Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 10/111/01. The contractual start date was in November 2010. The draft report began editorial review in May 2011 and was accepted for publication in August 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Vaccines used in the original study were manufactured by GlaxoSmithKline and Baxter, both of which donated the vaccine, but had no role in study planning or conduct. The vaccine used in this follow-on study was manufactured by GlaxoSmithKline, from whom it was purchased. AJP, AF, PTH and SF act as chief or principal investigators for clinical trials conducted on behalf of their respective NHS trusts and/or universities, sponsored by vaccine manufacturers, but receive no personal payments from them. KH has been an investigator for clinical trials sponsored by vaccine manufacturers, but received no personal payments from them. AJP, AF, PTH and SF have participated in advisory boards for vaccine manufacturers, but receive no personal payments for this work. MDS, PTH, SF, KH and AF have received financial assistance from vaccine manufacturers to attend conferences. All grants and honoraria are paid into accounts within the respective NHS trusts or universities, or to independent charities.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by de Whalley et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In 2009, a novel influenza A H1N1 strain (A/California/07/2009), first reported in Mexico, spread rapidly around the globe. 1,2 In the UK, the first cases were confirmed on 27 April 20093 and the World Health Organization declared a global pandemic on 11 June 2009. 4 The UK experienced two waves of infection in 2009, peaking in July and October. 5 There was a further resurgence of infection during the 2010–11 influenza season, peaking in late December 2010 and early January 2011. 6
Before the pandemic, children were less likely to possess protective antibody than adults and during the pandemic they experienced higher rates of infection. 7,8 In England, between 26 June 2009 and 22 March 2010, there were 70 deaths attributable to H1N1 influenza in those aged < 18 years. 9 Children also effectively transmit the virus. 10 For these reasons, children have been identified as a high-priority group for vaccination against pandemic influenza. 11
In 2009, the UK Government purchased two monovalent H1N1 vaccines: an AS03B-adjuvanted, split-virion vaccine, derived from egg culture (Pandemrix®, GlaxoSmithKline, Rixensart, Belgium), and a non-adjuvanted, whole-virion vaccine, derived from Vero cell culture (Celvapan®, Baxter, Vienna, Austria). The AS03B-adjuvanted vaccine was the predominant vaccine used for immunisation of both children and adults. Initially, from late October 2009, two doses of vaccine at least 3 weeks apart were offered to children > 6 months old who were in the clinical risk groups defining eligibility for seasonal influenza vaccine. 12 In the second phase of the vaccination programme, announced in November 2009, monovalent H1N1 vaccine was offered to all children from 6 months to 5 years of age, because of the frequency of hospital admission in this age group. 13 A single-dose regimen for immunocompetent children was adopted from December 2010. 14 By 31 March 2010, the overall uptake of the vaccine in this age group was 23.6% in England and 44.6% in Scotland. 5
The trivalent seasonal influenza vaccines used in the 2010–11 influenza season included the A/California/07/2009 (H1N1) strain. 15 These vaccines were offered to children in clinical risk groups only.
There are no previous reports of the persistence of antibody in children following vaccination for pandemic H1N1 influenza,16 and no previous data on the immunogenicity and reactogenicity of trivalent seasonal influenza vaccine given to those who have previously received pandemic H1N1 influenza vaccine. This information is important in assisting the formulation of vaccination policy. Knowledge of the persistence of antibody following vaccination is also valuable in the construction of disease transmission models, which are an important component of the UK’s pandemic influenza plan. 8
In an earlier study, conducted between September and December 2009, we compared the immunogenicity and reactogenicity of the two monovalent pandemic H1N1 influenza vaccines used in the UK, an AS03B-adjuvanted, split-virion vaccine and a non-adjuvanted, whole-virion vaccine. 17,18 Eight hundred and ninety-four children, aged from 6 months to 12 years, completed this study. The AS03B-adjuvanted vaccine was significantly more immunogenic than the non-adjuvanted vaccine, particularly in children < 3 years of age. For this age group, the seroconversion rate was 98.2% in those children who received the AS03B-adjuvanted vaccine, compared with 80.1% in recipients of the non-adjuvanted vaccine (p < 0.001). Seroconversion was defined as a fourfold rise in the microneutralisation (MN) titre to a value of ≥ 1 : 40, from before the first dose to 3 weeks after the second dose of vaccine. The AS03B-adjuvanted vaccine was also more reactogenic, with more local and systemic symptoms reported in the week following vaccination.
The present study followed up this cohort of children, 1 year later. The persistence of antibody was assessed by both MN and haemagglutination inhibition (HI) assays. A single dose of trivalent seasonal influenza vaccine was given and antibody titres were assessed 3 weeks later.
The study also provided an opportunity to monitor the long-term safety of the novel pandemic influenza vaccines and to store sera from children who received them. This could be particularly useful should a drifted strain of the virus emerge in the future, as it would allow rapid assessment of cross-protection. The sera are stored at the individual study sites.
Chapter 2 Methods
Participants
In the original study, children aged 6 months to 12 years were recruited by five UK sites (Southampton, Oxford, Bristol, London and Exeter). They were randomised (in a 1 : 1 ratio, with assignment by sequentially numbered, identical, opaque sealed envelopes) to receive two doses, 21 days apart, of either a non-adjuvanted whole-virion H1N1 influenza vaccine or an AS03B-adjuvanted split-virion H1N1 influenza vaccine. Children who completed the original study were invited to participate in this follow-on study, although those who had subsequently received a further dose of the H1N1 vaccine owing to an insufficient response to the original two doses of vaccine were excluded, as were those who had already received a dose of the 2010–11 trivalent seasonal influenza vaccine. Other exclusion criteria were severe allergic reaction following previous vaccination, suspected unexpected severe adverse reaction in the original study, current egg allergy, impaired immunity, receipt of blood products or > 1 week of systemic steroid treatment within the last 3 months, or participation in another clinical trial. Written informed consent was obtained from a parent or guardian, and verbal assent was sought from children aged ≥ 7 years. Enrolment took place in November and December 2010.
Study design
This was a multicentre, open-label, phase IV study, following on from a randomised trial. At the first study visit, a blood sample was taken to assess the persistence of antibody. A single dose of trivalent seasonal influenza vaccine was given by intramuscular injection (into the deltoid muscle). A second blood sample was taken 21 days later (protocol time window 14–35 days). For those who wanted to participate in the study, but who did not wish to receive the trivalent seasonal influenza vaccine, an option was available to consent to only the first blood test.
The study was approved by the UK Medicines and Healthcare products Regulatory Agency (EudraCT number 2010-022817-24), the Oxfordshire Research Ethics Committee A (10/H0604/81) and the local NHS organisations. The study was registered at ClinicalTrials.gov (NCT01239537).
Vaccines
The original study compared two novel H1N1 vaccines: a non-adjuvanted whole-virion H1N1 influenza vaccine (Celvapan) and an AS03B-adjuvanted split-virion H1N1 influenza vaccine (Pandemrix).
The non-adjuvanted, whole-virion vaccine was derived from Vero cell culture. Each dose (0.5 ml) contained 7.5 µg of haemagglutinin from influenza A/California/07/2009 (H1N1).
The AS03B-adjuvanted split-virion H1N1 influenza vaccine was derived from egg culture. Each dose (0.25 ml, half the adult dose) contained 1.875 µg of the haemagglutinin antigen and the oil-in-water emulsion-based adjuvant AS03B (containing 5.345 mg of squalene, 5.39 mg of dl-α-tocopherol, 2.43 mg of polysorbate 80 and thiomersal).
The present study used the 2010–11 trivalent seasonal influenza vaccine (Fluarix®, GlaxoSmithKline, Rixensart, Belgium). This contained an inactivated, split-virion influenza virus, propagated in fertilised hens’ eggs. Each 0.5-ml dose contained 15 µg of haemagglutinin for each of the three influenza strains [A/California/07/2009 (H1N1) derived strain (NYMC X-181), A/Perth/16/2009 (H3N2)-like strain (NYMC X-187, derived from A/Victoria/210/2009) and B/Brisbane/60/2008].
Laboratory analysis
Microneutralisation and haemagglutination assays were performed at the Centre for Infections, Health Protection Agency (London, UK), using previously described methods. 8 Sera were processed in 1 : 2 serial dilutions. For MN assays, the initial dilution was 1 : 10 and the final dilution 1 : 5120. For HI assays, the initial dilution was 1 : 8 and the final dilution 1 : 16,384.
Safety and reactogenicity assessments
Adverse events of special interest, defined by the European Medicines Agency19 (see Appendix 1 for further details), that had occurred in the year since receipt of the monovalent pandemic influenza vaccine were recorded.
For 7 days after receipt of the trivalent seasonal influenza vaccine, the following information was recorded in a diary card: daily axillary temperature, injection-site reactions, systemic symptoms and the use of antipyretic medication. Different systemic symptoms were solicited for children aged < 5 years than for older children, to accommodate their limited ability to articulate symptoms. All medical consultations occurring between receipt of the trivalent seasonal influenza vaccine and the second study visit were recorded.
Statistical analysis
For antibody persistence, data were analysed according to vaccine received, following a predetermined statistical plan. For response to trivalent seasonal vaccine, a modified intention-to-treat analysis was performed.
To enable an unbiased assessment of the persistence of antibody, a random sample of participants excluded owing to receipt of a third dose were included in the analysis, assuming that their (low) antibody titre was unchanged from its value after two doses of vaccine in the original study. 17,18 The number in this random sample was determined proportionately (the proportion of participants excluded owing to receipt of a third dose who were in the selected sample was equal to the proportion of participants in the original study who enrolled in the follow-on study).
For analysis, HI titres of < 8 were given a value of 4, MN titres of < 10 were given a value of 5 and MN titres of > 5120 were given a value of 10,240.
Comparisons between groups were made using Fisher’s exact test. Geometric means were compared using normal error regression on logged titres.
Additional analyses, examining the effect of different variables, were performed using multivariable logistic and normal error regression.
Statistical significance was set at 5%. Analysis was performed in Stata 10.0 (StataCorp LP, College Station, TX, USA).
Chapter 3 Results
Participants
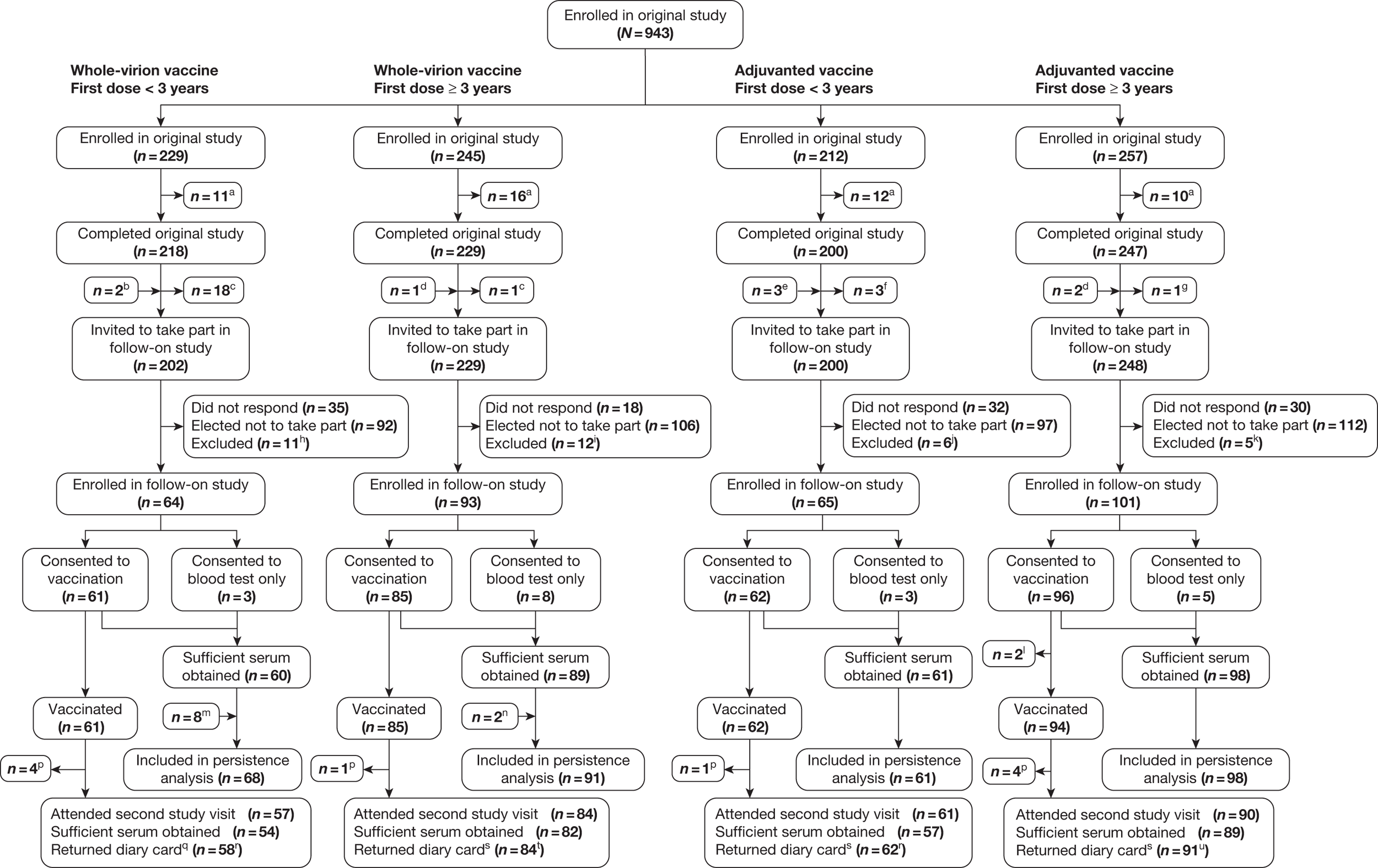
Figure 1 shows the flow diagram for study participants. Eight hundred and ninety-four children completed the original study. Of these, 115 could not be contacted, 49 were excluded and 407 elected not to take part in the follow-on study; 323 children enrolled in the follow-on study (36.1% of those completing the original study). Of these, 19 consented to one blood test only (but not to trivalent seasonal influenza vaccination); 290 children attended the second study visit.
FIGURE 1.
Flow diagram of participants. aDid not complete the original study. bOne invited in error; one randomised to receive the adjuvanted vaccine, but was given the whole-virion vaccine. cDid not fulfil the inclusion criteria because was known to have received a third dose of the vaccine. dInvited in error, as did not complete the original study. eTwo invited in error; one vaccinated just before third birthday, but assigned to the ≥ 3 years group in the original study in error. fOne invited in error; one did not fulfil inclusion criteria, owing to suspected unexpected severe adverse reaction in original study. One randomised to receive the adjuvanted vaccine, but was given the whole-virion vaccine. gOne vaccinated just before third birthday, but assigned to the ≥ 3 years group in the original study in error. hEight found to have received a third dose of pandemic influenza vaccine; two had already received 2010–11 seasonal influenza vaccine; one had received another vaccine in the exclusion period. iThree found to have received a third dose of pandemic influenza vaccine; nine had already received the 2010–11 seasonal influenza vaccine. jTwo had already received the 2010–11 seasonal influenza vaccine; two were too unwell to participate; one was taking part in another clinical trial of an investigation medical product; one was inadvertently excluded in error. kFive had already received the 2010–11 seasonal influenza vaccine. lWithdrew after consenting to vaccination but before receiving the vaccine. mEight extras, randomly chosen from those excluded owing to receipt of a third dose of pandemic influenza vaccine, to reduce bias in persistence analysis. nTwo extras, randomly chosen from those excluded owing to receipt of a third dose of pandemic influenza vaccine, to reduce bias in persistence analysis. pWithdrew after vaccination but before the second study visit. qIncludes two diary cards returned by participants who withdrew after vaccination. rAll diary cards for children < 5 years old. s Includes one diary card returned by a participant who withdrew after vaccination. tEight diary cards for children < 5 years old, 76 diary cards for children ≥ 5 years old. uNine diary cards for children < 5 years old and 82 diary cards for children ≥ 5 years old.
Persistence of antibody
Of the 323 participants enrolled, 15 were excluded from the persistence analysis (five did not have antibody assay results after the second dose of pandemic influenza vaccine in the original study and 10 had insufficient blood for the follow-on assay).
After the original study,17,18 28 children received a third dose of pandemic influenza vaccine, owing to both the MN and HI titres being below the protective threshold after two doses. These children were excluded from the follow-on study, as this study was designed to assess the persistence of antibody after two doses of the pandemic vaccine (not three). This introduced a potential bias, because poor responders to the vaccine in the original study were excluded. 17,18 In order to account for this, and to try to obtain an unbiased assessment of the persistence of antibody in a population representative of the participants in the original study, 10 of these 28 (36%), selected randomly, were included in the persistence analysis. They were not enrolled in (and therefore did not have a blood test in) the follow-on study, but it was assumed that their titres remained unchanged from the low values recorded after the second dose of pandemic influenza vaccine in the original study. Hence, a total of 318 children were included in the persistence analysis. Table 1 shows the demographic characteristics of these children.
| Group | ||||||
|---|---|---|---|---|---|---|
| Whole-virion vaccine | Adjuvanted vaccine | All | ||||
| First dose < 3 years | First dose ≥ 3 years | First dose < 3 years | First dose ≥ 3 years | |||
| Site | Bristol | 6 | 16 | 7 | 8 | 37 |
| Exeter | 8 | 3 | 2 | 9 | 22 | |
| Oxford | 24 | 27 | 17 | 28 | 96 | |
| Southampton | 26 | 32 | 31 | 36 | 125 | |
| St George’s, London | 4 | 13 | 4 | 17 | 38 | |
| Gender | Male | 31 | 39 | 31 | 52 | 153 |
| Female | 37 | 52 | 30 | 46 | 165 | |
| Age at original pandemic vaccination | Median months (range) | 21 (6–35) | 97 (37–155) | 24 (6–35) | 91 (36–150) | 51 (6–155) |
| Ethnicity | White | 60 | 86 | 60 | 91 | 297 |
| Indian | 0 | 0 | 0 | 0 | 0 | |
| Pakistani | 0 | 1 | 0 | 1 | 2 | |
| Asian – other | 0 | 0 | 0 | 0 | 0 | |
| Mixed | 8 | 1 | 1 | 2 | 12 | |
| Black African | 0 | 0 | 0 | 0 | 0 | |
| Black Caribbean | 0 | 0 | 0 | 2 | 2 | |
| Chinese | 0 | 2 | 0 | 1 | 3 | |
| Other | 0 | 1 | 0 | 1 | 2 | |
| Received previous seasonal vaccine | 2 | 6 | 1 | 4 | 13 | |
| Interval from blood sample taken after second dose of pandemic vaccine to persistence blood assay | Median days (range) | 359 (347–364) | 371 (351–384) | 361 (350–399) | 371 (351–384) | 371 (347–399) |
| Interval from second dose of pandemic vaccine to persistence blood assay | Median days (range) | 375 (365–396) | 392 (369–405) | 380 (365–413) | 392 (372–405) | 392 (365–413) |
Four groups were considered in the analysis, defined by the type of pandemic influenza vaccine received in the original study17,18 (AS03B-adjuvanted or whole-virion) and age at receipt of the first dose of pandemic vaccine (< 3 years or ≥ 3 years).
The median interval from the second dose of pandemic influenza vaccine in the original study to the blood draw to assess the persistence of antibody was 392 days (range 365–413 days). The median interval from the antibody assay after the second dose of pandemic influenza vaccine in the original study to the blood draw to assess the persistence of antibody was 371 days (range 347–399 days).
Table 2 shows the number (and percentage) of children with an MN titre ≥ 1 : 40 at 3 weeks and at 1 year after the second dose of pandemic influenza vaccine. Table 3 shows the MN geometric mean titre 1 year after the second dose of pandemic influenza vaccine. It was not possible to calculate a valid geometric mean titre for MN at 3 weeks after the second dose of the pandemic influenza vaccine because the upper limit of the MN titre measured in the original study was 1 : 320, and the MN titre was > 1 : 320 for many participants.
| Group | n/N, percentage (95% CI) with MN titre ≥ 1 : 40, 3 weeks after second pandemic vaccine dose | n/N, percentage (95% CI) with MN titre ≥ 1 : 40, 1 year after second pandemic vaccine dose |
|---|---|---|
| Whole-virion vaccine | ||
| 6 months to < 3 years | 56/68, 82.4% (71.2% to 90.5%) | 22/68, 32.4% (21.5% to 44.8%)a |
| 3–12 years | 86/91, 94.5% (87.6% to 98.2%) | 60/91, 65.9% (55.3% to 75.5%)a |
| Both age groups | 142/159, 89.3% (83.4% to 93.6%) | 82/159, 51.6% (43.5% to 59.6%)a |
| Adjuvanted vaccine | ||
| 6 months to < 3 years | 61/61, 100% (94.1% to 100%) | 61/61, 100% (94.1% to 100%)a |
| 3–12 years | 98/98, 100% (96.3% to 100%) | 95/98, 96.9% (91.3% to 99.4%)a |
| Both age groups | 159/159, 100% (97.7% to 100%) | 156/159, 98.1% (94.6% to 99.6%)a |
| Group | MN GMT (95% CI), 1 year after second pandemic vaccine dose |
|---|---|
| Whole-virion vaccine | |
| 6 months to < 3 years | 33.6 (23.8 to 47.5)a |
| 3–12 years | 66.9 (53.1 to 84.2)a |
| Both age groups | 49.8 (40.7 to 61.0)a |
| Adjuvanted vaccine | |
| 6 months to < 3 years | 411.9 (332.5 to 510.2)a |
| 3–12 years | 287.6 (230.5 to 358.9)a |
| Both age groups | 330.1 (281.3 to 387.4)a |
Table 4 shows the number (and percentage) of participants with an HI titre ≥ 1 : 32 at 3 weeks and at 1 year after the second dose of pandemic influenza vaccine. Table 5 shows the HI geometric mean titre at these time points. Table 6 shows the distribution of fold changes in the HI titre (change from HI titre 3 weeks after the second dose of pandemic influenza vaccine to HI titre 1 year after vaccination).
| Group | n/N, percentage (95% CI) with HI titre ≥ 1 : 32, 3 weeks after second pandemic vaccine dose | n/N, percentage (95% CI) with HI titre ≥ 1 : 32, 1 year after second pandemic vaccine dose |
|---|---|---|
| Whole-virion vaccine | ||
| 6 months to < 3 years | 40/68, 58.8% (46.2% to 70.6%) | 43/68, 63.2% (50.7% to 74.6%)a |
| 3–12 years | 82/91, 90.1% (82.1% to 95.4%) | 72/91, 79.1% (69.3% to 86.9%)a |
| Both age groups | 122/159, 76.7% (69.4% to 83.1%) | 115/159, 72.3% (64.7% to 79.1%)a |
| Adjuvanted vaccine | ||
| 6 months to < 3 years | 61/61, 100% (94.1 to 100%) | 60/61, 98.4% (91.2 to 100%)a |
| 3–12 years | 98/98, 100% (96.3 to 100%) | 95/98, 96.9% (91.3 to 99.4%)a |
| Both age groups | 159/159, 100% (97.7 to 100%) | 155/159, 97.5% (93.7 to 99.3%)a |
| Group | n | HI GMT (95% CI), 3 weeks after second pandemic vaccine dose | HI GMT (95% CI), 1 year after second pandemic vaccine dose | Fold change |
|---|---|---|---|---|
| Whole-virion vaccine | ||||
| 6 months to < 3 years | 68 | 35.6 (24.8 to 51.2) | 40.0 (27.9 to 57.6)a | 1.12 (0.78 to 1.61)a |
| 3 to 12 years | 91 | 110.8 (85.9 to 142.7) | 49.8 (39.0 to 63.5)a | 0.45 (0.35 to 0.58)a |
| Both age groups | 159 | 68.2 (54.3 to 85.6) | 45.4 (36.9 to 55.8)a | 0.67 (0.53 to 0.83)a |
| Adjuvanted vaccine | ||||
| 6 months to < 3 years | 61 | 520.8 (442.7 to 612.6) | 157.9 (125.8 to 198.3)a | 0.30 (0.24 to 0.38)a |
| 3 to 12 years | 98 | 455.6 (395.9 to 524.3) | 129.8 (105.7 to 159.5)a | 0.28 (0.23 to 0.35)a |
| Both age groups | 159 | 479.6 (431.4 to 533.2) | 140.0 (120.1 to 163.1)a | 0.29 (0.25 to 0.34)a |
| Group | ≥ 8-fold drop | 4- to 7.9-fold drop | 2- to 3.9-fold drop | < 2-fold change | 2- to 3.9-fold rise | 4- to 7.9-fold rise | ≥ 8-fold rise |
|---|---|---|---|---|---|---|---|
| Whole-virion vaccine | |||||||
| 6 months to < 3 years | 4 | 9 | 12 | 20 | 8 | 5 | 10 |
| 3–12 years | 18 | 22 | 19 | 19 | 8 | 2 | 3 |
| Adjuvanted vaccine | |||||||
| 6 months to < 3 years | 10 | 20 | 20 | 8 | 3 | 0 | 0 |
| 3–12 years | 21 | 34 | 28 | 12 | 1 | 1 | 1 |
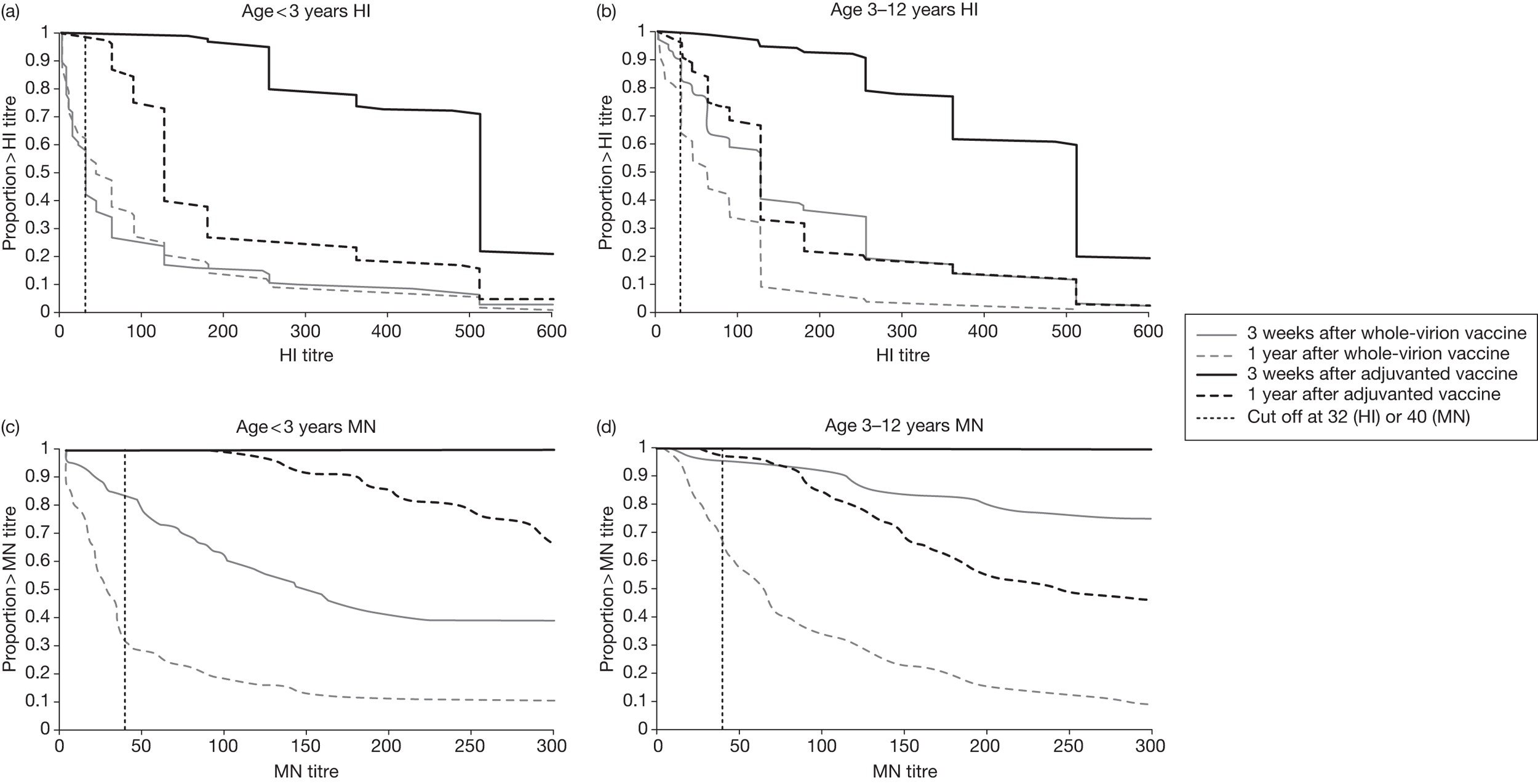
Figure 2 shows reverse cumulative distribution curves for HI and MN titres, at 3 weeks and at 1 year after receipt of a monovalent pandemic influenza vaccine, analysed according to vaccine given and age at first dose.
FIGURE 2.
Reverse cumulative distribution curves for HI and MN titres, at 3 weeks and at 1 year after receipt of a monovalent pandemic influenza vaccine, analysed according to vaccine given and age at first dose. MN titres are shown only to > 320, as this was the maximum dilution used in the assay at 3 weeks after vaccination.
The unexpected finding that the HI geometric mean titre was greater 1 year after vaccination than at 3 weeks after vaccination in children who had been given the whole-virion vaccine when < 3 years old prompted further analysis of the changes in titres in whole-virion vaccine recipients. Tables 7–9 compare those whose HI titre was ≥ 1 : 32 at 3 weeks after two doses of whole-virion vaccine with those whose HI titre was < 1 : 32 at this time point. For these two subgroups, Table 7 shows the HI geometric mean titre at 3 weeks and at 1 year after the second dose of the whole-virion vaccine, Table 8 shows the distribution of fold changes in the HI titre (change from HI titre 3 weeks after the second dose of pandemic influenza vaccine to HI titre 1 year after vaccination) and Table 9 shows the numbers and percentages with HI titres of ≥ 1 : 32 at 1 year after vaccination. Table 10 compares those recipients of the whole-virion vaccine whose MN titre was ≥ 1 : 40 at 3 weeks after vaccination with those whose MN titre was < 1 : 40, showing numbers and percentages with MN titre ≥ 1 : 40 at 1 year after vaccination.
| Group, age at first dose | n | HI GMT (95% CI), 3 weeks after second pandemic vaccine dose | HI GMT (95% CI), 1 year after second pandemic vaccine dose | Fold change (95% CI) |
|---|---|---|---|---|
| HI titre ≥ 1 : 32 | ||||
| 6 months to 3 years | 40 | 95 (67 to 135) | 73 (41 to 109) | 0.76 (0.48 to 1.20) |
| 3–12 years | 82 | 145 (118 to 177) | 56 (44 to 71) | 0.39 (0.31 to 0.49) |
| All ages | 122 | 126 (106 to 151) | 61 (50 to 75) | 0.48 (0.39 to 0.60) |
| HI titre < 1 : 32 | ||||
| 6 months to 3 years | 28 | 9 (7 to 11) | 19 (10 to 30) | 1.95 (1.11 to 3.43) |
| 3–12 years | 9 | 10 (5 to 18) | 17 (5 to 52) | 1.71 (0.54 to 5.40) |
| All ages | 37 | 9 (7 to 11) | 19 (11 to 27) | 1.89 (1.17 to 3.06) |
| Group, age at first dose | ≥ 8-fold drop | 4- to 7.9-fold drop | 2- to 3.9-fold drop | < 2-fold change | 2- to 3.9-fold rise | 4- to 7.9-fold rise | ≥ 8-fold rise |
|---|---|---|---|---|---|---|---|
| HI titre ≥ 1 : 32 | |||||||
| 6 months to 3 years | 4 | 7 | 9 | 8 | 6 | 2 | 4 |
| 3–12 years | 18 | 21 | 18 | 16 | 7 | 0 | 2 |
| HI titre < 1 : 32 | |||||||
| 6 months to 3 years | 0 | 1 | 3 | 12 | 2 | 3 | 6 |
| 3–12 years | 0 | 1 | 1 | 3 | 1 | 2 | 1 |
| Group, age at first dose | n/N, percentage (95% CI) with HI titre ≥ 1 : 32, 1 year after vaccination |
|---|---|
| HI titre ≥ 1 : 32 | |
| 6 months to 3 years | 32/40, 80% (64.4% to 90.9%) |
| 3–12 years | 68/82, 82.9% (73% to 90.3%) |
| All ages | 100/122, 82.0% (74% to 88.3%) |
| HI titre < 1 : 32 | |
| 6 months to 3 years | 11/28, 39.3 (21.5% to 59.4%) |
| 3–12 years | 4/9, 44.4% (13.7% to 78.8%) |
| All ages | 15/37, 40.5% (24.8% to 57.9%) |
| Group, age at first dose | n/N, percentage (95% CI) with MN titre ≥ 1 : 40, 1 year after vaccination |
|---|---|
| MN titre ≥ 1 : 40 | |
| 6 months to 3 years | 21/56, 37.5% (24.9% to 51.5%) |
| 3–12 years | 60/86, 69.8% (58.9% to 79.2%) |
| All ages | 81/142, 57.0% (48.5% to 65.3%) |
| MN titre < 1 : 40 | |
| 6 months to 3 years | 1/12, 8.3% (0.2% to 38.5%) |
| 3–12 years | 0/5, 0% (0% to 52.2%) |
| All ages | 1/17, 5.9% (0.1% to 28.7%) |
An additional analysis of the persistence of antibody, modelling the logged HI and MN titres 1 year after pandemic influenza vaccination, is shown in Tables 11 and 12. The variables considered were pandemic vaccine received, age at first dose (< 3 years or ≥ 3 years), gender, whether or not seasonal influenza vaccine had been previously given, interval between post-vaccination blood draw in the original study and the first blood draw in the follow-on study, and study site. Gender and receipt of previous seasonal influenza vaccine did not have a statistically significant effect and these variables are not shown in the tables. Table 11 shows the fold effect on HI and MN titres 1 year after pandemic influenza vaccination. Table 12 shows the fold effect on HI titres 1 year after pandemic influenza vaccination, including the HI titre recorded 3 weeks after pandemic vaccination as a covariate.
| Variable | Level | HI | MN | ||
|---|---|---|---|---|---|
| Fold effect (95% CI) | p-value | Fold effect (95% CI) | p-value | ||
| Age | < 3 years | Baseline | Baseline | ||
| ≥ 3 years | 1.16 (0.86 to 1.56) | 0.33 | 1.10 (0.81 to 1.49) | 0.54 | |
| Vaccine | Whole-virion | Baseline | Baseline | ||
| Adjuvanted | 3.10 (2.41 to 3.98) | < 0.001 | 6.63 (5.14 to 8.55) | < 0.001 | |
| Interval since blood draw for original post-vaccination titre | Effect per week | 0.86 (0.75 to 0.99) | 0.03 | 1.06 (0.92 to 1.22) | 0.39 |
| Site | Bristol | Baseline | Baseline | ||
| Exeter | 0.27 (0.14 to 0.49) | < 0.001 | 0.37 (0.20 to 0.69) | 0.002 | |
| Oxford | 0.88 (0.57 to 1.34) | 0.54 | 0.79 (0.51 to 1.23) | 0.30 | |
| Southampton | 1.01 (0.67 to 1.53) | 0.95 | 0.77 (0.50 to 1.18) | 0.23 | |
| St George’s, London | 1.26 (0.74 to 2.13) | 0.40 | 0.69 (0.40 to 1.19) | 0.18 | |
| Variable | Level | Fold effect (95% CI) | p-value |
|---|---|---|---|
| HI titre 3 weeks after vaccination | Per 2.7-fold change | 1.57 (1.41 to 1.75) | < 0.001 |
| Age | < 3 years | Baseline | |
| ≥ 3 years | 0.96 (0.73 to 1.26) | 0.78 | |
| Vaccine | Whole-virion | Baseline | |
| Adjuvanted | 1.29 (0.95 to 1.75) | 0.10 | |
| Interval since blood draw for original post-vaccination titre | Effect per week | 0.83 (0.74 to 0.94) | 0.004 |
| Site | Bristol | Baseline | |
| Exeter | 0.44 (0.25 to 0.78) | 0.005 | |
| Oxford | 0.87 (0.59 to 1.28) | 0.48 | |
| Southampton | 1.11 (0.76 to 1.63) | 0.57 | |
| St George’s, London | 1.40 (0.86 to 2.25) | 0.17 |
Safety of pandemic influenza vaccines
There were no reports of clinically significant adverse events related to the original pandemic influenza vaccination in any of the 323 children enrolled {0/157 [95% confidence interval (CI) 0% to 2.3%] in the whole-virion vaccine group and 0/166 (95% CI 0% to 2.2%) in the AS03B-adjuvanted vaccine group}. Clinically significant adverse events were determined by the investigators, from medical history given by the parents, and included hospitalisations, influenza-like episodes, febrile convulsions and adverse events of special interest.
Immunogenicity of trivalent seasonal influenza vaccine
A total of 302 children received the 2010–11 trivalent seasonal influenza vaccine in the follow-on study. Table 13 shows the demographic characteristics of these children. After vaccination, sufficient blood for analysis was obtained from 282 of these children. MN and HI titres for the H1N1 component of the vaccine were measured.
The median interval from vaccination to post-vaccination blood sampling was 21 days (range 11–44 days). For nine children, this interval was outside the range of 14–28 days (and for two of these children the interval was outside the range of 14–35 days). These children were included in the modified intention-to-treat analysis.
| Group | ||||||
|---|---|---|---|---|---|---|
| Whole-virion vaccine | Adjuvanted vaccine | All | ||||
| First dose < 3 years | First dose ≥ 3 years | First dose < 3 years | First dose ≥ 3 years | |||
| Site | Bristol | 5 | 16 | 7 | 8 | 36 |
| Exeter | 4 | 5 | 4 | 6 | 19 | |
| Oxford | 26 | 25 | 17 | 27 | 95 | |
| Southampton | 25 | 28 | 30 | 36 | 119 | |
| St George’s, London | 1 | 11 | 4 | 17 | 33 | |
| Gender | Male | 30 | 38 | 32 | 48 | 148 |
| Female | 31 | 47 | 30 | 46 | 154 | |
| Age at seasonal influenza vaccination | Median months (range) | 37 (19–48) | 112 (52–169) | 37 (18–49) | 105 (50–164) | 67 (18–169) |
| Ethnicity | White | 53 | 80 | 61 | 87 | 281 |
| Indian | 0 | 0 | 0 | 0 | 0 | |
| Pakistani | 0 | 1 | 0 | 1 | 2 | |
| Asian–other | 0 | 0 | 0 | 0 | 0 | |
| Mixed | 8 | 1 | 1 | 2 | 12 | |
| Black African | 0 | 0 | 0 | 0 | 0 | |
| Black Caribbean | 0 | 0 | 0 | 2 | 2 | |
| Chinese | 0 | 2 | 0 | 1 | 3 | |
| Other | 0 | 1 | 0 | 1 | 2 | |
| Received previous seasonal vaccine | 2 | 6 | 1 | 4 | 13 | |
| Interval from vaccination to blood assay | < 14 days | 0 | 0 | 0 | 1 | 1 |
| 14–28 days | 53 | 83 | 60 | 88 | 284 | |
| > 28 days | 3 | 2 | 1 | 2 | 8 | |
Table 14 shows the number (and percentage) of children with an MN titre ≥ 1 : 40 immediately before and 3 weeks after receipt of the seasonal influenza vaccine. A valid MN geometric mean titre could not be calculated, as titration was not performed beyond 1 : 5120 and the MN titre after vaccination was > 1 : 5120 for many participants.
| Group | n/N, percentage (95% CI) with MN titre ≥ 1 : 40 before vaccine | n/N, percentage (95% CI) with MN titre ≥ 1 : 40, 3 weeks after vaccine | n/N, percentage (95% CI) with ≥ 4-fold rise in MN titre |
|---|---|---|---|
| Whole-virion vaccine | |||
| 6 months to < 3 years | 21/60, 35.0% (23.1% to 48.4%) | 54/54, 100% (93.4% to 100%) | 51/53, 96.2% (87% to 99.5%) |
| 3–12 years | 52/81, 64.2% (52.8% to 74.6%) | 82/82, 100% (95.6% to 100%) | 77/78, 98.7% (93.1% to 100%) |
| Both age groups | 73/141, 51.8% (43.2% to 60.3%) | 136/136, 100% (97.3% to 100%) | 128/131, 97.7% (93.5% to 99.5%) |
| Adjuvanted vaccine | |||
| 6 months to < 3 years | 60/60, 100% (94% to 100%) | 57/57, 100% (93.7% to 100%) | 56/56, 100% (93.6% to 100%) |
| 3–12 years | 91/93, 97.8% (92.4% to 99.7%) | 89/89, 100% (95.9% to 100%) | 82/88, 93.2% (85.7% to 97.5%) |
| Both age groups | 151/153, 98.7% (95.4% to 99.8%) | 146/146, 100% (97.5% to 100%) | 138/144, 95.8% (91.2% to 98.5%) |
Table 15 shows the number (and the percentage) of participants with an HI titre ≥ 1 : 32, immediately before and 3 weeks after receipt of the seasonal influenza vaccine. Table 16 shows the HI geometric mean titres at these time points.
| Group | n/N, percentage (95% CI) with HI titre ≥ 1 : 32 before vaccine | n/N, percentage (95% CI) with HI titre ≥ 1 : 32, 3 weeks after vaccine | n/N, percentage (95% CI) with ≥ 4-fold rise in HI titre |
|---|---|---|---|
| Whole-virion vaccine | |||
| 6 months to < 3 years | 42/60, 70.0% (56.8% to 81.2%) | 54/54, 100% (93.4% to 100%) | 43/53, 81.1% (68% to 90.6%) |
| 3–12 years | 64/81, 79.0% (68.5% to 87.3%) | 82/82, 100% (95.6% to 100%) | 73/78, 93.6% (85.7% to 97.9%) |
| Both age groups | 106/141, 75.2% (67.2% to 82.1%) | 136/136, 100% (97.3% to 100%) | 116/131, 88.5% (81.8 % to 93.4%) |
| Adjuvanted vaccine | |||
| 6 months to < 3 years | 59/60, 98.3% (91.1% to 100%) | 57/57, 100% (93.7% to 100%) | 52/56, 92.9% (82.7% to 98%) |
| 3– 12 years | 90/93, 96.8% (90.9% to 99.3%) | 89/89, 100% (95.9% to 100%) | 76/88, 86.4% (77.4% to 92.8%) |
| Both age groups | 149/153, 97.4% (93.4% to 99.3%) | 146/146, 100% (97.5% to 100%) | 128/144, 88.9% (82.6% to 93.5%) |
| Group | HI GMT (95% CI) before vaccine | HI GMT (95% CI) 3 weeks after vaccine | Fold change |
|---|---|---|---|
| Whole-virion vaccine | |||
| 6 months to < 3 years | 50.2 (34.0 to 74.2) | 661.9 (524.9 to 834.6)a | 12.6 (8.3 to 19.1) |
| 3 to 12 years | 49.7 (38.6 to 64.1) | 846.6 (733.0 to 977.9)a | 16.7 (12.8 to 21.7) |
| Both age groups | 49.9 (40.1 to 62.1) | 767.8 (676.6 to 871.3)a | 14.9 (11.8 to 18.7) |
| Adjuvanted vaccine | |||
| 6 months to < 3 years | 159.4 (127.5 to 199.3) | 2611.9 (2238.1 to 3048.0)a | 16.2 (12.2 to 21.4) |
| 3 to 12 years | 131.4 (105.9 to 163.0) | 1425.8 (1244.9 to 1632.9)a | 10.7 (8.5 to 13.7) |
| Both age groups | 141.7 (121.2 to 165.8) | 1805.9 (1614.3 to 2020.3)a | 12.6 (10.5 to 15.1) |
Figure 3 shows reverse cumulative distribution curves for HI and MN titres immediately before and at 3 weeks after receipt of the trivalent seasonal influenza vaccine, analysed according to pandemic vaccine previously given and age at first dose.
FIGURE 3.
Reverse cumulative distribution curves for HI and MN titres immediately before (pre) and 3 weeks after (post) a dose of trivalent seasonal influenza vaccine (TIV), analysed according to pandemic influenza vaccine previously given and age at first dose. MN titres are shown to > 5120, as this was the maximum dilution used in the assay.
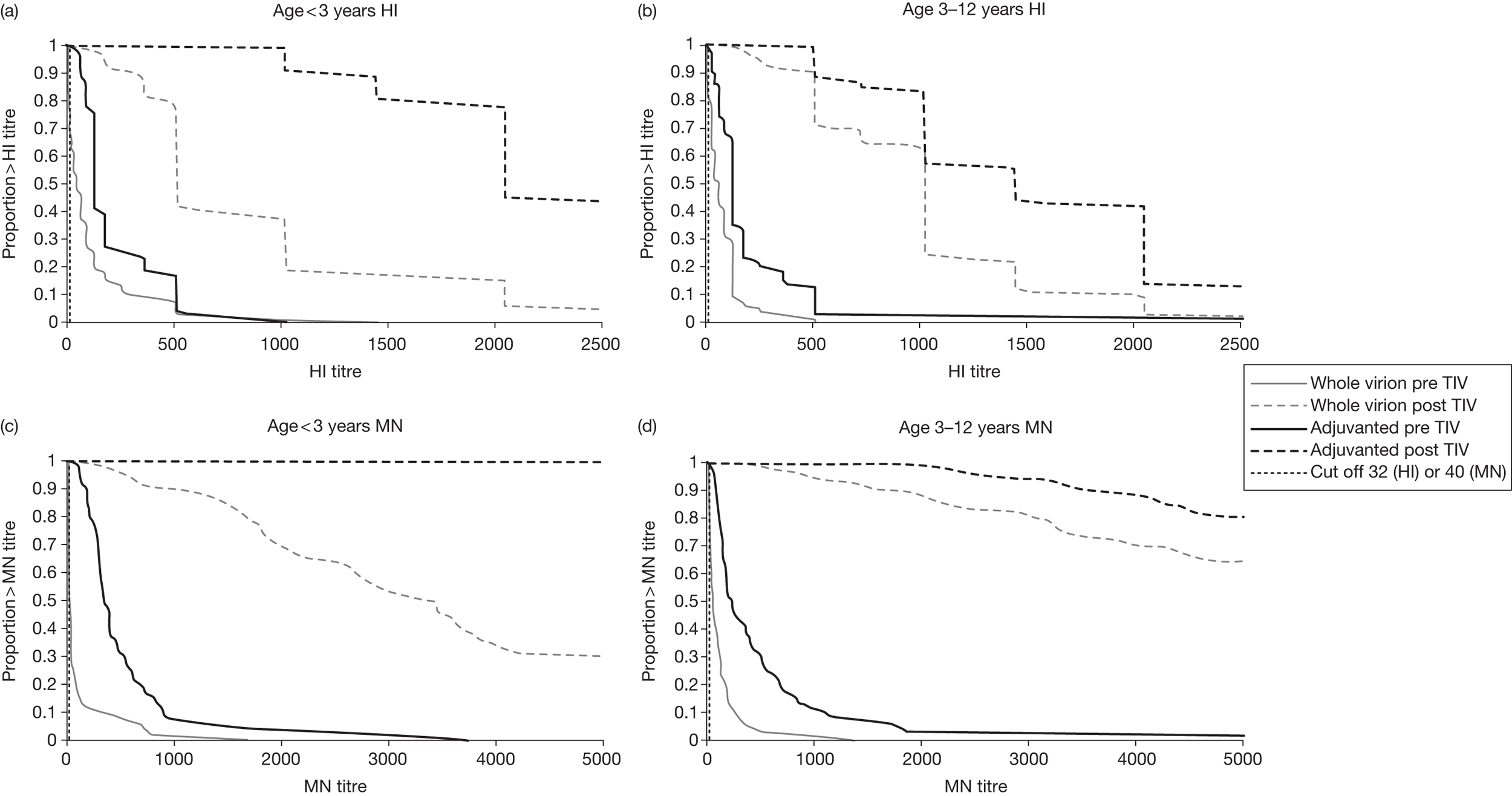
Table 17 shows the additional analysis, modelling HI titres 3 weeks after receipt of the trivalent seasonal influenza vaccine, split according to pandemic vaccine previously received. The variables of gender, previous receipt of seasonal influenza vaccine, interval from vaccination to blood assay and study site had no statistically significant effect. There was no age effect for the whole-virion vaccine group, but children given the AS03B-adjuvanted vaccine before 3 years of age had a significantly greater fold rise in HI titre in response to the trivalent influenza vaccine than did children who had received the AS03B–adjuvanted vaccine when ≥ 3 years old. HI titre 3 weeks after pandemic vaccination had a statistically significant effect on the response to the trivalent influenza vaccine, whereas HI titre immediately before giving the trivalent influenza vaccine did not.
| Variable | Level | Whole-virion vaccine | Adjuvanted vaccine | ||
|---|---|---|---|---|---|
| Fold effect (95% CI) | p-value | Fold effect (95% CI) | p-value | ||
| HI titre 3 weeks after pandemic vaccination | Per 2.7-fold change | 1.30 (1.18 to 1.43) | < 0.001 | 1.30 (1.10 to 1.53) | 0.002 |
| HI titre before trivalent seasonal influenza vaccine | Per 2.7-fold change | 1.03 (0.93 to 1.13) | 0.60 | 1.06 (0.96 to 1.18) | 0.26 |
| Age | < 3 years | Baseline | Baseline | ||
| ≥ 3 years | 0.95 (0.73 to 1.25) | 0.73 | 0.60 (0.49 to 0.74) | < 0.001 | |
| Site | Bristol | Baseline | Baseline | ||
| Exeter | 1.04 (0.53 to 2.03) | 0.91 | 1.51 (0.89 to 2.55) | 0.13 | |
| Oxford | 0.96 (0.66 to 1.38) | 0.82 | 1.04 (0.73 to 1.47) | 0.85 | |
| Southampton | 1.33 (0.92 to 1.91) | 0.13 | 1.20 (0.85 to 1.69) | 0.29 | |
| St George’s, London | 1.13 (0.68 to 1.89) | 0.64 | 0.91 (0.61 to 1.36) | 0.65 | |
Reactogenicity of trivalent seasonal influenza vaccine
Diary cards were returned by 295 out of the 302 children who received the trivalent seasonal influenza vaccine. Diary cards were completed during the 5 days after vaccination and most were returned at the second study visit. Five children who were vaccinated, but then withdrew from the study before their second visit returned diary cards.
Between vaccination and completion of (or withdrawal from) the study, there were no serious adverse events.
Table 18 shows local reactions reported by children < 5 years old. Table 19 shows local reactions reported by children ≥ 5 years old.
Table 20 shows systemic reactions reported by children < 5 years old. Table 21 shows local reactions reported by children ≥ 5 years old.
Table 22 shows local reactions, severe systemic symptoms and fever ≥ 38 °C reported by children across both age groups.
| Pandemic vaccine received in original study | Whole-virion vaccine | Adjuvanted vaccine | |||
|---|---|---|---|---|---|
| Total vaccinated | N = 69 | N = 72 | |||
| Number of diary cards available | n = 66 | n = 71 | |||
| Measurement | Level | Number | Percentage (95% CI) | Number | Percentage (95% CI) |
| Pain | Mild | 25 | 37.9% (26.2% to 50.7%) | 24 | 33.8% (23% to 46%) |
| Moderate | 1 | 1.5% (0% to 8.2%) | 7 | 9.9% (4.1% to 19.3%) | |
| Severe | 0 | 0% (0% to 5.4%) | 0 | 0% (0% to 5.1%) | |
| Any | 26 | 39.4% (27.6% to 52.2%) | 31 | 43.7% (31.9% to 56%) | |
| Redness | 1–24 mm | 15 | 22.7% (13.3% to 34.7%) | 16 | 22.5% (13.5% to 34%) |
| 25–49 mm | 1 | 1.5% (0% to 8.2%) | 3 | 4.2% (0.9% to 11.9%) | |
| ≥ 50 mm | 0 | 0% (0% to 5.4%)a | 10 | 14.1% (7% to 24.4%)a | |
| Any | 16 | 24.2% (14.5% to 36.4%)a | 29 | 40.8% (29.3% to 53.2%)a | |
| Swelling | 1–24 mm | 10 | 15.2% (7.5% to 26.1%) | 10 | 14.1% (7% to 24.4%) |
| 25–49 mm | 2 | 3% (0.4% to 10.5%) | 3 | 4.2% (0.9% to 11.9%) | |
| ≥ 50 mm | 1 | 1.5% (0% to 8.2%) | 5 | 7% (2.3% to 15.7%) | |
| Any | 13 | 19.7% (10.9% to 31.3%) | 18 | 25.4% (15.8% to 37.1%) | |
| Any local | Severe | 1 | 1.5% (0% to 8.2%)a | 10 | 14.1% (7% to 24.4%)a |
| Pandemic vaccine received in original study | Whole-virion vaccine | Adjuvanted vaccine | |||
|---|---|---|---|---|---|
| Total vaccinated | N = 77 | N = 84 | |||
| Number of diary cards available | n = 76 | n = 82 | |||
| Measurement | Level | Number | Percentage (95% CI) | Number | Percentage (95% CI) |
| Pain | Mild | 34 | 44.7% (33.3% to 56.6%) | 42 | 51.2% (39.9% to 62.4%) |
| Moderate | 16 | 21.1% (12.5% to 31.9%) | 19 | 23.2% (14.6% to 33.8%) | |
| Severe | 1 | 1.3% (0% to 7.1%) | 1 | 1.2% (0% to 6.6%) | |
| Any | 51 | 67.1% (55.4% to 77.5%) | 62 | 75.6% (64.9% to 84.4%) | |
| Redness | 1–24 mm | 19 | 25% (15.8% to 36.3%) | 22 | 26.8% (17.6% to 37.8%) |
| 25–49 mm | 3 | 3.9% (0.8% to 11.1%) | 7 | 8.5% (3.5% to 16.8%) | |
| ≥ 50 mm | 1 | 1.3% (0% to 7.1%) | 7 | 8.5% (3.5% to 16.8%) | |
| Any | 23 | 30.3% (20.2% to 41.9%) | 36 | 43.9% (33% to 55.3%) | |
| Swelling | 1–24 mm | 18 | 23.7% (14.7% to 34.8%) | 18 | 22% (13.6% to 32.5%) |
| 25–49 mm | 2 | 2.6% (0.3% to 9.2%) | 6 | 7.3% (2.7% to 15.2%) | |
| ≥ 50 mm | 0 | 0% (0% to 4.7%) | 1 | 1.2% (0% to 6.6%) | |
| Any | 20 | 26.3% (16.9% to 37.7%) | 25 | 30.5% (20.8% to 41.6%) | |
| Any local | Severe | 2 | 2.6% (0.3% to 9.2%) | 8 | 9.8% (4.3% to 18.3%) |
| Group | Whole-virion vaccine | Adjuvanted vaccine | |||
|---|---|---|---|---|---|
| Total vaccinated | N = 69 | N = 72 | |||
| Number of diary cards available | n = 66 | n = 71 | |||
| Measurement | Level | Number | Percentage (95% CI) | Number | Percentage (95% CI) |
| Decreased feeding | Mild | 14 | 21.2% (12.1% to 33%) | 10 | 14.1% (7% to 24.4%) |
| Moderate | 8 | 12.1% (5.4% to 22.5%) | 8 | 11.3% (5% to 21%) | |
| Severe | 1 | 1.5% (0% to 8.2%) | 4 | 5.6% (1.6% to 13.8%) | |
| Any | 23 | 34.8% (23.5% to 47.6%) | 22 | 31% (20.5% to 43.1%) | |
| Decreased activity | Mild | 12 | 18.2% (9.8% to 29.6%) | 14 | 19.7% (11.2% to 30.9%) |
| Moderate | 6 | 9.1% (3.4% to 18.7%) | 7 | 9.9% (4.1% to 19.3%) | |
| Severe | 2 | 3% (0.4% to 10.5%) | 3 | 4.2% (0.9% to 11.9%) | |
| Any | 20 | 30.3% (19.6% to 42.9%) | 24 | 33.8% (23% to 46%) | |
| Increased irritability | Mild | 18 | 27.3% (17% to 39.6%) | 15 | 21.1% (12.3% to 32.4%) |
| Moderate | 5 | 7.6% (2.5% to 16.8%) | 14 | 19.7% (11.2% to 30.9%) | |
| Severe | 4 | 6.1% (1.7% to 14.8%) | 2 | 2.8% (0.3% to 9.8%) | |
| Any | 27 | 40.9% (29% to 53.7%) | 31 | 43.7% (31.9% to 56%) | |
| Persistent crying | Mild | 18 | 27.3% (17% to 39.6%) | 15 | 21.1% (12.3% to 32.4%) |
| Moderate | 5 | 7.6% (2.5% to 16.8%) | 14 | 19.7% (11.2% to 30.9%) | |
| Severe | 4 | 6.1% (1.7% to 14.8%) | 2 | 2.8% (0.3% to 9.8%) | |
| Any | 27 | 40.9% (29% to 53.7%) | 31 | 43.7% (31.9% to 56%) | |
| Vomiting | Mild | 7 | 10.6% (4.4% to 20.6%) | 3 | 4.2% (0.9% to 11.9%) |
| Moderate | 1 | 1.5% (0% to 8.2%) | 1 | 1.4% (0% to 7.6%) | |
| Severe | 1 | 1.5% (0% to 8.2%) | 1 | 1.4% (0% to 7.6%) | |
| Any | 9 | 13.6% (6.4% to 24.3%) | 5 | 7% (2.3% to 15.7%) | |
| Diarrhoea | Mild | 11 | 16.7% (8.6% to 27.9%) | 11 | 15.5% (8% to 26%) |
| Moderate | 3 | 4.5% (0.9% to 12.7%) | 2 | 2.8% (0.3% to 9.8%) | |
| Severe | 1 | 1.5% (0% to 8.2%) | 0 | 0% (0% to 5.1%) | |
| Any | 15 | 22.7% (13.3% to 34.7%) | 13 | 18.3% (10.1% to 29.3%) | |
| Any severe systemic symptoms | 5 | 7.6% (2.5% to 16.8%) | 5 | 7% (2.3% to 15.7%) | |
| Fever ≥ 38 °C | 9 | 13.6% (6.4% to 24.3%) | 13 | 18.3% (10.1% to 29.3%) | |
| Group | Whole-virion vaccine | Adjuvanted vaccine | |||
|---|---|---|---|---|---|
| Total vaccinated | N = 77 | N = 84 | |||
| Number of diary cards available | n = 76 | n = 82 | |||
| Measurement | Level | Number | Percentage (95% CI) | Number | Percentage (95% CI) |
| Loss of appetite | Mild | 8 | 10.5% (4.7% to 19.7%) | 10 | 12.2% (6% to 21.3%) |
| Moderate | 0 | 0% (0% to 4.7%) | 0 | 0% (0% to 4.4%) | |
| Severe | 0 | 0% (0% to 4.7%) | 0 | 0% (0% to 4.4%) | |
| Any | 8 | 10.5% (4.7% to 19.7%) | 10 | 12.2% (6% to 21.3%) | |
| Generally unwell | Mild | 9 | 11.8% (5.6% to 21.3%) | 13 | 15.9% (8.7% to 25.6%) |
| Moderate | 7 | 9.2% (3.8% to 18.1%) | 14 | 17.1% (9.7% to 27%) | |
| Severe | 2 | 2.6% (0.3% to 9.2%) | 2 | 2.4% (0.3% to 8.5%) | |
| Any | 18 | 23.7% (14.7% to 34.8%) | 29 | 35.4% (25.1% to 46.7%) | |
| Headache | Mild | 17 | 22.4% (13.6% to 33.4%) | 15 | 18.3% (10.6% to 28.4%) |
| Moderate | 3 | 3.9% (0.8% to 11.1%) | 9 | 11% (5.1% to 19.8%) | |
| Severe | 1 | 1.3% (0% to 7.1%) | 1 | 1.2% (0% to 6.6%) | |
| Any | 21 | 27.6% (18% to 39.1%) | 25 | 30.5% (20.8% to 41.6%) | |
| Nausea/vomiting | Mild | 9 | 11.8% (5.6% to 21.3%) | 13 | 15.9% (8.7% to 25.6%) |
| Moderate | 1 | 1.3% (0% to 7.1%) | 1 | 1.2% (0% to 6.6%) | |
| Severe | 0 | 0% (0% to 4.7%) | 0 | 0% (0% to 4.4%) | |
| Any | 10 | 13.2% (6.5% to 22.9%) | 14 | 17.1% (9.7% to 27%) | |
| Diarrhoea | Mild | 5 | 6.6% (2.2% to 14.7%) | 9 | 11% (5.1% to 19.8%) |
| Moderate | 1 | 1.3% (0% to 7.1%) | 1 | 1.2% (0% to 6.6%) | |
| Severe | 0 | 0% (0% to 4.7%) | 0 | 0% (0% to 4.4%) | |
| Any | 6 | 7.9% (3% to 16.4%) | 10 | 12.2% (6% to 21.3%) | |
| Muscle pain | Mild | 15 | 19.7% (11.5% to 30.5%) | 21 | 25.6% (16.6% to 36.4%) |
| Moderate | 5 | 6.6% (2.2% to 14.7%) | 10 | 12.2% (6% to 21.3%) | |
| Severe | 1 | 1.3% (0% to 7.1%) | 0 | 0% (0% to 4.4%) | |
| Any | 21 | 27.6% (18% to 39.1%) | 31 | 37.8% (27.3% to 49.2%) | |
| Joint pain | Mild | 9 | 11.8% (5.6% to 21.3%) | 7 | 8.5% (3.5% to 16.8%) |
| Moderate | 2 | 2.6% (0.3% to 9.2%) | 3 | 3.7% (0.8% to 10.3%) | |
| Severe | 1 | 1.3% (0% to 7.1%) | 0 | 0% (0% to 4.4%) | |
| Any | 12 | 15.8% (8.4% to 26%) | 10 | 12.2% (6% to 21.3%) | |
| Any severe systemic symptoms | 2 | 2.6% (0.3% to 9.2%) | 2 | 2.4% (0.3% to 8.5%) | |
| Fever ≥ 38 °C | 1 | 1.3% (0% to 7.1%) | 1 | 1.2% (0% to 6.6%) | |
| Pandemic vaccine received in original study | Whole-virion vaccine | Adjuvanted vaccine | |||
|---|---|---|---|---|---|
| Total vaccinated | N = 146 | N = 156 | |||
| Number of diary cards available | n = 142 | n = 153 | |||
| Measurement | Level | Number | Percentage (95% CI) | Number | Percentage (95% CI) |
| Pain | Mild | 59 | 41.5% (33.3% to 50.1%) | 66 | 43.1% (35.2% to 51.4%) |
| Moderate | 17 | 12% (7.1% to 18.5%) | 26 | 17% (11.4% to 23.9%) | |
| Severe | 1 | 0.7% (0% to 3.9%) | 1 | 0.7% (0% to 3.6%) | |
| Any | 77 | 54.2% (45.7% to 62.6%) | 93 | 60.8% (52.6% to 68.6%) | |
| Redness | 1–24 mm | 34 | 23.9% (17.2% to 31.8%) | 38 | 24.8% (18.2% to 32.5%) |
| 25–49 mm | 4 | 2.8% (0.8% to 7.1%) | 10 | 6.5% (3.2% to 11.7%) | |
| ≥ 50 mm | 1 | 0.7% (0% to 3.9%)a | 17 | 11.1% (6.6% to 17.2%)a | |
| Any | 39 | 27.5% (20.3% to 35.6%)a | 65 | 42.5% (34.5% to 50.7%)a | |
| Swelling | 1–24 mm | 28 | 19.7% (13.5% to 27.2%) | 28 | 18.3% (12.5% to 25.4%) |
| 25–49 mm | 4 | 2.8% (0.8% to 7.1%) | 9 | 5.9% (2.7% to 10.9%) | |
| ≥ 50 mm | 1 | 0.7% (0% to 3.9%) | 6 | 3.9% (1.5% to 8.3%) | |
| Any | 33 | 23.2% (16.6% to 31.1%) | 43 | 28.1% (21.1% to 35.9%) | |
| Any local | Severe | 3 | 2.1% (0.4% to 6%)a | 18 | 11.8% (7.1% to 18%)a |
| Any symptoms | Severe | 7 | 4.9% (2% to 9.9%) | 7 | 4.6% (1.9% to 9.2%) |
| Fever | ≥ 38 °C | 10 | 7% (3.4% to 12.6%) | 14 | 9.2% (5.1% to 14.9%) |
Redness and severe local symptoms were reported more frequently in the children < 5 years old who had previously received the AS03B-adjuvanted pandemic influenza vaccine than in those children who had been given the whole-virion vaccine (p < 0.05). For all other solicited local and systemic symptoms, there was no statistically significant difference between the whole-virion and the AS03B-adjuvanted groups.
The relationship between reactogenicity and immune response was examined using logged HI titres in the multivariable model. Table 23 shows the effects of fever and severe local reactions on HI titre 3 weeks after trivalent seasonal influenza vaccination. Fever and severe local reactions were both associated with a greater HI titre response to the trivalent seasonal influenza vaccine in children who had previously received the AS03B-adjuvanted pandemic influenza vaccine, but not in those who had been given the whole-virion vaccine. No effect was seen for injection-site redness.
| Variable | Level | Whole-virion vaccine | Adjuvanted vaccine | ||
|---|---|---|---|---|---|
| Fold effect (95% CI) | p-value | Fold effect (95% CI) | p-value | ||
| Fever | ≥ 38 °C | 0.78 (0.42 to 1.43) | 0.41 | 1.60 (1.06 to 2.42) | 0.03 |
| Severe local | Yes | 1.28 (0.30 to 5.39) | 0.73 | 1.92 (1.24 to 2.98) | 0.004 |
Chapter 4 Discussion
Response to monovalent pandemic influenza vaccine
Nearly all children who received two doses of the AS03B-adjuvanted split-virion H1N1 monovalent pandemic influenza vaccine had antibody titres deemed protective (HI titre ≥ 1 : 32, MN titre ≥ 1 : 40) 1 year later. Children who received two doses of the whole-virion vaccine had lower titres than recipients of the AS03B-adjuvanted vaccine, both at 3 weeks after vaccination and at 1 year later.
One year after receipt of the AS03B-adjuvanted vaccine, the HI geometric mean titre had waned to about 30% of the baseline titre recorded 3 weeks after vaccination. Although AS03B-adjuvanted vaccine recipients had a greater percentage drop in HI geometric mean titre than children who had been given the whole-virion vaccine, their higher titres 3 weeks after vaccination led to higher titres 1 year later. A similar waning of antibody occurs after the trivalent influenza vaccine – in one study of children < 2 years old, the time taken for antibody to decay to one-half of the post-vaccination titre was calculated to be approximately 126 days for H1N1 and 258 days for H3N2. 20
In the group of children who received the whole-virion vaccine at < 3 years of age, an unexpected finding was that the HI geometric mean titre was higher at 1 year than at 3 weeks after vaccination. In this group of children, one-third (23 out of 68) had a more than twofold rise in HI titre, with 10 out of 68 having a more than eightfold rise in titre (see Table 6). This might be due to asymptomatic natural boosting of antibody following encounter with the virus. Alternatively, it might be due to the occurrence of symptomatic influenza infection (vaccine failure). These children did not report influenza-like illness in the year since receiving the pandemic influenza vaccine, indicating that natural boosting by asymptomatic exposure to the virus is the likely explanation. Subclinical infection with the virus was widespread – a seroepidemiological study in England indicated that many more children were infected during the first wave of 2009 pandemic H1N1 infection than had been estimated from surveillance of clinical cases. 8 We postulated that children with a low HI titre after vaccination were susceptible to asymptomatic infection, with consequent natural antibody boosting, whereas those with high HI titres after vaccination were unlikely to become infected. Further analysis of our results showed that children who had a poor HI titre response to the whole-virion vaccine were those most likely to have a higher HI titre 1 year later. The subgroup of children (of all ages) who had an HI titre < 1 : 32 at 3 weeks after receiving the whole-virion vaccine had a greater geometric mean titre at 1 year than at 3 weeks after vaccination (see Table 7), suggesting that some of them may have had subclinical infection in the intervening period. By contrast, the subgroup who had an HI titre ≥ 1 : 32 at 3 weeks after receiving the whole-virion vaccine, had a lower geometric mean titre 1 year after vaccination, indicative of waning antibody.
Our additional analysis of the persistence of antibody, modelling the HI and MN titres 1 year after pandemic influenza vaccination (see Tables 11 and 12), found that children from the Exeter site had statistically significantly lower HI titres 1 year after pandemic influenza vaccination than children at other sites, even allowing for their lower titres 3 weeks after vaccination. The explanation for these findings is unclear. One hypothesis might be that children in Exeter were less exposed to the virus than children at other sites, therefore having less pre-vaccination natural priming and less post-vaccination natural boosting. However, this hypothesis is not supported by serological evidence. Although it is known that influenza infection rates were highest during the second wave of the pandemic in two large metropolitan areas in England, London and the West Midlands, in other English regions rates did not differ from one another significantly. 8 Another hypothesis might be that the vaccines given in Exeter somehow differed from those given at the other sites. However, the batch numbers did not differ between sites and the Exeter vaccines were not subject to undue temperature deviation.
One year after receiving the whole-virion vaccine, fewer children had an MN titre ≥ 1 : 40 than had an HI titre ≥ 1 : 32, particularly in the younger age group. The HI titre specifically measures antibodies directed against the receptor-binding site of viral haemagglutinin, whereas the MN titre measures a broader range of neutralising antibodies. 21 It is currently unknown which antibody classes are predominantly detected by HI and MN assays, but experience with other sera which show discordance between HI and MN titres suggests that, in addition to detecting different antibody targets, the two tests might detect different antibody classes. This has been observed when comparing results from an HI assay with a single radial haemolysis technique – the latter appears unable to detect immunoglobulin A. 21 Expressed antibody class might affect the level of clinical protection. The HI assay is widely regarded as a surrogate measure for protection and is the assay used for licensure of influenza vaccines. 23,24 Correlation between MN titre and protection is less well documented. The threshold MN titre of 1 : 40 used in our analysis is speculative and was chosen because it is approximately fourfold greater than the MN titre observed in populations that have not been exposed to the virus or vaccinated against it.
Our findings in children are comparable with the results of a study of these two vaccines in adults, which also found the AS03B-adjuvanted vaccine to be more immunogenic than the whole-virion vaccine. 25 Both vaccines were found to be statistically significantly more immunogenic in adults from 18 to 44 years of age than in older individuals. This study assessed the persistence of antibody 6 months after vaccination. In the 18- to 44-year-old group, 6 months after a two-dose regimen, 98% of the AS03B-adjuvanted vaccine recipients had an HI titre ≥ 1 : 40, compared with 78% of the whole-virion vaccine recipients. In these adults, 6 months after vaccination, the HI geometric mean titre had declined to 41% of its post-vaccination value in those given the AS03B-adjuvanted vaccine and to 90% of the post-vaccination geometric mean titre in those given the whole-virion vaccine.
Vaccine effectiveness, assessed shortly after vaccination, for the AS03B-adjuvanted vaccine given to clinical risk groups in England was 77% (95% CI 11% to 94%) in children < 10 years old and 100% (95% CI 80% to 100%) in 10 to 24-year-olds, but considerably lower in older adults. 26 The vaccine was effective in preventing confirmed cases of pandemic H1N1 influenza infection from 7 days after vaccination. 27 A recent report provided estimates of the effectiveness of vaccination in preventing confirmed influenza A (H1N1) 2009 infection in the UK in the 2010–11 season. 28 The adjusted vaccine effectiveness was 34% (95% CI –10% to 60%) if vaccinated only with monovalent pandemic influenza vaccine during the 2009–10 season, 46% (95% CI 7% to 69%) if vaccinated only with trivalent influenza vaccine in the 2010–11 season and 63% (95% CI 37% to 78%) if vaccinated in both seasons. These data accord with our serological findings of waning antibody titre 1 year after receipt of a monovalent pandemic influenza vaccine, and also with our observation of effective boosting of antibody after a dose of the 2010–11 trivalent influenza vaccine.
Another oil-in-water adjuvant containing squalene, MF59, has been used in the formulation of influenza vaccines (Fluad® and Focetria®; Novartis, Marburg, Germany). Two doses of MF59-adjuvanted trivalent influenza vaccine given to children aged from 16 to 48 months resulted in higher HI titres, both at 3 weeks and at 1 year after vaccination, than in children given two doses of non-adjuvanted trivalent seasonal influenza vaccine. 29 An MF59-adjuvanted monovalent pandemic H1N1 influenza vaccine given to 101 children (two-thirds of whom were born at gestational age < 36 weeks) from 6 to 23 months of age, resulted in an HI titre ≥ 1 : 40 in 94% after one dose, and 100% after two doses. 30 Antibody titres induced by a single dose of either AS03B- or MF59-adjuvanted monovalent pandemic H1N1 influenza vaccine in immunocompetent children (from 6 months to 18 years old) were similar to HI and MN titres in unvaccinated children after natural infection. 31
None of the 323 children enrolled in our study reported clinically significant adverse events related to vaccination with either of the novel pandemic influenza vaccines, although the study was clearly too small to detect very rare adverse reactions. An increase in the incidence of narcolepsy in 4- to 19-year-old children and adolescents was recently reported in Finland, which appears to be associated with previous receipt of the AS03B-adjuvanted pandemic influenza vaccine. 32 This possible association has now also been reported in Sweden, France and Ireland, and is being investigated further. 33 Squalene-based adjuvants have been associated with autoimmune diseases in newborn rats. 34,35
Response to trivalent seasonal influenza vaccine
Our data show that the 2010–11 trivalent seasonal influenza vaccine, given to children who had received the pandemic influenza vaccine 1 year earlier, produced a marked serological response to the H1N1 component of the vaccine. All children in the study had an HI titre ≥ 1 : 32 and an MN titre ≥ 1 : 40 3 weeks after a dose of trivalent seasonal influenza vaccine. Nearly all had at least a fourfold rise in MN titre and most had at least a fourfold rise in HI titre. All groups showed at least a 10-fold increase in HI geometric mean titre from a high baseline. This is similar to the fold increase in HI geometric mean titre seen after the first dose of trivalent influenza vaccine in children who have not previously received an influenza vaccine, although in these children the baseline titre was low. 36,37
The additional analysis, modelling HI titres 3 weeks after receipt of the trivalent seasonal influenza vaccine (see Table 17), indicated that the HI titre 3 weeks after pandemic vaccination had a statistically significant effect on response to the trivalent influenza vaccine, but the HI titre immediately before giving the trivalent influenza vaccine did not. This might indicate that individuals who had a strong serological response to the monovalent pandemic influenza vaccine also tended to have a similar response to the trivalent seasonal influenza vaccine.
The trivalent seasonal influenza vaccine was well tolerated in this population of children. No serious adverse events occurred in the 3 weeks after vaccination. Reactogenicity to the trivalent seasonal influenza vaccine in our study was similar to that reported when trivalent vaccine was given to children who had not previously received an influenza vaccine. 36–38 In our study, redness and local reactions graded as severe were statistically significantly more frequent in children who had originally been given the AS03B-adjuvanted pandemic vaccine at < 3 years of age than in those who had received the whole-virion vaccine. In young children, previous receipt of an AS03B-adjuvanted vaccine seems to enhance local response to a dose of a non-adjuvanted vaccine given 1 year later. Our multivariable model indicated that fever and severe local reactions occurring after trivalent seasonal influenza vaccination were both associated with a greater serological response to the vaccine in children who had previously received the AS03B-adjuvanted pandemic influenza vaccine, but not in those who had previously been given the whole-virion vaccine (see Table 23). The mechanisms by which oil-in-water adjuvants mediate their immunological effects remain poorly understood. 39
After the trivalent vaccine, fever ≥ 38 °C was more commonly seen in children < 5 years old than in older children. It occurred in 18.3% of < 5 year old children who had previously received the AS03B-adjuvanted pandemic vaccine and in 13.6% of those who had received the whole-virion vaccine, but in only around 1% of older children. In our study, children < 5 years old developed post-vaccination fever more frequently than the rate reported in one study of influenza vaccine naive children < 3 years old who were given their first dose of trivalent influenza vaccine, and in whom 4/65 developed a fever ≥ 38 °C. 40 In Australia, an increase in the incidence of febrile convulsions occurred in children < 5 years old shortly after being given 2010 seasonal influenza vaccine. 41 This was subsequently found to be associated with one brand of vaccine (Fluvax®; CSL Biotherapies, Parkville, VIC, Australia), but not with the other brands of trivalent vaccine being used in Australia. 42 Similarly, in the USA, an increased risk of febrile convulsions has been reported with Fluzone® (Sanofi Pasteur, Swiftwater, PA, USA). 43 No child in our study experienced a febrile convulsion after the trivalent seasonal influenza vaccine.
Study limitations
This study investigates the persistence of putative protective antibody levels. It does not examine vaccine effectiveness.
The recruitment rate for our follow-on study, 36% of those completing the original study, was lower than anticipated (between 40% and 60%), which slightly reduced the power of the study to detect differences between groups. This was in part due to the need to complete recruitment to the study rapidly, prior to the start of the 2010–11 influenza season. The original study recruited participants at a time of very high media and public interest in pandemic influenza. The follow-on study recruited during a quiescent phase, with relatively few new cases, when there was a much lower level of public interest.
One limitation of our study is that a two-dose regimen of pandemic influenza vaccine was used. Our original study was designed when a two-dose schedule was planned for children. However, the majority of children in the UK vaccinated with pandemic influenza vaccine during the 2009–10 campaign were only given one dose. It is likely that these vaccinated children will have somewhat lower residual antibody titres than the children in our study. Our original study did not investigate the serological response after just one dose of pandemic vaccine. In young adults, the HI geometric mean titre was 30% higher 3 weeks after a second dose of AS03B-adjuvanted vaccine, and 23% higher after a second dose of whole-virion vaccine, than 3 weeks after a single dose of vaccine. 24
The addition of a control group, comprising children not previously vaccinated with pandemic influenza vaccine, would have strengthened the study design. This would have enabled direct comparison of antibody levels in children who had received the pandemic vaccines with children who had not, providing more robust data about the serological effects of the vaccines. A control group was not included because of time and budget limitations.
Chapter 5 Conclusions
Nearly all children who received two doses of the AS03B-adjuvanted split-virion pandemic H1N1 influenza vaccine had putative protective titres of antibody (HI titre ≥ 1 : 32, MN titre ≥ 1 : 40) 1 year later, although titres had waned. Children who received two doses of the whole-virion vaccine had lower titres, but many still had titres above the putative protective thresholds.
In children who had received either pandemic influenza vaccine 1 year earlier, the 2010–11 trivalent seasonal influenza vaccine produced a marked serological response to the H1N1 component of the vaccine.
Implications for health care
Children given two doses of pandemic influenza vaccines still have putative protective titres of antibody 1 year later, although persistence beyond 1 year remains unknown. In these children, administration of the trivalent vaccine, containing the pandemic strain as one component, effectively boosts antibody titre with an acceptable reactogenicity profile. The study provides serological evidence that a two-dose regimen of the AS03B-adjuvanted pandemic influenza vaccine may be sufficient to maintain protection across two waves of the same strain of virus.
Recommendations for future research
The inclusion of AS03B adjuvant has resulted in an antigen-sparing vaccine producing a marked antibody response, which persists a year after vaccination. The inclusion of this adjuvant in future seasonal influenza vaccines might enhance immunogenicity, particularly in children < 3 years old, and this warrants further investigation. It would be interesting to assess whether or not previous receipt of the AS03B-adjuvanted pandemic vaccine affected the serological response to the other two strains in the 2010–11 seasonal influenza vaccine. We propose to investigate this using stored serum. Further research is required to gain greater understanding of the immune response to AS03B adjuvant at a cellular level.
Assessment of the total duration of effective immunity after vaccination with either AS03B-adjuvanted or whole-virion pandemic influenza vaccines will require further study. It would be useful to assess the persistence of antibody after a single dose of these vaccines. There should be continuing surveillance of the long-term safety profile of these novel vaccines, especially in view of recent concerns regarding an association with narcolepsy in some countries. It is still unknown why two particular brands of trivalent seasonal influenza vaccine appeared to increase the risk of febrile convulsions in children < 5 years old in Australia and the USA, whereas other brands have not been implicated. It would be valuable to obtain further information on vaccine effectiveness, derived from cohort studies. Another priority should be the elucidation of the correlation between MN titre and protection from disease.
Acknowledgements
We thank the children and parents who participated in the study. We are grateful to all the doctors, nurses and administrative support staff who assisted with study visits. We thank Emma Plested, who co-ordinated study logistics at the Oxford site. Dr Shamez Ladhani and Dr Ifeanyichukwu Okike assisted in conducting the study at the St George’s site. We are grateful for the clinical and administrative assistance of the National Institute of Health Research (NIHR); the National Research Ethics Service, the Medicines and Healthcare products Regulatory Agency; the NHS; Thames Valley, Hampshire and Isle of Wight and South London NIHR Comprehensive Local Research Networks; the NIHR Medicines for Children Research Network; the Southampton University Hospitals NHS Trust Research and Development Office; the Child Health Computer Departments of Primary Care Trusts in Oxford, Southampton, Bristol, Exeter and London (Wandsworth); the NIHR Oxford Biomedical Research Centre; and the Health Protection Agency Centre for Infections for both administrative assistance and laboratory support. This study was funded by the NIHR Health Technology Assessment programme and was supported by the NIHR Oxford Comprehensive Biomedical Research Centre programme (including salary support for Matthew D Snape) and the Thames Valley, Hampshire and Isle of Wight and Western Comprehensive Local Research Networks. This study was adopted by the NIHR Medicines for Children Research Network and supported by the South West and London and South East Local Research Networks. AJP is a Jenner Institute Investigator and a James Martin Senior Fellow. PdW is a James Martin Fellow.
Contribution of authors
Dr Philip de Whalley (Clinical Research Fellow) contributed to design of the study, preparation of regulatory submissions, participant enrolment, data collection and interpretation. He drafted this report which has been reviewed by all the authors.
Dr Woolf Walker (Wellcome Trust Clinical Research Fellow, Paediatrics) contributed to the design of the study, participant enrolment and data interpretation.
Dr Matthew Snape (Consultant Paediatrician and Vaccinologist) contributed to the design of the study, preparation of regulatory submissions, participant enrolment and data interpretation. He was the principal investigator at the Oxford site.
Dr Clarissa Oeser (Clinical Research Fellow, Vaccines) contributed to participant enrolment and data collection.
Michelle Casey (Senior Research Nurse) contributed to participant enrolment and data collection.
Phoebe Moulsdale (Clinical Research Nurse) contributed to participant enrolment and data collection.
Caroline Harrill (Clinical Research Nurse) contributed to participant enrolment and data collection.
Nick Andrews (Senior Statistician) was responsible for statistical analysis and contributed to the design of the study and to data interpretation.
Katja Hoschler (Advanced Healthcare Scientist/Clinical Scientist) was responsible for laboratory analysis.
Ben Thompson (Project Manager) was responsible for project management and contributed to preparation of regulatory submissions.
Claire Jones (Postdoctoral Research Assistant) contributed to project management and was the laboratory liaison.
Jem Chalk (Software Developer/Computer Officer) was responsible for the computer database and contributed to data collection and analysis.
Simon Kerridge (Quality Assurance Manager) was responsible for data monitoring.
Dr Richard Tomlinson (Consultant Paediatrician) contributed to participant enrolment. He was the principal investigator at the Exeter site.
Dr Paul Heath (Executive Officer Paediatric Studies, Reader and Honorary Consultant Paediatrician) contributed to the design of the study, participant enrolment and data interpretation. He was the principal investigator at the St George’s, London site.
Professor Adam Finn (David Baum Professor of Paediatrics) contributed to the design of the study, participant enrolment and data interpretation. He was the principal investigator at the Bristol site.
Dr Saul Faust (Senior Lecturer in Paediatric Immunology and Infectious Diseases) contributed to the design of the study, participant enrolment and data interpretation. He was the principal investigator at the Southampton site.
Professor Elizabeth Miller (Head, Immunisation Department) contributed to the design of the study.
Professor Andrew Pollard (Professor of Paediatric Infection and Immunity) contributed to the design of the study, participant enrolment and data interpretation. He was the chief investigator.
Publication
Walker WT, de Whalley P, Andrews N, Oeser C, Casey M, Michaelis L, et al. H1N1 Antibody Persistence 1 Year After Immunization With an Adjuvanted or Whole-Virion Pandemic Vaccine and Immunogenicity and Reactogenicity of Subsequent Seasonal Influenza Vaccine: A Multicenter Follow-on Study. Clin Infect Dis 2012; in press. DOI: 10.1093/cid/cir905.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin Influenza A (H1N1) in Mexico. N Engl J Med 2009;361:680-9.
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. Pandemic potential of a strain of Influenza A (H1N1): early findings. Science 2009;324:1557-61.
- Donaldson LJ, Rutter PD, Ellis BM, Greaves FEC, Mytton OT, Pebody RG, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009;339.
- Chan M. World now at the start of 2009 influenza pandemic. World Health Organisation; 2009.
- McLean E, Pebody RG. Epidemiological Report of Pandemic (H1N1) 2009 in the UK 2010. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1284475321350 (accessed 1 August 2011).
- Health Protection Agency . HPA Weekly National Influenza Report 2011. www.hpa.org.uk/web/HPAweb%26HPAwebStandard/HPAweb_C/1287147913271.
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945-52.
- Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010;375:1100-8.
- Sachedina N, Donaldson L. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet 2010;376:1846-52.
- Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L, et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science 2009;326:729-33.
- Kuehn BM. CDC names H1N1 vaccine priority groups. JAMA 2009;302:1157-8.
- Salisbury DM. H1N1 influenza vaccination programme: information materials and vaccine schedule information. Department of Health; 2009.
- Donaldson L. Extending the H1N1 swine flu vaccination programme 2009/2010. Department of Health; 2009.
- Dalton I. A (H1N1) swine flu influenza: phase two of the vaccination programme; children over 6 months and under 5 years. Department of Health; 2009.
- World Health Organization . Recommended Viruses for Influenza Vaccines for Use in the 2010–2011 Northern Hemisphere Influenza Season 2010. www.who.int/csr/disease/influenza/201002_Recommendation.pdf (accessed 1 August 2011).
- Watson J, Peabody R. Pandemic influenza vaccines. BMJ 2011;342.
- Waddington CS, Andrews N, Hoschler K, Walker WT, Oeser C, Reiner A, et al. Open-label, randomised, parallel-group, multicentre study to evaluate the safety, tolerability and immunogenicity of an AS03B /oil-in-water emulsion-adjuvanted (AS03B) split-virion versus non-adjuvanted whole-virion H1N1 influenza vaccine in UK children 6 months to 12 years of age. Health Technol Assess 2010;14:1-130.
- Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months–12 years: open label, randomised, parallel group, multicentre study. BMJ 2010;340.
- European Medicines Agency . Guideline on Influenza Vaccines Prepared from Viruses With the Potential to Cause a Pandemic and Intended for Use Outside of the Core Dossier Context 2007. www.ema.europa.eu/pdfs/human/vwp/26349906enfin.pdf (accessed 1 August 2011).
- Wright P, Sannella E, Shi J, Zhu Y, Ikizler M, Edwards K. Antibody responses after inactivated influenza vaccine in young children. Pediatr Infect Dis J 2008;27:1004-8.
- Hardelid P, Andrews N, Hoschler K, Stanford E, Baguelin M, Waight P, et al. Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza AH1N1 2009. Health Technol Assess 2010;14:115-92.
- Russell S, McCahon D, Beare A. A single radial haemolysis technique for the measurement of influenza antibody. J Gen Virol 1975;27:1-10.
- Potter C, Oxford J. Determinants of immunity to influenza infection in man. Br Med Bull 1979;35:69-75.
- Wood JM, Levandowski RA. The influenza vaccine licensing process. Vaccine 2003;21:1786-8.
- Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis 2011;11:91-101.
- Andrews N, Waight P, Yung C-F, Miller E. Age specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis 2011;203:32-9.
- Hardelid P, Fleming D, McMenamin J, Andrews N, Robertson C, Sebastian Pillai P, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A(H1N1)2009 infection in England and Scotland 2009–2010. Eurosurveillance 2011;16.
- Pebody R, Hardelid P, Fleming D, McMenamin J, Andrews N, Robertson C, et al. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Eurosurveillance 2011;16.
- Vesikari T, Groth N, Karvonen A, Borkowski A, Pellegrini M. MF59® -adjuvanted influenza vaccine (FLUAD®) in children: Safety and immunogenicity following a second year seasonal vaccination. Vaccine 2009;27:6291-5.
- Esposito S, Pugni L, Daleno C, Ronchi A, Valzano A, Serra D, et al. Influenza A/H1N1 MF59-adjuvanted vaccine in preterm and term children aged 6 to 23 months. Pediatrics 2011;127:e1161-8.
- Meier S, Bel M, L’Huillier A, Crisinel P-A, Combescure C, Kaiser L, et al. Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. Vaccine 2011;29:3548-57.
- Kilpi T, Nohnyek H. Increased risk of narcolepsy observed among children and adolescents vaccinated with PandemrixR. National Institute for Health and Welfare; 2011.
- World Health Organization . Reviews on Narcolepsy Following Administration of Pandemrix Vaccine 2011.
- Carlson B, Jansson A, Larsson A, Bucht A, Lorentzen J. The endogenous adjuvant squalene can induce a chronic T-cell-mediated arthritis in rats. Am J Pathol 2000;156:2057-65.
- Satoh M, Kuroda Y, Yoshida H, Behney KM, Mizutani A, Akaogi J, et al. Induction of lupus autoantibodies by adjuvants. J Autoimmun 2003;21:1-9.
- Schmidt-Ott R, Schwarz T, Haase R, Sander H, Walther U, Fourneau M, et al. Immunogenicity and reactogenicity of a trivalent influenza split vaccine in previously unvaccinated children aged 6–9 and 10–13 years. Vaccine 2007;26:32-40.
- Neuzil K, Jackson L, Nelson J, Klimov A, Cox N, Bridges C, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. J Infect Dis 2006;194:1032-9.
- Neuzil K, Dupont W, Wright P, Edwards K. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J 2001;20:733-40.
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011;29:1812-23.
- Gonzalez M, Pirez M, Ward E, Dibarboure H, Garcia A, Picolet H. Safety and immunogenicity of a paediatric presentation of an influenza vaccine. Arch Dis Child 2000;83:488-91.
- Bishop J. Health authorities continue to put seasonal flu vaccine on hold for young children. Australian Government, Department of Health and Ageing; 2010.
- Bishop J. Seasonal flu vaccination for young children can be resumed. Australian Government, Department of Health and Ageing; 2010.
- US Food and Drug Administration . Fluzone Vaccine Safety 2011. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/ucm240037.htm (accessed 1 August 2011).
Appendix 1 Protocol
Appendix 2 Information booklet
Appendix 3 Consent form
Appendix 4 Diary card for children under 5 years of age
Appendix 5 Diary card for children over 5 years of age
List of abbreviations
- CI
- confidence interval
- HI
- haemagglutination inhibition
- MN
- microneutralisation
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington