Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 08/219/01. The assessment report began editorial review in July 2010 and was accepted for publication in September 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
Matthew Seymour (clinical advisor to the ERG) is a coinvestigator in a trial (321GO) that includes capecitabine as treatment for patients with advanced gastro-oesophageal cancer. The trial is peer reviewed and funded by Cancer Research UK, but also receives some supplementary financial support from Roche (£50,000 over 2 years). He also attended the American Society of Clinical Oncology (ASCO) conference last year as a guest of Roche. Daniel Swinson (clinical advisor to the ERG) is also a co-investigator on the 321GO study. Roche have also offered to sponsor his trip to ASCO this year.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into trastuzumab for the treatment of human epidermal growth factor receptor 2 (HER2)-positive metastatic adenocarcinoma of the stomach (mGC) or gastro-oesophageal junction. HER2 positivity is defined by immunohistochemistry (IHC)3+ or IHC2+/fluorescence in situ hybridisation (FISH)+. The decision problem addressed was the testing of the whole mGC population with IHC and, for IHC2+ patients, also with FISH, followed by treatment of HER2-positive patients with trastuzumab combined with cisplatin and either capecitabine or 5-fluorouracil (5-FU) [HCX (trastuzumab, cisplatin, capecitabine)/fluorouracil (F)] compared with current standard NHS therapy. The manufacturer’s submission contained direct evidence from the ToGA trial, a well-conducted, multinational, phase III randomised controlled trial (RCT) that compared HCX/F with cisplatin and a fluoropyrimidine alone [cisplatin, capecitabine (CX)/F]. HCX/F showed statistically significantly better overall survival in the European Medicines Agency-licensed population subgroup (74%) (hazard ratio 0.65, 95% confidence interval 0.51 to 0.83), corresponding to median survival of 16 months versus 11.8 months. No other evidence exists for the efficacy of any therapy in a known HER2-positive mGC population; other comparisons extrapolate from trials in mixed HER2 status populations. The ERG accepted the manufacturer’s view that a meaningful network meta-analysis to establish a comparison for HCX/F compared with current standard NHS therapy [epirubicin, cisplatin, capecitabine (ECX)/epirubicin, oxaliplatin, capecitabine (EOX)/epirubicin, cisplatin, 5-FU (ECF)] was not possible, but was unconvinced by arguments advanced in the alternative narrative synthesis. These involved disregarding evidence from a meta-analysis and interpreting non-significant results of small RCTs comparing epirubicin-containing triplets with cisplatin, 5-FU (CF)/capecitabine (X) doublets as evidence of no difference between triplet and doublet regimens. The high CX/F dose in the ToGA trial was an additional basis for the contention of equivalence. An appropriate de novo economic evaluation, including an economic model that separately compared HCX or trastuzumab, cisplatin, 5-FU (HCF) with the triplet regimens ECX, EOX and ECF, based on a simple, three-state cohort model (progression-free, disease, progression and death), was submitted. Utility weights were applied to estimate quality-adjusted life-years (QALYs). Costs were assessed from an NHS perspective, and incorporated the acquisition and monitoring costs of the alternative regimens, HER2 testing, adverse events and other supportive care costs. An 8-year time horizon was used to represent a lifetime analysis. Results from the ToGA trial were combined with a series of assumptions on relative treatment effects and testing strategies. The manufacturer’s results produced an incremental cost-effectiveness ratio (ICER) of £53,010 per QALY for HCX versus ECX. Although the manufacturer undertook a detailed set of sensitivity analyses, several alternative model assumptions were not evaluated. The ERG undertook a series of alternative base-case analyses. As a result of these analyses, EOX replaced ECX as the appropriate comparator, and the ICER for the comparison of HCX vs EOX increased to between £66,982 and £71,636 per QALY. The impact of implementation of alternative testing strategies remained unclear. There is also considerable uncertainty surrounding the true estimate of effectiveness for the comparison between triplet regimens containing epirubicin (ECX/ECF/EOX) and doublet CX/F regimens. Consequently, the view of the ERG was that there is insufficient evidence on the efficacy of HCX/F compared with current NHS standard therapy for an ICER to be determined with any degree of certainty.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor (in this instance, Roche). Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. 1 In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled Trastuzumab for the treatment of HER2 positive metastatic adenocarcinoma of the stomach or gastro-oesophageal junction. 2
Description of the underlying health problem
Gastric cancer is the 10th most commonly diagnosed cancer in the UK. Approximately 7000 cases are diagnosed each year in England and Wales3 and these account for around 4574 deaths. 4 For the 80% of patients unsuitable for curative surgery, palliative chemotherapy is an option, and it is estimated that just over one-half (around 2900) of the patients with advanced or metastatic adenocarcinoma of the stomach** (mGC) receive such treatment, which modestly improves survival as well as relieving disease-related symptoms. In the UK, the standard treatments for patients who are considered sufficiently fit are triplet regimens comprising a fluoropyrimidine [capecitabine (X) or 5-fluorouracil (F)], a platinum agent [cisplatin (C) or oxaliplatin (O)] and an anthracycline [epirubicin (E)]. Capecitabine is considered at least comparable to 5-fluorouracil (5-FU), and oxaliplatin at least comparable to cisplatin. 5,6 A proportion of patients with mGC have tumours that overexpress the human epidermal growth factor receptor 2 (HER2) receptor, meaning that they are potentially suitable for treatment with the monoclonal antibody trastuzumab (Herceptin®, Roche). The tests used to determine HER2 positivity are immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH).
The stomach is understood to include the gastro-oesophageal junction.
Scope of the evidence review group report
The decision problem addressed was the use of trastuzumab (H) in combination with cisplatin and either capecitabine or 5-FU (HCX/F) compared with current standard NHS therapy in patients with HER2-positive mGC or gastro-oesophageal junction cancer. HER2 positivity is defined as IHC3+ or IHC2+/FISH+. Such patients constituted 17.8% of the total screened population in the ToGA trial, which formed the basis of the manufacturer’s submission (MS). 7,8 The ERG considered that the decision problem comprised the testing of the whole mGC population with IHC, and for IHC2+ patients also with FISH, followed by treatment with trastuzumab in accordance with HER2 status, as specified by the licensed indication.
Methods
The ERG report comprised a critical review of the clinical effectiveness and cost-effectiveness of the technology based upon the MS to NICE as part of the STA process.
The ERG appraised the searches used to identify studies and the assessment tools used to critically appraise identified studies for both direct and indirect comparisons. The manufacturer’s decision not to use a network meta-analysis in the assessment of clinical effectiveness was reviewed, and the narrative synthesis submitted in place of such an analysis was scrutinised.
The ERG appraised the assumptions adopted in the economic model and reviewed the sources of the model data and the programming of the model. Following a response by the manufacturer to clarifications requested by the ERG, which included a revised model, the ERG further revised the base-case cost-effectiveness estimates to account for inconsistencies identified in the model. The ERG then carried out a series of sensitivity analyses to evaluate alternative assumptions, and produced an alternative base-case cost-effectiveness estimate based on equally plausible assumptions.
Results
Summary of submitted clinical evidence
The MS focused on direct evidence from the ToGA trial. 7 This was a phase III randomised controlled trial (RCT) that compared a doublet regimen of cisplatin plus a fluoropyrimidine [capecitabine or 5-FU (CX/F)] alone or in combination with trastuzumab (HCX/F) in patients with HER2-positive advanced adenocarcinoma of the stomach or gastro-oesophageal junction. The choice of fluoropyrimidine was at the discretion of the investigator; 87% of patients in each arm received capecitabine and 13% received 5-FU. The primary outcome was overall survival (OS). A subgroup of 74% of patients from this trial with IHC2+/FISH+ or IHC3+ mGC constituted the European Medicines Agency (EMA)-licensed population.
The hazard ratio (HR) for OS in the EMA subgroup [74% of the full analysis set (FAS) population (all randomised patients who received study medication at least once)] was 0.65 (95% confidence interval 0.51 to 0.83), corresponding to median survival of 16 months for the HCX/F group versus 11.8 months for the CX/F group. Progression-free survival (PFS) and response rates also showed evidence of a benefit of HCX/F (Table 1).
| Outcome | HCX/F | CX/F | Statistical results (HCX/F vs CX/F) |
|---|---|---|---|
| OS (median), (months) | 16.0 | 11.8 | HR 0.65 (95% CI 0.51 to 0.83) |
| PFS (median), (months) | 7.6 | 5.5 | HR 0.64 (95% CI 0.51 to 0.79) |
| Response rate (%)a | 47.3 | 34.5 | OR 1.70 (95% CI 1.22 to 2.38) |
| QoL | Graphical presentation of EORTC data only: ERG unable to form conclusions as to differences between the groups | ||
| Adverse eventsa |
Statistically significantly more grade 1 and grade 2 adverse events (multiple categories) in HCX/F group Statistically significantly more asymptomatic LVEF reductions in HCX/F group did not translate into increased symptomatic cardiac events No statistically significant differences in grade 3 or 4 events |
||
As doublet therapy with CX/F at the high doses evaluated in the ToGA trial7 is not used in an NHS context, the MS attempted to construct a network meta-analysis for the comparison of HCX/F with ECX (epirubicin, cisplatin, capecitabine), ECF (epirubicin, cisplatin, 5-FU) and EOX (epirubicin, oxaliplatin, capecitabine). It was decided, correctly in the view of the ERG, that construction of a meaningful network using the available clinical evidence was not possible. Therefore, a narrative discussion was presented, including the rationale for assumptions key to the economic model (see Commentary on the robustness of submitted evidence). The studies discussed in this narrative are detailed in Table 2.
| Study | n | Comparison | Estimate of OS: HR (95% CI) |
|---|---|---|---|
| RCTs | |||
| ToGA7 | 594 | HCX/F vs CX/F in patients who are IHC3+ or IHC2+/FISH+ for HER2 | 0.65 (0.51 to 0.83) |
| REAL-25 | 1002 |
(ECX + EOX) vs (ECF + EOF) (ECX + ECF) vs (EOF + EOX) |
0.89 (0.77 to 1.02) 0.95 (0.82 to 1.09) |
| Kim 200111 | 121 | ECF vs CF | 0.83 (0.42 to 1.61) |
| Tobe 199210 | 60 | ECF vs CF | 0.57 (0.27 to 1.20) |
| Ross 200213 | 580 | ECF vs MCF | 0.79 (0.62 to 0.95)a |
| Yun 201012 | 91 | ECX vs CX | NRb |
| Meta-analyses | |||
| Wagner 20109 | 501 |
ECF vs CF |
0.77 (0.62 to 0.95) |
| Okines 20096 |
(ECX or CX) vs (ECF or CF) |
0.87 (0.77 to 0.98) | |
Summary of submitted cost-effectiveness evidence
The MS did not identify any published cost-effectiveness studies of trastuzumab in HER2-positive patients with mGC. Therefore, the manufacturer’s de novo economic evaluation formed the basis of the submitted economic evidence.
The economic model included two separate trastuzumab regimens in combination with either cisplatin and capecitabine (HCX) or cisplatin and 5-FU (HCF). The trastuzumab regimens were compared with three other triplet regimens containing epirubicin in combination with either cisplatin and capecitabine (ECX), oxaliplatin and capecitabine (EOX) or cisplatin and 5-FU (ECF).
The manufacturer’s cost-effectiveness analysis was based on a simple, three-state cohort model (progression free, disease progression and death). Quality of life was quantified by applying utility weights to the separate model states in order to estimate quality-adjusted life-years (QALYs). Costs were assessed from an NHS perspective and incorporated the acquisition and monitoring costs of the alternative regimens, HER2 testing, adverse events and other supportive care costs associated with the management of progression-free disease and progressive disease. An 8-year time horizon was used and was considered to represent a lifetime analysis. Both one-way sensitivity analyses and probabilistic sensitivity analyses (PSAs) were undertaken.
In the absence of direct evidence comparing all five regimens, the manufacturer combined the results from the ToGA trial,7 which provided PFS and OS curves for HCF/X regimens and CF/X regimens, with a series of assumptions. As mentioned above, it was not possible to perform a robust network meta-analysis. The network of assumptions and the hazard rates used in the manufacturer’s economic model are illustrated in Figure 1.
FIGURE 1.
Network of assumptions used in the economic model presented in the manufacturer’s submission.
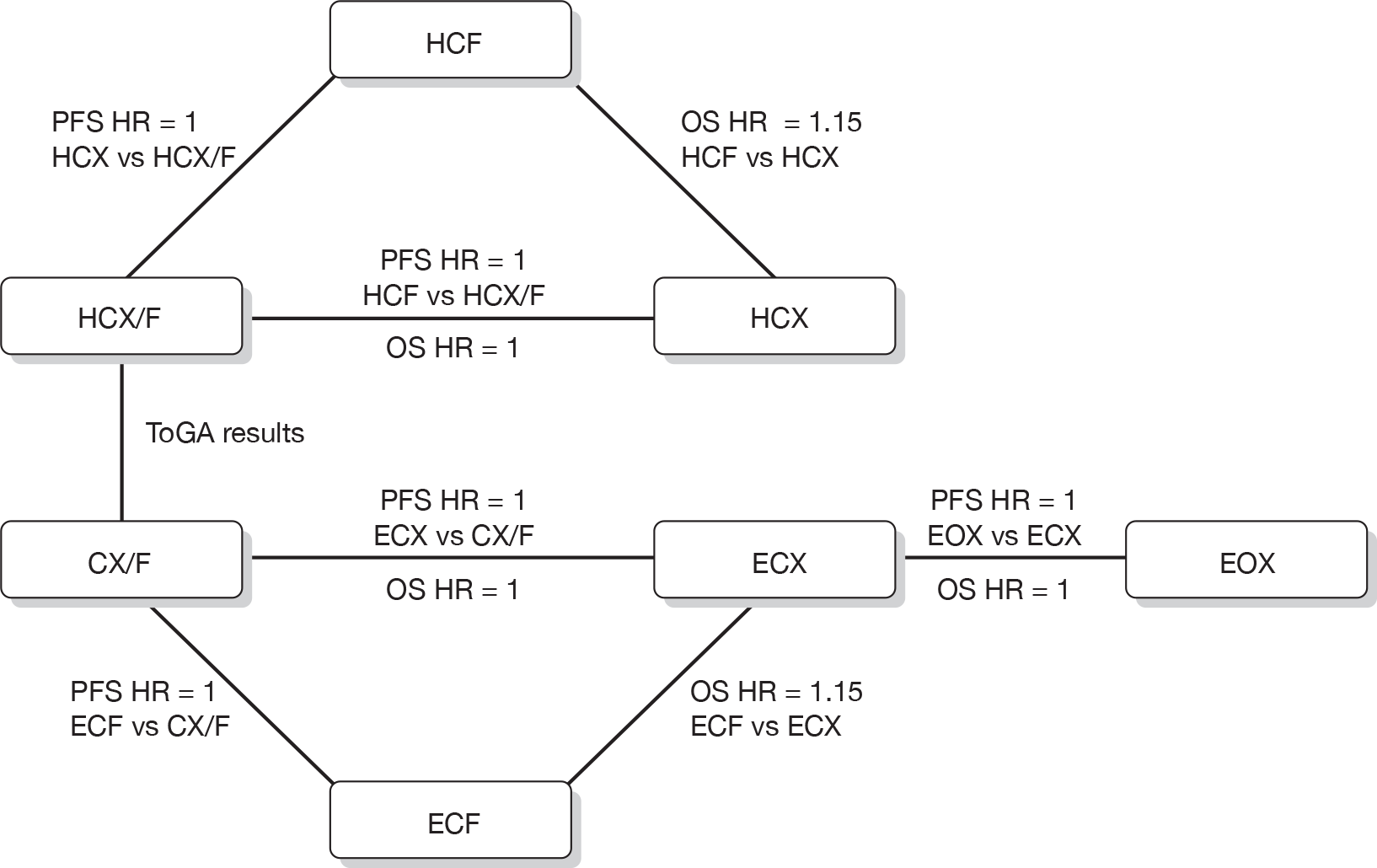
Key clinical effectiveness assumptions in the manufacturer’s model were:
-
CX/CF regimens with a higher dose of cisplatin had equal effectiveness to ECX/ECF regimens.
-
Capecitabine regimens had a survival benefit over 5-FU regimens.
-
Oxaliplatin regimens were equivalent to cisplatin regimens.
The manufacturer’s results showed that HCX resulted in a mean gain of 0.25 QALYs compared with ECX/EOX, 0.31 QALYs compared with ECF and 0.07 QALYs compared with HCF. ECX was the next most effective regimen after HCX that was not dominated (i.e. less effective and more costly) by another regimen, and the incremental cost-effectiveness ratio (ICER) of HCX versus ECX was £53,010 per QALY.
Commentary on the robustness of submitted evidence
The ToGA trial7 was a well-conducted, open-label phase III trial that directly compared trastuzumab in its licensed therapeutic combination with CX/F and CX/F alone. CX/F is considered to be standard therapy in other non-UK settings. The ToGA trial7 was appropriately randomised, and protocol amendments and termination took place on the advice of an independent data monitoring committee. While the evidence directly relevant to the decision problem is based on a subgroup of the ToGA trial,7 this constituted a clear majority of the trial population and was defined as a result of advances in the understanding of HER2 testing, giving it credibility as a distinct population. The use of subgroup data as the basis for the submission was therefore not considered problematic. Outcome assessors were unblinded, but, as the primary outcome was OS, this was not of major concern.
The economic model structure was considered to be appropriate for the decision problem, and the general approach used by the manufacturer to estimate lifetime cost-effectiveness met the requirements of the NICE reference case approach. Both one-way sensitivity analyses and PSA were used to reflect uncertainty in the model inputs and assumptions, and these were informative in exploring the robustness of the results and identifying potential key drivers of cost-effectiveness.
Two principal weaknesses were identified in the clinical effectiveness sections of the MS. First, as the HER2-positive mGC population has not been identified within previous trials, the efficacy of standard triplet regimens (or indeed any therapy) in this particular group is unknown. The comparator used in the ToGA trial7 (CX/F) is not the standard UK treatment and, where used in frailer patients, is used at lower doses. Indirect evidence is therefore required to assess the efficacy of HCX/F compared with current standard UK treatment for fit patients (ECX, ECF or EOX). This requires the assumption that the HER2-positive population is equivalent to a mixed HER2 population, containing an unknown proportion of HER2-positive patients. It is known that the rate of HER2 positivity varies with histological subtype; whether the histology seen in the ToGA trial7 is representative of the UK population is not clear, as the ToGA trial7 was primarily conducted in non-European settings.
The ERG considered as correct the manufacturer’s finding that it was not possible to create a network meta-analysis to compare HCX/F with triplet therapies using data from the general mGC population. However, the second major weakness of the MS was the approach of the narrative synthesis of relevant trials, in particular the argument that a meta-analysis of CF versus ECF regimens, which found an OS advantage for ECF,9 should be disregarded in favour of the results of individual small trials. 10–12 The ERG considered that the evidence of the meta-analysis, which was likely to be conservative to ECF and, by inference, to ECX, could not be disregarded (see Table 2). The alternative approach of the MS involved the argument that, as small RCTs did not show a statistically significant advantage of ECX/F over CX/F, this could be regarded as evidence of no advantage. An additional argument was that the higher dose of CX/F used in the ToGA trial7 provided additional efficacy over standard doses, giving comparable efficacy to epirubicin-based triplet regimens. The manufacturer therefore contended that the CX/F comparator in ToGA could be considered equivalent to ECX/F (and hence EOX on the basis of the evidence from the REAL-2 trial5). The ERG considered this argument to be unconvincing and non-conservative with respect to ECX/F. Further, they considered that there is a high level of uncertainty around the estimate of effect for the addition of epirubicin to CX/F regimens, and hence the estimate of effect for HCX/F versus triplet regimens.
From a cost-effectiveness perspective there were a number of additional weaknesses considered by the ERG. These stem largely from the lack of direct comparison of the different regimens incorporated in the economic analysis and the series of assumptions that were then necessary in order to estimate the incremental cost-effectiveness of the relevant regimens. Although the manufacturer undertook a detailed set of sensitivity analyses, several of the model assumptions were not incorporated.
The PSA did not include the uncertainty surrounding some of the estimates of effectiveness, yet the PSA still resulted in a wide range of estimates. Given this level of uncertainty and the number of assumptions required, scenario analyses could have been undertaken to demonstrate the combined effect of other plausible assumptions.
The ERG considered there to be equally plausible alternative estimates for the following significant assumptions used in the manufacturer’s base-case analysis:
-
relative effectiveness estimates of particular comparators
-
utility values applied during PFS
-
frequency of cardiac monitoring with trastuzumab and epirubicin.
In addition to these assumptions, the ERG also considered that there was insufficient discussion of the logistical issues of undertaking HER2 testing in this population and whether the effectiveness results from the ToGA trial7 (where parallel testing using IHC and FISH tests was used) could be generalised without any loss in treatment effect due to potential delays that could arise for IHC2+ patients, based on the sequential testing approach included in the model.
The ERG undertook a series of alternative base-case analyses to address these perceived weaknesses. As a result of these analyses, EOX replaced ECX as the next most effective regimen, after HCX, that was not dominated, and the ICER for the comparison of HCX versus EOX increased to between £66,982 and £71,636 per QALY. The REAL-2 trial5 indicated that EOX may be more effective than other triplet regimens. 5
Conclusions
There is considerable uncertainty surrounding the true estimate of effectiveness for the comparison between triplet regimens containing epirubicin (ECX/ECF/EOX) and doublet CX/F regimens. As a consequence of this, the estimate of effectiveness and hence the true ICER for HCX/F versus ECX/F and hence EOX is equally uncertain. Other areas of uncertainty include the generalisability of data to the HER2-positive subgroup; all trials other than ToGA7 were conducted in populations of mixed HER2 status. In the absence of a planned RCT of ECX/EOX versus HCX/F in HER2-positive patients, the ERG recommends that, if feasible, tissue samples from the REAL-2 trial5 be HER2 typed and correlated with outcome data. This would provide some indication of the efficacy of triplet therapies in the HER2-positive subpopulation. There is also some uncertainty as to the applicability of the ToGA trial,7 with its predominantly non-European population, to the UK mGC population.
Other areas of uncertainty involve the appropriate diagnostic testing strategy. The MS assumed that sequential testing would be performed. However, the effectiveness evidence related to parallel testing. The impact of delay in treatment arising from sequential testing has not been evaluated. The impact of the need for HER2 testing across the entire mGC population, 82% of whom will not be eligible for treatment with trastuzumab, is uncertain. In addition, the cost-effectiveness of the diagnostic testing used in the MS has not been demonstrated to be better than other ways of defining HER2 positivity and eligibility for trastuzumab.
Finally, the view of the ERG was that there was insufficient evidence as to the efficacy of HCX/F compared with current NHS standard therapy for an ICER to be determined with any degree of certainty.
Acknowledgements
Daniel Swinson, Medical Oncologist, St James’s Institute of Oncology, St James’s University Hospital, Leeds, UK.
Matthew Seymour, Professor of Gastrointestinal Cancer Medicine, Cancer Research UK Clinical Centre, University of Leeds, UK.
Ralph Crott, Senior Research Fellow, Centre for Reviews and Dissemination, University of York, UK.
Gerry Richardson, Senior Research Fellow, Centre for Health Economics, University of York, UK.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Summary of NICE guidance issued as a result of the STA
NICE guidance states that trastuzumab, in combination with cisplatin and capecitabine or 5-FU, is recommended as a treatment option for people with HER2-positive mGC who have not received prior treatement for their metastatic disease and who have tumours that express high levels of HER2, defined as IHC3+. This guidance was issued subsequent to the submission, following consultation on the appraisal consultation document, of additional analyses relating to this more narrowly defined subgroup by the manufacturer, and the ERG’s consideration of these data.
Key references
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Single Technology Appraisal Process 2009.
- Centre for Reviews & Dissemination (CRD) and Centre for Health Economics (CHE) Technology Assessment Group . Trastuzumab for the Treatment of HER2 Positive Metastatic Adenocarcinoma of the Stomach or Gastro-Oesophageal Junction: A Single Technology Appraisal 2010.
- Cancer Research UK . Stomach Cancer – UK Incidence Statistics n.d. http://info.cancerresearchuk.org/cancerstats/types/stomach/incidence/index.htm (accessed 18 February 2010).
- Stomach Cancer – UK Mortality Statistics n.d. http://info.cancerresearchuk.org/cancerstats/types/stomach/mortality/index.htm (accessed 18 February 2010).
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-4.
- Okines AFC, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 2009;20:1529-34.
- Van Cutsem E, Kang Y, Chung H, Shen L, Sawaki A, Lordick F. Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy (CT) in First-Line Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Advanced Gastric Cancer (GC) 2009.
- Bang YJ, Chung HC, Xu JM, Lordlick F, Sawaki A, Lipatov O, et al. Pathological Features of Advanced Gastric Cancer: Relationship to Human Epidermal Growth Factor Receptor 2 Positivity in the Global Screening Programme of the ToGA Trial n.d.
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev; 2010.
- Tobe T, Nio Y, Tseng CC, Shiraishi T, Tsubono M, Kawabata K, et al. A randomised, comparative-study of combination chemotherapies in advanced gastric-cancer – 5-fluorouracil and cisplatin (fp) versus 5-fluorouracil, cisplatin, and 4′-epirubicin (fpepir). Anticancer Res 1992;12:1983-8.
- Kim TW, Choi SJ, Ahn JH, Bang HS, Chang HM, Kang YK, et al. A prospective randomised phase III trial of 5-fluorouracil and cisplatin (FP) versus epirubicin, cisplatin, and 5-FU (ECF) in the treatment of patients with previously untreated advanced gastric cancer (AGC). Eur J Cancer 2001;37.
- Yun J, Lee J, Park SH, Park JO, Park YS, Lim HY, et al. A randomised phase II study of combination chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J Cancer 2010;46:885-91.
- Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomised trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002;20:1994-200.
- Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73.