 | |
| Rappeport 200783 |
FDG PET/CT, hepatic metastases, patient level |
26 | 0 | 3 | 2 | 0.929 | 1.000 | 1.000 | 0.600 |
| PET component of FDG PET/CT, hepatic metastases, patient level | 23 | 0 | 3 | 5 | 0.821 | 1.000 | 1.000 | 0.375 | |
| CT component of FDG PET/CT, hepatic metastases, patient level | 28 | 2 | 1 | 0 | 1.000 | 0.333 | 0.933 | 1.000 | |
| MRI, hepatic metastases, patient level | 28 | 2 | 1 | 0 | 1.000 | 0.333 | 0.933 | 1.000 | |
| FDG PET/CT, extrahepatic metastases, patient level | 10 | 1 | 22 | 2 | 0.833 | 0.957 | 0.909 | 0.917 | |
| PET component of FDG PET/CT, extraheptic metastases, patient level | NR | NR | NR | NR | |||||
| CT component of FDG PET/CT, extrahepatic metastases, patient level | NR | NR | NR | NR | |||||
| MRI, extrahepatic metastases, patient level | NR | NR | NR | NR | |||||
| FDG PET/CT, hepatic metastases, lesion level | 47 | 1 | 74 | 24 | 0.662 | 0.987 | 0.979 | 0.755 | |
| PET component of FDG PET/CT, hepatic metastases, lesion level | 38 | 1 | 74 | 33 | 0.535 | 0.987 | 0.974 | 0.692 | |
| CT component of FDG PET/CT, hepatic metastases, lesion level | 63 | 25 | 50 | 8 | 0.887 | 0.667 | 0.716 | 0.862 | |
| MRI, hepatic metastases, lesion level | 58 | 14 | 61 | 13 | 0.817 | 0.813 | 0.806 | 0.824 | |
| Coenegrachts 200984 |
FDG PET/CT, hepatic metastases, patient level |
23 | 0 | 0 | 1 | 0.958 | 0.000 | 1.000 | 0.000 |
| MRI (SPIO enhanced), hepatic metastases, patient level | 24 | 0 | 0 | 0 | 1.000 | 0.000 | 1.000 | 0.000 | |
| MRI (unenhanced), hepatic metastases, patient level | 24 | 0 | 0 | 0 | 1.000 | 0.000 | 1.000 | 0.000 | |
| FDG PET/CT, hepatic metastases, lesion level | 47 | 0 | 0 | 30 | 0.610 | 0.000 | 1.000 | 0.000 | |
| MRI (SPIO enhanced), hepatic metastases, lesion level | 69 | 0 | 0 | 8 | 0.896 | 0.000 | 1.000 | 0.000 | |
| MRI (unenhanced), hepatic metastases, patient level | 77 | 0 | 0 | 0 | 1.000 | 0.000 | 1.000 | 0.000 | |
| Wildi 200885 | FDG PET/CT, liver metastases, patient level | 17 | 4 | 0 | 10 | 0.630 | 0.000 | 0.810 | 1.000 |
| FDG PET/CT, plus IOUS, liver metastases, patient level | 25 | 3 | 1 | 2 | 0.926 | 0.250 | 0.893 | 2.000 | |
| FDG PET/CT, liver metastases, without pre-operative chemotherapy, patient level | 10 | 3 | 0 | 3 | 0.769 | 0.000 | 0.769 | 3.000 | |
| FDG PET/CT, plus IOUS, liver metastases, without pre-operative chemotherapy, patient level | 12 | 3 | 0 | 0 | 1.000 | 0.000 | 0.800 | 4.000 |
The majority of studies used a retrospective design,24,62,63,68,71,79,80,82,85,86 with only five studies using a prospective design70,81,83,84,87 and one being unclear whether the study was retrospective or prospective. 69 The majority of reports did not reveal the manner in which sample patients were recruited; only six reported taking a consecutive approach24,62,83,84,86,87 and one82 reported that the sample was not recruited consecutively.
Study setting and country in which the research was conducted
Most studies were conducted in institutes of nuclear medicine, university hospitals and specialist cancer centres in Europe62,63,79–81,83–85 and the USA. 24,68,82,86,87 One was conducted in a university hospital in India,70 one in a university hospital and China71 and one in a teaching and research medical centre in Israel. 69
Patient populations
The 16 included studies reported outcomes for 890 patients. When gender proportions were reported, men were usually in the majority. The mean ages of patients when reported were 58–65 years, with a range of 31–92 years. Two studies70,79 included people with different types of cancer in whom FDG PET/CT was used to detect metastases. The age and gender of the patients with CRC were not reported separately by the authors.
Indication for FDG PET/CT
In 13 studies,62,63,68–70,79–85,87 patients were indicated for an FDG PET/CT scan when hepatic metastases were suspected from other follow-up examinations, imaging tests and blood tests. In three studies,62,63,68 patients were investigated for a recurrence of CRC, but metastatic disease was detected.
FDG PET/CT equipment and patient preparation
Full details of all of the equipment used in the primary studies can be found in Table 7. FDG PET/CT equipment was manufactured by one of four companies: Philips Medical Systems, GE Healthcare CPS Innovations or Siemens Medical Solutions. The fasting duration prior to the scan was at least 4 hours in all studies that reported these data.
A wide range of injected FDG doses was reported, with units ranging from 296 to 740 MBq, and patients were scanned between 60 and 120 minutes after the administration of the radioactive tracer. In some studies patients received either oral68,69,84 or intravenous63,83–85 contrast agents with the CT component of the FDG PET/CT test.
Image interpretation
Differences in interpretation were identified between studies, with most studies involving a radiologist working with an expert in nuclear medicine. Some studies24,68,82,83 also used a qualitative judgement scoring grade [using scores of 0 to equate to no lesion, 1 = definitely benign, 2 = probably benign, 3 = possibly benign, 4 = possibly malignant, 5 = probably malignant, 6 = definitely malignant (1–3 benign, 4–6 malignant)], while others used focal increased FDG uptake compared with background lesions. 63,69,79,84,85,87
Only one study71 reported using SUVmax in the assessment of FDG PET/CT images. Lesions with a SUVmax of > 2.5 in the early imaging and a change in the SUVmax of > 20% in the delayed imaging were considered positive.
Anatomical delineation data from the CT component of the FDG PET/CT scan were used in the overall assessment in four studies,62,63,84,85 and lung nodules and sclerotic bone lesions on CT images even in the absence of increased FDG uptake were considered malignant, but otherwise abnormalities on CT without corresponding increased uptake were considered benign, in one report. 68 No information regarding the methods of assessment was given in five studies. 70,71,80,81,86
Reference standard
The studies reported the use of various methods as the reference standard, including surgically resected specimens, biopsy, other imaging modalities and clinical follow-up. None of the studies attributed a specific reference standard test to individual patients’ index tests. Most reported a period of follow-up, and this ranged from 1 month to 30 months. 62,63,68–70,79–85
Data synthesis – diagnostic performance
FDG PET/CT versus contrast-enhanced CT
The accuracy of FDG PET/CT versus contrast-enhanced CT was compared in six studies69,70,79–81,87 involving 362 patients. Only two reported exclusion criteria: patients who had received chemotherapy < 3 months before FDG PET/CT was performed,80 and intact primary tumour or previous treatment of liver metastases by radiotherapy or surgery. 81
Estimates of diagnostic accuracy (sensitivity and specificity) for the detection of liver metastases were published with both patients and lesions as the unit of analysis.
Patient-level accuracy estimates – hepatic metastases
Estimates of sensitivity for FDG PET/CT ranged from 87% to 100%, and estimates of specificity ranged from 75% to 100% in four studies70,79–81 that reported data with patients as the unit of analysis. The sensitivity of contrast-enhanced CT ranged from 75% to 98% and specificity from 25% to 100%. In two of the four studies, FDG PET/CT appeared to demonstrate greater diagnostic accuracy than contrast-enhanced CT;70,79 in one, FDG PET/CT and contrast-enhanced CT were found to be equally accurate in the detection of liver metastases;80 and a fourth found that FDG PET/CT detected two lesions that proved to be FPs, and that contrast-enhanced CT detected two lesions that were confirmed to be FNs. 81
Lesion-level accuracy estimates – hepatic metastases
Estimates of sensitivity of FDG PET/CT in the detection of hepatic metastases with lesions as the unit of analysis ranged from 49% to 98%, and estimates of specificity ranged from 76% to 100%. For contrast-enhanced CT, sensitivity ranged from 55% to 100% and specificity from 65% to 99%.
Patient-level accuracy estimates – extrahepatic metastases
One study87 presented accuracy estimates for FDG PET/CT and contrast-enhanced CT in the diagnosis of colorectal metastases that were not confined to the liver. FDG PET/CT had a sensitivity of 46% (95% CI 34% to 57%) and a specificity of 67% (95% CI 30% to 90%), and contrast-enhanced CT demonstrated a sensitivity of 37% (95% CI 26% to 50%) and a specificity of 92% (95% CI 68% to 98%) with the patient as the unit of analysis.
FDG PET/CT versus contrast-enhanced FDG PET/CT and MRI
One study82 compared FDG PET/CT with contrast-enhanced FDG PET/CT and MRI in the detection of colorectal metastases and presented lesion-level data showing the sensitivity of FDG PET/CT to be 67% (95% CI 57% to 75%) and the specificity to be 60% (95% CI 31% to 83%). Contrast-enhanced FDG PET/CT had a sensitivity of 85% (95% CI 76% to 90%) and a specificity of 100% (95% CI 72% to 100%), but MRI demonstrated greatest accuracy with a sensitivity of 98% (95% CI 93% to 99%) and a specificity of 100% (95% CI 72% to 100%).
FDG PET/CT versus MRI
One study84 compared FDG PET/CT with MRI including unenhanced single-shot spin-echo echo planar imaging (SS SE-EPI) and superparamagnetic oxide (SPIO) enhancement. FDG PET/CT had a sensitivity of 96% (95% CI 80% to 99%), MRI (SS SE-EPI) 100% (95% CI 86% to 100%) and MRI (SPIO) 100% (95% CI 86% to 100%) based on estimates with patients as the units of analysis. Sensitivities calculated from hepatic metastatic lesion-level data were 61%, 100% and 89% (95% CI not calculable) for FDG PET/CT, MRI (SS SE-EPI) and MRI (SPIO), respectively.
FDG PET/CT versus PET versus CT versus MRI
One study83 compared images taken with integrated FDG PET/CT equipment with the PET image alone, the CT image alone and MRI in 35 patients. Patient-level estimates of accuracy from the FDG PET/CT integrated equipment were reported to be superior to all other comparisons: FDG PET/CT sensitivity 93% (95% CI 77% to 98%) and specificity 100% (95% CI 43% to 100%); PET alone sensitivity 82% (95% CI 64% to 92%) and specificity 100% (95% CI 43% to 100%); CT alone sensitivity 100% (95% CI 88% to 100%) and specificity 33% (95% CI 6% to 79%); MRI sensitivity 100% (95% CI 88% to 100%) and specificity 33% (95% CI 61% to 79%).
FDG PET/CT versus FDG PET/CT plus intra-operative ultrasound
A study evaluating FDG PET/CT versus FDG PET/CT plus intra-operative ultrasound (IOUS) for colorectal liver metastases stratified patients into those who had received chemotherapy and those who had not. 85 The accuracy estimates were a sensitivity of 63% (95% CI 44% to 78%) (specificity not calculable) for FDG PET/CT and a sensitivity of 92% (95% CI 77% to 97%) and a specificity of 25% (95% CI 4% to 69%) for FDG PET/CT plus IOUS for data based on 31 patients as the units of analysis.
Patient-level data collected from 16 patients without pre-operative chemotherapy were as follows: FDG PET/CT sensitivity 77% (95% CI 50% to 92%) (specificity not calculable); FDG PET/CT plus IOUS sensitivity 100% (95% CI 75% to 100%) (specificity not calculable).
Studies with mixed indications for FDG PET/CT (primary, recurrent and metastatic disease)
FDG PET/CT versus PET alone
One study24 compared FDG PET/CT scans with solitary PET images from the PET component of the integrated equipment. The sensitivity of FDG PET/CT was 86% (95% CI 77% to 91%) and the specificity was 67% (95% CI 44% to 83%), which was slightly superior to the sensitivity [88% (95% CI 80% to 92%)] and specificity [56% (95% CI 33% to 75%)] of PET alone in detecting all lesions. Where patients had multiple liver lesions, a maximum of five were included in the analysis.
FDG PET/CT was shown to possess greater accuracy than PET alone in detecting extrahepatic intra-abdominal disease: FDG PET/CT had a sensitivity of 86% (95% CI 49% to 97%) and a specificity of 93% (95% CI 78% to 98%), while the sensitivity of the PET component was 71% (95% CI 36% to 92%) and the specificity was 90% (95% CI 74% to 96%).
FDG PET/CT alone
A study71 with no comparator reported PET/CT to have a sensitivity of 95% (95% CI 85% to 98%) and a specificity of 83% (95% CI 55% to 95%) in patients with a diagnosis of recurrent and metastatic disease undergoing postoperative follow-up.
FDG PET/CT versus FDG PET/CT plus dedicated CT
A study86 including patients with both primary and metastatic disease found that the diagnostic accuracy of FDG PET/CT was improved by the addition of a dedicated CT scan: FDG PET/CT sensitivity 91% (95% CI 82% to 96%) and specificity 63% (95% CI 45% to 78%) compared with FDG PET/CT plus CT sensitivity 97% (95% CI 91% to 99%) and specificity 100% (95% CI 87% to 100%).
Three studies in which the indication was suspicion of recurrent disease
FDG PET/CT versus multidetector CT
In one study62 involving 67 patients with a suspicion of recurrent CRC, FDG PET/CT was compared with multidetector CT and both tests showed a sensitivity of 100% (95% CI 81% to 100%) and a specificity of 100% (95% CI 92% to 100%) in the diagnosis of liver metastases. Eight patients received a diagnosis of lung metastases, and in these individuals FDG PET/CT demonstrated a sensitivity of 75% (95% CI 40% to 92%) compared with a sensitivity of 100% (95% CI not calculable) for multidetector CT.
FDG PET/CT versus PET (component of the integrated equipment)
In a study63 involving 84 patients, FDG PET/CT demonstrated a sensitivity of 88% (95% CI 73% to 95%) and a specificity of 94% (95% CI 84% to 97%) in diagnosing the presence of intra-abdominal and/or extrahepatic metastases compared with a sensitivity of 81% (95% CI 66% to 91%) and a specificity of 88% (95% CI 76% to 94%) for the use of the PET component of the integrated equipment by itself. For extra-abdominal and/or extrahepatic metastases, the sensitivity of FDG PET/CT was 94% (95% CI 75% to 99%) and the specificity was 100% (95% CI 94% to 100%), while PET alone showed a sensitivity of 74% (95% CI 51% to 88%) and a specificity of 88% (95% CI 77% to 93%).
In a second study68 comparing FDG PET/CT with the PET component of an integrated scanner, FDG PET/CT demonstrated a sensitivity of 87% (95% CI 62% to 96%) and a specificity of 100% (95% CI 89% to 100%), while PET alone had a sensitivity of 87% (95% CI 62% to 96%) and a specificity of 97% (95% CI 84% to 99%).
The accuracy of FDG PET/CT in detecting liver metastases
The accuracy data for FDG PET/CT in detecting liver metastases are derived from seven studies70,79,80,83–85,87 including 281 subjects (Figure 3). Only four reported exclusion criteria: patients who had received chemotherapy < 3 months before FDG PET/CT was performed,80 diabetes,83 contraindications for the comparison test (MRI) or > 3 months,84 second primary tumour. 85
FIGURE 3.
Accuracy of FDG PET/CT in the detection of hepatic metastases based on patient-level data.
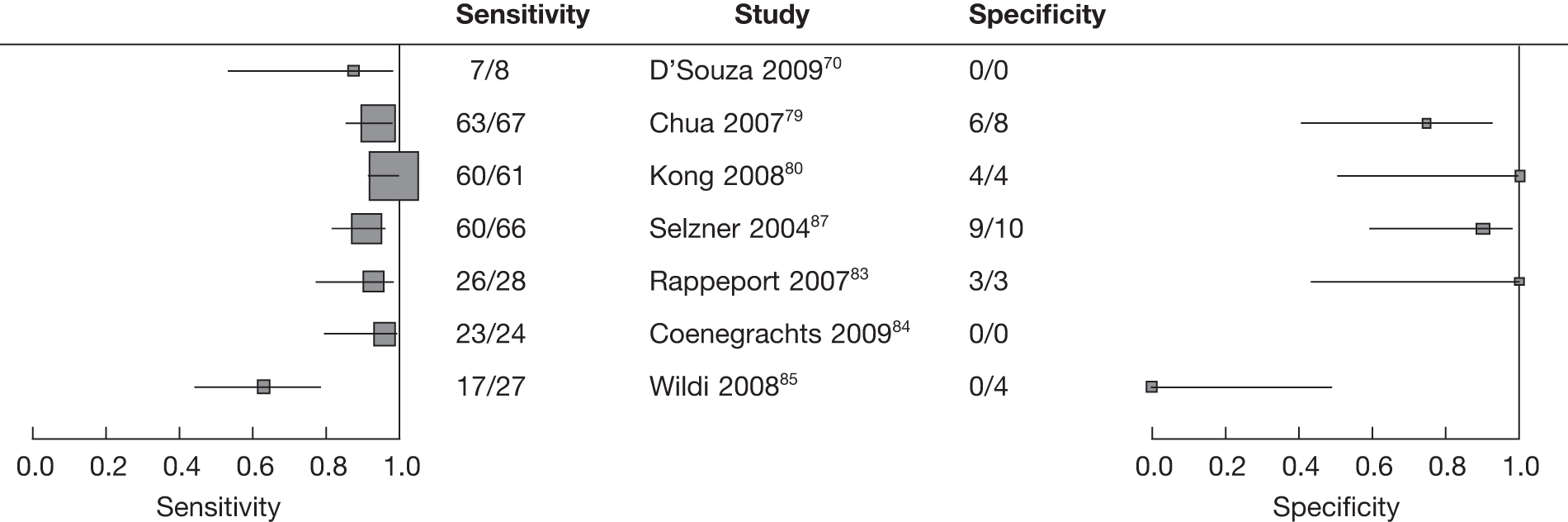
The bivariate HSROC method was not possible as the data did not allow adequate estimation of all the model parameters; therefore, two separate univariate meta-analyses for sensitivity and specificity were used. There was little evidence of heterogeneity in the sensitivity estimates, so fixed-effects meta-analysis was used. The overall estimate of sensitivity is 91% (95% CI 87% to 94%). There was evidence of some heterogeneity in the specificity estimates, so a random effects model was used and the overall estimate of specificity is 76% (95% CI 58% to 88%).
Quality assessment of included studies
Fourteen items from the QUADAS checklist were used to assess the methodological quality of the results, and the findings from this process are shown in Tables 9 and 10.
| Study | Spectrum of patients’ representative? | Selection criteria clearly described? | Reference standard likely to classify the target condition? | Time between the reference standard and index test short enough? | Whole or a random sample receive verification using a reference standard? | Patients received the same reference standard regardless of the index test result? | Reference standard independent of the index test? | Execution of the test described in sufficient detail to permit replication? | Execution of the reference standard described in sufficient detail? | Index test results interpreted without knowledge of the reference standard results? | Reference standard results interpreted without knowledge of the index test results? | Same clinical data available when tests results were interpreted as in clinical practice? | Uninterpretable intermediate test results reported? | Withdrawals from the study explained? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cantwell 200882 | N | Y | N | N | Y | N | Y | Y | UC | UC | UC | N | UC | Y |
| Chen 200771 | UC | N | UC | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Chua 200779 | UC | Y | UC | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Coenegrachts 200984 | Y | Y | UC | UC | Y | N | Y | Y | UC | Y | N | UC | UC | Y |
| Cohade 200324 | Y | Y | N | N | Y | N | Y | Y | UC | UC | UC | N | Y | Y |
| D’Souza 200970 | UC | N | UC | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Kamel 200486 | Y | N | UC | N | UC | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Kong 200880 | UC | N | UC | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Lubezky 200769 | UC | N | Y | Y | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Ramos 200881 | N | N | N | UC | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Rappeport 200783 | N | Y | Y | Y | N | N | Y | Y | UC | Y | UC | UC | UC | N |
| Selzner 200487 | Y | Y | Y | N | Y | N | Y | Y | UC | UC | UC | N | UC | UC |
| Wildi 200885 | N | N | UC | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Study | Spectrum of patients’ representative? | Selection criteria clearly described? | Reference standard likely to classify the target condition? | Time between the target condition and the reference standard short enough? | Whole or a random sample receive verification using a reference standard? | Patients received the same reference standard regardless of the index test result? | Reference standard independent of the index test? | Execution of the test described in sufficient detail to permit replication? | Execution of the reference standard described in sufficient detail? | Index test results interpreted without knowledge of the reference standard results? | Reference standard results interpreted without knowledge of the index test results? | Same clinical data available when tests results were interpreted as in clinical practice? | Uninterpretable intermediate test results reported? | Withdrawals from the study explained? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bellomi 200762 | Y | Y | Y | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Votrubova 200663 | UC | N | Y | N | Y | N | Y | Y | UC | UC | UC | UC | UC | Y |
| Kim 200568 | N | Y | Y | N | Y | N | Y | Y | UC | UC | Y | N | UC | Y |
In most studies it was unclear whether consecutive series of patients or a random sample of adults were undergoing staging for metastatic CRC; this was reported in only five studies. 24,62,84,86,87 We considered 6 weeks to be the time limit after which disease progression might occur, and the time between the reference standard and the index test was reported to be ≤ 6 weeks in only two studies. 69,83
A reference standard of surgically resected specimen, biopsy and/or clinical imaging follow-up of at least 6 months was reported in six studies. 62,63,68,69,83,87 Most studies reported that a whole or a random selection of the sample received verification using a reference standard of diagnosis. But FDG PET/CT results were verified using a variety of reference standards and, although these were independent of the index test in all studies, the index tests were interpreted without knowledge of the reference standard in only two studies83,84 and this item was not clearly reported in 14 studies. 24,62,63,68–71,79–82,85–87
The majority of studies did not clearly report whether the reference standard results were interpreted without knowledge of the index test results or vice versa. Only one study reported uninterpretable test results84 and this information was not clear in all of the others. 62,63,68–71,79–87
The validity of the conclusions from these studies was found to be compromised by the spectrum of patients, disease progression, differential verification and review bias.
Summary
-
The poor quality of the studies means that their conclusions should be interpreted cautiously, although overall it is clear that PET/CT is capable of identifying the small proportion of distant disease that is not detectable by conventional imaging modalities. The pooled accuracy data show FDG PET/CT to have a sensitivity of 91% (87% to 94%) and a specificity of 76% (95% CI 58% to 88%).
-
There are threats to the validity of these conclusions arising from retrospective (case series, audits) study designs and several types of bias. A major threat to the validity of these conclusions arises from the variation in the types of reference standard used (differential verification bias), which undermine the estimates.
-
Data to allow a cross-tabulation of results of different tests for patients contributing to the same study were not available and significance testing for differences between the sensitivity and the specificity of individual tests was not carried out.
-
PET/CT has been shown to be only slightly more accurate than PET alone in detecting metastases in patients with an indication of recurrent disease and in patients with an indication of metastatic disease. However, it has been shown to increase diagnostic certainty as to the type and site of disease.
Chapter 8 The therapeutic impact of FDG PET/CT in the pre-operative staging of colorectal cancer
Background
Evaluations of diagnostic tests tend to focus on accuracy but tests can be highly accurate without affecting therapy. 45 The extent to which diagnostic imaging techniques actually change clinical practice is a key consideration in assessing their cost-effectiveness: changes in practice might include decisions for or against progression to surgery, or reaching conclusions over aggressive versus conservative treatments. Although test accuracy is important, there is a need to examine whether or not new technologies have the potential to alter clinical decisions.
Therapeutic impact studies have reported high rates of treatment modifications when FDG PET (alone) is used in the pre-operative staging process. One systematic review44 summarised the effect of the use of FDG PET alone in the detection of metastatic disease of colorectal origin and reported pooled estimates of sensitivity and specificity of 88% and 96%, respectively, for hepatic disease and 91.5% and 95%, respectively, for extrahepatic disease. Additionally, the use of FDG PET led to a change in clinical management in 25% of cases. The authors concluded that FDG PET had a significant impact on clinical management compared with conventional diagnostic modalities for the assessment of the presence or absence of extrahepatic disease in patients being considered for resection of colorectal hepatic metastases.
We are unaware of any systematic review that evaluates the therapeutic impact of FDG PET/CT for the pre-operative staging of CRC. Integrated FDG PET/CT is increasingly used in the UK as the new equipment becomes more widely available, and an economic evaluation including its therapeutic impact in pre-operative staging of CRC is merited. 21
Aim
The aim of this chapter is to evaluate the therapeutic impact of FDG PET/CT in the pre-operative staging of patients with CRC.
Objectives
To identify changes to the treatment intent, the rate of modification of treatment plans, the nature and appropriateness of those changes and the effect on patient outcomes arising from the use of FDG PET/CT.
Methodological considerations
Diagnostic test accuracy studies commonly use a cross-sectional design,88 but studies evaluating the therapeutic impact of a test can utilise before-and-after as well as RCT designs. 45,46
Other methodological considerations in reviewing studies of diagnostic test accuracy and therapeutic impact relate to the intended role of FDG PET/CT; in all included diagnostic test accuracy studies, and in six of the seven included therapeutic impact studies, FDG PET/CT is treated as a replacement test;49,90–94 however, in one of the therapeutic impact studies its role as an add-on test is evaluated. 89 Because some of the diagnostic test accuracy studies also presented data for the therapeutic impact of FDG PET/CT, we present these data while recognising the important differences in study designs between diagnostic test accuracy and therapeutic impact studies, and recognising the two different roles for FDG PET/CT. 45,88
Results
Our search did not identify any systematic reviews to evaluate the therapeutic impact of integrated FDG PET/CT in the pre-operative staging of CRC.
The search identified seven therapeutic impact studies49,89–94 that sought to assess the value of FDG PET/CT in management. A further study published online as a pre-publication report was identified by one of our review team clinicians, and permission to include the study was obtained from the authors. 94
Nine studies included in the systematic review of diagnostic test accuracy also reported the therapeutic impact of FDG PET/CT, and we include them in this chapter. Two were concerned with the detection of recurrent disease64,66 and in all seven others patients had suspected metastases. 71,79–81,83,85,87
Study characteristics and study designs (therapeutic impact studies)
The therapeutic impact study characteristics are shown in Table 11, while the characteristics of the diagnostic test accuracy studies have been reported in Chapters 5–7. Four therapeutic impact studies reported the findings from retrospective case series;49,90,92,93 in one report the design was unclear;91 one reported using a prospective before-and-after design;89 and one used a case series with a nested before-and-after design. 94
| Study | Population | Index test, comparator(s), reference standard | Therapeutic impact of FDG PET/CT | Changes in management, planned management or treatment intent |
|---|---|---|---|---|
|
Davey 200889 Country: Italy Year: 2002–5 Study design: before and after; prospective study Setting: cancer centre Aim: to assess the incremental value of FDG PET/CT over conventional imaging in the management of primary rectal cancer |
83 patients (52 men, 31 women) Indication: staging Exclusion criteria: the most common reason was inability to obtain accurate follow-up information; no further details (not included n = 29/112) Disease: primary rectal adenocarcinoma Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery (GE Healthcare); fasting duration 6 hours; 300–400 MBq, time to scan 1 hour Conventional imaging Abdominal and pelvic CT (n = 83), chest radiography or CT (n = 76), and pelvic MRI (n = 54), endoanal ultrasound (n = 23) or both (n = 6) Reference standard: histology, operative findings |
FDG PET/CT had a high impact on management (change in treatment intent) in 12 patients (14%, 95% CI 8% to 24%) Change in treatment intent: n = 7 (curative → palliative n = 5, palliative → curative n = 2) Change in treatment modality: n = 10 |
FDG PET/CT had a high impact on management (change in treatment intent) in 12 patients (14%, 95% CI 8% to 24%) Change in treatment intent: n = 7 (curative → palliative n = 5, palliative → curative n = 2) Change in treatment modality: n = 10 (and intent n = 5, same intent n = 5): neoadjuvant chemotherapy + surgery → CRT alone due to detection of unresectable metastases (n = 5); neoadjuvant CRT → surgery alone due to detection of pelvic nodal spread (n = 3); neoadjuvant chemotherapy + surgery → surgery alone due to exclusion of pelvic nodal spread (n = 1); surgery alone → CRT alone due to detection of extensive iliac nodal disease in elderly patient (n = 1) |
|
Bassi 200890 Country: Italy Year: NR Study design: retrospective; consecutive sample: unclear Setting: department of radiology, university hospital Aim: to assess the impact of FDG PET/CT over CT on RT target volume and pre-operative staging of rectal cancer |
25 patients (19 men, 6 women), median age 65 years (44–79 years) Indication: pre-operative RT treatment planning Exclusion criteria: NR Disease: adenocarcinoma of the rectum Comorbidities: NR Previous treatment: NR |
FDG PET/CT Biograph (Siemens Medical Solutions); fasting duration at least 8 hours; FDG 5.18 MBq/kg, time to scan 60 minutes Contrast-enhanced FDG PET/CT CT component of FDG PET/CT Time between index and comparator tests: sequential Reference standard: proctoscopy, transrectal and liver ultrasound, and pelvic and abdominal CT scan |
4/25 cases FDG PET/CT affected tumour staging Additional nodal involvement in three and one additional metastasis in one of these cases One case showing potentially resectable LM on CT was shown to be several LM and inoperable with FDG PET/CT |
Mean clinical (RT) target volume (CTV): FDG PET/CT 737.3 cm3 (SD 121.7 cm3); PET NR; CT 708.3 cm3 (SD 124.6 cm3) FDG PET/CT CTV significantly > CT CTV (p = 0.00002) Mean difference between FDG PET/CT and CT CTV: 29.0 cm3 (95% CI 22.7 to 35.3 cm3) FDG PET/CT changed treatment intent in 1/25 patients from curative → palliative, due to detection of multiple hepatic lesions (CT showed a single potentially resectable lesion) |
|
Eglinton 201094 Country: Australia Year: 2006–7 Study design: case series with nested before-and-after design for a proportion of patients; consecutive sample: unclear Setting: university hospital Aim: to assess the role of FDG PET/CT in the initial staging of primary rectal cancer |
20 patients (14 men, 6 women), mean age 63 years (range 45–82 years) Indication: initial staging of primary rectal cancer Exclusion criteria: previous radiotherapy during involvement in another research study within 12 months Disease: rectal adenocarcinoma Comorbidities: NR Previous treatment: NR |
FDG PET/CT Gemini 16 (Philips Medical Systems); fasting duration NR; FDG 250 MBq, time to scan 45 minutes Conventional imaging Diagnostic (contrast-enhanced) CT of the chest, abdomen and pelvis, MRI rectum (n = 19/20) and colonoscopy (n = NR); one patient also had endorectal ultrasound. Equipment details: NR Reference standard: histology, conventional imaging, follow-up (average 16 months) |
FDG PET/CT correctly identified primary tumour in all 20 patients 11 discordant or incidental findings in nine patients, eight confirmed, seven in favour of FDG PET/CT result, one FP FDG PET/CT also detected (n = 3) or excluded (n = 2) extra-rectal neoplastic lesions in five other patients, but only two were confirmed: Increased uptake in prostate gland (n = 2), one confirmed by biopsy; increased uptake in right colon (n = 1, FP) No uptake in thoracic lymph node (n = 1) or paratracheal lymph node (n = 1), no histological confirmation |
Change in stage as a result of FDG PET/CT n = 6/9 with discordant findings Upstaging as a result of FDG PET/CT (n = 2): detection of liver metastases changed stage from III to IV (n = 1); internal iliac node uptake changed stage from II to III (n = 1); one patient with lung nodule uptake suspicious of metastases on FDG PET/CT awaiting verification by follow-up CT was not counted as upstaged Downstaging as a result of FDG PET/CT (n = 4): no liver metastases detected changed stage from IV to III (n = 3) or II (n = 1) No statistically significant difference in stage change according to conventional imaging stage or between low- or mid- and upper-level rectal tumours |
|
Engledow 200991 Country: France Year: NR Study design: unclear Setting: university hospital Aim: to evaluate the incremental value of FDG PET/CT over PET alone |
31 patients (18 men, 13 women), median age 68 years (range 29–77 years) Indication: suspected recurrence of CRC Exclusion criteria: NR Disease: recurrent CRC Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery LS (GE Medical Systems) PET PET component of FDG PET/CT equipment Reference standard: histology or routine clinical and radiological follow-up |
FDG PET/CT had an impact on planned surgical management in 6/31 patients: Downstaged: n = 2; upstaged: n = 2 Altered surgical incision with decreased morbidity (n = 2) due to improved anatomical localisation by FDG PET/CT |
FDG PET/CT had an impact on planned surgical management in 6/31 patients: Downstaged (n = 2): presumed liver lesion found to be physiological uptake in hepatic flexure (n = 1); presumed pelvic recurrence found to be physiological uptake in loop of small bowel (n = 1) Upstaged (n = 2) to inoperable owing to identification of occult intra-abdominal metastases (i.e. inappropriate liver resection avoided) Altered surgical incision with decreased morbidity (n = 2) due to improved anatomical localisation by FDG PET/CT |
|
Garin 200392 Country: France Year: NR Study design: retrospective Setting: nuclear medicine centre Aim: to assess the value of FDG PET/CT over conventional imaging in the management of recurrent CRC |
30 patients (19 men, 11 women) Indication: staging Exclusion criteria: NR Disease: recurrent CRC (colon n = 15, rectum n = 15) Comorbidities: NR Previous treatment: surgery |
FDG PET/CT Discovery (GE Healthcare); fasting duration 3 hours; FDG 5.18 MBq/kg, time to scan 1 hour PET PET component of FDG PET/CT equipment Reference standard: histopathology and/or follow-up |
Distinguished between bone and soft tissue in two cases Identified exact location between soft tissue and bone not detected with PET alone In total, FDG PET/CT had certain diagnosis in 6/20 (30%); 5/12 (42%) pelvic recurrence confirmed after it was suggested by other modalities or PET alone |
PET alone resulted in the modification of therapy: One had surgery, one had chemotherapy, one avoided surgery owing to the absence of a tumour. In three patients, PET alone did not help because it could not distinguish between bone and soft tissue. PET also suggested a hepatic metastasis, which when operated on was in the peritoneum PET wrongfully modified therapy after first-line chemotherapy, resulted in a delay before the second chemotherapy due to lack of identification (FN) PET showed soft-tissue changes in one patient not detected by FDG PET/CT For the group of patients thought to have operable metastases (n = 9) PET alone diagnosed metastases in six (1 TN, 5 TP) FDG PET/CT: Distinguished between bone and soft tissue in two cases Identified exact location between soft tissue and bone not detected with PET alone In total, FDG PET/CT had certain diagnosis in 6/20 (30%); 5/12 (42%) pelvic recurrence confirmed after it was suggested by other modalities or PET alone Of the group of 12 patients with raised CEA and a positive PET in the region of the pelvis, FDG PET/CT had a diagnostic impact in four |
|
Gearhart 200649 Country: USA Year: 2003–5 Study design: retrospective; consecutive sample: unclear Setting: teaching hospital Aim: to assess the incremental value of FDG PET/CT over standard initial evaluation of primary rectal cancer |
37 patients (26 men, 11 women) Indication: pre-treatment assessment Exclusion criteria: patients with early tumours not at risk of sphincter loss, or known metastatic disease Disease: primary rectal cancer Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery LS (GE Medical Systems); fasting duration 4 hours; 15–20 minutes; time between tracer and scan: NR Spiral CT Non-contrast-enhanced CT with oral contrast Reference standard: biopsy, follow-up or confirmatory imaging |
FDG PET/CT findings changed treatment plan in 10 patients | FDG PET/CT findings changed treatment plan in 10 patients: increased surgical margin as a result of identification of an additional colonic mass (n = 1); referred for neoadjuvant therapy as a result of identification of positive inguinal and/or pelvic lymph nodes (n = 2); positive inguinal or femoral lymph nodes included in RT field (n = 3); confirmatory lymph node biopsy at the time of surgery as a result of negative inguinal lymph nodes subsequently not included in RT fields (n = 1) or neoadjuvant therapy deferred (n = 1); liver resection as a result of identification of liver metastases (n = 2) |
|
Soyka 200893 Country: Switzerland Year: 2004–6 Study design: retrospective Setting: university hospital Aim: to investigate the value of contrast-enhanced FDG PET/CT as a first-line re-staging tool with special focus on the importance of intravenous contrast |
54 patients (37 men, 17 women), mean age 60.3 years (range 35–78 years) Indication: suspected recurrence of CRC Exclusion criteria: NR Disease: CRC Comorbidities: NR Previous treatment: all had resection of primary tumour; 45 had surgery plus chemotherapy or radiochemotherapy |
FDG PET/CT (contrast-enhanced FDG PET/CT versus non-contrast-enhanced FDG PET/CT) Discovery LS or Discovery ST (GE Healthcare); fasting duration 4 hours; 340–370 MBq; source NR; time between administration and performance of scan: 40–60 minutes Contrast-enhanced CT Contrast material (Ultravist 300) injected intravenously while patient remained on PET/CT table Reference standard: histopathology or clinical follow-up |
7/15 avoided surgery 6/15 had surgery where none had been scheduled 2/15 in the surgical procedure (strategy) was changed |
Change in therapeutic management according to additional findings, including change in management between (routine) non-contrast-enhanced FDG PET/CT and contrast-enhanced FDG PET/CT in patients who underwent liver surgery, in whom intravenous contrast agent was necessary for correct segmental assignment of liver lesions Contrast-enhanced CT versus non-contrast-enhanced FDG PET/CT 30/54 patients had inconclusive findings on contrast-enhanced CT and therefore received a ‘virtual’ referral for non-contrast-enhanced FDG PET/CT (routine clinical practice) In 20/30 patients, non-contrast-enhanced FDG PET/CT transformed indeterminate lesions to, or showed new lesions as, certainly benign or certainly malignant, i.e. provided correct additional information – and this had a effect on therapy in 15/20: avoided surgery (n = 7), surgery now considered appropriate (n = 6), altered the surgical strategy (n = 2; non-contrast-enhanced FDG PET/CT found an additional liver metastasis in one patient with known lung metastases and confirmed an additional local recurrence in one patient with known liver metastases); in 5/20 the correct additional information had no effect on therapy In 6/30 patients, non-contrast-enhanced FDG PET/CT provided no additional information In 2/30 patients, non-contrast-enhanced FDG PET/CT was FN (necrotic metastases due to previous chemotherapy) In 2/30 patients, non-contrast-enhanced FDG PET/CT was FP [granulomatous inflammation (n = 1), osteolysis in the sacrum (n = 1)] Overall, non-contrast-enhanced FDG PET/CT led to appropriate management decisions in 26/30 patients on equivocal contrast-enhanced CT 24/54 patients had conclusive findings on contrast-enhanced CT (not normally referred for non-contrast-enhanced FDG PET/CT in routine clinical practice) |
|
7/24 had correct additional information on non-contrast-enhanced FDG PET/CT – and this had a effect on therapy in 5/7: avoided surgery (n = 2), surgery now considered appropriate (n = 3); in 2/7 the correct additional information had no effect on therapy In 15/24 patients, non-contrast-enhanced FDG PET/CT provided no additional information |
Study setting and country in which the research was conducted (therapeutic impact studies)
All seven therapeutic impact studies were conducted in university teaching hospitals, specialist cancer centres or nuclear medicine centres. Five were conducted in Europe,89–93 one in Australia94 and one in the USA. 49
Patient populations (therapeutic impact studies)
The seven therapeutic impact studies reported outcomes for 280 patients in total. When gender proportions were reported, men were in the majority in all studies. The mean or median age of patients when reported was 60–68 years, with a range of 29–83 years.
The studies were all based on patients with either primary rectal cancer49,89,90,94 or recurrent CRC. 91–93
The diagnostic test accuracy studies reported in this chapter included studies focusing on patients with metastatic cancer.
Indication for FDG PET/CT
Suspected recurrence, pre-operative staging or a pre-treatment assessment was the indication for FDG PET/CT in all seven studies.
FDG PET/CT equipment and patient preparation
Full details of all of the equipment used in the primary studies can be found in Table 11. FDG PET/CT equipment was manufactured by one of three companies: GE Healthcare, Philips Medical Systems or Siemens Medical Solutions. The reported fasting duration prior to the scan was between 3 and 8 hours. Reported FDG doses either were 5.18 MBq/kg or ranged from 250 to 400 MBq, and patients were scanned between 40 and 60 minutes after the administration of the radioactive tracer. Contrast agent was given to patients in only one study. 94
Personnel interpreting the images
Images were most commonly interpreted by two physicians in our identified therapeutic impact studies – either radiologists or nuclear medicine physicians. In the study by Gearhart et al. 49 (a case series), a single nuclear medicine physician interpreted the images, while in the Davey et al. before-and-after study,89 images were interpreted at the time of the scan by physicians experienced in the use of FDG PET/CT to stage CRC; images were interpreted by incorporating FDG PET/CT findings with all other staging information. In the Eglinton et al. study94 (which used a case series with a nested before-and-after design), only patients with discordant results had their management plans reviewed by a multidisciplinary team (MDT) before and after FDG PET/CT results were made known. This limits the potential for therapeutic impact to be measured and, accordingly, we have excluded the data reported in this study from our report.
Techniques used for interpreting the images
Interpretation of FDG PET/CT scans is a complex process requiring a combination of clinical experience and judgement, pattern recognition and the application of guidelines (where they exist) over what constitutes an abnormal finding. A variety of what might be considered ‘qualitative’ and ‘quantitative’ techniques were used to assess the FDG PET/CT scans in the diagnosis of primary rectal tumours.
Among four studies49,89,90,94 that included patients with primary rectal cancer:
-
One reported that a focal uptake of FDG in the pelvis, chest or abdomen was considered positive only if the focal uptake was greater than the background uptake of the mediastinum and displayed definite structural abnormality on CT < 1 cm. 89
-
The Bassi et al. study90 defined positive lesions as those demonstrating a focus of activity significantly above the expected background not explained by a normal structure (e.g. lymph nodes > 15 mm or with increased FDG uptake).
-
Neither of the other two studies49,94 reported the process used for the assessment.
All studies including people suspected of CRC differed in their manner of image assessment:
-
In the Garin et al. study,92 lesions were judged positive if they demonstrated an increased intensity/avid tissue compared with the surrounding tissue, and, if images were considered equivocal, the assessors would form a bias in favour of malignancy.
-
In the Soyka et al. study,93 which included patients with recurrent disease, lesions identified with non-contrast-enhanced FDG PET/CT were reported as being malignant where soft-tissue masses were evident in conjunction with focally increased glucose metabolism. Lymph nodes were assessed for metastatic spread on the basis of glucose metabolism independent of their size. A diagnostic confidence score was also calculated based on the following categories: –2 = certainly benign; –1 = probably benign; 0 = indeterminate; 1 = probably malignant; 2 = certainly malignant. In the assessment of images using non-contrast-enhanced FDG PET/CT, the SUV was used to support the diagnosis of malignancy: SUVmax > 2.5 (extrahepatic lesions) or > 3.5 (intrahepatic lesions), but always in conjunction with the qualitative appearance. The Engledow et al. study91 of patients with suspected recurrence used a categorical scoring system for the anatomical localisation of lesions with the scores of 0 = unknown; 1 = probable; and 2 = definite. Engledow et al. also reported the degree of certainty of diagnosis by awarding a score: 0 = definitely benign; 1 = probably benign; 2 = equivocal; 3 = probably malignant; 4 = definitely malignant.
Therapeutic impact: pre-operative staging of rectal cancer
The results of the scans had varying influence on clinical decisions in the studies that we identified, and it was difficult to identify a consistent pattern.
Bassi et al.90
In 4/25 cases the findings on the FDG PET/CT scan changed the management of patients when FDG PET/CT was compared with contrast-enhanced FDG PET/CT:
-
FDG PET/CT identified additional nodal involvement in three patients and additional metastases in one patient.
-
FDG PET/CT upstaged one patient’s treatment from curative to palliative.
-
The radiotherapy clinical target volume was found to be statistically significantly greater on FDG PET/CT (737.3 cm3) than on CT (708.3 cm3).
Gearhart et al.49
Findings from FDG PET/CT changed management in 10/37 patients, seven of whom were upstaged and three downstaged. The details of these changes were as follows:
-
The identification of an additional colonic mass in one patient led to an increase in the surgical margin.
-
Two patients were referred for neoadjuvant therapy as a result of the identification of positive inguinal or pelvic lymph nodes.
-
Three patients with lymph node involvement were given radiotherapy while one patient was downstaged from lymph node radiation.
-
Neoadjuvant therapy was deferred in one patient.
-
Two patients had previously unidentified liver metastases resected.
The authors also reported that the impact on staging was significantly greater with tumours that were lower in the rectum (≤ 6 mm).
Eglinton et al.94
FDG PET/CT correctly identified the primary tumour in all 20 patients. Of those patients who had discordant findings on FDG PET/CT and CT scans (n = 9):
-
Six underwent a change in stage (two upstaged and four downstaged) but there was no statistically significant difference according to conventional imaging or the anatomical location of the rectal tumour (mid, low or upper).
-
Furthermore, FDG PET/CT had no effect on the surgical management of any of the patients who were included in the study.
Davey et al.89
In this study a stage and management plan was provided by each of the referring physicians based on conventional images, and these were compared with subsequent stage and management plans obtained from the same referring physicians after FDG PET/CT scans were made available. All imaging and management decisions were reviewed by an MDT at a tertiary referral centre for recurrent CRC and decisions were made in consensus:
-
PET/CT had a high impact on management and brought about changes in treatment intent in 12 patients (14%, 95% CI 8% to 24%).
-
For five patients, treatment modality was upstaged from curative to palliative, and in two the treatment changed from palliative to curative. The details of these changes were as follows:
-
– For five patients there was a change in treatment modality and a resultant change in treatment intent: planned neoadjuvant chemotherapy plus surgery was abandoned in favour of chemoradiotherapy (CRT) alone as a result of unresectable metastases.
-
– For a further five patients there was a change in treatment plan but no change in intent; in another five patients neoadjuvant chemotherapy was abandoned in favour of surgery alone.
-
-
FDG PET/CT detected extensive iliac lymph node involvement in one elderly patient who had CRT instead of surgery.
-
In one further patient the treatment intent changed from palliative to curative, and surgery was scheduled instead of neoadjuvant chemotherapy plus surgery because FDG PET/CT excluded pelvic nodal spread.
The authors reported that tumour height had no significant impact on the change in staging.
The effect on patient outcomes
Only the Eglinton et al. study94 provided information on actual patient outcomes. Patients were followed up for an average of 16 months (range 2–25 months), and two patients were reported to have died, one as a result of pneumonia.
Therapeutic impact: pre-operative staging of recurrent colorectal cancer
Therapeutic impact studies
Engledow et al.91
FDG PET/CT had an impact on planned surgical management in 6/31 patients:
-
Two patients had their disease downstaged because a presumed liver lesion was found to be a physiological uptake in hepatic flexure, and a presumed pelvic recurrence was found to be a physiological uptake in a loop of small bowel.
-
In two patients the disease was upstaged to inoperable as a result of identification of occult intra-abdominal metastases (i.e. inappropriate liver resections avoided).
-
The surgical incision was altered and decreased morbidity resulted in two patients as a result of improved anatomical localisation by FDG PET/CT.
Garin et al.92
In this study of patients suspected of having recurrent CRC, FDG PET/CT was compared with the PET component of the integrated equipment:
-
FDG PET/CT gave a definitive diagnosis in 6/20 (30%) patients, and in 5/12 (42%) patients it confirmed a pelvic recurrence after it was suggested by other modalities or PET alone.
-
PET/CT distinguished between bone and soft tissue in two cases and improved the anatomical localisation of the lesion by distinguishing between soft tissue and bone, which was not evident on the PET image.
-
In 12 patients who presented with a raised CEA and a positive PET scan of the pelvis, FDG PET/CT had a therapeutic impact in four cases but no details of these changes are available.
Soyka et al.93
In this study, which compared the therapeutic impact of non-contrast-enhanced FDG PET/CT and contrast-enhanced PET/CT in the detection of suspected recurrent CRC, non-contrast-enhanced FDG PET/CT had an effect on therapy in 15/20 patients:
-
Unnecessary surgery was avoided in 7/15.
-
Surgery when none had been scheduled was carried out in 6/15.
-
The planned surgical procedure altered in 2/15.
For 5/20 patients the additional correct information had no effect on therapy. The authors reported that contrast-enhanced FDG PET/CT was required in one case to allow the correct segmental assignment of liver lesions in a patient undergoing liver surgery.
Therapeutic impact: metastatic colorectal cancer (diagnostic test accuracy studies only)
Seven studies71,79–81,83,85,87 investigating the diagnostic test accuracy of FDG PET/CT for pre-operative staging of metastatic CRC and two studies64,66 concerned with the detection of recurrent disease also reported therapeutic impact outcomes (Table 12). Because the primary outcome of these studies was diagnostic test accuracy, their quality was assessed using QUADAS, and any threats to the validity of the studies is discussed in the previous chapters. The therapeutic impact data reported in these studies are included in this chapter for completeness.
| Study | Population | Index test, comparator(s), reference standard | Changes in management, planned management or treatment intent |
|---|---|---|---|
|
Davey 200889 Country: Italy Year: 2002–5 Study design: before and after; prospective study Setting: cancer centre Aim: to assess the incremental value of FDG PET/CT over conventional imaging on management of primary rectal cancer |
83 patients (52 men, 31 women) Indication: staging Exclusion: the most common reason was inability to obtain accurate follow-up information, no further details (not included n = 29/112) Disease: primary rectal adenocarcinoma Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery® (GE Medical Systems, Fairfield, CT); fasting duration 6 hours, 300–400 MBq; time to scan 1 hour Conventional imaging Abdominal and pelvic CT (n = 83), chest radiograph or CT (n = 76), and pelvic MRI (n = 54), endoanal ultrasound (n = 23) or both (n = 6) Reference standard: histology of operative findings |
FDG PET/CT had a high impact on management (change in treatment intent) in 12 patients (14%, 95% CI 8% to 24%) Change in treatment intent n = 7 (curative → palliative n = 5, palliative → curative n = 2) Change in treatment modality n = 10 (and intent n = 5, same intent n = 5): neoadjuvant chemotherapy + surgery → chemoradiotherapy alone due to detection of irresectable metastases (n = 5); neoadjuvant chemoradiotherapy → surgery alone due to detection of pelvic nodal spread (n = 3); neoadjuvant chemotherapy + surgery → surgery alone due to exclusion of pelvic nodal spread (n = 1); surgery alone → chemoradiotherapy alone due to detection of extensive iliac nodal disease in elderly patient (n = 1) |
|
Bassi 200890 Country: Italy Year: NR Study design: retrospective; consecutive sample: unclear Setting: department of radiology, university hospital Aim: to assess the impact of FDG PET/CT over CT on radio therapy target volume and pre-operative staging of rectal cancer |
25 patients (19 men, 6 women), Age: mean age median 65 years (range 44–79 years) Indication: pre-operative radio therapy treatment planning Exclusion criteria: NR Disease: adenocarcinoma of the rectum Comorbidities: NR Previous treatment: NR |
FDG PET/CT Biograph; fasting duration at least 8 hours; FDG 5 MBq/kg, time to scan 60 minutes Contrast-enhanced FDG PET/CT CT component of FDG PET/CT Time between index and comparator tests: sequential Reference standard: Proctoscopy, transrectal and liver ultrasound, and pelvic and abdominal CT scan |
Mean clinical (radio therapy) target volume: FDG PET/CT 737.3 cm3 (SD 121.7); PET NR; CT 708.3 cm3 (SD 124.6) FDG PET/CT clinical target volume significantly greater than CT (p = 0.00002) Mean difference between FDG PET/CT and CT clinical target volume: 29.0 cm3 (95% CI 22.7 to 35.3) FDG PET/CT changed treatment intent in 1/25 patient from curative → palliative, due to detection of multiple hepatic lesions (CT showed a single potentially resectable lesion) |
|
Eglinton 201094 Country: Australia Year: 2006–7 Study design: case series with nested before and after design for a proportion of patients; consecutive sample unclear Setting: university hospital Aim: to assess the role of FDG PET/CT in the initial staging of primary rectal cancer |
20 patients (14 men, 6 women) Age: 63 years (range 45–82 years) Indication: initial staging primary rectal cancer Exclusion criteria: previous RT during involvement in another research study within 12 months Disease: rectal adenocarcinoma Comorbidities: NR Previous treatment: NR |
FDG PET/CT Gemini 16; fasting duration NR; FDG 250 MBq; time to scan 45 minutes Conventional imaging protocol: diagnostic (contrast-enhanced) CT of the chest, abdomen and pelvis, MRI rectum (n = 19/20) and colonoscopy (n = NR); one patient also had endorectal ultrasound. Equipment details; NR Reference standard: histology, conventional imaging, follow-up (average 16 months) |
Change in stage as a result of FDG PET/CT, n = 6/9 with discordant findings Up staging as a result of FDG PET/CT (n = 2): detection of liver metastases changed stage from III to IV (n = 1); internal iliac node uptake changed stage from II to III (n = 1); NB, one patient with lung nodule uptake suspicious of metastases on FDG PET/CT awaiting verification by follow-up CT was not counted as upstaged Down staging as a result of FDG PET/CT (n = 4): no liver metastases detected changed stage from IV to III (n = 3) or II (n = 1) No statistically significant difference in stage change according to conventional imaging stage or between low or mid-to-upper level rectal tumours |
|
Engledow 200991 Country: France Year: NR Study design: unclear Setting: university hospital Aim: to evaluate the incremental value of FDG PET/CT over PET alone |
31 patients (18 men, 13 women) Age: median 68 years (range 29–77 years) Indication: suspected recurrence of colorectal cancer Exclusion: NR Disease: recurrent colorectal cancer Comorbidities: NR Previous treatment: NR |
FDG PET/CT Discovery LS PET PET component of FDG PET/CT equipment Reference standard: histology or routine clinical and radiological follow-up |
FDG PET/CT had an impact on planned surgical management in 6/31 patients Downstaged (n = 2): presumed liver lesion found to be physiological uptake in hepatic flexure n = 1, presumed pelvic recurrence found to be physiological uptake in loop of small bowel n = 1 Upstaged (n = 2) to inoperable due to identification of occult intra-abdominal metastases (i.e. inappropriate liver resection avoided) Altered surgical incision with decreased morbidity (n = 2) owing to improved anatomical localisation by FDG PET/CT |
|
Garin 200792 Country: France Year: NR Study design: retrospective Setting: nuclear medicine centre Aim: to assess the value of PET over FDG PET/CT conventional imaging on management of recurrent colorectal cancer |
30 patients (19 men, 11 women) Indication: staging Exclusion: NR Disease: recurrent CRC (colon n = 15, rectum n = 15) Comorbidities: NR Previous treatment: surgery |
FDG PET/CT Discovery; fasting duration 3 hours, FDG 5 MBq/kg; time to scan 1 hour PET PET component of FDG PET/CT equipment Reference standard: histopathology and/or follow-up |
PET alone resulted in the modification of therapy One had surgery One had chemotherapy One avoided surgery due to an absence of a tumour. In three patients PET alone did not help because it could not distinguish between bone and soft tissue. PET also suggested a hepatic metastases which when operated on was in the peritoneum PET wrongfully modified therapy after first-line chemotherapy resulted in a delay before the second-line chemotherapy due to lack of identification (FN) PET showed soft tissue changes in one patient not detected by FDG PET/CT For the group of patients thought to have operable metastases (n = 9) (see Table 4) the PET alone diagnosed metastases in six (one TN, five TP) FDG PET/CT distinguished between bone and soft tissue in two cases. Identified exact location between soft tissue and bone not detected with PET alone In total FDG PET/CT had certain diagnosis in 6/20 (30%) 5/12 (41%) pelvic recurrence confirmed after it was suggested by other modalities or the PET alone Of the group of 12 patients with raised cost-effectiveness acceptability and a positive PET in the region of the pelvis FDG PET/CT had a diagnostic impact in 4 |
|
Gearheart 200649 Country: USA Year: 2003–5 Study design: retrospective, consecutive sample; unclear Setting: teaching hospital Aim: to assess the incremental value of FDG PET/CT over standard initial evaluation primary rectal cancer |
37 patients (26 men, 11 women) Indication: pre-treatment assessment Exclusion: patients with early tumours not at risk of sphincter loss, or known metastatic disease Disease: primary rectal cancer |
FDG PET/CT Discovery LS; fasting duration 4 hours;15–20 ml; time between tracer and scan: NR Spiral CT Non-contrast enhanced CT with oral contrast Reference standard: biopsy, follow-up or confirmatory imaging |
FDG PET/CT findings changed treatment plan in 10 patients: increased surgical margin due to identification of an additional colonic mass (n = 1); referred for neoadjuvant therapy due to identification of positive inguinal and/or pelvic lymph nodes (n = 2); positive inguinal or femoral lymph nodes included in RT field (n = 3); confirmatory lymph node biopsy at the time of surgery owing to negative inguinal lymph nodes subsequently not included in RT fields (n = 1) or neoadjuvant therapy deferred (n = 1); liver resection due to identification of liver metastases (n = 2) |
|
Soyka 200893 Country: Switzerland Year: 2004–6 Study design: retrospective Setting: university hospital Aim: to investigate the value of contrast-enhanced FDG PET/CT as a first-line re-staging tool with special focus on the importance of intravenous contrast |
54 patients (37 men, 17 women) Age: 60.3 years (range 35–78 years) Indication: suspected recurrence of colorectal cancer Exclusion: NR Disease: colorectal cancer Comorbidities: NR Previous treatment: all had resection of primary tumour, 45 had surgery plus chemotherapy or radio chemotherapy |
FDG PET/CT (contrast-enhanced FDG PET/CT vs non-contrast-enhanced FDG PET/CT) Discovery LS or Discovery ST; 4 hours; 340–370 MBq; source NR; time between administration and performance of scan; 40–60 minutes Contrast-enhanced CT Contrast material (ultravist 300) injected intravenously while patient remained on T-CT table Reference standard: histopathology or clinical follow-up |
Change in therapeutic management according to additional findings; including change in management between (routine) non-contrast-enhanced-FDG PET/CT and contrast-enhanced-FDG PET/CT in patients who underwent liver surgery, in whom intravenous contrast agent was necessary for correct segmental assignment of liver lesions Contrast-enhanced CT vs non-contrast-enhanced FDG PET/CT n = 30/54 patients had inconclusive findings on contrast-enhanced CT and therefore received a ‘virtual’ referral for non-contrast-enhanced FDG PET/CT (routine clinical practice) In 20/30, non-contrast-enhanced FDG PET/CT transformed indeterminate lesions to, or showed new lesions as, certainly benign or certainly malignant, i.e. provided correct additional information – and this had a effect on therapy in 15/20 – avoided surgery (n = 7/15), surgery now considered appropriate (n = 6/15), altered the surgical strategy (n = 2/15: non-contrast-enhanced FDG PET/CT found an additional liver metastases in one patient with known lungs metastases; and confirmed an additional local recurrence in one patient with known liver metastases); in 5/20 the correct additional information had no effect on therapy n = 6/30 non-contrast-enhanced FDG PET/CT provided no additional information n = 2/30 non-contrast-enhanced FDG PET/CT was FN (necrotic metastases due to previous chemotherapy) n = 2/30 non-contrast-enhanced FDG PET/CT was FP [granulomatous inflammation (n = 1), osteolysis in the sacrum (n = 1)] Overall, non-contrast-enhanced FDG PET/CT led to appropriate management decisions in 26/30 patients on equivocal contrast-enhanced CT n = 24/54 patients had conclusive findings on contrast-enhanced CT (not normally referred for non-contrast-enhanced FDG PET/CT in routine clinical practice) n = 7/24 had correct additional information on non-contrast-enhanced FDG PET/CT – and this had a effect on therapy in 5/7: avoided surgery (n = 2/7), surgery now considered appropriate (n = 3/7); in 2/7 the correct additional information had no effect on therapy n = 15/24 non- contrast-enhanced FDG PET/CT provided no additional information |
|
Even-Sapir 200464 Country: Israel Year: NR Study design: retrospective; consecutive sample: unclear Setting: university hospital/cancer centre Aim: to assess the role of FDG PET/CT for detection of pelvic recurrence of rectal cancer |
62 patients (37 men, 25 women) Age: mean age 62 years (range 34–86 years) Indication: suspected recurrence of rectal cancer; increase in CEA (n = 16), suspected pelvic recurrence at CT (n = 19) or colonoscopy (n = 3), suspected extrapelvic recurrence or re-staging prior to surgical removal of presumed resectable metastases (n = 17), monitoring treatment response (n = 5), suspected second primary in lung (n = 1), unexplained anal pain (n = 1) Exclusion criteria: NR Disease: free of disease (n = 19), extrapelvic metastases with no evidence of pelvic recurrence (n = 19), extrapelvic metastases and pelvic recurrence (n = 8), pelvic recurrence only (n = 16) Comorbidities: diabetes (number NR) Previous treatment: abdominoperineal (n = 17) or anterior (n = 45) resection; neoadjuvant chemoradiation (n = 7); adjuvant chemotherapy (n = 16); post-surgical RT (n = 3) |
FDG PET/CT Discovery LS; fasting duration at least 4 hours; FDG 370–666 MBq, time to scan NR; CT: first 20 patients with oral contrast agent and 42 without Image interpretation Assessors: two experienced readers, in consensus; patients’ names were removed from reports; PET and FDG PET/CT images interpreted on separate days at least 1 week apart, presented in a different order Qualitative: uptake sites defined as malignant, benign or indeterminate on basis of shape, location and intensity; characterisation: 1 = benign, 2 = probably benign, 3 = equivocal, 4 = probably malignant, 5 = malignant Quantitative: SUVmax, no further details Comparator(s): PET component of FDG PET/CT, CT used for attenuation correction Image interpretation As index test Time between index and comparator tests: sequential Reference standard: histology (30/81 lesions) or clinical and imaging follow-up for at least 6 months (mean 8 ± 2.6 months); follow-up imaging included contrast-enhanced CT (n = 38), ultrasound (n = 4), MRI (n = 5), TRUS (n = 5), colonoscopy (n = 11), repeat FDG PET/CT (n = 9); two physicians, who did not participate in FDG PET/CT, PET interpretation, reviewed patient records together |
FDG PET/CT findings were of clinical relevance in 29/62 patients In patients investigated for unexplained increase in CEA (n = 16), FDG PET/CT detected pelvic recurrence (n = 4), extrahepatic metastases (n = 8) or both (n = 1) in 13 patients; 9/13 were referred for chemo, 4 for surgery In patients investigated for suspected recurrence at CT or colonoscopy, 5/13 with pelvic recurrence were not referred for surgery because of advanced locoregional disease and/or unsuspected nodal or distant metastases |
|
Kula 200466 Country: Poland Year: 2003–4 Study design: retrospective; consecutive sample: unclear Setting: hospital oncology centre Aim: to evaluate the usefulness of FDG PET/CT in the recurrence of colorectal cancer |
120 patients (44 men, 76 women), mean age 58.8 years women, 61.2 years men (range 24–80 years) Indication: suspected recurrence based on examination, routine imaging tests and raised CEA Exclusion criteria: > 8.4 mm/l glucose Disease: rectal cancer (n = 62), colon cancer (n = 28), hemicolorectomy left side (n = 12), hemicolorectomy right side (n = 10), not specified (n = 8) Comorbidities: NR Previous treatment: all had radical surgery; 62 rectal resection (51.7%) |
FDG PET/CT Biograph; fasting duration 1 hour; FDG 370 MBq, scan approximately 60–90 minutes later Image interpretation Assessors: NR Qualitative: NR Quantitative: NR Comparator(s): CEA and compared with all other tests Image interpretation 5 ng/ml threshold Time between index and comparator tests: 6 weeks before the FDG PET/CT scan Reference standard: histopathology and surgery; (n = 56) recurrence confirmed by surgical resection, (n = 24) recurrence was confirmed by histopathology Interpretation: (n = 10) liver, (n = 6) lungs, (n = 5) pelvis, (n = 3) postoperative scars Follow-up conducted within 12 months (average 6.3 months) |
10/38 patients’ surgery was abandoned |
|
Chua 200779 Country: UK Year: NR Study design: retrospective; consecutive sample: unclear Setting: university hospital (University College London) Aim: to compare FDG PET/CT and dedicated contrast-enhanced CT for detection of hepatic metastases |
Mixed cancer population, 75/131 CRC patients, age and gender not reported separately for CRC Indication: pre-operative staging (n = 2), assess suitability for liver resection (n = 21), assess suitability for radiofrequency therapy (n = 5), recurrence (n = 12), indeterminate CT findings (n = 12), suspected extrahepatic lesions on CT (n = 12), asymptomatic rise in tumour markers (n = 7), reassessment after chemotherapy/RT (n = 4) Exclusion criteria: NR Disease: primary site colorectal (n = 1), colon (n = 5), rectum (n = 2) Comorbidities: NR Previous treatment: some had undergone chemotherapy at least 6 months prior to imaging; at least four had chemotherapy/RT |
FDG PET/CT Discovery LS (GE Advance PET scanner and GE Lightspeed CT); fasting duration NR; FDG 350–370 MBq, scan 60 minutes later; non-contrast-enhanced CT Image interpretation Assessors: two experienced nuclear medicine physicians/radiologists, aware of clinical history, blinding not enforced Qualitative: visual assessment and maximum intensity tomographic data; negative: lesions not associated with focal increased uptake greater than background level Quantitative: NR Comparison(s): contrast-enhanced CT Siemens multidetector CT, oral and intravenous contrast agent Image interpretation Assessors: images reported under supervision of an experienced radiologist Qualitative: NR Quantitative: NR Time between index and comparator tests: within 6 weeks Reference standard: histopathology where available; clinical and radiological (both contrast-enhanced CT and FDG PET/CT) follow-up in discordant cases, duration at least 6 months |
FDG PET/CT resulted in a change in management in 18 CRC patients: Local recurrence seen on FDG PET/CT resulted in a change in surgical plans (n = 3) Multiple liver metastases (n = 4), multiple liver metastases and lung metastases (n = 1), multiple liver metastases and small bowel metastases (n = 1), multiple liver metastases and mesenteric lymphadenopathy (n = 1), mesenteric lymphadenopathy (n = 2), right iliac lymphadenopathy (n = 1), skeletal metastases (n = 2) seen on FDG PET/CT resulted in precluded hepatectomy (total n = 12) Findings on FDG PET/CT resulted in a change in favour of right hepatectomy (n = 2) Hepatic metastases on contrast-enhanced CT not seen on FDG PET/CT resulted in removal of primary colonic tumour (n = 1) |
|
Kong 200880 Country: UK Year: 2004–6 Study design: retrospective; consecutive sample: unclear Setting: specialist cancer centre (Royal Marsden) Aim: to compare FDG PET/CT with CT to identify extrahepatic disease, and with liver MRI to identify liver metastases |
65 patients (42 men, 23 women), median Age: 65 years (range NR) Indication: known or suspected potentially operable liver metastases Exclusion criteria: chemotherapy < 3 months before FDG PET/CT Disease: NR Comorbidities: NR Previous treatment: NR |
FDG PET/CT Gemini; fasting duration at least 4 hours; FDG 400 MBq, scan 60 minutes later; CT unenhanced Image interpretation Assessors: imaging results discussed at MDT meeting only, not directly reviewed Qualitative: NR Quantitative: NR Contrast-enhanced CT Lightspeed; intravenous contrast agent Liver MRI Maganese dipyridoxyl diphosphate (Mn-DPDP) contrast enhanced MRI; 1.5-T MRI System (Gyroscan Intera Master, Philips Medical Systems) Image interpretation As index test Time between index and comparator tests: median < 1month (range 0–49 days); 40/65 received liver MRI before FDG PET/CT Reference standard: histopathology (surgery or biopsy unclear) (n = 23); clinical/imaging follow-up (n = 42), median duration 13 months (range 1.5–30.3 months) |
Change in surgical management in all 11/65 patients in whom FDG PET/CT detected unexpected extrahepatic disease: upstaged and received palliative therapy (n = 6); required surgical intervention as well (n = 5) |
|
Ramos 200881 Country: Spain Year: 2006–7 Study design: prospective; consecutive sample: unclear Setting: university hospital Aim: to assess the additional value of FDG PET/CT versus conventional imaging for pre-surgical staging of liver metastases |
63 patients (41 men, 22 women), median Age: 61.8 years (range 38–78 years) Indication: referred for FDG PET/CT with a diagnosis of CRC liver metastasis on CT or MRI Exclusion criteria: intact primary tumour; previous treatment for liver metastases by surgery or RT Disease: primary tumour T4 (n = 13), T3 (n = 42), T2 (n = 7), T1 (n = 1); N0 (n = 21), one to three affected nodes (n = 24), more than three affected nodes (n = 18); synchronous metastasis (n = 31), metachronous metastasis (n = 32) Comorbidities: NR Previous treatment: chemotherapy in last 3 months, but discontinued at least 1 month before FDG PET/CT (n = 17) |
FDG PET/CT Discovery ST; fasting duration over 6 hours; FDG 307–7.4 MBq/kg (0.25 mg/kg intravenous furosemide 30 minutes after FDG), scan 50–90 minutes later; CT non-contrast enhanced, no further details Image interpretation Assessors: tumour staging done systematically, no further details Qualitative: NR Quantitative: NR Comparator(s): Contrast-enhanced CT 16-slice multidetector; intravenous contrast agent Liver MRI 1.5-T system (Phillips) Image interpretation As index test Time between index and comparator tests: between CT and FDG PET/CT median 16.8 days (range 1–62 days) Reference standard: an extension study including thoracoabdominal CT, FDG PET/CT and a colon study if none was available that was < 1 year old (protocol included MRI in patients with contrast agent allergy or steatosis), and manual abdominal exam of areas noted on intraoperative liver ultrasound; 1-year follow-up of all patients was planned to definitively confirm the results of the extension study – this report was based only on data available on completion of the extension study and the data from surgery only |
FDG PET/CT findings led to a change in the therapeutic decision in nine cases: one case of under staging, i.e. liver metastases ruled out (FP); new extrahepatic disease in eight cases, of which four were correct and four were FP |
|
Selzner 200487 Country: Switzerland and the USA Year: 2002–3 Study design: prospective; consecutive sample: yes Setting: university hospital Aim: to assess how FDG PET/CT would change the indications for surgery and the diagnostic accuracy |
76 patients (52 men, 24 women) mean age 63 years (range 35–78 years) Indication; consideration for liver resection Exclusion criteria: cases with synchronous metastases were not present in this series Disease: metastatic CRC Comorbidities: NR Previous treatment: 62 patients received chemotherapy after the colorectal surgery with a median interval of 3 months between last chemotherapy and FDG PET/CT |
FDG PET/CT Discovery LS; fasting duration 4 hours; FDG 10 mCi (370 MBq), time to scan 45 minutes; contrast-enhanced CT Image interpretation Assessors: single board registered radiologist and nuclear medicine physician viewed all images as co-registered on eNTEGRA software as well as separately Qualitative: based on the identification of regions with increased FDG uptake on PET images and the anatomical delineation of all FDG-avid lesions on the co-registered images Quantitative: liver lesions categorised into (a) unilobar disease up to three lesions, (b) unilobar disease more than four lesions, (c) bilobar disease Contrast-enhanced CT Multidetector row scanner (SOMATOM Volume Zoom Siemens Medical Solutions, Erlangen, Germany); intravenous contrast agent used Reference standard: biopsy, surgical histopathology, clinical and imaging follow-up, ultrasound and follow-up contrast-enhanced CT in all patients including those who did not undergo surgery |
Treatment plan formulated after contrast-enhanced CT and re-evaluated after FDG PET/CT 16/76 FDG PET/CT resulted in a treatment change: resection contraindicated (n = 10); change in surgical strategy (n = 6) |
|
Rappeport 200783 Country: Denmark Year: 2004–5 Study design: prospective; consecutive sample Setting: university hospital Aim: to compare the performance of the three modalities for detection of liver lesions |
35 patients (16 men, 19 women) Age: median age 62 years (range 33–74 years) Indication: patients referred for surgery for known or suspected CRC liver metastases Exclusion criteria: diabetes, MRI contraindications, imaging could not be performed before scheduled surgery Disease: NR Comorbidities: NR Previous treatment: chemotherapy within 1 month of FDG PET/CT (n = 4) |
FDG PET/CT Discovery LS; fasting duration NR; FDG 400 MBq, scan 50–90 minutes later; CT with intravenous contrast agent Image interpretation Assessors: a radiologist and a nuclear medicine physician with > 10 years experience, blind to other imaging results, in consensus; the radiologist had access to the complete CT data set Qualitative: assessors told to rely upon criteria from daily practice to decide whether a lesion was benign or malignant; characterisation: 1 = definitely benign, 2 = possibly malignant, 3 = definitely malignant; maximum diameter and segment location also recorded Quantitative: NR PET component of FDG PET/CT, CT used for attenuation correction CT component of FDG PET/CT MRI SPIO-enhanced: 1.5-T Horizon Sigma LX scanner (GE Medical Systems) Image interpretation Assessors: single observers, CT by a radiologist, and MRI by a different single observer, no further details Qualitative: suspicious: hypovascular liver lesion with low TI signal and high T2 signal not classified as haemangioma on contrast enhancement pattern Quantitative: NR Time between index and comparator tests: 1–2 days Reference standard: 28/31 surgical patients had liver metastases verified at surgery (23/28 histological confirmation, 5/28 verified intra-operatively), 2/31 had follow-up imaging; median time from scan to surgery was 7 days for FDG PET/CT, 12 days for MRI; data reviewed by three assessors, in consensus |
In all three patients for who FDG PET/CT detected additional sites liver surgery was cancelled Relying on FDG PET/CT only, one patient with no extrahepatic tumour (FP) would have been denied surgery |
|
Wildi 200885 Country: Switzerland Year: NR Study design: retrospective; consecutive sample: unclear Setting: tertiary referral centre Aim: to compare FDG PET/CT with FDG PET-C in combination with IOUS to determine the additional value of IOUS in pre-operative evaluation of hepatic metastases |
31 patients (16 men, 15 women) Age: mean age 63.5 years (range 53–82 years) Indication: potentially resectable metastatic disease by other imaging modalities (ultrasound, CT and/or MRI) Exclusion criteria: second primary tumour Disease: primary tumour sigmoid colon (n = 10), transverse colon (n = 2),ascending colon (n = 5), caecum (n = 3), rectum (n = 11); T2 (n = 29), T3 (n = 21), T4 (n = 28), N0 (n = 5), node positive (n = 26) Comorbidities: NR Previous treatment: adjuvant therapy for primary tumour (n = 25); pre-operative chemotherapy within 6 months of surgery for metastases (n = 15); surgery for recurrent metastatic disease (n = 7) |
FDG PET/CT Discovery LS; fasting duration NR; FDG dose NR, time to scan NR; CT with intravenous contrast agent (n = 8) or non-contrast enhanced (n = 23) Image interpretation Assessors: a certified radiologist, no further details Qualitative: based on identification of regions with increased uptake on PET and anatomic delineation of FDG-avid lesions on the co-registered CT; CT images also viewed separately to identify lesions without FDG uptake Quantitative: NR Comparison(s): FDG PET/CT as index test, plus IOUS (Nemio 30 scanner, Toshiba Medical Systems) Image interpretation Assessors: examinations performed by a certified gastroenterologist who had performed > 50 IOUSs in patients with liver metastases, not blind to other liver imaging results including FDG PET/CT Qualitative: NR Quantitative: NR Time between index and comparator tests: mean time between FDG PET/CT and IOUS (i.e. resection of the metastases) 22.6 days (range 1–56 days) Reference standard: histopathology, intra-operative frozen sections and pathologic specimen, and/or clinical follow-up, no further details |
Additional information from IOUS altered the surgical strategy in 11 cases |
| Mixed primary recurrent and metastatic CRC | |||
|
Chen 200771 Country: China Year: 2004–6 Study design: retrospective; consecutive sample: unclear Setting: university hospital Aim: to evaluate FDG PET/CT for detection of postoperative recurrence and/or metastasis of CRC |
68 patients (48 men, 20 women) Age: mean age 58 years (range 27–77 years) Indication: postoperative follow-up, no further details; time between operation and FDG PET/CT 4 months to 8 years (mean 2.5 years) Exclusion criteria: NR Disease: recurrent (n = 8), metastatic (n = 46) or both (n = 2) Comorbidities: NR Previous treatment: NR |
FDG PET/CT Biography Sensation® (Siemens Health Care Diagnostics, Surrey, UK); fasting duration NR; FDG 296–444 MBq, time to scan 50–60 minutes; CT: contrast-enhanced NR Image interpretation Assessors: more than two experienced nuclear medicine physicians and radiologists Qualitative: NR Quantitative: positive lesion: SUVmax > 2.5 in the early imaging and change in SUVmax > 20% in the delayed imaging; determination of malignancy simultaneously based on CT findings (reported as semi-quantitative) Comparator(s): none Reference standard: histopathology, colonoscopy, imaging and clinical follow-up, duration 5–28 months |
n = 11 cases had treatment plans altered based on FDG PET/CT n = 3 changed from surgery to chemotherapy n = 6 changed from chemotherapy and biotherapy to surgical resection n = 1 changed from chemotherapy to reoperation n = 1 changed from chemotherapy to CRT |
Diagnostic test accuracy studies
Even-Sapir et al.64
FDG PET/CT findings were of clinical relevance in 29/62 (47%) patients with suspected recurrent rectal cancer:
-
Among those patients investigated for an unexplained increase in CEA (n = 16), FDG PET/CT detected pelvic recurrence (n = 4), extrahepatic metastases (n = 8) or both (n = 1) in 13 patients; 9/13 were referred for chemotherapy and 4/13 for surgery.
-
Among patients who were investigated for suspected recurrence detected by CT or colonoscopy, 5/13 with pelvic recurrence were not referred for surgery because FDG PET/CT detected advanced locoregional disease and/or unsuspected nodal or distant metastases.
Kula et al.66
In this study, the findings of the FDG PET/CT scan led to an abandonment of the planned surgery for 10/38 patients with suspected rectal cancer.
The effect on patient outcomes
None of the studies examining pre-operative staging of CRC reported the effect that changes in management had on patient outcomes.
Chua et al.79
FDG PET/CT resulted in a change in management in 18 CRC patients:
-
Hepatectomy was precluded (n = 12) after the identification of multiple liver metastases (n = 4), multiple liver metastases and small bowel metastases (n = 1), multiple liver metastases and mesenteric lymphadenopathy (n = 1), mesenteric lymphadenopathy (n = 2), right iliac lymphadenopathy (n = 1) and skeletal metastases (n = 2) by FDG PET/CT.
-
Local recurrence seen on FDG PET/CT resulted in a change in surgical plans.
-
Hepatic metastases not seen on FDG PET/CT resulted in removal of primary colonic tumour.
The authors did not reveal the consequences of these changes (patient outcomes) or whether or not they were correct.
Kong et al.80
There was a change in surgical management in all 11/65 patients in whom FDG PET/CT detected unexpected extrahepatic disease:
-
Six patients were upstaged and received palliative therapy.
-
Five patients receiving palliative treatment required surgical intervention as well.
Ramos et al.81
FDG PET/CT findings led to a change in the therapeutic decision in nine cases:
-
One case of understaging, i.e. liver metastases ruled out.
-
New extrahepatic disease in eight cases, of which four were correct and four were FPs.
Selzner et al.87
In this study, the treatment plan was formulated after contrast-enhanced CT and re-evaluated after FDG PET/CT; 16/76 FDG PET/CT scans resulted in a treatment change:
-
Resection contraindicated (n = 10)
-
Change in surgical strategy (n = 6).
Rappeport et al.83
In all three patients for whom FDG PET/CT detected additional sites, liver surgery was cancelled; however, the authors reported a FP lesion on the FDG PET/CT scan of one patient with no extrahepatic tumour who would have been denied surgery had the FDG PET/CT scan been the only test used.
Wildi et al.85
This comparison of FDG PET/CT alone versus FDG PET/CT plus IOUS found that additional information from IOUS altered the surgical strategy in 11 cases.
The effect on patient outcomes
None of the studies examining metastatic CRC reported the effect that changes in management had on patient outcomes.
Quality assessment of the therapeutic impact studies
In the absence of an internationally recognised list of criteria against which to assess quality, included studies were judged against five components felt to be relevant: patient selection; description of initial treatment intent; independent/blind review; description of test on patient outcome; and withdrawals (Table 13):
-
Patients were reported to be selected as a consecutive sample undergoing staging for known or suspected primary cancer of the colon or the rectum in four studies90–93 and the patients were clearly described in three. 49,89,92
-
Only one study89 reported the intended management plan prior to the FDG PET/CT test, and in only two92,93 was the final confirmation method likely to correctly classify the disease.
-
The therapeutic impact attributed to FDG PET/CT was subject to independent review in only one study. 91
-
None of the studies showed that the therapeutic changes attributed to FDG PET/CT resulted in an improved patient outcome.
-
With the exception of one study, all explained withdrawals. 92
| Study | Spectrum of patients’ representative? | Selection criteria clearly described? | Was the intended management plan elicited before the FDG PET/CT scan was performed? | Was the final confirmation method likely to correctly classify disease status? | Was the therapeutic impact attributed to FDG PET/CT independent review? | Was the relationship between therapeutic changes attributed to FDG PET/CT clearly demonstrated ? | Withdrawals from the study explained? |
|---|---|---|---|---|---|---|---|
| Bassi 200890 | Y | N | N | UC | N | N | Y |
| Davey 200889 | UC | Y | Y | UC | N | N | Y |
| Gearhart 200649 | N | Y | N | UC | N | N | Y |
| Engledow 200991 | Y | N | N | UC | Y | N | Y |
| Garin 200392 | Y | Y | N | UC | UC | N | UC |
| Soyka 200893 | Y | UC | N | UC | N | N | Y |
| Eglinton 201094 | UC | N | Y | N | Y | N | Y |
Based on these observations, the overall quality of the therapeutic impact studies was deemed to be poor.
Conclusions
It is difficult to draw conclusions about therapeutic impact from the data available. Therapeutic impact was a secondary consideration in diagnostic test accuracy studies and, even in dedicated therapeutic impact studies, data were collected in very inconsistent ways. There is little or no agreement in the studies about the therapeutic impact or clinical decisions of interest. Further, we note that:
-
The FDG PET/CT images were assessed according to a variety of different criteria, which makes an overarching synthesis of study findings inappropriate.
-
Studies reported inconsistent findings about the effect that FDG PET/CT had on surgical management: some found no effect and others reported decreased morbidity from improved surgical techniques arising from increased precision in tissue identification.
-
In studies that reported changes in surgical management as a result of FDG PET/CT, upstaging and abandonment of surgery were more frequently reported than downstaging.
-
More decisions to undertake surgery were avoided when FDG PET/CT was used to eliminate patients from the surgical pathway (hepatectomy precluded after the identification of multiple liver metastases with FDG PET/CT).
-
Surgical resections with curative intent may be avoided, but some palliative operations will still be required.
-
The evidence regarding the effect that the tumour height and size has on staging is conflicting.
-
In all of the studies it was unclear whether the disease confirmation method would correctly classify the disease.
-
None reported the effect on patient outcomes and only one reported deaths. Little is therefore known about the effects of FDG PET/CT and the long-term outcomes, for those who receive an FDG PET/CT scan to stage their disease pre-operatively.
-
The quality of contrast-enhanced CT and liver MRI will have a substantial effect on the apparent therapeutic impact of FDG PET/CT.
-
It was suggested that contrast-enhanced FDG PET/CT is required to allow the correct segmental assignment of liver lesions.
-
There were reports of both FN and FP lesions with FDG PET/CT.
Although there is evidence that FDG PET/CT results influence clinical decisions in CRC, the direction and outcome of these decisions is unclear. The therapeutic impact appears to be dependent upon the nature of the disease, health-care context and a range of other factors.
Chapter 9 Safety
None of the 30 studies included in this review measured staff levels of radiation or reported adverse events or harms arising from the use of FDG PET/CT. Our separate search also failed to detect any studies that reported adverse events or harms arising from FDG PET/CT.
Two public assessment reports from the Medicines and Healthcare products Regulatory Agency Authority30,95 conclude that there is no evidence to suggest that FDG is toxic when used as a single-dose prescription-only medicine:95
Radiation exposure to patients derived from the administration of 18[F]-FDG is well within the limits of other radiological and nuclear medicine diagnostic procedures. As with any test involving ionising radiation, 18[F]-FDG should only be administered when the expected benefit (diagnostic yield) outweighs the risk.
A 4-year prospective questionnaire survey to identify the number of adverse events from PET procedures was conducted in 22 collaborating institutions in the USA. From a total of 47,876 PET radiopharmaceutical doses, the majority of which were FDG, no adverse events were recorded. 96
Minimising the overall dose of radiation to staff and other hospital patients requires specially shielded facilities and a carefully planned workflow to ensure that the staff are not in contact with patients who have received an FDG injection for longer than necessary. Similarly, ‘hot waiting rooms’ separate FDG PET/CT patients from other hospital patients.
As part of our systematic review, we sought advice from the Medical Physics Department of NHS Lothian regarding routine safety precautions for NHS staff and patients and were informed of work conducted by the Radiation Protection Service of NHS Grampian to assess risk for journeys made by various persons travelling with patients who have recently received an FDG PET/CT scan. As a result, NHS Lothian has produced advice for FDG PET/CT patients, their drivers and fellow passengers recommending that those who have recently had an FDG PET/CT scan should sit as far as possible from (diagonally opposite) the driver during car journeys. Of particular concern are those drivers who regularly transport patients who have received an FDG PET/CT scan. A copy of the NHS Lothian information leaflet can be obtained from the authors of this report.
Chapter 10 Economic evaluation
Aims and objectives
The aim of this chapter is to determine whether or not FDG PET/CT is cost-effective as an add-on test in comparison to routinely used imaging modalities for pre-operative staging in patients with primary, recurrent and metastatic CRC. Probabilistic decision-analytic modelling was undertaken (using Monte Carlo simulation) to address the following questions:
-
Is FDG PET/CT likely to be cost-effective as an add-on test for pre-operative staging in CRC compared with alternative methods of diagnosis and staging, given current evidence and uncertainty?
-
In which patient groups (i.e. primary rectal cancer, primary colon cancer, recurrent rectal cancer, recurrent colon cancer, metastatic disease) is it likely to be cost-effective?
-
Under what circumstances is it likely to be cost-effective?
A value of information analysis was also undertaken to help inform whether or not there is potential worth in undertaking further research.
Methods
The economic evaluation is based upon current evidence, utilising decision modelling techniques to synthesise data from numerous sources. 97,98 The evaluation is undertaken from the perspective of the UK NHS, reporting short-term outcomes in terms of the incremental cost per correct diagnosis, and longer-term outcomes in terms of the incremental cost per quality-adjusted life-year (QALY) gained. Reporting QALY outcomes enables the analysis to incorporate the potential patient management implications of accurate and inaccurate diagnoses, particularly the implications for patients’ quality of life.
FDG PET/CT and conventional imaging devices have been found to have different diagnostic test accuracies for staging primary, recurrent and metastatic CRC. As such, three separate economic models were designed to address the questions outlined in the aims. Patient management routes also differ between colon and rectal cancer, and so the primary and recurrent models were adapted to incorporate the specifics of rectal and colon cancer separately. The economic evaluation therefore involved the development of five models, based on the three cancer stages of interest. These five evaluations assessed the cost-effectiveness of FDG PET/CT as an add-on imaging device in pre-operative staging for (1) primary rectal cancer, (2) primary colon cancer, (3) recurrent rectal cancer, (4) recurrent colon cancer and (5) metastatic disease.
The following section outlines the various sources of evidence used in the analyses. This is followed by a description of the design, development and data used to populate each model.
Literature
The economic models were designed, developed and populated based on a variety of information sources (in particular published data sources) and literature, and in consultation with clinical experts.
Previous economic evaluations of imaging devices for CRC were used to aid the design of the models, while the preceding systematic reviews were used to derive diagnostic test accuracy evidence for FDG PET/CT and alternative imaging modalities. Economic and non-economic literature was required to inform specific model parameters, such as resource use, implications of diagnosis on patient management and therapeutic impact, quality of life and survival. Costing and resource use information was obtained from both the literature and UK NHS cost information sources such as the British National Formulary,99 Department of Health reference costs100 and the Personal Social Services Research Unit (PSSRU). 101
Papers that were considered to be potentially relevant for the health economic evaluation were identified by the systematic reviewers during their screening process and passed on to the health economists as first-line literature to inform the development of the economic models. These initial papers provided an indication of the types of literature that were available, and helped inform the design of the economic evaluations. Having established this first-line literature, a separate non-systematic literature search was undertaken in November 2009 to provide further information on the various parameters for the economic models. The objective was to search for and utilise information from economic evaluations and non-economic papers to develop and populate the economic models. Specifically, the search considered what evidence was available regarding the costs, treatment outcomes, management pathways, overall survival, quality of life and adverse events experienced by CRC patients undergoing pre-operative screening for primary, recurrent or metastatic CRC.
The following electronic databases were searched: MEDLINE, EMBASE, Web of Science, CINAHL Plus, The Cochrane Library [NHS Economic Evaluation Database (NHS EED), Health Technology Assessment (HTA), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effectiveness (DARE)], Health Management Information Consortium and the Cost-effectiveness Analysis Registry. Specific searches were constructed for four main areas (FDG PET/CT imaging for CRC, economics, adverse events or quality of life, and decision analysis) for each of the databases, as detailed in Appendix 3. Inclusion criteria were applied to include relevant publications in any language that provided information on the diagnostic imaging devices FDG PET/CT, contrast-enhanced CT or MRI for detecting CRC with regards to the topic areas of economic evaluation, costing, patient management and therapeutic impact, quality of life and overall survival. Papers that provided details only on diagnostic test efficacy were excluded. Conference proceedings and abstracts were also excluded. The search outputs are detailed in Table 14: a total of 51 papers deemed to be of relevance were identified from the search, plus an additional four quality of life papers identified through handsearching.
| Search stage | Search strategy/specified criteria | Number of papers |
|---|---|---|
| Initial search | Identified 902 papers after de-duplication. See Appendix 3 for search details for each database | 902 |
| Check titles and abstracts | Rejected 802 – all deemed irrelevant from title and abstract | 100 |
| Check full paper, apply inclusion criteria | Rejected 49 – irrelevant/unavailable/abstract only/conference proceeding (n = 21); irrelevant/efficacy data alone (n = 28) | 51 |
| Final papers |
Costing studies (n = 7)102–108 Economic evaluations (n = 10)51,58,109–116 Quality of life (n = 2)117,118 Management (n = 28)21–23,27,29,49,55,89,123–131,132–134,135–141 Additional quality-of-life papers previously identified through handsearching (n = 4)24,142–144 |
55 |
Information from this literature was used in consultation with the clinical experts involved with the project to design the models, in particular to identify appropriate comparators, management pathways and parameter estimates for each model.
The systematic review undertaken by the research team was intended to yield data on diagnostic test accuracy for the various imaging devices, which would be pooled in meta-analyses to inform the main parameters for the economic models. As discussed, the systematic review found inadequacies and reporting bias in published papers for all stages of CRC disease. Because of the lack of papers it was deemed inappropriate to undertake a meta-analysis in primary CRC. Meta-analyses were undertaken for recurrent and metastatic CRC; however, the pooled estimates for FDG PET/CT were considered to be an inaccurate reflection of diagnostic test accuracy and the CIs were tight around the pooled means, which is restrictive in terms of capturing a wide range of uncertainty. Therefore, the economic analyses considered the papers identified by the systematic review individually along with other literature identified through the economic search and considerable input from the clinical experts in order to decide which data to incorporate in the economic models.
Model structure
Each model was developed using a decision tree design. Decision trees are economic models that illustrate alternative decision options and their possible consequences. The decision trees were used to illustrate the patient pathway from suspected disease through to test outcome to distinguish accurate and inaccurate disease staging. The costs and diagnostic test accuracy of imaging devices were attributed to the appropriate branches in the trees, and then, dependent on the accuracy of the diagnostic test, a longer-term analysis followed to account for the costs, quality of life and survival impact of optimal versus received treatment.
Each model was analysed probabilistically, using Monte Carlo simulation, to determine the expected cost, outcomes (correct diagnoses and QALYs) and cost-effectiveness (cost per correct diagnosis and cost per QALY gained). The Monte Carlo simulations involved 2000 iterations for each model. The stability of the results was tested and found to be within reasonable bounds.
Primary disease
The evaluations considering the cost-effectiveness of FDG PET/CT as an add-on device in primary rectal (and primary colon) cancer relate to the initial pre-operative TNM staging of primary patients. The additional value of incorporating an FDG PET/CT scan to conventional imaging in this disease stage is through the identification of nodal and metastatic disease54,55 (clinical expertise). The only diagnostic test accuracy evidence available for FDG PET/CT in this context relates to the identification of lymph node involvement,54,55 and therefore the primary models were designed to evaluate the cost-effectiveness of FDG PET/CT as an add-on device in nodal staging.
Figure 4 depicts the basic decision tree structure used for the primary rectal (and colon) models. Because of the absence of economic models of FDG PET/CT in primary CRC in the literature (none was identified from the literature search), this model structure was informed primarily through consultation with clinical experts from the research team in order to accurately reflect the clinical pathway. The model was altered to include the disease-specific criteria for rectal and colon cancer separately. The model structure and parameters will be discussed in relation to rectal cancer, followed by a section detailing how and what parameters were altered for the colon model.
FIGURE 4.
Staging primary CRC. AJCC, American Joint Committee on Cancer.

The decision tree model begins with patients who have had an initial assessment (involving a clinical examination, colonoscopy or sigmoidoscopy, and a biopsy) that identified them as having primary rectal cancer. The standard procedure for patients suspected of having primary rectal cancer is to use contrast-enhanced CT scans of the chest, abdomen and pelvis and an MRI scan of the pelvis to diagnose and/or stage the extent of the disease. This conventional pathway is represented in the top half of the tree. Alternatively, patients will receive the standard work-up (contrast-enhanced CT and MRI) followed by an additional FDG PET/CT scan, which is depicted in the bottom half of the tree. The primary decision tree model has been designed using actual CRC disease status, splitting the patient population according to the true disease status before having the imaging scans, so that accurate and inaccurate scan diagnosis can be identified. The objective of the scan in this model is to assess whether or not there is any nodal spread and, therefore, after the initial decision node depicting the choice between conventional or add-on FDG PET/CT tests, the tree divides the population according to actual nodal spread disease status using the American Joint Committee on Cancer (AJCC) CRC staging system. 8 In the AJCC system, stages 1 and 2 have no nodal involvement, whereas stages 3 and 4 can have some nodal involvement. After dividing patients according to their true nodal spread disease status, the diagnostic tests are undertaken. Patients suspected of having rectal cancer who are in the conventional arm will have a contrast-enhanced CT scan of the chest, abdomen and pelvis and a pelvic MRI scan, which will identify either nodal involvement (test positive) or no nodal involvement (test negative). Having previously specified actual disease status, the top half of this branch represents primary rectal cancer with nodal spread (AJCC stages 3 and 4), and therefore the tree branch splits depending on whether the test was positive (accurately identified nodal involvement) or negative (inaccurately identifying no nodal involvement). These FN outcomes lead to inaccurate understaging, identifying no nodal involvement (AJCC 1 and 2) when the patients do have nodal involvement (AJCC 3 and 4). The bottom branch represents primary rectal cancer with no nodal spread (AJCC 1 and 2). The tree depicts the negative test outcomes that truly were negative (i.e. patients who are staged as AJCC 1 or 2 accurately) and also positive test outcomes that were inaccurate (FPs). These FP outcomes diagnose nodal involvement (AJCC 3 and 4), overstaging the extent of the disease, which is actually no nodal involvement (AJCC 1 and 2). In this way the decision tree separates out accurate and inaccurate diagnoses of nodal involvement.
Patients in the ‘conventional arm’ of the model will be staged using the standard diagnostic test work-up described above (contrast-enhanced CT of the chest, abdomen and pelvis and pelvic MRI), represented by ‘Test’ in the top half of the tree in Figure 4. Patients in the ‘intervention arm’ of the model are also given these conventional imaging tests, followed by the addition of a FDG PET/CT scan. This is represented in the bottom half of the tree, which has been abbreviated as its structure is identical to the structure of the top half. The diagnostic test accuracy of FDG PET/CT is influential as an add-on after the conventional test; therefore, in the model, a positive result from any of the scans incorporated in the ‘test’ strategy will be assumed to be a positive result (i.e. negative results from the conventional imaging tests that are refuted by the FDG PET/CT test are treated as positive). Results are treated as negative only when both the conventional and the FDG PET/CT test outcomes are negative.
The accurate and inaccurate nodal staging outcomes at the end of the decision tree branches for the conventional arm of the model (standard contrast-enhanced CT and MRI scans) are compared with those of the intervention arm of the model (standard contrast-enhanced CT and MRI scans plus FDG PET/CT) and assessed in terms of the incremental cost per accurate diagnosis.
These interim decision model outcomes of accurate and inaccurate diagnosis for the four AJCC stages were also used to undertake a longer-term analysis that assessed the impact of accurate and inaccurate staging on patient management, incorporating optimal treatments for each AJCC stage and measures of quality of life and overall survival, so that the conventional and intervention arms could also be compared in terms of the incremental cost per QALY gained.
The decision tree model is populated with parameters representing the prevalence of AJCC disease status and the diagnostic test accuracy of the conventional and intervention imaging devices and their associated costs. Table 15 details these parameters, along with the treatment, overall survival and quality of life parameters used in the longer-term analysis. The model parameters are now discussed including details of the longer-term modelling.
| Parameter | Primary rectal model | Primary colon model | Data source | ||||
|---|---|---|---|---|---|---|---|
| Point estimate | Standard error | Probabilistic distribution | Point estimate | Standard error | Probabilistic distribution | ||
| Cancer prevalence (AJCC stage) | |||||||
| AJCC 1 (T1, T2, no nodes, no metastases) | 0.19 | n = 541a | Dirichlet | 0.19 | n = 541a | Dirichlet | Clinical experts: Scottish CRC network data, February 2010. Further details in Tenesa et al. 2010145 |
| AJCC 2 (T3, T4, no nodes, no metastases) | 0.34 | n = 977a | Dirichlet | 0.34 | n = 977a | Dirichlet | |
| AJCC 3 (Any T, nodes, no metastases) | 0.31 | n = 891a | Dirichlet | 0.31 | n = 891a | Dirichlet | |
| AJCC 4 (Any T, nodes, metastases) | 0.15 | n = 429a | Dirichlet | 0.15 | n = 429a | Dirichlet | |
| Diagnostic test accuracy | |||||||
| Contrast-enhanced CT sensitivity | 0.55 | 0.06 | Beta | 0.55 | 0.06 | Beta | Bipat et al. 200439 |
| Contrast-enhanced CT specificity | 0.74 | 0.04 | Beta | 0.74 | 0.04 | Beta | Bipat et al. 200439 |
| MRI sensitivity | 0.66 | 0.06 | Beta | – | – | – | Bipat et al. 200439 |
| MRI specificity | 0.76 | 0.09 | Beta | – | – | – | Bipat et al. 200439 |
| FDG PET/CT sensitivity | 0.85 | 0.08 | Beta | 0.85 | 0.08 | Beta | Tateishi et al. 200754 |
| FDG PET/CT specificity | 0.42 | 0.10 | Beta | 0.42 | 0.10 | Beta | Tateishi et al. 200754 |
| Treatments | |||||||
| AJCC 1 – surgery | 1.00 | n = 541a | – | 1.00 | n = 541a | – | Clinical expertise and various references: Maroun et al. 2003,102 Davey et al. 2008,89 Gearhart et al. 200649 |
| AJCC 2 – surgery alone | 0.38 | n = 977a | Dirichlet | 0.80 | n = 977a | Dirichlet | |
| AJCC 2 – long-course CRT plus surgery | 0.46 | Dirichlet | – | – | – | ||
| AJCC 2 – surgery plus adjuvant chemotherapy | 0.15 | Dirichlet | 0.20 | Dirichlet | |||
| AJCC 3 – surgery alone | 0.34 | n = 891a | Dirichlet | 0.34 | n = 891a | Dirichlet | |
| AJCC 3 – long-course CRT plus surgery | 0.37 | Dirichlet | – | – | – | ||
| AJCC 3 – surgery plus adjuvant chemotherapy | 0.29 | Dirichlet | 0.66 | Dirichlet | |||
| AJCC 4 – surgery alone | 0.08 | n = 429a | Dirichlet | 0.09 | n = 429a | Dirichlet | |
| AJCC 4 – long-course CRT plus surgery | 0.11 | Dirichlet | – | – | – | ||
| AJCC 4 – surgery plus metastatic surgery | 0.16 | Dirichlet | 0.19 | Dirichlet | |||
| AJCC 4 – surgery followed by palliative care | 0.53 | Dirichlet | 0.63 | Dirichlet | |||
| AJCC 4 – palliative care alone | 0.13 | Dirichlet | 0.09 | Dirichlet | |||
| Overall survival | |||||||
| 5-year overall survival AJCC 1 | 0.95 | 0.01 | Beta | 0.95 | 0.01 | Beta | Clinical experts: Scottish CRC network data, February 2010. Further survival analysis details published in Tenesa et al. 2010145 |
| 5-year overall survival AJCC 2 | 0.86 | 0.01 | Beta | 0.86 | 0.01 | Beta | |
| 5-year overall survival AJCC 3 | 0.69 | 0.02 | Beta | 0.69 | 0.02 | Beta | |
| 5-year overall survival AJCC 4 | 0.13 | 0.02 | Beta | 0.13 | 0.02 | Beta | |
| Reduction in 5-year overall survival for AJCC 3 patients who fail to receive adjuvant chemotherapy | 0.25 | 0.05 | Beta | 0.25 | 0.05 | Beta | Assumption based on clinical advice |
| Quality of life/utility | |||||||
| Average 5-year utility AJCC 1 | 0.84 | 0.17 | Gamma (disutility) | 0.84 | 0.17 | Gamma (disutility) | Ramsey et al. 2000142 |
| Average 5-year utility AJCC 2 | 0.86 | 0.14 | Gamma (disutility) | 0.86 | 0.14 | Gamma (disutility) | Ramsey et al. 2000142 |
| Average 5-year utility AJCC 3 | 0.85 | 0.14 | Gamma (disutility) | 0.85 | 0.14 | Gamma (disutility) | Ramsey et al. 2000142 |
| Average 5-year utility AJCC 4 | 0.84 | 0.12 | Gamma (disutility) | 0.84 | 0.12 | Gamma (disutility) | Ramsey et al. 2000142 |
| Disutility for patients who fail to receive CRT or adjuvant chemotherapy | 0.20 | 0.08 | Gamma | 0.20 | 0.08 | Gamma | Assumption based on Tengs and Wallace 2000146 |
| Disutility for patients who fail to receive metastatic palliative care | 0.30 | 0.08 | Gamma | 0.30 | 0.08 | Gamma | Assumption based on Tengs and Wallace 2000146 |
| Utility during unnecessary long-course CRT | 0.74 | 0.14 | Gamma (disutility) | – | – | – | Ramsey et al. 2000142 |
| Utility during unnecessary adjuvant chemotherapy | 0.80 | 0.14 | Gamma (disutility) | 0.80 | 0.14 | Gamma (disutility) | Ramsey et al. 2000142 |
| Utility for unnecessary metastatic surgery | 0.74 | 0.21 | Gamma (disutility) | 0.74 | 0.21 | Gamma (disutility) | Langenhoff et al. 2006117 |
Primary colorectal cancer model parameters
The model incorporated nodal spread disease status using prevalence data from a Scottish network data set145 provided by the clinical experts in the research team. The data comprise detailed clinico-pathological and imaging staging data from an ongoing prospective study involving 2838 Scottish CRC patients (average age 61 years). The data set is a prospective series that identifies all cases of CRC in Scotland by direct clinical and nurse contact, through pathology department returns, managed clinical networks, cancer registration and death registration. This series is considered to represent the generality of CRC in the UK, as any differences in the epidemiology of CRC between Scotland and the rest of the UK will be marginal. The data set provided information on CRC disease status using the AJCC CRC staging system along with 5-year overall survival data for each of the four AJCC stages. This data set is discussed in full in a recent publication detailing the population background characteristics and survival analysis outcomes. 145 A previous analysis of a subset of the data set was published in 2006. 147
The AJCC stage prevalence data were incorporated into the model under the assumption that AJCC stages 1 and 2 represent patients with no nodal involvement (n = 1518, 53%), and AJCC stages 3 and 4 represent patients with nodal involvement (n = 1320, 47%). The prevalence and number of patients in each AJCC stage in the data set are detailed in Table 15. For the probabilistic analysis, Dirichlet distributions (a multinomial version of the beta distribution) were assigned using the total number of patients and AJCC stage prevalence.
Having merged the AJCC data to distinguish disease in terms of nodal involvement to synchronise with the diagnostic test outcomes, the decision tree then separates the data back into the individual AJCC stages in the final branches, in order to assign treatment strategies for each AJCC stage in the longer-term model. This was done based on an assumption that the extent of disease in the model is linked to the overall stage prevalence [i.e. within the nodal involvement arm (which was calculated by summing AJCC 3 and 4 prevalence), the proportion who are separated back out to stage AJCC 3 was calculated by dividing the AJCC 3 prevalence (31%) by the total nodal involvement prevalence – AJCC 3 + AJCC 4 (47%)]. Following this assumption, patients who are inaccurately staged are done so according to that disease stage prevalence, i.e. patients who have nodal involvement (AJCC 3 and 4) but who are understaged through FN test results are inaccurately staged as either AJCC 1 or 2 based on AJCC 1 and 2 prevalence. AJCC 2 is more prevalent than AJCC 1; as such, this assumption ensures that in the model when FNs inaccurately understage patients as AJCC 2 and 1 (instead of AJCC 3 and 4), a greater proportion of patients will be inaccurately staged as AJCC 2 than inaccurately staged as AJCC 1. It is also more likely that an AJCC 3 patient would be understaged to AJCC 2 than to AJCC 1. The prevalence of AJCC 3 is greater than that of AJCC 4, and therefore a greater proportion of inaccurate overstaging will be attributed to AJCC 3 than to AJCC 4. Similarly, a patient with no nodal involvement (AJCC 1 or 2) will be more likely to be mistaken as an AJCC 3 patient than as an AJCC 4 patient.
The systematic review for PET/CT in primary colorectal cancer (see Chapter 5), was intended to yield pooled data on diagnostic test accuracy for the main parameters in the economic model; however, only two papers were identified for FDG PET/CT in primary CRC,53,54 and there were inadequacies and reporting bias in the identified papers for all stages of CRC. Therefore, for the purpose of the economic analyses for primary CRC staging, papers identified by the systematic review were considered individually along with papers previously identified through the economic search; decisions were made to incorporate data that fit with the models.
With regards to the diagnostic test accuracy of contrast-enhanced CT, MRI and FDG PET/CT for staging primary CRC, the systematic review identified two papers53,54 that reported data for FDG PET/CT. The Tsunoda et al. 53 data were reported only at a lesion level and were therefore not useful for the model; however, the Tateishi et al. 54 paper (which compared FDG PET/CT with contrast-enhanced FDG PET/CT) reported patient-level data on the sensitivity and specificity of FDG PET/CT for staging nodal involvement and provided CIs. No distinction was made between colon and rectal cancer, and because of this and the lack of alternative information, the FDG PET/CT estimates were used in both models. The lower CI was used to calculate a standard error for use in the probabilistic analysis as it represented the widest range of uncertainty. We assumed an independent probability distribution for the sensitivity and specificity estimates, using beta distributions. Diagnostic test accuracy data for contrast-enhanced CT and MRI were taken from Bipat et al. ,39 who undertook a meta-analysis in primary CRC and reported diagnostic test accuracy estimates with CIs for these imaging modalities for staging nodal involvement. The lower CI was used to calculate a standard error for use in the probabilistic analysis. FDG PET/CT was not included in this meta-analysis; however, as the authors detail the sensitivity and specificity of contrast-enhanced CT and MRI specifically for nodal involvement, it is reasonable to enter these estimates into the primary models, to compare with the addition of FDG PET/CT using the Tateishi et al. estimates specifically for staging nodal involvement.
The primary rectal model used the diagnostic test accuracy estimates for MRI to represent the ‘conventional’ imaging arm as, overall, it has superior test performance characteristics for lymph node involvement, i.e. both the sensitivity and the specificity of MRI are superior to those of contrast-enhanced CT. 39 This approach of using superior test performance to represent joint imaging modalities has been used by others148 and is also reasonable given the evidence identified in the systematic review, which favoured MRI in the identification of nodal involvement. In the intervention arm, the diagnostic test accuracy for FDG PET/CT is added on after the conventional test, assuming that any outcome with a positive test is treated as such. Negative results from the conventional test that are refuted by the FDG PET/CT test are treated as positive. Results are treated as negative only when both the conventional and the FDG PET/CT test result are negative.
The economic models in our analyses were designed to incorporate the treatment impacts of accurate and inaccurate staging in primary CRC. The systematic review and the non-systematic economics search identified some literature on therapeutic impact and patient management in primary CRC. 49,89–93,102 This literature found that, although FDG PET/CT can have an impact in terms of more accurate staging of primary CRC, it had only a minor impact on changing patient management, as discussed in the therapeutic impact chapter of this report (see Chapter 8).
Optimal treatment combinations for each AJCC stage were determined through consideration of the literature49,89–93,102 and in consultation with clinical experts. It was assumed that, for primary rectal cancer, all AJCC1 patients receive primary surgery and no further treatment. AJCC 2 and 3 primary rectal patients will receive one of three options: surgery alone, long-course CRT prior to surgery or surgery followed by adjuvant chemotherapy. AJCC 4 patients will receive one of five treatment options: primary surgery alone, long-course CRT prior to primary surgery, primary surgery followed by metastatic surgery, primary surgery followed by palliative care or palliative care alone. Primary surgery refers to rectal excision with lymphadenectomy; metastatic surgery refers to surgery at the metastatic site; and palliative care represents an array of palliative treatments that may include chemotherapy. These optimal treatment profiles inform the costing and the utility weights in the model.
The proportions of patients receiving each treatment for each stage (detailed in Table 15) were assigned in consultation with the clinical experts on the research team to ensure consistency with the data set used. These were also compared with publications reporting treatment and therapeutic impacts for primary rectal and colon cancer27,49,89,102,109 to ensure that important treatments were included. Uncertainty was incorporated through a series of Dirichlet distributions, one for each AJCC stage, specified using the AJCC stage prevalence data from the Scottish data set and the probabilities of receiving a specific treatment option within each stage.
Assigning these optimal treatment options for each AJCC stage in the longer-term model means that patients in the decision tree who are accurately diagnosed will receive optimal treatment, whereas patients who are inaccurately staged (through FP or FN test outcomes) will receive suboptimal treatment [i.e. patients with no nodal involvement (AJCC 1 or 2 patients) who are inaccurately diagnosed as having nodal involvement (overstaged to either AJCC 3 or 4) will receive unnecessary AJCC 3 or 4 treatments]. In the case of inaccurate staging, the model assumes that patients will receive the treatments for their (mis)diagnosed stage, but within a year their true diagnosis will be correctly identified and optimal treatment will then be given. This assumption was made in consultation with clinical experts and is considered to be valid with 1 year as an appropriate time scale for encompassing most cases of understaging. This way the model accounts for the appropriate treatments and the treatments that are received unnecessarily or that initially fail to be received because of over- or understaging. No transitions between nodal status are allowed during the year.
The longer-term model incorporated overall survival in order to capture any potential impact on mortality over the lifetime of the patients.
The Scottish CRC network data set145 (2838 CRC patients, average age 61 years) detailed the 5-year overall survival of patients for each AJCC stage. These data were used to determine an annual mortality rate under the assumption of an exponential survivor function, used within a Markov simulation to estimate overall life expectancy for each AJCC stage. The Markov model assumed a starting age of 50 years and employed the mortality rate calculated from the data set for each stage for the first 10 years of the extrapolation; beyond 10 years patients were assumed to have survived their cancer and they were assumed to return to the average mortality rate for their age. 149 [The starting age of 50 years was used in the model as the data set is based on the Scottish CRC population aged ≥ 50 years (mean age 61 years). The models were also run using an older population (starting age 70 years), with the resultant effect of lowering life expectancy and quality-adjusted life expectancy for patients in each AJCC stage, but with no overall change to the incremental cost-effectiveness outcomes.]
Publications121,122,139 indicate that FDG PET/CT scanning (in comparison with conventional imaging modalities) has no impact on overall survival; however, consultation with clinical experts highlighted that patients with AJCC 3 stage cancer (nodal involvement but no metastases) who fail to receive adjuvant chemotherapy because of inaccurate staging may suffer a reduction in overall survival. This was incorporated into the model for AJCC 3 patients who were inaccurately understaged as AJCC 1 or 2 as a 25% reduction in overall survival.
The 5-year overall survival estimates for each AJCC stage are detailed in Table 15, along with an estimated 25% reduction in overall survival for AJCC 3 patients who were inaccurately diagnosed and failed to receive adjuvant chemotherapy. Beta distributions were applied for the probabilistic analysis.
A measure of quality of life (utility) was incorporated into the model, capturing the average quality of life experienced by patients in each AJCC stage, and incorporating disutility experienced by patients who receive unnecessary treatment or who fail to receive optimal treatments because of inaccurate staging. Average quality of life estimates for each of the four CRC stages were derived from data reported in Ramsey et al. 142 using the Health Utility Index. It was assumed that the average utility experienced by patients in a particular stage was constant for 5 years post diagnosis. Patients who were still alive 5 years post diagnosis were assigned age-specific utility weights based on UK population norms. 143 The quality of life estimates were combined in the survival analysis and discounted at 3.5%150 to derive discounted quality-adjusted life expectancies for each AJCC stage.
During the 5-year post-diagnosis stage, patients who were correctly diagnosed in the model received the average utility for their state, whereas patients incorrectly diagnosed received their true disease stage utility, but with a disutility relating to the inappropriate treatment they received for a specified duration. It was assumed that patients who were inaccurately staged and who failed to receive either long-course CRT pre-surgery or adjuvant chemotherapy post surgery received a disutility for a 6-month duration, whereas patients who were inaccurately diagnosed and who failed to receive metastatic treatments were assumed to receive disutility for 1 year, reflecting the large impact on quality of life for delayed treatment. Patients who received unnecessary long-course CRT, unnecessary adjuvant chemotherapy or unnecessary metastatic surgery received a lower utility during their unnecessary treatment.
Table 15 details the utility and disutility weights used in the model. The probabilistic analysis applied gamma distributions on disutility to represent uncertainty in the parameters.
The costs for the economic model are attributed to the cost of the alternative imaging devices (as a cost per scan) and the cost of the various treatment options for each AJCC stage. NHS reference cost data were used100,151 along with various other data sources for the AJCC stage treatment options. 99,101 The various cost items are detailed in Table 16, specifying unit costs and standard errors. Where appropriate, normal distributions were used to represent the uncertainty surrounding cost estimates in the probabilistic analysis.
The cost of the imaging devices was incorporated as a cost per scan, representing staff time and use of the imaging machinery. Cost details regarding contrast-enhanced CT and MRI scans were available in NHS reference costs;100 however, no details were provided for the cost of FDG PET/CT scanning in either the Department of Health100 or the Scottish Information Services Division151 reference costs. Various studies report the cost of an FDG PET/CT scan in the UK as between £750 and £1000 per scan. 103,151,152,155 It is also widely reported that FDG PET/CT scans generally have a duration of 20–40 minutes on equipment costing two to three times that of CT scanners, which can perform scans on a patient every 5–10 minutes;25 therefore, assigning a cost of £800 per FDG PET/CT scan seemed appropriate. A standard error for this baseline cost was derived using the upper and lower price range reported for an FDG PET/CT scan. 152
The cost of primary rectal surgery (rectal excision with lymphadenectomy) includes the cost of a distal colon procedure, an average hospital inpatient stay of 6 days and CRC surgery consultant follow-up. Long-course CRT treatment consisted of radiotherapy given over 5 weeks (45 Gy in 25 fractions) combined with a 12-week course of chemotherapy – intravenous 5-fluorouracil. 154,155 The adjuvant chemotherapy treatment consisted of a 6-month course of intravenous 5-fluorouracil plus oxaliplatin. 99,155 The cost of metastatic surgery was represented by the Information Services Division151 cost of surgical specialties in medical oncology, representing the cost of surgery, including theatre time, surgical consultation and follow-up, and an average inpatient stay of 10 days. Resource use and costs for palliative care were taken from a study that assessed the cost to the NHS of palliative care in CRC. 124 The costs of palliative care were reported at price year 2000/1, and therefore the hospital and community health services pay and price index101 was used to adjust this to price year 2009.
The average cost per AJCC stage was calculated using the proportion of patients receiving each treatment option within each AJCC stage. In the model, if a patient was staged accurately, he or she would receive his or her optimal treatment option and be assigned the average cost of treatment for that stage. The model also incorporates the extra costs incurred through inaccurate staging. If a patient is inaccurately diagnosed, he or she incurs the cost of the misdiagnosed treatment, followed by the discounted cost of treatment for his or her true stage the following year (i.e. it is assumed that the true disease stage will be identified within a year). Costs were discounted at 3.5%. 150
Primary colon model
The basics of the primary model structure were the same for both rectal and colon cancer. The parameters discussed above relate to rectal cancer; however, Tables 15 and 16 also detail the parameters that were used in the colon model. The specific aspects of the model that were altered for the colon model are discussed below.
| Item | Primary rectal model | Primary colon model | Data source | ||||
|---|---|---|---|---|---|---|---|
| Unit cost (£) | Standard error (£) | Probabilistic distribution | Unit cost (£) | Standard error (£) | Probabilistic distribution | ||
| Imaging devices | |||||||
| Contrast-enhanced CT scan (chest, abdomen, pelvis) | 143 | 22 | Normal | 143 | 22 | Normal | DoH reference costs 2009100 |
| MRI scan (pelvis) | 179 | 24 | Normal | – | – | – | DoH reference costs 2009100 |
| FDG PET/CT scan | 800 | 100 | Normal | 800 | 100 | Normal | DoH 2005,152 NCRI 2007153 |
| Treatments | |||||||
| Primary surgery (rectal excision with lymphadenectomy): includes cost of the distal procedure (including surgical consultation, theatre time, staff costs), inpatient stay in hospital for average 6 days and surgical follow-up consultation | 5637 | 677 | Normal | – | – | – | DoH reference costs 2009,100 ISD 2009151 |
| Primary surgery (colonic resection with lymphadenectomy): includes cost of the proximal colon procedure (including surgical consultation, theatre time, staff costs), inpatient stay in hospital for average 6 days and surgical follow-up consultation | – | – | – | 5893 | 746 | Normal | DoH reference costs 2009,100 PSSRU 2009101 |
| Long-course CRT [5 weeks’ radiotherapy combined with 3 months’ chemotherapy (5FU)]: includes cost of radiotherapy and chemotherapy drugs, administration and hospital stay | 13,721 | – | – | – | – | – | Royal College of Radiologists 200622 and 2008,155 BNF 58,99 ISD 2009,151 Cancer Research UK 2009154 |
| Adjuvant chemotherapy (6-month course post surgery: intravenous 5FU + oxaliplatin for 24 weeks in 12 × 2-weekly cycles): includes cost of chemotherapy drugs, administration and hospital stay | 11,532 | – | – | 11,532 | – | – | BNF 58,99 ISD 2009,151 Cancer Research UK 2009154 |
| Palliative care: average NHS cost per person for CRC palliative care; cost adjusted to price year 2008/9 using the HCHS pay and price index101 | 2468 | 494 | Normal | 2468 | 494 | Normal | Guest et al. 2006124 |
| Metastatic surgery (surgical specialties in medical oncology): inpatient cost per case, including surgical consultation, theatre time, staff costs and an average of 10 inpatient days | 9134 | 1827 | Normal | 9134 | 1827 | Normal | ISD 2009151 |
Magnetic resonance imaging is not used in the assessment of primary colon cancer and therefore the primary colon model incorporated only contrast-enhanced CT as the conventional imaging modality. As previously discussed, the diagnostic test accuracy literature made few distinctions between colon and rectal cancer, and therefore, because of this and the lack of alternative information, the FDG PET/CT and contrast-enhanced CT estimates were used in both models.
The intervention arm adopts the same approach to that described earlier, whereby the diagnostic test accuracy of FDG PET/CT is added on to the contrast-enhanced CT test outcomes, and any outcome with a positive test is treated as such. Negative results from the conventional test that are refuted by the FDG PET/CT test are treated as positive. Results are treated as negative only when both the contrast-enhanced CT and the FDG PET/CT test results are negative.
The AJCC treatment options for the colon model vary slightly. Primary surgery refers to a colonic resection with lymphadenectomy. The other major treatment change is that pre-operative long-course CRT is not used to treat primary colon cancer, and therefore the treatment options for AJCC stages 2, 3 and 4 were modified for the colon model. In the colon model, AJCC 2 and 3 primary colon patients receive one of two options: surgery alone or surgery followed by adjuvant chemotherapy. Patients with AJCC 4 disease may receive one of four treatment options: primary surgery alone, primary surgery followed by resection of metastases, primary surgery followed by palliative care or palliative care alone. [There is typically a fifth treatment option of primary surgery combined with concomitant resection of metastases; however, after consultation with clinical experts it was decided that this additional treatment option would result only in an unquantifiable and likely marginal effect on overall cost compared with primary surgery alone, hence these are considered together.] The same approach that was used for the rectal cancer model was used to determine the optimal treatment combination within each AJCC stage for colon cancer, i.e. through the literature and in consultation with clinical experts. Table 15 details the within-stage distributions that were assigned to each stage in the colon model.
There was no change to the survival analysis for the colon model; however, the utilities were amended to exclude the CRT-related utilities used in the rectal model. In the case of inaccurate staging, disutilities were still applied but were specifically for failing to receive adjuvant chemotherapy, or for patients who receive unnecessary adjuvant chemotherapy.
The costs were amended in line with the parameter modifications discussed above. In the colon model, conventional imaging involves only contrast-enhanced CT, and therefore it is the only imaging cost incorporated for the conventional arm (the costs of MRI are excluded). With regards to the treatment costs, the cost of primary surgery refers to a colonic resection. Cost data for a proximal colonic procedure, a hospital inpatient stay of 6 days and CRC surgery consultant follow-up were included. 100,101 The average cost of treatment per AJCC stage was calculated for the colon model in the same manner as for the rectal model, using the proportion of patients receiving each treatment option within each AJCC stage to calculate the average cost per AJCC stage.
Scenario analysis: contrast-enhanced FDG PET/CT as a lone technology
The previous chapters in this report found suggestions within the literature that in the future, as FDG PET/CT technology improves (i.e. with the development and introduction of contrast-enhanced FDG PET/CT scanners), it may be possible to use these higher-quality devices as an alternative to CT or contrast-enhanced CT in primary CRC rather than using FDG PET/CT as an add-on imaging device.
Although the scope of the current research was focused on FDG PET/CT as an add-on device, we have included a scenario analysis for the primary colorectal models in which contrast-enhanced FDG PET/CT is used as a replacement for conventional contrast-enhanced CT, rather than as an add-on device. The Tateishi et al. paper,54 which provided diagnostic test accuracy evidence for FDG PET/CT, also provided patient-level diagnostic test accuracy estimates for contrast-enhanced FDG PET/CT in nodal staging (with equivalent sensitivity to FDG PET/CT but improved specificity as reported in the diagnostic test accuracy tables for primary CRC in Table 2). These contrast-enhanced diagnostic test accuracy estimates and CIs were used in the scenario analysis to portray the future potential of improved FDG PET/CT imaging. For the primary rectal scenario, the conventional strategy (contrast-enhanced CT followed by MRI) was compared with a contrast-enhanced FDG PET/CT replacement strategy (contrast-enhanced FDG PET/CT followed by MRI); and for the primary colon scenario, conventional contrast-enhanced CT was compared with contrast-enhanced FDG PET/CT alone. All model parameters remain as above, with the exception of the diagnostic test accuracy estimates and the cost of contrast-enhanced FDG PET/CT. The contrast-enhanced FDG PET/CT diagnostic test accuracy estimates and CIs were used,54 and a cost for the contrast-enhanced FDG PET/CT scan was incorporated, assuming an increase of 20% to the FDG PET/CT scan cost to reflect the cost of this more expensive technology.
Recurrent disease
The recurrent model evaluations have been undertaken to assess the cost-effectiveness of FDG PET/CT as an add-on device in detecting recurrent rectal (and recurrent colon) cancer. The value of incorporating an FDG PET/CT scan in addition to conventional imaging in this disease stage is through the ability to confirm or refute local recurrence and potentially identify metastatic recurrence.
Figure 5 depicts the decision tree structure used for the recurrent rectal (and colon) models. This was altered to include the disease-specific criteria for rectal and colon cancer separately. The model structure was informed by the literature58,110 and was based on consultation with clinical experts. The parameters will be discussed in relation to rectal cancer, followed by a section detailing what elements and parameters were altered for the colon model.
FIGURE 5.
Staging recurrent CRC.
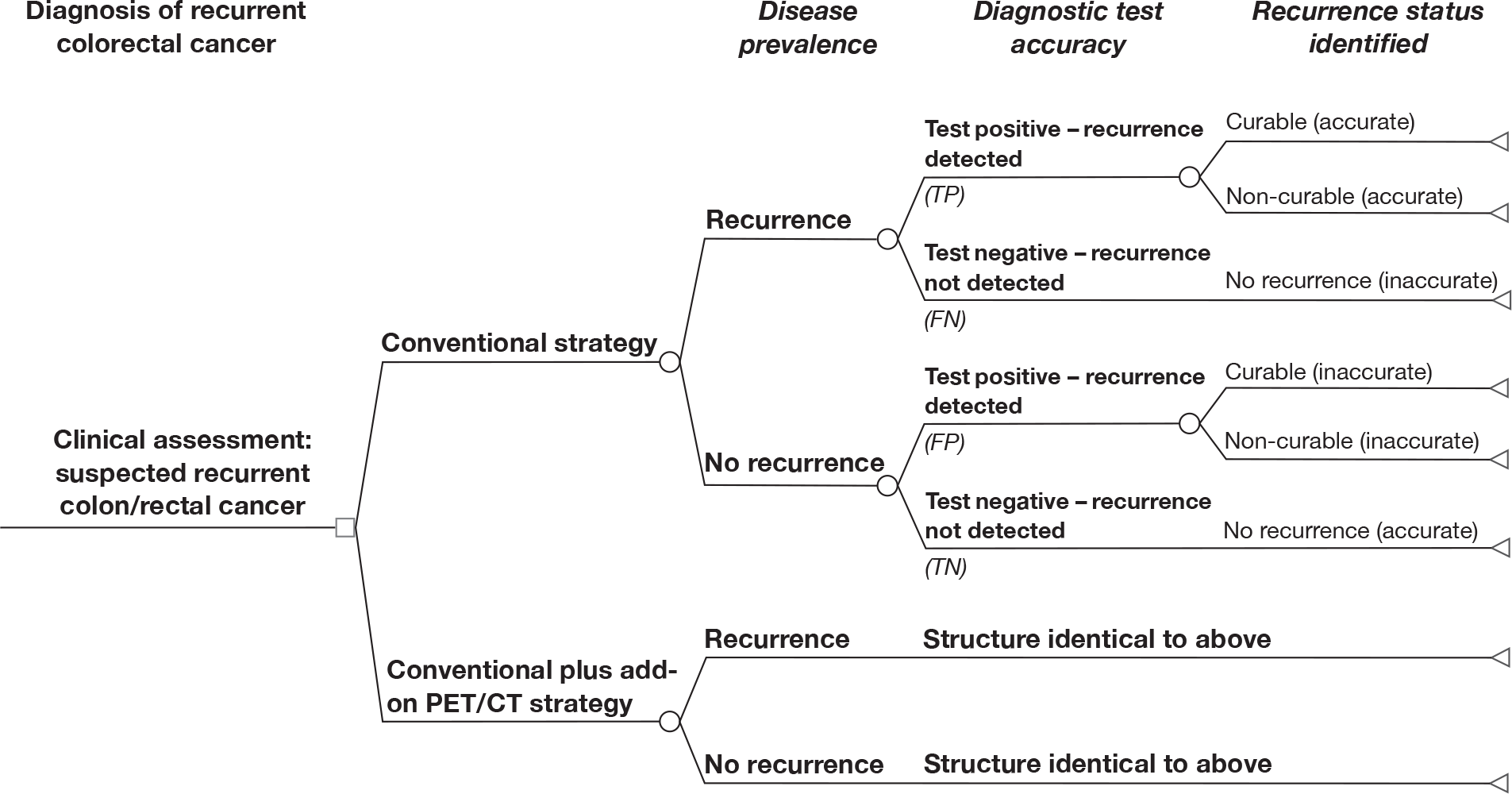
The recurrent decision tree model begins with patients who have previously had surgical treatment for primary rectal cancer and who, in a routine follow-up assessment (involving clinical examination, routine imaging and CEA testing), were found to have rising CEA levels, which identified them as potentially having recurrent rectal cancer. The decision tree then outlines the choice between conventional diagnostic testing and the add-on FDG PET/CT strategy. The standard procedure for patients suspected of recurrent rectal cancer involves contrast-enhanced CT scans of the chest, abdomen and pelvis and an MRI scan of the pelvis to confirm or refute rectal local recurrence and assess whether this is an isolated recurrence or associated with distant metastases. Similar to the structure used in the primary models, this decision tree model has been designed using actual disease status, and therefore the decision tree has split the patient population according to their true status before having the imaging scans, so that accurate and inaccurate diagnoses can be identified. The objective of the scan in this model is to assess whether or not there has been any recurrent disease, and therefore the tree divides into the recurrence (isolated local or local combined with distant metastases) and no recurrence populations. The standard work-up of diagnostic tests is then undertaken. Patients suspected of rectal recurrence in the conventional arm will have a contrast-enhanced CT scan of the chest, abdomen and pelvis and a pelvic MRI scan, which will identify either recurrence (test positive) or no recurrence (test negative). Having previously specified actual recurrence status, the top branch of the tree represents recurrent cancer, and therefore the tree branch splits depending on whether the test was positive (accurately identifying recurrence) or negative (inaccurately identifying no recurrence). Positively identified recurrence is then further separated into curable and non-curable recurrence, which will involve different treatment options in the longer-term model. Negative test outcomes indicate no recurrence and thus no treatment. In the top half of this branch, negative test outcomes represent FNs, which lead to patients being inaccurately diagnosed as having no recurrence. For the longer-term model it is assumed that patients will be accurately re-staged within a year.
The bottom branch in the top half of the tree represents the actual status of no recurrence, so negative test outcomes accurately indicate no recurrence. Positive test outcomes in this branch of the tree are FPs, which inaccurately diagnose recurrence when there is none. This population is further divided into curable and non-curable recurrence in order to determine what treatment patients receive unnecessarily in the longer-term model. In this way the decision tree separates out accurate and inaccurate diagnosis of recurrence.
Patients in the ‘conventional arm’ of the model will be staged using the standard diagnostic test work-up described above (contrast-enhanced CT of the chest, abdomen and pelvis and pelvic MRI), represented by ‘Test’ in Figure 5. Patients in the ‘intervention arm’ of the model will be given these conventional imaging tests, followed by the addition of a FDG PET/CT scan. This is represented in the bottom half of the tree, which has been abbreviated as the structure is identical to that of the top half.
The accurate and inaccurate identification of recurrence at the end of the decision tree branches for the conventional arm of the model (standard contrast-enhanced CT and MRI scans) are compared with the accurate and inaccurate identification of recurrence in the intervention arm of the model (standard contrast-enhanced CT and MRI scans plus the addition of FDG PET/CT) and assessed in terms of the incremental cost per accurate diagnosis. These interim outcomes of accurate and inaccurate diagnosis are then used to undertake a longer-term analysis that assesses the impact of accurate and inaccurate diagnoses of recurrence on patient management, incorporating optimal treatments for curable recurrence, non-curable recurrence and no recurrence, and modelling the impacts on quality of life and overall survival. In this way, the conventional and intervention arms can be compared in terms of the incremental cost per QALY gained.
This decision tree model is populated with parameters representing the prevalence of recurrent CRC and the diagnostic test accuracy of the conventional and intervention imaging devices for staging recurrent rectal (and colon) cancer and their associated costs. Table 17 details these parameters, along with the treatment, overall survival and quality of life parameters used in the longer-term analysis. The model parameters are discussed below, including details of the longer-term modelling.
| Parameter | Recurrent rectal model | Recurrent colon model | Data source | ||||
|---|---|---|---|---|---|---|---|
| Point estimate | Standard error | Probabilistic distribution | Point estimate | Standard error | Probabilistic distribution | ||
| Cancer prevalence (recurrence) | |||||||
| Local recurrence | 0.30 | – | Dirichlet | 0.30 | – | Dirichlet | Saunders et al. 2002123 |
| Metastatic recurrence | 0.40 | – | Dirichlet | 0.40 | – | Dirichlet | Saunders et al. 2002123 |
| No recurrence | 0.30 | – | Dirichlet | 0.30 | – | Dirichlet | Saunders et al. 2002123 |
| Recurrence curable | 0.30 | 0.10 | Beta | 0.30 | 0.10 | Beta | Lejeune et al. 200551 |
| Recurrence non-curable | 0.70 | – | 1 – above | 0.70 | – | 1 – above | Lejeune et al. 200551 |
| Diagnostic test accuracy | |||||||
| Contrast-enhanced CT sensitivity | 0.53 | 0.27 | Beta | 0.53 | 0.27 | Beta | Selzner et al. 2004,87 Ramos et al. 200881 |
| Contrast-enhanced CT specificity | 0.98 | 0.02 | Beta | 0.98 | 0.02 | Beta | Selzner et al. 2004,87 Ramos et al. 200881 |
| MRI sensitivity | 0.85 | 0.03 | Beta | – | – | – | Park et al. 2001109 |
| MRI specificity | 0.95 | 0.08 | Beta | – | – | – | Park et al. 2001109 |
| FDG PET/CT sensitivity | 0.93 | 0.10 | Beta | 0.93 | 0.10 | Beta | Selzner et al. 2004,87 Ramos. et al. 200881 |
| FDG PET/CT specificity | 0.98 | 0.03 | Beta | 0.98 | 0.03 | Beta | Selzner et al. 2004,87 Ramos et al. 200881 |
| Treatments | |||||||
| Surgery | 0.05 | – | Dirichlet | 0.05 | – | Dirichlet | Clinical expertise and various references: Maroun et al. 2003,102 Davey et al. 2008,89 Gearhart et al. 200649 |
| Surgery plus adjuvant chemotherapy | 0.10 | – | Dirichlet | 0.10 | – | Dirichlet | |
| Long-course CRT plus surgery | 0.25 | – | Dirichlet | – | – | – | |
| Surgery plus metastatic surgery | 0.10 | – | Dirichlet | 0.10 | – | Dirichlet | |
| Surgery plus adjuvant chemotherapy plus metastatic surgery | 0.15 | – | Dirichlet | 0.15 | – | Dirichlet | |
| Long-course CRT plus surgery plus metastatic surgery | 0.35 | – | Dirichlet | – | – | – | |
| Metastatic surgery followed by palliative care | 0.20 | 0.04 | Beta | 0.20 | 0.04 | Beta | MSAC 200858 |
| Palliative care alone | 0.80 | – | 1 – above | 0.80 | 1 – above | MSAC 200858 | |
| Wait and watch | 1.00 | – | – | 1.00 | – | – | Author assumption |
| 5-year overall survival | |||||||
| No recurrence | 0.85 | 0.01 | Beta | 0.85 | 0.01 | Beta | American Cancer Society 2005119 |
| Recurrence curable | 0.30 | 0.02 | Beta | 0.30 | 0.02 | Beta | American Cancer Society 2005119 |
| Recurrence non-curable | 0.10 | 0.01 | Beta | 0.10 | 0.01 | Beta | American Cancer Society 2005119 |
| Recurrence curable (fail to treat) | 0.20 | – | – | 0.20 | – | – | Author assumption |
| Quality of life/utility | |||||||
| No recurrence | 0.91 | 0.11 | Gamma (disutility) | 0.91 | 0.11 | Gamma (disutility) | Ramsey et al. 2000142 |
| Curable | 0.84 | 0.12 | Gamma (disutility) | 0.84 | 0.12 | Gamma (disutility) | Ramsey et al. 2000142 |
| Non-curable | 0.52 | 0.08 | Gamma (disutility) | 0.52 | 0.08 | Gamma (disutility) | Tengs and Wallace 2000146 |
| Disutility for patients who fail to receive curable treatment | 0.30 | 0.08 | Gamma | 0.30 | 0.08 | Gamma | Assumption based on Tengs and Wallace 2000146 |
| Disutility for patients who fail to receive non-curable treatment | 0.20 | 0.08 | Gamma | 0.20 | 0.08 | Gamma | Assumption based on Tengs and Wallace 2000146 |
| Utility during unnecessary curable treatment | 0.74 | 0.14 | Gamma (disutility) | 0.74 | 0.14 | Gamma (disutility) | Assumption based on Tengs and Wallace 2000146 |
| Utility during non-curable treatment | 0.61 | 0.20 | Gamma (disutility) | 0.61 | 0.20 | Gamma (disutility) | Langenhoff et al. 2006117 |
Recurrent colorectal cancer model parameters
The literature identified in the economics search and the systematic review was used to provide disease prevalence evidence for the recurrent model. Disease prevalence data on recurrence in CRC were based on estimates provided by Saunders et al. ,123 assigning a 30% probability of local recurrence and a 40% probability of metastatic recurrence for patients previously treated for primary CRC. It was assumed that a cohort of patients who were diagnosed as AJCC 1, 2 or 3 for primary CRC would be susceptible to recurrence. Using the Scottish network CRC data set145 to represent this cohort and assigning the probability of recurrence from Saunders et al. ,123 it was possible to apply Dirichlet distributions around these baseline estimates to incorporate uncertainty in the probabilistic analysis. Table 17 details these parameters.
This model structure is similar to the structure used in two other economic evaluations that assessed the value of using PET in the identification of recurrent CRC. 58,110 These two models also incorporated patient management and quality of life impacts by including a probability of curable and non-curable recurrence in the recurrent population. 58,110 Table 17 details the parameters, standard errors and probability distributions applied.
As reported in Chapter 6, a meta-analysis was undertaken using relevant papers identified from the systematic review to elicit pooled diagnostic test accuracy estimates of FDG PET/CT for recurrent CRC. As noted above, because of inadequacies and reporting bias in the identified papers, the pooled estimates for FDG PET/CT may not be an accurate reflection of the diagnostic test accuracy of FDG PET/CT. The pooled estimates give tight CIs that do not fully represent the wide uncertainty in the mean estimates. Therefore, papers identified by the systematic review were considered individually along with papers identified through the economic search to find reasonable estimates of diagnostic test accuracy for the economic models with wide uncertainty intervals.
Three papers provided diagnostic test accuracy evidence of FDG PET/CT as an add-on device for diagnosis of recurrent CRC. 65,81,87 Schmidt et al. 65 compared FDG PET/CT with whole-body MRI but, as reported in the systematic review chapter, there appeared to be reporting bias with this study. In addition, the diagnostic test accuracy for whole-body MRI was inappropriate for the model, which incorporates pelvic MRI rather than whole-body MRI. Ramos et al. 81 provide evidence for contrast-enhanced CT in comparison with FDG PET/CT, but the point estimates assigned appear to be biased in favour of FDG PET/CT (reporting a sensitivity of zero for contrast-enhanced CT, but with a CI range up to 0.65). Selzner et al. 87 provide diagnostic test accuracy evidence for contrast-enhanced CT in comparison with FDG PET/CT; however, they do not report any CIs or other measures of uncertainty. The point estimates from Selzner et al. 87 were deemed to be the best reflection of mean diagnostic test accuracy and were therefore used in the model along with the wide CIs from Ramos et al. 81 to ensure a suitably wide range to reflect the considerable uncertainty surrounding the mean diagnostic test accuracy estimates. The pooled meta-analysis diagnostic test accuracy estimates had more restrictive confidence limits and were therefore deemed inappropriate to accurately reflect uncertainty in the economic models. There were no reliable estimates of pelvic MRI diagnostic test accuracy for recurrent CRC reported; therefore, an estimate was taken from the diagnostic test accuracy of MRI used in the Park et al. 28 economic evaluation. Diagnostic test accuracy estimates, their standard errors and the distributions used in the probabilistic model are detailed in Table 17.
The recurrent CRC models were designed to incorporate the treatment impacts of accurate and inaccurate diagnoses of recurrent CRC.
Optimal treatment combinations for curable and non-curable recurrence were determined through the literature and in consultation with clinical experts. The model assumed that 40% of recurrent rectal cancer patients would have received radiotherapy as part of their treatment for primary cancer and therefore would not receive further radiotherapy, while the remaining 60% of those patients who subsequently developed local recurrence but who did not receive radiotherapy for their primary cancer would receive long-course CRT prior to surgery for recurrent disease. Patients with curable recurrence had one of six treatment options: local surgery alone, local surgery followed by adjuvant chemotherapy, long-course CRT prior to local surgery, local surgery followed by metastatic surgery, local surgery and adjuvant chemotherapy followed by metastatic surgery or long-course CRT prior to local surgery followed by metastatic surgery. Patients with non-curable recurrence had one of two treatment options: metastatic surgery followed by palliative care or palliative care alone. It was assumed that all patients with no recurrence would be treated with a wait and watch strategy in which they would be followed up annually.
The proportions of patients receiving each treatment for curable recurrence were assigned based on consultation with clinical experts and publications reporting treatment and therapeutic impacts for recurrent CRC. 71,93,102 The Scottish network CRC data set145 was used to derive a cohort of patients (AJCC 1–3) who would be susceptible to colorectal recurrence. A subset of this population was deemed to have curable recurrence, using the probability assigned in the model. A Dirichlet distribution was applied to this subset to capture the uncertainty surrounding the treatment allocation. The proportions of patients receiving each treatment for incurable recurrence were informed by the literature and previous economic models for recurrent CRC. 58,110 Beta distributions were applied to these estimates as there were only two options. Table 17 details the treatment options and the proportions.
The longer-term model incorporated overall survival in order to capture any potential impact on mortality over the lifetime of the patients.
The survival analysis was implemented employing an approach similar to that used in the primary model. Five-year overall survival estimates were determined from the literature for patients with no recurrence, recurrence that is curable and non-curable recurrence. 119 In addition, it was assumed that patients in the model who had curable recurrence but were inaccurately diagnosed and failed to receive treatment in the first year were assigned a 5-year overall survival midway between curable and non-curable survival estimates. These data were used to determine an annual mortality rate under the assumption of an exponential survivor function, and used within a Markov simulation to estimate overall life expectancy for no recurrence, curable recurrence, non-curable recurrence and curable recurrence when treatment is delayed. The Markov model assumed a starting age of 50 years and employed the mortality rate calculated for each group for the first 10 years of the extrapolation; beyond 10 years patients were assumed to have survived their cancer and were assumed to return to the average mortality rate for their age. 149
The cohort population of AJCC 1–3 patients derived from the Scottish network CRC data set145 was used to represent the recurrent model population.
The 5-year overall survival estimates for each of the model groups are detailed in Table 17. Beta distributions were applied for the probabilistic analysis.
Utility estimates were incorporated into the model, representing the average quality of life for patients in the no recurrence, curable recurrence and non-curable recurrence groups. Patients who were inaccurately diagnosed as no recurrence (FN test outcomes) and who failed to receive either curable or non-curable treatment in the first year were assigned a disutility for that year to account for the negative impact on their quality of life. Likewise, patients who were inaccurately diagnosed as having recurrent cancer (FP test outcomes) and who received unnecessary curative or non-curative treatments were assigned a lower utility status for that year to account for the negative impact of unnecessary treatment on quality of life.
The utility estimates were incorporated into the survival analysis as described previously in the primary models. It was assumed that the average utility experienced by patients in a particular stage was constant for 5 years post diagnosis. Patients who were still alive 5 years post diagnosis were assigned age-specific utility weights based on UK population norms. 143 The quality of life estimates were combined in the survival analysis and discounted at 3.5%150 to derive discounted quality-adjusted life expectancies for each of the model groups (no recurrence, curable recurrence and non-curable recurrence).
Table 17 details the utility and disutility weights used in the model. The probabilistic analysis applied gamma distributions on disutility to represent uncertainty in the parameters.
As in the primary models, the costs for the recurrent models are attributed to the alternative imaging devices (as a cost per scan) and the various treatment options assigned in the model. The various costs used in the recurrent models are detailed in Table 18, specifying unit costs, standard errors and the distributions used in the probabilistic analysis.
| Item | Recurrent rectal model | Recurrent colon model | Data source | ||||
|---|---|---|---|---|---|---|---|
| Unit cost (£) | Standard error (£) | Probabilistic distribution | Unit cost (£) | Standard error (£) | Probabilistic distribution | ||
| Imaging devices | |||||||
| Contrast-enhanced CT scan (chest, abdomen, pelvis) | 143 | 22 | Normal | 143 | 22 | Normal | DoH reference costs 2009100 |
| MRI scan (pelvis) | 179 | 24 | Normal | – | – | – | DoH reference costs 2009100 |
| FDG PET/CT scan | 800 | 100 | Normal | 800 | 100 | Normal | DoH 2005,152 NCRI 2007153 |
| Treatments | |||||||
| Local surgery (rectal excision with lymphadenectomy): includes cost of the distal procedure (including surgical consultation, theatre time, staff costs), inpatient stay in hospital for average 6 days and surgical follow-up consultation | 5637 | 677 | Normal | – | – | – | DoH reference costs 2009,100 PSSRU 2009101 |
| Local surgery (colonic resection with lymphadenectomy): includes cost of the proximal colon procedure (including surgical consultation, theatre time, staff costs), inpatient stay in hospital for average 6 days and surgical follow-up consultation | – | – | – | 5893 | 746 | Normal | DoH reference costs 2009,100 PSSRU 2009101 |
| Long-course CRT [includes 25 fractions of radiotherapy over 5 weeks combined with 3 months’ chemotherapy (5FU)]: includes cost of radiotherapy and chemotherapy drugs, administration and hospital stay | 13,721 | – | – | – | – | – | BNF 58,99 ISD 2009,151 Cancer Research UK,154 Royal College of Radiologists 200622 and 2008155 |
| Adjuvant chemotherapy (6-month course post surgery; intravenous 5FU + oxaliplatin for 24 weeks: 12 × 2-weekly cycles): includes cost of chemotherapy drugs, administration and hospital stay | 11,532 | – | – | 11,532 | – | – | BNF 58,99 ISD 2009,151 Cancer Research UK154 |
| Palliative care: average NHS cost per person for CRC palliative care; cost adjusted to price year 2008/9 using HCHS pay and price index101 | 2468 | 494 | Normal | 2468 | 494 | Normal | Guest et al. 2006124 |
| Metastatic surgery (surgical specialities in medical oncology): inpatient cost per case, including surgical consultation, theatre time, staff costs and an average 10-day inpatient stay | 9134 | 1827 | Normal | 9134 | 1827 | Normal | ISD 2009151 |
The costs of the imaging devices are the same as those used in the primary models. The treatment option combinations for the recurrent rectal model are different from those in the primary models; however, the costs of the component treatments were assigned in the same way. For example, the cost of recurrent rectal surgery was taken to be the same as the cost of primary surgery (summing the cost of a distal colon procedure, a 6-day hospital inpatient stay and CRC surgery consultant follow-up). The costs of long-course CRT treatment, adjuvant chemotherapy treatment, metastatic surgery and palliative care was also determined by the same means as in the primary models.
The expected costs in the no recurrence, recurrence curable and recurrence non-curable groups were calculated using the proportions of patients receiving each treatment option within each group. In the model, a patient who was diagnosed accurately would receive the optimal treatment option and incur the associated costs of that treatment. A patient who was inaccurately diagnosed would incur the cost of the diagnosed group treatment, followed by the discounted cost of treatment for his or her true diagnosis the following year (i.e. it is assumed that the true diagnosis would be identified within a year). Costs were discounted at 3.5%. 150
Recurrent colon model
The structure of the recurrent models was the same for both rectal and colon cancer. The parameters discussed above relate to rectal cancer; however, Tables 17 and 18 also detail the parameters that were used in the colon model. The specific aspects of the model that were altered for the colon model are discussed below.
Some publications indicate that local recurrence in rectal cancer is more common than local recurrence in colon cancer; however, data for the UK indicate only a very small difference in local recurrence for rectal and colon cancers. 155 Therefore, both the rectal and colon recurrent models assumed the same probability of recurrence. Both models incorporated a measure of the uncertainty around this estimate, which was applied in the probabilistic analysis.
The MRI imaging device is not used in the assessment of colon cancer and therefore the recurrent colon model incorporates only contrast-enhanced CT as the conventional imaging modality. The diagnostic test accuracy estimates for contrast-enhanced CT and FDG PET/CT were determined as discussed above for the recurrent rectal model.
The treatment options for the recurrent colon model vary slightly from those in the recurrent rectal model.
As with the primary colon model, CRT is not included as a treatment option for recurrent colon cancer. Patients with curable colon recurrence had one of four treatment options: local surgery alone, local surgery followed by adjuvant chemotherapy, local surgery followed by metastatic surgery, or local surgery and adjuvant chemotherapy followed by metastatic surgery. Patients with non-curable recurrence and no recurrence had the treatment options detailed above for the recurrent rectal model. The same approach was used to determine the optimal treatment combinations within curable colon recurrence as was used for the recurrent rectal model, i.e. through the literature and in consultation with clinical experts. Table 17 details the within-stage distributions that were assigned to each treatment group in the colon model.
There was no change to the survival analysis or to the quality of life parameters for the colon model.
The costs were amended in line with the parameter modifications discussed above. Because MRI is not used for colon cancer, in the recurrent colon model the cost of conventional imaging incorporates only contrast-enhanced CT, and the cost of the intervention arm incorporates only contrast-enhanced CT plus FDG PET/CT. With regards to the treatment costs, the cost of recurrent local surgery refers to a colonic resection. Cost data for a proximal colonic procedure, a 6-day hospital inpatient stay and CRC surgery consultant follow-up were included. 100,101 The average cost of treatment for each disease group (no recurrence, recurrence curable and recurrence non-curable) was calculated for the colon model in the same manner as for the rectal model, using the proportions of patients receiving each treatment option within each group.
Metastatic model
The metastatic model was undertaken to assess the cost-effectiveness of FDG PET/CT as an add-on device in detecting metastatic cancer. The added value of incorporating an FDG PET/CT scan in addition to conventional imaging in this disease stage is in its ability to detect unsuspected, metastatic disease and potentially identify unsalvageable extra metastases not detected by conventional imaging devices.
Figure 6 depicts the decision tree structure used for the metastatic model, informed by the literature51,109,111 and based on consultation with clinical experts. The metastatic decision tree begins with patients who have previously had surgical treatment for primary CRC and in a routine follow-up assessment (involving a clinical examination and CEA testing) were found to have rising CEA levels, and were identified as potentially having a metastatic recurrence. The decision node depicts the choice between the conventional or add-on FDG PET/CT arms. Similar to the structure used in the previous models, this decision tree has been designed using actual disease status, and therefore the decision tree has split the patient population according to their true disease status (metastatic recurrence or no metastatic recurrence) prior to applying the diagnostic test accuracy estimates for the tests, so that accurate and inaccurate diagnoses can be identified.
FIGURE 6.
Staging metastatic recurrence.
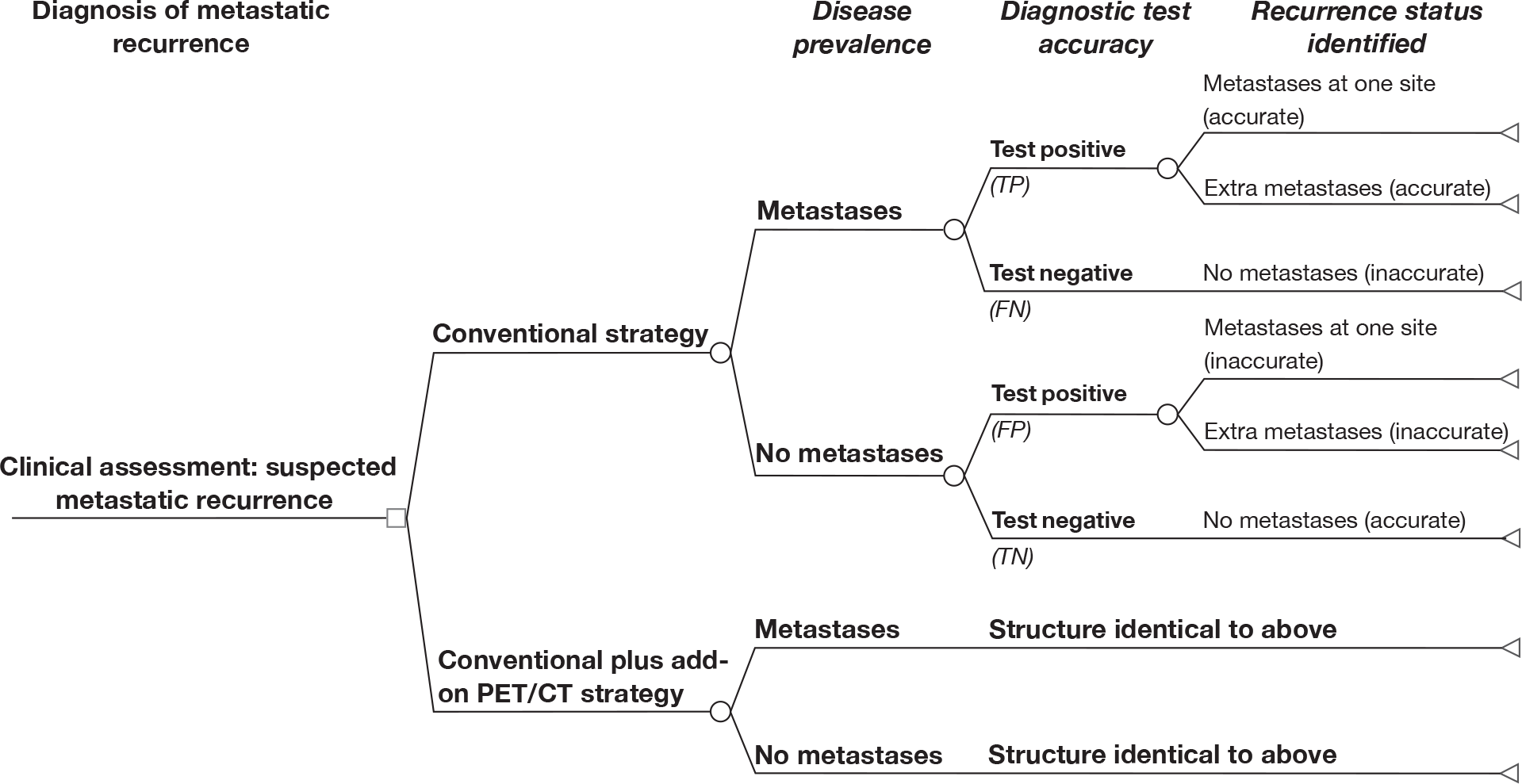
The conventional procedure for patients suspected of metastatic recurrence is to undertake a contrast-enhanced CT scan of the chest, abdomen and pelvis to confirm or refute metastatic recurrence and potentially identify additional sites of metastases. This scan will identify either metastases (test positive) or no metastases (test negative). In the conventional arm, having specified actual disease status, the top half of this branch represents metastatic recurrence, and therefore the tree branch splits depending on whether the test was positive (accurately identifying metastatic recurrence) or negative (inaccurately identifying no metastatic recurrence). Positive identification of metastatic recurrence is further separated in this model to distinguish between metastases at one site or extra metastases at numerous sites, as the extent of the metastatic recurrence will affect the treatment options in the longer-term model. The negative test outcomes in the top branch of the decision tree indicate a misdiagnosis of no metastatic recurrence (FN). For the longer-term model it is assumed that patients will be accurately re-staged within a year.
The bottom half of the conventional tree branch represents the status of no metastatic recurrence, so negative test outcomes accurately indicate no metastases. Positive test outcomes in the bottom half of the tree are FPs, which inaccurately diagnose metastatic recurrence when there is no recurrence. This population is then further divided to distinguish between inaccurate diagnosis of metastases at one site and inaccurate diagnosis of extra metastases at numerous sites. In this way the decision tree separates out accurate and inaccurate diagnoses of metastatic recurrence.
Patients in the ‘conventional arm’ of the model will be staged using the standard diagnostic test (contrast-enhanced CT of the chest, abdomen and pelvis), represented by ‘Test’ in the top half of Figure 6. Patients in the ‘intervention arm’ of the model will also be given the contrast-enhanced CT scan, followed by the addition of an FDG PET/CT scan. This is represented in the bottom half of the tree, but these branches have been abbreviated as the structure is identical to that of the top half.
The accurate and inaccurate identification of metastases at the end of the decision tree branches for the conventional arm of the model is compared with the accurate and inaccurate identification of metastases in the intervention arm and assessed in terms of the incremental cost per accurate diagnosis. These interim outcomes of accurate and inaccurate diagnosis are then used to undertake a longer-term analysis that assesses the impact of accurate and inaccurate diagnosis of metastases on patient management, incorporating optimal treatments for metastases at one site, extra metastases and no metastatic recurrence, and modelling the impacts on quality of life and overall survival. In this way the conventional and intervention arms can be compared in terms of the incremental cost per QALY gained.
This decision tree model is populated with parameters representing the prevalence of metastatic recurrence, the diagnostic test accuracy of contrast-enhanced CT and FDG PET/CT and the probability of having extra metastases (at more than one site). Table 19 details these parameters, along with the treatment, overall survival and quality of life parameters used in the longer-term analysis. The model parameters, including details of the longer-term modelling, are discussed below.
| Parameter | Point estimate | Standard error | Probabilistic distribution | Data source |
|---|---|---|---|---|
| Cancer prevalence (metastatic recurrence) | ||||
| No metastases | 0.60 | – | Dirichlet | Saunders et al. 2002123 |
| Metastases | 0.40 | – | Dirichlet | Saunders et al. 2002123 |
| Metastases at one site | 0.30 | 0.10 | Beta | Lejune et al. 200551 |
| Extra metastases | 0.70 | – | 1 – above | Lejune et al. 200551 |
| Diagnostic test accuracy | ||||
| Contrast-enhanced CT sensitivity | 0.91 | 0.05 | Beta | Chua et al. 200779 |
| Contrast-enhanced CT specificity | 0.70 | 0.15 | Beta | Selzner et al. 200487 |
| FDG PET/CT sensitivity | 0.94 | 0.04 | Beta | Chua et al. 200779 |
| FDG PET/CT specificity | 0.75 | 0.17 | Beta | Chua et al. 200779 |
| Treatments | ||||
| Metastases: pre-operative chemotherapy and metastatic surgery | 1.00 | – | – | Author assumption |
| Extra metastases: pre-operative chemotherapy and metastatic surgery | 0.20 | 0.04 | Beta | MSAC 200858 |
| Extra metastases: palliative care and chemotherapy | 0.80 | – | Beta | MSAC 200858 |
| Wait and watch | 1.00 | – | – | Author assumption |
| 5-year overall survival | ||||
| No metastases | 0.85 | 0.01 | Beta | American Cancer Society 2005119 |
| Metastases: surgery for cure | 0.24 | 0.03 | Beta | AJCC 2010120 |
| Extra metastases: metastatic surgery and palliative care | 0.12 | 0.04 | Beta | AJCC 2010120 |
| Extra metastases: palliative care | 0.06 | 0.04 | Beta | AJCC 2010120 |
| Quality of life/utility | ||||
| No metastases | 0.91 | 0.11 | Gamma (disutility) | Ramsey et al. 2000142 |
| Metastases: surgery for cure | 0.84 | 0.12 | Gamma (disutility) | Ramsey et al. 2000142 |
| Extra metastases: palliative care | 0.52 | 0.08 | Gamma (disutility) | Tengs and Wallace 2000146 |
| Extra metastases: metastatic surgery and palliative care | 0.74 | 0.21 | Gamma (disutility) | Langenhoff et al. 2006117 |
| Disutility for patients who fail to receive pre-operative chemotherapy plus surgery | 0.30 | 0.08 | Gamma | Assumption based on Tengs and Wallace 2000146 |
| Disutility for patients who fail to receive palliative treatment | 0.20 | 0.08 | Gamma | Assumption based on Tengs and Wallace 2000146 |
| Utility for patients who receive unnecessary metastatic surgery | 0.74 | 0.14 | Gamma (disutility) | Langenhoff et al. 2006117 |
| Utility for patients who receive unnecessary palliative treatment | 0.61 | 0.20 | Gamma (disutility) | Tengs and Wallace 2000146 |
Metastatic parameters
The literature identified in the economics search and the systematic review was used to provide disease prevalence evidence for the metastatic model. Estimates provided by Saunders et al. 123 were used for the prevalence of metastatic recurrence for patients previously treated for primary CRC. It was assumed that a cohort of patients who were diagnosed as AJCC 1, 2 or 3 for primary CRC would be susceptible to metastatic recurrence. Using the Scottish network CRC data set145 to represent this cohort, and assigning the probability of recurrence from Saunders et al. ,123 it was possible to apply a Dirichlet distribution to represent the uncertainty around the prevalence of metastatic recurrence in the probabilistic analysis. Table 19 details these parameters.
The model structure for this evaluation is similar to that used by previous economic evaluations assessing the cost-effectiveness of using add-on FDG PET/CT in the identification of metastatic disease. 29,51 Previous models have attempted to incorporate patient management and quality of life impacts by distinguishing between resectable and unresectable metastases51 or by distinguishing between hepatic and extra metastases. 29 Our evaluation distinguished between metastases at one site, and at multiple sites (extra metastases). Assigning a probability for each in the overall metastatic recurrence population. In this way the model could distinguish between metastatic (at one site) and extra-metastatic (at more than one site) disease, even though the diagnostic test accuracy estimate referred only to the identification of metastases.
As reported in Chapter 8, a meta-analysis was undertaken using relevant papers identified from the systematic review to elicit pooled diagnostic test accuracy estimates of FDG PET/CT for metastatic CRC. Because of inadequacies and reporting bias in the identified papers, these pooled estimates for FDG PET/CT may not be an accurate reflection of the mean diagnostic test accuracy. The CIs for the pooled estimates were also tight around the pooled mean, restricting the level of uncertainty represented. Therefore, the meta-analysis of diagnostic test accuracy data was deemed to be inappropriate for use in the economic model, and papers identified by the systematic review were considered individually, along with papers previously identified through the economic search, to find reasonable estimates of diagnostic test accuracy for the economic models.
Four papers provided diagnostic test accuracy evidence of FDG PET/CT in comparison with contrast-enhanced CT for diagnosing metastatic recurrence. 79,80,83,87 These diagnostic test accuracy papers were all deemed to be of poor quality and suffering from the reporting bias discussed in the systematic review (see Chapter 8). After considering the available evidence, the economic model incorporated the diagnostic test accuracy evidence for contrast-enhanced CT and FDG PET/CT from the Chua et al. paper,79 with an adjustment to the (low) point estimate for the specificity of contrast-enhanced CT and incorporating a wide range for the uncertainty based on the CI data from Selzner et al. 87 Diagnostic test accuracy estimates, their standard errors and the distributions used in the probabilistic model are detailed in Table 19.
The metastatic model was designed to incorporate the treatment impacts of accurate and inaccurate diagnosis of metastatic recurrence.
Treatment combinations for metastatic recurrence at one site, extra metastases and no metastatic recurrence were determined from the literature. Although extreme, the model assumes that all patients with metastases at a single site will receive pre-operative chemotherapy and metastatic surgery. Similarly, taking an extreme position, patients with extra metastases are assumed to be non-curable and will receive one of two treatment options: pre-operative chemotherapy followed by metastatic surgery and palliative care, or chemotherapy and palliative care. It was assumed that all patients identified as having no metastatic recurrence would be treated with a wait and watch strategy in which they would be followed up annually.
The proportions of patients receiving each of the two treatment options for extra metastases were determined from previous economic evaluations58,110 and uncertainty was represented by a beta distribution. Table 19 details these treatment options.
The longer-term model incorporated overall survival to capture any potential impact on mortality over the lifetime of the patients.
The survival analysis was implemented employing an approach similar to that used in the primary and recurrent models. Five-year overall survival estimates were determined from the literature for patients with no metastatic recurrence and patients with metastases at one site. 119,120 Patients with extra metastases were assigned different 5-year overall survival estimates dependent on the type of treatment that they received, i.e. patients with extra metastases who received metastatic surgery with palliative intent had a greater 5-year survival estimate than patients with extra metastases who received palliative care alone. 119,120 These data were used to determine an annual mortality rate under the assumption of an exponential survivor function, and used within a Markov simulation to estimate overall life expectancy for each group. The Markov simulation assumed a starting age of 50 years and employed the mortality rate calculated for each group for the first 10 years of the extrapolation; beyond 10 years patients were assumed to have survived their cancer and to return to the average mortality rate for their age. 149
The cohort population of AJCC 1–3 patients derived from the Scottish network CRC data set145 was used to represent the metastatic model population.
The 5-year overall survival estimates for each of the model groups are detailed in Table 19. Beta distributions were applied for the probabilistic analysis.
Utility estimates were incorporated into the model, representing the average quality of life for patients in the no metastatic recurrence, metastases at one site and extra metastases (at numerous sites) groups. Patients who were inaccurately diagnosed as no metastatic recurrence (FNs) and who therefore failed to receive treatment for metastases at either one or more than one site in the first year were assigned a disutility for that year to account for the negative impact on their quality of life. Likewise, patients who were inaccurately diagnosed as having metastases (FPs) and who received unnecessary metastatic surgery or treatments for extra metastases were assigned a lower utility status for that year to account for the negative impact of unnecessary treatment on their quality of life.
The utility estimates were incorporated into the survival analysis as previously described for the recurrent model. It was assumed that the average utility experienced by patients in a particular stage was constant for 5 years post diagnosis. Patients who were still alive 5 years post diagnosis were assigned age-specific utility weights based on UK population norms. 143 The quality of life estimates were combined in the survival analysis and discounted at 3.5%150 to derive discounted quality-adjusted life expectancies for each of the groups (no metastatic recurrence, metastases and extra metastases).
Table 19 details the utility and disutility weights used in the model. The probabilistic analysis applied gamma distributions on disutility to represent uncertainty in the parameters.
As in the recurrent model, the costs for the metastatic model are attributed to the alternative imaging devices (as a cost per scan) and the various treatment options assigned in the model. The various costs used in the metastatic model are detailed in Table 20, specifying unit costs, standard errors and the distributions used in the probabilistic analysis.
| Item | Metastatic model | Data source | ||
|---|---|---|---|---|
| Unit cost (£) | Standard error (£) | Probabilistic distribution | ||
| Imaging devices | ||||
| Contrast-enhanced CT scan (chest, abdomen, pelvis) | 143 | 22 | Normal | DoH reference costs 2009100 |
| MRI scan (pelvis) | 179 | 24 | Normal | DoH reference costs 2009100 |
| FDG PET/CT scan | 800 | 100 | Normal | DoH 2005,152 NCRI 2007153 |
| Treatments | ||||
| Chemotherapy (6-month course post surgery: intravenous 5FU + oxaliplatin for 24 weeks: = 12 × 2-weekly cycles): includes cost of chemotherapy drugs, administration and hospital stay | 11,532 | – | – | BNF 58,99 ISD 2009,151 Cancer Research UK 154 |
| Palliative care: average NHS cost per person for CRC palliative care; cost adjusted to price year 2008/9 using HCHS pay and price index101 | 2468 | 494 | Normal | Guest et al. 2006124 |
| Metastatic surgery (surgical specialties in medical oncology): inpatient cost per case, including surgical consultation, theatre time, staff costs and an average 10-day inpatient stay | 9134 | 1827 | Normal | ISD 2009151 |
| Wait and watch: cost of oncology follow-up | 60 | 13 | Normal | DoH reference costs 2009100 |
The costs of the imaging devices are the same as those used in the previous models. The treatment option combinations for the metastatic model are different to those in the primary and recurrent models; however, the costs of the component treatments were assigned in the same way. For example, the costs of metastatic surgery, palliative care and pre-operative chemotherapy were determined by the same means used in the primary and recurrent models.
The expected costs of treatment for the groups were calculated using the proportions of patients receiving each treatment option within each group. In the model, if a patient was diagnosed accurately, he or she would receive the optimal treatment option and incur the associated costs of that treatment. If a patient was inaccurately diagnosed, he or she would incur the cost of the treatment for the (mis)diagnosed group, followed by the discounted cost of treatment for his or her true diagnosis the following year (i.e. it is assumed that the true diagnosis would be identified within a year if the patient were still alive). Costs were discounted at 3.5%. 150
Results
Primary rectal cancer model
Table 21 details the expected costs of the imaging involved in the conventional and the intervention test strategies, the expected probability of a correct diagnosis under each strategy and the cost-effectiveness in terms of cost per correct diagnosis for primary rectal cancer. The addition of FDG PET/CT was dominated by the conventional strategy, i.e. FDG PET/CT was both more expensive and less effective.
| Diagnostic tool | Mean cost per scan (£) | Probability of correct diagnosis |
|---|---|---|
| MRI + CT | 322 | 0.71 |
| MRI + CT + FDG PET/CT | 1122 | 0.61 |
| Difference | 800 | –0.10 |
| ICER | MRI + CT dominates |
Table 22 details the expected costs of the imaging and treatment associated with the conventional and the intervention test strategies, the expected outcomes in terms of QALYs under each strategy and the cost-effectiveness in terms of cost per QALY gain for primary rectal cancer. On this basis, the addition of FDG PET/CT to the conventional strategy involved an additional cost of approximately £432,000 per QALY gained and would not be considered cost-effective under the usual definition [£20,000 per QALY < incremental cost-effectiveness ratio (ICER) < £30,000 per QALY]. 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| MRI + CT | 15,151 | 9.42 |
| MRI + CT + FDG PET/CT | 17,418 | 9.43 |
| Difference (95% CI) | 2267 (932 to 3602) | 0.01 (–0.02 to 0.03) |
| ICER | 431,691 |
Figure 7 illustrates the uncertainty surrounding the expected incremental costs and incremental QALYs for primary rectal cancer. The figure shows that there was considerable uncertainty about the extent, but not the existence, of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 7.
The cost-effectiveness plane for FDG PET/CT in primary rectal cancer.
The cost-effectiveness acceptability curve (CEAC) (Figure 8) illustrates the uncertainty in the cost-effectiveness estimate for primary rectal cancer. The CEAC shows the probability that FDG PET/CT was cost-effective as an add-on imaging device in comparison to CT and MRI at different values for the maximum acceptable cost-effectiveness ratio (λ). Figure 8 shows that, at a monetary threshold of < £100,000, the probability that the addition of FDG PET/CT was cost-effective is < 20%. Within the usual range of values for the maximum acceptable cost-effectiveness ratio (λ), the CEAC illustrates that the conventional CT and MRI devices have a (approximately) 100% probability of being cost-effective and the FDG PET/CT intervention has a (approximately) 0% probability of being cost-effective.
FIGURE 8.
The CEAC for primary rectal cancer.
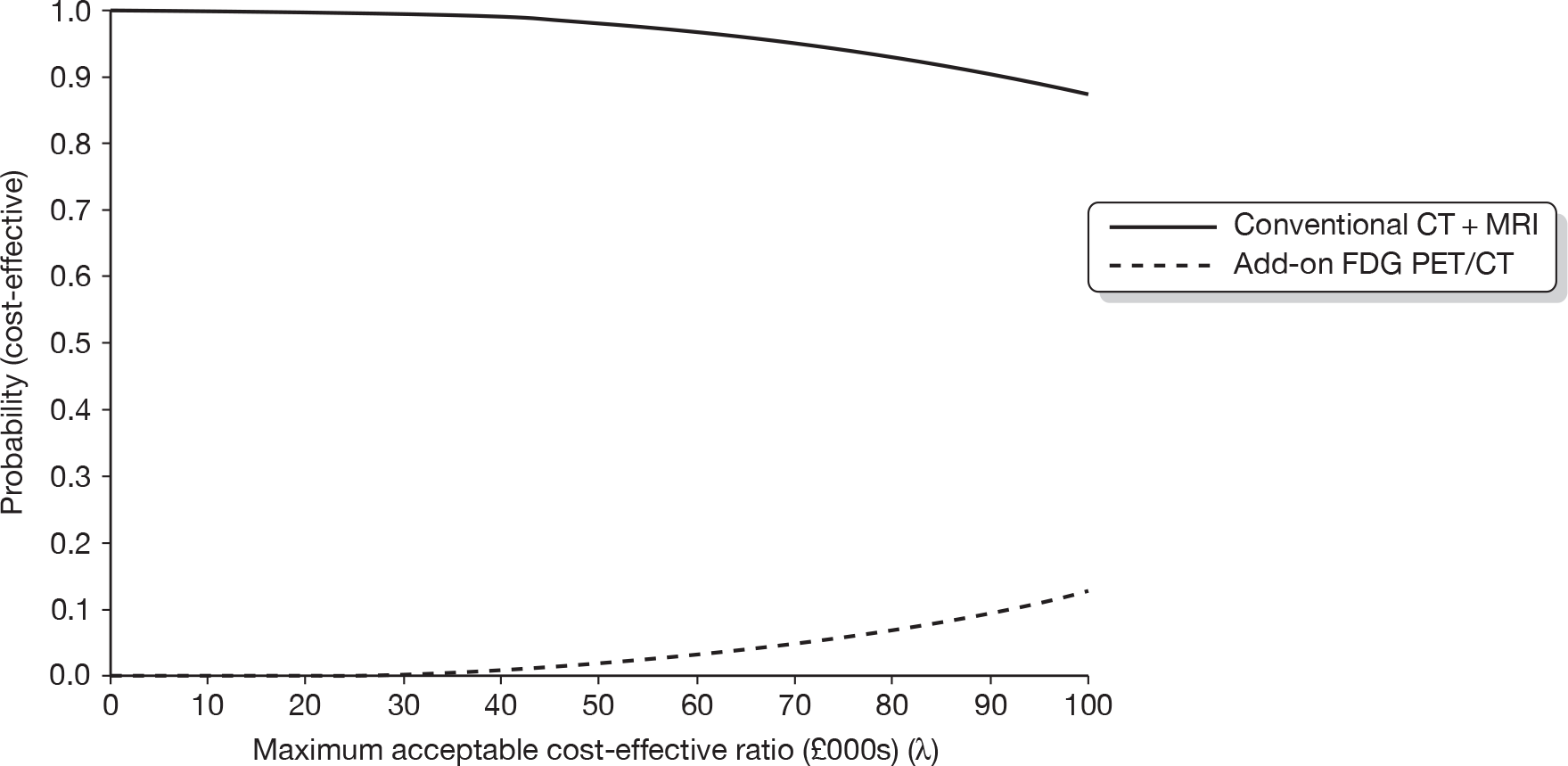
The expected value of perfect information (EVPI) analysis shows that, at a willingness-to-pay threshold of £30,000 per QALY, the EVPI per decision is < £2. To determine the overall population value of EVPI we assumed an annual incidence of 13,315156 cases and a time frame of 2 years (i.e. FDG PET/CT in its current form will be considered as an add-on for imaging for 2 years). This time frame was determined in part by the continual development and upgrading of FDG PET/CT, such that the estimates for diagnostic test accuracy are likely to change outside this time frame. Figure 9 details the results from the EVPI analysis at a population level. At a willingness-to-pay threshold of £30,000 per QALY, the EVPI for the population is approximately £34,000; thus it would not be worthwhile seeking additional information for FDG PET/CT for primary rectal cancer.
FIGURE 9.
The EVPI for primary rectal cancer – population level.
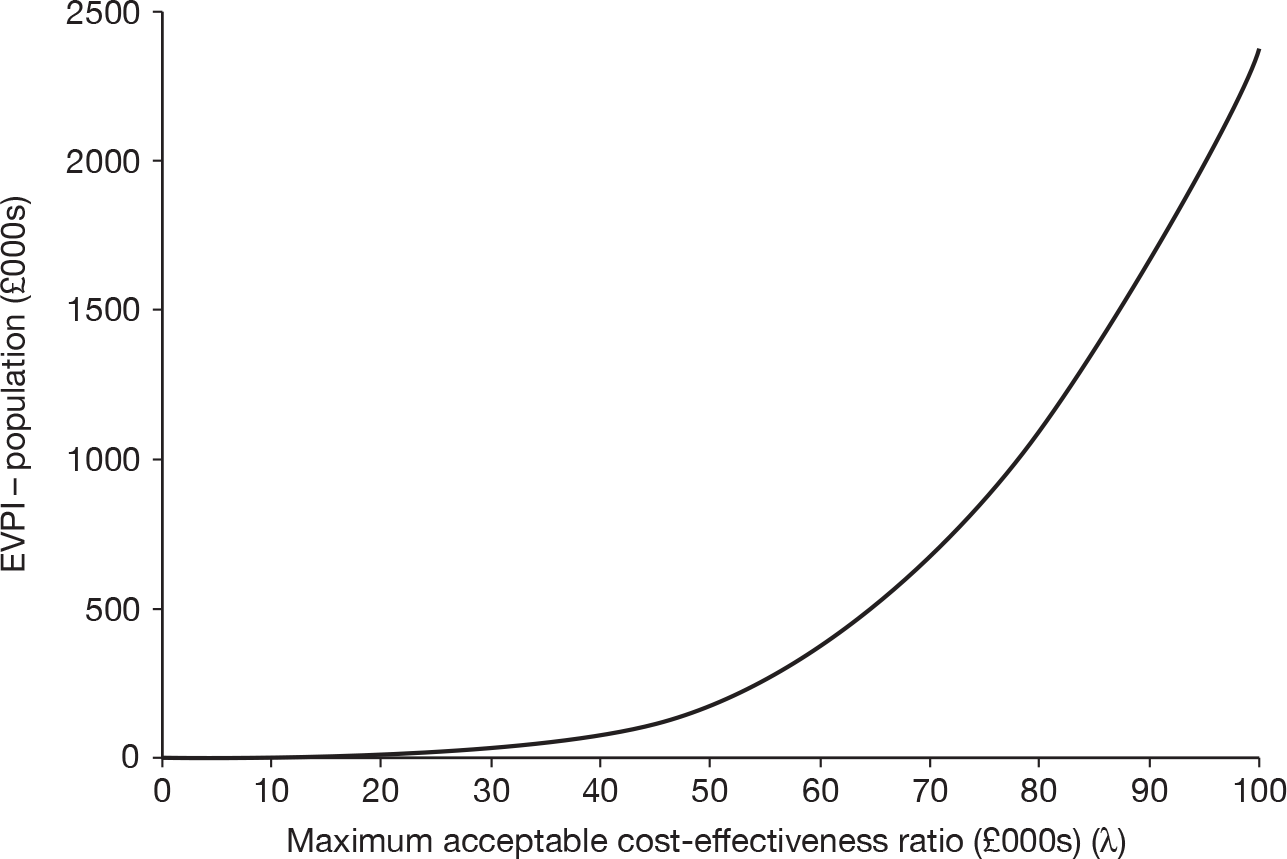
Primary colon cancer model
Table 23 details the expected costs of the imaging involved in the conventional and the intervention test strategies, the expected probability of a correct diagnosis under each strategy and the cost-effectiveness in terms of cost per correct diagnosis for primary colon cancer. On this basis, the addition of FDG PET/CT was dominated by the conventional strategy, i.e. FDG PET/CT was both more expensive and less effective.
| Diagnostic tool | Mean cost per scan (£) | Probability of correct diagnosis |
|---|---|---|
| CT | 143 | 0.65 |
| CT + FDG PET/CT | 943 | 0.60 |
| Difference | 800 | –0.05 |
| ICER | CT dominates |
Table 24 details the expected costs of the imaging and treatment associated with the conventional and the intervention test strategies, the expected outcomes in terms of QALYs under each strategy and the cost-effectiveness in terms of cost per QALY gain for primary colon cancer. On this basis, the addition of FDG PET/CT to the conventional strategy involved an additional cost of approximately £171,000 per QALY gained and would not be considered cost-effective under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| CT | 12,815 | 9.41 |
| CT + FDG PET/CT | 15,066 | 9.42 |
| Difference (95% CI) | 2253 (1195 to 3310) | 0.01 (–0.02 to 0.05) |
| ICER | 171,018 |
Figure 10 illustrates the uncertainty surrounding the expected incremental cost and incremental QALY results for primary colon cancer. The figure shows that there was considerable uncertainty about the extent, but not the existence, of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 10.
The cost-effectiveness plane for FDG PET/CT in primary colon cancer.
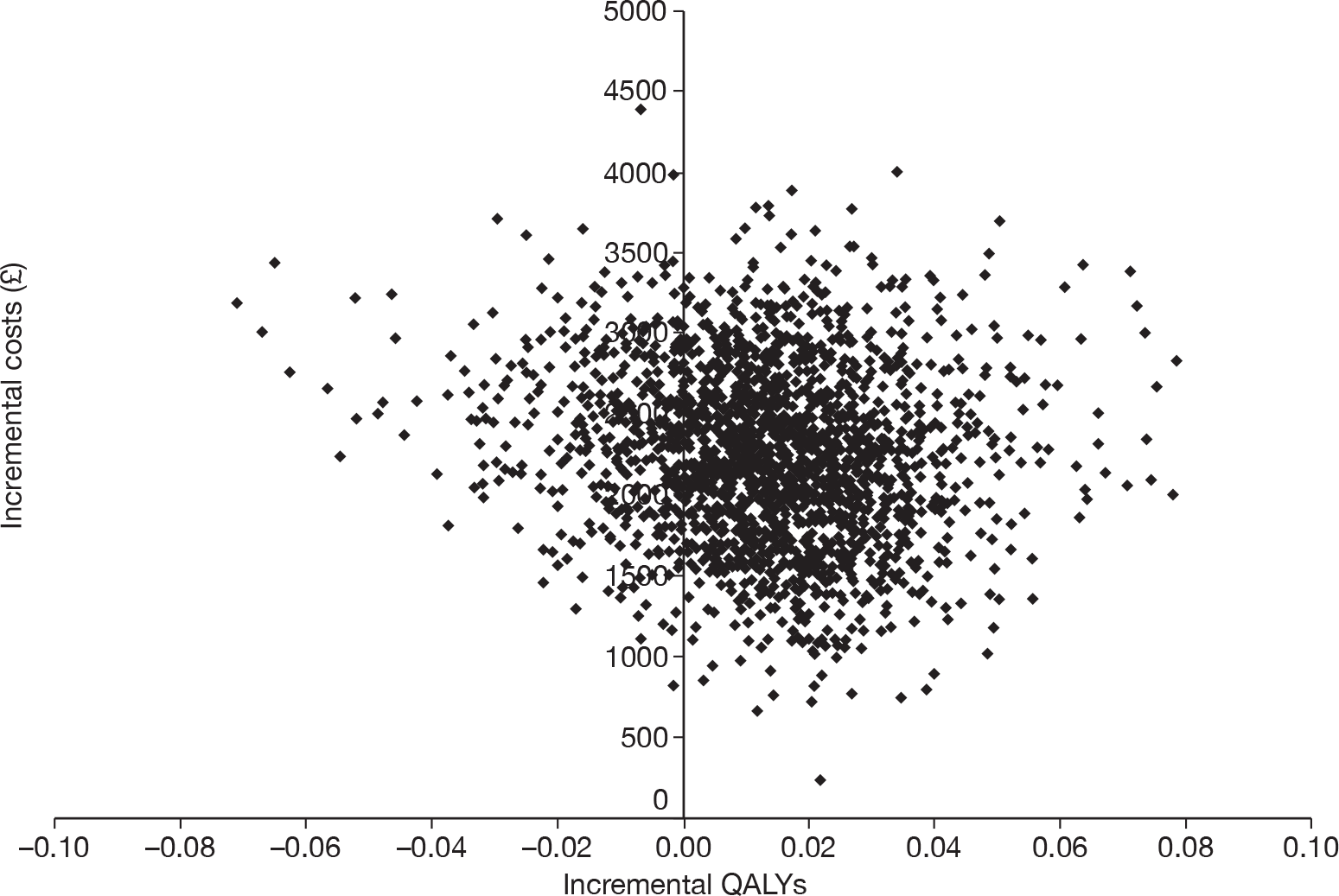
The CEAC (Figure 11) illustrates the uncertainty in the cost-effectiveness estimate for primary colon cancer. The figure shows that, at a monetary threshold of £100,000 per QALY, the probability that the addition of FDG PET/CT will be cost-effective is approximately 30%. At a threshold of £30,000 per QALY, the CEAC illustrates that the probability that FDG PET/CT will be cost-effective is approximately 1%. At this threshold the probability that the conventional CT strategy will be cost-effective is approximately 99%.
FIGURE 11.
The CEAC for primary colon cancer.
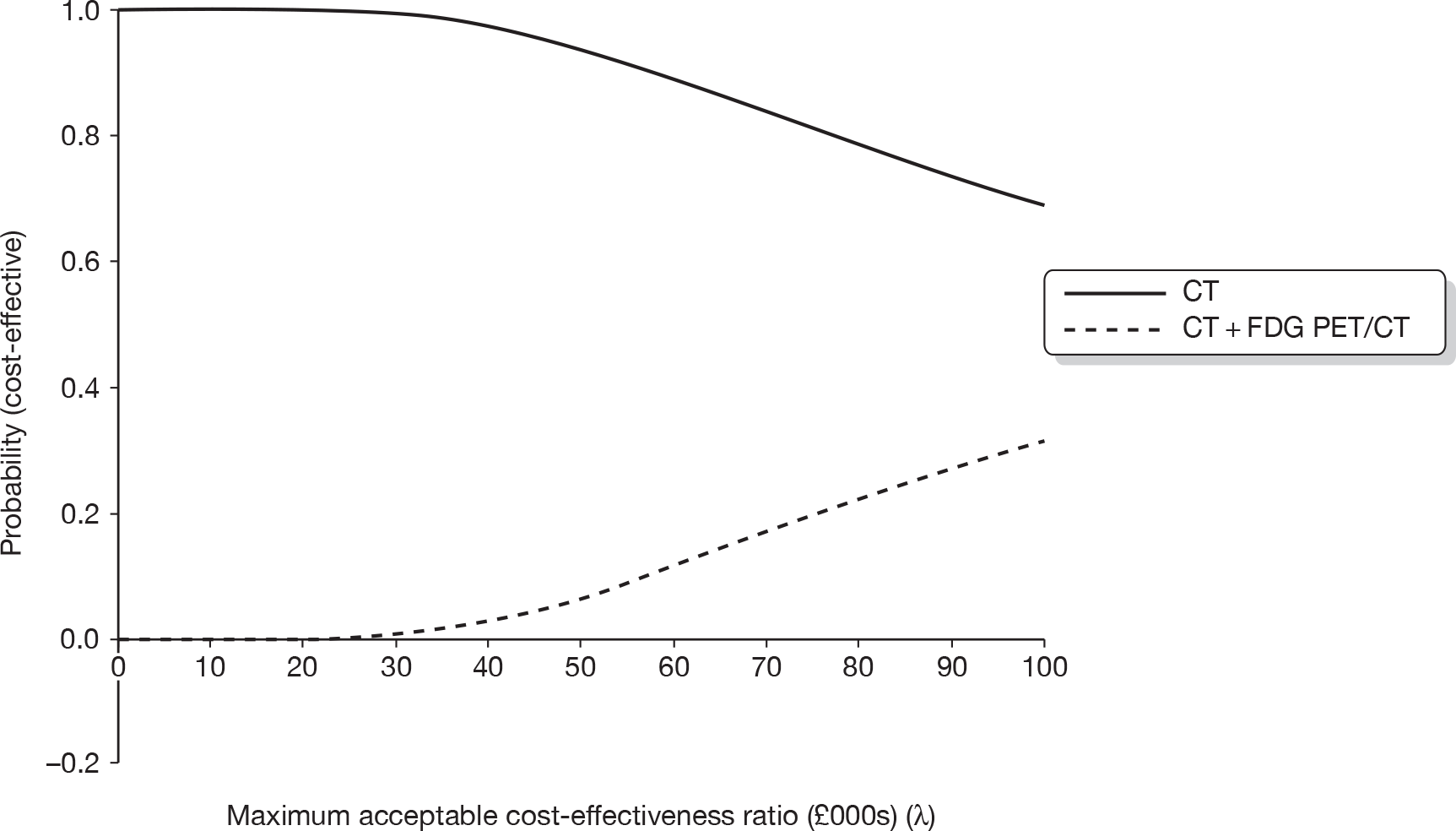
The EVPI results show that, at a willingness-to-pay threshold of £30,000 per QALY, the EVPI per decision is < £2. To determine the overall population value of the EVPI we assumed an annual incidence of 21,574156 cases and a time frame of 2 years (i.e. FDG PET/CT in its current form will be considered for imaging for 2 years). As noted above, this time frame was determined in part by the continual development and upgrading of FDG PET/CT, such that the estimates for diagnostic test accuracy are likely to change outside of this time frame. Figure 12 details the results from the EVPI analysis at a population level. At a willingness-to-pay threshold of £30,000 per QALY, the EVPI for the population is approximately £70,000; thus it would not be worthwhile seeking additional information for FDG PET/CT for primary colon cancer.
FIGURE 12.
The EVPI for primary colon cancer.
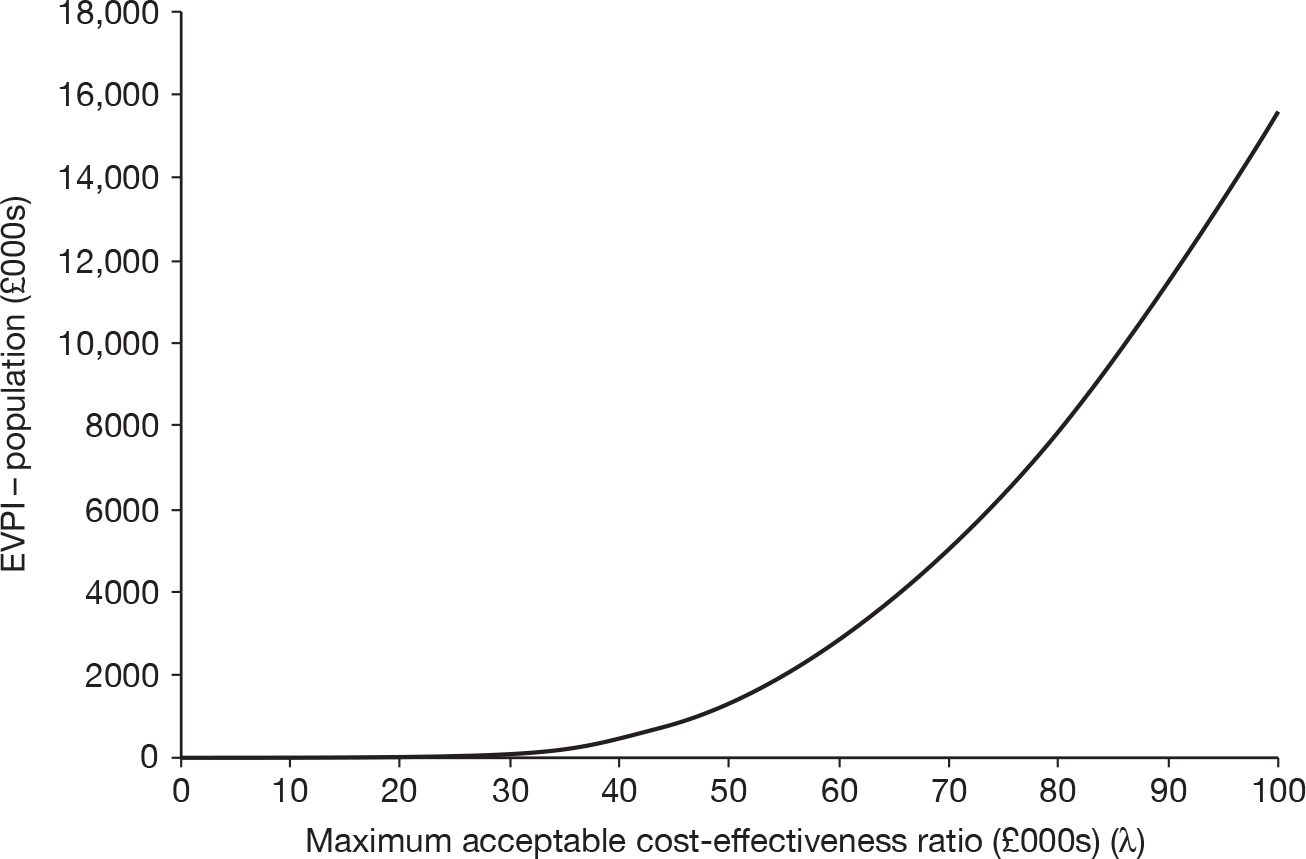
Scenario analysis: contrast-enhanced FDG PET/CT as a lone technology
Primary rectal cancer scenario
The results from the primary rectal cancer scenario, which replaced contrast-enhanced CT with contrast-enhanced FDG PET/CT in addition to an MRI scan, are detailed in Table 25. The results show an improvement in cost-effectiveness compared with the baseline add-on FDG PET/CT results (detailed in Table 21); however, with an ICER of £107,652 this potential future strategy of contrast-enhanced FDG PET/CT as a replacement for contrast-enhanced CT in primary rectal cancer would not be considered to be cost-effective under the usual definition of willingness to pay (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| MRI + CT | 15,120 | 9.43 |
| MRI + contrast-enhanced FDG PET/CT | 16,095 | 9.44 |
| Difference (95% CI) | 975 (–322 to 2271) | 0.01 (–0.01 to 0.03) |
| ICER | 107,652 |
Figure 13 illustrates the uncertainty surrounding the expected incremental cost and incremental QALY results for the primary rectal cancer scenario. The figure shows that there is considerable uncertainty about the extent and existence of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 13.
The cost-effectiveness plane for contrast-enhanced FDG PET/CT in the primary rectal cancer scenario.
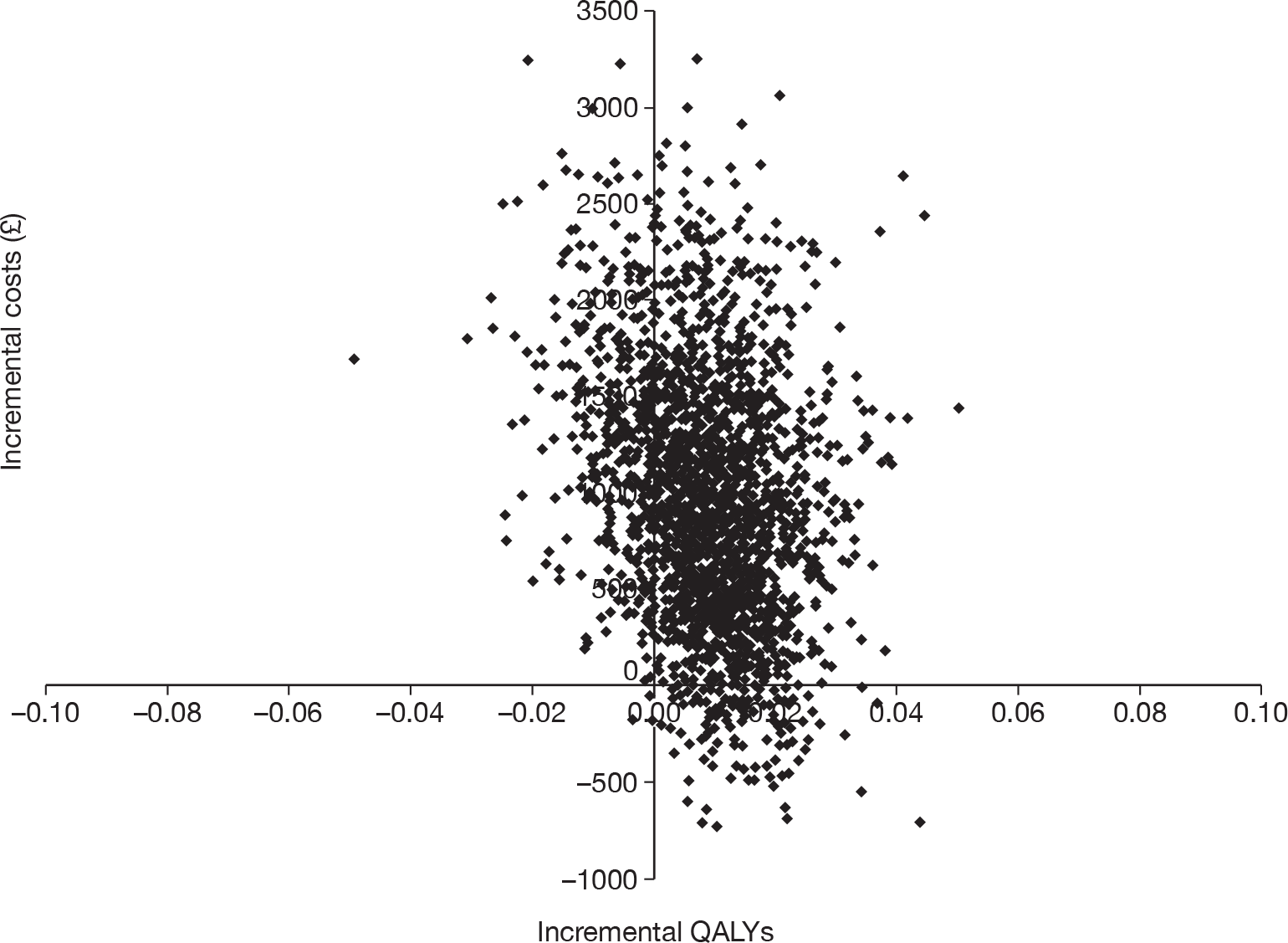
The CEAC (Figure 14) illustrates the uncertainty in the cost-effectiveness estimate for the primary rectal cancer scenario. The figure shows that, at a monetary threshold of £30,000 per QALY, the probability of contrast-enhanced FDG PET/CT and MRI being cost-effective in comparison with contrast-enhanced CT and MRI was < 20%.
FIGURE 14.
The CEAC for the primary rectal cancer scenario.
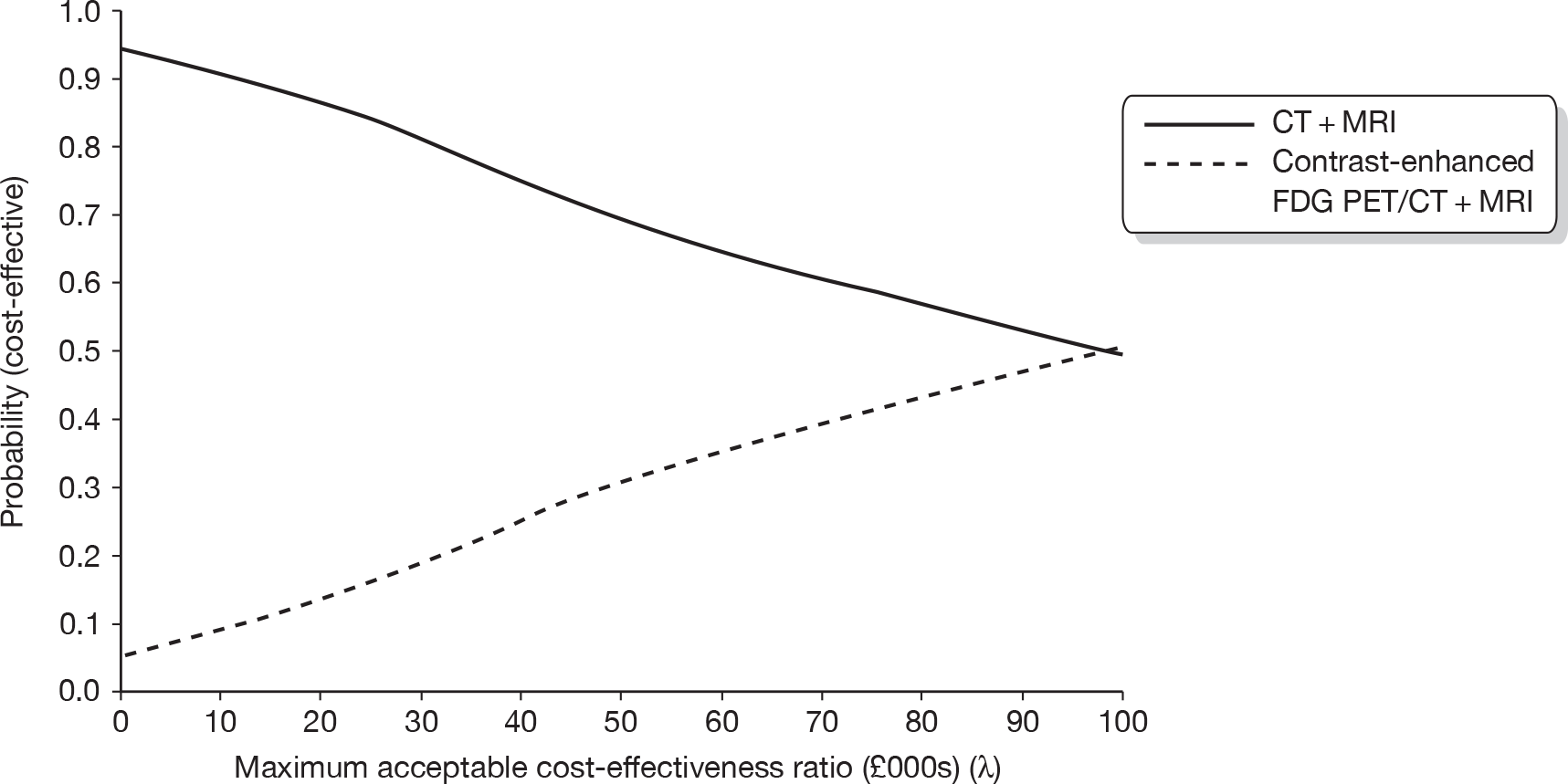
Figure 15 details the results from the EVPI analysis at a population level. The results indicate an EVPI per decision of £68, which translated to a population EVPI of £1.7M. Therefore, we concluded that it was potentially worthwhile to undertake further research to explore whether or not contrast-enhanced FDG PET/CT can be used as a replacement for contrast-enhanced CT in primary rectal cancer.
FIGURE 15.
The EVPI for the primary rectal cancer scenario.
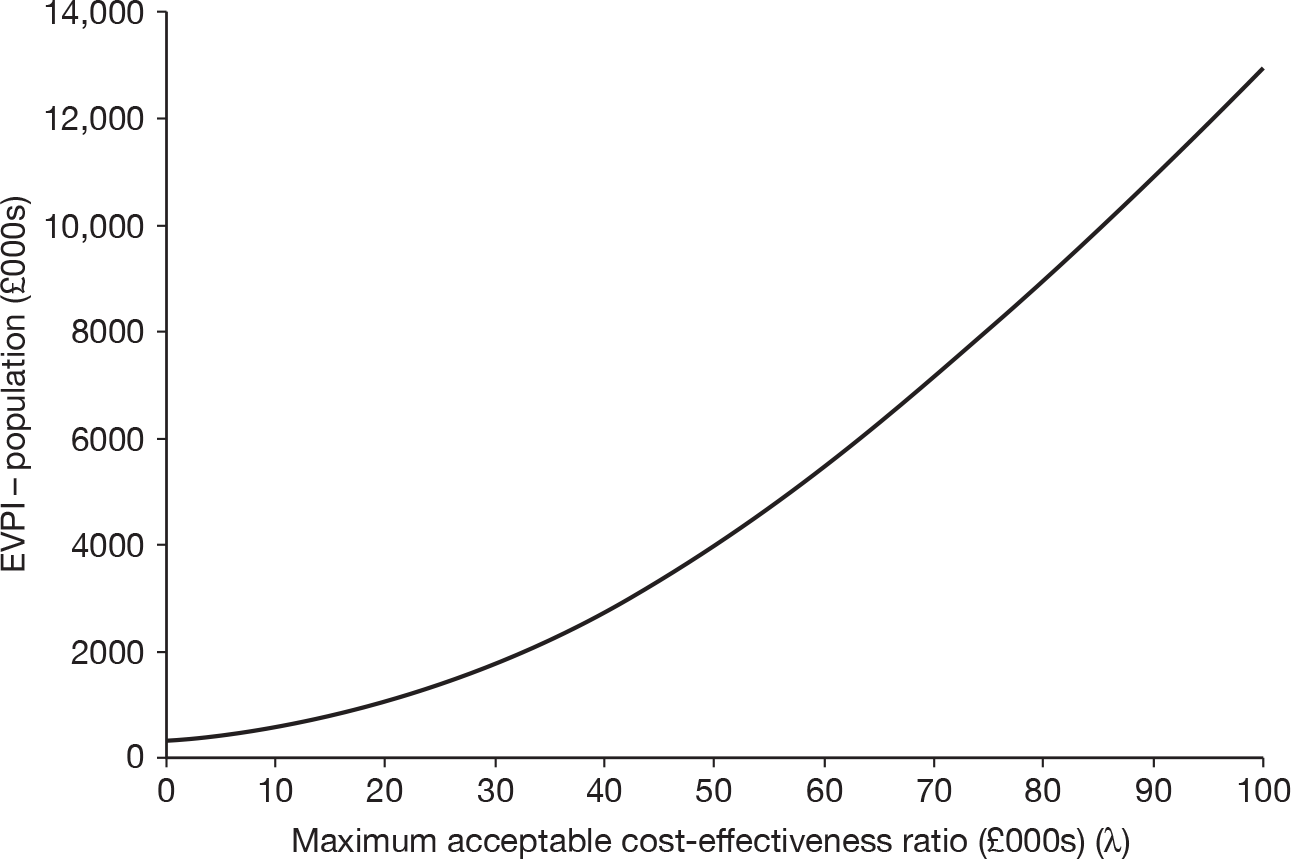
Primary colon cancer scenario
With regards to the primary colon cancer scenario, which compared conventional contrast-enhanced CT with contrast-enhanced FDG PET/CT alone, the results indicated that there was potential for this to be highly cost-effective. Table 26 shows that the ICER is £12,832, which is considerably below the usual definition of willingness to pay (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| CT | 12,766 | 9.45 |
| Contrast-enhanced FDG PET/CT | 12,972 | 9.47 |
| Difference (95% CI) | 206 (–1476 to 1887) | 0.02 (–0.0024 to 0.03) |
| ICER | 12,832 |
Figure 16 illustrates the uncertainty surrounding the expected incremental cost and incremental QALY results for the primary colon cancer scenario. The figure shows that there was considerable uncertainty about the extent and existence of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 16.
The cost-effectiveness plane for contrast-enhanced FDG PET/CT in the primary colon cancer scenario.
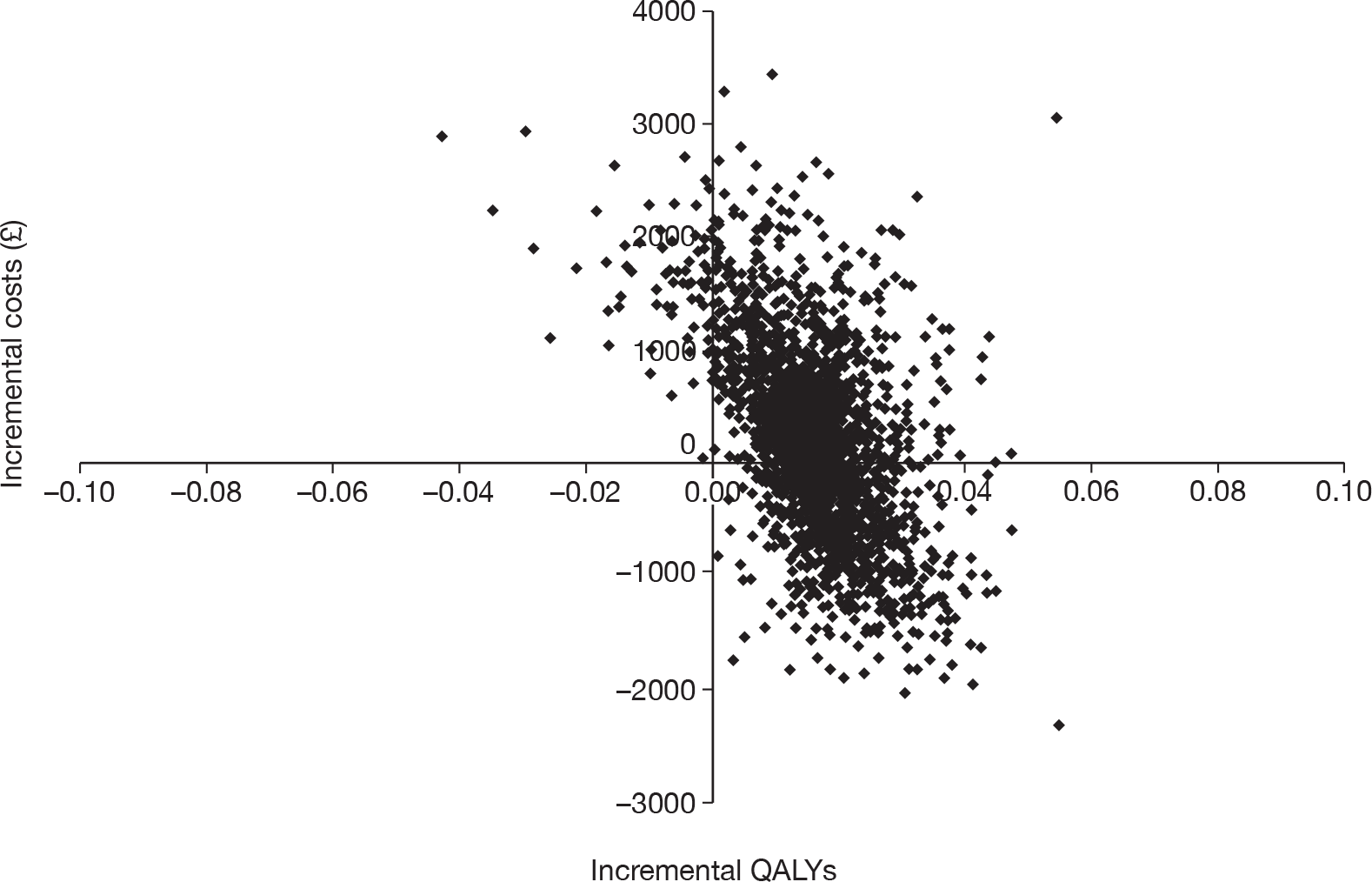
The CEAC (Figure 17) illustrates the uncertainty in the cost-effectiveness estimate for the primary rectal cancer scenario. The figure shows that, at a monetary threshold of £30,000 per QALY, there was a 60% probability of contrast-enhanced FDG PET/CT being cost-effective in comparison with contrast-enhanced CT.
FIGURE 17.
The CEAC for the primary colon cancer scenario.
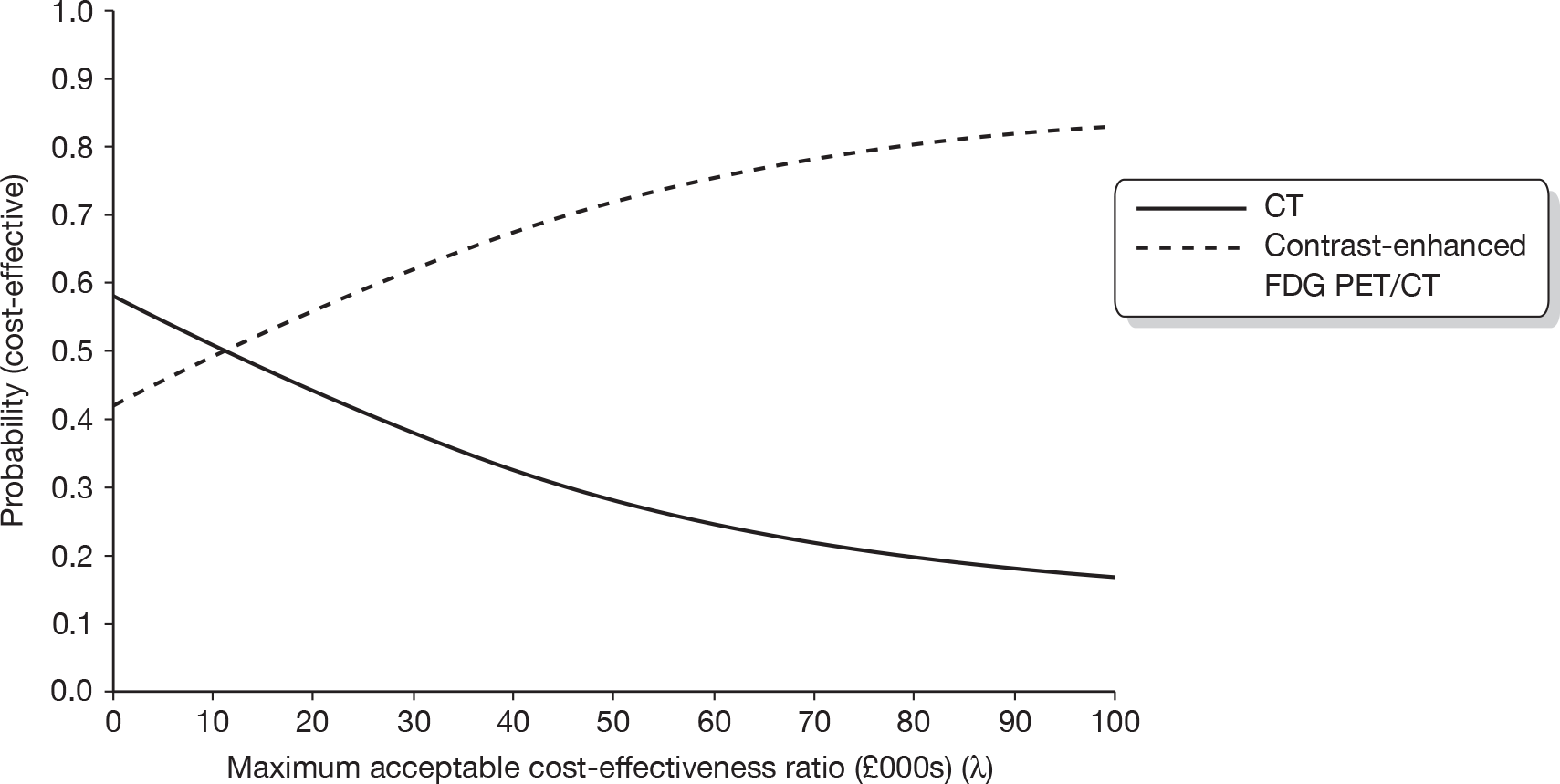
Figure 18 details the results from the EVPI analysis at a population level. The results indicated an EVPI per decision of £290, which translated to a population EVPI of £12.3M. Therefore, we concluded that it is potentially worthwhile undertaking further research to explore whether or not contrast-enhanced FDG PET/CT can be used as a replacement for contrast-enhanced CT in primary colon cancer.
FIGURE 18.
The EVPI for the primary colon cancer scenario.
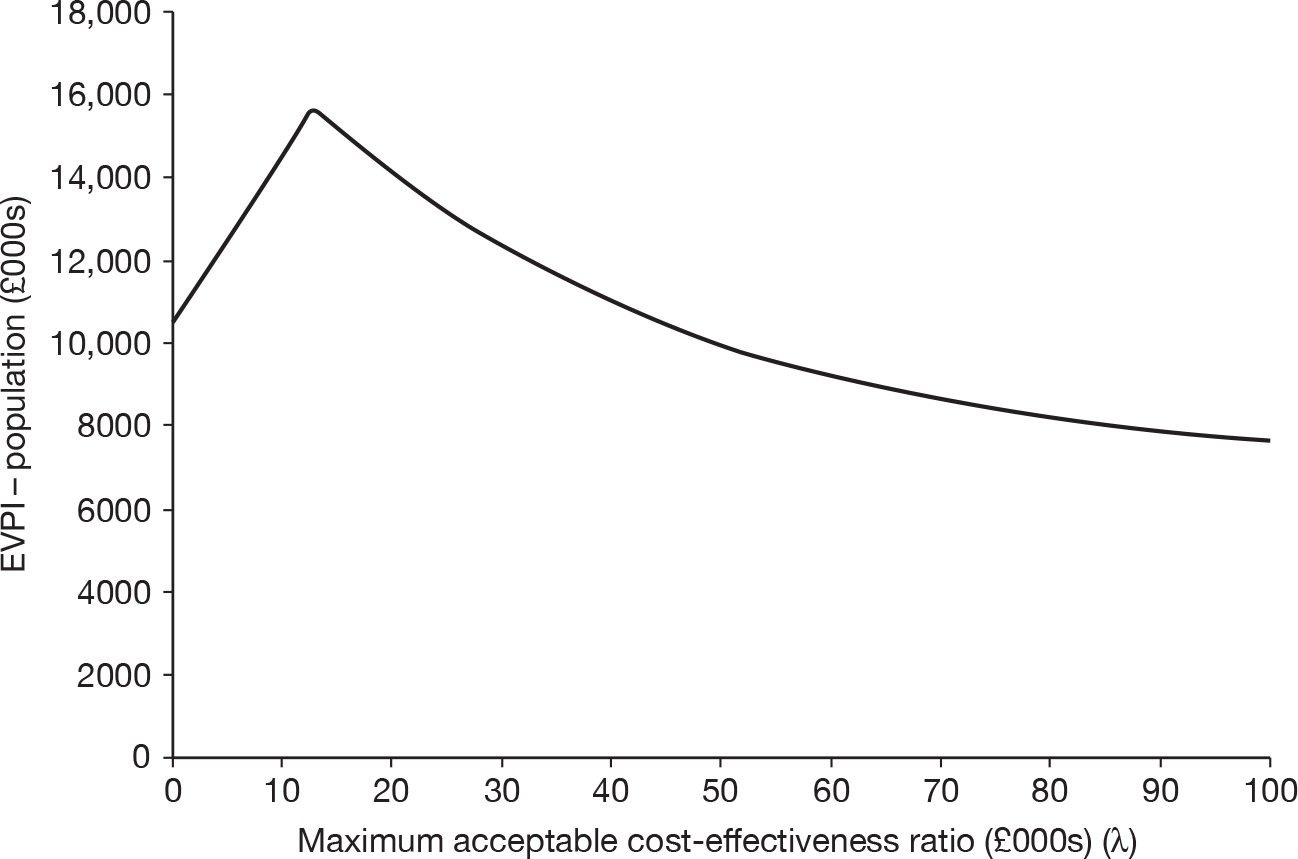
Recurrent rectal cancer model
Table 27 details the expected costs of the imaging involved in the conventional and the intervention test strategies, the expected probability of a correct diagnosis under each strategy and the cost-effectiveness in terms of cost per correct diagnosis for recurrent rectal cancer. On this basis, the addition of FDG PET/CT involved an additional cost of approximately £12,000 per correct diagnosis and would be considered cost-effective compared with the conventional strategy under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Mean cost per scan (£) | Probability of correct diagnosis |
|---|---|---|
| MRI + CT | 322 | 0.88 |
| MRI + CT + FDG PET/CT | 1122 | 0.95 |
| Difference | 800 | 0.07 |
| ICER | 11,713 |
Table 28 details the expected costs of the imaging and treatment associated with the conventional and the intervention test strategies, the expected outcomes in terms of QALYs under each strategy and the cost-effectiveness in terms of cost per QALY gain for recurrent rectal cancer. On this basis, the addition of FDG PET/CT to the conventional strategy involved an additional cost of £21,409 per QALY gained and was likely to be considered cost-effective under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| MRI + CT | 7243 | 4.56 |
| MRI + CT + FDG PET/CT | 7955 | 4.59 |
| Difference (95% CI) | 712 (185 to 1239) | 0.03 (–0.04 to 0.11) |
| ICER | 21,409 |
Figure 19 illustrates the uncertainty surrounding the expected incremental cost and incremental QALY result for recurrent rectal cancer. The figure shows that there was considerable uncertainty about the existence and extent of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 19.
The cost-effectiveness plane for FDG PET/CT in recurrent rectal cancer.
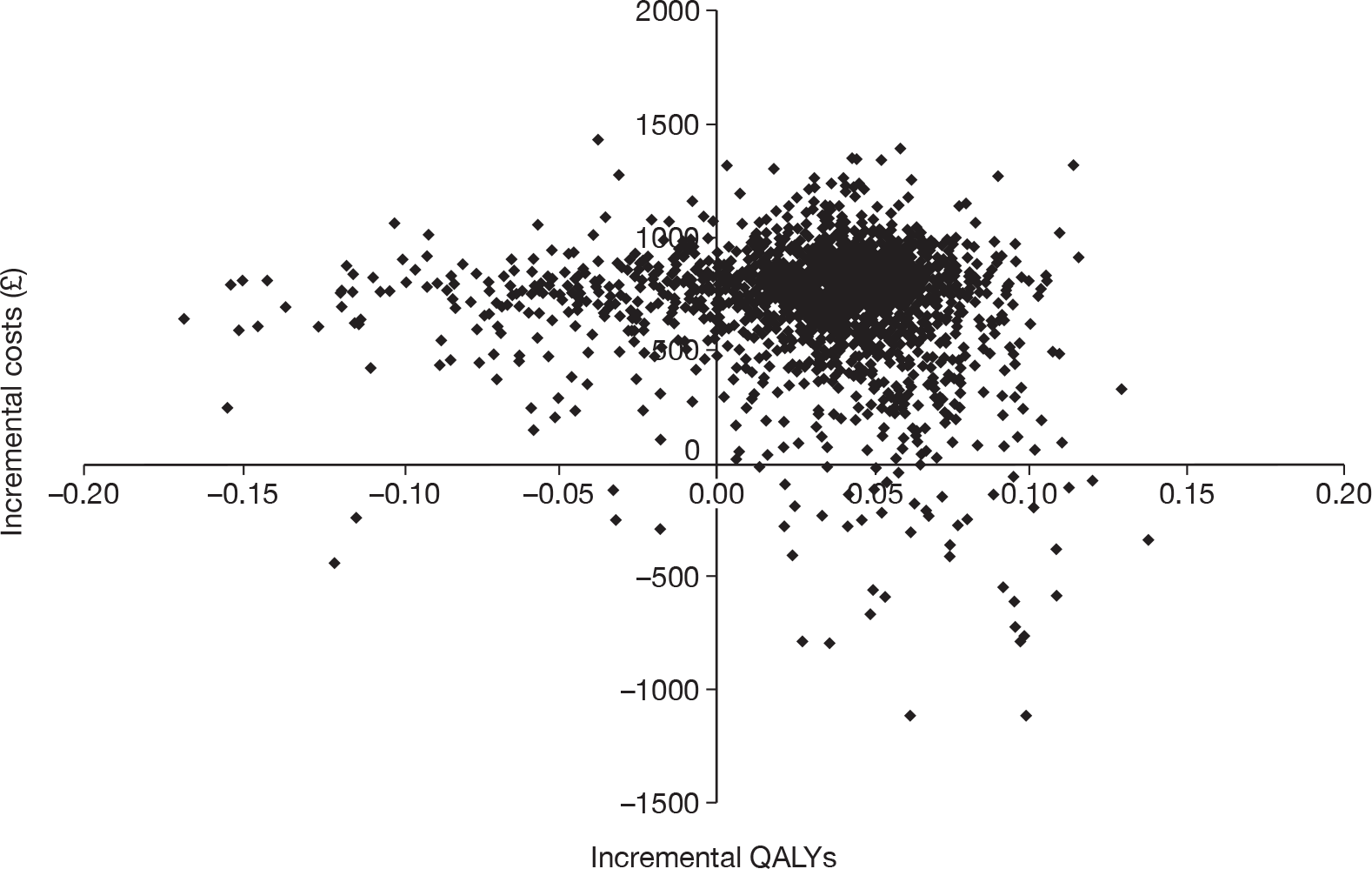
The CEAC (Figure 20) illustrates the uncertainty in the cost-effectiveness estimate for recurrent rectal cancer. The figure shows that, at a monetary threshold of < £20,000 per QALY, there was a greater probability that the conventional CT and MRI strategy was the most cost-effective, but at a monetary threshold of > £20,000 per QALY the add-on FDG PET/CT strategy had a greater probability of being the most cost-effective. At the £30,000 per QALY threshold recommended by the National Institute for Health and Clinical Excellence (NICE) the CEAC indicated an approximately 70% probability that FDG PET/CT would have been cost-effective in comparison with the conventional strategies.
FIGURE 20.
The CEAC for recurrent rectal cancer.
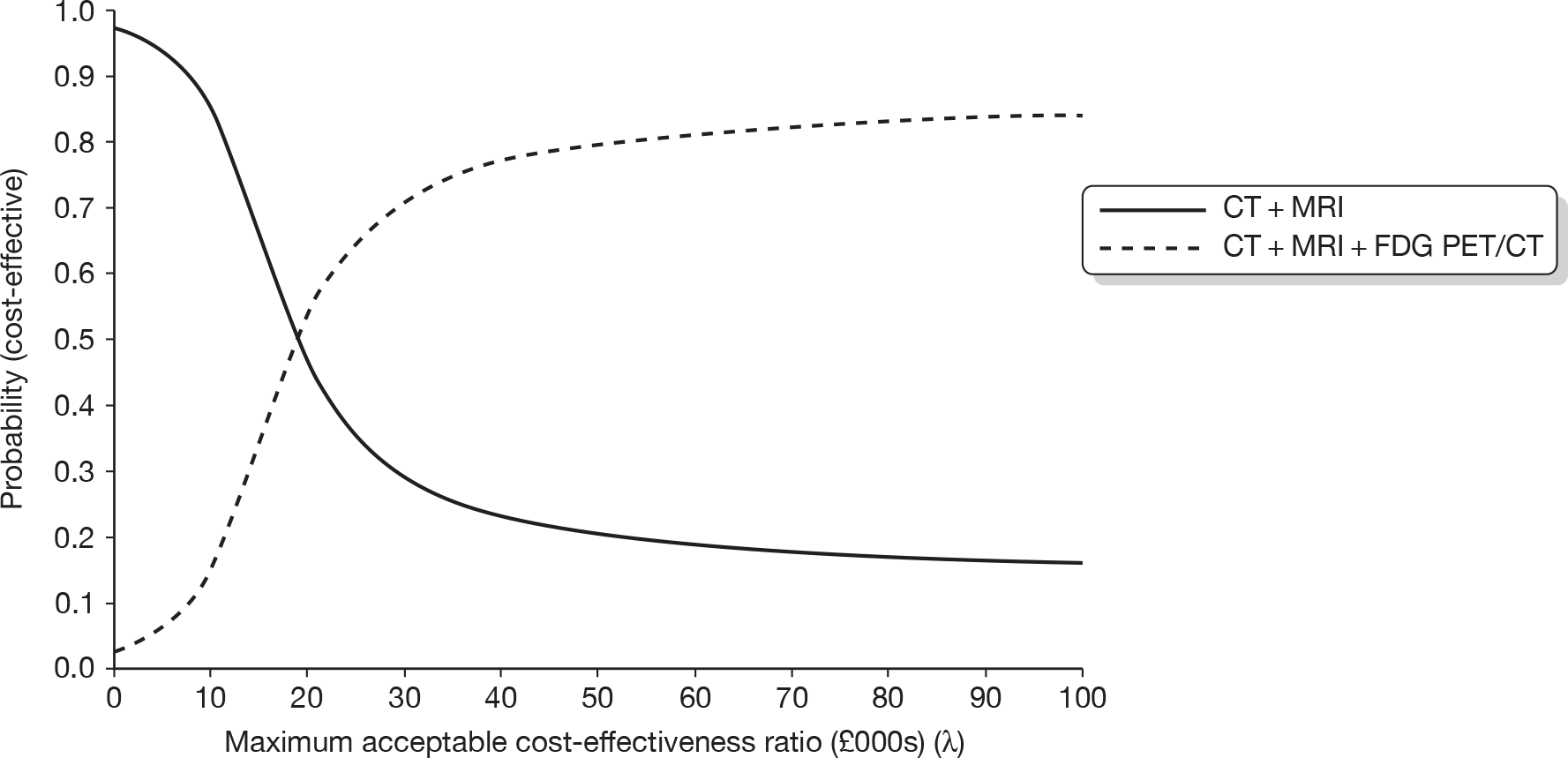
The EVPI results show that it is potentially worthwhile to collect more information about the use of FDG PET/CT for recurrent rectal cancer; at a willingness-to-pay threshold of £30,000 per QALY, the EVPI per decision is £316. To determine the overall population value of EVPI we assumed an annual incidence of 9054 cases (derived from the annual incidence of rectal cancer,156 assuming 70% recurrence123 and a death rate of 2.8% prior to recurrence diagnosis) and a time frame of 2 years (i.e. FDG PET/CT in its current form would be considered for imaging for 2 years). As noted above, this time frame was determined in part by the continual development and upgrading of FDG PET/CT, such that the estimates for diagnostic test accuracy would be likely to change outside this time frame. Figure 21 details the results from the EVPI analysis at a population level. At a willingness-to-pay threshold of £30,000 per QALY, the EVPI for the population was approximately £5.6M; thus it is potentially worthwhile seeking additional information for FDG PET/CT for recurrent rectal cancer.
FIGURE 21.
The EVPI for recurrent rectal cancer.
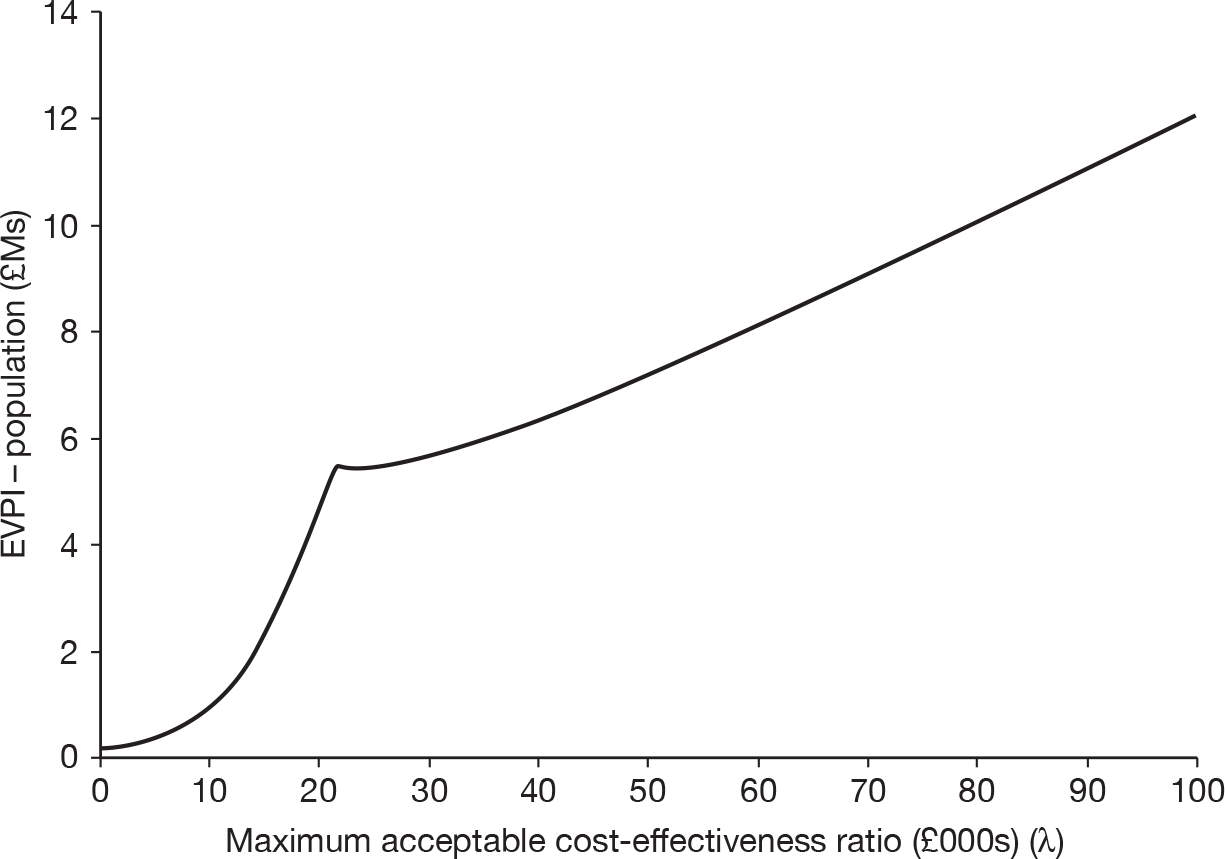
Recurrent colon cancer model
Table 29 details the expected costs of the imaging involved in the conventional and the intervention test strategies, the expected probability of a correct diagnosis under each strategy and the cost-effectiveness in terms of cost per correct diagnosis for recurrent colon cancer. On this basis, the addition of FDG PET/CT involved an additional cost of approximately £3000 per correct diagnosis and would be considered cost-effective compared with the conventional strategy under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY).
| Diagnostic tool | Mean cost per scan (£) | Probability of correct diagnosis |
|---|---|---|
| CT | 143 | 0.67 |
| CT + FDG PET/CT | 943 | 0.95 |
| Difference | 800 | 0.28 |
| ICER | 2857 |
Table 30 details the expected costs of the imaging and treatment associated with the conventional and the intervention test strategies, the expected outcomes in terms of QALYs under each strategy and the cost-effectiveness in terms of cost per QALY gain for recurrent colon cancer. On this basis, the addition of FDG PET/CT to the conventional strategy involved an additional cost of approximately £6000 per QALY gained and would be considered cost-effective under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| CT | 6677 | 4.44 |
| CT + FDG PET/CT | 7543 | 4.58 |
| Difference (95% CI) | 866 (562 to 1170) | 0.14 (–0.08 to 0.36) |
| ICER | 6189 |
Figure 22 illustrates the uncertainty surrounding the expected incremental cost and incremental QALY results for recurrent colon cancer. The figure shows that there was considerable uncertainty about the extent, but not the existence, of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 22.
The cost-effectiveness plane for FDG PET/CT in recurrent colon cancer.
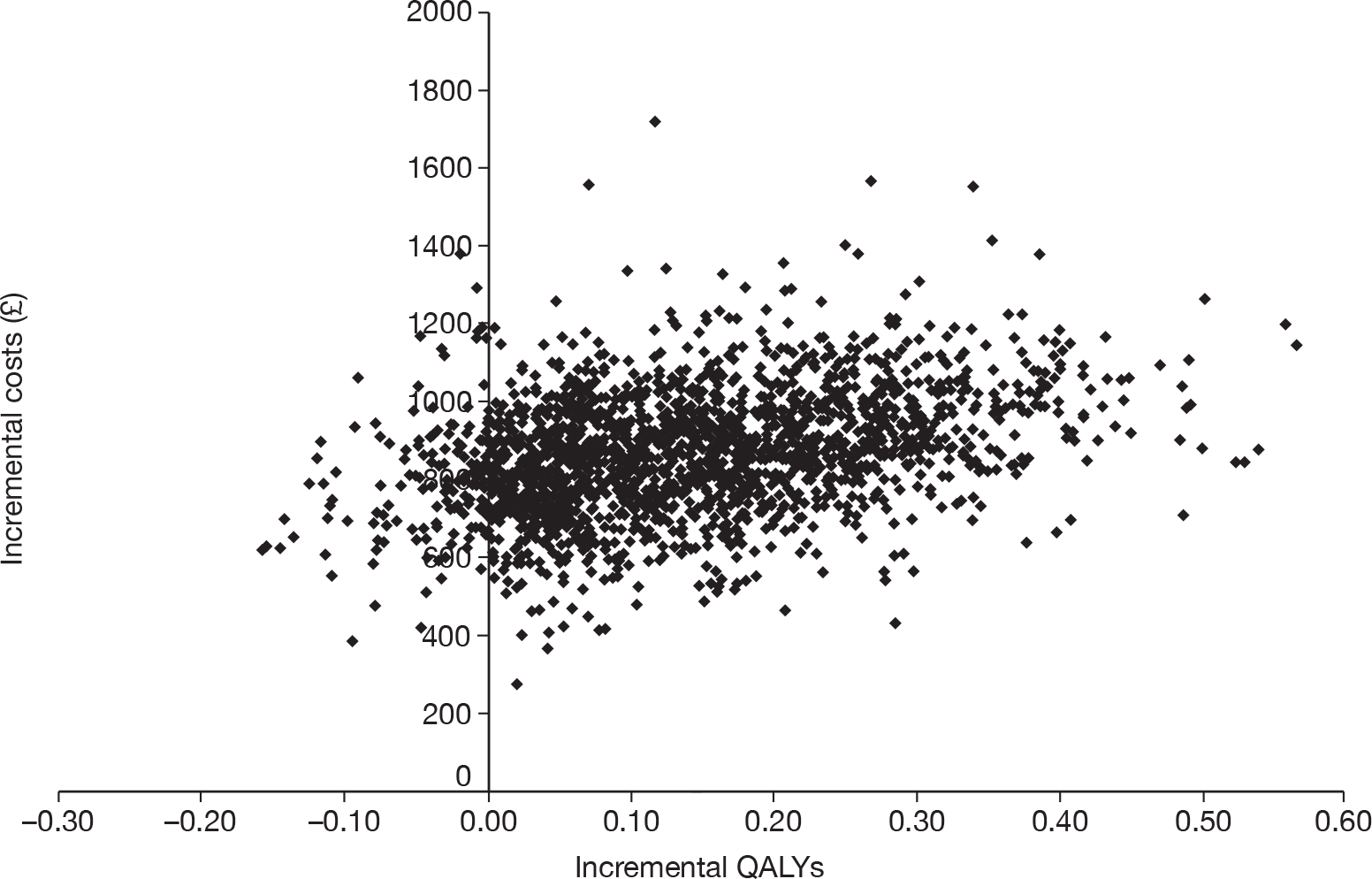
The CEAC (Figure 23) illustrates the uncertainty in the cost-effectiveness estimate for recurrent colon cancer. The figure shows that, at a monetary threshold > £6000 per QALY, the FDG PET/CT strategy had the greatest probability of being cost-effective. At the £30,000 per QALY threshold recommended by NICE, the CEAC indicated an approximately 85% probability that FDG PET/CT would be cost-effective in comparison with the conventional strategies.
FIGURE 23.
The CEAC for recurrent colon cancer.
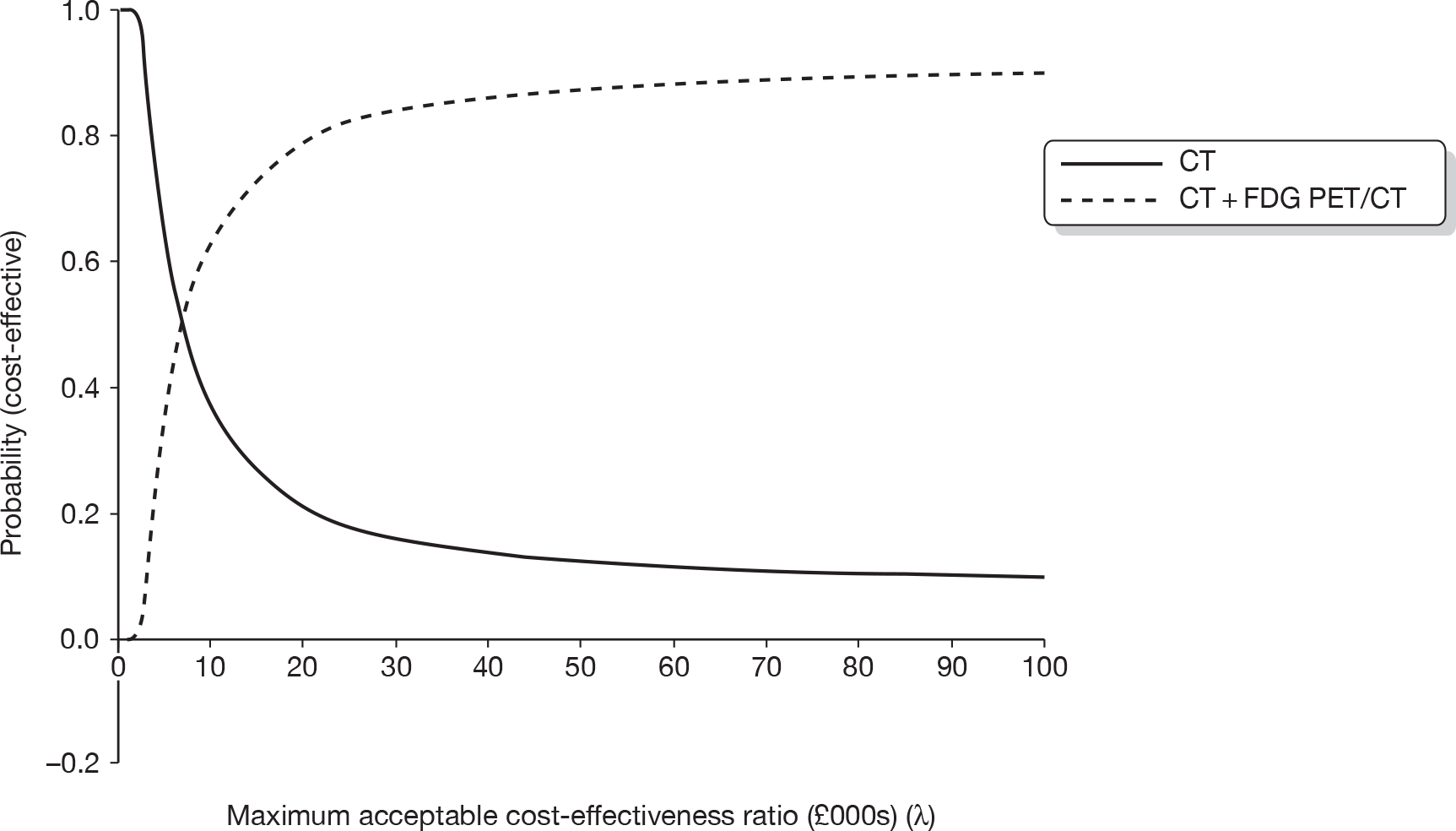
The EVPI results show that it would be potentially worthwhile collecting more information about the use of FDG PET/CT for recurrent rectal cancer; at a willingness-to-pay threshold of £30,000 per QALY, the EVPI per decision is £178. To determine the overall population value of the EVPI we assumed an annual incidence of 14,670 cases (derived from the annual incidence of colon cancer,156 assuming 70% recurrence123 and a death rate of 2.8% prior to recurrence diagnosis) and a time frame of 2 years (i.e. FDG PET/CT in its current form would be considered for imaging for 2 years). As noted above, this time frame was determined in part by the continual development and upgrading of FDG PET/CT, such that the estimates for diagnostic test accuracy are likely to change outside this time frame. Figure 24 details the results from the EVPI analysis at a population level. At a willingness-to-pay threshold of £30,000 per QALY, the EVPI for the population was approximately £5.1M; thus, it would be potentially worthwhile seeking additional information for FDG PET/CT for recurrent colon cancer.
FIGURE 24.
The EVPI for recurrent colon cancer.
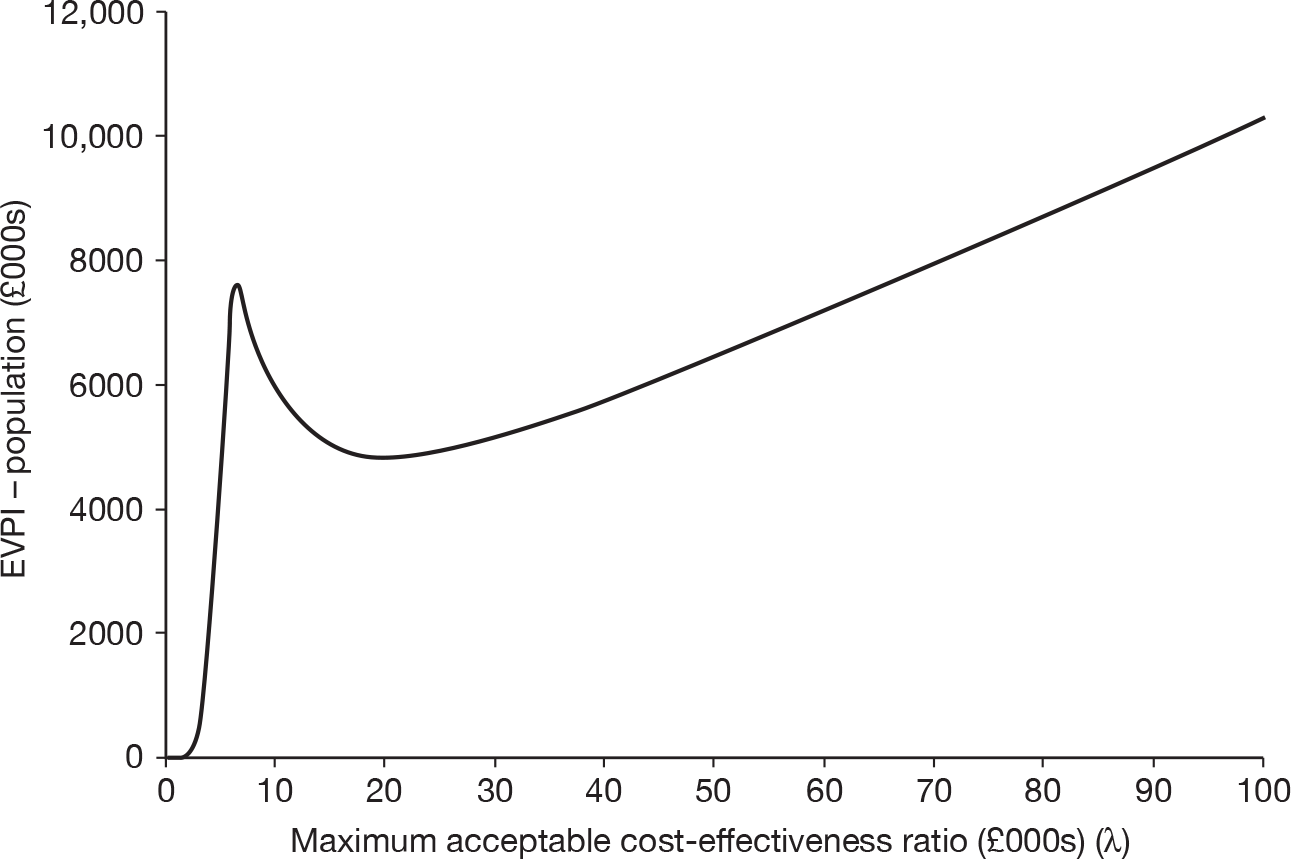
Metastatic cancer model
Table 31 details the expected costs of the imaging involved in the conventional and the intervention test strategies, the expected probability of a correct diagnosis under each strategy and the cost-effectiveness in terms of cost per correct diagnosis for metastatic CRC. On this basis, the addition of FDG PET/CT involved an additional cost of approximately £19,000 per correct diagnosis and would be considered cost-effective compared with the conventional strategy under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY).
| Diagnostic tool | Mean cost per scan (£) | Probability of correct diagnosis |
|---|---|---|
| CT | 143 | 0.78 |
| CT + FDG PET/CT | 943 | 0.83 |
| Difference | 800 | 0.04 |
| ICER | 19,048 |
Table 32 details the expected costs of the imaging and treatment associated with the conventional and the intervention test strategies, the expected outcomes in terms of QALYs under each strategy and the cost-effectiveness in terms of cost per QALY gain for metastatic CRC. On this basis, the addition of FDG PET/CT to the conventional strategy involved an additional cost of approximately £21,000 per QALY gained and would be considered cost-effective under the usual definition (£20,000 per QALY < ICER < £30,000 per QALY). 150
| Diagnostic tool | Cost per person (£) | QALY gain |
|---|---|---|
| CT | 10,184 | 7.48 |
| CT + FDG PET/CT | 10,460 | 7.49 |
| Difference (95% CI) | 276 (–4384 to 4937) | 0.01 (–0.08 to 0.10) |
| ICER | 21,434 |
Figure 25 illustrates the uncertainty surrounding the expected incremental cost and incremental QALY results for metastatic CRC. The figure shows that there was considerable uncertainty about the existence and extent of the additional expected costs (shown in the vertical plane) and the existence and extent of the additional expected QALYs (shown in the horizontal plane).
FIGURE 25.
The cost-effectiveness plane for FDG PET/CT in metastatic CRC.
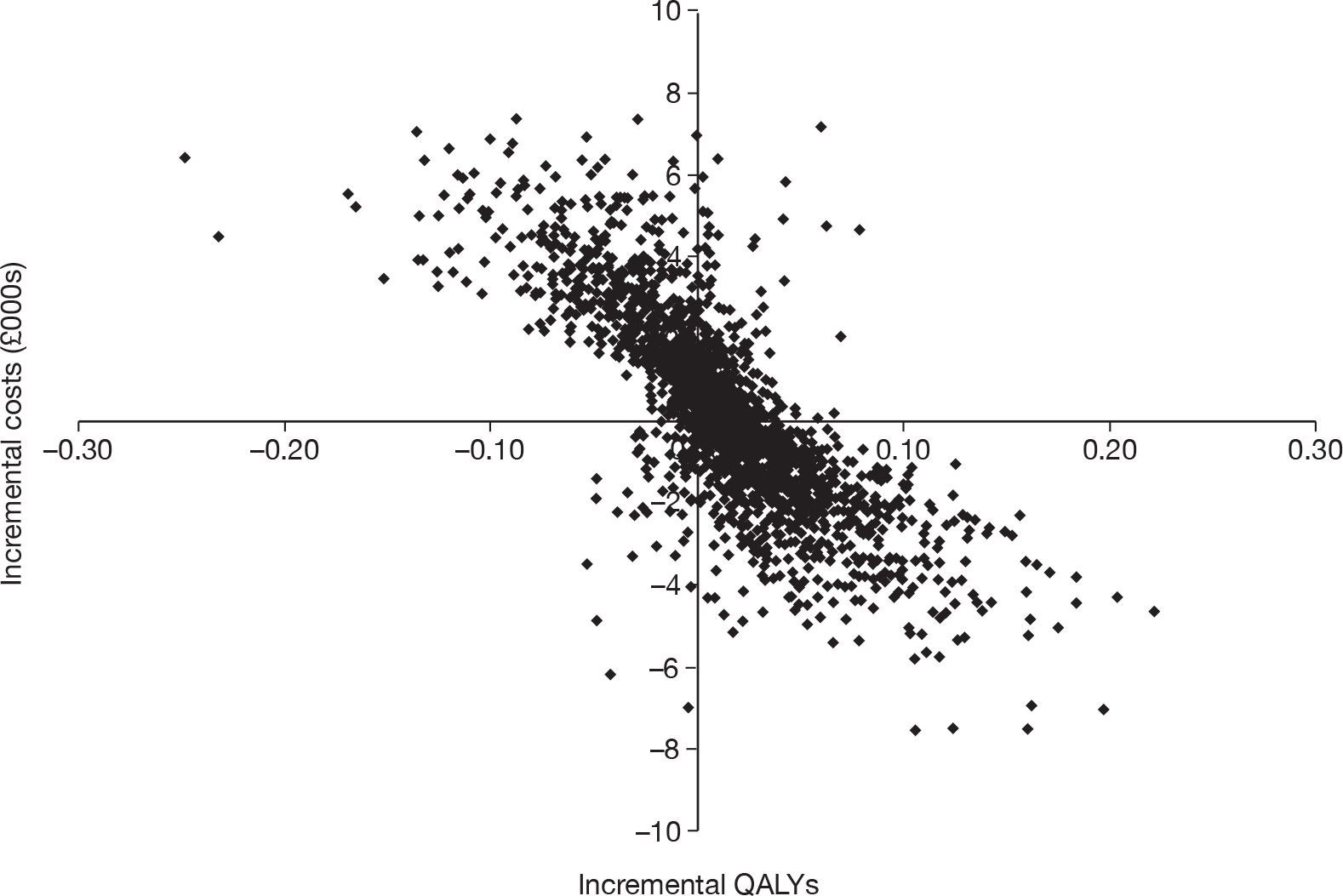
The CEAC (Figure 26) illustrates the uncertainty in the cost-effectiveness estimate for metastatic CRC. The figure shows that there was considerable uncertainty surrounding the cost-effectiveness of the FDG PET/CT strategy. At a monetary threshold of £21,000 per QALY, the probability that the FDG PET/CT intervention would be cost-effective was approximately 50%, as was the probability that CT would be cost-effective. At the £30,000 per QALY threshold recommended by NICE, the CEAC indicated that the FDG PET/CT intervention had a slightly greater probability of being cost-effective (52%).
FIGURE 26.
The CEAC for metastatic CRC.
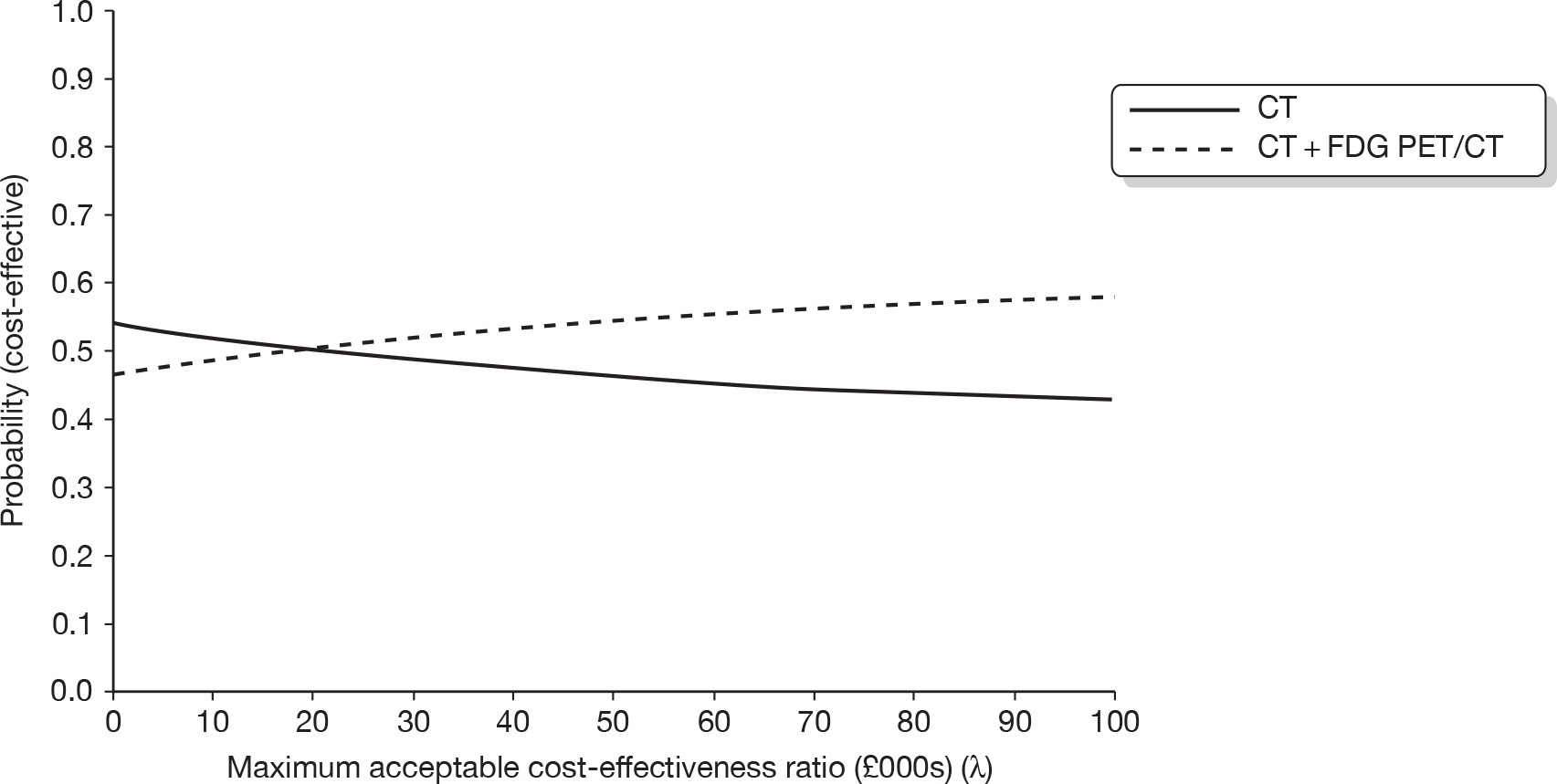
The EVPI results show that it is potentially worthwhile collecting more information about the use of FDG PET/CT for metastatic CRC. At a willingness-to-pay threshold of £30,000 per QALY, the EVPI per decision is £1328. To determine the overall population value of EVPI we assumed an annual incidence of 4000 cases (derived from the annual incidence of colon and rectal cancers,156 assuming a 70% likelihood of recurrence and a further 40% likelihood that the recurrence would be metastatic,123 and a death rate of 59% prior to metastatic diagnosis) and a time frame of 2 years (i.e. FDG PET/CT in its current form will be considered for imaging for 2 years). As noted above, this time frame was determined in part by the continual development and upgrading of FDG PET/CT, such that the estimates for diagnostic test accuracy are likely to change outside this time frame. Figure 27 details the results from the EVPI analysis for metastatic CRC at a population level. At a willingness-to-pay threshold of £30,000 per QALY, the EVPI for the population was approximately £10.5M; thus it is potentially worthwhile seeking additional information for FDG PET/CT for metastatic CRC.
FIGURE 27.
The EVPI for metastatic CRC.
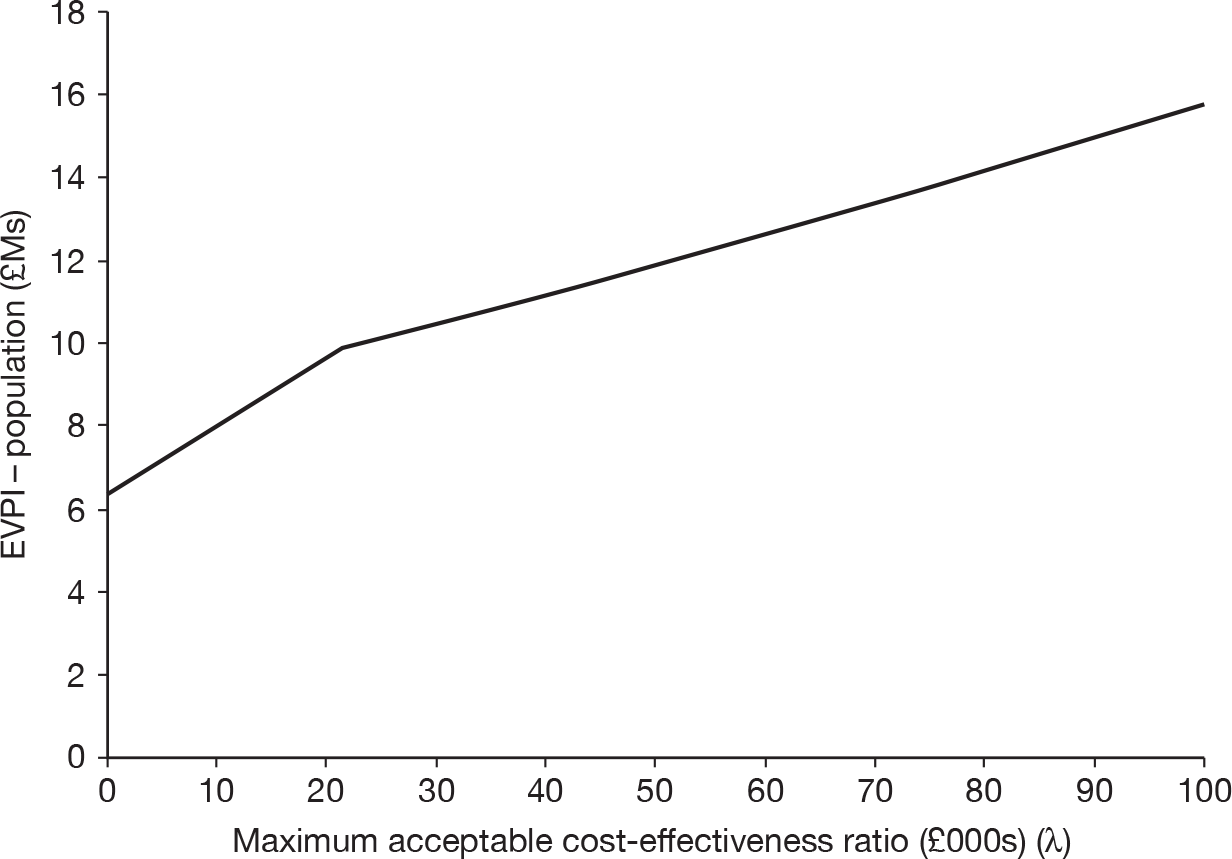
Discussion of the economic modelling
Primary colorectal cancer
To our knowledge, this is the first published evidence assessing the cost-effectiveness of FDG PET/CT as an add-on device for staging primary rectal and colon cancer.
Considering the cost per correct diagnosis outcomes, it is apparent that the diagnostic test accuracy estimates used in the models favour the conventional imaging modalities. This is a result of the use of FDG PET/CT as an add-on imaging device. In the primary model, FDG PET/CT is influential as an add-on after the conventional test; therefore, the model assumes any outcome with a positive test will be treated as such. Negative results from the conventional imaging tests that are refuted by the FDG PET/CT test are treated as positive. Results are treated as negative only when both the conventional and the FDG PET/CT test outcomes are negative. This results in an overall larger number of positive outcomes (both TPs and FPs) and a reduction in negative outcomes (fewer TNs identified) in the intervention strategy. Therefore, in both the rectal and colon primary models, the total proportion of correct diagnoses is greater using the conventional strategies, with no addition of FDG PET/CT. Adopting an alternative strategy in which an FDG PET/CT scan is undertaken only if the conventional tests identify negative outcomes may be a more cost-effective strategy. The model outcomes would be the same as in the strategy adopted in our analysis; however, the cost of an FDG PET/CT scan (which is approximately four times that of the conventional scans) would be incurred only when conventional imaging results are negative, reducing the overall cost of the strategy.
The longer-term analyses for the primary models showed that the low specificity of FDG PET/CT scans results in a greater number of FP outcomes, in which patients are overstaged and incur additional costs and suffer quality of life impacts for unnecessary treatments. This is reflected in the extremely high ICER outcomes in the primary models. Further, the therapeutic impact literature discussed earlier in this report27,49,89–93,102,109 found that, although FDG PET/CT may potentially affect accurate staging of primary CRC, it had only a minor impact in changing patient management. Both the rectal and colon primary models identified an incremental QALY gain of only 0.01, indicating that FDG PET/CT as an add-on imaging device in primary CRC does not have any overall impact on patient outcomes.
The cost per QALY results were extremely high for both the primary rectal and colon evaluations: > £400,000 and > £170,000, respectively, per QALY. As such, FDG PET/CT is not cost-effective in either primary rectal or primary colon cancer given the NICE recommended QALY threshold of £20,000–30,000 per QALY.
The cost-effectiveness plane shows that there is uncertainty surrounding both the incremental costs and incremental effects associated with FDG PET/CT; however, the CEACs show that this uncertainty does not translate into uncertainty about the cost-effectiveness of FDG PET/CT. For both primary rectal and colon cancer, the probability that FDG PET/CT as an add-on imaging device for staging is cost-effective is zero given the NICE recommended QALY threshold range of £20,000–30,000 per QALY. This translates into very small values for the EVPI. The results therefore show that the use of FDG PET/CT as an add-on imaging device for staging primary CRCs is not cost-effective and that there is no value associated with the collection of further information.
Earlier findings from the systematic review indicated that, as FDG PET/CT technology develops, there will be an increased potential in the future for this improved technology to be used as a lone device, replacing contrast-enhanced CT, as opposed to being utilised as an add-on imaging device. In primary rectal cancer, contrast-enhanced FDG PET/CT could potentially replace contrast-enhanced CT with the addition of an MRI scan, and in primary colon cancer, contrast-enhanced FDG PET/CT could be used alone as a replacement for contrast-enhanced CT. The two scenario analyses undertaken to explore this in primary CRC indicated that such an improved contrast-enhanced FDG PET/CT device is unlikely to be cost-effective for use in primary rectal cancer, but is likely to be very cost-effective for use in colon cancer. In primary rectal cancer, improved FDG PET/CT technology will not negate the necessity for an MRI scan, and therefore the potential incremental value of improved contrast-enhanced FDG PET/CT is limited by the strong diagnostic test accuracy achievable with MRI scanning. The colon cancer scenario analysis indicates substantial improvement in diagnostic test accuracy from contrast-enhanced FDG PET/CT compared with contrast-enhanced CT and improved efficiency through eliminating the need for an add-on test, thereby giving a highly cost-effective outcome. There remains considerable uncertainty in both these outcomes, which is highlighted in the value of information analyses, indicating potential value in further research with a population EVPI of £1.7M for the primary rectal population, and a value of £12.3M for the primary colon population. It must be noted that EVPI analysis can provide only an indication of potential worth for further information as any research undertaken will only reduce, rather than eliminate, uncertainty.
Recurrent colorectal cancer
The recurrent cancer models found FDG PET/CT as an add-on imaging device to have an ICER of £21,409 for rectal cancer and £6189 for colon cancer. Considering the NICE monetary threshold of £20,000–30,000 per QALY, these can be considered to be cost-effective.
The ICER for the recurrent colon cancer model is considerably lower than that for the recurrent rectal cancer model, indicating that FDG PET/CT is more cost-effective in the assessment of colon recurrence than in rectal recurrence. This difference is likely to be due to the sensitivity estimate for the CT diagnostic test parameter, which is considerably lower than the FDG PET/CT sensitivity estimate. The wide difference favours the accuracy of FDG PET/CT, and even though uncertainty around both these estimates was incorporated into the model, the strong influence of the choice of diagnostic test accuracy parameters on model outcomes is evident. The FDG PET/CT intervention does not have the same diagnostic test accuracy sensitivity advantage in the recurrent rectal model, as the MRI scan diagnostic test accuracy estimates are also incorporated. The MRI diagnostic test accuracy was superior to that of CT; therefore, in the recurrent rectal model, the conventional imaging diagnostic test accuracy estimates are closer to those of FDG PET/CT, limiting the incremental value of FDG PET/CT.
Meta-analyses were undertaken using relevant papers identified from the systematic review to elicit pooled diagnostic test accuracy estimates of FDG PET/CT for recurrent CRC. Because of inadequacies and reporting bias in the identified papers, the pooled estimates for FDG PET/CT were considered to be an inaccurate reflection of the diagnostic test accuracy of FDG PET/CT, and the CIs were tight around the pooled means, which was considered to be restrictive in terms of capturing a wide range of uncertainty. Therefore, expert judgement was used to determine point estimates and wide uncertainty intervals from the literature.
Most previous economic evaluations undertaken for recurrent CRC have been specifically interested in hepatic metastases. Two papers were identified that were interested in assessing recurrence. Sloka et al. 110 undertook a cost-effectiveness analysis of FDG PET/CT in comparison with CT for diagnosing colorectal recurrence, and the Medical Services Advisory Committee58 undertook a cost–consequence analysis of PET versus no PET for diagnosing local recurrence. Our models add to this literature, providing an assessment of the cost-effectiveness of FDG PET/CT as an add-on imaging device for diagnosing both recurrent rectal and recurrent colon cancer.
Sloka et al. 110 report cost savings with the FDG PET/CT approach through avoidance of unnecessary surgeries. The paper does not report the number of unnecessary surgeries avoided in each strategy, only the cost savings. After considering the parameter estimates used in their model, it can be seen that the diagnostic test accuracy estimates assigned to FDG PET/CT are superior to those in the CT comparator arm by a wide margin, so it is no surprise that the FDG PET/CT intervention was found to dominate CT. Our recurrent colon model assigned the same specificity values to contrast-enhanced CT and FDG PET/CT, and therefore there was no difference in unnecessary surgeries in our outcomes; however, our recurrent rectal model did indicate reductions in unnecessary surgeries with the FDG PET/CT intervention. The Medical Services Advisory Committee publication58 also reports cost savings through the use of PET in comparison with a no PET strategy; however, few details are provided as to what the no PET strategy entails.
In comparison with other economic evaluations undertaken in this disease area, our models appear to have adopted a more conservative approach in assigning diagnostic test accuracy estimates and through incorporating quality of life impacts and overall survival impacts in a cost per QALY outcome. This conservative approach attempted to minimise bias in the models to avoid unfairly favouring the intervention arm (add-on FDG PET/CT).
At a cost per QALY threshold of £30,000, the probability that the FDG PET/CT intervention will be cost-effective for recurrent colon cancer is 85%; this is lower (70%) for recurrent rectal cancer. This greater level of uncertainty in the recurrent models leads to a non-zero value for the EVPI (at a population level the EVPI is £5.6M for recurrent rectal cancer and £5.1M for recurrent colon cancer). At these levels there is potential worth in collecting further information to inform the decision regarding the use of FDG PET/CT in the future. It must be noted that EVPI analysis can provide only an indication of potential worth for further information, as any research undertaken will reduce rather than eliminate uncertainty.
Metastatic colorectal cancer
The metastatic model found FDG PET/CT as an add-on device to have an ICER of £21,434 per QALY gained. This ICER value is within the NICE monetary threshold range of £20,000–30,000 per QALY for determining cost-effectiveness.
Most of the existing publications that have undertaken economic evaluations of PET for CRC have been specifically interested in hepatic metastases. Park et al. 109 developed a decision model to determine the cost-effectiveness of PET and CT imaging in comparison with CT alone. They evaluated outcomes in terms of life-year gains and report an incremental cost per life-year gained of US$16,437. This paper is the most similar to our model, but does not incorporate quality of life impacts.
Other economic evaluations in metastatic CRC have been undertaken. Lejeune et al. 51 report cost savings of €2671 (US$3213) with no change in life expectancy when FDG PET/CT was compared with CT in staging metastatic CRC. Zubeldia et al. 111 assessed the cost-effectiveness of FDG PET/CT in comparison with CT for identifying the presence of extrahepatic metastases. They report a cost saving of US$5269 as a result of unnecessary surgeries avoided; however, they provide few details of how their model was constructed. Details were not provided of the diagnostic test accuracy estimates used in the model or how the impact on patient management was incorporated. None of these metastatic models used probabilistic analysis to incorporate uncertainty for each of the model parameters.
The CEAC for the metastatic model reflects uncertainty towards the cost-effectiveness of FDG PET/CT as an add-on strategy. The CEAC illustrates that beyond a threshold of £21,000 per QALY FDG PET/CT has a greater probability of being cost-effective than CT, although there is considerable uncertainty, with the probability that FDG PET/CT is cost-effective ranging between 40% and 60%. This level of uncertainty leads to an EVPI of £10.5M for the population. Thus, it is potentially worthwhile collecting further information to inform the decision regarding FDG PET/CT in the future. It must be noted that EVPI analysis can provide only an indication of potential worth for further information, as any research undertaken will reduce rather than eliminate uncertainty.
Future research
There is potential value in undertaking further research into the use of FDG PET/CT for:
-
staging recurrent colon cancer.
-
staging recurrent rectal cancer.
-
staging metastatic CRC.
There is the potential for contrast-enhanced PET/CT technology to be used as a replacement for contrast-enhanced CT in primary CRC, if and when this technology becomes available. Further research in this area is likely to be worthwhile, particularly for use in investigating primary colon cancer.
Chapter 11 Conclusions
Integrated FDG PET/CT equipment is the latest imaging technology to be used for the pre-operative staging of CRC, and these are the first systematic reviews of the diagnostic accuracy, therapeutic impact and cost-effectiveness of the technology in primary, recurrent and metastatic disease.
For staging primary CRC, the review found limited accuracy data to support the use of FDG PET/CT or contrast-enhanced FDG PET/CT, but FDG PET/CT was shown to identify nodal disease remote from the primary site. The studies used a reference standard likely to classify primary CRC, but there is a lack of clarity about the study populations. Although there may be a future role for FDG PET/CT in the pre-operative staging of primary rectal cancer, the lack of evaluations comparing its accuracy with other tests makes it difficult to place a value on its use in primary CRC. Consequently, there is little upon which to base a recommendation for the use of FDG PET/CT in the routine staging of all patients diagnosed with CRC.
The review of the use of FDG PET/CT for the detection of recurrent disease identified data from five retrospective studies from which a pooled sensitivity of 91% (95% CI 87% to 95%) and a pooled specificity of 91% (95% CI 0.85% to 95%) were observed. 61–65
There is a widely assumed benefit of increased accuracy from ‘new’ integrated FDG PET/CT scanners,21 and three studies63,64,68 that compared FDG PET/CT with FDG PET alone reported FDG PET/CT to be more accurate than FDG PET alone in staging recurrent CRC, but only one reported a statistically significant difference between data from these two tests. However, FDG PET/CT was also reported to be less accurate than multidetector CT, and of equivalent accuracy to MRI, in the detection of recurrent CRC. Although a comparison of FDG PET/CT with CEA estimation suggests that FDG PET/CT possesses greater accuracy, this is not a clinically valid comparison. We were unable to perform significance tests for any of these comparisons because cross-tabulation of results from different tests for patients contributing to the same study were not presented.
The largest yield of studies identified by our search activities related to evaluations of FDG PET/CT in the pre-operative staging of metastatic CRC, the literature reflecting its widespread use in staging advanced disease. The pooled accuracy data showed FDG PET/CT to have a sensitivity of 91% (95% CI 87% to 94%) and a specificity of 76% (95% CI 58% to 88%), but the poor quality of the studies means that the validity of these estimates is threatened by several biases, and once again the lack of paired data prevented statistical tests from eliminating chance findings.
The accuracy estimates from a single prospectively planned study that reported a consecutive series of patients with suspected metastatic disease and the use of a reference standard likely to correctly classify the disease found FDG PET/CT to have a sensitivity of 90% and a specificity of 90% in the detection of liver metastases. This was compared with contrast-enhanced CT, which showed a sensitivity of 95% and a specificity of 70%, but these observed differences were not reported to be statistically significantly different.
We found that the published research evaluating FDG PET/CT for primary and recurrent CRC did not reflect routine UK clinical practice at this time. Although FDG PET/CT is generally used as an add-on test in UK colorectal radiological practice, the accuracy studies included in the review compared FDG PET/CT with other imaging tests and considered its value as a replacement test. 88 This discord between UK routine staging practice and the test comparisons within the included studies was reinforced when none of the studies identified by our search strategy met our protocol objectives for primary or recurrent CRC staging. By deviating from the protocol and including all studies that aimed to assess the value of FDG PET/CT in staging CRC, regardless of the comparison tests, we have been able to consider the totality of evidence for the value of FDG PET/CT in staging CRC, and it is likely that this systematic review reflects a global variation in CRC staging practice.
The quality assessment of the included studies using the QUADAS criteria found them to be of generally poor quality and the data they report are highly susceptible to bias. A major threat to the validity of these conclusions arises from the variation in the types of reference standard used (differential verification bias), which undermines the estimates of the accuracy of FDG PET/CT. Only 9 of the 23 diagnostic test accuracy studies used reference standards that were likely to correctly classify the target condition. 37
In studies in which a composite reference standard was used (a surgically resected specimen, biopsy or follow-up), it was often unclear if this was applied to the whole sample of patients. In some studies it was reported that the reference standard was not a composite but verification was by any method available. The data from the included studies are also likely to be affected by partial verification bias, disease progression and review bias, all of which may have resulted in under- or overestimates of accuracy. 37
The patient populations recruited into the studies were not well described. The absence of FN or FP test results suggests that many of the study populations were a selected group who may not represent the wider CRC population. This may be a result of the study designs; studies that met the review eligibility criteria were mostly retrospective case series in which cases were obtained from hospital files. Finally, reporting bias was evident in some studies when only lesion-level data were presented and patient-level estimates were not.
Diagnostic accuracy data were often not reported in sufficient detail for statistical analysis, for example cross-tabulation of results of different tests for patients contributing to the same study were unavailable in all except one study. 70 This meant that significance testing for differences between sensitivity and specificity was not carried out and there remains clinical uncertainty about the diagnostic accuracy of FDG PET/CT relative to other tests.
In both meta-analyses a random effects method was used for the specificity estimates because of evidence of heterogeneity. However, in both cases a single estimate based on only four patients was largely responsible for the heterogeneity. 61,85 It is therefore pertinent to consider if these two studies differ in some way from the others, and if they should be removed from the meta-analysis. However, all the studies differed from one another in both clinical and methodological aspects, and also the fixed-effects meta-analyses of the same data produced very similar results to those presented in the random effects meta-analyses. Random effects meta-analyses are more conservative than fixed-effects meta-analyses. Heterogeneity may have arisen from the use of contrast agents (both oral and intravenous), differences in patient populations and different doses of FDG used in the scanning procedures.
The scanning procedures were well described and revealed quite marked differences in the amount of FDG administered (197–740 MBq). This could certainly explain differences in the accuracy of FDG PET/CT. The variation in the manner in which the FDG PET/CT scans were interpreted might also be associated with inaccurate estimates of the presence or absence of disease. However, meta-regression was not used to explore these possible relationships with diagnostic accuracy as there were multiple sources of heterogeneity, and many more studies than were found would be needed to make the results of any such analysis reliable.
The evidence that FDG PET/CT has a therapeutic impact on clinical practice is inconsistent. Some studies found no effect and others reported decreased morbidity from improved surgical techniques arising from increased precision in tissue identification. Studies reported inconsistent findings about the effect that FDG PET/CT had on surgical management. Critical to the apparent therapeutic impact of FDG PET/CT is the assiduousness of the search for metastatic disease and the accuracy of conventional staging modalities. Thus, MRI provides additional accuracy to liver contrast-enhanced CT in the assessment of suitability for hepatic resection. In some studies, FDG PET/CT was compared directly with CT alone, and so this is one example of a falsely enhanced apparent therapeutic impact for FDG PET/CT.
In studies that reported changes in surgical management as a result of FDG PET/CT assessment, upstaging and abandonment of surgery were more frequently reported than downstaging, i.e. more operations were avoided through FDG PET/CT assessment because patients were excluded from further consideration in the surgical pathway (e.g. hepatectomy abandoned after the identification of multiple liver metastases or extrahepatic metastases with FDG PET/CT). However, it was reported that some palliative operations still went ahead under these circumstances. The studies did not report the effect on patient outcomes or whether or not the changes in management were found to be correct, and, with the exception of one study, deaths were not reported. Little is therefore known about the effects of FDG PET/CT and the long-term outcomes for those who receive an FDG PET/CT scan to stage their disease pre-operatively.
As the systematic reviews found only a small amount of evidence to support the use of FDG PET/CT in the pre-operative staging of primary, recurrent and metastatic CRC and the data are generally divergent and the quality of research poor, the economic models were designed, developed and populated based on a variety of information sources including published data sources and literature, and in consultation with clinical experts.
None of our models reported cost savings, as has been seen in previous economic evaluations51,58,110 assessing the value of PET or FDG PET/CT in recurrent and metastatic CRC; however, the approach we adopted was conservative, in order to determine more reliable results given the lack of current information.
The economic evaluations reveal that, given the high degree of uncertainty in the models and results, FDG PET/CT as an add-on imaging device is cost-effective in recurrent colon, recurrent rectal and metastatic colorectal disease. There is value in undertaking further research in these disease areas. The evaluations also found that add-on FDG PET/CT is not cost-effective in primary colon or rectal cancer and further research is not worthwhile. However, the results of a scenario analysis suggest that future developments in FDG PET/CT technology to enhance the CT element, making it equivalent to standard CT, might make FDG PET/CT cost-effective as a replacement rather than as an add-on imaging device in primary colon cancer, although not in primary rectal cancer. Under this scenario further research was potentially cost-effective for both primary rectal and colon cancer.
Limitations
A potential limitation of the primary CRC economic model structure is that it does not allow for inaccuracies in staging between the AJCC 1 and 2 stages or between the AJCC 3 and 4 stages to be incorporated. This is because the model is based on the identification of nodal involvement. The diagnostic test accuracy evidence used in the model specifies only whether or not nodes are involved; therefore, the impact of under- and overstaging between AJCC stages with no nodal involvement and between AJCC stages with nodal involvement is not incorporated.
The primary limitations in the economic evaluations were due to uncertainty and the lack of available evidence from the systematic reviews for key parameters in each of the five models. To address this, a conservative approach was adopted in choosing diagnostic test accuracy estimates for the model parameters. Probabilistic analyses were undertaken for each of the models, incorporating wide levels of uncertainty, particularly for the diagnostic test accuracy estimates.
Recommendations for clinical practice
There is uncertainty about the value of using FDG PET/CT in CRC clinical practice, and those practitioners who access this imaging technology should routinely collect data to enable audits of patient outcomes, including detection rate and any changes in management resulting from its use.
Recommendations for research
Given the paucity of data and the observational nature of those which do exist, there would be value in undertaking an RCT with a concurrent economic evaluation in order to evaluate the therapeutic impact and cost-effectiveness of FDG PET/CT compared with conventional imaging (without PET) for the pre-operative staging of recurrent and metastatic CRC. The clinical end points should include inclusion/avoidance of operative intervention, inclusion/avoidance of chemotherapy or radiotherapy interventions, disease-free and overall survival and measures of morbidity and quality of life, and a concurrent economic evaluation is necessary to estimate the cost-effectiveness of the technology.
Currently, there is no value in undertaking further research in primary CRC. However, if FDG PET/CT technology improves (e.g. contrast-enhanced FDG PET/CT) then there would be potential value in undertaking further research to explore contrast-enhanced FDG PET/CT as a replacement for contrast-enhanced CT. A trial that addresses uncertainty in the diagnostic test accuracy and cost-effectiveness of advanced contrast-enhanced FDG PET/CT as a replacement for contrast-enhanced CT in primary CRC would be of value, particularly for primary colon cancer.
Acknowledgements
We are indebted to the following people for their help:
In translating non-English-language studies identified by the search strategy we were greatly assisted by Kyoko Atsumi (Japanese translations), Kathryn Beyer (German translation), Kaska Hemple (Polish translation), Puchan Liao (Chinese translations) and Madeleine Rooney (French translation).
Rosemary Porteous and Jan Bunyan for their administrative support throughout the duration of the project and assistance with the preparation of the final report.
Public partners from the South East Scotland Cancer Network were Terry Hegarty, Jeff Hurst and Stella McPherson, who generously donated their time, views and perspectives at steering committee meetings.
For advice regarding the safety considerations of FDG PET/CT, we thank Dr Jim Hannan, Medical Physicist at NHS Lothian, Edinburgh.
Contribution of authors
Each author made substantial contributions to the underlying scientific approaches used to answer the research questions: the research design, collection of data, analyses and the interpretation of the results. All contributed to the report writing and have approved the final draft.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Ferlay J, Bray F, Pisani P, Parkin DM. Cancer incidence, mortality and prevalence worldwide. Lyon: IARC; 2003.
- Cancer Research UK . Cancer Statistics 2009. http://info.cancerresearchuk.org/cancerstats/types/bowel/incidence/#source1 (accessed 1 December 2009).
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol 2007;8:784-96.
- Association of Coloproctology of Great Britain and Ireland (ACPGBI) . Guidelines for the Management of Colorectal Cancer 2007.
- Weller D, Coleman D, Robertson R, Butler P, Melia J, Campbell C, et al. The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer 2007;97:1601-5.
- Goodyear SJ, Leung E, Menon A, Pedamallu S, Williams N, Wong LS. The effects of population-based faecal occult blood test screening upon emergency CRC admissions in Coventry and north Warwickshire. Gut 2008;57:218-22.
- Dukes CE. Cancer of the rectum: an analysis if 1000 cases. J Pathol Bacteriol 1940;50:527-39.
- Edge SB, Byrd DR, Compton CC, Fritz GF, Greene FL, Trotti A. AJCC cancer staging manual. New York, NY: Springer; 2009.
- Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg 2009;96:280-90.
- National Bowel Cancer Audit Project (NBCAP) . National Bowel Cancer Audit Project Report 2009. www.ic.nhs.uk/services/national-clinical-audit-support-programme-ncasp/audit-reports/bowel-cancer (accessed 30 June 2011).
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46.
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40.
- Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients – a Dutch CRC group study. J Clin Oncol 2005;23:6199-206.
- Scott NA, Susnerwala S, Gollins S, Myint AS, Levine E. Preoperative neo-adjuvant therapy for curable rectal cancer – reaching a consensus 2008. Colorectal Dis 2009;11:245-8.
- Shihab OC, Heald RJ, Rullier E, Brown G, Holm T, Quirke P, et al. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol 2009;10:1207-11.
- Melton GB, Paty PB, Boland PJ, Healey JH, Savatta SG, Casas-Ganem JE, et al. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Dis Colon Rectum 2006;49:1099-107.
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet 2010;375:1030-47.
- Chin BB, Wahl RLC. 18F-Fluoro-2-deoxyglucose positron emission tomography in the evaluation of gastrointestinal malignancies 2003;52:23-9.
- National Institute for Health and Clinical Excellence (NICE). National Cancer Guidance Steering Group . Improving Outcomes in Colorectal Cancers. 2004. http://guidance.nice.org.uk/CSGCC (accessed 30 June 2011).
- Chang JM, Lee HJ, Goo JM, Lee HY, Chung JK, Im JG. False positive and false negative 18FDG-PET scans in various thoracic diseases. Korean J Radiol 2006;7:57-69.
- Herbertson RA, Scarsbrook AF, Lee ST, Tebbutt N, Scott AM. Established, emerging and future roles of 18FDG PET-CT in the management of colorectal cancer. Clin Radiol 2009;64:225-37.
- The Royal College of Radiologists . Recommendations for Cross-Sectional Imaging in Cancer Management 2006. www.rcr.ac.uk/docs/oncology/pdf/Cross_Sectional_Imaging_12.pdf (accessed 3 December 2009).
- The Royal College of Radiologists . A Strategy for 18FDG PET-CT in the UK 2005. www.rcr.ac.uk/docs/general/pdf/PETCT_final.pdf (accessed 22 November 2009).
- Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18)F-FDG PET and 18FDG PET-CT in patients with colorectal carcinoma. J Nucl Med 2003;44:1797-803.
- Hicks RJ, Ware RE, Lau EW. 18FDG PET-CT: will it change the way that we use CT in cancer imaging?. Cancer Imaging 2006;6:S52-62.
- Ell PJ. The contribution of 18FDG PET-CT to improved patient management. Br J Radiol 2006;79:32-6.
- Heriot AG, Hicks RJ, Drummond EG, Keck J, Mackay J, Chen F, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum 2004;47:451-8.
- Zanzonico P, Dauer L, St Germain J. Operational radiation safety for 18FDG PET-CT, SPECT-CT, and cyclotron facilities. Health Physics 2008;95:554-70.
- Park IJ, Kim HC, Yu CS, Ryu MH, Chang HM, Kim JH, et al. Efficacy of 18FDG PET-CT in the accurate evaluation of primary colorectal carcinoma. Eur J Surg Oncol 2006;32:941-7.
- MHRA . Public Assessment Report: Mutual Recognition Procedure: Steripet 250 MBq Ml Solution for Injection: Fludeoxglucose (18F) 2008. www.mhra.gov.uk/home/groups/l-unit1/documents/websiteresources/con025955.pdf (accessed 30 June 2011).
- Carson KJ, Young VAL, Cosgrove VP, Jarritt PH, Hounsell AR. Personal radiation dose considerations in the use of an integrated 18FDG PET-CT scanner for radiotherapy treatment planning. Br J Radiol 2009;82:946-9.
- Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult [update of Cochrane Database Syst Rev 2000; 2:CD001216]. Cochrane Database Syst Rev 2007;1.
- Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer [update of Cochrane Database Syst Rev 2002; 1:CD002200]. Cochrane Database Syst Rev 2007;1.
- Mijnhout GS, Hooft L, van Tulder MW, Deville WL, Teule GJ, Hoekstra OS. How to perform a comprehensive search for 18FDG-PET literature. Eur J Nucl Med 2000;27:91-7.
- Mijnhout GS, Riphagen II, Hoekstra OS. Update of the 18FDG PET search strategy. Nucl Med Commun 2004;25:1187-9.
- Ritchie G, Glanville J, Lefebvre C. Do published search filters to identify diagnostic test accuracy studies perform adequately?. Health Info Libr J 2007;24:188-92.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC. A unification of models of meta-analysis for diagnostic accuracy studies. Biostatistics 2007;1:1-21.
- Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging – meta-analysis. Radiology 2004;232:773-83.
- Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis – meta-analysis. Radiology 2005;237:123-31.
- Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartran CI, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 2005;237:893-904.
- Huebner RH, Park KC, Shepherd JE, Schwimmer J, Czernin J, Phelps ME, et al. A meta-analysis of the literature for whole-body 18FDG PET detection of recurrent colorectal cancer. J Nucl Med 2000;41:1177-89.
- Purkayastha S, Tekkis PP, Athanasiou T, Tilney HS, Darzi AW, Heriot AG. Diagnostic precision of magnetic resonance imaging for preoperative prediction of the circumferential margin involvement in patients with rectal cancer. Colorectal Dis 2007;9:402-11.
- Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 2005;104:2658-70.
- Guyatt GH, Tugwell PX, Feeny DH, Drummond MF, Haynes RB. The role of before-after studies of therapeutic impact in the evaluation of diagnostic technologies. J Chronic Dis 1986;39:295-304.
- Lord SJ, Irwig L, Simes J. When is measuring sensitivity and specificity sufficient to evaluate a diagnostic test, and when do we need randomized trials?. Ann Intern Med 2006;144:850-5.
- Cancer Research UK . Latest UK Cancer Incidence and Mortality Summary – Numbers 2009. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@sta/documents/generalcontent/crukmig_1000ast-2735.pdf (accessed 20 November 2009).
- Tutt AN, Plunkett TA, Barrington SF, Leslie MD. The role of positron emission tomography in the management of colorectal cancer. Colorectal Dis 2004;6:2-9.
- Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol 2006;13:397-404.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49.
- Lejeune C, Bismuth MJ, Conroy T, Zanni C, Bey P, Bedenne L, et al. Use of a decision analysis model to assess the cost-effectiveness of 18F-18FDG PET in the management of metachronous liver metastases of colorectal cancer. J Nucl Med 2005;46:2020-8.
- Weller D, Coleman D, Robertson R, Butler P, Melia J, Campbell C, et al. The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer 2007;97:1601-5.
- Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative diagnosis of lymph node metastases of colorectal cancer by 18FDG-PET-CT. Jpn J Clin Oncol 2008;38:347-53.
- Tateishi U, Maeda T, Morimoto T, Miyake M, Arai Y, Kim EE. Non-enhanced CT versus contrast-enhanced CT in integrated 18FDG PET-CT studies for nodal staging of rectal cancer. Eur J Nucl Med Mol Imaging 2007;34:1627-34.
- Israel O, Kuten A. Early detection of cancer recurrence: 18F-FDG PET/CT can make a difference in diagnosis and patient care. J Nucl Med 2007;48:28S-35S.
- Schaefer O, Langer M. Detection of recurrent rectal cancer with CT, MRI and 18FDG PET-CT. Eur Radiol 2007;17:2044-54.
- Zhang C, Chen Y, Xue H, Zheng P, Tong J, Liu J, et al. Diagnostic value of 18FDG-PET in recurrent colorectal carcinoma: a meta-analysis. Int J Cancer 2009;124:167-73.
- Medical Services Advisory Committee . Positron Emission Tomography for Recurrent Colorectal Cancer 2008.
- Segre D, Giuffrida MC, Dal Corso HM. Advanced rectal cancer: pelvic recurrence and liver metastases – the role of PET, CT scan, MRI and immunoscintigraphy. Proceedings of the Second Joint Meeting European Council of Coloproctology: First National Congress Italian Society of Colo-Rectal Surgery 2005:29-34.
- Karantanas AH, Yarmenitis S, Papanikolaou N, Gourtsoyiannis N. Preoperative imaging staging of rectal cancer. Dig Dis 2007;25:20-32.
- Sarikaya I, Bloomston M, Povoski SP, Zhang J, Hall NC, Knopp MV, et al. 18FDG-PET scan in patients with clinically and/or radiologically suspicious colorectal cancer recurrence but normal CEA. World J Surg Oncol 2007;5.
- Bellomi M, Rizzo S, Travaini LL, Bazzi L, Trifiro G, Zampino MG, et al. Role of multidetector CT and 18FDG-PET-CT in the diagnosis of local and distant recurrence of resected rectal cancer. Radiol Med 2007;112:681-90.
- Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, et al. The role of 18FDG-PET-CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging 2006;33:779-84.
- Even-Sapir E, Parag Y, Lerman H, Gutman M, Levine C, Rabau M, et al. Detection of recurrence in patients with rectal cancer: 18FDG PET-CT after abdominoperineal or anterior resection. Radiology 2004;232:815-22.
- Schmidt GP, Baur-Melnyk A, Haug A, Utzschneider S, Becker CR, Tiling R, et al. Whole-body MRI at 1.5 T and 3 T compared with 18FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol 2009;19:1366-78.
- Kula Z, Szefer J, Zuchora Z, Romanowicz G, Pietrzak T. Evaluation of positron emission tomography by using F-18-fluorodeoxyglucose in diagnosis of recurrent colorectal cancer. Pol Merkur Lekarski 2004;17:63-6.
- Strunk H, Bucerius J, Jaeger U, Joe A, Flacke S, Reinhardt M, et al. Combined 18FDG PET-CT imaging for restaging of colorectal cancer patients: impact of image fusion on staging accuracy. Rofo 2005;177:1235-41.
- Kim JH, Czernin J, Allen-Auerbach MS, Halpern BS, Fueger BJ, Hecht JR, et al. Comparison between 18FDG PET, in-line 18FDG PET-CT, and software fusion for restaging of recurrent colorectal cancer. J Nucl Med 2005;46:587-95.
- Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Klausner JM, et al. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (18FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg 2007;11:472-8.
- D’Souza MM, Sharma R, Mondal A, Jaimini A, Tripathi M, Saw SK, et al. Prospective evaluation of CECT and 18F-FDG-PET/CT in detection of hepatic metastases. Nucl Med 2009;30:117-25.
- Chen LB, Tong IL, Song HZ, Zhu H, Wang YC. 18FDG PET-CT in detection of recurrence and metastasis of colorectal cancer. World J Gastroenterol 2007;13:5025-9.
- Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55:1-8.
- Scheele J, Stang R, Altendorf Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995;19:59-71.
- Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99.
- Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag P, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16.
- National Institute for Health and Clinical Excellence . Cetuximab for First-Line Treatment of Metastatic Colorectal Cancer 2009.
- Rama N, Monteiro A, Bernardo JE, Eugenio L, Antunes MJ. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardiothorac Surg 2009;35:444-9.
- Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, et al. The impact of 18FDG PET-CT in patients with liver metastases. Eur J Nucl Med Mol Imaging 2007;34:1906-14.
- Kong G, Jackson C, Koh DM, Lewington V, Sharma B, Brown G, et al. The use of 18FDG PET-CT in colorectal liver metastases – comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging 2008;35:1323-9.
- Ramos E, Martinez L, Gamez C, Torras J, Valls C, Rafecas A, et al. Use of 18FDG PET-CT in pre-surgical staging of colorectal cancer hepatic metastases. Cir Esp 2008;84:71-7.
- Cantwell CP, Setty BN, Holalkere N, Sahani DV, Fischman AJ, Blake MA. Liver lesion detection and characterization in patients with colorectal cancer: a comparison of low radiation dose non-enhanced 18FDG PET-CT, contrast-enhanced 18FDG PET-CT, and liver MRI. J Comput Assist Tomogr 2008;32:738-44.
- Rappeport ED, Loft A, Berthelsen AK, von der Recke P, Larsen PN, Mogensen AM, et al. Contrast-enhanced 18FDG PET-CT vs. SPIO-enhanced MRI vs. 18FDG-PET vs. CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol 2007;48:369-78.
- Coenegrachts K, De Geeter F, ter Beek L, Walgraeve N, Bipat S, Stoker J, et al. Comparison of MRI (including SS SE-EPI and SPIO-enhanced MRI) and 18FDG PET-CT for the detection of colorectal liver metastases. Eur Radiol 2009;19:370-9.
- Wildi SM, Gubler C, Hany T, Petrowsky H, Clavien PA, Jochum W, et al. Intraoperative sonography in patients with colorectal cancer and resectable liver metastases on preoperative 18FDG PET-CT. J Clin Ultrasound 2008;36:20-6.
- Kamel IR, Cohade C, Neyman E, Fishman EK, Wahl RL. Incremental value of CT in 18FDG PET-CT of patients with colorectal carcinoma. Abdom Imaging 2004;29:663-8.
- Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel 18FDG PET-CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver?. Ann Surg 2004;240:1027-34.
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PMM. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149:889-97.
- Davey K, Heriot AG, Mackay J, Drummond E, Hogg A, Ngan S, et al. The impact of 18-fluorodeoxyglucose positron emission tomography-computed tomography on the staging and management of primary rectal cancer. Dis Colon Rectum 2008;51:997-1003.
- Bassi MC, Turri L, Sacchetti G, Loi G, Cannillo B, La Mattina P, et al. 18FDG PET-CT imaging for staging and target volume delineation in preoperative conformal radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:1423-6.
- Engledow AH, Bond-Smith GEL, Francis D, Pakzad F, Bomanji J, Groves A, et al. The incremental value of dual modality 18FDG PET-CT imaging over PET imaging alone in advanced colorectal cancer. Indian J Surg 2009;71:63-8.
- Garin E, Devillers A, Prigent F, Bouriel C, Girault S, Boudjema K, et al. Contribution of coregistrated 18FDG PET-CT for patients with suspected recurrence of colo-rectal cancer. Med Nucl 2003;27:665-75.
- Soyka JD, Veit-Haibach P, Strobel K, Breitenstein S, Tschopp A, Mende KA, et al. Staging pathways in recurrent colorectal carcinoma: is contrast-enhanced 18FDG PET-CT the diagnostic tool of choice?. J Nucl Med 2008;49:354-61.
- Eglinton T, Luck A, Bartholomeusz D, Varghese R, Lawrence M. Positron-emission tomography/computed tomography (PET-CT) in the initial staging of primary rectal cancer. Colorectal Dis 2010;12:667-73.
- MHRA . Public Assessment Report: Metatrace 18FDG Solution for Injection 300mBq Ml: Fludeoxyglucose (18-F) 2008. www.mhra.gov.uk/home/groups/l-unit1/documents/websiteresources/con2033925.pdf.
- Silberstein DHS. Prevalence of adverse reactions to positron emitting radiopharmaceuticals in nuclear medicine. J Nucl Med 1998;39:2190-2.
- Spiegelhalter D, Best N. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Stat Med 2003;22:3687-709.
- Brennan A, Akehurst R. Modelling in health economic evaluation: what is its place? What is its value?. Pharmacoeconomics 2000;17:445-59.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- Department of Health . NHS Reference Costs 2008–2009 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed 15 March 2010).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2009.
- Maroun J, Ng E, Berthelot J, Le Petit C, Dahrouge S, Flanagan W, et al. Lifetime costs of colon and rectal cancer management in Canada. Chronic Dis Can 2003;24:91-101.
- Price P, Laking G. How should we introduce clinical PET in the UK? The oncologists need to have a view. Clin Oncol 2004;16:172-5.
- Sloka JS, Hollett PD. Cost effectiveness of positron emission tomography in Canada. Med Sci Monit 2005;11:H1-6.
- Valk PE, Pounds TR, Tesar RD, Hopkins DM, Haseman MK. Cost-effectiveness of PET imaging in clinical oncology. Nucl Med Biol 1996;23:737-43.
- Kunkler I. Cure, palliation, and cost in cancer care. Lancet Oncol 2004;5.
- Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007;11.
- Bombardieri E, Crippa F, Rossetti C, Fazio F. The need of cost-effectiveness evaluation when using high-cost equipment in National Health Services. Tumori 1997;83:544-6.
- Park KC, Schwimmer J, Gambhir SS. Decision analysis for the cost-effective management of recurrent colorectal cancer. Ann Surg 2001;235:309-10.
- Sloka JS, Hollett PD, Mathews M. Cost-effectiveness of positron emission tomography in recurrent colorectal cancer in Canada. McGill J Med 2004;7:165-74.
- Zubeldia J, Bednarczyk E, Baker J, Nabi H. The economic impact of 18FDG positron emission tomography in the surgical management of colorectal cancer with hepatic metastases. Cancer Biother Radiopharm 2005;20:450-6.
- Miles KA. An approach to demonstrating cost-effectiveness of diagnostic imaging modalities in Australia illustrated by positron emission tomography. Australas Radiol 2001;45:9-18.
- Renehan A, Whynes D, O’Dwyer S. Cost effectiveness analysis of intensive versus conventional follow-up after curative resection for colorectal cancer. BMJ 2004 n.d.:328-81.
- Pickhardt P, Hassan C, Laghi A, Zullo A, Kim D, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography. Am Cancer Soc 2007;109:2213-21.
- Lansdorp-Vogelaar I, Van Ballegooijen M, Zauber A, Habbema JD, Kuipers E. Effect of raising chemotherapy costs on the cost savings of colon cancer screening. J Natl Cancer Inst 2009;101:1412-22.
- The current management of rectal cancer: history of surgical techniques for treatment of rectal carcinoma . Curr Probl Surg 2005;42:78-131.
- Langenhoff B, Krabbe P, Peerenboom L, Wobbes T, Ruers T. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg 2006:93-14.
- Sloan PA. The evolving role of interventional pain management in oncology. J Support Oncol 2004;2:491-503.
- American Cancer Society . Colorectal Cancer: Facts and Figures 2005.
- American Joint Committee on Cancer . Staging Manual. Colon and Rectum 2010. www.cancerstaging.org/.
- Capirci C, Rubello D, Chierichetti F, Crepaldi G, Fanti S, Mandoliti G, et al. Long-term prognostic value of 18F-FDG PET in patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy. Am J Roentgenol 2006;187:W202-8.
- Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18-fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438-47.
- Saunders TH, Mendes Ribeiro HK, Gleeson FV. New techniques for imaging colorectal cancer: the use of MRI, PET and radioimmunoscintigraphy for primary staging and follow-up. Br Med Bull 2002;64:81-99.
- Guest J, Ruiz F, Greener M, Trotman I. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. Eur J Cancer Care 2006;15:65-73.
- Ahmad MN, Zafar AM, Nadeem N. Where there is no PET/CT. Eur J Radiol 2009;70:463-4.
- Anderson C, Koshy M, Staley C, Esiashvili N, Ghavidel S, Fowler Z, et al. PET-CT fusion in radiation management of patients with anorectal tumors. Int J Radiat Oncol Biol Phys 2007;69:155-62.
- Anthony T. Colorectal cancer follow-up in 2005. Surg Oncol Clin N Am 2006;15.
- Arulampalam THA, Costa DC, Bomanji JB, Ell PJ. The clinical application of positron emission tomography to colorectal cancer management. Q J Nucl Med 2001;45:215-30.
- Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: a 2006 update. World J Gastroenterol 2006;12:3186-95.
- Chong PS, Finlay IG. Surgical options in the management of advanced and recurrent colorectal cancer. Surg Oncol Oxf 2007;16:25-31.
- Franc BL. PET and PET/CT for oncology applications in the abdomen and pelvis: update and future directions in the age of molecular medicine. Appl Radiol 2008;37:10-25.
- Ide M, Suzuki Y, Weckesser M, Schober O. Is whole-body FDG-PET valuable for health screening?. Eur J Nucl Med Mol Imaging 2005;32:339-43.
- Imdahl A, Reinhardt MJ, Nitzsche EU, Mix M, Dingeldey A, Einert A, et al. Impact of F-18-FDG-positron emission tomography for decision making in colorectal cancer recurrences. Langenbecks Arch Surg 2000;385:129-34.
- Institute for Clinical Evaluative Sciences (ICES) . Health Technology Assessment of Positron Emission Tomography in Oncology – a Systematic Review 2003.
- Jerusalem G, Hustinx R, Beguin Y, Fillet G. The value of positron emission tomography (PET) imaging in disease staging and therapy assessment. Ann Oncol 2002;13:227-34.
- Kalvin B, Fekeshazy A, Lengyel Z, Szakall S, Agoston P, Lengyel E, et al. Cost-effective PET investigations in oncology. Magy Onkol 2002;46:203-23.
- Kapse N, Goh V, Kapse N, Goh V. Functional imaging of colorectal cancer: positron emission tomography, magnetic resonance imaging, and computed tomography. Clin Colorectal Cancer 2009;8:77-8.
- Schoder H, Larson SM, Yeung HWD. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med 2004;45:72S-81S.
- Strasberg SM, Dehdashti F, Siegel BA, Drebin JA, Linehan D. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg 2001;233:293-9.
- Tanabe KK. Emerging therapies for metastatic carcinoma to the liver. Community Oncol 2006;3:567-73.
- Watson AJM, Lolohea S, Robertson GM, Frizelle FA. The role of positron emission tomography in the management of recurrent colorectal cancer: a review. Dis Colon Rectum 2006;50:102-14.
- Ramsey S, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal cancer. Am Cancer Soc 2000;88:1294-303.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Pickard S, Wilke C, Lin H, Llyod A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 2007;25:365-84.
- Tenesa A, Theodoratou E, Din FV, Farrington SM, Cetnarskyj R, Barnetson RA, et al. Ten common genetic variants associated with colorectal cancer risk are not associated with survival after diagnosis. Clin Cancer Res 2010;16:3754-9.
- Tengs T, Wallace A. One thousand health-related quality of life estimates. Med Care 2000;38:583-637.
- Barnetson R, Tenesa A, Farrington S, Nicholl I, Cetnarskyj R, Porteous M, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med 2006;354:2751-63.
- Pandharipande P, Choy G, del Carmen M, Gazelle SG, Russell A, Lee S. MRI and 18FDG PET-CT for triaging stage IB clinically operable cervical cancer to appropriate therapy: decision analysis to assess patient outcomes. Am J Roentgenol 2009;192:802-14.
- Office National Statistics . Deaths: Age and Sex, Numbers and Rates: Population Trends 2009. www.statistics.gov.uk.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 8 June 2009).
- Information Services Division . Scottish Health Service Costs Book 2009 2009. www.isdscotland.org/isd/797.html (accessed 20 November 2009).
- Department of Health . A Framework for the Development of Positron Emission Tomography (PET) Services in England 2005. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_110859.pdf (accessed 30 June 2011).
- National Cancer Research Institute . A Framework for PET Research in the UK 2007. www.ncri.org.uk/includes/Publications/reports/petreport_low.pdf.
- Cancer Research UK . Treating Bowel Cancer 2009. www.cancerhelp.org.uk/type/bowel-cancer/treatment/index.htm (accessed 25 February 2010).
- The Royal College of Radiologists . 18FDG PET-CT in the UK: A Strategy for Development and Integration of a Leading Edge Technology Within Routine Clinical Practice 2005. www.rcr.ac.uk/docs/general/pdf/PETCT_final.pdf (accessed 25 February 2010).
- Cancer Research UK . Cancer Stats: Large Bowel Cancer UK 2006. http://info.cancerresearchuk.org/cancerstats/keyfacts/bowel-cancer/ (accessed 20 February 2010).
- Heitman S, Manns B, Hilsden R, Fong A, Dean S, Romagnuolo J. Cost-effectiveness of computerized tomographic colonography versus colonoscopy for colorectal cancer screening 2005:173-8.
Appendix 1 Search strategies
MEDLINE (OvidSP) 1950 – May, week 4, 2009 (31 May 2009)
-
exp Colorectal Neoplasms/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
or/1-2
-
exp Liver Diseases/
-
liver disease*.ti,ab.
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) and metasta*).mp.
-
(4 or 5) and 6
-
3 or 7
-
exp Tomography, emission-computed/
-
positron emission tomography.ti,ab,rw,sh.
-
pet$.ti,ab,rw,sh.
-
animal/not (human/and animal/)
-
11 not 12
-
exp Deoxyglucose/
-
deoxyglucose.ti,ab,rw,sh.
-
deoxy-glucose.ti,ab,rw,sh.
-
fluorodeoxyglucose.ti,ab,rw,sh.
-
18fluorodeoxyglucose.ti,ab,rw,sh.
-
fludeoxyglucose.ti,ab,rw,sh.
-
18FDG$.ti,ab,rw,sh.
-
18FDG.ti,ab,rw,sh.
-
f-18-dg.ti,ab,rw,sh.
-
fluoro-2-deoxy-d-glucose.ti,ab,rw,sh.
-
2fluoro-2deoxyglucose.ti,ab,rw,sh.
-
fluoro-d-glucose.ti,ab,rw,sh.
-
or/9-10,13-25
-
8 and 26
-
animals/not (humans/and animals/)
-
27 not 28
EMBASE (OvidSP) 1980 – week 22, 2009 (31 May 2009)
-
exp anus tumor/or exp colon tumor/or exp rectum tumor/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
or/1-2
-
exp Liver Diseases/
-
liver disease*.ti,ab.
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) and metasta*).mp.
-
(4 or 5) and 6
-
3 or 7
-
exp computer assisted emission tomography/or exp positron emission tomography/or exp whole body tomography/
-
positron emission tomography.mp.
-
(pet* not (animal not (human and animal))).mp.
-
Deoxyglucose/
-
Fluorodeoxyglucose/
-
Fluorodeoxyglucose F18/
-
deoxyglucose.mp.
-
deoxy-glucose.mp.
-
fluorodeoxyglucose.mp.
-
18fluorodeoxyglucose.mp.
-
fludeoxyglucose.mp.
-
18FDG*.mp.
-
18FDG.mp.
-
f-18-dg.mp.
-
fluoro-2-deoxy-d-glucose.mp.
-
2fluoro-2deoxyglucose.mp.
-
fluoro-d-glucose.mp.
-
or/9-25
-
(rat or rats or mouse or mice or monkey* or rabbit* or hamster* or bovine or sheep).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
animal/or experimental animal/
-
27 or 28
-
8 and 26
-
30 not 29
Global Health (OvidSP) 1973 – 31 May 2009
-
colorectal cancer/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
or/1-2
-
exp Liver Diseases/
-
liver disease*.ti,ab.
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) and metasta*).mp.
-
(4 or 5) and 6
-
3 or 7
-
exp tomography/
-
positron emission tomography.mp.
-
(pet* not (animal not (human and animal))).mp.
-
Deoxyglucose/
-
Fluorodeoxyglucose/
-
Fluorodeoxyglucose F18/
-
deoxyglucose.mp.
-
deoxy-glucose.mp.
-
fluorodeoxyglucose.mp.
-
18fluorodeoxyglucose.mp.
-
fludeoxyglucose.mp.
-
18FDG*.mp.
-
18FDG.mp.
-
f-18-dg.mp.
-
fluoro-2-deoxy-d-glucose.mp.
-
2fluoro-2deoxyglucose.mp.
-
fluoro-d-glucose.mp.
-
exp tomography/
-
or/9-25
-
exp animals/not (man/and exp animals/)
-
(8 and 27) not 28
-
8 and 27
Web of Science all years, last update 30 May 2009 (31 May 2009)
Science Citation Index Expanded: 1900–present.
Social Sciences Citation Index: 1956–present.
Arts & Humanities Citation Index: 1975–present.
Conference Proceedings Citation Index-Science: 1990–present.
Topic=((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) same (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno-carcinoma* or adenom* or lesion* or CRC)) OR ((liver or hepatic) and metasta* and (rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal))) AND Topic=(((positron emission tomography or Fluorodeoxyglucose or 18fluorodeoxyglucose or deoxy-glucose or Deoxyglucose or fludeoxyglucose or 18FDG* or 18FDG or f-18-dg or fluoro-2-deoxy-d-glucose or 2fluoro-2deoxyglucose or fluoro-d-glucose or (PET* same (CT or computer tomography))) NOT Topic=(rat or rats or mouse or mice or monkey* or rabbit* or hamster* or bovine or sheep)
Biosis Previews via Institute for Scientific Information Web of Knowledge, 1926 – last update 28 May 2009 (31 May 2009)
Same as for Web of Science.
Topic=((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) same (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno-carcinoma* or adenom* or lesion* or CRC)) OR ((liver or hepatic) and metasta* and (rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal))) AND Topic=(((positron emission tomography or Fluorodeoxyglucose or 18fluorodeoxyglucose or deoxy-glucose or Deoxyglucose or fludeoxyglucose or 18FDG* or 18FDG or f-18-dg or fluoro-2-deoxy-d-glucose or 2fluoro-2deoxyglucose or fluoro-d-glucose or (PET* same (CT or computer tomography))) NOT Topic=(rat or rats or mouse or mice or monkey* or rabbit* or hamster* or bovine or sheep)
CENTRAL (31 May 2009) (same search used for DARE, NHS EED, HTA)
-
#1 MeSH descriptor Colorectal Neoplasms explode all trees
-
#2 (rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal) NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumour* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR CRC)
-
#3 MeSH descriptor Liver Diseases explode all trees
-
#4 (liver disease*)
-
#5 (rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal) AND metasta*
-
#6 (#1 OR #2 OR ((#3 OR #4) AND #5))
-
#7 MeSH descriptor Tomography, Emission-Computed explode all trees
-
#8 (positron emission tomography OR Fluorodeoxyglucose OR 18fluorodeoxyglucose OR deoxy-glucose OR Deoxyglucose OR fludeoxyglucose OR 18FDG* OR 18FDG OR f-18-dg OR fluoro-2-deoxy-d-glucose OR 2fluoro-2deoxyglucose OR fluoro-d-glucose OR (PET* NEAR/5 (CT OR computer tomography)))
-
#9 MeSH descriptor Deoxyglucose explode all trees
-
#10 (#7 OR #8 OR #9)
-
#11 (#6 AND #10)
CINAHL Plus via Ebsco (13 July 2009)
((MH “Colorectal Neoplasms+”) or (TX (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR CRC) AND (rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal)) ) AND ((MH “Tomography, Emission-Computed+”) or TX (“positron emission tomography” OR “18FDG PET/CT” OR “18FDG PET*”) or TX fluorodeoxyglucose or ((MH “Fludeoxyglucose F 18”)) )
Compendex Ei Village (31 May 2009)
((((({Positron emission tomography}) WN CV)) AND (1884-2009 WN YR)) OR ((($Fluorodeoxyglucose OR $18fluorodeoxyglucose OR $deoxy-glucose OR $Deoxyglucose OR $fludeoxyglucose OR 18FDG* OR $18FDG OR $f-18-dg OR $fluoro-2-deoxy-d-glucose OR $2fluoro-2deoxyglucose OR $fluoro-d-glucose OR PET*) WN ALL) AND (1884-2009 WN YR))) and ((((($rectal OR $rectum OR $colonic OR $colon OR $colorectal OR bowel* OR $sigmoid OR $anus OR $anal) WN ALL) AND (1884-2009 WN YR)) AND (((cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR $CRC) WN ALL) AND (1884-2009 WN YR))))
Inspec Ei Village (31 May 2009)
((((({positron emission tomography}) WN CV)) AND (1896-2009 WN YR)) OR ((($Fluorodeoxyglucose OR $18fluorodeoxyglucose OR $deoxy-glucose OR $Deoxyglucose OR $fludeoxyglucose OR 18FDG* OR $18FDG OR $f-18-dg OR $fluoro-2-deoxy-d-glucose OR $2fluoro-2deoxyglucose OR $fluoro-d-glucose OR PET*) WN ALL) AND (1896-2009 WN YR))) AND ((($rectal OR $rectum OR $colonic OR $colon OR $colorectal OR bowel* OR $sigmoid OR $anus OR $anal) WN ALL) AND (1896-2009 WN YR)) AND (((cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR $CRC) WN ALL) AND (1896-2009 WN YR))
Global Health Library regional indexes (LILACS, AFRO, EMRO, PAHO, WHOLIS, WPRO) (25 June 2009)
(cancer OR cancers OR carcinoma OR carcinomas OR neoplasm OR neoplasms OR neoplastic OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR CRC) AND (rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal) AND (positron emission tomography OR Fluorodeoxyglucose OR 18fluorodeoxyglucose OR deoxy-glucose OR Deoxyglucose OR fludeoxyglucose OR 18FDG* OR 18FDG OR f-18-dg OR fluoro-2-deoxy-d-glucose OR 2fluoro-2deoxyglucose OR fluoro-d-glucose OR PET*)
metaRegister of Current Controlled Trials (13 July 2009)
www.controlled-trials.com/mrct/
[comprised of ISRCTN Register, Action Medical Research, Leukaemia Research Fund, Medical Research Council (UK), National Health Service Research and Development Health Technology Assessment Programme (HTA), National Institutes of Health (NIH) (randomised trial records held on NIH ClinicalTrials.gov website), the Wellcome Trust, UK Clinical Trials Gateway]
colorectal AND (PET or “positron emission tomography”)
Index to Theses (13 July 2009)
(rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) and (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC) and (positron emission tomography or *18FDG* or PET or 18FDG PET/CT or *fluorodeoxyglucose*)
Dissertations and Theses (13 July 2009)
(rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal) AND (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma OR adenom* OR lesion* OR CRC) AND (positron emission tomography OR 18FDG* OR PET OR 18FDG PET/CT OR fluorodeoxyglucose*)
OpenSIGLE (13 July 2009)
(rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal) AND (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma OR adenom* OR lesion* OR CRC) AND (positron emission tomography OR 18FDG* OR PET OR 18FDG PET/CT OR fluorodeoxyglucose*)
National Technical Information Services (13 July 2009)
Colorectal AND (PET or Positron Emission Tomography)
UK Clinical Research Network Study Portfolio (13 July 2009)
All studies classified under:
UKCRN Study Portfolio > Cancer > Colorectal
Safety data
EMBASE (OvidSP)
-
ae.fs.
-
exp anus tumor/or exp colon tumor/or exp rectum tumor/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
2 or 3
-
positron emission tomography/
-
1 and 4 and 5
Appendix 2 Quality assessment operational definitions
| 1. Was the spectrum of patients representative of the patients who will receive the test in practice? |
Yes: The study included a consecutive series or random sample of adults undergoing staging for known or suspected primary cancer of the colon or rectum who received FDG PET/CT at any point in the pre-operative staging pathway, either as the first imaging investigation or referred following equivocal or suspicious findings on other tests including imaging, clinical examination and CEA testing No: The study included a non-consecutive or non-random sample, or there is clear evidence of selective sampling, e.g. restriction to patients with particular findings on FDG PET/CT Unclear: Insufficient information on the method of recruitment and selection criteria |
| 2. Were selection criteria clearly described? |
Yes: All relevant information regarding how participants were selected for inclusion in the study was provided No: Study selection criteria were not clearly reported Unclear: Insufficient information |
| 3. Is the reference standard likely to correctly classify the target condition? |
Yes: Surgical histopathology if surgical resection, or clinical/imaging follow-up of at least 6 months No: Criteria clearly not met Unclear: Insufficient information |
| 4. Is the time period between the index test and reference standard short enough to be reasonably sure that the target condition did not change between the two tests? |
Yes: If the time between FDG PET/CT and the reference standard is < 6 weeks. The clinical rational being a finding on PET CT in a patient with colon or rectal cancer should be acted upon within 6 weeks of study, or disease progression may occur. Similarly, a negative finding should really be confirmed within the same timescale No: ‘Yes’ criteria clearly not met Unclear: The time lapse between tests was not reported |
| 5. Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? |
Yes: It is reported that all or a random sample of the study participants did receive verification of their disease status using the reference standard defined in 2 above No: All participants did not receive verification using the reference standard and those who did were not selected randomly Unclear: Insufficient information |
| 6. Did patients receive the same reference standard regardless of the index test result? |
Yes: All FDG PET/CT results were verified by the same reference standard No: Some PET/CT results were verified by a different reference standard Unclear: Insufficient information |
| 7. Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? |
Yes: FDG PET/CT was not used in establishing the final diagnosis (i.e. FDG PET/CT was not a component of the reference standard) No: FDG PET/CT did form part of the reference standard. (Note this does not include the FDG PET/CT index test result being known when the reference standard diagnosis was made, only the specific incorporation of PET/CT as part of the reference standard test) Unclear: Insufficient information |
| 8. Was the execution of the index test described in sufficient detail to permit the replication of the test? |
Yes: Details of FDG PET/CT equipment and patient preparation was clearly described No: Information regarding the FDG PET/CT equipment and patient preparation was omitted from the report Unclear: Insufficient information |
| 9. Was the execution of the reference standard described in sufficient detail to permit its replication? |
Yes: Details of the reference standard(s) (surgically resected specimen +/– clinical/imaging follow-up) were clearly described No: Details of the reference standard(s) (surgically resected specimen +/– clinical/imaging follow-up) were not clearly described Unclear: Insufficient information |
| 10. Were the index test results interpreted without knowledge of the results of the reference standard? |
Yes: It was stated that the assessors were blind to the FDG PET/CT results No: It was stated that the assessors referred to the FDG PET/CT results during the interpretation Unclear: Insufficient information on which to judge whether the interpretation was blind |
| 11. Were the index test results interpreted without knowledge of the results of the reference standard? |
Yes: It is clearly stated that FDG PET/CT results were interpreted blind to the results of the reference standard; or the PET/CT results were clearly interpreted before the results of the reference standard were available No: It is clearly stated that FDG PET/CT results were interpreted with knowledge of the results of the reference standard Unclear: Insufficient information on which to judge whether the interpretation was blind |
| 12. Were the same clinical data available when the test results were interpreted as would be available when the test is used in practice? |
Yes: It is stated that the clinical information, including all previous test results, that would normally be available to the assessors when FDG PET/CT images are interpreted in practice was available when the index test was evaluated No: It is stated that the clinical information that would normally be available when PET/CT images are interpreted in practice was not available to the assessors when the index test was evaluated Unclear: Insufficient information |
| 13. Were uninterpretable/intermediate test results reported? |
Yes: It is clear that all test results including those that were uninterpretable, indeterminate or intermediate are reported, or it is clear there were none No: It is reported that uninterpretable, indeterminate or intermediate were excluded with no further information given, or there is reason to believe that such results occurred but were not reported Unclear: It is not clear whether there were any uninterpretable, indeterminate or intermediate results |
| 14. Were withdrawals from the study explained? |
Yes: All participants who were entered in the study either completed the study or were accounted for if they did not complete the study (reasons for withdrawal and loss to follow-up were given) No: Some participants who entered the study did not complete the study and were not accounted for Unclear: Insufficient information to judge whether or not all participants who entered the study were accounted for |
Appendix 3 FDG PET/CT economics, decision-making and quality of life search strategies
MEDLINE (OvidSP) 1950 – November, week 2, 2009
Base search for FDG PET/CT and colorectal cancer
-
exp Colorectal Neoplasms/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
or/1-2
-
exp Tomography, emission-computed/
-
positron emission tomography.ti,ab,rw,sh.
-
pet$.ti,ab,rw,sh.
-
animal/not (human/and animal/)
-
6 not 7
-
exp Deoxyglucose/
-
deoxyglucose.ti,ab,rw,sh.
-
deoxy-glucose.ti,ab,rw,sh.
-
fluorodeoxyglucose.ti,ab,rw,sh.
-
18fluorodeoxyglucose.ti,ab,rw,sh.
-
fludeoxyglucose.ti,ab,rw,sh.
-
18FDG$.ti,ab,rw,sh.
-
18FDG.ti,ab,rw,sh.
-
f-18-dg.ti,ab,rw,sh.
-
fluoro-2-deoxy-d-glucose.ti,ab,rw,sh.
-
2fluoro-2deoxyglucose.ti,ab,rw,sh.
-
fluoro-d-glucose.ti,ab,rw,sh.
-
or/4-5,8-20
-
animals/not (humans/and animals/)
-
(3 and 21) not 22
Economics search
-
exp “Costs and Cost Analysis”/
-
Economics/
-
Cost allocation/
-
Cost control/
-
Cost savings/
-
Cost of illness/
-
Cost sharing/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp “fees and charges”/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$or pharmacoeconomic$or price$or pricing).tw.
-
exp models, economic/
-
ec.fs.
-
or/1-31
Toxicity, adverse events, quality of life search
-
ae.xs.
-
“Quality of Life”/
-
mo.fs.
-
quality-adjusted life years/
-
“cost of illness”/
-
(QALY or QALM or Quality-Adjusted Life Month or DALY or Disability Adjusted Life-Years).mp
-
or/1-6
Decision-making search
-
Decision Trees/
-
algorithms/
-
exp decision making, computer-assisted/or exp decision support techniques/or decision support systems, clinical/
-
Decision Making/
-
exp Patient Care Planning/
-
or/1-5
EMBASE (OvidSP) 1980 – week 47, 2009
Base search for FDG PET/CT and colorectal cancer
-
exp anus tumor/or exp colon tumor/or exp rectum tumor/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
1 or 2
-
exp computer assisted emission tomography/or exp positron emission tomography/or exp whole body tomography/
-
positron emission tomography.mp.
-
(pet* not (animal not (human and animal))).mp.
-
Deoxyglucose/
-
Fluorodeoxyglucose/
-
Fluorodeoxyglucose F18/
-
deoxyglucose.mp.
-
deoxy-glucose.mp.
-
fluorodeoxyglucose.mp.
-
18fluorodeoxyglucose.mp.
-
fludeoxyglucose.mp.
-
18FDG*.mp.
-
18FDG.mp.
-
f-18-dg.mp.
-
fluoro-2-deoxy-d-glucose.mp.
-
2fluoro-2deoxyglucose.mp.
-
fluoro-d-glucose.mp.
-
exp tomography/
-
or/4-21
-
(rat or rats or mouse or mice or monkey* or rabbit* or hamster* or bovine or sheep).mp.
-
animal/or experimental animal/
-
23 or 24
-
(3 and 22) not 25
Economics search
-
Socioeconomics/
-
Cost benefit analysis/
-
Cost effectiveness analysis/
-
Cost of illness/
-
Cost control/
-
Economic aspect/
-
Financial management/
-
Health care cost/
-
Health care financing/
-
Health economics/
-
Hospital cost/
-
(fiscal or financial or finance or funding).tw.
-
Cost minimization analysis/
-
(cost adj estimate$).mp.
-
(cost adj variable$).mp.
-
(unit adj cost$).mp.
-
pe.fs.
-
or/1-17
Toxicity, adverse events, quality of life search
-
exp “Quality of Life”/
-
“cost of illness”/
-
(QALY or QALM or Quality-Adjusted Life Month or DALY or Disability Adjusted Life-Years).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
ae.fs.
-
to.fs.
-
or/1-5
Decision-making search
-
“decision tree”/
-
decision support system/
-
decision making/
-
algorithm/
-
clinical pathway/
-
or/1-5
Web of Science, all content up to 25 November 2009
Base search for FDG PET/CT and colorectal cancer
Topic=(((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) same (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno-carcinoma* or adenom* or lesion* or CRC)) AND (positron emission tomography or Fluorodeoxyglucose or 18fluorodeoxyglucose or deoxy-glucose or Deoxyglucose or fludeoxyglucose or 18FDG* or 18FDG or f-18-dg or fluoro-2-deoxy-d-glucose or 2fluoro-2deoxyglucose or fluoro-d-glucose or (PET* same (CT or computer tomography))) NOT (rat or rats or mouse or mice or monkey* or rabbit* or hamster* or bovine or sheep))
Economics search
Topic=(Economic* OR cost*)
Toxicity, adverse events, quality of life search
Topic= (toxic* or adverse or “quality of life” or QALY or “quality adjusted life years” or QALM or “quality adjusted life month” or DALY or “disability adjusted life years”)
Decision-making search
Topic= (decision* OR algorithm* OR pathway* OR (patient SAME management))
CINAHL Plus via Ebsco (30 November 2009)
Base search for FDG PET/CT and colorectal cancer
((MH “Colorectal Neoplasms+”) or (TX (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR CRC) AND (rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal))) AND ((MH “Tomography, Emission-Computed+”) or TX (“positron emission tomography” OR “18FDG PET-CT” OR “18FDG PET*”) or TX fluorodeoxyglucose or ((MH “Fludeoxyglucose F18”)))
Economics search
MW EC OR (TX cost or costs or economic* OR pharmacoeconomic* OR price* OR pricing*) OR (MH “Health Resource Utilization”) OR (MH “Health Resource Allocation”) OR (MH “Business+”) OR (MH “Financing, Organized+”) OR (MH “Financial Support+”) OR (MH “Financial Management+”) OR (MH “Economics+”)
Toxicity, adverse events, quality of life search
(QALY or QALM or DALY) OR quality adjusted life years OR quality adjusted life months OR disability adjusted life years OR (MH “Ferrans and Powers Quality of Life Index”) OR MW “AE” OR MW “TO” OR MW “MO” OR (MH “Quality of Life”)
Decision-making search
(MH “Decision Making+”) OR (MH “Algorithms”) OR (MH “Triage”) OR pathway* OR policy OR policies
Cochrane Library (NHS EED, HTA, CENTRAL, DARE) Issue 4, 2009
-
#1 MeSH descriptor Colorectal Neoplasms explode all trees
-
#2 (rectal OR rectum OR colonic OR colon OR colorectal OR bowel* OR sigmoid OR anus OR anal) NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumor* OR tumour* OR sarcoma* OR adenocarcinoma* OR adeno?carcinoma* OR adenom* OR lesion* OR CRC)
-
#3 MeSH descriptor Tomography, Emission-Computed explode all trees
-
#4 (positron emission tomography OR Fluorodeoxyglucose OR 18fluorodeoxyglucose OR deoxy-glucose OR Deoxyglucose OR fludeoxyglucose OR 18FDG* OR 18FDG OR f-18-dg OR fluoro-2-deoxy-d-glucose OR 2fluoro-2deoxyglucose OR fluoro-d-glucose OR (PET* NEAR/5 (CT OR computer tomography)))
-
#5 MeSH descriptor Deoxyglucose explode all trees
-
#6 ((#1 OR #2) AND (#3 OR #4 OR #5))
Health Management Information Consortium (OvidSP) (November 2009)
-
positron emission tomography.mp.
-
18FDG PET-CT.mp.
-
computed tomography scanners/or tomography/
-
colorectal cancer/
-
((rectal or rectum or colonic or colon or colorectal or bowel* or sigmoid or anus or anal) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or sarcoma* or adenocarcinoma* or adeno?carcinoma* or adenom* or lesion* or CRC)).mp.
-
1 or 2 or 3
-
4 or 5
-
6 and 7
Cost-effectiveness Analysis Registry
“positron emission tomography”
(Anything more detailed yielded nil results.)
Appendix 4 FDG PET/CT as an add-on imaging test versus routinely used imaging modalities for pre-operative staging in patients with primary, recurrent or metastatic colorectal cancer: a handsearch study
Systematic reviews adopt an approach of searching extensively for studies to ensure that as much available evidence as possible can inform the review, and to minimise bias, including publication bias. Handsearching has formed part of the extensive search approach for systematic reviews of effects. Handsearching is the page-by-page examination of the entire contents of a journal issue or conference proceedings to identify eligible reports of studies for a review. 1,2 Relevant information may appear in any part of a journal: articles, conference abstracts, news reports, letters, editorials, comments and other journal parts.
Guidance for conducting Cochrane systematic reviews of effects evidence and more recently for Cochrane diagnostic test accuracy studies recommends that handsearching should be considered to enhance the retrieval of relevant studies. 1,2 Handsearching is viewed as potentially valuable because it might identify additional reports of diagnostic test studies compared with searches of databases alone. 1,2 A systematic review of handsearching compared with database searches for randomised controlled trials (RCTs) has found that handsearching generates useful studies in addition to database searching. 3 A similar review is not yet available for diagnostic test accuracy studies, but single studies report that database searches alone can miss potentially relevant studies. This may be because those studies are not recorded in the database or are recorded but are not retrievable with the strategy used, perhaps because of inadequate or no indexing. 4–6 In addition, the variable quality of the reporting of diagnostic test accuracy studies has been noted by several authors and this is also likely to affect effective database retrieval. 1 However, the evidence for the benefits of handsearching for diagnostic test accuracy studies for systematic reviews remains to be established. 1
In the light of this sparse research evidence, we designed a complementary but distinct research project to exploit the opportunity to explore the value of handsearching to inform an imaging systematic review and to contribute to our understanding of the role of handsearching in the identification of reports of diagnostic test accuracy studies.
Aims and objectives
The aim of the sub-study was to investigate the contribution of handsearching to the identification of studies to inform a diagnostic test accuracy review.
The objectives of the handsearch project were:
-
to identify a range of relevant journals and handsearch them to identify diagnostic test accuracy studies, and more specifically diagnostic test accuracy studies of fluorine-18-deoxyglucose (FDG) positron emission tomography (PET)/computerised tomography (CT) imaging in colorectal cancer (CRC)
-
to assess the relative yield of handsearching compared with database searching in identifying reports of diagnostic test accuracy relating to FDG PET/CT imaging in CRC
-
to assess the costs of handsearching compared with those of database searching.
Methods
The first stage of the project was to identify high-yield journals to be handsearched for studies. A systematic review of handsearching studies to identify RCTs has reported several methods that have been used to identify and select journals to handsearch. 3 The most frequently mentioned approach is to identify which journals offer the highest yield of relevant studies for a specific review question and then to select a number of those journals that provide the largest number of studies as the candidate journals to handsearch. This approach is also endorsed by the Cochrane handbook for systematic reviews of diagnostic test accuracy. 1 Information retrieval advice in other subjects has also recommended this selection method. 7,8 High-yield journals were identified by selecting reviews of FDG PET for CRC retrieved by a search of Ovid MEDLINE in April 2009 and collecting the references of the studies included in those reviews. The references were obtained, de-duplicated and grouped by journal. From this frequency list, the 10 highest yielding journals to which we had electronic access were selected.
The high-yield journals were handsearched by accessing them in electronic form on their journal home sites. Journal issues from 2005 to June 2009 were handsearched.
Each article in each issue of each of the 10 journals was assessed for relevance by reading the title, abstract and as much of the paper as required to determine whether or not the paper reported the results of a diagnostic test accuracy study and in particular whether or not it focused on the diagnostic accuracy of FDG PET/CT imaging for CRC. In cases in which the journal issue contained conference abstracts in themed groups, only the sub-sections relevant to CRC or FDG PET were handsearched. Details of the journal, issue, time spent handsearching per issue and diagnostic test accuracy papers identified were recorded in an Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet.
The results from the handsearches were compared with the results of database searches conducted for the FDG PET/CT systematic review for the same years. The relative unique yields for handsearching and for database searching, and the overlap, were calculated. The average time spent searching per candidate study identified was calculated. The database searches and record assessment and selection were carried out independently from the handsearches.
Three handsearchers conducted the handsearch (MC, PL and JG). They participated in a 2-hour training session followed by several rounds of selection decision checking. An inter-relater reliability exercise was conducted to estimate the level of agreement between the two high-volume handsearchers (MC and JG) using a randomly selected 10% sample of journal issues.
In the final stage of the research, the studies included in the FDG PET/CT review were investigated to assess how far the journals in which they were published overlapped with the candidate journals that were handsearched. The journals in which the included studies were published were noted and the number of diagnostic test accuracy studies identified in each was collected. The two highest yield journals for included studies were then handsearched to identify any diagnostic test accuracy studies published in the period January 2005 to June 2009 inclusive. We also investigated the effect of removing the studies from the three more general reviews that contributed to the original frequency list to see if the order of candidate journals would have changed. We also analysed the disciplines of the candidate journals.
Results
The MEDLINE search for systematic reviews of FDG PET/CT for CRC identified 187 potentially relevant reviews. From these, six reviews were selected and their included studies were collected. 10–15 A total of 216 records of individual diagnostic test accuracy studies were sorted by journal name, and the journal names that appeared most frequently are shown in Table 1. Ten of the highest yielding 11 journals were selected for handsearching; the International Journal of Colorectal Diseases, which appears in the 10 highest yield journals, was not available to search free of charge through our institutional subscriptions.
| Journal name | Number of papers published 1985–98 | Number of papers published 1999–2005 | Papers in press | Total number of diagnostic test accuracy papers | Journal handsearched for this project |
|---|---|---|---|---|---|
| Radiology | 17 | 18 | 0 | 35 | Yes |
| Diseases of the Colon and Rectum | 15 | 11 | 0 | 26 | Yes |
| AJR: American Journal of Roentgenology | 8 | 5 | 1 | 14 | Yes |
| British Journal of Surgery | 10 | 4 | 0 | 14 | Yes |
| American Journal of Surgery | 2 | 5 | 0 | 7 | Yes |
| Gastrointestinal Endoscopy | 3 | 4 | 0 | 7 | Yes |
| International Journal of Colorectal Diseases | 6 | 0 | 0 | 6 | No |
| Abdominal Imaging | 3 | 2 | 0 | 5 | Yes |
| Archives of Surgery | 3 | 2 | 0 | 5 | Yes |
| Annals of Surgery | 2 | 2 | 0 | 4 | Yes |
| Gastroenterology | 1 | 3 | 0 | 4 | Yes |
A total of 573 journal issues from the 10 journals selected were handsearched. This process took 185 hours, not including the quality-checking process. The inter-rater reliability between the two high-volume handsearchers on record selection on a 10% random sample of 53 journal issues was found to have a Kappa value of 0.614 (p < 0.001), which falls just within the range of substantial agreement (0.61–0.8).
Through handsearching, 25 candidate diagnostic test accuracy records were identified (see Appendix, Table 4). Records were identified from full article, mixed full and abstract and abstract only journals. One candidate record was identified per 7.4 hours of handsearching. The time excludes the quality-checking process.
When the 25 records were checked against the database search results, seven of the handsearched records identified had not been identified by the database searches. However, once the full paper had been assessed, none of the seven studies fit the systematic review inclusion criteria. Some of the records were abstracts from the British Journal of Surgery and these were checked (searching on first and last authors) in MEDLINE for full reports, but none was identified at that time.
In addition to identifying diagnostic test accuracy studies of FDG PET/CT, this research also identified 855 potential diagnostic test accuracy studies for other conditions: 4.6 studies per handsearch hour.
The database searches for studies of FDG PET/CT had identified 30 studies that met the eligibility criteria. The journals in which these studies had been published were assessed for overlap with the journals that had been handsearched (Table 2). The 30 studies that met the FDG PET/CT review eligibility criteria appeared in 24 different journals, and three of these had been handsearched. None of the 21 non-handsearched journals had appeared as high-frequency journals in the original frequency analysis of the journals in which 200 relevant diagnostic test accuracy studies of FDG-PET had been published. The handsearch identified the one included study that was in a handsearched journal in the date range searched.
| Journal in which included studies were published | Number of included studies | Frequency of occurrence of studies from the frequency analysis of six reviews |
|---|---|---|
| European Journal of Nuclear Medicine and Molecular Imaging | 4 | 1 |
| Journal of Nuclear Medicine | 3 | 2 |
| European Radiology | 2 | 3 |
| Abdominal Imaging | 1 | 5 |
| Acta Radiologica | 1 | 3 |
| Annals of Surgery | 1 | 4 |
| Annals of Surgical Oncology | 1 | 3 |
| Cirugia Espanola | 1 | 0 |
| Colorectal Disease | 1 | 0 |
| Diseases of the Colon and Rectum | 1 | 26 |
| Indian Journal of Surgery | 1 | 0 |
| International Journal of Radiation Oncology, Biology, Physics | 1 | 1 |
| Japanese Journal of Clinical Oncology | 1 | 1 |
| Journal of Clinical Ultrasound | 1 | 0 |
| Journal of Computer Assisted Tomography | 1 | 3 |
| Journal of Gastrointestinal Surgery | 1 | 0 |
| Medecine Nucleaire | 1 | 0 |
| Nuclear Medicine Communications | 1 | 3 |
| Polski Merkuriusz Lekarski | 1 | 0 |
| Radiologia Medica | 1 | 1 |
| Radiology | 1 | 35 |
| Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin | 1 | 0 |
| World Journal of Gastroenterology | 1 | 0 |
| World Journal of Surgical Oncology | 1 | 0 |
The database searching and record selection process (excluding quality checking processes) took 126 hours and comprised the following activities:
-
20 hours spent searching, testing and downloading, de-duplicating and writing up
-
25 hours spent checking MEDLINE for full reports of conference proceedings
-
45 hours for selection of records from the database searches
-
36 hours spent checking the references of review articles and primary studies.
This represented one eligible study per 4.2 hours of searching and selection.
The two journals that published the highest number of included studies for the current review were then handsearched: the Journal of Nuclear Medicine and the European Journal of Nuclear Medicine and Molecular Imaging. The total time taken to handsearch the two additional journals (including the construction and population of the proformas) was 5.6 days. The European Journal of Nuclear Medicine and Molecular Imaging yielded a greater number of FDG PET/CT studies than the Journal of Nuclear Medicine (15 vs 3), although the total number of potentially relevant diagnostic test accuracy studies for all conditions was similar (108 vs 103). The mean time spent searching to identify a potentially relevant study in the two journals was 2.2 hours for FDG PET/CT and 0.2 hours for any diagnostic test accuracy study.
Of the 18 records identified by handsearching, six records had not already been retrieved by the database searches. All six records were conference abstracts. The records were assessed for relevance and checked in MEDLINE for full publications. One record was rejected because it was a study of separate PET and CT machines, three records had been published as full reports and are included in the reviews and two records do not seem to have full publications yet.
An analysis of the broad discipline categories of the journals that published diagnostic test accuracy studies is presented in Table 3.
| Review | Number of studies from the six FDG PET reviews per journal category | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer journals | Clinical journals | Colorectal journals | Gastrointestinal journals | General medical journals | Imaging journals | Surgical journals | Total number of studies | |
| Bipat 200410 | 2 | 2 | 23 | 1 | 3 | 30 | 26 | 87 |
| Bipat 200511 | 0 | 2 | 2 | 0 | 0 | 30 | 9 | 43 |
| Halligan 200512 | 0 | 2 | 0 | 5 | 9 | 24 | 2 | 42 |
| Huebner 200013 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 4 |
| Purkayastha 200714 | 0 | 0 | 6 | 0 | 1 | 0 | 3 | 10 |
| Wiering 200515 | 2 | 5 | 2 | 0 | 1 | 7 | 13 | 30 |
| Total number of studies | 4 | 11 | 34 | 6 | 14 | 92 | 55 | 216 |
The highest number of diagnostic test accuracy studies was reported in imaging journals, followed by surgical journals and then colorectal journals.
Discussion
We had expected that the handsearch would identify reports that had not been retrieved by the electronic searches. Although handsearching did identify publications that we had not previously encountered, it did not yield unique relevant diagnostic test accuracy studies. This result may be attributable to several factors. First, it may be a tribute to the sensitivity of the systematic review database searches and reference checking of reviews conducted for the project. It may also be the case that FDG PET/CT studies are relatively easy to retrieve in a sensitive way because the terms used to describe FDG PET/CT in abstracts and titles are reasonably standard. The search strategy development was informed by previous investigations of optimal searching for FDG PET conducted by Mijnhout et al. 16–17 and modified by a member of this team (MD) to maximise sensitivity.
There is little overlap between the journals that were handsearched and the journals in which the included studies were published: only three journals. The high-yield journal list used to identify journals to handsearch emerged from FDG-PET reviews. The journals in which the included studies selected for this review were published include some that did not yield any articles in previous reviews. This seems to indicate that FDG PET/CT studies are highly scattered in journals in many disciplines, and that it is difficult to predict where researchers may publish nuclear imaging diagnostic test accuracy studies and where handsearching may be most fruitful. This exercise may also indicate that the frequency analysis approach to identify journals for handsearching may not help when searching for imaging studies. It may be better to handsearch later in the review when included studies are emerging rather than basing the handsearch selection on previous reviews.
There is one area in which this handsearch was not comprehensive. Some journal issues contained conference abstracts. Where conference abstracts were grouped by session theme, only the relevant groups of abstracts were handsearched: sessions on CRC or imaging FDG-PET. This means that relevant abstracts may have been missed if they were categorised into different sessions from those we searched. In addition, non-FDG-PET diagnostic test accuracy conference abstracts in other sessions will also not have been captured.
It is possible that the choice of reviews to mine for included studies biased the journal frequency table. We investigated this by assessing the effect of removing some of the more general of the original reviews from the journal list, to see if this would have raised to prominence some lower-frequency journals from the original list. The reviews by Bipat et al. ,10 Halligan et al. 12 and Purkayastha et al. 14 consider techniques other than FDG PET and might, in retrospect, be deemed less pertinent to the final topic of this review. The references from those three reviews were removed from the initial journal frequency list to investigate the impact on high-frequency journals. The revised frequency list still ranked seven of the journals originally identified for handsearching in the top 10 (see the appendix, Table 5 for details). When the revised frequency list was compared with the journals in which the included studies for this review were published, the overlap was still four journals. Neither the revised or original frequency list highlighted the European Journal of Nuclear Medicine and Molecular Imaging as a candidate for handsearching, despite it yielding the highest number of included studies in this review.
In comparison with the other ‘high-yield’ journals searched, the European Journal of Nuclear Medicine and Molecular Imaging would appear to be an extremely good source of diagnostic test accuracy studies focusing on FDG PET/CT in CRC. Searching the two high-yield journals arising from the database searches had a substantial impact on the number of diagnostic test accuracy studies identified by handsearching. The total number of candidate records increased by approximately 70% (from 25 to 43) and the time spent handsearching to identify one candidate record fell by approximately 30% (from 7.4 to 5.2 hours).
The analysis of the journals by broad discipline shows that the publication of FDG PET/CT diagnostic test accuracy studies is widely scattered. The journal categories were very broad and approximate, with no overlap between categories allowed, and thus may not reflect the exact nature or encompass the true diversity of the journals. For example, the journal Radiology is not specific to pure imaging applications as it also reports studies relating to treatment. However, the exercise does illustrate that any search for diagnostic test accuracy studies for imaging cannot be easily limited to specific disciplines, and means that identifying journals to handsearch in this topic is problematic.
Conclusion
Handsearching is time-consuming and expensive. In this review, handsearching did not yield additional unique studies relevant to FDG-PET in addition to database searching and reference checking. Explanations for the low yield may be that the database searches were highly sensitive and FDG PET/CT studies tend to be consistently described and hence are easier to retrieve. The value of handsearching to identify studies of less clearly defined or reported diagnostic tests remains to be investigated. It was frequently difficult, during the preliminary identification of potentially relevant studies, to identify whether or not a paper was reporting a diagnostic test accuracy study from the abstract alone. Abstracts often allude to diagnosis or diagnostic issues, but only on reading the full paper does it become apparent that diagnostic test performance measures are not reported.
Handsearching did yield conference abstracts that may be useful clues or prompts to look for later published studies. Conference abstract publication rates may also give insight into publication bias in diagnostic tests.
Identifying the highest yield journals to handsearch for imaging studies may be difficult because of the scatter of studies over journals from many disciplines. The approach to identifying candidate journals to handsearch for imaging studies and diagnostic test studies more generally may require further investigation. Inevitably, in a topic in which there is publication in many different journals, the database and related searches are likely to provide the highest and most efficient yield of study reports.
Handsearching yields reports of many other diagnostic test accuracy studies in addition to those for the topic of interest, and this may be a useful byproduct of handsearching for a specific issue. It would be particularly valuable for future reviews in all topics if those studies identified by handsearching are contributed to the Cochrane Register of Diagnostic Test Accuracy Studies. As the Cochrane Register grows the need for handsearching should diminish.
References
- de Vet HCW, Eisinga A, Riphagen II, Aertgeerts B, Pewsner D. Cochrane handbook for systematic reviews of diagnostic test accuracy. Cochrane Collaboration; 2008.
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration; 2008.
- Hopewell S, Clarke M, Lefebvre C, Scherer RW. Handsearching versus electronic searching to identify reports of randomized trials. Cochrane Database Syst Rev 2007;2.
- Whiting P, Westwood M, Burke M, Sterne J, Glanville J. Systematic reviews of test accuracy should search a range of databases to identify primary studies. J Clin Epidemiol 2008;61:357-64.
- van der Weijden T, Ijzjermans CJ, Dinant GJ, van Duijn NP, de Vet R, Buntinx F. Identifying relevant diagnostic studies in MEDLINE. The diagnostic value of the erythrocyte sedimentation rate (ESR) and dipstick as an example. Fam Pract 1997;14:204-8.
- Vincent S, Greenley S, Beaven O. Clinical evidence diagnosis: developing a sensitive search strategy to retrieve diagnostic studies on deep vein thrombosis: a pragmatic approach. Health Info Libr J 2003;20:150-9.
- Armstrong R, Jackson N, Doyle J, Waters E, Howes F. It’s in your hands: the value of handsearching in conducting systematic reviews of public health interventions. J Public Health 2005;27:388-91.
- Torgerson C. Systematic reviews and meta-analysis. London: Continuum International Publishing; 2003.
- Hopewell S, Clarke M, Lusher A, Lefebvre C, Westby M. A comparison of handsearching versus MEDLINE searching to identify reports of randomized controlled trials. Stat Med 2002;21:1625-34.
- Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging – a meta-analysis. Radiology 2004;232:773-83.
- Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis – meta-analysis. Radiology 2005;237:123-31.
- Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 2005;237:893-904.
- Huebner RH, Park KC, Shepherd JE, Schwimmer J, Czernin J, Phelps ME, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med 2000;41:1177-89.
- Purkayastha S, Tekkis PP, Athanasiou T, Tilney HS, Darzi AW, Heriot AG. Diagnostic precision of magnetic resonance imaging for preoperative prediction of the circumferential margin involvement in patients with rectal cancer. Colorectal Dis 2007;9:402-11.
- Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 2005;104:2658-70.
- Mijnhout GS, Hooft L, van Tulder MW, Deville WL, Teule GJ, Hoekstra OS. How to perform a comprehensive search for FDG-PET literature. Eur J Nucl Med 2000;27:91-7.
- Mijnhout GS, Riphagen II, Hoekstra OS. Update of the FDG PET search strategy. Nucl Med Commun 2004;25:1187-9.
Appendix
Table 5 compares the numbers of studies retrieved from each journal depending on whether studies from six or three reviews were assessed. The revised frequency list ranked seven of the journals identified for handsearching (from the original list; marked ***) in the top 10. A further journal lost its top 10 ranking, while the three remaining journals failed to make the list. There was little change when considering the list of journal sources of studies included in the review (marked +). Neither the revised or original frequency list highlighted the European Journal of Nuclear Medicine and Molecular Imaging for handsearching, despite it yielding the highest number of included studies. The revised frequency list also failed to highlight Abdominal Imaging, the second highest yielding journal.
| Author | Journal title | Year | Volume | Issue | Pages | Single study or review (S/R) | Q = potentially relevant (e.g. may be insufficient details) |
|---|---|---|---|---|---|---|---|
| Dromain C | Abdominal Imaging | 2008 | 33 | 1 | 87–93 | S | Q |
| Rosenbaum SJ | Abdominal Imaging | 2006 | 31 | 1 | 25–35 | R | Q |
| Shin SS | Abdominal Imaging | 2008 | 33 | 3 | 270–7 | R | Q |
| Squillaci E | Abdominal Imaging | 2008 | 33 | 6 | 676–88 | S | Q |
| Badiee S | American Journal of Roentgenology | 2008 | 191 | 5 | 1436–9 | S | |
| Goodman LR | American Journal of Roentgenology | 2007 | 189 | 2 | 409–12 | S | Q |
| Gutman F | American Journal of Roentgenology | 2005 | 185 | 2 | 495–500 | S | |
| Nakamoto Y | American Journal of Roentgenology | 2007 | 188 | 1 | 257–67 | S | |
| Summers RM | American Journal of Roentgenology | 2008 | 191 | 1 | 168–74 | S | Q |
| Joyce DL | Archives of Surgery | 2006 | 141 | 12 | 1220–6 | S | Q |
| Wren SM | Archives of Surgery | 2006 | 141 | 12 | 1227 | S | Q – critique on Joyce |
| Gardner-Thorpe J | British Journal of Surgery | 2008 | 95 | S7 | Abstract ID500 | S | Q – conference |
| Pakzed F | British Journal of Surgery | 2006 | 93 | S1 | Poster 10055 | S | Q – conference |
| Rogers S | British Journal of Surgery | 2006 | 93 | S1 | Abstract 10957 | S | Q – conference |
| Skelly RT | British Journal of Surgery | 2006 | 93 | S1 | Abstract 10554 | S | Q – conference |
| Truant S | British Journal of Surgery | 2005 | 92 | 3 | 362–9 | S | |
| Davey K | Diseases of the Colon and Rectum | 2008 | 51 | 7 | 997–1003 | S | Q |
| Kristiansen C | Diseases of the Colon and Rectum | 2008 | 51 | 1 | 21–5 | S | Q |
| Nagata K | Diseases of the Colon and Rectum | 2008 | 51 | 6 | 882–90 | S | Q – FDG PET/CT colonography |
| Potter KC | Diseases of the Colon and Rectum | 2009 | 52 | 2 | 253–9 | S | |
| Watson AJM | Diseases of the Colon and Rectum | 2007 | 50 | 1 | 102–14 | R | |
| Lang L | Gastroenterology | 2007 | 132 | 2 | 473–4 | S | Q – news item |
| Blodgett TM | Radiology | 2007 | 242 | 2 | 360–85 | R | |
| Margolis DJA | Radiology | 2007 | 242 | 2 | 333–56 | R | |
| von Schulthess GK | Radiology | 2006 | 238 | 2 | 405–22 | R |
| Journal | Total studies retrieved from 1985–2005 | Ranking | ||
|---|---|---|---|---|
| Three reviews | Six reviews | New (three reviews) | Old (six reviews) | |
| Radiology+ | 18 | 35*** | 1 | 1*** |
| AJR American Journal of Roentgenology | 6 | 14*** | 2 | 3*** |
| Diseases of the Colon and Rectum+ | 5 | 26*** | 3 | 2*** |
| American Journal of Surgery | 4 | 7*** | 4 | 5*** |
| Archives of Surgery | 4 | 5*** | 4 | 8*** |
| Journal of Clinical Oncology | 3 | 3 | 6 | 18 |
| Nuclear Medicine Communications+ | 3 | 3 | 6 | 12 |
| Annals of Surgery+ | 2 | 4*** | 8 | 10*** |
| Annals of Surgical Oncology+ | 2 | 3 | 8 | 12 |
| British Journal of Surgery | 2 | 14*** | 8 | 3*** |
| Clinical Radiology | 2 | 3 | 8 | 12 |
| European Journal of Surgical Oncology | 2 | 2 | 8 | 20 |
| Journal of Nuclear Medicine+ | 2 | 2 | 8 | 20 |
| World Journal of Surgery | 2 | 2 | 8 | 20 |
| Abdominal Imaging+ | 1 | 5*** | 15 | 8*** |
| Acta Radiologica+ | 1 | 3 | 15 | 12 |
| The American Surgeon | 1 | 1 | 15 | 33 |
| Annals of Nuclear Medicine | 1 | 1 | 15 | 33 |
| Annals of the Royal College of Surgeons of England | 1 | 1 | 15 | 33 |
| Anticancer Research | 1 | 1 | 15 | 33 |
| Australian and New Zealand Journal of Surgery | 1 | 1 | 15 | 33 |
| Clinical Nuclear Medicine | 1 | 1 | 15 | 33 |
| Clinical Positron Imaging | 1 | 1 | 15 | 33 |
| European Journal of Cancer | 1 | 1 | 15 | 33 |
| European Journal of Nuclear Medicine | 1 | 1 | 15 | 33 |
| European Journal of Nuclear Medicine and Molecular Imaging+ | 1 | 1 | 15 | 33 |
| European Journal of Radiology+ | 1 | 3 | 15 | 12 |
| European Journal of Surgery | 1 | 2 | 15 | 20 |
| Journal of Computer Assisted Tomography+ | 1 | 3 | 15 | 12 |
| Journal of Magnetic Resonance Imaging | 1 | 2 | 15 | 20 |
| Langenbeck’s Archives of Surgery | 1 | 1 | 15 | 33 |
| MAGMA | 1 | 1 | 15 | 33 |
| Surgery | 1 | 2 | 15 | 20 |
| Tokai Journal of Experimental and Clinical Medicine | 1 | 1 | 15 | 33 |
| Total | 77 | 156 | ||
| Medium- to high-yielding journals not appearing on revised list | Total studies | Old ranking | ||
| Gastrointestinal Endoscopy | 7*** | 5 | ||
| International Journal of Colorectal Disease (not available to search) | 6*** | 7 | ||
| Gastroenterology | 4*** | 10 | ||
| European Radiology + | 3*** | 12 | ||
Appendix 5 Systematic review protocol: FDG PET-CT imaging for pre-operative staging in patients with primary colorectal cancer
Background
Colorectal cancer (cancer of the large bowel) is the third most common cancer worldwide and the second most common cause of cancer death in the Western world. The global incidence was estimated at over one million new cases diagnosed worldwide in 2002 (GLOBOSCAN 2002). Accurate staging to determine the extent of local and distant disease is important to inform the decision about treatment. Curative surgery is an option for most patients with localised disease, some patients with recurrent disease may be suitable for salvage surgery, and surgical removal of metastases (metastectomy) may be an option for some patients with advanced disease. Accurate pre-operative assessment of tumour stage, lymph node involvement and distant metastases is fundamental to informing clinical decisions about pre-operative adjuvant radiochemotherapy for localised rectal cancer and to guide patient selection for salvage surgery and metastectomy (Herbertson et al. 2009).
A number of imaging modalities are used in the pre-operative staging of colorectal cancers including ultrasound (US), computerised tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET). Largely due to its wider availability and relatively low cost, contrast enhanced CT is the technique most commonly used in conventional imaging for colorectal cancer diagnosis and tumour, lymph node and metastatic (TNM) staging. CT uses X-rays to produce images of anatomical (structural) changes due to malignancy and an intravenous contrast agent, such as iodine, is used to increase the contrast between tumour and normal tissue (contrast enhanced CT). Conventional CT provides high spatial resolution but is limited in its ability to accurately distinguish benign from malignant processes on the basis of structural information alone, and image interpretation can be difficult where normal anatomy is distorted, for example by local scarring (Chin 2008). Supplementary imaging is often needed to provide sufficient information to inform surgical decisions; however, US and MRI like CT produce purely anatomical images. For some patients the information obtained from imaging is still insufficient to avoid operative (surgical) evaluation to determine if curative resection is feasible (Hicks et al. 2006).
Positron emission tomography is a type of nuclear medicine imaging that involves introducing a radioactive tracer into the patient’s body, either by injection or oral ingestion, prior to the scanning procedure. The glucose analogue F-fluorine-18-deoxyglucose glucose (FDG) is currently the tracer most widely used in oncology. Tumours take up FDG through the natural process of glucose metabolism; the accumulated tracer emits gamma radiation that can be detected by the PET camera and appears as bright spots on the PET image. FDG PET imaging alone is limited by a lack of anatomical detail; furthermore, some colorectal cancers (such as mucinous adenocarcinomas) can be false negatives due to limited uptake of FDG and false positive uptake can occur, for example in the presence of inflammation (Herbertson et al. 2009). Modern scanners now combine PET and CT in a single machine that performs both imaging studies – these integrated PET-CT images combine the anatomical detail of CT with the functional detail of PET. Another advantage of integrated PET-CT is that the addition of the CT camera enables faster attenuation correction of the PET image, thereby increasing patient throughput. While most PET-CT scans are currently performed without contrast agents (non-contrast enhanced CT) the role of contrast enhanced PET-CT has been evaluated in more recent studies (Herbertson et al. 2009).
While the whole-body approach to PET/CT imaging allows broad coverage of multiple organs the radiation dose to the patient is potentially much higher than in conventional CT (Zanzonico et al. 2008). The radiation dose per full body PET-CT scan is around 15–25 mSv of which 3–15 mSv is attributable to CT radiation (Hays et al. 2002). The global average background dose of radiation is approximately 2.4 mSv per person per year (WHO 2004). Reduction in the parameters of the CT scan in PET-CT considerably reduces the effective radiation dose to the patient without compromising attenuation correction or registration of anatomical detail (Zanzonico et al. 2008). Regulatory agencies in the USA, UK and several other European countries have concluded that the use of FDG is safe for oncology patients undergoing PET (FDA 2000; MHRA 2008). Staff radiation exposure can be maintained below regulatory limits by appropriate design, particularly shielding, and workflow in PET-CT facilities (Zanzonico et al. 2008). Although the patient remains radioactive after the scan this reduces quickly over time (the half-life of fluorine-18 is 109 minutes); however, people in contact with the patient once they leave the hospital will receive a small dose of radiation. The associated risk of harm from such low doses is thought to be low although the risk to repeated contacts such as hospital drivers remains uncertain.
Several studies have shown PET-CT to be more accurate than diagnostic CT and stand-alone PET for cancer staging including of colorectal cancer (Hicks et al. 2006; Bar-Shalom et al. 2003; Cohade et al. 2003). Replacing diagnostic CT with PET-CT as the initial imaging investigation has considerable resource and cost implications. Currently, PET-CT is widely recommended for the assessment of suspected recurrence of colorectal cancer and in pre-operative staging prior to metastectomy, and it has an up-and-coming role in the initial staging of primary rectal cancer (Herbertson et al. 2009; Ell et al. 2006; Heriot et al. 2004). Clinical opinion on the role of PET-CT in the routine management of primary colon cancer varies: some investigators suggest that in certain clinical circumstances it should be considered as part of the standard pre-operative assessment (Park et al. 2006). Cost and resource considerations currently limit PET-CT use to an add-on test in most centres where the technology is available. A systematic review is warranted to inform how diagnostic accuracy, subsequent management and patient outcome are changed by the addition of PET-CT in the initial primary colorectal cancer staging pathway.
This systematic review is one of three Cochrane reviews of diagnostic test accuracy undertaken alongside a wider Health Technology Assessment of the clinical and cost-effectiveness of FDG PET-CT for staging primary, recurrent and metastatic colorectal cancer.
Objectives
The primary objective is to determine the diagnostic accuracy of integrated FDG PET-CT over (in addition to) conventional imaging for the pre-operative staging of primary colorectal cancer. The comparisons of interest are:
-
FDG PET-CT combined with pelvic MRI or routinely used imaging modalities versus routinely used imaging modalities (CT chest/abdomen/pelvis combined with pelvic MRI) for pre-operative staging of primary rectal cancer.
-
FDG PET-CT in addition to routinely used imaging modalities versus routinely used imaging modalities for pre-operative staging of primary colon cancer.
Secondary objectives
The secondary objective is to determine the impact of diagnostic information provided by FDG PET-CT over conventional imaging techniques on decisions about patient management. We will also assess adverse effects reported in the included studies.
Investigation of sources of heterogeneity
Several potential sources of heterogeneity have been identified in other (non-Cochrane) systematic reviews and meta-analyses of diagnostic imaging techniques in colorectal cancer (Bipat et al. 2004, 2005; Halligan et al. 2005; Huebner et al. 2000; Purkayastha et al. 2006; Wiering et al. 2005). These were considered by the clinical authors of this review who identified the factors most likely to affect diagnostic accuracy in studies of FDG PET-CT.
We will investigate the following potential sources of heterogeneity, using subgroup analysis where possible: academic (e.g. university hospital) versus non-academic setting; indication known or suspected; study conducted up to 2005 and post 2005 (reflecting differences in PET-CT technology); blinding of index and reference standard test interpretation or not.
Heterogeneity in the statistical analysis will initially be assessed graphically and where possible using meta-regression (see Investigations of heterogeneity, below).
Methods
Criteria for considering studies for this review
Types of studies
Prospective and retrospective patient series (diagnostic cohort) and randomised controlled trials (RCTs) will be eligible for inclusion. Both consecutive series and series that are not explicitly reported as consecutive will be included. Diagnostic case–control studies (two-gate design) will be excluded because clinically relevant estimates of specificity and sensitivity can only be derived from the clinical population and not healthy controls.
Participants
Adults with known or suspected primary cancer of the colon or rectum, undergoing pre-operative staging prior to curative surgery in a secondary care setting, will be eligible for inclusion. Patients with any stage of disease will be included. Studies solely in patients with anal cancer will be excluded because this rare cancer differs from colorectal cancers both biologically and in terms of the treatment pathway. Studies that include colon and rectal and anal cancer patients where data are not reported separately for the colon/rectal and anal cancer groups will be included in the review only if less than 20% had anal cancer (the effect of including any such studies will be explored using sensitivity analysis where possible).
Index tests
Integrated FDG PET-CT. Studies using both contrast enhanced and non-contrast enhanced CT will be included.
Comparator tests
Standard imaging tests including US, diagnostic CT, MRI and PET, alone or in combination.
Target conditions
Known or suspected primary colorectal cancer.
Reference standards
Surgical histopathology is the gold standard for colorectal cancer staging; however, patients who do not undergo surgical resection will have test results verified by an alternative standard. These include histopathology based on biopsy and clinical and imaging follow-up. Studies using these reference standards singly or mixed will be eligible for inclusion. Any duration of follow-up and frequency of testing will be included.
Search methods for identification of studies
Studies will be identified through searching a range of electronic databases shown to yield diagnostic test accuracy studies. In order to avoid language bias we will include studies published in all languages.
Electronic searches
Studies will be identified through searching a range of electronic databases shown to yield diagnostic test accuracy studies, including MEDLINE, EMBASE, Science Citation Index, BIOSIS and LILACS [CENTRAL, CINAHL (1982–2008), Compandex (1972–2008), EMBASE (1980–2008), Global Health (1972–2008), Inspec (1969–2008), LILACS (1982–2008), MEDLINE (1950–2008), Web of Sciences (1900–2008)].
The search strategy will involve a range of relevant database subject headings and text words
Database: OvidSP MEDLINE(R) 1996 – May, week 4, 2009
Search strategy:
-
exp Fluorodeoxyglucose F18/ (8115)
-
exp Tomography, Emission-Computed/ (35,123)
-
radiopharmaceuticals/ (22,612)
-
FDG PET$.ti,ab. (4903)
-
F-18-FDG PET$.ti,ab. (264)
-
(F-18 fluorodeoxyglucose adj3 pet$).ti,ab. (93)
-
positron emission tomography.ti,ab. (14,340)
-
or/1-7 (48905)
-
exp Colorectal Neoplasms/ (54,891)
-
exp Liver Diseases/ (128,417)
-
(rectal adj3 cancer$).ti,ab. (5440)
-
(colon$adj3 cancer$).ti,ab. (15,417)
-
(rectum adj3 cancer$).ti,ab. (409)
-
liver disease.ti,ab. (16,355)
-
or/9-14 (185,822)
-
8 and 15 (1958)
-
animals/not (humans/and animals/) (1,129,924)
-
16 not 17 (1834)
Database: OvidSP EMBASE 1980 – May, week 4, 2009
Searching other resources
Studies will also be identified through contact with experts in the field. In addition, information on studies in progress, unpublished research or research reported in the grey literature will be identified by searching databases including the UKCRN portfolio, the metaRegister of Current Controlled Trials, Clinicaltrials.gov, the (US) National Cancer Institute trials resource, NTIS, ISI Proceedings, OpenSIGLE, Digital Dissertations, and Index to Theses. Internet searches will also be conducted using specialist search engines such as OMNI. We will also search the web pages of key organisations including ASCO and ESTRO. We will also use Google Scholar and Science Citation Index to identify papers that cite (key) test reports. We will also search the reference lists of relevant studies and existing review articles for relevant studies.
Data collection and analysis
Selection of studies
One reviewer (FCr) will screen the titles and abstracts retrieved by the electronic searches and a second reviewer (FCh) will check the decisions on a random sample of 25%. Full papers will be obtained for potentially eligible studies. Two reviewers (HMc, FCr) will then independently apply the inclusion criteria to the full papers and resolve disagreements by discussion. The inclusion criteria will be applied in the same way to the full reports of studies identified through sources other than electronic databases. An overview of the selection process will be summarised in a flow diagram.
Data extraction and management
Data will be extracted by two reviewers (HMc, FCr) independently using a standard form, which will include the quality assessment criteria, and disagreements will be resolved by discussion. Data to populate 2 × 2 contingency tables consisting of the numbers of true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN) will be extracted as reported, including both patient-level and lesion-level data, and qualitative and quantitative definitions of diagnostic thresholds. Numbers of uninterpretable test results will also be extracted.
Data will also be extracted to describe the clinical characteristics of FP and FN PET-CT findings, and additional cases detected and cases re-staged by the use of PET/CT; and actual changes in planned management directed by PET/CT findings and the clinical consequences of the changes. Data on mortality, adverse events (including how these were monitored and recorded), and technical failures for both index and comparator tests will also be extracted.
To facilitate interpretation of the findings we will also extract data on comorbidities (e.g. diabetes) and previous treatment in the study population; the PET-CT system; fasting duration; FDG dose and time between administration of FDG and performance of the scan; comparator imaging test system(s), patient preparation and test interpretation; interval between index and comparator tests (more or less than 3 months); assessors (number, expertise, experience, consensus procedures and learning effect data); and in regard to the reference standard whether histopathology was by surgery or biopsy and the duration, frequency, type and interpretation of clinical and imaging follow-up tests, and the numbers of patients whose results were confirmed by each type of reference standard.
Assessment of methodological quality
Fourteen items from the QUADAS checklist will be used to assess the methodological quality of the included studies. Two reviewers (HMc, FCr) will apply the criteria independently and resolve disagreements by discussion. The results of the quality assessment will be used for descriptive purposes to provide an evaluation of the overall quality of the included studies and to investigate potential sources of heterogeneity (Whiting et al. 2004).
The classification of responses to each of the QUADAS items is summarised in Table 1.
| 1. Was the spectrum of patients representative of the patients who will receive the test in practice? |
Yes: The study included a consecutive series or random sample of adults undergoing staging for known or suspected primary cancer of the colon or rectum who received PET/CT at any point in the pre-operative staging pathway, either as the first imaging investigation or referred following equivocal or suspicious findings on other tests including imaging, clinical exam and carcinoembryonic antigen (CEA) test No: The study included a non-consecutive or non-random sample, or there is clear evidence of selective sampling, e.g. restriction to patients with particular findings on PET/CT Unclear: Insufficient information on the method of recruitment and selection criteria |
| 2. Is the reference standard likely to correctly classify the target condition? |
Yes: Surgical histopathology if surgical resection, or clinical/imaging follow-up of at least 6 months No: Criteria clearly not met Unclear: Insufficient information |
| 3. Is the time period between the index test and reference standard short enough to be reasonably sure that the target condition did not change between the two tests? |
Yes: If the time between PET/CT and the reference standard is less than 6 weeks, the clinical rational being a finding on PET/CT in a patient with colon or rectal cancer should be acted upon within 6 weeks of study, or disease progression may occur. Similarly a negative finding should really be confirmed within the same timescale No: ‘Yes’ criteria clearly not met Unclear: The time lapse between tests was not reported |
| 4. Did the whole sample or a random selection of the sample receive verification using a reference standard of diagnosis? |
Yes: It is reported that all or a random sample of the study participants did receive verification of their disease status using the reference standard defined in 2 above No: All participants did not receive verification using the reference standard and those who did were not selected randomly Unclear: Insufficient information |
| 5. Did patients receive the same reference standard regardless of the index test result? |
Yes: All PET/CT results were verified by the same reference standard No: Some PET/CT results were verified by a different reference standard Unclear: Insufficient information |
| 6. Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? |
Yes: PET/CT was not used in establishing the final diagnosis (i.e. PET/CT was not a component of the reference standard) No: PET/CT did form part of the reference standard (NB: this does not include the PET/CT index test result being known when the reference standard diagnosis was made, only the specific incorporation of PET/CT as part of the reference standard test) Unclear: Insufficient information |
| 7. Were the index test results interpreted without knowledge of the results of the reference standard? |
Yes: It is clearly stated that PET/CT results were interpreted blind to the results of the reference standard; or the PET/CT results were clearly interpreted before the results of the reference standard were available No: It is clearly stated that PET/CT results were interpreted with knowledge of the results of the reference standard Unclear: Insufficient information |
| 8. Were the reference standard results interpreted without knowledge of the results of the index test? |
Yes: It is clearly stated that the results of the reference standard were interpreted blind to the PET/CT results No: It is clearly stated that the results of the reference standard were interpreted with knowledge of the PET/CT results Unclear: Insufficient information |
| 9. Were the same clinical data available when the test results were interpreted as would be available when the test is used in practice? |
Yes: It is stated that the clinical information, including all previous test results, that would normally be available to the assessors when PET/CT images are interpreted in practice was available when the index test was evaluated No: It is stated that the clinical information that would normally be available when PET/CT images are interpreted in practice was not available to the assessors when the index test was evaluated Unclear: Insufficient information |
| 10. Were uninterpretable/intermediate test results reported? |
Yes: It is clear that all test results including those that were uninterpretable, indeterminate or intermediate are reported, or it is clear that there were none No: It is reported that uninterpretable, indeterminate or intermediate test results were excluded with no further information given, or there is reason to believe that such results occurred but were not reported Unclear: It is not clear whether there were any uninterpretable, indeterminate or intermediate results |
| 11. Were withdrawals from the study explained? |
Yes: All participants who were entered in the study either completed the study or were accounted for if they did not complete the study (reasons for withdrawal and loss to follow-up were given) No: Some participants who entered the study did not complete the study and were not accounted for Unclear: Insufficient information to judge whether all participants who entered the study were accounted for |
Statistical analysis and data synthesis
Results of the evaluation of the accuracy of PET-CT will be analysed in the following way.
Because of methodological problems, particularly those caused by the difficulty of estimating within-study variance where patients contribute more than one data point, and the individual patient data are not available, the 2 × 2 tables will be reported for the lesion-level data and the analyses will be restricted to the patient-level data.
The 2 × 2 tables for the patient-level data will be used to calculate sensitivity and specificity with confidence intervals. Data will be plotted graphically in both forest and receiver operating characteristic (ROC) plots. The forest plots will give an indication of the extent of heterogeneity between studies and the ROC plot if any heterogeneity is likely to be due to the threshold effect.
A random effects meta-analysis will be undertaken to fit the bivariate SROC curve model (Harbord et al. 2006) with the within-study variances fitted as binomial. The DiagMeta package in R will be used for all meta-analyses (www.diagmeta.info). If the data are not amenable to bivariate random effects meta-analyses, separate meta-analyses each for sensitivity and specificity will be presented instead, using fixed or random effects as appropriate depending on the degree of heterogeneity. Estimates will include the average sensitivity and specificity for each test and differences in sensitivity and specificity between PET-CT and each comparator.
Data for outcomes other than diagnostic test accuracy (changes in patient management, mortality) will be presented as relative and absolute risks, and, where appropriate, meta-analysis will be used to calculate pooled estimates using relative risk with 95% confidence intervals.
Investigations of heterogeneity
Heterogeneity due to individual diagnostic studies using different diagnostic thresholds will be explored as a standard part of fitting the bivariate SROC curve model. Heterogeneity due to other study characteristics will be explored with meta-regression, but only if the data are adequate for such analyses. In either case, graphical displays of estimates from individual studies grouped according to the prespecified sources of heterogeneity will be provided.
Sensitivity analyses
Where possible we will conduct sensitivity analysis by including only prospective studies (excluding retrospective), studies with explicitly consecutive samples (excluding non-consecutive or unclear), studies with a histopathology (surgery or biopsy) reference standard for all participants (excluding studies where some or all participants received only clinical or imaging follow-up as the reference standard) and studies that included only rectal and colon cancer patients (excluding anal cancer).
Assessment of reporting bias
A formal assessment of reporting bias will not be undertaken as there are as yet no accepted methods to do this (Brazzeli et al. 2009). However, the possibility of reporting bias will be highlighted and the results of any analysis interpreted cautiously.
Contributions of authors
Draft the protocol All review authors
Develop a search strategy Marshall Dozier and Julie Glanville
Search for trials Marshall Dozier
Select which trials to include Fay Crawford, Francesca Chappell and Heather McIntosh
Extract data from trials Fay Crawford and Heather McIntosh
Enter data into revman Fay Crawford and Heather McIntosh
Carry out the analysis Francesca Chappell
Interpret the analysis All review authors
Draft the final review All review authors
References
-
Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 2003;44:1200–9.
-
Bipat S, Glas AS, Slor FJM, Zwinderman AH, Bossuyt PMM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging? A meta-analysis. Radiology 2004;232:773–83.
-
Bipat S, Maarten S, van Leeuwen, Comans EFI, Pili MEJ, Bossuyt PMM, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis? Meta-analysis. Radiology 2005;237:123–31.
-
Brazzelli M, Lewis S, Deeks JJ, Sandercock P. No evidence of bias in the process of publication of diagnostic accuracy studies in stroke submitted as abstracts. J Clin Epidemiol 2009;62:425–30.
-
Chin BB. Clinical utility of combined 18F-fluoro-2-deoxyglucose positron emission tomography-computed tomography in the evaluation of gastrointestinal malignancies. Curr Med Imaging Rev 2008;4:255–69.
-
Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of 18F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med 2003;44:1797–803.
-
Ell PJ. The contribution of PET/CT to improved patient management. Br J Radiol 2006;79:32–6.
-
FDA. Federal Register Notice by FDA announcing safety of and approval of PET. Federal Register, 10 March 2000;65:13010–2.
-
GLOBOSCAN. Data held by the Descriptive Epidemiology Groups of IARC and provided by CANCER Mondial. 2002. URL: www-dep.iarc.fr
-
Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CL, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis and proposed minimum data set for studying level reporting. Radiology 2005;237:893–904.
-
Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC. A unification of models of meta analysis for diagnostic accuracy studies. Biostatistics 2006;1:1–21.
-
Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimation report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med 2002;43:210–4.
-
Herbertson RA, Scarsbrook AF, Lee ST, Tebbutt N, Scott AM. Established, emerging and future roles of PET/CT in the management of colorectal cancer. Clin Radiol 2009;64:225–37.
-
Heriot AG, Hicks RJ, Drummond EGP, Keck J, Mackay J, Chen F, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum 2004;47:451–8.
-
Hicks RJ, Ware RE, Lau EW. PET/CT: will it change the way we use CT in cancer imaging? Cancer Imaging 2006;6:S52–62.
-
Huebner RH, Park KE, Shepherd JE, Schwimmer J, Czernin J, Phelps ME, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med 2000;41:1177–89.
-
MHRA. Public assessment report: mutual recognition procedure: Steripet 250 MBq/ml solution for injection: fludeoxglucose (18F). 2008. URL: www.mhra.gov.uk/home/groups/l-unit1/documents/websiteresources/con025955.pdf
-
Park IJ, Kim HC, Yu CS, Ryu MH, Chang HM, Kim JH, et al. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur J Surg Oncol 2006;32:941–7.
-
Purkayastha S, Tekkis PP, Athanasiou T, Tilney HS, Darzi AW, Heriot AG. Diagnostic precision of magnetic resonance imaging for preoperative prediction of the circumferential margin involvement in patients with rectal cancer. Colorectal Dis 2006;9:402–11.
-
Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J. Development and validation of methods for assessing the quality of diagnostic test accuracy studies. Health Technol Assess 2004;8(25).
-
WHO. In Guidelines for drinking water quality. 3rd edn, vol. 1. 2004. URL: www.who.int/water_sanitation_health/dwq/gdwq3rev/en/ (accessed 30 June 2011).
-
Wiering B, Krabbe PFM, Jager GJ, Oyen WJG, Ruers TJM. The impact of fluor-18-doexgyglucose-positron emission tomography in the management of colorectal liver metastases. A systematic review and meta analysis. Cancer 2005;104:2658–70.
-
Zanzonico P, Dauer L, St Germain J. Operational radiation safety for PET-CT, SPECT-CT, and cyclotron facilities. Health Phys 2008;95:554–70.
Appendix 6 Excluded studies
| Author/year | Reason for exclusion |
|---|---|
| Abdel-Nabi 1990 | Not FDG PET/CT |
| Abdel-Nabi 1997 | Conference abstract |
| Abdel-Nabi 1998 | Non-integrated PET equipment |
| Abdel-Nabi 2002 | Review |
| Abir 2006 | Conference proceeding |
| Abouzied 2001 | Announcement of prize winners |
| Abouzied 2005 | Review |
| Adrianensen 2008 | Case study |
| Ak 2000 | Review |
| Akhurst 1996 | Conference abstract |
| Akhurst 1998 | Conference abstract |
| Akhurst 1999 | Review |
| Akhurst 2003 | Conference abstract |
| Akhurst 2005 | Non-integrated PET equipment |
| Alavi 2004 | Review |
| Alibazoglu 1999 | Review |
| Als 2002 | Conference proceeding |
| Altmann 2003 | Not staging |
| Ambrosini 2006 | Not staging |
| Amer 2003 | Conference abstract |
| Amthauer 2000 | Review |
| Amthauer 2004 | Non-integrated PET equipment |
| Amthauer 2006 | Non-integrated PET equipment |
| An 2008 | Not staging |
| Anderson 2007 | Review – radiotherapy planning |
| Anonymous 1994 | Conference proceeding |
| Anonymous 2000 | Conference proceeding |
| Anonymous 2002 | Conference proceeding |
| Anonymous 2003 | News report |
| Anonymous 2005 | News report |
| Anonymous 2007 | Review |
| Anonymous 2008 | Recommendation statement |
| Antoch 2004 | Data not available |
| Aparicio 2008 | Anal cancer |
| Arulampalam 2001 | Non-integrated PET equipment |
| Arulampalam 2001 | Review |
| Arulampalam 2001 | Review |
| Arulampalam 2004 | Not FDG PET/CT |
| Aubertin 2006 | Review |
| Avril 2003 | Review |
| Badiee 2008 | Mixed tumours, separate CRC data not available |
| Barba 2005 | Case report |
| Bares 1994 | Conference abstract |
| Barker 2005 | Review |
| Bar-Shalom 2000 | Unknown reference standard |
| Bashir 2008 | Review |
| Bauer 2004 | Conference abstract |
| Baum 1997 | Conference abstract |
| Beasley 2002 | Review |
| Bedi 2008 | Conference abstract |
| Beets 1994 | Non-integrated PET equipment |
| Beets-Tan 2004 | Review |
| Beets-Tan 2005 | Conference proceeding |
| Beller 2007 | Review |
| Berman 1998 | Review |
| Betler 2008 | Conference abstract |
| Biersack 2000 | PET alone |
| Bipat 2005 | Review |
| Bipat 2006 | Survey |
| Bipat 2007 | Review |
| Bipat 2008 | Review |
| Blahd 1996 | Review |
| Blend 1996 | Review |
| Bloeman 2008 | Conference proceeding |
| Blumstein 2004 | Conference abstract |
| Blumstein 2004 | Conference abstract |
| Bombardieri 1997 | Review |
| Bombardieri 2001 | Review |
| Bombardieri 2003 | Non-integrated PET equipment |
| Borrego 2004 | Non-integrated PET equipment |
| Bourguet 2007 | Review |
| Boykin 1999 | Non-integrated PET equipment |
| Brady 2008 | Review |
| Brennan 2001 | Review |
| Brihaye 2009 | Review |
| Brittenden 2007 | Review |
| Brown 2004 | Review |
| Brown 2005 | Review |
| Brown 2007 | Review |
| Bruna 2007 | Not staging |
| Bujenovic 2004 | Review |
| Burger 2002 | Conference abstract |
| Burvenich 2007 | Review |
| Bybel 2006 | Review |
| Cabello-Garcia 2007 | Conference proceeding |
| Calvo 2004 | PET alone |
| Capirci 2004 | Non-integrated PET equipment |
| Capirci 2005 | Conference abstract |
| Caprio 2006 | Conference abstract |
| Carillio 2005 | Case report |
| Carnaghi 2005 | Conference abstract |
| Carnaghi 2005 | Conference abstract |
| Carnaghi 2007 | Non-integrated PET equipment |
| Carneiro 2009 | Review |
| Castellucci 2007 | Conference abstract |
| Chan 2008 | Case report |
| Chang 2009 | Review |
| Charnsangavej 2006 | Consensus statement |
| Chen 2003 | Non-integrated PET equipment |
| Chessin 2005 | Review |
| Ching 2004 | Review |
| Cho 2007 | Conference abstract |
| Coha 2009 | Not FDG PET/CT |
| Cohade 2002 | Conference abstract |
| Cohen 1997 | Editorial |
| Collins 2007 | Review |
| Comans 2002 | Review |
| Cook 2004 | Review |
| Cook 2007 | Review |
| Coronado 2004 | Conference abstract |
| Cotter 2006 | Anal cancer |
| Czech 2000 | Review |
| Czernin 2002 | Review |
| Czernin 2002 | Review |
| Daenen 1996 | Conference abstract |
| Dahan 2008 | Review |
| Dane 2008 | Review |
| Danish Centre for Evaluation 2001 | Not FDG PET/CT |
| Decosterd 1989 | Laboratory-based study |
| De Gues-Oei 2006 | Not FDG PET/CT |
| De Gues-Oei 2006 | Non-integrated PET equipment |
| Delbeke 1996 | Conference abstract |
| Delbeke 1997 | Non-integrated PET equipment |
| Delbeke 1998 | Non-integrated PET equipment |
| Delbeke 1999 | Review |
| Delbeke 2001 | Non-integrated PET equipment |
| Delbeke 2004 | Review |
| Deleau 2008 | Not FDG PET/CT |
| Desai 2003 | Non-integrated PET equipment |
| De Winton 2009 | Anal cancer |
| Dias 2007 | Review |
| Dietlein 1999 | Review |
| Dietlein 2003 | Non-integrated PET equipment |
| Dietlein 2003 | Survey |
| Digby 2000 | Review |
| Dinter 2006 | Review |
| Dinter 2008 | Review |
| Dobos 2002 | Review |
| Drenth 2001 | Non-integrated PET equipment |
| Dromain 2004 | Review |
| Ducreux 2005 | Review |
| Eiland 2006 | Non-integrated PET equipment |
| Ellis 2006 | Review |
| Esnault 2006 | Conference abstract |
| Even-Sapir 2006 | Not staging |
| Falk 1994 | Non-integrated PET equipment |
| Faneyte 2008 | Data unavailable |
| Fasoli 2005 | Review |
| Figueras 2005 | Editorial |
| Filmont 2001 | Not FDG PET/CT |
| Filmont 2001 | Conference abstract |
| Finkelstein 2008 | Non-integrated PET equipment |
| Flamen 1998 | Conference proceeding |
| Flamen 1999 | Non-integrated PET equipment |
| Flamen 2000 | Review |
| Flamen 2008 | Review |
| Flanagan 1996 | Conference abstract |
| Flanagan 1998 | Non-integrated PET equipment |
| Fletcher 2008 | Review |
| Flynn 1996 | Review |
| Flynn 1996 | Review |
| Fong 1999 | Non-integrated PET equipment |
| Franc 2008 | Review |
| Francis 2003 | 2 × 2 data not available |
| Francis 2003 | Conference abstract |
| Francis 2004 | Response to therapy |
| Francis 2005 | Review |
| Franke 2000 | Not FDG PET/CT |
| Friedland 2004 | Conference abstract |
| Friedland 2005 | Non-integrated PET equipment |
| Fujimoto 2009 | Not FDG PET/CT |
| Fukunaga 2002 | Conference abstract |
| Furukawa 2006 | Non-integrated PET equipment |
| Furukawa 2008 | Review article |
| Gaa 2005 | Not FDG PET/CT |
| Gallego-Peinado 2007 | Conference abstract |
| Gambhir 1997 | Conference abstract |
| Gambhir 2001 | Conference proceeding |
| Gardner-Thorpe 2008 | Conference abstract |
| Gearhart 2005 | Conference proceeding |
| Ghosh 2007 | Letter to the editor |
| Gomez-Leon 2007 | Review |
| Goodman 2008 | Review |
| Gopalan 2002 | Not FDG PET/CT |
| Goshen 2006 | Case series |
| Graham 1999 | Conference abstract |
| Grande 2005 | Case report |
| Guan 2003 | Conference abstract |
| Guan 2005 | Non-integrated PET equipment |
| Gupta 1992 | Conference abstract |
| Gupta 1993 | Non-integrated PET equipment |
| Gupta 2005 | Case report |
| Haberkorn 2001 | Review |
| Habr-Gama 2008 | Non-integrated PET equipment |
| Haguet 2007 | Not FDG PET/CT |
| Haji 2007 | Review |
| Hallkar 1999 | Conference abstract |
| Hankins 2005 | Review |
| Harder 2008 | Not CRC |
| Herbertson 2009 | Review |
| Heriot 1999 | Review |
| Heriot 2004 | PET alone |
| Heriot 2007 | Conference proceeding |
| Hernandez 2005 | Review |
| Herrera 2007 | Two case reports |
| Hicks 2006 | Review |
| Hillner 2004 | Not FDG PET/CT |
| Hobbs 1995 | Review |
| Hoh 1997 | Review |
| Hojgaard 2003 | Review |
| Holstege 2006 | Review |
| Huebner 2000 | Review |
| Huguet 2007 | Not FDG PET/CT |
| Huguier 2006 | Non-integrated PET equipment |
| Hung 2001 | Non-integrated PET equipment |
| Hustinx 1998 | Non-integrated PET equipment |
| Hustinx 1999 | Non-integrated PET equipment |
| Hustinx 2004 | Non-integrated PET equipment |
| Iagaru 2007 | Review |
| Iagaru 2009 | Non-integrated PET equipment |
| Ide 2005 | Review |
| Ide 2006 | PET screening |
| Ike 2004 | Conference abstract |
| Imbriaco 1997 | Conference abstract |
| Imdahl 2000 | Non-integrated PET equipment |
| Institute for 2003 | Review |
| Ishikawa 2009 | Conference abstract |
| Ishimori 2005 | Staging diagnostic test accuracy not study purpose |
| Israel 2007 | Review |
| Ito 2002 | Review |
| Ito 2008 | Duplicate publication |
| Iwanicki-Caron 2006 | Not FDG PET/CT |
| Iyer 2006 | Review |
| Jadvar 1997 | Conference abstract |
| Jarnagin 1999 | Not staging |
| Jaruskova 2004 | Conference abstract |
| Jeong 2008 | Case report |
| Jerusalem 2002 | Review |
| Jerusalem 2003 | Review |
| Jerusalem 2003 | Review |
| Jiao 2007 | Non-integrated PET equipment |
| Johnson 2001 | Non-integrated PET equipment |
| Jones 2008 | Case report |
| Jorg 2002 | Review |
| Jorg 2002 | Review |
| Joyce 2006 | Non-integrated PET equipment |
| Kaerlev 2005 | Not a study of diagnostic test accuracy |
| Kaida 2006 | PET alone |
| Kamel 2002 | Conference abstract |
| Kamel 2004 | Mixed tumours, not separate data |
| Kang 2004 | Conference abstract |
| Kantorova 2003 | Non-integrated PET equipment |
| Kanyari 2005 | Non-integrated PET equipment |
| Kapse 2009 | Review |
| Karantanas 2007 | Review |
| Kato 2002 | Review |
| Kayani 2006 | Review |
| Kilbas 2007 | Conference abstract |
| Kim 2004 | Conference abstract |
| Kinkel 2002 | Review |
| Klaff 2002 | Non-integrated PET equipment |
| Kletter 2007 | Review |
| Klippenstein 2000 | Review |
| Koh 2006 | Review |
| Kojima 2003 | Review |
| Komori 2007 | Review |
| Koslin 2002 | Review |
| Kosugi 2008 | Non-integrated PET equipment |
| Krause 2004 | Conference abstract |
| Krengli 2008 | Anal cancer |
| Kubota 2001 | Review |
| Kuehl 2008 | Not staging |
| Kuehl 2008 | Not staging |
| Kuehl 2008 | For tumour progression |
| Kuehl 2008 | Letter to the editor |
| Kumar 2006 | Review |
| Lai 1996 | Not FDG PET/CT |
| Lang 1999 | Conference abstract |
| Lang 1999 | Conference abstract |
| Lang 1999 | Conference abstract |
| Lang 2000 | Conference proceeding |
| Lang 2007 | Journal news item |
| Langenhoff 2002 | Non-integrated PET equipment |
| Laupacis 2002 | Review |
| Layer 2008 | Review |
| Lee 2008 | Conference abstract |
| Lee 2008 | Case series |
| Lehner 1990 | Non-integrated PET equipment |
| Lejeune 2005 | Economic model |
| Liehn 1992 | Not PET |
| Lind 2003 | Review article |
| Liu 2001 | Conference abstract |
| Llamas-Elvira 2007 | Non-integrated PET equipment |
| Longo 2002 | Review |
| Lonneux 1996 | Conference abstract |
| Lonneux 1999 | Review |
| Lonneux 2001 | Conference abstract |
| Lonneux 2002 | Non-integrated PET equipment |
| Lonneux 2002 | Review |
| Lonneux 2003 | Review |
| Lonneux 2008 | Review |
| Low 2008 | Review |
| Macedon 2008 | Conference abstract |
| Maisey 2003 | Editorial |
| Makin 2001 | Review |
| Malyap 2004 | Conference abstract |
| Mann 2007 | Study does not evaluate role of FDG PET/CT |
| Manych 2007 | Review article |
| Martinez 2007 | Conference proceeding |
| Massardo 2007 | Review |
| Maublant 1998 | Review |
| Mavi 2006 | Conference abstract |
| Medea 2006 | Conference proceeding |
| Medical Services Advisory Committee 2007 | Review |
| Medical Services Advisory Committee 2008 | Review |
| Meijerink 2009 | Review |
| Messa 2006 | Review |
| Meta 2000 | Conference proceeding |
| Metser 2004 | Letter |
| Middleton 2002 | Review |
| Mitrakopoulou-Strauss 2003 | Not FDG PET/CT |
| Moadel 2008 | Review |
| Montravers 2001 | Not FDG PET/CT |
| Montravers 2002 | Conference abstract |
| Montravers 2004 | Compares two different PET systems |
| Moretti 2006 | Conference abstract |
| Mukai 2000 | Non-integrated PET equipment |
| Muthusamy 2007 | Review |
| Nachar 2002 | Review |
| Nagata 2008 | Not PET |
| Nagata 2008 | Colonography not tomography |
| Nahas 2008 | Non-integrated PET equipment |
| Nahas 2008 | Anal cancer |
| Nakamoto 2007 | Non-integrated PET equipment |
| Nakamoto 2008 | Lung cancer |
| Nakamoto 2009 | Not CRC, gastric cancer |
| Nakamura 2004 | Case study |
| Nanashima 2008 | Not FDG PET/CT |
| Nasu 2008 | Case study |
| National Coordinating Centre 2007 | Review |
| Nguyen 2008 | Anal cancer |
| O’Dwyer 2001 | Review |
| Ogunbiyi 1997 | Non-integrated PET equipment |
| Ondrak 2007 | Review |
| Ono 2007 | Non-integrated PET equipment |
| Ono 2009 | Non-integrated PET equipment |
| Osman 2003 | Letter |
| Ott 1999 | Not FDG PET/CT |
| Oyen 2000 | Conference proceedings |
| Oyen 2003 | PET alone |
| Pahlman 2002 | Review |
| Pakzad 2006 | Conference abstract |
| Palazzo 2000 | Review |
| Palomar 2006 | Conference abstract |
| Pandey 2005 | Not CRC |
| Pandit-Taskar 2004 | Non-integrated equipment |
| Pantaleo 2007 | Letter |
| Pantaleo 2008 | Not staging |
| Pantaleo 2008 | Review |
| Park 2005 | Conference proceeding |
| Park 2006 | Data not available |
| Paschos 2008 | Review |
| Paskeviciute 2009 | Not a study of diagnostic test accuracy |
| Pellet 2002 | Not FDG PET/CT |
| Pelosi 2004 | Not staging |
| Pelosi 2007 | Review |
| Perez 2007 | Conference proceeding |
| Pham 2002 | Review |
| Podoloff 2007 | Review |
| Redvanly 1998 | Conference abstract |
| Redvanly 1998 | Conference proceeding |
| Reerink 2004 | Review |
| Reske 1996 | Conference proceeding |
| Reske 1999 | Review |
| Reske 2001 | Review |
| Rodari 2007 | Conference abstract |
| Rogers 2006 | Conference abstract |
| Rohren 2002 | Non-integrated PET equipment |
| Rosa 2002 | Conference proceeding |
| Rosenberg 2007 | Conference abstract |
| Rosenberg 2009 | Not staging |
| Ruers 2002 | Non-integrated PET equipment |
| Ruhlmann 1996 | Case report |
| Ruhlmann 1997 | Non-integrated PET equipment |
| Sahani 2005 | Non-integrated PET equipment |
| Saunders 2002 | Review |
| Schaefer 2007 | Review |
| Schiepers 1995 | Non-integrated PET equipment |
| Schiepers 2003 | Comment |
| Schlag 2001 | Review |
| Schmidt 2004 | Interim report |
| Schmidt 2007 | Review |
| Schmidt 2007 | Indication unclear, does not meet histopath |
| Schreyer 1998 | Review |
| Schroder 1998 | Conference abstract |
| Scott 1994 | Conference abstract |
| Scott 1996 | Conference abstract |
| Scott 2002 | Review |
| Scott 2007 | Conference abstract |
| Scott 2008 | Non-integrated PET equipment |
| Segre 2005 | Conference proceeding |
| Selvaggi 2003 | Non-integrated PET equipment |
| Sharma 2008 | Review |
| Sheehan 2007 | Review |
| Sheehy 2007 | Review |
| Shin 2008 | Review |
| Simo 2002 | Non-integrated PET equipment |
| Skelly 2006 | Conference abstract |
| Sobhani 2008 | Non-integrated PET equipment |
| Sorensen 2007 | Non-integrated PET equipment |
| Speer 2001 | Non-integrated PET equipment |
| Squillaci 2008 | Data not available |
| Stelzner 2006 | Not staging |
| Storto 2006 | Conference abstract |
| Strasberg 2001 | Non-integrated PET equipment |
| Strasberg 2002 | Comment |
| Strasberg 2003 | Not CRC |
| Strauss 1991 | Review |
| Strauss 1993 | Review |
| Stroszczynski 2001 | Review |
| Sun 2008 | Review |
| Takahashi 2006 | Non-integrated PET equipment |
| Takeuchi 1999 | Non-integrated PET equipment |
| Talbot 2001 | Review |
| Tan 2000 | Review |
| Tang 2005 | Non-integrated PET equipment |
| Teague 2004 | Non-integrated PET equipment |
| Topal 2001 | Non-integrated PET equipment |
| Torricelli 2007 | Review |
| Touboul 2004 | Conference proceeding |
| Touboul 2007 | Review |
| Traeger 1999 | Conference abstract |
| Traeger 1999 | Conference proceeding |
| Trampal 1999 | Conference proceeding |
| Trampal 1999 | Non-integrated PET equipment |
| Travaini 2008 | Not staging |
| Truant 2005 | Non-integrated PET equipment |
| Tutt 2004 | Review |
| Tzimas 2004 | Review |
| Unidad de Evaluacion de Tecnologias Sanitarias 2004 | Economic evaluation |
| Uno 2003 | Single case |
| Valgaeren 2001 | Surveillance not staging |
| Valk 1996 | Conference abstract |
| Valk 1999 | Non-integrated PET equipment |
| Valotassiou 2006 | Conference abstract |
| Van 2001 | Review |
| Vandenbroucke 2008 | Review |
| Vander 2007 | Review |
| Van Kouwen 2005 | Non-integrated PET equipment |
| Veit 2004 | Conference abstract |
| Veit 2006 | Colonography |
| Veit-Haibach 2006 | Colonography not tomography |
| Vikram 2008 | Review |
| Vilstrup 2007 | Not FDG PET/CT |
| Vitola 1996 | Non-integrated PET equipment |
| Vogel 2005 | Review |
| Vogel 2007 | Not a study of diagnostic test accuracy |
| Vogl 2006 | Review |
| Von Gumppenberg 1993 | Review |
| Von Mallek 2006 | Prostate cancer |
| Von Schulthess 2008 | Review |
| Vuong 2006 | Conference proceeding |
| Wahl 1971 | Review |
| Wahl 2004 | Review |
| Wald 2006 | Review |
| Watson 2007 | Review |
| Whiteford 2000 | Non-integrated PET equipment |
| Wiering 2004 | Non-integrated PET equipment |
| Wiering 2005 | Review |
| Wiering 2007 | Not FDG PET/CT |
| Wiering 2007 | Not FDG PET/CT |
| Wiering 2008 | Review |
| Wilke 2002 | Non-integrated PET equipment |
| Witte 2007 | Review article |
| Woel 2008 | Conference abstract |
| Wu 2007 | Cancer of unknown primary |
| Yang 2003 | Non-integrated PET equipment |
| Yang 2007 | Not FDG PET/CT |
| Yasuda 1996 | Non-integrated PET equipment |
| Yasuda 1998 | Non-integrated PET equipment |
| Yasuda 1998 | Not FDG PET/CT |
| Yasuda 2001 | Non-integrated PET equipment |
| Yoshino 2008 | Conference abstract |
| Zervos 2001 | Non-integrated PET equipment |
| Zhang 2009 | Review |
| Zhuang 2000 | Non-integrated PET equipment |
| Zimmermann 2005 | Review |
| Zimmermann 2007 | Review |
| Zutshi 2005 | Review |
Glossary
- Confidence interval
- A range of values that it is possible to be confident includes the true value.
- Differential verification bias
- Use of a different reference standard to verify a proportion of the test results.
- Disease progression bias
- Delay in the timing of the index test and reference standard during which the disease status changes.
- False-negative test result
- Test is negative for the disease but the disease is truly present.
- False-positive test result
- Test is positive for the disease but the disease is truly absent.
- Fluorine-18-deoxyglucose (FDG)
- Radiopharmaceutical product used for injection in FDG positron emission tomography/computerised tomography scanning.
- Incorporation bias
- Use of the index test results in verifying the accuracy of the index test.
- Meta-analysis
- A method to combine the results of individual studies to increase power and precision in the estimate of intervention effects.
- Millisievert (mSv)
- Système International (SI) unit of radiation.
- Partial verification bias
- Incomplete confirmation of the study group’s diagnosis with the reference standard.
- QUADAS (Quality of Diagnostic Accuracy Studies)
- Evidence-based quality assessment tool.
- Review bias
- Results of the index tests influenced by knowledge of the reference standard, or results of the reference standard influenced by knowledge of the index test. Similar to ‘blinding’ in intervention studies.
- Sensitivity
- The probability of testing positive if the disease is truly present.
- Specificity
- The probability of testing negative if the disease is truly absent.
- Spectrum bias
- Differences between populations that may cause the accuracy of diagnostic tests to vary.
- True-negative test result
- Test is negative for the disease and the disease is truly absent.
- True-positive test result
- Test is positive for the disease and the disease is truly present.
List of abbreviations
- AJCC
- American Joint Committee on Cancer
- APR
- abdominoperineal resection
- CEA
- carcinoembryonic antigen
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRC
- colorectal cancer
- CRT
- chemoradiotherapy
- CT
- computerised tomography
- DARE
- Database of Abstracts of Reviews of Effectiveness
- EVPI
- expected value of perfect information
- FDG
- fluorine-18-deoxyglucose
- FN
- false negative
- FP
- false positive
- HSROC
- hierarchical summary receiver operating characteristic
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IOUS
- intraoperative ultrasound
- MDT
- multidisciplinary team
- MRI
- magnetic resonance imaging
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Clinical Excellence
- PET
- positron emission tomography
- PET/CT
- positron emission tomography/computerised tomography
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- RCT
- randomised controlled trial
- SCPRT
- short-course pre-operative radiotherapy
- SPIO
- superparamagnetic oxide
- SROC
- summary receiver operating characteristic
- SS SE-EPI
- single-shot spin-echo echo planar imaging
- SUV
- standardised uptake value
- TME
- total mesorectal excision
- TN
- true negative
- TNM
- tumour, node, metastasis
- TP
- true positive
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington