Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/62/01. The contractual start date was in December 2009. The draft report began editorial review in July 2010 and was accepted for publication in March 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draftdocument. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Stevenson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Alcohol-related liver disease (ALD) is the term used to describe changes within the liver that develop as a result of excess alcohol consumption. Included within this spectrum are hepatic steatosis (alcoholic fatty liver), alcoholic hepatitis, alcoholic cirrhosis, and hepatocellular cancer (HCC). 1–3 Although patients with cirrhosis may remain free of major complications for several years, once such complications develop survival is usually short. 4
In 2008, 4764 deaths registered in England and Wales were attributed to ALD. 5
Aetiology, pathology and prognosis
Aetiology
Alcohol-related liver disease is caused by the consumption of substantial quantities of alcohol. In the UK, alcoholic drinks are measured in units corresponding to approximately 10 ml (8 g) of ethanol. 6 Previously, the minimum alcohol intake associated with significant liver damage was thought to be around 20 units a day for 5 years and the likelihood of light to moderate drinkers developing cirrhosis was considered very remote. 7 More recently, it has been suggested that all people whose daily alcohol consumption exceeds 80 g (10 units) will eventually develop steatosis; 10–35% will develop alcoholic hepatitis, and approximately 10% will develop cirrhosis. 8 However, because steatosis is usually, and hepatitis and cirrhosis sometimes, asymptomatic, many will be unaware that they have ALD; indeed, 30–40% of cases of cirrhosis are not discovered until autopsy. 1
Pathology
As noted above, the term ALD covers a spectrum of illness. The majority of individuals who abuse alcohol will develop steatosis,9 a condition characterised by the accumulation of excess fat within the liver cells. 10 Steatosis is usually asymptomatic, but may manifest as changes in liver function test results; it is reversible if alcohol consumption is stopped or significantly reduced. 1 However, approximately 20% of people with alcoholic steatosis who continue long-term alcohol consumption are likely to develop fibrosis and cirrhosis. 2
In a minority (perhaps < 20%) of alcohol misusers, alcoholic steatosis evolves with the development of inflammation and cell death within the liver. This phase of injury is known as alcoholic hepatitis. 10 The majority of patients who develop alcoholic hepatitis are asymptomatic, but others suffer non-specific symptoms such as nausea, vomiting, and abdominal pain, whereas others present with acute alcoholic hepatitis (AAH), which is characterised by abdominal pain/discomfort, fever, a high white blood cell count, abnormalities of blood clotting, jaundice, and other features of liver failure. 1 Patients with mild alcoholic hepatitis will recover well, with reversal of the liver damage, if they stop drinking. Progression to cirrhosis is more likely in women than in men, and in individuals of both sexes who have severe alcoholic hepatitis on presentation, irrespective of their future drinking behaviour.
Repeated episodes of alcoholic hepatitis may result in the development of scar tissue known as fibrosis; this can be localised or diffuse, and can eventually lead to distortion of the architecture of the liver by broad fibrous bands and the development of regenerative nodules, which are the hallmark of cirrhosis. Patients with cirrhosis may be asymptomatic, and their liver function tests may show few if any abnormalities; such patients are described as having compensated disease. 10,11 In a recent Danish study, 24% of patients diagnosed with alcoholic cirrhosis had no complications. 12 Patients with cirrhosis may, however, develop a number of complications as a result of hepatocellular failure and the development of portal hypertension (PHt). Hepatocellular failure results in loss of the liver’s ability to deal with waste products (e.g. bilirubin from red blood cells, toxins produced in the bowel such as ammonia, and drugs) and impairment of its synthetic function leading to low levels of body proteins such as albumin and the factors responsible for blood clotting. PHt develops when the passage of blood from the intestine and the spleen, which is usually cleansed and detoxified in the liver, is impeded because of the presence of fibrosis. As a result, collateral vessels develop to bypass the liver to ensure that the blood gets back to the heart. These collateral vessels may develop in the stomach and oesophagus and may rupture when the pressure gradient within the portal system [the hepatic venous pressure gradient (HVPG)] exceeds 12 mmHg. 13 Hepatocellular failure and PHt result in the development of several problems, including jaundice, ascites, variceal bleeding, and hepatic encephalopathy (HE). 4 Patients with these complications are described as having decompensated cirrhosis. Each year, approximately 5–7% of patients with compensated cirrhosis will develop decompensated disease. 4 Approximately 20% of patients with cirrhosis will go on to develop HCC; this is more common in men than in women and in those who have stopped drinking (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication).
Alcoholic hepatitis and cirrhosis may co-exist within the same patient: thus, it has been reported that 50–60% of patients diagnosed with AAH already have cirrhosis at the time of diagnosis. 14
Measurement of disease
Traditionally, liver biopsy has been used to obtain histological samples from patients suspected of having ALD. These biopsy samples are used to confirm the diagnosis of ALD, exclude other or additional liver pathologies, and provide accurate staging of the degree of liver injury in order to enable the prediction of prognosis and inform treatment decisions.
Various semi-quantitative scoring systems have been developed to measure and categorise disease progression in biopsy samples. The most widely used system, the METAVIR system, has a grading system that scores the degree of inflammation from 0 to 4 (where 0 is no activity and 4 is severe activity) and a staging system that categorises the degree of architectural change and consequent severity of the underlying liver disease15 from none (F0) to mild (F1), moderate (F2), severe (F3), and cirrhosis (F4). It should be noted that METAVIR, and other similar systems, have been described as being, at best, ordered categorical data;16 they do not represent an arithmetic progression, and consequently, for example, a METAVIR score of F4 does not involve twice as much scarring as a score of F2. For further details of the various scoring systems referred to in this review, see Appendix 1.
In clinical practice, various boundaries are said to be significant. These are the boundaries between no-to-mild fibrosis (F0–F1) and significant fibrosis (F ≥ 2),17 and between mild-to-moderate fibrosis (F1–F2) and severe fibrosis or cirrhosis (F3–F4). 18 However, the former seems primarily relevant in viral hepatitis, where F2 fibrosis forms the threshold for the initiation of interferon therapy;19 it arguably has less relevance in ALD, where the boundary between F0–F3 and F4 appears more important as it forms the threshold for the initiation of regular screening for varices and HCC.
Prognosis
Prognosis in patients with ALD is strongly linked to the degree of disease progression. In an early UK study, Saunders et al. 20 found that 5-year survival in adults identified between 1959 and 1976 as having alcoholic cirrhosis was only 36%; most already had decompensated cirrhosis at diagnosis. More recently, Jepsen et al. 12 found that the median survival in Danish patients diagnosed between 1993 and 2005 with alcoholic cirrhosis without complications was 48 months. The 1-year mortality ranged from 17% in those who presented with no complications to 64% in those who presented with HE; the equivalent 5-year mortality figures were 58% and 85%, respectively. More generally, median survival times for patients diagnosed with compensated and decompensated cirrhosis have been estimated at over 12 years and around 2 years, respectively; most patients originally diagnosed with compensated cirrhosis die only after the development of decompensation. 4
In patients with cirrhosis, the presence of superimposed alcoholic hepatitis and the development of hepatocellular carcinoma significantly reduced survival. Verrill et al. 21 found that 30-day mortality in patients with cirrhosis and alcoholic hepatitis was 27%, while Orrego et al. 22 found that the 1-year and 5-year mortality were significantly higher than in those of patients who had alcoholic cirrhosis alone, and Chedid et al. 23 found that 65% of patients with concurrent alcoholic hepatitis and cirrhosis died within 4 years of diagnosis, mostly within the first year. Alcoholic cirrhosis substantially increases the risk of hepatocellular carcinoma,1 which, again, has a poor prognosis: 1-year survival after diagnosis of HCC is about 20%, 5-year survival about 5%,24 and approximately 15% of patients who develop alcohol-related cirrhosis will die of HCC. 9
However, the outcome for patients with alcohol-related cirrhosis is substantially determined not only by the degree of decompensation at presentation but also by subsequent drinking behaviour. In 2004, Tome and Lucey25 suggested that, in people with clinically compensated cirrhosis, the 5-year survival was about 90% in those who abstained compared with about 70% in those who did not, whereas in 2003 Mann et al. 1 suggested that, in patients with late-stage, decompensated cirrhosis, the 5-year survival was 60% in those who abstained, but only 35% in those who did not. In a recent study, Verrill et al. 21 found that patients who reported being abstinent from alcohol 30 days after receiving a diagnosis of alcoholic cirrhosis had a 7-year survival of 72% compared with 44% in those who continued to drink. A considerably older study by Saunders et al. 20 found that 10-year survival in patients who abstained completely from alcohol was nearly 60% in those who presented with compensated cirrhosis and around 50% in those who presented with decompensated cirrhosis, compared with around 30% and < 10%, respectively, in those who continued drinking. It is currently estimated that a middle-aged man who presents with compensated cirrhosis and subsequently abstains from alcohol has a 60–80% chance of being alive in 10 years, whereas a similar individual who presents with, for example, variceal bleeding, and who survives the initial presentation but who continues to drink, is unlikely to survive more than a year or two (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication).
A small study by Day,26 of 96 patients with biopsy-proven ALD and a weekly alcohol intake of 135 ± 8 units, suggests that a reduction in alcohol intake may also be beneficial. No difference was found between abstainers and mild/moderate drinkers in either mortality (total or liver-related) or the development of new liver complications; however, continued heavy drinking (> 50 units/week for men and > 35 units/week for women) was predictive of both total and liver-related mortality.
Alcohol consumption also has a marked impact on prognosis in patients with milder forms of ALD. With abstinence, hepatic steatosis appears to be completely reversed within several weeks and AAH usually improves. 25 Patients with hepatic steatosis who stop drinking have a near-normal lifespan; future drinking behaviour is the most important determinant of outcome in this patient group. Their prognosis has improved over time as the treatment of their complications has become more successful. Moreover, as Chedid et al. have shown,23 these patients tend to die as a result of their alcoholism rather than their liver disease. Thus, although the 2-year survival in patients with alcoholic steatosis is only 70%, those who die do so as a result of other alcohol-related events such as accidents, injuries, suicide, and homicide, not their liver disease. However, although some patients with alcoholic hepatitis who abstain from alcohol make a complete recovery, others develop cirrhosis despite abstinence. 10
Although only a minority of patients with ALD abstain from alcohol following diagnosis, the proportion may be increasing; although only 19% of patients in a UK study who were diagnosed with alcoholic cirrhosis between 1959 and 1976 abstained from alcohol after hospital discharge,20 36% of patients in a Danish study who received a hospital diagnosis of cirrhosis between 1993 and 2005 maintained abstinence,12 and 46% of participants in a UK study who were diagnosed with biopsy-proven alcohol-related liver cirrhosis between 1995 and 2000 reported abstinence both at 30 days post diagnosis and at follow-up a mean of 3.4 years later (although some admitted to lapses during that period). 21 However, the precise diagnosis given may influence subsequent drinking behaviour. Thus, Day26 studied 96 patients with biopsy-proven ALD referred to unit between August 1991 and August 1993: 76% had established cirrhosis or fibrosis, 23% had alcoholic hepatitis and 8% steatosis. At follow-up, 50% were still drinking heavily, although 21% were completely abstinent, and 21% had mild and 8% moderate alcohol intake. The only predictor of continued heavy intake was the absence of cirrhosis; continued heavy drinking was reported in 59% of patients without, and 38% of patients with, cirrhosis (p = 0.04). By contrast, a study of patients receiving liver transplants for ALD found that a number of factors were independently associated with a significantly increased risk of resumption of harmful alcohol consumption after transplantation: these were pre-transplant diagnosis of an anxiety or depressive disorder, a pre-transplant period of abstinence < 6 months, and a total score of > 3 on the high-risk alcoholism relapse (HRAR) scale (Table 1). Over a mean follow-up period of 61.2 months, the overall relapse rate was 11.9%; however, it varied from 5% to 100% depending on the number of relevant factors (Table 2). 27
| Item | Score |
|---|---|
| Duration of heavy drinking, in years | |
| ≤ 11 | 0 |
| 11–25 | 1 |
| ≥ 25 | 2 |
| Number of daily drinks (one drink = 12 g of ethanol) | |
| ≤ 9 | 0 |
| 9–17 | 1 |
| ≥ 17 | 2 |
| Number of prior alcoholism inpatient treatments | |
| 0 | 0 |
| 1 | 1 |
| ≥ 1 | 2 |
| Number of relevant factorsa | Number resuming harmful alcohol consumption | Rate % (95% CI) | Mean time to relapse (months) |
|---|---|---|---|
| 0 | 13/272 | 5 (2 to 7) | 45 |
| 1 | 16/92 | 17 (10 to 25) | 30 |
| 2 | 14/22 | 64 (44 to 84) | 32 |
| 3 | 3/3 | 100 | 23 |
It has therefore been suggested that, in patients with ALD, the degree of fibrosis may have less prognostic importance than other factors such as the severity of alcoholic hepatitis, severity of liver dysfunction as assessed by tools such as the Child–Pugh score, the MELD (Model for End-Stage Liver Disease) score, or the Glasgow score and subsequent drinking behaviour. 28
Epidemiology demographic factors (age, sex, ethnicity, income, and regional variation)
Current government guidelines state that women should drink no more than 2–3 units of alcohol a day, and men no more than 3–4 units; the recommended weekly limits are 14 and 21 units, respectively. 29 However, in 2007, a health survey for England found that 31% of women and 42% of men in England aged ≥ 16 years reported drinking more than the government recommended maximum on at least 1 day in the preceding week. 30,31 The figures reported to the Welsh health survey in 2008 were higher, at 38% of women and 52% of men. 32
Hazardous drinking has been defined as an established pattern of drinking that carries a risk of physical or psychological harm. Harmful drinking has been defined as drinking at a level where damage to physical or mental health is considered likely. 31 A score of 8–15 on the Alcohol Use Disorders Identification Test (AUDIT) is held to represent hazardous drinking, and an AUDIT score of ≥ 16 as harmful drinking (for the AUDIT tool, see Appendix 2). 31 A total of 20.4% of individuals in England aged ≥ 16 years living in private households who participated in the 2007 Adult Psychiatric Morbidity Survey reported established patterns of alcohol consumption that were categorised as hazardous, and a further 3.8% reported patterns categorised as harmful (Table 3). 31 These figures are probably underestimates as they are likely to under-represent both alcohol-dependent individuals, who are more likely than non-alcohol-dependent individuals to be homeless or living in an institution, and problem drinkers living in private households, who may be less likely than those who are not problem drinkers to participate in surveys. 31
| AUDIT score | Men (%) | Women (%) | All (%) |
|---|---|---|---|
| 0–7: not hazardous | 66.8 | 84.3 | 75.8 |
| 8–15: hazardous, not harmful | 27.4 | 13.8 | 20.4 |
| 16–40: harmful | 5.8 | 1.9 | 3.8 |
| 8–40: hazardous or harmful | 33.2 | 15.7 | 24.2 |
In England, rates of hazardous or harmful drinking vary by ethnic group, being highest in white people and lowest in people of South Asian origin31 (Table 4). However, there is evidence both that alcohol problems may be increasing in the South Asian community in the UK, and that they may be particularly vulnerable to ALD. 33,34
| AUDIT score | Ethnicity (%) | |||
|---|---|---|---|---|
| White | Black | South Asian | Othera | |
| Men | ||||
| 0–7: not hazardous | 64.2 | 81.4 | 88.0 | 84.1 |
| 8–15: hazardous, not harmful | 29.6 | 15.6 | 9.9 | 13.8 |
| 16–40: harmful | 6.2 | 3.0 | 2.1 | 2.1 |
| 8–40: hazardous or harmful | 35.8 | 18.6 | 12.0 | 15.9 |
| Women | ||||
| 0–7: not hazardous | 83.4 | 95.4 | 96.9 | 84.5 |
| 8–15: hazardous, not harmful | 14.5 | 4.6 | 3.1 | 13.9 |
| 16–40: harmful | 2.0 | 0 | 0 | 1.6 |
| 8–40: hazardous or harmful | 16.6 | 4.6 | 3.1 | 15.5 |
In England, levels of hazardous or harmful drinking vary by region. In 2007, they were lowest for women in the East of England (12.2%) and highest in Yorkshire and the Humber (21.1%), whereas they were lowest for men in the East Midlands (27.8%) and highest in the North East (42.4%) (Table 5). 31
| AUDIT score | Government Office region (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| North East | North West | Yorkshire and the Humber | East Midlands | West Midlands | East of England | London | South West | South East | |
| Men | |||||||||
| 0–7: not hazardous | 57.6 | 61.9 | 59.4 | 72.2 | 67.0 | 65.9 | 70.0 | 68.9 | 71.2 |
| 8–15: hazardous, not harmful | 32.2 | 31.7 | 34.4 | 23.8 | 26.2 | 27.9 | 25.6 | 25.5 | 23.4 |
| 16–40: harmful | 10.2 | 6.4 | 6.2 | 4.0 | 6.7 | 6.2 | 4.4 | 5.7 | 5.4 |
| 8–40: hazardous or harmful | 42.4 | 38.1 | 40.6 | 27.8 | 33.0 | 34.1 | 30.0 | 31.1 | 28.8 |
| Women | |||||||||
| 0–7: not hazardous | 79.2 | 80.6 | 78.9 | 82.9 | 84.5 | 87.8 | 86.2 | 85.7 | 87.7 |
| 8–15: hazardous, not harmful | 17.0 | 17.1 | 18.4 | 15.0 | 13.5 | 11.5 | 12.5 | 12.7 | 10.4 |
| 16–40: harmful | 3.7 | 2.3 | 2.7 | 2.2 | 2.0 | 0.6 | 1.3 | 1.7 | 1.9 |
| 8–40: hazardous or harmful | 20.8 | 19.4 | 21.1 | 17.1 | 15.5 | 12.2 | 13.8 | 14.3 | 12.3 |
Data about levels of hazardous or harmful drinking are not available for Wales. Although, in 2008, 45% of all adults in Wales reported drinking above the government-recommended sensible levels on at least 1 day in the preceding week,32 not all of these would necessarily have drinking patterns that equate to an AUDIT score of ≥ 8.
Incidence and prevalence
Alcohol-related liver disease is a major cause of death in England and Wales. In 2008, 4764 deaths registered in England and Wales were attributed to ALD5 – 0.95% of all deaths registered in people aged ≥ 20 years.
Although ALD mortality in England and Wales appears to have risen by 450% over the past 30 years,35 this rise in registrations may be at least in part because of the under-reporting of alcohol-related deaths during the earlier part of that period. This may be explained by the then requirement for post-mortem examinations and coroners’ inquests following alcohol-related deaths, and the potential invalidation of life insurance (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication). It may also be due in part to the increases in obesity and type 2 diabetes observed during the later part of the period. In a prospective study in France, Raynard et al. 36 found that body mass index (BMI) and fasting blood glucose were risk factors for histologically confirmed fibrosis in ALD, independent of age, gender, daily alcohol intake, and total duration of alcohol misuse, whereas, in Scotland, two prospective cohort studies found that raised BMI and alcohol consumption had a supra-additive effect on liver disease mortality. 37 The proportion of adults in England with a BMI ≥ 30 (i.e. categorised as obese or morbidly obese) rose from 13% of men and 18% of women in 1993, the first year for which data are available, to 25% and 28%, respectively, in 2008, whereas the proportions with raised waist circumference (i.e. > 102 cm for men and > 88 cm for women) rose from 20% of men and 26% of women in 1993 to 34% and 44%, respectively, in 2008,38 and, between 1991 and 2006, the prevalence of diagnosed type 2 diabetes increased by 65% in men and by 25% in women. 39
In 2008, over twice as many deaths were attributed to ALD in men than in women (Table 6).
| Age group (years) | Male | Female | ||
|---|---|---|---|---|
| Number of deaths | Percentage of total deaths | Number of deaths | Percentage of total deaths | |
| All ages | 3200 | 1.3 | 1564 | 0.6 |
| 20–24 | 2 | 0.2 | 5 | 1.1 |
| 25–29 | 25 | 1.8 | 10 | 1.7 |
| 30–34 | 83 | 4.9 | 36 | 4.2 |
| 35–39 | 173 | 6.5 | 85 | 6.0 |
| 40–44 | 339 | 9.0 | 172 | 7.2 |
| 45–49 | 408 | 7.8 | 217 | 6.4 |
| 50–54 | 562 | 8.0 | 252 | 5.2 |
| 55–59 | 534 | 5.0 | 256 | 3.6 |
| 60–64 | 471 | 2.9 | 225 | 2.0 |
| 65–69 | 289 | 1.4 | 143 | 1.1 |
| 70–74 | 176 | 0.6 | 105 | 0.5 |
| 75–79 | 89 | 0.2 | 41 | 0.1 |
| 80–84 | 32 | 0.07 | 10 | 0.02 |
| 85–89 | 14 | 0.04 | 7 | 0.01 |
| 90–94 | 3 | 0.02 | 0 | 0.0 |
| ≥ 95 | 0 | 0.0 | 0 | 0.0 |
Between 1992 and 2001, 3360 incident cases of cirrhosis in patients aged ≥ 25 years were reported to the UK General Practice Research Database, which contains data relating to over 13 million patients in the UK40 (approximately 20% of the UK population). Of the reported cases, 1287 (38.3%) were judged, on the basis of records of problem drinking in the GP notes, to be alcoholic cirrhosis. 40 These figures suggest an annual incidence of alcoholic cirrhosis in the UK of approximately 25,740. During the 10 years from 1992 to 2001, the incidence of alcoholic cirrhosis rose by 75% in men and by 34% in women,40 and in 2000 it was claimed that around 80% of all cases of liver cirrhosis seen in district general hospitals in the UK could be attributed to alcohol. 8
Impact of health problem
Significance for patients in terms of ill-health (burden of disease)
Alcohol-related liver disease is often asymptomatic. However, cirrhosis is associated with a number of complications, the more common of which include variceal bleeding, ascites, spontaneous bacterial peritonitis, and encephalopathy;41 these are associated with a significant burden of disease. Moreover, approximately 20% of patients with cirrhosis will go on to develop HCC (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication).
Portal hypertension is a major complication of chronic liver disease. It contributes to the development of ascites and HE, and forms a direct cause of variceal bleeding. Clinically significant PHt (i.e. PHt associated with a risk of such complications) has been defined as a HVPG measurement above about 10 mmHg. 42 Patients with clinically significant PHt should be offered treatment to reduce the risk of complications. 42
Approximately 40% of patients with liver cirrhosis have oesophageal varices, and approximately one-third of these will suffer variceal bleeding within 2 years of diagnosis. Such bleeding is associated with a mortality rate of 20–40% per episode43 and a 1-year mortality of 57%;4 nearly half of the deaths occur within 6 weeks of the initial episode of bleeding. 4
Over a 10-year period, approximately 50% of patients with compensated cirrhosis will develop ascites (excessive fluid within the peritoneal cavity). 44 This is associated with a poor quality of life, increased risk of infections, renal failure, and poor long-term outcomes. 45
Cirrhosis is also associated with HE, a condition that encompasses mental alterations ranging from mild (trivial lack of awareness, a shortened attention span, or euphoria or anxiety) to more obvious mental alterations including lethargy or apathy, occasional disorientation, personality change, and inappropriate behaviour, and in more severe cases to somnolence, confusion, gross disorientation and bizarre behaviour, and ultimately coma. Motor function is also impaired. 46 Patients who do not display any overt neurological abnormalities may nonetheless display subtle abnormalities of cognition and/or neurophysiological variables; this condition is termed minimal HE. Minimal HE appears to be associated with a reduction in health-related quality of life and in the ability to perform complex tasks such as driving a car. 46 It has been suggested that approximately 35–40% of cirrhotic patients will develop overt HE at some point, whereas approximately 20–60% with liver disease will develop minimal HE. 47
Significance for the NHS
The number of patients admitted to NHS hospitals in England with ALD has risen year on year from around 25,700 in 2002–3 to approximately 38,300 in 2007–8, an increase of around 49%. In approximately 14,300 of these patients (37%), ALD was the primary diagnosis (Table 7). 48
| Hospital admissions related to ALD | Number of admissions, rounded to nearest hundred | |||||
|---|---|---|---|---|---|---|
| 2002–3 | 2003–4 | 2004–5 | 2005–6 | 2006–7 | 2007–8 | |
| Patients admitted with ALD | 25,700 | 28,600 | 31,500 | 34,400 | 37,700 | 38,300 |
| Patients admitted primarily because of ALD | 11,500 | 12,200 | 13,100 | 13,800 | 14,500 | 14,300 |
| Alcoholic fatty liver | 100 | 200 | 200 | 200 | 200 | 200 |
| Alcoholic hepatitis | 1100 | 1200 | 1200 | 1300 | 1400 | 1400 |
| Alcoholic fibrosis and sclerosis of liver | 100 | 100 | 100 | 100 | 100 | 100 |
| Alcoholic cirrhosis of liver | 3100 | 3400 | 3800 | 4200 | 4800 | 4800 |
| Alcoholic hepatic failure | 800 | 800 | 900 | 1000 | 1100 | 1100 |
| Alcoholic liver disease, unspecified | 6300 | 6500 | 6800 | 7000 | 7000 | 6700 |
In 2006, liver disease was responsible for approximately 1600 hospital admissions in Wales. 49 No information was provided regarding the proportion of these admissions that could be attributed specifically to ALD.
The number of patients admitted to adult, general critical/intensive care units (ICUs) in England and Wales with ALD is estimated to have increased from 550 in 1996 to 1513 in 2005, and the number of ICU bed-days which they occupied to have increased from around 3100 to > 10,000. These figures are likely to be underestimates, as they exclude admissions to specialist liver critical-care beds and also any ICU patients with ALD who did not have ALD recorded as a primary or secondary reason for admission. 50
Current service provision
Diagnosis and subsequent management of disease
Diagnosis
The diagnostic pathways for suspected ALD are complex. Many people with ALD have no signs or symptoms of disease, the first indication of liver disease often coming from routine liver function tests. Others first come to medical attention when they report relatively mild symptoms (e.g. nausea, vomiting, abdominal discomfort, or diarrhoea). Sometimes, ALD is identified when people present voluntarily for detoxification, or when they require treatment for alcohol-related injuries or pneumonia, or alcoholic damage to organs other than the liver (e.g. the pancreas, heart, brain or peripheral nerves). 8 Yet other patients do not present until they have advanced liver disease characterised by more severe symptoms, such as jaundice, ascites, encephalopathy, or upper gastrointestinal bleeding. 8
The variability in the diagnostic pathways, caused by the varying reasons for presentation, is complicated by the absence of a single test that differentiates ALD from liver disease of other aetiologies. Rather, the patient’s history is obtained to identify risk factors (alcohol and other) for liver disease, and liver function tests, blood counts, and hepatitis serology are performed to exclude liver diseases of other aetiologies. 51 Ultrasound may also be used. 9 Because patients without clinical evidence of decompensated cirrhosis may have histologically advanced ALD but normal, or only mildly abnormal, liver function test results, liver biopsy may be required to confirm the diagnosis and the stage of the disease by assessing the degree of fibrosis. 9,52
There is a lack of consensus about the role and the timing of biopsy in patients with suspected ALD. 51 This derives in part from the absence of high-quality evidence for the accuracy of liver biopsy specifically in the diagnosis of ALD, together with the fact that its status as the ‘gold standard’ diagnostic and staging tool has been called into question for several reasons. There are also concerns relating to the safety of liver biopsy. For further details, see Summary of diagnostic tests.
Management of disease
Following a diagnosis of ALD, the aims of management are to treat the underlying cause, prevent disease progression, and manage complications. By far the most important management aim in all patients is to ensure long-term abstinence from alcohol. As noted in Aetiology, pathology and prognosis, a small UK study found that ≤ 50% of patients with ALD either abstained completely from alcohol or significantly reduced their intake following simple advice from a physician during their initial presentation. 26 Additional pharmacological or non-pharmacological interventions may also be used to promote abstinence. 8,52 Other aspects of management include lifestyle changes to reduce cigarette consumption and obesity,10 if relevant.
In patients with relatively mild alcoholic hepatitis, the focus of treatment is the achievement of abstinence. Corticosteroid therapy may be used to treat severe AAH,9 but there is no conclusive evidence that it leads to significant improvements in survival. 53 As most patients with AAH have some degree of malnutrition, nutritional therapy may also be offered. 25
A range of therapies may be used to treat the various complications of alcoholic cirrhosis. 8,52 Complications such as fluid retention, HE, and variceal bleeding are treated symptomatically. Because prophylactic treatment with non-selective beta-blockers or band ligation reduces the risk of first bleeding in patients with medium or large varices by 50%, all patients with cirrhosis should be screened regularly by endoscopy for signs of PHt (particularly the presence of oesophageal varices), and, if necessary, offered preventative treatment against gastrointestinal bleeding. Current guidance recommends that all patients diagnosed with cirrhosis should be offered such endoscopic screening every 2 years if they have no varices and annually if they have small varices54 or decompensated disease with or without varices. 42 Abdominal Doppler ultrasound may be used to screen for liver and portal abnormalities (especially HCC). 11
Orthotopic liver transplantation has a place in the management of patients with decompensated alcohol-related cirrhosis who have failed to improve despite well-documented abstinence from alcohol and expert medical treatment for a period of approximately 6 months. Survival rates are similar to those observed in patients transplanted for non-alcoholic cirrhosis, although recidivism rates are still unacceptably high in some centres. Transplantation is also considered the optimal treatment for early HCC. 55 However, the supply of donor livers is limited. 8
Current service cost
The current service cost has been limited to the cost of diagnostic liver biopsy, which has been estimated at £894 for a percutaneous biopsy56 and £1500 for a transjugular biopsy. 9 It is recognised that there are associated costs that have not been estimated, but that are assumed to be independent of the strategy employed.
No evidence has been identified regarding the number of diagnostic liver biopsies undertaken each year in England and Wales specifically as a consequence of suspected ALD.
Relevant national guidelines, including national service frameworks
The following relevant national guideline was issued in 2010:9
Alcohol use disorders: diagnosis and clinical management of alcohol-related physical complications.
The National Clinical Guideline Centre for Acute and Chronic Conditions
Variation in services and uncertainty about best practice
It became apparent, during the course of this project, that rates of referral to secondary care of patients with suspected ALD vary considerably in different parts of the country and that there is some uncertainty about best practice.
Description of technology under assessment
Summary of diagnostic tests
This review assesses four non-invasive tests for liver fibrosis: three blood tests [the Enhanced Liver Fibrosis (ELF™) test (Siemens Healthcare Diagnostic Inc., Tarrytown, NY, USA), FibroTest (BioPredictive, Paris, France) and FibroMAX (BioPredictive, Paris, France)] and transient elastography (FibroScan®; produced by EchoSens, Paris, France and distributed in the UK by Artemis Medical Ltd, Kent, UK). The reference standard with which they are generally compared is liver biopsy, but HVPG measurement and upper gastrointestinal endoscopy have also been considered. All these tests are discussed in turn below.
The Enhanced Liver Fibrosis Test
The Enhanced Liver Fibrosis Test is a blood test that uses an algorithm combining three biomarkers [hyaluronic acid (HA), tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) and aminoterminal propeptide of procollagen type III (PIIINP)] to assess the stage and rate of progression of liver fibrosis. 57 The biomarkers are direct markers of extracellular matrix metabolism/degradation indicative of liver fibrosis. A higher concentration of the individual biomarkers leads to a higher ELF score and indicates a higher probability of more severe fibrosis.
The test requires a minimum of 0.3 ml of serum. 58 Blood samples are collected in a clinic and analysed at a central laboratory. 51
As alcohol affects many of the variables used in the ELF test,59,60–62 individuals should ideally be abstinent before the blood sample is taken.
The ELF test was CE marked in May 2007. 51
Cautions and contraindications for the Enhanced Liver Fibrosis Test
No cautions and contraindications have been identified by the manufacturer. However, because the test uses direct markers of fibrogenesis (HA and TIMP-1), the results will be unreliable in patients with chronic diseases characterised by fibrogenesis in organs other than the liver. 63
FibroTest and FibroMAX
FibroTest and FibroMAX are proprietary algorithms that use serum biochemical markers to assess the stage of liver fibrosis. 64 FibroTest (marketed in the USA as FibroSure) combines one direct marker of extracellular matrix metabolism/degradation (alpha-2-macroglobulin) and four indirect markers (apolipoprotein A1, haptoglobin, total bilirubin and gamma-glutamyl transpeptidase) with the patient’s age and sex. 65
Blood samples are not analysed at a central laboratory, but it is recommended that the individual components of the test are analysed using the same techniques and analysers as used by the reference laboratory that developed FibroTest. 66 The individual values are then entered into BioPredictive’s website (www.biopredictive.com) and an algorithm calculates the results, which are presented as numeric estimates on a continuous scale ranging from 0.00 to 1.00. Table 8 displays the correspondence proposed by Morra et al. 67 between FibroTest scores and the fibrosis stages identified by the METAVIR, Knodell, and Ishak fibrosis staging systems.
| FibroTest | METAVIR | Knodell | Ishak |
|---|---|---|---|
| 0.00–0.21 | F0 | F0 | F0 |
| 0.22–0.27 | F0–1 | F0–1 | F1 |
| 0.28–0.31 | F1 | F1 | F2 |
| 0.32–0.48 | F1–2 | F1–3 | F2–3 |
| 0.49–0.58 | F2 | F1–3 | F5 |
| 0.59–0.72 | F3 | F3 | F4 |
| 0.73–0.74 | F3–4 | F3–4 | F5 |
| 0.75–1.00 | F4 | F4 | F6 |
FibroMAX combines the components of FibroTest with alanine aminotransferase, aspartate aminotransferase, total cholesterol, triglycerides, fasting blood glucose, height and weight, and presents on one sheet the scores for FibroTest, SteatoTest (which measures hepatic steatosis) and AshTest (which measures the degree of necroinflammatory activity of alcoholic origin). 68
FibroTest and FibroMAX are validated for use in patients with chronic viral hepatitis (B or C), ALD, and non-alcoholic fatty liver disease (NAFLD). The manufacturer recommends the use of FibroTest in patients with hepatitis B or C and FibroMAX in those with alcoholic or metabolic liver disease. 69
Although FibroTest may be performed on blood samples from fasting or non-fasting patients, FibroMAX must be performed on samples from fasting patients. 67 As in the case of the ELF test, alcohol affects many of the variables used in FibroTest; therefore, individuals should ideally be abstinent before the blood sample is taken (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication).
FibroTest and FibroMAX are said to yield consistent results, with a reproducibility > 95%. 70,71 However, this has been questioned. A small study by Gressner et al. 72 suggests that FibroTest scores may vary by up to two METAVIR stages as a result of possible interlaboratory differences in measurements of the individual test components, even when the laboratories fulfil BioPredictive’s technical requirements and the measurements lie within a quality-controlled, analytically acceptable range. Moreover, Poynard et al. ’s73 prospective analysis of discordance between the FibroTest and biopsy results in patients with hepatitis C demonstrated that critical interpretation of every FibroTest result is required in order to avoid false-positive (FP) or false-negative (FN) results.
The FibroTest and FibroMAX algorithms are patented, but not CE marked; they have not been published. 66 However, there are CE-marked kits for assessing the individual components.
Cautions and contraindications for FibroTest and FibroMAX
No cautions or contraindications relating to patient safety have been identified. However, for reasons relating to test accuracy, FibroTest is not recommended for use in patients who have intercurrent illness (particularly acute inflammation, haemolysis, or Gilbert’s syndrome), or who are taking medications that can cause elevated bilirubin levels11 (these include allopurinol, anabolic steroids, some antibiotics, antimalaria medications, azathioprine, chlorpropamide, cholinergics, codeine, diuretics, adrenaline, meperidine, methotrexate, methyldopa, monoamine oxidase inhibitors, morphine, nicotinic acid, birth control pills, phenothiazines, quinidine, rifampin, steroids, sulfonamides, and theophylline74).
FibroScan
FibroScan is produced by EchoSens, Paris, France, and distributed in the UK by Artemis Medical Ltd., Kent, UK75 It is a non-invasive test that uses a specialised probe, an ultrasound and elastography system, and specialised software. The probe is placed on the skin over the right lobe of the liver and generates a mechanical pulse that sends a shear wave to the liver through the intercostal spaces. 76,77 The wave’s velocity is measured by ultrasound and is determined by the stiffness of the liver, which is correlated with the degree of fibrosis. 76
The procedure, which is painless, can be performed by trained medical or paramedical staff. Each test typically takes < 15 minutes. 75 The result is the median of 10 individual ‘shots’, reported as a numerical measure in kilopascal (kPa);78 it is available immediately. 76 If a shot is unsuccessful, the machine provides no result and the whole process is deemed to have failed if no value is obtained after ≥ 10 shots. 79 In addition, the manufacturer recommends that, to be considered reliable, successful measurements should meet the following three criteria:
-
at least 10 valid shots
-
a success rate (the ratio of valid shots to total number of shots) of at least 60%
-
an interquartile range < 30% of the median value. 79
FibroScan values range from 2.5 to 75 kPa; normal values are around 5.5 kPa. 80 A cut-off value of about 12.5 kPa has been proposed as optimal for discriminating between fibrosis and cirrhosis in patients with chronic hepatitis C. 81 As the threshold for cirrhosis appears to be higher in patients with ALD, it is important that disease aetiology should be established before testing.
FibroScan is claimed to measure liver stiffness in a cylinder approximately 1 cm in diameter and 4 cm long, between 25 and 65 mm below the surface of the skin. 82 The volume of this cylinder is at least 100 times that of a percutaneous liver biopsy sample, and is therefore far more representative of the whole liver, reducing the risk of sampling error. However, the claim that it may be performed satisfactorily on non-fasting patients82 has recently been called into question by the finding that liver stiffness values increase substantially after food intake in both patients with hepatitis C infection and healthy controls. 83
FibroScan appears to have good reproducibility. In a series of 195 patients with chronic liver disease of various aetiologies and without ascites, using the FibroScan ultrasonography guide to identify a suitable portion of the liver for examination, Fraquelli et al. 84 found that overall agreement between two operators was 0.98 [95% confidence interval (CI) 0.977 to 0.987], and intraobserver agreement was 0.98 for both operators. Increased BMI (> 25 kg/m2), steatosis (> 24% of fatty liver cells), and histological evidence of no to mild fibrosis (METAVIR stage < F2) were all significantly associated with reduced interobserver agreement, and the most marked variability was seen in mild fibrosis (F0/F1), where interobserver agreement fell to 0.60 (95% CI 0.455 to 0.719).
FibroScan is CE marked. Because it is designed specifically to test for liver fibrosis, and does not produce an image, it cannot be used for any other diagnostic purpose. 56
Cautions and contraindications for FibroScan
No cautions or contraindications relating to patient safety have been identified. However, because elastic waves do not travel through liquids, FibroScan has no value in patients with ascites, even if it is clinically undetected. 77 Although this limitation has been claimed to be of little practical importance because the diagnosis of cirrhosis is clinically obvious in most patients with ascites,85 patients with portal vein or hepatic vein block may present with ascites although not having appreciable hepatic fibrosis (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication).
More importantly, it is difficult or impossible to use FibroScan in obese patients because the probe is calibrated for a specific distance between the liver and the chest wall,79 and the low-frequency vibration induced by the probe and/or the ultrasound wave can be strongly attenuated by the fatty tissue. 85 This limitation is particularly unfortunate as obese patients form an increasing proportion of the population and appear to be at increased risk of disease progression. However, a special probe with a measurement depth of 35–75 mm17 is currently being developed for use in patients who are morbidly obese. 77 It is also impossible to use FibroScan in patients whose intercostal spaces are too narrow for the 9-mm-diameter probe to fit between the costal bones. 85
Finally, FibroScan results may be influenced by factors such as acute liver injury, which will result in an overestimation of the degree of fibrosis. 79
Liver biopsy
The true gold standard for assessing the degree of liver fibrosis is histological analysis of the whole liver. As this is not possible in living patients, liver biopsy has been adopted as the reference standard.
Liver biopsy has a number of disadvantages. It is an invasive test: a hollow needle is used to remove a small sample of tissue from the patient for examination in the laboratory. It is performed in the fasting patient and is generally preceded by ultrasonography of the liver. 86 In most cases, liver biopsy is performed percutaneously, through the skin over the liver. However, in order to reduce the risk of complications associated with bleeding, it may be performed transvenously in patients with conditions such as ascites or coagulation defects, which are relatively common in ALD, and which form contraindications to percutaneous biopsy. In transvenous biopsy, a catheter carrying the biopsy needle is inserted through a vein (most commonly the jugular vein in the neck) and guided to the veins inside the liver. 87 Liver biopsy is also occasionally performed laparoscopically. 9
Liver biopsy often involves a hospital stay. Patients should undergo liver biopsy on an outpatient basis only if they have no conditions that may increase the risk of the procedure, and then only in locations with easy access to a laboratory, blood bank, and inpatient facilities, and with staff who can observe the patient for 6 hours following the procedure. In addition, the patient should be able to return easily within 30 minutes to the hospital where the biopsy was undertaken, and should have a reliable individual to stay with on the first night post biopsy. If any of these criteria cannot be met, biopsy must be undertaken on an inpatient basis. Moreover, patients who undergo outpatient biopsy should be hospitalised if there is any significant complication, including pain that requires more than one dose of analgesic. 88 In the USA, where liver biopsy is generally performed on an outpatient basis under local anaesthetic, 1–3% of patients subsequently require hospital care for the management of complications (predominantly pain or hypotension); 60% of these complications occur within 2 hours of the biopsy and 96% within 24 hours. 86
Because fibrosis is not evenly distributed throughout the liver, liver biopsy, which samples only 1/25,000–50,000 of the liver, carries a substantial risk of sampling error. Regev et al. 89 found a difference of at least one fibrosis stage between biopsy samples from the right and left hepatic lobes in 33% of patients with hepatitis C. Such sampling errors usually produce a low FP rate but a relatively high FN rate but, in the case of liver biopsy, inclusion of elements such as capsular or connective tissue will lead to overestimation of the degree of fibrosis. 16 Factors that affect the degree of sampling error include the length of the biopsy sample and the number of samples taken. Bedossa et al. 90 demonstrated experimentally in liver samples from patients with hepatitis C that correct staging was achieved for only 65% of cases when the biopsy sample was 15 mm in length; this figure rose to only 75% when samples 25 mm in length were analysed, and the proportion of samples which were correctly identified did not increase significantly with longer specimens. Abdi et al. 91 found that a single biopsy correctly identified cirrhosis in only 80% of cases (16/20) at post-mortem, but that 100% accuracy was achieved when three samples were taken,91 while Maharaj et al. 92 also found that a single sample was unreliable: when three consecutive samples were taken through a single entry site, all three samples identified cirrhosis in only 50% of cases.
In addition to sampling error, liver biopsy results may be affected by intra- and inter-pathologist variation in the interpretation of samples. 93 Levels of inter- and intra-observer discrepancies as high as 10–20%89,94 have been reported.
However, liver biopsy has the advantage that it provides information not only on liver fibrosis but also on other factors such as inflammation, necrosis, and steatosis, and permits the identification of potentially unexpected cofactors and comorbidities. 95
Cautions and contraindications for liver biopsy
As an invasive procedure, liver biopsy carries a risk of morbidity and mortality. Morbidity associated with liver biopsy may be broadly divided into minor complications, including transient discomfort at the biopsy site, post-procedural pain, and mild transient hypotension, and major complications, including more severe hypotension (systolic blood pressure < 90 mmHg), bleeding into the peritoneal or thoracic cavity, haemobilia, pneumothorax, perforation of the gall bladder or another organ, myocardial infarction, and death. 96
Murtagh and Foerster93 have suggested that one-third of patients who undergo liver biopsy report pain, and that complications occur in 3 per 1000 biopsies (0.3%) and death in 3 per 10,000 (0.03%). A systematic review of the adverse effects of liver biopsy undertaken as part of this project suggests that the overall rates of severe adverse events including death, and of death, in patients undergoing biopsy are higher at 0.81% (95% CI 0.71% to 0.90%) and 0.09% (95% CI 0.06% to 0.13%), respectively, for percutaneous biopsy, and 1.45% (95% CI 0.62% to 2.57%) and 0.18% (95% CI 0.02% to 0.85%), respectively, for transjugular biopsy (for details, see Appendix 3). The higher rates seen in patients undergoing transjugular biopsy presumably reflect the fact that they are at higher risk of adverse events because of the complications that form contraindications for percutaneous biopsy.
The systematic review found rates of any minor adverse events substantially lower than Murtagh and Foerster’s93 figure for pain alone, at 7.51% (95% CI 7.12% to 7.93%) for percutaneous biopsy and 9.14% (95% CI 6.51% to 12.37%) for transjugular biopsy. However, these results are less reliable than those for more severe adverse events because of greater variability in the definitions of minor adverse events used in the different studies (see Appendix 3). Moreover, the majority of included studies were retrospective audits of clinical notes that presumably only identified cases where the pain was sufficiently severe to require attention from medical or nursing staff.
The management of bleeding from internal biopsy sites has improved over the last 15–20 years because of the use of non-invasive arterial embolisation, which has a much lower associated morbidity and mortality than the open surgical procedures that were previously necessary to deal with this complication (Dr Marsha Morgan, Royal Free Hospital, London, 2010, personal communication). However, because of the particularly high risk of adverse effects in patients with alcoholic hepatitis and cirrhosis, both of which are associated with coagulation problems,8 current UK guidance recommends that biopsy is not required for confirmation of diagnosis in patients with a high clinical suspicion of ALD in whom other causes of liver disease have been excluded using blood tests, unless it is necessary to confirm a diagnosis of AAH in order to inform specific treatment decisions. 9 In other words, in these patients, liver biopsy should be performed only if the risks it poses are outweighed by the potential benefits of improved patient outcomes consequent on changes in management which would not be possible without information that could only be obtained by biopsy.
Hepatic venous pressure gradient measurement
Hepatic venous pressure gradient measurement is the gold standard for assessing the presence and severity of PHt in patients with alcohol-related cirrhosis, in whom splenomegaly, the clinical hallmark of PHt, is a less useful sign. HVPG measurement is an invasive procedure in which a balloon-tipped catheter is inserted into a hepatic vein via the femoral or jugular route. The pressure is measured with the balloon deflated, and again when it has been inflated to occlude the hepatic vein. The HVPG is the difference between the two measurements; a result > 5 mmHg indicates PHt, and a result > 10–12 mmHg indicates clinically significant PHt associated with a risk of complications such as ascites, HE, and variceal bleeding. 42
Hepatic venous pressure gradient measurement is reliable only when performed by an experienced operator. 97
Cautions and contraindications for hepatic venous pressure gradient measurement
Although invasive, HVPG measurement appears to be safe. No reports of mortality or serious complications have been published. The most common complications appear to be related to local injury to the vein used to gain access to the hepatic vein; they include leakage and haemorrhage. Other complications, such as vagal reactions and arteriovenous fistulae, are more rare. The risk of such complications is greatly reduced if deep-venous puncture is performed under Doppler ultrasound guidance, and this is particularly recommended in obese patients, or when arterial palpation is difficult. Finally, passage of the catheter through the right atrium may cause supraventricular arrhythmias; in over 90% of cases, these are self-limiting. 98
Upper gastrointestinal endoscopy
Upper gastrointestinal endoscopy involves the insertion of a thin, flexible viewing instrument, called an endoscope through the mouth into the oesophagus, stomach, and duodenum. It is used in patients with cirrhosis to identify medium or large varices in those areas so that prophylactic treatment may be initiated to reduce the risk of bleeding.
Endoscopy is expensive to perform. 99 Patients must have had nothing to eat or drink for 4–8 hours prior to the procedure.
Cautions and contraindications for upper gastrointestinal endoscopy
Upper gastrointestinal endoscopy is invasive and unpleasant for the patient if done without deep sedation. 100 When performed for diagnostic purposes, it has a small, but not insignificant, risk of complications. These include:
-
Cardiopulmonary complications related to sedation. These range from minor changes in vital signs to respiratory depression, shock/hypotension, and myocardial infarction, and account for approximately 40% of complications associated with diagnostic endoscopy.
-
Infectious complications resulting either from the procedure itself or from the use of contaminated equipment; these are relatively uncommon.
-
Perforation of the gastrointestinal tract; this is also relatively uncommon, but is associated with a mortality rate of approximately 25%.
-
Significant bleeding; this is rare, although individuals with thrombocytopenia and/or coagulopathy are at increased risk of bleeding. 101
Identification of important subgroups
Potentially important subgroups include:
-
obese patients (i.e. those with a BMI ≥ 30 kg/m2 or a waist circumference > 102 cm for men and > 88 cm for women)
-
patients with metabolic syndrome
-
patients with concurrent alcoholic hepatitis
-
patients who are not abstinent from alcohol.
All of these subgroups are important because their characteristics may affect the performance or results of the non-invasive tests assessed in this report; in addition, obese patients with ALD appear to be at increased risk of disease progression. Thus, FibroScan is more likely to fail, or to provide unreliable measurements, in patients with ALD who are obese or rotund, whereas it has been shown in apparently healthy individuals that liver stiffness measurements (LSMs) are higher in those with metabolic syndrome than in those without. 80 The inflammation associated with alcoholic hepatitis will result in an overestimation of the degree of fibrosis using FibroScan. 79 Finally, current alcohol consumption affects many components of the ELF test and FibroTest, so that the tests will provide different results in patients who are abstinent from alcohol and in those who are still drinking, even though they may have the same degree of fibrosis.
Current usage in the NHS
None of the four tests are currently routinely performed within the NHS. However, in April 2008, FibroScan machines were said to be installed in 12 NHS hospitals (implicitly in the UK rather than in England and Wales). 75 By 2009, this figure had risen to 17 NHS hospitals and five private hospitals in England, and six NHS hospitals in Scotland; there were none in Wales. 102 The majority of FibroScan machines in place in 2008 were funded by pharmaceutical companies and/or charitable donations. 75
In 2008, the manufacturers predicted that initial uptake would be confined to the major hepatology centres and would be limited to approximately 35 systems, but that FibroScan might subsequently expand into district general hospitals with gastroenterology departments. 75
Anticipated costs associated with non-invasive testing
There are no good data relating to the costs of non-invasive testing. The best data relate to the ELF test, where currently the favourable price for early adopters in the NHS is £45 per test for a volume of 100 tests a month (1200 a year), i.e. a total cost of £54,000/year (Dr Marsha Morgan, Royal Free Hospital, London 2010, personal communication). There is no indication as to what the price is likely to be for subsequent customers.
In 2007, Morra et al. 67 stated that the cost of FibroTest ranged from €90 to €300 and that of FibroMAX from €150 to €500. However, it seems likely that FibroTest will in the future be priced more competitively in relation to the ELF test. The cost of FibroMAX is likely to be somewhat higher than that of FibroTest as it incorporates more components and provides additional information.
The ancillary costs associated with the ELF test, FibroTest, and FibroMAX are those associated with any diagnostic blood test.
No cost data have been identified for FibroScan.
Chapter 2 Definition of the decision problem
Decision problem
Population
Patients with suspected liver fibrosis related to alcohol consumption.
Diagnostic tests under assessment
The non-invasive tests for liver fibrosis assessed in this review take the forms of either blood tests:
-
ELF test
-
FibroTest
-
FibroMAX
or transient elastography:
-
FibroScan.
Reference standard tests
The reference standard test for liver fibrosis is liver biopsy. However, HVPG measurement and upper gastrointestinal endoscopy are used to identify conditions associated with liver fibrosis, namely PHt and oesophageal varices respectively, which are of clinical importance and which can also be used as surrogates for the diagnosis of cirrhosis.
As noted in Chapter 1, Liver biopsy, liver biopsy carries a substantial risk of sampling error, and may also be affected by intra- and inter-observer variation in the interpretation of samples. It can, therefore, only be regarded as an imperfect reference standard.
Outcomes
Relevant outcomes include:
-
the diagnostic accuracy of the index test compared with the reference standard, as indicated by the area under the receiver operating characteristic curve (AUROC), for a specified fibrosis stage, and/or the sensitivity, specificity, positive predictive value, and negative predictive value, compared with the reference standard for the diagnosis of a specified fibrosis stage based on a specified cut-off point, or the data required to calculate those values [i.e. numbers of true-positive (TP), FN, true-negative (TN) and FP results]
-
numbers of test failures or other withdrawals
-
adverse effects associated with testing
-
long-term patient outcomes (disease progression, complications related to liver disease, need for liver transplantation, mortality)
-
health-related quality of life
-
cost-effectiveness and cost–utility.
Studies of diagnostic or predictive accuracy are included only if they report numbers of TP, FN, TN and FP results, or measures of diagnostic accuracy (e.g. sensitivity and specificity) calculated from those values, relating to the index test in comparison with either a reference standard test or a clinical outcome (e.g. survival or adverse clinical events).
Discussion of outcomes measuring test accuracy
In studies of diagnostic tests, patients are generally classified by the index test (i.e. the diagnostic test being investigated) as either positive or negative (i.e. as having or not having the condition that the test is designed to identify). The same patients are also assessed using the reference standard (an established diagnostic test assumed to have 100% sensitivity and specificity), and the latter result is taken to identify the patients’ true health status. If both the index test and the reference standard are positive, the result is described as a TP. If the index test is positive but the reference standard is negative, the result is termed a FP. If both the index test and the reference standard are negative, the result is termed a TN, whereas if the index test is negative and the reference standard is positive it is termed a FN. These results can be presented in a 2 × 2 table (Table 9).
| Index test result | Reference standard positive | Reference standard negative |
|---|---|---|
| Index test positive | TP | FP |
| Index test negative | FN | TN |
| Sensitivity = [TP/(TP + FN)]× 100 | Specificity = [TN/(TN + FP)] × 100 |
The sensitivity of a test (the proportion of patients with the condition of interest who have a positive test result – i.e. TPs), indicates how good it is at correctly identifying the condition of interest, whereas its specificity (the proportion of patients without the condition of interest who have a negative test result – i.e. TNs) indicates how good it is at correctly classifying people as free of that condition. The sensitivity and specificity are expressed as simple percentages (see Table 9). Both FP and FN results may be harmful: FP results may cause patients to undergo further tests or receive unnecessary treatment, whereas FN results may deprive them of the treatment they need. Ideally, therefore, diagnostic tests would have both high sensitivity and high specificity. In practice, however, they often have high sensitivity at the expense of low specificity or vice versa. The optimum balance between sensitivity and specificity varies from test to test because of differences in the relative consequences of FP and FN results in the context of the condition of interest.
In practice, the situation may be more complex than indicated in Table 9, as the results of the index test may be neither positive nor negative, but uninterpretable. It is important that such uninterpretable results should be included in the calculations. Their inclusion will lower the sensitivity and specificity of the index test (Table 10). In the absence of full data relating to uninterpretable results, sensitivity analyses may be used to explore the impact of such results on sensitivity and specificity.
| Index test result | Reference standard positive | Reference standard negative |
|---|---|---|
| Index test positive | TP | FP |
| Index test uninterpretable | Uninterpretable (U1) | Uninterpretable (U2) |
| Index test negative | FN | TN |
| Sensitivity = [TP/(TP + U1 + FN)] × 100 | Specificity = [TN/(TN + U2 + FP)] × 100 |
The positive predictive value of a test is the proportion of patients with positive test results who are correctly diagnosed. In clinical practice, this is generally the most important measure of test accuracy as it reflects the probability that a positive test reflects the underlying condition being tested for. Consequently, its value depends on the prevalence of the disease, which may vary, and indeed in real life situations may differ considerably from that seen in study populations. The negative predictive value is the proportion of patients with negative test results who are correctly diagnosed. The negative predictive value may be more important than the positive predictive value if the test is being used as a triage test to identify those patients who may have the condition of interest so that they may undergo further, more expensive or invasive, testing.
Although fibrosis has, for convenience, been divided into grades of severity using a number of different scales, the most common being the METAVIR scale (see Chapter 1, Measurement of disease), it actually forms a continuum that ranges from very mild fibrosis to severe fibrosis (cirrhosis). Consequently, the non-invasive tests for fibrosis reviewed in this report also yield continuous measurements and, therefore, diagnostic thresholds must be deliberately selected to define positive and negative results. The sensitivity and specificity of the tests will vary depending on the thresholds that have been selected. If several thresholds have been used in one data set, the diagnostic characteristics of the test in question may be illustrated using a receiver operating characteristic plot of the TP rate (sensitivity) against the FP rate (1 – specificity). 103
The AUROC may be used as an overall measure of the performance of a surrogate test when compared with the reference standard. Bedossa and Carrat104 state that the AUROC represents the probability that the surrogate will correctly rank two randomly chosen patients, one of whom has been classified by the reference standard as having, and the other as not having, the condition that the index and reference tests are intended to identify. Unlike sensitivity and specificity, the AUROC does not vary according to the threshold set for identification of a positive result. It therefore represents a loss of information, as compared with sensitivity and specificity, as it provides no indication of the degree to which any departure from 1.00 (a perfect result) is because of FPs, and the degree to which it is because of FNs. Moreover, Lambert et al. 105 have noted that, in the context of liver fibrosis, the AUROC has two main drawbacks:
-
Because it assumes that the reference standard yields a binary result whereas, as has been seen, liver biopsy uses an ordinal scoring system, the fibrosis stages have to be aggregated into two groups.
-
Because the proportion of each fibrosis stage in the sample can affect the AUROC, comparisons of AUROCs from populations with different distributions of fibrosis stages may be flawed. To overcome this, Poynard et al. 106 recommend standardising the AUROC according to the prevalence of fibrosis stages, but this method is complex and has not yet been statistically validated. 105
As noted above, calculation of the sensitivity and specificity of the index tests involves the assumption that the reference standard has 100% sensitivity and specificity. Unfortunately, this is not true of liver biopsy (see Chapter 1, Summary of diagnostic tests). If the results of the reference test are not very close to the truth, the performance of the index tests will be poorly estimated. 103 In an attempt to address this problem, a number of the studies included in this review have looked in detail at cases in which the index test and reference standard have yielded discrepant results, to determine which test is more likely to have provided the correct result in each individual case.
Another serious problem relating to the use of liver biopsy as the reference standard is its relevance to the different types of non-invasive tests. On the one hand, liver biopsy is arguably a more appropriate reference standard in relation to transient elastography than in relation to tests based on serum markers because it seeks directly to identify the degree of fibrosis present in the liver at a fixed point in time, whereas transient elastography measures the stiffness of the liver, a surrogate for fibrosis, also at a fixed point in time. By contrast, tests such as the ELF test and FibroTest, which are based on combinations of serum markers, seek to assess dynamic processes taking place within the liver. Consequently, their results may be discordant with the liver biopsy results either because the fibrotic process is highly active but fibrotic tissue has not yet developed (thus the serum tests will yield a higher result than the biopsy), or because fibrotic activity is temporarily discontinued even though there are clusters of fibrotic tissue in the liver (thus the serum tests will yield a lower result than the biopsy). 107 In such circumstances, the test results may be discordant even though both tests have yielded correct results in terms of the parameter that they set out to measure. On the other hand, it has been argued that liver biopsy is a more appropriate reference standard in relation to serum marker algorithms such as FibroTest and the ELF test, which have been designed to match histological stages of liver fibrosis as assessed by liver biopsy irrespective of biopsy accuracy, than in relation to transient elastography, which measures stiffness, a single genuine characteristic of liver tissue. 104
Study design
The best available level of evidence, with priority given to controlled studies, if available.
Overall aims and objectives of assessment
The overall aim of the assessment is to assess the diagnostic accuracy, effect on patient outcomes, and cost-effectiveness of the specified non-invasive tests for liver fibrosis in patients suspected of having liver fibrosis related to alcohol consumption. The tests are assessed firstly as a replacement for liver biopsy, and secondly as an additional test prior to liver biopsy.
The objectives of the assessment are:
-
To conduct a systematic review of the published evidence on the diagnostic accuracy and cost-effectiveness of the specified non-invasive tests for the assessment of liver fibrosis in patients suspected of having ALD.
-
To develop a decision model to investigate the benefits, harms, and cost-effectiveness of non-invasive testing, either as a replacement for liver biopsy or as an additional test in the diagnostic pathway for assessing liver fibrosis. Outcomes from the model will be expressed in terms of net health benefit and cost per quality-adjusted life-year (QALY).
Chapter 3 Cost-effectiveness: model structure and methodology
As previously discussed, the use of non-invasive liver tests (NILTs) for assessing the fibrosis levels of patients with suspected ALD has been posited owing to the fact that the current assessment method, biopsy, is associated with morbidity and mortality. If NILTs were of sufficient accuracy in determining the level of fibrosis, then they could be used cost-effectively to either filter those patients in whom biopsy would not be appropriate, or indeed replace biopsy for some patients. Henceforth strategies aimed at filtering patients will be referred to as ‘triaging strategies’ and strategies aimed at replacing biopsy will be referred to as ‘replacement strategies’.
The focus of the model is to evaluate the cost-effectiveness of different strategies involving NILTs when compared with biopsying all patients. Within this remit, it has been assumed that there is sufficient infrastructure for the identification and referral of patients with suspected ALD and subsequent treatment to be performed to a satisfactory level. Additional details on providing such services are contained in Alcohol use disorders: diagnosis, assessment and management of harmful drinking and alcohol dependence108 and the references contained therein.
During the process of undertaking the evaluation, it became apparent that data regarding the use of NILTs within primary care were extremely scant. Pivotal studies assessing test accuracy were all undertaken in secondary or tertiary care as a gold standard (liver biopsy) was needed. As it is unethical to undertake liver biopsy in those with minimal risk of fibrosis, the trials would be subject to considerable spectrum bias and the resultant sensitivities and specificities could not be assumed to apply in primary care. Clinical experts (comprising primary, secondary and tertiary care physicians) who provided advice to the assessment groups were unanimous that there was currently insufficient evidence to appraise the tests in primary care. This advice, in conjunction with the considerable uncertainty that is prevalent with regards to the cost-effectiveness of NILTs in secondary care, was the rationale for the results of this study to focus on the cost-effectiveness of NILTs solely within secondary and tertiary care.
Owing to the uncertainty in both management and prognosis following a diagnosis of cirrhosis, advanced fibrosis or of their absence, there were few data from the systematic reviews that could be utilised within the modelling evaluation. The key parameters that were used were the sensitivity and specificity of the tests.
The population simulated within the model will be those patients that a hepatologist would wish to biopsy. Guidance from the National Institute for Health and Clinical Excellence (NICE)9 indicates that biopsy, because of the potential for causing morbidity and mortality, should only be used when it would affect the management of the patient. It is assumed that within the model, management would only be altered where a patient had been diagnosed with cirrhosis, in which case the patient would be monitored for HCC, HE and oesophageal varices. In contrast, it is assumed that the management strategy would not change for those patients without cirrhosis, where the clinician would continue to stringently attempt to persuade the patient to become abstinent or reduce alcohol intake.
The prevention of further fibrosis (and ultimately cirrhosis) is of great importance and the model assumes that a proportion of those patients who continue to drink heavily will progress to cirrhosis, in which case the greater cost implications and reduced life expectancy will be taken into consideration.
A subset of patients will be suspected of having severe alcoholic hepatitis and/or decompensated liver disease; these will not be considered within the model. The rationale for this decision is twofold. Firstly, those patients with alcoholic hepatitis are likely to require treatment with steroids to reduce the risks of mortality; however, if the patient has decompensated cirrhosis, which can be determined by biopsy but not by any of the current NILT, then the course of steroids can cause mortality. Secondly, patients with alcoholic hepatitis will have inflammation of the liver, which can affect the validity of diagnosis provided by a NILT. There may be additional patients in whom the clinician believes that a biopsy would be unnecessary, for example where the clinical manifestations clearly indicate that the patient has cirrhosis; these patients are also not considered within the model with the assumption that the clinician would treat the patient as he or she deemed appropriate.
The strategies analysed
Ten strategies will be considered:
-
biopsy all patients (assumed current practice)
-
triage patients with FibroScan and biopsy all those in whom cirrhosis is indicated
-
triage patients with FibroTest and biopsy all those in whom cirrhosis is indicated
-
triage patients with the ELF and biopsy all those in whom cirrhosis is indicated
-
triage patients using clinical experience and biopsy all those in whom cirrhosis is indicated
-
use FibroScan and assume that the result is definitive
-
use FibroTest and assume that the result is definitive
-
use the ELF and assume that the result is definitive
-
use clinical experience and assume that the result is definitive
-
diagnose all patients as having cirrhosis.
Strategies 5, 9 and 10 are not considered as realistic recommendations for clinical practice, but are included to provide insight regarding whether or not a formal diagnostic test is required. These strategies were of particular relevance to an earlier version of the conceptual model, in which the quality of life impacts due to continued drinking in those without cirrhosis were assumed to be greater than subsequently used in the modelling following clinical advice. Using a high decrement resulted in strategies with poor specificity being more cost-effective as the rates of abstinence are assumed to be greater in those with diagnosed cirrhosis. Although these conclusions do not apply to the final model, strategies 5, 9 and 10 were included for completeness. FibroMax was excluded from the strategies analysed as there were no data found regarding sensitivity or specificity.
The assumed current clinical practice is shown in Figure 1. If a patient were shown to be cirrhotic, then he or she would receive monitoring for HCC and HE and prophylactic treatment for oesophageal varices. Patients not shown to be cirrhotic would receive lifestyle advice only, which would include the strong recommendation to become abstinent or to reduce alcohol consumption. This advice would also be given to those who received monitoring.
FIGURE 1.
Strategy 1: the assumed current practice.
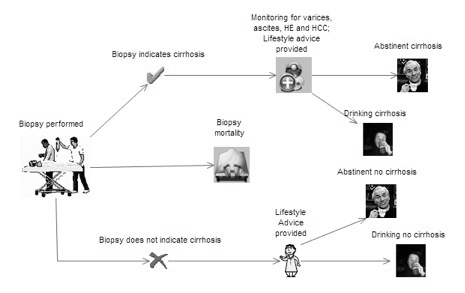
For those patients who were not diagnosed as cirrhotic, it is assumed that a proportion will progress and become cirrhotic (incurring substantial costs and reduced life expectancy if they continue to drink heavily). More detail is provided in Chapter 5. This pathway has not been included in Figure 1 to maintain clarity.
For the set of strategies where the NILT would be used to triage patients, it has been assumed that the management of patients would be as shown in Figure 2. If the NILT indicates that there is cirrhosis, then this would be confirmed with biopsy, with those shown to be cirrhotic on biopsy receiving monitoring for HCC and HE and provided with prophylactic treatment for oesophageal varices in addition to lifestyle advice. Those patients shown to be non-cirrhotic on biopsy would receive lifestyle advice only, which would include the strong recommendation to become abstinent or to reduce alcohol consumption. This advice would also be provided to patients who are not shown to be cirrhotic by the NILT. It is assumed that the knowledge of the result of the NILT would not affect the interpretation of the biopsy result, which would provide the same diagnosis when performed immediately on a patient or following a triage strategy.
FIGURE 2.
The use of a NILT in conjunction with a biopsy.
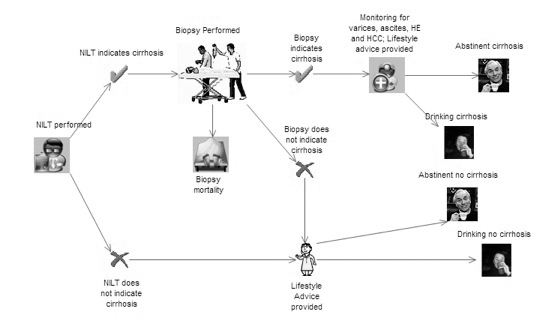
Depending on the sensitivity of the test, there is potential for patients to be diagnosed incorrectly as not having cirrhosis and not being offered appropriate monitoring for HCC, HE and prophylactic treatment for oesophageal varices.
For those patients who were not diagnosed as cirrhotic, it is assumed that a proportion will progress and become cirrhotic (incurring substantial costs and reduced life expectancy) if they continue to drink heavily. More detail is provided in Chapter 5. This pathway has not been included in Figure 2 to maintain clarity.
For the analyses where it is assumed that the patient’s management strategy would be determined by the NILT alone, the care pathway would be as shown in Figure 3.
FIGURE 3.
The use of a NILT to determine patient management.
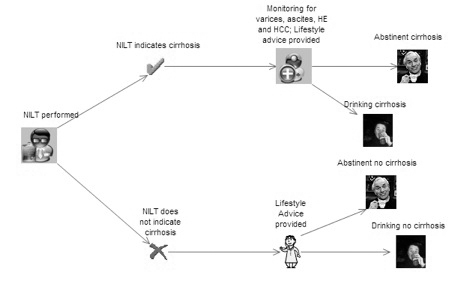
Depending on the sensitivity of the test, there is potential for patients to be diagnosed incorrectly as not having cirrhosis and not being offered the appropriate monitoring for HCC, HE and prophylactic treatment for oesophageal varices. Additionally, depending on the specificity, there is a possibility that patients are falsely diagnosed as having cirrhosis and will have unnecessary monitoring for a considerable period of time.
For those patients who were not diagnosed as cirrhotic, it is assumed that a proportion will progress and become cirrhotic (incurring substantial costs and reduced life expectancy if they continue to drink heavily). More detail is provided in Chapter 5. This pathway has not been included in Figure 3 to maintain clarity.
Liver transplant
In reality, there is a possibility that patients will have a liver transplant. However, this eventuality has been omitted from the model as current evidence shows that it is of borderline cost-effectiveness. Longworth et al. 109 report that liver transplants in patients with ALD may not be cost-effective. However, in the recent NICE guideline9 it was hypothesised that the cost per QALY gained estimated by Longworth et al. 109 may be overestimated as the selection of ALD patients for transplants may have improved, the study had not been extrapolated to patient lifetime and whether or not the full costs of pre-transplant costs should be included in the estimation of cost-effectiveness. Accordingly, the Guideline Development Group (GDG) concluded that ‘that liver transplantation in its current form is likely to be cost-effective for ALD patients, when long-term benefits and modern selection practices are taken into account’. 9 As there is no conclusive evidence on whether or not liver transplantation is cost-effective, the authors of this report have assumed that the cost-effectiveness of liver transplant in an ALD population is exactly on the threshold for cost per QALY chosen by the decision makers and that whether people do, or do not, have a liver transplant will not affect the decision on whether or not the use of a NILT is cost-effective. Given the great uncertainty in the results, owing to the lack of data on key variables within the model, the authors are not uncomfortable with this assumption.
Chapter 4 Assessment of clinical effectiveness
Methods for reviewing effectiveness
A systematic review was undertaken according to the general principles recommended in the quality of reporting of meta-analyses (QUOROM)110 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)111 statements.
Identification of studies
Extensive searches were undertaken for the comprehensive retrieval of studies of clinical effectiveness and cost-effectiveness relating to the research question. The concepts in the search strategies reflected the population and intervention categories of the Population, Intervention, Comparator, Outcome (PICO) model, namely patients with suspected liver fibrosis related to alcohol consumption and the specified non-invasive tests for the identification of fibrosis, respectively.
The search strategy comprised the following main elements:
-
searching of electronic databases
-
scrutiny of bibliographies of retrieved papers and previous systematic reviews
-
contact with experts in the field.
Sources searched
The electronic databases searched included MEDLINE, EMBASE, The Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Web of Knowledge (for details, see Appendix 5).
Search strategies
The MEDLINE search strategies are presented in Appendix 5. Search strategies for the other databases are available on request.
Search restrictions
Searches were not restricted by publication type, date of publication, or language.
Inclusion and exclusion criteria
Inclusion criteria
-
Participants: Patients with suspected liver fibrosis related to alcohol consumption. Studies that included patients with suspected liver fibrosis of other aetiologies were included if data relating to patients with suspected alcohol-related disease could be extracted separately.
-
Intervention: One of the specified non-invasive tests for liver fibrosis, namely:
-
– the ELF test
-
– FibroTest
-
– FibroMAX
-
– FibroScan.
-
-
Comparators: The primary comparator, or reference standard, was liver biopsy for the identification of liver fibrosis. Secondary reference standards were tests used to identify conditions associated with liver fibrosis, namely HVPG measurement for PHt, and upper gastrointestinal endoscopy for the identification of oesophageal varices.
-
Outcome measures: The primary outcome measure was the diagnostic accuracy of the index test compared with the reference standard in distinguishing patients with significant fibrosis, defined as METAVIR stages F2–F4, from patients without significant fibrosis, defined as METAVIR stages F0–F1. Other outcome measures were:
-
– the diagnostic accuracy of the index test compared with the reference standard in distinguishing patients with cirrhosis (METAVIR stage F4) from patients without cirrhosis (METAVIR stages F0–F3)
-
– the diagnostic accuracy of the index test compared with the reference standard in distinguishing patients with moderate-to-severe fibrosis (METAVIR stages F3–F4) from patients without moderate-to-severe fibrosis (METAVIR stages F0–F2)
-
– the diagnostic accuracy of the index test compared with the reference standard in distinguishing patients with fibrosis (METAVIR stages F1–F4) from patients without fibrosis (METAVIR stage F0)
-
– the diagnostic accuracy of the index test compared with the reference standards in distinguishing patients with and without the complications of fibrosis (PHt and oesophageal varices)
-
– the number of patients requiring referral to secondary care
-
– the number of patients requiring liver biopsy
-
– the number of patients giving up alcohol, or significantly reducing alcohol consumption, following receipt of a test result
-
– long-term patient outcomes (disease progression, complications related to liver disease, need for liver transplantation, mortality)
-
– adverse effects of testing
-
– health-related quality of life.
-
Only studies of the index tests that reported data relating to one of the outcome measures in relation to the population of interest were included in the review of clinical effectiveness. However, this criterion was relaxed for consideration of adverse events, where wider searches were undertaken to allow the inclusion of data relating to studies of the adverse effects of diagnostic venepuncture or transient elastography (see Appendix 5).
-
Study design: the best available level of evidence, with priority given to controlled studies, if available.
Exclusion criteria
The following publication types were excluded from the review:
-
animal models
-
preclinical and biological studies
-
narrative reviews, editorials, and opinions.
Systematic reviews of primary studies were excluded from the review of clinical effectiveness, but were scanned for potential additional relevant studies.
In addition, studies were excluded if:
-
they were considered methodologically unsound (specifically, if the reference standard was used in only a subset of study participants and the selection criteria used to identify that subset were not clear)
-
they were published as meeting abstracts only, and insufficient methodological details were reported to allow critical appraisal of study quality
-
they were meeting abstracts that had been superseded by later publications and did not contain any additional data.
Sifting
The references identified by the literature searches were sifted in three stages by a single reviewer. They were screened for relevance first by title and then by abstract. Those papers that seemed, from their abstracts, to be relevant were then read in full, as were all potentially relevant papers for which abstracts were not available. At each step, studies that did not satisfy the inclusion criteria were excluded.
Data extraction strategy
Data were extracted by one reviewer to customised data extraction forms. Where multiple publications of the same study were identified, data were extracted and reported as a single study.
Critical appraisal strategy
Study quality was assessed using a modified version of the quality assessment of diagnostic accuracy studies (QUADAS) checklist,112 a validated tool designed to assess the internal and external validity of studies of diagnostic accuracy. Definitions of some scoring criteria were adapted from the systematic review by Friedrich-Rust et al. 113 (for details, see Appendix 6). Where a study was reported in more than one publication, its quality was assessed on the basis of the combined data from all relevant publications.
The quality assessment of studies included in the review of clinical effectiveness was carried out by one researcher. Blinding of the quality assessor to author, institution, or journal was not considered necessary. 114,115
Methods of data synthesis
Studies that met the review’s entry criteria were eligible for inclusion in meta-analyses if this was appropriate in terms of comparability of the study populations, outcomes, and diagnostic thresholds, if the studies were unlikely to be biased,116 and if the numbers of TP, FP, TN, and FN results for each study were reported or could be obtained from the study authors. However, because of the degree of heterogeneity and the unavailability of full data from some studies, meta-analysis was not in fact considered appropriate. The presentation of results is therefore limited to a narrative review.
Where they were not reported by the original investigators, if data were available for the numbers of TPs, TNs, FPs and FNs, the reviewers independently calculated sensitivity, specificity and positive and negative predictive values, with CIs. This was done using beta distributions (alpha = TPs and beta = FNs for specificity; alpha = TNs and beta = FPs for specificity). If any number was < 5, a non-informative prior of 0.5 (equivalent to Jeffreys’ prior) was added to both the alpha and beta parameter.
Results
Quantity and quality of research available
The electronic literature searches identified 4039 potentially relevant citations. Of these, 3829 were excluded at the title or abstract stage, leaving 210 that were obtained for examination of the full text, together with five additional relevant articles that had been identified from other sources. Two of these five articles, those by Janssens et al. 117 and Mueller et al. ,118 had been published too recently to be identified by the electronic searches; they superseded abstracts119,120 that had been identified by those searches. A further two articles, by Rosenberg et al. 121 and Melin et al. ,122 were not identified by the electronic searches because they were not appropriately indexed in the electronic databases; they were supplied by the relevant manufacturers together with an unpublished paper by Parkes et al. 57
One hundred and ninety-eight citations were excluded at the full-text stage, leaving 17 articles that were included in the review (Figure 4). These 17 articles related to 14 studies: one study of the ELF test,57,121 four studies of FibroTest,13,123–126 eight studies of FibroScan97,117,118,122,127–131 and one study which used both FibroTest and FibroScan. 132 No studies of FibroMAX were identified.
FIGURE 4.
Clinical effectiveness: summary of study selection and exclusion.
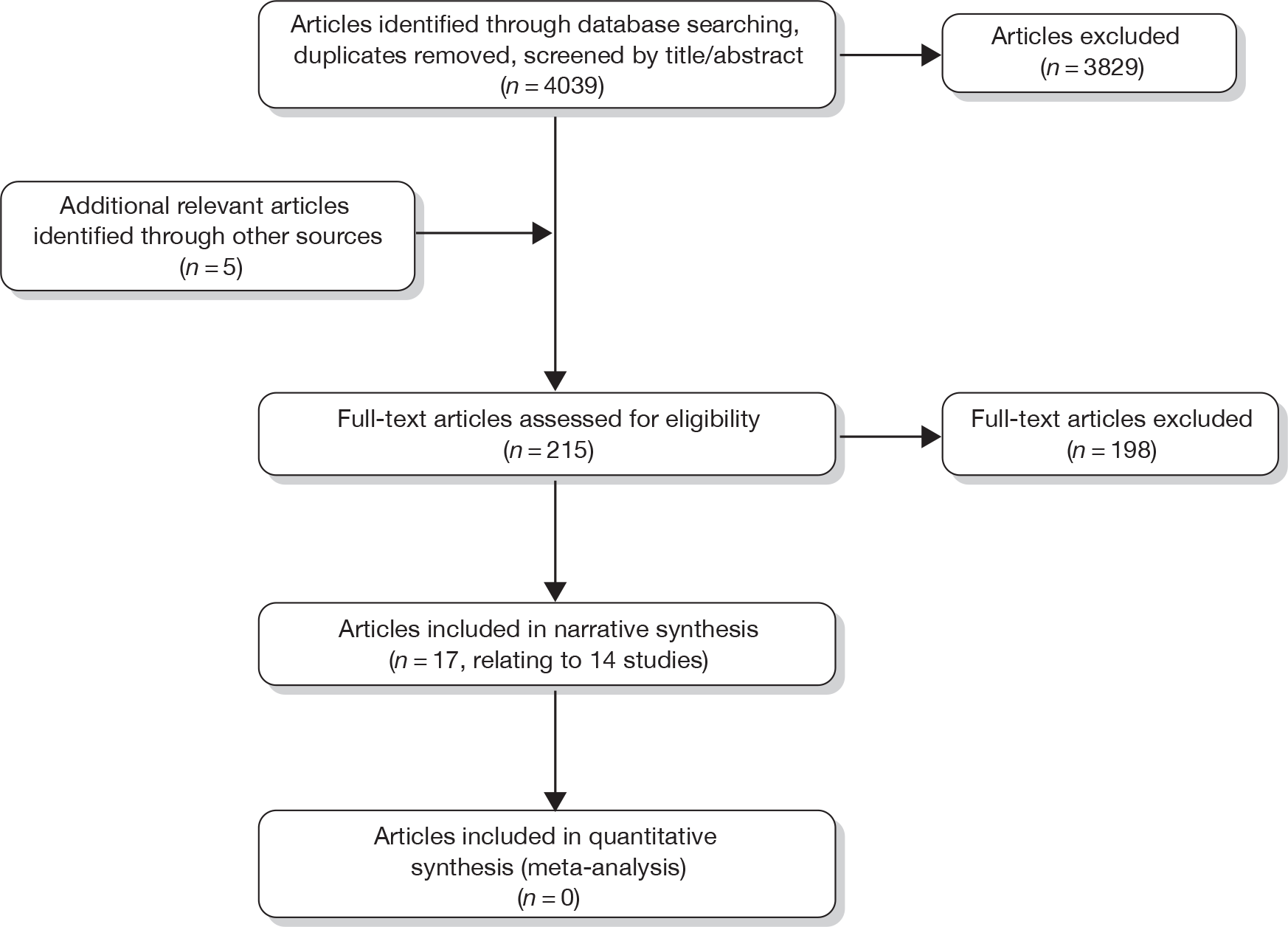
Number and type of studies included
The majority of articles that met the review inclusion criteria reported cross-sectional studies intended to confirm the performance of one of the non-invasive tests against a reference standard (liver biopsy, HVPG measurement, or endoscopic identification of oesophageal varices). A minority had a cohort design, following patients over time to assess how well the non-invasive test predicted adverse clinical outcomes. There were no randomised controlled trials (RCTs).
Number and type of studies excluded, with reasons
As may be seen from Quantity and quality of research available, a substantial number of the citations identified by the electronic searches related to studies that were excluded as part of the sifting process because they did not meet the inclusion criteria. Details are therefore given only of those citations that were excluded after a full reading, and then only if they were excluded for a reason other than a simple failure to meet the inclusion and exclusion criteria. Such citations are listed in Appendix 7, together with the reasons for their exclusion.
Study characteristics
Enhanced Liver Fibrosis Test
No studies were identified that assessed the ELF test as such. However, one study57 evaluated the European Liver Fibrosis Test, which was said to be essentially identical to the ELF test except for the inclusion of age in the algorithm. This study was, therefore, deemed to meet the inclusion criteria. Data from this study have been published in two articles. 57,121 The first article, by Rosenberg et al. ,121 assessed diagnostic test accuracy compared with liver biopsy in patients with chronic liver disease, some of whom had ALD. The second article, by Parkes et al. ,57 evaluated the ability of the test to predict survival and relevant adverse events in the cohort of patients with chronic liver disease of various aetiologies enrolled in the original study in English hepatology centres. The primary outcome measure used by Parkes et al. 57 was the first post-recruitment liver-related clinical event, defined as liver-related death, ascites, encephalopathy, oesophageal variceal haemorrhage confirmed by endoscopy, liver transplantation, or HCC. The presence of varices without haemorrhage was not included as an outcome because of the possibility that differences in the practice of endoscopy in the different centres may lead to ascertainment bias. Table 11 provides further details of study design.
| Study | Country/type of centre | Recruitment dates | Reference standard (fibrosis staging system) | Minimum biopsy length/minimum number of portal tracts/maximum time between index and reference test | Study design | Total patients with ALD/total study population | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Rosenberg 2004121 | Multinational/NR | 1998–2000 | Liver biopsy (Scheuer and Ishak systems, modified for conditions other than chronic viral or immune hepatitis) | 12 mm/≥ 5 portal tracts/tests performed on same day | Cross-sectional/prospective, consecutive |
Eligible: 64/1021 Assessed: 64a/300 |
Patients aged 18–75 years due to undergo liver biopsy to investigate chronic liver disease (defined as abnormal liver function tests for > 6 months) | Disorders associated with extrahepatic fibrosis (including rheumatic, renal, or lung disease); CVD or cancer; advanced cirrhosis with evidence of decompensation (Child–Pugh class C); hepatocellular carcinoma; drug-induced liver disease; regular aspirin consumption; inability to provide informed consent |
| Parkes 201057 | England/seven hepatology centres in secondary or tertiary care | 1998–2000 | Clinical outcomes | Not applicable | Cohort/prospective, consecutive |
Eligible: NR/NR Assessed: 85/457 |
Patients aged 18–75 years due to undergo liver biopsy to investigate chronic liver disease (defined as abnormal liver function tests for > 6 months) | Disorders associated with extrahepatic fibrosis (including rheumatic, renal, or lung disease); CVD or cancer; advanced cirrhosis with evidence of decompensation (Child–Pugh class C); hepatocellular carcinoma; drug-induced liver disease; regular aspirin consumption; inability to provide informed consent |
At first sight, the data relating to the number of patients with ALD included in the analyses by Rosenberg et al. 121 and Parkes et al. 57 are confusing. One thousand and twenty-one patients with chronic liver disease of any aetiology were eligible for inclusion in the original study, and 921 were recruited. Of these, 621 formed the training or derivation cohort used to identify the optimum combination of markers and algorithm (the European Liver Test) which was then assessed in the remaining 300 patients (the validation cohort). All 64 patients with ALD were included in the validation cohort (Professor William Rosenberg, University College London, 2010, personal communication). The follow-up study by Parkes et al. 57 states that 85 of the patients enrolled in the study in English centres alone had ALD (i.e. more patients with ALD than were said by Rosenberg et al. to have been included in the original study); this apparent discrepancy is attributed to the fact that some patients originally believed to have liver disease of a different aetiology were later found to have ALD (Professor William Rosenberg, University College London, 2010, personal communication).
FibroTest
Two studies of FibroTest were identified that specifically recruited patients with known or suspected ALD. Nguyen-Khac et al. 132 evaluated diagnostic test accuracy compared with liver biopsy, but did not include the full spectrum of ALD: patients with known or decompensated cirrhosis were excluded on the basis that they did not require further investigation. Naveau et al. 123 compared FibroTest and liver biopsy results in patients hospitalised either for complications of cirrhosis or for alcoholism. The study also assessed the ability of FibroTest to predict 5- and 10-year survival in 218 of the 292 patients (75%) enrolled in the study of test accuracy and followed up for a median period of 8.2 years (range 5 days to 11.8 years). 124
A further three studies,13,125,126 all by Thabut et al. , evaluated FibroTest in patients with liver disease of mixed aetiology, including ALD. To avoid any risk of double-counting, clarification was obtained from the author that no patient was included in more than one of these studies (Dr Dominique Thabut, Hôpital Pitié-Salpêtriére, Paris, 2010, personal communication). One study13 assessed the ability of FibroTest to identify PHt in patients undergoing transjugular liver biopsy for clinical reasons and also compared FibroTest and liver biopsy results in these patients. A second study125 assessed its ability to predict the presence of oesophageal varices in patients with chronic liver disease. The third study126 assessed FibroTest’s predictive value in relation to survival at 2 months and 6 months in patients with severe cirrhosis.
Table 12 provides further details of study design.
| Study | Country | Recruitment dates | Reference standard (fibrosis staging system) | Minimum biopsy length/minimum number of portal tracts/maximum time between index and reference test | Study design | Total patients with ALD/total study population | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Naveau 2005123 | France | February 1996 to May 2000 | Liver biopsy (modified METAVIR) | Minimum length and number of portal tracts NR [mean length 15 (SE 0.5) mm; mean number of portal tracts 14.4 (SE 0.7)]/31 days | Cross-sectional/prospective, consecutive |
Eligible: 292/292 Assessed: 221/221 |
Hospitalised for complications of cirrhosis (jaundice 12%, ascites 9%, digestive tract haemorrhage 3%) or alcoholism without complications of cirrhosis (76%); self-reported daily alcohol consumption in previous year ≥ 50 g pure ethanol (mean 146 ± 80 g/day for 17 years); available serum levels; ‘consistent’ liver biopsy | Concomitant liver diseases including hepatitis B or C; HIV antibodies; immunosuppression; serum or biopsy unavailable; > 31 days between biopsy and collection of serum |
| Naveau 2009124 | France | As above | 5- and 10-year survival | Not applicable | Cohort/prospective, consecutive |
Eligible: 292/292 Assessed: 218/218 |
||
| Nguyen-Khac 2008132 | France | April 2005 to January 2007 | Liver biopsy (METAVIR) | Minimum length and number of portal tracts NR (mean length 12.2 ± 3 mm; mean number of portal tracts 7.8 ± 2.7)/tests performed on same day | Cross-sectional/prospective |
Eligible: 160/160 Assessed: 103/103 |
Age ≥ 18 years; alcohol intake > 50 g/day for > 5 years; consulting hospital department of hepato-gastroenterology, alcoholism or internal medicine for detoxification and/or inpatient rehabilitation | Chronic viral hepatitis B or C; severe AAH (Maddrey score ≥ 32); known or decompensated alcoholic cirrhosis (ascites, oesophageal varices, prothrombin time < 70%, or imaging evidence of cirrhosis); pregnancy; refusal to undergo liver biopsy; failure to obtain valid transient elastography measurements; absence of consent |
| Thabut 2003125 | France | NR | Endoscopy | Not applicable/< 6 months | Cross-sectional/consecutive |
Eligible: 57/120a Assessed: 57/120 |
Chronic liver disease | History of liver transplantation |
| Thabut 2007a13 | France | Unspecified 8-month period | Liver biopsy (METAVIR), HVPG measurement | Minimum length and number of portal tracts NR/tests performed on same day | Cross-sectional/prospective, consecutive |
Eligible: NR/147 Assessed: 66/130 |
Undergoing transjugular liver biopsy for clinical reasons | History of liver transplantation; cardiac hepatopathy; pre-sinusoidal and extrahepatic PHt; renal disease |
| Thabut 2006126 | France | NR | Survival at 2 months and 6 months | Not applicable | Cross-sectional/prospective |
Eligible: NR/NR Assessed: 175/224a |
Severe cirrhosis; enrolled in placebo-controlled trial of pentoxifylline (Trental®, Sanofi-Aventis) | NR |
FibroMAX
No relevant studies of FibroMAX were identified.
FibroScan
Six studies117,118,122,127,128,132 were identified that specifically recruited patients with known or suspected ALD and assessed the diagnostic test accuracy of FibroScan relative to liver biopsy in these patients. In the studies by Kim et al. ,127 Mueller et al. ,118 Nahon et al. 128 and Nguyen-Khac et al. ,132 all patients who underwent FibroScan were also biopsied. However, in the studies by Janssens et al. 117 and Melin et al. ,122 biopsy was only undertaken in a subset of patients who underwent FibroScan. Janssens et al. 117 used FibroScan to identify those patients requiring alcohol detoxification or rehabilitation who had a score of ≥ 9.5 kPa; this threshold was chosen as it was thought to be indicative of severe fibrosis (F3–F4). Data relating to the test accuracy of FibroScan compared with biopsy and HVPG were therefore available only for patients with a FibroScan score of ≥ 9.5 kPa who then consented to liver biopsy and in whom both tests were conducted successfully. Similarly, Melin et al. 122 sought to compare the accuracy of FibroScan with biopsy in patients being treated for alcohol withdrawal who had a FibroScan score higher than 13 kPa; this threshold was apparently chosen because it was considered to be the appropriate threshold for the diagnosis of cirrhosis in patients with hepatitis C. Only 41 patients met this criterion; three of these refused biopsy and a further three had contraindications to biopsy.
A further three studies97,129,130 evaluated the diagnostic test accuracy of FibroScan in patients with liver disease of mixed aetiology, including ALD. Bureau et al. 129 studied the ability of FibroScan to predict significant PHt in patients undergoing transjugular liver biopsy for clinical reasons. Lemoine et al. 97 and Nguyen-Khac et al. 130 both specifically recruited patients with cirrhosis. Lemoine et al. 97 assessed the ability of FibroScan relative to HVPG measurement to predict significant PHt in patients with compensated cirrhosis, whereas Nguyen-Khac et al. 130 assessed its ability relative to upper intestinal endoscopy to predict the presence of large oesophageal varices in patients with cirrhosis of unspecified severity.
Table 13 provides further details of study design.
| Study | Country | Recruitment dates | Details of FibroScan assessment | Reference standard (fibrosis staging system) | Minimum biopsy length/minimum number of portal tracts/maximum time between index and reference test | Study design | Total patients with ALD/total study population | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|
| Bureau 2008129 | France | 15 November 2005 to 15 October 2006 | Right lobe of liver assessed through intercostal spaces. Reported result median of at least 10 validated measurements with a success rate ≥ 60% | Liver biopsy (METAVIR133); HVPG measurement; upper gastrointestinal endoscopy (patients with cirrhosis only) | Minimum length and number of portal tracts NR [mean length 15.5 (SE 7.1) mm]/tests performed on same day | Cross-sectional/prospective, consecutive |
Eligible: 51/150 Assessed: NR/144 |
Undergoing transjugular liver biopsy because of chronic liver abnormalities | Portal-vein thrombosis; Budd–Chiari syndrome; antiviral therapy or pharmacological treatment known to modify portal pressure |
| Janssens 2010117 | Belgium | 1 January 2006 to 29 February 2008 | Reported result median of at least 10 validated measurements, with a success rate ≥ 60% and interquartile range < 30%. Procedure said to be performed by an experienced examiner | Transjugular liver biopsy (METAVIR133); HVPG measurement | 15 mm; ≥ 6 portal tracts/circa 2 weeks | Cross-sectional/apparently prospective |
Eligible: 255/255 Biopsy offered to patients with FS score ≥ 9.5 kPa (72/239), and performed in 49 |
Admitted to unit for alcohol detoxification and rehabilitation; self-reported minimum daily alcohol intake ≥ 70 g/day | Declined to be rehospitalised for second week of programme; declined FS; FS unsuccessful; refused liver biopsy after successful FS |
| Kim 2009127 | Republic of Korea | August 2006 to April 2007 | Right lobe of liver assessed following ultrasound to identify appropriate location. Reported result apparently median of at least 10 validated measurements with a success rate ≥ 30% | Liver biopsy (Batts–Ludwig134) | 10 mm; > 10 periportal tracts/92 days | Cross-sectional/probably prospective and consecutive |
Eligible: 51/51 Assessed: 45/45 |
ALD (alcohol consumption ≥ 80 g/day (mean 150.7 ± 60.4 g, range 80–320 g; duration of alcohol consumption 20.8 ± 6.8 years, range 8–40 years) | Ultrasound evidence of ascites; factors other than viral liver diseases or ALD that could cause chronic liver disease; AAH |
| Lemoine 200897 | France | January 2004 to September 2006 | Right lobe of liver assessed through intercostal spaces. Reported result median of at least 10 validated measurements, with success rate ≥ 70% and interquartile range ≤ 30% of the median value. Measurements said to be obtained by two experienced operators |
HVPG measurement Liver biopsy (Chevallier et al. 135) |
Minimum length and number of portal tracts NR (mean length 15 mm)/tests performed on same day | Cross-sectional/prospective, consecutive |
Eligible: 48/92 Assessed: apparently 48/92 |
Histologically proven compensated cirrhosis (Child–Pugh class A) related to HCV or ALD (the latter defined by alcohol intake ≥ 50 g/day for ≥ 10 years) undergoing transjugular liver biopsy with HVPG measurement and transient elastography on the same day | Liver disease of other aetiologies, in particular hepatitis B or mixed aetiology (alcohol and HCV, or HIV co-infection); portal-vein thrombosis; ongoing beta-blocker therapy; ongoing or recent (< 6 months) antiviral therapy; recent gastrointestinal bleeding or endotherapy; liver cancer; cardiac failure |
| Melin 2005122 | France | Unspecified 6-month period | Right lobe of liver assessed through intercostal spaces following ultrasound to identify appropriate location. Reported result median of up to 10 validated measurements | Liver biopsy | NR | Cross-sectional/prospective, consecutive |
Eligible: 245/245 Biopsy offered to patients with FS score ≥ 13 kPa (41/227) and performed in 35 |
Patients seeking treatment for alcohol withdrawal; FS result ≥ 13 kPa | NR |
| Mueller 2010118 | Germany | NR | Right lobe of liver assessed through intercostal spaces. Reported result median of 10 procedures with a success rate ≥ 60% | Liver biopsy (Kleiner136) | 15 mm/minimum number of portal tracts and maximum time between tests NR | Cross-sectional (validation cohort only)/prospective, consecutive |
Eligible: 106/106 Assessed: 101/101 |
Histologically staged ALD (alcohol intake > 60 g/day120) | Biopsy < 15 mm; ultrasound results indicative of extrahepatic cholestasis, liver congestion, or liver tumours |
| Nahon 2008,128 Nahon 2007131 | France | November 2005 to November 2006 | Reported result median of at least 10 validated measurements with a success rate ≥ 50%. Procedure said to be performed by an experienced examiner | Liver biopsy (Brunt et al.137 Chevallier et al.135) | 10 mm/minimum number of portal tracts NR/tests performed on same day | Cross-sectional/prospective, consecutive |
Eligible: 174/174 Assessed: 147/147 |
New patients referred to hepatology units with suspected ALD (alcohol intake > 80 g/day for > 10 years) | Ascites; HIV; hepatitis B or C; refused FS or liver biopsy; FS and liver biopsy not performed on same day |
| Nguyen-Khac 2008132 | France | April 2005 to January 2007 | Reported result median of at least 10 validated measurements with a success rate ≥ 60% | Liver biopsy (METAVIR133) | Minimum length and number of portal tracts NR (mean length 12.2 ± 3 mm; mean number of portal tracts 7.8 ± 2.7)/tests performed on same day | Cross-sectional/prospective |
Eligible: 160/160 Assessed: 103/103 |
Age ≥ 18 years; alcohol intake > 50 g/day for > 5 years; consulting hospital department of hepato-gastroenterology, alcoholism or internal medicine for detoxification and/or inpatient rehabilitation | Chronic viral hepatitis B or C; severe AAH (Maddrey score ≥ 32); known or decompensated alcoholic cirrhosis (ascites, oesophageal varices, prothrombin time < 70%, or imaging evidence of cirrhosis); pregnancy; refusal to undergo liver biopsy; failure to obtain valid transient elastography measurements; absence of consent |
| Nguyen-Khac 2009130 | France | NR | NR | Upper intestinal endoscopy | Not applicable/maximum time between tests NR | Not clear |
Eligible: 103/183 Assessed: apparently 103/183 |
Cirrhosis | NR |
Study quality
Figures 5–9 provide an overview of the methodological quality of the included studies.
FIGURE 5.
The ELF test: methodological quality summary. Review authors’ judgements about each methodological quality item. –, no; +, yes; ?, unclear.
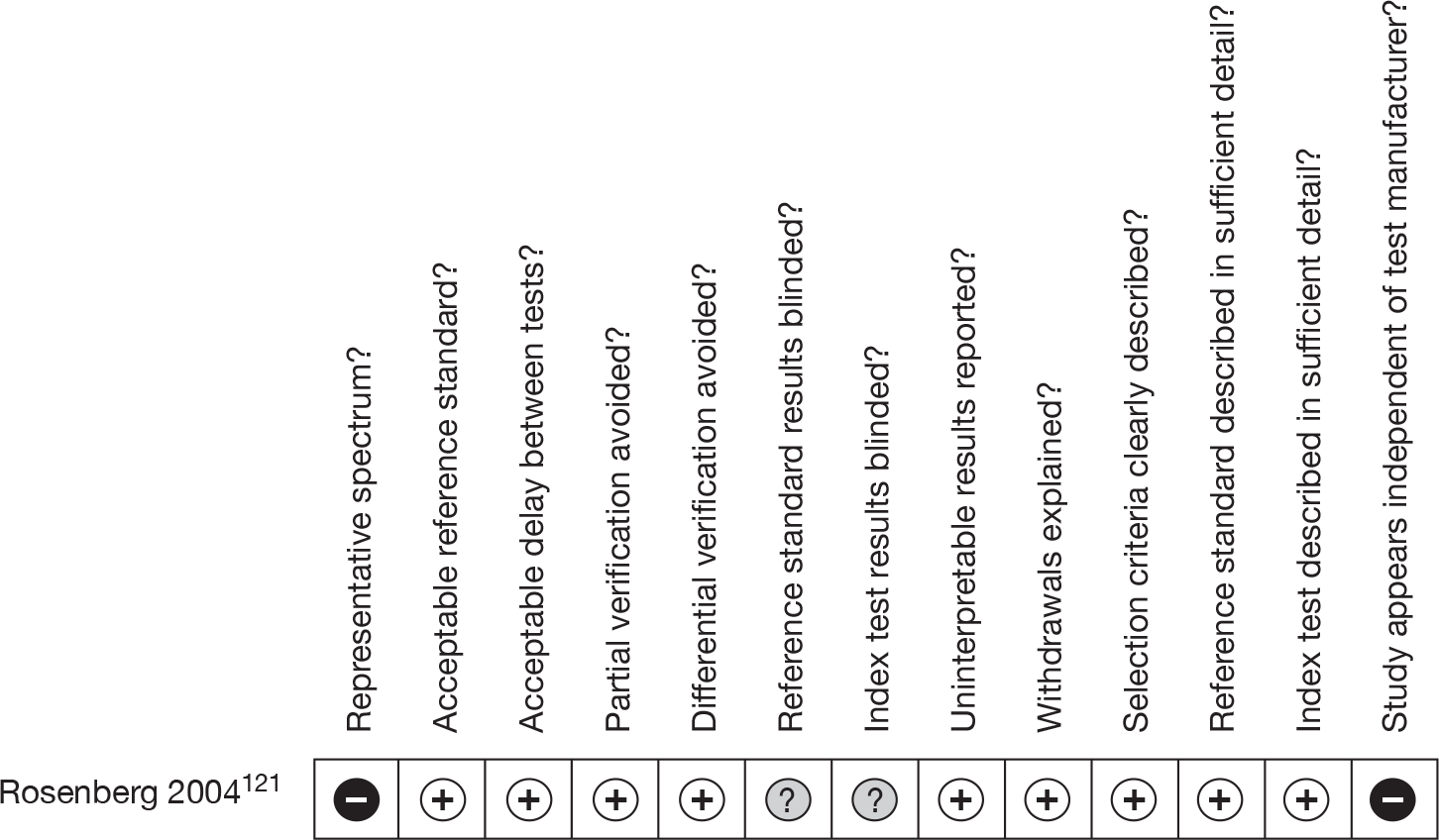
FIGURE 6.
FibroTest: methodological quality graph. Review authors’ judgements about each methodological quality item presented as percentages across all included studies.
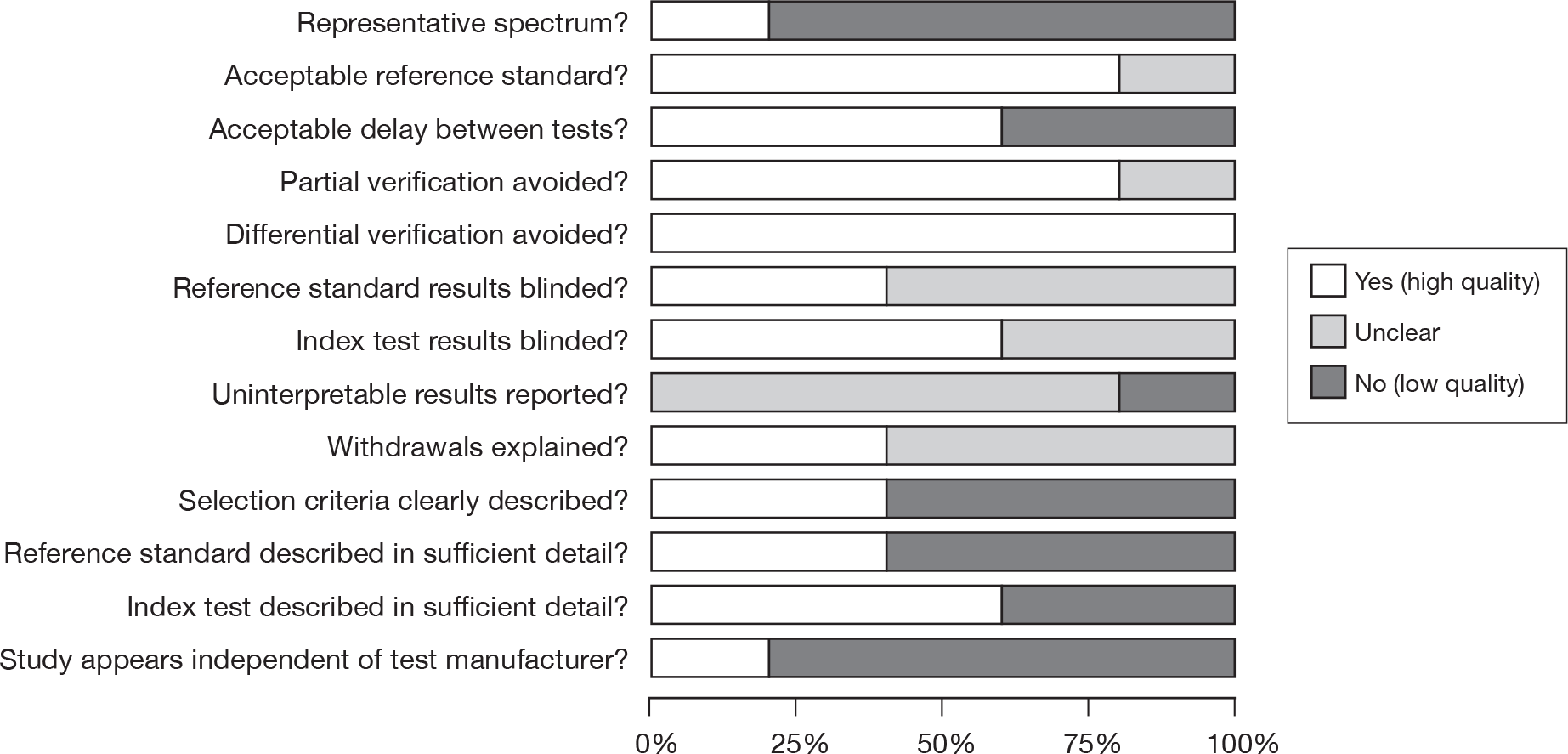
FIGURE 7.
FibroTest: methodological quality summary. Review authors’ judgements about each methodological quality item for each included study. –, no; +, yes; ?, unclear.
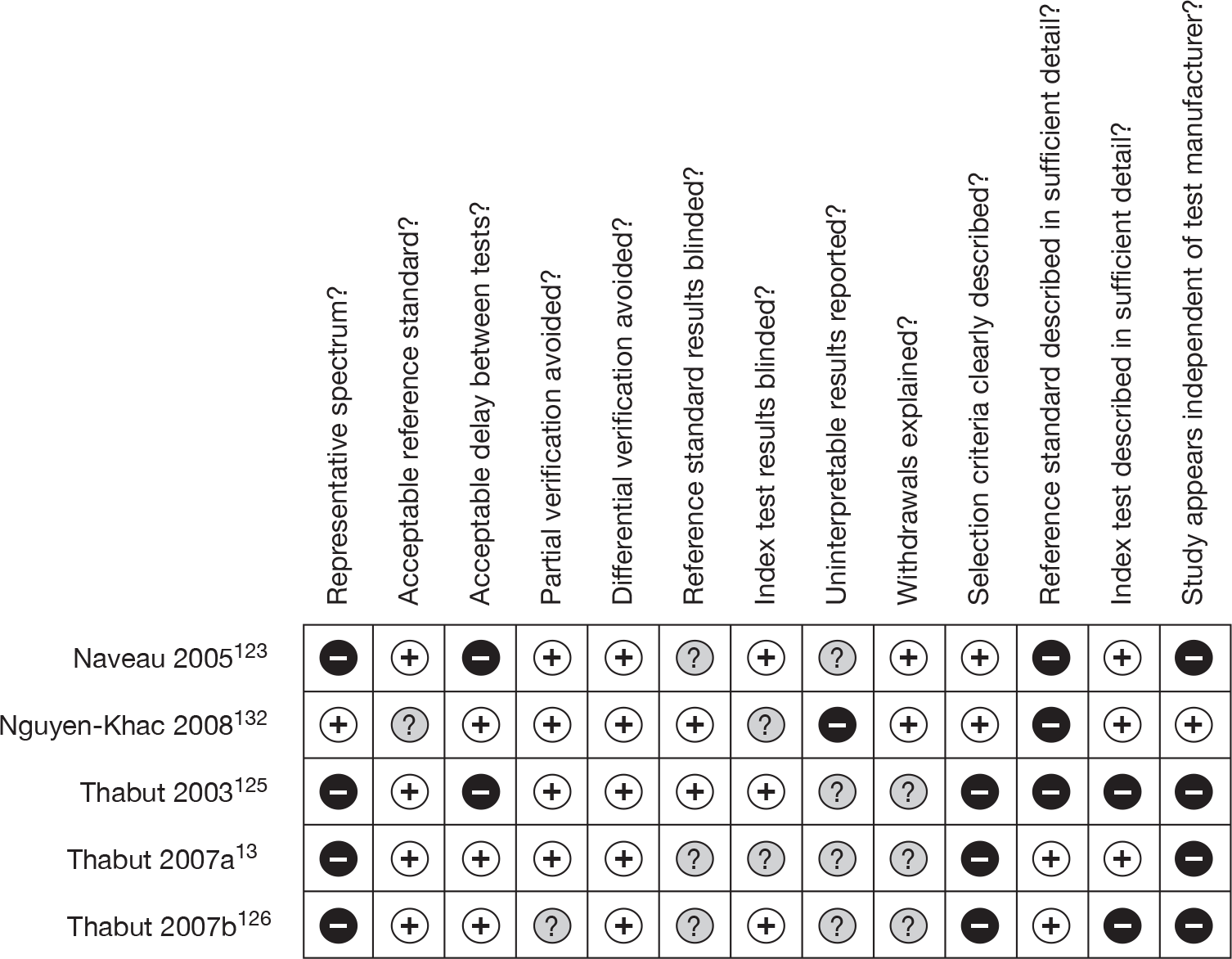
FIGURE 8.
FibroScan: methodological quality graph. Review authors’ judgements about each methodological quality item presented as percentages across all included studies.
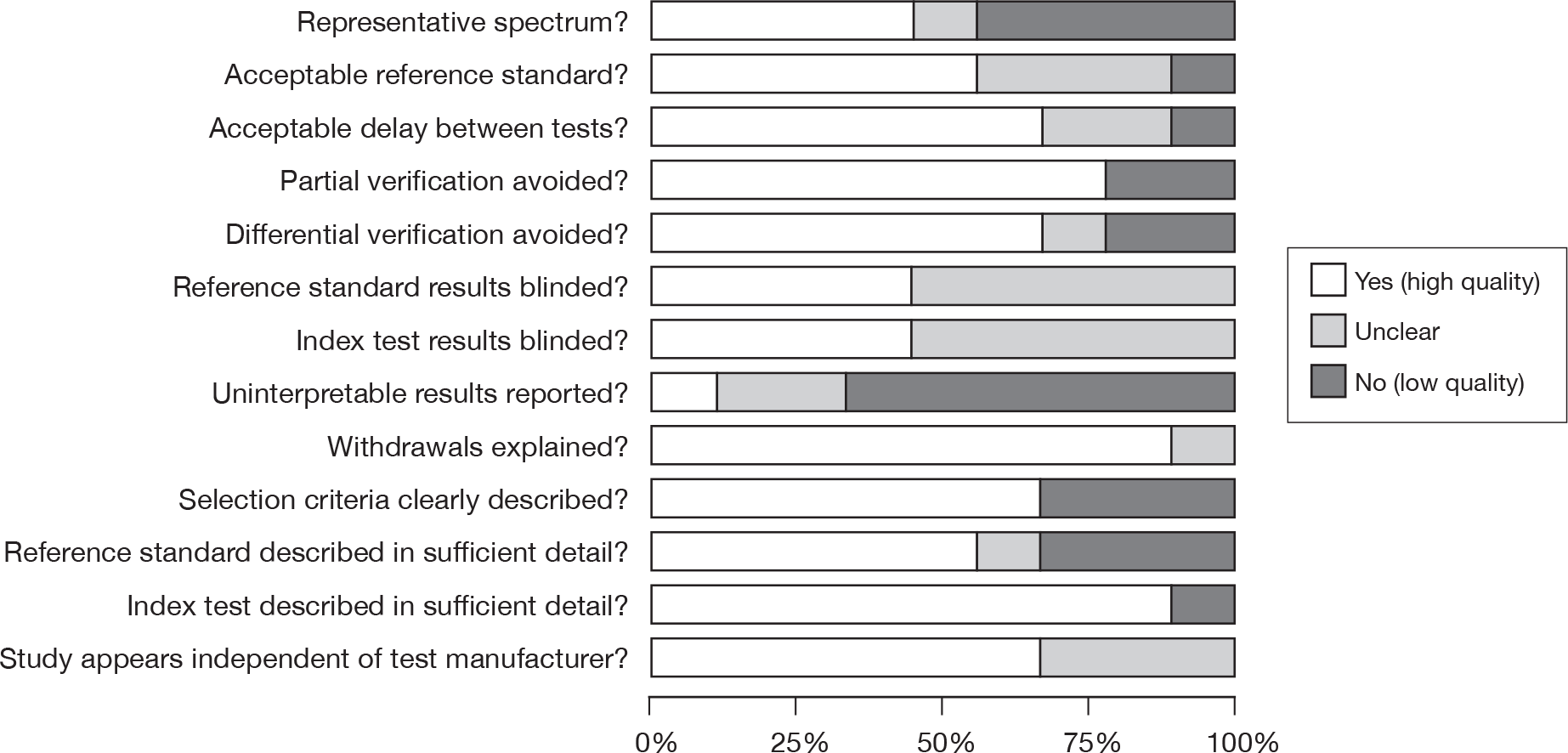
FIGURE 9.
FibroScan: methodological quality summary. Review authors’ judgements about each methodological quality item for each included study. –, no; +, yes; ?, unclear.
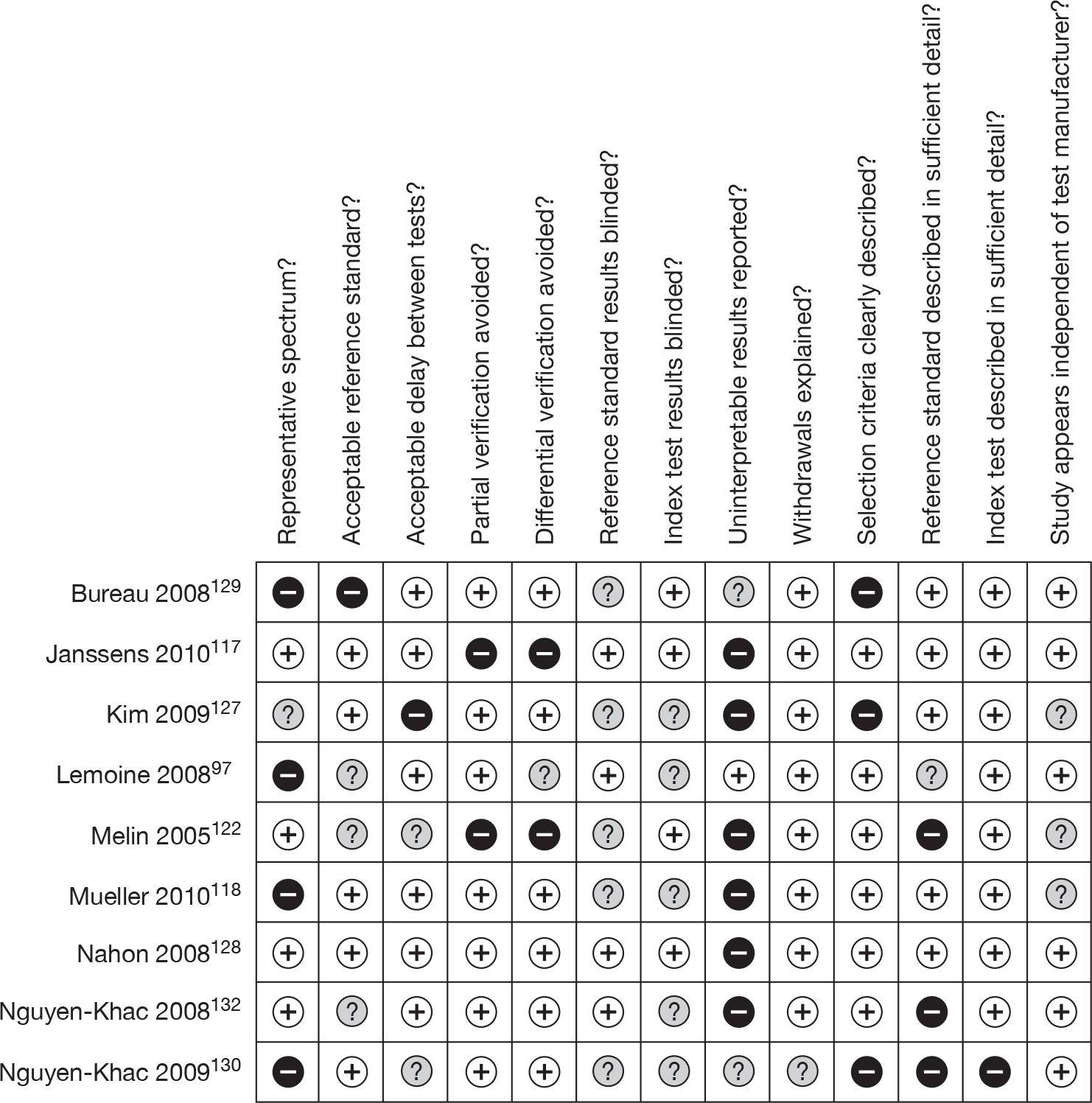
As may be seen, few studies presented results relating to a representative spectrum of patients suspected of having ALD. The majority recruited patients with relatively severe disease. Bureau et al. ,129 Rosenberg et al. 121 and Thabut et al. 2007a13 recruited patients who were due to undergo liver biopsy for clinical reasons, whereas Lemoine et al. ,97 Nguyen-Khac et al. 130 and Thabut et al. 2007b126 recruited patients known to have cirrhosis, Naveau et al. 123 recruited patients hospitalised for complications of cirrhosis or alcoholism, and Mueller et al. 118 and Thabut et al. 2003126 recruited those known to have, rather than suspected of having, chronic liver disease. Two studies, those by Janssens et al. 117 and Melin et al. ,122 recruited more representative populations, but displayed partial verification bias, using the reference standard only in patients scoring above a specific threshold on the index test (FibroScan in both cases).
In studies of test accuracy, it is clearly important that the interval between the performance of the index and reference tests should be as short as possible, to minimise the possibility of the patient’s condition altering significantly between the tests. However, several studies allowed a delay of > 2 weeks between the index test and reference standard. Naveau et al. 123 allowed an interval of up to 1 month, whereas Kim et al. 127 allowed the interval between transient elastography and liver biopsy to be as much as 92 days, and Thabut et al. 2003125 included patients in whom endoscopy was performed up to 6 months before or after FibroTest, although in this case the mean interval was only 5 days.
In approximately half of the included studies, it was not clear whether the reference standard results were interpreted without knowledge of the results of the index test (‘reference standard results blinded’) and vice versa (‘index test results blinded’). The remaining studies stated that blinding was used for either one or both tests. The index test was usually well described, but in many cases the execution of the reference standard was not described in sufficient detail to permit replication of the test precisely as performed by the study investigators.
Uninterpretable/intermediate results were generally poorly reported. Two studies, Rosenberg et al. ’s121 study of the ELF test and Lemoine et al. ’s97 FibroScan study, stated that patients were recruited prospectively and consecutively, and reported no uninterpretable/intermediate results, implying that there were none. Bureau et al. ’s129 FibroScan study reported the overall number of uninterpretable results, but did not specify how many related to patients with ALD; it is not clear whether or not they were included in the analyses. Janssens et al. ,117 Kim et al. ,127 Melin et al. ,122 Mueller et al. ,118 Nahon et al. 128 and Nguyen-Khac et al. 132 all reported the number of instances of FibroScan failure (i.e. no or uninterpretable results), but did not include them in their analyses. As none stated how many of these failures occurred in patients who tested positive and how many in patients who tested negative by the reference standard, their impact on test sensitivity and specificity could not be calculated.
The three tests for which evidence has been identified vary in the extent to which that evidence is independent of the test manufacturer. There is no wholly independent evidence relating to the ELF test: one of the investigators, Professor William Rosenberg, is the founder of, and holds stocks in, iQur Ltd, which holds a limited licence to conduct ELF assays on behalf of Siemens Healthcare Diagnostics. 57 Only one of the studies of FibroTest, that by Nguyen-Khac et al. ,132 appears to be independent. The remaining studies include in their authorship Thierry Poynard, a major stockholder in, and Mona Munteanu, an employee of, the manufacturers, BioPredictive. 125 However, six of the nine studies that provided data relating to FibroScan97,117,128–130,132 stated that the authors had no conflicts of interest in relation to the work; of the studies that did not include such a declaration, that by Mueller et al. 118 stated that it was funded from independent sources, and only the studies by Kim et al. 127 and Melin et al. 122 contained no relevant information on this point.
Two further indices of methodological quality proposed by Tsochatzis et al. 138 were not included in the QUADAS checklist as they were not applicable to all of the included studies. These were:
-
whether studies that used liver biopsy as the reference standard reported that it was performed to an acceptable standard (i.e. the specimen was at least 15 mm long and included at least six portal tracts)
-
whether studies of FibroScan reported that it was performed in accordance with the manufacturer’s instructions, i.e. using at least 10 valid shots, with a success rate (ratio of valid shots to total number of shots) of at least 60%, and an interquartile range < 30% of the median value. 79
Only one study that used liver biopsy as a reference standard reported using adequate criteria; this was the study by Janssens et al. 117 This was also one of only two studies which clearly stated that FibroScan was performed either in accordance with the manufacturer’s instructions or, in the case of the study by Lemoine et al. ,97 using more stringent criteria. The remaining studies failed either to meet or, more frequently, to report one or more of the standards (for details, see Table 14).
| Study | ≥ 10 valid shots | Success rate > 60% | Interquartile range < 30% of median LSM value |
|---|---|---|---|
| Bureau 2008129 | Yes | Yes | NR |
| Janssens 2010117 | Yes | Yes | Yes |
| Kim 2009127 | Yes | No (≥ 30%) | NR |
| Lemoine 200897 | Yes | Yes (≥ 70%) | Yes |
| Melin 2005122 | No (< 10) | NR | NR |
| Mueller 2010118 | Yes | Yes | No (< 40%) |
| Nahon 2008,128 Nahon 2007131 | Yes | No (≥ 50%) | NR |
| Nguyen-Khac 2008132 | Yes | Yes | NR |
| Nguyen-Khac 2009130 | NR | NR | NR |
Assessment of diagnostic and prognostic accuracy
Enhanced Liver Fibrosis Test: diagnostic and prognostic accuracy results
The evidence base for the ELF test is small, resting on a single study of the European Liver Fibrosis Test carried out in a population with chronic liver disease that included < 100 patients diagnosed with ALD. Moreover, the quality of that evidence is not ideal as liver biopsy was not performed to an acceptable standard (see Study quality).
This limited evidence suggests that the ELF test can generally distinguish patients with moderate-to-severe fibrosis (Scheuer stages 3–4) from those with milder or no fibrosis (Scheuer stages 0–2) in patients with ALD. Using a low threshold score of 0.087, the test showed 100% sensitivity, but only 16.7% specificity. Ninety-three per cent sensitivity and 100% specificity were achieved using a threshold score of 0.431 (Table 15). These threshold scores appear to have been derived from the AUROCs after data collection, rather than prospectively selected and validated. As the the investigators note, because the results rest on data from so few patients, the resulting positive and negative predictive values should be interpreted with caution. 121
| Condition of interest | Study | Number of assessed patients with known or suspected ALD | Prevalence of condition of interest (%) | Threshold | 2 × 2 data | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | AUROC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Moderate/severe’ fibrosis (Scheuer stages 3–4) | Rosenberg 2004121 | 64 | 71 | 0.087 | NR | 100 (97 to 100) | 16.7 (7 to 30) | 75.0 (67 to 82) | 100 (67 to 100) | 0.94 (0.84 to 1.00) |
| 0.431 | NR | 93.3 (87 to 97) | 100 (93 to 100) | 100 (97 to 100) | 85.7 (74 to 94) | |||||
| Probable or definite cirrhosis (Scheuer stage 4) | Rosenberg 2004121 | 64 | NR | NR | NR | NR | NR | NR | NR | 0.83 (0.73 to 0.92) |
| Liver-related clinical outcomes at 6 years | Parkes 201057 | 85 | 40 | NR | Not reported | NR | NR | NR | NR | 0.80 (0.70 to 0.98)a |
| All-cause mortality at 6 years | Parkes 201057 | 85 | 40 | NR | NR | NR | NR | NR | NR | 0.76 (0.65 to 0.82)a |
The ELF test appears to be less successful in distinguishing patients with probable or definite cirrhosis (Scheuer stage 4) from those with milder or no fibrosis (Scheuer stages 0–3) (see Table 15). As this result was only presented in the form of an AUROC, the sensitivity and specificity associated with a specific threshold score or scores could not be calculated. Discordant results were not discussed for either fibrosis range.
The evidence relating to the prognostic accuracy of the ELF is derived from data from 85 patients with ALD who were enrolled in the European Liver Fibrosis Test study in English centres and were followed up over a median period of 6.86 years (range 0–9 years). Thus, as for test accuracy, the evidence base is very small. During the follow-up period, 27 patients (32%) died of liver-related causes, a further seven (8%) suffered non-fatal liver-related clinical events, and seven (8%) died of non-liver-related causes. 57 Again, results are only presented in the form of an AUROC. Although this suggests that the ELF is predictive both of liver-related clinical outcomes and of all-cause mortality (for details, see Table 15), it should be noted that the sensitivity and specificity associated with a specific threshold score or scores could not be calculated and, more importantly, no information was presented on post-test alcohol consumption, although is likely to have been a substantial confounding factor.
FibroTest: diagnostic and prognostic accuracy results
The evidence base for FibroTest, although more substantial than that for the ELF test, derives from a total of only 622 patients enrolled in five small to medium-sized studies; although the evidence for test accuracy relative to liver biopsy is derived from a total of only 390 patients enrolled in three of those studies, none of which state that they stipulated a minimum biopsy length of 15 mm.
The available evidence suggests that FibroTest can distinguish between patients with cirrhosis and those with METAVIR stage F0–F3 fibrosis, and, with lesser accuracy, between those with stage F2–F4 and stage F0–F1 fibrosis, and between those with stage F3–F4 and stage F0–F2 fibrosis (Table 16). However, not only are these conclusions based on data from only three relatively small studies,13,123,132 in which some biopsy samples may not have met the recommended minimum standards, as noted above, but the prevalence of the condition of interest was high in each of the three studies, ranging from 63% to 98% for METAVIR stage F2–F4 fibrosis, 51% for METAVIR stage F3–F4 fibrosis, and from 31% to 92% for cirrhosis.
| Condition of interest | Study | Number of assessed patients with known or suspected ALD | Prevalence of condition of interest (%) | Threshold | 2 × 2 data | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | AUROC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mild–severe fibrosis (F1–F4) | Nguyen-Khac 2008132 | 103 | 92 | NR | NR | NR | NR | NR | NR | 0.77 (0.63 to 0.90) |
| Moderate–severe fibrosis (F2–F4) | Naveau 2005123 | 221 | 63 | 0.30 | TP: 125; FP: 28; FN: 23; TN: 54a | 84 (78 to 90) | 66 (55 to 76) | 82 (75 to 87) | 70 (60 to 80) | 0.83 (0.81 to 0.87) |
| 0.70 | TP: 81; FP: 6; FN: 67; TN: 76a | 55 (47 to 63) | 93 (86 to 97) | 93 (87 to 97) | 53 (45 to 61) | |||||
| Nguyen-Khac 2008132 | 103 | 75 | NR | NR | NR | NR | NR | NR | 0.79 (0.69 to 0.90) | |
| Thabut 2007a13 | 66 | 98 | 0.48b | TP: 62; FP: 1; FN: 3; TN: 0b | 95 c (88 to 99) | 0 c (0 to 85) | 98 c (93 to 100) | 0 c (0 to 54) | 0.83- ± –0.03b | |
| Severe fibrosis (F3–F4) | Nguyen-Khac 2008132 | 103 | 51 | NR | NR | NR | NR | NR | NR | 0.80 (0.70 to 0.91) |
| Cirrhosis (F4) | Naveau 2005123 | 221 | 31 | 0.30 | TP: 68; FP: 76; FN: 0; TN: 77 | 100 (96 to 100) | 50 (42 to 58) | 47 (39 to 55) | 100 (97 to 100) | 0.95 (0.94 to 0.96) |
| 0.70 | TP: 62; FP: 20; FN: 6; TN: 133 | 91 (83 to 97) | 87 (81 to 92) | 76 (66 to 84) | 96 (90 to 98) | |||||
| Nguyen-Khac 2008132 | 103 | 32 | NR | NR | NR | NR | NR | NR | 0.84 (0.72 to 0.97) | |
| Thabut 2007a13 | 66 | 92 | 0.74b | TP: 47; FP: 1; FN: 13; TN: 5b | 78 c (66 to 87) | 83 c (44 to 98) | 98 c (91 to 100) | 28 c (10 to 50) | 0.90 ± 0.04b | |
| HVPG ≥ 5 mmHg | Thabut 2007a13 | 66 | 94 c | 0.48b | TP: 48; FP: 1; FN: 14; TN: 3b | 77 c (65 to 87) | 75 c (28 to 97) | 98 c (91 to 100) | 18 c (5 to 40) | 0.92 ± 0.03b |
| HVPG ≥ 12 mmHg | Thabut 2007a13 | 66 | 86 c | 0.58b | TP: 53; FP: 2; FN: 4; TN: 7b | 93 c (84 to 98) | 87 c (59 to 99) | 98 c (92 to 100) | 67 c (39 to 88) | 0.84 ± 0.03b |
| Oesophageal varices (grade 2) | Thabut 2003125 | 58b | 76 c | 0.85b | TP: 39; FP: 7; FN: 5; TN: 7b | 89 c (78 to 96) | 50 c (25 to 77) | 85 c (73 to 94) | 58 c (31 to 83) | 0.73 ± 0.08b |
| All-cause mortality at 2 months | Thabut 2007b126 | 189 | 39b | NR | NR | NR | NR | NR | NR | 0.64 ± 0.05b |
| All-cause mortality at 6 months | Thabut 2007b126 | 189 | 54 | NR | NR | NR | NR | NR | NR | 0.58 ± 0.05b |
| Liver-related mortality at 5 years | Naveau 2009124 | 218 | 19 | ≥ 0.31 | NR | NR | NR | NR | NR | 0.79 (0.68 to 0.86) |
| 0.33–0.58 | NR | NR | NR | NR | NR | |||||
| ≥ 0.59 | NR | NR | NR | NR | NR | |||||
| All-cause mortality at 5 years | Naveau 2009124 | 218 | 39 | ≥ 0.31 | NR | NR | NR | NR | NR | 0.69 (0.61 to 0.76) |
| 0.33–0.58 | NR | NR | NR | NR | NR | |||||
| ≥ 0.59 | NR | NR | NR | NR | NR |
The largest study of test accuracy relative to liver biopsy, that by Naveau et al. ,123 also had the most representative population in that it included the lowest proportion of patients with F2–F4 fibrosis. It explored the impact of different threshold scores on sensitivity and specificity. At a threshold score of 0.30, FibroTest had reasonable sensitivity, but rather disappointing specificity in relation to moderate-to-severe (F2–F4) fibrosis; this situation was reversed when the threshold score was raised to 0.70. For cirrhosis, a threshold score of 0.30 produced 100% sensitivity but only 50% specificity, and the balance was improved using a threshold score of 0.70 (for details, see Table 16). Thabut et al. 2007a13 found that, at a threshold of 0.48, the specificity of FibroTest in relation to moderate-to-severe (F2–F4) fibrosis was 0% because the prevalence of that condition in the study population, as indicated by liver biopsy, was 98%. Similarly, although FibroTest displayed a sensitivity of 95% for the diagnosis of F2–F4 fibrosis, this result is not robust because 92% of the study population had biopsy results indicative of cirrhosis. The third study of test accuracy relative to liver biopsy, that by Nguyen-Khac et al. ,132 did not indicate what diagnostic thresholds were used; it did not report sensitivity and specificity, and the underlying data that would have allowed them to be calculated could not be obtained.
Both Naveau et al. 123 and Thabut et al. 2007a13 provided some discussion of discordant cases. Naveau et al. 123 reported a discordance of two or more fibrosis stages in 19% of assessed patients (42/221). On the basis of independent clinical, ultrasonographical, and endoscopic signs of cirrhosis, they attributed the error to the biopsy in 26 cases (14 FNs and 12 FPs), and to FibroTest in 13 cases (six FNs and seven FPs); three cases were unattributable. 123 Eighteen of the 42 discordant cases involved diagnoses of cirrhosis: three FNs and three possible FPs of FibroTest, three FPs and eight possible FNs of biopsy (the eight FNs of biopsy were all in poor-quality samples), and one unattributable case diagnosed as cirrhosis by FibroTest but not by biopsy. 123
Thabut et al. 2007a13 attributed discordant cases to failure of biopsy or FibroTest on the basis of clinical events (haemorrhage, ascites) and risk factors for FibroTest failure. Four of the 61 patients with cirrhosis (7%) had FN results on FibroTest; all had large ascites and low alpha-2-macroglobulin. No other discordant results were reported. 13
Two small studies by Thabut et al. (2007a and 2003),13,125 which included only 66 and 58 patients with ALD, respectively, suggest that FibroTest can also distinguish between patients with and without PHt and, with less accuracy, between those with and without oesophageal varices. However, these studies were also carried out in populations with a high prevalence of those conditions, and indeed the investigators noted that, as 86% of the population of Thabut et al. ’s 2007a study13 had HVPG results indicating clinically significant PHt (HVPG > 12 mmHg), the study findings should not be used as a basis for recommending the use of FibroTest alone to predict severe PHt in cirrhotic patients.
The study by Naveau et al. 124 and, to a lesser extent, that by Thabut et al. 2007b126 suggest that FibroTest may also be able, with relatively low accuracy, to predict liver-related mortality and all-cause mortality (see Table 16). In Naveau et al. ’s124 cohort study, 85 patients (39%) died during the follow-up period: 42 (19%) of liver-related causes (haemorrhage, HCC, and decompensation) and 43 (20%) of non-liver-related causes. FibroTest was predictive of survival or non-liver-related mortality and, to a lesser degree, of overall mortality (for details, see Table 16). Details of 5- and 10-year survival according to baseline FibroTest values are presented in Tables 17 and 18. The baseline FibroTest and biopsy results were concordant for 38 (90%) of the 42 liver-related deaths (29 with cirrhosis, nine without cirrhosis) and discordant for only four (10%; two FPs of FibroTest, and one FP and one FN of biopsy). 124
| Baseline FT value | n | Liver-related death | Survival or non-liver-related death (%) (95% CI) | Death, any cause | Overall survival (%) (95% CI) |
|---|---|---|---|---|---|
| 0.00–0.31 | 81 | 1 | 98.7 (96.0 to 100) | 9 | 88.9 (82.0 to 95.7) |
| 0.32–0.58 | 43 | 3 | 92.1 (83.5 to 100) | 7 | 83.4 (72.1 to 94.6) |
| 0.59–1.00 | 94 | 28 | 68.3 (58.5 to 78.0) | 39 | 58.4 (48.4 to 68.4) |
| All | 218 | 32 | 84.5 (79.5 to 89.4) | 55 | 74.7 (68.9 to 80.5) |
| Baseline FT value | n | Liver-related death | Survival or non-liver-related death: (%) (95% CI) | Death, any cause | Overall survival (%) (95% CI) |
|---|---|---|---|---|---|
| 0.00–0.31 | 81 | 5 | 92.0 (84.9 to 99.0) | 21 | 71.4 (60.7 to 82.2) |
| 0.32–0.58 | 43 | 4 | 87.5 (75.5 to 99.5) | 12 | 69.8 (55.2 to 84.4) |
| 0.59–1.00 | 94 | 32 | 62.6 (52.2 to 73.1) | 50 | 42.4 (31.1 to 53.6) |
| All | 218 | 41 | 78.5 (72.4 to 84.6) | 83 | 58.8 (51.7 to 65.9) |
Naveau et al. ’s124 cohort study also provided information on subsequent alcohol consumption in patients enrolled in their 2005 study of test accuracy. 124 Only 21% (46/218) were known to be abstinent during the follow-up period; 50% (108/218) were not abstinent, and the status of the remaining 29% (64/218) was not known. 124 Unfortunately, the authors did not link these data with test results, and thus it was not possible to determine whether or not the test results had an impact on subsequent alcohol consumption, or whether or not alcohol consumption affected survival.
FibroScan: diagnostic accuracy results
The evidence base for FibroScan is slightly larger than that for FibroTest, deriving from a total of approximately 868 patients enrolled in nine small to medium-sized studies (the total number of participants is approximate because, as indicated in Table 13, it is not always clear how many of the eligible patients were in fact assessed). The evidence for test accuracy relative to liver biopsy is derived from a total of only 480 patients enrolled in the studies by Janssens et al. ,117 Kim et al. ,127 Melin et al. ,122 Mueller et al. ,118 Nahon et al. ,128 and Nguyen-Khac et al. 132 In only one of these, that by Janssens et al. ,117 are the liver biopsy samples known to have met the recommended minimum standards.
The evidence that FibroScan can distinguish patients with METAVIR stage F1–F4 fibrosis from those without fibrosis (F0), and those with F2–F4 fibrosis from those with stage F0–F1, is not robust, being based on only one fairly small study by Nguyen-Khac et al. 132 in a population with a high prevalence of stage F2–F4 fibrosis. However, there is more substantial evidence that FibroScan can distinguish patients with stage F3–F4 from those with stage F0–F2 fibrosis, and those with cirrhosis from those with stage F0–F3 fibrosis (Table 19).
| Condition of interest | Study | Number of assessed patients with known or suspected ALD | Prevalence of condition of interest, % | Threshold (kPa) | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | AUROC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mild–severe fibrosis (F1–F4) | Nguyen-Khac 2008132 | 103 | 92 | 5.9 | NR | 83 (75 to 90) | 86 (55 to 99) | 99 a (94 to 100) | 35 a (14 to 50) | 0.84 (0.73 to 0.95) |
| Moderate–severe fibrosis (F2–F4) | Nguyen-Khac 2008132 | 103 | 75 | 7.8 | NR | 80 (70 to 88) | 90.5 (78 to 98) | 97 (90 to 99) | 60 (45 to 74) | 0.91 (0.85 to 0.97) |
| Severe fibrosis (F3–F4) | Janssens 2009,119 2010117 | 49 | 65 | 9.5 | TP: 32; FP 17; FN: 0; TN: 0 | 100 (93 to 100) | 0 (0 to 14) | 65 (52 to 78) | Not calculable (0 to 100) | 0.77 |
| 13.9 | NR | 81 (66 to 93) | 59 (35 to 80) | 79 (64 to 91) | 63 (38 to 84) | |||||
| 15.8 | NR | 75 (59 to 88) | 70 (48 to 89) | 83 (67 to 94) | 60 (38 to 80) | |||||
| 16.5 | NR | 72 (55 to 86) | 70 (48 to 89) | 82 (66 to 94) | 57 (36 to 77) | |||||
| 17.0 | NR | 72 (55 to 86) | 77 (53 to 91) | 85 (69 to 95) | 59 (38 to 78) | |||||
| 17.3 | NR | 69 (52 to 83) | 77 (53 to 91) | 85 (67 to 95) | 57 (36 to 76) | |||||
| Kim 2009127 | 45 | 80 | NR | TP: 19; FP: 1; FN: 10; TN: 15 | 66 (48 to 81) | 94 (74 to 99) | 95 (79 to 99) | 60 (41 to 78) | 0.98 (0.94 to 1.0) | |
| Mueller 2010118 | 101 | 45 | 8.0 | NR | 91 (80 to 97) | 75 (63 to 85) | 75 (62 to 85) | 91 (81 to 97) | 0.91 ± 0.03 | |
| Nahon 2008128 | 147 | 75 | 11.6 | NR | 87 (80 to 93) | 89 (76 to 96) | 96 (91 to 99) | 70 (57 to 82) | 0.94 (0.90 to 0.97) | |
| Nguyen-Khac 2008132 | 103 | 51 | 11.0 | NR | 87 (76 to 94) | 80.5 (69 to 90) | 82 (71 to 91) | 84 (74 to 94) | 0.90 (0.82 to 0.97) | |
| Cirrhosis (F4) | Janssens 2010117 | 49 | 41 | 12.5 | NR | NR | NR | NR | NR | 0.86 |
| 19.6 | NR | 80 (59 to 93) | 76 (59 to 89) | 70 (50 to 86) | 85 (67 to 95) | |||||
| 21.1 | NR | 75 (54 to 91) | 80 (63 to 92) | 71 (51 to 88) | 82 (66 to 94) | |||||
| 23.5 | NR | 65 (43 to 84) | 83 (67 to 94) | 72 (50 to 90) | 77 (61 to 90) | |||||
| Kim 2009127 | 45 | 64 | 28.5 | NR | 90 (75 to 97) | 87 (66 to 97) | 93 (79 to 98) | 82 (60 to 95) | 0.97 (0.93 to 1.0) | |
| Melin 2005122 | 35 | 97 | 13.0 | TP: 34; FP: 1; FN: 0; TN: 0 | 100 (93 to 100) | 0 (0 to 85) | 97 (87 to 100) | Not calculable (0 to 100) | NR | |
| Mueller 2010118 | 101 | 26 | 11.5 | NR | 100 (91 to 100) | 77 (67 to 86) | 60 (46 to 74) | 100 (96 to 100) | 0.92 (0.87 to 0.97) | |
| 12.5 | TP:25; FP: 15; FN: 1; TN: 60b | 96 (83 to 100) | 80 (70 to 88) | 63b (47 to 77) | 98b (93 to 100) | |||||
| Nahon 2008128 | 147 | 54 | 22.7 | NR | 84 (75 to 91) | 83 (74 to 91) | 86 (77 to 93) | 81 (71 to 89) | 0.87 (0.81 to 0.93) | |
| Nguyen-Khac 2008132 | 103 | 32 | 12.8 | NR | 100 (93 to 100) | 75 (64 to 84) | 65 (51 to 77) | 100 (95 to 100) | 0.92 (0.87 to 0.98) | |
| 19.5 | NR | 86 (71 to 95) | 84 (75 to 92) | 72 (57 to 85) | 92 (85 to 97) | |||||
| HVPG ≥ 10 mmHg | Bureau 2008129 | ≤ 51 | 51 | 21.0 | NR | NR | NR | NR | NR | 0.91 (0.76 to 1.01) |
| Janssens 2010117 | 49 | Not clear | 17.0 | NR | 80 | 55 | NR | NR | 0.81 | |
| 24.7 | NR | 67 | 80 | NR | NR | |||||
| Lemoine 200897 | 48 | 83 | 34.9 | NR | 90 (78 to 97) | 88 (55 to 99) | 97 (88 to 100) | 64 (35 to 86) | 0.94 ± 0.03 | |
| HVPG ≥ 12 mmHg | Janssens 2010117 | 49 | Not clear | 25.7 | NR | 75 | 80 | NR | NR | 0.80 |
| Large oesophageal varices | Nguyen-Khac 2009130 | 103 | 25 | 47.2 | NR | 85 (67 to 95) | 64 (53 to 74) | 41 (13 to 58) | 94 (83 to 97) | 0.77 (0.68 to 0.85) |
The data presented in Table 19 illustrate the impact of different threshold scores on the sensitivity and the specificity of FibroScan. Castéra et al. 81 originally suggested that, in patients with chronic hepatitis C, the optimal FibroScan threshold values for the identification of significant (F2–F4) fibrosis, advanced (F3–F4) fibrosis, and cirrhosis (F4) were 7.1, 9.5, and 12.5 kPa, respectively (Table 20). These values were used by some of the studies included in this review and were found to be less appropriate for use in patients with ALD. Melin et al. 122 achieved 100% sensitivity using a threshold score of 13 kPa to identify cirrhosis in patients with ALD, but could not estimate the specificity associated with this threshold because patients with a score < 13 kPa did not undergo liver biopsy. Other investigators specifically sought to identify the optimal threshold scores for use in patients with ALD (see Table 19). Janssens et al. 117 noted that, in such patients, the threshold score of 9.5 kPa proposed for hepatitis C had 100% sensitivity for identifying severe (F3–F4) fibrosis, but a PPV of only 65%; it overestimated the degree of fibrosis in 17 of the 49 patients (35%) who underwent liver biopsy, and in all but one of them did so by two or more stages. Janssens et al. ,117 therefore, suggested that more appropriate thresholds for the identification of severe (F3–F4) fibrosis in ALD would lie between 15.8 and 17.3 kPa. They did not report the sensitivity and the specificity of a threshold score of 12.5 kPa for identifying cirrhosis (F4) in patients with ALD, but suggested that a more appropriate threshold would lie between 19.6 and 23.5 kPa, the exact choice depending on the preferred balance between sensitivity and specificity. 117 This study was not ideally designed to establish specificity because biopsy was offered only to patients with a FibroScan score of ≥ 9.5 kPa.
| Degree of fibrosis | Optimal cut-off (kPa) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| F2–F4 | 7.1 | 67 | 89 | 95 | 48 |
| F3–F4 | 9.5 | 73 | 91 | 87 | 81 |
| F4 | 12.5 | 87 | 91 | 77 | 95 |
Three studies discussed discordant results. 97,117,128 Nahon et al. 128 found that, relative to biopsy, FibroScan underestimated and overestimated the degree of fibrosis in approximately equal proportions of patients. Fourteen per cent (11/79) of those with histologically proven cirrhosis had FibroScan scores < 22.6 kPa, whereas 16% (11/68) of those with FibroScan scores > 22.6 kPa had biopsy results that did not indicate cirrhosis, although the majority (10/11) displayed extensive fibrosis. Janssens et al. 117 found that FibroScan overestimated the degree of fibrosis in 7 of the 11 patients with severe steatosis (by two stages in five patients and by one stage in two patients). Of the six patients in the study with alcoholic hepatitis, FibroScan classified three as having cirrhosis, although their biopsy results indicated F3 fibrosis. In two of the remaining three, both FibroScan and biopsy results indicated cirrhosis, thus removing the potential for overestimation by FibroScan. Finally, Lemoine et al. 97 noted one discordant case in a 70-year-old patient who had been totally abstinent from alcohol for 12 months and had no histological indication of alcoholic hepatitis. The patient’s FibroScan score was 38.10 ± 10 kPa, suggestive of PHt, although his HVPG measurement was only 8 mmHg.
Like Janssens et al. ,117 Mueller et al. 118 found that in patients with inflammatory hepatitis, liver stiffness was increased independently of the degree of fibrosis. They, therefore, found that the diagnostic accuracy of FibroScan improved when patients with laboratory signs of alcoholic steatohepatitis [i.e. serum glutamic oxaloacetic transaminase (SGOT) levels above 100 units/litre (U/L)] were excluded. When patients with only mildly elevated SGOT (> 50 U/L) were excluded, diagnostic accuracy improved in relation to F3–F4 fibrosis, but not in relation to F4 (cirrhosis) (Table 21).
| Disease severity and SGOT status | Included patients (n) | AUROC | Standard error | Threshold value (kPa) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| F4 | 101 | 0.921 | 0.03 | 11.5 | 100 | 77 |
| 12.5 | 96 | 80 | ||||
| F4 without SGOT > 100 U/L | 86 | 0.944 | 0.02 | 11.5 | 100 | 84 |
| 12.5 | 95 | 90 | ||||
| F4 without SGOT > 50 U/L | 66 | 0.945 | 0.03 | 10.4 | 100 | 87 |
| 12.5 | 92 | 91 | ||||
| F3–F4 | 101 | 0.914 | 0.03 | 8.0 | 91 | 75 |
| F3–F4 without SGOT > 100 U/L | 80 | 0.922 | 0.03 | 8.0 | 87 | 87 |
| F3–F4 without SGOT > 50 U/L | 67 | 0.946 | 0.03 | 8.0 | 100 | 84 |
Three small studies97,117,129 suggest that FibroScan can generally distinguish between patients with and without PHt, whereas one smallish study130 suggests that it can distinguish with less success between patients with and without large oesophageal varices (see Table 19).
There are no long-term data relating FibroScan results to survival or other clinical outcomes.
Adverse events and failure rates
The Enhanced Liver Fibrosis Test and FibroTest: failure rates and adverse events
Both the ELF test and FibroTest use blood samples obtained by standard venepuncture. None of the included studies reported adverse events relating to this process. However, a systematic review of studies of adverse events in adults undergoing simple venepuncture for diagnostic or screening purposes indicates that between 14% and 45% of patients undergoing such venepuncture suffer pain and bruising, whereas between 0.9% and 3.4% suffer vasovagal reactions. Potentially disabling nerve injuries occur but, fortunately, appear to be very rare (for full details, see Appendix 8).
There are no data relating to test failure rates for the ELF test or FibroTest specifically in patients with ALD. The only relevant data come from Naveau et al. ’s study123 of FibroTest in which, for unspecified reasons, serum samples were unavailable for 17% (50/292) of the enrolled patients. In Rosenberg et al. ’s study121 of the ELF test, 4.4% (45/1021) of patients overall had incomplete clinical details or biochemical samples, compared with 5.6% (55/976) whose biopsy samples were considered inadequate; figures relating specifically to patients with ALD were not presented.
FibroScan: failure rates and adverse events
Only two of the included studies commented on the acceptability of FibroScan to patients. 117,132 Janssens et al. 117 found that only 2% (5/255) of patients entering hospital for alcohol detoxification and rehabilitation refused FibroScan, whereas 29% (21/72) of those with FibroScan results indicative of severe fibrosis or cirrhosis-refused liver biopsy. However, Nguyen-Khac et al. 132 found that 34% (55/160) of patients refused to participate in their study, which involved both transient elastography and venepuncture; it is not clear whether this reluctance to participate related specifically to one or other of those interventions, or to the perceived inconvenience of undergoing both.
Some studies reported the proportion of patients with ALD in whom FibroScan was unsuccessful: this ranged between 4.4% and 8.6% (Table 22). Other studies reported the number of potential participants who were excluded because of either failure to obtain a valid result or the presence of obesity or other factors likely to affect FibroScan performance. In the study by Kim et al. ,127 11.8% of patients were excluded because of the factors likely to affect test performance. Although obesity is the most frequently reported reason for FibroScan failure, Bureau et al. 129 found that one-third of test failures (2/6) in patients with liver disease of varied aetiology could not be attributed to obesity; their cause remained unclear.
| Study | Exclusion criteria that might affect FibroScan performance | FibroScan unsuccessful: patients with ALD (%) | Reasons for failure |
|---|---|---|---|
| Janssens 2010117 | None reported | 11/250 (4.4) | Obesity or ascites |
| Kim 2009127 | BMI ≥ 30; probability of successful testing < 30%: 6/51 (11.8%) excluded for these reasons | None reported | None reported |
| Lemoine 200897 | None reported | None reported | None reported |
| Melin 2005122 | None reported | 18/245 (7.3) | Obesity |
| Mueller 2010118 | None reported | 5/106 (4.7) | Measurements invalid or interquartile range > 40% |
| Nahon 2008128 | None reported | 15/174 (8.6) | Results inadequate; no reason given |
| Nguyen-Khac 2008132 | Failure to obtain valid measurements: 2/105 (1.9%) excluded, no more specific reason given | None reported | None reported |
| Nguyen-Khac 2009130 | None reported | None reported | None reported |
Two studies that sought specifically to identify the features associated with successful FibroScan use did not meet the review’s inclusion criteria, but nonetheless provide useful information in this context. 79,139 The largest prospective study of this nature, by Castéra et al. ,79 assessed 13,369 examinations performed by seven operators over a 5-year period in 7261 adult patients with chronic liver disease of varied aetiology. It recorded the prevalence of failure of LSM (defined, in accordance with the manufacturer’s recommendation, as failure to obtain any value after at least 10 shots) and unreliable results (defined, again in accordance with the manufacturer’s recommendations, as < 10 successful shots, a success rate < 60%, or an interquartile range > 30% of the median value). In 18.4% of examinations (2466/13,369), valid results could not be obtained. LSM failed in 3.1% (420/13,369), whereas in 15.8% (2046/12,949) of the remaining examinations the results were deemed unreliable. Although the number of patients in whom FibroScan could be used successfully could be increased with repeated examinations, there remained some for whom it was impossible to obtain either any result or a reliable result after five attempts (Table 23). 79
| Number of examinations undergone by patient | Percentage of patients with | |
|---|---|---|
| LSM failure | Unreliable results | |
| 1 | 4.0 | 17.0 |
| 2 | 2.4 | 15.2 |
| 3 | 2.2 | 14.5 |
| 4 | 1.2 | 14.3 |
| 5 | 1.2 | 9.6 |
Castéra et al. 79 found that the factor most strongly associated with both test failure and unreliable results at the first FibroScan examination was a BMI > 30 kg/m2 (Table 24); the rates of both failure and unreliable results increased in parallel with the BMI (Table 25). The rates of both failure and unreliable results were also substantially raised when the operator had performed < 500 examinations (Table 26). Such a high threshold was chosen to define operator experience because all the operators who participated in the study had already performed at least 100 FibroScan examinations. The failure rate ranged from 0.2% in lean young non-diabetic patients to 20.9% in elderly obese diabetic patients, while the rate of unreliable results ranged from 7.2% in lean young men without diabetes or hypertension to 60.4% in elderly obese women with diabetes and hypertension.
| Factor | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Failure of LSM | |||
| BMI > 30 kg/m2 | 7.5 | 5.6 to 10.2 | 0.0001 |
| Operator experience < 500 examinations | 2.5 | 1.6 to 4.0 | 0.0001 |
| Age > 52 years | 2.3 | 1.6 to 3.2 | 0.0001 |
| Type 2 diabetes (fasting serum glucose > 5.6 mmol/L or ongoing anti-diabetic treatment) | 1.6 | 1.1 to 2.2 | 0.009 |
| Unreliable results | |||
| BMI > 30 kg/m2 | 3.3 | 2.8 to 4.0 | 0.0001 |
| Operator experience < 500 examinations | 3.1 | 2.4 to 3.9 | 0.0001 |
| Age > 52 years | 1.8 | 1.6 to 2.1 | 0.0001 |
| Female gender | 1.4 | 1.2 to 1.6 | 0.0001 |
| Hypertension (defined as ongoing hypertensive pharmacological treatment) | 1.3 | 1.1 to 1.5 | 0.003 |
| Type 2 diabetes (fasting serum glucose > 5.6 mmol/L or ongoing anti-diabetic treatment) | 1.2 | 1.0 to 1.5 | 0.05 |
| BMI (kg/m2) | LSM failure (%) (n) | Unreliable results (%) (n) |
|---|---|---|
| < 25 | 1.0 (4172) | 12.0 (4130) |
| ≥ 25 | 8.1 (3089) | 24.3 (2838) |
| ≥ 28 | 12.4 (1568) | 31.2 (1373) |
| ≥ 30 | 16.9 (967) | 35.4 (804) |
| ≥ 35 | 24.9 (225) | 39.1 (169) |
| ≥ 40 | 41.7 (40) | 53.6 (28) |
| Operator experience | LSM failure | Unreliable results |
|---|---|---|
| < 500 examinations (%) | 8.3 | 30.5 |
| > 500 examinations (%) | 3.5 | 15.6 |
| p-value | < 0.0001 | < 0.0001 |
In a smaller study, Kettaneh et al. 139 failed to achieve the manufacturer’s recommendation of at least 10 successful LSMs in 8.4% (79/935) of patients with chronic hepatitis C. They found that success was directly related to increased operator experience, and inversely related to both patient age and patient BMI. However, with hindsight, they suggested that the limiting factor was not so much BMI per se as the presence of a fatty thoracic belt that made it technically impossible to obtain accurate results.
A pilot study conducted specifically in patients with a BMI of ≥ 30 kg/m2 found that the use of the XL specialised probe, which can measure to a depth of 35–75 mm below the skin surface, reduced rates of failure and unreliable results. However, even with this probe, no value could be obtained in 12% of patients and the recommended standard of at least 10 valid measurements could be achieved in only 76%. 140 These findings are particularly important in the light of the high proportion of the UK population with a BMI of ≥ 30 kg/m2 or a raised waist circumference (see Chapter 1, Incidence and prevalence).
Discussion of clinical effectiveness
Diagnostic and prognostic accuracy
Summary of diagnostic and prognostic accuracy of the Enhanced Liver Fibrosis Test
No studies were identified that specifically assessed the ELF test. One study121 (n = 64 patients) evaluated the diagnostic accuracy of the European Liver Fibrosis Test (essentially the ELF test with the addition of age to the algorithm) relative to liver biopsy, in identifying moderate-to-severe fibrosis and cirrhosis in patients with known or suspected ALD. The study found that, at a threshold of 0.431, the ELF test identified moderate-to-severe fibrosis with a sensitivity of 93% (95% CI 87% to 97%) and a specificity of 100% (95% CI 93% to 100%). The sensitivity and specificity for cirrhosis were not reported, but were presumably lower: the point estimate of the AUROC for cirrhosis was lower than that for moderate-to-severe fibrosis (0.83 vs 0.94), although the CIs overlapped. As the evidence base is very small, and acceptable minimum standards were not used for the biopsy sample, these findings are not robust.
A follow-up study57 (n = 85 patients) suggested that the test had a predictive value in relation to both liver-related clinical outcomes and all-cause mortality, but did not report sensitivity and specificity. Again, the findings are not robust because the evidence base is so small.
Summary of diagnostic and prognostic accuracy of FibroTest
Three studies13,123,132 assessed the diagnostic and prognostic accuracy of FibroTest in identifying moderate-to-severe fibrosis and cirrhosis in patients with known or suspected ALD (n = 390 patients). The largest of these studies, that by Naveau et al. 123 (n = 221 patients), also had the most representative population. The study found that, using a threshold score of 0.30, FibroTest could identify moderate-to-severe (F2–F4) fibrosis with a sensitivity of 84% (95% CI 78% to 90%) and a specificity of 66% (95% CI 55% to 76%), whereas, using a threshold score of 0.70, it could identify cirrhosis with a sensitivity of 91% (95% CI 83% to 97%) and a specificity of 87% (95% CI 81% to 92%). Evidence for FibroTest’s ability to distinguish between patients with and without fibrosis (F1–4 vs F0) is not robust, being based on only one fairly small study by Nguyen-Khac et al. 132 (n = 103 patients) in a population in whom the prevalence of fibrosis or cirrhosis was 92%.
A small study by Thabut et al. 2007a13 (n = 66 patients) found that FibroTest could identify clinically significant PHt (HVPG ≥ 12 mmHg) with a sensitivity of 93% (95% CI 84% to 98%) and specificity of 87% (95% CI 55% to 99%). However, because of the high prevalence of the condition in the study population, the investigators felt that this finding should not be used to support the use of FibroTest alone to predict severe PHt in cirrhotic patients.
A second small study by Thabut et al. 2003125 (n = 58 patients) found that, using a threshold of 0.85, FibroTest could predict the presence of grade 2 oesophageal varices with a sensitivity of 89% (95% CI 73% to 96%) and a specificity of 50% (95% CI 25% to 77%).
Finally, a study by Thabut et al. 2007b126 (n = 189 patients) suggested that FibroTest has a modest predictive value in relation to all-cause mortality at 2 and 6 months (AUROCs 0.64 ± 0.05 and 0.58 ± 0.05, respectively); sensitivity and specificity were not reported. A study by Naveau et al. 124 (n = 218 patients) suggested that FibroTest had a somewhat better predictive value in relation to both liver-related and all-cause mortality at 5 years [AUROCs 0.79 (95% CI 0.68 to 0.86) and 0.69 (95% CI 0.61 to 0.76), respectively], but, again, sensitivity and specificity were not reported.
Summary of diagnostic and prognostic accuracy of FibroMAX
No evidence of the diagnostic and prognostic accuracy of FibroMAX was identified.
Summary of diagnostic and prognostic accuracy of FibroScan
Six studies117,118,122,127,128,132 assessed the diagnostic and prognostic accuracy of FibroScan in identifying moderate-to-severe fibrosis and cirrhosis in patients with known or suspected ALD (n = 480 patients). The study with the most representative population, that by Nahon et al. ,128 found that, using a threshold score of 11.6, FibroScan could identify severe (F3–F4) fibrosis with a sensitivity of 87% (95% CI 80% to 93%) and a specificity of 89% (95% CI 76% to 96%), whereas, using a threshold score of 22.7, it could identify cirrhosis with a sensitivity of 84% (95% CI 75% to 91%) and specificity of 83% (95% CI 74% to 91%). As with FibroTest, evidence for FibroScan’s ability to distinguish between patients with and without fibrosis (F1–4 vs F0) is not robust, being based only on one fairly small study by Nguyen-Khac et al. 132 (n = 103 patients) in a population with a 92% prevalence of fibrosis or cirrhosis.
Two of the included studies, those by Janssens et al. 117 and Mueller et al. ,118 indicate that FibroScan may overestimate the degree of fibrosis in patients with inflammatory hepatitis. This is consistent with Sagir et al. ’s141 finding that 15 out of 20 patients with acute hepatitis of varying causes had FibroScan results indicative of cirrhosis although they had no other signs of cirrhosis, and with Arena et al. ’s142 finding that, in patients with chronic hepatitis C, necroinflammatory activity identified at biopsy was associated with increased liver stiffness at each fibrosis stage except cirrhosis. However, unlike Janssens et al. ,117 Arena et al. 142 found that the degree of steatosis did not influence FibroScan results.
Three studies95,117,129 (n ≤ 148 patients) assessed FibroScan’s ability to identify PHt. Only one of these, that by Lemoine et al. ,97 reported sensitivity and specificity. The study found that FibroScan could identify clinically significant PHt (HPVG ≥ 10 mmHg) with a sensitivity of 90% (95% CI 78% to 97%) and a specificity of 88% (95% CI 55% to 99%). 97
A small study by Nguyen-Khac et al. 130 (n = 103 patients) found that, using a threshold of 47.2 kPa, FibroScan could predict the presence of large oesophageal varices with a sensitivity of 85% (95% CI 67% to 95%) and a specificity of 64% (95% CI 53% to 74%).
Discussion of diagnostic and prognostic accuracy of the Enhanced Liver Fibrosis Test, FibroTest and FibroScan
The evidence relating to the diagnostic accuracy of the ELF test, FibroTest, and FibroScan in relation to liver fibrosis and cirrhosis is not robust, and does not support any attempt to differentiate between their performances in this respect. As Naveau et al. 124 note, indirect comparisons between the results of different studies of test accuracy are particularly hazardous, not least because of interstudy variability both in the prevalence of different stages of fibrosis and in biopsy lengths. Only one study was identified that compared two different non-invasive tests with liver biopsy in the same patients: this was the relatively small study by Nguyen-Khac et al. ,132 which presented data relating to both FibroTest and FibroScan. In this study, although the point estimates of the AUROCs were higher for FibroScan than for FibroTest, the CIs overlap and, therefore, it is not possible to conclude that FibroScan has better diagnostic accuracy than FibroTest (Table 27).
| Test | Condition of interest | |||||||
|---|---|---|---|---|---|---|---|---|
| Mild–severe fibrosis (F0–F4) | Moderate–severe fibrosis (F2–F4) | Severe fibrosis (F3–F4) | Cirrhosis (F4) | |||||
| AUROC | 95% CI | AUROC | 95% CI | AUROC | 95% CI | AUROC | 95% CI | |
| FibroTest | 0.77 | 0.63 to 0.90 | 0.79 | 0.69 to 0.90 | 0.80 | 0.70 to 0.91 | 0.84 | 0.72 to 0.97 |
| FibroScan | 0.84 | 0.73 to 0.95 | 0.91 | 0.85 to 0.97 | 0.90 | 0.82 to 0.97 | 0.92 | 0.87 to 0.98 |
All studies that compare non-invasive tests with liver biopsy in patients with ALD and present information on the interquartile ranges around the median test scores for the different METAVIR stages (i.e. the studies of FibroTest by Nahon et al. 128 and Naveau et al. ,123 and the studies of FibroScan by Janssens et al. ,117 Kim et al. ,127 Mueller et al. 118 and Nguyen-Khac et al. 132), display a substantial degree of overlap between those interquartile ranges. Thus, for any individual patient, whatever the non-invasive test score, there will be substantial uncertainty regarding their true fibrosis stage. Although this uncertainty may perhaps be due less to deficiencies in the non-invasive tests themselves than to issues related to liver biopsy (e.g. the use of inadequate samples) or differences between patients in the degree of necroinflammation and steatosis,143 until well-designed studies are conducted that take these factors into account, the clinical utility of the tests is not apparent.
The evidence relating to the diagnostic accuracy of the ELF test, FibroTest, and FibroScan in relation to PHt and oesophageal varices is weaker than that relating to fibrosis and cirrhosis, as it rests on even smaller patient numbers. Moreover, the use of FibroScan to identify cirrhotic patients at high risk of oesophageal varices is said to be inappropriate because, although varices form only when PHt is present, neither the presence of varices nor their size is directly correlated with the degree of portal pressure elevation. 144
Patient management and clinical outcomes
No studies were identified that reported data relating to the effect of the use of any of the four tests on patient management or clinical outcomes.
Adverse effects and contraindications
The non-invasive tests included in this review appear to be safe. No adverse effects were reported in any of the included studies and no additional evidence has been identified that indicates that transient elastography is specifically associated with any adverse effects. As noted in The Enhanced Liver Fibrosis Test and FibroTest: failure rates and adverse events, the ELF test, FibroTest, and FibroMAX, which utilise blood tests, will be associated with the same adverse effects as diagnostic venepuncture generally – primarily pain and bruising, with occasional vasovagal reactions, and very rarely potentially disabling nerve injuries. By contrast, liver biopsy is associated with a high level of morbidity and occasional mortality (see Chapter 1, Liver biopsy).
No contraindications have been specified for the ELF test. The contraindications specified for FibroTest, FibroMAX, and FibroScan all relate to the mode of operation of the test, and do not relate to any potential for harm in patients with the relevant characteristics. Moreover, there is evidence to suggest that FibroScan is generally acceptable to patients with ALD. As noted in FibroScan: failure rates and adverse events, Janssens et al. 117 found that only 2% of patients entering hospital for alcohol detoxification and rehabilitation refused FibroScan, although 34% refused to participate in the study by Nguyen-Khac et al. 132 which required them to undergo both FibroScan and blood tests. Finally, in a study of acceptability, Melin et al. 145 found that all 380 patients seen for alcohol problems during the course of a year agreed to undergo FibroScan; only 5% (2/44) of those who were offered liver biopsy because their FibroScan result indicated severe fibrosis or cirrhosis refused it, compared with 29% in the study by Janssens et al. 117
Internal and external validity
The results of the included studies summarised above suggest that the ELF test, FibroTest, and FibroScan can be used to identify patients with ALD who have fibrosis or cirrhosis. However, these results should be viewed with caution for a number of reasons. The most obvious reason is that they are not robust because they rest on data from relatively few patients with ALD; this is especially true of the ELF test.
Internal validity
As noted in Study quality above, study quality, as assessed using a modified version of the QUADAS checklist,112 is generally not high.
Most of the studies display spectrum bias because they recruited patients believed or known to have severe fibrosis or cirrhosis, rather than those representative of the whole spectrum of patients with suspected ALD. Such spectrum bias favours the index test: because the positive and negative predictive values of diagnostic tests depend critically on the prevalence of the condition being tested for in the population being tested,146 if the prevalence is considerably higher than would be expected in normal clinical practice, then the positive predictive value of the test will also be higher than it would be in normal clinical practice. Consequently, even if the studies indicate that the tests have high sensitivity and specificity, in normal use many of the positive results will be FPs. 146 Moreover, two of the studies that recruited a more representative patient sample, those by Janssens et al. 117 and Melin et al. ,122 used the reference standard only in those patients whose index test result was above a specific threshold. This use of the reference standard only in patients testing positive using the index test (verification bias) will result in overestimation of its sensitivity because the number of FN results is too low. 147 In the context of liver fibrosis, both spectrum bias and verification bias are probably due to valid ethical issues surrounding the use of biopsy in patients in whom it is not considered clinically necessary; however, they distort study results in such a way as to favour the index tests.
Conversely, however, studies that compare a non-invasive test with liver biopsy are disadvantaged by the fact that it is an imperfect reference standard; thus, discordance between the degree of fibrosis indicated by biopsy and by non-invasive testing may be because of an error in either test. Mehta et al. ,148 noted that liver biopsy is associated with such a degree of potential error that its use as the reference standard may make it impossible to differentiate between a perfect and an inadequate surrogate test. They calculated that, assuming that liver biopsy has a sensitivity and a specificity of 90% for the identification of significant liver fibrosis and that the prevalence of that condition in the population being tested is 40%, a perfect non-invasive test with an AUROC of 0.99 versus true disease can only achieve an AUROC of 0.90 versus liver biopsy. Indeed, Afdhal et al. 19 suggest that liver biopsy has a diagnostic accuracy of 80–90% and, in that case, any tests that are compared with liver biopsy cannot achieve an AUROC better than 0.9, and the results are likely to lie in the range 0.75–0.88, with a most likely value of 0.85. Thus, even if a non-invasive test is in fact a perfect non-invasive surrogate for liver biopsy, it may be impossible to prove this. 19
The use of liver biopsy as the reference standard is associated with a second problem. The non-invasive tests reviewed in this report all present a numeric result relating to a continuous measurement that is held to reflect, directly or indirectly, the degree of fibrosis in the liver. However, this result is then compared with a liver biopsy result expressed in terms of an ordinal scoring system: i.e. biopsy results are classified into a number of groups that have a natural ordering, in that they indicate progressively more severe liver damage, but do not represent a direct arithmetical progression. For example, the degree of fibrosis seen in METAVIR stage F4 is not necessarily twice that seen in METAVIR stage F2; instead, the different stages describe the pattern of deposition of fibrous tissue, as well as its extent. 149 Consequently, to permit comparison with liver biopsy results, a threshold value corresponding to each biopsy stage must be identified for each non-invasive test. In most of the included studies, the threshold values recommended as appropriate for the identification of the different stages of fibrosis and cirrhosis in patients with ALD have been derived statistically from the receiver operating characteristic curve after data collection. They have not been validated prospectively and, therefore, do not fulfil the standard criteria for the general use of a diagnostic test. 138
External validity
It is difficult to comment on the external validity of the included studies – i.e. the extent to which their populations and methods are generalisable to clinical practice in the UK – not least because of the lack of clarity surrounding the potential role of NILTs in clinical practice in the UK. The issues relate to the population in whom, and the purpose for which, such tests may be used; they are to some extent related.
In the original scope of this assessment, it was envisaged that NILTs would be used in primary care to enable more appropriate selection of patients with abnormal liver function tests and risk factors for chronic liver disease for referral to specialist care. By contrast, the included studies were conducted in secondary or tertiary care settings. Subsequently, clinical experts in the UK have suggested that it is unlikely, and possibly undesirable, that non-invasive tests will be used in primary care, and that most patients who are felt to need further investigation for suspected ALD should be referred to specialist care, where non-invasive tests will be performed if considered appropriate. However, even given this scenario, the range of disease severity is likely to be wider than that seen in the included studies, many of which were limited to patients believed to have relatively severe disease. Indeed, a number of studies recruited patients who not only required liver biopsy for clinical reasons but in whom that biopsy was performed transjugularly rather than percutaneously,13 suggesting the presence of decompensated cirrhosis.
In ALD, NILTs may be used for one of two main diagnostic purposes:
-
to identify patients with fibrosis, so that efforts may be made to prevent the development of cirrhosis
-
to identify patients with cirrhosis, enabling them to be monitored for the development of conditions such as oesophageal varices and HCC.
Assuming that non-invasive test results indicative of fibrosis are effective in influencing patients with ALD to abstain from alcohol – and no evidence for this has been identified – then the former use is of potentially greater clinical value as it would permit the identification of patients with ALD at a time when that disease was still reversible, whereas identification of patients with cirrhosis would only permit the initiation of monitoring to enable prompt treatment of symptoms of an incurable disease. However, only one study, that by Nguyen-Khac et al. ,132 reported on the ability of a non-invasive test to identify mild (METAVIR F1) as well as more severe (F2–F4) fibrosis in patients with suspected ALD, and in most studies the tests performed better when identifying cirrhosis (F4) than when identifying mild (F1), moderate (F2), or severe (F3) fibrosis. This clearly limits the clinical utility of the tests.
In tests that present results derived from a continuous scale, the intended purpose of that test will affect the choice of the threshold score. So, if the intended purpose of the NILTs reviewed in this report is to identify patients with cirrhosis to undergo further tests and monitoring, a threshold score should be chosen that maximises sensitivity (i.e. the proportion of patients who genuinely have the condition of interest who are correctly identified by the non-invasive test), as this will minimise the risk of patients with cirrhosis being mistakenly identified as not having the condition, and therefore not receiving further tests, monitoring, and treatment, as appropriate. However, if the intended purpose of the tests is to exclude patients without fibrosis, the threshold score should be chosen to maximise specificity (i.e. the proportion of people who genuinely do not have the condition of interest who are correctly identified by the non-invasive test), to reduce the risk of patients who do not have fibrosis undergoing costly and potentially invasive tests.
Test results may be influenced by factors other than the degree of fibrosis present in the liver. The included studies have shown that, for FibroScan, the optimum threshold values for fibrosis and cirrhosis are higher in patients with ALD than in patients with hepatitis C (and possibly other liver diseases), and it is therefore crucial that the aetiology of suspected liver disease is securely established before the test result is interpreted. 18 In addition, as noted in FibroScan: diagnostic accuracy results, in patients with a secure diagnosis of ALD, FibroScan may overestimate the degree of fibrosis if either steatosis or alcoholic hepatitis is present. Current drinking status is also relevant: Mueller et al. 118 have shown that, in patients with ALD, liver stiffness, as measured by FibroScan, decreases during alcohol detoxification independent of the fibrosis stage. Thus, consideration must also be given to the optimum timing of the tests.
Finally, it should be noted that, unlike liver biopsy, the non-invasive tests assessed in this report only seek to identify the degree of liver fibrosis. They cannot also provide additional useful information, for example by indicating the presence of another liver disease in addition to ALD, or by evaluating necroinflammation to assess whether that fibrosis is an ongoing process that may continue to develop or whether it results from a past event that has stabilised or even regressed. 104
Conclusions for clinical effectiveness
There is some evidence to suggest that, in patients with known or suspected ALD, the ELF test, FibroTest, and FibroScan can identify fibrosis with varying degrees of diagnostic accuracy; no evidence has been identified relating to FibroMAX, although this is recommended by the manufacturers in preference to FibroTest in patients with ALD. Although FibroTest and FibroScan appear to have greater accuracy in identifying cirrhosis rather than lesser degrees of fibrosis, the ELF test appears to perform less well in specifically identifying cirrhosis than in identifying the presence of moderate-to-severe fibrosis but, as the evidence base is very small and acceptable minimum standards were not used for the biopsy samples, this finding is not robust. Evidence for the ability of FibroTest and FibroScan to identify clinically significant PHt, and oesophageal varices, rests on extremely small studies, and again is not robust.
Moreover, the confidence that can be placed in the study results is reduced because most of the studies display spectrum bias, and the two studies that recruited a more representative sample display verification bias: both of these biases will favour the index test. In addition, the degree of error associated with liver biopsy is such that its use as the reference standard may make it impossible to judge with accuracy the adequacy of the surrogate test. Finally, the degree of overlap between the interquartile ranges around the median values relating to each METAVIR stage means that, for any individual patient, whatever their non-invasive test score, there will be substantial uncertainty regarding their true fibrosis stage, and this will substantially limit the clinical utility of the non-invasive tests.
Chapter 5 Cost-effectiveness: model parameters
This chapter details the parameters within the mathematical model and the sources used to provide the values assumed in the analyses. There was a considerable number of data that were not available and broad assumptions have been made to allow an estimation of the range of the potential cost-effectiveness of each NILT after considering the management and likely life expectancy of a patient following diagnosis. Sensitivity analyses have been performed in order to test the robustness of the results produced to changes in the input parameters.
Discount rates
In accordance with the NICE methods guide,150 both benefits and costs have been discounted at a rate of 3.5% per annum.
The sensitivity and specificity of non-invasive liver tests used within the economic model
As detailed, the sensitivity of each NILT, alongside that of biopsy, will determine the number of patients who have cirrhosis who are not appropriately diagnosed. The specificity of the NILT will determine the number of patients who may receive unnecessary biopsy or, if a triaging strategy is not pursued, the number of patients who are monitored unnecessarily. The advantage of a NILT is that fewer biopsies will be performed than in assumed current practice, which will be associated with reduced costs, and also reduced mortality and morbidity.
The estimated sensitivity and specificity for each NILT have been described previously in Tables 15, 16 and 19. No formal meta-analysis has been undertaken because of the potential heterogeneity within the trials in terms of the length of and the number of portal tracts examined within the liver biopsy, the number of days between the test and performing the biopsy, the level of current drinking within the cohort and the different cut-off thresholds for diagnosing cirrhosis/fibrosis. In order to provide an estimation of the cost-effectiveness of the NILTs, three scenarios for sensitivity and specificity are evaluated, which the authors have selected from the combinations of RCTs and cut-off thresholds for classification of cirrhosis.
These results will be indicative of the likely cost-effectiveness, although some caveats must be provided. These include:
-
The fact that the sensitivity and specificities reported are directly calculated from the trial data, whereas ideally the results would be calculated from a threshold that was specified in advance of the trial.
-
Inconsistency between reported trial data, for example, considering FibroTest, the study by Naveau et al. 124 has both a higher sensitivity and specificity at a 0.70 threshold than the study by Thabut et al. , 2007a13 which used a threshold of 0.74.
-
Small patient numbers and their fibrosis levels mean that there is reasonably large uncertainty. Where values for TPs, FNs, TNs and FPs were < 5, a non-informative prior of 0.5 was added to that value and the corresponding value when calculating sensitivity and specificity, which is equivalent to using Jeffreys’ prior. As such, the values for sensitivity and specificity may not match exactly those reported in the systematic review section.
-
The fact that the ELF did not report sensitivity and specificity for detecting only cirrhosis and that these have been inferred from a moderate/severe fibrosis population.
In addition to these caveats, there is the possibility that biopsy may not be a perfect gold standard and that sampling error may result in a FN being indicated by the test. As previously reported, data obtained when comparing liver biopsy with post-mortem examination showed that cirrhosis was detected in only 16 out of 20 cases when a single biopsy was taken, although this increased to 20 out of 20 when three biopsies were taken. 91 Further data show that when three biopsies were taken using the same entry site the sensitivity was 50%. 92 Exploratory results have been undertaken assuming that the sensitivity of biopsy in detecting cirrhosis is 80% rather than 100%. In this instance, further assumptions need to be made as the NILTs were compared with biopsy. Three scenarios have been explored, in a similar manner as previously used by Carlson et al. 151
-
That the NILTs also failed to detect the cirrhoses missed by the biopsy. This is termed a pessimistic scenario.
-
That the NILTs detected the cirrhoses missed by the biopsy, but these were recorded in the study as FPs. This is termed an optimistic scenario.
-
That the NILTs also failed to detect a proportion of the cirrhosis missed by the biopsy, with the remaining proportion being recorded as FPs. The proportion also missed would be assumed to be 1 – sensitivity reported in the trial. This is termed a stochastic scenario.
An example of this methodology is given below using data for detecting cirrhosis by FibroTest as reported by Naveau et al. 123 This trial reported (compared with a gold standard of biopsy) 62 TPs, 6 FNs, 133 TNs and 20 FPs, with a sensitivity of 91%. Biopsy detected 68 positives, but if the sensitivity of biopsy was 80% then biopsy would be expected to miss 17 (68/4) actual positives.
In the pessimistic scenario it would be assumed that 17 cases of TNs were actually FNs. Thus, the true distribution was 62 TPs, 23 FNs, 116 TNs and 20 FPs.
In the optimistic scenario it would be assumed that 17 cases of FNs were actually TPs. Thus, the true distribution was 79 TPs, 6 FNs, 133 TNs and 3 FPs.
In the stochastic scenario, it would be assumed that 91% of the 17 cases missed by biopsy would be detected and were classed as FPs; this number is rounded to the nearest integer and down if equidistant. This would result in 15 being initially recorded as FPs and 2 as TNs. Thus, the true distribution was 77 TPs, 8 FNs, 131 TNs and 5 FPs.
This approach was used for all test scenarios. In circumstances where the results could not meet the decision rules, for example if there were only three FNs but it was expected that biopsy would miss 10 that would all be diagnosed correctly in the optimistic scenario, then the maximum number that could be transferred from FPs to TPs would be transferred, which would be three in this example. When calculating the resultant sensitivities and specificities, if values for TPs, FNs, TNs and FPs were below 5, an uninformative prior of 0.5 was added to that value and the corresponding value when calculating sensitivity and specificity.
The sensitivities and specificities used in the model for each scenario are provided in Tables 28–31. It is noted that where there are few patients that biopsy rated as without cirrhosis, for instance in Thabut et al. 2007a,13 where only six patients were diagnosed as not cirrhotic by biopsy, the fluctuations in test characteristics can be large, and that in the pessimistic scenarios the sensitivities of the NILTs may become better than that for biopsy.
| NILT | Scenario | Calculated from | Threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| ELF | 1 | Rosenberg 2004121 | 0.431 | 92.4 | 97.4 |
| 2 | Rosenberg 2004121 | 0.087 | 98.9 | 18.4 | |
| 3 | Authors’ estimate correcting for a cirrhotic population only | 0.431 | 96.0 | 90.0 | |
| FibroTest | 1 | Naveau 2005123 | 0.70 | 91.2 | 86.9 |
| 2 | Naveau 2005123 | 0.30 | 99.3 | 50.3 | |
| 3 | Thabut 200713 | 0.74 | 78.3 | 78.6 | |
| FibroScan | 1 | Mueller 2010118 | 11.5 | 98.1 | 77.3 |
| 2 | Janssens 2010117 | 19.6 | 78.6 | 75.9 | |
| 3 | Nahon 2008128 | 22.7 | 83.5 | 83.6 | |
| Biopsy | – | Assumption | N/A | 100.0 | 100.0 |
| NILT | Scenario | Calculated from | Threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| ELF | 1 | Rosenberg 2004121 | 0.431 | 75.0 | 93.8 |
| 2 | Rosenberg 2004121 | 0.087 | 92.9 | 3.1 | |
| 3 | Authors’ estimate correcting for a cirrhotic population only | 0.431 | 78.0 | 87.0 | |
| FibroTest | 1 | Naveau 2005123 | 0.70 | 72.9 | 85.3 |
| 2 | Naveau 2005123 | 0.30 | 80.0 | 44.1 | |
| 3 | Thabut 200713 | 0.74 | 78.3 | 25.0 | |
| FibroScan | 1 | Mueller 2010118 | 11.5 | 81.3 | 75.3 |
| 2 | Janssens 2010117 | 19.6 | 64.0 | 70.8 | |
| 3 | Nahon 2008128 | 22.7 | 66.7 | 76.6 | |
| Biopsy | – | Assumption | N/A | 80.0 | 100.0 |
| NILT | Scenario | Calculated from | Threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| ELF | 1 | Rosenberg 2004121 | 0.431 | 92.4 | 97.4 |
| 2 | Rosenberg 2004121 | 0.087 | 99.1 | 43.8 | |
| 3 | Authors’ estimate correcting for a cirrhotic population only | 0.431 | 95.0 | 92.0 | |
| FibroTest | 1 | Naveau 2005123 | 0.70 | 92.9 | 97.4 |
| 2 | Naveau 2005123 | 0.30 | 99.4 | 56.6 | |
| 3 | Thabut 200713 | 0.74 | 78.7 | 91.7 | |
| FibroScan | 1 | Mueller 2010118 | 11.5 | 98.5 | 84.1 |
| 2 | Janssens 2010117 | 19.6 | 90.0 | 82.7 | |
| 3 | Nahon 2008128 | 22.7 | 85.6 | 99.1 | |
| Biopsy | – | Assumption | N/A | 80.0 | 100.0 |
| NILT | Scenario | Calculated from | Threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| ELF | 1 | Rosenberg 2004121 | 0.431 | 90.4 | 97.2 |
| 2 | Rosenberg 2004121 | 0.087 | 99.1 | 43.8 | |
| 3 | Authors’ estimate correcting for a cirrhotic population only | 0.431 | 94.0 | 90.0 | |
| FibroTest | 1 | Naveau 2005123 | 0.70 | 90.6 | 96.3 |
| 2 | Naveau 2005123 | 0.30 | 99.4 | 56.6 | |
| 3 | Thabut 200713 | 0.74 | 75.0 | 84.3 | |
| FibroScan | 1 | Mueller 2010118 | 11.5 | 98.5 | 84.1 |
| 2 | Janssens 2010117 | 19.6 | 80.0 | 86.0 | |
| 3 | Nahon 2008128 | 22.7 | 82.8 | 99.1 | |
| Biopsy | – | Assumption | N/A | 80.0 | 100.0 |
For all scenarios, the sensitivity of clinical experience alone was 81.3% and specificity was 89.2%. 9 For diagnosing all patients with cirrhosis sensitivity was 100% and specificity 0%.
Examination of Table 31, where a stochastic scenario has been assumed, shows that this is often identical or close to those predicted in the optimistic scenario (see Table 30), which produces results that are more favourable to the tests. Given this, for brevity reasons it was decided that results would not be produced using the stochastic scenario, with the authors comfortable that the remaining scenarios (100% sensitivity for biopsy, pessimistic and optimistic) provided a good indication of the uncertainty within the decision.
The prevalence of cirrhosis in the defined population
In addition to the test characteristics, the prevalence of cirrhosis in people whom a secondary care clinician would want to biopsy is needed to determine the absolute number of TPs, FPs, TNs and FNs. Based on clinical advice this value has been set to 35%.
The costs of biopsy and each non-invasive liver test
The costs used within the model are provided in Table 32. These have been inflated, where applicable, using the inflation indices reported in Curtis. 152 These costs are deemed additional to standard clinical practice.
| Test | Cost (£) | Source |
|---|---|---|
| Percutaneous biopsy | 894 | Stamuli 200956 |
| Transjugular biopsy | 1500 | An indicative figure for a biopsy requiring an overnight stay and possible transportation costs9 |
| ELF | 45 | Clinical input. This value comes from an early adopter quote provider to the Royal Free Hospital, London, UK for 100 ELFs per month. (Dr Marsha Morgan, Royal Free Hospital, London, UK, 2010, personal communication) |
| FibroScan (marginal cost) | 50 | Clinical input suggests that this is likely to be the price charged to the NHS per scan. This is preferred to the Stamuli 200956 estimated cost of £19.52 (range £12.44–33.94) |
| FibroTest | 50 | Set similar to the cost of the ELF as both are blood tests and are likely to be competitively priced. This estimate is preferred to a value of €90–300 reported by Morra 200767 |
| Clinical experience | 0 | Assumption |
| Diagnosing all with cirrhosis | 0 | Assumption |
Sensitivity analyses were undertaken to examine the robustness of the results to changes in the prices of diagnostic tests.
Adverse events related to each diagnostic test
It has been assumed that no NILT has adverse events, aside from a potential misdiagnosis of cirrhosis. Biopsy, however, owing to its invasive nature, is associated with both mortality and morbidity. From the systematic review undertaken in this study, we have assumed that percutaneous biopsy has a probability of 0.09% of causing mortality, with an additional risk of causing a serious adverse event of 0.72% (see Appendix 3). The corresponding values for transjugular biopsy are 0.18% and 1.27%, respectively (see Appendix 3).
A serious adverse event was deemed to be associated with a hospital stay, assumed to cost £1000, and a QALY decrement of 0.2 (equivalent to approximately 10 weeks with a utility of zero or a year with a utility decrement of 0.2). The QALY value was arbitrary, but was assumed to be a value that would be likely to disfavour biopsy; sensitivity analyses were undertaken to assess the robustness of the results to changes in both the costs and QALY decrements assumed to be related to serious adverse events.
Applying the base-case values to the risk of a serious adverse event results in an expected QALY decrement per patient of 0.000142 for a percutaneous biopsy and 0.000254 for a transjugular biopsy; the cost implications per patient would be £7 for a percutaneous biopsy and £13 for a transjugular biopsy. For all patients who die as a result of biopsy, the costs of the biopsy are assumed to be incurred, but no further QALYs will be accrued. It is uncertain whether or not patients undergoing a biopsy will suffer anxiety prior to the procedure; in order to address this issue and to assess the robustness of the results to this assumption, a sensitivity analysis was performed where the disutility associated with a biopsy was increased to 0.04 QALY (a value equivalent to approximately a fortnight with a utility of zero), which was deemed in consultations with clinicians to be an upper bound.
The proportion of tests that will produce results that cannot be used
The results of the NILTs can be confounded by patient characteristics such as obesity and concurrent drinking. This has been reflected within the model by assuming that the rates of tests that cannot be used are 20% for FibroScan and 25% for both blood tests (FibroTest and the ELF). The model assumes that when a test has produced a result that cannot be used, the patient will then receive a biopsy.
The outcomes associated with each final node within the economic model for diagnosis of cirrhosis
Figures 1–3 detail the assumed pathways within the model. These strategies have four common end points: (1) abstinent following a diagnosis of cirrhosis; (2) continuing to drink following a diagnosis of cirrhosis; (3) abstinent following a diagnosis of no cirrhosis; and (4) continuing to drink following a diagnosis of no cirrhosis. Those strategies that incorporate a biopsy also have the risk of biopsy mortality. Excluding biopsy mortality, these common end points represent an amalgamation of heterogeneous patient experiences into a long-term estimation of costs and QALYs. These end points are also further broken down into whether or not the diagnosis received was correct, which will differ depending on whether or not the diagnosis regarding cirrhosis is correct, increasing the actual number of end points within the model to eight.
Although not depicted within the model diagram, a proportion of patients who do not have cirrhosis at the time of the investigation but who continue to drink heavily are assumed to develop cirrhosis. These patients will be assigned the costs and QALYs associated with cirrhotic patients who continue to drink heavily rather than non-cirrhotic patients who continue to drink heavily.
Because of the likely heterogeneity of patients within each of the eight end points, the reliability of any estimate will be questionable. To populate the model we have collated data from various sources, including clinical input, and provided relatively broad estimates to guard against spurious accuracy. The sensitivity of the modelled results to these values is tested within sensitivity analyses. Each end point is discussed individually and, for reference, the cost and QALY values for each of the end points are collated in Table 33.
| End point | Costs (£) | QALYs |
|---|---|---|
| TPs for cirrhosis who abstain from drinking | 29,980 | 9.679 |
| TPs for cirrhosis who continue drinking | 39,474 | 4.399 |
| FPs for cirrhosis who abstain from drinking | 25,154 | 11.066 |
| FPs for cirrhosis who continue drinkinga | 25,154 | 11.066 |
| TNs for cirrhosis who abstain from drinking | 1000 | 11.066 |
| TNs for cirrhosis who continue drinkinga | 1000 | 11.066 |
| FNs for cirrhosis who abstain from drinking | 26,100 | 9.359 |
| FNs for cirrhosis who continue drinking | 36,100 | 3.744 |
End point 1: true-positives for cirrhosis who abstain from drinking
These patients will receive monitoring for HCC, HE and oesophageal varices. The estimated costs and QALYs associated with patients who are screened for HCC have been taken from a study by Thompson Coon et al. ,153 which reports that ALD patients with cirrhosis undergoing annual serum α-fetoprotein and 6-monthly ultrasound scans were estimated to accrue 9.410 QALYs at a cost of £27,400. This source was selected as it came from a health technology assessment that had explicitly divided the cost of surveillance for HCC into aetiology types, allowing values for ALD patients to be explicitly used.
End point 2: true-positives for cirrhosis who continue drinking
Clinical advice indicates that such patients will still receive monitoring for HCC, HE and varices despite their non-abstinence. Clinical advice also indicates that the costs associated with such patients will be much higher as the risk of progressing to more severe states, such as decompensated cirrhosis, is greater in the non-abstinent, as is the risk of HE and ascites. The literature has suggested that the cost per annum of decompensated cirrhosis is £9385 compared with £1171 per annum for compensated cirrhosis. 154 The costs of HE are £2718 per event (Code AA22Z),155 and the treatment of ascites has been reported to cost US$4048 per annum. 156 All historic costs have been inflated to 2008–9 costs. 152 The sources of these costs were selected as these were also used in the Thompson Coon et al. study153 to provide consistency. It was assumed that these costs would approximate to an additional £10,000 per person when compared with TPs who were abstinence and a cost of £37,400 was used.
For QALYs accrued, a study21 reported that the survival rate at 7 years for patients with cirrhosis was 72% for abstainers and 44% for those who continued to drink. For simplicity, it was assumed that these were results from exponential distributions; this would indicate that cirrhotic patients who continued to drink would have (on average) only 40% of the life expectancy of cirrhotic patients who abstained. Assuming that this value could also be used to downgrade QALYs, it is estimated that the QALYs accrued by TPs who continued to drink would be 3.764 (9.410 × 40%).
End point 3: false-positives for cirrhosis who abstain from drinking
These patients will receive monitoring for HCC, HE and oesophageal varices. The costs for these patients have been estimated from the costs of TPs who abstain (£27,400), but with a consideration that FPs are likely to incur fewer costs because of avoiding cirrhosis. It is assumed that FPs would be representative of the group termed by Wright et al. as having moderate disease,154 which was defined as having either a fibrosis score of between 3 and 5 or a necroinflammatory score of > 3. The cost per annum was £421 less in the group with moderate disease than in those with cirrhosis, a difference which has been increased to £469 per annum to incorporate inflation. Data from Wright et al. 154 were selected, as these were also used in the Thompson Coon et al. study153 and provided some consistency.
It is assumed that these costs would be applied for 20 years, resulting in a discounted cost reduction of £6899 and an overall cost of approximately £20,500. In the absence of further data, we have assumed that the QALYs gained will be equivalent to that of TNs, 11.066, using the methodology described later (see End point 5: true-negatives for cirrhosis who abstain from drinking).
End point 4: false-positives for cirrhosis who continue drinking
Few data are available for FPs who continue to drink. We have conservatively assumed that, for the majority of patients, the costs are equivalent for FPs who abstain (£20,500), as are the QALYs accrued (11.066). However, to acknowledge the fact that such patients may develop cirrhosis, a proportion are modelled to have the cost and QALY implications associated with TPs who continue to drink rather than those for FPs.
End point 5: true-negatives for cirrhosis who abstain from drinking
These patients will receive lifestyle advice only. A cost of £1000 per patient was estimated (based on clinical advice), to account for appropriate monitoring of the patient. In order to estimate the QALYs accrued we have assumed that the patients are the same age as those in Thompson Coon et al. 153 (53 years) and that they will have a reduced life expectancy compared with general population, but will live for a further 20 years. Using European Quality of Life-5 Dimensions (EQ-5D) population norms157 and assuming discounting at 3.5% per annum,150 an estimate of 11.066 QALYs was derived.
End point 6: true-negatives for cirrhosis who continue drinking
These patients will receive lifestyle advice only. We have assumed that the costs and QALYs accrued are the same as for TNs who are abstinent (£1000 and 11.066, respectively). It has been assumed that such patients may develop cirrhosis; thus, a proportion are modelled to have the cost and QALY implications associated with TPs who continue to drink rather than those for TNs.
Endpoint 7: false-negatives for cirrhosis who abstain from drinking
These patients will receive lifestyle advice only. The estimated costs and QALYs associated with ALD patients with cirrhosis who are not screened for HCC have been taken from a study by Thompson Coon et al. ,153 which reports that patients were estimated to accrue 9.359 QALYs at a cost of £26,100.
End point 8: false-negatives for cirrhosis who continue drinking
These patients will receive lifestyle advice only. It is uncertain how much additional cost would be incurred by this group compared with FNs who abstain; however, following the logic described for TPs, a value of £10,000 was assumed, resulting in a cost of £36,100. The QALYs accrued have been set at 40% of the level of FNs who abstain, using the reasoning described in the ‘TPs who continue drinking’ section. This would equate to 3.744 QALYs (9.359 × 40%).
The proportion of patients without cirrhosis who continue to drink heavily that will develop cirrhosis
On clinical advice this has been set to 20%.
Incorporating oesophageal varices bleeding
The analyses of screening for HCC undertaken by Thompson Coon et al. 153 did not include any costs or consequences associated with oesophageal varices bleeding. However, these can have significant morbidity and can also cause mortality. This has been included within the modelling by estimating the effects and costs associated with screening for varices, providing prophylaxis treatment where appropriate, and with bleeds in a separate model and then including these within our analyses. The varices model used the following assumptions, which were based, where possible, on recommendations provided by de Franchis. 158
-
All patients with cirrhosis would receive an endoscopy at a cost of £200 (data from the Royal Free Hospital, London, UK).
-
Forty per cent of those with cirrhosis will have varices,43 of which one-third are small, one-third moderate and one-third large (clinical advice).
-
The risk of bleeding in those who continue to drink is high, with a risk of 15% per annum,156,159 although this risk is reduced by 50% if prophylactic treatment is provided. 159
-
On clinical advice, the risk of bleeding among those who abstain was reduced to 5%, with a reduced risk of 2.5% in those taking prophylactic treatment.
-
Only those patients who have been diagnosed (correctly or incorrectly) with cirrhosis will be provided with prophylactic treatment. Patients with cirrhosis who were not correctly diagnosed will be provided with prophylactic treatment following an oesophageal varices bleed.
-
Prophylactic treatment with propranolol 40 mg twice daily costing £28 per year160 is provided to all patients with varices, excluding 50% of those with small varices (clinical advice).
-
The risk of mortality following a bleed is assumed to be 30%43 and unaffected by prophylactic treatment.
-
The cost of treating an oesophageal varices bleed is assumed to be £10,000. This has been estimated from a weighted average of costs presented by Thabut et al. ,161 which was €11,982; these costs are similar to figures reported in Wechowski et al. 162
-
An endoscopy once every 3 years to be provided for those without varices and every 2 years for those patients with small or moderate varices.
-
Those patients with large varices would have three sessions of band ligation costing £438 (US$674) each, with an endoscopy at 6 months and then at yearly intervals. 162
-
Patients falsely diagnosed as not having cirrhosis who have a subsequent variceal bleed would be prescribed propranolol 40 mg twice daily.
Compared with a no screening strategy, these assumptions provide an estimated net incremental cost of screening and subsequent treating to a patient with oesophageal varices who continues to drink of £400 and of £900 for those who abstain. The additional QALYs accrued from screening and providing subsequent treatment compared with a no screening strategy were 0.635 for those patients who continue to drink and 0.269 for abstinent patients. For simplicity within the model, these cost and QALY values were added to end point 1, for those people who abstain from alcohol, and end point 2 for those people who continue to drink heavily.
Detecting hepatic encephalopathy
An electroencephalogram [estimated to cost £220 (data obtained from the Royal Free Hospital, London, UK)] was assumed to be required annually to detect HE. These were estimated to have a discounted cost of £1674 (US$2578) over a lifetime for those patients diagnosed with cirrhosis.
The proportion of patients who continue to drink in relation to diagnosis by biopsy
One small study (n = 96), published in abstract form,26 reported that the level of continued drinking following biopsy-proven ALD was dependent on whether or not the patient had cirrhosis. Fifty-nine per cent of patients without cirrhosis had a heavy intake compared with 38% of those who had cirrhosis, a difference which was significant (p = 0.04). As it is plausible that the abstinence rate may be higher in patients with cirrhosis, these values were used within the model.
The proportion of patients who continue to drink in relation to diagnosis by a non-invasive liver test
It is conceivable that patients who have a less invasive test may be more reluctant than those who have a biopsy to become abstinent. Possible reasons for this include (1) the knowledge of the physician (and potentially a well-informed patient) that the NILT has low specificity; (2) the fact that inflammation and fat can be assessed directly following a biopsy (but not through a NILT), which can be used to inform the patient of an underlying disease progression that has not currently reached cirrhosis or severe fibrosis level; and (3) potentially the fact that a biopsy is invasive may also convince a patient that the disease is potentially life-threatening.
Exploratory results showed that, unsurprisingly, the model was very sensitive to the level of abstinence achieved in the ALD patients. In order to provide meaningful results, the abstinence rates were subjected to a threshold sensitivity analyses, where the percentage point increase in abstinence rates, for both those diagnosed with cirrhosis and those diagnosed without cirrhosis, required to change a decision regarding the cost-effectiveness of a strategy was calculated.
The benefit of biopsy in identifying liver disease that is not alcohol-related liver disease and confirming alcohol-related liver disease
Biopsy allows the aetiology of the liver disease to be established; this is not possible when NILTs alone are used. A diagnosis of liver disease that is not ALD would allow patients to receive alternative treatment for this condition rather than for ALD. It may also be the case that a patient may derive some underlying benefit from a definitive diagnosis of his or her condition. Initial exploratory results indicated that the results of the model were extremely sensitive to any gains in QALYs owing to a biopsy being performed. As such, this value has been left at zero for the analyses, but is subject to threshold sensitivity to determine the value that this would need to be greater than in order for the decision on which treatment was the most cost-effective to change.
Chapter 6 Cost-effectiveness model: results
During the course of the project, exploratory results were produced that showed that the model was relatively insensitive to some parameters, but was very sensitive to some parameters on which there were few data.
Those parameters that were shown to have little influence on the modelled results were subjected to univariate sensitivity analyses using a set of pre-defined ranges to check the robustness of the results regarding the cost-effectiveness of each. These results and the ranges used are shown in Table 34. These results assumed that the abstinence rates were independent of the diagnostic test and that a biopsy conferred no QALY gain and used scenario 1 (described in Table 35). In this scenario, only a FibroScan triage policy was more cost-effective than biopsying all patients, assuming a cost per QALY threshold of £20,000, where the cost-effectiveness of biopsying all patients had a cost per QALY of £71,327 compared with a FibroScan triage policy. The cost per QALY of biopsying all patients was £15,683 compared with an ELF triage policy and £17,702 compared with an ELF replacement policy, which were the two strategies where the cost-effectiveness of biopsying all patients was most uncertain.
| Parameter | Central estimate | Extreme values tested | Cost-effectivenessa |
|---|---|---|---|
| Prevalence of cirrhosis (%) | 35 | 25–45 | When set to 25%, the ELF triage strategy (£26,547) and the ELF replacement strategy (£28,783) became cost-effective |
| Cost of a percutaneous biopsy (£) | 894 | 600–1500 | When set to £1500, the ELF triage strategy (£26,581) and the ELF replacement strategy (£38,245) became cost-effective |
| Costs of FibroScan, ELF and FibroTest (£) | 50, 45 and 50 | 40–100 | No change |
| Simultaneous changing of all costs associated with the eight end points (%) | See Table 33 | –25–50 | When set to –25%, the ELF replacement strategy (£20,404) became cost-effective |
| Simultaneous changing of all QALYs associated with the eight end points (%) | See Table 33 | –25–50 | When set to –25%, the ELF triage strategy (£20,928) and the ELF replacement strategy (£23,641) became cost-effective |
| Mortality rate following percutaneous biopsy (%) | 0.09 | 0.05–0.20 | When set to 0.2%, the ELF replacement strategy (£27,293) became cost-effective |
| The average costs of dealing with complications following a biopsy (£) | 7 | 0–100 | No change |
| Percentage of biopsies that need to be repeated (%) | 0 | 0–5 | No change |
| Percentage of FibroScans that do not produce usable results (%) | 20 | 10–40 | No change |
| Percentage of FibroTests that do not produce usable results (%) | 25 | 10–40 | No change |
| Percentage of ELFs that do not produce usable results (%) | 25 | 10–40 | No change |
| The proportion of patients who progress to cirrhosis if they continue to drink heavily (%) | 20 | 10–40 | When set to 40%, the ELF replacement strategy (£23,440) became cost-effective |
| Scenario number | Biopsy sensitivity scenario | NILT accuracy scenario | Percutaneous or transjugular biopsy | Biopsy disutility |
|---|---|---|---|---|
| 1 | 100% | Scenario 1 | Percutaneous | Normal |
| 2 | 100% | Scenario 1 | Percutaneous | High |
| 3 | 100% | Scenario 1 | Transjugular | Normal |
| 4 | 100% | Scenario 1 | Transjugular | High |
| 5 | 100% | Scenario 2 | Percutaneous | Normal |
| 6 | 100% | Scenario 2 | Percutaneous | High |
| 7 | 100% | Scenario 2 | Transjugular | Normal |
| 8 | 100% | Scenario 2 | Transjugular | High |
| 9 | 100% | Scenario 3 | Percutaneous | Normal |
| 10 | 100% | Scenario 3 | Percutaneous | High |
| 11 | 100% | Scenario 3 | Transjugular | Normal |
| 12 | 100% | Scenario 3 | Transjugular | High |
| 13 | 80% pessimistic | Scenario 1 | Percutaneous | Normal |
| 14 | 80% pessimistic | Scenario 1 | Percutaneous | High |
| 15 | 80% pessimistic | Scenario 1 | Transjugular | Normal |
| 16 | 80% pessimistic | Scenario 1 | Transjugular | High |
| 17 | 80% pessimistic | Scenario 2 | Percutaneous | Normal |
| 18 | 80% pessimistic | Scenario 2 | Percutaneous | High |
| 19 | 80% pessimistic | Scenario 2 | Transjugular | Normal |
| 20 | 80% pessimistic | Scenario 2 | Transjugular | High |
| 21 | 80% pessimistic | Scenario 3 | Percutaneous | Normal |
| 22 | 80% pessimistic | Scenario 3 | Percutaneous | High |
| 23 | 80% pessimistic | Scenario 3 | Transjugular | Normal |
| 24 | 80% pessimistic | Scenario 3 | Transjugular | High |
| 25 | 80% optimistic | Scenario 1 | Percutaneous | Normal |
| 26 | 80% optimistic | Scenario 1 | Percutaneous | High |
| 27 | 80% optimistic | Scenario 1 | Transjugular | Normal |
| 28 | 80% optimistic | Scenario 1 | Transjugular | High |
| 29 | 80% optimistic | Scenario 2 | Percutaneous | Normal |
| 30 | 80% optimistic | Scenario 2 | Percutaneous | High |
| 31 | 80% optimistic | Scenario 2 | Transjugular | Normal |
| 32 | 80% optimistic | Scenario 2 | Transjugular | High |
| 33 | 80% optimistic | Scenario 3 | Percutaneous | Normal |
| 34 | 80% optimistic | Scenario 3 | Percutaneous | High |
| 35 | 80% optimistic | Scenario 3 | Transjugular | Normal |
| 36 | 80% optimistic | Scenario 3 | Transjugular | High |
In a number of univariate sensitivity analyses, there was a change in the cost-effectiveness of some strategies compared with biopsying all patients, although often this was because of the cost-effectiveness of biopsying all patients becoming slightly > £20,000 per QALY. Given the large impact on results caused by plausible changes in the remaining parameters, a decision was made to simplify the results produced by fixing the parameters in Table 34 at their central estimates, with the acknowledgement that uncertainty and interactions between these parameters were not considered, which would result in the results produced underestimating uncertainty.
Strategies with poor specificity for cirrhosis (diagnosing all with cirrhosis, and in some scenarios the ELF) produced the greatest number of QALYs owing to the assumed higher proportion of patients who became abstinent because of a diagnosis of cirrhosis. However, these relatively small QALY gains were not sufficient to be deemed cost-effective when the costs of follow-up in patients incorrectly diagnosed with cirrhosis were considered.
Key parameters that were identified as affecting the cost-effectiveness were:
-
The assumed sensitivity of biopsy and the assumption made regarding the accuracy of the NILTs in detecting these FPs. The scenarios analysed were 100% sensitivity, 80% sensitivity with pessimistic assumptions for the NILTs and 80% sensitivity with optimistic assumptions for the NILTs.
-
The assumed sensitivity and specificity of biopsy and of each NILT. These were the three scenarios detailed in Tables 28–30.
-
Whether or not the biopsy was undertaken using a percutaneous or transjugular method.
-
Whether or not the QALY loss associated with a biopsy would include any effects of anxiety. This was denoted as normal, which was the estimated value associated with severe adverse events, or high, a value of 0.04.
-
The assumed changes in abstinence rates dependent on the tests used to diagnose cirrhosis.
-
The assumed potential incidental QALY benefits of biopsy.
The fifth and sixth parameters within the list were evaluated using a threshold approach for each combination of the remaining four parameters. This resulted in 36 potential scenarios, which are detailed in Table 35. It is stressed that the scenarios regarding sensitivity and specificity for each of NILTs do not have to be compared directly, and that it is possible to compare scenario 1 for one NILT with scenario 2 for another, and with scenario 3 for a third. Similarly, when 80% sensitivity is assumed for biopsy, it may be plausible that one NILT could use an optimistic assumption, whereas a separate test, with a different modality, may use a pessimistic assumption.
Given the large uncertainty in the two parameters shown to be key in the exploratory analyses (the relationship between abstinence and the test used to diagnose cirrhosis and the gain in QALYs associated with biopsy), it was decided that carrying out a formal probabilistic sensitivity analyses163 would offer little additional insight.
The results in terms of costs, QALYs and predicted number of patients who receive a biopsy are presented in Appendix 10 for each of the 36 scenarios. These tables can be used to calculate incremental cost-effectiveness ratios for comparisons of all scenarios presented; the range of cost-effectiveness values is wide and can be seen to be favourable to the use of NILTs in some scenarios, with the use of NILTs producing more QALYs at a lower cost, but favourable to biopsy in other scenarios. However, it is stressed that these values assume that there is no decrease in abstinence rates when NILTs are used as a replacement for biopsy, that there are no incidental benefits of conducting a biopsy, and that the results could change markedly when values are assigned to these parameters.
Threshold analysis regarding the rates of abstinence
A threshold analysis was performed, which evaluated the change in the proportion of patients who remain abstinent required to change the conclusion so that biopsying all patients is the most cost-effective option. In this analysis, the increases were stepped in units of 0.1 percentage points until the test was no longer cost-effective, with this value reported as the threshold. For all strategies, only the abstinence rate associated with diagnosis by the NILT was altered, with the abstinence rates associated with biopsy remaining unaffected. Thus, for a triage strategy, those patients in whom the NILT was positive would progress to biopsy and would have an abstinence rate determined by biopsy; those patients in whom the NILT was negative would have an abstinence rate associated with a negative NILT diagnosis, rather than a biopsy diagnosis, and it is the former value that is altered in the threshold analyses. For replacement strategies, both the positive and negative abstinence rates would be altered as both would be determined by the NILT alone.
This is presented for all nine NILT strategies (four triaging, four replacement and one to diagnose all with cirrhosis). The figures have been commented on to provide the reader with an aid to interpret the results.
The threshold results are presented without comment on whether or not the thresholds are realistically achievable; no data were found on abstinence rate by method of diagnosis, and any reduction based on a non-invasive diagnostic technique is a matter of genuine clinical debate. Where the threshold is a zero decrease in abstinence rates, this indicates that, for that specific scenario, biopsying all patients was deemed a more cost-effective option than the relevant strategy with equal abstinence rates and no QALY gain from biopsy. These thresholds are shown graphically in Figures 10–27, assuming a cost per QALY threshold of £20,000, with more detailed results presented in Appendix 10. The thresholds can vary markedly; it is noted that in the scenarios most favourable to the NILT, the comparator is transjugular biopsy and a high degree of disutility prior to the biopsy is assumed.
FIGURE 10.
Threshold analyses on abstinence for FibroScan triage. The threshold for change in abstinence where biopsying all becomes more cost-effective than FibroScan triage.
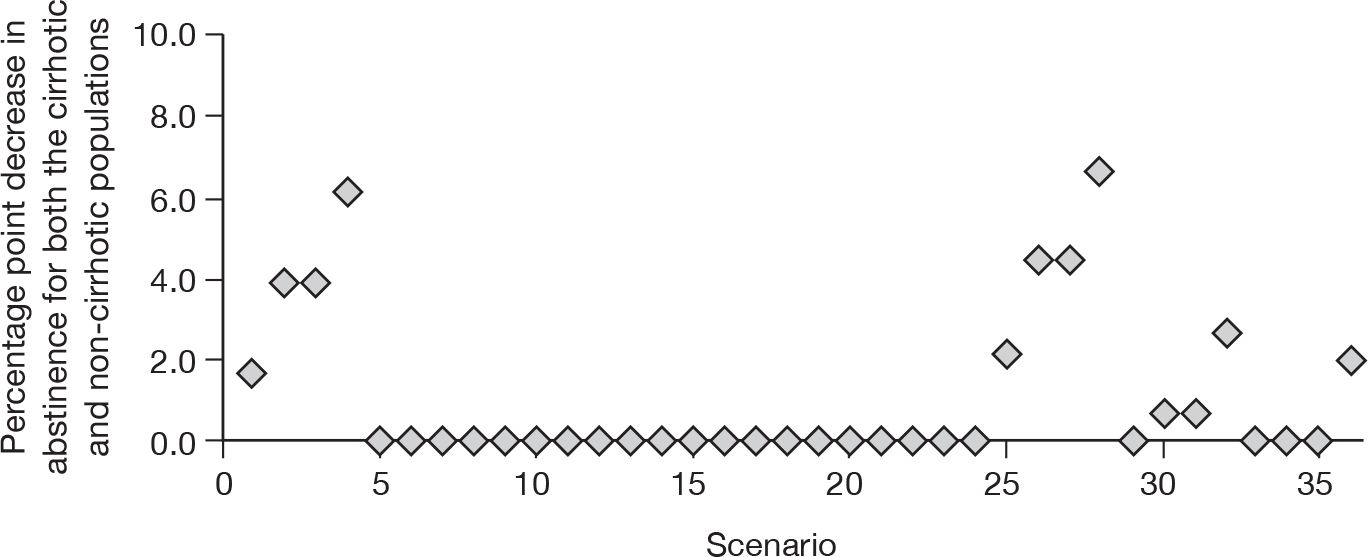
FIGURE 11.
Threshold analyses on abstinence for ELF triage. The threshold for change in abstinence where biopsying all becomes more cost-effective than ELF triage.
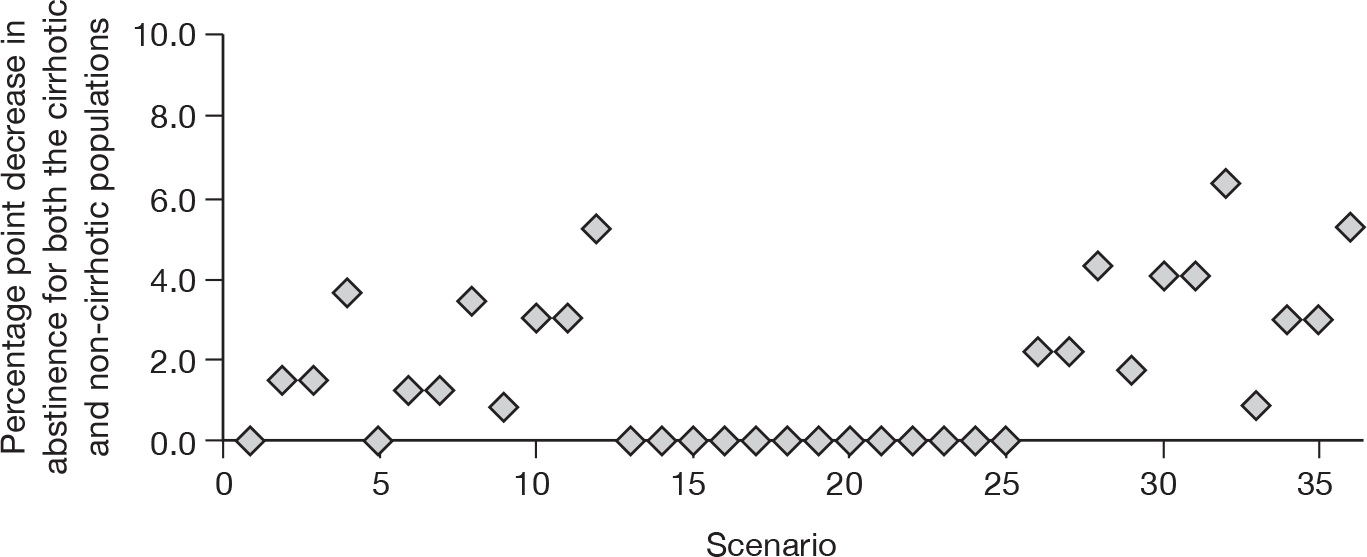
FIGURE 12.
Threshold analyses on abstinence for FibroTest triage. The threshold for change in abstinence where biopsying all becomes more cost-effective than FibroTest triage.
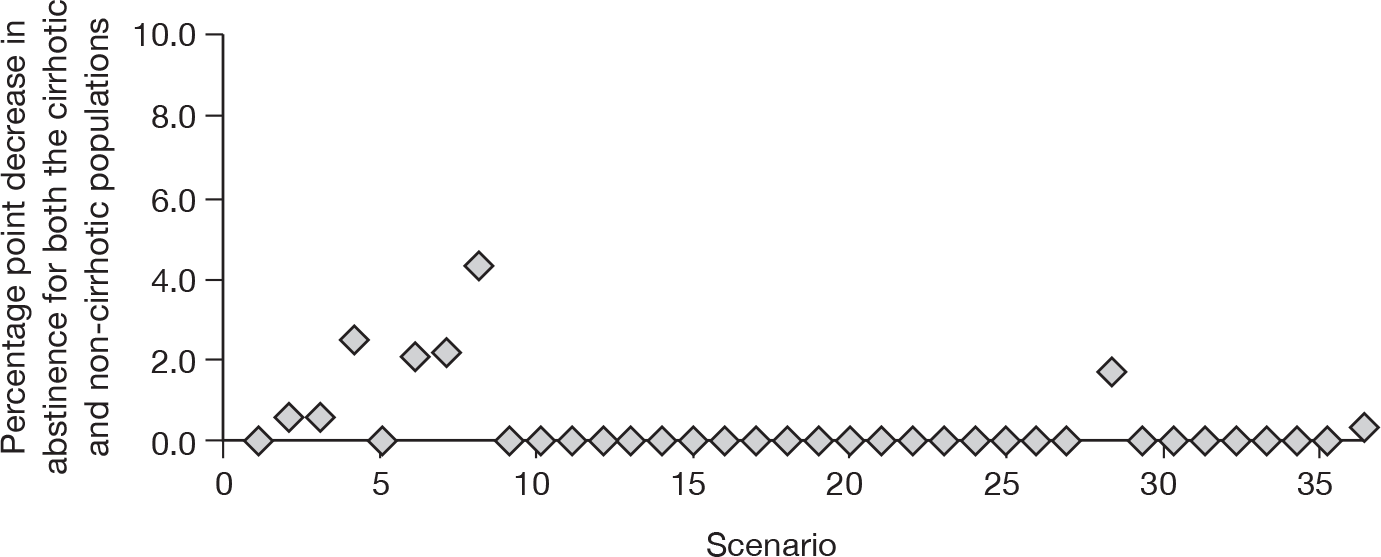
FIGURE 13.
Threshold analyses on abstinence for clinical experience triage. The threshold for change in abstinence where biopsying all becomes more cost-effective than clinical experience triage.
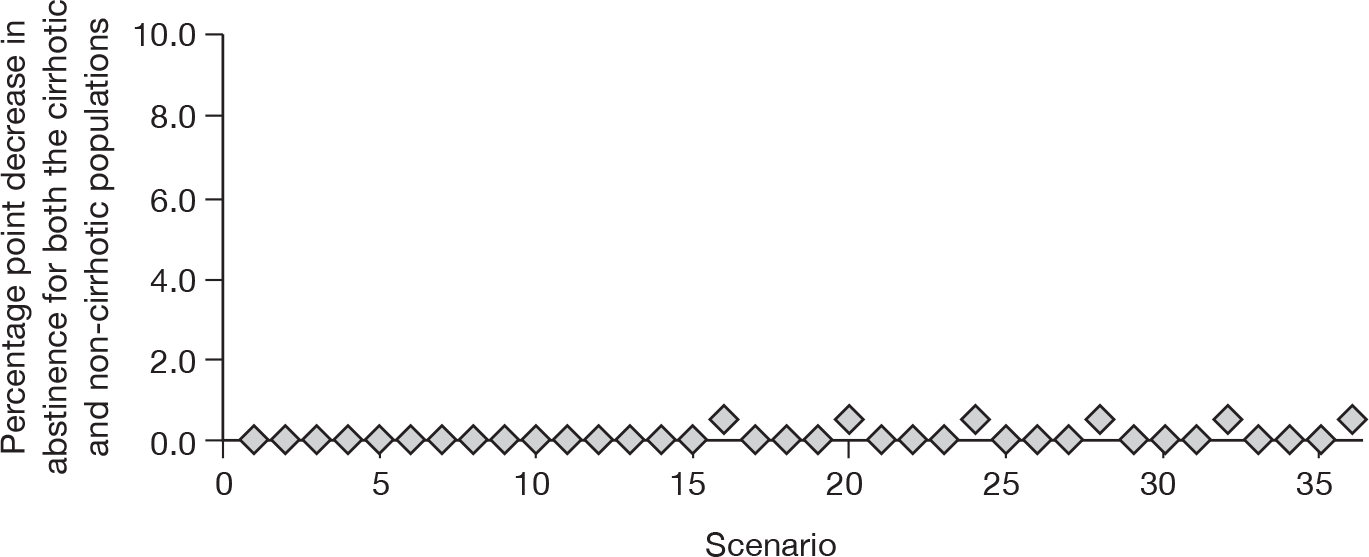
FIGURE 14.
Threshold analyses on abstinence for FibroScan replacement. The threshold for change in abstinence where biopsying all becomes more cost-effective than treating based on the FibroScan alone.
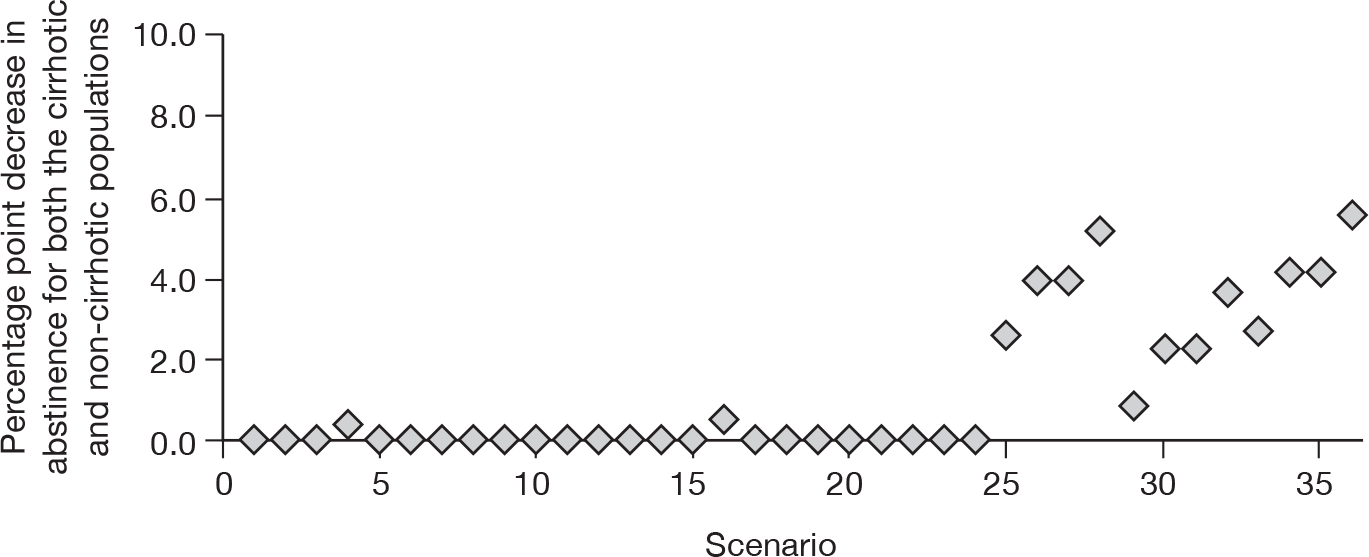
FIGURE 15.
Threshold analyses on abstinence for ELF replacement. The threshold for change in abstinence where biopsying all becomes more cost-effective than treating based on the ELF alone.
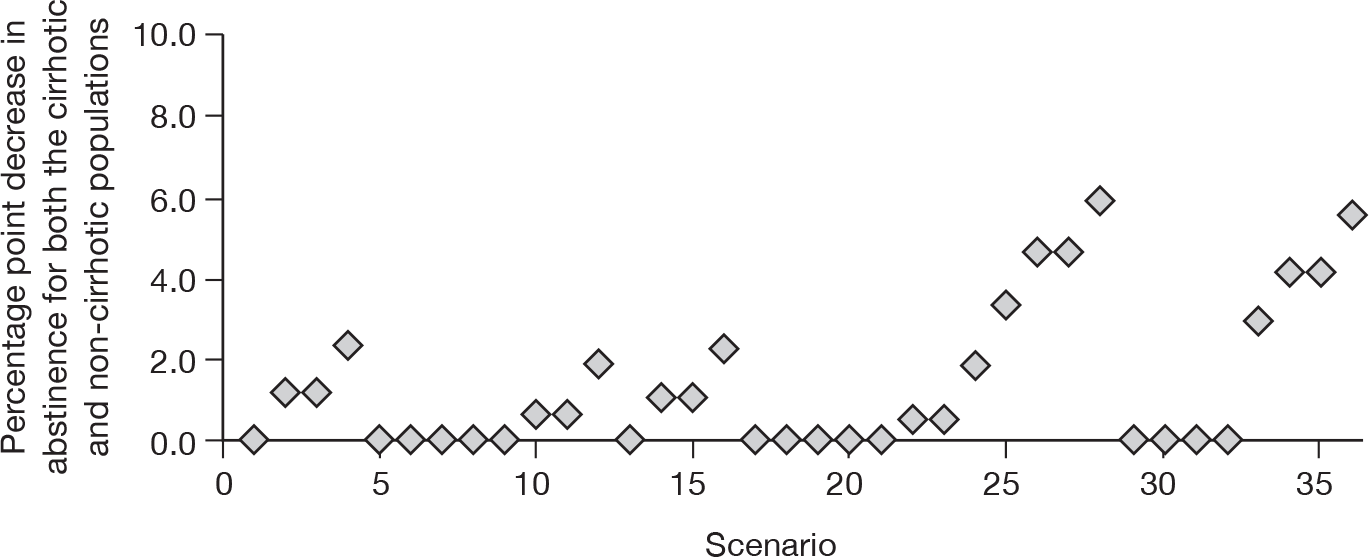
FIGURE 16.
Threshold analyses on abstinence for FibroTest replacement. The threshold for change in abstinence where biopsying all becomes more cost-effective than treating based on the FibroTest alone.
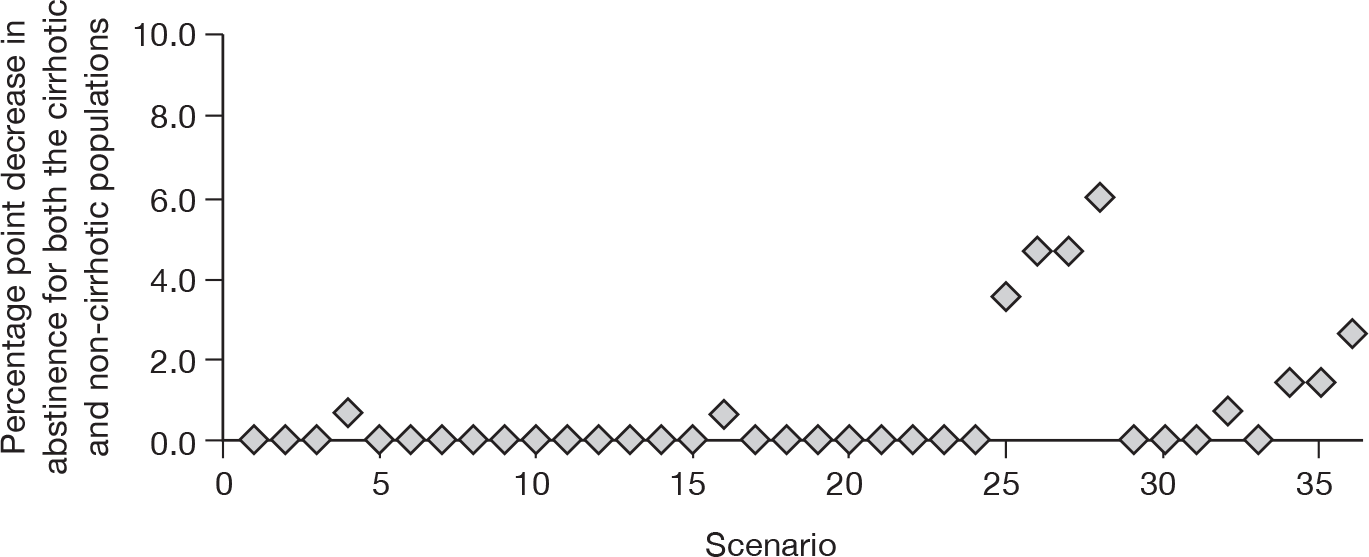
FIGURE 17.
Threshold analyses on abstinence for clinical experience replacement. The threshold for change in abstinence where biopsying all becomes more cost-effective than treating based on clinical experience alone.
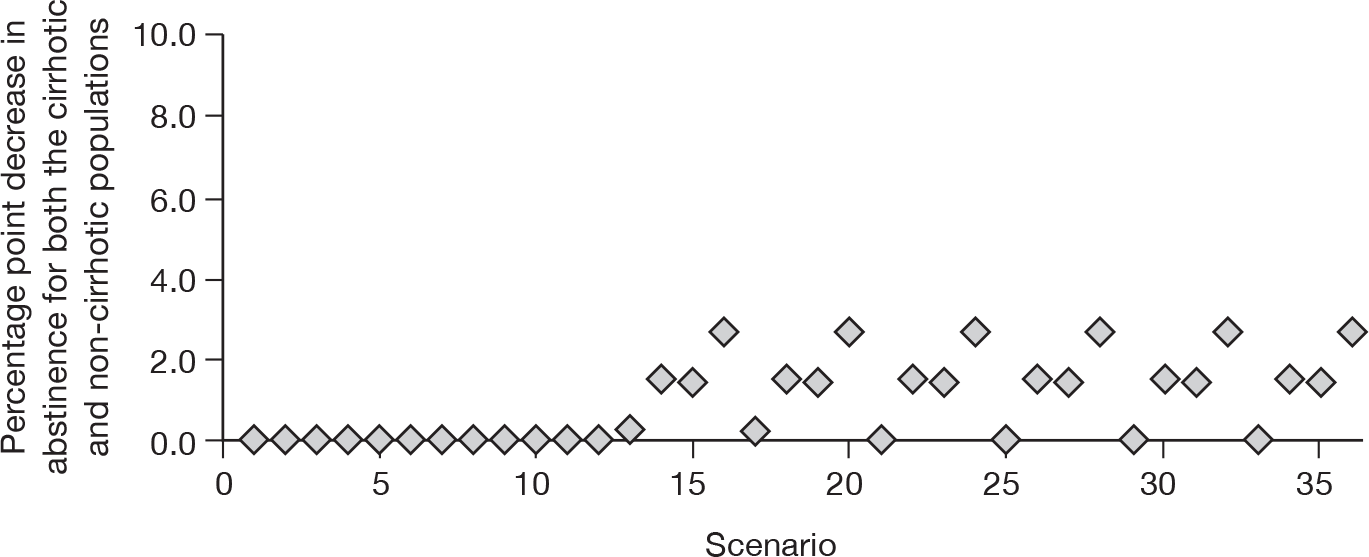
FIGURE 18.
Threshold analyses on abstinence for diagnosing all with cirrhosis replacement. The threshold for change in abstinence where biopsying all becomes more cost-effective than diagnosing all patients with cirrhosis.
FIGURE 19.
Threshold analyses on QALY gain associated with a biopsy for FibroScan triage. The threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than FibroScan triage.
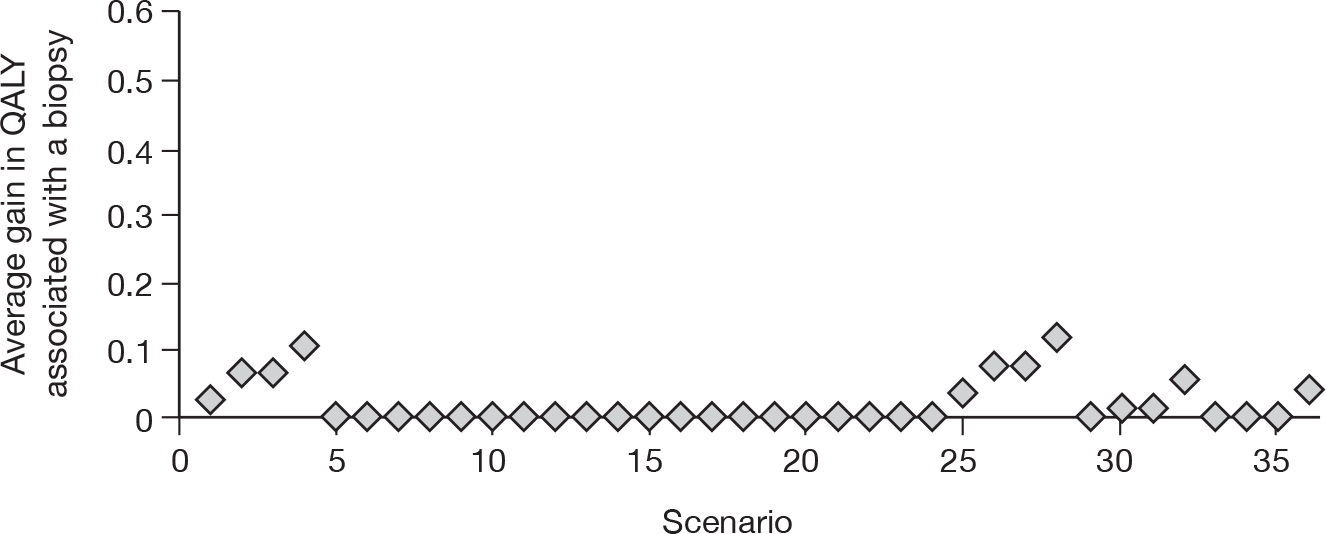
FIGURE 20.
Threshold analyses on QALY gain associated with a biopsy for ELF triage. The threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than ELF triage.
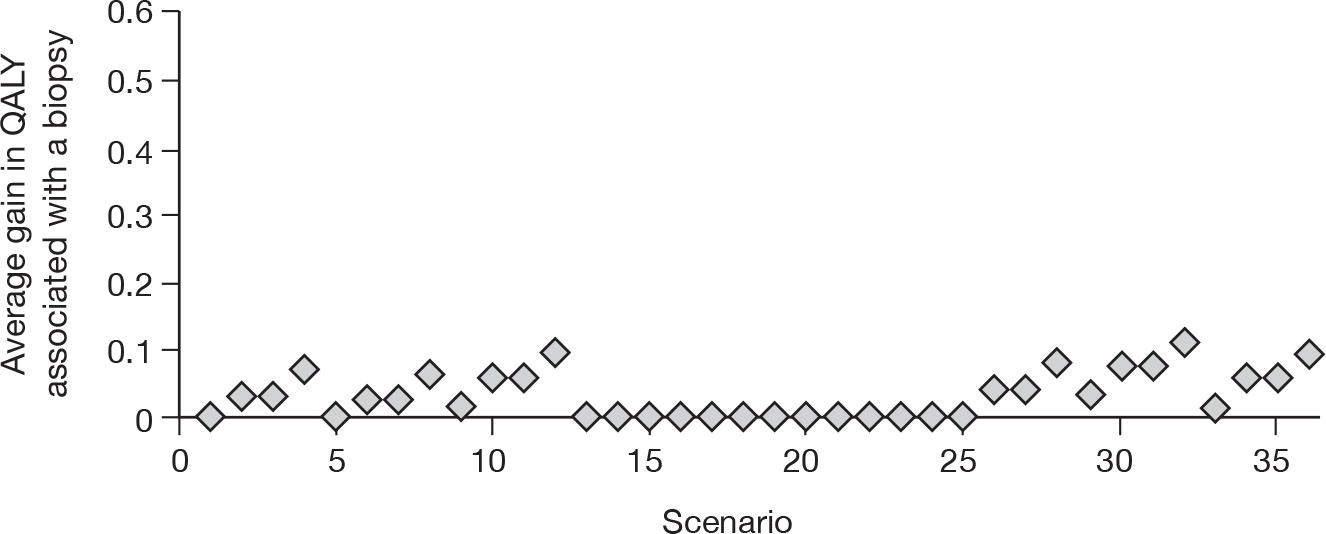
FIGURE 21.
Threshold analyses on QALY gain associated with a biopsy for FibroTest triage. The threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than FibroTest triage.
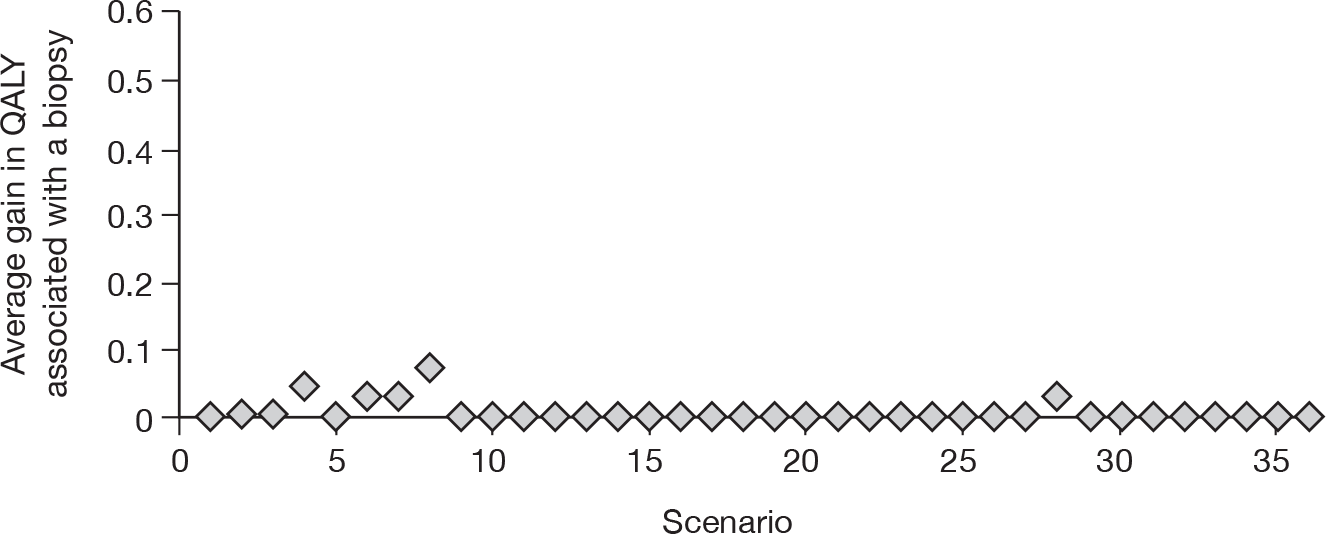
FIGURE 22.
Threshold analyses on QALY gain associated with a biopsy for clinical experience triage. The threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than clinical experience triage.
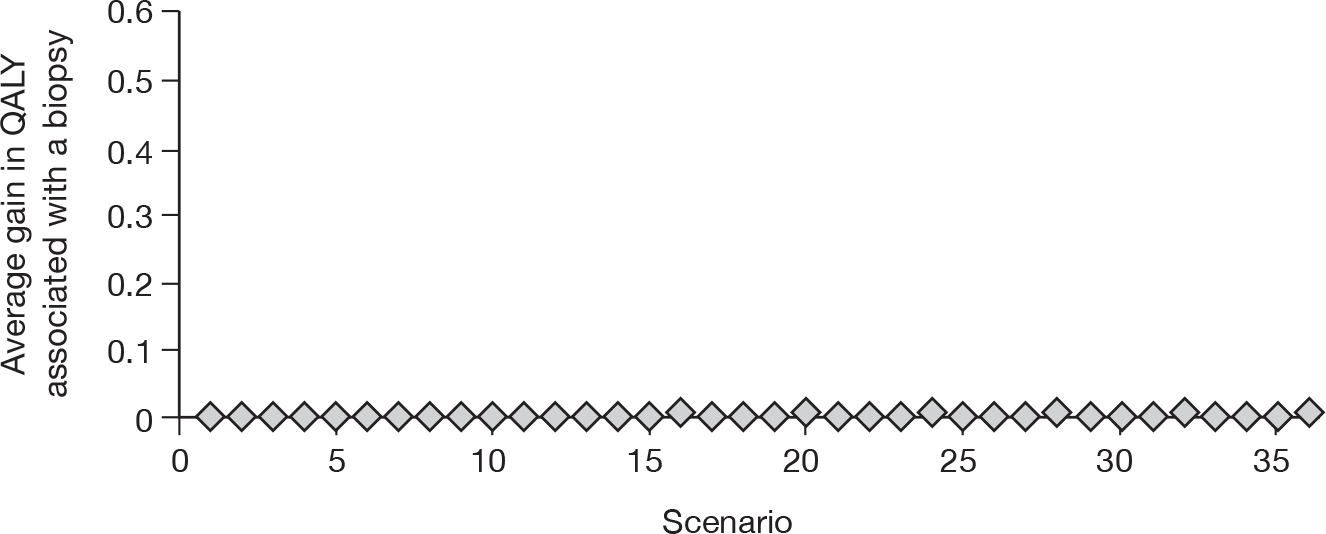
FIGURE 23.
Threshold analyses on QALY gain associated with a biopsy for FibroScan replacement. Threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than treating based on the FibroScan alone.
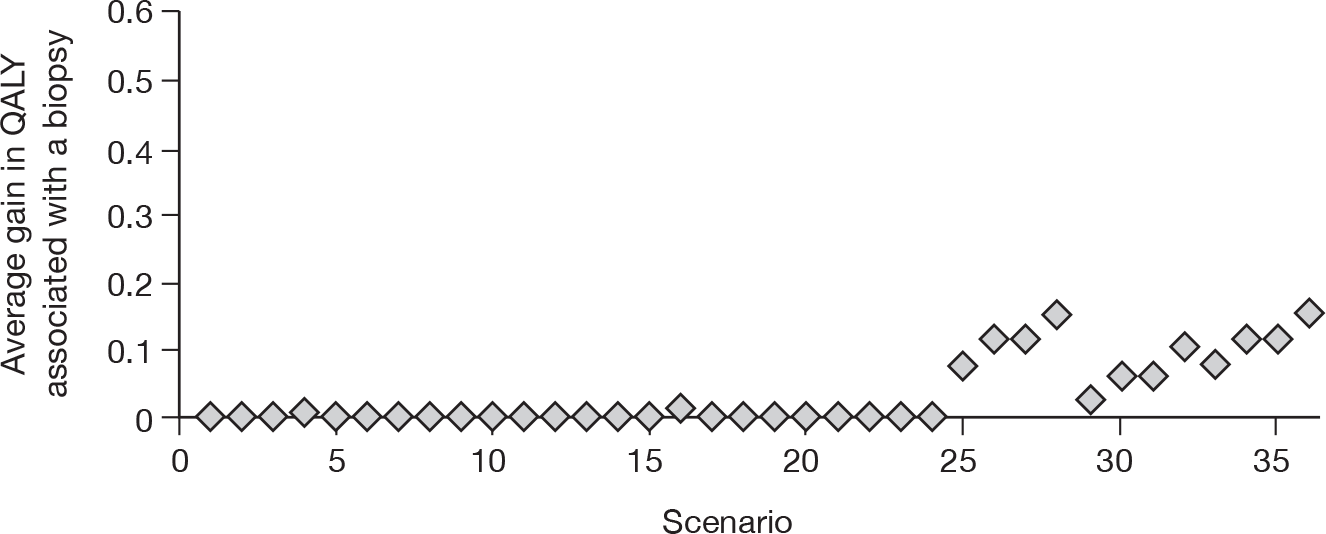
FIGURE 24.
Threshold analyses on QALY gain associated with a biopsy for ELF replacement. Threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than treating based on the ELF alone.
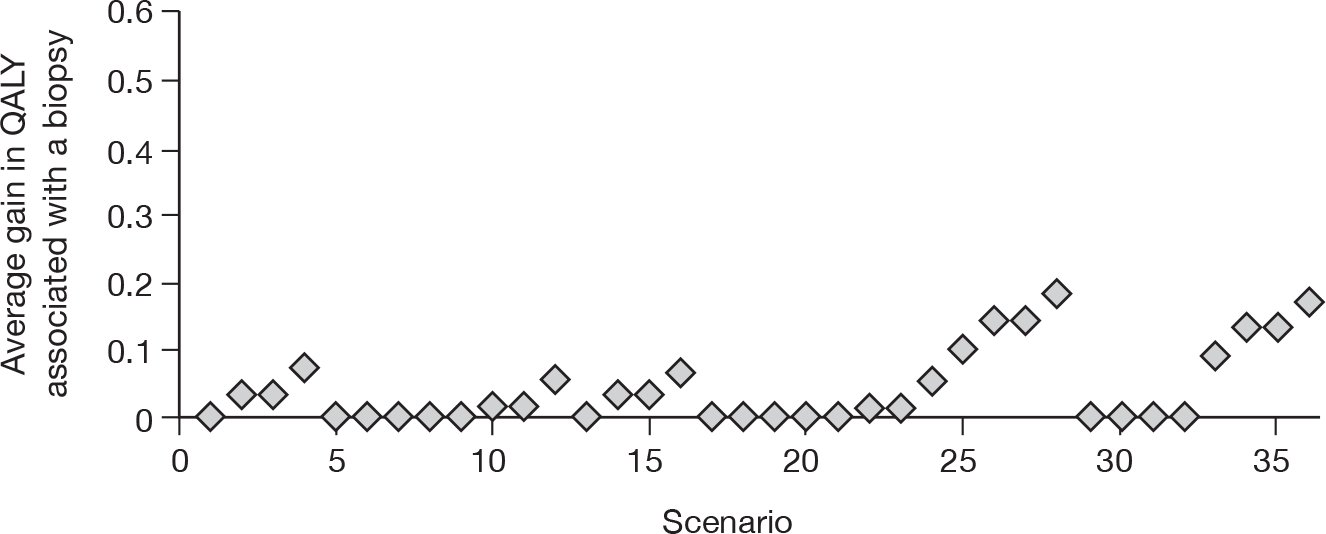
FIGURE 25.
Threshold analyses on QALY gain associated with a biopsy for FibroTest replacement. Threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than treating based on the FibroTest alone.
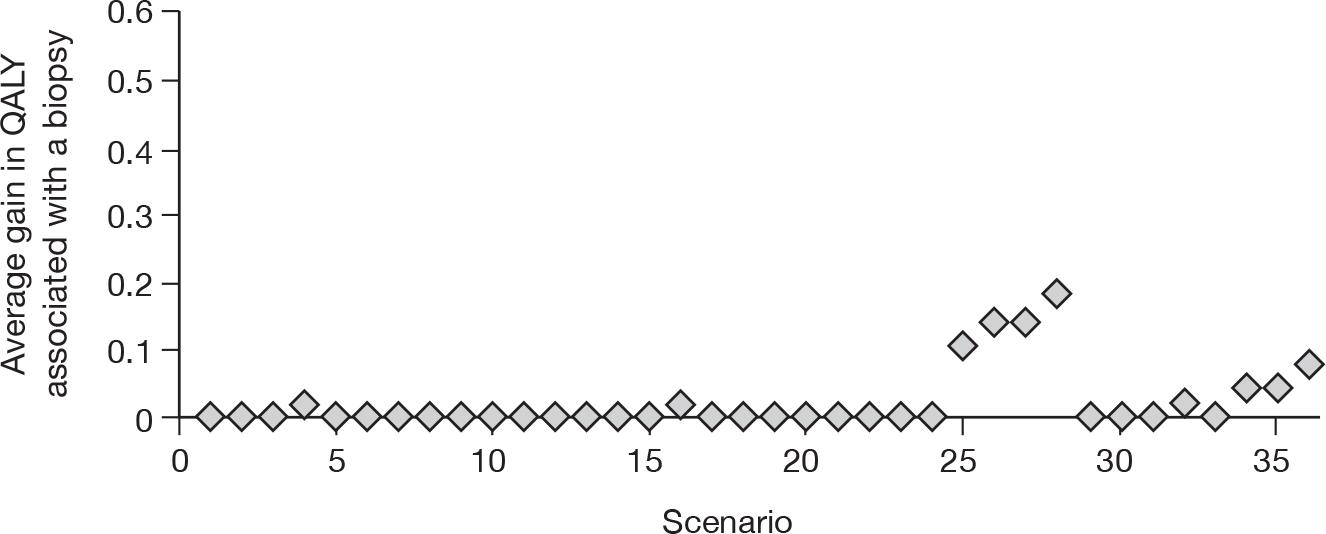
FIGURE 26.
Threshold analyses on QALY gain associated with a biopsy for clinical experience replacement. Threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than treating based on clinical experience alone.
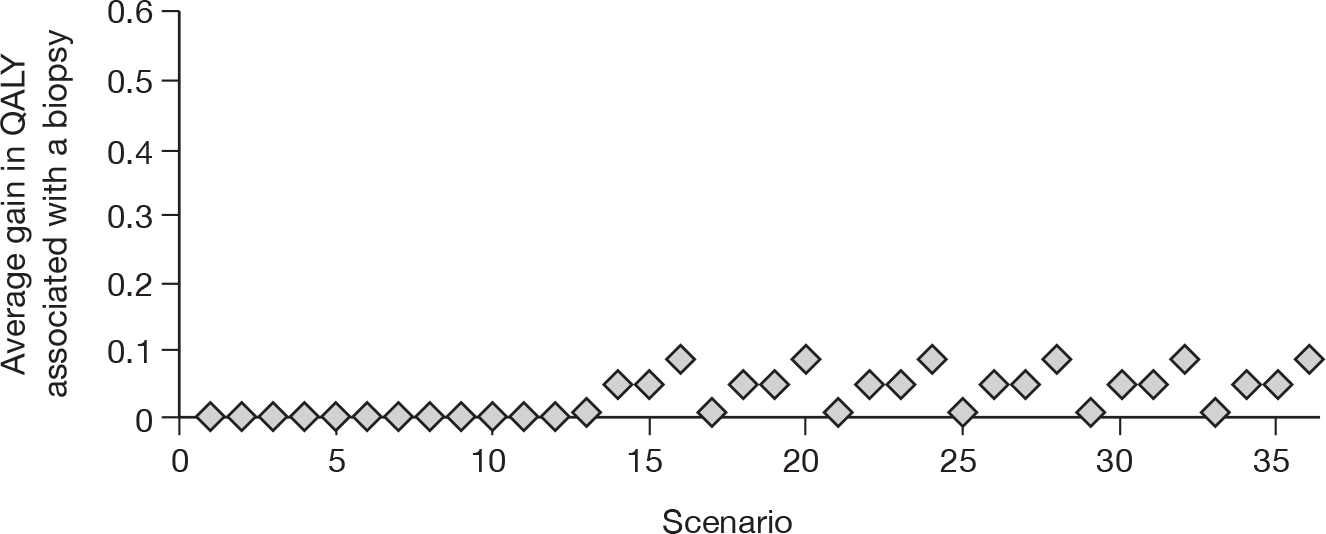
FIGURE 27.
Threshold analyses on QALY gain associated with a biopsy for diagnosing all with cirrhosis replacement. Threshold for QALY gain per biopsy performed when biopsy becomes more cost-effective than diagnosing all patients with cirrhosis.
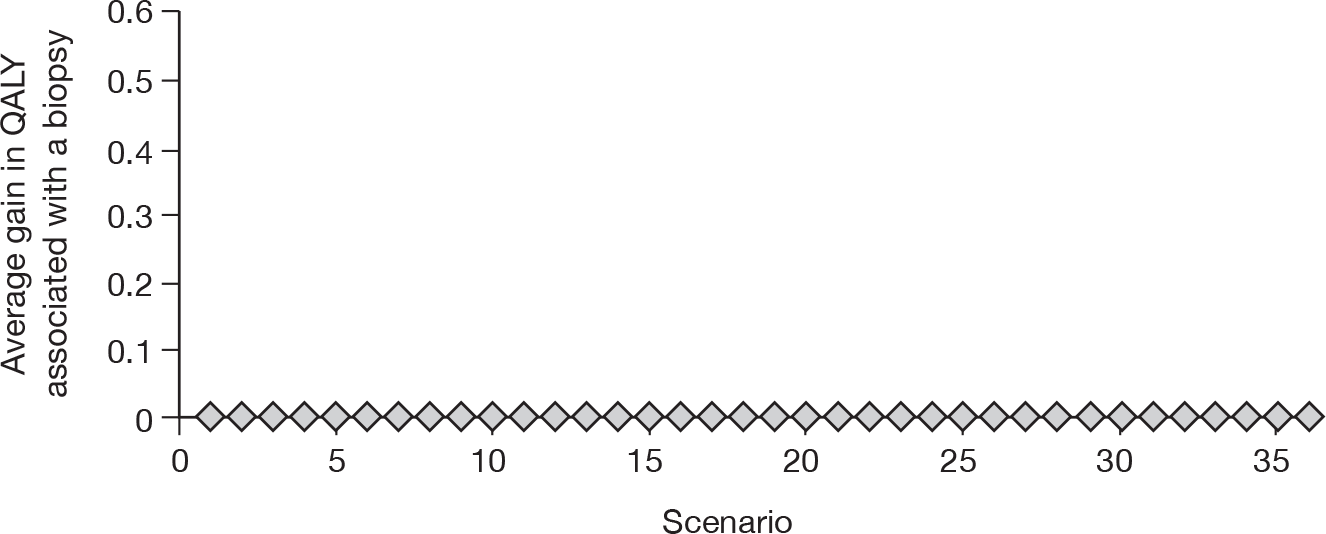
It is stressed that the threshold on abstinence is undertaken separately from the threshold on the potential benefits associated with a biopsy. Clearly the threshold for abstinence rates would decrease if there was a belief that there was also a QALY benefit associated with a biopsy.
No formal elicitation was undertaken to determine what the likely range of values for the decrease in abstinence rates when using a NILT or on the incidental benefits of a biopsy. The clinical input that was received suggested that these values would be highly uncertain.
The scenarios that are more favourable to FibroScan triage assume scenario 1 for test accuracy. In the most favourable scenario to FibroScan triage, the abstinence rate would need to decrease by ≥ 6.7 percentage points for biopsying all to be more cost-effective; alternative scenarios estimate that biopsying all patients is more cost-effective than FibroScan triage, even assuming the same rate in abstinence.
The ELF triage has a wide range of thresholds for decrease in abstinence levels, which can be as high as 6.3 percentage points in the most favourable scenarios, although often this value is < 2%. It is commented that the sensitivity and specificity for the ELF have not been taken from a cirrhotic population and are thus subject to more uncertainty than in the remaining tests.
For all scenarios, an increase in abstinence rates by ≥ 4.3 percentage points resulted in biopsying all patients being more cost-effective than FibroTest. However, in the majority of scenarios, biopsying all patients is indicated to be more cost-effective than FibroTest triage.
For all scenarios, an increase in abstinence rates of ≥ 0.5 percentage points resulted in biopsying all patients being more cost-effective than clinical experience triage. However, in the majority of scenarios, biopsying all patients is indicated to be more cost-effective than clinical experience triage.
For FibroScan replacement, the threshold values only rise > 1% when it is assumed that the sensitivity of biopsy in detecting cirrhosis is 80% and that a FibroScan would have detected these FNs as being cirrhotic. In this instance, the threshold level approaches 6 percentage points.
The threshold associated with ELF replacement varies markedly, but is relatively high and can reach 6 percentage points when it is assumed that the sensitivity of biopsy in detecting cirrhosis is 80% and that an ELF would have detected these FNs as being cirrhotic. It is commented that the sensitivity and the specificity for the ELF have not been taken from a cirrhotic population and are thus subject to more uncertainty than the remaining tests.
For the majority of scenarios, biopsying all patients is more cost-effective than a FibroTest replacement policy. The threshold is relatively high (reaching 6 percentage points) when it is assumed that the sensitivity of biopsy in detecting cirrhosis is 80% and that a FibroTest would have detected these FNs as being cirrhotic, and scenario 1 for FibroTest accuracy is used.
For all scenarios, an increase in abstinence rates by ≥ 2.8 percentage points resulted in biopsying all patients being more cost-effective than clinical experience replacement. For a sizeable proportion of scenarios, biopsying all patients was more cost-effective than a clinical experience replacement strategy.
For all scenarios, biopsying all patients was more cost-effective than a test that had 100% sensitivity and 0% specificity. Although the diagnose all strategy produced most QALYs, the large number of positives was associated with a much greater cost because of clinical follow-up following a diagnosis of cirrhosis.
Threshold analysis regarding the potential benefits associated with a biopsy
A threshold analysis was performed, which evaluated the average gain in QALY from a biopsy compared with a NILT to change the conclusion so that biopsying all patients is the most cost-effective option. In this analysis, the increases were stepped in units of 0.001 QALYs until the test was no longer cost-effective, with this value reported as the threshold.
This is presented for all nine NILT strategies (four triaging, four replacement and one to diagnose all with cirrhosis). The figures have been commented on to provide the reader with an aid to interpret the results.
The threshold results are presented without comment on whether or not the thresholds are realistically achievable; no quantitative data were found on the incidental benefits of biopsy compared with a NILT. Where the threshold is a zero decrease in abstinence rates, this indicates that, for that specific scenario, biopsying all patients was deemed a more cost-effective option than the relevant strategy with equal abstinence rates and no QALY gain from biopsy. These are shown graphically in the main report (see Figures 19–27), with the actual values presented in Appendix 10.
It is stressed that the threshold on abstinence is undertaken separately from the threshold on the potential benefits associated with rates of abstinence. Thus, the threshold for QALY gain from a biopsy would decrease if there was believed to be an increase in abstinence rates following biopsy. Similarly, the threshold for abstinence rates following biopsy would decrease if there was believed to be a QALY gain from biopsy.
It is seen that if the QALY increase associated with a biopsy is ≥ 0.12, then biopsying all patients is the most cost-effective option. For the majority of scenarios, biopsying all patients is the most cost-effective option.
It is seen that if the QALY increase associated with a biopsy is ≥ 0.11, then biopsying all patients is the most cost-effective option. It is commented that the sensitivity and the specificity for the ELF have not been taken from a cirrhotic population and are thus subject to more uncertainty than the remaining tests.
For all scenarios, a gain in biopsy of ≥ 0.08 QALYs resulted in biopsying all patients being more cost-effective than FibroTest triage. However, in the majority of scenarios, biopsying all patients is indicated to be more cost-effective than FibroTest triage.
For all scenarios, a gain in biopsy of ≥ 0.01 QALYs resulted in biopsying all patients being more cost-effective than clinical experience triage. In the majority of scenarios, biopsying all patients was estimated to be more cost-effective than clinical experience triage.
For FibroScan replacement, the threshold values are relatively greatest when it is assumed that the sensitivity of biopsy in detecting cirrhosis is 80% and that a FibroScan would have detected these FNs as being cirrhotic. The greatest threshold value seen is < 0.16 QALYs.
The greatest threshold values are produced when it is assumed that the sensitivity of biopsy in detecting cirrhosis is 80% and that an ELF would have detected these FNs as being cirrhotic. These thresholds are not estimated to be > 0.18 QALYs. It is commented that the sensitivity and the specificity for the ELF have not been taken from a cirrhotic population and are thus subject to more uncertainty than the remaining tests.
The results are seen to be more favourable to FibroTest replacement when it is assumed that the sensitivity of biopsy in detecting cirrhosis is 80% and that the FibroTest would have detected these FNs as being cirrhotic; in this instance, the threshold is 0.19 QALYs. However, in most scenarios, biopsying all patients is estimated to be more cost-effective than a FibroTest replacement strategy.
For all scenarios, a gain in QALY of ≥ 0.09 associated with a biopsy resulted in biopsying all patients being more cost-effective than clinical experience replacement.
In all scenarios, biopsying all patients was more cost-effective than a test with 100% sensitivity and 0% specificity, that is, diagnosing all with cirrhosis. Although the diagnose all strategy produced most QALYs, the large number of positives was associated with a much greater cost because of clinical follow-up following a diagnosis of cirrhosis.
Conclusions drawn from the cost-effectiveness results
It has been shown that the cost-effectiveness of each strategy is sensitive to relatively small values in the abstinence rates assumed to be associated with that strategy. For the triage policies, in the most favourable scenarios a decrease in abstinence of ≥ 6.7 percentage points in those diagnosed as without cirrhosis results in biopsying all to be the most cost-effective strategy; this value decreases to 4.5 percentage points if scenarios in which a biopsy is associated with anxiety and thus a high disutility are excluded, and falls to 2.2 percentage points when transjugular biopsies are further excluded. In general, the threshold values were typically < 2 percentage points, and in a sizeable number of scenarios biopsying all patients was more cost-effective than a NILT triaging strategy. FibroScan and the ELF appeared to perform better than the NILTs, although the small data set taken from a non-cirrhotic population casts more uncertainty regarding the ELF results. In some scenarios, however, FibroTest had relatively high thresholds.
For the replacement strategies, the greatest threshold for decreased abstinence rates was 6.0 percentage points, decreasing to 4.7 percentage points when scenarios in which a biopsy is associated with anxiety and thus a high disutility are excluded, and falling to 3.5 percentage points when transjugular biopsies are further excluded. FibroScan and the ELF appeared to perform better than the NILTs, although in some scenarios FibroTest had relatively high thresholds.
The conclusions from the threshold on abstinence rates also apply to the threshold analyses conducted on the QALY gains associated with a biopsy, with the cost-effectiveness results sensitive to relatively small changes in the QALY gain associated with a biopsy. For the triage policies, a QALY gain of ≥ 0.118 in those diagnosed as without cirrhosis results in biopsying all being the most cost-effective strategy; this value decreases to 0.078 if scenarios in which a biopsy is associated with anxiety and a high disutility are excluded, and reduces to 0.038 if transjugular biopsies are excluded.
For the replacement strategies, the greatest threshold for decreased abstinence rates is 0.186 QALYs, which decreases to 0.148 QALYs when scenarios in which a biopsy is associated with anxiety and thus a high disutility are excluded, and falls to 0.108 QALYs when transjugular biopsies are further excluded.
It is stressed that the threshold analyses on decreases in abstinence rates and in potential QALY gains from biopsies have been undertaken independently, and these thresholds would fall if both a decrease in abstinence rates and a gain from a biopsy were assumed.
The sensitivity of the model to these parameters, together with the absence of data on these issues, means that no reliable estimate can be provided for either the incremental cost or the incremental QALY of a strategy when compared with biopsying all patients, and thus also the incremental cost-effectiveness ratio. Until further data are obtained, no conclusion can be provided on the most cost-effective strategy; however, there is evidence that some strategies, such as using clinical experience or diagnosing all patients with cirrhosis, will not be the most cost-effective. Accordingly, there is not sufficient evidence to support a change from current best practice. 108
Chapter 7 Discussion
The estimation of the clinical effectiveness and the cost-effectiveness of NILTs for patients with suspected ALD has been difficult to conduct with precision owing to the paucity of data. As discussed in Chapter 4, Discussion of clinical effectiveness, there is insufficient robust evidence for the diagnostic and prognostic accuracy of NILTs in patients with suspected ALD. Moreover, even were such evidence available, there is no evidence linking test results to subsequent drinking behaviour, although long-term abstention from alcohol is known to be by far the most important management aim in patients with ALD.
The uncertainty in the clinical parameters resulted in 36 scenarios being evaluated with individual threshold analyses performed for two key variables within the conceptual model, which were the possibility of a decrease in abstinence rates associated with the NILTs compared with biopsy and the QALY gain that may be provided by a biopsy.
It is uncertain which, if any, of the 36 scenarios provide the best representation of reality, adding considerable uncertainty. The lack of data on the potential decreases in abstinence rates or QALY gains provided by a biopsy adds considerably more uncertainty, and it was seen that small changes in these values could alter the conclusion of whether or not a strategy was cost-effective compared with biopsying all patients. As such, it is not possible to provide a robust value for the incremental cost of a new strategy, the incremental QALYs of a new strategy and ultimately the incremental cost per QALY ratio. Scenarios exist in which each of the strategies analysed is more cost-effective than biopsying all patients and, in contrast, scenarios exist in which each strategy is less cost-effective than biopsying all patients.
It is plausible that patient behaviour may be affected if the specificity of the test is known, as could be the conviction with which a clinician would be able to tell the patient that they have cirrhosis if the positive predictive value of a NILT replacement strategy was low. A gain in QALYs associated with a biopsy, could also change the conclusion regarding the more cost-effective strategy when comparing biopsying all patients with diagnosing all patients with cirrhosis; if the gain per patient was > 0.19 QALYs, biopsying all patients would become the more cost-effective strategy in all scenarios.
Until better data are available for a large number of model parameters, no conclusion can be provided regarding the cost-effectiveness of NILTs in ALD. In particular, the following parameters need to be the subject of further research; however, this list does not indicate a priority order, which cannot be determined given the present limited data:
-
the sensitivity and specificity of biopsy compared with a gold standard of post-mortem assessment of fibrosis
-
the sensitivity and specificity of each NILT against a gold standard of post-mortem assessment of fibrosis (or failing that biopsy) at validated and pre-selected cut-off thresholds for the various degrees of liver damage
-
the influence of potential confounding variables, such as current drinking behaviour and the degree of hepatic inflammation, on the performance of NILTs
-
differential information on the percentage of alcohol misusers who will develop alcohol-related cirrhosis over time, by age at onset, gender and ethnic origin
-
the likelihood, and magnitude, of decreases in abstinence rates associated with a diagnosis of significant ALD by diagnostic modality compared with biopsy
-
the incidental gains in QALYs that may be associated with biopsy, owing to the determination of non-ALD-related aetiologies.
It is also noted that this report has addressed neither the issue of whether or not the provision of suitable care facilities, both before and after diagnosis, is sufficient nor the potential implications of regional variation in practice. These are key issues that would benefit from future research.
It is noted, as a limitation of the report, that study selection and data analysis were undertaken by one reviewer.
Acknowledgements
The authors acknowledge the clinical advice provided by Dr Erika Denton, Dr Dermot Gleeson, Dr Carsten Grimm, Dr Sally Hope, Helen Manley, Gerri Mortimore, Dr Phil Newsome, Dr Stephen Pereira, Dr Helen Reeves, Professor William Rosenberg and Dr Sandy Smith. The authors also wish to thank Andrea Shippam who organised the retrieval of papers and helped in preparing and formatting the report.
Contributions of authors
Matt Stevenson constructed the mathematical model and interpreted the results. Myfanwy Lloyd Jones performed the systematic review and interpretation of evidence. Marsha Morgan provided clinical advice throughout the project and contributed to the writing of the clinically related sections of the monograph. Ruth Wong performed the literature search. Both Myfanwy Lloyd Jones and Matt Stevenson were involved in writing the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health 2003;27:209-19.
- Levitsky J, Mailliard ME. Diagnosis and therapy of alcoholic liver disease. Semin Liver Dis 2004;24:233-47.
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18.
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31.
- Office for National Statistics . Mortality Statistics. Deaths Registered in 2008 2009. http://www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--deaths-registered-in-england-and-wales--series-dr-/2008/index.html (accessed 5 October 2011).
- Patient UK. Recommended Safe Limits of Alcohol 2010. http://www.patient.co.uk/health/Recommended-Safe-Limits-of-Alcohol.htm (accessed 5 October 2011).
- Department of Health . Sensible Drinking. The Report of an Inter-Departmental Working Group 1995. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4084702.pdf (accessed 5 October 2011).
- Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J 2000;76:280-6.
- The National Clinical Guideline Centre for Acute and Chronic Conditions . Use Disorders: Diagnosis and Clinical Management of Alcohol-Related Physical 2010.
- Marsano LS, Mendez C, Hill D, Barve S, McClain CJ. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health 2003;27:247-56.
- Fontaine H, Petitprez K, Roudot-Thoraval F, Trinchet J-C. Guidelines for the diagnosis of uncomplicated cirrhosis. Gastroenterol Clin Biol 2007;31:504-9.
- Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1-8.
- Thabut D, Imbert-Bismut F, Cazals-Hatem D, Messous D, Muntenau M, Valla DC, et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther 2007;26:359-68.
- Williams R. The pervading influence of alcoholic liver disease in hepatology. Alcohol Alcohol 2008;43:393-7.
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598-607.
- Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut 2006;55:569-78.
- Friedrich-Rust M, Zeuzem S. Reproducibility and limitations of transient elastography. Liver Int 2009;29:619-20.
- Cobbold JF, Morin S, Taylor-Robinson SD. Transient elastography for the assessment of chronic liver disease: ready for the clinic?. World J Gastroenterol 2007;13:4791-7.
- Afdhal NH, Curry M. Technology evaluation: a critical step in the clinical utilization of novel diagnostic tests for liver fibrosis. J Hepatol 2007;46:543-5.
- Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. BMJ 1981;282:263-6.
- Verrill C, Markham H, Templeton A, Carr NJ, Sheron N. Alcohol-related cirrhosis – early abstinence is a key factor in prognosis, even in the most severe cases. Addiction 2009;104.
- Orrego H, Blake JE, Blendis LM, Medline A. Prognosis of alcoholic cirrhosis in the presence and absence of alcoholic hepatitis. Gastroenterology 1987;92:208-14.
- Chedid A, Mendenhall CL, Gartside P, French SW, Chen T, Rabin L. Prognostic factors in alcoholic liver disease. Am J Gastroenterol 1991;86:210-16.
- Cancer Research UK . Statistics and Outlook for Liver Cancer 2009. http://cancerhelp.cancerresearchuk.org/type/liver-cancer/treatment/statistics-and-outlook-for-liver-cancer (accessed 5 October 2011).
- Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther 2004;19:707-14.
- Day CP, Gilvarry E, Butler TJ, James OFW. Moderate alcohol intake is not deleterious in patients with alcoholic liver disease. Hepatology 1996;24.
- De Gottardi A, Spahr L, Gelez P, Morard I, Mentha G, Guillaud O, et al. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med 2007;167:1183-8.
- Gleeson D. 2009 Annual Evidence Update on Alcoholic Liver Disease – Diagnosis 2009. http://www.library.nhs.uk/Gastroliver/ViewResource.aspx?resID=332177 (accessed 15 December 2009).
- Department of Health; Home Office; Department for Education and Skills; Department for Culture, Media and Sport . Safe. Sensible. Social. The Next Steps in the National Alcohol Strategy 2007. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_075219.pdf (accessed 5 October 2011).
- Fuller E, Craig R, Shelton N. Health Survey for England. Volume 1 Healthy lifestyles: knowledge, attitudes and behaviour. London: The Information Centre for Health and Social Care; 2008.
- Fuller E, Jotangia D, Farrell M, McManus S, Meltzer H, Brugha T, et al. Adult psychiatric morbidity in England, 2007. Results of a household survey. London: The Health and Social Care Information Centre; 2009.
- Statistics for Wales . Welsh Health Survey 2008 2009. http://wales.gov.uk/topics/statistics/publications/publication-archive/healthsurvey2008/?lang=en (accessed 5 October 2011).
- Gharial N. Alcohol as a problem for the south Asian community. UK Alcohol Alert 2007;1:21-2.
- Douds AC, Cox MA, Iqbal TH, Cooper BT. Ethinic differences in cirrhosis of the liver in a British city: alcoholic cirrhosis in South Asian men. Alcohol Alcohol 2003;38:148-50.
- British Liver Trust . Fighting Liver Disease: Facts about Liver Disease 2009. http://www.britishlivertrust.org.uk/home/about-us/media-centre/facts-about-liver-disease.aspx (accessed 10 December 2009).
- Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 2002;35:635-8.
- Hart CL, Morrison DS, Batty GD, Davey Smith G. Effect of body mas index and liver consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340.
- The information centre for health and social care . Health Survey for England – 2008. Adult Trend Tables 2009. http://www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles-related-surveys/health-survey-for-england/health-survey-for-england--2008-trend-tables (accessed 5 July 2010).
- National Heart Forum . Type 2 Diabetes 2006. http://www.heartforum.org.uk/AboutCHD_riskfac_type2diab.aspx (accessed 5 July 2010).
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008;49:732-8.
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51.
- de Franchis R, Dell’Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis 2008;40:312-17.
- Herrera JL, Rodriguez R. Medical care of the patient with compensated cirrhosis. Gastroenterol Hepatol 2006;2:124-33.
- Sandhu BS, Sanyal AJ. Management of ascites in cirrhosis. Clin Liver Dis 2005;9:715-32.
- Sargent S. The management and nursing care of cirrhotic ascites. Br J Nurs 2006;15:212-19.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004;19:253-67.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007;25:3-9.
- NHS Information Centre . Statistics on Alcohol: England, 2009 2009.
- Coles EC. Alcohol and health in Wales: a major public health issue. Cardiff: National Public Health Service for Wales; 2006.
- Welch C, Harrison D, Short A, Rowan K. The increasing burden of alcoholic liver disease on United Kingdom critical care units: secondary analysis of a high quality clinical database. J Health Serv Res Policy 2008;13:40-4.
- National Institute for Health Research National Horizon Scanning Centre . Enhanced Liver Fibrosis Test (ELF) for Evaluating Liver Fibrosis 2008.
- Stewart SF, Day CP. The management of alcoholic liver disease. J Hepatol 2003;38:S2-S13.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis – a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008;27:1167-78.
- Thabut D, Moreau R, Lebrec D. Screening for esophageal varices: endoscopy, other tools, or endoscopy and other tools?. Hepatology 2008;47:1434-6.
- Sotiropoulos GC, Beckebaum S, Lang H, Frilling A, Molmenti EP, Cicinnati VR, et al. Single-center experience on liver transplantation for hepatocellular carcinoma arising in alcoholic cirrhosis: results and ethical issues. Eur Surg Res 2008;40:7-13.
- Stamuli E, Kruger J, Hutton J. Cost-Effectiveness of Ultrasound Elastography in the Assessment of Liver Fibrosis 2009.
- Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 2010;59:1245-51.
- iQur Ltd . Enhanced Liver Fibrosis Test (ELFTM Test) 2006. http://www.iqur.com/Sampleprep.html (accessed 22 December 2009).
- Campbell S, Timms PM, Maxwell PR, Doherty EM, Rahman MZ, Lean ME, et al. Effect of alcohol withdrawal on liver transaminase levels and markers of liver fibrosis. J Gastroenterol Hepatol 2001;16:1254-9.
- Tsutsumi M, Urashima S, Takase S, Ueshima Y, Tsuchishima M, Shimanaka K, et al. Characteristics of serum hyaluronate concentrations in patients with alcoholic liver disease. Alcohol Clin Exp Res 7010;21:1716-21.
- Nouchi T, Worner TM, Sato S, Lieber CS. Serum procollagen type III N-terminal peptides and laminin Pl peptide in slcoholic liver disease. Alcohol Clin Exp Res 1987;11:7-287.
- Lieber CS, Weiss DG, Paronetto F. Veterans Affairs Cooperative Study 391 Group . Value of fibrosis markers for staging liver fibrosis in patients with precirrhotic alcoholic liver disease. Alcohol Clin Exp Res 2008;32:1031-9.
- Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango?. Gut 2010;59:861-6.
- National Institute for Health Research National Horizon Scanning Centre . Fibro-Test-ActiTest™ for Diagnosis and Monitoring of Fibrosis in Chronic Liver Conditions 2005.
- Poynard T, Halfon P, Castera L, Charlotte F, Le Bail B, Munteanus M, et al. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther 2007;25:733-9.
- Rosenthal-Allieri MA, Peritore ML, Tran A, Halfon P, Benzaken S, Bernard A. Analytical variability of the fibrotest proteins. Clin Biochem 2005;38:473-8.
- Morra R, Munteanu M, Imbert-Bismut F, Messous D, Ratziu W, Poynard T. FibroMAXTM: towards a new universal biomarker of liver disease?. Expert Rev Mol Diagn 2007;7:481-90.
- BioPredictive . Practice of FibroMax for Alcoholic Liver Disease 2010. http://www.biopredictive.com/intl/physician/physicians/fibromax-for-alcohol/ (accessed 8 July 2010).
- BioPredictive . Let’s Assess Our Liver 2009. http://www.biopredictive.com/intl/patient (accessed 22 December 2009).
- Halfon P, Imbert-Bismut F, Messous D, Antoniotti G, Benchetrit D, Cart-Lamy P, et al. A prospective assessment of the inter-laboratory variability of biochemical markers of fibrosis (FibroTest) and activity (ActiTest) in patients with chronic liver disease. Comp Hepatol 2002;1.
- Calès P, Veillon P, Konaté A, Mathieu E, Ternisien C, Chevailler A, et al. Reproducibility of blood tests of liver fibrosis in clinical practice. Clin Biochem 2008;41:10-8.
- Gressner OA, Beer N, Jodlowski A, Gressner AM. Impact of quality control accepted inter-laboratory variations on calculated Fibrotest/Actitest scores for the non-invasive biochemical assessment of liver fibrosis. Clin Chim Acta 2009;409:90-5.
- Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem 2004;50:1344-55.
- MedlinePlus . Bilirubin – Blood 2009. http://www.nlm.nih.gov/medlineplus/ency/article/003479.htm (accessed 5 January 2010).
- National Institute for Health Research National Horizon Scanning Centre . Transient Elastography (FibroScan) for Evaluating Liver Fibrosis 2008.
- Echosens . FibroScan 2009. http://www.echosens.com/pdf/3en.pdf (accessed 22 December 2009).
- Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005;41:48-54.
- Poynard T, Ingiliz P, Elkrief L, Munteanu M, Lebray P, Morra R, et al. Concordance in a world without a gold standard: a new non-invasive methodology for improving accuracy of fibrosis markers. PLoS ONE 2008;3.
- Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010;51:828-35.
- Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008;48:606-13.
- Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343-50.
- Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835-47.
- Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009;29:1500-6.
- Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007;56:968-73.
- Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Bio 2003;29:1705-13.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495-500.
- Patient UK. Liver Biopsy 2010. http://www.patient.co.uk/health/Biopsy-Liver.htm (accessed 29 April 2010).
- Neuberger J, Grant A, Day C, Saxseena S. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology; 2004.
- Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614-18.
- Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-57.
- Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Arch Intern Med 1979;139:667-9.
- Maharaj B, Maharaj RJ, Leary WP. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1986;1:523-5.
- Murtagh J, Foerster V. Transient elastography (FibroScan) for non-invasive assessment of liver fibrosis. Issues Emerg Health Technol 2006;90:1-4.
- The French METAVIR Cooperative Study Group . Intraobserver and interobserver variations liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15-20.
- Plebani M, Basso D. Non-invasive assessment of chronic liver and gastric diseases. Clin Chim Acta 2007;381:39-4.
- Van Thiel DH, Gavaler JS, Wright H, Tzakis A. Liver biopsy. Its safety and complications as seen at a liver transplant center. Transplantation 1993;55:1087-90.
- Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, et al. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther 2008;28:1102-10.
- Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn!. Indian J Gastroenterol 2008;27:74-80.
- Thabut D, Trabut JB, Massard J, Rudler M, Muntenau M, Messous D, et al. Non-invasive diagnosis of large oesophageal varices with FibroTest in patients with cirrhosis: a preliminary retrospective study. Liver Int 2006;26:271-8.
- Bosch J. Predictions from a hard liver. J Hepatol 2006;45:174-7.
- Eisen G, Baron TH, Dominitz J, Faigel DO, Goldstein JL, Johanson JF, et al. Complications of upper GI endoscopy. Gastrointest Endosc 2002;55:784-93.
- Artemis Medical . Echosens Fibroscan 2009. http://www.artemismedical.co.uk/fibroscan_installations.htm (accessed 29 April 2010).
- Deeks JJ, Egger M, Davey Smith G, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001.
- Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol 2009;50:1-3.
- Lambert J, Halfon P, Penaranda G, Bedossa P, Cacoub P, Carrat F. How to measure the diagnostic accuracy of noninvasive liver fibrosis indices: the area under the ROC curve revisited. Clin Chem 2008;54:1372-8.
- Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, et al. FibroPaca Group Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem 2007;53:1615-22.
- Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol 2005;42:S22-S36.
- National Institute for Health and Clinical Excellence (NICE) . Alcohol Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence 2010. http://www.nice.org.uk/nicemedia/live/11875/49448/49448.pdf (accessed 7 July 2010).
- Longworth L, Young T, Buxton MJ, Ratcliffe J, Neuberger J, Burroughs A, et al. CELT Project Team Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transpl 2003;9:1295-307.
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 1999;354:1896-900.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:332-9.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Friedrich-Rust M, Ong M, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960-74.
- Berlin JA. Does blinding of readers affect the results of meta-analyses?. Lancet 1997;350:185-6.
- Clark HD, Wells JA, Huet C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Clin Trials 1999;20:448-52.
- Deeks JJ. Systematic reviews in heatlh care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-62.
- Janssens F, de Suray N, Piessevaux H, Horsmans Y, de Timary P, Starkel P. Can transient elastography replace liver histology for determinatiaon of advanced fibrosis in alcoholic patients: a real-life study. J Clin Gastroenterol 2010;44:S75-82.
- Mueller S, Millonig G, Sarovska L, Friedrich S, Riemann FM, Pritsch M, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol 2010;16:966-72.
- Janssens F, de Surray N, Horsmans Y, Piessevaux H, de Timary P, Starkel P. Determination of severe fibrosis and cirrhosis in alcoholic patients by transient elastography: a prospective comparison with liver biopsy. J Hepatol 2009;50.
- Mueller S, Millonig G, Friedrich S, Welker A, Becker P, Reimann F, et al. Diagnosis of cirrhosis in alcoholic liver disease (ALD): is there a place for transient elastography?. Gastroenterology 2008;134:A307-8.
- Rosenberg WM, Voelker M, Thiel R. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704-13.
- Melin P, Schoeny M, Dacon A, Gauchet A, Diebold MD. Dépistage non invasif de la fibrose hépatique. Intérêt du FibroScan(R) en consultation d’alcoologie. Alcoologie Et Addictologie 2005;27:191-6.
- Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, Messous D, et al. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin Gastroenterol Hepatol 2005;3:167-74.
- Naveau S, Gaudé G, Asnacios A, Agostini H, Abella A, Barri-Ova N, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 2009;49:97-105.
- Thabut D, Trabut JB, Le Calvez S, Thibaut V, Massard J, d’Arondel C, et al. Diagnostic value of fibrosis biochemical markers (FibroTest) for the screening of oesophageal varices in patients with chronic liver disease. Hepatology 2003;38.
- Thabut D, Lebrec D, Oberti F, Perarnau J, Condat B, Barraud H, et al. Prognostic value of algorithms combining FibroTest®FibroSURE® (FT) and AshTest® (HT) in comparison with MELD and Pugh prognostic indexes in patients with severe cirrhosis. Hepatology 2007;46.
- Kim SG, Kim YS, Jung SW, Kim HK, Jang JY, Moon JH, et al. The usefulness of transient elastography to diagnose cirrhosis in patients with alcoholic liver disease. Korean J Hepatol 2009;15:42-51.
- Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Ledinghen V, Douvin C, et al. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol 2008;49:1062-8.
- Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther 2008;27:1261-8.
- Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL. Non-invasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis aetiology. Hepatology 2009;50.
- Nahon P, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Ganne-Carrie N, et al. Assessment of liver fibrosis using liver stiffness measurement (LSM) in patients with alcoholic liver disease. J Hepatol 2007;46:S278-S279.
- Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP, et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther 2008;28:1188-98.
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996;24:289-93.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 2010;19:1409-17.
- Chevallier M, Guerret S, Chossegros P, Gerard F, Grimaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology 1994;20:349-55.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94.
- Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 2010;54:650-9.
- Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007;46:628-34.
- de Ledinghen V, Fournier C, Foucher J, Miette V, Vergniol J, Rigalleau V, et al. New FibroScan probe for obese patients. A pilot study of feasibility and performances in patients with BMI ≥ 30 kg/m2. J Hepatol 2009;50.
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008;47:592-5.
- Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008;47:380-4.
- Ghany MG, Doo E. Assessment of liver fibrosis: palpate, poke or pulse?. Hepatology 2005;42:759-61.
- Bosch J. Towards the non-invasive diagnosis of cirrhosis: the nuts-cirrhosis connection. J Hepatol 2009;50:4-6.
- Melin P, Dacon A, Gauchet A, Schoeny M, Fournier C, Sandrin L, et al. Cirrhosis screening in alcoholism consultation using FibroScan (R). Hepatology 2005;42:492A-3A.
- Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ 1994;309.
- van Stralen KJ, Stel VS, Reitsma JB, Dekker FW, Zoccali C, Jager KJ. Diagnostic methods I: sensitivity, specificity, and other measures of accuracy. Kidney Int 2009;75:1257-63.
- Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol 2009;50:36-41.
- Rosenberg WM. Rating fibrosis progression in chronic liver diseases. J Hepatol 2003;38:357-60.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisals 2008. http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed October 2009).
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009;24:786-91.
- Curtis L. Unit costs of health and social care. Canterbury: PSSRU, University of Kent; 2009.
- Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer 2008;98:1166-75.
- Wright M, Main J, Goldin RD, Thomas HC. Health benefits from anti viral therapy for mild chronic hepatitis C. A UK multi-centre randomised controlled trial. Hepatology 2003;38.
- Department of Health . NHS Reference Costs 2007–08 2009.
- Huang E, Esrailian E, Spiegel BM. The cost-effectiveness and budget impact of competing therapies in hepatic encephalopathy – a decision analysis. Aliment Pharmacol Ther 2007;26:1147-61.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8.
- Arguedas MR, Heudebert GR, Eloubeidi MA, Abrams GA, Fallon MB. Cost-effectiveness of screening, surveillance, and primary prophylaxis strategies for esophageal varices. Am J Gastroenterol 2002;97:2441-52.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2010.
- Thabut D, Hammer M, Cai Y, Carbonell N. Cost of treatment of oesophageal variceal bleeding in patients with cirrhosis in France: results of a French survey (brief record). Eur J Gastroenterol Hepatol 2007;19:679-86.
- Wechowski J, Connolly M, Woehl A, Tetlow A, McEwan P, Burroughs A, et al. An economic evaluation of vasoactive agents used in the United Kingdom for acute bleeding oesophageal varices in patients with liver cirrhosis. Curr Med Res Opin 2007;23:1481-91.
- Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Economics 2005;14:339-47.
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431-5.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9.
- Scheuer PJ. Classification of chronic viral hepatitis. A need for reassessment. J Hepatol 1991;13:372-4.
- Beddy P, Lyburn IL, Geoghegan T, Buckley O, Buckley AR, Torreggiani WC. Outpatient liver biopsy: a prospective evaluation of 500 cases. Gut 2007;56.
- Caturelli E, Giacobbe A, Facciorusso D, Bisceglia M, Villani MR, Siena DA, et al. Percutaneous biopsy in diffuse liver disease: increasing diagnostic yield and decreasing complication rate by routine ultrasound assessment of puncture site. Am J Gastroenterol 1996;91:1318-21.
- Douds AC, Joseph AE, Finlayson C, Maxwell JD. Is day case liver biopsy underutilised?. Gut 1995;37:574-5.
- Firpi RJ, Soldevila PC, Abdelmalek MF, Morelli G, Judah J, Nelson DR. Short recovery time after percutaneous liver biopsy: should we change our current practices?. Clin Gastroenterol Hepatol 2005;3:926-9.
- Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut 1995;36:437-41.
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993;118:96-8.
- Lindor KD, Bru C, Jorgensen RA, Rakela J, Bordas JM, Gross JB, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996;23:1079-83.
- Little AF, Ferris JV, Dodd GD. Image-guided percutaneous hepatic biopsy: effect of ascites on the complication rate. Radiology 1996;199:79-83.
- Maharaj B, Bhoora IG. Complications associated with percutaneous needle biopsy of the liver when one, two or three specimens are taken. Postgrad Med J 1992;68:964-7.
- Manolakopoulos S, Triantos C, Bethanis S, Theodoropoulos J, Vlachogiannakos J, Cholongitas E, et al. Ultrasound-guided liver biopsy in real life: comparison of same-day prebiopsy versus real-time ultrasound approach. J Gastroenterol Hepatol 2007;22:1490-3.
- McVay PA, Toy PT. Lack of increased bleeding after liver biopsy in patients with mild hemostatic abnormalities. Am J Pathol 1990;94:747-53.
- Sheets PW, Brumbaugh CJ, Kopecky KK. Safety and efficacy of a spring-propelled 18-gauge needle for US-guided liver biopsy. J Vase Interv Radiol 1991;2:147-9.
- Smith BC, Desmond PV. Outpatient liver biopsy using ultrasound guidance and the biopty gun is safe and cost effective. Aust New Zeal J Med 1995;25:209-11.
- Thampanitchawong P, Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol 1999;5:301-4.
- van der Poorten D, Kwok A, Lam T, Ridley L, Jones DB, Ngu MC, et al. Twenty-year audit of percutaenous liver biopsy in a major Australian teaching hospital. Intern Med J 2006;36:692-9.
- Vivas S, Palacio MA, Rodriguez M, Lomo J, Cadenas F, Giganto F, et al. Biopsia hepática ambulatoria: complicaciones y evolución en 264 casos/ambulatory liver biopsy: complications and evolution in 264 cases. Rev Esp Enferm Dig 1998;90:175-82.
- Chevallier P, Ruitort F, Denys A, Staccini P, Saint-Paul MC, Ouzan D, et al. Influence of operator experience on performance of ultrasound-guided percutaneous liver biopsy. Eur Radiol 2004;14:2086-91.
- Colombo M, Del Ninno E, de Franchis R, De Fazio C, Festorazzi S, Ronchi G, et al. Ultrasound-assisted percutaneous liver biopsy: superiority of the Tru-Cut over the Menghini needle for diagnosis of cirrhosis. Gastroenterology 1988;95:487-9.
- Denzer U, Arnoldy A, Kanzler S, Galle PR, Dienes HP, Lohse AW. Prospective randomized comparison of minilaparoscopy and percutaneous liver biopsy: diagnosis of cirrhosis and complications. J Clin Gastroenterol 2007;41:103-10.
- Farrell RJ, Smiddy PF, Pilkington RM, Tobin AA, Mooney EE, Temperley IJ, et al. Guided versus blind liver biopsy for chronic hepatitis C: clinical benefits and costs. J Hepatol 1999;30:580-7.
- Papini E, Pacella CM, Rossi Z, Bizzarri G, Fabbrini R, Nardi F, et al. A randomized trial of ultrasound-guided anterior subcostal liver biopsy versus the conventional Menghini technique. J Hepatol 1991;13:291-7.
- Terjung B, Lemnitzer I, Dumoulin FL. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion 2003;67:138-45.
- Wawrzynowicz-Syczewska M, Kruszewski T, Boron-Kaczmarska A. Complications of percutaneous liver biopsy. Rom J Gastroenterol 2002;11:105-7.
- Weigand K. Percutaneous liver biopsy: retrospective study over 15 years comparing 287 inpatients with 428 outpatients. J Gastroenterol Hepatol 2009;24:792-9.
- Choh J, Dolmatch B, Safadi R, Long P, Geisinger M, Lammert G, et al. Transjugular core liver biopsy with a 19-gauge spring-loaded cutting needle. Cardiovasc Intervent Radiol 1998;21:88-90.
- Papatheodoridis GV, Patch D, Watkinson A, Tibballs J, Burroughs AK. Transjugular liver biopsy in the 1990s: a 2-year audit. Aliment Pharmacol Ther 1999;13:603-8.
- Soyer P, Fargeaudou Y, Boudiaf M, Rymer R. Transjugular liver biopsy using ultrasonographic guidance for jugular vein puncture and an automated device for hepatic tissue sampling: a retrospective analysis of 200 consecutive cases. Abdom Imaging 2008;33:627-32.
- Vlavianos P, Bird G, Portmann B, Westaby D, Williams R. Transjugular liver biopsy: Use in a selected high risk population. Eur J Gastroenterol Hepatol 1991;3:469-72.
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007;102:2589-600.
- Calès P, Oberti F, Rousselet MC, Gallois Y, Bedossa P. Usual biochemical tests are not suitable surrogate markers for the degree of liver fibrosis. J Hepatol 2002;36.
- Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konate A, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005;42:1373-81.
- Clevert D, Zachoval R, Reiser M. Acoustic radiation force impulse technology and Fibroscan in the evaluation of liver diseases. AJR Am J Roentgenol 2009;192.
- de Ledinghen V, Laharie D, Foucher J, Bernard P, Castera L, Adhoute X, et al. Assessment of cirrhosis and its severity by Fibroscan(R) and biochemical markers in alcoholic patients. 2006. Los Angeles; 2006.
- Foucher J, Castera L, Bernard PH, Adhoute X, Bertet J, Couzigou P, et al. Assessment of cirrhosis and its severity by Fibroscan and biochemical markers in alcoholic patients. J Hepatol 2006;44.
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8.
- Jimenez-Ridruejo JM, Gomez-Dominguez E, Otero RM. Measurement of hepatic elasticity: potential usefulness of Fibroscan. Gastroenterologia Y Hepatologia Continuada 2008;7:27-30.
- Laharie D, Foucher J, Bernard PH, Castera L, Adhoute X, Bertet J, et al. Assessment of cirrhosis and its severity by Fibroscan and biochemical markers in alcoholic patients. Gastroenterology 2006;130.
- Lee da M, Moon EJ, Hwang JA, Lee MS, Cheong JY, Cho SW, et al. Factors associated with liver stiffness in chronic liver disease. Korean J Hepatol 2009;15:464-73.
- Marin-Gabriel JC, De la Cruz JB, Tocado M, Rodriguez-Gil Y, Fernandez-Vazquez I, Martin-Algibez AM, et al. Failure of liver stiffness measurement with Fibroscan: prevalence and prognostic score. Gastroenterology 2008;134.
- Melin P, Dacon A, Gauchet A, Schoeny M, Diebold MD. Interest of the FibroScan in the screening of cirrhosis in patients attending alcoholism consultation. J Hepatol 2005;42.
- Morozov SV, Trufanova, Iu M, Pavlova TV, Isakov VA, Kaganov BS. Elastography for determination of hepatic fibrosis severity: registration study in Russia. Eksp Klin Gastroenterol 2008;2:40-7.
- Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann F, Pritsch M, et al. Alcoholic steatohepatitis increases liver stiffness independent of fibrosis stage: criteria for noninvasive fibrosis assessment. J Hepatol 2009;50.
- Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, Messous D, et al. Diagnostic value of biochemical markers (FibroTest) for the prediction of liver fibrosis in patients with chronic alcoholic liver disease (ALD). Hepatology 2003;38.
- Naveau S, Gaudé G, Asnacios A, Agostini H, Abella A, Barri-Ova N, et al. Diagnostic and 10-year prognostic values of non-invasive biomarkers of fibrosis in patients with Alcoholic liver disease (ALD). Hepatology 2008;48.
- Nguyen-Khac E, Robert B, Brevet M, Tramier B, Decrombecque C, Joly JP, et al. Assessment of asymptomatic liver fibrosis in alcohol abuser patients by transient elastography (Fibroscan). J Hepatol 2007;46.
- Parkes J, Roderick P, Harris S, Gough C, Wheatley M, Alexander GJ, et al. European liver fibrosis (ELF) panel of serum markers can predict clinical outcome in a cohort of patients from England with mixed aetiology chronic liver disease. Hepatology 2007;46.
- Rosenberg WM, Burt A, Hubscher S, Roskams T, Voelker M, Becka M, et al. Serum markers predict liver fibrosis. Hepatology 2001;34.
- Thabut D, Lebrec D, Imbert-Bismut F, Cazals-Hatem D, Moreau R, Messous D, et al. Diagnostic value of fibrosis biochemical markers (FibroTest) for the prediction of portal hypertension in chronic liver disease. Hepatology 2003;38.
- Galena HJ. Complications occurring from diagnostic venipuncture. J Fam Pract 1992;34:582-4.
- Horowitz SH. Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology 1994;44.
- Stitik TP, Foye PM, Nadler SF, Brachman GO. Phlebotomy-related lateral antebrachial cutaneous nerve injury. Am J Phys Med Rehabil 2001;80:230-4.
- Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord 2006;20:946-60.
- Godwin PG, Cuthbert AC, Choyce A. Reducing bruising after venepuncture. Qual Health Care 1992;1:245-6.
- Choffel C, Duterque M, Milleron B, Bernard A. Sarcoid nodules of the skin at the site of venipuncture. Nouv Presse Med 1981;10:697-9.
- Nouri M, Rozema C, Nouri M, Rouchet M, Bailly M. Radial neuropathy after peripheral venous puncture. Ann Fr Anesth Reanim 2000;19:39-41.
- Pradhan S, Gupta A. Iatrogenic median and femoral neuropathy. J Assoc Physicians India 1995;43.
- Saeed MA, Gatens PF. Anterior interosseous nerve syndrome: unusual etiologies. Arch Phys Med Rehabil 1983;64.
- Sander HW, Conigliari MF, Masdeu JC. Antecubital phlebotomy complicated by lateral antebrachial cutaneous neuropathy. N Engl J Med 1998;339.
- Vidal D, Barnadas M, Perez M, Coll P, Alomar A. Tuberculous gumma following venepuncture. Br J Dermatol 2001;144:601-3.
- Zubairy AI. How safe is blood sampling? Anterior interosseus nerve injury by venepuncture. Postgrad Med J 2002;78.
- Rodriguez Guerrero FJ, Seda Diestro J, Martin Llamas J. Aparición de hematomas asociados a la extracción de sangre venosa mediante vacío. Enferm Clin 2003;13:81-6.
- Berry PR, Wallis WE. Venepuncture nerve injuries. Lancet 1977;1:1236-7.
- Burgdorf WH, Hoxtell EO, Bart BJ. Sarcoid granulomas in venipuncture sites. Cutis 1979;24:52-3.
- Norcross WA, Shackford SR. Arteriovenous fistula. A potential complication of venipuncture. Arch Intern Med 1988;148:1815-6.
- Yuan RT, Cohen MJ. Lateral antebrachial cutaneous nerve injury as a complication of phlebotomy. Plast Reconstr Surg 1985;76:299-300.
- Wakita R, Ohno Y, Yamazaki S, Kohase H, Umino M. Vasovagal syncope with asystole associated with intravenous access. Oral Surge Oral Med Oral Pathol Oral Radiol Endod 2006;102:e28-e32.
- Horowitz SH. Venipuncture-induced causalgia: anatomic relations of upper extremity superficial veins and nerves, and clinical considerations. Transfusion 2000;40:1036-40.
- Newman BH, Waxman DA. Blood donation-related neurologic needle injury: evaluation of 2 years’ worth of data from a large blood center. Transfusion 1996;36:213-15.
Appendix 1 Categorisation of disease progression as identified by liver biopsy
In chronic liver disease, the liver may be affected by inflammation or fibrosis or both. The term ‘grading’ is conventionally used to describe the degree of inflammatory activity, whereas the term ‘staging’ is used to describe the degree of fibrosis and also architectural change. 15
A number of systems have been developed to measure and categorise these factors as identified by liver biopsy. These include the Knodell Histological Activity Index (HAI),164 the Ishak-modified HAI,165 and the Scheuer,166 Batts–Ludwig,134 Brunt,137 and METAVIR133 scoring systems. Some of these were developed for chronic liver disease of a specific aetiology, for example Brunt137 and Kleiner136 for NAFLD and METAVIR for hepatitis C. 94
The studies reviewed in this report that evaluated the test accuracy of NILTs using liver biopsy as their reference standard most commonly used the METAVIR staging system to measure the degree of fibrosis; however, some used other systems. For comparative purposes, the staging systems used in the included studies are summarised in Table 36. It should be noted that, although the staging systems have numeric labels that imply a linear increase in fibrosis severity between stages, they are in fact ordinal. In other words, although the stages follow a logical ordering in terms of disease severity, they do not represent an underlying continuous scale of measurement such that equal differences between values in the scale represent equivalent differences in the degree of fibrosis (so, for instance, METAVIR stage F4 does not indicate twice as much fibrosis as METAVIR stage F2). However, the degree of fibrosis is a continuous variable and the non-invasive tests reviewed in this report measure continuous variables (serum biochemical markers or liver stiffness) yielding results that may occur at any point on the relevant scale.
| Stage/degree of fibrosis | ||||||
|---|---|---|---|---|---|---|
| METAVIR94 | Scheuer 1991166 | Brunt 1999137 | Kleiner 2005136 | Batts–Ludwig 2010134 | Knodell 1981164 | Ishak modified HAI165 |
| 0: No fibrosis | 0: No fibrosis | 0: No fibrosis | 0: No fibrosis | 0: No fibrosis | 0: No fibrosis | 0: No fibrosis |
| 1: Stellate enlargement of portal tract, but without septa formation | 1: Enlarged fibrotic portal tracts | 1: Zone 3 perisinusoidal/pericellular fibrosis; focally or extensively present | 1: Perisinusoidal or periportal | 1: Portal fibrosis (fibrous portal expansion) | 1: Fibrous portal expansion | 1: Fibrous expansion of some portal areas, with or without short fibrous septa |
| 1A: Mild, zone 3, perisinusoidal | ||||||
| 1B: Moderate, zone 3, perisinusoidal | 2: Fibrous expansion of most portal areas, with or without short fibrous septa | |||||
| 1C: Portal/periportal | ||||||
| 2: Enlargement of portal tract with rare septa formation | 2: Periportal or portal–portal septa, but intact architecture | 2: Zone 3 perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis | 2: Perisinusoidal and portal/periportal | 2: Periportal fibrosis (periportal or rare P–P septa) | 2: Not allocated | 3: Fibrous expansion of some portal areas, with occasional P–P bridging |
| 3: Numerous septa without fibrosis | 3: Fibrosis with architectural distortion, but no obvious cirrhosis | 3: Zone 3 perisinusoidal/pericellular fibrosis and portal fibrosis with focal or extensive bridging fibrosis | 3: Bridging fibrosis | 3: Septal fibrosis (fibrous septa with architectural distortion, but no obvious cirrhosis) | 3: Bridging fibrosis (P–P or P–C linkage) | 4: Fibrous expansion of some portal areas, with marked bridging (P–P as well as P–C) |
| 4: Cirrhosis | 4: Probable or definite cirrhosis | 4: Cirrhosis | 4: Cirrhosis | 4: Cirrhosis | 4: Cirrhosis | 5: Marked bridging (P–P and/or P–C) with occasional nodules (incomplete cirrhosis) |
| 6: Cirrhosis, probable or definite | ||||||
Appendix 2 The Alcohol Use Disorders Identification Test
The AUDIT provides a score based on the following series of questions. 31
-
How often do you have a drink containing alcohol?
-
Never (0)
-
Monthly or less (1)
-
Two to four times a month (2)
-
Two to three times a week (3)
-
Four or more times a week (4)
-
-
How many drinks containing alcohol do you have in a typical day when you are drinking?
-
1 or 2 (0)
-
3 or 4 (1)
-
5 or 6 (2)
-
7 to 9 (3)
-
10 or more (4)
-
-
How often do you have six or more drinks on any one occasion?
-
Never (0)
-
Less than monthly (1)
-
Monthly (2)
-
Weekly (3)
-
Daily or almost daily (4)
-
-
How often during the last year have you found that you were not able to stop drinking once you had started?
-
Never (0)
-
Less than monthly (1)
-
Monthly (2)
-
Weekly (3)
-
Daily or almost daily (4)
-
-
How often during the last year have you failed to do what was normally expected of you because of drinking?
-
Never (0)
-
Less than monthly (1)
-
Monthly (2)
-
Weekly (3)
-
Daily or almost daily (4)
-
-
How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
-
Never (0)
-
Less than monthly (1)
-
Monthly (2)
-
Weekly (3)
-
Daily or almost daily (4)
-
-
How often during the last year have you had a feeling of guilt or remorse after drinking?
-
Never (0)
-
Less than monthly (1)
-
Monthly (2)
-
Weekly (3)
-
Daily or almost daily (4)
-
-
How often during the last year have you been unable to remember what happened the night before because you had been drinking?
-
Never (0)
-
Less than monthly (1)
-
Monthly (2)
-
Weekly (3)
-
Daily or almost daily (4)
-
-
Have you or someone else been injured because of your drinking?
-
No (0)
-
Yes, but not in the last year (2)
-
Yes, during the last year (4)
-
-
Has a relative, friend, doctor or other health worker been concerned about your drinking or suggested that you should cut down?
-
No (0)
-
Yes, but not in the last year (2)
-
Yes, during the last year (4)
-
Appendix 3 Liver biopsy: systematic review of adverse events
A systematic review was carried out with the aim of identifying studies that reported adverse events in adults undergoing either percutaneous or transjugular liver biopsy for any form of suspected liver disease. Because the aim was to assess the incidence of adverse events relating to each biopsy route, studies that included < 100 relevant patients undergoing biopsy by each route were excluded, as were any studies in which results were not provided separately for each route.
Details of the literature searches and the inclusion and exclusion criteria may be found in Appendix 4.
The searches were not restricted by study type, language, or date. However, because of the possibility of changes in standards of care over time, studies were excluded if they included data relating to biopsies undertaken prior to 1980. Moreover, because of time constraints, it was not possible to include non-English-language papers in the systematic review.
The same three-stage sifting process was used as in the systematic review of clinical effectiveness. Data were extracted directly to the tables included in the report.
The electronic literature searches identified 2289 potentially relevant articles. Of these, 12 met the review’s inclusion criteria (for PRISMA diagram, see Appendix 4). Seventeen additional relevant articles were identified from citations. 96,167–182
Data from these studies relating to adverse events, both minor and severe, and deaths associated with liver biopsy are set out in Tables 37 and 38. Because patients undergoing transjugular biopsy are at higher risk of complications than those undergoing percutaneous biopsy, the two techniques have been considered separately.
| Study | Brief description of patient group | Number of patients | Details of biopsy, setting, operator, etc. | Design/study country/date of biopsies | Minor adverse events related to biopsy | Severe adverse events (including deaths) related to biopsy | Deaths related to biopsy | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | n | % (95% CI) | Definition | n | % (95% CI) | n | % (95% CI) | |||||
| Beddy 2007167 | Platelet count > 50,000/mm3, prothrombin time prolonged by < 3 seconds, without severe ascites or intrahepatic biliary dilatation | 500 | Ultrasound-guided, outpatient, radiologist |
Prospective Multinational Not clear; presumed from publication date to post-date 1980 |
Pain requiring analgesia | 15 | 3.00 (1.64 to 4.53) | Hospital admission for haemorrhagic complications | 1 | 0.20 (0.02 to 0.93) | 0 | 0.00 (0.00 to 0.50) |
| Caturelli 1996168 | Suspected chronic liver disease, platelet count > 40,000/mm3 and/or prothrombin activity < 40% of control value | 753 | Ultrasound used to identify biopsy site, 88% outpatients, three skilled gastro-enterologists |
Retrospective audit Italy Not clear; presumed from publication date to post-date 1980a |
Pain or haematoma at biopsy site, shoulder pain | 33 | 4.40 (2.91 to 5.71) | Haemoperitoneum, vasovagal reaction requiring atropine | 4 | 0.53 (0.18 to 1.25) | 0 | 0.00 (0.00 to 0.33) |
| Chevallier 2004183 | Patients mainly had HCV or ALD; exclusions included ascites and clotting disorders | 732 | Ultrasound used to identify biopsy site, some outpatients, experienced and inexperienced radiologists |
Comparative (experienced vs inexperienced operators) France 1998–2000 |
Minor vasovagal reaction | 7 | 0.96 (0.38 to 1.76) | Severe vasovagal reaction, haemorrhage | 2 | 0.27 (0.06 to 0.87) | 0 | 0.00 (0.00 to 0.34) |
| Significant pain 6 hours after biopsy | 44 | 6.00 (4.16 to 7.40) | ||||||||||
| Colombo 1988184 | Diffuse liver disease, prothrombin and partial thromboplastin times within 3 SD of the normal mean, platelet count > 80,000/mm3, bleeding times < 7 minutes | 1192 | Ultrasound used to identify biopsy site, inpatient, four equally experienced physicians |
Prospective (RCT comparing two needles) Italy 1982–5 |
Significant pain requiring analgesia, > 5% haematocrit drop not requiring blood transfusion, mild transient vagal shock | 66 | 5.54 (4.08 to 6.54) | Acute abdomen (resolved in 24 hours without treatment) | 1 | 0.08 (0.01 to 0.39) | 0 | 0.00 (0.00 to 0.21) |
| Denzer 2007185 | Adults with chronic liver disease or liver disease of unknown aetiology, without severe coagulation defects | 415 | Ultrasound used to identify biopsy site, inpatient and outpatient, four experienced investigators |
Prospective (one arm of RCT) Germany NR |
Pain, vasovagal symptoms, agitation/disquiet, minor intra-abdominal bleeding | 19 | 4.58 (2.66 to 6.49) | Complications requiring hospital stay > 24 hours or new hospitalisation (intra-abdominal bleeding or haemobilia) | 4 | 0.96 (0.32 to 2.25) | 0 | 0.00 (0.00 to 0.60) |
| Douds 1995169 | Not specified | 453 | Ultrasound used to identify biopsy site, day case or inpatient, no details regarding operator |
Retrospective audit UK 1989–93 |
Abdominal pain | 7 | 1.55 (0.62 to 2.82) | Haemorrhage, pneumothorax | 5 | 1.10 (0.36 to 2.23) | 1 | 0.22 (0.02 to 1.02) |
| Farrell 1999186 | Patients with chronic hepatitis C; platelet count > 100,000/mm3, prothrombin time prolonged by < 2 seconds | 166 | Ultrasound guidance used in 53% of cases, apparently inpatient, blind biopsies performed by experienced gastroenterology registrar, guided biopsies by experienced radiology registrar or consultant radiologist |
Prospective comparison of blind vs guided biopsy Ireland 1993–5 |
Pain-associated vasovagal symptoms, persistent biopsy-site pain | 9 | 5.42 (2.39 to 8.86) | Not given | 0 | 0.00 (0.00 to 1.50) | 0 | 0.00 (0.00 to 1.50) |
| Firpi 2005170 | Any patients undergoing biopsy | 3214 | Ultrasound guidance used to identify biopsy site from February 2002, outpatient, no information regarding operator |
Retrospective audit USA 1995–2004 |
Mild/moderate pain requiring limited analgesia | 418 | 13 (11.84 to 14.16) | Events resulting in death or hospitalisation either immediately or up to 2 weeks after biopsy | 34 | 1.06 (0.73 to 1.42) | 2 | 0.06 (0.01 to 0.68) |
| Minor bleeding at biopsy site | 129 | 4.00 (3.32 to 4.68) | ||||||||||
| Nausea and vomiting | 16 | 0.50 (0.26 to 0.74) | ||||||||||
| Gilmore 1995171 | Any patients undergoing biopsy | 1500 | Pre-biopsy imaging (usually ultrasound) used in 84%; 96% inpatient; 34% of operators radiologists, 33% general internal medicine, 28% gastroenterology, 5% other |
Retrospective audit UK 1991 |
Minor complications NR | NR | Bleeding | 26 | 1.73 (1.12 to 2.41) | 5 | 0.33 (0.11 to 0.68) | |
| Janes 1993172 | Patients with chronic liver diseases | 405 | Apparently no ultrasound guidance, outpatient, gastro-enterologists (junior doctors or staff physician) |
Retrospective USA 1989–91 |
Minor complications NR | NR | Hospital admission | 13 | 3.21 (1.67 to 4.97) | 0 | 0.00 (0.00 to 0.62) | |
| Lindor 1996173 | Excluded patients with liver transplant, history of previous biopsy complications or difficulty obtaining tissue, suspected liver mass or known haemangiomas in right lobe, known bleeding tendencies or significant ascites | 836 | With or without ultrasound guidance, outpatient, consultants, trained fellows, and experienced associates |
Prospective, controlled USA/Spain 1992–4 |
Bleeding or hypotension not requiring hospitalisation | 16 | 1.91 (1.08 to 2.89) | Hospitalisation for pain with or without hypotension, lacerated gall bladder | 11 | 1.32 (0.65 to 2.16) | 0 | 0.00 (0.00 to 0.30) |
| Little 1996174 | Patients with suspected focal hepatic lesions, diffuse liver disease, or malignant portal vein thrombus | 476 | Ultrasound or computerised tomography guidance, inpatient and outpatient, no details regarding operators |
Retrospective audit USA 1992–4 |
Decrease in haemoglobin or haematocrit value not requiring treatment | 25 | 5.25 (3.26 to 7.06) | Bleeding necessitating blood transfusion or surgery, or resulting in death | 16 | 3.36 (1.87 to 4.99) | 0 | 0.00 (0.00 to 0.53) |
| McVay 1990177 | Patients with mild haemostatic abnormalities | 177 | Apparently no ultrasound guidance, inpatient, gastroenterology fellows, radiology residents or attending physicians |
Retrospective audit USA 1983–7 |
Bleeding complications not requiring transfusions | 2 | 1.13 (023 to 3.54) | Bleeding complications requiring transfusions | 6 | 3.39 (1.22 to 6.29) | 1 | 0.56 (0.06 to 2.60) |
| Maharaj 1992175 | Any patients undergoing biopsy | 2646 | Apparently no ultrasound guidance, inpatient, medical and surgical interns |
Retrospective (1984–7) Prospective (1988–90) South Africa |
Pain responding to analgesics | 31 | 1.17 (0.79–1.60) | Intraperitoneal bleeding, pain, symptomatic hypotension, biliary peritonitis | 32 | 1.21 (0.82 to 1.64) | 8 | 0.30 (0.13 to 0.54) |
| Manolakopoulos 2007176 | Patients with diffuse liver disease | 631 | Pre-biopsy or real-time ultrasound, inpatient, operator experienced gastro-enterologist for pre-biopsy ultrasound, radiologist for real-time ultrasound |
Prospective comparison (pre-biopsy vs real-time ultrasound) Greece 1995–2005 |
Minor complications not reported | NR | Need for blood transfusion, surgical intervention, or hospital stay > 48 hours post-biopsy | 1 | 0.16 (0.02 to 0.74) | 0 | 0.00 (0.00 to 0.40) | |
| Papini 1991187 | Women with suspected chronic liver disease (bleeding time < 4 minutes, platelet count > 50,000/mm3, prothrombin activity > 50%) | 340 | Blind intercostal or ultrasound-guided subcostal, 10% outpatient, three experienced hepatologists |
Prospective Italy 1985–9 |
Asymptomatic haematoma, hypotension, haemothorax, ileus, self-limiting bleeding in thoracic cavity | 8b | 2.35 (1.00 to 4.11) | Strong pain needing treatment | 10c | 2.94 (1.38 to 4.84) | 0 | 0.00 (0.00 to 0.40) |
| Sheets 1991178 | Patients with suspected diffuse liver disease | 114 (203 biopsies) | Ultrasound guidance used, no data regarding inpatient/outpatient status or operator |
Not clear USA 1988–9 |
Complications not requiring treatment (syncope, severe pain) | 2 | 1.75 (0.36 to 5.42) | Complications requiring treatment (syncope, bleeding requiring transfusion) | 4 | 3.51 (1.15 to 7.86) | 0 | 0.00 (0.00 to 2.17) |
| Smith 1995179 | Any patients | 250 | Ultrasound guidance used, 70% outpatient, gastro-enterology registrar |
Retrospective audit Australia 1991–3 |
Prolonged pain requiring narcotic administration without hospital admission | 1 | 0.40 (0.04 to 1.85) | Prolonged pain requiring narcotic administration and unplanned hospital admission | 3 | 1.20 (0.34 to 3.13) | 0 | 0.00 (0.00 to 1.00) |
| Terjung 2003188 | Any patients undergoing biopsy (40% had risk factors for biopsy-related bleeding) | 574 | Ultrasound used to identify biopsy site, inpatient, seven experienced hepatogastro-enterologists or five hepatogastro-enterology fellows under their supervision |
Retrospective audit Germany 1993–6 |
Fall in serum haemoglobin > 2 g/dl, haematoma assessed by ultrasound | 62 | 10.80 (7.57 to 12.17) | Clinically overt bleeding complication | 10 | 1.74 (0.83 to 2.91) | 3 | 0.52 (0.15 to 1.38) |
| Thampanitchawong 1999180 | Any patients | 484 biopsies | No data regarding use of ultrasound or inpatient/outpatient status, medical staff or residents (after 1994, all were well-trained gastroenterologists or residents under their supervision) |
Retrospective audit Thailand 1987–96 |
Minor bleeding (decrease in haematocrit ≥ 4% not requiring transfusion or surgery), transient hypotension | 18 | 3.72 (2.14 to 5.38) | Major bleeding (decrease in haematocrit ≥ 4% requiring transfusion or surgery) | 17 | 3.51 (1.99 to 5.14) | 6 | 1.20 (0.45 to 2.37) |
| Van der Poorten 2006181 | Any patients | 1398 | Ultrasound guidance used in 87.9%, 66% outpatient, mostly performed by radiologist or radiology registrar |
Retrospective audit Australia 1996–2005 |
Pain, nausea, vomiting, vasovagal episodes | 166 | 11.87 (9.14 to 12.19) | Significant haemorrhage, fall in haemoglobin of > 20 g/l, bile peritonitis, visceral perforation, death | 12 | 0.86 (0.44 to 1.39) | 3 | 0.21 (0.06 to 0.57) |
| Van Thiel 199396 | Patients attending liver transplant centre | 12,750 biopsies | Ultrasound guidance used in 0.4%, 95% inpatient, 67% performed by surgeons,33% by transplant physicians, 0.4% by radiologists |
Retrospective audit USA 1989–91 |
Minor complications NR | NR | – | Major complications including intra-abdominal haemorrhage | 26 | 0.20 (0.13 to 0.29) | 0 | 0.00 (0.00 to 0.02) |
| Vivas 1998182 | Patients with platelet count > 60,000/mm3, prothrombin time < 4 seconds above control, without ascites, HE or liver tumours | 378 | Ultrasound used to identify biopsy site, 70% outpatient, medical staff and residents in their third or fourth year of training |
Prospective Spain 1995–6 |
Pain requiring analgesia, but not hospital admission | 36 | 9.52 (6.18 to 11.59) | Pain or hypertension requiring hospital admission, abdominal bleeding requiring blood transfusion, subcapsular haematoma | 7 | 1.85 (0.74 to 3.37) | 0 | 0.00 (0.00 to 0.66) |
| Wawrzynowicz-Syczewska 2002189 | Patients with platelet count ≥ 50,000/mm3 and prothrombin activity ≥ 50% | 861 | Ultrasound only occasionally used to identify biopsy site, inpatient, no data regarding operator |
Retrospective audit Poland 1997–2001 |
Severe pain requiring intravenous analgesia or morphine | 60 | 6.97 (5.01 to 8.19) | Severe vasovagal reaction requiring atropine, haemoperitoneum, pneumothorax, renal puncture, haemothorax, septic shock | 12 | 1.39 (0.71 to 2.25) | 0 | 0.00 (0.00–0.29) |
| Weigand 2009190 | Patients without clotting disorders (prothrombin time < 70%, platelets < 80,000/ml) or anaemia (haemoglobin < 10 g/dl) | 715 | Ultrasound used, 60% outpatient, experienced investigators |
Retrospective audit Germany 1990–2005 |
Minor complications, most commonly mild pain | 63 | 8.81 (6.29 to 10.11) | Any event resulting in death, severe pain not resolving after oral analgesic, prolonged severe hypotension, or bleeding plus significant drop in haemoglobin level | 2 | 0.28 (0.06 to 0.89) | 0 | 0.00 (0.00 to 0.35) |
| Total | 31,960 | 1253/16,674 | 7.51 (7.11 to 7.91) | 259/31960 | 0.81 (0.71 to 0.91) | 29/31960 | 0.09 (0.06 to 0.12) | |||||
| Study | Brief description of patient group | Number of patients | Details of setting, operator, etc. | Design/study country/date of biopsies | Minor adverse events related to biopsy | Severe adverse events (including deaths) related to biopsy | Deaths related to biopsy | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | n | % (95% CI) | Definition | n | % (95% CI) | n | % (95% CI) | |||||
| Choh 1998191 | Patients with coagulopathy and/or ascites | 101 | No data regarding ultrasound guidance, inpatient status or operator |
Case series USA 1994–6 |
Minor neck haematoma | 2 | 1.98 (0.41 to 6.08) | Not given | 3 | 0.99 (0.82 to 7.50) | 0 | 0.00 (0.00 to 0.45) |
| Papatheodoridis 1999192 | Patients with acute liver disease, chronic liver disease or post-transplant disorder | 145 | No data regarding ultrasound guidance, inpatient status or operator |
Prospective UK 1995–7 |
Minor complications not reported | NR | Capsular perforations | 2 | 1.38 (0.28 to 4.29) | 0 | 0.00 (0.00 to 1.71) | |
| Soyer 2008193 | Any patients undergoing biopsy | 200 | Ultrasound guidance, inpatient, experienced radiologist aided by a technician |
Retrospective audit France 1995–2007 |
Pain at biopsy site or puncture site; mild haematoma at puncture site | 24 | 12.00 (7.02 to 15.07) | Not given | 0 | 0.00 (0.00 to 1.25) | 0 | 0.00 (0.00 to 1.25) |
| Vlavianos 1991194 | Patients with prothrombin time > 6 seconds above control, platelet count < 40,000/mm3 or tense ascites | 104 | Inpatient status NR, under supervision of an experienced hepatologist |
Prospective UK 1985–9 |
Perforation of liver capsule without clinical sequelae, tachycardia or small haemothorax which resolved spontaneously | 11 | 10.58 (4.92 to 15.54) | Ruptured subcapsular haematoma, perforation of liver capsule requiring blood transfusion, pneumothorax requiring drainage | 3 | 2.88 (0.80 to 7.29) | 1 | 0.96 (0.10 to 4.36) |
| Total | 550 | 37/405 | 9.14 (6.33 to 11.95) | 8/550 | 1.45 (0.45 to 2.45) | 1/550 | 0.18 (0.00 to 0.53) | |||||
The pooled data for percutaneous biopsy suggest that over 7% of patients suffer related minor adverse events, although < 1% suffer severe related adverse events (including death) and < 0.1% die (see Table 37). The comparable figures for transjugular biopsy are somewhat higher, at over 9%, 1.45%, and 0.18%, respectively (see Table 38). However, as may be seen, rates vary between individual studies. This is due, at least in part to, the use of varying definitions of minor and severe adverse events. Because of time constraints, it has not been possible to explore these issues, nor those relating to the use of ultrasound guidance for percutaneous biopsy, the clinical setting, and patient and clinician characteristics.
Appendix 4 Systematic review of the adverse effects of liver biopsy: search strategies
Sources searched
The electronic bibliographic databases that were searched are listed in Appendix 5. The searches were carried out in February 2010.
Search strategies
The MEDLINE search strategy may be found in Appendix 5.
Inclusion criteria
Population
-
Adults.
Intervention
-
Percutaneous or transjugular liver biopsy.
Outcomes
-
Adverse events probably or possibly caused by the liver biopsy.
Setting
-
Any country.
Study type
-
Any study design which presented data relating to over 100 patients.
Exclusion criteria
Population
-
Fewer than 100 participants (either overall or in either arm of a RCT comparing percutaneous with transjugular biopsy).
Intervention
-
Laparoscopic liver biopsy.
-
Biopsy undertaken prior to 1980.
Study type
-
Animal models.
-
Narrative reviews, editorials, opinions.
FIGURE 28.
Adverse effects of liver biopsy: summary of study selection and exclusion.
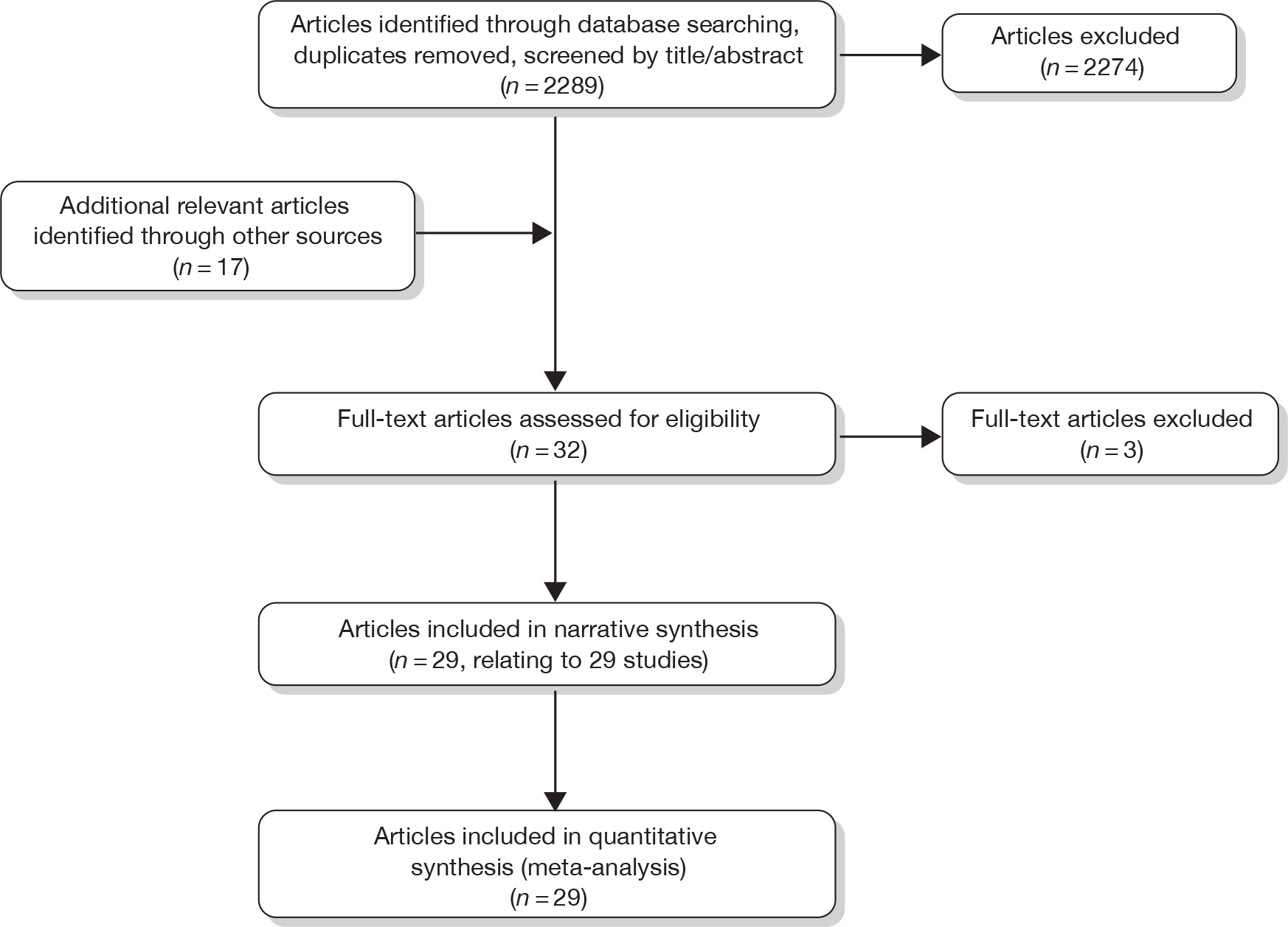
Appendix 5 Assessment of clinical effectiveness and cost-effectiveness, adverse effects, and quality of life: search strategies
The following electronic databases were searched:
-
MEDLINE via Ovid (1950 to present)
-
EMBASE via Ovid (1980 to present)
-
MEDLINE In-Process & Other Non-Indexed Citations via Ovid (1950 to present)
-
The Cochrane library:
-
Cochrane Database of Systematic Reviews (CDSR, 1996 to present)
-
Cochrane Central Register of Controlled Trials (CENTRAL, 1898 to present)
-
Database of Abstracts of Reviews of Effects (DARE, 1995 to present)
-
Cochrane Methodology Register (1904 to present)
-
Health Technology Assessment Database (1995 to present)
-
NHS Economic Evaluation Database (1995 to present)
-
-
CINAHL via EBSCO (1982 to present)
-
Web of Knowledge:
-
Science Citation Index (SCI, 1969 to present)
-
Conference Proceedings Citation Index (CPCI-S, 1990 to present)
-
BIOSIS Previews (1969 to present).
-
Search strategies
The search strategies shown below were used in Ovid MEDLINE (1950 to present), and were adapted for use across multiple databases.
Clinical effectiveness search strategy
To retrieve evidence of the diagnostic test reliability and accuracy, the broad and specific intervention terms (1–5, 9–15, 20–60) were combined with those of the clinical condition, namely liver fibrosis (6–7, 17–19), and then combined with the diagnostic filter (62–74). Search terms for the diagnostic test manufacturers were also included in the strategy (75–78).
-
(enhanced adj liver adj fibrosis).tw.
-
(elf adj test$).tw.
-
(elf and diagnos$).tw.
-
(elf and (fibros*s or cirrhos*s)).tw.
-
elf.tw.
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
5 and 6
-
1 or 2 or 3 or 4 or 7
-
FibroTest.tw.
-
fibrosure.tw.
-
fibromax.tw.
-
FibroScan.tw.
-
ashtest.tw.
-
(transient adj elastograph$).tw.
-
(elastograph$and liver).tw.
-
or/9-15
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
(fibros*s or cirrhos*s).tw.
-
17 or 18
-
Biological Markers/
-
(biomarker$or bio-marker$).tw.
-
(marker$and (biologic$or biochemical or serum or direct or indirect)).tw.
-
Algorithms/
-
algorithm$.tw.
-
(composite and blood).tw.
-
or/20-25
-
19 and 26
-
Hyaluronic Acid/
-
((hyaluronic adj acid) or (hyalauronate or hyaluronan)).tw.
-
28 or 29
-
(procollagen or piiinp or p3np or ppcp).tw.
-
((tissue and inhibitor and metalloproteinase$) or timps).tw.
-
30 and 31 and 32
-
30 or 31 or 32
-
34 and 19
-
Alpha-Macroglobulins/
-
((alpha and macroglobulin$) or (alpha adj 2m)).tw.
-
36 or 37
-
((apolipoprotein$adj a1) or apoa1).tw.
-
Haptoglobins/
-
haptoglobin$.tw.
-
40 or 41
-
(bilirubin$or hematoidin$).tw.
-
(gamma adj glutamyl adj transpeptidase$).tw.
-
(gamma adj glutamyltransferase$).tw.
-
((gamma adj gt) or ggt or ggtp).tw.
-
44 or 45 or 46
-
38 and 39 and 42 and 43 and 47
-
38 or 39 or 42 or 43 or 47
-
49 and 19
-
(alanine adj (aminotransferase$or aminotransaminase$)).tw.
-
(serum adj glutamic adj pyruvic adj transaminase$).tw.
-
sgpt.tw.
-
51 or 52 or 53
-
(aspartate adj (aminotransferase$or aminotransaminase$)).tw.
-
(serum adj glutamic adj oxaloacetic adj transaminase$).tw.
-
sgot.tw.
-
55 or 56 or 57
-
38 and 39 and 42 and 43 and 47 and 54 and 58
-
38 or 39 or 42 or 43 or 47 or 54 or 58
-
60 and 19
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/62-68
-
27 and 69
-
35 and 69
-
50 and 69
-
61 and 69
-
70 or 71 or 72 or 73
-
iqur.tw.
-
biopredictive.tw.
-
echosens.tw.
-
75 or 76 or 77
-
8 or 16 or 33 or 48 or 59 or 74 or 78
Cost-effectiveness search strategy
To retrieve evidence of cost-effectiveness, a costs filter (80–100) was added to the search strategy for the clinical effectiveness studies (1–79).
-
(enhanced adj liver adj fibrosis).tw.
-
(elf adj test$).tw.
-
(elf and diagnos$).tw.
-
(elf and (fibros*s or cirrhos*s)).tw.
-
elf.tw.
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
5 and 6
-
1 or 2 or 3 or 4 or 7
-
FibroTest.tw.
-
fibrosure.tw.
-
fibromax.tw.
-
FibroScan.tw.
-
ashtest.tw.
-
(transient adj elastograph$).tw.
-
(elastograph$and liver).tw.
-
or/9-15
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
(fibros*s or cirrhos*s).tw.
-
17 or 18
-
Biological Markers/
-
(biomarker$or bio-marker$).tw.
-
(marker$and (biologic$or biochemical or serum or direct or indirect)).tw.
-
Algorithms/
-
algorithm$.tw.
-
(composite and blood).tw.
-
or/20-25
-
19 and 26
-
Hyaluronic Acid/
-
((hyaluronic adj acid) or (hyalauronate or hyaluronan)).tw.
-
28 or 29
-
(procollagen or piiinp or p3np or ppcp).tw.
-
((tissue and inhibitor and metalloproteinase$) or timps).tw.
-
30 and 31 and 32
-
30 or 31 or 32
-
34 and 19
-
Alpha-Macroglobulins/
-
((alpha and macroglobulin$) or (alpha adj 2m)).tw.
-
36 or 37
-
((apolipoprotein$adj a1) or apoa1).tw.
-
Haptoglobins/
-
haptoglobin$.tw.
-
40 or 41
-
(bilirubin$or hematoidin$).tw.
-
(gamma adj glutamyl adj transpeptidase$).tw.
-
(gamma adj glutamyltransferase$).tw.
-
((gamma adj gt) or ggt or ggtp).tw.
-
44 or 45 or 46
-
38 and 39 and 42 and 43 and 47
-
38 or 39 or 42 or 43 or 47
-
49 and 19
-
(alanine adj (aminotransferase$or aminotransaminase$)).tw.
-
(serum adj glutamic adj pyruvic adj transaminase$).tw.
-
sgpt.tw.
-
51 or 52 or 53
-
(aspartate adj (aminotransferase$or aminotransaminase$)).tw.
-
(serum adj glutamic adj oxaloacetic adj transaminase$).tw.
-
sgot.tw.
-
55 or 56 or 57
-
38 and 39 and 42 and 43 and 47 and 54 and 58
-
38 or 39 or 42 or 43 or 47 or 54 or 58
-
60 and 19
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/62-68
-
27 and 69
-
35 and 69
-
50 and 69
-
61 and 69
-
70 or 71 or 72 or 73
-
iqur.tw.
-
biopredictive.tw.
-
echosens.tw.
-
75 or 76 or 77
-
8 or 16 or 33 or 48 or 59 or 74 or 78
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$adj2 (effective$or utilit$or benefit$or minimi$)).ab.
-
(economic$or pharmacoeconomic$or pharmaco-economic$).ti.
-
(price$or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/80-100
-
79 and 101
Adverse events searches
Venepuncture and transient elastography
A search strategy was developed to search for the adverse effects of venepuncture and transient elastography. This strategy included both subject headings with adverse effect subheadings for blood tests and imaging techniques (1–5) and free-text terms for adverse effects (7–13) combined with the statements for the diagnostic test interventions (15–25). The search was limited to the adult population (27–28).
-
exp Hematologic Tests/ae [Adverse Effects]
-
exp Serologic Test/ae [Adverse Effects]
-
Blood Specimen Collection/ae [Adverse Effects]
-
Phlebotomy/ae [Adverse Effects]
-
Elasticity Imaging Techniques/ae [Adverse Effects]
-
or/1-5
-
(adverse adj (event$or effect$or outcome$)).ab,ti.
-
risk$.ab,ti.
-
(safe or safety).ab,ti.
-
harm$.ab,ti.
-
complication$.ab,ti.
-
(treatment adj emergent).ab,ti.
-
tolerability.ab,ti.
-
7 or 8 or 9 or 10 or 11 or 12 or 13
-
(FibroTest or fibrosure or fibromax or ashtest or FibroScan).tw.
-
(transient adj elastograph$).tw.
-
(elastograph$and liver).tw.
-
(enhanced adj liver adj fibrosis).tw.
-
(elf adj test$).tw.
-
(elf and diagnos$).tw.
-
(elf and (fibros*s or cirrhos*s)).tw.
-
elf.tw.
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
22 and 23
-
15 or 16 or 17 or 18 or 19 or 20 or 21 or 24
-
14 and 25
-
adult/or aged/or middle aged/or young adult/
-
adult$.tw.
-
27 or 28
-
6 or 26
-
29 and 30
Liver biopsy
The search strategy which was developed to identify studies of the adverse effects of liver biopsy includes subject headings with adverse effect subheadings for biopsy (1–2) combined with the liver fibrosis terms (4–5). Free-text terms for adverse effects (8–14) were combined with the statement liver biopsy (16). The search was limited to the adult population (27–28).
-
Biopsy/ae [Adverse Effects]
-
exp Biopsy, Needle/ae [Adverse Effects]
-
1 or 2
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
(cirrhos*s or fibros*s).tw.
-
4 or 5
-
3 and 6
-
(adverse adj (event$or effect$or outcome$)).ab,ti.
-
risk$.ab,ti.
-
(safe or safety).ab,ti.
-
harm$.ab,ti.
-
complication$.ab,ti.
-
(treatment adj emergent).ab,ti.
-
tolerability.ab,ti.
-
8 or 9 or 10 or 11 or 12 or 13 or 14
-
(liver and biops$).tw.
-
15 and 16
-
6 and 17
-
7 or 18
-
letter.pt.
-
editorial.pt.
-
comment.pt.
-
20 or 21 or 22
-
19 not 23
-
adult/or aged/or middle aged/or young adult/
-
adult$.tw.
-
25 or 26
-
24 and 27
Quality of life searches
To search for evidence relating to the impact of the diagnostic tests on the well-being of patients with ALD, a quality-of-life search filter (80–115) was combined with the diagnostic test effectiveness searches (1–79). The quality-of-life filter consists of database subject headings and free-text terms associated with the quality of life, including generic, instrument specific, and methodological terms. In addition, searches were also carried out on the health-related quality of life of patients with the medical condition only (116–121).
-
(enhanced adj liver adj fibrosis).tw.
-
(elf adj test$).tw.
-
(elf and diagnos$).tw.
-
(elf and (fibros*s or cirrhos*s)).tw.
-
elf.tw.
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
5 and 6
-
1 or 2 or 3 or 4 or 7
-
FibroTest.tw.
-
fibrosure.tw.
-
fibromax.tw.
-
FibroScan.tw.
-
ashtest.tw.
-
(transient adj elastograph$).tw.
-
(elastograph$and liver).tw.
-
or/9-15
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
(fibros*s or cirrhos*s).tw.
-
17 or 18
-
Biological Markers/
-
(biomarker$or bio-marker$).tw.
-
(marker$and (biologic$or biochemical or serum or direct or indirect)).tw.
-
Algorithms/
-
algorithm$.tw.
-
(composite and blood).tw.
-
or/20-25
-
19 and 26
-
Hyaluronic Acid/
-
((hyaluronic adj acid) or (hyalauronate or hyaluronan)).tw.
-
28 or 29
-
(procollagen or piiinp or p3np or ppcp).tw.
-
((tissue and inhibitor and metalloproteinase$) or timps).tw.
-
30 and 31 and 32
-
30 or 31 or 32
-
34 and 19
-
Alpha-Macroglobulins/
-
((alpha and macroglobulin$) or (alpha adj 2m)).tw.
-
36 or 37
-
((apolipoprotein$adj a1) or apoa1).tw.
-
Haptoglobins/
-
haptoglobin$.tw.
-
40 or 41
-
(bilirubin$or hematoidin$).tw.
-
(gamma adj glutamyl adj transpeptidase$).tw.
-
(gamma adj glutamyltransferase$).tw.
-
((gamma adj gt) or ggt or ggtp).tw.
-
44 or 45 or 46
-
38 and 39 and 42 and 43 and 47
-
38 or 39 or 42 or 43 or 47
-
49 and 19
-
(alanine adj (aminotransferase$or aminotransaminase$)).tw.
-
(serum adj glutamic adj pyruvic adj transaminase$).tw.
-
sgpt.tw.
-
51 or 52 or 53
-
(aspartate adj (aminotransferase$or aminotransaminase$)).tw.
-
(serum adj glutamic adj oxaloacetic adj transaminase$).tw.
-
sgot.tw.
-
55 or 56 or 57
-
38 and 39 and 42 and 43 and 47 and 54 and 58
-
38 or 39 or 42 or 43 or 47 or 54 or 58
-
60 and 19
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/62-68
-
27 and 69
-
35 and 69
-
50 and 69
-
61 and 69
-
70 or 71 or 72 or 73
-
iqur.tw.
-
biopredictive.tw.
-
echosens.tw.
-
75 or 76 or 77
-
8 or 16 or 33 or 48 or 59 or 74 or 78
-
“Quality of Life”/
-
(qol or (quality adj2 life)).ab,ti.
-
(value adj2 (money or monetary)).tw.
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.tw.
-
(qaly$or qald$or qale$or qtime$).tw.
-
disability adjusted life.tw.
-
daly$.tw.
-
health status indicators/
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
(sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw.
-
health$year$equivalent$.tw.
-
health utilit$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
disutilit$.tw.
-
rosser.tw.
-
(quality adj2 wellbeing).tw.
-
qwb.tw.
-
(willingness adj2 pay).tw.
-
standard gamble$.tw.
-
time trade off.tw.
-
time tradeoff.tw.
-
tto.tw.
-
letter.pt.
-
editorial.pt.
-
comment.pt.
-
110 or 111 or 112
-
or/80-109
-
114 not 113
-
exp liver cirrhosis/or exp liver diseases, alcoholic/
-
(fibros*s or cirrhos*s).tw.
-
116 or 117
-
115 and 118
-
(pulmonary or cystic).tw.
-
119 not 120
-
79 and 115
-
122 or 121
Appendix 6 QUADAS: details of criteria for scoring studies
| 1. Was the spectrum of patients representative of the patients who will receive the test in practice? | |
| Yes | All patients had suspected ALD, and patients were recruited both prospectively and consecutively |
| No | Some patients were known to have liver fibrosis or cirrhosis, or patients were studied retrospectively or non-consecutively |
| Unclear | Insufficient details given about stage or recruitment methods to make a judgement about whether or not the patient spectrum would be scored ‘yes’ |
| 2. Is the reference standard likely to correctly classify the target condition? | |
| Yes | The reference standard was liver biopsy, HVPG measurement or endoscopy for oesophageal varices. The study excluded patients with liver biopsies < 10 mm in length195 |
| No | Some or all patients received a different reference standard. The study included patients with liver biopsies < 10 mm in length195 |
| Unclear | Reference standard is not stated; for liver biopsy, no data given on length of specimen or portal tracts113 |
| 3. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the performance of the two tests? | |
| Yes | Reference standard was performed within 2 weeks of the index test |
| No | Reference standard was performed more than 2 weeks before or after the index test |
| Unclear | The time between reference standard and index test is not stated |
| 4. Did the whole sample, or a random selection of the sample, receive verification using a reference standard? | |
| Yes | All patients, or a random selection of patients, who received the index test went on to receive verification of their disease status using a reference standard |
| No | Some patients who received the index test did not receive verification of their true disease state and the selection of patients to receive the reference standard was not random |
| Unclear | This information is not reported |
| 5. Did patients receive the same reference standard regardless of the index test result? | |
| Yes | Selection of reference standard was not determined by the index text result |
| No | Selection of reference standard was determined by the index test result |
| Unclear | It is not clear whether or not selection of reference standard was determined by the index test result |
| 6. Were the reference standard results interpreted without knowledge of the results of the index test? | |
| 7. Were the index test results interpreted without knowledge of the results of the reference standard? | |
| Yes | The index test was interpreted without knowledge of the results of the reference standard or vice versa. If the test was clearly interpreted before the results of the other test were available then this was scored as ‘yes’ |
| No | The person interpreting the index test was aware of the results of the reference standard or vice versa |
| Unclear | No information is provided regarding whether or not tests were interpreted blindly |
| 8. Were uninterpretable/intermediate test results reported and included in the analysis? | |
| Yes | There were uninterpretable/intermediate results and they were included in the analysis, or patients were recruited prospectively and consecutively and no uninterpretable/intermediate results were reported |
| No | There were uninterpretable/intermediate results and they were excluded from the analysis |
| Unclear | It is not clear whether or not there were any uninterpretable/intermediate test results (this includes studies which were not prospective or not consecutive which did not report these data) |
| 9. Were withdrawals from the study explained? | |
| Yes | All patients recruited to the study were accounted for |
| No | There appear to be patients who were recruited into the study who are not accounted for |
| Unclear | It is not clear whether or not any withdrawals occurred |
| 10. Were the selection criteria clearly described? | |
| Yes | All relevant information about how patients were selected for inclusion in the study was provided |
| No | Study selection criteria were not clearly reported |
| Unclear | Insufficient details given about stage or recruitment methods to make a judgement about whether or not the selection criteria would be scored ‘yes’ |
| 11. Was the execution of the reference standard described in sufficient detail to permit replication of the test? | |
| Yes | Sufficient details of the text execution are reported |
| No | Sufficient details are not reported |
| Unclear | Staging system given, but no inclusion criteria regarding the length of the liver biopsy or the number of portal tracts113 |
| 12. Was the execution of the index test described in sufficient detail to permit replication of the test? | |
| Yes | Sufficient details of the text execution are reported |
| No | Sufficient details are not reported |
| Unclear | Not applicable |
| 13. Did the study appear independent of the test manufacturer? | |
| Yes | Study authors state that they have no personal interests in the company |
| No | One or more of study authors known to have an interest in the company |
| Unclear | No information provided |
Appendix 7 Excluded studies
Table of studies identified by the electronic searches and other searches, and excluded at the full paper stage for reasons not immediately apparent from the full text.
| Study | Reason for exclusion |
|---|---|
| Calès 2002196 | Superseded by Calès 2005197 |
| Calès 2005197 | Clarification from the author that the validating population, in which FibroTest was used, was wholly composed of patients with hepatitis C (Dr Paul Calès, Université d’Angers, France, 2010, personal communication) |
| Clevert 2009198 | Not available within study timescale |
| De Ledinghen 2006199 | Information provided identical to that in Foucher 2006a200 |
| Foucher 2006a200 | Biopsy used only in a subset of 60 of patients in the study. The selection criteria for biopsy were not clear as 149 patients were said to have FibroScan results suggestive of cirrhosis |
| Foucher 2006b201 | Clarification from the author that the population overlaps with that of Foucher 2006a200 (Dr Juliette Foucher, Hôpital Haut-Lévèque, Pessac, France, 2010, personal communication), which reports data relating to substantially more patients with ALD; it is not clear how many patients with ALD underwent biopsy, and the selection criteria for biopsy were not clear, being described only as the ‘usual indications for liver biopsy’, and not in terms of the results of the FibroScan test |
| Jimenez-Ridruejo 2008202 | Not available within study timescale |
| Laharie 2006203 | Information provided identical to that in Foucher 2006a200 |
| Lee 2009204 | Not available within study timescale |
| Lieber 200862 | Although this study relates to the serum markers used in the ELF test, the tests do not appear to have been performed on an Immuno 1 machine, and therefore performance would be suboptimal (Professor William Rosenberg, University College London, 2010, personal communication) |
| Marin-Gabriel 2008205 | Not available within study timescale |
| Melin 2005206 | Superseded by Melin 2005122 |
| Morozov 2008207 | In Russian; no English abstract available |
| Mueller 2008120 | Superseded by Mueller 2010118 |
| Mueller 2009208 | Superseded by Mueller 2010118 |
| Nahon 2007131 | Superseded by Nahon 2008128 |
| Naveau 2003209 | Appears to be superseded by Naveau 2005123 (confirmation could not be obtained from the author) |
| Naveau 2008210 | Superseded by Naveau 2009124 |
| Nguyen-Khac 2007211 | Superseded by Nguyen-Khac 2008132 |
| Parkes 2007212 | Superseded by Parkes 201057 |
| Rosenberg 2001213 | Not available within study timescale |
| Thabut 2003214 | Data relating specifically to patients with ALD neither published nor available from study authors (Dr Thierry Poynard, Hôpital Pitié-Salpêtrière, Paris, 2010, personal communication) |
Appendix 8 Diagnostic venepuncture: systematic review of adverse events
A systematic review was carried out in order to identify studies reporting adverse events in adults undergoing simple venepuncture for diagnostic or screening purposes. Literature searches were performed in February 2010. Details of the electronic databases that were searched, the search strategies used and the inclusion and exclusion criteria may be found in Appendix 9.
Studies that related to blood donors were excluded because:
-
The withdrawal of larger volumes of blood makes it difficult to differentiate between vasovagal reactions and transient relative hypotension due to blood loss. 215
-
The use of needles with a larger bore than the 20–22 gauge generally used in blood sampling may increase the risk of injury,216 as may the fact that the needles are in place for a longer period of time. 217
Studies that used more invasive methods of blood collection (cannulation or catheterisation), or which collected arterial or capillary rather than venous blood samples, were also excluded.
The searches were not restricted by study type, language or date. The same three-stage sifting process was used as in the systematic review of clinical effectiveness. Data were extracted directly to the tables included in the report. Because many of the relevant studies took the form of case reports, a formal quality assessment was not undertaken. However, larger studies (observational or before-and-after studies) were deemed to be of higher quality than case series or case reports, and the latter were included only if they related to adverse events for which data were not available from the larger studies.
The electronic literature searches identified 979 potentially relevant articles. Of these, 11 met the review’s inclusion criteria (for PRISMA diagram, see Appendix 9). These articles were:
-
two observational studies, by Galena215 and Deacon and Abramowitz218
-
an uncontrolled before-and-after study by Godwin et al. 219
-
eight case reports by Choffel et al. ,220 Nouri et al. ,221 Pradhan and Gupta,222 Saeed and Gatens,223 Sander et al. ,224 Stitik et al. ,217 Vidal et al. 225 and Zubairy. 226
A possibly relevant article by Rodriguez Guerrero et al. 227 was excluded because it was published in Spanish.
Five additional relevant articles, by Berry and Wallis,228 Burgdorf et al. ,229 Horowitz,216 Norcross and Shackford230 and Yuan and Cohen,231 were identified from citations.
The adverse events identified by the included studies fall into three major categories:
-
vasovagal reactions
-
pain and bruising
-
nerve injuries.
These are discussed in turn below, as are the few studies relating to miscellaneous adverse events which do not fit into those categories.
Vasovagal reactions
Vasovagal reactions result from an abnormal reflex stimulation of the vagus nerve. The trigger factors may be emotional or somatic. 232 In most patients, the signs and symptoms (which may include pallor, sweating, nausea, dizziness or light-headedness) are light or moderate, and resolve spontaneously. However, some patients experience bradycardia with consequent hypotension, loss of consciousness, and, in very severe cases, death. 232 In the context of vasovagal reactions associated with venepuncture, it seems likely that the greatest risk is that, when blood is taken with the patient sitting or lying, resumption of the upright position results in a faint, as the subsequent fall may result in injury.
Because data relating to vasovagal reactions were available from two large observational studies, lower-quality studies (case reports and small case series) relating to such adverse events were excluded.
The larger observational study, that by Galena,215 recorded adverse effects associated with venepuncture carried out in outpatient settings between October 1988 and April 1991 on 4050 patients who were applying for life insurance. A 20- or 22-gauge needle was used to obtain a maximum of 30 ml blood from each patient. Delayed reactions were identified using telephone calls made an unspecified length of time after the venepuncture. Potentially serious vasovagal reactions were experienced by 3.4% of patients (Table 39); these were significantly more common in men than in women (4.0% vs 1.3%, p < 0,001). None of those who experienced convulsive syncope had a previous history of seizure disorder.
| Complication | Number (%, 95% CI) |
|---|---|
| Diaphoresis, near syncope | 105/4050 (2.6, 2.1 to 3.1) |
| Syncope | 24/4050 (0.6, 0.4 to 0.8) |
| Convulsive syncope | 6/4050 (0.10, 0.03 to 0.30) |
| Ventricular tachycardia | 1/4050 (0.02, 0.00 to 0.10) |
| Total | 136/4050 (3.4, 2.8 to 3.9) |
Deacon and Abramowitz218 found lower rates of vasovagal reactions in 3315 adults undergoing venepuncture in three hospital outpatient phlebotomy clinics over a 3-week period, even though 80% of their population had fasted prior to venepuncture (Table 40). Although the rate of vasovagal reactions indicated by the phlebotomists was higher, at 0.9%, than that reported by the patients, it was still substantially lower than the rate of 3.4% reported by Galena. 215
| Complication | Number (%, 95% CI) |
|---|---|
| Patient reported feeling very or extremely faint | 13/3315 (0.4, 0.2 to 0.6) |
| Patient reported losing consciousness | 7/3315 (0.2, 0.1 to 0.4) |
| Phlebotomist reported using strategies to manage fainting symptomsa with patient | 30/3315 (0.9, 0.6 to 1.2) |
Pain and bruising
Data relating to pain and bruising were available from one large observational study215 and one uncontrolled before-and-after study. 219 Lower-quality studies (case reports and small case series) relating to such adverse events were therefore excluded.
In Galena’s large observational study,215 14.2% of patients reported adverse events related to pain and bruising (Table 41). Such adverse effects were significantly more common in women than in men (38.1% vs 7.9%, p < 0,001), a result that Galena suggested was probably related to their narrower veins. No cases of local cellulitis or phlebitis were reported.
| Complication | Number (%, 95% CI) |
|---|---|
| Bruising | 416/4050 (10.3, 9.3 to 11.2) |
| Haematoma | 80/4050 (2.0, 1.6 to 2.4) |
| Pain | 80/4050 (2.0, 1.6 to 2.4) |
| Total | 576/4050 (14.2, 13.1 to 15.3) |
Godwin et al. 219 reported higher overall rates of bruising in a small before-and-after study that audited bruising in two groups of 100 consecutive medical and surgical inpatients aged ≥ 15 years who were not receiving anticoagulants and did not have extensive pre-existing bruises. Venepuncture was performed by phlebotomists using a pre-evacuated tube collection system to take blood from the antecubital fossa. A clean cotton wool ball was then taped to the venepuncture site. The phlebotomist instructed patients in the first group to apply pressure for a few minutes after the venepuncture, but remained with patients in the second group until the bleeding had stopped. The venepuncture site was then assessed 24 hours later. Bruising was less common in the second group (45% vs 25%, p < 0.01) and such bruises as occurred were also smaller in this group. The difference between the groups was more marked in older patients (Table 42) and the investigators suggested that this was perhaps because they were less able than younger patients to apply pressure to the venepuncture site;219 however, it is perhaps more likely to reflect the more fragile nature of the skin in the elderly.
| Patient age (years) | Number of patients with bruising (%, 95% CI) | |
|---|---|---|
| Patient pressure | Phlebotomist pressure | |
| < 60 | 11/37 (30, 15 to 44) | 7/42 (17, 5 to 28) |
| > 60 | 34/63 (54, 42 to 66) | 18/58 (31, 19 to 43) |
| Total | 45/100 (45, 35 to 55) | 25/100 (25, 17 to 33) |
Nerve injury
The potentially most serious adverse events associated with venepuncture relate to nerve injury. Such adverse events can have disabling consequences. The only identified publications that report venepuncture-associated nerve injuries sufficiently severe to be brought to medical attention take the form of case reports and one small case series. 216
The case series presented data relating to 11 patients who were referred to a specialist with a particular interest in nerve injuries because of causalgia following routine venepuncture. 216 However, only four of these patients had undergone venepuncture for blood sampling; in the remainder, the venepuncture was for blood donation, insertion of intravenous lines or intravenous medication. A later paper by Horowitz,233 which combined data relating to these 11 patients with data from 13 patients who had subsequently been evaluated, could not be utilised because it presented aggregated data from patients who had undergone venepuncture for blood sampling and patients who had undergone venepuncture for other reasons.
Data relating to the cases identified in the case reports, together with the four relevant patients from Horowitz’s233 case series, are summarised in Table 43. These data demonstrate that nerve damage consequent on venepuncture can cause long-lasting pain, and loss of muscle power and manual dexterity; it may also lead to clinical depression. Relatively few details of the venepuncture are reported, but in 4 of the 12 cases it was specifically said to have been difficult. The gauge of needle was specified in only two cases; in both cases, it was a 20-gauge needle.
| Study | Subject | Purpose and site of venepuncture | Diagnosis | Outcome | Comment |
|---|---|---|---|---|---|
| Berry 1977228 | 50-year-old woman | Blood grouping; left antecubital fossa | Injury to the medial cutaneous nerve | Pain and swelling in the forearm developed within 24 hours into hyperaesthesia in the whole forearm. A striking improvement was noted 24 hours after treatment with carbamazepine and 3 days later the only symptom was slight pain on moving the arm. Treatment was discontinued after 5 weeks, when the patient had no symptoms except slightly impaired touch sensation in the sensory distribution of the left medial cutaneous nerve | A 20-gauge needle used |
| Horowitz 1994216 | 61-year-old woman | Blood sampling; antecubital fossa | Causalgia affecting medial antebrachial cutaneous nerve | Increased symptoms and motor abnormalities of disuse, with joint contracture and psychiatric depression requiring antidepressant medication, observed at 7 years | |
| Horowitz 1994216 | 61-year-old man | Blood sampling; antecubital fossa | Causalgia affecting lateral antebrachial cutaneous nerve | Increased symptoms and motor abnormalities of disuse, with joint contracture and psychiatric depression requiring antidepressant medication observed at 4 years | |
| Horowitz 1994216 | 56-year-old woman | Blood sampling; antecubital fossa | Causalgia affecting medial antebrachial cutaneous nerve | Increased symptoms, with joint contracture and motor abnormalities of disuse observed at 18 months | |
| Horowitz 1994216 | 35-year-old man | Blood sampling; wrist | Causalgia affecting superficial radial nerve | The burning pain resolved spontaneously over a 2-week period, but hyperpathia and allodynia in the injured nerve distribution persisted at 2.5 years | |
| Nouri 2000221 | 59-year-old woman | Routine phlebotomy for pre-operative assessment; radial vein | Causalgia affecting radial nerve | Immediate acute pain and numbness; dysaesthesia, hyperaesthesia, allodynia, and loss of muscular power still persisted a year later. Following treatment with paroxetine, tramadol and capsaicin, and six nerve blocks, the pain in the arm and forearm was almost completely resolved, and that in the hand and wrist was somewhat reduced | A 20-gauge needle used. Venepuncture said to be difficult, requiring three attempts |
| Pradhan 1995222 | 32-year-old woman with a minor pyrexial illness | Routine blood testing; cubital vein | Median nerve | Immediate intense pain in whole of left arm persisting on the palmar aspect of the forearm and hand, and accompanied by weakness and tingling. The paraesthesia subsided in 2 months; mild anaesthesia in radial side of palm persisted for 4 months; muscle power returned to normal with physiotherapy, but minimal wasting was still observed after 1 year | Venepuncture said to be very difficult because of the non-visibility of veins |
| Saeed 1983223 | 47-year-old man | Preoperative phlebotomy; cubital vein | Anterior interosseous syndrome | Pain in forearm and inability to flex thumb noted 4 days after surgery. Surgical tendon transfer required 14 months later to enable appropriate movement of the thumb. | Venepuncture said to have been very difficult |
| Sander 1998224 | 64-year-old woman | Phlebotomy (purpose not stated); antecubital | Lateral antebrachial cutaneous neuropathy | Acute pain on insertion of needle followed by pain and numbness persisting, with some improvement, for 5 months | |
| Stitik 2001217 | 29-year-old man | Phlebotomy (purpose not stated); left cephalic vein, antecubital fossa | Lateral antebrachial cutaneous neuropathy | Shooting pain down forearm and into base of thumb at venepuncture; this initially resolved, but recurred the next morning together with dysaesthesias over the lateral aspect of the left distal forearm. At 6 months, dysaesthesias were reported in the entire left arm. The patient was then lost to follow-up | |
| Yuan 1985231 | 31-year-old man | Routine phlebotomy for preoperative blood tests; cubital vein | Laceration of the lateral antebrachial cutaneous nerve with neuroma formation | Excruciating pain followed by numbness noted during venepuncture, followed by pain and numbness in the forearm persisting for 3 weeks, and resistant to treatment with butazolidin; lidocaine and steroid injection did not produce lasting relief. Surgery was performed on two occasions; the first was ineffective and the second relieved the pain, but left permanent numbness. However, motor function was unimpaired | Repeated attempts at venepuncture were required |
| Zubairy 2002226 | 44-year-old woman | Routine postoperative blood sampling; cubital fossa | Severe anterior interosseus nerve lesion | Loss of function in the thumb and index finger; weakness of pronation. Management was conservative. The first sign of spontaneous recovery was observed at 20 months and normal function at 34 months after the injury |
The case studies summarised above do not provide any indication of the incidence of nerve injuries related to venepuncture, other than that they were rare. Some indication of the incidence can be obtained by considering only two studies from blood transfusion centres. In a New Zealand blood transfusion unit performing approximately 80,000 venepunctures a year, Berry and Wallis228 found that, over a 2-year period, six people suffered injuries to the median nerve or medial and lateral cutaneous nerves that were severe enough for them to seek medical attention – an overall rate of approximately 1 in 25,000 (0.004%). Of those six, only one (summarised in Table 43) was undergoing venepuncture for diagnostic purposes, using a 20-gauge needle; the remaining five were undergoing venepuncture for blood donation, using a larger 16-gauge needle. As this study gave no indication of the number or proportion of venepunctures undertaken for purposes of diagnosis rather than blood donation, it is not possible to calculate a rate of nerve injury specific to diagnostic venepuncture; however, it seems likely that it would be lower than the overall rate.
Newman and Waxman234 reported a higher nerve injury rate from a blood donation centre in the USA where nurses routinely reported all donor injuries. Over a 2-year period, 419,000 blood donations were collected using a 16-gauge needle and 66 cases of neurological nerve injury were identified from nursing records – a rate of 1 in 6300 (0.016%). This figure is not directly comparable with the New Zealand figure228 because it includes cases that were not brought to medical attention, but the data for donors who requested a physician consultation (17 of the 56 individuals with nerve injury for whom follow-up data were available) also indicate a rate of approximately 1 in 25,000 (0.004%) (Table 44). This is a conservative estimate as 9 of the 66 donors with nerve injury could not be contacted for telephone follow-up, and one was deliberately not contacted because of pending litigation. 234
| Recovery period | Number of donors with nerve injury and follow-up data
(n = 56) (% of total, 95% CI) |
Number requesting physician consultation(s) (% of category, 95% CI) |
Number with residual neurological defecta (% of category, 95% CI) |
|---|---|---|---|
| < 3 days | 22 (39, 27% to 52%) | 0 (0) | 0 (0) |
| 3–29 days | 17 (30, 18% to 42%) | 5 (29, 8% to 51%) | 0 (0) |
| 1–3 months | 13 (4, 0% to 8%) | 8 (62, 35% to 88%) | 2 (15, 0% to 35%) |
| 3–6 months | 2 (23, 12% to 34%) | 2 (100) | 1 (50, 0% to 100%) |
| > 6 months | 2 (23, 12% to 34%) | 2 (100) | 1 (50, 0% to 100%) |
Miscellaneous adverse events
Three case reports highlight the ability of venepuncture to provoke localised manifestations of underlying medical conditions. Burgdorf et al. 229 reported multiple sarcoid granulomas that developed following numerous venepunctures for diagnostic purposes in a 38-year-old woman who was subsequently diagnosed with sarcoidosis. Similarly, Choffel et al. 220 reported the formation of a sarcoid granuloma at the puncture site following venepuncture in a 56-year-old woman with sarcoidosis. Finally, Vidal et al. 225 reported that a tuberculous ulcerated nodule developed on the left wrist of an 88-year-old man with a history of pulmonary tuberculosis 2 years after venepuncture at that site; they considered the venepuncture to be the most likely cause of the reactivation of tuberculous infection.
Finally, a 27-year-old woman was aware of a ‘buzzing’ in the region of her left antecubital fossa following venepuncture performed by a gynaecologist rather than a medical technician. This buzzing became more pronounced over a 2-year period, and was eventually identified during a routine medical examination as an arteriovenous fistula which required surgical repair. 230
Summary
There is evidence that venepuncture may be associated with adverse effects. Vasovagal reactions were studied in two large observational studies, by Galena215 and Deacon and Abramowitz,218 which together included 7365 individuals. Data relating to pain and bruising were available from a total of 4250 patients included in Galena’s215 large observational study and the small before-and-after study by Godwin et al. 219 Unfortunately, data relating to direct nerve injuries in patients undergoing venepuncture specifically for diagnostic or screening purposes were available from only case series or case reports.
The most commonly reported adverse events were those related to pain and bruising, which affected between 14% and 45% of patients. Vasovagal reactions were rarer, affecting between 0.9% and 3.4%. There were no data regarding the incidence of nerve injuries associated with diagnostic venepuncture, but it seems likely that it would be lower than the 0.004% reported in blood donors. However, although such nerve injuries appear to be very rare, they are potentially disabling.
Appendix 9 Systematic review of the adverse effects of venepuncture: search strategies
Sources searched
The electronic bibliographic databases which were searched are listed in Appendix 5.
Search strategies
The MEDLINE search strategy may be found in Appendix 5.
Inclusion criteria
Population
-
Adults.
Intervention
-
Simple venepuncture for diagnostic or screening purposes.
Outcomes
-
Adverse events probably or possibly caused by the process of testing.
Setting
-
Any country.
Study type
-
RCTs.
-
Controlled non-randomised studies (egg cohort studies).
-
Case–control studies.
-
Case series.
-
Case reports.
-
Systematic reviews.
-
Economic evaluations.
Exclusion criteria
Population
-
Studies relating specifically to people receiving anticoagulation therapy, as their propensity to bruise would be significantly greater than that of patients not receiving anticoagulation therapy.
Intervention
Studies in which:
-
venepuncture was used either specifically to obtain blood donations, or to obtain both blood donations and smaller samples for diagnostic or screening purposes, but did not present separate data relating to the two uses
-
cannulation or catheterisation was used to obtain blood samples
-
the study related to the collection of arterial or capillary rather than venous blood samples.
Study type
-
Animal models.
-
Narrative reviews, editorials, opinions.
FIGURE 29.
Adverse effects of venepuncture: summary of study selection and exclusion.
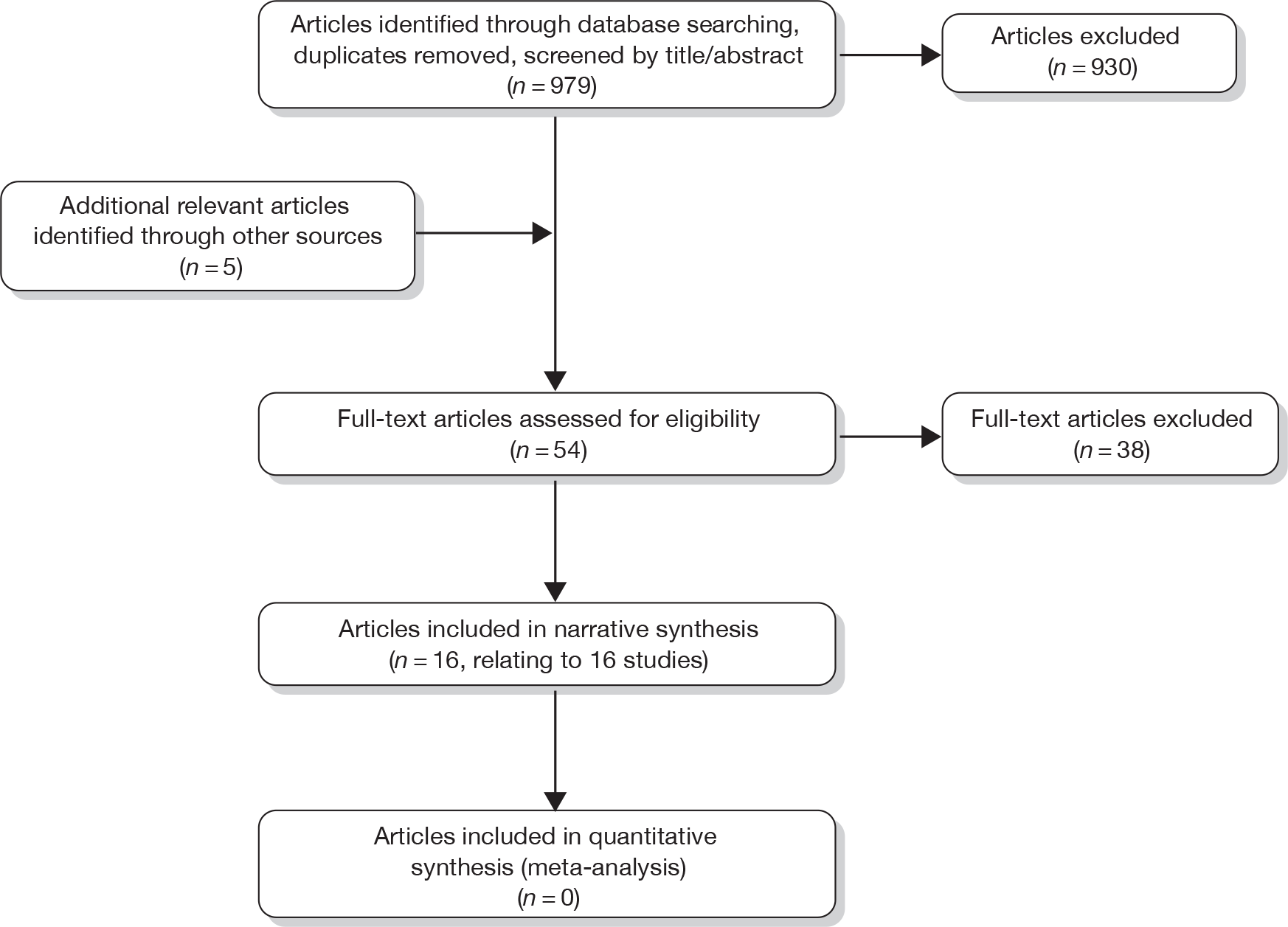
Appendix 10 The results from the cost-effectiveness analyses
Table 45 provides the costs and QALYs from each strategy assuming that there is no benefit associated with biopsy, nor any change in abstinence rates associated with diagnostic test. Table 46 provides the proportion of patients undergoing biopsy categorised by scenario number and diagnostic test.
| Scenario number | Biopsying all | FibroScan triage | ELF triage | FibroTest triage | Clinical experience triage | FibroScan replacement | ELF replacement | FibroTest replacement | Clinical experience replacement | Diagnose all as cirrhotic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | |
| 1 | 16,246 | 9.36 | 15,922 | 9.35 | 15,815 | 9.33 | 15,870 | 9.32 | 18,020 | 9.39 | 15,855 | 9.34 | 16,915 | 9.35 | 16,246 | 9.36 | 15,922 | 9.35 | 15,815 | 9.33 |
| 2 | 16,246 | 9.32 | 15,922 | 9.33 | 15,815 | 9.31 | 15,870 | 9.30 | 18,020 | 9.38 | 15,855 | 9.33 | 16,915 | 9.34 | 16,246 | 9.32 | 15,922 | 9.33 | 15,815 | 9.31 |
| 3 | 16,841 | 9.35 | 16,270 | 9.35 | 16,111 | 9.33 | 16,195 | 9.32 | 18,139 | 9.39 | 16,004 | 9.33 | 17,063 | 9.34 | 16,841 | 9.35 | 16,270 | 9.35 | 16,111 | 9.33 |
| 4 | 16,841 | 9.31 | 16,270 | 9.32 | 16,111 | 9.31 | 16,195 | 9.30 | 18,139 | 9.38 | 16,004 | 9.32 | 17,063 | 9.33 | 16,841 | 9.31 | 16,270 | 9.32 | 16,111 | 9.31 |
| 5 | 16,246 | 9.36 | 15,794 | 9.26 | 16,204 | 9.35 | 16,261 | 9.36 | 18,084 | 9.30 | 23,855 | 9.47 | 20,642 | 9.43 | 16,246 | 9.36 | 15,794 | 9.26 | 16,204 | 9.35 |
| 6 | 16,246 | 9.32 | 15,794 | 9.24 | 16,204 | 9.32 | 16,261 | 9.33 | 18,084 | 9.29 | 23,855 | 9.46 | 20,642 | 9.42 | 16,246 | 9.32 | 15,794 | 9.24 | 16,204 | 9.32 |
| 7 | 16,841 | 9.35 | 16,115 | 9.26 | 16,743 | 9.35 | 16,706 | 9.35 | 18,203 | 9.30 | 24,004 | 9.47 | 20,791 | 9.43 | 16,841 | 9.35 | 16,115 | 9.26 | 16,743 | 9.35 |
| 8 | 16,841 | 9.31 | 16,115 | 9.24 | 16,743 | 9.31 | 16,706 | 9.32 | 18,203 | 9.29 | 24,004 | 9.46 | 20,791 | 9.42 | 16,841 | 9.31 | 16,115 | 9.24 | 16,743 | 9.31 |
| 9 | 16,246 | 9.36 | 15,792 | 9.29 | 15,871 | 9.35 | 15,863 | 9.27 | 17,277 | 9.31 | 16,617 | 9.36 | 17,698 | 9.30 | 16,246 | 9.36 | 15,792 | 9.29 | 15,871 | 9.35 |
| 10 | 16,246 | 9.32 | 15,792 | 9.27 | 15,871 | 9.32 | 15,863 | 9.25 | 17,277 | 9.31 | 16,617 | 9.35 | 17,698 | 9.29 | 16,246 | 9.32 | 15,792 | 9.27 | 15,871 | 9.32 |
| 11 | 16,841 | 9.35 | 16,097 | 9.28 | 16,194 | 9.34 | 16,193 | 9.26 | 17,396 | 9.31 | 16,765 | 9.36 | 17,847 | 9.30 | 16,841 | 9.35 | 16,097 | 9.28 | 16,194 | 9.34 |
| 12 | 16,841 | 9.31 | 16,097 | 9.26 | 16,194 | 9.32 | 16,193 | 9.24 | 17,396 | 9.30 | 16,765 | 9.35 | 17,847 | 9.29 | 16,841 | 9.31 | 16,097 | 9.26 | 16,194 | 9.32 |
| 13 | 16,135 | 9.24 | 15,721 | 9.18 | 15,628 | 9.16 | 15,274 | 9.16 | 18,138 | 9.29 | 16,118 | 9.24 | 16,972 | 9.24 | 16,135 | 9.24 | 15,721 | 9.18 | 15,628 | 9.16 |
| 14 | 16,135 | 9.20 | 15,721 | 9.16 | 15,628 | 9.14 | 15,274 | 9.14 | 18,138 | 9.29 | 16,118 | 9.23 | 16,972 | 9.23 | 16,135 | 9.20 | 15,721 | 9.16 | 15,628 | 9.14 |
| 15 | 16,730 | 9.24 | 16,049 | 9.18 | 15,909 | 9.16 | 15,577 | 9.15 | 18,257 | 9.29 | 16,267 | 9.24 | 17,121 | 9.24 | 16,730 | 9.24 | 16,049 | 9.18 | 15,909 | 9.16 |
| 16 | 16,730 | 9.20 | 16,049 | 9.15 | 15,909 | 9.14 | 15,577 | 9.13 | 18,257 | 9.28 | 16,267 | 9.23 | 17,121 | 9.23 | 16,730 | 9.20 | 16,049 | 9.15 | 15,909 | 9.14 |
| 17 | 16,135 | 9.24 | 15,638 | 9.12 | 16,127 | 9.22 | 16,037 | 9.18 | 18,546 | 9.22 | 25,346 | 9.44 | 21,160 | 9.33 | 16,135 | 9.24 | 15,638 | 9.12 | 16,127 | 9.22 |
| 18 | 16,135 | 9.20 | 15,638 | 9.10 | 16,127 | 9.18 | 16,037 | 9.15 | 18,546 | 9.21 | 25,346 | 9.43 | 21,160 | 9.32 | 16,135 | 9.20 | 15,638 | 9.10 | 16,127 | 9.18 |
| 19 | 16,730 | 9.24 | 15,952 | 9.11 | 16,702 | 9.21 | 16,472 | 9.17 | 18,665 | 9.22 | 25,495 | 9.44 | 21,308 | 9.32 | 16,730 | 9.24 | 15,952 | 9.11 | 16,702 | 9.21 |
| 20 | 16,730 | 9.20 | 15,952 | 9.09 | 16,702 | 9.17 | 16,472 | 9.14 | 18,665 | 9.21 | 25,495 | 9.43 | 21,308 | 9.32 | 16,730 | 9.20 | 15,952 | 9.09 | 16,702 | 9.17 |
| 21 | 16,135 | 9.24 | 15,627 | 9.13 | 15,675 | 9.17 | 16,123 | 9.17 | 17,933 | 9.22 | 16,817 | 9.26 | 23,080 | 9.35 | 16,135 | 9.24 | 15,627 | 9.13 | 15,675 | 9.17 |
| 22 | 16,135 | 9.20 | 15,627 | 9.11 | 15,675 | 9.15 | 16,123 | 9.14 | 17,933 | 9.22 | 16,817 | 9.25 | 23,080 | 9.34 | 16,135 | 9.20 | 15,627 | 9.11 | 15,675 | 9.15 |
| 23 | 16,730 | 9.24 | 15,927 | 9.12 | 15,980 | 9.17 | 16,611 | 9.16 | 18,052 | 9.22 | 16,965 | 9.26 | 23,229 | 9.34 | 16,730 | 9.24 | 15,927 | 9.12 | 15,980 | 9.17 |
| 24 | 16,730 | 9.20 | 15,927 | 9.10 | 15,980 | 9.15 | 16,611 | 9.13 | 18,052 | 9.22 | 16,965 | 9.25 | 23,229 | 9.33 | 16,730 | 9.20 | 15,927 | 9.10 | 15,980 | 9.15 |
| 25 | 16,135 | 9.24 | 15,783 | 9.24 | 15,710 | 9.22 | 16,236 | 9.22 | 17,267 | 9.36 | 15,827 | 9.31 | 15,834 | 9.31 | 16,135 | 9.24 | 15,783 | 9.24 | 15,710 | 9.22 |
| 26 | 16,135 | 9.20 | 15,783 | 9.22 | 15,710 | 9.20 | 16,236 | 9.20 | 17,267 | 9.35 | 15,827 | 9.30 | 15,834 | 9.30 | 16,135 | 9.20 | 15,783 | 9.22 | 15,710 | 9.20 |
| 27 | 16,730 | 9.24 | 16,111 | 9.24 | 16,006 | 9.22 | 16,532 | 9.22 | 17,386 | 9.36 | 15,976 | 9.31 | 15,983 | 9.31 | 16,730 | 9.24 | 16,111 | 9.24 | 16,006 | 9.22 |
| 28 | 16,730 | 9.20 | 16,111 | 9.22 | 16,006 | 9.20 | 16,532 | 9.20 | 17,386 | 9.35 | 15,976 | 9.30 | 15,983 | 9.30 | 16,730 | 9.20 | 16,111 | 9.22 | 16,006 | 9.20 |
| 29 | 16,135 | 9.24 | 15,738 | 9.21 | 15,983 | 9.24 | 16,962 | 9.24 | 17,380 | 9.32 | 21,265 | 9.41 | 19,979 | 9.39 | 16,135 | 9.24 | 15,738 | 9.21 | 15,983 | 9.24 |
| 30 | 16,135 | 9.20 | 15,738 | 9.19 | 15,983 | 9.21 | 16,962 | 9.22 | 17,380 | 9.31 | 21,265 | 9.40 | 19,979 | 9.38 | 16,135 | 9.20 | 15,738 | 9.19 | 15,983 | 9.21 |
| 31 | 16,730 | 9.24 | 16,057 | 9.21 | 16,448 | 9.24 | 17,390 | 9.24 | 17,499 | 9.32 | 21,413 | 9.41 | 20,128 | 9.39 | 16,730 | 9.24 | 16,057 | 9.21 | 16,448 | 9.24 |
| 32 | 16,730 | 9.20 | 16,057 | 9.19 | 16,448 | 9.21 | 17,390 | 9.21 | 17,499 | 9.31 | 21,413 | 9.40 | 20,128 | 9.38 | 16,730 | 9.20 | 16,057 | 9.19 | 16,448 | 9.21 |
| 33 | 16,135 | 9.24 | 15,635 | 9.20 | 15,749 | 9.23 | 15,526 | 9.18 | 15,595 | 9.28 | 16,383 | 9.33 | 16,350 | 9.26 | 16,135 | 9.24 | 15,635 | 9.20 | 15,749 | 9.23 |
| 34 | 16,135 | 9.20 | 15,635 | 9.18 | 15,749 | 9.21 | 15,526 | 9.16 | 15,595 | 9.27 | 16,383 | 9.32 | 16,350 | 9.25 | 16,135 | 9.20 | 15,635 | 9.18 | 15,749 | 9.21 |
| 35 | 16,730 | 9.24 | 15,895 | 9.19 | 16,065 | 9.23 | 15,818 | 9.17 | 15,714 | 9.28 | 16,532 | 9.32 | 16,499 | 9.25 | 16,730 | 9.24 | 15,895 | 9.19 | 16,065 | 9.23 |
| 36 | 16,730 | 9.20 | 15,895 | 9.18 | 16,065 | 9.21 | 15,818 | 9.15 | 15,714 | 9.27 | 16,532 | 9.31 | 16,499 | 9.24 | 16,730 | 9.20 | 15,895 | 9.18 | 16,065 | 9.21 |
| Scenario numbers | Biopsying all (%) | FibroScan triage (%) | ELF triage (%) | FibroTest triage (%) | Clinical experience triage (%) |
|---|---|---|---|---|---|
| 1–4 | 100 | 49 | 34 | 35 | 40 |
| 5–8 | 100 | 43 | 88 | 35 | 67 |
| 9–12 | 100 | 40 | 40 | 35 | 41 |
| 13–16 | 100 | 45 | 30 | 35 | 35 |
| 17–20 | 100 | 41 | 96 | 35 | 64 |
| 21–24 | 100 | 39 | 36 | 35 | 76 |
| 25–28 | 100 | 45 | 34 | 35 | 34 |
| 29–32 | 100 | 43 | 71 | 35 | 63 |
| 33–36 | 100 | 31 | 38 | 35 | 33 |
Table 47 provides the threshold level for percentage point decrease in abstinence rates that would make biopsy all the more cost-effective strategy compared with each NILT; Table 48 provides the same detail, but the threshold value is the QALY gain associated with a biopsy
| Scenario number | FibroScan triage | ELF triage | FibroTest triage | Clinical experience triage | FibroScan replacement | ELF replacement | FibroTest replacement | Clinical experience replacement | Diagnose all as cirrhotic |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2 | 0.039 | 0.015 | 0.005 | 0.000 | 0.000 | 0.012 | 0.000 | 0.000 | 0.000 |
| 3 | 0.039 | 0.015 | 0.005 | 0.000 | 0.000 | 0.012 | 0.000 | 0.000 | 0.000 |
| 4 | 0.062 | 0.036 | 0.025 | 0.000 | 0.003 | 0.024 | 0.006 | 0.000 | 0.000 |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 6 | 0.000 | 0.013 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 7 | 0.000 | 0.013 | 0.021 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 8 | 0.000 | 0.034 | 0.043 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 9 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 10 | 0.000 | 0.030 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 |
| 11 | 0.000 | 0.030 | 0.000 | 0.000 | 0.000 | 0.006 | 0.000 | 0.000 | 0.000 |
| 12 | 0.000 | 0.052 | 0.000 | 0.000 | 0.000 | 0.019 | 0.000 | 0.000 | 0.000 |
| 13 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 |
| 14 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.011 | 0.000 | 0.016 | 0.000 |
| 15 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.011 | 0.000 | 0.015 | 0.000 |
| 16 | 0.000 | 0.000 | 0.000 | 0.005 | 0.005 | 0.023 | 0.006 | 0.028 | 0.000 |
| 17 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 |
| 18 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.016 | 0.000 |
| 19 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.015 | 0.000 |
| 20 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.028 | 0.000 |
| 21 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 |
| 22 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.016 | 0.000 |
| 23 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.015 | 0.000 |
| 24 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.018 | 0.000 | 0.028 | 0.000 |
| 25 | 0.022 | 0.001 | 0.000 | 0.000 | 0.025 | 0.034 | 0.035 | 0.003 | 0.000 |
| 26 | 0.045 | 0.022 | 0.000 | 0.000 | 0.039 | 0.047 | 0.047 | 0.016 | 0.000 |
| 27 | 0.045 | 0.022 | 0.000 | 0.000 | 0.039 | 0.046 | 0.047 | 0.015 | 0.000 |
| 28 | 0.067 | 0.043 | 0.017 | 0.005 | 0.052 | 0.059 | 0.060 | 0.028 | 0.000 |
| 29 | 0.000 | 0.018 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 | 0.003 | 0.000 |
| 30 | 0.007 | 0.041 | 0.000 | 0.000 | 0.022 | 0.000 | 0.000 | 0.016 | 0.000 |
| 31 | 0.007 | 0.041 | 0.000 | 0.000 | 0.022 | 0.000 | 0.000 | 0.015 | 0.000 |
| 32 | 0.027 | 0.063 | 0.000 | 0.005 | 0.036 | 0.000 | 0.007 | 0.028 | 0.000 |
| 33 | 0.000 | 0.009 | 0.000 | 0.000 | 0.027 | 0.030 | 0.001 | 0.003 | 0.000 |
| 34 | 0.001 | 0.030 | 0.000 | 0.000 | 0.042 | 0.043 | 0.014 | 0.016 | 0.000 |
| 35 | 0.001 | 0.030 | 0.000 | 0.000 | 0.041 | 0.042 | 0.014 | 0.015 | 0.000 |
| 36 | 0.020 | 0.052 | 0.003 | 0.005 | 0.055 | 0.055 | 0.026 | 0.028 | 0.000 |
| Scenario number | FibroScan triage | ELF triage | FibroTest triage | Clinical experience triage | FibroScan replacement | ELF replacement | FibroTest replacement | Clinical experience replacement | Diagnose all as cirrhotic |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.029 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2 | 0.069 | 0.028 | 0.008 | 0.000 | 0.000 | 0.037 | 0.000 | 0.000 | 0.000 |
| 3 | 0.069 | 0.029 | 0.009 | 0.000 | 0.000 | 0.036 | 0.000 | 0.000 | 0.000 |
| 4 | 0.109 | 0.068 | 0.048 | 0.000 | 0.008 | 0.076 | 0.017 | 0.000 | 0.000 |
| 5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 6 | 0.000 | 0.023 | 0.035 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 7 | 0.000 | 0.023 | 0.036 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 8 | 0.000 | 0.063 | 0.076 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 9 | 0.000 | 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 10 | 0.000 | 0.054 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 |
| 11 | 0.000 | 0.055 | 0.000 | 0.000 | 0.000 | 0.019 | 0.000 | 0.000 | 0.000 |
| 12 | 0.000 | 0.094 | 0.000 | 0.000 | 0.000 | 0.059 | 0.000 | 0.000 | 0.000 |
| 13 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 |
| 14 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.033 | 0.000 | 0.048 | 0.000 |
| 15 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.032 | 0.000 | 0.047 | 0.000 |
| 16 | 0.000 | 0.000 | 0.000 | 0.009 | 0.015 | 0.072 | 0.018 | 0.086 | 0.000 |
| 17 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 |
| 18 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.048 | 0.000 |
| 19 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.047 | 0.000 |
| 20 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 | 0.000 | 0.000 | 0.086 | 0.000 |
| 21 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 |
| 22 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.016 | 0.000 | 0.048 | 0.000 |
| 23 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.015 | 0.000 | 0.047 | 0.000 |
| 24 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 | 0.055 | 0.000 | 0.086 | 0.000 |
| 25 | 0.038 | 0.001 | 0.000 | 0.000 | 0.073 | 0.105 | 0.108 | 0.008 | 0.000 |
| 26 | 0.078 | 0.041 | 0.000 | 0.000 | 0.113 | 0.145 | 0.148 | 0.048 | 0.000 |
| 27 | 0.078 | 0.041 | 0.000 | 0.000 | 0.112 | 0.144 | 0.146 | 0.047 | 0.000 |
| 28 | 0.118 | 0.081 | 0.031 | 0.009 | 0.151 | 0.184 | 0.186 | 0.086 | 0.000 |
| 29 | 0.000 | 0.032 | 0.000 | 0.000 | 0.020 | 0.000 | 0.000 | 0.008 | 0.000 |
| 30 | 0.014 | 0.071 | 0.000 | 0.000 | 0.060 | 0.000 | 0.000 | 0.048 | 0.000 |
| 31 | 0.014 | 0.072 | 0.000 | 0.000 | 0.059 | 0.000 | 0.000 | 0.047 | 0.000 |
| 32 | 0.054 | 0.112 | 0.000 | 0.009 | 0.098 | 0.000 | 0.021 | 0.086 | 0.000 |
| 33 | 0.000 | 0.015 | 0.000 | 0.000 | 0.077 | 0.093 | 0.003 | 0.008 | 0.000 |
| 34 | 0.001 | 0.055 | 0.000 | 0.000 | 0.117 | 0.133 | 0.043 | 0.048 | 0.000 |
| 35 | 0.001 | 0.056 | 0.000 | 0.000 | 0.115 | 0.132 | 0.041 | 0.047 | 0.000 |
| 36 | 0.041 | 0.095 | 0.006 | 0.009 | 0.155 | 0.171 | 0.081 | 0.086 | 0.000 |
Appendix 11 Project protocol
Glossary
- Alcoholic fatty liver (hepatic steatosis)
- Accumulation of excess fat in the liver cells as a result of excess alcohol consumption.
- Alcoholic hepatitis
- An inflammatory condition involving liver damage.
- Ascites
- Excessive accumulation of fluid in the peritoneal cavity.
- Banding
- Use of elastic bands to tie off blood vessels.
- Beta-blocker
- A drug used to lower blood pressure. Propranolol, one of the beta-blockers, is used to treat portal hypertension and so to help prevent bleeding from oesophageal varices in patients with cirrhosis.
- Body mass index
- A measure of weight for height obtained by dividing a person’s weight in kilograms by the square of their height in metres.
- Cirrhosis
- Advanced fibrosis.
- Cohort study
- A study that follows groups of people with and without the condition of interest over time to study outcomes.
- Compensated cirrhosis
- Cirrhosis with no associated complications.
- Corticosteroids
- Drugs that reduce inflammation and can be used to treat severe alcoholic hepatitis.
- Cross-sectional study
- A study that measures the determinants of health in a population at a point in time or over a short period of time; in the context of diagnostic studies, a study that examines the accuracy of a test by comparison with a reference standard, rather than by following people who have undergone the test over time to compare their health outcomes with the test results.
- Decompensated cirrhosis
- Cirrhosis associated with complications such as ascites, peripheral oedema, jaundice, variceal bleeding, and hepatic encephalopathy.
- Ethanol
- Pure alcohol.
- Fibrosis
- Fibrous scar tissue.
- Haematocrit
- The percentage of red blood cells in a blood sample.
- Harmful drinking
- An established pattern of drinking at a level where damage to physical or mental health is considered likely.
- Hazardous drinking
- An established pattern of drinking that carries a risk of physical or psychological harm.
- Hepatic encephalopathy
- Symptom complex of neuropsychiatric abnormalities observed in patients with acute and chronic liver disease, and mainly affecting mental and motor function.
- Hepatic portal vein
- The vein that drains blood from the gastrointestinal tract and spleen to the liver.
- Hepatic steatosis
- Accumulation of excess fat within the liver cells.
- Hepatic venous pressure gradient
- The pressure gradient within the portal system.
- Hepatocellular carcinoma
- Primary cancer of the liver, generally as a consequence of either viral hepatitis or excessive alcohol consumption.
- High-Risk Alcoholism Relapse scale
- A scale designed to estimate the risk of alcoholism relapse following evaluation.
- Histological
- Related to the microscopic structure of tissue.
- Jaundice
- Yellow discoloration of the skin and eyes.
- Kilopascal (kPa)
- Metric unit of pressure.
- Laparoscopic
- Performed through small incisions in the abdomen.
- Liver biopsy
- Removal of a small sample of liver tissue using a hollow needle for examination in the laboratory.
- Liver fibrosis
- Formation of excessive fibrous scar tissue in the liver.
- Maddrey score
- A measure of severity of alcoholic hepatitis.
- Metabolic syndrome
- A combination of central obesity with any two of raised triglycerides, reduced high-density lipoprotein (HDL) cholesterol, raised blood pressure or raised fasting plasma glucose/previously diagnosed type 2 diabetes.
- Non-alcoholic fatty liver disease
- Liver disease characterised by the accumulation of fat in the liver cells of people who do not drink alcohol excessively.
- Non-alcoholic steatohepatitis
- A more advanced form of non-alcoholic fatty liver disease involving inflammation in and around the fatty liver cells. It may cause scarring of the liver and lead to cirrhosis.
- Obese
- Having a body mass index ≥ 30.
- Oesophageal varices
- Varicose veins in the lower end of the oesophagus which develop as a complication of cirrhosis.
- p-value
- The probability that an observed difference could have occurred by chance if the null hypothesis is correct. A p-value of < 0.05 is conventionally considered to be statistically significant.
- Percutaneous
- Performed through a needle puncture of the skin.
- Peripheral oedema
- Swelling of the feet, ankles and legs.
- Portal hypertension
- High blood pressure in the hepatic portal vein and its tributaries. It is often defined as a portal pressure gradient (the difference in pressure between the portal vein and the hepatic veins) of ≥ 5 mmHg. It may occur in people with or without chronic liver disease.
- Sclerotherapy
- Injection of a medication into blood vessels or blood vessel malformations to make them shrink.
- Serum
- The liquid component of blood (i.e. without the blood cells and clotting factors).
- Severe alcoholic hepatitis
- Alcoholic hepatitis with symptoms severe enough to require hospital admission.
- Shear wave
- An elastic wave which moves through the body of an object rather than over its surface.
- Spontaneous bacterial peritonitis
- Infection of the fluid which can accumulate in the abdomen (ascites) in patients with severe liver disease.
- Steatosis
- A condition characterised by the accumulation of excess fat within the liver cells.
- Transjugular
- Via the jugular vein in the neck.
- Unit of alcohol
- Quantity of any alcoholic drink which corresponds to approximately 10 ml (8 g) of ethanol.
- Variceal bleeding
- Gastrointestinal bleeding caused by rupture of the oesophageal/gastric varices.
- Varices
- Varicose veins usually in the stomach and lower end of the oesophagus (gullet) which develop as a complication of cirrhosis.
List of abbreviations
- AAH
- acute alcoholic hepatitis
- ALD
- alcohol-related liver disease
- AUDIT
- Alcohol Use Disorders Identification Test
- ASH
- alcoholic steatohepatitis
- AUROC
- area under the receiver operating characteristic curve
- BMI
- body mass index
- CI
- confidence interval
- ELF test
- Enhanced Liver Fibrosis test
- FN
- false-negative
- FP
- false-positive
- HA
- hyaluronic acid
- HCC
- hepatocellular cancer
- HE
- hepatic encephalopathy
- HRAR
- high-risk alcoholism relapse
- HVPG
- hepatic venous pressure gradient
- ICU
- intensive care unit
- LSM
- liver stiffness measurement
- NAFLD
- non-alcoholic fatty liver disease
- NICE
- National Institute for Health and Clinical Excellence
- NILT
- non-invasive liver test
- PHt
- portal hypertension
- PICO
- Population, Intervention, Comparator, Outcome
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QUADAS
- QUality Assessment of Diagnostic Accuracy Studies
- QUOROM
- QUality Of Reporting Of Meta-analyses
- RCT
- randomised controlled trial
- SGOT
- serum glutamic oxaloacetic transaminase
- TIMP-1
- tissue inhibitor of matrix metalloproteinase-1
- TN
- true-negative
- TP
- true-positive
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington