Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 09/99/01. The assessment report began editorial review in July 2010 and was accepted for publication in September 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of prucalopride for the treatment of women with chronic constipation in whom standard laxative regimens have failed to provide adequate relief. The ERG report is based on the manufacturer’s submission (MS) to the National Institute for Health and Clinical Excellence as part of the single technology appraisal process. In the submission, quality-of-life data [Patient Assessment of Constipation Quality of Life (PAC-QOL) and Patient Assessment of Constipation Symptoms (PAC-SYM) questionnaires] from trials of prucalopride were extrapolated to EQ-5D (European Quality of Life-5 Dimensions) data and used to inform effectiveness in an economic model. Response rates to prucalopride were derived from observed response rates in trials, defined as the proportion of patients achieving an average of three or more spontaneous complete bowel movements over the 4- or 12-week trial periods. Adult (18–64 years) and elderly (≥ 65 years) patients were considered separately in the model. Cost-effectiveness was determined from estimated improvements in EQ-5D and anticipated response rates, adjusted for baseline severity of chronic constipation. The ERG considered that the patients participating in these trials were not representative of those in the licensed indication. They were not all refractory to laxatives, and baseline EQ-5D scores showed a large spread in quality of life, with many patients experiencing little baseline dissatisfaction. The mapping of quality-of-life data from trials (PAC-QOL and PAC-SYM data) to EQ-5D was unclear and invalidated. The assumption of the long-term effectiveness and safety of prucalopride to 1 year was considered unjustified. There was no justification or sources given for coefficients used to predict effectiveness in the economic model, and no costs other than the cost of prucalopride were incorporated into the model. Owing to the many areas of uncertainty, particularly the effectiveness of prucalopride in the licensed patient group and its long-term effectiveness and safety, it was considered that the MS provided no evidence for whether prucalopride is effective or not in women with laxative-refractory chronic constipation. Further subgroup analysis of the actual patient group of interest may have better guided decision-making. However, long-term efficacy data, with validated estimates of quality of life incorporated in a well-founded model, would be important for an evidence-based judgement to be made.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled Prucalopride for the treatment of women with chronic constipation in whom standard laxative regimens have failed to provide adequate relief. 2
Description of the underlying health problem
Chronic constipation may be idiopathic or secondary to other causes, such as drug use or neuromuscular conditions. This submission relates to patients with idiopathic chronic constipation that is not secondary to other causes and is a long-term disease. Rates of chronic constipation are higher in women than in men. Clinical trials of chronic constipation include ∼90% women compared with ∼10% men,3–5 and this may be representative of the relative prevalence in men and women.
The majority of patients with chronic constipation are managed in primary care. Non-pharmacological measures, such as dietary modification and exercise, are recommended in the first instance, and, where these fail, pharmacological measures (a range of laxative treatments) can be prescribed. However, for a small proportion of these patients, laxative measures used over a long period of time fail to bring about bowel movements. These patients, with chronic constipation that is refractory to laxative treatments, are the population for whom prucalopride is licensed and the patient group for which guidance was to be made.
Estimates for the prevalence of chronic constipation vary and it is difficult to make a precise estimate of the size of this patient group. Furthermore, it is difficult to assess, from within this group, the number of laxative-refractory patients for whom prucalopride may be indicated. In the manufacturer’s submission (MS), it was estimated that the total eligible adult patient group that might benefit from prucalopride in the UK is 363,000 (estimated from a 47 million UK adult population, assuming an average prevalence of chronic constipation of 7.7% and that 10% of patients are dissatisfied with, or refractory to, laxatives).
Scope of the evidence review group report
Prucalopride is licensed in women with chronic constipation who are refractory to laxative medications. The licence is for daily doses of 2 mg for adult patients (18–64 years) and 1 mg for elderly patients (≥ 65 years), and treatment costs are £2.13 and £1.38 per day, respectively.
In the submission, data from nine trials were used to inform the assessment of clinical effectiveness, and four of these trials, along with data from six additional trials, were used to inform the economic evaluation. In trials, response to treatment was measured in terms of the number of spontaneous complete bowel movements (SCBMs) and by using quality-of-life questionnaires. The main outcome measure was number of patients achieving a mean of three or more SCBMs over the first 4 and 12 weeks of trials. Patient Assessment of Constipation Quality of Life (PAC-QOL) and Patient Assessment of Constipation Symptoms (PAC-SYM) surveys, designed by manufacturers for use in the prucalopride trials, were used to obtain quality-of-life data and SF-36 questionnaires (Short Form questionnaire-36 items) were also used in some of the trials. Data from PAC-QOL and PAC-SYM questionnaires were mapped to give quality of life in terms of EQ-5D (European Quality of Life-5 Dimensions). Results from SF-36 questionnaires were used in the development of regression equations for the mapping of PAC-QOL and PAC-SYM data to EQ-5D.
For the economic model, quality of life gained by responders was estimated by one of eight different regression equations. Scenarios varied, depending on the definition of patient response (three or more SCBMs per week or an increase of one or more SCBM per week), whether patients had previously been on laxative treatment and whether constipation severity at baseline was considered. The cost of prucalopride was the only cost included in the model, and incremental cost-effectiveness ratios (ICERs) were presented separately for the adult and elderly populations.
The ERG report aimed to assess the extent to which the clinical effectiveness and cost-effectiveness parts of the MS covered the appropriate population, intervention, comparators and outcomes, and the extent to which information used in the economic model was valid and incorporated in an appropriate way.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology, based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
Searches for studies of clinical effectiveness and cost-effectiveness were conducted. The clinical effectiveness part of the MS was assessed in terms of its coverage of relevant trials/studies, its relevance to the proposed drug indication and the quality of the presented data. The cost-effectiveness part of the submission was assessed in terms of the applicability of included data, the transparency in which model parameters were selected and the validity of assumptions used in the model.
Using the manufacturer’s economic model, the ERG performed additional analysis to investigate the effect of assuming that patients take prucalopride every day (instead of 220 days/year), incorporating an allowance for adverse events and reducing the estimated gain in quality of life.
Results
Summary of submitted clinical evidence
Results from nine trials were presented: three ‘pivotal’ trials,3–5 one trial in elderly patients,6 one re-treatment study,7 one trial in patients with opioid-induced chronic constipation8 and three long-term open-label studies. 9–11 Results for the three ‘pivotal’ trials3–5 (pooled by the ERG) and the trial in elderly patients6 are given in Table 1.
| Prucalopride | Placebo | |
|---|---|---|
| Proportion of patients with mean of three or more SCBMs/week: % (n/N) | ||
| Pivotal trialsa | 23.8 (151/635) | 11.4 (73/640) |
| Elderlyb | 39.5 (30/76) | 20.0 (14/70) |
| Proportion of patients with average increase of one or more SCBMs/week: % (n/N) | ||
| Pivotal trials | 43.2 (264/612) | 24.6 (155/630) |
| Elderly | 61.1 (44/72) | 33.8 (22/65) |
| Average number of SCBM/week: mean (mean change from baseline) | ||
| Pivotal trials | 1.9 (1.5) | 1.2 (0.7) |
| Elderly | 2.7 (1.9) | 1.7 (0.6) |
| Overall PAC-SYM symptoms score: mean (mean change from baseline) | ||
| Pivotal trials | 1.33 (–0.69) | 1.57 (–0.42) |
| Elderly | 0.88 (–0.53) | 1.22 (–0.23) |
| Overall PAC-QOL score: mean (mean change from baseline) | ||
| Pivotal trials | 1.33 (–0.77) | 1.68 (–0.44) |
| Elderly | 0.95 (–0.53) | 1.26 (–0.20) |
| SF-36 score: mean (mean change from baseline) | ||
| Pivotal trials | 48.2 (2.5) | 47.5 (1.9) |
| Elderly | Not measured | Not measured |
The open-label studies, conducted in patients from a mixture of different trials, showed that satisfaction with treatment in patients remaining in the study remained constant over the first year of treatment. However, 60% of patients had dropped out at 1 year (17% insufficient response, 8% adverse events). The re-treatment study showed that treatment with prucalopride in patients remaining in the study was just as effective. However, only data for 4 mg prucalopride were presented in the submission and only data from patients who did not drop out between study periods were used for the analysis. The trial in patients with opioid-induced chronic constipation was not relevant to this submission so was not assessed by the ERG.
Summary of submitted cost-effectiveness evidence
A cost-effectiveness model included costs for only prucalopride and no alternative treatment costs were incorporated. The quoted costs per responder were based on 292 days’ treatment (80% ‘compliance’) and estimated at £622 per adult patient and £402 per elderly patient per year on treatment. The base-case model predicted quality-adjusted life-year gains per responder of 0.0369 [standard deviation (SD) 0.0450] and 0.0342 (SD 0.1495) for adult and elderly patients, respectively, giving ICERs of £16,800 and £11,700, respectively.
Commentary on the robustness of submitted evidence
The submitted evidence was not considered to be robust and many factors remained unclear even after requests for clarification. There was poor transparency around the submission and the modelling process. The main specific areas for concern were:
-
The trials on which data for this submission were based were not conducted in patients with chronic constipation that was refractory to laxatives. This was evidenced in a number of ways:
-
Around 17.0% of patients in pivotal trials had found their previous treatment adequate.
-
Bisacodyl, a laxative, was used as a rescue medication in the trials and, on average, it induced one or more bowel movements per week in study participants.
-
Baseline EQ-5D scores (higher score, less severe) for adult (18–65 years) and elderly (≥ 65 years) patient data (Figure 1) suggest that these were not homogeneous patient groups and that many patients were not representative of the severe cases for whom prucalopride is licensed.
-
-
The extrapolation of data from PAC-QOL and PAC-SYM trial surveys to EQ-5Q data used in the economic model was unclear.
-
The model assumption that the relative advantage in quality of life in patients treated with prucalopride at the end of study follow-up (4 or 12 weeks) is maintained at 52 weeks is inappropriate owing to:
-
The high attrition in follow-up studies (> 60%). Patients remaining in the trial were likely to have been those who were relatively more satisfied.
-
Decreases in efficacy from the periods 1–4 weeks compared with 1–12 weeks in pivotal trials suggest that effectiveness was likely to decrease with time.
-
The lack of comparative data. If relative quality of life is to be compared, follow-up data in the placebo group would also be required.
-
Patients in long-term follow-up trials included patients who were not refractory to laxatives, and patient groups were mixtures of adult/elderly patients and patients with opioid-induced constipation.
-
This assumption is not tested in the manufacturer’s model. In order to test the effect of a reduction in quality-of-life gain over time, the ERG re-ran the model, considering a decrease in change in EQ-5D of 25%, 50% and 75% and the ICER was substantially increased.
-
-
There was no justification or explanation for the parameters used in the economic model. It was not possible to link the data that populated the model to the clinical trials. There was no way of discerning whether coefficients used in the model truly represented treatment effects.
-
No costs, other than the cost of prucalopride, were incorporated into the economic model.
-
In the model, the average use of prucalopride in responders has been assumed to be for 220 days per year but this assumption may not be justified. The ERG re-ran the model considering that all responders take treatment for the full year (365 days), and this made a substantial increase in the ICER.
-
No specific allowance was made for withdrawal from treatment at any time after 4 weeks.
-
Adverse events were not included in the model.
FIGURE 1.
Baseline EQ-5D scores for (a) adult (18–64 years) and (b) elderly (≥ 65 years) patient data used in the economic model.
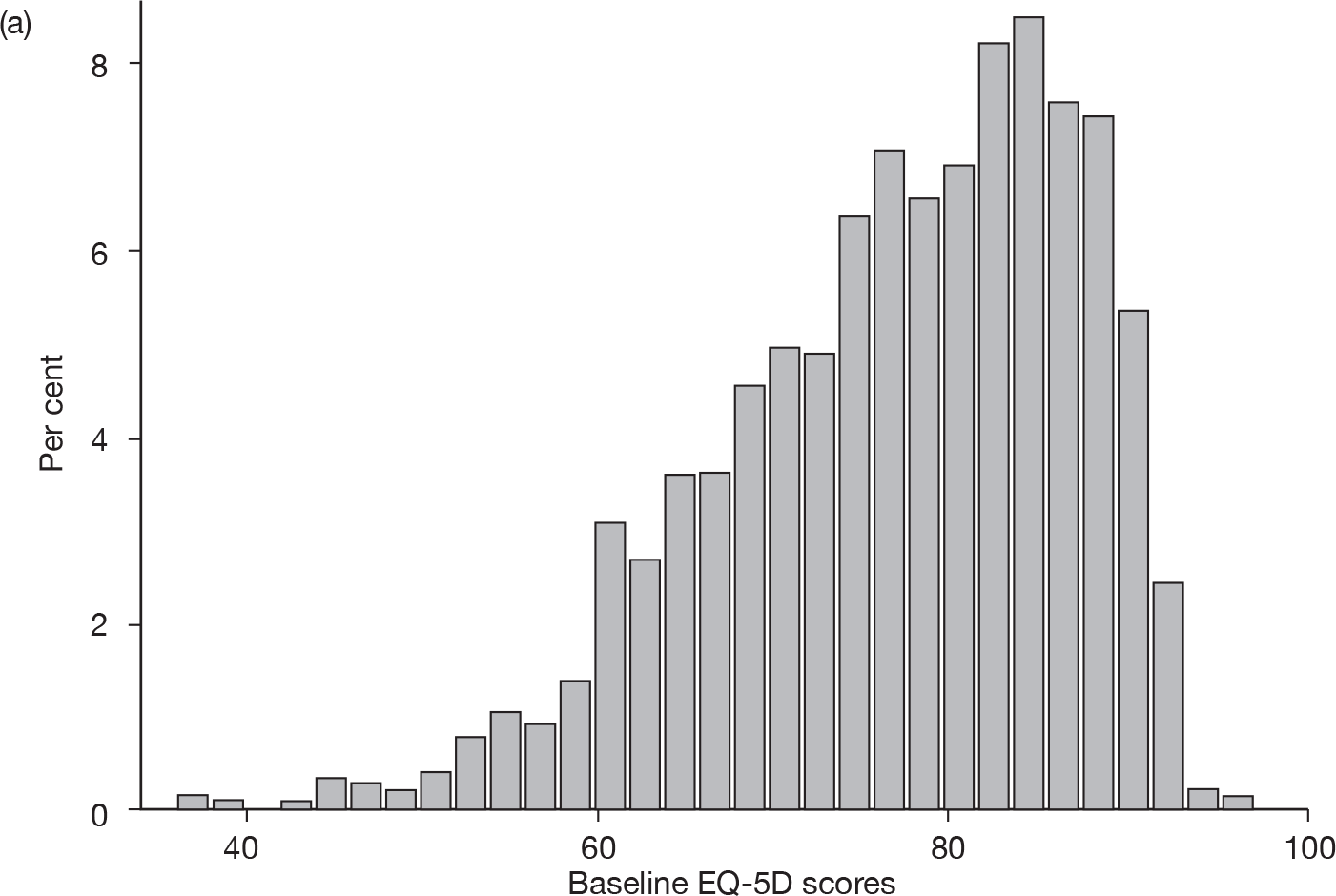
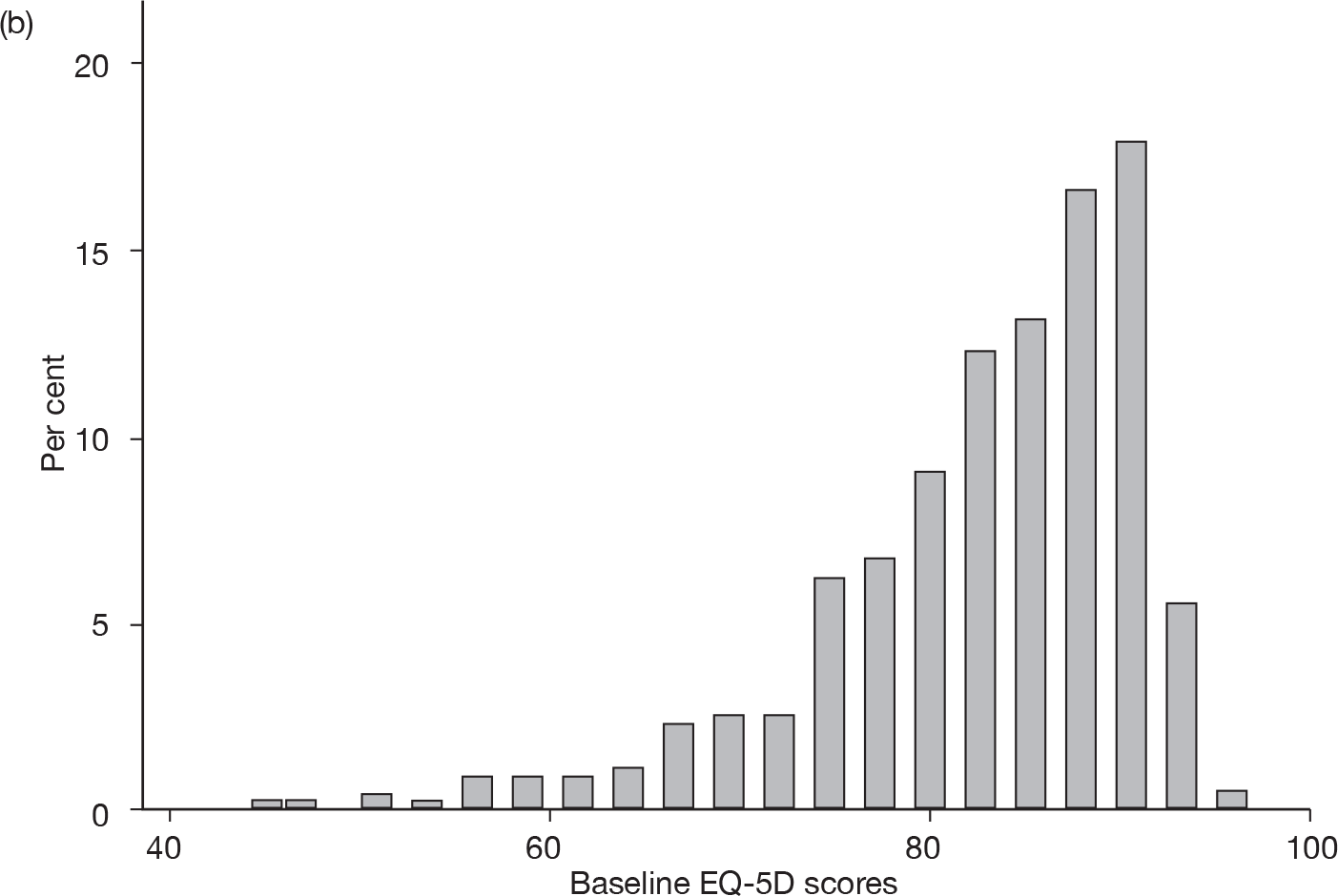
The population targeted in the scope of this technology appraisal is unlikely to be the same as that used to populate the economic model. Overall, it was felt that this submission provided no proper evidence on whether or not prucalopride is likely to be cost-effective compared with other treatment strategies in patients in the licensed indication.
Conclusions
Owing to the many areas of uncertainty, particularly the effectiveness of prucalopride in the licensed patient group and its long-term effectiveness and safety, it was considered that the MS provided no evidence for whether prucalopride is effective or not in women with laxative-refractory chronic constipation. Further subgroup analysis of the actual patient group of interest may have better guided decision-making. However, long-term efficacy data, with validated estimates of quality of life incorporated in a well-founded model, would be important for an evidence-based judgement to be made.
Summary of NICE guidance issued as a result of the STA
1.1 Prucalopride is recommended as an option for the treatment of chronic constipation only in women for whom treatment with at least two laxatives from different classes at the highest tolerated recommended doses for at least 6 months has failed to provide adequate relief and invasive treatment for constipation is being considered.
1.2 If treatment with prucalopride is not effective after 4 weeks, the patient should be re-examined and the benefit of continuing treatment reconsidered.
1.3 Prucalopride should only be prescribed by a clinician with experience of treating chronic constipation, who has supervised the woman’s previous courses of laxative treatments specified in 1.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide (accessed 1 May 2010).
- Pennant M, Orlando R, Barton P, Bayliss S, Routh K, Meads C. Prucalopride for the Treatment of Women With Chronic Constipation in Whom Standard Laxative Regimens Have Failed to Provide Adequate Relief. Evidence Review Group Report 2010.
- Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 2009;58:357-65.
- Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 2008;358:2344-54.
- Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation: a 12-week, randomised, double-blind, placebo-controlled study. Aliment Pharmacol Ther 2009;29:315-28.
- Movetis NV. Study PRU-INT-12. A Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety and Quality-of-Life of Prucalopride Tablets in Elderly Patients With Chronic Constipation 2007.
- Movetis NV. Study PRU-USA-28. A Two-Period, Double-Blind, Placebo-Controlled Study to Evaluate the Effects of Re-Treatment of Prucalopride on Efficacy and Safety in Patients With Chronic Constipation 2007.
- Movetis NV. Study PRU-INT-8. A Double-Blind, Placebo Controlled Trial to Evaluate the Efficacy and Safety of Prucalopride in Subjects With Chronic Non-Cancer Pain, Suffering from Opioid-Induced Constipation 2001.
- Movetis NV. Study PRU-INT-10. A Study to Evaluate the Long-Term Tolerability and Safety of Oral Prucalopride Administered to Patients With Chronic Constipation 2008.
- Movetis NV. Study PRU-USA-22. A Study to Evaluate the Long-Term Tolerability, Safety, Patient Satisfaction, Pharmacokinetics, and Use Pattern of Oral Prucalopride Tablets in Patients With Chronic Constipation 2008.
- Movetis NV. Study PRU-INT-17. A Study to Evaluate the Long-Term Tolerability and Safety and the Pattern of Use of Prucalopride in Patients With Chronic Pain (cancer and Non-cancer), Suffering from Opioid-Induced Constipation 2008.