Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/57/02. The contractual start date was in April 2009. The draft report began editorial review in October 2010 and was accepted for publication in January 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Harris et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
The need for improved dietary behaviour
The composition of habitual diets is associated with adverse or protective effects on health. 1–3 Specifically, diets high in saturated fats and sodium have been found to increase the risk of cardiovascular diseases (CVDs), whereas those high in fruit and vegetables and low in saturated fats have been linked with reductions in a range of diseases including some cancers, CVD and hypertension. 4–7 The World Health Organization reports that the consumption of up to 600 g per day of fruit and vegetables could reduce the total worldwide burden of disease by 1.8%, and reduce the burden of ischaemic heart disease and ischaemic stroke by 31% and 19%, respectively. 8 In the UK, the consumption of fruit, vegetables, dietary fibre, iron (pre-menopausal women only) and calcium are well below recommendations, whereas intakes of saturated fats and sodium exceed recommendations in large sections of the population. 9 Consequently, UK public health policy strongly advocates dietary change for the improvement of population health and emphasises the importance of individual empowerment to improve health,7,10 thereby shifting the focus of the NHS from treatment to prevention of illness. 11,12
Adaptive e-learning via interactive computerised interventions
A new and evolving area in the promotion of dietary behavioural change is ‘e-learning’, the use of interactive electronic media to facilitate teaching and learning on a range of issues including health. E-learning has grown out of recent developments in information and communication technology, such as the internet, interactive computer programs, interactive television and mobile telephones. 13–17 These technologies are rapidly becoming more accessible to the general population (e.g. an estimated 70% of the UK population has access to the internet and this percentage is likely to continue to grow18).
The high level of accessibility, combined with emerging advances in computer processing power, data transmission and data storage, makes interactive e-learning a potentially powerful and cost-effective medium for improving dietary behaviour. 19–21 It also has a number of potential advantages compared with traditional approaches for promotion of dietary behaviour change, such as the possibility of tailoring to individual circumstances,22 translating complex information through video, graphics and audio systems,23 and potential cost savings on face-to-face interventions involving health-care practitioners. The evidence that individualised, tailored e-learning approaches are more effective than traditional non-tailored interventions24 has given them a promising lead in health education. 25–27
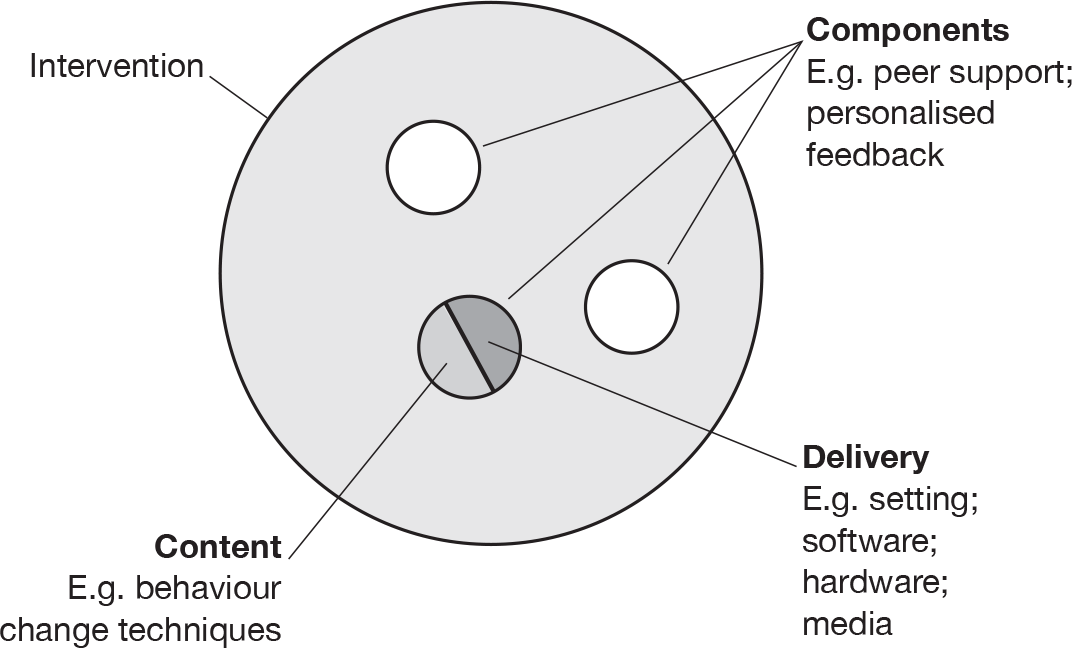
E-learning interventions may be classified into three ‘generations’, first-generation interventions use computers to tailor printed materials; second-generation interventions use interactive technology delivered on computers; and third-generation interventions use portable devices, such as mobile telephones, for more immediate interaction and feedback. 28 An exploration of the properties of different e-learning interventions is required in order to determine the possible effective components (where a component comprises both ‘content’ and ‘delivery’; Figure 1).
Figure 2 illustrates that dietary behaviours are likely to be heavily influenced by macro factors at the environmental level (e.g. access to shops selling fruit and vegetables at affordable prices); organisational level (e.g. energy-dense ‘junk’ food vending machines in schools or workplaces); population level (e.g. low income and unemployment); and sociocultural level (e.g. interpersonal influences, such as where one person is responsible for meals eaten by others in a household). These wider determinants of dietary behaviour are unlikely to be changed by individually targeted interventions such as e-learning. Individual-level factors such as self-efficacy, knowledge and intention may be subject to change by e-learning. An exploration of the potential cognitive and emotional mediators of individual dietary behaviour change is required in order to elicit potential mechanisms of action.
FIGURE 2.
Conceptual elements of behaviour and behaviour change (reproduced from Edwards et al. 29).
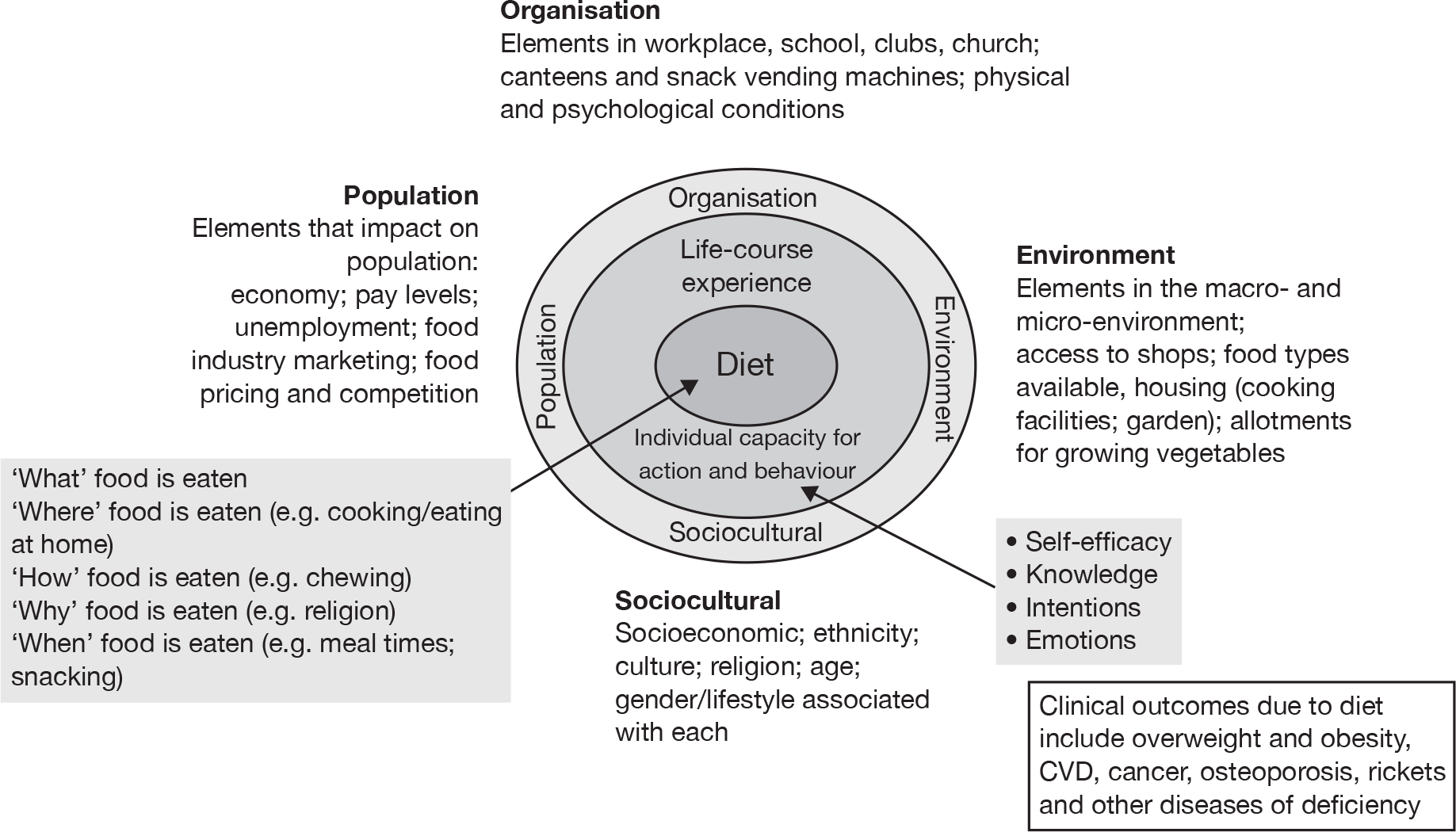
There is a risk that e-health and the use of new technologies in health care might widen health inequalities on either side of the ‘digital divide’. Experience suggests that there are two dimensions to the digital divide and its impact on health inequalities: access (to physical hardware and software) and accessibility (or the ability of people with differing literacy/health literacy/IT literacy to use or apply information and support supplied through e-learning). It has been shown that it is possible to deliver e-health interventions specifically designed for people with low literacy skills (e.g. Hispanics in southern USA,30 homeless drug users31 and single teenage mothers32). What remains less clear is the extent to which people with low literacy skills will feel comfortable using e-learning devices or will be able to act on information or advice provided through these media.
Interactive e-learning programs to promote positive dietary behavioural changes may have the potential to benefit population health. However, before e-learning can be considered as a dietary behaviour change intervention, the effective components and mechanisms of action of e-learning programs should be explored, and their cost-effectiveness established in different contexts.
Previous reviews
Three systematic reviews have examined the effectiveness of e-learning for dietary behaviour change. The first33 was restricted to first-generation interventions for dietary change and did not include any web- or internet-based interventions. The second34 examined a broad range of second-generation interactive interventions for dietary behaviour change. Both of these reviews reported studies published prior to 2006 that were carried out in a variety of settings. The third review28 was more recent, reviewing second- and third-generation interventions trialled up to 2008, but only in primary prevention in adults (no participants with diagnosed disease). All reviews were restricted to publications in the English language and limited their searches to relatively few databases, increasing the potential for publication bias. The conclusions drawn from these systematic reviews were that e-learning shows some promise for dietary behavioural change, although the findings were mixed. Interstudy heterogeneity with respect to study design, participants, measures and outcomes precluded meta-analysis to estimate pooled intervention effects. Moreover, the cost-effectiveness of e-learning was not evaluated in any review, nor was there an attempt to identify potential mechanisms of action.
Chapter 2 Methods for the descriptive analysis and systematic review of effectiveness
The protocol for this review has been published as Edwards et al. 29 and is available from www.biomedcentral.com/content/pdf/1471-2458-10-200.pdf.
Objectives
The aims of this systematic review were to assess the effectiveness and cost-effectiveness of adaptive e-learning for improving dietary behaviours. The specific objectives were to:
-
describe the range of e-learning technologies in use for promoting dietary behavioural change
-
evaluate the effectiveness of interactive e-learning in terms of improvement in dietary behaviour and clinical outcomes
-
analyse the e-learning interventions in order to determine the components contributing to the effects of e-learning interventions for dietary behavioural change
-
investigate potential explanations of dietary behavioural change, and mechanisms of action
-
evaluate cost-effectiveness compared with current standard interventions.
Design
The research consisted of a systematic review of the clinical and economic evidence.
Study eligibility criteria
Types of study
We included randomised controlled trials (RCTs) for evidence of effectiveness and economic evaluations for evidence of cost-effectiveness (including cost-effectiveness, cost–utility and cost–benefit analyses).
Types of population
We included adolescents or adults (mean sample age ≥ 13 years) who have participated in a study designed to evaluate the effectiveness of e-learning to promote dietary behavioural change. We included all clinical conditions for which dietary advice plays a major part in case management.
Types of intervention
Interventions were included if there were interactive computer software programs that tailored output according to user input (i.e. second- and third-generation interventions). These include interventions for which users enter personal data, or make choices about information, that alter pathways within programs to produce tailored material and feedback that is personally relevant. Users may interact with the programs as members of a small group, as well as individually. Programs should be available directly to users and allow independent access without the need for any expert facilitation.
Interventions were excluded if they were:
-
first-generation tailored ‘information only’ (e.g. providing a leaflet or PDF file)
-
simple information packages with no interactive elements
-
non-interactive mass media interventions (such as TV advertisements)
-
interventions designed to be used with others’ help (e.g. teacher or health professional)
-
interventions targeted at health professionals or teachers
-
computer-mediated delivery of individual health-care advice (e.g. online physicians)
-
electronic history-taking or risk assessment with no health promotion or interactive elements.
Outcome measures
We anticipated that most interventions would be aimed at dietary behaviours and were unlikely to have followed participants long enough to obtain changes in clinical measures. However, as measures of dietary behaviour tend to be based on self-report, they are prone to error (e.g. recall bias). Biological outcomes [e.g. body mass index (BMI)] tend to be measured more objectively (e.g. using measures of weight and height) and are also the necessary inputs to economic models of cost-effectiveness. We therefore specified dietary behaviour as our primary outcome, and also obtained data that allowed us to model the relationship between behaviours and clinical changes.
Primary outcome measures
Measures of dietary behaviours including estimated intakes or changes in intake of energy, nutrients, dietary fibre, foods or food groups. The dietary assessment tools or techniques used to estimate dietary behaviour were critically examined in terms of quality.
Secondary outcome measures
Objective measures that are likely to respond to changes in dietary behaviours and are associated with adverse clinical outcomes including measurements of anthropometric status and blood biochemistry.
Other data
We also sought data on economic outcomes, specifically the costs of providing the intervention and costs to the individual user, unintended adverse consequences of the interventions, and process outcomes (e.g. usage data). Data relating to potential cognitive and emotional mediators of dietary behaviour were also obtained.
Identification of eligible studies and data extraction
Search process
Our search comprised the following:
-
a search of electronic bibliographic databases for published work
-
a search of trial registers for ongoing and recently completed trials
-
inspection of the reference lists of all included studies and previously published reviews
-
contact with authors of included studies and e-health research groups to check for more trials.
There were no restrictions by language. The search strategy comprised two concepts: ‘computer-/internet-based interventions’ and ‘dietary behaviour’ (see Appendix 2 for full electronic search strategies).
Eleven electronic databases, two trials databases and two theses databases were searched using the search strategy (Table 1). Searches covered the period January 1990 to November 2009 (we assumed that any studies of e-learning conducted in the 1980s would be identified through inspection of the reference lists of all included studies).
| Database | Records | Dates |
|---|---|---|
| Australian Digital Theses | 67 | 1990–2009 |
| CINAHL | 6409 | 1990–2009 |
| ClinicalTrials.gov | 179 | 1990–2009 |
| Current Controlled Trials | 266 | 1990–2009 |
| Dissertation Abstracts | 2136 | 1990–2009 |
| EMBASE | 7843 | 1990–2009 |
| ERIC | 2990 | 1990–2009 |
| globalhealth.gov | 1447 | 1990–2009 |
| HEED | 18 | 1990–2009 |
| HMIC | 281 | 1990–2009 |
| Index to THESES | 214 | 1990–2009 |
| MEDLINE | 5483 | 1990–2009 |
| PsycINFO | 2376 | 1990–2009 |
| The Cochrane Library | 6448 | 1990–2009 |
| Web of Science | 222 | 1990–2009 |
| Total | 36,379 |
Selection process
All studies identified through the search process were exported to a bibliographic database (EndNote version X3; Thomson Reuters, CA, USA) for de-duplication and screening. Two review authors independently examined the titles, abstracts and keywords of electronic records according to the eligibility criteria above. Results of this initial screening were cross-referenced between the two review authors, and full-text records obtained for all potentially relevant reports of trials. These potentially eligible trials went through a secondary screening by each reviewer using a screening form based on the eligibility criteria (see Appendix 3) for final inclusion in the systematic review, with disagreements resolved by discussion with a third author.
Data extraction
Two review authors extracted relevant data into a Microsoft Access 2007 (Microsoft Corporation, Redmond, WA, USA) database specifically designed for the review (available from the authors on request). Corresponding authors of included studies were contacted directly by e-mail when required information or data were not reported in the published report, using a pre-notification e-mail followed by up to two contact attempts.
Methodological quality assessment
Two measures of methodological quality were used in the review: The Cochrane Collaboration’s risk of bias assessment,35 and the Effective Public Health Practice Project (EPHPP) quality assessment. 36 The Cochrane assessment requires a judgement to be made by the review authors on the likely risk of bias arising from six domains. Risk of bias is presented as a chart showing the proportion of studies judged to have ‘low risk of bias’ or ‘high risk of bias’ or those for which risk of bias is unclear, for each of the six domains. The EPHPP assessment provides an overall rating for each study (‘strong’, ‘moderate’ or ‘weak’), based on a series of questions about similar domains. The EPHPP assessment used in this review was unmodified, although it is a relatively new tool and there was some concern among the review authors that some of the questions were not relevant to e-learning interventions in particular (see Methodological quality of included studies). We chose to include EPHPP for its strengths regarding assessment of confounders, data collection methods, and withdrawal and dropouts (which are less well covered by the Cochrane tool).
Analysis
Descriptive analysis
We described all studies that met the inclusion criteria, including (where reported):
-
study design:
-
– study objectives (i.e. target outcomes)
-
– trial design and quality
-
– data collection methods, modes and techniques; validity of tools
-
-
participants:
-
– socioeconomic and demographic characteristics
-
– health status: diagnosed disease versus no diagnosed disease
-
-
intervention:
-
– components of the intervention, including delivery and content
-
– frequency, intensity and duration of the intervention
-
– behaviour change theories employed in intervention design, and postulated mediators
-
-
outcomes:
-
– primary and secondary outcomes measured
-
– information on process (ease of use) and usage (compliance).
-
Information on the sociodemographic characteristics of participants was used to address concerns over the ‘digital divide’. Where sufficient data were provided by the primary studies, we planned to undertake subgroup analyses of intervention effects in low-income and low-educational-status users.
Intervention content and mechanisms of action
In order to investigate the key behaviour change techniques’ contribution to intervention effects, we coded techniques according to a taxonomy developed by Abraham and Michie. 37 To investigate how interventions might change dietary behaviour, we documented the theories that were reported to account for the process of behaviour change. 38–40 Where theories had been used to inform intervention design in trials, we documented the potential mediators of behaviour change, such as knowledge, intention, self-efficacy and emotions.
Analysis of effectiveness
When studies reported the same outcome (e.g. servings of fruit and vegetables eaten per day, percentage of energy from fat), we pooled the results using a random effects model, with weighted mean differences (WMDs), and calculated 95% confidence intervals (CIs) and two-sided p-values for each outcome. When outcomes were assessed more than once during follow-up, the final assessment was used in analysis. In studies in which the effects of clustering were not taken into account, we adjusted the standard deviations (SDs) by the design effect, using intraclass coefficients if given in papers or using external estimates obtained from similar studies. 41
We assessed evidence for selection bias using Egger’s test for small study effects. Heterogeneity among the trial results was assessed using both a chi-squared test and the I2 statistic, the percentage of among-study variability that is due to true differences between studies (heterogeneity) rather than to sampling error. We considered an I2 value > 50% to reflect ‘substantial heterogeneity’. We conducted sensitivity analyses in order to investigate possible sources of heterogeneity including study quality (adequate vs inadequate allocation concealment, low vs high attrition) and sociodemographic factors that could act as effect modifiers [e.g. gender and socioeconomic status (SES)].
When studies reported more than one measure of a single outcome, the measure used was that for which greatest validity had been demonstrated [e.g. if a validated Food Frequency Questionnaire (FFQ) was used to measure the number of portions of fruit and vegetables eaten daily, as well as an unvalidated single item ‘how many portions of fruit and vegetables do you eat each day?’, we chose the former for inclusion in any subsequent meta-analysis].
Causes of heterogeneity and subgroup effects were assessed using random effects meta-analysis. This was implemented in Stata (StataCorp LP, College Station, TX, USA) using the ‘metareg’ command, and included trial characteristics as covariates. All statistical analysis was conducted using Stata statistical software version 11.
Chapter 3 Results of the descriptive analysis
Results of the literature search
A total of 36,379 titles were initially identified by the electronic searches, of which 2977 were duplicates and were removed. The remaining 33,402 titles (and abstracts where available) were screened independently by two review authors and the results compared. A total of 33,129 titles were excluded during this initial screening, primarily owing to not meeting our inclusion criteria for randomised trials of adaptive e-learning, yielding 273 potentially eligible studies for which full-text reports were sought.
A secondary screening process was undertaken on the full-text documents for the 273 studies, using the screening form provided in Appendix 3, by two review authors independently. A total of 233 studies were excluded through this process; a table of excluded studies is provided in Appendix 6 (see Table 28). Three new eligible titles were identified through searching reference lists of the included studies, yielding a total of 43 studies for inclusion in the review. See the PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow chart in Appendix 4 for details.
Overview of included studies
Included studies
Table 2 summarises the 43 studies included in the review.
| Study | Intervention | Comparatora |
|---|---|---|
| Agras (1990)42 | ||
| Country: USA | Setting: N/A | |
| Aim: weight down | Delivery mode: mobile device | |
| Description: | Tailored feedback for 12 weeks on intake and goals, plus an automated trainer to promote slow eating | A therapist-conducted weight-loss programme (behaviour therapy conducted in 10 sessions over a 12-week period) |
| Follow-up: baseline, 3 months, 6 months, 12 months | ||
| n = 90 | ||
| Alexander (2010)43 | ||
| Country: USA | Setting: N/A | |
| Aim: FV up | Delivery mode: internet | |
| Description: | Four sessions over 15 weeks on a tailored website providing a broad range of practical strategies, information and support for dietary behaviour change | Non-tailored website, same layout, containing generic information |
| Follow-up: baseline, 3 months, 6 months, 12 months | ||
| n = 2540 | n = 839 | n = 836 |
| Anderson (2001)44 | ||
| Country: USA | Setting: supermarket | |
| Aims: fat down, fibre up, FV up | Delivery mode: computer kiosk | |
| Description: | Weekly 10-minute sessions over 15 weeks, providing tailored information and planning and monitoring strategies for food purchases, including financial incentives (targeted food coupons) | No treatment |
| Follow-up: baseline, 3 months, 6 months | ||
| n = 296 | n = 148 | n = 148 |
| Beasley (2008)45 | ||
| Country: USA | Setting: N/A | |
| Aims: energy down, fat down, fibre up, weight down | Delivery mode: mobile device | |
| Description: | Food diary record to be completed three times daily for one month, providing tailored feedback on intake | Paper-based food diary; no feedback |
| Follow-up: baseline, 1 month | ||
| n = 174 | n = 80 | n = 79 |
| Blanson Henkemans (2009)46 | ||
| Country: Netherlands | Setting: N/A | |
| Aim: BMI down | Delivery mode: internet | |
| Description: | Access for 1 month to a website providing tailored feedback on intake and goals, provided by an automated computer assistant (iCat) | Used same website and undertook same activities, but did not have an automated computer assistant providing feedback |
| Follow-up: baseline, 1 month | ||
| n = 118 | n = 65 | n = 53 |
| Block (2004)47 | ||
| Country: USA | Setting: CD-ROM | |
| Aim: FV up | Delivery mode: community centre | |
| Description: | One 20-minute interaction with a computer program providing tailored feedback on intake, plus general nutrition information and goal setting | Similar interaction with a non-dietary CD-ROM programme on stress management |
| Follow-up: baseline, 2 months | ||
| n = 481 | n = 160 | n = 159 |
| Buller (2008)48 | ||
| Country: USA | Setting: N/A | |
| Aim: FV up | Delivery mode: internet | |
| Description: | Monthly sessions over 4 months on a website containing information and advice, broadly tailored to a local population | No intervention: delayed access to the intervention after the post-test |
| Follow-up: baseline, 4 months | ||
| n = 762 | n = 380 | n = 375 |
| Campbell (1999)49 | ||
| Country: USA | Setting: food stamp office | |
| Aim: fat down | Delivery mode: computer kiosk | |
| Description: | One 30-minute interaction with a multimedia intervention providing information and behaviour change strategies, including tailored feedback on intake and goal setting | No intervention |
| Follow-up: baseline, 1 month | ||
| n = 378 | n = 165 | n = 212 |
| Campbell (2004)50 | ||
| Country: USA | Setting: WIC clinic | |
| Aim: fat down, FV up | Delivery mode: computer kiosk | |
| Description: | One 25-minute interaction with a multimedia intervention providing information and behaviour change strategies, including tailored feedback on intake | No intervention: delayed access to the intervention after the post-test |
| Follow-up: baseline, 1 month | ||
| n = 307 | n = 141 | n = 166 |
| Carbone (1999)51 | ||
| Country: USA | Setting: primary care | |
| Aim: fat down, FV up | Delivery mode: CD-ROM | |
| Description: | One 30-minute interaction with an interactive game show, providing feedback tailored on participant knowledge |
Non-tailored video intervention (C1) Non-intervention control group (C2) |
| Follow-up: baseline, 2 months. | ||
| n = 201 | n = 70 | n = 71/60 |
| Cook (2007)52 | ||
| Country: USA | Setting: workplace | |
| Aim: weight down | Delivery mode: internet | |
| Description: | Access over 3 months to a website providing information and guidance, including tailored feedback on intake and BMI | Received high-quality commercially available print materials on the same topics (but not necessarily the same content) |
| Follow-up: baseline, 3 months | ||
| n = 419 | n = 209 | n = 210 |
| Cussler (2008)53 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI down, energy down, weight maintain | Delivery mode: internet | |
| Description: | Weekly access over 1 year to a website providing information, communications tools (e-mail, chat rooms) and progress monitoring tools (dietary log) to support maintenance of the weight loss phase | No further intervention (permitted to continue to meet and practice the principles learned during the weight loss phase) |
| Follow-up: baseline, 12 months | ||
| n = 135 | n = 66 | n = 69 |
| De Bourdeaudhuij (2007)54 | ||
| Country: Belgium | Setting: workplace | |
| Aim: fat down | Delivery mode: CD-ROM | |
| Description: | One interaction with an intervention providing tailored feedback on intake and knowledge |
Generic dietary information (C1) Non-intervention control group (C2) |
| Follow-up: baseline, 6 months | ||
| n = 539 | n = 192 | n = 197/150 |
| Delichatsios (2001)55 | ||
| Country: USA | Setting: home | |
| Aim: fat down, fibre up, FV up | Delivery mode: telephone (automated) | |
| Description: | 5- to 7-minute interaction weekly over 6 months with an intervention providing goals, information and automated counselling tailored to intake | Physical activity promotion counselling via the same technology with similar length of exposure |
| Follow-up: baseline, 6 months | ||
| n = 298 | n = 148 | n = 150 |
| Di Noia (2008)56 | ||
| Country: USA | Setting: community centre | |
| Aim: FV up | Delivery mode: CD-ROM | |
| Description: | Four 30-minute interactions over 1 month with an intervention providing feedback tailored on psychosocial variables | No intervention: participated in regular programmes offered at participating sites |
| Follow-up: baseline, 2 months | ||
| n = 507 | n = 117 | n = 390 |
| Ellrott (2005)57 | ||
| Country: Germany | Setting: N/A | |
| Aim: weight down | Delivery mode: mobile device | |
| Description: | Access for 12 weeks to a food diary record with tailored feedback on intake and nutrient content | Paper-based food diary and self-help manual |
| Follow-up: 1 month | ||
| n = 101 | n = 51 | n = 50 |
| Franko (2008)58 | ||
| Country: USA | Setting: school/college | |
| Aim: fat down, FV up | Delivery mode: internet | |
| Description: | Two 45-minute interactions with a website providing feedback tailored on intake, as well as information and goal setting |
Interactive anatomy education website (Second intervention group: use of the intervention with an additional booster session) |
| Follow-up: baseline, 3 months, 6 months | ||
| n = 476 | n = 165 | n = 147 (164) |
| Gow (2010)59 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI maintain, fat down, fibre up, FV up, weight maintain | Delivery mode: internet | |
| Description: | Weekly 45-minute sessions over 6 weeks with an intervention delivered via Blackboard©,a including information, self-assessments, discussion and experiential activities |
Feedback intervention arm does not participate in internet intervention, but receives weekly feedback on weight (C1) Non-intervention control group (C2) |
| Follow-up: baseline, 6 weeks | ||
| n = 159 | n = 40 | n = 39/40 |
| Haerens (2007)60 | ||
| Country: Belgium | Setting: school/college | |
| Aim: fat down | Delivery mode: CD-ROM | |
| Description: | One 50-minute interactive session delivered via Blackboard, including information and feedback tailored on intake and psychosocial data | Wait-list control |
| Follow-up: baseline, 3 months | ||
| n = 304 | n = 153 | n = 151 |
| Huang (2006)61 | ||
| Country: Australia | Setting: N/A | |
| Aim: fat down | Delivery mode: internet | |
| Description: | Access over 6 months to an intervention providing suggestions of lower-fat alternatives for each higher-fat item selected in an online shop | General advice about how to choose a diet lower in fat, as a static web page |
| Follow-up: 1 month, 6 months | ||
| n = 497 | n = 251 | n = 246 |
| Irvine (2004)62 | ||
| Country: USA | Setting: workplace | |
| Aim: fat down, FV up | Delivery mode: computer kiosk | |
| Description: | Access over 1 month to information and strategies for healthy eating tailored on intake, plus goal setting | Wait-list control |
| Follow-up: baseline, 1 month, 2 months | ||
| n = 517 | n = 260 | n = 257 |
| Jacobi (2007)63 | ||
| Country: Germany | Setting: N/A | |
| Aim: BMI maintain | Delivery mode: Internet | |
| Description: | Student Bodies software for prevention of eating disorders, adapted for German participants | Wait-list control |
| Follow-up: baseline, 3 months | ||
| n = 97 | n = 47 | n = 50 |
| Jones (2008)64 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI maintain, fat down | Delivery mode: internet | |
| Description: | Weekly access for 16 weeks to the Student Bodies software for prevention of eating disorders, including psycho-educational material, asynchronous discussion group, goal setting and a handbook for parents | Wait-list control |
| Follow-up: baseline, 4 months, 9 months | ||
| n = 105 | n = 52 | n = 53 |
| Kroeze (2008)65 | ||
| Country: Netherlands | Setting: home | |
| Aim: BMI down, energy down, fat down | Delivery mode: CD-ROM | |
| Description: | One-time access to an intervention providing information tailored on dietary habits | Non-tailored generic nutrition information |
| Follow-up: baseline | ||
| n = 442 | n = 151 | n = 141 |
| Low (2006)66 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI maintain | Delivery mode: internet | |
| Description: | Access over 8 weeks to the Student Bodies software for the prevention of eating disorders, including psycho-educational material and goal setting | Intervention plus: the program is accompanied by an asynchronous online discussion group with a clinical psychologist |
| Follow-up: baseline, 9 months | ||
| n = 61 | n = 14 | n = 14 |
| Oenema (2001)67 | ||
| Country: Netherlands | Setting: school/college, workplace | |
| Aim: fat down, FV up | Delivery mode: internet | |
| Description: | Information and feedback tailored on intake | Non-tailored nutrition information letter |
| Follow-up: baseline, 1 month | ||
| n = 204 | n = 102 | n = 102 |
| Oenema (2005)68 | ||
| Country: Netherlands | Setting: home, workplace | |
| Aim: fat down, FV up | Delivery mode: CD-ROM | |
| Description: | Information and feedback tailored on intake | Non-tailored nutrition information delivered on the web |
| Follow-up: baseline, 1 month | ||
| n = 782 | n = 261 | n = 260 |
| Oenema (2008)69 | ||
| Country: Netherlands | Setting: N/A | |
| Aim: fat down | Delivery mode: internet | |
| Description: | Access over 1 month to information and feedback tailored on intake | Non-intervention control group |
| Follow-up: baseline, 1 month | ||
| n = 2159 | n = 1080 | n = 1079 |
| Rothert (2006)70 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI down, weight down | Delivery mode: internet | |
| Description: | Access over 6 weeks to an intervention providing an action plan and information tailored on baseline assessment, plus opportunity to enrol a supportive ‘buddy’ | Information only: standard Kaiser Permanente member website (Kaiser Permanente, www.kaiserpermanente.org/, is a non-profit integrated health-care delivery system) |
| Follow-up: 3 months, 6 months | ||
| n = 2862 | n = 1475 | n = 1378 |
| Shapiro (2007)71 | ||
| Country: USA | Setting: home | |
| Aim: BMI down, FV up, weight down | Delivery mode: CD-ROM | |
| Description: | Access over 10 weeks to a program based on CBT providing information and psycho-education, and individualised CBT exercises |
10 weeks of group CBT treatment (C1) Wait-list control (C2) |
| Follow-up: baseline, 2 months | ||
| n = 66 | n = 22 | n = 22/22 |
| Sternfeld (2009)72 | ||
| Country: USA | Setting: workplace | |
| Aim: fat down, FV up | Delivery mode: internet | |
| Description: | Access over 16 weeks to an intervention providing information, feedback and goal setting tailored on intake | Wait-list control |
| Follow-up: baseline, 4 months, 8 months | ||
| n = 787 | n = 351 | n = 436 |
| Svetkey (2008)73 | ||
| Country: USA | Setting: home | |
| Aim: energy down, fat down, FV up, weight down | Delivery mode: internet | |
| Description: | Feedback and motivational messages tailored on intake, plus general information | Non-tailored nutrition and lifestyle information |
| Follow-up: baseline, 30 months | ||
| n = 1032 | n = 348 | n = 342 |
| Tate (2006)74 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI down, energy down, fat down, weight down | Delivery mode: internet | |
| Description: | Calorie-restricted diet based on baseline weight; meal replacements; feedback tailored on weight |
Human e-counselling (C1) No intervention control group (C2) |
| Follow-up: baseline, 3 months, 6 months | ||
| n = 192 | n = 61 | n = 67/64 |
| Trinh (2009)75 | ||
| Country: USA | Setting: N/A | |
| Aim: FV up | Delivery mode: internet | |
| Description: | Access over 5 weeks to an intervention providing feedback tailored on baseline assessment, plus information and strategies for healthy eating | Wait-list control |
| Follow-up: baseline, 2 months | ||
| n = 307 | n = 159 | n = 148 |
| Turnin (1992)76 | ||
| Country: France | Setting: home | |
| Aim: fat down, weight down | Delivery mode: Minitel | |
| Description: | Feedback tailored on intake, plus meal analysis and general dietary information | Wait-list control |
| Follow-up: baseline, 6 months | ||
| n = 105 | n = 54 | n = 51 |
| Turnin (2001)77 | ||
| Country: France | Setting: home | |
| Aim: BMI down, energy down, fat down | Delivery mode: Minitel | |
| Description: | Caloric recommendations tailored on baseline assessment, plus meal analysis and general dietary information | Usual care |
| Follow-up: baseline, 6 months, 12 months | ||
| n = 557 | n = 279 | n = 278 |
| Vandelanotte (2005)78 | ||
| Country: Belgium | Setting: school/college | |
| Aim: fat down | Delivery mode: personal computer | |
| Description: | One 50-minute session providing feedback tailored on intake and psychosocial variables, plus tips and suggestions on healthy eating | Wait-list control |
| Follow-up: – | ||
| n = 771 | ||
| Verheijden (2004)79 | ||
| Country: Canada | Setting: home, workplace | |
| Aim: BMI down, fat down | Delivery mode: internet | |
| Description: | Counselling messages tailored on intake and psychosocial variables, plus general information | Usual care |
| Follow-up: baseline, 6 months | ||
| n = 146 | n = 73 | n = 73 |
| Veverka (2003)80 | ||
| Country: USA | Setting: home, workplace | |
| aim: energy down, fat down, fibre up, FV up | Delivery mode: internet | |
| Description: | Monthly sessions over 6 months of an intervention providing information tailored by psychosocial variables | Non-intervention control group |
| Follow-up: baseline, 6 months | ||
| n = 39 | n = 20 | n = 19 |
| Winett (1991)81 | ||
| Country: USA | Setting: supermarket | |
| Aim: fat down, fibre up, FV up | Delivery mode: computer kiosk | |
| Description: | Weekly 8-minute sessions over 6 weeks of an intervention providing feedback concerning intended purchases, with prompts to encourage lower-fat and higher-fibre purchases and general nutritional information | Used the program to enter food purchases only; no feedback |
| Follow-up: baseline, 3 months | ||
| n = 77 | n = 40 | n = 37 |
| Winett (2007)82 | ||
| Country: USA | Setting: community centre | |
| Aim: fat down, fibre up, FV up, weight maintain | Delivery mode: internet | |
| Description: | Weekly 10-minute sessions over 12 weeks of an intervention providing individual goals with specific strategies, plus meal planner and general nutrition information | Wait-list control |
| Follow-up: baseline, 3 months, 6 months | ||
| n = 1071 | n = 364 | n = 364 |
| Wylie-Rosett (2001)83 | ||
| Country: USA | Setting: Health Maintenance Organisation | |
| Aim: BMI down, energy down, fat down, weight down | Delivery mode: computer kiosk | |
| Description: | Workbook, plus monthly 30-minute sessions over 12 months of an intervention providing feedback tailored in intake, plus goal setting and general nutrition information |
Workbook only (C1) Workbook + computer + staff consultation/therapy (C2) |
| Follow-up: baseline, 12 months | ||
| n = 588 | n = 236 | n = 116/236 |
| Zabinski (2001)84 | ||
| Country: USA | Setting: N/A | |
| Aim: BMI maintain | Delivery mode: internet | |
| Description: | Student Bodies software for the prevention of eating disorders, plus an electronic bulletin board | Wait-list control |
| Follow-up: baseline, 2 months | ||
| n = 62 | n = 31 | n = 31 |
Study design
A total of 43 RCTs were identified as eligible for inclusion in the review (see Table 2 for a summary of included studies; Appendix 5 for a full bibliography of included studies; and Appendix 4 for the PRISMA flow diagram detailing inclusion and exclusion during different stages of the review). Of the 43 included studies, one62 was a crossover trial, two79,82 were cluster randomised trials, whereas the rest were parallel-group RCTs.
Target outcomes of included studies
Although all interventions included in the review were designed to alter dietary behaviour, different interventions targeted different components of this behaviour and collected different outcome measures in order to measure success. Table 3 summarises the target outcomes of the included studies, whereas Table 15 summarises the dietary and clinical outcome measures used.
| Study | Increase fibre intake | Increase fruit and vegetable intake | Maintain BMI | Maintain weight | Reduce BMI | Reduce energy intake | Reduce fat intake | Reduce weight |
|---|---|---|---|---|---|---|---|---|
| Agras (1990)42 | ✓ | |||||||
| Alexander (2010)43 | ✓ | |||||||
| Anderson (2001)44 | ✓ | ✓ | ✓ | |||||
| Beasley (2008)45 | ✓ | ✓ | ✓ | ✓ | ||||
| Blanson Henkemans (2009)46 | ✓ | |||||||
| Block (2004)47 | ✓ | |||||||
| Buller (2008)48 | ✓ | |||||||
| Campbell (1999)49 | ✓ | ✓ | ||||||
| Campbell (2004)50 | ✓ | ✓ | ||||||
| Carbone (1999)51 | ✓ | ✓ | ||||||
| Cook (2007)52 | ✓ | |||||||
| Cussler (2008)53 | ✓ | ✓ | ✓ | ✓ | ||||
| De Bourdeaudhuij (2007)54 | ✓ | |||||||
| Delichatsios (2001)55 | ✓ | ✓ | ✓ | |||||
| Di Noia (2008)56 | ✓ | |||||||
| Ellrott (2005)57 | ✓ | |||||||
| Franko (2008)58 | ✓ | ✓ | ||||||
| Gow (2010)59 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Haerens (2007)60 | ✓ | |||||||
| Huang (2006)61 | ✓ | |||||||
| Irvine (2004)62 | ✓ | ✓ | ||||||
| Jacobi (2007)63 | ✓ | |||||||
| Jones (2008)64 | ✓ | ✓ | ||||||
| Kroeze (2008)65 | ✓ | ✓ | ||||||
| Low (2006)66 | ✓ | |||||||
| Oenema (2001)67 | ✓ | ✓ | ||||||
| Oenema (2005)68 | ✓ | ✓ | ||||||
| Oenema (2008)69 | ✓ | ✓ | ||||||
| Rothert (2006)70 | ✓ | |||||||
| Shapiro (2007)71 | ✓ | ✓ | ✓ | |||||
| Sternfeld (2009)72 | ✓ | ✓ | ✓ | ✓ | ||||
| Svetkey (2008)73 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Tate (2006)74 | ✓ | ✓ | ✓ | |||||
| Trinh (2009)75 | ✓ | |||||||
| Turnin (1992)76 | ✓ | ✓ | ||||||
| Turnin (2001)77 | ✓ | ✓ | ✓ | |||||
| Vandelanotte (2005)78 | ✓ | |||||||
| Verheijden (2004)79 | ✓ | ✓ | ||||||
| Veverka (2003)80 | ✓ | ✓ | ✓ | ✓ | ||||
| Winett (1991)81 | ✓ | ✓ | ✓ | |||||
| Winett (2007)82 | ✓ | ✓ | ✓ | ✓ | ||||
| Wylie-Rosett (2001)83 | ✓ | ✓ | ✓ | ✓ | ||||
| Zabinski (2001)84 | ✓ | |||||||
| TOTAL | 7 | 21 | 6 | 3 | 7 | 9 | 28 | 12 |
The majority of interventions sought to reduce fat intake44,45,49–51,54,55,58–62,64,65,67–69,72–74,76–83 (28 interventions) and/or to increase fruit and vegetable intake43,44,47–51,55,56,58,59,62,67,68,71–73,75,80–82 (21 interventions). Other interventions sought to increase fibre intake,44,45,55,59,80–82 reduce overall energy intake,45,53,65,72–74,77,80,83 reduce42,45,52,57,69–74,76,83 or maintain53,59,82 weight, or reduce46,53,71,73,77,79,83 or maintain53,59,63,64,66,84 BMI.
Comparators used in included studies
Table 4 shows a summary of comparators used in the studies. Fourteen studies44,48–51,53,54,56,59,69,74,77,79,80 trialled the intervention against a non-intervention control group. Three studies47,55,58 used attention controls, whereby participants in the control group received non-adaptive information on a similar topic, or received a similarly interactive intervention, but on an unrelated health topic. Thirteen studies48,50,62–64,71,72,75,76,78,82,84 used a wait-list control group that received the intervention after the data collection period. Two studies tested their intervention against a therapist-conducted intervention,42,74 or the e-learning intervention plus a therapist-conducted intervention. 66,83
| Comparator | Number of studiesa |
|---|---|
| Attention control, type 1: non-interactive information on a similar topic | 14 |
| No intervention (including usual care) | 13 |
| Wait-list control (intervention received at end of study) | 12 |
| Therapist-conducted intervention | 4 |
| Attention control, type 2: interactive intervention on an unrelated topic | 3 |
| Intervention plus therapist | 2 |
Interventions
Setting
Tables 5 and 6 summarise the countries and settings in which the interventions were delivered. All e-learning interventions were delivered in high-income countries, and the majority of interventions were delivered in the USA42–45,47–53,55,56,58,59,62,64,66,70–75,80–84 (29 interventions), the Netherlands46,65,67–69 (five studies) and Belgium54,60,78 (three studies). There are several distinct research groups dedicated to e-learning and diet research; hence, the geographic clustering of studies. For example, a single large e-learning research group produced all eight RCTs set in the Netherlands and Belgium. Interventions were also delivered in Australia,61 Canada,79 France76,77 and Germany. 57,63
| Country | Number of studies |
|---|---|
| USA | 29 |
| Netherlands | 5 |
| Belgium | 3 |
| France | 2 |
| Germany | 2 |
| Australia | 1 |
| Canada | 1 |
| Setting | Number of studiesa |
|---|---|
| N/Ab | 16 |
| Home | 9 |
| Workplace | 8 |
| Community centre | 3 |
| School/college | 4 |
| Supermarket | 2 |
| Food stamp office | 1 |
| Health Maintenance Organisation | 1 |
| Primary care | 1 |
| WIC clinic | 1 |
| Not specified | 1 |
Many interventions were offered over the internet or via a mobile device and without a specific setting. Of those that were delivered in a specific setting, nine were designed to be delivered in the home,55,65,68,71,73,76,77,79,80 eight in the workplace,52,54,62,67,68,72,79,80 three in community centres,47,56,82 four in schools/colleges58,60,67,78 and two in supermarkets. 44,81 Other settings included a food stamp office,49 a health maintenance organisation,83 a primary care clinic51 and a Women, Infants and Children Program clinic. 50 One study did not specify a setting. 84
Mode of delivery
Table 7 summarises the mode of delivery for interventions. Most interventions were delivered via the internet43,46,48,52,53,58,59,61,63,64,66,67,69,70,72–75,79,80,82,84 (22 interventions), compact disk read-only memory (CD-ROM)47,51,54,56,60,65,68,71 (eight interventions) and computer kiosks44,49,50,62,81,83 in specific locations (six interventions). Other modes of delivery included Minitel76,77 (an online system accessible through the telephone), mobile devices42,45,57 [such as personal digital assistants (PDAs)] and automated telephone services. 55 One study used personal computers, but did not specify how the intervention was delivered on the computer. 78
| Mode of delivery/hardware | Number of studies |
|---|---|
| Internet | 22 |
| CD-ROM | 8 |
| Computer kiosk | 6 |
| Mobile device | 3 |
| Minitel | 2 |
| Personal computer | 1 |
| Telephone (automated) | 1 |
Intervention usage and adherence
Although most studies reported the duration of the intervention, many did not report intended intensity or frequency, which precludes calculation of an intended ‘dose’ of the intervention. Of those that did, intended intensity of interventions varied enormously: several were one-day, one-off interactions with the intervention lasting under 1 hour,47,49–51,54,60,78 whereas the two longest studies lasted 1 year, with either weekly53 or monthly83 interaction with the intervention. The latter study had 12 sessions of 30 minutes over 1 year, providing a potential 6 hours of contact. Beasley et al. 45 required participants to use their mobile device three times a day for 28 days, with a potentially much longer contact time (minutes per session were not reported). The majority of interventions lasted between 1 and 6 months, with participants expected to interact with the intervention daily or weekly for 10–45 minutes at a time.
Twenty-four studies reported on actual use of intervention programs, either making use of automated logon or usage features within programs (16 studies42,48,53,59,62–64,66,69,71,74,77,79,80,82,84) or obtaining self-reported usage data from participants (three studies52,58,60). Five studies44,55,57,70,80 reported collecting usage data, but did not state how. The range of usage data collected varied: studies that specified an intended intensity or frequency of use calculated adherence to the intended programme; others reported number of logons or pages viewed, either as raw data or as a percentage of possible pages viewed. Reported adherence ranged from 43% to 85% in intervention groups.
Of the studies that assessed usage, few saw a drop-off over time. 42,62,74,77,84 In all five studies, compliance was high at onset but gradually declined from the second month. Usage was lowest in the final week in all but one study. 42,62,74,84 For example, in one study,42 the adherence to the treatment was high in the initial weeks for all the three groups until the 10th week, when it declined sharply in the intervention group, reaching 29% in the final (12th) week. In another study,62 the program usage was 14.7% and 7.5% in the second and third months, respectively. Tate et al. 74 found the median number of logins to be > 10 at the beginning of the study, but decreased to 5 by the second and third month, and finally to 2 by the sixth month. Turnin et al. 77 found the mean duration of intervention use was 260 minutes in the first month and 60 minutes in the next 2 months, and then gradually decreased to 30 minutes from the fourth month.
Components
This review sought to investigate the different components of e-learning interventions, in order to assess whether or not they were associated with effective behaviour change. Interventions were reported and described using widely varying terminology and level of detail, making direct comparisons difficult.
The frequency of reported components is summarised in Table 8 and is presented in detail for each study in Table 9.
| Component | Number of studiesa |
|---|---|
| Information, advice, tips (written on-screen or printed) | 31 |
| Entering consumption data/food diary | 28 |
| Feedback on intake (written or visual) | 21 |
| Goal setting/action plans; feedback on goals/plans | 15 |
| Incentives (vouchers; cash) | 15 |
| Psychological help/motivation/addressing barriers | 14 |
| Recipes; cooking tips; menus; meal plans | 12 |
| Communication tools (chat room; bulletin board; asynchronous discussion) | 9 |
| Automated reminder e-mails | 8 |
| Links to websites | 8 |
| Help with food purchasing/meal planning | 8 |
| Information (multimedia/interactive) | 6 |
| Entering clinical data; feedback on clinical data | 5 |
| Web guide/i-moderator | 4 |
| Technical support available | 4 |
| Knowledge test/quiz; feedback on knowledge | 3 |
| Assignments/homework/putting techniques into practice | 3 |
| Supportive ‘buddy’ | 3 |
| Presentation of a role model | 2 |
| Study | Reported components | Behaviour change techniques |
|---|---|---|
| Agras (1990)42 | Entering caloric data; goal setting; meal planning; trainer to promote slow eating; feedback and feedback graphs | Action planning; goal setting (behaviour); motivational interviewing; prompt review of behavioural goals; shaping |
| Alexander (2010)43 | Web program was divided into four intervention ‘sessions’ offered 1, 3, 13 and 15 weeks after enrolment; automated e-mails notified participants when a new website session was available. Each session included four to five pages of core content, illustrations, optional links to more detailed explanations and special features designed to supplement session content. Recipes and menus | Information on behaviour consequences in general; prompt identification as a role model; teach to use prompts/cues |
| Anderson (2001)44 | Prescriptive information; suggested planning and monitoring strategies for food purchases; personalised goal setting and feedback; incentives [targeted food coupons (US$8–12/week)] | General planning; goal setting (behaviour); information on behaviour consequences in general; information on when/where to perform behaviour; prompt review of behavioural goals; rewards contingent on successful behaviour; tailoring |
| Beasley (2008)45 | Food diary record (prompted by audible beep); personalised comparisons of intake to goal by meal and by day; recipes and meal plans | Goal setting (behaviour); prompt review of behavioural goals |
| Blanson Henkemans (2009)46 | Food diary; automated reminder e-mails; generated nutrient report; persuasive computer assistant (iCat) monitoring the diary and offering co-operative feedback; referral/linking to Dutch nutrition website for information | Goal setting (behaviour); information on behaviour consequences in general; motivational interviewing; prompt self-monitoring of behaviour; prompt self-monitoring of behavioural outcome |
| Block (2004)47 | Screening and feedback; recipes and cooking tips; goal setting; information and tips | Barrier identification/problem solving; goal setting (behaviour); tailoring |
| Buller (2008)48 | Routine e-mail notifications; information and advice; links to useful websites | Information on how to perform behaviour; normative information about others’ behaviour; prompt generalisation of target behaviour; provide information about others’ approval |
| Campbell (1999)49 | Informative soap opera; ‘infomercials’; feedback to knowledge quiz; comparison of individual intake to general goal intake; personal behavioural goal setting | Fear arousal; goal setting (behaviour); information on behaviour consequences in general; information on behaviour consequences to individual; model/demonstrate behaviour; tailoring |
| Campbell (2004)50 | The FoodSmart intervention included four main components: a full-motion video soap opera, interactive ‘infomercials’, tailored dietary and psychosocial feedback determined by baseline assessment questions, and take-home print materials | Information on behaviour consequences to individual; model/demonstrate behaviour |
| Carbone (1999)51 | Choice of two interactive game shows; feedback tailored on participant knowledge; low-fat recipe book | Tailoring |
| Cook (2007)52 | Interactive assessment of daily calorie and fat intake; calculation of BMI; assessment of user’s daily food categories based on the Healthy Eating Pyramid; video testimonials on benefits of good nutrition and weight management; information on popular diets (Atkins, South Beach, etc.); information and training; goal setting and progress tracking; meal planning and shopping strategies | Barrier identification/problem solving; emotional control training; general planning; goal setting (behaviour); information on behaviour consequences in general; model/demonstrate behaviour; normative information about others’ behaviour; prompt review of behavioural goals; prompt self-monitoring of behaviour |
| Cussler (2008)53 | Communications tools (e-mail, chat rooms); progress monitoring tools (bodyweight, dietary log); information; links to other websites | Goal setting (behaviour); prompt review of behavioural goals |
| De Bourdeaudhuij (2007)54 | Screening questionnaire with tailored feedback; written information | Information on behavioural consequences in general; information on behavioural consequences to individual; normative information about others’ behaviour; tailoring |
| Delichatsios (2001)55 | Food consumption questions; tailored goals; education, advice and counselling (automated) | Goal setting (outcome); prompt focus on past success; prompt review of outcome goals |
| Di Noia (2008)56 | Action planning; goal setting; information on behavioural consequences in general; information on behavioural consequences to individual; plan social support/social change | |
| Ellrott (2005)57 | Entry of foods/meals to a database; immediate feedback on nutrient content; information on, and comparison with, recommended intakes for the person’s age and sex; self-help manual (used only in weeks 2, 3, 4, 8 and 12); once a week to the clinic, for measurement of weight | Prompt self-monitoring of behaviour; provide feedback on performance; tailoring |
| Franko (2008)58 | (1) Three information links (Ask the Expert, Student Voices, College News); (2) rate myself assessment (questions that are part of the website that are used to provide feedback to the user); (3) four main topic pages (nutrition, eating on the run, weighing in, fitness); and (4) resources | Goal setting (behaviour) |
| Gow (2010)59 | Self-assessments, group discussions via the ‘Blackboard’ discussion board and experiential activities (e.g. mindful eating); homework assignments to encourage implementation of new skills; online discussion; weekly weight check and graphic feedback; groups were facilitated by the principal investigator | Barrier identification/problem solving |
| Haerens (2007)60 | Introduction page; diagnostic tool; intervention messages | Information on how to perform behaviour; normative information about others’ behaviour; plan social support/social change; tailoring |
| Huang (2006)61 | Suggestions of lower-fat alternatives for each higher-fat item selected in online shop | Tailoring |
| Irvine (2004)62 | On-screen eating habits assessment; eating strategies, recipes, barriers to eating healthy, eating habits assessment, information centre and quick tips | Barrier identification/problem solving; goal setting (behaviour); information on behaviour consequences in general; information on when and where to perform behaviour; model/demonstrate behaviour; prompt review of behavioural goals; tailoring |
| Jacobi (2007)63 | Student Bodies software, adapted for a German audience | |
| Jones (2008)64 | Psycho-educational material; self-monitoring journals; goals; asynchronous discussion group; a handbook for parents | Barrier identification/problem solving; environmental restructuring; goal setting (behaviour); information on when and where to perform behaviour; plan social support/social change; prompt self-monitoring of behaviour; relapse prevention/coping planning |
| Kroeze (2008)65 | Screening questionnaire; individualised computer-tailored information (personalised opening, general introduction to the topic); feedback on fat intake and feedback about the fat intake of peers; suggestions for how to change; feedback on how to lower fat intake in situations perceived as difficult; recipes | Barrier identification/problem solving; facilitate social comparison; information on behaviour consequences in general; information on how to perform behaviour; provide feedback on performance; tailoring |
| Low (2006)66 | Overview (included a description of the development and consequences of eating disorders); information (text, audio and video); on-line self-monitoring journals; behaviour change exercises; mandatory and optional assignments; discussion group | Information on behaviour consequences to individual; plan social support/social change; prompt self-monitoring of behaviour; tailoring |
| Oenema (2001)67 | Short introduction; tailoring questionnaire on dietary habits; tailored feedback (short introduction, feedback messages on the respondent’s own estimation of intake and the intake score computed from the baseline questionnaire); comparison of personal intake with recommended intake levels; messages on the most important sources of nutrients in the diet and possible alternatives; tips on altering consumption; recipes | Barrier identification/problem solving; facilitate social comparison; fear arousal; information on behavioural consequences to individual; information on how to perform behaviour; provide feedback on performance; tailoring |
| Oenema (2005)68 | Feedback on personal intake levels of fat, fruit and vegetables; feedback comparing intakes with recommendations and average intake of peers; tailored messages of encouragement; information about what changes to make and how to make changes; role model stories; recipe suggestions | Action planning; barrier identification/problem solving; facilitate social comparison; fear arousal; information on how to perform behaviour; model/demonstrate behaviour; provide feedback on performance; tailoring |
| Oenema (2008)69 | Assessment of perceived fat intake and perceived physical activity level; information modules | Fear arousal; information on behaviour consequences to individual; prompt self-monitoring of behaviour; provide feedback on performance; tailoring |
| Rothert (2006)70 | Individually tailored weight management plan; messages tailored to specific issues; opportunity to enrol a supportive ‘buddy’ | Action planning; barrier identification/problem solving; information on behaviour consequences to individual; plan social support/social change; prompt self-monitoring of behaviour; tailoring; teach to use prompts/cues |
| Shapiro (2007)71 | Personal stories (of fictional characters) to emphasise: reasonable portion sizes and non-emotional eating; healthy levels of regular exercise and increased daily non-exercise activity; psycho-education on unhealthy eating and weight-related practices; basic concepts and techniques of CBT; individualised and interactive exercises for practising CBT techniques; guidelines and exercises for relapse prevention | Model/demonstrate behaviour; tailoring |
| Sternfeld (2009)72 | Reports of intake of saturated fat, trans fats, added sugars, fruit and vegetables in relation to guidelines; feedback (including information on the participant’s top three sources of problematic nutrients); tailored goal setting and review (goal tracker); e-mail suggesting tailored goals; ‘personal home page’ containing tips, a goal tracker, health information and links to sites for additional information; graphic presentation of how much any specific change in diet (or physical activity) might move participants closer towards the recommended level | Barrier identification/problem solving; fear arousal; information on behaviour consequences in general; information on behaviour consequences to individual; plan social support/social change; prompt review of behavioural goals; prompt self-monitoring of behavioural outcome; provide feedback on performance; set graded tasks; tailoring; teach to use prompts/cues |
| Svetkey (2008)73 | Initial orientation; e-mail prompts; tailored motivational messages; relevant news and information; reminder to return to website (if necessary); self-monitoring data entry (food records, exercise, minimum daily calorie totals); tailored automated feedback; goal setting and action plan development; training modules for relapse prevention and problem solving; bulletin board discussions; e-mail with technical support ‘contact us’, reliable links, news and information, personal profiles, ‘ask the expert’ discussion group; telephone interactive voice response (if necessary) for encouragement to re-engage | Action planning; barrier identification/problem solving; plan social support/social change; prompt self-monitoring of behaviour; provide feedback on performance; tailoring; teach to use prompts/cues |
| Tate (2006)74 | Calorie-restricted diet according to weight at baseline; instruction on structured meals and meal replacement strategies; interactive website (weekly reporting and graphs of weight, e-mail prompts to report weight, weight loss tips via e-mail, recipes and a weight loss e-buddy network system for peer support for weight loss via e-mail); electronic diary (to report weight, daily caloric intake, use of meal replacements and exercise); message board to post messages to other study participants randomised to same group; weekly e-mail to remind to complete online diary and included a weekly behavioural lesson; summary of reported behaviours and weight loss progress, ongoing support, praise or motivation, and suggested next steps | Information on how to perform behaviour; plan social support/social change; prompt review of outcome goals; prompt self-monitoring of behavioural outcome; provide feedback on performance; rewards contingent on progress towards behaviour; tailoring; teach to use prompts/cues; use of follow-up prompts |
| Trinh (2009)75 | Online assessment; personalised, tailored feedback on number and size of servings for food groups; long-term goal setting and identification of first step to work towards goal for the next 7 days; online shopping list and food plan; weekly follow-up e-mail newsletters to support the messages and information provided on the website and to encourage further learning | Barrier identification/problem solving; goal setting (behaviour); information on how to perform behaviour; prompt review of behavioural goals; provide feedback on performance; set graded tasks; tailoring; teach to use prompts/cues |
| Turnin (1992)76 | Individualised counselling; general information; energy requirement calculation; individualised meal analysis; specially adapted menus; recipes; MONICA; general dietary information; e-mail; meal analysis; menus adapted to user’s energy requirements, pathology and seasonal food availability; recipes; general dietetic information | Information on behaviour consequences in general; information on how to perform behaviour; prompt review of behavioural goals; prompt self-monitoring of behaviour; provide feedback on performance; tailoring |
| Turnin (2001)77 | Individualised help in meal analysis and composition; access to general information about diet; individually tailored caloric recommendations; assessment of meal balance (analysis, nutritional advice and suggestions for balanced meals over the whole day); individualised daily menus; recipes; discussion forum | Information on how to perform behaviour; prompt self-monitoring of behaviour; provide feedback on performance; tailoring |
| Vandelanotte (2005)78 | Introduction page; tailored feedback (fat intake compared with current recommendations); tips and suggestions; feedback on participant’s psychosocial attitudes, perceived benefits and barriers, social support and self-efficacy related to physical activity or fat intake | Information on behaviour consequences in general; information on behaviour consequences to individual; information on how to perform behaviour; provide feedback on performance; tailoring |
| Verheijden (2004)79 | Monthly assessment tool to determine stage of change; information package for stage of change; self-assessment tool for dietary fat intake; recipes; bulletin board | Fear arousal; information on behavioural consequences to individual; information on how to perform behaviour; plan social support/social change; prompt self-monitoring of behavioural outcome; rewards contingent on progress towards behaviour; tailoring |
| Veverka (2003)80 | Website containing stage-matched health information; welcome screen; messages; survey (staging algorithm); tailored newsletter (topics in diet and physical activity), reinforcement of topic through feedback (quizzes and facts), ancillary hyperlinks to information from government and professional diet and exercise websites); e-mail to lead researcher for technical difficulties | Prompt review of behavioural goals; prompt self-monitoring of behaviour; tailoring |
| Winett (1991)81 | Video programmes; feedback concerning intended purchases; prompts to encourage lower-fat and higher-fibre purchases; preview of the following week’s programme; basic information and rationale for dietary change; information on reducing fat, increasing complex carbohydrates and fibre in supermarket purchases and meals; options for purchases and meals; ‘commit to’ new purchases and meals by use of touch screens with feedback (comprising suggested substitutes for higher-fat and lower-fibre items; praise for new purchases of lower-fat or higher-fibre products) | Information on behaviour consequences in general; information on how to perform behaviour; provide feedback on performance; rewards contingent on progress towards behaviour; rewards contingent on successful behaviour; set graded tasks; teach to use prompts/cues |
| Winett (2007)82 | Narrator guide; written information; weekly goals with specific strategies; positive and supportive feedback with suggested alternative strategies; ‘fast-food planner’ (virtually visit any restaurant and choose meal items < 750 kcal and 30 g of fat, with feedback); assessments of progress and feedback on meeting goals | Goal setting (behaviour); provide feedback on performance; rewards contingent on successful behaviour |
| Wylie-Rosett (2001)83 | Workbook; behavioural goals; review of goals; information and guidance regarding weight reduction; block fat screener; food guide pyramid | Barrier identification/problem solving; goal setting (behaviour); prompt review of behavioural goals; prompt self-monitoring of behaviour; tailoring |
| Zabinski (2001)84 | Student Bodies software and an electronic bulletin board (forum for discussion of readings and assignments) | Plan social support/social change |
A majority of interventions involved the presentation of nutrition, health and lifestyle information (32 studies43,44,46–54,57,58,60,62,65–73,75–77,79–83), the entering of food consumption data (25 studies42,45–47,49,50,52–55,57,58,65,67–69,72–79,83) and the presentation of personalised feedback on food and nutrient consumption (21 studies42,45–47,49,50,52,54,57,58,65,67–69,72–78). Other interventions focused more on the setting of goals and providing feedback on attainment of these goals (15 studies42,44–47,49,52,55,58,64,72,73,75,82,83). Several studies provided incentives for participation, mainly vouchers, but also cash or gifts (as opposed to incentives for providing follow-up data) (15 studies44,45,47–49,51,56,59,63,67,69,74,78,81,84). Several interventions also provided recipes or meal plans (20 studies42–45,47,51,52,62,65,67,68,70,73–77,79,81,82) or provided some kind of psychological support (14 studies59,62,64–68,70,71,73,74,78,82,84), for example motivational messages, help with overcoming barriers and forms of cognitive behavioural therapy (CBT).
Mechanism of action
The behavioural change theories reported to have been used in developing interventions are shown in Table 10 (relative frequency of use) and Table 11 (use of theories in each study).
| Behaviour change theory | Number of studies |
|---|---|
| Social cognitive theory | 12 |
| Transtheoretical model | 12 |
| CBT | 5 |
| Theory of planned behaviour | 5 |
| Precaution adoption process model | 4 |
| Health beliefs model | 2 |
| Theory of reasoned action | 2 |
| Attitude, social influence and self-efficacy model | 1 |
| Diffusion theory | 1 |
| Goal theory | 1 |
| Health communication theory | 1 |
| Social marketing | 1 |
| Study | Behaviour change theories |
|---|---|
| Agras (1990)42 | Not specified |
| Alexander (2010)43 | Health beliefs model |
| Social cognitive theory | |
| Transtheoretical model | |
| Anderson (2001)44 | Social cognitive theory |
| Beasley (2008)45 | Not specified |
| Blanson Henkemans (2009)46 | Not specified |
| Block (2004)47 | Not specified |
| Buller (2008)48 | Diffusion theory |
| Social cognitive theory | |
| Campbell (1999)49 | Social cognitive theory |
| Transtheoretical model | |
| Campbell (2004)50 | Social cognitive theory |
| Transtheoretical model | |
| Carbone (1999)51 | Not specified |
| Cook (2007)52 | Social cognitive theory |
| Transtheoretical model | |
| Cussler (2008)53 | Not specified |
| De Bourdeaudhuij (2007)54 | Theory of planned behaviour |
| Delichatsios (2001)55 | Social cognitive theory |
| Di Noia (2008)56 | Transtheoretical model |
| Ellrott (2005)57 | Not specified |
| Franko (2008)58 | Not specified |
| Gow (2010)59 | Social cognitive theory |
| Haerens (2007)60 | Attitude, social influence and self-efficacy model |
| Social cognitive theory | |
| Theory of planned behaviour | |
| Transtheoretical model | |
| Huang (2006)61 | Not specified |
| Irvine (2004)62 | Health communication theory |
| Social cognitive theory | |
| Theory of reasoned action | |
| Transtheoretical model | |
| Jacobi (2007)63 | Not specified |
| Jones (2008)64 | CBT |
| Kroeze (2008)65 | Precaution adoption process model |
| Theory of planned behaviour | |
| Low (2006)66 | CBT |
| Oenema (2001)67 | Precaution adoption process model |
| Oenema (2005)68 | Precaution adoption process model |
| Oenema (2008)69 | Precaution adoption process model |
| Rothert (2006)70 | Not specified |
| Shapiro (2007)71 | CBT |
| Sternfeld (2009)72 | Goal theory |
| Health beliefs model | |
| Social cognitive theory | |
| Social marketing | |
| Theory of reasoned action | |
| Svetkey (2008)73 | Motivational Interviewing |
| Tate (2006)74 | CBT |
| Trinh (2009)75 | Persuasive communication |
| Social cognitive theory | |
| Theory of planned behaviour | |
| Transtheoretical model | |
| Turnin (1992)76 | Not specified |
| Turnin (2001)77 | Not specified |
| Vandelanotte (2005)78 | Theory of planned behaviour |
| Transtheoretical model | |
| Verheijden (2004)79 | Transtheoretical model |
| Veverka (2003)80 | Transtheoretical model |
| Winett (1991)81 | Not specified |
| Winett (2007)82 | Not specified |
| Wylie-Rosett (2001)83 | CBT |
| Transtheoretical model | |
| Zabinski (2001)84 | Not specified |
Of the 26 studies that reported using behavioural theories, the most frequently used were social cognitive theory (12 studies43,44,48–50,52,55,59,60,62,72,75), the transtheoretical model (12 studies43,49,50,52,56,60,62,75,78–80,83), the theory of planned behaviour (five studies54,60,65,75,78), CBT (five studies64,66,71,74,83) and the precaution adoption process model (four studies65,67–69).
The relative frequency of use of behaviour change techniques used in interventions (shown in detail for each study in Table 9) is shown in Table 12. The most commonly used techniques were goal setting (behaviour) (14 studies42,44–47,49,52,53,58,62,64,75,82,83), provide feedback on performance (14 studies57,65,67–69,72–78,81,82), provide information on consequences of behaviour in general (13 studies43,44,46,49,52,54,56,62,65,72,76,78,81) or to the individual (11 studies49,50,54,56,66,67,69,70,72,78,79), barrier identification/problem solving (13 studies47,52,59,62,64,65,67,68,70,72,73,75,83), prompt self-monitoring of behaviour (14 studies46,52,57,64,66,69,70,72–74,76,77,80,83), provide instruction on how to perform the behaviour (12 studies48,60,65,67,68,74–79,81), prompt review of behavioural goals (11 studies42,44,45,52,53,62,72,75,76,80,83), and plan social support/social change (10 studies56,60,64,66,70,72–74,79,84). Tailoring of information or feedback to the individual based on personal data was an explicit inclusion criterion for this review of adaptive e-learning, and therefore all 43 studies provided some form of tailoring of information or feedback.
| Behaviour change techniquea | Number of studiesb | Behaviour change techniquea | Number of studiesb |
|---|---|---|---|
| Tailoring | 43 | Prompt review of outcome goals | 2 |
| Provide information on consequences of behaviour in general | 14 | Motivational interviewing | 2 |
| Goal setting (behaviour) | 14 | General planning | 2 |
| Provide feedback on performance | 14 | Prompting generalisation of a target behaviour | 1 |
| Barrier identification/problem solving | 13 | Provide information about others’ approval | 1 |
| Provide instruction on how to perform the behaviour | 12 | Shaping | 1 |
| Prompt self-monitoring of behaviour | 12 | Prompting focus on past success | 1 |
| Prompt review of behavioural goals | 11 | Use of follow-up prompts | 1 |
| Provide information on consequences of behaviour to the individual | 11 | Prompt identification as role model/position advocate | 1 |
| Plan social support/social change | 10 | Environmental restructuring | 1 |
| Teach to use prompts/cues | 7 | Relapse prevention/coping planning | 1 |
| Model/demonstrate the behaviour | 6 | Emotional control training | 1 |
| Fear arousal | 6 | Stimulate anticipation of future rewards | 0 |
| Action planning | 5 | Prompt practice | 0 |
| Provide normative information about others’ behaviour | 4 | Time management | 0 |
| Prompt self-monitoring of behavioural outcome | 4 | Prompt anticipated regret | 0 |
| Provide information on where and when to perform the behaviour | 3 | Prompt use of imagery | 0 |
| Set graded tasks | 3 | Prompt self-talk | 0 |
| Provide rewards contingent on effort or progress towards behaviour | 3 | Agree behavioural contract | 0 |
| Facilitate social comparison | 3 | Stress management | 0 |
| Goal setting (outcome) | 2 | General communication skills training | 0 |
| Provide rewards contingent on successful behaviour | 2 |
Participants
Baseline health and eligibility criteria
Several studies had specific eligibility criteria related to clinical health status. Of the 23 studies providing details of the study inclusion criteria, 12 offered the intervention only to those with BMI > 25 kg/m2;42,45,46,53,57,64,70,71,73,74,77,83 10 required participants to be generally healthy and free of diagnosed disease;43,47,52,53,55,59,63,73,78,80 one trialled the intervention in patients with type 1 or 2 diabetes;76 and one required participants to have at least one diagnosed risk factor for CVD. 79
Many studies recorded baseline measures of general health and nutrition status that were not primary outcomes of the research, in order to give a fuller picture of participants (Table 13). Fourteen studies46,53,59,63–66,70,74,77–79,83,84 recorded BMI, and five45,46,53,57,73 classified participants as overweight based on a BMI > 25kg/m2. Four studies61,75,79,80 assessed participants’ smoking status; two43,59 classified participants as ‘generally healthy’; and others looked at clinical measures such as family history of disease,73,74 history of binge eating disorder,63,64,71 hypertension,73,79 weight and health risk based on fat intake. 78
| Health measure | Number of studies |
|---|---|
| BMI | 14 |
| Obese/overweight | 5 |
| Smoking | 4 |
| Healthy | 2 |
| Binge eating disorder | 1 |
| Family history of disease | 1 |
| Fat intake/risk group | 1 |
| Hypertensive | 1 |
| Weight | 1 |
Age
Table 14 summarises the distribution of mean ages of participants; nine studies did not report the ages of the participants, although most stated an age range in their eligibility criteria. Of those papers reporting age of participants, three trialled interventions in adolescents under the age of 18 years. 56,60,64 Of those targeting adults, the majority of studies had participants with a mean age of 40–49 years. Only one study looked at adults with a mean age > 60 years. 79
| Mean age of participants (years) | Number of studies |
|---|---|
| < 18 | 3 |
| 18–29 | 6 |
| 30–39 | 3 |
| 40–49 | 17 |
| 50–59 | 4 |
| > 60 | 1 |
| Not reported | 9 |
Gender
Studies were predominantly mixed gender, although most studies contained more women than men; 15 studies included more than 75% women, and of these four were 100% women. 42,47,49,53 Nine studies45,48,52,64,65,70,72,74,76 did not report the gender of participants.
Race/ethnicity
Race/ethnicity was reported in all but three42,66,81 of the 29 studies conducted in the USA, and in none of the 12 studies conducted in Europe. In the USA, most study populations were ethnically mixed, and racial mix tended to be a function of study location; participants were generally a mixture of Caucasian, African American and Hispanic, with Asian and Native American participants depending on geographic location.
Socioeconomic status and education
Education level was reported with a highly diverse range of measures. Of the 22 studies that reported the educational levels of participants, some reported the average number of years in education, some reported the highest educational qualification achieved and some reported the highest educational institution (e.g. university) attended. Most studies contained a mix of educational attainment ranging from postgraduate level to completion of primary-level education only. Educational level was partly a function of the setting of the intervention; some studies focused on higher-attaining participants (particularly those set in the workplace), whereas others focused on those with lower educational level (for instance those set in community centres and government support offices).
Socioeconomic status was also reported using a diverse range of indicators. Only 11 studies44,45,47,52,55,56,61,62,75,77,78 reported any measure of SES, and these included income brackets, classification as low/high income, employment status and proportion of income over the ‘poverty line’. Of the studies that reported measures of SES, only two targeted predominantly low-income groups: Di Noia et al. ,56 in which 87% of participants were defined as low income; and Block et al. ,47 in which 68% of participants were below the poverty line. Campbell et al. 49,50 also stated that the intervention was trialled with low-income participants (although no measure of SES was reported) as these interventions targeted women receiving government support.
Outcomes
To measure achievement of dietary objectives, 17 studies45,49–51,54,57,59,60,64,65,68,69,74,76,78,80,81 measured total fat intake and eight studies45,55,61,65,67,69,72,80 measured saturated fat intake (either as grams consumed or as percentage of total energy consumed). Nineteen studies43,44,47,48,50,51,55,56,58,59,62,67,68,71,72,75,80–82 assessed servings of fruit and/or vegetables consumed per day. Other food and nutrient outcomes measured included energy intake (eight studies45,53,57,65,74,77,80,83) and fibre intake (five studies44,45,55,59,82). In terms of clinical outcomes, 13 studies46,53,59,63–66,71,73,77,79,83,84 measured BMI (kg/m2), 10 studies42,45,52,53,69,71,73,74,76,83 measured weight or weight loss (Table 15), three studies77,79,83 assessed cholesterol, three studies77,79,83 assessed triglycerides and two studies79,83 assessed blood pressure. Four studies63,64,66,84 concentrated on the avoidance of eating disorders and used scales of eating disorder risk alongside measures of BMI and/or weight. See Table 16 for outcome measures used in each study.
| Outcome measure | Number of studiesa |
|---|---|
| Food and nutrient outcomes | |
| Fruit and/or vegetables (servings) | 19 |
| Total fat (g) | 17 |
| Energy (kcal; kJ; MJ) | 8 |
| Saturated fat (g;% fat intake) | 8 |
| Dietary fibre (g) | 5 |
| Other foods/food groupsb | 4 |
| Other nutrientsc | 4 |
| Clinical outcomes | |
| Weight/weight loss (kg, lbs) | 10 |
| BMI (kg/m2) | 13 |
| Serum cholesterol (mmol/l) | 3 |
| Serum triglycerides (mmol/l) | 3 |
| Eating disorder outcomesd | 4 |
| Blood pressure (mmHg) | 2 |
| Other clinical outcomese | 2 |
| Study | Outcomes measured |
|---|---|
| Agras (1990)42 | Weight |
| Alexander (2010)43 | Fruit and vegetables |
| Anderson (2001)44 | % energy from fat |
| Dietary fibre | |
| Fruit and vegetables | |
| Beasley (2008)45 | Energy |
| Total fat; saturated fat | |
| Dietary fibre | |
| Weight | |
| Waist circumference | |
| Blanson Henkemans (2009)46 | BMI |
| Block (2004)47 | Fruit and vegetables |
| Buller (2008)48 | Fruit and vegetables |
| Campbell (1999)49 | Total fat |
| Campbell (2004)50 | Total fat |
| Fruit and vegetables | |
| Carbone (1999)51 | Total fat |
| Fruit and vegetables | |
| Cook (2007)52 | Weight |
| Cussler (2008)53 | Weight |
| BMI | |
| % body fat | |
| Energy | |
| De Bourdeaudhuij (2007)54 | % energy from fat |
| Total fat | |
| Delichatsios (2001)55 | Dietary fibre |
| Saturated fat | |
| Fruit; vegetables | |
| Red/processed meat | |
| Whole-fat dairy | |
| Wholegrain cereals | |
| Di Noia (2008)56 | Fruit and vegetables |
| Ellrott (2005)57 | Energy |
| Total fat | |
| % energy from fat | |
| Franko (2008)58 | Fruit and vegetables |
| % energy from fat | |
| Gow (2010)59 | BMI |
| Total fat | |
| Dietary fibre | |
| Fruit and vegetables | |
| Haerens (2007)60 | Total fat |
| Huang (2006)61 | Saturated fat |
| Irvine (2004)62 | Fruit and vegetables |
| Jacobi (2007)63 | BMI |
| EDI; EDE-Q; SCL-90-R | |
| Jones (2008)64 | BMI |
| EDE-Q | |
| Total fat | |
| Kroeze (2008)65 | BMI |
| Saturated fat | |
| Energy | |
| Total fat | |
| Low (2006)66 | BMI |
| EDI-DT; EDI-B; EDI-BD | |
| Oenema (2001)67 | Saturated fat |
| Fruit; vegetables | |
| Oenema (2005)68 | Total fat |
| Fruit; vegetables | |
| Oenema (2008)69 | Saturated fat; self-rated fat intake |
| Weight | |
| Rothert (2006)70 | % age of baseline weight loss |
| Shapiro (2007)71 | Fruit and vegetables |
| Fast foods | |
| BMI | |
| Weight | |
| Sternfeld (2009)72 | Fruit and vegetables |
| Saturated fat; trans fats | |
| Added sugars | |
| Svetkey (2008)73 | Weight |
| BMI | |
| Tate (2006)74 | Weight |
| Energy | |
| Total fat | |
| Trinh (2009)75 | Fruit |
| Vegetables | |
| Turnin (1992)76 | Total fat |
| Carbohydrate | |
| Caloric excess | |
| Protein | |
| Weight | |
| HbA1c | |
| Fructosamine | |
| Turnin (2001)77 | BMI |
| Total cholesterol; HDL; HDL2 | |
| Triglycerides; LDL cholesterol | |
| Energy | |
| Carbohydrate | |
| % energy from fat | |
| Protein | |
| Insulin | |
| Saccharose | |
| Vandelanotte (2005)78 | Total fat |
| % energy from fat | |
| Verheijden (2004)79 | BMI |
| Systolic BP; diastolic BP | |
| Total cholesterol; HDL; LDL | |
| Triglycerides | |
| Veverka (2003)80 | Energy |
| Total fat; MUFA; PUFA; saturated fat | |
| % energy from fat | |
| Fruit and vegetables | |
| Dietary fibre | |
| Winett (1991)81 | % energy from fat |
| Fruit and vegetables | |
| Wholegrain cereals | |
| Total fat | |
| Winett (2007)82 | % energy from fat |
| Fruit and vegetables | |
| Fibre | |
| Wylie-Rosett (2001)83 | Energy |
| % energy from fat | |
| Weight | |
| BMI | |
| Glucose | |
| Systolic BP; diastolic BP | |
| Total cholesterol; HDL; LDL | |
| Triglycerides | |
| Zabinski (2001)84 | BMI |
| EDE-Q; EDI-DT; EDI-B |
Usability
Almost two-thirds of included studies reported data on at least one aspect of process (accessibility, compliance, usability and acceptability). Table 17 presents the process measures reported in included studies. Accessibility was assessed in one study42 by collecting data on computer literacy. In the same study it was reported that prior to the intervention, 69% and 46% of the participants had access to a computer at work and home, respectively.
| Study | Accessibility | Compliance | Usability | Acceptability |
|---|---|---|---|---|
| Agras (1990)42 | ✓ | ✓ | ✓ | ✓ |
| Alexander (2010)43 | ✓ | ✓ | ||
| Anderson (2001)44 | ✓ | |||
| Beasley (2008)45 | ✓ | |||
| Blanson Henkemans (2009)46 | ✓ | ✓ | ||
| Block (2004)47 | ||||
| Buller (2008)48 | ✓ | |||
| Campbell (1999)49 | ✓ | |||
| Campbell (2004)50 | ✓ | |||
| Carbone (1999)51 | ||||
| Cook (2007)52 | ✓ | ✓ | ||
| Cussler (2008)53 | ✓ | |||
| De Bourdeaudhuij (2007)54 | ✓ | ✓ | ||
| Delichatsios (2001)55 | ✓ | |||
| Di Noia (2008)56 | ||||
| Ellrott (2005)57 | ✓ | |||
| Franko (2008)58 | ✓ | |||
| Gow (2010)59 | ✓ | |||
| Haerens (2007)60 | ✓ | ✓ | ||
| Huang (2006)61 | ||||
| Irvine (2004)62 | ✓ | |||
| Jacobi (2007)63 | ✓ | ✓ | ||
| Jones (2008)64 | ✓ | |||
| Kroeze (2008)65 | ✓ | |||
| Low (2006)66 | ✓ | |||
| Oenema (2001)67 | ||||
| Oenema (2005)68 | ✓ | ✓ | ✓ | |
| Oenema (2008)69 | ✓ | |||
| Rothert (2006)70 | ✓ | ✓ | ||
| Shapiro (2007)71 | ✓ | ✓ | ||
| Sternfeld (2009)72 | ✓ | |||
| Svetkey (2008)73 | ✓ | |||
| Tate (2006)74 | ✓ | |||
| Trinh (2009)75 | ||||
| Turnin (1992)76 | ✓ | |||
| Turnin (2001)77 | ✓ | ✓ | ||
| Vandelanotte (2005)78 | ✓ | ✓ | ||
| Verheijden (2004)79 | ✓ | |||
| Veverka (2003)80 | ✓ | |||
| Winett (1991)81 | ||||
| Winett (2007)82 | ✓ | |||
| Wylie-Rosett (2001)83 | ||||
| Zabinski (2001)84 | ✓ |
More than half of the included studies collected data on compliance of the participants to the intervention. Compliance to an intervention was assessed by monitoring the frequency of computer/website/system use/page visited in 19 studies. 42–44,48,53,55,59,62–64,66,69,74,76,77,79,80,82,84 One study45 used 24-hour dietary recall, two studies46,74 used a diary and another two studies52,68 used self-reported questions to capture compliance to an intervention. Four studies43,48,52,55 found a positive association between compliance with an intervention and increase in fruit and vegetable servings, and one study74 found a significant association between treatment compliance and weight loss.
Eleven studies42,46,52,54,60,63,65,68,70,77,78 assessed the usability of the intervention program using various methods, such as questionnaires in which the participants were required to give their responses using a five- or seven- or 10-point Likert scale. One common item assessed was whether or not the intervention material was easy to use. 46,52,65,68 Two studies46,52 achieved better ratings for this item than the control group, whereas in the other two studies65,68 this item was rated higher in the control group. In three studies,54,60,78 usability was evaluated in an earlier formative evaluation study85 which showed that the computer-tailored intervention was usable for reducing fat intake in a general population of adults in the Belgian Flanders. The usability was assessed on a 6-point scale ranging from 1 (very good) to 6 (very poor) in Jacobi et al. ,63 and the internet-based program was rated as good, having achieved a mean rating of 2. Most of the participants in the tailored expert system in the Rothert et al. study70 reported reading the web-based materials more often than the control group. The intervention group also found the materials more helpful, easy to understand and personally relevant. More than 60% of the participants in the Nutri-Expert group in the study by Turnin et al. 77 found the telematics system easy to use.
Acceptability of interventions was assessed in 11 studies. 42,43,49,50,52,54,60,68,70,71,78 Most studies that collected data on program acceptability reported that participants found the program to be very good and that they would recommend the program to others. 42,43,49,50,70 In Cook et al. ,52 the participants in the web group found the program materials more informative and helpful than the print group. In three studies,54,60,78 acceptability was evaluated in an earlier formative evaluation study85 that showed that the computer-tailored intervention was acceptable for reducing fat intake.
One study68 achieved positive ratings for all aspects of the process measures except for novelty on specific information on fat, fruit and vegetables. The same study achieved higher ratings than the control group in all aspects except for credibility. In one study,71 treatment acceptability was based on attrition rate, treatment choice (group vs CD-ROM) of individuals originally assigned to the waiting list condition, and treatment modality use (CD-ROM) after the program completion. Data on all three aspects favoured the CD-ROM, thus achieving good acceptability.
Overview of excluded studies
Table 28 in Appendix 6 lists the studies that were deemed potentially eligible through screening of study titles and abstracts, but which were subsequently found to fail one or more of the inclusion criteria for the systematic review. Of the 233 excluded studies, 81 were excluded because they were found not to be an RCT of an intervention (of which 11 may have been RCTs, but were earlier versions of reports published more fully elsewhere), five because participants were children, 126 because the intervention did not meet our criteria of ‘adaptive e-learning’, where program output/feedback was generated automatically based on user input (of which 33 may have involved adaptive e-learning, but also involved significant contact with a qualified therapist, rendering it impossible to determine the contribution of the e-learning component to any dietary or clinical change) and 21 because they did not assess any dietary or clinical outcomes relevant to this review. Two papers could not be obtained.
Methodological quality of included studies
Two methods to assess the methodological quality of studies were used in this review, the Cochrane Collaboration’s tool for assessing risk of bias35 and the EPHPP tool. 36
Risk of bias in included studies
Sequence generation
One trial49 was judged to be at high risk of bias for this domain. Of the 43 studies, 2742,43,45–48,50,52,59,61–67,69–74,78,79,81–83 used an adequate method of sequence generation (table of random numbers and computerised randomisation). The remaining 1544,51,53–58,60,68,75–77,80,84 were rated as unclear owing to no information being presented in the report or being available from the study author(s). Table 18 summarises the methods used to generate allocation sequences.
| Sequence generation | Number of studies |
|---|---|
| Not specified | 15 |
| Simple randomisation, number generator | 13 |
| Stratified randomisation | 5 |
| Simple randomisation, number table | 3 |
| Random permuted blocks | 2 |
| Drawing lots | 2 |
| Minimisation | 1 |
| By day registered | 1 |
Allocation concealment
Allocation concealment was adequate in 1643,46,48,50,52,59,61,65–67,69,71–74,78 of the 43 included studies. It was unclear in 24 studies,42,44,47,51,53–58,60,62–64,68,70,75–77,79–82,84 and inadequate in three studies. 45,49,78 Two studies45,78 used an open list to allocate the participants, whereas in the other study49 the participants were allocated according to the day on which they were entered in the trial. Thirteen studies43,46,48,50,52,59,61,65,69,72–74,83 achieved adequate allocation concealment as the allocation of the randomised sequence was determined by a computer. Three studies66,67,71 used a closed list to allocate the participants. The information on who generated the allocation concealment was specified in 15 studies;43,46,49,50,52,54,66,67,69,71–74,78,83 the information on who enrolled participants was known in 19 studies;43,45,48–50,54,58,62,64–69,71,72,74,78,81 and the information on who assigned participants to treatment groups was described in 23 studies. 43,45,46,48–50,52,54,59,62,64–67,69,71–74,78,79,81,83 Table 19 summarises the methods used to generate allocation, and to enrol and allocate participants.
| Implementation of allocation concealment | Methods | Number of studies |
|---|---|---|
| Allocation generation | Not specified | 28 |
| Computer | 7 | |
| Project team | 6 | |
| Independent researcher | 2 | |
| Enrolment | Not specified | 24 |
| Project team | 16 | |
| Independent researcher | 2 | |
| Self (via website) | 1 | |
| Assignment | Not specified | 18 |
| Self (via website)/computer | 9 | |
| Project team | 12 | |
| Independent researcher | 4 |
Blinding
Of the 43 studies, 1546,49,50,54,61,65–67,69–73,79,81 reported adequate blinding of outcome assessment although there was a high risk of bias because of inadequate blinding of outcome assessment in seven studies. 43,45,48,52,62,64,74 Methods of blinding were unclear in the remaining 21 studies. 42,44,47,51,53,55–60,63,68,75–78,80,82–84
Incomplete outcome data
Twenty-one studies42,45,48–50,52,53,55,56,59,61,63,64,67,69–73,75,78 were judged to be at a low risk of bias for this domain: there were no missing data in one trial,75 whereas the others used appropriate methods for imputing missing data. Four studies46,47,62,74 were judged as having a high risk of bias. There was insufficient information available to judge the risk of bias for the remaining 18 studies,43,44,51,54,57,58,60,65,66,68,76,77,79–84 which have been classified as unclear for this domain.
Selective outcome reporting
The trial protocols were available for two studies. 61,73 In both studies the final report contained data for all the pre-specified outcomes. Nine more studies44,53–55,60,70–72,80,84 were judged to be free from selective outcome reporting bias. In one study47 there was potential for risk of selective outcome reporting bias. It was unclear in the remaining 31 studies42,43,45,46,48–52,56–58,60,62–69,74–79,81–84 whether or not there was selective reporting of outcomes.
Conflict of interest
Authors of 2644,45,51–53,58,59,61,64,65,67–82 of the 43 studies provided a conflict of interest statement.
Figure 3 presents the Cochrane Collaboration summary figure for risk of bias across all included studies (the review authors’ judgement about the six bias items for each study is presented as overall percentages). Table 20 presents the judgements about risk of bias for each study.
FIGURE 3.
Cochrane Collaboration’s risk of bias summary. Figure produced using Cochrane Collaboration’s RevMan 5 software.
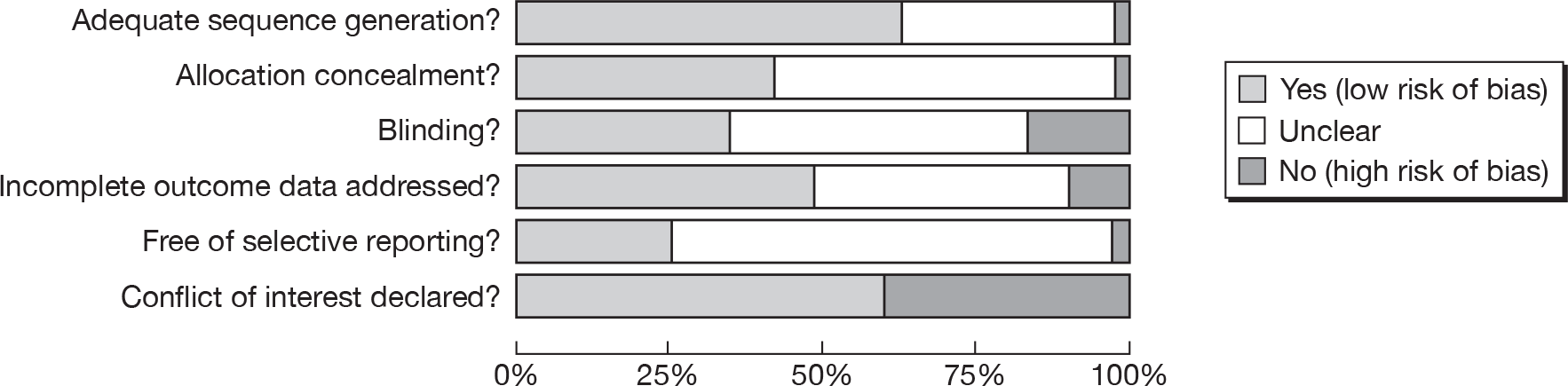
| Study | Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting bias | Conflict of interest statement |
|---|---|---|---|---|---|---|
| Agras (1990)42 | L | U | U | L | U | N |
| Alexander (2010)43 | L | L | H | U | U | N |
| Anderson (2001)44 | U | U | U | U | L | Y |
| Beasley (2008)45 | L | H | H | L | U | Y |
| Blanson Henkemans (2009)46 | L | L | L | H | U | N |
| Block (2004)47 | L | U | U | H | H | N |
| Buller (2008)48 | L | L | H | L | U | N |
| Campbell (1999)49 | H | H | L | L | U | N |
| Campbell (2004)50 | L | L | L | L | U | N |
| Carbone (1999)51 | U | U | U | U | U | Y |
| Cook (2007)52 | L | L | H | L | U | Y |
| Cussler (2008)53 | U | U | U | L | L | Y |
| De Bourdeaudhuij (2007)54 | U | U | L | U | L | N |
| Delichatsios (2001)55 | U | U | U | L | L | N |
| Di Noia (2008)56 | U | U | U | L | U | N |
| Ellrott (2005)57 | U | U | U | U | U | N |
| Franko (2008)58 | U | U | U | U | U | Y |
| Gow (2010)59 | L | L | U | L | L | Y |
| Haerens (2007)60 | U | U | U | U | U | N |
| Huang (2006)61 | L | L | L | L | L | Y |
| Irvine (2004)62 | L | U | H | H | U | N |
| Jacobi (2007)63 | L | U | U | L | U | N |
| Jones (2008)64 | L | U | H | L | U | Y |
| Kroeze (2008)65 | L | L | L | U | U | Y |
| Low (2006)66 | L | L | L | U | U | N |
| Oenema (2001)67 | L | L | L | L | U | Y |
| Oenema (2005)68 | U | U | U | U | U | Y |
| Oenema (2008)69 | L | L | L | L | U | Y |
| Rothert (2006)70 | L | U | L | L | L | Y |
| Shapiro (2007)71 | L | L | L | L | L | Y |
| Sternfeld (2009)72 | L | L | L | L | L | Y |
| Svetkey (2008)73 | L | L | L | L | L | Y |
| Tate (2006)74 | L | L | H | H | U | Y |
| Trinh (2009)75 | U | U | U | L | U | Y |
| Turnin (1992)76 | U | U | U | U | U | Y |
| Turnin (2001)77 | U | U | U | U | U | Y |
| Vandelanotte (2005)78 | L | H | U | L | U | Y |
| Verheijden (2004)79 | L | U | L | U | U | Y |
| Veverka (2003)80 | U | U | U | U | L | Y |
| Winett (1991)81 | L | U | L | U | U | Y |
| Winett (2007)82 | L | U | U | U | U | Y |
| Wylie-Rosett (2001)83 | L | L | U | U | U | N |
| Zabinski (2001)84 | U | U | U | U | U | N |
EPHPP assessment
The EPHPP tool consists of six criteria. Each criterion is rated as ‘strong’, ‘moderate’ or ‘weak’ depending on characteristics on each criterion reported in the study. From this rating, the overall assessment of strong, moderate or weak for each study is derived. A study that achieved at least four ‘strong’ ratings in each of the individual criteria with no ‘weak’ ratings would have received a ‘strong’ overall global rating. If fewer than four ‘strong’ ratings were achieved in the individual criteria and one ‘weak’ rating, then an overall rating of ‘moderate’ was achieved. If two or more criteria were rated ‘weak’ then an overall rating of ‘weak’ was given. Table 21 summarises the number of studies achieving the rating in each criterion (component rating) and Table 22 summarises the overall assessment of quality of the included studies.
| Component | Ratings | Studies |
|---|---|---|
| Selection bias | Strong | 1 |
| Moderate | 0 | |
| Weak | 42 | |
| Study design | Strong | 43 |
| Moderate | 0 | |
| Weak | 0 | |
| Confounders | Strong | 36 |
| Moderate | 0 | |
| Weak | 7 | |
| Blinding | Strong | 5 |
| Moderate | 14 | |
| Weak | 24 | |
| Data collection methods | Strong | 21 |
| Moderate | 11 | |
| Weak | 11 | |
| Attrition | Strong | 24 |
| Moderate | 12 | |
| Weak | 7 |
| Study | Selection | Allocation | Confounders | Blinding | Data collection | Attrition | Global rating |
|---|---|---|---|---|---|---|---|
| Agras (1990)42 | W | S | S | W | S | S | W |
| Alexander (2010)43 | W | S | S | M | M | M | M |
| Anderson (2001)44 | W | S | S | W | W | W | W |
| Beasley (2008)45 | W | S | W | W | M | S | W |
| Blanson Henkemans (2009)46 | W | S | W | S | W | W | W |
| Block (2004)47 | W | S | S | W | S | S | W |
| Buller (2008)48 | W | S | S | W | M | M | W |
| Campbell (1999)49 | W | S | W | S | M | M | W |
| Campbell (2004)50 | W | S | S | S | M | M | M |
| Carbone (1999)51 | W | S | S | W | W | W | W |
| Cook (2007)52 | W | S | S | W | W | S | W |
| Cussler (2008)53 | W | S | S | W | S | S | W |
| De Bourdeaudhuij (2007)54 | W | S | S | S | M | M | M |
| Delichatsios (2001)55 | W | S | S | W | M | W | W |
| Di Noia (2008)56 | W | S | S | W | M | S | W |
| Ellrott (2005)57 | W | S | S | W | W | W | W |
| Franko (2008)58 | W | S | S | W | S | S | W |
| Gow (2010)59 | W | S | S | W | M | M | W |
| Haerens (2007)60 | S | S | S | W | S | S | M |
| Huang (2006)61 | W | S | S | M | W | S | W |
| Irvine (2004)62 | W | S | S | W | M | S | W |
| Jacobi (2007)63 | W | S | S | W | M | S | W |
| Jones (2008)64 | W | S | S | W | S | M | W |
| Kroeze (2008)65 | W | S | S | S | S | S | M |
| Low (2006)66 | W | S | S | M | S | S | M |
| Oenema (2001)67 | W | S | S | W | S | S | W |
| Oenema (2005)68 | W | S | W | W | S | M | W |
| Oenema (2008)69 | W | S | S | M | S | M | M |
| Rothert (2006)70 | W | S | S | M | W | W | W |
| Shapiro (2007)71 | W | S | S | M | S | M | M |
| Sternfeld (2009)72 | W | S | S | M | S | W | W |
| Svetkey (2008)73 | W | S | S | M | S | S | M |
| Tate (2006)74 | W | S | S | M | S | S | M |
| Trinh (2009)75 | W | S | W | W | W | S | W |
| Turnin (1992)76 | W | S | W | W | W | S | W |
| Turnin (2001)77 | W | S | S | M | W | M | W |
| Vandelanotte (2005)78 | W | S | S | W | S | M | W |
| Verheijden (2004)79 | W | S | S | M | S | S | M |
| Veverka (2003)80 | W | S | S | M | S | S | M |
| Winett (1991)81 | W | S | S | M | W | S | W |
| Winett (2007)82 | W | S | S | W | S | S | W |
| Wylie-Rosett (2001)83 | W | S | W | M | S | S | M |
| Zabinski (2001)84 | W | S | S | W | S | S | W |
Selection bias
This part of the tool aims to assess the generalisability of study results by considering the likelihood that the selected participants are representative of the target population and participation rate.
Of the 43 studies, two60,64 were judged ‘very likely’, three69,72,79 as ‘somewhat likely’, and 3642–50,52–59,61–63,65–68,70,71,73–75,77,78,80–84 as ‘not likely’ to be representative of the target population. In nine studies,44,45,48,49,53,55,59,60,78 > 80% of the eligible participants agreed to participate in the trial, whereas in six studies,43,46,58,63,81,82 60–79% of the eligible participants took part. The participation rate was < 60% in 12 studies52,54,61,64,65,68,69,72,75,77,79,83 and it was not possible to ascertain the participation rate in 14 studies. 42,47,50,51,56,57,62,66,67,70,71,74,76,84
Study design
Of the 43 studies, one62 was a crossover trial and two79,82 were cluster randomised trials. The remainder were parallel-group RCTs. 42–61,63–78,80,81,83,84 The methods used to randomly allocate participants were described in 25 studies. 42,43,45–50,52,59,61–67,69,71–74,78,79,83
Confounders
Of the 43 studies, 3442–44,47,48,51–62,64–66,68–74,77–82,84 explicitly reported that the intervention and the comparator groups were balanced at baseline. Seven studies had differences between treatment groups at baseline (differences in stages of change and/or fat intake,49,83 race and ethnicity,50,75 eating disorder scales,63 awareness and intention to change fat intake68 and mean BMI45). It was not clear in two studies46,76 whether or not there were any differences at baseline. For the studies with differences at baseline, 80–100% of the confounders were controlled for in three studies,44,50,63 whereas < 60% of confounders were controlled for in two studies. 45,49 Five studies46,68,75,76,83 did not report how confounders were included in their analysis, or what proportion of confounders were addressed.
Blinding
Of the 43 studies, the outcome assessors were blind to participants’ treatment allocation in 11 studies. 43,46,49,50,54,65,72–74,79,81 It was unclear whether or not outcome assessors were aware of treatment allocation in 22 studies,42,44,47,51,53,55–60,63,67–69,75–78,82–84 although in the remaining 11 studies45,48,52,61,62,64,66,69–71,80 they were not blind to allocation. Blinding of participants to treatment allocation was achieved in eight studies. 46,49,50,54,61,65,70,83 It was unclear whether or not participants were aware of their intervention allocation in 24 studies,42–44,47,51,53,55–60,63,66–69,71,75,76,78,80,82,84 whereas in the remaining 11 studies45,48,52,62,64,72–74,77,79,81 the participants were not blind to allocation.
Withdrawals and dropouts
Attrition was reported in all 43 studies. In 24 studies,42,45,47,52,53,56,58,60–63,65–67,73–76,79–84 > 80% of participants completed the final data collection, and in 12 studies43,48–50,54,59,64,68,69,71,77,78 60–79% of participants completed the final data collection. Less than 60% of participants completed final follow-up in six studies. 44,46,55,57,70,72
Dietary assessment techniques
In the studies reviewed, dietary outcome variables were assessed in 39,42–62,64,65,67–69,71–83 using FFQs (n = 27; individual food items or food groups),43,44,48–51,54–56,58–60,62,64,65,67–69,72,74,78,80,82,83 24-hour recalls (n = 2),45,47 multiple-day food records (n = 6),45,46,53,57,76,77 questionnaires (n = 11)42,48,49,51,52,56,58,59,62,71,75 and shopping receipts/online food purchases (n = 4). 44,61,81,82 Eleven studies used two dietary assessment methods, combining a FFQ with a food pattern or screening FFQ (n = 2),43,55 a FFQ with one or two food habit/food pattern questions (n = 6),48,49,51,58,59,62 a FFQ with shopping receipts (n = 2)44,82 or a 24-hour recall with food records (n = 1). 45 Three studies63,66,84 used an Eating Disorder Inventory, which does not quantify energy, nutrient or food intakes. One study70 did not assess dietary outcomes. One study79 did not report its dietary outcomes (FFQ) because of concerns about data accuracy.
The gold standard dietary assessment technique is a prospective multiple-day diet record. 86 However, for evaluating intervention-related dietary change, the multiple-day 24-hour recall is recommended for populations for whom memory error is not a barrier, because prospectively recording food intakes (i.e. diet records) heightens one’s awareness of dietary practices and may result in compliance bias (i.e. an overestimation of the extent of dietary change). 87,88 Diet records can also lead to a simplification of dietary practices, because of the high respondent burden involved in recording food intakes. 86 The multiple-day 24-hour recall also has advantages over other dietary assessment methods: it is likely to be more responsive to dietary change than either the FFQ or food habit/food pattern questionnaires; and is likely to be more accurate than shopping receipts, because they do not account for household food distribution, food wastage or food gifts/purchases without receipts, which may differ pre and post intervention.
Although the multiple-day 24-hour recall is recommended for assessing intervention-related dietary change, it was not used in the studies reviewed. Two studies, however, used a 1-day 24-hour recall. 45,47 Of these two studies, only one used a 1-day 5-step pass 24-hour recall to reduce memory errors. 45 The primary outcomes of these two studies were intergroup comparisons of dietary intakes. For these outcomes, a 1-day 24-hour recall is adequate, because estimates of usual individual dietary intakes are not required. 86
In contrast to the studies using a 24-hour recall, six studies used diet records that assessed dietary intakes on more than 1 day. 45,46,53,57,76,77 The number of days of assessment ranged from 3 to 6, which is adequate for evaluating the outcomes of interest, i.e. intergroup differences in dietary outcomes. Further, to reduce compliance bias, one study used both a diet record and a 1-day 5-stage multiple-pass 24-hour recall. 45
The FFQ was the most common method used to assess dietary outcomes in the studies reviewed. Its accuracy depends on instrument characteristics such as the number and types of foods in the FFQ, the methods used to estimate serving sizes, the method of administration, the time required to complete the FFQ and methods of analysis. Characteristics of the target population are also important because the instrument is conceptually more abstract than other dietary assessment methods, i.e. the respondent must estimate ‘usual intakes’. In most of the studies reviewed, insufficient detail was provided to fully evaluate the FFQs. In particular, there were gaps in information regarding the average time it took to complete the FFQ (one reported it),55 the exact method of quantifying serving sizes (five reported it)43,49–51,78 and the time frame of the dietary assessment period (six reported it). 43,48,55,58,62,64 Most FFQs were self-administered (n = 19),43,44,54,55,58–60,62,64,65,67–69,72,74,78,80,82,83 including one study of adolescents64 despite its conceptual complexity for young people. 86 The number of items included in the FFQ ranged from 16 to 131. Nine studies did not report the number of items in their FFQ. 44,48,72–74,79,80,82,83 Eighteen FFQs had a limited number of items to reduce respondent burden (i.e. ranged from 16 to 35 foods/food groups), of which 12 were screening FFQs,43,48–51,55,58,59,64,67–69 three were focused solely on fat intakes54,60,78 and two were food group FFQs. 62,65 A screening FFQ will probably underestimate dietary change, especially if key food sources of target nutrients cannot be reported. 89 However, a social desirability bias may also occur with a screening FFQ,87 so the direction of bias is unpredictable.
A full-length FFQ of ≥ 90 items was used in three studies. 44,55,82 Three other studies did not provide sufficient detail to determine whether or not they had used a full-length or shortened FFQ. 72–74 A full-length FFQ is more likely to be responsive to dietary change than a screening or shortened FFQ, especially if it has been validated for the population and nutrients of interest. Full-length FFQs, however, tend to overestimate food consumption, especially when there are many food items within a food group. 86 To reduce this bias, seven studies combined a FFQ with a shortened food group FFQ or food patterns questions, adjusting the full-length FFQ estimates to achieve concurrence. 43,48,49,51,55,58,59
Sixteen studies reported using a validated FFQ. 43,48,49,54,55,58–60,62,64,65,67–69,72,78 However, in some studies, the FFQ was not validated for populations that were similar to their study population (n = 5),49,55,58–60 or for the dietary outcome variables of interest (n = 3). 43,48,49 These validation studies also did not assess the instrument’s accuracy for measuring dietary change.
Eleven studies used one or two questions to quantify the consumption of foods from one or two selected food groups (e.g. fruit and vegetables) within a more extensive questionnaire either alone (n = 5)42,52,56,71,75 or in combination with another dietary assessment technique (n = 6). 48,49,51,58,59,62 A recent validation study showed that, unlike a screening FFQ, a one-item question did not overestimate an intervention effect,87 presumably because less emphasis was placed on the desired dietary change in the questionnaire. The extent to which these results can be generalised to other studies is not known. One would expect that social desirability and compliance biases would limit the validity of a simple one- to two-item food group question for measuring intervention-related dietary change. A further limitation of these brief questions is that they do not describe intervention-related impacts on overall dietary patterns.
In conclusion, accurate measurements of dietary intakes are notoriously difficult to obtain. 86 Among the studies reviewed, those using a 1-day 24-hour recall (n = 2)45,47 or a combination of two or more dietary assessment methods (n = 11),43–45,48,49,51,55,58,59,62,82 especially two independent dietary assessment methods (n = 2),44,82 had the strongest dietary assessment methods for measuring dietary change. Those using a diet record alone (n = 5)46,53,57,76,77 would also be responsive to dietary change, as long as a compliance bias did not result in an overestimation of the intervention effect and dietary practices were not simplified to reduce respondent burden. The studies with the weakest dietary assessment methods were those using shopping receipts alone (n = 2),61,81 a screening FFQ alone (n = 3)50,64,83 or a one- or two-item food group question alone (n = 5). 42,52,56,71,75 Most studies did not adequately describe their dietary assessment techniques, and seven FFQs were not validated for the target population or nutrient of interest. 43,48,49,55,58–60
Chapter 4 Results of the systematic review of effectiveness
This chapter reports the results of meta-analyses and subgroup analyses undertaken to assess whether e-learning interventions were effective for improving dietary and clinical outcomes. Appendix 7 contains the results of further statistical analyses.
Dietary outcomes
Fruit and vegetable intake
Nineteen studies43,44,47,48,50,51,55,56,58,59,62,67,68,71,72,75,80–82 reported intake of fruit, vegetables, or fruit and vegetables combined as ‘servings per day’, of which 12 provided adequate data for meta-analysis (Figure 4). 43,44,48,50,51,56,58,59,62,71,72,80 There was substantial heterogeneity in the trial results (p < 0.001 and I2 = 83%). There was also a strong suggestion of publication bias (smaller studies were associated with larger effects on servings of fruit and vegetables per day; Egger’s test, p = 0.008). If the studies reporting fruit and vegetable servings per day are pooled in a random effects meta-analysis this would show a WMD of 0.24 servings (95% CI 0.04 to 0.44 servings; p = 0.019); about a quarter of a serving more was therefore reported in the intervention groups than in the control groups combined.
FIGURE 4.
Forest plot showing the effect of e-learning on the mean intake of fruit and vegetables (servings per day).
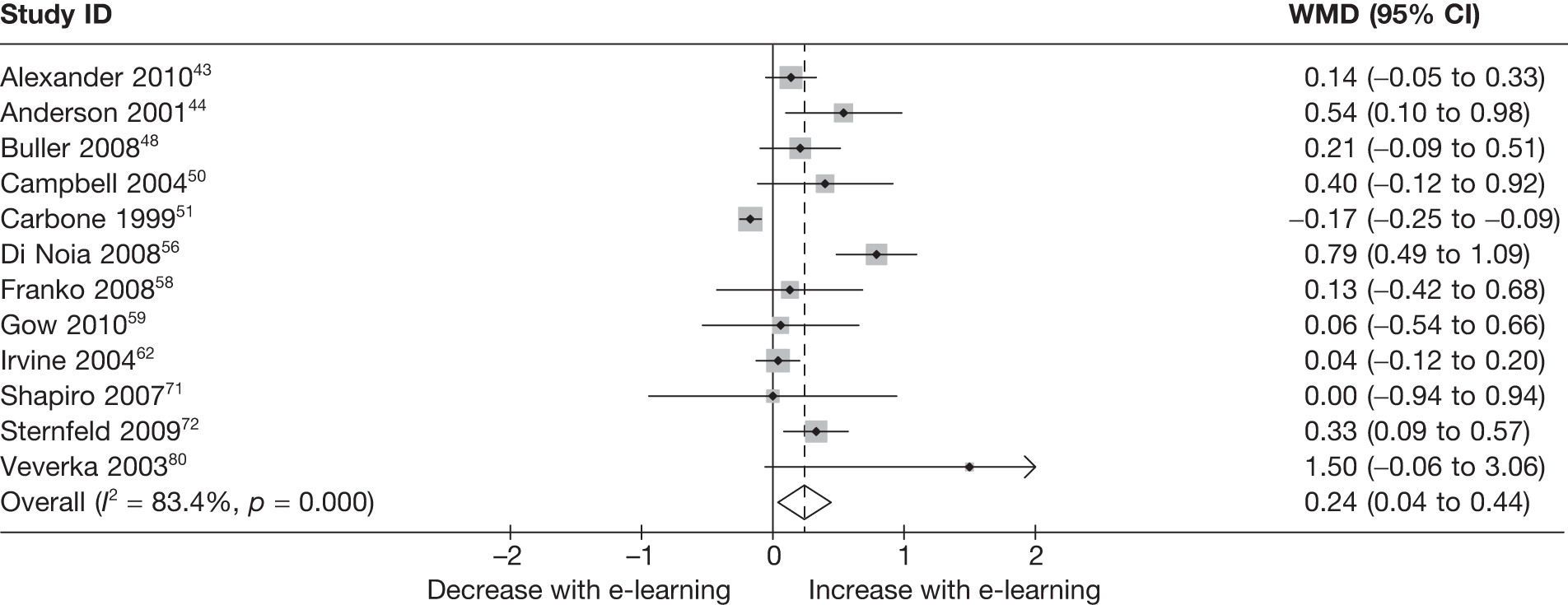
There was substantial heterogeneity in the results of studies which reported fruit intake alone (p = 0.09 and I2 = 65%), but not in those reporting vegetable intake alone (p = 0.2 and I2 = 38%). A slightly smaller effect (but not statistically significant) was estimated in the studies that reported fruit intake alone (WMD 0.10 servings; 95% CI –0.27 servings to 0.47 servings; p = 0.60), whereas a similar size of effect was shown in studies reporting vegetable intake alone (WMD 0.29 servings; 95% CI 0.07 servings to 0.51 servings; p = 0.011).
Subgroups
There was no evidence to suggest that estimates of effect on servings of fruit and vegetables were associated with study quality (EPHPP global rating ‘weak’ vs ‘moderate’, p = 0.82; level of attrition in studies ≤ 20% vs > 20%, p = 0.61; allocation adequately concealed from investigators ‘yes’ vs ‘unclear’, p = 0.80). There was some evidence that estimates of effect on daily servings of fruit and vegetables were larger in studies with participants from low-income groups (WMD 0.5 servings; 95% CI –0.01 to 1.00 servings; p = 0.053). None of the studies measuring average daily servings of fruit and vegetables included participants with a diagnosed illness.
There was no evidence that estimates of effect differed according to whether or not follow-up was earlier (within 3 months) or later (p = 0.56). Effects were not associated with whether or not the included participants were overweight (p = 0.67), whether or not the studies aimed to maintain or reduce BMI (p = 0.67) or whether or not interventions included a physical activity component (p = 0.60).
Fat intake
Seventeen studies45,49–51,54,57,59,60,64,65,68,69,74,76,78,80,81 looked at total fat, of which 12 provided sufficient data for meta-analysis (Figure 5),49–51,54,57,59,60,64,65,68,78,80 and eight45,55,61,65,67,69,72,80 at saturated fat intake, of which five provided sufficient data (Figure 6). 65,67,69,72,80 Fat intake was reported either as g/day or as a percentage of total energy consumed.
FIGURE 5.
Forest plot showing the effect of e-learning on the mean intake of fat (g/day).
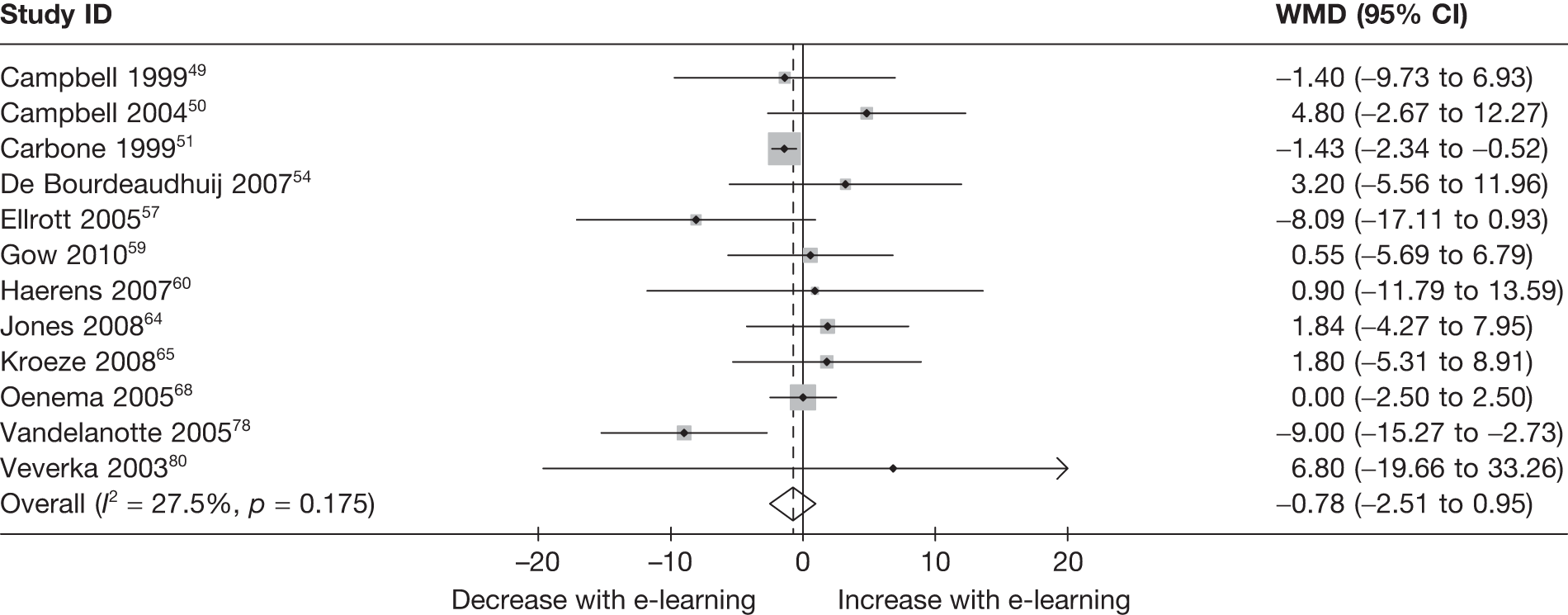
FIGURE 6.
Forest plot showing the effect of e-learning on the mean intake of saturated fat (g/day).
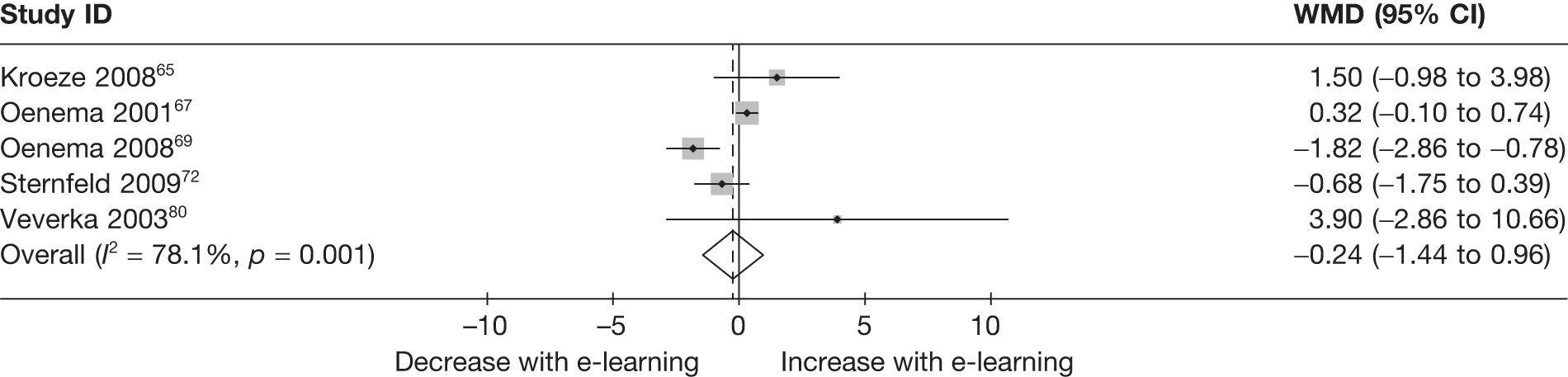
There was relatively little evidence for heterogeneity in the estimates of effect of e-learning on average grams of fat per day (p = 0.18 and I2 = 28%). There was no evidence that smaller studies were associated with a larger or smaller treatment effect (Egger’s test, p = 0.495). Random effects meta-analysis of all studies that reported grams of total fat consumed showed a WMD of –0.78 g (95% CI –2.5 to 0.95 g), which was not statistically significant (p = 0.38).
There was substantial heterogeneity in the estimates of effect on average saturated fat per day (p = 0.001 and I2 = 78%). There was no evidence that smaller studies were associated with a larger or smaller effect (p = 0.84). Studies reporting saturated fat intake in grams found no evidence for effect (WMD –0.24 g, 95% CI –1.44 to 0.96 g; p = 0.7).
Ten studies looked at percentage of total energy consumed from fat (Figure 7). 44,54,57,58,65,74,76–78,80 There was substantial heterogeneity in the results (p < 0.001 and I2 = 77%). There was no evidence that smaller studies were associated with larger or smaller treatment effects (Egger’s test, p = 0.203). Studies reporting percentage of total energy consumed from fat showed a WMD of –1.4% (95% CI –2.5% to –0.3%; p = 0.012).
FIGURE 7.
Forest plot showing the effect of e-learning on the mean percentage of energy from fat (%).
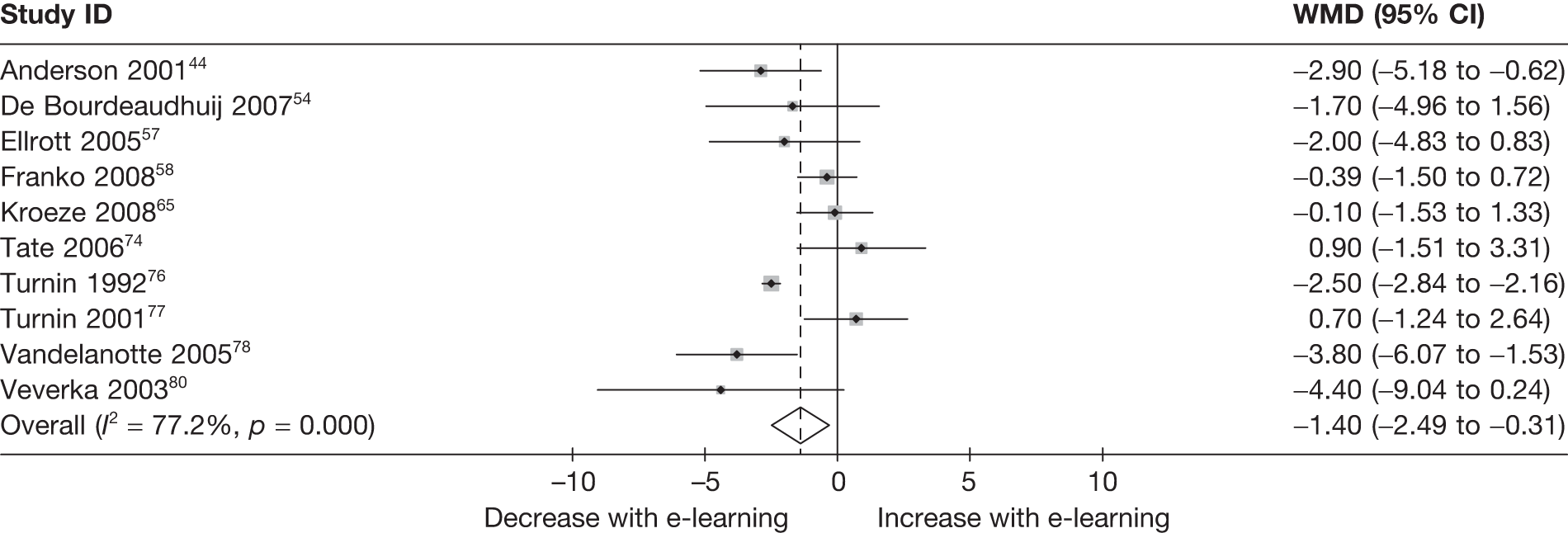
Subgroups
There was no good evidence to suggest that the estimates of effect on fat intake were associated with study quality (EPHPP global rating ‘weak’ vs ‘moderate’, p = 0.08; level of attrition in studies ≤ 20% vs > 20%, p = 0.50; allocation adequately concealed from investigators ‘yes’ vs ‘unclear’, p = 0.87). The results were similar for the estimates of effect on the percentage of fat intake (p = 0.39, p = 0.49 and p = 0.60, respectively).
There was no evidence that the estimates were associated with whether or not studies included participants from low-income groups (p = 0.39). None of the studies measuring percentage energy from fat included participants predominantly from low-income groups. There was no evidence that estimates of the effect on percentage of energy from fat differed according to whether or not participants had a diagnosed illness (p = 0.37). None of the studies measuring total fat intake included participants with a diagnosed illness.
There was no evidence that the estimates of effect differed according to whether or not follow-up was earlier (within 3 months) or later (average fat intake, p = 0.91; percentage energy from fat, p = 0.77). Effects were not associated with whether or not the included participants were overweight (average fat intake, p = 0.79; percentage energy from fat, p = 0.13) or whether or not the studies aimed to maintain or reduce BMI (average fat intake, p = 0.67; percentage energy from fat, p = 0.23).
There was some evidence that the estimates of effect on total fat intake were larger in studies in which interventions also included a physical activity component (WMD –7.1 g; 95% CI –14.1 to –0.1 g; p = 0.047). There was no evidence for a similar association for percentage energy from fat (p = 0.20).
Fibre intake
Five studies44,45,55,59,82 assessed average intake of fibre, reported as g/day, of which two provided sufficient data for meta-analysis (Figure 8). 44,59 There was some evidence for heterogeneity in the estimates (p = 0.11 and I2 = 60%). Meta-analysis of these studies showed a WMD of 1.45 g (95% CI –0.02 to 2.92 g; p = 0.053). A single study assessed average change in fibre intake and found a 0.7 g (95% CI 0.1 g to 1.4 g) larger change in the e-learning group than in the control group. Subgroups were not examined owing to too few studies.
FIGURE 8.
Forest plot showing the effect of e-learning on the mean intake of dietary fibre (g/day).
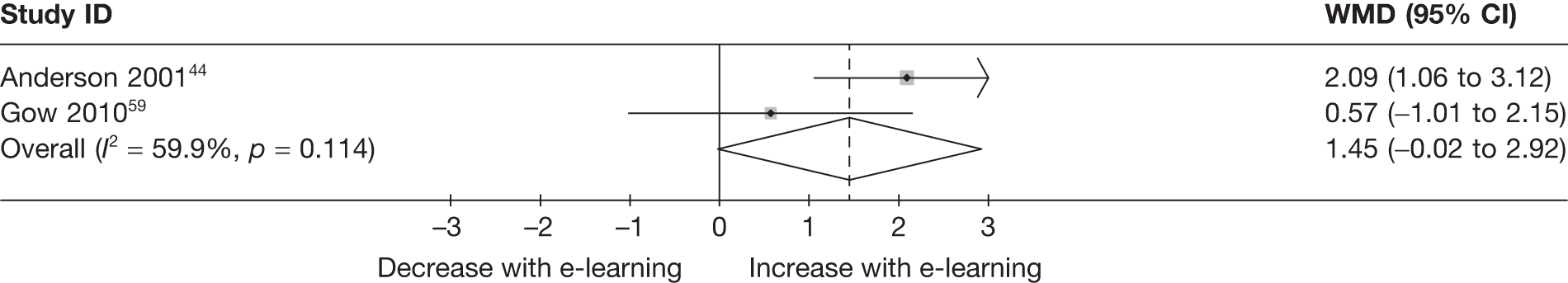
Energy intake
Mean energy intake, reported as kilocalories, was addressed in eight studies,45,53,57,65,74,77,80,83 five of which provided sufficient data for meta-analysis (Figure 9). 57,65,74,77,80 There was no evidence for heterogeneity in the estimates on difference in mean intake (p = 0.33 and I2 = 13%). Meta-analysis of these studies showed no evidence for an effect on daily energy intake (WMD 4 kcal; 95% CI –85 kcal to 93 kcal; p = 0.93).
FIGURE 9.
Forest plot showing the effect of e-learning on the mean energy intake (kcal/day).
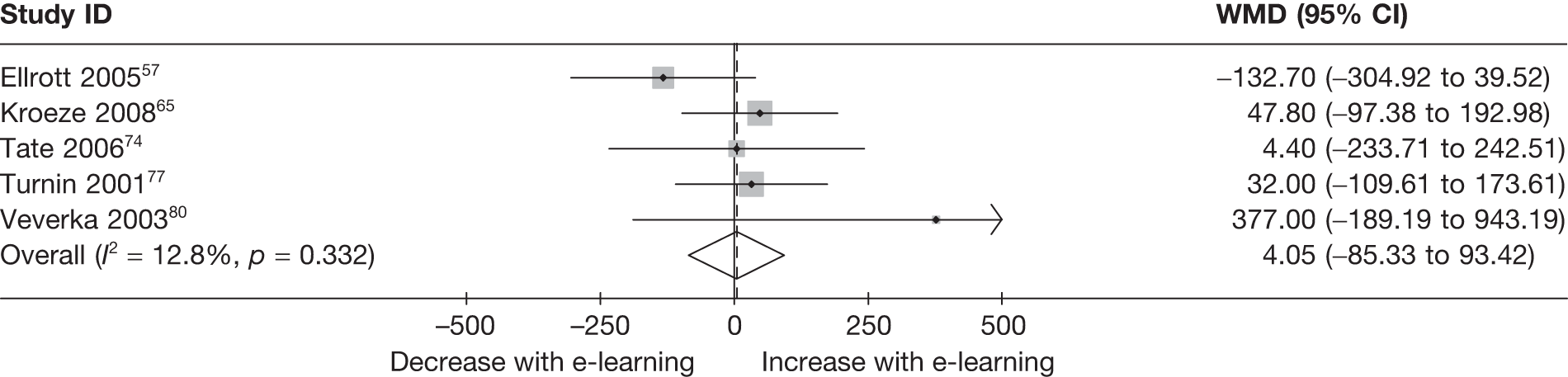
Subgroups
There was no evidence to suggest that estimates of effect on energy intake were associated with study quality (EPHPP global rating ‘weak’ vs ‘moderate’, p = 0.40; level of attrition in studies ≤ 20% vs > 20%, p = 0.40; allocation adequately concealed from investigators ‘yes’ vs ‘unclear’, p = 0.68). None of the studies measuring mean energy intake included participants predominantly from low-income groups or with a diagnosed illness.
There was no evidence that estimates of effect differed according to whether or not follow-up was earlier (within 3 months) or later (p = 0.18). Effects were not associated with whether or not the included participants were overweight (p = 0.37), whether or not the studies aimed to maintain or reduce BMI (p = 0.28) or whether or not interventions included a physical activity component (p = 0.61).
Clinical outcomes
Body mass index
Thirteen studies46,53,59,63–66,71,73,77,79,83,84 reported BMI as an outcome, nine46,59,63,64,66,71,73,77,84 of which reported mean BMI (with SDs; Figure 10) and three53,79,83 of which reported mean change in BMI (with SDs). There was no evidence for heterogeneity in the estimates of effect of e-learning on average BMI (p = 0.92 and I2 = 0%) and similarly no evidence that smaller studies were associated with larger effects (Egger’s test, p = 0.66). Random effects meta-analysis of all studies that reported mean BMI showed a WMD of –0.1 kg/m2 (95% CI –0.7 kg/m2 to 0.4 kg/m2) which was not statistically significant (p = 0.69).
FIGURE 10.
Forest plot showing the effect of e-learning on the mean BMI (kg/m2).
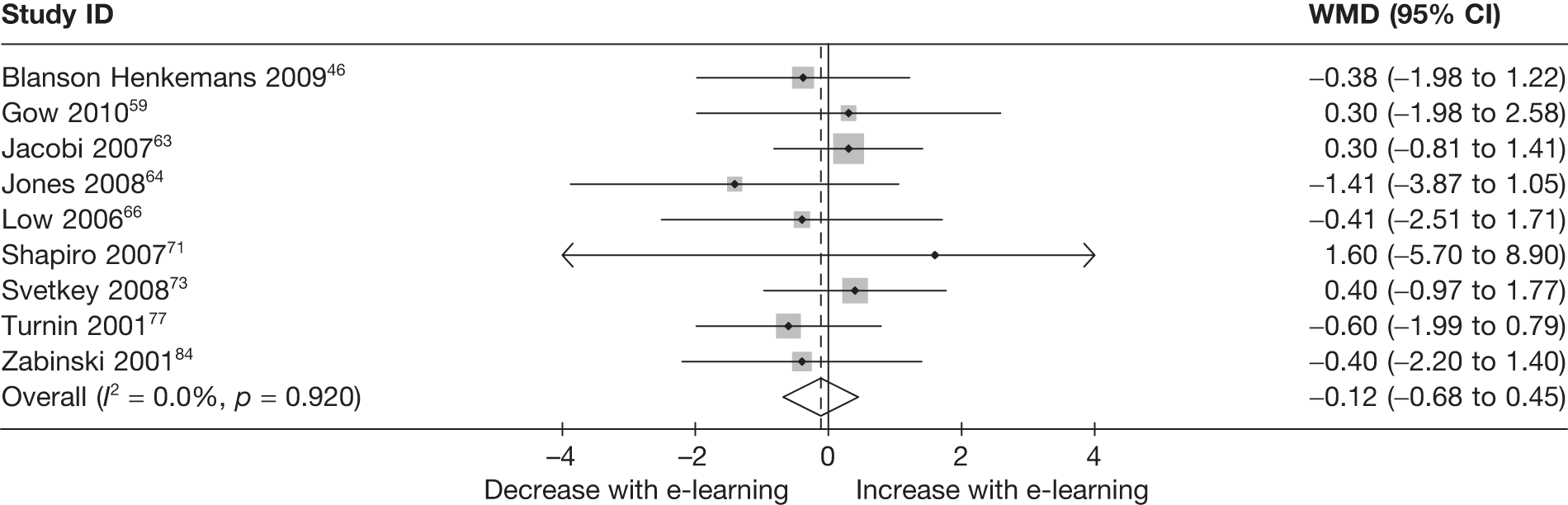
There was evidence for heterogeneity in the estimates of difference in mean change in BMI (p = 0.039 and I2 = 69%). Random effects meta-analysis of the three studies showed no evidence for a difference (–0.07 kg/m2; 95% CI –0.7 kg/m2 to 0.6 kg/m2; p = 0.82; Appendix 7).
Subgroups
There was no evidence to suggest that estimates of effect on BMI were associated with study quality (EPHPP global rating ‘weak’ vs ‘moderate’, p = 0.55; level of attrition in studies ≤ 20% vs > 20%, p = 0.29; allocation adequately concealed from investigators ‘yes’ vs ‘unclear’, p = 0.64). None of the studies measuring average BMI included participants predominantly from low-income groups or with a diagnosed illness.
There was no evidence that estimates of effect differed according to whether or not follow-up was earlier (within 3 months) or later (p = 0.59). Effects were not associated with whether or not the included participants were overweight (p = 0.58), whether or not the studies aimed to maintain or reduce BMI (p = 0.91) or whether or not interventions included a physical activity component (p = 0.91).
There were two studies that aimed to reduce BMI and did not include a physical activity component. 77,79 At final follow-up, Turnin et al. 77 estimated a reduction in BMI of –0.6 kg/m2 (95% CI –2.0 kg/m2 to 0.8 kg/m2) and Verheijden et al. 79 estimated no difference between mean change in the intervention and control groups (–0.01 kg/m2; 95% CI –1.6 kg/m2 to 1.6 kg/m2).
Weight
Ten studies42,45,52,53,69,71,73,74,76,83 reported weight as an outcome, four52,71,74,76 of which reported mean weight (with SDs; Figure 11) and three of which reported mean change in weight (with SDs). There was substantial heterogeneity in the trial results (p < 0.001 and I2 = 86%; p < 0.001 and I2 = 94%, respectively). Random effects meta-analysis of the four studies that reported mean weight found no evidence for effect (0.6 kg; 95% CI –3.5 kg to 4.6 kg; p = 0.78). 52,72,74,77
FIGURE 11.
Forest plot showing the effect of e-learning on the mean weight (kg).
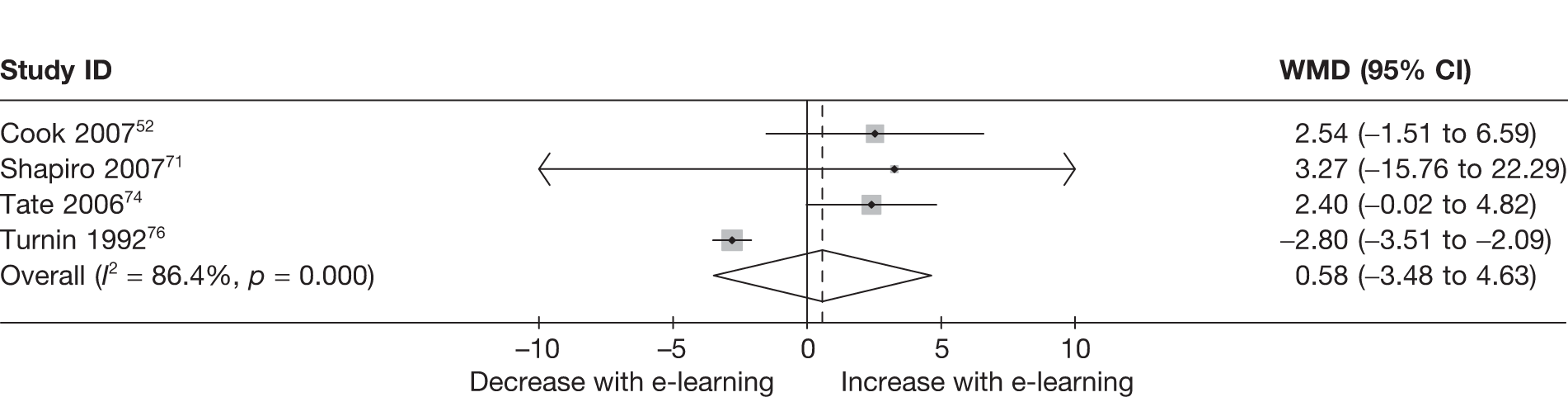
Random effects meta-analysis of the three studies53,73,83 that reported mean change in weight found no evidence for effect (–0.07 kg; –1.8 kg to 1.6 kg; p = 0.94; Appendix 7).
Subgroups
There was no evidence to suggest that estimates of effect on weight were associated with study quality (EPHPP global rating ‘weak’ vs ‘moderate’, p = 0.52; level of attrition in studies ≤ 20% vs > 20%, p = 0.82). There was some evidence to suggest that estimates of effect on weight were larger in the single study by Turnin76 in which the method of allocation concealment used was ‘unclear’ (p = 0.042) and in which participants had a diagnosed illness (p = 0.042). None of the studies measuring average weight included participants from low-income groups.
There was no evidence that estimates of effect differed according to whether or not follow-up was earlier (within 3 months) or later (p = 0.57). There was no evidence that the estimates of effect differed according to whether or not the included participants were overweight (p = 0.52).
Eating disorders
Four studies63,64,66,84 evaluated interventions that aimed to prevent eating disorders in high-risk college students. All four studies used the ‘Student Bodies program’90,91 to deliver the intervention. These studies measured a range of eating disorder outcomes: the Eating Disorder Inventory (EDI) scales, the Eating Disorder Examination-Questionnaire (EDE-Q), and Eating Disorder Examination-Body Dissatisfaction (EDI-BD) subscales. A further study59 used the EDI and EDE-Q to collect data on eating habits. We have not undertaken any analysis of these composite outcomes, as they were not included in our study aims and protocol; only BMI or weight outcomes were used from these studies.
Further analysis
Appendix 7 presents further analysis of effectiveness.
Appendix 8 presents the behavioural change techniques reported to have been used in interventions, tabulated against whether or not the study demonstrated evidence for effect (in the intended direction) on any outcome.
Chapter 5 Assessing the cost-effectiveness of e-learning devices for obesity
The aim of this chapter is to assess the cost-effectiveness of e-learning devices for obesity.
Published economic evaluations
A literature review revealed that no full economic evaluations of e-learning devices based on nutritional activities alone have been published. 83,92–94 However, one UK study that assessed the cost-effectiveness of a web-based support package for people who were obese, in which exercise was also a component, was identified. 93 The study was based on a 12-month RCT of a web-based e-learning device compared with ‘standard’ dietary advice alone. The results suggested that the dietary advice alone treatment arm was less costly and more effective than the e-learning device. The paper, however, has not been formally reviewed because the underlying RCT was excluded from the clinical review. However, it has been used as a basis to cost a generic ‘e-learning device’ in this analysis as it is relatively contemporary and UK-based and little other evidence was available.
The E-Learning Economic Evaluation Model
Because no directly relevant studies have been undertaken, a de novo economic decision model was built, referred to as the E-Learning Economic Evaluation Model (E-LEEM). The first stage in building the E-LEEM model was to examine other decision models for which the primary clinical aim was to promote weight loss or prevent further weight gain. In addition to the results of the clinical review, this process consisted of a non-systematic review of obesity-related studies submitted to the National Institute for Health and Clinical Excellence (NICE), since 2002, as part of either its technology appraisal or clinical guideline programme.
The decision problem
The (potential) role of e-learning devices
The place of e-learning devices in terms of an overall obesity pathway is somewhat unclear, as discussed already in this report. For example, they have been used to prevent obesity in the first instance and as a means of managing patient groups who are already considered to be (severely) obese (i.e. weight loss). Thus, the aim of intervention could feasibly be to promote weight loss, to prevent obesity in the first instance or both. On the balance of available clinical evidence, the decision was taken to estimate the costs and effects of e-learning devices as a method of promoting weight loss in obese people. All patients were also assumed to be non-smokers with no prior history of either type 2 diabetes (T2D) or CVD, with normal age-adjusted, systolic blood pressure levels, cholesterol ratios and a starting age of 50 years unless otherwise stated. These starting characteristics were varied in a number of sensitivity analyses.
Comparator intervention(s)
As most of the RCTs included a ‘traditional’ dietary advice alone arm, this was taken to represent the baseline treatment. However, in a separate sensitivity analysis, the use of a pharmacological treatment was also considered. The original intention was to include two NICE-approved treatments, orlistat and sibutramine. However, during the course of writing this report (January 2010), the Medicines and Healthcare products Regulatory Agency removed the marketing authorisation for the latter. Thus, only orlistat has been considered; note, no attempt has been made to use formal methods of performing mixed-treatment comparisons to assess relative treatment effects.
Description of the technology
Defining what e-learning devices actually consist of (in terms of resources) and how they should be used is difficult because they are idiosyncratic in terms of their design and platform base, not all are commercially available and they and their use were often poorly described in the clinical trials. Thus, for the purpose of the economic evaluation, a single hypothetical/generic package has been defined, largely based on the web-based McConnon et al. 93 intervention as previously discussed.
The model consists of a cost–utility analysis, with health outcomes expressed as quality-adjusted life-years (QALYs). Costs were included from a UK NHS perspective and expressed in 2009 prices. In all scenarios, the model was run until all patients died, implying a lifetime horizon for the analysis.
Heterogeneity versus individual variability
Various publications95 have suggested that there is a need to separate issues of patient heterogeneity from individual variability (first-order uncertainty) when undertaking economic evaluations to allow treatment costs and effects for individual patient (sub-) groups to be isolated and reported. For this reason, the model was run in the base-case analyses for people with fixed sets of starting characteristics – based broadly on the results of the clinical literature review, but also on clinical opinion of potential candidate patients (Table 23). Note, however, that the model can, if required, sample initial patient characteristics (such as age, gender and starting body weight/BMI level) rather than fixing them at the outset.
| Characteristic | Scenario | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| BMI (kg/m2) | 30 | 30 | 33 | 30 | 33 | 35 | 35 | 30 |
| Gender | Male | Male | Male | Female | Male | Female | Male | Female |
| Age (years) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Smoker | No | Yes | No | No | Yes | Yes | Yes | Yes |
| T2D | No | No | No | No | No | No | Yes | No |
Rationale for choice of modelling framework
A number of different modelling approaches were considered: decision trees, Markov models and discrete event simulations. A decision tree approach was discounted because this method is cumbersome when multiple health outcomes are important and events are considered over a relatively long period of time; both issues are important in the context of obesity. The non-systematic examination of associated NICE technology appraisals and clinical guidelines suggested that most obesity-related economic evaluations have been performed using a Markov approach. In a Markov-type analysis, individuals move between a set of pre-defined health states over a fixed unit of time according to a set of transition probabilities; they are often referred to as discrete time models for this reason. This is in contrast to discrete event simulations (DESs), where a set of possible events is defined (along with associated costs and health outcomes), but the time between each event is variable in a first-order sense. Thus, in effect, the purpose of a DES is to estimate times between events, with the sum of these intervals (typically) representing total life expectancy.
Discrete time and event models are both useful when treatment costs and benefits are likely to accrue over relatively long periods of times, as is likely to be the case herein. However, the limitation with Markov models is that the probability of moving from one health state to another is typically reliant only on an individual’s current state of health. Thus, no account is taken of an individual’s clinical history. This is arguably problematic in the context of evaluating obesity-related technologies as many issues are interlinked. For example, people who develop T2D are more likely to develop CVD than those who do not. A further limitation with Markov type models is that they become inefficient and demanding in a programming sense if multiple health outcomes are possible, as increasingly more complex sets of health states are required.
A DES potentially overcomes both of these problems; thus, it was judged to be the most appropriate modelling approach. This said, arguably the main limitation with DESs is that although second- and third-order simulations are technically feasible, they add significantly to the complexity of the programming and are computationally expensive. For these reasons, all reported results are based on probabilistic sensitivity analyses (PSA) using 1000 outer (second-order) simulations and 1000 inner (first-order) simulations, but the number of reported sensitivity analysis and expected value of perfect information (EVPI) calculations is limited (see Discussion section for more details).
Discrete event simulation model building considerations
As a basic aim of the evaluation was to link trial evidence to longer-term costs and outcomes, a priori consideration was given to the potential array of quantitative outcomes that relevant RCTs could report. It was suspected that the most useful outcomes would include weight change, percentage weight loss, change in BMI, change in systolic blood pressure and change in cholesterol level. Although a general approach was sought that could potentially incorporate all of these outcomes, the main favoured modelling approach was to link changes in body weight/BMI to changes in the timing of future events. For example, ceteris paribus increases in body weight/BMI were assumed to lead to shorter times to CVD, T2D and death. This approach was taken because, on a preliminary scan of the retrieved literature, BMI proved to be a reasonably frequently reported trial outcome. It is an approach which has been used before in other models and because, if required, costs and utilities for various body weight/BMI strata were available.
Model overview
All patients are assumed to be offered treatment for the first 12 months with either dietary advice alone or dietary advice through an e-learning device (Figure 12). Separate consideration was also given to the use of orlistat as an alternative, as previously discussed. During this time, people could experience an event (see Selecting events of interest, but including CVD), die or withdraw from treatment (modelled using an attrition rate). Those who experienced any one of these events were assumed to immediately stop treatment. They were also judged to be ‘treatment’ failures and to have put on weight over this time. On the other hand, patients who completed the 12-month treatment period were assumed to be treatment responders, and to have gained, lost or maintained weight. After the 12-month period, no further treatment is given in the base case, meaning that patients could not lose any further weight. Instead they were assumed to put on weight over time according to a specified function.
FIGURE 12.
Model schematic.
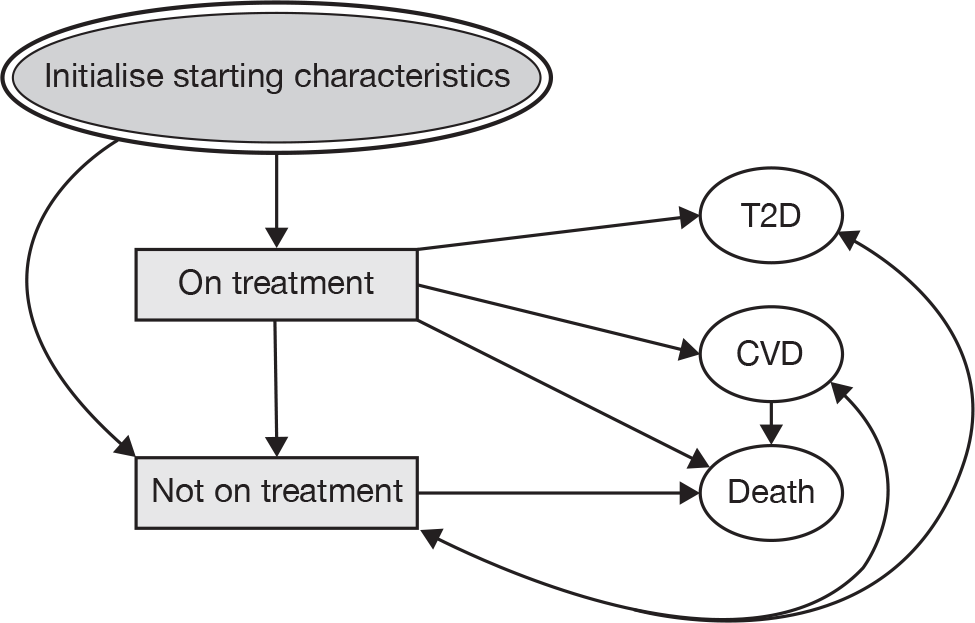
All changes in body weight were transformed into increases in BMI, which in turn are linked to changes in the risks of future health-related events and associated activities (increased costs, decreased health-related quality of life and increased risk of death). The model terminates only when all people have died.
This modelling approach means that treatment effects can conceptually be incorporated in three individual ways, through an improved attrition rate, lower weight gain for treatment failures or higher weight loss for treatment responders. However, owing to data limitations and the results from the meta-analysis, a decision was taken to include differences in weight gain (or, rather, BMI) only after 12 months in the base case. Other scenarios were explored in the sensitivity analysis.
Selecting events of interest
Obesity is associated with an increased risk of a number of health-related events. A brief examination of the published cost-effectiveness literature on obesity suggests that most models have focused on CVD, T2D or both. However, at least one model has also considered the costs and outcomes of colon cancer. The case for including both CVD and T2D in the model was clear, as the evidence supporting a relationship between these events and increased body weight is well documented. Moreover, the absolute risk of these conditions in obese patients is arguably high, they are costly to treat and are associated with significant morbidity. The original intention was to also include the possibility of developing colon cancer. However, evidence suggests that the absolute rate of developing disease over 5 years in relatively high-risk European males is approximately 200 cases per 100,000 subjects. Thus, a conservative estimate calculated using an exponential distribution suggests that, on average, relatively high-risk men develop colon cancer once every 2500 years. 96 For this reason, the possibility of developing colon cancer in the model was excluded. In addition to CVD, T2D and death, a BMI increase of 0.1 kg/m2 was also directly considered to be a possible event in order to model the natural history of the condition.
The probability of death
The basic method of calculating the probability of all-cause death was using a life-table approach, as outlined by Barton et al. 97 A normative age-adjusted data set from the UK’s Government Actuaries Department98 was used to calculate the time to death given current age and gender. In addition to this, 33%99 of patients who developed CVD were assumed to die immediately once the event had occurred (termed a fatal CVD event), with the likelihood of a CVD happening being partly conditional on whether or not patients had a recorded history of T2D. All deaths from T2D were considered to be attributable to cardiac problems; thus, they were not independently modelled. These approaches were used in the base case to avoid the possibility of double-counting the occurrences of death. However, sensitivity analyses were also run in which CVD and T2D were assumed to increase the age-related probability of death, by adjusting the life-tables using published BMI-related standardised mortality ratios (SMRs). In effect, this meant that the SMRs associated with CVD and T2D were equal to 1 in the base case, increasing to values > 1 in the sensitivity analyses.
The probability of developing cardiovascular disease
A number of algorithms with which to predict the timing of future CVD events are available. Perhaps the most frequently cited are the Framingham risk equations,100 a version of which is currently recommended for use in NICE’s lipid-lowering clinical guideline. 101 However, an updated algorithm constructed using UK data has more recently been published, with some evidence to suggest that it more accurately predicts 10-year risks of CVD events (the QRISK®2,102 University of Nottingham, Nottingham and Egton Medical Information Systems Limited, Leeds, UK). As it is UK-based and includes a number of parameters such as BMI that could possibly be reported by trials of e-learning devices and the presence/absence of T2D is included as an independent risk factor, it was chosen as the method of predicting the timing of future CVD events. Note, however, that a limitation of using the QRISK2 for this modelling exercise is that the primary outcome in the original study was the first recorded CVD event (angina, myocardial infarction, stroke or transient ischaemic attack), and individuals with pre-existing CVD were excluded from the study. Therefore, the model does not incorporate any additional risk of having a second (or subsequent) CVD event as a consequence of the original event. In the context of the decision model, this is likely to mean that the overall number of predicted CVD events per unit of time is conservative. Permission to reproduce the QRISK2 algorithm was sought and obtained from the authors.
The probability of developing type 2 diabetes
A number of risk equations for predicting T2D were identified that included BMI as an independent variable. The preferred algorithm was the UK-based QDScore®103 (ClinRisk Ltd, Leeds, UK), which was developed by the authors of the QRISK2 equation, using a similar methodology. However, the underlying algorithm is yet to be validated and published, so it was not possible to use it. None of the alternative algorithms was derived using UK data, and indeed related to North American104 and Finnish105 populations. Therefore, the rather arbitrary decision was made to use the Stern equation, but the Lindström model was used as an alternative in a sensitivity analysis.
Where gender = 0 for males and 1 for females.
The probability of developing T2D over 7.5 years = exp(A)1+exp(A)
The mean time to gaining body weight
All patients were assumed to put on weight after 12 months of treatment, quitting treatment early or developing either CVD or T2D, whichever occurred first. The 2005 NICE obesity guideline106 referenced Fine et al. ,107 stating that in the studied women, the average weight increase over 4 years was 1 kg per year. The guideline also stated that this finding is consistent with the findings of Heitmann et al. ,108 who performed a retrospective semi-longitudinal study to determine the pattern of weight changes over 11 years in a Danish population who became overweight in adulthood. Thus, all patients were assumed to put on an average of 1 kg per year, independent of initial BMI levels.
All weight gains were converted into increased BMIs by assuming that men were on average 1.75 m tall, whereas women were 1.62 m tall. This is equivalent to a 0.33 and a 0.38 unit increase in BMI, respectively, per 1 kg increase in weight [BMI = weight (kg)/height (m)2]. 109 The mean time to an increased threshold BMI was then calculated on this basis. For example, in the base case the threshold was set equal to a 0.1 unit increase, meaning that, on average, it would take a man 0.30 years (0.1/0.33) for his BMI to increase by this amount.
Baseline weight change
While on treatment, all body weights were assumed to change (decrease on average) in line with the RCT by McConnon et al. 93 kg (N ∼ 1.9, 0.63).
Intervention effectiveness
The base case relative effective of the e-learning devices compared with ‘standard care’ (i.e. dietary advice alone) was estimated using results from the systematic review and meta-analyses; weighted mean difference BMI (N∼ –0.115, 0.29) (see Chapter 4, Body mass index). Note, that there was no evidence of heterogeneity between studies for this outcome. The single RCT by Turnin et al. 77 was used as the estimate of treatment effect in a sensitivity analysis as it was the only study to use BMI as a primary outcome measure. Few other useful outcomes were reported in terms of being directly importable into the CVD and T2D risk functions, meaning that changes in BMI were the only treatment effect to be incorporated.
The expected costs and effects of orlistat were estimated in single sensitivity analysis. The mean difference (reduction) in body weight between the orlistat and placebo treatment arms (4.36 kg) was taken from Foxcroft,110 which in turn was based on individual patient-level data from three RCTs.
The mean time to stopping treatment
High attrition rates appear to be a defining feature of weight loss/preventing weight gain interventions. The Turnin et al. 77 RCT was (arbitrarily) used to estimate the base treatment effect. The results showed that 179/557 participants were lost to follow-up over the 12-month period (β∼ 179, 378). Differences in attrition rates between the treatment arms were judged to be negligible and were not included in the model.
Costs
Costs were broadly divided into two types, those associated with specific events and those relating to the initial 12 months of treatment (Table 24). All costs were inflated to 2009 prices using an NHS-specific index. The costs of CVD were taken from Warren et al. ,111 and were reported as one-off costs for fatal and non-fatal events, and as an annual cost for survivors. No other costs associated specifically with CVD were included. The annual cost of diagnosed T2D was taken from Ara and Brennan. 112 The cost included two GP visits per year, a specialist nurse visit, drug treatment for high blood pressure, statin therapy and treatment with metformin. The annual costs of ‘traditional’ dietary advice and those associated with e-learning devices were taken from a UK-based RCT of a web-based support package. 93 The costs for both interventions included resources such as drug costs and health-care visits and slimming clubs. The main difference between the two was that the web-based support package included an additional fixed cost per patient of £854 per annum for the actual web-based support (meaning that this cost was applied per patient irrespective of how long his or her treatment lasted in the base case). In a single scenario analysis, the costs and effects of orlistat were also estimated as a third mutually exclusive treatment option. The yearly cost of orlistat treatment was assumed to be £415 [based on 120-mg treatment three times per day, at a unit price per pill of £0.38 (£32.27/84)], plus the cost of five GP visits (£35 per visit).
| Description | Mean value | Distributiona | Source |
|---|---|---|---|
| CVD fatal event | £3058 | Gamma (9, 1/339) | bWarren et al.111 |
| CVD non-fatal event | £3648 | Gamma (9, 1/405) | bWarren et al.111 |
| Annual cost of non-fatal CVD event | £876 | Gamma (9, 1/97) | bWarren et al.111 |
| Annual cost of T2D | £724 | Gamma (9, 1/80) | Ara and Brennan,112 NICE bguideline106 |
| Annual cost attributable to orlistat | £715 | N/A | British National Formulary,113 Foxcroft110 |
| Annual cost of e-leaning device | £140 (SE £234) | Gamma (1402/2352, 140/2352) | cMcConnon et al.93 |
| Fixed cost of e-learning device | £854 | N/A | cMcConnon et al.93 |
| Annual cost of dietary advice | £226 (SE £329) | Gamma (2262/3292, 226/3292) | cMcConnon et al.93 |
Utilities
The main source for the evidence relating to utilities was Macran et al. 114 This study assessed the relationship between BMI and European Quality of Life-5 Dimensions utility scores in a UK population of approximately 12,000 people. Various statistical models based on ordinary least squares regression techniques are presented, but model F was chosen for the base-case analysis. Although this model related specifically to women, it demonstrated statistically significant differences in preference scores between different BMI strata, age bandings and long-standing illnesses, whereas in the model for men, the long-standing illness component was not significant. Thus, this model was chosen for both men and women; visual inspection of the various models suggests that differences in coefficients across the various models for men and women are arguably small. A maximum of two long-standing illnesses were permitted, representing the possibility of developing CVD and T2D. Note, however, that although these values have been used in the base case, Macran et al. are not specific about which long-standing illnesses were reported. The model was expressed as follows (and populated using the data in Table 25):
| BMI group (kg/m2) | Coefficient | Age group (years) | Coefficient | No. of LSIs | Coefficient |
|---|---|---|---|---|---|
| < 21 | –0.02 | 18–24 | 0 | 0 | 0 |
| 21–25 | 0 | 25–34 | 0.0005 | 1 | –0.115 |
| 26–30 | –0.02 | 35–44 | –0.01 | 2 | –0.196 |
| 31–39 | –0.04 | 45–54 | –0.02 | ||
| > 39 | –0.06 | 55–64 | –0.04 | ||
| 65–74 | –0.04 | ||||
| > 75 | –0.08 |
The report by Macran et al. 114 does not report standard errors for the coefficients. Therefore, for the PSA, rather than assume no associated parameter uncertainty, it was assumed that all coefficients could increase or decrease by a maximum of 10% by multiplying all values by a scaling factor that was using a uniformly distributed variable (U∼ 0.9, 1.1).
Discounting
All future costs and benefits were discounted using 3.5% per annum, using the following formulae:
One-off activity:
where c is the cost or benefit, λ = ln(discount rate) and t = time of the event in years.
Steady state activity:
where t is the time an event begins in years, over s years.
Model verification
A number of efforts were made to ensure that the model was technically correct, i.e. that the programming did exactly what it was intended to do. First, the T2D and CVD risk equations were entered into the TreeAge Pro 2009 program (TreeAge Software, Inc., Williamstown, MA, USA),115 run, then reprogrammed separately in Microsoft Excel 2007 (Microsoft Corporation) to ensure that the results matched. Results from the CVD risk algorithms were also cross-checked against the published clinical examples to ensure consistency. These processes identified a number of programming errors, which were subsequently corrected. TreeAge Pro 2009 also contains a facility to output calculations other than the individual total costs and benefits [using the Global(n) function]. This facility was used to ensure that the internal calculations for each model run looked plausible relative to the outputs so that no unusual events were occurring prior to the model terminating, such as decreases in BMI after the initial 12 months in the base case. The model was also tested to ensure it produced logical results, e.g. that higher starting body weights led to lower life expectancies and higher likelihoods of developing T2D and CVD.
Modelling software
The DES was built in TreeAge Pro 2009 using an approach outlined by Barton et al. 97 TreeAge Pro 2009 does not contain an explicit DES facility. Rather, the Markov node function is used as a cycling facility to resample times between possible events instead of indicating a movement between health states over a fixed period of time, which is implicit when using Markov models. Using the Markov function in this manner means that the inbuilt _stage function now in effect becomes a running total of the number of events that occur per individual rather than an indicator of time, and can no longer be used as a command in any meaningful way.
Other important aspects of the programming included the initiation of a series of tracker variables. Tracker variables are variables whose values can change over time, typically following some event. For example, a person’s current body weight was recorded using a tracker variable, as was a variable indicating whether or not a person had developed T2D. A set of tracker variables was also set to act as ‘clocks’, with each set recording the time between two or more events. For example, clocks were set to record the time from entering the model until death (equivalent to a person’s survival time) and the time between developing T2D and death. The full programming syntax is available on request.
There are a number of ways in which DESs can be run in terms of selecting the next event, as outlined by Barton et al. 97 The approach taken in this model was to independently sample times to the competing events using risk equations, mean times to events and a life-table, and to choose the event that was predicted to occur next. The remaining information on the time to the next event was discarded at this point, the tracker variables (including the clocks, total costs and QALYs) updated if required, and the process repeated until each patient had died. In all cases, the time to the next event was modelled assuming an exponential distribution after converting probabilities from the risk equations and life-table to rates, and by calculating the corresponding reciprocal value. These values were then multiplied through by a random draw from an exponential distribution with a lambda value of 1. The random draws were based on TreeAge’s ‘distforce’ function to ensure that times were resampled each time a new event was expected.
Example: say the predicted probability of developing CVD for a particular individual at a given point in time over 10 years is 0.2, as predicted using the QRISK2 equation. This is equivalent to a hazard rate of 0.022.
Converting this to an average time to a CVD event by calculating the reciprocal equals:
Multiplied by a random draw from an exponential distribution with a lambda value of 1, say 1.6, leads to a predicted time to a CVD event of 71.7 years. The random draw was repeated as many times as required for each of the 1000 hypothetical individuals. In the PSA component of the analysis, the underlying mean values were resampled and a further 1000 first-order simulations run.
Results
The base-case results are shown in Table 26 for a number of different patient starting characteristics. Although the absolute costs and QALYs vary across the scenarios, in each instance the incremental health gains were small, as indicated by the fact that very few additional cases of T2D or CVD were averted. The lowest reported incremental cost-effectiveness ratio (ICER) was approximately £102,000 per additional QALY. Scenarios run with women were associated with lower QALYs than those with men (e.g. scenario D compared with A). This was because lower rates and times spent with CVD for women were more than offset by higher rates and time spent with T2D.
| Scenario | Intervention | TyT2D | TyCVD | P T2D | P CVD | Cost (£) | QALYs | ICER (£) |
|---|---|---|---|---|---|---|---|---|
| A | Da | 5.372 | 3.611 | 0.443 | 0.559 | 4884 | 12.527 | |
| E-l | 5.352 | 3.608 | 0.441 | 0.558 | 5646 | 12.534 | 102,112 | |
| B | Da | 4.936 | 4.198 | 0.444 | 0.618 | 5364 | 12.093 | |
| E-l | 4.921 | 4.195 | 0.443 | 0.617 | 6129 | 12.100 | 121,856 | |
| C | Da | 6.142 | 3.709 | 0.511 | 0.577 | 5340 | 12.196 | |
| E-l | 6.123 | 3.707 | 0.510 | 0.576 | 6088 | 12.200 | 184,962 | |
| D | Da | 6.587 | 2.776 | 0.543 | 0.475 | 4035 | 11.703 | |
| E-l | 6.567 | 2.774 | 0.542 | 0.474 | 4732 | 11.708 | 125,891 | |
| E | Da | 5.632 | 4.290 | 0.494 | 0.635 | 5810 | 11.838 | |
| E-l | 5.615 | 4.287 | 0.493 | 0.634 | 6566 | 11.844 | 150,865 | |
| F | Da | 7.412 | 3.645 | 0.613 | 0.581 | 5201 | 11.209 | |
| E-l | 7.391 | 3.643 | 0.612 | 0.580 | 5902 | 11.214 | 151,142 | |
| G | Da | 19.745 | 5.677 | 1.000 | 0.786 | 15,014 | 10.910 | |
| E-l | 19.748 | 5.675 | 1.000 | 0.786 | 15,789 | 10.911 | 232,911 | |
| H | Da | 6.031 | 3.434 | 0.529 | 0.553 | 4469 | 11.500 | |
| E-l | 6.009 | 3.431 | 0.527 | 0.552 | 5186 | 11.506 | 112,628 |
The cost-effectiveness acceptability frontier for scenario A is shown in Figure 13. It shows that for up to about £200,000 per additional QALY, dietary advice alone is the preferred option. Note, that after this threshold value, e-learning becomes the preferred option, even though at most points the probability that it is the most cost-effective option is barely > 50%. It is, however, preferred because it is associated with the higher expected net benefits at these threshold values.
FIGURE 13.
Cost-effectiveness acceptability frontier relating to scenario.
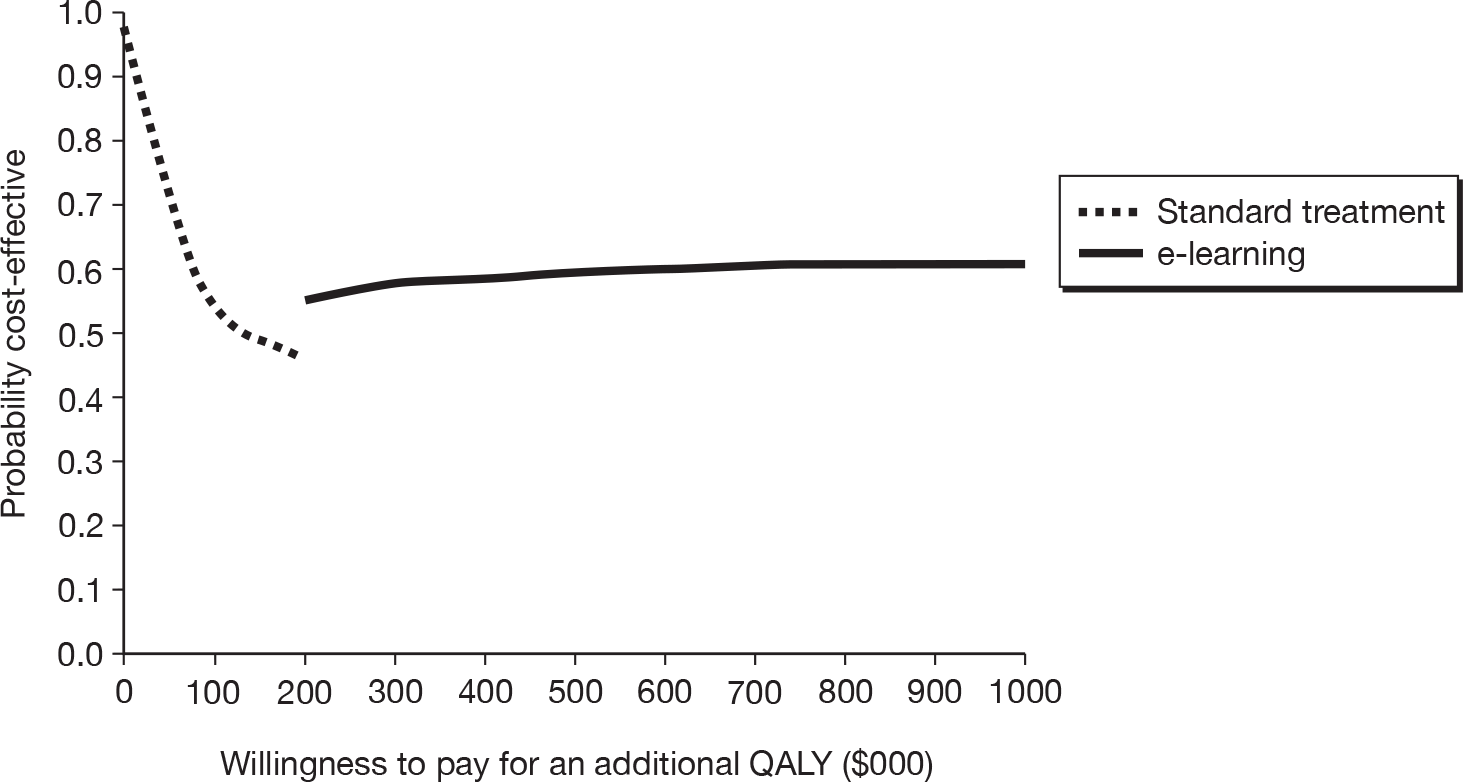
Sensitivity analysis
A number of one-way sensitivity analyses were performed. Perhaps the most important in terms of large changes to the ICER were the fixed initial cost associated with the e-learning devices, the relative treatment effect, the rate at which health outcomes were discounted and a longer duration of treatment effect (Table 27).
| Change | Dietary advice | E-learning | ICER | ||
|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | ||
| Doubling the time to a BMI increase of 0.1 kg/m2 after treatment stops | 4545 | 12.972 | 5302 | 12.978 | 122,125 |
| £0 initial cost for e-learning devices | 4903 | 12.475 | 4845 | 12.483 | Dom |
| Lindstrom T2D risk equation | 4248 | 12.812 | 5608 | 12.818 | 124,813 |
| Starting age of 60 years | 4577 | 9.749 | 5347 | 9.754 | 118,741 |
| Using estimate of relative treatment effect from Turnin et al.77 (N∼ –0.6, 0.71) | 4999 | 12.802 | 5704 | 12.837 | 20,053a |
| Doubling all T2D related costs | 6819 | 12.416 | 7561 | 12.435 | 83,306 |
| Doubling all CVD related costs | 7704 | 12.507 | 8458 | 12.513 | 100,480 |
| Standardised mortality ratio of 2 following T2D or a CVD event, or 4 for both events | 4307 | 12.550 | 5107 | 12.561 | 107,122 |
| Doubling the cost of dietary advice | 5115 | 12.482 | 5706 | 12.488 | 86,323 |
| 0% discount rate for health benefits | 4943 | 19.325 | 5699 | 19.338 | 58,869 |
| Halving the attrition rate for both treatments | 4871 | 12.799 | 5662 | 17.897 | 84,483 |
| Responders at 12 months continue to receive treatment for a maximum of a further 12 months, all other assumptions held constant | 5600 | 12.806 | 5719 | 12.817 | 64,487 |
| Time to next BMI change based on 0.5 instead of 0.1 kg/m2 | 4936 | 12.797 | 5711 | 12.805 | 103,627 |
In a number of scenarios, potentially counterintuitive results were produced. For example, when the costs of T2D and CVD were increased, the ICER associated with e-learning also increased. This is because people treated with e-learning devices on average live longer with these conditions, even though they are less likely to develop them in the first instance. The net result is an increase in the incremental cost and the associated ICER. Such seemingly counterintuitive results were also reported in the NICE obesity guideline,106 along with a similar explanation. Note, that the ICER increases when the time taken for a BMI increase of 0.1 kg/m2 is doubled. The main explanation for this is that although mean expected QALYs for all individuals have increased in this scenario, the overall difference in QALYs has slightly reduced. This is because the underlying likelihood of health-related events has also decreased. Thus, the potential impact of e-learning devices has lessened, resulting in fewer additional QALYs and a higher ICER.
When orlistat was included as a compactor, it dominated e-learning (that is, it was less costly and more effective than e-learning). Compared with dietary advice alone, orlistat cost approximately £3000 per additional QALY.
Expected value of perfect information analysis
The per person model-level EVPI was £13, rising to £453 at willingnesses to pay for an additional QALY of £0 and £100,000, respectively. The number of new obese individuals was difficult to assess, therefore the following assumptions were made. Prevalence data from the 2008 Health Survey for England Report,109 with obesity being classified as a BMI ≥ 30 kg/m2, and by assuming all obese people in 2007 remained obese in 2008, was used to calculate the annual incidence of obesity. Thus, the increase in the number of obese individuals, which was 308,000, is because of new incident cases. Arbitrarily assuming a 10-year lifespan for the technology and a 3.5% discount rate produces Figure 14 – with arguably large corresponding values at all positive willingnesses to pay.
FIGURE 14.
Expected value of perfect information and a single EVPPI analysis based on scenario A assumptions, and an incident obese population of 308,000 people every year for 10 years, discounted at 3.5% per annum.
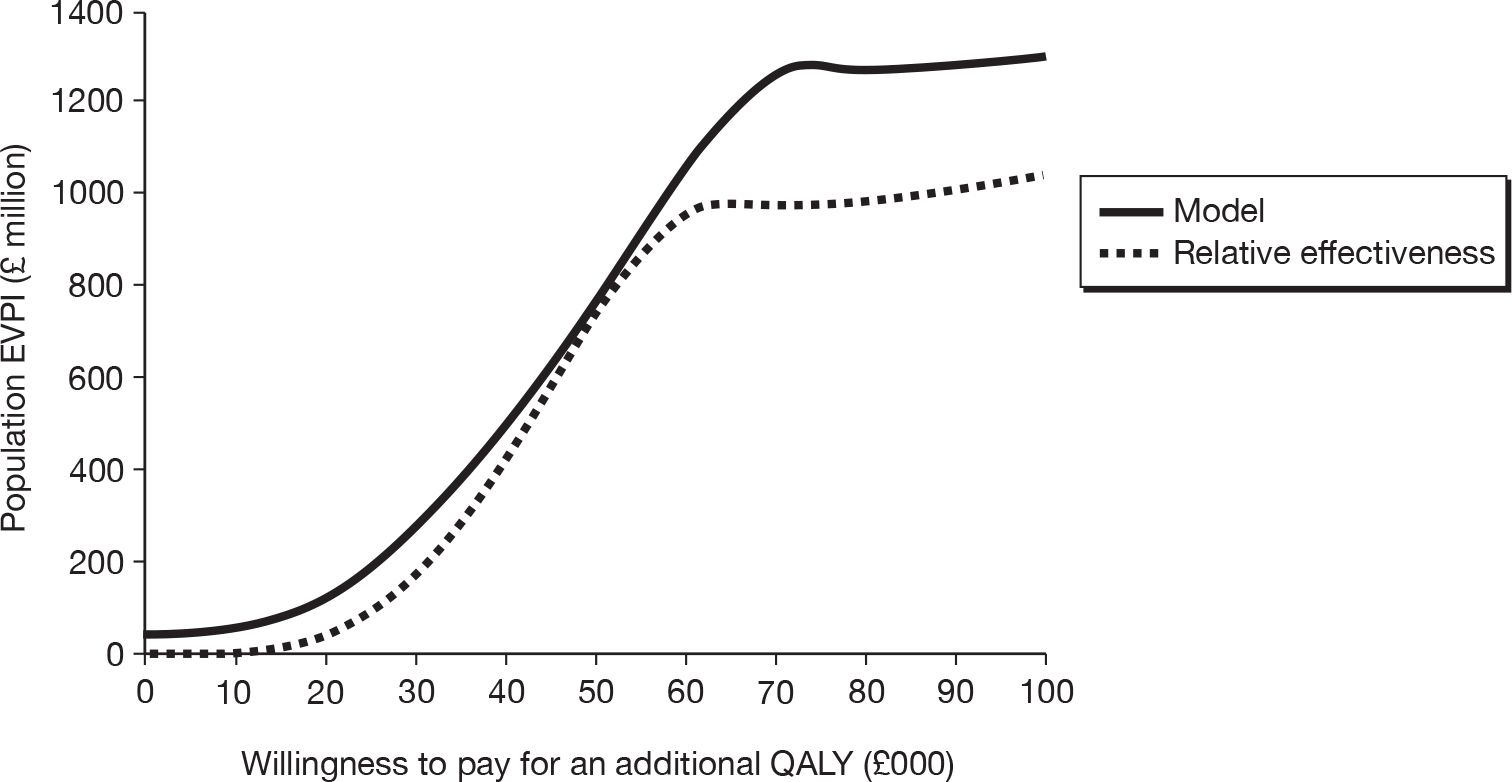
A single expected value of partial perfect information (EVPPI) analysis was undertaken on the relative treatment effect (WMD) associated with the two treatment options. The results suggest that at a willingness to pay of £20,000–30,000 per additional QALY, the maximum value of conducting a further RCT was between £37M and £170M.
Discussion
The aim of this chapter was to assess the cost-effectiveness of e-learning devices, for which the aim was to purely change dietary behaviours. No published economic evaluations on this subject were identified. One evaluation94 of a device based on a 12-month RCT that included a non-dietary component was, however, identified. In the base case, the results showed that the e-learning device was not cost-effective compared with standard care given the relatively large costs associated with the device. Indeed, although the results were not statistically significant, WMDs in weight loss were lower in the intervention arm and the costs were higher.
Because of the absence of published evidence, an economic model (E-LEEM) was constructed. It aimed to assess the cost-effectiveness of e-learning devices as a method of promoting weight loss in obese people relative to standard care (dietary advice alone). The model was based on a DES approach, and, although data from a number of sources were used to populate the model, the relative impact of the intervention (in terms of a difference in BMI) was based on the systematic review and meta-analysis presented in Chapter 4. The review suggested an advantage of e-learning devices compared with standard care; however, the results were not statistically significant and the mean difference is unlikely to suggest clinically significant differences in health. This conclusion is emphasised in the results of the economic evaluation: the differences in QALYs in all scenarios was arguably small. Moreover, because of the fixed initial cost that was assumed in the base case, the intervention was arguably not cost-effective at conventional willingness to pay levels. Only when the fixed cost of e-learning devices was removed, or substantially lowered, did e-learning devices appear to be cost-effective. But even in this circumstance, the probability it was cost-effective did not increase above 75%.
There are undoubtedly a number of (technical) limitations with the E-LEEM model. First, and perhaps most importantly, it was difficult to determine the cost associated with the device(s). This partly reflects the fact that most published clinical evaluations did not report resource/use costs associated with the technology at hand, the heterogeneous nature of the devices and because none appear to be commercially available. Thus, it is difficult to have any real idea how much they are likely to cost, particularly if rolled out for large numbers of patients (i.e. possible economies of scaling up use).
Another limitation was with the use of the QRISK2 risk equation. The equation is designed to assess the probability of developing a first CVD event. Thus, the model takes no account of the fact that individuals who have experienced any events are more likely to experience an additional event. However, given the relatively small difference in weight loss at 12 months derived from the literature review in the base case, it is considered unlikely that such an adjustment would have a significant impact on the results.
A further limitation of the modelling exercise relates to the choice of comparator technologies. The commissioned project scope related to the use of e-learning devices. The systematic review identified 43 RCTs. However, none compared the use of e-learning devices with anything other than (often poorly described) interventions based on other methods of providing ‘advice’. Although this is understandable in the context of performing specific trials, it is not helpful in a decision-making sense – there are a myriad of other potential interventions for obese people (for example, interventions based on promoting physical exercise and pharmacological treatments such as orlistat). With respect to the latter, although a formal systematic review and indirect treatment comparison has not been undertaken, a crude and unsystematic look at the evidence would suggest that orlistat could be less costly and more effective in this patient group. Indeed, NICE recommends the use of orlistat as a method of losing weight in obese people. 106 Although it is unclear whether or not this is indeed true in a relative sense, this does serve to emphasise the point that ‘cost-effectiveness’ is a relative term, and results depend on the choice of comparators. Thus, even if e-learning devices were considered to be cost-effective compared with dietary advice alone, it would remain unclear whether or not they are the optimal method of treating people. It is recommended that future assessments consider a broader project scope.
One particular difficulty was that the RCTs reported a number of different outcomes other than BMI, such as changes in fat and fruit consumption. Whereas it is not inconceivable that changes in these dietary behaviours could result in changes in longer-term health, it was not possible to quantitatively link the two in a robust manner. A related issue is that it is plausible that e-learning devices simultaneously affect more than just BMI levels. For example, NICE’s obesity guideline106 states that orlistat reduces blood pressure levels and BMI. The reviewed trials provided too little information for anything other than changes in BMI to be included in the E-LEEM model, but, clearly, if this is true, the cost-effectiveness of the e-learning devices has been underestimated.
The E-LEEM model was built using a DES approach because patients’ clinical histories were considered to be important in terms of predicting future events. The model was programmed to run in TreeAge Pro 2009. However, it transpired that running basic two-level simulations [i.e. first-order (1000 trials) and second-order (1000 samples) simulations] required almost 2 hours on a high-powered personal computer, meaning that the number of sensitivity analysis that could be run was constrained. It also meant that testing/validating the results was problematic and undertaking a series of EVPPI analyses with any degree of accuracy was impossible. Thus, although the population-level EVPI is arguably high at all positive willingnesses to pay, and the value of undertaking further research to assess the relative effectiveness of the two options is also arguably high, it remains unclear which other parameters are driving the results. At the time of writing this report, the company producing TreeAge Pro 2009 is beta testing an updated version of the software which can be run using Linux – which gives faster programming speeds. It is hoped to undertake extensive EVPPI when this update becomes available.
The EVPI analysis suggested that the value of further research was arguably large, despite the discouraging clinical results from the systematic review. This is because the incidence of obesity was estimated to be > 300,000 per year and the time horizon was assumed to be 10 years. Clearly, however, if either of these two estimates is believed to be too high, the value of the EVPI estimates will be sharply reduced.
In summary, the E-LEEM model was built to assess the cost-effectiveness of e-learning devices compared with dietary advice alone for people with obesity, as methods of promoting healthier eating and weight loss. The model contains a number of assumptions and necessarily draws on evidence from a number of different sources, although the estimate of relative treatment effect is based on a systematic review. The results from the review suggest only modest differences in outcomes (including BMI) between the approaches, suggesting that, even if statistically significant, clinical differences are likely to be minimal if not non-existent. This conclusion is clearly reflected in the cost-effectiveness results, where differences in QALYs were typically reported at only two decimal places and resulting ICERs were generally unfavourable from a UK NHS perspective. Costing the e-learning devices was difficult because they are idiosyncratic, most do not appear to be commercially available and the clinical studies rarely reported resource use or cost data. In terms of cost-effectiveness, and given the near equivalence of the clinical results, this is important, particularly whether or not there is a fixed initial cost of supplying each device. In the base case, a fixed initial cost of £854 based on the RCT by McConnon et al. 93 was assumed. The results showed that e-learning devices were the dominant option if the cost was removed. However, even if this cost was dramatically reduced it would remain unclear whether or not e-learning devices are more cost-effective, as the probability that they were cost-effective was only 59% in this analysis, and no other comparative interventions, such as exercise and pharmacological interventions, were evaluated. It is suggested that future evaluations of these devices consider a wider range of treatment options.
Chapter 6 Discussion
Explanations of effectiveness
Of the 43 interventions and trials identified, 1344,51,56,61,68–70,72,75,76,78,82,83 were found to be effective (with the intervention group demonstrating more positive change in the desired behaviour or outcome than the control group), 2742,43,46–50,52–55,57–60,62–66,71,74,77,79–81,84 showed no difference in effectiveness between the intervention and comparator and, in four trials,45,51,67,73 participants in the intervention group had less favourable change than those in the control group. In one trial, 51 participants in the intervention group showed a positive effect for one behaviour (reduced fat consumption), but a negative effect for another (increased fruit and vegetable consumption). We analysed the data with a view to determining reasons for the observed variability in effectiveness. We considered that potential reasons could include differences in:
-
target populations (low income, overweight, diagnosed illness)
-
target behaviours
-
intervention content
-
theoretical base
-
mode of delivery
-
‘dose’ of intervention (duration × intensity)
-
study quality.
Target populations
The target populations for the interventions varied: of 23 studies providing inclusion criteria, 10 were aimed at ‘healthy’ people, 12 aimed at overweight or obese people, one aimed at people with at least one risk factor for CVD, and one aimed at people with diabetes. We might speculate that people with a diagnosed condition (in this case diabetes) may be more motivated to change behaviour than people who consider themselves well; this seems intuitive, but studies included in this review were too weak to provide evidence either way. Although obesity is a clinically defined condition, many people who are obese do not consider themselves ill and may not have been motivated to change.
A defining feature of adaptive e-learning interventions delivered via the internet is that the user controls where, when and how often they use the tool. In view of this, users who are already highly motivated to change may be more likely to use the intervention as well as more likely to achieve change. Being diagnosed with a disease can often trigger a strong emotional reaction leading to a desire to change, which may not be triggered merely by being informed about having a risk factor. In contrast, while opting into an e-learning intervention delivered via mobile technologies requires motivation, ongoing programs may be sent to participants and do not require the ongoing motivation of individuals to seek the intervention. There were only two mobile technology-based interventions in this review. Further research may be warranted to explore whether or not the effects of mobile technology-based interventions are different from those of web-based interventions. One area in which further research may be warranted is exploring whether or not adaptive e-learning tools are better suited for disease management than for health promotion.
Target behaviours
The statistical meta-analyses demonstrated a small effect of increasing fruit and vegetable consumption, but no effect on reducing fat or energy intake or increasing fibre. It is worth speculating as to why this may be so. One possible reason is that it is easier to eat more of something seen as beneficial (and enjoyable), in this case fruit and vegetables, than to eat less of something seen as harmful. Eating less of something may be viewed as self-denial or self-punitive. Further research into people’s views and experiences of dietary change may be useful.
Intervention content
A comparison of effective and ineffective interventions did not show an association with content (which behaviour change techniques were included in the intervention; see Appendix 8). There was unexplained heterogeneity in the effects of intervention on several outcomes, including fruit and vegetable intake, fat intake and weight. However, our original study aim did not include an analysis of the impact of intervention content on statistical heterogeneity. It is possible that unexplained heterogeneity may be related to differences in intervention content, and we are planning further statistical analyses to explore this in a future publication.
Theoretical base
Of the 43 interventions, 2643,44,48–50,52,54–56,59,60,62,64–69,71,72,74,75,78–80,83 mentioned one or more behaviour theories and only eight44,56,68,69,72,75,78,83 of these analysed theoretically predicted mediators. This is of concern, given the importance of investigating not just whether or not interventions work, but also how they work. Understanding the mechanisms of action of interventions is key to developing more effective interventions. 117 Only one-third of interventions that are said to be theory based measure theoretically predicted mediators; without this, the statement that the intervention is theory based has limited scientific value. 118 Of the 13 effective interventions,44,51,56,61,68–70,72,75,76,78,82,83 eight44,56,68,69,72,75,78,83 mentioned one or more theories and only two44,68 of these analysed theoretically predicted mediators. Six studies56,69,72,75,78,83 measured putative mediators, but did not analyse their mediational role, only changes in the variables over time.
The first mediational analysis was within a trial44 of a theory-based intervention that included behaviour change techniques of planning and evaluation of goals. These were hypothesised on the basis of social cognitive theory to change self-efficacy and physical and social outcome expectations, which were in turn predicted to change behaviour. The intervention increased consumption of fibre and fruit and vegetables, and decreased fat consumption. This effect was mediated by two of the targeted constructs: self-efficacy and physical outcome expectancies.
The second mediational analysis was within a trial68 of a tailored information intervention which used theory, the Precaution Adoption Process Model, to select intermediate outcomes to measure. The theory identifies awareness of personal risk behaviour as an important step towards behaviour change. The intervention found no effects on fat or fruit intake and very small effects on vegetable intake and self-rated fat intake. It also found no difference on the theoretically derived mediators – awareness of personal fat, fruit and vegetable intakes. Although not theoretically based, the authors stated that they expected the tailored intervention to be mediated by perceived personal relevance, individualisation and interest: the first two were found to mediate, but on self-rated fat intake only.
Change scores, with no tests of mediation, were found for four of the six studies in which they were measured: self-efficacy to change diet,72 knowledge,76 ‘stages’ of change,56,72 and self–regulation strategies, although these were not associated with healthy eating. 82
In conclusion, only one44 of the 13 effective interventions45,51,56,61,68–70,72,75,76,78,82,83 drew on theory to identify intervention targets, measured the constructs theoretically hypothesised to bring about change and conducted a mediational analysis to evaluate whether or not intervention effects occurred as theoretically predicted. The intervention targeted self-efficacy and outcome expectancies and central constructs within social cognitive theory, and changed dietary behaviour at least partly by changing these constructs. We therefore have theoretically informed findings that we can apply to future intervention development from one of the studies reviewed.
Mode of delivery
As described earlier, the most common mode of delivery was the internet, followed by CD-ROM and computer kiosks. Mode of delivery did not appear to be related to effectiveness, with both effective and ineffective interventions delivered by the internet, CD-ROM and computer kiosks.
‘Dose’ of intervention
The intended duration and intensity of the intervention varied widely, from one-off single sessions lasting 20 minutes51 to repeated exposures (maximum was 30 minutes monthly for 12 months = 6 hours). 83 Where interventions were delivered over the internet, however, it was often not possible to determine how participants had actually used the intervention. For this reason it was not possible to determine whether or not there was a relationship between ‘dose’ of the intervention and effectiveness, nor could we even speculate on the nature of any relationship (e.g. threshold effect or linear relationship).
Study quality
According to the EPHPP study assessment method, there were 30 low-quality trials (global rating ‘weak’)42,44–49,51–53,55–59,61–64,67,68,70,72,75–78,81,82,84 and 13 medium-quality trials (global rating ‘moderate’). 43,50,54,60,65,66,69,71,73,74,79,80,83 Only two trials69,83 reporting a positive effect were of medium quality and 11 were of low quality. 44,51,56,61,68,70,72,75,76,78,82 Of the trials reporting no effect or a negative effect, 11 were of medium quality43,50,54,60,65,66,71,73,74,79,80 and 20 were of low quality. 42,45–49,51–53,55,57–59,62–64,67,77,81,84 It has previously been established that low trial quality is associated with an overestimation of effects. Specifically, effect estimates have been found to be higher where there is no allocation concealment or no blinding. 118 Further, it has been shown that effect estimates are higher in systematic reviews in which few trials report the same outcome than in systematic reviews in which a high proportion of trials report the same outcomes. 119 This is owing to selective reporting of outcomes in trials in which no statistically significant benefit is found. Our statistical assessment of the evidence suggests that trials with lower methodological quality (i.e. higher risk of bias owing to allocation concealment methods used and ‘weak’ EPHPP rating of study quality) may overestimate intervention effects.
Can e-learning interventions change dietary behaviour?
There are many factors affecting what foods people eat and why, and an intervention targeted at individual behaviour change can address only a selection of these. An individual’s food environment is composed of multiple connected factors acting at the macro level (e.g. national legislation and policy, regulation of food processers and vendors), the physical environment (access to and availability of healthy and unhealthy foods at schools/home/work, in shops, etc.) and the social environment (cultural food practices, social norms, role models). 120
Factors at all levels play a major role in determining whether or not individuals and populations consume a healthy diet. These factors can easily outweigh attempts by an individual to alter dietary behaviour.
E-learning has not proved itself to be more effective or cost-effective than other behaviour change approaches at an individual level for improving diet, or for preventing or reducing overweight or obesity. Nor is there any research comparing e-learning approaches with population-level approaches to tackling dietary change or reduce obesity. Some policy and population-level interventions, such as fat taxes or fruit and vegetable subsidies suggest that a carefully targeted tax on unhealthy foods could produce a modest but significant impact on population-level dietary intake and CVD that would be cost-effective. 121 However, a recent systematic review (produced for the World Health Organization) found that few policy and environmental interventions for diet have been properly evaluated in peer-reviewed studies. 122
Are e-learning interventions cost-effective to the NHS?
The review did not identify any published economic evaluations that perfectly fitted the project scope. That is, none was identified that assessed the cost-effectiveness of devices that were based purely on changing dietary behaviour. However, one recently published UK RCT-based study93 was identified that included a non-dietary component (physical exercise). In the base case, the results showed that the e-learning device was not cost-effective compared with standard care given the relatively large costs associated with the device. Indeed, although the results were not statistically significant, WMDs in weight loss were lower in the intervention arm and the costs were higher.
An economic model (E-LEEM) was built to assess the cost-effectiveness of the devices compared with nutritional advices alone delivered using standard approaches as a method of promoting weight loss. The results broadly suggest that the e-learning devices are unlikely to be cost-effective at conventional levels of £20,000–30,000 per QALY gained; the probability they are cost-effective was ≯ 25% at these threshold levels. A result that is perhaps not unsurprising given the relatively modest effects derived from the systematic review and meta-analysis. However, the results were sensitive to the assumptions regarding the initial fixed cost of the devices, lowering this value dramatically increased the cost-effectiveness of the devices.
What is the potential population health impact of e-learning interventions?
To what extent do statistical effects translate into health impact?
The evidence available suggests that e-learning interventions are not effective in changing dietary behaviours, apart from a possible small effect on increasing fruit and vegetable intake.
Fruit and vegetables
Dietary recommendations suggest five servings of fruit and vegetables per person per day; currently adults in the UK (aged 19–65 years) are eating on average 4.4 servings,123 so an increase of a quarter of a serving would still not raise the average intake to meet the guidelines.
Fat
Dietary recommendations suggest that no more than 35% of calories consumed should come from total fat, and ≯ 11% of calories from saturated fats. Currently, UK adults consume an average of 34–36% of energy from total fat, and 12.8% of energy from saturated fat,123 so a reduction of 1% would not facilitate achievement of guideline targets.
Fibre
Recommendations suggest an intake of 18 g of dietary fibre per day, with current intake in UK adults at around 14 g. 123 An increase of 1.5 g would therefore not facilitate achievement of the guideline.
Possible reasons for this lack of effect include that individual behavioural determinants may be relatively unimportant compared with organisational, environmental, economic and sociocultural factors; lack of adequate theoretical underpinning of theory; lack of clarity about potential target audiences; no perceived need for change by users; and high attrition rates leading to ineffective doses.
Budget impact analysis
If it assumed that e-learning devices are not currently provided by the NHS, then the estimated budget impact is zero, as the clinical and economic evidence suggests that they should not be used.
Limitations
The limitations of this review include potential reporting bias, incomplete retrieval of completed research studies and data extraction errors. It has been shown that trials showing no, or negative, effects are less likely to be offered for publication, and if offered are less likely to be accepted, resulting in a biased set of data available for review. Although every effort was made to conduct exhaustive electronic searches and to contact authors and researchers for further trials, it is possible that some studies were missed. Furthermore, it is possible that some papers were misclassified as not eligible for inclusion in the review. For some full-text reports that were retrieved it is possible that some data were incorrectly entered onto the review database, although some automatic controls were built into the database and data were double-checked in order to avoid extraction errors.
Further, it has been shown that intervention protocols document more behaviour change tools than are reported in study publications. However, we were unable to obtain the vast majority of intervention protocols. There was unexplained heterogeneity in trial results; our exploration of potential sources of heterogeneity was not exhaustive, owing to a limited number of studies in the review (and hence power). It is possible that individual trials reporting statistically significant results (with intervention effect sizes that would be of public health importance) are genuinely more effective than the pooled estimates of effects. Considering the heterogeneity in interventions, participants and outcomes, it might be considered inappropriate to pool the results of the included studies. In this systematic review, the decision to pool results using random effects meta-analysis was pre-specified in the study protocol. It may have been useful to plan an additional fixed effects meta-analysis, as a sensitivity analysis. However, in this review the use of random effects meta-analysis may be judged reasonable given the presence of substantial statistical heterogeneity among the intervention effects observed in different studies.
One final potential limitation for UK health purposes was that none of the studies were undertaken in the UK, although all were undertaken in similarly developed countries with functioning health systems. The context in which a complex intervention is used can be important, and a change in context may alter its effectiveness. The majority of the included studies were conducted in the USA, but as eating habits in the USA and UK are not dissimilar, it would be reasonable to consider that the overall findings are broadly generalisable to the UK.
Chapter 7 Conclusions
Effectiveness
Despite the availability of several good trials of e-learning interventions for dietary behaviour change, the evidence for effectiveness on dietary behaviours is weak. Although the pooled effects of interventions showed no effects of public health importance, there was high and unexplained heterogeneity in many trial results.
Neither the design nor the targeting of e-learning interventions can yet be informed by available evidence; the implication of these conclusions for policy and practice is that such interventions should not be introduced into routine practice at present. E-learning for dietary behaviour change may, however, have potential as one approach within wider intervention programmes to tackle poor diet and obesity in the population.
Cost-effectiveness
While the published evidence base was limited, the results from the modelling exercise suggest that e-learning devices to promote dietary behaviour change are unlikely to be cost-effective at conventional UK cost per additional QALY thresholds, unless they are much less costly to provide than assumed in the analysis, particularly the initial (fixed) setup costs. The current clinical and economic evidence base suggests that e-learning devices designed to promote dietary behaviour change will not produce clinically significant changes in dietary behaviour, and are at least as expensive as other individual behaviour change interventions.
Recommendations for future research
We identified 43 trials of e-learning for dietary behaviour change, and there are many more studies trialling e-learning interventions for altering different health behaviours. Despite the relatively high EVPI results from the cost-effectiveness modelling, we believe the implication for research is that further clinical trials of individual e-learning interventions should not be undertaken until theoretically informed work, which addresses the question of which characteristics of the target population, target behaviour, content and delivery of the intervention are likely to lead to positive results, is completed. This work would include:
-
reviews of available behaviour change theoretical frameworks and the empirical data to support each approach
-
research in behaviour change techniques (linking theory to techniques) to provide empirical data to help understand which techniques are effective, and under which conditions
-
cohort and other study designs which actively map and explore the pathways of change in outcomes among users of the intervention.
Acknowledgements
We would like to acknowledge the help of Ms Gabi Meineke and Ms Yvonne Gritschneder for their assistance with translation of text from German to English; Ms Soyun Jung for assistance with a Korean translation; and Ms Yusuke Shimakawa for assistance with a Japanese translation. Ms Eugenia Priedane assisted with the retrieval of some of the full-text reports.
Contributions of authors
Jody Harris (Research Assistant, Systematic Reviews in Nutrition) undertook screening, reviewing and data extraction, and produced the various tools to be used in the review. She also provided nutrition expertise, co-ordinated the writing of the report and publication of the study protocol.
Lambert Felix (Research Assistant, Systematic Reviews) undertook screening, reviewing and data extraction, and produced the various tools to be used in the review. He also undertook all database searching and contributed to the writing and editing of the review report.
Alec Miners (Lecturer, Health Economics) designed the economic model and undertook the economic analysis. He wrote the chapter on economic evaluation.
Elizabeth Murray (Reader, Primary Care) contributed materially to the design of the conceptual framework and the systematic review methods. She provided clinical expertise and contributed to the writing and editing of the review report.
Susan Michie (Professor, Health Psychology) contributed materially to the design of the conceptual framework and the systematic review methods. She provided expertise and guidance regarding behavioural theories to be investigated, and contributed to the writing and editing of the review report.
Elaine Ferguson (Senior Lecturer, Nutrition) provided expertise and guidance regarding nutrition tools and outcomes. She contributed to the writing and editing of the review report.
Caroline Free (Clinical Lecturer, Public Health and M-Health) contributed materially to the design of the conceptual framework and the systematic review methods.
Karen Lock (Senior Lecturer, Public Health) contributed materially to the design of the conceptual framework and the systematic review methods. She contributed to the writing and editing of the review report.
Jane Landon (Deputy Chief Executive, National Heart Forum) represented user groups in the design of the review and framing of the results.
Phil Edwards (Senior Lecturer, Statistics) managed the project, provided expertise in the design of the systematic review and designed and undertook the statistical analysis. He contributed to the writing and editing of the review report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Balcombe NR, Ferry PG, Saweirs WM. Nutritional status and well being. Is there a relationship between body mass index and the well-being of older people?. Curr Med Res Opin 2001;17:1-7.
- World Health Organization (WHO) . Reducing Risks, Promoting Healthy Life 2002.
- World Cancer Research Fund . American Institute of Cancer Research; Food, Nutrition and Physical Activity and the Prevention of Cancer: A Global Perspective 2007.
- Rolls BJ, Shide DJ. The influence of dietary fat on food intake and body weight. Nutr Rev 1992;50:283-90.
- Willet WC. Diet and health: What should we eat?. Science 1994;264:532-8.
- World Health Organization (WHO) . Diet, Nutrition, and the Prevention of Chronic Diseases 2003.
- Department of Health . Choosing Health: Making Healthier Choices Easier 2004.
- Lock K, Pomerleau J, Causer L, Altman DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ 2005;83:100-8.
- Hoare A, Henderson L, Bates CJ, Prentice A, Birch A, Swan G, et al. National Diet and Nutrition Survey: Adults Aged 19–64 Years. Summary Report 2004.
- Department of Health . Choosing a Better Diet: A Food and Health Action Plan 2005.
- Wanless D. Securing our future health: Taking a long-term view. London: HM Treasury; 2002.
- Wanless D. Securing good health for the whole population. London: HM Treasury; 2004.
- Brug J, Oenema A, Kroeze W, Raat H. The internet and nutrition education: challenges and opportunities. Eur J Clin Nutr 2005;59:S130-9.
- Martin Gould SM, Anderson J. Using interactive media nutrition education to reach low-income persons: an effectiveness evaluation. J Nutr Educ 2000;32:204-13.
- Tate DE, Jackvony EH, Wing RR. Effects of internet behavioral conuselling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA 2003;289:1833-6.
- Oenema A, Brug J, Lechner L. Web-based tailored nutrition education: results of a randomized controlled trial. Health Educ Res 2001;16:647-60.
- Eng TR. The eHealth landscape: a terrain map of emerging information and communication technologies in health and health care. Princeton, NJ: Robert Wood Johnson Foundation; 2001.
- Office for National Statistics . Statistical Bulletin: Internet Access. Households and Individuals 2009.
- Kanuga M, Rosenfeld WD. Adolescent sexuality and the internet: the good, the bad, and the URL. J Pediatr Adolesc Gynecol 2004;17:117-24.
- Patrick K, Robinson TN, Alemi F, Eng TR. the Science Panel on Interactive Communication and Health . Policy issues relevant to evaluation of interactive health communication applications. Am J Prev Med 1999;16:35-42.
- Williams P, Nicholas D, Huntington P, McClean F. Surfing for health: user evaluation of a health information web site. Part 1, Literature review. Health Inform Libr J 2002;19:98-108.
- Brug J, Oenema A, Campbell MK. Past, present, and future of computer-tailored nutrition education. Am J Clin Nutr 2003;77:S1028-34.
- Murray E. Internet-delivered treatments for long-term conditions: strategies, efficiency and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res 2008;8:261-72.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;2. 10.1002/14651858.CD001118.pub2.
- Stretcher VJ. Computer-tailored smoking cessation materials: a review and discussion. Patient Educ Counsel 1999;36:107-17.
- Skinner CS, Campbell MK, Rimer BK, Curry S, Prochaska JO. How effective is tailored print communication?. Ann Behav Med 1999;21:290-8.
- Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring health messages: customizing communication with computer technology. Mahwah, NJ: Lawrence Erlbaum Associates; 2000.
- Neville LM, O’Hara B, Milat AJ. Computer-tailored dietary behaviour change interventions: a systematic review. Health Educ Res 2009;24:699-720.
- Edwards P, Felix L, Harris J, Ferguson E, Free C, Landon J, et al. Assessing the effectiveness and cost effectiveness of adaptive e-Learning to improve dietary behaviour: protocol for a systematic review. BMC Public Health 2010;10.
- Valdez A, Banerjee K, Ackerson L, Fernandez M. A multimedia breast cancer education intervention for low-income Latinas. J Community Health 2002;27:33-51.
- Cashen MS, Sklar BM, Nguyen HH, Just M, Galzagorry G, Bakken S. Implementing a web-based information resource at an inner-city community church: lessons learned. Comput Inform Nurs 2002;20:244-50.
- Dunham PJ, Hurshman A, Litwin E, Gusella J, Ellsworth C, Dodd PW. Computer-mediated social support: single young mothers as a model system. Am J Community Psychol 1998;26:281-306.
- Kroeze W, Werkman A, Brug J. A systematic review of randomized trials on the effectiveness of computer-tailored education on physical activity and dietary behaviors. Ann Behav Med 2006;31:205-23.
- Norman GJ, Zabinski MF, Adams MA, Rosenberg DE, Yaroch AL, Atienza AA. A review of eHealth interventions for physical activity and dietary behavior change. Am J Prev Med 2007;33:336-45.
- Higgins JPT, Altman DG, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2: The Cochrane Collaboration. 2009.
- Effective Public Health Practice Project (EPHPP) . Quality Assessment Tool for Quantitative Studies 2009 n.d. www.ephpp.ca/tools.html (accessed 2009).
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87.
- Armitage CJ, Conner M. Social cognition models and health behaviour: A structured review. Psychol Health 2000;15:173-89.
- Ajken I. The theory of planned behaviour. Organ Behav Hum Decis Process 1991;50:179-211.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- Agras SW, Barr Taylor C, Feldman DE, Losch M, Burnett KF. Developing computer-assisted therapy for the treatment of obesity. Behav Ther 1990;21:99-109.
- Alexander GL, McClure JB, Calvi JH, Divine GW, Stopponi MA, Rolnick SJ, et al. A randomized clinical trial evaluating online interventions to improve fruit and vegetable consumption. Am J Public Health 2010;100:319-26.
- Anderson ES, Winett RA, Wojcik JR, Winett SG, Bowden T. A computerized social cognitive intervention for nutrition behavior: direct and mediated effects on fat, fiber, fruits, and vegetables, self-efficacy, and outcome expectations among food shoppers. Ann Behav Med 2001;23:88-100.
- Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr 2008;27:280-6.
- Blanson Henkemans OA, van der Boog PJ, Lindenberg J, van der Mast CA, Neerincx MA, Zwetsloot-Schonk BJ. An online lifestyle diary with a persuasive computer assistant providing feedback on self-management. Technol Health Care 2009;17:253-67.
- Block G, Wakimoto P, Metz D, Fujii ML, Feldman N, Mandel R, et al. A randomized trial of the Little by Little CD-ROM: demonstrated effectiveness in increasing fruit and vegetable intake in a low-income population. Prev Chronic Dis 2004;1.
- Buller DB, Woodall WG, Zimmerman DE, Slater MD, Heimendinger J, Waters E, et al. Randomized trial on the 5 a day, the Rio Grande Way Website, a web-based program to improve fruit and vegetable consumption in rural communities. J Health Commun 2008;13.
- Campbell MK, Honess-Morreale L, Farrell D, Carbone E, Brasure M. A tailored multimedia nutrition education pilot program for low-income women receiving food assistance. Health Educ Res 1999;14:257-67.
- Campbell MK, Carbone E, Honess-Morreale L, Heisler-Mackinnon J, Demissie S, Farrell D. Randomized trial of a tailored nutrition education CD-ROM program for women receiving food assistance. J Nutr Educ Behav 2004;36:58-66.
- Carbone E. LearnSmart: The Application of Adult Learning Theories to Nutrition Education 1999.
- Cook RF, Billings DW, Hersch RK, Back AS, Hendrickson A. A field test of a web-based workplace health promotion program to improve dietary practices, reduce stress, and increase physical activity: randomized controlled trial. J Med Internet Res 2007;9.
- Cussler EC, Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, et al. Maintenance of weight loss in overweight middle-aged women through the internet. Obesity 2008;16:1052-60.
- De Bourdeaudhuij I, Stevens V, Vandelanotte C, Brug J. Evaluation of an interactive computer-tailored nutrition intervention in a real-life setting. Ann Behav Med 2007;33:39-48.
- Delichatsios HK, Friedman RH, Glanz K, Tennstedt S, Smigelski C, Pinto BM, et al. Randomized trial of a “talking computer” to improve adults’ eating habits. Am J Health Promot 2001;15:215-24.
- Di Noia J, Contento IR, Prochaska JO. Computer-mediated intervention tailored on transtheoretical model stages and processes of change increases fruit and vegetable consumption among urban African-American adolescents. Am J Health Promot 2008;22:336-41.
- Ellrott T. Self-help programme for weight reduction with and without support by a handheld nutrition computer. Ernahrungs Umschau 2005;52:392-7.
- Franko DL, Cousineau TM, Trant M, Green TC, Rancourt D, Thompson D, et al. Motivation, self-efficacy, physical activity and nutrition in college students: randomized controlled trial of an internet-based education program. Prev Med 2008;47:369-77.
- Gow RW, Trace SE, Mazzeo SE. Preventing weight gain in first year college students: an online intervention to prevent the ’freshman fifteen’. Eat Behav 2010;11:33-9.
- Haerens L, Deforche B, Maes L, Brug J, Vandelanotte C, De Bourdeaudhuij I. A computer-tailored dietary fat intake intervention for adolescents: Results of a randomized controlled trial. Ann Behav Med 2007;34:253-62.
- Huang A, Barzi F, Huxley R, Denyer G, Rohrlach B, Jayne K, et al. The effects on saturated fat purchases of providing internet shoppers with purchase-specific dietary advice: a randomised trial. PLOS Clin Trial 2006;1.
- Irvine AB, Ary DV, Grove DA, Gilfillan-Morton L. The effectiveness of an interactive multimedia program to influence eating habits. Health Educ Res 2004;19:290-305.
- Jacobi C, Morris L, Beckers C, Bronisch-Holtze J, Winter J, Winzelberg AJ, et al. Maintenance of internet-based prevention: a randomized controlled trial. Int J Eat Disord 2007;40:114-19.
- Jones M, Luce KH, Osborne MI, Taylor K, Cunning D, Doyle AC, et al. Randomized, controlled trial of an internet-facilitated intervention for reducing binge eating and overweight in adolescents. Pediatrics 2008;121:453-62.
- Kroeze W, Oenema A, Campbell M, Brug J. The efficacy of web-based and print-delivered computer-tailored interventions to reduce fat intake: results of a randomized, controlled trial. J Nutr Educ Behav 2008;40:226-36.
- Low KG, Charanasomboon S, Lesser J, Reinhalter K, Martin R, Jones H, et al. Effectiveness of a computer-based interactive eating disorders prevention program at long-term follow-up. Eat Disord 2006;14:17-30.
- Oenema A, Brug J, Lechner L. Web-based tailored nutrition education: results of a randomized controlled trial. Health Educ Res 2001;16:647-60.
- Oenema A, Tan F, Brug J. Short-term efficacy of a web-based computer-tailored nutrition intervention: main effects and mediators. Ann Behav Med 2005;29:54-63.
- Oenema A, Brug J, Dijkstra A, de Weerdt I, de Vries H. Efficacy and use of an internet-delivered computer-tailored lifestyle intervention, targeting saturated fat intake, physical activity and smoking cessation: a randomized controlled trial. Ann Behav Med 2008;35:125-35.
- Rothert K, Strecher VJ, Doyle LA, Caplan WM, Joyce JS, Jimison HB, et al. Web-based weight management programs in an integrated health care setting: a randomized, controlled trial. Obesity 2006;14:266-72.
- Shapiro JR, Reba-Harrelson L, Dymek-Valentine M, Woolson SL, Hamer RM, Bulik CM. Feasibility and acceptability of CD-ROM-based cognitive-behavioural treatment for binge-eating disorder. Eur Eat Disord Rev 2007;15:175-84.
- Sternfeld B, Block C, Quesenberry CP, Block TJ, Husson G, Norris JC, et al. Improving diet and physical activity with ALIVE: a worksite randomized trial. Am J Prev Med 2009;36:475-83.
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139-48.
- Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med 2006;166:1620-5.
- Trinh L. The effect of an online nutrition intervention on fruit and vegetable intake among women. Fullerton, CA: California State University; 2009.
- Turnin MC. Telematic Expert System Diabeto – new tool for diet self-monitoring for diabetic patients. Diabetes Care 1992;15:204-12.
- Turnin MC, Bourgeois O, Cathelineau G, Leguerrier AM, Halimi S, Sandre-Banon D, et al. Multicenter randomized evaluation of a nutritional education software in obese patients. Diabetes Metab 2001;27:139-47.
- Vandelanotte C, De Bourdeaudhuij I, Sallis JF, Spittaels H, Brug J. Efficacy of sequential or simultaneous interactive computer-tailored interventions for increasing physical activity and decreasing fat intake. Ann Behav Med 2005;29:138-46.
- Verheijden M, Bakx JC, Akkermans R, van den Hoogen H, Godwin NM, Rosser W, et al. Web-based targeted nutrition counselling and social support for patients at increased cardiovascular risk in general practice: randomized controlled trial. J Med Internet Res 2004;6.
- Veverka DV, Anderson J, Auld GW, Coulter GR, Kennedy C, Chapman PL. Use of the stages of change model in improving nutrition and exercise habits in enlisted Air Force men. Mil Med 2003;168:373-9.
- Winett RA, Wagner JL, Moore JF, Walker WB, Hite LA, Leahy M, et al. An experimental evaluation of a prototype public access nutrition information system for supermarkets. Health Psychol 1991;10:75-8.
- Winett RA, Anderson ES, Wojcik JR, Winett SG, Bowden T. Guide to health: nutrition and physical activity outcomes of a group-randomized trial of an Internet-based intervention in churches. Ann Behav Med 2007;33:251-61.
- Wylie-Rosett J, Swencionis C, Ginsberg M, Cimino C, Wassertheil Smoller S, Caban A, et al. Computerized weight loss intervention optimizes staff time: the clinical and cost results of a controlled clinical trial conducted in a managed care setting. J Am Diet Assoc 2001;101:1155-62.
- Zabinski MF, Pung MA, Wilfley DE, Eppstein DL, Winzelberg AJ, Celio A, et al. Reducing risk factors for eating disorders: targeting at-risk women with a computerized psychoeducational program. Int J Eat Disord 2001;29:401-8.
- Vandelanotte C, De Bourdeaudhuij I, Brug J. Acceptability and feasibility of an interactive computer-tailored fat intake intervention in Belgium. Health Promot Int 2004;19:463-70.
- Gibson RS. Principles of nutritional assessment. Oxford: Oxford University Press; 2005.
- Peterson KE, Hebert JR, Hurley TG, Resnicow K, Thompson FE, Greene GW, et al. Accuracy and precision of two short screeners to assess change in fruit and vegetable consumption among diverse populations participating in health promotion intervention trials. J Nutr 2008;138:218S-25S.
- Buzzard IM, Faucett CL, Jeffery RW, McBane L, McGovern P, Baxter J, et al. Monitoring dietary change in a low-fat diet intervention study: Advantages of using 24-hour dietary recalls vs food records. J Am Diet Assoc 1996;96:574-9.
- Thomson CA, Giuliano A, Rock CL, Ritenbaugh CK, Flatt SW, Faerber S, et al. Measuring dietary change in a diet intervention trial: Comparing food frequency questionnaire and dietary recalls. Am J Epidemiol 2003;157:754-62.
- Winzelberg AJ, Eppstein D, Eldredge KL, Wilfley D, Dasmahapatra R, Dev P, et al. Effectiveness of an internet-based program for reducing risk factors for eating disorders. J Consult Clin Psychol 2000;68:346-50.
- Winzelberg AJ, Taylor CB, Sharpe T, Eldredge KL, Dev P, Constantinou PS. Evaluation of a computer-mediated eating disorder intervention program. Int J Eat Disord 1998;24:339-49.
- Dalziel K, Segal L. Time to give nutrition interventions a higher profile: cost-effectiveness of 10 nutrition interventions. Health Promot Int 2007;22:271-83.
- McConnon A, Kirk SFL, Cockroft JE, Harvey EL, Greenwood DC, Thomas JD, et al. The internet for weight control in an obese sample: Results of a randomised controlled trial. BMC 2007;7.
- Meenan RT, Stevens VJ, Funk K, Bauck A. Development and implementation cost analysis of telephone- and internet-based interventions for the maintenance of weight loss. Int J Technol Assess Health Care 2009;25:400-10.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2008.
- Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A. Body Size and Risk of Colon and Rectal Cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Canc Inst 2006;98:920-31.
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8.
- Government Actuaries Department . Life Tables 2010. www.statistics.gov.uk/StatBase/Product.asp?vlnk=14459 (accessed July 2010).
- Volimink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HAW. Coronary events and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. Heart 1998;80:40-4.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8.
- NICE . CG67. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease 2008.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82.
- Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 2009;338.
- Stern MP, Williams MS, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test?. Ann Int Med 2002;136:575-81.
- Lindstom J, Tuomilehto J. The diabetes risk score. Diabetes Care 2003;26:725-31.
- NICE . CG43. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children 2006.
- Fine J, Colditz G, Coakeley E, Moselay G, Manson J, Willet W. A prospective study of weight change and health related quality of life in women. JAMA 1999;282:2136-42.
- Heitmann B, Garby L. Patterns of long-term weight changes in overweight developing danish men and women aged between 30 and 60 years. Int J Obes Relat Metab Disord 1999;23.
- Department of Health . Health Survey for England 2008 2008. www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles-related-surveys/health-survey-for-england/health-survey-for-england--2008-trend-tables (accessed 1 January 2010).
- Foxcroft DR. Orlistat for the treatment of obesity: cost utility model. Obes Rev 2005;6:323-8.
- Warren E, Brennan A, Akehurst R. Cost-effectiveness of sibutramine in the treatment of obesity. Med Decis Making 2004;24:9-19.
- Ara R, Brennan A. The cost-effectiveness of sibutramine in non-diabetic obese patients: Evidence from four Western countries. Obes Rev 2007;8:363-71.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2010.
- Macran S. The relationship between body mass index and health-related quality of life. York: University of York; 2004.
- TreeAge Software . TreeAge Pro 2009 user’s Manual 2009.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660-80.
- Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol 2010;29:1-8.
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42-6.
- Furukawa TA, Watanabe N, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta-analyses. JAMA 2007;297:468-70.
- Chow CK, Lock K, Teo K, Subramanian SV, McKee M, Yusuf S. Environmental and societal influences acting on cardiovascular risk factors and disease at a population level: a review. Int J Epidemiol 2009;38:1580-94.
- Mytton O, Gray A, Rayner M, Rutter H. Could targeted food taxes improve health?. J Epidemiol Community Health 2007;61:689-94.
- World Health Organization (WHO) . Interventions on Diet and Physical Activity: What Works. Summary Report 2009.
- Food Standards Agency, Department of Health . National Diet and Nutrition Survey: Headline Results from Year 1 of the Rolling Programme (2008 2009) 2009.
Appendix 1 Research protocol
This protocol was previously published in Edwards P, et al. Assessing the effectiveness and cost effectiveness of adaptive e-Learning to improve dietary behaviour: protocol for a systematic review. BMC Public Health 2010;10:200.
Assessing the effectiveness and cost effectiveness of adaptive e-Learning to improve dietary behaviour: protocol for a systematic review
Abstract
Background
The composition of habitual diets is associated with adverse or protective effects on aspects of health. Consequently, UK public health policy strongly advocates dietary change for the improvement of population health and emphasises the importance of individual empowerment to improve health. A new and evolving area in the promotion of dietary behavioural change is e-Learning, the use of interactive electronic media to facilitate teaching and learning on a range of issues, including diet and health. The aims of this systematic review are to determine the effectiveness and cost-effectiveness of adaptive e-learning for improving dietary behaviours.
Methods/Design
The research will consist of a systematic review and a cost-effectiveness analysis. Studies will be considered for the review if they are randomised controlled trials, involving participants aged 13 or over, which evaluate the effectiveness or efficacy of interactive software programs for improving dietary behaviour. Primary outcome measures will be those related to dietary behaviours, including estimated intakes of energy, nutrients and dietary fibre, or the estimated number of servings per day of foods or food groups. Secondary outcome measures will be objective clinical measures that are likely to respond to changes in dietary behaviours, such as anthropometry or blood biochemistry. Knowledge, self-efficacy, intention and emotion will be examined as mediators of dietary behaviour change in order to explore potential mechanisms of action. Databases will be searched using a comprehensive four-part search strategy, and the results exported to a bibliographic database. Two review authors will independently screen results to identify potentially eligible studies, and will independently extract data from included studies, with any discrepancies at each stage settled by a third author. Standardised forms and criteria will be used.
A descriptive analysis of included studies will describe study design, participants, the intervention, and outcomes. Statistical analyses appropriate to the data extracted, and an economic evaluation using a cost-utility analysis, will be undertaken if sufficient data exist, and effective components of successful interventions will be investigated.
Discussion
This review aims to provide comprehensive evidence of the effectiveness and cost-effectiveness of adaptive e-Learning interventions for dietary behaviour change, and explore potential psychological mechanisms of action and the effective components of effective interventions. This can inform policy makers and healthcare commissioners in deciding whether e-Learning should be part of a comprehensive response to the improvement of dietary behaviour for health, and if so which components should be present for interventions to be effective.
Background
The need for improved dietary behaviour
The composition of habitual diets is associated with adverse or protective effects on health [1–3]. Specifically, diets high in saturated fats and sodium have been found to increase risk of cardiovascular diseases, while those high in fruit and vegetables and low in saturated fats have been linked with reductions in a range of diseases including certain cancers, cardiovascular disease and hypertension [4–7]. The WHO reports that the consumption of up to 600 g per day of fruit and vegetables could reduce the total worldwide burden of disease by 1.8%, and reduce the burden of ischaemic heart disease and ischaemic stroke by 31% and 19% respectively [8]. In the UK, the consumption of fruits and vegetables, dietary fibre, iron (pre-menopausal women only) and calcium are well below recommendations, whereas intakes of saturated fats and sodium exceed recommendations in large sections of the population [9]. Consequently, UK public health policy strongly advocates dietary change for the improvement of population health and emphasises the importance of individual empowerment to improve health [7, 10], thereby shifting the focus of the National Health Service from treatment to prevention of illness [11–12].
Adaptive e-Learning via interactive computerised interventions
A new and evolving area in the promotion of dietary behavioural change is e-Learning, the use of interactive electronic media to facilitate teaching and learning on a range of issues including health (see Additional file 1 for definitions of terms used in e-Learning). E-Learning has grown out of recent developments in information and communication technology, such as the Internet, interactive computer programs, interactive television, and mobile phones [13–17], technologies which are fast becoming more accessible to the general population. (For example, an estimated 70% of the population in the UK has access to the Internet and this percentage is likely to continue to grow [18].) This high level of accessibility with emerging advances in computer processing power, data transmission and data storage makes interactive e-Learning a potentially powerful and cost-effective medium for improving dietary behaviour [19–21]. It also has a number of distinct advantages compared with traditional approaches for the promotion of dietary behaviour change, such as the possibility of tailoring to individual circumstances [22], translating complex information through video, graphics, and audio systems [23], and potential cost savings on face-to-face interventions involving healthcare practitioners. The evidence that individualised, tailored e-Learning approaches are more effective than traditional non-tailored interventions [24] has given them a promising lead in health education [25–27]. E-Learning interventions have been classified into three generations: 1st generation interventions use computers to tailor print materials; 2nd generation interventions use interactive technology delivered on computers; and 3rd generation interventions use portable devices such as mobile phones, for more immediate interaction and feedback [28]. Exploration of the properties of different e-Learning interventions is now required in order to determine possible effective components (with each component comprising both delivery and content – see Figure 1). Potential cognitive and emotional mediators of dietary behaviour change should also be explored, in order to elicit potential mechanisms of action (see Figure 2).
There is a risk that e-Health and use of new technologies in health care might widen health inequalities on either side of the ‘digital divide’. Experience suggests that there are two dimensions to the digital divide and its impact on health inequalities: access (to physical hardware and software) and accessibility (or the ability of people with differing literacy/health literacy/IT literacy to use or apply information and support supplied through e-Learning). It has been shown that it is possible to deliver e-health interventions specifically designed for people with low literacy skills (e.g. Hispanics in Southern USA [29], homeless drug users [30] and single teenage mothers [31]). What remains less clear is the extent to which people with low literacy skills will feel comfortable using a computer, or will be able to act on information or advice provided over the Internet.
Interactive e-Leaning programs to promote positive dietary behavioural changes have the potential to benefit population health. However, before e-Learning can be hailed as a dietary behaviour change intervention of the future, the effective components and mechanisms of action of e-Learning programmes must be identified, and its cost-effectiveness established in different contexts.
Previous reviews
Three systematic reviews have examined the effectiveness of e-Learning for dietary behaviour change. The first [32] was restricted to first-generation interventions for dietary change and did not include any web or Internet-based interventions. The second [33] examined a broad range of second-generation interactive interventions for dietary behaviour change. Both of these reviews reported studies published prior to 2006 that were carried out in a variety of settings. The third review [28] was more recent, reviewing second- and third-generation interventions trialled up to 2008, but only in primary prevention in adults (no participants with diagnosed disease). All reviews were restricted to publications in the English language, and limited their searches to relatively few databases, increasing the potential for publication bias. The conclusions drawn from these systematic reviews were that e-learning shows some promise for dietary behaviour change, although the findings were mixed. Inter-study heterogeneity with respect to study design, participants, measures, and outcomes precluded meta-analysis to estimate pooled intervention effects. Moreover, the cost-effectiveness of e-Learning was not evaluated in any review, nor was there any attempt to identify potential mechanisms of action. The third review assessed internal and external validity of trials, and began to isolate effective components.
Our review will provide a comprehensive and up-to-date account of e-Learning technologies in use for promoting dietary behavioural change, and an evaluation of their effectiveness and cost-effectiveness in improving dietary behaviour as well as clinical outcomes. We will investigate the psychological theories that underlie the process of behaviour change [34–36], and look for key behaviour-change techniques that have been shown to be associated with healthy eating behaviours [37]. Where these have been used to inform intervention design in trials, we will explore potential mediators of behaviour such as knowledge, intention, self-efficacy and emotions with a view to understanding mechanisms of action. We will also explore the different components of trialled interventions, in order to find the effective components of successful e-Learning interventions for dietary change.
We will use a systematic search strategy (described below) to identify relevant studies and to reduce the potential for reporting biases, and use wider inclusion criteria than in previous reviews to enable a wider range of conclusions to be drawn. Preliminary literature searching, including the NHS’s Economic Evaluation Database, suggests that the published evidence on cost-effectiveness is extremely limited. Therefore, we will conduct a de novo economic evaluation of the intervention studies, looking at cost-effectiveness in England and Wales, if the required clinical effectiveness data are available from the primary trials. We will conclude with policy recommendations and recommendations for future primary research.
Aims of the review
The aims of this systematic review are to determine the effectiveness and cost-effectiveness of adaptive e-Learning for improving dietary behaviours. The specific objectives are to:
-
describe the range of e-Learning technologies in use for promoting dietary behavioural change
-
evaluate interactive e-Learning effectiveness in terms of improvement in dietary behaviour and clinical outcomes
-
explore the properties of different e-Learning interventions in order to determine possible effective components of successful e-Learning interventions for dietary behaviour change
-
investigate potential explanations of dietary behaviour change, and mechanisms of action
-
evaluate cost-effectiveness compared with current standard interventions, and likely budget impact in England & Wales.
Final outputs will be a report to the UK National Institutes of Health Research (NIHR) Health Technology Assessment (HTA) programme, and a peer-reviewed paper.
Methods/Design
Design
The research will consist of a systematic review and a cost-effectiveness analysis.
Criteria for considering studies
Types of study – We will include randomised controlled trials (RCTs) for evidence of effectiveness, and economic evaluations for evidence of cost-effectiveness.
Types of population – Adolescents or adults aged 13 years and above who have participated in a study designed to evaluate the effectiveness of e-Learning to promote dietary behavioural change. We shall include all clinical conditions where dietary advice plays a major part in case management.
Types of intervention – Interventions will be included if they are interactive computer software programs that tailor output according to user input (second and third generation interventions). These include those where users enter personal data or make choices about information that alter pathways within programs to produce tailored material and feedback that is personally relevant. Users may interact with the programs as members of a small group, as well as individually. Programs should be available directly to users and allow independent access without the need for any expert facilitation.
Interventions will be excluded if they are: first-generation tailored ‘information only’ (e.g. providing a leaflet or PDF); simple information packages with no interactive elements; non-interactive mass media interventions (such as TV advertisements); interventions designed to be used with others’ help (e.g. teacher or health professional); interventions targeted at health professionals or teachers; computer-mediated delivery of individual health-care advice (e.g. online physicians); or electronic history-taking or risk assessment with no health promotion or interactive elements.
Outcome measures – We anticipate that most interventions will be aimed at dietary behaviours, and are unlikely to have followed participants long enough to obtain clinical changes. However, as dietary behaviour tends to be self-reported it is prone to error (e.g. recall bias). Biological outcomes on the other hand are more objective and also more important for modelling purposes. We will therefore use dietary behaviour as our primary outcome, but we will attempt to obtain data that allow us to model the relationship between behaviours and clinical changes.
Primary outcome measures – The primary outcome variables will be those related to dietary behaviours. They will include estimated intakes or changes in intake of energy, nutrients, dietary fibre, foods or food groups. The dietary assessment tools or techniques used to estimate dietary behaviour will be critically examined in terms of quality.
Secondary outcome measures – Objective measures that are likely to respond to changes in dietary behaviours and are associated with adverse clinical outcomes will be examined, including measurements of anthropometric status and blood biochemistry.
Other data – We will also seek data on economic outcomes, including costs of providing the intervention and costs to the individual user; data on unintended adverse consequences of the interventions; and process outcomes (e.g. usage data). Data relating to potential cognitive and emotional mediators of dietary behaviour will also be extracted. These will only be extracted if primary and/or secondary outcome data are available.
Identification of eligible studies and data extraction
Search strategy
We have designed a four-part search strategy: firstly, we will search electronic bibliographic databases for published work (see below for databases to be searched). Secondly, we will search the grey literature for unpublished work. Thirdly, we will search trials registers for ongoing and recently completed trials. Finally, we will search reference lists of published studies and contact authors and e-health research groups to check for more trials. All databases will be searched from 1990 (any studies conducted in the 1980s will be identified by searching the reference lists of included studies). There will be no restrictions by language. To ensure the review is reasonably up-to-date at reporting, the searches will be re-run immediately prior to analysis and further studies retrieved for inclusion. The search strategy comprises two concepts: computer/internet-based interventions, and dietary behaviour (see Additional file 2 for full search strategies at www.biomed.central.com/147-2458/10/200).
The databases that will be searched are CINAHL, Cochrane Library, Dissertation Abstracts, EMBASE, ERIC, Global Health, HEED, HMIC, MEDLINE, PsychInfo, and Web of Science.
Screening and review process
All studies identified through the search process will be exported to a bibliographic database (EndNote version X3) for de-duplication and screening. Two review authors will independently examine the titles, abstracts, and keywords of electronic records for eligibility according to the inclusion criteria above. Results of this initial screening will be cross-referenced between the two review authors, and full-texts obtained for all potentially relevant reports of trials. Full-texts of potentially eligible trials will go through a secondary screening by each reviewer using a screening form based on the inclusion criteria (see Additional file 3 at www.biomed.central.com/147-2458/10/200)) for final inclusion in the review, with disagreements resolved by discussion with a third author. Reference lists of all eligible trials will be searched for further eligible trials.
Data extraction
Two review authors will independently extract relevant data using a standardised data extraction form (Additional file 4) in conjunction with a data extraction manual (Additional file 5). Trial managers will be contacted directly if the required data are not reported in the published study.
Analysis
Descriptive analysis
We will describe all studies that meet the inclusion criteria, including:
-
study design
-
trial design and quality
-
data collection methods, modes, and techniques; validity of tools
-
adherence to protocol (we will attempt to retrieve the protocols of eligible studies to examine the adherence to initial plans)
-
statistical and other analyses
-
conflict of interest
-
-
participants (intervention and control)
-
socioeconomic and demographic characteristics (e.g. age, ethnicity, education level)
-
health status: diagnosed disease (e.g. diabetes, cardiovascular disease, obesity) versus no diagnosed disease
-
technological literacy and access to technology
-
psychological characteristics (e.g. help seeking)
-
-
intervention
-
setting and recruitment methods
-
components of the intervention, including delivery and content
-
frequency, intensity and duration of the intervention
-
behaviour change theories employed in intervention design
-
-
outcomes
-
primary and secondary outcomes measured
-
information on process (ease of use) and usage (compliance).
-
Information on how access to the intervention was provided (e.g. free laptops/Internet access); the intended reading age (or other measure of technological literacy/skill required); and the sociodemographic characteristics of the participants will be used to address concerns over the digital divide. Where primary studies have included sub-group analyses of users with low-income or low educational status, we will note these. If sufficient data are provided by the primary studies we will consider undertaking sub-group analyses of intervention effects in low-income and low educational status users.
Statistical analysis
We will use statistical software (Stata version 11) for data synthesis. In the presence of sufficient homogeneity (i.e. comparable population, interventions and outcomes) we will pool the results of RCTs using a random-effects model, with standardised mean differences (SMDs) for continuous outcomes and odd ratios for binary outcomes, and calculate 95% confidence intervals and two sided p-values for each outcome. In studies where the effects of clustering have not been taken into account, we will adjust the SDs by the design effect, using intra-class coefficients if given in papers, or using external estimates obtained from similar studies [38]. In the absence of sufficient homogeneity, we will present tables of the quantitative results.
We will assess selection bias using Egger’s weighted regression method and Begg’s rank correlation test. Heterogeneity among the trials’ odds ratios will be assessed by using both χ2 test at a 5% significance level and the I2 statistic, the percentage of among-study variability that is due to true differences between studies (heterogeneity) rather than to sampling error. We will consider an I2 value greater than 50% to reflect substantial heterogeneity. We will conduct sensitivity analyses in order to investigate possible sources of heterogeneity including study quality (adequate vs inadequate allocation concealment; low vs high attrition) and sociodemographic factors that could act as effect modifiers (for example age, gender, sexuality and socioeconomic status). Details of each e-Learning program will be presented in a table of study characteristics, and we will conduct exploratory, descriptive analyses of data available on effective components and mechanisms of action.
Economic evaluation
A decision-analytic model will be built to assess cost-effectiveness, so that intervention effects identified by the systematic review can be extrapolated beyond the observed trial periods [39]. The aim of the evaluation will be to compare the cost-effectiveness of adaptive e-learning technologies against other dietary interventions available in England and Wales. We will combine the results of the systematic review with expert advice to identify the relevant e-learning technologies and appropriate comparators (e.g. group learning, individual contact with a dietitian) and model the costs associated with each.
The primary form of economic evaluation will be a cost-utility analysis, where health outcomes are expressed as quality-adjusted life-years (QALYs). The base case analysis will be performed from a NHS cost perspective. Future costs and health benefits will be discounted at 3.5% per annum. Results will be presented as expected costs, expected QALYs, incremental cost-effectiveness ratios, net benefit statistics and cost-effectiveness acceptability curves.
The model structure will be informed by: (i) reviewing previously published decision models where the immediate objective has been to evaluate technologies designed to help people change dietary behaviour and (ii) the results of the systematic review with respect to the recorded outcomes. For example, if the trials report changes in BMI, then a Markov model could be constructed, with the health states defined in terms of BMI groupings. Intervention costs [40] and effects could then be simulated by movements through these health states, with higher BMI being associated with increased health care costs (including costs of health outcomes such as cardiovascular disease, type 2 diabetes and cancer) and increased probabilities of all-cause mortality from sources such as the British Regional Heart Study [41].
Depending on the chosen model structure, other literature reviews will also be performed to identify evidence for other parameters, such as the increased costs and the dis-utility associated with increasing levels of obesity. Other variables for which additional searches might be required could include evidence linking increases in fruit or vegetable intake with weight loss and the reduction in the likelihood of cardiovascular disease following weight loss. Other important issues to incorporate in the model structure are likely to include attrition from the intervention, non-compliance and the need to retain a degree of flexibility as clinical studies are likely to report different outcomes (e.g. changes in behavioural and clinical outcomes).
If the primary systematic review identifies a ‘network’ of relevant RCTs, consideration will be given to performing formal mixed- or indirect-treatment comparisons to allow cost-effectiveness comparisons to be made across all programmes [42].
Stakeholder involvement
Involvement of non- governmental organisations who represent a range of potential user groups has been an important part of the project development. Jane Landon, Deputy Chief Executive of the National Heart Forum, is a member of the investigative team, attends steering group meetings with the other co-investigators, and contributes to decisions made as the study progresses. The National Heart Forum (NHF)is an alliance of over 60 national organisations representing professional, academic, consumer, charity and public sector organisations throughout the UK, and therefore represents a large population of potential users of e-Learning for dietary behaviour change. In our experience, user input is particularly valuable in considering outcomes of interest to users, and methods of disseminating results to user communities, thus contributing to public involvement in science.
Discussion
Strengths and limitations of the review
Strengths of this review include unambiguous definitions and inclusion criteria, and a clear and systematic approach to searching, screening and reviewing studies and extracting data using standardised forms and duplicating all stages. Our search area is large enough and our inclusion criteria broad enough to encompass the broadest range of interactive e-Learning interventions and dietary, clinical and behavioural outcomes, and so has the best chance of identifying effective components of effective interventions for translation into policy or further research. Our review will also pinpoint potential mechanisms of action in terms of psychological theories of behaviour change employed in interventions, which will further inform the future development of e-learning interventions. The final report to the HTA will allow for a comprehensive statistical, economic and subgroup analyses, as well as descriptive analysis not usually available given the limited space available in academic journals.
Although every effort will be made to locate unpublished trials our findings may still be vulnerable to selective reporting, and despite a pre-defined and systematic approach to screening and reviewing the study will still involve judgments made by review authors, either of which may lead to bias. This review will not look at cohort or other observational study designs, and therefore may not be able to evaluate acceptability or preference of e-Learning interventions.
Implications for policy and healthcare commissioning
This review aims to provide comprehensive evidence of the effectiveness and cost-effectiveness of adaptive e-Learning interventions for dietary behaviour change, and explore potential psychological mechanisms of action and the effective components of effective interventions. This can inform policy makers and healthcare commissioners in deciding whether e-Learning should be part of a comprehensive response to the improvement of dietary behaviour for health, and if so which components should be present for interventions to be effective.
Competing interests: none.
Authors’ contributions
PE will manage the project, and provided expertise in the design of the systematic review and statistical analysis; LF and JH will undertake screening, reviewing, and data extraction, and produced the various tools to be used in the review; LF will also undertake all database searching; JH will also provide nutrition expertise and coordinate writing and publication; EF provided expertise and guidance regarding nutritional outcomes to be investigated; CF, EM, SM and KL contributed materially to the design of the conceptual framework and the systematic review methods; SM also provided expertise and guidance regarding behavioural theories to be investigated; AM designed and will undertake the cost-effectiveness analysis; JL represented user groups in the design of the review. All authors contributed to the writing or editing of the protocol for publication, and will contribute to the final report and paper.
Acknowledgements
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 08/57/02) and will be published in full in Health Technology Assessment (see HTA Programme website for further project information). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
References
- Balcombe NR, Ferry PG, Saweirs WM. Nutritional status and well being. Is there a relationship between body mass index and the well-being of older people?. Curr Med Res Opin 2001;17:1-7.
- World Health Organization (WHO) . Reducing Risks, Promoting Healthy Life 2002.
- World Cancer Research Fund . American Institute of Cancer Research, Food, Nutrition and Physical Activity and the Prevention of Cancer: A Global Perspective 2007.
- Rolls BJ, Shide DJ. The influence of dietary fat on food intake and body weight. Nutr Rev 1992;50:283-90.
- Willet WC. Diet and health: What should we eat?. Science 1994:532-8.
- World Health Organization (WHO) . Diet, Nutrition, and the Prvention of Chronic Diseases 2003.
- Department of Health . Choosing Health: Making Healthier Choices Easier 2004.
- Lock K, Pomerleau J, Causer L, Altman DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bulletin of the World Health Organization 2005;83:100-8.
- Hoare A, Henderson L, Bates CJ, Prentice A, Birch A, Swan G, et al. National Diet and Nutrition Survey: Adults Aged 19–64 Years. Summary Report 2004.
- Department of Health . Choosing a Better Diet: A Food and Health Action Plan 2005:1-52.
- Wanless D. Securing our future health: Taking a long-term view. London: HM Treasury; 2002.
- Wanless D. Securing good health for the whole population. London: HM Treasury; 2004.
- Brug J, Oenema A, Kroeze W, Raat H. The internet and nutrition education: challenges and opportunities. European Journal of Clinical Nutrition 2005;59:S130-S139.
- Martin Gould SM, Anderson J. Using interactive media nutrition education to reach low-income persons: an effectiveness evaluation. Journal of Nutrition Education 2000;32:204-13.
- Tate DE, Jackvony EH, Wing RR. Effects of internet behavioral conuselling on weight loss in adults at risk for type 2 diabetes: a randomized trial. Journal of the American Medical Association 2003;289:1833-6.
- Oenema A, Brug J, Lechner L. Web-based tailorednutrition education: results of a randomized controlled trial. Health Education Research 2001;16:647-60.
- Eng TR. The eHealth landscape: a terrain map of emerging information and communication technologies in health and health care. Princeton NJ: Robert Wood Johnson Foundation; 2001.
- Office for National Statistics: Statistical Bulletin: Internet Access . Households and Individuals 2009.
- Kanuga M, Rosenfeld WD. Adolescent sexuality and the internet: the good, the bad, and the URL. Journal of Pediatric and Adolescent Gynecology 2004;17:117-24.
- Patrick K, Robinson TN, Alemi F, Eng TR. the Science Panel on Interactive Communication and Health: Policy issues relevant to evaluation of interactive health communication applications. American Journal of Preventative Medicine 1999;16:35-42.
- Williams P, Nicholas D, Huntington P, McClean F. Surfing for health: user evaluation of a health information web site. Part 1, Literature review. Health Information Library Journal 2002;19:98-108.
- Brug J, Oenema A, Campbell MK. Past, present, and future of computer-tailored nutrition education. American Journal of Clinical Nutrition 2003;77:S1028-S1034.
- Murray E. Internet-delivered treatments for long-term conditions: strategies, efficiency and cost-effectiveness. Expert Review of Pharmacoeconomics and Outcomes Research 2008;8:261-72.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2005.
- Stretcher VJ. Computer-tailored smoking cessation materials: a review and discussion. Patient Education and Counselling 1999;36:107-1.
- Skinner CS, Campbell MK, Rimer BK, Curry S, Prochaska JO. How effective is tailored print communication?. Annals of Bahavioral Medicine 1999;21:290-8.
- Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring Health Messages: Customising Communication with Computer Technology. Mahwah, NJ: Lawrence Erlbaum Associates; 2000.
- Neville LM, O’Hara B, Milat AJ. Computer-tailored dietary behaviour change interventions: a systematic review. Health Educ Res 2009;24:699-720.
- Valdez A BK, Ackerson L, Fernandez M. A multimedia breast cancer education intervention for low-income Latinas. J Community Health 2002;27:33-5.
- Cashen MS SB, Nguyen HH, Just M, Galzagorry G, Bakken S. Implementing a Web-based information resource at an inner-city community church: lessons learned. Comput Inform Nurs 2002;20:244-50.
- Dunham PJ HA, Litwin E, Gusella J, Ellsworth C, Dodd PW. Computer-mediated social support: single young mothers as a model system. Am J Community Psychol 1998;26:281-306.
- Kroeze W, Werkman A, Brug J. A systematic review of randomized trials on the effectiveness of computer-tailored education on physical activity and dietary behaviors. Annals of Behavioral Medicine 2006;31:205-23.
- Norman GJ, Zabinski MF, Adams MA, Rosenberg DE, Yaroch AL, Atienza AA. A review of eHealth interventions for physical activity and dietary behavior change. American Journal of Preventative Medicine 2007;33:336-45.
- Armitage CJ, Conner M. Psychology and Health 2000;15:173-89.
- Ajken I. The theory of planned behaviour. Organizational Behaviour and Human Decision Processes 1991:179-211.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3.
- Buxton MJ, Drummond MF, Van Hout BA, Prince RL, Sheldon TA, Szucs T, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Economics 1997;6:217-2.
- Hojgaard B, Gyrd-Hansen D, Olsen KR, Sogaard J, Sorensen TIA. Waist Circumference and Body Mass Index as Predictors of Health Care Costs. PloS One 2008;3.
- Shaper AG, Wannamethee SG, Walker M. Body weight: implications for the prevention of coronary heart disease, stroke, and diabetes mellitus in a cohort study of middle aged men. BMJ 1997;314:1311-7.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Kelly MP, Stewart E, Morgan A, Killoran A, Fischer A, Threlfall A, et al. A conceptual framework for public health: NICE’s emerging approach. Public Health 2009;123:e14-e20.
Appendix 2 Search strategies
Numbers in brackets indicate number of database hits.
MEDLINE
Dietary (A)
-
exp food/or exp beverages/ (892,524)
-
exp Diet/ (155,154)
-
exp nutrition processes/or exp nutritional requirements/or exp nutritional status/or exp nutritive value/ (160,564)
-
exp Nutrition Therapy/ (67,396)
-
exp nutrition assessment/ (17,368)
-
exp Body Weight/ (279,095)
-
exp Nutrition Disorders/ (197,372)
-
food$.ab,ti. (191,554)
-
nutri$.ab,ti. (177,742)
-
diet$.ab,ti. (288,751)
-
weigh$.ab,ti. (542,082)
-
(diet$adj3 behav$).ab,ti. (2698)
-
(eat$adj3 behav$).ab,ti. (4135)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 12 or 12 or 13 (A) – 1,950,164
E (B)
-
exp Electronics/ (18,202)
-
exp cybernetics/or exp reminder systems/or exp communications media/or exp computing methodologies/or computers/or exp informatics/ (635,512)
-
exp Audiovisual Aids/ (74,190)
-
exp Technology/ (219,462)
-
exp decision support techniques/ (43,790)
-
online.ab,ti. (14,628)
-
computer$.ab,ti. (178,320)
-
internet.ab,ti. (16,721)
-
(World wide web or world-wide-web or world-wide web or website$or internet$).ab,ti. (21,646)
-
(chat room$or chatroom$).ab,ti. (163)
-
(email or e-mail or electronic messag$).ab,ti. (3377)
-
(blog$or web-blog$or weblog$).ab,ti. (196)
-
(bulletin board$or bulletinboard$or message board$or message board$).ab,ti. (291)
-
(DVD or dvd).ab,ti. (378)
-
(CD-ROM or cd-rom or CDROM or cdrom).ab,ti. (966)
-
interactive health communicat$.ab,ti. (36)
-
interactive televis$.ab,ti. (72)
-
interactive video$.ab,ti. (404)
-
interactive technolog$.ab,ti. (63)
-
interactive multimedia.ab,ti. (214)
-
(E-health or ehealth or electronic health).ab,ti. (2078)
-
(surf or surf$or browse or brows$).ab,ti. (534,484)
-
(iphone or i-phone).ab,ti. (5)
-
(ipod or i-pod).ab,ti. (37)
-
(information kiosks or inform$kiosk$).ab,ti. (10)
-
(short messaging service or sms or text message or text$message or txt).ab,ti. (2181)
-
(multimedia messaging service or mms).ab,ti. (2549)
-
virtual reality.ab,ti. (2419)
-
15 or 16 or 17or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37or 38 or 39 or 40 or 41 or 42 (B) – 1,398,010
Study design (C)
-
Randomi#ed controlled trial.pt. (290,249)
-
controlled clinical trial.pt. (82,860)
-
Randomi#ed.ab. (233,631)
-
placebo.ab. (119,558)
-
clinical trials as topic.sh. (149,962)
-
randomly.ab. (140,912)
-
trial.ti. (85,200)
-
44 or 45 or 46 or 47 or 48 or 49 or 50 (683,732)
-
(animals not (humans and animals)).sh,ti. (3,414,928)
-
51 not 52 (C) – 634,766
Publication year (D)
-
(199$or 200$).yr. (D) – 10,151,288
A + B + C + D
-
14 (A) + 43(B) + 53 (C) + 54 (D) = 5483
CINAHL (Cumulative Index to Nursing and Allied Health Literature)
DIETARY (A)
-
(MH “Diet+”) (33,062)
-
(MH “Diet Records”) (3304)
-
(MH “Diet Therapy+”) (10,432)
-
(MH “Nutrition+”) or (MH “Nutrition Disorders+”) or (MH “Nutrition Services+”) or (MH “Nutritional Assessment”) or (MH “Nutritional Counseling”) or (MH “Nutritional Requirements+”) or (MH “Nutritional Status”) (80,466)
-
(MH “Nutrition Education”) (3603)
-
(MH “Eating Behaviour+”) (9299)
-
TI diet* (16,159)
-
AB diet* (20,894)
-
TI food* (11,959)
-
AB food* (15,241)
-
TI nutri* (18,746)
-
AB nutri* (21,060)
-
TI weigh* (12,948)
-
AB weigh* (36,173)
-
TI diet* N3 behavio?r (43)
-
AB diet* N3 behavio?r (89)
-
TI eat* N3 behavio?r (51)
-
AB eat* N3 behavio?r (126)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (A) – 157,321
E (B)
-
(MH “Informatics+”) (256,420)
-
(MH “Telecommunications+”) (41,066)
-
(MH “Communications Media+”) (199,431)
-
(MH “Information Technology+”) (7671)
-
(MH “Educational Technology”) (542)
-
(MH “Access to Information+”) (8277)
-
Cybernetics (9)
-
(MH “Blogs”) (323)
-
(MH “Information Retrieval+”) (4719)
-
(MH “Digital Divide”) (28)
-
TI online (6176)
-
AB online (5278)
-
TI computer* (9875)
-
AB computer* (15,217)
-
TI world-wide-web or TI website* (2051)
-
AB world-wide-web or AB website* (2235)
-
TI blog* or TI web-blog* or TI weblog* (401)
-
AB blog* or AB web-blog* or AB weblog* (164)
-
TI bulletin board* or TI bulletinboard* or TI message board* (939)
-
AB bulletin board* or AB bulletinboard* or AB message board* (126)
-
TI interactive health communicat* (9)
-
AB interactive health communicat* (10)
-
TI interactive televis* (30)
-
AB interactive televis* (42)
-
TI interactive video (93)
-
AB interactive video*–181)
-
TI interactive multimedia (62)
-
AB interactive multimedia (71)
-
TI surf* or TI brows* (3317)
-
AB surf* or AB brows* (13,219)
-
TI e-health or TI electronic –health (778)
-
AB e-health or AB electronic –health (538)
-
TI i-pod or TI i-phone (1)
-
TI inform* kiosk* (5)
-
AB inform* kiosk* (4)
-
TI short messaging service or TI sms or TI text message or TI txt message or TI txt (72)
-
AB short messaging service or AB sms or AB text message or AB txt message or AB txt (124)
-
TI multimedia messaging service or TI mms (9)
-
AB multimedia messaging service or AB mms (51)
-
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 (B) – 407,762
-
19 (A) and 59 (B) – 33,791
Study design (C)
-
(MH “Clinical Trials+”) (90,999)
-
(MH “Experimental Studies+”) (112,471)
-
PT Clinical trial (51,222)
-
TX Randomi?ed control* trial* (37,433)
-
AB randomly (20,411)
-
AB random assignment (387)
-
TX random* allocat* (335)
-
TX Placebo* (38,380)
-
TX Allocat* random* (195)
-
61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 (C) – 162,068
-
60 (A + B) and 70 (C) – 6415
Publication year (D)
-
A + B + C + D = 6409
MH = exact subject heading; TI = title; AB = abstract; TX = all text; PT = publication type.
PsycINFO
Dietary (A)
-
exp nutrition/or exp “beverages (nonalcoholic)”/or exp diets/or exp food/or exp food additives/ (14,533)
-
exp eating behaviour/or exp binge eating/or exp eating attitudes/or exp eating disorders/or exp food intake/or exp mealtimes/ (32,159)
-
((diet* or eat*) adj3 behav*).ti,ab. (5469)
-
“nutri*”.ti,ab. (11,209)
-
“food*”.ti,ab. (41,203)
-
“diet*”.ti,ab. (18,939)
-
“weigh*”.ti,ab. (49,146)
-
6 or 1 or 4 or 3 or 7 or 2 or 5 (117,495)
E (B)
-
exp internet/or exp communication systems/or exp information systems/or exp automated information processing/or exp computer applications/or exp computer mediated communication/or exp electronic communication/or exp online therapy/or exp telecommunications media/or exp telemedicine/or exp websites/or exp internet usage/ (62,091)
-
exp computer applications/or exp decision support systems/or exp electronic communication/or exp expert systems/or exp information science/or exp information technology/or exp intelligent agents/or exp knowledge management/or exp learning management systems/ (47,389)
-
exp human computer interaction/or exp virtual classrooms/or exp virtual teams/ (6943)
-
exp educational television/or exp educational audiovisual aids/or exp televised instruction/ (929)
-
exp computers/or exp artificial intelligence/or exp computer assisted design/or exp computer assisted therapy/or exp cybernetics/or exp databases/ (24,493)
-
exp computer games/or exp computer simulation/ (13,154)
-
exp communications media/ (29,968)
-
exp hypermedia/ (539)
-
computer*.ti,ab. (51,993)
-
(email or e-mail).ti,ab. (2466)
-
online.ti,ab. (9993)
-
(World wide web or world-wide-web or world-wide web or website* or www).ti,ab. (3291)
-
(E-health or ehealth or electronic health).ti,ab. (281)
-
“interactive health communicat*”.ti,ab. (28)
-
“interactive televis*”.ti,ab. (73)
-
“interactive video*”.ti,ab. (357)
-
“interactive technology*”.ti,ab. (48)
-
interactive multimedia.ti,ab. (182)
-
(surf or surf* or browse or brows*).ti,ab. (16,665)
-
(chat room* or chatroom*).ti,ab. (356)
-
(blog* or web-blog* or weblog*).ti,ab. (301)
-
(bulletin board* or bulletinboard* or message board* or message board*).ti,ab. (329)
-
(DVD or dvd).ti,ab. (284)
-
(CD-ROM or cd-rom or CDROM or cdrom).ti,ab. (499)
-
(i-phone or iphone).ti,ab. (3)
-
(i-pod or ipod).ti,ab. (25)
-
(information kiosks or inform*kiosk*).ti,ab. (5)
-
(short messaging service or sms or text message or text* message or txt).ti,ab. (453)
-
(multimedia messaging service or mms).ti,ab. (196)
-
virtual reality.ti,ab. (1451)
-
35 or 11 or 32 or 33 or 21 or 26 or 17 or 22 or 18 or 30 or 13 or 16 or 23 or 29 or 27 or 25 or 28 or 36 or 9 or 12 or 14 or 15 or 20 or 34 or 37 or 24 or 10 or 19 or 31 or 37 or 38 (144,714)
-
8 (A) and 38 (B) (4991)
Study design (C)
-
exp placebo/ (2403)
-
exp treatment effectiveness evaluation/ (10,902)
-
exp experimental design/ (40,065)
-
exp prospective studies/ (302)
-
“clinical trial*”.ti,ab. (10815)
-
controlled clinical trial.ti,ab. (608)
-
randomi?ed controlled trial.ti,ab. (4094)
-
randomi?ed.ti,ab. (23,653)
-
placebo.ab. (22,179)
-
randomly.ab. (35,218)
-
trial.ti. (9995)
-
((singl* or doubl* or trebl* or tripl*) adj3 (blind* or dummy or mask*)).ti,ab. (14,208)
-
((crossover or clin* or control* or compar* or evaluat* or prospectiv*) adj3 (trial* or studi* or study)).ti,ab. (106,973)
-
“exp*”.ti,ab. (1,062,801)
-
50 or 53 or 51 or 41 or 47 or 48 or 42 or 52 or 49 or 46 or 45 or 43 or 44 or 54 (1,194,568)
-
exp animals/ (207,822)
-
exp human females/ (86,263)
-
exp human males/ (27,625)
-
57 or 58 (107,294)
-
56 not (57 and 58) (487)
-
55 not 50 (1,194,324)
-
61 (C) and 40 (A+B) (2669)
Publication year (D)
-
(“1990” or “1991” or “1992” or “1993” or “1994” or “1995” or “1996” or “1997” or “1998” or “1999”).yr. (629,371)
-
“200*”.yr. (977,563)
-
63 or 64 (1,606,828)
A + B + C + D
-
62 (A + B + C)and 65 (D) = 2376
EMBASE
Dietary (A)
-
exp food/or exp nutrition/ (1,182,412)
-
food$.ti,ab. (174,537)
-
diet$.ti,ab. (283,160)
-
nutri$.ti,ab. (161,807)
-
weigh$.ti,ab. (538,793)
-
(diet$adj3 behav$).ti,ab. (2185)
-
(eat$adj3 behav$).ti,ab. (3827)
-
(feed$adj3 behav$).ti,ab. (4802)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (A) – 1,782,850
Electronics (B)
-
exp information system/ (38,399)
-
exp computer/ (58,488)
-
exp internet/or exp mass communication/ (129,648)
-
exp cybernetics/ (772)
-
exp educational technology/ (1107)
-
exp audiovisual equipment/ (35,989)
-
exp information processing/ (448,408)
-
computer$.ti,ab. (156,790)
-
online.ti,ab. (11,955)
-
(email or e-mail or electronic messag$).ti,ab. (4055)
-
(World wide web or world-wide-web or world-wide web or website$or www or internet connect$).ti,ab. (5821)
-
interactive health communicat$.ti,ab. (24)
-
interactive televis$.ti,ab. (23)
-
interactive video$.ti,ab. (207)
-
interactive technolog$.ti,ab. (37)
-
interactive multimedia$.ti,ab. (124)
-
(surf or surf$or browse or brows$).ti,ab. (535,139)
-
(chat room$or chatroom$).ti,ab. (140)
-
(blog$or web-blog$or weblog$).ti,ab. (142)
-
(bulletin board$or bulletinboard$or message board$or message board$).ti,ab. (197)
-
(DVD or dvd or video disk$).ti,ab. (336)
-
(CD-ROM or cd-rom or CDROM or cdrom).ti,ab. (677)
-
(iphone or i-phone).ti,ab. (5)
-
(ipod or i-pod).ti,ab. (32)
-
smartphone$.ti,ab. (26)
-
inform$kiosk$.ti,ab. (4)
-
(short messaging service or sms or text message or text$message or txt).ti,ab. (1978)
-
(multimedia messaging service or mms).ti,ab. (2318)
-
virtual reality.ti,ab. (1883)
-
second life.ti,ab. (40)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 37 (B) – 1,170,334
-
9 (A) and 40 (B) – 120,361
Study design (C)
-
exp clinical trial/ (583,349)
-
exp randomized controlled trial/ (178,474)
-
exp randomization/ (27,376)
-
exp single blind procedure/ (8733)
-
exp double blind procedure/ (79,416)
-
exp crossover procedure/ (22,265)
-
exp placebo/ (150,812)
-
randomi?ed controlled trial.ti,ab. (21,141)
-
rct.ti,ab. (3141)
-
random allocation.ti,ab. (729)
-
Randomly allocated.ti,ab. (10,942)
-
(allocated adj2 random).ti,ab. (723)
-
Single blind$.ti,ab. (8123)
-
double blind$.ti,ab. (97,446)
-
((treble or triple) adj blind$).ti,ab. (187)
-
placebo$.ti,ab. (123,895)
-
exp prospective study/ (88391)
-
42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 (774,721)
-
exp case study/ (15,716)
-
case report.ti,ab. (150,170)
-
(abstract report or letter).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (545,008)
-
60 or 61 or 62 (708,316)
-
59 not 63 (C) – 747,554
Publication year (D)
-
(199$or 200$).yr. (9,112,286)
A + B + C + D
-
9(A) + 40(B) + 64(C) + 65(D) = 7843
The Cochrane Library
Dietary (A)
-
MeSH descriptor Food and Beverages explode all trees (16,529)
-
MeSH descriptor Feeding Behaviour explode tree 2 (2995)
-
MeSH descriptor Nutrition Assessment explode all trees (453)
-
MeSH descriptor Food Labelling explode all trees (21)
-
MeSH descriptor Nutritional Requirements explode all trees (408)
-
MeSH descriptor Nutritional Status explode all trees (1129)
-
diet* or food* nutri* (25,028)
-
eat* behavio?r (337)
-
weigh* (35,289)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (A) – 57,374
E (B)
-
MeSH descriptor Computing Methodologies explode all trees (8032)
-
MeSH descriptor Telecommunications explode all trees (1969)
-
MeSH descriptor Cybernetics explode all trees (772)
-
MeSH descriptor Informatics explode all trees (49)
-
MeSH descriptor Medical Informatics explode all trees (6778)
-
MeSH descriptor Educational Technology explode all trees (1712)
-
MeSH descriptor Audiovisual Aids explode all trees (1697)
-
Computer* (14,503)
-
email or e-mail (3660)
-
DVD or dvd (55)
-
online (7582)
-
“World wide web” (153)
-
website* (1368)
-
“chat room*” (5)
-
“chatroom*” (2)
-
blog* (44)
-
weblog* (2)
-
“bulletin board*” (15)
-
“bulletinboard*” (2)
-
“message board*” (5)
-
“interactive health communicat*” (1)
-
“interactive television” (4)
-
“interactive video*” (35)
-
“interactive technology” (11)
-
“interactive multimedia” (59)
-
“E-health” (149)
-
ehealth (30)
-
“electronic health” (39)
-
surf (18)
-
surf* (10,603)
-
browse (263)
-
brows* (285)
-
ipod (2)
-
i-pod (1)
-
“information kiosks” (2)
-
“short messaging service” (8)
-
sms (130)
-
“text message” (11)
-
“text* message” (11)
-
txt (26)
-
MMS (93)
-
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (B) – 41,997
A + B
-
10 (A) and 52 (B) – 8279
Study design (C)
-
(randomly):ab or (trial):ti or (randomized controlled trial):pt or (controlled clinical trial):pt or (randomized):ab (329,582)
-
(placebo):ab (74,750)
-
clinical trial as topic (20,978)
-
(animals not (humans and animals)):ti (131)
-
(56 or 57 or 58) (338,150)
-
60 and not 59 (C) – 338,069
A + B + C
-
10 (A) + 52 (B) + 59 (C) = 6448
Global health
Dietary (A)
-
exp foods/ (96,400)
-
exp diet/or exp dietary guidelines/ (32,241)
-
exp nutrition/or exp nutrition information/or exp nutrition knowledge/or exp nutrition labeling/or exp nutrition planning/or exp nutrition research/or exp nutritional intervention/ (41,394)
-
“food*”.ab,ti. (188,659)
-
“diet*”.ab,ti. (229,658)
-
“nutri*”.ab,ti. (137,958)
-
(diet adj3 behavio#r).ab,ti. (200)
-
(eat adj3 behavio#r).ab,ti. (5)
-
“weigh*”.ab,ti. (173,600)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (A) – 566,683
E (B)
-
exp computers/or exp computer analysis/or exp computer assisted instruction/or exp computer graphics/or exp computer simulation/or exp computer software/or exp computer techniques/or exp electronics/or exp information systems/or exp information technology/ (6511)
-
exp telecommunications/ (1653)
-
exp mass media/ (1934)
-
exp information science/or exp information/or exp information needs/or exp information processing/or exp information services/ (33,902)
-
exp computer games/ (51)
-
exp cybernetics/ (5)
-
exp multimedia instruction/ (34)
-
exp educational technology/or exp educational innovation/or exp educational television/ (47)
-
(email or e-mail).ab,ti. (342)
-
“computer*”.ab,ti. (9723)
-
online.ab,ti. (1308)
-
(World wide web or world-wide-web or world-wide web or website$or internet$).ab,ti. (2714)
-
(E-health or ehealth or electronic health).ab,ti. (110)
-
“interactive health communicat*”.ab,ti. (3)
-
“interactive televis*”.ab,ti. (3)
-
“interactive video*”.ab,ti. (17)
-
“interactive tech*”.ab,ti. (11)
-
“interactive multimedia*”.ab,ti. (29)
-
(surf or surf* or browse or brows*).ab,ti. (66,004)
-
(chat room* or chatroom*).ab,ti. (32)
-
(blog* or web-blog* or weblog*).ab,ti. (13)
-
(bulletin board* or bulletinboard* or message board* or message board*).ab,ti. (27)
-
(DVD or dvd).ab,ti. (37)
-
(CD-ROM or cd-rom or CDROM or cdrom).ab,ti. (165)
-
(i-pod or ipod).ab,ti. (1)
-
(information kiosks or inform$kiosk$).ab,ti. (1)
-
(short messaging service or sms or text message or text$message or txt).ab,ti. (160)
-
(multimedia messaging service or mms).ab,ti. (99)
-
virtual reality.ab,ti. (24)
-
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 (B) – 116,166
-
10 (A) and 40 (B) – 25,317
Study design (C)
-
exp trials/or exp experimental design/or exp experiments/or exp feasibility studies/ (19,683)
-
“clinical trial*”.ab,ti. (14,110)
-
controlled clinical trial.ab,ti. (944)
-
randomi#ed controlled trial.ab,ti. (3778)
-
randomi#ed.ab,ti. (28,951)
-
trial.ti. (11,764)
-
((crossover or clin* or control* or compar* or evaluat* or prospectiv*) adj3 (trial* or studi* or study)).ab,ti. (127,130)
-
42 or 46 or 45 or 43 or 44 or 47 or 48 (C) – 146,517
-
41(A+B) and 49 (C) – 1742
Publication year (D)
-
(199* or 200*).yr. (1,147,042)
-
50 (A+B+C) and 51 (D) = 1447
Health Management Information Consortium
Diet (A)
-
exp food/ (2267)
-
exp diet/or exp diet therapy/or exp dietary advice/or exp dietary intake studies/or exp meals/or exp nutrients/or exp nutrition/or exp nutritional requirements/or exp nutritional value/ (2252)
-
diet$.ab,ti. (1841)
-
food$.ab,ti. (3322)
-
nutri$.ab,ti. (1528)
-
weigh$.ab,ti. (2463)
-
(eat$adj3 behav$).ab,ti. (56)
-
(diet$adj3 behav$).ab,ti. (72)
-
(eat$adj3 habit$).ab,ti. (68)
-
food habit$.ab,ti. (6)
-
(eat$adj3 disorder).ab,ti. (47)
-
6 or 11 or 3 or 7 or 9 or 2 or 8 or 1 or 4 or 10 or 5 (9261)
E (B)
-
exp information technology/or exp computer science/or exp computers/or exp expert systems/or exp information systems/or exp video discs/ (10,776)
-
exp telecommunications/or exp communication networks/ (1963)
-
exp communication media/ (859)
-
exp computer networks/ (1489)
-
exp online systems/ (332)
-
exp educational technology/ (13)
-
exp computer programs/ (127)
-
exp internet websites/ (536)
-
“computer*”.ab,ti. (4782)
-
(email or e-mail).ab,ti. (248)
-
online.ab,ti. (597)
-
(World wide web or world-wide-web or world-wide web or website$or internet$).ab,ti. (1723)
-
(E-health or ehealth or electronic health).ab,ti. (262)
-
interactive health communicat$.ab,ti. (4)
-
interactive televis$.ab,ti. (21)
-
interactive video$.ab,ti. (31)
-
interactive technolog$.ab,ti. (2)
-
interactive multimedia.ab,ti. (20)
-
(surf or surf$or browse or brows$).ab,ti. (449)
-
(chat room$or chatroom$).ab,ti. (10)
-
(blog$or web-blog$or weblog$).ab,ti. (9)
-
(bulletin board$or bulletinboard$or message board$or message board$).ab,ti. (22)
-
(DVD or dvd).ab,ti. (14)
-
(CD-ROM or cd-rom or CDROM or cdrom).ab,ti. (123)
-
(information kiosks or inform$kiosk$).ab,ti. (16)
-
(short messaging service or sms or text message or text$message or txt).ab,ti. (64)
-
(multimedia messaging service or mms).ab,ti. (3)
-
virtual reality.ab,ti. (34)
-
35 or 32 or 33 or 21 or 26 or 17 or 22 or 18 or 30 or 13 or 16 or 23 or 29 or 27 or 25 or 39 or 28 or 40 or 36 or 14 or 15 or 20 or 38 or 34 or 24 or 37 or 19 or 31 (16,076)
-
12 (A) and 41 (B) = 281
Web of Science
| Search field | Diet (A) | Electronics (B) | Study design (C) | A + B + C |
|---|---|---|---|---|
| Title | diet* or nutri* or food* or diet* intake or food intake or diet* behavio?r or eat* behavio?r or weigh* or food habit* or eat* habit* or eat* disorder or obesity or overweight | computer* or internet* or online or ‘e-health’ or ehealth or web* or interactive* or ‘communication systems’ or ‘information systems’ or ‘automated information processing’ or ‘computer applications’ or ‘computer mediated communication’ or ‘electronic communication’ or ‘online therapy’ or ‘educational audiovisual aids’ or ‘televised instruction’ or ‘artificial intelligence’ or ‘computer assisted design’ or ‘computer assisted therapy’ or cybernetics or ‘computer games’ or ‘computer simulation’ or ‘communications media’ or hypermedia or multimedia | experiment* or trial* or randomi$ed or evaluat* or prospect* or comparat* or crossover or random* or ‘clinical trial’ | 222 |
Health Economic Evaluations Database
| Search field | Diet (A) | Electronics (B) | A + B |
|---|---|---|---|
| Abstract | diet* or nutri* or food* or ‘diet intake’ within 3 or ‘food intake’ or ‘diet behaviour’ within 3 or ‘eat behaviour’ within 3 or weigh* or ‘food habit’ or ‘eat habit’ within 3 or ‘eat disorder’ within 3 or obesity or overweight | computer* or internet* or online or ‘e-health’ or ehealth or web* or interactive* or ‘communication systems’ or ‘information systems’ or ‘online therapy’ or ‘televised instruction’ or ‘artificial intelligence’ or cybernetics or ‘computer games’ within 4 or ‘computer simulation’ within 4 or ‘communications media’ or hypermedia or multimedia | 18 |
Education Resources Information Centre (1990–2009)
| Search field | Diet (A) | Electronics (B) | Study design (C) | A + B + C |
|---|---|---|---|---|
| Thesaurus descriptors | ‘Nutrition’ OR ‘Nutrition Instruction’ OR food OR ‘food habits’ | computer OR internet OR online | 2990 | |
| Title | food* OR nutri* OR weigh* | e-health OR ehealth OR ‘electronic health’ OR surf OR surf* OR browse OR brows* OR ipod OR i-pod OR ‘information kiosks’ OR ‘short messaging service’ OR sms OR ‘educational technology’ OR ‘audiovisual aids’ OR email OR e-mail OR ‘interactive health communication’ OR ‘interactive video’ OR ‘web based’ OR website OR ‘world wide web’ OR ‘interactive television’ OR ‘interactive multimedia’ OR computer* OR Internet OR online | ‘experimental design’ OR ‘prospective studies’ OR ‘clinical trial’ OR ‘controlled clinical trial’ OR ‘randomised controlled trial’ OR ‘randomised controlled trial’ OR random* OR trial OR ‘crossover trial’ OR ‘crossover study’ OR ‘comparative trial’ OR ‘comparative study’ OR ‘evaluative trial’ OR ‘evaluative study’ OR ‘prospective trial’ OR ‘prospective study’ |
Dissertation abstracts
| Diet (A) | Electronics (B) | Study design I (C1) | Study design II (C2) | A + B + C1 | A + B + C2 |
|---|---|---|---|---|---|
| diet or nutrition or food or diet* or eat* or nutri* or weigh* | computer* or online or internet or telecommunications or cybernetics or medical informatics | (experimental design OR prospective studies OR clinical trial* OR (controlled clinical trial) OR (randomi?ed controlled trial) OR randomi?ed OR random* OR trial) | ((crossover or clin* or control* or compar* or evaluat* or prospectiv*) W/3 (trial* or studi* or study)) | 659 | 303 |
| e-health or ehealth or ‘electronic health’ or surf or surf* or browse or brows* | 561 | 489 | |||
| ipod or i-pod or ‘information kiosks’ or ‘short messaging service’ or sms | 27 | 11 | |||
| ‘educational technology’ or ‘audiovisual aids’ or email or e-mail or DVD or CD-ROM or chatroom or blog or weblog* | 17 | 16 | |||
| ‘interactive health communicat*’ or ‘interactive video*’ OR interactive television or interactive multimedia | 8 | 2 | |||
| web-based or website or ‘world wide web’ | 25 | 18 | |||
| TOTAL | 1296 | 839 | |||
| TOTAL = (A + B + C1) + (A + B + C2) | 2136 | ||||
Appendix 3 Screening form
1. Design
| Is this a randomised controlled trial, evaluating the effectiveness of an intervention? | No | Yes | ? |
| Go to 5 | Go to 2 | Go to 2 |
Note: Exclude (but read for background and check reference list) any systematic or non-systematic reviews of interventions, and any non-RCT evaluations of e-learning interventions.
2. Participants
| Are participants adults or adolescents aged 13 years and above? | No | Yes | ? |
| Go to 5 | Go to 3 | Go to 3 |
Note: Interventions may be targeted to populations as a preventative measure, or to populations with clinical conditions for management of these conditions. Interventions may be targeted to individuals or their carers.
3. Intervention(s)
| Does the intervention seek to change behaviour through interactive software programs, delivered through electronic media, which tailor output according to user input? | No | Yes | ? |
| Go to 5 | Go to 4 | Go to 4 |
Note: Users may interact with the programme as members of a group or as individuals, but may not require expert facilitation. Multi-component interventions including other outcomes (e.g. physical activity) will be included only if the dietary component can be isolated.
4. Outcomes
| Does the study include any of the following as outcomes: dietary behaviour; food consumption; energy intake; nutrient or dietary fibre consumption; BMI; blood lipid levels; plasma vitamin or mineral levels or biomarkers of these; or a combination of these outcomes? | No | Yes | ? |
| Go to 5 | Go to 5 | Go to 5 |
5. Decisions
| If all 1–4 ‘Yes’ | Paper to be included. Data extraction form to be completed. Paper and form to be filed on computer. |
| If any 1–4 ‘No’ | Paper to be excluded. Reasons for exclusion to be entered on ‘exclusion’ database. Paper to be filed on computer. |
| If any 1–4 ‘?’ |
Reviewer 1 and 2 (and if necessary 3) to reach consensus; include/exclude as above. |
| Possible background papers | Reference lists to be checked. Paper to be filed on computer for reference. |
6. References
| Reference list checked? | Yes | No |
Appendix 4 PRISMA flow diagram
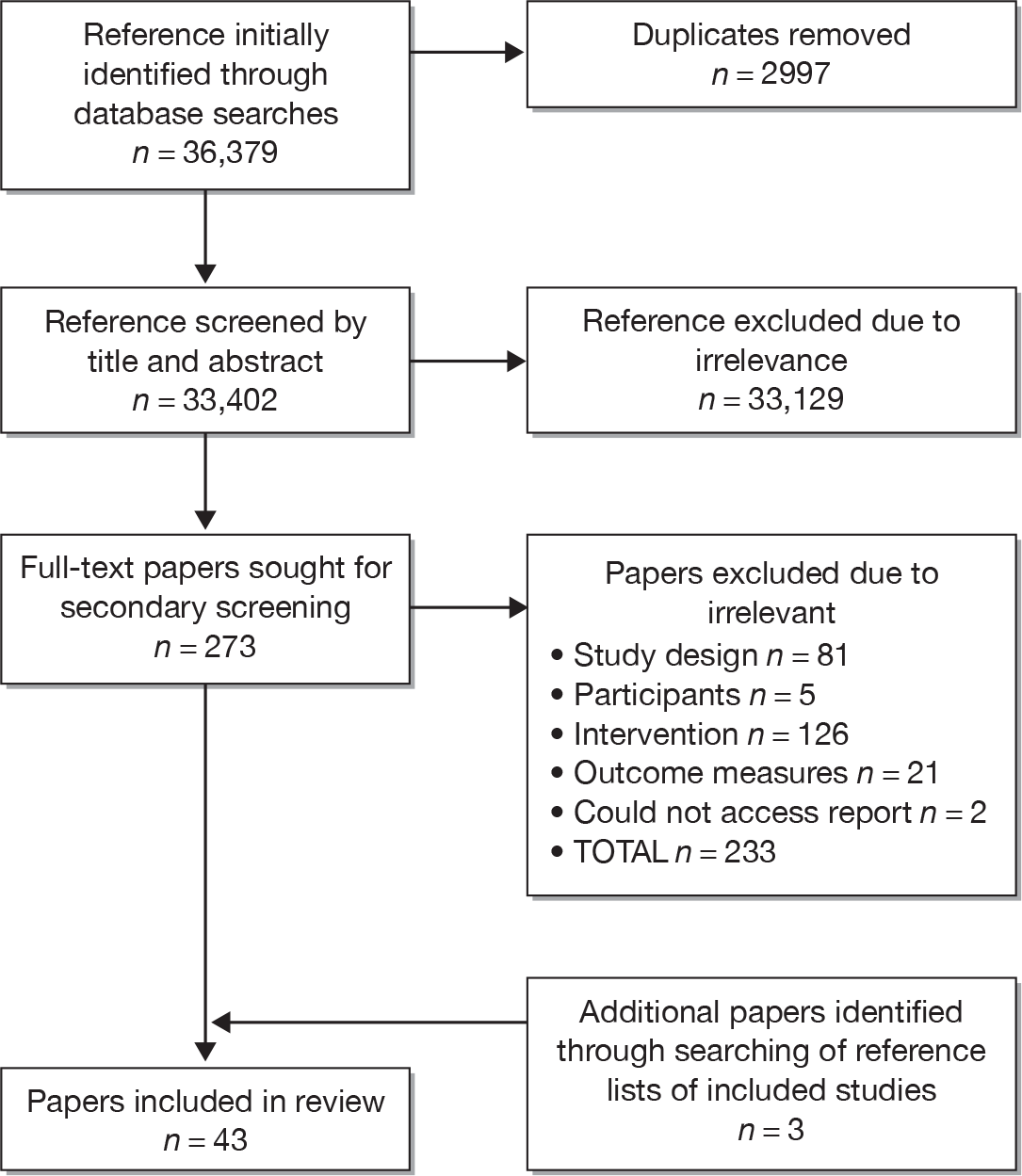
Appendix 5 Bibliography of included studies
-
Agras SW, Barr Taylor C, Feldman DE, Losch M, Burnett KF. Developing computer-assisted therapy for the treatment of obesity. Behav Ther 1990;21:99–109.
-
Alexander GL, McClure JB, Calvi JH, Divine GW, Stopponi MA, Rolnick SJ, et al. A randomized clinical trial evaluating online interventions to improve fruit and vegetable consumption. Am J Public Health 2010;100:319–26.
-
Anderson ES, Winett RA, Wojcik JR, Winett SG, Bowden T. A computerized social cognitive intervention for nutrition behavior: direct and mediated effects on fat, fiber, fruits, and vegetables, self-efficacy, and outcome expectations among food shoppers. Ann Behav Med 2001;23:88–100.
-
Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr 2008;27:280–6.
-
Blanson Henkemans OA, van der Boog PJ, Lindenberg J, van der Mast CA, Neerincx MA, Zwetsloot-Schonk BJ. An online lifestyle diary with a persuasive computer assistant providing feedback on self-management. Technol Health Care 2009;17:253–67.
-
Block G, Wakimoto P, Metz D, Fujii ML, Feldman N, Mandel R, et al. A randomized trial of the Little by Little CD-ROM: demonstrated effectiveness in increasing fruit and vegetable intake in a low-income population. Prev Chronic Dis 2004;1:A08.
-
Buller DB, Woodall WG, Zimmerman DE, Slater MD, Heimendinger J, Waters E, et al. Randomized trial on the 5 a day, the Rio Grande Way Website, a web-based program to improve fruit and vegetable consumption in rural communities. J Health Commun 2008;13:230–49.
-
Campbell MK, Carbone E, Honess-Morreale L, Heisler-Mackinnon J, Demissie S, Farrell D. Randomized trial of a tailored nutrition education CD-ROM program for women receiving food assistance. J Nutr Educ Behav 2004;36:58–66.
-
Campbell MK, Honess-Morreale L, Farrell D, Carbone E, Brasure M. A tailored multimedia nutrition education pilot program for low-income women receiving food assistance. Health Educ Res 1999;14:257–67.
-
Carbone E. LearnSmart: The application of adult learning theories to nutrition education. PhD thesis. Chapil Hill: University of North Carolina; 1999.
-
Cook RF, Billings DW, Hersch RK, Back AS, Hendrickson A. A field test of a web-based workplace health promotion program to improve dietary practices, reduce stress, and increase physical activity: randomized controlled trial. J Med Internet Res 2007;9:e17.
-
Cussler EC, Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, et al. Maintenance of weight loss in overweight middle-aged women through the Internet. Obesity 2008;16:1052–60.
-
De Bourdeaudhuij I, Stevens V, Vandelanotte C, Brug J. Evaluation of an interactive computer-tailored nutrition intervention in a real-life setting. Ann Behav Med 2007;33:39–48.
-
Delichatsios HK, Friedman RH, Glanz K, Tennstedt S, Smigelski C, Pinto BM, et al. Randomized trial of a “talking computer” to improve adults’ eating habits. Am J Health Promot 2001;15:215–24.
-
Di Noia J, Contento IR, Prochaska JO. Computer-mediated intervention tailored on transtheoretical model stages and processes of change increases fruit and vegetable consumption among urban African-American adolescents. Am J Health Promot 2008;22:336–41.
-
Ellrott T. [Self-help programme for weight reduction with and without support by a handheld nutrition computer.] Ernahrungs Umschau 2005;52:392–7.
-
Franko DL, Cousineau TM, Trant M, Green TC, Rancourt D, Thompson D, et al. Motivation, self-efficacy, physical activity and nutrition in college students: randomized controlled trial of an internet-based education program. Prev Med 2008;47:369–77.
-
Gow RW, Trace SE, Mazzeo SE. Preventing weight gain in first year college students: an online intervention to prevent the “freshman fifteen”. Eat Behav 2010;11:33–9.
-
Haerens L, Deforche B, Maes L, Brug J, Vandelanotte C, De Bourdeaudhuij I. A computer-tailored dietary fat intake intervention for adolescents: results of a randomized controlled trial. Ann Behav Med 2007;34:253–62.
-
Huang A, Barzi F, Huxley R, Denyer G, Rohrlach B, Jayne K, et al. The Effects on Saturated Fat Purchases of Providing Internet Shoppers with Purchase- Specific Dietary Advice: A Randomised Trial. PLOS Clin Trial 2006;1:e22.
-
Irvine AB, Ary DV, Grove DA, Gilfillan-Morton L. The effectiveness of an interactive multimedia program to influence eating habits. Health Educ Res 2004;19:290–305.
-
Jacobi C, Morris L, Beckers C, Bronisch-Holtze J, Winter J, Winzelberg AJ, et al. Maintenance of internet-based prevention: a randomized controlled trial. Int J Eat Disord 2007;40:114–19.
-
Jones M, Luce KH, Osborne MI, Taylor K, Cunning D, Doyle AC, et al. Randomized, controlled trial of an internet-facilitated intervention for reducing binge eating and overweight in adolescents. Pediatrics 2008;121:453–62.
-
Kroeze W, Oenema A, Campbell M, Brug J. The efficacy of Web-based and print-delivered computer-tailored interventions to reduce fat intake: results of a randomized, controlled trial. J Nutr Educ Behav 2008;40:226–36.
-
Low KG, Charanasomboon S, Lesser J, Reinhalter K, Martin R, Jones H, et al. Effectiveness of a computer-based interactive eating disorders prevention program at long-term follow-up. Eat Disord 2006;14:17–30.
-
Oenema A, Brug J, Dijkstra A, de Weerdt I, de Vries H. Efficacy and use of an internet-delivered computer-tailored lifestyle intervention, targeting saturated fat intake, physical activity and smoking cessation: a randomized controlled trial. Ann Behav Med 2008;35:125–35.
-
Oenema A, Brug J, Lechner L. Web-based tailored nutrition education: results of a randomized controlled trial. Health Educ Res 2001;16:647–60.
-
Oenema A, Tan F, Brug J. Short-term efficacy of a web-based computer-tailored nutrition intervention: main effects and mediators. Ann Behav Med 2005;29:54–63.
-
Rothert K, Strecher VJ, Doyle LA, Caplan WM, Joyce JS, Jimison HB, et al. Web-based weight management programs in an integrated health care setting: a randomized, controlled trial. Obesity 2006;14:266–72.
-
Shapiro JR, Reba-Harrelson L, Dymek-Valentine M, Woolson SL, Hamer RM, Bulik CM. Feasibility and acceptability of CD-ROM-based cognitive-behavioural treatment for binge-eating disorder. Eur Eat Disord Rev 2007;15:175–84.
-
Sternfeld B, Block C, Quesenberry CP, Jr., Block TJ, Husson G, Norris JC, et al. Improving diet and physical activity with ALIVE: a worksite randomized trial. Am J Prev Med 2009;36:475–83.
-
Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139–48.
-
Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an internet weight loss program. Arch Intern Med 2006;166:1620–5.
-
Trinh. The effect of an online nutrition intervention on fruit and vegetable intake among women. Fullerton: California State University; 2009.
-
Turnin MC. Telematic Expert System Diabeto – new tool for diet self-monitoring for diabetic patients. Diabetes Care 1992;15:204–12.
-
Turnin MC, Bourgeois O, Cathelineau G, Leguerrier AM, Halimi S, Sandre-Banon D, et al. Multicenter randomized evaluation of a nutritional education software in obese patients. Diabetes Metab 2001;27:139–47.
-
Vandelanotte C, De Bourdeaudhuij I, Sallis JF, Spittaels H, Brug J. Efficacy of sequential or simultaneous interactive computer-tailored interventions for increasing physical activity and decreasing fat intake. Ann Behav Med 2005;29:138–46.
-
Verheijden M, Bakx JC, Akkermans R, van den Hoogen H, Godwin NM, Rosser W, et al. Web-based targeted nutrition counselling and social support for patients at increased cardiovascular risk in general practice: randomized controlled trial. J Med Internet Res 2004;6:e44.
-
Veverka DV, Anderson J, Auld GW, Coulter GR, Kennedy C, Chapman PL. Use of the stages of change model in improving nutrition and exercise habits in enlisted Air Force men. Mil Med 2003;168:373–9.
-
Winett RA, Anderson ES, Wojcik JR, Winett SG, Bowden T. Guide to health: nutrition and physical activity outcomes of a group-randomized trial of an internet-based intervention in churches. Ann Behav Med 2007;33:251–61.
-
Winett RA, Wagner JL, Moore JF, Walker WB, Hite LA, Leahy M, et al. An experimental evaluation of a prototype public access nutrition information system for supermarkets. Health Psychol 1991;10:75–8.
-
Wylie-Rosett J, Swencionis C, Ginsberg M, Cimino C, Wassertheil Smoller S, Caban A, et al. Computerized weight loss intervention optimizes staff time: the clinical and cost results of a controlled clinical trial conducted in a managed care setting. J Am Diet Assoc 2001;101:1155–62.
-
Zabinski MF, Pung MA, Wilfley DE, Eppstein DL, Winzelberg AJ, Celio A, et al. Reducing risk factors for eating disorders: targeting at-risk women with a computerized psychoeducational program. Int J Eat Disord 2001;29:401–8.
Appendix 6 List of excluded studies
| Author and year | Not an RCT evaluation of an interventiona | Participants not adults or adolescents > 13 years old | Intervention does not seek to change behaviour exclusively through adaptive e-learningb | Study does not include dietary behaviour outcomes | a Data reported elsewhere (this is a dissertation or earlier paper) | b E-learning intervention, but involves significant contact with a therapist | Could not access the report |
|---|---|---|---|---|---|---|---|
| Abood 2008 | ✓ | ||||||
| Adachi 2004 | ✓ | ||||||
| Adachi 2007 | ✓ | ||||||
| Adachi 2009 | ✓ | ||||||
| Adams 2002 | ✓ | ✓ | |||||
| Anhoj 2004 | ✓ | ||||||
| Armitage 2001 | ✓ | ||||||
| Arsand 2008 | ✓ | ||||||
| Artal 2007 | ✓ | ||||||
| Ashfield-Watt 2002 | ✓ | ||||||
| Atienza 2008 | ✓ | ||||||
| Ayala 2002 | ✓ | ||||||
| Ayala 2006 | ✓ | ||||||
| Babazono 2007 | ✓ | ||||||
| Balas 1997 | ✓ | ||||||
| Bara-Carril 2004 | ✓ | ||||||
| Baranowski 2003 | ✓ | ||||||
| Baranowski 2003 | ✓ | ||||||
| Barkley 2003 | ✓ | ||||||
| Bauer 2009 | ✓ | ||||||
| Bechtel-Blackwell 2002 | ✓ | ||||||
| Bemelmans 2000 | ✓ | ||||||
| Bennett 2000 | ✓ | ||||||
| Bergh 2002 | ✓ | ||||||
| Biagioli 2007 | ✓ | ||||||
| Blissmer 2006 | ✓ | ||||||
| Block 2004 | ✓ | ||||||
| Block 2008 | ✓ | ||||||
| Bobroff 2003 | ✓ | ||||||
| Boeckner 1999 | ✓ | ||||||
| Bond 2007 | ✓ | ||||||
| Booth 2008 | ✓ | ||||||
| Booth 2008 | ✓ | ||||||
| Brantley 2008 | ✓ | ||||||
| Brotherton 2007 | ✓ | ||||||
| Brown 1995 | ✓ | ||||||
| Brown 1997 | ✓ | ||||||
| Brug 1996 | ✓ | ||||||
| Brug 1998 | ✓ | ||||||
| Brug 1999 | ✓ | ||||||
| Brug 1999 | ✓ | ||||||
| Bruning Brown 2004 | ✓ | ||||||
| Burden 2000 | ✓ | ||||||
| Burgess-Champouüt 2007 | ✓ | ||||||
| Burnett 1992 | ✓ | ||||||
| Calfas 2002 | ✓ | ||||||
| Campbell 1992 | ✓ | ✓ | |||||
| Campbell 1994 | ✓ | ||||||
| Campbell 1999 | ✓ | ||||||
| Campbell 1999 | ✓ | ||||||
| Campbell 1999 | ✓ | ||||||
| Campbell 2002 | ✓ | ||||||
| Campbell 2004 | ✓ | ||||||
| Carlton 2000 | ✓ | ||||||
| Carpenter 2004 | ✓ | ||||||
| Carroll 1996 | ✓ | ||||||
| Carroll 2007 | ✓ | ||||||
| Casazza 2006 | ✓ | ||||||
| Casazza 2007 | ✓ | ||||||
| Celio 2000 | ✓ | ||||||
| Celio 2002 | ✓ | ||||||
| Celio 2005 | ✓ | ||||||
| Cheskin 2008 | ✓ | ||||||
| Christian 2008 | ✓ | ||||||
| Clark 1997 | ✓ | ✓ | |||||
| Consoli 1994 | ✓ | ||||||
| Consoli 1995 | ✓ | ||||||
| Cook 2007 | ✓ | ||||||
| Cousineau 2005 | ✓ | ||||||
| Cousineau 2008 | ✓ | ||||||
| Coü 1998 | ✓ | ||||||
| Cullen 2008 | ✓ | ||||||
| Dalziel 2007 | ✓ | ||||||
| Dayton 2008 | ✓ | ||||||
| De Bar 2006 | ✓ | ||||||
| De Bar 2009 | ✓ | ✓ | |||||
| Dennison 1996 | ✓ | ||||||
| de Vet 2008 | ✓ | ||||||
| de Vries 2008 | ✓ | ||||||
| Dolhanty 2006 | ✓ | ✓ | |||||
| Doyle 2008 | ✓ | ||||||
| Dutton 1995 | ✓ | ✓ | |||||
| Dzator 2004 | ✓ | ||||||
| Dzator 2005 | ✓ | ✓ | |||||
| Eakin 2008 | ✓ | ||||||
| Eck 2005 | ✓ | ||||||
| Estabrooks 2005 | ✓ | ✓ | |||||
| Estabrooks 2008 | ✓ | ||||||
| Estabrooks 2009 | ✓ | ||||||
| Evers 2007 | ✓ | ||||||
| Ezendam 2007 | ✓ | ||||||
| Fernandez-Aranada 2009 | ✓ | ✓ | |||||
| Fitzgibbon 1995 | ✓ | ||||||
| Franko 2005 | ✓ | ||||||
| Frenn 2005 | ✓ | ||||||
| Friedman 1998 | ✓ | ||||||
| Gabriele 2009 | ✓ | ✓ | |||||
| Gadd 2000 | ✓ | ||||||
| Gans 2009 | ✓ | ||||||
| Glanz 2006 | ✓ | ||||||
| Glasgow 1995 | ✓ | ✓ | |||||
| Glasgow 1996 | ✓ | ✓ | |||||
| Glasgow 1997 | ✓ | ✓ | |||||
| Glasgow 2002 | ✓ | ||||||
| Glasgow 2003 | ✓ | ||||||
| Glasgow 2006 | ✓ | ||||||
| Glasgow 2006 | ✓ | ||||||
| Glasgow 2007 | ✓ | ||||||
| Gold 2007 | ✓ | ✓ | |||||
| Gollings 2006 | ✓ | ||||||
| Gould 2000 | ✓ | ||||||
| Goulis 2004 | ✓ | ||||||
| Greene 2008 | ✓ | ||||||
| Greenway 2005 | ✓ | ||||||
| Haerans 2006 | ✓ | ||||||
| Haerens 2006 | ✓ | ||||||
| Haerens 2007 | ✓ | ||||||
| Haerens 2007 | ✓ | ||||||
| Harvard Health Letter | ✓ | ||||||
| Harvey Berino 2002 | ✓ | ✓ | |||||
| Harvey-Berino 1998 | ✓ | ||||||
| Harvey-Berino 2004 | ✓ | ✓ | |||||
| Havas 1998 | ✓ | ||||||
| Heidal 2006 | ✓ | ||||||
| Heinicke 2007 | ✓ | ✓ | |||||
| Herrejon 2009 | ✓ | ||||||
| Horowitz 2005 | ✓ | ||||||
| Hung 2008 | ✓ | ||||||
| Hunter 2005 | ✓ | ✓ | |||||
| Hunter 2008 | ✓ | ✓ | |||||
| Jacobi 2005 | ✓ | ||||||
| Jacobi 2008 | ✓ | ||||||
| Jacobs 2002 | ✓ | ||||||
| Johnson 2008 | ✓ | ||||||
| Jones 2002 | ✓ | ||||||
| Kalten 2000 | ✓ | ||||||
| Kelders 2009 | ✓ | ||||||
| Kennedy 2008 | ✓ | ||||||
| Kerksick 2009 | ✓ | ||||||
| Kershaw 2001 | ✓ | ||||||
| Kim 2005 | ✓ | ||||||
| Kirk 2003 | ✓ | ||||||
| Kroeze 2008 | ✓ | ||||||
| Kroeze 2008 | ✓ | ||||||
| Kuhlmann 2008 | ✓ | ||||||
| Kypri 2005 | ✓ | ||||||
| Leefeldt 2007 | ✓ | ||||||
| Lindsay 2008 | ✓ | ||||||
| Lindsay 2009 | ✓ | ||||||
| Ljotsson 2007 | ✓ | ||||||
| Lutz 1996 | ✓ | ||||||
| Maddison 2009 | ✓ | ||||||
| McConnon 2007 | ✓ | ||||||
| McDoniel 2008 | ✓ | ||||||
| McDoniel 2009 | ✓ | ||||||
| McHugh 2008 | ✓ | ||||||
| McKay 2002 | ✓ | ||||||
| McVey 2009 | ✓ | ||||||
| Meyer 2004 | ✓ | ||||||
| Mhurchu 2007 | ✓ | ||||||
| Micco 2007 | ✓ | ✓ | |||||
| Miller 2007 | ✓ | ||||||
| Mitchell 2003 | ✓ | ||||||
| Mitchell 2008 | ✓ | ✓ | |||||
| Mobley 2006 | ✓ | ✓ | |||||
| Moore 2009 | ✓ | ||||||
| Morgan 2009 | ✓ | ✓ | |||||
| Murdy 2003 | ✓ | ✓ | |||||
| Nevonen 2006 | ✓ | ✓ | |||||
| Oenema 2003 | ✓ | ||||||
| Park 2008 | ✓ | ||||||
| Park 2009 | ✓ | ||||||
| Paüton 2007 | ✓ | ✓ | |||||
| Peng 2009 | ✓ | ||||||
| Plotnikoff 2005 | ✓ | ||||||
| Polzien 2005 | ✓ | ✓ | |||||
| Polzien 2007 | ✓ | ✓ | |||||
| Prochaska 2002 | ✓ | ||||||
| Prochaska 2004 | ✓ | ✓ | |||||
| Prochaska 2005 | ✓ | ||||||
| Pullen 2008 | ✓ | ||||||
| Raats 1999 | ✓ | ||||||
| Robertson 2007 | ✓ | ||||||
| Rubenfire 2006 | ✓ | ✓ | |||||
| Rubenfire 2006 | ✓ | ✓ | |||||
| Ruiter 2006 | ✓ | ||||||
| Schinke 1994 | ✓ | ||||||
| Schmidt 2006 | ✓ | ||||||
| Schmidt 2008 | ✓ | ||||||
| Schulz 2009 | ✓ | ||||||
| Shapiro 2008 | ✓ | ||||||
| Sheldon 1996 | ✓ | ||||||
| Sigrist 2004 | ✓ | ||||||
| Silk 2008 | ✓ | ||||||
| Smeets 2007 | ✓ | ||||||
| Stevens 2002 | ✓ | ✓ | |||||
| Stevens 2008 | ✓ | ||||||
| Struempler 2002 | ✓ | ||||||
| Tate 2001 | ✓ | ✓ | |||||
| Tate 2003 | ✓ | ||||||
| Tate 2003 | ✓ | ||||||
| Taylor 1991 | ✓ | ||||||
| Trepka 2008 | ✓ | ||||||
| Tsang 2001 | ✓ | ||||||
| Tsorbatzoudis 2005 | ✓ | ||||||
| Turner-McGrievy 2009 | ✓ | ||||||
| Turner-McGrievy 2009 | ✓ | ||||||
| Turnin 1994 | ✓ | ||||||
| Turnin 1995 | ✓ | ||||||
| Ueki 2009 | ✓ | ✓ | |||||
| Ueland 2009 | ✓ | ||||||
| van Assema 2006 | ✓ | ||||||
| Vandelanotte 2004 | ✓ | ✓ | |||||
| Vandelanotte 2005 | ✓ | ✓ | |||||
| Vandelanotte 2008 | ✓ | ✓ | |||||
| Volker 2008 | ✓ | ||||||
| Wangberg 2007 | ✓ | ||||||
| Webber 2008 | ✓ | ||||||
| White 2003 | ✓ | ✓ | |||||
| White 2004 | ✓ | ✓ | |||||
| Williamson 2003 | ✓ | ||||||
| Williamson 2005 | ✓ | ✓ | |||||
| Williamson 2006 | ✓ | ✓ | |||||
| Winett 1999 | ✓ | ||||||
| Wing 2006 | ✓ | ||||||
| Winzelberg 1998 | ✓ | ✓ | |||||
| Winzelberg 1999 | ✓ | ||||||
| Winzelberg 2000 | ✓ | ||||||
| Womble 2004 | ✓ | ||||||
| Yang 2004 | ✓ | ✓ | |||||
| Zabinski 2001 | ✓ | ||||||
| Zabinski 2004 | ✓ | ✓ | |||||
| Zabinski 2008 | ✓ | ||||||
| TOTAL | 81 | 5 | 126 | 19 | 10 | 34 | 2 |
Appendix 7 Additional analysis of effectiveness
This appendix includes additional analyses of outcomes, including assessment of evidence for publication bias and evidence for subgroup effects.
Table 29 presents an assessment of statistical heterogeneity in the results for outcomes reported in the included studies. The chi-squared test p-value quantifies the evidence against the null hypothesis of homogeneity (similarity) of effects estimated by the trials. I-squared (derived from the chi-squared statistic and degrees of freedom) quantifies the percentage of total variation in effects attributable to heterogeneity. The assessment of heterogeneity is shown in separate columns according to whether or not outcome measures were reported as means or as a mean change. Heterogeneity is not quantified for some outcomes for which the study reports did not provide SDs (and it was not possible to obtain these from authors), and for which an outcome was reported by a single study only.
| Outcomes | Studies | Difference in means | Difference in mean change | ||
|---|---|---|---|---|---|
| χ2 Q (df), p-value |
I 2 | χ2 Q (df), p-value |
I 2 | ||
| Fruit and vegetables | |||||
| Fruit and vegetables (servings per day) | 15 | 66.3 (11), p < 0.001 | 83.4% | – | – |
| Fruit (servings per day) | 4 | 2.8 (1), p = 0.09 | 64.9% | – | – |
| Vegetables (servings per day) | 4 | 1.6 (1), p = 0.20 | 38.0% | – | – |
| Fats | |||||
| Total fat (g/day) | 14 | 15.2 (11), p = 0.18 | 27.5% | – | – |
| Saturated fat (g/day) | 7 | 18.3 (4), p = 0.001 | 78.1% | – | – |
| % energy from fat | 13 | 39.4 (9), p < 0.001 | 77.2% | 5.9 (1), p = 0.015 | 83.1% |
| % energy from saturated fat | 2 | 2.2 (1), p = 0.13 | 55.6% | – | – |
| Whole-fat dairy (g/day) | 2 | No SD | – | No SD | – |
| % body fat | 1 | – | – | – | – |
| Poly-unsaturated fatty acid (g/day) | 1 | – | – | – | – |
| Mono-unsaturated fatty acid (g/day) | 1 | – | – | – | – |
| Trans fats (unsaturated fat) (g/day) | 1 | – | – | – | – |
| Fibre, proteins, sugars | |||||
| Dietary fibre (g/day) | 5 | 2.49 (1), p = 0.11 | 59.9% | – | – |
| Wholegrain cereals (g/day) | 3 | No SD | – | No SD | – |
| Red/processed meat (servings per day) | 1 | No SD | – | No SD | – |
| Protein (% of caloric intake) | 2 | 0.21 (1), p = 0.64 | 0.0% | – | – |
| Carbohydrate (% of caloric intake) | 2 | 11.8 (1), p = 0.001 | 91.5% | – | – |
| Saccharose (%) | 1 | – | – | – | – |
| Added sugars (g/day) | 1 | – | – | – | – |
| Glucose (mmol/l) | 1 | – | – | – | – |
| Fructosamine (%) | 1 | – | – | – | – |
| Energy | |||||
| Energy (kcal) | 9 | 4.6 (4), p = 0.33 | 12.8% | 19.7 (2), p < 0.001 | 89.9% |
| Caloric excess (kcal) | 1 | – | – | – | – |
| Weight | |||||
| BMI (kg/m2) | 12 | 3.2 (8), p = 0.92 | 0.0% | 6.5 (2), p = 0.039 | 69.2% |
| BMI z-score | 1 | – | – | – | – |
| Weight (kg) | 9 | 22.1 (3), p < 0.001 | 86.4% | 30.8 (2), p < 0.001 | 93.5% |
| % of baseline weight loss | 1 | – | – | – | – |
| Waist circumference (cm) | 1 | – | – | – | – |
| Blood pressure | |||||
| Systolic blood pressure (mmHg) | 2 | – | – | 2.3 (1), p = 0.13 | 57.1% |
| Diastolic blood pressure (mmHg) | 2 | – | – | 0.36 (1), p = 0.55 | 0.0% |
| Lipids, lipoproteins, etc. | |||||
| LDL (mmol/l) | 3 | – | – | 0.14 (1), p = 0.70 | 0.0% |
| HDL (mmol/l) | 3 | – | – | 0.02 (1), p = 0.89 | 0.0% |
| HDL2 (mmol/l) | 1 | – | – | – | – |
| Total cholesterol (mmol/l) | 3 | – | – | 8.1 (1), p = 0.004 | 87.6% |
| Triglycerides (mmol/l) | 3 | – | – | 0.03 (1), p = 0.87 | 0.0% |
| HbA1c (%) | 1 | – | – | – | – |
| Insulin (μU/ml) | 1 | – | – | – | – |
There was substantial heterogeneity (i.e. I2 > 50%) in the estimates of effect of e-learning interventions on many reported outcomes. There was relatively little evidence for heterogeneity in the estimates of effect of e-learning on total fat intake per day (p = 0.18 and I2 = 28%), energy intake per day (p = 0.33 and I2 = 13%) and BMI (p = 0.92 and I2 = 0%).
Fruit and vegetables
Figures 15–18 show analyses of estimates of effect on fruit and vegetables, fruit only and vegetables only.
FIGURE 15.
Forest plot showing the effect of e-learning on the mean intake of fruit and vegetables (servings per day).
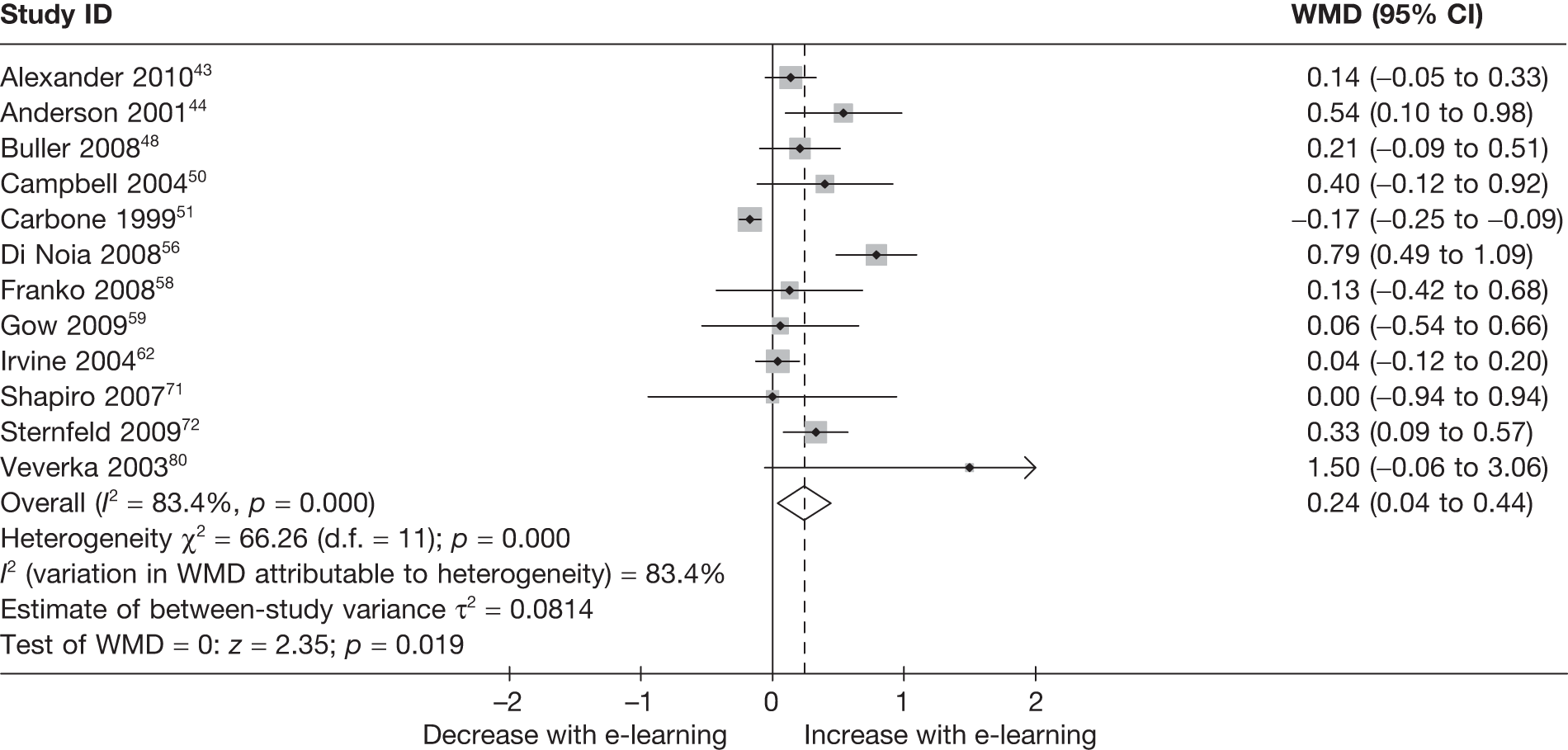
FIGURE 16.
Funnel plot showing estimates of the effect of e-learning on the mean intake of fruit and vegetables (servings per day).
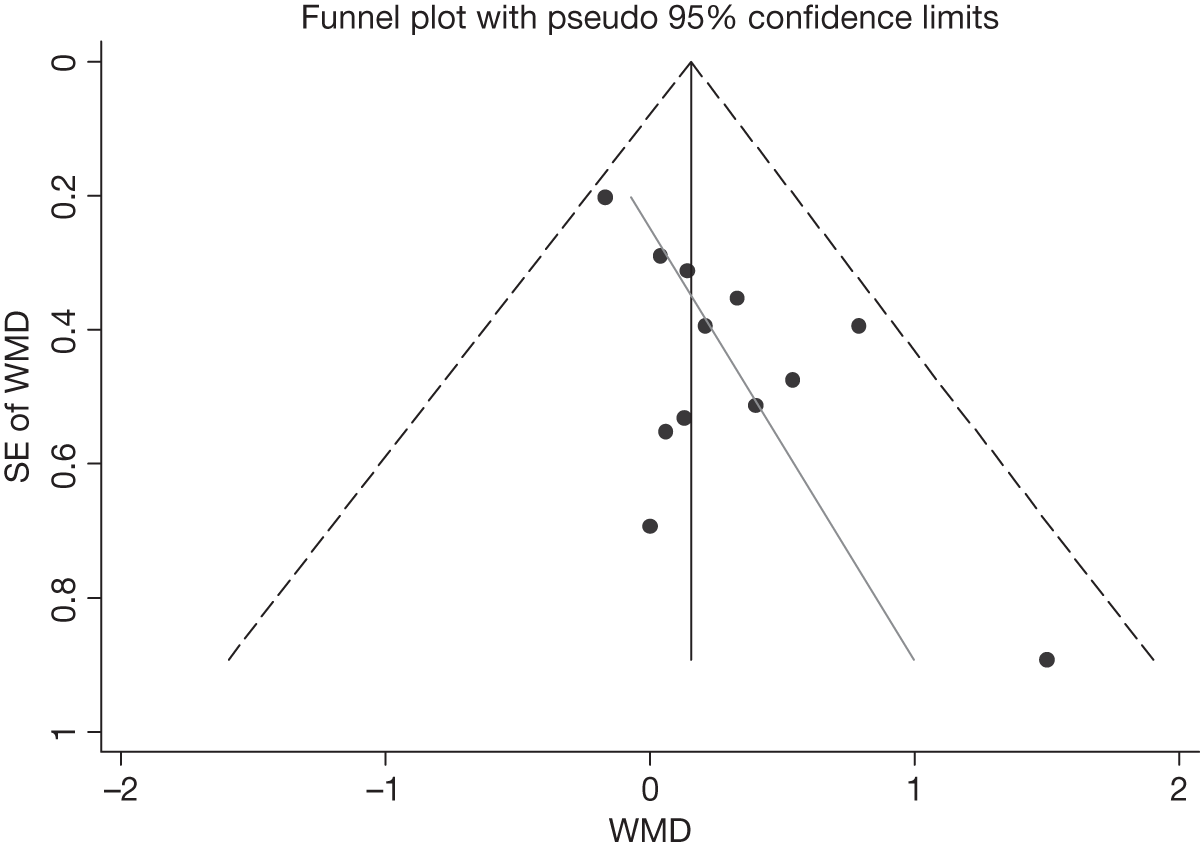
FIGURE 17.
Forest plot showing the effect of e-learning on the mean intake of fruit (servings per day).
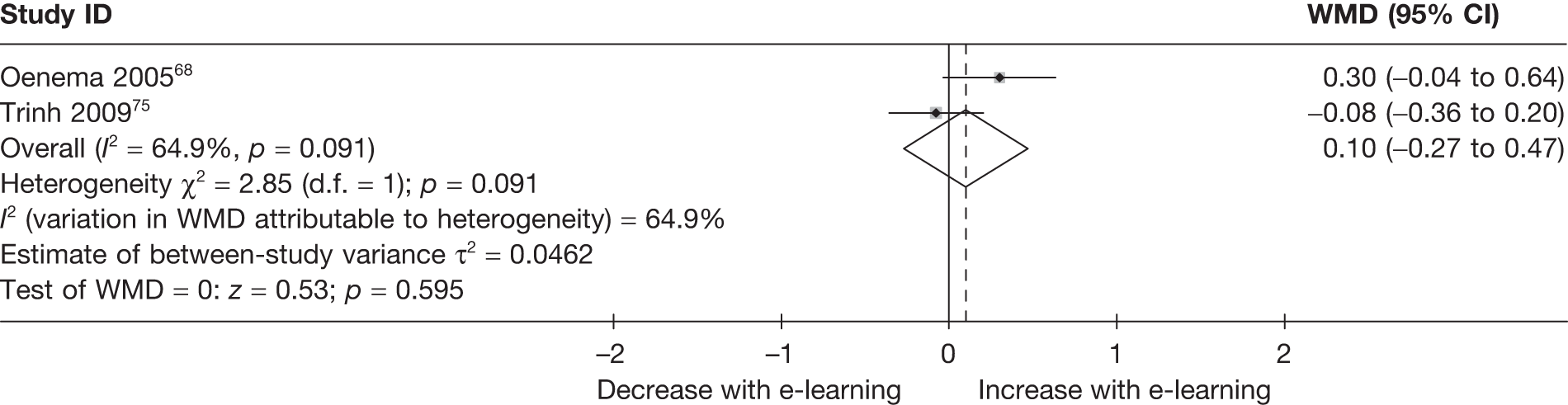
FIGURE 18.
Forest plot showing the effect of e-learning on the mean intake of vegetables (servings per day).
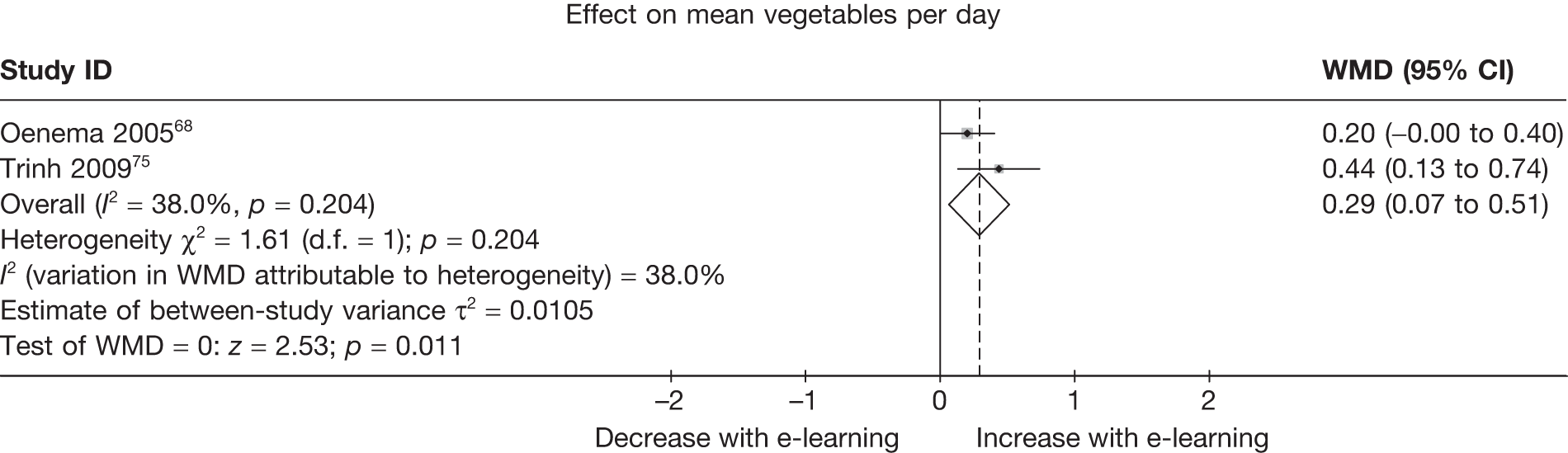
Egger’s test for small-study effects:
-
Number of studies = 12, root MSE = 1.781
| Std_Eff | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| Slope | –.2111621 | .0847431 | –2.49 | 0.032 | –.3999816 | –.0223426 |
| Bias | 2.616964 | .7948991 | 3.29 | 0.008 | .8458188 | 4.38811 |
Figure 16 shows the estimates of effect of e-learning on mean intake of fruit and vegetables (servings per day) plotted against their standard errors. Smaller studies are associated with a larger treatment effect (i.e. larger increase in servings of fruit and vegetables per day, p = 0.008).
Fats
Figures 19–26 show analyses of estimates of effect on total fat, saturated fat and percentage of energy from fat.
FIGURE 19.
Forest plot showing the effect of e-learning on the mean intake of fat (g/day).
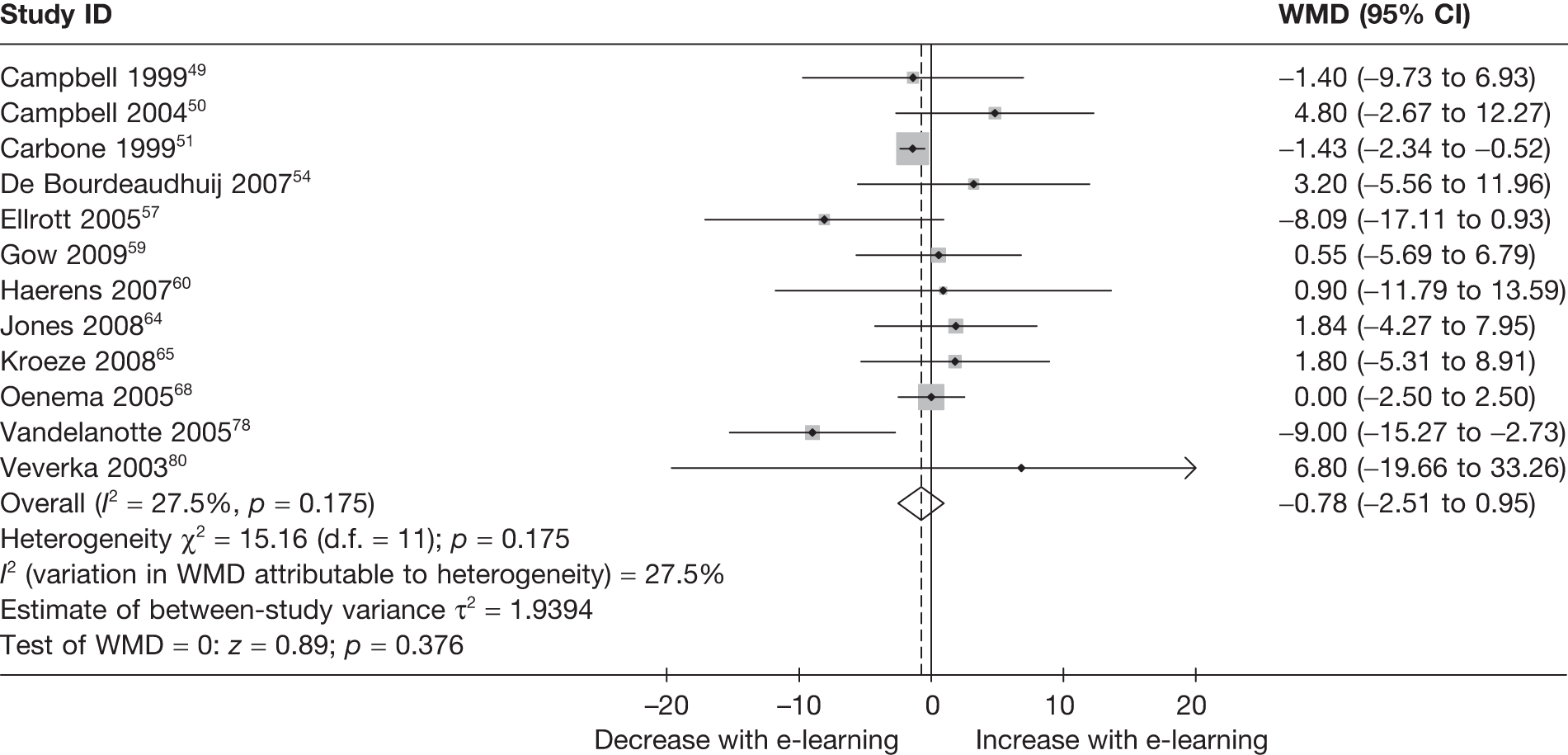
FIGURE 20.
Funnel plot showing estimates of the effect of e-learning on the mean intake of fat (g/day).
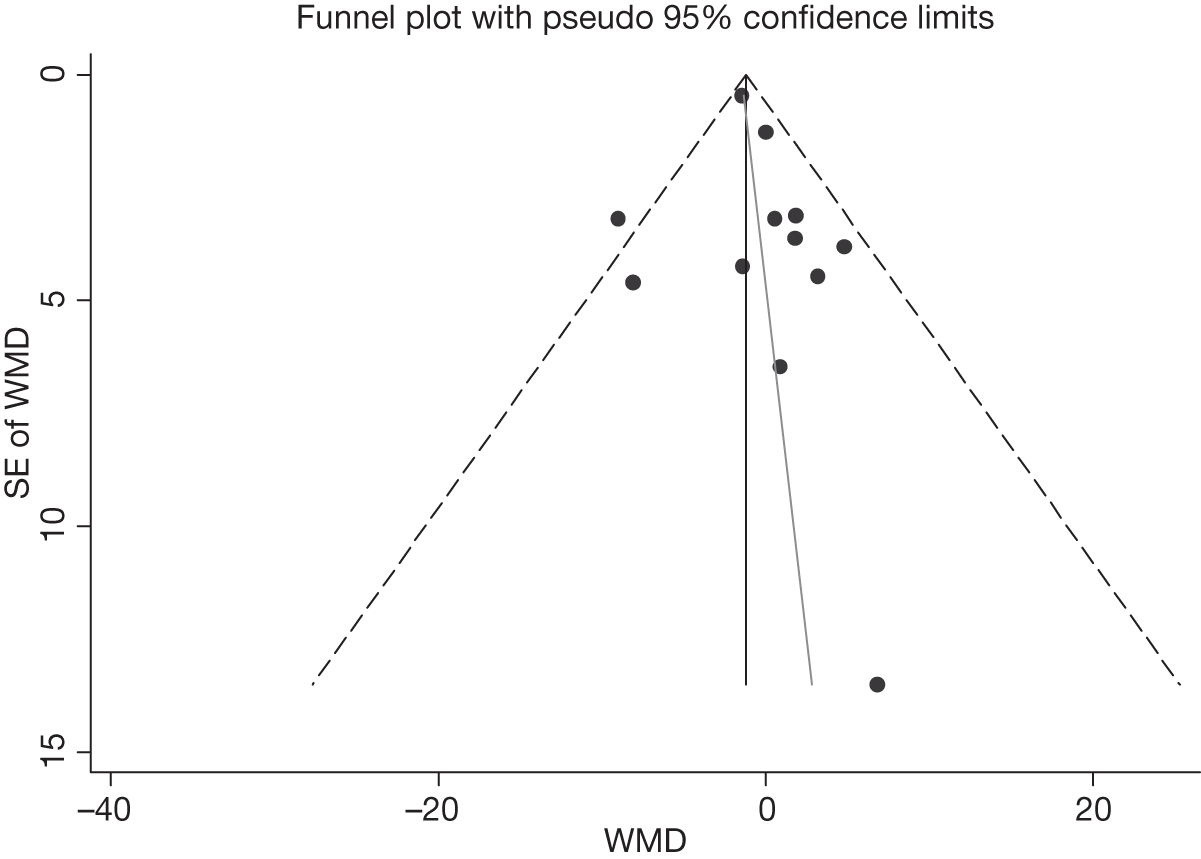
FIGURE 21.
Forest plot showing the effect of e-learning on the mean intake of saturated fat (g/day).
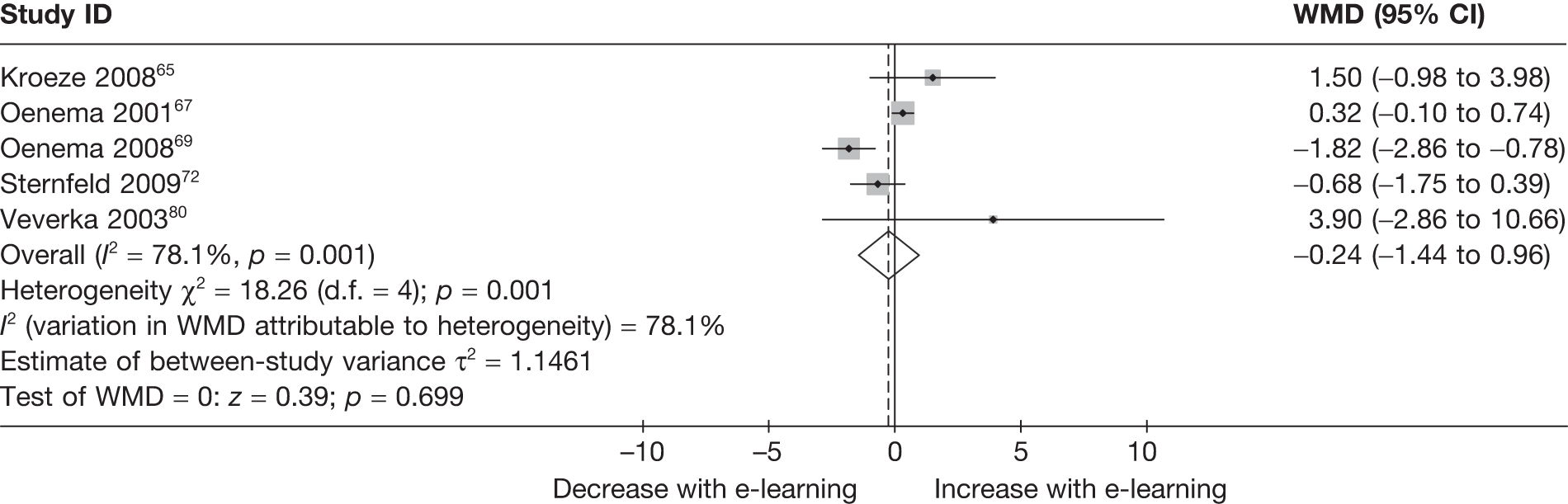
FIGURE 22.
Funnel plot showing estimates of the effect of e-learning on the mean intake of saturated fat (g/day).
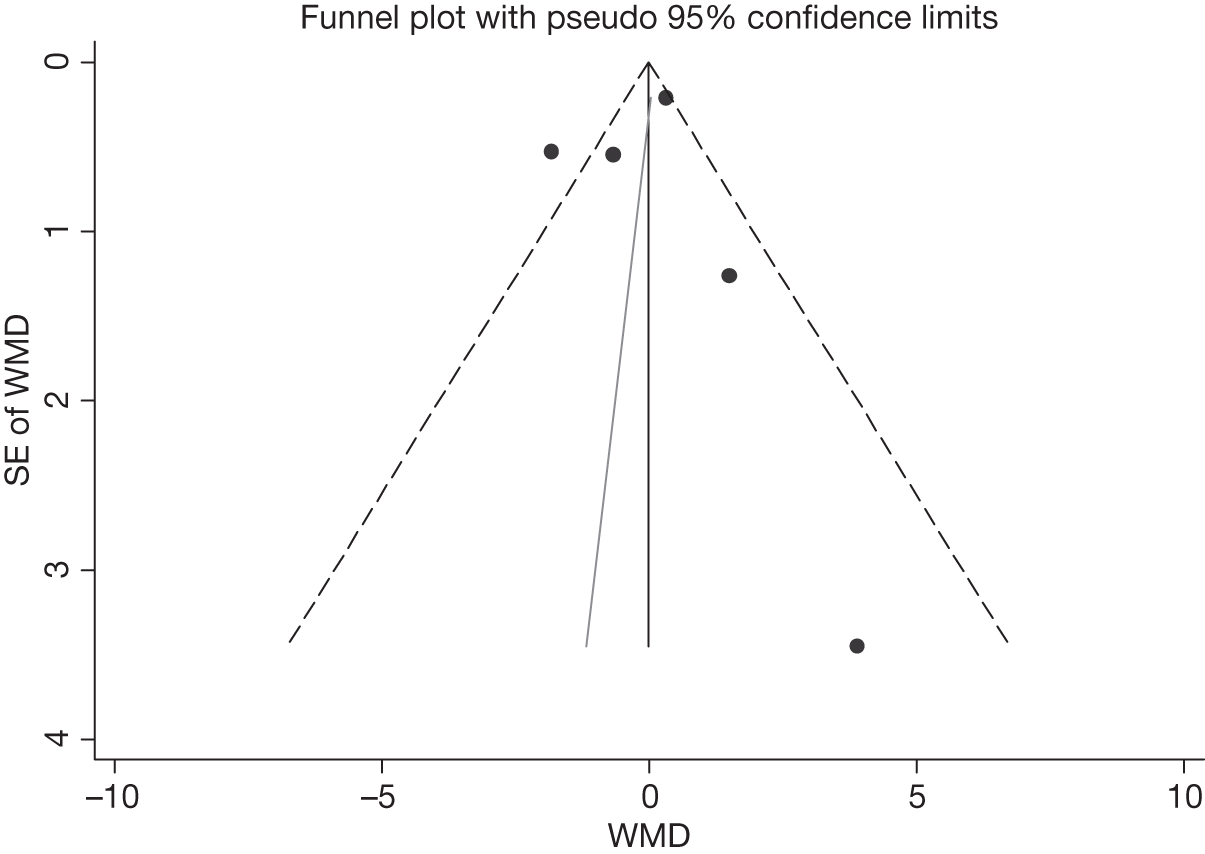
FIGURE 23.
Forest plot showing effect of e-learning on mean percentage of energy from fat (%).
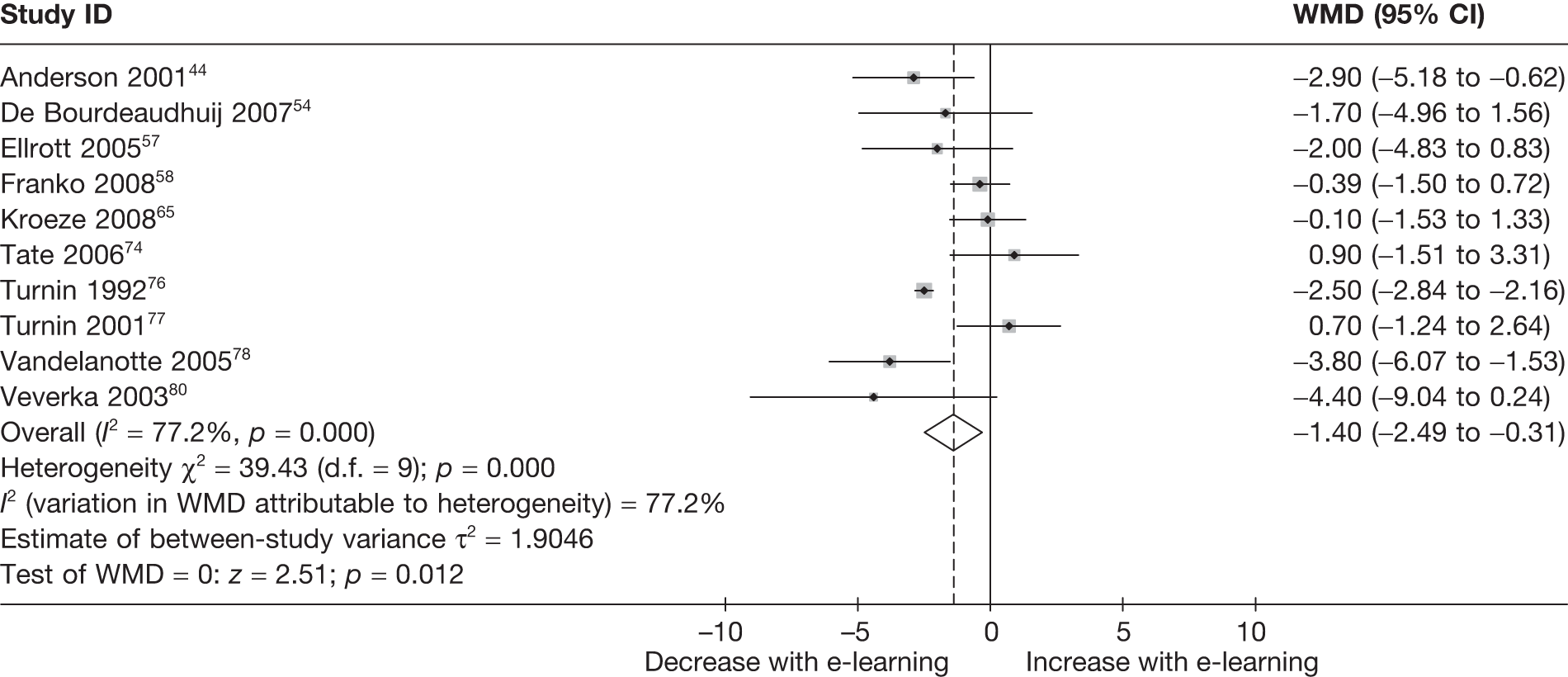
FIGURE 24.
Funnel plot showing estimates of the effect of e-learning on the mean percentage of energy from fat.
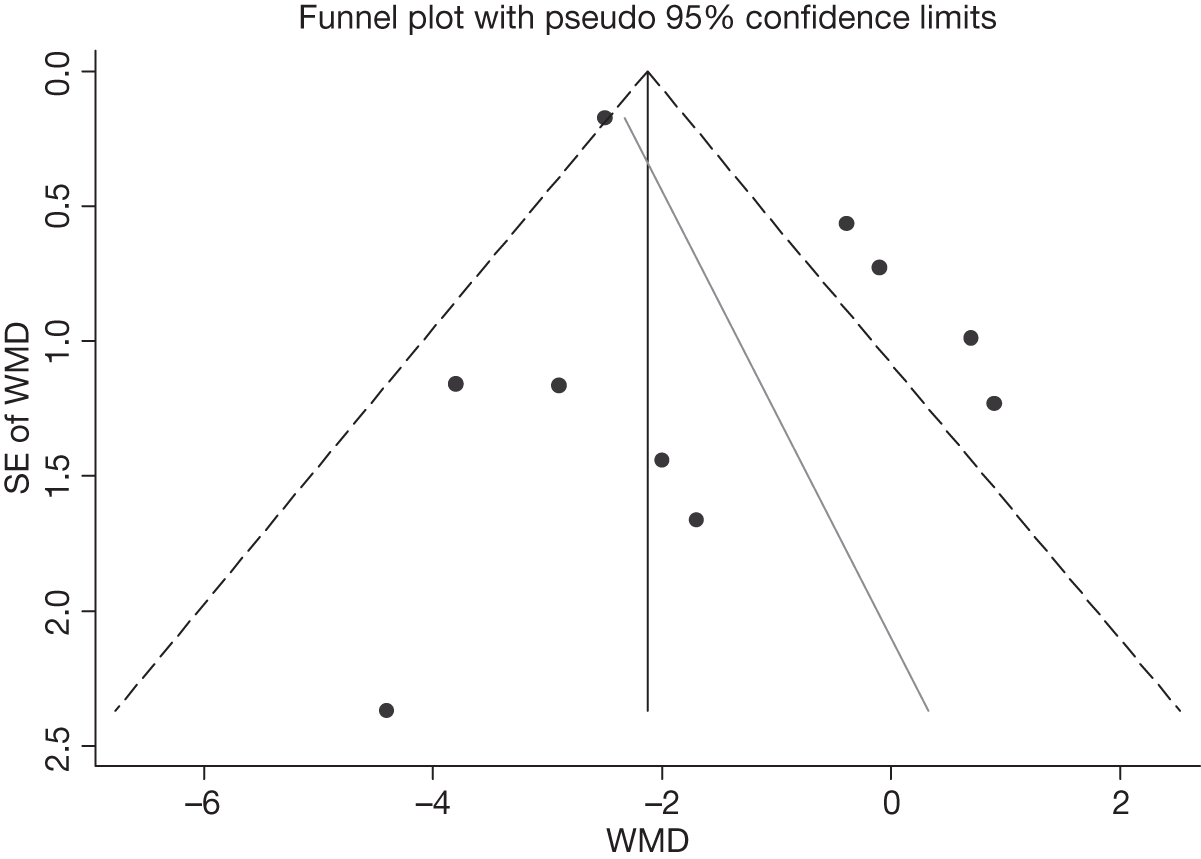
FIGURE 25.
Forest plot showing the effect of e-learning on the mean change in percentage of energy from fat (%).
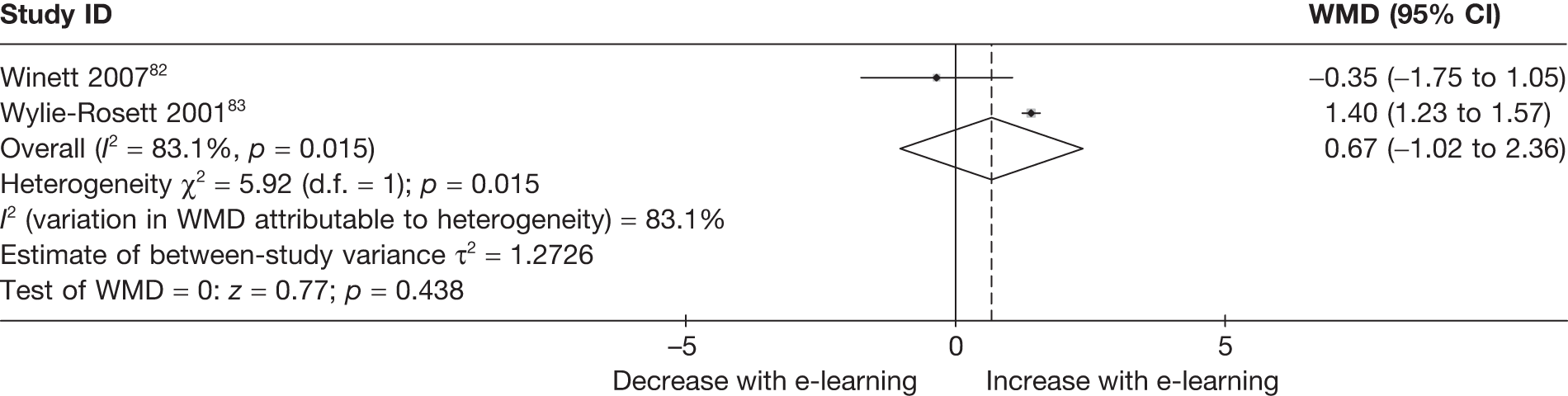
FIGURE 26.
Forest plot showing the effect of e-learning on the mean percentage of energy from saturated fat (%).
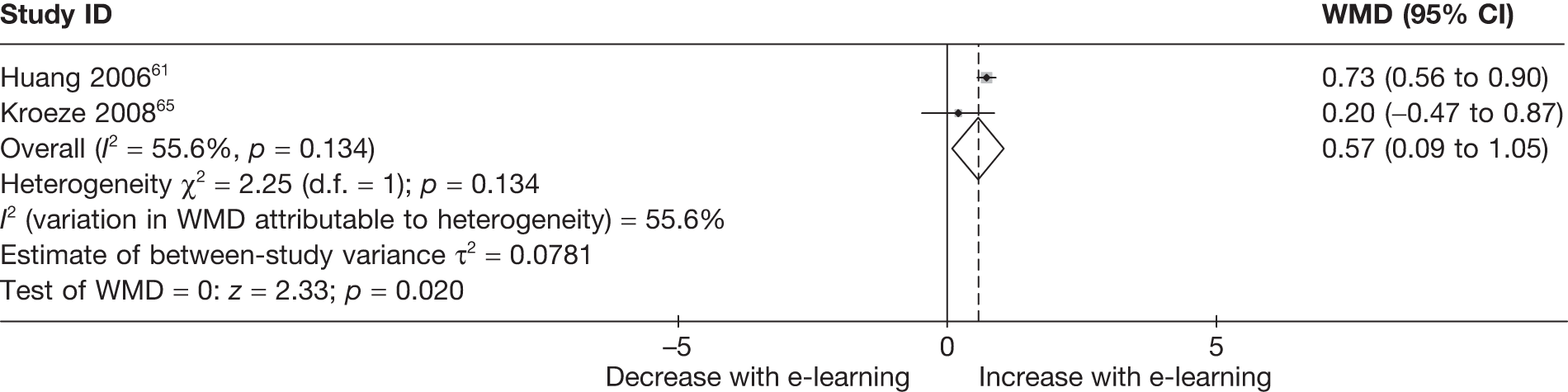
Egger’s test for small-study effects:
-
Regress standard normal deviate of intervention
-
Effect estimate against its SE
-
Number of studies = 12, root MSE = 1.202
| Std_Eff | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| Slope | –1.485873 | .6410574 | –2.32 | 0.043 | –2.914238 | -.0575083 |
| Bias | .3176107 | .4487315 | 0.71 | 0.495 | -.6822255 | 1.317447 |
Figure 20 shows the estimates of effect of e-learning on mean intake of fat (g/day) plotted against their standard errors. There was no evidence that smaller studies are associated with a larger or smaller treatment effect (p = 0.495).
Egger’s test for small-study effects:
-
Regress standard normal deviate of intervention
-
Effect estimate against its SE
-
Number of studies = 5, root MSE = 2.449
| Std_Eff | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| Slope | .1064684 | .7148023 | 0.15 | 0.891 | –2.168352 | 2.381288 |
| Bias | -.3716999 | 1.744761 | –0.21 | 0.845 | –5.924307 | 5.180907 |
Figure 22 shows the estimates of effect of e-learning on the mean intake of saturated fat (g/day) plotted against their standard errors. There was no evidence that smaller studies are associated with a larger or smaller treatment effect (p = 0.84).
Egger’s test for small-study effects:
-
Regress standard normal deviate of intervention
-
Effect estimate against its SE
-
Number of studies = 10, root MSE = 1.994
| Std_Eff | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| Slope | –2.535416 | .4271861 | –5.94 | 0.000 | –3.520509 | –1.550323 |
| Bias | 1.207866 | .8719168 | 1.39 | 0.203 | –.8027776 | 3.21851 |
Figure 24 shows the estimates of effect of e-learning on mean percentage of energy from fat plotted against their standard errors. There was no evidence that smaller studies are associated with a larger or smaller treatment effects (p = 0.203).
Fibre, proteins, sugars
Figures 27–29 show analyses of the estimates of effect on dietary fibre, percentage of energy from protein, and percentage of energy from carbohydrate.
FIGURE 27.
Forest plot showing the effect of e-learning on the mean intake of dietary fibre (g/day).
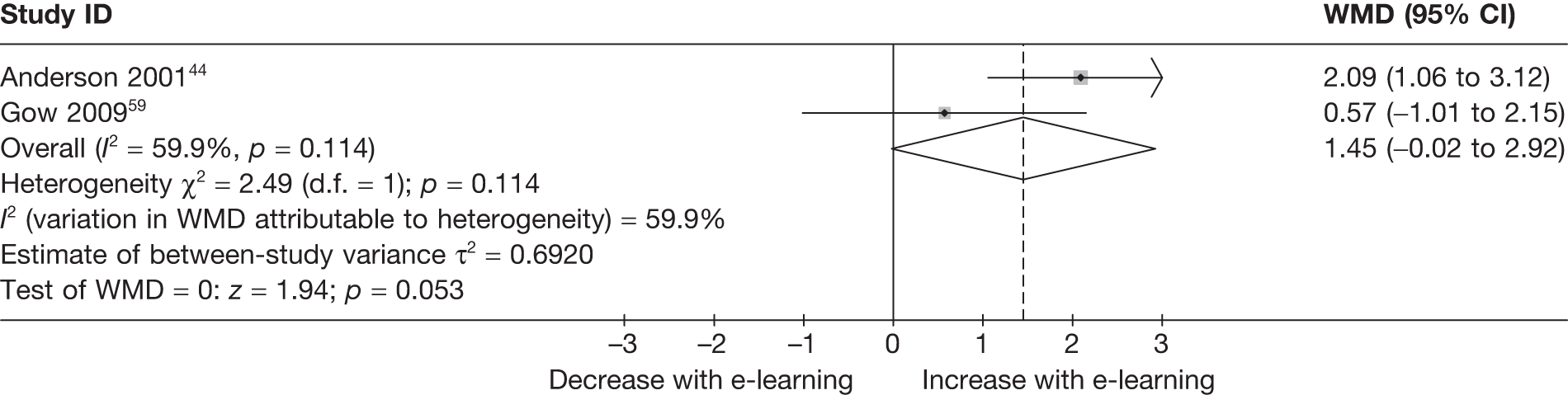
FIGURE 28.
Forest plot showing the effect of e-learning on the mean percentage of energy from protein (%).
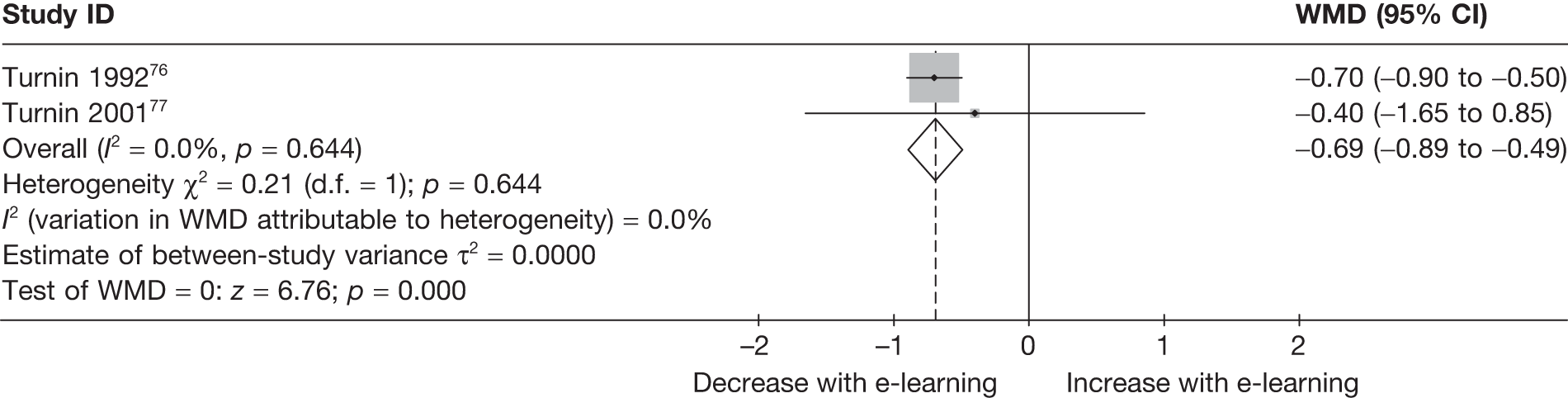
FIGURE 29.
Forest plot showing the effect of e-learning on the mean percentage of energy from carbohydrate (%).
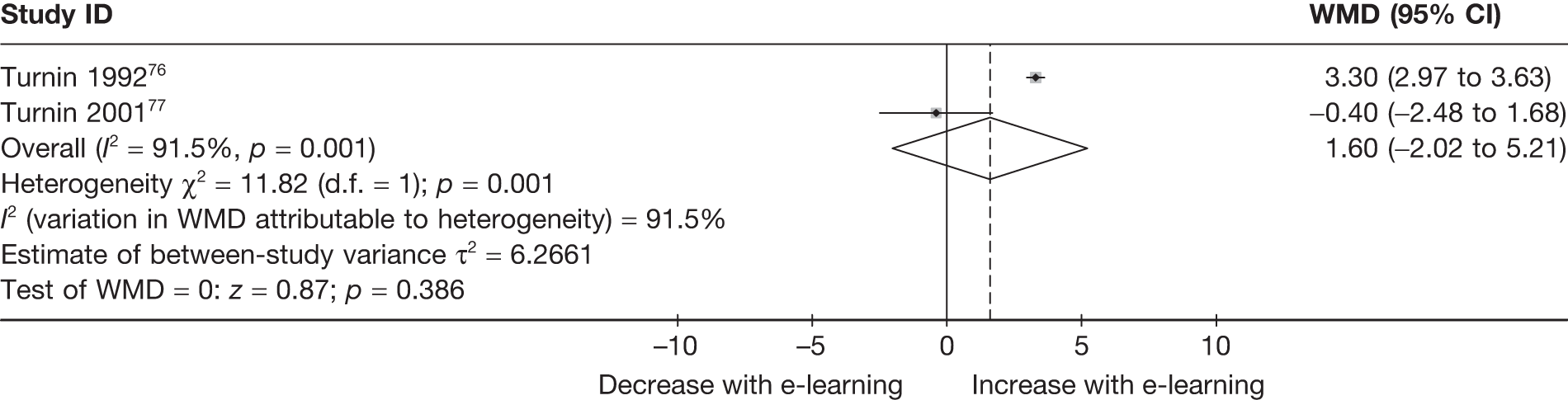
Energy
Figures 30 and 31 show analyses of estimates of effect on energy intake.
FIGURE 30.
Forest plot showing the effect of e-learning on the mean energy intake (kcal/day).
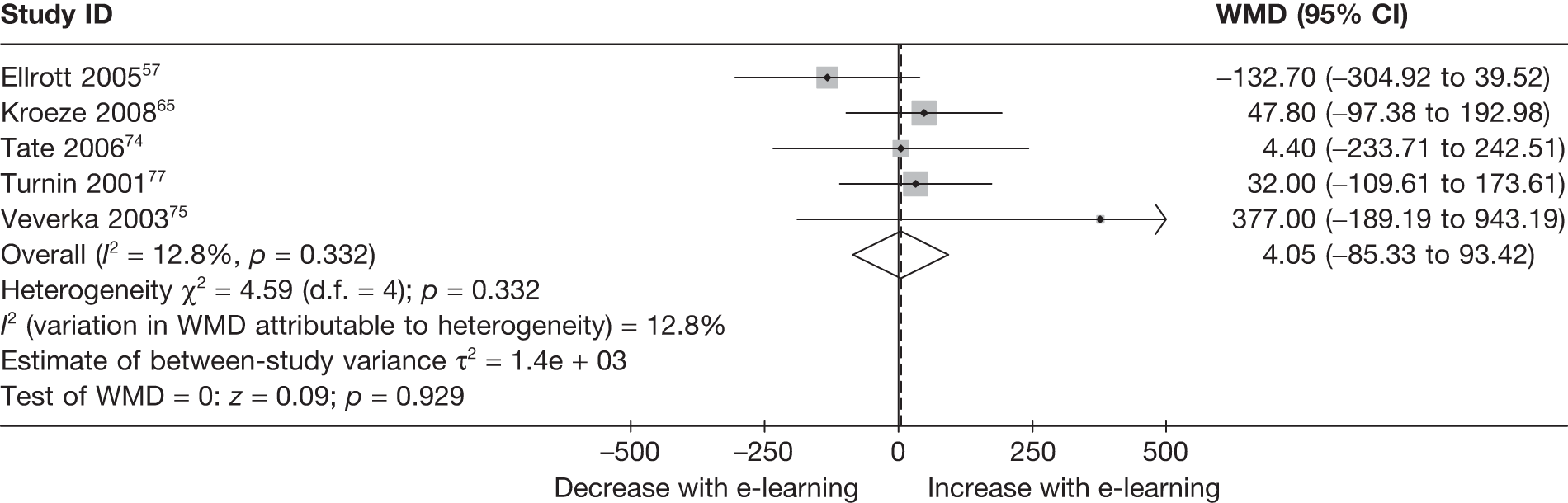
FIGURE 31.
Forest plot showing the effect of e-learning on the mean change in energy intake (kcal/day).
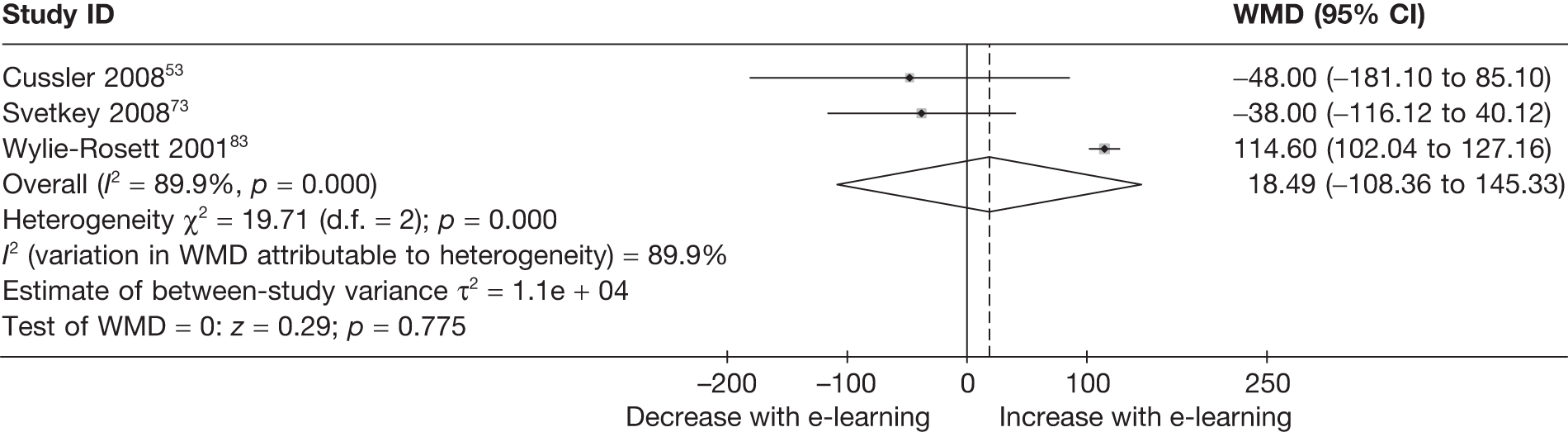
Body mass index/weight
Figures 32–36 show analyses of estimates of effect on BMI and weight.
FIGURE 32.
Forest plot showing the effect of e-learning on the mean BMI (kg/m2).
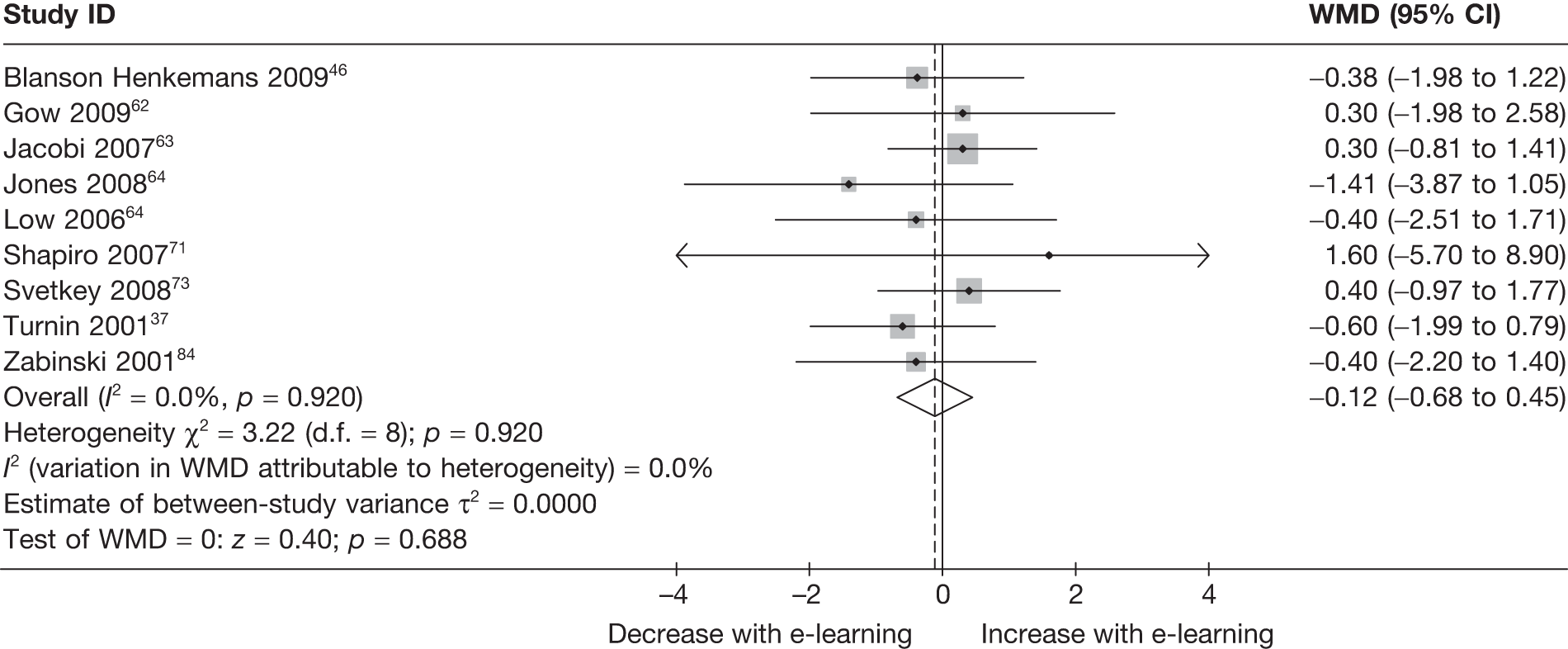
FIGURE 33.
Funnel plot showing estimates of the effect of e-learning on the mean BMI (kg/m2).
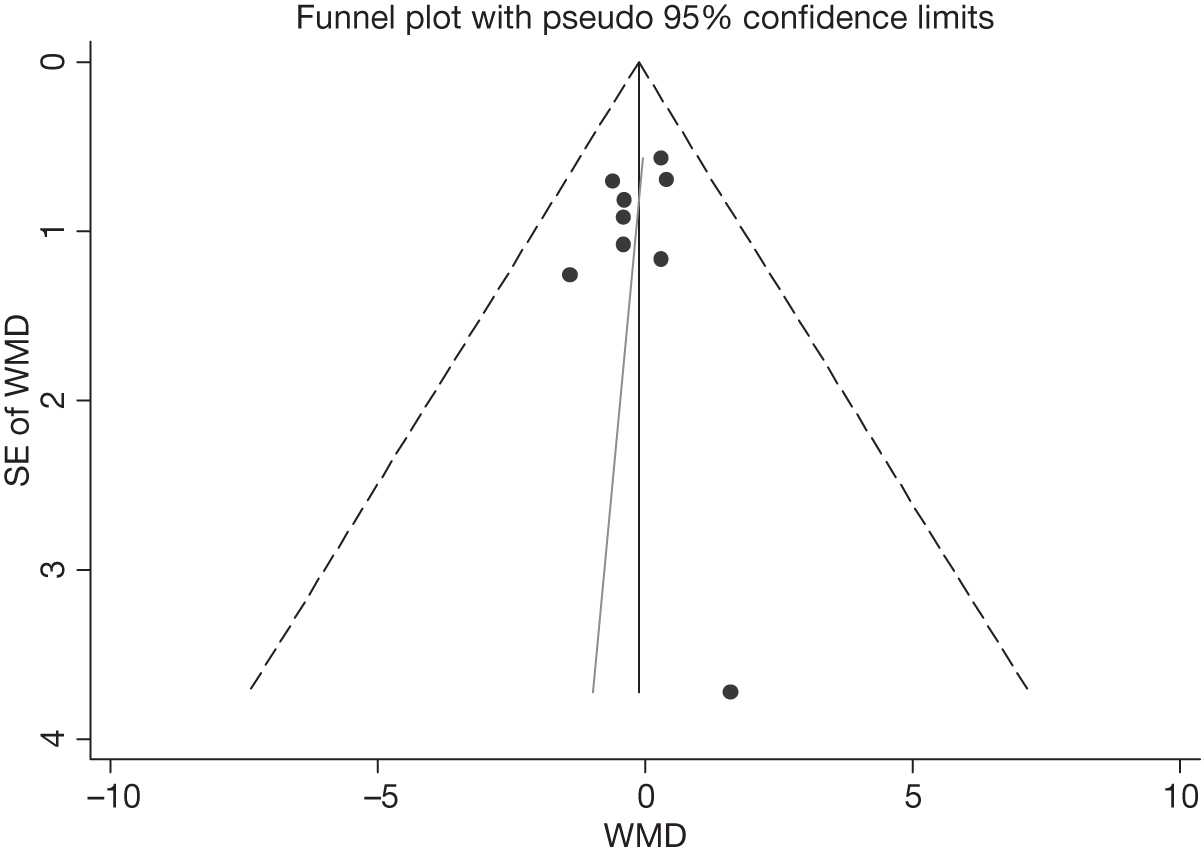
FIGURE 34.
Forest plot showing the effect of e-learning on the mean change in BMI (kg/m2).
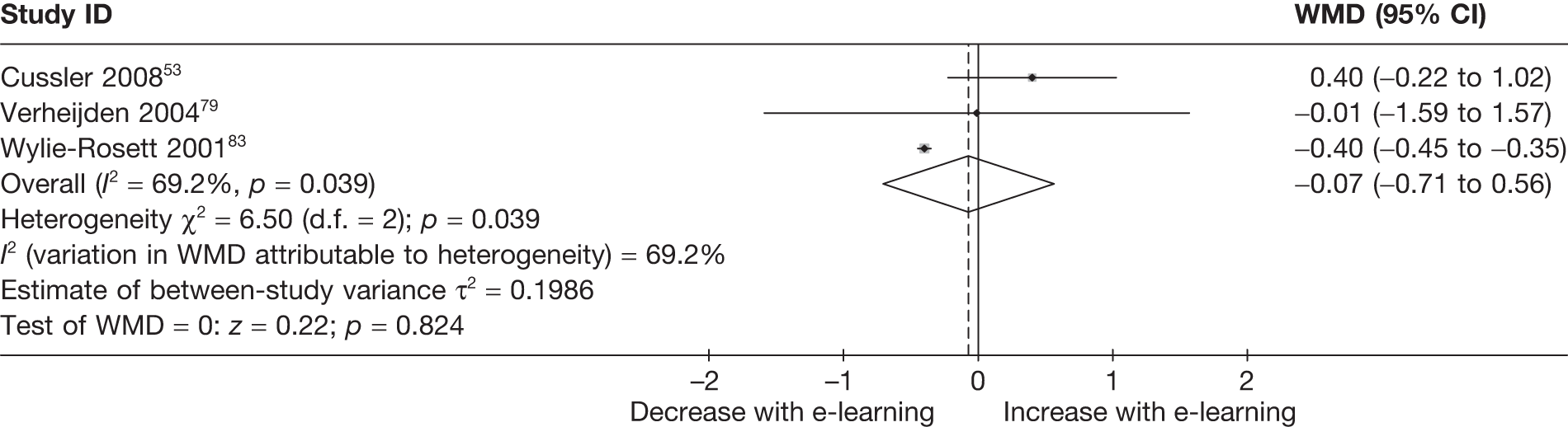
FIGURE 35.
Forest plot showing the effect of e-learning on the mean weight (kg).
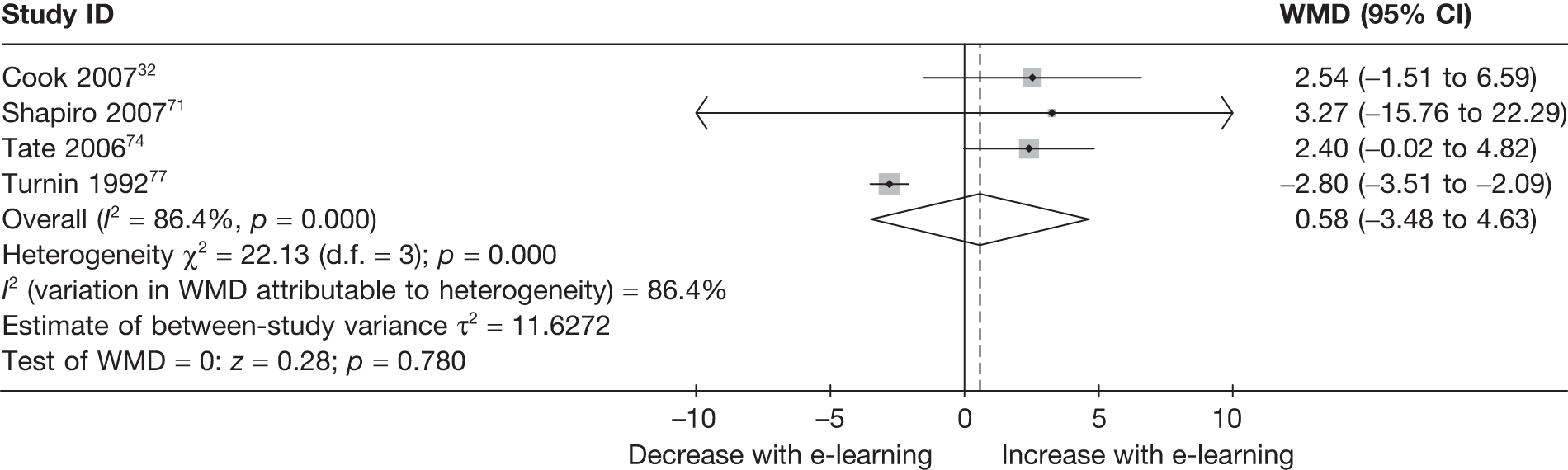
FIGURE 36.
Forest plot showing the effect of e-learning on the mean change in weight (kg).
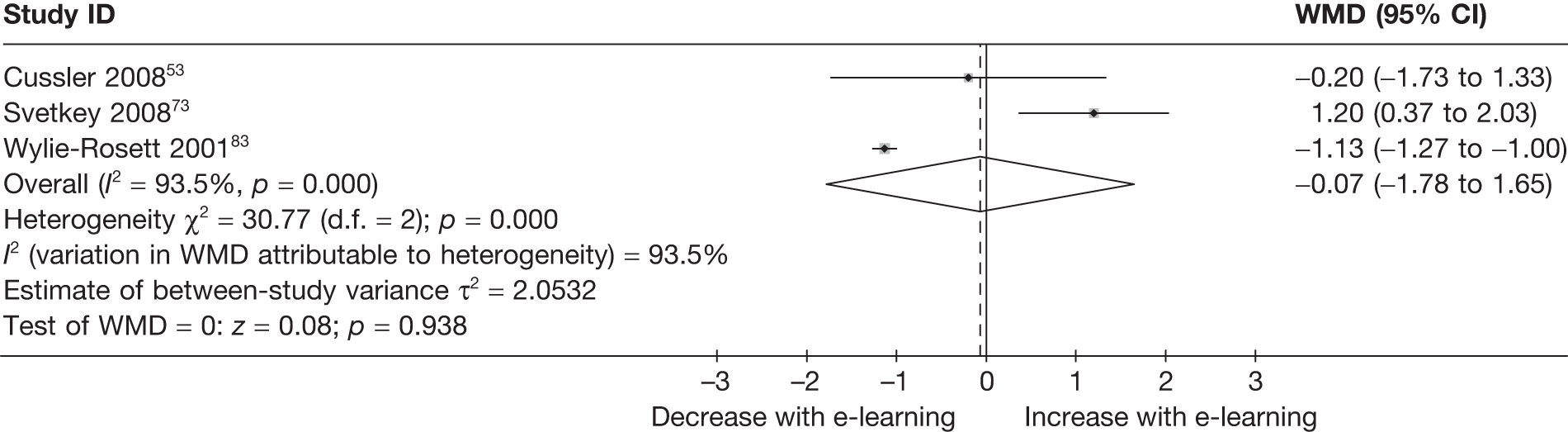
Egger’s test for small-study effects:
-
Regress standard normal deviate of intervention
-
Effect estimate against its SE
-
Number of studies = 9, root MSE = .6681
| Std_Eff | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| Slope | .1203499 | .5400326 | 0.22 | 0.830 | –1.156624 | 1.397324 |
| Bias | –.2923767 | .6274486 | –0.47 | 0.655 | –1.776057 | 1.191303 |
Figure 33 shows estimates of effect of e-learning on mean BMI plotted against their standard errors. There was no evidence that smaller studies are associated with larger treatment effects (i.e. larger decrease in BMI, p = 0.66).
Blood pressure
Figures 37 and 38 show analyses of the estimates of effect on systolic and diastolic blood pressure.
FIGURE 37.
Forest plot showing the effect of e-learning on the mean change in systolic blood pressure (mmHg).
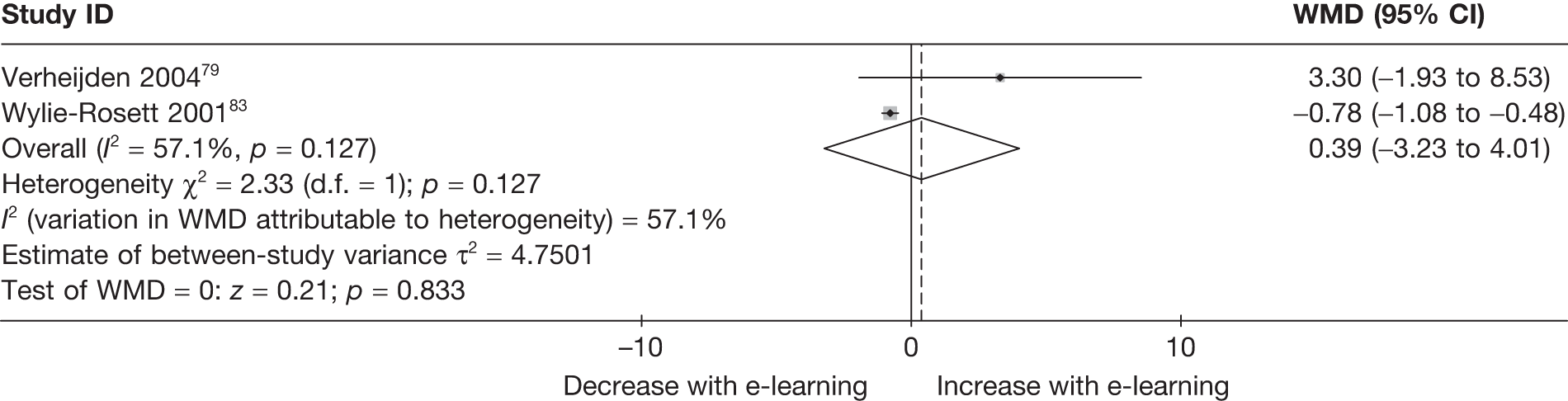
FIGURE 38.
Forest plot showing the effect of e-learning on the mean change in diastolic blood pressure (mmHg).
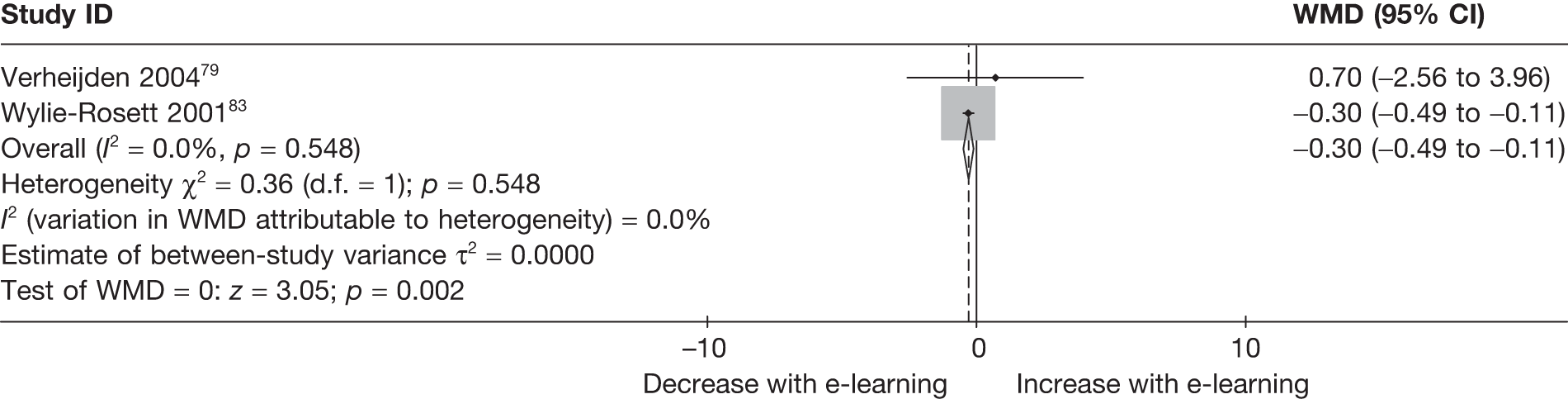
Lipids and lipoproteins
Figures 39–42 show analyses of estimates of effect on low-density lipids, high-density lipids, total cholesterol and triglycerides.
FIGURE 39.
Forest plot showing the effect of e-learning on the mean change in low-density lipids (mmol/l).
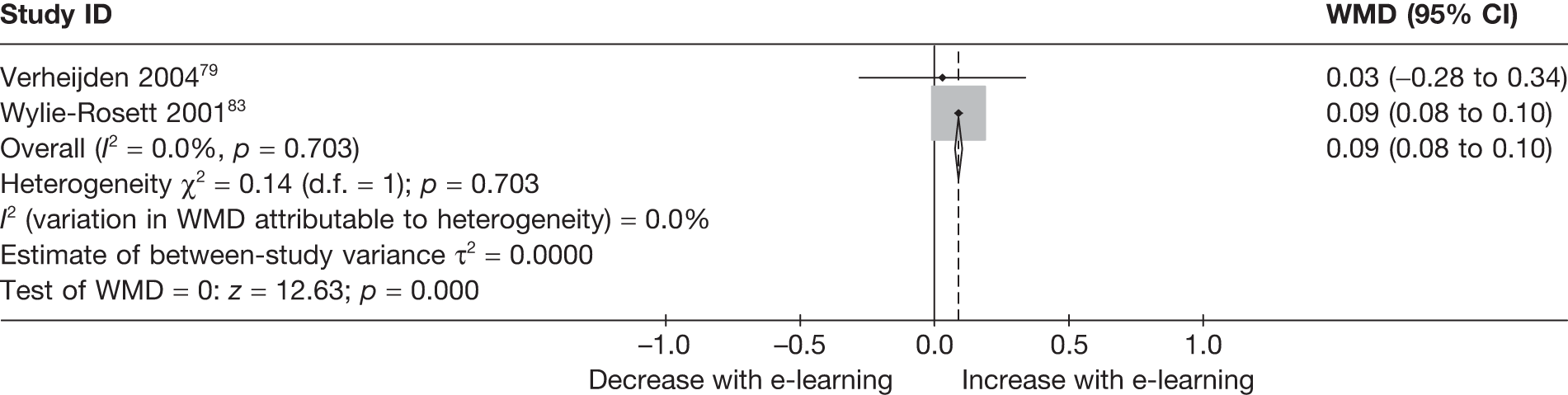
FIGURE 40.
Forest plot showing the effect of e-learning on the mean change in high-density lipids (mmol/l).
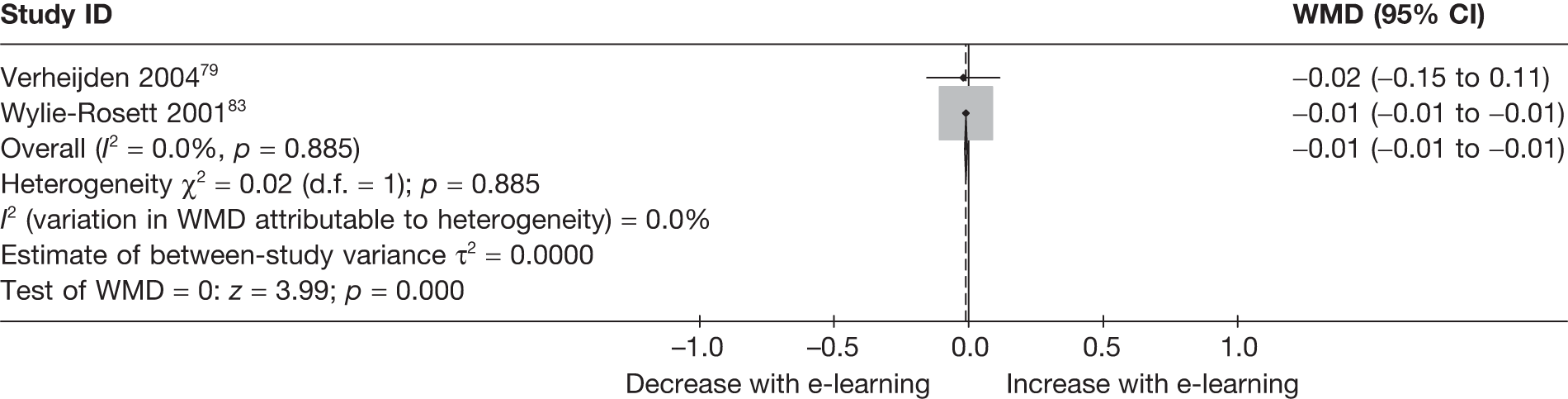
FIGURE 41.
Forest plot showing the effect of e-learning on the mean change in total cholesterol (mmol/l).
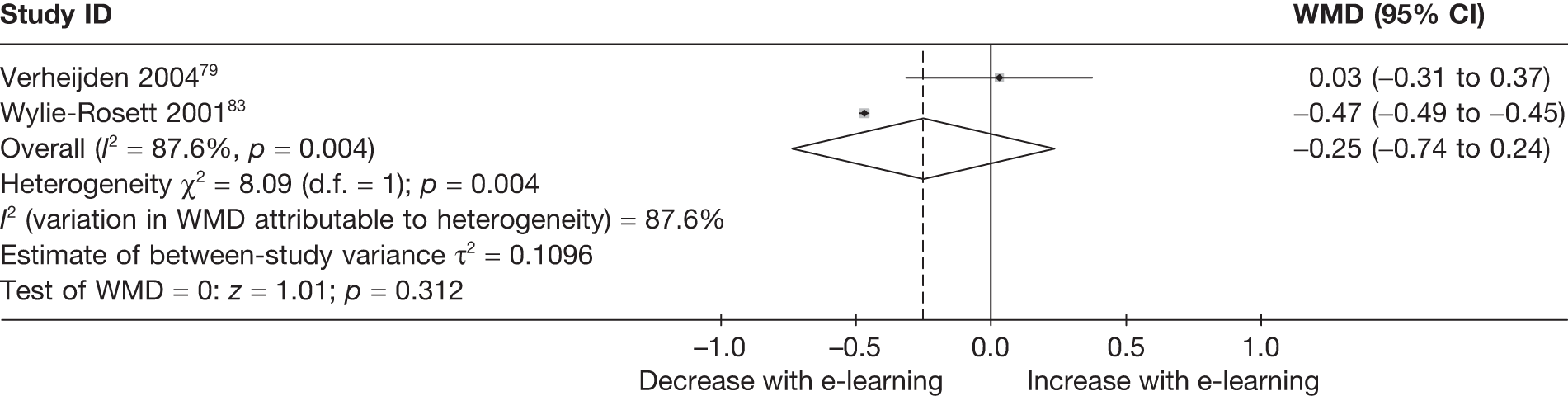
FIGURE 42.
Forest plot showing the effect of e-learning on the mean change in triglycerides (mmol/l).
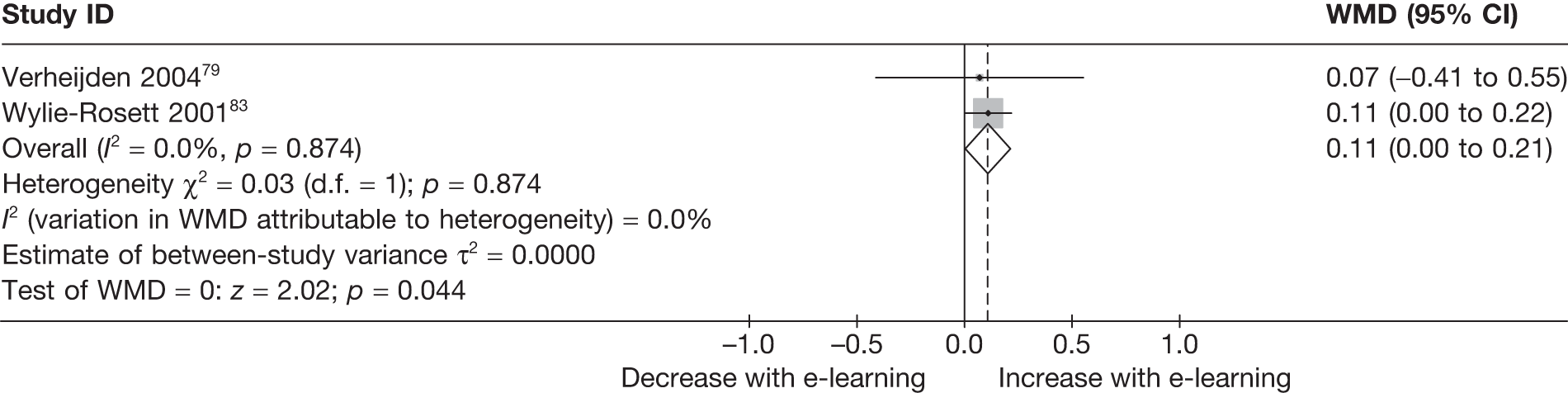
Subgroup analysis for selected outcomes
Our published study protocol stated that in order to investigate possible sources of heterogeneity we would conduct sensitivity analysis by study quality and by SES of participants. Subgroup analysis has been conducted where outcomes were reported by five or more studies. Subgroups were also investigated for our secondary outcomes of BMI and weight, according to whether or not studies aimed to maintain or reduce BMI (or weight), and whether or not studies included a physical activity component.
The Stata output is shown for each subgroup and an interpretation provided.
1. Study quality (EPHPP assessment)
Fruit and vegetables
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 61.4446
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.837
-
Moment-based estimate of between-study variance: τ2 = 0.0936
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iephpp_2 | –.0587248 | .2545131 | –0.23 | 0.822 | –.6258154 | .5083657 |
| _cons | .2898408 | .2220584 | 1.31 | 0.221 | –.2049361 | .7846178 |
There was no evidence to suggest that estimates of effect on servings of fruit and vegetables were associated with EPHPP global rating.
Total fat
-
. xi: metareg wmd i.ephpp, wsse(se) mm
-
i.ephpp _Iephpp_1–2 (_Iephpp_1 for ephpp = = MODERATE omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 10.9395
-
p > Q = 0.362
-
Proportion of variation due to heterogeneity I2 = 0.086
-
Moment-based estimate of between-study variance: τ2 = 0.4840
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iephpp_2 | –4.414625 | 2.273575 | –1.94 | 0.081 | –9.480465 | .6512156 |
| _cons | 3.059523 | 2.172567 | 1.41 | 0.189 | –1.781259 | 7.900304 |
There was weak evidence to suggest that studies with ‘weak’ EPHPP global rating on average found larger reductions on fat intake (p = 0.081).
Percentage energy from fat
-
. xi: metareg wmd i.ephpp, wsse(se) mm
-
i.ephpp _Iephpp_1–2 (_Iephpp_1 for ephpp = = MODERATE omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 28.5597
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.720
-
Moment-based estimate of between-study variance: τ2 = 1.6222
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iephpp_2 | –1.075684 | 1.170951 | –0.92 | 0.385 | –3.775902 | 1.624534 |
| _cons | –.6570533 | .9659334 | –0.68 | 0.516 | –2.8845 | 1.570393 |
There was no evidence to suggest that estimates of effect on percentage of energy fat were associated with the EPHPP global rating.
Energy
-
. xi: metareg wmd i.ephpp, wsse(se) mm
-
i.ephpp _Iephpp_1–2 (_Iephpp_1 for ephpp = = MODERATE omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 3.51876
-
p > Q = 0.318
-
Proportion of variation due to heterogeneity I2 = 0.147
-
Moment-based estimate of between-study variance: τ2 = 1.9e+03
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iephpp_2 | –91.91838 | 94.49451 | –0.97 | 0.402 | –392.6421 | 208.8053 | |
| _cons | 53.8557 | 69.5182 | 0.77 | 0.495 | –167.3822 | 275.0936 |
There was no evidence to suggest that estimates of effect on total energy intake were associated with EPHPP global rating.
Body mass index
-
. xi: metareg wmd i.ephpp, wsse(se) mm
-
i.ephpp _Iephpp_1–2 (_Iephpp_1 for ephpp = = MODERATE omitted)
-
Meta-regression number of studies = 9
-
Fit of model without heterogeneity (τ2 = 0): Q (7 d.f.) = 2.83317
-
p > Q = 0.900
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iephpp_2 | –.4149028 | .6658704 | –0.62 | 0.553 | –1.989436 | 1.15963 |
| _cons | .1978034 | .5780817 | 0.34 | 0.742 | –1.169143 | 1.564749 |
There was no evidence to suggest that estimates of effect on BMI were associated with the EPHPP global rating.
Weight
-
. xi: metareg wmd i.ephpp, wsse(se) mm
-
i.ephpp _Iephpp_1–2 (_Iephpp_1 for ephpp = = MODERATE omitted)
-
Meta-regression number of studies = 4
-
Fit of model without heterogeneity (τ2 = 0): Q (2 d.f.) = 6.48674
-
p > Q = 0.039
-
Proportion of variation due to heterogeneity I2 = 0.692
-
Moment-based estimate of between-study variance: τ2 = 9.4399
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iephpp_2 | –3.087439 | 3.944292 | –0.78 | 0.516 | –20.05836 | 13.88348 |
| _cons | 2.48286 | 3.149766 | 0.79 | 0.513 | –11.06949 | 16.03521 |
There was no evidence to suggest that estimates of effect on weight were associated with the EPHPP global rating.
2. Low attrition (0–20%) versus high attrition (> 20%)
Fruit and vegetables
-
. xi: metareg wmd i.CompleteEPHPP, wsse(se) mm
-
i.CompleteEPHPP _ICompleteE_1–2 (_ICompleteE_1 for Com∼P = = 60–79% omitted)
-
Meta-regression number of studies = 11
-
Fit of model without heterogeneity (τ2 = 0): Q (9 d.f.) = 25.0174
-
p > Q = 0.003
-
Proportion of variation due to heterogeneity I2 = 0.640
-
Moment-based estimate of between-study variance: τ2 = 0.0577
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IComplete∼2 | .1077183 | .2054918 | 0.52 | 0.613 | –.3571365 | .572573 |
| _cons | .2602589 | .1213245 | 2.15 | 0.061 | –.0141961 | .5347139 |
There was no evidence to suggest that estimates of effect on daily servings of fruit and vegetables were associated with the level of attrition in studies.
Total fat
-
. xi: metareg wmd i.CompleteEPHPP, wsse(se) mm
-
i.CompleteEPHPP _ICompleteE_1–2 (_ICompleteE_1 for Com∼P = = 60–79% omitted)
-
Meta-regression number of studies = 11
-
Fit of model without heterogeneity (τ2 = 0): Q (9 d.f.) = 13.4312
-
p > Q = 0.144
-
Proportion of variation due to heterogeneity I2 = 0.330
-
Moment-based estimate of between-study variance: τ2 = 5.4695
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IComplete∼2 | 2.718195 | 3.868409 | 0.70 | 0.500 | –6.032753 | 11.46914 |
| _cons | –.8227567 | 1.432562 | –0.57 | 0.580 | –4.063436 | 2.417923 |
There was no evidence to suggest that estimates of effect on total fat intake were associated with the level of attrition in studies.
Percentage energy from fat
-
. xi: metareg wmd i.CompleteEPHPP, wsse(se) mm
-
i.CompleteEPHPP _ICompleteE_1–2 (_ICompleteE_1 for Com∼P = = 60–79% omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 38.8865
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.794
-
Moment-based estimate of between-study variance: τ2 = 2.3678
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IComplete∼2 | .8740884 | 1.206316 | 0.72 | 0.489 | –1.907681 | 3.655858 |
| _cons | –1.890358 | .8921099 | –2.12 | 0.067 | –3.947567 | .1668514 |
There was no evidence to suggest that estimates of effect on percentage energy from fat were associated with the level of attrition in studies.
Energy
-
. xi: metareg wmd i.CompleteEPHPP, wsse(se) mm
-
i.CompleteEPHPP _ICompleteE_1–2 (_ICompleteE_1 for Com∼P = = 60–79% omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 3.51876
-
p > Q = 0.318
-
Proportion of variation due to heterogeneity I2 = 0.147
-
Moment-based estimate of between-study variance: τ2 = 1.9e+03
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IComplete∼2 | 91.91838 | 94.49451 | 0.97 | 0.402 | –208.8053 | 392.6421 |
| _cons | –38.06268 | 64.00337 | –0.59 | 0.594 | –241.75 | 165.6246 |
There was no evidence to suggest that estimates of effect on energy intake were associated with the level of attrition in studies.
Body mass index
-
. xi: metareg wmd i.CompleteEPHPP, wsse(se) mm
-
i.CompleteEPHPP _ICompleteE_1–2 (_ICompleteE_1 for Com∼P = = 60–79% omitted)
-
Meta-regression number of studies = 8
-
Fit of model without heterogeneity (τ2 = 0): Q (6 d.f.) = 1.76955
-
p > Q = 0.940
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IComplete∼2 | .7038533 | .6068578 | 1.16 | 0.290 | –.7810744 | 2.188781 |
| _cons | –.4702745 | .4494989 | –1.05 | 0.336 | –1.570159 | .6296096 |
There was no evidence to suggest that estimates of effect on BMI were associated with the level of attrition in studies.
Weight
-
. xi: metareg wmd i.CompleteEPHPP, wsse(se) mm
-
i.CompleteEPHPP _ICompleteE_1–2 (_ICompleteE_1 for Com∼P = = 60–79% omitted)
-
Meta-regression number of studies = 4
-
Fit of model without heterogeneity (τ2 = 0): Q (2 d.f.) = 21.7976
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.908
-
Moment-based estimate of between-study variance: τ2 = 12.1802
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IComplete∼2 | –2.791344 | 10.5395 | –0.26 | 0.816 | –48.13913 | 42.55644 |
| _cons | 3.266 | 10.31643 | 0.32 | 0.782 | –41.122 | 47.654 |
There was no evidence to suggest that estimates of effect on weight were associated with the level of attrition in studies.
3. Allocation concealment (Cochrane ‘low risk of bias’ vs other)
Fruit and vegetables
-
. xi: metareg wmd i.allocationconcealcoch, wsse(se) mm
-
i.allocationc∼h _Iallocatio_1–2 (_Iallocatio_1 for all∼h = = Unclear omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 52.3053
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.809
-
Moment-based estimate of between-study variance: τ2 = 0.0819
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iallocati∼2 | –.0546828 | .206455 | –0.26 | 0.796 | –.5146931 | .4053276 |
| _cons | .2688521 | .1447394 | 1.86 | 0.093 | –.0536475 | .5913517 |
There was no evidence to suggest that estimates of effect on daily servings of fruit and vegetables were associated with whether or not allocation was adequately concealed from investigators or was unclear.
Total fat
-
. xi: metareg wmd i.allocationconcealcoch, wsse(se) mm
-
i.allocationc∼h _Iallocatio_1–2 (_Iallocatio_1 for all∼h = = Unclear omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 15.1604
-
p > Q = 0.126
-
Proportion of variation due to heterogeneity I2 = 0.340
-
Moment-based estimate of between-study variance: τ2 = 2.8665
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iallocati∼2 | –.3916092 | 2.275972 | –0.17 | 0.867 | –5.46279 | 4.679572 |
| _cons | –.6184049 | 1.251933 | –0.49 | 0.632 | –3.407885 | 2.171075 |
There was no evidence to suggest that estimates of effect on total fat intake were associated with whether or not allocation was adequately concealed from investigators or was unclear.
Percentage energy from fat
-
. xi: metareg wmd i.allocationconcealcoch, wsse(se) mm
-
i.allocationc∼h _Iallocatio_1–2 (_Iallocatio_1 for all∼h = = Unclear omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 32.524
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.754
-
Moment-based estimate of between-study variance: τ2 = 1.9619
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iallocati∼2 | .669468 | 1.224406 | 0.55 | 0.599 | –2.154018 | 3.492954 |
| _cons | –1.609689 | .6854733 | –2.35 | 0.047 | –3.190393 | –.0289842 |
There was no evidence to suggest that estimates of effect on percentage of energy from fat were associated with whether or not allocation was adequately concealed from investigators or was unclear.
Energy
-
. xi: metareg wmd i.allocationconcealcoch, wsse(se) mm
-
i.allocationc∼h _Iallocatio_1–2 (_Iallocatio_1 for all∼h = = Unclear omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 4.14494
-
p > Q = 0.246
-
Proportion of variation due to heterogeneity I2 = 0.276
-
Moment-based estimate of between-study variance: τ2 = 4.2e+03
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iallocati∼2 | 50.12752 | 110.4682 | 0.45 | 0.681 | –301.4315 | 401.6866 |
| _cons | –17.01646 | 72.97764 | –0.23 | 0.831 | –249.2639 | 215.231 |
There was no evidence to suggest that estimates of effect on total energy intake were associated with whether or not allocation was adequately concealed from investigators or was unclear.
Body mass index
-
. xi: metareg wmd i.allocationconcealcoch, wsse(se) mm
-
i.allocationc∼h _Iallocatio_1–2 (_Iallocatio_1 for all∼h = = Unclear omitted)
-
Meta-regression number of studies = 9
-
Fit of model without heterogeneity (τ2 = 0): Q (7 d.f.) = 2.98302
-
p > Q = 0.887
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iallocati∼2 | .2828604 | .5793218 | 0.49 | 0.640 | –1.087018 | 1.652739 |
| _cons | –.2368358 | .3803496 | –0.62 | 0.553 | –1.13622 | .662548 |
There was no evidence to suggest that estimates of effect on BMI were associated with whether or not allocation was adequately concealed from investigators or was unclear.
Weight
-
. xi: metareg wmd i.allocationconcealcoch, wsse(se) mm
-
i.allocationc∼h _Iallocatio_1–2 (_Iallocatio_1 for all∼h = = Unclear omitted)
-
Meta-regression number of studies = 4
-
Fit of model without heterogeneity (τ2 = 0): Q (2 d.f.) = .010586
-
p > Q = 0.995
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _Iallocati∼2 | 5.246741 | 1.115829 | 4.70 | 0.042 | .4457161 | 10.04777 |
| _cons | –2.8 | .3627551 | –7.72 | 0.016 | –4.360809 | –1.239191 |
There was some evidence to suggest that estimates of effect on weight were larger in studies in which allocation was adequately concealed from investigators (p = 0.042). (Note: this analysis does not account for whether or not studies aimed to reduce or maintain weight.)
4. Low income group versus other
Fruit and vegetables
-
. xi: metareg wmd i.Low_Inc, wsse(se) mm
-
i.Low_Inc _ILow_Inc_0–1 (naturally coded; _ILow_Inc_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 38.2686
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.739
-
Moment-based estimate of between-study variance: τ2 = 0.0453
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _ILow_Inc_1 | .4975182 | .2271436 | 2.19 | 0.053 | –.0085892 | 1.003626 |
| _cons | .1452362 | .0912218 | 1.59 | 0.142 | –.0580186 | .348491 |
There was some evidence to suggest that estimates of effect on daily servings of fruit and vegetables were larger in studies with participants predominantly from low-income groups (p = 0.053).
Total fat
-
. xi: metareg wmd i.Low_Inc, wsse(se) mm
-
i.Low_Inc _ILow_Inc_0–1 (naturally coded; _ILow_Inc_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 13.8384
-
p > Q = 0.180
-
Proportion of variation due to heterogeneity I2 = 0.277
-
Moment-based estimate of between-study variance: τ2 = 1.9142
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _ILow_Inc_1 | 3.042827 | 3.369411 | 0.90 | 0.388 | –4.464689 | 10.55034 |
| _cons | –1.041848 | .9845122 | –1.06 | 0.315 | –3.235478 | 1.151781 |
There was no evidence to suggest that estimates of effect on total fat intake were associated with studies including participants predominantly from low-income groups.
Percentage energy from fat
None of the studies measuring percentage energy from fat included participants predominantly from low-income groups.
Energy
None of the studies measuring energy intake included participants predominantly from low-income groups.
Body mass index
None of the studies measuring BMI included participants predominantly from low-income groups.
Weight
None of the studies measuring weight included participants predominantly from low-income groups.
5. Early outcome (< 3 months) versus later outcome (≥ 3 months)
Fruit and vegetables
-
. xi: metareg wmd i.FU, wsse(se) mm
-
i.FU _IFU_0–1 (naturally coded; _IFU_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 47.6783
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.790
-
Moment-based estimate of between-study variance: τ2 = 0.0732
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IFU_1 | .1187708 | .1980919 | 0.60 | 0.562 | –.3226053 | .560147 |
| _cons | .1813987 | .1381617 | 1.31 | 0.219 | –.1264447 | .4892421 |
There was no evidence that estimates of effect on servings of fruit and vegetables differed according to time to follow-up.
Total fat
-
i.FU _IFU_0–1 (naturally coded; _IFU_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 15.159
-
p > Q = 0.126
-
Proportion of variation due to heterogeneity I2 = 0.340
-
Moment-based estimate of between-study variance: τ2 = 2.7992
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IFU_1 | –.2754214 | 2.40662 | –0.11 | 0.911 | –5.637705 | 5.086862 |
| _cons | –.6704323 | 1.204002 | –0.56 | 0.590 | –3.353117 | 2.012252 |
There was no evidence that estimates of effect on total fat intake differed according to time to follow-up.
Percentage energy from fat
-
. xi: metareg wmd i.FU, wsse(se) mm
-
i.FU _IFU_0–1 (naturally coded; _IFU_0 omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 39.4114
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.797
-
Moment-based estimate of between-study variance: τ2 = 2.0669
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IFU_1 | .6480017 | 2.137268 | 0.30 | 0.769 | –4.280546 | 5.576549 |
| _cons | –2 | 2.05116 | –0.98 | 0.358 | –6.729982 | 2.729982 |
There was no evidence that estimates of effect percentage of energy from fat differed according to time to follow-up.
Energy
-
. xi: metareg wmd i.FU, wsse(se) mm
-
i.FU _IFU_0–1 (naturally coded; _IFU_0 omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 1.46516
-
p > Q = 0.690
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IFU_1 | 176.0411 | 99.62481 | 1.77 | 0.175 | –141.0095 | 493.0917 |
| _cons | –132.7 | 87.86581 | –1.51 | 0.228 | –412.3282 | 146.9282 |
There was no evidence that estimates of effect on energy intake differed according to time to follow-up.
Body mass index
-
. xi: metareg wmd i.FU, wsse(se) mm
-
i.FU _IFU_0–1 (naturally coded; _IFU_0 omitted)
-
Meta-regression number of studies = 9
-
Fit of model without heterogeneity (τ2 = 0): Q (7 d.f.) = 2.90451
-
p > Q = 0.894
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IFU_1 | –.324209 | .5759164 | –0.56 | 0.591 | –1.686035 | 1.037617 |
| _cons | .0332676 | .3893472 | 0.09 | 0.934 | –.8873923 | .9539275 |
There was no evidence that estimates of effect on BMI differed according to time to follow-up.
Weight
-
. xi: metareg wmd i.FU, wsse(se) mm
-
i.FU _IFU_0–1 (naturally coded; _IFU_0 omitted)
-
Meta-regression number of studies = 4
-
Fit of model without heterogeneity (τ2 = 0): Q (2 d.f.) = 16.2713
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.877
-
Moment-based estimate of between-study variance: τ2 = 11.6652
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IFU_1 | –2.9805 | 4.481034 | –0.67 | 0.574 | –22.26083 | 16.29983 |
| _cons | 2.634944 | 3.721698 | 0.71 | 0.552 | –13.37823 | 18.64812 |
There was no evidence that estimates of effect on weight differed according to time to follow-up.
6. Overweight (BMI > 25 kg/m2) versus not overweight
Fruit and vegetables
-
. xi: metareg wmd i.Overweight, wsse(se) mm
-
i.Overweight _IOverweigh_0–1 (naturally coded; _IOverweigh_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 66.0638
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.849
-
Moment-based estimate of between-study variance: τ2 = 0.0835
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IOverweig∼1 | –.2510207 | .5715148 | –0.44 | 0.670 | –1.524435 | 1.022394 |
| _cons | .2510207 | .1057554 | 2.37 | 0.039 | .015383 | .4866585 |
There was no evidence that estimates of effect on servings of fruit and vegetables differed according to whether or not included participants were overweight.
Total fat
-
. xi: metareg wmd i.Overweight, wsse(se) mm
-
i.Overweight _IOverweigh_0–1 (naturally coded; _IOverweigh_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 15.1632
-
p > Q = 0.126
-
Proportion of variation due to heterogeneity I2 = 0.341
-
Moment-based estimate of between-study variance: τ2 = 2.6260
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IOverweig∼1 | –.9068454 | 3.274809 | –0.28 | 0.787 | –8.203574 | 6.389883 |
| _cons | –.6454614 | 1.091058 | –0.59 | 0.567 | –3.076491 | 1.785568 |
There was no evidence that estimates of effect on total fat intake differed according to whether or not included participants were overweight.
Percentage energy from fat
-
. xi: metareg wmd i.Overweight, wsse(se) mm
-
i.Overweight _IOverweigh_0–1 (naturally coded; _IOverweigh_0 omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 27.5027
-
p > Q = 0.001
-
Proportion of variation due to heterogeneity I2 = 0.709
-
Moment-based estimate of between-study variance: τ2 = 1.4112
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IOverweig∼1 | 1.918306 | 1.140995 | 1.68 | 0.131 | –.7128328 | 4.549444 |
| _cons | –1.891834 | .5879203 | –3.22 | 0.012 | –3.247581 | –.5360873 |
There was no evidence that estimates of effect on percentage of energy from fat differed according to whether or not included participants were overweight.
Energy
-
. xi: metareg wmd i.Overweight, wsse(se) mm
-
i.Overweight _IOverweigh_0–1 (naturally coded; _IOverweigh_0 omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 3.39924
-
p > Q = 0.334
-
Proportion of variation due to heterogeneity I2 = 0.117
-
Moment-based estimate of between-study variance: τ2 = 1.5e+03
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IOverweig∼1 | –102.272 | 98.09153 | –1.04 | 0.374 | –414.443 | 209.899 |
| _cons | 72.89176 | 80.47466 | 0.91 | 0.432 | –183.2145 | 328.9981 |
There was no evidence that estimates of effect on total energy intake differed according to whether or not included participants were overweight.
Body mass index
-
. xi: metareg wmd i.Overweight, wsse(se) mm
-
i.Overweight _IOverweigh_0–1 (naturally coded; _IOverweigh_0 omitted)
-
Meta-regression number of studies = 9
-
Fit of model without heterogeneity (τ2 = 0): Q (7 d.f.) = 2.88748
-
p > Q = 0.895
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IOverweig∼1 | –.3317409 | .5740735 | –0.58 | 0.581 | –1.689209 | 1.025727 |
| _cons | .0562025 | .4122949 | 0.14 | 0.895 | –.9187199 | 1.031125 |
There was no evidence that estimates of effect on BMI differed according to whether or not included participants were overweight.
Weight
-
. xi: metareg wmd i.Overweight, wsse(se) mm
-
i.Overweight _IOverweigh_0–1 (naturally coded; _IOverweigh_0 omitted)
-
Meta-regression number of studies = 4
-
Fit of model without heterogeneity (τ2 = 0): Q (2 d.f.) = 6.48674
-
p > Q = 0.039
-
Proportion of variation due to heterogeneity I2 = 0.692
-
Moment-based estimate of between-study variance: τ2 = 9.4399
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IOverweig∼1 | 3.087439 | 3.944292 | 0.78 | 0.516 | –13.88348 | 20.05836 |
| _cons | –.6045792 | 2.374113 | –0.25 | 0.823 | –10.81956 | 9.610406 |
There was no evidence that estimates of effect on weight differed according to whether or not included participants were overweight.
7. Primary prevention versus diagnosed illness
Fruit and vegetables
None of the studies measuring daily servings of fruit and vegetables included participants with a diagnosed illness.
Total fat
None of the studies measuring total fat intake included participants with a diagnosed illness.
Percentage energy from fat
-
. xi: metareg wmd i.Secondary, wsse(se) mm
-
i.Secondary _ISecondary_0–1 (naturally coded; _ISecondary_0 omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 18.9136
-
p > Q = 0.015
-
Proportion of variation due to heterogeneity I2 = 0.577
-
Moment-based estimate of between-study variance: τ2 = 1.4700
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _ISecondar∼1 | –1.350213 | 1.431508 | –0.94 | 0.373 | –4.651275 | 1.95085 |
| _cons | –1.149787 | .5965762 | –1.93 | 0.090 | –2.525494 | .2259199 |
There was no evidence that estimates of effect on percentage of energy from fat differed according to whether or not participants had a diagnosed illness.
Energy
None of the studies measuring energy intake included participants with a diagnosed illness.
BMI
None of the studies measuring BMI included participants with a diagnosed illness.
Weight
-
. xi: metareg wmd i.Secondary, wsse(se) mm
-
i.Secondary _ISecondary_0–1 (naturally coded; _ISecondary_0 omitted)
-
Meta-regression number of studies = 4
-
Fit of model without heterogeneity (τ2 = 0): Q (2 d.f.) = .010586
-
p > Q = 0.995
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _ISecondar∼1 | –5.246741 | 1.115829 | –4.70 | 0.042 | –10.04777 | -.4457161 |
| _cons | 2.446741 | 1.055217 | 2.32 | 0.146 | –2.093492 | 6.986975 |
There was some evidence to suggest that estimates of effect on weight were larger in studies with participants with a diagnosed illness (p = 0.042). (Note: this analysis does not account for whether or not studies aimed to reduce or maintain weight.)
8. Aimed to reduce versus maintain body mass index/weight
Fruit and vegetables
-
. xi: metareg wmd i.BMI_down, wsse(se) mm
-
i.BMI_down _IBMI_down_0–1 (naturally coded; _IBMI_down_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 66.0638
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.849
-
Moment-based estimate of between-study variance: τ2 = 0.0835
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IBMI_down_1 | –.2510207 | .5715148 | –0.44 | 0.670 | –1.524435 | 1.022394 |
| _cons | .2510207 | .1057554 | 2.37 | 0.039 | .015383 | .4866585 |
There was no evidence that estimates of effect on servings of fruit and vegetables differed according whether or not studies aimed to maintain or reduce BMI.
Total fat
-
. xi: metareg wmd i.BMI_down, wsse(se) mm
-
i.BMI_down _IBMI_down_0–1 (naturally coded; _IBMI_down_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 15.0849
-
p > Q = 0.129
-
Proportion of variation due to heterogeneity I2 = 0.337
-
Moment-based estimate of between-study variance: τ2 = 2.5378
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IBMI_down_1 | –1.535807 | 3.487471 | –0.44 | 0.669 | –9.306377 | 6.234762 |
| _cons | –.6056245 | 1.06856 | –0.57 | 0.583 | –2.986523 | 1.775275 |
There was no evidence that estimates of effect on total fat intake differed according whether or not studies aimed to maintain or reduce BMI.
Percentage energy from fat
-
. xi: metareg wmd i.BMI_down, wsse(se) mm
-
i.BMI_down _IBMI_down_0–1 (naturally coded; _IBMI_down_0 omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 36.9256
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.783
-
Moment-based estimate of between-study variance: τ2 = 2.7521
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IBMI_down_1 | 1.642182 | 1.275113 | 1.29 | 0.234 | –1.298234 | 4.582598 |
| _cons | –2.32978 | .9474181 | –2.46 | 0.039 | –4.51453 | –.1450303 |
There was no evidence that estimates of effect on percentage of energy from fat differed according whether or not studies aimed to maintain or reduce BMI.
Energy
-
. xi: metareg wmd i.BMI_down, wsse(se) mm
-
i.BMI_down _IBMI_down_0–1 (naturally coded; _IBMI_down_0 omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 2.88746
-
p > Q = 0.409
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IBMI_down_1 | –380.5853 | 291.8849 | –1.30 | 0.283 | –1309.493 | 548.3226 |
| _cons | 377 | 288.8699 | 1.31 | 0.283 | –542.313 | 1296.313 |
There was no evidence that estimates of effect on energy intake differed according whether or not studies aimed to maintain or reduce BMI.
Body mass index
-
. xi: metareg wmd i.BMI_down, wsse(se) mm
-
i.BMI_down _IBMI_down_0–1 (naturally coded; _IBMI_down_0 omitted)
-
Meta-regression number of studies = 9
-
Fit of model without heterogeneity (τ2 = 0): Q (7 d.f.) = 3.20983
-
p > Q = 0.865
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IBMI_down_1 | –.0619419 | .5753033 | –0.11 | 0.917 | –1.422318 | 1.298434 |
| _cons | –.0861856 | .391764 | –0.22 | 0.832 | –1.01256 | .840189 |
There was no evidence that estimates of effect on BMI differed according whether or not studies aimed to maintain or reduce BMI.
Weight
All studies that estimated mean weight as an outcome aimed to reduce weight.
9. Physical activity component in intervention versus none
Fruit and vegetables
-
. xi: metareg wmd i.PA, wsse(se) mm
-
i.PA _IPA_0–1 (naturally coded; _IPA_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 57.6919
-
p > Q = 0.000
-
Proportion of variation due to heterogeneity I2 = 0.827
-
Moment-based estimate of between-study variance: τ2 = 0.0800
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IPA_1 | .1512111 | .2807434 | 0.54 | 0.602 | –.4743242 | .7767464 |
| _cons | .2175574 | .1114721 | 1.95 | 0.080 | –.0308179 | .4659328 |
There was no evidence that estimates of effect on servings of fruit and vegetables differed according to whether or not interventions included a physical activity component.
Total fat
-
. xi: metareg wmd i.PA, wsse(se) mm
-
i.PA _IPA_0–1 (naturally coded; _IPA_0 omitted)
-
Meta-regression number of studies = 12
-
Fit of model without heterogeneity (τ2 = 0): Q (10 d.f.) = 10.0671
-
p > Q = 0.435
-
Proportion of variation due to heterogeneity I2 = 0.007
-
Moment-based estimate of between-study variance: τ2 = 0.0337
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IPA_1 | –7.125047 | 3.147323 | –2.26 | 0.047 | –14.13772 | –.1123749 |
| _cons | –1.033167 | .4412864 | –2.34 | 0.041 | –2.016414 | –.0499195 |
There was some evidence to suggest that estimates of effect on total fat intake were larger in studies in which interventions also included a physical activity component (p = 0.047).
Percentage energy from fat
-
. xi: metareg wmd i.PA, wsse(se) mm
-
i.PA _IPA_0–1 (naturally coded; _IPA_0 omitted)
-
Meta-regression number of studies = 10
-
Fit of model without heterogeneity (τ2 = 0): Q (8 d.f.) = 17.1085
-
p > Q = 0.029
-
Proportion of variation due to heterogeneity I2 = 0.532
-
Moment-based estimate of between-study variance: τ2 = 1.0507
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IPA_1 | –1.43327 | 1.032209 | –1.39 | 0.202 | –3.813548 | .9470078 |
| _cons | –.8038327 | .6508597 | –1.24 | 0.252 | –2.304718 | .6970525 |
There was no evidence that estimates of effect on percentage of energy from fat differed according to whether or not interventions included a physical activity component.
Energy
-
. xi: metareg wmd i.PA, wsse(se) mm
-
i.PA _IPA_0–1 (naturally coded; _IPA_0 omitted)
-
Meta-regression number of studies = 5
-
Fit of model without heterogeneity (τ2 = 0): Q (3 d.f.) = 4.29626
-
p > Q = 0.231
-
Proportion of variation due to heterogeneity I2 = 0.302
-
Moment-based estimate of between-study variance: τ2 = 3.7e+03
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IPA_1 | 78.65628 | 140.2495 | 0.56 | 0.614 | –367.6804 | 524.9929 |
| _cons | –9.159267 | 58.73057 | –0.16 | 0.886 | –196.0662 | 177.7476 |
There was no evidence that estimates of effect on total energy intake differed according to whether or not interventions included a physical activity component.
Body mass index
-
. xi: metareg wmd i.PA, wsse(se) mm
-
i.PA _IPA_0–1 (naturally coded; _IPA_0 omitted)
-
Meta-regression number of studies = 9
-
Fit of model without heterogeneity (τ2 = 0): Q (7 d.f.) = 3.20835
-
p > Q = 0.865
-
Proportion of variation due to heterogeneity I2 = 0.000
-
Moment-based estimate of between-study variance: τ2 = 0.0000
| WMD | Coef. | SE | t | p > |t| | (95% CI) | |
|---|---|---|---|---|---|---|
| _IPA_1 | .06573 | .5749834 | 0.11 | 0.912 | –1.29389 | 1.42535 |
| _cons | –.145655 | .3932479 | –0.37 | 0.722 | –1.075538 | .7842284 |
There was no evidence that estimates of effect on BMI differed according to whether or not interventions included a physical activity component.
Weight
All studies that estimated mean weight as an outcome included a physical activity component.
Appendix 8 Behaviour change techniques and effectiveness
| Behaviour change technique | Effective n = 13a | Ineffective n = 26b | Not clear n = 4c | Total n = 43d | |
|---|---|---|---|---|---|
| 1 | Provide information on consequences of behaviour in general | 6 | 7 | 1 | 14 |
| 2 | Provide information on consequences of behaviour to the individual | 5 | 6 | 11 | |
| 3 | Provide information about others’ approval | 1 | 1 | ||
| 4 | Provide normative information about others’ behaviour | 4 | 4 | ||
| 5 | Goal setting (behaviour) | 4 | 8 | 2 | 14 |
| 6 | Goal setting (outcome) | 1 | 1 | 2 | |
| 7 | Action planning | 3 | 1 | 1 | 5 |
| 8 | Barrier identification/problem solving | 5 | 7 | 1 | 13 |
| 9 | Set graded tasks | 2 | 1 | 3 | |
| 10 | Prompt review of behavioural goals | 5 | 5 | 1 | 11 |
| 11 | Prompt review of outcome goals | 1 | 1 | 2 | |
| 12 | Provide rewards contingent on effort or progress towards behaviour | 2 | 1 | 3 | |
| 13 | Provide rewards contingent on successful behaviour | 2 | 2 | ||
| 14 | Shaping | 1 | 1 | ||
| 15 | Prompting generalisation of a target behaviour | 1 | 1 | ||
| 16 | Prompt self-monitoring of behaviour | 4 | 8 | 12 | |
| 17 | Prompt self-monitoring of behavioural outcome | 1 | 3 | 4 | |
| 18 | Prompting focus on past success | 1 | 1 | ||
| 19 | Provide feedback on performance | 7 | 6 | 1 | 14 |
| 20 | Provide information on where and when to perform the behaviour | 1 | 2 | 3 | |
| 21 | Provide instruction on how to perform the behaviour | 4 | 7 | 1 | 12 |
| 22 | Model/demonstrate the behaviour | 1 | 5 | 6 | |
| 23 | Teach to use prompts/cues | 3 | 3 | 1 | 7 |
| 24 | Environmental restructuring | 1 | 1 | ||
| 25 | Agree behavioural contract | 0 | |||
| 26 | Prompt practice | 0 | |||
| 27 | Use of follow up prompts | 1 | 1 | ||
| 28 | Facilitate social comparison | 1 | 2 | 3 | |
| 29 | Plan social support/social change | 3 | 7 | 10 | |
| 30 | Prompt identification as role model/position advocate | 1 | 1 | ||
| 31 | Prompt anticipated regret | 0 | |||
| 32 | Fear arousal | 3 | 3 | 6 | |
| 33 | Prompt self-talk | 0 | |||
| 34 | Prompt use of imagery | 0 | |||
| 35 | Relapse prevention/coping planning | 1 | 1 | ||
| 36 | Stress management | 0 | |||
| 37 | Emotional control training | 1 | 1 | ||
| 38 | Motivational interviewing | 1 | 1 | 2 | |
| 39 | Time management | 0 | |||
| 40 | General communication skills training | 0 | |||
| 41 | Stimulate anticipation of future rewards | 0 | |||
| 42 | General planning | 1 | 1 | 2 | |
| 43 | Tailoringe | 13 | 26 | 4 | 43 |
Glossary
- Accessibility
- Extent to which an intervention reaches the target population (e.g. including those with differing literacy skills in use or application of information and support supplied through e-learning).
- Adaptive
- Requires contributions from users (e.g. entering personal data, making choices) which alters pathways within programs to produce tailored material and feedback that is personally relevant to users of the program.
- Computer kiosk
- A computer terminal that provides access to information via electronic methods.
- Diet
- Food that is eaten (may be categorised into food groups), including fortified or functional foods, but excluding supplements.
- Dietary behaviour
- Food intake (including what, where, how and when food is eaten).
- Dietary log
- Diary to keep track of one’s dietary intake.
- E-learning
- Anything electronic (e.g. SMS, digital TV, personal digital assistant, CD-ROM, internet, etc.) where the goal of the intervention is to improve knowledge/behaviour.
- European Quality of Life-5 Dimensions (EQ-5D)
- Standardised instrument used to measure health status.
- Metformin
- Drug used for treating diabetes.
- Minitel
- Videotex online service accessible through the telephone lines.
- Multimedia
- Media that contains a combination of different forms of content (e.g. text, audio, video, still images, animation, etc.).
- QDScore®
- Diabetes risk calculator.
- Orlistat
- Drug used for treating obesity.
- QRISK®2
- Cardiovascular disease risk calculator.
- Recall bias
- Systematic error due to differences in accuracy or completeness of recall to memory of past events or experiences.
- Statin therapy
- Drug used for preventing cardiovascular disease in patients with high levels of cholesterol.
- Usability
- Ease of use and satisfaction with an intervention.
List of abbreviations
- BMI
- body mass index
- CBT
- cognitive behavioural therapy
- CD-ROM
- compact disk read-only memory
- CI
- confidence interval
- CVD
- cardiovascular disease
- DES
- discrete event simulation
- E-LEEM
- E-Learning Economic Evaluation Model
- EDE-Q
- Eating Disorder Examination-Questionnaire
- EDI
- Eating Disorder Inventory
- EPHPP
- effective public health practice project
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- FFQ
- Food Frequency Questionnaire
- ICER
- incremental cost-effectiveness ratio
- MONICA
- Multinational MONItoring of trends and determinants in CArdiovascular disease
- NICE
- National Institute for Health and Clinical Excellence
- PDA
- personal digital assistant
- PRISMA
- preferred reporting items for systematic reviews and meta-analyses
- PSA
- probabilistic sensitivity analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SES
- socioeconomic status
- SMR
- standardised mortality ratio
- T2D
- type 2 diabetes
- WMD
- weighted mean difference
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington