Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 03/45/07. The contractual start date was in August 2006. The draft report began editorial review in October 2011 and was accepted for publication in October 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Chalder et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Depression is one of the leading reasons for disability in the UK and is the third most common reason for consulting a general practitioner (GP). There are now > 35 million prescriptions for antidepressants each year in primary care in England and Wales, which costs the NHS over £80M (www.ppa.org.uk). Despite their widespread use there is still reluctance among some patients to take antidepressants and concern on the part of GPs that they are medicalising emotional states that are part of normal experience. 1
Current treatment/management options in primary care
Although antidepressants are an effective treatment for the more severe depressions, there is some clinical uncertainly about their use, particularly in those patients with mild/moderate depression. Adherence to antidepressant treatment is often poor, and only about 20% of patients are understood to take their medication according to guidelines. 2,3 There is also widespread scepticism about the effectiveness of antidepressants among the general population, and this may contribute to an overall reluctance to consult a GP for depression. 4 Hence, there is a need to identify effective non-pharmacological interventions for the management of the common, less severe, forms of depression.
The main alternative to antidepressant medication is psychotherapy, particularly cognitive behavioural therapy (CBT). The availability of psychotherapies is improving thanks to the Improving Access to Psychological Therapies (IAPT) programme (www.iaptmds.co.uk/). However, the more intensive psychotherapies require professionals with high levels of training and frequent supervision. CBT can also be quite demanding of patients, requiring weekly hour-long appointments that can interfere with work and family commitments.
It is difficult to define ‘usual care’ of depression in the primary care setting. Some people with depression are managed without antidepressant medication, although often this group have a milder illness and could, in theory, be prescribed antidepressants if their symptoms persisted or worsened over time. Similarly, counselling and other psychotherapies can be used in primary care as an element of ‘usual care’, although often there is a delay to accessing such services because of the level of demand and the time needed for referral and assessment.
Possible health benefits of physical activity for depression
There has recently been an increased interest in the potential health benefits of physical activity in treating depression, following on from the well-documented success in managing heart disease, obesity and diabetes using similar methods. 5 ‘Exercise on prescription’ schemes have flourished within primary care in the UK, with over 800 such programmes being implemented in the past decade, designed primarily to improve physical health. The most recent guidance available to health-care providers on the treatment of depression within general practice is embodied in the National Institute for Health and Clinical Excellence (NICE) guidelines,6 which recommend a ‘structured’ physical activity programme for depression.
Possible mechanisms for how exercise might influence depression
Little is known about the possible mechanisms that might mediate any therapeutic effects of physical activity on depression. Suggested biological mechanisms include changes in neuroendocrine function, neurotransmission, core temperature, cerebral blood flow or muscular tension. Psychosocial mechanisms such as improvements in physical self-perception and self-confidence have been observed,7 and increased social interaction and perceived support from an exercise specialist or exercise group have also been suggested as possible therapeutic mechanisms.
Similarly, there is little evidence to indicate the type, intensity and duration of physical activity that might be most effective in reducing depression. The recent report5 by the Chief Medical Officer (CMO) concluded, on the basis of rather limited evidence, that aerobic exercise lasting between 20 and 60 minutes which involved large muscle groups, such as brisk walking, cycling and swimming, was likely to be most effective. A recent systematic review8 of physical activity and depression concluded that it was impossible to determine which types of activity provided the most benefit.
A physical activity intervention, if effective, is likely to improve depressive symptoms through some or all of the above pathways and it could be that the overall effectiveness of physical activity relies upon such multiple mechanisms. For this reason we think that the main question is whether or not physical activity, of whatever intensity, might improve outcomes for people with depression. We have chosen the term ‘physical activity’ to reflect this broad notion of what we wish to investigate. The term ‘exercise’ can, at least for some people, indicate vigorous and aerobic activity, which we suppose might exclude gentler activities, such as walking, that might still have psychological benefits.
Evidence for the effectiveness of physical activity in depression
A systematic review of randomised controlled trials (RCTs) by Lawlor and Hopker9 published in 2001 has now been updated. 8 They identified 28 RCTs that investigated physical activity for people with depression, and 23 trials (total n = 909) contributed to the meta-analysis in which physical activity was compared with a condition without active treatment. The results of the meta-analysis indicated that, on average, physical activity improved outcome immediately after treatment by 0.82 SMD [standardised mean difference; 95% confidence interval (CI) 0.51 to 1.12]. This is a large treatment effect but has to be treated with some caution because of a number of methodological concerns.
Lack of evidence in clinical populations
Twenty-one trials were conducted in non-clinical populations, the majority among community volunteers who responded to advertisements for an exercise in depression trial. In some studies there were financial or other incentives to participate. Volunteers are likely to have an extra degree of motivation compared with patients who present to primary care and so results might be difficult to apply to NHS settings. A recent systematic review10 of randomised trials in participants who had received a diagnosis of depression identified 13 such trials. The pooled SMD for these was –0.40 (95% CI –0.66 to –0.14) with evidence of heterogeneity between trials (I2 = 57.2%, p = 0.003). There was an inverse association between duration of intervention and magnitude of effect, with trials in which the intervention lasted for ≥ 10 weeks showing little evidence of a beneficial effect.
Short duration of follow-up
Only five of the trials studied whether or not any benefits of an exercise intervention outlasted the duration of the intervention. On average, the effect size was reduced at longer-term follow-up (between 4 and 26 months), with a SMD of 0.44 (95% CI 0.18 to 0.71) in the meta-analysis of the five studies. In the context of a chronic relapsing and remitting disease, it is important to estimate any long-term as well as short-term effects, although even a short-term benefit may still prove worthwhile and be cost-effective. In the subset of trials with participants with a clinical diagnosis of depression there was no evidence of a long-term benefit of physical activity. 10
Quality of trials
The majority of trials to date have used randomisation procedures that were inadequately concealed or failed to undertake intention-to-treat (ITT) analyses. In general, not reporting methodological characteristics of trials will tend to exaggerate the impact of an intervention. 11 There was also evidence of heterogeneity between trials, which suggests that results were not consistent across studies.
Small size of trials
The trials undertaken to date have all been far too small and underpowered to find anything other than a massive treatment effect. The largest of the trials12 included just 51 participants in the treatment arm and 49 in the control arm. Only six trials had > 50 participants.
Nature of the intervention
Only one trial12 described unsupervised physical activity as an intervention. In the remaining studies the nature of the intervention was either supervised or not described or reported. Blumenthal et al. 12 compared home-based unsupervised physical activity with supervised gym-based activity. Providing supervised physical activity seems costly and unrealistic as a health service intervention, unless offered in a group setting. None of the trials used an intervention that could be used in the NHS or other health service setting.
Two of the more recent trials are worthy of more detailed description. The DOSE study,13 based in the USA, reported a treatment response for the more intensive ‘dose’ of exercise. The intervention involved the participants attending a gym and carrying out supervised aerobic activity on an exercise bike. The more vigorous (17.5 kcal/kg/week) and more frequent (5 days) intervention appeared to have a benefit compared with the control group. However, the study was small and only 16 subjects were randomised to the most intensive group (80 in total to five groups with a Latin square design). The participants had very mild depression, with a mean Hamilton14 score of 16.2, although all met Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)15 criteria.
Another US study, by Blumenthal et al. ,12 carried out a comparison between two different exercise interventions and antidepressant and placebo treatments. They found a 9% difference in remission from depression in favour of home-based exercise when comparing home-based exercise and usual care at 16 weeks. This difference is slightly less than the difference we have used for the power calculations in the present study. However, we would expect a placebo effect in such trials and so we think that the plausible treatment difference between intervention and usual care would be greater than that reported in this trial. In addition, this was a relatively small trial with just over 40 participants in each group and so estimated these treatment differences with limited precision.
Another US trial, coincidentally also called TREAD, has begun and has published its protocol, but as far as we are aware it has not published any findings. 16 The intervention in this case is somewhat more pragmatic as it allows home-based as well as gym-based activity, modelled upon the more intensive intervention from the DOSE study. 13 The participants are sedentary at baseline and will be recruited by a combination of adverts and physician referral.
What kind of intervention could be implemented in the NHS?
Previous research has primarily been concerned with whether or not physical activity can improve outcome in people with depression. However, it is also important to consider the kind of intervention that could be implemented in the NHS. As far as we are aware, previous researchers have not fully adopted a pragmatic perspective and considered the nature of any subsequent implementation in much detail. As discussed, in many published articles there are few details about the intervention and often the participants were volunteers responding to advertisements, who might have a very different level of motivation from participants recruited in primary care. We therefore decided to develop our own intervention to increase physical activity in people with depression that matched the needs of the patient and also addressed issues of possible implementation in a primary care setting.
Previously published studies and reviews17–21 have suggested that consideration should be given to at least four aspects of any proposed intervention:
-
the intensity of the intervention
-
the theoretical model underpinning the intervention
-
who delivers the intervention
-
reducing the barriers to physical activity.
Intensity of the intervention
Previous reviews have concluded that there is little evidence to suggest that interventions designed to increase physical activity have led to long-term change. However, it is possible that this may have been because the interventions were not sufficiently intensive in terms of the amount of supervision and support provided to participants. Very few of the interventions would meet the NHS National Quality Assurance Framework (NQAF) guidelines for exercise referral schemes. 22,23 Many of the studies had only one contact with the patient and it is difficult to generalise from some of the US studies.
In a UK study, Harland et al. 24 concluded that progressively more intensive interventions, involving up to 6 counselling sessions and 30 free leisure centre vouchers, produced greater changes in physical activity up to 12 weeks; however, the effects were not sustained at 12 months. Taylor et al. ’s RCT in the UK7,25 reported increased activity and fitness at 26 weeks in response to a 10-week exercise referral scheme in a local leisure centre and improved physical self-perceptions at 9 months. However, in a recent systematic review26 of eight RCTs that evaluated exercise referral schemes there were no long-term differences in physical activity compared with usual care.
Most of the literature concerns interventions designed for patients with cardiovascular disease rather than depression. Depression is characterised by low motivation, fatigue and reduced self-esteem, and these symptoms are likely to make it difficult to increase physical activity levels. Almost all of the RCTs that have investigated the effects of physical activity on depression have used supervised physical activity sessions rather than advice. 8 This most likely reflects the understanding by those undertaking such studies that a less intensive intervention is unlikely to alter behaviour in those with depression. An intervention that has been relatively successful in changing behaviour in a cardiovascular disease group may not be intensive enough to produce an effect in a trial of people with depression.
Physical activity is still regarded with some scepticism as an effective treatment for depression and, at this stage of knowledge, it is important to ensure that any intervention has the best chance of changing behaviour. We want to avoid what Tones27 has called a ‘type 3’ error, in which lifestyle interventions have (correctly) failed to show an effect on outcome because the intervention itself was too weak to change behaviour. This suggests the need to lean towards greater intensity, such as offering more frequent contact over a longer period. On the other hand, if the intervention is too intensive, this will increase cost and reduce its cost-effectiveness and eventual adoption. It would be important to make an intervention for physical activity much less intensive, for example, than psychotherapies such as CBT.
Theoretical model underpinning intervention
Some of the reported trials have described an intervention based upon a theoretical framework. The most popular frameworks used were designed to influence exercise cognitions and behaviour based on stage of readiness to become more active (transtheoretical model). 28,29 A recent systematic review21 concluded that ‘stage of readiness’-based interventions have not, on the whole, been effective in increasing patients’ physical activity in primary care but, as mentioned above, many of these interventions were probably not sufficiently intensive. Little et al. 30 devised an intervention based upon the theory of planned behaviour (TPB) and reported a trend towards greater change in physical activity, but only at the 1-month follow-up and in patients without depression recruited through a postal request.
The existing research has not provided sufficient encouragement for the adoption of one approach over another. We chose to use self-determination theory,31 which proposes that real shifts in behaviour arise through heightened autonomy or personal ownership of behavioural success. Self-determination theory suggests that, in order to stimulate motivation, the psychological needs for competence, autonomy and relatedness must be met. Encouraging participants to take charge of their physical activity decisions and choices is therefore very important. This approach fits well with the principles of motivational interviewing,32 which is designed to lead to better adherence and better motivation. 33 It also supports the view that choice of physical activity option, as described later, should improve adherence, especially over longer time periods. 29,34,35 In practical terms, the key elements are likely to be an intervention that (1) assesses current attitudes to physical activity, perceived barriers and the readiness to change, (2) utilises motivational interviewing techniques32 to engage the patient’s own motivation rather than providing simple advice, (3) offers choice of physical activity and rate of improvement and (4) uses appropriate behavioural strategies that can increase self-efficacy and self-determination.
Who delivers the intervention
Evidence from primary care suggests that existing health professionals are very inconsistent at providing advice about physical activity. 36 For example, McKenna et al. 37 found that GPs and practice nurses typically did little to promote physical activity and that those who did were more likely to be active themselves. It appears that only health professionals with a commitment to physical activity tend to encourage an increase in activity in their patients. The intensity and nature of a physical activity intervention for depression suggests that individuals with both a commitment to the concept and a readiness to develop expertise are needed. If each general practice were to devote a health professional to promoting physical activity for people with depression the training would have to be less intensive and, as each professional would be seeing only a handful of patients, it might be difficult to develop expertise and commitment. Practice nurses already undertake a multiplicity of tasks and there are likely to be future shortages of health professionals, who are also expensive to employ. For these reasons many people have argued for a new type of health professional who has expertise in behavioural change, which we have described as a physical activity facilitator (PAF). If this model were to be adopted more widely, it would also be easier to implement, because it is much simpler to train one person who might cover 10 practices than to train a health professional from each of those practices.
Reducing barriers to physical activity
Many people are reluctant to engage in physical activity, not only because of financial barriers, but also because of their own perceptions about physical activity and preference for different forms of physical activity. The more traditional ‘exercise on prescription’ schemes in UK primary care have been termed ‘structured’ or ‘centre-based’ activity in which the patient attends formal group sessions at a leisure or community centre. In contrast, ‘lifestyle’ or ‘home-based’ activity allows individuals to develop their own physical activity programme from home, which often consists of walking or cycling. One issue facing ‘centre-based’ exercise is that many people find that initial visits to leisure centres and joining unfamiliar groups of exercisers are anxiety provoking. This may particularly be the case for those suffering from depression, who often have accompanying anxiety symptoms. In a recent Department of Health-commissioned review, Fox et al. 38 found no difference in adherence to these two programmes when patients were randomised. The critical issue is to maximise choice in order to increase chances of adherence. In some of the more progressive exercise schemes, such as those being delivered in Somerset, participants are referred to a trained exercise facilitator, who will establish exercise preferences.
As mentioned previously, we have chosen to use the term ‘physical activity’ to emphasise the broad range of activities that might be beneficial in depression and to try to prevent the idea that we wish only to encourage vigorous aerobic activities. This should help to reduce the perceived barriers to exercise. Related to this is the idea that if physical activity is to be sustained it has to be incorporated into a ‘routine’, for example by walking or cycling to work rather than using public transport or driving a car. This also has the effect of reducing the perceived barriers to physical activity.
Rationale for research
We are not aware of any interventions designed to increase the level of physical activity in depression that address the issues we have raised in this introduction. An important question is whether or not we can devise a relatively inexpensive intervention that can increase physical activity in people with depression and to investigate whether or not it in turn improves outcome in depression. This can be broken down into two related but distinct issues. First, does physical activity improve outcome in depression? Second, is an intervention that is designed to increase physical activity levels a cost-effective treatment for depression? We have chosen to answer the second question outlined here. It is a pragmatic question about whether or not the health service should introduce a physical activity intervention for depression. To investigate this question we propose a RCT in which the addition of the physical activity intervention to usual care is compared with usual care. Usual care for depression often, but not always, involves antidepressant medication but may also include counselling and other forms of psychological treatment. For the purposes of this trial, we suggest that usual care, in both arms of the trial, should allow all of these other treatments so that we are investigating any additional benefit of the physical activity intervention to usual care.
Aims and objectives
The TREAD (TREAting Depression with physical activity) study was funded by the National Institute of Health Research (NIHR), as part of its Health Technology Assessment (HTA) programme. The overall aim of the TREAD study was to use a RCT to evaluate a physical activity intervention that we designed for this trial to answer the primary research question: ‘Does an intervention designed to increase physical activity, in addition to usual care in primary health care, improve the outcome in depression and alter the subsequent use of antidepressant medication?’
The study comprised the following:
-
development of a physical activity intervention designed to increase physical activity levels in people with depression
-
a RCT in which the physical activity intervention in addition to usual care was compared with usual care alone
-
a nested qualitative study to explore patients’ and GPs’ expectations and experiences of physical activity and the physical activity intervention, with the particular aims of understanding:
-
– participants’ and GPs’ beliefs and attitudes to physical activity as a treatment for depression
-
– the acceptability and experience of the physical activity intervention
-
– how being in the usual care arm affected behaviour
-
-
an evaluation of the cost-effectiveness of providing the physical activity intervention.
Development of the TREAD intervention
The TREAD intervention was developed by Anne Haase, Ken Fox and Helen Thorp of the Centre for Exercise, Nutrition and Health Sciences, School of Policy Studies, University of Bristol, and Adrian Taylor in Sport and Health Sciences, University of Exeter. A description of the intervention and its theoretical rationale has already been published. 39
The intervention was designed to be delivered as an adjunctive treatment to ‘usual care’ in primary care and drew heavily on the NHS NQAF for Exercise Referral Schemes. Essentially, trial participants in receipt of the intervention were given access to a variety of local physical activity options, in addition to ongoing guidance and support from a PAF. Patients offered the intervention were still able to receive antidepressants, counselling or psychotherapy during the course of the trial if this was deemed necessary or desirable. The overall aim of the intervention was to maximise long-term and sustainable increases in physical activity.
The intervention consisted of a maximum of 13 sessions between the patient and the PAF. Three face-to-face interviews (one for 1 hour and two for 45 minutes) plus up to ten 10- to 20-minute phone sessions were typically distributed over a period of 6–8 months, often front loaded so that sessions occurred weekly for the first month and were then stretched out over the remaining time based flexibly on patient needs. PAFs helped patients set personal targets about incorporating physical activity into their lifestyle with the gradual building up of physical activity as a regular behaviour.
A range of techniques derived from motivational interviewing32 and behavioural strategies were used by the PAF within a collaborative approach. These included reflective listening, use of open questions, summarising, guided decision-making and exploration of ambivalence and confidence. Behavioural techniques involved breaking down plans to engage in physical activity into manageable and discrete steps or tasks and rating any mood change as a result of physical activity. As the sessions progressed, the overall aim was to help patients develop autonomous self-regulatory skills to manage barriers, engage in personal short-, medium- and long-term goal setting, increase perceptions of physical competence and confidence and move to a robust intrinsic motivational base. This approach to supporting depressed patients in physical activity behaviour change was patient centred, flexible in terms of mode of activity, frequency, duration and intensity of physical activity and flexible in choice of timing and progression of physical activity, as well as being based on sound theoretical constructs and best available evidence from behaviour change research.
The long-term goal of the intervention was to achieve the government’s recommendations for substantial health benefit of taking 30 minutes of moderate-intensity activity on at least 5 days each week. However, the volume of physical activity was guided by the patient and, if someone was doing little physical activity, even low-intensity activity such as strolling was still encouraged.
The PAFs who were employed were graduates of exercise science, psychology or related behavioural sciences and ideally had previous practical experience of working with clients. Following their engagement on the TREAD study, they received structured training on a range of topics including the nature of depression, pharmacological treatments, characteristics of depressed patients and working in primary care settings as well as motivational interviewing, health behaviour change techniques and physical activity facilitation. A specially developed training manual provided practical guidance on the principles used to underpin the TREAD intervention (see Appendix 1). PAFs were supervised regularly (approximately monthly) by some of the co-applicants (AH, KF, AT, GL).
Chapter 2 Trial design and methods
Study design
The TREAD study was set up to evaluate an innovative intervention for depression against established treatment options available within UK primary care. It was designed as a pragmatic, multicentre RCT with two treatment arms – TREAD physical activity intervention plus usual GP care versus usual GP care alone.
The main trial was supplemented with an economic evaluation to consider the cost-effectiveness of providing the intervention compared with usual GP care (see Chapter 4). There was also a qualitative study to explore the views and experiences of participants and GPs involved in the study, and this is described in Chapter 5. A description of the trial protocol has already been published. 40
Ethical approval and research governance
Ethical approval for the study was given by West Midlands Multicentre Research Ethics Committee (MREC) in October 2005 (reference number 05/MRE07/42). Local Research Ethics Committee (LREC) approval and the appropriate site-specific assessments were obtained from the primary care trusts (PCTs) covering the Bristol, south Gloucestershire, north Somerset and Devon areas. The trial was registered with the International Standard Randomised Controlled Trial Register (ISRCTN) under the reference number 16900744 and also with the National Research Register (NRR) under the reference number 2159. A summary of the changes made to the original protocol is given in Table 1.
| Change to protocol | Date |
|---|---|
| Refer to ‘physical activity’ rather than ‘exercise’ | 1 August 2007 |
| Increase lower age limit from 16 to 18 years | 1 August 2007 |
| Incorporate an additional follow-up at 18 months post randomisation | 1 August 2007 |
| Move the first follow-up point from 3 months to 4 months post randomisation | 1 August 2007 |
| Add an extra exclusion criterion: bipolar disorder | 1 August 2007 |
| Revise allocation strategy to allow stratification by antidepressant use and minimisation by severity of depression, recruiting centre and level of physical activity undertaken | 1 August 2007 |
| Shorten the intervention delivery period from 12 to 8 months | 1 February 2008 |
| Move the second follow-up point from 12 months to 8 months post randomisation in order to ‘match’ the timing of the intervention | 1 February 2008 |
| Reduce overall follow-up period from 24 to 12 months | 1 August 2008 |
| Extend the recruitment period from 15 to 27 months | 1 August 2008 |
| Reduce the target number of trial participants from 762 to 360 | 1 August 2008 |
| 12-month follow-up data are to be collected in person whenever possible, or by post when necessary, using telephone ‘reminders’ as needed | 1 February 2009 |
| ‘Nest’ qualitative element of study within main trial rather than operating as a ‘parallel’ enquiry. Work will take place in both recruitment areas rather than simply in Bristol | 1 February 2009 |
Participants
The study sought to recruit people with a recent first or new episode of mild/moderate depression from 65 general practices in the Bristol and Exeter areas.
Inclusion criteria
Patients were considered for inclusion if they:
-
were aged 18–69 years at the time of assessment
-
had a diagnosis of depression (F32, F33) according to the International Statistical Classification of Diseases and Related Health Problems, 10th Edition (ICD-10)41 using the revised Clinical Interview Schedule (CIS-R)42
-
scored ≥ 14 on the Beck Depression Inventory (BDI)43
-
were not taking antidepressants at the time of assessment or had been prescribed antidepressants within 4 weeks of assessment but had had an antidepressant-free period of 4 weeks prior to that.
Exclusion criteria
The study design excluded anyone:
-
unable to complete self-administered questionnaires in English
-
with medical contraindications to physical activity
-
being treated for psychosis or bipolar disorder
-
with a serious substance abuse problem
-
who was pregnant or breastfeeding at the time of assessment.
Women who became pregnant in the course of study participation were encouraged to continue their involvement under their GP’s supervision.
Recruitment procedure
The majority of practices chose to identify potential participants during routine consultations, when they were given a patient information leaflet by their GP and, if interested, asked to provide written authority to enable further contact by the research team. In some practices computer systems were also regularly searched for details of patients recently diagnosed as depressed or prescribed an antidepressant, in an effort to alert GPs to potentially eligible individuals. In this instance, patients were sent information about the study from their surgery and encouraged to respond to the research team directly, if interested, using a reply-paid envelope.
Once a referral was received or the research team had been contacted directly, a researcher telephoned the patient to introduce the study formally, make initial eligibility checks and arrange an appointment for the baseline assessment. Depending on patient preference, baseline assessments were conducted at a patient’s home, in the patient’s GP surgery or at the research office and were led by a researcher using the computer-based version of the CIS-R and a range of questionnaire-based self-report measures. Baseline assessments were conducted as soon as possible after referral, but were required to be undertaken within 4 weeks of referral if the inclusion criteria were to be satisfied.
Informed consent
Informed, written consent was obtained at two separate stages of the study: first, before undertaking the baseline assessment and, second, when appropriate, if trial eligibility was established. The original signed and dated consent forms were held securely as part of the trial site file, with copies sent to both the participant and their GP for their records. Patients deemed to be ineligible for inclusion in the study were informed of this outcome and encouraged to reconsult their GP, who was also informed about the outcome of the baseline assessment.
Randomisation, concealment and blinding
Eligible and consenting patients were individually randomised at the end of their baseline assessment to one of two treatment groups: usual GP care plus facilitated physical activity or usual GP care alone.
To conceal the allocation of treatment from those conducting the research, randomisation of individual participants to a particular treatment arm was undertaken using an automated telephone randomisation system, which was administered remotely and used a computer-generated code. The randomisation service was provided by the Bristol Randomised Trials Collaboration (BRTC), a United Kingdom Clinical Research Collaboration (UKCRC)-registered trials unit. Once the randomisation procedure had been completed, the outcome and further details about the allocated treatment were immediately communicated by the researcher to the participant. Because of the nature of the intervention, it was not possible to blind participants, GPs, researchers or the PAFs to the treatment allocation.
Randomisation was stratified to take account of antidepressant use at baseline (yes, no) and minimised by severity of depression (CIS-R score of ≤ 25, 26–33, > 34 at baseline), recruiting centre (Bristol, Exeter) and level of physical activity (based on the number of days per week recorded at baseline on which at least 30 minutes of moderate-intensity physical activity was being undertaken: ≤ 1, 2–3, > 4). The minimisation algorithm retained a probabilistic element (80 : 20) when allocating participants, in such a way as to minimise marginal imbalances in the above-mentioned variables.
Treatment group allocation
Usual care
Individuals allocated to the usual care arm of the trial were advised to follow the current advice of their GP regarding their depression and its treatment. This might have included antidepressant medication, counselling or referral to secondary mental health services.
Intervention
In addition to usual care from their GP, those allocated to the intervention arm were encouraged to work with their TREAD PAF.
Data collection and management
To standardise recruitment/retention processes across the trial sites and maximise data quality, researchers were trained to use detailed standard operating procedures (SOPs) for each stage of data collection. A number of cross-checks were routinely performed as a means of ensuring that any data inconsistencies arising from either baseline assessment or follow-up were identified and resolved at the earliest opportunity. Trial data were entered into a Microsoft Access 2003 database (Microsoft Corporation, Redmond, WA, USA) at each study centre, before being merged into one central database following the end of data collection. A range of data validation checks were carried out in both Microsoft Access 2003 and Stata 11.1 (StataCorp LP, College Station, TX, USA) to minimise erroneous or missing data.
Baseline assessment
As described earlier, baseline assessment for the trial comprised two elements: use of the computerised CIS-R to determine whether or not the individual met criteria for ICD-10 depression and a self-completion questionnaire. The majority of questions were of a closed format, requiring participants to choose one option from a limited selection of discrete responses. All of the follow-up assessments were also administered at baseline. All baseline assessments were conducted between 1 August 2007 and 31 October 2009.
Follow-up
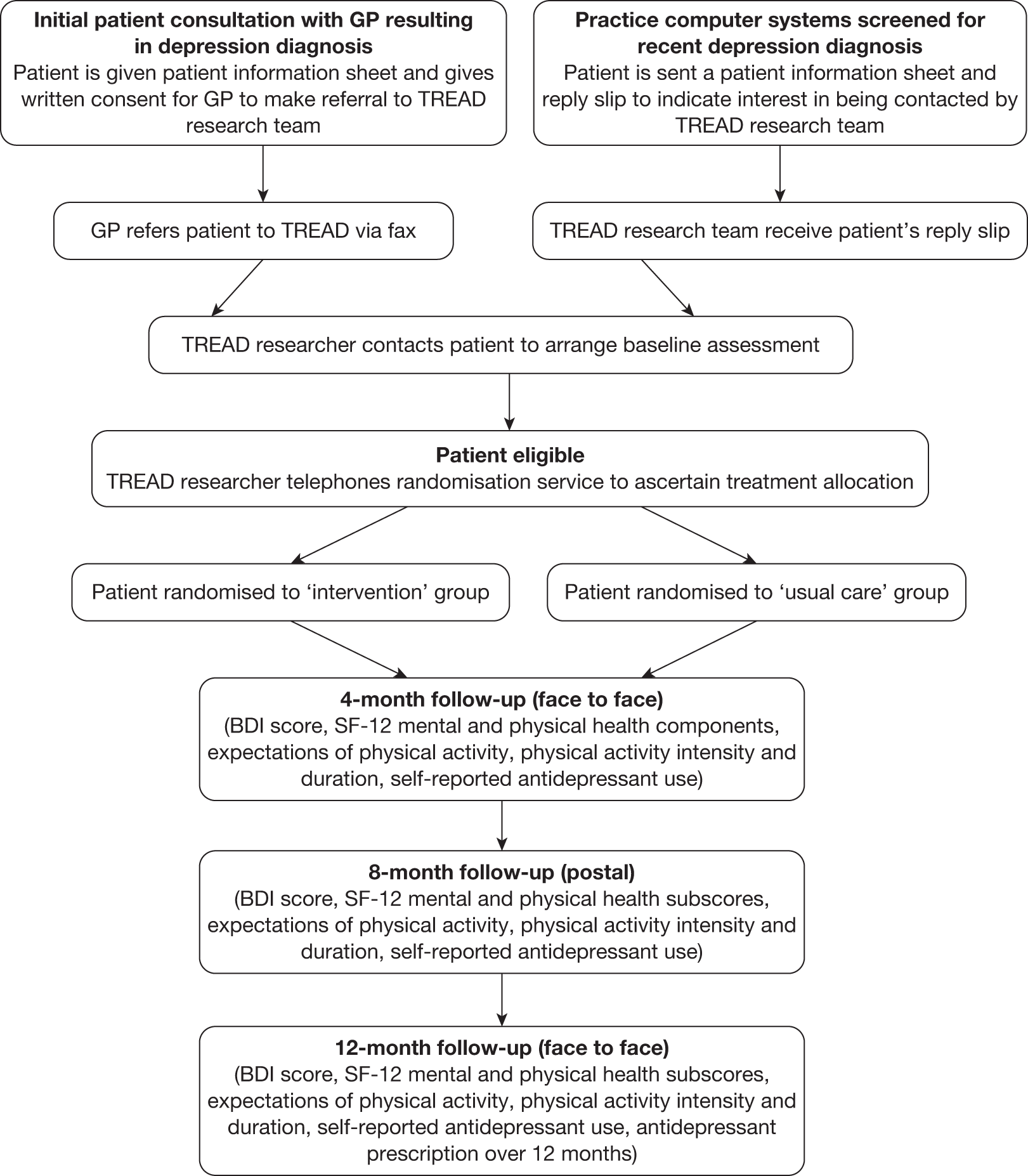
Follow-up data collection was scheduled to take place at three time points – 4, 8 and 12 months post randomisation – using the same self-completion questionnaire. The 8-month follow-up coincided approximately with the end of the intervention delivery period, whereas the 12-month follow-up was designed to enable the investigation of any longer-term effects on study outcomes. Whenever possible the researcher arranged to meet the patient at the 4- and 12-month follow-ups at the participant’s home, in his or her GP surgery or at the research office, with a small proportion of participants choosing to return the questionnaire by post. This approach was adopted because the response rate was higher in those instances when face-to-face data collection took place. The 8-month follow-up was conducted entirely by post. All follow-up data were collected between 1 August 2008 and 31 October 2010. A flow chart outlining TREAD recruitment and follow-up procedures is given in Figure 1.
FIGURE 1.
Flow chart outlining TREAD recruitment and follow-up procedures. SF-12, Short Form questionnaire-12 items.
Measures
Primary outcome
The BDI was used as a self-report measure of depression at the 4-month follow-up point. The 4-month follow-up was used as the measurement point for the primary outcome because it reflected the stage of the intervention at which the largest effect was expected. The BDI is a 21-item scale that has been widely used to measure depression outcome in randomised trials, particularly trials of cognitive psychotherapy, with higher scores indicating more severe depression. The score was treated as both a continuous (range 0–63) and a binary (where < 10 indicates recovery) outcome within the analysis, providing a quantitative measure of improvement and an estimate of the proportion of patients reaching symptomatic recovery.
Secondary outcomes
Depression symptoms
The BDI was used as a self-report measure of the longer-term effects of the intervention on depression at the 8- and 12-month follow-up points.
Physical activity
Physical activity was measured at the 4-, 8- and 12-month follow-up points using a new scale devised specifically for this study, as we wished to measure ‘bouts’ of time in which the participants had carried out some form of physical activity. On reviewing the existing physical activity measurement scales, none seemed suitable for a pragmatic trial for participants with depression. We therefore decided to use a modified 7-day recall diary measure in which the participants were asked to record 15-minute periods of ‘light’, ‘moderate’ and ‘vigorous’ activity, having considered a list of examples of each types of activity (see Appendix 3).
To take account of differences in intensity of physical activity undertaken, participants’ responses were transformed into MET minutes of physical activity per week, where MET is the metabolic equivalent of the task as a ratio to the basal metabolic rate. This was done by multiplying the reported total amount of ‘light’ physical activity in minutes by 2 METs, ‘moderate’ activity by 4.5 METs and ‘vigorous’ activity by 7.5 METs. 44 In the analysis, the total number of MET minutes per week for light, moderate and vigorous physical activity were combined and considered as a binary variable, with participants reporting ≥ 1000 MET minutes per week being classified as ‘active’ and those reporting < 1000 MET minutes per week deemed ‘inactive’. We chose to use this binary variable as the data were highly skewed. The classification was based upon the median value of MET minutes observed within the data. The sum of light, moderate and vigorous MET minutes was chosen as the intervention was designed to increase the number of episodes of activity, irrespective of the intensity. For example, the PAF encouraged walking in people who were not very active and this would count only as ‘light’ physical activity according to the above classification.
Health-related quality of life
Health-related quality of life was assessed at all three follow-up points using the physical and mental components of the Short Form questionnaire-12 items (SF-12). 45 Higher scores on either component denote better health.
Expectations of physical activity
Resnick et al. ’s46 Outcome Expectations for Exercise Scale was used at the 4-, 8- and 12-month follow-up points to assess beliefs about possible outcomes of undertaking physical activity. This scale included items such as ‘physical activity makes me feel less tired’ and ‘physical activity makes my mood better in general’. The 10 items of the scale were summed to create a score ranging from 10 to 50, with higher scores indicating that participants believed that physical activity was more beneficial.
Antidepressant use and prescription
Antidepressant use was assessed at all three follow-up points using a self-reported measure of medication adherence, whilst examination of GP records provided the number of days for which antidepressants had been prescribed for each participant over the 12-month follow-up period. This was coded as a binary variable for use in the analysis (0 = no prescription and 1 = at least one prescription issued).
Other variables used for baseline comparison
In addition to a range of sociodemographic characteristics, participants were asked, ‘How often do you have a drink containing alcohol?’, and the proportion using alcohol at least weekly was recorded. Respondents were also asked to indicate whether or not they were a current cigarette smoker.
Accelerometry
A subsample of the trial participants wore an accelerometer to provide an objective measure of the amount of physical activity undertaken and to validate the self-reported physical activity data elicited in the trial. Accelerometers record movement in such a way that it can be translated into a number of different outcomes, for example total step count, bouts of physical activity at specified intensities or energy expenditure. Every participant who completed the 4-month follow-up was offered an accelerometer to wear during waking hours for the next week, the aim being to gather data from 100 trial participants overall, taking into account their group allocation. At the end of the 7-day period, participants returned the monitor to the research team and completed the trial’s physical activity recall diary once more.
Accelerometry data were collected using Actigraph GT1Ms (Actigraph GT1M, Penascola, FL, USA) programmed to record using 10-second epochs. Data were reduced using MAH/UFFE Analyser v. 1.9.0.3 (MRC Epidemiology Unit, Cambridge, UK) set to ignore runs of 60 minutes of zeros. Days of data were matched with self-reported physical activity data, and cases with < 10 hours of Actigraph monitoring per day were also excluded from the analysis. Data were categorised as being of one of the following intensities:
-
minutes of sedentary [0–99 counts per minute (CPM)]
-
minutes of light inactive (100–499 CPM)
-
minutes of light active (500–1951 CPM)
-
minutes of moderate (1952–5723 CPM)
-
minutes of vigorous (> 5723 CPM).
Minutes spent at light and moderate intensities were multiplied by the median values (2 and 4.5 METs respectively) for the accepted MET ranges for these intensities (light > 1–3, moderate 3–5.99 METs). A value of 7.5 METs was used for minutes spent at vigorous intensity, for which the intensity classification is ≥ 6 METs, and was considered to be representative of vigorous activities likely to be performed by participants. 44 This calculation provided a number of MET minutes per day, which was then summed over the entire week. We calculated two summary measures: light, moderate and vigorous MET minutes of physical activity per week (LMVPA) and moderate and vigorous MET minutes of physical activity per week (MVPA).
Sample size
Original sample size justification
The original calculation for the research proposal estimated that 60% of participants in the usual care group and 73% in the intervention group would have recovered by the 4-month follow-up, that is, would score < 10 on the BDI. This difference of 13% in the proportion ‘recovered’ [equivalent to an odds ratio (OR) of 1.8] would be considered clinically worthwhile, being consistent with the lower end of treatment effects observed following treatment with antidepressants.
Assuming that 10% of the participants would not be taking antidepressants at the time of recruitment and so would be omitted from the originally planned primary analysis, 291 patients need to be recruited for each treatment group, with 90% power and 5% two-sided alpha. Previous studies using the BDI as a continuous outcome estimated a standard deviation of about 9 points and suggested a worthwhile target difference of 3–4 points. Thus, allowing for non-collection of primary outcome data of up to 15% at the 4-month follow-up point, the number required to be randomised was 762.
Revised sample size justification
After recruitment of 90 participants, we checked our assumptions in the original sample size calculation and found that the percentage of participants not on antidepressant treatment was closer to 50%, rather than the 10% originally anticipated. It was therefore proposed that all randomised participants should be included in the primary analysis rather than simply those on medication, with stratification by baseline antidepressant use to maximise balance between the trial arms. In addition, although the recovery rate of the participants in the usual care group was initially assumed to be around 60%, a recently concluded study conducted using similar methods47 found that the proportion of participants recovering in the equivalent group was nearer to 20% (95% CI 12.9% to 30.3%). Thus, the original power calculations were revised to reflect both the inclusion of all those randomised, irrespective of antidepressant use, and the change in proportion we expected to recover. The revised calculations – shown in Table 2 – indicate that, with 360 randomised participants, we would have adequate power to detect a 3-point difference in BDI total score and 80% power to detect a 15% difference in recovery between groups using the BDI as a binary variable.
| n randomised | n for primary analysisa | Power for 60% vs 73% (OR = 1.80)b | Power for 20% vs 33% (OR = 1.97)c | Detectable difference with 80% powerd | Power to detect 3-point BDI difference |
|---|---|---|---|---|---|
| 360 | 306 | 63% | 69% | 15% | 82% |
Statistical analysis
The analysis and reporting of this trial was undertaken in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines. 48 All statistical analysis was undertaken in Stata 11.1, following a predefined analysis plan agreed with the Trial Steering Committee. The primary comparative analyses between the randomised groups were conducted on an ITT basis without imputation of missing data.
Preliminary analyses
Descriptive statistics of the key clinical and sociodemographic variables were used to assess the baseline comparability of the two randomised groups and to enable additional adjustment of the primary and secondary analyses as appropriate.
Primary analyses
The primary outcome measure (BDI score at the 4-month follow-up point) was considered in both a binary and a continuous form. The continuous outcome was analysed in a linear regression model and presented as the adjusted difference in mean score between the intervention group and the usual care group with adjustment for baseline BDI score. The binary outcome was analysed in a logistic regression model and presented as an adjusted OR of recovery in the intervention group (with recovery denoted by a BDI score of < 10) compared with recovery in the usual care group. We calculated 95% CIs as well as p-values in interpreting both forms of the outcome measure. To account for the variables used for stratification and minimisation (the ‘design variables’) we also adjusted the primary analysis for antidepressant use, CIS-R score at baseline, recruiting centre and baseline level of physical activity.
Secondary analyses
The BDI score was also considered as a secondary outcome measure (in both binary and continuous forms) employing data from the 4-, 8- and 12-month follow-up points in a repeated measures analysis, using linear and logistic models as appropriate. This enabled investigation of whether or not between-group differences changed over time and estimated an average effect size over all three follow-up assessments in the absence of any time effect.
The other secondary outcome measures considered were SF-12 physical and mental health status, expectations of physical activity, physical activity levels and antidepressant use at the 4-month follow-up. All were analysed using the appropriate linear and/or logistic regression models with adjustment for the baseline value of that outcome as well as the design variables. The secondary outcomes were also subject to a repeated measures analysis using data from all three follow-up points. Finally, antidepressant prescription was analysed as a binary variable (0 = no prescription, 1 = at least one prescription issued) using all data available from the 12-month follow-up point in a logistic regression model. Accelerometer data were analysed in Stata using methods to allow clustering of observations in individuals. Means and CIs were calculated using the ‘svy’ commands.
Missing data
The potential influence on the analyses of missing data was investigated by identifying those variables associated with ‘missingness’ of data in the primary outcome measure (BDI score) and including them as covariates in the final regression models. We therefore considered the baseline characteristics of the participants with and without BDI outcome data at the 4-, 8- and 12-month follow-ups. This method for investigating the likely impact of missing data on outcome has been described by Carpenter and Kenward. 49
Potential clustering effects
Influence of practice
It is possible that participants attending the same general practice might have similarities in trial outcome and that, if this did occur, it could influence the variance estimates, showing a clustering effect. We therefore accounted for this possibility by using the robust variance techniques that are available for linear and logistic regression in Stata; however, Stata does not provide robust variance estimates for repeated measures logistic regression.
Influence of physical activity facilitator
Similarly, there may be variation in outcome dependent on the allocated PAF. Roberts and Roberts50 have suggested a method of accounting for this possibility using generalised linear and latent mixed regression to obtain a fully heteroscedastic model. Thus, it would be possible to determine whether or not the primary analyses at 4 months post randomisation were affected by incorporating any clustering effects according to PAF.
Subgroup analyses
Because both severity of depression and level of physical activity at baseline were identified as being potentially influential on the trial treatment effect, treatment by severity and treatment by physical activity interaction terms were added to the various regression models in order to ascertain their effects on BDI score at the 4-month follow-up point.
Treatment efficiency
Complier-average causal effect (CACE) analyses, using instrumental variable regression, were employed as a way of estimating unbiased treatment effects for the primary outcome. 51 The continuous primary outcome was analysed in a linear regression model, taking account of the CACE instrument, BDI score at baseline and the design variables. The result was presented as an adjusted difference in mean score comparing trial participants who received an ‘adequate dose’ of the intervention (i.e. completed at least five sessions with their PAF including one face-to-face appointment within the first 4 months of contact) with a comparable group of ‘would-be compliers’. The binary primary outcome was analysed and presented in a similar way using a probit regression model, with the effect estimate from the original ITT analysis remodelled using probit regression techniques to permit comparison with the CACE results.
Chapter 3 Trial results
Practice recruitment
A total of 101 general practices in the four PCTs across the Bristol and Exeter areas were initially approached to participate in the study. Of these, 65 practices agreed to support the research. Table 3 provides a summary of the main characteristics of the participating practices.
| Characteristic | Category | % (n = 65) |
|---|---|---|
| Centre | Bristol | 55 |
| Exeter | 45 | |
| PCT | Bristol | 37 |
| North Somerset | 11 | |
| South Gloucestershire | 8 | |
| Devon | 44 | |
| Geographic location | Urban | 35 |
| Suburban | 45 | |
| Rural | 20 | |
| Number of referred patients | 0–4 | 31 |
| 5–12 | 34 | |
| 13–20 | 14 | |
| 21+ | 21 | |
| Number of randomised participants | 0–4 | 54 |
| 5–12 | 34 | |
| 13–20 | 11 | |
| 21+ | 1 | |
| List size | 1–4999 | 14 |
| 5000–9999 | 49 | |
| 10,000–14,999 | 31 | |
| 15,000+ | 6 | |
| Number of GPs employed | 0–5 | 20 |
| 6–10 | 49 | |
| 11–15 | 27 | |
| 16+ | 4 |
Flow of participants in the trial
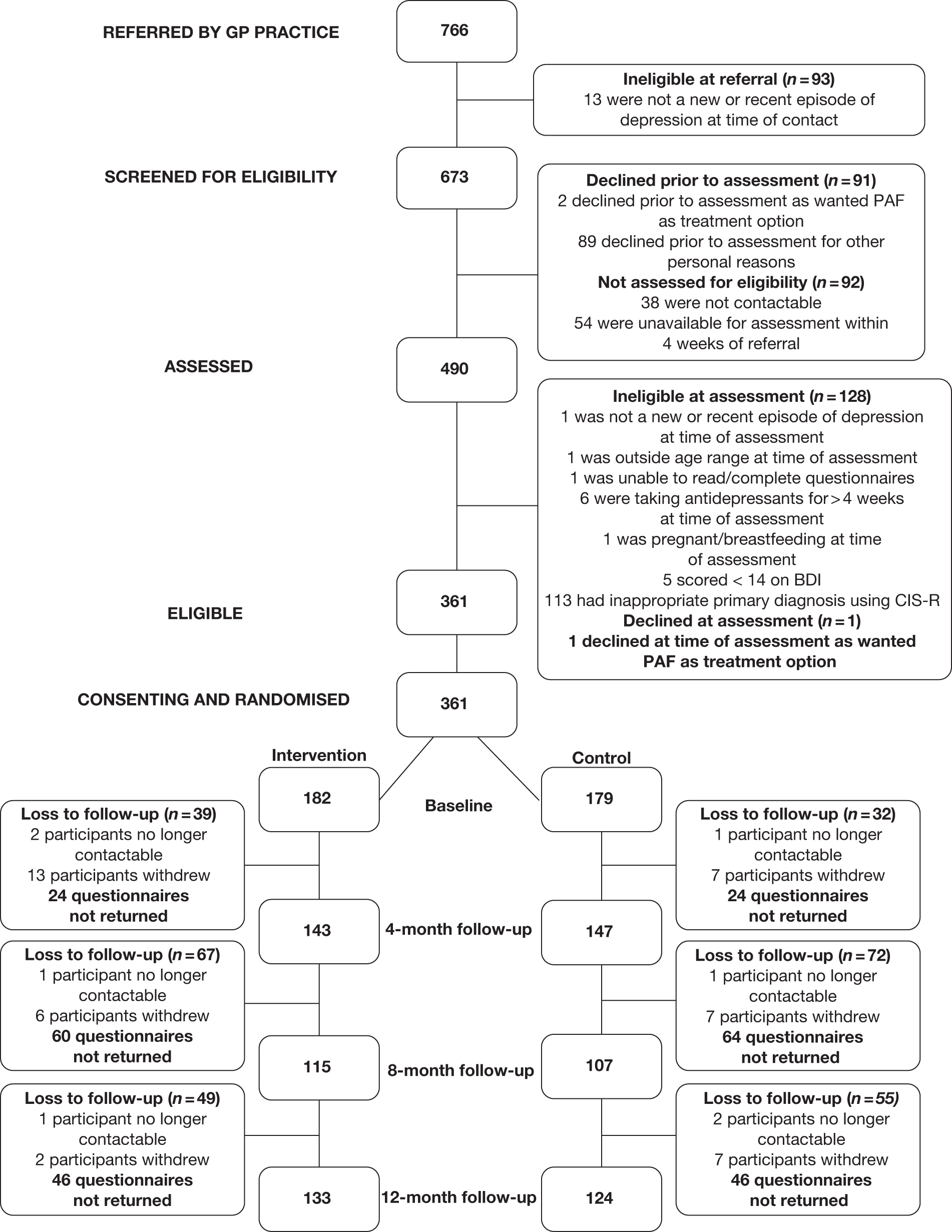
In total, 361 individuals were recruited to the TREAD trial, with 182 allocated to the intervention arm and 179 to the usual care group. Figure 2 presents the CONSORT flow diagram for the trial and summarises patient throughput from referral, through eligibility screening and randomisation on to completion of the 4-, 8- and 12-month follow-ups as appropriate. The diagram also reports numbers of patients who declined assessment, did not meet inclusion criteria, were excluded from the study, declined randomisation, withdrew following randomisation or were lost to follow-up at the 4-, 8- and 12-month follow-up points.
FIGURE 2.
CONSORT flow chart.
Baseline comparability
Table 4 presents a summary of the key descriptive statistics used to assess the baseline comparability of the randomised groups. The two groups were very similar, although there were slightly higher proportions of people receiving counselling and married/cohabiting individuals in the intervention group.
| Characteristic | Intervention (n = 182) | Usual routine care (n = 179) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Recruited via Bristol centre | 96 | 52.7 | 96 | 53.6 |
| Physically active at least 1 day per week | 92 | 50.5 | 82 | 45.8 |
| Mild/moderate depression according to CIS-R | 159 | 87.4 | 162 | 90.5 |
| Male | 59 | 32.4 | 63 | 35.2 |
| White | 170 | 93.4 | 166 | 92.7 |
| Married/cohabiting | 91 | 50 | 76 | 42.5 |
| Currently on antidepressant medication | 106 | 58.2 | 101 | 56.4 |
| Currently attending counselling | 38 | 20.9 | 27 | 15.1 |
| Currently employed, studying or training | 122 | 67.0 | 132 | 73.7 |
| Homeowner | 87 | 47.8 | 84 | 46.9 |
| Educated to A level or beyond | 93 | 51.1 | 98 | 54.7 |
| Current smoker | 55 | 30.2 | 65 | 36.3 |
| Drinking alcohol at least weekly | 69 | 37.9 | 72 | 40.2 |
| At least 1000 MET minutes of physical activity per week | 45 | 24.7 | 48 | 26.8 |
| Mean | SD | Mean | SD | |
| Age at referral (years) | 40.9 | 12.5 | 38.8 | 12.7 |
| SF-12 standardised physical health score | 51.4 | 9.8 | 50.3 | 9.9 |
| SF-12 standardised mental health score | 24.2 | 7.8 | 24.5 | 8.9 |
| BDI score | 32.1 | 9.0 | 32.1 | 9.5 |
| CIS-R score | 28.0 | 7.9 | 28.2 | 7.8 |
| Outcome Expectations for Exercise score | 36.3 | 5.2 | 37.2 | 6.4 |
Losses to follow-up
Follow-up data collection was scheduled to take place at three time points, 4, 8 and 12 months post randomisation, using the same self-completion questionnaire that was administered as part of the baseline assessment. Data collection at the 4-month follow-up resulted in 290 completed questionnaires – a retention rate of 80%. At the 8-month follow-up point 222 questionnaires were returned (61%), whereas at the 12-month follow-up point 257 questionnaires were completed (71%).
Adherence to intervention
Table 5 summarises the characteristics of the participants who were allocated to the intervention arm of the trial and offered the services of a PAF. In total, five PAFs delivered the intervention. The distribution of participants was fairly evenly spread, with four PAFs providing support to 14–20% each of those allocated the intervention and one PAF delivering the intervention to a larger proportion of participants (32%).
| Characteristic | Category | n | % |
|---|---|---|---|
| Recruiting centre | Bristol | 96 | 52.7 |
| Exeter | 86 | 47.3 | |
| PAF ID | 1 | 35 | 19.2 |
| 2 | 58 | 31.9 | |
| 3 | 36 | 19.8 | |
| 4 | 25 | 13.7 | |
| 5 | 28 | 15.4 | |
| Attendance at first intervention session | Within 1 week of randomisation | 35 | 19.2 |
| Within 2 weeks of randomisation | 65 | 35.7 | |
| Within 1 month of randomisation | 60 | 33.0 | |
| > 1 month from randomisation | 22 | 12.1 | |
| Time in receipt of intervention | Up to 1 month | 24 | 13.2 |
| 1–4 months | 33 | 18.1 | |
| 5 –8 months | 90 | 49.5 | |
| 9–12 months | 35 | 19.2 | |
| Total number of sessions received | 0 | 10 | 5.5 |
| 1 | 11 | 6.0 | |
| 2 | 13 | 7.1 | |
| 3 | 7 | 3.8 | |
| 4 | 8 | 4.4 | |
| 5 | 14 | 7.7 | |
| 6 | 11 | 6.0 | |
| 7 | 10 | 5.5 | |
| 8 | 13 | 7.1 | |
| 9 | 15 | 8.2 | |
| 10 | 19 | 10.4 | |
| 11 | 23 | 12.6 | |
| 12 | 14 | 7.7 | |
| 13 | 14 | 7.7 | |
| ‘Adequate dose’ of intervention receiveda | By 4-month follow-up point | 102 | 56.0 |
| Between 4- and 8-month follow-up point | 25 | 13.7 | |
| Between 8- and 12-month follow-up point | 2 | 1.1 | |
| Did not receive ‘adequate dose’ | 53 | 29.1 |
Missing data
The pattern of missing data was investigated by identifying those baseline variables associated with ‘missingness’ of data in the primary outcome measure (BDI score) at the three follow-up points. Table 6 presents a summary of all the key factors, although for reasons of brevity not every variable tested is listed.
| Characteristic | Missing | Present | p-value |
|---|---|---|---|
| 4-month follow-up | n = 73 | n = 288 | |
| Currently attending counselling | 6.9% | 20.5% | 0.01 |
| Homeowner | 38.4% | 49.7% | 0.08 |
| Educated to A level or beyond | 41.1% | 55.9% | 0.02 |
| Mean age at referral (years) | 35.6 | 40.9 | 0.001 |
| Current smoker | 38.4% | 32.5% | 0.35 |
| Drinking alcohol at least weekly | 37.0% | 40.3% | 0.61 |
| Mean SF-12 standardised physical component score | 48.5 | 51.4 | 0.04 |
| Male | 34.3% | 33.7% | 0.93 |
| Married/cohabiting | 42.5% | 47.2% | 0.47 |
| 8-month follow-up | n = 139 | n = 222 | |
| Currently attending counselling | 12.3% | 21.2% | 0.03 |
| Homeowner | 36.7% | 54.1% | 0.001 |
| Educated to A level or beyond | 45.3% | 57.6% | 0.02 |
| Mean age at referral (years) | 36.3 | 42.0 | 0.0001 |
| Current smoker | 41.7% | 28.6% | 0.01 |
| Drinking alcohol at least weekly | 46.0% | 35.5% | 0.04 |
| Mean SF-12 standardised physical component score | 49.0 | 52.0 | 0.08 |
| Male | 38.9% | 30.6% | 0.11 |
| Married/cohabiting | 39.6% | 50.5% | 0.16 |
| 12-month follow-up | n = 106 | n = 255 | |
| Currently attending counselling | 14.3% | 19.2% | 0.27 |
| Homeowner | 34.9% | 52.6% | 0.002 |
| Educated to A level or beyond | 43.4% | 56.9% | 0.02 |
| Mean age at referral (years) | 36.4 | 41.3 | 0.001 |
| Current smoker | 42.5% | 30.0% | 0.02 |
| Drinking alcohol at least weekly | 41.5% | 38.8% | 0.63 |
| Mean SF-12 standardised physical component score | 49.4 | 51.4 | 0.08 |
| Male | 34.0% | 33.7% | 0.97 |
| Married/cohabiting | 40.6% | 48.6% | 0.16 |
There was evidence which suggested that receiving counselling, more education, older age and homeownership and completion of the SF-12 physical component score were associated with fewer missing data. In contrast, weekly use of alcohol and current smoking were associated with more missing data. As a result, all regression analyses were adjusted for the following variables in order to investigate the possible impact of missing data on the findings: receipt of counselling, age, educational level, homeownership, weekly alcohol use, smoking and SF-12 physical component score. It should be noted that there was some variation in the number of observations included in each individual model because of varying numbers of missing data in one or more of the listed variables at different time points. However, the pattern of missing data was similar for all of the outcomes as it resulted from non-response to the follow-up questionnaires.
Primary outcomes
Depression symptoms
Beck Depression Inventory score as a continuous outcome measure at 4-month follow-up
The BDI score was considered first as a continuous variable and was analysed in a linear regression model using data from the 4-month follow-up. The results are presented in Table 7 as an adjusted difference in mean score when comparing the intervention and usual care groups, with a negative difference in means indicating better mood in the intervention group at the 4-month follow-up point.
| n | Mean | Difference in meansa (95% CI), p-value | Difference in meansb (95% CI), p-value | Difference in meansc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 142 | 16.12 | –0.54 (–3.06 to 1.99), 0.68 | –0.88 (–3.53 to 1.76), 0.51 | –0.88 (–3.54 to 1.77), 0.51 |
| Usual care | 146 | 16.87 | |||
| Total n | 288 | 288 | 271 | 271 |
There was no statistical evidence for a difference between the groups, although the intervention group had a slightly lower mean BDI score with narrow CIs for the difference in means. Adjustment for the variables associated with ‘missingness’ suggests that missing data might have led to an underestimation of the treatment effect, but only by about 0.3 BDI points, which is insufficient to change the interpretation of the results. There was no effect of clustering by practice on the confidence limits and, therefore, no impact on the findings.
Beck Depression Inventory score as a binary outcome measure at 4-month follow-up
The primary outcome at the 4-month follow-up was also considered as a binary variable in a logistic regression model. The results are presented in Table 8 as the OR of recovery in the intervention group compared with the usual care group, with recovery denoted by a BDI score of < 10. An OR of < 1 indicates that recovery was less likely in the intervention group at the 4-month follow-up point but there was no statistical evidence of a difference between the groups. After adjustment for the variables associated with ‘missingness’, there was a slight increase in the OR towards 1. The analysis that took account of clustering by practice increased the width of the CI but did not alter the overall result.
| n | % | ORa (95% CI), p-value | ORb (95% CI), p-value | ORc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 142 | 28.17 | 0.66 (0.40 to 1.11), 0.12 | 0.72 (0.42 to 1.25), 0.25 | 0.72 (0.37 to 1.42), 0.35 |
| Usual care | 146 | 35.62 | |||
| Total n | 288 | 288 | 271 | 271 |
Clustering effects by physical activity facilitator
To investigate the possibility of clustering by PAF we carried out an analysis that allows for individual estimates according to the PAF allocation. For the continuous outcome, BDI total score at the 4-month follow-up, the difference in means adjusted for design variables was 0.53 (95% CI –3.00 to 1.95) using this method. The equivalent result for the BDI binary outcome at the 4-month follow-up was an OR of 0.66 (95% CI 0.40 to 1.11). These results indicate that there was no evidence that clustering by therapist had any influence on the findings.
Subgroup analyses
There was no evidence to suggest that severity of depression or level of physical activity at baseline had any influence on the difference between intervention and usual care. This was studied by examining interaction terms in the regression models between randomised group and the baseline values of severity of symptoms (CIS-R strata used in randomisation) and the level of physical activity (MET minutes of LMVPA in three categories: 0, 1–999, 1000+). The p-values for tests of interaction in the logistic regressions were 0.73 and 0.79 respectively.
Treatment efficiency
The continuous BDI score was analysed in a linear instrumental variable regression model, using data from the 4-month follow-up, taking account of the CACE instrument, BDI score at baseline and the design variables. The results are presented in Table 9 as an adjusted difference in mean BDI score when comparing those trial participants who received an ‘adequate dose’ of the intervention with a comparable group of ‘would-be compliers’. A negative difference in means indicates better mood in the intervention group at the 4-month follow-up point.
| Analysis | Difference in meansa (95% CI), p-value | |
|---|---|---|
| Continuous BDI outcome | ITT | –0.54 (–3.06 to 1.99), 0.68 |
| CACE | –0.86 (–4.85 to 3.13), 0.67 |
The binary BDI score was analysed in a similar way using a probit instrumental variable regression model, using data from the 4-month follow-up, again taking account of the CACE instrument, BDI score at baseline and the design variables. For comparison, the original ITT model was also analysed using probit regression techniques. The results are presented in Table 10 as an adjusted difference in mean BDI score when comparing those trial participants who received an ‘adequate dose’ of the intervention with a comparable group of ‘would-be compliers’. A negative difference in means indicates better mood in the intervention group at the 4-month follow-up point. In summary, in none of the CACE analyses were there any marked differences from the results of the primary (ITT) analyses.
Secondary outcomes
Depression symptoms
Beck Depression Inventory score as a continuous outcome measure over the duration of the study
Table 11 summarises the mean BDI scores and difference in mean scores for both treatment groups at all three follow-up points. A decrease in mean score denotes better mood, whereas the difference in means indicates how many BDI points improvement the intervention group showed overall compared with the usual care group at that particular follow-up point.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 16.1 | 14.3 | 12.6 |
| Usual care | 16.9 | 16.1 | 13.5 |
| Difference in meansa (95% CI) | –0.54 (–3.06 to 1.99) | –1.69 (–4.64 to 1.26) | –1.06 (–3.56 to 1.45) |
| Total n | 288 | 222 | 255 |
The BDI score was considered as a continuous variable and analysed in a linear repeated measures model. The results are presented in Table 12 and can be interpreted as the average difference between the randomised groups over all three follow-up points, with a negative difference in means indicating better mood in the intervention group. There was no evidence that the difference between the intervention and the usual care groups changed over the three time periods (p-value for interaction between group and time = 0.61 with adjustments for design variables, baseline BDI score and variables associated with ‘missingness’).
| Outcomea (95% CI), p-value | Outcomeb (95% CI), p-value | Outcomec (95% CI), p-value | |
|---|---|---|---|
| Difference in means | –1.20 (–3.42 to 1.02), 0.29 | –1.58 (–3.89 to 0.73), 0.18 | –1.58 (–3.68 to 0.53), 0.14 |
| Total n | 308 | 288 | 288 |
Beck Depression Inventory score as a binary outcome measure over the duration of the study
Table 13 summarises the percentages recovered and ORs of recovery for both treatment groups at all three follow-up points. A higher percentage denotes a better recovery rate, whereas an OR of > 1 indicates that recovery was more likely in the intervention group than in the usual care group at that particular follow-up point.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 28.2% | 40.0% | 49.6% |
| Usual care | 35.6% | 36.5% | 45.1% |
| ORa (95% CI) | 0.66 (0.40 to 1.11) | 1.16 (0.66 to 2.02) | 1.26 (0.75 to 2.11) |
| Total n | 288 | 222 | 255 |
A repeated measures logistic regression analysis was performed using data from all three follow-up points. The results are presented in Table 14 as an OR of recovery, in which an OR of < 1 indicates a poorer recovery rate in the intervention group. It was not possible to calculate robust variances for this binary form of the outcome measure in Stata. There was some suggestion that the difference between the intervention and usual care groups varied across the three time periods (p-value for interaction between group and time = 0.04 with adjustments for design variables, baseline BDI score and variables associated with ‘missingness’); however, because this finding was not consistent with the more powerful continuous analysis, it is likely to be a chance finding.
Physical activity undertaken
Physical activity as a binary outcome measure at the 4-month follow-up
Physical activity was considered as a binary variable and analysed in a logistic regression model using data from the 4-month follow-up. At baseline, 26.3% (95% CI 21.8% to 31.2%) were considered to be physically active according to our criterion of 1000 MET minutes. The results are presented in Table 15 as the OR of being physically active in the intervention group at the 4-month follow-up point, at which physically active was deemed to mean having undertaken at least 1000 MET minutes of physical activity per week. An OR of > 1 indicates greater involvement in physical activity in the intervention group at the 4-month follow-up point. There was some weak evidence of an increase in physical activity levels in the intervention group at the 4-month follow-up point.
| n | % | ORa (95% CI), p-value | ORab (95% CI), p-value | ORac (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 136 | 51.5 | 1.58 (0.94 to 2.66), 0.08 | 1.58 (0.91 to 2.78), 0.11 | 1.58 (0.91 to 2.78), 0.11 |
| Usual care | 136 | 43.4 | |||
| Total n | 272 | 267 | 251 | 251 |
Physical activity as a binary outcome over the duration of the study
Table 16 summarises the percentages physically active and ORs of being physically active for both treatment groups at all three follow-up points. A higher percentage denotes a greater amount of physical activity being undertaken, whereas an OR of > 1 indicates greater involvement in physical activity by the intervention group than by the usual care group at that particular follow-up point.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 51.5% | 63.2% | 57.7% |
| Usual care | 43.4% | 49.4% | 40.4% |
| ORa (95% CI) | 1.58 (0.94 to 2.66) | 1.86 (1.0 to 3.46) | 2.17 (1.25 to 3.77) |
| Total n | 267 | 174 | 234 |
The data from all three follow-up points were analysed using a repeated measures logistic regression. The results are presented in Table 17 as an OR of being physically active in the intervention group compared with the usual care group and there is strong evidence of a statistically significant effect. There was no statistical evidence that the difference between the randomised groups increased over the duration of the study (p-value for interaction between group and time = 0.71 with adjustments for design variables and variables associated with ‘missingness’).
| Outcomea (95% CI), p-value | Outcomeb (95% CI), p-value | |
|---|---|---|
| OR | 2.27 (1.32 to 3.89), 0.003 | 2.39 (1.32 to 4.32), 0.004 |
| Total n | 293 | 275 |
Outcome expectations of physical activity
Outcome expectations of physical activity as a continuous outcome measure at the 4-month follow-up
Participants’ expectations of physical activity were considered as a continuous variable and analysed in a linear regression model using data from the 4-month follow-up. The results are presented in Table 18 as an adjusted difference in mean score when comparing the intervention and usual care groups, with a positive difference in means indicating higher outcome expectations in the intervention group at the 4-month follow-up point.
| n | Mean | Difference in meansa (95% CI), p-value | Difference in meansb (95% CI), p-value | Difference in meansc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 137 | 38.43 | 1.24 (–0.01 to 2.48), 0.05 | 1.29 (–0.04 to 2.63), 0.06 | 1.29 (–0.10 to 2.69), 0.07 |
| Usual care | 145 | 37.72 | |||
| Total n | 282 | 281 | 265 | 265 |
There was weak evidence that expectations of physical activity were greater in the intervention group. Adjustment for missing data tended to increase the apparent difference between the groups, although the confidence limits also widened. The clustering by practice also seemed to increase the width of the CI but did not alter the overall result.
Outcome expectations of physical activity as a continuous outcome measure over the duration of the study
Table 19 summarises the mean outcome expectations of physical activity scores for both treatment groups and adjusted difference in mean scores at all three follow-up points.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 38.4 | 39.0 | 39.8 |
| Usual care | 37.7 | 37.3 | 38.7 |
| Difference in meansa (95% CI) | 1.24 (–0.01 to 2.48) | 2.38 (0.97 to 3.78) | 1.89 (0.64 to 3.15) |
| Total n | 281 | 212 | 247 |
The data from all three follow-up points were analysed using a repeated measures linear regression. The results are presented in Table 20 as an adjusted difference in mean score when comparing the intervention group with the usual care group, with a positive difference in means indicating higher outcome expectations of physical activity in the intervention group over the duration of the study. There is strong evidence of an increase in outcome expectations in the intervention group. There is, however, no suggestion that the difference between the randomised groups changed across the three time periods (p-value for interaction between group and time = 0.57 with adjustments for design variables, expectations of physical activity score at baseline and variables associated with ‘missingness’).
| Outcomea (95% CI), p-value | Outcomeb (95% CI), p-value | Outcomec (95% CI), p-value | |
|---|---|---|---|
| Difference in means | 1.64 (0.60 to 2.69), 0.002 | 1.65 (0.54 to 2.77), 0.004 | 1.65 (0.60 to 2.71), 0.002 |
| Total n | 304 | 284 | 284 |
Antidepressant use
Antidepressant use as a binary outcome measure at the 4-month follow-up
Antidepressant use was considered as a binary variable and analysed in a logistic regression model using data from the 4-month follow-up. The results are presented in Table 21 as the OR of taking antidepressant medication in the intervention group compared with the usual care group. An OR of > 1 indicates a greater use of antidepressant medication in the intervention group at the 4-month follow-up point. There was no evidence to suggest a difference between the groups in respect of antidepressant use.
| n | % | ORa (95% CI), p-value | ORb (95% CI), p-value | ORc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 142 | 58.5 | 1.20 (0.69 to 2.08), 0.52 | 1.04 (0.57 to 1.90), 0.89 | 1.04 (0.59 to 1.85), 0.89 |
| Usual care | 147 | 53.1 | |||
| Total n | 289 | 289 | 271 | 271 |
Antidepressant use as a binary outcome measure over the duration of the study
Table 22 summarises the percentages using antidepressants in both treatment groups and ORs of using antidepressant medication in the intervention group compared with the usual care group at all three follow-up points. An OR of < 1 indicates a lower use of antidepressant medication in the intervention group at that particular follow-up point.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 58.5% | 41.7% | 34.6% |
| Usual care | 53.1% | 45.7% | 42.3% |
| ORa (95% CI) | 1.20 (0.69 to 2.08) | 0.82 (0.45 to 1.48) | 0.67 (0.39 to 1.15) |
| Total n | 289 | 220 | 256 |
The data from all three follow-up points were analysed using a repeated measures logistic regression. The results are presented in Table 23 as an adjusted OR of taking antidepressant medication in the intervention group compared with the usual care group. An OR of < 1 indicates a lower use of antidepressant medication by the intervention group over the duration of the study. However, there is no statistical evidence of a difference between the randomised groups. Neither is there any evidence of any differences across the three time periods (p-value for interaction between group and time = 0.22 with adjustments for design variables and variables associated with ‘missingness’).
Antidepressant prescription
Antidepressant prescription as a binary outcome measure over the duration of the study
Antidepressant prescription was considered as a binary variable and analysed using logistic regression. The results are presented in Table 24, with an OR of > 1 indicating higher prescription of antidepressant medication in the intervention group over the duration of the study. There is no evidence to suggest a difference in the issuing of antidepressant prescriptions between the randomised groups.
| n | % | ORa (95% CI), p-value | ORb (95% CI), p-value | ORc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 161 | 64.6 | 1.08 (0.65 to 1.81), 0.76 | 1.08 (0.69 to 1.69), 0.73 | 1.24 (0.77 to 2.02), 0.38 |
| Usual care | 163 | 62.6 | |||
| Total n | 324 | 324 | 303 | 303 |
Quality of life
SF-12 physical component score as a continuous outcome measure at the 4-month follow-up
The SF-12 physical component score was considered as a continuous variable and analysed in a linear regression model using data from the 4-month follow-up. The results are presented in Table 25 as an adjusted difference in mean SF-12 scores, with a negative difference in means indicating a lower physical component score in the intervention group at the 4-month follow-up point. There is no evidence for a difference in scores between the two groups.
| n | Mean | Difference in meansa (95% CI), p-value | Difference in meansb (95% CI), p-value | Difference in meansc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 130 | 50.6 | –0.32 (–2.27 to 1.63), 0.75 | –0.42 (–2.43 to 1.59), 0.68 | –0.42 (–3.12 to 2.27), 0.75 |
| Usual care | 143 | 49.7 | |||
| Total n | 273 | 263 | 258 | 258 |
SF-12 physical component score as a continuous outcome measure over the duration of the study
Table 26 summarises the mean SF-12 physical component scores for both treatment groups and difference in means at all three follow-up points. A higher mean score denotes better physical health and a positive difference in means indicates improved health in the intervention group at that particular follow-up point.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 50.6 | 50.7 | 50.5 |
| Usual care | 49.7 | 49.6 | 48.9 |
| Difference in meansa (95% CI) | –0.32 (–2.27 to 1.63) | –0.17 (–2.59 to 2.25) | 1.43 (–0.78 to 3.64) |
| Total n | 263 | 201 | 235 |
The data from all three follow-up points were analysed in a repeated measures logistic regression. The results are presented in Table 27 as an adjusted difference in mean SF-12 scores comparing the intervention and usual care groups over the duration of the study. There was no evidence of a difference between the randomised groups.
| Outcomea (95% CI), p-value | Outcomeb (95% CI), p-value | Outcomec (95% CI), p-value | |
|---|---|---|---|
| Difference in means | 0.30 (–1.28 to 1.87), 0.71 | 0.53 (–1.03 to 2.10), 0.50 | 0.53 (–1.33 to 2.40), 0.58 |
| Total n | 286 | 281 | 281 |
SF-12 mental component score as a continuous outcome measure at the 4-month follow-up
The SF-12 mental component score was considered as a continuous variable and analysed in a similar way to the SF-12 physical component score. The results are presented in Table 28 as an adjusted difference in mean SF-12 score, with a positive mean difference indicating a better mental health component score in the intervention group at the 4-month follow-up point. There is no evidence for any difference between the randomised groups.
| n | Mean | Difference in meansa (95% CI), p-value | Difference in meansb (95% CI), p-value | Difference in meansc (95% CI), p-value | |
|---|---|---|---|---|---|
| Intervention | 130 | 41.9 | 1.55 (–1.16 to 4.27), 0.26 | 1.48 (–1.38 to 4.35), 0.31 | 1.48 (–1.65 to 4.16), 0.35 |
| Usual care | 143 | 40.3 | |||
| Total n | 273 | 263 | 258 | 258 |
SF-12 mental component score as a continuous outcome measure over the duration of the study
Table 29 summarises the means and adjusted difference in mean SF-12 mental component scores for both treatment groups at all three follow-up points. A positive difference in means indicates better mental health in the intervention group at that particular follow-up point.
| 4-month follow-up | 8-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Intervention | 41.9 | 43.6 | 45.6 |
| Usual care | 40.3 | 41.5 | 44.6 |
| Difference in meansa (95% CI) | 1.55 (–1.16 to 4.27) | 2.06 (–1.33 to 5.45) | 1.32 (–1.72 to 4.35) |
| Total n | 263 | 201 | 235 |
The data from all three follow-up points were subjected to a repeated measures analysis using logistic regression. The results are presented in Table 30 as an adjusted difference in mean SF-12 scores, where a positive difference in means indicates better mental health in the intervention group over the duration of the study. There is no evidence to suggest a difference between the randomised groups.
| Outcomea (95% CI), p-value | Outcomeb (95% CI), p-value | Outcomec (95% CI), p-value | |
|---|---|---|---|
| Difference in means | 1.64 (–0.65 to 3.93), 0.16 | 1.51 (–0.84 to 3.86), 0.21 | 1.51 (–0.69 to 3.71), 0.18 |
| Total n | 286 | 281 | 281 |
Accelerometry
Data were available on 99 trial participants from both randomised groups who had at least 1 day with > 10 hours of usable accelerometer data, together with a recall diary entry completed for that same day. There were 84 participants with ≥ 5 days of paired data. The distribution of usable days for the respondents is given in Table 31.
| Number of days | n |
|---|---|
| 1 | 3 |
| 2 | 1 |
| 3 | 3 |
| 4 | 8 |
| 5 | 22 |
| 6 | 25 |
| 7 | 37 |
Table 32 gives details of the mean MET minutes for light, moderate and vigorous activity as recorded in participants’ recall diaries and the accelerometers. The CIs have taken account of any clustering in the data within individuals. This illustrates that the accelerometer was recording markedly more MET minutes than the recall diary, especially for light activity. The difference probably reflects the instruction for those completing the recall diary to record ‘bouts’ of activity lasting 15 minutes, whereas the accelerometer registered much shorter time periods of activity. The differences in the moderate and vigorous categories of activity were less marked.
| Activity | MET minutes per day | |||
|---|---|---|---|---|
| Recall diary | Accelerometer | |||
| Mean | 95% CI | Mean | 95% CI | |
| Light | 33.3 | 25.6 to 41.0 | 286.0 | 270.3 to 301.6 |
| Moderate | 21.1 | 15.1 to 27.1 | 31.6 | 27.5 to 35.6 |
| Vigorous | 6.8 | 2.4 to 11.1 | 3.5 | 1.9 to 5.1 |
| MVPAa | 27.9 | 19.5 to 36.3 | 35.0 | 30.2 to 39.9 |
| LMVPAb | 61.2 | 48.7 to 73.7 | 321.0 | 304.5 to 337.5 |
The association between the two measures was estimated using a random effects regression to take account of the clustering of the data within individuals. With the sum of light, moderate and vigorous activity on the accelerometer as the outcome, for each increase of 1 MET minute in the recall diary, there was an increase of 0.26 points (95% CI 0.13 to 0.40, p < 0.0001) in the accelerometer outcome. With the sum of moderate and vigorous MET minutes recorded by the accelerometer as the outcome, there was an increase of 0.12 points (95% CI 0.06 to 0.18, p < 0.0001) for each increase of 1 MET minute in the recall diary.
We also examined the agreement between the two types of physical activity measurement using the approach described by Bland and Altman,52 which takes account of any clustering by individual. Figure 3 gives the resultant plot and indicates that the difference between the two assessments increases as the participants become more active.
FIGURE 3.
Plot of the difference (accelerometer minus recall diary) between moderate and vigorous physical activity as recorded by accelerometer and moderate and vigorous physical activity as recorded by recall diary against the mean of both measures.
Chapter 4 Economic evaluation
Introduction
The aim of the economic evaluation was to assess the cost-effectiveness of the TREAD intervention for primary care patients with depression. The primary perspective was the health service provider, which was supplemented by information on the use of social and community services, time off work and the value of lost productivity. We included all health service resources used by participants from the time they were randomised into the study until final follow-up at 12 months post randomisation. The primary aim of the study as a whole was to examine the impact of the intervention on depression and the economic evaluation also took this stance. However, primary and community consultations can be complex and holistic and so it is often difficult to determine whether or not the reason for consultation was specifically and only for mental health issues. We therefore took an inclusive view for these types of resources, including all primary and community health-care resource use, irrespective of the reason. Secondary care, on the other hand, is specialist by nature and the reason is less ambiguous and so we were able to include only those secondary care consultations relating to mental health.
Methods
Health and social care resource use
Data on resource use came from two main sources. GP records were interrogated at the end of the 12-month follow-up period in order to collect information on all consultations in, by and through primary care; these were categorised according to who the consultation was with (GP, nurse, counsellor, etc.) and where the consultation took place (in the surgery, by telephone, etc.). Data on all medication prescribed to trial participants during the12 months were also extracted in this way.
A self-completion questionnaire was issued to participants as part of the follow-up assessments at 4, 8 and 12 months, which provided information about resource use not available from GP records. Included were questions about the use of secondary care services for a mental health problem, use of walk-in centres, NHS Direct and social and community services and any time off work because of mental health problems. The questionnaire was devised to be as user-friendly as possible. The main questionnaire asked a series of ‘filter’ questions that required a simple ‘yes’ or ‘no’ response according to whether or not a particular service was used. If a participant replied ‘yes’ to any service they were contacted by a researcher and asked for detailed information, for example which outpatient clinic or service was used, to help with the costing. This two-stage method of questionnaire delivery was designed to make data collection as efficient as possible and to reduce the ambiguity of participants either failing to respond to a question because they had not used a particular service or just failing to answer a question.
Valuing the resource use
The unit costs and their sources which were used to estimate total cost per participant are given in Table 33. For the most part primary and community service costs were based on those provided by Lesley Curtis. 53 We used the Department of Health reference costs54 for secondary care, and the British National Formulary (BNF)55 for prescribed medication. Published research was used to estimate the cost of an out-of-hours contact,56,57 a call to NHS Direct58 and a visit to a walk-in centre. 59 All resources were valued in pounds sterling at 2009 prices, adjusting for inflation where necessary using the Hospital and Community Health Services Pay and Price Index. 53 Time off work was valued using the human capital approach; we used the median gross weekly earnings by age and sex. 60
| Source | Resource | Unit cost (£) per encounter |
|---|---|---|
| Curtis53 | GP at the surgery | 31.00 |
| GP home visit | 103.00 | |
| GP telephone | 19.00 | |
| GP unknown | 29.16 | |
| Practice nurse at the surgery | 10.00 | |
| Practice nurse home visit | 13.00 | |
| Practice nurse telephone | 6.13 | |
| HCA/phlebotomist at the surgery | 6.92 | |
| HCA/phlebotomist home visit | 9.00 | |
| HCA/phlebotomist telephone | 4.24 | |
| Health visitor at the surgery | 12.00 | |
| Health visitor home visit | 36.00 | |
| Health visitor telephone | 7.35 | |
| District nurse at the surgery | 12.00 | |
| District nurse home visit | 24.00 | |
| District nurse telephone | 7.35 | |
| Midwife at the surgery | 12.00 | |
| Nurse practitioner | 14.00 | |
| Nurse practitioner telephone | 8.58 | |
| Graduate mental health worker | 32.00 | |
| Graduate mental health worker telephone | 19.61 | |
| Midwife home visit | 24.00 | |
| Counsellor at the surgery | 67.00 | |
| Mental health worker at the surgery | 32.00 | |
| Community psychiatric nurse | 32.00 | |
| Physiotherapist face to face | 16.00 | |
| Physiotherapist telephone | 16.00 | |
| Psychotherapist | 67.00 | |
| Department of Health reference costs54 | Paramedic | 253.00 |
| A&E | 68.00 | |
| Outpatients | By specialty | |
| National evaluations58,59 | Walk-in centre | 32.18 |
| NHS Direct | 20.65 | |
| Literature56,57 | Out-of-hours face to face | 23.64 |
| Out-of-hours home | 78.86 | |
| Out-of-hours telephone | 15.41 | |
| BNF55 | Prescribed medication | By item |
| Salaries paid in the study | PAF cost per hour | 32.00 |
The intervention
Resource use associated with the intervention was recorded by the PAFs. They noted details of all contacts with participants, face to face and by telephone; they also recorded time spent travelling to sessions, the number of non-contact phone calls, and letters, e-mails and text messages sent.
The principal cost of the intervention was the cost of the PAFs’ time spent directly with the participants and travelling to the sessions. In addition to this there were training sessions, ongoing supervision and some non-contact time. All time spent was valued according to salaries paid during the trial, except in the case of supervision. This was carried out by members of the trial team, but in the analysis it was costed at the rate of a senior nurse (Band 8) to reflect the likely situation if the intervention was widely adopted. Some participants received subsidies towards the cost of exercise; the PAFs noted these, the amount of the subsidy and the amount of administrative time spent dealing with them.
Quality-adjusted life-years
Data to estimate quality-adjusted life-year (QALY) gain were collected through the participant questionnaire, as described above. We used the European Quality of Life-5 Dimensions (EQ-5D),61 which was administered at baseline and at the 4, 8 and 12-month follow-ups. The EQ-5D is a generic measure of health-related quality of life, designed and widely used to estimate QALYs. The five domains of mobility, self-care, usual activities, pain/discomfort and anxiety/depression each have three possible levels of response relating to no problems, some problems and extreme problems. A subset of the 243 possible health states have been valued directly by a sample of the UK general population62 and these valuations have been used to derive a tariff of valuations for all possible health states. This tariff was used to estimate health-related quality of life for each participant at baseline and 4, 8 and 12 months and these were used to estimate QALY gain over the 12-month period.
Data analysis
The number of each item of resource used by participants in each group was investigated using descriptive statistics.
The total cost per participant was estimated by combining the number of each item of resource used with the unit cost of that item. This provided an estimate of mean cost per participant by group. The incremental cost of caring for participants in the intervention group compared with those in the control group is the difference in these mean costs and the CI indicates the level of uncertainty around this difference.
Quality-adjusted life-years were computed using the area under the curve approach, weighting the 12-month period by quality of life measured on a scale from 0 to 1. 63
Cost and QALY estimates were combined to provide an incremental cost-effectiveness ratio (ICER), showing the value of money spent on this intervention. We used the ‘bootstrapping’ technique to indicate the level of uncertainty around the ICER estimate. 64 A total of 5000 replicates of the ICER were generated and used to construct a cost-effectiveness plane and a cost-effectiveness acceptability curve. These indicate the probability that the intervention is cost-effective at any given level of willingness to pay for a QALY.
Base-case results used data with complete information, the ‘complete cases’. Missing cost and QALY data were imputed to allow comparison and to test the robustness of the base-case results. The multiple imputation by chained equation procedure was used. 65 The regression analyses used to impute missing cost data included age, gender, randomisation group, GP costs, secondary care costs and cost of the intervention as these were associated with missing outcome data. For the QALY regression, in addition to age, gender, randomisation group and EQ-5D scores, we also used the variables identified as being associated with missing data and included in the imputation of the clinical outcomes, namely baseline values for receipt of counselling, educational level, homeownership, weekly alcohol use, smoking and SF-12 physical component score (see Table 6).
Results
Data completeness
Data were missing either because permission to interrogate GP records was not given or because questionnaires were not fully completed at all time points. The most complete data were those obtained from GP records (86% complete) and data for the cost of the intervention, where data were recorded by the PAFs. Complete cost data were available for 156 (43%) participants – 72 from the usual care group and 84 from the intervention group. Complete EQ-5D data to estimate QALYs were available for 195 (54%) participants, 92 usual care and 103 intervention. Complete cost and QALY data were available for 152 (42%) participants, 71 usual care and 81 intervention. The base-case cost-effectiveness results use complete cases (n = 152) but we also include results for all available data where possible to maximise transparency and aid interpretation of results.
Resource use
The mean number of NHS contacts by participant is shown in Table 34. Results in this table are based on all available information for each participant, giving a variable number of observations in each category. Data on GP and nurse consultations were available for over 86% of participants; of these, 98% had some contact with a GP during the year and 64% had some contact with a nurse. The median number of each type of consultation was 8 for GPs and 1 for nurses. In total, 95% of participants were issued with at least one prescription, with a mean number of 8.2 and a median of 6. Participants accessed a wide range of services through primary care. Secondary care encounters were less common: 56 outpatient visits were reported during the year by 13 participants, and 2 participants visited an A&E department. There was no evidence of a difference between the usual care group and the intervention group in any category of resource use.
| Usual care | Intervention | Difference | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| GP at the surgery | 156 | 6.38 | 4.15 | 154 | 7.08 | 4.52 | 0.70 | –0.27 to 1.67 |
| GP home visit | 156 | 0.02 | 0.14 | 154 | 0.01 | 0.08 | –0.01 | –0.04 to 0.01 |
| GP telephone | 156 | 1.34 | 2.30 | 154 | 1.29 | 2.29 | –0.05 | –0.57 to 0.46 |
| GP unknown | 156 | 0.06 | 0.31 | 154 | 0.03 | 0.18 | –0.03 | –0.09 to 0.03 |
| Practice nurse at the surgery | 156 | 1.52 | 2.13 | 153 | 1.50 | 1.89 | –0.02 | –0.47 to 0.43 |
| Practice nurse home visit | 156 | 0.02 | 0.14 | 153 | 0.00 | 0.00 | –0.02 | –0.04 to 0.00 |
| Practice nurse telephone | 156 | 0.11 | 0.48 | 153 | 0.07 | 0.26 | –0.04 | –0.12 to 0.05 |
| Health visitor | 156 | 0.05 | 0.44 | 153 | 0.05 | 0.35 | –0.01 | –0.09 to 0.08 |
| District nurse | 156 | 0.01 | 0.16 | 153 | 0.00 | 0.00 | –0.01 | –0.04 to 0.01 |
| Other nurse (e.g. midwife, nurse practitioner) | 156 | 0.13 | 0.60 | 153 | 0.20 | 1.22 | 0.07 | –0.15 to 0.28 |
| HCA/phlebotomist | 156 | 0.31 | 0.83 | 153 | 0.63 | 1.82 | 0.31 | 0.00 to 0.63 |
| Other primary care (e.g. counsellor, community mental health team) | 156 | 0.19 | 0.98 | 147 | 0.26 | 0.80 | 0.07 | –0.14 to 0.27 |
| Out of hours | 158 | 0.23 | 0.59 | 151 | 0.23 | 0.75 | –0.01 | –0.16 to 0.14 |
| NHS Direct | 93 | 0.09 | 0.34 | 101 | 0.07 | 0.32 | –0.02 | –0.12 to 0.07 |
| Walk-in centre | 95 | 0.21 | 0.68 | 104 | 0.13 | 0.46 | –0.09 | –0.25 to 0.08 |
| Prescribed medications | 156 | 7.74 | 7.17 | 154 | 8.68 | 8.70 | 0.94 | –0.84 to 2.72 |
| Secondary care | 82 | 0.26 | 0.91 | 95 | 0.39 | 2.13 | 0.13 | –0.37 to 0.63 |
Data on the use of social and community services revealed very low use. Eight participants recorded contacts with a community support worker, a mental health nurse or a social worker; nine recorded having been to a day centre or being part of a self-help group; and nine recorded contact with ‘other services’, which included voluntary sector groups such as Lifecycle (cycling support group). As the reported use of these services was so low, they were excluded from the cost analysis to prevent a small number of observations having a disproportionate effect on the results.
Table 35a gives data on time off work by participants and their carers. Of those who provided data (n = 178, 41%), 65% reported no time off due to their illness, and for those who did take time away from work the median length of time away was 40 days. The mean amount of time off work by those in the intervention group was twice that of those in the usual care group (Table 35b).
| Number of working days off | Usual care | Intervention | ||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| Participant | ||||
| 0 | 57 | 69 | 59 | 62 |
| 1–5 | 6 | 7 | 4 | 4 |
| 6–10 | 4 | 5 | 5 | 5 |
| 11–15 | 0 | 0 | 1 | 1 |
| 16–20 | 1 | 1 | 2 | 2 |
| 21–40 | 7 | 8 | 3 | 3 |
| 41–80 | 2 | 2 | 8 | 8 |
| > 80 | 6 | 7 | 13 | 14 |
| Total | 83 | 100 | 95 | 100 |
| Carer | ||||
| 0 | 83 | 100 | 92 | 97 |
| 2 | 0 | 0 | 1 | 1 |
| 5 | 0 | 0 | 1 | 1 |
| 9 | 0 | 0 | 1 | 1 |
| Total | 83 | 100 | 95 | 100 |
| Usual care | Intervention | Difference | 95% CI | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Total number of days off (participant and carer) | 14 | 37 | 29 | 56 | 14 | –0.02 to 28.55 |
| Value of lost productivity (£) | 1424 | 3845 | 2942 | 6060 | 1517 | –9 to 3044 |
Cost of health-care services
Tables 36 and 37 provide information about the mean cost per participant by category of resource use. Table 36 is based on all available data and Table 37 is based on participants for whom we had complete cost data at all time points. Consultations by a GP accounted for > 60% of total health-care costs, excluding the cost of the intervention, and prescribed medication for > 20%. Comparing the two groups, the cost of primary care for those in the intervention group was greater than the cost for those in the usual care group, although these costs were very variable and there is no evidence of a statistical difference between the two groups. The cost of prescribed medication was also greater for those in the intervention group, giving a total difference in mean cost per participant of £39 (95% CI –£53 to £131).
| Usual care | Intervention | Difference | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| GP at the surgery | 156 | 197.92 | 128.54 | 154 | 219.62 | 140.03 | 21.69 | –8.34 to 51.73 |
| GP home visit | 156 | 1.98 | 14.19 | 154 | 0.67 | 8.30 | –1.31 | –3.91 to 1.29 |
| GP telephone | 156 | 25.46 | 43.78 | 154 | 24.43 | 43.49 | –1.03 | –10.78 to 8.73 |
| GP unknown | 156 | 1.87 | 9.18 | 154 | 0.95 | 5.19 | –0.92 | –2.59 to 0.75 |
| GP total | 156 | 227.23 | 144.10 | 154 | 245.66 | 153.96 | 18.43 | –14.89 to 51.75 |
| Practice nurse at the surgery | 156 | 15.19 | 21.27 | 153 | 14.97 | 18.92 | –0.22 | –4.73 to 4.28 |
| Practice nurse home visit | 156 | 0.25 | 1.79 | 153 | 0.00 | 0.00 | –0.25 | –0.53 to 0.03 |
| Practice nurse telephone | 156 | 0.67 | 2.92 | 153 | 0.44 | 1.59 | –0.23 | –0.75 to 0.30 |
| Health visitor | 156 | 1.69 | 15.51 | 153 | 1.12 | 9.09 | –0.58 | –3.43 to 2.28 |
| District nurse | 156 | 0.31 | 3.84 | 153 | 0.00 | 0.00 | –0.31 | –0.92 to 0.30 |
| Other nurse | 156 | 1.67 | 8.36 | 153 | 2.02 | 13.94 | 0.35 | –2.22 to 2.91 |
| All primary care nursing | 156 | 19.78 | 28.38 | 153 | 18.54 | 27.80 | –1.24 | –7.53 to 5.05 |
| HCA/phlebotomist | 156 | 2.17 | 5.71 | 153 | 4.32 | 12.57 | 2.15 | –0.03 to 4.33 |
| Other primary care professional | 153 | 14.48 | 63.64 | 150 | 8.54 | 38.72 | –5.94 | –17.88 to 6.00 |
| Out of hours | 156 | 5.13 | 15.67 | 153 | 5.53 | 14.86 | 0.40 | –3.02 to 3.82 |
| NHS Direct | 93 | 1.89 | 6.97 | 101 | 1.43 | 6.70 | –0.46 | –2.39 to 1.48 |
| Walk-in centre | 95 | 6.77 | 21.96 | 104 | 4.02 | 14.66 | –2.75 | –7.93 to 2.43 |
| All other primary care | 83 | 22.56 | 49.21 | 93 | 22.67 | 47.66 | 0.10 | –14.32 to 14.53 |
| All primary care consultations | 83 | 263.78 | 14.81 | 93 | 288.69 | 19.03 | 24.90 | –23.50 to 73.31 |
| Prescribed medication | 156 | 61.87 | 109.78 | 154 | 96.93 | 188.92 | 35.07 | 0.59 to 69.55 |
| Secondary care | 82 | 23.85 | 142.03 | 95 | 26.99 | 141.57 | 3.14 | –39.04 to 45.31 |
| All NHS | 72 | 355.61 | 258.19 | 84 | 394.49 | 314.43 | 38.89 | –53.08 to 130.85 |
| Usual care (n = 72) | Intervention (n = 84) | Difference | 95% CI | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| GP | 220 | 124 | 234 | 150 | 13 | –31 to 57 |
| All primary care nursing | 18 | 28 | 21 | 31 | 3 | –6 to 12 |
| All other primary care | 22 | 51 | 22 | 47 | –1 | –16 to 15 |
| All primary care consultations | 261 | 132 | 276 | 173 | 15 | –34 to 65 |
| Prescribed medication | 68 | 121 | 94 | 160 | 27 | –19 to 72 |
| All primary care | 328 | 209 | 371 | 265 | 42 | –34 to 119 |
| Secondary care | 27 | 151 | 24 | 141 | –3 | –50 to 43 |
| All NHS | 356 | 258 | 394 | 314 | 39 | –53 to 131 |
Cost of intervention
Table 38 gives the breakdown of time spent by the PAFs dealing with participants. Time spent on face-to-face sessions was slightly longer than that spent on phone contacts, but nearly as much time was spent on travel associated with face-to-face sessions as on the sessions themselves.
| Mean | SD | Max. | |
|---|---|---|---|
| Face-to-face sessions | 94 | 52 | 240 |
| Travel time | 170 | 93 | 270 |
| Phone contact | 80 | 66 | 405 |
| Total time on sessions | 344 | 187 | 825 |
| Non-contact phone calls | 14 | 12 | 120 |
| Writing letters | 2 | 4 | 15 |
| E-mailing | 4 | 13 | 110 |
| Sending texts | 13 | 17 | 96 |
| All non-contact time | 34 | 26 | 171 |
| Total time | 377 | 194 | 869 |
The mean total cost of the intervention, allowing for training and supervision, and subsidies given to some participants, was £220 per participant for all 182 participants (Table 39) and £252 for those in the complete cost and QALY set, which includes 81(45%) of those in the intervention group.
| All available data (n = 182) | Complete cost and QALY data (n = 81) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Training and supervision (fixed cost) | 14.57 | – | 14.57 | – |
| Cost of subsidies and related PAF time | 3.82 | 15.29 | 4.97 | 17.72 |
| Cost of PAF sessions excluding travel | 110.78 | 63.92 | 127.20 | 57.10 |
| Travel | 90.46 | 49.77 | 105.48 | 46.43 |
| Cost of PAF sessions | 201.24 | 103.25 | 232.68 | 88.09 |
| Total cost of intervention | 219.63 | 106.93 | 252.22 | 90.00 |
Value of lost productivity
The value of lost productivity due to time off work is given in Table 35b. The participants in the intervention group reported considerably more time off work than those in the usual care group, resulting in a difference in mean cost per participant between the two groups of £1517.
Quality-adjusted life-years
The EQ-5D scores for each measurement period are shown in Figure 4, for three levels of data completeness. The general pattern is that participants for whom we had the most complete data scored more highly than those with missing data, and those in the intervention group had a higher score at 12 months post randomisation than those in the usual care group. The slight imbalance across the groups at baseline for the set with complete QALY data is reflected in the QALYs shown in Table 40; the effect of the higher scores is mitigated by lower baseline scores.
FIGURE 4.
European Quality of Life-5 Dimensions (EQ-5D) scores by group.
| Usual care | Intervention | Difference | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| Complete QALY data | 92 | 0.784 | 0.149 | 103 | 0.781 | 0.181 | –0.004 | –0.05 to 0.04 |
| Complete cost and QALY data | 71 | 0.795 | 0.154 | 81 | 0.809 | 0.139 | 0.014 | –0.03 to 0.06 |
Cost-effectiveness
The point estimate of the incremental cost per QALY gain is £20,834 (Table 41) and the uncertainty around this estimate is illustrated in Figures 5 and 6. The probability that the intervention is cost-effective is 0.49 at a willingness to pay of £20,000 per QALY and 0.57 if the willingness to pay is £30,000 per QALY.
| Usual care (n = 71) | Intervention (n = 81) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| All primary care consultations (£) | 261 | 133 | 273 | 171 |
| Medication (£) | 63 | 117 | 96 | 162 |
| Secondary care (£) | 26 | 152 | 25 | 143 |
| NHS costs excluding intervention (£) | 350 | 256 | 394 | 317 |
| Cost of intervention (£) | 0 | 0 | 252 | 90 |
| Total cost (£) | 350 | 256 | 646 | 322 |
| QALYs | 0.795 | 0.15 | 0.809 | 0.14 |
| Incremental cost (£) (95% CI) | 296 (202 to 390) | |||
| Incremental benefit, QALY gain (95% CI) | 0.014 (–0.033 to 0.061) | |||
| ICER, incremental cost per QALY gain (£) | 20,834 | |||
| Median net monetary benefit (probability that net monetary benefit > 0) | ||||
| Willingness to pay = £20,000 per QALY | –9 (0.49) | |||
| Willingness to pay = £30,000 per QALY | 134 (0.57) | |||
FIGURE 5.
Cost-effectiveness plane showing bootstrapped replicates of the ICER.
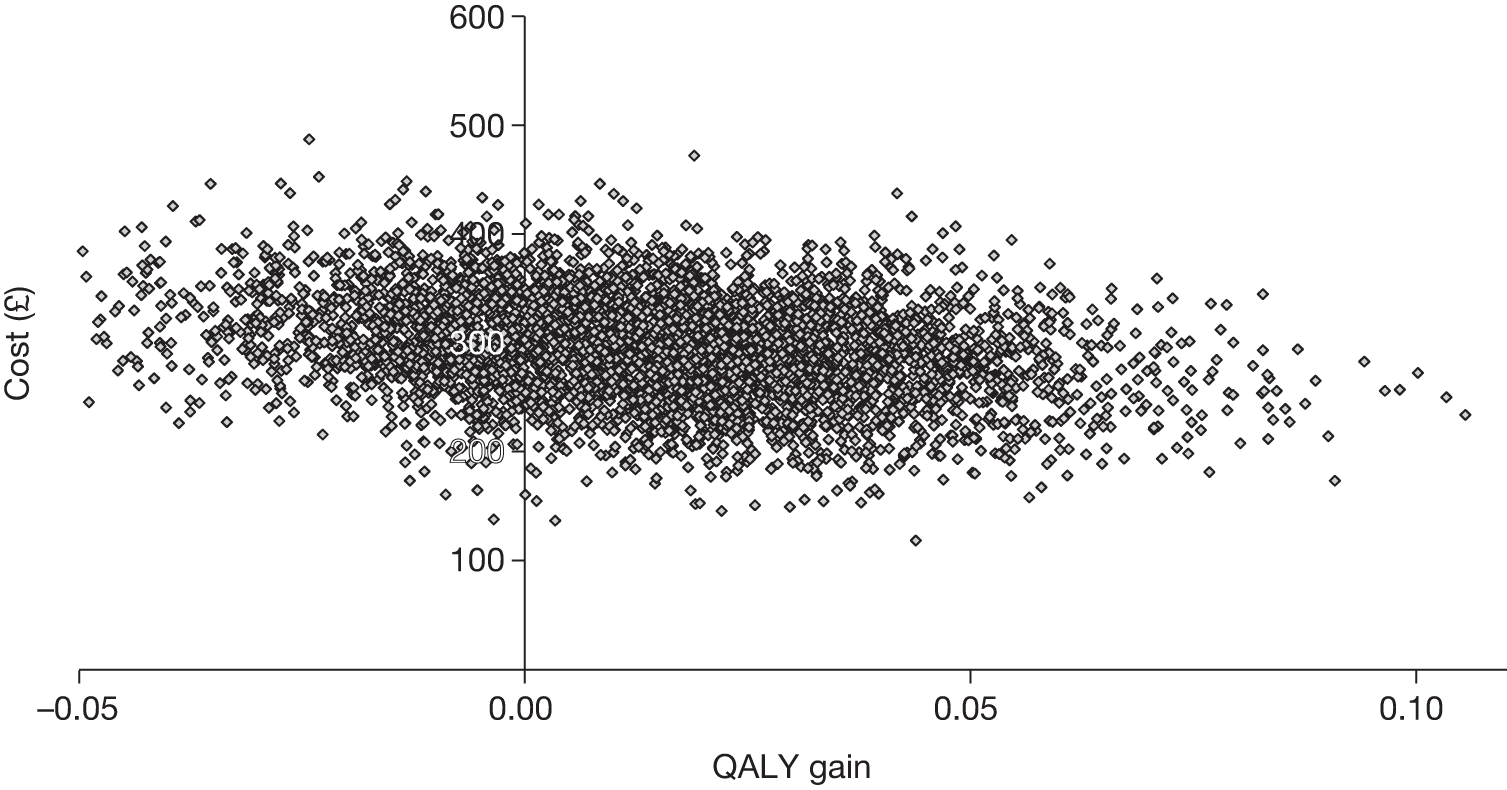
FIGURE 6.
Cost-effectiveness acceptability curve showing the probability that the intervention is cost-effective at different willingness-to-pay thresholds.
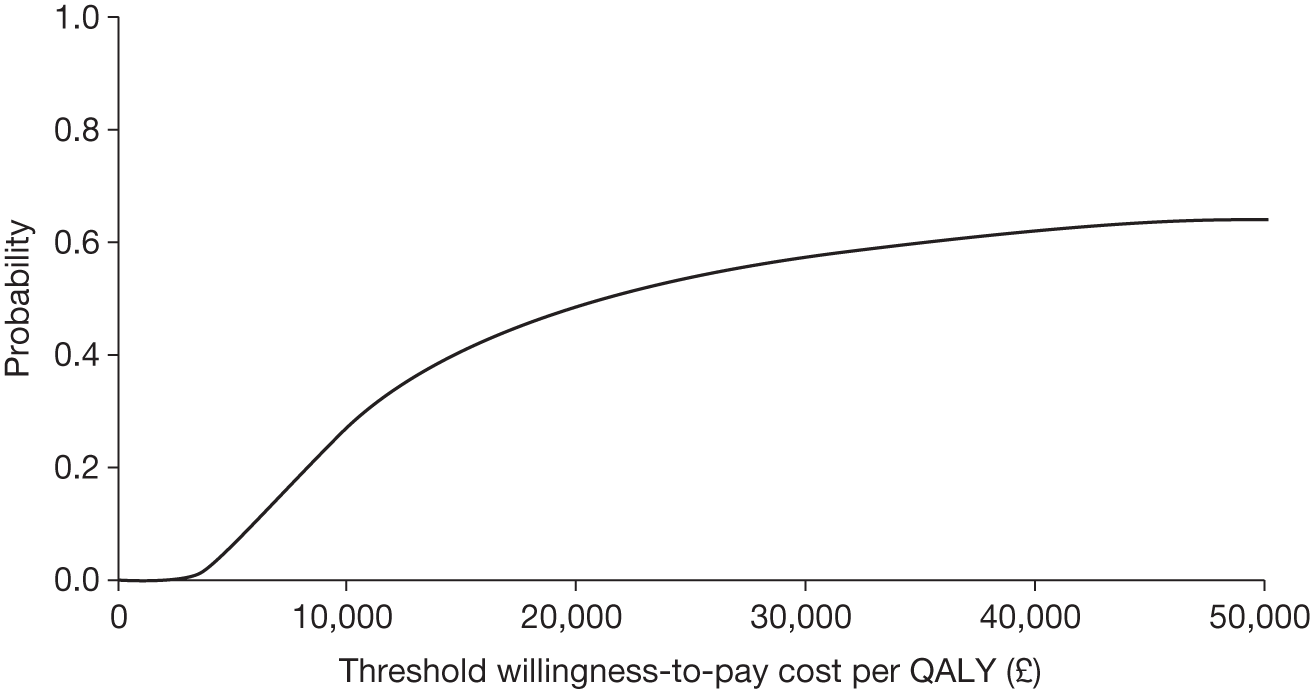
Sensitivity analysis: missing data
The results of imputing missing cost and QALY data are shown in Table 42. Compared with the complete case analysis, costs in the intervention group were lower because the participants for whom we did not have complete data (because of dropout, failure to complete questionnaires or withholding permission to interrogate GP records) were lower users of the intervention than those who provided complete data. QALY gain is lower in both groups although the difference between the two groups is unchanged.
| Usual care (n = 179) | Intervention (n = 182) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| NHS costs excluding intervention (£) | 352 | 241 | 402 | 269 |
| Cost of intervention (£) | 0 | 0 | 220 | 107 |
| Total cost (£) | 352 | 241 | 622 | 294 |
| QALYs | 0.745 | 0.172 | 0.759 | 0.174 |
| Incremental cost (£) (95% CI) | 270 (215 to 326) | |||
| Incremental benefit, QALY gain (95% CI) | 0.014 (–0.022 to 0.050) | |||
| ICER, incremental cost per QALY gain (£) | 19,394 | |||
| Median net monetary benefit (probability that net monetary benefit > 0) | ||||
| Willingness to pay = £20,000 per QALY | 4 (0.50) | |||
| Willingness to pay = £30,000 per QALY | 142 (0.60) | |||
The ICER for the intervention using the imputed data is £19,394, slightly lower than the complete case analysis (£20,834). The probabilities of a positive net monetary benefit are correspondingly slightly higher at 0.50 for the £20,000 threshold (compared with 0.49) and 0.60 for the £30,000 threshold (compared with 0.57) (see Table 42 and Figures 7 and 8).
FIGURE 7.
Cost-effectiveness plane showing bootstrapped replicates of the ICER – imputed data.
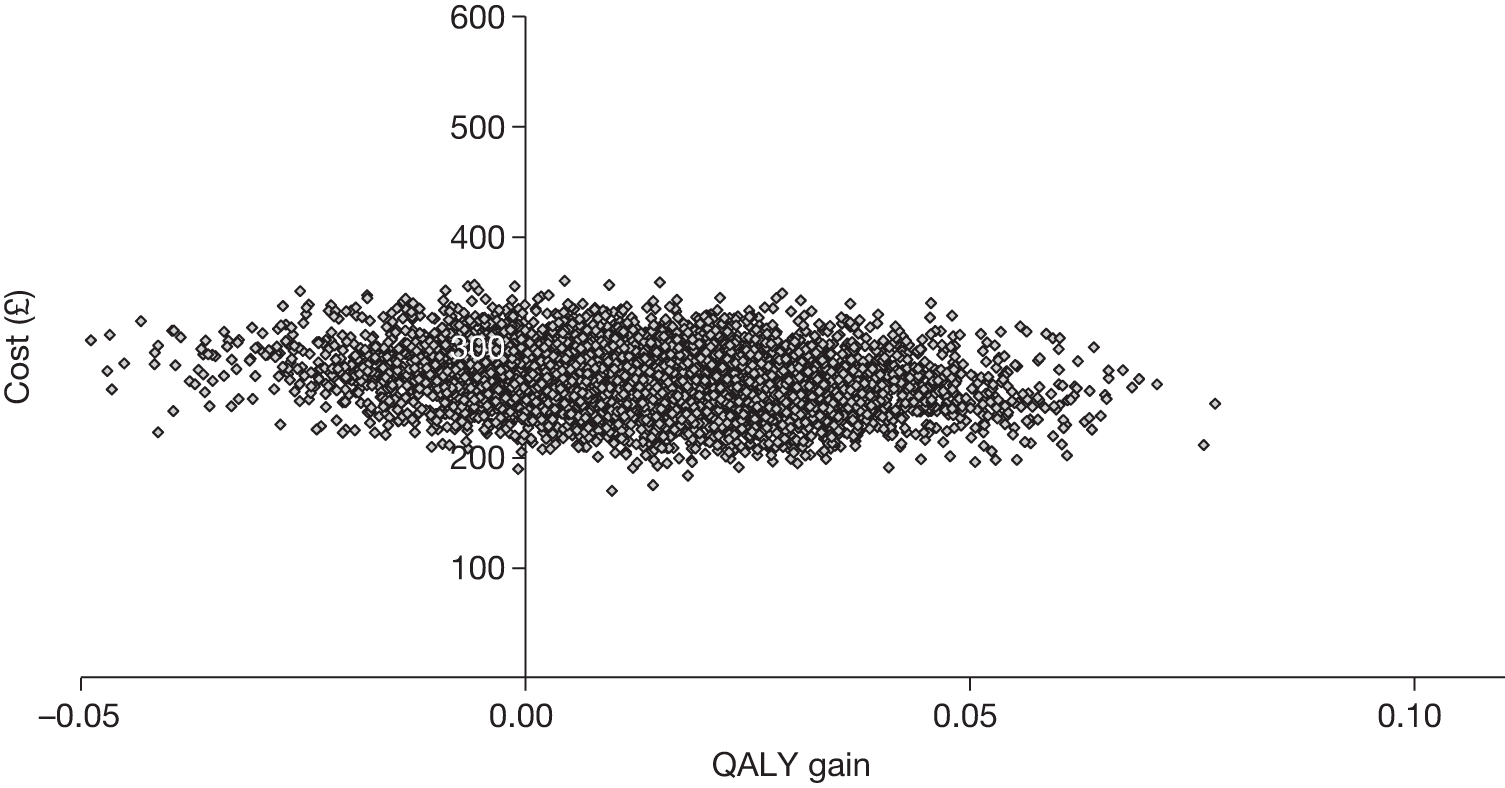
FIGURE 8.
Cost-effectiveness acceptability curve showing the probability that the intervention is cost-effectiveness at different willingness-to-pay thresholds – imputed data.
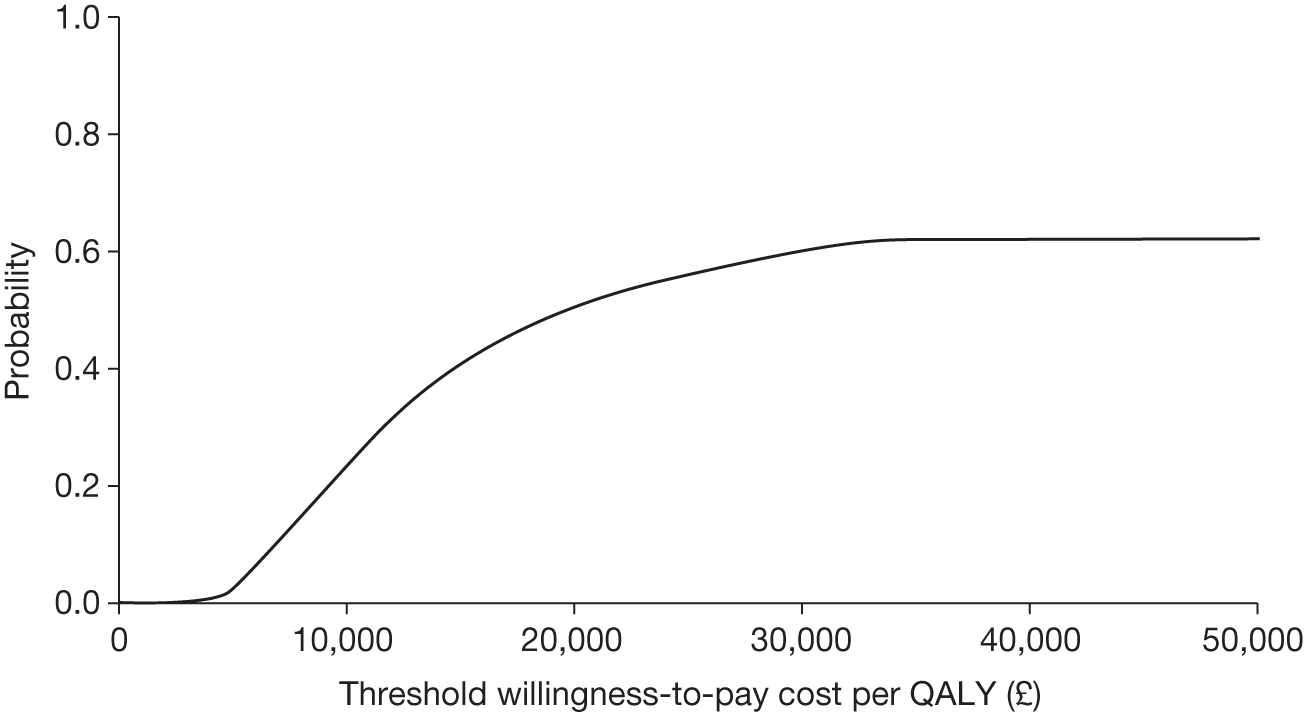
Chapter 5 Qualitative study
Introduction
Qualitative methods are increasingly being used within RCTs to explore the views and experiences of trial participants, and those of individuals involved in recruitment to the intervention being assessed. Data gathered can be an essential part of a trial’s evaluation and can highlight possible reasons for quantitative findings. The qualitative study nested within TREAD explored participants’ and GPs’ views and experiences of the trial and physical activity intervention. Some of the results presented here have already been published. 66 The specific aims were to investigate:
-
participants’ and GPs’ beliefs about and attitudes to physical activity as a treatment for depression
-
the acceptability and experience of the physical activity intervention
-
how being in the usual care arm affected behaviour.
We also report the views of patients and GPs about their participation in the research study.
Participants’ views and experiences
Methods
Recruitment and sampling
All trial participants had consented at baseline to being approached by a qualitative researcher. Using information collected during the baseline questionnaire, trial participants were purposively sampled to ensure that interviews were held with men and women in each arm of the trial who varied in age. Within this sampling approach, maximum variation was sought in relation to study centre, location of participants’ general practice (rural, suburban, urban), socioeconomic background according to housing status, educational attainment, level of physical activity and severity of depression at baseline. Participants sampled were contacted by telephone and invited for interview. If they were willing to be interviewed, a time and place was arranged. A letter confirming the interview arrangements was then posted along with an information leaflet about the qualitative study. Three participants declined an interview and one participant did not keep his interview appointment. These four participants gave time constraints as a reason for non-participation.
Interviews
Participants were interviewed at two time points: within a month of both their 4- and 12- month follow-up assessments once the primary and secondary outcome measures had been completed. Participants were interviewed at these two time points so that qualitative data could illuminate possible reasons for the quantitative findings resulting from analysis of data collected during the 4- and 12-month follow-ups. Participants were not interviewed before the 4-month follow-up in case the experience of being interviewed influenced their views of the trial and the treatment they received. The longitudinal design allowed participants’ experiences and views to be tracked over time and issues that were raised in the first interview, for example barriers to increasing levels of physical activity, to be discussed and explored again during the 12-month interview.
For both sets of interviews, a topic guide was used to ensure consistency across the interviews whilst allowing participants to raise issues that were salient to them. Two versions of the 4- and 12-month guides were developed, one for participants randomised to the intervention group and another for participants who were allocated to usual care (Table 43).
| Type of interview | Topic area |
|---|---|
| 4-month interview | Experience of recruitment to TREAD |
| Reasons for taking part in TREAD | |
| Physical activity as a treatment for depression | |
| Views of the trial | |
| Definition and experience of physical activity | |
| Barriers and supports to doing physical activity | |
| Experience and views of other treatments for depression | |
| Expectations of future physical activity levels | |
| 12-month interview | Current mood and well-being |
| Changes in physical activity levels and situation in last 8 months | |
| Current treatment for depression | |
| Current levels of physical activity | |
| Barriers and supports to doing physical activity | |
| Impact of physical activity on mental well-being | |
| Physical activity as a treatment for depression | |
| Expectations of future physical activity levels | |
| Intervention only at 4 and 12 months | Relationship with PAF |
| What was helpful/not helpful about PAF intervention | |
| Mode of delivery of intervention | |
| View on discontinuation of PAF intervention |
Of the 33 participants interviewed following their 4-month follow-up assessment (Table 44), 28 were interviewed on a face-to-face basis in their own homes, 2 in health-care centres and 3 on university premises. Of these 33 participants, 21 (12 intervention and 9 usual care) were then interviewed again following their 12-month follow-up assessment. Three participants were not contactable at 12 months, two had withdrawn from the study and seven were not able to commit to a second interview because of work and other commitments. Having previously developed a good rapport with participants through the 4-month interviews, the follow-up interviews were conducted by telephone at the convenience of the participants. Written consent to be interviewed was obtained at the first interview. The 4-month interviews were held between March and December 2009, and the 12-month interviews between December 2009 and August 2010. The 4-month interviews lasted between 30 and 120 minutes and the 12-month interviews between 20 and 40 minutes.
| Characteristic | Category | Intervention | Usual care |
|---|---|---|---|
| Centre | Bristol | 10 | 6 |
| Exeter | 9 | 8 | |
| Age range (years) | 19–69 | 21–65 | |
| Gender | Male | 8 | 6 |
| Female | 11 | 8 | |
| Employment status | Employed full-time | 8 | 6 |
| Employed part-time | 2 | 3 | |
| Unemployed | 3 | 1 | |
| Retired | 2 | 2 | |
| Permanent sick/full-time carer | 2 | 2 | |
| Training/education | 2 | 0 | |
| Educational attainment | Higher degree | 1 | 1 |
| Degree | 4 | 3 | |
| Diploma | 1 | 2 | |
| A level | 6 | 4 | |
| GCSE/O level | 3 | 2 | |
| Other | 2 | 1 | |
| None | 2 | 1 | |
| Ethnicity | White | 18 | 13 |
| Asian | 0 | 1 | |
| Mixed | 1 | 0 | |
| Married/cohabiting | Yes | 9 | 9 |
| Currently on antidepressants | Yes | 12 | 10 |
| History of depression | Yes | 13 | 10 |
| Physical comorbidity | Overall | 7 | 9 |
| Hypertension/heart disease | 2 | 4 | |
| Rheumatoid arthritis/fibromyalgia | 1 | 1 | |
| Cancer | 1 | 1 | |
| Back pain | 1 | 1 | |
| Stroke | 0 | 1 | |
| Diabetes | 1 | 0 | |
| Sarcoid of lung | 1 | 0 | |
| Visual loss | 0 | 1 | |
| Physical activity level | Low | 13 | 8 |
| Medium | 6 | 5 | |
| High | 0 | 1 | |
| CIS-R score | Mild | 5 | 2 |
| Moderate | 12 | 9 | |
| Severe | 2 | 3 | |
| BDI score | Range 15–57 | 18–45 | 15–57 |
| Mean = 29.6 | 33.8 | 31.7 | |
| Location of general practice | Urban | 12 | 7 |
| Suburban | 2 | 3 | |
| Rural | 5 | 4 |
Analysis of the interviews
All of the interviews were audiotaped and transcribed verbatim following written participant consent. Data collection and analysis were conducted in parallel to allow early analysis to inform the focus of later interviews. Throughout this period, transcripts were read and re-read by individual members of the research team to gain an overall understanding of the participants’ views and experiences and to identify emerging themes. Discussions were held between team members to debate the most appropriate analytical approach to use. It was decided that the interviews would be analysed thematically as this would allow comparisons to be made within and across the interviews and the views expressed in relation to a particular issue to be highlighted, for example participants’ views of physical activity as a treatment for depression.
Coding frames were developed for each data set. Three members of the research team independently coded transcripts and then met to discuss areas of consensus and discrepancy. This led to further codes being developed and to existing codes being defined more clearly. Transcripts were then imported into the software package ATLAS.ti (ATLAS.ti V5.O; Scientific Software Development, Berlin, Germany) to allow electronic coding and retrieval of data. Once all of the transcripts had been coded, data were systematically analysed using a framework approach. 67
Using this method, what participants had said in relation to specific issues was summarised in tables and comparisons were then made both within and across interviews to identify thematic patterns and deviant cases. The qualitative analysis was completed before analysis of the trial data so that the quantitative findings would not bias interpretation of the qualitative material.
Results
Participants’ views of physical activity as a treatment for depression
Definitions of physical activity
Participants varied in how they defined physical activity. Some described how they perceived physical activity as simply being able to function or encompassing low-intensity activities that could be built into everyday life, such as walking or gardening.
Physical activity for me would be, well, getting yourself out of bed in the morning, able to do things at work comfortably and not make it feel like a drain or a strain on you.
(Male, 44 years, intervention, moderate depression, medium active, urban, 4 months)
Well physical activity really just means moving around as much as possible, taking every opportunity. So its stairs instead of escalators, you know, parking your car further away from where you want to be to walk that little bit extra.
(Female, 48 years, intervention, moderate depression, low active, rural, 4 months)
Conversely, others reported more ‘purposeful’ or high-intensity activities such as running or swimming and structured classes such as Pilates and aerobics. These participants were usually individuals who were male and had a history of engaging in aerobic activity.
A level of intensity that brings you out in a sweat. A minimum of half an hour, more like an hour, I would consider that to be proper physical activity. It’s doing exercise for the sake of doing exercise, do you know what I mean.
(Male, 43 years, intervention, severe depression, medium active, rural, 4 months)
There was also a group of participants who defined physical activity in relation to their own physical capability. These individuals often had comorbid conditions that limited them physically, such as rheumatoid arthritis, coronary heart disease or fibromyalgia. These participants more often reported the benefits of a simple walk and that, overall, it had to be ‘something you enjoyed’, which highlighted the need for tailored activity.
I think you need to tailor the activity to your own personal abilities. It’s like with my condition [immune deficiency] I can’t obviously do aerobics, that would be too extreme. But if it’s tailored to your own needs, and if you’re capable of doing aerobics and that’s an interest to you, then that’s fine. Or just gardening, you know, walking, that way of doing physical activity.
(Female, 38 years, usual care, moderate depression, low active, urban, 4 months)
Physical activity as a treatment for depression
Most participants stated that they felt that physical activity could be an acceptable treatment for depression, and there was a general awareness that physical activity could be an effective means of managing depression. Evidence of this awareness was expressed by most participants, across both arms of the trial, and was informed anecdotally through the media and participants’ own experiences.
You read a lot things, and I remember reading that Ronnie O’Sullivan, the snooker player, he goes running as an antidote to the fact that he suffers from depression.
(Male, 43 years, intervention, severe depression, medium active, rural, 4 months)
I think it’s really a no-brainer. I always feel energised and elevated after I have done something that’s caused me hard work, my heart to beat faster. And usually when I’ve achieved something, you get a sense of euphoria. So I would have thought that exercise does help.
(Female, 63 years, usual care, severe depression, low active, suburban, 4 months)
When describing the possible mechanisms by which physical activity could relieve the symptoms of depression, participants mentioned biochemical pathways and physical activity providing a source of distraction from negative thoughts, and having wider lifestyle benefits and giving a sense of purpose.
There’s the physical well-being, feeling good, the emotional sense of challenge as I always push myself. The chemical high when you get back from a run and the emotional feel you get from actually pushing yourself into doing something.
(Male, 35 years, usual care, mild depression, low active, urban, 4 months)
I know that if you increase the amount of movement and your activity then your serotonin level is going to kick in and it’s going to make you feel better.
(Female, 48 years, intervention, moderate depression, low active, urban, 4 months)
Many of the participants who had been classified as having ‘medium’ or ‘high’ levels of physical activity at baseline stated that aerobic activities would be most helpful for depression. These individuals usually cited a biochemical imbalance in the brain as a cause of depression. In contrast, participants reporting low levels of activity at baseline, and participants citing situational factors as a cause of depression, usually mentioned lower-intensity activities as helpful. These participants detailed how they thought low-intensity activities could be helpful as a means of distraction.
I know how it [physical activity] can help distract me. And I switch then to positive thoughts, you know, positive thinking about much better things, so I know it works. I know when I’ve gone out walking with my friend, I’m concentrating on my surroundings which are great, I’m looking at different things, I’m not thinking about all the negative terrible things that I seem to have infested myself with. So I know it works, it makes you think much more positively.
(Female, 63 years, usual care, severe depression, low active, suburban, 4 months)
Some participants also reported that they experienced the benefits of physical activity through the interaction of a number of factors. For example, some participants reported that engaging in physical activity could facilitate social interaction, regulate sleep cycles and eating behaviour and control weight – a holistic experience that, in turn, could enhance self-confidence and esteem.
You’re feeling better about yourself and I think that lifts the mood and then the sleep pattern usually, you know, it has a knock on effect, eating wise you, you feel more like eating and more regularly.
(Male, 60 years, usual care, moderate depression, low active, urban, 4 months)
I’ve lost half a stone without really trying, and that’s very positive. Obviously the less weight your body carries, the more energy you have. So the less weight I’ve carried I’ve found myself able to keep going a lot longer and do more without the need to sit down for ten minutes or rest for ten minutes. And I’m sleeping better, which in turn, when I wake up in the morning you feel more optimistic. So it’s benefits all the way around I think.
(Female, 63 years, usual care, severe depression, low active, suburban, 4 months)
The benefits of engaging in physical activity could also be anticipated before engaging in an activity and could provide a sense of purpose that would lead to further positive actions.
I’m doing an activity that I’m satisfied with, that’s fine, I’m going to get self-esteem from it, so therefore when I’ve made the decision to go and do it the process has already begun before the activity has started.
(Male, 55 years, intervention, moderate depression, low active, rural, 4 months)
During the 4- and 12-month interviews, many participants in both trial arms reported that their mood had improved since joining the trial and partly attributed this to increases in levels of physical activity. However, more often enhanced mood was attributed to improvements in situational factors such as employment circumstances, finances and interpersonal relationships. Enhanced mood was also attributed to use of antidepressant medication, the passing of time and seasonal variation. Thus, it was often difficult for participants to delineate the reasons for enhanced mood and, for many, improved mood was a result of several factors converging together.
I’m a lot better; sort of normal is probably the best description. Occasionally I have a funny 5 minutes where I have a bit of a wobbly day, but on the whole, much better and much more normal feelings, normal enjoyment of stuff compared to a long time ago. It’s down to long-term medication and sort of gradual stabilisation of circumstances and getting used to it really, getting used to depression.
(Female, 24 years, usual care, moderate depression, low active, urban, 12 months)
I changed jobs. You know, time is a factor. Definitely getting out and doing the exercise has helped I’m sure. So I think it’s not just one thing, but certainly a whole host of things kind of came together at long last. But the job was definitely a big factor.
(Male, 43 years, intervention, severe depression, medium active, rural, 12 months)
Supports and barriers to undertaking physical activity
Many participants in both arms of the trial described both physical and mental symptoms of depression. The physical symptoms included lethargy and fatigue, whereas the mental symptoms included low confidence. Such symptoms were described as hindering efforts towards increasing activity and were evident across the study arms, gender, age range and all levels of activity and severity of depression.
You feel as though you are walking through a bog in the fog, like you’re dragging your limbs around – you need to get out of that stage in order to start doing something.
(Male, 55 years, intervention, moderate depression, low active, rural, 4 months)
You find yourself withdrawing until you find a comfortable place, like your home, and you just want to be safe in that place. And you feel that if you venture out of that through physical activity you feel more vulnerable, until you get to a point when you’re beginning to come through the worst of it, and then physical activity is not so daunting, you feel more positive.
(Female, 38 years, usual care, moderate depression, low active, urban, 4 months)
Although the positive aspects of activity were emphasised, some participants described negative aspects of attempting to engage in physical activity, which could be demotivating. These negative aspects mainly related to perceived physical ability, confidence to engage in an activity and self-image. Such views tended to be reported by female, low-active participants:
I didn’t enjoy rock climbing at all indoors. I have absolutely no upper body strength so it was – I didn’t like it, it made me feel like I was inadequate.
(Female, 24 years, usual care, moderate depression, low active, urban, 4 months)
I was finding, you know, like walking, I’d get out of puff and a bit achy. I don’t enjoy ageing, and finding that my strength and my stamina doesn’t last like it used to can be quite demotivating. Sometimes it leaves my legs shaking, sometimes I ache the next day. Sometimes I discover muscles, especially if it’s a bit of exercise I haven’t done for a few months, that I didn’t know I’d got.
(Female, 63 years, usual care, moderate depression, low active, urban, 4 months)
I would say that it’s just a self-conscious doubt in yourself that, you want to do it, but you just feel conscious about your whole appearance.
(Female, 40 years, usual care, moderate depression, low active, urban, 4 months)
One female participant felt that it was social conditioning to be a caregiver that was a prominent barrier:
I’ve got other responsibilities, sort of, mentally they’ve got to come first, like caring for the kids, I’ve got a teenager with a baby . . . I should put myself first, do something for me, so I’m in a better sort of state myself to be able to help her. Maybe as a female that’s kind of how you are conditioned – you’ve always got work to do and looking after people.
(Female, 45 years, intervention, moderate depression, low active, urban, 4 months)
Support from significant others was considered to be very important for many participants for initiating and maintaining an interest in physical activity. This support was often found among friends or relatives, or in the sense of companionship experienced in attending classes or other activities. It could be emotional rather than practical in nature:
There’s no practical support, I guess, no sort of practical guidance like ‘I’ll start running with you if you want someone to run with’, or anything like that. They’re [a relative] more the kind of, ‘Tell me when you’re feeling down’, that sort of more talking support I guess.
(Female, 24 years, usual care, moderate depression, low active, urban, 4 months)
However, a degree of self-motivation was still seen to be important:
I can be self-motivated, but also influenced by others as well, I’d say. Because I go with two friends quite regularly, it’s almost like a little thing of competing against each other sometimes. But I feel like you need your own self-motivation to be involved in that. Yeah you’ve got your peer pressure, but if you didn’t have the self-motivation you’d just walk away. So it’s almost a mixture of both.
(Male, 21 years, usual care, moderate depression, medium active, urban, 4 months)
Participants reported practical issues such as cost, time and access to facilities as barriers to engaging in physical activity:
Opportunity gets less, the cost, you know, the money is a big issue of course. Well it’s those two factors isn’t it, money and time really? And I would like to say for somebody like myself, that it doesn’t matter, but it obviously does, it’s quite a big deal to me.
(Male, 35 years, usual care, moderate depression, low active, urban, 4 months)
It’s not like taking a tablet is it? You’ve actually got to do it, and find time to do it. I really find it hard to make the time to do anything.
(Female, 45 years, intervention, mild depression, low active, urban, 4 months)
Physical activity compared with other treatments
Participants in both arms of the trial were asked how physical activity compared with their experience of other treatments for depression. A few stated that the effectiveness of physical activity to manage depression would depend on the severity of depression and whether depression was thought to have a biochemical basis or be due to situational factors.
I suppose it’s difficult because without sort of having antidepressants or counselling or whatever, it’s difficult to know whether that [physical activity] would be enough on its own. I suppose it depends on the level of depression and issues that may be causing it.
(Female, 40 years, usual care, severe depression, low active, urban, 4 months)
Most participants stated a preference for physical activity over other treatments, particularly antidepressants, expressing a desire for some autonomy in the longer-term management of their depression, which they felt could be gained from engaging in physical activity.
I am increasing my confidence, physical activity and some of these more complementary things need to take over from perhaps some traditional medication, you know. Because I’ve got perhaps 30 years to live, do you know what I mean? And I don’t want to be considering taking long-term medication.
(Female, 48 years, intervention, moderate depression, low active, urban, 4 months)
However, one participant who was seeing a counsellor emphasised that she felt that there would still be a need for counselling, whereas another participant suggested that physical activity was not enough by itself and a deeper level of understanding emotions was required to help with depression. Both participants were in the usual care arm of the trial and classified as being low active and with moderate depression.
I find that seeing a counsellor is useful to getting things off your chest that maybe you wouldn’t want to talk to your family about . . . So I feel still being able to talk to somebody, I feel is useful for me. It may be the case of physical activity for other people is enough, but I’m not sure, my personal experience is that I couldn’t do it without other help.
(Female, 38 years, usual care, moderate depression, low active, urban, 4 months)
I know that physical activity is so helpful to how you feel and moving out of depression, or to help manage it, but it doesn’t just go away by going off and jumping around for a couple of hours in an exercise class. You’ve got to learn to, sort of understand how things make you feel.
(Female, 66 years, usual care, moderate depression, low active, suburban, 12 months)
In addition, acknowledgement of the difficulties of engaging in physical activity such as low motivation and confidence meant that some participants viewed physical activity as an adjunct to medication, particularly individuals experiencing more severe episodes of depression. Indeed, medication was seen to have a ‘time and place’ such that it could assist participants in initiating physical activity.
I think I’ve reached the stage with fluoxetine where it’s kick-started the process [engaging in activity]. I hope I have, I feel as though I have.
(Male, 55 years, intervention, moderate depression, low active, rural, 4 months)
A few participants suggested that taking antidepressants could lead to dependency, although it was not clear whether they meant physical or emotional dependency.
Yeah and I didn’t want to [take tablets]. Because obviously I know a few people with depression and who are actually on antidepressants, and they’ve got it for quite a few years and it’s hard to get off.
(Male, 38 years, intervention, moderate depression, low active, urban, 4 months)
However, some saw medication as a more reliable and stable treatment in terms of adherence because of the ease of taking a pill compared with the motivation and commitment required for engaging in physical activity.
There’s no excuse for you not being able to take them [tablets], whereas for exercise you have to be a bit more sporadic about it sometimes, there are other things that will consume your time.
(Female, 24 years, usual care, moderate depression, low active, urban, 12 months)
Only two participants reported a risk of dependency on physical activity. Both of them suggested it could become an obsessive pursuit.
You can almost become obsessive with trying to feel good all the time, so you end up exercising yourself, you know, like a lunatic, just trying to achieve that high all the time. So yeah I think you can go to the extreme.
(Female, 48 years, intervention, moderate depression, low active, rural, 4 months)
I became too obsessive, it was all or nothing approach and I would wear myself out.
(Female, 45 years, intervention, mild depression, low active, urban, 12 months)
Physical activity undertaken by participants during the trial
During the 4- and 12-month interviews, participants in both arms of the trial were asked about any current physical activity they were doing, the type of activity and if they attributed any change in activity levels to trial participation. There did not appear to be clear differences between the levels and types of activity undertaken by participants in each arm of the trial. Participants in both arms of the trial described being more aware of the need to be more physically active and having made an effort to be so. In addition, the intervention and usual care participants who were engaging in physical activity tended to describe undertaking low-intensity activities, such as walking.
Many participants in both arms of the trial detailed how, through their participation in TREAD, they had developed an increased awareness of their activity levels. There was some evidence to suggest that this was an artefact of completing activity logs at baseline and for follow-up for the main study.
I think if anything it [the trial] has made me more encouraged to do more, even though I am not on that side of the study, it’s sort of still made me think, well I’ve taken a good look at how much activity I do, and I know I won’t do enough so it’s made me feel I want to push that little bit further to get myself fit and active again and feel a whole person instead of the half a person I feel at times.
(Female, 38 years, usual care, moderate depression, low active, urban, 4 months)
I think it [the trial] focused my mind a little bit in terms of thinking about exercise. What it really did, and what I found very interesting, was particularly recording. When we were looking at recording how much exercise you’re doing in a week on that chart, [I realised] how little I was doing [laughs].
(Male, 35 years, usual care, mild depression, low active, urban, 4 months)
A few participants had been regular exercisers before their episode of depression and were motivated to get back into a regular pattern of activity.
At the start of the TREAD study it was nothing, and it gradually picked up. So, it’s gradually becoming more and more frequent now. So I am trying to get back to where I was before but it’s a big gap to go from almost 4 months of non-participating to try and get it all back.
(Male, 21 years, usual care, moderate depression, medium active, urban, 4 months)
There were also a few intervention participants who had a strong history of engagement in physical activity who had returned to more specialised or higher-intensity activities after receiving facilitation.
The karate has become the main thing and it’s you know, it’s part of the routine now, so it happens. As I say, it has returned, so at least I’m getting kind of, you know, 2, 2.5 hours training a week, um which has to be beneficial. Um so yeah, I think it [PAF intervention] has been a significant factor without a doubt.
(Male, 43 years, intervention, moderate depression, medium active, rural, 12 months)
One participant in the usual care arm had started engaging in activity and attributed her engagement with activity to factors outside the trial, such as making a new year’s resolution to be more active and noting that a friend with depression always seemed better having been for a run or to the gym. Another usual care participant partly attributed his engagement in activity as a response to the boredom experienced from being signed off work as a result of having depression.
It was a knock-on effect. I had to do something, because I couldn’t sit at home and just fester away like I was. So, just going for walks, you know, if I wanted something from down-town I’d walk instead of jumping in the car, and things like that. And it just sort of morphed into bigger walks.
(Male, 46 years, usual care, mild depression, medium active, urban, 4 months)
Participants who received the intervention reported at both time points making efforts to be more physically active mainly as a result of their contact with the PAF, and how the input they had received had provided a ‘way in’ to making changes in physical activity.
I think that simply having the oversight of the facilitator has encouraged me to keep going with things.
(Male, 55 years, intervention, moderate depression, low active, rural, 4 months)
It had come to a stop really, apart from the odd walk. But what I did was to make a conscious effort to do something every day, or every other day, which is the promise I made [to the PAF]. And I more or less kept to that.
(Female, 66 years, intervention, moderate depression, low active, suburban, 4 months)
The PAF has been really good, and really helped a lot with the way I see things and do things although I have not been able to do a lot – but it has changed, it has actually changed what I do and made me question what I do. And part of me is thinking if I can start feeling a bit better within myself I might be more likely to do exercise or find the time to do exercise.
(Female, 45 years, intervention, moderate depression, medium active, suburban, 12 months)
Participants in the intervention arm, particularly women with children, detailed how they had incorporated activity into family life.
I’m trying to get out with the dog for nearly an hour every day. I have also been going out for run/walks with the kids – because the kids aren’t averse – they like to come out as well because I used to run and they would cycle and so I’ve been trying to build it around the children’s activities.
(Female, 40 years, intervention, mild depression, medium active, suburban, 4 months)
However, there were also a few male participants in the intervention group who reported that they did not make an effort to be more physically active as they were in manual occupations such as the construction industry and were often too tired to contemplate any additional activity and considered their daily tasks as adequate activity:
I am obviously quite active in work, lifting and walking all day. I’m usually too tired – by the time I get home from work I’m too knackered to do anything.
(Male, 38 years, intervention, mild depression, low active, suburban, 4 months)
I’m doing everyday tasks like gardening, walking, washing the car and I’ve also got work to keep me occupied.
(Male, 40 years, intervention, severe depression, low active, rural, 12 months)
Although many participants in both arms of the trial reported an intention to do more physical activity when interviewed at 4 months, there was little evidence during the 12-month interviews to suggest that levels of activity had increased over the intervening 8 months. Many participants in both arms reported that their activity level had remained at a similar level to that reported in the initial interview. Participants reported barriers such as time, work and family commitments as reasons for not engaging in activity.
Participants’ experiences of the facilitated physical activity intervention
Views and experiences of the physical activity facilitator
During the 4- and 12-month interviews, virtually all intervention participants described how they had felt comfortable with their assigned facilitator and had benefited from having contact with a PAF. The approach of the PAFs was described as being tailored on an individual basis. It was apparent that such tailoring had a significant bearing on the extent to which participants felt they were able to engage with the intervention. Many participants appreciated the idea of verbally exploring the pros and cons of engaging in physical activity with the guidance of the PAF. The PAFs were described as being non-judgemental and objective, giving encouragement and guidance, enabling reflection and providing inspiration. Participants also found it helpful if the PAF could help them consider different options and encourage them to start taking responsibility for the amount of activity they were doing.
I think it’s just the making you sit down and take the time to think about options, and have somebody that’s, you know, going to offer you options. It just gives you that additional impetus to say, ‘Well actually I must go and do such and such.’
(Male, 43 years, intervention, severe depression, medium active, rural, 12 months)
I just think thinking it through and talking it through and being motivated to do something about it has sort of helped quite a lot. Because you’re not quite so alone, you’ve got someone there trying to help you and sort of get you doing something about it, and putting the responsibility back with myself really to do it.
(Female, 45 years, intervention, moderate depression, low active, urban, 12 months)
Many participants felt that the ‘non-judgemental’ approach taken by the PAFs had been a positive and helpful experience. In addition, the ability of the PAF to listen objectively and take an interest in what the participants had to say about their situation and attempts to be more active was also deemed to be very important.
Just the general friendly attitude, and when you actually talked to her she was actually listening and – taking it in, and not just like there thinking, ‘Oh alright, if I let him talk for a little while then, you know, I’ve done me job’, sort of thing. You felt like – the person was interested in what you had to say and in your problem, and not just there for the sake of it.
(Male, 44 years, intervention, mild depression, medium active, urban, 4 months)
Participants expressed their appreciation for the guidance that their PAF had given them and which they had delivered in a non-prescriptive context in which individual autonomy and participant choice were sought.
I mean they set the framework in which I made the decisions about what it was I was going to attempt to do. It was not directive in any way about saying how I was going to do it, ‘What you’re going to do, I don’t expect you to go to the gym, I don’t expect you to do this. What do you feel that you can do?’ Definitely non-prescriptive, and allowed me to make a commitment which I felt then that I had to keep up.
(Male, 69 years, intervention mild depression, medium active, urban, 12 months)
It’s at your own pace and I think it was initiated by myself as well really otherwise it doesn’t work. Yes, again with that self-initiation is that somebody is sort of supporting you through that. I know it’s her job and things, but then you do see it as, you know, I need to get this done for my own [sake].
(Female, 45 years, intervention, mild depression, low activity, urban, 4 months)
I don’t know whether it’s specifically tailored for me. But it might have been, whatever she was saying, and the way she was, not leading, but doing the questions that she needed to get out, or answers, I don’t know, it was just done in a way where you just quite freely flowed with it.
(Male, 44 years, intervention, moderate depression, medium active, urban, 4 months)
However, one participant reported that her PAF had been quite directive and specific, describing how the PAF had encouraged her to increase the frequency of visits to the gym.
I suppose it was kind of led by me, but with her direction if that makes sense. Initially it was a 15 minute walk every day. And she sort of tried to pin me down to going to the gym at the weekend. Some weeks I did and some weeks I didn’t. And then she kind of upped the ante a bit by trying to make me go in the middle of the week as well by persuading me it will be good and talking to me about how I felt about it and whether I thought it would be good, and how I could achieve that.
(Female, 54 years, intervention, mild depression, low active, urban, 12 months)
Many participants had been active in the past. These participants reported that their PAFs were helpful in re-evaluating their choice of activity or setting realistic goals.
I’m the kind of person that likes to plan things, and they made me sit back and think about what I’d been doing. And to a certain extent I think with constantly struggling on with the running, the expression, ‘flogging a dead horse’ came to mind. And I guess I kind of did view it as an opportunity to consider an alternative.
(Male, 43 years, intervention, severe depression, medium active, rural, 4 months)
She would ask me what my goals were, and talk them through, and check with me that they were realistic and that I wasn’t sort of expecting to do too much. And then whether or not I’d achieved what I planned to achieve the week before, which kind of made me think about – so when I set goals and I’d said, ‘These are my goals’, I sort of felt, ‘Oh I need to go and do those because someone’s going to check up on me’.
(Female, 36 years, intervention, moderate depression, low activity, urban, 12 months)
Although participants receiving the intervention generally described positive experiences, less positive experiences were also mentioned. These included feelings of guilt or embarrassment and not wanting to let the PAF down. However, such uncomfortable feelings were often overcome with time and the non-judgemental approach maintained by the PAF.
Well, she did say like that when I didn’t [meet goals] I didn’t answer the phone to her for a week, she did say that was actually very common for people to do, you know, to make a spurt and then drop back a bit and then . . .
(Male, 56 years, intervention, moderate depression, medium active, urban, 12 months)
One participant was disappointed that on having ‘opened up’ to the PAF they were not willing to discuss personal issues or take on a more formal counselling role.
I’ve got to know the PAF now, I actually do open up so they know a lot of personal things now of me. A good listener but like today, they phoned me this morning, and some things I talked to them about. They can’t really advise me and talk about it, can’t give advice on this situation.
(Male, 29 years, intervention, mild depression, low active, urban, 4 months)
Another participant explained that he would also have benefited from a more ‘hands-on’ approach, akin to the role of a ‘personal trainer’, that is, someone who would accompany him when exercising. A more proactive participant suggested that she had an expectation of a different approach to that experienced.
Well the PAF visited me twice I think, and phoned me a couple of times. But I mean obviously because I’ve done a lot and suggested a lot of things that I’m going to do, she hasn’t really sort of done anything apart from talk through what I’m doing and how I’m doing it and when I’m doing it, and what I propose to do in the future, in the month, in between seeing or speaking to her. So apart from that it’s been quite informal really.
(Female, 48 years, intervention, moderate depression, low active, rural, 4 months)
However, being assigned to the PAF did enable this participant to gauge her progress, which was a reaffirming experience for her.
It’s made me think about the fact that when I put in my diary that, the PAF is going to ring me I think, ‘Right, you know, from the last time we spoke, I want to report a better place to be.’ So she sort of reiterates what the conversation was last time, what the main points were, and then we go over like, ‘Have you achieved these?’ I kind of want to do that, because I want to hear it from myself that I’ve actually moved on.
(Female, 48 years, intervention, moderate depression, low active, rural, 4 months)
Finally, one participant reported being underwhelmed by the support he had received from the PAF and suggested that someone with less determination than himself may not have fared so well in receiving the facilitation.
I think on reflection, I don’t want to sound personal, but I wasn’t too impressed with what the PAF was giving me to do. In the sense that, ‘Here’s a sheet, write down what you’re going to do in a week, come back and tell me’, sort of business. Or ring me up on the phone, and they would ring me some days, and then forget to do it, and then ring me three days later, and all that sort of stuff. I think somebody who was less determined than I might not have kept going in quite the same way as I did, without a bit more personal support, you know, ‘Hello John, how are you getting on, what are you doing?’
(Male, 69 years, intervention, severe depression, medium active, rural, 12 months)
Mode of delivery and acceptability of facilitated physical activity intervention
Most participants stated that both modes of delivery of the intervention (face to face and telephone calls) were important to them. The face-to-face sessions were important for ‘putting a name to a face’, gaining trust and building a rapport with the PAF. Participants also reported feeling reassured by the visual cues and body language they experienced from having face-to-face contact with another person. There was a suggestion that the face-to-face meetings were tailored on an individual basis and there was a greater awareness that the PAF was listening to them. In addition, having the initial face-to-face contact made the transition to telephone contact more acceptable to participants.
To start with the face to face, because then you could see the reaction when you’re speaking to the other person. But after that the back-up was – was good, just to reassure you that someone was there, but you didn’t need to see them because you’re sort of left to get on with it a bit on yourself, which you need to do anyway.
(Male, 44 years, intervention, mild depression, low active, urban, 12 months)
The telephone calls were described as friendly reminders in the background that served to help the continuity of the relationship with the PAF and focus on physical activity in relation to mood. They were also described as providing a regular motivational prompt for self-reflection and a channel for sharing experiences and as a form of affirmation with regard to any progress made towards engaging in physical activity. It was also apparent that many participants had become quite reliant on the telephone calls as a means of monitoring progress and for the continued provision of guidance in making plans and setting goals.
Well there was always the fact that someone was going to ring me up and ask me what I’d been doing [laughs], which is quite a good motivator. At least certainly to start with, I wanted to have something to say that I’d done positive. But also talking through what might be, you know, easy to fit into my life, and helping to make plans and set goals.
(Female, 36 years, intervention, moderate depression, medium active, urban, 4 months)
Actually the most helpful thing for me was when he rung like once a fortnight that actually prompted me to think about it a bit more and also like the analysing of it afterwards. It helped me to see that I was making progress. I just liked looking at things in a fresh way. It was nice not to be stuck in a rut if that makes sense.
(Female, 26 years, intervention, severe depression, medium active, urban, 12 months)
Acceptability of activity logs and worksheets
Participants were given a number of worksheets to assist both the participant and the PAF in the facilitation of physical activity. The worksheets were primarily aimed at raising awareness of present levels of physical activity and the advantages and disadvantages of engaging in physical activity and helping to plan physical activity sessions and set realistic goals (see PAF manual in Appendix 1). Participants were also encouraged to keep a log of any activity they achieved on a weekly basis, including their emotional response before, during and after activity.
Most participants described how the worksheets were useful for planning and initiating activity, and for placing some perspective on their efforts to be more active. The logs were useful for gauging motivation and recording feelings associated with engaging in physical activity.
I suddenly realised that there wasn’t a vision of the perfect level of exercise, there was something that related to me, and then I realised it would have specific benefits for me. At the stage when I started writing this down [log], and I started to walk a bit more and I started to feel more positive, and I thought, well, yeah this is working. I can feel the benefits physically and can also feel the benefits emotionally, this makes me feel good. And it was at that point that I thought, well, we’re onto something here and I suddenly realised I needed to latch onto something more constructive.
(Male, 69 years, intervention, mild depression, medium active, urban, 4 months)
The worksheets and logs were not favoured by all participants, but even these participants expressed how they were useful in terms of increasing their awareness of how much activity they were doing.
I thought it was tedious [the log] but it was very helpful because it demonstrated what little I was doing. The only advantage was that sitting there doing it for yourself was quite powerful in terms of illustrating what I actually did or didn’t [do].
(Male, 69 years, intervention, mild depression, medium active, urban, 12 months)
Utility of the facilitated physical activity intervention in relation to future well-being
Many participants receiving the intervention spoke of the perceived success of the input from the facilitator in terms of what they had learned or could take away for future reference. For example, some reported that they were able to be more self-reflective and were now able to reframe negative thoughts in a more positive way. Other participants described how they could use what they had learned from the PAF as coping strategies or as a means of preventing future depressive episodes. They could utilise these strategies alongside mood changes and could anticipate the onset of a depressive episode.
You sort of feel better able to cope perhaps look back and think about how you coped in the past and things, and use techniques and things, that’s helped. Yeah sort of things that initially I wrote down and things at the start of the TREAD, and talking to the PAF and bringing up things that have worked in the past as well before I met her. I suppose you would say it was a case of nipping things in the bud a bit. Because the winter months are sort of not good for me in the past, and sometimes allowing myself permission to feel like that sometimes as well, you know.
(Female, 45 years, intervention, mild depression, low active, urban, 12 months)
Participants also reported having a deeper understanding of depression, the ability to be reflective with a greater awareness of the triggers for depression. For some, such insight also assisted in the development of coping strategies that could be expressed by becoming more physically active.
I feel a lot better. I feel I have achieved a huge amount, bearing in mind, as I say, I was quite happy just to give everything up really. I feel I’ve got potential to have a future, which includes some of the things that I used to do when I was, you know, younger and fitter and so on, I can do them proportionately in the future. So yeah, I feel a lot more positive. But I’m also very aware of what the triggers are or understanding my depression a bit more so it’s not so much the enemy, I can work with it.
(Female, 48 years, intervention, moderate depression, low active, rural, 12 months)
I do understand now that there is a relationship between, like if I continue to do nothing for too long, then I will sink, you know, and it’s no good to do that. But I do at some point have to go, ‘Right, I’m going to have to actually go and do something’, and move, physically, and actually do something, and – it does counteract it, you know.
(Male, 56 years, intervention, mild depression, medium active, urban, 4 months)
View of impending loss of physical activity facilitator and suggested improvements to facilitation
During the 12-month interviews participants did not report negative experiences of losing contact with the PAF after the 8-month intervention period. In fact, many felt that they had experienced the benefits of the intervention delivered by the PAF and had now moved on with regard to depression.
It’s been fine because I’ve made that step to move on anyway – I was getting there anyway, so it was good.
(Female, 45 years, intervention, moderate depression, low active, urban, 12 months)
However, a few participants did express a desire for continued contact with a facilitator or felt that the intervention could have offered something different. One participant stated a need for more counselling and a cognitive approach to assist ‘constructive and progressive thinking’. Conversely, other participants expressed a desire for a more direct approach to encouraging physical activity such as on a one-to-one basis akin to having a personal trainer.
It would be nice just to have somebody there sort of motivating me by just being there. Now that I am making progress it would be nice to have somebody to tell.
(Male, 55 years, intervention, moderate depression, low active, rural, 12 months)
It’s not just one thing, you know, it’s not just like you go out and you do lots of physical things and you’re going to suddenly – it’s going to help, but I think it’s a mixture. There has to be a certain amount of counselling, and positive thinking. Um not really positive, that’s not the right word. Sort of constructive, like progressive thinking, like that actually gets somewhere instead of just thinking stuff.
(Male, 56 years, intervention, mild depression, medium active, urban, 12 months)
Participants’ views and experiences of the usual care arm
Usual care participants’ view of not receiving facilitated activity intervention
Some participants in the usual care arm expressed disappointment at not receiving the intervention, as they felt that they would have benefited from having contact with someone who was able to encourage and help them to become more physically active.
I would have found it very useful, giving me that encouragement and assistance, I probably would have been doing more physical activity than perhaps I am because if somebody could have tailored something more to my specific needs and abilities it could have probably encouraged me to do more than I actually am. You know, there would have been that guidance there.
(Female, 38 years, usual care, moderate depression, low active, urban, 4 months)
A few participants had been offered exercise prescription schemes by their GP as ‘consolation’ for not being randomised to facilitated physical activity, but these schemes were not always viewed as appropriate.
Yeah, there was another programme that she [GP] could put me on. The problem with that was you could only go twice a week, which was fine, but it was set days. It was Mondays and Thursdays, you couldn’t go any other time. And you had to be out of the gym by 12 o’clock midday. So it was very restricting and [small laugh] with depression you can’t always guarantee that you’re going to feel right on those particular days.
(Male, 60 years, usual care, mild depression, low active, urban, 4 months)
Other usual care participants, having not been allocated to the trial intervention, had taken action of their own volition in an attempt to enhance their mood. These actions included meditation and counselling, in addition to taking antidepressant medication.
I have done meditation, as an additional thing, it’s based on a Buddhist technique, but it’s taking some of what they do. It’s not teaching you Buddhism but it’s taking some of their techniques and tailoring it into a meditation technique to help you to, I suppose, relax yourself.
(Female, 38 years, usual care, moderate depression, low active, urban, 4 months)
I’ve had counselling through work. To start with I saw her every week, and now I just go monthly just for a regular chat really now. Um there was a lot of help to start with. I wasn’t getting on too well to start with, so I also took – well I still am taking fluoxetine antidepressant.
(Male, 21 years, usual care, moderate depression, medium active, urban, 4 months)
However, one participant stated that she did not expect to get any therapeutic benefit from participation in the trial, thus randomisation to usual care was not an issue for her. However, despite this declaration, this participant had engaged in high-intensity physical activity.
I’m not looking for anything for me. I think it would be unrealistic for it to treat me. Because I have been more active since I started the study, and it has made a difference I think with the running, especially with the running actually. So if you can glean anything from that and it can be useful to anybody else then that’s fine.
(Female, 24 years, usual care, moderate depression, low active, urban, 4 months)
Reasons for participation in TREAD
Participants reported many reasons for taking part in TREAD. The most common reason reported by participants in both trial arms was altruism – participating for the greater good and the sense of contributing to research that may help not only them but also others with depression. No participants mentioned that their involvement in the study was influenced by the possibility of superior clinical care or additional attention. However, some did say that they felt that the study would offer an alternative to the prescription of antidepressants. These participants were usually from the intervention arm.
I’m a great believer that if people with problems don’t volunteer for research, how are you ever going to solve the problems? I kind of looked at it and thought, well, I don’t know whether it’s likely to be effective and I don’t have an inkling as to what research has currently been done, but it was clearly written, you know all the information was well presented, and I followed it through. I think you get to a point where, from my perspective, all I ever get from the doctor is a pack of pills.
(Male, 44 years, intervention, moderate depression, low active, urban, 4 months)
I thought exercise would help – I was looking for ways to manage my depression without pills, to have things I could do when I came off pills again. I hate suffering with depression and I wouldn’t wish it on anybody, and if I can help to develop things that will help other people then that is a good thing as well.
(Female, 36 years, intervention, moderate depression, medium active, urban, 4 months)
Others simply wanted to cooperate with the recommendations of their GP or were demonstrating a high degree of trust in their relationship with their GP.
I was looking for anything that might help really, and my doctor said ‘give it a go’ – that’s all you have to go by really. If your doctor thinks it’s a good idea, then she’s probably right, you know. So, yeah, I thought, I’ll give it a go.
(Male, 35 years, usual care, moderate depression, low active, urban, 4 months)
A few participants participated because they were glad to have someone independent to talk to. There were also a few participants who had been active in the past and saw an opportunity to re-engage with activities that they had previously enjoyed which had diminished as a consequence of their depression.
There’s a factor there that made me think, yeah, this is probably the right kind of study for me because I used to enjoy this and would like to get back to enjoying it and whether that made it easier for me or not, I don’t know.
(Male, 43 years, intervention, severe depression, medium active, urban, 4 months)
Finally, there were some participants who detailed how they saw the study as a potential means of gaining general health benefits, such as weight loss, from being encouraged to become more physically active.
Discussion
Strengths and limitations of the qualitative study
Interviewing participants in a trial of physical activity may have meant we interviewed a select group of participants who held particularly positive views about physical activity as a treatment for depression. The content of the patient information sheets may have had a priming effect for participants by introducing thoughts about physical activity that had not previously existed. However, such priming may not necessarily influence their ability to undertake activity and its acceptability among other treatments offered for depression in primary care. It is possible that some participants may have found physical activity more accessible when interviewed for the study at 4 months compared with 12 months because, for example, they had been signed off work by their GPs and thus had more time in which to engage in physical activity. Additionally, as intervention participants were followed up 4 months after receiving the last meeting or telephone call from the facilitator, the accuracy of memories of activity level and impact of facilitation may have diminished. Subsequently, this may have led to a misattribution with regard to engagement with physical activity or what they felt they had gained from the facilitation in terms of increases in activity and improvements in mood.
The 4-month interviews were largely conducted in participants’ homes with a researcher who was independent of the trial in which they participated. This may have helped to elicit a high degree of candour and openness from participants. The 12-month interviews were conducted by telephone. Participants appeared to remember the researcher when being contacted about the second interview, and we are aware that well-planned telephone interviews can gather the same material as those conducted face to face. 68 Although data collection was undertaken by one individual, several team members were involved in development of the coding frame and analysis of the data. By employing a purposeful sampling approach, we ensured that interviews were held with men and women in both trial arms who varied in terms of age and socioeconomic background. The generalisability of our findings, however, may be limited as participants were primarily white British, and depression is known to affect individuals of all ethnic backgrounds, who may have different views.
Summary of findings and implications
Participants in both arms of the trial perceived physical activity as an acceptable treatment that could enhance mood for a wide range of individuals with depression. The mechanisms by which participants believed that physical activity could enhance mood included biochemical pathways, distraction from negative thoughts and providing a sense of structure to daily life. Participants also reported other benefits of engaging in activity, such as facilitating social interaction, weight loss and regulation of sleep or eating patterns, which could lead to a holistic sense of well-being, which in turn increased self-esteem. However, the perceived cause of depression had a bearing on the extent to which participants thought that activity might be helpful, with it being suggested that it was less beneficial if depression was believed to be a function of situational factors as opposed to being of biochemical origin. In addition, participants who thought that depression was due to a biochemical imbalance rather than to situational factors tended to state that physical activity had to be aerobic in nature in order to be effective, whereas those believing that their depression was related to situational or adverse life events tended to report the benefits of less intense aerobic activities, such as walking.
There was some evidence that participants may prefer the ‘self-help’ aspect of engaging in physical activity as opposed to the passive nature of taking antidepressant medication, despite the fact that many were currently being treated with antidepressant medication. Previous work has shown that physical activity is primarily seen by patients as an alternative coping mechanism – a credible next step after prior stabilisation with medication. 69 In addition, physical activity may be viewed in a ‘self-regulatory’ way as a means of providing temporary relief from symptoms rather than as a cure for depression. 70 However, some participants suggested that prescribed medication had enabled them to initiate and maintain physical activity. It had helped them to overcome barriers to activity such as low levels of motivation and confidence, which may be a particular problem in patients with depression. It was with reference to these barriers that participants felt that it would be easier to take a pill than participate in regular activity, implying a recognition of problems overcoming the initiation and maintenance of physical activity as a treatment.
Most participants receiving the facilitated physical activity intervention stated that they were making a conscious effort to do more activity at both time points. However, it was also apparent that some participants in the usual care arm had made efforts to be more active in the trial period, suggesting that participation in the trial and exposure to information sheets and activity logs could have influenced behaviour in the usual care arm.
For many participants, motivation was an issue and there was a reliance on other parties for engaging in physical activity. Indeed, support for physical activity was often found in the sense of companionship experienced in attending classes or other activities with friends or relatives. Even if activity was being undertaken, access was an overarching barrier to physical activity for participants in both the usual care and intervention arms and was primarily related to cost and time. In fact, for many participants there was an assumption that to undertake physical activity there would be a need for gym membership or to sign up for structured classes held at sports centres.
The experience of the majority of patients receiving the intervention was very positive. For many the facilitation was considered to be tailored to the individual and gave participants someone who would listen objectively and in a non-judgemental manner. Most participants stated that both modes of delivery of the intervention (face to face and telephone calls) were of equal importance to them, but often for different reasons. The face-to-face sessions were important for putting a name to a face, building trust and developing a rapport with the PAF and could also be a reaffirming experience in a time of emotional distress and were therefore appropriate in this study involving participants with depression. With regard to the telephone calls over the 8-month programme it was clear that this method of delivery served to help the continuity of the relationship with the PAF. The telephone calls were for the most part seen as ‘friendly reminders’ that enabled participants to focus on their endeavours to be physically active and to discuss ways of overcoming barriers to activity. The telephone contacts also provided the participants with the opportunity for a more informal discussion of their emotional well-being in relation to receiving the intervention.
Finally, with regard to the paperwork elements of the intervention, the worksheets enabled participants to evaluate their work/life or family balance and the development of life skills. The physical activity logs also served a useful role in delineating the extent of physical activity undertaken and setting future goals. During the 12-month interviews participants did not talk of using the worksheets and logs during the later stages of the intervention. This was the case even for those who found them useful at 4 months, suggesting that such tools had served their purpose in the early stages of the intervention and their use by participants was not sustained throughout the intervention period.
Facilitated physical activity for managing depression
Participants in the intervention arm described how their PAF had provided emotional support and encouragement, given guidance, been sympathetic to their situation and listened carefully and objectively to what they had to say. Participants reported emotional benefits from the contact with the PAF. In addition, participants described how contact with their PAF had encouraged them to be more physically active. Physical activity was reported as leading to improvements in mood, and it was apparent that participants could experience these benefits both during and after an activity. Some participants even described feeling more mentally positive when simply planning to be active. However, participants who described improvements in mood usually attributed this to situational factors, such as changes in employment circumstances, or use of medication and the passing of time. Thus, physical activity was only one of various factors described as influencing mood and was not usually reported as the key factor. Yet participants who had received the intervention felt that they had gained a greater awareness of depression and had learned to reframe negative thoughts or behaviours in a more positive way. For some, such insight had assisted in the development of coping strategies that they could utilise in the future once the input from the PAF had ended.
General practitioners’ views and experiences
Methods
Recruitment and sampling
General practitioners from practices participating in TREAD were invited to take part in a telephone interview to give their views regarding physical activity as a potential treatment for depression and their experiences of referring patients to TREAD. We aimed to maximise the spread of geographical location of the practice (suburban, urban and rural) together with indication of the socioeconomic status of the patient list based on observations made by researchers who had visited these practices. Practices were also selected on whether the practice referred to the trial directly or through record searches. Within these practices, we aimed to maximise variation with regard to position/role of the GP (salaried GP, partner, research lead) and number of personal referrals made to the study (none to multiple referrals). The GPs who were sampled were sent a letter enclosing an invitation and information about the qualitative study. A total of 40 GPs were approached from 20 practices in the Bristol area and 20 from the Exeter site. All 40 GPs were followed up with a telephone call within 2 weeks of sending the approach letter. GPs were contacted up to three times by telephone to gain consent for interview. This procedure yielded 18 agreements to be interviewed; however, 3 GPs did not keep their appointments and were unable to reschedule and therefore we interviewed 15 GPs in total. Consent forms for participation in the study were faxed to consenting GPs and signed copies were returned either by fax or by post.
Interviews
General practitioners were interviewed by telephone because the team’s previous research experience suggested that this would encourage participation. Semistructured interviews were conducted over the telephone using a topic guide. The interviews covered:
-
views of physical activity as a treatment for depression
-
understanding of the TREAD trial and facilitated physical activity
-
views of referral to study and introducing the study to patients
-
general views of the research in the context of primary care.
Referring GPs were also asked about which patients they referred to the study and to provide any information they had regarding why patients had declined participation. The interviews were conducted between January and April 2010 once recruitment to the trial had ended. On average interviews lasted for 15 minutes.
Analysis of interviews
All interviews were audiotaped and transcribed verbatim. Transcripts were read and reread for familiarisation of the data and identification of emerging themes. Emerging themes were then discussed with MCal until consensus was achieved. A coding frame was developed with reference to the emerging themes and all transcripts were imported and stored in ATLAS.ti for coding.
Results
Views expressed by GPs were classified within four principal domains during the analyses. These were:
-
practitioners’ views of physical activity as a treatment for depression
-
promotion of physical activity in the consultation
-
practitioners’ experiences of referring patients’ to TREAD and patient feedback
-
impact of facilitated physical activity on general practice.
General practitioners’ views of physical activity for the treatment of depression
Most GPs reported that physical activity could have a therapeutic role in the management of depression but there were also GPs who were ambivalent in their views regarding its efficacy for depression and who suggested that there was still a need for robust evidence to advocate its use (Table 45). If GPs did refer to evidence it was mostly anecdotal and many GPs disclosed their own experience to illustrate their belief that physical activity could enhance mood.
| GP type | Practice type | View of physical activity |
|---|---|---|
| Female, GP, referred to trial | Bristol, urban, deprived | ‘Pro physical activity’. Anecdotal evidence. Does cite evidence base for efficacy of physical activity. Could be used as an independent treatment as ‘watchful waiting’ |
| Female, GP, referred to trial | Bristol, urban, affluent | ‘Pro physical activity’. Does not cite evidence base. Considered to be an ‘underprescriber’ of medication. Little awareness of or access to exercise prescription schemes |
| Male, GP partner, referred to trial | Bristol, suburban, mixed | ‘Pro physical activity’. Believes that there is an accumulating evidence base. Advocate of using as many ways as possible to treat depression |
| Male, GP partner, referred to trial | Exeter, urban, affluent | ‘Ambivalent’ view of physical activity. Described evidence base as not conclusive. Rarely refers to exercise prescription schemes as patient motivation an issue |
| Male, GP partner, referred to trial | Exeter, urban, affluent | ‘Pro physical activity’. Does not cite evidence base. Recommends physical activity to patients in ‘ad hoc’ fashion if they are signed off work |
| Male, GP partner/research lead, referred to trial | Exeter, urban, mixed | ‘Pro physical activity’. Does cite evidence base and discloses own experience of using physical activity. Against medication, would not use it himself |
| Male, GP, referred to trial | Exeter, urban, mixed | ‘Champion’ of physical activity. Does not cite evidence base. Socially oriented outlook of cause and treatment of depression. Against medication |
| Male, GP partner, did not refer to trial | Exeter, rural, affluent | ‘Pro physical activity’. Does not cite own experience or evidence base |
| Male, GP partner, referred to trial | Bristol, urban, deprived | ‘Champion’ of physical activity. Refers to growing evidence base. Self-disclosure of activity. Socially oriented outlook of cause and treatment of depression |
| Male, GP partner/research lead, did not refer to trial | Bristol, suburban, mixed | ‘Pro physical activity’. Positive view of physical activity based on anecdotal evidence and self-disclosure of physical activity for depression |
| Female, GP partner, referred to trial | Bristol, suburban, deprived | ‘Pro physical activity’. Does not cite evidence base. Uses exercise prescription schemes for obesity. People with depression have problems accessing schemes |
| Female, GP, did not refer to trial | Bristol, urban, affluent | ‘Champion’ of physical activity. Does cite evidence base. Known for promoting activity. One of many tools used for recovery from depression but also need to address underlying causes |
| Male, GP partner, referred to trial | Bristol, rural, affluent | ‘Ambivalent’ view of physical activity. Not aware of evidence base but thinks physical activity would be a useful adjunct and for patients less keen on medication. Limited experience of exercise referral schemes |
| Female, GP partner, referred to trial | Bristol, urban, deprived | ‘Pro physical activity’. Does cite evidence base for recommending physical activity. Broad scope of activity, suitable for all groups, based on personal experience and NICE guidance. Patient-led approach if not wanting to take medication |
| Male, GP partner, did not refer to trial | Exeter, suburban, affluent | ‘Ambivalent’ view of physical activity. Not aware of evidence base. Thinks any activity would be beneficial for depression, could be artistic endeavour |
I believe, personally, that exercise is good generally so I can’t see any reason why it shouldn’t be good for depression, presumably that’s why you’re doing the trial. I wasn’t really aware of the literature in many respects, and from what I’ve heard the literature wasn’t conclusive. Either way I think as a GP there are hundreds and hundreds of things you come across and that was just one of the ones that hadn’t really sort of impacted on me, but I guess that was because of the lack of evidence really.
(GP4, male, urban, affluent)
I’m sure there is some evidence that exercise is effective, and anecdotally it certainly has helped many patients of mine.
(GP1, female, urban, deprived)
Only a few GPs were aware of relevant scientific literature or made references to current clinical guidance.
Oh, very, very keen on it. I would quite often ask patients who are depressed – ‘Look there’s some evidence that exercise will actually help you with your depression, so I’d recommend that you get out and try and do some exercise’.
(GP6, male, urban, mixed)
You go on experience but also, you know, on what’s advised by NICE and other depression studies one looks at – or depression advice, not studies particularly, but sort of meta-analyses and advice given.
(GP14, female, urban, deprived)
The reasons that GPs gave for how physical activity might be useful in managing depression often reflected reasons given by patients. For example, the idea of a biochemical pathway was often reported, as was a multifactorial view that physical activity could provide social interaction and structure to daily life, raise self-esteem, aid sleep and engender a sense of autonomy in one’s life.
I think you’ve not just got your physical benefits, but there are psychological benefits to it, in that it does seem to help lift mood. I talk to them about, you know, a bit of an adrenaline relief. But I think the satisfaction they get from achieving something as well is added in on that. You’re getting them out of the house and doing something, so a bit of fresh air and exercise sometimes or even going to the gym where again there’s a bit more social interaction there, so there’s the stimulation from it as well.
(GP12, female, urban, affluent)
There was some debate as to what constitutes physical activity and what level of intensity or type of activity is required to enhance mental well-being. Physical activity did not necessarily have to be an aerobic or a high-intensity activity; the process of getting out of the house and into another environment was seen to be a positive component in improving well-being.
Well, what is physical activity? Physical activity for me, I mean obviously there’s aerobic exercise that certain people can do, but there’s other things that people who are not particularly – you know, the old dears could – just to get them out of their little flats . . . I mean the physical exercise might be just getting out the house to get the bus to get down to the place where they can then play bridge, if you see what I mean.
(GP 7, male, urban, mixed)
Virtually all GPs saw physical activity as an adjunct treatment for managing depression rather than as a sole treatment, although there was an awareness that for many patients medication was not a favoured form of treatment.
I have to say very often it’s used alongside other treatments. But some people just do not want to take tablets, so then one would be more emphasising the non-medication side of things.
(GP14, female, urban, deprived)
A few GPs saw physical activity as having a maintenance role in the management of depression such that the patient could be autonomous after initial treatment with medication and/or counselling.
I’m a great believer in people being able to talk, you know, talking treatments primarily. I think that antidepressants and whatever have a positive role as well. But I’d like to see that combined with increasing physical activity, because I think it’s a useful tool to go on in life, rather than just for a period of time you’re either on the antidepressant or you’re talking to a counsellor and then it finishes.
(GP9, male, urban, deprived)
Promotion of physical activity in the consultation
General practitioners were also asked about the extent to which they promoted or recommended physical activity in consultations with depressed patients. Many felt that it was important for GPs to validate physical activity as a viable non-medical treatment option.
Sort of slightly de-medicalising it, because whenever you offer these things, we may have diagnosed depression but people really like the idea that there is a sort of non-pharmacological, or its considered important [that you refer them], even if they are going to take a drug, that the life change or exercise or whatever is considered is promoted and considered by me, and considered by the patient.
(GP10, male, suburban, mixed)
A few GPs stated that there was a need to be careful when recommending physical activity as it could have a negative effect on some more vulnerable patients or those perceived as less able.
I think you have to be careful for those people who are very negative and very self-critical, that if you set unrealistic expectations and they can’t manage, that’s an issue. So particularly say if people are very overweight or if they’ve got an injury that prevents them undertaking physical activity, so one looks to tailor it a bit accordingly, because you don’t want to set unrealistic expectations and then that actually just reinforces the negative rather than brings positive benefits. So yeah I think it needs to be used with a degree of common sense.
(GP12, female, urban, affluent)
Many barriers were reported with regard to recommending physical activity. These barriers included access, time and perceived ability to engage in physical activity.
Well I think it’s only certain groups. Because basically it’s not just about TREAD and depression, but exercise in general. Because there are so many people who could benefit, from physical activity all across the clinical spectrum. But lots of them just don’t enjoy it, or can’t fit it in, or physically can’t do it, you know, just all the barriers.
(GP10, male, suburban, mixed)
Referral to TREAD and patient feedback
Some GPs felt that it was difficult to introduce the TREAD trial to patients during initial consultations for depression because of the emotional state of the patient. Some GPs also expressed difficulty in providing adequate information during a short initial consultation to support the patient in making an informed decision to consent to recruitment.
We actually now have most of the trials now on our Intranet, so they are instantly available, and so we can print out. I mean just before this interview I printed out the GP information leaflet for the TREAD trial, and I did that in a matter of probably 15 seconds. Now that is acceptable. But if you have a pile of written information on your desk, your chances of finding it in 15 seconds is much less. And so it’s first of all having the information instantly available. And then often at the time of patients coming in, particularly their initial visit, they are extremely tearful, and the last thing they want to do is discuss things other than their particular sort of symptoms. And therefore it is actually quite difficult to get them out of that mindset to think about that, and to actually feel that you’ve got informed consent. It’s quite easy to get written consent, but it’s much more difficult to get what you really consider to be informed consent.
(GP3, male, suburban, mixed)
Other GPs stated that recommending physical activity is always on the agenda for consultations with depressed patients and therefore introducing the trial did not feel like an additional burden as long as there was the time to do it.
I think first of all you’re making a diagnosis of depression, and then by being aware that there is a study to be had, if there’s time to introduce it then I would certainly do that. It’s going to be mentioned in part of my spiel anyway. So it does partly depend upon time. And it might not be the first time I meet them that I have got the ability to mention the study, but it could have been the second time that I met them as well, or not at all, in the sense of just absolutely not having time and feeling that other things were more important.
(GP14, female, urban, deprived)
In addition to time barriers, other barriers to recruitment mentioned were GPs remembering the existence of the trial and what the trial criteria were for patient eligibility.
Well the key thing was remembering to do it, because sometimes you just forget about TREAD. I know it’s a big thing for you, but we’re sort of trying to juggle in our head about 30 different things all at one point. There’s that, and then there’s have you got time to do it? I think those are the two most important things – rather than, how severe the depression was. I think they had to reach a certain criteria, to have a PHQ-9 of more than something, didn’t they?
(GP7, male, suburban, mixed)
Conversely, some GPs commented that patients may make a point of asking for alternative treatments to antidepressants, which would act as a prompt to introduce the study.
I have to say very often it’s used alongside other treatments. But some people just do not want to take tablets, so then one would be more emphasising the non-medication side of things.
(GP14, female, urban, deprived)
GPs were asked to describe who they referred to the study and whether or not they had received any feedback from referred patients. Many GPs made a decision to introduce the study based on a value judgement according to the visual appearance of the patient and how they presented in the consultation rather than on what they knew about the patient.
I think I would have looked at the person in front of me and sort of thought, ‘Well, what would I be encouraging them to do?’ And it would be, you know, looking at that person and thinking, ‘Well this person I’m sure would respond, could respond to that’. If someone is elderly, say, and infirm, that’s a slightly different matter isn’t it?
(GP2, female, urban, affluent)
I suppose they were probably of the younger end of the age range, under 60. I would tend to have put it out to them. And I suppose new presentations rather than the current. But I suppose you make a sort of value judgement about, you know, this is the sort of person who might engage.
(GP8, male, rural, affluent)
Most GPs had not received any direct feedback from patients participating in the study. When feedback was received it was often ‘neutral’ such that they did not critically appraise any aspect of their participation in the trial.
Well I’ve had a few patients who have said that they’ve you know, that they’ve been seen. I’ve had fairly neutral comments, I haven’t had people saying, ‘Oh it was fantastic’ and I haven’t had people saying, ‘It was a load of rubbish’. So actually it’s been fairly neutral feedback. I’ve had patients who have said, ‘I’ve been referred, I’m being seen’, but no more than that.
(GP3, male, suburban, mixed)
In addition, some GPs were of the impression that patients may have misinterpreted what facilitated physical activity would entail.
I think it’s hard because there may have been one or two or three, I can’t remember. I think feedback would have been mixed because I’m not sure whether I or they were expecting more actual input from an individual. I mean I think what we didn’t appreciate perhaps it was my advice I gave them – that it wouldn’t be somebody holding your hand and taking you to do the exercise, it would be sort of making sure that you had a place to go to, the exercise.
(GP2, female, urban, affluent)
Impact of facilitated physical activity on general practice
Some GPs stated that facilitated physical activity would have a significant impact on primary care services. Many GPs reported that there was a tendency to prescribe antidepressants as the first line of treatment for depression and that there were few alternative treatments. A few GPs were more explicit and stated that there was an absence of an adequate or effective alternative to medical intervention.
I think it [physical activity] would be brilliant, it would be really, really good. I mean we’re so short of non-pharmacological ways of treating depression. And every day I send people to the voluntary sector, counselling organisations, to Right Steps, which is a new sort of telephone-based primary care mental health organisation, but it’s just quite slow and not effective. I would really value it as a GP in terms of giving patients options.
(GP10, male, suburban, mixed)
Only two GPs made a reference to the cost-effectiveness of providing physical activity facilitation within primary care services. These GPs expressed the need for facilitated physical activity to be cost-effective and that it would require appropriate financial resources for it to be operationalised. It was also stated that, if operationalised, facilitated physical activity should be accessible and user-friendly from both the patient and the GP perspective.
Well if there were the resources to back it, and an easy way in for patients to access it, I think it could be really very helpful and very beneficial for us in terms of the burden of minor mental health problems. So I think that would be very good. I can’t see it happening.
(GP9, male, urban, deprived)
Well I would see it fitting in, provided that there was some good evidence of its cost-effectiveness and efficacy, full stop . . . I think, yes, my impression is that it would probably be certain patients that this may help a great deal, and there are others that certainly wouldn’t benefit much. I think we’d probably use it, providing it was user-friendly, and not just – I don’t just mean the patient, I mean for the referral process and the feedback process and all the rest of it. I think we’d probably have an impact, it would be another thing to offer.
(GP13, male, rural, affluent)
One GP felt that for most cases it was not necessary to see a medical professional at all. It was suggested that the enhancement of life skills was the most important element of care and would provide the most benefit to patients. The same GP also stated that a well-managed exercise programme could reduce demands on both primary and secondary levels of health care.
You know, it doesn’t really need a doctor for 70% or 60 or certainly a high percentage of what we see. It’s the common sense counselling, a pastor, a vicar or somebody that will just sit there and spend some time, and encourage a bit of life skills, you know, time for yourself, time with your family, and get the balance between work, rest and play and exercise – although that’s play isn’t it? I think TREAD, an exercise programme, that it would reduce hopefully demands on the GP. So I think a properly managed exercise programme could reduce demands on the National Health Service at primary and secondary care level.
(GP7, male, urban, mixed)
Summary and discussion
The majority of GPs felt that although physical activity could be helpful in treating depression it was best utilised as an adjunct treatment with antidepressant medication. Many GPs reported that they felt that the benefits of engaging in physical activity with respect to improving symptoms of depression would be through several mechanisms acting together. These included biochemical pathways, distraction, social interaction and providing structure to the day. Physical activity could be of low intensity such as walking and that could be a sufficient means of distraction from more ruminative forms of depression. GPs were aware of many barriers associated with recommending physical activity to depressed patients. There were practical barriers such as time and access to facilities and the burden of comorbid conditions. In addition, GPs described issues relating to patients’ levels of motivation, confidence and self-efficacy such that a recommendation to be active may exacerbate feelings of low self-esteem if a patient was not successful in initiating or maintaining an activity.
There was also awareness among GPs that many patients were looking for alternative approaches to treating depression, or at least would like to be more autonomous in their care and less reliant on medication. Such views are also in alignment with patients’ views of physical activity for depression. 67 Previous work has shown that physical activity is primarily seen by patients as an alternative coping mechanism – a credible next step after prior stabilisation with medication. 69 In addition, physical activity may be viewed in a ‘self-regulatory’ way as a means of providing temporary relief from symptoms rather than as a cure for depression. 70 Thus, together, these studies demonstrate some acceptability of promoting physical activity as a treatment in primary care for mild depression from the perspective of both doctors and patients. With regard to the utility of facilitated activity, some GPs cited a lack of alternative and effective strategies for dealing with mild to moderate depression, suggesting that facilitated activity might fill the gap.
Finally, the extent of positive views of physical activity for managing depression, particularly when combined with the fact that few cited any scientific evidence for its effectiveness, would suggest that bias may be present in the sample. Although all GPs were from practices participating in the trial, not all had referred patients to the study. This could indicate variation in commitment to physical activity as a treatment for depression. However, it could also reflect variation between the GPs in their attitudes towards recruiting participants for trials, how frequently they saw depressed patients meeting the criteria for our trial or how busy they were.
Implications for primary care
The management of depression in primary care has generated much research in recent years. 71,72 Indeed, Johnston et al. 72 suggest that GPs need greater awareness of the extent to which their goals for the management of depression are perceived as relevant or achievable by patients. These studies and our data would suggest that GPs should explore patients’ perceptions of physical activity as a treatment before recommending physical activity either alone or as an adjunct treatment. Our findings also have implications for the first step of a ‘stepped-care’ approach outlined in current NICE guidance. 6 The principal tenet of stepped care is patient-led self-help with limited intervention from professionals. 73 A stepped-care approach would suggest that physical activity is promoted before approaches based on antidepressant medication.
Patients may also benefit from individually tailored physical activity counselling that entails a progressive approach using motivational interviewing and behavioural strategies. Such an approach also has the potential to fit within a ‘stepped-care’ approach to treating depression, in which physical activity counselling is considered as a low-intensity option within the Increasing Access to Psychological Therapies initiative. Finally, it is highlighted that the trial was undertaken before the recent government directive regarding GP commissioning of resources. In the light of these changes it is clear that some GPs are enthusiastic about promoting physical activity for the management of depression and may do so without reference to evidence-based practice.
Chapter 6 Discussion and conclusions
Summary of findings
The results of the TREAD trial are very clear. There was no evidence that the physical activity intervention improved outcome for depression when used in conjunction with usual care and compared with usual care. This was true when we examined all of the mental health outcomes both at our primary outcome at 4 months post randomisation and also over the 12-month duration of the study. We used our primary outcome, depressive symptoms measured by the BDI, as both a continuous measure and a binary measure but neither approach suggested that our intervention was effective. Although considerable improvement in mood was seen in both treatment groups over the course of the study, there was no evidence that the two randomised groups differed at all in terms of depressive symptoms.
We have also considered whether or not our result was sufficiently precise to rule out the possibility of a beneficial effect. The most statistically powerful analysis was in using the BDI as a continuous outcome. The results for our primary analysis were a difference of means of –0.5 (95% CI –3.1 to 2.0). It is difficult to estimate a clinically important difference in BDI score, although the NICE guideline panel74 has suggested that this corresponds to about 3 points [0.35 standard deviations (SDs)] on the Hamilton Depression Rating Scale. 14 The equivalent difference on the BDI total score would be 4.1 points (SD at 4 months 11.8 points). These rough calculations therefore suggest that we have excluded the possibility, at least in statistical terms, that the intervention added to usual care is clinically effective in improving depressive symptoms compared with usual care alone.
We also examined whether the intervention had any impact on the use or prescription of antidepressants. There was no evidence to suggest a difference in the issuing of antidepressant prescriptions or in the self-reported use of antidepressants between the two randomised groups over the course of the trial. This supports our main finding on the primary outcome of depressive symptoms.
Economic evaluation
The cost-effectiveness analysis suggests that there was no evidence in support of the physical activity intervention offering value for money for treating depression as there is a < 50% chance of it being cost-effective at current willingness-to-pay thresholds. There was no evidence that the increased resources provided by the PAFs were offset by any reduction in the use of other health-care resources. If anything, more health-care resource was used by those allocated to the intervention, although this increase was compatible with chance. Furthermore, there was no evidence of a difference in QALY gain between the two groups so it is unlikely that the intervention is cost-effective as a treatment for depression using current willingness-to-pay thresholds, which lie between £20,000 and £30,000 per QALY.
The main limitation of the economic analysis was the presence of missing data, and we have investigated the possible influence of this by using an established method of imputation. This did not alter our conclusions. Most missing data resulted from a low response rate to the 8-month questionnaire, which was carried out by post. Because of the cumulative nature of cost data, one missing observation renders all other data for that participant unusable in a complete case analysis. We had complete data on intervention costs for all participants, and data from GP records for 86% of participants, and these two elements together constituted the majority of total cost. However, the presence of missing other observations reduced the complete case cost analysis to n = 156 (43%). The imputation, which takes account of information lost in the complete case analysis (particularly the information on the cost of the intervention), gives a more realistic estimate of true cost and uses all of the available data.
Similarly, QALY data were affected by the low responses to the EQ-5D in the 8-month questionnaire. Taken as a whole, we had 1119 responses out of a possible total of 1444 for the EQ-5D (4 for each of the 361 participants), representing 77% completeness. Thus, although QALY estimates were possible only for 54% of participants using the raw data, considerably more observed data were available once the imputation had been performed and these data would also lead to the missing at random assumption being more reasonable.
The resource use at a primary care level included both mental health and physical health consultations because of the holistic nature of primary care. At a secondary care level we chose to restrict our collection of resource-use data to activities related to the participants’ mental health. Therefore, the questionnaire asked participants to record secondary care resource use only for mental health problems. We do not think that this will have materially affected our results as we were interested in the effectiveness of our intervention in improving mental health.
The physical activity intervention was less expensive than many of the psychological therapies that are used to treat depression. A course of CBT, for example, for which there is evidence of effectiveness, is likely to cost between £10075 and £30076 more per participant than this intervention. The travel costs for the face-to-face sessions with the participants were high in this trial, which may be because of the geographical spread of practices in the trial. If this service were to be implemented in the NHS it is likely that some efficiencies would occur, although excluding travel time from the analysis completely only improves the net benefit to £96 at a willingness to pay of £20,000 (probability of net benefit > 0 = 58%) and £239 at a willingness to pay of £30,000 (probability of net benefit > 0 = 63%).
Effectiveness and acceptability of the intervention
Despite the negative findings in relation to our clinical outcome, there was evidence to support the effectiveness of the intervention we developed in increasing the physical activity levels of the participants. This was most apparent when self-reported physical activity at all three follow-up points was considered. There was no evidence that the increase in physical activity reduced over time. If anything, there was a slight increase in the apparent effectiveness of the intervention over the course of the trial. Many of the participants had completed their contact with the PAF at around the 8-month point; therefore, it appears that the influence of the intervention on physical activity levels outlived the duration of the actual intervention. This is unusual in trials designed to increase physical activity, both with depressed and with non-depressed participants.
The intervention was also viewed very positively by the participants and GPs. The intervention was developed to improve long-term adherence by encouraging autonomy and allowing the participants to choose their own physical activity and their own pace of change. The qualitative study of the participants supported the acceptability of the intervention and confirmed that the participants’ own experience was of a non-judgemental approach that was encouraging and gave people choice. These findings confirmed that the PAFs in the trial were following the approach outlined in the manual. The quantitative and qualitative results, therefore, support the approach that was taken and suggest that, at least for participants with depression, this approach was acceptable and effective in increasing physical activity levels.
The adherence to the intervention reflected the perceived acceptability of this approach. Participants were recruited directly from primary care and were not selected on the basis of their interest in or motivation to engage in physical activity. However, it is possible that only those participants with relatively positive views of physical activity would have agreed to take part. In total, 95% of the participants allocated to the intervention attended at least one session and about two-thirds received at least five sessions, including a face-to-face meeting. We also conducted a CACE analysis to obtain an unbiased estimate of the effectiveness of the intervention in those who adhered to at least five sessions of the intervention before the primary outcome collection at 4 months. These results suggested that the difference in BDI score between the groups increased slightly, but not to an extent that it altered our conclusions from the ITT analysis.
Strengths and limitations
The conduct and reporting of the trial have followed the CONSORT recommendations. 48 All participants were recruited directly from within primary care and this should improve the generalisability of the findings. The study used a remote telephone randomisation system to conceal allocation and conducted the primary analyses on an ITT basis and according to a predefined analysis plan agreed with the Trial Steering Committee.
Baseline comparability of the two treatment groups was very good. Response rates were 80% at 4 months, 61% at 8 months and 71% at 12 months. Such high retention rates will have reduced the impact of any missing data on our findings although missing data did affect the complete case economic analysis. We also carried out an analysis in which we investigated whether or not the variables associated with ‘missingness’ altered our results from the analysis of the outcomes. There was no evidence to suggest that missing data had any influence on our findings. For the economic analysis, we carried out a multiple imputation that allowed use of all of the observed data. Again, it did not alter our findings. It is worth noting that we attribute the lower response rate at the 8-month follow-up to the use of postal reminders rather than arranging to meet the participants face to face.
During the course of the study it became apparent that we were not able to meet our original recruitment target. As a result we revised our power calculation and approach towards the analysis as described in the Methods (see Chapter 2, p. 15). Although we were concerned about the loss of power, it is clear from the discussion above that, in the event, our results were sufficiently precise to be able to draw quite firm conclusions about the lack of effectiveness of our intervention. We therefore think that the lower than hoped for recruitment did not prevent us answering the study aims.
In our original proposal we had intended to exclude those people at baseline who were taking antidepressants as we were concerned that the intervention might influence antidepressant use and make the results difficult to interpret. We changed our approach as about half of our participants were on antidepressants, and the IPCRESS47 trial, which had included participants who were taking antidepressants, reported that their intervention (CBT delivered via the internet) did not influence whether or not antidepressants were used. Our own results suggested that 57% of participants were taking antidepressants at baseline. There was a decline in their use over the duration of the trial, with 38% still reporting antidepressant use at the 12-month follow-up. However, there was no evidence at follow-up that there was differential use of antidepressants between the two randomised groups. The use of antidepressants by participants is therefore unlikely to have influenced our findings.
One of the strengths of the study was the recruitment of patients from primary care who had recently consulted their GP about depression. As a result, the patients included here were suffering from relatively severe depression, with a mean BDI total score of approximately 32, and half were on antidepressant medication. We think that this is the appropriate group of participants to study with a physical activity intervention if we wish to generalise to UK primary care. Furthermore, we did not find any evidence to suggest that the difference between treatment effect varied according to the baseline severity of depression. Nevertheless, it is possible that this group of patients had more severe depression than in previous trials in this area and there is always a possibility that physical activity might be of value in people with milder depression.
Because the TREAD study allocation was not blind for the participants or researchers, we used self-reported information for the assessment of outcome to eliminate any observer bias. However, the responses of participants could have been influenced by their knowledge of their allocation. For example, those in the intervention arm might have altered their responses to the self-reported questions on depressive symptoms or physical activity. This potential bias could not explain the lack of influence on the BDI scores but it is of course possible that there was a differential influence on the physical activity measure but not on depressive symptoms.
Measurement of physical activity and possible contamination
Physical activity is notoriously difficult to measure. In a subset of the participants we compared the results of the physical activity recall diary with a more objective measure of activity derived from an accelerometer. For the diary, participants were asked to record 15-minute episodes when they engaged in light, moderate or vigorous activity (see Appendix 3). In contrast, the accelerometer recorded activity during 10-second epochs. This seems the likely reason why the number of MET minutes of light activity recorded by the accelerometer was very much larger than the number obtained using the recall diary. There are also other reasons why the accelerometer results might differ from the recall diary. The movement associated with cycling is not recorded appropriately by an accelerometer and an accelerometer cannot be used when swimming. For many apparently vigorous activities, such as tennis or football, there are still periods when a person might be stationary. Despite these differences, there was still evidence of some agreement between the recall diary and the accelerometer; however, in those who were more vigorous, there was an increasing level of disagreement between the two measures. The impact of the intervention on physical activity has, therefore, to be treated with some caution, although any random measurement error could not explain the differences between the randomised groups observed in our study.
Other methodological issues
All of the participants recruited to the trial were aware of the design and the purpose of the study. The qualitative study indicated that, for some people, taking part in the trial and being allocated to the usual care arm led to an effort to be more active. Both groups reported an increase in physical activity between baseline and the 4-month follow-up and it is possible that some of this increase in the usual care group resulted from participation in the trial. There was also a suggestion that the difference between the two arms increased over the duration of the trial and this might also reflect some initial increase in activity in the usual care arm. On the other hand, the participants were recovering from depression during the first 4 months and this would also have presumably led to increased levels of physical activity in both arms.
We investigated whether or not the clustering by practice could have affected our statistical power. There was little evidence that any clustering by practice had much influence on our results or conclusions. Finally, there could also be an influence on the findings of the non-random allocation to PAFs. We used a method that allowed for non-random allocation of PAFs, but, again, it had no effect on the results.
Interpretation of results
Previous findings reviewed in Chapter 1 and summarised in the review by Mead et al. 8 have suggested that physical activity can improve outcome in depression. Our results are in stark contrast to the results of this meta-analysis, which indicated an effect size of 0.8 SDs at the end of the intervention. This result may well be an overestimate as the effect size was half this in the review that restricted analysis to trials in which the participants had received a diagnosis of depression. 10 Also, in trials with longer-term follow-up of around 16 weeks, there was little evidence of any influence. 10 It could therefore be argued that our finding is not as inconsistent with previous findings as might appear at first sight. Nevertheless, we wished to consider three further explanations for this apparent discrepancy, apart from those already described weaknesses in the literature.
First, it is possible that the physical activity intervention did not have a sufficiently large impact on the amount of physical activity undertaken to lead to an improvement in depressive symptoms. The absolute difference between the groups in the category meeting our threshold of MET minutes was about 15%. If physical activity were an effective treatment, the difference in the ‘active ingredient’ between the groups was therefore quite modest. Some of the other studies have used rather less pragmatic interventions such as supervised exercise. Nevertheless, it is possible that they generated a much larger difference in physical activity between their groups. We might therefore have been underpowered to find what might have been a relatively modest treatment effect considering the need for any treatment effect to be mediated by physical activity. However, such a treatment effect would not be of clinical importance as our results have excluded the possibility of a clinically important improvement in depressive symptoms. So this potential explanation still does not alter the conclusion that our intervention would not be clinically effective.
The second possibility we have considered is that only vigorous aerobic activity might have benefits for depressive symptoms. There is experimental evidence from health volunteers that affect improves during light physical activity and shortly after finishing very vigorous activity – the ‘runners’ high’ – possibly resulting from endogenous opioid activity. 77 Our intervention encouraged all types of activity, including ‘light’ activities such as walking. This was a pragmatic decision, often taken by the PAF. Even though the PAF was trying to encourage more vigorous activity this was often not possible in the population recruited to the trial. It is often difficult to influence people to change their behaviour and particularly to increase their level of vigorous activity. Even if this explanation were true, it still raises the issue of how the information can be used in a practical way by the NHS. Such an explanation is of theoretical importance but we are still left with uncertainty about how we would be able to use this information to improve human health.
Finally, it is possible that our participants differ in a very important way from those of previous studies. Many of the previous studies relied upon advertising whereas we recruited directly from primary care. Respondents to adverts might already have an interest in physical activity and regard it as an enjoyable pastime. Encouraging those people might help them to restore activities that have been abandoned as a result of their depressive illness. For many people with depression for whom physical activity has never played an important part in their life perhaps encouraging physical activity is not beneficial.
Implications for health care
-
We can be confident in concluding that our physical activity intervention does not benefit outcome in depressive illness when used as an adjunct to usual care and it is very unlikely to be cost-effective. Given the intensity of the intervention and its lack of effectiveness, we think it unlikely that advising patients with depression will improve their outcome.
-
The TREAD physical activity intervention did increase physical activity, an effect that lasted beyond the duration of the intervention. Our approach was patient centred, putting emphasis on choice and autonomy. It relied not simply upon giving advice or instruction but upon a range of behaviour change techniques. These might well offer GPs and other health professionals different methods of helping patients with or without depression to increase activity when indicated.
Future research implications
-
Future research would be useful if it were to identify and explain the mechanisms by which physical activity might affect mood in healthy volunteers. We have referred to evidence on the improvement in mood after vigorous activity and further understanding of the possible biological or other mechanisms would be of value.
-
It is possible that only vigorous physical activity leads to benefit in depression. Further smaller-scale ‘proof of concept’ or experimental medicine studies could be used to investigate the optimal type, intensity and duration of physical activity that might be required to produce a therapeutic effect. The effect on mood at different severities of depression could also be investigated using such methods.
-
The TREAD physical activity intervention successfully increased physical activity in people with depression, a population in which a number of factors would have been expected to make this task more difficult. It would be useful to examine the cost-effectiveness of the intervention in other areas of medicine where an increase in physical activity might be beneficial, for example in obesity and cardiovascular disease.
Acknowledgements
We would like to thank the external members of the Trial Steering Committee for their advice and support for the project: Professor Michael King (University College London), Professor Margaret Thorogood (University of Warwick), Professor Chris Dowrick (University of Liverpool) and Mr Paul Lanham as our service user representative. Our thanks go also to the Data Monitoring and Ethics Committee comprising Professor Helen Lester (Manchester University), Professor Kerry Hood (Cardiff University) and Professor Keith Lloyd (Swansea University).
We are grateful to the participants and GPs who supported the study, giving so generously of their time and sharing their experiences with us. Likewise, the practice managers and administrative staff at all of the collaborating practices and PCTs who provided valuable assistance to us throughout the study.
We would like to thank a number of people who helped towards the successful completion of the study. Sofie Sherlock, Caroline Jenkinson, Amanda Burston and Susan Bryant contributed to collecting data for the study. Ceire Costelloe helped with the analysis. We would also like to thank Julia Carver, Sarah Dawkins, Linda Mottram, Alison Wellingham and Kate Button who provided administrative support to the trial. Nathan Filer, Alice Garrood and Davina Chauhan [Mental Health Research Network (MHRN) Clinical Support Officers] helped in both the recruitment and the follow-up phases of the trial. Anne Laure-Donskoy provided advice from a service user perspective on the preparation of the protocol and on the management group.
The following delivered the intervention as PAFs: Helen Thorp, Joanna Yarham, Georgina Bentley (University of Bristol), Charlotte Hale and Tom Thompson (Exeter University).
Ethical approval
Ethical approval for the study was given by West Midlands MREC in October 2005 (reference number 05/MRE07/42).
Local Research Ethics Committee approval and the appropriate site-specific assessments were obtained from the PCTs covering the Bristol, South Gloucestershire, North Somerset and Devon areas and all study staff held honorary contracts for working within the relevant NHS trusts.
The trial was registered with the ISRCTN (reference number 16900744) and the NRR (reference number 2159).
Funding
This study was funded by the NIHR HTA programme (project number 03/45/07).
The University of Bristol agreed to act as sponsor for the research and the study was adopted by both the MHRN and Primary Care Research Network (PCRN).
Excess treatment costs and service support were provided by a central subvention from the NIHR and the local PCTs.
Contribution of authors
GL had overall responsibility for the study and MC was responsible for the day-to-day operationalisation and management of the study, conducting the analysis and drafting the final report. The co-applicants JC, MCal, KF, AH, SH, DL, AM, TP, DS, AT and NW were involved in all stages of the work: the design, analysis and commenting upon and drafting sections of the final report. KT took over the leadership of the qualitative element from MCal after the funding had been received and also contributed to all stages of the work. AH, KF, AT and HT were responsible for developing the physical activity intervention and for its description in the report. AS carried out the qualitative fieldwork, performed the analysis with the assistance of KT and MCal and drafted the qualitative chapters of the report. SH led on the design, analysis and drafting of the economic analyses. HB, RW and CW were involved in the design and conduct of the study and commented on the final report. MD was involved in the design of the accelerometer study and the analysis of the data and drafting of and commenting on the report.
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
Publications
-
Baxter H, Winder R, Chalder M, Wright C, Sherlock S, Haase A, et al. Physical activity as a treatment for depression: the TREAD randomised trial protocol. Trials 2010;11:105.
-
Haase AM, Taylor AH, Fox KR, Thorp H, Lewis, G. Rationale and development of the physical activity counselling intervention for a pragmatic TRial of Exercise and Depression in the UK (TREAD-UK). Ment Health Phys Act 2010;3:85–91.
-
Searle A, Calnan M, Lewis G, Campbell J, Taylor A, Turner K. Patients’ views of physical activity as treatment for depression in primary care: a qualitative study. Br J Gen Pract 2011;61:14–56.
-
Searle A, Calnan M, Turner KM, Lawlor DA, Campbell J, Chalder M, et al. General Practitioners’ beliefs about physical activity for managing depression in primary care. Ment Health Phys Activ 2012; in press.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Heath I. Commentary: there must be limits to the medicalisation of human distress. BMJ 1998;318:436-40.
- Donoghue M, Tylee A. The treatment of depression: prescribing patterns in primary care in the UK. Br J Psychiatry 1996;168:164-8.
- Demyttenaere K. Risk factors and predictors of compliance in depression. Eur Neuropsychopharmacol 2003;13:69-75.
- Prior L, Wood F, Lewis G, Pill R. Stigma revisited, disclosure of emotional problems in primary care consultations in Wales. Soc Sci Med 2003;56:2191-200.
- Department of Health . At Least Five a Week: Evidence on the Impact of Physical Activity and Its Relationship to Health – a Report from the Chief Medical Officer 2004.
- National Institute for Health and Clinical Excellence . Depression: The Treatment and Management of Depression in Adults 2009.
- Taylor AH, Fox KR. Changes in physical self-perceptions: findings from a randomised controlled study of a GP exercise referral scheme. Health Psychol 2004;24:11-2.
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev 2009;3.
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001;322:1-8.
- Krogh J, Nordentoft M, Sterne JA, Lawlor DA. The effect of exercise in clinically depressed adults: systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry 2010;72:529-38.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12.
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007;69:587-96.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005;28:1-8.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV 1994.
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Galper DI, Sunderajan P, et al. TREAD: TReatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials 2006;3:291-305.
- Eakin EG, Glasgow RE, Riley KM. Review of primary care-based physical activity intervention studies: effectiveness and implications for practice and future research. J Fam Pract 2000;49:158-68.
- Eaton CB, Menard LM. A systematic review of physical activity promotion in primary care office settings. Br J Sports Med 1998;31:11-6.
- Lawlor DA, Hanratty B. The effect of physical activity advice given in routine primary care consultations. J Public Health Med 2001;23:219-26.
- Simons-Morton DG, Calfas KJ, Cooper A. Effects of interventions in health care settings on physical activity or cardio-respiratory fitness. Am J Prev Med 1998;15:413-30.
- van Sluijs EM, van Poppel MN, van Mechelen W. Stage-based lifestyle interventions in primary care: are they effective?. Am J Prev Med 2004;26:330-43.
- Department of Health . Exercise Referral Systems: A National Quality Assurance Framework 2001.
- Craig AC, Dinan S, Smith A, Taylor AH, Webborn NJ. National Quality Assurance Framework will guide best value and best practice in GP exercise referral schemes (letter). BMJ 2000;320.
- Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: a randomised controlled trial of methods to promote physical activity in primary care. BMJ 1999;319:828-32.
- Taylor AH, Doust J, Webborn ADJ. Randomised controlled trial to examine the effects of a GP exercise referral programme in East Sussex, UK on modifiable coronary heart disease risk factors. J Epidemiol Commun Health 1998;52:595-601.
- Pavey TG, Anokye N, Taylor AH, Trueman P, Moxham T, Fox KR, et al. Clinical effectiveness and cost-effectiveness of exercise referral schemes. Health Technol Assess 2011;15.
- Tones K. Evaluating health promotion: a tale of three errors. Patient Educ Couns 2000;39:227-36.
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking; toward an integrated model of change. J Consult Clin Psychol 1983;51:390-5.
- Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health behaviour change in primary care. Am J Prev Med 1999;17:275-84.
- Little P, Dorward M, Gralton S, Hammerton L, Pillinger J, White P, et al. A randomised controlled trial of three pragmatic approaches to initiate increased physical activity in sedentary patients with risk factors for cardiovascular disease. Br J Gen Pract 2004;54:189-95.
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behaviour. New York, NY: Plenum Press; 1985.
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change. New York, NY: Guilford Press; 2002.
- Markland DM. Self-determination moderates the effects of perceived competence on intrinsic motivation in an exercise setting. J Sport Exerc Psychol 1999;21:351-61.
- Biddle SJH, Biddle SJH, Fox KR, Boutcher SH. Physical activity and psychological well-being. London: Routledge; 2000.
- Leith LM, Taylor AH. Behaviour modification and exercise adherence: a literature review. J Sport Behav 1992;15:60-74.
- Mental Health Foundation . Moving on up 2009.
- McKenna J, Naylor P-J, McDowell N. Barriers to physical activity promotion by general practitioners and practice nurses. Br J Sports Med 1998;32:242-7.
- Fox KR, Fitzsimmons K, Haase AM, Riddoch CJ. An appraisal of the evidence supporting new public health messages for the promotion of physical activity. London: Department of Health; 2004.
- Haase A, Taylor A, Fox KR, Thorp H, Lewis G. Rationale and development of the physical activity counselling intervention for a pragmatic TRial of Exercise and Depression in the UK (TREAD-UK). Ment Health Phys Act 2010;3:85-91.
- Baxter H, Winder R, Chalder M, Wright C, Sherlock S, Haase A, et al. Physical activity as a treatment for depression: the TREAD randomised trial protocol. Trials 2010;11.
- World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders 1992.
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardised assessment for use by lay interviewers. Psychol Med 1992;22:465-86.
- Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498-504.
- Stewart AD, Hays RD, Ware JE. The MOS short-form General Health Survey. Med Care 1988;26:724-32.
- Resnick B, Zimmerman SI, Orwig D, Furstenberg ALMJ. Outcome expectations for exercise scale: utility and psychometrics. J Gerontol Soc Sci 2000;55B:S352-6.
- Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340.
- Carpenter J, Kenward M. Guidelines for Handling Missing Data in Social Science Research 2008. www.missingdata.org.uk.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95.
- Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007;17:571-82.
- Curtis L. Unit costs of health and social care 2009. Canterbury: PSSRU, University of Kent; 2009.
- Department of Health . National Schedule of Reference Costs 2009–10 for NHS Trusts and PCTs Combined 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123459 (accessed January 2012).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2008. www.bnf.org/bnf/.
- Scott A, Simoens S, Heaney D, O’Donnell C, Thomson H, Moffat K, et al. What does GP out of hours care cost? An analysis of different models of out of hours care in Scotland. Scott Med J 2004;49:61-6.
- National Audit Office . The Provision of Out-of-Hours Care in England 2006.
- Munro J, Nicholl J, O’Cathain A, Knowles E, Morgan A. Evaluation of NHS Direct First Wave Sites: Final Report of the Phase 1 Research 2001.
- Salisbury C, Chalder M, Manku-Scott T, Nicholas R, Deave T, Noble S, et al. The National Evaluation of NHS Walk-in Centres. Final Report 2002.
- Office for National Statistics . Annual Survey of Hours and Earnings 2009. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2009-revised/index.html (accessed January 2012).
- Brooks R. EuroQol Group . Euroqol: the current state of play. Health Policy 1996;37:53-72.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. 1–9–1995 n.d. www.york.ac.uk/inst/che/pdf/DP138.pdf (accessed January 2012).
- Drummond M, Sculpher M, Torrance GW, O’Brien B, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Thompson S, Barber J. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94.
- Searle A, Calnan M, Lewis G, Campbell J, Taylor A, Turner K. Patients’ views of physical activity as treatment for depression: a qualitative study. Br J Gen Pract 2011;61:149-56.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing qualitative data. London: Routledge; 1994.
- Taylor AW, Wilson DH, Wakefield M. Differences in health estimates using telephone and door-to-door survey methods – a hypothetical exercise. Aust N Z J Public Health 1998;22:223-6.
- Faulkner G, Biddle SJH. Exercise and depression: considering variability and contextuality. J Sport Exerc Psychol 2004;26:3-18.
- White KT. Why Does Physical Activity Alleviate Depression? Identifying Potential Mediators and Understanding the Process of Change 2008.
- Hyde J, Calnan M, Prior L, Lewis G, Kessler D, Sharp D. Reluctant medicalisers? A qualitative study exploring how general practitioners decide to prescribe antidepressants. Br J Gen Pract 2005;55:755-62.
- Johnston O, Kumar S, Kendall K, Peveler R, Gabbay J, Kendrick T. Qualitative study of depression management in primary care: GP and patient goals, and the value of listening. Br J Gen Pract 2007;57:872-9.
- Davison GC. Stepped care: doing more with less?. J Consult Clin Psychol 2000;68:580-5.
- National Institute for Health and Clinical Excellence . Depression: Management of Depression in Primary and Secondary Care 2004.
- McCrone P, Knapp M, Kennedy T, Seed P, Jones R, Darnley S, et al. Cost-effectiveness of cognitive behaviour therapy in addition to mebeverine for irritable bowel syndrome. Eur J Gastroenterol Hepatol 2008;20:255-63.
- Hollinghurst S, Peters TJ, Kaur S, Wiles N, Lewis G, Kessler D, et al. Cost-effectiveness of therapist-delivered online cognitive-behavioural therapy for depression: randomised controlled trial. Br J Psychiatry 2010;197:297-304.
- Ekkekakis P, Hall EE, Petruzzello SJ. Variation and homogeneity in affective responses to physical activity of varying intensities: an alternative perspective on dose–response based on evolutionary considerations. J Sports Sci 2005;23:477-500.
Appendix 1 TREAD PAF manual
Appendix 2 TREAD study protocol
HTA no: 03/45/07
ISRCTN no: 16900744
UKCRN study ID: 2159
MREC no: 05/MRE07/42
Funded by the NHS R & D Health Technology Assessment Programme
A pragmatic randomised controlled trial to evaluate physical activity as a treatment for depression
TREAD
1. PLANNED INVESTIGATION
1a) Research objectives
The primary research question is ‘Does physical activity, in addition to usual care in primary health care, change the outcome in depression and alter the subsequent use of antidepressant medication?’
1b) Existing research
Depression is one of the leading reasons for disability in the UK, as elsewhere, and is the third most common reason for consulting a general practitioner. There are now over 25m prescriptions of antidepressants each year in primary care in England that cost £80m (www.ppa.org.uk). Antidepressants are an effective treatment for the more severe depressions but there is uncertainty and concern about the use of antidepressants, especially in those with mild depression. Adherence to antidepressant treatment is often poor and only about 20% of patients will take medication according to guidelines. 1,2 There is widespread scepticism about the effectiveness of antidepressants amongst the general population and this may contribute to a reluctance to consult general practitioners for depression. 3 Hence, there is a need to identify effective non-pharmacological interventions for the management of the common, less severe forms of depression and as a potential means of reducing the length of treatment with pharmacological agents.
Health benefits of physical activity
There has recently been an increased interest in the potential health benefits of physical activity in heart disease, obesity and diabetes. 4 In the past decade ‘exercise on prescription’ schemes have become popular in primary care in the UK. However, there has also been a suggestion that exercise (or it’s more widely-defined counterpart, physical activity) could be an effective treatment of depression. Many of the 800 or so schemes in the UK receive referrals for people with depression (Wright Foundation Survey 2003).
A systematic review of randomised controlled trials (RCTs) by one of the Co-applicants5 found evidence to support the effectiveness of exercise for depression, but it also identified some important methodological limitations which mean it is still uncertain whether physical activity is effective in the management of patients with depression in primary care. These issues can be summarised as follows:
(a) lack of evidence in clinical populations
The majority of RCTs have been conducted in non-clinical community volunteers who have responded to advertisements concerning an exercise in depression trial. In some studies, there were financial or other incentives to participate. Results from such trials are difficult to generalise to patients who present to primary care as volunteers are likely to have an extra degree of motivation.
(b) short duration of follow-up
Only one of the trials studied whether any benefits of an exercise intervention outlasted the duration of the intervention. In the meta-regression,5 the length of follow-up was an important source of heterogeneity, with those of shortest duration reporting the largest effects suggesting that effects may be weak or non-existent over the long-term. In the context of a chronic relapsing and remitting disease, it is important to estimate the long-term as well as the short-term effects, though even a short term benefit may still be cost-effective.
(c) poor quality of trials
The majority of trials to date used randomisation procedures that were inadequately concealed, failed to undertake intention-to-treat analyses or followed protocols involving unblinded treatment allocation. In general, these failures will tend to exaggerate treatment effects. 6
(d) small size of trials
The trials undertaken to date were all far too small and underpowered to find anything apart from a massive treatment effect. The largest of the trials included just 36 participants in the treatment arm and 28 in the control arm; most trials had fewer than 20 participants in total.
In a recent update of the Lawlor systematic review, a number of new studies were reported.
The DOSE study7 found a treatment response for the more intensive ‘dose’ of exercise in their trial. This intervention involved the participants attending a gym and carrying out aerobic activity on an exercise bike. The more vigorous (17.5 Kcal/kg/wk) and more frequent (five days) form of intervention appeared to have a benefit when compared to the control group. However, the study was small, with only sixteen subjects randomised to the most intensive group – overall there were eighty participants allocated to five groups with a Latin square design. Additionally, participants had very mild depression, with a mean Hamilton8 score of 16.2, although all met DSM-IV9) criteria. The fact that this was a non-pragmatic trial in a different health care setting to TREAD means that vital issues about treating depression with physical activity remain unexplored.
Blumenthal et al10 have also carried out a comparison, in the US, between two different exercise interventions, with antidepressant and placebo treatments. They found a 9% difference between remission rates as a result of home-supervised exercise and placebo at sixteen weeks. This difference is slightly smaller than that which we have used for the TREAD power calculations. However, we would expect a placebo effect in such trials, so we think that the plausible treatment difference between intervention and usual care would be greater than that reported in this particular trial. In addition, this was a relatively small trial with just over 40 participants in each group and so probably estimated these differences with little accuracy.
Another US trial, coincidentally also called TREAD, is underway and has published its protocol. 11 The intervention in this case is modelled upon the more intensive intervention from the DOSE study, although it is somewhat more pragmatic since it allows home-based as well as gym-based activity. However, participants are sedentary at baseline and there were reported difficulties in implanting the intervention. The protocol paper does not state the overall sample size nor does it undertake any form of economic analysis.
Whilst the above-mentioned studies address some of the previously mentioned methodological concerns, there is no suggestion that they substantially change our original conclusions. None of them address the same aims as TREAD, nor do they deal with issues in a way which relates to the NHS. Thus, the scientific questions of our study are still relevant and of importance, particularly if we want to contribute to UK policy and practice.
How might exercise work?
Little is known about the possible mechanisms that might mediate any therapeutic effects of physical activity on depression. Suggested biological mechanisms include changes in neuro-endocrine function, neurotransmission, core temperature, cerebral blood flow, or muscular tension. Psychosocial mechanisms such as improvements in physical self-perceptions and self-confidence have been observed12 and increased social interaction and perceived support from an exercise specialist or exercise group have also been suggested.
Similarly, there is little evidence to indicate the type, intensity and duration of physical activity that might be most effective in reducing depression. The recent CMO report4 concluded, on the basis of rather limited evidence, that aerobic exercise lasting between 20 and 60 minutes which involved large muscle groups, such as brisk walking, cycling and swimming, was likely to be most effective. The Dunn13 trial was designed to compare 180 and 80 minutes of moderate activity per week over 5 and 3 days (for each dose) and a stretching group, over 12 weeks.
A physical activity intervention, if effective, is likely to improve depressive symptoms through some or all of these pathways and it could be that the overall effectiveness of physical activity relies upon such multiple mechanisms. We, therefore, think that the priority, at this stage, is to determine whether physical activity might improve outcomes in patients with depression. The exploratory analyses and the qualitative elements of the proposed research will be of value in planning future, more detailed investigations.
How can we best encourage an increase in physical activity?
In order to investigate our research question, we need to design an intervention that will lead to a sustainable change in physical activity patterns. There are some systematic reviews14–18 of RCTs that have investigated the ability of exercise promotion interventions to increase activity levels. In planning an intervention for depressed participants, there are four issues to consider:
1) intensity of the intervention
The reviews concluded that there is little evidence to suggest that the investigated interventions led to a long-term change in physical activity. However, this may have been because the interventions were not intensive enough in terms of the amount of supervision and support provided to participants. Very few of the interventions would meet the more recent NHS National Quality Assurance Framework (NQAF) guidelines for exercise referral schemes. 19,20 Many of the studies only had one contact with the patient and it is probably difficult to generalise from some of the US studies. In a UK study, Harland21 concluded that progressively more intensive interventions, involving up to 6 counselling sessions and 30 free leisure centre vouchers, produced greater changes in physical activity up to 12 weeks. However, the effects were not sustained at 12 months. Taylor’s RCT in the UK,12,22 reported increased activity and fitness at 26 weeks in response to a 10-week exercise referral scheme in a local leisure centre and improved physical self-perceptions at 9 months.
Most of the literature concerns interventions designed for patients with cardiovascular disease, rather than depression. Depression is characterised by low motivation, fatigue and reduced self-esteem and these are likely to make it difficult to increase physical activity levels. All the RCTs that have investigated the effects of physical activity on depression have used supervised physical activity sessions rather than advice. 5 This most likely reflects the understanding by those undertaking such studies that a less intensive intervention is unlikely to alter behaviour in those with depression. An intervention that has been relatively successful in changing behaviour in a cardiovascular disease group may not, therefore, be intensive enough for a trial of people with depression. Physical activity is still regarded with some scepticism as an effective treatment for depression and, at this stage of knowledge, it would be important to ensure that any intervention gives the best chance for changing behaviour. We want to avoid what Tones23 has called a ‘type 3’ error, in which lifestyle interventions have (correctly) failed to show an effect on outcome because the intervention itself was too weak to change behaviour.
2) theoretical model underpinning intervention
Only half of the reported trials have described an intervention based upon a theoretical framework. The most popular frameworks used were designed to influence exercise cognitions and behaviour based on stage of readiness to become more active (Transtheoretical Model). 24,25 A recent systematic review18 concluded that ‘stage of readiness’ based interventions have not, on the whole, been effective in increasing patient’s physical activity in primary care but, as mentioned above, many of these interventions were probably not sufficiently intensive. Little26 devised an intervention based upon the Theory of Planned Behaviour (TPB) and other behavioural techniques found that counselling sessions produced a trend towards greater change in physical activity, but only at one month follow-up and in patients without depression recruited through a postal request.
The wide range of components used within this intervention, as with others, makes it difficult to attribute any change in behaviour to the use of the TPB or any one particular theoretical model. Furthermore, TPB seems to be more useful in predicting more intense physical activity rather than the more modest levels likely to be seen in this population. In this situation, using the model of self-determination theory (SDT) would seem more appropriate, with self-determination being based on the perception of choice in engaging in any behaviour. 27 Self-determination theory proposes that real shifts in behaviour arise through heightened autonomy or personal ownership of behavioural success. Self-determination theory also suggests that steady incremental improvement in self-efficacy occurs through achievement of personally directed goals. It also maintains that autonomous change of this nature provides the basis of self-esteem improvement. Encouraging participants to take charge of their physical activity decisions and choices is, therefore, very important. This approach fits well with the principles of motivational interviewing28 leading to better adherence and better motivation. 29 It also supports the view that choice of physical activity option, as described later, should improve adherence, especially over the longer term. 30
It would seem both sensible and pragmatic to base an intervention for depressed subjects on an appropriate theoretical model25,31 within the frameworks of self-determination theory. 27 In practical terms, the key elements are likely to be an intervention that (a) assesses current attitudes to physical activity, perceived barriers and the readiness to change (b) utilises motivational interviewing techniques28 to engage the patients own motivation rather than providing simple advice (c) offers choice of physical activity and rate of improvement and (d) that uses appropriate behavioural strategies that can increase self-efficacy31 and self-determination. 27 We will check if participants’ baseline expectancy of a treatment effect on depression predicts adherence to physical activity and an improvement in depression.
3) who delivers the intervention
Evidence from primary care suggests that existing health professionals are very inconsistent at providing advice about exercise and physical activity. For example, McKenna et al32 found that GPs and practice nurses typically did little to promote physical activity and those who did were up to four times more likely to be active themselves. It appears that only health professionals with a commitment to physical activity tend to encourage an increase in activity in their patients. The intensity and nature of a physical activity intervention for depression suggests that individuals with both a commitment to the concept and a readiness to develop expertise are needed. If each practice were to devote a health professional to this task, the training would have to be less intensive and, since each professional would only be seeing a handful of patients, it would be difficult to develop any expertise in the area. For such reasons, many people, within and outside the Department of Health, have argued for a new type of health professional who has expertise in behavioural change, that we shall call a Physical Activity Facilitator (PAF).
There is already a multiplicity of tasks for practice nurses and there are likely to be future shortages of health professionals as a result of the recent NHS plan. It, therefore, makes sense to expand the NHS workforce by recruiting Physical Activity Facilitators from those who are now emerging from undergraduate and postgraduate courses. If this model were to be adopted more widely, it would also be easier to implement since it is much simpler to train one person who might cover ten practices, than to have to train a health professional from each of those practices. The establishment of the Register for Exercise Professionals (www.reps-uk.org/welcome.asp), alongside the NQAF launch in 2001, has helped to ensure that staff employed in exercise referral schemes have appropriate training, insurance and abide by a code of ethics comparable to professions allied to medicine.
4) reducing barriers to physical activity
Many people are reluctant to engage in physical activity, not only because of financial barriers, but also because of their own perceptions about physical activity and preference for different forms of physical activity. The more traditional ‘exercise on prescription’ schemes in UK primary care, have been termed structured or centre-based activity where the patient attends formal group sessions at a leisure or community centre. In contrast, lifestyle or home-based activity allows the individual to develop their own physical activity programme from home which primarily consists of brisk walking or cycling. Of course, many individuals combine both. In a recent Department of Health commissioned review, Fox et al33 found no difference in adherence to these two programmes where patients were randomised. The critical issue is to maximise choice in order to increase chances of adherence. In some of the more progressive physical activity schemes, such as those being delivered in Somerset, participants are referred to a trained facilitator who will establish activity preferences, needs and fitness levels. The Somerset scheme has some 25 different physical activity options including individual programmes of walking and callisthenics in addition to leisure-centre based courses.
Summary
In summary, there is only limited direct evidence at this point to inform the design of interventions or services that might lead to long-term increases in physical activity in the UK primary care setting among depressed patients. There is no well worked out ‘off the shelf’ intervention. The Co-applicants intend to base the intervention on the principles outlined above. In particular, the intervention should (1) provide relatively intensive contact and support (2) be based upon established psychological models of behavioural change (3) be administered by a trained Physical Activity Facilitator (4) provide choice and (5) a financial subsidy, where needed, to reduce barriers. As part of the research, we will provide a standardised training and a manual both to ensure consistent delivery of the intervention in the study and to aid wider implementation if that were indicated. The intervention will be based upon the Somerset scheme and the NHS National Quality Assurance Framework for Exercise Referral Schemes. 19
2. RESEARCH METHODS
2a) Study design
We are proposing a two-arm, multi-centre, pragmatic, randomised controlled trial with randomisation at the level of the individual participant.
Definition of usual care
It is difficult to define ‘usual care’ of depression in primary care. Some people with depression are managed without antidepressant medication but we suspect that this group have a milder illness,34 would be prescribed antidepressants if their symptoms persisted and would be more difficult to recruit to a study, since GPs might not have discussed the diagnosis with their patients. Any pragmatic trial of physical activity would be non-blinded, so if participants were not receiving antidepressants at the outset this could lead to a difference in antidepressant prescription between groups. For example, if participants who are not currently treated with antidepressants are randomised to usual care, the general practitioner (GP) might be more likely to prescribe antidepressants in view of the need to provide the individual with something more tangible in the way of treatment.
We are also interested in the use of medication over a longer term follow-up such as that specified in the research brief. The use of medication will doubtless be very different between individuals who are on antidepressants at the beginning of the trial and those who are not. For these reasons, we propose that the randomisation should be stratified according to whether the patient is taking antidepressants prescribed by their GP at the entry of the trial. The proposed analysis will also take these matters into account. Counselling and, on occasions, other psychotherapies, is used in primary care as an element of ‘usual care’. This will also affect the design and interpretation of the proposed trial. However, the delay that inevitably occurs before counselling or psychotherapy are received will make it unlikely that this would affect our primary outcome or have a major impact on the trial. For this reason, we will not use it as a minimisation factor in our analysis.
Contamination
In an individually randomised trial, there is a possibility that subjects not randomised to physical activity will inadvertently have a form of usual care in which physical activity is given far more prominence or that participants on usual care would pursue their own programme of physical activity. This ‘contamination’ of the usual care group could reduce any observed treatment effect. Subjects randomised to usual care will not have access to the Physical Activity Facilitator (PAF). General advice from the GP to patients about physical activity is a common element in health promotion. However, in this trial, patients would only receive the advice of the Physical Activity Facilitator and access to many of the physical activity options if they meet the Physical Activity Facilitator. This is an advantage of the TREAD intervention over one that uses practice staff. In this trial, we do not think that contamination will be a serious problem but we will measure activity levels in both arms as a means of monitoring this aspect.
2b) Recruitment
Recruitment will take place over a 27-month period, predominantly in general practices that are currently part of the well established primary care R & D networks in Bristol (Avon Consortium – 20 practices) and Exeter (PenRen – 40 practices). We will ask GPs to refer patients whom they have just started on antidepressant medication for depression and also depressed patients who are not currently on antidepressant medication but who wish to pursue further treatment for their condition. We will use a variety of means to encourage referral to the trial. These will include stickers, posters, newsletters and other publicity. We will also screen practice computer systems for people who have recently been diagnosed as depressed or given an antidepressant, in order to recruit additional participants who have not been referred by their GP to the trial via consultation.
The two GP research networks we plan to use have a strong track record in carrying out research and both have experience and commitment to mental health trials. We will be providing a well thought out package as the intervention and this will help to gain the confidence of the GPs and encourage recruitment. The GP will ask patients diagnosed with depression if they are interested in taking part in the trial and suggest that they release their personal details to the research team for further contact. Once given permission to contact the patient, a Research Assistant will perform the baseline assessment, confirm eligibility and obtain informed consent. For participants randomised to the intervention arm, an appointment will be made to meet the Physical Activity Facilitator in the general practice, at the research office, or in the patient’s home. For those randomised to the control arm, the participant will be asked to continue with their usual GP care.
Blinding and other forms of bias
It is not possible to blind participants or their GPs to their allocation of treatment. As far as possible, we propose to use self-administered measures to assess outcomes, in order to eliminate any observer bias. We have, therefore, chosen not to use clinician-based measures of outcome such as the Hamilton Rating Scale for Depression. 8 We propose to minimise selection bias by recruiting participants from a variety of practices based in rural, urban, affluent and deprived areas. Bristol and Exeter provides a whole range of environments from the deprived and ethnically-mixed inner city of Bristol to market towns and rural areas. We will also aim to keep exclusion criteria to a minimum.
Allocation to trial groups
The study will use individual allocation from a central telephone randomisation service controlled by an administrator in Bristol. Allocation will be stratified by antidepressant use (yes, no), and minimised by severity of depression (CIS-R score of ≤ 25, 26–33, 34+ at baseline), recruiting centre (Bristol, Exeter) and level of physical activity (≤ 1, 2–3, 4+ days per week where at least 30 minutes of moderate intensity physical activity is undertaken.) We do not think it is practicable to stratify by practice, age or receipt of psychotherapy since this would then have too many strata for the randomisation. In any case, minimising by centre will ensure balance in terms of local factors including any co-interventions, and will ensure proportionate workload for the Physical Activity Facilitators. Stratification by use of antidepressant medication is justified as this may have an important bearing on the trial outcome as explained above. Minimisation by the other variables will ensure balance between the arms of the study and help with power since these factors, particularly baseline severity of depression, are likely to be important predictors of outcome.
2c) Planned interventions
The principles behind the intervention have been described already in Section 1b. We propose to develop an intervention manual, based on the NHS National Quality Assurance Framework for Exercise Referral Schemes (NQAF) and the existing referral scheme in Somerset (in operation for over 10 years) which involves a trained exercise facilitator. The patients in receipt of the intervention will be given a list of local physical activity options, in addition to support from the Physical Activity Facilitator.
Physical Activity Facilitators
Two part-time Physical Activity Facilitators at each site will be required, two each for Bristol and Exeter. They would be graduates of existing undergraduate and MSc courses and would have some practical experience of similar facilitation processes. They will have two days additional training in the nature of depression, pharmacological treatment, characteristics of depressed patients and working in primary care settings. The Departments of Exercise, Nutrition and Health Sciences at Bristol and the School of Sport and Health at Exeter will provide professional supervision, support and resources for their professional development. Each Physical Activity Facilitator will be employed by the relevant academic institution and cover a number of local practices. Physical Activity Facilitators will be instructed not to discuss any non-intervention patients with any other staff in the practice.
Frequency of contact
The goal of the Physical Activity Facilitator is to maximise long-term increases in physical activity over a period of eight months. There would be an initial face-to-face assessment meeting lasting around 45 minutes followed by a series of up to ten further telephone contacts and two further face-to-face 30-minute meetings over the 8-month intervention period. These further contacts would follow a protocol, depending upon whether the person was meeting agreed goals. For example, contacts would be less frequent and intense if the person was successfully implementing the physical activity plan.
Contents of manual
The manual will provide practical guidance on the principles outlines in the introduction. It will also provide a structure for the assessment interview to include physical activity history, motives and barriers to undertaking physical activity as well as scoping patient needs and preferred options. The facilitator would agree an activity plan with the patient and set both short and long-term goals. Simple psychological and behavioural techniques would be described to help people adhere to a physical activity plan, including the use of diaries to record physical activity. Background information about depression would also be provided.
Physical activity advice
The physical activity advice given to participants in the intervention will be individually tailored to take account of current levels of fitness, motivation and previous experience of physical activity. Short-term goals will be tailored to the patient’s recent physical activity history with the long-term goal of achieving the recent recommendations;4 i.e. 30 minutes (in one or more sessions) of moderate intensity activity (e.g. brisk walking) on at least five days each week. The emphasis will be on frequency of daily activity in the first instance, followed by increasing duration of sessions. A recent review of existing trials has shown no difference in adherence to programmes of physical activity using shorter versus long bouts. 33
Monitoring intervention
A random sample of the face-to-face and telephone contacts for all Physical Activity Facilitators will be recorded to ensure adherence to the model and consistency of delivery.
Usual care
The usual care group will be advised to follow the current advice of their general practitioner regarding their depression and its treatment.
Loss to follow-up
Our sample size calculation has allowed a 15% loss to follow-up at 4-months post-randomisation. A recent randomised controlled trial conducted by two of the Co-applicants35 achieved an 81% follow-up rate at 6 weeks. In a recent, mild depression trial (MRC G9304472) we had an 88% follow-up rate at 6 weeks and 81% at 12 weeks. In order to minimise attrition, data collection at 4 and 12-month follow-up will be conducted face-to-face wherever possible.
Acceptability
There are two aspects to the acceptability of the trial. The first is the acceptability of the physical activity intervention. We suspect that some individuals will refuse to consider the trial because they do not like or want to carry out any physical activity. As discussed above, we will ensure, as far as possible, that the intervention is acceptable and individually tailored to the participants and this will be explained by the Research Assistant. The second issue is the acceptability of the randomisation procedure. In the trial, there will be no interference with usual care. The GP and patient can decide on any additional treatments including antidepressants, counselling or referral to secondary care, as they feel appropriate. However, half the people entering the trial will not be randomised to the physical activity intervention. We do not, however, think that this will reduce the overall acceptability of the trial, since it will provide an extra treatment option that is not widely used in the two areas in which the study will be conducted.
2d) Planned inclusion/exclusion criteria
We seek to recruit people with mild and moderate depression who are beginning a new episode of depression. We will, therefore, include people aged 18–69 who have either recently started antidepressants (within 4 weeks of their assessment and following an antidepressant free period of at least 1 month) or who are not currently on antidepressants but have recently consulted their GP for depression. The baseline assessment will use the revised Clinical Interview Schedule (CIS-R)36 administered by computer in order to make an ICD-10 diagnosis of depression (F32), a criteria for inclusion in the trial. The participants will also have to score 14 or more on the Beck Depression Inventory37 (BDI), in order to ensure that there is room for improvement in our primary outcome. Other exclusions will cover any medical contraindications to physical activity,38 inability to complete self-administered questionnaires in English, psychosis, bipolar disorder and any serious drug or alcohol abuse. Women who are pregnant at the time of recruitment will automatically be excluded from the trial but those who become pregnant during the trial may continue, providing they have approval and permission to do so from their GP. We will request consent from patients referred by the GP to use basic demographic information as a means of describing those who are excluded from the trial in comparison with those who do take part
2e) Ethical issues
We do not think this trial will raise any particular ethical issues. We are not interfering with the usual clinical care of participants. The physical activity intervention is an extra intervention in addition to GP usual care. We are obtaining valid informed consent from the subjects. The lack of a clear effect of physical activity in the treatment of depression from the most up-to-date systematic review5 shows that there is clinical equipoise. Finally, participants can still receive antidepressants, counselling or psychotherapy during the course of the trial, if this proves necessary or desirable.
2f) Proposed baseline and outcome measures
Primary outcome
The primary outcome will be clinical symptoms of depression assessed using the Beck Depression Inventory (BDI). 37 In the analysis, BDI will be treated as both a continuous and binary (< 10 or ≥ 10) outcome. The continuous outcome will give a measure of improvement and the binary an estimate of the proportion that has symptomatic recovery. Both are important clinical outcomes and we will power the study to detect differences in both. The primary follow-up will be at 4-months post randomisation as we would expect the maximum impact at 4-months. This corresponds broadly to the time-frame that was used in previous trials. 5 The primary analysis that we, therefore, propose is the BDI score at 4-months, after adjustment for BDI score at baseline.
Secondary outcomes
Other depression and anxiety measures
It is difficult to measure episodes of depression retrospectively, so number of days prescribed an antidepressant during the 12-month follow-up period will be a secondary outcome. This will be measured by searching the GPs’ computerised records and by using a self-reported measure of medication adherence. We will also use BDI (both continuous and binary) at the 8 and 12-month follow-ups in order to measure longer term effects of the intervention on our outcomes. The Physical Activity Facilitator will maintain contact with the participant for approximately eight months (though at a reduced level) whilst the 12-month data collection will allow investigation of any longer term sustained effects on outcomes. We will also ask about any depressive episodes between the follow-up times but recognise that this information is likely to be inaccurate.
Quality of life
Quality of life will be assessed using the SF-1239 at baseline, 4, 8 and 12 months. This is a widely used scale that examines a range of items concerned primarily with functional status. The EQ-5D40 will also be used in the economic analysis.
Measuring adherence to physical activity programme
We will measure adherence to the physical activity programme at baseline and all follow-up points using a self-reported questionnaire comprising a variety of previously validated and specially drafted measures. Because of the known error in self-reported physical activity, and the need to monitor activity levels in the usual care group, we are proposing the use of accelerometers in a sub-sample of participants. Accelerometers are matchbox-sized computers that are worn, during waking hours, on a belt at the hip. They provide minute-by-minute estimates of movement. This movement can be translated into number of steps walked and percentage of time spent in different intensities of activity. They can also identify sustained sessions of activity at various intensities, including sedentary time and thus providing a comprehensive activity profile. Additionally, movement counts can also produce estimates of energy expenditure. 41,42 These accelerometers will be used to record a week of activity by a random sample of the patients at 4-month follow-up. We will carry out these tests on 50 subjects in each treatment group with the aim of validating the self-reported activity data. This sample size is based upon current advice43 for reliability testing to give reasonably precise (± 0.15) estimates of the reliability coefficient. It should enable us to detect, at 80% power and 5% significance, a difference of 0.4 SD in the mean activity levels between the two randomised groups.
Other measures
Because of the possible link between physical activity and other psychosocial variables we will measure social support (using ONS Psychiatric survey scales),44 physical self perceptions45 and physical activity self-efficacy46 at baseline and all follow-up points. Personality variables are also very important prognostic indicators and we will, therefore, use the Big Five inventory to investigate these. 47 A discrete choice experiment (DCE) in questionnaire format will also be included, in order to examine patients’ preferences for different aspects of the physical activity intervention.
Baseline assessment
This will consist primarily of the CIS-R, BDI, SF-12, EQ-5D, self-report physical activity questionnaire, as well as questions on social support, physical self-perceptions, physical activity self-efficacy, previous psychiatric history and socio-demographics.
2g) Economic data & analysis
The aim of the economic evaluation is to compare the costs and benefits of physical activity in addition to usual care with usual care alone for primary care patients with depression. These two proposed methods of patient care will be compared from the viewpoint of: (i) the National Health Service (NHS) and personal social services (PSS), (ii) patients and carers, and (iii) society. 48 The analysis will be based on the costs incurred over the 12 months following randomisation, measured at baseline, 4, 8 and 12 months.
Resources used by all patients will be identified, measured and valued. The principal costs to the health care provider will relate to the cost of the intervention, primary and secondary health care contacts, and medication. Patients and carers are likely to incur travel costs, use of alternative therapies, loss of income, and home support costs such as childcare. Societal costs will relate to lost production due to time off work. We will collect patient level data from routine sources such as practice records, as well as a patient questionnaire based on the Client Service Receipt Inventory,49 which has been used elsewhere to assess the costs of treating mental illness. This will be adapted to suit this study, this patient group and for postal administration.
Health care resources will be valued using published national sources, for example, Unit Costs of Health and Social Care and the British National Formulary. The cost of the intervention will be based on the cost of its provision in the trial, but any protocol-driven research costs will be excluded. Informal care giving will be valued using the principle of opportunity cost, so the shadow price of informal care will be estimated as the unit cost of a home care worker. In valuing lost production, we will follow the recommendations of Drummond. 50 Productivity losses will be reported separately and measured in terms of days lost. We will estimate the value of lost production using the ‘friction’ approach, a variation of the ‘human capital’ approach, which includes only the resources required to replace the employee. Costs and outcomes at 12 months will be discounted at the recommended rate of 3.5%. 50 Costs will be related to the primary clinical outcome of the trial (BDI) and quality of life as measured by the EQ-5D. 40
It is our intention that incremental cost-effectiveness ratios will be formed comparing (i) the cost per extra patient recovering; (ii) the cost per depression free days; and (ii) the cost per QALY gain, for each of the proposed treatments. Sensitivity analyses will be conducted in those areas where there is uncertainty around assumptions about resource use measurement and/or valuation. Patient variation in resource use and the effectiveness of the intervention will be captured using ‘bootstrapping’ to construct a cost effectiveness acceptability curve. 51
2h) Feasibility Phase
Aims of feasibility phase
Given the novelty of the trial, we propose carrying out a feasibility phase study in order to:
-
estimate recruitment rate
-
pilot and refine physical activity intervention
-
investigate acceptability of the recruitment procedure and physical activity intervention.
1) estimate recruitment rate
In our original proposal, we estimated our recruitment rate as 2.5 participants per practice per month, using a 12-month recruitment period. If we extend the recruitment period by 3 months, the required recruitment rate drops to 2 per practice per month. We are currently recruiting for a trial of antidepressants in depression (GenPod MRC G0200243) and initial impressions are that it is realistic to recruit at that rate. However, we recognise that this is a challenging recruitment rate and its achievement will depend upon how potential recruits view the acceptability of the physical activity intervention. The feasibility phase will, therefore, provide us with a more precise estimate of the recruitment rate to this trial.
2) pilot and refine physical activity intervention
The physical activity intervention will be piloted and refined during the feasibility phase. As we have argued, a physical activity intervention for people with depression has somewhat different requirements to a generic physical activity intervention. Though we have already given a very clear idea of these requirements, an extended developmental phase would be valuable since it will allow the Physical Activity Facilitators to gain experience of delivering the intervention before the main phase of the trial starts. One element of this work will be to create a local list, for both the Bristol and Exeter sites, of community-based physical activity opportunities that are not provided in local leisure centres.
3) investigate acceptability of the recruitment procedure and physical activity intervention
We will additionally investigate the acceptability of the recruitment procedure and the physical activity intervention using qualitative methods. This will provide more systematic evidence with which to revise the recruitment process and the intervention. In-depth interviews will be carried out with participants, practice managers, general practitioners (GPs) and key trial personnel such as the Trial Co-ordinator, Research Assistants and Physical Activity Facilitators. We will also invite potential participants who have refused to take part in the randomised trial to be interviewed, although we recognise that they may be reluctant to take part in the qualitative interviews. The interviews with GPs, practice managers and participants will focus upon their experience of recruitment and the perceived acceptability of the recruitment process as well as considering their own views about clinical equipoise in relation to the trial. We will ask them to describe the reasons why they decided to take part (or not if more appropriate) and how they weighed up the advantages and disadvantages of doing so.
For participants allocated to the intervention arm, we will also ask them about physical activity. We will attempt to interview anyone who drops out of the physical activity treatment arm as well as those who continue with it. The interviews will focus on the reasons behind their decision to continue or stop the physical activity intervention as well as exploring those aspects they found most and least helpful. The Trial Co-ordinator would carry out the recruitment for individuals who would then be interviewed using qualitative methods by the Research Assistant. Interviews will be carried out with approximately 4 GPs, 2 practice managers and up to 12 participants, unless the results indicate that further interviews are required. All interviews will be audio-taped and transcribed. Data collection and analysis will run in parallel. Transcripts will be studied in detail and a list of common themes and concepts drawn up. The analysis of the data will follow the principles outlined in the main trial methods section. The resulting data will enable us to modify the recruitment method and, if necessary, the intervention.
2i) Sample size justification
Our original power calculation anticipated that about 60% of participants in the usual care group and 73% in the intervention group would have recovered by 4-month follow-up – scoring < 10 on the BDI. This difference of 13% in the proportion ‘recovered’, equivalent to an odds ratio of 1.8, is consistent with the lower end of treatment effects observed with antidepressant medication, but is still substantial and worth detecting for a common condition such as depression and for an intervention with other possible health benefits such as physical activity. With 90% power and 5% two-sided alpha, this would require 291 patients per group.
When using the BDI as a continuous outcome, previous studies have estimated a standard deviation of about 9 points,52 and have suggested that a worthwhile and feasible target difference is about 3–4 points. With 5% two-sided alpha, a sample size of 291 per group will afford 98 to > 99% power to detect a difference of 0.33 to 0.44 standard deviations. Furthermore, it will yield a derived margin of error for the difference between the randomised groups of approximately 0.16 standard deviations, equivalent to 95% CIs on the BDI scale of approximately 1.5 to 4.5 and 2.5 to 5.5 for estimated differences of 3 and 4 respectively.
The table below presents original estimates for numbers required to be recruited for different powers and proportions of patients not on antidepressants at baseline, allowing for an attrition rate of 15%, and based on detecting an odds ratio of 1.8 with 5% two-sided alpha.
| Power | % untreated at baseline | n for analysis | Derived margin of error for primary comparison | ||
|---|---|---|---|---|---|
| 0% | 5% | 10% | |||
| 80% | 520 | 548 | 578 | 442 | 1.68 |
| 85% | 592 | 622 | 658 | 502 | 1.57 |
| 90% | 686 | 722 | 762 | 582 | 1.46 |
Assuming that 10% of the sample would not be on antidepressant treatment when recruited, and that, overall, there would be a 10–15% attrition rate, the sample size specification above required 291 per group to be available for analysis. In order to achieve this, the total number that will need to be recruited, depending on the attrition rate, would be between 720 and 762 [(291*2)/(0.9*0.9) to (291*2)/(0.9*0.85)]. We, therefore, proposed to recruit 762 participants at the outset.
However, our initial calculation made a number of assumptions about:
-
recruitment rate
-
antidepressant use
-
recovery rate at 4-month follow-up
-
follow-up rate
Recruitment rate
In our original proposal, we predicted that we could randomise two participants per practice per month. In fact, the recruitment rate achieved in the first few months of the trial was far lower, at around 0.3 participants per practice per month or approximately 18 participants per month for each of the 60 practices we initially planned to recruit. As a result, it became clear that we were unlikely to meet our original recruitment target within the original timeframe. Indeed, if we continued to recruit to the original schedule, we would only expect to recruit between 180 and 198 participants overall. However, if we extend recruitment by an additional twelve months, and assume the same recruitment rate of between 15 and 18 per month, we would expect to recruit between 360 and 414 participants in total.
Antidepressant use
Originally, we were concerned that patients not on treatment i.e those allocated to the usual care arm, were more likely to be prescribed antidepressants during the trial and that this might lead to a marked reduction in any expected treatment effect. We anticipated, at the outset, that the proportion of patients not on treatment would be approximately 10% of those recruited. We, therefore, proposed to power the study and conduct the primary and main secondary analyses on the 90% of recruited patients who we anticipated would be on antidepressant treatment at the point of randomisation. However, data from the early stages of the trial suggests that about 50% of randomised patients were taking antidepressants. On this basis, and taking into account the fact that randomisation is stratified by antidepressant use at baseline, we are reassured that we should include all randomised participants in the primary analysis, irrespective of antidepressant use.
Recovery rate at 4-month follow-up
The original power calculation had assumed that 60% of participants would recover within four months. However, the proportion of patients seen to recover in the recently concluded IPCRESS study was found to be somewhat lower, with only 20% of participants recovering in the waiting list group (19/92 = 20.6%; 95% CI 12.9–30.3). Our revised power calculations, therefore, examine the difference between 20% and 33%, maintaining the original absolute difference in rates from our original power calculation. This will not have any effect on the estimates of precision in relation to continuous outcomes.
Follow-up rate
In the original protocol, we had assumed an 85% retention rate at 4-month follow-up i.e. our primary outcome. Rate of follow-up is difficult to estimate accurately but current confidence limits do include this value. We are, therefore, confident that we can meet our target of 85% and have continued to use this assumption in our revised power calculations. Our original protocol outlined postal follow-up at 4-months but other studies have shown that collecting follow-up data in person can improve follow-up rates. We, therefore, propose to carry out both the 4-month and 12-month assessments in face-to-face mode wherever possible.
The table below provides a summary of our revised power calculations assuming a 27-month recruitment period. We have given figures for both our most conservative estimate of recruiting fifteen randomised patients per month and a more optimistic upper bound of recruiting eighteen randomised patients per month. We conclude that the revised sample size will still give us adequate power for our primary analysis using the continuous outcome. Whilst there will inevitably be some reduction in power for the categorical outcome, we will still be able to detect a 14% or 15% difference with 80% power.
| Monthly recruitment rate | Total n randomised | n for primary analysis | Power for 73% vs 60% (OR = 1.80) | Power for 20% vs 33% (OR=1.97) | Detectable difference with 80% power1 | Error factor2 for odds ratio | Power to detect 3 BDI point difference | Standard error3 on BDI |
|---|---|---|---|---|---|---|---|---|
| 15 | 360 | 306 | 63% | 69% | 15% | 1.68 | 82% | 1.03 |
| 18 | 414 | 354 | 70% | 76% | 14% | 1.62 | 87% | 0.96 |
2j) Statistical analysis
The analysis and presentation of this pragmatic randomised trial data will be in accordance with CONSORT guidelines,53 with the primary comparative analyses being conducted on an intention-to-treat basis and due emphasis placed on confidence intervals for the between-arm comparisons. Descriptive statistics of socio-demographic and clinical measures will be used to detect any marked imbalance between the arms at baseline. As described previously, we intend to make full use of BDI as both a binary and continuous outcome measure in the interpretation of the results of this trial. Although unusual we, therefore, specify two primary analyses. These primary comparative analyses will employ multivariable logistic or linear regression, as appropriate, to investigate differences between the groups, adjusting for minimisation variables and baseline BDI amongst those medicating at baseline. For the binary outcome, the comparison will be presented as an odds ratio of recovery (scoring < 10 on the BDI) in the intervention group compared with the control group. For BDI as a continuous measure, the comparison will be presented as a difference in group mean scores. For both outcomes we will also present 95% confidence intervals and p-values.
Sensitivity analyses, making different assumptions such as ‘best’ and ‘worst’ case scenarios as well as imputation models of ‘missingness’, will be conducted to investigate the potential impact of missing data. We will also investigate the extent and impact on the results of clustering by general practice and possibly Physical Activity Facilitator; although the small number of facilitators will mean that investigation of such effects will be limited. In the absence of adequate power to formally test for differential effects according to antidepressant therapy at baseline, we will investigate the patterns of confidence intervals for both subsets of patients separately and combined.
In addition to carrying out the same analyses for the secondary outcomes (where p-values will be adjusted to account for multiple testing), and to repeating any such primary analyses adjusted also for any variables exhibiting marked imbalance at baseline, the secondary analyses for this trial will take three general forms:
-
investigation of process measures such as adherence to the physical activity programme, use of antidepressants, counselling and social support. Mostly these will be descriptive analyses, but this information will be used to investigate whether adherence to the physical activity programme is associated with ‘recovery’, and will also be employed within the economic evaluation.
-
some of this process data will also be employed in secondary, explanatory analyses that attempt to explain the comparisons between the two treatment arms from the intention-to-treat analyses. This will be investigated by adjusting for factors such as adherence to the physical activity programme in the intervention group, but also reported activity levels in both groups. The models employed will be essentially the same as those for the primary analyses. In addition, we will investigate the patterns of BDI scores (as a continuous measure) between the groups at the 4, 8 and 12- month follow-up using repeated measures (random effects) linear regression, adjusting for baseline BDI, minimisation factors, and any other variables displaying imbalances at baseline. Divergence or convergence between the two groups over time will also be investigated using appropriate interaction terms.
-
thirdly, appropriate interaction terms will be entered into the primary regression analyses for BDI as both a binary and continuous outcome. in order to conduct pre-specified subgroup analyses according to baseline severity of depression (CIS-R score of ≤ 25, 26–33, 34+ at baseline) and baseline physical activity level (≤ 1, 2–3, 4+ days per week where at least 30 minutes of moderate intensity physical activity is undertaken). Since the trial is powered to detect overall differences between the groups rather than any interactions terms, the results of these exploratory analyses will be presented using confidence intervals as well as p-values, and interpreted with due caution.
2k) Qualitative Study
Qualitative methods can be an essential part of a trial’s evaluation and can provide another perspective on a trial’s results from those provided by the quantitative analysis. Within TREAD, results from a qualitative study could help us understand why the physical activity intervention, in addition to usual GP care, was or was not effective in changing the outcome of depression and/or altering subsequent use of antidepressants. We, therefore, suggest that the qualitative work is not restricted to the feasibility phase of the trial but also extends into the main trial.
The main aim of the qualitative study will be to explore patients’, health professionals’ and Physical Activity Facilitators’ views and experiences of the physical activity intervention, in order to assess its acceptability and to illuminate possible reasons for the quantitative results. Study objectives are to:
-
assess patients’ views and experiences of the physical activity intervention
-
identify patients’ reasons for accepting, declining, adhering to or withdrawing from the intervention
-
explore health professionals’ views of the intervention and its impact on general practice
-
assess the Physical Activity Facilitators’ views and experiences of providing the intervention
Design
We intend to carry out the qualitative study in practices that are already participating in the main trial, with access to the same intervention and the same Physical Activity Facilitators. However, since in-depth qualitative interviews have some similarities to supportive counselling, we propose to only interview trial participants after they have provided follow-up data on the primary outcome, in order to avoid unduly influencing the main trial. The qualitative study will entail conducting interviews with trial participants, health professionals and the Physical Activity Facilitators. It will also involve recording patients’ reasons for declining to take part in the trial and for not adhering to the intervention.
Interviews with trial participants
Using information collected during the baseline questionnaire, participants will be purposively sampled to ensure that interviews are held with men and women of varying age, who differ in terms of what their level of physical activity had been at baseline (i.e. low, medium or high). Within this sampling approach, we will also aim for maximum variation in relation to level of depression, history of depression, socio-economic background, and whether individuals live in rural or urban areas. Interviews will be carried out with trial participants recruited in both Bristol and Exeter, and in both arms of the trial.
All participants taking part in the trial will have consented at baseline to being approached by a qualitative researcher. Thus, the researcher employed to conduct the interviews will telephone individuals who have been sampled for the qualitative study to ask if they would be willing to take part in an interview. The researcher will explain the aims and design of the qualitative study and answer any questions the participant might have. If the participant is willing to take part in an interview, an interview time and place will be arranged. A letter confirming the interview arrangements will then be posted to the participant. This letter will be accompanied by an information leaflet about the study.
Participants will be interviewed on two occasions: within a month of the 4 month follow-up and at 12 months post-randomisation, i.e. once the primary and final outcome measures have been completed. The interviews will take place at a time that suits the individual, at a location of his/her choice. Prior to interview, both written and verbal consent to be interviewed will be secured from the participant.
The four-month interview with participants in the intervention arm will explore their reasons for taking part in the trial; their views about physical activity as a treatment for depression; what physical activity they were undertaking prior to TREAD; their experiences of the intervention; their relationship with their Physical Activity Facilitator; their experiences of usual care and what other treatments they have tried or are using for their depression; barriers and supports to increasing levels of physical activity; how they think their views towards physical activity have changed; how they think physical activity has affected their depression; and whether or not they think physical activity has become more integrated into their lives. Participants in the control group will also be asked about their reasons for taking part in the trial, their views on physical activity as a treatment for depression and what physical activity they were undertaking prior to TREAD. In addition, they will be asked about their experiences of usual care and what treatments they have used to manage their depression.
The twelve-month interview with participants in the intervention arm will explore their experiences in the later stages of the intervention; whether or not they have managed to maintain changes made whilst in contact with a Physical Activity Facilitator; and what factors have supported or prevented further changes or changes being maintained. Interviews with those in the control group will assess their experiences of usual care and what treatments they have used to manage their depression.
Data collection will continue until saturation of key themes has been reached. It is predicted that this will mean about 50 individuals will be interviewed in total, i.e. 20 from the control group and 30 from the intervention group. Interviewing about 50 individuals at the 4-month point will also ensure that we have adequate numbers of participants at 12-months post-randomisation to make this second data set meaningful.
Recording of reasons for declining to take part in the trial
Patients who have agreed to have their contact details passed on to the research team may still decline to take part in the trial. They may decline on being contacted by the research team or at the baseline assessment prior to randomisation. It is important that we explore why individuals decline to take part in the trial, as these individuals may have specific views towards physical activity as a treatment of depression, particularly in terms of its acceptability and effectiveness. Thus, in situations where an individual declines to take part in the trial, the researcher conducting the initial telephone ‘screen’ or baseline assessment will invite him/her to explain his/her decision. Any reasons given will be noted.
Recording of reasons for withdrawing from the intervention
Some participants randomised to the intervention arm may decide not to continue with the intervention. Like the individuals who decline to take part in the trial, these individuals may hold particular views toward the intervention and, therefore, provide important insights into its acceptability. Where possible, the Physical Activity Facilitator or researcher in touch with these individuals will invite them to explain the rationale behind their decision to discontinue treatment. Any reasons given will be noted.
Interviews with health professionals
Interviews will be held with GPs who have been involved with the trial. We will sample GPs in both Bristol and Exeter, GPs who have and have not referred to the trial, and GPs working in areas of varying levels of affluence/deprivation and urbanisation. GPs sampled will be sent a letter inviting them to take part in an interview. This letter will be accompanied by an information sheet. The qualitative researcher will then telephone or email the GP a week later to ask if s/he would be willing to take part in an interview. To encourage participation, GPs will be given the choice of being interviewed at their place of work, at home or over the telephone. The interviews will explore GPs’ views on physical activity as a treatment for depression, their use and implementation of the physical activity programme, their views on its impact on general practice, and their reasons for referring or not referring patients to the trial. GPs who did refer patients will also be asked about which patients they referred to the study and any information they have on why patients had refused to take part. These interviews will be held once recruitment to the trial has ended. It is predicted that about 10 to 15 GPs will be interviewed in total. Prior to interview, both written and verbal consent to take part in an interview will be secured. In practices where others have been involved with the recruitment process, e.g. Practice Managers, once recruitment to the trial has ended, interviews will also be held with these professionals to explore their views on physical activity as a treatment for depression. These individuals will be invited and consented for interview using the same approach and paperwork used for recruiting GPs. It is predicted about 5 such interviews will be held.
Interviews with Physical Activity Facilitators
The Physical Activity Facilitators in both Bristol and Exeter will be asked to take part in an interview, once they have finished delivering the intervention. The qualitative researcher will explain to them that the purpose of the interview will be to explore their views and experiences of delivering the intervention, their understanding of the aims of the intervention and the rationale behind its design, and how they translated key elements in to practice. The researcher will also provide the Physical Activity Facilitators with an information leaflet that provides more details about the interviews. The researcher will then contact each Physical Activity Facilitator a week later to ask if she would be willing to take part in an interview. The interviews will take place at a time that suits the facilitator, at a location of her choice. Prior to interview, both written and verbal consent to take part in an interview will be secured.
Data analysis
With participant consent, all the interviews will be audio-taped, fully transcribed and anonymised. Notes taken by members of the research team about reasons for declining or not adhering to the intervention will also be typed up. Data collection and analysis will run in parallel. Transcripts will be read and re-read in order to gain an overall understanding of each interviewee’s views and experiences. This process will also be used to develop a coding frame, to identify common themes and concepts. The coding frame will be developed and refined as additional material emerges. Each transcript will be imported into a software package, such as ATLAS.ti, to allow electronic coding and retrieval of data. Transcripts will be coded by two independent researchers in order to maintain reliability of coding. The analysis will rely upon ‘constant comparison’ and will continue until no new themes emerge. Data collected from trial participants might also be analysed using a biographical approach so that we can identify developments between the first and second interview, in terms of behavioural changes, participants’ knowledge and attitudes.
2l) Management and supervision of trial
Many of the Bristol Co-applicants are based in the Department of Community based Medicine at Bristol University (GL, DS, TP, NW, AM, SH) whilst the Department of Exercise, Nutrition and Health Science (KF, AH) is on the same University precinct, as is the Department of Social Medicine (MCal, DL). Exeter (JC, AT) is 75 minutes drive from Bristol, with regular train services between the two cities. We will have a full-time Trial Co-ordinator based in Bristol who has overall responsibility for the trial. The Trial Co-ordinator will develop the detailed protocol, finalise baseline and follow-up assessments, manage the Research Assistants, maintain the central database and coordinate meetings of the management group and Trial Steering Committee. They will also take the lead in the data analysis and preparation of final reports with the assistance of the Co-applicants when needed. A number of Research Assistants will be based in both Bristol and Exeter. Their primary role will be to conduct baseline assessments, obtain consent and activate the randomisation procedure. The administrators will arrange appointments and send out the mailings for follow-up assessments, working alongside the Research Assistants, for the Trial Co-ordinator. A research management group comprising GL, NW, JC, AM, SH, the Trial Co-ordinator, Research Assistants and administrators will meet monthly. KF, AH, AT, DL will attend regular meetings, when required, to supervise the physical activity element of the trial. A Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) will be appointed based upon MRC guidelines and after approval from the HTA.
The Co-applicants are all in WestHub, part of the Mental Health Research Network (MHRN). The study will be adopted by both the MHRN and PCRN and this will give us access to the infrastructure to help with ethics applications, research governance and recruitment. The study will also benefit from the experience gained from existing HTA and MRC treatment trials of depression in Bristol.
3. PROJECT TIMETABLE and MILESTONES
The original timetable has been modified to include delays to the start-up of the study. The final study schedule is shown below:
Timetable: 5 years, 3 months
0–9 months: Secure MREC and NHS research governance approval. Recruit staff.
10–14 months: Begin recruiting practices. Develop protocol, questionnaires and SOPs.
15–21 months: Conduct feasibility phase. Finalise protocol. Continue recruiting practices.
22 months: Start of main trial. Begin to recruit patients. Carry out baseline assessments.
26 months: Begin 4-month follow-up assessments.
30 months: Begin 8-month follow-up assessments.
34 months: Begin 12-month follow-up assessments.
39 months: Start of qualitative component.
54–56 months: Extract GP computer record data.
57–61 months: Conduct data analysis.
62–63 months: Prepare final report.
4. EXPERTISE
The study team has psychiatry (Glyn Lewis), physical activity (Ken Fox, Adrian Taylor, Anne Haase, Debbie Lawlor), primary care (Debbie Sharp, John Campbell, Debbie Lawlor), randomised clinical trial (Glyn Lewis, Debbie Sharp, Tim Peters, Alan Montgomery, Nicola Wiles, Debbie Lawlor, Melanie Chalder), statistical (Tim Peters, Alan Montgomery), health economics (Sandra Hollinghurst) and qualitative (Mike Calnan, Adrian Taylor, Katrina Turner) research expertise. Anne Laure-Donskoy, a member of our local service user group SURF, has contributed to the proposal and a number of other lay members have been involved in the drafting of the trial documentation and management. We will make use of two well-established and active primary care research networks based in Bristol and Exeter. We have recently completed three randomised controlled trials of depression funded by the MRC, HTA and BUPA Foundation in Bristol and this study will also benefit from the management experience of our well-established Trial Co-ordinators group. The Department of Exercise, Nutrition and Health Science in Bristol has participated in three randomised trials of physical activity/exercise for other health related conditions.
5. DISSEMINATION
The results will be published in peer review journals and presented to the relevant conferences, nationally and internationally. The production of a manual for the physical activity intervention will enable us to provide specific guidance on the training that would be needed and the nature of the intervention, if it proved cost-effective.
Reference list
- Donoghue M, Tylee A. The treatment of depression: prescribing patterns in primary care in the UK. Br J Psychiatry 1996;168:164-8.
- Demyttenaere K. Risk factors and predictors of compliance in depression. Eur Neuropsychopharmacol 2003;13:69-75.
- Prior L, Wood F, Lewis G, Pill R. Stigma revisited, disclosure of emotional problems in primary care consultations in Wales. Soc Sci Med 2003;56:2191-200.
- Department of Health . At Least Five a Week: Evidence on the Impact of Physical Activity and Its Relationship to Health – a Report from the Chief Medical Officer 2004.
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001;322:1-8.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005;28:1-8.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV 1994.
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007;69:587-96.
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Galper DI, Sunderajan P, et al. TREAD: TReatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials 2006;3:291-305.
- Taylor AH, Fox KR. Changes in physical self-perceptions: findings from a randomised controlled study of a GP exercise referral scheme. Health Psychol 2004;24:11-2.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. The DOSE study: a clinical trial to examine efficacy and dose response to exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
- Eakin EG, Glasgow RE, Riley KM. Review of primary care-based physical activity intervention studies: effectiveness and implications for practice and future research. J Fam Pract 2000;49:158-68.
- Eaton CB, Menard LM. A systematic review of physical activity promotion in primary care office settings. Br J Sports Med 1998;31:11-6.
- Lawlor DA, Hanratty B. The effect of physical activity advice given in routine primary care consultations. J Public Health Med 2001;23:219-26.
- Simons-Morton DG, Calfas KJ, Cooper A. Effects of interventions in health care settings on physical activity or cardio-respiratory fitness. Am J Prev Med 1998;15:413-30.
- van Sluijs EM, van Poppel MN, van Mechelen W. Stage-based lifestyle interventions in primary care: are they effective?. Am J Prev Med 2004;26:330-43.
- Department of Health . Exercise Referral Systems: A National Quality Assurance Framework 2001.
- Craig AC, Dinan S, Smith A, Taylor AH, Webborn NJ. National Quality Assurance Framework will guide best value and best practice in GP exercise rferral schemes (letter). BMJ 2000;320.
- Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: a randomised controlled trial of methods to promote physical activity in primary care. BMJ 1999;319:828-32.
- Taylor AH, Doust J, Webborn ADJ. Randomised controlled trial to examine the effects of a GP exercise referral programme in East Sussex, UK on modifiable coronary heart disease risk factors. J Epidemiol Commun Health 1998;52:595-601.
- Tones K. Evaluating health promotion: a tale of three errors. Patient Educ Couns 2000;39:227-36.
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking; toward an integrated model of change. J Consult Clin Psychol 1983;51:390-5.
- Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health behaviour change in primary care. Am J Prev Med 1999;17:275-84.
- Little P, Dorward M, Gralton S, Hammerton L, Pillinger J, White P, et al. A randomised controlled trial of three pragmatic approaches to initiate increased physical activity in sedentary patients with risk factors for cardiovascular disease. Br J Gen Pract 2004;54:189-95.
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behaviour. New York: Plenum Press; 1985.
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change. New York: Guilford Press; 2002.
- Markland DM. Self-determination moderates the effects of perceived competence on intrinsic motivation in an exercise setting. J Sport Exerc Psychol 1999;21:351-61.
- Biddle SJH, Biddle SJH, Fox KR, Boutcher SH. Physical activity and psychological well-being. London: Routledge; 2000.
- Leith LM, Taylor AH. Behaviour modification and exercise adherence: a literature review. J Sport Behav 1992;15:60-74.
- McKenna J, Naylor P-J, McDowell N. Barriers to physical activity promotion by general practitioners and practice nurses. Br J Sports Med 1998;32:242-7.
- Fox KR, Fitzsimmons K, Haase AM, Riddoch CJ. An appraisal of the evidence supporting new public health messages for the promotion of physical activity. Department of Health; 2004.
- Kisely SR, Linden M, Bellantuono C, Simon G, Jones J. Why are patients prescribed psychotropic drugs by general practitioners? Results of an international study. Psychol Med 2000;30:1217-25.
- Thomas HV, Lewis G, Sharp D, Watson M, Bell TS, Lyons I, et al. Computerised patient-specific guidelines for management of common mental disorder in primary care: a randomised controlled trial. Br J Gen Pract 2004;54:832-7.
- Lewis G. Assessing psychiatric disorder with a human interviewer or a computer. J Epidemiol Commun Health 1994;48:207-10.
- Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71.
- American College of Sports Medicine . American College of Sports Medicine Guidelines for Exercise Testing and Prescription 1995.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I: conceptual framework and item selection. Med Care 1992;30.
- Williams A, Kind P, Hopkins A. Measures of the quality of life and the uses to which such measures may be put. London: RCP Publications; 1992.
- Cooper AC, Page AS, Fox KR, Misson J. Physical activity patterns in normal, overweight and obese individuals using minute-by-minute accelerometry. Eur J Clin Nutrition 2000;54:887-94.
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. acceleometer. Med Sci Sports Exerc 1998;30:777-81.
- Streiner DL, Norman GR. Health measurement scales. Oxford: Oxford Medical Publications; 1989.
- Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H. Psychiatric morbidity among adults living in private households. London: The Stationery Office; 2001.
- Fox KR, Corbin CB. The Physical Self-Perception Profile: development and preliminary validation. J Sport Exerc 1989;11:408-30.
- McAuley E, Talbot H-M, Martinez S. Manipulating self-efficacy in the exercise environment in women: influences on affective responses. Health Psychol 1999;18:288-94.
- John OP, Donahue EM, Kentle RL. The ‘Big Five’ inventory – version 4a and 54. Berkeley: Institute of Personality and Social Research, University of California, Berkeley; 1991.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Techonology Appraisal 2004.
- Beecham J, Knapp M, Thornicroft G, Brewin CR, Wing J. Measuring mental health needs. Oxford: Oxford University Press; 2004.
- Drummond MF, O’Brien B, Stoddard GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- Briggs A, Drummond M, McGuire A. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001.
- Elkin I, Shea T, Watkins JT, Imber SD, Sotsky SM, Collins JF, et al. National Institute of Mental Health Treatment of Depressionn Collaborative Research Program: general effectiveness of treatments. Arch Gen Psychiatry 1989;46:971-83.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Lancet 2001;357:1191-4.
Appendix 3 TREAD physical activity recall diary
List of abbreviations
- BDI
- Beck Depression Inventory
- BNF
- British National Formulary
- CACE
- complier-average causal effect
- CBT
- cognitive behavioural therapy
- CI
- confidence interval
- CIS-R
- revised Clinical Interview Schedule
- CMO
- Chief Medical Officer
- CONSORT
- Consolidated Standards of Reporting Trials
- CPM
- counts per minute
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D
- European Quality of Life-5 Dimensions
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- ICD-10
- International Classification of Diseases and Related Health Problems, 10th Edition
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Randomised Controlled Trial Register
- ITT
- intention to treat
- LMVPA
- light, moderate and vigorous physical activity
- MET
- metabolic equivalent of the task as a ratio to the basal metabolic rate
- MHRN
- Mental Health Research Network
- MREC
- Multicentre Research Ethics Committee
- MVPA
- moderate and vigorous physical activity
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute of Health Research
- NQAF
- National Quality Assurance Framework
- NRR
- National Research Register
- OR
- odds ratio
- PAF
- physical activity facilitator
- PCRN
- Primary Care Research Network
- PTA
- pre-trial assessment
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SMD
- standardised mean difference
- SOP
- standard operating procedure
- TPB
- theory of planned behaviour
- TREAD
- TREAting Depression with physical activity
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington