Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 95/02/99. The contractual start date was in July 2005. The draft report began editorial review in December 2010 and was accepted for publication in May 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Abdominal aortic aneurysm
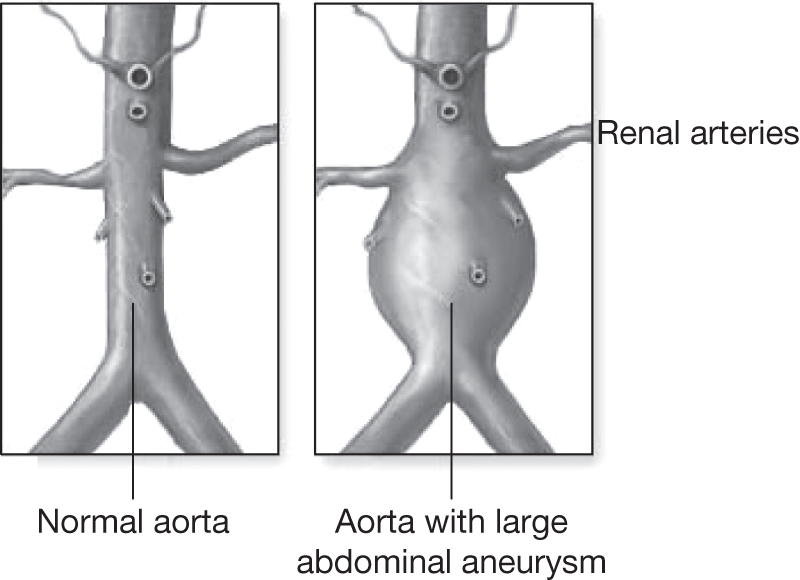
Abdominal aortic aneurysm (AAA) is a condition in which the abdominal segment of the aorta below the diaphragm becomes weakened and balloons outwards. Figure 1 shows a typically diseased aorta. This dilatation can continue for many years and in some cases it can lead to catastrophic rupture, which commonly results in death from internal haemorrhage unless emergency surgery can be performed in time to repair the damaged aorta. The aneurysmal dilatation of the aorta is commonly found below the renal arteries but expansion can also be found in the suprarenal segment and can sometimes extend upwards into the thoracic segment of the aorta above the diaphragm, and also downwards beyond the aortic bifurcation into the common iliac arteries.
FIGURE 1.
Abdominal aortic aneurysm.
The normal diameter of adult human abdominal aorta ranges from 1.0 to 2.5 cm at the level of the renal arteries and tapers as it approaches the aortic bifurcation. 1 Normal aortic diameters tend to be mainly dependent upon gender and body habitus, with narrower vessels in women and adults of smaller frame,2 although there is also some evidence to suggest variation between racial groups. 3–6 There have been a number of attempts to define the presence of an AAA7,8 but a common definition developed by McGregor et al. 9 classifies the abdominal aorta as aneurysmal if the diameter measures > 3.0 cm. Others have argued that a relative increase in diameter when compared with a proximal segment should be regarded as aneurysmal. 10,11 Currently, no universally accepted definition exists but, in clinical terms, if left untreated, AAAs have been known to grow to very large sizes, for example ≥ 15 cm, and in rare cases others have ruptured at more modest diameters as small as 3–4 cm.
Diagnosis of the condition is usually incidental as most aneurysms are asymptomatic. In some cases the aneurysm is known to become tender or lead to lower abdominal or back pain, and this can be exacerbated if the abdomen is pressed firmly. When the aneurysm becomes fairly large, AAA can be diagnosed by examining the abdomen of the supine patient and feeling for a large pulsatile mass, although diagnostic accuracy is reduced in obese subjects. Most AAAs are found when the patient is scanned for other conditions in the abdominal or pelvic areas. Given the asymptomatic nature of the disease, a considerable number of cases present as an emergency following rupture, which is thought to have only a 10–20% survival rate. 12 This is predominantly because many patients die rapidly in the community and only about a half of cases make it to hospital, with even fewer surviving an emergency operation.
Once diagnosed, non-ruptured aneurysms can be repaired surgically as a planned procedure but the successful management of AAA patients depends on the clinician finding the correct balance between careful surveillance of the aneurysm diameter until it enlarges to a point at which the risk of rupture exceeds the risk of death from elective surgery. However, making these kinds of predictions for an individual patient is extremely difficult and there is currently no proven medical therapy for primary prevention, cure or even retardation of expansion of the aneurysm.
Epidemiology of abdominal aortic aneurysm
Abdominal aortic aneurysm predominantly presents in later life and occurs in at least 5% of men aged > 65 years. 13 Larger screening studies in men aged between 65 and 85 years have found similar figures ranging from 4.5% to 7.7%. 14–18 Age appears to be the strongest factor relating to development of the disease, with the prevalence in males starting at about 2.6% in those aged 60–64 years and increasing to 6% in those aged 65–74 years and 9% in men aged ≥ 75 years. 19 However, these rates do not apply to women as the condition is three to four times more common in men than in women. 6,20,21 The reasons for this are not fully understood but are thought possibly to relate to the same biological mechanisms that lead to the higher rate of atherosclerotic disease in men than in premenopausal women. Further research has shown that, although women are rarely diagnosed with AAA, those who are found to have one experience significantly higher rupture, growth and operative mortality rates than men,20,22–25 and one laboratory study has shown a reduction in the tensile strength of female aortic tissue relative to male. 26
The prevalence of AAA is thought to differ between countries and racial groups, with the Asian subcontinent population exhibiting the lowest prevalence and Caucasians the highest. 27,28 One study from the USA suggests that although AAA is more prevalent in the white population, Afro-Americans with AAA show a higher mortality from the condition than Caucasians when adjusted for age. 5 Other studies have shown that the Asian population who tend to be of smaller stature than western Caucasians may be disadvantaged when being considered for endovascular aneurysm repair (EVAR), as the presence of smaller vessels is not conducive to easy deployment or long-term durability of the grafts. 29,30
The incidence of AAA presenting as elective or emergency cases has also been shown to have increased in England and Wales31 over the last 40 years, a trend that, it has been concluded, cannot be fully explained by improvements in scanning modalities and training in elective surgical techniques. Other research from Sweden corroborates this finding by demonstrating a marked increase in the incidence of ruptured AAA from 5.6 to 10.6 per 100,000 person-years between 1971 and 2004 despite a 100% increase in the number of elective repairs. 32 Similar trends have also been shown in the USA5 and Australia. 33
Possibly the most important environmental factor associated with the development and prognosis of AAA is smoking. A number of studies have demonstrated a strong relationship between smoking and the development of an AAA, and this strength of association is even higher than that found between smoking and cardiovascular disease. 34–38 The odds ratio (OR) between smokers and non-smokers for development of at least a 4.0-cm aneurysm has been measured to be as high as 5.57 [95% confidence interval (CI) 4.24 to 7.31]. 39 Furthermore, once the AAA has been diagnosed, smoking has been shown to increase the rate of expansion of the AAA as well as the risk of rupture. 40,41 There is also some evidence of a dose-related effect as development of AAA has been shown to be significantly positively associated with the number of years of smoking as well as significantly negatively associated with the number of years after smoking cessation. 35 These are all strong arguments for encouraging the cessation of smoking.
A host of other risk factors such as greater height, high cholesterol, hypertension and poor lung function have been suggested to increase the risk of AAA development, but not all have not demonstrated consistent results in other cohorts. 39,42,43 Another notable observation is that patients with diabetes appear to have a reduced incidence of AAA,39,44 and this is particularly interesting given that the prevalence of diabetes is higher in the Asian population relative to the Caucasian population. 45 Also, diabetes has been associated with a slower AAA growth rate. 40
Management and treatment of abdominal aortic aneurysm
Detection, screening and surveillance
Most conventional scanning modalities can be used for the diagnosis and follow-up of AAA; however, the most common methods are computerised tomography (CT) or B-mode ultrasound scanning. In recent years duplex ultrasound (B-mode with colour flow imaging) has become the main imaging choice for surveillance of the aneurysm, but many aneurysms are still detected incidentally on CT scan. Although there is good correlation between AAA diameters measured by duplex and CT, there is not good agreement, and differences in sizes have been estimated to be as large as 5 mm between modalities. 46,47 Over the last 10 years, the development of EVAR has meant that CT scans have become essential for the planning of the EVAR procedure and many argue that this should remain the optimal method for post-EVAR surveillance despite the increase in radiation dosage to the patient. Increased radiation exposure and use of potentially nephrotoxic contrast agents have prompted some to move to duplex ultrasound surveillance after EVAR, but there is little evidence to justify this practice, and the sensitivity and specificity when compared with CT have been shown to be suboptimal. 48 Magnetic resonance imaging is also possible but tends to be limited for post-EVAR surveillance, as a number of endovascular stents contain ferrous material. Aortography is also used but this is felt to be too invasive for routine use and tends to be selected in an emergency situation or if postoperative graft problems are suspected.
Over the last 20 years, the efficacy and feasibility of aneurysm screening has gained ground. In the UK, a national screening programme has been instigated for AAA in 65-year-old men. At present, this is being undertaken in a number of pilot centres in England and it is anticipated that a full national programme will be rolled out over the next 5–10 years. Initially, the feasibility of such a programme was demonstrated by the Gloucester Aneurysm Screening Programme (GASP), which has been running in the UK since 1990. 49,50 Subsequently, good evidence became available to support the implementation of a national screening programme for AAA, with two UK randomised trials demonstrating both clinical benefit (significant reduction in aneurysm-related mortality) as well as good cost-effectiveness with an incremental cost-effectiveness ratio (ICER) falling well within the limits of affordability recommended by the National Institute for Health and Clinical Excellence (NICE). 14,51–54 Similar clinical benefits and cost-effectiveness conclusions were drawn from another randomised screening trial based in Denmark. 55,56 In Western Australia, a further randomised trial also demonstrated clinical benefit but concluded that a national programme might be justified only in those who are at higher risk of developing an aneurysm, for example those with a family history of AAA or heavy smoking history. 17,57 A systematic review of the evidence for AAA screening was published in 200558 and a Cochrane review of all randomised trials was published in 2007. 59 Although there was some variation in the prevalence of AAA seen between studies, an overall clinical benefit was evident with a pooled 40% reduction in aneurysm-related mortality in the screened group. 59 Despite this, there is little evidence to suggest a reduction in all-cause mortality in any of the studies. In practical terms, aneurysms can be detected with good sensitivity and specificity using small portable ultrasound equipment in local general practitioner (GP) clinics. 60,61 Other research has shown that the optimal age for screening should be 65 years in men, as the probability of developing an aneurysm later in life is very low in aortas of normal size at this age. 19 There is come controversy over aneurysm screening in women, who have a three- to fourfold lower incidence of aortic aneurysm than men. Randomised evidence on aneurysm screening in women does not support the implementation of a national programme. 59,62 However, some have argued that screening might be cost-effective in women over time. 63
Medical therapy
A number of medical therapies have been proposed for the treatment of AAA but none has provided any consistent or sufficiently powerful evidence for the prevention or treatment of the disease. Given that a number of studies have found that hypertension is associated with the development of AAA, it is not surprising that antihypertensive therapies have been postulated as a potential medication for AAA. 64 One of the earliest groups of drugs to be tested for any association with AAA growth or rupture were beta-blockers, but although laboratory models65,66 provided encouraging evidence of a beneficial effect this has not translated convincingly into the general AAA population,67 although some benefit has been seen in patients with Marfan syndrome. 68 Angiotensin converting enzyme (ACE) inhibitors are also thought to provide benefit to patients with AAA69–71 but there is little evidence on their relationship with rupture and growth rates,64,72 and there is some evidence to suggest that the use of ACE inhibitors may be harmful in these patients. 73,74 Non-steroidal anti-inflammatory drugs, and, in particular, cyclo-oxygenase-2 (COX-2) inhibitors, have also been suggested as an agent for reducing AAA growth rate75 but this finding has not been reproduced consistently in other patient series. 76 Given the inflammatory nature of AAA, a number of randomised trials have investigated the impact of antibiotics on progression of the disease and a few small studies have generated encouraging results; however, larger studies are required to determine whether real benefit can be shown and whether long-term antibiotic use can be tolerated by most patients. 77–79
Currently, some of the most compelling evidence points towards statins as a potential treatment, with AAA growth rates shown to be reduced in patients taking statins,76,80 as well as a significant reduction in all-cause and cardiovascular mortality if patients are treated with statins prior to non-cardiac vascular surgery. 81 The mechanism for these effects is not understood, particularly as lipids have not been shown to have any impact on the development or progression of aneurysmal disease. 40,82 One study investigating the effects of statins has already been closed prematurely as recruitment of a sufficient number of control patients not taking statins was unfeasible. 83 In the absence of evidence from randomised trials on the effectiveness of statins it is difficult to draw any strong conclusions and, given the multiple unexplained coincidental benefits that statins appear to offer, it is unlikely that such a trial will ever be performed, particularly in elderly patients with other comorbidities.
A recent systematic review and meta-analysis investigating the impact of various medical therapies on growth rates of AAA has demonstrated little strong evidence for reduction in growth rates across a range of pharmaceutical products, including beta-blockers, other antihypertensive therapies, antibiotics and anti-inflammatory agents, including statin use. 84 Statins were the only therapy that showed encouraging results, with a random effects meta-analysis pooled difference in growth rate of –2.97 mm/year (95% CI –5.83 to –0.11 mm/year) between patients prescribed statins and control subjects.
Thus, given the lack of any proven benefit to medical therapy, current treatment methods are limited to interventional procedures, and at present three are available for the treatment of AAA: open surgical, endovascular or laparoscopic repair treatment.
Open surgical repair
This method is currently regarded as the standard surgical intervention for AAA and has been used since the early 1950s when Dubost et al. 85 presented the first case. The patient requires a general anaesthetic while a midline abdominal (or retroperitoneal) incision is made and the aneurysm is exposed. A clamp is fixed above the aneurysm, just below the renal arteries, and the aneurysmal sac is opened so that a synthetic piece of graft material, usually made from Dacron, can be sutured into place. The distal fixation is dependent upon the amount of aneurysmal disease and how far it extends beyond the aortic bifurcation. In most cases a straight tube graft is inserted, even if there is mild dilatation in the iliac system, but in some cases bifurcated or uni-iliac grafts are sutured beyond the aortic bifurcation. The old aneurysmal tissue is then loosely sewed back over the graft before surgical closure. The operation is regarded as a major procedure and carries a relatively high risk of mortality and morbidity, particularly in terms of cardiovascular end points.
However, elective repair is preferable to emergency repair, for which operative mortality rates have been estimated to range between 30% and 60%. 86–88 Many studies have estimated that the 30-day mortality of elective open repair and figures vary considerably within the UK and between countries. 89–94 Probably the most reliable source of unbiased data is randomised controlled trials (RCTs) but even here there is discrepancy between the UK Small Aneurysm Trial (UKSAT), which quotes a 30-day mortality of 5.6%,95 and the US Aneurysm Detection And Management (ADAM) trial, which quotes 2.7%. 96 National figures for 30-day operative mortality in the UK have been shown to be as high as 12% in district hospitals,91 whereas other cohorts from single-centre vascular specialist centres have quoted very small risks of < 2%. 97,98 Much of this variation is thought to relate to study design and measurement within hospital- or population-based cohorts;99 however, a review combining results from 64 studies estimated an average mortality rate of 5.5%. 100 Surgical training, operator experience and hospital volume are thought to be important factors, but UK practice at present allows open aneurysm repair to be performed by general surgeons who are not necessarily specialists in vascular surgery. 101–105 The Vascular Society of Great Britain and Ireland quotes the risk of 30-day death rate as 5% in their patient information documents,106 but it is stressed that there is considerable variation among patients as well as among hospitals within the UK. The most recent publication by Aylin et al. 107 compared the in-hospital elective AAA repair mortality using a number of sources, including the Hospital Episode Statistics (HES) database and the National Vascular Database (NVD), and found alarmingly high rates of 6.8% in the NVD group and 8.7% in the HES group.
Further difficulty lies in disentangling the influence of individual patient selection, which is also thought to be very important. In particular, patient fitness for general anaesthesia is an influential factor, principally in terms of cardiac and respiratory disease; however, renal function also appears to play an important role and is consistently included in the numerous risk scores that have been developed for prediction of postoperative death after AAA repair. 108–114
Following the open procedure, most patients require a relatively long period of convalescence, typically up to 3 months. Beyond this time, open repair is regarded as durable and the patient can be discharged without long-term follow-up, as the graft is expected to last for the remainder of the patient’s life. Nevertheless, there remains a small risk of other related complications, including incisional hernia, aortoenteric fistula, impotence, graft thrombosis, graft infection and, in rare cases, graft rupture. However, there is a suspicion that many complications may remain unreported, as demonstrated by a recent publication reporting Medicare data in the USA, which found the rate of laparotomy-related complications to be as high as 10% at 4 years. 115 Despite this, postoperative complications are thought to be infrequent and mandatory long-term follow-up is not felt to be necessary.
Endovascular aneurysm repair
In the early 1990s, a new endovascular method for correction of AAA emerged. Two independent endovascular pioneers, Volodos et al. in the Ukraine116 and Parodi et al. in Argentina,117 each developed a stent–graft for correction of the aneurysm in patients who were not thought to be fit enough for an open surgical repair. The method is less invasive than open repair, as it requires only two small incisions in the groin to expose the femoral arteries. The stent–graft system is then fed into the aorta via catheters and guidewires so that it can be positioned correctly above and below the aneurysmal segment of aorta. The location of the graft is imaged using radiological methods, with patients being exposed to relatively large doses of radiation and contrast agent. The fixation mechanism for the stent–graft is held within a removable sheath and, as this is pulled back, the fixation devices open and become lodged within the aortic wall. Some grafts use hooks and barbs to take hold of the aortic wall, whereas others use expandable stents that can be either self-expanding or require balloon angioplasty to ensure a good seal with the aortic wall.
Since the early 1990s, EVAR technology has developed intensely, with manufacturers becoming the main producers of stent–graft systems, and some would argue that for relatively simple anatomy the technology is now reaching a plateau. However, there are still anatomical constraints and not all patients are suited to the devices available on the market at present. Defining suitability for EVAR is a complex issue and is dependent on both manufacturer guidelines as well as individual clinician judgement. Numerous studies have shown varying degrees of suitability for EVAR, ranging from 25% to 75%;118–124 however, most studies struggle when trying to collect data for a reliable consecutive series of patients with AAA. Suitability for EVAR at the proximal end of the device is predominantly dependent on having an adequately long aortic neck between the top of the aneurysm and the bottom of the lowest renal artery as well as a neck that is no more than approximately 2–3 cm in diameter, depending on which graft manufacturer is selected. Other considerations include assessment of neck angulation, as well as the extent of thrombus or calcification in the section where the stent is to be deployed. Similar anatomical considerations are required in the distal segments of the iliac arteries, and the tortuosity of the vessels, as well as the minimum vessel diameter for access of the device, is also important. More recently, fenestrated and branched graft designs have become available for aortas with more challenging anatomy but these are expensive and do not reflect current standard EVAR practice. 125–127
Over the last 15 years, various manufacturers have developed a number of grafts, but all of these have required some form of technical revision and some have been withdrawn from the market due to high complication rates. 128 Given that EVAR is still a relatively young treatment modality, the long-term efficacy remains unknown and this has meant that most clinicians still monitor their patients following EVAR. At present, most patients are followed indefinitely until there is good evidence to justify discharge. There is considerable speculation about the best method of surveillance following EVAR, with some clinicians believing that duplex ultrasonography with a plain radiograph is sufficient, whereas others argue that CT scanning should remain compulsory until the long-term durability is known. 129,130
Many studies have reported the 30-day operative mortality of EVAR, and this appears to be lower than that reported for open repair. However, a recent meta-analysis of 163 studies has estimated a pooled rate of 3.3% (95% CI 2.9% to 3.6%), with wide variation between studies ranging from close to zero up to over 10%. 131 When compared with open surgical repair, there tends to be a relatively shorter convalescence following EVAR, with less need for intensive care or high-dependency unit (HDU) stays. 121 However, hospital costs can escalate later if reinterventions are required to correct any graft complications. The main disadvantage of EVAR is that the long-term durability of the grafts remains uncertain. Certainly the risks of leaks and other graft complications appear to be higher in patients undergoing EVAR treatment than in those patients undergoing open-repair treatment. 132
The first report on the use of EVAR in the emergency situation was published in 1994133 and, since then, certain specialist centres have reported promising results for operative mortality when compared with the 40–50% rates seen following open emergency repair. 134–139 However, the results from one small RCT that was forced to close early suggest that the benefit is marginal and generalisable only to haemodynamically stable patients, with logistical difficulties making the method difficult to offer in all cases. 140 The anatomical limitations of EVAR still exist in the emergency situation, although they tend to be less stringent, and there remains a need for rapid radiological assessment or CT scanning to determine suitability for the device and 24-hour radiological staff, which are not usually available in current routine practice. A number of other randomised trials are in progress, in particular the National Institute for Health Research (NIHR) Health Technology Assessment (HTA)-funded Immediate Management of the Patient with Rupture: Open Versus Endovascular repair (IMPROVE) trial, which started recruitment in 2009 and will be the largest trial (600 patients) comparing EVAR with open repair for ruptured AAA.
Laparoscopic repair
The method of laparoscopic aneurysm repair was first published in the early 1990s by Dion et al. 141 but has not penetrated the vascular surgical world to the same extent as EVAR. The technique requires a high degree of skill and, despite encouraging results with very low operative mortality,142 is still performed in only a few specialist centres. The work presented in this report does not include any research on laparoscopic repair and thus will not be detailed further.
Size threshold for repair of abdominal aortic aneurysm
Currently, there is clear agreement that very small aneurysms measuring < 4.0 cm in diameter do not require surgical intervention, as the risk of rupture has been shown to be very low and certainly < 1% per year. 143,144 Small aneurysms in the larger range of sizes, typically between 4.0 and 5.5 cm, three large multicentre randomised trials – one in the UK, one in the USA and another in Canada – were instigated to determine whether or not open surgical repair should be offered to patients with small aneurysms. 145,146 The Canadian trial was forced to close after recruitment of just 100 patients but the UKSAT and the ADAM trial subsequently met recruitment targets and have published both short- and long-term results. 95,96,145,147,148 Both studies concluded that for people with small AAAs measuring between 4.0 and 5.5 cm, regular ultrasound surveillance until the aneurysm reached 5.5 cm, became tender or grew fast (> 1.0 cm per year) was a safe and less expensive management policy than immediate elective surgery. One meta-analysis has combined the results from the UKSAT and the ADAM trial with pooled hazard ratios (HRs) for all-cause and AAA-related mortality of 1.01 (95% CI 0.77 to 1.32) and 0.78 (95% CI 0.56 to 1.10), respectively. 149 There is little evidence to suggest any detrimental impact on quality of life in patients under surveillance and a reduction in impotence was also seen in this group. 150 Despite the findings of these trials, there are still those who feel that repair of small AAA was justified,151 and one cost-effectiveness modelling analysis performed in the USA has inferred that surgery may be cost-effective in patients aged < 72 years with AAAs between 4.5 and 5.5 cm in diameter. 152 During the 1990s, the use of EVAR became increasingly popular and some argued that EVAR may be justified in small AAA. This speculation led to the instigation of two further trials – the European Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) trial153 and the American Positive Impact of endoVascular Options for Treating Aneurysm earLy (PIVOTAL) trial154 – both of which were company-funded randomised trials comparing EVAR against surveillance in patients with small AAAs (4.0–5.5 cm). The results from these trials have been released recently with no evidence to support EVAR in small AAA. 155,156 Thus, current evidence suggests that intervention for the aneurysm may be delayed until the aneurysm reaches 5.5 cm, becomes tender or grows fast (> 1.0 cm per year).
Current trials comparing treatments for large abdominal aortic aneurysm
The results of the trials in small aneurysms have provided evidence that small AAAs of < 5.5 cm can be monitored safely. The current debate relating to large aneurysms is, first, whether they should be treated with open or endovascular repair and, second, whether endovascular repair is justified in patients when open repair is not an option, usually on the grounds of poor anaesthetic fitness. A number of trials have been instigated to try and answer the first question but only one randomised trial (EVAR trial 2) has been set up to assess the role of EVAR in patients considered unfit for open repair. This report focuses on the results from the UK EVAR trials 1 and 2 but a brief summary of the other three trials follows, with Table 1 summarising all of the trials.
| EVAR trial 1 (UK) | DREAM trial (Netherlands) | ACE trial (France) | OVER trial (USA) | |
|---|---|---|---|---|
| Recruitment period | 1999–2004 | 2000–3 | 2003–8 | 2002–7 |
| Recruitment target | 900 | 400 | 600 | 900 |
| Final recruitment | 1252 | 351 | 306 | 881 |
| Age entry criteria | ≥ 60 years | Any | Any | Any |
| Gender entry criteria | Both | Both | Both | Mainly male |
| AAA diameter entry criteria | ≥ 5.5 cm | ≥ 5.0 cm | ≥ 5.0 cm for men | ≥ 5.0-cm AAA |
| ≥ 4.5 cm for women | ≥ 3.0-cm CIA | |||
| ≥ 4.5-cm AAA with fast growth | ||||
| Other entry criteria | None | Life expectancy > 2 years | Neck length > 15 mm | None |
| Neck angle < 60° |
The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial
Soon after the EVAR trials began, a trial of similar protocol to EVAR trial 1 was started in the Netherlands and the trial methods have been published. 157 The target trial recruitment was 400 patients from 24 Dutch and four Belgian hospitals, but recruitment closed when only 351 patients had been randomised to receive either EVAR (n = 173) or open repair (n = 178). Trial entry criteria differed slightly from EVAR trial 1, with slightly smaller aneurysms (at least 5.0 cm) being eligible for inclusion. Operative mortality and longer-term results have been published. 158–160 Further data published on sexual dysfunction after each type of operation have shown that both treatments lead to some reduction in sexual function but this recovers more quickly following EVAR;161 however, this benefit is moderated somewhat by other data demonstrating a significant quality of life benefit in the open-repair group after 6 months. 162
The French Anévrisme de l’aorte abdominale, Chirurgie versus Endoprothèse (ACE) trial
This trial commenced in 2003 after experiencing significant bureaucratic start-up delays. 163 The trial struggled with recruitment, which was further hindered by the publication of favourable 30-day mortality results for EVAR in both EVAR trial 1 and the DREAM trial in 2004. EVAR funding issues continued to hamper recruitment, which eventually closed in 2008 when just over 300 patients had been recruited. In contrast to the three other trials there was no difference in operative mortality between the open and the endovascular repair arms, 0.6% versus 1.2%, respectively. 164
Open Versus Endovascular Repair (OVER) trial
This US trial recruited patients across 43 centres between October 2002 and 2008. Patients who were considered fit for a general anaesthetic with AAAs measuring at least 5.0 cm and who were anatomically suitable for EVAR were recruited from the Veterans Affairs Program and randomised to receive either EVAR (n = 444) or open repair (n = 437). The protocol is similar to the EVAR and DREAM trials, although the patients are predominantly male, marginally younger and have smaller aneurysms. Operative mortality and 2-year outcomes were published in 2009,165 and long-term results are due for release in 2013.
Registry data
Registries act as an important and necessary complement to RCTs and this is certainly the case with developing technologies such as EVAR. Numerous registries have been set up around the world, usually to monitor national case load and outcome; however, there is enthusiasm to collaborate on an international registry that has recently tested the practicalities of managing such an extensive database by starting with AAA repairs. 166 In the UK, generic national registries for all treatments include the HES database, as well as the Dr Foster registry, which provides data on clinicians and hospitals across the UK. In 2000, The Vascular Society of Great Britain and Ireland instigated the NVD, which is specific to vascular surgery, with reports available online. 167 There are also a number of registries that are exclusively for endovascular repair of AAA. The Registry for Endovascular Treatment of Aneurysms (RETA) was based in Sheffield, overseen by the Registry Committee of the Vascular Society of Great Britain and Ireland. It began collating data on endovascular repairs performed in the UK in 1996 and reports were available via the Vascular Society website. Follow-up has now closed for this registry but the results have been published widely and the organisers were very involved in the setting up of the UK EVAR trials. 168–170 One of the largest registries that started in 1996 is The EUROpean collaborators on Stent–graft Techniques for abdominal aortic Aneurysm Repair (EUROSTAR), which collates EVAR data from over 20 European countries and has been used extensively as a data source for many publications. 171,172 The EUROSTAR Secretariat is based in Eindhoven, the Netherlands, and data on over 6000 EVAR cases have been collected.
Other international registries include the commercially funded Lifeline registry in the USA, which has been running since 1998 and concentrates on pooling the data from trials on different manufactured EVAR devices, but it also holds data on corresponding open surgical controls. 173–175 In Australia in 1999, the Medical Services Advisory Committee (MSAC) and the Australian Government Department of Health and Ageing recommended that a registry, rather than a RCT, should be used to monitor the impact of endovascular repair in their country. This is managed at present by the Australian Safety and Efficacy Register of New Interventional Procedures–Surgical (ASERNIP-S) and, to date, just under 1000 cases have been registered, with regular data reports available on the internet. 176
Although these registries are very helpful in providing summary data and preliminary results about the performance of hospitals, surgeons and types of procedure, none of them is mandatory and selection bias is a common problem with registry data. The reliability of the data is often further compromised by insufficient funding, which can lead to poor data collection and reduced enthusiasm of the participants to submit new cases or follow up old ones. Despite validation of the databases, none have been able to document all cases of interest, and a recent audit of the NVD reported that only about a half of all vascular cases have been submitted. 107 It has also been suggested that missing cases tend not to be missing at random, with the worst outcome data often excluded. 177 For these reasons, registries are not able to answer all the pertinent questions relating to treatments but, in combination with well-conducted RCTs, are likely to provide the best evidence for making public health decisions.
Objectives of the UK EVAR trials
In 1996, the Department of Health issued a call for research into the efficacy of EVAR. This was followed by a number of years of consultation on study design and ethical issues, and in July 1999 the UK EVAR trials were commissioned by the NHS Research and Development Health Technology Assessment Programme, now renamed as the NIHR Health Technology Assessment programme. Initially, the trials were funded for 4 years, from July 1999 to 2003. A 2-year extension was granted to ensure that recruitment targets were met and subsequently a long-term follow-up grant was awarded for a further 5 years of follow-up until July 2010. The trial objectives were to assess the safety and efficacy of EVAR against current standard treatment in the management of large AAAs measuring at least 5.5 cm in diameter according to a CT scan. Two trials were instigated: EVAR trial 1 would compare EVAR against open repair in patients who were considered fit and suitable for both procedures and EVAR trial 2 would compare EVAR against no intervention for patients who were considered suitable for EVAR but unfit for open repair. The primary outcome was mortality for both trials with secondary outcomes of graft-related complications and reinterventions as well as health-related quality of life (HRQoL), adverse events, renal function, costs and cost-effectiveness.
Chapter 2 Methods for UK EVAR trials
Organisational structure of the trials and relevant committees
The trials are a joint collaboration of many surgeons, radiologists, clinical trials specialists and vascular health professionals. A full list of trial participants is provided in Appendix 1. The trials were managed centrally by the principal investigator (Professor Roger Greenhalgh), the trial manager (Dr Louise Brown) and Professor Janet Powell (co-applicant), who are based at the Charing Cross Hospital site of Imperial College London. Statistical expertise was provided by Professor Simon Thompson, Director of the Medical Research Council Biostatistics Unit in Cambridge, and costs and cost-effectiveness expertise were provided by Professor Mark Sculpher and Mr David Epstein from the University or York, with input from Professor Martin Buxton from the Centre for Health Economics at the University of Brunel. Figure 2 presents the structure of the trial committees in relation to the sponsor and regulatory bodies. The minutes of all of the committee meetings are archived at the central trial office. Dates of the meetings are provided in Appendix 2. The protocol is provided in Appendix 3.
FIGURE 2.
Structure of EVAR trial committees.
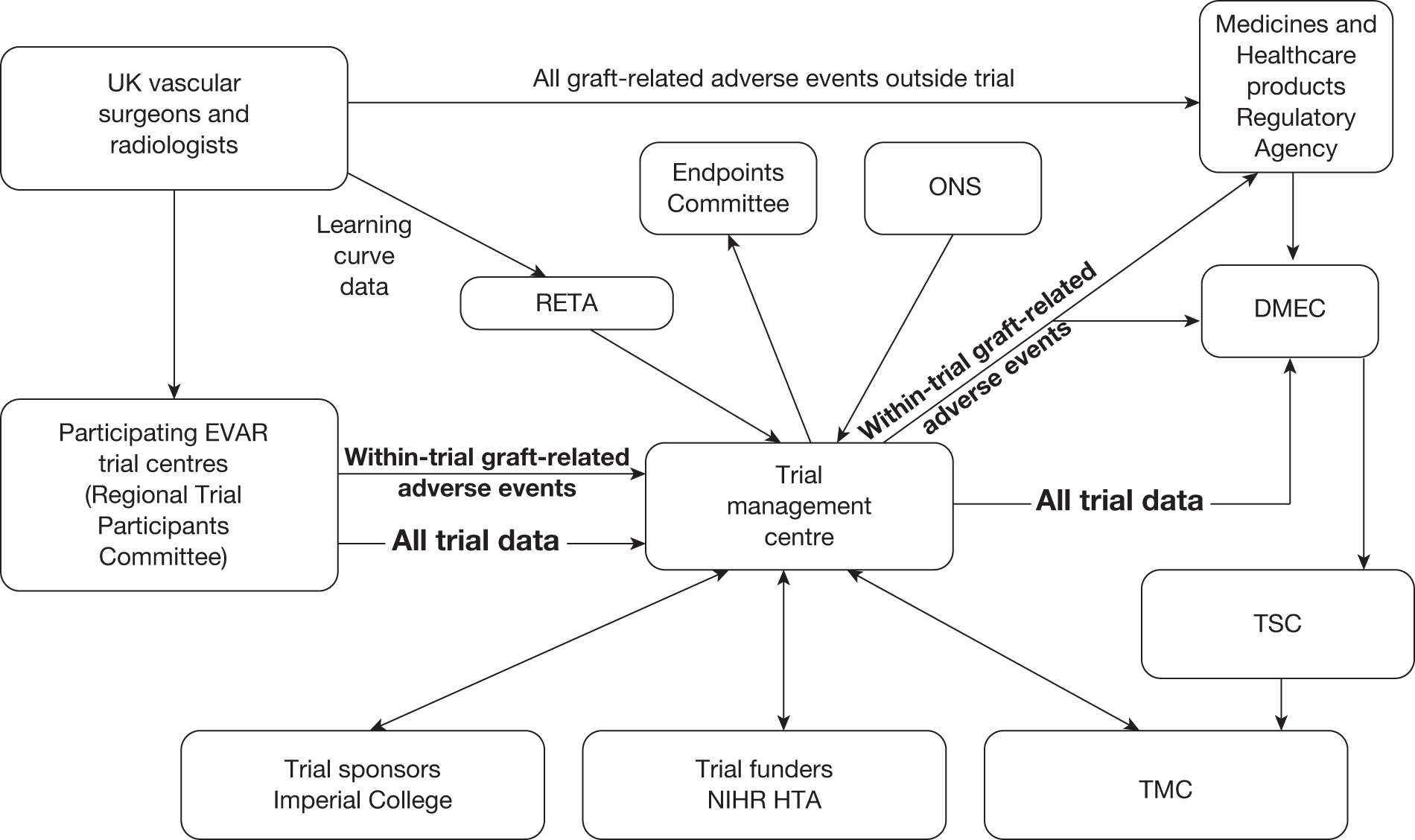
Data Monitoring and Ethics Committee
We are indebted to the late Professor PA Poole-Wilson (Professor of Cardiology, National Heart & Lung Institute, Imperial College London), who chaired this committee on behalf of the trials. Membership included two representatives of The Vascular Society of Great Britain and Ireland, namely Professor CV Ruckley (Edinburgh) and Mr WB Campbell (Exeter) and also two representatives of The British Society of Interventional Radiology (BSIR), namely Dr MRE Dean (Shrewsbury) and Dr MST Ruttley (Cardiff), as agreed with their councils. Dr EC Coles (Cardiff) acted as the statistical representative for the Data Monitoring and Ethics Committee (DMEC). Data on trial progress as well as mortality results at prespecified time points were provided to DMEC by the trial manager and audit of these data was confidential and never disclosed outside the committee. The DMEC communicated with the Trial Steering Committee (TSC).
Trial Steering Committee
This was chaired by Professor Richard Lilford (University of Birmingham) and included Roger Greenhalgh for the applicants and Trial Management Committee (TMC), as well as surgical and radiological input supplied by Professor Sir Peter Bell (Leicester) and Dr Simon Whitaker (Nottingham). The role of the committee was to liaise between the DMEC and TMC and oversee any issues relating to the progress of the trials or needs for additional funding.
Trial Management Committee
This was concerned with the day-to-day running of the EVAR trials and related to both the DMEC and TSC committees. It was chaired by Roger Greenhalgh and included Simon Thompson (statistics), Janet Powell (vascular biology), Ian Russell (HRQoL), Jonathan Beard (RETA), Peter Harris (EUROSTAR), John Rose (interventional radiology) and Martin Buxton (costs). During the course of the trial, Ian Russell moved to another institution and his role was replaced by Mark Sculpher and his colleague David Epstein from the University of York, who collaborated with Martin Buxton on the cost and cost-effectiveness issues relating to the trials. Louise Brown (Trial Manager) attended all meetings to present on trial progress and any problematic issues.
Regional Trial Participants Committee
This included a surgical and radiological representative as well as a co-ordinator from each participating centre and was convened at the request of trial centres or the trial management centre whenever the need arose, but usually the members met at the annual meetings of The Vascular Society and BSIR to update participants on trial progress or obtain feedback on any pragmatic running issues.
Endpoints Committee
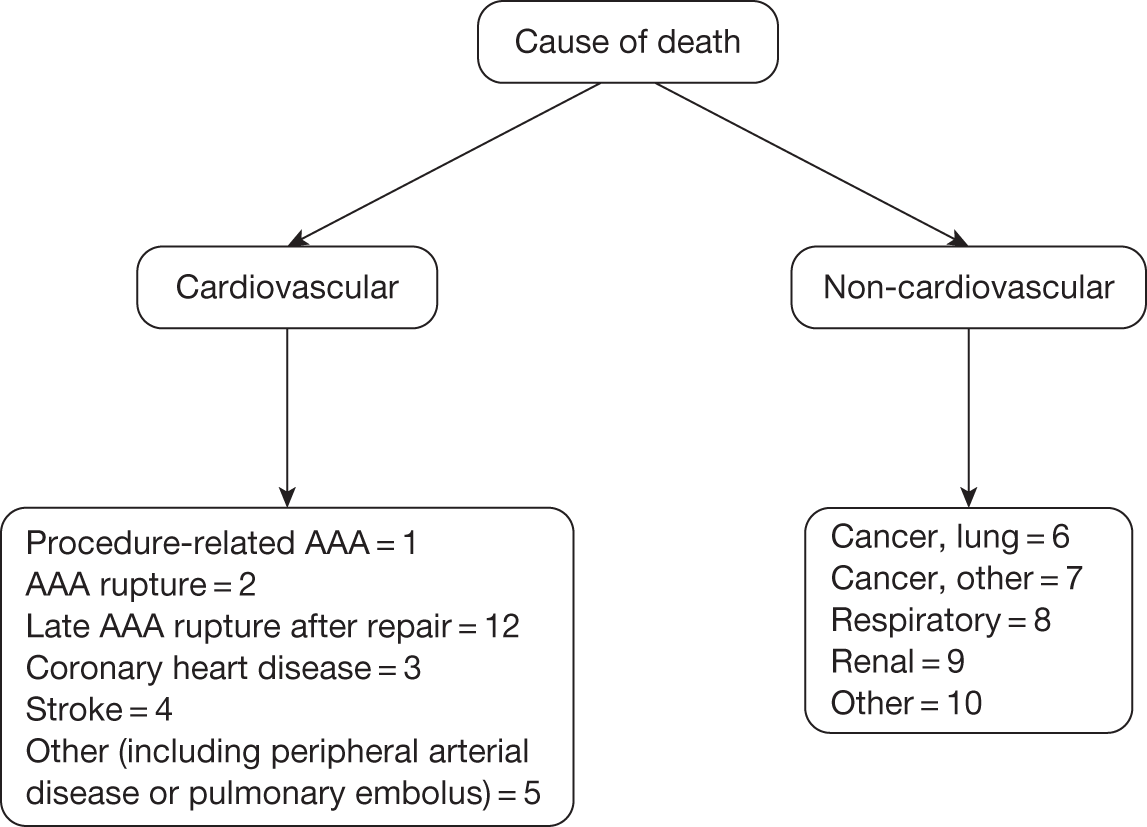
This committee was chaired by Professor Janet Powell and consisted of an independent vascular surgeon who was not participating in trial recruitment (Professor Alison Halliday) and a consultant cardiologist (Dr Simon Gibbs). All death certificates were centrally coded at the Office for National Statistics (ONS) and these were reviewed by this committee in relation to any aneurysm-related procedures. The committee were blinded to randomised group but all available data relating to the death and a primary underlying cause of death were classified according to the groupings presented in Figure 3, where death codes 1, 2 and 12 were classified as aneurysm related. Aneurysm-related deaths were defined as all deaths occurring within 30 days of the primary AAA repair or any reintervention for a graft-related complication unless over-ruled by post-mortem findings or a separate procedure (unrelated to the AAA) that took place between the aneurysm intervention and death (code 1); all deaths from rupture of an unrepaired AAA (code 2) and all deaths from rupture of a repaired AAA, usually endograft rupture (code 12). In addition, late complications of AAA repair, such as aortoduodenal fistula or bowel obstruction, were recorded as procedure-related deaths (code 1).
FIGURE 3.
Classification of deaths codes assigned by the Endpoints Committee.
Learning curve and eligibility of participating centres
The setting up of the trials used invaluable data provided by the two main registries that had been running since 1996 and monitoring the performance of EVAR in the UK and the rest of Europe, namely the RETA and the EUROSTAR collaboration. There was representation from both of these registries on the EVAR TMC.
The UK Registry for Endovascular Treatment of Aneurysms
The national RETA registry, based at the Northern General Hospital in Sheffield, was initiated in January 1996 to audit ‘in-house’ and commercially available EVAR systems deployed within the UK. Annual audits were conducted and reports made available to the EVAR TMC, principally to be advised when centres were trained. As the EVAR technique was felt to be highly operator and hospital dependent, it was felt that a learning curve of training should be established to ensure that basic expertise had been acquired by the operators before EVAR could compete realistically with open repair as part of a trial comparison. Four specialist vascular centres were nominated as training hospitals to offer expertise and support in getting other hospitals through this learning curve, namely The Queens Medical Centre in Nottingham, The Royal Liverpool University Hospital, The Freeman Hospital in Newcastle and Leicester Royal Infirmary. Thus, the EVAR TMC met regularly to monitor progress of the trials and demanded that each centre had performed at least 20 EVAR procedures according to RETA before they were able to participate in the trials. It was also felt strongly that endovascular repair would achieve the best results if it was regarded as a multidisciplinary procedure with good collaboration between vascular surgeons and interventional radiologists. Therefore, each centre was required to nominate a vascular surgeon, interventional radiologist and trial co-ordinator as trial participants for their hospital. At the start of the trials in 1999, only 13 centres were eligible for participation in the trials but during the 5 years of recruitment a further 28 centres met the eligibility criteria. However, although 41 centres were eligible, only 38 centres actually entered patients into the trials before recruitment closed in August 2004.
EUROpean collaborators on Stent–graft Techniques for abdominal aortic Aneurysm Repair (EUROSTAR)
The EUROSTAR project was launched in 1996 to audit prospectively the performance of EVAR across 14 European countries. 171 At the start of the EVAR trials, a long-term durability analysis of EVAR (up to 4 years of follow-up) was performed on 2464 patients from the EUROSTAR registry and this demonstrated a 1% annual rupture rate for EVAR devices deployed in small and large aneurysms across Europe. 178 A similar rupture rate had been observed during surveillance of patients randomised in the UKSAT,179 and although these cohorts of patients were quite different there was concern that EVAR may do little to improve upon the natural history of AAA. Subsequent reports from EUROSTAR and other EVAR series have not provided any evidence of a substantial reduction in this rupture rate and there is concern that the long-term rupture rate may be even higher. 180,181
The role of the UK Small Aneurysm Trial (UKSAT)
The results of the UKSAT have been reported in a number of publications over the last 10 years, with the final statement on the role of early elective open repair in small AAA being published recently. 148 This trial was instrumental in defining the aneurysm diameter for inclusion in the EVAR trials, as it had shown that aneurysms could be safely monitored until they grew to 5.5 cm, when intervention could be considered. The trial showed that although there was a slight increase in the 30-day operative mortality for patients who underwent elective or emergency surgery later on in the surveillance group (7.2%), this increase was not statistically significantly different from that seen in patients who went for immediate elective surgery soon after randomisation into the trial (5.5%), χ2 (p-value = 0.28). Further corroborating evidence has also come from the ADAM96 trial and thus the aneurysm diameter for inclusion in the trials was set at 5.5 cm, although the measurement modality had switched from ultrasonography in the UKSAT to CT scan in the EVAR trials.
The need for two separate trials: EVAR trials 1 and 2
Endovascular repair was originally intended for use in patients who were regarded as too unfit for a conventional open procedure but, as the technology advanced, clinicians started offering it to fitter patients as the use of hospital facilities and the length of postoperative recovery seemed to be much improved over the conventional open operation. By the time the EVAR trials began in 1999 it was estimated that approximately 75% of patients undergoing EVAR were fit, whereas the remaining 25% were considered unfit for an open repair. Consequently, it was thought appropriate to pose two separate questions: first, whether or not EVAR was at least as good as open repair in patients considered fit for an open repair, and, second, whether or not EVAR with best medical therapy offered any benefit over best medical therapy alone in patients considered unfit for an open repair. Unfortunately, by the time EVAR trial 2 reported in 2005, it was clear that best medical therapy had not been implemented very successfully, with only 56% of patients on aspirin and 39% on a statin. Thus, it was decided that the description ‘EVAR versus no intervention’ was more appropriate for the EVAR trial 2 comparison.
Outcome measures
The primary end point for both trials was mortality, which included assessment of all-cause, aneurysm-related and 30-day operative mortalities.
All-cause mortality for EVAR trial 1
When the EVAR trials were being devised, the UKSAT patient data were used to estimate expected mortality rates for patients with AAA. Patients randomised to open repair in the UKSAT experienced an annual all-cause mortality of 7.1%. In the EVAR trials, patients were undergoing AAA repair for larger aneurysms and thus an annual mortality rate of 7.5% was assumed. If EVAR could reduce this mortality to 5% per year then EVAR might be justified as a viable treatment alternative for AAA. Similar mortality results had been reported in the EUROSTAR and RETA registries. At the start of the trials, funding was requested for follow-up to April 2005 and this would accumulate an average of 3.33 years’ follow-up per patient. To achieve 80% power at the 5% significance level, a total of 900 patients would be required to detect this 2.5% difference in annual mortality between the groups.
All-cause mortality for EVAR trial 2
Patients with large AAAs who were considered unfit for open repair in the UKSAT had been followed up for AAA growth and rupture and were shown to have an annual all-cause mortality of 25%. The RETA registry provided data on patients who were considered unfit for open repair and who had been treated with EVAR and these data showed that such patients experienced an annual all-cause mortality of 15%. To be consistent with EVAR trial 1, it was decided that patient follow-up would continue until April 2005, when an average of 3.33 years’ follow-up had been accrued per patient. To achieve 95% power at the 5% significance level, a total of 280 patients would be required to detect this 10% difference in annual mortality between the groups.
Thirty-day operative mortality
From the UKSAT data, 30-day operative mortality was calculated for patients who were randomised to observation but whose aortic aneurysms subsequently grew to > 5.5 cm, at which point surgery was performed (n = 191). Eleven were dead at 30 days, leading to a 30-day operative mortality of 5.8%. Power calculations for 30-day operative mortality in EVAR trial 1 were based on 90% power at the 5% significance level using 5.8% for open repair and 1.5% for EVAR, and these indicated that 443 patients would be required in each arm, leading to a total of 900 patients to detect this difference, should it exist.
Aneurysm-related mortality
To increase the power of the trials, it was decided that disease-specific mortality should also be used as an outcome measure to complement the all-cause mortality results, as this is often a more sensitive measure of effect. An Endpoints Committee was convened to scrutinise all of the death certificates and ascribe the cause of death according to a predefined protocol. The underlying cause of death on the death certificates provided by the ONS was centrally coded by ONS according to the International Classification of Diseases and Health Related Problems, Version 10 (ICD-10). The Endpoints Committee defined an aneurysm-related death as a death from any cause within 30 days of any intervention for the aneurysm or if the underlying cause of death on the death certificate was coded using ICD-10 codes 713–719, which includes ruptured AAA.
Graft durability
The incidence of graft-related complications and reinterventions was monitored for both types of AAA repair. Annual CT scans were selected as the method of surveillance to record the aneurysm sac and other postoperative aortic and iliac measurements for all patients in the trials.
Endoleaks in EVAR patients were classified according to an amended version of the White and May classification:182
-
endoleak type 1 perigraft leak, perigraft channel or graft-related endoleak at proximal or distal end
-
endoleak type 2 retrograde endoleak, collateral flow, retroleak or non-grade-related endoleak, leak from patent lumbar, inferior mesenteric or intercostal arteries
-
endoleak type 3 fabric tear, modular disconnection or poor seal between subparts, stent fracture or separation
-
endotension presence of continued sac expansion without any detected graft complication.
Incidence of graft migration, rupture, anastomotic aneurysm, thrombosis, stenosis, graft infection and renal infarction was also monitored. Collaboration with the Medicines and Healthcare products Regulatory Agency (MHRA) was instigated early in the trial. At the time the trials commenced, the reporting of graft-related complications to the MHRA was not mandatory and in order to ensure complete reporting of these events it was agreed that the trial manager would send details of any graft-related complications detected in the trials to the MHRA, which also established links with DMEC to alert them of any potentially important safety issues relating to particular EVAR devices.
Renal function
Serum creatinine was measured at baseline and annually for all patients in both trials to investigate whether or not the use of contrast agents in the deployment of EVAR devices has a detrimental effect on renal function.
Health-related quality of life
The HRQoL assessment was completed by patients in the form of a full questionnaire at recruitment and subsequently 1, 3 and 12 months after surgery or at the beginning of medical treatment as appropriate. For long-term economic evaluation, a EuroQol questionnaire continued to be completed each year until follow-up closed at the end of 2009. The full questionnaire includes three generic instruments: the Short-Form questionnaire-36 items (SF-36) Health Survey,183 European Quality of Life-5 Dimensions (EQ-5D) version 2 (visual analogue scale and utility index),184 and the State–Trait Anxiety questionnaire, selected to assess patient anxiety. Unfortunately there is no specific instrument designed to measure HRQoL in patients suffering from AAA. Thus, it was proposed that the most relevant specific instrument would be the Patient-Generated Index (PGI). This quasi-specific HRQoL instrument focuses on the concerns of the individual patient with a given condition rather than the concerns derived by the investigator for the typical patient with that condition. 185
Economic evaluation
Hospital inpatient data for aneurysm-related procedures were collected for all patients from randomisation. Resource use was estimated from the results of a survey questionnaire that was sent to trial centres in May 2004 requesting information on the costs of their chosen endovascular devices, theatre occupation time, blood products used, contrast agent used, radiological and theatre facility costs (including staff and consumables), and costs of stay on standard wards and in intensive treatment units and HDU. These costs were applied to patient-specific data for the primary AAA repair as well as any subsequent aneurysm-related inpatient procedures. Given the limited trial resources for data collection, we were not able to collect data for non-aneurysm-related admissions or for the number of GP, outpatient or day-case appointments. Similarly, data on admissions for laparotomy-related complications after open repair, such as incisional hernia or wound infections, were excluded.
Trial recruitment procedure
Ethical approval and informed consent procedure
The trials are registered with international trial number ISRCTN 55703451. National ethical approval was obtained from the North West Multicentre Research Ethics Committee (MREC), subsequently to become the Integrated Research Application System (IRAS), based in Manchester (MREC references 98/8/26 and 98/8/27). Once approved, all participating centres were required to obtain local ethical approval and copies of the approval documents were sent to the main trial office at Charing Cross Hospital before any patient could be entered into the trials. Patients were provided with a patient information sheet and counselled regarding their possible recruitment into the trial. In addition, they were encouraged to spend as much time as they wished discussing their involvement in the trial with family, friends and their GP, and asked to sign their consent form only when they fully understood the implications of the trial. Patients could not be entered into the trial until a signed copy of the consent form had been received at the central trial office. The patient information sheets and consent forms are provided as Appendices 4 and 5.
Generalisability and the EVAR study
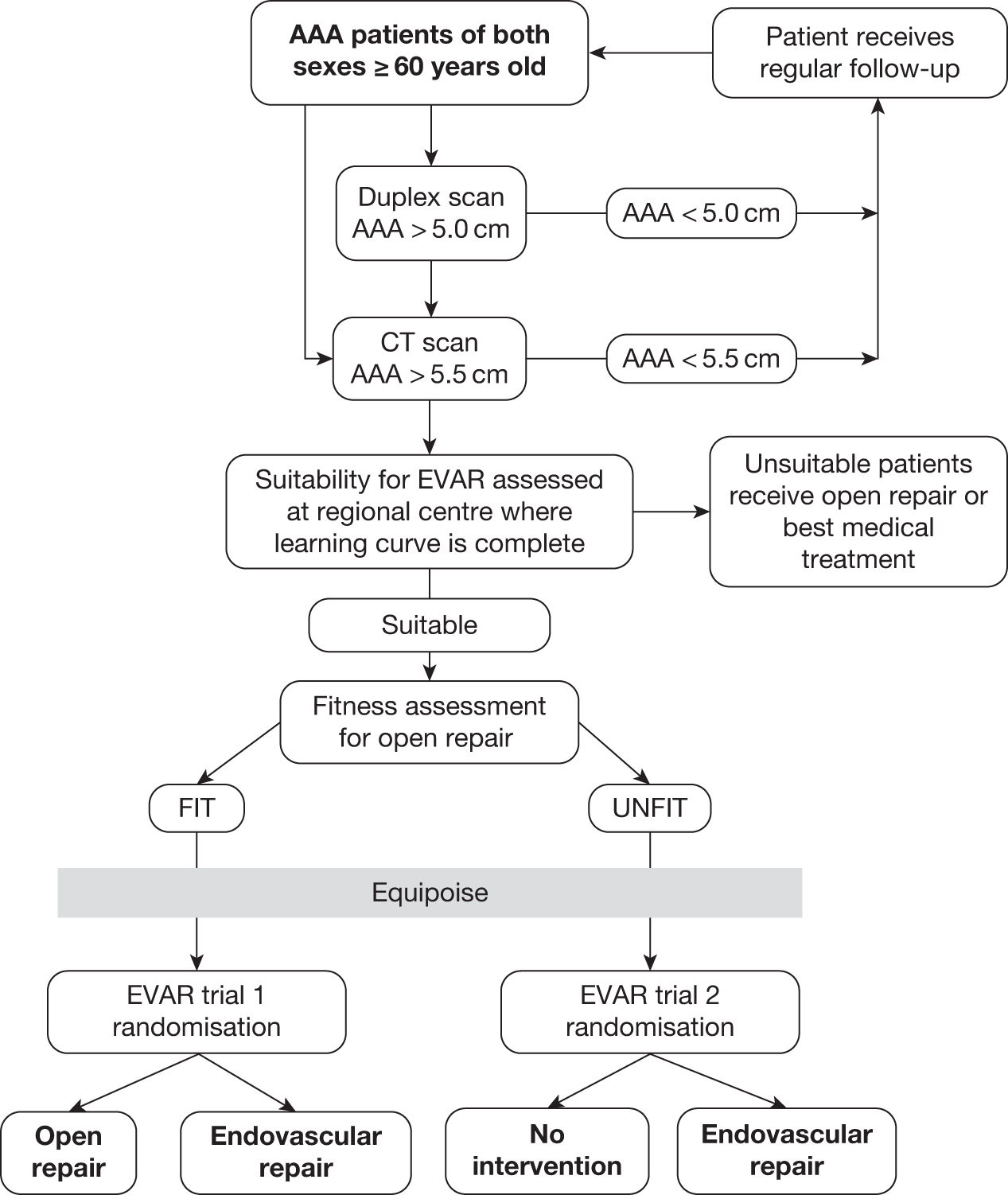
It was of particular importance that patients found to be unsuitable for an EVAR device were recorded. Numbers of unsuitable patients were logged and reasons for unsuitability were recorded in order to determine what proportion of patients with AAA were anatomically suitable for an EVAR device at the national level. Thus, all patients registered for assessment of anatomical suitability for an EVAR device formed the ‘EVAR Study’ and trial patients were drawn from this pool of patients with AAA. Some of the eligible centres acted as both the ‘local’ and ‘regional specialist’ centres for their area. Figure 4 demonstrates the trial recruitment procedure.
FIGURE 4.
Summary of recruitment procedure for EVAR trials.
Entry criteria
Age of at least 60 years
A minimum age of 60 years was chosen, as surgeons often manage patients of < 60 years in a different way because there may be an associated genetic reason why expansion rates and extent of aortic aneurysm may be extreme, such as Marfan syndrome. No upper age limit was thought to be necessary as it was felt that very elderly patients may benefit from the use of an EVAR device and their additional recruitment would be important for achieving the numbers required.
Size of abdominal aortic aneurysm
The criterion for entry into both trials was an aneurysm diameter measuring ≥ 5.5 cm according to a CT scan. However, reproducibility differences between duplex ultrasound and CT scanners can lead to significant variation in AAA diameters. Duplex scanning can produce smaller AAA diameters than CT scanning and therefore it was recommended that patients presenting with a ≥ 5.0-cm aneurysm on duplex should be sent for a CT scan to determine whether or not the aneurysm was ≥ 5.5 cm in any diameter on CT scan and thus suitable for EVAR trial entry. It was decided that tender aortic aneurysms or contained ruptures could be included providing the aneurysm measured at least 5.5 cm on a CT scan and suitable EVAR equipment was available at short notice.
Anatomical suitability for EVAR
This was assessed by spiral CT, conventional CT or, if necessary, with conventional angiography where a marked catheter could be used to measure aortic length. The trial co-ordinator was required to work closely with the local radiologist and document how the aneurysm was assessed and how the size and type of EVAR device were selected.
Fitness for open surgery
This was determined locally by the surgeon, radiologist, anaesthetist and cardiologist. It was originally thought that American Society of Anesthesiology (ASA) grades I, II and III would indicate entry to EVAR trial 1, and ASA grade IV patients would permit entry into EVAR trial 2. However, despite the simplicity of ASA grading it can be open to wide interpretation at each centre and thus it proved too difficult to use as a classification system for EVAR trial 1 or 2. It had also been appreciated during the UKSAT that fitness ‘inflation’ emerged with respect to the size of aneurysm. Patients who were earlier described as ‘unfit for open repair’ and later developed a larger aneurysm were suddenly deemed ‘fit for the procedure’. It was believed that this could happen equally for these trials and for the purposes of pragmatism, fitness was determined at the local level. Recommended cardiac, respiratory and renal guidelines were provided as outlined in Figure 5, and baseline data were collected to allow assessment of patient fitness in the final analyses. It was felt that these guidelines would help provide some conformity of fitness classification for EVAR trial 1 or 2. Furthermore, randomisation was stratified by centre and this would also ensure that any differences in assignment of fitness status between centres would not lead to any considerable differences between randomised groups. In hindsight, it would appear that these guidelines were good at separating patients into the EVAR 1 and 2 cohorts, and further assessment on classification of fitness will be made in Chapter 4, Results for EVAR trial 1, Chapter 5, Results for EVAR trial 2 and Chapter 9, Discussion.
FIGURE 5.
Recommended guidelines for assessment of patient fitness for open repair and suitability for EVAR trial 1 or 2. PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen.
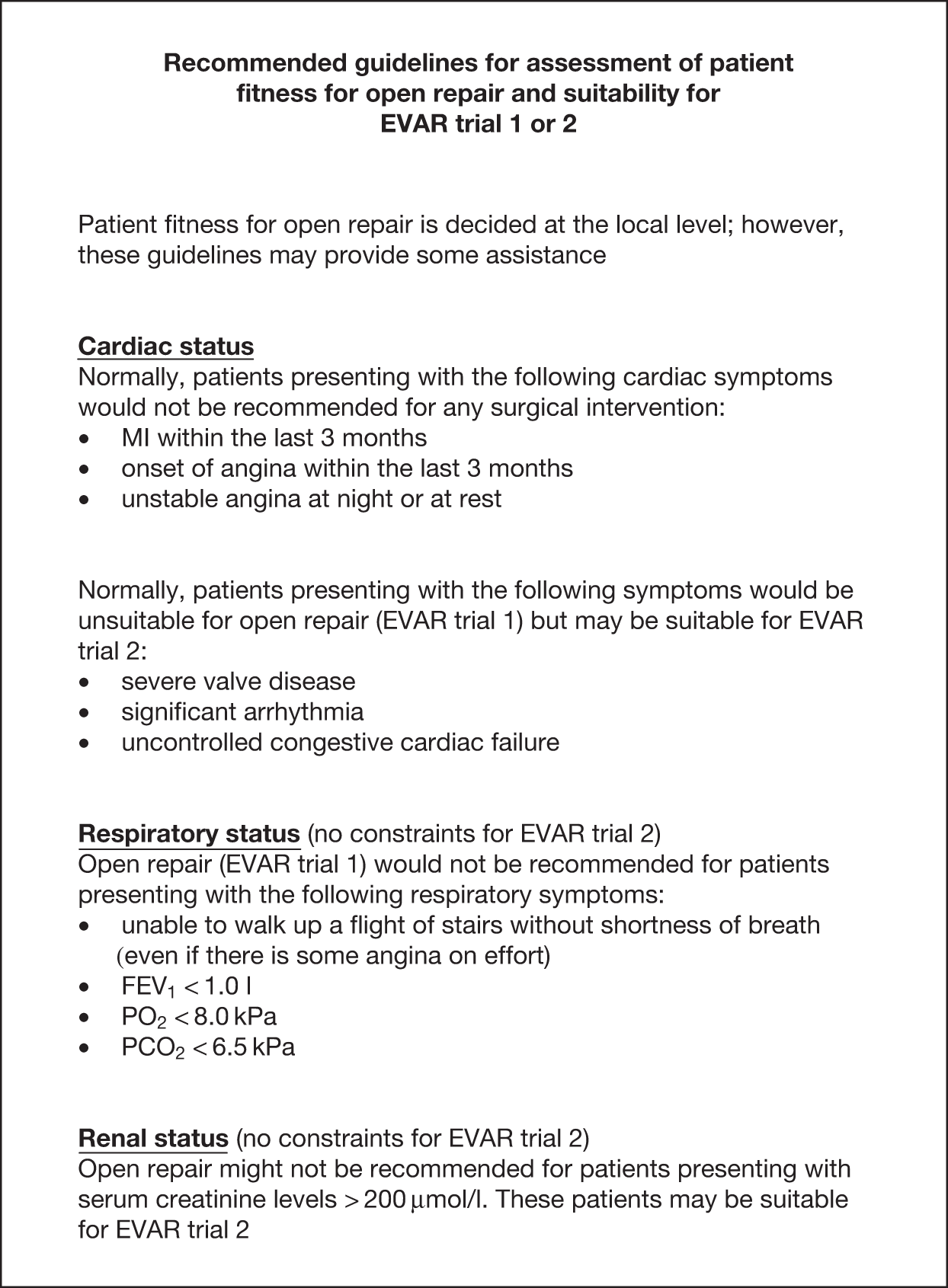
Baseline assessment
Patients who met the entry requirements of the trial underwent a full baseline assessment, during which data were collected for basic demographics (age, gender, postcode, occupation, level of education, source of referral and marital status), physical fitness in terms of cardiac disease [history of myocardial infarction (MI), angina, cardiac revascularisation, severe cardiac valve disease, uncontrolled congestive cardiac failure or significant arrhythmia sourced from hospital notes], respiratory disease [forced expiration volume in 1 second (FEV1) and forced vital capacity from a hand-held spirometer] and renal function (serum creatinine from trial hospital laboratory), as well as other markers of mortality such as body mass index (BMI), ankle–brachial pressure indices (ABPIs) (ratio of blood pressure in ankle to arm), blood pressure (standard cuff sphygmomanometry), pulse rate, total serum cholesterol (from trial hospital laboratory), smoking status (patient reported), diabetes (insulin controlled or not), and medication history for aspirin, non-steroidal anti-inflammatory drugs, cholesterol-lowering drugs, statins and beta-blockers. These baseline data were subsequently used to calculate the customised probability index (CPI) score for each patient. This score is a validated prognostic score for fitness for open repair and uses data on cardiac, renal and respiratory function, as well as use of medical therapies, to generate a score such that higher scores indicate poorer fitness. 108,109 This score was used as a marker of general fitness for all the patients. A full collection of anatomical aortic measurements was also taken from the baseline CT scan.
Randomisation
Randomisation was performed for each trial using 1 : 1 ratio randomly permuted block sizes constructed by the Stata package version 7.0 (StataCorp, College Station, TX, USA). Randomisation was stratified by centre and performed only when all necessary baseline data had been received at the central trial office based at Charing Cross Hospital, London. This enabled patients to be randomised into the relevant trial and simultaneously flagged for mortality at the ONS. Centres were encouraged to perform surgery within 1 month of randomisation.
Choice of EVAR device and reimbursement of treatment costs
Participating centres were free to decide which commercial or ‘in-house’ device to use, although the use of commercially available devices was favoured. These all carry the CE (Conformité Européenne) mark and are therefore freely available on the market and have undergone certain checks before being released. It was assumed that each centre would take the time to discuss the evidence for the safety of each device with the company. The anatomical suitability of EVAR devices would therefore be very centre specific depending on the number of devices that they chose to use in that hospital. It was not feasible for the trial protocol to intrude on the choice of device at each centre and this was left as a pragmatic decision for the participating clinicians.
It had been anticipated that the cost of the EVAR procedure would incur significantly greater treatment costs over open repair and there was concern that this may impede recruitment into the trials as local trusts would refuse to pay these additional costs. Following negotiations with the NHS Executive (North Thames London Region) it was agreed that treatment costs may be reimbursed to each trial centre on randomisation to an EVAR device. It was agreed that additional service costs would not be funded, as EVAR may be associated with a reduction in length of stay and particularly intensive treatment unit (ITU) and HDU usage. An assessment of costs was carried out to ascertain the excess treatment expenditure associated with an EVAR repair over an open repair for EVAR trial 1 and the additional costs of EVAR over medical treatment alone in EVAR trial 2. Estimates were made and a fixed figure was agreed with the Department of Health such that randomisation to an EVAR device in EVAR trial 1 triggered £5418 of additional funding and randomisation to an EVAR device in EVAR trial 2 triggered £8102 of additional funding. It is thought that this payment incentive contributed greatly to achieving the excellent recruitment rates into both trials.
Trial follow-up protocol
All trial patients were flagged for mortality at the UK ONS, which provided death certificates on which the underlying cause of death was assigned using ICD-10 codes. A trial Endpoints Committee was convened to confirm this underlying cause of death as well as determine whether or not the death was aneurysm related.
All centres were required to nominate a local trial co-ordinator, who was responsible for all aspects of trial recruitment and follow-up at that hospital. The co-ordinator was required to attend a 1-day training course in trial protocol, recruitment and data collection procedures at the main trial headquarters at Charing Cross Hospital. All patients were required to have baseline CT scan and fitness assessment data collected prior to randomisation and these data needed to be sent to the central trial office where randomisation was performed. After randomisation, data were collected for the primary AAA repair operation as well as for any further admissions for aneurysm-related complications that required at least one night in hospital. Admission details were obtained on theatre time and blood product usage as well as length of stay in ITU, HDU and standard bed wards. HRQoL data were collected at baseline and then at 1, 3 and 12 months following treatment with a further EuroQol questionnaire annually until the end of the trial to be used for cost-effectiveness evaluation. Further data were collected on the incidence of any of the following adverse events: ruptured AAA for patients without AAA repair, conversion from EVAR to open repair, MI, stroke, renal failure and amputation (above or below knee). Annual creatinine measurements were taken to assess renal function over time. CT scans were used for assessment of growth rates, persistent endoleaks and graft durability with all graft-related adverse events for EVAR patients reported to the MHRA. Centres were encouraged to provide data from as many CT scans as possible, but the minimum requirement for CT scan follow-up was at 1 and 3 months post EVAR procedure for EVAR patients and then annual scans for all randomised patients in each arm of both trials. Centres were free to utilise any additional imaging modality beyond CT scan if it is felt appropriate; however, data were not collected for any additional imaging, as the CT scan form could be used to record any problems that had been identified with the AAA or graft.
Data collection and management
Data were entered into an Access database version 10 (2002) (Microsoft Corporation, Redmond, WA, USA) by the trial manager based at Charing Cross Hospital, who remained as the trial manager for the whole duration of the trial and was the only person responsible for data entry. Data entry errors were assessed using consistency checks on time between dates and on unreasonable or outlier values. The trial case record forms are provided in Appendix 6. To encourage good data retrieval, the departments of each trial co-ordinator were paid a small amount of money on receipt of clean and complete data at Charing Cross Hospital. The payment could be spent at the discretion of each local centre, but centres were encouraged to use the funds as an incentive for the co-ordinator, for example as funding to attend conferences or relevant training courses. An estimate was made of the length of time a trial co-ordinator would take to complete the forms (1 hour for a baseline assessment and 20 minutes for a follow-up appointment). A £25 payment was made for each complete baseline assessment and a further £25 payment for any operation or reintervention forms. A £25 payment was also made on receipt of each complete set of follow-up data.
Quality assurance and data audit
To check that all adverse events, graft-related complications and reinterventions had been reported, a data clerk was employed to audit the trial case record notes (completed by the local co-ordinators) against the local hospital notes. Two periods of audit were conducted: one in 2007 and one in 2009. A total of 1052 (84%) patient notes were audited in EVAR trial 1 with the remaining 200 sets of notes unavailable in archive. A total of 308 (76%) patient notes were audited in EVAR trial 2, with the remaining 96 sets of notes unavailable in archive. All reported events were confirmed and a small number of unreported events were detected and included in the main database.
Methods specific to renal function analyses
For details, see Chapter 4, Renal function, Chapter 5, Renal function and Chapter 6, Factors associated with development of serious graft-related complications and reinterventions.
Serum creatinine measurements were collected for all patients at baseline and as part of their annual follow-up. Available measurements were included up to March 2008, when the analyses were undertaken. For this investigation, a priori inclusion and exclusion criteria were defined as follows: (1) only elective cases of aneurysm repair would be included as the impact of emergency repair on renal function may distort the results; (2) for similar reasons, renal function data collected after non-compliance with randomised management would be excluded; and (3) creatinine measurements during the 6-month period after the AAA repair were not included in the analysis to allow renal function to stabilise after any acute kidney injury associated with the initial procedure.
In both trials, the analyses were timed from randomisation as the baseline creatinine measurements had been collected at that time. Patients without a baseline and at least one follow-up estimated glomerular filtration rate (eGFR) measurement were excluded. As the trial protocol specified that creatinine measurements needed to be collected only annually, survival to 1 year became an indirect inclusion criterion for the analysis. However, these analyses focused on the long-term consequences of different aneurysm management policies on renal function, relevant only to those who survive beyond 1 year. In EVAR trial 1, annual follow-up measurements were used to compare changes in eGFR over time between those who received an elective EVAR in the EVAR randomised group and those who received an elective open repair in the open-repair randomised group. In EVAR trial 2, changes in eGFR over time were compared between those who received an elective EVAR in the EVAR group with those who remained under surveillance in the no-intervention group. Patients without AAA repair in the EVAR group were excluded and eGFR measurements after any AAA repair in the no-intervention group were excluded. For both trials, patients who required chronic renal dialysis during the course of follow-up were censored at the time of commencing dialysis, as their creatinine results would be unreliable after this date.
Assessment of renal function
Estimated glomerular filtration rate was selected to represent renal function as it has been recommended as a more sensitive determinant of renal function in patients with AAA. 186 As the Cockcroft–Gault equation requires weight at each creatinine measurement (and only baseline weight was available), we used the Modification of Diet in Renal Disease calculation,187 sourced from the website of the Renal Association for UK professional renal physicians and scientists,188 which includes creatinine in units of micromoles per litre, age in years, sex and ethnicity:
Data on ethnicity were not available in the EVAR trials’ data sets, but after consultation with local co-ordinators it was clear that very few patients (< 1%) were of black origin and application of the 1.210 correction factor to all of their eGFR measurements would be unlikely to change the overall results or the within-patient changes over time. Another potential source of error is the fact that laboratory standards for measurement of creatinine vary across the UK. As creatinine was measured by the same hospital for each patient, this would not affect the analyses based upon within-patient changes over time. Once eGFR was calculated, patients were classified according to the National Kidney Foundation Kidney Dialysis Outcomes Quality Initiative (KDOQI) staging for renal impairment. 189 Statistical methods for the renal analyses are provided below (see Multilevel modelling statistical methods for renal function analyses).
Methods specific to analysis of cardiovascular mortality and events
For details, see Chapter 4, Cardiovascular mortality and events and Chapter 5, Cardiovascular events.
The local trial co-ordinators had collected data prospectively on MI and stroke events throughout the trial, although World Health Organization (WHO) criteria were not required. Two outcomes were analysed: time from randomisation to first cardiovascular event (fatal or non-fatal MI or stroke) and time from randomisation to cardiovascular death (which was defined according to ICD-10). In addition, the numbers of multiple cardiovascular events in patients were summated to calculate a crude overall event rate.
Definition of fatal MI Primary cause of death on the death certificate assigned under ICD-10 MI codes I210 to I238.
Definition of non-fatal MI Any report of a non-fatal MI from the co-ordinator at the participating hospital or any mention of ICD-10 codes I210 to I238 on the death certificate, providing that they were not attributed as the original underlying cause of death. In the latter cases, the date of death was used as the date of event and the events were audited by two independent assessors blinded to randomised group.
Definition of fatal stroke Primary cause of death on the death certificate assigned under cerebrovascular disease leading to stroke, ICD-10 codes I600 to I640.
Definition of non-fatal stroke Any report of a non-fatal stroke from the co-ordinator at the participating hospital or any mention of ICD-10 codes I600 to I640 on the death certificate using the same criteria as those for non-fatal MIs.
Definition of cardiovascular death All death certificates were reviewed by an Endpoints Committee, who ascribed the following underlying primary causes of death as cardiovascular: death within 30 days of an aneurysm-related procedure, aortic aneurysm rupture (before or after aneurysm repair), cardiac (including all coronary deaths), cerebrovascular disease or stroke, other cardiovascular disease such as peripheral vascular disease or pulmonary embolism.
The timing of events and censoring of patients was slightly different for cardiovascular events and deaths, and predefined according to the following rules.
-
For patients with a new cardiovascular event recorded since randomisation:
-
If the patient had a baseline/follow-up appointment or had been audited within 18 months prior to a first recorded event then the event was defined as the first event.
-
If the patient had a baseline/follow-up appointment or had been audited more than 18 months prior to the event then it could not be assumed that this was the first event and the patients were censored without an event on the date last seen or audited. This removed events in a small number of patients (n = 3) who were not seen for at least 18 months and then died with a fatal or non-fatal mention of MI or stroke on their death certificate.
-
-
For patients without any new cardiovascular event recorded since randomisation, censoring occurred at the latest of these dates:
-
For patients who were alive on 1 September 2009, the date of last follow-up appointment or the date of audit.
-
For patients who were dead, the date of death (without mention of MI or stroke cause) was used providing that the death occurred within 18 months after the last follow-up or date of audit. For patients dying more than 18 months after their last follow-up or date of audit, the date of follow-up or audit was used for censoring.
-
-
For patients with a cardiovascular death by 1 September 2009, the date of death was used for their event. For patients without a cardiovascular death by 1 September 2009, censoring occurred either at non-cardiovascular death or on the date of last follow-up for those lost to follow-up or on 1 September 2009.
Cox regression modelling was used to compare time to first cardiovascular event and time to cardiovascular death between randomised groups. HRs were presented overall as well as during three prespecified time periods: 0–6 months, 7–24 months and > 24 months after randomisation. The early 6-month period was selected to allow for the delay between randomisation and surgery, as well as to present event rates during the early postoperative phase. The second time period of 6–24 months was selected after inspection of the published all-cause mortality curves from EVAR trial 1, the DREAM trial and the Medicare comparative study. 115,132,159 Kaplan–Meier methods were used to display survival curves and estimates at 6 months and 2 and 8 years after randomisation.
Statistical methods
All analyses were performed according to predetermined statistical analysis plans with a priori lists of agreed variables for analysis. In some cases, post hoc analyses were conducted for exploratory purposes but these are indicated in the text when performed. All analyses were conducted using Stata statistical software versions 7.0, 8.0 or 10.0. The methods described in this section are generic to all of the analyses performed. Any additional analyses specific to a results chapter are described separately in that chapter.
Descriptive statistics
Continuous data were checked for outliers using scatterplots and approximation to the normal distribution using normal plots. Data were transformed where necessary, particularly in the case of creatinine, which was always strongly positively skewed and required log transformation. Continuous data were compared between groups using Student’s t-tests for normally distributed data or using Mann–Whitney U-tests when transformation was unable to normalise the distribution of the data. Categorical data were compared between groups using chi-squared tests.
Regression modelling
Data on the incidence of mortality, complications, reinterventions or rupture were analysed using Cox regression modelling and data on operative mortality were analysed using logistic regression modelling. Prespecified baseline covariates used for adjustment are specified in the relevant chapters. Survival estimates were presented graphically using Kaplan–Meier methods and, where appropriate, log-rank tests were performed between stratified groups. For the Cox models, deviation from the proportional hazards assumption was checked by regressing scaled Schoenfeld residuals against the logarithm of time. 190 For the analysis investigating factors associated with endograft rupture in Chapter 6, Factors associated with endograft ruptures, time to complication was included as a time-dependent variable so that rates of rupture could be compared before and after the detection of a complication.
Whenever possible, all regression models included continuous data in non-stratified format but data were usually presented above and below the median values to display directions of effect.
Handling of missing data
In general, data were very complete in the trials but to maximise power and inclusion of all patients, data were assumed to be missing at random191 and two primary strategies were used for handling of missing data (usually performed as sensitivity analyses). First, for comparisons between randomised groups, logistic regression models were used to derive a propensity score of being randomised to the EVAR group for each patient according to the list of covariates selected for the adjusted model. For those patients in whom a propensity score could not be calculated owing to missing data, the patient was included in the model using the missing indicator method. 192 Second, for comparisons that were not between randomised groups, multiple imputation using chained equations (MICE) was used to derive estimates for missing covariates. When time-to-event outcomes were investigated using Cox regression, the data were imputed using models that included terms for whether or not the patient had experienced the event, as well as a term for the log of time to the event or to censoring. 193,194 Seven imputation cycles were performed and the results were combined using Rubin’s rules. 191,195
Multilevel modelling statistical methods for renal function analyses
For the investigation into renal function changes over time, it was necessary to analyse the data using a hierarchical approach to account for the different number of creatinine measurements (converted to eGFR) provided by each patient during their different lengths of follow-up. Therefore, random effects multilevel modelling was used to analyse the eGFR measurements over time within each patient. 196,197 A random slopes and intercepts model was applied and fixed and random effects terms were combined to calculate a predicted eGFR measurement for each follow-up, as well as a rate of change in eGFR over time for each patient. Normal plots were used to check that the distribution of the random effects slopes and intercepts were approximately normally distributed. A correlation coefficient between the random effects slopes and intercepts was used to determine whether or not baseline eGFR was related to subsequent rate of decline in eGFR. Histograms were plotted to observe the distribution of rates of change in eGFR for all the patients.
For the investigations comparing randomised groups within EVAR trials 1 and 2 separately, additional terms for randomised group as well as its interaction with time were included in the model to assess the crude effect of randomised group on eGFR and on the rate of decline in eGFR. These estimates were adjusted further for two sets of predefined potential confounding baseline variables. Primary adjustment was made for age, sex, AAA diameter, systolic blood pressure, diabetes, smoking status, cholesterol, use of non-steroidal anti-inflammatory drugs and use of statins. Secondary adjustment was made for all the primary variables, as well as aortic neck diameter at the level of the lowest renal artery, neck length, BMI, previous cardiac disease (MI, angina, cardiac revascularisation, valve disease, significant arrhythmia or uncontrolled congestive cardiac failure), FEV1, use of aspirin, use of beta-blockade and mean of ABPI for both legs.
For the analysis investigating the impact of graft-related complications after EVAR the patients in the open-repair group of EVAR trial 1 and the no-intervention group of EVAR trial 2 were excluded. Complications were defined as any of the following: graft rupture, migration, infection, endoleak type 1, 2 or 3, graft kinking, thrombosis, distal embolisation or endotension. Reinterventions were defined as any intervention for any of the complications listed above. Four new variables were defined, taking the values:
-
‘1’ for patients with a complication at any time during follow-up, ‘0’ otherwise
-
‘1’ for eGFR measurements after a complication was detected, ‘0’ otherwise
-
‘1’ for patients with a reintervention at any time during follow-up, ‘0’ otherwise
-
‘1’ for eGFR measurements after a reintervention, ‘0’ otherwise.
Multilevel models were repeated, including these new variables and their interactions with time, to investigate the impact of complications and reinterventions on eGFR and rate of change of eGFR over time. All models were adjusted for whether the patient was in EVAR trial 1 or 2.
Chapter 3 Trial recruitment, patient flow and completeness of follow-up
Trial recruitment
The trials began recruitment on 1 September 1999 and three phases of randomisation followed. The first phase was the planned phase according to the original trial protocol and this was completed on 31 December 2003 at which time 1082 patients had been randomised into EVAR trial 1 and 338 into EVAR trial 2, in both cases about 20% higher than their targets of 900 and 280 patients, respectively. The second phase of recruitment continued until 31 August 2004, when the first results of the trials were published, on 30-day operative mortality for the 1082 patients recruited into EVAR trial 1 during phase 1. At this stage the results indicated a threefold reduction in operative mortality between EVAR and open repair, and it was felt that equipoise for both patients and clinicians would probably no longer exist. However, the power calculations for the trial were based on all-cause mortality after at least 1 year of follow-up for all patients and thus results for the primary outcome would not be available until June 2005. Therefore, a third period of randomisation continued between 1 September 2004 and 30 June 2005 but data collection for patients in this third phase was minimal, as these extra patients would be used only if the additional power was thought to be necessary. Figure 6 shows the milestones for recruitment and follow-up during the course of the trials and Figures 7 and 8 demonstrate cumulative recruitment into both trials up to the end of phase 2 in August 2004. All analyses presented in this report are based upon patients recruited during phases 1 and 2 of recruitment: 1252 in EVAR trial 1 and 404 in EVAR trial 2 (see Figure 6). Follow-up closed on 31 December 2009.
FIGURE 6.
Milestones for recruitment and follow-up in the EVAR trials.
FIGURE 7.
Cumulative recruitment into EVAR trial 1 between 1 September 1999 and 31 August 2004.
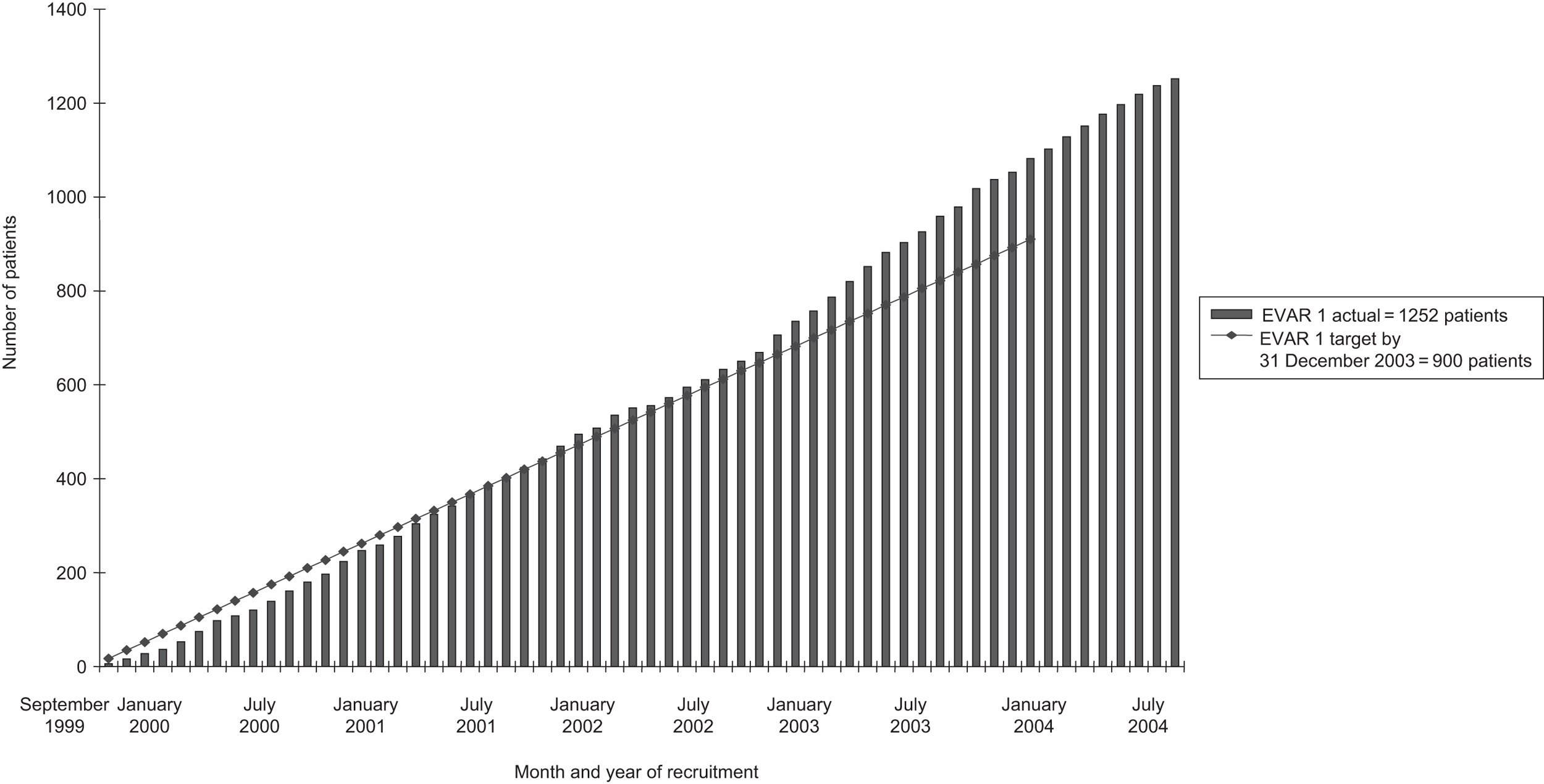
FIGURE 8.
Cumulative recruitment into EVAR trial 2 between 1 September 1999 and 31 August 2004.
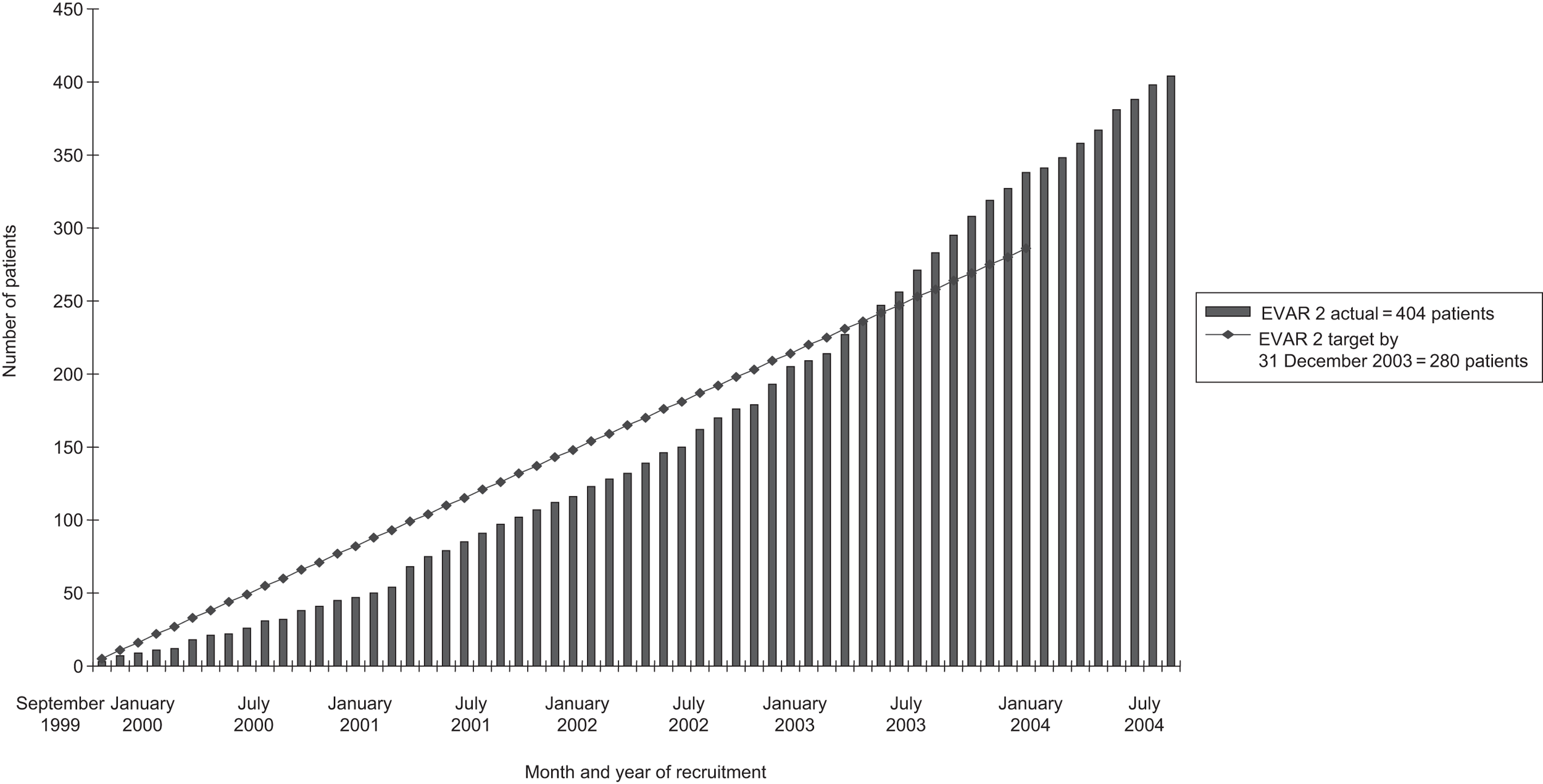
By 31 December 2003, 4799 patients had been registered into the EVAR study (see next section for generalisability of trials in relation to screened patients). Beyond this date, registration into the EVAR study was closed, as only randomisation continued until June 2005. Suitable patients were drawn from this pool of EVAR study registrations and randomised into EVAR trial 1 or 2. Patients who did not enter the trials were excluded for various reasons, including unsuitability for an EVAR device, AAA < 5.5 cm on CT scan, refusal to enter into either trial or refusal to undergo CT scan or further treatment. Recruitment rates varied considerably between the 41 centres, with some hospitals more enthusiastic about EVAR trial 2 than others. EVAR trial 1 recruited almost continuously ahead of target, whereas recruitment into EVAR trial 2 struggled in the early phase of the trial but accelerated during the last 2 years such that the target was exceeded by the end of recruitment in August 2004.
Generalisability of the trials and patient flow through each trial
Figure 9 presents the numbers of patients who were screened for the trials, and Figures 10 and 11 present the flow of patients through each trial. Of the 4799 patients screened for eligibility for the trials by 31 December 2003, 894 had an aneurysm of < 5.5 cm or had refused assessment or had missing CT data for EVAR suitability. Of the remaining 3905 patients, 1795 were deemed to be anatomically unsuitable for EVAR (46% of the 3905 assessed). The remaining 2110 patients proceeded to an assessment of their fitness for open repair: 230 anatomically suitable patients were still having their fitness assessed by 31 December 2003 and could not be enrolled by that date, 1423 were considered to be fit for open repair and offered entry into EVAR trial 1, and 457 patients were deemed to be unfit for open repair and offered entry into EVAR trial 2. A total of 341 patients refused to enter or to be entered into EVAR trial 1, with 1082 consenting to be randomised into the trial by 31 December 2003. A total of 119 patients refused to enter or to be entered into EVAR trial 2, with 338 consenting to be randomised by 31 December 2003. Beyond this time data were not collected for screened patients but only for patients entered into the trial, and an additional 170 patients were recruited into EVAR trial 1 and 66 into EVAR trial 2 by 31 August 2004 (see Trial recruitment for full description of recruitment periods).
FIGURE 9.
Trial profile showing patients screened and entered into EVAR trials.
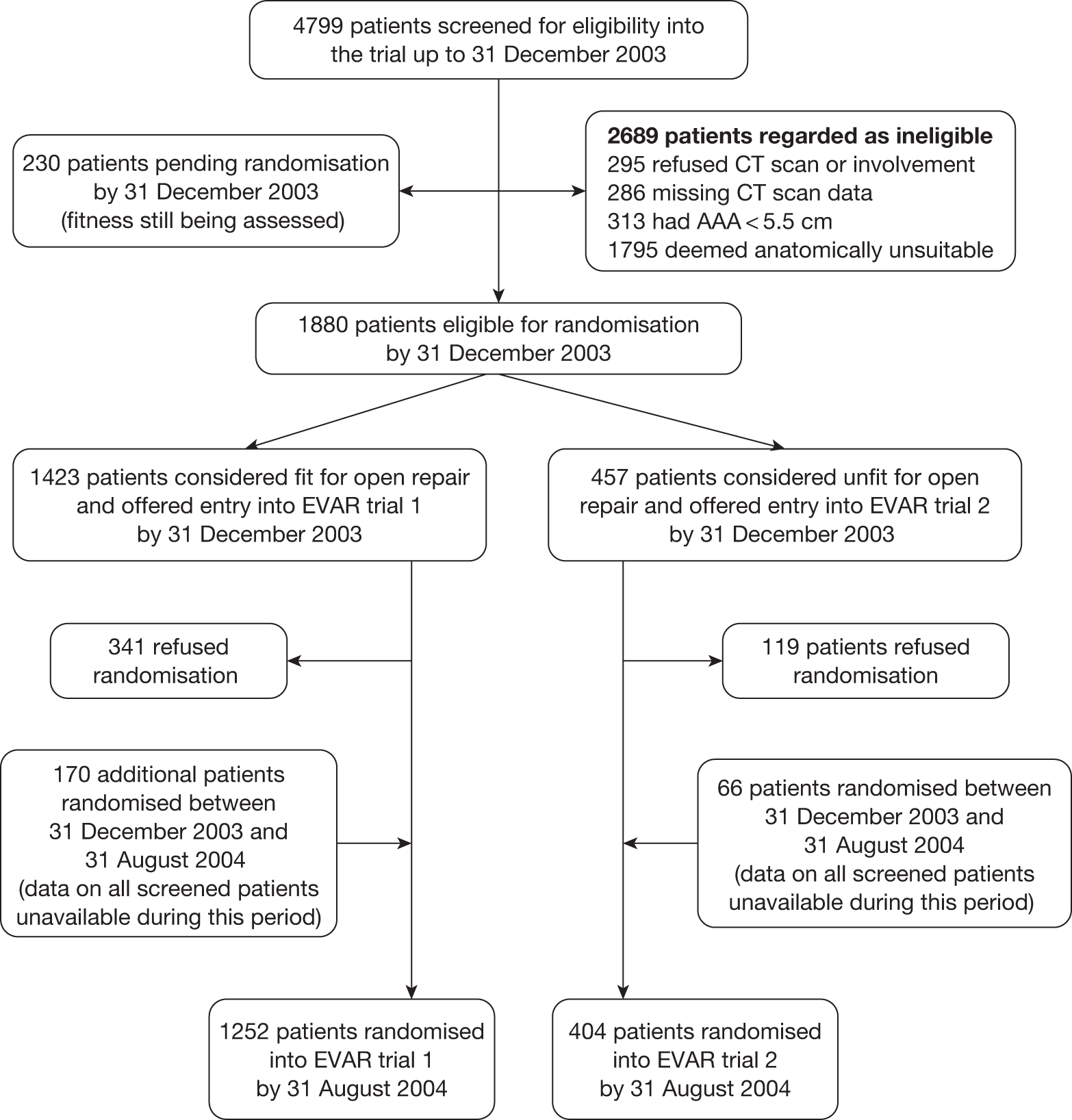
FIGURE 10.
Trial profile showing flow of patients entered into EVAR trial 1 (per-protocol patients marked with an asterisk, 93% overall). OR, open repair.
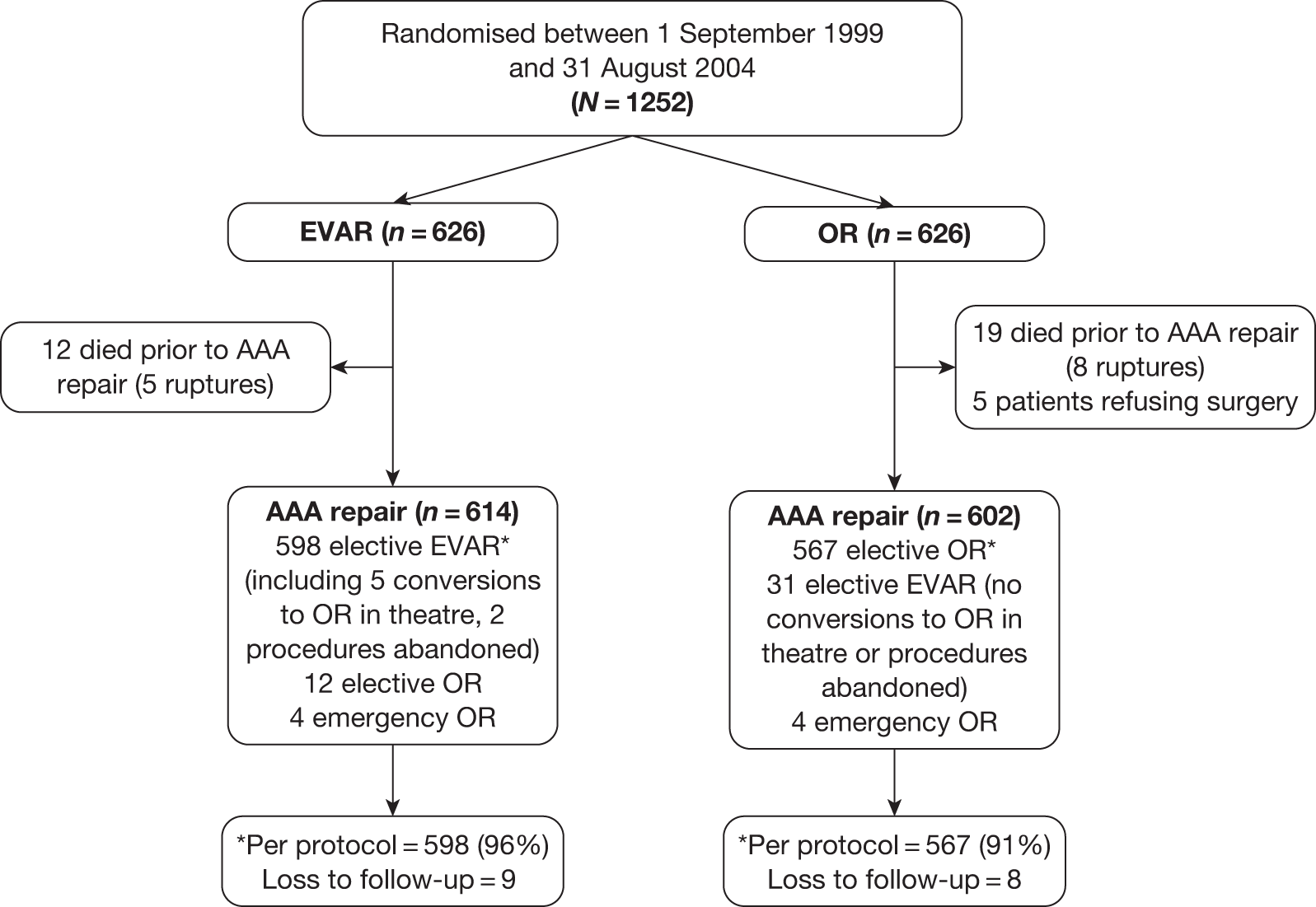
FIGURE 11.
Trial profile showing flow of patients entered into EVAR trial 2 (per-protocol patients marked with an asterisk, 84% overall). OR, open repair.
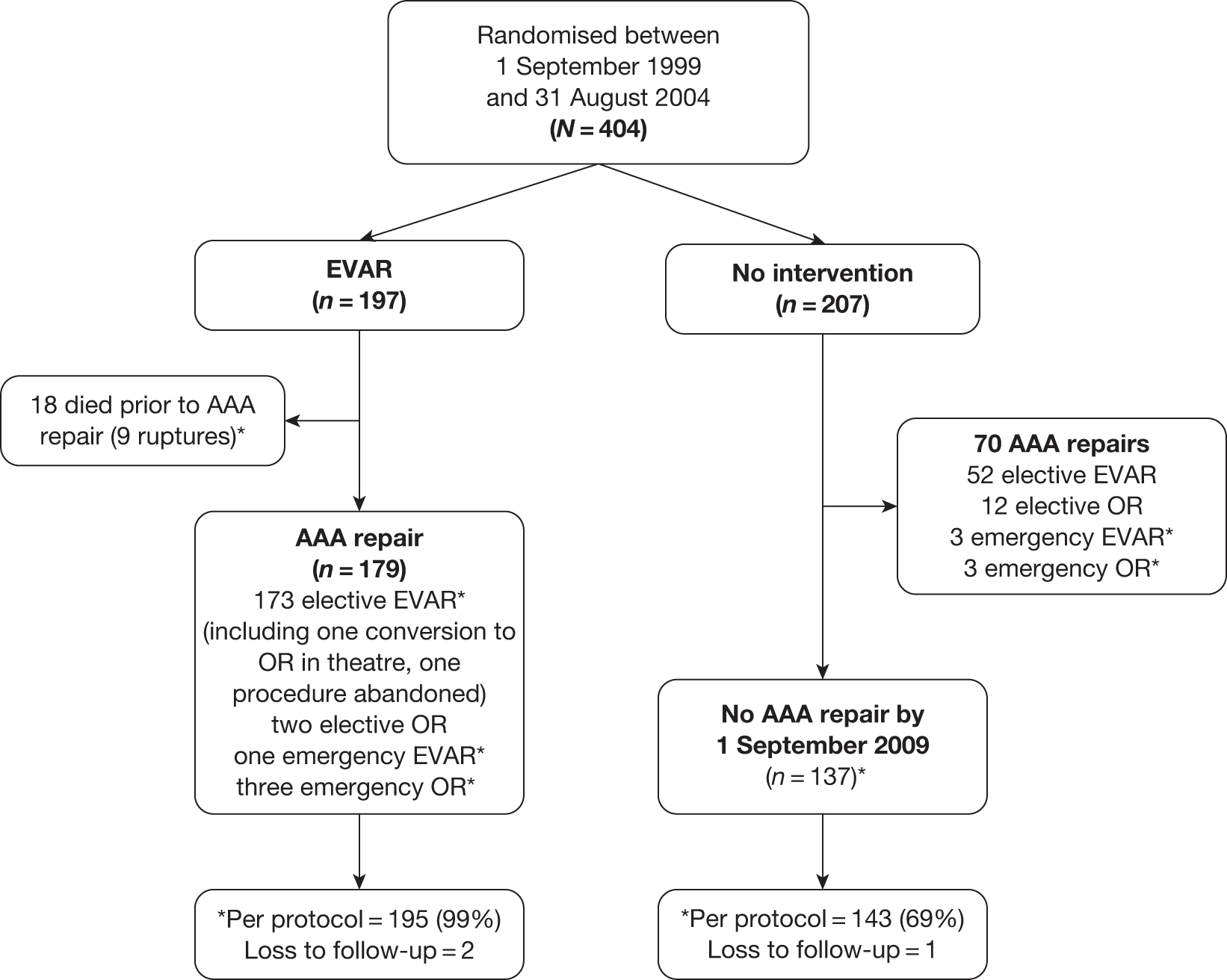
Completeness of follow-up
Primary outcome – mortality
All patients were flagged at the ONS but as there is a delay of a couple of months for notification of death from ONS, it was agreed that mortality follow-up would close on 1 September 2009 (5 years after the first randomisation), so that the last few months of 2009 could be used to make personal contact with all patients thought to be alive. On 1 December 2009, letters were sent to all 815 patients without any notification of death from ONS. By 18 January 2010, 684 (84%) patients had responded and can be assumed to have been alive on the end of follow-up date of 1 September 2009. The remaining 131 patients were chased by phone calls to their home, GP or local co-ordinator: 18 patients had died (three patients before 1 September 2009, and death certificates were subsequently received and processed), 56 patients had been seen in an outpatients appointment or by their GP after 1 September 2009 so were alive at the end of follow-up, 37 patients had been seen by the trial co-ordinator in 2009 but before 1 September 2009 so were censored as alive at their last follow-up, and 20 patients had not responded to their letter or been seen by their GP or local trial co-ordinator in 2009 and they were censored on the date of their last trial follow-up. Total person-years accrued were 6904 in EVAR trial 1 and 1413 in EVAR trial 2 (total = 8317).
Secondary outcomes – adverse events, graft-related complications and reinterventions
Follow-up for these outcomes was more complicated, as it required patient attendance as well as completion of the CRF documentation by the local co-ordinator and radiologist. The data audit, described in Chapter 2,Quality assurance and data audit, had checked the hospital notes of 1360 of the 1656 randomised patients (82%). Two periods of audit were conducted – one in 2007 and a more recent one in 2009. Figures 12 and 13 summarise the completeness of the data for the secondary outcomes in each trial and the following censoring criteria were used for non-mortality outcomes:
-
Alive patients were censored on the date of last follow-up or the date of audit.
-
Dead patients were censored on the date of death, providing that it occurred within a year of the last follow-up or audit, otherwise the date of last follow-up or audit was used for censoring.
-
For non-audited patients who had not been followed up in 2009 or in the year prior to their death, the date of last follow-up was used for censoring or the date of discharge from hospital if they had AAA repair and no subsequent follow-up.
FIGURE 12.
Summary of completeness of secondary outcome data for EVAR trial 1. OR, open repair.
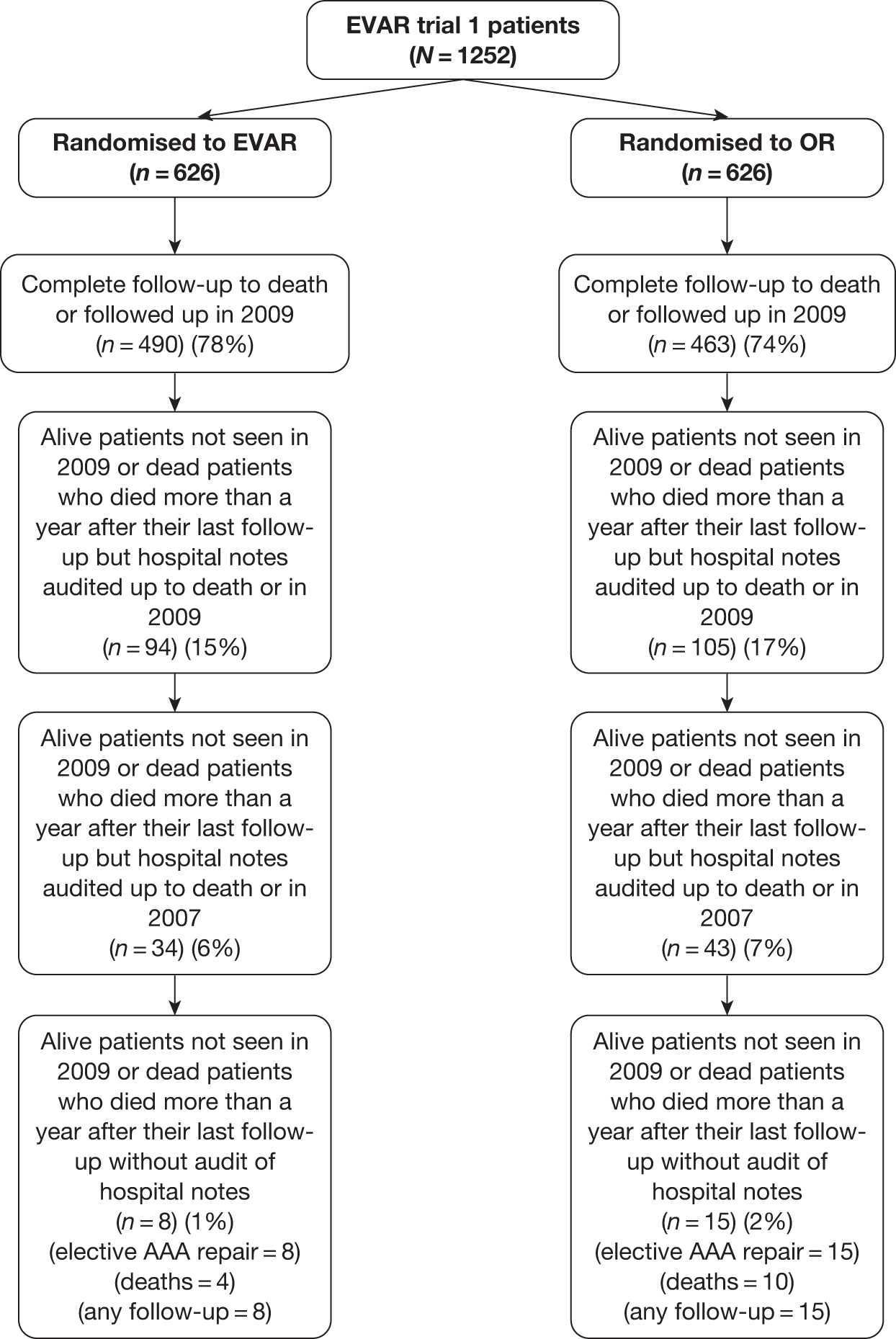
FIGURE 13.
Summary of completeness of secondary outcome data for EVAR trial 2.
Using these censoring criteria, total person-years for non-mortality outcomes were 6690 in EVAR trial 1 and 1351 in EVAR trial 2 (total = 8041). Thus, a total of 276 person-years of follow-up (3%) were lost owing to the more rigorous censoring of the secondary outcome data.
Chapter 4 Results for EVAR trial 1
Descriptive results
Patients were recruited across 37 hospitals and followed for a minimum of 5 years until 1 September 2009 (average 7.5 years) for mortality and the end of December 2009 for graft-related complications, reinterventions and adverse events. Baseline characteristics between the randomised groups are given in Table 2, with no apparent differences between them. The overall mean [standard deviation (SD)] age was 74.1 (6.1) years and 1135 (91%) were men. The mean (SD) aneurysm diameter was 6.4 cm (0.9 cm).
| Baseline characteristica | EVAR (n = 626) | Open repair (n = 626) |
|---|---|---|
| Age (years) | 74.1 (6.1) [0] | 74.0 (6.1) [0] |
| No. of males (%) | 565 (90) [0] | 570 (91) [0] |
| AAA diameter (cm) | 6.4 (0.9) [0] | 6.5 (1.0) [1] |
| BMI (kg/m2) | 26.5 (4.6) [1] | 26.5 (4.3) [6] |
| Diabetes (%) | 61 (10) [2] | 68 (11) [6] |
| Smoking status (%) | (1) | (1) |
| Current | 134 (21) | 136 (22) |
| Past | 419 (67) | 444 (71) |
| Never | 72 (12) | 45 (7) |
| History of cardiac diseaseb (%) | 269 (43) [0] | 261 (42) [0] |
| Single cardiac morbidity | 122 | 116 |
| Two or more comorbidities | 147 | 145 |
| Angina | 184 | 178 |
| Unstable angina | 8 | 12 |
| Previous MI | 151 | 158 |
| Coronary revascularisation | 92 | 86 |
| Valve disease | 6 | 10 |
| Arrhythmia | 32 | 21 |
| Congestive cardiac failure | 4 | 0 |
| Systolic blood pressure (mmHg) | 148 (22) [5] | 147 (21) [2] |
| Diastolic blood pressure (mmHg) | 82 (12) [7] | 82 (13) [3] |
| ABPI (mean of both legs) | 1.01 (0.18) [13] | 1.03 (0.18) [27] |
| FEV1 (l) | 2.1 (0.7) [8] | 2.2 (0.7) [4] |
| Serum creatinine (μmol/l)a | 102 (91–118) [1] | 102 (90–120) [4] |
| Serum cholesterol (mmol/l) | 5.1 (1.2) [18] | 5.1 (1.1) [25] |
| Statin use (%) | 216 (35) [7] | 224 (36) [3] |
| Aspirin use (%) | 338 (54) [0] | 325 (52) [0] |
| CPI scorec | 3.6 (9.3) [39] | 3.7 (9.5) [39] |
A Consolidated Standards of Reporting Trials (CONSORT) diagram is provided in Figure 10 in Chapter 3, Generalisability of the trials and patient flow through each trial. A total of 1216 AAA repairs occurred (eight emergency repairs). The median [interquartile range (IQR)] time from randomisation to AAA repair was 44 (29–70) days for the patients randomised to EVAR and 35 (20–57) days for those randomised to open repair. Of the 12 patients who did not have AAA repair in the EVAR group, seven died within 6 months of randomisation (three ruptures), three became unfit, one refused and one became anatomically unsuitable for either operation. Of the 24 patients who did not have AAA repair in the open-repair group, seven died within 6 months of randomisation (three ruptures), seven became unfit and eight refused (three now dead); in two cases the reason for delay is unknown but both patients are now dead. The choice of graft manufacturer for the 629 EVAR procedures was split primarily between three device manufacturers – Zenith (Cook, Copenhagen, Denmark) 337 (54%), Talent (Medtronic, Minneapolis, MN) 199 (32%) and Excluder (Gore, Flagstaff, AZ) 40 (6%) – with the rest of the devices split between AneuRx (Medtronic, Minneapolis, MN) 18 (3%), ‘other’ 32 (4%) and ‘unknown’ for three procedures (1%).
Primary outcome results – mortality
Operative mortality
A total of 37 deaths occurred within 30 days of AAA repair (3.0%) and 50 deaths occurred in hospital prior to discharge (4.1%). Table 3 presents the elective and total 30-day and in-hospital mortality results from logistic regression analysis in the 1216 patients undergoing AAA repair.
| Outcome | EVAR (n = 614) (four emergency) | Open repair (n = 602) (four emergency) | Crude OR (95% CI) (p-value) | Primarya adjusted OR (95% CI) (p-value) | Secondaryb adjusted OR (95% CI) (p-value) |
|---|---|---|---|---|---|
| 30-day (%) | |||||
| Total | 11/614 (1.8) | 26/602 (4.3) | 0.40 (0.20 to 0.83) (0.013) | 0.44 (0.21 to 0.92) (0.029) | 0.39 (0.18 to 0.87) (0.021) |
| Elective | 10/610 (1.6) | 25/598 (4.2) | 0.38 (0.18 to 0.80) (0.011) | 0.42 (0.20 to 0.90) (0.026) | 0.37 (0.16 to 0.85) (0.020) |
| In hospital (%) | |||||
| Total | 14/614 (2.3) | 36/602 (6.0) | 0.37 (0.20 to 0.69) (0.002) | 0.39 (0.21 to 0.74) (0.004) | 0.39 (0.20 to 0.76) (0.006) |
| Elective | 12/610 (2.0) | 33/598 (5.5) | 0.34 (0.18 to 0.67) (0.002) | 0.37 (0.18 to 0.73) (0.004) | 0.36 (0.18 to 0.75) (0.006) |
All-cause and aneurysm-related mortality
During 6904 person-years of follow-up, 524 deaths (76 aneurysm related) occurred. Table 4 presents the all-cause and aneurysm-related mortality results from Cox regression analysis by randomised group and time period. The crude all-cause mortality rates were 7.5 and 7.7 deaths per 100 person-years for the endovascular and open-repair groups, respectively [secondary adjusted HR 1.03 (95% CI 0.86 to 1.23, p = 0.72]. The crude aneurysm-related mortality rates were 1.0 and 1.2 per 100 person-years for the endovascular and open-repair groups, respectively [secondary adjusted HR 0.92 (95% CI 0.57 to 1.49), p = 0.73]. There was evidence of deviation from the proportional hazards assumption for aneurysm-related mortality (p = 0.004) with an early benefit of endovascular repair during the first 6 months [adjusted HR > 0.47 (95% CI 0.23 to 0.93), p = 0.03] counteracted by an increase in aneurysm-related deaths beyond 4 years [adjusted HR 4.85 (95% CI 1.04 to 22.72), p = 0.05]. There was no significant evidence of deviation from the proportional hazards assumption for all-cause mortality (p = 0.11). Figure 14 presents the Kaplan–Meier curves for all-cause and AAA-related mortality truncated at 8 years when 210 patients remained at risk. Kaplan–Meier estimates at 8 years for all-cause mortality were 54% (95% CI 50% to 59%) and 54% (95% CI 49% to 59%) for the EVAR and open-repair groups, respectively. Kaplan–Meier estimates at 8 years for AAA-related mortality were 93% (95% CI 90% to 95%) and 93% (95% CI 91% to 95%) for the EVAR and open-repair groups, respectively. Figure 14 demonstrates that all-cause mortality converged during the first 2 years, with aneurysm-related mortality converging at 6 years. Results for the tests of interaction between randomised group and age, sex, AAA diameter and CPI (as a marker of patient fitness) are given in Table 5 and causes of death are given in Table 6.
| Outcome | EVAR (n = 626): deaths/patients (crude rate per 100 person-years) | Open repair (n = 626): deaths/patients (crude rate per 100 person-years) | Crude HR (95% CI) (p-value) | Primarya adjusted HR (95% CI) (p-value) | Secondaryb adjusted HR (95% CI) (p-value) |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Total | 260/626 (7.5) | 264/626 (7.7) | 0.98 (0.82 to 1.16) (0.788) | 1.02 (0.86 to 1.22) (0.801) | 1.03 (0.86 to 1.23) (0.721) |
| 0–6 months | 26/626 (8.5) | 45/626 (15.0) | 0.57 (0.35 to 0.92) (0.022) | 0.62 (0.38 to 1.01) (0.056) | 0.61 (0.37 to 1.02) (0.058) |
| 6 months to 4 years | 125/599 (6.7) | 116/581 (6.3) | 1.06 (0.82 to 1.37) (0.645) | 1.10 (0.85 to 1.41) (0.478) | 1.12 (0.86 to 1.45) (0.389) |
| > 4 years | 109/472 (8.4) | 103/461 (7.9) | 1.04 (0.80 to 1.37) (0.753) | 1.11 (0.84 to 1.47) (0.469) | 1.09 (0.82 to 1.44) (0.567) |
| AAA-related mortality | |||||
| Total | 36/626 (1.0) | 40/626 (1.2) | 0.89 (0.57 to 1.39) (0.606) | 0.98 (0.62 to 1.56) (0.929) | 0.92 (0.57 to 1.49) (0.731) |
| 0–6 months | 14/626 (4.6) | 30/626 (10.0) | 0.46 (0.24 to 0.87) (0.017) | 0.52 (0.27 to 0.99) (0.046) | 0.47 (0.23 to 0.93) (0.031) |
| 6 months to 4 years | 12/599 (0.6) | 8/581 (0.4) | 1.48 (0.60 to 3.61) (0.393) | 1.76 (0.69 to 4.49) (0.236) | 1.46 (0.56 to 3.82) (0.442) |
| > 4 years | 10/472 (0.8) | 2/461 (0.2) | 4.96 (1.09 to 22.65) (0.039) | 4.73 (1.01 to 22.07) (0.048) | 4.85 (1.04 to 22.72) (0.045) |
| Outcomea | EVAR: deaths/patients (%) or (crude rate per 100 person-years) | Open repair: deaths/patients (%) or (crude rate per 100 person-years) | Crude odds or HR (95% CI) (p-value) | Primaryb adjusted odds or HR (95% CI) (p-value) | p-value from test of interaction in primary adjusted modela |
|---|---|---|---|---|---|
| 30-day mortality | |||||
| n = 614 | n = 602 | ORs | |||
| Age (years) | |||||
| < 74 | 2/300 (0.7) | 11/309 (3.6) | 0.18 (0.04 to 0.83) (0.027) | 0.17 (0.03 to 0.86) (0.032) | 0.222 |
| ≥ 74 | 9/314 (2.9) | 15/293 (5.1) | 0.55 (0.24 to 1.27) (0.160) | 0.65 (0.27 to 1.57) (0.342) | |
| Sex | |||||
| Males | 9/554 (1.6) | 22/548 (4.0) | 0.39 (0.18 to 0.87) (0.020) | 0.45 (0.20 to 1.01) (0.054) | 0.888 |
| Females | 2/60 (3.3) | 4/54 (7.4) | 0.43 (0.08 to 2.45) (0.343) | 0.28 (0.04 to 1.92) (0.194) | |
| AAA diameterc (cm) | |||||
| < 6.3 | 5/326 (1.5) | 10/314 (3.2) | 0.47 (0.16 to 1.40) (0.177) | 0.48 (0.15 to 1.53) (0.216) | 0.197 |
| ≥ 6.3 | 6/288 (2.1) | 16/287 (5.6) | 0.36 (0.14 to 0.93) (0.036) | 0.40 (0.15 to 1.05) (0.062) | |
| CPI scored | |||||
| < 4 | 3/307 (1.0) | 12/286 (4.2) | 0.23 (0.06 to 0.81) (0.022) | 0.22 (0.06 to 0.79) (0.021) | 0.088 |
| ≥ 4 | 7/268 (2.6) | 9/279 (3.2) | 0.80 (0.30 to 2.19) (0.671) | 0.81 (0.29 to 2.27) (0.683) | |
| All-cause mortality | |||||
| n = 626 | n = 626 | HRs | |||
| Age (years) | |||||
| < 74 | 83/306 (4.5) | 100/320 (5.4) | 0.82 (0.62 to 1.10) (0.191) | 0.81 (0.60 to 1.09) (0.170) | 0.481 |
| ≥ 74 | 177/320 (10.9) | 164/306 (10.3) | 1.08 (0.87 to 1.34) (0.475) | 1.15 (0.93 to 1.43) (0.194) | |
| Sex | |||||
| Males | 231/565 (7.3) | 241/570 (7.8) | 0.94 (0.79 to 1.13) (0.517) | 0.99 (0.82 to 1.19) (0.925) | 0.267 |
| Females | 29/61 (9.3) | 23/56 (7.2) | 1.44 (0.83 to 2.50) (0.198) | 1.43 (0.81 to 2.53) (0.217) | |
| AAA diameterc (cm) | |||||
| < 6.3 | 120/330 (6.5) | 118/327 (6.4) | 1.02 (0.79 to 1.32) (0.874) | 1.07 (0.83 to 1.39) (0.604) | 0.564 |
| ≥ 6.3 | 140/296 (8.6) | 146/298 (9.3) | 0.93 (0.74 to 1.17) (0.538) | 0.97 (0.77 to 1.23) (0.822) | |
| CPI scored | |||||
| < 4 | 107/313 (5.9) | 112/294 (6.8) | 0.87 (0.67 to 1.14) (0.322) | 0.92 (0.71 to 1.21) (0.564) | 0.186 |
| ≥ 4 | 134/274 (9.3) | 129/293 (8.0) | 1.17 (0.92 to 1.48) (0.214) | 1.21 (0.95 to 1.54) (0.125) | |
| AAA-related mortality | |||||
| n = 626 | n = 626 | HRs | |||
| Age (years) | |||||
| < 74 | 6/306 (0.3) | 18/320 (1.0) | 0.34 (0.14 to 0.86) (0.022) | 0.38 (0.15 to 0.98) (0.045) | 0.142 |
| ≥ 74 | 30/320 (1.9) | 22/306 (1.4) | 1.31 (0.76 to 2.28) (0.332) | 1.50 (0.85 to 2.67) (0.162) | |
| Sex | |||||
| Males | 29/565 (0.9) | 33/570 (1.1) | 0.87 (0.53 to 1.43) (0.584) | 1.00 (0.59 to 1.68) (0.996) | 0.993 |
| Females | 7/61 (2.3) | 7/56 (2.2) | 0.96 (0.34 to 2.74) (0.936) | 0.84 (0.28 to 2.49) (0.748) | |
| AAA diameterc (cm) | |||||
| < 6.3 | 13/330 (0.7) | 15/327 (0.8) | 0.86 (0.41 to 1.80) (0.682) | 1.04 (0.48 to 2.28) (0.913) | 0.644 |
| ≥ 6.3 | 23/296 (1.4) | 25/298 (1.6) | 0.90 (0.51 to 1.59) (0.728) | 0.95 (0.53 to 1.70) (0.858) | |
| CPI scored | |||||
| < 4 | 16/313 (0.9) | 17/294 (1.0) | 0.87 (0.44 to 1.73) (0.694) | 0.96 (0.48 to 1.92) (0.909) | 0.281 |
| ≥ 4 | 18/274 (1.2) | 16/293 (1.0) | 1.22 (0.62 to 2.40) (0.561) | 1.25 (0.64 to 2.47) (0.515) | |
| Cause of death | EVAR (n = 260) (36) | Open repair (n = 264) (40) | Total (n = 524) (76) |
|---|---|---|---|
| Prior to AAA repair | |||
| AAA rupture | 5 | 8 | 13 |
| IHD | 1 | 4 | 5 |
| Stroke | 0 | 1 | 1 |
| Other PAD | 1 | 1 | 2 |
| Cancer (lung) | 5 (0) | 2 (0) | 7 |
| Respiratory | 0 | 2 | 2 |
| Other | 0 | 1 | 1 |
| Total | 12 | 19 | 31 |
| Within 30 days of AAA repair | |||
| Procedure related (elective AAA repair) | 8 | 25 | 33 |
| Procedure related (emergency AAA repair) | 1 | 1 | 2 |
| Graft rupture after EVAR deploymentb | 2 | 0 | 2 |
| Total | 11 | 26 | 37 |
| Between 30 days and 4 years of AAA repair | |||
| Procedure related | 1 | 1 | 2 |
| Procedure related (emergency AAA repair) | 1 | 1 | 2 |
| Graft rupture after EVAR deploymentb | 8 | 2 | 10 |
| IHD | 31 | 25 | 56 |
| Stroke | 11 | 6 | 17 |
| Other PAD | 7 | 6 | 13 |
| Cancer (lung) | 38 (20) | 47 (20) | 85 (40) |
| Respiratory | 10 | 21 | 31 |
| Renal | 6 | 1 | 7 |
| Other | 16 | 9 | 25 |
| Unknown | 1 | 0 | 1 |
| Total | 130 | 119 | 249 |
| Beyond 4 years after AAA repair | |||
| Procedure related | 4 | 2 | 6 |
| Graft rupture after EVAR deploymentb | 6 | 0 | 6 |
| IHD | 27 | 26 | 53 |
| Stroke | 11 | 11 | 22 |
| Other PAD | 7 | 4 | 11 |
| Cancer (lung) | 22 (9) | 29 (9) | 51 (18) |
| Respiratory | 15 | 17 | 32 |
| Renal | 4 | 2 | 6 |
| Other | 11 | 9 | 20 |
| Total | 107 | 100 | 207 |
FIGURE 14.
Kaplan–Meier estimates for all-cause and AAA-related mortality by randomised group in EVAR trial 1.
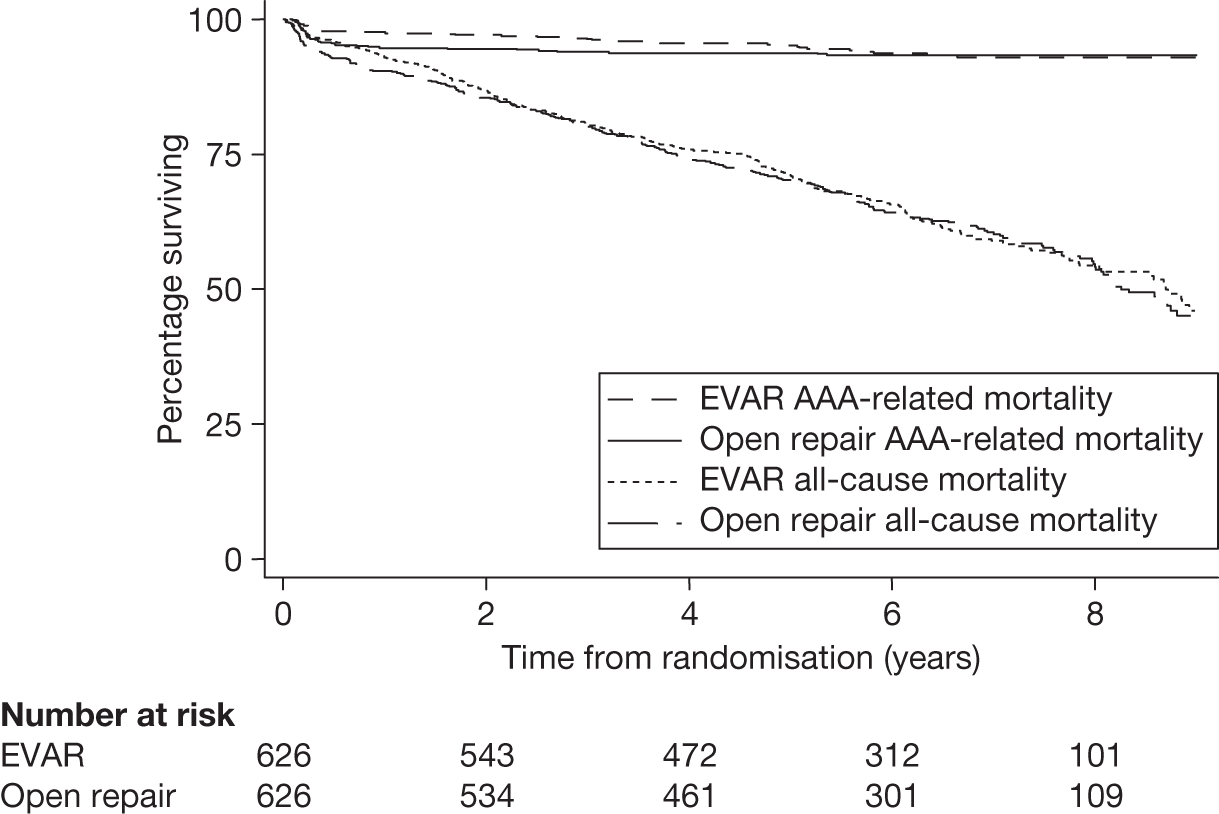
In order to assess whether or not there was any evidence of a significant difference in treatment effect across hospitals, a shared frailty term was added to the Cox model, which allowed centre to be included as a random effect term. There was no evidence to suggest a significant difference in outcome across the hospitals, with p-values from the secondary adjusted model of 0.500 for both the all-cause and AAA-related mortality models.
Per-protocol analyses for all-cause and aneurysm-related mortality
A per-protocol analysis was performed for the 1165 patients highlighted with an asterisk in Figure 10. A total of 469 deaths occurred (56 aneurysm related) in the per-protocol group. Crude all-cause mortality rates were 7.2 (endovascular repair) and 7.1 (open repair) per 100 person-years [adjusted HR 1.05 (95% CI 0.87 to 1.27), p = 0.61]. Crude aneurysm-related mortality rates were 0.9 (endovascular repair) and 0.8 (open repair) per 100 person-years [adjusted HR 1.06 (95% CI 0.60 to 1.88), p = 0.85].
Sensitivity analyses for missing data in mortality outcomes
Missing indicator method:
-
all-cause mortality secondary adjusted HR = 0.99 (95% CI 0.83 to 1.17), p = 0.889
-
AAA-related mortality secondary adjusted HR = 0.90 (95% CI 0.57 to 1.41), p = 0.650.
Multiple imputation method:
-
all-cause mortality secondary adjusted HR = 1.00 (95% CI 0.84 to 1.19), p = 0.977
-
AAA-related mortality secondary adjusted HR = 0.90 (95% CI 0.57 to 1.41), p = 0.636.
Secondary outcome results
Graft-related complications
During 5309 person-years of follow-up, a total of 567 graft complications were reported in 360 patients. Figure 15 presents the distribution of total number of complications detected for each patient and Table 7 presents the types of first graft complications by type of AAA repair completed during the primary procedure [not by intention to treat (ITT)], with total numbers of each complication in brackets in the first column. The number of complications reported was more than the number of reinterventions reported (see Figures 16 and 18).
FIGURE 15.
Distribution of the number of complications detected for each patient in EVAR trial 1.
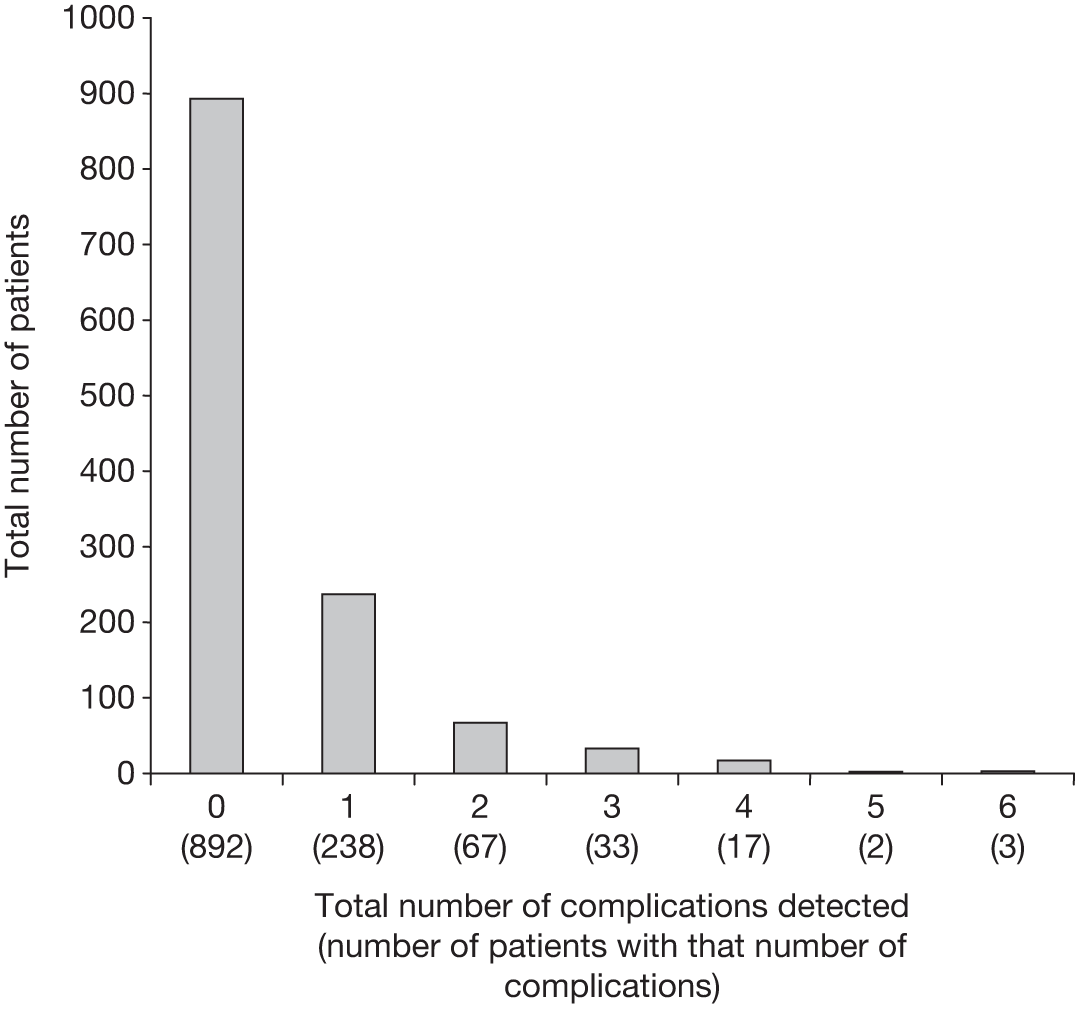
| Complication (total no. of particular complication)a | Successful EVARs completed (n = 624)b | Open repairs completed (n = 592)b |
|---|---|---|
| Graft rupture (25) | 9 | 0 |
| Deployment difficulties or conversion to open repair after primary procedure (25) | 8 | 5b |
| Graft infection (4) | 2 | 2 |
| Migration (48) | 29 | 0 |
| Type 1 endoleak (62)c | 40 | 0 |
| Type 3 endoleak (28)c | 13 | 0 |
| Kinking (24) | 10 | 1 |
| Sac, neck or iliac expansion (46) | 15 | 12 |
| Type 2 endoleakc + sac, neck or iliac expansion (34) | 17 | 1 |
| Type 2 endoleakc (122) | 91 | 2 |
| Graft thrombosis (41) | 20 | 2 |
| Graft stenosis (10) | 4 | 1 |
| Distal embolisation (2) | 1 | 0 |
| Renal infarction (5) | 2 | 0 |
| Anastomotic or false aneurysm (10) | 1 | 6 |
| Re-exploration of open repair (17) | 0 | 17 |
| Other surgery during primary admission (29) | 15 | 13 |
| Unclassifiable endoleak (6) | 5 | 0 |
| Haematoma (2) | 0 | 1 |
| Other (27) | 6 | 9 |
| Total (567) | 288 | 72 |
FIGURE 16.
Kaplan–Meier estimates for time to first complication by randomised group in EVAR trial 1.
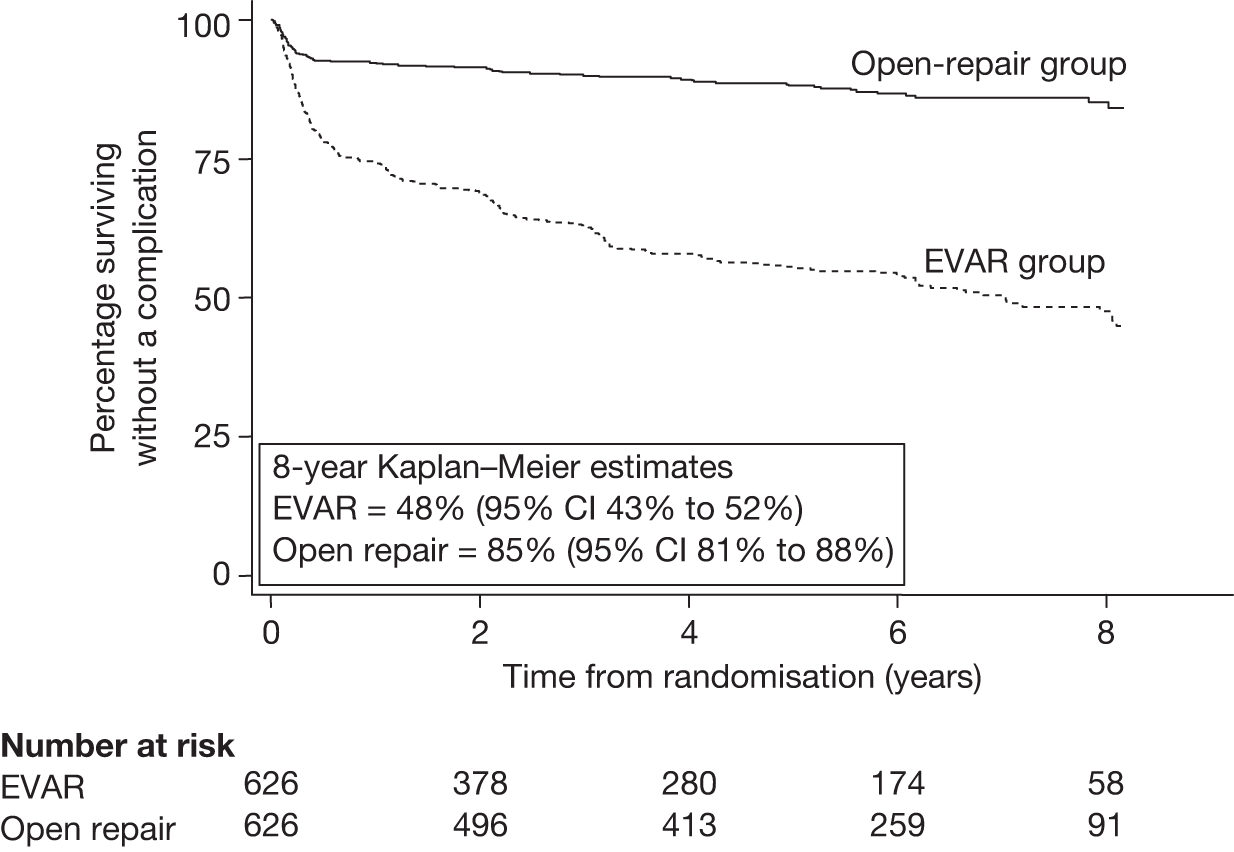
Table 8 presents the results of Cox regression analysis of time to first complication timed from randomisation by randomised group. Figure 16 shows the Kaplan–Meier estimates for time to first complication, truncated at 8 years. There was some evidence to suggest deviation from the proportional hazards assumption for randomised group (p = 0.011). Sensitivity analyses for missing data in the complications outcome did not demonstrate markedly different results: missing indicator method – time to first complication secondary adjusted HR 4.33 (95% CI 3.36 to 5.56), p < 0.0001; multiple imputation method – time to first complication secondary adjusted HR 4.44 (95% CI 3.45 to 5.72), p < 0.0001.
| Outcomea | EVAR (n = 626): events/patients (crude rate per 100 person-years) | Open repair (n = 626): events/patients (crude rate per 100 person-years) | Crude HR (95% CI) (p-value) | Primarya adjusted HR (95% CI) (p-value) | Secondaryb adjusted HR (95% CI) (p-value) |
|---|---|---|---|---|---|
| Complications | |||||
| Total | 282/626 (12.6) | 78/626 (2.5) | 4.38 (3.41 to 5.63) (< 0.0001) | 4.37 (3.39 to 5.63) (< 0.0001) | 4.39 (3.38 to 5.70) (< 0.0001) |
| 0–6 months | 132/626 (48.7) | 45/626 (15.6) | 3.08 (2.20 to 4.33) (< 0.0001) | 3.13 (2.22 to 4.41) (< 0.0001) | 3.18 (2.23 to 4.52) (< 0.0001) |
| 6 months to 4 years | 114/473 (9.0) | 18/550 (1.1) | 8.37 (5.09 to 13.76) (< 0.0001) | 8.21 (4.99 to 13.53) (< 0.0001) | 7.92 (4.80 to 13.09) (< 0.0001) |
| > 4 years | 36/280 (5.1) | 15/413 (1.4) | 3.65 (2.00 to 6.67) (< 0.0001) | 3.54 (1.92 to 6.51) (< 0.0001) | 3.33 (1.76 to 6.29) (< 0.0001) |
| Reinterventions | |||||
| Total | 145/626 (5.1) | 55/626 (1.7) | 2.78 (2.04 to 3.80) (< 0.0001) | 2.86 (2.09 to 3.92) (< 0.0001) | 2.86 (2.08 to 3.94) (< 0.0001) |
| 0–6 months | 66/626 (22.9) | 40/626 (13.8) | 1.65 (1.12 to 2.44) (0.012) | 1.69 (1.13 to 2.51) (0.010) | 1.75 (1.16 to 2.63) (0.007) |
| 6 months to 4 years | 55/537 (3.4) | 6/555 (0.3) | 9.97 (4.29 to 23.15) (< 0.0001) | 9.95 (4.28 to 23.1) (< 0.0001) | 9.12 (3.90 to 21.3) (< 0.0001) |
| > 4 years | 24/377 (2.4) | 9/428 (0.8) | 3.12 (1.47 to 6.80) (0.003) | 3.39 (1.56 to 7.41) (0.002) | 3.24 (1.48 to 7.11) (0.003) |
Graft-related reinterventions
During 6015 person-years of follow-up, a total of 257 reinterventions occurred in 200 patients. Figure 17 presents the distribution of total number of reinterventions for each patient and Table 8 presents the results of Cox regression analysis of time to first reintervention timed from randomisation by randomised group. There were a total of 25 graft ruptures (18 deaths within 30 days and no additional in-hospital deaths). There were 25 conversions to open repair (four deaths within 30 days and two additional in-hospital deaths beyond 30 days). Figure 18 shows the Kaplan–Meier estimates for time to first reintervention, truncated at 8 years. There was strong evidence to suggest deviation from the proportional hazards assumption for randomised group, p = 0.0001. Sensitivity analyses for missing data in the reinterventions outcome did not demonstrate markedly different results: missing indicator method – time to first reintervention secondary adjusted HR 2.79 (95% CI 2.04 to 3.80), p < 0.0001; multiple imputation method – time to first reintervention secondary adjusted HR 2.86 (95% CI 2.09 to 3.90), p < 0.0001.
FIGURE 17.
Distribution of the number of reinterventions for each patient in EVAR trial 1.
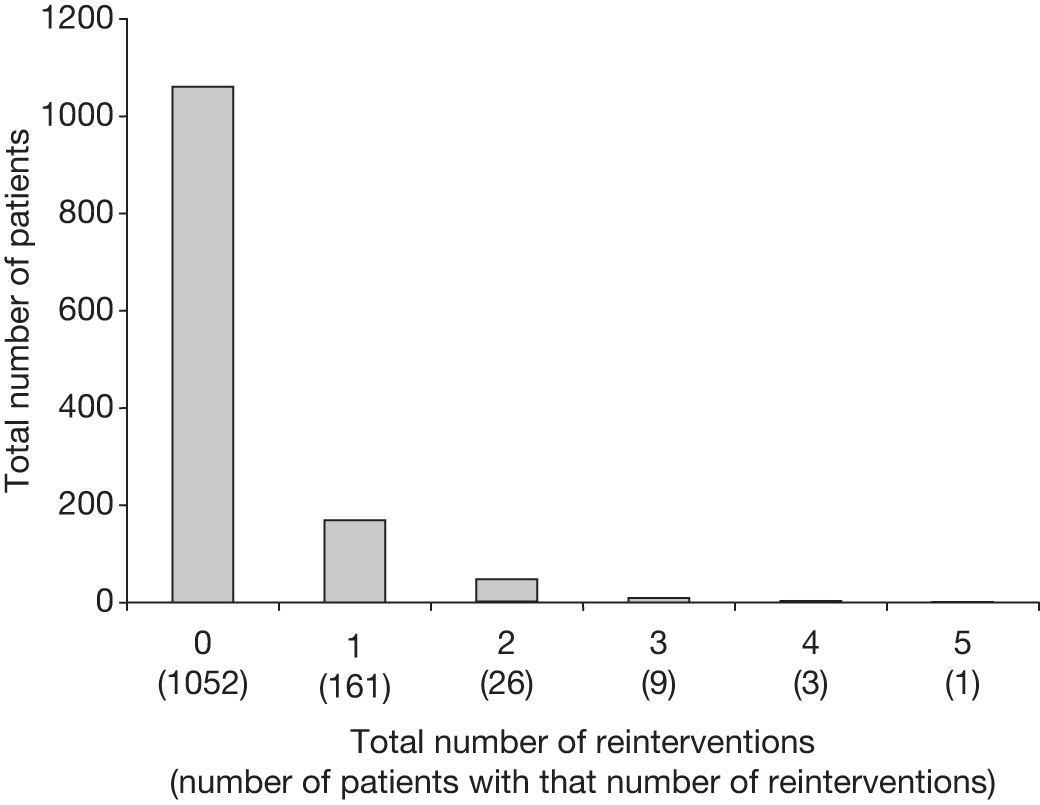
FIGURE 18.
Kaplan–Meier estimates for time to first reintervention by randomised group in EVAR trial 1.
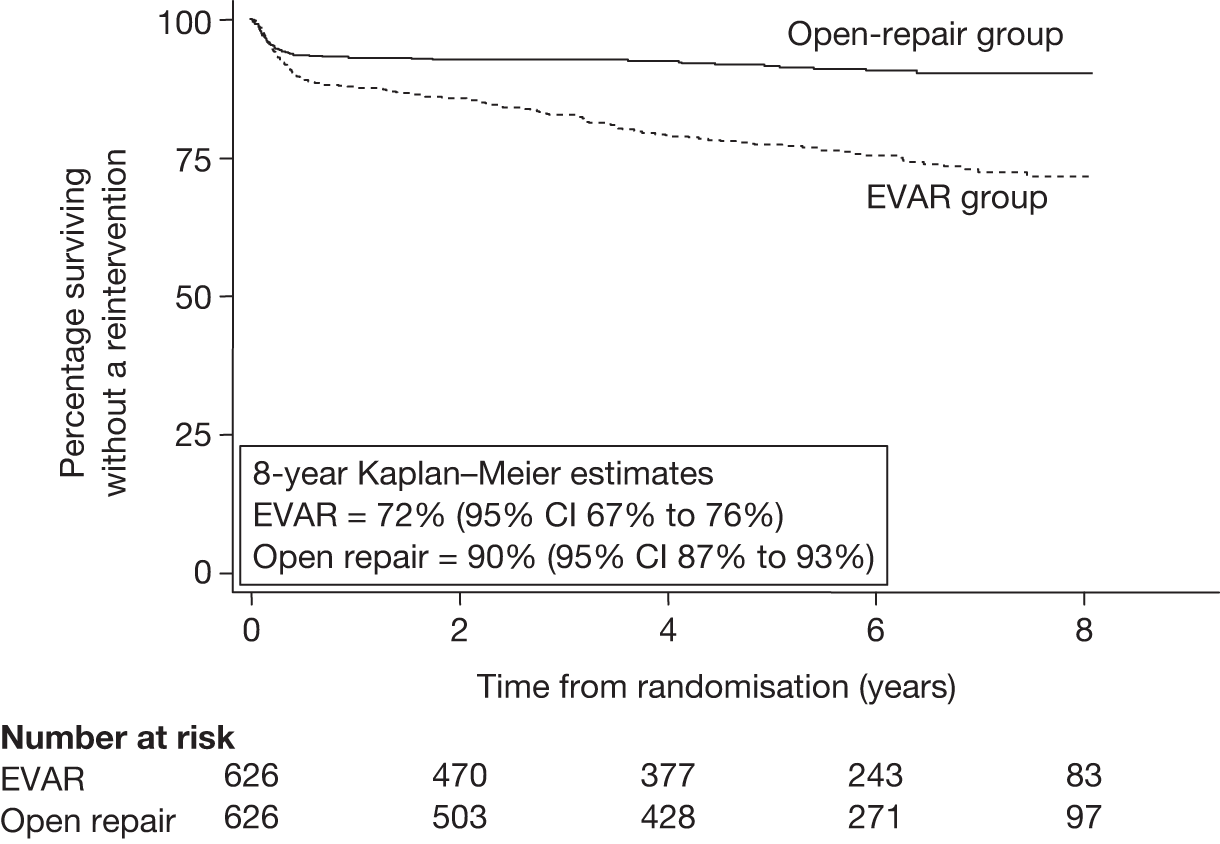
Adverse events
Table 9 presents a breakdown of the numbers of adverse events reported by randomised group. A more detailed analysis of cardiovascular events is given in the next section (see Subsidiary analyses, Renal function).
| Event | EVAR group (n = 626): no. of events (no. of patients) | Open-repair group (n = 626): no. of events (no. of patients) | Total: no. of events (no. of patients) |
|---|---|---|---|
| MI | 26 (24) | 35 (32) | 61 (56) |
| Stroke | 30 (26) | 40 (34) | 70 (66) |
| Renal failure | 12 (12) | 9 (9) | 21 (21) |
| Amputation | 6 (6) | 2 (2) | 8 (8) |
| Total | 74 (68) | 86 (77) | 160 (145) |
Health-related quality of life
Full quality-of-life data were collected only during the first year for the 1082 patients recruited during the planned recruitment phase up to 31 December 2003. However, the EuroQol (EQ-5D) questionnaire was collected annually throughout the trial for cost-effectiveness assessment. Table 10 presents the results of an analysis of covariance comparing the EQ-5D (scale and visual indices) and the Short-Form (SF-36) Physical Component Summary (PCS) and the Mental Component Summary (MCS) scores between randomised groups at 1, 3 and 12 months after randomisation. Table 11 presents the same analysis for each of the eight dimensions of the SF-36 score. There were no clear differences between the randomised groups apart from an anticipated significant decrease in physical functioning in the open-repair group during the first month. The data collected for the PGI and State–Trait Anxiety Index (STAI) questionnaire have not been analysed because of a lack of staff with adequate specialist understanding of these instruments.
| HRQL measure | EVAR (n = 543): mean (SD) (no. of patients) | Open repair (n = 539): mean (SD) (no. of patients) | Crude difference: mean (SE) | Difference adjusted for baseline: mean (SE) (no. of patients) (p-value) |
|---|---|---|---|---|
| EQ-5D scale index | ||||
| Baseline | 0.75 (0.22) (541) | 0.74 (0.23) (531) | 0.01 (0.01) | – |
| 1 month | 0.73 (0.21) (238) | 0.67 (0.25) (245) | 0.06 (0.02) | 0.05 (0.02) (482) (0.01) |
| 3 month | 0.71 (0.25) (476) | 0.73 (0.23) (414) | –0.01 (0.02) | –0.01 (0.01) (885) (0.37) |
| 12 month | 0.74 (0.24) (398) | 0.75 (0.25) (371) | –0.01 (0.02) | –0.02 (0.02) (764) (0.29) |
| EQ-5D visual index | ||||
| Baseline | 70.82 (16.66) (542) | 70.78 (16.92) (537) | 0.04 (1.02) | – |
| 1 month | 70.20 (16.25) (240) | 64.09 (19.12) (246) | 6.11 (1.61) | 5.60 (1.52) (486) (0.0002) |
| 3 month | 69.69 (18.10) (481) | 71.36 (16.98) (419) | –1.67 (1.18) | –1.37 (1.07) (899) (0.20) |
| 12 month | 71.29 (18.02) (397) | 72.53 (16.50) (374) | –1.24 (1.25) | –1.35 (1.12) (771) (0.23) |
| SF-36 PCS score | ||||
| Baseline | 39.92 (5.92) (533) | 39.83 (5.90) (534) | 0.08 (0.36) | – |
| 1 month | 37.82 (5.92) (225) | 36.14 (5.45) (242) | 1.68 (0.53) | 1.66 (0.50) (462) (0.001) |
| 3 month | 37.77 (5.73) (466) | 37.81 (5.84) (394) | –0.05 (0.40) | 0.04 (0.37) (849) (0.91) |
| 12 month | 38.17 (5.83) (359) | 38.33 (5.78) (339) | –0.16 (0.44) | –0.15 (0.40) (692) (0.71) |
| SF-36 MCS score | ||||
| Baseline | 43.59 (6.79) (533) | 43.95 (6.73) (534| | –0.35 (0.41) | – |
| 1 month | 43.86 (7.02) (225) | 44.04 (7.31) (242) | –0.18 (0.66) | –0.05 (0.66) (462) (0.94) |
| 3 month | 44.64 (6.67) (466) | 44.18 (6.81) (394) | 0.46 (0.46) | 0.41 (0.45) (849) (0.36) |
| 12 month | 44.54 (6.43) (359) | 44.76 (6.81) (339) | –0.22 (0.50) | –0.29 (0.49) (692) (0.56) |
| HRQL measure | EVAR (n = 543): mean (SD) (no. of patients) | Open repair (n = 539): mean (SD) (no. of patients) | Crude difference: mean (SE) | Difference adjusted for baseline: mean (SE) (no. of patients) (p-value) |
|---|---|---|---|---|
| Physical function | ||||
| Baseline | 67.02 (23.93) (540) | 66.01 (24.01) (537) | 1.02 (1.46) | – |
| 1 month | 60.21 (25.35) (238) | 51.15 (25.00) (246) | 9.06 (2.29) | 7.96 (1.92) (483) (< 0.0001) |
| 3 month | 59.09 (26.06) (477) | 60.13 (24.68) (415) | –1.05 (1.71) | –0.88 (1.36) (889) (0.52) |
| 12 month | 60.26 (26.88) (364) | 62.09 (25.70) (350) | –1.83 (1.97) | –2.97 (1.56) (713) (0.06) |
| Role – physical | ||||
| Baseline | 68.03 (29.52) (539) | 69.03 (29.71) (535) | –1.00 (1.81) | – |
| 1 month | 46.78 (27.71) (234) | 33.33 (24.89) (245) | 13.44 (2.40) | 13.36 (2.35) (476) (< 0.0001) |
| 3 month | 53.65 (30.52) (473) | 52.45 (29.46) (407) | 1.20 (2.03) | 1.84 (1.91) (875) (0.34) |
| 12 month | 62.24 (30.07) (368) | 63.28 (29.66) (346) | –1.04 (2.24) | –0.48 (2.05) (712) (0.81) |
| Role – mental | ||||
| Baseline | 74.85 (27.18) (539) | 77.23 (26.17) (535) | –2.38 (1.63) | – |
| 1 month | 66.76 (29.81) (234) | 60.53 (31.85) (243) | 6.23 (2.83) | 6.63 (2.75) (474) (0.02) |
| 3 month | 71.29 (28.38) (470) | 70.45 (28.08) (401) | 0.85 (1.92) | 1.05 (1.84) (867) (0.57) |
| 12 month | 73.20 (26.54) (369) | 74.82 (27.02) (347) | –1.62 (2.00) | –1.82 (1.96) (713) (0.35) |
| Social functioning | ||||
| Baseline | 48.84 (11.60) (541) | 48.77 (11.42) (537) | 0.08 (0.70) | – |
| 1 month | 48.90 (13.63) (239) | 48.93 (11.63) (245) | –0.03 (1.15) | –0.05 (1.15) (484) (0.97) |
| 3 month | 49.27 (11.44) (477) | 49.10 (12.81) (416) | 0.17 (0.81) | 0.13 (0.81) (892) (0.87) |
| 12 month | 48.82 (10.57) (370) | 49.32 (12.28) (351) | –0.51 (0.85) | –0.50 (0.85) (721) (0.56) |
| Mental health | ||||
| Baseline | 61.41 (9.65) (542) | 61.60 (10.40) (536) | –0.18 (0.61) | – |
| 1 month | 63.04 (9.35) (235) | 63.33 (10.51) (245) | –0.28 (0.91) | –0.20 (0.90) (480) (0.83) |
| 3 month | 63.04 (9.53) (474) | 63.17 (10.38) (415) | –0.14 (0.67) | 0.02 (0.65) (887) (1.00) |
| 12 month | 63.15 (10.32) (370) | 62.84 (9.43) (348) | 0.30 (0.74) | 0.35 (0.72) (717) (0.62) |
| Energy/vitality | ||||
| Baseline | 55.39 (10.65) (542) | 54.51 (11.37) (536) | 0.88 (0.67) | – |
| 1 month | 57.43 (11.38) (234) | 57.91 (10.15) (245) | –0.47 (0.98) | –0.59 (0.97) (479) (0.54) |
| 3 month | 56.65 (9.59) (474) | 55.01 (9.82) (415) | 1.64 (0.65) | 1.36 (0.63) (887) (0.03) |
| 12 month | 54.90 (10.88) (370) | 54.62 (9.50) (348) | 0.28 (0.76) | 0.28 (0.75) (717) (0.71) |
| Pain | ||||
| Baseline | 23.64 (23.25) (541) | 25.80 (25.35) (535) | –2.16 (1.48) | – |
| 1 month | 33.32 (23.54) (239) | 40.30 (22.94) (245) | –6.98 (2.11) | –5.88 (1.99) (482) (0.003) |
| 3 month | 30.24 (26.05) (474) | 29.98 (23.80) (411) | 0.26 (1.69) | 0.65 (1.56) (882) (0.68) |
| 12 month | 25.42 (24.35) (370) | 25.74 (24.04) (347) | –0.32 (1.81) | 0.17 (1.67) (715) (0.92) |
| General health | ||||
| Baseline | 56.67 (13.86) (540) | 56.00 (14.19) (536) | 0.66 (0.86) | – |
| 1 month | 56.81 (13.79) (233) | 61.32 (13.54) (244) | –4.51 (1.25) | –4.03 (1.14) (477) (0.001) |
| 3 month | 58.33 (14.11) (472) | 58.03 (13.20) (410) | 0.30 (0.92) | –0.13 (0.84) (878) (0.87) |
| 12 month | 57.13 (13.59) (367) | 56.19 (12.95) (346) | 0.93 (1.00) | 0.62 (0.94) (711) (0.51) |
Subsidiary analyses
Renal function
Figure 19 describes which patients were included and excluded from the renal analyses and Table 12 describes their baseline characteristics. Excluded patients were older (p < 0.001) and less fit in terms of mean ABPI (p = 0.003), FEV1 (p = 0.041) and eGFR (p < 0.001); this is perhaps a consequence of survival to 1 year being an inclusion criterion. Table 13 presents the baseline characteristics of the patients included by randomised group, with little evidence of any significant differences between them. A total of 972 patients were available – 509 in the EVAR group and 463 in the open-repair group – who provided a total of 4250 eGFR measurements during a mean (SD) follow-up of 3.9 (1.7) years. The mean (SD) volume of contrast agent used during the primary EVAR procedures was 203 ml (105 ml). Only a small number of patients developed end-stage kidney disease requiring dialysis during the course of follow-up (nine in each randomised group), with eGFR measurements excluded at and beyond this time. Approximately half of these 18 patients had shown some indication of renal impairment with baseline creatinine measurements of > 200 µmol/l. There was substantial correlation between the baseline and follow-up eGFR measurements, with correlation coefficients typically ranging from 0.6 to 0.85. Normal plots demonstrated reasonable approximation to the normal distribution for all the baseline variables and for the random effects slopes and intercepts generated from the multilevel model. Figure 20 presents the patients with their baseline renal function classified according to the KDOQI stages of renal impairment. Figure 21 demonstrates the distribution of rates of change seen across all patients with a mean rate of change of –1.03 ml/minute/1.73 m2 per year (range –8.1 to 7.5 ml/minute/1.73 m2 per year), with only eight patients exhibiting a renal function deterioration faster than –5 ml/minute/1.73 m2 per year (four in each randomised group).
| Baseline characteristic | Included in analysis (N = 972) | Excluded from analysis (N = 280) | p-value for comparisona |
|---|---|---|---|
| Age (years) | 73.7 (6.1) | 75.2 (6.1) | 0.0003 |
| Sex (% male) | 880 (91) | 255 (91) | 0.786 |
| AAA diameter (cm) | 6.4 (0.9) | 6.5 (1.0) | 0.091 |
| Top aortic neck diameter (cm) | 2.3 (0.3) | 2.4 (0.3) | 0.073 |
| Aortic neck length (cm) | 2.9 (1.2) | 2.8 (1.1) | 0.258 |
| BMI (kg/m2) | 26.6 (4.5) | 26.2 (4.4) | 0.280 |
| Diabetes: n (%) | 99 (10) | 30 (11) | 0.794 |
| Smoking status (%) | |||
| Current | 206 (21) | 64 (23) | 0.737 |
| Past | 675 (70) | 188 (67) | |
| Never | 89 (9) | 28 (10) | |
| Previous history of cardiac diseaseb (%) | 408 (42) | 121 (43) | 0.712 |
| Systolic blood pressure (mmHg) | 148 (21) | 146 (22) | 0.200 |
| Diastolic blood pressure (mmHg) | 82 (13) | 82 (11) | 0.876 |
| Treated for hypertension (%) | 496 (52) | 152 (57) | 0.171 |
| ABPI (mean of both legs) | 1.03 (0.17) | 0.99 (0.19) | 0.003 |
| FEV1 (l) | 2.2 (0.7) | 2.1 (0.7) | 0.041 |
| Serum creatinine (μmol/l)c | 101 (89–118) | 106 (92–126) | 0.0001 |
| Serum eGFR (ml/minute/1.73 m2) | 64.9 (17.1) | 60.7 (18.4) | 0.0004 |
| Serum cholesterol (mmol/l) | 5.1 (1.1) | 5.2 (1.2) | 0.313 |
| Aspirin use (%) | 515 (53) | 148 (53) | 0.970 |
| Statin use (%) | 332 (34) | 108 (39) | 0.160 |
| Non-steroidal anti-inflammatory drug use (%) | 63 (6) | 17 (6) | 0.802 |
| Beta-blocker use (%) | 269 (28) | 81 (29) | 0.663 |
| Baseline characteristic | EVAR group (N = 509) (2262 eGFR measurements) | Open-repair group (N = 463) (1988 eGFR measurements) | p-value for comparisona |
|---|---|---|---|
| Age at randomisation (years) | 73.8 (6.1) | 73.6 (6.1) | 0.613 |
| No. of males: n (%) | 459 (90) | 421 (91) | 0.689 |
| AAA diameter (cm) | 6.4 (0.9) | 6.5 (0.9) | 0.488 |
| Top aortic neck diameter (cm) | 2.3 (0.3) | 2.3 (0.3) | 0.599 |
| Aortic neck length (cm) | 2.8 (1.2) | 2.9 (1.3) | 0.718 |
| BMI (kg/m2) | 26.6 (4.6) | 26.5 (4.4) | 0.823 |
| Diabetes: n (%) | 44 (9) | 55 (12) | 0.087 |
| Smoking status: n (%) | |||
| Current | 107 (21) | 99 (22) | 0.172 |
| Past | 346 (68) | 329 (71) | |
| Never | 55 (11) | 34 (7) | |
| Previous history of cardiac disease:b n (%) | 210 (41) | 198 (43) | 0.634 |
| Systolic blood pressure (mmHg) | 149 (22) | 147 (21) | 0.225 |
| Diastolic blood pressure (mmHg) | 82 (12) | 82 (13) | 0.413 |
| Treated for hypertension: n (%) | 257 (52) | 239 (52) | 0.803 |
| ABPI (mean of both legs) | 1.02 (0.17) | 1.04 (0.17) | 0.077 |
| FEV1 (l) | 2.16 (0.71) | 2.20 (0.68) | 0.366 |
| Serum creatinine (μmol/l)c | 102 (90–117) | 101 (89–118) | 0.887 |
| Serum eGFR (ml/minute/1.73 m2) | 64.8 (16.5) | 65.1 (17.8) | 0.787 |
| Serum cholesterol (mmol/l) | 5.1 (1.2) | 5.1 (1.1) | 0.783 |
| Aspirin use: n (%) | 270 (53) | 245 (53) | 0.968 |
| Statin use: n (%) | 169 (34) | 163 (35) | 0.615 |
| Non-steroidal anti-inflammatory drug use: n (%) | 28 (6) | 35 (7) | 0.190 |
| Beta-blocker use: n (%) | 141 (28) | 128 (28) | 0.999 |
FIGURE 19.
Flow chart describing patients included and excluded from the renal function analysis in EVAR trial 1.
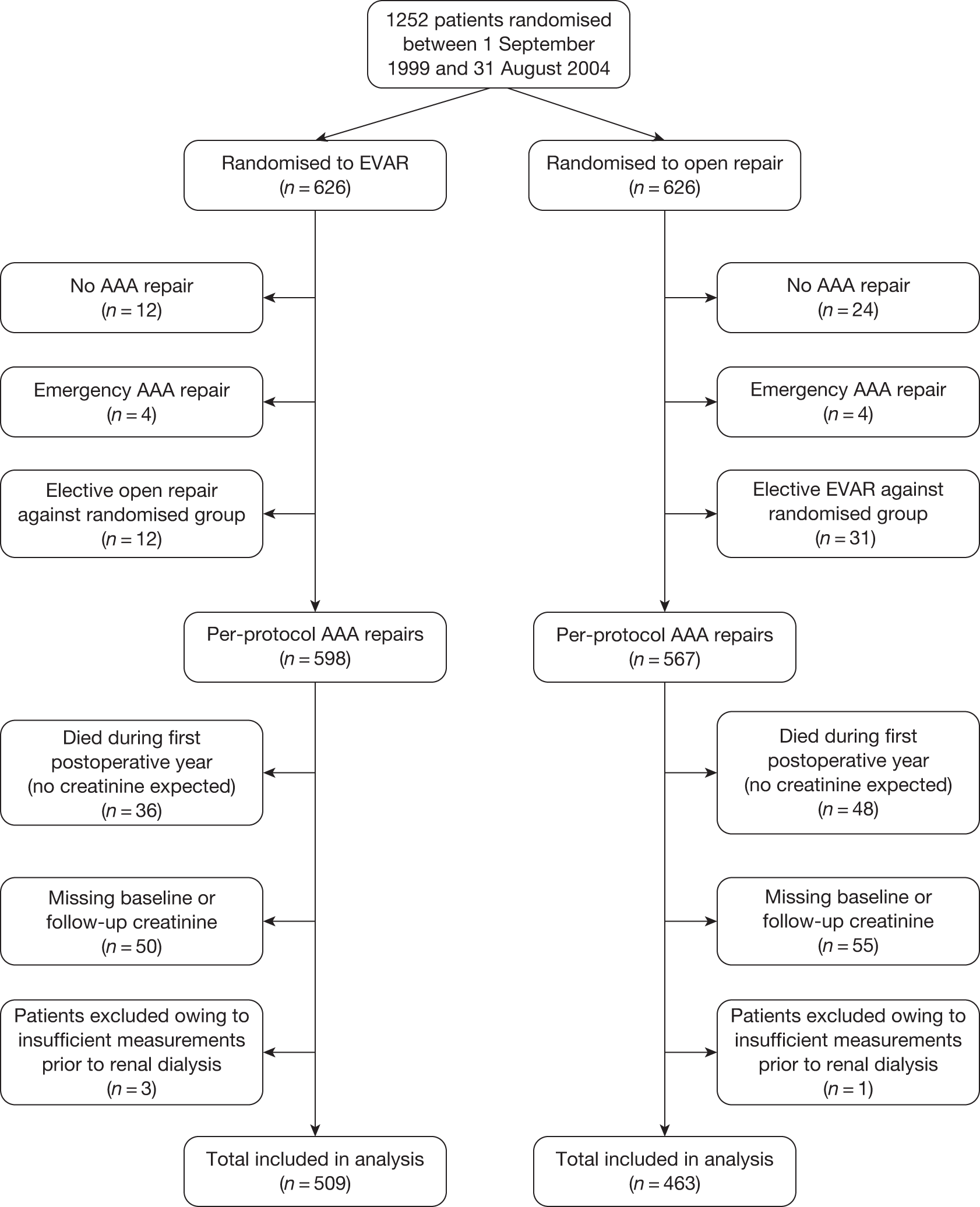
FIGURE 20.
Classification of baseline renal impairment staging according to the National Kidney Foundation KDOQI in EVAR trial 1. a, Stages 1 and 2, eGFR > 60 ml/minute/1.73 m2 – normal or mild impairment; stage 3, eGFR 30–59 ml/minute/1.73 m2 – moderate impairment; stage 4, eGFR 15–29 ml/minute/1.73 m2 – severe impairment; stage 5, eGFR < 15 ml/minute/1.73 m2 – renal failure, referred for dialysis.
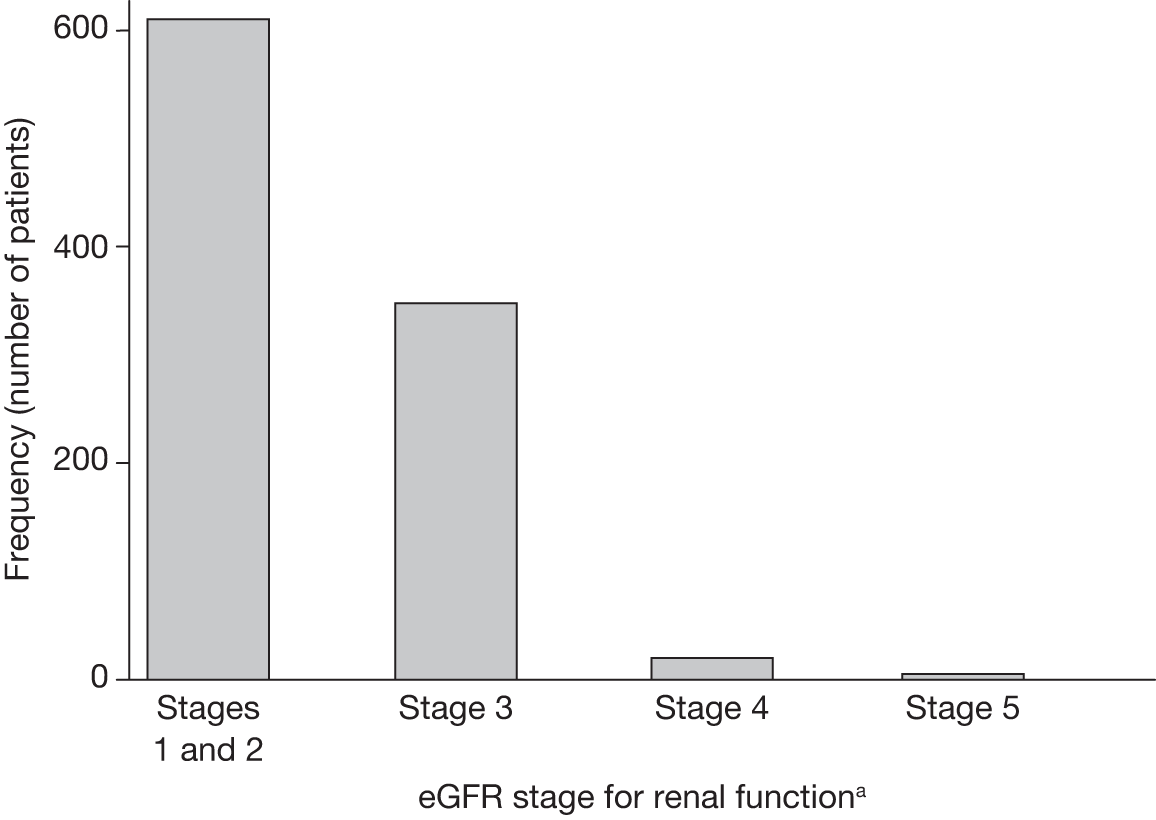
FIGURE 21.
Histogram of rates of change in eGFR for 972 patients included in renal function analysis in EVAR trial 1.
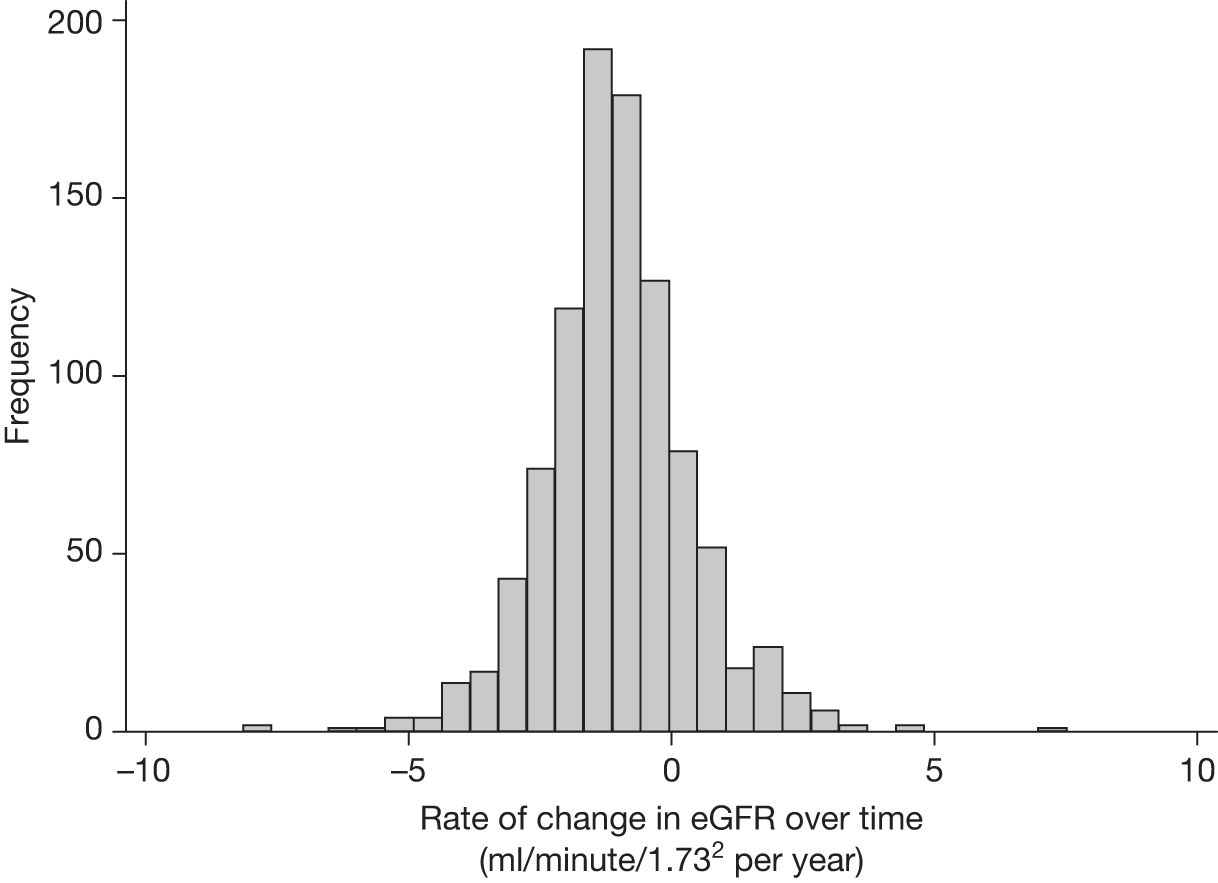
The mean (SD) rates of change of eGFR in the EVAR and open-repair groups were –1.13 ml/minute/1.73 m2 per year (1.43 ml/minute/1.73 m2 per year) and –1.00 ml/minute/1.73 m2 per year (1.43 ml/minute/1.73 m2 per year), respectively, but this difference was not statistically significant in the crude, primary or secondary adjusted models – p = 0.275, p = 0.208, p = 0.286, respectively.
Cardiovascular mortality and events
Figure 14 (see Primary outcome results – mortality, All-cause and aneurysm-related mortality) demonstrates an early separation between the all-cause mortality curves, which is driven by the two-thirds reduction in 30-day operative mortality seen after EVAR compared with open repair. However, the curves converge during the first 2 years, with no significant difference in all-cause mortality beyond this time. This also has been demonstrated in other series of published data comparing EVAR with open repair. 115,160 One hypothesis to explain this convergence is that patients with significant cardiac or carotid artery disease who survived the initial EVAR procedure subsequently died of this cardiovascular disease during the early postoperative years. 159 In the equivalent group in the open-repair arm of the trial, more died during the early postoperative period as a result of the greater stress response to major open surgery. Therefore, it was proposed to use the EVAR trial 1 data to investigate whether or not cardiovascular events (MI and stroke) differed between EVAR and open repair, and whether or not an excess of cardiovascular deaths after endovascular repair explained the 2-year convergence in survival curves between the groups.
The types of first cardiovascular event and death are presented in Table 14. A total of 187 first cardiovascular events occurred during an average of 5.1 years of follow-up. Of the 187 patients, 30 patients had one additional event and three patients had two additional events generating a total of 223 events: crude overall rate 3.5 (robust 95% CI 3.1 to 4.0) events per 100 person-years. Five cardiovascular events occurred before any aortic repair had been performed (one in the EVAR group and four in the open-repair group) and 32 events occurred within 30 days of aneurysm repair (10 in the EVAR group and 22 in the open-repair group). By September 2009, a total of 524 deaths had occurred (see Table 6) during an average of 5.5 years of follow-up, with 256 classified as cardiovascular: crude rate of 3.7 (95% CI 3.3 to 4.2) cardiovascular deaths per 100 person-years.
| Event type | EVAR (n = 626) | Open repair (n = 626) |
|---|---|---|
| Cardiovascular events | ||
| Fatal MIa | 25 | 18 |
| Non-fatal MIb | 23 | 32 |
| Fatal strokea | 14 | 17 |
| Non-fatal strokeb | 24 | 34 |
| Total | 86 | 101 |
| Cardiovascular deaths | ||
| AAA procedure related | 13 | 28 |
| Rupture of unrepaired AAA | 7 | 10 |
| Cardiac | 59 | 55 |
| Stroke | 22 | 18 |
| Other vascular | 15 | 11 |
| Endograft rupture | 16 | 2 |
| Total | 132 | 124 |
Comparisons of event rates by randomised group and time periods are shown in Table 15, with Kaplan–Meier estimates in Figure 22. There was some evidence of deviation from the proportional hazards assumption for both cardiovascular events (p = 0.084) and cardiovascular deaths (p = 0.049). The overall rate of cardiovascular events was non-significantly lower in the EVAR than the open-repair group (2.6 vs 3.2 per 100 person-years, respectively) – adjusted HR 0.83 (95% CI 0.62 to 1.10), p = 0.199. The observation of a lower cardiovascular event rate in the EVAR group in the early 0–6 months period demonstrated borderline significance, whereas after 2 years the event rates appeared to be similar between groups. Overall, there was no difference in terms of cardiovascular mortality: adjusted HR 1.06 (95% CI 0.83 to1.36), p = 0.638. There was a significantly lower incidence of cardiovascular deaths (mainly operative) in the endovascular group during the first 6 months. Beyond 6 months, there was an apparent excess of cardiovascular deaths in the endovascular group although this was not statistically significant during either the 6–24 months period [adjusted HR 1.44 (95% CI 0.79 to 2.62), p = 0.237] or beyond 2 years [adjusted HR 1.25 (95% CI 0.91 to 1.72), p = 0.172]. To investigate whether the difference between EVAR and open repair differed between patients with or without a previous history of cardiac disease, a test of interaction was performed between this baseline variable and randomised group. There was no evidence to suggest a significant interaction for either cardiovascular events (adjusted p = 0.937) or deaths (adjusted p = 0.473). A post hoc analysis combined the cardiovascular events with the cardiovascular deaths, generating a total of 335 events (164 and 171 in the EVAR and open-repair groups, respectively). Cox regression for this combined outcome also did not demonstrate any difference between the groups [adjusted HR 0.92 (95% CI 0.74 to 1.14), p = 0.466].
| Time period | EVAR (n = 626): no. of events/patients (rate per 100 person-years) | Open repair (n = 626): no. of events/patients (rate per 100 person-years) | Crude HR (95% CI) p-value | Adjusted hazarda ratio (95% CI) p-value |
|---|---|---|---|---|
| Cardiovascular events | ||||
| Total follow-up | 86/626 (2.6) | 101/626 (3.2) | 0.82 (0.61 to 1.09) 0.164 | 0.83 (0.62 to 1.10) 0.199 |
| 0–6 months | 18/626 (6.0) | 29/626 (10.0) | 0.60 (0.34 to 1.09) 0.093 | 0.60 (0.33 to 1.09) 0.095 |
| 7–24 months | 18/584 (2.2) | 22/564 (2.8) | 0.78 (0.42 to 1.46) 0.442 | 0.81 (0.43 to 1.51) 0.503 |
| > 24 months | 50/523, (2.4) | 50/502 (2.5) | 0.95 (0.64 to 1.41) 0.798 | 0.96 (0.65 to 1.43) 0.853 |
| Cardiovascular deaths | ||||
| Total follow-up | 132/626 (3.8) | 124/626 (3.6) | 1.05 (0.82 to 1.35) 0.674 | 1.06 (0.83 to 1.36) 0.638 |
| 0–6 months | 20/626 (6.5) | 37/626 (12.3) | 0.53 (0.31 to 0.92) 0.023 | 0.52 (0.30 to 0.91) 0.021 |
| 7–24 months | 27/599 (3.1) | 18/581 (2.1) | 1.46 (0.81 to 2.66) 0.210 | 1.44 (0.79 to 2.62) 0.237 |
| > 24 months | 85/543 (3.7) | 69/534 (3.0) | 1.23 (0.89 to 1.69) 0.206 | 1.25 (0.91 to 1.72) 0.172 |
FIGURE 22.
Kaplan–Meier curves for (a) time to first cardiovascular event and (b) time to cardiovascular death by randomised group in EVAR trial 1.
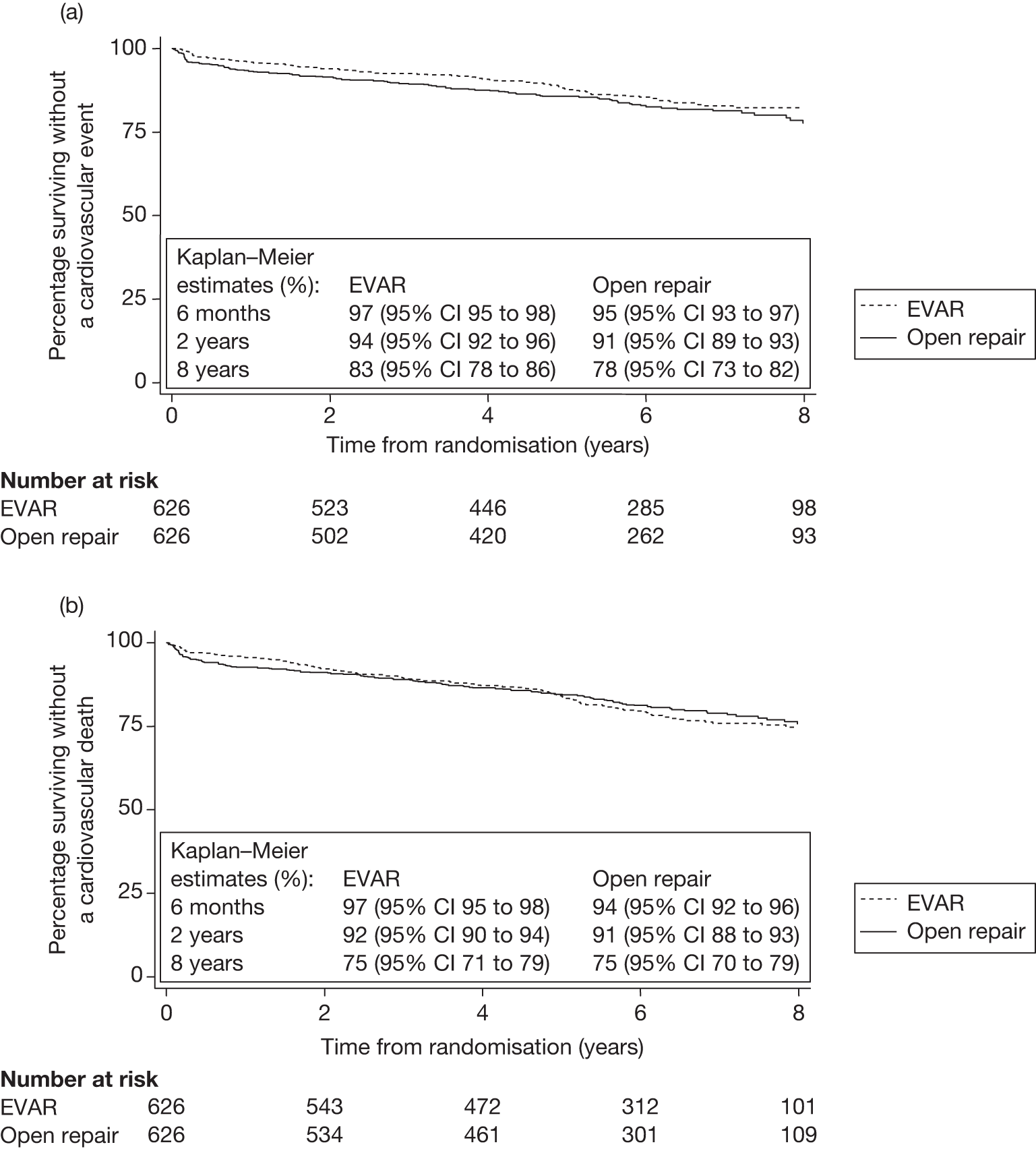
Chapter 5 Results for EVAR trial 2
Descriptive results
Patients were recruited across 33 hospitals and followed for a minimum of 5 years until 1 September 2009 (average 7.5 years) for mortality and until the end of December 2009 for graft-related complications, reinterventions and adverse events. The baseline characteristics of the randomised groups are given in Table 16; there were no apparent differences between the groups. The overall mean (SD) age was 76.8 (6.5) years and 347 (86%) were men. The mean (SD) aneurysm diameter was 6.7 (1.0) cm. The ascribing of patients’ fitness, and thus eligibility for either trial 1 or 2, had been earmarked as potentially very important when designing the trials, which is why recommended guidelines had been integrated into the case record forms at the start of the trials (see Figure 5). Table 17 presents the specific questions asked on the case record forms, as well as the numbers of patients who recorded a positive response in EVAR trial 2. Thus, this classification appeared to work rather well, with the equivalent percentages in EVAR trial 1 being considerably lower: 530/1252 (42%) for cardiac disease, 51/1252 (4%) for respiratory disease and 33/1252 (3%) for renal disease. Table 17 also suggests that there were a small number of patients entered into EVAR trial 2 in whom ‘yes’ had been recorded for questions 1–3 and it is likely that further preoperative optimisation might have been required for these patients, leading to considerable delays before any EVAR could be performed.
| Baseline characteristica | EVAR (n = 197) | No intervention (n = 207) |
|---|---|---|
| Age (years) | 77.2 (6.3) [0] | 76.4 (6.7) [0] |
| No. of males (%) | 168 (85) [0] | 179 (86) [0] |
| AAA diameter (cm) | 6.8 (1.0) [0] | 6.7 (1.0) [0] |
| BMI (kg/m2) | 26.4 (5.0) [1] | 26.5 (4.4) [1] |
| Diabetes (%) | 30 (15) [2] | 29 (14) [2] |
| Smoking status (%) | (0) | (0) |
| Current | 33 [17] | 37 [18] |
| Past | 152 [77] | 156 [75] |
| Never | 12 [6] | 14 [7] |
| History of cardiac diseaseb (%) | 132 (67) [0] | 153 (74) [0] |
| Single cardiac morbidity | 38 | 57 |
| Two or more comorbidities | 94 | 96 |
| Angina | 107 | 121 |
| Unstable angina | 10 | 8 |
| Previous MI | 90 | 97 |
| Coronary revascularisation | 45 | 36 |
| Valve disease | 16 | 22 |
| Arrhythmia | 47 | 46 |
| Congestive cardiac failure | 19 | 13 |
| Systolic blood pressure (mmHg) | 140 (20) [0] | 139 (23) [0] |
| Diastolic blood pressure (mmHg) | 79 (12) [0] | 79 (12) [3] |
| ABPI (mean of both legs) | 0.99 (0.20) [10] | 0.98 (0.19) [8] |
| FEV1 (l) | 1.6 (0.6) [7] | 1.7 (0.7) [4] |
| Serum creatinine (μmol/l)a | 107 (90–134) [0] | 112 (94–140) [2] |
| Serum cholesterol (mmol/l) | 4.8 (1.2) [13] | 4.8 (1.1) [7] |
| Statin use (%) | 82 (42) [1] | 86 (42) [0] |
| Aspirin use (%) | 114 (58) [1] | 114 (55) [0] |
| CPI scorec | 10.8 (12.3) [19] | 9.4 (10.5) [12] |
| CRF question | No. of patients with positive response in EVAR trial 2 (n = 404) |
|---|---|
| Cardiac status | |
| 1. Has the patient had a MI within the last 3 months? | 4 |
| 2. Has the patient experienced onset of angina within last 3 months? | 44 |
| 3. Does the patient have unstable angina at night or at rest? | 18 |
| If yes to any of questions 1–3, entry unlikely into either trial at this stage | |
| 4. Is there a past history of MI? | |
| 5. Is there a history of cardiac revascularisation? | |
| 6. Is there a past history of angina pectoris? | |
| 7. Is there severe heart valve disease? | |
| 8. Is there significant arrhythmia? | |
| 9. Is there uncontrolled congestive cardiac failure? | |
| If ‘yes’ to any of questions 4–9, patient may be more suitable for EVAR trial 2 | 285 (71%) |
| If ‘no’ to all of questions 4–9, patient may be suitable for EVAR trial 1 | 119 |
| Respiratory status | |
| 10. Is FEV1 < 1.0 l? | |
| If ‘yes’ to question 10, patient may be more suitable for EVAR trial 2 | 65 (16%) |
| If ‘no’ to question 10, patient may be suitable for EVAR trial 1 | 339 |
| Renal status | |
| 11. Is serum creatinine > 200 μmol/l? | |
| If ‘yes’ to question 11, patient may be more suitable for EVAR trial 2 | 34 (8%) |
| If ‘no’ to question 11, patient may be suitable for EVAR trial 1 | 370 |
| Confirmation of decision to offer EVAR trial 1 or 2 | |
| 12. Having answered questions 1–11, in the views of your anaesthetist and surgeon, is your patient fit for open repair? | Yes/No |
| 13. If not, is your patient suitable for EVAR trial 2? | Yes/No |
| 14. Which trial has the patient been offered? | EVAR trial 1/EVAR trial 2 |
| 15. Is the abdomen hostile such that open repair is not an option? | Yes/No |
A CONSORT diagram is provided in Figure 11. A total of 249 AAA repairs occurred (10 emergency). The median (IQR) time from randomisation to AAA repair was 55 (38–77) days for the patients randomised to EVAR and 244 (83–643) days for those randomised to no intervention. Of the 18 patients who died prior to AAA repair in the EVAR group, seven died within 6 months of randomisation (two ruptures), eight became too unfit or unsuitable for EVAR, one refused AAA repair and for two the reason is unknown. Of the 64 patients having elective repair in the no-intervention group, 14 became tender, eight demonstrated fast growth, one experienced symptoms, one was incorrectly entered into trial 2 rather than trial 1, 24 refused surveillance and for 16 no reason was provided. By January 2010, 11 patients remained alive without AAA repair. The choice of graft manufacturer for the 229 EVAR procedures was split primarily between four device manufacturers – Cook/Zenith 140 (61%), Medtronic/Talent 52 (23%) and Gore/Excluder 11 (5%), Medtronic/AneuRx 10 (4%) – and ‘other’ for 11 (5%) and ‘unknown’ for five procedures (2%).
Primary outcome results – mortality
Operative mortality
In the EVAR group, a total of 13 deaths occurred within 30 days of AAA repair (7.3%) and 15 occurred in hospital prior to discharge (8.4%). These values drop to 10/175 (5.7%) and 11/175 (6.3%), respectively, for elective AAA repairs. These figures are somewhat lower than those published in the mid-term results of 2005 (9% for total 30-day mortality),198 as none of the 31 additional patients randomised to EVAR between January and August 2004 died within 30 days of surgery (0/29). Additional analyses comparing the 338 patients randomised before 31 December 2003 with the 66 randomised after demonstrated significantly older age, lower creatinine, lower cholesterol and higher statin use in the 66 additional patients. However, these differences have not influenced the overall differences between randomised groups seen in Table 16.
In the no-intervention group, a total of two deaths occurred within 30 days of AAA repair (2.9%) and three occurred in hospital prior to discharge (4.3%). These values drop to 1/64 (1.6%) and 2/64 (3.1%), respectively, for elective AAA repairs.
All-cause and aneurysm-related mortality
During 1413 person-years of follow-up, 305 deaths (78 aneurysm related) occurred. Table 18 presents the all-cause and aneurysm-related mortality results from Cox regression analysis by randomised group and time period. The crude all-cause mortality rates were 21.0 and 22.1 deaths per 100 person-years for the endovascular and no-intervention groups, respectively [secondary adjusted HR 0.99 (95% CI 0.78 to 1.27), p = 0.967]. The crude aneurysm-related mortality rates were 3.6 and 7.3 per 100 person-years for the endovascular and no-intervention groups, respectively [secondary adjusted HR 0.53 (95% CI 0.32 to 0.89), p = 0.015]. There was strong evidence of deviation from the proportional hazards assumption for aneurysm-related mortality (p < 0.001) with an early detriment of endovascular repair during the first 6 months [adjusted HR 1.78 (95% CI 0.75 to 4.21), p = 0.188] being counteracted by a decrease in aneurysm-related deaths beyond this time [adjusted HR between 6 months and 4 years 0.34 (95% CI 0.16 to 0.72), p = 0.005, and no events in the EVAR group beyond 4 years]. There was only borderline evidence of deviation from the proportional hazards assumption for all-cause mortality (p = 0.07). Figure 23 presents the Kaplan–Meier curves for all-cause and AAA-related mortality truncated at 8 years. The Kaplan–Meier estimates at 6 years for all-cause mortality were 30% (95% CI 24% to 37%) and 26% (95% CI 20% to 32%) for the EVAR and no-intervention groups, respectively. The Kaplan–Meier estimates at 6 years for AAA-related mortality were 86% (95% CI 79% to 90%) and 64% (95% CI 55% to 72%) for the EVAR and no-intervention groups, respectively. Results for the tests of interaction between randomised group and age, sex, AAA diameter and CPI are given in Table 19 and causes of death are given in Table 20.
| Outcome | EVAR (n = 197): deaths/patients (crude rate per 100 person-years) | No intervention (n = 207): deaths/patients (crude rate per 100 person-years) | Crude HR (95% CI) (p-value) | Primarya adjusted HR (95% CI) (p-value) | Secondaryb adjusted HR (95% CI) (p-value) |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Total | 145/197 (21.0) | 160/207 (22.1) | 0.95 (0.76 to 1.19) (0.661) | 0.98 (0.78 to 1.24) (0.879) | 0.99 (0.78 to 1.27) (0.967) |
| 0–6 months | 24/197 (26.0) | 19/207 (19.0) | 1.38 (0.76 to 2.52) (0.295) | 1.48 (0.80 to 2.71) (0.209) | 1.32 (0.68 to 2.54) (0.410) |
| 6 months to 4 years | 92/173 (21.4) | 108/188 (23.6) | 0.90 (0.69 to 1.20) (0.481) | 0.96 (0.72 to 1.29) (0.800) | 1.02 (0.75 to 1.37) (0.921) |
| > 4 years | 29/81 (17.3) | 33/80 (20.0) | 0.86 (0.52 to 1.42) (0.560) | 0.77 (0.48 to 1.30) (0.327) | 0.72 (0.42 to 1.24) (0.237) |
| AAA-related mortality | |||||
| Total | 25/197 (3.6) | 53/207 (7.3) | 0.50 (0.31 to 0.81) (0.005) | 0.55 (0.34 to 0.89) (0.015) | 0.53 (0.32 to 0.89) (0.015) |
| 0–6 months | 15/197 (16.3) | 9/207 (9.0) | 1.82 (0.80 to 4.16) (0.156) | 1.93 (0.84 to 4.46) (0.122) | 1.78 (0.75 to 4.21) (0.188) |
| 6 months to 4 years | 10/173 (2.3) | 35/188 (7.6) | 0.31 (0.15 to 0.62) (0.001) | 0.33 (0.16 to 0.68) (0.003) | 0.34 (0.16 to 0.72) (0.005) |
| > 4 years | 0/81 (0) | 9/80 (5.5) | c | c | c |
| Outcomea | EVAR (n = 197): deaths/patients (crude rate per 100 person-years) | No intervention (n = 207): deaths/patients (crude rate per 100 person-years) | Crude HR (95% CI) (p-value) | Primaryb adjusted HR (95% CI) (p-value) | p-value from test of interaction in primary adjusted modela |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Age (years) | |||||
| < 77 | 67/98 (18.0) | 74/104 (18.2) | 0.98 (0.71 to 1.37) (0.920) | 1.01 (0.72 to 1.44) (0.936) | 0.871 |
| ≥ 77 | 78/99 (24.5) | 86/103 (27.1) | 0.93 (0.68 to 1.26) (0.632) | 0.96 (0.70 to 1.33) (0.826) | |
| Sex | |||||
| Males | 122/168 (20.6) | 138/179 (21.8) | 0.95 (0.75 to 1.22) (0.699) | 0.96 (0.75 to 1.24) (0.774) | 0.984 |
| Females | 23/29 (23.0) | 22/28 (24.3) | 0.95 (0.53 to 1.71) (0.871) | 0.90 (0.48 to 1.68) (0.737) | |
| AAA diameter (cm) | |||||
| < 6.5 | 68/98 (18.4) | 84/110 (21.2) | 0.88 (0.64 to 1.21) (0.424) | 0.94 (0.67 to 1.33) (0.734) | 0.103 |
| ≥ 6.5 | 77/99 (24.1) | 76/97 (23.2) | 1.03 (0.75 to 1.42) (0.843) | 1.01 (0.72 to 1.41) (0.948) | |
| CPI score | |||||
| < 11 | 66/95 (18.0) | 78/105 (20.4) | 0.90 (0.65 to 1.25) (0.521) | 0.82 (0.59 to 1.15) (0.252) | 0.320 |
| ≥ 11 | 67/83 (26.8) | 75/90 (26.2) | 1.03 (0.74 to 1.44) (0.847) | 1.11 (0.79 to 1.56) (0.558) | |
| AAA-related mortality | |||||
| Age (years) | |||||
| < 77 | 9/98 (2.4) | 26/104 (6.4) | 0.38 (0.18 to 0.81) (0.012) | 0.38 (0.18 to 0.84) (0.017) | 0.528 |
| ≥ 77 | 16/99 (5.0) | 27/103 (8.5) | 0.61 (0.33 to 1.14) (0.122) | 0.70 (0.37 to 1.33) (0.276) | |
| Sex | |||||
| Males | 21/168 (3.6) | 42/179 (6.6) | 0.54 (0.32 to 0.91) (0.022) | 0.60 (0.35 to 1.03) (0.063) | 0.376 |
| Females | 4/29 (4.0) | 11/28 (12.2) | 0.36 (0.11 to 1.12) (0.079) | 0.32 (0.10 to 1.04) (0.058) | |
| AAA diameter (cm) | |||||
| < 6.5 | 13/98 (3.5) | 22/110 (5.6) | 0.66 (0.33 to 1.31) (0.233) | 0.71 (0.34 to 1.45) (0.345) | 0.952 |
| ≥ 6.5 | 12/99 (3.8) | 31/97 (9.5) | 0.39 (0.20 to 0.76) (0.006) | 0.41 (0.21 to 0.82) (0.011) | |
| CPI scorec | |||||
| < 11 | 10/95 (2.7) | 27/105 (7.0) | 0.40 (0.19 to 0.82) (0.012) | 0.36 (0.17 to 0.74) (0.006) | 0.217 |
| ≥ 11 | 14/83 (5.6) | 23/90 (8.0) | 0.71 (0.37 to 1.39) (0.321) | 0.74 (0.37 to 1.46) (0.382) | |
| Cause of death | EVAR (n = 145) (25) | No intervention (n = 160) (53) | Total (n = 305) (78) |
|---|---|---|---|
| Between randomisation and 6 months | |||
| Procedure related | 8 | 1 | 9 |
| AAA rupture | 7 | 8 | 15 |
| IHD | 2 | 5 | 7 |
| Stroke | 0 | 2 | 2 |
| Cancer (lung) | 1 (1) | 0 | 1 (1) |
| Respiratory | 5 | 1 | 6 |
| Renal | 0 | 1 | 1 |
| Other | 1 | 1 | 2 |
| Total | 24 | 19 | 43 |
| 6 months to 4 years | |||
| Procedure related | 3 | 0 | 3 |
| AAA rupture | 6 | 35 | 41 |
| Graft rupture after EVAR deployment | 1 | 0 | 1 |
| IHD | 30 | 32 | 62 |
| Stroke | 3 | 2 | 5 |
| Other PAD | 0 | 3 | 3 |
| Cancer (lung) | 21 (7) | 14 (3) | 35 (10) |
| Respiratory | 19 | 9 | 28 |
| Renal | 2 | 3 | 5 |
| Other | 7 | 8 | 15 |
| Unknown | 0 | 2 | 2 |
| Total | 92 | 108 | 200 |
| Beyond 4 years | |||
| Procedure related | 0 | 2 | 2 |
| AAA rupture | 0 | 7 | 7 |
| IHD | 10 | 9 | 19 |
| Stroke | 1 | 1 | 2 |
| Cancer (lung) | 6 (3) | 7 (3) | 13 (6) |
| Respiratory | 7 | 4 | 11 |
| Other | 5 | 3 | 8 |
| Total | 29 | 33 | 62 |
FIGURE 23.
Kaplan–Meier estimates for all-cause and AAA-related mortality by randomised group in EVAR trial 2.
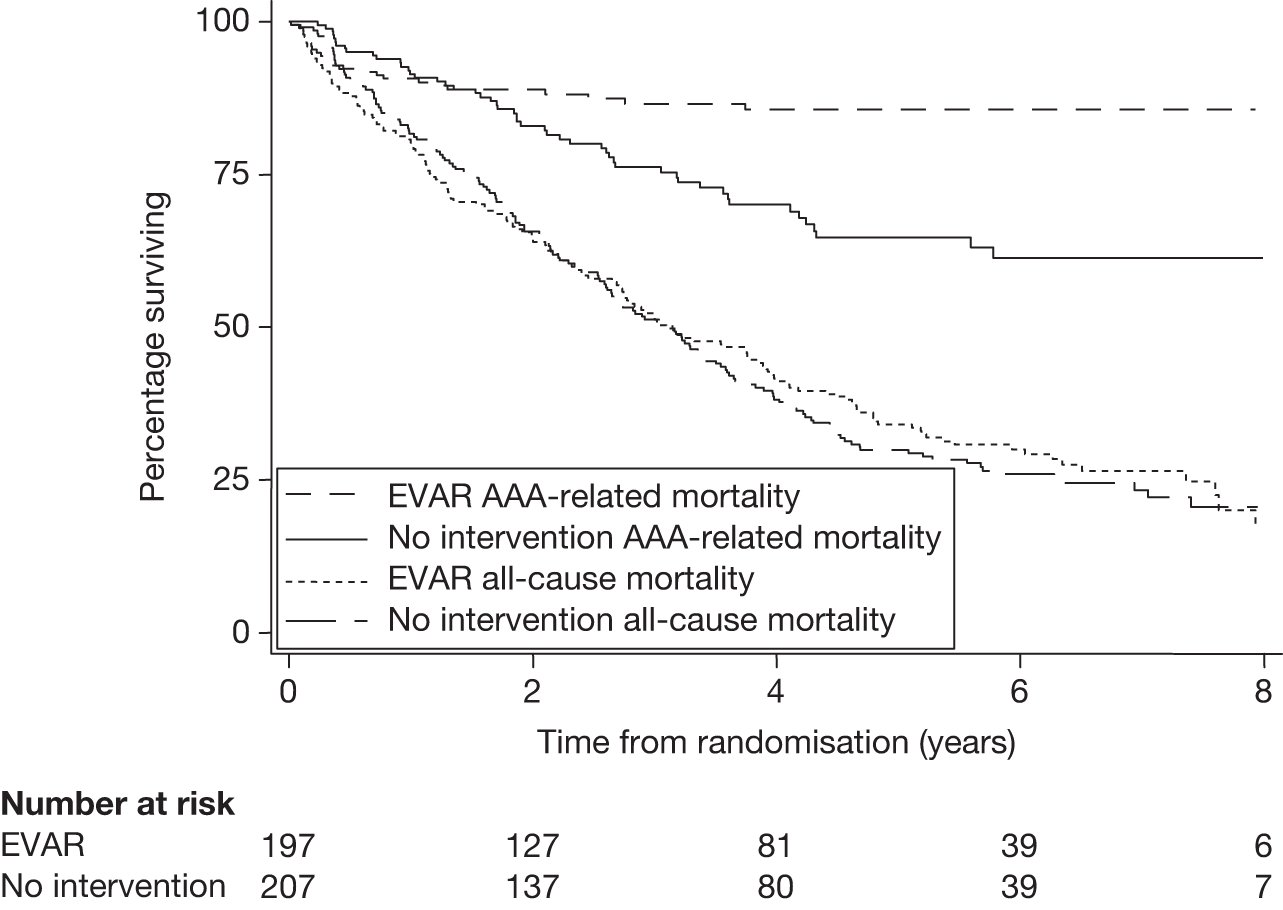
In order to assess whether there was any evidence of a significant difference in treatment effect across hospitals, a shared frailty term was added to the Cox model, which allowed centre to be included as a random effect term. There was weak evidence to suggest a significant difference in outcome across the hospitals for all-cause mortality (p-value from secondary adjusted model = 0.071). There was no evidence to suggest any significant difference across centres for AAA-related mortality (p-value from secondary adjusted model = 0.333).
Per-protocol analyses for all-cause and aneurysm-related mortality
A per-protocol analysis was defined in the analysis plan prior to inspection of any results and performed on the patients who had complied with their randomised allocation (see Figure 11). In the group randomised to EVAR, per-protocol patients were defined as those in whom elective EVAR was attempted, even if the surgeon subsequently changed to open repair during the primary procedure in theatre. Patients who died without undergoing aneurysm repair or who had an emergency repair were included as per-protocol patients. Patients who had elective open repair in the EVAR group were censored at aneurysm repair. In the group randomised to no intervention, per-protocol patients were defined as those who remained without aneurysm repair at the end of the study or who had emergency repair as a result of rupture. Patients undergoing any type of elective aneurysm repair in the no-intervention group were censored at the time of repair. The results for both all-cause (269 deaths) and aneurysm-related mortality (75 deaths) moved marginally in favour of EVAR: secondary adjusted HR for all-cause mortality 0.82 (95% CI 0.63 to1.07), p = 0.140; secondary adjusted HR for aneurysm-related mortality 0.41 (95% CI 0.24 to 0.69), p = 0.001. Figure 24 presents the per-protocol Kaplan–Meier curves truncated at 8 years.
FIGURE 24.
Kaplan–Meier estimates for per-protocol analysis of all-cause and AAA-related mortality by randomised group in EVAR trial 2.
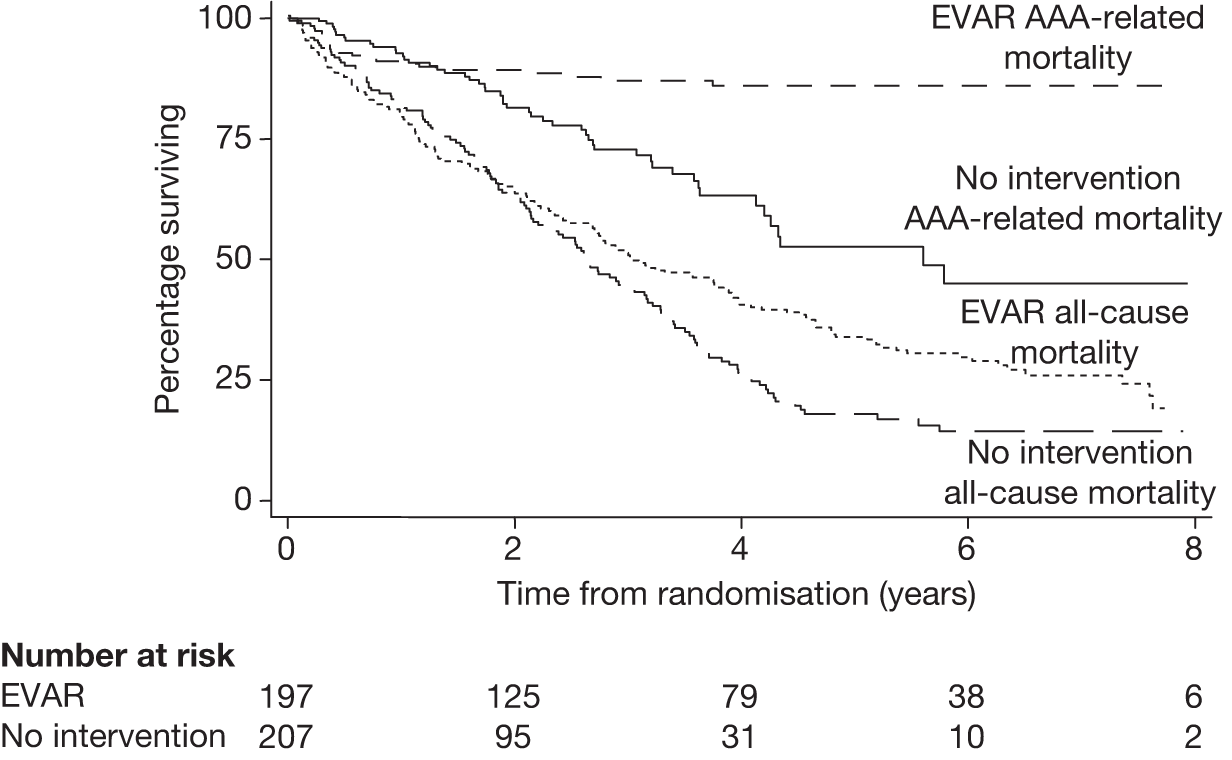
Given that a considerable number of patients in the no-intervention group crossed over and had aneurysm repair, a post hoc analysis was performed comparing the baseline fitness of the 70 patients who had aneurysm repair in the no-intervention group with the 179 patients who had aneurysm repair in the EVAR group. The CPI was used to ascribe patient fitness. 108,109 This is a validated prognostic score for operative mortality after open repair but it was used in this instance as a marker of patient fitness, with higher values indicating worse fitness. The mean (SD) CPI score was 5.8 (9.5) for the 70 non-compliant patients compared with 10.5 (11.8) for the 179 compliant patients (Student’s t-test, p-value = 0.004). Thus, patients who crossed over from the no-intervention group appeared to be fitter at baseline. Unfortunately, data are not available to determine their fitness level at the later time of aneurysm repair.
Sensitivity analyses for missing data in mortality outcomes
-
Missing indicator method:
-
– all-cause mortality secondary adjusted HR 0.92 (95% CI 0.73 to 1.16), p = 0.478
-
– AAA-related mortality secondary adjusted HR 0.47 (95% CI 0.29 to 0.77), p = 0.002.
-
-
Multiple imputation method:
-
– all-cause mortality secondary adjusted HR 0.99 (95% CI 0.78 to 1.24), p = 0.918
-
– AAA-related mortality secondary adjusted HR 0.51 (95% CI 0.32 to 0.83), p = 0.007.
-
Secondary outcome results
Rupture of non-repaired aneurysms
There were a total of 68 ruptures across both randomised groups (55 in the no-intervention group). Emergency repair was performed for 10 patients (six in the no-intervention group) of whom five survived (all in the no-intervention group). After censoring at non-rupture death or elective AAA repair, the crude rate of rupture in the no-intervention group was 12.4 (95% CI 9.6 to 16.2) ruptures per 100 person-years. The Kaplan–Meier estimates for time to rupture are presented in Figure 25, demonstrating that the rate was approximately constant over time.
FIGURE 25.
Kaplan–Meier estimates for AAA rupture in the 207 patients randomised to no intervention in EVAR trial 2.
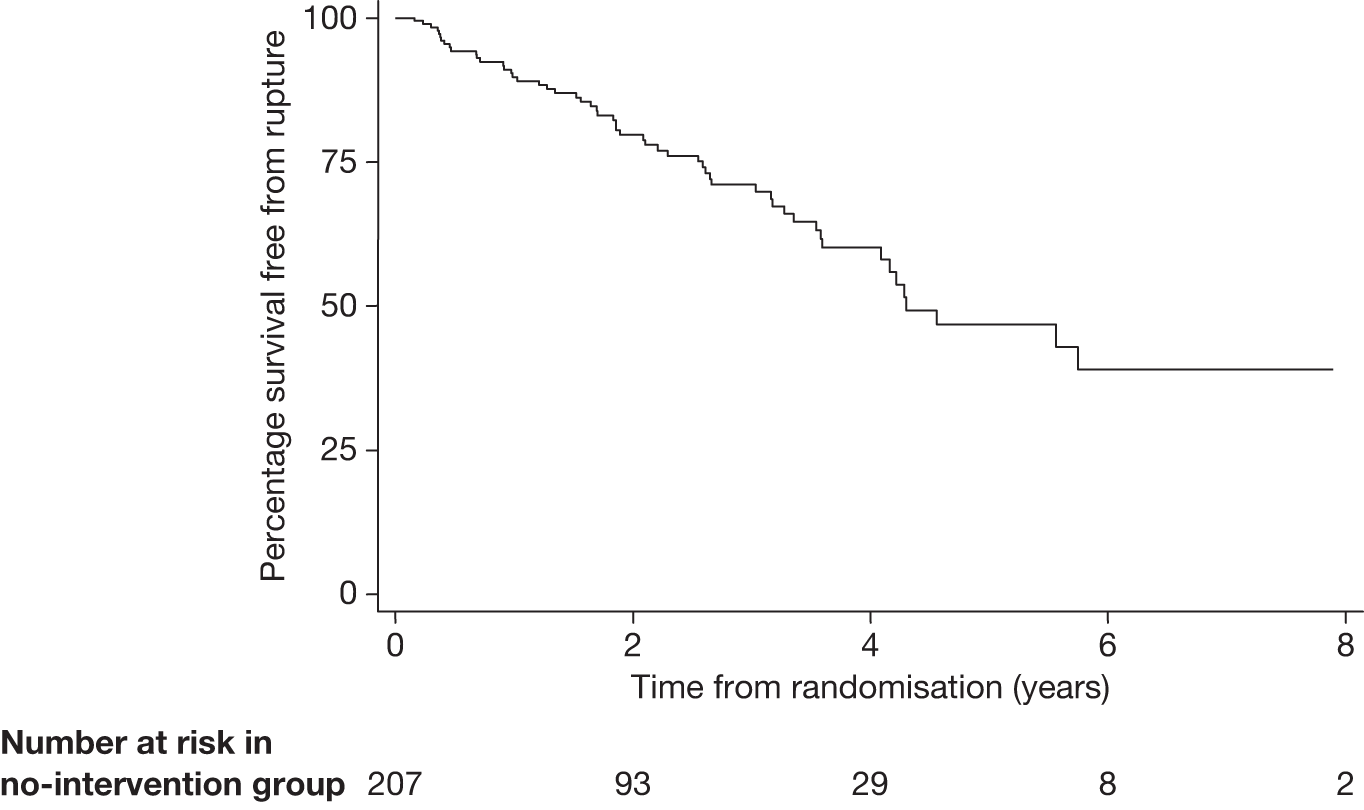
Graft-related complications and reinterventions
During 1084 person-years of follow-up, a total of 158 graft complications were reported in 97 patients. Fifty-two patients had just one complication, 33 patients had two complications, eight patients had three complications and four patients had four complications. Table 21 presents the types of first graft complications occurring after the EVARs performed in each randomised group with total numbers of each complication in brackets in the first column. Among the 20 open repairs performed across both arms of the trial, a total of five complications occurred (one thrombosis, one graft infection and three re-explorations of the open repair). Graft rupture occurred in two patients after the placement of an endograft (one patient underwent insertion of a stent on an emergency basis and survived, and the other underwent attempted conversion to open repair but died). Conversions to open repair occurred for other reasons in an additional two patients, and both survived. A total of 66 graft-related reinterventions were performed in 55 patients, with one reintervention in 48 patients, two reinterventions in three patients and three reinterventions in four patients. Figure 26 presents the Kaplan–Meier curves for cumulative incidence of first complications and reinterventions in the EVAR group.
| Complication (total no. of particular complication)b | EVARs in EVAR group (n = 174) | EVARs in no-intervention group (n = 55) |
|---|---|---|
| Graft rupture (2) | 0 | 0 |
| Deployment difficulties or conversion to open repair after primary procedure (3) | 2 | 1 |
| Graft infection (3) | 0 | 0 |
| Migration (6) | 1 | 0 |
| Type 1 endoleak (25)c | 11 | 6 |
| Type 3 endoleak (11)c | 5 | 1 |
| Kinking (4) | 1 | 2 |
| Sac, neck or iliac expansion (11) | 3 | 1 |
| Type 2 endoleakc + sac, neck or iliac expansion (11) | 9 | 1 |
| Type 2 endoleakc (40) | 18 | 6 |
| Graft thrombosis (16) | 3 | 2 |
| Graft stenosis (1) | 0 | 0 |
| Renal infarction (2) | 2 | 0 |
| Anastomotic or false aneurysm (1) | 0 | 1 |
| Other surgery during primary admission (11) | 8 | 1 |
| Unclassifiable endoleak (3) | 1 | 0 |
| Other (5) | 3 | 0 |
| Unknown (3) | 2 | 1 |
| Total (158) | 69 | 23 |
FIGURE 26.
Kaplan–Meier estimates for cumulative incidence of complications and reinterventions for the 197 patients randomised to EVAR in EVAR trial 2.
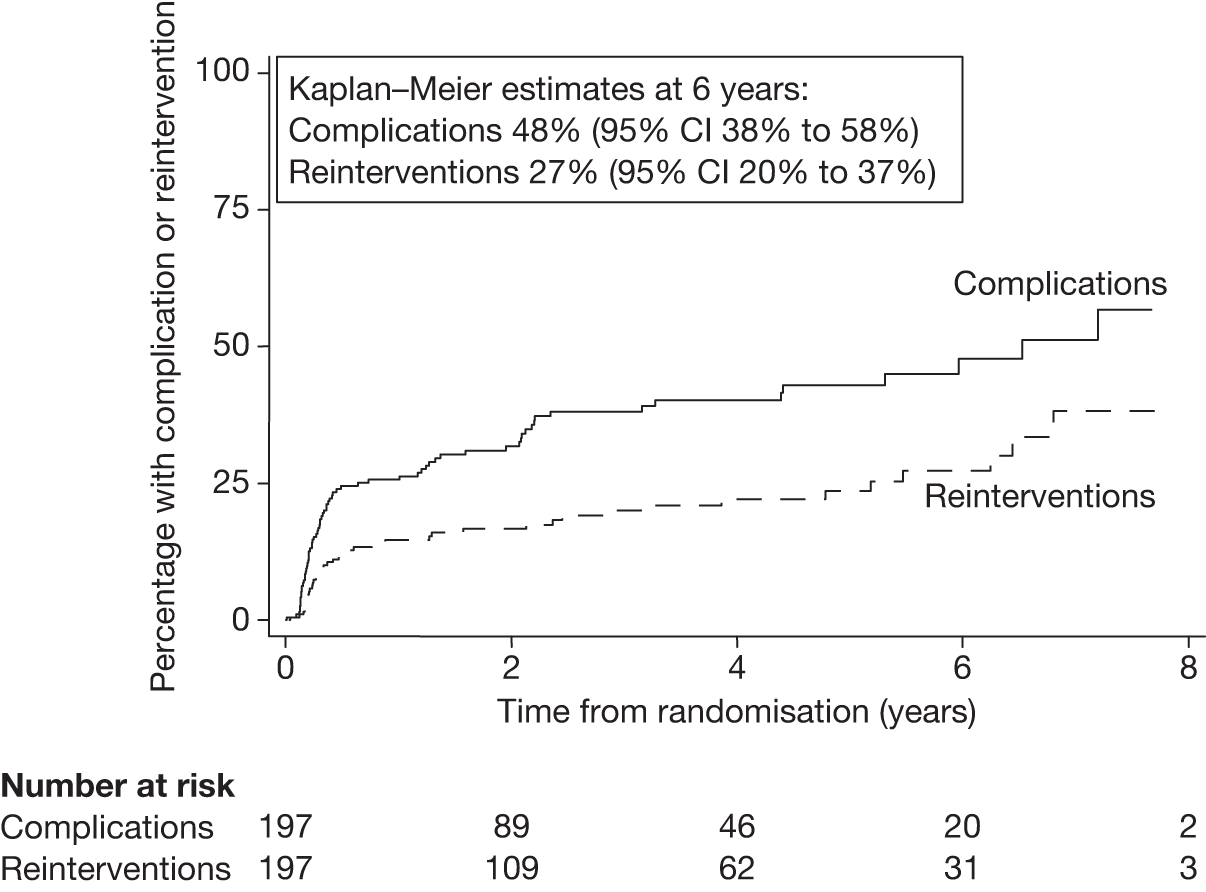
It was decided that a comparison of complication and reintervention rates between the randomised groups of EVAR trial 2 would not be very informative as so few of the no-intervention group had undergone AAA repair. Therefore, a comparison of rates of complications and reinterventions was made between the EVAR groups of EVAR trials 1 and 2 to see whether or not the different classifications of fitness would alter the rate of graft-related complications or reinterventions. Figure 27 presents the Kaplan–Meier curves for time to first complication and reintervention in the EVAR groups of each trial. There was no evidence to suggest any difference in the rates of graft-related events between the trials, despite the considerable disparity in fitness. The crude rates of complications were 12.6 and 15.8 per 100 person-years in trials 1 and 2, respectively [crude Cox HR 1.02 (95% CI 0.79 to 1.32), p = 0.867]. The crude rates of reinterventions were 5.1 and 7.3 per 100 person-years in trials 1 and 2, respectively [crude Cox HR 1.20 (95% CI 0.85 to 1.70), p = 0.305].
FIGURE 27.
Kaplan–Meier estimates for time to first complication and reintervention in the EVAR groups of EVAR trials 1 and 2. (a) Percentage with complication; (b) percentage with reintervention.
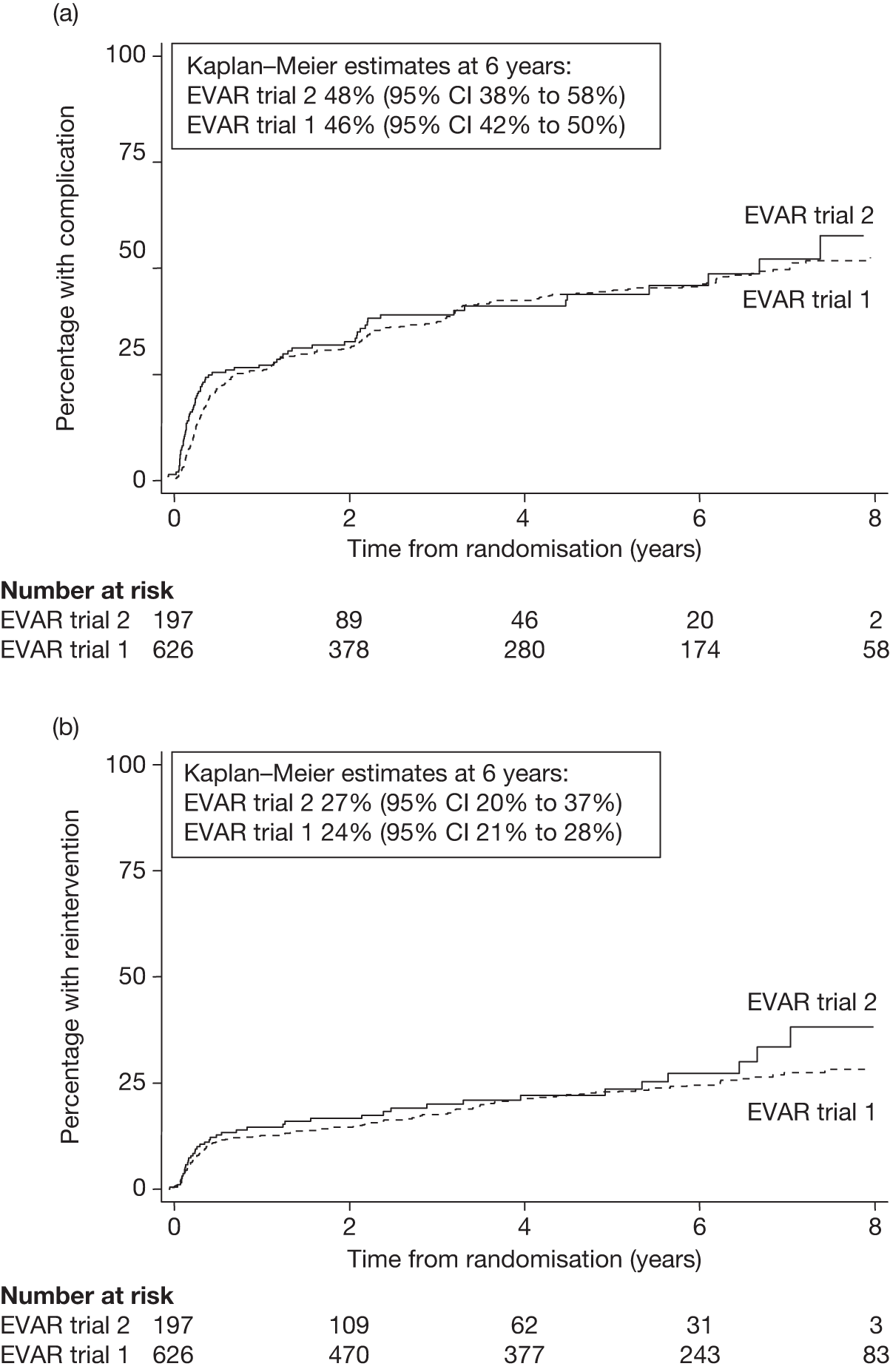
Adverse events
Table 22 presents a breakdown of the numbers of all non-fatal adverse events reported by randomised group up to December 2009. A more detailed analysis of cardiovascular events (fatal and non-fatal MI and stroke) reported up to July 2009 is provided below (see Subsidiary analyses, Cardiovascular events).
| Event | EVAR (n = 197): no. of events (no. of patients) | No intervention (n = 207): no. of events (no. of patients) | Total (n = 404): no. of events (no. of patients) |
|---|---|---|---|
| MI | 11 (10) | 1 (1) | 12 (11) |
| Stroke | 8 (8) | 4 (4) | 12 (12) |
| Renal failure | 2 (2) | 2 (2) | 4 (4) |
| Amputation | 1 (1) | 0 (0) | 1 (1) |
| Total | 22 (21) | 7 (7) | 29 (28) |
Health-related quality of life
Full quality-of-life data were collected during the first year only for the 338 patients recruited during the planned recruitment phase up to 31 December 2003. However, the EQ-5D questionnaire was collected annually throughout the trial for cost-effectiveness assessment. Table 23 presents the results of an analysis of covariance comparing the EQ-5D (scale and visual indices) and the SF-36 PCS and the MCS scores between randomised groups at 1, 3 and 12 months after randomisation. Table 24 presents the same analysis for each of the eight dimensions of the SF-36 score. There were no clear differences between the randomised groups although the EVAR group appeared to have improved EQ-5D visual scales across all the time points.
| HRQL measure | EVAR (n = 166): mean (SD) (no. of patients) | No intervention (n = 172): mean (SD) (no. of patients) | Crude difference: mean (SE) | Difference adjusted for baseline: mean (SE) (no. of patients) (p-value) |
|---|---|---|---|---|
| EQ-5D scale index | ||||
| Baseline | 0.58 (0.31) (164) | 0.63 (0.28) (171) | –0.05 (0.03) | – |
| 1 month | 0.57 (0.28) (48) | 0.56 (0.29) (92) | 0.01 (0.05) | 0.03 (0.05) (139) (0.51) |
| 3 month | 0.64 (0.28) (122) | 0.60 (0.26) (120) | 0.04 (0.03) | 0.06 (0.03) (241) (0.06) |
| 12 month | 0.65 (0.24) (88) | 0.60 (0.30) (68) | 0.05 (0.04) | 0.04 (0.04) (156) (0.30) |
| EQ-5D visual index | ||||
| Baseline | 57.02 (17.47) (165) | 59.08 (19.16) (172) | –2.05 (2.00) | – |
| 1 month | 59.00 (16.04) (50) | 52.75 (19.98) (91) | 6.25 (3.29) | 6.86 (2.93) (140) (0.02) |
| 3 month | 60.47 (17.60) (122) | 57.09 (18.94) (121) | 3.38 (2.35) | 3.79 (1.99) (242) (0.19) |
| 12 month | 62.28 (15.05) (90) | 59.14 (18.53) (72) | 5.14 (2.64) | 5.52 (2.40) (162) (0.02) |
| SF-36 PCS score | ||||
| Baseline | 35.47 (6.63) (160) | 35.12 (6.23) (171) | 0.35 (0.71) | – |
| 1 month | 33.96 (5.13) (46) | 35.60 (5.70) (89) | –1.64 (1.00) | –1.86 (0.88) (134) (0.04) |
| 3 month | 34.33 (6.10) (116) | 35.12 (6.42) (111) | –0.78 (0.83) | –1.11 (0.77) (224) (0.15) |
| 12 month | 34.54 (5.89) (71) | 36.01 (6.92) (60) | –1.47 (1.12) | –0.64 (1.04) (130) (0.54) |
| SF-36 MCS score | ||||
| Baseline | 45.13 (7.92) (160) | 46.31 (6.97) (171) | –1.18 (0.82) | – |
| 1 month | 45.76 (8.65) (46) | 44.03 (7.78) (89) | 1.73 (1.47) | 2.30 (1.38) (134) (0.10) |
| 3 month | 44.76 (7.21) (116) | 44.84 (7.85) (111) | –0.08 (1.00) | 0.94 (0.95) (224) (0.32) |
| 12 month | 45.36 (7.20) (71) | 44.67 (7.93) (60) | 0.70 (1.32) | 0.50 (1.29) (130) (0.70) |
| HRQL measure | EVAR (n = 166): mean (SD) (no. of patients) | No intervention (n = 172): mean (SD) (no. of patients) | Crude difference: mean (SE) | Difference adjusted for baseline: mean (SE) (no. of patients) (p-value) |
|---|---|---|---|---|
| Physical function | ||||
| Baseline | 38.56 (25.84) (166) | 42.72 (25.03) (172) | –4.16 (2.77) | – |
| 1 month | 36.39 (22.40) (50) | 38.21 (25.90) (92) | –2.82 (4.34) | 0.65 (3.15) (142) (0.84) |
| 3 month | 39.19 (25.88) (122) | 39.84 (25.55) (118) | –0.65 (3.32) | 1.77 (2.44) (240) (0.47) |
| 12 month | 39.57 (25.34) (76) | 44.02 (28.76) (61) | –4.44 (4.63) | –0.14 (3.64) (137) (0.97) |
| Role – physical | ||||
| Baseline | 51.18 (33.58) (164) | 53.92 (33.46) (172) | – | – |
| 1 month | 38.30 (31.10) (47) | 44.57 (31.71) (91) | –6.28 (5.66) | –5.99 (4.98) (137) (0.23) |
| 3 month | 40.78 (28.92) (118) | 43.26 (31.58) (114) | –2.47 (3.97) | –2.39 (3.69) (231) (0.52) |
| 12 month | 48.84 (33.71) (74) | 48.02 (32.21) (60) | 0.82 (5.64) | 1.88 (5.24) (134) (0.72) |
| Role – mental | ||||
| Baseline | 69.39 (31.43) (162) | 74.22 (29.69) (171) | –4.83 (3.35) | – |
| 1 month | 66.67 (31.66) (47) | 59.36 (33.12) (89) | 7.30 (5.88) | 10.44 (5.53) (135) (0.06) |
| 3 month | 64.02 (30.37) (118) | 63.41 (32.68) (115) | 0.61 (4.13) | 4.88 (3.96) (231) (0.22) |
| 12 month | 66.78 (31.54) (72) | 67.78 (30.12) (60) | –1.00 (5.40) | –2.10 (5.20) (130) (0.69) |
| Social functioning | ||||
| Baseline | 47.65 (14.97) (165) | 48.11 (12.61) (172) | –0.46 (1.51) | – |
| 1 month | 47.75 (14.44) (50) | 48.76 (11.79) (91) | –1.01 (2.25) | –1.13 (2.25) (141) (0.62) |
| 3 month | 47.05 (12.91) (123) | 49.89 (13.66) (119) | –2.84 (1.71) | –2.83 (1.72) (241) (0.10) |
| 12 month | 47.53 (10.41) (76) | 50.42 (13.21) (60) | –2.88 (2.03) | –2.39 (1.97) (135) (0.23) |
| Mental health | ||||
| Baseline | 60.30 (11.42) (164) | 61.37 (9.73) (172) | –1.07 (1.16) | – |
| 1 month | 61.13 (12.97) (50) | 61.09 (10.71) (92) | 0.04 (2.03) | 0.60 (1.95) (142) (0.76) |
| 3 month | 61.23 (11.17) (122) | 62.63 (10.61) (118) | –1.40 (1.41) | –0.60 (1.31) (239) (0.65) |
| 12 month | 62.41 (11.01) (76) | 60.51 (10.24) (61) | 1.90 (1.83) | 2.43 (1.80) (137) (0.18) |
| Energy/vitality | ||||
| Baseline | 56.54 (11.01) (164) | 56.66 (10.30) (172) | –0.12 (1.16) | – |
| 1 month | 58.75 (10.94) (50) | 57.29 (10.70) (92) | 1.46 (1.89) | 1.25 (1.88) (192) (0.51) |
| 3 month | 56.81 (10.76) (122) | 56.36 (10.43) (118) | 0.46 (1.37) | 0.51 (1.36) (239) (0.71) |
| 12 month | 56.50 (10.68) (76) | 55.02 (12.49) (61) | 1.48 (1.98) | 1.54 (1.95) (137) (0.43) |
| Pain | ||||
| Baseline | 34.59 (26.85) (165) | 29.17 (24.83) (172) | 5.42 (2.82) | – |
| 1 month | 36.85 (24.16) (48) | 38.32 (26.31) (91) | –1.46 (4.57) | –3.38 (3.82) (139) (0.38) |
| 3 month | 33.14 (26.71) (123) | 35.66 (25.72) (116) | –2.52 (3.39) | –3.38 (3.09) (239) (0.28) |
| 12 month | 27.78 (24.92) (76) | 35.12 (25.89) (60) | –7.34 (4.30) | –7.10 (3.92) (136) (0.07) |
| General health | ||||
| Baseline | 63.46 (14.46) (164) | 62.47 (14.89) (172) | 0.99 (1.60) | – |
| 1 month | 65.29 (13.24) (49) | 62.90 (13.91) (92) | 2.38 (2.42) | 2.19 (2.03) (141) (0.28) |
| 3 month | 63.10 (13.03) (122) | 64.38 (13.55) (117) | –1.27 (1.72) | –0.74 (1.47) (238) (0.62) |
| 12 month | 64.31 (14.21) (76) | 62.70 (14.23) (61) | 1.62 (2.44) | 1.52 (2.22) (137) (0.49) |
Subsidiary analyses
Renal function
Figure 28 describes which patients were included and excluded from the renal analyses and Table 25 describes their baseline characteristics. The groups were remarkably similar despite the considerable number of exclusions. The main difference was seen in AAA diameter: the larger AAA in the excluded group may be partially explained by some of the patients with larger aneurysms in the no-intervention group crossing over to AAA repair. Borderline differences indicated that the excluded group had slightly poorer respiratory function, a higher proportion of patients with cardiac disease and fewer patients taking statins but, in contrast, this group had fewer past smokers, more never smokers and slightly lower systolic blood pressure. Table 26 presents the baseline characteristics of the patients included within each randomised group. The groups were reasonably well balanced apart from renal function (both eGFR and creatinine), aortic neck length and use of beta-blockers, and these had already been included as adjustment variables in the analysis plan. The median (IQR) time between randomisation and surgery for those included in the EVAR group was 57 (40–73) days. The mean (SD) volume of contrast agent used during the primary EVAR procedures was 200 (103) ml. During the course of trial follow-up for all 404 randomised patients, two patients in the EVAR group and two patients in the no-intervention group went into chronic renal failure and required long-term dialysis. There was substantial correlation between the baseline and follow-up eGFR measurements, with correlation coefficients typically ranging from 0.53 to 0.90. Normal plots demonstrated reasonable approximation to the normal distribution for all the baseline variables and for the random effects slopes and intercepts generated from the multilevel model. Figure 29 presents the patients with their baseline renal function classified according to the KDOQI stages of renal impairment. Figure 30 demonstrates the distribution of rates of change seen across all patients in EVAR trial 2 analysis with a mean rate of change of –0.87 ml/minute/1.73 m2 per year (range –5.3 to 4.4 ml/minute/1.73 m2 per year) and only two patients having a renal function deterioration faster than –5 ml/minute/1.73 m2 per year (both in the EVAR group).
FIGURE 28.
Flow chart describing patients included and excluded from the renal function analysis in EVAR trial 2.
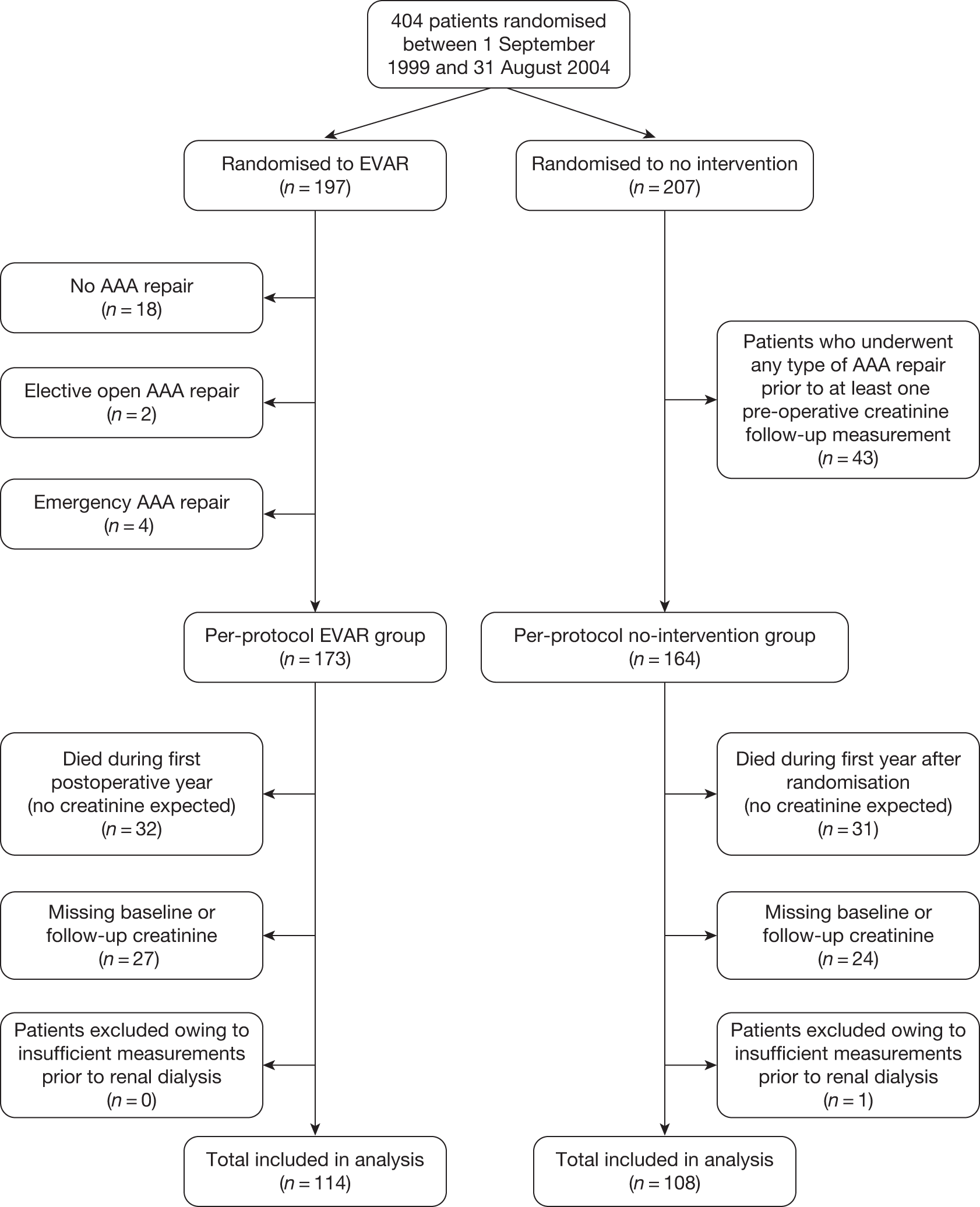
| Baseline characteristic | Included in analysis (N = 222) | Excluded from analysis (N = 182) | p-value for comparisona |
|---|---|---|---|
| Age at randomisation (years) | 76.6 (6.6) | 77.1 (6.4) | 0.404 |
| No. of males: n (%) | 190 (86) | 157 (86) | 0.846 |
| AAA diameter (cm) | 6.5 (1.0) | 6.9 (1.0) | 0.0004 |
| Top aortic neck diameter (cm) | 2.4 (0.3) | 2.4 (0.3) | 0.580 |
| Aortic neck length (cm) | 2.7 (1.2) | 2.7 (1.3) | 0.925 |
| BMI (kg/m2) | 26.6 (4.6) | 26.3 (4.9) | 0.500 |
| Diabetes: n (%) | 34 (15) | 25 (14) | 0.691 |
| Smoking status: n (%) | |||
| Current | 37 (17) | 33 (18) | 0.181 |
| Past | 175 (79) | 133 (73) | |
| Never | 10 (4) | 16 (9) | |
| Previous history of cardiac disease:b n (%) | 150 (68) | 135 (74) | 0.147 |
| Systolic blood pressure (mmHg) | 141 (21) | 137 (22) | 0.134 |
| Diastolic blood pressure (mmHg) | 79 (11) | 78 (12) | 0.370 |
| Treated for hypertension: n (%) | 142 (65) | 116 (65) | 0.955 |
| ABPI (mean of both legs): n (%) | 0.98 (0.20) | 0.99 (0.19) | 0.442 |
| FEV1 (l) | 1.7 (0.7) | 1.6 (0.7) | 0.186 |
| Serum creatinine (μmol/l)c | 109 (94–138) | 109 (90–135) | 0.811 |
| Serum eGFR (ml/minute/1.73 m2) | 57.3 (19.2) | 58.3 (22.1) | 0.626 |
| Serum cholesterol (mmol/l) | 4.8 (1.2) | 4.8 (1.1) | 0.863 |
| Aspirin use: n (%) | 130 (59) | 98 (54) | 0.374 |
| Statin use: n (%) | 99 (45) | 69 (38) | 0.190 |
| Non-steroidal anti-inflammatory drug use: n (%) | 14 (6) | 14 (8) | 0.575 |
| Beta-blocker use: n (%) | 77 (35) | 66 (36) | 0.735 |
| Baseline characteristic | EVAR (N = 114) (406 eGFR measurements) | No intervention (N = 108) (306 eGFR measurements) | p-value for comparisona |
|---|---|---|---|
| Age at randomisation (years) | 76.5 (6.6) | 76.6 (6.7) | 0.938 |
| No. of males: n (%) | 97 (85) | 93 (86) | 0.828 |
| AAA diameter (cm) | 6.6 (1.0) | 6.5 (1.0) | 0.334 |
| Top aortic neck diameter (cm) | 2.4 (0.3) | 2.5 (0.3) | 0.411 |
| Aortic neck length (cm) | 2.6 (1.2) | 2.9 (1.2) | 0.097 |
| BMI (kg/m2) | 26.5 (4.9) | 26.7 (4.2) | 0.770 |
| Diabetes : n (%) | 17 (15%) | 17 (16%) | 0.841 |
| Smoking status: n (%) | |||
| Current | 23 (20) | 14 (13) | 0.353 |
| Past | 86 (75) | 89 (82) | |
| Never | 5 (5) | 5 (5) | |
| Previous history of cardiac disease:b n (%) | 74 (65) | 76 (70) | 0.385 |
| Systolic blood pressure (mmHg) | 140 (20) | 141 (22) | 0.730 |
| Diastolic blood pressure (mmHg) | 79 (11) | 80 (11) | 0.797 |
| Treated for hypertension: n (%) | 73 (66) | 69 (64) | 0.771 |
| ABPI (mean of both legs) | 0.98 (0.21) | 0.97 (0.19) | 0.611 |
| FEV1 (l) | 1.7 (0.6) | 1.7 (0.7) | 0.639 |
| Serum creatinine (μmol/l)c | 106 (91–131) | 113 (97–146) | 0.019 |
| Serum eGFR (ml/minute/1.73 m2) | 59.8 (17.9) | 54.6 (20.2) | 0.044 |
| Serum cholesterol (mmol/l) | 4.8 (1.1) | 4.9 (1.2) | 0.479 |
| Aspirin use: n (%) | 69 (61) | 61 (56) | 0.541 |
| Statin use: n (%) | 53 (46) | 46 (43) | 0.559 |
| Non-steroidal anti-inflammatory drug use: n (%) | 8 (7) | 6 (6) | 0.654 |
| Beta-blocker use: n (%) | 33 (29) | 44 (41) | 0.072 |
FIGURE 29.
Classification of baseline renal impairment staging according to the National Kidney Foundation: KDOQI in EVAR trial 2. Stages 1 and 2, eGFR > 60 ml/minute/1.73 m2 – normal to mild impairment; stage 3, eGFR 30–59 ml/minute/1.73 m2 – moderate impairment; stage 4, eGFR 15–29 ml/minute/1.73 m2 – severe impairment; stage 5, eGFR < 15 ml/minute/1.73 m2 – renal failure, referred for dialysis.
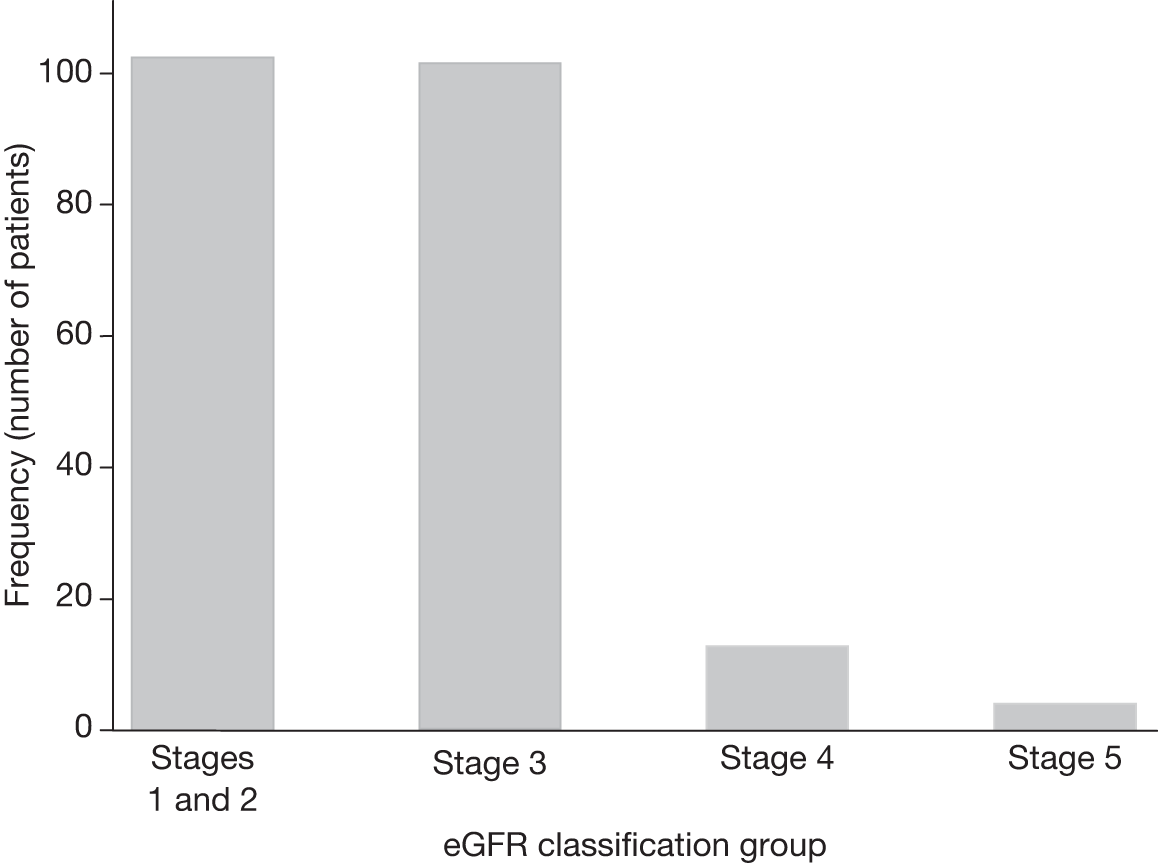
FIGURE 30.
Histogram of rates of change in eGFR for 222 patients included in renal function analysis in EVAR trial 2.
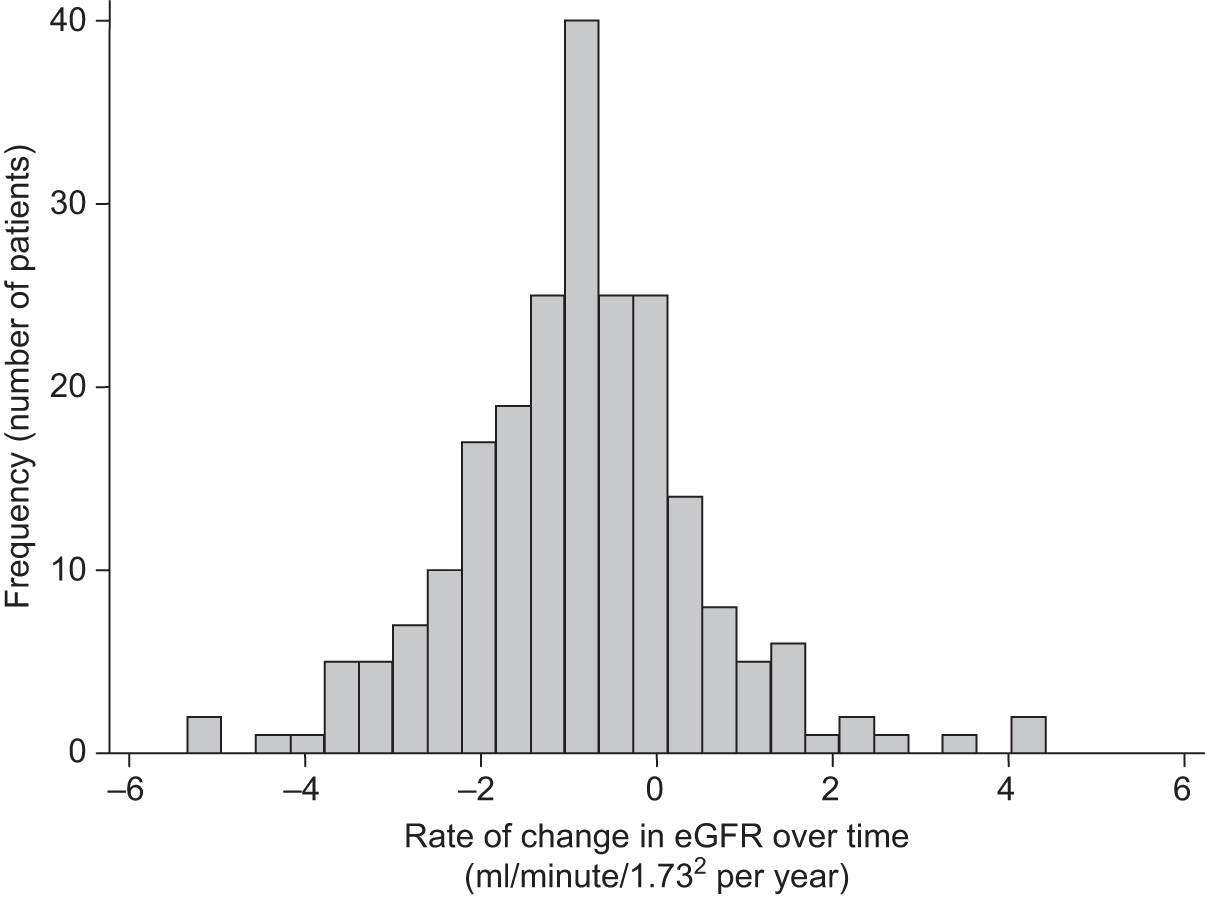
The mean (SD) rate of change in eGFR was –0.98 ml/minute/1.73 m2 (1.49 ml/minute/1.73 m2) and –0.76 ml/minute/1.73 m2 (1.30 ml/minute/1.73 m2) for the EVAR and no-intervention groups, respectively, but this difference did not achieve statistical significance (crude, primary and secondary adjusted models, p = 0.100, p = 0.087, p = 0.139, respectively).
Cardiovascular events
Given the poor fitness level of patients in EVAR trial 2, it was decided to compare the rates of serious cardiovascular events (fatal and non-fatal MI and stroke) between the groups to investigate whether or not the EVAR group experienced a higher rate of cardiovascular events. This analysis was performed in July 2009, 6 months before the official close of trial follow-up at the end of 2009, and therefore includes a slightly different number of events from those reported in Table 22. Data were analysed from the time of randomisation to the first event for those who experienced one, or were censored at death (not from MI or stroke) or end of follow-up for those without an event. A total of 67 first cardiovascular events occurred during an average of 2.85 years of follow-up; a breakdown of the type of events is given in Table 27. Of these 67 patients, two went on to have a second event and one had a third event, generating a total of 70 events in an average of 2.86 years of follow-up: crude overall rate 6.1 (robust 95% CI 4.7 to 7.6) events per 100 person-years. In the EVAR group, three cardiovascular events were reported before the EVAR procedure and 10 occurred within 30 days of the EVAR, with the remaining 23 occurring more than 30 days after EVAR. In the no-intervention group, nine events occurred after AAA repair in the 63 patients who had been reported as having aneurysm repair against protocol by July 2009 (none within 30 days). Of the 319 patients who were still complying with their randomised allocation at that time, 33 (19%) patients in the EVAR group and 22 (15%) patients in the no-intervention groups experienced a cardiovascular event during follow-up.
| Event type | EVAR (n = 197 patients) | No intervention (n = 207 patients) |
|---|---|---|
| Fatal MIa | 14 | 20 |
| Non-fatal MIb | 10 | 2 |
| Fatal strokea | 5 | 3 |
| Non-fatal strokeb | 7 | 6 |
| Total events | 36 | 31 |
By July 2009, there were a total of 36 first events in the EVAR group (crude rate 6.6 per 100 person-years) and 31 in the no-intervention group (crude rate 5.1 per 100 person-years). The patients in the EVAR group experienced a higher rate of cardiovascular events, but this did not reach statistical significance in a Cox regression analysis [crude HR 1.31 (95% CI 0.81 to 2.12), p = 0.272; adjusted HR 1.42 (95% CI 0.87 to 2.34), p = 0.156]. Figure 31 shows the Kaplan–Meier estimates for time to first event truncated 6 years after randomisation.
FIGURE 31.
Kaplan–Meier estimates for time to first cardiovascular event stratified by randomised group in EVAR trial 2.
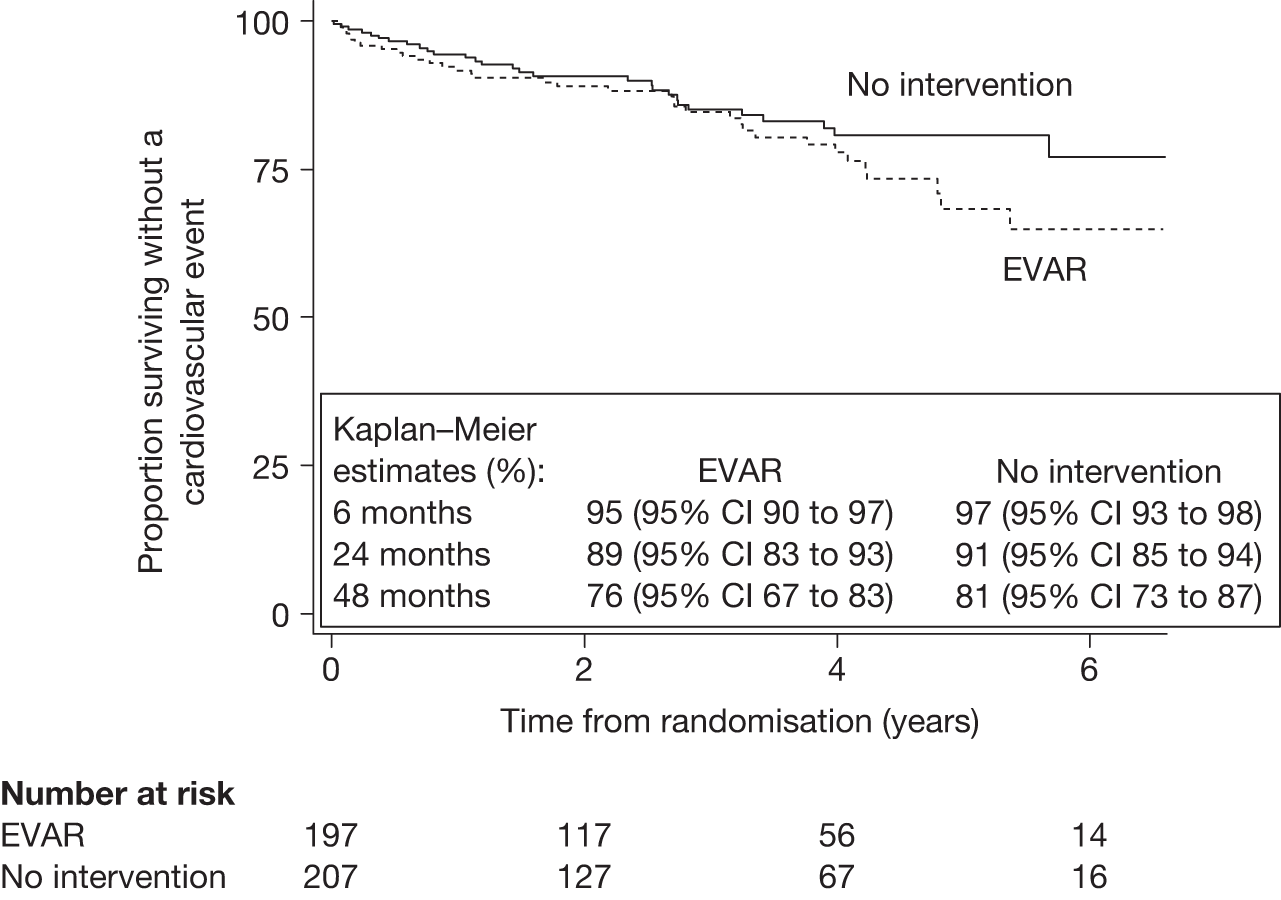
Chapter 6 Results for combined analyses of EVAR trials 1 and 2
Factors associated with endograft ruptures
Objective
During the course of the trials, a total of 27 graft ruptures occurred (rupture of the aorta despite the presence of an aneurysm repair graft). It was noted that none of these ruptures had occurred in the patients who had been treated with open repair and therefore it was decided to audit the 27 cases of endograft rupture and see whether or not there were any factors that might predispose patients to this serious occurrence.
Methods
The case record forms were inspected to compile a narrative for each of the 27 cases of endograft rupture and further information was sought from the local trial co-ordinator where necessary. Given that there were just 27 ruptures, analysis of factors associated with graft rupture was limited to no more than four. These four potentially important factors were selected as part of a statistical analysis plan agreed before the data were analysed. However, the types of complications present in the 27 ruptures were known prior to the agreement of the analysis plan as the narrative of each case had been summarised previously. It was agreed that these four factors would be investigated:
-
Previous CT diagnosis of these specific complications Endoleak type 1, type 2 with sac growth of ≥ 5 mm, type 3, migration or kinking.
-
Top neck diameter Aortic diameter at the level of the lower renal artery.
-
Neck length Distance from the lower renal artery to the start of the aneurysm.
-
Common iliac diameter Maximum of both legs.
Cox regression analysis was used to investigate whether or not these factors were associated with an increased risk of endograft rupture. Analyses were timed from the endovascular procedure and follow-up was truncated in December 2009. Patients were excluded in this analysis if the EVAR was performed for an emergency repair or if conversion to open repair occurred in theatre during the primary procedure (but not as a result of a graft rupture in theatre). Non-ruptured patients were censored at death, date of last follow-up or date of audit of hospital notes, whichever occurred latest. The complications variable was included as a time-dependent variable that accounted for the time between aneurysm repair, onset of complication and subsequent time of rupture. Three levels of model were performed:
-
a univariate model for each of the four factors separately adjusted for trial 1 or 2
-
a second model including all four factors together adjusted for trial 1 or 2
-
a third model including all four factors adjusted for trial 1 or 2, baseline age, sex, maximum aneurysm diameter, length of procedure (as an indicator of difficulty of repair), time of endovascular procedure since 1 September 1999 (as an indicator of early or late iterations of device), shape of graft (straight or uni-iliac vs bi-iliac) and graft manufacturer (Cook/Zenith, Medtronic/Talent, Gore/Excluder or other).
Results – narrative description of 27 cases of endograft rupture
A total of 624 patients receiving endovascular repair in EVAR trial 1 and 224 receiving endovascular repair in EVAR trial 2 were combined to yield a total of 848 EVARs, which were followed for an average of 4.8 years. The mean (SD) age at baseline was 75 (6) years and 758 (89%) were male. The mean (SD) aneurysm size at baseline was 6.5 (0.9) cm. Of the 27 ruptures that occurred, 25 were in EVAR trial 1 and two in EVAR trial 2 [crude rates of 0.8 (95% CI 0.5 to 1.1) and 0.2 (95% CI 0.1 to 1.0) ruptures per 100 person-years, respectively]. Five ruptures occurred during the first 30 postoperative days and 22 after 30 days: crude rates of 7.2 (95% CI 3.0 to 17.4) and 0.6 (95% CI 0.4 to 0.9) per 100 person-years, respectively. Beyond the perioperative period ruptures appeared to occur at a constant rate over the years. Open surgical repair was used to treat seven of the ruptured patients, five of whom survived beyond 30 days. An endovascular approach was used to treat five patients (three limb extensions, one cuff insertion, one failed repeat EVAR), four of whom survived beyond 30 days. The remaining 15 patients died before aneurysm repair could be attempted but the reasons for absence of intervention were not reported. Overall, 18 patients (67%) died within 30 days of rupture, a further five died 1 year or more later, and four remained alive at the end of follow-up in December 2009.
After detailed inspection of the trial case record forms, the 27 patients were grouped into three subsets according to the timing of the rupture and whether prior complications had been reported.
Group A – perioperative ruptures
Five (18.5%) of the aneurysm ruptures occurred in the perioperative period (≤ 30 days). Three of these occurred in-hospital and two at home after discharge. Three of the five patients died within 30 days of the rupture.
In one patient, the aneurysm ruptured after an unremarkable endograft deployment on the same day. Urgent open repair was performed and the patient survived. Two patients experienced rupture at home, one on day 3 and one on day 8, after an unremarkable endograft deployment. No additional post-procedural imaging was performed before discharge. Urgent open repair and survival occurred in one patient and death in the other. In the case of the third patient there were numerous attempts at deployment during a long, 5-hour procedure and eventually the endovascular repair was abandoned with a view to performing open repair soon after. The aneurysm ruptured on day 5 while waiting for this procedure. Urgent open repair was performed; however, the patient died of sepsis on day 18. The last patient in this group received a uni-iliac graft with a short limb. While waiting for the limb extension to be delivered from the manufacturer, the graft ruptured on day 8 and the patient died in theatre during an attempted conversion to open repair.
Group B – late ruptures without prior complications
Five (18.5%) patients presented with ruptures > 30 days after the repair without any previously reported complications or signs of failed endovascular treatment. These occurred at various times (32 days to 3.6 years after the initial procedure). Four of the five patients died within 30 days of the rupture.
One patient ruptured at home on day 32, 25 days after discharge but only 2 days outside the definition for ‘perioperative ruptures’. No post-procedural imaging was performed before discharge and the 1-month follow-up scan was just about to be performed. Three patients had an unremarkable follow-up CT scan within 12 months prior to rupture. All had shown sac shrinkage and no complications had been identified. The last patient in this group had shown sac shrinkage and no complications during the first 2 years, but had missed the 3-year follow-up scan. Thus, even if a pre-discharge scan had been part of the EVAR trials’ protocol, there would still have been three unexplained ruptures (3/27 = 11%).
Group C – late ruptures with prior complications
Seventeen (63%) patients presenting with rupture had previously reported complications or signs of failed EVAR > 30 days after the repair. Eleven of these 17 patients died within 30 days of the rupture. Sac growth had been observed in 15 of the 17 patients, with endoleaks identified in 12 of these 15. Of the remaining two patients, without sac expansion, one had migration and one had an endoleak of undefined origin documented. Three cases had a type 2 endoleak as the initially reported complication. All these experienced concomitant sac growth before rupture. Twelve patients with a complication underwent a secondary intervention prior to rupture. One patient refused all CT follow-up scans. An endoleak of undefined origin was detected by duplex ultrasound on day 15 and again 2.9 years after endograft deployment without indication of sac growth. Rupture occurred 3 weeks after the second ultrasound. One patient experienced two ruptures: first, there was sac growth in the presence of a type 2 endoleak then rupture (3.4 years after the endovascular repair), which was treated with endovascular insertion of a cuff. Thereafter, the patient did not attend follow-up scans or appointments, with final rupture and death occurring 5 years after the original procedure.
Results – analysis of factors associated with endograft rupture
Table 28 presents the results of the analysis of factors associated with endograft rupture. There was a strongly significant association between rupture and previous detection of these serious complications (endoleak type 1, type 2 with sac expansion, type 3, migration or kinking) with a crude rate of rupture before detection of 0.4 ruptures per 100 person-years compared with a crude rate of 2.4 ruptures per 100 person-years after detection [adjusted multivariate HR 8.83 (95% CI 3.76 to 20.76), p < 0.0001]. The HR for neck diameter in Table 28 represents increase in hazard per cm increase in neck diameter. Neck length and common iliac diameter were positively skewed and required log transformation, meaning that HRs represent change in hazard per 2.7 cm increase in these covariates. There was no strong evidence to suggest that the three anatomical factors selected were associated with graft rupture but, given that only 27 cases occurred, power was limited. However, a non-significant trend was observed for top neck diameter, with the risk of rupture doubling for each centimetre increase. There was no suggestion of a significant difference in rupture rates between graft manufacturers (number of ruptures/patients, crude rate per 100 person-years): Cook/Zenith: 11/469, 0.5; Medtronic/Talent: 13/250, 1.1; Gore/Excluder 2/51, 0.7; other 1/70, 0.3; and 0/8 unknown.
| Covariatea | No. of ruptures/patients (crude rate per 100 person-years) | Univariateb HR (95% CI) p-value | Multivariateb HR for all four factors (95% CI) p-value | Adjustedb multivariate HR for all four factors (95% CI) p-value |
|---|---|---|---|---|
| Top neck diameter (cm) | 1.91 | 1.71 (0.50 to 5.82) 0.392 | 2.07 (0.59 to 7.20) 0.253 | |
| < 2.4 | 13/434 (0.6) | (0.55 to 6.59) | (0.50 to 5.82) | (0.59 to 7.20) |
| ≥ 2.4 | 14/412 (0.7) | 0.308 | 0.392 | 0.253 |
| Neck length (cm)c | 0.87 | 0.88 | 0.82 | |
| < 2.6 | 13/428 (0.6) | (0.34 to 2.22) | (0.34 to 2.28) | (0.28 to 2.38) |
| ≥ 2.6 | 14/416 (0.7) | 0.763 | 0.794 | 0.711 |
| Maximum common iliac diameter (cm)d | 1.38 | 1.07 | 0.97 | |
| < 1.7 | 15/444 (0.7) | (0.47 to 4.02) | (0.33 to 3.54) | (0.30 to 3.17) |
| ≥ 1.7 | 12/399 (0.6) | 0.55 | 0.908 | 0.956 |
| Complicationd | 8.94 | 8.70 | 8.83 | |
| Before | 13/676 (0.4) | (3.88 to 20.57) | (3.77 to 20.11) | (3.76 to 20.76) |
| After | 14/172 (2.4) | < 0.0001 | < 0.0001 | < 0.0001 |
Post hoc inspections of the HRs for the adjustment variables suggested that older patients may experience increased rates with the risk of rupture increasing by 10% per 1-year increase in age [HR 1.10 (95% CI 1.03 to 1.19), p = 0.008]. Also, the rupture rate in EVAR trial 2 appeared to be about 75% lower than that seen in EVAR trial 1 patients [HR 0.26 (95% CI 0.06 to 1.18), p = 0.081]. However, the results from a sensitivity analysis that included only patients in EVAR trial 1 did not demonstrate any marked differences with the main results, with previous diagnosis of the serious complications selected still proving important [adjusted HR 7.8 (95% CI 3.2 to 18.6), p< 0.0001].
Factors associated with development of serious graft-related complications and reinterventions
Objective
In 2009, NICE published an appraisal document on the use of EVAR in the UK NHS. 199 It concluded that EVAR should be offered to all patients who are suitable for both EVAR and open repair, but highlighted the need for identifying more cost-effective subgroups in which EVAR performed particularly well. Therefore, it was decided to use data from both EVAR trials 1 and 2 to investigate whether or not any baseline factors were associated with the subsequent rate of serious graft-related complications and reinterventions after EVAR implantation, as this may help to identify a subgroup of patients in whom EVAR performs particularly well.
Methods
The analysis was performed on patients randomised to EVAR in either trial who had an elective EVAR within 6 months of randomisation. To maximise the power of the analysis, trial 1 and 2 patients were combined but all analyses were adjusted for trial to account for any differences between them. Time to first complication or reintervention was timed from the EVAR procedure and patients were followed until August 2009 (minimum 5 years), when the analysis was performed. Patients without a complication or reintervention were censored on the latest of three dates: date of last follow-up, date of audit of hospital notes or date of death (providing it occurred within 18 months of last follow-up or audit, otherwise the date of last follow-up or audit was used for censoring).
Definition of serious graft-related complications
Complications and reinterventions were recorded during the primary admission and during subsequent follow-up after discharge. Graft-related complications were reported by local radiologists and classified according to the revised White and May guidelines170,182 (see case record form in Appendix 6). For the purposes of this analysis, only serious complications were investigated (listed below). This includes graft rupture, other complications that have been shown to increase the risk of graft rupture, clinically serious events such as graft infection or renal infarction, which can precipitate conversion to open repair, and any technical complications or conversions to open repair for any complication. Type 2 endoleaks were excluded not because they are unimportant, but because the prevailing current practice is to monitor them and intervene only if the sac enlarges appreciably over time. Furthermore, during the earlier phase of the EVAR trials, intervention for type 2 leaks was far more common than is now standard practice and therefore the natural history of type 2 leaks in this series is not representative of the present day. Unexplained sac enlargement (also known as endotension) would also tend to be regarded as a serious complication but standardised definitions, as well as validated measurement protocols, had not been developed for this at the start of the EVAR trials and thus the reporting of this outcome is less secure. Nevertheless, given that endotension is of concern to most clinicians, a sensitivity analysis was also performed that included all cases of endotension (as reported by the local radiologists) and these results were compared with the main analysis to check whether or not inclusion of endotension altered the findings.
Thus, for the main analysis, serious graft complications were defined as any of the following:
-
graft rupture
-
graft migration – proximal or distal
-
type 1 endoleak – proximal or distal
-
type 3 endoleak – loss of structural integrity, modular disconnection, stent fracture, fabric tear or holes
-
graft kinking or thrombosis
-
graft infection
-
renal infarction
-
unsuccessful deployment
-
conversion to open repair for any complication, including type 2 leaks or endotension
-
endotension included as part of a sensitivity analysis.
The first occurrence of any of these serious complications was used even if some developed in severity after the first time of detection.
Definition of reinterventions
This was defined as the first reintervention for any of the serious complications listed above. This included reinterventions occurring either during the primary admission or during follow-up where the patient was readmitted for an overnight stay in hospital. Day cases for investigations such as angiograms were excluded. A similar sensitivity analysis to the complications analysis was performed that included cases of endotension.
Selection of variables for assessment
Provisional inspection of the data set indicated that approximately 180 serious complications and 120 reinterventions for serious complications had occurred and therefore the analysis was restricted to investigating 12–18 baseline factors (10 events per factor analysed). Variables were selected on the basis that they described aortic anatomy or were related to vessel integrity, for example extent of calcification or perfusion to the aorta. Data on morphological variables such as extent of thrombus, calcification and angulation were not available. The variables were split into three related blocks.
Block 1 – clinical parameters relating to fitness and survival
Age, sex, smoking status (current, past or never), previous history of cardiac disease (MI, angina, cardiac revascularisation, significant valve disease, significant arrhythmia or uncontrolled congestive cardiac failure), systolic blood pressure, lowest ABPI of both legs, diabetes, eGFR calculated from serum creatinine (adjusted for age and sex but not ethnicity as < 1% of patients in EVAR trials were black187,188) and FEV1. A quadratic effect of age was also investigated.
Block 2 – anatomical parameters
Maximum aneurysm sac diameter in any plane, top neck diameter at the level of the lowest renal artery, neck length (distance from the lowest renal artery to the top of the aneurysmal sac), neck conicality [(bottom neck diameter – top neck diameter)/neck length] and maximum common iliac diameter at the internal iliac bifurcation (largest of both limbs).
Block 3 – medical therapies relating to survival
Aspirin use and statin use. Cox regression modelling was used to investigate time to first complication or reintervention from date of EVAR deployment. Continuous variables were included in the models in continuous format but stratified above and below the median for presentation purposes. A comparison of hazards between EVAR trials 1 and 2 showed very similar results, so the trials were combined but all models were adjusted for trial. First, univariate analyses were performed for each variable. Second, a final model was developed by inspecting the results within each block separately and dropping variables from blocks 3, 2 and 1 (in that order) that were individually non-significant (p > 0.2). Once the final model had been derived, further adjustment was made for the choice of graft (stratified into Cook/Zenith, Medtronic/Talent, Gore/Excluder and other), shape of device (straight and uni-iliac vs bifurcated), early or late time of deployment (calculated as time between 1 August 1999 and deployment to account for any differences between the earlier and later iterations of grafts) and centre as a random effect (shared frailty term). The number of missing data was small, with 90% of patients having a complete set of covariates, but imputation for missing data in the remaining 10% was performed to maximise the power of the analysis. Results from an analysis of the 90% of patients with complete data demonstrated almost identical results to those based upon imputed data.
Results
Of the 823 patients randomised to EVAR, 756 received an elective EVAR within 6 months of randomisation by the time the analysis was performed in August 2009 (588 in EVAR trial 1 and 168 in EVAR trial 2). The mean (SD) age and AAA diameter were 74.6 (6.3) years and 6.5 (0.9) cm, respectively, and 677 (90%) were male. The patients in EVAR trial 2 had their EVAR slightly later than those in EVAR trial 1: median (IQR) time from randomisation to surgery was 54 (39–74) days in EVAR trial 2 and 43 (28–68) days in EVAR trial 1. Graft use consisted of Cook/Zenith 421 (55%), Medtronic/Talent 218 (29%), Gore/Excluder 45 (6%), Other 67 (9%) and unknown 5 (1%).
In total, 179 first serious graft complications were reported during an average 3.7 years’ follow-up [crude rate 6.5 per 100 person-years (95% CI 5.6 to 7.5 per 100 person-years)], together with 114 first reinterventions [crude rate 3.8 per 100 person-years (95% CI 3.2 to 4.6 per 100 person-years)]. Table 29 shows the types of complications and numbers with reinterventions and Figure 32 shows the Kaplan–Meier estimates for survival without serious complication or reintervention up to 6 years. Five patients converted to open repair in theatre during the primary procedure and 39 of the 179 complications occurred during the primary admission with the remaining 140 occurring during follow-up after discharge from hospital. These results report time to first complication and a number of these complications developed in severity; for example, although only 12 ruptures occurred as a first event, an additional 15 ruptures occurred as secondary events after diagnosis of an earlier serious complication (see previous section, Factors associated with endograft ruptures). Similarly, although 10 conversions to open repair occurred as a first serious complication in this analysis, an additional 18 conversions to open repair occurred after diagnosis of other serious complications. As part of the sensitivity analysis, a further 16 cases of endotension were included as a serious complication (six reinterventions). The criteria for reintervention for any complications were not specified in the trial protocol and left as a pragmatic local decision. Reasons for no intervention included patient refusal, lack of a feasible treatment option and death prior to intervention. Cox regression analysis of event rates comparing EVAR trials 1 and 2 did not indicate any significant difference between them: HR for complications 0.99 (95% CI 0.67 to 1.44), p = 0.946; HR for reinterventions 1.04 (95% CI 0.64 to 1.68), p = 0.878. In addition, the time between diagnosis of complication and reintervention was compared between the trials using a Mann–Whitney test and no significant difference was shown, with median (IQR) times of 21 (0–117) days and 10 (1–168) days for trials 1 and 2, respectively (p = 0.571). Thus, the trials were combined but all analyses were adjusted for trial.
| Complication | No. of complications (reinterventions) | ||
|---|---|---|---|
| EVAR trial 1 | EVAR trial 2 | Total | |
| Graft rupture | 10 (5) | 0 (0) | 10 (5) |
| Graft infection | 2 (1) | 1 (0) | 3 (1) |
| Migration | |||
| Proximal | 11 (6) | 2 (1) | 13 (7) |
| Distal | 5 (3) | 1 (0) | 6 (3) |
| Unspecified | 13 (8) | 0 (0) | 13 (8) |
| Type 1 endoleak | |||
| Proximal | 15 (9) | 4 (4) | 19 (13) |
| Distal | 17 (12) | 4 (3) | 21 (15) |
| Unspecified | 11 (9) | 5 (3) | 16 (12) |
| Type 3 endoleak | 15 (7) | 6 (4) | 21 (11) |
| Kinking or thrombosis | 31 (21) | 6 (4) | 37 (25) |
| Renal infarction | 4 (0) | 2 (0) | 6 (0) |
| Problematic deployment | 4 (4) | 0 (0) | 4 (4) |
| Conversion to open repair | 8 (8) | 2 (2) | 10 (10) |
| Total | 146 (93) | 33 (21) | 179 (114) |
FIGURE 32.
Kaplan–Meier estimates for time to first serious complication or first reintervention for a serious complication for 756 patients in EVAR trials 1 and 2.
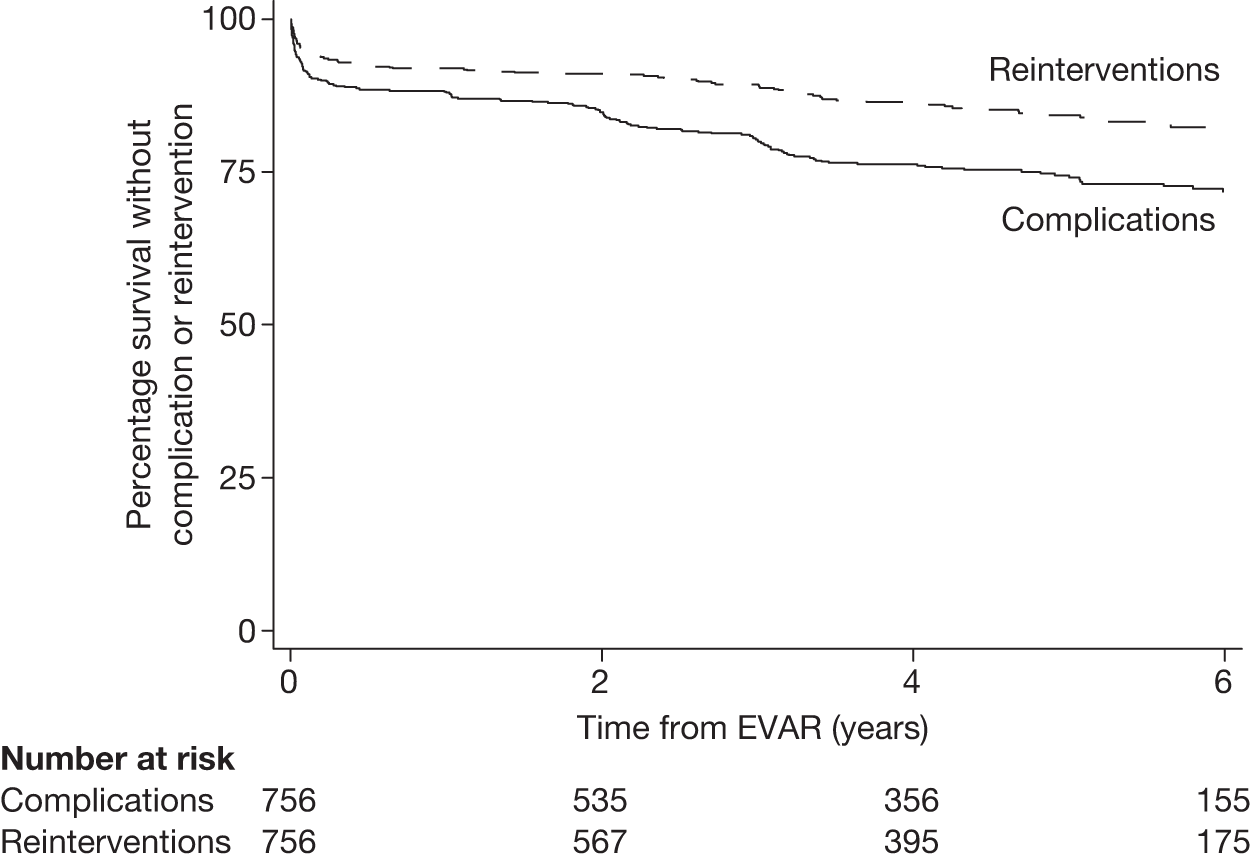
Table 30 presents the rates of complications and reinterventions within four prespecified time periods, with the highest rate occurring during the first 30 days after EVAR deployment and remaining relatively high for the first 6 months. There was some indication of an increase in rates beyond 2 years, corresponding to the drop in the Kaplan–Meier curves after 2 years in Figure 32. A post hoc regression of rates against time from 6 months onwards indicated a significant increase in reinterventions (p = 0.019) but no significant increase in complications (p = 0.843).
| Time period | No. of complications/patients, rate per 100 person-years (95% CI) | No. of reinterventions/patients, rate per 100 person-years (95% CI) |
|---|---|---|
| Total follow-up | 179/75 | 114/756 |
| 6.5 (5.6 to 7.5) | 3.8 (3.2 to 4.6) | |
| EVAR to 30 days | 60/756 | 39/756 |
| 103 (80 to 133) | 66 (48 to 90) | |
| 30 days to 6 months | 23/684 | 16/703 |
| 8.4 (5.6 to 12.6) | 5.6 (3.4 to 9.2) | |
| 6 months to 2 years | 27/638 | 10/663 |
| 3.0 (2.1 to 4.4) | 1.1 (0.6 to 2.0) | |
| > 2 years | 69/534 | 49/567 |
| 4.4 (3.5 to 5.6) | 2.9 (2.2 to 3.8) |
Table 31 presents the crude and adjusted results for the factors that remained in the final Cox regression models for complications and reinterventions. There was no evidence to suggest violation of the proportional hazards assumption in the final adjusted models (p = 0.293 for complications and p = 0.112 for reinterventions). Older age and larger AAA diameters were both significantly associated with increased incidence of both complications and reinterventions (Figure 33). The rate of increase with age diminished as patients aged, corresponding to a significant quadratic effect of age (p = 0.043 for complications and p = 0.034 for reinterventions). It should be stressed that the AAA diameter relationship relates to baseline measurements, and not to changes in sac size after EVAR deployment. There was weaker evidence to suggest that women and patients with larger neck diameters have a higher rate of complications and reinterventions but this was statistically unconvincing, particularly when the multiple testing of 16 covariates was taken into consideration. There was some slightly stronger evidence to suggest that larger common iliac diameters were associated with a higher rate of complications but not reinterventions. The modelling results from the sensitivity analysis, including cases of endotension as a serious complication, generated very similar results although the association between complications and top neck diameter was diminished. For complications, final adjusted HRs (p-values) were 1.83 (0.020) for age, 1.44 (0.123) for sex, 1.34 (< 0.0001) for AAA diameter, 1.31 (0.290) for neck diameter and 1.69 (0.011) for maximum common iliac diameter. For reinterventions, final adjusted HRs (p-values) were 2.24 (0.025) for age, 1.52 (0.164) for sex, 1.45 (< 0.0001) for AAA diameter and 1.26 (0.483) for neck diameter.
| Covariateb | No. of events/patients (rate/100 person-years) | Crude model:c HR (95% CI) p-value | Final model:c HR (95% CI) p-value | Final adjusted model:c HR (95% CI) p-value |
|---|---|---|---|---|
| Complications | ||||
| Age (per year) | 1.80 | 1.81 | 1.72 | |
| < 75 | 86/378 (5.6) | (1.08 to 3.00) | (1.08 to 3.04) | (1.02 to 2.89) |
| ≥ 75 | 93/378 (7.4) | 0.024 | 0.024 | 0.040 |
| Age squared (per year2) | 0.99 (0.99 to 1.00) 0.028 | 0.99 (0.99 to 1.00) 0.027 | 0.99 (0.99 to 1.00) 0.043 | |
| Sex | ||||
| Male | 158/677 (6.3) | 158/677 (6.3) | Reference group | Reference group |
| Female | 21/79 (7.6) | 21/79 (7.6) | 1.48 (0.93 to 2.37) 0.101 | 1.46 (0.91 to 2.36) 0.120 |
| AAA diameter (per cm) | 1.33 | 1.29 | 1.32 | |
| < 6.2 | 74/384 (5.0) | (1.15 to 1.54) | (1.11 to 1.50) | (1.13 to 1.54) |
| ≥ 6.2 | 105/372 (8.1) | < 0.0001 | 0.001 | < 0.001 |
| Top neck diameter (per cm) | 1.77 | 1.56 | 1.48 | |
| < 2.3 | 81/393 (5.5) | (1.10 to 2.84) | (0.96 to 2.54) | (0.89 to 2.45) |
| ≥ 2.3 | 98/362 (7.6) | 0.018 | 0.074 | 0.131 |
| Maximum common iliac diameter (cm)b | 1.88 | 1.70 | 1.69 | |
| < 1.6 | 78/398 (5.1) | (1.27 to 2.78) | (1.14 to 2.53) | (1.13 to 2.54) |
| ≥ 1.6 | 100/354 (8.3) | 0.002 | 0.009 | 0.011 |
| Reinterventions | ||||
| Age (per year) | 2.35 | 2.31 | 2.16 | |
| < 75 | 57/378 (3.5) | (1.16 to 4.76) | (1.14 to 4.69) | (1.06 to 4.37) |
| ≥ 75 | 57/378 (4.2) | 0.018 | 0.020 | 0.033 |
| Age squared (per year2) | 0.99 (0.99 to 1.00) 0.020 | 0.99 (0.99 to 1.00) 0.021 | 0.99 (0.99 to 1.00) 0.034 | |
| Sex | ||||
| Male | 100/677 (3.7) | Reference group | Reference group | Reference group |
| Female | 14/79 (4.9) | 1.29 (0.73 to 2.25) 0.380 | 1.64 (0.92 to 2.93) 0.095 | 1.60 (0.89 to 2.88) 0.116 |
| AAA diameter (per cm) | 1.44 | 1.44 | 1.47 | |
| < 6.2 | 43/384 (2.7) | (1.21 to 1.72) | (1.20 to 1.72) | (1.23 to 1.77) |
| ≥ 6.2 | 71/372 (5.1) | < 0.0001 | < 0.001 | < 0.001 |
| Top neck diameter (per cm) | 1.68 | 1.63 | 1.47 | |
| < 2.3 | 52/393 (3.3) | (0.93 to 3.04) | (0.88 to 3.00) | (0.78 to 2.79) |
| ≥ 2.3 | 62/362 (4.4) | 0.087 | 0.119 | 0.235 |
FIGURE 33.
Crude rates of serious complications and reinterventions across increasing quartile groups of (a) age and (b) AAA diameter.
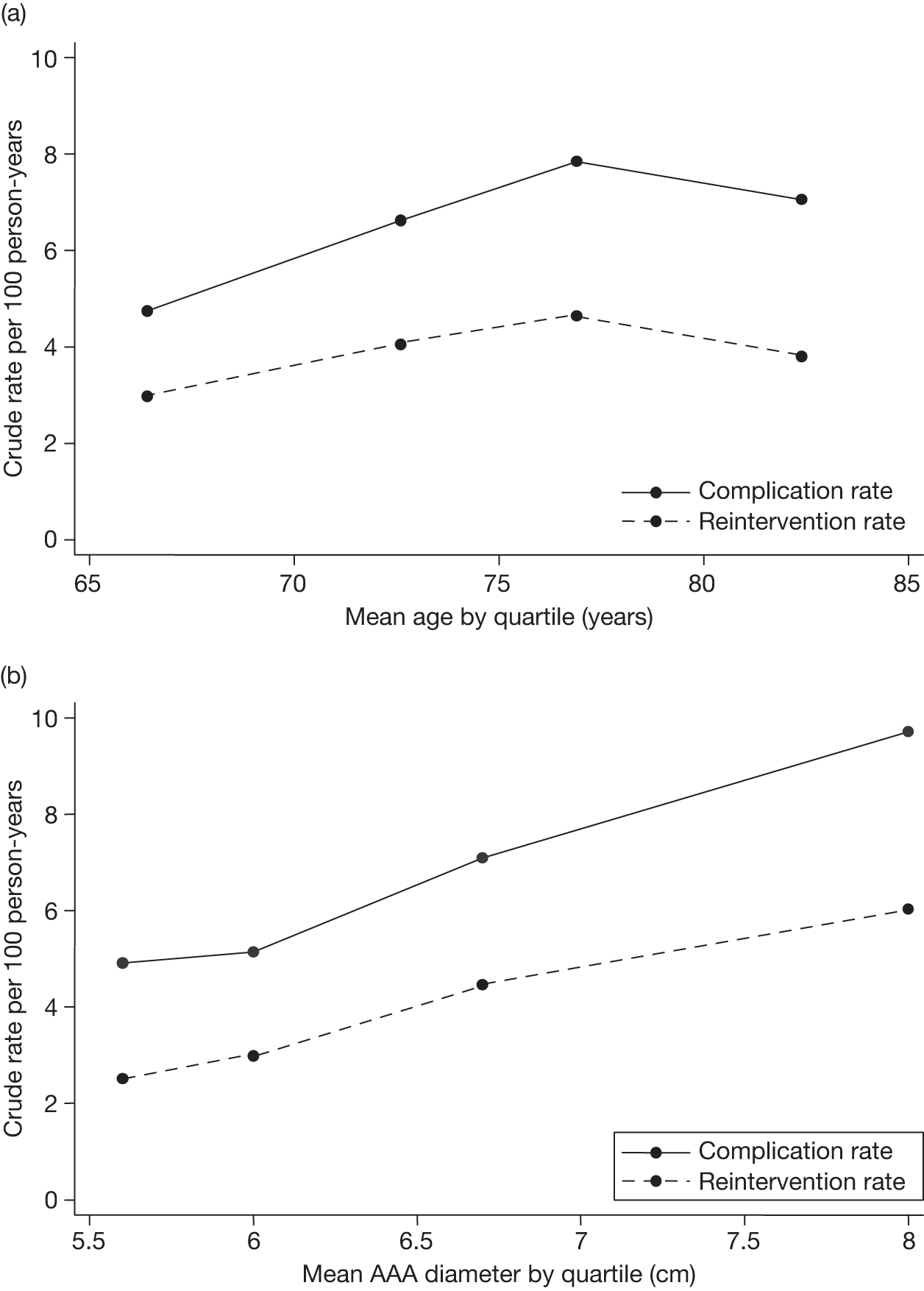
Influence of graft type
For the prespecified adjustment variables, there was some evidence to suggest that patients with a Gore/Excluder graft experienced significantly lower rates of complications and reinterventions than those with the other graft types (Table 32). This was confirmed by significant post hoc likelihood ratio tests on three degrees of freedom between the four graft groups in the final adjusted model (p = 0.022 for complications and p = 0.006 for reinterventions). However, the number of Gore grafts used was small and particular to a subgroup of 11 centres (although results have been adjusted for centre). There was no evidence to suggest any change in rates of events with chronological time since the start of the trial despite all the three main graft brands modifying their grafts with iterative improvements. Straight and uni-iliac grafts demonstrated slightly higher rates of events than bifurcated grafts, but this was not significant for either the adjusted complications model [HR 1.22 (95% CI 0.72 to 2.08), p = 0.455] or the adjusted reinterventions model [HR 1.49 (95% CI 0.80 to 2.80), p = 0.211]. For both complications and reinterventions models there was some borderline evidence to suggest that the rates differed significantly across centres (p = 0.099 for complications and p = 0.041 for reinterventions from shared frailty models including centre as a random effect).
| Graft manufacturer | No. complications/ patients (rate per 100 person-years) | Adjustedb HR for complications (95% CI) p-value | No. reinterventions/patients (rate per 100 person-years) | Adjustedb HR for reinterventions (95% CI) p-value |
|---|---|---|---|---|
| Gore/Excluder | 4/45 (1.9) | Reference group | 1/45 (0.5) | Reference group |
| Cook/Zenith | 100/421 (6.5) | 3.22 (1.16 to 8.88) 0.024 | 68/421 (4.2) | 9.86 (1.35 to 72.0) 0.024 |
| Medtronic/Talent | 54/218 (7.0) | 3.52 (1.25 to 9.89) 0.017 | 32/218 (3.8) | 8.66 (1.17 to 64.3) 0.035 |
| Other | 21/67 (8.7) | 4.48 (1.50 to 13.3) 0.007 | 13/67 (4.7) | 12.2 (1.57 to 94.3) 0.017 |
Impact of graft complications and reinterventions on renal function
Objectives
The EVAR trials 1 and 2 offered the first opportunity to investigate longitudinal changes in renal function in a cohort of patients with large aneurysms. Annual creatinine measurements had been collected for all patients since the start of the trials in 1999 and this had produced a large database in which renal function could be investigated further. There has been much speculation on whether or not the use of EVAR has a more detrimental effect on renal function, partly as a result of the primary procedure but also from the increased imaging intensity and high number of reinterventions that occur after EVAR. Therefore, in addition to the comparisons of renal function between the randomised groups of each trial documented in Chapter 4, Renal function and Chapter 5, Renal function, this analysis investigated the impact of graft-related complications and reinterventions on subsequent renal function after EVAR.
Methods
Patients undergoing EVAR in either EVAR trial 1 or 2 were selected as described in Chapter 4, Renal function (509 from EVAR trial 1) and Chapter 5, Renal function (114 from EVAR trial 2). This produced a combined total of 623 patients randomised to EVAR and having elective EVAR with a baseline and at least one eGFR measurements collected during their post-EVAR follow-up. The statistical methods have already been described in Chapter 2, Multilevel modelling statistical methods for renal function analyses.
Results
Of the total of 623 patients (2668 eGFRs), 279 patients had a complication reported at some time during follow-up, with 471 eGFR measurements before detection and 754 afterwards. A total of 344 patients did not have a complication detected during their follow-up and they provided a total of 1443 eGFR measurements. The mean rates of decline in eGFR were –1.08 ml/minute/1.73 m2 per year for patients without a complication versus a significantly higher rate of –1.41 ml/minute/1.73 m2 per year for patients with a complication at any time (see Table 32). Among patients with a complication, the decline was greater (slope = –2.61, p < 0.001) before the complication but then reduced (slope = –0.19, p < 0.001) after the complication. All of these differences were highly significant even after adjustment for a list of predefined factors thought to be associated with renal function decline (Table 33; see footnote b). To investigate how the timing of the diagnosis of complication influenced renal function, the average profiles of eGFR were plotted at baseline, before and after diagnosis of the complication and then at the final follow-up measurement (Figure 34). A possible explanation for the improvement after diagnosis is treatment or reintervention for the complication so further analysis was performed into the impact of any reinterventions. A total of 143 patients had a reintervention at some time during follow-up, with 329 eGFR measurements before the reintervention and 311 afterwards. A total of 480 patients did not have a reintervention during their follow-up (including 136 patients with an untreated complication) and they provided a total of 2028 eGFR measurements. The reintervention group demonstrated higher eGFR measurements and a faster rate of decline of eGFR over time: –1.67 versus –1.08 ml/minute/1.73 m2 per year than the group without any reinterventions (Table 34). However, inclusion of terms for reintervention in the model investigating the impact of complications made only a minor difference to the results, implying that the deceleration in renal function decline after diagnosis of a complication was only partially explained by reintervention. The crude model estimates are provided below and it is clear from the last four terms describing the effects of reinterventions that they do not influence eGFR or its subsequent rate of decline as strongly as the presence of a complication. Moreover, the last two terms demonstrate little difference in eGFR or rate of decline in eGFR before and after the reintervention. None of these last four terms were statistically significant in the model while the terms relating to complications retained strong statistical significance.
| Renal outcome | Complications (n = 279 patients, n = 1225 eGFRs) | No complications (n = 344 patients, n = 1443 eGFRs) | Crude coefficienta (95% CI), p-value | Final coefficientb (95% CI), p-value | |
|---|---|---|---|---|---|
| Before complication (n = 471) | After complication (n = 754) | ||||
| Mean (SD) eGFR for all measurements (ml/minute/1.73 m2) | 62.2 (18.2) | 60.1 (17.7) | 60.1 (18.1) | 3.96 (1.37 to 6.55) < 0.001 | 4.49 (1.91 to 7.06) 0.001 |
| Mean (SD) rate of decline in eGFR (ml/minute/1.73 m2 per year) | –1.41 (1.14) | –1.08 (1.73) | –1.56 (–2.48 to –0.63) 0.001 | –1.59 (–2.52 to –0.66) 0.001 | |
FIGURE 34.
Profiles of observed average eGFR against average time at baseline and final follow-up for patients without any complications (left plot) and at baseline, last eGFR prior to complication diagnosis, first eGFR after complication diagnosis and final follow-up for patients with complications (right plot).
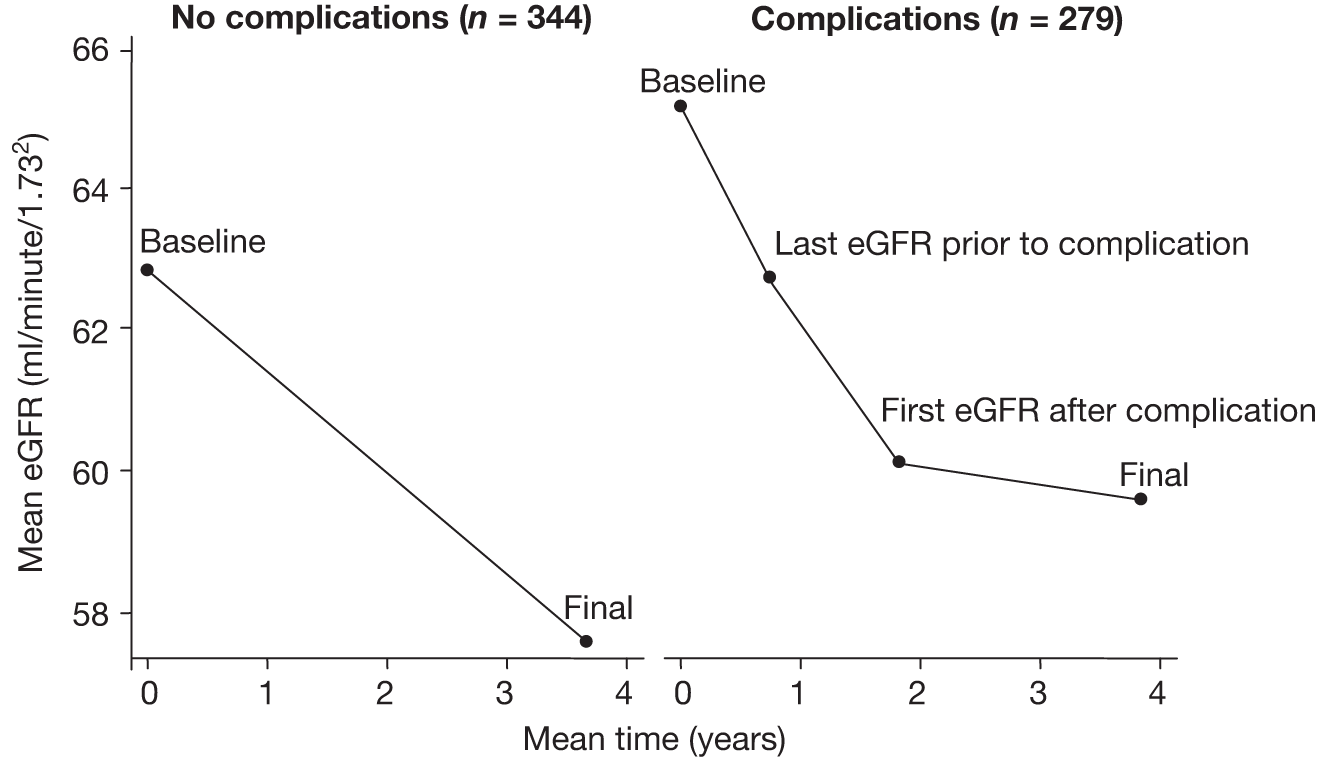
| Renal outcome | Reinterventions (n = 143 patients, n = 640 eGFRs) | No reinterventions (n = 480 patients, n = 2028 eGFRs) | Crude coefficienta (95% CI), p-value | Final coefficientb (95% CI), p-value | |
|---|---|---|---|---|---|
| Before reintervention (n = 329) | After reintervention (n = 311) | ||||
| Mean (SD) eGFR for all measurements (ml/minute/1.73 m2) | 61.8 (17.1) | 60.0 (18.0) | 60.3 (18.2) | 2.99 (–0.08 to 6.06) 0.056 | 3.55 (0.55 to 6.55) 0.021 |
| Mean (SD) rate of decline in eGFR (ml/minute/1.73 m2 per year) | –1.67 (0.92) | –1.08 (1.56) | –1.16 (–2.05 to –0.26) 0.011 | –1.17 (–2.07 to –0.28) 0.010 | |
Chapter 7 Costs and cost-effectiveness analysis of EVAR versus open repair
Introduction
This chapter estimates the costs and cost-effectiveness of endovascular repair versus open repair for AAA. There have been a number of recent published economic evaluations of these treatments. This chapter reviews the methods and data used in those studies, highlights the key uncertainties about the cost-effectiveness of the treatments, and updates a previously published decision model200 in the light of the recently available mid- and long-term results of the relevant clinical trials: the EVAR trial 1,201 the DREAM160 and the OVER trials. 165
Review of recently published economic models of EVAR versus open repair
There are several published economic models comparing EVAR with open repair. 200,202–207 Chambers et al. 208 present a complete review. Of these, the most relevant and internally valid are those studies that incorporated comparisons of treatment effects based exclusively on RCTs. 160,165,201 These models are:
-
a 1-year cost-effectiveness analysis based on the DREAM207 trial
-
participants of EVAR (2008), a model based on the 4-year results of EVAR trial 1132
-
the submission made by Medtronic (manufacturer of Talent and AneuRx stent–grafts) to NICE appraisal 167199,208
-
the base case of the Assessment Report by the University of York to NICE appraisal 167199,208
-
the version of the model accepted in the final appraisal document (FAD) by NICE appraisal 167. 199,208
Table 35 sets out the main similarities and differences in terms of structure, inputs and results. Prinssen et al. 207 took a 1-year time horizon, with costs and effectiveness based on the DREAM trial. 207 This study found that survival at 1 year was slightly better after EVAR. However, HRQoL (measured by the EQ-5D) tended to be higher following open repair after the first 3 months, although the difference was small and non-significant. Overall, EVAR was associated on average with lower quality-adjusted life-years (QALYs) and higher costs than open repair over 1 year. However, the short time horizon in the analysis may be biased, as it assumes no difference in survival (or costs) beyond 1 year. The EVAR model (2008)200 was a lifetime analysis extrapolating from the 4-year results of EVAR trial 1. This study concluded that EVAR was unlikely to be cost-effective, given the assumptions that patients faced a continuing elevated risk (compared with open repair) of late AAA mortality and reinterventions after EVAR for the rest of their lives and that there was no difference in overall survival after 4 years. The recent NICE appraisal of endovascular stents considered that EVAR was likely to be cost-effective. 199 This was based on expert opinions that the relative risks of late AAA mortality and reinterventions after aneurysm repair in current practice were more favourable to EVAR than those estimated by EVAR trial 1 and that, with current devices and surgical practice, there is now little difference in initial costs between the procedures. The new cost-effectiveness analysis presented in this chapter updates the previous models in the light of the 8-year results. 160,201 The main uncertainties leading to differences in results between the models are:
-
Does EVAR offer any benefit compared with open repair in terms of the overall probability of survival in the medium term?
-
What are the risks of AAA-related mortality in the medium and long term after the procedures?
-
What are the risks of reinterventions in the medium and long term?
-
What are the medium- and long-term requirements (and cost) for surveillance?
-
What are the relative costs of the procedures with current devices and practice?
| Parameter | Prinssen et al. (2007)207 | BJS paper (2008)200 | Medtronic (2009)208 | York Report (2009)208 | NICE FAD (2009)208 |
|---|---|---|---|---|---|
| Model structure and states | |||||
| Structure | Within-trial economic analysis (1-year time horizon) | Initial procedure (30-day operative mortality or conversion to open repair). Survivors pass into Markov model | Initial procedure (30-day operative mortality or conversion to open repair). Survivors pass into Markov model | Initial procedure (30-day operative mortality or conversion to open repair). Survivors pass into Markov model | Initial procedure (30-day operative mortality or conversion to open repair). Survivors pass into Markov model |
| Health states | Not applicable | Symptom-free survival, non-fatal stroke or MI, non-fatal secondary readmission (tunnel), death from AAA, cardiovascular or other causes | Symptom-free survival, non-fatal stroke or MI, non-fatal secondary readmission (tunnel), death from AAA, cardiovascular or other causes | Symptom-free survival, non-fatal secondary readmission (tunnel), death from AAA or other causes | Symptom-free survival, non-fatal secondary readmission (tunnel), death from AAA or other causes |
| Parameter values | |||||
| 30-day operative mortality (excluding pre-operative deaths) | Open repair: 0.056 | Open repair: 0.05 | Open repair: 0.042 | Open repair: 0.05 | Open repair: 0.062 |
| EVAR: 0.012 | EVAR: 0.016 | EVAR: 0.016 | EVAR: 0.016 | EVAR: 0.021 | |
| OR: 0.30 | OR: 0.30 | OR: 0.35 | OR: 0.30 | OR: 0.35 | |
| Conversion to open repair during primary EVAR | NR | 4/500 = 0.008 | 0.002 | 0.008 | 0.008 |
| AAA mortality rate after initial procedure from 6 months to 4 years | NR | Open repair: 0.0008/year | No parameter in base case (assumed to be included in all-cause mortality) | EVAR: 0.0048/year. HR EVAR vs open repair 1.5 | EVAR: 0.0048/year. HR EVAR vs open repair 1.5 |
| EVAR: 0.0048/year | |||||
| HR EVAR vs open repair = 6.0 | |||||
| Long-term relative risk of AAA mortality after 4 years | NR | HR EVAR vs open repair = 6.0 for lifetime | No parameter in base case (assumed to be included in all-cause mortality) | HR EVAR vs open repair = 1.5 until year 8, no difference thereafter | HR EVAR vs open repair = 1.5 until year 8, no difference thereafter |
| All-cause survival curves meet? | All-cause deaths at 1 year: open repair 12/170; EVAR 10/170; p = 0.7 | Survival curves meet at 2 years | Higher all-cause mortality after EVAR for 4 years. Survival curves do not meet | Survival curves meet at 3 years | Survival curves meet at 3 years |
| SMR for non-AAA death after AAA repair compared with general population | NR | SMR of about 1.1 for non-AAA mortality relative to general population (71% survive 4 years after open repair) | SMR of about 1.1 for non-AAA mortality relative to general population | SMR of about 1.1 for non-AAA mortality relative to general population | SMR of about 1.1 for non-AAA mortality relative to general population (54% survive 8 years after open repair) |
| Reinterventions for graft-related causes 0–6 months | Severe complications for any cause at 1 year: open repair 37/170; EVAR 33/170 | Open: 0.016/year, HR EVAR vs open repair 6.7 | Open: 0.010/month, EVAR 0.017/month | Open: 0.016/year, HR 6.7 | Open: 0.016/year, HR 1.5 |
| Reinterventions for graft-related causes after 6 months plus | NR | Open: 0.004/year (declining Weibull), HR 6.7 | Open: 0, EVAR 0.003/month | Open: 0.004/year (declining Weibull), HR 6.7 | Open: 0.004/year (declining Weibull), HR 1.5 |
| Systemic complications (renal/stroke/MI) after first 30 days | NR | Proportional to rate of cardiovascular mortality | Stroke/MI equal; higher rate of renal failure in open repair | Not included in model | Not included in model |
| Costs of primary procedure including stent–graft (excluding conversions from EVAR to open repair) | EVAR €2940 more expensive than open repair | EVAR £1148 more expensive than open repair | EVAR £623 less than open repair | EVAR costs £523 more than open repair | Costs of procedures are the same |
| Follow-up surveillance | Cost during first year: EVAR €1295; open repair €995 | Additional cost per year after EVAR: £194 (CT + OP visit per year) | Additional cost £108 per year after EVAR (CT only) | Additional cost £98 per year after EVAR (CT only) | Additional cost £54 per year after EVAR (CT every 2 years) |
| Loss of utility compared with normal person for 3 months after procedure/reintervention | Graphs show a difference in favour of EVAR at 3 months, and subsequently a difference in favour of open repair | Open repair 0.027, EVAR 0.094 | Open repair 0.027, EVAR 0.094 | Open repair 0.027, EVAR 0.094 | Open repair 0.027, EVAR 0.094 |
| Results | |||||
| Incremental costs (EVAR less open) | €4239 (at 1 year) | £3578 | £1098 | £2002 | £534 |
| Incremental QALY (EVAR less open) | –0.01 (at 1 year) | –0.020 | 0.076 | 0.041 | 0.043 |
| ICER | EVAR dominated | EVAR dominated | £14,506/QALY | £48,990/QALY | £12,305/QALY |
Methods
Overview
The model compares EVAR with open repair in patients who are considered fit for open repair. The cost perspective is that of the NHS, and the price year is 2008–9. Health effects are quantified in terms of QALYs, and the annual discount rate for costs and QALYs is 3.5%.
Model structure
The model structure (Figure 35) is similar to the York Assessment Report for NICE – Technology Assessment appraisal 167. 208 However, the structures differ in one important respect. All previous models began at the initial AAA procedure (EVAR or open repair). Survivors passed into a Markov model to estimate lifetime costs and QALYs. This is not an ITT analysis, because patients did not have surgery immediately after randomisation. In EVAR trial 1, the median (IQR) time from randomisation to surgery was 43 (28–70) days in the EVAR group and 36 (20–59) days in the open-repair group. 121 The wait for repair and differences in the waiting time between treatments may be unavoidable, given constraints on the health service. Starting the model at the time of surgery may lead to bias, for several reasons:
FIGURE 35.
Structure of the cost-effectiveness model comparing EVAR with open repair.
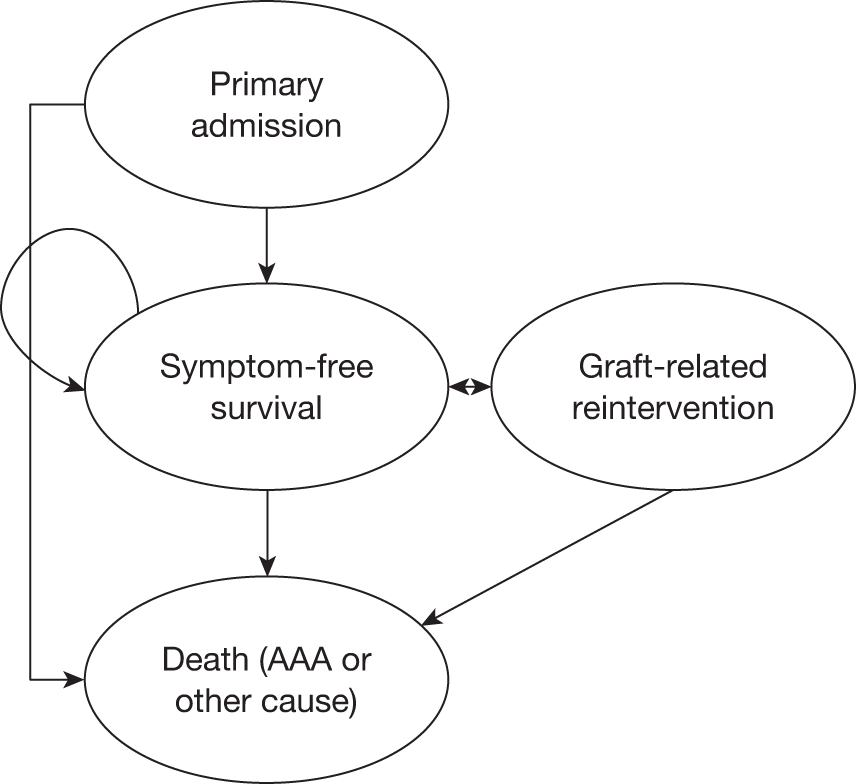
-
It ignores deaths during the waiting time, which might be considered as arising from the treatment strategy.
-
Estimates of relative risks of events after the procedure may be biased because they are not based on baseline randomisation groups. The frailest patients are most likely to die during the waiting time.
-
Splitting the follow-up time into up to 30 days post-procedure and after 30 days may be considered somewhat arbitrary. Some patients are discharged from hospital after 30 days, and adverse events (AAA deaths and reinterventions) are most likely to occur during the initial 6 months. Estimates of HRs that include events in the first 6 months may not be relevant for the purposes of extrapolation to the long term.
The model in this chapter improves on previous models by carrying out a strictly ‘ITT’ analysis. The follow-up time is divided into four periods: randomisation to 6 months, 6 months to 4 years, 4–8 years, and after 8 years. This structure and parameterisation of the model is consistent with the secondary analyses reported by EVAR trial 1. 201 As there are limited data beyond 8 years, estimates of these very long-term rates of events must be obtained from observational data or expert opinion. Given that 54% of patients survive more than 8 years after AAA repair (Figure 14), these very long-term estimates are likely to be important for the model results.
The weakness of this ITT approach is that other clinical trials, the DREAM160 and the OVER165 trials did not report outcomes in this way. These trials report 30-day operative mortality and overall probability of death at the end of the trial. This makes synthesis of all available evidence difficult. To attempt to incorporate all relevant evidence, a secondary analysis is undertaken with the model structure of Chambers et al. 208 using pooled treatment effects for 30-day mortality from all the randomised trials.
In the current model, the cycle length is 6 months, and the time horizon is 25 years. The primary admission is assumed to take place during the first 6 months, during which patients incur procedure costs and diminished HRQoL. Survivors pass into the long-term model, where it is assumed that, if they have no complications requiring reintervention, they will achieve the HRQoL of the general population of that age, although they are assumed to require ongoing surveillance. Graft-related reinterventions can occur in any cycle and incur diminished HRQoL and hospital costs for that cycle. Patients can die of aneurysm-related or other causes in any cycle. Other possible systemic complications (such as renal failure, MIs, etc.) were included in some earlier modelling studies. 200,208 They are not included in the current model as no significant evidence of any difference was apparent (see Chapter 4, Adverse events and Chapter 4, Cardiovascular mortality and events).
Model parameters
Abdominal aortic aneurysm-related mortality
The model inputs include estimates of AAA-related mortality rates and HRs for EVAR versus open repair, classified by time since randomisation (Table 36). The 10-year follow-up from the EVAR trial found a continued statistically significant higher risk of AAA mortality after EVAR than open repair after 4 years, although the absolute rate of deaths after EVAR is < 1 per 100 patient-years of follow-up. The data from the EVAR arm of the trial can be compared with the EUROSTAR registry,171 a longitudinal database of outcomes in patients following endovascular repair until October 2007 [unpublished analysis undertaken by the authors using the individual patient data (IPD)]. In patients who are fit for open repair, the EUROSTAR data estimate a much higher rate of AAA mortality after EVAR than EVAR trial 1. This maybe because the register includes patients with older generations of devices, but might also be because of patient selection into EVAR trial 1. The EUROSTAR data indicate that the risk of AAA mortality diminishes over time, and appear to confirm that the absolute risk may be small after 8 years. The base case assumes that there is no difference in the rate of AAA deaths between treatments after 8 years. This assumption is likely to be in favour of EVAR, and alternatives to this scenario are explored in sensitivity analysis. The OVER trial reported four AAA deaths after discharge from hospital after EVAR (in about 800 patient-years of follow-up, a mean rate of about 0.5 per 100 patient-years) and no AAA deaths after hospitalisation following open repair. 165
| Time after enrolment to register | EUROSTAR | EVAR trial 1 | ||||
|---|---|---|---|---|---|---|
| Events during period | No. at risk at start of period | Person-years at risk during period | Rate per 100 person-years | Rate per 100 person-years | HR for EVAR vs open repair (95% CI) | |
| 0–6 months | 258 | 8076 | 3213 | 8.0 | 4.6 | 0.47 (0.23 to 0.93) |
| 6 months to 4 years | 600 | 5367 | 13,791 | 4.4 | 0.6 | 1.46 (0.56 to 3.82) |
| 4–8 years | 192 | 1152 | 7108 | 2.7 | 0.8 | 4.85 (1.04 to 22.72) |
| > 8 years | 17 | 115 | 1074 | 1.6 | NA | NA |
Both the DREAM and the OVER trials reported relative risks for AAA mortality up to 30 days after aneurysm repair. Table 37 shows the estimated pooled value of the OR for EVAR versus open repair across the three trials. Guidelines for economic analysis recommend that treatment effects should be estimated by a synthesis of all available evidence. 209 However, both the OVER and the DREAM trials did not report relative risks for up to 6 months and after 6 months from randomisation as required by the base-case model. Therefore, the relative risks in the base-case model are taken exclusively from EVAR trial 1, rather from a meta-analysis. Moreover, the participants recruited to the trials may represent different populations. The OVER trial recruited younger, fitter patients with smaller aneurysms and this may at least partly explain the more favourable OR in this trial. The results of the model parameterised by the outcomes of the OVER trial (treatment effects and costs) are shown as a sensitivity analysis.
| Trial | Deaths at 30 days | EVAR vs open repair, OR (95% CI) | |
|---|---|---|---|
| EVAR | Open repair | ||
| DREAM160 | 2/171 | 8/174 | 0.297 (0.085 to 1.043) |
| EVAR trial 1201 | 9/532 | 25/518 | 0.368 (0.186 to 0.729) |
| OVER165 | 1/442 | 10/436 | 0.188 (0.057 to 0.612) |
| Total (Peto) | 1145 | 1128 | 0.309 (0.181 to 0.528) |
| Heterogeneity test chi-squared | p = 0.631 | ||
| Total DREAM and EVAR trial 1 only | 703 | 692 | 0.35 (0.19 to 0.64) |
| Heterogeneity test | p = 0.77 | ||
Other-cause mortality
Rates of all-cause mortality are higher after successful AAA repair than would be expected in the general population. The standardised mortality ratio (SMR) has been estimated as 1.36 in men and 1.82 in women for those who survive > 30 days after aneurysm repair. 148 In EVAR trial 1, 54% of patients survived 8 years. After excluding the elevated risk of AAA mortality, this implies a SMR for men in the EVAR trial 1 population of about 1.1, relative to the general population. Furthermore, there was no observed difference between EVAR and open repair in overall survival after 2 years in DREAM or EVAR trial 1, or after 3 years in the US Medicare registry. 115 This cannot be entirely explained by the higher rate of AAA mortality following EVAR after 6 months. Given an initial benefit for endovascular repair, then in order for the survival curves to meet at 2 years there must be an offsetting increase in mid-term mortality after endovascular repair, relative to open repair. In EVAR trial 1, this appears to arise from cardiovascular causes. 210 To incorporate these results in the model, other-cause (non-AAA) mortality is calibrated so that the all-cause survival curves meet at 2 years after randomisation. It is assumed that after 2 years there is no further difference in non-AAA mortality, although there remains a difference in AAA mortality. This ‘catch-up’ in mortality is varied in sensitivity analysis, including a scenario in which there is no excess mortality. The OVER trial found that the early advantage of endovascular repair was not, on average, offset by increased all-cause mortality in the first 2 years, i.e. overall mortality remained lower in the EVAR group, although the difference in survival was not statistically significant (Table 38).
| Trial | Percentage alive | Difference in percentage alive (95% CI) | HR for rate of death for any cause (95% CI) | |
|---|---|---|---|---|
| EVAR | Open | EVAR less open repair | EVAR vs open repair | |
| DREAM (6 years)160 | 68.9 | 69.9 | –1.0 (–10.8 to 8.8) | NR |
| EVAR trial 1 (8 years)201 | 54 | 54 | 0 (95% CI NA) | 1.03 (0.86 to 1.23) |
| OVER (2 years)165 | 93.0 | 90.2 | 2.8 (95% CI NA) | 0.7 (0.4 to 1.1) |
Graft-related reinterventions
The base-case model estimates the rate of aneurysm-related reinterventions after endovascular repair, and the relative risk for open repair versus endovascular repair, from EVAR trial 1 for different times (Table 39). There was little consistency across the three randomised trials in the definition of a reintervention and the format in which they were reported. The rate of events and HR from the DREAM trial were not reported, but these parameters can be approximately inferred from the reported probability of survival free of reinterventions at 6 years. The mean HR will be approximately 5.6/3.1 = 1.8 (see Table 39 for details of calculation), although the standard error (SE) is not available. The overall rate of reintervention in the DREAM trial after endovascular repair is similar to EVAR trial 1, but the rate in the open-repair group is higher. This may be due to differences between the trials in the types of outcomes recorded. The rates of reinterventions or HR were not reported in the OVER trial, but it is stated that the difference in secondary therapeutic procedures was not statistically significant.
| Trial | Percentage surviving without reintervention | Rate of reinterventions per 100 person-years (95% CI) | Secondary therapeutic procedure | |||||
|---|---|---|---|---|---|---|---|---|
| EVAR | Open repair | Diff. EVAR – open repair | EVAR | Open repair | HR EVAR vs open repair | EVAR | Open repair | |
| EVAR trial 1 (mean over 8 years) | 72 | 90 | –18 (95% CI NR) | 5.1 | 1.7 | 2.86 (2.08 to 3.94) | NR | NR |
| EVAR trial 1 (0–6 months) | 22.9 | 13.8 | 1.65 (1.12 to 2.49) | |||||
| EVAR trial 1 (6 months to 4 years) | 3.4 | 0.3 | 9.97 (4.29 to 23.15) | |||||
| EVAR trial 1 (4–8 years) | 2.4 | 0.8 | 3.12 (1.47 to 6.80) | |||||
| DREAM (mean > 6 years) | 71.4 | 82.9 | –11.5 (–21.0 to 2.0) | 5.6a | 3.1a | 1.8 (95% CI NR) | NR | NR |
| OVER (mean > 2 years) | NR | NR | NR | NR | NR | NR | 61/444 | 55/437 |
Costs
In the base case, the mean cost of the admission for primary aneurysm repair was estimated from EVAR trial 1. 201 These costs include the graft or prosthesis, theatre time, anaesthetic, consumables, blood products, radiation exposure, postoperative interventions, conversions to open repair and length of stay on wards, ITUs and HDUs (Table 40). Unit costs were obtained from routine national sources211–213 and from the results of questionnaires sent to trial centres in May 2004,132 updated for inflation214 (Table 41).
| Description | Resource use of primary admission | Cost of primary admission (ITT) (£) | ||||
|---|---|---|---|---|---|---|
| EVAR repair (n = 614) | Open repair (n = 602) | EVAR repair (n = 626) | Open repair (n = 626) | |||
| Mean | SD | Mean | SD | |||
| Device and consumables | 6124 | 782 | ||||
| Theatre occupation time (minutes) | 191 | 62 | 215 | 68 | 3255 | 3647 |
| Duration of fluoroscopy (minutes) | 25 | 13 | 2 | 9 | 82 | 5 |
| Blood products (ml) | 141 | 471 | 863 | 1018 | 43 | 258 |
| Preoperative stay (days) | 1.81 | 2.34 | 2.16 | 3.15 | 477 | 558 |
| Postoperative stay (days) | 6.53 | 12.33 | 9.25 | 13.42 | 1719 | 2385 |
| ITU (days) | 0.59 | 3.68 | 2.47 | 0.46 | 672 | 2767 |
| HDU (days) | 0.83 | 2.02 | 1.88 | 2.80 | 675 | 1504 |
| Total | 13,019 | 11,842 | ||||
| Description | Measure | Unit cost (£) | Source |
|---|---|---|---|
| EVAR stent and parts | Per patient | 5219 | NICE appraisal 2008208 |
| Dacron graft, open surgery | Per graft | 285 | EVAR trial survey 2004208 |
| Consumables, EVAR | Per patient | 460 | EVAR trial survey 2004208 |
| Consumables, open surgery | Per patient | 89 | EVAR trial survey 2004208 |
| General anaesthetic consumables, open surgery | Per patient | 137 | EVAR trial survey 2004208 |
| Blood | ml | 0.325 | National Blood Centre 2007–8213 |
| HDU | Day | 832 | NHS Reference Costs 2007–8211 |
| ITU | Day | 1165 | NHS Reference Costs 2007–8211 |
| Vascular surgery ward | Day | 268 | NHS Reference Costs 2007–8211 |
| Operation room | Hour | 1055 | NHS Scotland 2008–9212 |
| Fluoroscopy, 1 –20 minutes | Session | 49 | NHS Reference Costs 2007–8211 |
| Fluoroscopy, 21–40 minutes | Session | 94 | NHS Reference Costs 2007–8211 |
| Fluoroscopy, > 40 minutes | Session | 138 | NHS Reference Costs 2007–8211 |
| CT | Session | 108 | NHS Reference Costs 2007–8211 |
| Vascular surgery outpatient | Attendance | 88 | NHS tariff 2008–9211 |
The costs of the primary aneurysm repair estimated from EVAR trial 1 in an ITT analysis were £13,019 in the endovascular-repair group (N = 626) and £11,842 in the open-repair group (N = 626) (mean difference £1177; 95% CI £−374 to £2728). These values were used in the economic model. Table 40 shows these costs in more detail, and Table 42 shows the mean costs of reinterventions after EVAR. The costs of reinterventions that occur during the primary admission are included in the estimate of the primary procedure cost. Therefore, in the model these must be excluded from the estimate of reinterventions during the first 6 months to avoid double counting the costs of reinterventions. From EVAR trial 1 data, it was assumed that 38/40 (95%) reinterventions during the first 6 months after open repair occurred during the primary admission, and 42/66 (64%) after endovascular repair were during the primary admission.
| Description | Resource use (N = 160) | Cost (£) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Device and consumables (n/N) | 10/160 | 297 | 1219 | |
| Theatre occupation time (minutes) | 179 | 66.63 | 3148 | 1171 |
| Duration of fluoroscopy (minutes) | 14 | 17.42 | 93 | 55 |
| Blood products (ml) | 383 | 827.77 | 119 | 258 |
| Preoperative stay (days) | 1.60 | 2.45 | 429 | 657 |
| Postoperative wards (days) | 6.67 | 15.80 | 1789 | 4248 |
| ITU (days) | 1.21 | 4.96 | 1405 | 5782 |
| HDU (days) | 0.31 | 1.18 | 255 | 983 |
| Total | 7536 | 10,679 | ||
In-hospital costs were also reported by the DREAM207 trial and resource use was reported in the OVER trial. 165 The DREAM trial estimated the mean in-hospital costs in the Netherlands as €14,915 after EVAR and €11,975 after open repair, a difference of €2940. The OVER trial, conducted in the USA, appears to show that EVAR uses fewer hospital resources than in EVAR trial 1 (Table 43). However, there are likely to be important differences between the health-care systems in the UK, the Netherlands and the USA that make intracountry comparison unreliable. Furthermore, the populations in these trials were slightly different than in EVAR trial 1. Both the DREAM and the OVER trials included patients with aneurysms of < 5.5 cm, and patients in the OVER trial were on average younger and fitter than in EVAR trial 1, and this is also likely to influence costs.
| Description | Measure | Resource | |||
|---|---|---|---|---|---|
| EVAR | Open repair | ||||
| Median | IQR | Median | IQR | ||
| Theatre occupation time | Hours | 2.9 | 2.3–3.7 | 3.7 | 2.9–4.7 |
| Duration of fluoroscopy | Minutes | 23 | 17.0–31.0 | 0 | |
| Blood loss | ml | 200 | 150–400 | 1000 | 650–2000 |
| Hospital stay | Days | 3 | 2.0–5.0 | 7 | 6.0–10.0 |
| ITU | Days | 1 | 1.0–2.0 | 4 | 3.0–6.0 |
The EVAR trial 1 required surveillance after AAA repair at 1 month and 3 months and yearly thereafter (see Chapter 2, Trial follow-up protocol). However, this protocol may not reflect standard clinical practice, particularly after open repair. In the base case, based on the results of the survey in May 2004,132 patients are assumed to require one outpatient visit and CT after open repair, with no further routine surveillance. Patients are assumed to require one outpatient visit and CT every year after EVAR for the rest of their life. In clinical practice, the frequency of surveillance will depend on many variables, for example patients with diagnosed, untreated complications may have more frequent surveillance and more costly scans and the European guidelines for vascular surgery recommend duplex scans at 5, 10 and 15 years after open repair. 215
Health-related quality of life
The EVAR trial 1 found that patients incur a greater loss of HRQoL following open repair than EVAR for the first 3 months, but there are no significant differences in HRQoL after this time. Patients are assumed to incur similar loss of HRQoL following a secondary reintervention as the primary reintervention. This parameter is varied in sensitivity analyses.
Cost-effectiveness analysis
Deterministic analysis
In the deterministic model, the parameters are point estimates of their mean values. The model calculates mean costs and QALYs associated with each treatment, given these inputs. If one treatment has a higher mean cost and lower mean QALY than the other then it is dominated. Otherwise, the ICER is calculated as the ratio of mean incremental costs divided by mean incremental QALYs. Conventionally the cost-effectiveness threshold against which the ICER is assessed is £20,000–30,000 per QALY gained in England. 209
Probabilistic sensitivity analysis
In the probabilistic model, the parameters are stochastic, i.e. each is characterised by a probability distribution rather than a point estimate of the mean. Monte Carlo simulation runs 1000 iterations of the model, and the costs and QALYs associated with each treatment are recorded at each simulation. The mean incremental cost and mean incremental QALY of endovascular repair less open repair are estimated over the 1000 simulations. The probability that endovascular repair is cost-effective is estimated as the proportion of the 1000 simulations for which endovascular repair would be cost-effective, over a range of values for the threshold cost per QALY. 216
Univariate sensitivity analyses
Table 44 shows the mean values of the parameters in the base case and in three alternative scenarios: a scenario using the inputs estimated in EVAR 2008,200 a scenario based on the NICE FAD208 and a scenario based on the results for in-hospital mortality, overall survival and reinterventions of the OVER trial. 165 To identify which parameters had the most influence on the model results, the values in the base-case model were varied one at a time to correspond with these alternative scenarios in a series of univariate sensitivity analyses.
| No. | Population | Base case | Value | ||||
|---|---|---|---|---|---|---|---|
| Value | Measure of uncertainty | EVAR 2008200 | NICE FAD208 | OVER165 | |||
| 1 | Age | 74 | 74 | 74 | 70 | ||
| 2 | Gender (% male) | 90 | 90 | 90 | 95 | ||
| 3 | AAA size (cm) | 6.5 | 6.5 | 6.5 | 5.8 | ||
| Model structure | |||||||
| 4 | Length of first cycle (months) | 6 | 1 | 1 | 1 | ||
| Parameter | |||||||
| Mean | n/N | Mean | Mean | Mean | |||
| 5 | Probability AAA death (EVAR) 0–6 months | 0.022 | 14 | 626 | NA | NA | |
| 6 | Probability AAA death (EVAR) 30 days after admission or during hospitalisation | NA | 0.016 | 0.021 | 0.0045 | ||
| 7 | Rate AAA mortality (EVAR) 6 months to 4 years/year | 0.006 | 0.005 | 0.009 | 0.006 | ||
| 8 | Rate AAA mortality (EVAR) > 4 years/year | 0.008 | 0.005 | 0.009 | 0.008 | ||
| Mean | 95% CI | ||||||
| 9 | OR AAA deaths (EVAR vs open repair) during first model period | 0.47 | 0.23 | 0.93 | 0.31 | 0.35 | 0.19 |
| 10 | HR AAA deaths (EVAR vs open repair) 6 months to 4 years | 1.46 | 0.56 | 3.82 | 6.0 | 1.5 | 1.46 |
| 11 | HR AAA deaths (EVAR vs open repair) 4–8 years | 4.85 | 1.04 | 22.72 | 6.0 | 1.5 | 4.85 |
| 12 | SMR relative to general population for age 74 years | 1.1 | 1.4 | 1.1 | 1.1 | ||
| 13 | Time to converge of all cause survival (excess non-AAA mortality) (years) | 2 years | 3 years | 3 years | No excess non-AAA mortality | ||
| Value | Lower range | Upper range | |||||
| 14 | Number of years after the initial procedure during which AAA mortality is higher after EVAR than open repair | 8 years | 6 years | 20 years | 20 years | 8 years | 8 years |
| Mean | 95% | CI | |||||
| 15 | Procedure cost difference (including conversion to open repair and other reinterventions during the initial admission) (£) | 1177 | –374 | 2728 | 1613 | 0 | 1177 |
| 16 | Surveillance cost (£)/year | 196 | 194 | 54 | 194 | ||
| 17 | Rate of reinterventions EVAR 0–6 months/year | 0.115a | 0.106b | 0.106b | 0.115a | ||
| 18 | Rate of reinterventions EVAR 6 months to 4 years/year | 0.034 | 0.034b | 0.034b | 0.034 | ||
| 19 | Rate of reinterventions EVAR > 4 years/year | 0.024 | 0.024b | 0.024b | 0.024 | ||
| 20 | HR reinterventions (EVAR vs open repair) 0–6 months | 1.65 | 1.12 | 2.49 | 6.75 | 1.5 | 1.0 |
| 21 | HR reinterventions (EVAR vs open repair) 6 months to 4 years | 9.97 | 4.29 | 23.15 | 6.75 | 1.5 | 1.0 |
| 22 | HR reinterventions (EVAR vs open repair) > 4 years | 3.24 | 1.47 | 6.8 | 6.75 | 1.5 | 1.0 |
Results
Base-case deterministic analysis
Table 45 shows the results of the base-case model. The predicted probabilities of survival for AAA and all causes at 8 years are consistent with the clinical results. 201 The model predicts a difference in life expectancy and QALYs in favour of open repair (mean difference in QALY, –0.042), with higher lifetime costs after EVAR (mean difference £3521). EVAR is, therefore, on average dominated by open repair. In the base-case analysis life expectancy and QALYs are greater after open repair because it is assumed that the all-cause survival curves meet at 2 years, and there is a greater hazard of late AAA deaths after EVAR up to 8 years. This means that the predicted all-cause survival curves cross and, by 8 years, the initial advantage in AAA survival has been almost entirely offset by late AAA mortality (Figure 36).
| Outcome | EVAR | Open repair | Difference |
|---|---|---|---|
| Life expectancy (undiscounted years) | 8.921 | 9.029 | –0.108 |
| Probability of aneurysm-related survival (8 years) | 0.938 | 0.937 | 0.001 |
| Probability of survival from any cause (8 years) | 0.531 | 0.547 | –0.018 |
| Mean QALYs (lifetime discounted) | 5.391 | 5.433 | –0.042 |
| Mean costs (lifetime discounted, £) | 15,784 | 12,263 | 3521 |
| ICER EVAR vs open repair | EVAR dominated (ICER cannot be calculated) |
FIGURE 36.
Predicted survival from AAA and any cause by treatment.
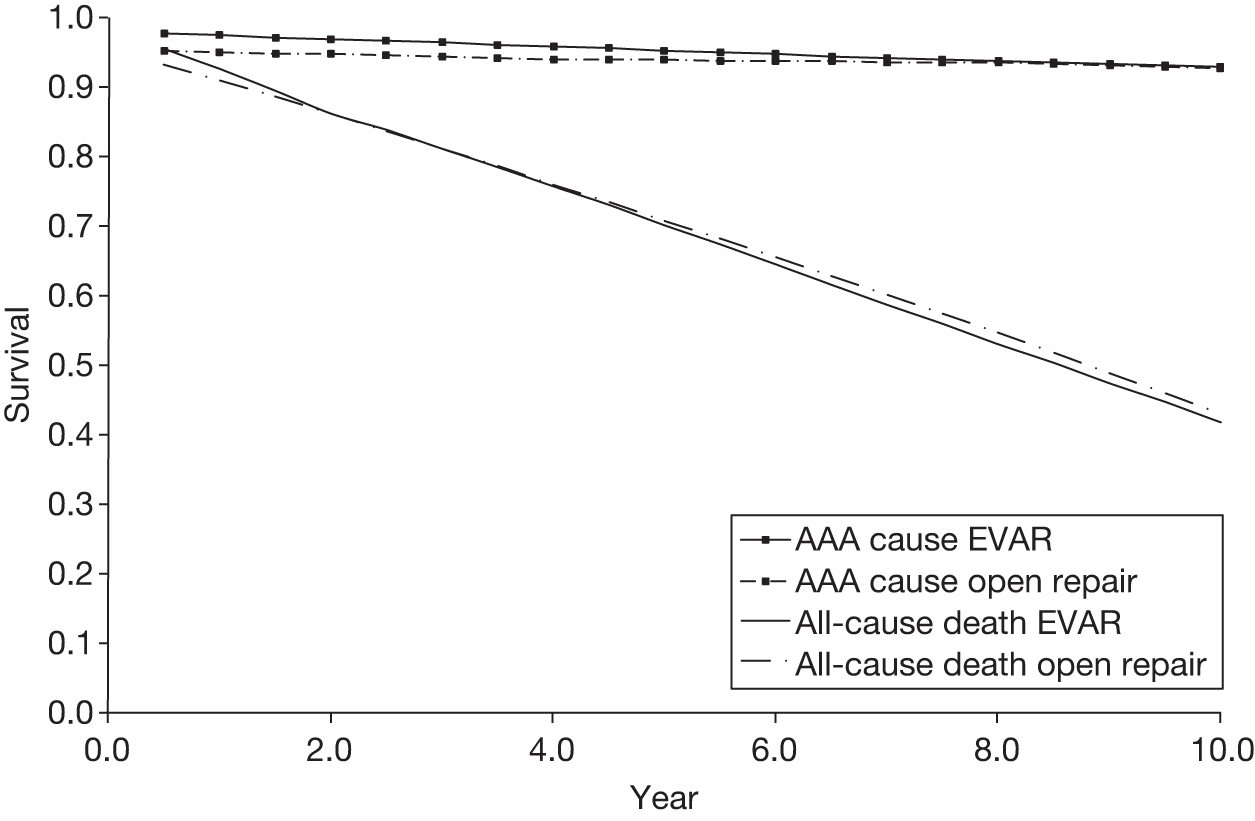
Base-case probabilistic analysis
Probabilistic sensitivity analysis using the parameters of the base-case model estimated the mean difference in costs to be £3519 (95% CI £1919 to £5053) and the mean difference in QALYs to be –0.032 (–0.117 to 0.096). The probability of EVAR being cost-effective was 0.01 at a threshold of £20,000 per QALY and 0.02 at £30,000 per QALY.
Sensitivity analyses
Comparison of the base-case model with the NICE final appraisal document (2009)
The first set of sensitivity analyses explores the parameters that contribute most to the difference in results between the NICE FAD208 and the base case. The NICE FAD found that the difference in costs between treatments was £534 and the difference in QALYs was 0.043, with an ICER of about £12,000 per QALY. Table 46 shows a series of univariate sensitivity analyses, varying the parameters of the model one at a time, while keeping the others at their base-case values. The mean values used in the base-case model and the sensitivity analysis are shown. The parameters that differ between the two models and which affect costs most are the initial procedure cost, the annual surveillance cost (£54 per year in NICE FAD vs £196 in base case) and the HR of reinterventions after 6 months [HR (EVAR vs open repair) 1.5 in NICE FAD vs 3.2 in base case].
| Parameter number | Sensitivity analysis | Base-case mean | NICE FAD mean | Diff. cost (£) | Diff. QALY | ICER |
|---|---|---|---|---|---|---|
| Base case | 3521 | –0.042 | Dom | |||
| 4 | Length of first cycle (months) | 6 months | 1 month | 3613 | –0.050 | Dom |
| 5 | Probability AAA mortality (EVAR) 0–6 months | 0.022 | 0.021 | 3526 | –0.046 | Dom |
| 7 and 8 | Rate AAA mortality/year (EVAR) | 6 months to 4 years: 0.006 | 0.0048 | 3498 | –0.015 | Dom |
| > 4 years: 0.008 | ||||||
| 9 | Relative risk AAA deaths (EVAR vs open repair) 0–6 months | 0.47 | 0.35 | 3502 | –0.015 | Dom |
| 10 and 11 | HR AAA deaths (EVAR vs open repair) | 6 months to 4 years: 1.46 | 1.5 | 3524 | –0.009 | Dom |
| > 4 years: 4.85 | ||||||
| 13 | Time to converge of all-cause survival (years) | 2 | 3 | 3528 | –0.031 | Dom |
| 15 | Procedure cost difference (£) | 1177 | 0 | 2344 | –0.042 | Dom |
| 16 | Surveillance cost (£)/year | 196 | 54 | 2647 | –0.042 | Dom |
| 20–22 | HR reinterventions (EVAR vs open repair) | 0–6 months: 1.65 | All times: 1.5 | 2831 | –0.041 | Dom |
| 6 months to 4 years: 9.97 | ||||||
| > 4 years: 3.24 | ||||||
| All above | Total NICE FAD | 534 | 0.043 | 12,305 |
The parameters that differ between the models and affect QALYs most are the OR of early AAA deaths (0.35 in NICE FAD in the 30 days after surgery vs 0.47 in the base case in the 6 months from randomisation), the HR of late AAA deaths after 4 years (1.5 in the NICE FAD vs 4.85 in the base case), the time at which the survival curves are assumed to converge (3 years in the NICE FAD vs 2 years in the base case) and the rate of late AAA deaths after EVAR (0.0048 per person-year in the NICE FAD vs 0.0060–0.0080 in the base case).
Figure 37 illustrates the importance of each of these parameters in a tornado plot. In order to compare the impact of each parameter on the model, incremental QALYs are multiplied by £20,000 (the conventional cost-effectiveness threshold209). The overall incremental net benefit (INB) of EVAR versus open repair estimated by the base-case model (given an incremental EVAR cost of £3521 and incremental QALY of –0.042) is then (–0.042 × £20,000) – £3521 = –£4361. A negative INB indicates that EVAR is not cost-effective given these parameters.
FIGURE 37.
Change in INB of EVAR less open repair (compared with base-case model) resulting from changing each parameter to correspond with the NICE FAD. 199 Positive (negative) values on the horizontal axis indicate that the change in the parameter from the base case to that in the sensitivity analysis has increased (decreased) the cost-effectiveness of EVAR compared with open repair, holding all other parameters at their base-case values.
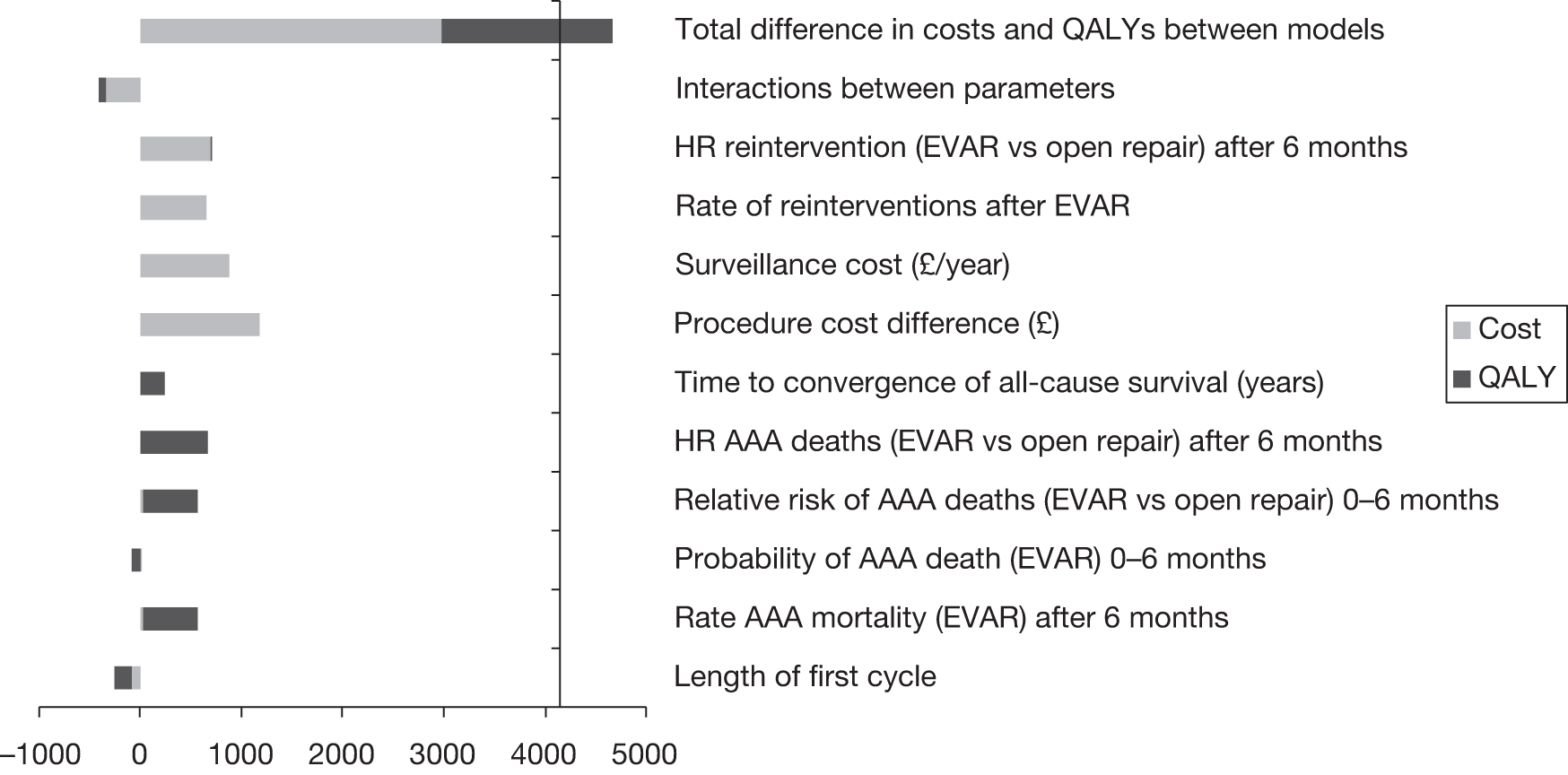
The overall INB estimated by the NICE model is (0.044 × £20,000) – £534 = £346, i.e. a positive net benefit in favour of EVAR. The overall difference in INB between the models is then valued at £346 – (–£4361) = £4707. Figure 37 shows the contribution of each parameter individually and independently to this difference in INBs between the models. Positive values on the horizontal axis indicate that the change in the parameter from the base case to that in the sensitivity analysis has increased the cost-effectiveness of EVAR. The vertical axis crosses the horizontal at £4361, indicating the change to the parameter(s) that is required for EVAR to be considered cost-effective at the threshold of £20,000 per QALY. The tornado plot shows that, starting from the base case, changing any one of the model parameters to correspond with the NICE FAD (and holding the others at their base-case values) would not change the conclusion that EVAR is not cost-effective. However, changing all the parameters simultaneously would lead to the conclusion that EVAR is cost-effective at a threshold of £20,000 per QALY.
Comparison of base-case model with the OVER trial
The OVER165 trial results differed from EVAR trial 1 and the DREAM trial in several outcomes. The results suggest a very low OR of 30-day operative mortality, that the survival curves did not converge (by 2 years) with no statistically significant difference in reinterventions. The relative risk of AAA mortality after 6 months is not calculable (as these are zero after open repair) but there is no reason to assume it is different from EVAR trial 1. The costs of the hospital procedure after EVAR may be lower in the OVER trial than in EVAR trial 1, but intracountry comparisons of in-hospital resource use are likely to be unreliable, and therefore the resource use estimates from the OVER trial are not used in the UK model. A sensitivity analysis is carried out with these assumptions, individually and together (Table 47). Overall, a model based on these assumptions together finds EVAR to be more effective than open repair (difference in QALYs 0.018 in favour of EVAR), but EVAR is not cost-effective in this scenario, as the ICER is £148,000 per QALY. Figure 38 shows the contribution of each individual parameter to this result, holding all other inputs at their base-case values. EVAR is estimated to be effective in this case because of the favourable OR and the assumption that the rates of other-cause mortality are equal. However, the overall expected difference in QALYs is quite modest because the initial advantage from operative mortality is gradually offset by late AAA deaths. This means the all-cause survival curves are predicted to cross at around 6 years.
| Parameter number | Sensitivity analysis | Base-case value | Sensitivity mean | Diff. cost (£) | Diff. QALY | ICER |
|---|---|---|---|---|---|---|
| Base case | 3521 | –0.042 | Dom | |||
| 5 and 9 | Probability AAA mortality (EVAR and open repair) 0–6 months | EVAR 0.022 | EVAR 0.0045 | 3576 | –0.053 | Dom |
| Open 0.047 | Open 0.023 | |||||
| 13 | Time to converge of all-cause survival (years) | 2 | 6 | 3575 | 0.061 | 59018 |
| 20–22 | HR reinterventions (EVAR vs open repair) | 0–6 months: 1.65 | All times: 1.0 | 2328 | –0.042 | Dom |
| 6 months to 4 years: 9.97 | ||||||
| > 4 years: 3.24 | ||||||
| 1 | Age (years) | 74 | 70 | 3689 | –0.056 | Dom |
| All above | Total OVER model | 2668 | 0.018 | 147,882 |
FIGURE 38.
Change in INB of EVAR less open repair (compared with base-case model) resulting from changing each parameter to correspond with the OVER trial. Positive (negative) values on the horizontal axis indicate that the change in the parameter from the base case to that in the sensitivity analysis has increased (decreased) the cost-effectiveness of EVAR compared with open repair, holding all other parameters at their base-case values.
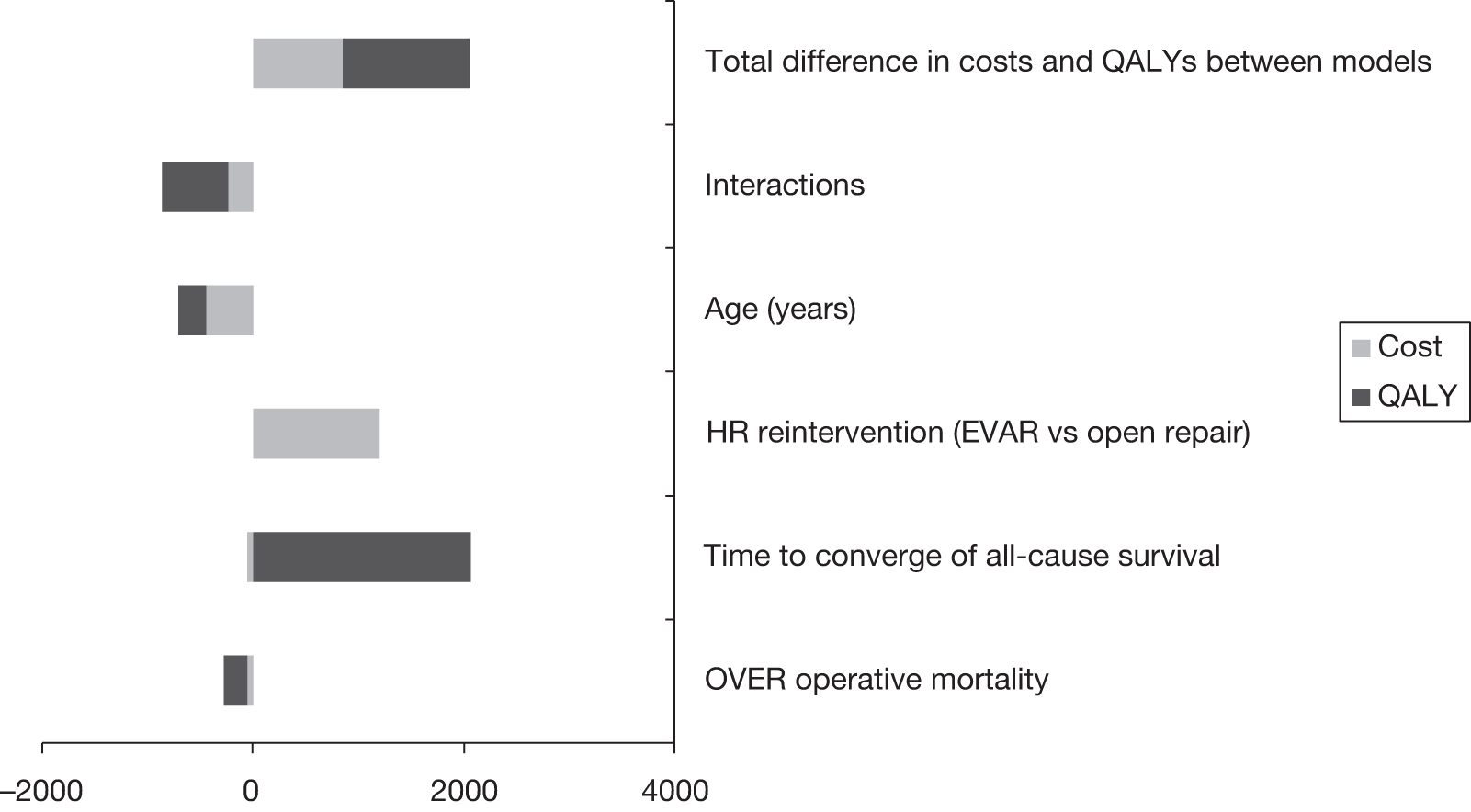
Comparison of base case with EVAR (2008) model
The EVAR (2008) model200 extrapolated the 4-year results of EVAR trial 1132 to predict outcomes over patients’ lifetimes. In that model, EVAR was associated with lower QALYs (–0.02) and higher lifetime costs (£3578) than open repair. Table 48 shows univariate sensitivity analyses changing the parameters of the base-case model one at a time to correspond with the value in the EVAR (2008) model. The EVAR (2008) model used some input values that were less favourable to EVAR than the base case. The base case assumes no difference in late AAA deaths for EVAR versus open repair after 8 years (i.e. the AAA-related survival curves meet but do not cross), whereas EVAR (2008) assumed a lifetime excess risk (and the AAA-related survival curves cross). The estimate of the difference in procedure cost between EVAR versus open repair was slightly lower in the 8-year data201 than in the 4-year data. 132 EVAR (2008)200 used other input values that were more favourable to EVAR than the base case. EVAR (2008)200 used a slightly lower estimate of the rate of late AAA deaths after EVAR. To estimate early deaths, EVAR (2008)200 used the 30-day OR of death after the primary operation of 0.30,132 whereas the base case uses the HR over the first 6 months of 0.47. 201 EVAR (2008)200 used a slightly higher estimate of the SMR after AAA repair than the base case. Other things being equal, as long as expected survival is > 2 years, EVAR would appear to be more cost-effective in patients with lower life expectancy, because there is less time for complications to develop and lower lifetime costs of surveillance.
| Parameter number | Sensitivity analysis | Base-case value | Sensitivity mean | Diff. cost (£) | Diff. QALY | ICER |
|---|---|---|---|---|---|---|
| Base case | 3521 | –0.042 | Dom | |||
| 5 and 9 | Probability AAA mortality 0–6 months | EVAR 0.022 | EVAR 0.016 | 3613 | –0.033 | Dom |
| Open 0.047 | Open 0.051 | |||||
| 7 and 8 | Rate AAA deaths per year (EVAR) | 6 months to 4 years: 0.006 | 0.0048 | 3554 | –0.014 | Dom |
| > 4 years: 0.008 | ||||||
| 13 | Time to converge of all-cause survival (years) | 2 | 3 | 3528 | –0.031 | Dom |
| 10 and 11 | HR AAA deaths (EVAR vs open repair) | 6 months to 4 years: 1.46 | 6.00 | 3528 | –0.075 | Dom |
| 4–8 years: 4.85 | ||||||
| 16 | Excess AAA mortality after EVAR | Up to 8 years | Up to 20 years | 3518 | –0.086 | Dom |
| 12 | SMR compared with general population | 1.1 | 1.4 | 3268 | –0.032 | Dom |
| 20–22 | HR reinterventions (EVAR vs open repair) | 0–6 months: 1.65 | All times: 6.75 | 3626 | –0.042 | Dom |
| 6 months to 4 years: 9.97 | ||||||
| > 4 years: 3.24 | ||||||
| 15 | Difference in procedure cost including conversion to open repair (£) | 1177 | 1613 | 4017 | –0.042 | Dom |
| All above | Total BJS 2008 model | 3578 | –0.020 | Dom |
Chapter 8 Costs and cost-effectiveness results for EVAR trial 2
Introduction
This chapter compares the cost-effectiveness of endovascular repair versus no surgery in patients clinically ineligible for open repair. These strategies have been previously compared in a modelling study. 205 The study found EVAR to be cost-effective with an ICER of < £10,000 per QALY. However, estimates of relative effectiveness were based on observational data because the study was undertaken before the results of EVAR trial 2 were known. The current study is a within-trial cost-effectiveness analysis based on IPD from EVAR trial 2.
Methods
Overview
Health outcomes are measured in QALYs, and the costs, from a NHS perspective, include those for the main procedure, graft-related reinterventions and surveillance after the procedure, at 2008–9 prices. Costs and outcomes are discounted at 3.5% per year. The primary analysis (base case) analyses patients according to their randomised treatment group (ITT). A secondary analysis is conducted on a per-protocol basis. The base case is based on a ‘within-trial’ analysis, which uses a time horizon of 8 years. As not all patients have died by this time, this limited time horizon may underestimate the difference in life expectancy between the treatments. Therefore, in another secondary analysis, a decision model was constructed to extrapolate estimates of the rate of mortality from the trial in order to estimate mean life expectancy and QALYs associated with the treatments.
Health outcomes
Health outcomes are measured in QALYs, which are calculated as the health state of each individual multiplied by the time spent in that state. The health state of each individual in the study was measured using the EQ-5D. 184 The five dimensions of the EQ-5D are mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Patients respond to each dimension in terms of whether they have no problems, some problems or severe problems. Therefore, in responding to the EQ-5D, patients can ‘locate’ themselves into one of 243 different health states (245, including dead and unconscious). These states have been valued on a scale from 1 (full health) to zero (equivalent to dead), although negative values exist for health states considered worse than death by a sample of the UK general population. 217
Costs
Hospital inpatient costs for aneurysm-related procedures were calculated for a period of 8 years from randomisation. Resource use collected in EVAR trial 2 included the endovascular device, theatre occupation time, blood products used, radiation exposure time, postoperative interventions, length of stay on wards, ITUs and HDUs for the primary aneurysm procedure, and inpatient graft-related reinterventions. Unit costs were obtained from routine national sources211–213 and from the results of questionnaires sent to trial centres in May 2004,132 updated for inflation214 (Table 49). Patients were expected to attend regular follow-up with CT in both arms of EVAR trial 2, before and after aneurysm repair. However, the study did not record CTs undertaken in other hospitals, and the surveillance protocol followed in the trial may not reflect clinical practice. This analysis does not include the costs of surveillance. This omission is unlikely to materially bias the analysis because surveillance would be required in both strategies.
| Description | Cost (£) | Source |
|---|---|---|
| EVAR stent | 5000 | EVAR trial survey 2003–4208 |
| Dacron graft, open surgery | 240 | EVAR trial survey 2003–4208 |
| Consumables, EVAR | 387 | EVAR trial survey 2003–4208 |
| Consumables, open surgery | 75 | EVAR trial survey 2003–4208 |
| General anaesthetic consumables, open repair | 115 | EVAR trial survey 2003–4208 |
| Blood/ml | 0.31 | NHS National Blood Service 2007–8213 |
| HDU/day | 797 | NHS Reference Costs (weighted mean), 2007–8211 |
| ITU/day | 1116 | NHS Reference Costs (weighted mean), 2007–8211 |
| Vascular surgery ward/day | 257 | NHS Reference Costs 2007–8211 |
| Operation rooma/minute | 17.58 | NHS Scotland Costs Book 2008–9212 |
| Radiology, 1–20 minutes | 47 | NHS Reference Costs 2007–8211 |
| Radiology, 21–40 minutes | 90 | NHS Reference Costs 2007–8211 |
| Radiology, > 40 minutes | 132 | NHS Reference Costs 2007–8211 |
| CT | 103 | NHS Reference Costs 2007–8211 |
Missing data and imputation
Follow-up interviews to collect EQ-5D, interventions and health-care resource use were scheduled at baseline, 3 months and 12 months, and yearly thereafter. Considerable effort was made to minimise missing data in the trial (see Chapter 3 for completeness of data). Nevertheless, only a minority of patients completed every question at every scheduled follow-up, and therefore the analysis must deal with the various types of missing data in the IPD.
Missing data in the trial were categorised into these types:
-
Data are right-censored (see Chapter 3, Secondary outcomes – adverse events, graft-related complications and reinterventions for the censoring criteria). Time in the study varies between patients due to different times of entry.
-
A patient is interviewed at baseline or a scheduled follow-up, but one item (dimension) of the EQ-5D questionnaire has not been completed.
-
Two or more dimensions of EQ-5D at baseline are missing.
-
Two or more dimensions of the EQ-5D questionnaire have not been completed for a scheduled follow-up.
-
Missing one or more scheduled follow-up interviews after the baseline interview and before the date of death or censoring.
-
One or more items of resource use for an AAA-related procedure are missing.
All imputation and analysis was carried out using Stata version 9.2.
Type 1 – administrative censoring
Administrative censoring arises because of differential times of entry into the trial. If these data can be considered ‘missing completely at random’ (MCAR) then a complete-case analysis would be unbiased, but there are very few patients with 8 years of follow-up and, therefore, this method would be very inefficient. 191 Inverse probability weighting is used to estimate costs and QALYs at 8 years, taking account of administrative censoring. 218 This method also assumes data are MCAR, but is a much more efficient estimator than complete case analysis. Censoring criteria were the same as those used for the analysis of graft-related complications and reinterventions (described in Chapter 3, Secondary outcomes – adverse events, graft-related complications and reinterventions). The follow-up time was split into 10 periods after randomisation: up to month 1, month 1 to month 3, month 3 to month 12, and seven yearly periods thereafter. Data for patients who are observed are weighted by the reciprocal of the probability of being censored during the period. Mean costs and QALYs for each period are estimated using linear regression, adjusting for baseline EQ-5D. The mean difference in total costs and QALYs between treatment groups is then the sum of the mean difference in costs and QALYs in each period. The uncertainty in the results was estimated from 1000 bootstrap replicates of the IPD, calculating the incremental mean costs and QALYs for each simulation. 219 The incremental mean costs and QALYs at 8 years are then estimated by the means of the 1000 bootstrap estimates, and CIs for the means are estimated by the 2.5th and 97.5th percentiles. The probability that EVAR is cost-effective is estimated over a range of values for the cost-effectiveness threshold from the empirical joint distribution of the incremental mean costs and QALYs. 220
Type 2 – missing one dimension of EQ-5D at baseline or follow-up
If one dimension of EQ-5D was missing, the missing variable was imputed using the univariate stochastic imputation (‘uvis’) procedure in Stata version 9.2. This assumes that these data are missing at random (MAR). 191 Each of the dimensions of the EQ-5D can take three values: no problems, some problems or major problems. An ordered probit regression model was used to predict the relation between this variable, the four observed dimensions of EQ-5D at that time point, the randomised treatment indicator and an indicator for the period of follow-up. An imputed value of the missing EQ-5D dimension was predicted from the posterior distribution of the regression coefficients, allowing for uncertainty in these coefficients, and conditional on the covariates. 195
Type 3 – missing two or more dimensions of EQ-5D at baseline
Baseline EQ-5D data are essential to the subsequent analysis, because this is one of the prediction variables for other missing data, and an adjustment variable in the analysis. Given that the dimensions of the EQ-5D are likely to be correlated, imputing more than one missing dimension at any time point for an individual would require multiple imputation. 195 In principle, one could simplify the problem of imputing baseline EQ-5D (and avoid multiple imputation) by imputing the EQ-5D index value at baseline, rather than the individual dimensions (as is done to impute type 4 missing data). However, because the participants in this case also had missing data at subsequent time points, this approach would still require multiple imputation. Multiple imputation is somewhat complicated in this analysis because of the use of inverse probability weighting to account for administrative censoring. Although in principle it might be feasible to combine these methods, this was considered to introduce unnecessary complexity. Instead, given the few individuals in this case, these participants were excluded from the cost-effectiveness analysis.
Type 4 – missing two or more dimensions of EQ-5D at a follow-up
If two or more dimensions of EQ-5D were missing at a follow-up interview, the index value of the EQ-5D was imputed for that time point for that individual using the ‘uvis’ procedure (Stata version 9.2). An ordinary least-squares regression model was used to estimate the relation between this variable, baseline EQ-5D index score and randomised treatment, and an imputed value of the missing EQ-5D index score was predicted from the posterior distribution of the regression coefficients, given the covariates.
Type 5 – missing one or more scheduled follow-up interviews after the baseline interview and before the date of death or censoring
The same method as for type 4 missing data was used to impute EQ-5D index in cases where a participant missed a scheduled follow-up before the date of death or date of censoring for that individual. For example, a participant may have attended a baseline interview, an interview at around month 3, and a final interview at around the end of year 3. In this case, EQ-5D are missing for scheduled follow-ups at the end of year 1 and the end of year 2, and these data were imputed using univariate stochastic imputation.
Type 6 – missing one or more items of resource use for an AAA-related surgical intervention
Resource use for the primary aneurysm procedure and inpatient graft-related reinterventions was recorded for each patient by a member of the surgical team or the trial co-ordinator in each centre. The small number of missing data were stochastically imputed using univariate stochastic imputation, regressing the observed values of the variable on treatment received using ordinary least squares to predict the missing values.
Estimation of difference in mean survival and lifetime quality-adjusted life-years
The ITT cost-effectiveness analysis estimates QALYs over 8 years; 17% of patients in the EVAR group and 18% in the control group were alive at this time. 221 Truncating the analysis at 8 years assumes no difference in the proportion alive at this time and thereafter.
Therefore, a secondary analysis was undertaken to estimate mean survival and lifetime QALYs in each group. Parametric survival analysis was carried out to estimate the survival function in each ITT group. A parsimonious approach to estimating proportional hazards models (exponential, Weibull or Gompertz) might begin by carrying out a test for proportional hazards. If this was not rejected at some significance level for type 1 error (usually 5%) then a single function would be fitted to the whole data set with a covariate (dummy) representing the treatment group. However, the aim of this analysis is not to estimate a parsimonious model for inference but, rather, to extrapolate beyond the observed data, i.e. to predict survival in each arm as accurately as possible in order to estimate the difference in mean survival and QALYs. The trial data indicate that the Kaplan–Meier survival curves cross at about 2 years. 201 Estimates of survival from a single parametric function that assumed a constant treatment effect would not predict that the survival curves cross. Therefore, separate functions were fitted to each treatment arm, regardless of the p-value of a test for proportional hazards. Age (centred at the mean age in the trial) and sex (male = 0, female = 1) were included as covariates. Various parametric functions were considered: exponential, Weibull, log-normal, log-logistic, Gompertz and gamma. The overall goodness-of-fit estimates with the data were compared using the Akaike information criterion (AIC). 222 However, a close fit with the observed data does not necessarily mean that the function is the most appropriate to predict the unobserved mortality. One way of informing the choice of distribution is to compare the performance of the models using long-term observational data with longer follow-up and larger sample size than the randomised trials. The EUROSTAR database is a register of time to death and other outcomes after endovascular repair. 171 In October 2007, 2391 patients who were assessed as unfit for open repair had contributed data, followed for up to 10 years (unpublished analysis undertaken by the authors using the IPD). Each of the survival distributions was fitted to the EUROSTAR data, with age and sex as covariates, and the AIC was calculated.
The survival distribution that was assessed as a good fit and most appropriate was used to predict mean survival and QALYs over the lifetime of a male patient with the mean age of participants in EVAR trial 2 (75 years). This was undertaken in a spreadsheet model. The probability of survival was predicted from the parametric function in intervals of 3 months. Uncertainty in these predictions was estimated from 1000 Monte Carlo simulations of the data, incorporating the correlation between the coefficients of the parametric model through the Cholesky decomposition of the estimated covariance matrix. 223 The predicted probabilities were discounted at 3.5% per year, and adjusted for quality of life using the mean EQ-5D in each group at each follow-up estimated from EVAR trial 2 IPD. Mean QALYs are then calculated as the area under the discounted quality-weighted survival curve. A similar approach was used to estimate the lifetime difference in mean survival and QALYs for the per-protocol analysis.
Results
Missing data and imputation
Type 1 – administrative censoring
Table 50 shows the number of follow-up interviews conducted per patient in EVAR trial 2. The number of follow-ups is not significantly different in the two arms. A slightly greater proportion of patients in the no-surgery group had two or three follow-ups than in the EVAR group, and a slightly greater proportion of patients in EVAR arm had 4–10 follow-ups. The method of inverse probability weighting218 used to account for this censoring assumes that data are MCAR, where patients who die are not considered to be ‘missing’. The assumption of MCAR appears justified given that the small differences observed between treatment arms could be due to chance and could, in part, be related to the differences in the proportion alive during the trial follow-up period.
| No. of follow-ups per participant | EVAR (%) | No surgery (%) | Total |
|---|---|---|---|
| 1 (baseline) | 197 (100) | 207 (100) | 404 |
| 2 | 162 (82) | 177 (86) | 339 |
| 3 | 143 (73) | 158 (76) | 301 |
| 4 | 118 (60) | 113 (55) | 231 |
| 5 | 83 (42) | 73 (35) | 156 |
| 6 | 55 (28) | 47 (23) | 102 |
| 7 | 36 (18) | 27 (13) | 63 |
| 8 | 24 (13) | 19 (9) | 43 |
| 9 | 16 (8) | 15 (7) | 31 |
| 10 | 9 (5) | 9 (4) | 18 |
| 11 | 4 (2) | 4 | |
| 12 | 4 (2) | 4 | |
| 13 | 1 (0.5) | 1 |
Types 2–4 – missing one or more dimensions of EQ-5D at baseline or follow-up
Table 51 shows the number of records with zero, one, two, three and four missing dimensions of the EQ-5D (out of a maximum of five) at baseline and follow-up interviews. These data were imputed using univariate stochastic methods. Two patients with insufficient baseline EQ-5D were excluded from the analysis.
| No. of missing dimensions of EQ-5D (maximum five) | EVAR | No surgery | Total | Type of missing data | Method of handling missing data |
|---|---|---|---|---|---|
| At baseline | |||||
| 0 | 195 | 206 | 401 | Not missing | |
| 1 | 1 | 0 | 1 | Type 2 | Univariate stochastic imputation of missing dimension |
| 2 | 1 | 1 | 2 | Type 3 | Patients excluded from analysis |
| 3 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | ||
| 197 | 207 | 404 | |||
| At follow-up | |||||
| 0 | 633 | 635 | 1268 | Not missing | |
| 1 | 9 | 10 | 19 | Type 2 | Univariate stochastic imputation of missing dimension |
| 2 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | ||
| 4 | 1 | 0 | 1 | Type 4 | Univariate stochastic imputation of missing EQ-5D index value |
| 643 | 645 | 1288 | |||
Type 5 – missing one or more scheduled follow-up interviews up to the date of death or censoring
Table 52 shows the proportion of patients who had ‘gaps’ in their scheduled follow-up, i.e. who missed one or more scheduled follow-up interviews up to their last date of analysis in the study. These missing EQ-5D index data were imputed using univariate stochastic imputation.
| No. of missing follow-ups | EVAR (n) | EVAR (n/N) (%) | No surgery (n) | No surgery (n/N) | Total (n) | (n/N) |
|---|---|---|---|---|---|---|
| 0 | 4 | 2.0 | 10 | 4.9 | 14 | 3.5 |
| 1 | 44 | 22.4 | 49 | 23.8 | 93 | 23.1 |
| 2 | 67 | 34.2 | 41 | 19.9 | 108 | 26.9 |
| 3 | 36 | 18.4 | 49 | 23.8 | 85 | 21.1 |
| 4 | 25 | 12.8 | 23 | 11.2 | 48 | 11.9 |
| 5 | 10 | 5.1 | 16 | 7.8 | 26 | 6.5 |
| 6 | 6 | 3.1 | 8 | 3.9 | 14 | 3.5 |
| 7 | 0 | 0 | 3 | 1.5 | 3 | 0.7 |
| 8 | 4 | 2.0 | 3 | 1.5 | 7 | 1.7 |
| 9 | 0 | 0 | 3 | 1.5 | 3 | 0.7 |
| 10 | 0 | 0 | 1 | 0.5 | 1 | 0.2 |
| 196 | 100 | 206 | 100 | 402 | 100 |
Differences in health-related quality of life between treatment groups
Figure 39 shows the mean difference in EQ-5D index score between the treatment groups, adjusting for baseline score. No imputation has been undertaken in this analysis. There were no clear and consistent differences in HRQoL at any time. 198 However, HRQoL measured by EQ-5D tended to be higher during the first 3 years after EVAR, and tended to be higher during the subsequent years after no surgery, although with increasingly wide CIs as the sample size diminishes.
FIGURE 39.
Mean difference (95% CI) in EQ-5D index score between EVAR and no surgery randomised groups at each follow-up, without imputation, after adjusting for baseline EQ-5D index score. Note: A negative difference is in favour of EVAR.
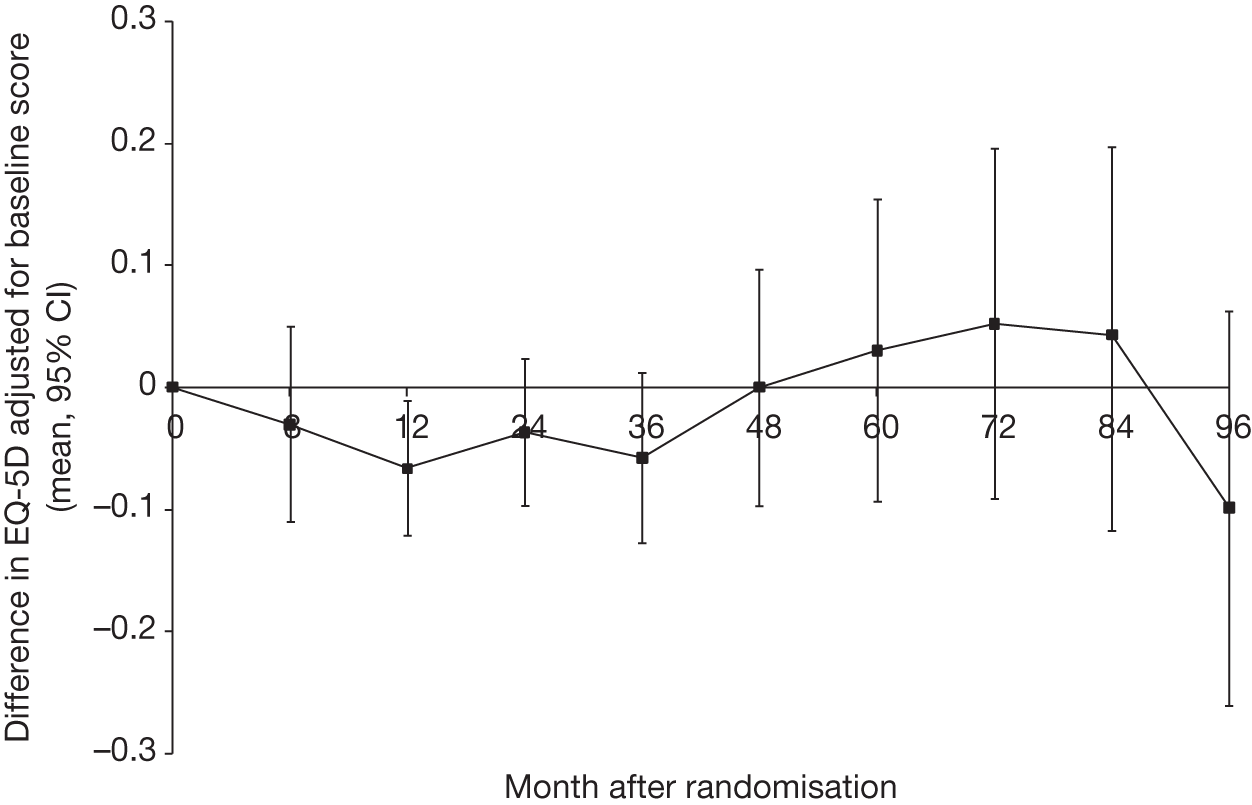
Results of the cost-effectiveness analysis: intention to treat
Table 53 shows the estimated costs and health outcomes over 8 years, based on ITT. The expected costs of the EVAR group are considerably greater than the no-surgery group (mean difference £10,596, 95% CI £8183 to £12,660). Figure 40 shows that about 90% of the total costs per patient were accrued in the first 2 years of the trial. As these costs include only AAA repair and graft-related reinterventions and surveillance, all of the costs in the control group arise from patients who ‘crossed over’ to have AAA surgery. Discounted life expectancy is on average lower in the EVAR group at 8 years. However, there is a 0.04 (95% CI –0.26 to 0.35) difference in favour of EVAR in quality-adjusted life expectancy. This is because of the trend towards greater HRQoL in the EVAR group, measured by EQ-5D. The ICER is £10,596/0.04 = £264,900 per QALY. The probability that EVAR is cost-effective (calculated by the bootstrap method) is zero at a threshold of £20,000 per QALY and 0.01 at a threshold of £30,000 per QALY.
| Outcome | EVAR (n = 197) | No surgery (n = 207) | Diff. | |||
|---|---|---|---|---|---|---|
| Mean | SE | Lower | Upper | |||
| Costs (£) | ||||||
| Main procedure | 14,561 | 4347 | 10,214 | 928 | 8420 | 12,037 |
| Readmissions | 1129 | 747 | 382 | 628 | –1033 | 1465 |
| Total | 15,690 | 5094 | 10,596 | 1135 | 8183 | 12,660 |
| Health outcomes | ||||||
| Life-years | 3.228 | 3.360 | –0.132 | 0.26 | –0.632 | 0.414 |
| QALYs | 1.846 | 1.809 | 0.037 | 0.155 | –0.261 | 0.350 |
FIGURE 40.
Cumulative costs (ITT analysis) over 8 years by randomised treatment group [mean (£) and 95% CI].
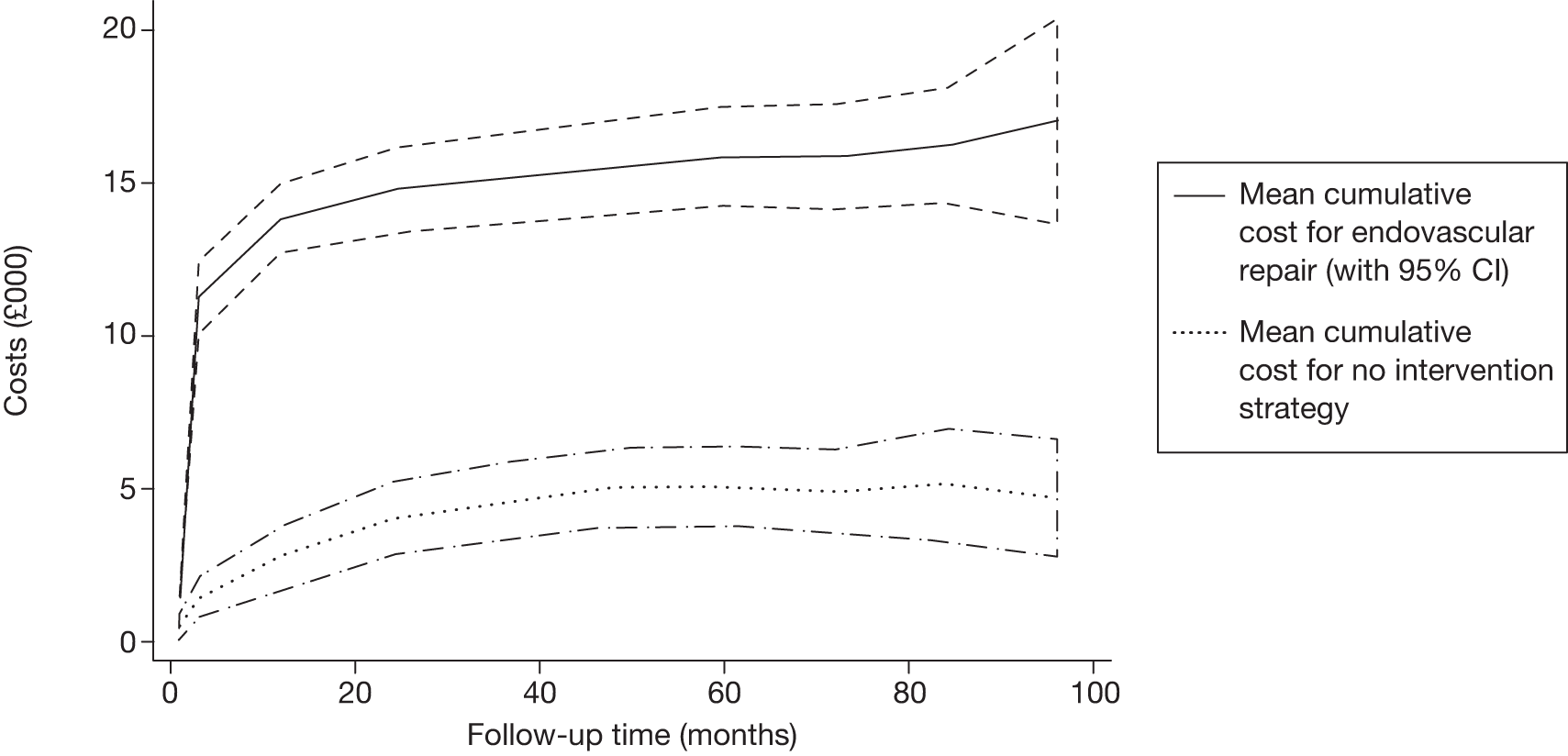
Results of the cost-effectiveness analysis – per protocol
Table 54 shows the estimated costs and health outcomes over 8 years in the per-protocol analysis. There are almost no costs in the ‘no-surgery’ group, which is expected, given that this analysis includes only these patients up to the time of any AAA repair. The mean difference in cost is £14,066 (95% CI £12,515 to £15,593). There is a much greater and significant difference in QALYs (mean difference 0.40, 95% CI 0.10 to 0.72) than in the ITT analysis. This is plausibly because the estimate of the difference in survival in the ITT analysis is ‘diluted’ by patients undergoing EVAR in the control arm. The ICER is £14,066/0.399 = £35,253 per QALY. The probability that EVAR is cost-effective is 0.03 at a threshold of £20,000 per QALY, 0.33 at a threshold of £30,000 per QALY and 0.61 at a threshold of £40,000 per QALY.
| Outcome | EVAR (n = 197) | No surgery (n = 207) | Diff. | |||
|---|---|---|---|---|---|---|
| Mean | SE | Lower | Upper | |||
| Costs (£) | ||||||
| Main procedure | 13,580 | 565 | 13,015 | 729 | 11,607 | 14,411 |
| Readmissions | 1083 | 32 | 1051 | 335 | 421 | 1772 |
| Total | 14,662 | 596 | 14,066 | 862 | 12,515 | 15,593 |
| Costs (£)>/Health outcomes | ||||||
| Life-years | 3.299 | 2.700 | 0.599 | 0.247 | 0.131 | 1.073 |
| QALYs | 1.892 | 1.493 | 0.399 | 0.163 | 0.097 | 0.715 |
Goodness-of-fit of parametric survival models
Table 55 shows the AIC scores for each of the survival distributions, for the ITT analysis and for the per-protocol analysis of EVAR trial 2. The survival curves were fitted independently to each arm of the trial. The Weibull distribution has the lowest AIC for the no-surgery arm and the exponential has the lowest AIC for the EVAR arm. However, assuming a constant rate of mortality over the lifetime of the whole cohort may lack face validity. Typically, one would expect higher rates of mortality in the early years after surgery as the frailer patients are likely to die first (although rates of mortality may increase again in the longer term, as the surviving patients age). The fit of the parametric survival models was also assessed using the EUROSTAR data. The gamma, followed by the Weibull, distribution showed the best fit to the EUROSTAR data. The exponential has a very poor fit with EUROSTAR. Therefore, the Weibull distribution was preferred for all analyses, as it shows a good fit to EVAR trial 2 data in both groups, a reasonable fit with the EUROSTAR data, and has better face validity in this patient population than the exponential. From the AIC values, the gamma model might be a satisfactory alternative to the Weibull and this was used in a sensitivity analysis.
| Distribution | ITT, EVAR trial 2 | Per protocol, EVAR trial 2 | EUROSTAR | ||
|---|---|---|---|---|---|
| EVAR | No surgery | EVAR | No surgery | EVAR | |
| Exponential | 616 | 604 | 612 | 464 | 3212 |
| Weibull | 618 | 602 | 614 | 456 | 3140 |
| Gompertz | 618 | 604 | 620 | 470 | 3210 |
| Log-normal | 626 | 616 | 614 | 562 | 3190 |
| Log-logistic | 622 | 606 | 616 | 498 | 3158 |
| Gamma | 620 | 604 | 616 | 456 | 3128 |
Table 56 shows the coefficients of the Weibull model in EVAR trial 2 data. The log-shape parameter is less than zero in the EVAR group, indicating declining rate of death over time, whereas the value is greater than zero in the no-surgery group, indicating increasing rate of death. In all cases, the shape parameter is non-significant at the 5% level. Nevertheless, it is included in the model because it improves the mean prediction of the probability of survival, compared with the exponential (constant rate) model.
| Analysis | EVAR | No surgery | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| ITT | ||||
| Covariate | ||||
| Age (centred on mean) | 0.023 | 0.013 | 0.032 | 0.012 |
| Sex (male = 0, female = 1) | 0.061 | 0.228 | 0.042 | 0.236 |
| Constant | –3.863 | 0.287 | –4.538 | 0.315 |
| Log shape parameter | –0.047 | 0.072 | 0.137 | 0.067 |
| Per-protocol analysis | ||||
| Covariate | ||||
| Age (centred on mean) | 0.0255 | 0.013 | 0.028 | 0.014 |
| Sex (male = 0, female = 1) | 0.048 | 0.228 | –0.063 | 0.249 |
| Constant | –3.802 | 0.285 | –4.751 | 0.346 |
| Log shape parameter | –0.065 | 0.072 | 0.238 | 0.071 |
Validation of the parametric model against the non-parametric analysis
Figure 41 compares the Kaplan–Meier estimates of the probability of survival with Weibull and gamma parameterisations, for each treatment group. The parametric curves appear to closely fit with the Kaplan–Meier estimates in the early years, but appear to estimate a greater difference in the probability of survival than the Kaplan–Meier estimates in the later years of the trial. This difference between the treatments is continued in the extrapolation beyond the trial data. The gamma model gave almost identical estimates as the Weibull model for the survival function and the difference in mean QALYs.
FIGURE 41.
Comparison of Kaplan–Meier, gamma and Weibull survival curves, for ITT analysis.
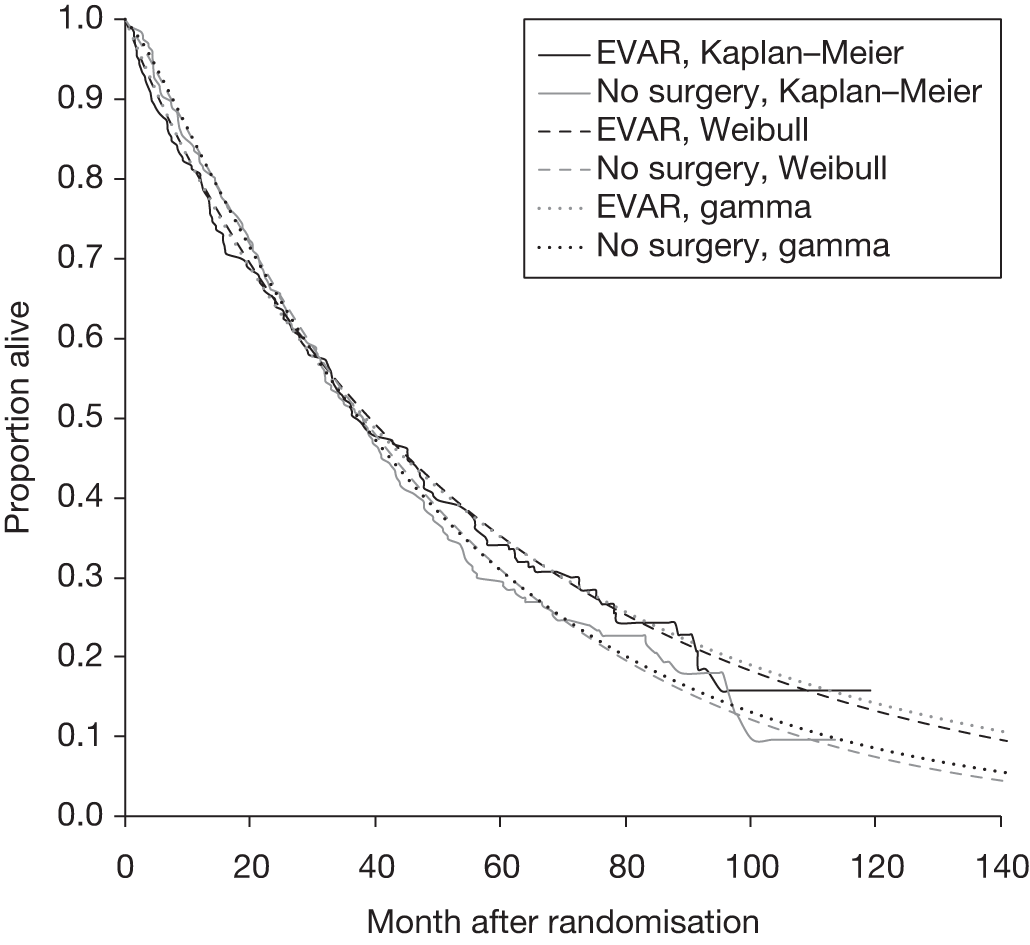
Table 57 shows the predictions of the Weibull parametric model, in terms of mean survival and QALYs for 8 years, which can be compared with Tables 53 and 54. The ITT parametric model appears to overestimate the difference in life-years and QALYs at 8 years. However, both the parametric and the non-parametric analyses estimate wide CIs that cross zero. These results do not necessarily show that the parametric model should be rejected but highlight that the parametric model is an imperfect fit with the observed data and that the model may be overestimating the difference in survival. The per-protocol parametric model gives much closer estimates to the non-parametric model of the difference in life-years (mean difference: 0.62 parametric model vs 0.60 in non-parametric model) and QALYs (mean difference: 0.45 parametric vs 0.40 non-parametric). In both models these estimates are statistically significant.
| Outcome | ITT | Per protocol | ||||||
|---|---|---|---|---|---|---|---|---|
| EVAR | No surgery | Diff. | 95% CI | EVAR | No surgery | Diff. | 95% CI | |
| Health outcomes at 8 years | ||||||||
| Proportion alive | 0.19 | 0.13 | 0.06 | –0.02 to 0.14 | 0.20 | 0.06 | 0.14 | 0.07 to 0.22 |
| Life-years | 3.64 | 3.52 | 0.12 | –0.38 to 0.61 | 3.65 | 3.04 | 0.61 | 0.11 to 1.13 |
| QALYs | 2.24 | 2.09 | 0.15 | –0.15 to 0.44 | 2.25 | 1.80 | 0.45 | 0.14 to 0.76 |
| Health outcomes over lifetime | ||||||||
| Life-years | 4.26 | 3.81 | 0.45 | –0.38 to 1.27 | 4.31 | 3.12 | 1.19 | 0.44 to 2.02 |
| QALYs | 2.62 | 2.27 | 0.35 | –0.15 to 0.84 | 2.64 | 1.85 | 0.79 | 0.34 to 1.29 |
Estimates of mean survival, lifetime quality-adjusted life-years and cost-effectiveness from the parametric model
The mean lifetime difference in QALYs was estimated to be 0.35 (95% CI –0.15 to 0.84) in the ITT analysis and 0.79 (95% CI 0.34 to 1.29) in the per-protocol analysis (see Table 57). Given that most of the costs are incurred in the first 2 years (see Figure 40), no further costs were assumed in the model after 8 years in addition to the estimates in Tables 53 and 54 (mean difference £10,596 in the ITT analysis and £14,066 in the per-protocol analysis). The estimated incremental cost per QALY for EVAR versus no surgery using the parametric model is £10,596/0.35 = £30,274 in the ITT analysis. The estimated probability that EVAR is cost-effective is 0.23 at a threshold of £20,000 per QALY and 0.49 at £30,000 per QALY. In the per-protocol analysis using the parametric model the ICER is £14,066/0.79 = £17,805 per QALY, and the probability that EVAR is cost-effective is 0.61 at £20,000 per QALY and 0.91 at £30,000 per QALY.
Chapter 9 Discussion
Discussion of EVAR trial 1
Mortality
After up to 10 years of patient follow-up, the principal benefit of EVAR versus open repair was the threefold reduction in operative mortality. Within 2 years this survival benefit had been eroded with respect to all-cause mortality and within 6 years the aneurysm-related mortality benefit had been eroded: more detailed investigations have revealed some of the reasons for these observations. The EVAR trials were designed in 1996, and recruited between 1999 and 2004; they provide the most comprehensive long-term follow-up of patients receiving EVAR compared with open repair. Thus, they supply a crucial complement to other registry data, such as EUROSTAR, which also provide some long-term follow-up results, although these are based on incomplete reporting of late events. Although the mean age at patient entry in EVAR trial 1 was 74 years, after 8 years 54% of patients remained alive, which stresses the need for durable aneurysm repair and long-term follow-up. Newer devices are now available and it is hoped that these will perform better.
The final mortality results presented here, and described in our final mortality publication,201 demonstrate that endovascular repair of AAA in patients considered to be fit enough for open repair, and with large aneurysms deemed to be anatomically suitable for EVAR, is associated with a threefold reduction in operative mortality and an improved aneurysm-related survival during the early years. However, this early benefit is lost in the longer term, with aneurysm-related mortality beyond 4 years being substantially higher after endovascular repair than after open repair. No significant all-cause mortality differences were demonstrated between the two groups as early separation of the curves, driven by the lower operative mortality in the endovascular group, was not sustained, with convergence of the curves at 2 years (see Figure 14). These results are broadly similar to those published by the other randomised trials comparing EVAR with open repair in fit patients but there are some slight differences. The DREAM trial has published long-term findings recently, which are very similar to those presented in this report with a 4.6% operative mortality in the open-repair group versus 1.2% in the endovascular group – risk ratio 0.26 (95% CI 0.03 to 1.11). 158 The trial also demonstrated lower aneurysm-related and all-cause mortalities in the EVAR group during the early years but with no difference in the longer term. 159,160 The American OVER trial demonstrated lower operative mortalities in both groups (0.2% and 2.3% for the EVAR and open repair groups, respectively) and an even greater reduction in mortality in the EVAR group (OR 0.10; 95% CI 0.01 to 0.76). 165 The French ACE trial also showed slightly lower operative mortalities but no strong evidence of a statistically significant reduction in operative mortality with EVAR; however, power is limited in this trial, which was forced to close early because of poor recruitment. 163 Publication of the 2-year ACE trial results are currently in press during the writing of this report so further comment is not possible. In addition to the randomised trials, the US Medicare registry has been used to compare EVAR with open repair and demonstrated a similar reduction in operative mortality with EVAR as well as a mortality ‘catch-up’. 115
The slight dissimilarities seen between the results for EVAR trial 1 and the other studies may just be due to chance but could also be explained by the varying inclusion criteria and subsequent baseline characteristics between them as well as different eras of device. It is possible that particular subgroups of patients do better with EVAR than others, but all the tests of interactions performed in these trials [with age, sex, AAA diameter and a fitness score (CPI) in EVAR trial 1] were non-significant (see Table 5), as was also the case in the OVER trial subgroup results. 165 However, results by some subgroups in EVAR trial 1 merit further discussion. Table 5 demonstrates that the fitness of the patient may be an important factor in terms of operative mortality, with the fitter patients benefiting more from EVAR over open repair than those of worse fitness (adjusted ORs of 0.22 versus 0.81, respectively, with the test of interaction being of borderline significance; p = 0.088). A similar finding is seen for age although the p-value for the test of interaction is less significant (p = 0.222). These observations are echoed in the results for aneurysm-related mortality in Table 5 and described more fully in our publication of the impact of fitness on survival in EVAR trial 1. 224 Furthermore, these findings are in accord with the results of the OVER trial, which recruited younger, fitter patients and demonstrated an even greater benefit of EVAR over open repair than that seen in EVAR trial 1. 165 Patients in the DREAM were similar to those in EVAR trial 1, although they were slightly younger (mean age 70 years) and had smaller aneurysms (mean diameter 6.0 cm) but their results for operative, aneurysm-related and all-cause mortality were very similar.
Very few of the EVAR trial 1 patients either violated treatment protocol or were lost to follow-up, with few missing data. Per-protocol analysis yielded very similar results to the ITT analysis, as did sensitivity analyses that included patients with missing baseline covariate data. Table 6 shows the causes of death in EVAR trial 1. After the postoperative period, just under half of all deaths were attributed to cardiovascular disease (including aneurysm), a slightly lower proportion than that reported for the 4-year results,132 which may reflect improving medical therapy. 225 Just over one-quarter of deaths were attributed to cancer. After the postoperative period, there were 20 and six aneurysm-related deaths in the endovascular and open-repair groups, respectively; two of the late deaths in the open-repair group were from graft rupture in patients who had violated protocol and undergone endovascular repair. In total, 25 secondary aneurysm ruptures were reported in EVAR trial 1, and 18 (72%) of these ruptures were fatal.
Subsequent analyses of EVAR trial 1 data have demonstrated two main additional mortality findings. First, endograft rupture would appear to explain the convergence in the aneurysm-related mortality curves at 6 years (see Chapter 6, Factors associated with endograft ruptures). Second, cardiovascular mortality does appear to contribute to the catch-up in all-cause mortality seen during the first 2 years (see Chapter 4, Cardiovascular mortality and events). Both of these findings cast doubt on the later efficacy of EVAR but improvements in the use of medical therapy and the development of endograft design may lead to better outcomes for patients who are treated with an endovascular approach in future. These two issues will now be discussed separately.
Patients with aortic aneurysm are known to be at greater risk of mortality than the age- and sex-matched population. 226 Much of this increase is thought to be from cardiovascular disease and it has been shown to persist beyond repair of the aneurysm. 148 The convergence of the all-cause mortality curves during the early years after aneurysm repair has been demonstrated in other studies: after 1 year in the DREAM in the Netherlands160 and after 3 years in the Medicare registry in the USA,115 although not after 2 years of follow-up in the American OVER trial. 165 One hypothesis to explain this convergence is that patients with significant cardiac or carotid artery disease who survived the initial EVAR procedure subsequently died of this cardiovascular disease during the early postoperative years. 159 In the equivalent group in the open repair arm of the trial, more died during the early postoperative period as a result of the greater stress response to major open surgery. In Chapter 4, Cardiovascular mortality and events, we tested this hypothesis by investigating the impact of different interventions on cardiovascular event and death rates and showed that there is no strong evidence to suggest any differences between EVAR and open repair, except during the first 6 months, when both cardiovascular events and deaths were lower in the EVAR group. This accords with other research demonstrating reduced cardiac stress during the early postoperative phase after EVAR compared with open repair. 227 Beyond 6 months, although the EVAR group continued to experience a lower rate of cardiovascular events than the open-repair group, they appeared to have a higher rate of cardiovascular death, particularly during the 6- to 24-month period. This is counterintuitive but may be explained by the fact that we include only data on a subset of cardiovascular events (MI and stroke), while all cardiovascular deaths are reported. Therefore, there is some evidence that cardiovascular deaths are partially responsible for some of the convergence in all-cause mortality between the EVAR and open-repair groups during the first 2 years. However, it must be stressed that the HRs beyond 6 months are not statistically significant and other factors may be contributory. Nevertheless, it is possible that subgroups of patients with varying degrees of cardiovascular disease may benefit more from EVAR than from open repair, particularly if cardioprotective medication is managed more rigorously both before and after aneurysm repair. Use of cardioprotective medication in the EVAR trials was suboptimal; at baseline only 53% and 36% of patients were taking aspirin and statins, respectively, without differences between the EVAR and open-repair groups. Improved medical therapy of patients with aneurysm could reduce overall cardiovascular event rates in the future228 and may limit the extent of mortality catch-up between the EVAR and open-repair groups. In addition, these results suggest that careful preoperative cardiac investigations and treatments should be standard before aneurysm repair, regardless of whether open repair or the less invasive EVAR is being considered. The importance of preoperative optimisation is now being stressed in the European Society of Vascular Surgery guidelines for preoperative care. 215
The second convergence in survival curves presented in Figure 14 occurred at about 6 years for aneurysm-related mortality. By the time follow-up closed at the end of December 2009, a total of 27 endograft ruptures had been reported across both trials (25 in EVAR trial 1) with a high mortality of 67% within 30 days of rupture. This alarming occurrence, which was not reported for any of the patients undergoing open repair, prompted an audit of these ruptures as described in Chapter 6, Factors associated with endograft ruptures and more fully described in our recent publication. 229 Five of the ruptures occurred during the early postoperative period and could be classified as related to technical problems. Thus, it would seem prudent for a pre-discharge CT scan to be undertaken always in order to identify any early problems. In the trial era this was not always done and reliance was erroneously placed on the flush angiogram at completion on the operating table. In retrospect, this was unwise. The audit also demonstrated the importance of patients adhering to their surveillance protocol as two patients died of rupture after refusing to attend follow-up at which potentially correctable complications might have been detected. For 17 of the ruptures, a complication had been detected previously and, for 15 of these, the complication was accompanied by sac growth. Analysis of pre-selected baseline factors provided strong evidence that detection of any of these complications (endoleaks type 1, type 3 or type 2 with sac growth, migration or kinking) was strongly associated with endograft rupture (adjusted HR 8.83; 95% CI 3.76 to 20.76; p < 0.0001). Therefore, further work is required to determine what the most optimal reintervention protocol may be for patients with these complications. It is also perhaps a wake-up call to intervene if possible with sealing stents and, if not, to lower the threshold for consideration of conversion to open repair, particularly if the complication is not resolved and the patient is fit enough for this step. However, the risks of such an approach will need to be evaluated prospectively as there is a high mortality risk of rupture if nothing is done and an uncertain mortality risk by converting to open repair. The most worrying group of patients (only three) were those whose graft ruptured despite them adhering to their surveillance protocol and without detection of any complications on their CT scans. Fortunately, this is a rare occurrence.
It is hoped that these long-term results will inform an update to the current UK NICE guidelines on the use of EVAR. 199 In addition, the results of these trials may have repercussions for the UK national screening programme for AAA, which is currently in its pilot phase but is expected to be rolled out nationally over the next 5 years. This programme was instigated on the basis that randomised trials have shown that screening men in the age range of 65–74 years is associated with a significant reduction in aneurysm-related mortality, which is highly cost-effective. 14,51–54 The trials were based upon intervention using predominantly open repair when the aneurysm reached 5.5 cm and the cost-effectiveness of screening should perhaps be re-evaluated under the alternative scenario that EVAR is used for intervention. The operative mortality is lower but the cost is higher and this may have implications for the future cost of the screening programme.
Graft-related complications and reinterventions
The most notable disadvantage associated with EVAR over open repair is the high rate of graft-related complications and reinterventions that continue to be reported up to 8 years after the procedure. Secondary rupture after aneurysm repair was reported only after endovascular repair and appeared to explain the increase in aneurysm-related mortality in the longer term. In contrast, open repair was very durable, but was associated with a higher operative mortality. Table 8 and Figures 15 and 17 show that the rate of complications after EVAR was greatest during the first 6 months and that the crude rates within subsequent time periods appear to have reduced. However, it is alarming that cumulatively, over 8 years, over 50% of all patients experienced some kind of graft-related complication, with 28% requiring some kind of reintervention, although fortunately the latter appeared to be associated with a very low operative mortality. Nevertheless, this unrelenting occurrence of graft-related complications and reinterventions after EVAR emphasises the need for continuing surveillance, and these clinical episodes contribute to an increasing lifetime cost of aneurysm-related events after endovascular repair. A streamlined postrepair surveillance algorithm to minimise patient radiation exposure but not limit the future detection and management of potentially dangerous complications associated with graft failure may enhance cost-effectiveness. Therefore, in order for EVAR to compete with open repair in terms of cost-effectiveness, these rates of graft-related events must be reduced and it is hoped that future graft design development will achieve this. Currently, patient preference is strongly in favour of endovascular repair. 230,231 However, these preferences were declared on the basis of early and mid-term evidence alone. Although there is still early mortality benefit with endovascular repair and it is a less invasive procedure than open repair, it is difficult to predict what effect these recent, long-term findings will have on patient preference. Ultimately, these long-term results have implications for the selection of patients for endovascular repair, patient choice, postrepair surveillance and cost-effectiveness. The results also confirm that careful long-term follow-up of surgical innovations is essential, as discussed in the Idea-Development-Exploration-Assessment-Long-term (IDEAL) study statement. 232
Comparing the rates we present in this report with others in the literature is difficult as many other studies present percentage risks233–236 rather than rates. The study most contemporaneous with the EVAR trials is based on EUROSTAR registry data on 2846 patients undergoing EVAR between 1999 and 2004 and reported Kaplan–Meier estimates for reintervention at 1, 2, 3 and 4 years of 6%, 9%, 12% and 14%, respectively;237 these are rather low compared with the rates of 12%, 14%, 17% and 21% that we present for EVAR trial 1. More recently, Schermerhorn et al. 115 found even lower rates after EVAR at these time points: 2.7%, 4.8%, 7.0% and 9.0%, respectively. The DREAM report a 6-year reintervention estimate of 30%,160 which is slightly higher than the 24% seen at 6 years in EVAR trial 1. The 2-year results of the OVER trial did not demonstrate a higher rate of reinterventions after EVAR but the authors chose to pool all reinterventions (not just graft-related ones) and thus a higher number of reinterventions were apparent in the open-repair group. 165 The largest series of data comes from a meta-analysis of 28,862 patients, which quotes an absolute risk of rupture, endoleak or conversion to open repair of approximately 30%; however, average length of follow-up is not reported, making it difficult to compare this figure with our results. 131 This meta-analysis also provided some evidence to suggest that complication rates declined between 1994 and 2002, but this was not shown to be the case in the EVAR trials. Other work comparing uni-iliac and bifurcated grafts has found a non-significantly higher rate of complications in the uni-iliac group,233 and this is confirmed in our analysis in Chapter 6, Factors associated with development of serious graft-related complications and reinterventions.
Types of complications and factors associated with increased rates of graft-related events
Table 7 presents the types of complications that were reported during the course of follow-up after EVAR and open repair separately. A large proportion (32%) of the first complications seen after EVAR were type 2 leaks without any notable sac expansion, and current practice tends to be just monitoring these leaks rather than intervening. Type 2 leaks tend to be regarded as more serious if they are accompanied by sac expansion, and this occurred in only 6% of first complications reported after EVAR. In Chapter 6, Factors associated with development of serious graft-related complications and reintervention, a more detailed analysis was undertaken to investigate which baseline factors might predispose certain patients to an increased rate of serious complications and reinterventions. 238 This analysis was restricted to serious complications (excluding type 2 leaks), which were defined according to whether they may relate to subsequent graft rupture or to the need to convert to open repair. Some might argue that the decision to exclude type 2 endoleaks is controversial as the true fate of untreated type 2 leaks is still unknown. However, a sensitivity analysis that also included all cases of sac growth (which included the more serious cases of type 2 leaks with sac growth) did not alter the findings markedly. Overall, the results clearly showed that both older age and larger aneurysm diameters increased the rates of serious complication and reintervention. Therefore, a subgroup of younger patients with AAA diameters between about 5.5 and 6.0 cm may experience particularly low graft-related event rates (shown in Figure 33). This potentially bodes well for patients in countries, such as the UK, where national aneurysm screening programmes are being implemented and where patients are referred promptly for consideration of repair when the aneurysm reaches a threshold diameter, commonly 5.5 cm. There was some evidence to suggest that patients with larger common iliac diameters had higher rates of complications, but this did not appear to influence the rate of reinterventions (p = 0.334 in adjusted model). Complications in the iliac segments are possibly regarded as less serious than those in the proximal neck region and are sometimes more difficult to treat given the tortuosity and smaller vessel size below the aortic bifurcation. There was weaker evidence of an association between complications and larger top neck diameters, whereas neck length and conicality were not apparently influential. Interestingly, there was also a hint that women do worse after EVAR than men, with graft-related event rates being approximately 50% higher in women, but this was not statistically significant. Similar findings have been published recently in a large cohort of 3662 patients (18% female) in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set236 but these findings are in conflict with data from the Australian Safety and Efficacy Register of New Interventional Procedures (A-SERNIP) EVAR registry, which show females appearing to do better than males for a number of outcomes. 239
The analysis presented in Chapter 6 generated some important incidental observations to suggest that, within the trials, both the type of endograft and the centre for aneurysm repair may influence results. The variation across these 37 hospitals in both complication and reintervention rates may reflect the wide range of experience and skills, varied intervention and patient selection policies, different device choices for a given anatomy, or variation in the quality of care delivered in different centres. In terms of graft manufacturer, there was some evidence to suggest that patients with a Gore Excluder graft experienced significantly lower rates of complications and reinterventions than those receiving the other graft types (see Table 32). However, the number of Gore grafts used was small and confined to a subgroup of 11 centres (although results have been adjusted for centre). An earlier comparison of mortality and reintervention rates between the two main graft types used in the EVAR trials (Zenith and Talent) did not demonstrate any convincing differences. 240 It is possible that the inclusion of both the Excluder device (which appears to perform well) and the earlier grafts (including some now withdrawn because of poor performance and used in only a few centres) has revealed graft-specific and centre effects, both of which merit further investigation in future research. There was no evidence to suggest any change in rates of events with chronological time since the start of the trial despite all of the three main graft brands modifying their grafts with iterative improvements. Although the EVAR trials commenced in 1999, these results should still be relevant to current practice, particularly as 90% of the grafts used were second- and third-generation devices and very few of the other grafts used in the trials were models that have been removed from the UK market because of potential safety issues (AneuRx = 28, Vanguard = 0).
Any interpretation of the results presented in Chapter 6 needs to account for the multiple testing of 16 variables on two outcomes, and it is only aneurysm diameter that stands out as the most convincing finding. However, it is important to stress that these results cannot be extrapolated to justifying EVAR in patients with aortas that are < 5.5 cm, for which regular surveillance is known to be the optimum management policy, as AAA rupture rates are very low below this threshold. 96,148,155,156 Furthermore, all of the results presented in Chapter 6 are generalisable only to patients who have already been deemed anatomically suitable for EVAR, as this was an entry criterion for the trials. If the anatomical selection criteria for EVAR are relaxed then the complication and reintervention rates may be even higher and appropriate selection of anatomically suitable cases remains paramount.
Health-related quality of life
Full quality of life assessment according to the EQ–5D and SF-36 questionnaires did not provide any strong evidence of a difference in quality of life between the EVAR and open-repair groups, apart from an anticipated detriment in terms of pain and physical functioning during the first few months after open repair (see Tables 10 and 11). At baseline, the EQ-5D scores were similar in both groups and similar to the age- and sex-matched population norms. 217 Secondary analyses based upon time from surgery rather than randomisation did not alter the findings. Unfortunately, resources for collection of full SF-36 data were available only during the first year, so it is not possible to determine the impact of each treatment policy on long-term quality of life is not possible. The DREAM has reported data on the these instruments for up to 1 year, with very similar findings, although its data suggested a significant improvement in the EQ-5D score after 6 months in the open-repair group. 162 The OVER trial has assessed quality of life for longer, but results up to 2 years did not suggest any differences between the groups at any time since randomisation. 165 In addition, both these trials compared changes in erectile dysfunction between the groups with neither demonstrating any significant differences between EVAR and open repair. 161,165
Renal function
In Chapter 4, Renal function and Chapter 5, Renal function, we have addressed the role of renal function, which is an important, but sometimes overlooked, aspect of patient fitness. The striking findings for both EVAR trials 1 and 2 were the stability of renal function in patients with aortic aneurysm who survive for longer than 12 months after presentation, the minimal effect of treatment modality on renal function and the more rapid deterioration of renal function before a complication is diagnosed (for patients treated with EVAR). The observed overall mean rates of decline in renal function in all patients in both arms of each trial ranged between –0.76 and –1.13 ml/minute/1.73 m2 per year, and these are similar to those found in a 10-year study of patients (median age 75 years) with stage 3 chronic kidney disease in Tromsø, Norway (–1.03 ml/minute/1.73 m2 per year). 241 For nephrologists, concern is aroused if a patient’s annual rate of deterioration is more than about 5 ml/minute/1.73 m2 per year or if a 15% per year fall in eGFR is demonstrated. 242 The stable renal function seen in both trials is perhaps surprising. Given that patients with AAA often have a history of smoking and usually present with various other comorbidities, such as hypertension cardiovascular disease and other smoking-related illnesses, a more rapid deterioration in eGFR might have been expected. Yet the EVAR trial patients appear to be little different from the population without AAA. If anything, the extent of renal impairment seen in this study compares favourably with a recent study of over 13,000 elderly patients in the UK, of whom 56% demonstrated eGFR measurements of < 60 ml/minute/1.73 m2 (KDOQI stage 3 or less). 243 Other smaller studies comparing renal function after EVAR or open repair have also shown a deterioration in renal function, with one study demonstrating similar decline between types of repair244 and two others showing greater deterioration after EVAR. 245,246 Several studies have compared suprarenal versus infrarenal fixation during EVAR, and these support a deterioration in renal function after EVAR247–253 but demonstrate little difference between suprarenal or infrarenal fixation. A meta-analysis of these studies provided conflicting results according to the analytical method used and concluded that the data were insufficient to draw any strong conclusions on the impact of suprarenal fixation. 254 None of the studies assessing renal function after AAA repair has used multilevel modelling to calculate annual rates of change in eGFR, and most have used creatinine or creatinine clearance rather than eGFR so it is difficult to compare results directly with those presented in this report. However, all studies have concluded that the reasons for renal function deterioration are likely to be multifactorial. Overall, it is encouraging that the use of EVAR does not appear to have a more deleterious impact on renal function than open repair even although the presence of graft-related complications (which are common after EVAR) did appear to have a strong influence on the rate of renal function decline. This impact of complications may have been ameliorated as faster decline is only apparent prior to detection of the complication, with much slower rates after this time. However, it must be stressed that these conclusions can only be made for patients who survive beyond 1 year as creatinine measurements were only collected annually and therefore survival to 1 year became as indirect inclusion criterion for the analysis. Nevertheless, the number of deaths as a result of renal failure was very low (see Tables 6 and 20) and these analyses aimed to focus on the long-term consequences of different aneurysm management policies on renal function, relevant only to those who survived beyond 1 year.
Costs and cost-effectiveness
Chapter 7 estimated the cost-effectiveness of endovascular repair versus open repair for AAA using a decision model. The base-case model found that the difference in lifetime costs was £3519 (95% CI £1919 to £5053) higher with EVAR and there was only a very small difference in QALYs [–0.032 (95% CI –0.117 to 0.096) in favour of open repair, estimated by Monte Carlo simulation], and therefore EVAR is, on average, dominated. EVAR is estimated to be less effective than open repair over the long term because the initial benefit of EVAR is offset by higher mortality from other-cause deaths (assumed to be up to 2 years after aneurysm repair) and more AAA deaths (assumed to be up to 8 years after aneurysm repair). EVAR is estimated to be more costly than open repair because of the lifetime greater incidence of reinterventions and the need for annual surveillance. In addition, the acquisition cost of the endovascular graft appears to be greater than savings to the NHS from fewer days in hospital and shorter time in surgery. This was also the conclusion of the DREAM,207 although intracountry comparisons of hospital resource use must be treated with caution. The model can be used to indicate alternative scenarios in which EVAR might be cost-effective. To be cost-effective, any more costly technology must be more effective over the long term than the comparator. The recent NICE appraisal199 considered that the difference in QALY might be 0.043 in favour of endovascular repair. This was based on a series of assumptions that individually do not greatly affect the decision but cumulatively are more optimistic towards EVAR than the current base-case model. First, deaths while waiting for initial AAA repair were not counted in the NICE appraisal, and the OR during the initial period was measured by deaths up to 30 days after AAA repair. The current base case measures deaths during the first 6 months after randomisation. Counting events from the date of AAA repair assumes that deaths are equally distributed during the waiting period, and allows a pooled treatment effect on operative mortality to be estimated from all of the RCTs. Counting events from the date of randomisation has higher internal validity and is more consistent with the ITT analysis of the clinical trial.
Secondly, the rate of late AAA mortality in the NICE appraisal was estimated (from 4-year data) as six deaths in 1250 patient-years of follow-up, or 0.0048. Longer-term data from EVAR trial 1 estimated a higher rate of AAA deaths of 0.008 after 4 years. Thirdly, the HR of late AAA deaths for EVAR versus open repair was estimated in the NICE appraisal from expert opinion to be 1.5, whereas the longer term EVAR trial 1 data estimated a higher relative risk of 4.85. The NICE appraisal was also more optimistic about costs than the current analysis. The latest estimate from EVAR trial 1 was that the endovascular procedure (including the device) cost £1177 more than open repair. The NICE appraisal considered the cost data outdated, as the devices were mainly implanted between 1999 and 2004, and that with current devices and techniques there would be no difference in initial procedure cost. The NICE appraisal also considered that there were fewer reinterventions in current practice than EVAR trial 1 and the HR for reintervention would be 1.5 (EVAR vs open repair). Long-term EVAR trial 1 data estimated a relative risk of 3.24 after 4 years. Finally, the NICE appraisal considered that surveillance would cost on average £54 per year (reflecting use of duplex ultrasound and/or less frequent follow-up rather than annual CT and outpatient visit costing £196, as assumed in the base-case model).
The inputs to the base-case analysis were mainly estimated from the results of EVAR trial 1. Guidelines for economic analysis recommend that data from all relevant sources are incorporated in the model. 209 The results of the DREAM are quite similar to those of EVAR trial 1 and so inclusion of treatment effects from this trial would not change the main conclusions. Some of the results of the OVER trial differ considerably from those of EVAR trial 1, particularly the rates of in-hospital mortality, the overall difference in survival and the rates of reintervention. Comparison between EVAR trial 1 and the OVER trial is not straightforward because the OVER trial was conducted in a younger, fitter group with a lower probability of operative mortality in both groups than in EVAR trial 1. Nevertheless, the results may be informative for UK policy, at least in this subpopulation. The OVER trial found a lower OR for in-hospital mortality than EVAR trial 1. However, absolute risk after open repair was also lower in this study. Consequently, the absolute difference in operative mortality is similar to than in EVAR trial 1 (mean absolute reduction in risk of death during hospitalisation 3.1% in EVAR trial 1 vs 2.5% in the OVER trial) and it is absolute differences in mortality that drive estimates of life expectancy, QALYs and hence cost-effectiveness. These results indicate that, even if EVAR was considered to be relatively more effective (a lower OR) in a subgroup with low operative risk, this would not translate into improved cost-effectiveness unless the absolute risk reduction was increased. Furthermore, if a fitter patient had longer life expectancy, this would reduce the cost-effectiveness of EVAR given a continued need for surveillance and lifetime risk of reinterventions.
The authors of the OVER trial stated that they did not observe increased mid-term mortality after EVAR, implying that the initial benefit of EVAR is continued for at least 2 years (although not statistically significant). Under the assumption of no ‘catch-up’ in other-cause mortality, the model predicts a small positive lifetime difference in QALY in favour of EVAR (mean difference 0.018 QALYs). However, given UK estimates of costs, EVAR would still not be cost-effective in this scenario, with an ICER of about £148,000 per QALY.
In conclusion, the economic analysis did not find that EVAR is cost-effective compared with open repair, but there is great uncertainty in many of the variables in the base-case model, particularly those that are associated with long-term outcomes. There are scenarios in which EVAR might be cost-effective. For example, the NICE FAD considered that current devices would have lower rates of complications than EVAR trial 1, and procedure costs, surveillance costs, AAA mortality and reintervention rates would be lower. The likelihood of these scenarios jointly being true may be limited.
Discussion of EVAR trial 2
Mortality
In 2005, when the 4-year results of EVAR trial 2 were presented, there was little evidence to support the use of EVAR in this very unfit group of patients. 198 With longer follow-up, there is now some evidence in favour of EVAR. However, these patients have limited life expectancy, with the Kaplan–Meier survival curves in Figure 24 falling steeply for all patients and few remaining alive after 8 years. Patients considered unfit for open repair are vulnerable to many comorbidities, and this is reflected by the relatively high operative mortality seen after EVAR in this trial (7.3%), which is considerably higher than that reported in the fit patients of EVAR trial 1 (1.8%). 201 The mid-term results for EVAR trial 2198 reported a slightly higher operative mortality of 9%, which appears to be attenuated with the recruitment of an additional 66 patients. The use of statins appears to have increased during the course of the trials (from 39% before December 2003 to 53% afterwards), and this may have reduced operative mortality. 255,256 Similarly, other improvements in clinical practice and optimising fitness may have been implemented. 257 The analyses presented in Chapter 5, Cardiovascular events have demonstrated a higher cardiovascular event rate (MIs and strokes) in the EVAR group although this difference was not strongly statistically significant. 258 Thus, the previous recommendation198 that optimisation of fitness should be prioritised ahead of endograft deployment remains valid in the light of these long-term results; the checklist of fitness parameters recommended in Table 17 seems to have worked well in allocating patients into EVAR trial 1 and EVAR trial 2 groups. Sadly, EVAR trial 2 is unique and therefore comparison with other trials is not possible. However, when the mid-term results were published in 2005, a number of subsequent studies from the USA tried to compare the mortality of EVAR trial 2 patients with cohorts of patients who had been classified as ‘high risk’,259–261 concluding that EVAR was justified in these patients. The mortality data for these cohorts were further compared with those seen across all the EVAR trial patients and this comparison demonstrated that the US ‘high-risk’ patients represent a different group who are more similar to the less-fit patients of EVAR trial 1. Therefore, it is important to make the distinction between patients regarded as ‘high risk’, in whom an open repair may be attempted but with an anticipated higher operative mortality, and those patients regarded as ‘unfit’ for open repair, in whom the procedure would not be attempted. The results we present for EVAR trial 2 patients are generalisable to the latter group.
The most striking finding of these long-term EVAR trial 2 results is that, if patients survive long enough, placement of an endograft does appear to lead to a significant reduction in aneurysm-related mortality, primarily through prevention of aneurysm rupture in the long term. This had not been foreseen in the mid-term results of 2005. 198 This finding is corroborated somewhat by the tests of interaction for AAA-related mortality presented in Table 19, which show that the benefit of EVAR is greater in the younger and fitter patients. Similarly, it is perhaps unsurprising that the patients with larger AAA experience a greater benefit with EVAR, but it must be stressed that none of the tests of interaction was statistically significant. Unfortunately, although EVAR prevents aneurysm rupture, it does not appear to lead to an improvement in overall survival. The rupture rate of 12.4 per 100 person-years seen in the no-intervention group is somewhat lower than that seen in other cohorts of unfit patients with large aneurysm. 179,262 Previous work has suggested that anatomical suitability may impart some protection against rupture. 263 Also, the aneurysm repairs that occurred against protocol may have led to a reduced number of ruptures, and this rate may not reflect the true natural history of large aneurysms if left untreated in the long term.
Although compliance was very good in the EVAR group (99%), it was not good in the no-intervention group (69%), with both clinicians and patients losing equipoise during the course of the trial (see Figure 11). Table 17 shows that, among the 18 patients who did not receive their EVAR soon after randomisation in the EVAR group and subsequently died prior to any aneurysm repair, there were a small number of patients who were very unwell, particularly in terms of cardiac disease, and delay of their procedure seems justified while their comorbidities were treated. Conversely, a post hoc analysis comparing baseline fitness (CPI score) in the 70 patients who had an aneurysm repair in the no-intervention group with the 179 patients who had an aneurysm repair in the EVAR group demonstrated that the patients who crossed over in the no-intervention group were significantly fitter. After censoring patients at elective aneurysm repair in the no-intervention group, per-protocol analyses showed potentially greater benefit in terms of both aneurysm-related and all-cause mortality in the patients treated with EVAR, but the difference in all-cause mortality remained non-significant. Interpretation is problematic, however, as the analysis is not by randomised group and therefore is potentially biased. Regardless of these considerations, the rate of crossover in the trial suggests that it may prove difficult to withhold endovascular repair in future.
Graft-related complications and reinterventions
As seen for EVAR trial 1, complications and reinterventions remained common after EVAR in trial 2 but they do not appear to be associated with increased mortality, with very few procedure-related deaths occurring beyond the early 6-month primary procedure period (see Table 20). Figure 27 shows that, despite gross differences in fitness and overall mortality between EVAR trials 1 and 2 cohorts, the rates of complications and reinterventions are remarkably similar, suggesting that anaesthetic suitability for open repair appears to be of little relevance to the development of subsequent graft-related events. In addition, one might have expected a lower reintervention rate than in EVAR trial 1 as EVAR trial 2 patients were frailer and less fit, but this does not seem to have influenced the decision to intervene. The results in Chapter 6, Factors associated with development of serious graft-related complications and reinterventions, which combined patients with EVAR from both trials, demonstrated that older age and larger aneurysm diameter appear to be influential. 264 However, the modest differences in these factors between EVAR trials 1 and 2 do not appear to have led to different rates of graft-related events. This may be explained partially by the high mortality attrition in EVAR trial 2, leaving less time for patients to develop complications. This may also be why so few endograft ruptures occurred in trial 2 (just two) compared with EVAR trial 1 (25). In terms of the other types of complications, comparison of Tables 7 and 21 shows that the distribution is fairly similar between the two trials, although there was a higher proportion of type 2 endoleaks with sac growth in EVAR trial 2 (13%) than in EVAR trial 1 (6%) but a lower proportion of cases of migration (1% and 10%, respectively). Overall, the cost and inconvenience of these complications and reinterventions need to be weighed up against the longer-term prevention of AAA rupture and each patient is likely to prioritise these differently.
Health-related quality of life
The baseline EQ-5D and SF-36 scores in EVAR trial 2 (see Tables 23 and 24) were substantially lower than for patients randomised in EVAR trial 1 (see Tables 10 and 11). There were no clear or consistent differences in HRQoL demonstrated between the two randomised groups of EVAR trial 2, whether timed at 0–3, 3–12 or 12–24 months after randomisation, and similar results were found when timed at 1, 3 and 12 months after operation. Many of these patients live with a number of serious comorbidities that are far more life-limiting than the presence of an aortic aneurysm and therefore it is perhaps unsurprising that the correction of their aneurysm does not appear to have had a beneficial impact on their quality of life.
Renal function
In addition to the points raised above (see Discussion of EVAR trial 1, Renal function), which applied to both trials, in EVAR trial 2 the effect of EVAR versus no intervention provided some weak evidence to suggest that patients experienced a greater deterioration in renal function after EVAR, but the difference between groups was small and unlikely to be of great clinical importance or have any significant impact on renal services, particularly given the relatively short life expectancy for the very unfit patients in EVAR trial 2. When comparing the KDOQI classifications at baseline between EVAR trial 1 (see Figure 20) and EVAR trial 2 (see Figure 29), the poorer renal function in EVAR trial 2 is clearly apparent, with a much higher proportion of patients being classified with stage 3 impairment.
Although a sizable proportion of patients were excluded from renal function analyses (see Figure 28), these exclusions did not generate any major differences between groups in the analyses. However, as the comparisons were no longer by ITT, some bias may have been introduced into these analyses that even adjustment for baseline variables cannot remove. The exclusion of 43 patients in the no-intervention group of EVAR trial 2 who underwent AAA repair before any follow-up creatinine measurements could be obtained potentially provides the greatest source of bias as these are likely to be the fitter patients with better renal function. Thus, the ‘per-protocol’ patients remaining in the no-intervention group are likely to be those with worse renal function, and this may explain the significant differences in baseline eGFR seen in Table 26. Although only 18 patients were excluded from the EVAR group of EVAR trial 2 because they did not have their AAA repair, this non-compliance with randomised allocation may also relate to their renal function. All of these factors may affect the generalisability of these results to all patients with AAA.
Costs and cost-effectiveness
Chapter 8 conducted an economic evaluation of EVAR versus no surgery using data from EVAR trial 2. The primary analysis was on an ITT within-trial basis with results at 8 years. The analysis found that mean survival (truncated at 8 years) was higher in the no-surgery group, with a wide CI (mean difference –0.13, 95% CI –0.63 to 0.41). The expected difference in QALYs (truncated at 8 years) was very small (0.04, 95% CI –0.26 to 0.35). The difference in QALYs is greater than the difference in mean life expectancy because EVAR patients tended to report better HRQoL, measured by the EQ-5D, during the first 3 years, although this difference in EQ-5D did not reach statistical significance. The mean difference in costs was £10,596 (95% CI £8183 to £12,660), and the ICER was £265,000 per QALY. This cost per QALY would not be considered cost-effective in the UK (NICE 2008)208.
The non-parametric within-trial analysis is limited to 8 years. This may underestimate the lifetime relative benefits of EVAR. Survival analysis was used to estimate parametric survival curves to predict life expectancy and QALYs over the patients’ lifetimes. A Weibull model was used to extrapolate from the trial data. This model indicated that, in the ITT analysis, the difference in mean QALYs was 0.35 (95% CI –0.15 to 0.84) and the ICER for EVAR versus no surgery was about £32,000 per QALY. However, this model has considerable uncertainty in the functional form used to extrapolate from the trial data, and assumes that the difference in costs does not change from 8 years. The non-parametric analysis might be considered ‘pessimistic’ towards EVAR in the sense that it assumes that no benefits accrue after 8 years. The parametric analysis might be considered ‘optimistic’ in the sense that it assumes no further increment in cost and that those who survive up to 8 years will continue to benefit. Therefore, these analyses might represent the range of ‘modelling uncertainty’ for the ICER. 265
About 30% of the patients in the control arm of EVAR trial 2 had AAA repair, and this may have diluted the estimate of the benefit of EVAR. A per-protocol analysis was conducted by including patients in the control arm up to the date of surgery. At 8 years, the difference in costs was £14,066 (95% CI £12,515 to £15,593) and the difference in QALYs was 0.40 (95% CI 0.10 to 0.72). The ICER for EVAR versus no surgery was about £35,000 per QALY in the per-protocol analysis at 8 years. If mortality rates are extrapolated using the Weibull distribution (with no change in total costs), the ICER for EVAR versus no surgery was estimated to be about £18,000 per QALY in the per-protocol analysis over a lifetime. The per-protocol analysis was post hoc, and may be biased. Those who crossed over were significantly fitter than average. Therefore, as with the clinical per-protocol analyses (see Chapter 5, Per-protocol analyses for all-cause and aneurysm-related mortality), these results should be interpreted with caution.
The costs included in this analysis include the primary operation, reinterventions for graft-related reasons and surveillance with CT after endovascular repair. There may be other relevant categories of cost that were excluded. The trial did not collect all types of reintervention, for example hernia repairs were not included. The trial did collect data on the incidence of systemic complications such as renal disease, infarctions, stroke and amputations. The costs of these were not included in the current analysis because this would have required assumptions to be made about the long-term costs associated with these conditions, which did not seem appropriate for a primarily within-trial analysis. In any event, the incidence of these complications did not differ significantly between the arms (see Table 27). The analysis did not include the costs of surveillance. It is likely that any bias arising from these omissions will be small.
Although follow-up was extremely thorough in this trial, there were a considerable number of missing data, mainly because of administrative censoring (staggered recruitment into the trial), and not all patients attended every scheduled follow-up interview, leaving gaps in some patients’ records. The former type of missing data was handled by the estimation of inverse probability weights,218 whereas the latter was imputed. 195 These methods necessarily require modelling assumptions, primarily that the missing data mechanism is ignorable, i.e. patients do not miss interviews because of their current health state. It is difficult to verify if this assumption is valid. However, excluding patients with missing data would not be an efficient option and, in any case, excluding patients would also assume that the data are MCAR.
In conclusion, the base-case analysis finds that EVAR is not likely to be considered cost-effective with 8 years’ follow-up, with an ICER of £265,000 per QALY, which is well above NICE’s cost-effectiveness threshold of £20,000 to £30,000 per QALY gained. 209 An indicative model to extrapolate beyond the trial suggested that the ICER might be about £32,000 per QALY over a lifetime, but this model makes optimistic assumptions about both treatment effect and cost. A per-protocol analysis suggested that the ICER for EVAR versus no surgery might be about £35,000 per QALY over 8 years, and modelling suggested that the ICER might be £18,000 per QALY over a lifetime. However, the per-protocol analysis is post hoc and may be biased.
Limitations of the EVAR trials
There are some limitations that relate to the interpretation of our findings in the EVAR trials. First, although the trial used principally second- and third-generation endografts, later iterations of grafts would now be the more common choices of device. The long-term durability of these later iterations of endografts has not been evaluated but it is hoped that they would be associated with lower complication rates. Second, investigators were allowed to enter patients into the trial after they had completed 20 EVAR procedures, irrespective of the number of aortic procedures performed in a centre. Later evidence suggests that both a longer learning curve and larger volume centres are associated with improved outcomes for aneurysm-repair patients. Third, the trial started 3 years before the standardised reporting of graft-related complications,266 and reporting of complications relied on radiologists in the participating centres and was not evaluated in a core laboratory. Fourth, we did not record day-case procedures, which will have included minor procedures such as diagnostic angiograms often performed after endovascular repair to obtain more detailed information on any potential complications. A corresponding underestimation of reintervention rates (and costs) may also have occurred for the open-repair group, as readmission data were not collected for abdominal hernias or other open-repair-related complications. Fifth, we did not record changes in medication during follow-up, particularly for those medications associated with cardiovascular risk reduction.
There are also limitations to the methods applied in our investigation of cardiovascular events. First, non-fatal cardiovascular events may have been under-reported, particularly if patients were not treated at the same hospital where aneurysm repair and follow-up were conducted. Second, the ascertainment of non-fatal events from death certificates is unconventional, but this captured a small number of additional events (5%) taking place after last follow-up. The timing of such events at date of death will have led to a small underestimation in event rates. Third, clinical confirmation of non-fatal events according to WHO criteria was available for only just over half of patients. However, these limitations apply equally between the groups being compared, and therefore are offset by the strengths of the randomised design. In addition, our results are in keeping with the only previously published data reporting longer-term cardiovascular event rates after aneurysm repair. 267
Recommendations for further work
-
These trials closed follow-up at the end of December 2009 to allow all of the results to be analysed and reported before funding ceased at the end of December 2010. However, the continued occurrence of endograft ruptures remains a concern. The authors are aware of three new cases of endograft rupture that have been reported to the central trial office since the trial closed follow-up in December 2009. Therefore, it is unfortunate that data on AAA-related mortality beyond the 8 years that we present in Figure 14 will not be forthcoming. If the lines remain parallel then this would not be a concern but it is possible that more future endograft ruptures could lead to the survival curves crossing for AAA-related mortality, such that mortality after EVAR may exceed that for open repair. Collection of further data beyond 10 years would be required to investigate this possibility.
-
An IPD meta-analysis of all the randomised trials comparing EVAR with open repair should be undertaken. All of these trials have now closed recruitment but the OVER trial is still in the follow-up phase. All trial principal investigators have agreed to collaborate once follow-up and publication of long-term results have been released for each trial. This would yield a total of 2834 patients in whom more powerful subgroup analyses could be undertaken. This may help to determine whether there are any subgroups in whom EVAR performs particularly well or poorly and permit analysis of gender-specific effects.
-
A systematic review of operative mortality after EVAR needs to be performed, leading to an IPD meta-analysis of factors associated with 30-day or in-hospital mortality. This could be used to develop a prognostic risk score, which could be applied to all patients being considered for AAA repair. Ideally, this will be validated externally in an independent data set but internal validation may be an alternative if the power of the score development process is to be maximised.
-
An optimal and cost-effective surveillance protocol after EVAR needs to be developed and tested prospectively ensuring an appropriate balance between detection of potentially serious complications and safe levels of exposure to radiation and contrast agent.
-
In order to prevent the most serious of all complications, endograft rupture, a surveillance and intervention protocol needs to be tested prospectively in patients who have been diagnosed with any of the complications that were found to be associated with endograft rupture, namely endoleaks type 1, type 3, or type 2 with sac growth, migration and kinking.
-
Following on from recommendation no. 4, the long-term morphological changes of the aorta and iliac arteries need to be investigated after EVAR implantation. The tendency of most aortoiliac segments in patients with aneurysm is for the dilating process to continue. Thus, the endograft landing zones may no longer be sealed, giving rise to an increase in sac diameter. Nor is open repair necessarily free from the ongoing dilatation problem. Late ruptures can be expected as the aortic neck or iliac vessels outgrow the Dacron anastomoses. At present, sac growth is regarded as an indicator that the endograft has not excluded the aneurysm sac and changes in sac size after implantation may hold the key to understanding why grafts fail and how future device design might be improved.
Chapter 10 Conclusions
For patients with large AAA, who are deemed anatomically suitable for EVAR and anaesthetically fit for open repair, EVAR is associated with a significantly lower operative mortality but late endograft ruptures appear to erode this early aneurysm-related survival benefit such that no differences are seen in all-cause or aneurysm-related mortality in the long term. There is little difference between these groups in terms of cardiovascular events, quality of life or renal function decline. However, EVAR is associated with increased rates of graft-related complications and reinterventions, and requires continued surveillance to prevent the catastrophic event of endograft rupture. Thus, it is a more costly treatment option and unlikely to be cost-effective in all patients. It is possible that there are subgroups of patients in whom EVAR performs particularly well. For example, younger patients with smaller AAA close to 5.5 cm have the lowest rates of complications and younger, fitter patients appear to experience the greatest benefit of EVAR relative to open repair in terms of a relative reduction in operative mortality. However, the absolute difference in operative mortality between endovascular and open repair remains about 3% for younger and fitter patients, similar to the difference for the population as a whole. It is absolute, not relative, differences that determine gains in survival and life expectancy (which are important to patients) and ultimately drive cost-effectiveness. Improvements in endograft design, more rigorous implementation of medical therapies and better optimisation of fitness prior to AAA repair should improve outcomes and cost-effectiveness for EVAR.
For patients with large AAA who are deemed anatomically suitable for EVAR but too unfit to be considered for open repair, EVAR offers a significant long-term benefit over no intervention in terms of aneurysm-related mortality, but all-cause mortality is apparently unaffected. There are no benefits in terms of quality of life and high rates of adverse events, complications and reinterventions after EVAR contribute to increased costs and thus poor cost-effectiveness. The outcome for patients unfit for open repair is high mortality risk, whether or not an endograft is deployed, but for those who survive long enough EVAR is successful in reducing the risk of death from aneurysm rupture.
Acknowledgements
These trials are a collaborative effort and their success is a result of the enormous enthusiasm and support from all of the committee members and participating centres. A full list of the EVAR trial participants is given in Appendix 1. We would also like to thank all of the other clinicians from local supporting hospitals who were responsible for referral of almost one-third of all patients to the participating trial centres.
In addition to thanking all of those who dedicated their valuable time to sitting on the trial committees, we would also like to send our gratitude to other specialists whom we approached for external help and advice on a number of the subsidiary projects. In particular, we would like to thank Dr Simon Howell (Consultant Anaesthetist, Leeds General Infirmary) for his advice on anaesthetic fitness for AAA repair, Professor Edwina Brown (Consultant Renal Physician, Imperial College, London) for her advice on renal disease, and Dr Thomas Wyss (Surgical Research Fellow, University of Bern, Switzerland) for his work on the endograft rupture assessment.
Contribution of authors
Louise Brown drafted and compiled the report as trial manager, referring always to the other authors.
Janet Powell critically reviewed the manuscript, especially cardiovascular risk, morbidity and mortality issues.
Simon Thompson critically reviewed the manuscript, especially statistical issues.
Mark Sculpher provided critical input to Chapter 8.
David Epstein wrote Chapter 8.
Roger Greenhalgh had overall responsibility, co-ordination and supervision, with special responsibility for clinical issues.
Ethical approval
National ethical approval was obtained from the North West Multicentre Research Ethics Committee (MREC), subsequently to become the Integrated Research Application System (IRAS), based in Manchester (MREC reference 98/8/26 and 98/8/27).
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Liddington MI, Heather BP. The relationship between aortic diameter and body habitus. Eur J Vasc Surg 1992;6:89-92.
- Sonesson B, Lanne T, Hansen F, Sandgren T. Infrarenal aortic diameter in the healthy person. Eur J Vasc Surg 1994;8:89-95.
- Johnson G, Avery A, McDougal EG, Burnham SJ, Keagy BA. Aneurysms of the abdominal aorta. Incidence in blacks and whites in North Carolina. Arch Surg 1985;120:1138-40.
- Lamorte WW, Scott TE, Menzoian JO. Racial differences in the incidence of femoral bypass and abdominal aortic aneurysmectomy in Massachusetts: relationship to cardiovascular risk factors. J Vasc Surg 1995;21:422-31.
- Gillum RF. Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol 1995;48:1289-98.
- Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg 1997;26:595-601.
- Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991;13:452-8.
- Collin J. A proposal for a precise definition of abdominal aortic aneurysm. A personal view. J Cardiovasc Surg (Torino) 1990;31:168-9.
- McGregor JC, Pollock JG, Anton HC. The value of ultrasonography in the diagnosis of abdominal aortic aneurysm. Scott Med J 1975;20:133-7.
- Sterpetti AV, Schultz RD, Feldhaus RJ, Cheng SE, Peetz DJ. Factors influencing enlargement rate of small abdominal aortic aneurysms. J Surg Res 1987;43:211-19.
- Alcorn HG, Wolfson SK, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1996;16:963-70.
- Ingoldby CJ, Wujanto R, Mitchell JE. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. Br J Surg 1986;73:551-3.
- Collin J, Araujo L, Walton J, Lindsell D. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet 1988;2:613-15.
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360:1531-9.
- Heather BP, Poskitt KR, Earnshaw JJ, Whyman M, Shaw E. Population screening reduces mortality rate from aortic aneurysm in men. Br J Surg 2000;87:750-3.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005;330.
- Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004;329.
- Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, Quick CR, et al. Incidence among men of asymptomatic abdominal aortic aneurysms: estimates from 500 screen detected cases. J Med Screen 1999;6:50-4.
- Scott RA, Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA. The long-term benefits of a single scan for abdominal aortic aneurysm (AAA) at age 65. Eur J Vasc Endovasc Surg 2001;21:535-40.
- Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg 2001;34:122-6.
- Cornuz J, Sidoti PC, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health 2004;14:343-9.
- Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation 2007;115:2865-9.
- Stenbaek J, Granath F, Swedenborg J. Outcome after abdominal aortic aneurysm repair. Difference between men and women. Eur J Vasc Endovasc Surg 2004;28:47-51.
- Mofidi R, Goldie VJ, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg 2007;94:310-14.
- Hultgren R, Granath F, Swedenborg J. Different disease profiles for women and men with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2007;33:556-60.
- Vande Geest JP, Dillavou ED, Di Martino ES, Oberdier M, Bohra A, Makaroun MS, et al. Gender-related differences in the tensile strength of abdominal aortic aneurysm. Ann N Y Acad Sci 2006;1085:400-2.
- Hobbs SD, Sam RC, Bhatti A, Rehman A, Wilmink AB, Adam DJ, et al. The low incidence of surgery for non-cardiac vascular disease in UK Asians may be explained by a low prevalence of disease. Eur J Vasc Endovasc Surg 2006;32:494-9.
- Spark JI, Baker JL, Vowden P, Wilkinson D. Epidemiology of abdominal aortic aneurysms in the Asian community. Br J Surg 2001;88:382-4.
- Masuda EM, Caps MT, Singh N, Yorita K, Schneider PA, Sato DT, et al. Effect of ethnicity on access and device complications during endovascular aneurysm repair. J Vasc Surg 2004;40:24-9.
- Cheng SW, Ting AC, Ho P, Poon JT. Aortic aneurysm morphology in Asians: features affecting stent-graft application and design. J Endovasc Ther 2004;11:605-12.
- Fowkes FG, Macintyre CC, Ruckley CV. Increasing incidence of aortic aneurysms in England and Wales. BMJ 1989;298:33-5.
- Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg 2006;44:237-43.
- Norman PE, Castleden WM, Hockey RL. Prevalence of abdominal aortic aneurysm in Western Australia. Br J Surg 1991;78:1118-21.
- McGill HC. The cardiovascular pathology of smoking. Am Heart J 1988;115:250-7.
- Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg 1999;30:1099-105.
- Tonstad S, Andrew JJ. Cardiovascular risks associated with smoking: a review for clinicians. Eur J Cardiovasc Prev Rehabil 2006;13:507-14.
- Lee AJ, Fowkes FG, Carson MN, Leng GC, Allan PL. Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur Heart J 1997;18:671-6.
- Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg 2003;38:329-34.
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997;126:441-9.
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004;110:16-21.
- Roake JA. Vs04 risk/benefit assessment for elective abdominal aortic aneurysm surgery. ANZ J Surg 2007;77.
- Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg 2000;87:195-200.
- Fowkes FG, Anandan CL, Lee AJ, Smith FB, Tzoulaki I, Rumley A, et al. Reduced lung function in patients with abdominal aortic aneurysm is associated with activation of inflammation and hemostasis, not smoking or cardiovascular disease. J Vasc Surg 2006;43:474-80.
- Mattes E, Davis TM, Yang D, Ridley D, Lund H, Norman PE. Prevalence of abdominal aortic aneurysms in men with diabetes. Med J Aust 1997;166:630-3.
- Dodson PM, Kritzinger EE, Clough CG. Diabetes mellitus and retinal vein occlusion in patients of Asian, west Indian and white European origin. Eye 1992;6:66-8.
- Ellis M, Powell JT, Greenhalgh RM. Limitations of ultrasonography in surveillance of small abdominal aortic aneurysms. Br J Surg 1991;78:614-16.
- Ellis M, Cuming R, Laing S, Vashisht R, Franks PJ, Greenhalgh RM, et al. The reproducibility of colour-coded duplex scanning in measuring arterial wall dimensions. Eur J Vasc Surg 1992;6:386-9.
- Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther 2005;12:297-305.
- O’Kelly TJ, Heather BP. General practice-based population screening for abdominal aortic aneurysms: a pilot study. Br J Surg 1989;76:479-80.
- Lucarotti M, Shaw E, Poskitt K, Heather B. The Gloucestershire Aneurysm Screening Programme: the first 2 years’ experience. Eur J Vasc Surg 1993;7:397-401.
- Multicentre Aneurysm Screening Study Group . Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ 2002;325.
- Kim LG, Scott RA, Ashton HA, Thompson SG. A sustained mortality benefit from screening for abdominal aortic aneurysm. Ann Intern Med 2007;146:699-706.
- Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg 2007;94:696-701.
- Kim LG, Thompson SG, Briggs AH, Buxton MJ, Campbell HE. How cost-effective is screening for abdominal aortic aneurysms?. J Med Screen 2007;14:46-52.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms. Results from a randomised population screening trial. Eur J Vasc Endovasc Surg 2002;23:55-60.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Preliminary ten year results from a randomised single centre mass screening trial for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2006;32:608-14.
- Spencer CA, Jamrozik K, Norman PE, Lawrence-Brown MM. The potential for a selective screening strategy for abdominal aortic aneurysm. J Med Screen 2000;7:209-11.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2005;142:203-11.
- Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2007;2.
- Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999;17:472-5.
- Thomas PR, Shaw JC, Ashton HA, Kay DN, Scott RA. Accuracy of ultrasound in a screening programme for abdominal aortic aneurysms. J Med Screen 1994;1:3-6.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002;89:283-5.
- Wanhainen A, Lundkvist J, Bergqvist D, Bjorck M. Cost-effectiveness of screening women for abdominal aortic aneurysm. J Vasc Surg 2006;43:908-14.
- Wilmink AB, Vardulaki KA, Hubbard CS, Day NE, Ashton HA, Scott AP, et al. Are antihypertensive drugs associated with abdominal aortic aneurysms?. J Vasc Surg 2002;36:751-7.
- Slaiby JM, Ricci MA, Gadowski GR, Hendley ED, Pilcher DB. Expansion of aortic aneurysms is reduced by propranolol in a hypertensive rat model. J Vasc Surg 1994;20:178-83.
- Moursi MM, Beebe HG, Messina LM, Welling TH, Stanley JC. Inhibition of aortic aneurysm development in blotchy mice by beta adrenergic blockade independent of altered lysyl oxidase activity. J Vasc Surg 1995;21:792-9.
- Propranolol Aneurysm Trial Investigators . Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002;35:72-9.
- Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med 1994;330:1335-41.
- Kurzencwyg D, Filion KB, Pilote L, Nault P, Platt RW, Rahme E, et al. Cardiac medical therapy among patients undergoing abdominal aortic aneurysm repair. Ann Vasc Surg 2006;20:569-76.
- Lloyd GM, Newton JD, Norwood MG, Franks SC, Bown MJ, Sayers RD. Patients with abdominal aortic aneurysm: are we missing the opportunity for cardiovascular risk reduction?. J Vasc Surg 2004;40:691-7.
- Kinikini D, Sarfati MR, Mueller MT, Kraiss LW. Meeting AHA/ACC secondary prevention goals in a vascular surgery practice: an opportunity we cannot afford to miss. J Vasc Surg 2006;43:781-7.
- Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet 2006;368:659-65.
- Lederle FA, Taylor BC. ACE inhibitors and aortic rupture. Lancet 2006;368.
- Sweeting MJ, Thompson SG, Brown LC, Greenhalgh RM, Powell JT. Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J Vasc Surg 2010;52:1-4.
- Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, et al. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation 1999;100:48-54.
- Schouten O, van Laanen JH, Boersma E, Vidakovic R, Feringa HH, Dunkelgrun M, et al. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg 2006;32:21-6.
- Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 2001;34:606-10.
- Vammen S, Lindholt JS, Ostergaard L, Fasting H, Henneberg EW. Randomized double-blind controlled trial of roxithromycin for prevention of abdominal aortic aneurysm expansion. Br J Surg 2001;88:1066-72.
- Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Kent KC, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg 2002;36:1-12.
- Sukhija R, Aronow WS, Sandhu R, Kakar P, Babu S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am J Cardiol 2006;97:279-80.
- Leurs LJ, Visser P, Laheij RJ, Buth J, Harris PL, Blankensteijn JD. Statin use is associated with reduced all-cause mortality after endovascular abdominal aortic aneurysm repair. Vascular 2006;14:1-8.
- Lindholt JS, Heegaard NH, Vammen S, Fasting H, Henneberg EW, Heickendorff L. Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2001;21:51-6.
- Evans J, Powell JT, Schwalbe E, Loftus IM, Thompson MM. Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur J Vasc Endovasc Surg 2007;34:302-3.
- Guessous I, Periard D, Lorenzetti D, Cornuz J, Ghali WA. The efficacy of pharmacotherapy for decreasing the expansion rate of abdominal aortic aneurysms: a systematic review and meta-analysis. PLoS ONE 2008;3.
- Dubost C, Allary M, Oeconomos N. Treatment of aortic aneurysms;removal of the aneurysm;re-establishment of continuity by grafts of preserved human aorta. Mem Acad Chir 1951;77:381-3.
- Visser P, Akkersdijk GJ, Blankensteijn JD. In-hospital operative mortality of ruptured abdominal aortic aneurysm: a population-based analysis of 5593 patients in The Netherlands over a 10-year period. Eur J Vasc Endovasc Surg 2005;30:359-64.
- Jibawi A, Hanafy M, Guy A. Is there a minimum caseload that achieves acceptable operative mortality in abdominal aortic aneurysm operations?. Eur J Vasc Endovasc Surg 2006;32:273-6.
- Morishita Y, Arikawa K, Yamashita M, Shimokawa S, Ohzono H, Saigenji H, et al. Ruptured abdominal aortic aneurysm: factors influencing operative mortality. Jpn J Surg 1986;16:272-6.
- Aune S, Amundsen SR, Evjensvold J, Trippestad A. Operative mortality and long-term relative survival of patients operated on for asymptomatic abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1995;9:293-8.
- Amundsen S, Skjaerven R, Trippestad A, Soreide O. Abdominal aortic aneurysms. Is there an association between surgical volume, surgical experience, hospital type and operative mortality? Members of the Norwegian Abdominal Aortic Aneurysm Trial. Acta Chir Scand 1990;156:323-7.
- Berridge DC, Chamberlain J, Guy AJ, Lambert D. Prospective audit of abdominal aortic aneurysm surgery in the northern region from 1988 to 1992. Northern Vascular Surgeons Group. Br J Surg 1995;82:906-10.
- Hallett JW, Naessens JM, Ballard DJ. Early and late outcome of surgical repair for small abdominal aortic aneurysms: a population-based analysis. J Vasc Surg 1993;18:684-91.
- Olsen PS, Schroeder T, Agerskov K, Roder O, Sorensen S, Perko M, et al. Surgery for abdominal aortic aneurysms. A survey of 656 patients. J Cardiovasc Surg 1991;32:636-42.
- Johnston KW. Nonruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg 1994;20:163-70.
- The UK Small Aneurysm Trial Participants . Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998;352:1649-55.
- Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002;346:1437-44.
- Hertzer NR, Mascha EJ, Karafa MT, O’Hara PJ, Krajewski LP, Beven EG. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg 2002;35:1145-54.
- Zarins CK, Harris EJ. Operative repair for aortic aneurysms: the gold standard. J Endovasc Surg 1997;4:232-41.
- Blankensteijn JD, Lindenburg FP, Van der GY, Eikelboom BC. Influence of study design on reported mortality and morbidity rates after abdominal aortic aneurysm repair. Br J Surg 1998;85:1624-30.
- Hallin A, Bergqvist D, Holmberg L. Literature review of surgical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2001;22:197-204.
- Hannan EL, Kilburn H, O’Donnell JF, Bernard HR, Shields EP, Lindsey ML, et al. A longitudinal analysis of the relationship between in-hospital mortality in New York State and the volume of abdominal aortic aneurysm surgeries performed. Health Serv Res 1992;27:517-42.
- Holt PJ, Poloniecki JD, Loftus IM, Michaels JA, Thompson MM. Epidemiological study of the relationship between volume and outcome after abdominal aortic aneurysm surgery in the UK from 2000 to 2005. Br J Surg 2007;94:441-8.
- Tu JV, Austin PC, Johnston KW. The influence of surgical specialty training on the outcomes of elective abdominal aortic aneurysm surgery. J Vasc Surg 2001;33:447-52.
- Pearce WH, Parker MA, Feinglass J, Ujiki M, Manheim LM. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg 1999;29:768-76.
- Eckstein HH, Bruckner T, Heider P, Wolf O, Hanke M, Niedermeier HP, et al. The Relationship Between Volume and Outcome Following Elective Open Repair of Abdominal Aortic Aneurysms (AAA) in 131 German Hospitals. Eur J Vasc Endovasc Surg 2007;34:260-6.
- Parvin SD. Patient Information – Aortic Aneurysm. The Operation Explained 2007. www.vascularsociety.org.uk/patient/aaaop.html (accessed 20 August 2007).
- Aylin P, Lees T, Baker S, Prytherch D, Ashley S. Descriptive study comparing routine hospital administrative data with the Vascular Society of Great Britain and Ireland’s National Vascular Database. Eur J Vasc Endovasc Surg 2007;33:461-5.
- Kertai MD, Boersma E, Klein J, van SM, Schouten O, van UH, et al. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med 2005;165:898-904.
- Kertai MD, Steyerberg EW, Boersma E, Bax JJ, Vergouwe Y, van Urk H, et al. Validation of two risk models for perioperative mortality in patients undergoing elective abdominal aortic aneurysm surgery. Vasc Endovascular Surg 2003;37:13-21.
- Biancari F, Heikkinen M, Lepantalo M, Salenius JP. Glasgow Aneurysm Score in patients undergoing elective open repair of abdominal aortic aneurysm: a Finnvasc study. Eur J Vasc Endovasc Surg 2003;26:612-17.
- Steyerberg EW, Kievit J, de Mol Van Otterloo JC, van Bockel JH, Eijkemans MJ, Habbema JD. Perioperative mortality of elective abdominal aortic aneurysm surgery. A clinical prediction rule based on literature and individual patient data. Arch Intern Med 1995;155:1998-2004.
- Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043-9.
- Shuhaiber JH, Hankins M, Robless P, Whitehead SM. POSSUM for the prediction of mortality and morbidity in infra-renal abdominal aortic aneurysm repair. The Hastings experience. Eur J Vasc Endovasc Surg 2001;22:180-2.
- Prytherch DR, Sutton GL, Boyle JR. Portsmouth POSSUM models for abdominal aortic aneurysm surgery. Br J Surg 2001;88:958-63.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008;358:464-74.
- Volodos NL, Karpovich IP, Troyan VI, Kalashnikova Y, Shekhanin VE, Ternyuk NE, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. VASA 1991;33:S93-5.
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491-9.
- Arko FR, Filis KA, Seidel SA, Gonzalez J, Lengle SJ, Webb R, et al. How many patients with infrarenal aneurysms are candidates for endovascular repair? The Northern California experience. J Endovasc Ther 2004;11:33-40.
- Armon MP, Yusuf SW, Latief K, Whitaker SC, Gregson RH, Wenham PW, et al. Anatomical suitability of abdominal aortic aneurysms for endovascular repair. Br J Surg 1997;84:178-80.
- Woodburn KR, Chant H, Davies JN, Blanshard KS, Travis SJ. Suitability for endovascular aneurysm repair in an unselected population. Br J Surg 2001;88:77-81.
- EVAR Trial Participants . Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843-8.
- Nasim A, Thompson MM, Sayers RD, Bolia A, Bell PR. Endovascular repair of abdominal aortic aneurysm: an initial experience. Br J Surg 1996;83:516-19.
- Yusuf SW, Baker DM, Hind RE, Chuter TA, Whitaker SC, Wenham PW, et al. Endoluminal transfemoral abdominal aortic aneurysm repair with aorto-uni-iliac graft and femorofemoral bypass. Br J Surg 1995;82.
- Andrews SM, Cuming R, MacSweeney ST, Barrett NK, Greenhalgh RM, Nott DM. Assessment of feasibility for endovascular prosthetic tube correction of aortic aneurysm. Br J Surg 1995;82:917-19.
- Greenberg RK, West K, Pfaff K, Foster J, Skender D, Haulon S, et al. Beyond the aortic bifurcation: branched endovascular grafts for thoracoabdominal and aortoiliac aneurysms. J Vasc Surg 2006;43:879-86.
- O’Neill S, Greenberg RK, Haddad F, Resch T, Sereika J, Katz E. A prospective analysis of fenestrated endovascular grafting: intermediate-term outcomes. Eur J Vasc Endovasc Surg 2006;32:115-23.
- Haulon S, Greenberg RK, Pfaff K, Francis C, Koussa M, West K. Branched grafting for aortoiliac aneurysms. Eur J Vasc Endovasc Surg 2007;33:567-74.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Endovascular Aortic Stent Device Alerts n.d. www.mhra.gov.uk/2007 (accessed 23 August 2007).
- Raman KG, Missig-Carroll N, Richardson T, Muluk SC, Makaroun MS. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 2003;38:645-51.
- AbuRahma AF. Fate of endoleaks detected by CT angiography and missed by color duplex ultrasound in endovascular grafts for abdominal aortic aneurysms. J Endovasc Ther 2006;13:490-5.
- Franks SC, Sutton AJ, Bown MJ, Sayers RD. Systematic review and meta-analysis of 12 years of endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2007;33:154-71.
- EVAR Trial Participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86.
- Yusuf SW, Whitaker SC, Chuter TA, Wenham PW, Hopkinson BR. Emergency endovascular repair of leaking aortic aneurysm. Lancet 1994;344.
- Ohki T, Veith FJ. Endovascular grafts and other image-guided catheter-based adjuncts to improve the treatment of ruptured aortoiliac aneurysms. Ann Surg 2000;232:466-79.
- Greco G, Egorova N, Anderson PL, Gelijns A, Moskowitz A, Nowygrod R, et al. Outcomes of endovascular treatment of ruptured abdominal aortic aneurysms. J Vasc Surg 2006;43:453-9.
- Vaddineni SK, Russo GC, Patterson MA, Taylor SM, Jordan WD. Ruptured abdominal aortic aneurysm: a retrospective assessment of open versus endovascular repair. Ann Vasc Surg 2005;19:782-6.
- Lachat ML, Pfammatter T, Witzke HJ, Bettex D, Kunzli A, Wolfensberger U, et al. Endovascular repair with bifurcated stent-grafts under local anaesthesia to improve outcome of ruptured aortoiliac aneurysms. Eur J Vasc Endovasc Surg 2002;23:528-36.
- Peppelenbosch N, Geelkerken RH, Soong C, Cao P, Steinmetz OK, Teijink JA, et al. Endograft treatment of ruptured abdominal aortic aneurysms using the Talent aortouniiliac system: an international multicenter study. J Vasc Surg 2006;43:1111-23.
- Hinchliffe RJ, Braithwaite BD, Hopkinson BR. The endovascular management of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2003;25:191-20.
- Hinchliffe RJ, Bruijstens L, MacSweeney ST, Braithwaite BD. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm: results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg 2006;32:506-13.
- Dion YM, Katkhouda N, Rouleau C, Aucoin A. Laparoscopy-assisted aortobifemoral bypass. Surg Laparosc Endosc 1993;3:425-9.
- Cau J, Ricco JB, Marchand C, Lecis A, Habbibeh H, Guillou M, et al. Total laparoscopic aortic repair for occlusive and aneurysmal disease: first 95 cases. Eur J Vasc Endovasc Surg 2006;31:567-74.
- Limet R, Sakalihassan N, Albert A. Determination of the expansion rate and incidence of rupture of abdominal aortic aneurysms. J Vasc Surg 1991;14:540-8.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997;157:2064-8.
- The UK Small Aneurysm Trial Participants . The UK Small Aneurysm Trial: design, methods and progress. Eur J Vasc Endovasc Surg 1995;9:42-8.
- Lederle FA, Wilson SE, Johnson GR, Littooy FN, Acher C, Messina LM, et al. Design of the abdominal aortic Aneurysm Detection and Management Study. ADAM VA Cooperative Study Group. J Vasc Surg 1994;20:296-303.
- The UK Small Aneurysm Trial Participants . Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002;346:1445-52.
- Powell JT, Brown LC, Forbes JF, Fowkes FG, Greenhalgh RM, Ruckley CV, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg 2007;94:702-8.
- Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med 2007;146:735-41.
- Lederle FA, Johnson GR, Wilson SE, Acher CW, Ballard DJ, Littooy FN, et al. Quality of life, impotence, and activity level in a randomized trial of immediate repair versus surveillance of small abdominal aortic aneurysm. J Vasc Surg 2003;38:745-52.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003;37:1106-17.
- Schermerhorn ML, BirKaplan Meiereyer JD, Gould DA, Cronenwett JL. Cost-effectiveness of surgery for small abdominal aortic aneurysms on the basis of data from the United Kingdom small aneurysm trial. J Vasc Surg 2000;31:217-26.
- Cao P. Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) trial: study design and progress. Eur J Vasc Endovasc Surg 2005;30:245-51.
- Ouriel K. Does the availability of endovascular grafts change the size threshold for repair?. Endovascular Today; 2005.
- Ouriel K, Clair DG, Kent KC, Zarins CK. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010;51:1081-7.
- Cao P, Verzini F, De Rango P, Parlani G, Romano L, Cieri E. Results from the CAESAR Trial Comparing Observation With EVAR for Small AAAs 2009. www.veithsymposium.org/pdf/vei/2671.pdf (accessed November 2009).
- Prinssen M, Buskens E, Blankensteijn JD. The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial. Background, design and methods. J Cardiovasc Surg 2002;43:379-84.
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607-18.
- Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 2005;352:2398-405.
- De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1881-9.
- Prinssen M, Buskens E, Nolthenius RP, van Sterkenburg SM, Teijink JA, Blankensteijn JD. Sexual dysfunction after conventional and endovascular AAA repair: results of the DREAM trial. J Endovasc Ther 2004;11:613-20.
- Prinssen M, Buskens E, Blankensteijn JD. Quality of life endovascular and open AAA repair. Results of a randomised trial. Eur J Vasc Endovasc Surg 2004;27:121-7.
- Becquemin JP. The ACE trial: a randomized comparison of open versus endovascular repair in good risk patients with abdominal aortic aneurysm. J Vasc Surg 2009;50:222-4.
- Becquemin JP, Pillet JC, Lescalie F, Sapoval M, Goueffic Y, Lermusiaux P, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg 2011;53:1167-73.
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009;302:1535-42.
- Bjorck M, Gibbons CP, Jensen LP, Laustsen J, Lees T, Moreno-Carriles R, et al. Vascular registries join to create a common international dataset on AAA surgery. Eur J Vasc Endovasc Surg 2007;34:257-9.
- National Vascular Database Commitee . National Vascular Database Information n.d. www.vascularsociety.org.uk/committees/audit.asp (accessed 23 August 2007).
- Thomas SM, Gaines PA, Beard JD. Vascular surgical society of great britain and ireland: RETA: the registry of endovascular treatment of abdominal aortic aneurysms. Br J Surg 1999;86.
- Thomas S, Beard J, Wyatt MG, Watkinson AF. Endovascular interventions: current controversies. Shrewsbury: TFM Publishing Ltd; 2004.
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004;27:372-81.
- Harris PL, Buth J, Mialhe C, Myhre HO, Norgren L. The need for clinical trials of endovascular abdominal aortic aneurysm stent-graft repair: The EUROSTAR Project. EUROpean collaborators on stent-graft techniques for abdominal aortic aneurysm repair. J Endovasc Surg 1997;4:72-7.
- Harris PL, Buth J. An update on the important findings from the EUROSTAR EVAR registry. Vascular 2004;12:33-8.
- Lifeline Registry of Endovascular Aneurysm Repair Steering Committee . Lifeline registry: collaborative evaluation of endovascular aneurysm repair. J Vasc Surg 2001;34:1139-46.
- Lifeline Registry of Endovascular Aneurysm Repair Steering Committee . Lifeline registry of endovascular aneurysm repair: registry data report. J Vasc Surg 2002;35:616-20.
- Lifeline Registry of EVAR Publications Committee . Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg 2005;42:1-10.
- Research and Audit Division of the Royal Australasian College of Surgeons (RACS) . Australian Audit of Endovascular Aneurysm Repair n.d. www.surgeons.org/Content/NavigationMenu/Research/ASERNIPS/ASERNIPSAudits/Audits.htm (accessed 23 August 2007).
- Elfstrom J, Stubberod A, Troeng T. Patients not included in medical audit have a worse outcome than those included. Int J Qual Health Care 1996;8:153-7.
- Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van MC, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European collaborators on stent/graft techniques for aortic aneurysm repair. J Vasc Surg 2000;32:739-49.
- Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial participants. Ann Surg 1999;230:289-96.
- Vallabhaneni SR, Harris PL. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aortic aneurysm repair. Eur J Radiol 2001;39:34-41.
- Schlosser FJ, Gusberg RJ, Dardik A, Lin PH, Verhagen HJ, Moll FL, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted?. Eur J Vasc Endovasc Surg 2009;37:15-22.
- White GH, May J, Greenhalgh RM. The durability of vascular and endovascular surgery. London: WB Saunders; 1999.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Ruta DA, Garratt AM, Leng M, Russell IT, MacDonald LM. A new approach to the measurement of quality of life. The Patient-Generated Index. Med Care 1994;32:1109-26.
- Azizzadeh A, Sanchez LA, Miller CC, Marine L, Rubin BG, Safi HJ, et al. Glomerular filtration rate is a predictor of mortality after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2006;43:14-8.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70.
- The Renal Association . Calculation for Conversion of Creatinine to Estimated Glomerular Filtration Rate n.d. www.renal.org/eGFRcalc/GFR.pl.
- KDOQI clinical practice guidelines for chronic kidney disease . Evaluation, classification and stratification. Am J Kidney Dis 2002;39:S17-31.
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515-26.
- Little RJA, Rubin DB. Statistical analysis with missing data. London: John Wiley & Sons; 2002.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007.
- Clark TG, Altman DG. Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol 2003;56:28-37.
- Moons KG, Donders RA, Stijnen T, Harrell FE. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006;59:1092-101.
- Royston P. Multiple imputation of missing values: update of ice. Stata J 2005;5:527-36.
- Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. College Station, TX: Stata; 2008.
- Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med 2002;21:3291-315.
- EVAR Trial Participants . Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187-92.
- National Institute for Health and Clinical Excellence (NICE) . Endovascular Stent Grafts for the Treatment of Abdominal Aortic Aneurysms n.d. www.nice.org.uk/nicemedia/pdf/TA167Guidance.pdf (accessed 1 February 2009).
- Epstein DM, Sculpher MJ, Manca A, Michaels J, Thompson SG, Brown LC, et al. Modelling the long-term cost-effectiveness of endovascular or open repair for abdominal aortic aneurysm. Br J Surg 2008;95:183-90.
- The UK EVAR Trial Investigators . Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71.
- Bowen J, de Rose G, Hopkins R, Novick T, Blackhouse G, Tarride JE. Systematic Review and Cost Effectiveness Analysis of Elective Endovascular Repair Compared to Open Repair of Abdominal Aortic Aneurysm. Final Report 2005.
- Bowen J, de Rose G, Novick T, Blackhouse G, Hopkins R, Tarride JE. Systematic Review and Cost Effectiveness Analysis of Elective Endovascular Repair Compared to Open Repair of Abdominal Aortic Aneurysm. Final Report 2007.
- Bosch JL, Kaufman JA, Beinfeld MT, Adriaensen ME, Brewster DC, Gazelle GS. Abdominal aortic aneurysms: cost-effectiveness of elective endovascular and open surgical repair. Radiology 2002;225:337-44.
- Michaels JA, Drury D, Thomas SM. Cost-effectiveness of endovascular abdominal aortic aneurysm repair. Br J Surg 2005;92:960-7.
- Patel ST, Haser PB, Bush HL, Kent KC. The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: a decision analysis model. J Vasc Surg 1999;29:958-72.
- Prinssen M, Buskens E, de Jong SE, Buth J, Mackaay AJ, van Sambeek MR, et al. Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2007;46:883-90.
- Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al. Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model. Health Technol Assess 2009;13.
- National Institute for Health and Clinical Excellence (NICE) . Methods for the Evaluation of Health Technologies 2008.
- Brown LC, Thompson SG, Greenhalgh RM, Powell JT. on behalf of the EVAR Trial Participants . Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm in the randomized EVAR Trial 1. Br J Surg 2011;98:935-42.
- NHS . NHS Trust Reference Cost Schedules 2007–08 2009.
- NHS Scotland . Scottish Health Service Costs (Costs Book) 2008 2009 2009.
- National Blood Service (NBS) . National Blood & Components Price List 2009 10 2009.
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit; 2009.
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011;41:S1-S58.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19.
- Hardman G, Kind P, Macran S. UK population norms for EQ-5D. York: University of York; 1999.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45.
- Efron B, Tibshirani R. An introduction to the bootstrap. New York, NY: Chapman & Hall; 1993.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford: Oxford University Press; 2007.
- The UK EVAR Trial Investigators . Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010;362:1872-80.
- Cleves M, Gould W, Gutierrez R. An introduction to survival analysis using Stata. College Station, TX: Stata Press; 2004.
- Briggs AH, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York, NY: Oxford University Press; 2006.
- Brown LC, Greenhalgh RM, Howell S, Powell JT, Thompson SG. Patient fitness and survival after abdominal aortic aneurysm repair in patients from the UK EVAR trials. Br J Surg 2007;94:709-16.
- Kertai MD, Boersma E, Westerhout CM, van Domburg R, Klein J, Bax JJ, et al. Association between long-term statin use and mortality after successful abdominal aortic aneurysm surgery. Am J Med 2004;116:96-103.
- Brady AR, Fowkes FG, Thompson SG, Powell JT. Aortic aneurysm diameter and risk of cardiovascular mortality. Arterioscler Thromb Vasc Biol 2001;21:1203-7.
- Feringa HH, Karagiannis S, Vidakovic R, Noordzij PG, Brugts JJ, Schouten O, et al. Comparison of the incidences of cardiac arrhythmias, myocardial ischemia, and cardiac events in patients treated with endovascular versus open surgical repair of abdominal aortic aneurysms. Am J Cardiol 2007;100:1479-84.
- Heart Protection Study Collaborative Group . Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007;45:645-54.
- Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR Trials. Ann Surg 2010;252:805-12.
- Winterborn RJ, Amin I, Lyratzopoulos G, Walker N, Varty K, Campbell WB. Preferences for endovascular (EVAR) or open surgical repair among patients with abdominal aortic aneurysms under surveillance. J Vasc Surg 2009;49:576-81.
- Reise JA, Sheldon H, Earnshaw J, Naylor AR, Dick F, Powell JT, et al. Patient preference for surgical method of abdominal aortic aneurysm repair: postal survey. Eur J Vasc Endovasc Surg 2010;39:55-61.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12.
- Jean-Baptiste E, Batt M, Azzaoui R, Koussa M, Hassen-Khodja R, Haulon S. A comparison of the mid-term results following the use of bifurcated and aorto-uni-iliac devices in the treatment of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2009;38:298-304.
- Drury D, Michaels JA, Jones L, Ayiku L. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg 2005;92:937-46.
- Pitoulias GA, Schulte S, Donas KP, Horsch S. Secondary endovascular and conversion procedures for failed endovascular abdominal aortic aneurysm repair: can we still be optimistic?. Vascular 2009;17:15-22.
- Abedi NN, Davenport DL, Xenos E, Sorial E, Minion DJ, Endean ED. Gender and 30-day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. J Vasc Surg 2009;50:486-91.
- Hobo R, Buth J. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg 2006;43:896-902.
- Brown LC, Greenhalgh RM, Powell JT, Thompson SG. Use of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2010;97:1207-17.
- Barnes M, Boult M, Maddern G, Fitridge R. A model to predict outcomes for endovascular aneurysm repair using preoperative variables. Eur J Vasc Endovasc Surg 2008;35:571-9.
- Brown LC, Greenhalgh RM, Kwong GP, Powell JT, Thompson SG, Wyatt MG. Secondary interventions and mortality following endovascular aortic aneurysm repair: device-specific results from the UK EVAR trials. Eur J Vasc Endovasc Surg 2007;34:281-90.
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006;69:375-82.
- Joint Specialty Committee on renal medicine of the Royal College of Physicians and the Renal Association and the Royal College of General Practitioners . Chronic Kidney Disease in Adults. UK Guidelines for Identification, Management and Referral 2006:23-4. www.renal.org/CKDguide/full/CKDprintedfullguide.pdf (accessed 5 May 2008).
- Roderick PJ, Atkins RJ, Smeeth L, Nitsch DM, Hubbard RB, Flectcher AE, et al. Detecting chronic kidney disease in older people;what are the implications?. Age Ageing 2008;37:179-86.
- Greenberg RK, Chuter TA, Lawrence-Brown M, Haulon S, Nolte L. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA Endovascular Graft) in contrast to open surgical repair. J Vasc Surg 2004;39:1219-28.
- Gawenda M, Brunkwall J. Renal response to open and endovascular repair of abdominal aortic aneurysm: a prospective study. Ann Vasc Surg 2008;22:1-4.
- Mills JL, Duong ST, Leon LR, Goshima KR, Ihnat DM, Wendel CS, et al. Comparison of the effects of open and endovascular aortic aneurysm repair on long-term renal function using chronic kidney disease staging based on glomerular filtration rate. J Vasc Surg 2008;47:1141-9.
- Lalka S, Johnson M, Namyslowski J, Dalsing M, Cikrit D, Sawchuk A, et al. Renal interventions after abdominal aortic aneurysm repair using an aortic endograft with suprarenal fixation. Am J Surg 2006;192:577-82.
- Alsac JM, Zarins CK, Heikkinen MA, Karwowski J, Arko FR, Desgranges P, et al. The impact of aortic endografts on renal function. J Vasc Surg 2005;41:926-30.
- Parmer SS, Carpenter JP. Endovascular aneurysm repair with suprarenal vs infrarenal fixation: a study of renal effects. J Vasc Surg 2006;43:19-25.
- Cayne NS, Rhee SJ, Veith FJ, Lipsitz EC, Ohki T, Gargiulo NJ, et al. Does transrenal fixation of aortic endografts impair renal function?. J Vasc Surg 2003;38:639-44.
- Alric P, Hinchliffe RJ, Picot MC, Braithwaite BD, MacSweeney ST, Wenham PW, et al. Long-term renal function following endovascular aneurysm repair with infrarenal and suprarenal aortic stent-grafts. J Endovasc Ther 2003;10:397-405.
- Cotroneo AR, Iezzi R, Giancristofaro D, Santoro M, Pierro A, Spigonardo F, et al. Endovascular abdominal aortic aneurysm repair and renal complications: a comparison between suprarenal and infrarenal fixation of stent grafts. Radiol Med (Torino) 2007;112:252-63.
- Lau LL, Hakaim AG, Oldenburg WA, Neuhauser B, McKinney JM, Paz-Fumagalli R, et al. Effect of suprarenal versus infrarenal aortic endograft fixation on renal function and renal artery patency: a comparative study with intermediate follow-up. J Vasc Surg 2003;37:1162-8.
- Walsh SR, Boyle JR, Lynch AG, Sadat U, Carpenter JP, Tang TY, et al. Suprarenal endograft fixation and medium-term renal function: systematic review and meta-analysis. J Vasc Surg 2008;47:1364-70.
- Schouten O, Boersma E, Hoeks SE, Benner R, van UH, van Sambeek MR, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med 2009;361:980-9.
- Durazzo AE, Machado FS, Ikeoka DT, De Bernoche C, Monachini MC, Puech-Leao P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004;39:967-75.
- Poldermans D, Bax JJ, Boersma E, De Hert S, Eeckhout E, Fowkes G, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery: the task force for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery of the European Society of Cardiology (ESC) and endorsed by the European Society of Anaesthesiology (ESA). Eur Heart J 2009;30:2769-812.
- Brown LC, Greenhalgh RM, Thompson SG, Powell JT. Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair? Results from the randomised EVAR Trial 2. Eur J Vasc Endovasc Surg 2010;39:396-402.
- Greenberg RK, Francis C, Katz E, Geiger L, Roizen MF, Clair DG, et al. Survival following endovascular aneurysm repair in high-risk patients: a prospective analysis of 7 year data n.d.
- Bush RL, Hedayati N, Johnson ML, Henderson WG, Khuri SF, Lin PH, et al. Endovascular aortic aneurysm repair should be performed in high risk patients: results from the VA National Surgery Quality Improvement Program n.d.
- Sicard GA, Zwolak RM, Sidawy AN, White RA, Siami FS. Endovascular abdominal aortic aneurysm repair: long-term outcome measures in patients at high-risk for open surgery. J Vasc Surg 2006;44:229-36.
- Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Blebea J, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002;287:2968-72.
- Powell JT, Brown LC, Greenhalgh RM, Thompson SG. The rupture rate of large abdominal aortic aneurysms: is this modified by anatomical suitability for endovascular repair?. Ann Surg 2008;247:173-9.
- Brown LC, Greenhalgh RM, Powell JT, Thompson SG. on behalf of the UK EVAR Trial Participants . Use of baseline factors to predict complications and reinterventions after repair of endovascular abdominal aortic aneurysm. Br J Surg 2010;97:1207-17.
- Manning W, Fryback D, Weinstein M, Gold M, Siegel J, Russell L, et al. Cost effectiveness in health and medicine. New York, NY: Oxford University Press; 1996.
- Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048-60.
- Onohara T, Komori K, Kume M, Ishida M, Ohta S, Takeuchi K, et al. Increased plasma fibrinogen level and future risk of coronary artery disease after repair of abdominal aortic aneurysm. J Am Coll Surg 2000;191:619-25.
Appendix 1 The UK EVAR trial participants
Applicants
Professor RM Greenhalgh (lead applicant), Professor DJ Allison, Professor PRF Bell, Professor MJ Buxton, Professor PL Harris, Professor BR Hopkinson, Professor JT Powell, Professor IT Russell, Professor SG Thompson.
Data and trial management
Dr LC Brown (Trial Manager).
Statistical and costs analyses
Dr LC Brown, Mr DM Epstein, Professor MJ Sculpher, Professor SG Thompson.
Trial Management Committee
Professor RM Greenhalgh (Chair), Mr JD Beard, Professor MJ Buxton, Mr PL Harris, Professor JT Powell, Dr JDG Rose, Professor IT Russell, Professor MJ Sculpher, Professor SG Thompson.
Trial Steering Committee
Professor RJ Lilford (Chair), Professor Sir PRF Bell, Professor RM Greenhalgh, Dr SC Whitaker.
Data Monitoring and Ethics Committee
Professor PA Poole-Wilson (Chair), Professor CV Ruckley, Professor WB Campbell, Dr MRE Dean, Dr MST Ruttley, Dr EC Coles.
Endpoints Committee
Professor JT Powell (Chair), Miss A Halliday, Dr S Gibbs.
Data audit
Miss Heather Dorricott.
Regional Trial Participants Committee
Represented by one surgeon, radiologist and co-ordinator per centre. (Number in parentheses indicates the number of patients entered into both trials.)
Mr K Varty, Dr C Cousins, Addenbrookes Hospital, Cambridge (10)
Mr RJ Hannon, Dr L Johnston, Belfast City Hospital, Belfast (53)
Professor AW Bradbury, Dr MJ Henderson, Birmingham Heartlands Hospital, Birmingham (8)
Mr SD Parvin, Dr DFC Shepherd, Bournemouth General Hospital, Bournemouth (68)
Professor RM Greenhalgh, Dr AW Mitchell, Charing Cross Hospital, London (27)
Professor PR Edwards, Dr GT Abbott, Countess of Chester Hospital, Chester (15)
Mr DJ Higman, Dr A Vohra, Coventry and Walsgrave Hospital, Coventry (8)
Mr S Ashley, Dr C Robottom, Derriford Hospital, Plymouth (2)
Mr MG Wyatt, Dr JDG Rose, Freeman Hospital, Newcastle (121)
Mr D Byrne, Dr R Edwards, Gartnavel General Hospital, Glasgow (12)
Mr DP Leiberman, Dr DH McCarter, Glasgow Royal Infirmary, Glasgow (19)
Mr PR Taylor, Dr JF Reidy, Guy’s & St. Thomas’ Hospital, London (124)
Mr AR Wilkinson, Dr DF Ettles, Hull Royal Infirmary, Hull (29)
Mr AE Clason, Dr GLS Leen, James Cook University Hospital, Middlesborough (19)
Mr NV Wilson, Dr M Downes, Kent and Canterbury Hospital, Kent (1)
Mr SR Walker, Dr JM Lavelle, Lancaster General Infirmary, Lancaster (12)
Mr MJ Gough, Dr S McPherson, Leeds General Infirmary, Leeds (38)
Mr DJA Scott, Dr DO Kessell, Leeds St. James’s Hospital, Leeds (11)
Professor R Naylor, Mr R Sayers, Dr NG Fishwick, Leicester Royal Infirmary, Leicester (148)
Professor PL Harris, Dr DA Gould, Liverpool Royal Hospital, Liverpool (143)
Professor MG Walker, Dr NC Chalmers, Manchester Royal Infirmary, Manchester (96)
Mr A Garnham, Dr MA Collins, New Cross Hospital, Wolverhampton (1)
Mr JD Beard, Dr PA Gaines, Northern General Hospital, Sheffield (77)
Mr MY Ashour, Dr R Uberoi, Queen Elizabeth Hospital, Gateshead (18)
Mr B Braithwaite, Dr SC Whitaker, Queen’s Medical Centre, Nottingham (116)
Mr JN Davies, Dr S Travis, Royal Cornwall Hospital, Truro (26)
Mr G Hamilton, Dr A Platts, Royal Free Hospital, London (42)
Mr A Shandall, Dr BA Sullivan, Royal Gwent Hospital, Newport (1)
Mr M Sobeh, Dr M Matson, Royal London Hospital, London (7)
Mr AD Fox, Dr R Orme, Royal Shrewsbury Hospital, Shrewsbury (7)
Mr W Yusuf, Dr T Doyle, Royal Sussex County Hospital, Brighton (6)
Professor M Horrocks, Dr J Hardman, Royal United Hospital, Bath (34)
Mr PHB Blair, Dr PK Ellis, Royal Victoria Hospital, Belfast (46)
Mr Gareth Morris, Dr A Odurny, Southampton General Hospital, Southampton (39)
Mr R Vohra, Dr M Duddy, Selly Oak Hospital, Birmingham (22)
Professor M Thompson, Mr TML Loosemore, Dr AM Belli, Dr R Morgan, St George’s Hospital, London (54)
Mr M Adiseshiah, Dr JAS Brookes, University College Hospital, London (69)
Professor CN McCollum, Dr R Ashleigh, University Hospital of South Manchester, Manchester (127).
Trial co-ordinators
Marion Aukett, Sara Baker, Emily Barbe, Nicky Batson, Jocelyn Bell, Jo Blundell, Dee Boardley, Sheila Boyes, Oliver Brown, Jennie Bryce, Michelle Carmichael, Tina Chance, Joanne Coleman, Chryz Cosgrove, Gail Curran, Trez Dennison, Carol Devine, Nikki Dewhirst, Barry Errington, Hannah Farrell, Cathy Fisher, Paul Fulford, Moira Gough, Chris Graham, Rona Hooper, Gill Horne, Liz Horrocks, Bet Hughes, Tracey Hutchings, Marilyn Ireland, Claire Judge, Linda Kelly, Julie Kemp, Alison Kite, Milla Kivela, Michelle Lapworth, Chris Lee, Lorraine Linekar, Asif Mahmood, Linda March, Janis Martin, Nick Matharu, Kathy McGuigen, Phyl Morris-Vincent, Shirley Murray, Allison Murtagh, Gareth Owen, Vish Ramoutar, Chris Rippin, Jane Rowley, Julie Sinclair, Sarah Spencer, Victoria Taylor, Cindy Tomlinson, Sue Ward, Vera Wealleans, Julia West, Karen White, Jenny Williams, Lesley Wilson.
Appendix 2 Dates of meetings of EVAR trial Committees
Minutes for all meetings are archived at the central trial office.
Data Monitoring and Ethics Committee
-
7 June 2001.
-
18 July 2002.
-
28 May 2003.
Trial Steering Committee
-
30 November 1999.
-
30 January 2001.
-
31 October 2001.
-
7 June 2002.
-
8 August 2003.
Trial Management Committee
-
14 December 1998.
-
22 December 1998.
-
30 November 1999.
-
26 June 2000.
-
10 July 2000.
-
8 November 2000.
-
3 April 2001.
-
31 October 2001.
-
28 May 2002.
-
15 October 2002.
-
8 April 2003.
-
8 October 2003.
-
5 May 2004.
-
8 September 2004.
-
23 February 2005.
-
15 June 2005.
-
14 September 2005.
-
15 November 2005.
-
22 June 2006.
-
20 June 2007.
-
16 November 2007.
-
29 January 2008.
-
18 April 2008.
-
2 April 2009.
-
23 September 2009.
Regional Trial Participants Committee
-
5 May 1999.
-
10 November 1999.
-
26 November 1999.
-
1 November 2000.
-
30 November 2000.
-
6 November 2002.
-
21 November 2002.
-
4 November 2003.
-
27 November 2003.
-
25 November 2004.
-
23 November 2005.
-
22 November 2006.
-
29 November 2007.
Trial Endpoints Committee
-
3 March 2005.
-
31 January 2006.
-
15 May 2007.
-
17 April 2008.
-
5 May 2009.
-
17 December 2009.
Appendix 3 Protocol for EVAR trials
Summary
Training centres for the use of endovascular stent grafts for abdominal aortic aneurysm (AAA) repair will be established and progress audited in a National Society Registry. Trial co-ordinators at initially 13 UK centres will be trained at Charing Cross Hospital in correct protocol procedures and collection of health-related quality of life (HRQL). Trained operators will enter patients undergoing AAA repair into randomised trials of (1) EVAR vs. Open repair (OR) in fit patients and (2) EVAR plus best medical treatment vs. best medical treatment in patients unfit for OR. Each trial will compare EVAR against current best alternative in terms of mortality, durability, safety and costs as well as generic and patient specific health-related quality of life (HRQL). 1180 patients will be entered over 4 yrs, 900 in trial 1 and 280 in trial 2.
Benefits the proposed investigation will bring to the NHS
The investigation will support the findings of the Joint Working Party for the Vascular Surgical Society of Great Britain & Ireland (VSS) and the British Society of Interventional Radiologists (BSIR), to bring the disciplines together for the introduction of endovascular grafting of abdominal aortic aneurysm and maintain the Registry of Endovascular Treatment of Aneurysms (RETA) which was initiated on the 1st January 1996 by Mr Jonathan Beard of the Sheffield Vascular Institute. Centres will be provided in Nottingham, Leicester, Liverpool and Newcastle to train surgeons and radiologists together (the operators) according to the VSS and BSIR Guidelines. Trainee learning will be by open audit (RETA) with feedback and provide a model for future surgical and interventional radiological technology assessment during development. Learning curves of both operators and newly introduced stent graft systems can be thus checked before introduction. Currently, trainers are finding that approximately 20 EVAR procedures are needed for training the surgeon and radiologist working together. Trial findings will indicate degree of safety, efficacy and durability of new EVAR systems as they are introduced and in fit patients to establish the value of EVAR against conventional abdominal aortic aneurysm (AAA) open repair (OR) with respect to mortality, durability, safety, costs, and quality of life. The investigation should also show if EVAR has any place in the management of patients with AAA unfit for conventional open repair (OR). Findings could markedly reduce the costs for treatment of all AAAs and provide potential to reduce bed occupancy and increase patient satisfaction. A Cochrane Review will be initiated.
Background to the project
The incidence of abdominal aortic aneurysm in England and Wales has been increasing. From 1950 to 1984 age standardised mortality rose twenty fold in men to 47.1 per 100,000 population and eleven fold in women to 22.2 per 100,000. 1 The authors concluded that the trends were not wholly compatible with increases in diagnosis and surgery because there were inconsistencies by age and sex and increases had occurred in the number of complicated as well as uncomplicated cases. Similarities to the trends were noted in North America, elsewhere in Europe and Australasia and so the authors concluded that there was a true increase in the incidence of abdominal aortic aneurysms. At the beginning of this decade Parodi, Palmaz and Barone in Argentina2 and Volodos in the Ukraine introduced EVAR in sicker patients with shorter hospital stay. These pioneers used hand made stent graft systems beginning with a repair to lie entirely within the abdominal aorta (aorto-aortic graft). Subsequently it has been shown that the aorto-aortic EVAR can be used in less than 10% of patients and bifurcation systems have been developed which enable approximately 25% of AAA to be managed by an EVAR method. 3 “Home-made systems” have been introduced in this country in Nottingham4 and Leicester. 5 These systems have employed an aorto uni iliac EVAR system. The second side is occluded using a Dacron sac and stent and the procedure completed with a femorofemoral crossover graft just leaving the patient with 2 small incisions in the groins and minimum pain. The Nottingham group4 have shown recently that using their system, 75% of all AAA could be managed by EVAR.
The applicants are ideally placed to carry out the proposed research for a number of reasons. The MRC supported multicentre Femoropopliteal Bypass Trial and UK Small AAA Trial6 have given valuable experience in multi-centre vascular surgical trials in Britain. There is an excellent network of collaboration in vascular surgery in Britain and the applicants are well placed in the VSS (Bell, President Elect 1999, Greenhalgh, President Elect 2000). The collaboration extends through the joint working party to the officers of the BSIR (President 1999 Professor A. Adam). Such national collaboration is no better established in any other country at present but other European countries will be encouraged to copy our trial protocols with a view to the possible pooling of data. There is also interest in Canada and Australia to enter patients into our trial. The applicants have demonstrated their ability in the UK Small AAA Trial to recruit according to schedule, document carefully and achieve a result (published in The Lancet November 1998). Facilities are in place to assess costs (Brunel) and Health-related quality of life (York). The UK Small AAA Trial has indicated that we can expect to recruit about 1000 patients fit for conventional surgery (OR) over 4 years and during that time approximately 70 patients per annum will be seen with AAA who are unfit for OR. Outside the UK small AAA trial, patients deemed unfit for surgery had a 22% mortality at 10 months (vide infra) and 50% mortality at 2 years with best medical treatment.
The Registry for Endovascular Treatment of Aneurysms (RETA)
The National RETA registry was initiated in January 1996 to audit “home-made” and commercially available EVAR systems deployed within the UK. Annual audits have been conducted and reports are available to the EVAR Trial Management Committee, principally to be advised when centres are trained.
According to the 1998 data, patients have been classified as either fit or unfit for open repair (OR). The proportions of each are given in Figure 1 and represent the distribution of patients that would enter EVAR Trial 1 (fit for OR) or EVAR Trial 2 (unfit for OR). It is clear that the operative mortality at 30 days is significantly worse for unfit patients (χ2 = 23.4, p < 0.001).
FIGURE 1.
RETA data for 30 day EVAR mortality (1998) according to fitness for Open Repair (OR), (n = 239).
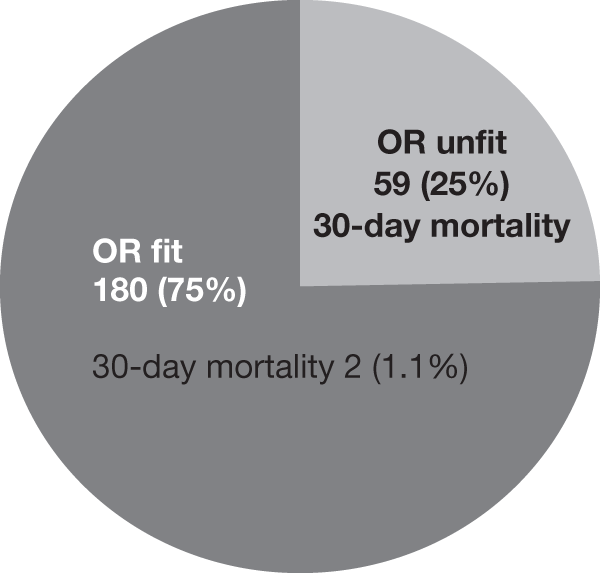
In patients suitable for open repair the data for 1996, 1997 and 1998 (Figure 2) show decreasing 30 day mortality. It must be remembered that not all EVAR procedures in the UK are recorded in these data.
FIGURE 2.
30 day post operative mortality for patients classified as fit for open repair (OR). Data taken from RETA annual reports from 1996 to 1998 (n = number of EVARS performed in that year).
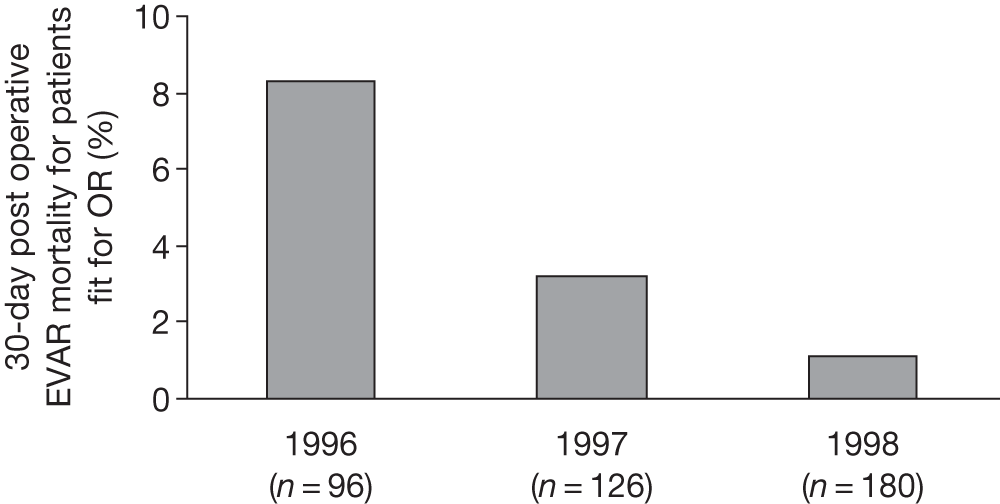
EVAR is currently being used both for fit for OR patients (75%) and unfit for OR patients (25%). Consequently it is appropriate to pose the question of the original NHS R&D HTA commissioning brief what is the cost-effectiveness ofaortic stenting -v- other innovative methods -v- OR for elective AAA’s? Currently the accepted alternative to EVAR is open repair (OR) in patients who are fit enough for the procedure. For those that are not fit for OR, EVAR is currently being used as an adjunct to best medical treatment. Should it be? Can best medical treatment be “innovative”? We have shown that smoking increases the growth rate of small abdominal aortic aneurysms7 and so after EVAR one can no longer expect the aortic dimensions proximal and distal to the stents to remain constant if a patient continues to smoke. Consequently innovative best medical treatment could involve the setting up of smoking advice clinics using nicotine replacement therapy in the trial centres with measurement of smoking markers for compliance. Careful control of blood pressure including reduction in pulse pressure should be advocated. EVAR procedures are being performed in the UK on patients less than fit for OR and this is a potentially expensive exercise for the NHS and the appropriate trial would be to assess any adjuvant benefit of EVAR beyond current best medical practice, particularly any treatment which can slow the expansion of the aortic aneurysm.
In considering a random allocation trial EVAR v OR, it is argued that the operative mortality for the commercially available stent grafts is very low. Blum et al. in Freiburg, Germany using the Mintek System in 140 patients, reported a 0.7% 30d mortality. 8 Moore et al. 9 in North America reported a 33% 30d mortality in 30 patients using another commercially available device. The Eurostar Audit of Systems in Europe has data on 400 procedures with a 30d mortality of 4% for mainly commercially available systems. 10 Presently commercially available systems can only be used in up to 25% of AAA and generally in the less diseased or extensive AAA with suitable anatomical dimensions. We had no alternative but to base our calculations on the pilot data of RETA which included aorto-uni iliac data of “home-made systems” which brings to 75% the proportion of patients correctable by EVAR. 11
The UK Small Aneurysm Trial
The results of The UK Small Aneurysm Trial were reported in two back-to-back papers published in The Lancet on November 21st 1998. 6 During the 4 years of recruitment from August 1991 to 1995, 1090 patients aged 60 to 76 presenting with asymptomatic, infrarenal AAA sized between 4.0 and 5.5 cm were randomised either to regular ultrasound surveillance or elective open repair. Patients were followed for a further 3 years in terms of mortality, cost-effectiveness and health-related quality of life. Kaplan–Meier survival analysis indicated that surgical intervention for abdominal aortic aneurysm was not justified in terms of all-cause mortality, cost-effectiveness or health-related quality of life. Survival was similar in both groups and regular surveillance was found to be a safe and reliable mode of treatment to monitor the aneurysm until it grew to 5.5 cm, became tender, grew fast (> 1.0 cm/year) or ruptured. The 30 day operative mortality for patients randomised to elective surgery in the UK Small Aneurysm Trial was 5.8% and an annual rupture rate of 1% was found. Accordingly, no benefit was found for early surgical intervention (within 3 months of randomisation for AAA 4.0 – 5.5 cm). Instead, surveillance to 5.5 cm was seen to be better. We see no reason to modify these findings for EVAR at this stage and AAA ≥ 5.5 cm will be considered for surgery. From the Freeman Hospital, Newcastle, Berridge et al reported a 5 year prospective audit on 1,131 patients undergoing surgery for AAA from 1988 to 1992. 12 The teaching hospital 30d mortality was 3.9% and DGH mortality 12.0%. The audit showed a far greater mortality for over 80 year olds (23.8%), compared with under 80 (7.6%). Hopkinson in Nottingham has also found higher mortality in > 85 year old patients undergoing EVAR. 11
Plan of investigation including research methodology proposed
Trial management structure
Data Monitoring and Ethics Committee (DMEC)
This has been convened by Professor Philip Poole-Wilson, (Professor of Cardiology, National Heart & Lung Institute, Brompton Hospital) who has kindly agreed to chair the DMEC. Membership includes 2 representatives of The Vascular Surgical Society of Great Britain & Ireland (VSSGBI), namely Professor CV Ruckley (Edinburgh) and Mr WB Campbell (Exeter) and also 2 representatives of The British Society of Interventional Radiology (BSIR), namely Dr MRE Dean (Shrewsbury) and Dr MST Ruttley (Cardiff) as agreed with their councils. Dr EC Coles (Cardiff) has agreed to act as the statistical representative for DMEC. The DMEC will communicate with the Trial Steering Committee (TSC). The DMEC and Trial Management Committee (TMC) will together discuss stopping rules. Audit of the data is “closed” as well as being device, operator and centre specific. Information from EVAR procedures elsewhere may be fed into the DMEC and the manufacturer will be able to feed in details of product modification. The DMEC may wish to meet EVAR manufacturers from time to time as an EVAR comparative audit will be performed as subgroup analyses by DMEC (TRACKER TRIALS).
Trial Steering Committee (TSC)
This will meet as required. Professor Richard Lilford has accepted the chair. The TSC would include Roger Greenhalgh for the applicants and Trial Management Committee. Surgical and radiological input will be supplied by the operators at the participating centres who will serve on the TSC on an annual rotation basis. There should be patient representation on this committee which will receive constant input from the DMEC and TMC. It is expected that patient representation will involve participation from patients treated with both open repair and EVAR.
Trial Management Committee (TMC)
This is concerned with the day to day running of the EVAR trials and relates to both the DMEC and Trial Steering Committee. It will be chaired by Roger Greenhalgh and includes Simon Thompson (statistics), Ian Russell (HRQL), Jonathan Beard (RETA), Janet Powell (best medical), Martin Buxton (costs). There is also one participating surgeon and radiologist or representatives of them who serve on this committee on an annual rotation basis. The committee is convened by Louise Brown.
Regional Trial Participants Committee (RTPC)
This includes a surgical and radiological representative of each participating centre and is convened by Louise Brown as required and requested by trial centres, training centres and the trial co-ordinating centre whenever the need arises but usually at annual meetings such as the VSS and BSIR.
The training of surgeons and radiologists (operators) and trial co-ordinators
Surgeons and interventional radiologists (Operators) will be trained in Nottingham (Hopkinson) and Leicester (Bell) for home-made aorto-uni iliac systems. Training for the commercially available ‘Vanguard’ bifurcation system (Boston Scientific) will be in Liverpool (Harris) and Newcastle (Wyatt). In addition Gough (Leeds) has offered training for the Endovascular Technology (EVT) device and Adiseshiah (UCL) could train for the World Medical Talent Graft. In addition to the six training centres mentioned, and the National Registry (RETA) in Sheffield and the Trial Co-ordinating Centre in London (Charing Cross), the following centres are trained and have agreed to take part in the trial: Bournemouth (Parvin), Guy’s (Taylor), Hull (Wilkinson), Manchester Royal (Walker), Manchester Withington (McCollum). Other centres can come on stream when trained and will submit experience to Sheffield.
The success of the new technique is thought to be highly device, operator and centre dependant and therefore hospitals need to demonstrate competence at performing the new procedure before it can realistically compete with current alternative best medical or surgical practice. Trial co-ordinators at centres entering patients will be trained before the first patient is entered and skills will be checked during the trial and compared between centres.
Role of supporting hospitals
It is important that patient recruitment is as high as possible. Each trained regional centre also acts as a specialist centre in its own area. It may be possible for surrounding non-vascular specialist hospitals to support recruitment by referring vascular patients believed to be suitable for the EVAR Trials to that regional centre. If anatomically suitable for EVAR and agreeable to randomisation the patient receives treatment at the regional centre. Thus all EVAR, OR, best medical treatment and follow-up is performed at the regional centre.
Generalisability
It is of particular importance that patients found to be unsuitable for an EVAR device are recorded at initial consultation. Numbers of unsuitable patients and reasons for this will determine what proportion of AAA patients are anatomically suitable for an EVAR device at the national level. It is thought that certain centres, e.g. Liverpool, Leicester, Sheffield and Bournemouth act as both the “DGH” and “regional centre” for their area. These centres could be ideal for assessing generalisability according to postcode of patient being treated. These centres could give more reliable population information about the proportion of patients across the land who could be treated by EVAR.
Entry criteria
Age at least 60 years
A minimum age of 60 years is chosen as surgeons may wish to manage patients under 60 years in a different way because frequently there is an associated genetic cause where expansion rates and extent of AAA may be extreme, such as Marfan syndrome. No upper age limit is thought necessary as very elderly patients may benefit from the use of an EVAR device and their recruitment will be important for achieving the numbers required.
Size of AAA
The criterion for entry into both trials is an AAA diameter measuring ≥ 5.5 cm according to a CT scan. The UK Small Aneurysm Trial has shown that it is safe to leave abdominal aortic aneurysms until they reach this size. However, reproducibility differences between Duplex Ultrasound and CT scanners can lead to significant variation in AAA diameters. Duplex scanning tends to produce AAA diameters smaller than CT scanning and therefore we recommend that patients presenting with a ≥ 5.0 cm AAA on Duplex should be sent for a CT scan to determine whether the AAA is ≥5.5 cm in any diameter on CT scan and thus suitable for EVAR Trial entry. Tender AAA and contained ruptures may be included provided the AAA measures at least 5.5 cm on a CT scan and suitable EVAR equipment is available at such short notice. Tender AAA < 5.5 cm requiring surgery will only have the options of open repair or surveillance.
Anatomical suitability for EVAR
This is assessed usually by spiral CT or conventional CT combined with conventional angiography with a marked catheter to enable the calculation of length. The training centres differ in their methods of measuring the tortuous length of the abdominal aorta. This measurement is extremely important in calculating the precise length of the EVAR system used. The learning curve of every operator indicates that there is a repeated tendency for a graft system to be chosen too short. A surgeon is used to fixing the upper end at open repair and cutting the prosthetic graft to length before fixing the lower end. With EVAR the lengths must be carefully measured in the pre-operative period and even then errors can occur. The precise measurement particularly of the axial length of the aneurysm is critical for good results. The trial centre radiologist will require special training in these calculations which will be checked at training centres and by the commercial companies involved until proficiency is achieved. The trial co-ordinator must work closely with the local radiologist and appropriate training centre and document how the AAA was assessed and how the size and type of EVAR device was selected.
Patients found to be unsuitable for an EVAR device are not flagged for mortality at The Office of National Statistics (ONS) but reasons for unsuitability are collected. Patients referred from supporting hospitals are returned there for treatment.
Fitness for surgery
This is determined locally by the surgeon, radiologist, anaesthetist and cardiologist. It was originally thought that ASA grades I, II and III would indicate entry to EVAR Trial 1 and ASA IV patients would permit entry into EVAR Trial 2. However, despite the simplicity of ASA grade it can be open to different interpretation at each centre and has proved too difficult to use as a classification system for EVAR Trial 1 or 2. Recently, more sophisticated tests have not been good predictors of outcome in vascular surgery. 13 It has been appreciated during the UK Small Aneurysm Trial that fitness “inflation” has emerged with respect to the size of aneurysm. Patients who were earlier described as “unfit for OR” and later developed a larger aneurysm were suddenly deemed “fit for the procedure”. This could equally happen for these current trials. For the purposes of pragmatism, fitness is determined at the local level for these trials. Recommended guidelines on cardiac, respiratory and renal status have been provided as outlined in Figure 3 and baseline data will be used to assess fitness of randomised patients at the final analysis. These guidelines may help provide some conformity of fitness classification for EVAR Trial 1 or 2.
FIGURE 3.
Recommended guidelines for assessment of patient fitness for open repair and suitability for EVAR trial 1 or 2.
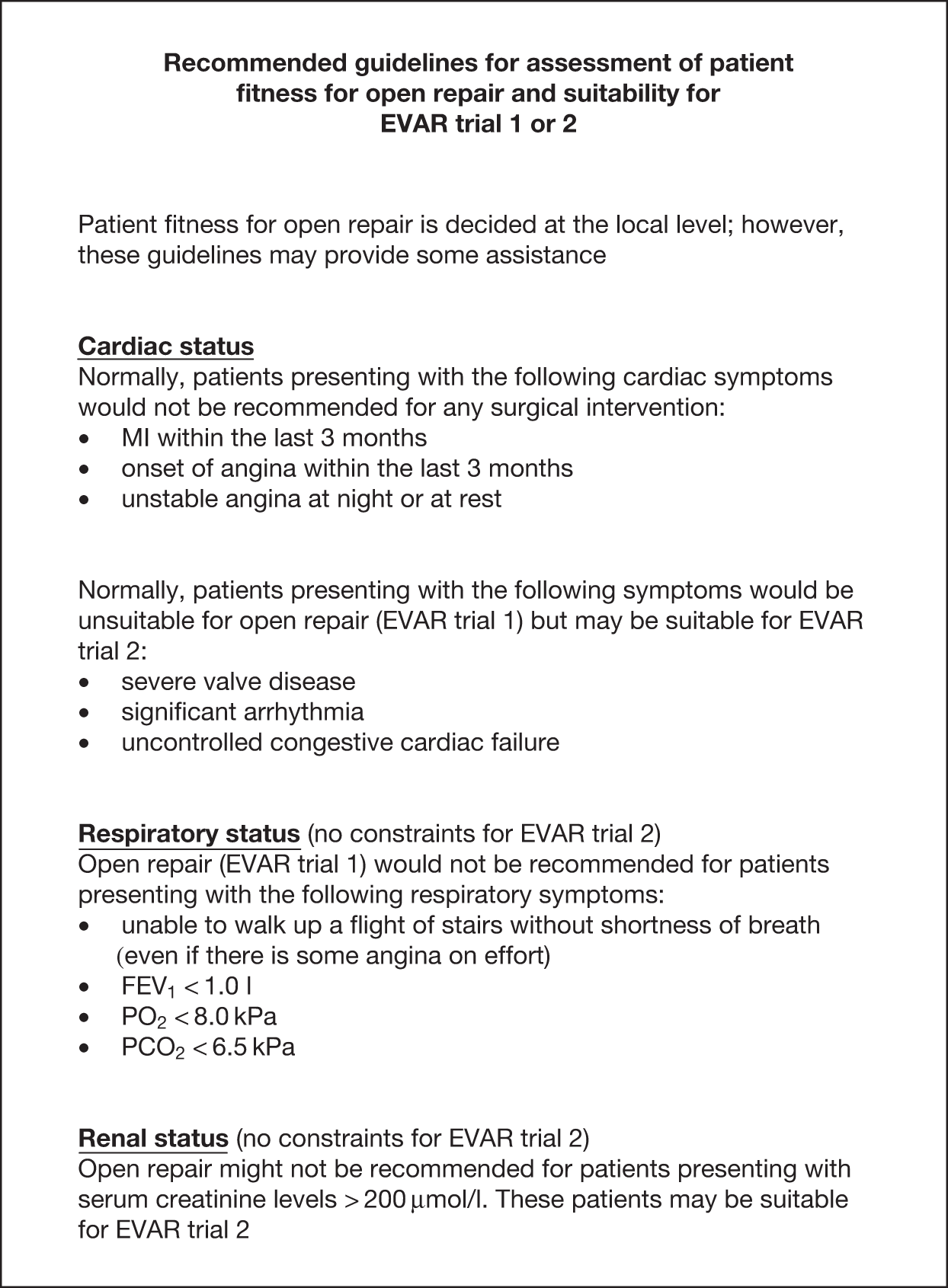
Randomisation
This is performed at Charing Cross, where randomisation tables have been produced using the Stata 6.0 statistical package. Randomisation is stratified by centre.
Trial 1
Consideration has been given to whether we should seek patient preference but the majority view is that trialists are truly uncertain of whether OR or EVAR is preferable for patients short term or long term and so the equipoise position will exist from which randomisation to OR or EVAR can occur. We aim not to introduce the matter of patient preference but hope for maximum recruitment into 50 : 50 random allocation. However if patient preference emerges we shall respect it and note outcomes. It is our understanding that the EVAR device is currently not available on the NHS except as part of these randomised controlled trials. We feel that on balance, if we introduce the concept of patient preference, this could lose randomised numbers and tend to bias patients when in fact trialists truly do not know which procedure is better.
Trial 2
For the OR unfit group the ethical considerations are more difficult. The trialists are inclined to pursue a randomised trial here because we are being pressed to use EVAR in these patients. Randomisation should be between EVAR and best medical treatment against best medical treatment alone. Best medical treatment will be offered to the whole group. Smoking advice will be given and hypertension will be carefully controlled and monitored. The patients will be asked if they will be prepared to have an EVAR device in the future and if so to have CT scan or angiogram to see if their aorta would be potentially suitable for correction by an endovascular device should this be required. Patients will then be randomised to receive EVAR or not. The risks of EVAR and the potential for needing to correct by urgent open repair would be described. Undoubtedly some patients would not wish to undergo randomisation and this patient preference would be respected. Others will press for EVAR and trialists believe we should see if we can recruit patients prepared to be randomised. If trialists explain to patients that EVAR could be beneficial but that there is no certainty, equipoise could be achieved with some difficulty. The alternative is that some surgeons will just put them in and other centres will put in no EVAR devices. The role of a monitoring committee would be vital here as it must be possible to say to a patient that outcomes are being monitored and if EVAR looks beneficial it will be offered to that patient later. It is considered that patient preference should not formally be sought but if during the discussion before randomisation, a strong patient preference emerges this will, of course, be recognised and randomisation only applied to the equipoise patients, but no NHS funding is available for EVAR devices except as part of the randomised controlled trials, EVAR 1 & 2.
Figure 4 demonstrates the entry protocol for patients into both trials.
FIGURE 4.
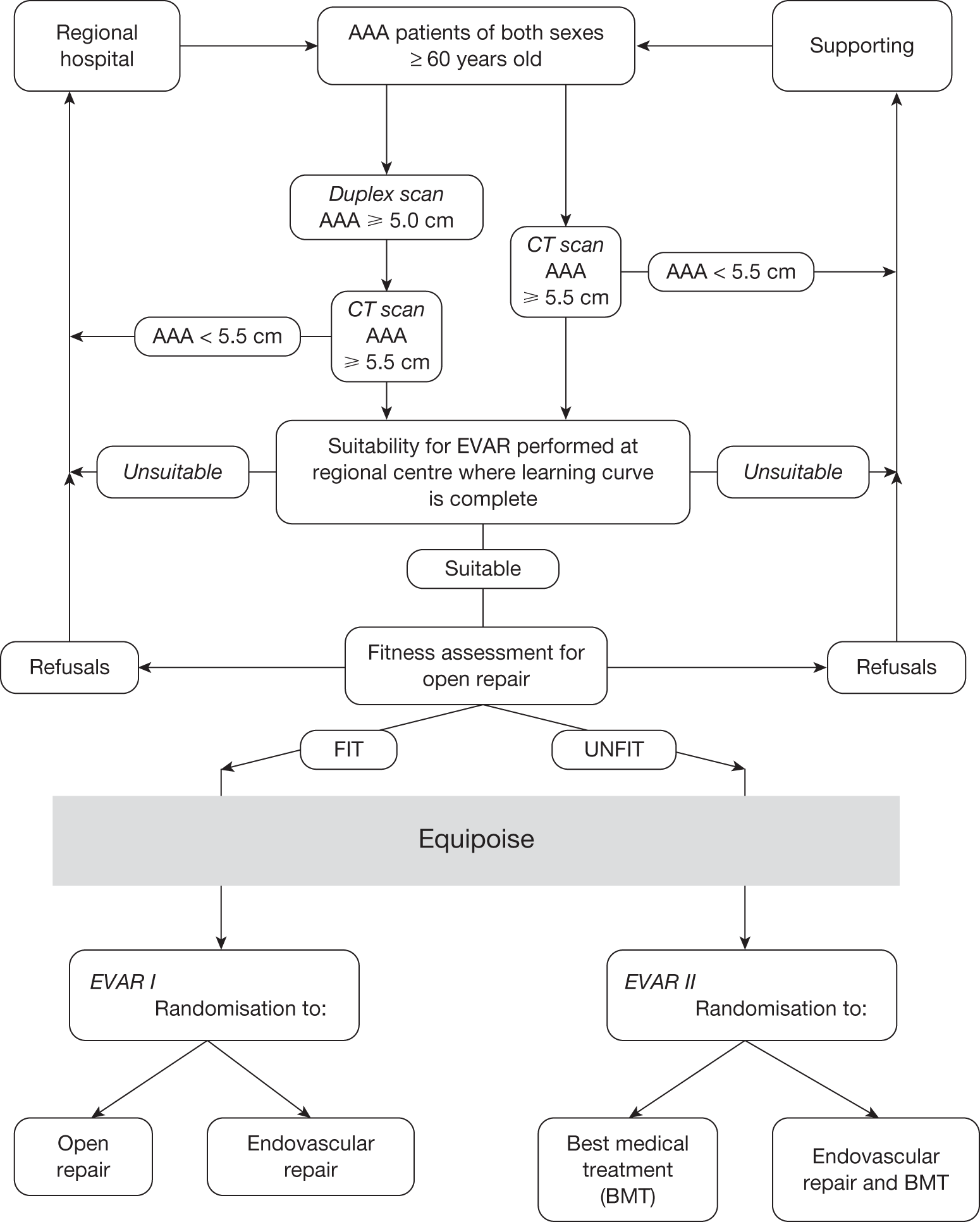
Triggering of treatment costs on randomisation to an EVAR device
The use of stents over open repair carries a significant increase in treatment costs. Following negotiations with The NHS Executive (North Thames London Region) it was agreed that treatment costs may be reimbursed to each trial centre on randomisation for an EVAR device. Service costs are unlikely to be funded. An assessment of costs was carried out to ascertain the excess treatment cost expenditure associated with an EVAR repair over an open repair (OR) or best medical treatment. According to Höltzenbein et al. 14 80% of costs associated with AAA repair can be accounted for by, 1) total length of stay, 2) days in ITU, days in HDU, 3) theatre costs. Estimates were made and are given in Figures 5 and 6. Thus, a patient randomised for EVAR in EVAR Trial 1 will require £6,465 additional funding triggered to the relevant NHS provider Trust on a named patient, named operator and named centre basis. Similarly, a patient randomised to EVAR in EVAR Trial 2 will require £9,139 of triggered funding.
FIGURE 5.
Treatment costs of EVAR (EVAR trial 2) and net costs over OR (EVAR trial 1).
| Treatment costs | EVAR | OR |
|---|---|---|
| Theatre, surgeon, anaesthetist, nurse, sutures, current device* | 3.7 hours = £924 | 3.7 hours = £924 |
| AAA repair device** | £5,000 | 0 |
| Wires, catheters for radiologists** | £800 | 0 |
| Consultant radiologist*** | £378 | 0 |
| (2 day) | ||
| Senior radiographer grade I*** | £146 | 0 |
| (2 day) | ||
| Radiology nurse, grade F*** | £141 | 0 |
| (2 day) | ||
| Post operative CT scans (£250 each) at 1/12, 3/12, 6/12, 1, 2, 3, 4 years | £1,750 | £1,750 |
| Totals | £9,139 | £2,674 |
FIGURE 6.
Outline of service costs for EVAR and Open Repair (OR).
| Service costs | EVAR | OR |
|---|---|---|
| Pre operative duplex and CT scans* | £513 | £513 |
| Pre operative assessment days** | 2 days = £224 | 1 day = £112 |
| (standard rate, £112 per day)* | ||
| Post operative ITU days** | 0 | 1 day = £797 |
| (standard rate, £797 per day)* | ||
| Post operative HDU days** | 7 days = £2,786 | 0 |
| (standard rate, £398 per day)* | ||
| Post operative standard days** | 0 | 9 days = £1,008 |
| (standard rate, £112 per day)* | ||
| Totals | £3,523 | £2,430 |
Financial provision for complete data collection
It is essential that high quality data is collected for all patients randomised in the EVAR Trials. To encourage good data retrieval, trial co-ordinators based at each of the 13 participating centres will be paid an additional amount of money on receipt of clean and complete data at Charing Cross. An estimate has been made of the length of time a trial co-ordinator will take to complete the case report forms, (1 hour for a baseline assessment and 20 minutes for a follow up appointment). A £25 payment will be made for each complete baseline assessment and a further £25 payment for the operation data. A £25 payment will also be made on receipt of each complete set of follow-up data.
Outcome measures
Mortality
The primary endpoint for both trials is all-cause mortality.
All-cause mortality for EVAR Trial 1
Patients randomised to open repair in the UK Small Aneurysm Trial experienced an annual all-cause mortality of 7.1%. In the EVAR Trials patients are undergoing AAA repair for larger aneurysms and we have assumed an annual mortality rate of 7.5%. If EVAR can reduce this mortality to 5% per year then EVAR might be justified as a viable treatment alternative for AAA. By the end of the recruitment phase we need to randomise 900 patients into EVAR Trial 1. Patients will be followed until April 2005 and this will accumulate an average follow-up of 3.33 years per patient. This produces 80% power at the 5% significance level.
All-cause mortality for EVAR Trial 2
Patients with large AAA considered unfit for open repair in the UK Small Aneurysm Study were followed up for AAA growth and rupture and were shown to have an annual all-cause mortality of 25%. The RETA registry has shown that patients considered unfit for open repair who have been treated with EVAR have an annual all-cause mortality of 15%. By the end of the recruitment we need to randomise 280 patients into EVAR Trial 2. Patients will be followed until April 2005 and this will accumulate an average follow-up of 3.33 years per patient. This produces 90% power at the 5% significance level to detect a difference of 10% between the two treatment regimes.
30-day operative mortality in EVAR Trial 1
From The UK Small Aneurysm Trial data, 30 day operative mortality was calculated for patients who were randomised to observation but whose aortic aneurysms subsequently grew to > 5.5 cm when surgery was performed (n=191). 11 were dead at 30 days leading to a 30-day operative mortality of 5.76%. Power and sample size calculations were performed using 5.76% for open repair 30-day mortality and the RETA 30 day mortality figures for 1996 to 1998. Figure 7 shows that with 900 patients randomised into EVAR Trial 1 we should also have 90% power at the 5% significance level to detect a difference in 30 day operative mortality of 5.76% in the open repair arm compared to 1.5% in the EVAR arm.
FIGURE 7.
Numbers of patients required for EVAR trial 1 to detect a difference in 30 day operative mortality between Open Repair (OR) at 5.76% and EVAR mortality figures according to year of RETA audit.
| Open repair [UK Aneurysm Trial] | EVAR [Original grant application] | EVAR [RETA 1996 data] | EVAR [RETA 1997 data] | EVAR [RETA 1998 data] | |
|---|---|---|---|---|---|
| Number dead at 30 days | 11 | 6 | 8 | 4 | 2 |
| Total operated | 191 | 91 | 96 | 126 | 180 |
| 30 day operative mortality | 5.76% | 6.6% | 8.3% | 3.2% | 1.1% |
| Numbers required per group (total recruitment) to detect difference between EVAR and OR | 17,504 (35,008) | 2,205 (4,410) | 1,448 (1,896) | 361 (722) | |
Sample size calculations are calculated to provide 90% power at the 5% significance level.
The incidence of endoleaks from EVAR (safety of procedure)
A CT scan is performed on all EVAR patients in the first month after operation seeking endo-leak. Endoleak is extremely important to find particularly at the top end where blood flow between the stent graft system and the aortic wall can increase pressure on the aortic wall, greater than if the stent graft system was not in place. If uncorrected, mortality follows. Endoleak is checked at the time of the procedure with contrast radiography but if the upper end works loose, endoleak from there could occur and is best detected (at this state of knowledge) by CT scan with contrast. Additional procedures to correct endoleak such as the use of additional stents with covered grafts will be carefully noted. This is an important outcome measure and critical to assure safety and efficacy of the procedure. It will also affect costs and patient anxiety. Endoleak is conveniently classified in the manner suggested by Geoffrey White of Sydney, Australia:15
-
endoleak type I perigraft leak at proximal or distal end
-
endoleak type II retrograde endoleak from patent lumber artery, inferior mesenteric artery, intercostal artery or other (renal, internal iliac, subclavian etc.)
-
endoleak type III fabric tear
-
endoleak type IV graft porosity
-
endoleak type V endopressure.
Health-related quality of life (HRQL)
In measuring HRQL a combination of specific and generic instruments is recommended e.g. 16 specific instruments are useful for clinical evaluation; their narrow focus makes them more responsive to small but clinically important changes in health. Generic instruments are useful for economic evaluation and for comparisons across groups of patients; their comprehensive nature also enables them to detect unforeseen effects of treatment. There are two main types of generic instrument – health profiles and utility measures. Health profiles measure HRQL across a number of distinct dimensions and thus assess the effect of health care on different aspects of HRQL. Utility measures incorporate the values that individuals attach to HRQL and thus produce a single index of HRQL suitable for economic evaluation.
The portfolio of instruments to measure HRQL in the proposed trials is designed to be comprehensive yet brief. It will be completed by patients in the form of a questionnaire – at recruitment and subsequently one, three and 12 months after surgery or the beginning of medical treatment as appropriate. The questionnaire will include two generic instruments – the Short-Form 36-item (SF-36) Health Survey and the EuroQol. The SF-36 is a health profile comprising eight distinct scales including physical and social functioning, role limitation, mental health, vitality, pain and general health. 17 It has been shown to be valid, reliable and responsive to changes in health in British patients. 18–20 The EuroQol is a validated utility measure comprising five items covering mobility, self care, usual activities, pain, anxiety and depression. 21 The HRQL states defined by the various combinations of responses to these items have been valued by the general public for use in cost-utility analysis. Unfortunately we know of no specific instrument designed to measure HRQL in patients suffering from AAA; this has been confirmed by a recent systematic search of MEDLINE. One likely reason for this lack is the wide range of effects that this condition has on patients. In these circumstances we propose to use the State Trait Anxiety Inventory (STAI)22 which encompasses both the state form (transitory feelings of fear or worry) and the trait form (the stable tendency to respond anxiously to stressful situations or proneness). The STAI measures in-built tendency to anxious response and current feelings of anxiety. It enables the investigator to distinguish between the transitory (state) and the dispositional types of anxiety.
We also propose to use The Patient Generated Index (PGI). This is a quasi-specific HRQL instrument that focuses on the concerns of the individual patient with a given condition rather than concerns derived by the investigator for the typical patient with that condition. 23 Patients nominate and rate on a scale the five most important aspects of their lives affected by their health. The final score represents the gap between their current health status and their expectations in those areas of their lives in which they would most value an improvement. Thus the PGI measures the effect of the condition on quality of life as defined by the patient. There is good evidence for the acceptability, validity, reliability and responsiveness of this instrument.
Economic evaluation
Within each of the two sub-trials the type and extent of economic evaluation will depend crucially upon the clinical outcome of that trial:
-
If one technology produces a clinically better outcome than another at significantly lower cost, then clinical and financial criteria both lead to the same conclusion.
-
If there is no clinically significant difference in outcome between two technologies under comparison, then the least cost option is preferable (cost minimisation analysis24).
-
If one technology produces a clinically better outcome than another at higher cost, then we shall undertake marginal cost-utility analysis24 (based on mortality and the EuroQol21) and, if appropriate, marginal cost-effectiveness analysis24 (based on the Patient Generated Index23).
-
NHS costs will be collected. These will include the length of time in hospital (subdivided into intensive care, high-dependency care, acute care and convalescent care), and theatre costs (subdivided into the length of operation and the use of staff, tests and drugs).
Under scenario 3 we shall use the EuroQol to estimate changes in health utility. One advantage of using the EuroQol is that it expresses changes in HRQL on a ratio scale. Thus cost–utility ratios in the form of cost per quality-adjusted life year (QALY) can be constructed from changes in mortality (if any) and in HRQL. Comparisons can then be made with other health care interventions. If there is no significant change in mortality, however, care will be needed because the EuroQol is less responsive to change than most condition-specific measures. To reduce the possibility of a Type II error, we shall also undertake a cost-effectiveness analysis based on the PGI.
We shall subject our results to extensive sensitivity analysis. First we shall identify the critical components of the cost and outcome by varying all estimated parameters in the analysis individually, to see how the economic findings are affected. Those parameters which lead to substantial changes in these findings will be varied over plausible ranges in combination to see whether the main conclusions are altered. 25
As this economic evaluation is being undertaken alongside a randomised trial, both cost and outcome data will be subject to random variation. Therefore we shall estimate confidence intervals for costs, outcomes, cost–utility ratios and cost-effectiveness ratios. The last two will use the resampling technique known as bootstrapping. 26
Follow-up
All trial patients will be ONS flagged for mortality. HRQL data will be collected at 1, 3 and 12 months following treatment for those allocated to an operation. However, for patients randomised to best medical treatment in EVAR Trial 2 we have incorporated a 1 month delay for the early follow-up in these patients. This takes into account the estimated 1 month delay patients will experience waiting for their EVAR procedure in the EVAR arm of trial 2. For cost-effectiveness the 2 page EuroQol questionnaire will also be collected annually throughout the period of follow-up.
Cost evaluation will be based on operation costs and in patient admissions during the course of follow up. The incidence of any adverse events will also be collected at every follow-up appointment, e.g. tender AAA, ruptured AAA, conversion to open repair, myocardial infarction, stroke, renal failure and amputation. CT scan will be used for assessment of growth rates, persistent endoleaks and durability which could vary with stent graft type. CT scan follow-up will be at 1 and 3 months, 1 year, 2 years, 3 years and 4 years for EVAR patients in trial 1 or 2. CT scan follow up will be performed annually for patients randomised to EVAR Trial 1 OR. CT scan follow up will be annually for best medical treatment patients in EVAR Trial 2. Creatinine will be recorded annually for all patients to assess any changes in renal function between the randomised groups. Figure 8 illustrates the treatment procedure for each patient.
FIGURE 8.
Patient treatment procedure within EVAR trials.
| Data to be collected at each follow up appointment | ||||
|---|---|---|---|---|
| EVAR Trial 1 | EVAR Trial 2 | |||
| Follow up interval | EVAR Follow-up from operation | Open repair Follow-up from operation | EVAR + best medical treatment Follow-up from operation | Best medical treatment Follow-up from randomisation |
| 1 month | CT scan | HRQL | CT scan | None |
| HRQL | HRQL | |||
| 2 months | None | None | None | HRQL |
| 3 months | CT scan | HRQL | CT scan | None |
| HRQL | HRQL | |||
| 4 months | None | None | None | HRQL |
| 1 year | CT scan | CT scan | CT scan | CT scan |
| HRQL | HRQL | HRQL | HRQL | |
| Creatinine | Creatinine | Creatinine | Creatinine | |
| 2 years | CT scan | CT scan | CT scan | CT scan, EuroQol |
| Creatinine | Creatinine | Creatinine | Creatinine | |
| 3 years | CT scan | CT scan | CT scan | CT scan, EuroQol |
| Creatinine | Creatinine | Creatinine | Creatinine | |
| 4 years | CT scan | CT scan | CT scan | CT scan, EuroQol |
| Creatinine | Creatinine | Creatinine | Creatinine | |
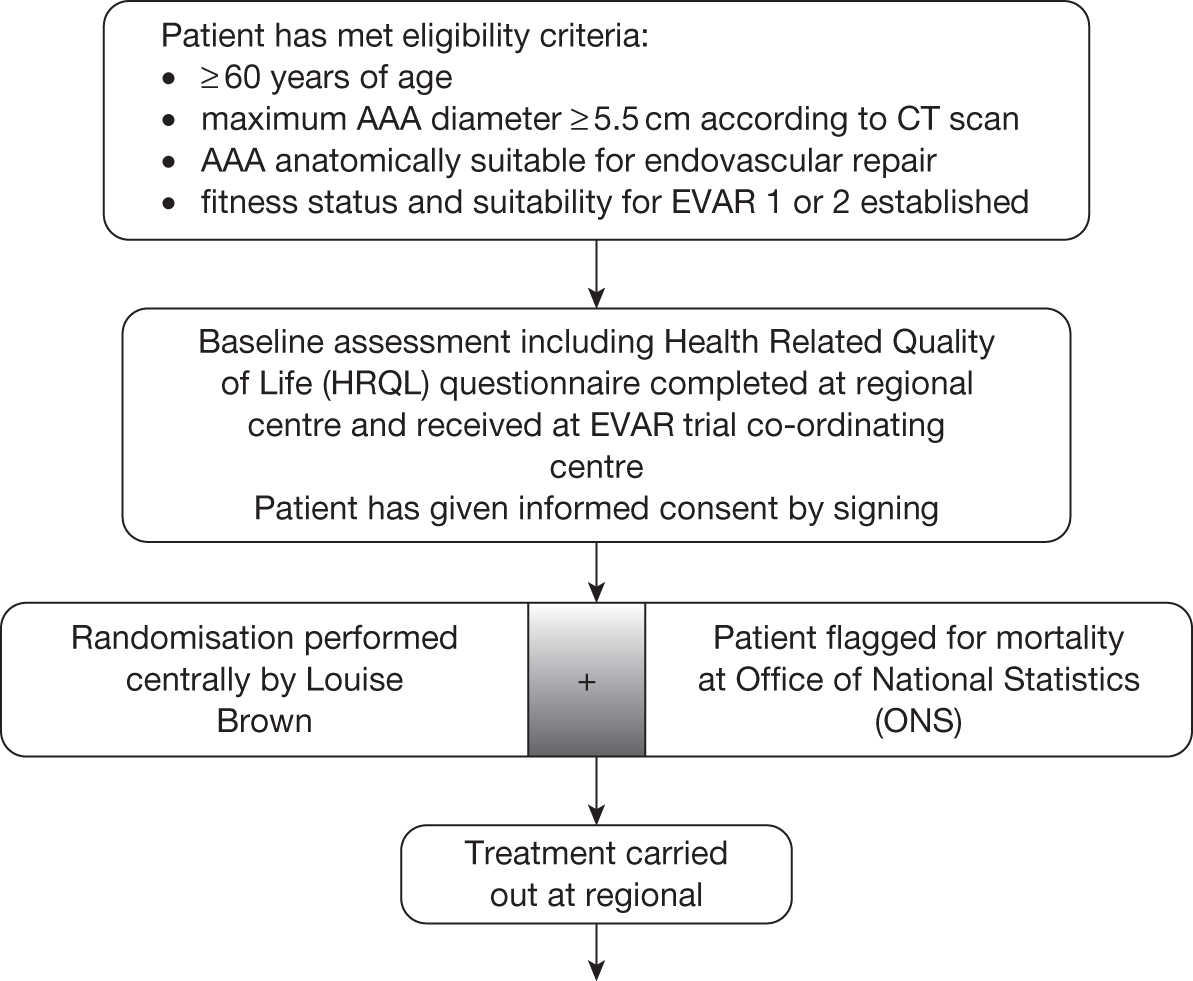
Project milestones of the program grant
Operator learning curves are completed for 13 centres. The RETA registry has recommended that these centres should form the initial regional trial participants. The 13 co-ordinators have been trained at Charing Cross trial centre in London. Patients will be recruited to both EVAR trials for the whole of the four year period. Follow-up commences from discharge of the first patients and exceed three years for the early patients entered. Evaluation and appraisal of data will be undertaken during the fourth year during which there would need to be close liaison with the DMEC. This committee would play a vital role in both trials. In Trial 1 the monitoring committee will determine and track mortality in OR v EVAR and be in a position to predict if a result is likely and if so when. During the period of this investigation safety, efficacy and durability of EVAR in that trial will be established. If there is any possible chance of showing a difference in mortality in favour of EVAR, EVAR would potentially be the most cost effective method of treating AAA within the NHS. Much shorter hospital stays and reduced pain from absence of the large abdominal incision would be clear advantages. In Trial 2 the monitoring committee review the mortality closely in the two arms and apply stopping rules if EVAR is clearly showing no adjuvant benefit beyond best medical treatment. If EVAR was abandoned for unfit for OR patients this would constitute great savings to the NHS. If the trial is not performed, we believe that there will be operator pressure for the NHS to provide EVAR in these patients, as these are the type of patients first treated successfully by EVAR. The NHS has funded EVAR Trials 1 and 2 with the intention that NHS money for EVAR procedures will only be available within these trials until an answer is known.
Methods for disseminating and implementing research results
Results will be presented to the Cochrane Research Group for Vascular Disease in Edinburgh and the NHS Centre for Review and Dissemination in York. We would certainly follow the guidelines of the Research and Development Directorate for reporting research results in the NHS. Results would be presented to National and International peer reviewed journals and offered for presentation at national and international societies. In this regard the applicants are well placed within key societies and various discipline groups in the UK and Europe.
References
- Fowkes FGR, MacIntyre CCA, Ruckley CV. Increasing incidence of AA in England and Wales. Brit Med J 1989;298:33-5.
- Parodi JC, Palma JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysm. Annals of Vascular Surgery 1991;5:491-7.
- Andrews SM, Cuming R, MacSweeney ST, Barrett NK, Greenhalgh RM, Nott DM. Assessment of feasibility for endovascular prosthetic tube correction of aortic aneurysm. British Journal of Surgery n.d.
- Yusef SW, Baker DM, Hind RE, . Endoluminal transfemoral abdominal aortic aneurysm repair with aorto-uni-iliac graft and femorofemoral bypass. British Journal of Surgery n.d.
- Nasim A, Thompson MM, Sayers RD, Bolia A, Bell PRF. Endovascular repair of abdominal aortic aneurysm: an initial experience. British Journal of Surgery 1996;83:516-9.
- The UK Small Aneurysm Trial Participants . Results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms (Mortality results, health service costs and quality of life). Lancet n.d.:352-1660.
- MacSweeney STR, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and Growth rate of small abdominal aortic aneurysms. Lancet 1994;344:651-52.
- Blum U, Voshage G, . Endoluminal stent grafts for infra-renal abdominal aortic aneurysms. N Eng J Med 1997;336:13-20.
- Moore WS, Rutherford RB. for the EVT investigators . Transfemoral endovascular repair of abdominal aortic aneurysms. Results of the North American EVT phase 1 Trial. J Vasc Surg 1996;23:543-5.
- Harris P. The need for clinical trials of endovascular abdominal aortic aneurysms stent/graft repair: The Eurostar Project. J Endovasc Surg 1997;4:72-7.
- Yusef SW, Whitaker SC, Chuter TAM, . Early results of Endovascular abdominal aortic aneurysm repair with aorto uni iliac graft and femoro femoral bypass. J Vasc Surg 1997;25:165-72.
- Berridge DC, Chamberlain J, Guy AJ, Lambert D. on behalf of the Northern Vascular Surgeons Group . Prospective audit of abdominal aortic aneurysm surgery in the northern region from 1988–1992. Brit J Surg 1995;82:906-10.
- D’Angelo J, Pappalo D, Farber A, Murphy AE, Faust GR, Cohen Jon R. Is preoperative cardiac evaluation for abdominal aortic aneurysm repair necessary?. J Vasc Surg 1997;25:152-6.
- Höltzenbein J, Kretschmer G, Glanzl R, Schon A, Thurnher S, . Endovascular AAA treatment: Expensive prestige or economic alternative?. European J Vascular &Amp; Endovascular Surgery 1997;14:265-72.
- White GH, May J, Greenhalgh RM. The Durability of Vascular and Endovascular Surgery. WB Saunders Publishing Co. Ltd; 1999.
- Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. Oxford: Oxford University Press; 1989.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Medical Care 1992;30:473-83.
- Garratt AM, Ruta DA, Abdalla MI, Buckingham KJ, Russell IT. The SF-36 health survey questionnaire: an outcome measure suitable for routine use within the NHS?. BMJ 1993;306:1440-4.
- Ruta DA, Abdalla MI, Garratt AM, Russell IT. The SF-36 health survey questionnaire: reliability in patient populations. Quality in Health Care 1994;3:180-5.
- Garratt AM, Russell IT, Abdalla MI. The SF-36 health survery questionnaire: responsiveness to changes in health status in four common clinical conditions. Quality in Health Care 1994;3:186-92.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Speilberger CD, Gorush RL, Luchene RE, . Manual for the State-Trait Anxiety Inventory. Palo Alto CA: Consulting Psychologists Press; 1983.
- Ruta DA, Garratt AM, Leng M, Macdonald LM, Russell IT. A new approach to the measurement of quality of life – the Patient-Generated Index. Medical Care 1994;11:1109-26.
- Drummond MF. Economic analysis alongside clinical trials. London: Department of Health; 1994.
- Torgerson DJ, Spencer A. Marginal costs and benefits. British Medical Journal 1996;312:35-6.
- Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM 1999;92:177-82.
Appendix 4 Patient information and consent forms for EVAR trial 1
Appendix 5 Patient information and consent forms for EVAR trial 2
Appendix 6 Trial case record forms
Initial consultation form (1 page)
CT findings for anatomical suitability for EVAR form (two pages)
Baseline assessment form to determine fitness for open repair (four pages)
Full health-related quality of life (HRQoL) assessment form (seven pages)
Randomisation form for EVAR trial 1 (one page)
Randomisation form for EVAR trial 2 (one page)
Operative procedure information form (two pages)
General follow-up form 1 (one page)
Adverse events follow-up form 2 (one page)
CT scan follow-up form 3 (one page)
CT scan incidents follow-up form 4 for patients who have had an EVAR (one page)
CT scan incidents follow-up form 4 for patients who have had an open repair (one page)
Patient refusal form (one page)
Appendix 7 List of publications arising from the EVAR trials
All publications acknowledge the funding of the NIHR HTA and include an NHS disclaimer. 1–17
References
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004;27:372-81.
- EVAR Trial Participants . Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843-8.
- EVAR Trial Participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86.
- EVAR Trial Participants . Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187-92.
- Greenhalgh RM, Brown LC, Powell JT. High risk and unfit for open repair are not the same. Eur J Vasc Endovasc Surg 2007;34:154-5.
- Brown LC, Greenhalgh RM, Howell S, Powell JT, Thompson SG. Patient fitness and survival after abdominal aortic aneurysm repair in patients from the UK EVAR trials. Br J Surg 2007;94:709-16.
- Brown LC, Greenhalgh RM, Kwong GP, Powell JT, Thompson SG, Wyatt MG. Secondary interventions and mortality following endovascular aortic aneurysm repair: device-specific results from the UK EVAR trials. Eur J Vasc Endovasc Surg 2007;34:281-90.
- Powell JT, Brown LC, Greenhalgh RM, Thompson SG. The rupture rate of large abdominal aortic aneurysms: is this modified by anatomical suitability for endovascular repair?. Ann Surg 2008;247:173-9.
- Epstein DM, Sculpher MJ, Manca A, Michaels J, Thompson SG, Brown LC, et al. Modelling the long-term cost-effectiveness of endovascular or open repair for abdominal aortic aneurysm. Br J Surg 2008;95:183-90.
- Rodway AD, Powell JT, Brown LC, Greenhalgh RM. Do abdominal aortic aneurysm necks increase in size faster after endovascular than open repair?. Eur J Vasc Endovasc Surg 2008;35:685-93.
- Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG. on behalf of the UK EVAR Trial Participants . Renal function and abdominal aortic aneurysm: the impact of different management strategies on long-term renal function in the UK EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 2010;251:966-75.
- Brown LC, Greenhalgh RM, Thompson SG, Powell JT. Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair? Results from the randomised EVAR Trial 2. Eur J Vasc Endovasc Surg 2010;39:396-402.
- Brown LC, Greenhalgh RM, Powell JT, Thompson SG. Use of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2010;97:1207-17.
- The UK EVAR Trial Investigators . Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010;362:1872-80.
- The UK EVAR Trial Investigators . Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71.
- Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR Trials. Ann Surg 2010;252:805-12.
- Brown LC, Thompson SG, Greenhalgh RM, Powell JT. on behalf of the EVAR Trial participants . Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm: results from the randomised EVAR Trial 1. Br J Surg 2011;98:935-42.
List of abbreviations
- ASERNIP-S
- Australian Safety and Efficacy Register of New Interventional Procedures – Surgical
- AAA
- abdominal aortic aneurysm
- ABPI
- ankle–brachial pressure index
- ACE
- angiotensin converting enzyme
- ACE trial
- Anévrisme de l’aorte abdominale, Chirurgie versus Endoprothèse trial
- ADAM
- Aneurysm Detection And Management trial
- AIC
- Akaike Information Criterion
- BMI
- body mass index
- BSIR
- British Society of Interventional Radiology
- CI
- confidence interval
- CPI
- Customised Probability Index
- CT
- computerised tomography
- DMEC
- Data Monitoring and Ethics Committee
- DREAM
- Dutch Randomised Endovascular Aneurysm Management trial
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- European Quality of Life-5 Dimensions
- EUROSTAR
- The EUROpean collaborators on Stent–graft Techniques for abdominal aortic Aneurysm Repair
- EVAR
- endovascular aneurysm repair
- FAD
- final appraisal document
- FEV1
- forced expiratory volume in 1 second
- GP
- general practitioner
- HDU
- high-dependency unit
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICD-10
- International Classification of Diseases and Health Related Problems, Version 10
- INB
- incremental net benefit
- IPD
- individual patient data
- IQR
- interquartile range
- ITT
- intention to treat
- ITU
- intensive treatment unit
- KDOQI
- Kidney Dialysis Outcomes Quality Initiative
- MCAR
- missing completely at random
- MCS
- Mental Component Summary (SF-36)
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MI
- myocardial infarction
- NICE
- National Institute for Health and Clinical Excellence
- NVD
- National Vascular Database
- ONS
- Office for National Statistics
- OR
- odds ratio
- OVER
- Open Versus Endovascular Repair trial
- PCO2
- partial pressure of carbon dioxide
- PCS
- Physical Component Summary (SF-36)
- PGI
- Patient-Generated Index
- PO2
- partial pressure of oxygen
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RETA
- Registry for Endovascular Treatment of Aneurysms
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SMR
- standardised mortality ratio
- TMC
- Trial Management Committee
- TSC
- Trial Steering Committee
- UKSAT
- UK Small Aneurysm Trial
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington