Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 09/120/01. The assessment report began editorial review in October 2010 and was accepted for publication in November 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
MS has a financial interest in a consulting company that has undertaken work for Abbott, Schering-Plough and Wyeth, but not relating to psoriatic arthritis, and he has not personally participated in this consultancy work. He has personally undertaken paid consultancy for some of the comparator manufacturers, again not relating to psoriatic arthritis.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the use of golimumab for the treatment of psoriatic arthritis (PsA). The main clinical effectiveness data were derived from a single phase III randomised controlled trial (RCT: GO-REVEAL) that compared golimumab with placebo for treating patients with active and progressive PsA who were symptomatic despite the use of previous disease-modifying antirheumatic drugs or non-steroidal anti-inflammatory drugs. The 14-week data showed that, compared with placebo, golimumab 50 mg significantly improved joint disease response as measured by American College of Rheumatology (ACR) 20 [relative risk (RR) 5.73, 95% confidence interval (CI) 3.24 to 10.56] and Psoriatic Arthritis Response Criteria (PsARC) (RR 3.45, 95% CI 2.49 to 4.87), and skin disease response as measured by the Psoriasis Area and Severity Index (PASI) 75 (RR 15.95, 95% CI 4.62 to 59.11). The 24-week absolute data showed that these treatment benefits were maintained. There was a significant improvement in patients’ functional status as measured by the Health Assessment Questionnaire (HAQ) change from baseline at 24 weeks (–0.33, p < 0.001). The open-label extension data showed that these beneficial effects were also maintained at 52 and 104 weeks. However, PASI 50 and PASI 90 at 14 weeks, and all of the PASI outcomes at 24 weeks, were not performed on the basis of intention-to-treat analysis. Furthermore, analyses of the 24-week data were less robust, failing to adjust for treatment contamination due to patient crossover at week 16. The manufacturer conducted a mixed treatment comparison (MTC) analysis. The ERG considered the assumption of exchangeability between the trials for the purpose of the MTC analysis to be acceptable, and the statistical approach in the MTC analysis to be reliable. Regarding the safety evaluation of golimumab, the manufacturer failed to provide longer-term data or to consider adverse event data of golimumab from controlled studies in other conditions, such as rheumatoid arthritis and ankylosing spondylitis. Although the adverse effect profile of golimumab appears similar to other anti-tumour necrosis factor (TNF) agents, the longer-term safety profile of golimumab remains uncertain. The manufacturer’s submission presented a decision model to compare etanercept, infliximab, golimumab and adalimumab versus palliative care for patients with PsA. In the base-case model, 73% of the cohort of patients were assumed to have significant psoriasis (> 3% of body surface area). Estimates of the effectiveness of anti-TNF agents in terms of PsARC, HAQ change and PASI change were obtained from an MTC analysis of RCT data. The manufacturer failed to calculate incremental cost-effectiveness ratios (ICERs) correctly by comparing golimumab with palliative care instead of the most cost-effective alternative (etanercept). Despite the manufacturer’s claim that golimumab is a cost-effective treatment option, the manufacturer’s own model showed that golimumab is not cost-effective compared with other biologics when the ICERs are correctly calculated. None of the sensitivity analyses carried out by the manufacturer or the ERG regarding uncertainty in the estimates of clinical effectiveness, the acquisition and administration cost of drugs, the cost of treating psoriasis and the utility functions estimated to generate health outcomes changed this conclusion. However, a key area in determining the cost-effectiveness of anti-TNF agents is whether they should be treated as a class. If all anti-TNF agents are considered equally effective then etanercept, adalimumab and golimumab have very nearly equal costs and equal quality-adjusted life-years (QALYs), and all have an ICER of about £15,000 per QALY versus palliative care, whereas infliximab with a higher acquisition cost is dominated by the other biologics.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor (Schering-Plough). Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. 1 In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled Golimumab for the treatment of psoriatic arthritis. 2
Description of the underlying health problem
Psoriatic arthritis (PsA) is defined as a unique inflammatory arthritis affecting the joints and connective tissue and is associated with psoriasis of the skin or nails. 3 The prevalence of psoriasis in the general population has been estimated at between 2% and 3%,3 and the prevalence of inflammatory arthritis in patients with psoriasis has been estimated to be up to 30%. 4 PsA affects males and females equally. The figures for the UK have estimated the adjusted prevalence of PsA in the primary care setting to be 0.3%. 5 Severe PsA with progressive joint lesions can be found in at least 20% of patients with psoriasis. 6
The current UK treatment for PsA aims to improve psoriasis, arthritis or both. Non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs) are widely used to relieve symptoms, slow disease progression and prevent disability. For active and progressive patients with PsA, who have responded inadequately to at least two DMARDs, NICE clinical guideline 199 recommends three licensed anti-tumour necrosis factor (TNF) agents (etanercept, infliximab and adalimumab) as standard biological therapies. 7
Scope of the evidence review group report
The scope specified by NICE was the use of golimumab (Simponi®, Merck & Co.) for the treatment of active and progressive PsA that has responded inadequately to previous DMARDs. Golimumab is licensed for the treatment of active and progressive PsA. 8 The NICE scope specified the following comparators to be of interest: (1) alternative TNF-α inhibitors and (2) conventional management strategies for active and progressive PsA that has responded inadequately to previous DMARD therapy excluding TNF-α inhibitors.
The outcome measures considered were pain and other symptoms, functional capacity, effect on concomitant skin condition, joint damage, disease progression (e.g. imaging), adverse effects of treatment, and health-related quality of life (HRQoL). The outcome of economic evaluation was incremental cost per quality-adjusted life-year (QALY) gained.
Methods
The ERG report comprised a critical review of evidence for clinical evidence and cost-effectiveness of the technology based upon the manufacturer’s submission (MS) to NICE as part of the STA process. The ERG appraised the literature searches and carried out a search for ongoing trials. The systematic review methodology was appraised. The ERG also performed quality assessment of included trials using the Centre for Reviews and Dissemination guidelines for the critical appraisal of randomised controlled trials (RCTs).
The manufacturer’s model was checked for any discrepancies and the results were validated. A series of sensitivity analyses was also conducted. A critical appraisal of the submission was conducted with the aid of a checklist9 to assess the quality of economic evaluations and a narrative review to highlight key assumptions and possible limitations.
Results
Summary of submitted clinical evidence
The main clinical effectiveness data were derived from a single phase III RCT (GO-REVEAL10,11) that compared golimumab with placebo for treating active and progressive patients with PsA who were symptomatic despite the use of current or previous DMARDs or NSAIDs. The 14-week data (Table 1) showed that, compared with placebo, golimumab 50 mg significantly improved joint disease response as measured by ACR 20 [relative risk (RR) 5.73, 95% confidence interval (CI) 3.24 to 10.56] and the Psoriatic Arthritis Response Criteria (PsARC) (RR 3.45, 95% CI 2.49 to 4.87), and skin disease response as measured by the Psoriasis Area and Severity Index (PASI) 75 (RR 15.95, 95% CI 4.62 to 59.11). The 24-week absolute data showed that these treatment benefits were maintained (see Table 1). There was a statistically significant improvement in patients’ functional status as measured by the Health Assessment Questionnaire (HAQ) change from baseline at 24 weeks (–0.33, p < 0.001), thereby achieving the minimum clinically significant threshold for PsA (–0.3). 12 Golimumab 100 mg significantly achieved a similar magnitude of treatment effects at 14 and 24 weeks. The open-label extension data showed that these beneficial effects were also maintained at 52 and 104 weeks.
| Duration | Outcomes | Golimumab (n, %) | Placebo (n, %) | Golimumab (RR or mean difference, 95% CI) | ||
|---|---|---|---|---|---|---|
| 50 mg | 100 mg | 50 mg | 100 mg | |||
| 14 weeks | PsARC | 107/146 (73.3) | 105/146 (71.9) | 24/113 (21.2) | 3.451 (2.49 to 4.87) | 3.386 (2.43 to 4.80) |
| ACR 20 | 74/146 (50.7) | 66/146 (45.2) | 10/113 (8.8) | 5.727 (3.24 to 10.56) | 5.108 (2.86 to 9.48) | |
| ACR 50 | 44/146 (30.1) | 41/146 (28.1) | 2/113 (1.8) | 17.027 (4.81 to 63.32) | 15.866 (4.47 to 59.11) | |
| ACR 70 | 18/146 (12.3) | 25/146 (17.1) | 1/113 (0.9) | 13.932 (2.46 to 81.82) | 19.349 (3.48 to 112.44) | |
| HAQ change from baseline, mean (SD) | NA | NA | NA | – | – | |
| PASI 50a | 63/106 (59.4) | 83/107 (77.6) | 7/73 (9.6) | 6.198 (3.22 to 12.7) | 8.089 (4.38 to 16.04) | |
| PASI 75a | 44/109 (40.4) | 63/108 (58.3) | 2/79 (2.5) | 15.945 (4.62 to 59.11) | 23.042 (6.85 to 84.59) | |
| PASI 90a | 22/106 (20.8) | 26/107 (24.3) | 0/73 (0.0) | ∞ (4.21 to ∞) | ∞ (4.95 to ∞) | |
| 24 weeks | PsARC | 102/146 (69.9) | 124/146 (84.9) | 33/113 (29.2) | 2.392 (1.81 to 3.20) | 2.908 (2.28 to 3.68) |
| ACR 20 | 76/146 (52.1) | 89/146 (61.0) | 14/113 (12.4) | 4.202 (2.60 to 7.03) | 4.920 (3.09 to 8.13) | |
| ACR 50 | 47/146 (32.2) | 55/146 (37.7) | 4/113 (3.5) | 9.094 (3.62 to 23.94) | 10.642 (4.27 to 27.85) | |
| ACR 70 | 27/146 (18.5) | 31/146 (21.2) | 1/113 (0.9) | 20.897 (3.77 to 121.19) | 23.993 (4.35 to 138.68) | |
| HAQ change from baseline, mean (SD) | 0.33 ± 0.55, p < 0.001 | 0.39 ± 0.50, p < 0.001 | –0.01 ± 0.49 | – | – | |
| PASI 50a | 77/102 (75.5) | 87/106 (82.1) | 6/73 (8.2) | 9.185 (4.69 to 19.45) | 9.986 (5.21 to 20.76) | |
| PASI 75a | 57/102 (55.9) | 70/106 (66.0) | 1/73 (1.4) | 40.794 (7.86 to 232.88) | 48.208 (9.44 to 274.39) | |
| PASI 90a | 33/102 (32.4) | 34/106 (32.1) | 0/73 (0.0) | ∞ (6.65 to ∞) | ∞ (6.59 to ∞) | |
In the absence of head-to-head comparisons of the relative efficacy between different anti-TNF agents, the manufacturer conducted a mixed treatment comparison (MTC) analysis to estimate the relative efficacy of the four relevant anti-TNF agents: golimumab, etanercept, adalimumab and infliximab. The results of MTC analyses in the MS were marked as confidential and therefore cannot be reported. Table 2 presents the ERG’s recalculated results of the MTC analyses based on the data provided in the MS. These results are generally similar to the results of MTC analyses from the MS. The results (see Table 2) show that infliximab appears to be the most effective of the four anti-TNF agents, being associated with the highest probabilities of response in terms of joint and skin disease outcomes. Golimumab achieves the second highest PsARC response (joint disease), and golimumab has the third highest response for skin disease in terms of PASI change from baseline. In those patients who achieved a PsARC response, the highest mean improvement in the functional status (HAQ) is seen with infliximab (–0.659), and the lowest mean improvement in HAQ is seen with golimumab (–0.440). For all four of the anti-TNF agents, the changes in HAQ for those patients who did not achieve a PsARC response are below the minimum clinically significant threshold (–0.3). 12 The credible intervals of most outcomes for all four anti-TNF agents overlap each other.
| Outcomes | Placebo | Infliximab | Etanercept | Adalimumab | Golimumab | |
|---|---|---|---|---|---|---|
| PsARC response | Mean (SD) | 0.247 (0.036) | 0.793 (0.057) | 0.712 (0.070) | 0.585 (0.070) | 0.764 (0.065) |
| 95% CrI | 0.175 to 0.318 | 0.001 to 0.799 | 0.562 to 0.832 | 0.441 to 0.716 | 0.622 to 0.871 | |
| HAQ change from baseline, in PsARC responders | Mean (SD) | –0.2663 (0.044) | –0.659 (0.709) | –0.635 (0.091) | –0.4818 (0.065) | –0.4404 (0.085) |
| 95% CrI | –0.3555 to –0.1816 | –1.026 to –0.286 | –0.8144 to –0.4563 | –0.6053 to –0.3488 | –0.6088 to –0.2756 | |
| HAQ change from baseline, in PsARC non-responders | Mean (SD) | 0 | –0.1981 (0.073) | –0.1949 (0.099) | –0.136 (0.068) | –0.0308 (0.088) |
| 95% CrI | 0 | –0.3382 to 0.056 | –0.3917 to 0.00023 | –0.2684 to 0.0017 | –0.2608 to 0.1418 | |
| PASI change from baseline, in patients ≥ 3% BSA psoriasis at baseline | Mean (SD) | – | –7.2168 | –2.5044 | –5.17769 | –4.486 |
| 95% CrI |
Short-term radiographic data from the GO-REVEAL trial10,11 indicated that golimumab 50 mg significantly slowed joint disease progression during the 24 weeks. There was a lack of follow-up radiographic data to determine whether these effects persisted in the longer term.
The limited available evidence for the safety evaluation from the single GO-REVEAL trial10,11 suggested that the most frequently reported adverse events associated with golimumab therapy were infections and infestations, upper respiratory tract infection and nasopharyngitis. Serious adverse events including serious infection and malignancy were rare. No active tuberculosis in any treatment arm was observed.
Summary of submitted cost-effectiveness evidence
The MS included a decision model to compare etanercept, infliximab, golimumab and adalimumab versus palliative care for patients with PsA. In the base-case model, 73% of the cohort of patients were assumed to have significant psoriasis (> 3% body surface area). Estimates of the effectiveness of anti-TNF agents in terms of PsARC, HAQ change and PASI change were obtained from an MTC analysis of RCT data.
Patients in the model were assumed to continue with biologic therapy after 12 weeks if they achieved a PsARC response (Figure 1). HRQoL and costs were estimated as a function of HAQ and PASI score. The acquisition costs of anti-TNF agents (other than golimumab) were taken from the British National Formulary. 13 The acquisition, administration and monitoring costs of golimumab were stated by the manufacturer to be equivalent to the list price of adalimumab. The unit price for golimumab is £774.58 for a 0.5-ml pre-filled pen/syringe containing 50 mg of golimumab. The annual drug acquisition cost is £9294.96.
FIGURE 1.
Model structure. NatHist, natural history; Pall, palliative care.
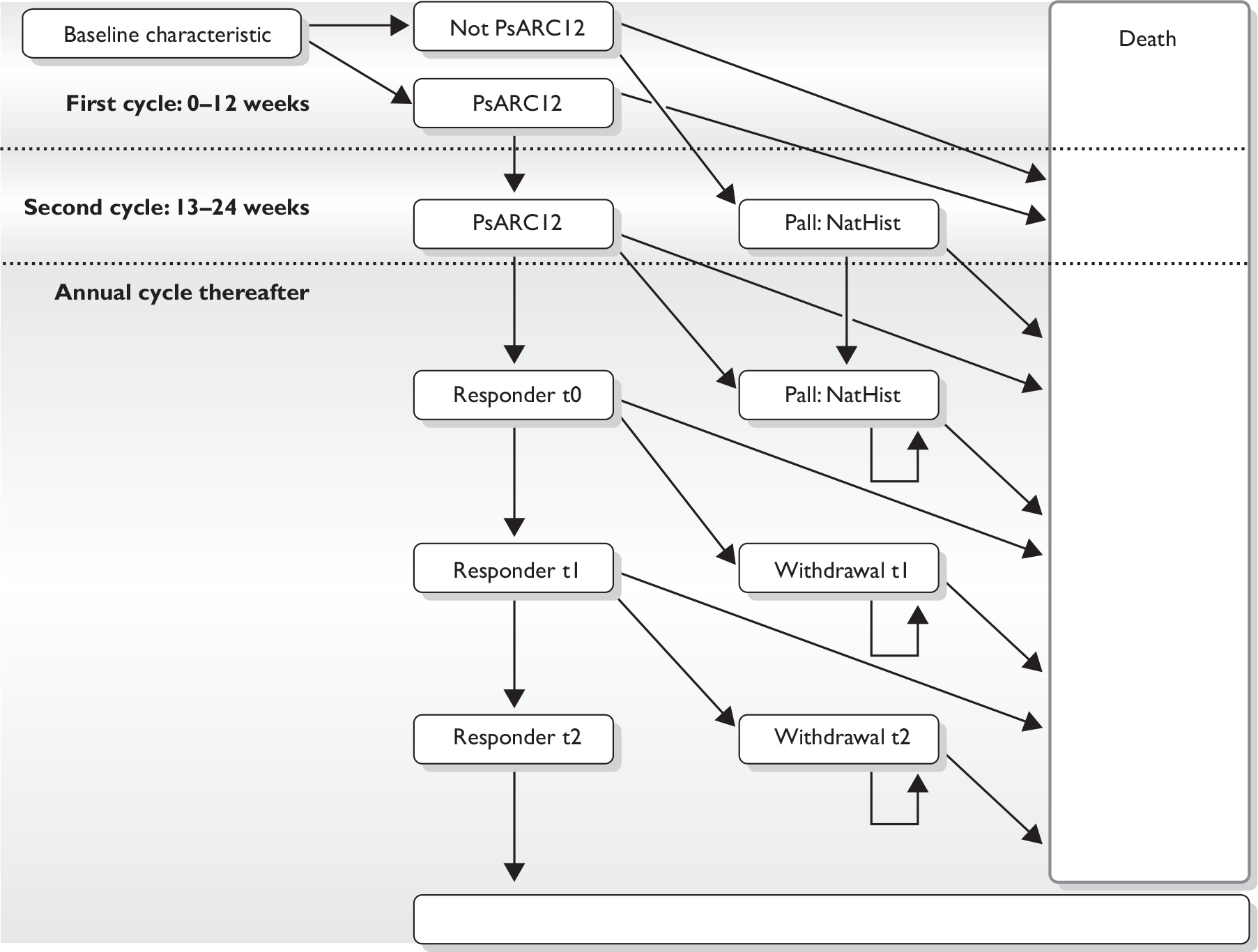
The original MS base-case model was revised following requests for clarifications from the ERG. The revised MS model amended the functional form of the utility algorithm linking HAQ and PASI to HRQoL. The revised model also assumed that infliximab was administered without vial sharing.
The revised decision model found that the incremental cost-effectiveness ratio (ICER) of golimumab versus palliative care was just under £20,000 per QALY. However, the comparison with palliative care does not meet the NICE requirement for an incremental cost-effectiveness analysis to be conducted, in which each strategy should be compared with the next best alternative.
Commentary on the robustness of submitted evidence
Strengths of the manufacturer’s submission
The manufacturer’s systematic review identified the single double-blind phase III RCT (GO-REVEAL10,11) that was conducted in a relevant population, and the dosing regimen (including dose adjustment) for the golimumab 50 mg group was generally reflective of clinical practice. The results from the 14-week data analyses of this trial were considered to be robust.
The degree of clinical heterogeneity between the included trials in the MTC was considered reasonable, and the assumption of exchangeability between the trials for the purpose of the MTC analysis was acceptable. The ERG also considered the statistical approach in the manufacturer’s MTC analysis to be reliable.
For the economic evaluation, the manufacturer’s model took account of all important elements of the decision problem, in terms of the rules for continuation of biological therapy, natural history of arthritis and psoriasis in these patients, the treatment effects, the relationship between psoriasis, arthritis and HRQoL, and its associated costs.
Weaknesses of the manufacturer’s submission
The manufacturer did not adequately apply the intention-to-treat approach for all outcomes in the efficacy analysis in the MS. Based on the revised data table provided by the manufacturer, PASI 50 and PASI 90 at 14 weeks and all the PASI outcomes at 24 weeks were also not performed on the basis of intention-to-treat analysis. Such analyses may have potentially compromised the internal validity of the results in terms of these skin disease outcomes.
There was further concern about the robustness for the analyses on the 24-week data in the GO-REVEAL trial,10,11 which failed to adjust the treatment contamination due to patients crossing over at week 16. This may have threatened the internal validity of trial results for all the efficacy and safety outcomes at 24 weeks.
In terms of safety evaluation, the manufacturer did not present data to facilitate a comparison between the adverse events of golimumab with those of the comparator anti-TNF agents. The longer-term follow-up safety data (e.g. at 52 and 104 weeks) from the GO-REVEAL trial10,11 were not available. Furthermore, the manufacturer failed to consider adverse event data of golimumab from controlled studies in other conditions, such as rheumatoid arthritis and ankylosing spondylitis.
Regarding the cost-effectiveness analysis, there was some concern about the robustness of the estimates of the cost associated with psoriasis. This was based on a survey of 22 dermatologists. The manufacturer stated that, based on the results from survey, the cost per PASI point was £53 per year if phototherapy is excluded and £167 per PASI point per year if phototherapy is included as a treatment for psoriasis. This implies that reducing PASI from, for example, 9.9 to 3.3 (a reduction of 6.6 points estimated for infliximab) would reduce the expected cost of treating psoriasis by £1100 per year if phototherapy was used and by £350 per year if phototherapy was not used. However, the MS provided insufficient detail of these calculations for the ERG to check whether or not these estimated costs were valid. No estimates of variability or sampling uncertainty were provided. The manufacturer provided raw data from the survey of dermatologists on request for clarification, but these data did not show the unit costs or details of how the results of the survey were synthesised to generate the mean cost per PASI point.
The MS did not correctly calculate the ICERs used to compare the cost-effectiveness of the treatments. The MS did not exclude extendedly dominated alternatives. The ERG recalculated the ICERs using the results of the MS model. The corrected ICER from the MS model for etanercept versus palliative care is about £17,000 per QALY. According to the MS model, with the ICERs correctly calculated, other anti-TNF agents (golimumab, adalimumab and infliximab) are not cost-effective because they are either dominated or extendedly dominated by etanercept.
Areas of uncertainty
While MTC analyses provide evidence of the relative efficacy of these anti-TNF agents, those findings may be considered more uncertain than would be provided in head-to-head RCTs. In particular, there were substantial uncertainties for the estimates of PASI change from baseline owing to a small sample size of patients evaluable for psoriasis.
No trial specified the failure to respond to at least two DMARDs (patients whom the current British Society for Rheumatology guidelines and NICE guidance for etanercept, infliximab and adalimumab consider eligible for the biologic treatment) as a recruitment criterion. As trial participants were not precisely representative of the active and progressive PsA population recommended for anti-TNF agents by the current guidelines, it remains unclear that the beneficial effects observed in these trial participants were similar in those treated in routine clinical practice.
Other areas of uncertainty that were explored in sensitivity analyses by the ERG were the effects of alternative estimates of clinical effectiveness in terms of PsARC; HAQ change and PASI change from the ERG evidence synthesis; the cost of administration of drugs; alternative values for NHS cost of psoriasis, measured by PASI; alternative utility functions; and the possibility of increasing the dose of golimumab (to 100 mg) for patients who do not achieve adequate response at 12 weeks, in accordance with the licence. None of these sensitivity analyses changed the conclusion that golimumab is extendedly dominated by etanercept. Further analyses were also conducted using the ERG model developed by the York Assessment Group during the recent appraisal of etanercept, infliximab and adalimumab. These analyses were used to validate the MS model by comparing the results with an independently constructed model. The MS model and the ERG alternative model have a broadly similar structure and data inputs, and gave similar results.
A key area of uncertainty is whether the anti-TNF agents should be considered equally clinically effective, i.e. to treat them as a class. This was the position adopted by the recent guidance issued by NICE regarding the previous appraisal of etanercept, adalimumab and infliximab for PsA. 7 If all anti-TNF agents are considered equally effective (in terms of PsARC, HAQ and PASI responses) then etanercept, adalimumab and golimumab have very nearly equal costs and QALYs, and all have an ICER of about £15,000 per QALY versus palliative care, whereas infliximab, with a higher acquisition cost, is dominated by the other biologic.
The licence for golimumab indicates that patients who are > 100 kg in weight and who fail to respond to golimumab 50 mg at 3 months can be trialled on a higher dose of 100 mg. A full economic analysis of this option could not be undertaken because of a lack of clinical data for this subgroup of patients. The ERG notes that if patients are titrated and maintained on a higher dose then the additional acquisition costs will be around £2145 per 3 months. However, the clinical adviser to the ERG suggests that, in practice, this scenario is unlikely because of the additional cost; eligible patients are more likely to be tried on an alternative biologic agent.
A remaining source of uncertainty is the annual cost of treating psoriasis. Although the MS conducted a survey of dermatologists and presented the raw data from the survey, there was no detail of the statistical methods used to calculate the mean costs from the raw data and, therefore, the ERG could not validate the calculations. However, the ERG conducted sensitivity analysis on the PASI cost using the ERG model. Doubling or halving the cost per PASI point of £167 per year did not materially affect the results of the ERG model.
Conclusions
The data from the GO-REVEAL trial10,11 provide evidence to suggest that golimumab appears to be an efficacious treatment for patients with active and progressive PsA despite the use of previous DMARDs or NSAIDs. The effect sizes of point estimates of joint and skin disease response and functional status were moderate to large, implying that these treatment effects could be clinically significant. However, the analyses for efficacy outcomes were limited to only one RCT (GO-REVEAL10,11) with limited sample size. In particular, few patients provided data on the psoriasis response to golimumab treatment.
The ERG further considered the evidence for safety evaluation of golimumab to be inadequate. The evidence was exclusively based on 24-week data from the single RCT with patients with PsA (GO-REVEAL10,11). The manufacturer failed to provide longer-term data or to consider adverse event data of golimumab from controlled studies in other conditions, such as rheumatoid arthritis and ankylosing spondylitis. Although the adverse effects profile of golimumab appears similar to other anti-TNF agents, the longer-term safety profile of golimumab remains uncertain. Given these limitations and uncertainties, the manufacturer’s conclusion that golimumab is a safe treatment option similar to other anti-TNF agents may be premature and may not be reliable.
Despite the claim made by the manufacturer that golimumab is a cost-effective treatment option, the manufacturer’s own model showed that golimumab is not cost-effective when the ICERs are correctly calculated. None of the sensitivity analyses carried out by the manufacturer or the ERG regarding uncertainty in the estimates of clinical effectiveness, the acquisition and administration cost of drugs, the cost of treating psoriasis and the utility functions estimated to generate health outcomes changed this conclusion.
However, a key area in determining the cost-effectiveness of anti-TNF agents is whether they should be treated as a class. If all anti-TNF agents are considered equally effective (in terms of PsARC, HAQ and PASI responses) then etanercept, adalimumab and golimumab are all cost-effective, whereas infliximab is dominated by the other biologic agents.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in April 2011 states that:
Golimumab is recommended as an option for the treatment of active and progressive psoriatic arthritis in adults only if:
it is used as described for other tumour necrosis factor (TNF) inhibitor treatments in ‘Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis’ (NICE technology appraisal guidance 199)7 and
the manufacturer provides the 100 mg dose of golimumab at the same cost as the 50-mg dose.
When using the Psoriatic Arthritis Response Criteria (PsARC; as set out in NICE technology appraisal guidance 199), health-care professionals should take into account any physical, sensory or learning disabilities, or communication difficulties that could affect a person’s responses to components of the PsARC and make any adjustments they consider appropriate.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- Schering-Plough Ltd . Golimumab for Psoriatic Arthritis: Submission to National Institute of Health and Clinical Excellence. Single Technology Appraisal (STA) [industry Submission] 2010.
- Yang H, Epstein D, Bojke L, Craig D, Light K, Bruce I, et al. Evidence Review group’s Report: Golimumab for the Treatment of Psoriatic Arthritis 2010. www.hta.ac.uk/erg/reports/2320.pdf (accessed 22 March 2011).
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64:14-7.
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 2003;4:441-7.
- Kay LJ, Parry-James JE, Walker DJ. The prevalence and impact of psoriasis and psoriatic arthritis in the primary care population in North East England. Arthritis Rheum 1999;42.
- Feuchtenberger M, Kleinert S, Tony H-P, Kneitz C. Psoriatic arthritis: therapeutic principles. Clin Dermatol 2008;26:460-3.
- National Institute for Health and Clinical Excellence . Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis. Includes a Review of NICE Technology Appraisal Guidance 104 and 125 2010. http://guidance.nice.org.uk/TA199/Guidance/pdf/English (accessed 17 September 2010).
- European Medicines Agency . Summary of Product Characteristics – Simponi 50 Mg Solution for Injection in Pre-Filled Pen (Centocor B.V.) 2010. www.ema.europa.eu/humandocs/PDFs/EPAR/simponi/emea-combined-h992en.pdf (accessed 7 July 2010).
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Centocor Ltd . A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Golimumab, a Fully Human Anti-TNFα Monoclonal Antibody, Administered Subcutaneously in Subjects With Active Psoriatic Arthritis 2007.
- Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976-86.
- Mease P, Ganguly R, Wanke L, Yu E, Singh A. How much improvement in functional status is considered important by patients with active psoriatic arthritis: applying the outcome measures in rheumatoid arthritis clinical trials (OMERACT) group guidelines. Ann Rheum Dis 2004;63.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.