Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/16/01. The contractual start date was in March 2010. The draft report began editorial review in January 2011 and was accepted for publication in May 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Shyangdan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
What is non-alcoholic fatty liver disease?
‘Non-alcoholic fatty liver disease’ (NAFLD) is a term used for a group of inter-related chronic liver disorders causing a wide spectrum of liver damage associated with increased fat content in the liver in the absence of increased alcohol intake [< 10 g of alcohol per day for women, < 20 g per day for men (around 9–18 units of alcohol per week, respectively)]. 1,2 By definition, to have NAFLD, > 5% of the liver weight must be due to accumulation of fat. 2 NAFLD has become recognised as an important problem only recently and was relatively unknown prior to 1980. 3,4 Population-based screening studies suggest that the prevalence of NAFLD is in the region of 17–33% in the Western world. 5 The prevalence of non-alcoholic steatohepatitis (NASH) is not known because it currently requires a liver biopsy to confirm the diagnosis, but it has been estimated to be around 3% in the total population. 5
The spectrum of potentially progressive liver damage can include:
-
simple (hepatic) steatosis (fatty accumulation in the liver, also known as ‘fatty liver’)
-
NASH
-
advanced fibrosis
-
cirrhosis
-
hepatocellular carcinoma (HCC), hepatoma, liver cancer.
The term ‘NASH’ is sometimes used to describe the three subsequent stages after hepatic steatosis,6 but in this report will be used only for the inflammatory stage that follows simple steatosis.
Non-alcoholic fatty liver disease is the most common cause of abnormal blood tests of liver function. 7 The liver damage seen in NAFLD is similar to the changes seen in alcohol-related liver disease,3 but, by definition, individuals with NAFLD do not consume increased quantities of alcohol.
Not every individual who develops simple steatosis (which is asymptomatic) progresses to the subsequent stages of liver damage, but some will progress to chronic liver failure (inability of the liver to fulfil its role in detoxifying the blood and synthesising various compounds necessary for the body to function) with potential subsequent acute decompensation. Chronic liver failure is associated with a very poor prognosis. 8 Finally, some individuals can go on to develop HCC after reaching the cirrhotic stage. 9 Data on disease progression are reviewed later in the chapter.
Although more cases of cirrhosis are due to alcohol than to NAFLD,10 more people have some form of NAFLD than alcohol-related fatty liver disease (ALD). 11 NAFLD is strongly linked to insulin resistance, and thus associated with conditions such as type 2 diabetes mellitus (T2DM) and obesity. Therefore, as the prevalence of these two conditions increases, it is likely that there will be a marked rise in NAFLD, making NAFLD a growing issue for health-care providers. NAFLD is also a risk factor for the development of T2DM. 5
What causes non-alcoholic fatty liver disease and its progression?
The key feature in NAFLD is the accumulation of fat in the liver. As mentioned above, simple fat deposition occurs at the beginning and is relatively benign; however, this can progress to inflammatory change (steatohepatitis, NASH), with the possible formation of scar tissue (fibrosis) and further structural change associated with the reduction in liver function (cirrhosis).
The accumulation of liver fat
Hepatic steatosis (accumulation of fat within liver cells) is the first stage of NAFLD. There is no single mechanism leading to hepatic steatosis, but rather the combination of a number of pathologies that ultimately disrupt normal lipid [fat-rich products, mainly triglycerides (TGs) – fatty acid molecules] movement through the liver cell and cause lipid accumulation. The fat in the liver can be traced to three sources – dietary intake, de novo synthesis and circulating non-esterified fatty acids (NEFAs) derived from body fat stores. 5 It is known that high-fat diets can lead to hepatic steatosis, but it appears that NEFAs are the main source of liver fat, with 60% in individuals with NAFLD who have a normal fat-containing diet. 12
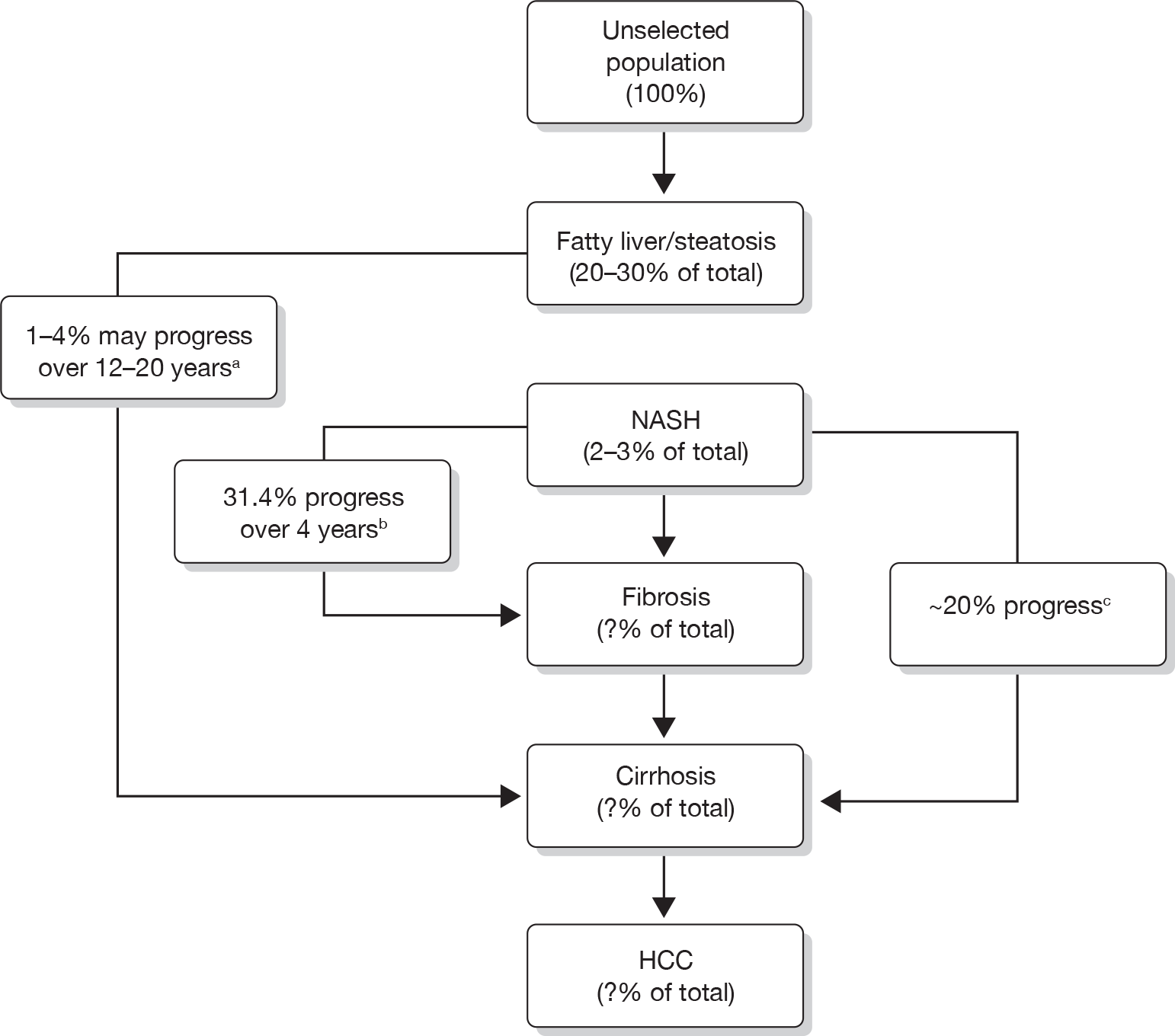
The body’s resistance to the effects of the hormone insulin (required for the uptake of glucose – the main sugar derived from dietary carbohydrate by liver, fat and muscle cells) is thought to play a key role in increasing levels of NEFAs, particularly the insulin resistance shown by fat and muscle cells (Figure 1). Once lipid starts to accumulate in the liver this can, in fact, worsen the body’s insulin resistance, reducing the beneficial effects of insulin, leading to a vicious cycle. 13–15
FIGURE 1.
The effects and potential clinical indicators of accumulating liver fat. The presence of ectopic fat in the liver cell leads to hepatic insulin resistance following the accumulation of intracellular lipid by-products, which leads to disturbed glucose metabolism. Gluconeogenesis is the production of glucose from protein and fat breakdown, and glycogen is the form of complex carbohydrate that glucose is stored as. BMI, body mass index; FPG, fasting plasma glucose. Adapted from Preiss and Sattar. 5
Inflammation of the fatty liver – non-alcoholic steatohepatitis
McCullough16 has reviewed the pathophysiology of NASH. Hepatic steatosis is considered to have a benign course in most cases. 17 This may be because of associated counter-regulatory protective mechanisms, which means that liver tissue changes (other than steatosis) and liver function may remain normal. NASH represents the stage when the fatty liver starts to show inflammatory change. It is the development of NASH and progression to fibrosis and cirrhosis that is responsible for the liver-specific morbidity and mortality of NAFLD.
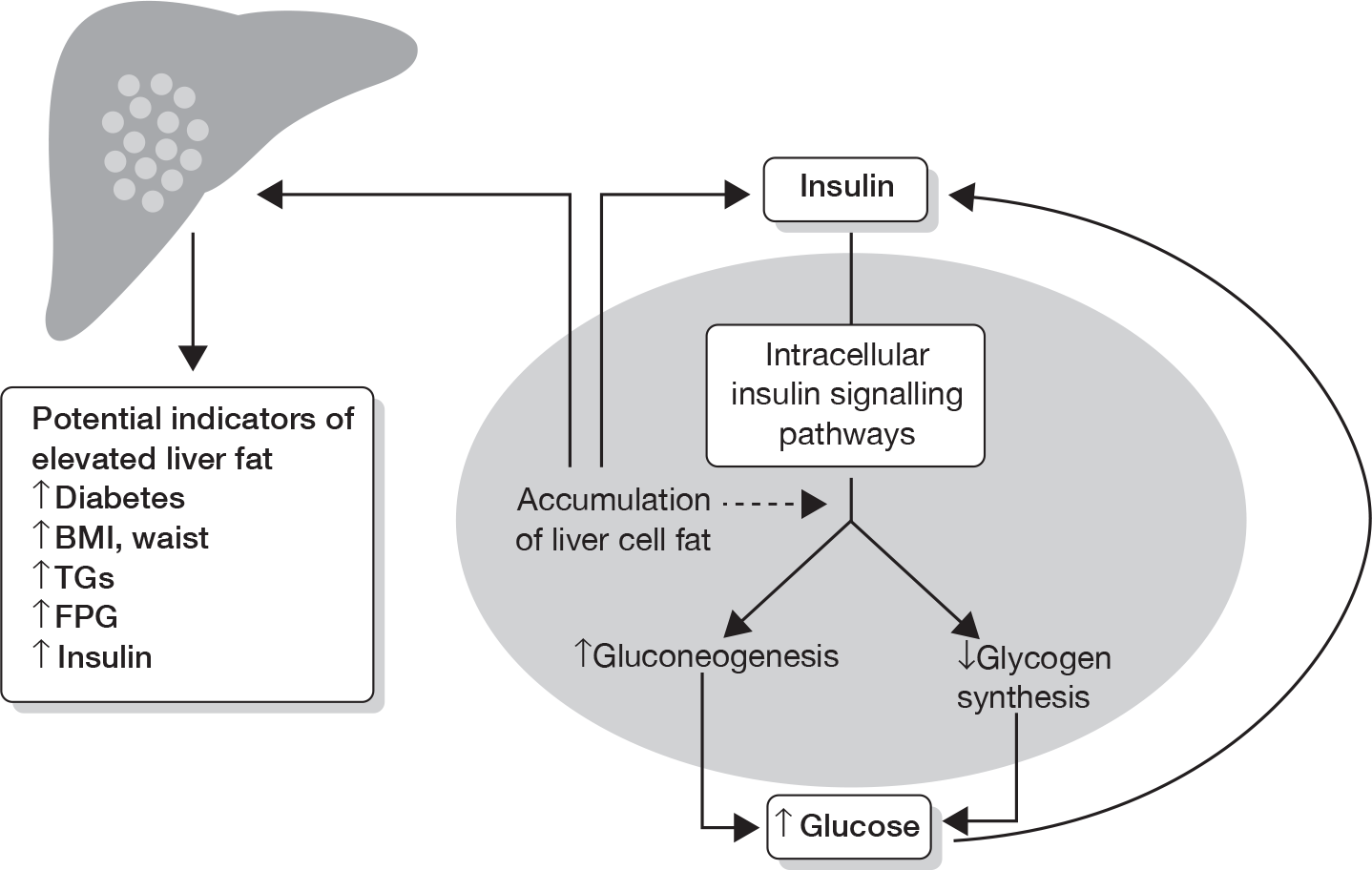
It is unclear why only approximately 25–30% of patients with simple steatosis develop NASH. 16 The counter-regulatory processes occurring as a means to maintain liver cell (hepatocyte) health forms the basis of the older ‘two-hit’ theory in the development of NASH. A newer theory suggests that NASH occurs by liver fat directly causing inflammation.
In the two-hit theory, the first hit refers to the accumulation of liver fat. It has been proposed the hepatocytes act, in addition, as a reservoir of toxic agents and are most susceptible to a ‘second hit’ – oxidative stress18,19 – caused by endogenous compounds within liver cells and by environmental toxins. 20,21 The cells in the body constantly react with inhaled oxygen, producing energy – oxidation. As a consequence of this activity, highly reactive molecules (free radicals) are produced. Free radicals interact with other molecules within cells. This can cause damage – oxidative stress – to proteins, membranes and genes. Patients with NASH have increased levels of oxidative stress when compared with patients with steatosis alone. 22,23
In addition to endogenous toxins, three factors have been proposed as potential causative mechanisms for producing this oxidative stress: increased insulin levels, lipid peroxidation and liver iron content (Figure 2).
FIGURE 2.
The two-hit theory of NASH. This involves insulin resistance, which causes hepatic steatosis, a process enhanced by obesity and/or T2DM. Once developed, hepatic steatosis may remain in a benign state or progress to NASH via the mechanisms discussed. Reproduced with permission from AJ McCullough. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 2006;40(Suppl. 1):17–29. 16
Insulin may injure the liver both directly and indirectly. 24,25 The indirect effects are related to the hyperinsulinaemic state increasing liver fat accumulation, as described earlier. The direct effect may be due to insulin’s ability to generate oxidative stress. 25 It also appears to have direct effects by stimulating scar tissue formation, especially in the presence of increased glucose levels. 26 This may explain the observation that NAFLD patients with T2DM have a particularly poor prognosis. 17,27 Insulin may be directly involved in causing stress on parts of the liver cell that lead to cell death (apoptosis). This, in itself, may exacerbate insulin resistance. 28
Increased lipid peroxidation (breakdown of NEFAs, causing oxidative stress) has been demonstrated in patients with NAFLD. 22,23,29–31 As previously described, patients with NAFLD have increased breakdown of fat stores and increased delivery of NEFAs to the liver. 22,32 The products of NEFA oxidation are capable of generating oxidative stress and subsequent lipid peroxidation, setting up a vicious cycle.
In response to oxidative stress, there is usually an increased synthesis of antioxidants. However, NAFLD patients have decreased antioxidants (glutathione). 33 Therefore, patients with NAFLD have an impaired ability to produce sufficient antioxidants. A deficiency in antioxidants is also supported by a preliminary report that demonstrated that betaine (a naturally occurring antioxidant) improved the microscopic images of liver tissue and liver function tests (LFTs) in patients with NAFLD. 34
The role of iron as a pro-oxidant in NASH is unclear. In McCullough’s review,16 there is mention of patients with NAFLD having increased ferritin levels, and a relationship between hepatic iron and insulin resistance. However, there is acknowledgement that there is no strong evidence associating iron overload with NAFLD. Therefore, for now, iron is likely to be important in only a minority of patients with NASH and more research is required on iron as a pathophysiological factor.
There is also good evidence to support a one-hit theory of liver fat causing chronic inflammation directly, leading to direct NAFLD progression. 5 A key player in steatosis formation appears to be nuclear factor-κB (NF-κB). This is a transcription factor – an intracellular protein required for the transcription of deoxyribonucleic acid to form proteins. In animal models it has been shown that a high-fat diet with resultant hepatic steatosis leads to increased NF-κB signalling in the liver. 35 This then induces the production of chemicals involved with causing inflammation, which may play a role in the progression of NAFLD. The transcription factor also leads to activation of specific cells (Kupffer cells and macrophages) within liver tissue, which are known to cause further damage to liver tissue. In the same study35 there is evidence that inflammation, in the form of isolated increased NF-κB expression in rat liver, can lead directly to insulin resistance. 35
Another factor that may be involved in the process is adiponectin, a polypeptide produced in adipocytes (fat cells) and which may be protective via an insulin-sensitising effect in the liver. Polyzos et al. 36 reviewed the evidence on the role of adiponectin and noted that it was reduced in NAFLD and NASH, and increased by most interventions which improved NAFLD, including weight loss, bariatric surgery and pioglitazone, but not metformin. However, formal interventions and links to adiponectin genotypes would be required to prove a causal relationship.
The advanced stages – fibrosis and cirrhosis
As NAFLD progresses, more advanced forms can occur – fibrosis and cirrhosis. Liver fibrosis is the formation of excess fibrous tissue in the liver37 and is a reparative or reactive process, as a result of NASH,38 or, in a few cases, direct progression from simple steatosis. 39 Liver fibrosis can lead to loss of function.
Cirrhosis of the liver is advanced fibrosis associated with regenerative nodules (an attempt at repair). 40 Cirrhosis is associated with variable and usually irreversible loss of liver function. When liver function is minimally or not significantly compromised clinically, it is often termed ‘compensated cirrhosis’; however, when there is clinical evidence that the cirrhotic liver is unable to function properly, it is termed ‘decompensated cirrhosis’. Whereas decompensation can be reversible if due to an acute insult (e.g. infection), decompensation is often progressive, resulting in liver failure and death. Hui et al. 41 in their prospective cohort study followed up 23 patients with NASH-associated cirrhosis for a mean duration of 84 months (range 5–177 months) and found that 9 out of 23 cases developed liver-related morbidity (eight developed ascites and/or encephalopathy, one developed variceal bleeding). The authors then found that probability of complication-free survival was 83%, 77% and 48% at 1, 3 and 10 years, respectively, and the cumulative probability of overall survival at 1, 3 and 10 years was 95%, 90% and 84%, respectively. 41
Other conditions associated with non-alcoholic fatty liver disease
As described above, other diseases and physiological states are associated with the development, severity and progression of NAFLD. The main ones are diabetes mellitus (particularly T2DM42), insulin resistance, obesity or overweight, and increased levels of TGs in the blood (hypertriglyceridaemia). 7 Insulin resistance, obesity and hypertriglyceridaemia are key components of the (multiply defined) topical metabolic syndrome,43 a ‘syndrome’ associated with increased cardiovascular events. 44 A substantial number of studies have shown the increased prevalence of these three conditions in patients with NAFLD, and some data are summarised in Table 1. 45 More data relating to these conditions and NAFLD will be commented on in the next section.
| Author | No. of patients | Diabetes (%) | Obesity (%) | Hypertriglyceridaemia (%) |
|---|---|---|---|---|
| Ludwig (1980) | 20 | 25 | 90 | 67 |
| Diehl (1988) | 39 | 88 | 71 | – |
| Lee (1989) | 49 | 51 | 69 | 4 |
| Powell (1990) | 42 | 36 | 93 | 81 |
| Bacon (1990) | 33 | 21 | 39 | 21 |
| Matteoni (1999) | 132 | 33 | 70 | 92 |
| Angulo (1999) | 144 | 28 | 60 | 27 |
Although all three conditions are associated with the development of NAFLD, it is also likely that NAFLD, associated with elevations in liver enzymes, has a causal role in development of T2DM,46–49 as previously highlighted in Figure 1.
Prevalence and natural history of non-alcoholic fatty liver disease
Estimates of prevalence are variable, because of differences in the method used to diagnose the various stages of NAFLD and the variation in sample selection and size. As the histological (microscopic tissue) features of NAFLD may be indistinguishable from those of alcoholic liver disease, the diagnosis requires the exclusion of known excessive alcohol intake.
International data
As previously mentioned, screening studies using serum liver tests and ultrasonography (USG) suggest that the prevalence of NAFLD ranges from 17% to 33% in the general population of the Western world. 50 Imaging using magnetic resonance spectroscopy (MRS) gives NAFLD a prevalence of 34%. 51 The prevalence of NASH is less well known, as a liver biopsy is required to confirm the diagnosis. Estimates of the prevalence are in the range of approximately 3% in the general population and higher among obese persons. 52 The average age at diagnosis lies between 45 and 55 years,45 and has a slight female preponderance. 45
Natural history
Good long-term data (including UK data) on the natural history of NAFLD from simple steatosis to more advanced stages are lacking, for a number of reasons. NAFLD has only recently been characterised in detail. There are few long-term follow-up studies of well-defined patient cohorts, and follow-up liver biopsies have been performed in only a limited number of patients. The diagnostic method for fatty liver – blood tests ± ultrasound ± liver biopsy (the current gold standard)50 – is not uniform, and studies using ultrasound to diagnose steatosis will give higher numbers than liver biopsy. Furthermore, it is possible that the long-term complications of NAFLD may be under-recognised and under-reported, as the characteristic features of fatty liver, such as steatosis, may disappear in the late stages of the disease, leading to a picture of ‘bland’ cirrhosis, frequently described as ‘cryptogenic’, rather than NAFLD-related cirrhosis, which is now recognised as the most common cause of cryptogenic cirrhosis. 53 Finally, most studies to date that have studied the natural history of NAFLD have been retrospective analyses (e.g. clinical follow-up from cohort studies) or case series in which selected patients with a diagnosis of NASH underwent subsequent liver biopsies. 6
Despite these limitations, the following diagrams indicate the current thinking on the prevalence and progression of NAFLD (Figures 3 and 4).
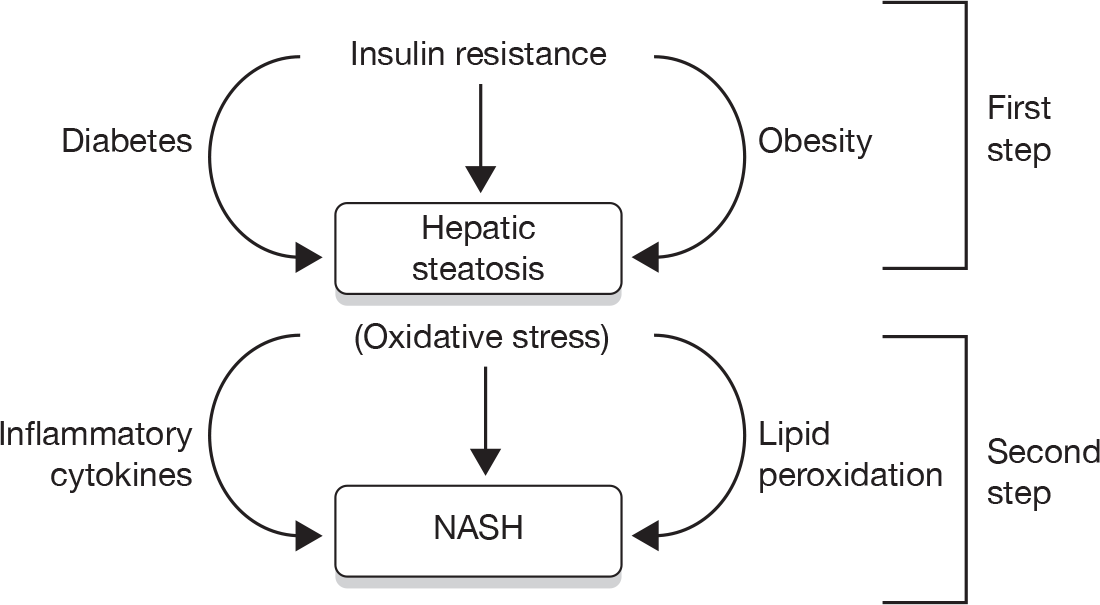
FIGURE 4.
The natural history and clinical outcomes of NASH. OLTX, orthotopic liver transplantation. Reproduced with permission from AJ McCullough. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 2006;40(Suppl. 1):17–29. 16
Hence, it is difficult to determine what proportion of an unselected population will develop NAFLD-related cirrhosis and subsequent HCC. One study estimated that 7% of cases of HCC were related to underlying NAFLD or cryptogenic cirrhosis but such data are, at best, approximates. 9
UK data
The UK data on NAFLD are limited, with no nationwide data. The incidence of NAFLD in a hepatology catchment area in England of 200,000 residents, based on referrals to a secondary care setting, was calculated at 29 per 100,000 population. 55 This was subdivided into 23.5 per 100,000 population for non-cirrhotic NAFLD and 5.5 per 100,000 population for cirrhotic NAFLD. There are no satisfactory prevalence data.
Associations with type 2 diabetes mellitus and obesity
Numerous studies have reported the increased prevalence of NAFLD in individuals with T2DM,56 and increased risk and severity of NAFLD in T2DM. 57–59 In 939 randomly selected people with T2DM in Edinburgh, ultrasound-detected steatosis was present in 73.9% of subjects. 60 A recent study in the USA reported the prevalence of ultrasound-determined NAFLD to be 69.5%. 56 The prevalence of NAFLD in obese individuals (ultrasound determined) has been estimated to be high as 80%. 50 One thing is certain – as the incidence and prevalence of obesity and T2DM increase,61 the incidence and prevalence of fatty liver, and hence NASH and more severe forms of NAFLD, are also likely to increase.
Cardiovascular risk
In addition to the organ-specific related morbidity and mortality of NAFLD, NAFLD has also been linked with increased cardiovascular risk, largely through the components of the metabolic syndrome. A detailed review in 2007 on NAFLD and cardiovascular risk62 showed that this increased risk was related to the presence of known cardiovascular risk factors, several of which (insulin resistance, obesity and dyslipidaemia/hypertriglyceridaemia) are also well associated with NAFLD. 63
Similar conclusions were reached in a more recent review by Ghouri et al. ,64 who concluded that the presence of NAFLD was an indication for screening for T2DM, but that it did not add useful data on CVD risk compared with traditional risk factors.
Hence, it appears that NAFLD itself is not an independent contributor to CVD risk, but that it is associated with adverse risk factors.
A more recent review by Targher et al. 65 also addressed the question of whether or not NAFLD increased the risk of cardiovascular disease, independent from its association with traditional risk factors. They concluded that:
Although additional research is required to draw a definite conclusion, these observations raise the possibility that NAFLD – especially its necroinflammatory variant, NASH – not only is a marker of cardiovascular disease but may also be involved in its pathogenesis. This process may occur through the systemic release of pro-atherogenic mediators from the steatotic and inflamed liver or through the contribution of NAFLD itself to insulin resistance and atherogenic dyslipidaemia.
One key issue noted by Targher et al. 65 is that cardiovascular disease is a greater threat to people with NAFLD than liver disease.
Associations with type 1 diabetes
A recent study66 has reported a high prevalence of NAFLD in 202 patients with type 1 diabetes in Italy. NAFLD was diagnosed by history and liver ultrasound. Over half of the group were classed as having NAFLD. Those who did were older, had suffered from diabetes longer and had higher body mass indices (BMIs) than those who did not.
Clinical features of non-alcoholic fatty liver disease
Given that NAFLD is a spectrum of (often progressive) liver damage, the clinical presentation can vary depending on the stage of presentation. Simple hepatic steatosis or fatty liver is often asymptomatic and is picked up only following investigations of abnormal blood LFTs. Symptoms, when present, may include fatigue and right upper quadrant pain and the most commonly reported clinical finding is hepatomegaly (enlarged liver on examination). 67 Often these features are more apparent in individuals with NASH or early cirrhosis. If advanced cirrhosis eventually develops prior to diagnosis, presentation is similar to that of cirrhosis from other causes, with clinical signs including ascites (fluid collecting in the abdomen), variceal haemorrhage (bleeding from large veins in the gastrointestinal tract), splenomegaly (enlarged spleen on examination), bruising and eventual jaundice.
As mentioned above, NAFLD is associated with several other metabolic disorders, and therefore people with NAFLD can exhibit clinical features of these conditions as well. It is beyond the scope of this report to go into this aspect in detail.
Diagnosis of non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease can be reasonably accurately diagnosed from clinical history and ultrasound. However, staging is more difficult, and the current consensus is that NASH can be diagnosed only after liver biopsy. This could be a major hindrance to any trials that need to recruit large number of patients. Liver biopsy can have complications, such as bleeding.
For detecting liver fibrosis, various non-invasive alternatives to liver biopsy have been suggested, including combinations of blood tests (‘serum marker panels’) and either transient or real-time elastography (a form of ultrasound). For detecting NAFLD, ultrasound and magnetic resonance imaging (MRI)/MRS have been suggested.
The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme is commissioning a full review of evidence on non-invasive methods for the assessment and monitoring of liver fibrosis and cirrhosis (HTA no. 09/07),68 and so this review does not duplicate that. However, we review the diagnosis of lesser degrees of liver damage in Chapter 3.
Biochemical picture associated with non-alcoholic fatty liver disease
Table 2 summarises the biochemical pattern in NAFLD, and has been compared with ALD, the other most common cause of liver problems. 5
| Feature | NAFLD | ALD |
|---|---|---|
| ALT | ↑ | → |
| AST | → | ↑ |
| ALT/AST ratio | > 1.0 | < 1.0 |
| GGT | →↑ | ↑↑ |
| Mean corpuscular volume | → | ↑ |
| FPG | →↑ | → |
| HDL-cholesterol | ↓ | ↑↑ |
| TGs | ↑ | ↑ or ↑↑ |
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) [and sometimes gamma-glutamyl transferase (GGT)] are components of the broader group of liver function blood tests known as the LFTs. It should be noted that the AST concentration can be higher than ALT in cirrhosis, and thus in individuals with known NAFLD a rising AST concentration (and reversal of the ALT/AST ratio) is potentially a bad prognostic sign, suggesting necrosis (liver cell death associated with inflammation). 5
Predicting non-alcoholic fatty liver disease
There are published algorithms that have attempted to detect individuals with a higher likelihood of NASH. 69,70 Factors such as age (> 50 years), BMI (> 28 kg/m2), hypertriglyceridaemia and elevated ALT level were risk factors that were independently associated with liver fibrosis. A scoring system combining these factors could reduce the number of patients requiring liver biopsy. 69
Treatment of non-alcoholic fatty liver disease
There is currently no approved treatment (medical or surgical) for NAFLD. Given that NAFLD is a group of inter-related progressive liver disorders, the aim and type of treatment will depend on the stage at which the diagnosis is made. There is no available prospective evidence showing the outcome of any form of treatment on important long-term outcomes, such as the development of cirrhosis or HCC. Treatment is aimed at reducing future risk of diabetes, or optimising control in patients with diabetes, treating obesity and reducing cardiovascular risk. Interventional studies have tended to use improvements in LFTs and liver histology as surrogate end points, based on the assumption that these will reflect subsequent reductions in morbidity from NAFLD.
Lifestyle changes
Reduced calorie intake and increased physical activity are viewed as logical methods to reduce liver fat content. Two studies have shown that a reduction in weight by 10% significantly reduced elevated LFTs compared with subjects with NAFLD who did not lose as much weight. 71,72 Park et al. 72 from Republic of Korea reported a marked reduction in liver enzymes (AST and ALT) in those who lost weight, but not in those who did not. Ueno et al. 73 from Japan reported that effects of intensive lifestyle modification over a 3-month period in overweight patients with NAFLD produced an improvement in the microscopic changes in liver tissue, but such changes did not attain significance. 73 However, a reduction of three units in BMI normalised previously elevated AST and ALT levels.
Weight loss drugs
Published pilot data on orlistat, a weight loss drug that reduces fat absorption, have shown improvements in LFTs, ultrasound findings and microscopic changes in liver tissue. 74,75 A further small study76 compared orlistat with another drug – sibutramine, an appetite suppressant – and the results showed an improvement in LFTs and reduced liver fat on ultrasound in both groups.
In a detailed review of the effects of lifestyle modification with and without the use of weight loss drugs in patients with steatosis and NASH, Harrison and Day77 concluded that a weight loss of 10% of body weight not only improved the biochemical measures seen in NAFLD, but also improved the histological changes seen in NASH. They also concluded that the evidence from a number of studies suggested that physical activity alone, or with only modest (3%) weight loss, was also effective in improving insulin sensitivity.
More recently, a high-quality systematic review by Musso et al. 78 reviewed all treatments for NAFLD. The authors also concluded that weight loss improved or reversed NASH, but that it appeared from two randomised controlled trials (RCTs) that weight loss had to be at least 7% or 9% for histological features to measurably improve. Musso et al. 78 came to the same conclusion as Harrison and Day77 (but based on different studies) – that the evidence suggested that physical activity improved NAFLD independently of weight loss. These data, in turn, concur with evidence for an independent association of higher activity levels, albeit assessed by questionnaire, with lower GGT levels in a cohort study of British women. 79
Drugs that control blood glucose levels
Trials using drugs that improve the body’s sensitivity to the effects of insulin have been performed in individuals with NAFLD. Two main drugs – metformin and thiazolidinediones (TZDs) (glitazones) – have been investigated. Metformin reduces glucose production in the liver and improves the uptake of circulating glucose in the blood by fat and muscle, whereas the glitazones improve the uptake of circulating glucose in the blood and are also believed to redistribute fat away from ectopic sources (particularly the liver) to subcutaneous areas, often with an overall increase in weight (substantial in some individuals). 80
The glitazones have adverse effects, including oedema, higher risk of fracture and in addition the development and worsening of heart failure;81,82 and rosiglitazone moderately increases cardiovascular risk83,84 and has recently been suspended in Europe.
The evidence on metformin and the glitazones is reviewed in Chapter 2.
Non-alcoholic fatty liver disease and other drugs
Patients with NAFLD are likely to be considered for lipid-lowering statin therapy owing to their elevated lipid levels and increased cardiovascular risk. Statin therapy in NAFLD certainly appears safe and should not be avoided because of mildly abnormal LFTs. 85 There is also some evidence of improvement in liver histology on statin therapy from a small placebo-controlled study that was recently published. 86 Other drugs studied have included vitamin E and fibrates. Vitamin E therapy has produced variable results87,88 and fibrate therapy has not shown benefit thus far. 89 In the only placebo-controlled studies so far, combination therapy with the bile component ursodeoxycholic acid (UDCA) with vitamin E for 2 years resulted in a significant reduction in steatosis. 90 UDCA therapy alone did not improve changes in liver tissue, although there was an improvement in LFTs. In two small studies, treatment with the angiotensin II blocker, losartan, also led to improvements in liver histology. 91,92
A review of all drugs for NAFLD was not in our remit, but we note the findings of the recent review by Musso et al. ,78 who found that:
-
There is some evidence of benefit from treatment with polyunsaturated fatty acids. Trials are in progress.
-
Fibrates had no effect.
-
The evidence on statins was sparse, but there was some evidence of benefit from atorvastatin.
-
The lipid-lowering drug probucol lowered ALT, but also high-density lipoprotein (HDL), which might increase cardiovascular risk.
-
UDCA had little or no effect.
-
There were mixed results with antioxidants.
-
Pentoxifylline appeared to be of benefit; two RCTs are under way.
-
Telmisartan (an angiotensin receptor blocker) appeared to reduce fibrosis, the only drug to do that. It improved steatosis, ballooning and lobular inflammation. It led to a reduction in insulin resistance, and TG and total cholesterol (TC) levels. It is noteworthy that the effects of valsartan were less despite similar blood pressure effects. The liver effects of telmisartan may be related to its peroxisome proliferators-activated receptor (PPAR) gamma activity.
-
There was some evidence of benefit for l-carnitine.
-
Orlistat added to diet resulted in little difference – weight loss of 8% instead of 6% on diet alone.
Surgery
Recently, data on surgery for morbid obesity with 18- to 24-month follow-up have been published. There are two main types of surgery for obesity. One procedure, gastric bypass surgery, in essence involves reducing the size of the stomach, by bypassing a large section of the stomach and connecting it to the small intestine, and this can be done laparoscopically (keyhole surgery). The second procedure is adjustable gastric banding, and involves placing an adjustable band over the top part of the stomach, creating a pouch that reduces the size of the stomach, and is also done laparoscopically. Studies have shown improvement of NAFLD staging or even complete disease resolution following surgery. Mummadi et al. 93 carried out a systematic review to explore effectiveness of bariatric surgery in patients with NAFLD and found that the procedure was safe, with resolution of steatosis in 91.6% of patients, improvement of steatohepatitis and fibrosis in 81.3% and 65.5% of patients, respectively, and resolution of NASH in 69.5%. 93
The effects of bypass surgery and concomitant weight loss (mean loss 50 kg), evaluated by liver biopsy, found considerable improvements in the prevalence of steatosis (90% preoperatively to 2.9% post-operatively), hepatocellular ballooning (swelling of liver cells: 58.9% preoperatively to 0% post-operatively) and fibrosis (50% down to 25%). 94 In a similar study,95 there were similarly impressive reductions in steatosis, fibrosis and hepatocellular ballooning. In this latter study on a group of 18 patients, NASH resolved in 84% of patients and steatosis in 75%. Laparoscopic adjustable gastric banding with resultant weight loss has also led to promising improvements in liver histology. 96,97 As obesity surgery is relatively novel, long-term outcome data are still limited, but should become more available as more of these procedures are performed.
Musso et al. 78 concluded that the RCTs of surgery had insufficient follow-up as yet, noting that liver enzymes fluctuated and did not always correlate with fibrosis and NASH. However, a review of bariatric surgery by Kushner and Noble98 noted that two studies had shown resolution or improvement in NASH and fibrosis after bariatric surgery.
Conclusion
In summary, NAFLD is a chronic liver condition with a spectrum from simple steatosis to liver failure, associated with metabolic disturbances that result in organ-specific and cardiovascular morbidity and mortality. It appears to be increasing in prevalence. Unless this trend is reversed, this is likely to lead to increased demands on NHS resources in the years to come.
Decision problems
The HTA commissioning brief for this review identified the main issues as being the clinical effectiveness and cost-effectiveness of the insulin sensitisers in NAFLD, with the expectation that a trial might be required.
The first aim of this review is therefore to assess the current evidence base, with possible outcomes as follows:
-
There may be enough evidence to show that these drugs are effective, and that a further trial is not necessary. Alternatively, there may be sufficient evidence to show that, though effective, the effect size is too small to make them worth pursuing as a therapy for NAFLD.
-
There may be insufficient evidence for use in therapy, but enough to justify a large trial.
If there were to be a large trial, one problem would be how to identify eligible patients. It would be impractical to carry out liver biopsies on large numbers of people, so we would need a non-invasive screening test. Possible options are reviewed in Chapter 3.
Another consideration, which is outwith the scope of this review, is whether or not other drugs might be better options. The number of drugs that have been used suggests that none has been strikingly effective. Chekhov’s comment may be relevant: ‘When a lot of remedies are suggested for a disease, that means it can’t be cured’. 99
However, the evidence suggests that NAFLD can be cured by sufficient weight loss. Unfortunately, adherence to lifestyle change is frequently poor.
Chapter 2 Clinical effectiveness
Methodology
Criteria for considering studies for this review
Types of studies
Systematic reviews and RCTs. There was no size restriction on the number of patients in trials, as those with inadequate numbers, and hence power, might be useful when combined in a meta-analysis. Observational studies were considered for data on safety.
Types of participants
Participants of any age, sex or ethnic origin with NAFLD proven by liver biopsy.
Types of interventions
Metformin, pioglitazone or rosiglitazone given at any dose or any duration, given separately or in combination versus no intervention, placebo or other pharmacological interventions.
Types of outcome measures
Measures of disease progression such as:
-
fibrosis and cirrhosis
-
other hepatic-related morbidity, such as variceal bleeding or liver failure
-
cardiovascular events
-
quality of life (QoL)
-
new diabetes
-
adverse events.
Search strategy
Comprehensive systematic searches of electronic databases were performed in order to retrieve relevant papers.
Searches were conducted in the following sources to identify both published studies and meeting abstracts:
-
MEDLINE, 1950 to June 2010; EMBASE, 1980 to June 2010; Science Citation Index – Expanded, June 2010 (limited to meeting abstracts only); Conference Proceedings Citation Index – Science, June 2010; The Cochrane Library, 2005–2010; and authors’ reference lists. Websites such as ‘ClinicalTrials.gov’ were systematically searched to find any ongoing trials.
The following MEDLINE search strategy (Ovid) was adapted for use with the other databases:
-
fatty liver/
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFLD or NAFLD or NASH).tw.
-
4 and 3 and 2
-
6 or 1 or 5
-
exp Metformin/
-
exp Thiazolidinediones/
-
(insulin sensit* or metformin or pioglitazone or rosiglitazone or thiazolidinedione* or glitazone*).tw.
-
18 or 9 or 10
-
7 and 11.
All of the relevant systematic reviews were searched for additional studies. No attempt was made to find unpublished studies. No language restriction was applied to the search strategy.
Details of the electronic search strategies used for the review of clinical effectiveness are given in Appendix 1.
Identification of studies
Abstracts and titles retrieved by the search strategy were assessed independently by two researchers and screened for inclusion and exclusion. Full texts of studies considered possible inclusions were obtained and each was examined by two reviewers independently. Any discrepancies between the two were resolved by discussions and with involvement of a third reviewer when necessary. The papers that did not meet the inclusion criteria were excluded.
Data extraction strategy
Two reviewers independently extracted data in a specially designed form, and data regarding study design and characteristics, details of the intervention and patient characteristics and outcomes were recorded in the form for each study. Differences in data extraction were resolved by discussion, referring back to the original papers and with involvement of a third reviewer when necessary.
Quality assessment strategy
To assess the quality of the RCTs, the following criteria were used: (1) method and description of randomisation; (2) allocation concealment; (3) blinding; (4) intention-to-treat (ITT) analysis; (5) percentage who completed the trial; (6) power calculation; and (7) similarity of group participants at baseline.
Best practice for each of the criteria would be as follows:
-
randomisation random assignment generated by computer
-
concealment of allocation those at point of implementing random allocation to treatment do not know to what the next patient will be allocated
-
blinding those assessing outcomes (e.g. the pathologist looking at biopsies) should not know which treatment patients were on
-
intention to treat patients remained on their allocated treatments throughout with no crossover
-
loss to follow-up all patients completed the trial with no losses to follow-up
-
baseline matching randomisation ensured that prognostically important variables were equally distributed across the arms.
Studies meeting most of these criteria were regarded as high-to-moderate quality.
Analysis
Meta-analyses of the outcomes were not possible, as outcomes were reported incompletely and in a variety of ways. Hence, all of the results are presented in text and tables.
Results
Result of the searches
A total number of 1842 titles and abstracts were retrieved by the searches (Figure 5). The titles and abstracts were screened for inclusion and exclusion; 49 were considered possible inclusions and full texts of these were obtained. Out of these, 34 papers were excluded because of not meeting the inclusion criteria, not reporting outcomes of interest or not being RCTs. Details of the reasons for exclusion are given in Figure 5.
FIGURE 5.
Flow chart of search results.
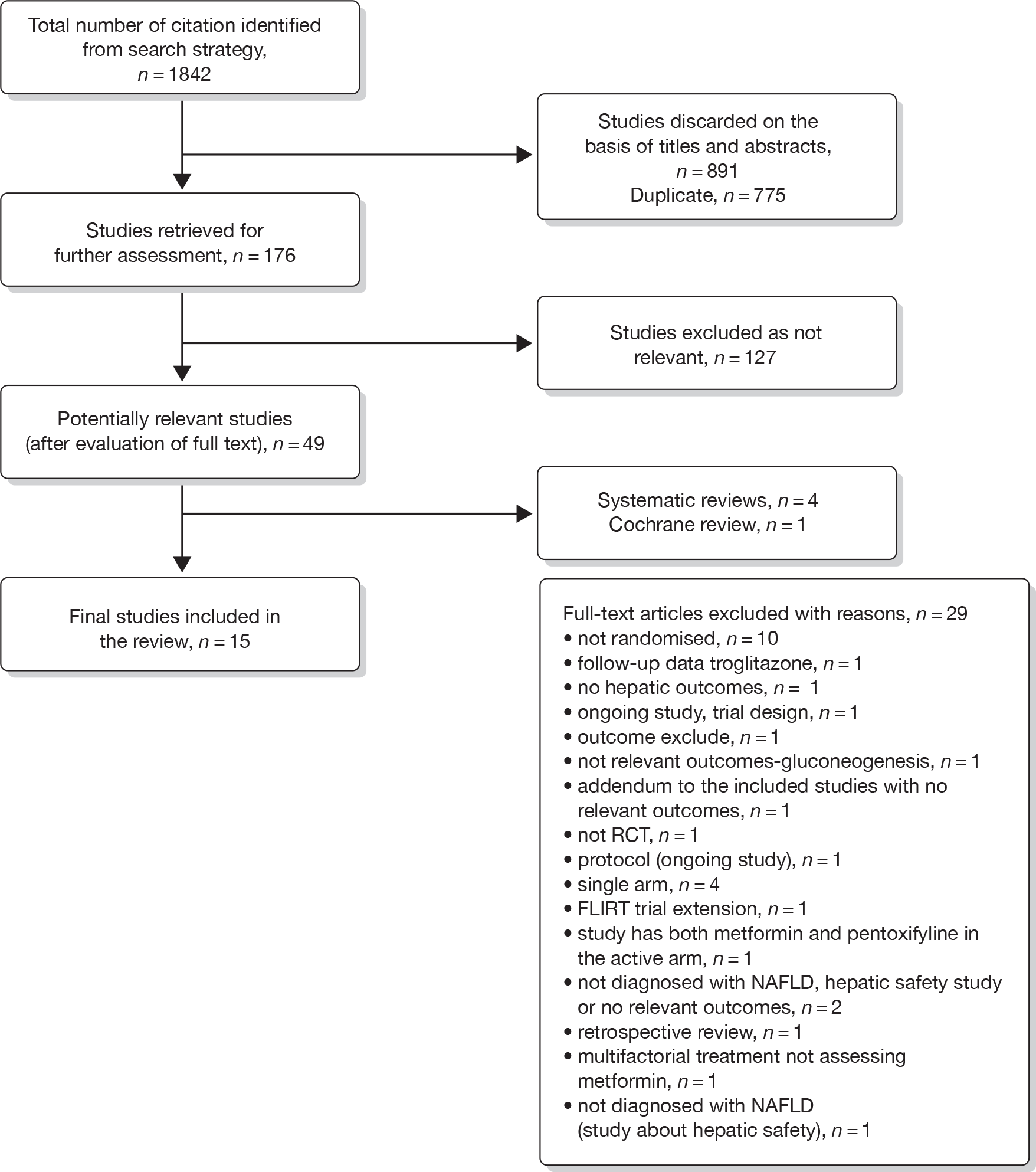
A total of 15 RCTs (14 full texts and one abstract100) fulfilled the inclusion criteria and were included in the review. Of the 15 studies included, four examined pioglitazone,101–104 eight metformin,87,100,105–110 one rosiglitazone111 and two trials compared the effects of metformin and rosiglitazone. 112,113
A search for ongoing trials or reviews was carried out. An out-of-date Cochrane review on insulin sensitisers by Angelico et al. ,114 in Rome, was found. It excluded people with T2DM, and included only three trials: two of metformin and one of pioglitazone. Four systematic reviews were also found but none included all of the trials now available. A systematic review by Musso et al. 78 also included fully published trials and abstracts that had explored the efficacy of non-pharmacological interventions, UDCA, lipid-lowering drugs, antioxidants, anti-tumour necrosis factor alpha agents (pentoxifylline), anti-hypertensive drugs, endocannabinoid receptor antagonists, l-carnitine and bariatric surgery among patients with NASH/NAFLD.
Descriptions of included studies
The included trials (identified by first author and year) are reviewed in this section. Further details of these can be found in the data extraction tables in Appendix 2. Table 3 gives a summary of all of the included studies.
| Study ID | Sample size (completed/randomised) | Interventions | Comparators | Comparisons | Duration of trial | Country (settings) | Diabetes |
|---|---|---|---|---|---|---|---|
| Pioglitazone | |||||||
| Pioglitazone vs placebo | |||||||
| Aithal et al. 2008101 |
n Pio: 31/37 n Pbo: 30/37 |
Pioglitazone + diet/exercise | Placebo + diet/exercise | Pio vs Pbo | 1-year run-in period; 3 months before randomisation; treatment period, 12 months | UK (Queens Medical Centre in Nottingham and Derby City General Hospital) | People with NASH and without diabetes |
| Belfort et al. 2006102 |
n Pio: 18/26 n Pbo: 22/26 |
Pioglitazone + hypocaloric diet | Placebo + hypocaloric diet | Pio vs Pbo | 6 months: October 2002 to November 2004; run-in period, 4 weeks; treatment, 6 months | USA, TX | 55 with impaired glucose tolerance test or diabetes |
| Sanyal et al. 2004103 |
n Pio: 8/10 n Pbo: 10/10 |
Pioglitazone + vitamin E | Vitamin E | Pio vs Pbo | 6 months; follow-up before randomisation, 3 months; treatment, 6 months | USA (NAFLD clinic within the general clinical research centre at the Virginia University) | People with NASH and without diabetes |
| Sanyal et al. 2010104 |
n Pio: 80 n Pbo: 83 |
Pioglitazone | Placebo | Pio vs Pbo | 96 weeks; 24 weeks’ follow-up | USA, multiple centres | People with NASH and without diabetes |
| Pioglitazone vs vitamin E | |||||||
| Sanyal et al. 2010104 |
n Pio: 80 n Vitamin E: 84 |
Pioglitazone | Vitamin E | Pio vs Vit E | 96 weeks; 24 weeks’ follow-up | USA, multiple centres | People with NASH and without diabetes |
| Metformin | |||||||
| Metformin vs placebo | |||||||
| Garinis et al. 2010105 |
n Met: 15/20 n Pbo: 25/25 |
Metformin + hypocaloric diet | Hypocaloric diet | Met vs Pbo | 6 months | Italy (Endocrine Unit of University Magna Graecia of Cantanzora) | People with NAFLD and without diabetes |
| Haukeland et al. 2009106 |
n Met: 20/24 n Pbo: 24/24 |
Metformin + advice on healthy lifestyle | Placebo + advice on healthy lifestyle | Met vs Pbo | 6 months | Norway (four university hospitals) | Impaired glucose tolerance or T2DM |
| Idilman et al. 2008112 |
n Met: 24/24 n C: 25/25 |
Metformin + diet and exercise | Diet and exercise only | Met vs Pbo | 48 weeks: December 2004 to October 2005; treatment, 48 weeks; follow-up, 6 months | Turkey, (Ankara, outpatient clinic) | People with NASH and without diabetes |
| Nadeau et al. 2009107 |
n Met: 28/37 n Pbo: 10/13 |
Metformin + lifestyle modifications | Placebo + lifestyle modifications | Met vs Pbo | 6 months; treatment, 6 months | USA | People without diabetes |
| Nar and Gedik 2009108 |
n Met: 19/19 n Pbo: 15/15 |
Metformin + diet and exercise | Diet and exercise only | Met vs Pbo | 6 months | Turkey, Ankara | 34 patients with newly diagnosed diabetes with NAFLD without anti-diabetic medication |
| Shields et al. 2009109 |
n Met: 9/9 n Pbo: 7/10 |
Metformin + diet and exercise | Placebo + diet and exercise | Met vs Pbo | 1 year | USA (Gastroenterology clinics at the Naval Medical Centre, San Diego, CA) | People with NASH and without diabetes |
| Uygun et al. 2004110 |
n Met: 15/17 n Pbo: 17/17 |
Metformin + diet | Diet alone | Met vs Pbo | 6 months: August 1999 to June 2001 | Turkey, Ankara | People with NASH and without diabetes |
| Metformin vs diet | |||||||
| Bugianesi et al. 200587 |
n Met: 55/55 n C: prescriptive diet 27/27 |
Metformin | Prescriptive diet only | Met vs prescriptive diet | 1-year treatment; 12 months | Italy (two units, Bologna and Turin) | People with NAFLD and without diabetes |
| Metformin vs vitamin E | |||||||
| Bugianesi et al. 200587 |
110: n Met: 55/55 n Pbo: vitamin E 28/28 |
Metformin | Vitamin E | Met vs Vit E | 1 year | Italy (two units: Bologna and Turin) | People with NAFLD and without diabetes |
| Metformin vs rosiglitazone + metformin | |||||||
| Omer et al. 2010113 |
n Met: 19/22; n Met + Rosi: 22/22 |
Metformin | Metformin + rosiglitazone | Met vs Met + Rosi | 12 months | Turkey (outpatient clinics) | Impaired glucose tolerance or T2DM with NAFLD |
| Rosiglitazone | |||||||
| Rosiglitazone vs placebo | |||||||
| Idilman et al. 2008112 |
n Rosi: 24/25 n C: 25/25 |
Rosiglitazone + diet and exercise | Diet and exercise only | Rosi vs Pbo | 48 weeks | Turkey (Ankara, outpatient clinic) | People with NASH and without diabetes |
| Ratziu et al. 2008, FLIRT111 |
n Rosi: 32/32 n Pbo: 31/31 |
Rosiglitazone | Placebo | Rosi vs Pbo | 1 year; January 2003 to November 2004; treatment, 12 months; follow-up, 4 months | France | People with NASH and without diabetes |
| Rosiglitazone vs met formin + rosiglitazone | |||||||
| Omer et al. 2010113 |
n Rosi: 20/20 n Met + Rosi: 22/22 |
Rosiglitazone | Rosiglitazone + metformin | Rosi vs Rosi + Met | 12 months | Turkey (outpatient clinics) | Impaired glucose tolerance or T2DM with NAFLD |
| Torres et al. 2009100 (abstract) |
108 randomised (till date); 49 completed; n Rosi: 15 n Met + Rosi: 16; n Rosi + Los: 18 (excluded) |
Rosiglitazone | Rosiglitazone + metformin | Rosi vs Rosi + Met | 1 year | NR (presumably USA – San Antonio, TX); Centre: NR | People with NASH, diabetes not reported |
| Rosiglitazone vs metformin | |||||||
| Omer 2010 et al.113 |
n Rosi: 20/20 n Met: 19/22 |
Rosiglitazone | Metformin | Rosi vs Met | 12 months | Turkey (outpatient clinics) | Impaired glucose tolerance or T2DM with NAFLD |
Pioglitazone
Aithal (2008)101 conducted a randomised, double-blind, placebo-controlled trial in 74 non-diabetic patients, aged 18–70 years, diagnosed with NASH, confirmed by liver biopsy and ultrasound. The participants were randomised into two groups, one receiving pioglitazone 30 mg/day, whereas the second group had placebo tablets. Both of these groups also received advice on diet and exercise. Mean BMI at baseline was 29.8 kg/m2 [standard deviation (SD) 3.0 kg/m2] in the pioglitazone group and 30.8 kg/m2 (SD 4.1 kg/m2) in the control group. The treatment was carried out for 1 year. The primary aim of the study was to measure changes in liver histology by assessing liver biopsy specimens using staging and grading criteria proposed by Brunt et al. 115
Belfort (2006)102 conducted a randomised, double-blind, placebo-controlled trial, to explore the effects of pioglitazone plus a calorie-restricted diet in patients with NASH. The treatment group received 30 mg of pioglitazone per day for the first 2 months, which was increased to 45 mg/day and remained unchanged until end of the study, whereas the control group received placebo pills. These interventions were combined with dietary advice in all the participants. They were advised to reduce their calorie intake by 500 kcal per day. Mean BMI at baseline was 33.5 kg/m2 (SD 4.9 kg/m2) in the treatment group and 32.9 kg/m2 (SD 4.4 kg/m2) in the control group. The primary outcome measure was histological changes in liver assessed by the NASH histological scoring system proposed by Kleiner et al. 116
Sanyal (2004),103 in a pilot study, compared the effects of pioglitazone along with an antioxidant (vitamin E) in 20 participants with NASH. In this prospective RCT, the first group took pioglitazone 30 mg/day in combination with vitamin E 400 international units (IU)/day, whereas the other group were on vitamin E only. The treatment was given for 6 months. Mean BMI at baseline was 32.5 kg/m2 (SD 4.3 kg/m2) in the pioglitazone group and 30.7 kg/m2 (SD 4.7 kg/m2) in the control group. The primary aim of the study was to explore the changes in liver histology using modified Brunt score.
Sanyal (2010)104 conducted a multicentre, randomised, double-blind, placebo-controlled trial to compare vitamin E and pioglitazone with placebo in NASH. A total of 247 participants were randomised into three groups, i.e. pioglitazone, vitamin E and placebo. Pioglitazone was given in a dose of 30 mg/day, vitamin E 400 (IU)/day, and placebo resembling either pioglitazone or vitamin E. The treatment was given for 96 weeks, with an additional 24 weeks’ follow-up. Mean BMI at baseline was 34.0 kg/m2, 34.0 kg/m2 and 35.0 kg/m2 in pioglitazone, vitamin E and placebo group, respectively. The primary aim of the study was to explore the changes in liver histology.
Metformin
Bugianesi (2005)87 recruited 110 participants with NAFLD confirmed by liver biopsy. This was an open-label trial conducted in two units in Italy and the participants were followed up for 12 months. At one centre, metformin was compared with vitamin E and in the other it was compared against a prescriptive diet. One group of participants received metformin, with a maximum dose of 2000 mg/day, the second group either received vitamin E 400 IU twice a day (b.i.d.)/day or a weight-reducing prescriptive diet to determine a caloric deficit of 500 kcal per day. In addition, all of the participants were encouraged to walk or to jog at least 30 minutes a day. Mean BMI at baseline in metformin group was 28.7 kg/m2 (SD 3.6 kg/m2) (in both centres), 29.1 kg/m2 (SD 2.7 kg/m2) in the vitamin E group and 28.2 kg/m2 (SD 3.6 kg/m2) in the prescriptive diet group. The primary aim of this study was to compare the effects of metformin against vitamin E or prescriptive weight-reducing diet in terms of ALT normalisation, histological changes in liver, changes in liver enzymes and insulin resistance.
Garinis (2010)105 recruited 50 participants in an open-label trial and randomised them into two groups; one group (n = 25) received metformin plus hypocaloric diet (1300 kcal/day) and the second received hypocaloric diet only. Mean age of the participants in the metformin and placebo groups was 40.8 and 45.8 years, respectively. Mean BMI at baseline was 36.5 kg/m2 in the metformin group and 34.7 kg/m2 in the second. The participants were followed up for 6 months to explore the changes in liver steatosis confirmed by USG.
Haukeland (2009)106 recruited 48 participants with NAFLD in a double-blind RCT. The mean ages of the participants in the placebo and metformin group was 49.9 years and 44.3 years, respectively. All of the participants received general advice about a healthy lifestyle that included taking 30 minutes of physical activity a day, and a diet low in fat, especially saturated fat, and refined carbohydrates. Mean BMI at baseline in the placebo and metformin groups was 31.4 kg/m2 and 30.3 kg/m2, respectively. The intervention was given for 6 months. Changes in steatosis, confirmed by liver biopsy, were measured.
Nadeau (2009)107 conducted a double-blind RCT and recruited 55 participants with fatty liver and elevated liver-associated enzymes. The participants were adolescents aged between 12 and 18 years and received either metformin or placebo capsules. Mean age of the participants was 15.1 years. Both groups underwent a dietary assessment and watched a standardised video about healthy eating habits. The participants were treated for 6 months and the progression or regression of the fatty liver was measured by liver ultrasound and other biochemical examination. Mean BMI at baseline was 39.6 kg/m2 [standard error (SE) 0.98 kg/m2)] and 40.2 kg/m2 (SE 1.8 kg/m2) in the metformin and placebo groups, respectively.
Nar (2009)108 recruited 34 participants with newly diagnosed T2DM and NAFLD (diagnosed by ultrasound) to explore the effect of metformin on plasma leptin levels. The participants on metformin were given the maximum dose of 1700 mg of metformin per day, whereas the other group was only on diet and exercise. Dietary advice was given by a dietician and the recommended exercise was walking for a minimum of 30 minutes at least 3 days a week. The progression of liver disease was measured by liver ultrasound and biochemical examinations. Mean BMI at baseline was 31.0 kg/m2 (SE 4.0 kg/m2) in the metformin group and 33.7 kg/m2 (SE 6.0 kg/m2) in the control group.
Shields (2009)109 conducted a pilot study of a prospective, randomised, placebo-controlled trial conducted in 19 participants with insulin resistance and NASH. The intervention group received 500 mg of metformin daily titrated to 1000 mg, and dietary counselling with recommendation to lose weight and take 30 minutes of aerobic exercise four times a week. The control group received the same diet and exercise advice. The primary aim of the study was to assess the histological changes in liver using criteria proposed by Brunt et al. 115 Mean BMI at baseline in the treatment group was 32.2 kg/m2 (SD 4.9 kg/m2) and 32.8 kg/m2 (SD 4.9 kg/m2) in the control group. The participants were treated for 1 year.
Torres (2009)100 conducted an open-label, randomised trial (reported as an abstract only) and recruited 108 participants with NASH to compare the effects of rosiglitazone against rosiglitazone and metformin in combination. Details given are scarce and the abstract gives results for the first 49 (of 108) participants who have completed the trial to date. The third group of participants receiving rosiglitazone and losartan was not considered in the present review. Mean age of the participants was 48.9 years. Mean BMI at baseline was 33.3 kg/m2.
Uygun (2004)110 conducted an RCT of metformin in 36 participants with NASH, insulin resistance and elevated liver enzyme concentrations. The treatment group received metformin in the dose of 850 mg twice daily plus dietary treatment, whereas the control group received only dietary treatment. In addition, all obese participants were advised to lose weight with a restriction of calorie intake to 1600–1800 calories per day. The interventions were given for 6 months and the groups were followed up for another 6 months. The primary aim of the study was to compare the effects of these interventions on liver histology and insulin resistance. Mean BMI of the treatment and control groups at baseline was 30.1 kg/m2 (SD 3.4 kg/m2) and 28.4 kg/m2 (SD 3.9 kg/m2), respectively.
Rosiglitazone
Ratziu [Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) trial] (2008)111 recruited 64 participants with NASH to a double-blind, placebo-controlled RCT. The treatment group received rosiglitazone 4 mg daily for the first month, and then 8 mg daily until end of the trial. Both groups were instructed to lose weight, to follow a healthy diet and to exercise at least twice a week, if they were obese or overweight. No specialised nutritional counselling was implemented. The primary aim of the study was to assess if there had been a > 30% reduction or even a complete disappearance of hepatic steatosis at end of the study compared with the baseline findings. The progression of the liver histology was assessed using the Brunt scoring system. Mean BMI at baseline in the rosiglitazone group was 31.5 kg/m2 (SD 6 kg/m2), whereas it was 30.5 kg/m2 (SD 4.4 kg/m2) in the placebo group.
Metformin and rosiglitazone
Idilman (2008)112 recruited participants over 18 years with newly diagnosed NASH to compare the effect of metformin and rosiglitazone. The participants were randomised into three groups: one received diet and exercise plus metformin 850 mg b.i.d., the second group diet and exercise plus rosiglitazone 8 mg per day, and the third group was on diet and exercise alone. The intervention was carried out for 48 weeks with 6 months of post-intervention follow-up. The primary aim of the study was unclear; however, the authors compared the effects of these interventions in terms of metabolic, biochemical and histological parameters. The progression or regression of the liver disease was assessed by liver biopsy using criteria proposed by Brunt et al. 115 and a NAFLD activity score (NAS) by Kleiner et al. 116 Mean BMI at baseline was 31.2 kg/m2 (SD 3.6 kg/m2) in the insulin sensitisers group and 32.2 kg/m2 (SD 5.1 kg/m2) in the diet and exercise group.
Omer (2010)113 conducted an open-label, single-centre RCT and recruited 64 participants with NAFLD and T2DM or impaired glucose metabolism. The participants were randomised into three different groups and received metformin or rosiglitazone or both. All the groups also received dietary counselling from an endocrinologist and dieticians, and were also encouraged to do regular exercise 12 weeks prior to the intervention and also during the study period. The intervention was given for 12 months and at the end histological changes were assessed using Kleiner et al. ’s grading system. 116 The mean age of the participants receiving metformin or rosiglitazone or both was 48 years, 49.3 years and 49.6 years, respectively. Mean BMI at baseline was 30.8 kg/m2 in the metformin group, 28.4 kg/m2 in the rosiglitazone group and 32.5 kg/m2 in the metformin and rosiglitazone group.
Quality of included studies
A summary of the quality of the studies is given in Table 4. Full details are given in Appendix 3.
| Study | Method of randomisation | Allocation concealment | Blinding | ITT data analysis | Percentage who completed trial | Power calculation | Similarity of groups at baseline | Sponsorship/author affiliation | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | |||||||||
| Aithal 2008101 | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | 4 |
| Belfort 2006102 | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | 5 |
| Sanyal 2004103 | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | 4 |
| Sanyal 2010104 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| Metformin | |||||||||
| Bugianesi 200587 | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | 4 |
| Garinis 2010105 | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | 2 |
| Haukeland 2009106 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | 7 |
| Nadeau 2009107 | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | 4 |
| Nar 2009108 | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | 2 |
| Shields 2009109 | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | 5 |
| Torres 2009100 | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | 1 |
| Uygun 2004110 | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | 3 |
| Rosiglitazone | |||||||||
| Ratziu 2008 (FLIRT trial)111 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | 7 |
| Idilman 2008112 | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | 3 |
| Omer 2010113 | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | 2 |
Internal validity
Randomisation
The majority of the studies described the randomisation procedure. In some100,105,107,108,112,113 the descriptions were unclear or not given. Aithal (2008),101 Sanyal (2004)103 and Ratziu (2008)111 used block randomisation. Belfort (2006),102 Haukeland (2009)106 and Shields (2009)109 used computerised allocation. Bugianesi (2005)87 used random sequence and the randomisation was centralised in one centre. Sanyal (2010)104 randomised participants centrally by the Data Coordinating Centre (DCC) and the participants were assigned in permuted blocks of treatments.
The methods of allocation concealment were reported in only four studies;87,104,106,111 in the remaining papers the method of allocation concealment was either not clear or not reported.
Sample size
Descriptions of study power were given in only four studies. 101,104,111,117 The remaining studies did not report if the power was calculated. Two of the 15 trials103,109 were pilot studies.
Similarity of groups at baseline
In the majority of the studies, patients in the arms were similar to each other at baseline. Aithal (2008)101 reported that all other parameters were similar between groups except that the pioglitazone group had lower alkaline phosphatase and fasting insulin levels than the placebo group. Sanyal (2010)104 reported that all three groups were similar in demographic, clinical, laboratory results, and the NASH scores. However, about 17%, 18% and 28% in the placebo, vitamin E and pioglitazone groups, respectively, did not have hepatocellular ballooning on assessment of their initial biopsy specimen. Shields (2009)109 stated that the treatment group was older and predominantly male. Haukeland (2009)106 reported that the participants on metformin were younger, less often treated for hypertension and slightly more obese. Omer (2010)113 reported that all groups were similar at baseline, except that baseline serum insulin was significantly higher in the metformin group and in the group that was on a combination of metformin and rosiglitazone than in the rosiglitazone group.
Intention-to-treat analysis
Only three studies87,104,109 conducted an ITT analysis. Haukeland (2009)106 had done per-protocol analysis. The descriptions of completion rate, loss to follow-up and withdrawals were given in all the studies except those by Nar (2009)108 and Torres (2009). 100 Torres (2009) has published results only for the first 49 participants that have completed the trial and in addition reported that a total of 108 participants have been randomised to date. Three studies87,111,112 had no dropouts.
Detection bias
Out of 15 trials, only six101,102,104,106,107,111 were double-blind, placebo-controlled trials. The remaining trials were unblinded. In the unblinded trials, the pathologists reviewing liver biopsies100,103,106,107,109,111,112 and radiologists conducting ultrasound108 were blinded to treatment arms.
External validity
The trials were conducted in the UK, France, Italy, Turkey, Norway and the USA, with none of them in Asian or African countries.
Main results
The following outcomes are summarised in this section:
-
liver histology
-
glycosylated haemoglobin (HbA1c)
-
fasting plasma glucose (FPG)
-
weight/BMI
-
blood pressure
-
lipid parameters
-
liver biochemistry
-
insulin sensitivity/resistance
-
QoL
-
conversion to diabetes/impaired glucose tolerance/metabolic syndrome.
Details of all outcomes are given in the tables below (see Tables 5–12).
| Study | Outcome | Change from baseline | p-value (from baseline to end) | p-value (between groups) |
|---|---|---|---|---|
| Pioglitazone | ||||
|
Aithal 2008101 (Brunt et al. 115): n (%) |
Steatosis |
Decrease: Pio: 15/31 (48%); Pbo: 11/30 (37) Increase: Pio: 1/31 (3.2%); Pbo: 3/30 (10) |
Not given | p = 0.19 |
| Hepatocellular injury |
Decrease: Pio: 10/31 (32); Pbo: 3/30 (10) Increase: Pio: 4/31 (13); Pbo: 12/30 (40) |
p = 0.005 | ||
| Lobular inflammation |
Decrease: Pio: 14/31 (45); Pbo: 8/30 (27%) Increase: Pio: 4/31 (13); Pbo: 3/30 (10) |
p = 0.25 | ||
| Portal inflammation |
Decrease: Pio: 8/31 (26); Pbo: 7/30 (23) Increase: Pio: 8/31 (26); Pbo: 11/30 (37%) |
p = 0.67 | ||
| Mallory bodies |
Decrease: Pio: 8/31 (26); Pbo: 1/30 (3) Increase: Pio: 0/31 (0); Pbo: 3/30 (10) |
p = 0.004 | ||
| Fibrosis |
Decrease: Pio: 9/31 (29); Pbo: 6/30 (20) Increase: Pio: 0/31 (0); Pbo: 6/30 (20) |
p = 0.05 | ||
|
Belfort 2006102 (Kleiner et al. 116) |
Steatosis |
Improvement: Pio: 65%; Pbo: 38% Reduction in score of ≥ 2: Pio: 9/21 (43%); Pbo: 0/14 (0%) |
Not given | Improvement: p = 0.003; reduction in score of ≥ 2: p = 0.004 |
| Ballooning necrosis | Improvement: Pio: 54%; Pbo: 24% | p = 0.02 | ||
| Lobular inflammation | Improvement: Pio: 65%; Pbo: 29% | p = 0.008 | ||
| Combined necroinflammation |
Improvement: Pio: 85%; Pbo: 38% Patients with reduction in score of ≥2: Pio: 11/24 (46); Pbo: 3/21 (14) |
Improvement: p = 0.001; reduction in score of ≥ 2: p = 0.02 | ||
| Fibrosis |
Improvement: Pio: 46%; Pbo: 33% Reduction in score: Pio: 5/12 (42%); Pbo: 1/6 (17%) |
Improvement:0.08; reduction in score of ≥ 2: p = 0.31 | ||
| Sanyal 2004103 (modified Brunt score) | Steatosis grade | Pio: –1.4; Vit E: –0.8 | Pio: p = 0.002; Vit E: p = 0.02 | p = NS |
| Cytological ballooning | Pio: –1; Vit E: –0.7 | Pio: p = 0.01; Vit E: p = 0.055 | p = 0.002 | |
| Mallory’s hyaline | Pio: –0.7; Vit E: –0.2 | Pio: p = 0.02; Vit E: p = 0.055 | p = 0.03 | |
| Pericellular fibrosis | Pio: –0.7; Vit E: –0.3 | Pio: p = 0.03; Vit E: NS | p = NS | |
| Inflammation | Not given | Not given | p = 0.001 | |
| Portal fibrosis | Pio: –0.2; Vit E: –0.1 | Pio: NS; Vit E: NS | p = NS | |
| Sanyal 2010104 (Brunt et al.115/Kleiner et al.116) | Fibrosis (%) | Pio: –0.4; Vit E: –0.3; Pbo: –0.1 | Not given | Pio vs Pbo: p = 0.10; Vit E vs Pbo: p = 0.19; Vit E vs Pio: p = 0.78 |
| Steatosis (%) | Pio: –0.8; Vit E: –0.7; Pbo: –0.1 | Pio vs Pbo: p < 0.0001; Vit E vs Pbo: p < 0.0001; Vit E vs Pio: p = 0.41 | ||
| Amount (foci) of lobular inflammation (%) | Pio: –0.7; Vit E: –0.6; Pbo: –0.2 | Pio vs Pbo: p = 0.0009; Vit E vs Pbo: p = 0.008; Vit E vs Pio: p = 0.59 | ||
| Portal, chronic inflammation (%) | Not given | Not given | ||
| Ballooning degeneration (%) | Pio: –0.4; Vit E: –0.5; Pbo: –0.2 | Pio vs Pbo: p = 0.01; Vit E vs Pbo: p = 0.03; Vit E vs Pio: p = 0.59 | ||
| Metformin | ||||
|
Bugianesi 200587 (modified Brunt score): n (%) |
Fat | Met: –20 | Met: p = 0.004 | Not given |
| Necroinflammation | Met: –0.65 | Met: p = 0.012 | ||
| Fibrosis | Met: –0.7 | Met: p = 0.012 | ||
| NASH index | Met: –2.06 | Met: p < 0.0001 | ||
| Garinis 2010105 (liver ultrasound) | Liver steatosis |
Improved/disappeared: Met: 5/20 (25%); Pbo: 6/25 (24%) Moderate to mild: Met: 2/20 (10%); Pbo: 5/25 (20%) Disappeared: Met: 3/20 (15%); Pbo: 1/25 (4%) |
Met: p < 0.0001; Pbo: p = 0.029 | Not given |
| Haukeland 2009106 (Kleiner score) | Steatosis | Proportion with improvement: Met: 25%; Pbo: 38% | Met: p = 0.10; Pbo: p = 0.033 | p = 0.52 |
| Ballooning necrosis | Proportion with improvement: Met: 5%; Pbo: 13% | Met: p = 0.058; Pbo: p = 1.0 | p = 0.61 | |
| Lobular inflammation | Proportion with improvement: Met: 15%; Pbo: 33% | Met: p = 0.21; Pbo: p = 0.59 | p = 0.29 | |
| Fibrosis | Proportion with improvement: Met: 5%; Pbo: 17% | Met: p = 1.0; Pbo: p = 0.56 | p = 0.066 | |
| Steatosis as % of hepatocytes with fat, (mean) | Met: –8; Pbo: –7 | Met: p = 0.024; Pbo: p = 0.052 | p = 0.09 | |
| NAS |
Met: 0.3; Pbo: –0.42 Proportion with improvement: Met: 20%; Pbo: 50% |
Met: p = 0.23; Pbo: p = 0.12 | p = 0.066; proportion with improvement (p = 0.060) | |
| Nadeau 2009107 (liver ultrasound) | Fatty liver by ultrasound (n,%) | Met: –4 (13%); Pbo: 2 (15%) | Met: p < 0.04 | p < 0.04 |
| Nar 2009108 (liver ultrasound) | Grade of hepatic steatosis | Results unclear – report decreases in liver echogenicity, but table suggests increase? | Met: p = NS; Pbo: p = NS | p = 0.043 |
| Shields 2009109 (Brunt scores) | Grade | Met: –0.11; Pbo: –0.35 | Not given | p = 0.67 |
| Steatosis | Met: –0.09; Pbo: –0.65 | p = 0.23 | ||
| Ballooning | Met: –0.22; Pbo: –0.28 | p = 0.967 | ||
| Intra-acinar inflammation | Met: 0.11; Pbo: –0.12 | p = 0.478 | ||
| Portal tract inflammation | Met: –0.22; Pbo: –0.08 | p = 0.523 | ||
| Fibrosis | Met: –0.05; Pbo: 0.2 | p = 0.447 | ||
| NAS | Met: –0.9; Pbo: –1.2 | p = 0.108 | ||
| Torres 2009100 | Steatosis | Met + Rosi: –23.1%; Rosi: –24.5% | Not given | Not given |
| Inflammation grade | Met + Rosi: –8.7%; Rosi: –12.5% | |||
| Fibrosis grade | Met + Rosi: –45.6%; Rosi: –15% | |||
| Uygun 2004110 (Brunt scores) | Necroinflammatory score | Met: –0.26; Pbo: –0.11 | Met: p = 0.31; Pbo: p = 0.62 | Not given |
| Fibrosis | Met: –0.02; Pbo: 0.07 | Met: p = 0.96; Pbo: p = 0.91 | ||
| Steatosis (USG abdomen) | Met: –0.64; Pbo: –0.25 | Met: p = 0.038; Pbo: p = 0.17 | ||
| Rosiglitazone | ||||
| Ratziu 2008111 (Brunt scores) | Steatosis grade | Reduction, mean (%): Rosi: –20 (26%); Pbo: –5 (23%) | Not given | p = 0.02 |
| Hepatocyte ballooning | Rosi: 0.13, SD 0.71; Pbo: 0.23, SD 0.8 | p = 0.61 | ||
| Lobular necrosis and inflammation | Rosi: –0.09, SD 0.73; Pbo: –0.13, SD 0.81 | p = 0.86 | ||
| Mallory bodies | Not given | Not given | ||
| Perisinusoidal fibrosis | Rosi: –0.03, SD 0.54; Pbo: –0.06, SD 0.63 | p = 0.83 | ||
| Fibrosis (stage) | Rosi: 0.03, SD 0.95; Pbo: –0.18, SD 1.14 | p = 0.43 | ||
| NAS score, median (IQR) | Not given | p = 0.60 | ||
| Metformin vs rosiglitazone | ||||
| Idilman 2008112 (Brunt et al.115) | Steatosis | Met: –1; Rosi: –1; Pbo: –0.5 | Met: p = NS; Rosi: p < 0.05; Pbo: p = NS | Not given; p < 0.05 insulin sensitisers vs baseline |
| Lobular inflammation | Met: 0; Rosi: 0; Pbo: 0 | Met: p = NS; Rosi: p = NS; Pbo: p = NS | ||
| Ballooning | Met: 0; Rosi: –1; Pbo: 0 | Met: p = NS; Rosi: p = NS; Pbo: p = NS | Not given; p < 0.05 insulin sensitisers vs baseline | |
| Portal inflammation | Met: 0.5; Rosi: 1; Pbo: –1 | Met: p = NS; Rosi: p = NS; Pbo: p = NS | Not given | |
| Fibrosis | Met: 0; Rosi: 1; Pbo: 0 | Met: p = NS; Rosi: p = NS; Pbo: p = NS | ||
| NAS | Met: –1; Rosi: –2; Pbo: 1 | Met: p = NS, Rosi: p = NS; Pbo: p = NS | NR; p < 0.05 insulin sensitisers vs baseline | |
| Brunt’s grade | Met: –0.5; Rosi: –1; Pbo: 0 | Met: p = NS; Rosi: p = NS; Pbo: p = NS | Not given | |
| Omer 2010113 (Kleiner et al.116) | NAS | Not given | Met: p = 0.726; Rosi: p = 0.012; Met + Rosi: p = 0.026 | Not given |
| Study | HbAlc levels (%) | p-value (from baseline to end) | p-value between groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End of study | Change from baseline/end minus baseline | |||||||
| Pioglitazone | |||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | ||||||
| Aithal 2008101 | 5.8, SD 0.6 | 5.9, SD 1.0 | 5.6, SD 0.4 | 6.0, SD 1.2 | Pio: –0.2; Pbo: +0.1 | Pio: p = 0.06; Pbo: p = 0.04 | p = 0.01 | ||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | ||||||
| Belfort 2006102 | 6.2, SD 1.5 | 6.2, SD 1.1 | 5.5, SD 0.8 | 6.1, SD 0.9 | Pio: –0.7; Pbo: –0.1 | Pio: p < 0.001; Pbo: p = 0.73 | p = 0.008 | ||
| Metformin | |||||||||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | ||||||
| Haukeland 2009106 | 5.7, SD 0.6 | 5.8, SD 0.4 | 5.5, SD 0.4 | 5.9, SD 0.6 | Met: –0.23, SD 0.36; Pbo: 0.1 | Met: p = 0.020; Pbo: p = 0.017 | p = 0.001 | ||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | ||||||
| Nar 2009108 | 6.9, SE 1.4 | 6.1, SE 1.1 | 5.7, SD 1.1 | 5.5, SD 0.3 | Met: –1.2; Pbo: –0.6 | Met: p = 0.001; Pbo: p = 0.010 | p = NS | ||
| Rosiglitazone | |||||||||
| Rosi (32) | Pbo (31) | Rosi (32) | Pbo (31) | ||||||
| Ratziu 2008111 | 5.6, IQR 1.03 | 5.6, IQR 0.9 | Not given | Not given | Rosi: –0.18, SD 0.98; Pbo: 0.17, SD 0.53 | Not given | p = 0.078 | ||
| Metformin vs rosiglitazone | |||||||||
| Met (22) | Rosi (20) | Met + Rosi (22) | Met(22) | Rosi (20) | Met + Rosi (22) | ||||
| Omer 2010113 | 5.8, SD 1.3 | 6.0, SD 0.7 | 6.9, SD 2.1 | 5.8, SD 0.7 | 5.8, SD 0.6 | 6.9, SD 1.9 | Met: 0; Rosi: –0.2; Met + Rosi: 0 | Met: NS; Rosi: p = NS; Met + Rosi: p = NS | p = NS |
| Study | FPG (mmol/l) | p-value (from baseline to end) | p-value between groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End of study | Change from baseline/end minus baseline | |||||||
| Pioglitazone | |||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | ||||||
| Aithal 2008101 | 5.7, SD 1.6 | 5.6, SD 0.9 | 5.3, SD 0.6 | 6.0, SD 1.3 | Pio: –0.4; Pbo: +0.4 | Pio: p = 0.78; Pbo: p = 0.002 | p = 0.02 | ||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | ||||||
| Belfort 2006102 | 6.61, SD 1.94 | 6.38, SD 1.55 | 5.5, SD 0.88 | 6.44, SD 1.72 | Pio: –1.11; Pbo: +0.06 | Pio: p = 0.004; Pbo: p = 0.75 | p = 0.011 | ||
| Pio (80) | C (84/83) | Pio (80) | C (84/83)) | ||||||
| Sanyal 2010104 | 5.11 |
Vit E: 5.28 Pbo: 5.28 |
Not given | Not given | Pio: –0.17; Vit E: 0.10; Pbo: 0.10 | Not given | Pio vs Pbo: p = 0.006; Vit E vs Pbo: p = 0.81 | ||
| Metformin | |||||||||
| Met (55) | C (28/27) | Met (55) | C (28/27) | ||||||
| Bugianesi 200587 |
BU: 5.61, SD 2.33 TU: 5.89, SD 1.94 |
Vit E: 5.17, SD 0.67 Diet: 5.56, SD 2.22 |
Not given | Not given | Met: –0.56; C: –0.28 | Not given | p = 0.125 | ||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | ||||||
| Haukeland 2009106 | 5.5, SD 0.8 | 5.7, SD 1.0 | 5.2, SD 0.6 | 5.8, SD 0.9 | Met: –0.34, SD 0.70; Pbo: +0.1 | Met: p = 0.051; Pbo: p = 0.30 | p = 0.032 | ||
| Met (37) | Pbo (13) | Met (37) | Pbo (13) | ||||||
| Nadeau 2009107 | 5.06, SE 0.12 | 4.93, SE 0.14 | 4.91, SE 0.09 | 5.12, SE 0.21 | Met: –0.15; Pbo: +0.19 | Not given | p = NS | ||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | ||||||
| Nar 2009108 | 9.58, SD 3.35 | 8.02, SD 1.54 | 6.39, SD 1.59 | 6.22, SD 0.42 | Met: –3.19; Pbo: –1.8 | Met: p = 0.001; Pbo: p = 0.002 | Not given | ||
| Met (17) | Pbo (17) | Met (17) | Pbo (17) | ||||||
| Uygun 2004110 | 4.83, SD 0.79 | 5.36, SD 1.07 | 4.48, SD 0.37 | 5.15, SD 0.87 | Met: –0.35, SD 0.62 (7.2%); Pbo: –0.21, SD 0.39 (3.8%) |
Met: p = 0.033 Pbo: p = 0.04 |
p = 0.38 | ||
| Rosiglitazone | |||||||||
| Rosi (32) | Pbo (31) | Rosi (32) | Pbo (31) | ||||||
| Ratziu 2008111 | 5.3, IQR 1.9 | 5.4, IQR 2.2 | Not given | Not given | Rosi: –0.93, SD 1.72; Pbo: 0.55, SD 1.74 | Not given | p = 0.001 | ||
| Metformin vs rosiglitazone | |||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | ||||
| Idilman 2008112 | 5.86, SD 1.09 | 5.83, SD 1.06 | 5.53, SD 0.86 | 5.26, SD 0.72 | 5.13, SD 0.77 | 5.68, SD 3.07 | Met: –0.26; Rosi: –0.7; Pbo: +0.15 | Met: p = 0.005; Rosi: p < 0.001; Pbo: p > 0.05 | Not given |
| Met (22) | Rosi (20) | Met + Rosi (22) | Met (22) | Rosi (20) | Met + Rosi (22) | ||||
| Omer 2010113 | 6.56, SD 1.29 | 6.46, SD 1.49 | 7.81, SD 2.71 | 6.61, SD 1.07 | 5.68, SD 1.13 | 7.39, SD 3.23 | Met: +0.05; Rosi: –0.78; Met + Rosi: –0.42 | Met: p = NS; Rosi: p = 0.029; Met + Rosi: p = NS | Not given |
| Study | Baseline | End of study | Change from baseline/end minus baseline | p-value (from baseline to end) | p-value between groups | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | |||||||||
| Weight (kg) | |||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | ||||||
| Aithal 2008101 | 88.6, SD 10.7 | 92.8, SD 21.1 | 91.2, SD 12.6 | 89.3, SD 18.6 | Pio: 2.6; Pbo: –3.5 | Pio: p = 0.005; Pbo: p = 0.69 | p = 0.02 | ||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | ||||||
| Belfort 2006102 | 93.7, SD 18.1 | 90.2, SD 15.4 | 96.2, SD 19.6 | 89.7, SD 14.8 | Pio: 2.5; Pbo: –0.5 | Pio: p < 0.001; Pbo: p = 0.53 | p = 0.003 | ||
| Pio (80) | C (84/83) | Pio (80) | C (84/83) | ||||||
| Sanyal 2010104 | 97 |
Vit E: 94 Pbo: 99 |
Only baseline values reported | Pio: 4.7; Vit E: 0.4; Pbo: 0.7 | Not given |
Pio vs Pbo: p < 0.0001 Vit E vs Pbo: p = 0.65 |
|||
| Metformin | |||||||||
| Weight (kg) | |||||||||
| Met (20) | C (25) | Met (20) | C (25) | ||||||
| Garinis 2010105 | 85.5, SD 13.4 | 83.7, SD 10.5 | 78.8, SD 15 | 78.5, SD 10.9 | Met: –6.70; C: –5.20, |
Met: p = 0.0001; C: p = 0.0001 |
Not given | ||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | ||||||
| Haukeland 2009106 | 97.1, SD 17.6 | 91.5, SD 12.1 | 92.8, SD 17.3 | 91.8, SD 13.0 | Met: –4.3, SD 4.3; Pbo: +0.3, SD 2.2 | Met: p < 0.001; Pbo: p = 0.45S | p < 0.001 | ||
| Met + Rosi (16) | Rosi (15) | Met + Rosi (16) | Rosi (15) | ||||||
| Torres 2009100 | Not given | Not given | Not given | Not given | Met + Rosi: –3.3%; Rosi: –1.3% | Not given | Not given | ||
| Rosiglitazone | |||||||||
| Weight (kg) | |||||||||
| Rosi (32) | Pbo (31) | Rosi (31) | Pbo (31) | ||||||
| Ratziu 2008111 | Not given | Not given | Not given | Not given | Rosi: +1.5, SD 5.2; Pbo: –1, SD 0.5 | Not given | p = 0.03 | ||
| Pioglitazone | |||||||||
| BMI (kg/m2) | |||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | ||||||
| Aithal 2008101 | 29.8, SD 3.0 | 30.8, SD 4.1 | 30.5, SD 3.9 | 30.8, SD 4.5 | Pio: 0.7; Pbo: 0 | Pio: p = 0.31; Pbo: p = 0.90 | p = 0.45 | ||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | ||||||
| Belfort 2006102 | 33.5, SD 4.9 | 32.9, SD 4.4 | 34.6, SD 5.7 | 32.7, SD 4.5 | Pio: 1.1; Pbo: –0.2 | Pio: p < 0.001; Pbo: p = 0.62 | p = 0.005 | ||
| Pio (10) | Pbo (10) | Pio (10) | Pbo (10) | ||||||
| Sanyal 2004103 | 32.5, SD 4.3 | 30.7, SD 4.7 | 32.5, SD 7 | 31.2, SD 3.6 | Pio: 0; Pbo: 0.5 | Not given | p = NS | ||
| Pio (80) | C (84/83) | Pio (80) | C (84/83) | ||||||
| Sanyal 2010104 | 34 |
Vit E: 34 Pbo: 35 |
Not given | Not given | Pio: 1.8; Vit E: 0.1; Pbo: 0.4 | Not given |
Pio vs Pbo: p = 0.0001 Vit E vs Pbo: p = 0.50 |
||
| Waist–hip ratio | |||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | ||||||
| Aithal 2008101 | 0.95, SD 0.06 | 0.97, SD 0.13 | 0.95, SD 0.07 | 0.96, SD 0.07 | Pio: 0; Pbo: –0.01 | Pio: p = 0.84; Pbo: p = 0.12 | p = 0.23 | ||
| Pio (10) | Pbo (10) | Pio (10) | Pbo (10) | ||||||
| Sanyal 2004103 | 0.92, SD 0.08 | 0.94, SD 0.08 | 0.94, SD 0.1 | 0.94, SD 0.08 | Pio: 0.02; Pbo: 0 | Not given | p = NS | ||
| Fat (%) | |||||||||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | ||||||
| Belfort 2006102 | 33.7, SD 5.6 | 35.7, SD 8.8 | 35.2, SD 6.1 | 34.9, SD 8.8 | Pio: 1.5; Pbo: –0.8 | Not given | p = 0.005 | ||
| Pio (10) | Pbo (10) | Pio (10) | Pbo (10) | ||||||
| Sanyal 2004103 | 36.5, SD 9 | 34.7, SD 9 | 33.6, SD 14 | 30.3, SD 7 | Pio: –2.9; Pbo: –4.4 | Not given | p = NS | ||
| Pio (80) | C (84/83) | Pio (80) | C (84/83) | ||||||
| Sanyal 2010104 | 40 |
Vit E: 39 C: 40 |
Not given | Not given | Pio: 2.7; Vit E: 0.4; Pbo: 0.0 | Not given |
Pio vs Pbo: p < 0.0001 Vit E vs Pbo: p = 0.50 |
||
| Metformin | |||||||||
| BMI (kg/m2) | |||||||||
| Bugianesi 200587 | Met (55) | C (28/27) | Met (55) | C (28/27) | |||||
| 28.7, SD 3.5 | C (Vit E/Pbo): 28.8, SD 3.2 | 27.4, SD 3.4 | C (Vit E/Pbo): 27.8, SD 2.9 | Met: –1.3; C: –1 | Met: p < 0.0001; C: p = 0.0003 | Met vs C: p = 0.113 | |||
| Met (20) | C (25) | Met (20) | C (25) | ||||||
| Garinis 2010105 | 36.5, SD 4.9 | 34.7, SD 3.5 | 33.3, SD 5.4 | 32.9, SD 3.6 | Met: –3.20; C: –1.80 |
Met: p = 0.0001; C: p = 0.0001 |
Not given | ||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | ||||||
| Haukeland 2009106 | 31.4, SD 3.9 | 30.3, SD 3.3 | 30.1, SD 4.2 | 30.4, SD 3.4 | Met: –1.3; Pbo: 0.1 |
Met: p < 0.001 Pbo: p = 0.59 |
p < 0.001 | ||
| Met (37) | Pbo (13) | Met (37) | Pbo (13) | ||||||
| Nadeau 2009107 | 39.6, SE 0.98 | 40.2, SE 1.8 | 39.2, SE 1.3 | 41.7, SE 3.1 | Met: –0.4; Pbo: +1.5 | Not given | p = NS | ||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | ||||||
| Nar 2009108 | 31.0, SD 4.0 | 33.7, SD 6.0 | 28.8, SD 3.85 | 31.2, SD 5.75 | Met: –2.2; Pbo: –2.5 |
Met: p = 0.001 Pbo: p = 0.001 |
p = NS | ||
| Met (9) | Pbo (10) | Met (9) | Pbo (10) | ||||||
| Shields 2009109 | 32.2, SD 4.9 | 32.8, SD 4.9 | Not given | Not given | Met: –0.9; Pbo: –1.7 | Not given | p = 0.514 | ||
| Met (17) | Pbo (17) | Met (17) | Pbo (17) | ||||||
| Uygun 2004110 | 30.1, SD 3.4 | 28.4, SD 3.9 | 27.7, SD 2.5 | 26.5, SD 3.7 | Met: –2.4, SD 1.9 (7.9%); Pbo: –1.9, SD 2.1 (6.7%); | Met: p = 0.001; Pbo: p = 0.01 | p = 0.01 | ||
| Waist–hip ratio | |||||||||
| Met (20) | C (25) | Met (20) | C (25) | ||||||
| Garinis 2010105 | 0.86, SD 0.05 | 0.88, SD 0.05 | 0.82, SD 0.02 | 0.85, SD 0.07 | Met: –0.04; C: –0.0 | Met: p = 0.33; C: p = 0.11 | Not given | ||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | ||||||
| Nar 2009108 | 0.90, SD 0.07 | 0.89, SD 0.09 | 0.88, SD 0.07 | 0.84, SD 0.07 | Met: –0.02; Pbo: –0.05 |
Met: p = 0.035 Pbo: p = 0.019 |
p = NS | ||
| Waist circumference (cm) | |||||||||
| Met (20) | C (25) | Met (20) | C (25) | ||||||
| Garinis 2010105 | 108.4, SD 12.5 | 102.8, SD 8.8 | 101.4, SD 12.7 | 95.6, SD 8.5 | Met: –7.00; C: –7.20 | Met: p = 0.0009; C: p = 0.0001 | Not given | ||
| Metformin vs rosiglitazone | |||||||||
| BMI (kg/m2) | |||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | ||||
| Idilman 2008112 | 30.8, SD 3.9 | 31.5, SD 3.4 | 32.2, SD 5.1 | 29.0, SD 3.5 | 30.9, SD 3.2 | 31.5, SD 5.3 | Met: –1.8; Rosi: –0.6; Pbo: –0.7 | Met: p < 0.001; Rosi: p = 0.09; Pbo: p = 0.002; no significant change in either group during the 6-month follow-up | Not given |
| Met (22) | Rosi (20) | Met + Rosi (22) | Met (22) | Rosi (20) | Met + Rosi (22) | ||||
| Omer 2010113 | 30.8, SD 6.6 | 28.4, SD 4.1 | 32.5, SD 7.0 | 27.6, SD 2.8 | 28.1, SD 3.8 | 31.2, SD 6.7 | Met: –3.2; Rosi: –0.3; Met + Rosi: –1.3 |
Met: p = 0.002 Rosi: p = NS Met + Rosi: p = 0.006 |
Not given |
| Waist circumference (cm) | |||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | ||||
| Idilman 2008112 | 101.9, SD 9.7 | 102.7, SD 7.3 | 104.3, SD 8.5 | 95.3, SD 8.9 | 100.2, SD 7.7 | 101.9, SD 9.2 | Met: –6.6; Rosi: –2.5; Pbo: –2.4 | Met: p < 0.001; Rosi: p = 0.061; Pbo: p = 0.023; no significant change in either group during the 6-month follow-up | Not given |
| Met (22) | Rosi (20) | Met + Rosi (22) | Met (22) | Rosi (20) | Met + Rosi (22) | ||||
| Omer 2010113 | 99.6, SD 11.6 | 97.9, SD 9.0 | 106.4, SD 12.1 | 95.4, SD 6.8 | 96.0, SD 8.7 | 103.5, SD 11.8 | Met: –4.2; Rosi: –1.9; Met + Rosi: –2.9 |
Met: p = 0.022 Rosi: p = NS Met + Rosi: p = 0.031 |
Not given |
| Hip circumference (cm) | |||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | ||||
| Idilman 2008112 | 100.9, SD 5.9 | 103.1, SD 6.8 | 105.1, SD 10.2 | 99.3, SD 7.4 | 102.1, SD 6.2 | 104.3, SD 10.1 | Met: –1.6; Rosi: –1; Pbo: –0.8 (end minus baseline) | Met: p = 0.040; Rosi: p = 0.067; Pbo: p = 0.003; no significant change in either group during a 6-month follow-up | Not given |
| Body fat content (%) | |||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | ||||
| Idilman 2008112 | 31.4, SD 6.2 | 34.0, SD 6.6 | 33.4, SD 7.5 | 29.5, SD 6.9 | 33.1, SD 6.7 | 31.4, SD 8.3 | Met: –1.9; Rosi: –0.9; Pbo: –2 | Met: p = 0.017; Rosi: p = 0.205; Pbo: p = 0.019; no significant change in either group during a 6-month follow-up | Not given |
| Study | Outcome | Baseline | End of study | Change from baseline/end minus baseline | p-value (from baseline to end) | p-value between groups | ||
|---|---|---|---|---|---|---|---|---|
| Pioglitazone | ||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | |||||
| Aithal 2008101 | SBP (mmHg) | 144.6, SD 17.6 | 141, SD 18.7 | 139.4, SD 15.8 | 139, SD 20.4 | Pio: –5.2; Pbo: –2 | Pio: p = 0.32; Pbo: p = 0.74 | p = 0.70 |
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | |||||
| Aithal 2008101 | DBP (mmHg) | 93.3, SD 9.0 | 89.1, SD 10.4 | 85.3, SD 9.3 | 78.7, SD 11.1 | Pio: –8; Pbo: –10.4 | Significant reduction in both groups from baseline Pio: p = 0.008; Pbo: p < 0.001 | p = 0.36 |
| Study | Outcome | Baseline | End of study | Change from baseline/end minus baseline | p-value (from baseline to end) | p-value between groups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | ||||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | |||||||
| Aithal 2008101 | TC (mmol/l) | 5.6, SD 1.2 | 5.6, SD 1.3 | 5.5, SD 1.4 | 5.4, SD 1.3 | Pio: –0.1; Pbo: –0.2 | Pio: p = 0.26; Pbo: p = 0.17 | p = 0.88 | ||
| LDL cholesterol (mmol/l) | 3.3, SD 1.0 | 3.4, SD 1.1 | 3.2, SD 1.2 | 3.2, SD 1.1 | Pio: –0.1; Pbo: –0.2 | Pio: p = 0.04; Pbo: p = 0.44 | p = 0.17 | |||
| HDL cholesterol (mmol/l) | 1.3, SD 0.3 | 1.3, SD 0.3 | 1.3, SD 0.3 | 1.3, SD 0.4 | Pio: 0; Pbo: 0 | Pio: p = 0.77; Pbo: p = 0.93 | p = 0.99 | |||
| TGs (mmol/l) | 2.4, SD 2.1 | 2.1, SD 1.2 | 2.2, SD 1.2 | 1.8, SD 0.9 | Pio: –0.2; Pbo: –0.3 | Pio: p = 0.79; Pbo: p = 0.19 | p = 0.35 | |||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | |||||||
| Belfort 2006102 | TC (mmol/l) | 4.86, SD 0.85 | 4.89, SD 1.06 | 4.99, SD 0.93 | 4.94, SD 1.06 | Pio: 0.13; Pbo: 0.05 | Pio: p = 0.48; Pbo: p = 0.62 | p = 0.79 | ||
| LDL cholesterol (mmol/l) | 3.05, SD 0.80 | 3.03, SD 0.96 | 3.10, SD 0.91 | 2.97, SD 0.93 | Pio: 0.05; Pbo: –0.06 | Pio: p = 0.68; Pbo: p = 0.65 | p = 0.58 | |||
| HDL cholesterol (mmol/l) | 1.03, SD 0.23 | 0.96, SD 0.23 | 1.11, SD 0.23 | 1.01, SD 0.23 | Pio: 0.08; Pbo: 0.05 | Pio: p = 0.004; Pbo: p = 0.22 | p = 0.31 | |||
| TGs (mmol/l) | 1.76, SD 0.98 | 1.95, SD 1.60 | 1.49, SD 0.95 | 2.34, SD 1.78 | Pio: –0.27; Pbo: 0.39 | Pio: p = 0.17; Pbo: p = 0.001 | p = 0.003 | |||
| Pio (80) | C (84/83) | Pio (80) | C (84/83) | |||||||
| Sanyal 2010104 | TC (mmol/l) | 5.04 |
Vit E: 5.04 Pbo: 5.15 |
Not given | Not given | Pio: –0.29; Vit E: –0.35; Pbo: –0.25 | Not given |
Pio vs Pbo: p = 0.50 Vit E vs Pbo: p = 0.25 |
||
| LDL cholesterol (mmol/l) | 3.10 |
Vit E: 3.08 Pbo: 3.23 |
Not given | Not given | Pio: –0.21; Vit E: –0.31; Pbo: –0.15 | Not given |
Pio vs Pbo: p = 0.26 Vit E vs Pbo: p = 0.07 |
|||
| HDL cholesterol (mmol/l) | 1.16 |
Vit E: 1.14 Pbo: 1.11 |
Not given | Not given | Pio: 0.03; Vit E: –0.02; Pbo: –0.05 | Not given |
Pio vs Pbo: p = 0.008 Vit E vs Pbo: p = 0.51 |
|||
| TGs (mmol/l) | 1.83 |
Vit E: 1.87 Pbo: 1.86 |
Not given | Not given | Pio: –0.22; Vit E: –0.01; Pbo: –0.08 | Not given |
Pio vs Pbo: p = 0.16 Vit E vs Pbo: p = 0.45 |
|||
| Metformin | ||||||||||
| Met (55) | C (28/27) | Met (55) | C (28/27) | |||||||
| Bugianesi 200587 | HDL cholesterol (mmol/l) |
BU: 1.14, SD 0.28; TU: 1.24, SD 0.28 |
Vit E: 1.09, SD 0.31; Diet: 1.14, SD 0.21 | Not given | Not given | p = NS | ||||
| TGs (mmol/l) |
BU: 2.00, SD 1.06; TU: 1.61, SD 0.98 |
Vit E: 2.62, SD 1.40; Diet: 1.61, SD 0.78 | Not given | Not given | p = NS | |||||
| Met (20) | C (25) | Met (20) | C (25) | |||||||
| Garinis 2010105 | TC (mmol/l) | 5.03, SD 1.0 | 5.45, SD 0.88 | 5.06, SD 1.01 | 5.22, SD 0.95 | Met: 0.03, C: –0.23, | Met: p = 0.35; C: p = 0.21 | Not given | ||
| LDL cholesterol (mmol/l) | 3.34, SD 0.87 | 3.61, SD 0.90 | 3.30, SD 0.75 | 3.43, SD 0.99 | Met: –0.04; C: –0.18 | Met: p = 0.70; C: p = 0.81 | Not given | |||
| HDL cholesterol (mmol/l) | 1.35, SD 0.39 | 1.51, SD 0.35 | 1.50, SD 0.38 | 1.46, SD 0.36 | Met: 0.15; C: –0.05, | Met: p = 0.63; C: p = 0.95 | Not given | |||
| TGs (mmol/l) | 1.09, SD 0.4 | 1.28, SD 0.87 | 1.06, SD 0.48 | 1.15, SD 0.38 | Met: –0.03; C: –0.13, | Met: p = 0.99; C: p = 0.53 | Not given | |||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | |||||||
| Haukeland 2009106 | TC (mmol/l) | 5.9, SD 1.0 | 5.4, SD 1.3 | 5.4, SD 1.3 | 5.4, SD 1.2 | Met: –0.57, SD 0.86; Pbo: –0.02, SD 0.47 | Met:p = 0.008; Pbo: p = 0.64 | p = 0.004 | ||
| LDL cholesterol (mmol/l) | 3.9, SD 0.9 | 3.3, SD 0.9 | 3.2, SD 1.1 | 3.2, SD 1.0 | Met: –0.73, SD 0.82; Pbo: –0.07, SD 0.34 | Met: p = 0.001; Pbo: p = 0.34 | p < 0.001 | |||
| HDL cholesterol (mmol/l) | 1.25, SD 0.35 | 1.23, SD 0.26 | 1.24, SD 0.32 | 1.21, SD 0.26 | Met: –0.01; Pbo: –0.02 | Met: 0.92; Pbo: p = 0.43 | p = 0.80 | |||
| TGs (mmol/l) | 1.8, SD 0.8 | 2.0, SD 1.5 | 2.2, SD 1.0 | 2.4, SD 2.4 | Met: 0.4; Pbo: 0.4 | Met: p = 0.007; Pbo: p = 0.42 | p = 0.27 | |||
| Met (37) | Pbo (13) | Met (37) | Pbo (13) | |||||||
| Nadeau 2009107 | TC (mmol/l) | 4.39, SE 0.13 | 4.17, SE 0.23 | 4.19, SE 0.12 | 4.16, SE 0.29 | Met: –0.2; Pbo: –0.01 | Not given | p = NS | ||
| LDL cholesterol (mmol/l) | 2.58, SE 0.11 | 2.41, SE 0.21 | 2.57, SE 0.14 | 2.45, SE 0.23 | Met: –0.01; Pbo: 0.04 | Not given | p = NS | |||
| HDL cholesterol (mmol/l) | 1.02, SE 0.04 | 1.12, SE 0.04 | 1.02, SE 0.04 | 1.07, SE 0.07 | Met: 0; Pbo: –0.05 | Not given | p = NS | |||
| TGs (mmol/l) | 1.75, SE 0.11 | 1.45, SE 0.31 | 1.45, SE 0.08 | 1.42, SE 0.33 | Met: –0.3; Pbo: –0.03 | Met: p = 0.03; Pbo: Not given | p = NS | |||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | |||||||
| Nar 2009108 | TC (mmol/l) | 5.68, SD 0.95 | 4.88, SD 1.01 | 5.18, SD 0.61 | 4.67, SD 0.31 | Met: –0.5; Pbo: –0.21 | Met: p = 0.085; Pbo: p = 0.412 | p = NS | ||
| LDL cholesterol (mmol/l) | 3.55, SD 0.77 | 2.90, SD 0.74 | 2.95, SD 0.59 | 2.76, SD 0.26 | Met: –0.6; Pbo: –0.14 | Met: p = 0.002; Pbo: p = 0.470 | Unclear | |||
| HDL cholesterol (mmol/l) | 1.17, SD 0.19 | 1.16, SD 0.20 | 1.26, SD 0.22 | 1.15, SD 0.06 | Met: 0.09; Pbo: –0.01 | Met: p = 0.035; Pbo: p = 0.257 | Unclear | |||
| TGs (mmol/l) | 2.05, SD 0.91 | 1.94, SD 0.93 | 2.03, SD 1.39 | 1.69, SD 1.15 | Met: –0.02; Pbo: –0.25 | Met: p = 0.851; Pbo: p = 0.053 | p = NS | |||
| Met (17) | Pbo (17) | Met (17) | Pbo (17) | |||||||
| Uygun 2004110 | TC (mmol/l) | 4.89, SD 1.01 | 5.12, SD 1.03 | 4.42, SD 1.29 | 4.48, SD 0.82 | Met: 0.48, SD 0.97 (9.7%); Pbo: 0.63, SD 1.06 (12.3%) | Met: p = 0.05; Pbo: p = 0.01 | p = 0.9 | ||
| TGs (mmol/l) | 2.01, SD 0.80 | 2.29, SD 0.77 | 1.94, SD 0.74 | 2.09, SD 0.58 | Met: 0.07, SD 0.55 (3.5%); Pbo: 0.20, SD 0.44 (8.7%) | Met: p = NS; Pbo: p = NS | p = 0.8 | |||
| Rosiglitazone | ||||||||||
| Rosi (32) | Pbo (31) | Rosi (32) | Pbo (31) | |||||||
| Ratziu 2008111 | TC (mmol/l) | Not given | Not given | Not given | Not given | Rosi: 0.45, SD 0.67; Pbo: –0.24, SD 0.75 | Not given | p = 0.0013 | ||
| LDL cholesterol (mmol/l) | Not given | Not given | Not given | Not given | Rosi: 0.41, SD 0.79; Pbo: –0.32, SD 0.87 | Not given | p = 0.0014 | |||
| HDL cholesterol (mmol/l), median (IQR) | 1.22, IQR 0.42 | 1.27, IQR 0.49 | Not given | Not given | Rosi: 0.05, SD 0.27; Pbo: 0.01, SD 0.27 | Not given | p = 0.72 | |||
| TGs (mmol/l), median (IQR) | 1.34, IQR 0.9 | 1.65, IQR 1.21 | Not given | Not given | Rosi: 0.21, SD 1; Pbo: 0.2, SD 1.26 | Not given | p = 0.94 | |||
| Metformin vs rosiglitazone | ||||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | |||||
| Idilman 2008112 | Cholesterol (mmol/l) | 5.93, SD 1.35 | 5.25, SD 1.30 | 5.57, SD 0.80 | 5.69, SD 1.43 | 4.89, SD 1.09 | 5.05, SD 1.10 | Met: –0.24; Rosi: –0.16; Pbo: –0.52 | Met: p = 0.287; Rosi: p = 0.171; Pbo: p = 0.001 | p = NS |
| TGs (mmol/l) | 2.66, SD 1.21 | 1.91, SD 0.83 | 1.87, SD 0.72 | 2.24, SD 1.06 | 1.63, SD 0.76 | 1.71, SD 0.74 | Met: –0.42; Rosi: –0.28; Pbo: –0.16 | Met: p = 0.082; Rosi: p = 0.120; Pbo: p = NS | Not given | |
| Study | Outcome | Baseline | End of study | Change from baseline/end minus baseline | p-value (from baseline to end) | p-value between groups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | ||||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | |||||||
| Aithal 2008101 | ALT (U/l) | 93.6, SD 61.3 | 84.1, SD 37.7 | 55.9, SD 25.7 | 77.2, SD 43.0 | Pio: –37.7; Pbo: –6.9 | Pio: p < 0.001; Pbo: p = 0.02 | p = 0.009 | ||
| Pio (26) | Pbo (21) | Pio (26) | Pbo (21) | |||||||
| Belfort 2006102 | ALT (U/l) | 67, SD 26 | 61, SD 33 | 28, SD 12 | 40, SD 17 | Pio: –39; Pbo: –21 | Pio: p < 0.001; Pbo: p = 0.033 | p < 0.001 | ||
| Pio (10) | Pbo (10) | Pio (10) | Pbo (10) | |||||||
| Sanyal 2004103 | ALT (U/l) | 121, SD 76 | 111, SD 45 | 56, SD 67 | 36, SD 15 | Pio: –65; Pbo: –75 | Not given | p = NS | ||
| Pio (80) | C (84/83) | Pio (80) | C (84/83) | |||||||
| Sanyal 2010104 | ALT (U/l) | 82 |
Vit E: 86 Pbo: 81 |
Not given | Not given | Pio: –40.8; Vit E: –37.0; Pbo: –20.1 | Not given |
Pio vs Pbo: p < 0.0001 Vit E vs Pbo: p = 0.001 |
||
| Metformin | ||||||||||
| Met (55) | C (28/27) | Met (55) | C (28/27) | |||||||
| Bugianesi 200587 | ALT (U/l) |
BU: 99, SD 52 TU: 91, SD 52 |
Vit E: 83, SD 29 Diet: 90, SD 47 |
45.5, SE 5 (estimated from graph) |
Vit E: 70.3, SE 8 Diet: 59.5, SE 10 (estimated from graph) |
Vit E: –12.7; Diet: –30.5 | Not given | p < 0.0001 | ||
| ALT within normal range (< 40 U/l) | n = 31 | n = 12 | p = 0.0006 | |||||||
| Met (20) | C (25) | Met (20) | C (25) | |||||||
| Garinis 2010105 | ALT (U/l) | 26.1, SD 12.1 | 23.0, SD 10.1 | 24.0, SD 17.5 | 18.9, SD 5.9 | Met: –2.10, C: –4.10 | Met: p = 0.75; C: p = 0.29 | Not given | ||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | |||||||
| Haukeland 2009106 | ALT (U/l) | Not given | Not given | Not given | Not given | Met: median reduction –15 U/l; Pbo: median reduction –22 U/l | Met: p = 0.025; Pbo: p = 0.025 | Not given | ||
| Met (37) | Pbo (13) | Met (37) | Pbo (13) | |||||||
| Nadeau 2009107 | ALT (IU/l) | 61.5, SE 4 | 52.9, SE 8.2 | 46, SE 3 | 36.8, SE 2.8 | Met: –15.5; Pbo: –16.1 | Met: p < 0.006; Pbo: Not given | p = NS | ||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | |||||||
| Nar 2009108 | ALT (U/l) | 37.4, SD 17.7 | 32.3, SD 11.0 | 21.4, SD 8.9 | 25.5, SD 13.4 | Met: –16; Pbo: –6.8 | Met: p = 0.015; Pbo: p = 0.047 | p = NS | ||
| Met (9) | Pbo (10) | Met (9) | Pbo (10) | |||||||
| Shields 2009109 | ALT (IU/l) | 79.4, SD 26.1 | 97.5, SD 45.6 | Not given | Not given | Met: –21.5; Pbo: –40.7 | Overall change: p = 0.014 | p = NS | ||
| Met + Rosi (16) | Rosi (15) | Met + Rosi (16) | Rosi (15) | |||||||
| Torres 2009100 | ALT(U/l) | Not given | Not given | Not given | Not given | Met + Rosi: –46.5%; Rosi: –46.3% | Not given | Not given | ||
| Met (17) | Pbo (17) | Met (17) | Pbo (17) | |||||||
| Uygun 2004110 | ALT (U/l) | 83.5, SD 24.6 | 72.8, SD 31.2 | 46.4, SD 23.3 | 55.4, SD 16.3 | Met: –37.1, SD 22.2 (44%); Pbo: –17.4, SD 14.1 (24%); | Met: p = 0.0001; Pbo: p = 0.001 | p = 0.003 | ||
| Rosiglitazone | ||||||||||
| Rosi (32) | Pbo (31) | Rosi (32) | Pbo (31) | |||||||
| Ratziu 2008111 | ALT (U/l) | 69, SD 40 | 84, SD 38 | Not given | Not given | Normal ALT level: Rosi: 12 (38%); Pbo: 2 (7%), | Not given | p = 0.005 | ||
| Metformin vs rosiglitazone | ||||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | |||||
| Idilman 2008112 | ALT(U/l) | 82.9, SD 52.9 | 73.6, SD 39.3 | 66.9, SD 28.9 | 50.0, SD 37.1 | 44.6, SD 32.7 | 42.0, SD 16.2 | Met: –32.9; Rosi: –29; Pbo: −24.9 | Met: p = 0.017; Rosi: p = 0.001; Pbo: p < 0.001; no significant change in either group during a 6-month follow-up | p = NS for insulin sensitisers vs control |
| Met (22) | Rosi (20) | Met + Rosi (22) | Met (22) | Rosi (13) | Met + Rosi (12) | |||||
| Omer 2010113 | ALT(U/l) | 63.1, SD 24.2 | 64.9, SD 23.5 | 72.6, SD 31.9 | 46.4, SD 28.5 | 28.7, SD 18.2 | 49.9, SD 28.1 | Met: –16.7; Rosi: –36.2; Met + Rosi: –22.7 | Met: p = NS; Rosi: p < 0.0001; Met + Rosi: p = 0.017 | Not given |
| Study | Outcome | Baseline | End of study | Change from baseline/end minus baseline | p-value (from baseline to end) | p-value between groups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | ||||||||||
| Pio (31) | Pbo (30) | Pio (31) | Pbo (30) | |||||||
| Aithal 2008101 | HOMA–IR | 4.3, SD 3.4 | 5.2, SD 2.8 | 4.8, SD 4.6 | 5.1, SD 2.9 | Pio: 0.5; Pbo: –0.1 | Pio: p = 0.87; Pbo: p = 0.09 | p = 0.26 | ||
| Pio (80) | C (84/83) | Pio (80) | C (84/83) | |||||||
| Sanyal 2010104 | HOMA–IR | 5.0 |
Vit E: 5.2 Pbo: 5.5 |
Not given | Not given | Pio: –0.7; Vit E: 0.4; Pbo: 0.4 | Not given |
Pio vs Pbo: p = 0.03 Vit E vs Pbo: p = 0.80 |
||
| Metformin | ||||||||||
| Met (55) | C (28/27) | Met (55) | C (28/27) | |||||||
| Bugianesi 2005 87 | HOMA (%) |
BU: 3.5, SD 1.3 TU: 5.0, SD 2.4 |
Vit E: 3.6, SD 1.9 Diet: 3.9, SD 2.8 |
Not given | Not given | Met: –1.5%; C: –0.5% | Not given | p = 0.002 | ||
| Met (20) | C (25) | Met (20) | C (25) | |||||||
| Garinis 2010105 | HOMA–IR | 3.3, SD 1.6 | 3.2, SD 1.6 | 2.4, SD 1.2 | 2.8, SD 1.1 | Met: –0.90; C: –0.40 | Met: p = 0.003; C: p = 0.15 | Not given | ||
| Met (24) | Pbo (24) | Met (24) | Pbo (24) | |||||||
| Haukeland 2009106 | HOMA–IR | 1.88, SD 0.88 | 2.18, SD 1.24 | 2.41, SD 1.96 | 2.81, SD 2.17 | Met: 0.53; Pbo: 0.63 | Met: p = NS; Pbo: p = 0.067 | p = 0.71 | ||
| Met (19) | Pbo (15) | Met (19) | Pbo (15) | |||||||
| Nar 2009108 | HOMA–IR | 3.1, SD 2.0 | 3.2, SD 1.8 | 2.6, SD 1.1 | 2.7, SD 1.1 | Met: –0.5; Pbo: –0.5 | Met: p = 0.038; Pbo: p = 0.035 | p = NS | ||
| Met (9) | Pbo (10) | Met (9) | Pbo (10) | |||||||
| Shields 2009109 | HOMA–IR | 6.14, SD 4.5 | 4.02, SD 3.99 | Not given | Not given | Met: –1.58; Pbo: –1.14 | Overall change: p = 0.002 | p = 0.886 | ||
| Met + Rosi (16) | Rosi (15) | Met + Rosi (16) | Rosi (15) | |||||||
| Torres 2009100 | HOMA–IR | Not given | Not given | Not given | Not given | Met + Rosi: –51.8%; Rosi: –50.2% | Not given | Not given | ||
| Met (17) | Pbo (17) | Met (17) | Pbo (17) | |||||||
| Uygun 2004110 | HOMA (%) | 2.53, SD 0.98 | 1.83, SD 0.74 | 1.38, SD 0.71 | 1.81, SD 0.67 | Met: –1.15; Pbo: –0.02 | Met: p = 0.0001; Pbo: p = 0.18 | p = 0.001 | ||
| Rosi (32) | Pbo (31) | Rosi(32) | Pbo (31) | |||||||
| Ratziu 2008111 | HOMA, median (IQR) | 4.57, IQR 4.66 | 4.21, IQR 4.32 | Not given | Not given | Rosi: –1.4, IQR 2.6; Pbo: 0.61, IQR 5.5 | Not given | p < 0.001 | ||
| Metformin vs rosiglitazone | ||||||||||
| Met (10) | Rosi (11) | Pbo (8) | Met (10) | Rosi (11) | Pbo (8) | |||||
| Idilman 2008112 | HOMA–IR | 4.9, SD 3.9 | 5.7, SD 3.8 | 3.8, SD 3.1 | 2.8, SD 2.9 | 2.8, SD 1.8 | 3.9, SD 2.0 | Met: –2.1; Rosi: –2.9; Pbo: 0.1 | Met: p = 0.002; Rosi: p = 0.001; Pbo: p = NS; No significant change in either group during 6-month follow-up | Not given |
| Met (22) | Rosi (20) | Met + Rosi (22) | Met (22) | Rosi (13) | Met + Rosi (12) | |||||
| Omer 2010113 | HOMA–IR | 4.4, SD 2.5 | 2.9, SD 1.9 | 5.7, SD 4.3 | 3.6, SD 3.8 | 1.8, SD 0.9 | 4.1, SD 3.8 | Met: –0.8; Rosi: –1.1; Met + Rosi: –1.6 | Met: p = NS; Rosi: p = 0.003; Met + Rosi: p = NS | p = NS |
Liver histology
Pioglitazone
All four studies101–104 collected liver biopsy specimens at baseline and at the end of the study. The studies used different grading and staging methods to report the progression or regression of liver histology. Aithal (2008)101 used a NASH histological grading system developed by Brunt et al.,115 Sanyal (2004)103 used modified Brunt scores, whereas Belfort (2006)102 followed criteria proposed by Kleiner et al. 116 to report on histological changes of liver with pioglitazone. Sanyal (2010)104 used a grading system proposed by Brunt et al. 115 and Kleiner et al. 116
Aithal (2008)101 followed up participants for 12 months and at the end of the study found that steatosis, hepatocellular injury, lobular inflammation, Mallory bodies and fibrosis were all improved with pioglitazone 30 mg/day. There was some improvement with placebo, but this was limited to hepatic steatosis, whereas all other parameters worsened during the study period. The reduction in hepatocellular injury, Mallory bodies and fibrosis was statistically significant with pioglitazone compared with placebo (Table 5). Belfort (2006)102 reported that at 6-months’ follow-up, the only improvement seen in the placebo group was a reduction in inflammation, whereas with pioglitazone there were improvements in all parameters and the differences were statistically significant compared with placebo (see Table 5). Sanyal (2004)103 found that there were significant histological changes from baseline to end with both vitamin E and pioglitazone (see Table 5). However, there were no significant improvements in inflammation and fibrosis with vitamin E. The changes in cytological ballooning, Mallory’s hyaline and inflammation were statistically significant with pioglitazone compared with vitamin E (see Table 5). Sanyal (2010)104 found significant changes in most histological parameters with both vitamin E and pioglitazone compared with placebo; however, the difference between vitamin E and pioglitazone was not significant for any of the parameters.
Metformin
Eight studies examined the effect of metformin on liver histology. 87,100,105–110 Five studies87,100,106,109,110 carried out liver biopsy at baseline and at the end of the study to report histological changes of the liver while three105,107,108 used liver ultrasound.
Out of the five87,100,109,110,115 that used liver biopsy, three studies87,109,110 used the scoring system proposed by Brunt et al. 115 and the other106 used criteria proposed by Kleiner et al. 116 One study100 did not mention which grading system was used to assess liver histology.
Bugianesi (2005)87 carried out a second liver biopsy only in participants treated with metformin, but not in the control groups because of ‘ethical issues’. Metformin significantly reduced the percentage of fat in liver and also reduced necroinflammation, fibrosis and the NASH index (see Table 5). Haukeland (2009)106 found a slight reduction in all the parameters of liver histology with both metformin and placebo, but no significant difference between arms (see Table 5). Shields (2009)109 compared the efficacy of metformin and diet and exercise in participants with NASH, and found no statistically significant differences between the groups in regards to NAS, individual components of NAS or fibrosis. However, the authors reported that both the interventions had caused improvements in steatosis and NAS.
Uygun (2004)110 compared metformin against dietary treatment alone and found no significant differences between the groups in terms of NAS and fibrosis. There was slight decrease in the necroinflammatory activity with metformin, whereas no changes were seen with dietary treatment. The frequency of participants achieving improvements was significantly greater with metformin (46% vs 10%) than with diet alone and the difference between the two was reported to be statistically not significant (p = 0.17). In addition to liver biopsy, Uygun (2004)110 had carried out liver ultrasound to explore the changes in liver steatosis with the two treatments and found the changes from baseline to end was significant (p = 0.038) with metformin than with diet alone.
Three trials105,107,108 used only liver ultrasound to report the progression or regression of NAFLD. Garinis (2010)105 reported that the proportions of participants in whom liver steatosis improved or disappeared were not different between the two groups, i.e. one taking metformin plus hypocaloric diet and the second only on dietary treatment (25% vs 24%). In some, the liver steatosis disappeared completely, and this was more common in the metformin group than in the control group (15% vs 4% – p-value not given). Nadeau (2009)107 quantified the severity of fatty liver using a scoring system of 0, 1, 2 and 3, where ‘0’ meant absence of fatty liver and ‘1’, ‘2’ and ‘3’ represented mild, moderate or severe fatty liver, respectively. There was some improvement in fatty liver with metformin (74% in baseline vs 61% at the end; p < 0.04) and in some (three participants) it completely resolved, whereas the participants on placebo had no improvements. The difference between the two groups was significant (p < 0.04). Nar (2009)108 reported that both groups, i.e. metformin with lifestyle interventions versus diet and exercise alone, had significant improvements (p < 0.05) in grades of liver echogenicity at end of the study, but the difference between the two groups was not significant.
In Torres (2009),100 the combination of metformin and rosiglitazone had no greater effect than rosiglitazone alone in terms of steatosis (–23.1% with combination, –24.5% with rosiglitazone) and inflammation (–8.7% with combination, –12.5% with rosiglitazone), but more effect on fibrosis (–45.6% with combination vs –15% with rosiglitazone, p-value not reported).
Rosiglitazone
One trial111 compared rosiglitazone with placebo. The Brunt scoring system115 was used to assess liver histology, and the modified Kleiner et al. 116 criteria for steatosis.
Ratziu (2008)111 found a significantly greater reduction (> 30%) in hepatic steatosis (47% vs 16%; p = 0.014) with rosiglitazone than with placebo. There were some improvements in ballooning, inflammation and fibrosis, but the changes were not significantly different between treatments. The authors found no significant difference in the mean variation of the composite NAS, but the ranked assessment of pretreatment and end-of-treatment liver biopsy specimens showed a significantly greater effect with rosiglitazone than with placebo. The proportion of participants progressing to hepatocyte ballooning, portal inflammation and overall fibrosis was lower with rosiglitazone than with placebo (p < 0.05).
Metformin vs rosiglitazone
Two trials112,113 compared the effects of metformin and rosiglitazone. Idilman (2008)112 assessed liver histology by the Brunt et al. 115 scoring system and Omer (2010)113 by Kleiner et al. 116 Idilman (2008)112 also assessed NAS by using the criteria proposed by Kleiner et al. 116 (see Table 5). Idilman (2008)112 performed a second biopsy at the end of the study in 29 participants with NASH (eight in the diet and exercise group and 21 in the treatment group) and found greater improvements in hepatic steatosis, ballooning and NAS score in the treatment groups than in the diet and exercise group. Omer (2010)113 found that the NAFLD score significantly decreased in the groups that were on combination treatment (p = 0.026) and rosiglitazone (p = 0.012), whereas no significant changes were observed in the metformin group. None of the treatments had a significant effect on fibrosis.
Glycosylated haemoglobin
Out of 15 trials, only six101,102,106,108,111,113 reported changes in HbA1c levels (Table 6). The reduction in HbA1c levels was greater with insulin sensitisers than with placebo. The changes ranged from –0.2% to –0.7% with pioglitazone, –0.23% to –1.2% with metformin, –0.18% to –1.2% with rosiglitazone and from +0.17% to –0.6% with placebo. The reductions with pioglitazone101,102 and metformin106,108 were significantly greater than with placebo. Rosiglitazone111,113 led to a greater reduction in HbA1c levels than metformin in one head-to-head trial. 113
Fasting plasma glucose
Eleven trials87,101,102,104,106–108,110–113 reported FPG changes (Table 7). In most trials, the reduction in FPG levels with insulin sensitisers was significantly greater than with placebo. In the placebo group, FPG levels increased in most trials. The changes ranged from –0.17 to –1.11 mmol/l with pioglitazone, from +0.05 to –3.19 mmol/l with metformin and from –0.78 to –0.93 mmol/l with rosiglitazone. Omer (2010)113 found a greater reduction in FPG with rosiglitazone (–0.78 mmol/l) than with a combination of rosiglitazone and metformin (–0.42 mmol/l), whereas there was an increment in FPG with metformin (+0.05 mmol/l). In Idilman (2008),112 there was reduction in FPG with rosiglitazone (–0.7 mmol/l) and metformin (–0.26 mmol/l), whereas there was an increment with diet and exercise (+0.15 mmol/l). Similarly, in Ratziu (2008),111 rosiglitazone led to a significantly greater reduction in FPG levels than placebo (–0.93 mmol/l, SD 1.72, vs +0.55mmol/l, SD 1.74).
Weight-related outcomes
Three pioglitazone trials101,102,104 reported this outcome (Table 8). In all three trials, participants taking pioglitazone gained weight (+2.5 to +4.7 kg), whereas those on placebo lost weight (–0.5 to –3.5 kg); however, in one trial104 the participants in the control group also gained weight. In the latter trial, the participants in the control group were taking either vitamin E or placebo. Two trials105,106 reported changes with metformin. Participants lost between 4.3 and 6.7 kg with metformin, whereas those in the placebo arm gained 0.3 kg in one trial106 and those on a hypocaloric diet in the other trial lost 5.2 kg. 105 Two trials100,111 reported changes with rosiglitazone and the results varied between the two. In Torres (2009),100 weight was reduced in the rosiglitazone-only group (–1.3%); however, there was no significant difference to the reduction in the combined metformin and rosiglitazone group (–3.3%). In contrast, participants on rosiglitazone in the trial by Ratziu (2008)111 gained weight (+1.5 kg, SD 5.2 kg), whereas those on placebo lost weight (–1 kg, SD 0.5 kg).
With pioglitazone, BMI increased with changes ranging between +0.7 to +1.8 kg/m2, whereas with metformin and rosiglitazone BMI was reduced and the changes ranged between –0.4 and –3.2 kg/m2 and between –0.3 and –0.6 kg/m2, respectively. The reduction also occurred in the participants taking a combination of rosiglitazone and metformin (–1.3 kg/m2)113 and the change was greater than with rosiglitazone. In all of the pioglitazone trials, except Belfort (2006),102 the BMI of the participants in the control group increased. All of the participants in Belfort (2006)102 were also on a hypocaloric diet, whereas the participants in Aithal (2008)101 received diet and exercise advice. In the remaining pioglitazone trials none of the participants received any other interventions. In most of the metformin trials, reduction in BMI also occurred in the control group and the changes ranged between +1.5 and –2.5 kg/m2. In two head-to-head trials,112,113 reduction in BMI was greater with metformin (–1.8 to –3.2 kg/m2), followed by those taking a combination of metformin and rosiglitazone (–1.3 kg/m2) and finally those only on rosiglitazone (–0.3 to –0.6 kg/m2). The changes with metformin and a combination of metformin and rosiglitazone were significant from baseline to end, but were not significant with rosiglitazone alone.
Some studies used other parameters such as waist–hip ratio, waist or hip circumference and body fat content. Only two pioglitazone101,103 and one metformin108 study reported waist–hip ratio. The change in waist–hip ratio with pioglitazone was very small (0 to +0.02 with pioglitazone vs 0 to –0.01 with placebo). There was reduction in waist–hip ratio with metformin, but not greater than the reduction in the control group (–0.02 to –0.04 in metformin group vs –0.05 in control group; p = not significant). 105,108 The change in body fat content with pioglitazone was inconclusive. One trial103 found a reduction in both the pioglitazone and control groups (vitamin E) (greater reduction in the control group; –4.4% vs –2.9%), but there were increases with pioglitazone in the other two trials. 102,104 Only one head-to-head trial112 reported this outcome and it found that the reduction in body fat was higher in the participants on diet and exercise (–2%), followed by metformin (–1.9%) and rosiglitazone (–0.9%). In the same trial, it was found that the reduction in waist circumference was greater with metformin (–6.6 cm) than with rosiglitazone (–2.5 cm) or diet and exercise (–2.4), and the findings were not different for hip circumference (–1.6 cm with metformin, –1 cm with rosiglitazone and –0.8 cm with a combination of metformin and rosiglitazone). Another head-to-head trial113 reported that the reduction in waist circumference was greater with metformin (–4.2 cm) than with rosiglitazone (–1 cm) or a combination of metformin and rosiglitazone (–2.9 cm).
Blood pressure
Only Aithal (2008),101 and none of the metformin trials, reported this outcome (Table 9). Pioglitazone led to reductions in both systolic blood pressure (SBP) and diastolic blood pressure (DBP), but changes also occurred in the placebo group with no significant difference between the groups.
Lipid profiles
Eleven of the 15 trials reported the changes in lipid profiles (Table 10). 87,101,102,104–108,110–112 The changes with pioglitazone were not significant for any of the parameters when compared with control except for TG levels (p = 0.003) in Belfort (2006). 102 In Sanyal (2010),104 vitamin E led to greater reduction in most lipid parameters than pioglitazone and placebo; however, the difference was significant only for low-density lipoprotein (LDL) cholesterol. There was a reduction in some of the lipid parameters with metformin, but the changes were significant only in Haukeland (2009). 106 The participants in this trial were on either metformin or placebo, and all of the participants received advice on healthy lifestyle. The reduction in TC levels and LDL levels were significantly greater with metformin than with placebo. With rosiglitazone, the findings were mixed. Idilman (2008)112 (where all the participants were also on diet and exercise) found a greater reduction in TC and TGs with metformin than with rosiglitazone (–0.24 vs –0.16 mmol/l for TC, –0.42 vs –0.28 mmol/l for TG), but the reduction in TC, but not TGs, was greater with diet and exercise alone (–0.52 mmol/l for TC and –0.16 mmol/l for TG). In contrast to these findings, Ratziu (2008)111 (where none of the participants was on any form of diet or exercise regime) found an increment in all parameters of the lipid profile with rosiglitazone, whereas there was a reduction in TC and LDL cholesterol (–0.24 vs +0.45 mmol/l for TC, –0.32 vs +0.41 mmol/l for LDL) with placebo, and the changes were significant between the groups.
Alanine aminotransferase
There was a significantly greater reduction in ALT levels with pioglitazone in all the trials except in the trial by Sanyal (2004)103 (Table 11). In Sanyal (2004),103 the reduction was greater in the control group (vitamin E) than the pioglitazone group [–75 unit/litre (U/l) vs –65 U/l; p = not significant (NS)]. In contrast to this finding, in Sanyal 2010,104 the reduction with vitamin E was not greater than the reduction seen with pioglitazone (–40.8 U/l with pioglitazone, –37 U/l with vitamin E and -20.1 U/l with placebo). The changes in ALT levels with pioglitazone ranged between –37.7 and –65 U/l, –37.0 U/l and –75 U/l with vitamin E, and –6.9 and –21 U/l with placebo. The changes were significantly different between the groups.
In all of the trials, a significant reduction from baseline to end occurred with metformin (see Table 11). The reductions ranged between –15 and –37.1 U/l in the metformin group and between –6.8 and –40.7 U/l in the control group. The change in ALT with rosiglitazone was not different to those caused by a combination of metformin and rosiglitazone (Torres 2009100) (–46.3% vs –46.5%). Metformin led to a greater reduction in ALT levels than rosiglitazone. 112
Insulin sensitivity
Most trials reporting insulin sensitivity used homeostatic model assessment–insulin resistance (HOMA–IR) (Table 12). Pioglitazone showed varying results, with Aithal (2008)101 reporting an increase in insulin resistance with pioglitazone and Sanyal (2010)104 reporting a reduction with pioglitazone and an increase with vitamin E or placebo. Aithal (2008)101 argued that the increment in insulin resistance with pioglitazone could have occurred because all of the participants were non-diabetic and a relatively low dose of pioglitazone was used. 101 In most trials, metformin led to a significant reduction in insulin resistance from baseline to end, but there was no significant difference with placebo. In one trial (Haukeland 2009106), both metformin and placebo (+0.58% with metformin and +0.63% with placebo) led to an increase in insulin resistance. However, the difference between the two was not statistically significant (p = 0.71). In Idilman (2008)112 (–2.9 HOMA–IR score with rosiglitazone, –2.1 score with metformin and +0.1 score with placebo) and Omer (2010)113 (–1.1 score with rosiglitazone, –0.8 score with metformin), rosiglitazone led to a greater reduction in insulin resistance than metformin. In Ratziu (2008),111 there was a reduction with rosiglitazone and an increase in the placebo group [–1.41 interquartile range (IQR) vs +0.61 IQR]. Two trials (Omer 2010113 and Torres 2009100) reported that the changes in insulin resistance were greater with a combination of metformin and rosiglitazone than with either rosiglitazone or metformin alone (–1.6 with combination in Omer;113 in Torres100 –51.8% with a combination vs –50.2% with rosiglitazone).
Quality of life
Only Sanyal (2010)104 reported health-related QoL, assessed using the Short Form questionnaire-36 items (SF-36). There was reduction in both the physical and mental components with pioglitazone (reduction by a mean of –0.9 in the physical component of SF-36 and a reduction by a mean of –1.9 in the mental component of SF-36), but only in the physical component with placebo (mean score of –0.3 physical component and mean score of +0.4 mental component), and only in the mental component (mean score of –0.5 mental component and mean score of +0.4 physical component) with vitamin E. However, the changes were not significantly different between the groups.
Conversion to diabetes/impaired glucose tolerance/metabolic syndrome
Two studies (Idilman 2008112 and Sanyal 2010104) reported frequency of new-onset diabetes at the end of the study, and one study (Garinis 2010105) compared the total number of participants with impaired glucose tolerance at baseline and end of the study. In Sanyal (2010),104 two participants in the vitamin E group and none in the placebo or pioglitazone group developed new-onset diabetes. Idilman (2008)112 reported that two participants in the group on diet and exercise alone and a total of six (combined number for both rosiglitazone and metformin) in the remaining groups developed diabetes by the end of the study. Garinis (2010)105 found that the number of participants who had impaired glucose tolerance at baseline reduced at the end of the study in the group taking metformin (three at baseline and two at end), whereas the number remained unchanged in the control group (three at baseline and three at end). The numbers in some of these groups are quite small.
Discussion
Summary
Fifteen RCTs were included in this review. Four trials examined the effects of pioglitazone, seven examined metformin, one rosiglitazone and three compared metformin against rosiglitazone. The duration of most trials ranged between 6 months and 1 year, and only one trial104 lasted 96 weeks with a 24-week follow-up. The total number of participants analysed in this review was 881 (751 excluding participants who received rosiglitazone in combination with other drugs or rosiglitazone on its own). In all of the pioglitazone trials, the dose of pioglitazone was 30 mg once a day. The dose of metformin varied between 1 and 2.5 or 3 g/day, with most trials using 1.7 g/day. The dose of rosiglitazone ranged between 4 and 8 mg/day. In most trials, the participants in the control group as well as in the intervention group also received other measures including counselling on diet and exercise, use of a hypocaloric diet and lifestyle modifications.
Pioglitazone was found to improve all parameters of liver histology, was better than placebo or diet and exercise or a hypocaloric diet, but no better than vitamin E.
Results with metformin were mixed. In comparison with placebo or diet and exercise, some studies suggested that metformin led to some improvements in steatosis and necroinflammation, but not in fibrosis. However, in most studies the changes seen with metformin were not significantly different from those in the control arms. The Bugianesi trial87 reported clear improvements in histology with metformin, but no biopsies were done in the control arm. The ultrasound trials suggest that metformin led to improved liver echogenicity compared with diet and exercise. Uygun et al. 110 found significant differences with ultrasound (p = 0.038), but not with biopsy.
Rosiglitazone showed significant improvement in hepatic steatosis and very little improvement in any of the other parameters. In head-to-head trials comparing metformin and rosiglitazone, the results varied. Idilman (2008)112 found greater improvements in hepatic steatosis, ballooning and fibrosis with metformin than diet and exercise alone. ‘Rosiglitazone only’ led to a reduction in hepatic steatosis. The overall improvement in NAFLD score from baseline to end was better with either metformin or rosiglitazone than with diet and exercise (Idilman 2008112). Omer (2010)113 found significant improvement in NAFLD score with rosiglitazone and a combination of rosiglitazone and metformin and no significant changes occurred with metformin alone.
Pioglitazone was better than any control groups (placebo, diet or exercise) in terms of improvement of ALT, insulin sensitivity, and FPG, but led to weight gains of 2.5–4.7 kg, and increases in BMI of 0.7–4.7 kg/m2. The changes in waist–hip ratio, waist or hip circumference and body fat with pioglitazone were not significant. Metformin led to greater reduction in weight, BMI and waist or hip circumference than rosiglitazone or a combination of the two. Although there were reductions in lipid parameters, ALT and insulin resistance with metformin, the changes were similar to those with placebo or lifestyle modifications or rosiglitazone.
Rosiglitazone was better than metformin in terms of HbA1c levels, FPG and insulin sensitivity, but the changes in weight were inconclusive, with one trial (Ratziu 2008111) showing better results with rosiglitazone and the other with a combination of rosiglitazone and metformin (Torres 2009100). The reductions in BMI, body fat and waist or hip circumference were not greater with rosiglitazone. The changes in lipid profiles with rosiglitazone were inconclusive. A combination of metformin and rosiglitazone was found to be similar to rosiglitazone alone in terms of ALT reduction and insulin resistance (Torres 2009100).
Strengths and limitations
Strengths
We carried out an up-to-date systematic search to identify all trials that explored the effectiveness of insulin sensitisers in patients with NAFLD. We included only RCTs either published in full or as an abstract. There were no language restrictions.
Limitations
There were only two head-to-head trials comparing the effects of different insulin sensitisers against each other and, therefore, no firm conclusions can be made about their relative effectiveness. Given recent evidence, pioglitazone would have been a more useful comparator than rosiglitazone because of its better cardiovascular risk profile. There are also doubts about comparisons of pioglitazone and metformin against vitamin E. Although Sanyal (2010)104 found that effect of vitamin E was similar to pioglitazone in terms of histological changes to the liver, no firm conclusions can be made that vitamin E would be as effective as insulin sensitisers.
The trials were not long enough to make firm conclusions that insulin sensitisers would be effective for long periods of time. Only one trial lasted 96 weeks, and the comparison was against vitamin E, whose effect on NAFLD is not proven.
Because the data were inadequately reported, meta-analyses could not be done. Therefore, our results are reported only as texts and tables. Out of 15 studies, only three87,104,109 had carried out an ITT analysis. We made no attempts to find unpublished studies.
There are also doubts about some of the findings. Bugianesi (2005)87 conducted a follow-up biopsy only in the participants who were on metformin, but not in the control groups, because the physicians taking care of the participants in the control group did not support a second one. The findings showed a marked improvement in liver steatosis with metformin, but without a control biopsy the findings are inconclusive. It is noteworthy that Haukeland (2009),106 with a much larger sample size, found improvements also in the placebo group. Torres (2009)100 reported findings of only the first 49 participants who completed a trial that is still ongoing. The preliminary findings, however, suggest that rosiglitazone alone was as effective as when combined with metformin.
There are also doubts about the generalisability of the findings. Three of the trials were conducted in Turkey, one each in the UK and Italy, and the rest in the USA. The total number of participants in most trials was small, i.e. < 50, and, in addition, the methodological quality of some trials was low. We did not explore the safety and tolerability of these drugs as these are well documented. In some trials there were concerns about loss to follow-up. In three trials107,109,110 one or more arms had loss to follow-up of > 20%, raising the risk of bias and generalisability of the results. 118
Other systematic reviews114,119,120 on the effectiveness of insulin sensitisers in patients with NAFLD were identified, but they differed from this review. Angelico et al. 114 included only three RCTs. Chavez-Tapia et al. 119 included non-RCTs and Socha et al. 120 also included trials that compared efficacy of non-insulin sensitisers in patients with NAFLD. A systematic review by Musso et al. 78 included trials that compared the efficacy of non-pharmacological interventions and all drugs in patients with NASH/NAFLD. The findings from these reviews were inconclusive regarding the effectiveness of insulin sensitisers and suggested that more trials were needed. Our findings showed improvements in some outcomes with insulin sensitisers. There is need for more head-to-head trials with relevant comparators and a bigger sample size with participants followed up for longer durations of time.
Conclusions
There were some improvements in liver histology and other parameters with pioglitazone, but there was a lack of evidence that metformin improved liver histology, compared with placebo or diet and exercise, in mainly short-term trials. There were only two head-to-head trials comparing rosiglitazone with metformin.
Chapter 3 Diagnosis of non-alcoholic fatty liver disease and its stages
Introduction
This chapter examines the ways in which NAFLD is diagnosed and the differentiation of simple steatosis from NASH. The main interest is in doing this in a non-invasive manner.
Liver biopsy has traditionally been viewed as being necessary for accurately diagnosing and staging NAFLD. 121–123 However, there has been much interest in alternative means of diagnosing the condition for the following reasons:
-
Prevalence of NAFLD It is believed that NAFLD affects approximately 25–30% of adults in Western countries. 123–126 Performing liver biopsies on such a large number of people is not feasible. 127
-
Risks of liver biopsy Biopsy is an invasive procedure and is associated with complications including haemorrhage and death, although the risk of these complications is very low (mortality risk about 0.1%). 123,126 After a review of the literature, Gaidos et al. 128 estimated the risk of death after liver biopsy to be around 0.01%.
-
Costs of biopsy Biopsy is not an inexpensive test. Therefore, its use in a large number of trial people is not feasible. 126
-
Accuracy of biopsy ‘Biopsy-only’ samples a small amount of hepatic tissue. It is recognised, however, that pathological changes in chronic liver disease may not be evenly distributed throughout the liver. Therefore, sampling a relatively small area of tissue could give rise to false-negative results. 121,123,125,126,129
If there is to be a large trial to provide better evidence on the effectiveness of insulin sensitisers, it will need to be based on a diagnostic method other than liver biopsy, especially if patients are to be recruited from the more advanced stages than simple steatosis. Ultrasound at baseline, combined with alcohol history, could establish a diagnosis of NAFLD but not the stage, such as differentiating steatosis from NASH.
Methods
A systematic search was undertaken on electronic bibliographic databases, MEDLINE (1950 to June 2010) and EMBASE (1980 to June 2010). Searches were developed by using relevant medical subject heading (MESH) terms with explosion of the MESH terms when necessary.
The following search strategy was used:
-
Liver.tw
-
(non-alcoholic or non alcoholic or nonalcoholic).tw
-
(fatty or steato*).tw.
-
(NAFL or NAFLD or NASH).tw
-
exp *Diagnosis/
-
diagnos*.tw.
-
Fatty Liver/di [Diagnosis].
The searches were limited to the English language. Search results from both databases were imported into Reference Manager 12 (Thomson Reuters, Philadelphia, PA, USA). Duplicates were removed before two authors (NW and RH) scanned the list independently to identify relevant papers. Any discrepancies were resolved by consensus.
Full texts of all potentially relevant studies were obtained.
Study selection
Articles for review were selected, based on whether or not they seemed likely to have data on non-invasive alternatives to liver biopsy, ideally with liver biopsy used as a comparator. Reviews were selected in the first instance.
Quality assessment
The intention was to assess the quality of review articles using recognised appraisal frameworks, for example the Critical Appraisal Skills Programme (CASP)130 or the Scottish Intercollegiate Guidelines Network (SIGN). 131 However, the majority of reviews did not describe the search strategy adopted, their inclusion and exclusion criteria or how included studies were appraised. Therefore, this section aims to simply summarise their findings and conclusions.
Diagnosis of non-alcoholic fatty liver disease
Guidelines recently published by the Italian Association for the Study of the Liver (AISF)125 recommend that NAFLD can be diagnosed based on clinical history, clinical examination, laboratory tests [e.g. full blood count (FBC), LFTs, fasting glucose, etc.] and an abdominal ultrasound scan. The AISF guidelines recommend that liver biopsy be performed on individuals believed to be at high risk of NASH (e.g. age > 45 years, obesity, diabetes/insulin resistance, etc.), although the guidance does not state how many of these risk factors should be present before considering biopsy. 125 The guideline also recommends that biopsy should be considered if a patient still has evidence of steatosis on abdominal ultrasound scan or deranged LFTs 6–12 months after commencing lifestyle modifications following diagnosis. 125
Myers126 also advocates a multifaceted approach to the diagnosis of NAFLD, stating that individual parameters may be misleading. 126 People with NAFLD may have no or non-specific symptoms, and laboratory tests may also be of limited value when used on their own (e.g. ALT levels may vary in people with NAFLD).
Myers126 states that abdominal ultrasound scans are the most frequently used imaging tool for diagnosing NAFLD in the USA. However, it is recognised that ultrasound is also subject to limitations. It may fail to detect mild steatosis,126,127,132–134 suffers from poor performance when used on morbidly obese individuals134 and is vulnerable to inter- and intra-observer variability. 134 Such variability may reflect factors such as differences in the criteria used to diagnose steatosis and differences in ultrasound transducers as well as differences in the populations studied. 132 Furthermore, ultrasound scans cannot distinguish between steatosis and NASH. 126,127,132–134
Therefore, it might be argued that abdominal ultrasound may not add enough to the combination of clinical history, clinical examination and laboratory tests to influence clinical management, because of the lack of proven treatment options, other than weight loss. 5 However, it could still be used to screen patients for trials.
Imaging with modalities other than ultrasound has also been explored. Computerised tomography (CT) scanning is also able to detect steatosis and has been said to be ‘… more reliable for detecting and grading fatty infiltration of the liver than ultrasonography.’132 However, CT scanning requires the use of ionising radiation, reducing its usefulness. 123,125 In addition, CT scanning does not appear to have a role in distinguishing between steatosis and NASH. 5,122,129,135
Magnetic resonance imaging has also been used as a means of diagnosing NAFLD, with Ma et al. 133 describing MRI as ‘… one of the most sensitive modalities for detection and characterisation of fatty infiltration of the liver’.
The AISF guideline states that MRS is ‘… probably the most accurate and fastest method of detecting liver fat’. 125 The technique allows detailed examination of particular areas of interest within the liver. However, MRS is expensive125,126 and may not be routinely available in radiology departments. 123,125,126
Chemical shift-based MRI techniques have also been reported as being able to diagnose steatosis. 5,122,123,135 Chemical shift imaging techniques have been modified during recent years, and have been described as ‘… the most common MRI modality to evaluate NAFLD…’ in clinical practice. 122 In comparison with MRS, chemical shift imaging is said to have ‘… a shorter acquisition time, can measure fat content throughout the liver instead on in one or just a few voxels, no special misregistration errors, as well as easier and faster processing’. 122 Cassidy et al. 121 recommend the use of chemical shift imaging as opposed to MRS in clinical practice, stating that ‘in most clinical situations, chemical shift imaging provides adequate detection in a fraction of time’. However, they state that the sensitivity of chemical shift imaging appears to increase with increasing steatosis,121 although Charatcharoenwitthaya and Lindor132 claim that ‘… gradient-echo techniques have been shown to accurately quantify the hepatic fat fraction at low or near normal level’. 132
Biochemical tests have also been suggested for the diagnosis of NAFLD. The SteatoTest (BioPredictive, Paris, France) combines several demographic variables (e.g. age, gender) with laboratory analyses (e.g. cholesterol, TGs, glucose, ALT, etc.) to assess steatosis. 126,135 An initial study that recruited individuals with a range of chronic liver conditions (e.g. hepatitis C, alcoholic liver disease) in addition to NAFLD reported favourable results. 126 Further independent studies involving people with NAFLD are required to assess the utility of the test. 126,135
In summary, there appears to be an emerging view that steatosis can be diagnosed without the need for liver biopsy on the basis of clinical history, clinical examination and laboratory tests, supported by imaging. Abdominal ultrasound scanning appears to be the most commonly used modality, although it is subject to several limitations. CT and MRI also appear to be useful tools, although their use may be limited by concerns regarding exposure to ionising radiation and cost, respectively.
Diagnosis of non-alcoholic steatohepatitis
Although it appears that NAFLD may be diagnosed without liver biopsy, the recently published guidelines produced by AISF describe biopsy as being ‘… an irreplaceable diagnostic tool to differentiate NASH from NAFLD’,125 a view shared by others. 127,132 Note that the terminology can be confusing. The term NAFLD is sometimes used to cover the whole spectrum, but at other times to refer to the stages before NASH.
However, as previously described, the prevalence of NAFLD together with the invasive nature of, and the risks associated with, liver biopsy has led to interest in developing new means of differentiating between these conditions. Some of these are described below.
Laboratory markers of fibrosis
The guideline recently issued by AISF states that several biochemical indicators of fibrosis have been investigated as means of reducing the number of people requiring liver biopsy. 125 However, AISF describes these as having not yet been rigorously tested and validated, as well as being unavailable within many laboratories. 125 Therefore, AISF states that these ‘do not avoid the need to perform liver biopsy in clinical practice.’125
Myers126 categorised such markers as signifying oxidative stress, inflammation or hepatocyte apoptosis. 126 With respect to the evaluation of markers of oxidative stress, he describes the results as generally being ‘… disappointing and/or inconsistent …’, concluding that ‘… additional studies are necessary before markers of oxidative stress can be used clinically to differentiate simple steatosis from NASH’.
With respect to biomarkers of inflammation, Myers126 states that a marker which has been ‘… validated and is ready for clinical use is not available.’ With respect to hepatocyte apoptosis, Myers discussed research concerning the use of caspase-generated fragments of cytokeratin-18 (CK-18). Although encouraging, Myers126 states the findings require validation ‘… in large-scale, multicentre studies that include more diverse patient populations.’
Clinical scores for fibrosis
Several clinical scoring systems, or fibrosis marker panels, have been developed with the aim of differentiating NASH from NAFLD (e.g. NAFLD fibrosis score, European Liver Fibrosis panel, etc.). 125,126 Some of these are subject to patent. 126
These scoring systems attempt to identify people at risk of fibrosis through measuring indicators such as BMI together with various biochemical parameters. The biochemical parameters can be described as:
-
indirect markers of fibrosis in that they ‘… reflect alterations in hepatic function, but do not directly reflect extracellular matrix (ECM) metabolism (e.g. liver biochemistry, platelets)’126
-
direct markers of fibrosis in that they ‘… reflect the dynamics of the ECM [extracellular matrix] turnover (e.g. matrix metalloproteinases and their inhibitors, collagens, hyaluronic acid)’. 126
Both indirect and direct markers are subject to limitations. For example, changes in some indirect markers may be observed only in the advanced stages of chronic liver disease. 136 Changes in direct markers may be due to non-hepatic disease. 136
In many cases, the biochemical parameters within fibrosis marker panels are a mixture of direct and indirect markers. 126 Many of these panels were developed for use among patients with chronic hepatitis C infection, but their use in other conditions, including NAFLD, is being explored. 126
Poynard et al. 137 undertook a review of biomarkers of liver fibrosis associated with chronic hepatitis C, chronic hepatitis B, alcoholic liver disease, NAFLD and mixed causes. They identified 14 biomarkers, nine of which were not patented and five that were. Only patented biomarkers were considered in detail within the review. Two of the authors have connections to a company marketing one of the patented biomarkers considered – FibroTest™ (Biopredictive, Paris, France). They found no significant differences between the five patented tests. With respect to performance in relation to the aetiology of fibrosis, the authors stated that most studies had been performed among patients with chronic hepatitis C, with only one test – FibroTest – having ‘… been investigated specifically in the four most frequent chronic liver disease(s)’. Only three of the studies of patented biomarkers included in the review – two involving FibroTest (267 patients) and one involving the European Liver Fibrosis panel (61 patients) – examined the use of biomarkers among individuals with NAFLD. The authors stated that ‘neither biomarkers nor biopsy are sufficient alone to take definitive decision(s) in a given patient …’ and suggested ‘… a moratorium on liver biopsy as a first line procedure while awaiting studies demonstrating biopsy cost-utility vs that of biomarkers’.
Poynard137 and another author138 contributing to the above review (both of whom have connections to the company marketing FibroTest) contributed to a further review focusing on FibroTest as a measure of liver fibrosis. The majority of studies identified assessed the use of the test among patients with chronic hepatitis C. Only two studies examining the use of the test among patients with NAFLD were identified, both of which were included in the earlier review. 137 Similar to the conclusions of the earlier review, the authors stated: ‘FibroTest™ may be an alternative to liver biopsy in the four more common liver diseases – namely, HCV, HBV, NAFLD and ALD. However, neither biomarkers nor biopsy alone are sufficient to allow definitive decisions to be made for a given patient, and all clinical and biological data must be taken into account.’
The AISF guideline125 did not refer to FibroTest, although it referred to several other fibrosis marker panels not included within the Poynard review137 [e.g. BAD (BMI, age, T2DM), BAAT (BMI, age, ALT, TG), HAIR (hypertension, ALT, insulin resistance), BARG (BMI, age, AST/ALT ratio, HbA1c or glucose), NAFLD Fibrosis Score, BARD (BMI, AST/ALT ratio, T2DM), Clinical Scoring System for Predicting NASH]. Studies describing the use of some of these were published outwith the time period covered by Poynard et al. 137 (February 2001 to July 2007). The AISF guideline states: ‘These indices, particularly the NAFLD fibrosis score, should be considered in evaluating patients for liver biopsy.’125
The majority of studies evaluating the use of fibrosis marker panels have involved people with chronic hepatitis C. Some marker panels have been investigated among patients with NAFLD, although for some the findings of initial studies require to be confirmed by further research. 135,136 At the current time, the relative ability of these panels to differentiate NASH from NAFLD is unclear, and comparisons are difficult as a consequence of studies using a variety of scoring systems and end points in the assessment of fibrosis. 135
At present, the consensus view appears to be that fibrosis panels should be used as an adjunct to liver biopsy rather than as a substitute. Myers126 states:
‘Most experts would agree that the most rational approach is to use these markers as a complement to liver biopsy on a case-by-case basis. It would be unrealistic to expect any index to completely replace liver biopsy which offers a wealth of additional information … It is important to remember that up to a third of patients suspected of having NAFLD have another cause of liver enzyme elevations identified by liver biopsy.’126
Imaging
Ultrasound scanning
Ultrasound scanning is said to be unable to distinguish between steatosis and steatohepatitis. 122,123,132 However, initial studies of contrast-enhanced ultrasound have been more encouraging, although further evaluation involving larger numbers of patients with NAFLD is required. 127,132
Transient elastography
Transient elastography (TE) is a technique that may supplement traditional USG. TE aims to measure liver stiffness, a variable that is believed to be associated with the degree of fibrosis. A mechanical pulse is generated at the skin surface, which is propagated through the liver. The velocity of the wave is measured by ultrasound. The velocity is directly related to the stiffness of the liver, which, in turn, affects the degree of fibrosis: the stiffer the liver is, the greater the degree of fibrosis. 139 The technique has been used in a range of liver conditions, but relatively few studies have described its use in NAFLD. 126,135,136
The results of TE are influenced by factors including elevated ALT levels124,125,136 and possibly by the degree of steatosis. 125,126,136 In addition, it is technically difficult in the presence of obesity. 126,127,132,135,136,140 Furthermore, cut-off points between stages of fibrosis have been said to be unclear, and may vary with aetiology. 126 As a consequence, Myers126 states: ‘In light of these unresolved issues, and despite the widespread use of TE in patients with NAFLD, much further investigation is necessary to guide the optimal incorporation of this promising technology into routine clinical practice’,126 a sentiment echoed by Charatcharoenwitthaya and Lindor. 132 Browning,127 however, is less optimistic: ‘Further study is required to determine if FibroScan [a commercially available TE machine] is a viable method for differentiating patients who have NAFLD with no fibrosis from those who have minimal or no fibrosis … However, based upon data available for staging other forms of liver disease, this seems unlikely.’127
Computed tomography scanning
The AISF guideline states that, in contrast to individuals with simple steatosis, those with NASH ‘… have a greater liver span and increased caudate-to-right-lobe-ratio.’125 Charatcharoenwitthaya and Lindor, however, state that although some studies have reported differences between patients with NAFLD and NASH that are evident on CT scanning, in their opinion the technique is unable to reliably differentiate between steatosis and steatohepatitis,132 a view also shared by others. 122,127,135
Studies have examined the use of dynamic contrast-enhanced CT as a means of diagnosing fibrosis. 127,140 However, the majority of studies have been conducted among people with cirrhosis,127,140 so it is uncertain if the technique will be able to diagnose earlier stage fibrosis. 140 Nevertheless, Browning believes that based upon current knowledge of liver perfusion in NAFLD ‘… the utility of this imaging modality in staging NAFLD is questionable.’127
Therefore, the current consensus appears to be that CT scanning does not have a role to play in distinguishing NAFLD from NASH.
Magnetic resonance imaging
Browning states that standard MRI cannot differentiate between NAFLD and NASH,127 with Castera140 describing its ability to diagnosis early-stage fibrosis as being ‘limited’. 135 Although a small number of studies have described changes relating to liver fibrosis on MRI, its sensitivity or specificity has not been reported. 140
Contrast-enhanced MRI has been suggested as a means of diagnosing fibrosis, and a limited number of studies having shown encouraging results in staging of advanced hepatic fibrosis. 140 Browning127 states that ‘Much more work is needed, however, to fully evaluate the utility and limitations of such contrast-based methodologies’. Furthermore, the possibility of allergic reactions associated with contrast agents140 may also prove a barrier to the future use of the technique.
Diffusion-weighted MRI is another technique that has been investigated as a non-invasive means of diagnosing liver fibrosis. 140 It appears that the technique can distinguish between normal and cirrhotic liver, but its ability to differentiate earlier stages of fibrosis has been less positive. 140
Magnetic resonance spectroscopy allows an assessment of the ‘… concentrations of different chemical components within tissues’. 140 However, the results of the technique are sensitive to movement, and require the patient being examined to lie as still as possible during the examination. 140
Bonekamp140 states that although proton MRS appears to be of limited use in distinguishing NAFLD from NASH, initial studies evaluating phosphorous MRS have shown some promise, although the technique requires more thorough evaluation. In addition, the need for special equipment, together with considerable operator experience, makes it unlikely that it will be a widely accessible technique in the short term, even if it is proved to be effective. 140
The use of magnetic resonance elastography (MRE) has also been suggested as a means of differentiating NAFLD from NASH. In contrast to conventional TE, MRE is said to be unaffected by obesity, and may also be unaffected by steatosis. 126 However, although there appear to be some encouraging results using this technique, Myers126 counsels that: ‘Future studies will be necessary to validate these findings and confirm the cost-effectiveness of MRE before it gains widespread clinical use.’126 Similarly, Bonekamp et al. state ‘… more studies including larger patient populations are needed to confirm the sensitivity and specificity of MRE and to standardise the technique to make results comparable.’140
Other considerations
Ability to compare markers
Interpreting the current data on the effectiveness of non-invasive markers of liver fibrosis is challenging as a consequence of:
-
small number of patients recruited in some studies135
-
assessment of fibrosis varies across studies135
-
the performance of tests being dependent on the prevalence of severe fibrosis in the study population. 136
Area under receiver operating characteristic curve (AUROC) estimates are a commonly used measure of the effectiveness of diagnostic and screening tests. However, Poynard et al. ,137 with reference to biochemical markers of liver fibrosis, have discussed the need for standardisation when comparing the AUROC estimates to take account of differences in the prevalence of stage of fibrosis between studies. 137
Quality of reviews studied
Many of the reviews studied in the preparation of this section referred to an extensive body of research. However, as described above, methodological issues associated with primary studies limit the strength of the conclusions that they can draw.
Furthermore, with respect to identifying non-invasive means of distinguishing NAFLD from NASH, although a large number of techniques have been studied, in general the volume of evidence underpinning individual approaches among individuals with NAFLD appears limited. There is, therefore, a need for additional high-quality research in this area, focusing on those approaches assessed as holding greatest promise.
As described, many of the reviews studied in the preparation of this section referred to a large number of papers. However, the majority contained no description of how these papers were identified, inclusion and exclusion criteria or appraisal methods. In the majority of reviews, it was not clear if the conclusions were based upon an assessment of the quality of the primary studies referred to. Therefore, there is also a need for rigorous systematic reviews of methodologically robust primary research.
Conclusions
There appears to be an emerging view that simple steatosis can be diagnosed without the need for liver biopsy, on the basis of clinical history, clinical examination and laboratory tests, and ultrasound.
Although several non-invasive means of distinguishing simple steatosis from NASH have been studied, at the current time it appears that liver biopsy is required to differentiate between these conditions. However, this issue has been the focus of much research and further studies may help to identify if any of the approaches discussed, or novel techniques, could become acceptable alternatives. However, there is a need for high-quality research to enable approaches to be compared against liver biopsy and each other.
Implications for trials and diagnostic research needs
If we assume that (1) a definitive trial of drug intervention in NAFLD will require a large number of patients followed for several years and (2) liver biopsy will not be feasible or perhaps ethical, then we have to consider other approaches.
One consideration concerns who should be included in a trial. It would be desirable to intervene in time to prevent fibrosis, so the most important aspect of diagnosis might be to distinguish between those without fibrosis and those with. Progression to fibrosis might be a key end point.
Non-invasive methods of distinguishing those with simple steatosis from those with NASH are, as yet, unproven. However, it could be argued that inclusion of patients with simple steatosis in a trial of an old tried and tested drug, such as metformin, would do them no harm, and might well do good (e.g. reducing the chance of progression to diabetes), and that therefore a screening test that lacked specificity could be allowed. The Italian guideline panel of tests might be used, perhaps combined with MRS or MRE for follow-up, with reduction in steatosis as one outcome. Unfortunately, in trials to date, metformin does not appear to improve histology, and trials might be better done using newer and more expensive drugs such as liraglutide.
Several diagnostic measures appear promising, but require further evaluation. One is TE, except in the more obese. NFS has been reported to be reliable in the LEAD (Liraglutide Effect and Action in Diabetes) trials. 141 MRE appears promising but requires further evaluation and standardisation. Even if too expensive for routine use, it could be trialled against liver biopsy in a small trial and, if reliability is proven, it could then be used as the reference standard in trials of treatment to assess the accuracy of TE and NAFLD Fibrosis Score (NFS).
This chapter was only a rapid review of recent evidence on diagnostic options, and a full systematic review would be worthwhile as a prelude to primary diagnostics research.
Chapter 4 Review of economics studies
The search strategy used is in Appendix 1.
Few studies were found.
Baumeister et al. 142 set out to estimate the inpatient and outpatient costs for people with fatty liver disease (FLD), using data from the Study of Health in Pomerania database. They used ultrasound hyperechogenicity (‘bright liver’) and ALT levels to identify people with FLD. However, although they identified people with ‘at-risk’ drinking of alcohol, they did not provide data broken down by lesser levels of alcohol use, and so it is not possible to extract data on the costs of NAFLD. One problem with such studies is that people with FLD are at higher risk of other metabolic conditions such as diabetes and hypertension. Baumeister et al. 142 adjusted for comorbidities and found that people with FLD still had higher outpatient costs.
A study from the USA by Younossi and Singer,143 available at present only as an abstract, used Markov modelling to estimate lifetimes costs of NAFLD and reported that NAFLD led to costs of several billion US dollars (undiscounted).
Dan et al. 144 surveyed health-related QoL in people with NAFLD who had been referred to a Liver Service. This implies that they were symptomatic, and may have been on the more severely affected end of the NAFLD spectrum. Dan et al. 144 compared the NAFLD group with patients with chronic hepatitis B or C (but who were not on treatment with interferon, which reduces QoL while patients are taking it). The NAFLD group had the poorest QoL. They differed from the hepatitis groups by being much more likely to be obese (76% vs 14% and 36%), diabetic (26% vs 4% and 8%) and hypertensive (39% vs 9% and 14%). However, Dan et al. 144 reported that the reduced QoL in the NAFLD group persisted after adjusting for obesity (which itself reduces QoL).
David et al. 145 from the NASH Clinical Research Network (CRN) Research Group administered the SF-36 to 713 people enrolled in two studies, one an observational cohort, the other the PIVENS (Pioglitazone Versus Vitamin E Versus Placebo for the Treatment of Non-diabetic Patients with Non-alcoholic Steatohepatitis) trial. Most (61%) had NASH, and 28% had fibrosis or cirrhosis. All had had liver biopsy. So as with the Dan study,144 there is some selection towards the severe end of the NAFLD spectrum. The results in the NAFLD group were compared with the general US population and adjusted for age. Because the general population also have chronic diseases (including, at the relevant age, 22% with diabetes and 27% with hypertension), David et al. 145 compared the NAFLD group with the whole general population and after excluding those in the general population with chronic diseases. In the NAFLD/NASH group, 72% were obese.
The NASH groups had poorer SF-36 scores than the general population, by about five points (SF-36 is a 100-point scale) when compared with the whole general population, and by 11 points when compared with the general population with those with chronic diseases excluded. Within the NASH groups, there was a gradient, so that, overall, QoL declined along the spectrum: general population > NAFLD > NASH > fibrosis > cirrhosis.
Physical health status was reduced more than mental health, with vitality/fatigue most affected. As the authors note, in a cross-sectional study cause and effect cannot be determined, so either NAFLD reduces physical activity or those with reduced physical activity are more likely to develop NAFLD.
Another study146 from the NASH CRN Network looked at the QoL in children aged 5–17 years with NAFLD, mean age 12.6 years, mean BMI 33, with nearly all obese (defined as BMI ≥ 95th percentile). The patients were recruited from the observational NAFLD database, and from a trial group [TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children), see Chapter 5]. All had biopsy-proven NAFLD. The measure used was the Pediatric Quality of Life Inventory (PedsQL), with healthy children used as control subjects. The NAFLD children had poorer QoL scores (mean 73) than the healthy children (mean 84). Thirty-nine per cent of the NAFLD group had reduced QoL. Interestingly, the parents of the NAFLD children scored them lower (65) than the children did themselves. The study did not adjust for the effect of obesity, so much of the QoL deficit might have been due to obesity. The authors called for a study of QoL in obese children without NAFLD.
Similar findings were reported by Kerkar et al. 147 from a pilot study in 17 children aged 8–18 years, also using PedsQL, but reported in abstract form only at present.
The value of early liver biopsy has been assessed by Gaidos et al. 128 by decision analysis modelling. They carried out modelling comparing two options, early biopsy and no biopsy. They assumed that patients with confirmed NASH would be treated with insulin sensitisers or bariatric surgery. Patients in the no-early-biopsy arm would not receive treatment until there was evidence of disease progression. They assumed that mortality from liver biopsy was 1 in 1000 biopsies, but that 3% would have sufficient complications to require a hospital stay. They concluded that an early biopsy policy would reduce mortality at 5 years from 0.53% to 0.32%, and progression to severe disease from 4.4% to 2.4%.
The conclusions from the sparse evidence available are that:
-
More advanced degrees of NAFLD reduce QoL.
-
NASH and its sequelae are costly to health services.
-
Future studies need to disentangle the effects on QoL of NAFLD itself, from co-conditions such as obesity and diabetes.
-
We need longer follow-up to assess frequency and speed of progression, in order to base modelling on more data and fewer assumptions.
Chapter 5 Discussion
Statement of principal findings
-
NAFLD in its various stages is becoming more common and is an increasing health problem, because of the rising prevalence of obesity and insulin resistance.
-
Three insulin-sensitising drugs were covered in this review. Rosiglitazone is no longer relevant because it has been withdrawn from use. Metformin is still used, but its effect on liver histology is modest. Pioglitazone was more effective in improving liver histology, but can have adverse effects, including oedema, weight gain, precipitation of heart failure and fractures.
-
Weight loss by diet and exercise is effective in reducing liver fat, but compliance is often an issue. Bariatric surgery is highly effective.
-
Simple steatosis can be diagnosed by non-invasive means, but, at present, liver biopsy remains the only proven method of distinguishing between steatosis and NASH. Newer non-invasive methods show promise, but further research is required. This seems to be the highest research priority at present because of the impracticality of doing large trials of new agents if liver biopsies are required at entry and end of trial.
-
Of the newer agents, the angiotensin receptor blocker telmisartan (which also has PPAR gamma activity) and the glucagon-like peptide-1 (GLP-1) analogues show sufficient promise to be considered for trials.
-
Trials should probably be in NASH rather than the relatively benign simple steatosis.
-
As one of the (anonymous) NIHR HTA programme referees noted, an overall finding is a shortage of high-quality evidence, including health economics aspects.
-
Lifestyle measures remain worthy of consideration. Weight loss by calorie restriction has been shown to be effective in reducing hepatic steatosis77 but, as usual, the problem is compliance. When compliance is ensured by bariatric surgery, leading to the loss of large amounts of weight, there is improvement or resolution of NAFLD in up to 90% of patients. 98 There is general pessimism about the long-term success of non-surgical management of obesity,148 but some successes have been achieved. Goodpaster et al. 149 found that severely obese people (mean BMI 44 kg/m2) lost on average 12 kg over 12 months by a combination of calorie restriction and physical activity, with the activity (moderate intensity equivalent to brisk walking for 300 minutes a week) having an additive effect to calorie restriction. Lazo et al. 150 also had good results in a study of diet and physical activity in 96 people with T2DM. The patients had MRS at baseline and after 12 months. NAFLD was defined as steatosis of over 5.5%. The patients aimed to achieve 10% weight loss by 12 months. The mean reduction in BMI was 2.7% (34.7 kg/m2 baseline, 32 kg/m2 at 12 months). The proportion with liver fat content under 5.5% rose from 67% at baseline to 80% at end, with an impressive fall in those with 10–20% steatosis from 17.4% at baseline to 6.5% at 12 months.
-
The usual caveats apply to both the Lazo and Goodpaster studies149,150 – 12 months is a short time in lifestyle change and the effect may not be sustained after the intervention ends.
Some issues
Defining ‘non-alcoholic’
Vuppalanchi and Chalasani151 have pointed out the lack of a consistent definition of the level of alcohol consumption compatible with a definition of NAFLD.
Progression to diabetes
The trials have been too short to assess whether or not treatment with any drugs will prevent progression to diabetes.
Which drug?
If there is to be a large trial, would insulin sensitisers be the best drug to test? Rosiglitazone would not be included, because its cardiovascular risk profile makes pioglitazone the drug of choice in the TZDs, and rosiglitazone has been withdrawn from use in the UK. However, pioglitazone is not without risk – oedema, heart failure, fractures. The safety of the glitazones was reviewed in a recent report published in the Health Technology Assessment journal. 84
Metformin, which has been used for more than 50 years, has been proven to be safe in those who can tolerate it – a small proportion have to stop because of gastrointestinal symptoms.
Evidence from the LEAD trials is that liraglutide is effective in improving NAFLD. Armstrong et al. ,141 so far, by abstract only, have reported that many patients in the LEAD trials had advanced NAFLD, but that liraglutide 1.8 mg reduced the NFS score. However, there were no data on the 1.2-mg dose in this abstract. The National Institute for Health and Clinical Excellence (NICE) in a recent appraisal concluded that the 1.8 mg was not recommended by NICE because the marginal benefits did not justify the large increase in marginal cost. 152 In passing, it should be noted that most of these patients were also on metformin, which casts doubt on its long-term effectiveness.
Two recent abstracts provide further information on the effects of GLP-1 analogues on NAFLD. Gardner et al. 153 in a small study gave an unspecified incretin mimetic to seven diabetic patients. Liver fat was reduced from mean 29% to 16%.
Horton et al. 154 pooled data from two DURATION (Diabetes therapy Utilization: Researching changes in A1c, weight and other factors Through Intervention with Exenatide ONce weekly) trials on 223 patients on once-weekly exenatide and reported improvements in ALT and AST, although values are not given in the abstract.
A trial in non-alcoholic fatty liver disease/steatosis
If a trial is restricted to the steatosis stage of NAFLD, diagnosis could be on the basis of clinical history (particularly around alcohol intake, history of hypertension, obesity, T2DM, hyperlipidaemia, cardiovascular diseases), clinical examination (BMI, hypertension or stigmata of other liver disease), laboratory tests (the usual LFTs plus exclusion of viral hepatitis, autoimmune disease, haemochromatosis, Wilson’s disease, and perhaps those with alpha-1 antitrypsin deficiency), plus some form of imaging to confirm the excess of fat in the liver, such as transabdominal ultrasound.
There would have to be long duration of follow-up because simple steatosis is fairly benign.
A trial in non-alcoholic steatohepatitis
The main problem with any trial in NASH is that at present we have insufficient knowledge that any other diagnostic test could replace liver biopsy. Various options such as panels of test could be assessed against liver biopsy (note that some panels are patented and affordability could be a problem). A NASH trial would also have to be long term (perhaps 10 years?). Assessment of diagnostic options would involve assessing surrogate markers to see how well they detected progression or regression of inflammatory infiltrates and fibrosis. It is worth noting that these are still surrogates whose importance is the risk of progression to liver failure or hepatocellular cancer.
The group in which intervention might be most likely to be cost-effective comprises those with NASH and fibrosis. End points would be similar to a NASH-alone group, but a trial would need smaller numbers. Unfortunately, to identify this group, we would currently need liver biopsy. Ultrasound elastography is becoming more available, but is less reliable in the more overweight patients, such as those with BMI > 30 or 35 kg/m2. (There is a special probe for the more obese, but it is currently expensive.)
As reported in Chapter 3, other options such as MRI elastography appear promising, but cost and availability would cause problems.
Hence the main research need appears to be to identify reliable and affordable non-invasive alternatives to liver biopsy. It may be that methods such as MRI elastography, although not being affordable in routine care, could be trialled against liver biopsy, and if reliable as assessed against the histological gold standard, could then be used in larger trials to assess the reliability of less expensive non-invasive methods.
A Health Technology Assessment trial?
An HTA trial may therefore be a combined diagnostic and therapeutic one, possibly with the following features:
-
It should be in a higher risk group, such as those with NASH.
-
Biopsy would be done, in a least a subgroup, and compared with other diagnostic options.
-
The drugs to be trialled might be pioglitazone versus a weekly GLP-1 agonist?
-
There should also be more research into how to promote adherence to weight loss.
The main cause of death in people with NAFLD is cardiovascular disease rather than cirrhosis.
Current trials
Searches for current trials have identified the following from the Clinical Trials Register. Several rosiglitazone trials are still registered, but will probably be discontinued.
Pioglitazone
NCT00633282
A Phase II RCT of pioglitazone 15 mg every day (q.d.) for 16 weeks, compared with a lifestyle arm (aerobic exercise and calorie restriction) and berberine, in patients with T2DM of no more than 1 year’s duration, and fatty liver confirmed by ultrasound. Start date March 2008, end date September 2011. Primary outcome measure is glycaemic control. Secondary outcomes include liver fat content by nuclear magnetic resonance spectroscopy.
NCT00681733
Pentoxifylline Versus Pioglitazone In Non-Alcoholic Steatohepatitis (NASH). This trial may have been completed because the estimated completion date was December 2008.
NCT00994682 (University of Texas Health Science Center, San Antonio, TX, USA; Pentoxifylline Versus Pioglitazone In Non-Alcoholic Steatohepatitis trial)
This is reported to be a Phase IV RCT of pioglitazone 30 mg per day orally for 8 weeks, increased if well tolerated, titrated to 45 mg per day until end of study (18 and 36 months), compared with placebo, in patients with or without T2DM. Start date December 2008, end date July 2013. Primary outcome measure includes liver histology at 18 and 36 months. Secondary outcomes include measurement of liver fat content by MRS at 18 and 36 months.
NCT01002547
This seems to be a similar Phase IV RCT of pioglitazone 30 mg per day for 8 weeks, if well tolerated titrated to 45 mg per day until end of study (next 3 years) in combination with vitamin E 400 IU orally twice daily, compared against placebo in combination with vitamin E 400 IU orally twice daily in patients with T2DM. Start date June 2010, end date July 2015. Primary outcome measure is liver histology at 18 and 36 months. Secondary outcomes include measurement of liver fat content by MRS at 18 and 36 months.
NCT01068444
A Phase II RCT of pioglitazone 30 mg per day for 6 months and then 3 months’ follow-up after treatment, compared against placebo in patients with the diagnosis of NASH with or without cirrhosis confirmed by liver biopsy. Start date April 2009, end date July 2012. Primary outcomes include comparison of steatosis and LFT at 9 months. Secondary outcomes include comparison of necroinflammation and fibrosis.
Metformin
NCT00063635 (Treatment of Nonalcoholic Fatty Liver Disease in Children; TONIC)
A Phase III RCT of metformin 500 mg twice daily compared with vitamin E 400 IU twice daily and matching dose of placebo, in patients with NAFLD confirmed by biopsy. Start date August 2005, end date April 2010 (last updated January 2010). Primary outcome measures include sustained reduction in ALT to either 50% of baseline value or < 40 U/l at 96 weeks. Secondary outcomes include reduction in AST, GGT, change in liver histology and change in insulin resistance indices.
NCT00081328 (TODAY; Treatment Options for type 2 Diabetes in Adolescents and Youth)
A Phase III RCT of metformin 1000 mg b.i.d. compared against metformin 100 mg b.i.d. + rosiglitazone 4 mg b.i.d. or metformin 1000 mg b.i.d. + behavioural TODAY lifestyle programme in patients aged 10–17 years with diabetes for < 2 years. Start date May 2004, end date February 2013. Primary outcome measures include loss of glycaemic control at 6 months. Secondary outcome measures include safety and other complications.
NCT00134303
A Phase IV RCT of metformin compared against placebo in patients receiving bariatric surgery for obesity. Start date June 2005, end date December 2010 (last updated June 2010). Primary outcome measures include number of patients with histological amelioration of NASH after 1 year. Secondary outcome measures include number of patients with normalisation of ALT and normalisation of steatosis on ultrasound after 1 year.
NCT00247117
An open-label, non-RCT of metformin in patients with NASH (types 2–4) and ALT level more than two times normal range. Start date January 2004, estimated to complete by August 2005 (last updated October 2006). Primary outcome includes histological and biochemical change in 1 year. Secondary outcome: none.
NCT00303537
A Phase II and III RCT of metformin compared against placebo in patients with histologically proven NAFLD of < 18 months. Start date November 2004, estimated to complete by June 2008 (last updated June 2007). Primary outcome measure is grade of steatosis during repeat biopsy at 6 months. Secondary outcome measures include grade of necroinflammation by repeat biopsy, liver density by computer scan and serum ALT level (all at 6 months).
NCT00736385
A Phase IV RCT of metformin 2000 mg (Glucophage) daily for 12 months compared against placebo 2000 mg daily in patients with biopsy-proven NAFLD within 12 months of study initiation. Start date April 2009, end date July 2011. Primary outcome measures include measurements of insulin sensitivity, hepatic insulin clearance, and altered parameters of lipid metabolism, changes in the histological features that define NAFLD, and quantitative measurements of visceral and peripheral fat during 24 months. Secondary outcome measures include insulin sensitivity and lipid metabolism during 24 months.
Conclusions
Given the number of pioglitazone and metformin studies listed above, the priority now is probably for trials involving newer agents such as the GLP-1 agonists, or possibly the less expensive and orally administered dipeptidyl peptidase-4 (DPP-4) inhibitors, the gliptins.
The most important question might be whether or not it is possible to prevent progression of NASH to fibrosis, but a study to determine that would be difficult because the timescale required would be uncertain (as we lack data on how many progress and over what timescale) and because of the need for liver biopsies to establish NASH and to monitor progression.
Acknowledgements
We would like to thank Lynn Robertson, NHS Grampian, for carrying out initial searches.
Contribution of authors
Chapter 1 was drafted by Nazim Ghouri, Chapter 2 by Deepson Shyangdan and Christine Clar, Chapter 3 by Rob Henderson, and Chapters 4 and 5 by Norman Waugh. Tara Gurung carried out literature searches. Data extraction of trials was carried out by Deepson Shyangdan and Christine Clar. David Preiss, Naveed Sattar and Andrew Fraser provided expert advice and comments on the full draft report. The section on research needs was drafted mainly by Andrew Fraser and Norman Waugh. All authors reviewed the draft final report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Nonalcoholic steatohepatitis clinical research network . Hepatology 2003;37.
- Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci 2009;116:539-64.
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434-8.
- McCullough AJ, Farrell GC, George J, de la M Hall P, McCullough AJ. Fatty liver disease: NASH and related disorders. Oxford: Blackwell; 2005.
- Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci 2008;115:141-50.
- Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol 2006;40:S11-16.
- Ahmed MH, Byrne CD, Byrne CD, D Wild S. Metabolic syndrome. Chichester: John Wiley & Sons; 2005.
- Propst A, Propst T, Zangerl G, Ofner D, Judmaier G, Vogel W. Prognosis and life expectancy in chronic liver disease. Dig Dis Sci 1995;40:1805-15.
- Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134-40.
- Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: Part I. Diagnosis and evaluation. Am Fam Physician 2006;74:756-62.
- Bedogni G, Miglioli L, Masutti F, Castiglione A, Croce LS, Tiribelli C, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology 2007;46:1387-91.
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343-51.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis 2007;11:75-104.
- London RM, George J. Pathogenesis of NASH: animal models. Clin Liver Dis 2007;11:55-74.
- Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA 2001;98:7522-7.
- McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 2006;40:S17-S29.
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413-19.
- Day CP, James OF. Steatohepatitis: a tale of two ‘hits’?. Gastroenterology 1998;114:842-5.
- Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party?. Hepatology 1998;27:1463-6.
- Dong W, Simeonova PP, Gallucci R, Matheson J, Fannin R, Montuschi P, et al. Cytokine expression in hepatocytes: role of oxidant stress. J Interf Cytok Res 1998;18:629-38.
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA 1997;94:2557-62.
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183-92.
- Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci 2004;106:261-8.
- Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol 2001;35:297-306.
- Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 2005;54:311-21.
- Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001;34:738-44.
- Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262-5.
- Muoio DM. Insulin resistance takes a trip through the ER. Science 2004;306:4285-426.
- Garcia-Monzon C, Martin-Perez E, Iacono OL, Fernandez-Bermejo M, Majano PL, Apolinario A, et al. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol 2000;33:716-24.
- Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci 2004;34:57-62.
- Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, et al. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol 2001;35:568-74.
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844-50.
- Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall’Agata M, et al. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology 2001;33:808-15.
- Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol 2001;96:2711-17.
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nature Med 2005;11:183-90.
- Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab 2010;12:365-83.
- Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J 2008;411:1-18.
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43:S99-112.
- Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology 2005;129:375-8.
- Friedman SL, Schiff ER, Sorrell MF, Maddrey WC. Schiff’s diseases of the liver. Philadelphia, PA: Lippincott-Raven; 1999.
- Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 2003;38:420-7.
- Gaiani S, Avogaro A, Bombonato GC, Bolognesi M, Amor F, Vigili de Kreutzenberg S, et al. Nonalcoholic fatty liver disease (NAFLD) in nonobese patients with diabetes: Prevalence and relationships with hemodynamic alterations detected with Doppler sonography. J Ultrasound 2009;12:1-5.
- Preiss D, Sattar N. Metabolic syndrome, dysglycaemia and vascular disease: making sense of the evidence. Heart 2007;93:1493-6.
- Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007;49:403-14.
- McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol 2002;34:255-62.
- Sattar N, Scherbakova O, Ford I, O’Reilly DS, Stanley A, Forrest E, et al. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes 2004;53:2855-60.
- Sattar N, McConnachie A, Ford I, Gaw A, Cleland SJ, Forouhi NG, et al. Serial metabolic measurements and conversion to type 2 diabetes in the west of Scotland coronary prevention study: specific elevations in alanine aminotransferase and triglycerides suggest hepatic fat accumulation as a potential contributing factor. Diabetes 2007;56:984-91.
- Sattar N, Wannamethee SG, Forouhi NG. Novel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilities?. Diabetologia 2008;51:926-40.
- Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008;51:1781-9.
- Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology 2003;124:248-50.
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387-95.
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990;12:1106-10.
- Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 2003;289:3000-4.
- Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004;40:820-6.
- Whalley S, Puvanachandra P, Desai A, Kennedy H. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med 2007;7:119-24.
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of Nonalcoholic Fatty Liver Disease and Its Association With Cardiovascular Disease Among Type 2 Diabetic Patients. Diabetes Care 2007;30:1212-18.
- Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol 2005;16:421-7.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221-31.
- Day CP. Non-alcoholic fatty liver disease: current concepts and management strategies. Clin Med 2006;6:19-25.
- Williamson RM, Price JF, Glancy S, Butcher I, Perry E, Nee LD, et al. Prevalence of and risk factors for hepatic steatosis and non-alcoholic fatty liver disease in type 2 Diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes 2009;58.
- Gonzalez ELM, Johansson S, Wallander MA, Rodriguez LAG. Trends in the prevalence and incidence of diabetes in the UK: 1996–2005. J Epidemiol Community Health 2009;63:332-6.
- Loria P, Lonardo A, Bellentani S, Day CP, Marchesini G, Carulli N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: An open question. Nutr Metab Cardiovasc Dis 2007;17:684-98.
- Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men. Diabetes Care 2004;27:2498-500.
- Ghouri N, Preiss D, Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology 2010;52:1156-61.
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341-50.
- Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia 2010;53:1341-8.
- Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002;123:1705-25.
- NIHR Health Technology Assessment programme . Non-Invasive Methods for the Assessment and Monitoring of Liver Fibrosis and Cirrhosis 2011. www.hta.ac.uk/search/results.asp?Action=Search%26QU=09%2F07%26go=Go (accessed 1 January 2011).
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology 2000;118:1117-23.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356-62.
- Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 1990;99:1408-13.
- Park HS, Kim MW, Shin ES. Effect of weight control on hepatic abnormalities in obese patients with fatty liver. J Korean Med Sci 1995;10:414-21.
- Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 1997;27:103-7.
- Harrison SA, Fincke C, Helinski D, Torgerson S, Hayashi P. A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Aliment Pharmacol Ther 2004;20:623-8.
- Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. IJG 2004;23:131-4.
- Sabuncu T, Nazligul Y, Karaoglanoglu M, Ucar E, Kilic FB. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol 2003;12:189-92.
- Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut 2007;56:1760-9.
- Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010;52:79-104.
- Lawlor DA, Sattar N, Smith GD, Ebrahim S. The associations of physical activity and adiposity with alanine aminotransferase and gamma-glutamyltransferase. Am J Epidemiol 2005;161:1081-8.
- Martens FM, Visseren FL, Lemay J, de Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs 2002;62:1463-80.
- Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. N England J Med 2007;357:28-3.
- Zib I, Jacob AN, Lingvay I, Salinas K, McGavock JM, Raskin P, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 2007;55:230-6.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457-71.
- Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14.
- Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology 2006;44:466-71.
- Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol 2007;47:135-41.
- Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:1082-90.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003;98:2485-90.
- Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology 1996;23:1464-7.
- Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2006;4:1537-43.
- Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004;40:1222-5.
- Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis 2007;16:39-46.
- Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2008;6:1396-402.
- Liu X, Lazenby AJ, Clements RH, Jhala N, Abrams GA. Resolution of nonalcoholic steatohepatits after gastric bypass surgery. Obesity Surgery 2007;17:486-92.
- Furuya CK, Jr, de Oliveira CP, de Mello ES, Faintuch J, Raskovski A, Matsuda M, et al. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol 2007;22:510-14.
- Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 2004;39:1647-54.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obesity Surgery 2006;16:1278-86.
- Kushner RF, Noble CA. Long-term outcome of bariatric surgery: an interim analysis. Mayo Clin Proc 2006;81:S46-51.
- Chekhov AP. Chekhov: five major plays. New York, NY: Bantam Books; 1982.
- Torres DM, Jones FJ, Williams CD, Harrison S. The effect of 48 weeks of rosiglitazone alone versus combination rosigilitazone and metformin (avandamet) versus combination rosiglitazone and losartan in the treatment of nonalcohoholic steatohepatitis (NASH): a open-label prospective randomized clinical trial. Hepatology 2009;50.
- Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135:1176-84.
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297-307.
- Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2004;2:1107-15.
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-85.
- Garinis GA, Fruci B, Mazza A, De SM, Abenavoli S, Gulletta E, et al. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes 2010;34:1255-64.
- Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol 2009;44:853-60.
- Nadeau KJ, Ehlers LB, Zeitler PS, Love-Osborne K. Treatment of non-alcoholic fatty liver disease with metformin versus lifestyle intervention in insulin-resistant adolescents. Pediatr Diabetes 2009;10:5-13.
- Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol 2009;46:113-18.
- Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Ther Adv Gastroenterol 2009;2:157-63.
- Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2004;19:537-44.
- Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) Trial. Gastroenterology 2008;135:100-10.
- Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, et al. Clinical trial: Insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2008;8:200-8.
- Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2010;22:18-23.
- Angelico F, Burattin M, Alessandri C, Del BM, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev 2007;1.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467-74.
- Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21.
- Haukeland-JW KZLE. A randomized, placebo controlled trial with metformin in patients with NAFLD. Hepatology 2008;48.
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies?. Arch Dis Child 2008;93:458-61.
- Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Sanchez-Avila F, Montano-Reyes MA, Uribe M. Insulin sensitizers in treatment of nonalcoholic fatty liver disease: systematic review. World J Gastroenterol 2006;12:7826-31.
- Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr 2009;48:587-96.
- Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics 2009;29:231-60.
- Fabbrini E, Conte C, Magkos F. Methods for assessing intrahepatic fat content and steatosis. Curr Opin Clin Nutr Metab Care 2009;12:474-81.
- Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009;51:433-45.
- Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol 2009;25:230-7.
- Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis 2010;42:272-82.
- Myers RP. Noninvasive diagnosis of nonalcoholic fatty liver disease. Ann Hepatol 2009;8:S25-33.
- Browning JD. New imaging techniques for non-alcoholic steatohepatitis. Clin Liver Dis 2009;13:607-19.
- Gaidos JK, Hillner BE, Sanyal AJ. A decision analysis study of the value of a liver biopsy in nonalcoholic steatohepatitis. Liver Int 2008;28:650-8.
- Ali R, Cusi K. New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann Med 2009;41:265-78.
- CASP . 10 Questions to Help You Make Sense of Reviews 2010. www.sph.nhs.uk/sph-files/S.Reviews%20Appraisal%20Tool.pdf/?searchterm=review (accessed 15 June 2010).
- SIGN . Methodology Checklist 1: Systematic Reviews and Meta-Analyses 2010. www.sign.ac.uk/guidelines/fulltext/50/checklist1.html (accessed 15 June 2010).
- Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 2007;11.
- Ma X, Holalkere NS, Kambadakone RA, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics 2009;29:1253-77.
- Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis 2008;28:386-95.
- Castera L. Non-invasive diagnosis of steatosis and fibrosis. Diabetes Metab 2008;34:674-9.
- Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol 2008;5:95-106.
- Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, et al. Biomarkers of liver fibrosis. Adv Clin Chem 2008;46:131-60.
- Halfon P, Munteanu M, Poynard T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol Clin Biol 2008;32:22-39.
- The Princess Grace Hospital . Patient Information on the FibroScan 2010. www.fibroscan.co.uk/fibroscan_leaflet.pdf (accessed 21 December 2010).
- Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately?. J Hepatol 2009;50:17-35.
- Armstrong MJ, Falahati A, Braun OO, Schmidt WE, Gough S, Newsome PN. High prevalence of advanced NAFLD in type 2 diabetic patients with normal liver enzymes and effect of liraglutide on NAFLD: meta-analysis of the LEAD program. Diabetes 2010;59.
- Baumeister SE, Volzke H, Marschall P, John U, Schmidt CO, Flessa S, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology 2008;134:85-94.
- Younossi ZM, Singer ME. Lifetime medical and economic impact of patients with non-alcoholic fatty liver disease (NAFLD) in the United States (US). Hepatology 2006;44.
- Dan AA, Kallman JB, Wheeler A, Younoszai Z, Collantes R, Bondini S, et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2007;26:815-20.
- David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009;49:1904-12.
- Kistler KD, Molleston J, Unalp A, Abrams SH, Behling C, Schwimmer JB. Symptoms and quality of life in obese children and adolescents with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2010;31:396-40.
- Kerkar N, Kochin IN, Miloh TA, Arnon R, Suchy FJ, Annunziato R. A pilot study to assess quality of life in children with nonalcoholic fatty liver disease. Hepatology 2008;48.
- Ryan DH, Kushner R. The state of obesity and obesity research. JAMA 2010;304:1835-6.
- Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010;304:1795-802.
- Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156-63.
- Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology 2009;49:306-17.
- Cummins E, Royle P, Shyangdan D, Waugh N. Evidence Review: Liraglutide for the Treatment of Type 2 Diabetes: A Single Technology Appraisal. 2010. www.hta.ac.uk/erg/reports/2157.pdf (accessed 20 December 2010).
- Gardner CJ, Irwin A, Goenka N, Furlong N, Adams V, Kemp G, et al. The effect of incretin mimetics on liver fat in type 2 diabetes. Diabet Med 2011;28.
- Horton E, Walsh B, Han J, Lutz K, Taylor K, Magg D. Improvements in glycaemic control, body weight and markers of cardiovascular risk and hepatic injury are demonstrated after 52 weeks’ treatment with exenatide once weekly. Diabet Med 2011;28.
- Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004;39:188-96.
- Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med 1983;139:43-50.
Appendix 1 Search strategy
Literature search strategy
For the introduction
-
We checked NICE, HTA, SIGN and NHS evidence.
-
Searched MEDLINE for general reviews.
-
Aetiology, pathology, natural history – searched MEDLINE, limits clinical queries.
-
Checked guidelines from the following professional bodies:
-
– American Gastroenterological Association
-
– American Association for the Study of the Liver; The British Association for the Study of the Liver
-
– The British Society of Gastroenterology
-
– The European Association for the Study of the Liver.
-
Epidemiology (prevalence and incidence)
-
MEDLINE 1990 – current and EMBASE 1990 – current.
-
Non-alcoholic fatty liver disease terms plus (incidence or prevalence).tw.
-
Web of Science for abstracts.
-
Google search for grey literature.
Ovid MEDLINE(R) 1996 to week 3 August 2009
-
exp *fatty liver/
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty and liver).tw.
-
3 and 2
-
steato*.tw.
-
2 and 5
-
(NAFL or NAFLD or NASH).tw.
-
6 or 4 or 1 or 7
-
limit 8 to (English language and yr=“1999 -Current” and “diagnosis (specificity)”).
Clinical effectiveness search strategy
Ovid MEDLINE(R) 1950 to week 2 June 2010
-
fatty liver/
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFLD or NAFLD or NASH).tw.
-
4 and 3 and 2
-
6 or 1 or 5
-
exp Metformin/
-
exp Thiazolidinediones/
-
(insulin sensit* or metformin or pioglitazone or rosiglitazone or thiazolidinedione* or glitazone*).tw.
-
8 or 9 or 10
-
7 and 11.
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations, 22 June 2010
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFLD or NAFLD or NASH).tw.
-
1 and 2 and 3
-
4 or 5
-
(insulin sensit* or metformin or pioglitazone or rosiglitazone or thiazolidinedione* or glitazone*).tw.
-
6 and 7.
EMBASE 1980 to 2010 week 24
-
*fatty liver/
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFLD or NAFLD or NASH).tw.
-
2 and 3 and 4
-
1 or 5 or 6
-
exp metformin/
-
exp thiazolidinediones/
-
(insulin sensit* or metformin or pioglitazone or rosiglitazone or thiazolidinedione* or glitazone*).tw.
-
8 or 9 or 10
-
7 and 11.
Science Citation Index – Expanded plus Conference Proceedings Citation Index – Science, 1970 to June 2010
The following conferences were also covered:
-
American Gastroenterological Association (Gastroenterology)
-
American Association for the Study of Liver Diseases (Hepatology)
-
European Association for the Study of the Liver (J Hepatol)
-
American College of Gastroenterology (Am J Gastroenterol).
-
TS=liver
-
TS=(non-alcoholic or non alcoholic or ‘nonalcoholic’)
-
TS=(fatty or steato*)
-
#3 and #2 and #1
-
TS=(NAFL or NAFLD or NASH)
-
#5 OR #4
-
TS= (insulin sensit* or metformin or piolitazone or rosiglitazone or thiazolidinedione*)
-
#7 AND #6
-
#7 AND #6 Refined by: Document Type=(MEETING ABSTRACT).
Cochrane
Up to June 2010
-
(liver):ti,ab,kw and (non-alcoholic or non alcoholic or nonalcoholic):ti,ab,kw and (fatty or steato*):ti,ab,kw
-
(NAFL or NASH or NAFLD):ti,ab,kw
-
(#1 OR #2)
-
MeSH descriptor Metformin explode all trees
-
MeSH descriptor Thiazolidinediones explode all trees
-
(insulin sensit* or metformin or pioglitazone or rosiglitazone or glitazone* or thiazolidinediones*):ti,ab,kw
-
(#4 OR #5 OR #6)
-
(#3 AND #7).
Search alerts
Search alerts were set up in MEDLINE and EMBASE to run daily.
Diagnostics search strategy
Ovid MEDLINE(R) 1950 to June 2010
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFL or NAFLD or NASH).tw.
-
exp *Diagnosis/
-
diagnos*.tw.
-
Fatty Liver/di [Diagnosis]
-
1 and 3 and 2
-
8 or 4
-
6 or 5
-
10 and 9
-
11 or 7
-
limit 12 to English language.
June 2010
EMBASE 1980 to June 2010
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFL or NAFLD or NASH).tw.
-
exp *Diagnosis/
-
diagnos*.tw.
-
Fatty Liver/di [Diagnosis]
-
1 and 3 and 2
-
8 or 4
-
6 or 5
-
10 and 9
-
11 or 7
-
limit 12 to English language.
Cost effectiveness search strategy
Ovid MEDLINE(R) 1950–2010
-
Fatty Liver/
-
liver.tw.
-
(non-alcoholic or non alcoholic or nonalcoholic).tw.
-
(fatty or steato*).tw.
-
(NAFLD or NAFLD or NASH).tw.
-
2 and 3 and 4
-
1 or 5 or 6
-
“Costs and Cost Analysis”/
-
“Cost of Illness”/
-
exp Economics/
-
exp Health Status/
-
exp “Quality of Life”/
-
Quality-Adjusted Life Years/
-
(health state* or health status).tw.
-
(qaly$or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36).tw.
-
(markov or time trade off or standard gamble or hrql or hrqol or diabilit$).tw.
-
(quality adj2 life).tw.
-
(decision adj2 model).tw.
-
(pharmacoeconomics$or pharmaco-economic$or economics$or cost-effective* or cost benefit).tw.
-
(utilit* adj3 (cost* or analys* or score* or health or value* or assessment*)).tw.
-
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
-
7 and 21.
Web of Science 2010 (meeting abstracts)
TS=liver
TS=(non-alcoholic or nonalcoholic or ‘non alcoholic’)
TS=(fatty or steato*)
#3 AND #2 AND #1
TS=(NAFL or NAFLD or NASH)
#5 OR #4
TS=quality of life
TS=(pharmacoeconomic* or pharmaco-economic* or economic* or cost-effective* or cost–benefit or decision model or health state* or health status)
TS= (qaly$or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or decision model or utilit*)
#9 OR #8 OR #7
#10 AND #6
#10 AND #6 Refined by: Document Type=(MEETING ABSTRACT).
Appendix 2 Characteristics of included studies
| Study/design | Participants | Interventions | Outcome measures |
|---|---|---|---|
| Pioglitazone | |||
|
Aithal 2008 101 Country: UK Focus: effect of pioglitazone in non-diabetic participants with NASH Design: randomised, double-blind, placebo-controlled trial Multicentre: two hospitals (Nottingham, Derby) Duration: 1 year Follow-up: no post-intervention follow-up |
Total number: 74 n Pio: 37; 31 completed n Pbo: 37; 30 completed Inclusion criteria: age 18–70 years, NASH Diagnosis: liver biopsy (standard protocol) and ultrasound; NASH: histology, presence of fat plus evidence of hepatocyte injury and inflammation or fibrosis Definition of non-alcoholic: Men < 210 g/week, women < 140 g/week IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: alcohol excess, other liver diseases, diabetes mellitus, only hepatic steatosis, weight-reduction medication, pregnant or lactating women, current or previous heart failure, renal impairment; taking drugs associated with fatty liver, e.g. methotrexate, amiodarone, tamoxifen, valproate, etc.); diabetes mellitus diagnosed before or at the time of recruitment Age: Pio: 52 years (28–71 years); Pbo: 55 years (27–73 years) Sex: Pio: 30% female; Pbo: 49% female BMI: Pio: 29.8 kg/m2, SD 3.0; Pbo: 30.8 kg/m2, SD 4.1 Ethnicity: NR Fibrosis: (Brunt scores) Pio: none: 26%, 1; 7%, 2; 45%, 3; 16%, 4; 6% Pbo: none: 17%, 1; 7%, 2; 40%, 3; 23%, 4; 13% Stage/severity of NAFLD: NASH (see Results section for detailed Brunt scores) Previous treatment: NR Comorbidities: NR |
Pio: 30 mg/day pioglitazone Pbo: placebo tablets Both groups: diet/exercise advice Adherence: 1/61 missed 3.3% of tablets, 60/61 were > 99% adherent; 18/30 in placebo group and 14/31 in pioglitazone group fully compliant with lifestyle advice, the rest partly compliant Run-in period: 3 months, seen by a dietician, instructed to reduce calorie intake by 500 kcal/day and to perform modest exercise 30–40 minutes per day at least 5 days/week Co-interventions/co-medications: four patients on lipid-lowering drugs (stable dose for 3 months); advice on diet/exercise reinforced at each follow-up visit |
Primary: liver histology Progression/regression of liver disease: liver histology; liver biopsy specimens graded according to the NASH histological scoring system (Promrat et al. 2004,155 Brunt et al. 1999115) IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: yes FPG: yes Weight change: weight, BMI, waist–hip ratio Blood pressure: diastolic, systolic BP Lipid parameters: TC, HDL, LDL, TGs Liver biochemistry: bilirubin, albumin, ALT, GGT, ferritin Insulin resistance: yes, HOMA Health-related QoL: no Mortality: no Other: C-peptide, fasting insulin, adiponectin, leptin, resistin Timing of assessment: at 2-month intervals throughout the study |
|
Belfort 2006 102 Country: USA Focus: effect of pioglitazone plus a calorie-restricted diet in participants with NASH Design: randomised, double-blind, placebo-controlled trial Multicentre: three centres in San Antonio, TX (University Health Centre; Audie L Murphy Division, South Texas Veterans Health Care System; Brooke Army Medical Center) Duration: 6 months Follow-up: 4 weeks |
Total number: 55 with impaired glucose tolerance or diabetes enrolled; control group of 10 healthy participants with normal glucose tolerance and without fatty liver n Pio: 26; 23 completed n Pbo: 21; 17 completed Inclusion criteria: NASH Diagnosis: liver biopsy (standard clinical indications), medical history, physical examination, routine blood tests Definition of non-alcoholic: > 12–15 g of alcohol per day, or > 12 oz of beer, 5 oz of wine, or 1.5 oz of distilled spirits IGT/diabetes (including diagnostic criteria): impaired glucose tolerance or T2DM (75-g oral glucose tolerance test) Exclusion criteria: levels of plasma ALT and AST ≥ 2.5 upper limit of normal; FPG ≥ 13.3 mmol/l; type 1 diabetes, heart disease, hepatic disease (other than NASH), renal disease; receiving metformin, TZDs or insulin Age: Pio: 51 years, SD 7.0; Pbo: 51 years, SD 10.0 Sex: Pio: 46% female; Pbo: 67% female BMI: Pio: 33.5 kg/m2, SD 4.9; Pbo: 32.9 kg/m2, SD 4.4 Ethnicity: NR Fibrosis: (Kleiner scores) Pio: none: 8%, 1; 46%, 2; 19%, 3; 27%, 4; 0% Pbo: none: 28.5%, 1; 43%, 2; 19%, 3; 9.5%, 4; 0% Stage/severity of NAFLD: NASH (see Results section for detailed Kleiner scores) Diabetes duration: NR Previous treatment: NR Comorbidities: NR |
Pio: 30 mg/day pioglitazone for the first 2 months and then increased to 45 mg/day until the end of the study Pbo: placebo pills Both groups: diet (reduce calorie intake by 500 kcal per day) Adherence: assessed by means of a pill count on follow-up visits Run-in period: 4 weeks, interviewed by research dietician, instructed not to change the calorie content of their diet or their level of physical activity Co-interventions/co-medications: advice on diet reinforced at each follow-up visit |
Primary: liver histology Progression/regression of liver disease: liver histology; liver biopsy specimens graded according to the NASH histological scoring system (Kleiner et al. )116 IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: yes FPG: yes Weight change: weight, BMI, fat (%) Blood pressure: no Lipid parameters: TC, HDL, LDL, TGs Liver biochemistry: ALT, AST Health-related QoL: no Insulin resistance/sensitivity: yes Mortality: no Other: hepatic fat content (MRS), whole body fat, lipid insulin, free fatty acids, cytokine, adiponectin concentrations, tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), insulin secretion, endogenous glucose production, and the rate of glucose disappearance after a 75-g oral glucose load (oral glucose tolerance test) Timing of assessment: metabolic variables assessed at baseline at the end of the study, liver biopsy at baseline and after 6 months; every 2 weeks participants seen at the general clinic and vital signs, physical examination, the results of home glucose monitoring, compliance with the study drug (confirmed by pill count) and adverse events were assessed |
|
Sanyal 2004 103 Country: USA Focus: effect of insulin sensitiser (pioglitazone) along with an antioxidant (vitamin E) in participants with NASH Design: prospective RCT, single centre Duration: 6 months Run-in before randomisation: 3 months Treatment: 6 months Follow-up: no post-intervention follow-up |
Total number: 20 n Pio + Vit E: 10; 8 completed n Vit E: 10; all completed Inclusion criteria: NASH Diagnosis: liver biopsy; included if macrovesicular steatosis, and (1) ≥ 1 of the following: cytological ballooning, Mallory’s hyaline, pericellular fibrosis, and (2) varying degrees of inflammation and portal fibrosis Definition of non-alcoholic: non-alcoholic induced nature of the disease established by clinical interview of the participants IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: age < 18 years; diabetes mellitus; cirrhosis; weight gain or loss of > 5 lb in past month; severe comorbid conditions limiting life expectancy to < 1 year; pregnancy; symptomatic gallstone disease; those being considered for or who had bariatric surgery; iatrogenic NASH; concomitant presence of other causes of liver disease (e.g. hepatitis C); participants on stable doses of drugs for hyperlipidaemia for > 6 months could continue, those requiring dose modification or starting drugs within this time frame were excluded Age: Pio + Vit E: 47.0 years, SD 12.0; Vit E: 46.0 years, SD 13.0 Sex: Pio + Vit E: 40% female; Vit E: 60% female BMI: Pio + Vit E: 32.5 kg/m2, SD 4.3; Vit E: 30.7 kg/m2, SD 4.7 Ethnicity: all Caucasians Fibrosis: Pio + Vit E: (from graph) pericellular fibrosis grade 1.3, SD 0.3; portal fibrosis grade 1, SD 0.3 Vit E: (from graph) pericellular fibrosis grade 1.2, SD 0.3; portal fibrosis grade 0.7, SD 0.3 Stage/severity of NAFLD: NASH (details see results section) Previous treatment: drugs for hyperlipidaemia |
Pio + Vit E: pioglitazone 30 mg/day + Vitamin E 400 IU orally every day Vit E: Vitamin E 400 IU orally every day Both groups: standardised recommendations about diet and exercise Adherence: not reported Run-in period: followed up for 3 months prior study to ensure that everyone had a stable weight and had been given similar diet and exercise prescriptions. Co-interventions/co-medications: participants receiving stable doses of drugs for hyperlipidaemia for more than 6 months continued to take these |
Primary: improvement in liver histology Progression/regression of liver disease: liver histology; liver biopsy specimens graded according to the NASH histological scoring system (modified Brunt scores) IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: no FPG: no Weight change: BMI, waist–hip ratio, fat (%) Blood pressure: no, (hypertension) Lipid parameters: TC, HDL, TG Liver biochemistry: ALT, AST, bilirubin Health-related QoL: no Insulin resistance/sensitivity: metabolic clearance of glucose measured by glucose infusion rate during low-dose insulin infusion, fasting insulin level and fasting free fatty acid level Mortality: no Other: metabolic end points included: overall insulin sensitivity; sensitivity of lipolysis to insulin; lipid and carbohydrate oxidation rates; mitochondrial fatty acid oxidation; hepatic glucose output and its sensitivity to insulin Timing of assessment: after randomisation, seen initially at week 2 and then at week 4, subsequently seen at months 3 and 6. Laboratory studies were obtained at monthly intervals and reviewed by the principal investigator. End of the study, participants underwent a euglycaemic hyperinsulinaemic clamp and then after 1–2 weeks a liver biopsy examination was performed |
|
Sanyal 2010 104 Country: USA Focus: effects of TZDs and vitamin E in NASH Design: multicentre, randomised, placebo-controlled, double-masked clinical trial Centre: multicentre Duration: 96 weeks Follow-up: additional 24 weeks |
Total number: 247 randomised n Pio: 80; 70 completed n Vit E: 84; 80 completed n Pbo: 83; 72 completed Inclusion criteria: ≥ 18 years; liver biopsy obtained within 6 months before randomisation Diagnosis: liver biopsy; definite or possible NAS of ≥ 5, or definite steatohepatitis (confirmed by two pathologists) with NAS of 4, a score of at least 1 for hepatocellular ballooning was required in all cases Definition of non-alcoholic: alcohol consumption of more than 20 g per day in women and more than 30 g in men of at least three consecutive months during the previous 5 years, as assessed with the use of the Lifetime Drinking History questionnaire of Skinner et al. and the self-administered Alcohol Use and Disorders Identification Test (AUDIT) IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: adults with diabetes; alcohol consumption as given above; cirrhosis, hepatitis C or other liver diseases, heart failure (NYHA class II or IV), receiving drugs known to cause steatohepatitis Age: Pio: 47.0 years, Vit E: 46.6 years, Pbo: 45.5 years Sex: Pio: 58.8% female, Vit E: 61.9% female, Pbo: 57.8% female BMI: Pio: 34.0 kg/m2, Vit E: 34 kg/m2, Pbo: 35 kg/m2 Ethnicity: Hispanic Pio: 18.8%, Vit E: 19.0%, Pbo: 7.2%; Non-White Pio: 18.8%, Vit E: 15.2%, Pbo: 11.0% Fibrosis: Pio: 86.2%, Vit E: 83.1%, Pbo: 80.7% Stage/severity of NAFLD: see Results section Previous treatment: NR Comorbidities: NR |
Pio: pioglitazone 30 mg once daily Vit E: 800 IU, natural form, once daily Pbo: placebo identical to Pio once daily or placebo identical to Vitamin E Both groups: all participants were given a standardised set of pragmatic recommendations about lifestyle changes and diet; discouraged from adding other drugs that are used for nonalcoholic steatohepatitis to their regimen Adherence: NR Run-in period: participants were not allowed to use any prescription or over-the-counter medication or herbal remedy taken with an intent to improve or treat NASH or liver disease or obesity or diabetes for the 3 months before liver biopsy as well as the 3 months before randomisation Co-interventions/co-medications: NR |
Primary: Improvement in histological findings, which required an improvement by 1 or more points in the hepatocellular ballooning score; no increase in the fibrosis score; and either a decrease in the activity score for NAFLD to a score of 3 or less or a decrease in the activity score of at least 2 points, with at least a 1-point decrease in either the lobular inflammation or steatosis score Progression/regression of liver disease: liver biopsy (analysis based on Kleiner scores) IGT → diabetes: no NGT → IGT/diabetes: yes HbA1c: no FPG: yes Weight change: weight, BMI, waist circumference, waist–hip ratio, triceps skinfold, mid-upper arm circumference, trunk (% fat), total (% fat) Blood pressure: no Lipid parameters: TG, TC, HDL, LDL Liver biochemistry: ALT, AST, GGT, bilirubin, alkaline phosphatase Health-related QoL: yes Insulin resistance/sensitivity: HOMA–IR Mortality: yes Timing of assessment: Participants were seen at 8 weeks (2-month intervals) after randomisation through 96 weeks; a follow-up liver biopsy was conducted at the week 96 visit |
| Metformin | |||
|
Bugianesi 2005 87 Country: Ankara, Turkey Focus: effect of metformin, prescriptive diet or vitamin E in participants with NAFLD Design: open-label RCT Multicentre: two units – Bologna (BU) and Turin (TU), Italy Duration: 12 months Follow-up: no post-intervention follow-up |
Total number: 110 participants with NAFLD n Met: 55; 55 completed n Vit E: 28; 28 completed n diet: 27; 27 completed Inclusion criteria: NAFLD Diagnosis: liver biopsy (Brunt criteria with minor modifications) Definition of non-alcoholic: alcohol consumption < 20g/day IGT/diabetes (including diagnostic criteria): oral glucose tolerance test Met: IGT: Bologna 4%, Turin15%; diabetes: Bologna 10%, Turin 12% Vit E: IGT: 7%; diabetes: 4% Diet: IGT: 7%; diabetes: 4% Exclusion criteria: evidence of advanced clinical or biochemical liver disease or cirrhosis, alcohol consumption > 20 g/day, positive screening for viral hepatitis B and C, autoimmune phenomena indicating autoimmune hepatitis or celiac disease, presence of gene markers of familial haemochromatosis, previously diagnosed diabetes, severe or morbid obesity (≥ 35 kg/m2) Age: Met: Bologna 42 years, SD 10.0, Turin 45 years, SD 10.0; Vit E: 40 years, SD 10.0; Diet: 41 years, SD 10.0 Sex: Met: Bologna 24% female, Turin 31% female; Vit E: 0% female; Diet: 15% female (p = 0.006) BMI: Met: 28.7 kg/m2, SD 3.6; Vit E: 29.1 kg/m2, SD 2.7; Diet: 28.2 kg/m2, SD 3.6 Ethnicity: NR Fibrosis: Met: 93%; Vit E: 91%; Diet: 77% Stage/severity of NAFLD: NASH index (details see Results section) Previous treatment: NR |
Met: metformin daily maximum dose of 2000 mg/day, the dose was progressively increased from 250 mg b.i.d. to the maximum dose at 500-mg weekly intervals Vit E: vitamin E 400 IU b.i.d. Diet: weight reducing prescriptive diet to determine a caloric deficit of 500 kcal per day (determining a weight loss of approximately 500 g/week) All groups: 2 hours nutritional counselling by an experienced dietician on the basis of a dietary investigation; all participants encouraged to walk or to jog at least 30 minutes daily Adherence: compliance tested by pill counts in participants on metformin and vitamin E; in participants on prescriptive diet only weight loss was considered as a compliance marker; compliance always exceeded 90% of prescribed dose Run-in period: none Co-interventions/co-medications: NR |
Primary: ALT normalisation Progression/regression of liver disease: liver histology IGT → diabetes: no NGT → IGT/diabetes: change in proportion of participants with metabolic syndrome HbA1c: yes FPG: yes Weight change: BMI, waist–hip ratio Blood pressure: diastolic and systolic BP Lipid parameters: TC, HDL, TGs Liver biochemistry: ALT level, AST level, GGT, alkaline phosphatase Health-related QoL: no Mortality: no Other: insulin resistance (HOMA), assessment of iron status (serum iron, transferrin saturation, ferritin), lactic acid Timing of assessment: biochemical and clinical control visits every 3 months; second liver biopsy only on 17 metformin-treated participants and in none of the two control groups |
|
Garinis 2010 105 Country: Italy Focus: comparison of the efficacy of low-dose metformin plus dietary treatment vs dietary treatment alone Design: prospective, randomised study Centre: Endocrine Unit of University Magna Graecia of Cantanzaro for treatment of obesity and overweight Duration: 6 months Follow-up: no post-intervention follow-up |
Total number: 50 participants n Met: 20; 15 completed n C: 25; 25 completed Inclusion criteria: BMI > 25 kg/m2, liver steatosis Diagnosis: USG Definition of non-alcoholic: alcohol intake < 20 g/day Exclusion criteria: participants with evidence of heart disease, renal failure and diabetes, even if newly discovered, smoking habits, alcohol intake of more 20 g per day, signs of hepatic virus infection (participants were tested for hepatitis B antigen or hepatitis C antibodies), the presence of clinical or biochemical evidence of autoimmune, metabolic or genetic liver diseases and use of drugs known to induce liver steatosis Age: Met: 40.8 years, SD 13; C: 45.8 years, SD 13.6 Sex: Met: 90% female; C: 80% female BMI: Met: 36.5 kg/m2, SD 4. 9; diet alone: 34.7 kg/m2, SD 2.5 Ethnicity: NR Fibrosis: NR Stage/severity of NAFLD: see Results section Previous treatment: NR Comorbidities: NR |
Met: Metformin was started at a dose of 250 mg b.i.d. and was increased after a week to the final dose of 500 mg b.i.d. C: dietary treatment alone Both groups: hypocaloric (1300 kcal) diet Adherence: NR Run-in period: no Co-interventions/co-medications: none |
Primary: liver steatosis amelioration or disappearance, as evaluated by USG Progression/regression of liver disease: USG (liver steatosis graded as mild, moderate and severe: mild, mild increase in liver echogenicity; moderate, increased liver echogenicity that obscured hepatic and portal vein walls; severe, posterior attenuation of the deep liver parenchyma) IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: no FPG: yes Weight change: BMI, waist–hip ratio, waist circumference (cm) Blood pressure: no Lipid parameters: TC, HDL, TGs, Liver biochemistry: ALT level, AST level, alkaline phosphatase Health-related QoL: no Mortality: no Other: Insulin resistance (HOMA), HOMA–IR, assessment of iron status (serum iron, transferrin, ferritin concentration) Timing of assessment: once in every 8 weeks |
|
Haukeland 2009 106 Country: Norway Focus: effect of metformin on liver histology in participants with NAFLD Design: randomised, double-blind, placebo-controlled trial Multicentre: Four University hospitals Duration: 6 months Follow-up: no post-intervention follow-up |
Total number: 48 n Met: 24; 20 completed n Pbo: 24; 24 completed Inclusion criteria: adults with NAFLD Diagnosis: histologically verified NAFLD within 18 months prior to inclusion; participants with simple steatosis where included if they had elevated transaminases (> ULN) and impaired glucose tolerance or T2DM Definition of non-alcoholic: alcohol consumption < 24 g/day (alcohol history assessed by the physicians who admitted the participants based on all alcohol-containing drinks ingested during a typical 4-week period) IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: weight change > 5 kg since biopsy, previous or ongoing treatment with insulin, metformin or TZDs, kidney failure, pharmacologically treated heart failure, significant coronary heart disease (NYHA classes III and IV), moderate to severe chronic obstructive lung disease, liver cirrhosis or liver diseases other than NAFLD and alcohol consumption > 24 g/day Age: Met: 44.3 years, SD 9.0; Pbo: 49.9 years, SD 12.8 Sex: Met: 20% female; Pbo: 33% female BMI: Met: 31.4 kg/m2, SD 3.9; Pbo: 30.3 kg/m2, SD 3.3 Ethnicity: Caucasian Met: 85%; Pbo: 88% Fibrosis: Met: 40%; Pbo: 58% Stage/severity of NAFLD: see Results section for detailed Kleiner scores Previous treatment: NR Comorbidities: Met: 45% abnormal glucose tolerance, 20% T2DM, 25% hypertension; Pbo: 50% abnormal glucose tolerance, 33% T2DM, 54% hypertension |
Met: metformin maximal daily dose 2500 mg or 3000 mg (if body weight was > 90 kg); starting dose 500 mg/day, medication was increased every week until maximum dose was reached after 4 or 5 weeks (if side effects occurred, dose was transiently or permanently reduced to a dose tolerated by the patient) (average dose at 4 or 5 weeks was 2.6, SD 0.4 g/day) Pbo: equivalent dosing of placebo tablets Both groups: all participants received general advice about a healthy lifestyle i.e. physical activity at least 30 min daily and diet low in fats, particularly saturated fat, and refined carbohydrates Adherence: pill counting Run-in period: none Co-interventions/co-medications: NR |
Primary: changes in liver steatosis between the index biopsy and the second biopsy Progression/regression of liver disease: liver biopsy, histopathological changes assessed according to the criteria defined by Kleiner et al. ;116 liver steatosis by CT; NAS IGT → diabetes: NR NGT → IGT/diabetes: NR HbA1c: yes FPG: yes Weight change: BMI, body weight Blood pressure: yes Lipid parameters: TC, HDL, TGs, TGs Liver biochemistry: AST level, ALT level, GGT Health-related QoL: NR Mortality: NR Other: Insulin resistance (HOMA–IR), insulin, leptin, adiponectin, tumour necrosis factor Timing of assessment: participants were seen monthly at the research units for blood sampling and assessment of the compliance (pill counting), body weight and adverse events; second liver biopsy taken at the end of treatment |
|
Nadeau 2009 107 Country: USA Focus: effect of metformin and lifestyle recommendations in adolescents with fatty liver and elevated liver associated enzymes Design: double-blind design Centre: urban health-care system including school-based health centres in Denver, CO, USA Duration: 6 months Follow-up: no post-intervention follow-up |
Total number: 55 enrolled n Met: 37; 28 completed n Pbo: 13; 10 completed Inclusion criteria: adolescents aged 12–18 years; two of the three of the following: acanthosis nigricans, BMI > 30 kg/m2 or > 95% for age, or family history of T2DM Diagnosis: liver ultrasound Definition of non-alcoholic: adolescents with current or prior alcohol consumption excluded IGT/diabetes (including diagnostic criteria): insulin resistance (fasting insulin level > 25 microunits/ml or homeostasis model assessment > 3.5) Exclusion criteria: pre-existing diabetes; pregnancy; serum creatinine > 1.5 mg/dl; significant heart disease; liver disease other than NAFLD Age: 15.1 years (range 12–18 years) Sex: Met: 68% female; Pbo: 62% female BMI: Met: 39.6 kg/m2, SE 0.98; Pbo: 40.2 kg/m2, SE 1.8 Ethnicity: Met: 32% African American, 62% Hispanic, 3% Caucasian, 3% Asian/Pacific Islanders; Pbo: 38% African American, 46% Hispanic, 8% Caucasian Fibrosis: NR Stage/severity of NAFLD: see Results sections for details Previous treatment: NR Comorbidities: NR Subgroups: fatty vs normal liver parameters |
Met: metformin capsules, started initially with 500 mg once daily, increased to 500 mg twice daily at 1 month and then to 850 mg twice daily at 2 months Pbo: placebo capsules, equivalent dosing scheme to metformin Both groups: underwent a dietary assessment and watched a standardised video about healthy eating habits. Wellness education emphasised balanced meals with modest calorie reduction; decreased fat and simple sugar consumption; increased fibre, fruit and vegetable intake; and regular aerobic exercise. Participants chose three specific dietary or exercise goals and were given a calendar to record progress on their goals and medication compliance Adherence: pill count and review of the participants’ pill calendar to assess compliance (pill counts similar between groups) Run-in period: none Co-interventions/co-medications: NR |
Primary: fatty liver Progression/regression of liver disease: liver ultrasound; severity of fatty liver was quantitated using a scoring system (0 = no fatty liver, 1 = mild fatty liver, 2 = moderate fatty liver and 3 = severe fatty liver); participants with an ultrasound reading of 1, 2 or 3 were considered to have NAFLD IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: no FPG: yes Weight change: BMI Blood pressure: no Lipid parameters: Cholesterol, HDL, LDL, TG Liver biochemistry: GGT, AST, ALT Health-related QoL: no Insulin resistance/sensitivity: insulin, 2 hours glucose, 2 hours insulin Mortality: no Other: insulin, total protein, creatinine Timing of assessment: outcomes reported for screening and 6-month visit |
|
Nar 2009 108 Country: Turkey Focus: effect of metformin on plasma leptin levels in obese patients with T2DM and NAFLD Design: parallel RCT, single centre Duration: 6 months Follow-up: no post-intervention follow-up |
Total number: 34 n Met: 19/19 completed (presumably) n C: 15/15 completed (presumably) Inclusion criteria: newly diagnosed T2DM, no anti-diabetic medications, obese (BMI > 30 kg/m2) Diagnosis: ultrasonic evidence of NAFLD (both liver-kidney contrast and vascular blurring) Definition of non-alcoholic: 30 patients with no alcohol consumption, 4 patients with < 100 g of ethanol/month IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: suspected acute or chronic viral hepatitis, autoimmune hepatitis, history of malignancy, impaired renal function, haemodynamic instability, diseases or pituitary, adrenal glands or pancreas, prolonged use of corticosteroids or sex hormones, use of anti-hyperlipidaemic agents, use of anti-obesity medication Age: Met: 49.4 years SE 8.6; C: 44.5 years SE5.9 Sex: Met: 79% female; C: 67% female BMI: Met: 31.0 kg/m2, SE 4.0; C: 33.7 kg/m2, SE 6.0 Ethnicity: NR Fibrosis: NR Stage/severity of NAFLD (grade of hepatic steatosis): Met: normal: 21%, low: 63%, moderate: 16%; C: normal: 27%, low: 53%, moderate: 20% Diabetes duration: newly diagnosed Previous treatment: NR Comorbidities: Met: macroangiopathy: 26.7%, autonomic neuropathy: 5.6%, peripheral neuropathy: 26.7%, retinopathy: 35.7%; C: macroangiopathy: 15.4%, autonomic neuropathy: 7.1%, peripheral neuropathy: 15.4%, retinopathy: 25.0% |
Met: 1700 mg/day metformin (starting at 850 mg/day in week 1 and increased to 1700 mg/day thereafter) plus diet/exercise C: diet/exercise only Both groups: exercise: walking a minimum of 30 minutes at least 3 days/week; consultation with dietician Adherence: adherence to metformin (pill count) and diet/exercise; 74% with good dietary/exercise adherence Run-in period: none Co-interventions/co-medications: NR |
Primary: plasma leptin Progression/regression of liver disease: USG: low grade (< 30%), moderate grade (30 to 50%), high grade (> 50%) (Yajima et al. 1983)156 IGT → diabetes: NR NGT → IGT/diabetes: NR HbA1c: yes FPG: yes Weight change: yes, BMI, waist–hip ratio Blood pressure: no Lipid parameters: yes, total and HDL, LDL cholesterol, TGs Liver biochemistry: ALT, AST Insulin resistance: yes, HOMA Health-related QoL: no Mortality: no Other: leptin, insulin Timing of assessment: every 2 months |
|
Shields 2009 109 Country: USA Focus: effect of diet, exercise and metformin in participants with insulin resistance and NASH Design: prospective, randomised, placebo-controlled trial Centre: gastroenterology clinics at the Naval Medical Centre, San Diego, CA Duration: 1 year Follow-up: no post-intervention follow-up |
Total number: 19 n Met: 9; 9 completed n Pbo: 10; 7 completed Inclusion criteria: NASH and one of the following: BMI > 27 kg/m2, a fasting blood sugar between 6.11 and 6.94 mmol/l; a diagnosis of polycystic ovarian syndrome; metabolic syndrome; age > 17 years, an unremarkable serological evaluation for chronic liver Diagnosis: histological diagnosis of NASH defined by the presence of cytological ballooning and inflammation in addition to steatosis, liver biopsy within 18 months of enrolment Definition of non-alcoholic: > 20 g/day or 80 g/week IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: known diabetes mellitus (type 1 or 2), a fasting blood sugar > 6.94 mmol/l, prior history of alcoholic liver disease, any other known chronic liver disease, renal insufficiency (serum creatinine > 1.2), a known allergic reaction to metformin, prior use of an insulin-sensitising agent such as metformin or a TZD, gastric bypass within 2 years, untreated thyroid disease, coagulopathy, chronic thrombocytopenia, significant alcohol use during previous 2 years Age: Met: 50.2 year, SD 9.1; Pbo: 44.4 years, SD 12 Sex: Met: 11% female; Pbo: 50% female BMI: Met: 32.2 kg/m2, SD 4.9; Pbo: 32.8 kg/m2, SD 4.9 Ethnicity: Met: Hispanic 11%, Caucasian 67%, Asian 11%, African American 11%; Pbo: Hispanic 10%, Caucasian 50%, Asian 40%, African American 0% Fibrosis: (Brunt scores) Met: 1.61; Pbo: 1.7 Stage/severity of NAFLD: Kleiner score, see Results section Previous treatment: NR Comorbidities: NR |
Met: metformin 500 mg daily, titrated to 1000 mg daily if serum amino transferases did not show improvement Pbo: placebo Both groups: referred to a dietician for a dietary counselling (DASH-Dietary Approaches to Stop Hypertension), recommendations for weight loss and 30 minutes aerobic exercise four times a week Adherence: NR Run-in period: none Co-interventions/co-medications: NR |
Primary: liver histology Progression/regression of liver disease: liver histology; liver biopsy specimens graded according to the NASH histological scoring system (Brunt scores); in addition all biopsies were given a NAS as proposed by Kleiner et al. 116 IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: no FPG: no Weight change: yes Blood pressure: no Lipid parameters: yes Liver biochemistry: yes Health-related QoL: no Insulin resistance/sensitivity: yes Mortality: no Other: overall improvement in BMI, HOMA–IR and serum aminotransferase Timing of assessment: participants seen 2 weeks after enrolment and at 6-week intervals thereafter; repeat liver biopsy was performed after completion of the therapy |
|
Uygun 2004 110 Country: Turkey (Ankara) Focus: effect of metformin in participants with NASH, insulin resistance and elevated liver enzyme concentrations Design: RCT Centre: outpatient clinics Duration: 6 months Follow-up: 6 months – MET group |
Total number: 36 participants enrolled n Met: 17; 15 (88%) completed in 6 months and 11 (65%) in 12 months n Pbo: 17; 17 completed Inclusion criteria: Diagnosis: liver biopsy and histopathological examination; ultrasound of the abdomen; a detailed clinical and laboratory evaluation, including liver enzyme concentrations, hepatitis markers and autoantibodies Definition of non-alcoholic: < 100 g ethanol per month; diagnosed by history and also participants spouse or one of the family members asked about the alcohol intake IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: contraindication for metformin, possible liver disease other than NASH, history of malignant disease, impaired renal function (serum creatinine > 1.5 mg/dl), heart failure, history of lactic acidosis, severe infection, hypoxic status, serious acute and chronic illnesses, homodynamic instability, age > 70 years, diabetes mellitus or the current use of any drugs that may affect the results; elevated gamma-glutamyl transpeptidase activity (> 75 U/l, indicating possible concealed alcohol intake) Age: Met: 39.8 years, SD 10.6 (range 22–64); Pbo: 41.5 years, SD 9.1 (range: 23–61) Sex: Met: 35% female; Pbo: 41% female BMI: Met: 30.1 kg/m2, SD 3.4; Pbo: 28.4 kg/m2, SD 3.9 Ethnicity: NR Fibrosis: (Brunt fibrosis scores) Met: 0.94, SD 1.02; Pbo: 1.05, SD 1.1 Stage/severity of NAFLD: see Results section for Brunt scores Previous treatment: NR Comorbidities: Met: obesity (6), hyperlipidaemia (2), obesity and hyperlipidaemia (8) and undefined (1); Pbo: obesity (5), hyperlipidaemia (3), obesity and hyperlipidaemia (7) and undefined (2) |
Met: metformin 850 mg twice daily plus dietary treatment Pbo: dietary treatment alone Both groups: consultation with dietician for restriction of patients’ intake of lipids and non-complex carbohydrates; all obese and overweight participants were advised to lose weight with a restriction of daily calorie intake to 1600–1800 calories per day Adherence: assessed by questionnaire; in both groups nearly one-half of patients reported good compliance with their dietary recommendations whereas the compliance was moderate or poor in the others Run-in period: none Co-interventions/co-medications: NR |
Primary: unclear Progression/regression of liver disease: liver histology; liver biopsy specimens graded according to the NASH histological scoring system (Brunt scores) IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: no FPG: yes Weight change: BMI Blood pressure: no Lipid parameters: cholesterol, TGs Liver biochemistry: ALT, AST, Health-related QoL: no Insulin resistance/sensitivity: HOMA (%) Mortality: no Other: insulin, C-peptide, Timing of assessment: participants in the metformin group contacted by telephone in the first and second weeks of treatment and questioned about any side effects attributed to the drug; also warned of possible hypoglycaemia and gastrointestinal side effects due to metformin; in both groups all serum parameters (and, in the metformin group, the serum lactate level) were repeated at the end of the first, third and sixth months of treatment; ultrasound of the abdomen and liver biopsy at baseline and at the end of the study if informed consent had been obtained. In both groups, the participants were advised to maintain their dietary recommendations after the study period and were recalled for clinical evaluation and liver enzyme concentrations every 3 months Four participants in Met group and seven in the dietary-alone group refused the control liver biopsy, even though written consent was given prior the study. Therefore, control biopsy was done in 13 in the Met and 10 in the diet-alone group |
| Rosiglitazone | |||
|
Ratziu 2008 (FLIRT trial) 111 Country: France Focus: efficacy and safety of rosiglitazone in participants with NASH Design: randomised, double-blind, placebo-controlled trial Centre: single institution (presumably hospital) Duration: 12 months Follow-up: 4 months |
Total number: 64 n Rosi: 32; 32 completed n Pbo: 32; 31 completed Inclusion criteria: 18–75 years, histological diagnosis of NASH Diagnosis: liver biopsy performed within 12 months before inclusion; steatosis > 20% and the presence of either hepatocyte ballooning or intralobular hepatocyte necrosis, all signs with centrolobular accentuation; elevated ALT level was defined as a baseline level > 28 IU/l in women and 35 IU/l in men and at least two other abnormal values in the 6 months before baseline Definition of non-alcoholic: daily alcohol consumption of < 30g in men and 20 g in women whether current or in the past IGT/diabetes (including diagnostic criteria): NR Exclusion criteria: presence of bland steatosis on liver biopsy or steatosis with non-specific inflammation; any cause of liver disease other than NASH, including suspicion of drug-induced liver injury; treatment with insulin for diabetes or with UDCA; cardiac insufficiency (NYHA Class > 1); current or past treatment with drugs that can induce steatohepatitis; neoplastic disease; Child class B or C cirrhosis; pregnancy; organ transplantation; haemoglobin level < 10 g/dl; polymorphonuclear count < 750/mm;3 platelet count < 50,000/mm3 Age: Rosi:53.1, SD 11.5, Pbo: 54.1, SD 10.4 Sex: Rosi: 41% female, Pbo: 42% female BMI: Rosi: 31.5, SD 6 kg/m2, Pbo: 30.5, SD 4.4 kg/m2 Ethnicity: NR Fibrosis: (Brunt scores) Rosi: 91%, Pbo: 97% Stage/severity of NAFLD: see Results section Previous treatment: History of treatment with diabetes – metformin: 16 (Rosi: 7, Pbo: 9); sulfamide: 10 (Rosi: 7, Pbo: 3); none: 3 (Rosi: 1, Pbo: 2) Comorbidities: NR |
Rosi: rosiglitazone 4 mg daily for the first month, 8 mg daily until end of treatment and then discontinued Pbo: placebo Both groups: instructed to lose weight if they were obese or overweight, to follow a healthy diet and to exercise at least twice a week; no specialised nutritional counselling was implemented Adherence: NR Run-in period: no Co-interventions/co-medications: NR |
Primary: reduction in steatosis > 30% between baseline and end of treatment or disappearance of steatosis at end of treatment Progression/regression of liver disease: liver histology; liver biopsy specimens graded according to the NASH histological scoring system (Brunt scores); steatosis semi-quantitatively assessed by the per cent of hepatocyte involved within a lobule (0–100% steatosis score) and by using a four-grade classification modified from Kleiner et al. ; non-alcoholic fatty liver disease activity score (NAS) calculated according to Kleiner et al. 116 IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: yes FPG: yes Weight change: BMI, waist circumference, Blood pressure: no (but arterial hypertension) Lipid parameters: TGs, HDL Liver biochemistry: ALT, AST, GGT level, bilirubin level Health-related QoL: no Insulin resistance/sensitivity: HOMA index and quantitative insulin-sensitivity check index (QUICKI) Mortality: no Other: serum leptin, adiponectin, insulin level, metabolic syndrome (%) Timing of assessment: during treatment, participants were followed up at months 1 and 2 and then every 2 months until end of treatment when liver biopsy was performed. At the end of the follow-up, all participants were asked to participate in a 2-year, open-label, extension trial of rosiglitazone irrespective of what they received during the 1-year, double-blinded phase of the trial |
| Rosiglitazone/metformin | |||
|
Idilman 2008 112 Country: Turkey Focus: insulin sensitisers (metformin, rosiglitazone) in the treatment of NASH Design: parallel RCT, single centre Duration: 48 weeks Follow-up: 6 months post-intervention |
Total number: 74 n Met: 24; 24 completed n Rosi: 25; 24 completed n C: 25; 25 completed Inclusion criteria: newly diagnosed NASH, age > 18 years Diagnosis: diagnosis based on biochemical, radiological and histological criteria, including abnormal serum ALT levels, abnormal ECHO pattern on sonography consistent with fatty infiltration; liver biopsy documenting steatosis and ballooning degeneration, with or without necroinflammatory activity, portal inflammation, fibrosis or cirrhosis Definition of non-alcoholic: women < 15 g/day, men < 20 g/day IGT/diabetes (including diagnostic criteria): unclear Exclusion criteria: confounding disease including acute hepatitis (A, B, C) and/or chronic viral hepatitis; heart or renal disease, other forms of liver disease (e.g. autoimmune, drug induced, metabolic) Age: insulin sensitisers: 47.9 years, SD 8.3; C: 45.8 years, SD 10.4 Sex: insulin sensitisers: 56% female; C: 64% female BMI: insulin sensitisers: 31.2 kg/m2, SD 3.6; C: 32.2 kg/m2, SD 5.1 Ethnicity: NR Fibrosis: unclear Stage/severity of NAFLD (NAS): insulin sensitisers: 5 (3 to 8); C: 4 (3 to 8) Previous treatment: NR Comorbidities: insulin sensitisers: 21% hyperlipidaemia; C: 20% hyperlipidaemia |
Met: diet and exercise plus metformin 850 mg b.i.d. (1700 mg) Rosi: diet and exercise plus rosiglitazone 8 mg/day C: diet and exercise alone All groups: diet and exercise programme: exercise included walking (initially 300 steps/day for 3 days, then adding 500 steps at 3-day intervals until 10,000 steps attained) and jogging (20 minutes b.i.d.), improvement in associated conditions such as moderate/severe hyperlipidaemia, discontinuation of potentially hepatotoxic drugs; diet: 25 kcal/kg × ideal body weight, three meals containing 60% carbohydrate, 25% fat and 15% protein provided for each individual; adherence to diet and exercise encouraged during 6-month follow-up Adherence: adherence to diet and exercise monitored; adherence was judged to be insufficient in both groups Run-in period: none Co-interventions/co-medications: 15 patients with hyperlipidaemia (five in diet/exercise-only group and 10 in insulin sensitiser group) were on lipid-reduced diet and anti-hyperlipidaemic agents and continued this treatment throughout the study |
Primary: unclear Progression/regression of liver disease: liver histology; liver biopsy performed before intervention and after 48 weeks; histological features interpreted according to the criteria given by Brunt et al. ,115 NAS according to Kleiner et al. 116 IGT → diabetes: no NGT → IGT/diabetes: no HbA1c: no FPG: yes Weight change: BMI, body fat content, waist and hip circumference Blood pressure: no Lipid parameters: cholesterol, TGs Liver biochemistry: ALT, AST, GGT, ALP, bilirubin Insulin resistance: yes, HOMA Health-related QoL: NR Mortality: NR Other: insulin, serum iron, ferritin, copper, ceruloplasmin; anti-nuclear, anti-smooth muscle and anti-mitochondrial antibodies Timing of assessment: baseline, after 4 weeks, then at 3-month intervals |
|
Omer 2010 113 Country: Turkey Focus: effects of insulin-sensitising agents in participants with NAFLD with T2DM or impaired glucose metabolism Design: open-label, randomised, single-centre study Multicentre: hepatology and endocrinology outpatients clinic Duration: 12 months Follow-up: no post-intervention follow-up |
Total number: 64 n Met: 22; 19 completed n Rosi: 20; 20 completed n Met + Rosi: 22; 22 completed Inclusion criteria: NAFLD; being on a dietary and exercise programme for at least 12 weeks before enrolment Diagnosis: elevated ALT for at least 6 months before enrolment; NAS at least five in liver biopsy performed within 6 months before enrolment Definition of non-alcoholic: alcohol consumption < 20 g/day IGT/diabetes (including diagnostic criteria): patients with impaired glucose metabolism (T2DM or impaired glucose tolerance) Exclusion criteria: use of oral anti-diabetics, insulin or a chemotherapeutic agent; presence of other chronic liver diseases, such as metabolic liver diseases, autoimmune liver diseases, and chronic viral hepatitis B or C, HIV infection; pregnancy or lactation; being a candidate for organ transplantation; presence of a malignancy; renal function impairment (serum creatinine > 1.5 mg/dl in men and > 1.4 mg/dl in women), and clinically significant systematic illness Age (years): Met: 48.0 years, SD 9.8; Rosi: 49.3 years, SD 6; Met + Rosi: 49.6 years, SD 9.1 Sex: Met: 32% female; Rosi: 55% female, Met + Rosi: 50% female BMI: Met: 30.8 kg/m2, SD 6.6; Rosi: 28.4 kg/m2, SD 4.1; Met + Rosi: 32.5 kg/m2, SD 7.0 Ethnicity: NR Fibrosis: proportion not reported Stage/severity of NAFLD: NAS – see Results section Previous treatment: NR Comorbidities: NR |
Met: metformin 1700 mg/day Rosi: rosiglitazone 4 mg/day Met + Rosi: metformin 1700 mg/day and rosiglitazone 4 mg/day Both groups: dietary counselling by endocrinologists and dieticians, and encouraged to do exercise for 12 weeks before study medication and also to continue this programme during the entire study Adherence: NR Run-in period: 12 weeks with exercise programme Co-interventions/co-medications: NR |
Primary: histological changes in liver Progression/regression of liver disease: Liver biopsy, assessed according to criteria by Kleiner et al. ;116 NAS; total NAFLD score IGT → diabetes: NR NGT → IGT/diabetes: NR HbA1c: yes FPG: no Weight change: BMI, waist circumference Blood pressure: NR Lipid parameters: TC, HDL, LDL, TGs Liver biochemistry: AST level, ALT level, GGT, alkaline phosphates Health-related QoL: NR Insulin sensitivity/resistance: HOMA–IR Mortality: NR Other: transferrin saturation, ferritin, insulin, blood urea nitrogen, creatinine Timing of assessment: follow-up every months for the first 3 months and then every 3 months |
|
Torres 2009 100 Country: NR (presumably USA –San Antonio, TX) Focus: effect of rosiglitazone or rosiglitazone and metformin in combination after 1 year of therapy in participants with NASH Design: randomised open-label trial Multicentre: NR Duration: 1 year Follow-up: no post-intervention follow-up |
Total number: 108 randomised, preliminary results for 49 participants reported n Rosi: 15 completed n Rosi + Met: 16 completed Rosi + Los: 18 completed (not considered here) Inclusion criteria: screened for other aetiologies of chronic liver disease including daily alcohol intake of 20 g Definition of non-alcoholic: < 20 g per day IGT/diabetes (including diagnostic criteria): Exclusion criteria: Age (years): mean age 48.9? SD 9.2 years BMI: mean BMI 33.3? SD 5.1 kg/m2 Ethnicity: NR Fibrosis: NR Stage/severity of NAFLD: NR Previous treatment: NR Comorbidities: NR |
Rosi: 4 mg rosiglitazone twice daily Rosi + Met: 4 mg rosiglitazone and 500 mg metformin twice daily Rosi + Los: 4 mg rosiglitazone and 50 mg losartan once daily for 48 weeks (not considered here) Both groups: NR Adherence: NR Run-in period: NR Co-interventions/co-medications: NR |
Primary: histopathological changes in liver Progression/regression of liver disease: liver biopsy IGT → diabetes: NR NGT → IGT/diabetes: NR HbA1c: no FPG: no Weight change: weight Blood pressure: no Lipid parameters: no Liver biochemistry: AST level, ALT level, Health-related QoL: no Insulin sensitivity/resistance: mean HOMA–IR Mortality: NR Timing of assessment: NR |
Appendix 3 Quality of included studies
| Study | Method of randomisation | Allocation concealment | Blinding | ITT data analysis | Percentage who completed trial | Power calculation | Similarity of groups at baseline | Sponsorship/author affiliation | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | |||||||||
| Aithal 2008101 | Computer randomisation in blocks of four | Not given | Double blind | Not given |
I: 84% C: 81% |
Yes (requiring 33 in each group) | Most parameters; lower alkaline phosphatase and fasting insulin in pioglitazone group at baseline | Unclear, investigator-initiated study, but Takeda UK provided pioglitazone and placebo tablets; one author received funding by Takeda to attend meetings | 4 |
| Belfort 2006102 | Computer generation by research pharmacy | Not given | Double blind | Not given |
Pio: 23/26 (88%) Pbo: 17/21 (81%) |
No | Yes |
Grants from the National Centre for Research Resources, Takeda Pharmaceuticals, and the Veterans Affairs Medical Research Fund One of the authors is a member of the speakers bureau of Eli Lilly, one a consultant to Eli Lilly and the third a member of the advisory board and speakers bureau of Takeda Pharmaceuticals. No other conflict of interest |
5 |
| Sanyal 2004103 | By an independent statistician in random blocks of two, four and six | No | Pathologist blinded | No |
Pio: 8/10 (80%) Vit E: 10/10 (100%) |
No | Yes | Supported in part by a grant from the National Institutes of Health to the General Clinical Research Centre at Virginia Commonwealth University and by an award to AJS | 4 |
| Sanyal 2010104 | Administered centrally by the DCC, web-based application; assigned participants in permuted blocks of treatments stratified by clinical centre | Randomisation centrally, request made by a web based application and participants randomised in permuted blocks | Double blind | Yes |
Pio: 70/80 (88%) Vit E: 80/84 (95%) Pbo: 72/83 (87%) |
Yes | Yes |
Supported by National Institute of Diabetes and Digestive and Kidney Disease Additional funding by Takeda Pharmaceuticals North America Inc. |
8 |
| Metformin | |||||||||
| Bugianesi 200587 | Randomisation procedure was centralised in Bologna, and based on a random sequence | Sealed envelopes were used to conceal randomisation | Both patients and investigator were not blind to the treatment | ITT, LOCF analysis |
I: 100% C: 100% |
No | Most parameters; significant difference in sex between groups (100% men in Vit E group) and in cholesterol levels (significantly lower in diet group) | Not given | 4 |
| Garinis 2010105 | Not given | Not given | Open label | Not given |
I: 15/20(75%) C: 25/25 (100%) Five participants dropped out because of non-compliance to the treatment |
Not given | Two treatment groups showed similar clinical and biochemical variables | Not clear | 2 |
| Haukeland 2009106 | Computer-assisted process of minimalisation (to minimalise baseline difference in serum ALT and liver histology) | Allocation code was blinded to participants and investigators; two pathologists blinded | Double blind | Per-protocol analyses (44 participants that completed the trial) |
I: 83% C: 100% Four participants treated with metformin did not complete the study due to gastrointestinal side effects (n = 1), development of exanthema (n = 1), withdrawal of consent (n = 1) and erroneous use of study medication (n = 1) |
Yes, based on liver steatosis, at least 36 participants in each group to have 80% power to detect a significant difference between Met and placebo and 10% mean reduction; only 44 participants completed the trial, the power to detect a significant difference between treatment groups was reduced to 60% | Metformin-treated participant were younger, less often treated for hypertension and slightly more obese (significance not reported) | Eastern Norway Regional Health Authority (grant) and Merck Santé (delivery of study medication); no conflicts of interest | 7 |
| Nadeau 2009107 | Research pharmacist. Stratified by ethnic group as well as insulin level > 40 or < 40 to assure similar distributions of drug and placebo groups | No | Double blind; study radiologist blinded | No |
I: 28/37 (76%) C: 10/13 (77%) |
No | Yes | Grant by General Clinical Research Centers Program, National Centers for Research Resources, Insulin Control of Fat Regulation and Exercise in Teens and the Kettering Family Foundation | 4 |
| Nar 2009108 | Not given | Not given | Operator performing ultrasound blind to treatment | Not given but presumably all patients completed the study | Not given, presumably 100% | Not given | Yes | Not given | 3 |
| Shields 2009109 | Randomised by the pharmacy using a computer-generated program into group A or B | No | Two study pathologists were blinded | Yes |
I: 7/10 (70%) C: 9/9 (100%) |
No | Yes; two groups similar regarding their laboratory and anthropometric data; treatment group was older and predominantly male; (but difference not significant) | No conflict of interest; funding source not reported | 5 |
| Torres 2009100 | Not given; randomised | Not given |
Open label; pathologist blinded |
Not given | Total number of participants allocated in each group is reported in the abstract as 108 till date | Not given | Not given | Not given | 1 |
| Uygun 2004110 | Random sampling numbers | No | Unblinded | No |
I: 15/17 (88%) completed in 6 months and 11/17 (65%) in 12 months; liver biopsies at study end by 13 only C: 17/17 (100%); liver biopsies at study end by 10 only |
No | Yes | Not given | 3 |
| Rosiglitazone | |||||||||
| Ratziu 2008111 | Randomisation (pre-sealed envelopes) conducted by blocks of four and stratified on metformin use | Pre-sealed envelopes |
Double blind Pathologist blinded |
No |
I: 32/32 (100%) C: 31/31 (100%) |
The inclusion of 29 participants per arm was deemed necessary for rejecting the null hypothesis with 80% power and a type I error rate of 0.05. The total number of included participants was set at 32 per arm to account for 20% lost to follow-up | Yes | GlaxoSmithKline partly funded the trial, investigator-initiated trial. One of the authors is a consultant to Astellas, Gilead, Pfizer, Sanofi-Aventis, and Trophos. Another author is a consultant for, and owns 15% of, BioPredictive, a company that markets FibroTest and SteatoTest. None of the authors has a personal conflict of interest with the manufacturer of any of the marketed TZDs | 7 |
| Metformin/rosiglitazone | |||||||||
| Idilman 2008112 | Random assignment 1 : 2, method not reported | Not given | Pathologist assessing liver biopsies blinded to patient and treatment | No (but only one patient did not complete the study) |
Met: 100% Rosi: 96% C: 100% |
Not given | Yes | Stated that there was no conflict of interest with respect to funding | 3 |
| Omer 2010113 | Not given | Not given | Open label | Not given |
Met: 86% Rosi: 100% Met + Rosi: 100% |
Not given | All three groups were similar at baseline except baseline serum insulin level (significantly higher in the metformin group and metformin-plus-rosiglitazone group compared with the rosiglitazone group) | Committee of Ege University Medical School, Department of Gastroenterology and Endocrinology | 2 |
Appendix 4 Protocol
Technology Assessment Report commissioned by the NIHR HTA programme on behalf of the National Institute for Health and Clinical Excellence
Project 09/16
Final protocol 23 February 2010
Title Insulin sensitizers in treatment of non-alcoholic fatty liver disease
Aberdeen TAR team
-
Contact
Professor Norman Waugh
Section of Population Health
Polwarth Building
Foresterhill
Aberdeen AB25 2ZD
n.r.waugh@abdn.ac.uk
Plain English summary
Non-alcoholic fatty liver disease (NAFLD) is a common disease due to a build up of fat in the cells of the liver. It can range from causing no symptoms at all, to severe damage (cirrhosis) of the liver, and death. Liver disease is common in those who drink to excess, but liver disease can also occur in people who drink little or no alcohol (defined as less than one unit, 10g, a day), especially if they are fat.
NAFLD is becoming more common because of the rise in obesity, and it is estimated that about 20% of people in the USA have it. It is also the most common cause of liver disease in children.
In the early stages of NAFLD, the liver is simply full of fat (steatosis), but this can progress to inflammation (steatohepatitis), and then to scarring and cirrhosis. It used to be seen typically in middle age, but with increasing levels of obesity in children, cases have been reported in children under 10.
Most people who get NAFLD are overweight or obese, and there is a close association with insulin resistance. More than half of the people with NAFLD will also have type 2 diabetes, and many will have high cholesterol levels. There is an increased risk of heart disease.
Treatment should start with diet and weight loss, aided by physical activity, and if sufficient weight is lost, the condition will improve. However adherence to lifestyle changes is often poor.
Because NAFLD is usually seen in people who have insulin resistance, a group of drugs which improve the body’s sensitivity to insulin have been tried. These drugs are called the insulin sensitisers – metformin, pioglitazone and rosiglitazone.
This review will examine the evidence for the effectiveness of these drugs in NAFLD.
Decision problem
-
Key question: what is the clinical and cost-effectiveness of metformin, rosiglitazone and pioglitazone in NAFLD?
-
Should the HTA Programme seek to commission further primary research in the value of insulin-sensitisers in NAFLD?
It will be assumed that first-line treatment will be with lifestyle changes (diet, physical activity and weight loss), and that the insulin-sensitisers will be used as a second-line addition to those. A Cochrane review on dietary interventions by Rex Wang and colleagues is in progress, and we will not examine the literature on that.
Given recent evidence on the relative vascular risks of pioglitazone and rosiglitazone (summarised in the HTA monograph on newer drugs for type 2 diabetes), our prior position will be that pioglitazone is preferred. We will exclude any studies which used troglitazone, an earlier glitazone which is no longer used because it caused liver damage.
The population of interest will be those with diagnosed NAFLD, and the HTA Programme commissioning brief specifies that the patient group of most interest is people with evidence of fibrosis.
Sub-groups will include:
-
Those with type 2 diabetes.
-
Children and adolescents.
-
Those with other features of the metabolic syndrome such as hypertension and hyperlipidaemia.
-
Those with and without fibrosis.
-
Ethnic groups at higher risk.
Diagnosis
There is a problem with the diagnosis of NAFLD. The current consensus is that it can only be diagnosed on the basis of a liver biopsy. This could be a major hindrance to any trials which need to recruit large numbers of patients, perhaps especially if young people are involved. Liver biopsy can have complications, such as bleeding, at any age.
Hence it would not be feasible to mount a large trial of insulin-sensitisers if the diagnosis has to be based on liver biopsy. We are aware that research into alternative methods of diagnosis, such as panels of liver tests, ultrasound and MRI, is underway. For detecting NAFLD, ultrasound and MRI have been suggested. For detecting liver fibrosis, various non-invasive alternatives to liver biopsy have been suggested, including combinations of blood tests (“serum marker panels”), and either transient or real-time elastography.
The HTA Programme is commissioning a full review of evidence on non-invasive methods for the assessment and monitoring of more advanced stages, liver fibrosis and cirrhosis (HTA number 09/07), and so this review will not duplicate that. This review is more concerned with a trial which would seek to prevent people reaching those stages.
The aim for diagnosis is therefore to distinguish those patients with simple steatosis from those who have steatohepatitis.
We will therefore carry out a brief review of alternatives to biopsy at earlier stages, such as NASH.
What we will try to do is identify non-invasive tests which could be used to recruit patients to a trial, even if that meant accepting that the tests were sensitive but not specific. The safety and adverse effects of the drugs under review are well-known and that the drugs are well tolerated and safe. Hence a case could be made that using a test which had good sensitivity but not very good specificity, would be suitable for identifying patients for a trial, on the grounds that including some people who had fatty livers but had not progressed to NASH, would do them no harm, but possibly some good.
However it might reduce the power of the study by reducing the frequency of adverse outcomes in the placebo arms.
Our aim will not be to make a firm recommendation as to what diagnostic tests should be used in a trial, but rather to suggest non-invasive options which the HTA Programme could include in the vignette and then the CB. It would then be up to bidders to justify their choices.
Report methods for synthesis of evidence of clinical effectiveness
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles recommended in NHS CRD Report No.4.
Criteria for considering studies for this review
Types of studies: systematic reviews and randomised clinical trials. There will be no size restriction on number of patients in trials, since those with inadequate numbers and hence power, might be useful when combined in a meta-analysis. Observational studies may be used for data on safety and for assessing diagnostic methods.
We note the Cochrane review on insulin sensitisers by Francesco Angelico and colleagues in Rome. It excluded people with type 2 diabetes, and only included three trials, two of metformin and one with pioglitazone. Our scoping searches suggest that there may be another nine trials which need to be considered.
Types of participants: Participants of any age, sex, or ethnic origin with NAFLD proven by liver biopsy or other methods.
Types of interventions: Metformin, pioglitazone, or rosiglitazone at any dose or duration, given separately or in combination versus no intervention, placebo, or other pharmacological interventions of proven effectiveness.
Types of outcome measures: Measures of disease progression such as fibrosis and cirrhosis, other hepatic-related morbidity such as variceal bleeding liver failure, hepatic-related and all-cause mortality, cardiovascular events, quality of life, new diabetes, adverse events. We include some of these for completeness but do not expect studies to be large enough or long enough to report on all of these outcomes.
We will check the diagnostic methods used in previous trials, and if data permit, we will compare the findings of liver biopsy with those of non-invasive tests. We will carry out searches on diagnostic methods other than liver biopsy. Ideally, these would compare new tests with liver biopsy as the gold standard.
Search methods for identification of studies
We will search the following sources
-
MEDLINE
-
EMBASE
-
The Cochrane Library (all sections)
-
Science Citation Index Expanded (SCI expanded) and Conference Proceedings Citation Index- Science (CPCI-S)
-
Contact with experts in the field
-
Scrutiny of bibliographies of retrieved papers
We will search for articles published since 2005, since a Cochrane review included studies found by searches to February 2006. No language restrictions will be applied to the search strategy, but we may not be able to translate studies in languages other than English, German and French.
Data collection and analysis
Study Selection
Study selection will be made independently by two reviewers. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
Data extraction
Data will be extracted by one reviewer, using a standardised data extraction form, and checked by a second. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
The quality of the individual studies will be assessed by one reviewer, and independently checked for agreement by a second reviewer. Any disagreements will be resolved by consensus and if necessary a third reviewer will be consulted. The quality of the clinical effectiveness studies will be assessed according to criteria based on NHS CRD Report No.4.
Existing systematic reviews will be quality assessed, summarised and results compared. Reasons for differences between the reviews will be investigated and possible reasons for conflicting results will be investigated in a narrative review.
RCTs published since the existing systematic reviews will be added and included if appropriate in a new meta-analysis. If not, evidence synthesis of all RCTs which meet our inclusion criteria will be done using a narrative review.
Searches will be carried out for on-going research.
We will contact the authors of the Cochrane review and if they are updating it in our timescale, will offer collaboration. If they are not doing it in our timescale, we will invite them to act as peer reviewers of the unpublished draft final report.
Report methods for synthesising evidence of cost-effectiveness
We will review the literature on cost-effectiveness but will not undertake any de novo modelling.
Products
The main product from this review will be a short report for publication in the HTA monograph series, but as requested in the commissioning brief, we will also produce a vignette on the desirability of new primary research for the Pharmaceutical Panel of the HTA Programme. We will also aim to submit a version suitable for publication in an appropriate journal. We will contact the authors of the Cochrane review with a view to helping them update their review.
Competing interests of authors
None.
Details of TAR team
-
Dr Deepson Shyangdan
Section of Population Health
Polwarth Building
Foresterhill
Aberdeen AB25 2ZD
-
Dr Christine Clar
Researcher in Systematic Reviews
Hasenheide 67
D-10697 Berlin
Germany
clar@cc-archie.de
-
Dr Tara Gurung
Section of Population Health
Polwarth Building
Foresterhill
Aberdeen AB25 2ZD
-
Dr Nazim Ghouri
BHF Glasgow Cardiovascular Research Centre
126 University Place
University of Glasgow
Glasgow G12 8TA
-
Professor Naveed Sattar
Professor of metabolic medicine
BHF Glasgow Cadiovascular Research Centre
126 University Place
University of Glasgow
Glasgow G12 8TA
-
Dr Ewen Cummins
Health Economist
McMaster Development Consultants
46 Polwarth Street
Glasgow G12 9TJ
ecummins@mcmdc.com
-
Norman Waugh
Professor of Public Health
University of Aberdeen
Tel. 01224 555998
n.r.waugh@abdn.ac.uk
An expert in liver disease is being approached to join the team.
Timetable/milestones
Assessment Report to be delivered by end July 2010.
List of abbreviations
- AISF
- Italian Association for the Study of the Liver
- ALD
- alcohol-related fatty liver disease
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- AUROC
- area under receiver operating characteristic curve
- BAAT
- BMI, age, ALT, TG
- BAD
- BMI, age, T2DM
- BARD
- BMI, AST/ALT ratio, T2DM
- BARG
- BMI, age, AST/ALT ratio, HbA1c or glucose
- b.i.d.
- twice a day
- BMI
- body mass index
- CASP
- Critical Appraisal Skills Programme
- CK-18
- cytokeratin-18
- CRN
- Clinical Research Network
- CT
- computerised tomography
- DBP
- diastolic blood pressure
- DCC
- Data Coordinating Centre
- DURATION
- Diabetes therapy Utilization: Researching changes in A1c, weight and other factors Through Intervention with Exenatide ONce weekly
- FBC
- full blood count
- FLD
- fatty liver disease
- FLIRT
- Fatty Liver Improvement with Rosiglitazone Therapy
- FPG
- fasting plasma glucose
- GGT
- gamma-glutamyl transferase
- GLP-1
- glucagon-like peptide-1
- HAIR
- hypertension, ALT, insulin resistance
- HbA1c
- glycosylated haemoglobin
- HBV
- hepatitis B virus
- HCC
- hepatocellular carcinoma
- HCV
- hepatitis C virus
- HDL
- high-density lipoprotein
- HOMA–IR
- homeostatic model assessment–insulin resistance
- HTA
- Health Technology Assessment
- IQR
- interquartile range
- ITT
- intention to treat
- IU
- international unit
- LDL
- low-density lipoprotein
- LEAD
- Liraglutide Effect and Action in Diabetes
- LFT
- liver function test
- MRE
- magnetic resonance elastography
- MRI
- magnetic resonance imaging
- MRS
- magnetic resonance spectroscopy
- NAFL
- non-alcoholic fatty liver
- NAFLD
- non-alcoholic fatty liver disease
- NAS
- NAFLD activity score
- NASH
- non-alcoholic steatohepatitis
- NEFA
- non-esterified fatty acid
- NFS
- NAFLD Fibrosis Score
- NGT
- normal glucose tolerance
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- PedsQL
- Pediatric Quality of Life Inventory
- PPAR
- peroxisome proliferators-activated receptor
- QoL
- quality of life
- RCT
- randomised controlled trial
- SBP
- systolic blood pressure
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- T2DM
- type 2 diabetes mellitus
- TC
- total cholesterol
- TE
- transient elastography
- TG
- triglyceride
- TONIC
- Treatment of Non-alcoholic Fatty Liver Disease in Children
- TZD
- thiazolidinedione
- USG
- ultrasonography
- UDCA
- ursodeoxycholic acid
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington