Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 03/36/01. The contractual start date was in December 2003. The draft report began editorial review in December 2010 and was accepted for publication in June 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The National Institute for Health Research awarded a grant to the London School of Hygiene and Tropical Medicine to carry out this research. The Queen’s University Belfast, Royal Liverpool and Broadgreen University Hospitals Trust were reimbursed for some of the time of Professor Chakravarthy (chief investigator) and SP Harding (principal investigator). Professor Reeves (principal investigator) was employed by the London School of Hygiene and Tropical Medicine until 2005 and part of his salary was paid by the grant. Part or all of the salaries of other authors employed by the London School of Hygiene and Tropical Medicine institution were also paid by the grant for all or part of the duration of the study. The Central Angiographic Research Facility and the three reading centres were funded by a separate grant from the NHS, which disbursed funding to the institutions of Professors Chakravarthy and Harding and Dr Peto. Professor Chakravarthy has received funding from Novartis for her membership of an advisory board on dry age-related macular degeneration. Her institution has received funding from Bausch and Lomb, Novartis, Pfizer, Oraya Therapeutics and Bayer for participation in studies on age-related macular degeneration. Professor Harding has received funding from Pfizer (unrestricted educational grant) and Novartis (conference attendance). His institution has received funding from Novartis for participation in studies on agerelated macular degeneration.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Reeves et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and rationale
Macular degeneration (MD) is the commonest cause of visual impairment in the developed world. 1,2 It mainly affects central vision, which underpins the ability to do tasks that require fine detail to be resolved, for example reading, watching television and recognising faces. The commonest cause of MD is ageing, although there are many other less frequent causes. Age-related MD (AMD) causes visual loss principally through the development of new vessels, which form in the choroid under the retina and leak fluid, bleed and eventually fibrose (choroidal neovascularisation, CNV). A less frequent site for the development of new vessels is in the retina itself, and this is termed retinal angiomatous proliferation (RAP). The process of neovascularisation is often referred to as ‘wet’ or neovascular AMD (nAMD) to distinguish it from a less aggressive or ‘dry’ form. CNV is identified and categorised using fundus fluorescein angiography (FA) into ‘classic’ and ‘occult’ forms based on patterns of fluorescence and location under the retina. RAP is identifiable using FA but more readily using indocyanine green angiography. At the onset of nAMD, a progressive fall in vision in the affected eye generally occurs over weeks and months and is more rapid with classic than occult CNV.
Verteporfin is a light-sensitive drug which is used in combination with an infrared laser to treat abnormal blood vessels proliferating under or within the macular retina in patients with nAMD and, less frequently, in some other eye conditions. The drug is injected intravenously and binds selectively to endothelial cells in the abnormal retinal vessels. The drug is activated by low-energy laser radiation, causing the abnormal vessels to regress. These two steps, that is initial infusion of verteporfin followed by laser exposure, are known as verteporfin photodynamic therapy (VPDT). Over time the vessels frequently reopen and so treatment usually needs to be repeated 3-monthly.
Verteporfin photodynamic therapy has been shown in randomised clinical trials to be better than sham treatment in maintaining sight in patients with nAMD. In the clinical trials of VPDT carried out for licensing, the Treatment of Age-related macular degeneration with Photodynamic therapy (TAP) trials and the Verteporfin in Photodynamic therapy (VIP) trials,3–5 the overall difference between treatment and placebo groups was small except in a subgroup of patients in whom ≥ 50% of the area of CNV was classified as classic by FA; in this subgroup, the benefit was larger. 4
There are no established criteria for stopping a course of treatment. The treatment protocol used in the TAP trials required 3-monthly reviews of FA for 2 years with retreatment if active CNV was observed. 3
The cost of a course of therapy has been estimated to be between £6000 and £8000. This relatively high cost arises largely because of the frequency of retreatment episodes in research cohorts. Cost-effectiveness was not estimated alongside the clinical trials used for licensing. In 2003, the National Institute for Clinical Excellence (NICE; now the National Institute for Health and Clinical Excellence) recommended treatment in the UK NHS for patients with nAMD ‘who have a confirmed diagnosis of classic with no occult subfoveal choroidal neovascularisation’. 6 However, NICE did not recommend VPDT for people with predominantly classic CNV ‘except as part of on-going or new clinical studies that are designed to generate robust and relevant outcome data, including data on optimum treatment regimens, long-term outcomes, quality of life and costs’ (Box 1). 6
1.1 Photodynamic therapy (PDT) is recommended for the treatment of wet age-related macular degeneration for individuals who have a confirmed diagnosis of classic with no occult subfoveal choroidal neovascularisation (CNV) (that is, whose lesions are composed of classic CNV with no evidence of an occult component) and best-corrected visual acuity 6/60 or better. VPDT should be carried out only by retinal specialists with expertise in the use of this technology.
1.2 PDT is not recommended for the treatment of people with predominantly classic subfoveal CNV (that is, 50% or more of the entire area of the lesion is classic CNV but some occult CNV is present) associated with wet age-related macular degeneration, except as part of on-going or new clinical studies that are designed to generate robust and relevant outcome data, including data on optimum treatment regimens, long-term outcomes, quality of life and costs.
1.3 The use of VPDT in occult CNV associated with wet age-related macular degeneration was not considered because the photosensitising agent (verteporfin) was not licensed for this indication when this appraisal began. No recommendation is made with regard to the use of this technology in people with this form of the condition.
Uncertainties about the effectiveness of VPDT were highlighted by the NICE technology appraisal. 6 Around the time of publication of the NICE guidance, the NHS R&D Health Technology Assessment programme, on behalf of the Department of Health, approached potential investigators about carrying out further research on VPDT to address these uncertainties. The overall aim was to characterise the cohort of patients referred for and treated with VPDT and to collect data about their visual acuity outcomes, quality of life and use of health and social care resources.
Initially, it was envisaged that this research should focus on the group of patients referred to in paragraph 1.2 of the NICE guidance (see Box 1), that is patients with ‘predominantly classic’ CNV lesions. However, discussions with the regional NHS commissioners and the Royal College of Ophthalmologists led to the scope of the research being expanded to include all patients treated with VPDT, irrespective of the subtype of the CNV. The VPDT cohort study was set up to meet these objectives. It built on surveillance programmes and research proposals active at the time and allowed patients with nAMD to access VPDT through the NHS.
The VPDT cohort study investigators aimed to address a number of questions which were relevant to NICE and to the NHS which were unanswered at the time of its guidance in 2003. These are set out in Chapter 2. However, the study was also of interest to other stakeholders, for example commissioners of NHS care. There is a need to develop robust methods of managing the introduction of new technologies into the NHS, especially when these are expensive, and the VPDT cohort study is one model by which this might be achieved. At the outset, there was the ambition that establishing a treatment register would allow a new technology (in this case, VPDT) to be introduced to a pre-specified service standard ensuring best possible care for patients, its use to be monitored effectively by commissioners and uncommon/rare adverse events (AEs) not identified in pre-licensing trials to be detected. A further benefit might be training clinical sites in research methods and processes to facilitate future clinical trials and research relevant to the NHS.
Visual acuity and health-related quality of life
A limitation of many randomised controlled trials (RCTs) of ophthalmological interventions is that the researchers conventionally choose best-corrected monocular distance visual acuity (BCVA) as the primary outcome. Reporting the effect of a new treatment as the average BCVA benefit relative to a control group allows ophthalmologists to consider the probable value of the new treatment compared with the best existing treatment (or alternative treatments) for the same condition. However, the limitations of clinical measures of outcome are now widely appreciated and many governmental and non-governmental organisations emphasise the importance of patient-reported outcomes or health-related quality of life (HRQoL) for measuring treatment effectiveness and health-care performance. 7,8 Moreover, the benefits of ophthalmic interventions are difficult to compare with other health-care interventions without being able to describe them in a common currency, for example quality-adjusted life-years (QALYs, see below and The health and social service costs of nAMD and associated treatments). 9
Health-related quality of life is a complex concept. A spectrum of instruments have been designed to measure HRQoL, from ones focused closely on functional performance to those assessing broader domains and rating the importance to an individual patient of a perceived loss of physical, social or emotional function. 10 This spectrum can be investigated specifically with respect to the condition affecting a respondent (condition-specific instruments) or to his or her wider life experience (generic instruments). The National Eye Institute Visual Function Questionnaire (NEIVFQ), which lies towards the functional performance end of the HRQoL spectrum, is perhaps the most widely used vision-specific HRQoL instrument. 11
A subset of generic HRQoL instruments explicitly recognises underlying preferences for different health states. These preference-based measures, such as the European Quality of Life-5 Dimensions (EQ-5D) and the Short Form questionnaire-6 Dimensions (SF-6D),12,13 report HRQoL on a scale with ‘anchors’ at 0 for death and 1 for perfect health. Preference-based measures of HRQoL are important because they can be combined with the relative effects of interventions on life expectancy to report QALYs. 9 QALYs allow comparison of interventions that may improve HRQoL but not life expectancy (such as many ophthalmic interventions) with interventions in other disease areas that can improve life expectancy but have little effect on HRQoL (e.g. statins to prevent coronary heart disease). Such comparisons, using HRQoL measures that take preference weights (i.e. societal values) or utilities from the general population,14,15 underpin health policy in many publicly funded health systems. They enable policy-makers to decide the relative worth of a new treatment in a wider context, that is compared with the value of health-care treatments for all other conditions that compete for funding from a finite budget.
Many studies have examined cross-sectional associations between visual acuity and HRQoL using a variety of HRQoL instruments including preference-based measures. 16,17 However, few have examined these associations longitudinally. Also, several studies that have reported preference-based measures of HRQoL have reported utilities elicited directly from patients18 rather than by the recommended process of taking these preferences from the general population. 14,15 Previous studies that have attempted to use preference-based measures of HRQoL to assess the gains from ophthalmic interventions for policy-making purposes have highlighted the deficiencies in existing studies. 19
The VPDT cohort study collected clinical measures of vision, measures of HRQoL and measures of resource use to achieve its principal aims of estimating the effectiveness and cost-effectiveness of VPDT in routine clinical practice. 20,21 Understanding the relationships between visual function and vision-specific and generic HRQoL was central to achieving these aims. 22
The health and social service costs of neovascular (wet) age-related macular degeneration and associated treatments
Neovascular AMD is potentially associated with high costs to health services and society. 23–25 Interventions for nAMD may improve HRQoL and reduce the costs associated with declining vision. 26 Cost-effectiveness analysis (CEA) is a powerful tool to evaluate and prioritise health-care interventions according to their relative effectiveness and cost. In many publicly funded health systems, policy-makers require CEA to assess whether or not a new intervention has sufficient gain to justify additional costs before recommending adoption. Decision-makers in predominantly privately funded health systems have recently shown interest in using CEA. 27
Previous CEAs of VPDT for nAMD have been contradictory. Some studies have reported that VPDT is ‘highly cost-effective’ and others that it is ‘definitely not cost-effective’. 26,28 For CEAs to provide a sound basis for decision-making, they must meet certain methodological standards. 15,29 The previous CEA of VPDT did not meet these standards on three grounds. 19 Firstly, intervention costs were based on treatment frequencies reported in the TAP trial, which are higher than those for routine practice. 3,20,30 Secondly, the HRQoL measures used took inappropriate preference weightings from patients with nAMD rather than generic measures such as the SF-6D that take health-state preferences from the general population. 19,31 Because CEAs are used to compare health gain across disease areas, the HRQoL measures used should weight different health states according to valuations taken from the general population rather than any specific patient group, for example patients with AMD. Thirdly, costing studies have reported that, compared with the general population, patients with AMD are more likely to use residential care and social services and to take antidepressants. 32,33 However, previous CEAs either ignored costs associated with vision loss or relied on expert opinion, rather than collecting appropriate patient-level costs. 19,26,32,33 The VPDT cohort study was commissioned to address some of these limitations.
Chapter 2 Aims and objectives
The overarching aim of the VPDT cohort study was to broaden the understanding of the pathogenesis of CNV and its treatment with VPDT through a longitudinal analysis of outcomes in patients undergoing VPDT for CNV.
The a priori objectives of the study were set out in the manual of operations (see Appendix 1). These were:
-
to estimate the prevalence and incidence of patients with CNV being referred photodynamic therapy (PDT) who meet the eligibility criteria for treatment
-
to describe the clinical management of patients with CNV being referred for VPDT who meet the eligibility criteria for treatment
-
to characterise changes over time in clinical outcomes, self-reported visual functioning (SRVF), generic quality of life and the societal costs of illness in patients receiving VPDT who meet the eligibility criteria for treatment
-
to describe the relationship between clinical outcomes, SRVF and HRQoL
-
to estimate incremental cost-effectiveness, cost–utility and cost impact on the NHS (using data estimated for objectives 1–4 of implementing VPDT in the NHS for patients who meet the eligibility criteria for treatment.
During the first year of the project and following review of progress by the Health Technology Assessment programme, but before any analyses of data collected in the study, these objectives were updated to characterise better the uncertainties experienced by the NICE appraisal committee:
-
Is VPDT in the NHS provided as in randomised trials?
-
Is ‘outcome’ the same in the NHS as in randomised trials?
-
Is ‘outcome’ the same for patients who would have been ineligible for randomised trials?
-
Is VPDT safe when provided in the NHS?
-
How effective and cost-effective is VPDT?
These objectives will be referred to in sequence in the rest of the report.
Chapter 3 Methods
Study design
The VPDT cohort study was designed as a longitudinal treatment register (case series) of all patients treated with VPDT in the UK. The study received research ethics committee approval (reference MREC/03/11/103).
The manual of operations, describing the study design and methods, including standard protocols for all measurements, was prepared before recruitment started and was updated periodically. The final version of this manual (version 2.1, December 2005) is included as Appendix 1.
Setting
When the VPDT cohort study was conceived, VPDT was not widely used in the NHS. Local Specialised Commissioning Groups (SCGs) recommended that VPDT should be provided only in designated ophthalmology departments in NHS hospitals. The Royal College of Ophthalmologists and clinicians involved with the VPDT cohort study took responsibility for providing a training programme for ophthalmologists and other health-care staff in hospitals that were newly implementing the provision of VPDT. Thus, the setting for the study was all designated hospitals (DHs) providing VPDT in the NHS.
After the majority of DHs had been identified and their personnel had been trained, the study team organised meetings of investigators. These meetings described progress with the study, discussed aspects of compliance with the study protocol, data submission and quality, and provided additional training. This training covered:
-
for participating ophthalmologists, retreatment decision-making and interpretation of angiograms
-
for independent grading for ophthalmic photographers and technicians, acquisition of angiograms and their subsequent submission to the Network of Ophthalmic Reading Centres in the UK (NetwORC UK, see Network of Ophthalmic Reading Centres in the UK)
-
for nurses, optometrists and site co-ordinators, assessment of visual and other study procedures.
Participants
The manual of operations described the criteria for eligibility for treatment according to the NICE guidance:
-
Best corrected visual acuity in the eye being considered for treatment must be equal to or better than Snellen 6/60, approximately equivalent to seeing one or more letters on the line corresponding to a logarithm of the minimum angle or resolution (logMAR) of 1.0, or > 30 letters when measured with an Early Treatment Diabetic Retinopathy Study (ETDRS) distance visual acuity chart (see Outcomes).
-
Choroidal neovascularisation must be wholly or predominantly classic (i.e. ≥ 50% of the entire lesion must consist of classic CNV).
-
Patients with subfoveal CNV due to nAMD or any other disorder are eligible for inclusion in the VPDT study.
All patients referred for assessment at a VPDT clinic in a designated treatment centre, whether eligible or not, made up the reference population. As part of the assessment, the ophthalmologist in charge of the patient made a decision on eligibility for treatment (above). There were no a priori exclusion criteria for people in the reference population. Participating hospitals were asked to submit a full set of data at the screening visit for all ineligible patients seen in person at the VPDT clinic, together with the FA used for decision-making, irrespective of whether the FA was carried out by the participating centre or by a referring hospital.
The study population consisted of all patients treated with VPDT at participating centres irrespective of CNV aetiology. (The decision whether or not to include all patients treated with VPDT, irrespective of aetiology, was made by the SCGs.) Participants were asked to give written informed consent for the collection of data and use of these data for the research.
Treatment with verteporfin photodynamic therapy
Participating centres were requested to classify CNV as had been done in previous RCTs3,5,34 in order to decide whether or not patients were eligible for treatment (Table 1).
| A. Identify morphological features Use stereos of colour and angiographic frames to assist in recognition of the following lesion components |
B. Assess total lesion size | C. Categorise lesion subtype |
|---|---|---|
|
1. CNV lesion components Fluorescein leakage associated with CNVFeatures contiguous to CNV which prevent determination of the extent of leakage and which therefore constitute part of the lesion2. Other features associated with CNV which are NOT used to define the boundaries of the lesion3. Other features which help with categorisation of CNV or which may modify natural history |
|
|
Participating centres were also requested to review patients at 3-month intervals, carrying out ophthalmological and angiographic examinations to determine whether or not repeat therapy was needed. Two algorithms to guide retreatment decisions were included in the study manual (Figure 1 and Table 2). Investigators were also referred to the retreatment criteria developed by an international expert consensus group, the Verteporfin Round Table. 37
FIGURE 1.
Example of flow chart for making retreatment decisions – Belfast retreatment criteria. VA, visual activity.
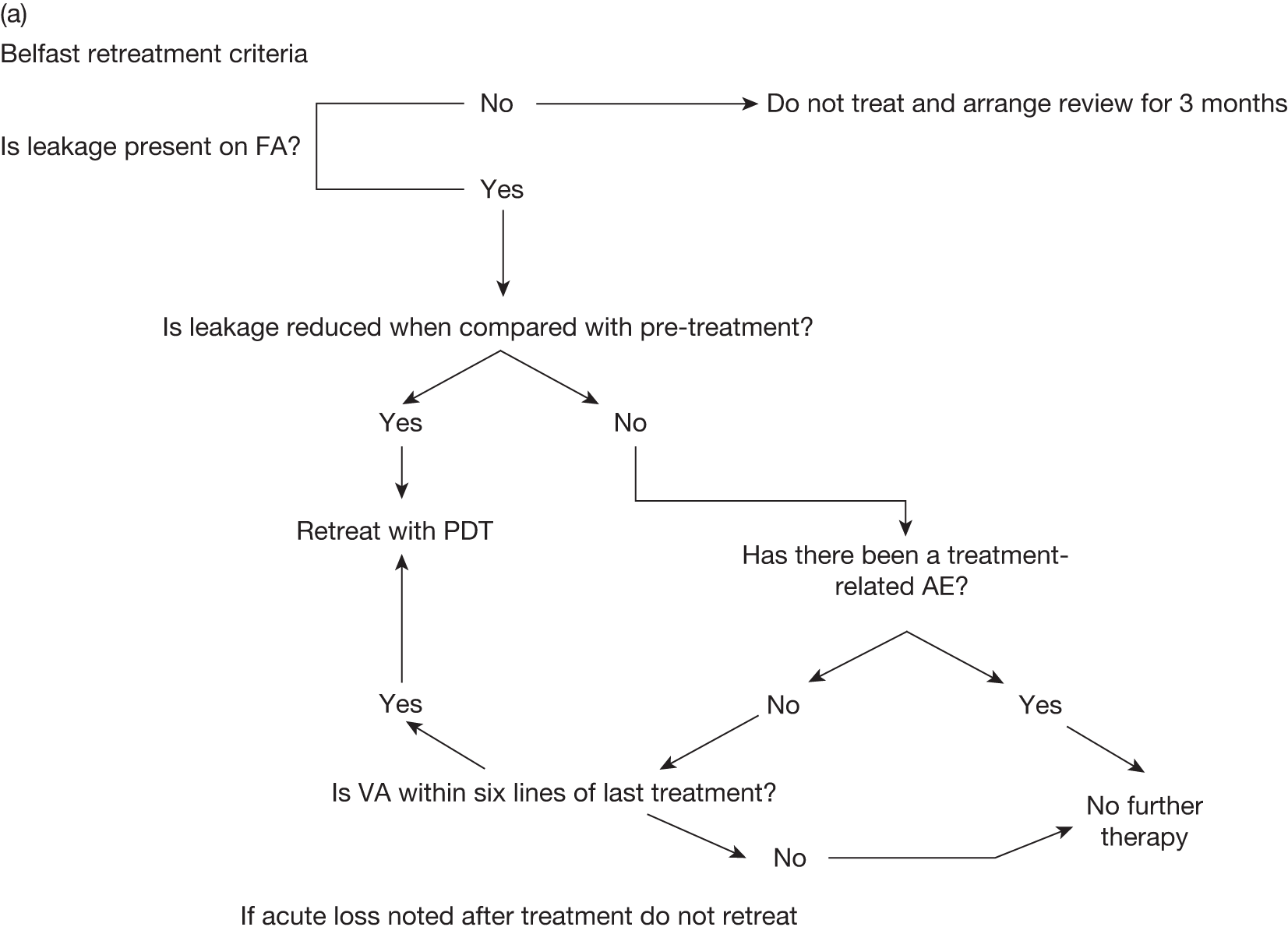
| Parameter | Retreat | Do not retreat |
|---|---|---|
| FA | Leakage | No leakage/no leakage at centre |
| Visual acuity | Dropping | Stable or < 20 letters |
| Subretinal fluid | Persistent | Cleared |
| Haemorrhage | New | Cleared |
| CNV | Extension | Inactive |
| Next visit | 3 months | ≥ 9 monthsa |
Outcomes
The primary outcome was defined as BCVA, measured on a logMAR scale using the ETDRS distance visual acuity chart. 38
Secondary outcomes included:
-
safety, that is adverse reactions (ARs) and AEs
-
contrast sensitivity (CS) measured with the Pelli–Robson chart at 1 m39
-
generic HRQoL measured using the Short Form questionnaire-36 items (SF-36),40 from which SF-6D scores were also derived13
-
vision-specific HRQoL measured using the NEIVFQ11
-
independently graded morphological changes in treated lesions, that is total lesion size, total CNV leakage, classic leakage and fibrosis
-
health and social services (HSS) resource use measured using a custom-designed questionnaire administered to patients at the time of hospital visits for treatment or review.
Collecting data to characterise ARs and AEs was an important objective of the study because, at the outset, there was concern that such events experienced in licensing trials of VPDT may not be representative of the events observed in usual practice. The manual of operations specified that all ARs (during or just after treatment) or AEs (between treatment visits) should be recorded in the database. Any AR or AE considered to be serious and possibly, probably or definitely associated with treatment had to be reported to the Data Management Centre within 24 hours in accordance with good clinical practice in clinical research.
Other predictors of visual function
Data were collected at baseline to characterise study participants. The data included variables that were considered important for describing the study population and potential predictors of BCVA that might confound associations investigated in the analyses:
-
age
-
gender
-
baseline BCVA and CS
-
CNV composition, that is lesion area and proportion of the lesion graded as classic and occult CNV.
The collection of additional potential predictors of visual function was instituted after the study started to recruit (see Chapter 4, Collection of additional predictors of visual function).
Network of Ophthalmic Reading Centres in the UK
The baseline morphological characteristics of CNV lesions were defined in the protocol as potentially important covariates/confounding factors (see Other predictors of visual function) with respect to the primary outcome of visual acuity (see Outcomes). For example, classic compared with occult CNV is associated with more rapid loss of vision but greater responsiveness to treatment. 4 Changes in the morphological characteristics of CNV lesions over time, with or without treatment, were also defined as secondary outcomes (see Outcomes).
Considerable training is required to distinguish the various components of CNV lesions reliably, and, although the training put in place by the Royal College of Ophthalmologists and the research team included a session on lesion composition, this was not considered sufficient for research purposes. When designing the study, the research team was concerned that judgements about CNV composition made by the ophthalmologists treating patients might be unreliable and potentially biased. Unreliable judgements about CNV composition would have biased observed associations to the null. More importantly, given that the study was to some degree the means by which patients with predominantly classic CNV lesions could access treatment, there was a possibility that ophthalmologists might tend to overdiagnose the presence of classic CNV in order to classify patients as eligible.
The research context was also important. The TAP trials that were reporting at the time the study was being designed had taken great care to assure the quality of photographic images and the grading of these images. All photographers were accredited at the outset and reaccredited annually. All photographic images (colour fundus and FA) were submitted to a central reading centre for independent grading. 3 The VPDT cohort study research team wanted to establish the same standards to ensure that the results of the study were credible.
Therefore, NetwORC UK was established with the capacity to carry out independent grading of, potentially, > 5000 angiograms per year. Three geographically distinct centres, in Belfast, London and Liverpool, with facilities to grade stereoscopic fundus colour images and FAs were combined into a single network with a management facility in Belfast (Central Angiographic Resource Facility; CARF) to co-ordinate the administrative and technical issues. CARF managed the collection and archiving of images from designated VPDT treatment centres, performed consistency checks, certification and training of photographers, and transmitted images electronically to the three reading centres using a customised software platform. Regular training and concordance exercises were organised to ensure consistency between the reading centre grading staff and minimise grading protocol discordance.
The large volume of FAs to be graded precluded a double grading. Therefore, quality assurance was built in as an integral feature of the grading process. One in every eight FAs was randomly selected for regrading by the same reading centre, and 1 in 80 FAs was randomly selected for regrading by one of the other reading centres. All graders were masked to whether a particular grading was the original grading or a regrading.
Stereoscopic colour images and FAs were graded by the three reading centres that made up NetwORC UK using previously published definitions and protocols. 35,36 Grading involved the delineation and measurement of the area of classic and occult CNV and other lesion components contiguous to CNV, for example fibrosis and haemorrhage.
Data collection and management
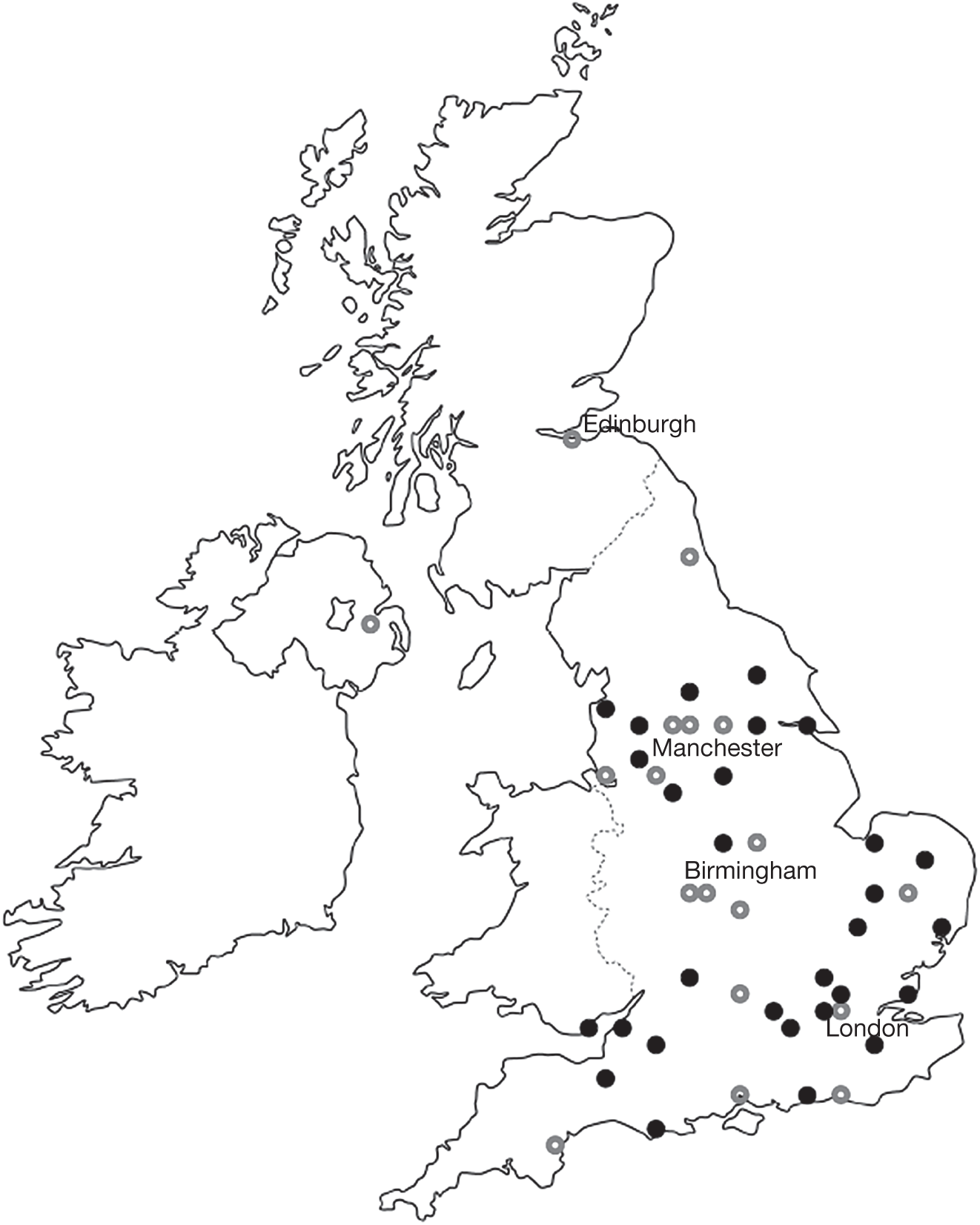
Table 3 shows the schedule of data collection at follow-up visits. A decision was taken to collect HRQoL and HSS data in a subset of centres because of the workload involved and because not all primary care trusts (PCTs) paid the full tariff covering the costs of data collection. There was a strong desire to ensure that these data were collected in a representative population. Therefore, the research team reviewed fully funded sites and their geographic disposition and recommended to the Steering Committee that 18 centres collect these data. The geographic distribution of all sites is shown in Figure 2, distinguishing between the sites which collected only the clinical data set and the sites which also collected HRQoL and HSS data.
| Activity | Screening visit | Month 0 | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | Month 21 | Month 24 | Month 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum data set | |||||||||||
| Informed consent | ✓ | ||||||||||
| Clinical history | ✓ | ||||||||||
| Refractiona | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| BDVA in the study eye | ✓ | ✓ | ✓ | ✓ | ✓* | ✓ | ✓* | ✓ | ✓* | ✓ | ✓ |
| Binocular distance visual acuity with habitual correction | ✓ | ✓ | ✓ | ✓ | ✓* | ✓ | ✓* | ✓ | ✓* | ✓ | ✓ |
| Ophthalmic exam | ✓ | ||||||||||
| Stereo colour photography and angiographyc,d | ✓ | ✓ | ✓ | ✓ | ✓* | ✓* | ✓* | ✓* | ✓* | ✓* | ✓* |
| Extended data set | |||||||||||
| Contrast sensitivity test (Pelli–Robson) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Quality of life and resource use questionnaires | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
FIGURE 2.
Map of the UK showing sites which participated in the VPDT cohort study. Sites shown as open circles were selected to collect the extended data set, including contrast sensitivity, HRQoL and resource use.
To meet its objectives, the VPDT study had to collect data from all of the hospitals designated for providing VPDT identified at the outset. These hospitals had been selected to form a network of specialist retinal practitioners. When planning the study, we estimated that these hospitals would enrol about 7000 patients each year and that each patient would make, on average, three clinic visits per year. The study was planned to run for 3 years so that, in total, data would be collected for 21,000 patients who, between them, would make up to 168,000 clinic visits.
The study aimed to collect data for all patients who were referred to a participating VPDT clinic and who gave consent for their data to be collected. This population included those who were subsequently found to be ineligible for VPDT treatment, so that an accurate estimate could be made of the proportion of all referrals who were subsequently found to be eligible for treatment. This proportion, and the way in which it changed over time, was of interest because of uncertainty about the ability of referring practitioners to diagnose CNV lesions that were eligible for treatment. With experience and training, we expected the proportion of referrals found to be eligible for treatment to increase.
At each patient visit, data had to be collected on BCVA, any VPDT treatment given, ARs resulting from VPDT treatment given at the current visit and any AE possibly resulting from VPDT treatment given at the previous visit. Demographic and referral data were also collected at the patient’s first clinic visit.
Imaging of the fundus of the eye was carried out at each clinic visit, yielding colour images and FAs. Grading of these images was co-ordinated by CARF (see Network of Ophthalmic Reading Centres in the UK) and the resulting data then had to be transmitted electronically to the London School of Hygiene and Tropical Medicine (LSHTM) and linked with all other data.
Based on a previous postmarketing surveillance study sponsored by the manufacturer of verteporfin (Novartis), the cohort study adopted a strategy of requiring ophthalmologists, optometrists, nurses and administrative staff to collect and enter data in the course of providing treatment at each PDT clinic. It was felt that this approach would maximise data quality, reduce the burden of data collection upon a small number of individuals and avoid the need for additional staff to be appointed.
The information technology (IT) strategy for data collection required a robust, networked method of electronic data entry at each site. When the strategy was planned in 2003, web-based methods of longitudinal data collection were not easily available at a reasonable cost and this, combined with the complexity of the clinical data being recorded, meant that a database installed on the local network within each hospital was the preferred solution to allow data entry at multiple points of care. Participating sites were provided with a copy of a uniform Microsoft Access (Microsoft Corporation, Redmond, WA, USA) database developed by a third party. This database was a modification of the database used for the previous postmarketing surveillance study. Where possible, the database was installed on the hospital network. A minority of the initial 30 DHs had taken part in this study and were familiar with the database.
The strategy was based on electronic data entry taking place at each site, in real time, during the course of each patient visit. Thus, records clerks and reception staff would enter initial demographic data, optometrists the BCVA measurements and ophthalmologists the data relating to disease assessment and treatment. The use of paper case record forms would be avoided, and at the end of each session data would be transmitted electronically to the data management centre at the LSHTM. As the number of data entered at each clinic grew, so it was hoped that the data sources would become administrative tools in their own right, and be used routinely to access and review patient information. We expected regular review and appraisal of data to help to improve the accuracy of data collected for the study.
Data transmitted electronically to the LSHTM were placed into a secure central database and were ‘queried’ extensively with respect to data ranges and consistency. The data queries that resulted from these checks were e-mailed as a report to the relevant centres, which were asked to make corrections to the data held in their own local database or confirm that the original data were indeed correct. Corrections were part of the next data transmission, and queries which had been successfully resolved were labelled as such in the central database and removed from the subsequent data monitoring report.
The majority of data being collected was information that we expected to be collected in the course of usual care, although investigations were required to be carried out according to the manual of operations. Additional data were required about potential predictors of visual function outcome – possible ARs and AEs that might otherwise have been considered ‘expected occurrences’ (e.g. back pain). Additional data collection (CS, HRQoL and resource use) was carried out in the subset of centres.
Although the data collection strategy strove to avoid using paper case record forms, there were two elements of the study in which these were to be used. The first was the recording of visual acuity onto paper data sheets by optometrists at each clinic. This paper record was intended to act as a validation for the BCVA data (the primary outcome), which were also being collected electronically, and the paper forms were sent directly to the LSHTM for data entry and comparison with the electronic records. Paper forms were also used to collect HRQoL and resource use by the subset of 18 participating centres.
Risk of biases
Risk of bias is described below for the main bias domains identified in the Cochrane Handbook for Systematic Reviews of Interventions41 when reviewing primary studies of the effectiveness of interventions.
Selection bias
The VPDT cohort study focused on patients who were treated with VPDT. Although we attempted to collect baseline data for all patients referred for VPDT, including those subsequently found to be ineligible, ineligible patients were clearly different from treated ones and we never planned to compare outcomes in these groups.
The potential remained for selection bias when comparing outcome between subgroups in the treated cohort, for example according to patients’ classification with respect to the inclusion criteria for the TAP trials or baseline measurements of classic and occult CNV. Therefore, we attempted to characterise a range of potential confounding factors in order to minimise the risk of confounding (see Other predictors of visual function).
Detection bias
The ophthalmologist collecting clinical data was not masked, so these data were potentially at risk of detection bias. At each visit, the ophthalmologist judged the lesion composition and the change in lesion composition since the previous visit (from a retinal examination or from an FA performed on the day of review), with knowledge of whether or not VPDT had been administered at the last visit. The study manual described a strict protocol (similar to a forced choice psychometric task) for the measurement of BCVA, the primary outcome, to try to minimise detection bias. However, the BCVA was measured by an optometrist or nurse who was not blinded to treatment status and so was also potentially at risk of detection bias for the same reason.
All analyses involving lesion characteristics (including the categorical variable ‘TAP eligibility’) used the independently graded data provided by NetwORC UK, which were not fed back to participating centres.
Performance bias
Although all clinical staff were aware of treatment status, the study was not described as a treatment comparison to health-care staff, so we judged that the risk of performance bias was low.
Attrition bias
With an elderly study population and a treatment requiring regular review, the risk of attrition bias was high. We used mixed regression models, taking account of the hierarchical nature of the data set, to include data for as many patients as possible (see Plan of analysis and Chapter 4, B: Is ‘outcome’ the same in the NHS as in randomised trials?), irrespective of compliance with the data collection schedule. However, this analytic strategy did not remove the risk of informative censoring.
Sample size considerations
Because the UK Specialised Services Commissioning Group originally intended that treatment in the UK should be conditional on recruitment and participation in the study, the study population was defined in the manual of operations simply as the number of patients recruited during the study period. Uncertainties (e.g. about the proportion of patients likely to be referred, the proportion of referred patients found to be ineligible, the proportions of eligible patients categorised as having different CNV subtypes and the precise ways in which control data were to be modelled) made it difficult to provide in advance a clear sample size justification.
For illustrative purposes, we considered a simple comparison of a continuously scaled outcome, that is BCVA, between two subgroups of patients with different types of CNV lesions. 6 The following assumptions were made for this illustration: (a) equal sample sizes for the two groups; (b) analysis adjusted for baseline BCVA; (c) standard deviation (SD) of changes in BCVA = 0.1 logMAR; (d) two-tailed significance level of 0.01; and (e) power = 0.95. Such a comparison would require only about 50 subjects in each group to detect a difference of 0.1 logMAR in the mean change between groups.
We acknowledged that other outcomes might have a larger SD and that subgroups might not have equal sample sizes. For example, comparing a continuously scaled outcome with SD = 0.3 in two subgroups with sample sizes as unequal as 4 : 1 would require a total of about 1200 (960 : 240) subjects. These simple illustrations did not take into account the added precision from the longitudinal nature of the data but also did not consider dependencies between patients treated by the same medical retina teams.
Plan of analysis
The manual of operations recognised that the data set for the VPDT cohort study would have a complex structure, with varying numbers of visits/duration of follow-up across patients up to about eight visits/3 years of follow-up. It was also recognised that patients would be ‘nested’ within groups of retinal specialists and DHs. Therefore, we planned to analyse the data set by mixed regression with multilevel modelling, an extension of conventional regression methods to take into account statistical dependency between observations that are ‘clustered’ in the data structure, for example observations within patients or patients within retinal teams.
Follow-up of patients throughout the study period allowed repeated measurements of outcomes and changes over time to be described in detail. The main outcomes (BCVA, CS, HRQoL and lesion characteristics) were continuously scaled and could be analysed by mixed regression with multilevel modelling. We also planned to use similar models to quantify associations between clinical outcomes and HRQoL. The analysis plan did not provide details of additional analyses but envisaged that outcomes might also be analysed in different ways, for example by dichotomising the change in BCVA to describe a deterioration of ≥ 15 ETDRS letters or not (a deterioration expected to occur in about 50% of participants) or using survival analysis to describe the cumulative probability of a deterioration of this degree with increasing duration of follow-up.
The analysis plan stated that, because of the complexity of the data set and the likelihood that the composition of the cohort would influence the nature of the analysis, a detailed plan of analyses would be written after carrying out preliminary descriptive analyses. The preliminary descriptive analyses would characterise baseline clinical and treatment characteristics of patients recruited to the cohort but not involve any comparative analyses. A number of baseline factors were expected to influence outcomes independently following photodynamic therapy, including BCVA at presentation, CNV composition and fellow eye comorbidities, and the analysis plan specified that analyses should take all of these factors into account.
Consideration of predictors of outcome in the analyses
As described in Other predictors of visual function, known predictors of outcome were identified and collected. The use of adjunctive treatments was also documented, although it was not known if these would influence outcome. The plan of analysis recognised that it would be important to take into account differences in these predictors between subgroups that were of interest to compare.
Estimating the effectiveness and cost-effectiveness of verteporfin photodynamic therapy
The objective of estimating effectiveness and cost-effectiveness required comparisons to be made with untreated patients. At the outset, we recognised that the lack of a concurrent control group was an important limitation of the study and a number of strategies were discussed to estimate outcomes for untreated patients. We proposed to use the following three methods and to investigate the impact of using different methods on estimates of effectiveness, cost-effectiveness and cost–utility:
-
Extrapolation from trial data Existing trials of VPDT provide estimates of effectiveness. Longitudinal data for BCVA, CS and HRQoL outcomes also exist from a previously conducted UK-based clinical trial of CNV of AMD in which the intervention was not effective at the specified outcome points. 42 Self-reported use of HSS resources in relation to AMD were also collected in the VPDT cohort study (see Appendix 2). We proposed to use these data, together with the characteristics of participants, to model indirect comparisons between treated and untreated patients.
-
Extrapolate use of health and social service resources The use of health and personal resources can be extrapolated from associations between the use of resources and visual function and other outcomes in the groups documented in the study. For example, if a relationship between the use of resources and amount of deterioration over time were observed in the study, the use of resources could be extrapolated to the level of deterioration in acuity expected without treatment, based on published data for sham/no treatment groups from previous randomised or non-randomised studies.
-
Estimate use of health and social service resources from the cohort This method assumed that resource use for an untreated control group would be similar to that for patients observed in the cohort who received VPDT but who showed no benefit (i.e. whose BCVA and Pelli–Robson Contrast Sensitivity outcomes deteriorate in a similar way to patients in the control groups in trials). This method required estimates to be adjusted for any difference in clinical characteristics between patients who showed no benefit in the cohort study and patients in the control groups of trials.
We stated that cost-effectiveness estimates would be calculated by combining the estimates of effectiveness with utilities derived from SF-6D scores and the association between use of resources and visual function.
Data management and statistical analyses
Treating centres submitted clinical and HRQoL data to an independent data management centre at the LSHTM. The imaging data were submitted to the central angiographic resource facility which managed the grading of the angiograms by NetwORC UK.
Chapter 4 Key changes to the protocol
The scope and duration of the VPDT cohort study were atypical. In particular, its longitudinal nature with visits at regular intervals at which retreatment decisions were made, and the fact that many participating sites had to establish VPDT provision, distinguished it from many earlier treatment registries or comparative outcome studies. 43–45 Its set-up also involved negotiations between many stakeholders, namely ophthalmologists at participating sites, the Royal College of Ophthalmologists, SCGs, PCTs and the Department of Health. Consequently, amendments to the protocol and the study procedures were required during the course of the study. These included the submission to the Ethics Committee for a protocol amendment, addition of study sites, revision of the data set and the methods of data collection, and changes to the image grading procedures.
Protocol amendments submitted to the Research Ethics Committee
One formal protocol amendment was approved by the Research Ethics Committee during the course of the study.
An amendment was submitted for approval in September 2005 requesting approval for five changes:
-
to obtain an anonymised minimum data set for all patients considered for VPDT
-
to include presenting binocular visual acuity as part of the data set
-
to adopt a modified patient information sheet (PIS)
-
to allow nested RCTs as a secondary objective
-
to approve one such trial (comparing combined triamcinolone and VPDT vs VPDT only), for which a detailed protocol was submitted.
The request for an amendment was rejected because of the amendments describing nested RCTs. The amendment was resubmitted without items 4 and 5 in December 2005 and was finally approved in February 2006.
The reasons for seeking these amendments were as follows. Patients receiving VPDT in the NHS had to give informed consent for their data to be included. We wanted to describe the characteristics of all patients considered for VPDT, by eligibility for treatment and by willingness to take part in the study, so that we could comment on the representativeness of the study population. We wanted to collect presenting binocular visual acuity (i.e. binocular visual acuity with a patient’s habitual spectacle correction, rather than BCVA) because we reasoned that this was likely to be the visual function parameter most strongly associated with a patient’s self-reported vision-specific HRQoL. We requested approval to adopt a much simpler PIS because patients reported to us that the PIS initially approved (which included possible side effects of having VPDT) was too complex and discouraged participation. The proposed simpler PIS distinguished procedural consent for treatment (independent of the study) from research consent to use the data collected in the course of treatment.
These amendments did not alter the overall study design, the setting, the eligible study population or the outcomes.
Changes to study procedures relating to participating sites
As the study progressed, more hospitals were designated to provide VPDT. At the start of the study, because of the novel and complex nature of the treatment, there was professional concern to restrict the provision of VPDT to designated centres in which specified doctors and other personnel had been accredited by attending appropriate training, provided by the Royal College of Ophthalmologists. As time passed, this policy became more difficult to sustain because of the inconvenience to patients in sparsely populated geographic areas. Some hospitals, together with their commissioning PCTs which wanted the hospitals to provide VPDT, were unwilling to participate in the study. Unfortunately, despite the fact that policy-makers provided a lot of the impetus to carry out the study, there was no NHS directive requiring centres to participate. It also became clear at quite an early stage that participating sites were not complying with the follow-up schedule; this required the principal investigators to adapt the planned statistical analyses.
Collection of additional predictors of visual function
The data set being collected for the study was reviewed after 12 months. Because of evidence about the influence of other factors on BCVA, the Steering Committee accepted the recommendation of the principal investigators to ask centres to collect information on additional potential confounding factors:
These factors were likely not to vary over time for a participant, and we asked centres to collect the data at the next visit for participants who had already been recruited. At the time of this change to the data set, there was only a small minority of existing participants who had already been discharged from treatment or lost to follow-up.
Smoking status was classified as current smoker, ex-smoker or never smoked. The visual status of the fellow eye (worse or better BCVA) was assigned based on BCVA data collected across the duration of the study. If the better-seeing eye varied across visits, that is both eyes had similar BCVA, the fellow eye status was classified as uncertain.
Network of Ophthalmic Reading Centres in the UK
Images from the VPDT cohort study were graded from 2004 to 2008 by NetwORC UK. Regular training and concordance exercises within the three designated reading centres ensured the reproducibility and reliability of grading outputs. During this period, improvements occurred in image acquisition systems which led to an expansion in the knowledge of the different phenotypes of nAMD. The expanded phenotypic spectrum was incorporated into the grading vocabulary, and the grading protocols were also appropriately amended.
Data collection and management
Changes in the data collection strategy
Several factors were responsible for the study recruiting substantially fewer people than anticipated. However, one was the failure of the original data collection strategy to function in the manner intended. The strategy failed for four main reasons:
-
The database supplied by the third party could not be adapted in a satisfactory way to the needs of the study.
-
One particular aspect of the inadequacy of the database was the perceived lack of security of electronic data submission. The study coincided with considerable investment in IT modernisation in the NHS and greater awareness among IT managers of national guidance about the confidentiality of patient data held in electronic form. 52
-
Some sites refused to install the local database on their local computer networks.
-
Despite the investment in modernising IT in the NHS, many participating sites did not have reliable local computer networks to support data collection at the multiple points of care involved in the management of patients being treated with VPDT. Also, some sites provided VPDT in clinics that were remote from the main ophthalmology department.
There were two main consequences of the failure of the strategy. Data collection at most sites was carried out on paper forms, using forms developed and recommended by the co-ordinating centre (Figure 3) or custom forms developed by a site. Using paper forms often represented duplication of the recording of most of the clinical data and required an unexpected time commitment locally for entry of data into the database. There was also a general reluctance to use the adapted database and difficulties in submitting data at some sites.
FIGURE 3.
Paper data collection forms recommended by the data management centre.


Concerns about the third-party database became sufficiently grave that, during the summer of 2005, the local database was completely rewritten by LSHTM staff, retaining only the table structure of the original so that data from old and new databases could be combined with relative ease. A new data transmission protocol was also developed by the LSHTM, in which data were transmitted to a secure web address and were, therefore, powerfully encrypted by Secure Socket Layer technology. This revised data transmission protocol met with the requirements of the NHS Information Authority, which the original database could not do, allowing the sites that had refused to submit data electronically to do so; it also persuaded some IT managers who had previously been reluctant to do so to install the database on the local computer network. The revised database and data transmission protocol also allowed implementation of submission of the anonymised minimum data set for patients who had treatment but from whom consent had not been obtained for participation in the cohort study (see Protocol amendments submitted to the Research Ethics Committee).
All centres were provided with the revised database. Clinics which had already collected data via the original system were able to retain the original data tables and have the revised database added as a new ‘front end’. Setting up the new databases required every site to receive a visit from a member of the LSHTM staff, during which the updated database was installed and staff were trained. The first of these site visits took place in August 2005, with the majority of upgrades taking place during the 12 months from September 2005. The database upgrade also required additional investment by the data management centre at the LSHTM, which had to recruit an extra full-time member of staff for 12 months.
The fundamentally different design of the revised clinical database was welcomed by the vast majority of clinics and overcame a lot of the reluctance to collect data. However, it could not overcome inadequacies in local computer networks or the refusal to install the database at some sites. In two cases the database could be installed only on a stand-alone personal computer with no network/internet connection. Other obstacles which made networking the database difficult included a virtual private network at one clinic, a complex arrangement of virtual servers at another, specialist optometry software which altered the configuration of dates and an unwillingness to install the software which the database required to operate. These cases highlighted that the hardware infrastructure at clinics was far from standard.
Centres generated a data report by executing a standard query on their local database and submitted the data periodically to the co-ordinating centre by the secure internet link, except for the two sites without an internet connection which submitted data by computer disk sent by registered post. The co-ordinating centre implemented data validation checks and sent back data queries to sites centres, as originally planned (see Chapter 3, Data collection and management).
Collection of health-related quality-of-life data
The protocol specified that participants should complete HRQoL questionnaires in large-print versions at baseline and every 6 months thereafter. An assisted self-administration approach was specified, described in detail in the manual of operations. Some centres were unable to follow this approach because of a lack of resources. Therefore, it was agreed that selected patients who had sufficiently good vision to read the questions and who, for example, had already completed a set of questionnaires using the assisted self-administration method could be given the questionnaires to complete at home and return by post.
Bias
Attrition was very much worse than expected in that many patients were not followed up as described in the protocol. Consequently, we were required to rethink the approach to the analysis plan (see Chapter 3, Plan of analysis). The revised analysis plan allowed us to include data for all of the observation time/documented visits in the analysis plan, irrespective of compliance with the schedule, but did not address the risk of attrition bias/informative censoring.
Sample size considerations
The sample size considerations remained the same as described in Chapter 3, Sample size considerations. However, we originally expected the study to document VPDT in about 20,000–25,000 patients over 3 years. Although the rate of recruitment increased substantially over the course of the study, it was quickly apparent that the difficulties in ensuring that DHs participated would mean that the actual sample recruited would be considerably smaller.
Detailed consideration of possible biases also led us to decide to exclude from the main analyses of BCVA patients who were within 1 year of their first treatment, unless their treatment episodes were completed (see Plan of analysis). We used this strategy because the TAP trials reported 1-year outcome and suggested that outcomes continue to improve with repeated treatment up to 1 year.
Despite these limitations, the study still had considerable precision (greater than in the TAP trials) when estimating treatment outcomes after 1 year by virtue of the continuously scaled outcomes of BCVA and HRQoL and their repeated measurement over time in the study. These attributes of the outcomes contrast with primary outcome in the TAP trials, namely the percentage of patients losing > 15 ETDRS letters at a particular time point.
Plan of analysis
The objectives of the study were reformulated prior to analytical comparisons as described in Chapter 2.
A detailed description of our approach to derivation of new variables for the analyses from the data collected and analysis plans to address each of the objectives of the study are described in the ensuing sections. These data management decisions and analysis plans were established by exploring the accumulating data descriptively and before the key analytic comparisons were carried out. Methods of fitting of final models could not be completely pre-specified, but evolved primarily to optimise the fit of the models given the limitations of the data set.
Data analysis decisions and definitions of derived analysis variables
Close of data collection for the data analyses
Centres were told in advance that recruitment to the study and documentation of study visits would stop for the data analyses on 14 September 2007. An exception to this rule was made for centres that did not submit their final data download on or after this date. For these centres, the cut-off date for calculating whether or not a patient had a completed treatment episode was the date of the last data submission.
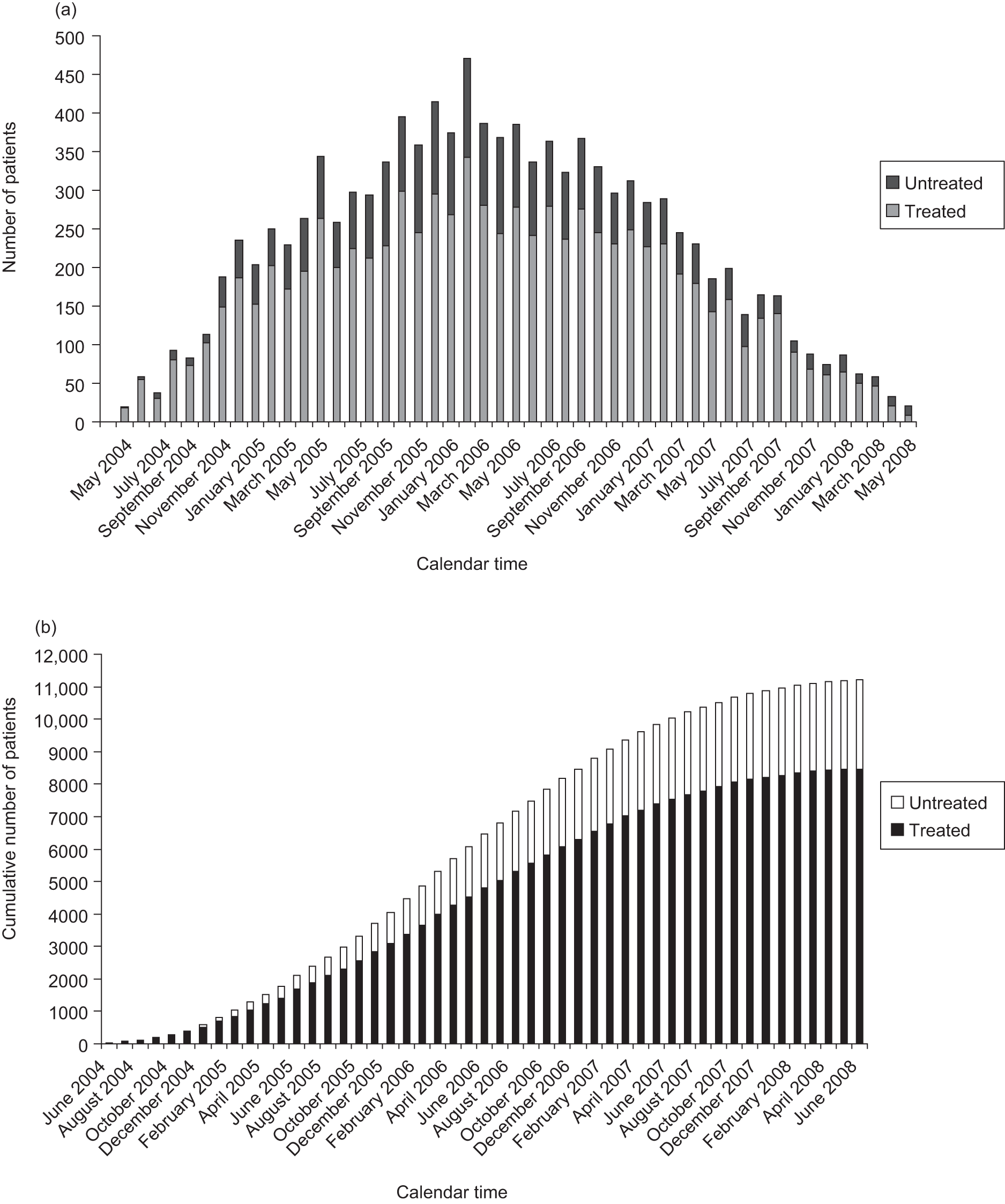
Because the NICE guidance stated that patients with predominantly classic CNV lesions with some occult CNV should be treated only in a research study, the VPDT cohort study was funded to continue to collect data up to 31 March 2008. This allowed sites to continue to address data validation queries and missing data for visits that took place up to 14 September 2007. Data submitted for visits after 14 September 2007 were excluded from the analyses in this report. Few new patients were recruited, and few additional visits took place during this period for patients who were already recruited, because new treatments, primarily drugs that inhibited vascular endothelial growth factor (VEGF), were supplanting VPDT (Figure 4).
FIGURE 4.
Recruitment to the VPDT cohort study for the UK. (a) Monthly recruitment up to the end of June 2008. (b) Cumulative monthly recruitment up to the end of June 2008.
Definition of eligible patients and eligible eyes
A patient was eligible for inclusion in the analysis if he/she had at least one eligible eye and had consented to VPDT and to submission of his/her data to the study. Although eligibility for treatment was also defined with respect to visual acuity and lesion composition (see Chapter 3, Participants), these criteria were not always adhered to (judged by BCVA data submitted by the site and by independent grading of the baseline FA).
An eye was defined as eligible for inclusion in the analysis if it had been treated with VPDT at least once and had BCVA recorded at the first treatment visit and at least one follow-up visit. If a patient had had both eyes treated with VPDT, the first treated eye was included in preference to the second because it was more likely to have longer follow-up, or to have a completed treatment episode, that is to be eligible for inclusion. If the first treated eye of a patient was ineligible, the second treated eye was included if it met the above criteria. If both eyes were treated at same time and both were eligible, one eye was chosen at random. Some treated eyes with missing BCVA at baseline or no BCVA measurements after treatment could not contribute to the analysis and were excluded. Untreated patients were excluded from all analyses, except the description of the overall cohort.
Definition of year 1 and year 2
In order to compare the number of treatments administered in the VPDT cohort study with the number of treatments administered in the TAP trials, we needed to classify visits as occurring in year 1 or year 2 of follow-up. Cut-off dates for year 1 and year 2 were defined, respectively, as ≤ 350 days and > 350 and ≤ 715 days after the date of first treatment on the assumption that scheduled visits would tend to slip over time, and were unlikely to occur at shorter time intervals than scheduled.
Classification of treatment as active or completed
For objective A (see Chapter 2), we needed to define episodes of treatment as active or completed because, by including patients still receiving active treatment, we would have underestimated the number of treatments administered. This distinction was complicated by the fact that many participants were discharged from or lost to follow-up before 1 year. Distinguishing between active and completed treatment was also important when estimating BCVA 12 months after starting treatment (a prerequisite for addressing objectives B, C and E). We wanted to include in these analyses data for patients classified as having completed their treatment before 12 months. However, patients who had not reached 12 months’ follow-up and who were still having active treatment could have experienced additional benefit from ongoing treatment up to 12 months.
Patients were classified as having completed treatment for year 1 if they satisfied one of the following sets of conditions:
-
visit with BCVA follow-up data ≥ 350 days after the first treatment
-
no visit with BCVA follow-up data ≥ 350 days after the first treatment and no visit recorded in the 150 days before 14 September 2007 (or the last date of data submission, if earlier)
-
no visit with BCVA follow-up data ≥ 350 days after the first treatment and visit in the 150 days before 14 September 2007 and explicit reason for loss to follow-up (planned discharge, treatment failure, etc.).
Other participants, that is those with no data for BCVA follow-up ≥ 350 days after the first treatment and a visit in the 150 days before 14 September 2007 and a further visit booked (or no reason for not booking a further visit, e.g. explicit reason for loss to follow-up), were classified as having ‘active treatment, with continuing follow-up’. Classification as active or completed treatment was mutually exclusive.
Definition of ‘TAP eligibility’
We decided that the independent, reading centre gradings of baseline angiograms should be the basis for the classification of patients as ‘eligible for the TAP trials’ (EFT) or ‘not eligible for the TAP trials’ (IFT). As described in Chapter 3, Network of Ophthalmic Reading Centres the UK, this decision was made because the research team was concerned that ophthalmologists’ in vivo clinical gradings might be biased in order to allow a patient to be classified as eligible for treatment (e.g. percentage of classic CNV overestimated).
This concern was substantiated by an unpublished interim subanalysis comparing ophthalmologists’ classifications with reading centre gradings for 2441 eyes which showed that, on average, the former classified a higher percentage of patients as having predominantly classic CNV lesions (Table 4). Agreement was poor (although substantially better than expected by chance: κ = 0.093, standard error 0.010, p < 0.0001). Many more eyes were classified as predominantly classic with occult by ophthalmologists than by independent grading; conversely, fewer eyes were classified as minimally classic (with or without occult) by ophthalmologists, that is as ineligible for VPDT according to the NICE guidance. 6
| CNV classification by ophthalmologist | CNV classification by reading centre | ||||
|---|---|---|---|---|---|
| CNO | PCO | MC | ONC | Total | |
| CNO | 1085 | 29 | 275 | 66 | 1455 |
| PCO | 538 | 22 | 198 | 93 | 851 |
| MC | 14 | 2 | 18 | 6 | 40 |
| ONC | 31 | 1 | 27 | 36 | 95 |
| Total | 1668 | 54 | 518 | 201 | 2441 |
At the time of first treatment, eyes were classified into mutually exclusive categories based on the proportion of classic and occult CNV (predominantly classic, minimally classic or occult no classic) as independently graded. We grouped patients into three categories based on whether or not the treated eye met the following eligibility criteria for the TAP trials:
-
BCVA > 33 and < 74 letters at first treatment AND
-
evidence on FA of at least some classic CNV (> 1% of lesion) AND
-
total CNV area ≥ 50% of the lesion AND
-
CNV under the geometric centre of foveal avascular zone. 3
Thus, each treated eye was classified as:
-
meeting these eligibility criteria (EFT)
-
not meeting the criteria (IFT)
-
not classifiable owing to the absence of gradable baseline FA (‘unclassifiable’; UNC).
A: Is verteporfin photodynamic therapy in the NHS provided as in randomised trials?
We aimed to address objective A by describing the following aspects of VPDT provision:
-
distribution of the number of treatments received in years 1 and 2 by patients classified as having completed their treatment for year 1/2
-
time to stopping treatment among patients classified as having completed their treatment
-
rate of treatment (per eye) among patients classified as having completed their treatment (treatments/year)
-
reasons for loss to follow-up before 1 year.
Item 3 was subsequently omitted because of the substantial loss to follow-up in the first 2 years after starting treatment. Item 4 was omitted because reasons for loss to follow-up were frequently not reported by participating centres.
In order to investigate numbers of treatments administered, we had to distinguish clinical follow-up visits from visits solely for the purposes of the study. In addition to the criteria for a completed treatment episode described in Classification of treatment as active or completed, treatment was defined as complete (despite continuing follow-up) if > 150 days (approximately 5 months) had elapsed between subsequent visits, except when a gap of > 150 days occurred between consecutive treatment visits. This criterion allowed for slippage in a scheduled 3-month visit, or one missed 3-month visit, and classified a 6-month follow-up visit without treatment as follow-up for the purposes of the study in accordance with the data collection schedule.
For item 1, the primary outcome was the number of applications of VPDT in years 1 (≤ 350 days) and 2 (> 350 and ≤ 715 days). Treatment frequencies were cross-tabulated with TAP eligibility and tested for significance using chi-squared statistics. We also compared treatment frequencies in year 1 with treatment frequencies reported for the TAP trials. 30 For item 2, we calculated the time until the first treatment episode was completed (see Classification of treatment as active or completed) or 350 days, whichever was later. These times were described as a Kaplan–Meier curve (see Figure 6), estimating the duration of follow-up when 50% of participants had completed their treatment.
In order to make the comparison with the TAP trials, the cohort for this analysis was restricted to patients with a CNV lesion diagnosed as nAMD and who had completed their treatment or who had completed follow-up for 1 or 2 years after the first treatment. The analysis was also limited to one eye per patient.
B: Is ‘outcome’ the same in the NHS as in randomised trials?
Objective B focused on patients who would have been EFT. We aimed to address this objective by estimating BCVA 1 year after the first treatment in patients classified as having completed their treatment for year 1. We fitted a mixed regression model to estimate the BCVA trajectory during the first year, using data up to 2 years where available. This method of analysis allowed all visit data for an eligible eye to be included irrespective of adherence to the data collection schedule. The duration of follow-up (‘time’) was a covariate in the model; interactions of other covariates with time represented non-parallel trajectories.
A single model was used to answer objectives B and C and included the following covariates: age, gender, baseline BCVA, TAP eligibility, CNV composition, smoking status and whether or not the fellow eye was the better-seeing eye. Coefficients from the model were used to estimate BCVA at 1 year for the EFT [objective (B)], IFT [objective (C)] and UNC subgroups. Inclusion of covariates was necessary because they were potential confounding factors when comparing outcome across the EFT, IFT and UNC subgroups. The influence of the covariates in such a large cohort was also intrinsically of interest; inclusion of the UNC subgroup increased the precision of the analysis with respect to estimating the influence of the covariates.
Because of substantial loss to follow-up in year 2, we again restricted our main analysis to estimating BCVA at 12 months for the cohort of patients described above for objective A (see A: Is verteporfin photodynamic therapy in the NHS provided as in randomised trials?).
C: Is ‘outcome’ the same for patients ineligible from randomised trials?
Objective C focused on patients who would have been IFT. A single model was used to address objectives B and C (see B: Is ‘outcome’ the same in the NHS as in randomised trials?).
D: Is verteporfin photodynamic therapy safe when provided in the NHS?
Adverse reactions and AEs were not classified as required for good clinical practice, although such events were promptly notified to the Data Management Centre at the LSHTM in accordance with good clinical practice. Attribution of ocular AEs to VPDT is difficult because such events may occur as part of the natural history of nAMD. An AR was defined as an ocular or systemic reaction at the time of treatment which was recorded on the same day as the treatment with other clinical data for that visit. An AE was defined as any other ocular or systemic AE reported at the next visit after a treatment or retreatment visit. The association of an AE with the previous treatment visit was coded during data management and, therefore, was associated with the corresponding treatment visit, not the visit on which it was reported.
The probability of a treatment visit giving rise to an AR or AE by site and visit was estimated using a logistic regression model, fitting participating site as a random effect. The distributions of centre-specific probabilities were examined carefully because of concern about the extent to which sites had adhered to the instructions for collecting data about ARs and AEs. To contextualise the overall probability of an AR or AE, we also described the probabilities for a site which had the largest number of treated patients and which we believed had collected such data better than average. We also investigated whether any site had a site-specific upper 95% confidence limit below the lower 95% confidence limit for the entire cohort; where this was the case, a sensitivity analysis was rerun omitting the site.
E: How effective and cost-effective is verteporfin photodynamic therapy?
Different approaches to estimate effectiveness were proposed in the manual of operations (see Chapter 3, Estimating the effectiveness and cost-effectiveness of verteporfin photodynamic therapy). For reasons outside our control, we were able to use only the second of these methods, that is to investigate associations between the use of resources and visual function and other outcomes in the study. This method is described in more detail in How effective is verteporfin photodynamic therapy?, below. This method also underpinned the second element of objective E, that is estimation of the cost-effectiveness of VPDT.
The first method depended on obtaining individual patient data from other researchers, including the TAP triallists. We were able to obtain some data for studies which had academic or public sponsors, but were unable to obtain the data for the key RCTs of VPDT (TAP and VIP trials3,5), even though the manufacturer of verteporfin (Novartis) was represented on the Steering Committee. Without these data, we judged that the first method was not feasible.
The third method depended on being able to characterise an untreated control group in the cohort of patients recruited for the study. However, it quickly became apparent that we were not capturing adequate data for patients who were not treated (either by choice or because of ineligibility) and that untreated patients represented in the database were not similar across sites because of the varied arrangements in place for triaging patients before referral to VPDT clinics.
How effective is verteporfin photodynamic therapy?
The estimates of BCVA outcome at 1 year were used to derive indirect estimates of the effectiveness of VPDT by comparing the estimates with the reported BCVA outcomes at 1 year in the treatment and sham treatment groups of the TAP trials.
The strategy for estimating the HRQoL benefit from VPDT was as follows:
-
to estimate the extent to which HRQoL changes per unit change in BCVA
-
to ‘translate’ the observed difference in BCVA in the TAP trials into HRQoL, based on the association quantified by step 1
-
to ‘translate’ the observed change in BCVA in the VPDT cohort study over time minus the change in BCVA observed in the sham treatment arms of the TAP trials into HRQoL, based on the association quantified by step 1.
In addition to estimating the overall associations between BCVA and HRQoL, we also sought to test two pre-specified subhypotheses. One concerned the shape of the association. We hypothesised that the associations would be sigmoid, with a relatively shallow gradient at the extremes of the visual function continuum. We reasoned that HRQoL would vary a relatively small amount (shallow gradient) among people above and below visual function thresholds for being easily able, and completely unable, to carry out tasks that depend on vision; conversely, we reasoned that HRQoL would drop sharply over the range of visual function when people’s ability to do such tasks also deteriorated markedly. The second subhypothesis concerned adaptation over time to poorer visual function. We hypothesised that the gradients of the relationships would decrease with increasing time from first treatment, as patients adapted to their residual vision.
We used BCVA and CS measurements from the better-seeing eye and HRQoL data for corresponding visits. Visits were classified using the following time intervals: 0 months (first treatment date), 3 months (> 77 to ≤ 168 days), 6 months (> 168 to ≤ 259 days), 9 months (> 259 to ≤ 350 days), 12 months (> 350 to ≤ 442 days), 15 months (> 441 to ≤ 533 days), 18 months (> 533 to ≤ 624 days), 21 months (> 624 to ≤ 715 days) or 24 months (> 715 to ≤ 807 days) after the date of first treatment. Intervals were not symmetrical around the 3-monthly schedule because follow-up visits tended to shift towards longer rather than shorter intervals.
Mixed regression models were used to allow all available visits to contribute to the analysis, taking into account multiple visits by the same patients and visits without HRQoL data. To allow for the correlation of the data, an unstructured covariance matrix was used where possible, otherwise random intercepts and slopes were fitted.
To address our second objective, that relationships are sigmoid, we fitted a range of putative models; these included linear, quadratic, cubic and spline functions. We addressed our third objective, that gradients decrease with time since first treatment, by modelling time in 3-month intervals (see above). The analyses investigated both time interval and the interaction between BCVA and time, allowing the gradient of the relationship to vary with time.
We also fitted a range of covariates (including age, gender, participating centre, smoking status and whether or not the fellow eye was the better-seeing eye). Covariates did not materially alter the shape or gradient of the relationships between visual function and HRQoL, and their effects are not described.
We judged that the cause of CNV was very unlikely to influence the association between BCVA and HRQoL. There was also no reason why the association would be influenced by whether or not a patient had completed treatment. By virtue of modelling BCVA in the better-seeing eye, the issue of treatment in both eyes did not arise. Therefore, the cohort for this analysis included all patient visits for which visual function data (BCVA or CS) and HRQoL data (NEIVFQ or SF-36) were reported.
How cost-effective is verteporfin photodynamic therapy?
The CEA element of objective E consisted of three parts: (a) estimation of the costs of delivering VPDT in routine clinical practice; (b) development of a regression model to quantify changes in HSS for a given change in visual function (i.e. BCVA); and (c) assessment of the cost-effectiveness of VPDT versus best supportive care (BSC) using the findings from (a) and (b).
Overview of the cost-effectiveness analysis
The VPDT cohort study was designed to assess the costs and HRQoL of VPDT and to report the cost-effectiveness of VPDT versus BSC. The CEA was undertaken in accordance with current methodological standards. It took a health and personal social services perspective and so included all relevant costs to HSS. 28 The main assumptions underlying this CEA are reported in Box 2.
The target population was patients treated with VPDT in routine NHS practice
VPDT treatment was given to the better-seeing eye
nAMD costs and HRQoL only varied according to BCVA which, in turn, depends on whether treatment is or is not given and changes over time as described in the TAP trials
Treatment frequency was as observed in VPDT study
For patients having both eyes treated, the treatment costs were assumed to be the twice the costs for patients having a single eye treated
To calculate QALYs from HRQoL, it was assumed that there was no mortality
Costs and QALYs in year 2 were discounted at 3.5%
The study used the BCVA measures reported in the TAP trial to assess the cost-effectiveness of VPDT versus BSC. 3 The effect of VPDT on BCVA was taken from the subgroup of eyes with predominantly classic lesions in the TAP trials; the effectiveness of VPDT was largest in this subgroup of eyes (mean difference in BCVA letters lost from baseline of 11 letters at 2 years), which was the basis for the previous NICE recommendations. 3,6,30 This report combines these data from the TAP trials with estimates from the VPDT cohort study of (a) the relationship between BCVA and HRQoL (see How effective is verteporfin photodynamic therapy?), (b) treatment frequency in routine practice (see A: Is verteporfin photodynamic therapy in the NHS provided as in randomised trials?) and (c) the relationship between BCVA and HSS cost (using the same methods as when estimating the relationship between BCVA and HRQoL, described in How effective is verteporfin photodynamic therapy?). This report also estimates the costs of VPDT in routine practice and the cost-effectiveness of PDT versus BSC over 2 years.
Costs of verteporfin photodynamic therapy and best supportive care
The VPDT cohort study recorded the number of outpatient visits, tests performed (colour photography or FA) and VPDT treatments administered. For each patient, the treatment costs were measured from the date on which the first eligible eye was treated for up to 2 years. Costs were categorised as falling in year 1 or year 2 (Definition of year 1 and year 2). Because the probability of receiving one FA for each visit (whether a treatment or follow-up visit) exceeded 0.95 for > 90% of study centres, it was assumed that there was one FA for each visit.
We assumed that one vial of verteporfin was used per treated eye as stipulated in the licence. Verteporfin costs were taken from the British National Formulary (£860 per treatment) and excluded value added tax. 53 The numbers of treatment and follow-up visits were combined with national unit costs (£113 and £67 for treatment and follow-up visits respectively). 54
The costs of BSC were estimated by assuming plausible costs for follow-up without VPDT. In the base-case analysis, it was assumed that there would be on average 1 and 1.5 low-vision assessments scheduled in years 1 and 2 respectively.
Health and social sciences use and costs related to vision loss
The cost of HSS for patients affected by nAMD was estimated from the HSS use questionnaires which were administered every 6 months to patients attending a subset of 18 of the participating sites. The questionnaires elicited the use of HSS relating to the patient’s eye condition in the preceding 3 months. This included unscheduled low-vision appointments, use of antidepressants, visits to the general practitioner (GP), visits from social services (mainly home carers) and time in nursing homes, residential care or sheltered housing. The patient-reported HSS use at each time point was combined with national unit costs to give an estimate of the costs in the 3 months preceding the visit that were ‘attributable’ to the patients’ vision. 54 All costs (inflated to 2007 prices) were summed across years 1 and 2 to give total costs per patient and were reported in UK pound sterling. 55
Estimating incremental quality-adjusted life-years, and incremental costs for verteporfin photodynamic therapy versus best supportive care
The incremental costs of VPDT versus BSC comprised the mean differences in both intervention and HSS costs between the VPDT and BSC groups. The association of BCVA with HSS cost was estimated using regression models, following the same strategy used to measure the association between BCVA and HRQoL. Estimates for the CEA were taken from a model which included just BCVA (or CS) as an independent variable because other covariates did not improve model fit.
Because a substantial proportion of the sample of patients incurred zero HSS costs, we used a ‘two-part’ model. 56 The first part modelled all observations in a logistic regression, with use (or not) of any service in the 3 months preceding the visit as the dependent variable and BCVA as the independent variable. The second part of the model included only those observations for which HSS was used and fitted a linear regression with the HSS cost per user as the dependent variable and BCVA as an independent variable. The resultant conditional probabilities of HSS use and HSS costs per user were combined to predict overall HSS costs with varying BCVA.
The CEA then combined the 3-monthly BCVA data from the TAP trial with the association between BCVA and HSS cost to report the incremental HSS costs for VPDT versus BSC. These costs were added to the incremental intervention costs of VPDT and BSC to give the overall incremental costs of VPDT at 1 and 2 years.
This association between BCVA and SF-6D was combined with the differential decline in BCVA from baseline for the VPDT and placebo groups in TAP to derive differences in HRQoL between VPDT and BSC at 3-monthly intervals. The incremental QALYs for VPDT versus BSC were calculated as the average HRQoL difference for each 3-monthly time point multiplied by 0.25 years and summed over 1 or 2 years. The CEA reported incremental (mean VPDT – mean BSC) costs, QALYs and costs per QALY.
Sensitivity analyses
A probabilistic sensitivity analysis was undertaken to recognise the sampling uncertainty surrounding the key parameters (BCVA, association between BCVA and HRQoL, intervention costs and association between BCVA and HSS costs), and to report the probability that VPDT is cost-effective compared with BSC at different levels of willingness to pay for a QALY gain (e.g. £20,000 per QALY). 28,57
Further sensitivity analyses assessed the robustness of the results to the main methodological assumptions and data sources used in the base case. Five alternative scenarios were considered:
-
The treatment frequency was taken from TAP trials rather than the VPDT cohort study.
-
BCVA data for the VPDT group were taken from the cohort study (post- vs pre-VPDT) rather than TAP.
-
The relationships of cost and HRQoL with CS rather than BCVA were used.
-
To assess whether or not the results were sensitive to the costs of BSC, which may be higher when financed under private health insurance, the BSC costs were assumed to be 10-fold higher than in the base case.
-
Cost-effectiveness was estimated over 5 rather than 2 years, assuming that the difference in BCVA between the treatment groups observed at 2 years applied for years 2–5.
As when quantifying the association between BCVA and HRQoL, the analysis to quantify the relationship between BCVA and resource use included all patient visits for which visual function data (BCVA or CS) and resource use data (NEIVFQ or SF-36) were reported. The treatment frequency data were restricted to the group of patients with predominantly classic lesions who had completed treatment or who had follow-up data to 1 year.
Chapter 5 Results (1) – study cohort
Participating centres
The number of participating sites increased over time. We originally planned for 30 but, by the time that data collection started, 48 DHs had been identified by SCGs. A few more DHs were identified as the study progressed; this number is imprecise because, if DHs refused to register for the study, we sometimes did not receive definitive information about whether or not a prospective DH actually started to provide VPDT. Additional DHs joined the study up until May 2007. A total of 49 sites registered to take part in the VPDT service but only 47 contributed data to the study (see Figure 2). The first participating site gained the necessary approval to enrol patients on 21 May 2004. Approval for other sites progressed steadily throughout 2004–5. Twenty-one sites were submitting data by May 2005, 38 by May 2006 and 45 by May 2007. Some sites submitted data early during the course of the study but did not continue to do so throughout.
Study population
The first patient was enrolled on 3 June 2004. The rate of recruitment increased to a peak in April 2006 and then declined steadily (see Figure 4). The numbers of patients recruited by each site are shown in Table 5. The numbers ranged from 5 to 593 for all patients treated at any time, but only from 3 to 351 for patients with CNV caused by nAMD, baseline BCVA and at least one follow-up BCVA assessment and not under active treatment or > 1 year since the first treatment (i.e. patients included in the main analyses; Figure 5 and Chapter 4, Definition of ‘TAP eligibility’ and B: Is ‘outcome’ the same in the NHS as in randomised trials?).
| Centre | All patients treated at any time | Patients treated at any time with BCVA | Patients with BCVA and not under active treatmenta | Patients with AMD, BCVA and not under active treatmenta | ||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | % | Number of patients | % | Number of patients | % | Number of patients | % | |
| 1 | 52 | 0.67 | 42 | 0.68 | 21 | 0.46 | 19 | 0.47 |
| 2 | 106 | 1.37 | 90 | 1.45 | 56 | 1.23 | 51 | 1.26 |
| 3 | 39 | 0.50 | 35 | 0.56 | 22 | 0.48 | 21 | 0.52 |
| 4 | 5 | 0.06 | 3 | 0.05 | 3 | 0.07 | 3 | 0.07 |
| 5 | 165 | 2.13 | 147 | 2.36 | 102 | 2.23 | 92 | 2.28 |
| 6 | 186 | 2.40 | 155 | 2.49 | 128 | 2.80 | 94 | 2.33 |
| 7 | 84 | 1.08 | 61 | 0.98 | 16 | 0.35 | 14 | 0.35 |
| 8 | 566 | 7.31 | 457 | 7.35 | 355 | 7.77 | 307 | 7.59 |
| 9 | 134 | 1.73 | 115 | 1.85 | 90 | 1.97 | 84 | 2.08 |
| 10 | 125 | 1.61 | 114 | 1.83 | 93 | 2.04 | 89 | 2.20 |
| 11 | 60 | 0.77 | 44 | 0.71 | 31 | 0.68 | 25 | 0.62 |
| 12 | 55 | 0.71 | 39 | 0.63 | 39 | 0.85 | 37 | 0.92 |
| 13 | 117 | 1.51 | 95 | 1.53 | 62 | 1.36 | 58 | 1.43 |
| 14 | 49 | 0.63 | 24 | 0.39 | 10 | 0.22 | 8 | 0.20 |
| 15 | 255 | 3.29 | 224 | 3.60 | 161 | 3.53 | 153 | 3.78 |
| 16 | 114 | 1.47 | 1 | 0.02 | – | – | – | – |
| 17 | 281 | 3.63 | 215 | 3.46 | 130 | 2.85 | 119 | 2.94 |
| 18 | 23 | 0.30 | 19 | 0.31 | 14 | 0.31 | 14 | 0.35 |
| 19 | 46 | 0.59 | 12 | 0.19 | 7 | 0.15 | 7 | 0.17 |
| 20 | 292 | 3.77 | 194 | 3.12 | 148 | 3.24 | 129 | 3.19 |
| 21 | 101 | 1.30 | 90 | 1.45 | 55 | 1.20 | 48 | 1.19 |
| 22 | 42 | 0.54 | 28 | 0.45 | 5 | 0.11 | 4 | 0.10 |
| 23 | 133 | 1.72 | 117 | 1.88 | 111 | 2.43 | 105 | 2.60 |
| 24 | 113 | 1.46 | 92 | 1.48 | 65 | 1.42 | 64 | 1.58 |
| 25 | 110 | 1.42 | 80 | 1.29 | 61 | 1.34 | 57 | 1.41 |
| 26 | 258 | 3.33 | 227 | 3.65 | 179 | 3.92 | 151 | 3.73 |
| 27 | 586 | 7.56 | 513 | 8.25 | 399 | 8.74 | 344 | 8.51 |
| 28 | 77 | 0.99 | 49 | 0.79 | 48 | 1.05 | 43 | 1.06 |
| 29 | 263 | 3.39 | 221 | 3.55 | 120 | 2.63 | 105 | 2.60 |
| 30 | 323 | 4.17 | 263 | 4.23 | 232 | 5.08 | 202 | 5.00 |
| 31 | 593 | 7.65 | 536 | 8.62 | 393 | 8.61 | 351 | 8.68 |
| 32 | 50 | 0.65 | 28 | 0.45 | 27 | 0.59 | 23 | 0.57 |
| 33 | 320 | 4.13 | 282 | 4.53 | 195 | 4.27 | 180 | 4.45 |
| 34 | 76 | 0.98 | 38 | 0.61 | 35 | 0.77 | 33 | 0.82 |
| 35 | 168 | 2.17 | 141 | 2.27 | 98 | 2.15 | 76 | 1.88 |
| 36 | 48 | 0.62 | 30 | 0.48 | 23 | 0.50 | 23 | 0.57 |
| 37 | 360 | 4.65 | 329 | 5.29 | 247 | 5.41 | 225 | 5.57 |
| 38 | 102 | 1.32 | 48 | 0.77 | 34 | 0.74 | 31 | 0.77 |
| 39 | 192 | 2.48 | 123 | 1.98 | 100 | 2.19 | 88 | 2.18 |
| 40 | 88 | 1.14 | 83 | 1.33 | 63 | 1.38 | 55 | 1.36 |
| 41 | 147 | 1.90 | 128 | 2.06 | 104 | 2.28 | 95 | 2.35 |
| 42 | 14 | 0.18 | 7 | 0.11 | – | – | – | – |
| 43 | 209 | 2.70 | 184 | 2.96 | 156 | 3.42 | 127 | 3.14 |
| 44 | 126 | 1.63 | 113 | 1.82 | 82 | 1.8 | 78 | 1.93 |
| 45 | 350 | 4.52 | 297 | 4.77 | 187 | 4.1 | 157 | 3.88 |
| 46 | 60 | 0.77 | 24 | 0.39 | 7 | 0.15 | 7 | 0.17 |
| 47 | 85 | 1.10 | 64 | 1.03 | 52 | 1.14 | 47 | 1.16 |
| Total | 7748 | 100 | 6221 | 100 | 4566 | 100 | 4043 | 100 |
FIGURE 5.
Consolidated Standards of Reporting Trials style diagram showing the patients and eyes treated in the VPDT cohort study.
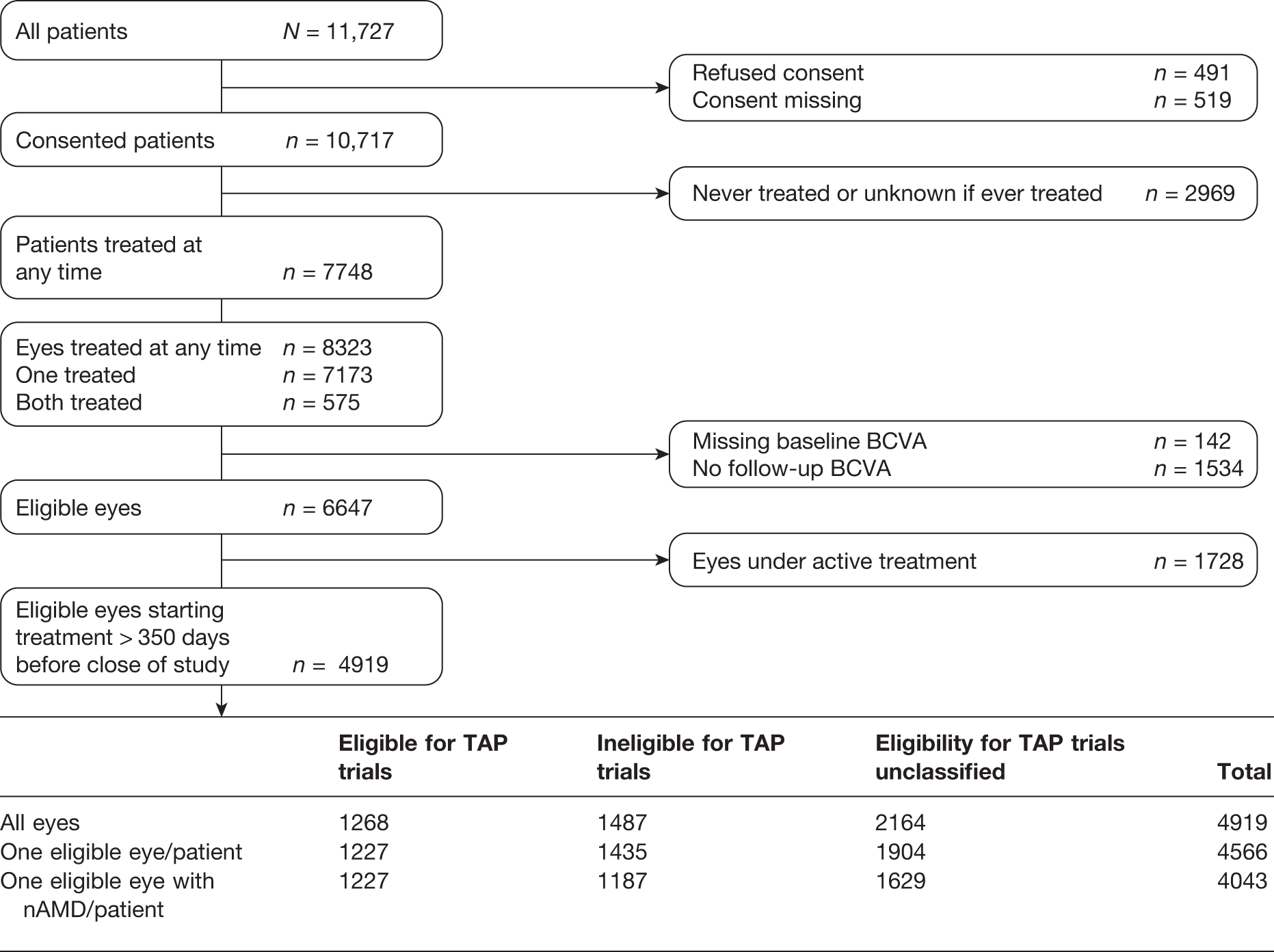
The flow of recruited patients in the study with respect to consent, treatment, inclusion of one or both eyes and whether or not an eye was considered under active treatment when the database was locked is shown in Figure 5. Between June 2004 and September 2007, data on 11,727 patients were submitted. A total of 7748 patients were recorded as having been treated at any time; 575 patients were treated and contributed data for both eyes, giving a total of 8323 eyes. Data were submitted for 31,640 clinic visits in these 7748 patients. The referral mechanisms adopted by sites varied considerably; for example, some had systems for initial triaging of patients with respect to criteria determining eligibility for treatment. Therefore, the data on patients found to be ineligible when attending VPDT clinics could not be interpreted and were not analysed further.
Missing BCVA for 1527 patients resulted in the exclusion of 1676 eyes (142 missing at baseline and 1534 at follow-up). The characteristics of the remaining 6221 patients are shown in Table 6. Their median age was 78 years (interquartile range from 72 to 83 years). The majority were female (3620/6202, 58.4%). The majority (55.4%) were current (832/5282, 15.8%) or ex-smokers (2092/5282, 39.6%).
| Patient characteristic | Number of patients first treated > 350 days before the end of the study | Number of patients first treated ≤ 350 days before the end of the study | Total |
|---|---|---|---|
| Total eligible patients (see Figure 3) | 4566 | 1655 | 6221 |
| Gender | |||
| Male | 1893 (41.5%) | 689 (41.6%) | 2582 (41.5%) |
| Female | 2658 (58.2%) | 962 (58.1%) | 3620 (58.2%) |
| Missing | 15 (0.3%) | 4 (0.2%) | 19 (0.3%) |
| Age (years) | |||
| Median | 78 | 79 | 78 |
| Interquartile range | 72–83 | 73–84 | 72–83 |
| Range | 8–102 | 14–102 | 8–102 |
| Age group | |||
| < 50 years | 203 (4.6%) | 61 (3.7%) | 264 (4.2%) |
| ≥ 50 to < 65 years | 345 (7.6%) | 120 (7.3%) | 465 (7.5%) |
| ≥ 65 to < 75 years | 993 (21.7%) | 319 (19.3%) | 1312 (21.1%) |
| ≥ 75 to < 85 years | 2180 (47.7%) | 805 (48.6%) | 2985 (48.0%) |
| ≥ 85 years | 845 (18.5%) | 350 (21.1%) | 1195 (19.2%) |
| Smoking history | |||
| Unknown | 775 (17.0%) | 164 (9.9%) | 939 (15.1%) |
| Current smoker | 617 (13.5%) | 215 (13.0%) | 832 (13.4%) |
| Ex-smoker | 1543 (33.8%) | 549 (33.2%) | 2092 (33.6%) |
| Never smoked | 1631 (35.7%) | 727 (43.9%) | 2358 (37.9%) |
Of the 6221 patients, 426 (6.8%) were treated and contributed data for both eyes, giving a total of 6647 eyes treated at least once with valid BCVA data at baseline and at least one follow-up assessment; 1728 eyes in 1655 patients had been first treated ≤ 350 days (study definition equivalent to < 1 year) before the database was locked and the study was closed, leaving 4919 eyes in 4566 patients. Patients with CNV caused by nAMD made up 88.5% of these. The characteristics of the 6647 eyes are summarised in Table 7. Very few had had prior laser photocoagulation. The mean BCVA at baseline was 50.4 letters and the mean CS was 22.7 letters. The characteristics of the treated CNV lesions that were independently graded are summarised in Table 8. The majority of lesions had a greatest linear dimension of fewer than three disc areas (1892/2957, 64.0%), were subfoveal or juxtafoveal (2459/2756, 89.2%), were composed of > 50% classic CNV (1943/2777, 70.0%) and were without occult CNV (2243/2773, 80.9%).
| Visual function measure | Number of eyes first treated > 350 days before the end of the study | Number of eyes ≤ 350 days before the end of the study | Total |
|---|---|---|---|
| Total eligible eyes (see Figure 3) | 4919 | 1728 | 6647 |
| Visual acuity at baseline (first treatment visit) | |||
| Mean BCVA (SD) | 50.4 (16.0) | 50.6 (15.5) | 50.4 (15.9) |
| Median (interquartile range) | 51 (39–62) | 51 (40–62) | 51 (40–62) |
| Number of ETDRS letters read | |||
| > 73 | 356 (7.2%) | 122 (7.1%) | 478 |
| > 53 to ≤ 73 | 1838 (37.4%) | 640 (37.0%) | 2478 |
| > 33 to ≤ 53 | 2089 (42.5%) | 767 (44.4%) | 2856 |
| ≤ 33 | 626 (12.7%) | 195 (11.3%) | 821 |
| CF, HM, PL, NPL | 10 (0.2%) | 2 (0.1%) | 12 |
| Contrast sensitivity | |||
| Mean contrast sensitivity (SD) | 22.8 (7.6) | 22.4 (7.6) | 22.7 (7.6) |
| Median (interquartile range) | 24 (18–28) | 24 (18–28) | 24 (18–48) |
| Number of CS letters read | |||
| 0 | 45 (0.9%) | 26 (1.5%) | 71 |
| > 0 and ≤ 18 | 684 (13.9%) | 224 (13.0%) | 908 |
| > 18 and ≤ 24 | 828 (16.8%) | 300 (7.2%) | 1128 |
| > 24 and ≤ 28 | 580 (11.8%) | 188 (17.4%) | 768 |
| > 28 | 679 (7.2%) | 210 (12.1%) | 889 |
| Missing | 2103 (13.8%) | 780 (45.1%) | 2883 |
| Evidence of prior laser photocoagulation | 12 | 3 | 15 |
| Lesion characteristic | Number of eyes first treated > 350 days before the end of the study | Number of eyes first treated ≤ 350 days before the end of the study | Total |
|---|---|---|---|
| Total eligible eyes (see Figure 3) | 4919 | 1728 | 6647 |
| Eligible eyes with an independently graded baseline angiogram | 3182 (64.7%) | 943 (54.6%) | 4125 (62.1%) |
| Lesion area, disc areas | |||
| ≤ 3 | 2225 (69.9%) | 669 (70.9%) | 2894 (70.2%) |
| > 3 to ≤ 6 | 400 (12.6%) | 140 (14.8%) | 540 (13.1%) |
| > 6 to ≤ 9 | 94 (3.0%) | 33 (3.5%) | 127 (3.1%) |
| > 9 | 54 (1.7%) | 18 (1.9%) | 72 (1.7%) |
| Missing or could not be graded | 409 (12.9%) | 83 (8.8%) | 492 (11.9%) |
| Greatest linear dimension, disc area | |||
| ≤ 3 | 1892 (59.5%) | 549 (58.2%) | 2441 (59.2%) |
| > 3 to ≤ 6 | 966 (30.4%) | 310 (32.9%) | 1276 (30.9%) |
| > 6 to ≤ 9 | 90 (2.8%) | 26 (2.8%) | 116 (2.8%) |
| > 9 | 9 (0.3%) | 4 (0.4%) | 13 (0.3%) |
| Missing or could not be graded | 225 (7.1%) | 54 (5.7%) | 279 (6.8%) |
| Lesion area composed of CNV | |||
| 0% | 1 (0.0%) | 0 (0.0%) | 1 (0.0%) |
| > 0% and < 50% | 355 (11.2%) | 121 (12.8%) | 476 (11.5%) |
| ≥ 50% and < 100% | 1305 (41.0%) | 377 (40.0%) | 1682 (40.8%) |
| 100% | 1031 (32.4%) | 352 (37.3%) | 1383 (33.5%) |
| Missing or could not be graded | 490 (15.4%) | 93 (9.9%) | 583 (14.1%) |
| CNV location | |||
| Extrafoveal | 297 (9.3%) | 99 (10.5%) | 396 (9.6%) |
| Juxtafoveal | 408 (12.8%) | 94 (10.0%) | 502 (12.2%) |
| Subfoveal | 2051 (64.5%) | 660 (70.0%) | 2711 (65.7%) |
| Missing or could not be graded | 426 (13.4%) | 90 (9.5%) | 516 (12.5%) |
| Lesion area composed of classic CNV | |||
| ≥ 50% to < 100% | 1943 (61.1%) | 585 (62.0%) | 2528 (61.3%) |
| > 0% to < 50% | 588 (18.5%) | 200 (21.2%) | 788 (19.1%) |
| 0% | 246 (7.7%) | 75 (8.0%) | 321 (7.8%) |
| Missing or could not be graded | 405 (12.7%) | 83 (8.8%) | 488 (11.8%) |
| Evidence of occult CNV | |||
| ≥ 50% | 402 (12.6%) | 142 (15.1%) | 544 (13.2%) |
| > 0 and < 50% | 128 (4.0%) | 22 (2.3%) | 150 (3.6%) |
| 0% | 2243 (70.5%) | 696 (73.8%) | 2939 (71.2%) |
| Missing or could not be graded | 409 (12.9%) | 83 (8.8%) | 492 (11.9%) |
| Lesion included blood | |||
| Yes | 1285 (40.4%) | 367 (38.9%) | 1652 (40.0%) |
| No | 1647 (51.8%) | 522 (55.4%) | 2169 (52.6%) |
| Questionable | 34 (1.1%) | 7 (0.7%) | 41 (1.0%) |
| Missing or could not be graded | 216 (6.8%) | 47 (5.0%) | 263 (6.4%) |
| Lesion with blocked hypofluorescence not caused by visible blood | |||
| Yes | 655 (20.6%) | 154 (16.3%) | 809 (19.6%) |
| No | 2298 (72.2%) | 740 (78.5%) | 3038 (73.6%) |
| Questionable | 13 (0.4%) | 2 (0.2%) | 15 (0.4%) |
| Missing or could not be graded | 216 (6.8%) | 47 (5.0%) | 263 (6.4%) |
| Serious pigment epithelial detachment | |||
| Yes | 117 (3.8%) | 44 (4.7%) | 161 (3.9%) |
| No | 2842 (89.3%) | 852 (90.3%) | 3694 (89.6%) |
| Questionable | 7 (0.2%) | 0 (0.0%) | 7 (0.2%) |
| Missing or could not be graded | 216 (6.8%) | 47 (5.0%) | 263 (6.4%) |
Subgroup of patients with choroidal neovascularisation caused by neovascular (wet) age-related macular degeneration
As described previously (see Classification of treatment as active or completed), patients still under active treatment when the study closed were not included in the main analyses because of the risk of bias. It was challenging to include second eyes in the main analyses because most second eyes developed nAMD and received a first treatment at varying times after the first. Thus, a second eye was often still under active treatment or it was ≤ 350 days since the first eye had completed treatment or underwent a first treatment > 350 days before the study was closed. Restricting the analysis to one eye per patient excluded a further 8% of treated eyes. Given the marketing authorisation for verteporfin, CNV lesions caused by nAMD were of particular interest; after excluding treated eyes with non-AMD aetiology, a total of 4043 eyes remained.
The baseline characteristics of this subset of patients and eyes are shown in Table 9, which breaks down the data according to whether patients/eyes were classified as EFT, IFT or UNC. In the EFT group, predominantly classic CNV was present in 86.7% (1064/1227) and minimally classic in 13.3% (163/1227). In the IFT group, predominantly classic CNV was present in 52.9% (628/1187) and minimally classic in 47.1% (559/187). The mean baseline logMAR BCVA was 50 letters (20/100) in the treated eye, which was very similar to study eyes of patients randomised in the TAP trials (53 letters). 3
| Baseline characteristics | EFTb (n = 1227) | IFT (n = 1187) | UNC (n = 1629) | Total (n = 4043) |
|---|---|---|---|---|
| Mean age, years (SD) | 78.8 (7.17) | 78.3 (8.31) | 78.7 (8.66) | 78.6 (8.13) |
| Male, n (%) | 513 (41.9%) | 612 (42.8%) | 768 (40.5%) | 1893 (41.6) |
| Smoking status, n (%) | ||||
| Current smoker | 170 (16.4%) | 158 (16.5) | 211 (15.5) | 539 (16.1) |
| Ex-smoker | 437 (42.1%) | 415 (43.5) | 574 (42.1) | 1426 (42.5) |
| Never smoked | 432 (41.6%) | 382 (40.0) | 579 (42.5) | 1393 (41.5) |
| BCVA, letters (SD) | 50.6 (10.4) | 50.2 (19.0) | 48.7 (15.7) | 49.7 (15.5) |
| BCVA group, n (%) | ||||
| > 73 ETDRS letters | – | 144 (12.1) | 79 (4.9) | 223 (5.5) |
| 73–34 ETDRS letters | 1227 (100%) | 766 (64.5) | 1311 (80.5) | 3304 (81.7) |
| < 34 ETDRS letters | – | 277 (23.3) | 239 (14.7) | 516 (12.0) |
| CS, letters (SD)a | 22.4 (6.89) | 23.0 (7.67) | 22.1 (7.59) | 22.5 (7.38) |
| Lesion area, median mm2 (interquartile range) | ||||
| All lesions | n = 1215 | n = 1158 | n = 25 | n = 2398 |
| 3.81 (1.8–6.8) | 2.58 (0.9–6.4) | 4.20 (1.8–5.0) | 3.28 (1.4–6.6) | |
| Predominantly classic | n = 1058 | n = 621 | ||
| 3.46 (1.7–6.2) | 1.80 (0.6–4.7) | |||
| Minimally classic | n = 157 | n = 537 | ||
| 6.66 (4.2–11) | 3.90 (1.6–8.4) | |||
| Lesion area, n (%) | ||||
| Predominantly classic | n = 1064 | n = 628 | ||
| ≤ 3 DA | 868 (81.6%) | 557 (88.7%) | ||
| > 3 DA ≤ 6DA | 152 (14.3%) | 56 (9.0%) | ||
| > 6 DA ≤ 9 DA | 28 (2.6%) | 13 (2.1%) | ||
| > 9 DA | 16 (1.5%) | 2 (0.3%) | ||
| Minimally classic | n = 163 | n = 559 | ||
| ≤ 3 DA | 90 (55.2%) | 389 (69.6%) | ||
| > 3 DA ≤ 6 DA | 50 (30.7%) | 114 (20.4%) | ||
| > 6 DA ≤ 9 DA | 14 (8.6%) | 35 (6.3%) | ||
| > 9 DA | 9 (5.5%) | 21 (3.8%) | ||
| CNV location, n (%) | ||||
| n = 1227 | n = 1187 | n = 2414 | ||
| Subfoveal | 1227 (100%) | 586 (49.4%) | 1813 (75.1%) | |
| Juxtafoveal | 0 (0%) | 349 (29.4%) | 349 (14.5%) | |
| Extrafoveal | 0 (0%) | 252 (21.2%) | 252 (10.4%) | |
| Lesion % classic CNV, n (%) | ||||
| n = 1227 | n = 1187 | n = 2414 | ||
| ≥ 50% | 1064 (86.7%) | 628 (53.0%) | 1692 (70.1%) | |
| > 0% < 50% | 163 (13.3%) | 351 (29.6%) | 514 (21.3%) | |
| 0% | 0 (0.0%) | 208 (17.5%) | 208 (8.6%) | |
| Occult CNV present, n (%) | n = 1227 | n = 1187 | n = 2414 | |
| 197 (16.1%) | 303 (25.5%) | 500 (20.7%) | ||
| Blood present in lesion, n (%) | n = 1227 | n = 1187 | n = 2414 | |
| 600 (49.9%) | 532 (44.9%) | 1132 (46.9%) | ||
| SPED present in lesion, n (%) | n = 1227 | n = 1187 | n = 2414 | |
| 11 (0.9%) | 86 (7.3%) | 97 (4.0%) | ||
Chapter 6 Results (2) – objectives A, B, C and D
A: Is verteporfin photodynamic therapy in the NHS provided as in randomised trials?
The analysis for objective A focused on provision of VPDT in the year following the first treatment. Given that TAP trials required prospective participants to have CNV from nAMD, the analysis was based on only the subgroup of patients with CNV from nAMD who were classified as having completed their treatment (n = 4043), with one eye per patient.
Is treatment administered at the same frequency as in the randomised trials?
The numbers of VPDT treatments administered in years 1 and 2 by TAP eligibility status (i.e. groups EFT, IFT and UNC) are shown in Tables 10 and 11. In year 1 of the VPDT cohort study (≤ 350 days after the first treatment), fewer treatments were administered (average 2.35) than in year 1 in the TAP trials (average 3.4). We compared the numbers of patients having one, two, three and four treatments in year 1 in the VPDT cohort study and in the TAP trials, which differed significantly (χ2 = 615.2, degrees of freedom 4, p < 0.0001). 4,30 The average number of treatments for each of the TAP eligibility groups in the VPDT cohort study was EFT 2.47, IFT 2.31 and UNC, 2.29. The numbers of patients having one, two, three and four treatments in year 1 also differed significantly between groups (χ2 = 364.3, degrees of freedom 8, p < 0.0001).
| Treatments in year 1 | EFT (N = 1227) | IFT (N = 1187) | UNC (N = 1629) | Total patients (N = 4043) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 1 | 255 | 20.8 | 307 | 25.9 | 425 | 26.1 | 987 | 24.4 |
| 2 | 377 | 30.7 | 400 | 33.7 | 571 | 35.1 | 1348 | 33.3 |
| 3 | 364 | 29.7 | 292 | 24.6 | 384 | 23.6 | 1040 | 25.7 |
| 4 | 224 | 18.3 | 181 | 15.3 | 229 | 14.1 | 634 | 15.7 |
| 5 | 6 | 0.5 | 7 | 0.6 | 18 | 1.1 | 31 | 0.8 |
| 6 | 1 | 0.1 | 0 | 0.0 | 2 | 0.1 | 3 | 0.1 |
| Treatments in year 2 | EFT (N = 533) | IFT (N = 478) | UNC (N = 600) | Total patients (N = 1611)a | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 0 | 392 | 73.6 | 348 | 72.8 | 425 | 70.8 | 1165 | 72.3 |
| 1 | 90 | 16.9 | 89 | 18.6 | 112 | 18.7 | 291 | 18.1 |
| 2 | 33 | 6.2 | 35 | 7.3 | 46 | 7.7 | 114 | 7.1 |
| 3 | 14 | 2.6 | 4 | 0.8 | 14 | 2.3 | 32 | 2.0 |
| 4 | 4 | 0.8 | 2 | 0.4 | 3 | 0.5 | 9 | 0.6 |
When considering treatment frequencies in year 2, we had data on 1611 patients who had completed treatment for year 2 of study (see Chapter 4, Definition of year 1 and year 2). The average number of treatments administered to these patients was 0.40, compared with 2.2 in the TAP trials. In year 2, the numbers of treatments administered cannot be compared because the distribution of treatments in the TAP trials in year 2 was not reported. The average number of treatments for each of the TAP eligibility groups was EFT 0.40, IFT 0.37 and UNC, 0.43. Unlike year 1, the numbers of patients having one, two, three and four treatments in year 2 did not differ significantly between groups (χ2 = 6.62, degrees of freedom 6, p = 0.36).
Is treatment duration the same as in the randomised trials?
As described in Chapter 3, Data collection and management, the VPDT manual of operations set out a schedule for follow-up and retreatment. This schedule was intended to approximate the follow-up and retreatment guidance provided in the TAP trials, that is patients should be expected to be observed over a period of 2 years with retreatment every 3 months if required. The treatment frequencies described in Is treatment administered at the same frequency as in the randomised trials? show that much less treatment was administered in the study than would have been expected on the basis of the TAP trials.
Based on our definition of a completed treatment episode (see Chapter 4, Classification of treatment as active or completed), we constructed a Kaplan–Meier curve describing ‘survival’ until completion of the treatment episode for the 4566 patients who had data for one eye starting treatment > 350 days before the close of the study, shown in Figure 6. This figure shows that just over 50% of eyes completed the treatment episode in < 1 year. Thus, not only were fewer treatments administered in the study than in the TAP trials, but also the duration of review of patients’ CNV status was shorter than in the TAP trials for the majority of treatment episodes.
FIGURE 6.
Time to completion of treatment episode; Kaplan–Meier graph showing the cumulative proportion of eyes completing the first treatment episode.
B: Is ‘outcome’ the same in the NHS as in randomised trials?
This analysis included 4043 eyes of the 4043 patients who had a diagnosis of nAMD and a first treatment > 350 days before the close of the study. As described in Chapter 4, Definition of ‘TAP’ eligibility, eyes were classified as EFT, IFT or UNC. BCVA outcome in the EFT subgroup was of primary interest in addressing objective B. In order to maximise the power of the analysis, all three subgroups were included in one mixed regression analysis that included the TAP eligibility subgroup and baseline lesion classification (predominantly classic, minimally classic, occult only; Table 12). The possibility of a differing gradient of change over time by subgroup was tested by fitting an interaction of TAP eligibility subgroup and time, but this was found to be non-significant and was excluded from the final model.
| Covariate | Regression coefficient | 95% CI | p-value |
|---|---|---|---|
| Time (per year) | –15.68 | –17.78 to –13.59 | < 0.0001 |
| Time2 (per year) | 3.33 | 2.74 to 3.92 | < 0.0001 |
| TAP eligibility | |||
| EFT group | 0.00 | – | |
| IFT group | 1.42 | 0.17 to 2.67 | 0.026 |
| % of lesion classified as classic | 0.014b | ||
| ≥ 50% | 0.00 | – | – |
| > 0% and < 50% | 1.13 | –0.44 to 2.70 | 0.158 |
| 0% | 3.43 | 1.04 to 5.83 | 0.005 |
| Unknown % classic | 1.50 | 0.29 to 2.72 | 0.016 |
| % of lesion classified as classic × timea | 0.031b | ||
| ≥ 50% | 0.00 | – | – |
| > 0% and < 50% | 2.08 | 0.38 to 3.77 | 0.016 |
| 0% | 2.79 | 0.20 to 5.38 | 0.035 |
| Unknown % classic | 0.77 | –0.45 to 1.98 | 0.218 |
| Age (year) | –0.20 | –0.26 to –0.14 | < 0.0001 |
| Age × timea | –0.29 | –0.36 to –0.22 | < 0.0001 |
| Baseline ETDRS7 (per letter) | 0.743 | 0.70 to 0.77 | < 0.0001 |
| Baseline ETDRS7 (per letter) × timea | –0.20 | –0.23 to –0.16 | < 0.0001 |
| Gender | |||
| Male | 1.00 | – | – |
| Female | 1.76 | 0.85 to 2.67 | 0.0002 |
| Smoking status | 0.091b | ||
| Never smoked | 1.00 | – | – |
| Ex-smoker | 1.89 | 0.34 to 3.44 | 0.017 |
| Current smoker | 1.59 | 0.01 to 3.18 | 0.049 |
| Unknown smoking status | 2.03 | 0.10 to 3.96 | 0.039 |
| Smoking status × timea | 0.002b | ||
| Never smoked | 1.00 | – | – |
| Ex-smoker | 1.09 | –0.66 to 2.83 | 0.223 |
| Current smoker | 3.03 | 1.28 to 4.78 | 0.0007 |
| Unknown smoking status | 1.21 | –0.78 to 3.20 | 0.233 |
| BCVA in fellow eye | < 0.0001b | ||
| Fellow worse | 1.00 | – | – |
| Fellow similar to treated eye | –2.12 | –3.46 to –0.77 | 0.002 |
| Fellow better | –2.57 | –3.73 to –1.40 | < 0.0001 |
| BCVA in fellow eye x timea | < 0.0001b | ||
| Fellow worse | 1.00 | – | – |
| Fellow similar to treated eye | –1.53 | –3.04 to –0.02 | 0.0471 |
| Fellow better | –5.16 | –6.52 to –3.79 | < 0.0001 |
We compared descriptively the change in BCVA in the two angiographic subtypes in our study with those previously reported for treatment and sham treatment arms in the TAP trials (Figure 7). 4,30 Both the fitted trajectory and the absolute changes in BCVA over time for patients with predominantly classic lesions in the EFT group were similar to those for patients in the TAP treatment arm. For eyes with minimally classic lesions in the EFT group, the trajectory of BCVA was parallel to that observed for the minimally classic subgroup of the TAP treatment arm but showed less absolute loss of BCVA (Figure 8). 4,30
FIGURE 7.
Change in BCVA from baseline in eyes classified as EFT with predominantly classic CNV. Confidence intervals are not shown for TAP groups because they were not reported. 30
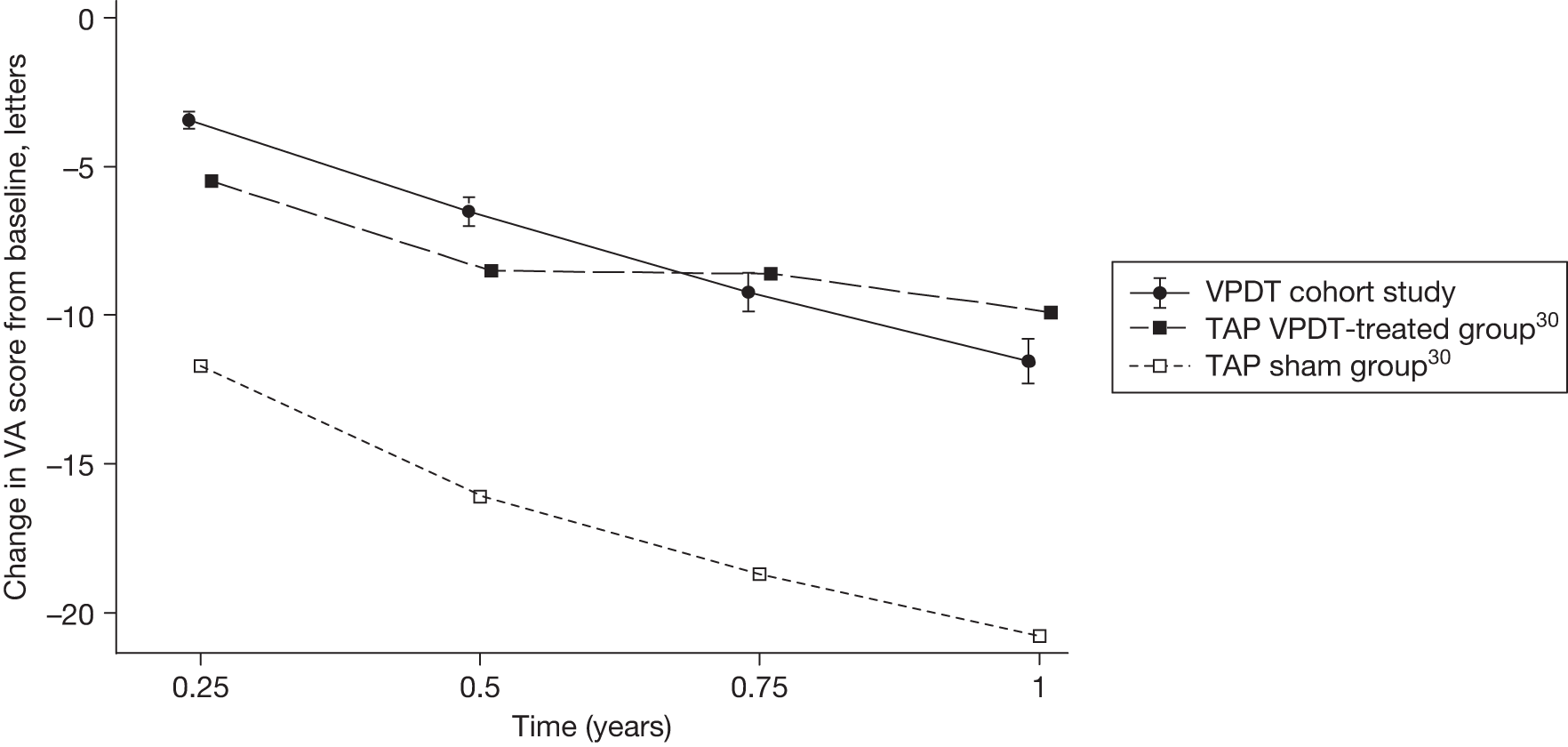
FIGURE 8.
Change in BCVA from baseline in eyes classified as EFT with minimally classic CNV. Confidence intervals are not shown for TAP groups because they were not reported. 30
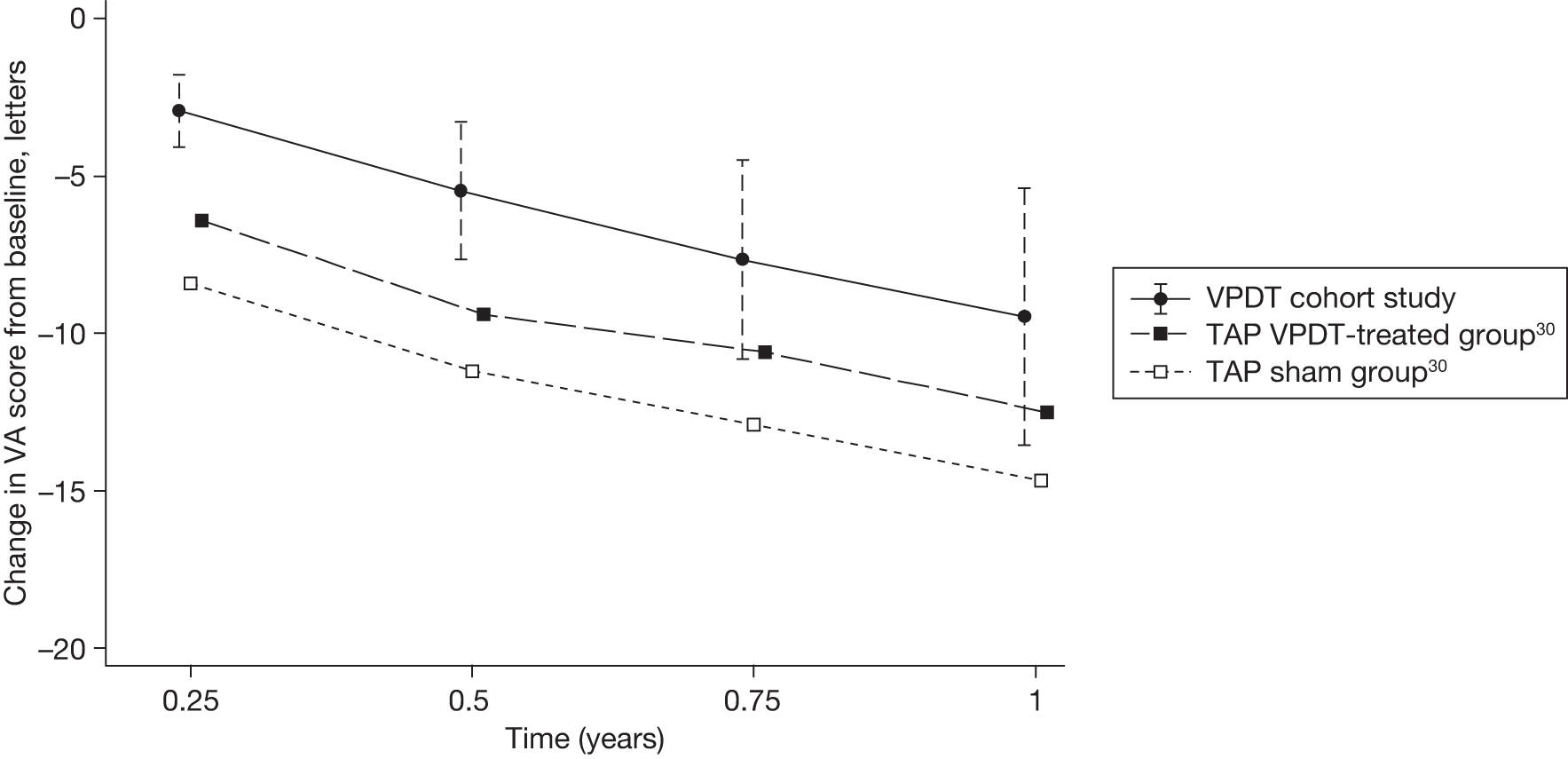
Baseline lesion classification influenced outcome. Within the EFT group, eyes with minimally classic CNV had better BCVA at baseline (+1.13 letters; see Table 12) and deteriorated more slowly than eyes with predominantly classic CNV (+1.13 + 2.08 = +3.2 letters at 1 year; see Table 12).
The influences of several covariates on BCVA were also investigated, partly because the covariates could have confounded the influences of the TAP eligibility subgroup and baseline lesion classification, and partly because their possible influences were of interest in their own right. The coefficients from the final mixed regression model, shown in Table 12, show that a number of baseline covariates did indeed influence BCVA. None of these statistically significant covariates interacted with the TAP eligibility subgroup, so they can be considered to apply equally to the EFT, IFT and UNC subgroups.
The rate of deterioration of BCVA was influenced by baseline acuity, with faster decline in BCVA over time in eyes with better starting acuity; a patient who read five letters more than average at baseline read only 2.7 letters more at 1 year. BCVA deteriorated faster in older patients; after 1 year of follow-up, BCVA was two letters worse for a person 10 years older than average (88 vs 78 years). Women presented with better baseline BCVA and maintained this difference during follow-up (+1.8 letters). Ex-smokers and those who had never smoked presented with better baseline BCVA (+1.6 and +1.8 letters respectively) and deteriorated more slowly than current smokers. The decrease in BCVA by 1 year was one letter fewer in treated eyes of ex-smokers, and three letters fewer in treated eyes of never smokers, than in treated eyes of current smokers. If the fellow eye had better BCVA than the treated eye, BCVA in the treated eye was worse at baseline (+2.6 letters) and deteriorated faster; the decrease in BCVA by 1 year was five letters worse than if the treated eye was classified as the better-seeing eye. The decrease in BCVA over 1 year was 8 to 16 letters depending on patients’ characteristics and lesion factors (see C: Is ‘outcome’ the same for patients excluded from randomised trials? below).
C: Is ‘outcome’ the same for patients ineligible from randomised trials?
The mixed regression analysis addressing this objective was the same as the one used to address objective B given that we did not find an interaction of TAP eligibility subgroup and time. BCVA outcome in the IFT subgroup, and to a lesser extent the UNC subgroup, was of primary interest in addressing objective C.
Eyes classified as IFT presented with better BCVA (+1.4 letters; see Table 12) than EFT eyes with predominantly or minimally classic CNV; UNC eyes also presented with better BCVA (+1.5 letters) than EFT eyes and deteriorated more slowly (+1.50 + 0.77 = +2.3 letters at 1 year; see Table 12); these eyes were UNC because there were no corresponding independently graded FA findings, and so, by definition, they had unknown lesion percentages classified as classic and occult. Eyes with occult only lesions (a subgroup of IFT eyes) were better by +3.4 letters at baseline and were observed to deteriorate more slowly (+3.43 + 2.79 = +6.2 letters at 1 year).
D: Is verteporfin photodynamic therapy safe when provided in the NHS?
The analysis was carried out on the cohort of patients who were treated at any time, that is n = 7748 (see Figure 5). This larger cohort was used to address objective D because ARs and AEs were collected at all visits and did not require patients to have achieved a particular duration of follow-up. If a patient had only one treatment, then an AR documented at the time of treatment or at a subsequent follow-up visit was considered relevant. Despite defined fields in the database and on the data collection form provided (see Figure 2), and a stated requirement in the manual of operations that these fields should be completed at all visits, information about ARs and AEs was often missing. For example, question 8 (‘Has there been an AE since the last visit or an AR at this visit?’) was often not completed or, when one of these fields was ticked ‘yes’, the specific AR/AE form was not completed.
The analysis of safety was complex because both ARs (at the time of treatment) and AEs (documented at the subsequent visit and attributed to the previous treatment) could in principle be either ‘systemic’, affecting the whole patient, or ‘ocular’, affecting a particular eye. However, ARs tended to be systemic (e.g. systemic AR = back pain, a known side effect of VPDT and an AR that was documented in the TAP trials) and AEs tended to be ocular (e.g. ocular AE = drop in BCVA > 20 letters within 7 days of treatment or intraretinal haemorrhage).
The cohort of 7748 patients had a total of 31,640 visits documented. Treatment was administered at 17,809 (56.3%) visits. ARs and AEs were reported infrequently, with total numbers of 216 (1.2%) and 253 (1.4%) respectively. The distributions of ARs and AEs by visit (with AEs attributed to the previous treatment visit) are shown in Figures 9 and 10. The high frequency of ARs and AEs associated with the first treatment is, to some extent, explained by the fact that the number of patients having a first treatment was higher than the number of patients having a subsequent visit. However, the proportion of treated visits at which ARs and AEs were reported/attributed declined from a maximum of 1.4% and 2.0%, respectively, for visit 1 to 0.3 and 0.9%, respectively, by visit 4 with no ARs or AEs being reported beyond visit 8. Thus, an AR or AE was much more likely to be reported or attributed to visit 1 than to subsequent visits. Therefore, analysis focused only on ARs and AEs reported at visit 1 because treatments on visit 1 were most numerous and most likely to be representative of the reference population of patients who were eligible for VPDT.
FIGURE 9.
Distribution of all ARs over time since eyes were first treated.
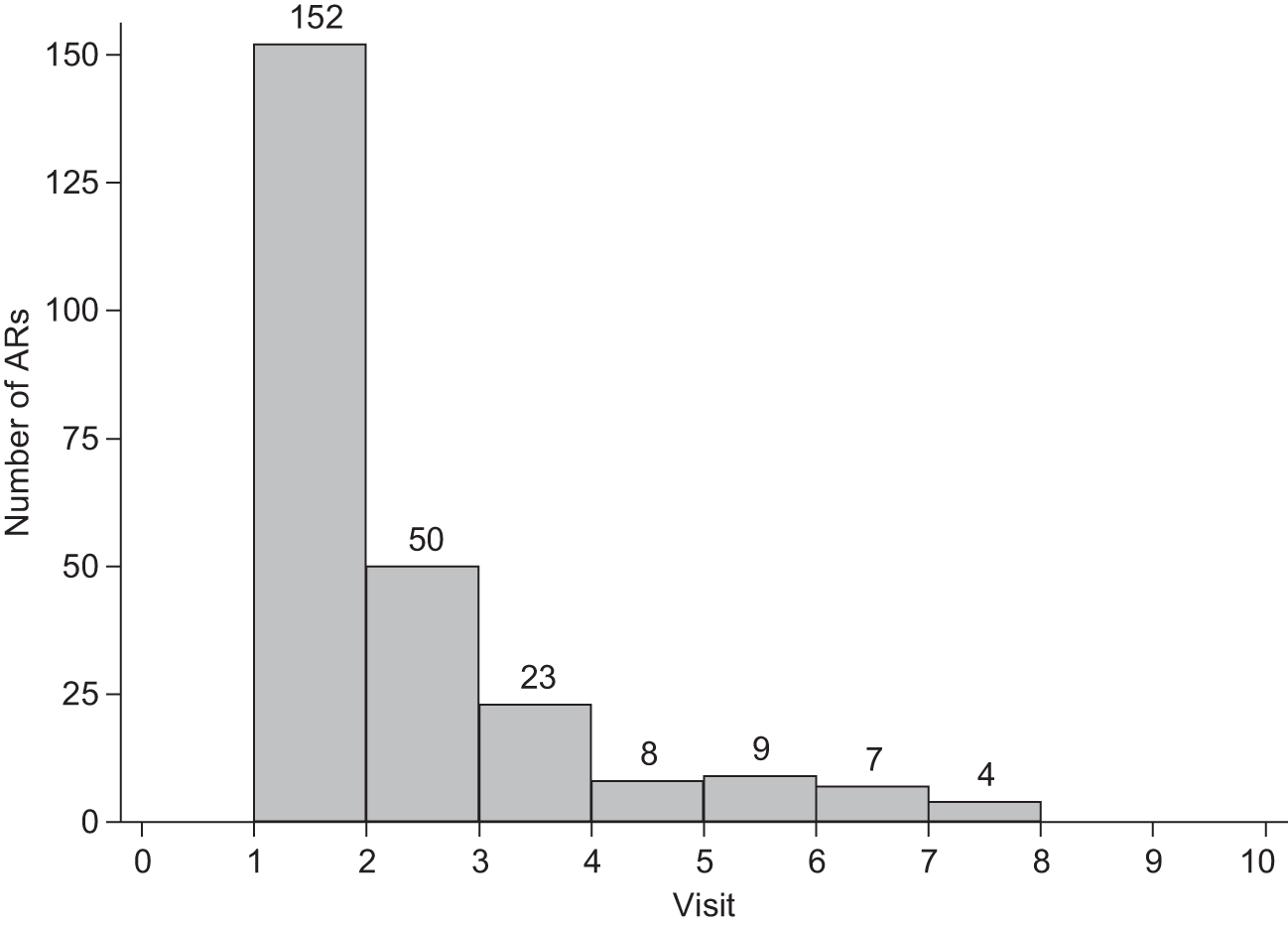
FIGURE 10.
Distribution of all AEs over time since eyes were first treated.
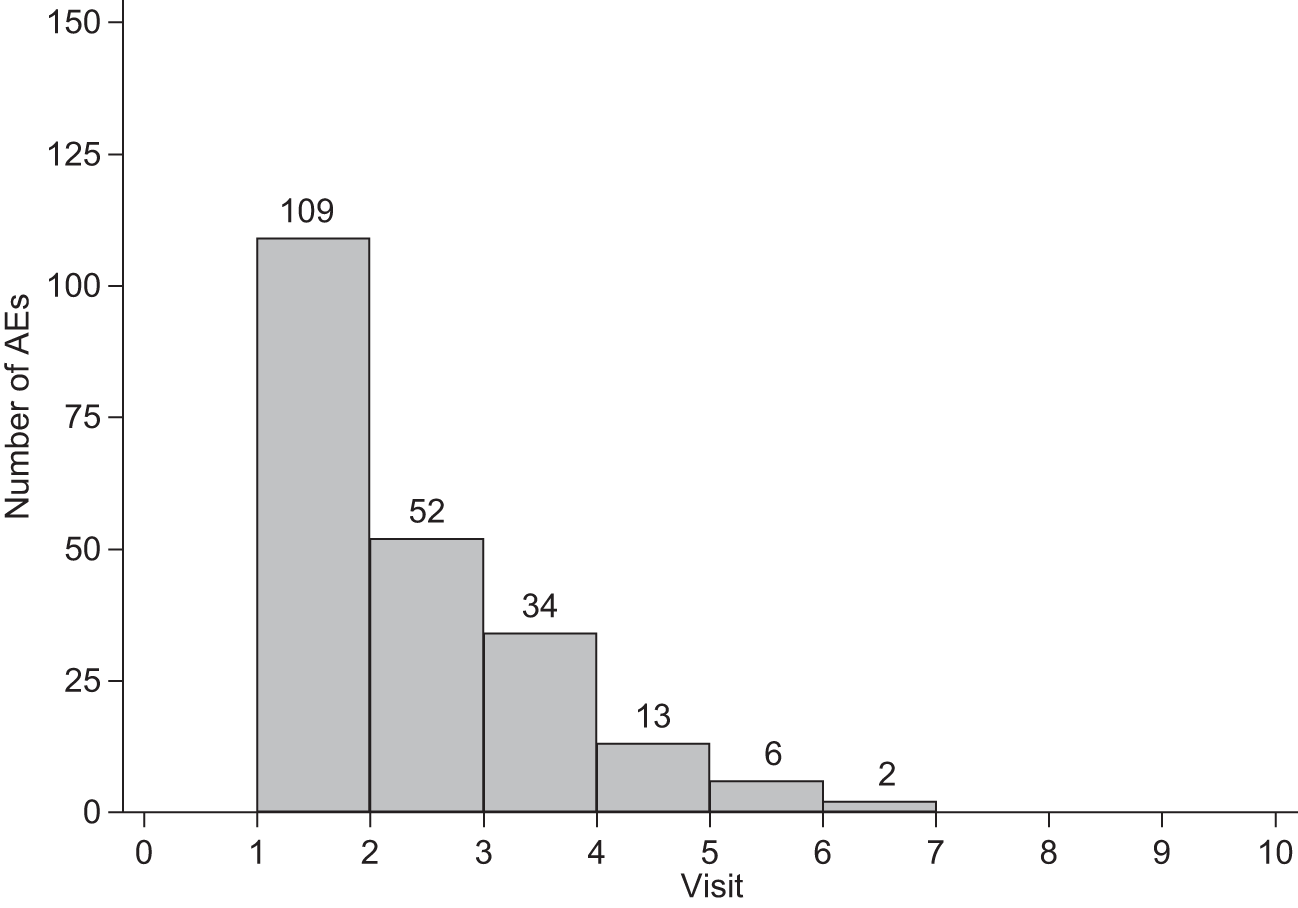
The proportion of first treatment visits at which an AR was reported varied from 0% to 7.8% across centres (Figure 11); the overall proportion was 1.4% [exact 95% confidence interval (CI) 1.2% to 1.6%]. Back pain was documented in 86 of 7748 first treatments (1.1%); 58% of these reactions were mild, 27% moderate and 15% severe. Pain at the injection site (n = 2) and extravasation (n = 1) were rarely reported. Fifteen centres (albeit with relatively few documented treatment visits) reported no AR.
FIGURE 11.
Probability of an AR at the time of the first VPDT administration. Each line represents one participating site, ordered by the mean site-specific probability. The vertical dashed lines represent the overall mean probability and 95% confidence interval across all participating sites. The solid vertical line represents the mean probability for the site which submitted data for the most treated patients and which was judged to have complied relatively well with the instructions for recording AEs.
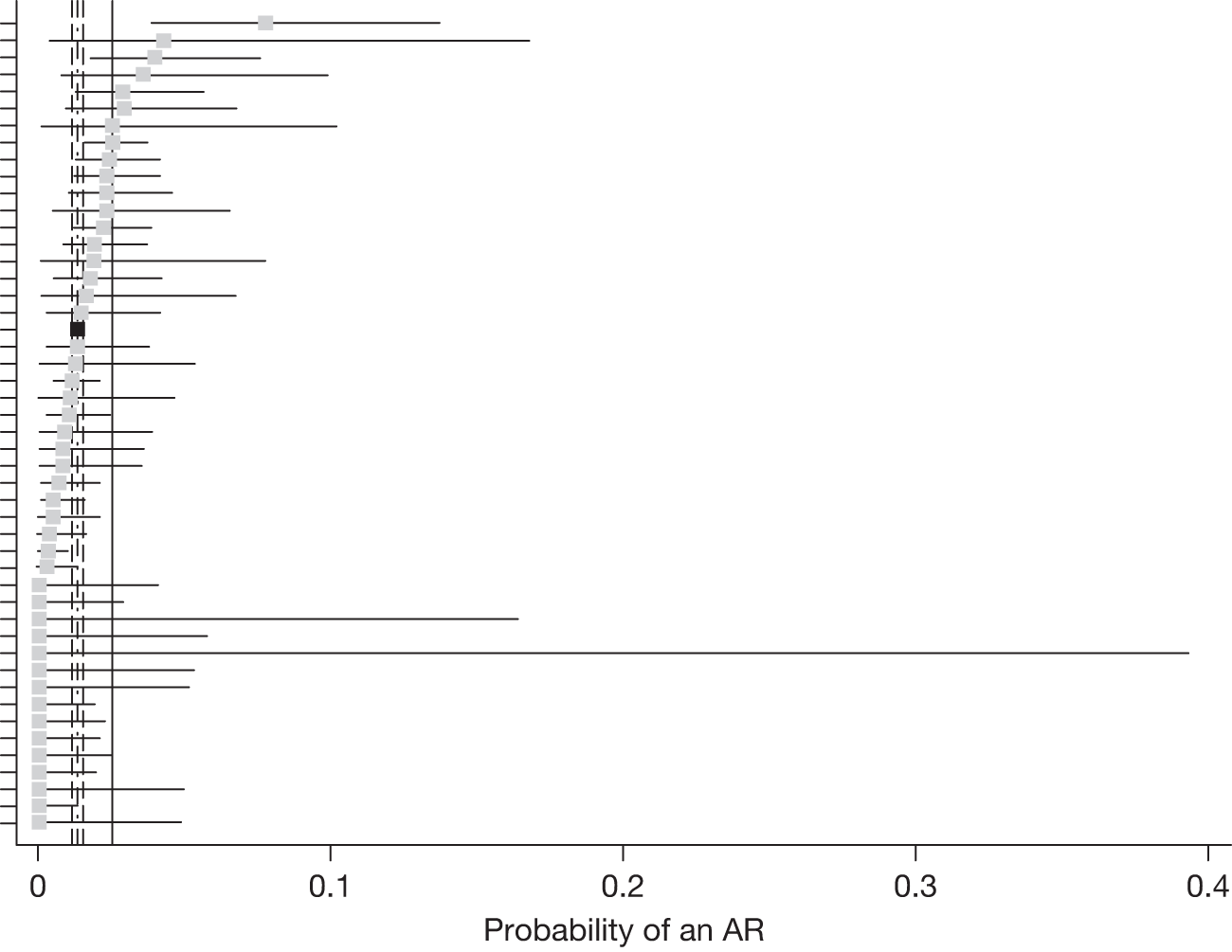
We were concerned about the compliance of centres with reporting ARs and, consequently, the danger of underestimating the risk of an AR. For comparison, the probability of an AR in the centre with the most documented treatment visits (1603) was 2.6% (exact 95% CI 1.8% to 3.5%). ARs were reported less frequently than in the TAP trials in which, for example, back pain was documented in association with 2.2% of VPDT treatment administrations, photosensitivity reactions with 3.0% and ‘adverse events at the site of injections’3 with 13.4% (compared with 3.4% for sham treatments).
Ocular AEs were also reported infrequently. The proportion of first treatment visits at which an AE was reported varied from 0% to 14.3% across centres (Figure 12); the overall proportion was 2.0% (exact 95% CI 1.7% to 2.2%). AEs included a sudden fall in vision reported by the patient or a documented loss of ≥ 20 letters within 7 days of treatment in 25 of 7748 first treatments (0.3%), a tear of the retinal pigment epithelium in 5 (0.1%) and diverse other AEs in 121 (1.6%). AEs, like ARs, were reported less frequently than in the TAP trials, in which ‘visual disturbance’ was documented in association with 17.7% of VPDT treatment administrations compared with 11.6% of sham treatments, and vitreous haemorrhage with 1.0% (compared with 0.5% of sham treatments). 3
FIGURE 12.
Probability of an AE associated with the first VPDT administration. Each line represents one participating site, ordered by the mean site-specific probability. The vertical dashed lines represent the overall mean probability and 95% CI across all participating sites. The solid vertical line represents the mean probability for the site which submitted data for the most treated patients and which was judged to have complied relatively well with the instructions for recording AEs.
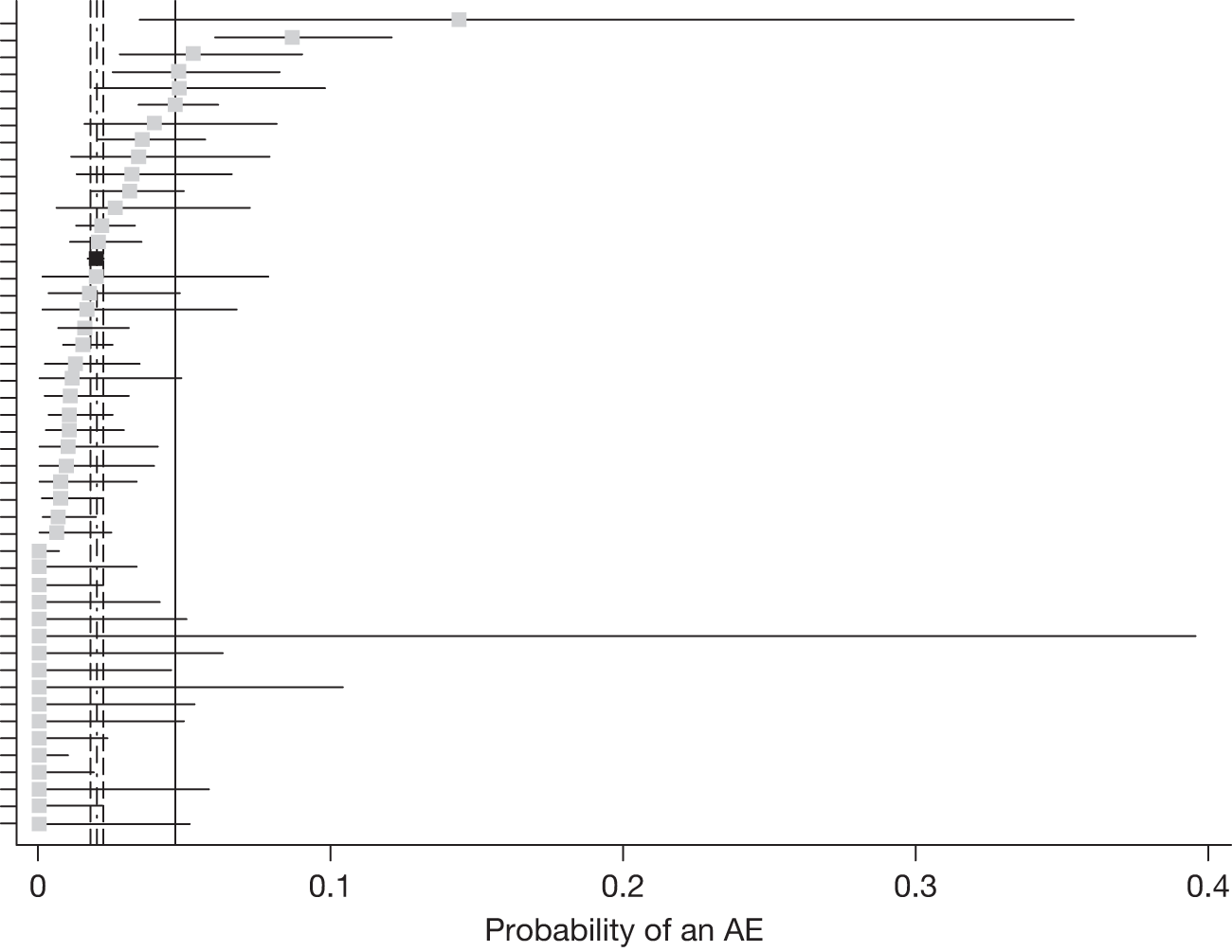
The variation between centres in the proportion of treatment visits in which an AE was reported was greater than expected; 70% (33/47; 95% CI 55% to 83%) had a centre-specific proportion below the overall proportion. We attributed this to poor compliance in reporting AEs that would have biased downwards the overall estimate of the probability of an AE. In order to estimate a more representative overall proportion, we excluded data for two centres which reported a proportion for visit 1 with a centre-specific upper 95% confidence limit below the lower 95% confidence limit for the entire cohort. The overall proportion increased to 2.1% (exact 95% CI 1.9% to 2.4%). For comparison, the probability of an AE in the centre with the most documented treatment visits (1603) was 4.6% (exact 95% CI 3.6% to 5.8%).
Chapter 7 Results (3) – objective E
How effective is verteporfin photodynamic therapy with respect to best-corrected monocular distance visual activity?
Chapter 6 reports outcomes of VPDT in comparison with the TAP trials, that is the change in BCVA over time. As the VPDT cohort study did not have a control group, effectiveness could be estimated only indirectly. Although we considered three ways to do this, we were able to apply only the second method (see Chapter 4, E: How effective and cost-effective is verteporfin photodynamic therapy?).
In terms of BCVA, this method was, in effect, the comparison in change in BCVA over time reported in Chapter 6. Both the fitted trajectory and the average absolute change in BCVA over time for patients with predominantly classic lesions in the EFT group were similar to those for patients in the TAP treatment arm. For eyes with minimally classic lesions in the EFT group, the trajectory of BCVA was parallel to that observed for the minimally classic subgroup of the TAP treatment arm, but showed less absolute loss of BCVA (see Figures 7 and 8).
For HRQoL outcomes, no similar comparison could be made because HRQoL data were not reported for participants in the TAP trials.
How effective is verteporfin photodynamic therapy with respect to health-related quality of life?
Absence of HRQoL outcome in the TAP trials was a major limitation with respect to the NICE technology appraisal of VPDT. Therefore, describing the change in HRQoL with treatment was a key objective of the study. We aimed to do this by quantifying the extent to which BCVA predicted HRQoL.
The subgroup of 18 centres collected and submitted BCVA, CS and HRQoL data for 3262 visits for 1829 patients (Tables 13 and 14). Most data were available for visits at 0, 6 and 12 months, as planned, but data for many patients were also available for 3 and 9 months; 53% of patients had data for two or more visits [one visit, 47%; two visits, 33%; three visits, 16%; more than three visits (maximum six), 4%].
| HRQoL instrument | Duration of follow-up in months | Total | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | > 12 | ||
| SF-36 – PCS | 1196 | 116 | 680 | 158 | 459 | 383 | 2992 |
| SF-36 – MCS | 1196 | 116 | 680 | 158 | 459 | 383 | 2992 |
| SF-6D | 1156 | 117 | 683 | 152 | 469 | 385 | 2962 |
| NEIVFQ – composite | 1270 | 130 | 739 | 174 | 504 | 435 | 3252 |
| NEIVFQ – distance activities | 1267 | 129 | 736 | 174 | 504 | 434 | 3244 |
| NEIVFQ – near activities | 1268 | 130 | 739 | 174 | 504 | 435 | 3250 |
| HRQoL instrument | Duration of follow-up (months) | Total | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | > 12 | ||
| SF-36 – PCS | 1122 | 82 | 599 | 108 | 392 | 320 | 2623 |
| SF-36 – MCS | 1122 | 82 | 599 | 108 | 392 | 320 | 2623 |
| SF-6D | 1084 | 81 | 605 | 104 | 401 | 325 | 2600 |
| NEIVFQ – composite | 1187 | 89 | 649 | 119 | 428 | 356 | 2828 |
| NEIVFQ – distance activities | 1184 | 88 | 647 | 119 | 428 | 355 | 2821 |
| NEIVFQ – near activities | 1185 | 89 | 649 | 119 | 428 | 356 | 2826 |
Generic health-related quality of life
Best-corrected monocular distance visual acuity: in the better-seeing eye strongly predicted SF-6D, physical component score (PCS) and mental component score (MCS) (p < 0.0001 for all three HRQoL measures). For each HRQoL measure, the best-fitting models were linear. No evidence was found to support the hypothesis of a sigmoid relationship. We also did not observe any tendency at all for gradients to decrease with the duration of follow-up. The relationship between BCVA in the better-seeing eye and the SF-6D utility score is shown in Figure 13, with the fitted regression superimposed on a scatterplot of the raw data. Predicted changes in SF-6D, PCS and MCS for 5- and 100-letter reductions in BCVA are shown in Table 15.
FIGURE 13.
Scatterplot showing SF-6D preference-based measure of health (equivalent to utility score) compared with better-seeing eye BCVA. The fitted regression line (see Table 15) is superimposed on a scatterplot of the raw data. Values of BCVA < 0 (–10 and –20) represent ‘counting fingers’ and ‘hand movements’ levels of vision respectively.
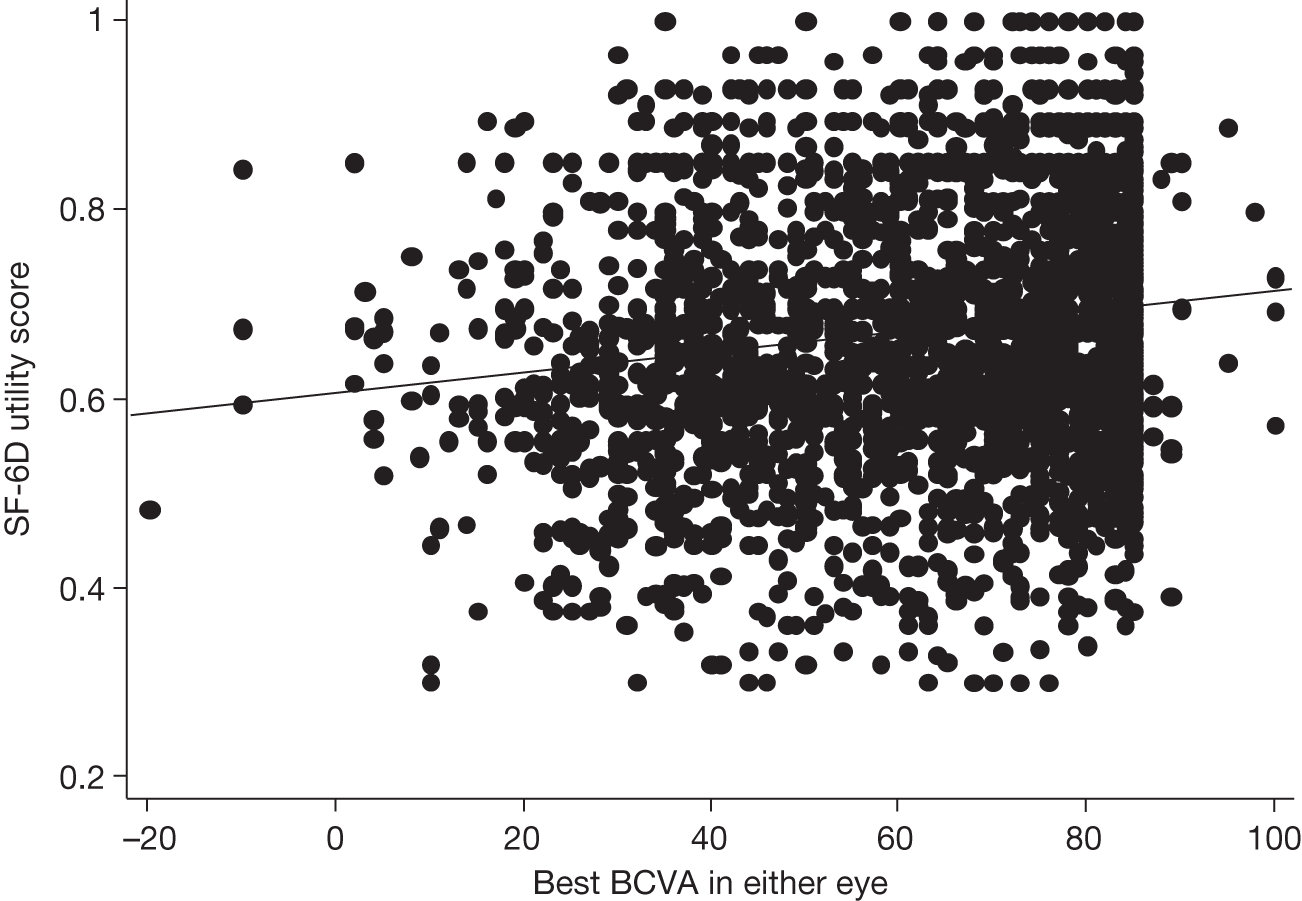
| HRQoL score | HRQoL scale | Linear regression coefficient (95% CI) | Quadratic regression coefficient (95% CI) | HRQoL change per five letters (≡ one chart line)a | HRQoL change per 100 letters (i.e. whole chart range)a |
|---|---|---|---|---|---|
| BCVA | |||||
| SF-6D | 0–1 | 0.0012 (0.0009 to 0.0014) | – | 0.0058 | 0.116 |
| SF-36 PCSb | Mean = 50 | 0.049 (0.025 to 0.073) | – | 0.245 | 4.906 |
| SF-36 MCSb | Mean = 50 | 0.109 (0.078 to 0.140) | – | 0.546 | 10.920 |
| HRQoL change per three letters (i.e. one contrast sensitivity triad)a,c | HRQoL change per 48 letters (i.e. whole chart range)a | ||||
| CS | |||||
| SF-6D | 0–1 | –0.0016 (–0.0041 to 0.0009) | 0.0001 (0.00003 to 0.00015) | 0.014 | ~0.14 |
| SF-36 PCS | Mean = 50 | –0.269 (–0.476 to –0.062) | 0.008 (0.003 to 0.013) | 0.792 | ~7.7 |
| SF-36 MCS | Mean = 50 | –0.120 (–0.382 to 0.143) | 0.008 (0.002 to 0.016) | 1.155 | ~12.1 |
Contrast sensitivity in the better-seeing eye predicted SF-6D, PCS and MCS (p < 0.01 for all three HRQoL measures) but less strongly. The relationship between CS and the SF-6D utility score is shown in Figure 14, with the fitted regression superimposed. For all HRQoL measures, the best-fitting models were positive and quadratic, with the fitted values tending to an asymptote when < 15 letters could be read (see Figure 14). As with BCVA, no evidence was found to support the prior hypothesis of a sigmoid relationship. Predicted changes in SF-6D, PCS and MCS for three-letter (one ‘triad’) and 48-letter reductions in BCVA are shown in Table 15. The latter predicted changes are described as approximate because the quadratic models sometimes caused fitted values to increase slightly when very few letters were read. The predicted changes reported are the fitted value for 48 letters minus the minimum fitted value.
FIGURE 14.
Scatterplot showing SF-6D preference-based measure of health (equivalent to utility score) compared with better-seeing eye CS. The fitted regression line (see Table 13) is superimposed on a scatterplot of the raw data.
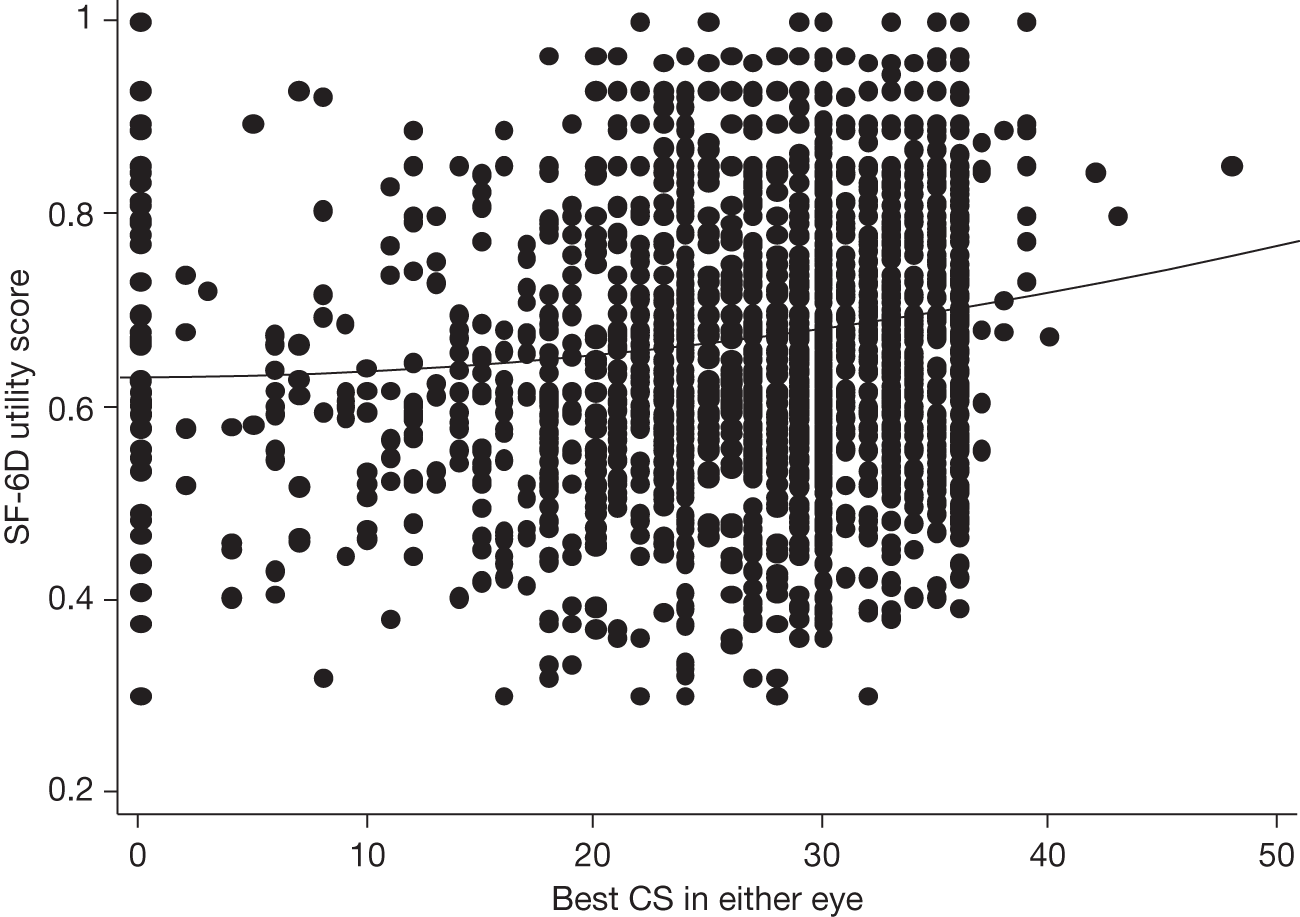
For predominantly classic nAMD lesions, VPDT was observed to confer a net benefit of 11 ETDRS and 5 CS letters after 1 year. 4,30 Based on the best-fitting model for SF-6D, these visual function benefits ‘translate’ into utility differences of 0.013 and 0.022 respectively (on a scale of 0–1). In the VPDT cohort study, the net BCVA benefit compared with the TAP sham VPDT group was slightly smaller, at about nine letters (see Figure 7); this BCVA benefit ‘translates’ into a utility difference of about 0.011.
For minimally classic nAMD lesions, VPDT was observed to confer a net benefit of four ETDRS letters after 1 year. 4,30 Based on the best-fitting model for SF-6D, these visual function benefits ‘translate’ into a utility difference of 0.005. The net BCVA benefit observed in the study, of about five letters (see Figure 8), ‘translates’ into a utility difference of about 0.006.
Vision-specific health-related quality of life
In the better-seeing eye, BCVA also strongly predicted the composite total NEIVFQ score, and the distance and near activity subscales (p < 0.0001 for all three NEIVFQ scores). The relationship between BCVA in the better-seeing eye and NEIVFQ composite total score is shown in Figure 15; similar relationships were observed for distance and near NEIVFQ scores. Relationships were positive and quadratic and had steeper gradients (in relation to the range of BCVA) than those seen for SF-6D and SF-36 component scores. Gradients were steeper for the distance and near activities subscores than for the composite total score. Predicted changes in composite total, distance and near NEIVFQ scores for 5- and 100-letter reductions in BCVA are shown in Table 16. Because the functions were quadratic, the predicted NEIVFQ changes for 100-letter reductions are described as approximate.
FIGURE 15.
Scatterplot showing the NEIVFQ composite total score compared with better-seeing eye BCVA. The fitted regression line (see Table 16) is superimposed on a scatterplot of the raw data. Values of BCVA < 0 (–10 and –20) represent ‘counting fingers’ and ‘hand movements’ levels of vision respectively.
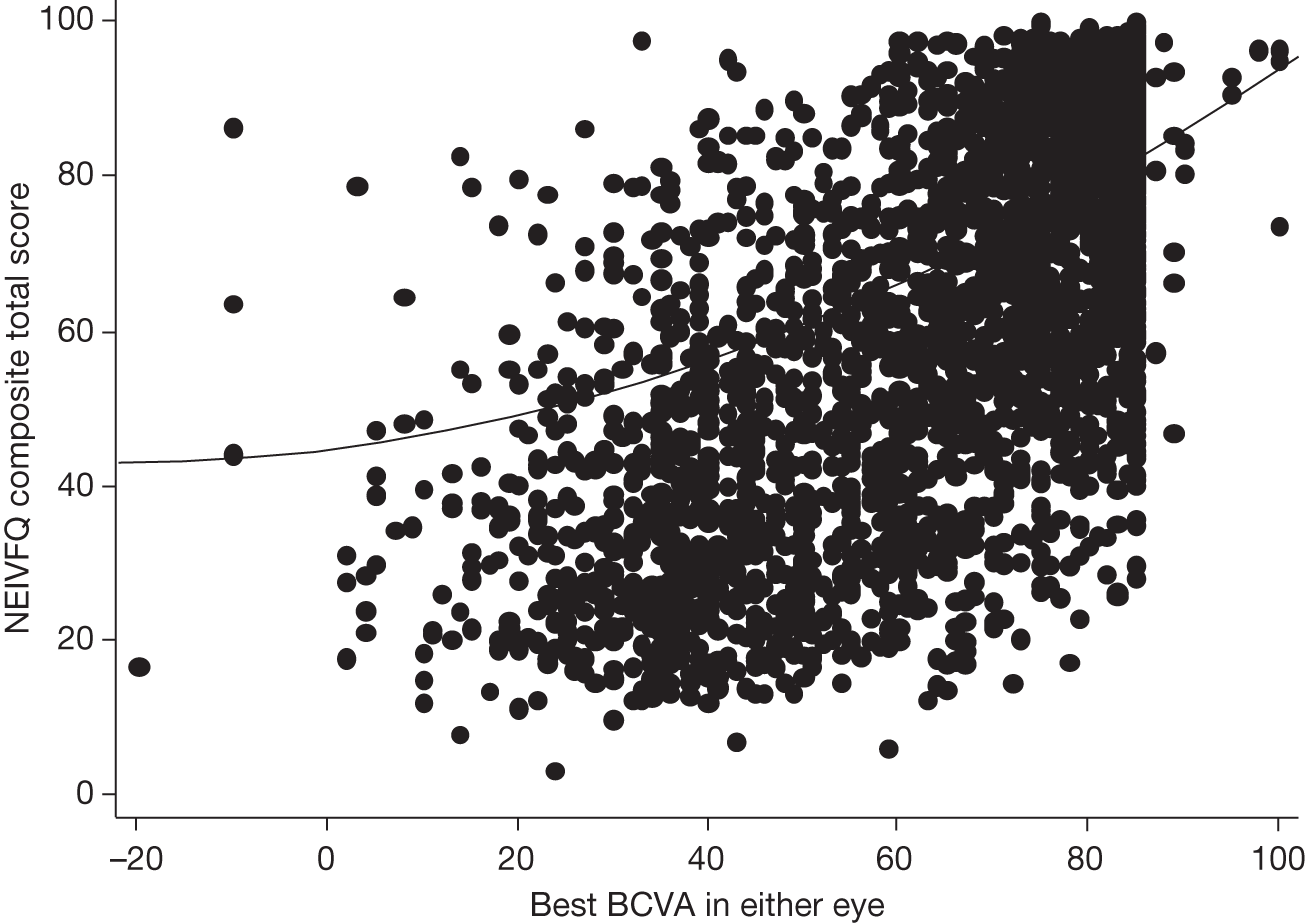
Similarly, CS in the better-seeing eye strongly predicted the composite total, distance and near activity NEIVFQ score (p < 0.0001 for all three HRQoL measures, but less strongly than BCVA). The relationship between CS and NEIVFQ core total is shown in the scatterplot in Figure 16; the relationships were similar for distance and near NEIVFQ scores. As for BCVA, the relationships were positive and quadratic and had steeper gradients than those observed for SF-6D, PCS and MCS scores. Predicted changes in composite total, distance and near NEIVFQ scores for 3-letter (one triad) and 48-letter reductions in CS are shown in Table 16. Because the functions were again quadratic, the predicted NEIVFQ changes for 48-letter reductions are again described as approximate.
FIGURE 16.
Scatterplot showing the NEIVFQ composite total score compared with better-seeing eye CS. The fitted regression line (see Table 16) is superimposed on a scatterplot of the raw data.
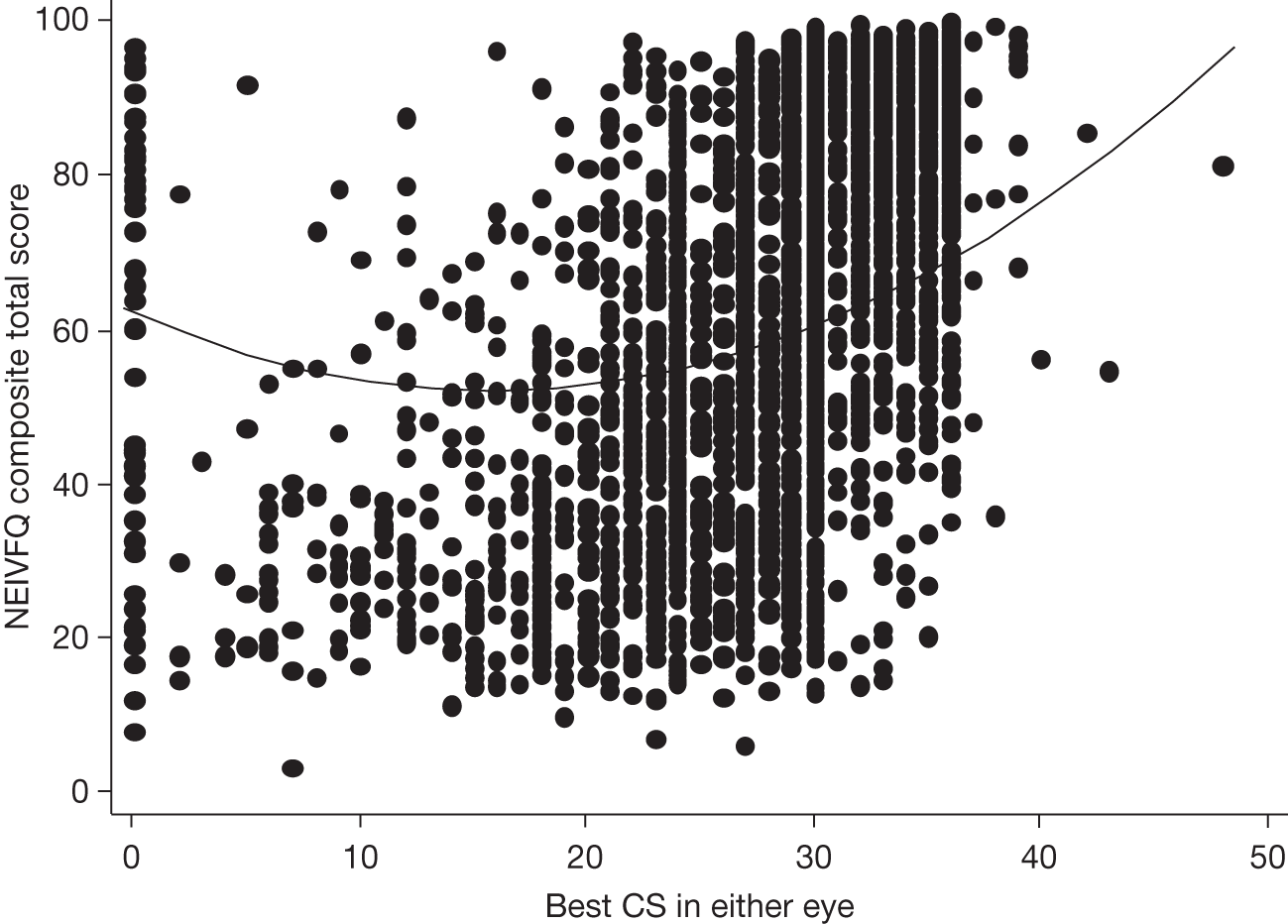
| HRQoL score | HRQoL scale | Linear regression coefficient (95% CI) | Quadratic regression coefficient (95% CI) | HRQoL change per five letters (≡ one chart line)a,b | HRQoL change per 100 letters (i.e. whole chart range)a |
|---|---|---|---|---|---|
| BCVA | |||||
| NEIVFQ composite | 0–100 | –0.129 (–0.342 to 0.083) | 0.0067 (0.0049 to 0.0085) | 3.90 | ~55.0 |
| NEIVFQ distance activities | 0–100 | 0.00002 (–0.236 to 0.236) | 0.0075 (0.0054 to 0.0096) | 5.08 | ~72.5 |
| NEIVFQ near activities | 0–100 | 0.397 (–0.626 to –0.127) | 0.0111 (0.0090 to 0.0131) | 5.48 | ~72.8 |
| HRQoL change per three letters (≡ one contrast sensitivity triad)a,b | HRQoL change per 48 letters (i.e. whole chart range)a | ||||
| CS | |||||
| NEIVFQ composite | 0–100 | –1.285 (–1.646 to –0.924) | 0.0540 (0.0454 to 0.0625) | 6.99 | ~44.4 |
| NEIVFQ distance activities | 0–100 | –1.737 (–2.181 to –1.292) | 0.0694 (0.0589 to 0.0799) | 8.74 | ~57.6 |
| NEIVFQ near activities | 0–100 | –1.967 (–2.406 to –1.527) | 0.0757 (0.0653 to 0.0860) | 9.32 | ~64.7 |
Based on the best-fitting model for the NEIVFQ composite total score and an average presenting BCVA of 50 letters and CS of 23 letters, the differences in BCVA and CS observed in the TAP trials for predominantly classic nAMD lesions (11 ETDRS and 5 CS letters, respectively)4,30 ‘translate’ into differences in score of about 9 and 12 respectively (on a scale of 0 to 100). In the VPDT cohort study, the net BCVA benefit was slightly smaller, at about nine letters (see Figure 7); this BCVA benefit ‘translates’ into a difference in NEIVFQ composite total score of about 7.
For minimally classic nAMD lesions, the visual benefit observed in the TAP trials of four ETDRS letters after 1 year4,30 ‘translates’ into a difference in NEIVFQ composite total score of about 3. The larger net BCVA benefit observed in the study, of about five letters (see Figure 8), ‘translates’ into a difference in NEIVFQ composite total score of about 4.
How cost-effective is verteporfin photodynamic therapy?
Verteporfin photodynamic therapy and best supportive care costs
Data describing VPDT treatments were available for 4566 patients in year 1 and 1834 patients in year 2 (Table 17). The mean number of VPDT treatments per patient was 2.4 in year 1, falling to 0.4 in year 2. The mean intervention cost of VPDT was £3026 in year 1 and £845 in year 2. The main cost component was the drug cost, which, on average, was 60% of the year 1 costs. The corresponding mean intervention costs for BSC were £166 for year 1 and £101 for year 2, giving incremental intervention costs of £2860 and £744 respectively.
| Item | Year 1 (n = 4566) | Year 2 (n = 1834) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| VPDT resource use | ||||
| VPDT treatment visits | 2.41 | 1.17 | 0.43 | 0.81 |
| Follow-up visits | 1.30 | 1.02 | 1.59 | 1.25 |
| VPDT costs (£) | ||||
| FA | 591 | 152 | 325 | 234 |
| Verteporfin and disposables | 2074 | 1003 | 365 | 698 |
| VPDT treatment visit | 272 | 132 | 48 | 92 |
| Follow-up visit | 87 | 69 | 107 | 83 |
| Total | 3026 | 1182 | 845 | 944 |
Health and social services use and costs related to vision loss
Health and social services and BCVA costs were available for a total of 3435 visits in 1764 patients. As in the case of HRQoL data, most resource use questionnaires were completed in association with visits at 0, 6 and 12 months (Table 18). All visits with data were included in the analysis. Only about 10% of patients reported using a health service, such as an unscheduled low-vision appointment or seeing their GP, while < 0.5% of patients reported moving into a nursing home, residential home or sheltered accommodation (Table 19). The mean annual total costs related to the patients’ eye conditions were low (approximately £300) relative to the VPDT intervention costs. Although the highest cost item was social service costs, the mean cost for this item was driven by the 1% of patients who received high levels of support from social service home carers costing > £3500 per year.
| Parameter | Duration of follow-up (months) | Total | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | > 12 | ||
| Resource use and BCVA | 1350 | 136 | 848 | 171 | 528 | 402 | 3435 |
| Resource use and CS | 1276 | 96 | 764 | 127 | 466 | 356 | 3085 |
| Item | % using service in last 3 months | Unit cost | Mean (SD) annual cost per patient (£) |
|---|---|---|---|
| Low vision appointment | 9.96 | 83 | 51.2 (259.2) |
| Visits to GP | 10.88 | 44 | 22.1 (70.5) |
| Social services | 1.14 | 35 | 129.0 (1531) |
| Day centre | 1.21 | 32 | 26.3 (256) |
| Nursing home stay | 0.06 | 648 | 19.6 (812) |
| Residential care | 0.12 | 443 | 26.8 (785) |
| Sheltered housing | 0.20 | 140 | 14.8 (328) |
| Antidepressant use | 0.52 | 10 | 0.64 (8.8) |
| Other | NA | NA | 10.0 (53) |
| Total | NA | NA | 297 (2078) |
There was a negative relationship between BCVA and annual HSS cost. Figure 17 shows that the gradient of this relationship, like that for HRQoL, was shallow. For example, for a five-letter decrease in BCVA for patients with baseline of 50 letters, the predicted increase in mean annual costs was about £28. For those patients who used a service, a five-letter decrease in BCVA was associated with a statistically significant increase in mean annual costs of £111 (95% CI ∼£48 to ∼£174). This association between BCVA and SF-6D was combined with the differential decline in BCVA from baseline for the VPDT and placebo groups in TAP to derive differences in HRQoL between VPDT and BSC at 3-monthly intervals (Figure 18). 30
FIGURE 17.
Scatterplot showing the annual cost of HSS resource use compared with better-seeing eye BCVA. The regression line superimposed on a scatterplot of the raw data is derived from fitting the two-part model. Values of BCVA < 0 (–10 and –20) represent ‘counting fingers’ and ‘hand movements’ levels of vision respectively.
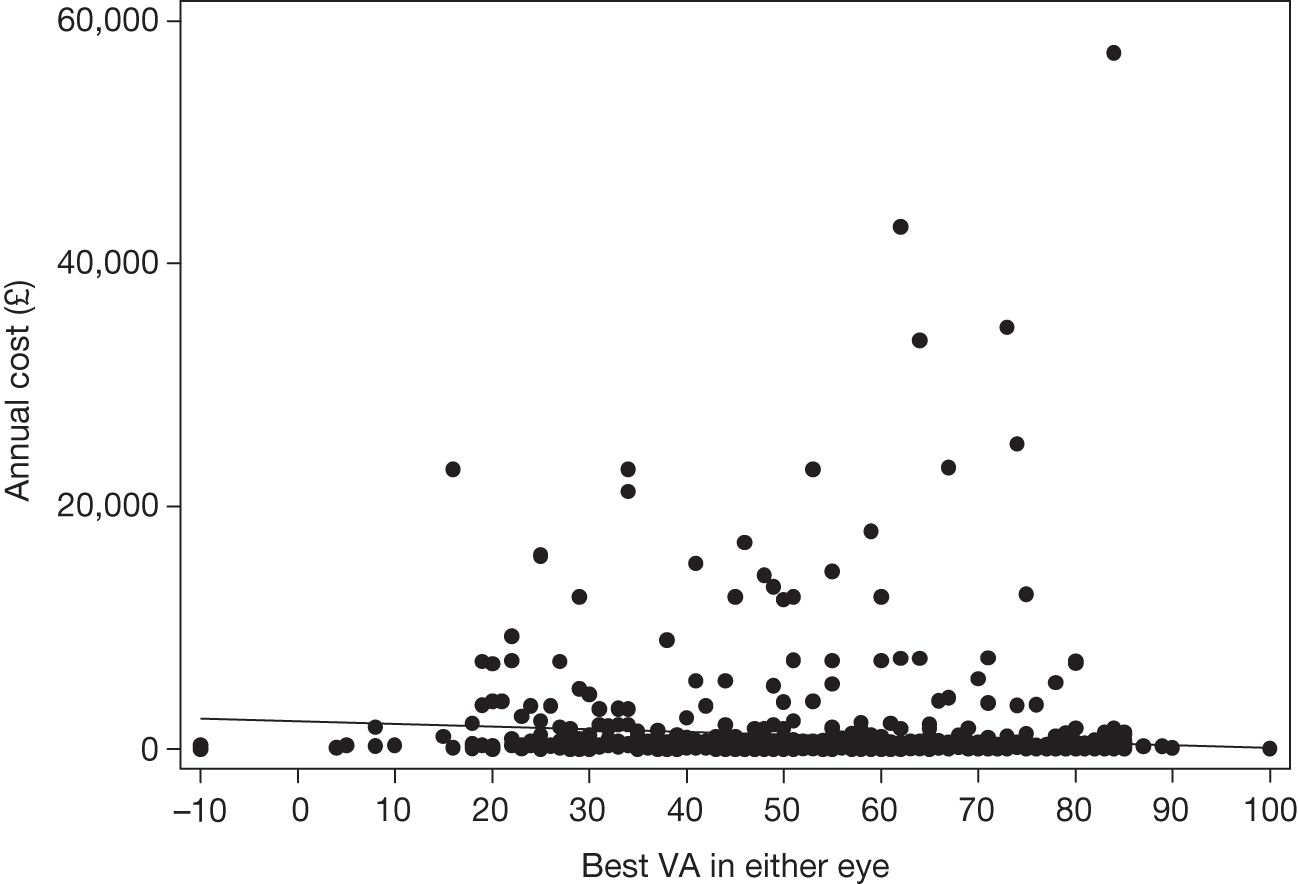
FIGURE 18.
Mean predicted change in HRQoL at 3-monthly time points for VPDT vs BSC for eyes with predominantly classic lesions that would have been EFT. Predictions combine VPDT map of HRQoL and visual acuity with visual acuity for VDPT treatment and placebo groups reported in the TAP study.
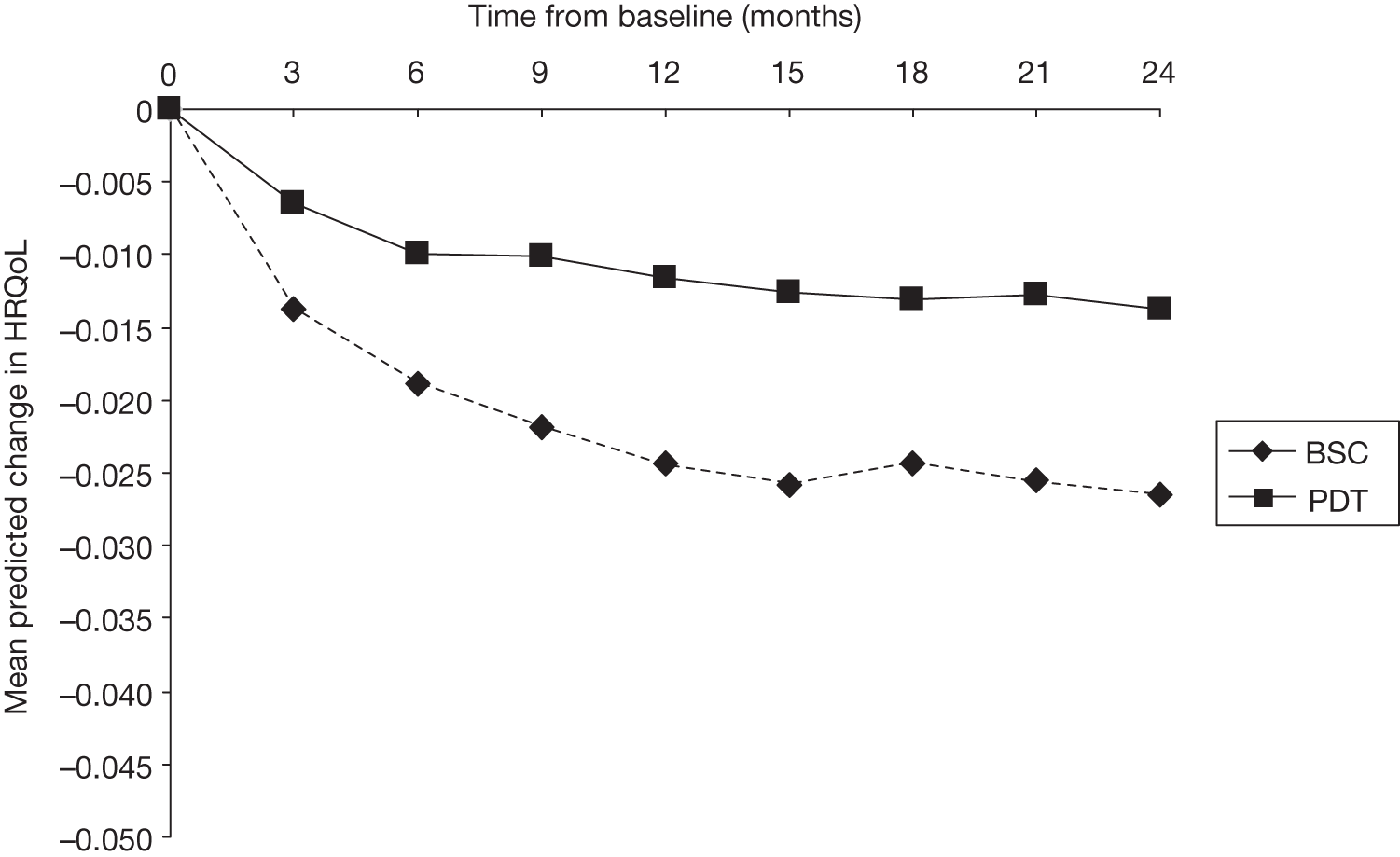
Cost-effectiveness
There was a small reduction in vision-related HSS costs following VPDT compared with BSC because of the smaller vision loss (year 1: mean costs of £320 for BSC group vs £260 for VPDT group; year 2: £382 vs £290). This reduction in vision-related costs offsets the additional costs of the intervention only to a small extent, and the incremental total costs of VPDT were positive (Table 20). The incremental QALYs for VPDT versus BSC were positive but small (Table 20). The base-case cost-effectiveness results showed that combining the small positive incremental QALYS gained for VPDT with the relatively high additional costs gave a cost per QALY of £170,000 over 2 years (i.e. £3514/0.0207).
| Item | Year 1 | Year 2 | (Year 1 + 2) |
|---|---|---|---|
| Incremental intervention costs (£) | 2860 | 744 | 3604 |
| Incremental costs of HSS (£) | –59 | –92 | –151 |
| Incremental total costs (£) | 2884 | 630 | 3514 |
| Incremental QALY | 0.00866 | 0.01212 | 0.02071 |
| Incremental cost/QALY (£) | 333,000 | 52000 | 170,000 |
Sensitivity analyses
The sensitivity analyses found that the results were robust to the methodological assumptions and data sources used in the base-case analysis (Table 21). The probability that VPDT is cost-effective is zero unless the willingness to pay for a QALY gain exceeds £100,000 per QALY (Figure 19). The further sensitivity analyses showed that:
-
If the treatment frequency was taken from TAP rather than the VPDT cohort study, the incremental costs increased to £5946 and the cost per QALY rose to £288,000.
-
If the pre–post BCVA difference from the cohort study was used rather than the difference between arms in the TAP trials, then the estimated QALY gain was higher (0.0212 vs 0.0207), but the cost per QALY gained still exceeded £150,000.
-
If the relationships of cost and HRQoL with CS rather than BCVA were used, this led to a small increase in QALY gain compared with the base case (0.0220 vs 0.0207), but again the cost per QALY exceeded £150,000.
-
If the costs of BSC were 10-fold those assumed in the base case, the cost per QALY was £91,000.
-
If the difference in BCVA observed for VPDT compared with BSC at 2 years was maintained until 5 years, the cost per QALY was £94,000.
| Sensitivity analysis | Incremental QALY | Incremental cost | Incremental cost/QALYa |
|---|---|---|---|
| Base case | 0.02066 | 3514 | 170,000 |
| TAP study treatment frequency | 0.02066 | 5946 | 288,000 |
| VPDT cohort study VA results | 0.02122 | 3511 | 165,000 |
| Contrast and sensitivity map | 0.02201 | 3412 | 155,000 |
| 10-fold increase in BSC costs | 0.02066 | 1891 | 91,000 |
FIGURE 19.
Cost-effectiveness acceptability curve for VPDT vs BSC.
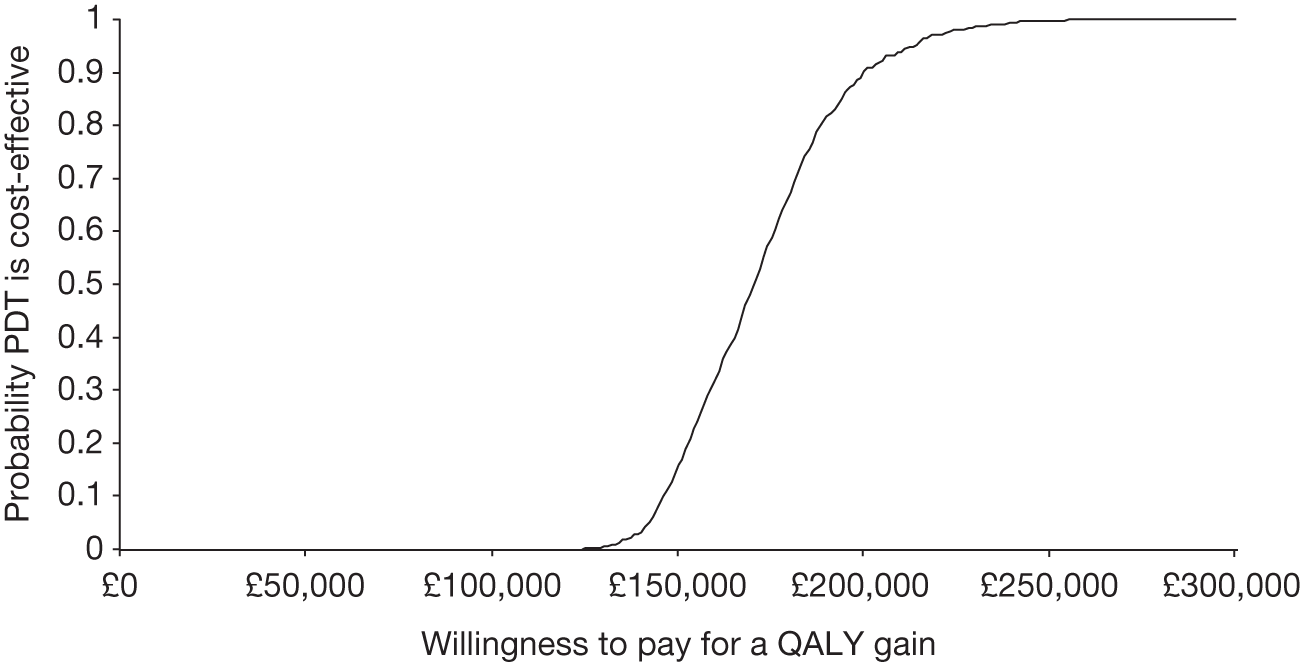
Chapter 8 Discussion of results
The VPDT cohort study broke new ground in that it was a publicly funded postlicensing study of an expensive new technology that was beneficial in the management of subfoveal nAMD. Previously, this condition had been largely untreatable. The technology involved the use of the drug verteporfin (a photosensitiser) and its activation within the eye with an infrared non-thermal laser (thus avoiding direct physical injury to the neural retina). Its application to routine practice necessitated significant investment in expensive equipment and dissemination of new knowledge to ophthalmologists in the interpretation of retinal imaging outputs for diagnosis, case selection, treatment initiation and retreatment decision-making.
Although the appraisal by NICE found the treatment to be clinically effective, there was a recognition that evidence about its cost-effectiveness was based on multiple assumptions which were not robust. Hence, the Department of Health recommended a limited and managed introduction of this technology with collection of robust visual function and resource utilisation data to assess whether or not its clinical effectiveness and cost-effectiveness matched those observed in the licensing trials; it was also recommended that a measure of its impact on HRQoL should be obtained.
Thus, the size and the scope of the VPDT study was far more extensive than any previous study of nAMD and acted as a treatment registry containing data acquired on patients receiving VPDT in the clinical sites that were selected to provide VPDT. Protocol-based BCVA and CS were measured at multiple time points and HRQoL instruments were administered to patients at about half of the clinical sites. Collection of these data made it possible to investigate relationships between clinical measures of vision and HRQoL as continuous scales (in contrast to many previous studies).
Key findings
The key findings from the VPDT cohort study are outlined below:
-
The change in BCVA was similar to that observed in the treatment groups in the pivotal licensing trials, although the deterioration in BCVA over time may have been underestimated (see Strengths and weaknesses of the verteporfin photodynamic therapy cohort study).
-
The BCVA benefit was achieved with fewer treatments.
-
In addition, non-treatment-related baseline covariates influenced change in BCVA over the study period.
-
In the better-seeing eye, BCVA and CS were highly significant predictors of SF-6D utility, SF-36 component scores and NEIVFQ scores. The change in utility for a unit change in BCVA was less than several previous estimates.
-
Realistic estimates of the cost-effectiveness of VPDT were obtained and were consistent with higher previous estimates, although these high estimates are almost certainly too low because of the assumption (common to all CEAs) that the better-seeing eye is being treated.
Strengths and weaknesses of the verteporfin photodynamic therapy cohort study
Despite its observational nature, the VPDT cohort study has many strengths. These include its size, pragmatic nature, and systematic collection of standardised data on acuity and lesion characteristics. This was the largest study of its kind to date, giving new insights into the HRQoL of people with nAMD and new data that will be pivotal to future studies of effectiveness in this population. Many aspects of the data collected in this study were robust. Protocol-based BCVA and CS were measured and HRQoL instruments administered at multiple time points, which allowed us to investigate in detail the relationships between clinical measures of vision and HRQoL. The large sample gave us reasonable power to test secondary hypotheses about the shape of the relationships and adaptation to vision loss over a 2-year period, even though the proportion of patients with data for more than three visits was small.
The VPDT cohort study was the first with concurrent concurrent collection of data on HRQoL and patient-level data on HSS resource use, and, hence, first to have undertaken a systematic CEA using data acquired during treatment rather than data stipulated by a trial protocol. Our CEA also tackled three major methodological concerns not previously addressed in CEAs of interventions for nAMD. Thus, our work extends the literature on the costs and cost-effectiveness of interventions for nAMD, and its findings will assist future CEA.
Set against these strengths, there were a number of limitations. These include the observational nature of the study, the loss to follow-up of large proportions of the original sample, missing data and the lack of a BSC comparison group of current relevance. There were major logistical challenges in establishing the study, which were discussed at a project review meeting about 18 months after the contract for the study started; key observations and recommendations from this meeting are set out in Appendix 3.
Unlike the pivotal trials, in which almost all patients were followed up for 24 months,3,4,30 about half of the patients included in our analyses did not have 1-year follow-up. Because poor data quality is a well-recognised limitation of observational studies, we undertook computerised data validation checks on an on-going basis and when compiling the final data set. We checked whether or not data were missing for some visits by (a) matching records from paper and electronic systems for collecting BCVA and (b) requesting that centres should check explicitly whether or not additional visits had taken place for selected patients. The results from these checks implied that data had been submitted for > 95% of completed visits.
The exact reasons for loss to follow-up are not known. We attempted to collect information about reasons for completing a treatment episode early or for patients being lost to follow-up, but participating centres did not report reasons reliably. Anecdotally, we became aware that some hospitals had a policy of not rebooking appointments for patients who missed a visit, and some ophthalmologists were put under pressure to discharge patients who did not require active treatment rather than to continue to review them. The context for these policies was the extremely overstretched nature of macular clinics. It should be remembered that providing VPDT required hospitals to make regular appointments (up to four per year) to review a large number of patients who had previously had fewer than one; reviewing a patient required FA and, if treatment was required, administration of VPDT. Irrespective of whether or not funding was available to pay for these resources, the expert workforce needed to provide VPDT could not be expanded rapidly.
Patients who were not followed lost the opportunity to be retreated if reactivation occurred; this could have led to worse BCVA outcomes with treatment in everyday practice than with treatment in the licensing trials. However, the BCVA outcome in the VPDT cohort study was generally similar to that observed in the treatment arm of the TAP trials.
The loss to follow-up introduced uncertainty to the data analyses, which was taken into account by using a mixed regression model to predict BCVA at 1 year in different subgroups. This approach allows all of the available data to be modelled but does not prevent attrition bias. We observed that patients who were lost to follow-up tended to have poorer BCVA at baseline (data available from the authors). Because follow-up data were more likely to be missing with increasing duration after first treatment, and patients with a poor outcome were more likely to be lost to follow-up, the model may have tended to underestimate deterioration in BCVA over time. The regression model for BCVA trajectory assumed that BCVA deteriorated steadily (on the principles of parsimony and ‘best fit’); this assumption is unlikely to be valid when BCVA is poor because the neovascular process burns out, causing a ‘floor’ effect. Attrition bias and a floor effect would have affected the results in opposite directions. Consequently, it is uncertain whether or not the BCVA deterioration over time in the study was truly similar to that observed in the TAP trials or underestimated because of selective attrition.
Although loss to follow-up is a scientific limitation, our experience also demonstrates vividly the difficulties associated with follow-up when treatments requiring multiple visits over an extended period of time in an older age group are introduced into routine clinical practice. Such data are invaluable to health service planners and are rarely available. We believe that patients and ophthalmologists became disheartened with eyes that experienced deterioration of vision during treatment and follow-up, causing treatment to be discontinued before the recommended time point of 2 years. We did not attempt to predict outcome at 2 years because the data were sparse.
A further limitation of the study was the inability to classify 40% of the lesions at baseline with respect to TAP eligibility (eyes classified as UNC), either because an angiogram was not submitted or because the submitted angiogram could not be graded. This group was retained in the model and had parameter estimates which tended to lie between those for EFT and IFT groups and between those for predominantly and minimally classic lesions. Thus, there was no reason to believe that these eyes represented a biased selection with respect to eligibility for the TAP trials or their lesion composition.
In the CEA, we were unable to use a direct control group, instead we relied on the control group in the TAP trials. Extrapolating relative effects across different populations is potentially problematic; for example, trials often report different estimates of effect from those given by observational studies. 58 Nevertheless, in this cohort study, BCVA at first treatment and at 1 and 2 years were similar to the predominantly classic subgroup studied in the TAP trials.
Interpretation
Comparison of outcomes in the verteporfin photodynamic therapy cohort study versus TAP
Visual acuity outcome
The effectiveness of VPDT in reducing deterioration in BCVA in the context of RCTs has been confirmed by a systematic review in which the chosen outcomes were step changes in BCVA over 24 months, for example loss of three or more lines of visual acuity. 59 VPDT was estimated to reduce the risk of losing more than three lines by 20% (risk ratio 0.80, 95% CI 0.73 to 0.88).
The VPDT study protocol specified that BCVA should be measured at 3-monthly intervals. We could not express the outcomes of treatment with VPDT in the study with the outcomes of treatment in the systematic review because of the loss to follow-up. Instead, we estimated the change in BCVA over time (modelling baseline BCVA as a covariate) and observed that at 12 months it was not dissimilar to that reported in the key licensing trials, although we may have underestimated BCVA deterioration at 1 year because of attrition. Notably, for the participants in the VPDT study who would have qualified for entry into the TAP study, the trajectory of change in BCVA was highly similar to that observed in the TAP trials. 4,30 This finding may be interpreted as providing some corroboration of the clinical effectiveness of the technology in terms of better preserved BCVA in the treated eye.
Factors not related to treatment that influenced change in visual acuity over time
Overall in the VPDT cohort study, BCVA in the treated eye declined over time. This finding was consistent with the outcomes observed in the key licensing trials. However, the size of the cohort study allowed us to examine with high statistical power the influence of a number of baseline covariates on the change in BCVA. Although treatment was associated with a lower rate of decline of BCVA, several other factors influenced change in BCVA. Those that contributed to deterioration included older age, poorer BCVA at commencement of treatment and being a current or ex-smoker. One factor was associated with a better outcome and this was having a fellow eye with better vision than the treated eye.
Although our findings are consistent with clinical wisdom, experience and intuition, this is the first study to quantify the effects of these factors on visual change. For example, the eyes of older participants tended to deteriorate faster than those of younger participants with better BCVA. The magnitudes of the interactions between smoking status and vision in the fellow eye with time, estimated here for the first time, are quite striking. Also, our finding of a better outcome when the treated eye is the better-seeing eye is important and has been overlooked in previous studies. This finding is consistent with a previous report that suggested that an eye with nAMD does not achieve its full visual potential unless it is the better-seeing eye,51 and with previous findings of improvements in adult amblyopic eyes when vision in the fellow eye is lost. 60 The modest size of the effect, and its consistency across conditions, suggests that it may arise from a shift in decision criterion. 61
The number of verteporfin photodynamic therapy treatments administered
A striking feature of the VPDT cohort study was the much smaller number of treatments that were administered, an average of 2.3 and 0.4 treatments respectively in years 1 and 2, even though it was specified that ophthalmologists should retreat as in the TAP trials. By comparison, in the TAP trials30 an average of 3.4 treatments were administered in the first year and 2.2 in the second year; these frequencies are not surprising because treatment was mandated if leakage was judged to be present on an FA at the 3-monthly review visits. It is also notable that in the TAP trials the FAs had to be performed to standardised protocols and scrutinised by an accredited angiogram-reading centre, thus ensuring consistency of interpretation for retreatment decision-making.
Our findings suggest that ophthalmologists do not adhere to treatment algorithms that are used in key licensing trials and that decisions to treat are influenced more by subsequent experience gained from treating large numbers of patients. Thus, a matter of increasing unease is the applicability to routine practice of the treatment protocols specified in pivotal licensing trials and subsequent marketing authorisations. The need for regular review combined with invasive and time-consuming imaging procedures followed by administration of treatment can impose significant burdens on already stretched health-care systems. Traditionally, these factors have not been considered when implementing new therapies into routine practice. With VPDT, however, the question was raised about whether or not efficacy might be diminished if treatment was not administered at the recommended frequency or under the conditions determined by the licensing trials. Even minor diminution of efficacy could have resulted in VPDT becoming cost-ineffective given the borderline benefit of VPDT. Thus, providers and purchasers of health care need to be aware that the characteristics of treatment in routine practice may be substantially different to the treatment recommendations made on the basis of licensing trials. Our findings also highlight that commercial trials may recommend more treatment than necessary or at a treatment frequency that is not deliverable across eligible populations. This fact is also of importance to researchers when designing pragmatic phase 3 trials. 62,63
Visual function and health-related quality of life
The large sample in the VPDT study gave us adequate power to test the secondary objectives on HRQoL relationships and adaptation to vision loss over a 2-year period, even though the proportion of patients with data for more than three visits was small. Approximately half of the centres provided data on a second measure of vision, namely CS, and also administered structured and validated instruments to ascertain visual functioning, HRQoL and resource utilisation. Thus, only a subset of participants in the VPDT study contributed the data for the analysis of the secondary outcomes as they were necessarily a selected sample and had to be attending one of these centres. These limitations may have led to selection in terms of socio-economic status or age. However, previously published work shows that these factors do not influence HRQoL or visual functioning. 18,31
Our analyses of the associations between BCVA and HRQoL identified three main features:
-
Health-related quality of life (SF-36 and the NEIVFQ) decreased with deteriorating visual function (BCVA or CS in the better-seeing eye) over a wide range of visual function.
-
The relationship between visual function and HRQoL measures was not sigmoid but tended to plateau at low levels of visual function.
-
The gradient of the relationships did not change over time up to 2 years after first treatment.
Health-related quality of life is a ‘whole-patient’ outcome, which is dependent on patients’ binocular visual experience on a day-to-day basis using their habitual correction (if any) and in their customary environment. Previous studies have demonstrated weak relationships between declining visual function and generic HRQoL instruments and stronger relationships with visual functioning instruments such as the NEIVFQ. However, the association between the most commonly used surrogate marker for visual function, that is BCVA, and the NEIVFQ is at best moderate, with the majority of the variation remaining unexplained. Reasons for the lack of a strong association include that (a) BCVA itself is a psychophysical test and is influenced by patient factors; (b) BCVA subserves only foveal function and does not reflect the more complex aspects of overall vision such as reading text, depth perception, movement detection, colour and contrast processing and field of vision; and (c) self-reported HRQoL has an in-built variability which is dependent on the respondent’s mood and emotional status.
Clinical tests to obtain estimates of additional or more global aspects of visual function are not easily measured in routine practice and were beyond the scope of the VPDT study. Instead, we attempted to collect presenting binocular BCVA with the participant wearing the habitual correction, if any. We reasoned that this measure would better reflect the usual state of visual functioning and, thus, the HRQoL response. However, one-third of the data describing presenting binocular BCVA were missing and many of the remaining data were collected in a variety of formats, making meaningful interpretation of the findings difficult. Therefore, like previous researchers, we were forced to select BCVA in the better-seeing eye as the proxy for binocular visual performance. 18,31,64 Validation of the BCVA data showed that BCVA had been collected in a far more robust and reproducible manner.
Our analyses revealed that the decrease in generic HRQoL for a clinically significant deterioration in vision, as measured by BCVA, was small. The predicted decreases for a five-letter drop in BCVA in the better-seeing eye were 0.0058, 0.245 and 0.546 for the SF-6D, PCS and MCS respectively (all p < 0.0001). We also had CS data from a subset of participants and therefore were able to estimate changes in HRQoL for both BCVA and CS and relate these to the findings observed in the TAP trial. However, when interpreting these associations, it is very important to recognise that the predicted HRQoL changes do not describe the average benefit in a representative sample of patients, given that a proportion (47% of the VPDT cohort) will have had treatment to the first affected eye and had a normally sighted fellow eye.
The gradient of change in visual functioning measured by the NEIVFQ instrument was not influenced by the duration of follow-up. Models predicting distance, near and composite NEIVFQ scores from BCVA were quadratic. The predicted decreases for a five-letter drop in BCVA in the better-seeing eye were 5.08, 5.48 and 3.90 for the distance and near domains and for the composite instrument respectively. The Submacular Surgery Trials Research Group quantified the association between BCVA and NEIVFQ scores using the change in BCVA. 65 The observed change in NEIVFQ composite score per 100 letters was 18 points, very much less than our estimate of 55 letters. Their lower estimate may be due, in part, to the assumption of a linear relationship or to their much smaller sample size. The VPDT cohort study has enabled more precise quantification of these relationships, particularly those between BCVA and HRQoL. These relationships constitute an important source of robust information for modelling the cost-effectiveness of current and future interventions for nAMD.
The shape of the relationship between visual function and HRQoL had also not been previously investigated. Even in a study as large at the VPDT cohort study, the analyses had limited power to detect departures from a linear relationship. The tendency for some functions to plateau with severe loss of visual function is clearly not a floor effect and supports the prior hypothesis that HRQoL decreases less steeply when visual function is very poor. Our failure to observe a plateau when vision is excellent may have arisen because few patients achieved BCVA < 0.0 logMAR (6/6 Snellen) in the best-seeing eye, either because patients could not achieve better acuity or because they were not encouraged to do so. However, this explanation is not consistent with the observation that visually demanding tasks such as fluent reading and driving ability can be performed with BCVA = 0.3 logMAR (6/12 Snellen) in the best-seeing eye.
Previous researchers have investigated which of BCVA or CS in the better-seeing eye is the stronger predictor of HRQoL. Based on a multivariable regression, Bansback et al. 66 reported that CS was a better predictor of HRQoL than BCVA and, using the Health Utilities Index mark 367 (HUI3), estimated a change in utility of 0.14 per log unit. In contrast, we found that, in this large data set, BCVA was a consistently stronger predictor of HRQoL than CS.
Patients’ adaptation to loss of visual function was investigated by Brown. 31 In a sample of 237 patients with mixed causes of visual loss there was a tendency for those who had had visual loss for longer (over a time frame of 5 years) to report better HRQoL. 31 In a sample of 72 patients who had had AMD for up to 20 years (35 for < 1 year, 19 for 1–3 years and 18 for 3–20 years), patients with durations of visual loss ≥ 1 year had better HRQoL than those with durations < 1 year (p < 0.005). 18 This association was potentially affected by the small number of patients with very longstanding disease. Also, these studies did not assess HRQoL longitudinally in the same patients, so the observed finding is less confidently attributed to the duration of disease and it is not clear whether or not the differences in HRQoL with duration of visual loss were adjusted for the extent of visual loss, that is BCVA. However, our data were obtained over relatively short durations of follow-up and it is possible that adaptation occurs only over longer periods of time.
Narrative reviews have concluded that utility decreases with deteriorating visual function,16,17,68 but few studies have systematically quantified the relationships between these measures for patients with nAMD (Table 22). 18,64 Our estimate of ∼0.1 change in utility per 100 letters is consistent with utility estimates based on the SF-6D or the EQ-5D. It is lower than the estimates based on the HUI364 (see below) and those based on preferences elicited directly from patients. 18,65 The distributions of preferences elicited directly from AMD patients were markedly skewed in contrast to scores on preference-based utility measures derived from societal valuations (acknowledging that this contrast is both between source of valuation, i.e. patients vs society, and between measure, i.e. directly elicited preference by time trade-off vs preference-based utility measure). This observation is consistent with some patients refusing to trade years of life for improved vision,18,31 and raises concern about the validity of the method. More fundamentally, as generic measures of HRQoL are used to make broad comparisons across interventions in different disease areas, it is more appropriate to value health states with preference weights from the general population rather than specific groups. 13
| Study | Instrument/method used | Source of utility values | Visual acuity and utility observations | Approximate utility change per 100 letters |
|---|---|---|---|---|
| Brown et al., 200018 | Time trade-off | Patients | 20/20 to 20/400 (0.0–1.3 logMAR or 70 letters ≈ utility 0.89 to 0.52 ≈ 0.47 difference | 0.67 |
| Espallargues et al., 200564 | EQ-5D | General population | ≤ 0.3 to > 2.0 logMAR or 120 letters ≈ utility 0.75–0.63 ≈ 0.12 difference | 0.10 |
| Espallargues et al., 200564 | SF-6D | General population | ≤ 0.3 to > 2.0 logMAR or 120 letters ≈ utility 0.70–0.63 ≈ 0.07 difference | 0.06 |
| Espallargues et al., 200564 | HUI3 | General population | ≤ 0.3 to > 2.0 logMAR or 120 letters ≈ utility 0.50–0.10 ≈ 0.40 difference | 0.33 |
| Espallargues et al., 200564 | Visual analogue scale | Patients | ≤ 0.3 to > 2.0 logMAR or 120 letters ≈ utility 0.71–0.59 ≈ 0.12 difference | 0.10 |
| Espallargues et al., 200564 | Time trade-off | Patients | ≤ 0.3 to > 2.0 logMAR or 120 letters ≈ utility 0.73–0.47 ≈ 0.26 difference | 0.22 |
| VPDT cohort study | SF-6D | General population | Regression coefficient, 0.0012 per letter | 0.12 |
Is the Short Form questionnaire-6 Dimensions an appropriate measure of generic health-related quality of life?
A generic HRQoL measure chosen for comparing health gain across disease areas should have a descriptive system that covers all the important dimensions of health. The SF-6D, like the EQ-5D, has a descriptive system that purports to meet the World Health Organization definition of health: ‘complete physical, mental and social well-being and not merely the absence of disease or infirmity’. 69 Utilities measured using the SF-6D are similar to those measured using the EQ-5D. 70 By contrast, the HUI3 is based on a narrower, ‘within the skin’ definition of health focusing on impairment and not on the social context of the impairment. 71 Thus, the HUI3 consists of items that tap self-reported functioning more directly than the EQ-5D and SF-6D. The HUI3 is, therefore, likely to be ‘more sensitive’71 to visual loss than the SF-6D. 64 However, the HUI3 has been criticised for using this relatively narrow description of health. 9
A further concern that has been previously expressed is that the utilities for the HUI3 have been derived from a power transformation of values from a visual analogue scale,72 rather than by direct valuation with choice-based methods such as the standard gamble (used for the SF-6D) or the time trade-off (used for the EQ-5D). Our approach of using the SF-6D follows the recommendations of policy-makers such as NICE. 15 Therefore, it is not surprising that our findings are consistent with previous studies that have used the EQ-5D. 64
In terms of an internationally recognised measure of HRQoL appropriately based on societal preferences, the gradient of the decrease in HRQoL with deteriorating visual function is small. The estimated gain in HRQoL (utility) from VPDT is about 0.02 (in terms of BCVA, a difference of about 11 letters after 2 years, assuming that only the best-seeing eye is being treated), and from ranibizumab is about 0.04 (a difference of about 21 letters after 2 years,73,74 under the same assumption). Gains in utility (over varying time horizons) for other common interventions for chronic conditions (Table 23) show that the utility gain associated with VPDT is relatively small compared with other competing interventions. 75,76 These utilities measured over the appropriate time horizon, and combined with relative effects on life years gained, translate into QALYs and inform health policy decisions.
| Interventiona | Utility gainb | Duration of follow-upc | Measure usedd |
|---|---|---|---|
| Cataract surgery | 0.03 | 3 months | EQ-5D |
| Groin hernia repair | 0.06 | 3 months | EQ-5D |
| Total hip replacement | 0.42 | 6 months | EQ-5D |
| Varicose vein surgery | 0.10 | 3 months | EQ-5D |
| Total knee replacement | 0.31 | 6 months | EQ-5D |
| Coronary artery bypass grafting | 0.21 | 6 years | EQ-5D |
| VPDTe | Year 1: 0.009; year 2: 0.012; total: 0.021 | 2 years | SF-6D |
Cost of illness and resource utilisation
The VPDT cohort study, unlike most other studies used to estimate cost-effectiveness, collected data concurrently on both resource utilisation and HRQoL. Thus, it was able to report on the cost-effectiveness of VPDT versus BSC under the assumption that BSC involved scheduled visits to the ophthalmology clinic to monitor patients’ vision and no other treatment.
The main empirical finding from the CEA is that the costs of providing VPDT for patients included in the UK VPDT cohort study were relatively high compared with the projected QALY gain. The incremental cost per QALY was £170,000 over 2 years for the base case. Even if the gain from the intervention was extrapolated over 5 years, the incremental cost per QALY was still approximately £100,000, five times higher than the threshold that NICE uses to identify interventions that are ‘relatively cost-effective’. 15 The cost-effectiveness ratios reported in previous CEA of VPDT for nAMD have ranged from US$30,000 to US$250,000 (£20,000 to £166,667 assuming an exchange rate of US$1.5 to the UK pound). 26,28 Unlike previous CEA of VPDT for patients with predominantly classic CNV secondary to nAMD, we were able to include all appropriate costs and account for HRQoL associated with vision loss. This resulted in improved patient-centred estimates that showed that VPDT is unlikely to be cost-effective.
There are three other features of our CEA that are relevant to future CEA of interventions for nAMD. Firstly, the study used data on the use of a treatment in routine practice rather than data collected in accordance with a trial protocol. Despite the much lower treatment frequency, we observed similar visual outcomes to those observed in the TAP trials. In the case of our CEA, it was more appropriate to use the lower treatment frequency observed in the VPDT cohort study, and previous CEAs that used treatment frequencies from the TAP trials overstated the incremental costs of the VPDT intervention compared with BSC. 19,26,77 The lesson for future CEA is that treatment intensities observed in licensing trials may overestimate the treatment intensity used in routine practice and, hence, overestimate the costs of treatment in the usual care setting.
Second, previous CEAs either excluded costs associated with declining vision or estimated these costs based on expert opinion. 19,26,28,77–79 By contrast, by collecting patient-level data on HSS resource use and BCVA, our CEA was able to incorporate the cost of declining vision. Our data showed that the HSS costs for patients with nAMD were low (e.g. a mean of £320 for BSC group in year 1), and hence the reduction in these costs after VPDT was relatively small (£151 over 2 years). The costs were lower than those reported by a recent observational study assessing the economic burden of nAMD by self-reported use from 400 patients across five different countries23 and from studies of Medicare costs based on claims data. 80,81 However, these studies were based on aggregated costing approaches, which tend to overstate costs. The morbidity costs observed in the VPDT cohort study relied entirely on patient recall and therefore may have under-represented the true cost, and also they may reflect the relatively poor availability of low-vision services in the UK; previous studies have found a similarly low use of vision-related services in the UK. 82,83 However, this low use of vision services has been reported in other countries that have used patient-level data, which suggests that the current findings may be more widely applicable. 24 The lesson for future CEA is that the source and robustness of data describing costs associated with declining vision need to appraised with care.
The third feature of our CEA that is relevant to future CEA concerns the importance of using HRQoL measures based on preference weights from the general population rather than patients with nAMD.
A final issue for future CEAs which emerged from the VPDT study but which was not incorporated into our CEA was the relationship between BCVA and HRQoL. The regression models which investigated this relationship found that the rate of change in HRQoL with varying BCVA was not influenced by whether the better- or the worse-seeing eye was being treated. However, a key assumption in this and other CEA is that it is always the better-seeing eye that is being treated, as the HRQoL gain is ‘credited’ for all treated eyes. Unless policy-makers rule that worse-seeing or ‘first’ eyes should not be treated (an option considered but rejected by NICE during its deliberations prior to issuing its technology appraisal6), the worse-seeing eye will be treated in a proportion of patients (48% in the VPDT cohort study). Therefore, the CEA reported here (and other CEAs) has overstated the QALY gain and the cost-effectiveness of VPDT. It is important for future CEAs to assess the proportion of worse-seeing eyes that present for treatment in routine practice. We did not incorporate the proportion from the cohort study because it will almost certainly have changed over time as treatments and referral pathways for nAMD became more established.
Implications for practice
The main implications do not relate to the use of VPDT to treat nAMD because VPDT has now been superseded by the introduction of new treatments (see Current status of verteporfin photodynamic therapy and future research).
-
The fact that a much smaller number of treatments was administered than in the TAP trials suggests that treatment regimens receiving marketing authorisation may overestimate the intensity of treatment required. This observation may apply to other new health technologies.
-
Our ability to estimate effectiveness was limited by substantial loss to follow-up, which prevented comparisons with outcomes in RCTs and a systematic review of RCTs. This limitation should be carefully considered in the design of similar future studies if the effectiveness of an intervention is not dramatic and treatment or follow-up is scheduled over many months (conditions which we suspect may cause clinicians or patients not to adhere to the treatment regimen).
-
Verteporfin photodynamic therapy is less effective than newer technologies in treating nAMD. Its use should be limited to circumstances in which these newer technologies are contraindicated or refused by patients, and to categories of AMD such as polypoidal choroidopathy or other diseases with neovascularisation arising from the choroid, for example high myopia.
-
Licensing trials generally involve only one eye, and the benefit to a person from effective treatment for an eye will depend on whether or not the person’s visual function is limited by the vision in the treated eye. In terms of cost-effectiveness, an appraisal of a technology that benefits one eye should evaluate the benefit at the level of a person, not an eye.
-
The gradients of the relationships (a) between BCVA and EQ-5D utility and (b) between BCVA and HSS resource use/cost were shallower than most previous estimates. The consequences of these relationships for the appraisal of other technologies to treat eye diseases that impair vision need to be considered carefully.
Current status of verteporfin photodynamic therapy and future research
Verteporfin photodynamic therapy as a monotherapy for nAMD has been superseded by the introduction of molecular biologicals that target VEGF, a key molecule that promotes neovascularisation in AMD. The latter technology was introduced in 2006 following demonstration that monthly intravitreal injections of ranibizumab, a monoclonal antibody that inhibits VEGF, is vastly superior to both BSC and PDT. 72,73 Anti-VEGF therapies have also been shown to result in discernible improvements in HRQoL, outcomes which were not demonstrable with VPDT. VPDT combined with anti-VEGF therapy has been investigated, but the results suggest only a marginally improved benefit in terms of fewer treatments and no benefit in terms of visual acuity for nAMD. 84
Research recommendations outside the context of nAMD are:
-
Benefit from VPDT has been shown for visual acuity outcomes in specific nAMD variants such as polypoidal choroidopathy. VPDT continues to be used as first-line treatment in the management of other conditions such as neovascularisation due to myopia, inflammation and certain other choroidal diseases including central serous chorioretinopathy. Further studies are required to investigate the effectiveness of VPDT in these disease conditions. Similarly, the methods used in this study could be applied, cautiously, in order to estimate the cost-effectiveness of VPDT for these other eye conditions.
-
We observed differences in the rate of change in BCVA over time for a number of covariates. It would be interesting to investigate whether or not the effectiveness of new interventions for nAMD also varies in a similar way in relation to these covariates.
-
The study demonstrates the value of estimating the relationship between a common clinical outcome, that is BCVA, and other outcomes less often measured in RCTs but which are important for technology appraisals, for example HRQoL or HSS resource use. Because of the size of the study population, these relationships were estimated relatively precisely. Once established, relationships of this kind should be able to be applied to modelling of the effectiveness of other technologies, on the basis of estimates of effect from RCTs for the common clinical outcome. Further research could investigate how widely these relationships can be applied, for example to other diseases that reduce BCVA.
Chapter 9 Conclusions
The most notable finding of the VPDT cohort study was that a visual outcome comparable to that reported in the licensing trial was achieved, although we may have underestimated BCVA deterioration at 1 year because of attrition. This visual outcome was achieved despite a considerably lower retreatment frequency. This finding highlights that treatment regimens that receive marketing authorisation may overestimate the intensity of treatment required. Similar questions have arisen in the last 5 years about other technologies, for example the duration of treatment with Herceptin? (Roche) that women require after primary surgery and chemotherapy to achieve the benefits seen in licensing trials.
The VPDT study clearly showed that the small beneficial differences in BCVA between the treatment and control groups in the TAP licensing trials would translate into small gains in generic and vision-specific HRQoL and that the cost of achieving these outcomes was similar to the largest previously published estimate. The associations between BCVA and HRQoL, and between BCVA and HSS costs, represent an enduring legacy of the VPDT cohort study that are likely to be applicable to future evaluations of interventions to treat nAMD.
Even though VPDT is no longer the first line of treatment for nAMD, our findings continue to have relevance to clinical practice. In particular, the quantification of the influence of key covariates of age, cigarette smoking and status of the fellow eye on the trajectory of vision loss has added to our knowledge and suggests that these factors should be considered in the design and analysis of trials of treatments for nAMD. The findings will also continue to be of relevance and provide a reference framework for the estimation of cost-effectiveness in future trials and technology appraisals.
The future role of studies like the VPDT cohort study needs to considered carefully. The authorities that desired the VPDT cohort study did not state its purpose clearly in advance of commissioning the study, unlike other research commissioned by the National Institute for Health Research. For example, there was ambiguity in the wider health community about whether the study was being set up (a) to allow selected ophthalmologists, with the resources to participate, to treat patients with predominantly classic CNV lesions or (b) to provide a vehicle to allow such patients to receive treatment given the guidance by NICE. Despite a strong desire by the authorities for the study to achieve its aim of providing a quality-controlled implementation of VPDT, this was not matched by unequivocal guidance to commissioners, designated hospitals and ophthalmologists that participation was not voluntary. This lack of direction meant that the flow of resources needed to carry out the study at a participating site was perceived to be discretionary by some PCTs and hospital managers. Some ophthalmologists were also hostile to the study despite strong support from the Royal College of Ophthalmologists. If in the future the introduction of a new technology is made contingent on undertaking postlicensing data collection and research, a more explicit commitment from commissioners and policy-makers is needed.
The model of the VPDT cohort study can be used to meet the many needs of the NHS when considering how to implement an expensive therapy, for example provision of the education and training required to implement the therapy, treatment protocols to assure the quality of treatment, infrastructure for auditing implementation through standardised data acquisition and measures of the amount of treatment given and safety in routine practice. During the implementation of the cohort study, significant weaknesses in the capabilities of clinicians, clinical teams and trusts to understand the principles and practicality of gathering systematic information were identified. However, undertaking the study has created a network of resources and research competent sites currently participating in other portfolio studies.
Acknowledgements
The VPDT cohort study group thanks the following, without whose help the study would not have happened:
-
patients who participated
-
chairpersons of the VPDT Cohort Study Steering Committee (Mr Nick Astbury and Ms Gilli Vafidis); members of the Steering Committee (Professor Alan Bird, Mr Richard Wormald, Dr Daphne Austin, Mr Tom Bremridge, Mr Derek Busby)
-
grading staff at the network of angiogram-reading centres in the UK (Belfast Reading Centre: Andrew Milburn, Frank Picton, John Conlon, Roisin McQuade, Barbre Hamill; Liverpool Reading Centre: Stanley Spencer, David Parry, John Deane; Moorfields Reading Centre: Irene Leung, Momina Khatum, Meike Walcha)
-
members of staff at the LSHTM who helped with study and data management: Parminder Dhiman, Roli Gostelow, Sheila Harvey, Michele Intorcia, Abigail Taylor, Annette Powell, Shirley Stanley.
Contribution of authors
-
Professor BC Reeves designed the study with Professors U Chakravarthy and SP Harding and Dr R Grieve. He led the Data Management Centre at the LSHTM and oversaw the data analyses and their interpretation from an epidemiological perspective. He drafted the report with Professors Chakravarthy and Harding.
-
Professor SP Harding designed the study with Professors Chakravarthy and Reeves and Dr Grieve. He contributed to the initiation and recruitment of sites and the management of the project, including the training of research staff at sites. With colleagues, he led the establishment and management of NetwORC UK, developed specifically for this project. He contributed to the interpretation of the results and drafted the report with Professors Chakravarthy and Reeves.
-
Ms J Langham was the study manager at the Data Management Centre. She liaised with sites about protocol or data queries and managed the study on a day-to-day basis. She contributed to data management and descriptive statistics about the study cohort.
-
Dr R Grieve designed and conducted the CEA, interpreted the cost-effectiveness study and drafted this section of the report. He also contributed to the design of the overall study, and to the interpretation and reporting of the analysis of HRQoL.
-
Mr K Tomlin designed and developed the databases used for VPDT data collection at each of the participating hospitals and provided technical and data management support to each of the clinical teams. He contributed to data management, especially linking the clinical and grading data from NetwORC UK, and descriptive statistics about the study cohort.
-
Ms J Walker carried out the regression modelling of visual outcomes and the relationships between visual outcomes, HRQoL and resource use under the supervision of Dr Carpenter.
-
Dr C Guerriero helped to design the CEA and to interpreting and drafting the sections of the report on the CEA.
-
Dr J Carpenter advised on the statistical aspects of the study, at both the design and analysis stages, especially the regression modelling and the handling of missing data.
-
Dr WP Patton contributed to the setting up of NetwORC UK and managed all of the data coming from the reading centres to the Data Management Centre. He worked on the quality assurance processes, training and validation exercises, and contributed to the analyses.
-
Dr KA Muldrew contributed to the setting up of NetwORC UK. She designed the grading protocols with clinician colleagues, developed training and validation programmes and trained staff employed in the reading centres. She worked on the quality assurance processes and contributed to the analyses.
-
Dr T Peto is Head of the Moorfields Eye Hospital Reading Centre, part of NetwORC UK. She was involved in the setting up, design and execution of the imaging and grading protocol. She also contributed to the training and certification of graders, and advised clinicians about image interpretation, together with the clinical leads from the Belfast and the Liverpool reading centres.
-
Professor U Chakravarthy designed the study with Professors Harding and Reeves and Dr Grieve. She was the principal applicant in the effort to secure funding. She led the study and, with colleagues, the establishment and management of NetwORC UK, developed specifically for this project. She contributed to the interpretation of the results and drafted the report with Professors Harding and Reeves.
All authors contributed to the reporting of the study through this report and other peer-reviewed publications arising from the study.
Verteporfin photodynamic therapy cohort study contributors
Mr Douglas Newman, Addenbrookes Hospital, Cambridge.
Mr Martin Harris, General Hospital, Barnet, London.
Mr Richard Antcliffe, Royal United Hospital, Bath.
Ms Salwa Abugreen, Royal Blackburn Hospital, Blackburn.
Ms Clare Bailey, Bristol Eye Hospital, Bristol.
Professor Usha Chakravarthy, Royal Victoria Hospital, Belfast.
Mr Simon Morgan, Blackpool Victoria Hospital, Blackpool.
Mr Jonathon Gibson, Birmingham and Midlands Eye Hospital, Birmingham.
Mr Faruque Ganchi, Bradford Royal Infirmary, Bradford.
Mr Anthony Casswell, Sussex Eye Hospital, Brighton.
Mr Shafiq Rehman, Calderdale Royal Hospital, Calderdale, Yorkshire.
Mr Robert Johnston, Cheltenham General Hospital, Cheltenham.
Mr Sergio Pagliarini, University Hospitals Coventry and Warwickshire, Coventry.
Mr Hean-Choon Chen, Derbyshire Royal Infirmary, Derby.
Mrs Geeta Menon, Frimley Park Hospital, Frimley Park.
Mr Sakkaf Aftab and Mr Satish Kotta, Northern Lincolnshire and Goole NHS Trust, Goole.
Mr Gavin Walter, Harrogate District Hospital, Harrogate.
Mr Kevin Gregory-Evans, Western Eye Hospital, London.
Mr Nicholas Lee Hillingdon Hopsitals NHS Trust. Middlesex.
Mr Meen Zaman, Hull Royal Infirmary, Hull.
Mr Clive Edelsten, Ipswich Hospital NHS Trust, Ipswich.
Mr Victor Chong, King’s College Hospital, London.
Mr R J Pushpanathan, Queen Elizabeth Hospital, King’s Lynn.
Mr Simon Horgan, Kingston Hospital, Kingston.
Mr Martin McKibbin, Leeds Teaching Hospital, Leeds.
Professor Simon Harding, Royal Liverpool University Hospital, Liverpool.
Mr Frank Ahfat, Maidstone Hospital, Maidstone.
Mr Paulo Stanga, Spire Manchester Hospital, Manchester.
Mr Adnan Tufail, Moorfields Eye Hospital, Moorfields, London.
Mr James Talks, Newcastle Upon Tyne Hospitals NHS Trust, Newcastle.
Mr Andrew Glenn, Norfolk and Norwich University Hospital, Norwich.
Mr Alexander Foss, Queen’s Medical Centre, Nottingham.
Ms Susan Downes, Oxford Eye Hospital, Oxford.
Professor Bal Dhillon, Princess Alexandra Eye Pavillion, Edinburgh.
Ms Sarah-Lucie Watson, Royal Berkshire Hospital, Reading.
Mr Christopher Brand, Royal Hallamshire Hospital, Sheffield.
Mr Niral Karia, Southend Hospital, Southend.
Professor Andrew Lotery, Southampton University Hospital, Southampton.
Mr Myer Mark Yodaiken, Stepping Hill Hospital, Stockport.
Ms Consuela Moorman, Stoke Mandeville Hospital, Aylesbury.
Mr Michael Cole, Torbay Hospital, Torbay.
Mr Salim Natha, Leigh Infirmary, Wigan.
Mr Yit C Yang, Wolverhampton Eye Infirmary, Wolverhampton.
Mr Sal Rassam, Worthing Hospital, Worthing.
Mr Gavin Walters, Harrogate District Hospital, Harrogate.
Publications
Harding SP, Tomlin K, Reeves BC, Langham J, Walker J, Carpenter J, et al. Verteporfin photodynamic therapy cohort study: report 1: effectiveness and factors influencing outcomes. Ophthalmology 2009;116:e1–8.
Grieve R, Guerriero C, Walker J, Tomlin K, Langham J, Harding S, et al. Verteporfin photodynamic therapy cohort study: report 3: cost effectiveness and lessons for future evaluations. Ophthalmology 2009;116:2471–7.
Reeves BC, Langham J, Walker J, Grieve R, Chakravarthy U, Tomlin K, et al. Verteporfin photodynamic therapy cohort study: report 2: clinical measures of vision and health-related quality of life. Ophthalmology 2009;116:2463–70.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health
References
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1992;99:933-43.
- Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, et al. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology 1995;102:205-10.
- TAP Study Group . Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with verteporfin: one-year results of 2 randomised clinical trials. TAP report 1. Arch Ophthalmol 1999;117:1239-45.
- TAP Study Group . Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with verteporfin: two-year results of 2 randomised clinical trials. TAP report 2. Arch Ophthalmol 2001;119:198-207.
- Verteporfin in Photodynamic Therapy Study Group . Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial – VIP report no. 1. Ophthalmology 2001;108:841-52.
- National Institute for Clinical Excellence . The Clinical Effectiveness and Cost Effectiveness of Photodynamic Therapy for Age-Related Macular Degeneration; 2003. www.nice.org.uk/guidance/TA68 (accessed 30 November 2010).
- Bren L. The importance of patient-reported outcomes … It’s all about patients. FDA Consumer Magazine 2006;40.
- UK Department of Health . Guidance on the Routine Collection of Patient Reported Outcome Measures (PROMs); 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_092647 (accessed 30 November 2010).
- Brazier J, Ratcliffe J, Saloman JA, Tsuchiya A. In Measuring and valuing health benefits for economic evaluation. Oxford: Oxford University Press; 2007.
- Bradley C. Importance of differentiating health status from quality of life. Lancet 2001;357:7-8.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050-8.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Pol 1990;16:119-208.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92.
- Gold M, Siegel J, Russel L, Weinstein M, Gold MR, Siegel JE, et al. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996.
- National Institute for Clinical Excellence . Updated Guide to the Methods of Technology Appraisal; 2008. www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guidetothemethodsoftechnologyappraisal.jsp (accessed 4 October 2009).
- Stelmack J. Quality of life of low-vision patients and outcomes of low-vision rehabilitation. Optom Vis Sci 2001;78:335-42.
- Margolis MK, Coyne K, Kennedy-Martin T, Baker T, Schein O, Revicki DA. Vision-specific instruments for the assessment of health-related quality of life and visual functioning: a literature review. Pharmacoeconomics 2002;20:791-812.
- Brown GC, Sharma S, Brown MM, Kistler J. Utility values and age-related macular degeneration. Arch Ophthalmol 2000;118:47-51.
- Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2008;12.
- Harding SP, Tomlin K, Reeves BC, Langham J, Walker J, Carpenter J, et al. Verteporfin photodynamic therapy cohort study: report 1: effectiveness and factors influencing outcomes. Ophthalmology 2009;116:e1-8.
- Grieve R, Guerriero C, Walker J, Tomlin K, Langham J, Harding S, et al. Verteporfin photodynamic therapy cohort study: report 3: cost effectiveness and lessons for future evaluations. Ophthalmology 2009;116:2471-7.
- Reeves BC, Langham J, Walker J, Grieve R, Chakravarthy U, Tomlin K, et al. Verteporfin photodynamic therapy cohort study: report 2: clinical measures of vision and health-related quality of life. Ophthalmology 2009;116:2463-70.
- Cruess AF, Zlateva G, Xu X, Soubrane G, Pauleikhoff D, Lotery A, et al. Economic burden of bilateral neovascular age-related macular degeneration: multi-country observational study. Pharmacoeconomics 2008;26:57-73.
- Garattini L, Castelnuovo E, Lanzetta P, Viscarra C, Ricci E, Parazzini F, et al. Direct medical costs of age-related macular degeneration in italian hospital ophthalmology departments. A multicenter, prospective 1-year study. Eur J Health Econ 2004;5:22-7.
- Greiner RA. Cost of care for patients with age-related macular degeneration in Switzerland and cost-effectiveness of treatment with verteporfin therapy. Semin Ophthalmol 2001;16:218-22.
- Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C. Clinical effectiveness and cost-utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2003;7.
- Stirling B, Shoshanna S, Taryn S, Gold M. Has the time come for cost-effectiveness analysis in US health care?. Health Econ Pol Law 2009;4:425-43.
- Brown GC, Brown MM, Campanella J, Beauchamp GR. The cost–utility of photodynamic therapy in eyes with neovascular macular degeneration – a value-based reappraisal with 5-year data. Am J Ophthalmol 2005;140:679-87.
- Gold MR, Patrick DL, Torrance GW, Fryback DG, Hadorn DC, Kamlet MS, et al. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996.
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group . Verteporfin therapy of subfoveal choroidal neovascularization in patients with age related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes. Arch Ophthalmol 2002;120:1443-54.
- Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999;97:473-511.
- Meads C, Hyde C. What is the cost of blindness?. Br J Ophthalmol 2003;87:1201-4.
- Wright SE, Keeffe JE, Thies LS. Direct costs of blindness in australia. Clin Exp Ophthalmol 2000;28:140-2.
- Hart PM, Chakravarthy U, Mackenzie G, Chisholm IH, Bird AC, Stevenson MR, et al. Visual outcomes in the subfoveal radiotherapy study: a randomized controlled trial of teletherapy for age-related macular degeneration. Arch Ophthalmol 2002;120:1029-38.
- Ali F, Chan WC, Stevenson MR, Muldrew KA, Chakravarthy U. Morphometric analysis of angiograms of exudative lesions in age-related macular degeneration. Arch Ophthalmol 2004;122:710-15.
- Hogg R, Curry E, Muldrew A, Winder J, Stevenson M, McClure M, et al. Identification of lesion components that influence visual function in age-related macular degeneration. Br J Ophthalmol 2003;87:609-14.
- Verteporfin Roundtable 2000 and 2001 Participants . Treatment of age-related macular degeneration with photodynamic therapy (TAP) study group principal investigators; Verteporfin in photodynamic therapy (VIP) study group principal investigators. Guidelines for using verteporfin (visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina 2002;22:6-18.
- Early Treatment Diabetic Retinopathy Study Research Group . Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS Report Number 7. Ophthalmology 1991;98:741-56.
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci 1988;2:187-99.
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36):I. Conceptual framework and item selection. Med Care 1992;30:473-83.
- Higgins JPT, Altman DG, Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley; 2008.
- Stevenson MR, Hart PM, Chakravarthy U, Mackenzie G, Bird AC, Owens SL, et al. Subfoveal radiotherapy study. Visual functioning and quality of life in a randomised controlled clinical trial SFRADS report 2. Br J Ophthalmol 2005;89:1045-51.
- Desai P, Minassian DC, Reidy A. National cataract survey 1997–8: a report of the results of the clinical outcomes. Br J Ophthalmol 1999;83:1336-40.
- Browne JP, Hopkins C, Slack R, Topham J, Reeves BC, Lund V, et al. Health-related quality of life after polypectomy with and without additional surgery. Laryngoscope 2006;116:297-302.
- Langham J, Reeves BC, Lindsay KW, van der Meulen JH, Kirkpatrick PJ, Gholkar AR, et al. Variation in outcome after subarachnoid hemorrhage: a study of neurosurgical units in UK and Ireland. Stroke 2009;40:111-18.
- Maguire MG, Klein ML, Chamberlin JA. Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Macular Photocoagulation Study Group. Arch Ophthalmol 1986;104:503-12.
- Vingerling JR, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration and smoking. The Rotterdam Study. Arch Ophthalmol 1996;114:1193-6.
- Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye 2005;19:935-44.
- Guymer RH, Chiu AW, Lim L, Baird PN. HMG CoA reductase inhibitors (statins): do they have a role in age-related macular degeneration?. Surv Ophthalmol 2005;50:194-206.
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108:697-704.
- Doris H, Hart PM, Chakravarthy U, McCleland J, Stevenson M, Hudson C, et al. Relation between macular morphology and visual function in patients with choroidal neovascularisation of age related macular degeneration. Br J Ophthalmol 2001;85:184-8.
- Hendy J, Fulop N, Reeves BC, Hutchings A, Collin S. Implementing the National Programme for Information Technology: a qualitative study of progress in acute trusts. BMJ 2007;334:1360-4.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- Department of Health . NHS Reference Costs 2002. Appendix 1E. National Schedule of Reference Costs: NHS Trust Outpatient HRG Data. Ophthalmology HRG Label 2002.
- Curtis L. Unit costs of health and social care. University of Kent, Canterbury: Personal Social Service Research Unit; 2008.
- Mullahy J. Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J Health Econ 1998;17:247-81.
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation: a practical approach. Med Decis Making 1985;5:157-77.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Wormald R, Evans JR, Smeeth LL, Henshaw KS. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD002030.pub3.
- El Mallah MK, Chakravarthy U, Hart PM. Amblyopia: is visual loss permanent?. Br J Ophthalmol 2000;84:952-6.
- Swets J, Tanner WP, Birdsall TG. Decision processes in perception. Psychol Rev 1961;68:301-40.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464-75.
- Zwarenstein MJ, Treweek S. What kind of randomized trials do we need?. CMAJ 2009;180:998-1000.
- Espallargues M, Czoski-Murray CJ, Bansback NJ, Carlton J, Lewis GM, Hughes LA, et al. The impact of age-related macular degeneration on health status utility values. Invest Ophthalmol Vis Sci 2005;46:4016-23.
- Submacular Surgery Trials Research Group . Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST Report Number 19. Ophthalmic Epidemiol 2007;14:205-15.
- Bansback N, Czoski-Murray C, Carlton J, Lewis G, Hughes L, Espallargues M, et al. Determinants of health related quality of life and health state utility in patients with age related macular degeneration: the association of contrast sensitivity and visual acuity. Qual Life Res 2007;16:533-43.
- Health Utilities Inc . Health Utilities Index Mark 3 2008. www.healthutilities.com/hui3.htm (accessed 30 November 2010).
- Chakravarthy U, Stevenson M. Self-reported visual functioning and quality of life in age-related macular degeneration. Curr Opin Ophthalmol 2005;16:179-83.
- World Health Organization . Constitution of the World Health Organization. Basic Documents 2006. www.who.int/governance/eb/who_constitution_en.pdf (accessed 30 November 2010).
- Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and the SF-6D across seven patient groups. Health Econ 2004;13:873-84.
- Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the Health Utilities Index Mark 3 system. Med Care 2002;40:113-28.
- Stevens KJ, McCabe CJ, Brazier JE. Mapping between Visual Analogue Scale and Standard Gamble data: results from the UK Health Utilities Index 2 valuation survey. Health Econ 2007;15:527-33.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-44.
- Browne J, Jamieson L, Lewsey J, Van der Meulen J, Black N, Cairns J, et al. Patient Reported Outcome Measures (PROMs) in Elective Surgery 2007. www.lshtm.ac.uk/hsru/research/PROMs-Report-12-Dec-07.pdf (accessed 4 October 2009).
- Griffin SC, Barber JA, Manca A, Sculpher MJ, Thompson SG, Buxton MJ, et al. Cost effectiveness of clinically appropriate decisions on alternative treatments for angina pectoris: prospective observational study. BMJ 2007;334.
- Smith DH, Fenn P, Drummond M. Cost effectiveness of photodynamic therapy with verteporfin for age related macular degeneration: the UK case. Br J Ophthalmol 2004;88:1107-12.
- Fletcher EC, Lade RJ, Adewoyin T, Chong NV. Computerized model of cost-utility analysis for treatment of age-related macular degeneration. Ophthalmology 2008;115:2192-8.
- Sharma S, Brown GC, Brown MM, Hollands H, Shah GK. The cost-effectiveness of photodynamic therapy for fellow eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 2001;108:2051-9.
- Coleman AL, Yu F. Eye-related Medicare costs for patients with age-related macular degeneration from 1995 to 1999. Ophthalmology 2008;115:18-25.
- Smiddy WE. Relative cost of a line of vision in age-related macular degeneration. Ophthalmology 2007;114:847-54.
- Birchall W. Minimising the impact of visual impairment: training in use of low vision aids is important. BMJ 1999;319.
- Margrain TH. Minimising the impact of visual impairment: low vision aids are a simple way of alleviating impairment. BMJ 1999;318.
- Kiss CG, Simader C, Michels S, Schmidt-Erfurth U. Combination of verteporfin photodynamic therapy and ranibizumab: effects on retinal anatomy, choroidal perfusion and visual function in the protect study. Br J Ophthalmol 2008;92:1620-7.
Appendix 1 Protocol
Appendix 2 Verteporfin photodynamic therapy cohort study for the UK
Appendix 3 Key observations and recommendations arising from an interim project review of the study (1 July 2005)
It is worth noting that it was not possible to undertake a truly comprehensive review. In the writing up of this report, a number of other issues have come to light and been discussed but these have not been included. If the NCCHTA wish to further explore some of these issues then it would be possible to seek additional feedback from the individuals most closely involved both in the implementation of the Cohort Study and the service within the NHS.
Key observations
-
This is an example of how a number of influential people and organisations anticipated that there could be major problems with the introduction of a new treatment and aimed to deal with the issues proactively. Despite this foresight and attempts to avoid problems arising, introducing both the treatment and the cohort study has been one of the most difficult exercises.
-
The model developed in the cohort study can be used to meet many of the needs of the NHS in the introduction of an expensive therapy by providing postmarketing surveillance, a managed introduction to standard treatment protocols, evidence of effectiveness in routine clinical practice and data for optimisation of treatment protocols.
-
Two important communications problems arose which complicated implementation:
-
The NICE guidance did not explicitly refer to the cohort study.
-
The Department of Health did not explicitly support the cohort study nor did it issue clear directions in relation to it. In particular, this left those tasked with setting up the cohort study without any mandate to implement it in the NHS.
It is not clear why NICE and the DOH did not feel able to provide this explicit support.
-
-
It is not clear:
-
Why NICE did not recommend a RCT and why the National Coordinating Centre for Health Technology Assessment was willing to support a cohort study instead of a RCT.
-
Why the cohort study was seen as the best option.
-
-
There was initial hostility to the cohort study from many of the clinicians, in part because of their general reluctance to gather clinical data and in part because they perceived the cohort study to be a threat to their clinical freedom. Many, but not all, have since changed that view.
-
During the implementation of the cohort study, significant weaknesses in the capabilities of clinicians, clinical teams and trusts in understanding the principles and practicality of gathering systematic information were identified.
-
Commissioners were initially unsupportive of the cohort study. This probably stemmed from a lack of understanding of what it was trying to achieve. Some have remained indifferent to it. The lack of clarity about the aims and objectives of the cohort study meant that interests of commissioners were never made explicit.
-
Both patient groups and the manufacturer (Novartis) were also initially hostile to the cohort study, although patient groups were not initially averse to the idea of a RCT. From the manufacturer’s perspective, the findings of the cohort study could at best only confirm the status quo; at worst, the findings could seriously damage their commercial interest.
-
The size of the task was underestimated by all.
-
The designation processes undertaken locally were very confused and varied greatly.
Recommendations
-
The NHS needs to better anticipate the requirements needed to manage the introduction of new treatments particularly where establishing a new treatment is likely to be complex. (Note that a recommendation has already been made through the Specialised Commissioning Group. This recommendation proposes that NICE, for example, involves ‘on the ground’ NHS staff from both commissioning and provider organisations in discussion of the practical issues that may arise from specific pieces of guidance.)
-
Establishing postlicensing studies to evaluate the introduction and use of new treatments should not be considered without the explicit support of the Department of Health. This support needs to be transparent and public.
-
A clear set of aims and objectives must be published and made widely available before commencement of any cohort study to ensure that all stakeholders are fully informed and take ownership.
-
There needs to be better understanding of the role of cohort studies together with that of registries and databases.
-
Clinicians should be better informed of the need for robust, continuing postmarketing evaluation of clinical therapies after marketing authorisation in order to ensure that results from RCTs carried out for the purposes of licensing are generalizable in everyday clinical practice after the RCTs have ended. The importance of continuing to acquire data on the use of treatments in everyday practice must be stressed and clinicians strongly encouraged to collect these data particularly when introducing high cost medicines into the NHS.
-
The NHS could benefit from the development of good practice guidance in relation to designation and accreditation processes. (Some work on this has already begun in the West Midlands.)
-
The NHS could benefit from the development of good practice guidance on postlicensing collection of data on new treatments.
Overall, despite very serious obstacles, the cohort study has been established and has achieved the majority of its objectives with a large number of trusts providing good data. Wide geographical access is being provided to a high standard.
The review group strongly supports the development of on-going assessment of new treatments and for the health community and policy-makers to gain as much experience from the cohort study as possible. In spite of a complex set of problems that have been overcome to lesser or greater extent, the model that has been developed by the cohort study team provides a useful model for the future.
Current status of the cohort study
The study is running effectively with good rates of recruitment and data being submitted by most designated providers.
There has been slippage estimated at around 1 year at present. It was expected to reach a full recruitment rate by June 2004 but in reality only a handful of patients had been recruited by then. A year later, 1200–1500 patients had been recruited. Since the project review, a year’s extension to data collection has been provisionally funded. The study is expected to recruit until November 2007.
The study team continue to deal with issues around NHS implementation requiring a major time investment to sort out on-going problems.
Funding for treatment was not considered a major issue at the time of the review other than for the establishment of some dedicated clinics to promote rapid referral. However, since then it has become apparent that the current financial situation has resulted in clinical and nursing posts being frozen, waiting lists being allowed to develop and planned service developments being put on hold. In addition, the effects of national tariffs under Payment By Results are causing concern and generating uncertainties in the minds of managers in designated provider trusts. Indeed the whole issue of funding flows is confused. It is likely that there are problems at all levels: failure of some commissioners to fully fund the cohort study, failure of some trusts not to forward funds given for the cohort study and treatment to clinical teams and also some trusts receiving money for the cohort study but not entering patients into the study.
Compliance among clinicians with respect to the collection and submission of high-quality data continues to be an important problem. Some providers are receiving funding to provide photodynamic therapy but are not participating in the study. The Steering Group constantly monitor this situation.
Data collection is still being established at some sites.
A number of centres are reporting considerable delays in getting patients to treating centres – such that a significant percentage of referrals are deemed ineligible for treatment owing to the poor level of visual acuity in the eye being assessed, suggesting that earlier referral would have resulted in the patient receiving treatment.
Alternative treatments with antiangiogenic drugs with equivalent efficacy to VPDT and, potentially, wider application are due to be licensed for use in this condition during 2006–7. It can be anticipated that the implementation of these new treatments will present a major problem for the NHS as well as a threat to the cohort study.
List of abbreviations
- AE
- adverse event
- AMD
- age-related macular degeneration
- AR
- adverse reaction
- BCVA
- best-corrected monocular distance visual acuity
- BSC
- best supportive care
- CARF
- Central Angiographic Resource Facility
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- CNV
- choroidal neovascularisation
- CS
- contrast sensitivity
- DH
- designated hospital
- EFT
- eligible for TAP trial (as later defined)
- EQ-5D
- European Quality of Life-5 Dimensions
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- FA
- fundus fluorescein angiography
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HSS
- health and social services
- HUI3
- Health Utilities Index mark 3
- IFT
- ineligible for TAP trial (as later defined)
- IT
- information technology
- LogMAR
- logarithm of the minimum angle of resolution
- LSHTM
- London School of Hygiene and Tropical Medicine
- MCS
- mental component score (derived from the SF-36)
- MD
- macular degeneration
- nAMD
- neovascular (wet) age-related macular degeneration
- NEIVFQ
- National Eye Institute Vision Functioning Questionnaire
- NetwORC UK
- Network of Ophthalmic Reading Centres in the UK
- NICE
- National Institute for Health and Clinical Excellence (previously National Institute for Clinical Excellence)
- PCS
- physical component score (derived from the SF-36)
- PCT
- primary care trust
- PIS
- patient information sheet
- QALY
- quality-adjusted life-year
- RAP
- retinal angiomatous proliferation
- RCT
- randomised controlled trial
- SCG
- Specialised Commissioning Group
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SRVF
- self-reported visual functioning
- TAP
- Treatment of Age-related macular degeneration with Photodynamic therapy
- UNC
- unclassifiable with respect to TAP eligibility (as previously defined)
- VEGF
- vascular endothelial growth factor
- VIP
- verteporfin in photodynamic therapy
- VPDT
- verteporfin photodynamic therapy
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington