Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 09/92/01. The protocol was agreed in April 2010. The assessment report began editorial review in November 2010 and was accepted for publication in May 2011. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Squires et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Peripheral arterial disease (PAD), also known as peripheral vascular disease, is a condition in which there is blockage of the arteries that carry blood to the legs and arms. The cause of PAD is atherosclerosis, which is the narrowing of the arteries (stenosis), caused by fatty deposits on the arterial walls.
There are four stages of PAD, as described by the Fontaine Classification scheme. 1 The disease can be asymptomatic (Fontaine Classification stage I) or symptomatic (Fontaine Classification stages II–IV). 1 The commonest symptom of PAD is intermittent claudication (IC) (stage II), characterised by pain in the legs on walking that is relieved with rest. People with severe PAD experience pain at rest (stage III), and this can then progress to necrosis and gangrene (stage IV). 1 Other symptoms of PAD include cold or numbness in the feet, hair loss and non-healing sores on the legs, feet or toes. 2
Intermittent claudication is the consistent presence of muscle fatigue, cramping pain or aching experienced by patients when walking. 3 This pain results from the inadequate blood flow to leg muscles caused by PAD, limiting the increase in blood flow needed for muscle metabolism. 3 This pain is relieved with rest, as a result of normalisation of blood flow. 3 The restriction of mobility caused by IC can impair health-related quality of life (HRQoL). 2
Aetiology, pathology and prognosis
Intermittent claudication is most commonly experienced in the calf and is often then associated with PAD of the femoropopliteal segment. 2 If PAD is present at the aortoiliac level, this can result in pain in the thigh, hip or buttock, rather than/or in addition to calf claudication. 2 Rarely, IC may be located in the foot. 3
The major risk factors for developing PAD are similar to risk factors for coronary heart disease. 4 Up to 68% and 50% of patients with PAD will also have coronary and cerebrovascular disease, respectively, as these diseases have the same underlying pathology. 2,5 The major risk factors for PAD are smoking and diabetes mellitus. 3 Other risk factors are hypertension, hypercholesterolaemia, obesity, renal insufficiency, hyperhomocysteinaemia, raised C-reactive protein and a sedentary lifestyle. 3,4
Intermittent claudication is not itself life-threatening, but it is estimated that 40–68% of affected individuals have coronary artery disease as well. 6 Patients with IC are at higher risk of cardiovascular mortality than patients with PAD who do not have claudication. 7 People with PAD are approximately two to three times more likely to suffer myocardial infarction (MI) or stroke than other people of their gender and age. 2,8 Risk of cardiovascular mortality is approximately the same in patients with PAD as for patients with coronary or cerebrovascular disease. 2 There is an increased risk of disease progression in patients with multilevel arterial involvement, low ankle–brachial pressure index (ABPI), chronic renal insufficiency or diabetes mellitus. 8 Few patients with IC progress to critical limb ischaemia. 3 Fewer than 5% of patients per 5 years deteriorate to a level requiring peripheral arterial endovascular treatments or surgery. 9
Epidemiology and prevalence
The annual incidence of PAD is difficult to measure3 and has not been quantitated in any documentation identified. It has been estimated (Edinburgh Artery Study10) that approximately 20% of people aged from 55 to 75 years have evidence of PAD in the legs, and the prevalence of IC in this age group has been estimated as 4.5%. Prevalence of PAD increases with age, from around 2% at age 55 years to around 7% at age 74 years. 3 In younger age groups, IC is more common in men than in women, but in older age groups prevalence of IC is similar in both genders. 3 The prevalence of IC also increases with lower social class10 and PAD has a higher prevalence in people of black ethnicity than white ethnicity. 3
Impact of health problem
Significance for patients in terms of ill health (burden of disease)
Patients with IC, by definition, suffer pain only during physical activity. However, this has wide-ranging effects on their health status, daily living and quality of life. Within studies of patients with IC whose health status was assessed with the Short Form questionnaire-36 items (SF-36), this population had significantly worse scores than published norms across all domains, i.e. physical and social function, physical and emotional role, vitality, bodily pain, general health and mental health. 11,12 This translates into quality-of-life detriments [as measured by the World Health Organization Quality of Life (WHOQoL) instrument], affecting overall health, social relationships, levels of independence, opportunities for acquiring new information and skills, and recreation and leisure. 12
Significance for the NHS
Patients with IC may require treatment in primary or secondary care. It is estimated from population-based studies that around only 50–90% of patients with IC present for medical attention,3 as a large proportion of people assume it is a natural part of ageing. Although PAD is a chronic disease, only around one-quarter of patients with IC will ever significantly deteriorate. Therefore, for the majority of patients, the burden on the NHS is in terms of the initial diagnosis and treatment aimed at reducing the risk from cardiovascular events. This includes smoking cessation, cholesterol lowering, glycaemic control, weight reduction and blood pressure control. Antiplatelet and statin therapy may be given as long-term prophylaxis against MI and stroke. The management of claudication symptoms includes the recommendation to exercise and may include vasoactive drugs. For patients with severe disability or deteriorating symptoms, further evaluation with imaging (with magnetic resonance angiography, computerised tomography angiography, duplex ultrasound or conventional arteriography) is required within secondary care to assess the potential for treatment with angioplasty or bypass surgery. Around 1–3.3% of patients with IC will need major amputation over a 5-year period. 3
Measurement of disease
Not all patients with PAD will experience classic claudication symptoms and it is estimated that the ratio of symptomatic to asymptomatic patients is in the range of 1 : 3 to 1 : 4. 3 Often, patients will not know they have the disease until they have a heart attack or stroke. Equally, not all claudication pain is caused by PAD, and diseases such as deep vein thrombosis, hip, foot or ankle arthritis, sciatica and spinal stenosis (narrowing of the spinal canal) can cause similar symptoms. 3 Those with exercise-induced lower limb pain should undergo investigations to confirm that the cause is PAD. The patient’s ABPI can be measured. This is done using a sphygmomanometer cuff and a Doppler (ultrasound) instrument to measure the pressure of arteries in the arm and ankle. Diagnostic criteria vary, but the recent UK primary care guidelines13 consider a ABPI of 0.9 as confirmation of PAD. For those with ABPI between 0.91 and 1.30 and classic PAD symptoms, referral to hospital for exercise ABPI testing or other investigations is recommended. Although PAD is usually indicated by an ABPI below the normal value of 1, a high ABPI may also indicate PAD because concomitant calcification of the vessels can elevate the ABPI. As such, patients with an ABPI of > 1.3 should be referred to a vascular specialist for assessment. When the ABPI indicates PAD, this does not rule out the possibility that coexisting conditions, such as arthritis and spinal stenosis, may be contributing to the patient’s pain.
For the purposes of publications and clinical trials, claudication pain is often classified according to the Fontaine Classification,1 as described above, or by the Rutherford Classification14 (Table 1). Both of these classifications use pain-free walking distance (PFWD) to stage the disease. Maximal walking distance (MWD) and PFWD are usually assessed with the use of a graded treadmill test. 3 In primary care, the use of treadmills is not considered practical,15 and, instead, a clinical diagnosis of IC (Fontaine stage II: mild, moderate or severe claudication by the Rutherford scale) may be simplified to the presence of pain upon exercise. 13 The Edinburgh Claudication Questionnaire16 is a sensitive tool for identifying those with symptomatic IC. It asks patients to indicate the type, location and pattern of pain upon walking and during rest to assess whether or not their pain is consistent with a diagnosis of IC. Classical IC symptoms are the presence of reproducible leg muscle pain on exercise which is relieved by rest within 10 minutes. 17 Pain usually occurs in the calf, as the reduced blood supply is only adequate to serve the buttock and thigh, although, rarely, pain can occur in the buttocks and thigh and even more rarely in the foot. In those with no pain, walking impairment may still occur. 3
| Fontaine | Rutherford | |||
|---|---|---|---|---|
| Stage | Clinical | Grade | Category | Clinical |
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | IC, PFWD > 200 m | I | 1 | Mild claudication, completion of treadmill test, after-exercise ABPI > 50 mmHg and < 20 mmHg lower than resting value |
| IIb | IC, PFWD < 200 m | 2 | Moderate claudication, in between categories 1 and 3 | |
| 3 | Severe claudication, cannot complete standard treadmill exercise, with after-exercise ABPI < 50 mmHg | |||
| III | Ischaemic rest pain | II | 4 | Ischaemic rest pain |
| IV | Ulceration or gangrene | III | 5 | Minor tissue loss |
| 6 | Major tissue loss | |||
To ensure that the pain is from claudication due to PAD, a PAD diagnosis should be confirmed by assessment of the patient’s peripheral pulses and measuring the ABPIs at rest.
Maximal walking distance (also known as absolute claudication distance) is a measure of how far a patient can walk before IC no longer allows walking. PFWD (also known as initial claudication distance) is a measure of distance walked before IC causes pain. The European Medicines Agency (EMA) recommends treadmill tests to assess claudication distances. 18 The EMA specifies two internationally recognised treadmill protocols:18 constant-workload treadmill protocols involve the treadmill being set at a fixed slope at a fixed speed;18 graded test treadmill protocols (also known as variable load or progressive workloads) involve the treadmill being set at a fixed speed with the slope being increased by a pre-set amount at regular intervals. 18 Both of these types of test are valid but they are not interchangeable, i.e. trials should use the same protocol throughout. 18
Current service provision
Management of disease
Treatment within England and Wales is variable and there is limited published evidence of current practice. Patients may present with IC to primary or secondary care and a number of interventions are used for the conventional management of IC. Treatment is targeted at reducing the risk from cardiovascular events, such as smoking cessation, cholesterol lowering, glycaemic control, weight reduction and blood pressure control. Antiplatelet and statin therapy may be given as a long-term prophylaxis against MI and stroke. The management of claudication symptoms includes the recommendation to exercise. Supervised exercise programmes are the most effective form of exercise therapy,19 but are not generally available across England and Wales. The vasoactive drugs being assessed within this report may also be used for the management of symptoms, although current usage is variable. For patients with severe disability or deteriorating symptoms, further evaluation with imaging is usually performed to assess the potential for treatment with angioplasty or bypass surgery.
Vasoactive drugs for PAD can be provided within both primary and secondary care. Provision does not usually require additional management, as these drugs would be provided alongside a range of other treatments for PAD. Their use is generally for symptom relief only and does not impact upon disease progression. Therefore, the burden upon the NHS is generally in terms of the drug acquisition cost only. Within England and Wales these drugs are generally available to be prescribed to patients with IC, although there may be restrictions to their use due to local policies (Steven Thomas, Jonathan Michaels and Gerard Stansby, University of Sheffield, September 2010, personal communication).
Clinical practice is variable between clinicians for prescribing vasoactive drugs for IC patients whose symptoms continue despite a period of conservative management. Some clinicians will assess whether or not angioplasty is appropriate within this patient group and, if so, undertake this immediately. If angioplasty either is not appropriate or fails, then those patients may receive vasoactive drugs. Alternative practice is for patients with IC to be offered vasoactive drugs whether or not they may be considered for angioplasty. If the drugs are unsuccessful, patients may then be considered for angioplasty if this is an appropriate option, but, if successful, vasoactive drugs for PAD may negate or delay the need for angioplasty.
Relevant national guidelines, including National Service Frameworks
Within England and Wales there is currently no guidance around the use of the vasoactive drugs considered in this report for PAD. The development of National Institute for Health and Clinical Excellence (NICE) guidance is currently under way regarding the diagnosis and management of lower limb PAD in adults; this is due to be published in October 2012. 20 NICE guidance has also been developed for clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events (review of Technology Appraisal guidance No. 90),21 within which patients with PAD are considered as a subgroup.
The Scottish Intercollegiate Guidelines Network (SIGN) has developed and published guidelines around the diagnosis and management of PAD within Scotland. 2 This recommends that patients with IC, in particular over a short distance, should be considered for treatment with cilostazol (Pletal®, Otsuka Pharmaceuticals). If cilostazol is ineffective after 3 months, or if adverse effects prevent compliance with therapy, the drug should be stopped. It also recommends that patients with IC and a poor quality of life may be considered for treatment with naftidrofuryl oxalate (Praxilene®, Merk Serono).
Description of technology under assessment
Summary of intervention
Four vasoactive drugs for IC are considered within this review. All are pharmacological agents for the symptomatic relief of IC secondary to PAD. Once a patient’s diagnosis of both IC and PAD have been confirmed, treatment is twofold, namely management of associated cardiovascular risk factors and symptomatic relief. Symptomatic relief is addressed through exercise and lifestyle advice, and, where this is not effective, pharmacological agents may be used. Where pharmacological agents are effective, they are likely to be administered for the lifetime of the patient or until symptoms worsen and require surgery.
The four vasoactive drugs for PAD are as follows.
Cilostazol
-
Brand name, manufacturer Pletal®, Otsuka Pharmaceuticals. 22
-
Other manufacturers None.
-
Therapeutic classification Phosphodiesterase III inhibitor, which acts as a direct arterial vasodilator and also inhibits platelet aggregation. 23
-
Dosage, length of treatment and route Oral, at a dose of 100 mg twice daily (200 mg daily dose), 30 minutes before or 2 hours after food. Treatment for 16–24 weeks can result in a significant improvement in walking distance. Some benefit may be observed following treatment for 4–12 weeks.
-
Licensed indications Cilostazol has a UK marketing authorisation for the improvement of the MWD and PFWD in patients with IC who do not have rest pain and who do not have evidence of peripheral tissue necrosis (PAD Fontaine stage II). 22
-
Contraindications Known hypersensitivity to cilostazol or to any of the excipients; severe renal impairment – creatinine clearance of 25 ml/minute; moderate or severe hepatic impairment; congestive heart failure (CHF); pregnancy; any known predisposition to bleeding [e.g. active peptic ulceration, recent (within 6 months) haemorrhagic stroke, proliferative diabetic retinopathy, poorly controlled hypertension]; with any history of ventricular tachycardia, ventricular fibrillation or multifocal ventricular ectopics, whether or not adequately treated, and prolongation of the QTc interval. 22
-
Warnings Patients should be warned to report any episode of bleeding or easy bruising while on therapy. It is possible that an increased bleeding risk occurs in combination with surgery. There have been rare or very rare reports of haematological abnormalities. Caution is advised when cilostazol is co-administered with inhibitors or inducers of Cytochrom P enzymes CYP3A4 and CYP2C19 or with CYP3A4 substrates, when prescribing cilostazol along with any other agent that has the potential to reduce blood pressure for patients with atrial/ventricular ectopy and patients with atrial fibrillation or flutter or when co-administering cilostazol with any other agents that inhibit platelet aggregation. See the Summary of Product Characteristics22 for further details.
Naftidrofuryl oxalate
-
Brand name, manufacturer (Praxilene®, Merk Serono). 2
-
Other manufacturers Actavis UK, Kent Pharmaceuticals, Mylan, Teva UK. 24
-
Therapeutic classification Peripheral vasodilator that selectively blocks vascular and platelet 5-hydroxytryptamine (5-HT2) receptors. 23
-
Dosage, length of treatment and route Oral, one or two 100-mg capsules three times daily (300 mg or 600 mg daily dose) for a minimum of 3 months or at the discretion of the physician. 22
-
Licensed indications Naftidrofuryl oxalate has a UK marketing authorisation for peripheral vascular disorders including IC. 22
-
Indications not included in this review Peripheral vascular disorders – night cramps, rest pain, incipient gangrene, trophic ulcers, Raynaud’s syndrome, diabetic arteriopathy and acrocyanosis; cerebral vascular disorders – cerebral insufficiency and cerebral atherosclerosis, particularly where these manifest themselves as mental deterioration and confusion in the elderly. 22
-
Contraindications Hypersensitivity to the drug. Patients with a history of hyperoxaluria or recurrent calcium-containing stones. 22
-
Warnings A sufficient amount of liquid should be taken during treatment to maintain an adequate level of diuresis. 22
Pentoxifylline
-
Brand name, manufacturer Trental 400®, Sanofi-aventis. 22
-
Other manufacturers Apotex UK. 24
-
Therapeutic classification Peripheral vasodilator, which is derived from methylxanthine. 23
-
Dosage, length of treatment and route Recommended initial dose, one tablet (400 mg) three times daily (1200 mg daily dose); two tablets daily may prove sufficient in some patients (800 mg daily dose), particularly for maintenance therapy. Tablets should be taken with or immediately after meals, and swallowed whole with plenty of water. In patients with impairment of renal function (creatinine clearance < 30 ml/minute), a dose reduction by approximately 30–50% may be necessary, guided by individual tolerance. 22
-
Licensed indications UK marketing authorisation for the treatment of PAD, including IC and rest pain. 22
-
Contraindications: Not suitable for children; known hypersensitivity to the active constituent, pentoxifylline other methylxanthines or any of the excipients; patients with cerebral haemorrhage, extensive retinal haemorrhage, acute MI and severe cardiac arrhythmias. 22
-
Warnings Use with caution in patients with hypotension or severe coronary artery disease, and particularly careful monitoring is required in patients with impaired renal function. See the Summary of Product Characteristics22 for further details.
Inositol nicotinate
-
Brand name, manufacturer Hexopal®, Genus Pharmaceuticals. 22
-
Other manufacturers Mylan. 24
-
Therapeutic classification Peripheral vasodilator thought to work by slowing the release of nicotinic acid. 23
-
Dosage, length of treatment and route The usual dose is two 500-mg tablets three times daily (3 g daily dose). The dose may be increased to 4 g daily if necessary.
-
Licensed indications UK marketing authorisation for the symptomatic relief of severe IC.
-
Indications not included in the review Raynaud’s phenomenon.
-
Contraindications Recent MI or acute phase of a cerebrovascular accident; hypersensitivity to ingredients.
-
Warnings Use with caution in the presence of cerebrovascular insufficiency or unstable angina.
Identification of important subgroups
No specific subgroups have been identified for consideration within the effectiveness review. However, there is a subgroup of patients with more severe IC who may be more likely to be offered angioplasty. If effective, these drugs may prevent the need for angioplasty for some patients within this small subgroup. This would impact upon cost-effectiveness and hence an exploratory subgroup analysis is undertaken within the cost-effectiveness analysis.
Current usage in the NHS
Within England and Wales the vasoactive drugs being assessed within this report are generally available for prescribing to patients with IC. However, there may be restrictions to their use due to local policies (Steven Thomas, Jonathan Michaels and Gerard Stansby, personal communication). The only evidence available around current usage of the vasoactive drugs for PAD within England and Wales is the Prescription Costs Analysis England 2009,25 from which it is estimated that the proportionate market shares for cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate are 29%, 52%, 4% and 15%, respectively.
Anticipated costs associated with intervention
As described in Current service provision, the only additional costs associated with the vasoactive drugs compared with no vasoactive drugs for PAD are the acquisition costs. These are shown in Table 2. 26 Where there is more than one licensed dose available, the cost of the drug was based upon the doses used within the randomised controlled trials (RCTs) identified within the clinical effectiveness review. Naftidrofuryl oxalate is available both as a generic drug, at a lower price, and as Praxilene by the original patent-holder.
| Drug | Licensed dose | Dose used for estimating costs (mg/day) | Quantity | Drug specification (manufacturer) | Price (£) | Weekly costs (£) |
|---|---|---|---|---|---|---|
| Cilostazol | 100 mg twice daily (30 minutes before or 2 hours after food), i.e. 200 mg per day | 200 | 56 | 100-mg tablets (Pletal) | 35.31 | 8.83 |
| Naftidrofuryl oxalate | 100–200 mg three times daily, i.e. 300 mg or 600 mg per day | 600 | 84 | 100-mg capsules (generic) | 4.52 | 2.26 |
| 100 | 100-mg capsules (Praxilene) | 9.83 | 4.13 | |||
| Pentoxifylline | 400 mg two to three times daily, i.e. 800 mg or 1200 mg per day | 1200 | 90 | 400-mg tablets (Trental 400) | 19.68 | 4.59 |
| Inositol nicotinate | 3 g daily in two or three divided doses; maximum 4 g daily (tablets 500 mg or 750 mg) | 4000 | 100 | 500-mg tablets (Hexopal) | 30.76 | 17.23 |
Chapter 2 Definition of the decision problem
This review will assess the clinical effectiveness and cost-effectiveness of vasoactive drugs for the treatment of IC due to PAD in adults whose symptoms continue despite a period of conservative management. Conventional management usually involves 3–6 months of conservative treatment that would consist of risk modification, usually with a statin, aspirin, smoking cessation advice and advice to exercise (Steven Thomas, Jonathan Michaels and Gerard Stansby, University of Sheffield, July 2010, personal communication).
Decision problem
The decision problem has been specified as follows.
Interventions
-
Cilostazol (Pletal).
-
Naftidrofuryl oxalate (Praxilene/generic).
-
Pentoxifylline (Trental 400).
-
Inositol nicotinate (Hexopal).
Population
The population will include people with IC due to PAD whose symptoms continue despite a period of conservative management. No relevant subgroups have been identified for consideration within the review; however, an exploratory analysis around a subgroup of patients with more severe IC who may receive angioplasty was carried out within the economic model. Subgroups of CVD risk factor would have been considered if data were available.
Relevant comparators
The vasoactive drugs will be compared with each other and with no vasoactive drugs.
Outcomes
-
Maximal walking distance.
-
Pain-free walking distance.
-
Ankle–brachial pressure index.
-
Vascular events (including interventions and requirement of hospitalisation).
-
Mortality.
-
Adverse effects of treatment.
-
Health-related quality of life.
Overall aims and objectives of assessment
The review has the following aims:
-
to evaluate the clinical effectiveness of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of IC due to PAD in adults whose symptoms continue despite a period of conservative management
-
to evaluate the adverse effect profile of the vasoactive drugs for PAD
-
to estimate the incremental cost-effectiveness of the vasoactive drugs for PAD
-
to identify key areas for primary research
-
to estimate the possible overall cost in England and Wales for vasoactive drugs for PAD.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Identification of studies
A comprehensive search was undertaken to systematically identify clinical effectiveness literature concerning cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate within their licensed indications for the treatment of IC in people with PAD.
The search strategy comprised the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
The following databases were searched for published trials and systematic reviews:
-
MEDLINE: Ovid, 1950 to present
-
MEDLINE In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
EMBASE: Ovid, 1980 to present
-
The Cochrane Library: Wiley Interscience
-
– Cochrane Database of Systematic Reviews (CDSR), 1996 to present
-
– Database of Abstracts of Reviews of Effects (DARE), 1995 to present
-
– Cochrane Central Register of Controlled Trials (CCRCT), 1995 to present
-
– Cochrane Methodology Register, 1904 to present
-
– Health Technology Assessment Database (HTA), 1995 to present
-
– NHS Economic Evaluation Database (NHS EED), 1995 to present
-
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL): EBSCO, 1982 to present
-
Web of Knowledge Science Citation Index, 1899 to present
-
Conference Proceedings Citation Index (CPCI): Web of Knowledge, 1990 to present
-
BIOSIS Previews: Web of Knowledge, 1969 to present.
Additional searches were carried out for unpublished studies (e.g. ongoing, completed):
-
The National Research Register (NRR): NIHR, 2000–7
-
The metaRegister of Controlled Trials (mRCT): Springer Science+Business Media, 2000 to present.
Industry submissions, as well as any relevant systematic reviews, were also hand searched in order to identify any further clinical trials.
The MEDLINE search strategy is presented in Appendix 1. The search strategies were translated across all databases. No date (from the start of database coverage date to present) or language restrictions were applied to all searches. Literature searches were conducted from April to June 2010. References were collected in a bibliographic management database, and duplicates removed.
Inclusion and exclusion criteria
Inclusion criteria
Inclusion criteria were taken from the scope provided by NICE,23 outlined below.
Interventions
The following vasoactive drugs were included if administered within their licensed indications:
-
cilostazol
-
naftidrofuryl oxalate
-
pentoxifylline
-
inositol nicotinate.
Population
-
People with IC due to PAD, whose symptoms continue despite a period of conservative management.
Comparators
-
Placebo.
-
Usual care of PAD without vasoactive drugs.
-
Vasoactive drugs compared with each other.
Outcomes
-
Maximal walking distance.
-
Pain-free walking distance.
-
Ankle–brachial pressure index.
-
Cardiovascular events (including interventions and requirement of hospitalisation).
-
Mortality.
-
Adverse effects of treatment.
-
Health-related quality of life.
Study types
Randomised controlled trials were included. Data from non-randomised studies were not included, as evidence for relevant populations and outcomes was available from RCTs.
Systematic reviews were included if they provided additional data for RCTs meeting the inclusion criteria (i.e. unavailable from published trial reports). Other systematic reviews identified were not included but were checked for RCTs that met the inclusion criteria of this review.
Exclusion criteria
Studies based on animal models; preclinical and biological studies; editorials, opinion pieces; reports published as meeting abstracts only, where insufficient details were reported to allow inclusion; studies only published in languages other than English; studies with vasoactive drugs not within their licensed indications; studies in which the population was not restricted to Fontaine stage II, unless data for just this population were presented; and studies that did not present data for the included outcomes.
Studies retrieved for full-paper screening that were excluded are listed in Appendix 2 with reasons for exclusion. Based on the above inclusion/exclusion criteria, study selection was conducted by one reviewer, with involvement of a clinical advisor when necessary.
Data abstraction and critical appraisal strategy
Data were extracted with no blinding to authors or journal. Quality relating to study design was assessed according to criteria based on NHS Centre for Reviews and Dissemination (CRD) Report No. 4,27 and quality relating to studies of PAD was assessed according to criteria developed by EMA. 27 The quality assessment forms are shown in Appendix 3. The purpose of such quality assessment was to provide a narrative account of trial quality for the reader. Data were extracted by one reviewer using a standardised form, shown in Appendix 4, and checked by a second reviewer.
Data synthesis methods
Prespecified outcomes were tabulated and discussed within a descriptive synthesis. MWD and PFWD were synthesised across studies using meta-analysis models. Separate analyses were conducted for the evaluation of cilostazol on MWD, based on the studies described in the Cochrane review by Robless et al. ,28 and for MWD and PFWD for all studies that formed a network of evidence.
The analyses used a random effects model (to allow for heterogeneity in treatment effect across studies) implemented using Winbugs software (MRC Biostatistics Unit, Cambridge, UK);29 details of the statistical model are described in Appendix 5. The summary statistics that were analysed were the absolute mean change from baseline in MWD compared with week 24 for studies included in the Cochrane review,28 the logarithm (log) of the geometric mean change from baseline in MWD compared with week 24, and the log of the geometric mean change from baseline in PFWD compared with week 24.
Individual studies generally reported treatment effects in terms of the ratio of the geometric mean change from baseline. Taking the log of the geometric means meant that the transformed sample statistics were additive on the log scale. Studies that reported results only in terms of the arithmetic mean change from baseline were not transformed to the log scale because taking the log of arithmetic means does not produce additive results on the log scale.
Results were reported in terms of the mean difference and 95% credible interval for the mean difference for each intervention relative to placebo. Finally, a random effects model places a random component on the treatment by study interaction term in the model and acknowledges the fact that the effect of treatment varies across studies. Therefore, the posterior mean of the between-study standard deviation (SD) together with the 95% credible interval is also presented.
Results
Quantity and quality of research available
Quantity of research available
The search for clinical effectiveness literature yielded 1867 article citations after duplicates had been removed. Figure 1 shows study selection. Citations presenting purely economic analyses were not included in this chapter. Trials excluded at full-paper screening stage (see Figure 1) are shown in Appendix 2.
FIGURE 1.
Flow diagram of study inclusion [adapted from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA30)].
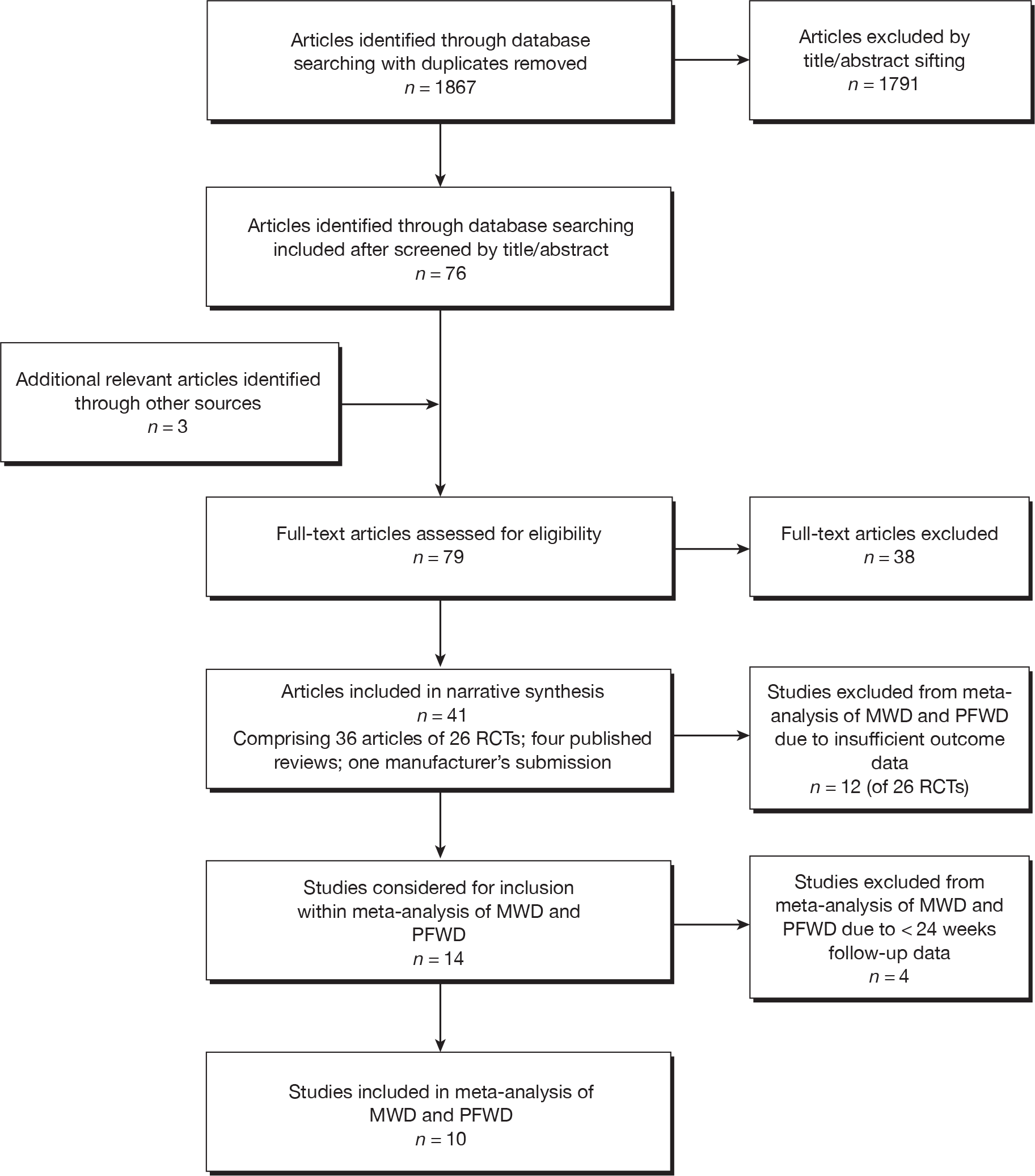
Twenty-six RCTs were identified that met the inclusion criteria for this review. There were 36 published articles describing these 26 RCTs (Table 3).
| Trial name | Treatment group 1, daily dose | Treatment group 2, daily dose | Treatment group 3, daily dose | Treatment group 4 | Groups not relevant to this review |
|---|---|---|---|---|---|
|
CASTLE |
Cilostazol 200 mg | Placebo | |||
| O’Donnell 200951,53–55,83 | Cilostazol 200 mg | Placebo | |||
| Strandness 200256,57 | Cilostazol 200 mg | Placebo | Cilostazol 100-mg daily dose | ||
| Dawson 200058–60 | Cilostazol 200 mg | Placebo | Pentoxifylline 1200 mg | ||
| Beebe 199961 | Cilostazol 200 mg | Placebo | Cilostazol 100-mg daily dose | ||
| Otsuka 21-94-30134 | Cilostazol 200 mg | Placebo | Pentoxifylline 1200 mg | ||
| Otsuka 21-98-21334 | Cilostazol 200 mg | Placebo | Pentoxifylline 1200 mg | ||
| Money 199862 | Cilostazol 200 mg | Placebo | |||
| Dawson 199864 | Cilostazol 200 mg | Placebo | |||
| Elam 199864 | Cilostazol 200 mg | Placebo | |||
| Otsuka 21-95-20134 | Cilostazol 200 mg | Placebo | Cilostazol 300 mg | ||
| Spengel 200247 | Naftidrofuryl oxalate 600 mg | Placebo | |||
| Kieffer 200165 | Naftidrofuryl oxalate 600 mg | Placebo | |||
| Adhoute 198666 | Naftidrofuryl oxalate 600 mg | Placebo | |||
| Trubestein 198467 | Naftidrofuryl oxalate 600 mg | Placebo | |||
| Ruckley 197868 | Naftidrofuryl oxalate 300 mg | Placebo | |||
| Dettori 198969 | Pentoxifylline 1200 mg | Placebo | Acenocoumarol (dose adjusted according to INR) plus placebo; acenocoumarol plus pentoxifylline 1200 mg | ||
| Creager 200870 | Pentoxifylline 1200 mg | Placebo | Iloprost 100 µg plus placebo; iloprost 200 µg plus placebo; iloprost 300 µg plus placebo | ||
| Lindgarde 198971 | Pentoxifylline 1200 mg | Placebo | |||
| Porter 1982, and Gillings 198772–75 | Pentoxifylline 1200 mg | Placebo | |||
| Gallus 198576 | Pentoxifylline 1200 mg | Placebo | |||
| Di Perri 198377 | Pentoxifylline 1200 mg | Placebo | |||
| O’Hara 198878,79 | Inositol nicotinate 4 g | Placebo | |||
| Kiff 198880 | Inositol nicotinate 4 g | Placebo | |||
| Head 198681 | Inositol nicotinate 4 g | Placebo | |||
|
INEXACT Hobbs 200782 |
Cilostazol 200 mg | Cilostazol 200 mg plus supervised exercise | Supervised exercise | Usual care |
Four published systematic reviews28,31–33 were included in this review, as they provided additional data from the included RCTs that were unavailable from the published trial reports. In addition, the manufacturer’s submission to NICE of cilostazol34 also provided additional data from the included RCTs which were not available in the trial reports.
Other published systematic reviews were not included in this review as they did not provide additional trial data, but they were checked for RCTs meeting the inclusion criteria of this review. 35–46 No additional RCTs were identified from these excluded reviews.
Twenty-six RCTs34,47–82 were included in this review. One of these was a pooled analysis of three RCTs, run as a study programme by Spengel et al. 47 The three individual RCTs were not considered separately. The included trials and their treatment groups are shown in Table 3. Eligibility criteria and baseline characteristics were similar across trials, with clinically diagnosed, stable IC, patients of both genders included, and age ranges within 35–86 years. Further details of these included trials, including baseline characteristics of the study population, outcome measures used, details of withdrawals and study results, are shown in Appendix 4.
Three of the included studies have not been published (to date) as trial reports: Otsuka 21-94-301; Otsuka 21-98-213; Otsuka 21-95-201. Information about these trials was available from three published reviews28,31,33 and the manufacturer’s submission to NICE. 34 Additional information on naftidrofuryl oxalate trials was available from one published systematic review. 32
Placebo-controlled RCTs were available for all four of the vasoactive drugs for PAD assessed within this report. The only head-to-head comparison was that of cilostazol versus pentoxifylline. Studies with more than two trial arms provided data for more than one comparison.
The included studies provided data for the following comparisons:
-
cilostazol 200 mg versus placebo (11 trials)
-
naftidrofuryl oxalate 600 mg versus placebo (four trials)
-
naftidrofuryl oxalate 300 mg versus placebo (one trial)
-
pentoxifylline 1200 mg versus placebo (nine trials)
-
inositol nicotinate 4 g versus placebo (three trials)
-
cilostazol 200 mg versus pentoxifylline 1200 mg (three trials)
-
cilostazol 200 mg (with or without supervised exercise) versus usual care (with or without supervised exercise) (one trial).
The number of patients and outcomes reported for these comparisons are shown in Tables 4–10. Treatment duration is also shown in these tables, and it can be seen that only two studies had a treatment duration of more than 24 weeks (CASTLE,49 Dettori et al. 69). The 11 trials comparing cilostazol versus placebo (see Table 4) were the same 11 trials included in the manufacturer’s submission to NICE. 34
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes reported | |
|---|---|---|---|---|
| Cilostazol | Placebo | |||
|
CASTLE Hiatt 200849 |
Up to 144 | 717 | 718 | Mortality, cardiovascular events, AEs |
| O’Donnell 200951 | 24 | 51 | 55 | MWD, PFWD, AEs, HRQoL |
| Strandness 200256 | 24 | 133 | 129 | MWD, PFWD, mortality, cardiovascular events, AEs, HRQoL |
| Dawson 200058 | 24 | 227 | 239 | MWD, PFWD, ABPI, mortality, cardiovascular events, AEs, HRQoL |
| Beebe 199961 | 24 | 175 | 170 | MWD, PFWD, mortality, cardiovascular events, AEs, HRQoL |
| Otsuka 21-94-30134 | 24 | 123 | 124 | MWD, PFWD, cardiovascular events, AEs |
| Otsuka 21-98-21334 | 24 | 260 | 260 | MWD, PFWD, mortality, AEs |
| Money 199862 | 16 | 119 | 120 | MWD, PFWD, ABPI, mortality, cardiovascular events, AEs, HRQoL |
| Dawson 199863 | 12 | 54 | 27 | MWD, PFWD, mortality, cardiovascular events, AEs |
| Elam 199864 | 12 | 95 | 94 | MWD, PFWD, ABPI, mortality, cardiovascular events, AEs, HRQoL |
| Otsuka 21-95-20134 | 12 | 72 | 70 | MWD, PFWD, mortality, AEs, HRQoL |
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes | |
|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | |||
| Spengel 200247 | 24 | 382 | 372 | PFWD, mortality, AEs, HRQoL |
| Kieffer 200165 | 24 | 98 | 98 | MWD, PFWD, ABPI, cardiovascular events, AEs |
| Adhoute 198666 | 24 | 64 | 54 | PFWD, ABPI, AEs |
| Trubestein 198467 | 12 | 54 | 50 | MWD, PFWD, AEs |
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes | |
|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | |||
| Ruckley 197868 | 12 | 25 | 25 | PFWD, AEs |
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes | |
|---|---|---|---|---|
| Pentoxifylline | Placebo | |||
| Dettori 198969 | 52 | 37 | 37 | PFW time, ABPI, mortality, cardiovascular events |
| Creager 200870 | 24 | 86 | 84 | MWD, PFWD, mortality, cardiovascular events, AEs, HRQoL |
| Dawson 200058 | 24 | 232 | 239 | MWD, PFWD, ABPI, mortality, cardiovascular events, AEs |
| Lindgarde 198971 | 24 | 76 | 74 | MWD, PFWD, AEs |
| Porter 198274 | 24 | 67 | 61 | MWD, PFWD, cardiovascular events that lead to withdrawal, AEs |
| Otsuka 21-94-30134 | 24 | 123 | 124 | MWD, PFWD, cardiovascular events, AEs |
| Otsuka 21-98-21334 | 24 | 262 | 262 | MWD, PFWD, mortality, AEs |
| Gallus 198576 | 8 | 25 | 23 | MWD, PFWD, mortality, cardiovascular events that lead to withdrawal |
| Di Perri 198377 | 8 | 12 | 12 | MWD |
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes | |
|---|---|---|---|---|
| Inositol nicotinate | Placebo | |||
| O’Hara 198878 | 12 | 62 | 58 | PFW paces, mortality, cardiovascular events that lead to withdrawal, AEs that lead to withdrawal |
| Kiff 198880 | 12 | 40 | 40 | MWD, ABPI, cardiovascular events that lead to withdrawal, AEs that lead to withdrawal |
| Head 198681 | 12 | 51 | 62 | Time to claudication, cardiovascular events that lead to withdrawal, AEs that lead to withdrawal |
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes | |
|---|---|---|---|---|
| Cilostazol | Pentoxifylline | |||
| Dawson 200058 | 24 | 227 | 232 | MWD, PFWD, ABPI, mortality, cardiovascular events, AEs |
| Otsuka 21-94-30134 | 24 | 123 | 123 | MWD, PFWD, cardiovascular events, AEs |
| Otsuka 21-98-21334 | 24 | 260 | 260 | MWD, PFWD, mortality, AEs |
| Trial | Treatment duration (weeks) | No. in analysis | Outcomes | |
|---|---|---|---|---|
| Cilostazol | Usual care | |||
|
INEXACT Hobbs 200782 |
24 | 16 | 18 | MWD, PFWD |
The location of the trials and the number of participants from the UK are shown in Table 11. There are only six UK trials, including assessments of cilostazol (O’Donnell et al. 51,53–55,83 and Hobbs et al. 82), naftidrofuryl oxalate (Ruckley et al. 68) and inositol nicotinate (O’Hara et al. ,78,79 Kiff and Quick 198880 and Head 198681). Most cilostazol studies took place in the USA, whereas studies of pentoxifylline and naftidrofuryl oxalate mostly took place in the USA and Europe.
| Trial name | Treatment and dose | Location | No. of participants from UK |
|---|---|---|---|
|
CASTLE |
Cilostazol 200 mg | USA | 0 |
| O’Donnell 200951,53–55,83 | Cilostazol 200 mg | UK (Northern Ireland) | 106 |
| Strandness 200256,57 | Cilostazol 200 mg | USA | 0 |
| Dawson 200058–60 | Cilostazol 200 mg, pentoxifylline 1200 mg | USA | 0 |
| Beebe 199961 | Cilostazol 200 mg | USA | 0 |
| Otsuka 21-94-30134 | Cilostazol 200 mg, pentoxifylline 1200mg | USA | 0 |
| Otsuka 21-98-21334 | Cilostazol 200 mg, pentoxifylline 1200 mg | USA | 0 |
| Money 199862 | Cilostazol 200 mg | USA | 0 |
| Dawson 199863 | Cilostazol 200 mg | USA | 0 |
| Elam 199864 | Cilostazol 200 mg | USA | 0 |
| Otsuka 21-95-20134 | Cilostazol 200 mg | USA | 0 |
| Spengel 200247 | Naftidrofuryl oxalate 600 mg | Germany, France, Belgium | 0 |
| INEXACT Hobbs 200782 | Cilostazol 200 mg, cilostazol 200 mg plus supervised exercise | UK | 38 |
| Kieffer 200165 | Naftidrofuryl oxalate 600 mg | USA | 0 |
| Adhoute 198666 | Naftidrofuryl oxalate 600 mg | France | 0 |
| Trubestein 198467 | Naftidrofuryl oxalate 600 mg | Germany | 0 |
| Ruckley 197868 | Naftidrofuryl oxalate 300 mg | UK | 50 |
| Dettori 198969 | Pentoxifylline 1200 mg | Italy | 0 |
| Creager 200870 | Pentoxifylline 1200 mg | USA | 0 |
| Lindgarde 198971 | Pentoxifylline 1200 mg | Sweden, Denmark | 0 |
| Porter 1982, Gillings 198772–75 | Pentoxifylline 1200 mg | USA | 0 |
| Gallus 198576 | Pentoxifylline 1200 mg | Australia | 0 |
| Di Perri 198377 | Pentoxifylline 1200 mg | Italy | 0 |
| O’Hara 198878,79 | Inositol nicotinate 4 g | UK | 120 |
| Kiff 198880 | Inositol nicotinate 4 g | UK | 80 |
| Head 198681 | Inositol nicotinate 4 g | UK | 123 |
Quality of research available
Details of the quality assessment scores for each trial are listed in Appendix 3. Across the four sets of studies, CRD items that relate to study quality (as listed in the tables in Appendix 3) were largely fulfilled. Treatment groups were generally comparable, blinding was usually maintained, intention-to-treat (ITT) analysis was usually undertaken, and at least 80% of participants were followed up in most cases. However, sequence generation and allocation concealment were poorly reported, and there may be some problems with imbalances between dropouts, as this was poorly reported. In some cases there is evidence of selective reporting of outcomes.
European Medicines Agency items were, however, less well adhered to. EMA items are specific to PAD and aim to minimise confounding factors. Criteria regarding diagnosis and length of having IC are included to avoid inclusion of patients who were misdiagnosed or have unstable symptoms. These items were usually met, except in the case of inositol nicotinate. The EMA recommends that the treatment period should be a minimum of 24 weeks. Treatment period was a problem in some cases. The use of concomitant treatments was rarely reported and stratification for diabetes, as recommended by EMA, was rare. A placebo run-in period is also recommended, where all patients are given a placebo for between 2 and 6 weeks. The lack of placebo run-ins was an issue in studies of cilostazol and inositol nicotinate, but less problematic in studies of naftidrofuryl oxalate and pentoxifylline. Treadmill testing is the preferred method of assessing walking distances and should follow a standardised protocol. Treadmill use was widespread and usually standardised (although different protocols were used), except in studies of inositol nicotinate. Some patients exhibit highly variable walking distances, which might introduce unwanted noise in these data. The use of two treadmill tests separated by at least a week at baseline and the selection of patients with < 25% change in baseline is recommended by EMA to minimise the effect these types of patients may have on results. These items were adhered to only sometimes and may therefore introduce variability to these data.
Cilostazol
For CRD quality assessment items, studies scored well in most cases and for most items, with some exceptions. Sequence generation and allocation concealment both scored poorly across studies, with most studies failing to report on these items. Imbalances between dropouts was poorly reported (Elam et al. ,64 Dawson et al. ,63 Money et al. ,62 Hiatt et al. ,49 Otsuka 21-95-20134 and Otsuka 21-94-30134) and may be a source of bias. There was some evidence of selective reporting in the Strandness et al. ,56 Elam et al. 64 and Dawson et al. 58 trials, as additional data were found in published systematic reviews. These were mostly AE data and have been incorporated in this review, but they highlight the possibility that there are additional unreported data. It is unclear how this may affect current estimates of the study outcomes. For EMA items, quality was largely good, although there is potential for some problems, mainly due to poor reporting. Treatment duration varied between studies, with only the Strandness et al. ,56 Beebe et al. ,61 Hiatt et al. ,64 O’Donnell et al. ,51 Dawson et al. ,58 Otsuka 21-94-30134 and Otsuka 21-98-21334 trials treating patients for at least 24 weeks (this will not affect the meta-analysis, which considers only studies that treated patients for 24 weeks). The use of concomitant treatment was poorly reported, and studies did not generally state that they had stratified for diabetes. Less than half of the studies stated that only patients with a < 25% change in baseline walking distances were selected, and this may introduce unwanted variability to the results. However, there does not appear to be clinical evidence to suggest that these patients respond differently to treatment. A placebo run-in period was reported only by Dawson et al. 63 and Hiatt et al. 49 – for between 2 and 6 weeks in both cases. All studies reporting walking distance outcomes used a standardised treadmill test. There was, however, heterogeneity in tests between protocols, which is discussed elsewhere in this report.
Naftidrofuryl oxalate
Studies of naftidrofuryl oxalate scored moderately well overall for both CRD and EMA items. Items that scored poorly were sequence generation and allocation concealment, as most studies scored unclear for these items. Baseline characteristics may influence results, as Trubestein et al. 67 and Ruckley et al. 68 did not score positively according to CRD criteria in that the former had more smokers in the intervention group and the latter did not report baseline characteristics. It is unclear what effects this would have on results. For EMA items, more specific problems with patient characteristics were identified as follows: concomitant treatment was unclear in every case; the distribution of diabetics was stratified only in the Kieffer et al. 65 trial and proportions of diabetics are unknown for the Adhoute et al. ,66 Trubestein et al. 67 and Ruckley et al. 68 trials; only Kieffer et al. 65 and Adhoute et al. 66 selected patients with a < 25% change at baseline measurements. Other EMA items were generally well addressed.
Pentoxifylline
Overall, studies were of mixed quality and some items may impact on estimates of treatment effect. Among the CRD quality items, sequence generation and allocation concealment may present problems, with two-thirds of studies scoring unclear. Items that were of mixed quality included the use of an ITT analysis, follow-up of at least 80% of participants, imbalances between dropouts and selective reporting of outcomes. EMA items were also only partially fulfilled. Although diagnosis, history of condition and treatment duration were mostly good, other items were mixed. Among items relating to patient characteristics, it was largely unclear whether or not concomitant treatment was comparable across groups; there may have been imbalances in the numbers of diabetics, and patients may not have always been selected on the basis of having a < 25% change in baseline assessments. Outcomes may also have been affected by the lack of a 2- to 6-week placebo run-in. 34,38,58
Inositol nicotinate
Overall, studies of inositol nicotinate scored well for most CRD quality assessment items but very poorly for the EMA items. This reflects the age of the studies and is likely to introduce a considerable degree of inaccuracy to the study findings. Among the CRD quality assessment items, methods of randomisation and treatment allocation were poorly reported in every case. Baseline characteristics were not similar in the study by Head. 81 All studies stated that they were double blind. An ITT analysis was provided in every case, and at least 80% of participants were followed up in the final analysis. Imbalances in dropouts were not reported or did not occur, and this seems unlikely to affect results. There is no evidence of selective reporting within the studies. Several EMA items scored poorly or were unclear. Only Kiff and Quick80 stated that IC was objectively diagnosed, and only this same study stated that patients had a 6-month history of the condition. None of the studies treated patients for 24 weeks or longer, and it was unclear in every case whether or not concomitant treatments were comparable across groups. Kiff and Quick80 and Head81 did not stratify for diabetes, and did not report how many were diabetic in each group. Although MWD and/or PFWD were reported in O’Hara et al. 78 and Head,81 neither of these studies used a treadmill test, although the alternative walking distance tests used did follow a standard protocol.
Assessment of effectiveness
Results of the clinical effectiveness review are presented for each outcome, organised by comparison.
Maximal walking distance
Maximal walking distance narrative summary
Details of MWD results, where reported, are shown in Tables 12–17 (from published trial reports) and Appendix 4 (which includes details from reviews and the manufacturer’s submission). Across trials, there was a tendency for all groups, including placebo groups, to show improvement with time. Of the 10 studies of cilostazol 200 mg versus placebo comparison, seven significantly favoured cilostazol over placebo,56,58,61–64,83 whereas three trials (the three unpublished Otsuka trials34) did not find any significant difference between groups. As patient populations were similar across trials, in terms of disease, diabetes, hypertension, smoking and age range, these characteristics cannot explain any significant differences between treatment groups. Other issues of trial design were similar across trials: all were blinded, randomised and presented ITT analyses, and all measured baseline walking distance with two treadmill tests. As the graded test encourages longer walking distances than the constant-load protocol, absolute mean walking distance in metres is not directly comparable between protocols (see Chapter 3, Measurement of disease). 28 The use of the treadmill protocol (see Appendix 4) may go some way to explaining the presence of heterogeneity across trials. All three trials using the graded test treadmill protocol reported a significantly greater improvement in the cilostazol group than in the placebo group: Dawson et al. 58 at 24 weeks’ follow-up (p = 0.0005), Money et al. 62 at 16 weeks’ follow-up (p < 0.05) and Elam et al. 64 at 12 weeks’ follow-up (p = 0.004). However, treadmill protocol does not explain why some trials report significant differences and others do not, as this differs between trials using the same treadmill protocol (the constant treadmill protocol). Trials with non-significant results do not have shorter follow-up or smaller sample sizes than trials with significant results.
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in MWD (%) | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Placebo | Cilostazol group | Placebo group | ||||
| O’Donnell 200951 | 24 | 51 | 55 | Constant | 161.7 mean improvement | 79 mean improvement | p = 0.048 |
| Strandness 200257 | 24 | 133 | 129 | Constant | Mean difference (m): 76.2 improvement | Mean difference (m): 21.1 improvement | p = 0.0003 |
| Dawson 200058 | 24 | 227 | 239 | Graded | Mean difference (m): 107 (SD 158) improvement | Mean difference (m): 65 (SD 135) improvement | p = 0.0005 |
| Beebe 199961 | 24 | 175 | 170 | Constant | Mean difference (m): 129.1 improvement | Mean difference (m): 26.8 improvement | p < 0.001 |
| Money 199862 | 16 | 119 | 120 | Graded | Mean difference (m): 96.4 improvement | Mean difference (m): 31.4 improvement | p < 0.05 |
| Dawson 199863 | 12 | 54 | 27 | Constant | 30.5 improvement | –9.3 change (worsening) | p < 0.01 |
| Elam 199864 | 12 | 95 | 94 | Graded | Mean difference (m): 72.7 improvement | Mean difference (m): 25.8 improvement | p = 0.004 |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in MWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | Naftidrofuryl oxalate group | Placebo group | ||||
| Kieffer 200165 | 24 | 98 | 98 | Constant | Mean difference (m): 158.7 improvement | Mean difference (m): 28.1 improvement | p < 0.001 |
| Trubestein 198467 | 12 | 54 | 50 | Constant | Mean difference (m): 122 improvement | Mean difference (m): 90 improvement | Non-significant |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in MWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Pentoxifylline | Placebo | Pentoxifylline group | Placebo group | ||||
| Creager 200870 | 24 | 86 | 84 | Graded | 13.90% improvement | 3.30% improvement | p = 0.039 |
| Dawson 200058 | 24 | 232 | 239 | Graded | Mean difference (m): 64 improvement | Mean difference (m): 65 improvement | p = 0.82 |
| Lindgarde 198971 | 24 | 76 | 74 | Constant | Geometric mean 50% improvement (SE 9) | Geometric mean 29% improvement (SE 8) | p = 0.094 |
| Porter 198273 | 24 | 67 | 61 | Constant | Geometric mean 33% improvement (SE 8) | Geometric mean 20% improvement (SE 7) | Two-sided p = 0.316, one-sided p = 0.049 |
| Gallus 198576 | 8 | 25 | 23 | Constant | Geometric mean 23% improvement | Geometric mean 17% improvement | Ratio of per cent change from baseline (pentoxifylline/placebo) 1.05 (95% CI 0.81 to 1.36), non-significant |
| Di Perri 198377 | 8 | 12 | 12 | Not treadmill, horizontal ground | Mean difference (m): 136 improvement | Mean difference (m): 6 improvement | p < 0.01 |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in MWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Pentoxifylline | Cilostazol group | Pentoxifylline group | ||||
| Dawson 200058 | 24 | 227 | 232 | Graded | Mean difference (m): 107 (SD 158) improvement | Mean difference (m): 64 (SD 127) improvement | p = 0.0002 |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in MWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Inositol nicotinate | Placebo | Cilostazol group | Pentoxifylline group | ||||
| Kiff 1988 | 12 | 40 | 40 | Patient walked at own pace on a constant slope | Mean difference (m): 65.4 improvement | Mean difference (m): 102.8 improvement | Non-significant |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in MWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Usual care | Cilostazol group | Usual care group | ||||
| INEXACT Hobbs 200782 | 24 | 16 (seven with exercise, nine without) | 18 (nine with exercise, nine without) | Constant | Plus exercise mean ratio 2.58 (SD 1.39), without exercise mean ratio 1.69 (SD 0.59) improvement | Plus exercise mean ratio 1.45 (SD 0.80), without exercise mean ratio 1.09 (SD 0.34) improvement | Difference in effect 1.64 (p = 0.005) |
Of the seven trials using the constant-workload treadmill protocol, four56,61,63,83 reported a significantly greater improvement in the cilostazol group than in the placebo group, although for one of these trials83 significance was only borderline; three of these had follow-up time of 24 weeks [O’Donnell et al. 83 (p = 0.048), Strandness et al. 56 (p = 0.0003), Beebe et al. 61 (p < 0.001)], and the fourth trial, Dawson et al. ,63 had a follow-up time of 12 weeks (p < 0.01). The three trials34 that did not find any significant difference between groups used the constant-workload treadmill protocol, and two of these trials had follow-up times of 24 weeks (Otsuka 21-94-301, p = 0.06;34 Otsuka 21-98-213, p = 0.9134), and the other had a follow-up time of 12 weeks (Otsuka 21-95-201, p = 0.9034). Lack of significant treatment effect cannot be explained by sample size, as these three trials did not have smaller sample sizes than the other trials (see Appendix 4).
The review by Pande et al. 31 included nine industry-sponsored trials, of which six trials (Otsuka trials56,57,61–64) found a significant difference between treatment groups, and three trials (the three trials without published trial reports) found no significant difference between cilostazol 200 mg and placebo groups (Otsuka trials 21–94–301, 21–98–213, 21–95–201 et al. 34). 31 The Pande et al. review31 presented a pooled analysis of these nine trials as a ratio of geometric means, and calculated an estimate of treatment effect31 of 1.15 95% [confidence interval (CI) 1.11 to 1.19], which significantly favoured cilostazol over placebo. 31 This analysis31 did not include the O’Donnell et al. trial,51 which found a borderline significant treatment effect for the whole trial population (p = 0.048) but found no significant difference between treatment groups when considering the subgroups of patients with diabetes (p = 0.09, n = 26)53 or without diabetes (p = 0.27, n = 80),83 which may reflect the small sample sizes rather than lack of actual treatment effect. The cilostazol versus placebo comparison trials that reported significant treatment effect for MWD generally also reported significant treatment effect for PFWD (see Chapter 5, Pain-free walking distance) and vice versa; however, there were a couple of exceptions in that the O’Donnell et al. 83 and Elam et al. 64 trials, found a significant treatment effect for MWD but did not find a significant treatment effect for PFWD.
Two trials for the naftidrofuryl oxalate 600 mg versus placebo comparison reported MWD; Kieffer et al. 65 reported significantly greater improvement for naftidrofuryl oxalate 600 mg versus placebo (p < 0.001), and Trubestein et al. 67 found no significant difference between groups. It may be that this difference could be explained in terms of length of follow-up, in that Kieffer et al. 65 had a follow-up of 24 weeks, whereas Trubestein et al. 67 had a follow-up of 12 weeks. These trials both used the constant-workload treadmill protocol and designs were similar in terms of having a placebo run-in, being randomised, presenting ITT analyses, measuring baseline walking distance with two tests and being blinded. There was little difference between these two trials in baseline MWD (see Appendix 4). However, both naftidrofuryl oxalate trials (Kieffer et al. ,65 Trubestein et al. 67) had higher baseline MWD than the cilostazol trials that used the constant workload treadmill protocol (Strandness et al. ,57 Beebe et al. ,61 Dawson et al. ,63 O’Donnell et al. ,51 Otuska trials 21–94–301, 21–98–213, 21–95–201) (see Appendix 4). 34,83 The Kieffer65 trial found a significant treatment effect for PFWD (see Chapter 5, Pain-free walking distance) as well as for MWD. However, the Trubestein et al. 67 trial, which had not found a significant treatment effect for MWD, did report a significant effect for PFWD favouring naftidrofuryl oxalate (see Chapter 5, Pain-free walking distance).
Of the eight trials34,58,70,71,73,76,77 comparing pentoxifylline versus placebo in terms of MWD, two trials significantly favoured pentoxifylline over placebo: Creager et al. (p = 0.039)70 and Di Perri et al. (p < 0.01). 77 Of these, the Di Perri77 trial did not use a treadmill protocol, instead measuring the distance that a patient could walk on a horizontal level at metronome controlled speed of 120 steps per minute, with a follow-up at 8 weeks. The Creager et al. 70 trial used a graded treadmill test protocol, and found a significant effect on MWD at 24 weeks. Of the six trials finding no significant difference between groups for MWD, one of these used the graded test (Dawson et al. ;58 p = 0.82) and had a follow-up of 24 weeks. The five trials using the constant-workload treadmill protocol all found no statistically significant difference between pentoxifylline and placebo groups (Gallus et al. ,76 Lindgarde et al. ,71 Porter et al. ,73 Otsuka 21-98-21334 and Otsuka 21-94-30134). Of these, the Gallus et al. 76 study had a follow-up of only 8 weeks [ratio of percentage change from baseline 1.05 (95% CI 0.81 to 1.36), which was non-significant], and the other studies had follow-up of 24 weeks: Lindgarde et al. 71 (p = 0.09), Porter et al. 73 (two-sided p = 0.32), Otsuka 21-98-21334 (p = 0.24) and Otsuka 21-94-30134 (p = 0.29). The pentoxifylline-versus-placebo comparison trials that reported significant treatment effect for MWD generally also reported significant treatment effect for PFWD (see Chapter 5, Pain-free walking distance) and vice versa; however, there were a couple of exceptions in that the Creager et al. 70 trial, which found a significant treatment effect for MWD, did not find a significant treatment effect for PFWD, and the Dawson et al. 58 trial did not find an effect for MWD but did find a treatment effect for PFWD (see Chapter 5, Pain-free walking distance).
For the comparison of inositol nicotinate 4 g versus placebo, only the Kiff and Quick trial80 reported MWD. This trial found no significant difference between inositol nicotinate and placebo groups at 12 weeks for MWD measured by patients walking at their most comfortable speed on a treadmill set at a 10% gradient. 80
Three trials reported MWD for the comparison of cilostazol versus pentoxifylline, all with 24 weeks’ follow-up (Dawson et al. ,58 Otsuka 21-98-21334 and Otsuka 21-94-30134). Two trials found no significant difference between the cilostazol and pentoxifylline groups (Otsuka 21-98-213,34 p = 0.65; Otsuka 21-94-301,34 p = 0.87), both using the constant-workload treadmill protocol. One trial (Dawson et al. 58), which used the graded test treadmill protocol, found a significantly greater improvement in MWD (p = 0.0002) in the cilostazol group than in the pentoxifylline group. 58
In the one trial (Hobbs et al. 82) comparing cilostazol (with or without supervised exercise) versus usual care (with or without supervised exercise), all treatment groups improved, but there was significantly more improvement for cilostazol added to supervised exercise or usual care (p = 0.005). 82 This trial used the constant workload treadmill protocol and measured MWD at 24 weeks.
Maximal walking distance meta-analysis
The reanalysis of the cilostazol trials included within the Cochrane review28 is presented in Table 18, in terms of change from baseline in absolute mean walking distance.
| Study | Placebo: mean (SD), n | Cilostazol: mean (SD), n |
|---|---|---|
| Dawson 199863 | 4.56 (61.5,) 25 | 84.6 (144.94), 52 |
| Elam 199864 | 36.1 (141.55), 94 | 79.05 (134.5), 95 |
| Money 199862 | 47.1 (124.88), 120 | 101.1 (154.9), 119 |
| Beebe 199961 | 26.82 (148.5), 140 | 129.1 (463.3), 140 |
| Dawson 200058 | 64.7 (134.61), 226 | 107.36 (158.4), 205 |
| Strandness 200256 | 23.2 (78.26), 125 | 96.41 (200.44), 124 |
| Otsuka 21-95-20134 | 38.1 (69.7), 60 | 35.2 (72.05), 54 |
The posterior distribution of a parameter is a weighted compromise between the prior information and the sample data. In particular, if for some value of the likelihood function (expressing what is known about a parameter based on the sample data) the likelihood of that value is small, so that these data suggest that this value is implausible, then the posterior distribution will also give small probability to this value. Similarly, if for some value of the prior distribution (expressing what is known about a parameter in addition to the sample data) the prior probability of that value is small, so that the prior information suggests that this value is implausible, then, again, the posterior distribution will also give small probability to this value. In general, the posterior probability will be high for some value only when both information sources support that value. The posterior mean treatment effect for the cilostazol studies, together with the 95% credible interval, is shown in Table 19. Table 19 also shows the posterior mean of the between-study SD, together with the 95% credible interval.
| Treatment effect | Mean (95% credible interval) |
|---|---|
| Cilostazol random effects | 57.27 (24.93 to 86.57) |
| Cilostazol predictive distribution | 57.28 (–16.40 to 127.40) |
| Between-study SD | 25.16 (1.46 to 72.75) |
The random effects meta-analysis of the change from baseline in absolute mean walking distance showed that treatment with cilostazol resulted in an increase of 57.27 m (95% credible interval 24.93 to 86.57 m) compared with placebo.
For the overall comparison of the treatment options, of the 26 studies identified by the systematic literature review, 12 studies were excluded from the meta-analysis of MWD for the reasons provided within Table 20.
| Study | Drug assessed | Reason for exclusion |
|---|---|---|
| Di Perri 198377 | Pentoxifylline | This study was excluded because it was an 8-week study |
| Gallus 198576 | Pentoxifylline | This study was excluded because it was an 8-week study |
| Head 198681 | Inositol nicotinate | This study was excluded because it was a 12-week study and provided no information on percentage change from baseline |
| Kiff 198880 | Inositol nicotinate | This study was excluded because it was a 12-week study and provided no information on percentage change from baseline |
| O’Hara 198878 | Inositol nicotinate | This study was excluded because it was a 12-week study and provided no information on MWD or PFWD |
| Detorri 198969 | Pentoxifylline | This study was excluded because MWD or PFWD was not collected in the study |
| Otsuka 21–98–21448–50 | Cilostazol | This study provided no information on MWD or PFWD |
| Adhoute 198666 | Naftidrofuryl oxalate | This study provided no information on MWD and no PFWD data suitable for inclusion in the network meta-analysis |
| Trubestein 198467 | Naftidrofuryl oxalate | This study provided no information on percentage change from baseline in MWD or PFWD |
| Ruckley 197868 | Naftidrofuryl oxalate | This study was excluded because it was a comparison of naftidrofuryl oxalate 300 mg daily and provided no information on percentage change from baseline in MWD or PFWD |
| Spengel 200247 | Naftidrofuryl oxalate | This study provided no information on MWD and no information on PFWD by treadmill test; PFWD was presented as patient estimates |
| Hobbs 200782 | Cilostazol | This study used Best Medical Treatment as the comparator (may be alongside supervised exercise) |
The evidence base for the log of the geometric mean change from baseline in MWD and PFWD generates a network of trials comparing different pairs or triplets of treatments, as shown in Figure 2. The numbers within Figure 2 represent the number of times that specific treatment arms are compared within studies.
FIGURE 2.
Network of evidence used in the analysis of the change from baseline in log mean walking distance (log m).
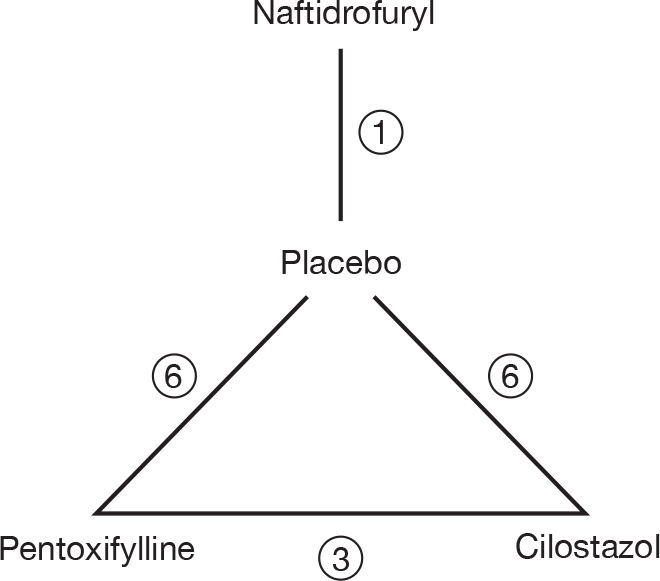
The 10 studies (leading to 16 comparisons) included within the meta-analysis of MWD, represented in Figure 2, are the seven two-arm and three three-arm 24-week studies that are described in Table 21. Three 12-week studies34,63,64 and one 16-week study62 in which there were data on MWD available, as described in Table 21, were excluded from this analysis as the outcomes from these studies with a shorter follow-up period are not directly comparable.
| Study | Placebo: mean (SD), n | Cilostazol: mean (SD), n | Pentoxifylline: mean (SD), n | Naftidrofuryl oxalate: mean (SD), n |
|---|---|---|---|---|
| a,bDawson 199863 | –0.098 (0.847),c 25 | 0.266 (0.847),c 52 | ||
| a,bElam 199864 | 0.218 (0.438), 94 | 0.304 (0.438), 95 | ||
| a,dMoney 199862 | NAe (0.358),h 120 | NAe (0.358),h 119 | ||
| aBeebe 199961 | 0.140 (0.464),c 140 | 0.412 (0.464),c 140 | ||
| aStrandness 200256 | 0.184 (0.441), 125 | 0.578 (0.441), 124 | ||
| a,bOtsuka 21-95-20134 | 0.262 (0.396), 66 | 0.247 (0.396), 60 | ||
| aO’Donnell 200983 | 0.582 (0.993),f 55 | 0.962 (0.993),f 51 | ||
| Porter 198272 | 0.148 (NA), 61 | 0.285 (NA), 63 | ||
| Lindgarde 198971 | 0.215 (0.608),f 74 | 0.405 (0.608),f 76 | ||
| Creager 200870 | 0.032 (0.256),f 84 | 0.130 (0.256),f 86 | ||
| Kieffer 200165 | 0.130 (NA), 92 | 0.603 (NA), 89 | ||
| Dawson 200058 | 0.293 (NA), 226 | 0.432 (NA), 205 | 0.262 (NA), 212 | |
| aOtsuka 21-94-30134 | 0.351 (0.302),g 132 | 0.519 (0.302),g 123 | 0.501 (0.302), 118 | |
| aOtsuka 21-98-21334 | 0.346 (0.226), 260 | 0.362 (0.226), 260 | 0.413 (0.226), 260 |
Table 22 also shows the estimated treatment effect of cilostazol relative to placebo in the study by Money et al. ,62 for which individual arm data were not available. This study was excluded from the meta-analysis because of the 16-week follow-up.
| Study | Difference, cilostazol–placebo, mean (SE) |
|---|---|
| a,bMoney 199862 | 0.255 (0.045)c |
Goodness-of-fit was assessed by calculating the arm-specific and total residual deviance. The total residual deviance was 23.03, which compares favourably with the 23 data points being analysed. The arm-specific deviance terms were not indicative of any particular sample mean, being poorly represented by the model. The posterior mean treatment effect for these studies, together with the 95% credible interval, is shown in Table 23. Table 23 also shows the posterior mean of the between-study SD, together with the 95% credible interval.
| Intervention | Treatment effect | Mean (95% credible interval) |
|---|---|---|
| Cilostazol | Random effects | 0.220 (0.108 to 0.337) |
| Predictive distribution | 0.220 (–0.072 to 0.511) | |
| Pentoxifylline | Random effects | 0.101 (–0.016 to 0.217) |
| Predictive distribution | 0.101 (–0.195 to 0.383) | |
| Naftidrofuryl oxalate | Random effects | 0.472 (0.181 to 0.762) |
| Predictive distribution | 0.472 (0.087 to 0.865) | |
| Between-study SD | 0.125 (0.068 to 0.220) |
The random effects meta-analysis of the change from baseline in log walking distance showed that treatment with naftidrofuryl oxalate had the greatest effect [60.3% = 1 – exp(0.472)] relative to placebo, followed by cilostazol (24.6%) and pentoxifylline (10.6%).
The 95% credible intervals suggest that treatment with naftidrofuryl oxalate and cilostazol produces real increases in the percentage change from baseline walking distance relative to placebo, although there was some uncertainty as to the true effect.
Table 24 gives a matrix of results from the network meta-analysis (see Table 23), where the upper right cells are the pair-wise posterior means (i.e. the direct effects) and the lower left cells are the network meta-analysis posterior means. Only four direct effects were estimable, and in three cases the mean results were essentially the same as the results from the network meta-analysis but with greater uncertainty, as expected. There was a greater difference in the results for the comparison between cilostazol and pentoxifylline, with the direct effect giving approximately 4% improvement in MWD in favour of cilostazol and the network meta-analysis result giving an approximately 11% improvement in favour of cilostazol, although there was considerable uncertainty as to the true effect based on the direct evidence alone.
| Intervention | Placebo (95% credible interval) | Cilostazol (95% credible interval) | Pentoxifylline (95% credible interval) | Naftidrofuryl oxalate (95% credible interval) |
|---|---|---|---|---|
| Placebo | – | 0.222 (0.038 to 0.415) | 0.096 (–0.001 to 0.195) | 0.472a (–0.170 to 1.111) |
| Cilostazol | 0.220 (0.108 to 0.337) | – | –0.045 (–0.889 to 0.790) | NA |
| Pentoxifylline | 0.101 (–0.016 to 0.217) | –0.119 (–0.280 to 0.037) | – | NA |
| Naftidrofuryl oxalate | 0.472 (0.181 to 0.762) | 0.252 (–0.062 to 0.563) | 0.371 (0.053 to 0.681) | – |
There was moderate between-study variation, which suggests that the treatment effect varied depending on the characteristics of the study. The trial by Strandness et al. 56 had the largest observed effect of cilostazol compared with placebo (0.394) and the Otsuka 21-98-21334 trial had the smallest observed cilostazol effect compared with placebo (0.016). The trial by Lindgarde et al. 71 had the largest observed pentoxifylline effect compared with placebo (0.190) and the trial by Dawson et al. 58 had the smallest observed pentoxifylline effect compared with placebo (–0.031).
Forest plots are shown in Figure 3.
FIGURE 3.
Posterior distribution for the change from baseline in log mean MWD for cilostazol, naftidrofuryl oxalate and pentoxifylline vs placebo.
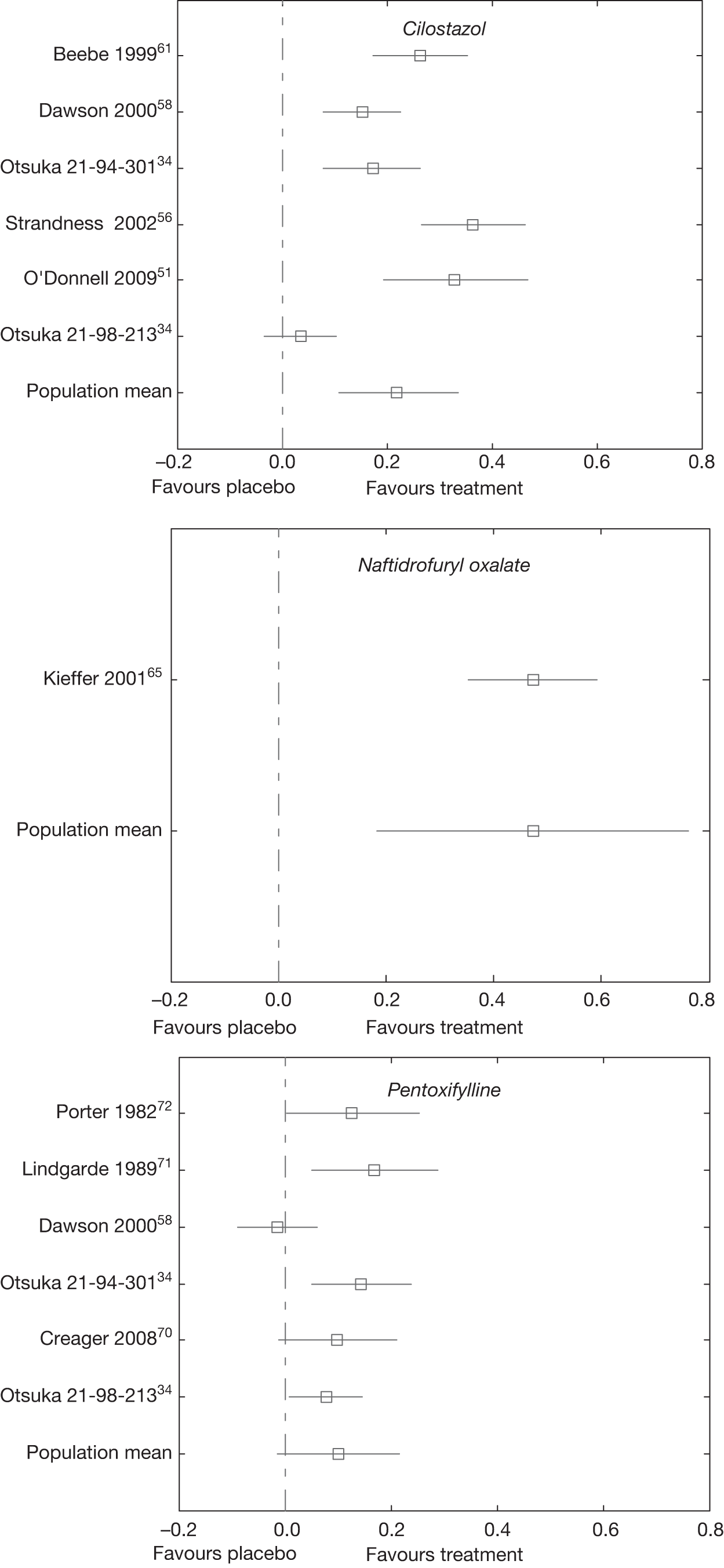
Figure 3 suggests that the relative treatment effects are unrelated to the date of the study. Furthermore, of the trials used within the meta-analysis it is only some of the pentoxifylline trials that pre-date the last 12 years. All trials of cilostazol and naftidrofuryl oxalate were published within the last 12 years.
The percentage change from baseline to 24 weeks in MWD, estimated by projecting the treatment effects from the network meta-analysis on to an estimated placebo response, is shown in Table 25.
| Intervention | Mean (95% credible interval) |
|---|---|
| Placebo | 27.6 (13.7 to 43.2) |
| Cilostazol | 59.2 (35.4 to 87.1) |
| Pentoxifylline | 41.4 (19.8 to 65.9) |
| Naftidrofuryl oxalate | 106.7 (49.9 to 177.5) |
Tables 26–28 give the results of five sensitivity analyses, which allow for more informative prior distributions for the between-study SD and treatment effects. Tables 26 and 27 provide the results of the sensitivity analyses for the placebo mean and the treatment effects relative to placebo, respectively, whereas Table 28 provides the results for the between-study standard SD.
| Prior distribution | Mean | SD | 2.5 percentile | 97.5 percentile |
|---|---|---|---|---|
| τ ∼ U(0, 10), μ ∼ N(10,000) | 0.242 | 0.0582 | 0.129 | 0.359 |
| τ ∼ U(0, 0.5), μ ∼ N(10,000) | 0.242 | 0.0580 | 0.126 | 0.360 |
| τ ∼ U(0, 0.5), μ ∼ N(1000) | 0.243 | 0.0591 | 0.125 | 0.361 |
| τ ∼ U(0, 0.25), μ ∼ N(1000) | 0.242 | 0.0535 | 0.133 | 0.349 |
| τ ∼ U(0, 0.2), μ ∼ N(100) | 0.242 | 0.0490 | 0.143 | 0.341 |
| Mean | SD | 2.5 percentile | 97.5 percentile | |
|---|---|---|---|---|
| Cilostazol | ||||
| τ ∼ U(0, 10), μ ∼ N (10,000) | 0.220 | 0.0576 | 0.108 | 0.337 |
| τ ∼ U(0, 0.5), μ ∼ N (10,000) | 0.221 | 0.0577 | 0.109 | 0.340 |
| τ ∼ U(0, 0.5), μ ∼ N (1000) | 0.220 | 0.0574 | 0.106 | 0.337 |
| τ ∼ U(0, 0.25), μ ∼ N (1000) | 0.219 | 0.0569 | 0.108 | 0.335 |
| τ ∼ U(0, 0.2), μ ∼ N (100) | 0.220 | 0.0544 | 0.114 | 0.333 |
| Pentoxifylline | ||||
| τ ∼ U(0, 10), μ ∼ N (10,000) | 0.101 | 0.0584 | –0.016 | 0.217 |
| τ ∼ U(0, 0.5), μ ∼ N (10,000) | 0.101 | 0.0576 | –0.012 | 0.216 |
| τ ∼ U(0, 0.5), μ ∼ N (1000) | 0.100 | 0.0580 | –0.014 | 0.215 |
| τ ∼ U(0, 0.25), μ ∼ N (1000) | 0.101 | 0.0579 | –0.016 | 0.214 |
| τ ∼ U(0, 0.2), μ ∼ N (100) | 0.100 | 0.0559 | –0.012 | 0.213 |
| Naftidrofuryl oxalate | ||||
| τ ∼U(0, 10), μ ∼ N (10,000) | 0.472 | 0.1464 | 0.181 | 0.762 |
| τ ∼ U(0, 0.5), μ ∼ N (10,000) | 0.471 | 0.1422 | 0.188 | 0.751 |
| τ ∼ U(0, 0.5), μ ∼ N (1000) | 0.472 | 0.1454 | 0.182 | 0.758 |
| τ ∼ U(0, 0.25), μ ∼ N (1000) | 0.474 | 0.1441 | 0.187 | 0.759 |
| τ ∼ U(0, 0.2), μ ∼ N (100) | 0.472 | 0.1363 | 0.200 | 0.746 |
| Between-study SD | ||||
|---|---|---|---|---|
| Mean | SD | 2.5 percentile | 97.5 percentile | |
| τ ∼ U(0, 10), μ ∼ N (10,000) | 0.125 | 0.0399 | 0.068 | 0.220 |
| τ ∼ U(0, 0.5), μ ∼ N (10,000) | 0.125 | 0.0387 | 0.068 | 0.219 |
| τ ∼ U(0, 0.5), μ ∼ N (1000) | 0.125 | 0.0392 | 0.070 | 0.220 |
| τ ∼ U(0, 0.25), μ ∼ N (1000) | 0.124 | 0.0360 | 0.069 | 0.211 |
| τ ∼ U(0, 0.2), μ ∼ N (100) | 0.120 | 0.0305 | 0.068 | 0.185 |
Table 26 shows that the placebo mean is insensitive to relatively informative prior distributions [i.e. sensitivity analysis 5 (SA5)] compared with the results based on weak prior distributions (i.e. SA1).
Table 27 shows that the treatment effects are insensitive to relatively informative prior distributions compared with the results based on weak prior distributions.
Table 28 shows that the between-study SD is relatively insensitive to more informative prior distributions than the results based on weak prior distributions, although the mean from SA5 is smaller than the results of the other analysis. This is a consequence of the prior distribution in SA5 excluding estimates considered plausible in the other sensitivity analyses, i.e. posterior estimates > 0.2.
Pain-free walking distance
Pain-free walking distance narrative summary
Tables 29–33 show PFWD results as reported by the trials. This may be reported as the difference in mean PFWD between baseline and final measurement, the change from baseline as a percentage or as an effect size. Details of treadmill protocols are shown in Appendix 4. As the graded test encourages longer walking distances than the constant-load protocol, absolute mean walking distance in metres is not directly comparable between protocols. 28
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in PFWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Placebo | Cilostazol group | Placebo group | ||||
| O’Donnell 200951 | 24 | 51 | 55 | Constant | 67% improvement | 51.6% improvement | p = 0.63 |
| Strandness 200257 | 24 | 133 | 129 | Constant | 22% (favours cilostazol) | ||
| Dawson 200058 | 24 | 227 | 239 | Graded | Mean difference (m): 94 (SD 127) | Mean difference (m): 57 (SD 93) | p = 0.0001 |
| Beebe 199961 | 24 | 175 | 170 | Constant |
Mean difference (m): 67.5 59% improvement |
Mean difference (m): 23.1 20% improvement |
p < 0.001 |
| Money 199862 | 16 | 119 | 120 | Graded | p < 0.05 | ||
| Dawson 199863 | 12 | 54 | 27 | Constant | 31.7% improvement | –2.5% change (worsening) | p < 0.01 |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in PFWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | Naftidrofuryl oxalate group | Placebo group | ||||
| Spengel 200247 | 24 | 382 | 372 | Not treadmill – patient estimate only | Mean difference (m): 204 (SD 433) | Mean difference (m): 51 (SD 455) | p < 0.001 |
| Kieffer 200165 | 24 | 98 | 98 | Constant | Mean difference (m): 158.2 | Mean difference (m): 29.9 | p < 0.001 |
| Adhoute 198666 | 24 | 64 | 54 | Constant | Mean difference (m): 201.41 | Mean difference (m): 98.03 | p < 0.02 |
| Trubestein 198467 | 12 | 54 | 50 | Constant | Mean difference (m): 93 | Mean difference (m): 36 | p < 0.02 |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in PFWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Pentoxifylline | Placebo | Pentoxifylline group | Placebo group | ||||
| Creager 200870 | 24 | 86 | 84 | Graded | 34.30% | 21.20% | Non-significant |
| Dawson 200058 | 24 | 232 | 239 | Graded | Mean difference (m): 74 (SD 106) | Mean difference (m): 57 (SD 93) | p = 0.07 |
| Lindgarde 198971 | 24 | 76 | 74 | Constant | Geometric mean 80% improvement (SE 12) | Geometric mean 60% improvement (SE 11) | p = 0.268 |
| Porter 198273 | 24 | 67 | 61 | Constant | 47% (SE 10) by geometric mean | 26% (SE 9) by geometric mean | Two-sided p = 0.042, one-sided p = 0.01 |
| Gallus 198576 | 8 | 25 | 23 | Constant | 55% improvement by geometric mean | 26% improvement by geometric mean | Ratio of per cent change from baseline (pentoxifylline/placebo) 1.23 (95% CI 0.86 to 1.77); p < 0.3) |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in PFWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Pentoxifylline | Cilostazol group | Pentoxifylline group | ||||
| Dawson 200058 | 24 | 227 | 232 | Graded | Mean difference (m): 94 (SD 127) | Mean difference (m): 74 (SD 106) | p = 0.02 |
| Trial | Treatment duration (weeks) | No. in analysis | Treadmill protocol | Change in PFWD | Comparison between groups | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Usual care | Cilostazol group | Usual care group | ||||
| INEXACT Hobbs 200782 | 24 | 16 (seven with exercise, nine without) | 18 (nine with exercise, nine without) | Constant |
Mean ratio plus exercise 3.84 (SD 3.62) Without exercise, mean ratio 3.34 (SD 4.23) |
Mean ratio plus exercise 2.22 (SD 2.71) Without exercise, mean ratio 1.23 (SD 0.73) |
Difference in effect 2.07 p = 0.090 |
For the 10 studies comparing cilostazol 200 mg with placebo, five did not find any significant difference between groups (O’Donnell et al. ,83 Otsuka trials 21–94–301, 21–98–213, 21–95–201,34 Elam et al. 64), and five significantly favoured cilostazol over placebo (Strandness et al. ,57 Dawson et al. ,58,63 Beebe et al. ,61 Money et al. 62). Table 29 shows the PFWD data from the published trial reports. The review by Pande et al. 31 included nine industry-sponsored trials, of which five trials (Strandness et al. ,57 Dawson et al. ,58,63 Beebe et al. ,61 Money et al. 62) found a significant difference between treatment groups, and four trials (including the three trials without published trial reports) found no significant difference between cilostazol 200 mg and placebo groups (Otsuka trials 21–94–301, 21–98–213, 21–95–201,34 Elam et al. 64). The five trials finding a significant difference between treatment groups reported this in published trial reports (see Table 29). The Pande et al. review31 presented a pooled analysis of these nine trials as a ratio of geometric means, and calculated an estimate of treatment effect of 1.15 (95% CI 1.10 to 1.20), which significantly favoured cilostazol over placebo. 31 This analysis31 did not include the O’Donnell et al. trial. 51 The O’Donnell et al. trial51 did not find any significant treatment effect, with both the cilostazol and placebo groups showing improvement in PFWD (see Table 29). O’Donnell et al. 83 also found no significant difference between treatment groups when considering the subgroups of patients with diabetes (p = 0.14, n = 26)53 or without diabetes (p = 0.63, n = 80), although, as described above (see Maximum walking distance), this may be due to the small sample sizes.
As patient populations were similar across trials, in terms of disease, diabetes, hypertension, smoking and age range, these characteristics do not appear to explain whether or not, significant differences between treatment groups were found and nor does sample size. Of the trials using the constant-workload treadmill protocol, three out of seven favoured cilostazol over placebo (Strandness et al. ,56 Beebe et al. ,61 Dawson et al. 63), whereas four out of seven were non-significant (O’Donnell et al. ,83 Otsuka trials 21–94–301, 21–98–213, 21–95–20134). Of the trials using the graded test treadmill protocol, two out of three favoured cilostazol over placebo (Dawson et al. ,58 Money et al. 62), whereas one was non-significant (Elam et al. 64). For the graded test protocol trials, the trial with the non-significant result (Elam et al. 64) was the trial with the shortest follow-up, at 12 weeks, whereas the trials with significant results had follow-up periods of 16 weeks (Money et al. 62) and 24 weeks (Dawson et al. 58). However, length of follow-up cannot explain the difference between significant and non-significant results in the constant-workload protocol trials. For the constant-workload protocol trials with 24 weeks’ follow-up, three were non-significant in terms of PFWD comparing cilostazol versus placebo (O’Donnell et al. ,83 Otsuka trials 21–94–301, 21–98–21334), whereas two were significant (Strandness et al. ,56 Beebe et al. 61). Two of the constant-workload protocol trials had follow-up of 12 weeks, and, of these, one produced significant results (Dawson et al. 63), whereas the other one (Otsuka 21-95-20134) did not find any difference between treatment groups. Only Dawson et al. 63 specified administration of placebo during the run-in period of the study. These trials had similar designs: all were blinded, randomised and presented ITT analyses, and all measured baseline walking distance with two tests.
For the naftidrofuryl oxalate 600 mg versus placebo comparison (see Table 30), the three trials using constant-workload treadmill protocol – Kieffer et al. 65, Adhoute et al. 66 and Trubestein et al. 67 – all reported significantly greater improvement in PFWD in the naftidrofuryl oxalate group than in the placebo group. These trials had similar designs: all had placebo run-in, were randomised, presented ITT analyses, measured baseline walking distance with two tests, and were blinded with the exception that the clinicians in the Adhoute et al. trial66 were not blinded to treatment group. The three naftidrofuryl oxalate 600 mg trials using constant-workload treadmill protocol – Kieffer et al. ,65 Adhoute et al. 66 and Trubestein et al. 67 – had some variation across trials in baseline PFWD; however, all had higher baseline PFWD (see Appendix 4) than the constant-workload cilostazol trials (Strandness et al. ,56 Beebe et al. ,61 Dawson 1998 et al. ,63 O’Donnell et al. ,83 Otsuka trials 21–94–301, 21–98–213, 21–95–20134). The Spengel et al. trial47 reported significantly greater improvement in claudication distance in the naftidrofuryl oxalate 600 mg group than in the placebo group; however, this was based on patient estimates of PFWD at baseline and 24 weeks, not treadmill testing. The Ruckley et al. trial68 of naftidrofuryl oxalate 300 mg versus placebo reported that there was no significant difference between groups for PFWD at 12 weeks’ follow-up,68 as measured by patients’ normal walking pace on a level.
Of the seven trials comparing pentoxifylline 1200 mg versus placebo in terms of PFWD, five found no significant difference between treatment groups (Creager et al. ,70 Lindgarde et al. ,71 Gallus et al. 76 and Otsuka 21-98-213, 21–94–30134), whereas two significantly favoured pentoxifylline over placebo for PFWD (Dawson et al. 58 and Porter et al. 73). The Gallus et al. 76 study had a follow-up of only 8 weeks, whereas the other studies had a follow-up of 24 weeks. Three published trials (see Table 31) comparing pentoxifylline with placebo – Creager et al. ,70 Lindgarde et al. 71 and Gallus et al. 76 – reported no significant difference between treatment groups in PFWD. Two trials without published trial reports – Otsuka 21-98-21334 and Otsuka 21-94-30134 – also found no significant difference between the pentoxifylline and placebo groups in PFWD. Two trials (reported in Table 31) – Dawson et al. 58 and Porter et al. 73 – reported significantly greater improvement in PFWD for the pentoxifylline group than for the placebo group. Five of the trials comparing pentoxifylline with placebo used constant workload treadmill protocols, of which four found no significant treatment effect (Lindgarde et al. ,71 Gallus et al. ,76 Otsuka 21-98-21334 and Otsuka 21-94-30134), and one favoured pentoxifylline (Porter et al. 73). Of the two trials using a graded test protocol, one found a significant treatment effect (Dawson et al. 58), whereas the other did not (Creager et al. 70).
For the comparison of inositol nicotinate 4 g versus placebo, none of the trials reported PFWD. However, O’Hara et al. 78 measured pain-free walking paces and claudication time, and reported that there was no significant difference between groups in claudication time, and that pain-free walking paces improved significantly in both groups, with inositol nicotinate showing significantly greater improvement (p < 0.05) than the placebo group at 12 weeks. 78 The Head81 trial reported improved claudication times for both treatment groups at 12 weeks, but there was a significant difference between treatment groups (p < 0.001) only for patients with moderate disease, among whom the inositol nicotinate group (n = 24) had a significantly greater improvement than the placebo group (n = 28).
For the comparison of cilostazol 200 mg versus pentoxifylline 1200 mg (see Table 32), Dawson et al. 58 found a significantly greater improvement in PFWD for the cilostazol group than for the pentoxifylline group. Two trials without published trial reports – Otsuka 21-98-21334 and Otsuka 21-94-30134 – found no significant difference between cilostazol and pentoxifylline groups in PFWD. The Dettori et al. 69 trial did not report PFWD, but did report pain-free walking time, which was statistically more improved for patients taking pentoxifylline (p < 0.05) in an analysis including all four trial arms of the study (see Table 3 for summary table of included studies) at 1-year follow-up.
For the trial comparing cilostazol 200 mg (with or without supervised exercise) versus usual care (with or without supervised exercise) (see Table 33), all treatment groups improved but there was no significant effect of cilostazol added to supervised exercise or usual care. 82
Pain-free walking distance meta-analysis
The 10 studies included within this analysis are the same as for those used in the meta-analysis of MWD, shown in Table 21. Table 34 shows the change from baseline in log mean PFWD, including the three studies that are excluded from the meta-analysis because they had follow-up times of < 24 weeks.
| Study | Placebo: mean (SD), N | Cilostazol: mean (SD), N | Pentoxifylline: mean (SD), N | Naftidrofuryl oxalate: mean (SD), N |
|---|---|---|---|---|
| a,bDawson 199863 | –0.025 (NA), 25 | 0.275 (NA), 52 | ||
| a,bElam 199864 | 0.322 (NA,) 94 | 0.513 (NA), 95 | ||
| aBeebe 199961 | 0.182 (NA), 140 | 0.464 (NA), 140 | ||
| aStrandness 200256 | 0.320 (NA), 125 | 0.611 (NA), 124 | ||
| a,bOtsuka 21-95-20134 | 0.419 (0.406), 66 | 0.457 (0.406), 60 | ||
| aO’Donnell 200983 | 0.416 (0.581), 55 | 0.513 (0.581), 51 | ||
| Porter 198272 | 0.166 (NA), 40 | 0.385 (NA), 42 | ||
| Lindgarde 198971 | 0.470 (NA), 74 | 0.588 (NA), 76 | ||
| Creager 200870 | 0.192 (NA), 84 | 0.295 (NA), 86 | ||
| Kieffer 200165 | 0.155 (NA), 92 | 0.651 (NA), 89 | ||
| Dawson 200058 | 0.588 (0.602),c 226 | 0.663 (0.602),c 205 | 0.554 (0.602),c 212 | |
| aOtsuka 21-94-30134 | 0.464 (0.474), 122 | 0.467 (0.474), 123 | 0.548 (0.474), 118 | |
| aOtsuka 21-98-21334 | 0.501 (0.580), 260 | 0.521 (0.580), 260 | 0.578 (0.580), 260 |
Goodness-of-fit was assessed by calculating the arm-specific and total residual deviance. The total residual deviance was 23.07, which compares favourably with the 23 data points being analysed. The arm-specific deviance terms showed that the data from the Beebe et al. 61 and Strandness et al. 56 studies had the largest deviances.
The posterior mean treatment effects for these studies, together with the 95% credible intervals, are given in Table 35. Table 35 also gives the posterior mean of the between-study SD, together with the 95% credible interval.
| Intervention | Treatment effect | Mean (95% credible interval) |
|---|---|---|
| Cilostazol | Random effects | 0.126 (0.024 to 0.226) |
| Predictive distribution | 0.126 (–0.107 to 0.359) | |
| Pentoxifylline | Random effects | 0.088 (–0.017 to 0.195) |
| Predictive distribution | 0.087 (–0.153 to 0.326) | |
| Naftidrofuryl oxalate | Random effects | 0.495 (0.231 to 0.764) |
| Predictive distribution | 0.496 (0.157 to 0.845) | |
| Between-study SD | 0.095 (0.032 to 0.184) |
The random effects meta-analysis of the change from baseline in log walking distance showed that treatment with naftidrofuryl oxalate had the greatest effect [64.2% = 1 – exp(0.496)] relative to placebo, followed by cilostazol (13.4%) and pentoxifylline (9.2%).
The 95% credible intervals suggest that treatment with naftidrofuryl oxalate and cilostazol produces real increases in the percentage change from baseline PFWD relative to placebo, although there was some uncertainty as to the true effect.
There was moderate between-study variation, which suggests that the treatment effect varied depending on the characteristics of the study. The trial by Strandness et al. 56 had the largest observed effect of cilostazol effect compared with placebo (0.291) and the Otsuka 21-94-30134 trial had the smallest observed cilostazol effect compared with placebo (0.003). The trial by Porter et al. 72 had the largest observed pentoxifylline effect compared with placebo (0.219) and the trial by Dawson et al. 58 had the smallest observed pentoxifylline effect compared with placebo (–0.034).
Forest plots are shown in Figure 4.
FIGURE 4.
Posterior distribution for the difference from placebo in change from baseline log mean PFWD for cilostazol, naftidrofuryl oxalate and pentoxifylline vs placebo.
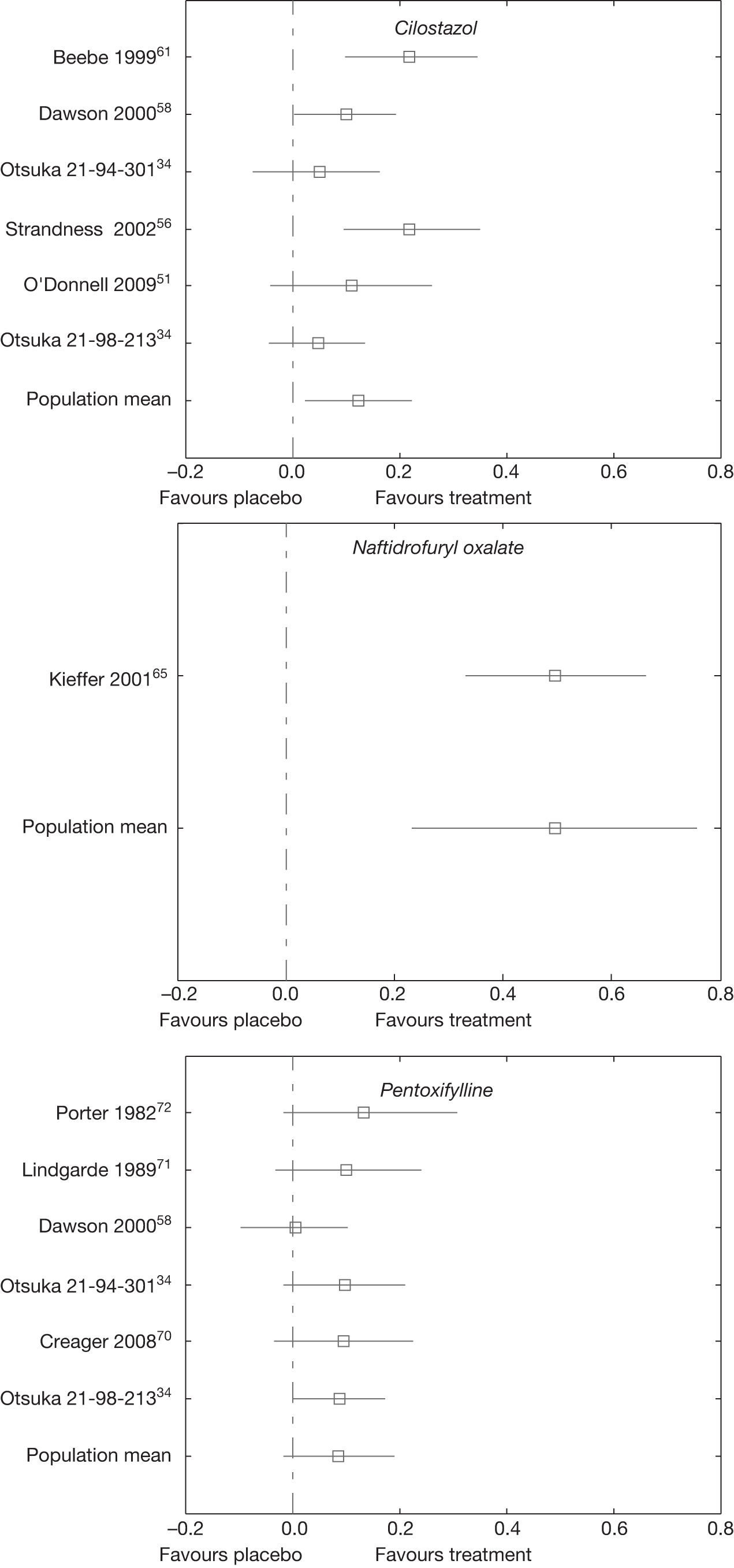
The percentage change from baseline to 24 weeks in PFWD, estimated by projecting the treatment effects from the network meta-analysis on to a estimated placebo response, is shown in Table 36.
| Intervention | Mean (95% credible interval) |
|---|---|
| Placebo | 42.2 (25.1 to 60.7) |
| Cilostazol | 61.4 (36.8 to 88.3) |
| Pentoxifylline | 55.4 (31.6 to 82.8) |
| Naftidrofuryl oxalate | 135.3 (74.9 to 212.6) |
Ankle–brachial pressure index
Tables 37–40 show ABPI results as reported by the trials as difference in mean between baseline and final measurement or change from baseline as a percentage. Across all treatment groups in all trials, where reported, differences from baseline to final measurement were slight.
| Trial | Follow-up | No. of patients in analysis | Change in ABPI | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Cilostazol | Placebo | Cilostazol group | Placebo group | |||
| Dawson 200058 | 24 | 205 | 226 | Difference in means 0.04 | Difference in means –0.01 | p < 0.01a |
| Money 199862 | 16 | 119 | 120 | 9% increase | 1% increase | p = 0.0125a |
| Elam 199864 | 12 | 95 | 94 | 9.03% increase | 1.2% increase | p < 0.001a |
| Trial | Follow-up | No. of patients in analysis | Change in ABPI | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | Naftidrofuryl oxalate group | Placebo group | |||
| Kieffer 200165 | 24 | 89 | 92 | Difference in means 0.03 | Difference in means 0.04 | Non-significant |
| Adhoute 198666 | 24 | 42 | 40 | Difference in means 0.02 | Difference in means 0.01 | Non-significant |
| Trial | Follow-up | No. of patients in analysis | Change in ABPI | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Pentoxifylline | Placebo | Pentoxifylline group | Placebo group | |||
| Dettori 198969 | 52 | 29 | 30 |
Post-exercise 8.3% At rest 2.5% |
Post-exercise 9.4% At rest –3.1% |
Post-exercise ABPI p = 0.09a At rest ABPI non-significant |
| Dawson 200058 | 24 | 212 | 226 | Difference in means 0.05 | Difference in means –0.01 | Non-significant |
| Trial | Follow-up | No. of patients in analysis | Change in ABPI | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Cilostazol group | Pentoxifylline group | Cilostazol group | Pentoxifylline group | |||
| Dawson 200058 | 24 | 205 | 212 | Difference in means 0.04 | Difference in means 0.05 | Non-significant |
For the cilostazol 200 mg versus placebo comparison (see Table 37), only three trials reported ABPI, and these all reported significantly more improvement in the cilostazol treatment group than in the placebo group. 58,62,64 Only in the Dawson et al. 58 trial did the placebo group’s ABPI slightly worsen, with the Money et al. 62 and Elam et al. 64 trials showing improvement in both groups.
For the naftidrofuryl oxalate 600 mg versus placebo comparison (see Table 38), the two trials65,66 reporting ABPI found no significant difference between the naftidrofuryl oxalate and placebo groups, with both groups in both the Kieffer et al. 65 and Adhoute et al. 66 trials showing a small, non-significant improvement. Trubestein et al. 67 recorded ankle pressure and found no significant change for either treatment group.
For the pentoxifylline 1200 mg versus placebo comparison (see Table 39), Dawson et al. 58 did not find any significant difference between groups. 58 The Dettori et al. trial69 with follow-up of 1 year found that, by geometric mean, there was no significant difference between pentoxifylline and placebo groups for ABPI measured at rest or post-exercise. For both of these trials,58,69 there was a slight worsening of the placebo group and small improvement for the pentoxifylline group.
For the comparison of inositol nicotinate 4 g versus placebo, the Kiff and Quick80 trial reported that there was no significant change, from baseline to final measurement at 12 weeks, in ABPI for either treatment group.
For the comparison of cilostazol 200 mg versus pentoxifylline 1200 mg (see Table 40), the Dawson et al. 58 study did not find any significant difference between groups for ABPI. The mean change was slightly larger in the pentoxifylline group than in the cilostazol group for this trial; however, there was greater variability in the pentoxifylline group. This resulted in a lack of significance in the comparison of pentoxifylline and placebo within this trial, but a significant difference between the cilostazol and placebo groups.
Mortality
Tables 41–45 show mortality results reported by trials. Across studies, there were no significant differences in mortality rates between treatment groups. No mortality was directly attributed to the vasoactive drugs. However, follow-up times were relatively short and hence very few deaths occurred. Only two studies had follow-up of over 24 weeks. 49,69 The CASTLE study49 of cilostazol 200 mg versus placebo (which included some patients taking pentoxifylline in both groups) reported mortality of approximately 7% in both groups by ITT analysis at 144 weeks. The Dettori et al. study69 of pentoxifylline 1200 mg versus placebo, at 1 year, found no mortality in the pentoxifylline group and a mortality rate of 5.4% in the placebo group, although this was based on only two deaths.
| Trial | Treatment duration (weeks) | No. in analysis | Cilostazol group | Placebo group | |||
|---|---|---|---|---|---|---|---|
| Cilostazol | Placebo | Mortality (n) | Mortality (%) | Mortality (n) | Mortality (%) | ||
| CASTLE, Hiatt 200849 | Up to 144 | 717 | 718 | 49 | 6.8 | 52 | 7.2 |
| Strandness 200256 | 24 | 133 | 129 | 2 | 1.5 | 0 | 0 |
| Dawson 200058 | 24 | 227 | 239 | 2 | 0.8 | 1 | 0.4 |
| Beebe 199961 | 24 | 175 | 170 | 3 | 1.2 | 2 | 1.2 |
| Otsuka 21-98-21334 | 24 | 260 | 260 | 0 | 0 | 2 | 0.8 |
| Money 199862 | 16 | 119 | 120 | 1 | 0.8 | 1 | 0.8 |
| Dawson 199863 | 12 | 54 | 27 | 0 | 0 | 1 | 3.7 |
| Elam 199864 | 12 | 95 | 94 | 1 | 1.1 | 1 | 1.1 |
| Otsuka 21-95-20134 | 12 | 72 | 70 | 0 | 0 | 2 | 2.9 |
| Trial | Treatment duration (weeks) | No. in analysis | Naftidrofuryl oxalate group | Placebo group | |||
|---|---|---|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | Mortality (n) | Mortality (%) | Mortality (n) | Mortality (%) | ||
| Spengel 200247 | 24 | 382 | 372 | 1 | 0.26 | 5 | 1.30 |
| Trial | Treatment duration (weeks) | No. in analysis | Pentoxifylline group | Placebo group | |||
|---|---|---|---|---|---|---|---|
| Pentoxifylline | Placebo | Mortality (n) | Mortality (%) | Mortality (n) | Mortality (%) | ||
| Dettori 198969 | 52 | 37 | 37 | 0 | 0 | 2 | 5.4 |
| Creager 200870 | 24 | 86 | 84 | 1 | 1.20 | 1 | 1.20 |
| Dawson 200058 | 24 | 232 | 239 | 3 | 1 | 1 | 0.4 |
| Otsuka 21-98-21334 | 24 | 260 | 260 | 3 | 1.2 | 2 | 0.8 |
| Gallus 198576 | 8 | 25 | 23 | 0 | 0 | 1 | 4 |
| Trial | Treatment duration (weeks) | No. in analysis | Inositol nicotinate group | Placebo group | |||
|---|---|---|---|---|---|---|---|
| No. in analysis Inositol nicotinate | No. in analysis, placebo | Mortality (n) | Mortality (%) | Mortality (n) | Mortality (%) | ||
| O’Hara 198878 | 12 | 62 | 58 | 0 | 0 | 1 | 1.70 |
Cardiovascular events
Across studies, there were no significant differences in cardiovascular event rates in between treatment groups within trials. Further details are provided in Appendix 4.
Only two studies had follow-up of over 24 weeks. 49,69 The CASTLE study of 144 weeks49 of cilostazol versus placebo (which included some patients taking pentoxifylline in both groups) reported no significant difference in cardiovascular mortality between the cilostazol and placebo groups, with a hazard ratio (HR) for cilostazol of 0.852 (95% CI 0.515 to 1.410; p = 0.533) by ITT analysis. This was based on 28 events (3.9%) in the cilostazol group and 33 events (4.6%) in the placebo group. 49 The CASTLE study49 also found no significant difference between groups when using on-treatment analysis, with 14 cardiovascular deaths in each treatment group. The Dettori et al. study69 found one non-fatal cardiovascular event (2.7%) at 1-year follow-up in the pentoxifylline group, and three cardiovascular events (of which one was fatal) in the placebo group (8.1%).
Eight of the cilostazol 200 mg versus placebo trials (Strandness et al. ,57 Dawson et al. 58,63 Beebe et al. ,61 Money et al. ,62 Elam et al. 64) were included in an analysis by Pratt,28,33 which reported a cardiovascular event rate of 6.5% (20/308) for the cilostazol 200 mg groups and 7.7% (23/299) for the placebo groups. This analysis also reported cardiovascular mortality within 30 days of drug administration as 0.67% (7/1048) for cilostazol 200 mg and 0.1% (1/973) for placebo. 33
For the naftidrofuryl oxalate 600 mg versus placebo comparison, the Kieffer et al. 65 trial reported that 2% (n = 2) of the naftidrofuryl oxalate group and 3% (n = 3) of the placebo group were referred for vascular intervention with endovascular or surgical treatment.
For the pentoxifylline 1200 mg versus placebo comparison, Creager et al. 70 reported serious cardiovascular events for 7% (n = 6) of the pentoxifylline group and 12% (n = 10) of the placebo groups. The Porter and Bauer74 and Gallus et al. 76 trials reported only cardiovascular events that led to withdrawal from the studies, with Porter and Bauer74 reporting cardiovascular events for 1.5% (n = 1) of the pentoxifylline group and 4.8% (n = 3) of the placebo group, and Gallus et al. 76 reporting 12% (n = 3) for the placebo group but no events for the pentoxifylline group.
The inositol nicotinate 4 g trials reported only cardiovascular events that led to withdrawal from the studies. The O’Hara et al. trial78 reported a 2% (n = 1) cardiovascular event rate in both the inositol nicotinate and placebo groups, Kiff and Quick80 reported a 2.5% (n = 1) event rate for the inositol nicotinate group and no events for the placebo group, and Head81 reported a 1.6% (n = 1) event rate for the placebo group and no events for the inositol nicotinate group.
Adverse events and serious adverse events
Tables 46–50 show numbers of patients experiencing at least one adverse event (AE) or serious adverse event (SAE) according to results reported by the trials. Further details, including types of AEs, are provided in Appendix 4. Differences in reporting across trials, including that some trials reported only AEs leading to discontinuation or had unclear clinical criteria for AEs, precluded meta-analysis.
| Trial | Treatment duration (weeks) | No. in analysis | Cilostazol group | Placebo group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with one or more SAE | Patients with one or more AE | Patients with one or more SAE | Patients with one or more AE | ||||||||
| Cilostazol | Placebo | n | % | n | % | n | % | n | % | ||
| Strandness 200256 | 24 | 133 | 129 | 25 | 18.8 | 124 | 93.2 | 20 | 15.5 | 99 | 76.7 |
| Dawson 200058 | 24 | 227 | 239 | 27 | 11.9 | 201 | 88.5 | 31 | 13 | 188 | 78.7 |
| Beebe 199961 | 24 | 175 | 170 | 23 | 13.1 | 159 | 90.9 | 29 | 17.1 | 150 | 88.2 |
| Otsuka 21-94-30134 | 24 | 123 | 124 | 16 | 13 | 116 | 94 | 11 | 9 | 103 | 83 |
| Otsuka 21-98-21334 | 24 | 260 | 260 | 32 | 12.3 | 207 | 79.6 | 31 | 11.9 | 197 | 75.8 |
| Money 199862 | 16 | 119 | 120 | 14 | 11.8 | 98 | 82.4 | 11 | 9.2 | 90 | 75 |
| Dawson 199863 | 12 | 54 | 27 | 7 | 13 | 47 | 87 | 1 | 4 | 20 | 74 |
| Elam 199864 | 12 | 95 | 94 | 6 | 6.3 | 79 | 83.2 | 7 | 7.4 | 76 | 80.9 |
| Otsuka 21-95-20134 | 12 | 72 | 70 | 4 | 5.6 | 60 | 83.3 | 10 | 14.3 | 52 | 74.3 |
| Trial | Treatment duration (weeks) | No. in analysis | Naftidrofuryl oxalate group | Placebo group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with one or more SAE | Patients with one or more AE | Patients with one or more SAE | Patients with one or more AE | ||||||||
| Naftidrofuryl oxalate | Placebo | n | % | n | % | n | % | n | % | ||
| Kieffer 200165 | 24 | 98 | 98 | 12 | 12 | 18 | 18 | 13 | 13 | 21 | 21 |
| Adhoute 198666 | 24 | 64 | 54 | NR | NR | 5 | 7.80 | NR | NR | 4 | 7.80 |
| Trubestein 198467 | 12 | 54 | 50 | NR | NR | 2 | 4 | NR | NR | 2 | 4 |
| Trial | Treatment duration (weeks) | No. in analysis | Naftidrofuryl oxalate group | Placebo group | |||
|---|---|---|---|---|---|---|---|
| Naftidrofuryl oxalate | Placebo | Patients with one or more AE | Patients with one or more AE | ||||
| n | % | n | % | ||||
| Ruckley 197868 | 12 | 25 | 25 | 6 | 24 | 4 | 16 |
| Trial | Treatment duration (weeks) | No. in analysis | Pentoxifylline group | Placebo group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with one or more SAE | Patients with one or more AE | Patients with one or more SAE | Patients with one or more AE | ||||||||
| Pentoxifylline | Placebo | n | % | n | % | n | % | n | % | ||
| Creager 200870 | 24 | 86 | 84 | 12 | 14 | 59 | 69 | 14 | 17 | 49 | 58 |
| Dawson 200058 | 24 | 232 | 239 | 31 | 13.4 | 200 | 86.2 | 31 | 13 | 188 | 78.7 |
| Lindgarde 198971 | 24 | 76 | 74 | NR | NR | 17 | 22 | NR | NR | 10 | 14 |
| Porter 198272 | 24 | 63 | 61 | NR | NR | 37 | 55 | NR | NR | 24 | 39 |
| Otsuka 21-94-30134 | 24 | 123 | 124 | 22 | 18 | 104 | 85 | 11 | 9 | 103 | 83 |
| Otsuka 21-98-21334 | 24 | 260 | 260 | NR | NR | 208 | 80 | 31 | 11.9 | 197 | 75.8 |
| Trial | Treatment duration (weeks) | No. in analysis | Cilostazol group | Pentoxifylline group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with one or more SAE | Patients with one or more AE | Patients with one or more SAE | Patients with one or more AE | ||||||||
| Cilostazol | Pentoxifylline | n | % | n | % | n | % | n | % | ||
| Dawson 200058 | 24 | 227 | 232 | 27 | 11.9 | 201 | 88.5 | 31 | 13.4 | 200 | 86.2 |
| Otsuka 21-94-30134 | 24 | 123 | 123 | 16 | 13 | 116 | 94 | 22 | 18 | 104 | 85 |
| Otsuka 21-98-21334 | 24 | 260 | 260 | 32 | 12.3 | 207 | 79.6 | 28 | 10.8 | 208 | 80 |
Only two studies had follow-up of over 24 weeks. 49,69 The CASTLE study49 of cilostazol versus placebo (which included some patients taking pentoxifylline in both groups), with a follow-up period of 144 weeks, reported higher frequency of headaches, diarrhoea and palpitations in the cilostazol group, and a higher frequency of bronchitis in the placebo group than in the cilostazol group, although none of these events had a rate higher than 11%. Most SAEs reported by the CASTLE study were cardiovascular (see Cardiovascular events, above), but there was also dyspepsia occurring in 1% of the cilostazol group and 0.4% of the placebo group. 49 The Dettori et al. 69 study reported only SAEs leading to withdrawal from study drug, and these were all cardiovascular in nature (see Cardiovascular events, above).
Eight of the cilostazol 200 mg versus placebo trials (Strandness et al. ,57 Dawson et al. ,58,63 Beebe et al. ,61 Money et al. ,62 Elam et al. 64) were included in an analysis by Pratt33 that reported higher frequency of headaches, diarrhoea, peripheral oedema and palpitations in the cilostazol groups than in the placebo groups. Although this analysis33 included cilostazol doses of 100 mg and 300 mg (excluded from the current report for not being the licensed dose), as well as the cilostazol 200 mg groups, this pattern is reflected in the published trial reports (see Appendix 4).
For both naftidrofuryl oxalate 600 mg and 300 mg compared with placebo, rates of AEs or SAEs were similar between treatment groups (see Tables 47 and 48). The Kieffer et al. trial65 additionally reported SAEs, including cardiovascular and non-cardiovascular events, for 24 weeks following treatment cessation and again found no significant difference in rates between the naftidrofuryl oxalate (6%) and placebo (7%) groups. 65 Non-SAEs were mostly gastrointestinal in nature (see Appendix 4).
For the pentoxifylline versus placebo trials (see Table 49) event rates were similar between treatment groups. Lower rates in the Lindgarde et al. 71 trial than in the Creager et al. ,70 Dawson et al. ,58 Porter et al. ,72 Otsuka 21-94-30134 and Otsuka 21-98-21334 trials are likely to be due to the patient self-reporting AEs, as populations were similar across trials. Non-SAEs were mostly headaches or gastrointestinal complaints (see Appendix 4).
The inositol nicotinate 4 g versus placebo trials reported only AEs that led to withdrawal from trials, and these were similar between treatment groups (see Appendix 4) and mostly related to difficulty swallowing or gastrointestinal problems. 79–81
The cilostazol versus pentoxifylline trials reported similar rates of SAEs and AEs across treatment groups. 33,58
Health-related quality of life
Several different outcome measures have been used to assess quality of life in study participants, and no single measure has been used to assess all four treatments. The most commonly used quality-of-life measure was the SF-36,84 with data available for cilostazol and pentoxifylline. There are also data available for the Walking Impairment Questionnaire (WIQ85) for both cilostazol and pentoxifylline, although this measure aims to assess walking impairment and not quality of life. Other outcome measures used include the claudication outcome measure (COM –a measure developed by the funders but which had not undergone validation,61 and does not appear to have been published), vascular quality of life (VascuQoL),86 an independent measure that has been validated, and the Claudication Scale (CLAU-S), another independent, extensively validated tool. 87 Tables 51–54 summarise the evidence around HRQoL.
| Trial | Duration (weeks) | No. of patients in analysis | Change in HRQoL | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Cilostazol | Placebo | Cilostazol group (0–100 scale) | Placebo group | |||
| SF-36a | ||||||
| Strandness 200256 | 24 | Unclear | Unclear | NR | NR |
Physical health summary (physical function, bodily pain and role–physical), non-significant trend Physical function p = 0.048 |
| Mean change from baseline | Mean change from baseline | |||||
| Beebe 199961 | 24 | 137 | 141 |
Physical health Physical function 7.1 Role–physical 5.3 Bodily pain 7.2 |
Physical health Physical function 2.0 Role–physical –2.8 Bodily pain –1.8 |
Physical health Physical function, significant Bodily pain, significant Role–physical, positive trend |
|
Mental health Social function 1.0 Role–emotional 2.9 Mental health 2.5 |
Mental health Social function 0.4 Role–emotional –1.66 Mental health 0.9 |
Mental health Non-significant |
||||
| Mean change from baseline | Mean change from baseline | |||||
| Money 199862 | 16 | Unclear, probably 119 | Unclear, probably 120 |
Physical health summary 2.99 Physical function 8.3 Other subscales change NR |
Physical health summary 0.12 Physical function 2.3 Other subscales change NR |
Physical health summary p = 0.0059 Physical function p = 0.0024 Bodily pain p = 0.0772 General health p = 0.436 Role–physical p = 0.061 Mental components non-significant |
| Difference between median at baseline and 24 weeks (calculated by reviewer) | Difference between median at baseline and 24 weeks (calculated by reviewer) | |||||
| O’Donnell 2009 (diabetics)53 | 24 | 12 | 14 |
Physical function 5.2 Role–physical 0 Body pain 3.4 General health 2.2 Total SF-36, 3.7 Significance of change from baseline (unclear if median or mean) Physical component p = 0.043 Vitality p = 0.016 Others NR |
Physical function 0.5 Role–physical 3.7 Body pain 0 General health 0.2 Total SF-36 1 Significance of change from baseline (unclear if median or mean) No significant changes |
Physical function p = 0.42 Role–physical p = 0.72 Body pain p = 0.31 General health p = 0.93 Total SF-36 p = 0.40 |
| Mean change from baseline or significance of change from baseline NR | Mean change from baseline or significance of change from baseline NR | |||||
| O’Donnell 2009 (non-diabetics)83 | 24 | 39 | 41 |
Physical health summary p = 0.044 Physical function p = 0.013 Role–physical p = 0.62 Body pain, p = 0.21 General health p = 0.48 Other subsets and summary non-significant Total SF-36 p = 0.50 |
||
| Dawson 200058 | 24 | 205 | 226 | NR | NR | Mental health summary, general health perception, physical health summary, and vitality scores all non-significant |
| Otsuka 21-98-21334 | 24 | NR | NR | NR | NR |
Only week 12 statistics reported Physical health summary significant at 12 weeks |
| WIQb | ||||||
| Strandness 200256 | 24 | Unclear | Unclear | NR | NR |
Non-significant trends General health perception Walking distance |
| Beebe 199961 | 24 | 137 | 141 | NR | NR | Walking speed and walking distance improved, unclear if significant |
| Money 199862 | 16 | Unclear, probably 119 | Unclear, probably 120 | NR | NR |
Walking speed, p = 0.0331 Walking distance, non-significant |
| O’Donnell 2009 (non-diabetics)83 | 24 | 12 | 14 |
Distance p = 0.014 Speed p = 0.021 |
Distance p = 0.81 Speed p = 0.74 |
Non-significant |
| Dawson 200058 | 24 | 205 | 226 | NR | NR | Non-significant |
| COMc | ||||||
| Mean change from baseline (0–4 scale) | Mean change from baseline (0–4 scale) | |||||
| Beebe 199961 | 24 | 137 | 141 |
Change in pain/discomfort 2.8 Pain/discomfort daily activities 0.4 Pain/discomfort physical activities 0.5 Pain/discomfort social activities 0.3 Walking pain/discomfort 0.7 Worry/concern due to pain 0.8 |
Change in pain/discomfort 2.4 Pain/discomfort daily activities 0.2 Pain/discomfort physical activities 0.2 Pain/discomfort social activities 0.3 Walking pain/discomfort 0.4 Worry/concern due to pain 0.5 |
Statistically significant Walking pain/discomfort Change in walking pain/discomfort Walking pain/discomfort physical activities All other domains and subscales not significant |
| VascuQold | ||||||
| O’Donnell 2009 (diabetics)53 | 24 | 12 | 14 | NR | NR |
Activity p = 0.59 Symptom p = 0.025 (significantly more increase for placebo) Pain p = 0.08 Emotion p = 0.013 Social p = 0.06 Total p = 0.04 |
| O’Donnell 2009 (non-diabetics)83 | 24 | 39 | 41 |
Significant improvement in pain (p = 0.005) All others non-significant |
No significant changes for placebo group |
Activity p = 0.34 Symptom p = 0.34 Pain p = 0.89 Emotion p = 0.63 Social p = 0.67 Total p = 0.78 |
| Trial | Duration (weeks) | No. of patients in analysis | Summary physical health | Physical function | Bodily pain | Role–physical | General health | Summary mental health | Vitality | Social functioning | Role–emotional | Mental health | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cilostazol | Placebo | ||||||||||||
| SF-36a | |||||||||||||
| Strandness 200256 | 24 | Unclear | Unclear | Non-sig | Sig | Non-sig | Non-sig | Non-sig | NR | NR | NR | NR | NR |
| Beebe 199961 | 24 | 137 | 141 | NR | Sig | Sig | Non-sig | NR | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig |
| Money 199862 | 16 | Unclear, probably 119 | Unclear, probably 120 | Sig | Sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig |
| O’Donnell 2009 (diabetics)53 | 24 | 12 | 14 | NR | Non-sig | Non-sig | Non-sig | Non-sig | NR | NR | NR | NR | NR |
| O’Donnell 2009 (non-diabetics)83 | 24 | 39 | 41 | NR | Sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig |
| Dawson 200058 | 24 | 205 | 226 | Non-sig | NR | NR | NR | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig | Non-sig |
| Trial | Duration (weeks) | No. of patients in analysis | Change in HRQoL | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Naftidrofuryl oxalate | Naftidrofuryl oxalate group (0–100 scale) | Placebo | Placebo group | |||
| Mean change from baseline read from graph/calculated from tables | Mean change from baseline read from graph/calculated from tables | |||||
| Spengel 200247 | 24 | 358 | 351 |
Daily living 7.5/7.5 Pain 8.4/6.4 Social life 3.1/3.1 Disease–specific anxiety 0.2/1.9 Mood 3.5/3.5 |
Daily living –1.3/–1.4 Pain –0.4/–0.4 Social life –2.4/–2 Disease specific anxiety 0.2/1.1 Mood –1.3/–1.2 |
Daily living p < 0.001 Pain p < 0.001 Social life p = 0.001 Disease-specific anxiety non-significant Mood p = 0.03 |
| Trial | Duration (weeks) | No. of patients in analysis | Change in HRQoL | Comparison between groups | ||
|---|---|---|---|---|---|---|
| Pentoxifylline | Pentoxifylline group (0–100 scale) | Placebo | Placebo group | |||
| SF-36a | ||||||
| Otsuka 21-98-21334 | NR | NR | Physical health summary not significant | |||
| Creager 200870 | 24 | 86 | 84 | NR | NR | Non-significant in any component |
| WIQb | ||||||
| Creager 200870 | 24 | 86 | 84 | NR | NR | Non-significant |
Cilostazol
Table 51 summarises the HRQoL data for cilostazol, and Table 52 further summarises SF-36 scales and subscales. Strandness et al. ,56,57 Beebe et al. ,61 Money et al. ,62 O’Donnell et al. ,53,83 (diabetics and non-diabetics), Dawson et al. ,58,63 and Otsuka 21-98-21334 (diabetic and non-diabetic participants were reported separately by one author but are drawn from one study)53,83 assessed the quality of life of study participants in trials of cilostazol using the SF-36. However, not all studies reported significance values for all summary measures and subscales, as can be seen from Table 51. It is likely that only scales that were significant were reported. Assuming this to be the case, no summary measure or subscale shows a consistent positive outcome for physical or mental health. The subscale physical function improved significantly in Strandness et al. ,56 Beebe et al. ,61 Money et al. 62 and O’Donnell et al. 83 (non-diabetics), although, of these, the magnitude of the change is only reported in Beebe et al. 61 and the effect does not seem to be strong enough to lead to significant changes in the summary physical function score. No study reported significant differences for between-group comparisons for any mental health component of the SF-36. COM was reported only by Beebe et al. ,61 and the results of this corresponded with the SF-36 results reported for the same trial.
VascuQoL was used in one study that reported diabetic (O’Donnell et al. 53) and non-diabetic (O’Donnell et al. 83) patients separately. VascuQoL scores did not correspond with SF-36 scores in either diabetic or non-diabetic patients. VascuQoL is a disease-specific measure designed for use with critical as well as chronic limb ischaemia,87 and as such may have different psychometric properties. Across the five studies that used WIQ, results were conflicting, with some significant results reported in Beebe et al. 61 and Money et al. ,62 significant trends reported in Strandness et al. 56 but no significant changes reported for the remaining two studies, O’Donnell et al. 83 (non-diabetics) and Dawson et al. 58
Naftidrofuryl oxalate
-
Only Spengel et al. 47 reported HRQoL for naftidrofuryl oxalate versus placebo. The outcome measure used was CLAU-S, and results were significant across four domains (daily living, pain, social life and mood) but not for the disease-specific anxiety domain.
Pentoxifylline
Inositol nicotinate
-
No studies reported HRQol data.
Summary
There is some evidence that cilostazol affects the physical function subscale of the SF-36, which suggests that there are some tangible improvements in physical function for the patient. This is somewhat supported by mixed evidence from WIQ, which suggests that patients perceive improvements in walking speed and distance in some cases. These health status improvements do not appear to translate into an overall improvement in HRQoL, with no changes in the mental health components, such as social functioning and the role–emotional subscale. The very limited evidence for naftidrofuryl oxalate suggests that improvements in pain are associated with improvements in daily living, social life and mood, but not with improvements in anxiety. The very limited evidence for pentoxifylline suggests that it does not improve HRQoL.
Discussion
Clinical effectiveness data were available from 26 RCTs. Blinded RCTs were available for all vasoactive drugs assessed within this report compared with placebo. The only vasoactive drugs for PAD compared head to head were cilostazol and pentoxifylline. Most of the trials were short term with follow-up of 24 weeks, with the exception of two trials. In practice most patients would take the vasoactive drugs for longer. Trial quality was generally good, with treatment groups within trials being comparable, blinding being maintained, and trials presenting ITT analysis.
The trial populations reported were relevant to UK practice. Populations across trials were very similar, having well-defined disease, with similar severity and duration of symptoms. Some trials specified that no specific advice was given about smoking cessation, diet and exercise, whereas others did not. Smoking tended to be balanced between treatment groups at baseline. Information about diet and exercise for participants was not reported, although there was no reason to believe that differences existed between treatment groups within trials.
For MWD and PFWD, all patients, including those in placebo groups, tended to show improvement on average. There was some evidence that walking distance outcomes were improved by cilostazol and naftidrofuryl oxalate to a significantly greater extent than improvement in placebo groups.
For walking distance data, most trials used standardised treadmill protocols, with the exception of RCTs of inositol nicotinate. Some trials used constant workload and others used graded test protocols, but treadmill protocols alone, across studies, do not seem to explain the difference between studies in whether or not a treatment effect was found. With a few exceptions, trials reporting a significant effect for MWD also reported a significant effect for PFWD.
Previously published Cochrane reviews found more improvement in MWD and PFWD compared with baseline for cilostazol than for placebo,28 and in PFWD compared with baseline for naftidrofuryl oxalate than for placebo. 32 The Cochrane cilostazol review28 included seven cilostazol versus placebo trials, all of which are included in this review. The Cochrane naftidrofuryl oxalate PFWD analysis32 included six trials, of which three were excluded from this review because either the naftidrofuryl oxalate dose was not in line with UK marketing authorisation or the population included patients with Fontaine stage III.
The meta-analysis of MWD and PFWD included 10 studies. Several studies were excluded from the meta-analysis that had been included within the narrative synthesis because the published reports did not provide data in a form that was suitable for comparison across trials. In the analysis, it was assumed that data from the studies were missing at random and that the lack of usable data was not related to the observed treatment effect. Based upon evidence from the excluded studies, there is no evidence of publication bias for the naftidrofuryl oxalate trials. In addition, the studies that were excluded owing to short follow-up suggested a similar direction of effect.
Adverse events were minor and included headaches and gastrointestinal difficulties. Minor AEs were more frequent for cilostazol and pentoxifylline than for naftidrofuryl oxalate. Incidence of SAEs including cardiovascular events was not increased by the vasoactive drugs for PAD compared with placebo; however, most studies had relatively short follow-up time (up to 24 weeks) to address this outcome. Across studies, mortality rates had no significant differences between treatment groups; however, these were mostly based on relatively short follow-up times. Only two studies had follow-up of over 24 weeks:49,69 the CASTLE study49 of cilostazol versus placebo (which included some patients taking pentoxifylline in both groups) with a follow-up period of 3.5 years, and the Dettori et al. study69 of pentoxifylline versus placebo with a follow-up period of 1 year. Neither of these trials reported treatment group differences for mortality or cardiovascular events. There were no trials of naftidrofuryl oxalate or inositol nicotinate with follow-up of over 24 weeks. ABPI was not reported by many trials, but mostly there was a non-significant trend to improve in both treatment groups, with some suggestion that cilostazol may improve ABPI more than placebo. HRQoL was measured in different ways across studies, making it difficult to compare treatments. There is some evidence that there are some tangible improvements in physical function for patients taking cilostazol, but these do not appear to translate into overal improvements in quality of life. Evidence for naftidrofuryl oxalate is very limited, but indicates that there may be improvements in both physical function and overall quality of life. Pentoxifylline does not seem to have HRQoL benefits but evidence is limited, and there was no evidence for inositol nicotinate.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
Searches
A systematic literature search was undertaken to identify economic evaluations of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate compared with each other or no vasoactive drugs for the treatment of IC in people with PAD.
Appendix 1 reports details of the search strategy used and databases searched. None of the manufacturers submitted an economic model to evaluate the cost-effectiveness of the drugs.
Study selection, data extraction and quality assessment strategy
The inclusion and exclusion criteria applied to the searches are shown in Table 55. A health economic modeller applied the inclusion and exclusion criteria (YM), with checking by a second health economic modeller (HS). The quality of the economic evaluation studies that met the inclusion criteria was assessed using an adapted version of the Drummond and Jefferson British Medical Journal criteria for economic evaluation (Drummond et al. 88) and the Consensus on Health Economic Criteria (CHEC)-list (Evers et al. 89). Papers remaining in the review were read in detail and data extracted using a predesigned data extraction form (shown in Appendix 6). Data on the following were sought:
-
study characteristics, such as the study question, study design, population, comparators, interventions, perspective, time horizon and type of modelling method used
-
clinical effectiveness and cost parameters, such as effectiveness data, health-state utilities, cost and resource use data, discounting and other key assumptions
-
baseline results and SA.
| Study design | Cost–consequence analysis, cost–benefit analysis, cost-effectiveness analysis or cost–utility analysis |
|---|---|
| Population | PAD patients with IC |
| Intervention | Cilostazol, naftidrofuryl oxalate, pentoxifylline and/or inositol nicotinate |
| Comparator | Placebo, exercise, surgical procedure and/or any vasoactive drug |
| Outcome | Cost-effectiveness |
Results
The literature searches identified 187 potentially relevant citations. Only 25 of these appeared to relate to the economic evaluations of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate. From these, five full papers were reviewed, and two studies met the inclusion criteria: one is a published journal paper (Guest et al. 90) and one is a conference poster presentation with only an abstract (Ratcliffe91). Figure 5 shows the summary of the study selection and exclusion. The evaluation of the full paper met 9 out of the 10 Drummond and Jefferson quality assessment criteria and 15 out of the 19 CHEC-list criteria. The abstract met fewer assessment criteria due to limited information. Full details can be found in Appendix 6.
FIGURE 5.
Summary of economic evaluation selection and exclusion.
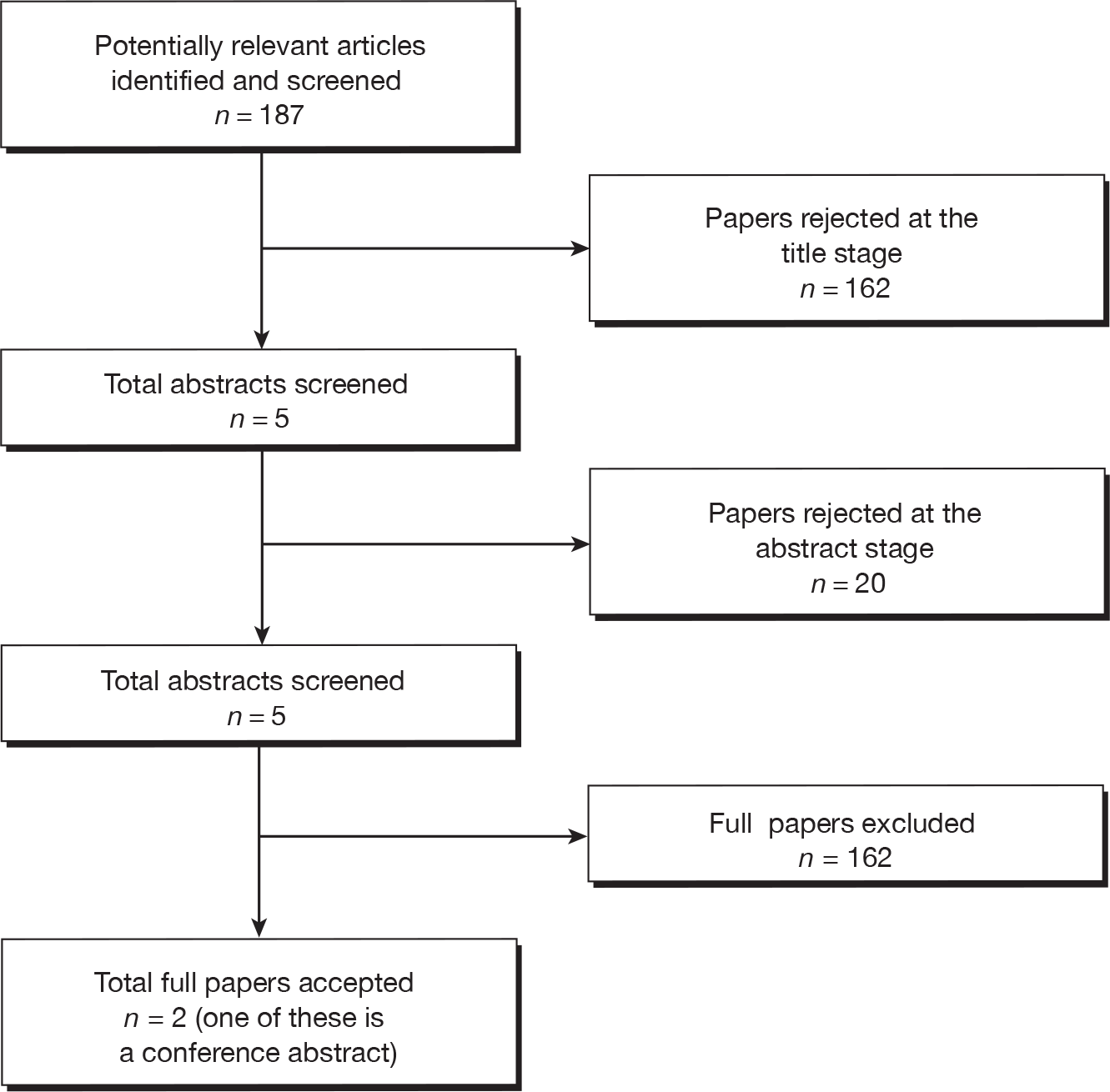
The characteristics and the main results of the economic evaluations are summarised in Table 56.
| Study | Author | |
|---|---|---|
| Guest et al.90 | Ratcliffe (abstract)91 | |
| Country and year of publication | UK, 2005 | UK, 2005 |
| Sponsor | Otsuka Pharmaceuticals | Unclear |
| Type of analysis | Cost-effectiveness (improvement in MWD at 24 weeks) | Cost–utility |
| Health economic perspective | NHS in the UK | NHS in Scotland |
| Model type | Decision tree | Unclear |
| Software used | Data Professional (TreeAge Software, Inc., Williamstown, MA, USA) and Crystal Ball (Oracle, Montreal, QC, Canada) | Unclear |
| Intervention(s) | Cilostazol | Cilostazol |
| Comparator(s) | Naftidrofuryl oxalate and pentoxifylline | Placebo |
| Population characteristics | Patients in the UK (aged 40 years) who have 24 weeks of symptomatic IC, secondary to PAD | Patients in Scotland with IC |
| Time horizon | 24 weeks | 24 weeks |
| Effectiveness data | Six published RCTs; panel of 12 vascular surgeons in the UK | Two published RCTs |
| Cost year and currency | 2002–3, UK £ | Unclear |
| Health economic outcomes | Change in the percentage improvement in MWD vs change in costs | Cost per QALY |
| Base-case results |
Cilostazol vs naftidrofuryl oxalate: 32% increase in the percentage improvement in MWD for a 12% increase in costs Cilostazol vs pentoxifylline: 67% increase in the percentage improvement in MWD for a 2% decrease in costs Naftidrofuryl oxalate vs pentoxifylline: 27% increase in the percentage improvement in MWD for a 14% decrease in costs |
ICER: £12,500 per QALY |
Guest et al. 90 presented the methods and results of a cost-effectiveness analysis comparing cilostazol [100 mg twice daily (b.i.d.)], naftidrofuryl oxalate [300 mg b.i.d. or 200 mg three times daily (t.i.d.)] and pentoxifylline (400 mg t.i.d.) within UK patients who are 40 years of age and have had at least 6 months of IC. A decision tree model was developed in Data Professional (TreeAge Software Inc., Williamstown, MA, USA) to model the management of IC patients over a period of 24 weeks. The analysis was carried out from the UK NHS perspective and the outcome of the model was the change in the percentage improvement in MWD versus the change in costs. HRQoL was not considered within the model.
The decision tree model considered the decision by a vascular surgeon to initially treat a patient with either cilostazol, naftidrofuryl oxalate or pentoxifylline. Within the model, a patient may continue the initial treatment for 24 weeks or discontinue the initial treatment. Patients who do not continue with the initial treatment for 24 weeks may either switch to another drug or discontinue drug treatment. Additionally, patients may undergo an angioplasty or bypass surgery.
The effect of each drug in improving MWD for 24 weeks was derived from six published double-blind, placebo-controlled RCTs,61,65,92–95 all of which were identified for inclusion within this clinical effectiveness review. RCTs that were not blinded or did not report treadmill speeds were excluded. Studies that used varying treadmill speeds or gradients were also excluded. The probabilities of continuing/discontinuing treatment were obtained from published studies. An assumption was made that patients who stop receiving treatment will achieve the same improvement of MWD as those patients on placebo. In the case of patients who switched drugs and would have been on the new drug for 12 or 18 weeks, it was assumed that these patients would achieve the same improvement in MWD at these time points as the drug-treated patients in the trials.
Costs included diagnosis of IC, drug costs, follow-up visits by the vascular surgeon and/or general practitioner (GP), supervised exercise, angioplasty and bypass surgery. The frequency of surgeon and GP visits and the probabilities of using different types of diagnostic techniques, switching to other drugs and undergoing supervised exercise, angioplasty or bypass surgery were based on interviews of 12 vascular surgeons in the UK. Unit resource costs were obtained from NHS reference costs, drug tariff and published studies. All costs were presented in 2002–3 UK pounds sterling. Costs and outcomes were not discounted due to the short period of modelled time.
Probabilistic sensitivity analyses (PSAs) were undertaken with uncertainty around parameters of percentage improvement in MWD, probabilities and resource use.
The results of the model suggested that starting treatment with cilostazol instead of naftidrofuryl oxalate increases the percentage improvement in MWD by 32% (from 57% to 75%) for a 12% increase in costs (from £801 to £895). Starting treatment with cilostazol instead of pentoxifylline was found to increase the percentage improvement of MWD by 67% (from 45% to 75%) and reduce costs by 2% (from £917 to £895). Starting treatment with naftidrofuryl oxalate instead of pentoxifylline was found to increase the percentage improvement in MWD by 27% (from 45% to 57%) and decrease costs by 13% (from £917 to £801). The sensitivity analyses suggest that the variability around the incremental cost-effectiveness was driven by the uncertainty in the probabilities of continuing treatment for 24 weeks, the percentage improvement in MWD and the probability of having diagnostic tests among patients who complete 24 weeks of treatment.
The study has several limitations:
-
There was not a ‘no vasoactive drug’ comparator.
-
The time horizon was 24 weeks.
-
Effectiveness was evaluated only in terms of improvement in MWD. HRQoL (utilities) was not evaluated.
-
No model validation was reported.
Ratcliffe91 presented a brief description of the methods and results of a cost–utility analysis of cilostazol (100 mg) versus placebo for Scottish patients with IC. The study was only available in an abstract as a conference poster and no full paper was available. The assessment group attempted to contact the author but this was unsuccessful. A decision analytical model (type of model was not specified) was built to evaluate the cost-effectiveness of cilostazol versus placebo over a period of 24 weeks. The analysis was carried out from the Scottish NHS perspective and the outcome of the model was the incremental cost per quality-adjusted life-year (QALY).
Effectiveness was based on two published 24-week RCTs of cilostazol versus placebo (not referenced in the abstract). HRQoL was measured in the trials using the SF-36. The scores were converted into utilities using a validated mapping algorithm (not referenced in the abstract). Costs included drug costs and treatment costs. Treatment costs were based on an independent survey of expert clinical opinion in Scotland. Both costs and QALYs were not discounted due to the short time horizon of the model.
The model results suggested that the incremental cost–utility ratio for cilostazol over placebo was estimated to be £12,500 per QALY gained. SA suggested that the results were most sensitive to the cost of an angiography, the utility values estimated and the price of cilostazol. Detailed evaluation of the model was not possible because there was no published full paper available.
Summary
There are currently no economic evaluations of cilostazol, naftidrofuryl oxalate, pentoxifylline or inositol nicotinate, which consider long-term costs and outcomes. Only one economic evaluation of cilostazol considered outcomes in terms of a cost per QALY and this was reported in a non-peer-reviewed conference abstract only. A de novo economic evaluation is therefore required.
Independent economic assessment
Methods
This section provides details of a model developed by the assessment team and used to evaluate the cost-effectiveness of each vasoactive drug for PAD within its licensed indication compared with no vasoactive drug and with the remaining vasoactive drugs for PAD.
Model description
Patient population
A Markov model was developed in Excel® (Microsoft Corporation, Redmond, WA, USA) to determine the cost-effectiveness of each drug compared with no vasoactive drugs for PAD and with the remaining vasoactive drugs. The population considered was patients who have stable (at least for the past 3 months, which is the inclusion criterion for most of the RCTs identified) and symptomatic IC, secondary to PAD. Furthermore, only patients whose symptoms continue despite a period of conservative management, such as advice to cease smoking or do more exercise, were considered by the model. The model did not distinguish between patients who are in primary care and secondary care, because no published evidence was identified to support this classification. The model also did not distinguish between patients with different severity of the disease, because the considered patient population was already narrowly defined (i.e. patients with stable IC who fail conservative management) and no published evidence was identified to support a subgroup analysis. However, an exploratory subgroup analysis was undertaken around patients with more severe IC who might receive angioplasty following drug discontinuation.
Interventions and comparators
The four drugs considered within their licensed indications for IC were cilostazol (200 mg per day), naftidrofuryl oxalate (600 mg per day), pentoxifylline (1200 mg per day) and inositol nicotinate (4 g per day). These were compared with each other and no vasoactive drugs for PAD. As it was not possible to include inositol nicotinate within the meta-analysis of MWD or PFWD, this drug has not been included within the main analysis. However, inositol nicotinate has been included within a threshold analysis to assess how many QALYs would be required for it to have a cost per QALY gained below £20,000 and £30,000 compared with no vasoactive drug.
Outcomes
The model outcome is the incremental cost per QALY gained. Owing to lack of evidence around the utilities of patients having naftidrofuryl oxalate, pentoxifylline and inositol nicotinate, change in MWD was used as a surrogate measure to estimate the change in utilities based on a regression model. MWD was selected as the surrogate because it is the primary outcome of most identified RCTs. More detail about this regression is provided below (see Estimate of model parameters). The life-years gained outcome is not presented within this analysis because the drugs are not expected to have an impact on life-years, only on patient quality of life.
Model structure
The structure of the decision model is presented in Figure 6. The model includes three main health states: vasoactive drug treatment (where patients receive one of the four drugs under evaluation); no vasoactive drug treatment (where patients receive none of the four drugs or have discontinued); and death. Patients begin in the vasoactive drug treatment state and have a weekly probability of moving to the no vasoactive drug treatment state by discontinuing. Patients also have a weekly probability of dying of any cause from both the vasoactive drug treatment state and the no vasoactive drug treatment state. Patients who do not receive any of the four drugs have zero time in the vasoactive drug treatment state. All patients are in Fontaine stage II and have had persistent IC symptoms despite a period of conservative management to be eligible to receive the vasoactive drugs for PAD. The health states are classified according to whether or not the patients are receiving vasoactive drugs for PAD rather than by progression through different disease stages (i.e. Fontaine stages II–IV), as the drugs are for symptom relief and it is assumed that they do not have an impact on disease progression.
FIGURE 6.
Diagram of the structure of the decision model.
Patients in the vasoactive drug treatment state could improve their quality of life as a result of the treatment effect. In the base-case analyses, the only extra cost for patients receiving the vasoactive drugs for PAD compared with no vasoactive drugs is the drug acquisition cost.
Patients may discontinue the drug because of AEs, death or other reasons of non-compliance. Switching to another vasoactive drug after discontinuation and returning to the same vasoactive drug after discontinuation were not considered within the model owing to lack of published evidence. In addition, expert clinical opinion suggested that this would not be standard practice within England and Wales (Steven Thomas, Jonathan Michaels and Gerard Stansby, personal communication).
Patients who have discontinued the drug therapy were assumed to incur no extra costs compared with those patients initially having no treatment. It was also assumed that the drugs are only effective while they are being given, as suggested by the study by Keiffer et al. ,65 which recorded MWD for 2 months beyond treatment discontinuation. 65 Therefore, patients discontinuing a vasoactive drug will have no extra health gains (regarding utility, walking distance or disease progression) compared with no vasoactive drug.
Time horizon
The time horizon of the model was 100 years to ensure that all differences in costs and benefits are captured within the model, and a starting age of 66 years was used to represent the average age of patients with IC. The starting age was based on the average age of patients within the CASTLE study,49 which has the longest follow-up period and the largest sample size of all RCTs (Hiatt et al. 49). A time cycle of 1 week was chosen as being sufficiently short to capture the effect of treatment, and the time period was in line with that used in the trials for measurement of walking distance and quality of life.
Discounting
All costs and QALYs were discounted at a rate of 3.5% per year.
Estimate of model parameters
Maximal walking distance and utilities
The majority of studies reported the change in MWD for the vasoactive drug and control arms, but only some stated that quality-of-life data were collected and only two RCTs (both for cilostazol)61,83 reported quantitative data for SF-36 quality-of-life outcomes that can be converted to utilities using published algorithms. 96 No studies of naftidrofuryl oxalate, pentoxifylline or inositol nicotinate provided sufficient quality-of-life evidence to estimate utility outcomes associated with these drugs. However, in order to compare the cost-effectiveness of these vasoactive drugs with other interventions routinely funded by the NHS, quality-of-life estimates are required for all drugs being assessed so that an incremental cost per QALY can be calculated. Given the limited published data around quality-of-life outcomes, the authors of the identified RCTs were contacted to ask for the patient-level or summary SF-36 data if the paper mentioned that the SF-36 questionnaire was used within the RCT. The aim of this was to attempt to determine a relationship between the change in MWD and the change in utility scores that could be used to estimate the utility gains associated with the drugs being assessed for which there were only MWD data and no utility data. MWD was chosen to be linked to utility because MWD is the primary outcome of most identified RCTs. Most of the authors responded (80%) but could not provide these data. One author (O’Donnell) provided a complete set of patient-level data (N = 106) for MWD and SF-36 scores based on a recent RCT in the UK comparing cilostazol and no vasoactive drug for PAD. 83
The SF-36 conversion algorithm, as defined by Ara and Brazier,96 was applied to the patient-level data from O’Donnell et al. 83 to calculate the utilities of each patient at week 0 and week 24 (the period of the RCT). These patient-level data were then used to test for a correlation between change in MWD and change in utilities from week 0 to week 24, which is the trial period of most identified RCTs. The correlation coefficient of the absolute difference in MWD on the log scale (log of MWD in week 24 minus log of MWD in week 0) and absolute difference in utilities (utility in week 24 minus utility in week 0) was 0.39 and the scatterplot is presented in Figure 7. A linear regression model was fitted to these data to predict the absolute change in utilities from the absolute change in MWD on the log scale during the RCT period. A linear relationship was assumed owing to lack of evidence of other types of underlying relationship. One regression model was fitted to the placebo and cilostazol data combined and this maximised the sample size for the regression analysis. The underlying assumption was that the relationship between MWD and utility is independent of treatment, such that the relationship can be applied to all of the vasoactive drugs being evaluated and the no vasoactive drug comparator. This was tested by including an additional term in the regression analysis representing the treatment effect which was not significant. The fitted regression based on the O’Donnell et al. patient-level data83 and used in the economic model is:
FIGURE 7.
The relationship between the absolute change in utilities and the absolute change in MWD on log scale based on patient-level data (O’Donnell et al. 83).
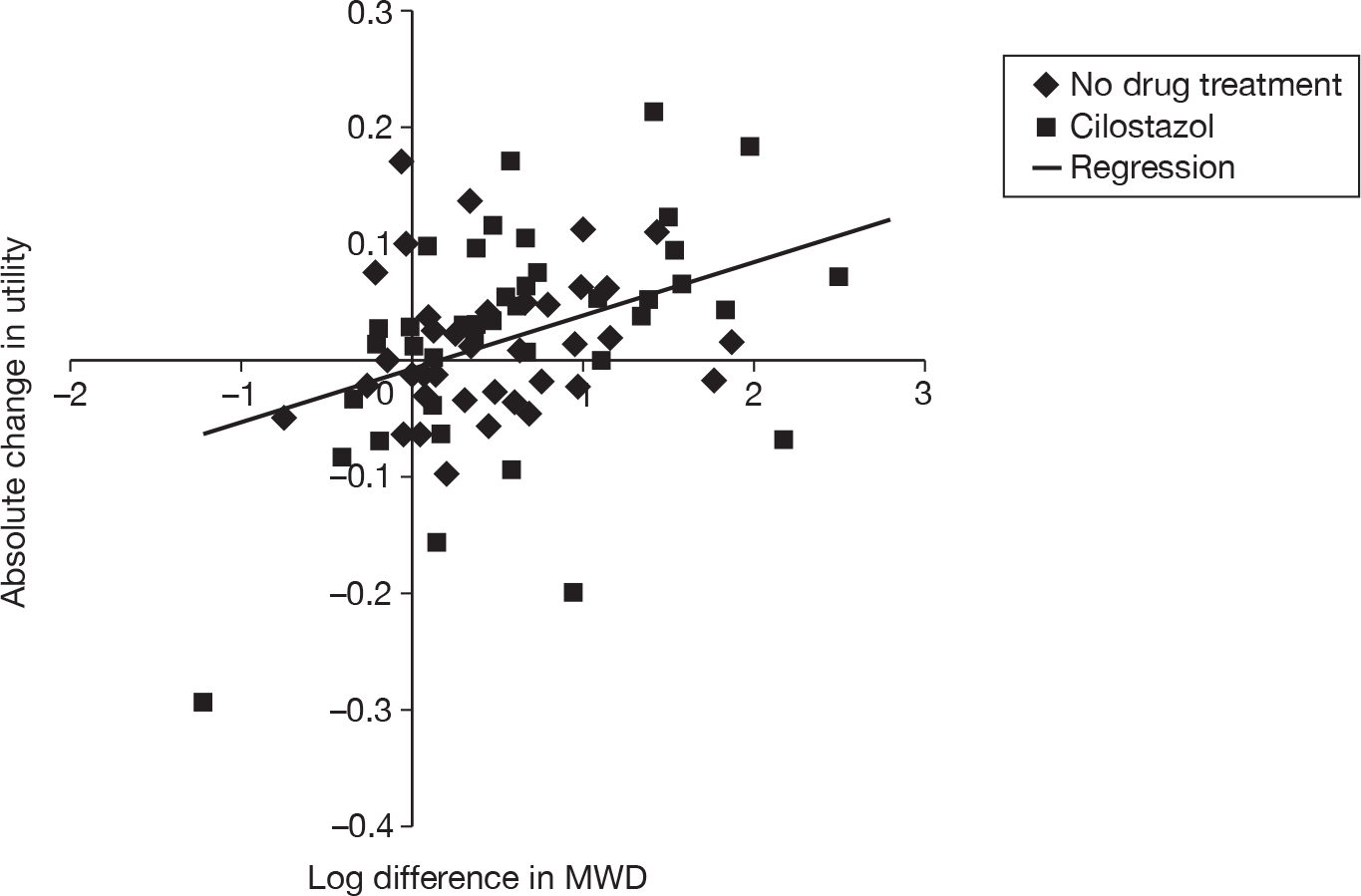
It is reassuring that the constant within the regression model is very close to zero, which means that when there is no change in MWD, there is also no change in the utility score. The variance–covariance matrix of the slope and the intercept of the regression model is presented in Table 57. To represent the uncertainty of the regression model, the matrix was used to sample the two coefficients of the regression model in the PSA. By using the variance–covariance matrix within the PSA, the uncertainty around the relationship between MWD and utility is propagated through the model and represented within the model results. However, this uncertainty does not account for any differences in this relationship between the vasoactive drugs.
| Slope | Intercept | |
|---|---|---|
| Slope | 0.00015001 | |
| Intercept | –0.0000813 | 0.000111 |
The estimate of treatment effect that is generally reported in the RCTs of the vasoactive drugs for PAD is the percentage change between treatments based on the geometric mean change from baseline. The reason for this is because the raw data are analysed on the log scale and anti-logging the sample means on the log scale produces sample geometric means. The motivation for transforming these data to the log scale is to produce a scale on which the treatment effects can be assumed to be linear. We use a similar rationale when relating the log of the difference in MWD to the absolute difference in utilities, with treatment effects in terms of utilities assumed to be linear on the absolute scale.
The regression model was applied to all four drugs and to no vasoactive drug treatment to estimate the absolute change in utilities given a certain change in MWD from week 0 to week 24 on the log scale. The baseline utilities, i.e. the utilities for patients at week 0, were also estimated from the patient-level data. 83 All estimated absolute changes in utilities were applied to the baseline utilities. The estimated mean of the baseline utilities was 0.4838 and the estimated SD was 0.1001. The patient-level data used for this analysis were collected within the latest reported clinical trial on cilostazol and were based on patients in the UK. 83 Therefore, the mean baseline utilities should reflect the quality of life of patients with stable IC in the UK NHS context. SA was performed to test alternative baseline utilities.
The SF-36 data were also available at week 6 for each patient; and the mean utility change over time (at weeks 0, 6 and 24) for all patients is presented in Figure 8. Several RCTs of cilostazol, naftidrofuryl oxalate and pentoxifylline reported the change in MWD over time, which also suggested a linear increase. 58,61,65,66 In the absence of any additional evidence, this suggests that a linear model may be appropriate when representing the increase in utilities over the first 24 weeks. For patients who receive a vasoactive drug beyond 24 weeks, it was assumed the utility remains constant from week 24 onwards, due to the lack of published evidence beyond this time point. For patients who discontinue treatment, it was assumed the utility returns to the level of the no vasoactive drug group at the time of discontinuation.
FIGURE 8.
Mean utility change over time (at weeks 0, 6 and 24) based on patient-level data (O’Donnell et al. 83).
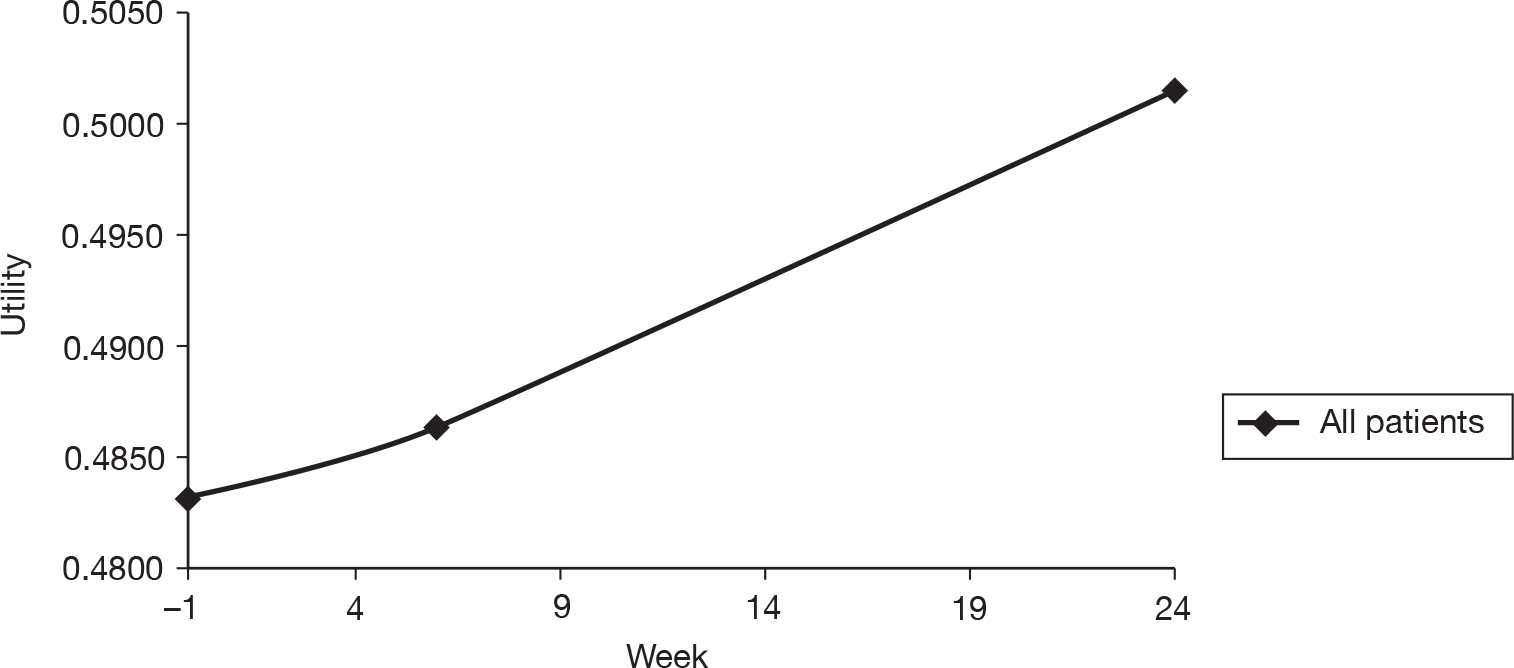
Given that the HRQoL of the general population is dependent upon age, it is important to take this into account in the model. General population utility estimates from Ara and Brazier97 were applied using a regression analysis of utility versus age. The age-related utility was calculated by the following formula:
where A = –0.0001728, B = –0.000034 and C = 0.9584588.
The ratio between the utility at age 66 years using this formula for the general population and the utility at age 66 years for IC patients estimated using the regression above was calculated. The age-related utility within the general population was then adjusted to account for the lower average utility associated with IC patients by multiplying it by this ratio for each age within the model.
Given the limited evidence in terms of utilities, a threshold analysis has also been undertaken to assess the QALY gain required for each of the drugs to be considered to be cost-effective compared with no vasoactive drug at willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained. The threshold analysis is the only way in which the cost-effectiveness of inositol nicotinate is assessed owing to the lack of effectiveness data available for this drug.
Table 58 presents the predicted mean utility values for each vasoactive drug and no vasoactive drug treatment at week 24.
| Drug | Mean utility at week 24 |
|---|---|
| No vasoactive drug treatment | 0.4873 |
| Cilostazol | 0.4973 |
| Naftidrofuryl oxalate | 0.5088 |
| Pentoxifylline | 0.4919 |
Adverse events
Within the trials identified within the systematic review, rates of SAEs were similar between the treatment groups and the placebo groups, and rates of minor AEs were similar between naftidrofuryl oxalate and placebo and inositol nicotinate and placebo. The trials of cilostazol and pentoxifylline reported higher rates of minor AEs within the treatment groups than the placebo groups, which were mainly headaches, diarrhoea, peripheral oedema and palpitations (see Chapter 3, Adverse events and serious adverse events, above, for further details). Clinical expert advice suggests that these patients are unlikely to require additional treatment as they would discontinue the vasoactive drugs, as suggested by the trials that demonstrate higher discontinuation rates for cilostazol and pentoxifylline. This means that there are unlikely to be any additional costs incurred as a result of these AEs. As these minor AEs would generally be experienced for a short time period, and, given that these patients already have a lower utility on average than that experienced by the general population, the impact of these minor AEs upon utilities is expected to be minimal (i.e. unlikely to affect total QALYs to fewer than three decimal places). The quality of life of patients will therefore be affected within the model only by the patients discontinuing and hence having a lower quality of life than if they remain on treatment.
Discontinuation of treatment
The rates of discontinuation for the first 24 weeks were based on meta-analyses of all identified RCTs. The long-term discontinuation rate (i.e. beyond 24 weeks) was reported in only one study of cilostazol (Hiatt et al. 49), which reported that 68% of patients in the cilostazol arm discontinued with the drug by 36 months. Expert clinical opinion suggests that many discontinuations beyond 24 weeks are likely to be due to the patients’ condition improving or mortality and hence the patients no longer require the drug, rather than discontinuations being because of any AEs associated with the drugs. Therefore, given the lack of published evidence, the long-term discontinuation rates of the remaining three drugs were assumed to be the same as for cilostazol.
Mortality
It was assumed that all drugs are for symptomatic relief rather than having an impact on the progression of the disease (Steven Thomas, Jonathan Michaels and Gerard Stansby, personal communication). Therefore, all patients within the model have the same overall mortality rates. General population mortalities were based on the latest life tables of the general population in England and Wales [Office for National Statistics (ONS)]. 98 The mortalities of the patient population in the model were calculated by multiplying the general population mortality by the relative risk of mortality of IC patients, which was assumed to be 1.6 based upon a study of the risk of mortality and cardiovascular disease associated with ABPI by Heald et al. 99
Resource use and costs
The cost of each drug was based on the latest drug tariff updated in October 2010. 26 Where there is more than one licensed dose available, the cost of the drug was based upon the doses used within the RCTs identified within the clinical effectiveness review, which are also current practice in the UK. Inositol nicotinate is supplied in two packs: 100 tablets of 500 mg at a price of £30.76 (6.152p per 100 mg) and 112 tablets of 750 mg at a price of £51.03 (6.075p per 100 mg). The former pack was used for estimating costs as it has a higher unit price (in terms of 100 mg) and the identified inositol nicotinate RCTs used a dose of 4 g per day (which can not be divided by 750-mg tablets). Naftidrofuryl oxalate is available both as a generic drug at a price of 5.38p per 100 mg and produced by the manufacturer that held the original patent at a higher price of 9.83p per 100 mg. The former cost was used in the base-case model because this is expected to be the acquisition cost in practice. Drug costs included within the model are presented in Table 59.
| Drug | Licensed dose | Brand name | Dose used for estimating costs (mg/day) | Drug specification (manufacturer) | Quantity | Price (£) | Weekly costs (£) | Remarks |
|---|---|---|---|---|---|---|---|---|
| Cilostazol | 100 mg twice daily (30 minutes before or 2 hours after food), i.e. 200 mg per day | Pletal | 200 | Cilostazol 100-mg tablets (Pletal) | 56 | 35.31 | 8.83 | |
| Naftidrofuryl oxalate | 100–200 mg three times daily, i.e. 300 or 600 mg per day | Generic | 600 | Naftidrofuryl oxalate 100-mg capsules | 84 | 4.52 | 2.26 | |
| Praxilene | 600 | Naftidrofuryl oxalate 100-mg capsules (Praxilene) | 100 | 9.83 | 4.13 | |||
| Pentoxifylline | 400 mg two or three times daily, i.e. 800 or 1200 mg per day | Trental 400, Pentofin, Oxpentifylline | 1200 | Pentoxifylline 400-mg modified-release tablets (Trental 400) | 90 | 19.68 | 4.59 | |
| Inositol nicotinate | 3 g daily in two or three divided doses; maximum 4 g daily (tablets 500 mg or 750 mg) | Hexopal, Hexopal Forte, Hexanicotol | 4000 | Inositol nicotinate 500-mg tablets (Hexopal) | 100 | 30.76 | 17.23 | The drug is also supplied as 750-mg tablets in quantities of 112 at £51.03 (manufactured by Hexopal) |
Assessment of cost-effectiveness
The main results are an estimate of the total costs and total QALYs of each intervention and the comparator, and the incremental cost-effectiveness ratios (ICERs). In incremental analyses, one intervention may be dominated or extendedly dominated by the comparator. Dominance is defined as an intervention being less effective and more expensive than its comparator. Extended dominance is present if the ICER for a given treatment alternative is higher than that of the next more effective comparator. In total, 10,000 PSA runs were implemented to estimate the expected costs and QALYs. A cost-effectiveness acceptability curve (CEAC) and a cost-effectiveness plane are included to give a measure of the uncertainty reflected by the model. A range of univariate sensitivity analyses were performed to explore the sensitivity of the model results to key parameters and assumptions.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was applied to the following input parameters to represent the uncertainty around the model inputs:
-
discontinuation rates for the four drugs within 24 weeks
-
discontinuation rates for cilostazol beyond 24 weeks (assumed to be equivalent for all other vasoactive drugs for PAD)
-
change in MWD on the log scale for the vasoactive drugs and no vasoactive drug
-
baseline utilities for patients at week 0
-
coefficients (constant and slope) of the regression model to predict the change in utility from the change in MWD.
Table 60 summarises the input parameters and their base-case mean values and distributions (used for PSA) for the model.
| Parameters | Mean | Distribution (parameters) | Source |
|---|---|---|---|
| Age | 66 years | Fixed | Hiatt et al. 200849 |
| Discount rate (costs and utilities) | 3.5% | NICE 2008100 | |
| Relative risk of mortality for patients with IC | 1.6 | Heald et al. 200699 | |
| Discontinuation rates (%) | |||
| Proportion of patients discontinuing cilostazol within 24 weeks | 27.8 | Normal (27.8% to 1.5%) | Based on meta-analysis of RCTs reported in Chapter 5 |
| Proportion of patients discontinuing naftidrofuryl oxalate within 24 weeks | 11.1 | Normal (11.1% to 2.5%) | |
| Proportion of patients discontinuing pentoxifylline within 24 weeks | 29.1 | Normal (29.1% to 1.8%) | |
| Proportion of patients discontinuing inositol nicotinate within 12 weeksa | 20.0 | Normal (20.0% to 6.3%) | |
| Proportion of patients discontinuing cilostazol (and other vasoactive drugs for PAD) within 36 months | 68 | Normal (68.0% to 1.7%) | Hiatt et al. 200849 |
| Drug costs (£) | |||
| Weekly costs of cilostazol | 8.83 | Fixed | Drug Tariff, October 2010 (www.drugtariff.co.uk)26 |
| Weekly costs of naftidrofuryl oxalate | 2.26 | ||
| Weekly costs of pentoxifylline | 4.59 | ||
| Weekly costs of inositol nicotinate | 17.23 | ||
| Baseline utility | |||
| Baseline utility | 0.4838 | Beta (11.58 to 12.36) | Based on patient-level data from O’Donnell et al.83 |
| Change in MWD on the log scale | |||
| Change in MWD on the log scale for no vasoactive drug (week 0 to week 24) | 0.2419 | The joint posterior distribution from the random effects network meta-analysis analysed in Winbugs | Meta-analysis reported in Chapter 5 |
| Change in MWD on the log scale for cilostazol (week 0 to week 24) | 0.4615 | ||
| Change in MWD on the log scale for naftidrofuryl oxalate (week 0 to week 24) | 0.7134 | ||
| Change in MWD on the log scale for pentoxifylline (week 0 to week 24) | 0.3427 | ||
| Change in MWD on the log scale for inositol nicotinate (week 0 to week 24)b | NA | ||
| Regression model | |||
| Intercept of the regression model | –0.0283 | Based on the variance–covariance matrix of the intercept and slope (see Table 57) | Based on patient-level data from O’Donnell et al.83 |
| Slope of the regression model | 0.0995 | ||
Univariate sensitivity analysis
Univariate SA can be used to assess the impact of alternative assumptions upon the model results. This means that if there is uncertainty around any of the assumptions within the base-case model, it is possible to understand whether or not they are important in terms of the impact upon the results. The following univariate sensitivity analyses were performed to explore the uncertainty of model assumptions.
Sensitivity analysis 1: utility remains the same as when on the drug if discontinuation occurs after 24 weeks
Clinicians suggest that a proportion of patients discontinue with the drug after 24 weeks because their condition has improved. The SA assumes that if the patients discontinue the drug after 24 weeks, the utility remains the same over this subgroup of patients’ remaining lifetime as when on the drug at the time of discontinuation.
Sensitivity analysis 2: alternative baseline utility
Two RCTs of cilostazol were identified within the clinical effectiveness review, which presented SF-36 data that could be converted to utilities. 61,83 The SF-36 data were converted into utilities for both studies and found to be very different from each other: a utility of 0.4838 versus 0.7562. The former utility was used within the base-case analysis, and the latter was tested within this SA. Utility would also be variable between patients in practice. The relationship between baseline utility and utility at 24 weeks is assumed to remain the same within this analysis.
Sensitivity analysis 3: alternative cost for naftidrofuryl oxalate
In the base-case model, the cost of generic naftidrofuryl oxalate is used. The drug is also produced by the manufacturer that held the patent (with a brand name of Praxilene), but at a higher cost of £4.13 per week compared with £2.26 per week in the base case. This SA was performed to test the impact on cost-effectiveness results using the alternative drug cost.
Sensitivity analysis 4: shorter time horizon
Most of the evidence on change in MWD, change in utility and discontinuation rates is based on RCTs that have a follow-up period of < 24 weeks. Beyond 24 weeks, a number of assumptions have been made within the model around the change in utilities and the drug discontinuation rates owing to a lack of published evidence. This SA tests a shorter time horizon of 24 weeks where data are most robust.
Sensitivity analysis 5: alternative starting age
The base-case model assumes that the cohort of patients within the model begin treatment at age 66 years. This SA assesses whether or not the age at which patients begin treatment affects the cost-effectiveness of the drugs. A starting age of 55 years (the age at which the disease begins being prevalent in the population) was applied to test the robustness of the results to starting age.
Sensitivity analysis 6: alternative long-term discontinuation rates
No evidence on the long-term discontinuation rates of pentoxifylline, naftidrofuryl oxalate or inositol nicotinate was identified. The base case assumes that the discontinuation rates of these vasoactive drugs beyond 24 weeks are the same as the discontinuation rates of cilostazol, as clinicians suggest that the reasons for discontinuation beyond 24 weeks would be more likely related to improvements in disease than to AEs, as for the first 24 weeks. However, in order to test the impact of alternative discontinuation rates beyond 24 weeks, the SA assumes that the long-term discontinuation rates of pentoxifylline and naftidrofuryl oxalate maintain the same relative ratios compared with cilostazol within the 24 weeks (i.e. as the discontinuation rate of pentoxifylline and naftidrofuryl oxalate are, respectively, 5% more and 60% less than that of cilostazol within the first 24 weeks. It is assumed that beyond 24 weeks the discontinuation rates of pentoxifylline and naftidrofuryl oxalate are also, respectively, 5% more and 60% less than the long-term discontinuation rate of cilostazol).
Sensitivity analysis 7: angioplasty procedure for patients discontinuing within 24 weeks
Clinical practice regarding prescribing of vasoactive drugs for IC patients whose symptoms continue despite a period of conservative management varies among clinicians. Some clinicians will assess whether angioplasty is appropriate within this patient group and if so undertake this immediately. If angioplasty either is not appropriate or fails then those patients may receive vasoactive drugs. Alternative practice is for IC patients to be offered vasoactive drugs whether or not they may be considered for angioplasty. If the drugs are unsuccessful, patients may then be considered for angioplasty if this is an appropriate option, but, if successful, these vasoactive drugs may negate or delay the need for angioplasty. The former clinical practice is considered within the base-case analysis. This SA concerns the latter of these two alternative clinical practices.
The subgroup of patients who would be potentially offered angioplasty may be prespecified as they tend to have a worse prognosis. It may be that the cost-effectiveness of the assessed drugs is different within this subgroup of patients and hence an exploratory analysis has been undertaken around this subgroup. The analysis is considered to be exploratory, as there is no published evidence reporting the costs and outcomes associated with this subgroup and hence it is mainly based upon personal communication with the team of clinical advisors (Steven Thomas, Jonathan Michaels and Gerard Stansby, personal communication). Those patients with a worse prognosis in whom angioplasty is potentially appropriate were estimated to represent around 15% of the overall patient group included within this assessment (Steven Thomas, Jonathan Michaels and Gerard Stansby, personal communication).
A set of simplified assumptions were made for this SA:
-
Patients who discontinue the vasoactive drugs within 24 weeks will have angioplasty.
-
Patients in the comparator group with no vasoactive drug treatment will have angioplasty at week 0.
-
The costs of angioplasty include two hospital visits (£99.03 per visit),90 one imaging (£189.90)101 and the angioplasty procedure (£925.58). 90 All costs were adjusted to 2009–10 prices. 102
-
Owing to lack of comparative evidence around the utility associated with angioplasty, it will be varied over the largest plausible range within this analysis and will be related to the utility associated with naftidrofuryl oxalate (the drug associated with the highest utility). The lower bound of the utility increase due to angioplasty is assumed to be zero. The upper bound is assumed to be the same as the utility of the general population used in the model. The utility associated with angioplasty is therefore assumed to be:
-
– equivalent to the utility associated with naftidrofuryl oxalate (the drug associated with the highest utility)
-
– 20% higher than the utility associated with naftidrofuryl oxalate
-
– 40% higher than the utility associated with naftidrofuryl oxalate
-
– equivalent to the utility of the general population used in the model, which is around 60% higher than the utility associated with naftidrofuryl oxalate.
-
-
Patients who have no vasoactive drugs and have angioplasty will have the utility described above for 1 year. The utility will then decrease to that associated with placebo.
-
Patients who have angioplasty after discontinuation of the vasoactive drugs will have the utility described above until the end of the first year; it will then decrease to the level of utility associated with placebo.
-
The baseline utility for these patients in practice will be lower, as they have a worse prognosis by definition within this subgroup analysis. However, the impact of baseline utility is tested within SA3 and hence is not altered here.
Sensitivity analysis 8: informative prior distributions within network meta-analysis
The impact of more informative prior distributions for the between-study SD and treatment effects within the network meta-analysis upon the results of the meta-analysis was tested and reported (see Chapter 3, Maximal walking distance meta-analysis). The results of the network meta-analysis when an informative prior distribution from SA5 was used [τ ∼ U(0, 0.2), μ ∼ N(100)] and tested within the model.
Results
All results presented within this section are discounted.
Cost–utility analysis – base case
The total costs, the total QALYs and the ICERs associated with the base case are presented in Table 61.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 4.975 | – | |
| Pentoxifylline | 493 | 4.984 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 964 | 4.994 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 298 | 5.024 | 6070 |
The base-case results suggest that naftidrofuryl oxalate has the lowest additional costs (£298) compared with no vasoactive drug, and cilostazol has the highest additional costs (£964), whereas the additional cost of pentoxifylline is £493. In terms of total QALYs, naftidrofuryl oxalate is estimated to increase QALYs by 0.049 (from 4.975 to 5.024) compared with no vasoactive drug for PAD. Pentoxifylline is estimated to have the smallest QALY gains (0.009) compared with no vasoactive drug. Cilostazol increases QALYs by 0.019 compared with no vasoactive drug. Overall, the results show that both pentoxifylline and cilostazol are dominated by naftidrofuryl oxalate, which has both higher total QALYs and lower additional costs. The ICER associated with naftidrofuryl oxalate compared with no vasoactive drug is estimated to be £6070 in the base-case scenario, based upon the discounted expected values.
The CEAC is presented in Figure 9, which shows the probability of each vasoactive drug and the comparator being optimal given a range of willingness-to-pay thresholds (thresholds from £0 to £100,000 were tested). The probability of cilostazol or pentoxifylline being most cost-effective at any willingness-to-pay threshold is < 1%. Naftidrofuryl oxalate has the highest probability of being most cost-effective above willingness-to-pay thresholds of around £6000 per QALY gained.
FIGURE 9.
Cost-effectiveness acceptability curve for the base-case model results.
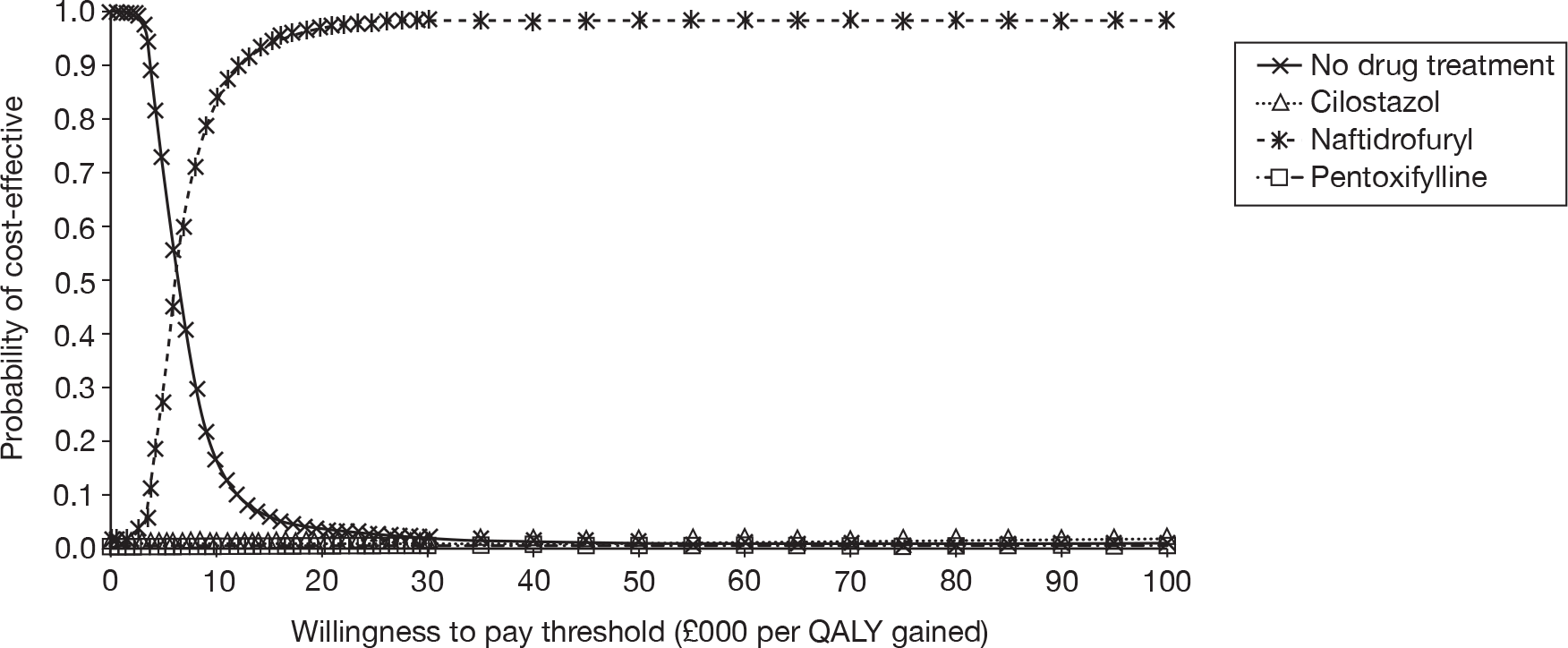
To further demonstrate the cost-effectiveness of each drug compared with no vasoactive drug and the uncertainties around the cost-effectiveness results, the cost-effectiveness plane is presented in Figure 10, which shows the incremental effectiveness and incremental costs of each of the drugs versus no vasoactive drug for PAD. The willingness-to-pay thresholds of £10,000, £20,000 and £30,000 per QALY gained compared with no drug treatment are also shown on the plane. The figure shows why naftidrofuryl oxalate dominates both cilostazol and pentoxifylline, as the cluster representing naftidrofuryl oxalate is associated with higher incremental effectiveness and lower incremental costs. The figure also shows that naftidrofuryl oxalate has a cost per QALY gained below £10,000 compared with no vasoactive drug, as more points lie below the threshold line. However, naftidrofuryl oxalate is associated with the greatest uncertainty in terms of incremental effectiveness (from around –0.05 to around 0.2) and cilostazol is the least uncertain regarding incremental effectiveness. Therefore, based upon current evidence, it is possible for cilostazol to be more effective than naftidrofuryl oxalate; however, cilostazol is unlikely to have an incremental cost per QALY gained below £20,000 compared with no vasoactive drug due to the higher costs associated with this drug.
FIGURE 10.
Cost-effectiveness plane showing incremental effectiveness and costs of the vasoactive drugs vs no vasoactive drug (base case).
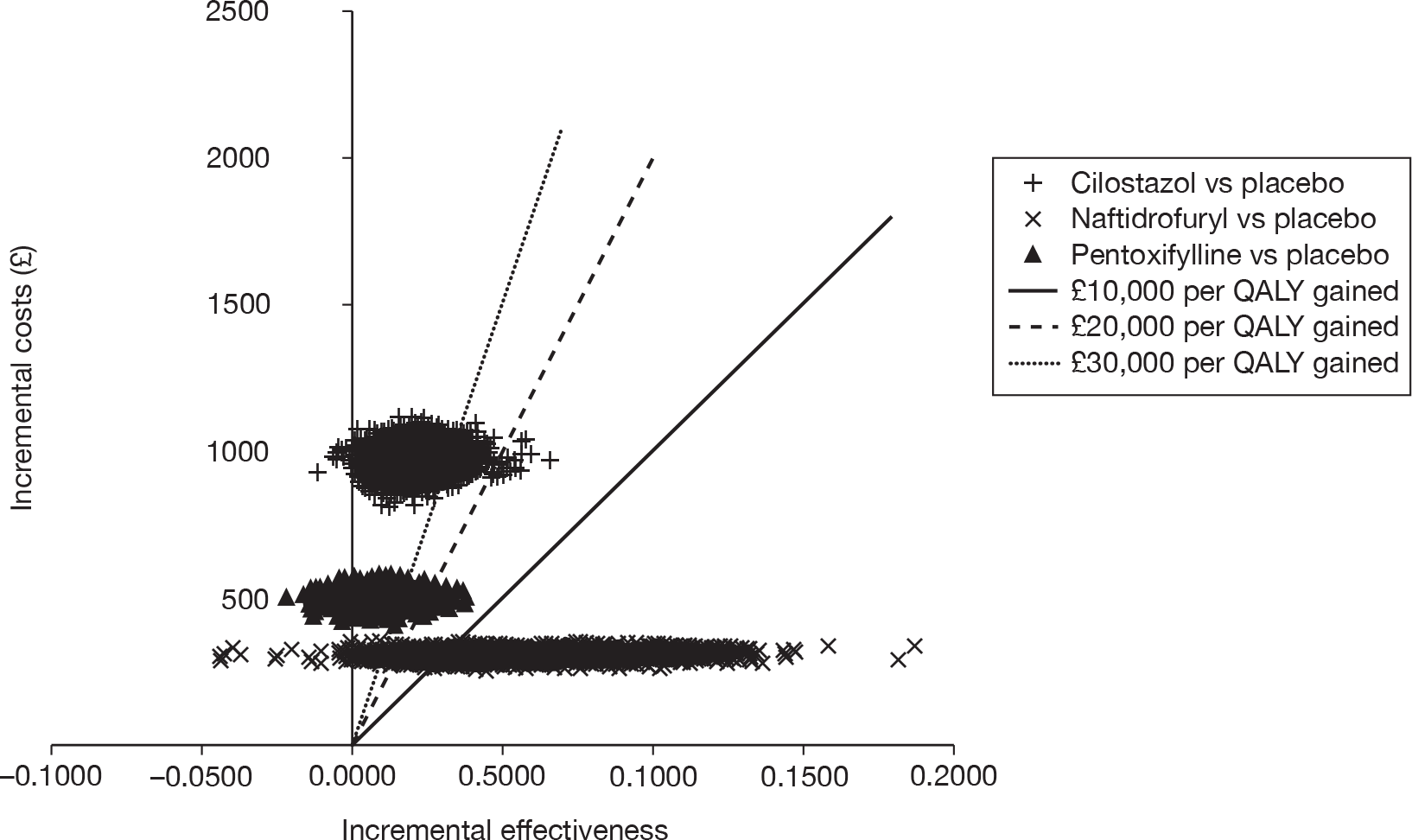
The figure also shows that all three drugs have a small probability of being more costly and less effective than no vasoactive drug (i.e. points located in the northwest quadrant), of which cilostazol has the smallest probability, of 0.11%, compared with 0.18% for naftidrofuryl oxalate and 4.05% for pentoxifylline.
Results of univariate sensitivity analyses
Sensitivity analysis 1: utility remains the same as when on the drug if discontinuation occurs after 24 weeks
The SA assumes the effectiveness of the vasoactive drug continues when patients discontinue the drug after 24 weeks. The incremental cost-effectiveness results of the SA are presented in Table 62. The results show that the effectiveness of all drugs increase significantly compared with no vasoactive drug. For example, the total QALYs of naftidrofuryl oxalate increase from 5.024 in the base case to 5.174. The base-case cost-effectiveness conclusions are not changed. Both pentoxifylline and cilostazol are dominated by naftidrofuryl oxalate. The ICER of naftidrofuryl oxalate compared with no vasoactive drug decreases from £6070 in the base case to £1538, which is more favourable to the intervention.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 4.980 | – | |
| Pentoxifylline | 493 | 5.013 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 963 | 5.053 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 298 | 5.174 | 1538 |
Sensitivity analysis 2: alternative baseline utility
This SA applies an increased baseline utility of 0.7562 compared with 0.4838. The incremental cost-effectiveness results of the SA are presented in Table 63. The base-case cost-effectiveness conclusions and the ICER of naftidrofuryl oxalate compared with no vasoactive drug are similar to the base-case results, which demonstrates that the model is not sensitive to different baseline utilities.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 7.764 | – | |
| Pentoxifylline | 493 | 7.773 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 963 | 7.783 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 298 | 7.813 | 6053 |
Sensitivity analysis 3: alternative cost for naftidrofuryl oxalate
This SA applies a higher cost for naftidrofuryl oxalate, which is £4.13 per week (using the cost of the brand name Praxilene), compared with £2.26 per week (generic) in the base case. The incremental cost-effectiveness results of the SA are presented in Table 64. The results show that cilostazol is dominated by naftidrofuryl oxalate, which has lower costs and higher total QALYs. Pentoxifylline is extendedly dominated by naftidrofuryl oxalate because the ICER associated with pentoxifylline is higher than that associated with naftidrofuryl oxalate. This is due to the drug acquisition cost of naftidrofuryl oxalate becoming substantially higher than that of pentoxifylline. The ICER of naftidrofuryl oxalate compared with no vasoactive drug is £11,058 per QALY gained, which is higher than the base case of £6070, because of the cost increase of naftidrofuryl oxalate.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 4.980 | – | |
| Pentoxifylline | 493 | 4.988 | Extendedly dominated by naftidrofuryl oxalate | |
| Cilostazol | 963 | 4.999 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 545 | 5.029 | 11,058 |
Sensitivity analysis 4: shorter time horizon
This SA considers a time horizon of 24 weeks. The incremental cost-effectiveness results of the SA are presented in Table 65. The base-case cost-effectiveness conclusions are not changed. The ICER of naftidrofuryl oxalate compared with no vasoactive drug increases from £6070 in the base case to £10,733.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 0.220 | – | |
| Pentoxifylline | 92 | 0.221 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 178 | 0.222 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 51 | 0.225 | 10,733 |
Sensitivity analysis 5: alternative starting age
This SA assumes that patients begin treatment with these drugs at age 55 years. The incremental cost-effectiveness results of the SA are presented in Table 66. The base-case cost-effectiveness conclusions and the ICER of naftidrofuryl oxalate compared with no vasoactive drug are similar to the base-case results, which demonstrates that the model is not sensitive to the starting age of patients.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 6.878 | – | |
| Pentoxifylline | 493 | 6.886 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 963 | 6.897 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 298 | 6.927 | 6033 |
Sensitivity analysis 6: alternative long-term discontinuation rates
This SA assumes that the long-term discontinuation rates of pentoxifylline and naftidrofuryl oxalate maintain the same relative ratios compared with cilostazol within the 24 weeks. The incremental cost-effectiveness results of the SA are presented in Table 67. The results show that cilostazol is dominated by naftidrofuryl oxalate, which has lower costs and higher total QALYs. Pentoxifylline is extendedly dominated by naftidrofuryl oxalate because the drug acquisition cost of naftidrofuryl oxalate becomes substantially higher than that of pentoxifylline, owing to the lower long-term discontinuation rate of naftidrofuryl oxalate compared with the base case. The ICER of naftidrofuryl oxalate compared with no vasoactive drug is £5899 per QALY gained, which is similar to the base case.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 4.980 | – | |
| Pentoxifylline | 473 | 4.988 | Extendedly dominated by naftidrofuryl oxalate | |
| Cilostazol | 963 | 4.999 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 646 | 5.089 | 5899 |
Sensitivity analysis 7: angioplasty procedure for patients discontinuing within 24 weeks
This subgroup analysis assumes that patients who have more severe IC and discontinue with the drugs within 24 weeks will receive an angioplasty procedure that will improve HRQoL of these patients on average. These patients who receive no vasoactive drug for PAD are assumed to have an angioplasty procedure at the start of the model and experience the improved HRQoL immediately. Owing to lack of comparative evidence around the utility associated with angioplasty, four scenarios were tested: the utility associated with angioplasty is equivalent to the utility associated with naftidrofuryl oxalate, 20% and 40% higher than the utility associated with naftidrofuryl oxalate, and equivalent to the utility of general population used in the model.
The incremental cost-effectiveness results of the SA are presented in Tables 68–71. Unlike the base case and other sensitivity analyses, in which the cost of no vasoactive drug is zero, the comparator of no vasoactive drug is associated with a significant cost which is £1313, which represents the costs of angioplasty.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) | Dominance |
|---|---|---|---|---|
| Pentoxifylline | 862 | 4.993 | Dominated by naftidrofuryl oxalate | |
| No vasoactive drug (baseline technology, all patients receive angioplasty) | 1313 | 4.996 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 1315 | 5.003 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 431 | 5.032 | – |
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| Pentoxifylline | 862 | 5.019 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 1315 | 5.028 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 431 | 5.044 | – | |
| No vasoactive drug (baseline technology, all patients receive angioplasty) | 1313 | 5.093 | 17,992 |
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) | Dominance |
|---|---|---|---|---|
| Pentoxifylline | 862 | 5.044 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 1315 | 5.052 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 431 | 5.056 | – | |
| No vasoactive drug (baseline technology, all patients receive angioplasty) | 1313 | 5.191 | 6545 |
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) | Dominance |
|---|---|---|---|---|
| Pentoxifylline | 862 | 5.066 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 431 | 5.067 | – | |
| Cilostazol | 1315 | 5.074 | Dominated by no vasoactive drug | |
| No vasoactive drug (baseline technology, all patients receive angioplasty) | 1313 | 5.282 | 4094 |
When it is assumed that the utility associated with angioplasty is equivalent to the utility associated with naftidrofuryl oxalate, naftidrofuryl oxalate dominates pentoxifylline, cilostazol and no vasoactive drug. When it is assumed that the utility associated with angioplasty is 20% and 40% higher than the utility associated with naftidrofuryl oxalate, no vasoactive drug (all patients receive angioplasty) is associated with the highest total QALYs. In both scenarios, pentoxifylline and cilostazol are dominated by naftidrofuryl oxalate and the ICERs of no vasoactive drug (all patients receive angioplasty) compared with naftidrofuryl oxalate are £17,992 and £6545 per QALY gained, respectively.
When it is assumed that the utility associated with angioplasty is equivalent to the utility of the general population within the model (around 60% higher than the utility associated with naftidrofuryl oxalate), no vasoactive drug (all patients receive angioplasty) is associated with the highest total QALYs. Naftidrofuryl oxalate is associated with fewer total QALYs than cilostazol because more patients discontinue with cilostazol and could therefore benefit from the angioplasty procedure. Pentoxifylline is dominated by naftidrofuryl oxalate and cilostazol is dominated by no vasoactive drug (all patients receive angioplasty). The ICER of no vasoactive drug (all patients receive angioplasty) compared with naftidrofuryl oxalate is £4094 per QALY gained.
Therefore, this exploratory analysis suggests that if angioplasty is associated with an increase in quality of life compared with the vasoactive drugs, vasoactive drugs are unlikely to be considered to be economically attractive at a willingness-to-pay threshold of £20,000 per QALY gained for this small subgroup of patients. However, this subgroup analysis is largely based upon clinical advice owing to lack of evidence. This is therefore an exploratory and highly uncertain analysis, and hence these results should be treated with caution.
Sensitivity analysis 8: informative prior distributions within network meta-analysis
This SA assesses the impact of allowing more informative prior distributions for the between-study SD and treatment effects within the network meta-analysis. The incremental cost-effectiveness results of the SA are presented in Table 72. The results suggest that changes to the prior distributions for the between-study SD and treatment effects have very little impact upon the model results.
| Interventions and comparator | Total costs (additional to no vasoactive drug treatment) (£) | Total QALYs | ICER (cost per QALY gained) (£) | Dominance |
|---|---|---|---|---|
| No vasoactive drug (baseline technology) | 0 | 4.978 | – | |
| Pentoxifylline | 493 | 4.986 | Dominated by naftidrofuryl oxalate | |
| Cilostazol | 964 | 4.996 | Dominated by naftidrofuryl oxalate | |
| Naftidrofuryl oxalate | 298 | 5.027 | 6072 |
Threshold analyses
Given the uncertainties around the quality-of-life evidence and the uncertain long-term outcomes, threshold analyses were carried out to determine the required QALYs gained for each drug for it to be associated with a cost per QALY gained below £20,000 and £30,000 compared with no vasoactive drug. The additional discounted costs for each drug compared with no vasoactive drug over the lifetime of the patients were based on the base-case PSA results of the economic model. The costs associated with the vasoactive drugs for PAD are associated with much less uncertainty than the QALYs, with the biggest uncertainty relating to the costs being the long-term discontinuation rates. Table 73 summarises the results of this analysis.
| Interventions and comparator | Additional costs compared with no vasoactive drug (95% CI) (£) | Required QALYs gained for threshold of: | |
|---|---|---|---|
| £20,000 (95% CI) | £30,000 (95% CI) | ||
| No vasoactive drug (baseline technology) | 0 | ||
| Cilostazol | 964 (892 to 1040) | 0.048 (0.045 to 0.052) | 0.032 (0.030 to 0.035) |
| Naftidrofuryl oxalate | 298 (273 to 325) | 0.015 (0.014 to 0.016) | 0.010 (0.009 to 0.011) |
| Pentoxifylline | 493 (454 to 535) | 0.025 (0.023 to 0.027) | 0.016 (0.015 to 0.018) |
| Inositol nicotinate | 1695 (1242 to 2200) | 0.085 (0.062 to 0.110) | 0.056 (0.041 to 0.073) |
The threshold analysis suggests that for a willingness-to-pay threshold of £20,000 and £30,000 per QALY gained, naftidrofuryl oxalate requires a QALY gain of 0.015 and 0.010, respectively, as this is the cheapest vasoactive drug. Pentoxifylline requires QALY gains of 0.025 and 0.016, respectively, to make the drug cost-effective at willingness-to-pay thresholds of £20,000 and £30,000. The QALYs gained required for cilostazol to be cost-effective at willingness-to-pay thresholds of £20,000 and £30,000 are 0.048 and 0.0322, respectively. Inositol nicotinate requires the biggest QALYs gained for it to be considered to be cost-effective. For willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, the required QALYs gained are 0.085 and 0.056, respectively.
Discussion
Summary of key results
The economic evaluation suggests that naftidrofuryl oxalate dominates cilostazol and pentoxifylline and has an incremental cost per QALY gained of around £6070 compared with no vasoactive drug. This result is reasonably robust to changes within the key model assumptions; however, the method for estimating utilities based upon MWD and long-term discontinuation rates is uncertain. A threshold analysis was undertaken to assess the QALY gains required for naftidrofuryl oxalate to have an incremental cost per QALY gained of £20,000 compared with no vasoactive drug, which suggested that an estimated 0.015 QALYs gained would be required.
Sensitivity analyses suggest that the base-case ICER of naftidrofuryl oxalate compared with no vasoactive drug does not change substantially with alternative baseline utility (SA2), alternative starting age (SA5), alternative long-term discontinuation rates (SA6) and informative prior distributions within the network meta-analysis (SA8). When it is assumed that the effectiveness associated with the vasoactive drugs continues over a patient’s lifetime when patients discontinue the drug after 24 weeks (SA1), the ICER of naftidrofuryl oxalate compared with no vasoactive drug decreases from £6070 in the base case to £1538 per QALY gained. When the patented manufacturer’s cost for naftidrofuryl oxalate is used (SA3) and when a shorter time horizon of 24 weeks is used (SA4), the ICER of naftidrofuryl oxalate compared with no vasoactive drug increases to £11,058 and £10,733 per QALY gained, respectively. In all of these SAs, both cilostazol and pentoxifylline are dominated or extendedly dominated (for pentoxifylline in SA3 and SA6) by naftidrofuryl oxalate.
Exploratory subgroup analyses which assume that patients who discontinue with the drugs within 24 weeks will receive angioplasty (SA7) suggest that the effectiveness of the drugs depends on the assumed utility associated with angioplasty. When it is assumed that the utility associated with angioplasty is equivalent to the utility associated with naftidrofuryl oxalate, naftidrofuryl oxalate dominates pentoxifylline, no vasoactive drug and cilostazol. However, when it is assumed that the utility is higher than the utility associated with naftidrofuryl oxalate, no vasoactive drug is associated with the highest total QALYs, and the ICERs of no vasoactive drug compared with naftidrofuryl oxalate are < £20,000 per QALY gained. Cilostazol and pentoxifylline are dominated either by naftidrofuryl oxalate or by no vasoactive drug. However, this subgroup analysis is highly uncertain and hence these results should be treated with caution.
Given the current evidence around effectiveness, naftidrofuryl oxalate is estimated to dominate both cilostazol and pentoxifylline in the base case and in most sensitivity analyses. It was not possible to estimate the QALY gains associated with inositol nicotinate owing to lack of data around MWD at 24 weeks. Inositol nicotinate was therefore not included within the main analysis. However, the threshold analysis suggests that inositol nicotinate would have to demonstrate considerably greater impacts upon quality of life than the other vasoactive drugs being assessed for it to have an estimated incremental cost per QALY gained of < £20,000 compared with no vasoactive drug, because of its more expensive acquisition cost. An estimated QALY gain of 0.085 would be required using a willingness-to-pay threshold of £20,000 per QALY gained, compared with a 0.015 QALY gain required for naftidrofuryl oxalate. Therefore, it is unlikely that inositol nicotinate would be considered to be economically attractive compared with no vasoactive drug or with the other drugs being assessed, given the available effectiveness evidence.
Generalisability of results
There is no evidence to suggest that the results of the analysis cannot be generalised across all patients who have stable (at least for the past 3 months) and symptomatic IC, secondary to PAD, whose symptoms continue despite a period of conservative management. There may, however, be a subgroup of patients with more severe IC in whom treatment with these drugs may prevent the need for angioplasty. In this subgroup of patients, no vasoactive drug (all patients receive angioplasty) may have a favourable ICER compared with any of the vasoactive drugs; however, this analysis is highly uncertain owing to lack of evidence and hence further research is required around the effectiveness of angioplasty in these patients.
Strengths and limitations of analysis
The economic evaluation has several strengths compared with previous studies. To our knowledge, it is the first study to model the lifetime of the patients who take the drugs and it is also the first study to incorporate utility in the economic evaluation by predicting the change in utility from the change in MWD, based on patient-level data from a RCT.
There are several limitations of the study. There is uncertainty regarding the change in utility and discontinuation rate beyond 24 weeks because most RCTs do not have follow-up beyond this time point. In the base case, it was assumed that utility remains at the same level after 24 weeks if patients continue the drug or that it decreases to the level of no vasoactive drug if patients discontinue the drug. This was tested within a SA that did not alter the conclusions. Any additional effectiveness of naftidrofuryl oxalate beyond discontinuation would improve cost-effectiveness. It was also assumed that discontinuation rates of other drugs are the same as cilostazol beyond 24 weeks. There is evidence that once patients discontinue the drug, the MWD decreases to that of no vasoactive drug for PAD. A SA was carried out to test alternative long-term discontinuation rates, which did not alter the conclusions.
The regression model fitted to predict the change in utility from the change in MWD was based on patient-level data from a RCT of cilostazol with a sample size of 106 patients in the UK. 83 The underlying assumption of this analysis is that the same relationship applies for all drugs and no vasoactive drug between MWD and utilities. An analysis was undertaken using the patient-level data which suggested that there was no significant treatment effect for cilostazol versus placebo. However, this was based upon a relatively small sample of patients, and there may be some difference between treatment groups. Cilostazol is generally associated with more minor AEs; hence these may affect this relationship. Direct long-term utility data associated with each of the drugs would provide less uncertain estimates of cost-effectiveness. A value of information analysis has not been undertaken due to the uncertainties associated with the utility outcomes, which were not possible to fully quantitate within the PSA.
Cardiovascular AEs are common for the patient population considered in the study. The model assumes that the drugs are for symptom relief and have no impact on the progression of disease or serious cardiovascular events. The long-term safety of cilostazol was tested in a good-quality trial,49 which suggests that there is very little difference between cardiovascular outcomes for cilostazol and placebo (for the ‘on-treatment’ group there was no difference in the number of cardiovascular mortalities and similar numbers of cardiovascular AEs). Personal communication with the team of clinical advisors (Steven Thomas, Jonathan Michaels and Gerard Stansby, University of Sheffield, August 2010, personal communication) suggests that there is no clinical reason why these vasoactive drugs for PAD would impact upon the number of cardiovascular events and hence this small difference in cardiovascular events is thought to be due to random variation. There are, however, no long-term safety studies on naftidrofuryl oxalate, pentoxifylline or inositol nicotinate, and if there was a small increase or reduction in the incidence of cardiovascular events when patients are on these drugs, the results could alter substantially because of the otherwise small impact on costs and quality of life associated with these drugs.
The economic evaluation identified within the literature review by Guest et al. 90 included costs of diagnosis of IC, follow-up visits, supervised exercise, angioplasty and bypass surgery in addition to the drug acquisition costs. Within our model the cost of diagnosis and follow-up visits was assumed to be unchanged by the vasoactive drugs, as all patients will be diagnosed and patients will be followed up for other treatment they are receiving for PAD whether or not they are receiving vasoactive drugs. The team of clinical advisors (Steven Thomas, Jonathan Michaels and Gerard Stansby, University of Sheffield, July 2010, personal communication) suggested that in practice supervised exercise programmes are currently unavailable in many regions of England and Wales and that the use of vasoactive drugs is unlikely to affect whether or not a patient requires bypass surgery. Vasoactive drugs may prevent the need for angioplasty in a small subgroup of patients who have more severe IC when clinical practice is to provide angioplasty following discontinuation of vasoactive drugs. Owing to the limited evidence base around the long-term comparative effectiveness of angioplasty in this patient population, this was treated as an exploratory subgroup analysis within this report, the results of which are described above.
The economic evaluation performed by Guest et al. 90 suggested that cilostazol is more cost-effective for improving MWD at 24 weeks than naftidrofuryl oxalate or pentoxifylline. The conclusion is different from this evaluation, which suggests that naftidrofuryl oxalate is most cost-effective compared with the other vasoactive drugs and no vasoactive drug. The main reason for the difference is the estimates of the changes in MWD for the vasoactive drugs. Guest et al. 90 estimated the mean percentage changes in MWD from baseline to be 82.6% and 59.9% for cilostazol and naftidrofuryl oxalate, respectively. The estimates were not based on a network meta-analysis, but were calculated independently for each drug as a weighted mean based on the sample size of the identified trials. Two trials of cilostazol56,61 and three trials of naftidrofuryl oxalate65,93,94 were included. The two included trials of cilostazol were the two trials demonstrating the greatest effectiveness of cilostazol at that time (see Figure 3), whereas two of the three trials of naftidrofuryl oxalate included within the analysis assess a dose that is not currently licensed within England and Wales. In comparison, this evaluation applied a random effects network meta-analysis, considering all trials identified by the systematic review, providing that they reported relevant outcomes and had a follow-up period of at least 24 weeks (see Chapter 3, Maximal walking distance meta-analysis, above). The estimated percentage changes from this analysis in terms of MWD from baseline for cilostazol and naftidrofuryl oxalate were 59.2% and 106.7%, respectively.
Chapter 5 Assessment of factors relevant to the NHS and other parties
The vasoactive drugs assessed within this report are generally currently available to be prescribed to patients with IC within England and Wales for symptom relief, although there may be restrictions to their use due to local policies. The only evidence available around current usage of the vasoactive drugs for PAD within England and Wales is the Prescription costs analysis England 2009. 25 Based upon this, assuming that all patients receive the licensed doses of the vasoactive drugs as outlined within this report for the whole year, 11,540 patients are currently estimated to be prescribed these vasoactive drugs for PAD within the community within England. The calculated proportional split of the usage of these vasoactive drugs based upon these data is shown in Table 74. However, it should be noted that these estimates are highly uncertain.
| Drug | Proportionate market share (from community prescriptions) (%) |
|---|---|
| Cilostazol | 29 |
| Naftidrofuryl oxalate | 52 |
| Pentoxifylline | 4 |
| Inositol nicotinate | 15 |
The costs associated with providing the vasoactive drugs for PAD are the acquisition costs of the drugs only; there are not expected to be any additional management costs due to the health-care requirements already incurred by this patient group. The estimated annual cost for each of the vasoactive drugs for PAD provided to this patient population is shown in Table 75. This is calculated using the graph of prevalence by age from the study by Norgren et al. 3 and England and Wales population statistics by age from the ONS. 98 This results in an estimated 703,403 prevalent cases of IC within England and Wales. Of these, it is assumed that 70% will seek medical help, based upon the mid-point of the range provided by Norgren et al. ,3 and that, of these, 20% would require the vasoactive drugs after a period of conservative management. This results in an estimated 98,476 people within England and Wales requiring treatment with a vasoactive drug.
| Drug | Annual cost (£) |
|---|---|
| Cilostazol | 45,340,641 |
| Naftidrofuryl oxalate (generic) | 11,604,739 |
| Naftidrofuryl oxalate (Praxilene) | 21,206,891 |
| Pentoxifylline | 23,568,918 |
| Inositol nicotinate | 88,473,301 |
As some patients are already receiving these vasoactive drugs for PAD, the additional cost to the NHS of recommending one or more of these drugs is likely to be lower than predicted here. As an approximation, based upon the estimated current number of prescriptions dispensed within the community in England and the estimated proportionate market share of the vasoactive drugs for PAD shown in Table 74, the current cost of treatment in England is estimated to be just over £1.5M, although this estimate is highly uncertain.
Chapter 6 Discussion
Statement of principal findings
Clinical effectiveness data were available from 26 RCTs. There was some evidence that walking distance outcomes were improved by cilostazol and naftidrofuryl oxalate. The 95% credible intervals estimated from the network meta-analysis for the difference from placebo in the log mean change MWD from baseline were –0.016 to 0.217 for pentoxifylline, 0.108 to 0.337 for cilostazol and 0.181 to 0.762 for naftidrofuryl oxalate. Based upon this analysis, the percentage change in MWD from baseline to 24 weeks for placebo, pentoxifylline, cilostazol and naftidrofuryl oxalate can be estimated as 27.6%, 41.4%, 59.2% and 106.7%, respectively. It was not possible to include inositol nicotinate within the meta-analysis of MWD and PFWD owing to the lack of 24-week data; however, the shorter-term data did not suggest a significant effect. AEs were minor, and included headaches and gastrointestinal difficulties. The incidence of SAEs, including cardiovascular events and mortality, was not shown to be increased or decreased by the vasoactive drugs compared with placebo; however, most studies had relatively short follow-up time to address this outcome.
The economic evaluation suggests that it is unlikely that cilostazol, pentoxifylline or inositol nicotinate would have an incremental cost per QALY gained below £30,000 compared with no vasoactive drug. Naftidrofuryl oxalate is associated with an estimated incremental cost per QALY gained of around £6000 compared with no vasoactive drug. There are, however, uncertainties around the long-term effectiveness of the drugs. Naftidrofuryl oxalate would need to be associated with an estimated 0.0271 QALYs gained in order to have an estimated incremental cost per QALY gained of £20,000 compared with no vasoactive drug.
Strengths and limitations of the assessment
The main strengths of the review are that the literature search was comprehensive and that the included studies were of relevance to UK practice in terms of populations. In addition, all included trials prescribed medications in line with UK marketing authorisations. However, most of the trial data had follow-up of 24 weeks, which is relatively short-term compared with practice. Relevant trials that were not published in English may have been missed; however, methodology studies have indicated that language restrictions do not often influence the results of systematic reviews of conventional medicines. 103–105
Within the meta-analysis of MWD and PFWD, several studies were excluded because the published reports did not provide data in a form that was suitable for inclusion. In the analysis, we assumed that the data from the studies were missing at random and that the lack of usable data was not related to the observed treatment effect. The existing evidence on naftidrofuryl oxalate which was excluded from the analysis does not suggest that publication bias is a problem. Furthermore, a review of existing trial databases was undertaken by De Backer et al. ,106 which suggests that there is no evidence of any publication bias. There are no head-to-head data comparing naftidrofuryl oxalate with any other vasoactive drug; the results of the analysis depended upon a network meta-analysis.
Within the health economic model, there is uncertainty regarding the utility estimates and discontinuation rate beyond 24 weeks because most RCTs do not have follow-up beyond this time point. The analysis takes the conservative assumption that there is no benefit of the vasoactive drugs following discontinuation. Therefore, any additional effectiveness of naftidrofuryl oxalate beyond discontinuation would improve cost-effectiveness of this drug. A SA was undertaken to test alternative long-term discontinuation rates, which did not alter the conclusions.
The regression model fitted to predict the change of utility from the change in MWD within the health economic model was based on patient-level data from a RCT of cilostazol with a sample size of 106 patients in the UK. 83 The underlying assumption of this analysis is that there is the same relationship for all drugs and no vasoactive drug between MWD and utilities. An analysis was undertaken using the patient-level data, which suggested that there was no significant treatment effect for cilostazol versus placebo. However, this was based upon a relatively small sample of patients, and there may be some difference between treatment groups. Cilostazol is generally associated with more minor AEs; hence, these may affect this relationship. Direct long-term utility data associated with each of the drugs would provide less uncertain estimates of cost-effectiveness. A threshold analysis was undertaken to address this issue. In addition, there was insufficient evidence around inositol nicotinate to assess this within the base-case analysis, hence, this was assessed only within a threshold analysis. A value of information analysis has not been undertaken owing to the uncertainties associated with the long-term outcomes, which were not possible to fully quantitate within the PSA.
Cardiovascular AEs are common among the patient population considered in the study. The model assumes that the drugs are prescribed for symptom relief and have no impact on the progression of disease or serious cardiovascular events. The long-term safety of cilostazol was tested in a good-quality trial49 which found that there is very little difference in cardiovascular outcomes between cilostazol and placebo (among the ‘on-treatment’ group there was no difference in the number of cardiovascular mortalities and similar numbers of cardiovascular AEs). Personal communication with the team of clinical advisors (Steven Thomas, Jonathan Michaels and Gerard Stansby, August 2010) suggests that there is no clinical reason why these vasoactive drugs for PAD would impact upon the number of cardiovascular events and hence this small difference in cardiovascular events is thought to be due to random variation. There are, however, no long-term safety studies on naftidrofuryl oxalate, pentoxifylline or inositol nicotinate, and if there was a small increase or reduction in the incidence of cardiovascular events when patients are on these drugs, the results could alter substantially due to the otherwise small impact on costs and quality of life associated with these drugs.
Uncertainties
The key uncertainties associated with this evaluation are:
-
long-term quality-of-life impacts of the drugs
-
long-term discontinuation rates
-
the number of people using the drugs
-
any long-term AEs or benefits associated with naftidrofuryl oxalate, pentoxifylline and inositol nicotinate.
Other relevant factors
Naftidrofuryl oxalate could potentially be prescribed to more patients than cilostazol, as CHF is not contraindicated for naftidrofuryl oxalate and it has fewer drug interactions.
Chapter 7 Conclusions
Naftidrofuryl oxalate and cilostazol are both effective treatments for this patient population, with minimal SAEs; however, naftidrofuryl oxalate is the only treatment with an incremental cost per QALY gained below £20,000 compared with no vasoactive drug, with an estimated incremental cost per QALY gained of £6070.
Implications for service provision
Provision of these drugs does not usually engender significant additional management costs, as these drugs would be provided alongside a range of other treatments for PAD and its risk factors and there is no evidence that they impact upon disease progression. Therefore, the burden upon the NHS is generally in terms of the drug acquisition cost only. Within England and Wales the vasoactive drugs assessed within this report are available to be prescribed to patients with IC, although there may be restrictions to their use due to local policies. Therefore, if these drugs were to be recommended, prescription rates of the drugs may rise considerably.
Suggested research priorities
A trial comparing the long-term effectiveness (beyond 24 weeks) of cilostazol, naftidrofuryl oxalate and placebo would be beneficial, which should collect utility data as well as walking distance outcomes. The health economic model currently assumes that the effectiveness of the vasoactive drugs is maintained while the patients are taking the drugs; however, this should be tested within a trial. It would also be useful to compare the outcomes associated with naftidrofuryl oxalate with those associated with supervised exercise programmes and other treatments, such as angioplasty. Importantly, there are currently no long-term safety trials for naftidrofuryl oxalate; however, clinical experts suggest that the mechanism of the drugs is such that no long-term impacts on cardiovascular events or mortality would be expected. Any such trials are likely to be costly due to the sample size and length of follow-up required to detect any differences between the two arms for these events.
Acknowledgements
The authors would like to thank Dr Mark O’Donnell for providing patient-level data from a trial of cilostazol. The authors would also like to thank Andrea Shippam for her help in preparing and formatting the report, and Dr Matt Stevenson and Dr Eva Kaltenthaler for peer reviewing the report.
Contributions of authors
Hazel Squires was the Assessment Group lead, who advised on the cost-effectiveness modelling and developed the budget impact model. Emma Simpson and Sue Harnan undertook the clinical effectiveness review. Yang Meng undertook the cost-effectiveness review and developed the cost-effectiveness model. John Stevens undertook the quantitative meta-analysis and Ruth Wong performed the literature searches. Steve Thomas, Jonathan Michaels and Gerard Stansby provided clinical advice throughout. All authors contributed to the report.
About the School of Health and Related Research
The School of Health and Related Research (ScHARR) is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of the public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR Health Technology Assessment programme on behalf of a range of policy-makers, including NICE. ScHARR-TAG is part of a wider collaboration of six units from other regions. The other units are Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsular Technology Assessment Group (PenTAG), University of Exeter; NHS CRD, University of York; and West Midlands Health Technology Assessment Collaboration (WMHTAC), University of Birmingham.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helvetica Chirurgica Acta 1954;21:499-533.
- Scottish Intercollegiate Guidelines Network (SIGN) . Diagnosis and Management of Peripheral Arterial Disease. A National Clinical Guideline 2006.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007;33:1-75.
- Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2008;4.
- Hiatt WR. The US experience with cilostazol in treating intermittent claudication. Atherosclerosis 2005;6:21-3.
- Dormandy J, Heeck L, Vig S. Lower-extremity arteriosclerosis as a reflection of a systemic process: implications for concomitant coronary and carotid disease. Semin Vasc Surg 1999;12:118-22.
- Hooi JD, Stoffers HE, Knottnerus JA, van Ree JW. The prognosis of non-critical limb ischaemia: a systematic review of population-based evidence. Br J Gen Pract 1999;49:49-55.
- Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edn). Chest 2008;133:S815-43.
- Peripheral Arterial Diseases Antiplatelet Consensus Group . Antiplatelet therapy in peripheral arterial disease. Consensus statement. Eur J Vasc Endovasc Surg 2003;26:1-16.
- Fowkes FGR, Housley E, Cawood EHH, MacIntyre CCA, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384-92.
- Pell JP. Impact of intermittent claudication on quality of life. The Scottish Vascular Audit Group. Eur J Vasc Endovasc Surg 1995;9:469-72.
- Breek JC, Hamming JF, De Vries J, Aquarius AE, Van Berge Henegouwen DP, Breek JC, et al. Quality of life in patients with intermittent claudication using the World Health Organization (WHO) questionnaire. Eur J Vasc Endovasc Surg 2001;21:118-22.
- NHS Primary Care Commissioning . Primary Care Service Framework: Peripheral Arterial Disease 2009. www.pcc.nhs.uk/primary-care-service-frameworks (accessed February 2010).
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517-38.
- Dobesh PP, Stacy ZA, Persson EL. Pharmacologic therapy for intermittent claudication. Pharmacotherapy 2009;29:526-53.
- Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol 1992;45:1101-9.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Int Angiol 2007;26:82-157.
- EMA . The European Agency for Evaluation of Medicinal Products. Note for Guidance on Clinical Investigations of Medicinal Products in the Treatment of Chronic Peripheral Arterial Occlusive Disease 2002.
- Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2006;2.
- Lower Limb Peripheral Arterial Disease. Guidelines in Progress 2010. http://guidance.nice.org.uk/CG/Wave23/5.
- Greenhalgh J, Bagust A, Boland A, Martin Saborido C, Oyee J, Blundell M, et al. Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events (review of Technology Appraisal No. 90): a systematic review and economic analysis. Health Technol Assess 2011;15.
- Abbot Healthcare Products Ltd . Summary of Product Characteristics 2010.
- National Institute for Health and Clinical Excellence (NICE) . Cilostazol, Naftidrofuryl Oxalate, Pentoxifylline and Inositol Nicotinate for the Treatment of Intermittent Claudication in People With Peripheral Arterial Disease 2010. www.nice.org.uk/nicemedia/live/12265/48291/48291.pdf (accessed February 2010).
- National Institute for Health and Clinical Excellence (NICE) . Cilostazol, Naftidrofuryl Oxalate, Pentoxifylline and Inositol Nicotinate for the Treatment of Intermittent Claudication in People With Peripheral Arterial Disease 2010. www.nice.org.uk/nicemedia/live/12265/48292/48292.pdf (accessed April 2010).
- The NHS Information Centre, Prescribing Support Unit . Prescription Cost Analysis England 2009 2010.
- NHS Drug Tariff . Drug Tariff 2010. www.ppa.org.uk/edt/October_2010/mindex.htm.
- Centre for Reviews and Dissemination (CRD) . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev 2008;1.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS: a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Moher D, Liberati A, Tetzlaff J, Altman DG. and the PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med 2009;151:65-94.
- Pande RL, Hiatt WR, Zhang P, Hittel N, Creage MA. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med 2010;15:181-8.
- de Backer-Tine LM, Vander SR, Lehert P, Van Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database Syst Rev 2008;2.
- Pratt CM. Analysis of the cilostazol safety database. Am J Cardiol 2001;87:D28-33.
- Otsuka Pharmaceuticals Submission to NICE . Cilostazol for the Symptomatic Treatment of Intermittent Claudication Secondary to Peripheral Arterial Disease 2010.
- Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol 2002;90:1314-19.
- Regensteiner JG, Ware JE, McCarthy WJ, Zhang P, Forbes WP, Heckman J, et al. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc 2002;50:1939-46.
- Momsen AH, Jensen MB, Norager CB, Madsen MR, Vestersgaard-Andersen T, Lindholt JS. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463-74.
- de Backer TL, Vander Stichele RH, Warie HH, Bogaert MG. Oral vasoactive medication in intermittent claudication: utile or futile?. Eur J Clin Pharmacol 2000;56:199-206.
- Hood SC, Moher D, Barber GG. Management of intermittent claudication with pentoxifylline: meta-analysis of randomized controlled trials. CMAJ 1996;155:1053-9.
- Girolami B, Bernardi E, Prins MH, Ten Cate JW, Hettiarachchi R, Prandoni P, et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta-analysis. Arch Intern Med 1999;159:337-45.
- Rowlands TE, Donnelly R. Medical therapy for intermittent claudication. Eur J Vasc Endovasc Surg 2007;34:314-21.
- Uchiyama S, Demaerschalk BM, Goto S, Shinohara Y, Gotoh F, Stone WM, et al. Stroke prevention by cilostazol in patients with atherothrombosis: meta-analysis of placebo-controlled randomized trials. J Stroke Cerebrovasc Dis 2009;18:482-90.
- Goldsmith DR, Wellington, K. Naftidrofuryl: a review of its use in the treatment of intermittent claudication. Drugs Aging 2005;22:967-77.
- Hashiguchi M, Ohno K, Saito R. Studies on the effectiveness and safety of cilostazol, beraprost sodium, prostaglandin E1 for the treatment of intermittent claudication. Yakugaku Zasshi (J Pharm Soc Japan) 2004;124:321-32.
- Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs 2003;3:117-38.
- Cameron HA, Waller PC, Ramsay LE. Drug treatment of intermittent claudication: a critical analysis of the methods and findings of published clinical trials, 1965-1985. Br J Clin Pharmacol 1988;26:569-76.
- Spengel F, Clement D, Boccalon H, Liard F, Brown T, Lehert P. Findings of the Naftidrofuryl in Quality of Life (NIQOL) European study program. Int Angiol 2002;21:20-7.
- Stone WM, Demaerschalk BM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. J Stroke Cerebrovasc Dis 2008;17:129-33.
- Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (cilostazol: a study in long-term effects). J Vasc Surg 2008;47:330-6.
- Hiatt WR, Brass EP, Money S. Long-term safety of cilostazol in patients with peripheral artery disease. Circulation 2007;116.
- O’Donnell ME, Badger SA, Sharif MA, Makar RR, McEneny J, Young IS, et al. The effects of cilostazol on exercise-induced ischaemia-reperfusion injury in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 2009;37:326-35.
- ADIS R&D Insight Profile . EGb 761: Ginkgo biloba extract, Ginkor. Drugs in R and D 2003;4:188-93.
- O’Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee, B, et al. The vascular and biochemical effects of cilostazol in diabetic patients with peripheral arterial disease. Vasc Endovasc Surg 2009;43:132-43.
- O’Donnell ME, Badger SA, Makar RR, McEneny J, Young IS, Lau LL. Randomised, controlled trial assessing the effects of cilostazol on exercise-induced ischaemia–reperfusion in patients with peripheral arterial disease. Ann Roy Coll Surg 2009;91:172-3.
- O’Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The effects of cilostazol on peripheral neuropathy in diabetic patients with peripheral arterial disease. Angiology 2008;59:695-704.
- Strandness DE, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, et al. Effect of cilostazol in patients with intermittent claudication: a randomized, double-blind, placebo-controlled study. Vasc Endovasc Surg 2002;36:83-91.
- Strandness DE, Dalman R, Panian S, Rendell M, Comp P, Bortey EB. Two doses of cilostazol versus placebo in the treatment of claudication: results of a randomized, multicenter trial. Circulation 1998;98:1-12.
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW, Martin JD, Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med 2000;109:523-30.
- Dawson DL, Zheng Q, Worthy SA, Charles B, Bradley DV. Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication. Angiology 2002;53:509-20.
- Dawson DL, DeMaioribus CA, Hagino RT, Light JT, Bradley DV, Britt KE, et al. The effect of withdrawal of drugs treating intermittent claudication. Am J Surg 1999;178:141-6.
- Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE, Bortey EB, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med 1999;159:2041-50.
- Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, et al. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg 1998;27:267-74.
- Dawson DL, Cutler BS, Meissner MH, Strandness DE. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation 1998;98:678-86.
- Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, et al. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arteriosclerosis Throm Vas 1998;18:1942-7.
- Kieffer E, Bahnini A, Mouren X, Gamand S. A new study demonstrates the efficacy of naftidrofuryl in the treatment of intermittent claudication. Findings of the Naftidrofuryl Clinical Ischemia Study (NCIS). Int Angiology 2001;20:58-65.
- Adhoute G, Bacourt F, Barral M, Cardon JM, Chevalier JM, Cuny A, et al. Naftidrofuryl in chronic arterial disease. Results of a six month controlled multicenter study using Naftidrofuryl tablets 200 mg. Angiology 1986;37:160-7.
- Trubestein G, Bohme H, Heidrich H. Naftidrofuryl in chronic arterial disease. Results of a controlled multicenter study. Angiology 1984;35:701-8.
- Ruckley CV, Callam MJ, Ferrington CM, Prescott RJ. Naftidrofuryl for intermittent claudication: a double-blind controlled trial. Br Med J 1978;1.
- Dettori AG, Pini M, Moratti A, Paolicelli M, Basevi P, Quintavalla R, et al. Acenocoumarol and pentoxifylline in intermittent claudication. A controlled clinical study. The APIC Study Group. Angiology 1989;40:237-48.
- Creager MA, Pande RL, Hiatt WR. A randomized trial of iloprost in patients with intermittent claudication. Vasc Med 2008;13:5-13.
- Lindgarde F, Jelnes R, Bjorkman H, Adielsson G, Kjellstrom T, Palmquist I, et al. Conservative drug treatment in patients with moderately severe chronic occlusive peripheral arterial disease. Scandinavian Study Group. Circulation 1989;80:1549-56.
- Porter JM, Cutler BS, Lee BY, Reich T, Reichle FA, Scogin JT, et al. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am Heart J 1982;104:66-72.
- Gillings D, Koch G, Reich T, Stager WJ. Another look at the pentoxifylline efficacy data for intermittent claudication. J Clin Pharmacol 1987;27:601-9.
- Porter JM, Bauer GM. Pharmacologic treatment of intermittent claudication. Surgery 1982;92:966-71.
- Reich T, Cutler BC, Lee BY, Porter JM, Reichle FA, Scogin JT, et al. Pentoxifylline in the treatment of intermittent claudication of the lower limbs. Angiology 1984;35:389-95.
- Gallus AS, Gleadow F, Dupont P, Walsh J, Morley AA, Wenzel A, et al. Intermittent claudication: a double-blind crossover trial of pentoxifylline. Aust NZ J Med 1985;15:402-9.
- Di Perri T, Guerrini M. Placebo controlled double blind study with pentoxifylline of walking performance in patients with intermittent claudication. Angiology 1983;34:40-5.
- O’Hara J, Jolly PN, Nicol CG. The therapeutic efficacy of inositol nicotinate (Hexopal) in intermittent claudication: a controlled trial. Br J Clin Pract 1988;42:377-83.
- O’Hara JA. Double-blind placebo-controlled study of Hexopal in the treatment of intermittent claudication. J Int Med Res 1985;13:322-7.
- Kiff RS, Quick CR. Does inositol nicotinate (Hexopal) influence intermittent claudication? A controlled trial. Br J Clin Pract 1988;42:141-5.
- Head A. Treatment of intermittent claudication with inositol nicotinate. Practitioner 1986;230:49-54.
- Hobbs SD, Marshall T, Fegan C, Adam D J, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in intermittent claudication: a randomized controlled trial. J Vasc Surg 2007;45:65-70.
- O’Donnell ME, Badger SA, Sharif MA, Young IS, Lee B, Soong CV. The vascular and biochemical effects of cilostazol in patients with peripheral arterial disease. J Vasc Surg 2009;49:1226-34.
- Ware JE. SF-36 Health survey: manual interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993.
- Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Bio 1990;2:142-50.
- Morgan M, Crayford T, Murrin B, Fraser S. Developing the vascular quality of life questionnaire: a new disease-specific quality of life measure for use in lower limb ischaemia. J Vasc Surg 2001;33.
- Mehta T, Venkata Subramaniam A, Chetter I, McCollum P. Disease-specific quality of life assessment in intermittent claudication: review. Eur J Vasc Endovasc Surg 2003;25:202-8.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Guest JF, Davie AM, Clegg JP. Cost effectiveness of cilostazol compared with naftidrofuryl and pentoxifylline in the treatment of intermittent claudication in the UK. Curr Med Res Opin 2005;21:817-26.
- Ratcliffe A. Cost-utility of cilostazol for the treatment of intermittent claudication in Scotland. Value Health 2005;8:A113-14.
- Strandness DE, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, et al. Effect of cilostazol in patients with intermittent claudication: a randomized, double-blind, placebo-controlled study. Vasc Endovasc Surg 2002;36:83-91.
- Adhoute G, Andreassian B, Boccalon H, Cloarec M, Di Maria G, Lefebvre O, et al. Treatment of stage II chronic arterial disease of the lower limbs with the serotonergic antagonist naftidrofuryl: results after 6 months of a controlled, multicenter study. J Cardiovasc Pharmacol 1990;16:S75-80.
- Moody AP, al-Khaffaf HS, Lehert P, Harris PL, Charlesworth D. An evaluation of patients with severe intermittent claudication and the effect of treatment with naftidrofuryl. J Cardiovasc Pharmacol 1994;23:S44-7.
- Lindgarde F, Labs KH, Rossner M. The pentoxifylline experience: exercise testing reconsidered. Vasc Med 1996;1:145-54.
- Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 2008;11:1131-43.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18.
- Office for National Statistics (ONS) . Mid-2009 Population Estimates: England and Wales; Estimated Resident Population by Single Year of Age and Sex 2010.
- Heald CL, Fowkes FGR, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis 2006;189:61-9.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Department of Health (DoH) . NHS Reference Costs 2008-2009 2009.
- Personal Social Services Research Unit (PSSRU) . Unit Costs of Health and Social Care 2009 2009.
- Egger M. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7.
- Moher D, Pham P, Klassen TP, Schulz KF, Berlin JA, Jadad AR, et al. What contributions do languages other than English make on the results of meta-analyses?. J Clin Epidemiol 2000;53:964-72.
- Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol 2005;58:769-76.
- De Backer T, Stichele RV, Lehert P, Van Bortel L. Naftidrofuryl for intermittent claudication: meta-analysis based on individual patient data. BMJ 2009;338.
- Belcaro G, Nicolaides AN, Griffin M, De Sanctis MT, Cesarone MR, Incandela L, et al. Intermittent claudication in diabetics: treatment with exercise and pentoxifylline: a 6-month, controlled, randomized trial. Angiology 2002;53:S39-43.
- Bieron K, Kostka-Trabka E, Starzyk D, Goszcz A, Grodzinska L, Korbut, R. Bencyclane: a new aspect of the mechanism of action in patients with peripheral arterial occlusive disease. Open-label, prospective, randomized trial. Acta Angiol 2005;11:157-72.
- Boccalon H, Lehert P, Mosnier, M. Effect of naftidrofuryl on physiological walking distance in patients with intermittent claudication. Annales De Cardiologie Et d’angéiologie 2001;50:175-82.
- Bollinger A, Frei C. Double-blind study of pentoxifylline against placebo in patients with intermittent claudication. Pharmatherapeutica 1977;1:557-62.
- Chacon-Quevedo A, Eguaras MG, Calleja F, Garcia MA, Roman M, Casares J, et al. Comparative evaluation of pentoxifylline, buflomedil, and nifedipine in the treatment of intermittent claudication of the lower limbs. Angiology 1994;45:647-53.
- Ciocon JO, Galindo DJ. A comparison between aspirin and pentoxifylline in relieving claudication due to peripheral vascular disease in the elderly. Angiology 1997;48:237-40.
- Clyne CA, Galland RB, Fox MJ, Gustave R, Jantet GH, Jamieson CW. A controlled trial of naftidrofuryl (Praxilene) in the treatment of intermittent claudication. Br J Surg 1980;67:347-8.
- de Albuquerque RM, Virgini-Magalhaes CE, Lencastre Sicuro F, Bottino DA, Bouskela E. Effects of cilostazol and pentoxifylline on forearm reactive hyperemia response, lipid profile, oxidative stress, and inflammatory markers in patients with intermittent claudication. Angiology 2008;59:549-58.
- De Sanctis MT, Cesarone MR, Belcaro G, Nicolaides AN, Griffin M, Incandela L, et al. Treatment of long-distance intermittent claudication with pentoxifylline: a 12-month, randomized trial. Angiology 2002;53:S13-17.
- Diehm C, Kuhn A, Strauss R, Hubsch-Muller C, Kubler W. Effects of regular physical training in a supervised class and additional intravenous prostaglandin E1 and naftidrofuryl infusion therapy in patients with intermittent claudication: a controlled study. Vasa 1989;28.
- Donaldson DR, Hall TJ, Kester RC, Ramsden CW, Wiggins PA. Does oxpentifylline (‘Trental’) have a place in the treatment of intermittent claudication?. Curr Med Res Opin 1984;9:35-40.
- Hentzer E. Meso-inositol hexanicotinat (Hexanicit) treatment of intermittent claudication as studied by a double blind technique employing xenon 133 clearance method. Scand J Lab Invest 1965;17.
- Jaffe G. Double blind comparison of bradilan (Tetranicotinoylfructose) tablets and hexopal (Inositol nicotinate) tablets in the treatment of intermittent claudication. J Int Med Res 1975;3:428-30.
- Karnik R, Valentin A, Stollberger C, Slany J. Effects of naftidrofuryl in patients with intermittent claudication. Angiology 1988;39:234-40.
- Kriessmann A, Neiss A, Kriessmann A, Neiss A. Clinical effectiveness of naftidrofuryl in intermittent claudication. Vasa 1988;24:27-32.
- Milio G, Coppola G, Novo S. The effects of prostaglandin E-1 in patients with intermittent claudication. Cardiovasc Hematol Disord Drug Targets 2006;6:71-6.
- Reilly DT, Quinton DN, Barrie WW. A controlled trial of pentoxifylline (Trental 400) in intermittent claudication: clinical, haemostatic and rheological effects. N Z Med J 1987;100:445-7.
- Roekaerts F, Deleers L. Trental 400 in the treatment of intermittent claudication: results of long-term, placebo-controlled administration. Angiology 1984;35:396-40.
- Rosas G, Cerdeyra C, Lucas MA, Parano JR, Villa JJ. Comparison of safety and efficacy of buflomedil and naftidrofuryl in the treatment of intermittent claudication. Angiology 1981;32:291-7.
- Schubotz R. Double blind trial of pentoxifylline in diabetics with peripheral vascular disorders. Pharmatherapeutica 1976;1:172-9.
- Soga Y, Yokoi H, Kawasaki T, Nakashima H, Tsurugida M, Hikichi Y, et al. Efficacy of cilostazol after endovascular therapy for femoropopliteal artery disease in patients with intermittent claudication. J Am Coll Cardiol 2009;53:48-53.
- Spitzer S, Kiesewetter H, Jung F, Gerhards M, Leipnitz G. Walk training and drug treatment in patients with peripheral arterial occlusive disease: a randomised, prospective, placebo-controlled double-blind study. Adv Vasc Pathol 1989;1.
- Strano A, Davi G, Avellone G, Novo S, Pinto A. Double-blind, crossover study of the clinical efficacy and the hemorheological effects of pentoxifylline in patients with occlusive arterial disease of the lower limbs. Angiology 1984;35:459-66.
- Trubestein G, Trubestein R, Duong QD. Comparative evaluation of the effectiveness of buflomedil and pentoxifylline in patients with arterial occlusive disease. Angiology 1981;32:705-10.
- Tyson VC. Treatment of intermittent claudication. Practitioner 1979;223:121-6.
- Waters KJ, Craxford AD, Chamberlain J. The effect of naftidrofuryl (Praxilene) on intermittent claudication. Br J Surg 1980;67:349-51.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
Appendix 1 Literature search strategies
Search strategies were developed to retrieve both RCTs and systematic reviews.
Randomised controlled trials
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
Intermittent Claudication/
-
claudication.tw.
-
1 or 2
-
exp Peripheral Vascular Diseases/
-
(peripheral adj arterial adj disease$).tw.
-
(peripheral adj vascular adj disease$).tw.
-
(atherosclero$ and (PAD or PVD)).tw.
-
((arterial adj disease$) and (PAD or PVD)).tw.
-
or/4-8
-
Atherosclerosis/dt, th [Drug Therapy, Therapy]
-
Vascular Diseases/dt, th [Drug Therapy, Therapy]
-
Vasodilator Agents/
-
vasodilator$.tw.dh
-
Platelet Aggregation Inhibitors/
-
(platelet adj aggregation adj inhibitor$).tw.
-
Phosphodiesterase Inhibitors/
-
(phosphodiesterase adj inhibitor$).tw.
-
Tetrazoles/tu [Therapeutic Use]
-
or/10-18
-
3 and 9 and 19
-
cilostazol$.tw.
-
(pletal or pletaal).tw.
-
OPC-13013.tw.
-
73963-72-1.rn.
-
or/21-24
-
3 and 25
-
9 and 25
-
Nafronyl/
-
naftidrofuryl$.tw.
-
naphtidrofuryl.tw.
-
(nafronyl or naftifurin).tw.
-
praxilene.tw.
-
(dusodril or iridus).tw.
-
3200-06-4.rn.
-
or/28-34
-
3 and 35
-
9 and 35
-
Pentoxifylline/
-
pentoxifylline.tw.
-
trental.tw.
-
oxpentifylline.tw.
-
(pentoxil or pentofin).tw.
-
bl-191.tw.
-
6493-05-6.rn.
-
or/38-44
-
3 and 45
-
9 and 45
-
Nicotinic Acids/
-
(inositol adj (nicotinate or hexanicotinate)).tw.
-
(inositol adj niacinate).tw.
-
hexopal.tw.
-
(dilexpal or mesotal or palohex or hexanicotol or esantene or hexanicit or linodil or mesonex or dilcit).tw.
-
6556-11-2.rn.
-
or/48-53
-
3 and 54
-
9 and 54
-
26 or 36 or 46 or 55
-
27 or 37 or 47 or 56
-
57 or 58
-
20 or 59
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
or/61-69
-
(clinic$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
(allocated adj2 random).tw.
-
or/71-75
-
70 or 76
-
Case report.tw.
-
Letter/
-
Historical article/
-
78 or 79 or 80
-
77 not 81
-
60 and 82
-
exp Animals/
-
Humans/
-
84 not 85
-
83 not 86
Broad drug class terms (10–18) were combined with both IC (1–2) and PAD statements (4-8). In addition, terms relating to the drug interventions (synonyms, alternative proprietary names, CAS registry numbers) were combined with either IC (1–2) or PAD terms (4–8). A RCT filter (61–86) was applied to retrieve the highest level of evidence.
Systematic reviews
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
61. meta-analysis as topic/
-
62. (meta analy$ or metaanaly$).tw.
-
63. Meta-Analysis/
-
64. (systematic adj (review$1 or overview$)).tw.
-
65. “Review Literature as Topic”/
-
66. or/61-65
-
67. (cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or b.i.ds or cancerlit).ab.
-
68. ((reference adj list$) or bibliograph$ or hand-search$ or (relevant adj journals) or (manual adj search$)).ab.
-
69. ((selection adj criteria) or (data adj extraction)).ab.
-
70. “review”/
-
71. 69 and 70
-
72. comment/ or editorial/ or letter/
-
73. Animals/
-
74. Humans/
-
75. 73 and 74
-
76. 73 not 75
-
77. 72 or 76
-
78. 66 or 67 or 68 or 71
-
79. 78 not 77
-
80. 60 and 79
Search statements 1–60 of the RCT search strategy above were combined with a systematic reviews methodology filter (statements 61–79).
Economic studies
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
61. exp “Costs and Cost Analysis”/
-
62. Economics/
-
63. exp Economics, Hospital/
-
64. exp Economics, Medical/
-
65. Economics, Nursing/
-
66. exp models, economic/
-
67. Economics, Pharmaceutical/
-
68. exp “Fees and Charges”/
-
69. exp Budgets/
-
70. budget$.tw.
-
71. ec.fs.
-
72. cost$.ti.
-
73. (cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
74. (economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
75. (price$ or pricing$).tw.
-
76. (financial or finance or finances or financed).tw.
-
77. (fee or fees).tw.
-
78. (value adj2 (money or monetary)).tw.
-
79. quality-adjusted life years/
-
80. (qaly or qalys).af.
-
81. (quality adjusted life year or quality adjusted life years).af.
-
82. or/61-81
-
83. 60 and 82
To retrieve evidence of cost-effectiveness studies, an economics filter was applied in place (61–82) of the RCT/SR search strategies above.
Adverse events
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
Nafronyl/ae, po, to
-
Pentoxifylline/ae, po, to
-
Nicotinic Acids/ae, po, to
-
or/1-3
-
cilostazol$.tw.
-
(pletal or pletaal).tw.
-
OPC-13013.tw.
-
73963-72-1.rn.
-
naftidrofuryl$.tw.
-
naphtidrofuryl.tw.
-
(nafronyl or naftifurin).tw.
-
praxilene.tw.
-
(dusodril or iridus).tw.
-
3200-06-4.rn.
-
pentoxifylline.tw.
-
trental.tw.
-
oxpentifylline.tw.
-
(pentoxil or pentofin).tw.
-
bl-191.tw.
-
6493-05-6.rn.
-
(inositol adj (nicotinate or hexanicotinate)).tw.
-
(inositol adj niacinate).tw.
-
hexopal.tw.
-
(dilexpal or mesotal or palohex or hexanicotol or esantene or hexanicit or linodil or mesonex or dilcit).tw.
-
6556-11-2.rn.
-
or/5-25
-
(adverse adj2 (event$ or effect$ or reaction$ or outcome$)).ti,ab.
-
(adrs or adr or complication$ or harm$ or harmful or risk$ or safe or safety or tolerability or tolerance or tolerate or toxic or toxicity).ti.
-
((side or undesirable) adj2 effect$).ti,ab.
-
(treatment adj2 emergent).ti.
-
or/27-30
-
26 and 31
-
4 or 32
-
exp Animals/
-
Humans/
-
34 not 35
-
33 not 36
Two approaches were used in the search for AEs of the four interventions. First, the AE subheadings that are linked to indexed drug names (1–3) and second, free-text terms relating to AEs (27–31) were combined with the intervention terms (5–26).
Quality-of-life studies
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
61. “Quality of Life”/
-
62. (qol or (quality adj2 life)).ab,ti.
-
63. (value adj2 (money or monetary)).tw.
-
64. value of life/
-
65. quality adjusted life year/
-
66. quality adjusted life.tw.
-
67. (qaly$ or qald$ or qale$ or qtime$).tw.
-
68. disability adjusted life.tw.
-
69. daly$.tw.
-
70. health status indicators/
-
71. (SF-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
72. (sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
73. (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
74. (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen).tw.
-
75. (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
76. (euroqol or euro qol or eq5d or eq 5d).tw.
-
77. (hql or hqol or h qol or hrqol or hr qol).tw.
-
78. (hye or hyes).tw.
-
79. health$ year$ equivalent$.tw.
-
80. health utilit$.tw.
-
81. (hui or hui1 or hui2 or hui3).tw.
-
82. disutilit$.tw.
-
83. rosser.tw.
-
84. (quality adj2 wellbeing).tw.
-
85. qwb.tw.
-
86. (willingness adj2 pay).tw.
-
87. standard gamble$.tw.
-
88. time trade off.tw.
-
89. time tradeoff.tw.
-
90. tto.tw.
-
91. letter.pt.
-
92. editorial.pt.
-
93. comment.pt.
-
94. 91 or 92 or 93
-
95. or/61-90
-
96. 95 not 94
-
97. 60 and 96
Search statements 1–60 in the RCT search strategy were combined with the quality-of-life methodology filter (statements 61–96).
Quality-of-life of intermittent claudication
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
Intermittent Claudication/
-
claudication.tw.
-
1 or 2
-
“Quality of Life”/
-
(qol or (quality adj2 life)).ab,ti.
-
(value adj2 (money or monetary)).tw.
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.tw.
-
(qaly$ or qald$ or qale$ or qtime$).tw.
-
disability adjusted life.tw.
-
daly$.tw.
-
health status indicators/
-
(SF-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
(sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw.
-
health$ year$ equivalent$.tw.
-
health utilit$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
disutilit$.tw.
-
rosser.tw.
-
(quality adj2 wellbeing).tw.
-
qwb.tw.
-
(willingness adj2 pay).tw.
-
standard gamble$.tw.
-
time trade off.tw.
-
time tradeoff.tw.
-
tto.tw.
-
letter.pt.
-
editorial.pt.
-
comment.pt.
-
34 or 35 or 36
-
or/4-33
-
38 not 37
-
3 and 39
Searches for studies of patients with IC without treatment were carried out. Terms for IC (1–2) were combined with the quality-of-life filter as shown above (4–39). Records retrieved from the quality-of-life searches with interventions form a sub-set of the records retrieved from these searches.
Quality of life of advanced intermittent claudication
MEDLINE and MEDLINE(R) In-Process & Other Non-Indexed Citations: Ovid, 1950 to present
-
Intermittent Claudication/
-
claudication.tw.
-
(advance$ or severe).tw.
-
(1 or 2) and 3
-
critical limb isch?emia.tw.
-
isch?emic rest pain.tw.
-
((CLI or IRP) and (peripheral arterial disease or PAD)).tw.
-
advanced peripheral arterial disease.tw.
-
or/4-8
-
“Quality of Life”/
-
(qol or (quality adj2 life)).ab,ti.
-
(value adj2 (money or monetary)).tw.
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.tw.
-
(qaly$ or qald$ or qale$ or qtime$).tw.
-
disability adjusted life.tw.
-
daly$.tw.
-
health status indicators/
-
(SF-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
(sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw.
-
health$ year$ equivalent$.tw.
-
health utilit$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
disutilit$.tw.
-
rosser.tw.
-
(quality adj2 wellbeing).tw.
-
qwb.tw.
-
(willingness adj2 pay).tw.
-
standard gamble$.tw.
-
time trade off.tw.
-
time tradeoff.tw.
-
tto.tw.
-
letter.pt.
-
editorial.pt.
-
comment.pt.
-
40 or 41 or 42
-
or/10-39
-
44 not 43
-
9 and 45
Search terms for advanced IC (1–9) were combined with the quality-of-life methodology filter (9–45).
Appendix 2 Table of excluded studies with rationale
| Trial | Comparison | Reason for exclusion |
|---|---|---|
| Adhoute 199093 | Naftidrofuryl fumarate vs placebo | Not licensed |
| Belcaro 2002107 | Pentoxifylline 1600 mg vs placebo | Not licensed dose |
| Bieron 2005108 | Intravenous pentoxifylline vs intravenous bencyclane | Not licensed |
| Boccalon 2001109 | Naftidrofuryl oxalate 200 mg t.i.d. vs placebo | Population includes Fontaine stage III, non-English language |
| Bollinger 1977110 | Pentoxifylline 600 mg vs placebo | Not licensed dose |
| Chacon-Quevedo 1994111 | Pentoxifylline 1200 mg vs buflomedil 600 mg | Comparator not relevant |
| Ciocon 1997112 | Pentoxifylline 400 mg t.i.d. vs aspirin 325 mg daily | Comparator not relevant |
| Clyne 1980113 | Naftidrofuryl oxalate 400 mg vs placebo | Not licensed dose |
| de Albuquerque 2008114 | Cilostazol 100 mg b.i.d. vs pentoxifylline 600 mg b.i.d. vs placebo | No comparative data between treatment groups for any of the outcomes included in this review |
| De Sanctis 2002115 | Pentoxifylline 1600 mg vs placebo | Not licensed dose |
| Diehm 1989116 | Intravenous naftidrofuryl oxalate 600 mg vs prostaglandins of the E1 type | Not licensed |
| Donaldson 1984117 | Pentoxifylline 600 mg vs placebo | Not licensed dose |
| Hentzer 1965118 | Inositol 1.8 g vs placebo | Not licensed dose |
| Jaffe 1975119 | Inositol 3 g vs bradilan 1500 mg | Comparator not relevant |
| Karnik 1988120 | Naftidrofuryl oxalate 400 mg b.i.d. vs placebo | Not licensed dose |
| Kriessman 1988121 | Naftidrofuryl oxalate 400 mg vs placebo | Non-English language |
| Milio 2006122 | Intravenous pentoxifylline and buflomedil vs postaglandins of the E1 type | Not licensed |
| Moody 199494 | Naftidrofuryl fumarate vs placebo | Not licensed |
| Reilly 1987123 | Pentoxifylline 400 mg vs placebo | Not licensed dose |
| Roekaerts 1984124 | Pentoxifylline 1200 mg vs placebo | Population includes Fontaine stage III |
| Rosas 1981125 | Naftidrofuryl oxalate 300 mg vs buflomedil 500 mg | Comparator not relevant, population includes Fontaine stage III |
| Schubotz 1976126 | Pentoxifylline 800 mg vs placebo | Population includes Fontaine stages I–III |
| Soga 2009127 | Cilostazol 200 mg daily for 2 years vs oral ticlopidine for 4 weeks | Excluded population –| all patients underwent endovascular therapy on day of starting study drug, some of patients in both groups had been taking cilostazol up to randomisation |
| Spitzer 1989128 | Intravenous pentoxifylline vs placebo | Not licensed |
| Strano 1984129 | Pentoxifylline 800 mg vs placebo | Population includes Fontaine stage III |
| Trubestein 1981130 | Pentoxifylline 300 mg vs buflomedil 450 mg | Not licensed dose, comparator not relevant |
| Tyson 1979131 | Inositol nicotinate vs placebo | Non-randomised study |
| Waters 1980132 | Naftidrofuryl oxalate 200 mg t.i.d. vs placebo | No comparative data between treatment groups for any of the outcomes included in this review |
Appendix 3 Quality assessment
| Trial (first author, year, trial number if known) | Strandness 200256 | Beebe 199961 | Elam 199864 | Dawson 199863 | Money 199862 | CASTLE,48–50 Stone 200848 | O’Donnell 200983 | Otsuka 21-95-20134 | Hobbs 200782 | Dawson 200058 | Otsuka 21-94-30134 | Otsuka 21-98-21334 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data from peer-reviewed journal(s) | Ref. no.s 56 and 57 | Ref. no. 61 | Ref. no. 64 | Ref. no. 63 | Ref. no. 62 | Ref. no.s 48–50 | Ref. no.s 51, 53, 55, 83 and 133 | Unpublished trial from Otsuka34 | Ref. no. 82 | Ref. no. 58 | Unpublished trial from Otsuka34 | Unpublished trial from Otsuka34 |
| Data from peer-reviewed systematic review(s) | Ref. no. 42 | Ref. no. 42 | Ref. no. 42 | Ref. no. 42 | Ref. no.s 35 and 42 | |||||||
| Data from industry submission | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | ||||
| What method was used to generate the randomised allocation sequence? | U | a, b | U | U | U | U | U | b | c | U | U | |
| Was the method used to generate the allocation sequence to treatment groups adequate? | U | Y | U | U | U | U | U | U | U | Y | U | U |
| What method was used to conceal treatment allocation? | U | c | U | U | U | U | d | U | U | e | U | U |
| Was the allocation of treatment concealed adequately? | U | Y | U | U | U | U | Y | U | U | Y | U | U |
| Were the treatment groups comparable at baseline? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were clinicians blind to treatment? | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Were participants blind to treatment? | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| If independent outcome assessors were used, were they blind to treatment? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Were participants analysed in their allocated treatment groups, in accordance with the ITT principle? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were at least 80% of the participants originally randomised followed up in the final analysis? | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Were there any imbalances in dropouts between groups? | N | N | U | U | U | U | N | U | U | N | U | N |
| If so, were these imbalances in dropouts adjusted for in analyses? | NA | NA | NA | NA | NA | U | NA | U | NA | NA | U | NA |
| Is there any evidence of selective reporting of outcomes (i.e. that the authors measured more outcomes than reported)? | Yf | N | Yf | N | N | N | N | NA | N | Yf | NA | NA |
| Trial (first author, year, trial number if known) | Strandness 200256 | Beebe 199961 | Elam 199864 | Dawson 199863 | Money 199862 | CASTLE,48–50 Stone 200848 | O’Donnell 200983 | Otsuka 21-95-20134 | Hobbs 200782 | Dawson 200058 | Otsuka 21-94-30134 | Otsuka 21-98-21334 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data from peer-reviewed journal(s) | Ref. no.s 56 and 57 | Ref. no. 61 | Ref. no. 64 | Ref. no. 63 | Ref. no. 62 | Ref. no.s 48–50 | Ref. no.s 51, 53, 55 and 133 | Unpublished trial from Otsuka34 | Ref. no. 82 | Ref. no. 58 | Unpublished trial from Otsuka34 | Unpublished trial from Otsuka34 |
| Data from peer-reviewed systematic review(s) | Ref. no. 42 | Ref. no. 42 | Ref. no. 42 | Ref. no. 42 | Ref. no.s 35 and 42 | |||||||
| Data from industry submission | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | Ref. no. 34 | ||||
| Was IC diagnosed by objective evidence (e.g. reduced ankle systolic blood pressure)? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Did patients have a history of at least 6 months of IC? | Y | Y | Y | Y | Y | U | N | Y | N | Y | Y | Y |
| Was the treatment period at least 24 weeks’ duration? | Y | Y | N | N | N | Y | Y | N | U | Y | Y | Y |
| Was concomitant treatment comparable across treatment groups? | U | U | Y | U | U | Y | Y | U | Y | U | U | U |
| If the study included diabetics and non-diabetics, was there stratification for diabetes? | U | N | U | N | U | U | Y | N | N | N | N | U |
| Was there a placebo run-in phase? | U | N | N | Y | N | Y | N | N | N | N | N | U |
| If so, did the placebo run-in phase last 2–6 weeks? | U | NA | NA | Y | NA | Y | NA | NA | NA | NA | NA | U |
| Did reported outcomes include MWD and/or PFWD? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Did the study use a clearly designed protocol for the treadmill test? | Y | Y | Y | Y | Y | NA | Y | Y | Y | Y | Y | Y |
| If not a treadmill test, was there a clearly defined protocol for the walking distance test? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| For placebo run-in phase or baseline, were there at least two treadmill tests with an interval of at least 1 week? | U | Y | Y | U | U | NA | Y | Y | N | U | Y | U |
| If so, did patients have a baseline MWD with < 25% change? | U | Y | U | N | Y | NA | U | Y | NA | Y | Y | U |
| Trial (first author, year, trial number if known) | Dawson 200058 | Otsuka34 | Otsuka 21-98-21334 | Lindegarde 198971 | Porter 1982,72 Gillings 198773 | Gallus 198576 | Di Perri 198377 | Dettori 198969 | Creager 200870 |
|---|---|---|---|---|---|---|---|---|---|
| Data from peer-reviewed journal | Ref. no. 58 | Unpublished trial from Otsuka | Unpublished trial from Otsuka | Ref. no. 71 | Ref. no.s 72–75 | Ref. no. 76 | Ref. no. 77 | Ref. no. 69 | Ref. no. 70 |
| Data from peer-reviewed systematic review(s) | Ref. no. 42 | Ref. no. 35 | |||||||
| Data from industry submission | Ref. no. 34 | Ref. no. 34 | |||||||
| What method was used to generate the randomised allocation sequence? | Permuted block design | U | U | U | U | Random number sequence | U | Computer-generated random numbers | U |
| Was the method used to generate the allocation sequence to treatment groups adequate? | Y | U | U | U | U | Y | U | Y | U |
| What method was used to conceal treatment allocation? | a | U | U | U | U | b | U | c | U |
| Was the allocation of treatment concealed adequately? | Y | U | U | U | U | Y | U | Y | U |
| Were the treatment groups comparable at baseline? | Y | Y | Y | Y | Y | Y | U | Y | Y |
| Were clinicians blind to treatment? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Were participants blind to treatment? | Y | Y | Y | Y | Y | Y | Y | N | Y |
| If independent outcome assessors were used, were they blind to treatment? | NA | NA | NA | NA | NA | NA | NA | Y | NA |
| Were participants analysed in their allocated treatment groups, in accordance with the ITT principle? | Y | Y | Y | Y | U | N | Y | N | Y |
| Were at least 80% of the participants originally randomised followed up in the final analysis? | Y | Y | Y | U | N | N | Y | Y | Y |
| Were there any imbalances in dropouts between groups? | N | U | N | U | U | N | N | N | U |
| If so, were these imbalances in dropouts adjusted for in analyses? | NA | U | NA | NA | U | NA | NA | NA | NA |
| Is there any evidence of selective reporting of outcomes (i.e. that the authors measured more outcomes than reported)? | Yd | NA | NA | Y | Y | N | N | Y | N |
| Trial (first author, year, trial number if known) | Dawson 200058 | Otsuka 21–94–30134 | Otsuka 21–98–21334 | Lindegarde 198971 | Porter 1982,72 Gillings 198773 | Gallus 198576 | Di Perri 198377 | Dettori 198969 | Creager 200870 |
|---|---|---|---|---|---|---|---|---|---|
| Data from peer-reviewed journal | Ref. no. 58 | Unpublished trial from Otsuka | Unpublished trial from Otsuka | Ref. no. 71 | Ref. no.s 72–75 | Ref. no. 76 | Ref. no. 77 | Ref. no. 69 | Ref. no. 70 |
| Data from peer-reviewed systematic review(s) | Ref. no. 42 | Ref. no. 35 | |||||||
| Data from industry submission | Ref. no. 34 | Ref. no. 34 | |||||||
| Was IC diagnosed by objective evidence (e.g. reduced ankle systolic blood pressure)? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Did patients have a history of at least 6 months of IC? | Y | Y | Y | Y | Y | Y | Y | U | N |
| Was the treatment period at least 24 weeks’ duration? | Y | Y | Y | Y | Y | N | N | Y | Y |
| Was concomitant treatment comparable across treatment groups? | U | U | U | U | Y | U | Y | U | U |
| If the study included diabetics and non-diabetics, was there stratification for diabetes? | N | N | U | NA | N | N | NA | N | N |
| Was there a placebo run-in phase? | N | N | U | Y | Y | Y | N | Y | Y |
| If so, did the placebo run-in phase last 2–6 weeks? | NA | NA | U | Y | Y | Y | NA | Y | Y |
| Did reported outcomes include MWD and/or PFWD? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Did the study use a clearly designed protocol for the treadmill test? | Y | Y | Y | Y | Y | Y | N | Y | Y |
| If not a treadmill test, was there a clearly defined protocol for the walking distance test? | NA | NA | NA | NA | NA | NA | Y | NA | NA |
| For placebo run-in phase or baseline, were there at least two treadmill tests with an interval of at least 1 week? | U | Y | U | Y | Y | Y | N | U | Y |
| If so, did patients have a baseline MWD with < 25% change? | Y | Y | U | N | Y | U | NA | U | Y |
| Trial (first author, year, trial number if known) | Kieffer 200165 | Adhoute 198666 | Trubestein 198467 | Ruckley 197868 | Spengel 200247 |
|---|---|---|---|---|---|
| Data from peer-reviewed journal(s) | Ref. no. 65 | Ref. no. 66 | Ref. no. 67 | Ref. no. 68 | Ref. no. 47 |
| What method was used to generate the randomised allocation sequence? | Computer generated | U | NR | U | Computer-generated list |
| Was the method used to generate the allocation sequence to treatment groups adequate? | Y | U | U | U | Y |
| What method was used to conceal treatment allocation? | U | U | U | Coded container | U |
| Was the allocation of treatment concealed adequately? | U | U | U | U | U |
| Were the treatment groups comparable at baseline? | Y | Y | U | N | Y |
| Were clinicians blind to treatment? | Y | N | Y | Y | Y |
| Were participants blind to treatment? | Y | Y | Y | Y | Y |
| If independent outcome assessors were used, were they blind to treatment? | NA | NA | NA | NA | NA |
| Were participants analysed in their allocated treatment groups, in accordance with the ITT principle? | Y | Y | Y | U | Y |
| Were at least 80% of the participants originally randomised followed up in the final analysis? | Y | N | Y | Y | Y |
| Were there any imbalances in dropouts between groups? | N | N | N | U | N |
| If so, were these imbalances in dropouts adjusted for in analyses? | NA | NA | NA | NA | NA |
| Is there any evidence of selective reporting of outcomes? | Y | N | N | N | N |
| Trial (first author, year, trial number if known) | Kieffer 200165 | Adhoute 198666 | Trubestein 198467 | Ruckley 197868 | Spengel 200247 |
|---|---|---|---|---|---|
| Data from peer-reviewed journal(s) | Ref. no. 65 | Ref. no. 66 | Ref. no. 67 | Ref. no. 68 | Ref. no. 47 |
| Was IC diagnosed by objective evidence (e.g. reduced ankle systolic blood pressure)? | Y | Y | Y | U | Y |
| Did patients have a history of at least 6 months of IC? | Y | Y | Y | U | N |
| Was the treatment period at least 24 weeks’ duration? | Y | Y | N | N | Y |
| Was concomitant treatment comparable across treatment groups? | U | U | U | U | U |
| If the study included diabetics and non-diabetics, was there stratification for diabetes? | Y | U | U | N | N |
| Was there a placebo run-in phase? | Y | Y | Y | N | Y |
| If so, did the placebo run-in phase last 2–6 weeks? | Y | Y | Y | NA | Y |
| Did reported outcomes include MWD and/or PFWD? | Y | Y | Y | Y | Y |
| Did the study use a clearly designed protocol for the treadmill test? | Y | Y | Y | U | NA |
| If not a treadmill test, was there a clearly defined protocol for the walking distance test? | NA | NA | NA | NA | N |
| For placebo run-in phase or baseline, were there at least two treadmill tests with an interval of at least 1 week? | Y | Y | Y | N | N |
| If so, did patients have a baseline MWD with < 25% change? | Y | Y | N | NA | NA |
| Trial (first author, year, trial number if known) | O’Hara 198878 (O’Hara 1985 same study) | Kiff 198880 | Head 198681 |
|---|---|---|---|
| Data from peer-reviewed journal(s) | Ref. no. 78 | Ref. no. 80 | Ref. no. 81 |
| What method was used to generate the randomised allocation sequence? | U | U | U |
| Was the method used to generate the allocation sequence to treatment groups adequate? | U | U | U |
| What method was used to conceal treatment allocation? | U | U | U |
| Was the allocation of treatment concealed adequately? | U | U | U |
| Were the treatment groups comparable at baseline? | Y | Y | N |
| Were clinicians blind to treatment? | Y | Y | Y |
| Were participants blind to treatment? | Y | Y | Y |
| If independent outcome assessors were used, were they blind to treatment? | NA | NA | NA |
| Were participants analysed in their allocated treatment groups, in accordance with the ITT principle? | Y | Y | Y |
| Were at least 80% of the participants originally randomised followed up in the final analysis? | Y | Y | Y |
| Were there any imbalances in dropouts between groups? | N | N | U |
| If so, were these imbalances in dropouts adjusted for in analyses? | NA | NA | NA |
| Is there any evidence of selective reporting of outcomes? | Y | Y | Y |
| Trial (first author, year, trial number if known) | O’Hara 198878 (O’Hara 1985 same study) | Kiff 198880 | Head 198681 |
|---|---|---|---|
| Data from peer-reviewed journal(s) | Ref. no. 78 | Ref. no. 80 | Ref. no. 81 |
| Was IC diagnosed by objective evidence (e.g. reduced ankle systolic blood pressure)? | U | Y | U |
| Did patients have a history of at least 6 months of IC? | U | Y | U |
| Was the treatment period at least 24 weeks’ duration? | N | N | N |
| Was concomitant treatment comparable across treatment groups? | U | U | U |
| If the study included diabetics and non-diabetics, was there stratification for diabetes? | U | N | N |
| Was there a placebo run-in phase? | N | N | N |
| If so, did the placebo run-in phase last 2–6 weeks? | NA | NA | NA |
| Did reported outcomes include MWD and/or PFWD? | Y | Y | N |
| Did the study use a clearly designed protocol for the treadmill test? | N | N | NA |
| If not a treadmill test, was there a clearly defined protocol for the walking distance test? | Y | NA | Y |
| For placebo run-in phase or baseline, were there at least two treadmill tests with an interval of at least 1 week? | N | N | N |
| If so, did patients have a baseline MWD with < 25% change? | NA | NA | NA |
Appendix 4 Data abstraction tables
Data are as reported in the primary publication listed in the ‘publication type’ row, unless indicated otherwise by square brackets [ ]. These data are taken from a secondary publication, as referenced.
Two-arm trials of cilostazol versus placebo
| Strandness 200256 | |
|---|---|
| Study details | |
| Publication type | Strandness 2002,56 full report in peer-reviewed journal |
| Additional sources of data | Strandness 1998,57 Thompson 2002,35 Cochrane review 2008,28 Pande 2010,31 Otsuka Pharmaceuticals submission to NICE34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups |
Cilostazol 200 mg (100 mg b.i.d.) Placebo Cilostazol 100 mg (50 mg b.i.d.) – this dose is not licensed in the UK and has been excluded from analysis |
| Comparator | Placebo |
| Run-in phase | 3 weeks, non-placebo |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 2 weeks, 4 weeks, then every 4 weeks until 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2.0 mph (3.2 km/hour) at a constant 12.5% grade PFWD: as MWD AEs: patient self-report HRQoL: SF-36, WIQ, COM |
| Notes on statistics |
Raw data: arithmetic mean, mean change and per cent change Analysis: last observation carried forward, analysis of variance of the log (distance at week 24/baseline). Between-group analysis by estimated treatment effect, calculated as ratio of geometric mean (antilog of the difference in mean of cilostazol change from baseline minus mean of placebo change from baseline) |
| Population | |
| Eligibility criteria | Age ≥ 40 years; stable, PAD-induced IC of at least 6 months’ duration; no significant change in symptom severity for at least 3 months; diagnosis of PAD required Doppler measurement of an ABPI £ 0.90; resting ABPI < 0.90 and at least a 10 mmHg decrease in ankle systolic blood pressure in the reference leg at the completion of testing MWD on two consecutive prerandomisation treadmill tests varied by < 20%; walking distance 30–200 m. For subjects with equivalent bilateral disease, the limb with the lowest resting ABPI was analysed. Excluded if rest pain: Buerger’s disease; ischaemic tissue necrosis; surgical or endovascular procedures within 3 months; unstable coronary artery disease or a coronary intervention within 6 months; deep vein thrombosis within 3 months; symptomatic cardiac arrhythmias; conditions other than claudication that limited exercise capacity, or other medical conditions likely to preclude completing the study; women of childbearing age not using a reliable birth control method; patients receiving anticoagulants or using > 81 mg/day aspirin or > 1200 mg/day ibuprofen; gross obesity; hypertension (> 200 mmHg systolic or > 100 mmHg diastolic supine resting pressures), malignancy or metastatic malignancy, exercise-limiting cardiac disease, history of bleeding tendencies, and concomitant use of antiplatelet, anticoagulant, haemorheological or non-steroidal anti-inflammatory agents |
| Concomitant interventions allowed or excluded |
Allowed: occasional use of diclofenac sodium Disallowed: antiplatelet, anticoagulant, haemorheological or non-steroidal anti-inflammatory agents. No specific counselling regarding smoking cessation, diet or exercise was given |
| Power calculation | Powered at 90%, based on a 5% significance level (two-sided) |
| N randomised to treatments included in review | 262 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 133 | 129 |
| Baseline characteristics | ||
| Age | Mean 63.1 years (SE 10.2 years) | Mean 64.4 years (SE 10.2 years) |
| Gender | M 76.7%; F 23.3%a | M 77.5%; F 22.5% |
| Smokers | 50.4% current smokers | 48.1% current smokers |
| Diabetics | 23.3% | 17.1% |
| Hypertension/blood pressure | NR | NR |
| Hyperlipidaemia | NR | NR |
| Obesity or weight | Mean weight 80.1 (SE 14.8) kg | Mean weight 80.1 (SE 15.1) kg |
| Angina | NR | NR |
| History of vascular therapy | ||
| Other | Currently drinks alcohol 61.7% | Currently drinks alcohol 55.0% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | Nine did not have at least one post-randomisation treadmill test; 22.6% withdrew owing to AEs | Four did not have at least one post-randomisation treadmill test; 10.1% withdrew owing to AEs |
| Results | ||
| MWD n in analysis | 124 at 24 weeks | 125 at 24 weeks |
| MWD baseline | Mean 119.4 m | Mean 120.1 m |
| MWD follow-up | Mean 195.6 m | Mean 141.2 m |
| MWD change | Mean 76.2 m (63.82%) | Mean 21.1 m (17.6%) |
| MWD between-group comparison |
Estimated treatment effect 1.21 (95% CI 1.09 to 1.35) p = 0.0003 |
|
| PFWD n in analysis | ||
| PFWD baseline | [Otsuka submission34 arithmetic mean 63.6] | [Otsuka submission34 arithmetic mean 67.5] |
| PFWD follow-up | ||
| PFWD change | [Robless 2008:28 mean 58.5 (SD 128.3)] [Otsuka submission34 arithmetic mean 47.2 (84.3%)] | [Robless 2008:28 mean 17.2 (SD 43.6)] [Otsuka submission34 arithmetic mean 19.8 (37.7%)] |
| PFWD between-group comparison | [Strandness 1998:57 22% net improvement] [Otsuka submission34 estimated treatment effect (geometric mean ratio) 1.22, p = 0.0015] | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 265 | 129 |
| Vascular events follow-up | 24 weeks | |
| Vascular events included | NR | |
| Vascular events reported | n = 12 | n = 5 |
| Vascular events between-group comparison | NR | |
| AEs n in analysis | 133 | 129 |
| AEs follow-up | 24 weeks | |
| AEs included | ||
| AEs reported |
Headache 40.6%; infection 18%; leg pain 11.3%; diarrhoea 16.5%; abnormal stools 19.5%. Serious treatment-emergent AEs 18.8% Potentially cilostazol-related AEs (n = 7) 5.3% |
Headache 12.4%; infection 12.4%; leg pain 14.0%; diarrhoea 6.2%; abnormal stools 5.4% Serious treatment-emergent AEs 15.5% |
| AEs between-group comparison | NR | |
| Mortality reported | 2 | 0 |
| Mortality between-group comparison | Log-rank test on the Kaplan–Meier estimates of survival, no significant differences among treatment groups (p = 0.6723) in the probability of having a cardiovascular event or dying throughout the course of the study | |
| HRQoL n in analysis | Unclear | Unclear |
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | Statistically significant improvement in the physical function scale at week 24 for the cilostazol group compared with placebo (p = 0.048). Non-significant trend favouring cilostazol over placebo for physical health concept scales (physical function, bodily pain and role–physical), general health perception score and walking distance score on the WIQ | |
| Beebe 199961 | |
|---|---|
| Study details | |
| Publication type | Beebe 1999,61 full report in peer-reviewed journal |
| Additional sources of data | Cochrane review 2008,28 Uchiyama 2009,42 Rowlands 2007,41 industry submission34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 mg (100 mg b.i.d.) |
| Comparator | Placebo |
| Run-in phase | 3 weeks, non-placebo |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 4, 8, 12, 16, 20 and 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2.0 mph (3.2 km/hour) at a constant 12.5% grade PFWD: as MWD Vascular events: method NR AEs: patient self-report Mortality: method NR HRQoL: SF-36, WIQ, COM |
| Notes on statistics | Log transformation of the data was used for walking distances |
| Population | |
| Eligibility criteria | Age ≥ 40 years; stable, PAD-induced IC of at least 6 months’ duration; no significant change in symptom severity for at least 3 months; diagnosis of PAD required Doppler measurement of an ABPI ≤ 0.90 and a ≥ 10 mmHg decrease in ankle artery blood pressure following the onset of MWD; PFWD 30–200 m on two consecutive pre-randomisation treadmill tests (12.5% incline, 3.2 km/hour) varied by < 20%. Excluded if rest pain; obesity; hypertension(> 200 mmHg systolic or > 100 mmHg diastolic supine resting blood pressure), current metastatic malignant neoplasm; conditions other than claudication that limited exercise capacity, or other medical conditions likely to preclude completing the study; women of childbearing age not using a reliable birth control method; history of bleeding tendencies |
| Concomitant interventions allowed or excluded |
Allowed: [Otsuka submission,34 diclofenac sodium as clinically indicated] Disallowed: anticoagulant, antiplatelet, vasoactive, haemorheological or non-steroidal anti-inflammatory agents |
| Power calculation | Powered at 80% to detect a doubling of the cardiovascular morbidity and all-cause mortality event rate, based on a 5% significance level (two-sided) |
| N randomised to treatments included in review | 345 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 175 | 170 |
| Baseline characteristics | ||
| Age | Mean 64.3 years (SD 8.5 years) | Mean 65.1 years (SD 9.3 years) |
| Gender | M 74.3%; F 25.7% | M 77.1%; F 22.9% |
| Smokers | 34.9% | 44.1% |
| Diabetics | 26.3% | 28.2% |
| Hypertension/blood pressure | ||
| Hyperlipidaemia | ||
| Obesity or weight | Weight mean 78.6 (SD 16.1) kg range 41.8–115.0 kg | Weight mean 78.8 (SD 16.0) kg range 47.7–129.4 kg |
| Angina | ||
| History of vascular therapy | ||
| Other | Currently drinks alcohol 60.6% | Currently drinks alcohol 57.1% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | 26 withdrew for AEs, 11 for other reasons | 24 withdrew for AEs, five for other reasons |
| Results | ||
| MWD n in analysis | 140 | 140 |
| MWD baseline | Geometric mean 129.7 m | Geometric mean 147.8 m |
| MWD follow-up | Geometric mean 258.8 at 24 weeks (at 16 weeks 216.0) | Geometric mean 174.6 at 24 weeks (at 16 weeks 161.9) |
| MWD change | Geometric mean change from baseline 1.51 at 24 weeks (at 16 weeks = 1.41); difference (258.8–129.7 = 129.1) [129.1 (463.3)]28 [Rowlands 2007:41 mean change 51%] | Geometric mean change from baseline 1.15 at 24 weeks (at 16 weeks = 1.11); difference 26.82 [26.82 (148.5)]28 [Rowlands 2007:41 mean change 15%] |
| MWD between-group comparison | p < 0.001 at 24 weeks (p < 0.001 at 16 weeks) | |
| PFWD n in analysis | 140 | 140 |
| PFWD baseline | Geometric mean 70.4 m | Geometric mean 72.4 m |
| PFWD follow-up | Geometric mean 137.9 at 24 weeks (at 16 weeks = 112.4) | Geometric mean 95.5 at 24 weeks (at 16 weeks = 91.9) |
| PFWD change | Geometric mean change from baseline 1.59 at 24 weeks (at 16 weeks = 1.43); difference 67.5 [Robless 2008:28 67.5 (130.4)] [Rowlands 2007:41 mean change 59%] | Geometric mean change from baseline 1.20 at 24 weeks (at 16 weeks = 1.15); difference 23.04 [Robless 2008:28 23.04 (63.78) [Rowlands 2007:41 mean change 20%] |
| PFWD between-group comparison | p < 0.001 at 24 weeks (p < 0.001 at 16 weeks) | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 175 | 170 |
| Vascular events follow-up | 24 weeks | |
| Vascular events included |
|
|
| Vascular events reported, n (%) |
|
|
| Vascular events between-group comparison | No statistically significant differences between treatment groups in the probability of survival without cardiovascular morbidity or all-cause mortality during 24 weeks of therapy (p = 0.71) | |
| AEs n in analysis | 175 | 170 |
| AEs follow-up | 24 weeks | |
| AEs included | ||
| AEs reported |
Headache 34.3%; abnormal stool samples 14.9%; diarrhoea 12.0%; dizziness 10.3%; palpitations 11.4% Withdrew due to headache n = 4; due to palpitations n = 4 |
Headache 14.7%; abnormal stool samples 3.5%; diarrhoea 4.1%; dizziness 4.7%; palpitations 0% |
| AEs between-group comparison | ||
| Mortality reported | n = 2, 1.1% | n = 2, 1.2% |
| Mortality between-group comparison | ||
| HRQoL n in analysis | 137 | 141 |
| HRQoL baseline | ||
| HRQoL follow-up | ||
| Mean score (mean change from baseline) | Mean score (mean change from baseline) | |
| HRQoL change | SF-36 physical health (score range 0–100): physical function 61.6 (7.1); role–physical 61.3 (5.3); bodily pain 62.9 (7.2); mental health (score range 0–100) social function 86.3 (1.0); role–emotional 91.7 (2.9); mental health 82.2 (2.5) | SF-36 physical health (score range 0–100): physical function 53.8 (2.0); role–physical 49.8 (–2.8); bodily pain 54.0 (–1.8); mental health (score range 0–100) social function 82.5 (0.4); role–emotional 84.2 (–1.66); mental health 79.6 (0.9) |
| HRQoL between-group comparison | For the physical health concepts domain of the SF-36, cilostazol was significantly superior to placebo at week 24 in the physical function and bodily pain scales. There was no significant difference between cilostazol and placebo for the mental health concepts domain. For the WIQ at week 24, both cilostazol groups were superior to placebo for walking speed and walking distance. Statistically significant improvements were seen in the following COM scales: walking pain/discomfort, change in walking pain/discomfort, and walking pain/discomfort related to ability to perform physical activities. For all other domains and subscales, the cilostazol groups were not significantly different from the placebo group | |
| Elam 199864 | |
|---|---|
| Study details | |
| Publication type | Elam 1998,64 full report in peer-reviewed journal |
| Additional sources of data | Thompson 2002,35 Cochrane review 2008,28 Uchiyama 2009,42 Otsuka Pharmaceuticals submission to NICE34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 mg (100 mg b.i.d.) |
| Comparator | Placebo |
| Run-in phase | |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, then every 4 weeks until 12 weeks |
| Outcomes and measures |
MWD: graded test, constant speed [Thompson 2002:35 2.0 mph (3.2 km/hour), at 0% grade with a 3.5% increase in grade every 3 minutes] ABPI: Doppler AEs: patient self-report |
| Notes on statistics | Arithmetic means used for MWD and PFWD |
| Population | |
| Eligibility criteria | Documented chronic, stable, symptomatic IC secondary to PAD. PAD was defined as an ABPI ≤ 0.90; termination of walking on a variable-load, constant-speed treadmill due to IC (between 54 and 805 m); and a Doppler-measured drop of ≥ 10 mmHg in blood pressure of one ankle after the treadmill test. For patients without a qualifying ABPI, a 20 mmHg drop in post-exercise ankle artery pressure was required for entry. Patients with documented IC underwent two fasting blood draws (at least 1 week apart) in which plasma triglyceride concentration (average of two determinations) was < 350 mg/dl, and plasma low-density lipoprotein cholesterol was between 100 and 190 mg/dl in all subjects. Women were not of child-bearing potential (either surgically sterilised or at least 1 year post-menopause). Exclusions: gross obesity (> 60% above ideal body weight), poorly controlled hypertension (systolic pressure > 200 mmHg; diastolic pressure > 100 mmHg), poorly controlled diabetes, a history of malignancy, current alcohol or drug abuse, renal disease (creatinine > 2.5 mg/dl), or bleeding tendencies; patients taking antiplatelet, anticoagulant, vasoactive, haemorheological or lipid-modifying medications |
| Concomitant interventions allowed or excluded |
Allowed: therapy with beta-blockers and thiazide diuretics was allowed if held at a constant dose for 8 weeks before the trial and if the dosage was maintained during the 12-week treatment period Disallowed: specific counselling regarding smoking cessation, diet or exercise |
| Power calculation | Powered at 80%, based on a 5% significance level (two-sided) |
| N randomised to treatments included in review | |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 95 | 94 |
| Baseline characteristics | ||
| Age | Mean 66.7 years | Mean 65.8 years |
| Gender | M 87.4%; F 12.6% | M 80.9%; F 19.1% |
| Smokers | ||
| Diabetics | 18.9% | 20.2% |
| Hypertension/blood pressure | 55.8% | 60.6% |
| Hyperlipidaemia | ||
| Obesity or weight | Weight mean 81.7 kg | Weight mean 81.1 kg |
| Angina | 8.4% | 10.6% |
| History of vascular therapy | More CABG in placebo than cilostazol group, figures | |
| Other | NR Prior MI 10.6% | Prior MI 17.1% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | 13.7% did not complete study. Four discontinued due to headache, one discontinued due to diarrhoea | 6.4% did not complete study |
| Results | ||
| MWD n in analysis | Unclear, could be all 95 with imputed data (as for lipid outcomes), 82 completed study | Unclear, could be all 94 with imputed data (as for lipid outcomes), 88 completed study |
| MWD baseline | Mean 262.3 m (SE 17 m) | Mean 278.2 m (SE 17 m) |
| MWD follow-up | 335 (SE 24) | 304 (SE 23) |
| MWD change | 35.5% mean change; difference 72.7 [Robless 2008:28 79.05] [Otsuka submission34 has 76.9 (35%)] | 24.3% mean change; difference 25.8 [Robless 2008:28 36.1] [Otsuka submission34 has 23.8 (18%)] |
| MWD between-group comparison | Cilostazol improved significance over placebo (p = 0.004) | |
| PFWD n in analysis | ||
| PFWD baseline | Mean 122.2 m | Mean 142.3 m |
| PFWD follow-up | ||
| PFWD change | [Otsuka submission34 has 75.0 (67%)] | [Otsuka submission34 has 48.8 (38%)] |
| PFWD between-group comparison | [Otsuka submission34 has p = 0.0035] | |
| ABPI n in analysis | Unclear, could be all 95 with imputed data (as for lipid outcomes), 82 completed study | Unclear, could be all 94 with imputed data (as for lipid outcomes), 88 completed study |
| ABPI baseline | Mean 0.66 (SE 0.02) | Mean 0.65 (SE 0.02) |
| ABPI follow-up | 0.73 (0.02) | 0.65 (0.02) |
| ABPI change | Mean change 9.03% [difference mean 0.07] | Mean change 1.2% (as reported, even though baseline and final scores are the same) [difference mean 0.00] |
| ABPI between-group comparison | Cilostazol improved significance over placebo p < 0.001 [Otsuka submission:34 has p = 0.0008)] | |
| Vascular events n in analysis | 95 | 94 |
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | [Uchiyama 2008:42 no coronary vascular events; no cerebral vascular events; one serious bleeding, 1.1%] | [Uchiyama 2008:42 no coronary vascular events; no cerebral vascular events; one serious bleeding, 1.1%] |
| Vascular events between-group comparison | ||
| AEs n in analysis | 95 | 94 |
| AEs follow-up | ||
| AEs included | ||
| AEs reported | Headache 32.6%; diarrhoea 18.9%; musculoskeletal pain 14.7%; abnormal stools 13.7%; dizziness 12.6%; peripheral oedema 11.6% | Headache 12.8%; diarrhoea 8.5%; musculoskeletal pain 11.7%; abnormal stools 7.4%; dizziness 4.3%; peripheral oedema 5.3% |
| AEs between-group comparison | Headache p < 0.05, all others non-significant | |
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Dawson 199863 | |
|---|---|
| Study details | |
| Publication type | Dawson 1998,63 full report in peer-reviewed journal |
| Additional sources of data | Cochrane review 2008,28 Uchiyama 2009,42 Otsuka Pharmaceuticals submission to NICE34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 mg (100 mg b.i.d.) |
| Comparator | Placebo |
| Run-in phase | |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, then every 4 weeks until 12 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2.0 mph (3.2 km/hour) at a constant 12.5% grade PFWD: as MWD ABPI: continuous wave Doppler ultrasound and cuff occlusion AEs: patient self-report |
| Notes on statistics | Log transform for walking distances, last observation carried forward for missing data [Otsuka submission34 states arithmetic mean used for MWD and PFWD] |
| Population | |
| Eligibility criteria | Stable symptoms of IC secondary to chronic occlusive arterial disease from atherosclerosis (symptoms present for at least 6 months and not significantly changed within the past 3 months). Clinical diagnoses of chronic occlusive arterial disease were supported with objective criteria from non-invasive vascular tests, including an PFWD on the treadmill between 30 and 200 m and a minimum post-exercise drop in Doppler-measured ankle systolic blood pressure of ≥ 20 mmHg. Exclusions: limb-threatening chronic limb ischaemia, manifested by ischaemic rest pain, ulceration or gangrene, lower-extremity surgical or endovascular arterial reconstructions or sympathectomy in the preceding 6 months, uncontrolled hypertension, inability to complete the treadmill walking test for reasons other than claudication, recent MI (within 6 months), recent deep vein thrombosis (within 3 months), severe concomitant diseases, substance abuse and gross obesity |
| Concomitant interventions allowed or excluded |
Allowed: antihypertensive agents, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, or calcium channel blockers, or the occasional use of nitroglycerin. Dosages of all concomitant medications were kept constant throughout the study when feasible. Acetaminophen and diclofenac sodium Disallowed: antiplatelet agents (including aspirin), anticoagulants, vasoactive agents (papaverine, isoxsuprine, nylidrin, cyclandelate, and niacin derivatives), haemorheological agents (pentoxifylline), and non-steroidal anti-inflammatory drugs. No specific counselling regarding smoking cessation, diet or exercise was provided |
| Power calculation | [Otsuka submission:34 powered at 90%, based on a 5% significance level (two sided, assuming > 40% difference in MWD or PFWD)] |
| N randomised to treatments included in review | 81 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 54 | 27 |
| Baseline characteristics | ||
| Age | Mean 66 years (SE 1.1 years) | Mean 67 years (SE 2.0 years) |
| Gender | M 70%; F 30% | M 89%; F 11% |
| Smokers | 40.7% | 55.6% |
| Diabetics | 25.9% | 14.8% |
| Hypertension/blood pressure | ||
| Hyperlipidaemia | ||
| Obesity or weight | Weight mean 79.1 (SE 2.3) kg | Weight mean 84.3 (SE 2.9) kg |
| Angina | ||
| History of vascular therapy | ||
| Other |
Duration of symptomatic chronic arterial occlusive disease mean years 6.8 (SE 0.82) Current alcohol use 35.2% |
Duration of symptomatic chronic arterial occlusive disease mean years 5.7 (SE 0.83) Current alcohol use 55.6% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | Total 18.5%, n = 10. Five adverse drug reaction, two marked deterioration in clinical status, two ineligible for study, one laboratory abnormalities | Total 18.5%, n = 5. One adverse drug reaction, one marked deterioration in clinical status, one ineligible for study, two other reasons |
| Results | ||
| MWD n in analysis | 52 | 25 |
| MWD baseline | Mean 141.9 (SE 21.0) m | Mean 168.6 (SE 33.1) m |
| MWD follow-up | 231.7 (SE 36.9) | 152.1 (SE 23.9) |
| MWD change | Change from baseline least mean squares 88.9 (SE 22.7). Per cent change from baseline by geometric means 30.5%; difference 89.8 [Robless 2008:28 84.6] [Otsuka submission34 has arithmetic mean change (per cent change) 88.9 (60%), geometric mean per cent change 30.5%] | Change from baseline least mean squares –16.9 (SE 32.6). Per cent change from baseline by geometric means –9.3%; difference –16.5% [Robless 2008:28 4.56] [Otsuka submission34 has arithmetic mean change (per cent change) 168.6 (–16.9%), geometric mean per cent change –9.3%] |
| MWD between-group comparison | p = 0.002. Per cent change from baseline by geometric means p < 0.01 (at follow-ups prior to week 12 non-significant) | |
| PFWD n in analysis | 52 | 25 |
| PFWD baseline | Mean 71.2 (SE 6.0) m | Mean 77.7 (SE 8.4) m |
| PFWD follow-up | 112.5 (SE 13.8) | 84.6 (SE 13.7) |
| PFWD change | Change from baseline least mean squares 42.6 (SE 8.2). Per cent change from baseline by geometric means 31.7%; difference 41.3 [Robless 2008:28 38.9] [Otsuka submission34 has arithmetic mean change per cent change) 42.6 (55%), geometric mean per cent change 31.7%] | Change from baseline least mean squares 3.5 (SE 11.7). Per cent change from baseline by geometric means –2.5%; difference 6.9 [Robless 2008:28 8.3] [Otsuka submission34 has arithmetic mean change (per cent change) 3.5 (11%), geometric mean –2.5%] |
| PFWD between-group comparison | p = 0.007. Per cent change from baseline by geometric means p < 0.01 | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | There was no significant change in resting or post-exercise ABPI | |
| Vascular events n in analysis | 54 | 27 |
| Vascular events follow-up | 12 weeks | |
| Vascular events included | NR | |
| Vascular events reported | One stenosis, one MI, one angina, one TIA (also in AEs) [Uchiyama 2008:42 two coronary vascular events, 3.7%; one serious bleeding, 1.9%] | One death from MI (also in AEs) [Uchiyama 2008:42 one coronary vascular event, 3.7%; one serious bleeding, 3.7%] |
| Vascular events between-group comparison | ||
| AEs n in analysis | ||
| AEs follow-up | ||
| AEs included | (The US Food and Drug Administration defines a SAE as an occurrence that is fatal, life-threatening, disabling, or requires hospitalisation; or a drug overdose, congenital anomaly, or cancer) | |
| AEs reported |
SAEs: n = 6 hospitalisations of cilostazol-treated patients [subclavian artery stenosis, unstable angina, pneumonia (n = 2), MI, and TIA] Non-SAEs: 44% gastrointestinal complaints, headaches 20% |
SAEs: n = 1 death from MI in the placebo group Non-SAEs: 15% gastrointestinal complaints, headaches 15% |
| AEs between-group comparison | ||
| Mortality reported | One death from MI | |
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Money 199862 | |
|---|---|
| Study details | |
| Publication type | Money 1998,62 full report in peer-reviewed journal |
| Additional sources of data | Cochrane review 2008,28 Uchiyama 2009,42 Otsuka Pharmaceuticals submission to NICE34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | NR, but one of the centres was Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 (100 b.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | 2-week screening, non-placebo |
| Treatment duration | 16 weeks |
| Outcome(s) | |
| Follow-up | Baseline, then every 4 weeks until 16 weeks |
| Outcomes and measures |
MWD: graded test, 2.0 mph (3.2 km/hour), at 0% grade with a 3.5% increase in grade every 3 minutes PFWD: as MWD ABPI: Doppler HRQoL: SF-36, WIQ |
| Notes on statistics | Log transform for walking distances, last observation carried forward [Otsuka submission34 uses arithmetic mean and geometric mean comparison for MWD and PFWD] |
| Population | |
| Eligibility criteria |
More than 40 years of age, PAD for at least 6 months with no change in symptoms in the previous 3 months. Diagnosis of PAD verified by a Doppler-measured ABPI of ≤ 0.90 after 10 minutes of rest and by a reduction in the blood pressure of at least one ankle artery by a minimum of 10 mmHg when measured 1 minute after claudication-limiting treadmill testing, or a decrease of at least one ankle artery blood pressure by a minimum of 20 mmHg when measured 1 minute after treadmill testing. Baseline initial claudication distance (PFWD) of at least 54 m (corresponding to 1 minute on the treadmill), a reproducible absolute claudication distance (MWD) (variance no greater than 20% between the two screening visits), and a maximum allowable absolute claudication distance of 805 m (corresponding to 15 minutes). Exclusion limb-threatening PAD, including gangrene or ischaemic rest pain; surgical or endovascular procedures in the preceding 3 months; gross obesity; hypertension, > 200 systolic or > 100 diastolic (mmHg); current malignancy (except basal cell carcinoma or in situ carcinoma); Buerger’s disease or deep venous thrombosis in the previous 3 months; inability to complete treadmill testing for reasons unrelated to IC; or bleeding problems |
| Concomitant interventions allowed or excluded | Disallowed: warfarin, heparin and pentoxifylline, and antiplatelet agents, such as aspirin, persantine, ticlopidine, and non-steroidal anti-inflammatory agents |
| Power calculation | Powered at 80%, based on a 5% significance level (two-sided) |
| N randomised to treatments included in review | 239 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 119 | 120 |
| Baseline characteristics | ||
| Age | Mean 64.8 years (SD 9.4 years) | Mean 64.5 years (SD 8.8 years) |
| Gender | M 75.6%; F 24.4% | M 75.0%; F 25.0% |
| Smokers | 36.1% | 40.0% |
| Diabetics | 25.2% | 30.8% |
| Hypertension/blood pressure | ||
| Hyperlipidaemia | ||
| Obesity or weight | Weight mean 82.5 (SD 16.6) kg, range 42–130 kg | Weight mean 79.6 (SD 14.9) kg, range 49–127 kg |
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up | (104 completed study) n = 2 discontinued due to headaches, n = 1 discontinued due to dizziness. 15 withdrawals, 12 of which for AEs | (108 completed study) n = 1 discontinued due to headaches. 12 withdrawals, 10 of which for AEs |
| Results | ||
| MWD n in analysis | 119 | 120 |
| MWD baseline | Mean trough 236.9 (SE 13.6) m; peak 211.4 (SE 12.4) m | Mean trough 244.3 (SE 13.7) m; peak 219.3 (SE 12.9) m |
| MWD follow-up | Trough 332.6 (SE 20.0) m; peak 306.9 (SE 19.1) m [at 12 weeks trough 313.4 (SE 19.9) m] | Trough 281.1 (SE 19.2) m; peak 267.5 (SE 18.5) m [at 12 weeks trough 279.2 (SE 18.3) m] |
| MWD change | At 16 weeks mean 96.4 m, p < 0.05 [Robless 2008:28 101.1] [Otsuka submission34 has arithmetic mean change (per cent change), trough 96.4 m (47.4%), peak 96.2 m (56.1%)] | At 16 weeks mean 31.4 m, p < 0.05; [Robless 2008:28 47.1] [Otsuka submission:34 has arithmetic mean change (per cent change), trough 31.4 m (12.9%), peak 44.4 m (25.4%)] |
| MWD between-group comparison | Difference between cilostazol and placebo, by geometric mean per cent change at 16 weeks, trough 32%, peak 27%, p < 0.05 (at 12 weeks trough 21%, p < 0.05 between groups). (The small subgroup size precluded the derivation of inferential statistics.) [Otsuka submission34 has arithmetic mean change trough p = 0.0001 and peak p = 0.0003; ratio of geometric mean trough 1.29, p = 0.0001, peak 1.21, p = 0.0005] | |
| PFWD n in analysis | 119 | 120 |
| PFWD baseline | [Otsuka submission:34 arithmetic mean trough 130.4, peak 118.5] | [Otsuka submission:34 arithmetic mean trough 138.7, peak 129.9] |
| PFWD follow-up | ||
| PFWD change | [Robless 2008:28 85.9] [Otsuka submission:34 arithmetic mean change per cent change) trough 76.8 (68.3%), peak 80.7 (87.1%)] | [Robless 2008:28 has 54.2] [Otsuka submission:34 arithmetic mean change (per cent change) trough 47.6 (38.5%), peak 53.1 (49.7%)] |
| PFWD between-group comparison | Difference between cilostazol and placebo, by geometric mean per cent change, at 16 weeks, 27% trough, 32% peak, p < 0.05 [Otsuka submission34 has arithmetic mean change trough, p = 0.0019, peak p = 0.0035, ratio of geometric mean trough 1.2, p = 0.0049, peak 1.2, p = 0.0074] | |
| ABPI n in analysis | Unclear | Unclear |
| ABPI baseline | Mean 0.64 (SD 0.02) | Mean 0.68 (SD 0.02) |
| ABPI follow-up | 0.70 (0.02) | 0.69 (0.02) |
| ABPI change | 9% increase [70/64 = 1.09375] [difference mean 0.06] | [69/68 = 1.01470, so 1% increase] [difference mean 0.01] |
| ABPI between-group comparison | p = 0.0125 | |
| Vascular events n in analysis | 119 | 120 |
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | One patient died of MI 6 days after stopping cilostazol [Uchiyama 2008:42 one coronary vascular events, 0.8%; no cerebral vascular events; no serious bleeding] | [Uchiyama 2008:42 one coronary vascular events, 0.8%; no cerebral vascular events; no serious bleeding] |
| Vascular events between-group comparison | ||
| AEs n in analysis | 119 | 120 |
| AEs follow-up | ||
| AEs included | ||
| AEs reported | Headaches (30.3%), abnormal stools (16.0%), diarrhoea (12.6%) and dizziness (12.6%). SAEs 11.8% (n = 13) | Headaches (9.2%), abnormal stools (5.0%), diarrhoea (6.7%) and dizziness (5.0%). SAEs 9.2% (n = 11) |
| AEs between-group comparison | ||
| Mortality reported | One patient died of MI 6 days after stopping cilostazol | One patient died while on placebo |
| Mortality between-group comparison | ||
| HRQoL n in analysis | Unclear | Unclear |
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | SF-36 physical component scale score increased by 2.99 points. WIQ improved 20% | |
| HRQoL between-group comparison | SF-36: cilostazol improved vs placebo physical component scale score, p = 0.0059. Bodily pain (p = 0.0772), general health (p = 0.436), and role–physical (p = 0.061). Non-significant for mental components. WIQ significantly better for cilostazol, p = 0.0331 [Otsuka submission:34 physical function score p = 0.0024, WIQ significant improvements in walking speed and specific measures of walking difficulty] | |
| CASTLE, Hiatt 200749,50/Stone 200848 | |
|---|---|
| Study details | |
| Publication type | Stone 2008,48 Hiatt 2008 (RM22),49 Hiatt 2007 (RM 2195).50 Full reports in peer-reviewed journals |
| Additional sources of data | |
| Trial design | RCT, Phase IV (post-marketing), multicentre |
| Country | USA |
| Dates of participant recruitment | Up to November 2004 |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 (100 b.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | 30 days, single blind |
| Treatment duration | Up to 36 months |
| Outcome(s) | |
| Follow-up | Every 26 weeks up to 3 years |
| Outcomes and measures | AEs: mortality, cardiovascular deaths. Categorisation of the event by the study sponsor according to standard definitions from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines. All AEs were recorded when patients were on treatment through 14 days after discontinuation of treatment. Non-fatal AEs were not monitored after drug discontinuation. Serious adverse bleeding events were defined as haemorrhages that were fatal, life-threatening, required or prolonged hospitalisation, caused significant disability or were medically significant in the judgement of the site investigator |
| Notes on statistics | Given the high discontinuation rate of the study medication and that most deaths occurred 30 days after discontinuation of study drug, the committee determined that the original ITT analysis would not provide a full assessment of cilostazol safety or risk. Therefore, the committee used a primary analysis based on deaths that occurred while patients were taking the study medication plus a 30-day period designed to capture deaths that might have resulted from exposure to the study medication; hereafter, this is regarded as the ‘on-treatment’ period. The original, prospectively defined ITT population was also evaluated and defined as all randomised patients who received at least one dose of study medication. Also tabulated were deaths occurring in the ITT population during the entire study period, including those 30 days after study medication discontinuation |
| Population | |
| Eligibility criteria | Aged at least 17 years with a history of IC secondary to PAD as diagnosed by a physician (specific ABPI criteria for inclusion were not defined). Exclusion criteria included women who were pregnant or breastfeeding, patients currently or previously using of cilostazol, use of an investigational drug in the past 30 days, consumption of grapefruit juice, or patients found to be non-compliant during the 30-day single-blind, run-in phase. Patients with current CHF of any severity, as assessed by the site investigator, were excluded, but those with a history of heart failure who had recovered were eligible for enrolment. Subjects who failed to comply with at least 70% of placebo run-in prescribed regimen were withdrawn from the study |
| Concomitant interventions allowed or excluded | Allowed: patients taking aspirin, clopidogrel, pentoxifylline, or anticoagulants were eligible for participation |
| Power calculation | By 34 months after the first patient was randomised, less than half of the projected number of deaths had occurred and the discontinuation rate from study drug was high, which led to study termination in November 2004, as already described. As a result, the study was underpowered to meet its primary end point, but inferences with respect to cilostazol effects on mortality could be described by the 95% CI of the HR |
| N randomised to treatments included in review | 1435 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 717 | 718 |
| Baseline characteristics | ||
| Age | Mean 66.5 years (SD 10.2 years) | Mean 65.9 years (SD 10.5 years) |
| Gender | M 65.6% | M 65.5% |
| Smokers | 28.6% | 31.3% |
| Diabetics | 37.8% | 33.7% |
| Hypertension/blood pressure | 82.4% | 81.1% |
| Hyperlipidaemia | (Hypercholesterolaemia 82.0%) | (Hypercholesterolaemia 78.0%) |
| Obesity or weight | Weight mean 84.6 (SD 19.5) kg | Weight mean 84.6 (SD 18.8) kg |
| Angina | ||
| History of vascular therapy | ||
| Other | MI 29.3%; stroke 10.3%; CHF 4.7% | MI 29.8%; stroke 10.6%; CHF 4.9% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | Probability of discontinuation from the study was 68% in the cilostazol group | Probability of discontinuation from the study was 64% in the placebo group |
| Results | ||
| MWD n in analysis | ||
| MWD baseline | ||
| MWD follow-up | ||
| MWD change | ||
| MWD between-group comparison | ||
| PFWD n in analysis | ||
| PFWD baseline | ||
| PFWD follow-up | ||
| PFWD change | ||
| PFWD between-group comparison | ||
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 717 | 718 |
| Vascular events follow-up | Up to 144 weeks | |
| Vascular events included | ||
| Vascular events reported | ITT cardiovascular mortality n = 28; event rate per person-year 1.89. On-treatment analysis n = 14, event rate per person-year 1.34 [Uchiyama 2008:42 126 coronary vascular events, 17.6%; 18 cerebral vascular events 2.5%; 18 serious bleeding, 2.5%] | ITT cardiovascular mortality n = 33; event rate per person-year 2.22. On-treatment analysis n = 14, event rate per person-year 1.28 [Uchiyama 2008:42 132 coronary vascular events, 18.4%; 34 cerebral vascular events 4.7%; 22 serious bleeding, 3.1%] |
| Vascular events between-group comparison | HR for cardiovascular deaths was 1.054 (95% CI 0.502 to 2.210; p = 0.89) in the on-treatment population and 0.852 (95% CI 0.515 to 1.410; p = 0.533) in the ITT population | |
| AEs n in analysis | 717 | 718 |
| AEs follow-up | Up to 144 weeks | |
| AEs included | ||
| AEs reported |
Minor events, n (%) Headache 75 (10.5) Palpitations 38 (5.3) Diarrhoea 78 (10.9) Bronchitis 23 (3.2). Serious events, n (%) Dyspnoea 7 (1.0) Cerebrovascular accident 7 (1.0) Carotid artery stenosis 5 (0.7) Femoral artery occlusion 3 (0.4) Cardiac arrest 2 (0.3) Events leading to discontinuation, n (%) Oedema 10 (1.4) Headache 15 (2.1) Diarrhoea 20 (2.8) Serious bleeding events 18 (2.5) |
Minor events, n (%) Headache 35 (4.9) Palpitations 18 (2.5) Diarrhoea 48 (6.7) Bronchitis 37 (5.2) Serious events, n (%) Dyspnoea 3 (0.4) Cerebrovascular accident 15 (2.1) Carotid artery stenosis 11 (1.5) Femoral artery occlusion 7 (1.0) Cardiac arrest 7 (1.0) Events leading to discontinuation, n (%) Oedema 0 (0) Headache 2 (0.3) Diarrhoea 5 (0.7) Serious bleeding events 22 (3.1) |
| AEs between-group comparison | ||
| Mortality reported | ITT all-cause mortality n = 49; event rate per 100 person-years 3.31. On-treatment analysis n = 18, event rate per person-year 1.72 | On-treatment analysis mortality HR of 0.99 (95% CI 0.52 to 1.88, p = 0.97). ITT all-cause mortality HR for cilostazol compared with placebo was 0.94 (95% CI 0.64 to 1.39, p = 0.77) |
| Mortality between-group comparison | On-treatment analysis mortality HR of 0.99 (95% CI 0.52 to 1.88, p = 0.97). ITT all-cause mortality HR for cilostazol compared with placebo was 0.94 (95% CI 0.64 to 1.39, p = 0.77) | |
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| O’Donnell 200951 | |
|---|---|
| Study details | |
| Publication type | O’Donnell 2009,51 full report in peer-reviewed journal |
| Additional sources of data | O’Donnell 200983 (non-diabetic subgroup), O’Donnell 200855 (diabetic subgroup), O’Donnell 2009,54 O’Donnell 2009 (RM2126)53 (diabetic subgroup) |
| Trial design | RCT, single centre |
| Country | Northern Ireland |
| Dates of participant recruitment | 2004–6 |
| Sources of funding | Funded by the Belfast City Hospital Vascular Research Fund and the Daisy Hill Hospital research fellowships and research grants from the Insulin Dependant Diabetes Trust and the Royal College of Surgeons, Edinburgh. Otsuka Pharmaceuticals provided the placebo for the study and have supported the corresponding author in presenting the results at research conferences |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 (100 b.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | No, but two baseline assessments 4 weeks apart |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 6 and 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 3.2 km/hour (2 mph) 10% gradient PFWD: as MWD AEs: patient self-report HRQoL: SF-36, VascuQoL |
| Notes on statistics | [Otsuka submission:34 The Mann–Whitney U-test was used for between-group differences. The Wilcoxon signed-rank test was used for within-group differences. All statistics were two sided and a p-value of < 0.05 was considered significant] |
| Population | |
| Eligibility criteria |
Male and female (non-pregnant) patients between the ages of 30 and 90 years, IC defined as reproducible muscle discomfort in the lower limb produced by exercise and relieved by rest, with an ABPI of < 0.9, which had been stable on optimal medical therapy that included antiplatelet and lipid-lowering medication, cardiovascular risk assessment and treatment (e.g. hypertension) and smoking cessation therapy combined with the provision of exercise advice for a period of 3 months Exclusions current or previous acute or critical limb ischaemia, severe claudication that prohibited the use of treadmill testing as determined during pre-recruitment vascular assessments, an endovascular or surgical procedure within the preceding 6 months or a non-atherosclerotic comorbidity that had limited their walking before the onset of claudication pain, predisposition to bleeding, a history of uncontrolled cardiac, respiratory, renal or liver disease |
| Concomitant interventions allowed or excluded |
Allowed: aspirin, clopidogrel, warfarin, statin, ACE inhibitors, ACE II antagonists, beta-blocker, calcium antagonist diuretic Disallowed: omeprazole and diltiazem |
| Power calculation | 30 patients per treatment group completing the trial would have a 90% power to detect a statistically significant (p < 0.05, two-tailed) difference in the change in MWD, between groups, of a magnitude of 45 m. assumed that approximately 20% of patients would withdraw from the study, a total of 144 patients were required |
| N randomised to treatments included in review | 106 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 51 | 55 |
| Baseline characteristics | ||
| Age | Median 64.2 (range 37–86) years | Median 66.1 (range 39–80) years |
| Gender | M 67% | M 71% |
| Smokers | 45% | 55% |
| Diabetics | 23.5% | 25.5% |
| Hypertension/blood pressure | 62.7% | 67.3% |
| Hyperlipidaemia | Hypercholesterolaemia 76.5% | Hypercholesterolaemia 76.4% |
| Obesity or weight | ||
| Angina | 13.7 | 5.5 |
| History of vascular therapy | CABG 5.9%, carotid endarterectomy 3.9%, vascular arterial bypass/endovascular intervention 7.8% | CABG 9.1%, carotid endarterectomy 5.5%, vascular arterial bypass/endovascular intervention 10.9% |
| Other | MI 17.6%, CVA 5.9%, abdominal aortic aneurysm 0% | MI 12.7%, CVA 5.5%, abdominal aortic aneurysm 1.8% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | n = 8 (15.7%) owing to side effects n = 6 [six non-diabetics withdrew, four due to AEs] [Otsuka submission34 one withdrew due to non-compliance, six due to AEs, one due to other reasons] | n = 7 (12.7%) owing to side effects n = 2 [three non-diabetics withdrew] [Otsuka submission34 two withdrew due to non-compliance, two due to AEs, three due to other reasons] |
| Results | ||
| MWD n in analysis | 51 | 55 |
| MWD baseline | Median 144.4 (IQR 99.7 to 204.3) m; non-diabetics median 144.4 m, diabetics 118.5 m | Median 138.6 (IQR 101.7 to 193.8) m; non-diabetics median 138.6 m, diabetics 115.6 m |
| MWD follow-up | Non-diabetics median 286.1 m at 24 weeks, diabetics 158.3 m | Non-diabetics median 227.1 m at 24 weeks, diabetics 157.8 m |
| MWD change | 161.7% mean change, non-diabetics median 173.1% change, diabetics 143.1% | 79.0% mean change, non-diabetics median 92.1% change, diabetics 23.2% |
| MWD between-group comparison | p = 0.048: non-diabetics non-significant, p = 0.27; diabetics non-significant, p = 0.086 | |
| PFWD n in analysis | 51 | 55 |
| PFWD baseline | Median 69.7 (IQR 50.1 to 94.8) m; non-diabetics median 69.7 m, diabetics 69.3 m | Median 63.9 (IQR 45.2 to 85.8) m; non-diabetics median 63.5 m, diabetics 66.2 m |
| PFWD follow-up | Non-diabetics median 82.7 m at 24 weeks, diabetics 82.3 m | Non-diabetics median 85.0 m at 24 weeks, diabetics 55.9 m |
| PFWD change | 67% mean change, non-diabetics median 84.8% change, diabetics 21.1% | 51.6% mean change, non-diabetics median 66.5% change, diabetics –4.4% change |
| PFWD between-group comparison | p = 0.63 non-significant: non-diabetics non-significant, p = 0.63; diabetics non-significant, p = 0.14 | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | [O’Donnell 200983 diabetic subgroup 12] | [O’Donnell 200983 diabetic subgroup 14] |
| AEs follow-up | 24 weeks | |
| AEs reported | [O’Donnell 2009:83 diabetics 14 side effects (12 within first 6 weeks), this is number of events rather than number of patients with an event, events were headache, diarrhoea or palpitations] | [O’Donnell 2009:83 diabetics seven side effects (all within first 6 weeks), this is number of events rather than number of patients with an event, events were headache, diarrhoea or palpitations] |
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | (O’Donnell 2009:83 non-diabetics 39) | (O’Donnell 2009:83 non-diabetics 41) |
| HRQoL baseline | ||
| HRQoL follow-up |
Mean (SE) SF-36 (%): Physical function 11.0 (4.5) Role–physical 7.8 (4.3) Body pain 3.7 (3.3) General health 2.7 (3.5) PCS 11.4 (3.2) Total 1.8 (3.2) VascuQol activity 7.3 (4.6) Symptom 3.1 (3.0) Pain 10.4 (5.1) Emotion 5.7 (4.1) Social 1.1 (5.9) Total 5.5 (3.5) Diabetics [O’Donnell 2009 53 ]: at 24 weeks median (IQR) SF-36 (%): Physical function 38.1 (29.7 to 41.3) Role–physical 34.8 (28.7 to 43.4) Body pain 46.1 (33.2 to 50.8) General health 42.4 (31.7 to 45.8) Total 42.5 (34.8 to 46.2) VascuQol activity 3.9 (3.4 to 5.0) Symptom 5.5 (5.4 to 6.1) Pain 5.0 (4.4 to 5.6) Emotion 5.6 (4.5 to 6.6) Social 5.0 (4.5 to 6.5) Total 5.2 (4.3 to 5.6) |
Mean (SE) SF-36 (%): Physical function –0.3 (3.1) Role–physical 5.4 (3.9) Body pain 10.5 (3.5) General health –1.0 (2.5) PCS 5.1 (3.4) Total 1.4 (1.7) VascuQol activity 1.8 (2.9) Symptom 3.2 (2.6) Pain 13.2 (4.3) Emotion 1.8 (4.0) Social 3.4 (5.2) Total 3.0 (2.1) Diabetics [O’Donnell 2009 53 ]: at 24 weeks median (IQR) SF-36 (%): Physical function 27.6 (24.5 to 40.2) Role–physical 37.3 (25.0 to 45.9) Body pain 37.2 (33.0 to 43.8) General health 41.0 (38.2 to 47.0) Total 37.8 (31.2 to 46.3) VascuQol activity 4.4 (2.8 to 4.7) Symptom 5.3 (3.9 to 5.4) Pain 4.3 (3.4 to 4.8) Emotion 3.7 (3.0 to 5.0) Social 4.0 (3.5 to 5.0) Total 4.3 (3.2 to 4.9) |
| HRQoL change | ||
| HRQoL between-group comparison |
Non-diabetics at 24 weeks mean (SE) SF-36 (%): Physical function p = 0.013 significantly more improvement for cilostazol Role physical p = 0.62 Body pain p = 0.21 General health p = 0.48 PCS p = 0.044 significantly more improvement for cilostazol Total p = 0.50 VascuQol activity p = 0.34 Symptom p = 0.34 Pain p = 0.89 Emotion p = 0.63 Social p = 0.67 Total p = 0.78 WIQ – non-significant between-groups distance p = 0.41, speed p = 0.88 (even though cilostazol group had significantly improved and placebo group had non-significant improvement) Diabetics [RM2126] at 24 weeks SF-36 (%): Physical function p = 0.42 Role–physical p = 0.72 Body pain p = 0.31 General health p = 0.93 Total p = 0.40 VascuQol activity p = 0.59 Symptom p = 0.025 (significantly more increase for placebo, cilostazol more improved) Pain p = 0.08 Emotion p = 0.013 (significantly more increase for cilostazol, cilostazol more improved) Social p = 0.06 Total p = 0.05 (significantly more increase for cilostazol, cilostazol more improved) |
|
| Otsuka 21-95-20134 | |
|---|---|
| Study details | |
| Publication type | Thompson 2002,35 systematic review in peer-reviewed journal |
| Additional sources of data | Cochrane review 2008,28 Uchiyama 2009,42 Otsuka Pharmaceuticals submission to NICE34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 (100 b.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | No, but there was a screening phase |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, then every 4 weeks until 12 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2.0 mph (3.2 km/hour) at a constant 12.5% grade PFWD: as MWD Vascular events: unclear HRQoL: [Otsuka submission:34 SF-36, WIQ] |
| Notes on statistics | |
| Population | |
| Eligibility criteria | Age ≥ 40 years; stable, PAD induced IC of at least 6 months’ duration; no significant change in symptom severity for at least 3 months; diagnosis of PAD required Doppler measurement of an ABPI ≤ 0.90; MWD on two consecutive prerandomisation treadmill tests varied by < 20%. Excluded if rest pain: Buerger’s disease; ischaemic tissue necrosis; surgical or endovascular procedures within 3 months; unstable coronary artery disease or a coronary intervention within 6 months; deep vein thrombosis within 3 months; symptomatic cardiac arrhythmias; conditions other than claudication that limited exercise capacity, or other medical conditions likely to preclude completing the study; women of childbearing age not using a reliable birth control method |
| Concomitant interventions allowed or excluded |
Allowed: [Otsuka submission:34 paracetamol] Disallowed: patients receiving anticoagulants or using > 81 mg/day of aspirin or > 1200 mg/day of ibuprofen. No specific counselling regarding smoking cessation, diet, or exercise was given |
| Power calculation | [Otsuka submission:34 based on results from a previous study, 60 patients per group was calculated to provide > 90% power on the log and the raw scale, based on a 5% (two-sided) significance level] |
| N randomised to treatments included in review | 142 |
| Treatment group | Cilostazol 100 mg b.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 72 | 70 |
| Baseline characteristics | ||
| Age | [Robless 2008:28 mean age 68 years] [Otsuka submission34 has mean age 67.6 years (SD 8.8 years)] | [Robless 2008:28 mean age 66 years] [Otsuka submission34 has mean age 65.6 years (SD 7.4 years)] |
| Gender | [Robless 2008:28 M 75%; F 25%] | [Robless 2008:28 M 81%; F 19%] |
| Smokers | [Otsuka submission34 has 38.1%] | [Otsuka submission34 has 38.6%] |
| Diabetics | [Otsuka submission34 has 30.6%] | [Otsuka submission34 has 34.3%] |
| Hypertension/blood pressure | ||
| Hyperlipidaemia | ||
| Obesity or weight | [Otsuka submission34 has weight 78.8 (SD 15.7) kg] | [Otsuka submission34 has weight 84.3 (SD 16.8) kg] |
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up | [Otsuka submission34 has 17 withdrawals: failed screening, one; marked deterioration, one; AE, 14; other, one] | [Otsuka submission34 has eight withdrawals: lack of response, one; AE, six; other, one] |
| Results | ||
| MWD n in analysis | [Otsuka submission34 has 60] | [Otsuka submission34 has 66] |
| MWD baseline | [Otsuka submission34 has mean 121.9] | [Otsuka submission34 has mean 123.4] |
| MWD follow-up | ||
| MWD change | Approximately 28% (estimated from figure 1, Thompson 200235) [Robless 2008:28 mean 35.2 (SD 72.05)] [Otsuka submission34 has arithmetic mean change 37.5 (59.4%)] | Approximately 30% (estimated from figure 1, Thompson 200235) [Robless 2008:28 mean 38.1 (SD 69.7)] [Otsuka submission34 has arithmetic mean change 33.9 (59.6%)] |
| MWD between-group comparison | Non-significant [Otsuka submission34 has 0.8585 ratio of geometric mean change 1.02 (CI 0.88 to 1.18), p = 0.7925] | |
| PFWD n in analysis | [Otsuka submission34 has 60] | [Otsuka submission34 has 66] |
| PFWD baseline | [Otsuka submission34 has mean 65.7] | [Otsuka submission34 has mean 67.4] |
| PFWD follow-up | ||
| PFWD change | Approximately 58% (estimated from figure 2, Thompson 200235) [Robless 2008:28 mean 41.4 (SD 63.2)], [Otsuka submission34 has arithmetic mean change 37.5 (59.4%)] | Approximately 52% (estimated from figure 2, Thompson 200235) [Robless 2008:28 mean 34.4 (SD 57.3)], [Otsuka submission34 has arithmetic mean change 33.9 (59.6%)] |
| PFWD between-group comparison | Non-significant [Otsuka submission34 has 0.4818 ratio of geometric mean change 1.18 (CI 1.02 to 1.37), p = 0.0309] | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 145 (including 150-mg b.i.d. group, which was excluded from other analyses) | 70 |
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | [Uchiyama 2008:42 three coronary vascular events, 2.1%; no cerebral vascular events; no serious bleeding] | [Uchiyama 2008:42 one coronary vascular events, 1.4%; one cerebral vascular events 1.4%; no serious bleeding] |
| Vascular events between-group comparison | ||
| AEs n in analysis | ||
| AEs follow-up | ||
| AEs reported | ||
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | [Otsuka submission34 has SF-36 positive trend in favour of cilostazol with regards to role–physical scores. WIQ showed a trend towards improvement with respect to walking difficulty secondary to pain] | |
Three-arm trials of cilostazol, pentoxifylline and placebo
| Dawson 200058 | |
|---|---|
| Study details | |
| Publication type | Dawson 2000,58 full report in peer-reviewed journal |
| Additional sources of data | Cochrane review 2008,28 Uchiyama 200942 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups |
Cilostazol 200 mg (100 mg b.i.d.) plus placebo Pentoxifylline 1200 mg daily dose (400 mg t.i.d.) plus placebo |
| Comparator | Placebo |
| Run-in phase | No, but 2- to 3-week baseline assessment period |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 2 weeks, 4 weeks, then every 4 weeks until 24 weeks |
| Outcomes and measures |
MWD: treadmill with graded test, 2.0 mph (3.2 km/hour), at 0% gradient with a 3.5% increase in gradient every 3 minutes PFWD: as MWD ABPI: Doppler AEs: patient self-report HRQoL: SF-36, WIQ |
| Notes on statistics | Geometric mean change in MWD was determined. This change was expressed as a log of the quotient of the post-treatment MWD divided by the baseline MWD value |
| Population | |
| Eligibility criteria | Stable, PAD-induced IC of at least 6 months’ duration; no significant change in symptom severity for at least 3 months; diagnosis of PAD required Doppler measurement of an ABPI ≤ 0.90; MWD on two consecutive pre-randomisation treadmill tests varied by < 20%; baseline PFWD more than or equal to 53.6 m; MWD ≤ 537.6 m. Excluded if rest pain; Buerger’s disease; lower extremity arterial reconstruction (surgical or endovascular) or sympathectomy within the previous 3 months, exercise capacity limited by conditions other than IC |
| Concomitant interventions allowed or excluded |
Allowed: aspirin at a dose of no more than 81 mg per day, up to 1200 mg per day of ibuprofen Disallowed: anticoagulants or other antiplatelet agents, non-steroidal anti-inflammatory drugs |
| Power calculation | Two hundred patients per treatment group would provide > 95% power at a 5% significance level to detect a difference between cilostazol and pentoxifylline, based on these values and a SD of 68% |
| N randomised to treatments included in review | 698 |
| Treatment group | Cilostazol 100 mg b.i.d. | Pentoxifylline 400 mg t.i.d. | Placebo |
|---|---|---|---|
| N randomised to treatment | 227 | 232 | 239 |
| Baseline characteristics | |||
| Age | Mean 66 years (SD 9 years) | Mean 66 years (SD 9 years) | Mean 66 years (SD 9 years) |
| Gender | M 76% | M 78% | M 74% |
| Smokers | 41% | 33% | 38% |
| Diabetics | 32% | 28% | 31% |
| Hypertension/blood pressure | 73% | 69% | 72% |
| Hyperlipidaemia | Hypercholesterolaemia 65% | Hypercholesterolaemia 67% | Hypercholesterolaemia 67% |
| Obesity or weight | Weight 81 (SD 16) kg | Weight 82 (SD 15) kg | Weight 81 (SD 15) kg |
| Angina | |||
| History of vascular therapy | |||
| Other | |||
| Withdrawals | |||
| Withdrawals/loss to follow-up | n = 39 (no significant differences in the baseline demographic or clinical features of patients who withdrew from the study before completion compared with those who completed the study) due to AEs 16% | n = 40 due to AEs 19% | n = 25 due to AEs 9% |
| Results | |||
| MWD n in analysis | 205 | 212 | 226 |
| MWD baseline | Mean 241 (SD 123) m | Mean 238 (SD 119) m | Mean 234 (SD 119) m |
| MWD follow-up | Mean 350 (SD 209) m | Mean 308 (SD 183) m | Mean 300 (SD 180) m |
| MWD change | Mean 107 (SD 158) m [Robless 2008:28 107.36 (158.4) m] | Mean 64 (SD 127) m [Robless 2008:28 64.7 (134.61) m] | Mean 65 (SD 135) m [Robless 2008:28 64.4 (126.6) m] |
| MWD between-group comparison | Cilostazol vs placebo p = 0.0005; pentoxifylline vs placebo 0.82; cilostazol vs pentoxifylline p = 0.0002 | ||
| PFWD n in analysis | 205 | 212 | 226 |
| PFWD baseline | Mean 124 (SD 81) m | Mean 126 (SD 79) m | Mean 122 (SD 69) m |
| PFWD follow-up | Mean 218 (SD 149) m | Mean 202 (SD 139) m | Mean 180 (SD 115) m |
| PFWD change | Mean 94 (SD 127) m [Robless 2008:28 93.6 (127.4) m] | Mean 74 (SD 106) m [Robless 2008:28 56.5 (93.1) m] | Mean 57 (SD 93) m [Robless 2008:28 73.6 (93.1) m] |
| PFWD between-group comparison | Cilostazol vs placebo p = 0.0001; pentoxifylline vs placebo 0.07; cilostazol vs pentoxifylline p = 0.02 | ||
| ABPI n in analysis | 205 | 212 | 226 |
| ABPI baseline | Mean 0.66 (SD 0.18) | Mean 0.66 (SD 0.21) | Mean 0.68 (SD 0.42) |
| ABPI follow-up | Mean 0.70 (SD 0.18) | Mean 0.71 (SD 0.24) | Mean 0.67 (SD 0.19) |
| ABPI change | [Difference in means 0.04] | [Difference in means 0.05] | [Difference in means –0.01] |
| ABPI between-group comparison | Significantly more improvement in cilostazol than placebo p < 0.01. Non-significant between other groups | ||
| Vascular events n in analysis | |||
| Vascular events follow-up | |||
| Vascular events included | |||
| Vascular events reported | [Uchiyama 2008:42 two coronary vascular events, 0.9%; three cerebral vascular events 1.3%; no serious bleeding] | [Uchiyama 2008:42 two coronary vascular events, 0.8%; no cerebral vascular events; no serious bleeding] | |
| Vascular events between-group comparison | |||
| AEs n in analysis | 227 | 232 | 239 |
| AEs follow-up | |||
| AEs reported | n (%) | n (%) | n (%) |
| Patients with at least one event 201 (86) | Patients with at least one event 200 (86) | Patients with at least one event 188 (79) | |
| Headache 63 (28) | Headache 26 (11) | Headache 28 (12) | |
| Pain 30 (13) | Pain 38 (16) | Pain 33 (14) | |
| Diarrhoea 43 (19) | Diarrhoea 18 (8) | Diarrhoea 13 (5) | |
| Pharyngitis 22 (10) | Pharyngitis 32 (14) | Pharyngitis 17 (7) | |
| Peripheral vascular disorder 13 (6) | Peripheral vascular disorder 22 (10) | Peripheral vascular disorder 26 (11) | |
| Abnormal stools 33 (15) | Abnormal stools 12 (5) | Abnormal stools 7 (3) | |
| Palpitation 39 (17) | Palpitation 5 (2) | Palpitation 3 (1) | |
| SAEs 27 (12) | SAEs 31 (13) | SAEs 31 (13) | |
| AEs between-group comparison | Withdrawal due to AEs similar in cilostazol (16%) and pentoxifylline (19%), significantly less in placebo (9%). Headache, diarrhoea and abnormal stools were significantly more common in cilostazol than other groups | ||
| Mortality reported | 0.8%, n = 2 | 1%, n = 3 | 0.4%, n = 1 |
| Mortality between-group comparison | NR | ||
| HRQoL n in analysis | |||
| HRQoL baseline | |||
| HRQoL follow-up | |||
| HRQoL change | |||
| HRQoL between-group comparison | None of the treatments significantly affected the Medical Outcomes Scale Short Form-36 scores on Mental Health Concepts, General Health Perception, Physical Health Concepts or Vitality Scores. There were also no significant differences in patient-reported walking distance or speed as determined by the WIQ | ||
| Otsuka 21-94-30134 | |
|---|---|
| Study details | |
| Publication type | Thompson 2002,35 systematic review in peer-reviewed journal |
| Additional sources of data | Uchiyama 2009,42 Otsuka Pharmaceuticals submission to NICE34 |
| Trial design | RCT, multicentre |
| Country | UK |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 (100 b.i.d.) mg, pentoxifylline 1200 (400 t.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 2 weeks, 4 weeks, then every 4 weeks until 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2.0 mph (3.2 km/hour) at a constant 12.5% grade PFWD: as MWD Vascular events |
| Notes on statistics | [Otsuka submission:34 to reduce the impact of variability in walking distances, log transformation was employed. Treatment differences were assessed in the efficacy ITT population as the estimated treatment effect of cilostazol 100 mg b.i.d. vs placebo and cilostazol 100 mg b.i.d. vs pentoxifylline 400 mg t.i.d. Secondary analyses were performed for absolute claudication distance and PFWD with last visit and time point analyses using last observation carried forward, completers, and categorical analysis. Continuous efficacy measures: analysis of variance and the Wilcoxon rank-sum test. Categorical efficacy measures: van Elteren test and Cochran–Mantel–Haenszel test. For the primary and secondary efficacy analyses, values of test statistics were considered statistically significant if p < 0.025 and p < 0.05, respectively] |
| Population | |
| Eligibility criteria | Age ≥ 40 years; stable, PAD-induced IC of at least 6 months’ duration; no significant change in symptom severity for at least 3 months; diagnosis of PAD required Doppler measurement of an ABPI ≤ 0.90; MWD on two consecutive prerandomisation treadmill tests varied by < 20%. Excluded if rest pain; Buerger’s disease; ischaemic tissue necrosis; surgical or endovascular procedures within 3 months; unstable coronary artery disease or a coronary intervention within 6 months; deep vein thrombosis within 3 months; symptomatic cardiac arrhythmias; conditions other than claudication that limited exercise capacity; or other medical conditions likely to preclude completing the study; women of childbearing age not using a reliable birth control method |
| Concomitant interventions allowed or excluded |
Allowed: 81 mg/day aspirin, 1200 mg/day ibuprofen Disallowed: anticoagulants, no specific counselling regarding smoking cessation, diet or exercise was provided |
| Power calculation | [Otsuka submission:34 sample size was based on the results of previous studies of cilostazol and placebo. Estimating mean walking distances (percentage increase from baseline) as 35% for cilostazol, 25% for pentoxifylline and 15% for placebo, with a SD of about 37, it was originally estimated that 100 patients per group would provide approximately 90% power to detect the above-mentioned differences, based on a 5% two-sided significance level. Based on 100 completed patients, the actual power to detect differences is 91% for the cilostazol vs placebo comparison and is 34% for the cilostazol vs pentoxifylline comparison] |
| N randomised to treatments included in review | 370 |
| Treatment group | Cilostazol 100 mg b.i.d. | Pentoxifylline 400 mg t.i.d. | Placebo |
|---|---|---|---|
| N randomised to treatment | 123 | 123 | 124 |
| Baseline characteristics | |||
| Age | [Otsuka submission34 has mean 66 (SD 8.3) years] | [Otsuka submission34 has mean 66.4 (SD 8.2) years] | [Otsuka submission34 has mean 65.9 (SD 8.8) years] |
| Gender | [Otsuka submission34 has M 69.9%; F 30.1%] | [Otsuka submission34 has M 72.4%; F 27.6%] | [Otsuka submission34 has M 73.4%; F 26.6%] |
| Smokers | [Otsuka submission34 has 29%] | [Otsuka submission34 has 32.5%] | [Otsuka submission34 has 35.5%] |
| Diabetics | [Otsuka submission34 has12.2%] | [Otsuka submission34 has10.6%] | [Otsuka submission34 has 12.1%] |
| Hypertension/blood pressure | |||
| Hyperlipidaemia | |||
| Obesity or weight | [Otsuka submission34 has weight (n = 121) 73.9 (SD 13.6) kg] | [Otsuka submission34 has weight 73.1 kg (SD 11.7) kg] | [Otsuka submission34 has weight 72.4 (SD 11.5) kg] |
| Angina | |||
| History of vascular therapy | |||
| Other | |||
| Withdrawals | |||
| Withdrawals/loss to follow-up | [Otsuka submission34 has 34 withdrew. Non-compliance, one; marked deterioration, one; AE, 30; death, one; other, one] | [Otsuka submission34 has 37 withdrew. Non-compliance, two; marked deterioration, zero; AE, 33; death, zero; other, two] | [Otsuka submission34 has 19 withdrew. Non-compliance, two; marked deterioration, zero; AE, 14; death, one; other, two] |
| Results | |||
| MWD n in analysis | [Otsuka submission34 has n = 123] | [Otsuka submission34 has n = 118] | [Otsuka submission34 has n = 122] |
| MWD baseline | [Otsuka submission34 has mean 128.1] | [Otsuka submission34 has mean 135.4] | [Otsuka submission34 has mean 128.1] |
| MWD follow-up | |||
| MWD change | Approximately 68% (estimated from Figure 1 Thompson 200235) [Otsuka submission:34 arithmetic mean change 86.3 (54.9%)] | Approximately 65% (estimated from Figure 1 Thompson 200235) [Otsuka submission:34 arithmetic mean change 86.7 (64.0%)] | Approximately 42% (estimated from Figure 1 Thompson 200235) [Otsuka submission:34 arithmetic mean change 52.7 (46.1%)] |
| MWD between-group comparison | Non-significant. [Otsuka submission34 has arithmetic mean change, cilostazol vs pentoxifylline p = 0.4827, cilostazol vs placebo p = 0.4382, pentoxifylline vs placebo p = 0.1421; ratio of geometric mean, cilostazol vs pentoxifylline 0.99 (CI 0.88 to 1.11) p = 0.8700, cilostazol vs placebo 1.06 (CI 0.94 to 1.18) p = 0.3616, pentoxifylline vs placebo 1.07 (CI 0.95 to 1.20) p = 0.2876] | ||
| PFWD n in analysis | [Otsuka submission34 has n = 123] | [Otsuka submission34 has n = 118] | [Otsuka submission34 has n = 122] |
| PFWD baseline | [Otsuka submission34 has mean 77.7] | [Otsuka submission34 has mean 81.4] | [Otsuka submission34 has mean 74.3] |
| PFWD follow-up | |||
| PFWD change | Approximately 68% (estimated from Figure 2, Thompson 200235) [Otsuka submission34 has arithmetic mean change 52.3 (59.5%)] | Approximately 59% (estimated from Figure 2, Thompson 200235) [Otsuka submission34 has arithmetic mean change 46.6 (72.9%)] | Approximately 50% (estimated from Figure 2, Thompson 200235) [Otsuka submission34 has arithmetic mean change 36.5 (59.1%)] |
| PFWD between-group comparison | Non-significant. [Otsuka submission34 has arithmetic mean change, cilostazol vs pentoxifylline p = 0.3017, cilostazol vs placebo p = 0.8528, pentoxifylline vs placebo p = 0.2245; ratio of geometric mean, cilostazol vs pentoxifylline 0.98 (CI 0.87 to 1.11), p = 0.7217, cilostazol vs placebo 1.01 (CI 0.90 to 1.14), p = 0.0.8258, pentoxifylline vs placebo 1.04 (CI 0.92 to 1.17), p = 0.5678] | ||
| ABPI n in analysis | |||
| ABPI baseline | |||
| ABPI follow-up | |||
| ABPI change | |||
| ABPI between-group comparison | |||
| Vascular events n in analysis | 123 | 124 | |
| Vascular events follow-up | |||
| Vascular events included | |||
| Vascular events reported | [Uchiyama 2008:42 two coronary vascular events, 1.6%; two cerebral vascular events 1.6%; one serious bleeding, 0.8%] | [Uchiyama 2008:42 three coronary vascular events, 2.4%; no cerebral vascular events; no serious bleeding] | |
| Vascular events between-group comparison | |||
| AEs n in analysis | [Otsuka submission34 has n = 123] | [Otsuka submission34 has n = 123] | [Otsuka submission34 has n = 124] |
| AEs follow-up | |||
| AEs reported |
[Otsuka submission34 has one or more AEs, 116. AEs that occurred in > 10% patients: headache 47 (38.2%), abnormal stools 17 (13.8%), diarrhoea 33 (26.8%), dyspepsia 12 (9.8%), nausea 14 (11.4%) pain 10 (8.1%), pharyngitis 12 (9.8%)] |
[Otsuka submission34 has one or more AEs, 104. AEs that occurred in > 10% patients: headache 14 (11.4%), abnormal stools 7 (5.7%), diarrhoea 11 (8.9%), dyspepsia 14 (11.4%), nausea 20 (16.3%), pain 10 (8.1%), pharyngitis 14 (11.4%)] |
[Otsuka submission34 has one or more AEs, 103. AEs that occurred in > 10% patients: headache 19 (15.3%), abnormal stools 3 (2.4%), diarrhoea 8 (6.5%), dyspepsia 11 (8.9%), nausea 14 (11.3%), pain 18 (14.5%), pharyngitis 6 (4.8%)] |
| AEs between-group comparison | There was a greater number of withdrawals due to AEs in the two active treatment groups than in the placebo group (p = 0.0061) | ||
| Mortality reported | 1 | 0 | 1 |
| Mortality between-group comparison | |||
| HRQoL n in analysis | |||
| HRQoL baseline | |||
| HRQoL follow-up | |||
| HRQoL change | |||
| HRQoL between-group comparison | |||
| Otsuka 21-98-21334 | |
|---|---|
| Study details | |
| Publication type | Pande 2010,31 systematic review in peer-reviewed journal |
| Additional sources of data | Otsuka industry submission34 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Otsuka America Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups |
|
| Comparator | Placebo |
| Run-in phase | NR |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, every 4 weeks until 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2.0 mph (3.2 km/hour) at a constant 12.5% grade PFWD: as MWD Vascular events: AEs: patient self-report Mortality: HRQoL: SF-36, WIQ, COM |
| Notes on statistics | [Otsuka submission:34 for the primary efficacy analyses, values of test statistics were considered statistically significant if p ≤ 0.05. Continuous efficacy measures were analysed by analysis of variance and the Wilcoxon rank-sum test. Categorical efficacy measures were analysed by the van Elteren test and the Cochran–Mantel–Haenszel test. Centre 138 data were excluded from all efficacy analyses due to their unreliability based on the results of a site audit] |
| Population | |
| Eligibility criteria |
40 years or older, with PAD and IC with stable symptoms for the preceding 3 months. PAD diagnosed as an abnormal resting ABPI [Otsuka submission:34 ABPI ≥ 0.4 and ≤ 0.9 in the reference leg], with addition decline in postexercise ABPI ≥ 10 mmHg as confirmation. Symptomatic patients with normal resting ABPI but with pressure drop of > 20 mmHg were also eligible. MWD varied by no more than 20% on two or three consecutive treadmill tests Exclusion: limb-threatening ischaemia, limb revascularisation within 3 months, unstable coronary artery disease, coronary revascularisation within 6 months, thromboangiitis obliterans, deep vein thrombosis within 3 months, symptomatic arrhythmia and conditions other than PAD that might limit exercise ability or preclude completion of the study. CHF |
| Concomitant interventions allowed or excluded |
Allowed: aspirin at up to 81 mg/day Disallowed: aspirin > 81 mg/day, high-dose ibuprofen (> 1200 mg/day) |
| Power calculation | [Otsuka submission:34 based on the results of study 21–96–202, the between-group difference in the change from baseline in the log (absolute claudication distance) was expected to be 0.14, with a SD of 0.45. In order to detect this difference with 90% power at a 5% significance level (two sided), at least 218 patients were required per treatment arm. Therefore, a recruitment target was set at 260 patients per treatment arm or a total of 780 patients |
| N randomised to treatments included in review | [Otsuka submission:34 785] |
| Treatment group | Cilostazol 200 mg t.i.d. | Pentoxifylline 400 mg t.i.d. | Placebo |
|---|---|---|---|
| N randomised to treatment | [Otsuka submission:34 261] | [Otsuka submission:34 262] | [Otsuka submission:34 262] |
| Baseline characteristics | |||
| Age (years) | [Otsuka submission:34 66.7 ± 9.9 | [Otsuka submission:34 67.4 ± 9.4 | [Otsuka submission:34 67.1 ± 10.0] |
| Gender | [Otsuka submission:34 M 75.4%; F 24.6%] | [Otsuka submission:34 M 76.9%; F 23.1%] | [Otsuka submission:34 M 75.4%; F 24.6%] |
| Smokers | [Otsuka submission:34 31.5%] | [Otsuka submission:34 33.8%] | [Otsuka submission:34 31.9%] |
| Diabetics | |||
| Hypertension/blood pressure | |||
| Hyperlipidaemia | |||
| Obesity or weight | [Otsuka submission:34 (n = 258) mean 83.2 (SD 15.2) kg] | [Otsuka submission:34 (n = 260) mean 79.6 (SD 15.3) kg] | [Otsuka submission:34 (n = 260) mean 82.9 (SD 15.8) kg] |
| Angina | |||
| History of vascular therapy | |||
| Other | |||
| Withdrawals | |||
| Withdrawals/loss to follow-up | [Otsuka submission:34 35.4% overall. Non-compliance, 2.7%; AEs, 24.6%; other, 8.1%] | [Otsuka submission:34 31.5% overall. Non-compliance, 3.5%; AEs, 18.8%; other, 9.2%] | [Otsuka submission:34 26.9% overall. Non-compliance, 4.2%; AEs, 12.7%; other, 10%] |
| Results | |||
| MWD n in analysis | [Otsuka submission:34 260] | [Otsuka submission:34 260] | [Otsuka submission:34 260] |
| MWD baseline | [Otsuka submission:34 arithmetic mean 138.2] | [Otsuka submission:34 arithmetic mean 148.0] | [Otsuka submission:34 arithmetic mean 141.4] |
| MWD follow-up | |||
| MWD change | [Otsuka submission:34 arithmetic mean 60.4 (43.6%)] | [Otsuka submission:34 arithmetic mean 75.6 (51.2%)] | [Otsuka submission:34 arithmetic mean 59.0 (41.4%)] |
| MWD between-group comparison |
Cilostazol vs placebo mean difference 1.3 (SE 11.7) m, p = 0.910. Estimated treatment effect 1.03 (95% CI 0.95 to 1.12) [Otsuka submission:34 arithmetic means: cilostazol vs placebo p = 0.7502; pentoxifylline vs placebo p = 0.2774, cilostazol vs pentoxifylline p = 0.4490; estimated treatment effects: cilostazol vs placebo 1.03 (95% CI 0.95 to 1.12), p = 0.4749; pentoxifylline vs placebo 1.05 (95% CI 0.97 to 1.14), p = 0.2385, cilostazol vs pentoxifylline 0.98 (95% CI 0.90 to 1.07), p = 0.6491] |
||
| PFWD n in analysis | [Otsuka submission:34 260] | [Otsuka submission:34 260] | [Otsuka submission:34 260] |
| PFWD baseline | [Otsuka submission:34 arithmetic mean 74.9] | [Otsuka submission:34 arithmetic mean 77.1] | [Otsuka submission:34 arithmetic mean 75.5] |
| PFWD follow-up | |||
| PFWD change | [Otsuka submission:34 arithmetic mean 47.3 (62.6%)] | [Otsuka submission:34 arithmetic mean 62.6 (86.0%)] | [Otsuka submission:34 arithmetic mean 45.3 (65.0%)] |
| PFWD between-group comparison |
Cilostazol vs placebo 1.02 (95% CI 0.92 to 1.13) [Otsuka submission:34 arithmetic means: cilostazol vs placebo p = 0.8322; pentoxifylline vs placebo p = 0.1363, cilostazol vs pentoxifylline p = 0.0923; estimated treatment effects: cilostazol vs placebo 1.02 (95% CI 0.92 to 1.13), p = 0.7692; pentoxifylline vs placebo 1.08 (95% CI 0.97 to 1.19), p = 0.1517; cilostazol vs pentoxifylline 0.94 (95% CI 0.85 to 1.05), p = 0.2602] |
||
| ABPI n in analysis | |||
| ABPI baseline | |||
| ABPI follow-up | |||
| ABPI change | |||
| ABPI between-group comparison | |||
| Vascular events n in analysis | |||
| Vascular events follow-up | |||
| Vascular events included | |||
| Vascular events reported | |||
| Vascular events between-group comparison | |||
| AEs n in analysis | [Otsuka submission:34 260] | [Otsuka submission:34 260] | [Otsuka submission:34 260] |
| AEs follow-up | 24 weeks | ||
| AEs reported |
[Otsuka submission:34 79.6% patients had one or less AE AEs occurring in > 10% of patients Pharyngitis 9.6% Headache 16.5% Diarrhoea 13.1% Pain 8.1% Palpitation 10%] |
[Otsuka submission:34 80% patients had one or less AE AEs occurring in > 10% of patients Pharyngitis 15% Headache 10.8% Diarrhoea 11.2% Pain 8.8% Palpitation 1.5%] |
[Otsuka submission:34 75.8% patients had one or less AE AEs occurring in > 10% of patients Pharyngitis 11.2% Headache 6.2% Diarrhoea 6.2% Pain 11.5% Palpitation 2.7%] |
| AEs between-group comparison | |||
| Mortality reported | 0 | 3 | 2 |
| Mortality between-group comparison | |||
| HRQoL n in analysis | |||
| HRQoL baseline | |||
| HRQoL follow-up | |||
| HRQoL change | |||
| HRQoL between-group comparison | [Otsuka submission:34 the physical component score of the SF-36 was statistically significantly better with cilostazol 100 mg than with placebo (at week 12). Pentoxifylline was not significantly different from placebo with respect to the SF-36 physical component score] | ||
Two-arm trials of naftidrofuryl oxalate and placebo
| Kieffer 200165 | |
|---|---|
| Study details | |
| Publication type | Kieffer 2001,65 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT, multicentre |
| Country | France |
| Dates of participant recruitment | NR |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Naftidrofuryl oxalate 600 (200 t.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | 4 weeks |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 8 weeks, 16 weeks, 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 3.2 km/hour, 10% incline PFWD: as MWD ABPI: mode of measurement NR Vascular events AEs: recorded whether or not considered treatment related |
| Notes on statistics | Log transform for walking distances |
| Population | |
| Eligibility criteria | Outpatients of both genders, aged 35–85 years, with moderately severe chronic, stable IC of at least 6 months and which had been clinically stable during the last 3 months and the diagnosis of which was confirmed by arteriography or duplex scan. All patients had already undergone a course of exercise therapy. PFWD and MWD between 100 and 300 m (treadmill 3.2 km/hour, 10% slope), did not vary by more than 25% during placebo run-in phase. Exclude Fontaine stage I, III or IV; non-vascular leg pain; revascularisation within last 6 months or likely to be needed within 6 months; severe or unstable hypertension; exercise-limiting condition or medication; pregnancy or childbearing potential; poor (< 70%) compliance with medication during placebo run-in |
| Concomitant interventions allowed or excluded |
Allowed: NR Disallowed: NR |
| Power calculation | Minimum 100 patients per group required to detect difference of 20% (alpha error 0.5, beta error 0.1) in treadmill walking distance |
| N randomised to treatments included in review | 196 |
| Treatment group | Naftidrofuryl oxalate 200 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 98 | 98 |
| Baseline characteristics | ||
| Age | Mean 67.5 (SD 10.1) years | Mean 66.3 (SD 10.9) years |
| Gender | M 78.7%; F 21.3%a | M 81.5%; F 18.5% |
| Smokers | 83.1% | 89.1% |
| Diabetics | 19.1% | 20.6% |
| Hypertension/blood pressure | 51.7% | 42.4% |
| Hyperlipidaemia | 35.2% | 37.0% |
| Obesity or weight | BMI mean 25.9 (SD 4.3) | BMI mean 24.5 (SD 3.4) |
| Angina | ||
| History of vascular therapy | Prior vascular surgery 25.8% | Prior vascular surgery 22.8% |
| Other | Hypercholesterolaemia 36.4% | Hypercholesterolaemia 37.0% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | Nine randomised to naftidrofuryl oxalate did not supply any more data (five patient refusals, two reported AE, two lost to follow-up). A further 13 withdrew during 6-month study (six patient refusals, four lost to follow-up, three not specified) | Six randomised to placebo did not supply any more data (four patient refusals, one reported AE, one did not meet eligibility criteria). A further 16 withdrew during 6-month study (five patient refusals, six lost to follow-up, five not specified) |
| Results | ||
| MWD n in analysis | 89 | 92 |
| MWD baseline | Geometric mean 191.9 m, arithmetic mean 202 (SD 62) m | Geometric mean 203.0 m, arithmetic mean 213 (SD 63) m |
| MWD follow-up | At 24 weeks, geometric mean 350.6. Arithmetic means: 16 weeks 322, 24 weeks 385, 32 weeks (2 months without treatment) 296 | At 24 weeks, geometric mean 231.1. Arithmetic means: 16 weeks 266, 24 weeks 259, 32 weeks (2 months without treatment) 265 |
| MWD change | At 24 weeks by geometric mean 82.7%. Subgroup geometric means: diabetics 87.2% change, non-diabetics 81.6% change | At 24 weeks by geometric mean 13.9%. Subgroup geometric means: diabetics 9.5% change, non-diabetics 15.0% change |
| MWD between-group comparison | At 24 weeks by geometric mean p < 0.001. Arithmetic means 16 weeks p < 0.01, 24 weeks p < 0.001 (at 8 weeks non-significant) | |
| PFWD n in analysis | 89 | 92 |
| PFWD baseline | Geometric mean 172.3, arithmetic mean 182 (SD 64) m | Geometric mean 177.9, arithmetic mean 189 (SD 63) m |
| PFWD follow-up | At 24 weeks, geometric mean 330.5. arithmetic means 16 weeks 298, 24 weeks 367, 32 weeks (2 months without treatment) 281 | At 24 weeks, geometric mean 207.8. arithmetic means 16 weeks 244, 24 weeks 237, 32 weeks (2 months without treatment) 240 |
| PFWD change | At 24 weeks by geometric mean 91.8%. Subgroup geometric means diabetics 103.0% change, non-diabetics 89.2% change [RM1987 has mean 156.35 (SD 104.88)] | At 24 weeks by geometric mean 16.8%. Subgroup geometric means diabetics 17.3% change, non-diabetics 16.7% change [RM1987 has mean 39.67 (SD 83.84)] |
| PFWD between-group comparison | At 24 weeks by geometric mean p < 0.001. arithmetic means 16 weeks p < 0.01, 24 weeks p < 0.001, 32 weeks (2 months without treatment) p < 0.05 (at 8 weeks non-significant) | |
| ABPI n in analysis | 89 | 92 |
| ABPI baseline | Mean 0.55 (SD 0.35) | Mean 0.55 (SD 0.37) |
| ABPI follow-up | Mean 0.58 (SD 0.33) | Mean 0.59 (SD 0.33) |
| ABPI change | Difference 0.03 | Difference 0.04 |
| ABPI between-group comparison | Non-significant | |
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | (Two vascular surgery, also listed in AEs) | (Three vascular surgery, also listed in AEs) |
| Vascular events between-group comparison | ||
| AEs n in analysis | 98 | 98 |
| AEs follow-up | ||
| AEs reported | Number of patients with at least one AE 18. Number of AEs 21 (of which 12 serious: two vascular surgery and two hospitalisation for other diseases and two surgery for other condition). Non-serious possibly treatment-related one mild digestive disorder | Number of patients with at least one AE 21. Number of AEs 25 (of which 13 serious: three vascular surgery and six hospitalisation for other diseases and one surgery for other condition). Non-serious possibly treatment-related – three |
| AEs between-group comparison | Non-significant | |
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Adhoute 198666 | |
|---|---|
| Study details | |
| Publication type | Adhoute 1986,66 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT, multicentre |
| Country | France |
| Dates of participant recruitment | NR |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Naftidrofuryl oxalate 600 (200 t.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline after 4-week run-in, 3 months, 6 months |
| Outcomes and measures |
PFWD: treadmill with constant workload 3 km/hour, 10% slope ABPI: ultra sonographic measure AEs: patient self-report |
| Notes on statistics | No adjustment due to homogeneity of groups |
| Population | |
| Eligibility criteria | Patients of both genders between 40 and 70 years with Fontaine stage II PAD, IC for at least 6 months, diagnosis confirmed by angiography or Doppler velocimetry examination, PFWD (at 3 km/hour, 10% slope) 150–300 m and after a wash-out period of 1 month up to 20% variation in PFWD. Exclude vascular surgery or specific physical training within 6 months, recent MI, angina pectoris, myocardial/renal/hepatic insufficiency, labile diabetes, non-treated arterial hypertension |
| Concomitant interventions allowed or excluded |
Allowed: patients given rules about smoking and physical training Disallowed: all other treatments for arterial disease |
| Power calculation | NR |
| N randomised to treatments included in review | 154 |
| Treatment group | Naftidrofuryl oxalate 200 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | NR. 64 remained at end of study | NR. 54 remained at end of study |
| Baseline characteristics | ||
| Age | Mean 58.53 (± 8.35) years | Mean 59.62 (± 8.35) years |
| Genders | M 86%; F 14% | M 93%; F 7% |
| Smokers | 63% | 63% |
| Diabetics | ||
| Hypertension/blood pressure | ||
| Hyperlipidaemia | 31% | 33% |
| Obesity or weight | ||
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up |
(Whole study 118 remained of 154 randomised) Naftidrofuryl oxalate group reasons for withdrawal included surgery (n = 2), pathology, patient refusal or treatment intolerance (n = 3, gastralgia) Placebo group reasons for withdrawal included surgery (n = 3), pathology, patient refusal or treatment intolerance (n = 2, nausea or cutaneous rash) |
|
| Results | ||
| MWD n in analysis | ||
| MWD baseline | ||
| MWD follow-up | ||
| MWD change | ||
| MWD between-group comparison | ||
| PFWD n in analysis | 64 | 54 |
| PFWD baseline | 214.95 m mean (SD 58.33 m) | 214.98 m mean (SD 57.92 m) |
| PFWD follow-up | 335.21 m mean (SD 193.11 m) at 12 weeks; at 24 weeks 416.36 (SD 273.58) m | 274.24 m mean (SD 124.55 m) at 12 weeks; at 24 weeks 313.01 (SD 169.56) m |
| PFWD change | At 24 weeks 201.37 (SD 254.80) significantly improved p < 0.02; [RM1987 has mean 199.63 (SD 247.91)] | At 24 weeks 98.33 (SD 145.65) significantly improved p < 0.02; [RM1987 has mean 106.54 (SD 182.66)] |
| PFWD between-group comparison | At 12 weeks naftidrofuryl oxalate significantly more improved than placebo p < 0.05; at 24 weeks naftidrofuryl oxalate significantly more improved than placebo p < 0.02 | |
| ABPI n in analysis | ||
| ABPI baseline | 0.65 (SD 0.24) | 0.61 (SD 0.20) |
| ABPI follow-up | 0.67 (SD 0.23) | 0.62 (SD 0.17) |
| ABPI change | Non-significant | Non-significant |
| ABPI between-group comparison | Non-significant | |
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | 64 | 54 |
| AEs follow-up | ||
| AEs reported | Gastric, 5 | Gastric, 6 |
| AEs between-group comparison | ||
| Mortality reported | One death due to MI. Does not specify if during run-in period, or, if randomised, to which group | |
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Trubestein 198467 | |
|---|---|
| Study details | |
| Publication type | Trubestein 1984,67 full report in peer-reviewed journal |
| Additional sources of data | de Backer-Tine 2008 (RM1987)32 |
| Trial design | RCT, multicentre |
| Country | Germany |
| Dates of participant recruitment | 1981–3 |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Naftidrofuryl oxalate 600 (200 t.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | 4 weeks |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 8 and 12 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload 5 km/hour, 10% slope. Performed twice with at least 20 minutes interval PFWD: as MWD ABPI: Doppler ultrasound (venous occlusion plethysmography) AEs: method of data collection not reported |
| Notes on statistics | Log transform for MWD and PFWD |
| Population | |
| Eligibility criteria | IC patients between 40 and 65 years, PAD of femoral artery, with IC for at least 6 months and maximum 5 years, no physical training for at least 6 months, diagnosis confirmed with angiography, baseline PFWD (at 5 km/hour, 10% slope) of 100–300 m, after 4-week run-in no more than 30% change. Exclude beta-blockers, defibrinogenating enzymes, antiplatelets, anticoagulants; non-vascular exercise limiting diseases, coronary heart disease within 6 months, myocardial/respiratory/renal insufficiency, severe hypertension systolic 180 mmHg, diastolic 110 mmHg, vascular surgery within 6 months |
| Concomitant interventions allowed or excluded |
Allowed: therapy allowed Disallowed: beta-blockers, defibrinogenating enzymes, antiplatelets, anticoagulants |
| Power calculation | |
| N randomised to treatments included in review | 104 |
| Treatment group | Naftidrofuryl oxalate 200 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 54 | 50 |
| Baseline characteristics | ||
| Age | ||
| Gender | ||
| Smokers | 63% | 44% |
| Diabetics | ||
| Hypertension/blood pressure | ||
| Hyperlipidaemia | ||
| Obesity or weight | ||
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up | ||
| Results | ||
| MWD n in analysis | 54 | 50 |
| MWD baseline | 220 m | 224 m |
| MWD follow-up | 342 m | 314 m |
| MWD change | ||
| MWD between-group comparison | Non-significant between groups. For subgroup stenosis femoral artery, naftidrofuryl oxalate group significantly more improvement than placebo p < 0.02; non-significant between groups for occlusion femoral or tibial arteries | |
| PFWD n in analysis | 54 | 50 |
| PFWD baseline | 137 m | 135 m |
| PFWD follow-up | 230 m | 171 m |
| PFWD change | Difference 93 m [de Backer-Tine32 mean 82.2 (SD 144.39)] | Difference 36 m [de Backer-Tine32 mean 32.48 (SD 68.49)] |
| PFWD between-group comparison | p < 0.02. For subgroups stenosis femoral artery and occlusion tibial arteries, naftidrofuryl oxalate group significantly more improvement than placebo p < 0.01; non-significant between-groups for occlusion femoral artery; tibial arteries | |
| ABPI n in analysis | 54 | 50 |
| ABPI baseline | 98 (SD 3.7) mmHg [unclear if mean and SD] | 93 (SD 3.2) mmHg |
| ABPI follow-up | 101 (SD 3.98) mmHg (non-significant) | 92 (SD 3.9) mmHg (non-significant) |
| ABPI change | ||
| ABPI between-group comparison | Non-significant change for either group | |
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | 54 | 50 |
| AEs follow-up | ||
| AEs reported | n = 2 gastric disorders or erythema | n = 2 gastric disorders or erythema |
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Spengel 200247 | |
|---|---|
| Study details | |
| Publication type | Spengel 2002,47 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | Meta-analysis of three multicentre RCTs (Liard 1997, Spengel 1999 and D’Hooge 2001) |
| Country | Germany, France, Belgium |
| Dates of participant recruitment | NR |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Naftidrofuryl oxalate 600 (200 t.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | 1 month |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 12 and 24 weeks |
| Outcomes and measures |
PFWD: Claudication distance as estimated by patient at baseline and at the end of the study AEs: AEs were reported by the patients, in response to indirect questions from the investigator, who assessed their relationship to treatment. Reported as death, serious, minor HRQoL: CLAU-S (five dimensions – daily living, pain, social life, disease-specific anxiety, mood) |
| Notes on statistics |
Individual patient data meta analysis, study block factor added. Many other technical details reported CLAU-S multivariate analysis of covariance using the five dimensions at baseline as the multivariate covariate. If this showed effect, univariate analysis of covariance conducted. Multivariate analysis of covariance adjusted for baseline values, study effect and first order study treatment interaction |
| Population | |
| Eligibility criteria | IC (Fontaine stage II), age 40–80 years, history of IC > 3 months, stable over the previous 3 months, subjective PFWD of 50–500 m, ABPI of ≤ 0.85. In addition, it is not clear if only patients who completed the 1-month run-in (included those who had not undergone any surgical intervention during the previous 3 months nor was any surgical intervention planned and that they did not have any difficulty in understanding, or completing the questionnaire) and patients whose ABPI remained ≤ 0.85 and whose tablet compliance was > 70% were randomised |
| Concomitant interventions allowed or excluded | NR for trial, though some patients excluded for taking non-permitted concomitant medication. For run-in period, no concomitant treatment with vasoactive or rheologically active substances was permitted, basic rules pertaining to hygiene, diet, tobacco consumption and physical exercise were explained to the patients |
| Power calculation | NR |
| N randomised to treatments included in review | 754 |
| Treatment group | Naftidrofuryl oxalate 200 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 382 | 372 |
| Baseline characteristics | ||
| Age (years) | Mean 66.2 ± 9.5 | Mean 65.7 ± 9.1 |
| Gender | M, 70.4%; F, 29.6% | M, 73.8%; F, 26.2% |
| Smokers | Ex and current 72.3% | Ex and current 70.9% |
| Diabetics | 17.9% (of 510 cases for whom information available) | 15.3% (of 510 cases for whom information available) |
| Hypertension/blood pressure | ||
| Hyperlipidaemia | 36% | 32.8% |
| Obesity or weight | 23.7%, BMI (mean ± SD) 26.1 ± 3.8 | 19.1%, BMI (mean ± SD) 25.9 ± 3.9 |
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up | 24 – baseline data only – excluded from analysis | 21 – baseline data only – excluded from analysis (two further not analysed, for PFWD, but HRQoL data available) |
| 16 – lost to follow-up | 14 – lost to follow-up | |
| Nine – did not comply with treatment protocol/had concomitant medication | 12 – did not comply with treatment protocol/had concomitant medication | |
| Four – referral to hospital | Six – referral to hospital | |
| Results | ||
| MWD n in analysis | ||
| MWD baseline | ||
| MWD follow-up | ||
| MWD change | ||
| MWD between-group comparison | ||
| PFWD n in analysis | 358 | 349 |
| PFWD baseline | Mean 389 (SD 389) m | Mean 424 (SD 432) m |
| PFWD follow-up | Mean 593 (SD 500) m | Mean 476 (SD 476) m |
| PFWD change | Mean 204 (SD 443) m | Mean 51 (SD 455) m |
| PFWD between-group comparison |
Final absolute value p = 0.002 Difference p < 0.001 |
|
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | One death from MI | Unclear |
| Vascular events between-group comparison | ||
| AEs n in analysis | Unclear (states ‘whole study population’ for deaths, but not clear if withdrawals were followed up for AEs, and presumably those lost to follow-up would not have been included) | Unclear (states ‘whole study population’ for deaths, but not clear if withdrawals were followed up for AEs, and presumably those lost to follow-up would not have been included) |
| AEs follow-up | Assume 6 months | |
| AEs reported |
One death 33 serious (one considered to be in relation to the treatment) 11 minor (11 gastrointestinal, five skin reactions) |
Five deaths 34 serious [two considered to be in relation to the treatment (assume assessor was blinded)] 12 minor (eight gastrointestinal, four skin events) |
| AEs between-group comparison | ||
| Mortality reported | One also reported in AEs | Five also reported in AEs |
| Mortality between-group comparison | ||
| HRQoL n in analysis | 358 | 351 |
| HRQoL baseline | Daily living, 65.8 (SD 23.7); pain, 65.6 (SD 18.9); social life, 86.9 (SD 19.8); disease-specific anxiety, 81.1 (SD 20.3); mood, 79.3 (SD 20.1) | Daily living, 66.9 (SD 23); pain, 65 (SD 19.2); social life, 86.1 (SD 20.2); disease-specific anxiety, 80.9 (SD 20.2); mood, 80.7 (SD 18.5) |
| HRQoL follow-up | Daily living, 73.3 (SD 25); pain, 72 (SD 19.2); social life, 90.0 (SD 16.9); disease-specific anxiety, 83 (SD 20.3); mood, 82.8 (SD 18.5) | Daily living, 65.5 (SD 26.2); Pain, 64.6 (SD 23.1); social life, 84.1 (SD 24.6); disease-specific anxiety, 82 (SD 19.3); mood, 79.5 (SD 22.4) |
| HRQoL change | (Read from graph/calculated from tables): daily living, 7.5/7.5; pain, 8.4/6.4; social life, 3.1/3.1; disease-specific anxiety, 0.2/1.9; mood, 3.5/3.5 | (Read from graph/calculated from tables): daily living,–1.3/–1.4; pain, –0.4/–0.4; social life, –2.4/–2; disease-specific anxiety, 0.2/1.1; mood, –1.3/–1.2 |
| HRQoL between-group comparison | ANCOVA: daily living, p < 0.001; pain, p < 0.001; social life, p = 0.001; disease-specific anxiety, non-significant; mood, p = 0.03 | |
| Ruckley 197868 | |
|---|---|
| Study details | |
| Publication type | Ruckley 1978,68 short report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | Unclear if RCT or clinical trial |
| Country | UK |
| Dates of participant recruitment | NR |
| Sources of funding | Lipha Pharmaceuticals UK |
| Intervention(s) and comparator | |
| Treatment groups | Naftidrofuryl oxalate 300 (100 t.i.d.) mg |
| Comparator | Placebo |
| Run-in phase | No |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 2 weeks, 4 weeks, then every 4 weeks until 24 weeks |
| Outcomes and measures |
PFWD: not explicit that treadmill was used, but likely that it was. Categorised as < 100 yards = severe, 100–200 yards = moderate, > 200 yards = mild AEs: patient self-report |
| Notes on statistics | Wilcoxon rank-sum test |
| Population | |
| Eligibility criteria | Consecutive patients attending a peripheral vascular clinic with stable claudication |
| Concomitant interventions allowed or excluded | Allowed: all patients asked to take regular exercise |
| Power calculation | NR |
| N randomised to treatments included in review | 50 |
| Treatment group | Naftidrofuryl oxalate 100 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | ||
| Baseline characteristics | ||
| Age | ||
| Gender | ||
| Smokers | ||
| Diabetics | ||
| Hypertension/blood pressure | ||
| Hyperlipidaemia | ||
| Obesity or weight | ||
| Angina | ||
| History of vascular therapy | ||
| Other | Severity: 15 mild, three moderate, seven severe | Severity: nine mild, six moderate, 10 severe |
| Withdrawals | ||
| Withdrawals/loss to follow-up | One patient failed to attend final test, NR which group | |
| Results | ||
| MWD n in analysis | ||
| MWD baseline | Severity: 15 mild, three moderate, seven severe | Severity: nine mild, six moderate, 10 severe |
| MWD follow-up | ||
| MWD change | ||
| MWD between-group comparison | Not significant at p = 0.05 | |
| PFWD n in analysis | ||
| PFWD baseline | ||
| PFWD follow-up | ||
| PFWD change | ||
| PFWD between-group comparison | ||
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | 25 | 25 |
| AEs follow-up | 12 weeks | |
| AEs reported |
Vertigo 8% Nausea 8% Slight insomnia 8% |
Epigastric pain 4% Indigestion 4% Constipation 4% Headache and nausea 4% |
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
Trials of pentoxifylline and placebo
| Lindgarde 198971 | |
|---|---|
| Study details | |
| Publication type | Lindgarde 1989,71 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT, multicentre (two Sweden, one Denmark) |
| Country | Sweden, Denmark |
| Dates of participant recruitment | NR |
| Sources of funding | Drugs supplied by Hoechst AG Werk Albert |
| Intervention(s) and comparator | |
| Treatment groups | Pentoxifylline 1200-mg daily dose (400 mg t.i.d.) |
| Comparator | Placebo |
| Run-in phase | 4–6 weeks |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline (after run-in) then every 4 weeks until 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 2 mph (3.2 km/hour), 12.5% inclination PFWD: as MWD AEs: recorded at each follow-up |
| Notes on statistics | Efficacy results reported after adjustment for study site. Comparison of treatment effects was performed with the extended Mantel–Haenszel test with stratification adjustment for site and standardised rank scores. Geometric means of per cent change from baseline and CI calculated. ANOVA to test treatment groups and background variables, Wilcoxon signed-rank test for changes in normal/abnormal lab tests, chi-squared test for side effects. All tests two sided, p < 0.05 significance |
| Population | |
| Eligibility criteria | At least 40 years of age, suffering from moderately severe chronic obstructive pulmonary airways disease with a PFWD of between 50 and 200 m, as tested on a treadmill set at a speed of 2 mph (3.2 km/hour) and an inclination of 12.5% (7.1°). History of IC of at least 6 months in duration. The diagnosis of chronic obstructive pulmonary disease was established by clinical examination and by Doppler pressure assessment at rest and after exercise. Diagnosis confirmed by angiography. PFWD stable for the last two visits of run-in phase (difference of < 35% in patients with baseline PFWD up to 100 m, < 25% in patients with baseline PFWD 101–200 m. Excluded if: complete occlusion of the aortoiliac segment, femoral bifurcation, or popliteal artery without angiographically proven distal refilling of the segment; vascular reconstruction or sympathectomy within the last 12 months; peripheral neuropathy; Buerger’s disease; marked postphlebotic syndrome; diabetes; cardiac failure or sever rhythm disorders; major infections; abnormal values for platelets; prothrombin index or partial thromboplastin time; history of xanthine hypersensitivity; addiction to analgesics; malignant disease, or any other condition that limits walking ability or full understanding of study procedure |
| Concomitant interventions allowed or excluded | NR |
| Power calculation | NR |
| N randomised to treatments included in review | |
| Treatment group | Naftidrofuryl oxalate 200 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 76 | 74 |
| Baseline characteristics | ||
| Age | Mean 65 (SD 7) years | Mean 64 (SD 8) years |
| Gender | M 79%; F 21% | M 80%; F20% |
| Smokers | 63% | 59% |
| Diabetics | 0% | 0% |
| Hypertension/blood pressure | 37% | 35% |
| Hyperlipidaemia | 26% | 30% |
| Obesity or weight | 1.03 (SD 0.1) (as reported, note that value is not within standard BMI range) | 1.05 (SD 0.2) (as reported, note that value is not within standard BMI range) |
| Angina | 26% | 24% |
| History of vascular therapy | ||
| Other | MI, 24%; isolated iliac or iliofemoropopliteal lesions, 17%; isolated femoropopliteal or femoropopliteal/lower leg lesions, 72% | MI, 18%; isolated iliac or iliofemoropopliteal lesions, 12%; isolated femoropopliteal or femoropopliteal/lower leg lesions, 68% |
| Withdrawals | ||
| Withdrawals/loss to follow-up | NR | NR |
| Results | ||
| MWD n in analysis | 76 | 74 |
| MWD baseline | Geometric mean 132 (SEM 9) m | Geometric mean 155 (SEM 11) m |
| MWD follow-up | 50% improvement (SEM 9%) (crude calculation, 198 m) | 24% improvement (SEM 7%) (crude calculation, 192.2 m) |
| MWD change | Crude calculation, 66 m | Crude calculation, 37.2 m |
| MWD between-group comparison | Non-significant, p = 0.094 | |
| PFWD n in analysis | 76 | 74 |
| PFWD baseline | Geometric mean 77 (SEM 4) m | Geometric mean 79 (SEM 4) m |
| PFWD follow-up | 80% improvement (SEM 12%) (crude calculation, 138.6 m) | 60% improvement (SEM 11%) (crude calculation, 126.4 m) |
| PFWD change | Crude calculation, 61.6 m | Crude calculation, 47.4 m |
| PFWD between-group comparison | ||
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | ||
| AEs follow-up | ||
| AEs reported | 22% (13 reported gastrointestinal complaints, other mild events were not defined) | 14% (seven reported gastrointestinal complaints, other mild events were not defined) |
| AEs between-group comparison | Gastrointestinal complaints non-significant | |
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Porter 198272 | |
|---|---|
| Study details | |
| Publication type | Porter 1982,72 full report in peer-reviewed journal |
| Additional sources of data |
Gillings 1987 (RM265),73 post hoc ITT analysis Porter 1982 (RM294),74 Reich 1984 (RM287)75 |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | NR |
| Sources of funding | Drugs supplied by Hoechst–Roussel Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Pentoxifylline 600-mg daily dose (200 mg t.i.d.) for first week, increased in a stepped manner to 1200-mg daily dose (assume 400 mg t.i.d.) by fourth week |
| Comparator | Placebo |
| Run-in phase | 4–6 weeks |
| Treatment duration | 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 2, 4, 6, 8, 12, 16, 20 and 24 weeks |
| Outcomes and measures |
MWD: [Porter 1982:74 at each visit two treadmill tests were performed at 30- to 60-minute intervals and the mean of the two tests used. Treadmill set to 1.5 mph, 7°] PFWD: as MWD AEs: brief physical examination and careful monitoring of observed and reported unwanted effects. ECG and routine blood analysis performed once or more during the trial and again at the end. Audiograms and ophthalmic examinations were only repeated at the final visit Vascular events: reported as part of AE analysis |
| Notes on statistics |
PFWD and MWD analysed with repeat measures two way analysis of variance with interaction (investigator, intervention, investigator and intervention). Transformed into per cent change (= geometric mean of response value/baseline value –1 × 100) to limit undue influence of outlying values. After 24 weeks were analysed by the extended Mantel–Haenszel procedure for ordered contingency tables by classifying patients into one of four categories (< 25% change, 25–49% change, 50–100% change, > 100% change). Mantel–Haenszel results not extracted [RM 265: as above for log of (distance/baseline) ratios. Gives equations in statistical appendix. ITT analysis was of all patients who completed at least one follow-up. Extended Mantel–Haenszel procedure with log-rank scores, provides a two-sided non-parametric test. Fisher procedure also with log-rank scores gives one-sided test] |
| Population | |
| Eligibility criteria |
Included: patients with IC secondary to chronic obstructive pulmonary disease. Chronic obstructive pulmonary disease diagnosed by arteriography or by the absence of diminution of one or more lower limb pulses as determined by palpation. IC must have been experienced for at least 6 months prior to a patient’s enrolment. IC characterised by pain, muscular ache, cramps or severe fatigue involving one or both lower limbs when walking. Patients had to be able to walk on the treadmill for at least 50 m at a speed of 1.5 mph and a grade of 7° without experiencing claudication, but not for > 510 m in 9.5 minutes at a speed of 2 mph before claudication. MWD had to be stable in last two visits during placebo run-in, i.e. within 20% of one another. [Reich 1984:75 patients had to demonstrate compliance with protocol] Excluded: patients with severe chronic obstructive pulmonary disease (pain at rest, ulceration, gangrene), sympathectomy within previous 6 months, severe peripheral neuropathy, chronic infection or any hypersensitivity to methylxanthines (caffeine, theophylline, theobromine) and women who were pregnant/of childbearing potential/using oral contraceptives |
| Concomitant interventions allowed or excluded |
Allowed: NR Disallowed: all current treatment for peripheral vascular disease was stopped for 2 weeks before placebo run-in phase |
| Power calculation | NR |
| N randomised to treatments included in review | 127 (one randomised twice, therefore authors treat total number as 128) |
| Treatment group | Naftidrofuryl oxalate 200 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 66 (67 if include placebo patient randomised a second time) | 61 |
| Baseline characteristics | ||
| Age (years) | Mean 62 | Mean 63.5 |
| Gender |
M 82.1%; F 17.9% [Gillings 1987:73 n = 124, M 81%; F 19%] |
M 82%; F 18% [Gillings 1987:73 n = 124, M 82%; F 18%] |
| Smokers |
67.2% [Gillings 1987:73 n = 124, 67%] |
68.9% [Gillings 1987:73 n = 124, 69%] |
| Diabetics |
22.4% [Gillings 1987:73 n = 124, 22%] |
24.6% [Gillings 1987:73 n = 124, 25%] |
| Hypertension/blood pressure | [Gillings 1987:73 mean diastolic BP 81 mmHg] | [Gillings 1987:73 mean diastolic BP 82 mmHg] |
| Hyperlipidaemia | ||
| Obesity or weight | ||
| Angina | [Reich 1984:75 10/63 (15.9%)] | [Reich 1984:75 6/61 (9.8%)] |
| History of vascular therapy | ||
| Other |
Mean duration of chronic obstructive airways disease, 3.0 years [Gillings 1987:73 mean duration of chronic obstructive pulmonary disease, 3.4 years] [Reich 1984:75 occasional exercise, 29/63 (46.0%), regular exercise 25/63 (39.7%)] |
Mean duration of chronic obstructive airways disease, 2.8 years [Gillings 1987:73 mean duration of chronic obstructive pulmonary disease 4.3 years] [Reich 1984:75 occasional exercise, 28/61 (45.9%), regular exercise 19/61 (31.1%)] |
| Withdrawals | ||
| Withdrawals/loss to follow-up |
Patients excluded from non-ITT analysis (25/67): already randomised, 1; did not keep visit schedule, 8; prescribed improper medication, 2; trial closed before patient completed 24 weeks, 4; intercurrent medical problem, 5 [Gillings 1987:73 ITT analysis: only four excluded: discontinued study before first follow-up, 3; previously randomised to placebo, 1] |
Patients excluded from non-ITT analysis (21/61): treadmill entry criteria violated, 2; did not keep visit schedule, 7; refused medication, 2; prescribed improper medication, 2; trial closed before patient completed 24 weeks, 1; intercurrent medical problem, 4 [Gillings 1987:73 ITT analysis: no withdrawals] |
| Results | ||
| MWD n in analysis |
42 [Gillings 1987:73 63] |
40 [Gillings 1987:73 61] |
| MWD baseline | 172 m [Gillings 1987:73 147 (SE 9 m)] | 181 [Gillings 1987:73 161(SE 10 m)] |
| MWD follow-up | 268 m | 250 m |
| MWD change | 38% (calculated: 96 m) [Gillings 1987:73 33 (SE 8 m)] | 25% (calculated: 69 m) [Gillings 1987:73 16 (SE 5 m)] |
| MWD between-group comparison |
p = 0.035 by repeat measures two-way analysis of variance with interaction of the study data [Gillings 1987:73 extended Mantel–Haenszel p = 0.316, one-sided p = 0.049] |
|
| PFWD n in analysis | 42 | 40 |
| PFWD baseline | 111 m [Gillings 1987:73 95 (SE 6 m)] | 117 m [Gillings 1987:73 102 (SE 6 m)] |
| PFWD follow-up | 195 m | 180 m [RM265: 147 (SE 9 m)] |
| PFWD change | 59% (calculated: 84 m) [Gillings 1987:73 47 (SE 10 m)] | 36% (calculated: 63 m) [Gillings 1987:73 18 (SE 6 m)] |
| PFWD between-group comparison | p = 0.016 by repeat measures two-way analysis of variance with interaction of the study data. [Gillings 1987:73 extended Mantel–Haenszel p = 0.042, one-sided p = 0.1] | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 66 (67) | 61 |
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | One angina | One MI, one cerebrovascular accident, one cardiac surgery |
| Vascular events between-group comparison | ||
| AEs n in analysis | ||
| AEs follow-up | ||
| AEs reported | (Also listed in withdrawals): 37 (55%) experienced some AEs including: nausea, 24 (35.8%); depression of the central nervous system symptoms, 15 (22.4%). Other AEs not detailed | (Also listed in withdrawals): 24 (39%) experienced some AEs including: nausea, 3; depression of the central nervous system symptoms, 7; blurred vision, 1; weakness, 1. Other AEs not detailed |
| AEs between-group comparison | Nausea p < 0.05, depression of the central nervous system and others not significant | |
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Gallus 198576 | |
|---|---|
| Study details | |
| Publication type | Gallus 1985,76 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT crossover (extract up to crossover) |
| Country | Australia |
| Dates of participant recruitment | NR |
| Sources of funding | Hoechst Australia supported trial |
| Intervention(s) and comparator | |
| Treatment groups | Pentoxifylline 800-mg daily dose (400 mg b.i.d.) for first week, increased to 1200-mg daily dose (400 mg t.i.d.) |
| Comparator | Placebo |
| Run-in phase | 4 weeks |
| Treatment duration | 8 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 8 weeks |
| Outcomes and measures |
MWD: treadmill with constant speed of 4 km/hour and a slope of 10° PFWD: as MWD Vascular events Mortality |
| Notes on statistics | Geometric means used. Log transformation was used to normalise apparently log-normal distribution of several variables, including all treadmill distances. Student’s t-test with confidence limits of 95% were calculated according to Armitage for the ‘therapeutic effects ratio’ obtained by dividing the observed pentoxifylline effect on treadmill claudication or walking distance by the observed placebo effect |
| Population | |
| Eligibility criteria |
Include: patients who estimated they could walk < 750 m before the onset of leg pain. Stable claudication distance for over 6 months, the presence of peripheral vascular disease documented through clinical examination by a vascular surgeon and supplemented by angiography or non-invasive testing, age > 50 years, a pledge not to change smoking habits during the trial and informed consent Exclude: those with vascular surgery or sympathectomy within the previous 6 months, ischaemic leg ulcer or rest pain, exercise tolerance limited by conditions other than peripheral vascular disease and treatment with lipid-lowering or antiplatelet drugs |
| Concomitant interventions allowed or excluded |
Allowed: unspecified non-trial drugs allowed Disallowed: lipid-lowering or antiplatelet drugs not allowed |
| Power calculation | NR |
| N randomised to treatments included in review | 47 |
| Treatment group | Pentoxifylline 800-mg daily dose (400 mg b.i.d.) for first week, increased to 1200-mg daily dose (400 mg t.i.d.) | Placebo |
|---|---|---|
| N randomised to treatment | 25 | 23 |
| Baseline characteristics | ||
| Age | Not including five withdrawals: mean 68 (SD 6) years | Not including four withdrawals: mean 66 (SD 6) years |
| Gender | Not including five withdrawals: M 89.5%; F 10.5% | Not including four withdrawals: M 73.7%; F 26.3% |
| Smokers | Not including five withdrawals: 52.6% | Not including four withdrawals: 36.8% |
| Diabetics | Not including five withdrawals: 15.8% | Not including four withdrawals: 10.5% |
| Hypertension/blood pressure | Not including five withdrawals, supine BP ( mmHg): mean systolic 167 (SD 30); mean diastolic 88 (SD 12) | Not including four withdrawals, supine BP ( mmHg): mean systolic 165 (SD 27); mean diastolic 90 (SD 12) |
| Hyperlipidaemia | ||
| Obesity or weight | NR, weight mean 76 (SD 11) kg | NR, weight mean 74 (SD 12) kg |
| Angina | Not including five withdrawals: 26.3% | Not including four withdrawals: 26.3% |
| History of vascular therapy | Not including five withdrawals: vascular reconstruction 31.6%; sympathectomy 15.8% | Not including four withdrawals: vascular reconstruction 31.6%; sympathectomy 26.3% |
| Other | Not including five withdrawals: MI 21.1% cerebral ischaemia 10.5%; symptom duration (geometric mean ± 1 SD) 53 ± 23–122 months | Not including four withdrawals: MI 10.5%, cerebral ischaemia 26.3%; symptom duration (geometric mean ± 1 SD) 24 ± 9–59 months |
| Withdrawals | ||
| Withdrawals/loss to follow-up | Five withdrawals, only two before crossover: nausea and vomiting, one; breathless with effort, one. Three who withdrew after crossover: R on T extra systoles with effort (as reported), one; uninterpretable exercise ECG, one; onset of effort angina, one.) Missing data in results (Table 3) not explained, though probably due to exclusion of patients with < 10 m baseline claudication distance | Four withdrawals, all before crossover: death (MI), one; myocardial infarct/stroke, one; angina with exercise, one; technical, one. Missing data in results (Table 3) not explained, though probably due to exclusion of patients with < 10 m baseline claudication distance |
| Results | ||
| MWD n in analysis | 19 at baseline, 16 at 8 weeks | 19 at baseline, 16 at 8 weeks |
| MWD baseline | Geometric mean 90.4 m | Geometric mean 99.8 m |
| MWD follow-up | ||
| MWD change | Per cent change from baseline (× 100) 1.23 | Per cent change from baseline (× 100) 1.17 |
| MWD between-group comparison | Ratio of per cent change from baseline (pentoxifylline/placebo) 1.05 (95% CI 0.81 to 1.36) | |
| PFWD n in analysis | 18 at baseline, 16 at 8 weeks | 19 at baseline, 16 at 8 weeks |
| PFWD baseline | Geometric mean 47.7 m | Geometric mean 48.3 m |
| PFWD follow-up | ||
| PFWD change | Per cent change from baseline (× 100) 1.55 | Per cent change from baseline (× 100) 1.26 |
| PFWD between-group comparison | Ratio of per cent change from baseline (pentoxifylline/placebo) 1.23 (95% CI 0.86 to 1.77) | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | No withdrawals due to vascular events | Three withdrawals due to vascular events (one fatal MI, one MI, one angina) |
| Vascular events between-group comparison | ||
| AEs n in analysis | ||
| AEs follow-up | ||
| AEs reported | ||
| AEs between-group comparison | ||
| Mortality reported | 0 | 1 |
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Di Perri 198377 | |
|---|---|
| Study details | |
| Publication type | Di Perri 1983,77 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT crossover (extract up to crossover) |
| Country | Italy |
| Dates of participant recruitment | NR |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Pentoxifylline 1200-mg daily dose (400 mg t.i.d.) |
| Comparator | Placebo |
| Run-in phase | No |
| Treatment duration | 8 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 8 weeks |
| Outcomes and measures |
MWD: measured absolute walking distance (m). The absolute distance which the individual patient was able to cover by walking on horizontal level at metronome-controlled speed of 120 steps/minute under supervision of a medical doctor. At each time point the walking test was performed three times and a mean taken AEs: unclear how recorded |
| Notes on statistics | Student’s t-test and two-way analysis of variance were used |
| Population | |
| Eligibility criteria | Outpatients suffering from peripheral arterial occlusive disease with IC. Fontaine’s classification stage II severity. Walking capacity between 100 m and 400 m. Free from pain at rest and skin lesions. Excluded diabetes mellitus, severe hypertension (> 180/110 mmHg) and CHF |
| Concomitant interventions allowed or excluded | None allowed |
| Power calculation | NR |
| N randomised to treatments included in review | 24 |
| Treatment group | Pentoxifylline 400 mg t.i.d. | Placebo |
|---|---|---|
| N randomised to treatment | 12 | 12 |
| Baseline characteristics | ||
| Age | Mean 59.3 years | Mean 59.3 years |
| Gender | M 83.3%; F 16.7% | M 75%; F 25% |
| Smokers | ||
| Diabetics | 0% | 0% |
| Hypertension/blood pressure | 0% | 0% |
| Hyperlipidaemia | 0% | 0% |
| Obesity or weight | ||
| Angina | ||
| History of vascular therapy | ||
| Other | 12 across the two groups displayed symptoms of moderate coronary heart disease and/or cerebrovascular disorders | |
| Withdrawals | ||
| Withdrawals/loss to follow-up | 0 | 0 |
| Results | ||
| MWD n in analysis | 12 | 12 |
| MWD baseline | Mean 223 ± 20 m (SD or SE NR). Also reported as ± 29 m | Mean 208 ± 24.6 m |
| MWD follow-up | Mean 359 ± 29 m (SD or SE NR) | Mean 215 ± 25 m |
| MWD change | 136 m (reported) | 6 m (reported) |
| MWD between-group comparison | Student’s t-test of the individual increases discloses significant superiority in the pentoxifylline group (p < 0.01) | |
| PFWD n in analysis | ||
| PFWD baseline | ||
| PFWD follow-up | ||
| PFWD change | ||
| PFWD between-group comparison | ||
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | 12 | 12 |
| AEs follow-up | ||
| AEs reported | 0 | 0 |
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Dettori 198969 | |
|---|---|
| Study details | |
| Publication type | Dettori 1989,69 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT, multicentre, factorial |
| Country | Italy |
| Dates of participant recruitment | Between March 1983 and February 1985 |
| Sources of funding | Hoechst Italia |
| Intervention(s) and comparator | |
| Treatment groups | Pentoxifylline 1200-mg daily dose (400 mg t.i.d.) |
| Comparators |
1. acenocoumarol 4-mg tablets (adjusted to patient) 2. 1200 mg pentoxifylline daily dose (400 mg t.i.d.) plus acenocoumarol 4-mg tablets (adjusted to patient) 3. placebo |
| Run-in phase | 4 weeks |
| Treatment duration | 52 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 13 weeks, 26 weeks, 39 weeks, 52 weeks |
| Outcomes and measures |
PFW time: speed of 3 km/hour, 10% elevation. PFW time recorded. For those who could walk for 30 minutes without experiencing pain, a higher speed was used in the second test (5 km/hour) ABPI: Doppler ultrasound. Measured on both lower limbs, highest value measure used as denominator |
| Notes on statistics | Analysis of variance to compare baseline characteristics. Chi-squared test for PFWD, by categorising patients into improved (≥ 25% from baseline), not improved (–25% to +25% from baseline), deteriorated (> –25% from baseline). Also assessed by means of the analysis of variance for repeated measures. ABPI compared by means of Mann–Whitney test. Fisher’s exact test used to compare frequency of relevant clinical events |
| Population | |
| Eligibility criteria | |
| Concomitant interventions allowed or excluded |
Allowed: advice to quit smoking and to perform daily walks Disallowed: anticoagulants, other medications unless authorised by the physicians involved in the study |
| Power calculation | 80%, p < 0.05 |
| N randomised to treatments included in review | 146 |
| Treatment group | Pentoxifylline 1200-mg daily dose (400 mg t.i.d.) | Acenocoumarol 4-mg tablets (adjusted to patient) | 1200-mg pentoxifylline daily dose (400 mg t.i.d.) plus acenocoumarol 4-mg tablets (adjusted to patient) | Placebo |
|---|---|---|---|---|
| N randomised to treatment | 37 | 36 | 36 | 37 |
| Baseline characteristics | ||||
| Age | (m bar = mean?) 62 ± SD 5 years | (m bar = mean?) 58 ± SD 7 years | (m bar = mean?) 60 ± SD 6 years | (m bar = mean?) 59 ± SD 8 years |
| Gender | M 89.2%; F 10.8% | M 91.7%; F 8.3% | M 91.7%; F 8.3% | M 94.6%; F 5.4% |
| Smokers | ||||
| Diabetics | 10.8% | 8.3% | 13.9% | 24.3% |
| Hypertension/blood pressure | 32.4% | 27.8% | 36.1% | 35.1% |
| Hyperlipidaemia | ||||
| Obesity or weight | ||||
| Angina | ||||
| History of vascular therapy | 0% | 0% | 0% | 0% |
| Other | Heart disease: 13.5%; median duration of symptoms, 8 months | Heart disease: 22.2%; median duration of symptoms, 7.5 months | Heart disease: 19.4%; median duration of symptoms, 12 months | Heart disease: 13.5%, median duration of symptoms, 12 months |
| Withdrawals | ||||
| Withdrawals/loss to follow-up |
Angina, one; unrelated diseases, three; intolerance, two; refusal, two Total = eight |
Non-fatal bleeding, two; angina, one; unrelated diseases, three Total = six |
Fatal bleeding, two; non-fatal bleeding, one; angina, one; unrelated diseases, one; intolerance, two Total = seven |
Fatal MI, two; reversible ischaemic neurological deficit, one; unrelated diseases, one; refusal, three Total = seven |
| Results | ||||
| MWD n in analysis | ||||
| MWD baseline | ||||
| MWD follow-up | ||||
| MWD change | ||||
| MWD between-group comparison | ||||
| PFWD n in analysis | 29 | 30 | 29 | 30 |
| PFWD baseline | Geometric mean 112 (range 25–660) seconds | Geometric mean 121 (range 13–395) seconds | Geometric mean 138 (range 45–480) seconds | Geometric mean 144 (range 45–758) seconds |
| PFWD follow-up | Geometric mean 324 (range 50–1800) seconds | Geometric mean 406 (range 115–1800) seconds | Geometric mean 468 (range 118–1800) seconds | Geometric mean 349 (range 60–1800) seconds |
| PFWD change | +189% categorisation: improved, 25; unchanged, three; worse, one | +236% categorisation: improved, 26; unchanged, four; worse, zero | +239% categorisation: improved, 28; unchanged, zero; worse, one | +149% categorisation: improved, 20; unchanged, seven; worse, three |
| PFWD between-group comparison | Two-way contingency, grouping T1 and T3 (pentoxifylline groups) together, and T2 and T4 (no pentoxifylline) together gave a statistically significant difference between improved vs not improved (worse + unchanged) for pentoxifylline (χ2 = 4.73, p < 0.05) and acenocoumarol (χ2 = 5.08, p < 0.05). Analysis of variance for repeated measures was non-significant | |||
| ABPI n in analysis | 29 | 30 | 29 | 30 |
| ABPI baseline |
At rest: (m bar = mean?) 0.68 (SD 0.14) After exercise: (m bar = mean?) 0.57 (SD 0.22) |
At rest: 0.68 (SD 0.18) After exercise:0.54 (SD 0.23) |
At rest: 0.69 (SD 0.20) After exercise:0.56 (SD 0.27) |
At rest: 0.67 (SD 0.14) After exercise: 0.57 (SD 0.19) |
| ABPI follow-up |
At rest: (m bar = mean?)0.71 (SD 0.17) After exercise: 0.62 (SD 0.21) |
At rest: 0.75 (SD 0.20) After exercise: 0.61 (SD 0.24) |
At rest: 0.73 (SD 0.16) After exercise: 0.65 (SD 0.22) |
At rest: 0.65 (SD 0.13) After exercise: 0.52 (SD 0.19) |
| ABPI change |
At rest: +2.5% After exercise: +8.3% |
At rest: +9.7% After exercise:+16.1% |
At rest: +8.7% After exercise: +20.6% |
At rest: –3.1% After exercise: –9.4% |
| ABPI between-group comparison |
At rest: T2 compared with placebo significant (p = 0.04), T3 compared with placebo borderline (p = 0.07) After exercise: T1 vs placebo p = 0.09, T2 vs placebo p = 0.05, T3 vs placebo p = 0.01. Differences between active drugs non-significant |
|||
| Vascular events n in analysis | 37 | 36 | 36 | 37 |
| Vascular events follow-up | ||||
| Vascular events included | Fatal bleeding, non-fatal bleeding, angina, reversible ischaemic neurological deficit | |||
| Vascular events reported | One | Three | Four | One (plus two deaths from MI, not included in statistical comparison between groups) |
| Vascular events between-group comparison | Only compared acenocoumarol with non-acenocoumarol groups (T2, T3 vs T4, T1): non-significant difference | |||
| AEs n in analysis | 37 | 36 | 36 | 37 |
| AEs follow-up | Negative end points were defined as death, acute MI, onset of angina pectoris, stroke or TIA, cerebral haemorrhage. Other side effects (such as epigastric pain) were all recorded | |||
| AEs reported |
Angina, one; unrelated diseases, three; intolerance, two; refusal, two Total = eight |
Non-fatal bleeding, two; angina, one; unrelated diseases, three Total = six |
Fatal bleeding, two; non-fatal bleeding, one; angina, one; unrelated diseases, one; intolerance, two Total = seven |
Fatal MI, two; reversible ischaemic neurological deficit, one; unrelated diseases, one; refusal, three Total = seven |
| AEs between-group comparison | NR for pentoxifylline | |||
| Mortality reported | Zero | Zero | Two | Two |
| Mortality between-group comparison | NR | |||
| HRQoL n in analysis | ||||
| HRQoL baseline | ||||
| HRQoL follow-up | ||||
| HRQoL change | ||||
| HRQoL between-group comparison | ||||
| Creager 200870 | |
|---|---|
| Study details | |
| Publication type | Creager 2008,70 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT, multicentre |
| Country | USA |
| Dates of participant recruitment | February 1998 to October 1999 |
| Sources of funding | Berlex Pharmaceuticals |
| Intervention(s) and comparator | |
| Treatment groups | Pentoxifylline 1200-mg daily dose (400 mg t.i.d.) |
| Comparator |
|
| Run-in phase | 4–6 weeks |
| Treatment duration | 26 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 26 weeks |
| Outcomes and measures |
MWD: graded treadmill, speed at a constant 2 mph. Graduation started at 0% and increased by 2% every 2 minutes. Primary measure was walking time, converted to distance PFWD: as MWD AEs: reports those that affected > 5% of any group with a ratio > 2.0 or < 0.5 compared with placebo. SAEs reported (death, permanent substantial disability, inpatient hospitalisation or prolongation of existing inpatient hospitalisation, or an AE that was life-threatening or was a congenital anomaly, cancer or overdose) are those that affected > 1% HRQoL: WIQ and SF-36 |
| Notes on statistics |
Primary analysis: mean per cent change from baseline between T4 and T2. Efficacy analysis based on ITT (only those 370 participants with baseline treadmill, at least one dose after randomisation, and one follow-up treadmill assessment). Two-way analysis of covariance. Last observation carried forward Secondary analysis: individual comparisons between placebo and T1, T3, T4 and T5. No adjustment for multiple comparisons. Additional analyses used graded threshold criteria (25%, 25–50% and 50% from baseline). Cochran–Mantel–Haenszel method based on rank (Van Elteren) was applied, stratified by baseline diabetic status. Also done for secondary efficacy variables. All tests were two-tailed and performed at p = 0.05. Pair-wise testing of placebo vs drug and pentoxifylline vs iloprost. Subgroup analysis included age, gender, race, smoking status, duration of PAD, prior intervention, antiplatelet medication, absolute claudication distance at baseline and diabetic status |
| Population | |
| Eligibility criteria |
Men and women aged ≥ 40 years, with PAD and IC (Fontaine stage II) were eligible for participation. Stable claudication for at least 3 months prior to entry, despite standard care, which included cardiovascular risk factor modification and exercise training. Absolute claudication distance between 50 and 800 m on a baseline eligibility exercise test. ABPI of ≤ 0.9 in the symptomatic leg. In addition, a > 20% fall in ABPI within 1 minute following cessation of exercise served as confirmation of a diagnosis of PAD. In patients with non-compressible vessels (ABPI > 1.50), the TBI at rest had to be < 0.70. Run-in phase requirements: MWD measured by exercise treadmill test on two to three occasions at an interval of 7–14 days had to be within 20% of the MWD measured at the previous test (up to three tests to meet this requirement), drug compliance had to be 80–120% Exclusions: ischaemic rest pain, ulcers, gangrene (Fontaine stage III or IV), evidence of non-atherosclerotic PAD, and peripheral neuropathy that impaired walking ability, revascularisation for PAD within the preceding 3 months, sympathectomy within 6 months, type 1 diabetes mellitus, MI or major cardiac surgery within 3 months, unstable angina and heart failure |
| Concomitant interventions allowed or excluded |
Allowed: aspirin alone or warfarin alone Disallowed: warfarin in combination with aspirin, or any drug specific to the treatment of IC, low molecular weight heparin |
| Power calculation | Based on comparison of placebo and iloprost 100 μg t.i.d., assuming 20% improvement of MWD in placebo group, and total 55% improvement for iloprost group; 80 patients per group would give 90% power at p = 0.05 level using two-tailed t-test |
| N randomised to treatments included in review | 430 |
| Treatment group | Pentoxifylline 1200-mg daily dose (400 mg t.i.d.) | Placebo | Iloprost 50 μg twice daily plus placebos to make up to three capsules t.i.d. | Iloprost 100 μg twice daily (increased in second week from 50 μg twice daily) plus placebos to make up to three capsules t.i.d. | Iloprost 150 μg twice daily (increased to 150 μg by 50 μg/week from 50 μg twice daily in first week) plus placebos to make up to three capsules t.i.d. |
|---|---|---|---|---|---|
| N randomised to treatment | 86 | 84 | 87 | 86 | 87 |
| Baseline characteristics | |||||
| Age (years) | 67.2 | 66.5 | 67.1 | 66.6 | 67.3 |
| Gender | M 78%; F 22% | M 82%; F 18% | M 83%; F 17% | M 86%; F 14% | M 77%; F 23% |
| Smokers | Currently smoking 31.4% | Currently smoking 33.3% | Currently smoking 31% | Currently smoking 38.4% | Currently smoking 27.6% |
| Diabetics | 24.4% | 33.3% | 31% | 23.3% | 29.9% |
| Hypertension/blood pressure | 72.1% | 71.4% | 71.3% | 68.6% | 75.9% |
| Hyperlipidaemia | 70.9% | 70.2% | 64.4% | 73.3% | 74.7% |
| Obesity or weight | |||||
| Angina | 30.2% | 31% | 32.2% | 32.6% | 26.4% |
| History of vascular therapy | Previous intervention (not defined further): 32.6% | Previous intervention (not defined further): 32.1% | Previous intervention (not defined further): 31.0% | Previous intervention (not defined further): 32.6% | Previous intervention (not defined further): 32.2% |
| Other |
History of MI: 30.2% Aspirin use: 75.6% Mean duration of claudication: 65.9 months |
History of MI: 34.5% Aspirin use: 72.6% Mean duration of claudication: 80.4 months |
History of MI: 29.9% Aspirin use: 71.3% Mean duration of claudication: 61.4 months |
History of MI: 27.9% Aspirin use: 74.4% Mean duration of claudication: 65.5 months |
History of MI: 36.8% Aspirin use: 70.1% Mean duration of claudication: 74.6 months |
| Withdrawals | |||||
| Withdrawals/loss to follow-up | SAEs leading to discontinuation, 15% (headache, 2%; pain in extremity, 0%; vasodilation, 0%; dyspepsia, 1%) | SAEs leading to discontinuation, 14% (headache, 1%; pain in extremity, 1%; vasodilation, 0%; dyspepsia, 1%) | SAEs leading to discontinuation, 31% (headache, 14%; pain in extremity, 6%; vasodilation, 1%; dyspepsia, 0%) | SAEs leading to discontinuation, 57% (headache, 36%; pain in extremity, 6%; vasodilation, 2%; dyspepsia, 0%) | SAEs leading to discontinuation, 53% (headache, 26%; pain in extremity, 6%; vasodilation, 2%; dyspepsia, 3%) |
| Results | |||||
| MWD n in analysis | NR (86 originally randomised, unclear how many dropped out of this group) | NR (84 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) | NR (86 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) |
| MWD baseline | Mean 316 (SD 191) m | Mean 292 (SD 161) m | Mean 244 (SD 164) m | Mean 312 (SD 193) m | Mean 289 (SD 171) m |
| MWD follow-up | NR | NR | NR | NR | NR |
| MWD change | 13.9% | 3.3% | 7.7% | 8.8% | 11.2% |
| MWD between-group comparison | Statistically significant (p = 0.039) difference for pentoxifylline only | ||||
| PFWD n in analysis | NR (86 originally randomised, unclear how many dropped out of this group) | NR (84 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) | NR (86 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) |
| PFWD baseline | Mean 118 (SD 83) m | Mean 120 (SD 88) m | Mean 105 (SD 81) m | Mean 124 (SD 96) m | Mean 129 (SD 88) m |
| PFWD follow-up | NR | NR | NR | NR | NR |
| PFWD change | 34.3% | 21.2% | 24% | 28.9% | 31.2% |
| PFWD between-group comparison | No significant difference | ||||
| ABPI n in analysis | |||||
| ABPI baseline | |||||
| ABPI follow-up | |||||
| ABPI change | |||||
| ABPI between-group comparison | |||||
| Vascular events n in analysis | NR (86 originally randomised, unclear how many dropped out of this group) | NR (84 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) | NR (86 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) |
| Vascular events follow-up | 26 weeks | ||||
| Vascular events included | Cardiovascular events that affected > 1% of any group with a ratio > 2.0 or < 0.5 in treatment groups compared with placebo | ||||
| Vascular events reported | 7% | 12% | 8% | 2% | 2% |
| Vascular events between-group comparison | Not numerically different | ||||
| AEs n in analysis | NR (86 originally randomised, unclear how many dropped out of this group) | NR (84 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) | NR (86 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) |
| AEs follow-up | 26 weeks (assumed) | ||||
| AEs reported | 69% | 59% | 77% | 88% | 90% |
| AEs between-group comparison | Statistical significance NR. Dose–response-like results seen for iloprost and headache and flushing. Other AEs occurred more frequently in iloprost groups: pain in extremities, jaw pain, nausea, diarrhoea. Mild dyspepsia occurred more frequently in pentoxifylline group. No meaningful numerical differences among groups in any specific cardiovascular events (angina, CHF, MI) | ||||
| Mortality reported | One (1.2%) | One (1.2%) | Zero | Zero | Zero |
| Mortality between-group comparison | Not numerically different | ||||
| HRQoL n in analysis | NR (86 originally randomised, unclear how many dropped out of this group) | NR (84 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) | NR (86 originally randomised, unclear how many dropped out of this group) | NR (87 originally randomised, unclear how many dropped out of this group) |
| HRQoL baseline | NR | NR | NR | NR | NR |
| HRQoL follow-up | NR | NR | NR | NR | NR |
| HRQoL change | Only differences seen in stair-climbing ability; 9% improvement compared with placebo | NA | Only differences seen in stair-climbing ability; 11% improvement compared with placebo | NR | Only differences seen in stair-climbing ability; 16% improvement compared with placebo |
| HRQoL between-group comparison | Stair-climbing ability statistically significant improvement for T1, T3 and T5. All other outcomes not statistically significant for WIQ and SF-36 | ||||
Trials of inositol nicotinate and placebo
| O’Hara 198878 | |
|---|---|
| Study details | |
| Publication type | O’Hara 1988,78 full report in peer-reviewed journal |
| Additional sources of data | O’Hara 198579 |
| Trial design | RCT, multicentre |
| Country | UK |
| Dates of participant recruitment | NR |
| Sources of funding | Winthrop Laboratories, for drugs and statistical analysis |
| Intervention(s) and comparator | |
| Treatment groups | Inositol nicotinate 4-g daily dose (4 × 500-mg tablets b.i.d.) |
| Comparator | Placebo |
| Run-in phase | No |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 12 weeks |
| Outcomes and measures |
PFWD: training device (pair of stirrups which moved in opposition in a near vertical plane by means of an interconnecting belt and pulley mechanism in a supporting metal frame), which simulated box-stepping. Elapsed time and number of steps to claudication were recorded. (Some information from O’Hara 1985. 79) Time to recovery from claudication pain was recorded. Waist-band pedometer to record ‘similar weekly walks’ Vascular events: not systematically reported. Some given in withdrawals AEs: Subjective complaints were sought by the question ‘How did the medication suit you?’ |
| Notes on statistics | Wilcoxon matched pairs signed-rank and two-sample tests, Student’s t-tests (paired and unpaired), or chi-squared test as appropriate |
| Population | |
| Eligibility criteria | Male or female with clinical diagnosis of IC, which limited walking to 500 yards (457 m). Aged 50–75 years. Weighing 40–100 kg. Exclusions: insulin-dependent diabetes, severe angina, rest pain or gangrene, non-vascular causes of IC, symptomatic treatment for claudication pain within the month preceding entry to the study, malignant diseases, gross renal or hepatic impairment and arterial surgery for claudication within previous 3 years |
| Concomitant interventions allowed or excluded | NR |
| Power calculation | NR |
| N randomised to treatments included in review | 120 |
| Treatment group | Inositol nicotinate 4-g daily dose | Placebo |
|---|---|---|
| N randomised to treatment | 62 | 58 |
| Baseline characteristics | ||
| Age | Mean 66.2 (SE 0.7) years | Mean 65.6 (SE 1.0) years |
| Gender | M 64.5%; F 35.5% | M 72.4%; F 27.6% |
| Smokers | 64.5% | 50% |
| Diabetics | 4.8% | 5.2% |
| Hypertension/blood pressure | Mean 161.4 (SE 2.4)/87.6 (SE 1.4) | Mean 152.7 (SE 2.5)/84.7 (SE 1.2) |
| Hyperlipidaemia | ||
| Obesity or weight | Weight mean 69.3 (SE 1.3) kg | Weight mean 71.8 (SE 1.0) kg |
| Angina | ||
| History of vascular therapy | ||
| Other | Duration mean 2.3 (SE 0.4) years | Duration mean 2.8 (SE 0.5) years |
| VAS pain score mean 62.1 (SE 2.1) mm | VAS pain score mean 56.7 (SE 2.4) mm | |
| No. of cigarettes smoked per day mean 16.1 (SE 1.2) | No. of cigarettes smoked per day mean 18.3 (SE 1.6) | |
| Withdrawals | ||
| Withdrawals/loss to follow-up | {O’Hara 1985:79 five withdrawals [personal choice (two), stroke (one), gastrointestinal complaints (one), and ‘too many tablets’ (one)]} | {O’Hara 1985:79 seven withdrawals [personal choice (two), persistent illness (one), death (O’Hara 198878 suggests this was unrelated to IC) (one), MI (one), general malaise (one), rash (one)]} |
| Results | ||
| MWD n in analysis | ||
| MWD baseline | ||
| MWD follow-up | ||
| MWD change | ||
| MWD between-group comparison | ||
| PFWD n in analysis | 57 | 51 |
| PFWD baseline |
Free walking paces (weekly): mean 455.2 (SE 78.5) Claudication time (s): mean 129.2 (SE 16) |
Free walking paces (weekly): mean 617.2 (131.3) Claudication time (s): mean 102.4 (SE 12.2) |
| PFWD follow-up | (Only reported as change from baseline – see below) | (Only reported as change from baseline – see below) |
| PFWD change |
Free walking paces (weekly): mean 469.6 (SE 183.7) Claudication time (s): mean 43.3 (SE 21) |
Free walking paces (weekly): mean 325.4 (SE 220.6) Claudication time (s): mean 28.6 (SE 17.9) |
| PFWD between-group comparison | Free walking paces: within group comparisons significant for both T1 and T2. Between-group comparisons only significant for T1. Claudication time: between-group comparisons of change from baseline were not significant at p = 0.05. Within group comparisons of change from baseline were significant for inositol at 3 months, but not for placebo | |
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 62 | 58 |
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | Stroke, one – also reported in withdrawals | MI, one – also reported in withdrawals |
| Vascular events between-group comparison | ||
| AEs n in analysis | 62 | 58 |
| AEs follow-up | ||
| AEs reported | [O’Hara 1985:79 16.1% patients reported minor side effects, mostly related to difficulty in swallowing tablets] | [O’Hara 1985:79 19.0% patients reported minor side effects, mostly related to difficulty in swallowing tablets] |
| AEs between-group comparison | ||
| Mortality reported | Zero | One – also reported in withdrawals |
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Kiff 198880 | |
|---|---|
| Study details | |
| Publication type | Kiff 1988,80 full report in peer-reviewed journal |
| Additional sources of data | Unclear whether or not the patients are the same as some patients in O’Hara 198878 and O’Hara 1985.79 Different outcomes reported using different techniques |
| Trial design | RCT |
| Country | UK |
| Dates of participant recruitment | March 1984 to January 1986 |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Inositol nicotinate 4-g daily dose (2 g b.i.d.) |
| Comparator | Placebo |
| Run-in phase | No |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 12 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 10% gradient ABPI: Doppler ultrasound flow detector and sphygmomanometer at rest |
| Notes on statistics | Wilcoxon matched pairs signed-rank test or student’s paired t-tests as appropriate |
| Population | |
| Eligibility criteria |
Inclusion: stable IC (duration of symptoms of at least 6 months), PAD confirmed by resting ankle pressure index of < 0.9 or a drop in ankle pressure with exercise of > 30 mmHg. All patients had palpable femoral pulses and could walk between 35 and 500 m on a treadmill. Any medication for IC stopped 1 month before trial Exclusion: walking distance on treadmill > 500 m, serious medical disease, rest pain or gangrene, treatment with beta-blockers which was not stabilised or arterial surgery for claudication within the previous 3 months |
| Concomitant interventions allowed or excluded | NR |
| Power calculation | NR |
| N randomised to treatments included in review | 80 |
| Treatment group | Inositol nicotinate 4-g daily dose (2-g b.i.d.) | Placebo |
|---|---|---|
| N randomised to treatment | 40 | 40 |
| Baseline characteristics | ||
| Age | Mean 61.5 (SD 9.3) years | Mean 62.8 (SD 7.3) years |
| Gender | M 82.5%; F 17.5% | M 77.5%; F 22.5% |
| Smokers | 57.5% | 72.5% |
| Diabetics | ||
| Hypertension/blood pressure | Mean 153.6 (SD 23.9) mmHg/87.5 (SD 10.6) mmHg | Mean 152.9 (SD 24.1) mmHg/88.3 (SD 10.5) mmHg |
| Hyperlipidaemia | ||
| Obesity or weight | ||
| Angina | ||
| History of vascular therapy | ||
| Other | Duration mean 2.5 (SD 1.8) years | Duration mean 1.6 (SD 1.1) years |
| VAS pain score mean 49.1 (SD 22.6) mm | VAS pain score mean 53.4 (SD 17.8) mm | |
| Estimate of free walking mean 330.6 (SD 219) yards | Estimate of free walking mean 309.1 (SD 239.7) yards | |
| Withdrawals | ||
| Withdrawals/loss to follow-up | Eight withdrawals [reasons were eight out of: moved from district (three), family problems (two), felt unwell taking tablets (two), personal choice (four), referred for surgery (one), hospitalised for an unrelated condition (one)] | Seven withdrawals [reasons were nausea and vomiting (one), constipation (one) and five out of: moved from district (three), family problems (two), felt unwell taking tablets (two), personal choice (four), referred for surgery (one), hospitalised for an unrelated condition (one)] |
| Results | ||
| MWD n in analysis | Initially 40 – assume 12 weeks minus withdrawals (32) | Initially 40 – assume 12 weeks minus withdrawals (33) |
| MWD baseline | Mean 131.7 (SD 80.4) (n = 40) | Mean 118.4 (SD 70.9) (n = 40) |
| MWD follow-up | Mean 197.1 (SD 125.7) (assume n = 32) | Mean 221.2 (SD 154.2) (assume n = 33) |
| MWD change | Calculated: 65.4, p < 0.05 | 102.8, p < 0.05 |
| MWD between-group comparison | No statistically significant difference between the groups | |
| PFWD n in analysis | ||
| PFWD baseline | ||
| PFWD follow-up | ||
| PFWD change | ||
| PFWD between-group comparison | ||
| ABPI n in analysis | Initially 40 – assume minus withdrawals (32) at 12 weeks | Initially 40 – assume minus withdrawals (33) at 12 weeks |
| ABPI baseline | Mean 0.718 (SD 0.144) m | Mean 0.694 (SD 0.215) m |
| ABPI follow-up | NR | NR |
| ABPI change | Not significant | Not significant |
| ABPI between-group comparison | Not significant | |
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | As for withdrawals | As for withdrawals |
| AEs follow-up | ||
| AEs reported | ||
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
| Head 198681 | |
|---|---|
| Study details | |
| Publication type | Head 1986,81 full report in peer-reviewed journal |
| Additional sources of data | |
| Trial design | RCT, multicentre |
| Country | UK |
| Dates of participant recruitment | NR |
| Sources of funding | NR |
| Intervention(s) and comparator | |
| Treatment groups | Inositol nicotinate 4-g daily dose (1-g q.i.d.) |
| Comparator | Placebo |
| Run-in phase | No |
| Treatment duration | 12 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 12 weeks |
| Outcomes and measures |
PFWD: time to claudication was recorded: a metronome was set at 80 beats/minute and each patient was instructed to climb up and down the first two steps of a standard ladder with a rung interval of 19 cm. Patients climbed one step at a time to the beat of the metronome, leading with the worse leg and bringing the other leg up before proceeding to the next step and then returning to the ground in a similar fashion. The time to onset of calf pain was recorded using a stopwatch, and pressure readings repeated AEs: elicited by question ‘How did the tablets suit you?’ |
| Notes on statistics | NR |
| Population | |
| Eligibility criteria | Patients with clinical diagnosis of IC due to vascular insufficiency. Male or female, aged between 18 and 80 years, weigh between 40 and 100 kg and be judged suitable to receive a 3-month course of inositol nicotinate 1-g q.d. or matching placebo |
| Concomitant interventions allowed or excluded | NR |
| Power calculation | NR |
| N randomised to treatments included in review | 123 |
| Treatment group | Inositol nicotinate 4-g daily dose | Placebo |
|---|---|---|
| N randomised to treatment | 51 (plus unspecified number who withdrew) | 62 (plus unspecified number who withdrew) |
| Baseline characteristics | ||
| Age | Severe (IC < 60 seconds): mean 68.6 (SD 7.7) | Severe (IC < 60 seconds): mean 64.3 (SD 7.6) |
| Moderate (IC 60–120 seconds): mean 67.0 (SD 6.7) | Moderate (IC 60–120 seconds): mean 64.8 (SD 7.7) | |
| Mild (IC > 120 seconds): mean 65.0 (SD 14.4) | Mild (IC > 120 seconds): mean 61.6 (SD 13.4) | |
| Gender | Severe (IC < 60 seconds): M 78.9%; F 21.1% | Severe (IC < 60 seconds): M 66.7%; F 33.3% |
| Moderate (IC 60–120 seconds): M 84.6%; F 15.4% | Moderate (IC 60–120 seconds): M 81.3%; F 18.7% | |
| Mild (IC > 120 seconds): M 66.7%; F 33.3% | Mild (IC > 120 seconds): M 55.6%; F 44.4% | |
| Smokers | Severe (IC < 60 seconds): 57.9% | Severe (IC < 60 seconds): 47.6% |
| Moderate (IC 60–120 seconds): 73.1% | Moderate (IC 60–120 seconds): 46.9% | |
| Mild (IC > 120 seconds): 33.3% | Mild (IC > 120 seconds): 44.4% | |
| Diabetics | Severe (IC < 60 seconds): 15.8% | Severe (IC < 60 seconds): 4.8% |
| Moderate (IC 60–120 seconds): 0% | Moderate (IC 60–120 seconds): 3.1% | |
| Mild (IC > 120 seconds): 0% | Mild (IC > 120 seconds): 0% | |
| Hypertension/blood pressure | All in mmHg: | All in mmHg: |
| Severe (IC < 60 seconds): mean 162.1 (SD 23.3)/85.7 (SD 8.2) | SEVERE (IC < 60 seconds): mean 164.3 (SD 19.9)/92.6 (SD 10.1) | |
| Moderate (IC 60–120 seconds): mean 159.4 (SD 21.1)/88.6 (SD 12.3) | Moderate (IC 60–120 seconds): mean 163.3 (SD 29.8)/89.7 (SD 16.6) | |
| Mild (IC > 120 seconds): mean 160 (SD24.5)/83.0 (SD 12.2) | Mild (IC > 120 seconds): mean 155.7 (SD 13.2)/85.3 (SD 8.5) | |
| Hyperlipidaemia | ||
| Obesity or weight | Severe (IC < 60 seconds): mean 69.3 (SD 13.4) kg | Severe (IC < 60 seconds): mean 68.0 (SD 11.3) kg |
| Moderate (IC 60–120 seconds): mean 72.0 (SD 11.7) kg | Moderate (IC 60–120 seconds): mean 73.4 (SD 11.7) kg | |
| Mild (IC > 120 seconds): mean 69.6 (SD 4.8) kg | Mild (IC > 120 seconds): mean 72.3 (9.7) kg | |
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up | Broken ankle, one; inability to swallow, one; constipation, one; non-compliance, one | Cerebrovascular accident, one; thrombophlebitis, one; gastrointestinal upset, two; personal reasons, one |
| Also, 10 patients were excluded from analysis, unclear which groups they were from | Also, 10 patients were excluded from analysis, unclear which groups they were from | |
| Reasons were: congestive cardiac failure, three; osteoarthritis, two; severe leg pain at rest, one; carcinoma of the stomach with secondaries in the liver, one; failure to return, one; leukaemia, one; rheumatoid arthritis, one | Reasons were: congestive cardiac failure, three; osteoarthritis, two; severe leg pain at rest, one; carcinoma of the stomach with secondaries in the liver, one; failure to return, one; leukaemia, one; rheumatoid arthritis, one | |
| Results | ||
| MWD n in analysis | ||
| MWD baseline | ||
| MWD follow-up | ||
| MWD change | ||
| MWD between-group comparison | ||
| PFWD n in analysis | 47 | 57 |
| PFWD baseline | PFW time (s): | PFW time (s): |
| Severe: mean 44.42 (SD 14.78) | Severe: mean 44.33 (SD 14.81) | |
| Moderate: mean 85.23 (SD 15.96) | Moderate: mean 88.53 (SD 17.21) | |
| Mild: mean 183.5 (SD 66.67) | Mild: mean 156.9 (SD 19.71) | |
| PFWD follow-up | PFW time (s): | PFW time (s): |
| Severe: mean 59.59 (SD 28.08) | Severe: mean 64.86 (SD 36.70) | |
| Moderate: mean 105.50 (SD 36.71) | Moderate: mean 97.11 (SD 36.25) | |
| Mild: mean 156.2 (SD 40.87) | Mild: mean 194.6 (SD 93.49) | |
| PFWD change | PFW time (s): | PFW time (s): |
| Severe: p < 0.05 | Severe: p < 0.01 | |
| Moderate: p < 0.01 | Moderate: p < 0.01 | |
| Mild: non-significant | Mild: non-significant | |
| PFWD between-group comparison |
PFW time (s): Severe: non-significant Moderate: significant between-group comparison p < 0.001 Mild: non-significant |
|
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | 51 | 62 |
| Vascular events follow-up | ||
| Vascular events included | Taken from AEs | |
| Vascular events reported | Zero | Cerebrovascular accident, one; thrombophlebitis, one – also reported in AEs |
| Vascular events between-group comparison | ||
| AEs n in analysis | Baseline, 51; 12 weeks, 47 | Baseline, 62; 12 weeks 57 |
| AEs follow-up | ||
| AEs reported | 4/51 (7.8%). Broken ankle, one (2%); inability to swallow, one (2%); constipation, one (2%); non-compliance, one (2%) | 5/62 (8.1%). Cerebrovascular accident, one (1.6%); thrombophlebitis, one (1.6%); gastrointestinal upset, two (3.2%); personal reasons, one (1.6%) |
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
Trials testing intervention against other treatments
| Hobbs 2007,82 INEXACT | |
|---|---|
| Study details | |
| Publication type | Hobbs 2007,82 full report in peer-reviewed journal |
| Additional sources of data | None |
| Trial design | RCT, single centre |
| Country | UK |
| Dates of participant recruitment | NR |
| Sources of funding | S Hobbs is supported by a British Heart Foundation Junior Research Fellowship and the Royal College of Surgeons of England ‘Lea Thomas’ Research Fellowship |
| Intervention(s) and comparator | |
| Treatment groups | Cilostazol 200 mg (100 mg b.i.d.). If side effects, dosing halved for 1 week, with or without exercise |
| Comparator | Usual care, with or without exercise |
| Run-in phase | No |
| Treatment duration | Unclear: 3 or 6 months. Follow-up 24 weeks |
| Outcome(s) | |
| Follow-up | Baseline, 12 weeks, 24 weeks |
| Outcomes and measures |
MWD: treadmill with constant workload, 3 km/hour at a 10% incline PFWD: as MWD AEs: patient self-report |
| Notes on statistics | None |
| Population | |
| Eligibility criteria | IC diagnosed by Edinburgh claudication questionnaire and reduced ABPI < 0.9, reviewed after 3–6 months; MWD 20–500 m. Excluded: significant aortoiliac disease; unable to complete treadmill assessment to absolute claudication distance; MI, TIA, CVA or PTCA in past 3 months; GFR 20 ml/minute, CHF, known predisposition for bleeding |
| Concomitant interventions allowed or excluded |
Allowed: antiplatelets, statins, antihypertensives, ACE inhibitor Disallowed: CYP3A4 or CYP2C19 inhibitors (cimetidine, diltiazem, erythromycin, ketoconazole, lansoprazole, omeprazole and human immunodeficiency virus 1 protease inhibitors) |
| Power calculation | 32 subjects were required to detect a 50% reduction in thrombin–antithrombin complex (outcome NR in this review) in the treatment groups with 80% power and a p-value of < 0.05 |
| N randomised to treatments included in review | 34 |
| Treatment group | Cilostazol 100 mg b.i.d. | Usual care |
|---|---|---|
| N randomised to treatment | 16 (nine cilostazol alone, seven cilostazol plus exercise) | 18 (seven usual care alone, nine usual care plus exercise) |
| Baseline characteristics | ||
| Age | Mean 58 (52 to 71) years | Mean 67 (63.5 to 74) years |
| Gender | M 89% | M 78% |
| Smokers | 33% | 22% |
| Diabetics | ||
| Hypertension/blood pressure | (n = 6 on antihypertensives) | (n = 8 on antihypertensives) |
| Hyperlipidaemia | ||
| Obesity or weight | ||
| Angina | ||
| History of vascular therapy | ||
| Other | ||
| Withdrawals | ||
| Withdrawals/loss to follow-up | [NR by group. Of 38 participants recruited, four subjects withdrew after randomisation (three no longer wished to continue to participate in the trial, and one subject sustained a fractured ankle unrelated to trial participation)] | |
| Results | ||
| MWD n in analysis | 16 | 18 |
| MWD baseline | ||
| MWD follow-up | ||
| MWD change | p = 0.008 mean ratio 1.69 (SD 0.59) | p = 0.635 mean ratio 1.09 (SD 0.34) |
| MWD between-group comparison | Cilostazol vs no cilostazol (combined groups, not just usual care group) effect 1.64, p = 0.005 | |
| PFWD n in analysis | ||
| PFWD baseline | ||
| PFWD follow-up | ||
| PFWD change | ||
| PFWD between-group comparison | ||
| ABPI n in analysis | ||
| ABPI baseline | ||
| ABPI follow-up | ||
| ABPI change | ||
| ABPI between-group comparison | ||
| Vascular events n in analysis | ||
| Vascular events follow-up | ||
| Vascular events included | ||
| Vascular events reported | ||
| Vascular events between-group comparison | ||
| AEs n in analysis | ||
| AEs follow-up | ||
| AEs reported | ||
| AEs between-group comparison | ||
| Mortality reported | ||
| Mortality between-group comparison | ||
| HRQoL n in analysis | ||
| HRQoL baseline | ||
| HRQoL follow-up | ||
| HRQoL change | ||
| HRQoL between-group comparison | ||
Walking distance and HRQoL outcome measures used in included studies
| Trial name | Treatment and dose | Outcome measures for PFWD and MWD | Outcome measures for HRQoL |
|---|---|---|---|
| CASTLE, Hiatt 200848–50 | Cilostazol 200 mg | NR | NR |
| O’Donnell 200951,53–55,83 | Cilostazol 200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 10% gradient |
SF-36 VascuQoL |
| Strandness 200256,57 | Cilostazol 200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
SF-36 WIQ COM |
| Dawson 200058–60 | Cilostazol 200 mg, pentoxifylline 1200 mg |
Treadmill with graded test: 3.2 km/hour (2 mph) 0% gradient with a 3.5% increase in gradient every 3 minutes |
SF-36 WIQ |
| Beebe 199961 | Cilostazol 200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
SF-36 WIQ COM |
| Otsuka 21-94-30134 | Cilostazol 200 mg, pentoxifylline 1200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
NR |
| Otsuka 21-98-21334 | Cilostazol 200 mg, pentoxifylline 1200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
SF-36 WIQ COM |
| Money 199862 | Cilostazol 200 mg |
Treadmill with graded test: 3.2 km/hour (2 mph) 0% gradient with a 3.5% increase in gradient every 3 minutes |
SF-36 WIQ |
| Dawson 199863 | Cilostazol 200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
NR |
| Elam 199864 | Cilostazol 200 mg |
Treadmill with graded test: 3.2 km/hour (2 mph) 0% gradient with a 3.5% increase in gradient every 3 minutes |
NR |
| Otsuka 21-95-20134 | Cilostazol 200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
SF-36 WIQ |
| INEXACT, Hobbs 200782 | Cilostazol 200 mg, cilostazol 200 mg plus supervised exercise |
Treadmill with constant workload: 3 km/hour 10% gradient |
NR |
| Spengel 200247 | Naftidrofuryl oxalate 600 mg | Estimated by patient | CLAU-S |
| Kieffer 200165 | Naftidrofuryl oxalate 600 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 10% gradient |
NR |
| Adhoute 198666 | Naftidrofuryl oxalate 600 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 10% gradient |
NR |
| Trubestein 198467 | Naftidrofuryl oxalate 600 mg |
Treadmill with constant workload: 5 km/hour 10% gradient, performed twice with at least 20 minutes interval |
NR |
| Ruckley 197868 | Naftidrofuryl oxalate 300 mg |
Unclear if treadmill used < 100 yards = severe 100–200 yards = moderate > 200 yards = mild |
NR |
| Dettori 198969 | Pentoxifylline 1200 mg |
Treadmill with varied workload: 3 km/hour. If PFW > 30 minutes, higher speed was used in the second test (5 km/hour) 10% gradient |
NR |
| Creager 200870 | Pentoxifylline 1200 mg |
Treadmill with graded test: 3.2 km/hour (2 mph) 0% gradient, increased by 2% every 2 minutes |
SF-36 WIQ |
| Lindgarde 198971 | Pentoxifylline 1200 mg |
Treadmill with constant workload: 3.2 km/hour (2 mph) 12.5% gradient |
NR |
| Porter 198272,74 and Gillings 198773,75 | Pentoxifylline 1200 mg |
Treadmill with constant workload: 1.5 mph 7° gradient, two treadmill tests were performed at 30- to 60-minute intervals and the mean of the two tests used |
NR |
| Gallus 198576 | Pentoxifylline 1200 mg |
Treadmill with constant workload: 4 km/hour 10° gradient |
NR |
| Di Perri 198377 | Pentoxifylline 1200 mg | Absolute distance covered by walking on horizontal level at metronome controlled speed of 120 steps/minute. Walking test was performed three times and a mean taken | NR |
| O’Hara 198878,79 | Inositol nicotinate 4 g | Training device (pair of stirrups in a metal frame), which simulated box-stepping. Elapsed time and number of steps to claudication and time to recovery were recorded. Waist-band pedometer to record ‘similar weekly walks’ | NR |
| Kiff 198880 | Inositol nicotinate 4 g |
Treadmill with constant workload: 10% gradient |
NR |
| Head 198681 | Inositol nicotinate 4 g | Time to claudication. Patients climbed up and down the first two steps of a standard ladder in time with a metronome set at 80 beats/minute leading with the worse leg | NR |
Appendix 5 Statistical methods used within meta-analysis
We present the basic details for the meta-analysis of the data described in this report.
For treatment j in study i, we have an observation vector, yij, such that:
where x¯ij is the sample mean for treatment j in study i, and sij/nij is the standard error for treatment j in study i.
We assume that the sample means, x¯ij, are normally distributed such that:
and that μij = ɸ1 + θij.
ɸi is the effect of study i, and θij is the effect of treatment j in study i.
We treat the ɸi as nuisance parameters with fixed (but unknown) study effects and give them weak prior distributions such that ɸ1 ∼ N(0,10,000).
We assume a random (treatment) effects model in which the θij are assumed to come from a common population distribution such that θij ∼ N(μθ1,τ2). To make the parameters identifiable, we set μθ1 = 0 so that ɸi is the effect of the control group in study i, and μθ1 is the population mean effect of treatment j relative to treatment 1.
We give μθ1, j ≠ 1 a weak prior distribution such that θij ∼ N(μθ1,τ2).
τ represents the between-study SD, which we give a prior uniform distribution, τ ∼ U(0,10).
We assume that the sample variances, sij2, are gamma distributed such that:
The model is completed by giving the log of the population SD a prior uniform distribution such that log(σ) ∼ U(0,10).
The model for the network meta-analyses differs from this basic model in two particular ways. First, the estimates of treatment effect within each study are represented as functions of each treatment effect relative to placebo. Second, it is acknowledged that three of the studies are multi-arm studies in which there will be correlation between treatment effects.
For each study it was assumed that the sample SDs were the same in each treatment arm of the study within study.
Sample SDs on the log scale generally had to be derived. In some cases, these were derived from the mean and CI for the difference between treatments in geometric mean change from baseline; in others it was derived from the treatment mean changes from baseline and the p-value for the comparison between treatments.
Appendix 6 Economic evaluation checklist
Drummond adapted criteria
For details see Drummond and Jefferson. 133
| Criteria | Guest 200590 | Ratcliffe 200591 |
|---|---|---|
| 1. Was a well-defined question posed in answerable form? | Yes | Yes |
| 2. Was a comprehensive description of the competing alternatives given? | Yes | Unclear |
| 3. Was the effectiveness of the programme or services established? | Yes | Yes |
| 4. Were all the important and relevant costs and consequences for each alternative identified? | Yes | Unclear |
| 5. Were costs and consequences measured accurately in appropriate physical units? | Yes | Unclear |
| 6. Were the cost and consequences valued credibly? | Yes | Unclear |
| 7. Were costs and consequences adjusted for differential timing? | Not available | Not available |
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Yes |
| 9. Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Unclear |
| 10. Did the presentation and discussion of study results include all issues of concern to users? | Yes | Unclear |
| Consensus on Health Economic Criteria list (Evers 2005134) | ||
| 11. Is the study population clearly described? | Yes | Yes |
| 12. Are competing alternatives clearly described? | Yes | Yes |
| 13. Is a well-defined research question posed in answerable form? | Yes | Yes |
| 14. Is the economic study design appropriate to the stated objective? | Yes | Yes |
| 15. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes |
| 16. Is the actual perspective chosen appropriate? | Yes | Yes |
| 17. Are all important and relevant costs for each alternative identified? | Yes | Unclear |
| 18. Are all costs measured appropriately in physical units? | Yes | Unclear |
| 19. Are costs valued appropriately? | Yes | Unclear |
| 20. Are all important and relevant outcomes for each alternative identified? | Yes | Unclear |
| 21. Are all outcomes measured appropriately? | Yes | Yes |
| 22. Are outcomes valued appropriately? | Not available | Yes |
| 23. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | Yes |
| 24. Are all future costs and outcomes discounted appropriately? | Not available | Not available |
| 25. Are all important variables, whose values are uncertain, appropriately subjected to SA? | Yes | Unclear |
| 26. Do the conclusions follow from the data reported? | Yes | Yes |
| 27. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | Unclear |
| 28. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Yes | Unclear |
| 29. Are ethical and distributional issues discussed appropriately? | No | Unclear |
Glossary
Technical terms and abbreviations are used throughout this report. The meaning is usually clear from the context, but a glossary is provided for the non-specialist reader.
- Arithmetic mean
- A measure of central tendency calculated as the sum of all of the numbers in a series divided by the count of all numbers in the series.
- Dominated (simple)
- Where an intervention is less effective and more expensive than its comparator.
- Dominated (extended)
- Where the incremental cost-effectiveness ratio for a given treatment alternative is higher than that of the next more effective comparator.
- Geometric mean
- A measure of central tendency calculated by multiplying a series of numbers and taking the nth root of the product, where n is the number of items in the series.
- Meta-analysis
- A statistical method by which the results of a number of studies are pooled to give a combined summary statistic.
- Posterior distribution
- A representation of the knowledge associated with the true value of a population parameter after combining the prior distribution with sample data.
- Prior distribution
- A representation of the knowledge associated with the true value of a population parameter in addition to any sample data.
- Relative risk
- Ratio of the probability of an event occurring in an exposed group relative to a non-exposed or control group.
List of abbreviations
- ABPI
- ankle–brachial pressure index
- AE
- adverse event
- b.i.d.
- twice a day
- CEAC
- cost-effectiveness acceptability curve
- CHEC
- Consensus on Health Economic Criteria
- CHF
- congestive heart failure
- CI
- confidence interval
- COM
- claudication outcome measure
- CRD
- Centre for Reviews and Dissemination
- EMA
- European Medicines Agency
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- IC
- intermittent claudication
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- log
- logarithm
- MI
- myocardial infarction
- MWD
- maximal walking distance
- NICE
- National Institute for Health and Clinical Excellence
- ONS
- Office for National Statistics
- PAD
- peripheral arterial disease
- PFWD
- pain-free walking distance
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SA
- sensitivity analysis
- SAE
- serious adverse event
- ScHARR
- School of Health and Related Research
- ScHARR-TAG
- ScHARR Technology Assessment Group
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- t.i.d.
- three times a day
- VascuQoL
- vascular quality of life
- WHOQoL
- World Health Organization Quality of Life
- WIQ
- Walking Impairment Questionnaire
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington