Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/01/01. The protocol was agreed in December 2010. The assessment report began editorial review in November 2011 and was accepted for publication in November 2011. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Hockenhull et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Clarification of research question and scope
Pharmalgen® products (ALK Abelló) are used for the diagnosis and treatment of immunoglobulin E (IgE)-mediated allergy to bee and wasp venom. The aim of this systematic review was to assess whether use of Pharmalgen products is of clinical value when providing VIT to individuals with a history of severe reaction to bee and wasp venom, and whether it would be considered cost-effective compared with alternative treatment options available in the NHS in England and Wales.
Description of health problem
Aetiology, pathology and prognosis
Apidae (bees), Vespidae (wasps and hornets) and Formicidiae (ants) form part of the order Hymenoptera. Bees and wasps have a modified ovipositor at the terminal end of their abdomen that gives them the ability to sting other organisms. Bees possess a barbed stinger, which, together with their venom sac, remains in their victim’s skin after they sting. This means that bees are able to sting only once, and die soon afterwards. Wasps’ stingers are not barbed and they are therefore capable of delivering more than one venom-injecting sting in their lifetime. Bee and wasp stings contain allergenic proteins. In wasps, these are predominantly phospholipase A1,1 hyaluronidase1 and antigen 52 and, in bees, phospholipase A2 and hyaluronidase. 3 It has been estimated that each bee sting contains 147 μg of venom and each wasp sting contains 17 μg of venom. 4
The symptoms produced following a sting can be classified into non-allergic and allergic reactions. All envenomated individuals are likely to experience local burning and pain followed by erythema (redness) and a small area of oedema (swelling) at the site of the sting. These are caused by vasoactive components of venom and the mechanism is toxic rather than allergic. 4
Following an initial sting, some individuals generate an immune response, which produces antibodies of the IgE class. These antibodies sensitise cells, particularly histamine-containing mast cells, so that allergen re-introduced by a subsequent exposure can bind to the preformed IgE molecules, triggering the cells to produce a rapid inflammatory response (this is referred to as a ‘type 1’ or ‘immediate-type’ hypersensitivity reaction). These allergic reactions in venom-sensitised individuals can be local or systemic, can vary in severity and are typically of rapid onset. 5–8 The term ‘anaphylaxis’ is applied to the most severe reactions. These frequently occur within 15 minutes of a sting; initial symptoms are usually cutaneous (flushing, urticaria, angioedema) followed by hypotension (with light-headedness, fainting or collapse) and/or respiratory symptoms (due to an asthma-like response or laryngeal oedema). Progression to fatal cardiorespiratory arrest can occur within several minutes. 5 Anaphylaxis occurs more commonly in males and in people under 20 years of age,6 and the species that cause the most frequent allergic reactions in humans following a sting are the Apidae (bees) and the Vespidae (wasps and hornets). 7
In addition to local and systemic allergic reactions, individuals may also experience allergic reactions due to circulating immune complexes or delayed hypersensitivity reaction. This is uncommon, and presents as skin rashes and sickness-like symptoms occurring within 3 days to 2 weeks post sting. 5
Severity of systemic reactions to Hymenoptera venom can be measured using the Mueller grading system,8 which is summarised in Table 1. The grading system classifies the reaction to a sting according to the severity of symptoms. Severity ranges from grade 1 (symptoms of skin and mucous membranes) to grade 4 (cardiovascular symptoms).
| Grade | Description | Signs and symptoms |
|---|---|---|
| 1: Slight general reaction | Skin and mucous membrane symptoms | Generalised urticaria or erythema, itching, malaise or anxiety |
| 2: General reaction | Gastrointestinal symptoms | Any of the above plus two or more of generalised oedema, constriction in chest, wheezing, abdominal pain, nausea and vomiting, dizziness |
| 3: Severe general reaction | Respiratory symptoms | Any of the above plus two or more of dyspnoea, dysarthria, hoarseness, weakness, confusion, feeling of impending disaster |
| 4: Shock reaction | Cardiovascular symptoms | Any of the above plus two or more of loss of consciousness, incontinence of urine or faeces, cyanosis |
Epidemiology
In the UK, insect stings are the second most frequent cause of anaphylaxis outside of medical settings,9 and Hymenoptera venoms are one of the three main causes of fatal anaphylaxis in both the USA and the UK. 10 It is estimated that the prevalence of bee and wasp sting allergy is between 0.4% and 3.3%. 11
The prevalence rates of large local reactions (LLRs) in the general population have been estimated at between 2.4% and 26.4%, and up to 38% in beekeepers. 10 Children are reported to have lower rates of both large local and systemic reactions to Hymenoptera stings, at between 11.5% and 19% and between 0.15% and 0.8%, respectively. 5 After a LLR, 5–15% of people will go on to develop a systemic reaction when next stung. 12
The prevalence of systemic reactions to Hymenoptera venom is not reliably known, but estimates range from 0.5% to 3.3% in the USA,12,13 and from 0.3% to 7.5% in Europe. 10 Differences in rates of systemic allergic reactions in children and adults have been reported: up to 3% of adults and almost 1% of children have a medical history of severe sting reactions. 11,13 In people with a mild systemic reaction, the risk of subsequent systemic reactions is thought to be between 14% and 20%. 12 Within the USA, severe life-threatening reactions occur in 0.4–0.8% of children and 3% of adults. 14
UK data
Between two and nine people in the UK die each year as a result of anaphylaxis due to having experienced reactions to bee and wasp stings. 15 Once an individual has experienced an anaphylactic reaction, the risk of having a recurrent episode has been estimated to be between 60% and 79%. 12 In 2000, the register of fatal anaphylactic reactions in the UK from 1992 to 2000 was reported by Pumphrey and Roberts. 16 Of the 56 postmortems carried out during this period, 19 deaths (33.9%) were recorded as reactions to Hymenoptera venom. A retrospective study in 200417 examined all deaths from anaphylaxis in the UK between 1992 and 2001 and estimated 47/212 (22.2%) to have resulted from reactions to Hymenoptera venom during this period. This further breaks down into 29/47 (61.7%) from reactions to wasp stings and 4/47 (8.5%) from reactions to bee stings, the remaining 14/47 being caused by unidentified Hymenoptera stings (29.8%). 17
Current diagnostic options
Currently, individuals can be tested to determine if they are at risk of systemic reactions to bee and wasp venom. The primary diagnostic method for allergic sensitisation to bee and/or wasp stings is venom skin testing.
Venom skin testing involves skin prick testing (SPT) and/or intradermal skin testing (IDT) by injection with Hymenoptera venom protein extracts at concentrations in the range of 0.001–1.0 μg/ml. This establishes the minimum concentration giving a positive result. Guidelines produced by the American Academy of Allergy, Asthma and Immunology (AAAAI), the American College of Allergy, Asthma and Immunology (ACAAI) and the European Academy of Allergy and Clinical Immunology (EAACI)12,18,19 recommend that SPT be the first line of investigation to diagnose Hymenoptera venom allergy, and be performed 2 weeks after the sting reaction. IDT should be used when the results of SPT are negative, as IDT is 90% more sensitive than SPT at a concentration of 1 μg/ml. 12 As venom tests show unexplained variability over time,20 and as negative skin tests can occur following recent anaphylaxis, if an individual displays a history of systemic reactions but his or her skin tests are negative it is recommended that tests should be repeated 1–2 months later, along with serum-specific IgE measurement. 12
Another method of diagnosis is direct measurement of allergen-specific IgE antibodies in serum (previously, and sometimes still, referred to as radioallergosorbent testing, or RAST, although this is now an anachronistic misnomer). This test is less sensitive than a skin test but is useful when skin tests cannot be carried out, for example in people with skin conditions. 21,22
Current treatment options
For treatment of symptoms in the event of being stung, people can be provided with an emergency kit. 23 The contents can be tailored to the perceived risk of a severe reaction but the options include an H1-blocking high-dose antihistamine (HDA), a corticosteroid, a bronchodilator and an adrenaline auto-injector (AAI).
Injected adrenaline (a sympathomimetic drug that acts on both alpha- and beta-adrenoceptors), administered as part of hospital treatment, is regarded as the emergency treatment of choice for cases of acute anaphylaxis as a result of Hymenoptera stings. 24 For adults, the recommended dose is between 0.3 mg and 0.5 mg via intramuscular injection, and 0.01 mg/kg via intramuscular injection for children. AAIs available in the UK for carriage by individuals at risk of anaphylactic reactions, and designed for immediate self-administration, include EpiPen® (Mylan Inc.) and Anapen® (Lincoln Medical Ltd). These AAIs must be prescribed by a clinician. People and their relatives/carers receive training in using the AAI, and are advised to practise regularly using a suitable training device. 25
In addition to emergency treatments, preventative measures include education (avoidance advice) on how to avoid bee and/or wasp stings. Additionally, education includes advice on recognising the early symptoms of anaphylaxis so that individuals summon help quickly and are prepared to use their emergency medication. All those at high risk should consider wearing a device such as a bracelet (e.g. MedicAlert) that provides information about their history of anaphylactic reaction to bee and/or wasp venom. 25
Venom immunotherapy
In addition to the measures detailed above, people with a history of a systemic allergic reaction to Hymenoptera venom can be considered for specific allergen immunotherapy. It is recommended that venom immunotherapy (VIT) is considered ‘when positive test results for specific IgE antibodies correlate with suspected triggers and patient exposure’. 26 VIT is intended to prevent or reduce the severity of future systemic allergic reactions and can be administered using a variety of products and according to a variety of protocols. Currently, the only products licensed for use in the UK are Pharmalgen products (Table 2).
| Drug | Manufacturer | Licensed in the UK? |
|---|---|---|
| Pharmalgen bee venom | ALK Abelló | Yes |
| Pharmalgen wasp venom | ALK Abelló | Yes |
| Aquagen® | ALK Abelló | No |
| Alutard SQ® | ALK Abelló | No |
| Alyostal® | Stallergenes | No |
| VENOMENHAL® | HAL Allergy | No |
| Venomil® | Hollister-Stier Laboratories LLC | No |
Venom immunotherapy consists of subcutaneous injections of increasing amounts of venom, and treatment is divided into two periods: the updosing phase and the maintenance phase. VIT is normally discontinued after 3–5 years, but adjustments to the treatment regime may be necessary when treating people with intense allergen exposure (such as beekeepers) or those with individual risk factors for severe reactions. There are 44 centres across the UK that provide PhVIT to people for bee and wasp sting allergy. 27 From the findings of the latest UK audit,14 it is clear that there is no single standard approach to the delivery of PhVIT; different centres appear to follow different dosing and administration protocols and every treatment package is tailored to the requirements of the individual patient.
In 1978, the first randomised controlled trial (RCT)28 assessing the effectiveness of VIT in the treatment of insect venom allergy was published, in which people were randomised to either VIT or placebo. Systemic reactions following re-sting occurred in 7 of 12 people receiving placebo and in 1 of 18 people receiving VIT. As a direct result of this study, it is now considered unethical to randomise people eligible for VIT to receive placebo treatment.
Assessing the effectiveness of venom immunotherapy
The impact of VIT can be assessed using both clinical and psychological outcomes. Clinical outcomes relate to the effectiveness of VIT in reducing the rate of reaction to subsequent stings and the psychological outcomes relate to quality of life (QoL) and anxiety related to fear of future stings.
The effectiveness of VIT has been assessed using various methods. A method frequently used in clinical trials is that of a hospital sting challenge (SC), in which a patient is purposely stung, in a controlled environment, by a living insect of the species to which they have been desensitised. Any reaction to the sting is then reported and treated if necessary. Another measure of effectiveness is that of patient-reported reactions to accidental field stings (FSs). Other methods include the measurement of serum IgE and skin tests similar to those used in the diagnosis of venom allergy. However, there is no completely reliable method of predicting which people will be at risk of further anaphylactic reactions and which will remain anaphylaxis free in the long term, following VIT. 26
Local or systemic adverse reactions (ARs) may occur as a result of VIT. They normally develop within 30 minutes of the injection, but occasionally delayed reactions can occur after several hours. Each patient is monitored closely following each injection to check for ARs. These reactions inform the rate of progression to increased doses during the updosing phase of treatment.
Relevant national guidelines
Emergency treatment
The Resuscitation Council of the UK updated guidelines for the emergency treatment of anaphylactic reactions in 2008. 25 These guidelines detail the diagnosis, treatment, investigation and follow-up of people who have had an anaphylactic reaction, including those reacting to Hymenoptera venom. Emergency treatment with 0.5 mg of intramuscular adrenaline is recommended for people experiencing an anaphylactic reaction. Intravenous adrenaline is recommended only for occasional use by experienced specialists; subcutaneous or inhaled adrenaline is not recommended. Treatment with the highest concentration of oxygen available via a mask, and loading with 500–1000 ml of fluids (for adults) is also recommended, in addition to adrenaline.
High-dose antihistamines are recommended as a second-line treatment for anaphylaxis to help counter histamine-mediated vasodilatation and bronchoconstriction. 25 For adults, chlorphenamine 10 mg intramuscularly or intravenously is recommended. People experiencing an anaphylactic reaction should be treated and then observed for at least 6 hours in a clinical area with facilities for treating life-threatening breathing complications.
The Resuscitation Council of the UK25 also recommends that all people presenting with anaphylaxis should be referred to an allergy clinic to determine the cause of the reaction and to prepare the patient to be able to manage future episodes themselves.
Preventative measures
The AAAAI guidelines for the management and prevention of stinging insect hypersensitivity were first produced in 1999,29 and were subsequently updated in 200430 and 2011. 18 They recommend that people who have experienced a systemic reaction to an insect sting should be referred to an allergist–immunologist for skin testing or in vitro testing for venom-specific IgE antibodies. A positive IDT response to insect venom at a concentration of ≤ 1.0 μg/ml demonstrates the presence of specific IgE antibodies, and VIT is recommended. If people have a negative skin test despite a history of anaphylaxis, in vitro testing for IgE antibodies or repeat skin testing is recommended before concluding that VIT is not indicated.
Venom immunotherapy in adults is usually recommended for all individuals who have experienced systemic reactions, but is generally not necessary for individuals who have had only an LLR because of low risk of a systemic reaction to a subsequent sting. The AAAAI18 recommends that, once started, VIT should be continued for at least 3–5 years. During this time, and in people who did not commence VIT, it is recommended that people carry an AAI at all times.
The technology
Pharmalgen products are produced by ALK Abelló and have had UK marketing authorisation for the diagnosis (using skin testing/intracutaneous testing) and treatment (using PhVIT) of IgE-mediated allergy to bee venom (Pharmalgen Bee Venom) and wasp venom (Pharmalgen Wasp Venom) since March 1995 (marketing authorisation number PL 10085/0004). 31 The active ingredient is freeze-dried Apis mellifera venom in Pharmalgen bee venom and partially purified, freeze-dried Vespula spp. venom in Pharmalgen wasp venom, each provided with a solvent to prepare for injection.
Before treatment is considered, allergy to bee or wasp venom must be confirmed by case history and diagnostic testing as outlined previously. Treatment with Pharmalgen bee or wasp venom is performed by subcutaneous injection. The treatment is carried out in two phases: the updosing phase and the maintenance phase.
In the updosing phase, the dose is increased stepwise until the maintenance dose (the maximum tolerable dose before an allergic reaction, or a maximum dose of 100 μg, whichever is the smaller) is achieved. ALK Abelló recommends the following dosage protocols: ‘conventional’, ‘modified rush’ (clustered) and ‘rush’ updosing. In conventional updosing, the patient receives one injection every 3–7 days. In modified rush (clustered) updosing, the patient receives two to four injections once a week. If necessary, this interval may be extended up to 2 weeks. The two to four injections are given with an interval of 30 minutes. In rush updosing, while hospitalised, the patient receives injections at 2-hour intervals and a maximum of four injections per day may be given in the updosing phase. An ultra-rush protocol has also been used in some studies in which hospitalised patients receive all injections in one day at 30-minute intervals. 32
The updosing phase ends when the individual maintenance dose has been attained and the interval between the injections is increased by 2, 3 or 4 weeks. This is called the maintenance phase, and the maintenance dose is then given every 4–6 weeks for at least 3 years.
In the UK, treatment is carried out in hospital, either as an outpatient for conventional updosing or as an inpatient for rush protocols. Treatment is administered by a specialist, and emergency resuscitation equipment should be available in case it is required to treat any systemic reaction. Venom from ALK Abelló is used in most clinics in the UK, with 92% of clinics employing the conventional 12-week updosing protocol and the remainder employing a clustered (7- to 8-week) updosing protocol. 14
For bee venom-sensitised people, the relevant PhVIT preparation costs £54.81 during the updosing phase and then £15.94 per injection during the maintenance phase. For wasp venom-sensitised people, PhVIT costs £67.20 during the updosing phase and then £20.51 per injection during the maintenance phase.
Contraindications/warnings
The Pharmalgen summary of product characteristics (SmPC)31 lists several contraindications to PhVIT treatment. These are immunological diseases (e.g. immune complex diseases and immune deficiencies), chronic heart/lung diseases, treatment with beta-blockers and severe eczema. Side effects include superficial wheal and flare, local swelling (which may be immediate or delayed up to 48 hours), mild general reactions (urticaria, erythema, rhinitis or mild asthma) and moderate or severe general reactions (more severe asthma, angioedema or anaphylactic reaction with hypotension and respiratory embarrassment and possible death). 31
Chapter 2 Definition of the decision problem
Decision problem
The remit of this review is to assess the clinical effectiveness and cost-effectiveness of PhVIT in providing immunotherapy to individuals with a history of type 1 IgE-mediated systemic allergic reaction to bee and wasp venom. Table 3 shows the key elements of the decision problem of the appraisal.
| Intervention(s) | Pharmalgen for the treatment of bee and wasp venom allergy |
|---|---|
| Population(s) | People with a history of type 1 IgE-mediated systemic allergic reactions to bee venom and/or wasp venom |
| Comparators | Alternative treatment options available in the NHS without VIT including
|
| Study design |
Randomised controlled trials Systematic reviews Economic evaluations Revised inclusion criteria |
| Outcomes | Outcome measures to be considered include
|
| Other considerations | If the evidence allows, considerations will be given to subgroups of people according to their
|
Following completion of the review protocol and preliminary searches, revisions were made to the review protocol so as to include any VIT as a comparator to PhVIT, and comparative studies in addition to RCTs, systematic reviews and economic evaluations. These are reflected in the revised decision problem set out in Table 3.
This review, for the National Institute for Health and Clinical Excellence (NICE), was limited to Pharmalgen, which is the only licensed venom product for use in VIT in the UK. At the time of writing, a systematic review of all VIT was being undertaken by the Cochrane Skin Group, to be published in 2011. 33 To place the current review in the context of the overall literature on the clinical effectiveness of VIT, the Assessment Group (AG) worked in collaboration with the Cochrane Skin Group to provide the best available summary of the evidence for the use of VIT in the treatment of Hymenoptera allergy.
Overall aims and objectives of assessment
The aim of this review was to assess the clinical effectiveness and cost-effectiveness of Pharmalgen in providing immunotherapy to individuals with a history of type 1 IgE-mediated systemic allergic reaction to bee and wasp venom. The review considered the effectiveness of PhVIT when compared with alternative treatment options available in the NHS, including advice on the avoidance of bee and wasp stings, and HDA and AAI prescription and training. The review also examined the existing health economic evidence and identified the key economic issues related to the use of PhVIT in UK clinical practice and developed a de novo economic model.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
The methods used for reviewing both the clinical effectiveness and the cost-effectiveness literature are described in this section.
Search strategy
A comprehensive search strategy using a combination of index terms (e.g. Pharmalgen) and free-text words (e.g. allerg$) was developed and used to interrogate the following electronic databases:
-
EMBASE (1980 to 2011 Week 4)
-
MEDLINE (1948 to February Week 3 2011)
-
The Cochrane Library (February 2011).
The results were entered into an Endnote X4 library (Thomson Reuters, CA, USA) and the references were de-duplicated. Full details of the search strategies and the number of citations returned for each search are presented in Appendix 1.
Inclusion and exclusion criteria
The identified citations were assessed for inclusion through two stages and disagreements were resolved through discussion. In stage 1, two reviewers (JH/GC) independently screened all titles and abstracts and identified the potentially relevant articles to be retrieved. In stage 2, full-paper manuscripts of identified studies were assessed independently by two reviewers (JH/GC) for inclusion using the criteria as outlined in the decision problem (Table 3) and described below. Studies that did not meet the criteria were excluded from the review and their bibliographic details are listed alongside reasons for their exclusion in Appendix 2. Bibliographic details of included studies are shown in Appendix 3.
Study design
Any comparative studies were included in the assessment of clinical effectiveness of PhVIT. Full economic evaluations were included in the assessment of cost-effectiveness. The Evidence Review Group also identified and assessed the quality of existing systematic reviews to cross-check for additional studies. A summary and critique of relevant systematic reviews is presented in Comparative studies of venom immunotherapy other than Pharmalgen.
Intervention
The use of Pharmalgen within its licensed indication was assessed. Where non-PhVIT was administered and compared with non-VIT interventions, these studies were identified but excluded from the review.
Comparator(s)
All of the studies describing the clinical effectiveness of PhVIT compared with any alternative treatment options available in the NHS without VIT, that is, advice on avoidance of bee and wasp venom or HDA or AAI prescriptions and training, were considered for inclusion. These criteria were later widened to include any comparator to PhVIT, including non-PhVIT and different PhVIT dosing protocols and administration methods. These changes are reflected in the decision problem in Table 3.
Population
To be included studies must have investigated people with a history of type 1 IgE-mediated systemic allergic reactions to bee venom and/or wasp venom determined by a history of a systemic reaction to a sting and a positive skin test and/or positive tests for the detection of serum IgE.
Outcomes
Data on any of the following outcomes were included in the assessment of clinical effectiveness: reaction to subsequent stings (assessed through accidental FS or SC), anxiety related to the possibility of future allergic reactions, reported ARs to treatment and QoL. For the assessment of cost-effectiveness, outcomes considered were incremental cost per quality-adjusted life-year (QALY) gained.
Data abstraction strategy
Data relating to both study design and quality were extracted by one reviewer (JH) into a Microsoft Access 2007 database (Microsoft Corporation, Redmond, WA, USA) and were cross-checked by a second reviewer (GC). Where multiple publications of the same study were identified, data were extracted and reported as a single study.
Critical appraisal strategy
The quality of the included clinical effectiveness studies was assessed by one reviewer (JH) and checked by a second reviewer (GC) according to criteria based on CRD (Centre for Reviews and Dissemination) Report 4. 34 The checklist used to critically appraise the included studies is specific to RCTs; for the non-RCT studies a modified version of this checklist was used. All relevant information was tabulated and summarised within the text of the report. Full details and results of the quality assessment strategy for clinical effectiveness studies are reported in Appendix 4.
Methods of data synthesis
The results of the data extraction are summarised in structured tables and as a narrative description. A standard meta-analysis was planned if sufficient clinically and statistically homogeneous data were available from the included studies. The primary outcomes identified for our evidence synthesis were systemic reaction to FS or SC during treatment and/or ARs to VIT. Secondary outcomes included LLR to VIT, LLR to FS or SC, number of stings and deaths.
We planned to extract number of events for each outcome and total number of people in each treatment arm in order to calculate odds ratios and the corresponding 95% confidence intervals for each study. Studies with no events in both arms would be excluded from analysis. All analyses were planned based on the intention-to-treat (ITT) population where possible. Where appropriate, the levels of clinical and methodological heterogeneity would be investigated, and statistical heterogeneity would be assessed using Q- and I2-statistics. 35,36 Given the small number of trials available, a fixed-effects model was planned using the ‘metan’ command within Stata Version 9.2 (StataCorp LP, College Station, TX, USA) where pooling was appropriate.
If the data allowed, a mixed-treatment comparison (MTC) of relevant comparators to PhVIT would be considered. A MTC analysis allows for the synthesis of data from direct and indirect comparisons, and allows for the ranking of different treatments in order of efficacy and estimation of the relative treatment effect of competing interventions. This approach assumes ‘exchangeability’ of treatment effect across all included trials, such that the observed treatment effect for any comparison could have been expected to arise if it had been measured in all other included trials. This approach fulfils the objective of providing simultaneous comparison of all of the relevant treatment alternatives, and can provide information about the associated decision uncertainty or sufficient information for economic evaluation. Hence, for the purposes of decision-making, a Bayesian MTC framework would be adopted to synthesise information on all technologies simultaneously using Markov chain Monte Carlo (MCMC) methods to estimate the posterior distributions for our outcomes of interest. The MCMC simulation begins with an approximate distribution and, if the model is a good fit to the data, the distribution converges to the true distribution. As with all meta-analyses, MTC may be conducted using either fixed- or random-effects models. Random-effects models allow for the possibility that the true treatment effect may differ between trials. The model fit will be assessed based on residual deviance and deviance information criteria.
WinBUGS version 1.4 statistical software37 (MRC Biostatistics Unit, Cambridge, UK) was planned for use in the MTC. 38 Two chains would be used to ensure that model convergence was met after 50,000 iterations with a burn-in of 100,000. Formal convergence of the models would be assessed using trace plots and the Gelman–Rubin approach39 and through inspection of the history plots.
Data would be pooled only if it was felt that the studies were measuring the same effects and if the studies had the same study design. When meta-analysis was considered unsuitable for the data that were identified (e.g. because of the heterogeneity of the studies, or because no reliable data were presented in the report), a narrative synthesis approach would be employed.
Results
Quantity and quality of research available
The electronic searches identified 1397 citations, which, after de-duplication, included 1065 individual papers, of which 799 were excluded after scanning titles and abstracts in stage 1. The full papers of 266 references were obtained and screened using the previously described inclusion criteria. Of the 266 papers screened at stage 2, 11 papers (nine studies) met the revised inclusion criteria. Of the remaining 255 excluded papers, the majority (161) were not comparative studies of PhVIT; other reasons for exclusion included inappropriate outcomes and irrelevant patient populations (Figure 1).
FIGURE 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart.
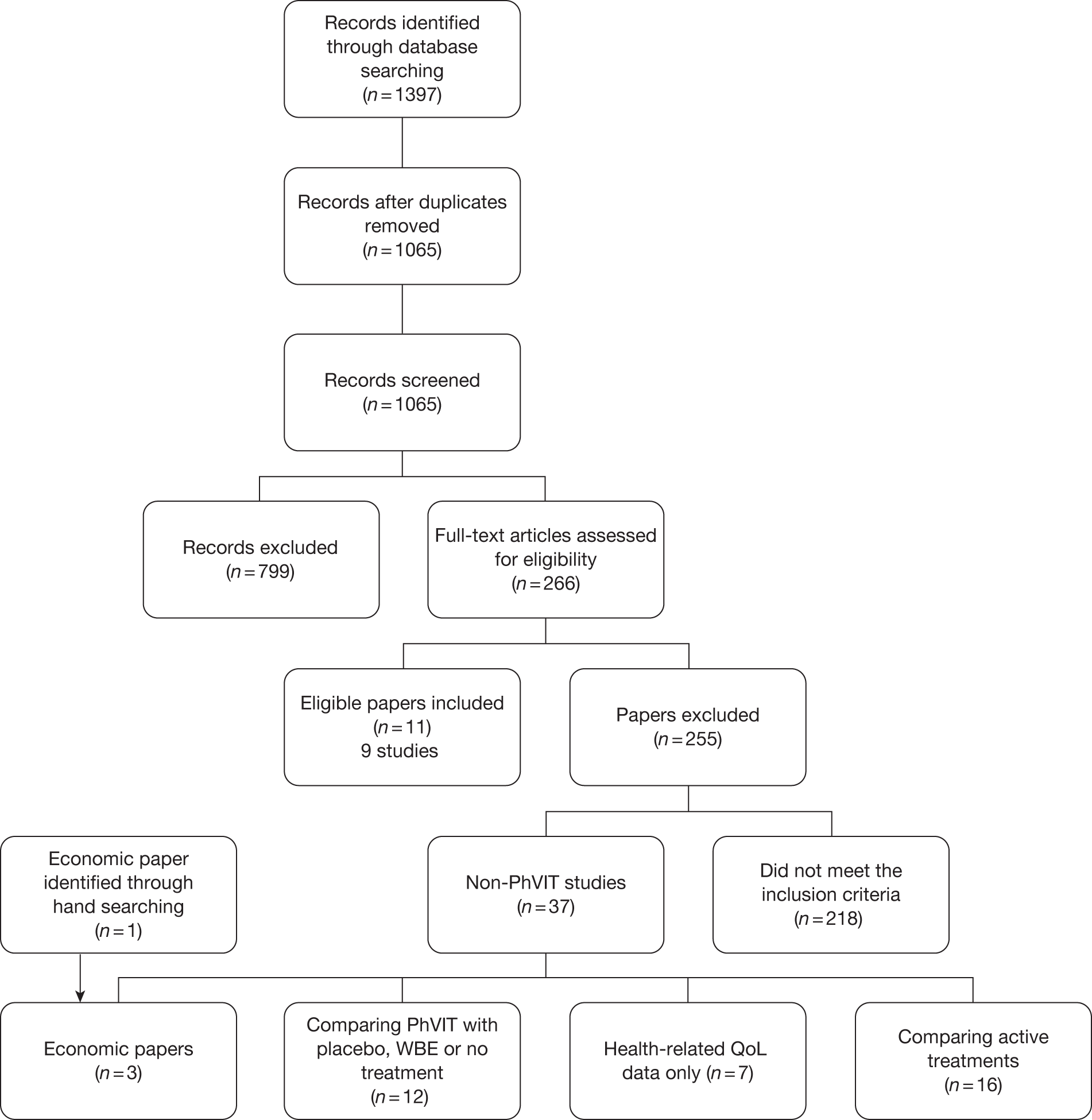
There were 38 excluded papers that require further mention in this report as they met the majority of the inclusion criteria but were studies of non-PhVIT. These 38 papers included 16 papers that compared two non-PhVIT treatments and 12 papers that compared non-PhVIT with no VIT [placebo, AAI prescriptions or whole bee extract (WBE)] and are described in the clinical effectiveness section (see Comparative studies of venom immunotherapy other than Pharmalgen). Seven papers provided data on QoL and three were economic papers (see Figure 1).
Nine comparative studies, reported in 11 publications,32,40–49 met the inclusion criteria for this review. The references discussed in the text refer to the primary papers and any other publications of the study are listed by study in Appendix 3. A summary of the included studies is shown in Table 4.
| Study ID | Intervention (no. of patients at end of study) | Comparator (no. of patients at end of study) | Design | Outcome data | ARs | |||
|---|---|---|---|---|---|---|---|---|
| FS/SC | Systemic reaction | LLR | Other | |||||
| RCTs | ||||||||
| Golden 198041,44 | Pharmalgen: rush therapy (18) |
Pharmalgen: step therapy (19) Pharmalgen: slow therapy (19) |
RCT | FS/SC | Yes | No | No | Systemic reaction, LLR |
| Mosbech 198645 | Pharmalgen: aqueous induction and maintenance (3) |
Alutard: depot induction and maintenance (7) Aquagen: aqueous induction and maintenance (9) |
RCT | SC | Yes | No | No | Systemic reaction, LLR |
| Müller 198746,47 | Pharmalgen or Reless: HBV (14) | Modified Pharmalgen: monomethoxy polyethylene glycol-coupled HBV (17) | RCT | SC | Yes | Yes | No | Systemic reaction |
| Quercia 200148 | Pharmalgen: cluster (20) |
Pharmalgen: rush (20) Depot cluster (15) |
RCT | NA | No | No | No | Systemic reaction, LLR |
| Non-RCTs | ||||||||
| Cadario 200440 | Pharmalgen: aqueous induction and maintenance (18) | Alutard: depot induction and maintenance (27) | Quasi-experimental: interventions alternated in consecutive subjects | FS | Yes | No | local reaction | Systemic reaction, local reaction |
| Golden 198143 | Pharmalgen: 50 µg maintenance (19) |
Pharmalgen: 100 µg maintenance45 (18) In-house venom: 100 µg maintenance28 (19) |
Historical control group | SC | Yes | No | No | LLR |
| Golden 198142 | Pharmalgen: 6-weekly maintenance (29) |
Pharmalgen: 4-weekly maintenance a (42) Pharmalgen: 4-weekly maintenance b (56) |
Randomly selected patients from larger cohort compared with historical controls (some overlap of people) | SC | Yes | No | No | Systemic reaction, LLR |
| Patriarca 200832 | Pharmalgen: ultra-rush SCIT (20) | Aquagen: ultra-rush SLIT (17) | Case–control: people who declined SCIT were given SLIT | FS | Yes | Yes | No | Systemic reaction, LLR |
| Thurnheer 198349 | Pharmalgen: conventional | Pharmalgen: rush | Quasi-experimental: groups determined by season | FS | Yes | No | No | Systemic reaction, LLR |
| Total for both arms (40) | ||||||||
Quality assessment
Of the nine studies identified, four were RCTs. Studies included small sample sizes at recruitment (range 30–65) and one study48 did not report on the effectiveness of PhVIT but rather reported ARs only. Six studies used SC to assess the effectiveness of PhVIT and three studies32,40,49 considered a subsequent FS, thereby further decreasing the final number of people assessed in these three studies.
The results of the quality assessment of included trials using CRD Report 434 are reported in Appendix 4. None of the RCTs44,45,47,48 described the randomisation method used, so it was not possible to ascertain whether the method of allocation and its concealment were adequate.
Baseline comparability was achieved in eight studies. One study45 reported the severity of reaction to initial sting across the groups but otherwise did not comment on the comparability of groups.
All studies reported their eligibility criteria and no co-interventions were identified. Only one46 of the studies was blinded and, although the authors described it as a double-blind study, details of who was blinded were not reported.
All studies reported on the number of withdrawals but only one study45 reported more than 20% dropout. Two studies40,48 reported zero dropouts and one study47 reported dropout for the experimental group but not for the historical control group. Where dropouts were reported there was imbalance in the rate of dropout between the arms for all but one study49 and these imbalances were not explained or adjusted for. There was no evidence of more outcomes measured than reported.
Clinical effectiveness
Trial characteristics
The nine included studies compared PhVIT with an active treatment. Five compared PhVIT with a differing dose or protocol of PhVIT,42–44,48,49 one compared PhVIT with a modified form of PhVIT47 and three compared PhVIT with non-PhVIT. 32,40,45 Information on trial characteristics is presented in Table 4.
Four of the studies were RCTs,44,45,47,48 two compared an intervention group with historical controls42,43 and three were quasi-experimental with people allocated to groups by differing means. 32,40,49 Cadario et al. 40 alternated treatments in consecutive people, Patriarca et al. 32 offered sublingual PhVIT to those who had refused subcutaneous PhVIT, and Thurneer et al. 49 administered PhVIT in a rush protocol through the insect flying season and in a conventional protocol out of the insect flying season.
All but one study48 reported the result of subsequent stings. Five of the studies42–45,47 used a SC performed on all people to determine the effectiveness of treatment, thereby ensuring that outcome data were available for all people, and three studies reported the effects of accidental FSs. 32,40,49 Only three studies32,40,47 reported on outcomes other than systemic reaction, that is, LLRs and local reactions (see Table 4). No studies reported on mortality although this is likely to be because there were no deaths rather than a failure of reporting. Data on ARs were available from all studies. Eight studies32,40,42–45,47,49 reported details of systemic reaction to PhVIT and seven reported data on LLRs. 32,40,42,44,45,48,49 One study reported data on local reactions. 41
Details of further trial characteristics are reported in Table 5. None of the studies was conducted in the UK and outcomes were measured at different time points between 4 days and > 3 years. Sponsorship was not reported in any studies, but four studies40,45,47,48 were co-authored by the manufacturer and three42–44 stated that the venom was provided by the manufacturer. Two studies32,49 reported that the venom was provided by the manufacturer and the studies were co-authored by the manufacturer. No studies selected special populations although one40 stated that people selected had to have ‘significant risks of subsequent exposure whether in terms of actual physical risk of severe reactions or socially relevant impairment of the QoL due to fear of subsequent stings’; however, in their description of people included in the study they report on people with ‘low risk’.
| Study ID | Setting | Country | Design | Duration of trial | Sponsorship | Special population |
|---|---|---|---|---|---|---|
| RCTs | ||||||
| Golden 198041,44 | NR | USA | RCT | 20 weeks | Manufacturer provided venom | No |
| Mosbech 198645 | Two allergy clinics | Denmark | RCT | 2.5–3 years | One author from Allergologisk Laboratorium A/S (producers of ALK Aquagen) | No |
| Müller 198746,47 | NR | Switzerland and South Africa | RCT | 14 weeks | One author from ALK Abelló | No |
| Quercia 200148 | NR | Italy | RCT | 4 days–6 weeks | One author from ALK Abelló | No |
| Non-RCTs | ||||||
| Cadario 200440 | Eight medical care units, outpatient | Italy | Interventions alternated in consecutive subjects | ≥ 3 years | One author from ALK Abelló | Noa |
| Golden 198143 | NR | USA | Historical control group | 20 weeks | Manufacturer provided venom | No |
| Golden 198142 | NR | USA | Randomly selected patients from larger cohort compared with historical controls (some overlap of people) | 2.5–2.75 years | Manufacturer provided venom | No |
| Patriarca 200832 | Allergy department | Italy | People who declined SCIT were given SLIT | 2 years | Manufacturer provided venom and one author from ALK Abelló | No |
| Thurnheer 198349 | Hospital with maintenance at family doctor | Switzerland | Quasi-experimental: groups determined by season | 3 years | Manufacturer provided venom and one author from Pharmacia | No |
Inclusion/exclusion criteria
All studies recruited people who were shown to be allergic to Hymenoptera venom determined through skin tests and seven confirmed this diagnosis with IgE testing (the majority using RAST). No studies used a SC as a diagnostic tool or selected people on the duration of their allergy or particular demographics such as age or sex. Five studies40,42–44,49 did not select people on species of venom allergy, two32,45 selected wasp venom-allergic people only and two47,48 included bee venom-allergic patients only. Severity of reaction was an inclusion criterion for three studies. 40,48,49 Two studies40,48 included only people with a grade 2 or higher reaction as determined by an adapted Mueller grading system. 50 One study44 stated that people with sting-related anaphylaxis had been included. Only two studies reported any exclusion criteria, these being beta-blocker therapy, cardiovascular, renal or respiratory disease or pregnancy in one study32 and no previous VIT in the other study45 (Table 6).
| Study ID | Inclusion criteria | Exclusion criteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin testing | IgE | Diagnostic SC | Severity of condition | Duration of condition | Demographics | Species | Other/recent treatments | Other illness | Other criteria | |
| RCTs | ||||||||||
| Golden 198041,44 | Intradermal | RAST | No | Sting-related anaphylaxis | No | No | Hymenoptera | No | No | No |
| Mosbech 198645 | Skin prick test | RAST | No | None | No | No | Yellow jacket (wasp) | No | No | No VIT previously |
| Müller 198746,47 | Intradermal | Yes | No | None | No | No | Honey bee | No | No | No |
| Quercia 200148 | Skin prick test and intracutaneous | RAST | No | ≥ grade 2 Mueller8 | NR | No | Apis mellifera (honey bee) | No | No | No |
| Non-RCTs | ||||||||||
| Cadario 200440 | Skin prick test and intradermal | RAST | No | ≥ grade 2 Mueller8 (revised by Wuthrich)51 | No | No | Hymenoptera | No | No | No |
| Golden 198143 | Intradermal | No | No | None | No | No | Hymenoptera | No | No | No |
| Golden 198142 | Intradermal | No | No | None | No | No | Hymenoptera | No | No | No |
| Patriarca 200832 | Skin prick test and intradermal | UniCAP® (Pharmacia) | No | None | No | No | Vespula (wasp) | Beta-blocker therapy | Cardiovascular, renal or respiratory disease | Pregnancy |
| Thurnheer 198349 | Intradermal | RAST | No | Grades 1–4 Mueller50 with modifications by Huber52 | No | No | Hymenoptera | No | No | No |
Intervention characteristics
Details of the dosing protocols for each of the studies are described in Table 7. As many of the studies were looking at different regimens, the updosing protocols differed between the studies, with PhVIT given in between 6 and 35 doses over 3 hours to 16 weeks. The maintenance dosing protocols were more similar across the studies, with most studies reporting a maintenance dose of 100 μg every month/4 weeks. The exceptions to this were the studies by Golden et al. , one43 of which compared a monthly 100-μg maintenance dose with a monthly maintenance dose of 50 μg and one42 of which compared a 6-weekly 100-μg maintenance protocol with two historical groups who received a 100-μg maintenance dose every 4 weeks, and that by Müller et al. ,47 which compared a monthly maintenance dose of 200 μg with one of 100 μg. Outcomes were measured at between 2 weeks and 5 years of maintenance therapy. No trial reported pretreatment with a HDA; two studies stated that no pretreatment was used.
| Study ID | Intervention | Updosing: frequency, dose(s) received on initial visit | Maintenance: dose and frequency | Duration of maintenance at time of reporting | Trade name/supplier | Pretreatment |
|---|---|---|---|---|---|---|
| RCTs | ||||||
| Golden 198041,44 | Slow therapy |
14 doses in 14 visits (weekly), total 14 weeks Week 1: 0.01 μg |
Week 17: 100 μg, week 20: 100 μg | 6 weeks | Pharmalgen, Pharmacia | NR |
| Step therapy |
10 doses in 8 visits, total 11 weeks Initial: 1, 5, 10 μg (every 30 minutes) |
Week 13: 100 μg, week 15: 100 μg, week 18: 100 μg | 9 weeks | Pharmalgen, Pharmacia | NR | |
| Rush therapy |
6 doses in 4 visits (every 2 weeks), total 6 weeks Initial: 1, 5, 10 μg (every 30 minutes) |
100 μg every 4 weeks | 14 weeks | Pharmalgen, Pharmacia | NR | |
| Mosbech 198645 | Pharmalgen |
26 doses in 13 visits (twice weekly), total 13 weeks (> one injection per visit initially until local swelling exceeded 5 cm in diameter) Initial: 0.2 ml of 0.001 μg/ml concentration |
100 μg or the dose four times giving local swelling > 5 cm, 4 ± 1 weeks | 2.5–3 years | Pharmalgen, Pharmacia | NR |
| Alutard |
19 doses in 19 visits (weekly), total 19 weeks Initial: 0.02 μg |
100 μg or the dose four times giving local swelling > 8 cm, 6 ± 2 weeks | 2.5–3 years | Alutard, ALK Abelló | NR | |
| Aquagen |
26 doses in 13 visits (twice weekly), total 13 weeks (> one injection per visit initially until local swelling exceeded 5 cm in diameter) Initial: 0.2 ml of 0.001 μg/ml concentration |
100 μg or the dose four times giving local swelling > 5 cm, 4 ± 1 weeks | 2.5–3 years | Aquagen, ALK Abelló | NR | |
| Müller 198746,47 | HBV |
9 doses in 7 visits (weekly), total 6 weeks Week 0: 0.1, 1.0, 3.0 μg |
100 μg weeks 7, 9, 12 and 16 then monthly | NR | Pharmalgen or Reless, Pharmacia | NR |
| Monomethoxy polyethylene glycol-coupled HBV |
7 doses in 5 visits (weekly), total 4 weeks Week 0: 0.5, 5.0, 10.0 μg |
200 μg weeks 7, 8, 9 and 11 then monthly | NR | Pharmalgen, Pharmacia | NR | |
| Quercia 200148 | Pharmalgen: cluster |
12 doses in 6 visits (every week), total 6 weeks Week 1: five doses, 0.01, 0.1, 1.0, 3.0, 6.0 μg (hourly) |
100 μg per visit weeks 2, 3 and 4 then every 4 weeks | 5 years | Pharmalgen, ALK Abelló | No |
| Pharmalgen: rush |
13 doses in 4 visits (every day), total 4 days Day 1: four doses, 0.01, 0.1, 1.0, 2.0 μg (hourly) |
100 μg per visit at weeks 2, 3 and 4 then every 4 weeks | 5 years | Pharmalgen, ALK Abelló | No | |
| Depot cluster |
12 doses in 5 visits (weekly), total 5 weeks Week 1: four doses, 0.03, 0.1, 0.3, 1.0 μg (hourly) |
100 μg per visit at weeks 2, 3 and 4 then every 4 weeks | 5 years | Alutard, ALK Abelló | No | |
| Non-RCTs | ||||||
| Cadario 200440 | Aqueous induction and aqueous maintenance |
12 doses in 8 visits (weekly), total 8 weeks Week 1: 0.01, 0.1 μg (30 minutes between) |
100 μg monthly | 3 years | Pharmalgen, ALK Abelló | No |
| Depot induction and depot maintenance |
15 doses in 15 visits (weekly), total 15 weeks Week 1: 0.02 μg |
100 μg monthly | 3 years | Alutard, ALK Abelló | No | |
| Golden 198143 | 50 μg maintenance |
6 doses in 6 visits (weekly), total 6 weeks Initial: 1 μg on first day |
50 μg monthly | 14 weeks | Pharmalgen, Pharmacia | NR |
| 100 μg maintenance44 | 6 doses in 4 visits every 2 weeks, total 6 weeks | 100 μg monthly | 14 weeks | Pharmalgen, Pharmacia | NR | |
| 100 μg maintenance28 | 12 doses in 9 visits, total 4 weeks | 100 μg monthly | 2 weeks | In-house venom | NR | |
| Golden 198142 | 4-weekly maintenance a | NA | 100 μg every 4 weeks | 2 years | Pharmalgen, Pharmacia | NR |
| 6-weekly maintenance | NA | 100 μg every 4 weeks for 2 years then 100 μg every 6 weeks | 2 years + 25–36 weeks | Pharmalgen, Pharmacia | NR | |
| 4-weekly maintenance b | NA | 100 μg every 4 weeks | 1 year | Pharmalgen, Pharmacia | NR | |
| Patriarca 200832 | Ultra-rush SCIT |
6 doses in 1 visit (every 30 minutes), total 3 hours Day 1: 0.1 μg |
100 μg monthly | 2 years | Pharmalgen, ALK Abelló | NR |
| Ultra-rush SLIT |
10 doses in 1 visit (every 20 minutes), total 3 hours Initial dose dilution: 1 : 10,000, one drop |
10 drops of pure extract given three times a week | 2 years | Aquagen, ALK Abelló | NR | |
| Thurnheer 198349 | Conventional |
24 doses in 10 visits (weekly), total 10 weeks Day 1: 0.1 ml (0.0001 μg/ml), 0.1 ml (0.001 μg/ml), 0.1 ml (0.01 μg/ml), |
1.0 ml twice a week for 4 weeks, 1.0 ml weekly for 4 weeks, 1.0 ml every 2 weeks for 8 weeks, 1.0 ml monthly | 3 years | Pharmalgen, ALK Abelló | NR |
| Rush |
35 doses in 10 visits (daily), total 10 days Day 1: 0.1, 0.2, 0.4, 0.8 ml (0.0001 μg/ml) |
1.0 ml twice a week for 4 weeks, 1.0 ml weekly for 4 weeks, 1.0 ml every 2 weeks for 8 weeks, 1.0 ml monthly | 3 years | Pharmalgen, ALK Abelló | NR | |
Patient characteristics
The number of people recruited to the studies ranged from 30 to 65, and the number included in the final analyses ranged from 19 to 56. The average age of participants was similar across studies and ranged from 35 to 49 years. All studies reported a higher percentage of males than females (between 57% and 88%). The severity of systemic reaction to the initial sting was reported in terms of Mueller grades50 in four studies32,40,48,49 and not at all by one study. 42 The remaining studies43–45,47 reported severity by clinical symptoms (Table 8).
| Study ID | Name of intervention | n | Age (range), years | Male, n (%) | Severity, n (%) | Loss to follow-up | |||
|---|---|---|---|---|---|---|---|---|---|
| Reason | Total n (%) | Final n | ITT | ||||||
| RCTs | |||||||||
| Golden 198041,44 | Slow therapy | 22 | NR | NR |
Cutaneous signs only: 7/64 (10.9) Urticaria: 44/64 (68.8) Dizziness or hypotension: 43/64 (67.2) Throat swelling or hoarseness: 32/64 (50.0) Dyspnoea: 31/64 (48.4) Loss of consciousness: 19/64 (29.7) Wheezing: 5/64 (7.8) |
2 no SC: not reached maintenance due to systemic reaction and local reaction to VIT 1 no SC: not reached maintenance as 2-month interruption in therapy |
3 (13.6) | 19 | No |
| Step therapy | 20 | 1 no SC because of cardiac status | 1 (5.0) | 19 | No | ||||
| Rush therapy | 22 |
2 no SC because of illness or cardiac status 1 no SC: only treated with Polistes wasp venom 1 no SC: anti-venom IgE was in doubt at the time |
4 (18.2) | 18 | No | ||||
| Mosbech 198645 | Pharmalgen | 10 | 46 (21–62) | NR |
Urticaria/angioedema: 8/10 (80%) Respiratory symptoms: 6/10 (60%) CNS symptoms: 5/10 (50%) |
1 immunotherapy with bee venom 1 local and systemic side effects 1 other disease 1 lack of time 3 no SC: reason unclear |
7 (70.0) | 3 | No |
| Alutard | 12 | 41 (29–79) | NR |
Urticaria/angioedema: 11/12 (91.7%) Respiratory symptoms: 7/12 (58.3%) CNS symptoms: 9/12 (75%) |
1 psychic reactions 1 other disease 1 unknown 1 emigration 1 no SC: reason unclear |
5 (41.7) | 7 | No | |
| Aquagen | 10 | 40 (24–60) | NR |
Urticaria/angioedema: 7/10 (70) Respiratory symptoms: 3/10 (30) CNS symptoms: 7/10 (70) |
1 no SC: reason unclear | 1 (10.0) | 9 | No | |
| Müller 198746,47 | HBV | 17 | 34.5 (17–57) | 15 (88.2) |
Urticaria/angioedema: 3/17 (17.6) Respiratory: 10/17 (58.8) Shock: 4/17 (23.5) |
2 side effects 1 went abroad |
3 (17.6) | 14 | No |
| Monomethoxy polyethylene glycol-coupled HBV | 17 | 34.6 (17–70) | 13 (76.5) |
Urticaria/angioedema: 5/17 (29.4) Respiratory: 9/17 (52.9) Shock: 3/17 (17.6) |
None | 0 (0) | 17 | No | |
| Quercia 200148 | Pharmalgen: cluster | 20 | 46.35 (28–76) | 16/20 (80) |
Grade 1: 0 (0.0) Grade 2: 10 (50.0) Grade 3: 5 (25.0) Grade 4: 5 (25.0) |
NA | 0 (0) | 20 | NA |
| Pharmalgen: rush | 20 | 48.5 (18–73) | 16/20 (80) |
Grade 1: 1 (5.0) Grade 2: 5 (25.0) Grade 3: 11 (55.0) Grade 4: 3 (15.0) |
NA | 0 (0.0) | 20 | NA | |
| Depot cluster | 15 | 41.47 (18–68) | 13/15 (86.7) |
Grade 1: 1 (7.7) Grade 2: 4 (30.8) Grade 3: 6 (46.2) Grade 4: 4 (30.8) |
NA | 0 (0.0) | 15 | NA | |
| Non-RCTs | |||||||||
| Cadario 200440 | Aqueous induction and aqueous maintenance | 18 | 42.6 (19–69) | 15 (83.3) |
Grade 2: 9 (50.0) Grade 3: 0 (0.0) Grade 4: 9 (50.0) |
NA | 0 (0.0) | 18 | NA |
| Depot induction and depot maintenance | 27 | 39.0 (15–68) | 19 (70.4) |
Grade 2: 5 (18.5) Grade 3: 9 (33.3) Grade 4: 13 (48.1) |
NA | 0 (0.0) | 27 | NA | |
| Golden 198143 | 50 μg maintenance | 23 | NR | 14 (60.9) |
Cutaneous signs only: 10/65 (15.4) Urticaria: 50/65 (77) Dizziness or hypotension: 41/65 (63.1) Throat swelling or hoarseness: 26/65 (40) Dyspnoea/wheezing: 27/65 (41.5) Loss of consciousness: 22/65 (33.8) |
4 not available for SC | 4 (17.4) | 19 | No |
| 100 μg maintenance44 | 22 | 13 (59.1) |
2 no SC: illness or cardiac status 1 no SC: only treated with Polistes wasp venom 1 no SC: anti-venom IgG was in doubt at the time |
4 (18.2) | 18 | No | |||
| 100 μg maintenance28 | 20 | 13 (65.0) | 1 no SC: could not tolerate maintenance dose | 1 (5.0) | 19 | No | |||
| Golden 198142 | 4-weekly maintenance a | NR | NR | NR | NR |
1 not available for SC None others stated |
NR | 42 | No |
| 6-weekly maintenance | 30 | NR | NR | NR | 1 not available for SC | 1 (3.3) | 29 | No | |
| 4-weekly maintenance b | NR | NR | NR | NR |
1 not available for SC None others stated |
NR | 56 | No | |
| Patriarca 200832 | Ultra-rush SCIT | 20 | 35 (±14) | 16/20 (80) |
Grade 1: 1 (5) Grade 2: 9 (45) Grade 3: 4 (20) Grade 4: 6 (30) |
NA | 0 (0.0) | 20 | No |
| Ultra-rush SLIT | 21 | 38 (±16) | 15/21 (71.4) |
Grade 1: 3 (14.3) Grade 2: 11 (52.4) Grade 3: 3 (14.3) Grade 4: 4 (19.0) |
2 lack of compliance 2 continued but did not have other outcomes measured |
4 (19.0) | 17 | No | |
| Thurnheer 198349 | Conventional | 21 | 36.3 (±15.4) (6–69) | 12/21 (57.1) |
Grade 1: 2 (9.5) Grade 2: 3 (14.3) Grade 3: 11 (52.4) Grade 4: 5 (23.8) |
1 pregnancy 1 treatment failure |
2/42 (4.8) | 40 | No |
| Rush | 21 | 36.1 (±19.3) (11–70) | 13/21 (61.9) |
Grade 1: 1 (4.8) Grade 2: 5 (23.8) Grade 3: 9 (42.9) Grade 4: 6 (28.6) |
NA | ||||
Outcomes
Although it was not their primary outcome, all but one study48 reported clinical effectiveness outcomes; the study not reporting on clinical effectiveness reported only on ARs. The other eight studies reported the number of systemic reactions to re-stings and two reported the number of LLRs. For three studies32,40,49 re-stings were FS and therefore not all people had been re-stung. The percentages of people re-stung in these studies were 24%,40 35%32 and 60%. 49 The remaining studies used SC. The time point of any re-sting (FS or SC) varied between studies but all occurred during treatment.
The incidence of systemic reaction to re-sting ranged from 0.0%40,44,45 to 36.4%49 (Table 9). Two studies42,43 compared the rate of systemic reaction across the arms of the study and neither reported a significant difference between the arms.
| Study IDa | Name of intervention | FS or SC (n) | Time point | Final n | Re-stung, n (%) | Systemic reaction, n (%) | p-valueb |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| Golden 198041,44 | Slow therapy | FS (4), SC (52) | 18–20 weeks of VIT | 19 | 19 (100) | 0 (0.0) | NR |
| Step therapy | 19 | 19 (100) | |||||
| Rush therapy | 18 | 18 (100) | |||||
| Mosbech 198645 | Pharmalgen | SC | 2.5–3 years | 3 | 3 (100) | 0 (0.0) | NR |
| Alutard | 7 | 7 (100) | 0 (0.0) | ||||
| Aquagen | 9 | 9 (100) | 0 (0.0) | ||||
| Müller 198746,47 | HBV | SC | ~14 weeks | 14 | 14 (100) | 2 (14.3) (angioedema) | NR |
| Monomethoxy polyethylene glycol-coupled HBV | SC | ~14 weeks | 17 | 17 (100) | 4 (23.5) [urticaria: 1 (5.9), respiratory: 3 (17.6), shock: 2 (11.8), gastrointestinal: 2 (11.8)] | ||
| Non-RCTs | |||||||
| Cadario 200440 | Aqueous induction and aqueous maintenance | FS | 3 years | 18 | 5 (27.8) | 0 (0.0) | NR |
| Depot induction and depot maintenance | FS | 3 years | 27 | 6 (22.2) | 0 (0.0) | ||
| Golden 198143 | 50 μg maintenance | SC | 20 weeks of VIT | 19 | 19 (100) | 4 (21.1) | 0.0587 |
| 100 μg maintenance44 | SC | 20 weeks of VIT | 18 | 18 (100) | 0 (0.0) | ||
| 100 μg maintenance28 | SC | 6 weeks of VIT | 19 | 19 (100) | NR | NR | |
| Golden 198142 | 4-weekly maintenance a | SC | 2 years | 42 | 42 (100) | 1 (2.4) | > 0.05 |
| 6-weekly maintenance | SC | 2 years + 6–9 months | 29 | 29 (100) | 1 (3.4) | ||
| 4-weekly maintenance b | SC | 1 year | 56 | 56 (100) | 1 (1.8) | ||
| Patriarca 200832 | Ultra-rush SCIT | FS | During treatment | 20 | 9 (45) | 1 (11.1) (dizziness) | NR |
| Ultra-rush SLIT | FS | During treatment | 17 | 4 (23.5) | 1 (25.0) [2/6 (33.3%) stings at 12 and 24 months (throat constriction)] | ||
| Thurnheer 198349 | Conventional | FS (22), SC (2) | NR | 40 | 24 (60)c | 4 (36.4)c [3 (27.3) patients diminished systemic reaction (mild symptoms), 1 (9.1) patient same systemic reaction) | NR |
| Rush | NR | 3 (27.3)c [diminished systemic reaction (mild symptoms)] | NR | ||||
Large local reactions were reported in two studies (Table 10). The frequency of LLRs was similar in the two arms of the Müller study47 (35.7% and 41.2%) and differed between PhVIT administered subcutaneously and PhVIT administered sublingually in the Patriarca study32 (88.9% and 50.0% respectively).
| Study ID | Name of intervention | SC or FS | Time point | Final n | Re-stung, n (%) | LRR, n (%) |
|---|---|---|---|---|---|---|
| Müller 198746,47 | HBV | SC | ~14 weeks | 14 | 14 (100) | 5 (35.7) |
| Monomethoxy polyethylene glycol-coupled HBV | SC | ~14 weeks | 17 | 17 (100) | 7 (41.2) | |
| Patriarca 200832 | Ultra-rush SCIT | FS | During treatment | 20 | 9 (45.0) | 8 (88.9) |
| Ultra-rush SLIT | FS | During treatment | 17 | 4 (23.5) | 2 (50.0) [2/6 (33.3%) stings at 1 and 12 months] |
Adverse reactions
Details of ARs during treatment were reported by eight studies: one study during induction only,40 five during treatment (induction and maintenance)32,44,47–49 and two during maintenance only. 42,45
Systemic reactions during induction were reported in two studies. Cadario et al. 40 reported no difference in the frequency of systemic reactions in the aqueous and depot arms (11.1% and 7.4% respectively). Mosbech et al. 45 reported no systemic reactions in the PhVIT and non-PhVIT (Aquagen) arms; however, 3/10 people in the non-PhVIT (Alutard) arm experienced systemic reactions during the induction phase. Five studies32,44,47–49 reporting the frequency of systemic reactions during the whole treatment period reported frequencies of between 0.0% and 38.1%. The statistical difference between arms was calculated in two of these studies32,44 and no statistically significant difference was found. A third study reported the same rates in each arm (Table 11). 49
| Study ID | Name of intervention | Definition | Timing | n (%) | p-value |
|---|---|---|---|---|---|
| RCTs | |||||
| Golden 198041,44 | Slow therapy | Systemic reaction | During VIT | 4/22 (18.2) patients, 7/450 (1.6) doses | > 0.05 |
| Step therapy | 2/20 (10.0) patients, 4/260 (1.5) doses | ||||
| Rush therapy | 4/22 (18.2) patients, 4/233 (1.7) doses | ||||
| Mosbech 198645 | Pharmalgen | Systemic reaction | Updosing and maintenance | 0/10 (0.0) patients, 0/3 (0.0) patients | NR |
| Alutard | 3/10 (33.3) patients, 0/7 (0.0) patients | ||||
| Aquagen | 0/12 (0.0) patients, 0/9 (0.0) patients | ||||
| Müller 198746,47 | HBV | Objective systemic reaction | During VIT | 4/14 patients (28.6) | NR |
| Monomethoxy polyethylene glycol-coupled HBV | 2/17 patients (11.8) | ||||
| Non-RCTs | |||||
| Cadario 200440 | Aqueous induction and aqueous maintenance |
During induction systemic reactionb,c Clinician reported using criteria of Lockey et al. 53 and Mueller50 |
Early = within 60 minutes Late = after 60 minutes |
All: 2/18 (11.1) patients, 9/216 (4.1) doses; early: 2/18 (11.1) patients, 9/216 (4.1) doses; late: 0/18 (0.0) patients, 0/216 (0) doses | All: 0.3205 (patients), 0.0339 (doses) |
| Depot induction and depot maintenance | All: 2/27 (7.4) patients, 7/405 (1.7) doses; early: 0 (0.0) patients, 0 (0.0) doses; late: 2/27 (7.4) patients, 7/405 (1.7) doses | ||||
| Golden 198142 | 4-weekly maintenance a | Systemic reaction | During maintenance | NR | NR |
| 6-weekly maintenance | 0/30 (0.0) | ||||
| 4-weekly maintenance b | NR | NR | |||
| Patriarca 200832 | Ultra-rush SCIT | Mild general side effects (dysphagia, itching, headache and stomach ache | During VIT | 1/20 (5) patients | > 0.05 |
| Ultra-rush SLIT | 2/21 (9.5) patients | ||||
| Quercia 200148 | Pharmalgen: cluster | Systemic reaction | During VIT | 1/20 (5.0) patients | Unclear |
| Pharmalgen: rush | Grades 1–4 Mueller | 7/20 (35.0) patients | |||
| Depot cluster | 0/15 (0.0) patients | ||||
| Thurnheer 198349 | Conventional |
All systemic reaction grades Systemic reaction grades 1–2 Systemic reaction grades 3–4 |
During 3-year treatment | All: 8/21 (38.1) patients; grades 1–2: 7/21 (33.3) patients; grades 3–4: 1/21 (4.8) patients | NR |
| Rush | All: 8/21 (38.1) patients; grades 1–2: 5/21 (23.8) patients; grades 3–4: 3/21 (14.3) patients | ||||
Two studies42,45 reported the rates of systemic reactions during maintenance therapy. In one,42 no reactions were reported, and in the other45 3/10 people experienced a systemic reaction (see Table 11).
Cadario et al. 40 reported general local reactions during induction and found a significantly higher rate of local reactions in the aqueous treatment arm [7/18 (38.9%) patients, 13/216 (6.0%) doses] than in the depot arm [4/27 (14.8%) patients, 5/405 (1.2%) doses] [p = 0.0328 (patients), p = 0.0004 (doses)] (Table 12).
| Study IDa | Name of intervention | Definition | Timing | n (%) | p-value |
|---|---|---|---|---|---|
| RCTs | |||||
| Golden 198041,44 | Slow therapy | LLR | During VIT | 9/22 (40.9) patients, 37/450 (8.2) doses | |
| Step therapy | 12/20 (60.0) patients, 31/260 (11.9) doses | ||||
| Rush therapy | 11/22 (50.0) patients, 22/233 (9.4) doses | ||||
| Mosbech 198645 | Pharmalgen | LLR | During maintenance | 1/10 (10.0) patients | NR |
| Alutard | 0/12 (0.0) patients | ||||
| Aquagen | 0/10 (0.0) patients | ||||
| Non-RCTs | |||||
| Cadario 200440 | Aqueous induction and aqueous maintenance |
During induction local reactionb Clinician reported using criteria of Lockey et al. 53 and Mueller50 |
Early = reactions within 60 minutes Late = reactions after 60 minutes |
All: 7/18 (38.9) patients, 13/216 (6.0) doses; early: 1/18 (5.6) patients, 1/216 (0.5) doses; late: 6/18 (33.3) patients, 12/216 (5.6) doses | All: 0.0328 (patients), 0.0004 (doses) |
| Depot induction and depot maintenance | All: 4/27 (14.8) patients, 5/405 (1.2) doses; early: 1/27 (3.7) patients, 1/405 (0.2) doses; late: 3/27 (11.1) patients, 4/405 (1.0) doses | ||||
| Golden 198142 | 4-weekly maintenance a | LLR | During maintenance | 6 per 100 injections | > 0.05 |
| 6-weekly maintenance | LLR | 2 per 100 injections | |||
| 4-weekly maintenance b | LLR | NR | NR | ||
| Patriarca 200832 | Ultra-rush SCIT | LLR | During VIT | 3/20 (15) patients | NR |
| Ultra-rush SLIT | 0/21 (0.0) | ||||
| Quercia 200148 | Pharmalgen: cluster | LLR (erythema > 10 cm) | During VIT | 4/20 (20.0) patients | Unclear |
| Pharmalgen: rush | 4/20 (20.0) patients | ||||
| Depot cluster | 1/15 (6.7) patients | ||||
| Thurnheer 198349 | Conventional | LLR | During 3-year treatment | 5/21 (23.8) patients | NR |
| Rush | 3/21 (14.3) patients | ||||
The four studies32,44,48,49 reporting LLRs during treatment reported frequencies of LLR from subcutaneous PhVIT of between 6.7% and 60.0%. People receiving sublingual PhVIT32 reported no LLRs. The difference in LLRs between arms was reported in one study;41,44 no difference in rates between the arms was observed. Of the two studies42,45 reporting LLRs during the maintenance phase of treatment, one42 reported LLRs on average of 6 per 100 injections for the 4-weekly maintenance programme and 2 per 100 injections for the 6-weekly maintenance programme. The second study45 reported that no LLRs occurred in any of the treatment arms (see Table 12).
Indirect analysis and mixed-treatment comparisons
The possibility of conducting a MTC was investigated when no head-to-head studies were identified that compared PhVIT and alternative treatment options available in the NHS without VIT such as advice on the avoidance of bee and wasp venom, HDA and AAI prescription and training. It was planned that studies that investigated non-VIT against non-PhVIT would be used in the MTC analysis to estimate the indirect treatment effect for PhVIT versus non-VIT; however, given the small number of trials and lack of head-to-head comparisons of PhVIT versus any intervention, pooling of all outcomes using standard meta-analysis was not possible. Any indirect analysis comparing PhVIT with any other intervention (including different doses and administration protocols of PhVIT) would be inappropriate because of sparse data and heterogeneity in the study designs and the characteristics of non-PhVIT and non-VIT interventions.
Additional data
Because of the lack of relevant comparative data on PhVIT, observational non-comparative studies of PhVIT have also been considered as well as comparative studies of non-PhVIT.
Observational studies of Pharmalgen
In addition to the comparative studies of PhVIT included in this review the searches identified 17 observational studies of PhVIT in the treatment of bee and wasp venom allergy (Table 13). It is likely that some of these papers are multiple publications from the same studies but in the following description they are assumed to be independent. All 17 studies assessed the rate of systemic reactions to subsequent stings, either FS or SC, after or during PhVIT.
| Study ID | Country | Maintenance dose | n | No. of re-stings | No. of systemic reactionsa | Timing of stings | Type of sting | Comments | Special population |
|---|---|---|---|---|---|---|---|---|---|
| Carballada 200370 | Spain | 100 μg/ml | 241 | 84 | 12 | During or after treatment | FS | 84 stings in 58 patients | |
| Carballada 200955 | Spain | 100 μg/ml | 21 | 7 patients | 0 | During maintenance or after | FS | 4–16 years old | |
| Carballada 201058 | Spain | 100 μg/ml | Bee 438, wasp 124 | Bee 130, wasp 68 | Bee 5, wasp 0 | During treatment | FS |
6 patients had a maintenance dose of 200 μg/ml and 7 people could not tolerate Pharmalgen and were changed to Aquagen Do not distinguish between people or re-stings |
|
| Bee 62, wasp 14 | Bee 3, wasp 0 | After treatment | |||||||
| Fricker 199766 | Switzerland | 100 μg/ml | 10 | 9 | 1 | During treatment | 3 FS, 6 SC | 9 stings in 6 patients | Confirmed urticaria pigmentosa |
| Graft 198756 | USA | 100 μg/ml | 66 | 200 | 4 | During or after treatment | 130 FS, 70 SC | 200 stings in 49 children | 4–17 years old |
| 68 | 0 | After at least 2 years of treatment | |||||||
| Haeberli 200359 | Switzerland | 100 μg/ml | Bee 158, wasp 101 | 161; bee 104 (21 early), wasp 57 | 41; bee 34, wasp 7 | During treatment | SC | 21 bee venom-allergic patients had an SC within 6 months of treatment | Some patients heavily exposed to bees/wasps |
| Haugaard 199167 | Denmark | 100 μg/ml | 25 | 28 | 0 | After treatment (mean 25.2 months, range 12–36 months) | SC | 2 patients could tolerate only 60 μg/ml, and 1 only 20 μg/ml | |
| Kalogeromitros 201065 | Greece | 100 μg/ml | 49 | 59 | 1 | During maintenance | FS | 59 stings in 14 patients | |
| Kochuyt 199460 | Belgium | 100 μg/ml | 217 | 290; bee 213, wasp 77 | 1; bee 1, wasp 0 | During 12-week maintenance (19 months’ treatment + bees mean 25 months (range 5–76) wasps mean 31.5 months (range 3–96) | FS | 290 stings in 65 patients; bees 213 stings in 17 patients, wasps 77 stings in 48 patients | |
| Lerch 199861 | Switzerland | 100 μg/ml | 358 | 200; bee 120, wasp 80 | 25; bee 19, wasp 6 | After ≥ 3 years of treatment stopped | FS | ||
| Müller 198963 | Switzerland | 100 μg/ml | 67 | 67 (29 early, 38 late) | 15 (7 early) | During treatment | SC | 18 patients had a 200 μg/ml maintenance dose; 29 patients had an SC in the first year of VIT (mean 4.41 ± 2.29 months), the remainder had an SC later in VIT treatment (mean 60.6 ± 21.3 months) | All bee allergic |
| Müller 199262 | Switzerland | 100 μg/ml | Bee 148, wasp 57 | Bee 148 (36 early), wasp 57 | Bee 34 (6 early), wasp 5 | During treatment | SC | 31 patients had a maintenance dose of 200 μg/ml; 36 beekeepers had an SC early into maintenance and the rest after at least 3 years of VIT | |
| Ramirez 198154 | USA | 100 μg/ml | 22 | 12 patients | 1 patient | During maintenance | SC | Itchy eyes and ears 20 minutes after sting | |
| Sanchez-Machin 201064 | Spain | 100 μg/ml | 54 | 3 patients | 0 | During maintenance | FS | All bee allergic | |
| Schiavino 200468 | Italy | 100 μg/ml | 57 | 23 patients | 0 | After treatment | FS | ||
| Szymanski 199569 | Poland | 100 μg/ml | 21 | 9 patients | 0 | After treatment | SC | 12 patients did not have SC because of contraindications or lack of consent | |
| Urbanek 198557 | Germany | 100 μg/ml | 66 | 29 patients | 1 patient | 1 year after treatment | SC | 2 years after treatment 2/14 mild systemic reaction | 4–20 years old |
All but one study54 was conducted in Europe and all studies used a maintenance dose of 100 μg/ml Pharmalgen. The number of people receiving treatment ranged from 10 to 562 and the number of re-stings reported in each study ranged from 3 to 290. Three studies55–57 included only children. Five studies58–62 split results by insect venom type and a further two63,64 reported outcomes only for individuals with a bee venom allergy.
The timing of the sting differed between studies and as such has an important bearing on the rates of systemic reaction reported. Four54,60,64,65 reported re-sting during maintenance, four59,62,63,66 during updosing and maintenance, five57,61,67–69 after PhVIT, two55,70 during or after PhVIT and two56,58 during and after PhVIT.
The reported rates of systemic reaction ranged between 0.0% and 32.7%. This large range reflects differences in the timing of re-stings, with 12 studies reporting data on re-stings before the completion of PhVIT. For the studies reporting systemic reactions after PhVIT, three smaller studies67–69 reported no systemic reactions, two larger studies reported 4/200 (2.0%)56 and 8/274 (2.9%)58 systemic reactions, and the remaining two studies reported 1/29 (3.4%)57 and 25/200 (12.5%)61 systemic reactions.
Comparative studies of venom immunotherapy other than Pharmalgen
Although the remit of this review was to assess the clinical effectiveness and cost-effectiveness of PhVIT for the treatment of bee and wasp venom allergy, as discussed in Chapter 1, Venom immunotherapy, there are other VIT products that are available to treat bee and wasp venom allergy. The searches for this review identified one meta-analysis71 and two systematic reviews33,72 reporting on comparative studies of non-PhVIT products in the population of interest, and an overview of the publications is given in Table 14.
| Publication year | Ross71 | Watanabe72 | Elremeli33 |
|---|---|---|---|
| 2000 | 2010 | In press | |
| Databases searched (dates) | MEDLINE (1966–96) | MEDLINE; LILACS; EMBASE; SciSearch; SciELO; Cochrane Database of Systemic Reviews (all searched from beginning to 2008) |
CENTRAL (2010 issue 4–); MEDLINE (2005–10); EMBASE (2007–10); PsycINFO (1806–2010); AMED (1985–2010); LILACS (1982–2010); SIGLE EAACI (2008–10), AAAAI (2008–11) Plus details of ongoing trials were searched using the mRCT; the World Health Organization International Clinical Trials Registry platform; the Australian and New Zealand Clinical Trials Registry; the US National Institutes of Health Ongoing Trials Register; the Ongoing Skin Trials Register |
| No. of included studies | 8 | 4 | 8 |
| References of included studies | Graft 1984,73 Hunt 1978,28 Müller 1979,74 Schuberth 1983,75 Thurneer 1983,49 Tsicopoulos 1988,76 Wyss 1993,77 Yunginger 197978 | aBrown 2003,79 Hunt 1978,28 Schuberth 1983,75 Valentine 199080 | aBrown 2003,79 Golden 2009,81 Hunt 1978,28 Oude Elberink 200182/Oude Elberink 200283/bOude Elberink 2006,84 Oude Elberink 2009,85 Schuberth 1983,75 Severino 2008,86 Valentine 199080 |
| Design of included studies | Seven of the eight were open trials and all were ‘comparisons of the people’s history with post-treatment experience’ | RCTs comparing Hymenoptera VIT with placebo or emergency treatment | RCTs comparing VIT with placebo, no treatment or back-up treatment for prevention of fatal insect sting anaphylaxis such as education and provision of self-administered adrenaline were included |
| Other inclusion criteria | Full papers in English in refereed journals. Studies of subcutaneous VIT | None |
All participants with a previous systemic reaction or LLR to any insect sting and a positive skin test and/or serum-specific IgE to insect venom were included in this review, regardless of age, gender, ethnicity or duration of insect sting allergy Studies using standardised venom extract in any form of immunotherapy (subcutaneous or sublingual) were included. All appropriate allergens were included at all doses and all durations of treatment. It was also planned to include studies that used a mix of different extracts, e.g. bee and wasp together Placebo, no treatment or back-up treatment for prevention of fatal insect sting anaphylaxis such as education and provision of self-administered adrenaline were included. In RCTs comparing more than one treatment arm to control group, only the treatment arm using standard venom extract compared with a control group was included in the analysis |
| Exclusion criteria | Studies of oral, sublingual or other routes of administration | Other routes of administration such as sublingual or oral were excluded | No other exclusion criteria |
| Reported outcomes | Protection against a major systemic reaction, specific IgE, IgG tiers, ARs | Changes in clinical manifestation after SC or accidental stings, indication for VIT, changes in levels of venom-specific IgE or IgG antibodies | Systemic reaction to FS or SC, local reaction to FS or SC, QoL, ARs |
| Conclusions | The findings of this meta-analysis support the conclusion that specific immunotherapy is effective in the treatment of Hymenoptera venom hypersensitivity | Specific immunotherapy should be recommended for adults and children with moderate to severe reactions, but there is no need to prescribe it for children with skin reactions alone, especially if the exposure is very sporadic. On the other hand, the risk–benefit relation should always be assessed in each case | Review in progress |
The AG assessed the systematic reviews33,72 for quality using the Database of Abstracts of Reviews of Effects (DARE) quality assessment tool. 88 Both were shown to be of high quality (Table 15). One of the high-quality reviews was a Cochrane review that is ongoing, and the AG have worked in collaboration with this group on a number of systematic reviews.
| Watanabe72 | Cochrane33 | |
|---|---|---|
| Are inclusion/exclusion criteria reported that address the review questions? | Good | Good |
| Is there evidence of a substantial effort to search for all relevant research literature? | Good | Good |
| Is the validity of included studies adequately assessed? | Good | Good |
| Is sufficient detail of the individual studies presented? | Good | Good |
| Are the primary studies summarised appropriately? | Good | Good |
Both of the systematic reviews33,72 and the meta-analysis71 conclude that VIT is effective in preventing future systemic reactions to venom in venom-allergic people.
Health-related quality of life
Although some studies have assessed the clinical efficacy of VIT, less research has been conducted on the psychological effects of VIT and Hymenoptera venom allergy. Frequency of re-sting in individuals who have undergone VIT is varied, and some individuals may not be stung again post VIT. However, these individuals may experience anxiety related to the possibility of a future sting, which may impact on their QoL. QoL has been assessed in a series of papers by Oude Elberink,82–84,87 and a tool has been developed to specifically measure this: the Vespid Allergy Quality of Life Questionnaire (VQLQ). 84 The VQLQ has been found to have adequate cross-sectional and longitudinal validity. 89
None of the included studies in our review reported data on the anxiety levels or the QoL of people receiving PhVIT. However, in the wider literature there have been several papers published looking at the effect of VIT on people’s anxiety levels and their QoL. The current Cochrane review of VIT for the prevention of allergic reactions to insect stings33 is investigating the evidence related to the QoL of VIT.
The Cochrane group searches identified four publications of RCTs reporting QoL data (Table 16). The relationship between the different publications (Oude Elberink et al. 82–84,87) is not clear and it is possible that one publication reports data on people who are also included in another publication. Therefore, for the purpose of this review it is assumed that the publications of Oude Elberink relate to two separate RCTs, one RCT of VIT for the treatment of adults with a history of anaphylactic reaction to yellow jacket sting (Oude Elberink et al. 82–84) and one RCT of VIT for the treatment of adults with a history of cutaneous reaction to yellow jacket sting (Oude Elberink et al. 87).
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| Oude Elberink 200182 | Oude Elberink 200283 | Oude Elberink 200684 | Oude Elberink 200987 | |
| Methods | ||||
| Design | Randomised, open-label, controlled parallel group trial | Randomised, open-label, controlled parallel group trial | Randomised, open-label, controlled parallel group trial | Randomised, open-label, controlled parallel group trial |
| Participants | ||||
| Country | The Netherlands | The Netherlands | The Netherlands | The Netherlands |
| Age range | Not stated | Adults (18–65 years) | Adults (18–65 years) | Adults (≥ 18 years) |
| Total n | 101 | 74 | 94 | 29 |
| Treatment group; loss to follow-up | 50; not clear | 36 (16 men); 2 | 47; 0 | 15 (9 men); 0 |
| Control group; loss to follow-up | 51; not clear | 38 (18 men); 3 | 47; 1 | 14; 1 |
| Species of insect venom(s) to which participants were allergic | Yellow jacket | Yellow jacket | Yellow jacket | Yellow jacket |
| Inclusion criteria | History of systemic reaction to yellow jacket sting and ‘sensitised to yellow jacket venom’ | History of one or more anaphylactic reactions after yellow jacket stings and positive SPT or serum IgE test | History of one or more anaphylactic reactions after yellow jacket stings and positive SPT or serum IgE test | One or more dermal reactions following yellow jacket stings and positive SPT or serum IgE test |
| Exclusion criteria | Not stated | Beta-blocker therapy or if there was a need to carry an EpiPen for other reasons, mastocytosis or serious medical or surgical illness and pregnancy | Beta-blocker therapy or if there was a need to carry an EpiPen for other reasons, mastocytosis or serious medical or surgical illness and pregnancy | Beta-blocker therapy or if there was a need to carry an EpiPen for other reasons, mastocytosis or serious medical or surgical illness and pregnancy |
| Interventions | ||||
| Treatment | Subcutaneous injections of VIT | Subcutaneous injections of VIT | Subcutaneous injections of VIT | Subcutaneous injections of VIT |
| VIT | Pharmalgen/Alutard (ALK Abelló) | Pharmalgen/Alutard (ALK Abelló) | Pharmalgen/Alutard (ALK Abelló) | Pharmalgen/Alutard (ALK Abelló) |
| Duration | 1 year | 1 year | 1 year | 1 year |
| Updosing | Modified semi-rush protocol over approximately 6-week period | Modified semi-rush protocol over approximately 6-week period | Modified semi-rush protocol over approximately 6-week period | Modified semi-rush protocol over approximately 6-week period |
| Maintenance dose | 100 µg every 6 weeks | 100 µg every 6 weeks | 100 µg every 6 weeks | 100 µg every 6 weeks |
| Control | EpiPen | EpiPen | EpiPen | EpiPen |
| Outcomes | Systemic reaction to accidental insect sting | Systemic reaction to accidental insect sting | ||
| Quality of life using a 7-point health-related QoL score | Quality of life assessment using VQLQ at 1 year | Quality of life assessment using BOT questionnaire at 1 year | Quality of life assessment using VQLQ at 1 year | |
| Notes | May be some overlap with people in Oude Elberink 200283 and 200684 publications | |||
Both trials randomised consenting people to either VIT or an EpiPen. At the end of the treatment period those who had been randomised to an EpiPen were given the opportunity to receive VIT. People were asked to complete the VQLQ, the State-Trait Anxiety Inventory (STAI) and a burden of treatment (BOT) question [people were asked to weigh the advantages and disadvantages of their treatment on a 7-point scale, ranging from extremely positive (score 1) to extremely negative (score 7)]. All measures were taken before treatment and after 1 year of treatment. Oude Elberink et al. 84 also reported results of accidental re-stings after 1 year of treatment.
Additional information
Vespid Allergy Quality of Life questionnaire
In a study of 29 people with a history of LLR to yellow jacket sting, Oude Elberink et al. 87 reported that 53% had a significant improvement in QoL score (an increase of at least 0.5 points in VQLQ) at 1 year in the immunotherapy group compared with 8% in the control group. Mean VQLQ at the end of treatment was 5.84 in the immunotherapy group and 4.53 in the control group. In an abstract publication82 the same research group reported a mean difference in QoL score change of a 0.96-point improvement on a 1–7 scale after 1 year of yellow jacket immunotherapy in 50 people compared with a 0.37-point deterioration in a control group of 51 people, all of whom had a history of systemic allergic reaction. A further publication83 by the same research group in 69 people with a history of systemic reaction to yellow jacket sting reported that 74% had a significant improvement in QoL score (an increase of at least 0.5 points in VQLQ) at 1 year with immunotherapy compared with 9% in the control group. Mean VQLQ at the end of treatment was 4.35 in the immunotherapy group and 2.90 in the control group. A meta-analysis of the two studies for the outcome change in VLQL over time significantly favoured VIT over EpiPen (test for overall effect: z = 36.25 p < 0.00001).
Acceptability of treatment
The studies of Oude-Elberink et al. 82–84,87 reported patient views of the burden of treatment in both VIT and control arms using a 7-point scale in which a score of 1–3 was classed as a ‘positive’ view of treatment and a score of 4–7 as negative or neutral view of treatment. In their 2006 study84 of people with a history of systemic reaction to yellow jacket sting, 44/47 (94%) immunotherapy-treated people had a positive overall assessment of their treatment after 1 year compared with 22/46 (48%) people in the control group (p < 0.001); similarly, in their 2009 study87 of people with a history of LLR to yellow jacket sting, 93% of immunotherapy-treated people and 42% of those in the control group had a positive overall assessment of their treatment at 1 year.
Summary of clinical evidence
Pharmalgen venom immunotherapy studies: comparative
-
Nine studies of PhVIT were identified for inclusion in the review; none of the study comparators was a non-VIT intervention.
-
One study compared PhVIT with non-PhVIT; the others compared PhVIT with PhVIT.
-
Four of the included studies were RCTs and five were quasi-experimental studies.
-
None of the studies was carried out in the UK.
-
Dosing protocols and administration protocols of PhVIT varied across studies.
-
Where re-sting data were available, the rate of systemic reactions ranged from 0.0%40,44,45 to 36.4%49 and the timing of re-sting varied across studies.
-
Systemic reactions were reported at rates of between 0.0% and 38.1% and none was fatal.
-
None of the included studies reported QoL data.
Pharmalgen venom immunotherapy studies: non-comparative
-
Seventeen non-comparative studies of PhVIT were identified for inclusion in the review.
-
Reported rates of systemic reactions following re-sting ranged from 0.0% to 32.7%; 12 studies reported re-sting data before completion of VIT.
-
Post-PhVIT systemic reaction rates ranged from 2.0% to 12.5%.
-
None of the included studies reported QoL data.
Health-related quality of life
-
QoL not reported in any PhVIT study.
-
Two RCTs looked at QoL in people receiving a combination of PhVIT and Alutard VIT (crossover trial) versus EpiPen.
-
Data showed that QoL of people receiving VIT improved more than those receiving an EpiPen.
Non-Pharmalgen venom immunotherapy studies: comparative
-
Two systematic reviews and one meta-analysis assessed the clinical effectiveness of VIT versus non-VIT; none included any trials of PhVIT.
-
All three studies concluded that VIT was effective in reducing systemic reactions to re-stings when compared with non-VIT interventions.
Discussion of clinical results and key issues
The aim of this clinical review was to evaluate the clinical effectiveness and cost-effectiveness of PhVIT in preventing future systemic reactions to bee and wasp venom in venom-sensitised people. To achieve this aim, comparisons were sought between PhVIT and any comparator (i.e. non-PhVIT and other non-VIT such as AAIs, HDAs and advice on the avoidance of bee and wasp stings).
No studies comparing PhVIT with non-VIT interventions were identified. Our search of the clinical effectiveness literature identified nine trials for inclusion in the review. Five clinical trials compared PhVIT with PhVIT (different doses and administration protocols) and four studies compared PhVIT with non-PhVIT. Several RCTs have been published comparing VIT with non-VIT interventions; however, none of these studies has used PhVIT. The current PhVIT literature is therefore limited to RCTs (n = 4) and quasi-experimental studies (n = 5) comparing different methods of administering PhVIT, different PhVIT dosing protocols and other non-PhVIT. Cohort studies reporting ARs to PhVIT and/or the effectiveness of PhVIT in reducing systemic reactions to subsequent re-stings have also been published; 17 non-comparative studies of PhVIT were identified for inclusion in the systematic review.
The results of this review have been limited by the decision problem set by NICE, which is focused on the use of PhVIT. Only studies that include PhVIT as the intervention of interest were therefore included in the systematic review. Not only are there very few published studies of PhVIT but the AG is very much aware that the nine comparative studies included in the systematic review do not accurately reflect, in terms of updosing and/or maintenance programmes, the dosing and administration protocols described in terms of the EU licence and may or may not reflect current UK clinical practice.
The quality of the included clinical trials was poor; all of the trials were small, with none including > 65 participants (range 6–65), and none was carried out in the UK. The authors of the included studies did not describe the method of randomisation used and there were imbalances in the rates of dropout between arms in all but one study. 49 There was heterogeneity among studies in the outcomes reported, the timing of re-stings, the type and length of treatment and the proportion of people being re-stung. Differences were also found among studies in maintenance dosing protocols. Health outcomes were measured at between 2 weeks and 5 years of maintenance therapy, thus making accurate comparison of data between studies difficult. The quality of the non-comparative studies was not assessed by the AG.
Venom immunotherapy with PhVIT carries with it a significant risk of systemic allergic reaction, with ARs reported in up to 38% of those treated in studies included in this review. However, these ARs were treatable and transient, and none was fatal.
Fatal sting anaphylaxis is estimated to occur in between two and nine individuals in the UK each year,15 and because of the rarity of this outcome it is therefore not possible to conclude from the data presented in either the current review or previous systematic reviews33,71,72 whether PhVIT prevents fatal sting anaphylaxis.
Because of the low occurrence of FS, the clinical effectiveness of VIT is generally assessed by SC, that is, the number of subsequent re-stings in controlled circumstances that lead to systemic ARs. Of the eight included studies reporting re-sting rates, three32,40,49 reported FS, with the proportion of people being stung ranging from 24% to 60%. This clinical evidence suggests that there may be a degree of protection following PhVIT against systemic reaction to subsequent stings, as the systemic reaction rates in these studies following (field) re-sting ranged from 0.0% to 36.4%, which is lower than those rates reported in ‘natural history’ studies of untreated people. However, unless all patients are re-stung (FS), true assessment of clinical effectiveness is uncertain.
The non-comparative studies generally support the results of the comparative studies in terms of rates of ARs to PhVIT and reductions in systemic reactions following re-sting.
Only one study83 was identified that compared a combination of PhVIT/Alutard with a non-VIT comparator (EpiPen); the study’s main outcome was QoL and limited re-sting data were reported by the authors. It is not therefore possible to directly report on the clinical effectiveness of PhVIT versus EpiPen.
Two systematic reviews33,72 and a meta-analysis71 have concluded that VIT is effective in preventing future systemic reactions to venom in venom-allergic people; however, these studies included all types of VIT and it may not be possible to generalise the findings of these reviews to PhVIT because of differences in venom extracts and concentrations and differences in administration methods. The AG notes that venom products for use in VIT are manufactured by several different companies, and some companies produce more than one venom product.
It was not possible for the AG to undertake meta-analyses or a MTC of PhVIT versus non-PhVIT because of the small number of published RCTs and the lack of head-to-head studies available.
The AG is of the opinion that there are limited clinical data to support the use of PhVIT in the treatment of patients with a history of type 1 IgE-mediated systemic allergic reactions to bee and/or wasp venom. Whether or not the results of the clinical review are generalisable to the UK population is unknown as current clinical practice in the UK with PhVIT is varied. Clinical experts have advised the AG that PhVIT is always tailored to the needs of the individual as specified in the SmPC,31 which means that it may be inappropriate to focus on a single standardised programme of PhVIT. Interpretation of the clinical effectiveness data assessing PhVIT is problematic because of discrepancies in timing and delivery (FS vs SC) of re-sting.
Other systematic reviews33,72 comparing VIT with non-VIT indicate that VIT may be more effective than non-VIT in the treatment of patients with a history of allergic reaction to bee and/or wasp venom.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
A systematic review of the economic literature was conducted to identify the existing evidence assessing the cost-effectiveness of PhVIT for the treatment of bee and wasp venom allergy. The search strategy shown in Chapter 3 was used to identify the relevant studies for inclusion in the review. Three studies were identified; two were full papers90,91 and one was in abstract format. 92 None of the studies compared PhVIT with AAIs, HDAs or avoidance advice, the studies were USA based and costs were expressed in US dollars. The AG was unable to apply any systematic review evaluation checklist to the identified studies and therefore brief summaries of each study are reported below.
The study by Bernstein et al. 90 was a 10-year observational study that reported the safety of using rapid VIT compared with modified rush VIT for people with Hymenoptera anaphylaxis. In the study, patient mean age was 36.6 years; ten and four people received single honey bee and wasp VIT, respectively, and eight people were injected with three different venoms at the same time (honey bee, wasp and mixed vespids). The paper showed that the use of rapid VIT was safe and time saving for people to reach the dose for maintenance phase compared with modified rush VIT. A cost analysis was conducted and indicated that rapid VIT is cheaper than modified rush VIT mainly because of reduced inpatient costs.
The study by Shaker91 in 2007 was a cohort simulation study that evaluated prophylactic self-injectable adrenaline alone for the prevention of fatalities in mild childhood venom anaphylaxis. The cost-effectiveness analysis assumed that the baseline annual risk of venom fatality rate was 0.44 per 100,000 persons, and the estimated incremental cost-effectiveness ratio (ICER) was US$469,459 per life-year saved and therefore not cost-effective. Sensitivity analyses were conducted to explore alternative scenarios. When the fatality rate reached 2.2 per 100,000 persons at risk, the ICER was US$97,146 per life-year saved and self-injectable adrenaline appeared to be cost-effective; self-injectable adrenaline was increasingly cost-effective with higher fatality rates. Age variation was also explored in the sensitivity analysis; the therapy became more expensive as the cohort aged, with the ICER remaining well above the usual thresholds even for a cohort of 3-year-olds (US$459,645).
The study by Brown et al. 92 was published in abstract format and reported only the cost-effectiveness analysis of VIT used as cure and prevention in children experiencing severe anaphylaxis. A Markov model was used taking into account clinical likelihood, QALYs saved, reduced deaths and costs in US dollars; however, very limited data were available in the abstract. The paper concluded that VIT was cost-effective when it was used for risk reduction (US$7876 per life-year saved) and cure (US$2278 per life-year saved) in patients with a history of severe venom anaphylaxis at a greater risk of severe reactions.
Independent economic assessment
The results of the systematic review of cost-effectiveness literature revealed that there were no published economic evaluations relevant to the decision problem set by NICE. The manufacturer of PhVIT did not submit any clinical effectiveness or cost-effectiveness evidence to NICE. The AG developed a de novo economic model designed specifically to compare the cost-effectiveness of PhVIT with the cost-effectiveness of currently available NHS interventions in people with a history of type 1 IgE-mediated systemic allergic reactions to bee and wasp venom.
Overview of Assessment Group model
An overview of the AG’s de novo economic model is given in Table 17.
| Attribute | Economic model developed by the AG |
|---|---|
| Decision problem | The model has been structured to match the decision problem defined by NICE |
| Intervention |
PhVIT (the model assumes that 92% of people receive conventional updosing and 8% use modified rush) The economic model considered PhVIT + HDA + AAI as the technology of interest as PhVIT is typically administered in combination with HDA + AAI |
| Comparator(s) |
Comparators included according to NICE scope: HDA, AAI, avoidance advice only The economic model considered (1) HDA + AAI and (2) avoidance advice only as the two treatment alternatives of interest (based on clinical opinion) |
| Population |
Individuals with previous systemic reactions to bee and/or wasp venom as well as positive test results for specific IgE antibodies Average age of 37 years is applied in the base case and a range of 5–55 years is explored in sensitivity analyses. Gender is not considered a significant parameter in the economic model because of its lack of impact on clinical effectiveness and cost; this assumption is tested in the sensitivity analysis |
| Type of model | 1-year cohort decision tree model, which can be extrapolated to have a horizon of multiple years. The only changes are reductions in the size of the cohort at the end of each year as a result of sting-related death or death from other causes |
| Perspective costs | Costs from NHS Reference Costs93 and PSSRU94 are used |
| Drug costs |
Drug costs from BNF 6195 are applied: Pharmalgen bee venom: £54.81 (updosing pack) and £63.76 (maintenance pack) Pharmalgen was venom: £67.20 (updosing pack) and £82.03 (maintenance pack) |
| Economic evaluation | Cost-effectiveness analysis |
| Time horizon | Base case assumes a 10-year horizon while 5, 15, 20 and 25 years are explored in the sensitivity analysis |
| Outcome measure | QALYs |
| Discount rate | An annual rate of 3.5% is applied to both costs and health effects in the base case; 0% and 5% discount rates are applied in scenario analysis |
| Subgroup analysis | ‘High Risk of Sting Patients’ and ‘PhVIT Anxiety QoL Improvement’ (which assumes that PhVIT is not effective at reducing systemic reactions to sting compared with HDA and AAI but does improve QoL) are the only two subgroups considered |
| Sensitivity analysis | Sensitivity of several model parameters is tested (see Table 25) |
| Scenario analysis | Several model scenarios are explored (see Table 26) |
Methods
Economic model
The economic model is constructed as a 1-year cohort decision tree that can be extrapolated to have a horizon of multiple years with the only changes being a reduction in the size of the cohort at the end of each year as a result of sting-related death or death from other causes. The average age of the cohort increases with the time horizon of the model with all-cause mortality rates changing as the average age of the cohort increases. 96 Development of a Markov model was not appropriate for disease modelling of the decision problem. To illustrate, with the exception of death, there is no transition into a state that results in changes to the key parameters, for example being stung does not change the probability of experiencing a systemic reaction from future stings.
The available evidence for the key pathway parameters (likelihood of sting, resulting systemic reaction under different treatment arms and the likelihood of death following systemic reaction) is weak. As such, construction of probability distributions around these parameters was not feasible. Instead, a deterministic model was produced using the best available estimates with sensitivity and scenario analyses employed to test the impact of changing the parameters within plausible ranges.
A schematic of the first year of the model for PhVIT + AAI + HDA is shown in Figure 2. The schematic for subsequent years is identical with the exception that the updosing phase of VIT is no longer present and after PhVIT has stopped the maintenance phase ends. The model then simplifies into the number of stings per patient per year with resulting systemic reactions and the number of deaths from other causes. For the other treatment arms the model is essentially this simplified version of the intervention arm. The cohort is defined as 1000 patients who receive a full course of PhVIT; any extra costs due to non-adherence to treatment are considered implicitly if maintenance continues for 5 years rather than 3 years as described in the sensitivity analysis.
FIGURE 2.
The AG’s de novo economic model in the first year.
Treatment options to be evaluated
To provide evidence on treatment pathways we sent out 97 electronic questionnaires to immunology clinicians in allergy clinics in the UK to gather information to inform the economic modelling. The survey and summary results are presented in Appendix 5. This survey identified that approximately 97% (n = 200) of people receiving PhVIT in the responding clinics were provided with an emergency kit that included an AAI and sometimes a HDA.
The intervention of interest is not considered to be PhVIT in isolation but rather PhVIT in combination with an emergency kit of an AAI and a HDA. The emergency kit is assumed to be provided to the patient during PhVIT treatment and for the lifetime of the patient after treatment has ended. The comparators of interest are (1) an emergency kit of an AAI and a HDA or (2) avoidance advice. It is assumed that avoidance advice is provided to all people regardless of receipt of PhVIT or an emergency kit.
Treatment pathways were determined through reviewing the included evidence on effectiveness of PhVIT in Chapter 3, a published audit of allergy clinics in the UK,14 published guidelines97 and our own survey (for results see Appendix 5).
For the PhVIT + AAI + HDA base case, the patient pathway is assumed to start after the individual has been assessed to be suitable for PhVIT. There are two phases to PhVIT – updosing and maintenance. During PhVIT an individual may experience local and systemic ARs. As the cost and QoL considerations for anything but systemic reactions are considered to be zero (discussed below), the pathway and model consider only systemic ARs by Mueller grade50 (details of Mueller grade can be found in Table 1). The cost of treatment of ARs is assumed to vary by Mueller grade.
The patient pathway assumes that each patient will experience an average number of sting events per year during or after PhVIT. A proportion of these stings result in systemic reactions of one of the four Mueller grades. A proportion of the grade 4 systemic reactions can result in death. There is also a probability that each year a patient can die as a result of causes unrelated to his or her sting allergy, which is dependent on the age of the patient.
Patient population
The patient population considered includes people who would be considered for PhVIT as a result of their previous systemic reaction to bee and/or wasp sting and who have positive test results for specific IgE antibodies. This reflects both the licensed indication and the study populations described in the available effectiveness evidence.
The average age of people starting PhVIT is taken from our survey of clinicians in UK allergy clinics. The survey was returned by 32 out of 97 clinics (33.0%), of which 16 responded that they used PhVIT. In these clinics approximately 200 people commence PhVIT for wasp and/or bee sting each year.
For simplicity of completion of the survey, an estimate of the percentage of PhVIT patients starting in the clinic was requested for three age bands. Assuming that people were on average in the middle of each age band (aged 50 years in the 40+ band), a simple average age across responding clinics was estimated to be 37 years. This age is comparable to the average age reported in the trials included in the effectiveness review shown in Table 8. Sensitivity analysis was used to explore how the age of the individual when starting PhVIT influenced results, with a range between 5 and 55 years being explored.
Evidence from published studies suggests that the majority of people undertaking PhVIT are male. In the base case 80% of people are assumed to be male. As effectiveness and cost are not linked to gender, and age-related QoL norms vary only marginally by gender, it was not anticipated that this would have a significant bearing on results. To test this assumption, two scenarios were created: one in which all people were male (‘100% male’) and one in which all people were female (‘100% female’).
Model parameters
The choice of parameters and their values used in the model is based on the available published literature, discussion with UK clinicians and the results of the short economics survey of UK allergy clinics (see Appendix 5).
Annual number of stings for people in receipt of Pharmalgen venom immunotherapy
The model requires an estimate of the annual number of times an individual receiving PhVIT will be stung. No data were available from the UK, but six studies32,40,49,83,98,99 identified in the literature search did contain data on FS during/following treatment with VIT. These studies provided detailed information on the number of FS events over a specified time period and included more than 10 people in each study. Other studies, notably observational studies, did provide information on FS but either were too small (≤ 10 people) or did not provide a specific length of follow-up over which the FS occurred. Findings from the six included studies are summarised in Table 18.
| Study | Country | No. of people | No. of people with re-stings | No. of years | Stings per year per person |
|---|---|---|---|---|---|
| Haye 200598 | Norway | 315 | 201 | 5 | 0.128 |
| Roesch 200899 | Germany | 146 | 65 | 6.5 | 0.068 |
| Oude Elbrink 200283 | Netherlands | 148 | 2 | 1 | 0.014 |
| Cadario 200440 | Italy | 45 | 11 | 3 | 0.081 |
| Patriarca 200832 | Italy | 41 | 13 | 2 | 0.159 |
| Thurnheer 198349 | Switzerland | 40 | 22 | 3 | 0.183 |
| Total | 735 | 314 | 4.09 (weighted average) | 0.095 |
None of the studies listed above is significantly methodologically stronger than the others and as such a simple pooling of the studies through a weighted average was used to generate an average number of stings per year (0.095); this rate compares favourably to the rates of FS reported by Cadario40 (see Chapter 3, Clinical effectiveness). In the base case this value (0.095) is used. Sensitivity analysis varies the annual number of stings between lowest and highest published rates (0.014–0.183). The lower value addresses the issue that bee and wasp stings are not separated in the above data and that no evidence was found in the review detailing how people with wasp allergy react with bee sting and vice versa. If people allergic to one of the venoms are no more likely to have an allergic reaction to another venom from a different insect then the reference rate of sting used in the base case in the economic model may overestimate the actual rate and so the number of stings to which PhVIT people could have an allergic reaction to could be lower than in the base case.
Findings from these studies and from the observational studies indicated that there were people who experienced multiple stings. For example, although Kochuyt and Stevens60 did not provide detailed information on length of follow-up, as this varied, the study found that 17 people suffered 213 bee stings during follow-up, whereas 18 people had no FS during follow-up. This could be explained by differential follow-up periods, but could also suggest that there are some people undergoing VIT who are at significantly higher risk of sting than others. This is supported by the fact that one of the factors in considering suitability for PhVIT is that an individual has an occupation or lifestyle that substantially increases their risk of sting.
A subgroup analysis (‘High Risk of Sting Patients’) was used to explore how people with substantially increased rates of sting affected the model findings. This subgroup has a base number of five stings per year with sensitivity analysis exploring the impact of 1–10 stings per year.
Systemic adverse reactions due to Pharmalgen venom immunotherapy
Systemic ARs due to PhVIT are included in the model as the likelihood of systemic AR following each PhVIT injection. Non-systemic ARs are not included in the base case as evidence from the effectiveness review suggests that local reactions, even if large, are short-lived and, based on discussion with clinical experts, do not incur any cost beyond the occasional use of topical or oral antihistamines. In a scenario analysis (‘PhVIT Local Adverse Reactions’), we explored the impact of ignoring local reactions by assuming that 25%, 50%, 75% and 100% of post-injection ARs results in local reactions that require the administration of an antihistamine cream.
Evidence from studies described in Chapter 3 states that the rate of systemic ARs per patient due to PhVIT is between 0% and 38.1% during treatment. However, only two papers provided the dose risk of systemic reaction. During the updosing phase, Golden et al. 44 suggests a dose risk of systemic ARs of 1.6%, and a rate of 2.6% is taken from Cadario et al. 40 A pooled estimate across people within these trials suggests a dose risk of systemic AR during updosing of 2.0%, and this is used in the base case. Sensitivity analysis explores rates between 0% and 2.6%; the model has therefore explored what happens with higher or lower plausible values for systemic ARs no matter the reason for the increase/decrease (e.g. if there are more/fewer systemic ARs with bee PhVIT compared with wasp PhVIT).
No studies were found that reported any dose risk leading to systemic AR during the maintenance phase. However, Haye and Dosen98 in a cohort study of 315 people receiving VIT found that 138 people had a systemic AR during the updosing phase and 59 during the maintenance phase. Insufficient detail was provided to calculate the number of injections to which this related. However, our base case assumes that over 3 years with a 4-week interval at maintenance and a 12-injection updosing phase (conventional protocol) there are approximately three times as many injections during maintenance as updosing. If the same updosing to maintenance injection ratio is applied in the model as described in the Haye and Dosen98 study, 7.8 systemic ARs would occur during updosing for every one during maintenance. Applying this ratio to our base-case dose risk of systemic ARs during updosing suggests a dose risk during maintenance of 0.26%, and this is used in the model base case. A scenario analysis assumes a dose risk in maintenance that is equal to that in updosing (‘Equal AE risk Updosing/Maintenance’) and sensitivity analysis explores dose risk values in maintenance of 0–1%.
Thurnheer et al. ’s49 is the only study that provides information on the grade of systemic AR. This study reported that 75% of systemic ARs are grades 1–2 and 25% grades 3–4. Data on sting systemic reaction in people without VIT (Table 19) suggest that only a very small percentage of systemic reactions are grade 4 (1.1%). As such, we assumed that grade 1–2 reactions are split evenly between grades so in the base case grade 1 and grade 2 reactions are each 37.5% of the total.
| Grade | Systemic reaction following a sting without VIT (%) | Systemic reaction following a sting with VIT (%) |
|---|---|---|
| 1 | 6.5 | 38.5 |
| 2 | 80.3 | 54.0 |
| 3 | 12.1 | 7.5 |
| 4 | 1.1 | 0 |
Scenario analysis explored 100% of reactions being grade 1 (‘ARs all Grade 1’) and all of the grade 3/4 reactions being grade 4 (‘25% ARs Grade 4’).
Death due to PhVIT was not reported in any published study we identified and was assumed to be zero in the base case and was not varied in either sensitivity analysis or scenario analysis.
Systemic reactions due to stings
The model requires estimates of systemic reaction due to a sting for the three treatment arms: PhVIT + HAD + AAI versus HAD + AAI versus avoidance advice only.
For avoidance advice only, although the risk of sting may be reduced (which is accounted for by looking at the rate of sting in people who have received PhVIT – all of whom are assumed to have been given vespid sting avoidance advice), the rate of systemic reaction following sting is assumed to be equal to that of allergic people suitable for PhVIT but with no treatment.
Bilo et al. 12 report repeat anaphylactic risk rates following sting, assuming an episode in the past, of between 60% and 79%. This appears to be a lifetime risk rather than a per-sting risk. Reisman100 reported the results of a survey of 220 people who had not received VIT but who had experienced a systemic reaction to sting in the past and had received a second sting since the first event. There were 124 of these people who had a systemic reaction on second sting. This suggests that the probability of systemic reaction in people with previous history of systemic reaction following sting but without PhVIT is 56.4% per sting, and this is used as the base-case value for the avoidance advice arm.
The grade of systemic reaction following sting without VIT is taken from a survey by Roesch et al. 99 in Germany (see Table 19).
The risk of systemic reaction following PhVIT was calculated by pooling the sting data from the available trial data described in Chapter 3. The pooled data suggest that of 337 people stung following PhVIT there were 22 systemic reactions, a rate of 6.5% per sting; from the data available it was not possible to estimate a more accurate systemic reaction rate per sting as the systemic reaction rates are reported at different times in PhVIT studies. This rate is supported by the evidence from the observational studies included in Chapter 3 (see Observational studies of Pharmalgen). In sensitivity analysis the rate of systemic reaction explored following PhVIT ranges from 5% to 15%.
Although some authors report effectiveness of 100%, these studies are small and, given that other studies have found systemic reactions with PhVIT, the balance of evidence does not support suggesting 100% effectiveness for PhVIT in stopping systemic reactions. Evidence that effectiveness declines over time is mixed so in the base case it is assumed that there is no decline in effectiveness over time. A scenario analysis assumes that effectiveness declines smoothly from 5% at the end of therapy year 1 to 15% at 10 years following the end of therapy (‘Declining VIT Effectiveness’).
Evidence on effectiveness of PhVIT suggests that the severity of systemic reaction following sting is reduced with PhVIT, but trials that actually reported the grade of systemic reaction were too small to establish the actual impact on grade.
The survey by Roesch et al. 99 that provided grade of systemic reaction to sting for people before VIT also provided the grade of systemic reaction for the same people following sting after having received VIT (see Table 19). Although these are observational rather than trial data, in the absence of more robust data it is the best evidence available for use in the model.
High-dose antihistamine is given as an emergency treatment following a sting to reduce the possibility and severity of systemic reaction. The results of our survey found that clinicians advise the use of AAI following a sting only if symptoms of systemic reaction occur. Therefore, AAI can only reduce the severity of systemic reaction. However, for both HDA and AAI there is no published evidence to support the use of these interventions in the treatment of systemic allergic reactions. 101,102 Effectiveness therefore has to be assumed. For simplicity, in the base case, HDA is assumed to be 25% as effective as VIT at reducing the likelihood of systemic reaction, meaning that the risk of systemic reaction is 43.9% with no reduction in severity of reaction. AAI is assumed to reduce the number of grade 3 and grade 4 systemic reactions by half of the reduction with VIT with these reactions evenly distributed between grade 1 and grade 2 reactions, but AAI does not reduce the possibility of systemic reaction.
The addition of AAI and HDA to PhVIT is assumed to not alter the effectiveness of stopping or reducing the severity of systemic reaction compared with PhVIT alone.
As these assumptions are without an evidence base, it is important that scenario analysis is used to explore how important these assumptions are to model findings. Therefore, a scenario is used in which AAI + HDA is assumed to be no more effective than avoidance advice only, that is, they make no difference to the likelihood or severity of systemic reaction following sting (‘AAI + HDA No Systemic Reaction Effectiveness’). A separate scenario analysis assumes that AAI + HDA are as effective at reducing the likelihood and severity of systemic reaction as PhVIT, although an increase in QoL through reduced sting anxiety with PhVIT is introduced. This is discussed further in the section on QoL in the model.
Local reactions to sting are assumed to be trivial in terms of both cost and QoL impact and so are excluded from the model.
Deaths following sting
Deaths following sting are rare in the UK (and the rest of the world) so making an estimate of the death rate following sting is difficult. Although deaths due to sting are recorded, it is not known how many of these people received VIT or how many sting events they relate to.
To provide an estimate of sting death rate, an indirect approach was taken based upon the findings from Pumphrey and Pumphrey. 103 The survey reported an average of 20 deaths due to allergic anaphylaxis (all causes) per year in the UK. Hospital episode statistics (HES)104 data suggest that there are approximately 1600 inpatient episodes due to anaphylaxis each year. Combining these facts suggests a death rate following anaphylaxis (which we assume in the model to be a Mueller grade 4 reaction) of 1.25%. This rate is used in the model in the base case by assuming that death from allergic anaphylaxis is independent of the allergen.
As the probability of grade 4 reaction with PhVIT is assumed to be 0% then, by default, the death rate with PhVIT due to bee/wasp sting is assumed to be 0%.
With no published range of fatality rates following sting, sensitivity analysis undertaken around this parameter explores the effect of the value being 50% higher and lower than in the base case.
Quality of life
The model estimates the number of deaths and life-years under each treatment arm over the time horizon chosen. The life-years are adjusted to calculate QALYs by using age-dependent European Quality of Life-5 Dimensions (EQ-5D) Weighted Heath Status Index population norms published by the University of York. 105
Evidence83,87 presented in Chapter 3 shows that fear of sting in some people not receiving VIT reduces QoL and this is at least partly negated by PhVIT. However, no evidence is available to support this finding using a validated utility measure such as EQ-5D. 105 As such, in the base case no change in utility due to anxiety is assumed. Having a systemic reaction could potentially impact on QoL and different severities of reaction could impact on QoL differently. Unfortunately, there is no evidence on utility levels during a systemic reaction, and as such the QoL differences resulting from the number of systemic sting reactions in different treatment arms are not included in the model. This means that any health benefits from VIT are entirely due to its effectiveness in reducing systemic reactions from sting and resulting deaths.
A separate subgroup analysis assumes that fear of sting does affect the utility of some people and that VIT reduces this anxiety and so negates this loss in QoL (‘VIT Anxiety QoL Improvement’). The survey of EQ-5D norms105 by the University of York suggested that a level 2 ‘anxiety/depression’ health state induces a detriment to utility of 0.07 per year. A level 2 interference with ‘usual activities’ health state induces a utility decrement of 0.036. The actual reduction would not make a significant difference to the findings of the economic model, but provides an indication of the likely scale of the positive benefit from PhVIT.
The actual reduction in utility per person per year is unlikely to exceed 0.106 in total if the fear of sting both causes a reduction in utility due to anxiety and interferes with usual activities. As a cautious estimate we assume that the actual reduction in utility due to fear of sting is approximately 40% of the potential 0.106 per person per year maximum and that this is alleviated by PhVIT by approximately 40%. This means that having PhVIT increases utility by 0.01 per person per year.
This can be interpreted as a cautious estimate of the impact of PhVIT on utility. Sensitivity analysis explores increases in utility from PhVIT of between 0.004 and 0.04 (10–100% of assumed decrease in utility due to anxiety) per person per year.
As stated previously, a separate scenario analysis explores the cost-effectiveness of PhVIT assuming that it is not effective at reducing systemic reaction to sting compared with AAI + HDA but does improve utility (‘PhVIT Anxiety QoL Improvement Only’).
Cost of treatment and health states
The model requires estimates of the costs of treatment in the different intervention arms as well as health-care costs in different health states, specifically from systemic ARs to PhVIT and systemic reactions to sting.
To produce these estimates a range of unit costs is applied to resource use. The resources considered in the model and the unit costs are provided in Table 20.
| Resource | Unit | Unit cost (£) | Source | |
|---|---|---|---|---|
| A&E attendance | Per attendance | 103 | NHS Reference Costs93 (code: TA and EMSNA) | |
| Inpatient stay | Per day | 350 | NHS Reference Costs93 (code: WA16Y) | |
| AAI (EpiPen) | Per injector | 28.77 | BNF 6195 | |
| Ampoule of adrenaline | Per 1 ml ampoule | 0.57 | BNF 6195 | |
| Syringe and needle | Per syringe/needle | 0.10 | Assumed | |
| HDA | Per dose | 0.14 | BNF 6195 (average of four most commonly used HDAs) | |
| Allergy clinic nurse specialist | Per minute | 1.07 | PSSRU94 | |
| Pharmalgen bee venom | Per kit | Initial pack | 54.81 | BNF 6195 |
| Maintenance pack | 63.76 | BNF 6195 | ||
| Pharmalgen wasp venom | Per kit | Initial pack | 67.20 | BNF 6195 |
| Maintenance pack | 82.03 | BNF 6195 | ||
Cost of drugs and drug administration
Following published clinical guidelines,97 administration of PhVIT was assumed to include the use of a syringe and a prophylactic HDA, the time involved in a pre-injection health check, venom injection preparation and post-injection observation (this has been defined as individuals staying in the consulting room with specialists to be seen if any immediate reactions manifest). No published information was available on the actual resource usage of these individual elements so values were assumed by the AG and then verified by a consultant in an allergy clinic.
The model assumes that bee, wasp, and bee plus wasp PhVIT are equally effective. However, the cost of PhVIT for these treatments varies. Our survey of UK allergy clinic clinicians suggested that approximately 23% of people are bee allergic, 70% of people are wasp allergic and 7% are both. These proportions are used in our base case but scenario analysis explores the difference in findings if people are 100% bee allergic, 100% wasp allergic or 100% both.
According to the manufacturer’s SmPC,31 conventional updosing is carried out weekly for 12 weeks with one injection per visit. A modified rush protocol is made up of 16 injections over a period of 7 weeks. The published allergy clinic survey suggests that 92% of people receive conventional updosing and 8% use modified rush. These values are used in the model and scenario analysis is used to explore the importance of the type of protocol (‘100% Conventional’ and ‘100% Modified Rush’).
In the base case the maintenance phase is assumed to be 3 years following updosing. This is varied in the sensitivity analysis between 3 years and 5 years. The interval between injections during the maintenance phase is 4 weeks, as per available guidelines, but sensitivity analysis explores the impact of intervals of between 5 weeks and 8 weeks.
The resources used and costs associated with PhVIT administration are shown in Table 21.
| Resource | Unit | Usage (sensitivity analysis) | Cost (£) |
|---|---|---|---|
| Prophylactic HDA | Per visit | 1 dose | 0.14 |
| Pre-injection health check (nurse specialist time) | Per visit | 15 minutes (10–20 minutes) | 16 (10.67–21.33) |
| Venom injection preparation (nurse specialist time) | Per dose | 5 minutes (3–7 minutes) | 5.33 (3.20–7.47) |
| Post-injection observation (nurse specialist time) | Per dose | 3 minutes (2–4 minutes) | 3.20 (2.13–4.27) |
| PhVIT costs updosing | Updosing phase | 1 kit | 68.19a |
| PhVIT costs maintenance | Per injection | Quarter of a kit | 20.57 |
The emergency kit is assumed to comprise a HDA and an AAI. The AAI is assumed to be EpiPen and to have a shelf life of 18 months, after which a new one is issued. The HDA in the emergency kit is assumed to be replaced annually. Avoidance advice is assumed to constitute a 60-minute consultation with a nurse specialist at a cost of £64 from PSSRU. 94 As these costs are added equally to all three intervention arms, the actual cost incurred should make no difference to the results of the incremental analysis and so no sensitivity analysis was performed around these values.
Treatment of systemic adverse reactions to Pharmalgen venom immunotherapy
For local ARs to PhVIT the costs of treatment are considered to be trivial, involving the administration of an antihistamine cream or ice pack. The model focuses on systemic reactions.
No data were available describing the resources used to treat systemic ARs; therefore assumptions were made and then checked with an allergy clinic clinician. In the base case we assume that in all cases of systemic AR to PhVIT an HDA would be given and an ampoule of adrenaline drawn and administered by a nurse. The clinician suggested that in all cases of systemic AR people would be observed closely for at least 30 minutes following emergency treatment. It is assumed that all grade 4 systemic reactions result in close observation by a nurse for 60 minutes and 50% of people require a hospital inpatient stay for overnight observation. The resource use associated with systemic ARs is provided in Table 22. Scenario analysis explores the cost of systemic reaction that is 50% higher (‘50% Higher Systemic AR Cost’) and 50% lower (‘50% Lower Systemic AR Cost’) and a scenario analysis also explores the impact of no grade 4 systemic reactions resulting in an inpatient stay (‘No Admissions Due to Systemic Adverse Reactions’).
| Grades 1–3 | Grade 4 | |||
|---|---|---|---|---|
| Resource use | Cost (£) | Resource use | Cost (£) | |
| Antihistamine | 1 dose | 0.14 | 1 dose | 0.14 |
| Adrenaline | 1 ampoule | 0.57 | 1 ampoule | 0.57 |
| Needle/syringe for adrenaline | 1 | 0.10 | 1 | 0.10 |
| Observation time in unit (nurse specialist time) | 30 minutes | 32.00 | 60 minutes | 64.00 |
| Inpatient stay (1 day) | 0% of patients | 0.00 | 50% of patients | 175.00 |
| Total cost | 32.81 | 239.81 | ||
Treatment of systemic reactions to sting
Resource use and costs related to systemic reactions to sting are displayed in Tables 23 and 24.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | |
| A&E visit | 100% of patients | 103 | 100% of patients | 103 | 100% of patients | 103 | 100% of patients | 103 |
| Inpatient stay | 0% of patients | 0 | 10% of patients | 35 | 30% of patients | 105 | 50% of patients | 175 |
| Antihistamine | 1 dose | 0.14 | 1 dose | 0.14 | 1 dose | 0.14 | 1 dose | 0.14 |
| EpiPen | 1 | 28.77 | 1 | 28.77 | 1 | 28.77 | 1 | 28.77 |
| Total cost | 131.91 | 166.91 | 236.91 | 306.91 | ||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | |
| A&E visit | 100% of patients | 103 | 100% of patients | 103 | 100% of patients | 103 | 100% of patients | 103 |
| Inpatient stay | 0% of patients | 0 | 10% of patients | 35 | 30% of patients | 105 | 50% of patients | 175 |
| Antihistamine | 1 dose | 0.14 | 1 dose | 0.14 | 1 dose | 0.14 | 1 dose | 0.14 |
| Adrenaline | 1 ampoule | 0.67 | 1 ampoule | 0.67 | 1 ampoule | 0.67 | 1 ampoule | 0.67 |
| Total cost | 103.81 | 138.81 | 108.81 | 178.81 | ||||
It was considered that all individuals experiencing a systemic sting reaction visit the A&E department regardless of treatment arm. This is confirmed by our survey, in which we asked for avoidance advice given to people by clinicians should they be stung and all said that those experiencing a systemic reaction to sting are told to attend A&E. We assume that all people are able to attend A&E without the need for ambulatory care, which we accept potentially acts as a deflator to the actual cost of treating systemic reactions. However, no data were available on the number of people being stung and requiring paramedic assistance.
For people with an emergency kit, the model assumes that all people with a systemic reaction would use the AAI and HDA. For people receiving avoidance advice only, adrenaline is administered via ampoule in the A&E department.
There is a risk of delayed anaphylactic shock with sting and we assume that a proportion of people with systemic reactions would be observed overnight in hospital as an inpatient. We have no data on the likelihood of an inpatient stay so we asked for clinician advice on likely values for this parameter. In the base case the model assumes that 50% of those with a grade 4 systemic reaction would be held overnight for observation; it is also assumed that 30% of those with a grade 3 reaction, 10% with a grade 2 reaction and no-one with a grade 1 reaction would be held overnight for observation.
Scenario analysis explores the cost of systemic reaction that is 50% higher (‘50% Higher Systemic Sting Treatment Cost’) and 50% lower (‘50% Lower Systemic Sting Treatment Cost’). Scenario analysis is also used to explore the impact of no inpatient stays regardless of grade of systemic reaction (‘No Systemic Reaction Inpatient Stay’) and 100% stay for those with a grade 4 systemic reaction (‘100% Grade 4 Systemic Reaction Inpatient Stay’).
Time horizon
In the base case the time horizon is 10 years. This was chosen as there is evidence that PhVIT is still effective up to 10 years after maintenance but no studies could be found that had looked at periods beyond this. Results over 5, 15, 20 and 25 years are also estimated based upon the assumption that PhVIT is equally effective over all of these periods.
Discount rate
Discount rates of 3.5% per annum are applied to both costs and benefits in the base case. Scenario analysis is used to explore the impact of no discount rate for costs and benefits and a discount rate of 5% per annum.
Other model assumptions
There are several assumptions made to make the model tractable that have not previously been mentioned.
The efficacy of bee and wasp PhVIT is assumed to be the same in terms of reducing the probability and severity of systemic reaction following sting.
Adverse reactions per dose and efficacy of PhVIT are assumed to be independent of the type of updosing phase used or length of maintenance phase (provided the maintenance phase is at least 3 years as suggested by the available evidence).
In the clinical effectiveness literature identified through the systematic review, there was no mention of ARs related to AAIs and HDAs; therefore, ARs are assumed to be zero. If there are significant ARs to either AAIs or HDAs then the costs of systemic reaction to sting are likely to be higher than we have suggested in the model. This is explored in sensitivity analysis by raising the costs of systemic reactions to sting by 50%.
Model validation
Internal validation of Assessment Group model
During model construction the algorithms within the model were checked using extreme value analysis for parameters to ensure that results generated were within acceptable bounds. To verify the accuracy of the model, key algorithms within the model were checked by an independent statistician. On completion, the model was assessed and validated by a team of external economists and statisticians.
External validation of Assessment Group model
The model was also cross-checked by an external consultant. The economic model was checked for functionality, clarity, accuracy, consistency and validity. Validation of calculated parameters within the model was carried out where possible against observational studies; however, given that this is a de novo economic model, it was not possible for the external consultant to conduct validation regarding final results.
Model parameters and values used in the base case, sensitivity analysis and scenario analysis
Table 25 summarises the parameters that can vary within the model and the values applied in the base case and sensitivity analyses.
| Parameter | Base-case values (sensitivity analysis) |
|---|---|
| No. of stings per year for PhVIT patients | 0.095 (0.014–0.183) |
| No. of stings per year for subgroup ‘High Risk of Sting Patients’ | 5 (1–10) |
| Pre-injection health check (nurse specialist time) | 15 (10–20) minutes |
| Venom injection preparation (nurse specialist time) | 5 (3–7) minutes |
| Post-injection observation (nurse specialist time) | 3 (2–4) minutes |
| Proportion of PhVIT doses leading to systemic AR |
Updosing: 0.02 (0–0.026) Maintenance: 0.0026 (0–0.01) |
| Grade of systemic AR due to VIT dose | Grade 1: 37.5%, grade 2: 37.5%, grade 3: 21.9%, grade 4: 3.1% |
| Proportion of stings that lead to systemic reaction (advice only) | 0.56 |
| Grade of systemic reaction following sting (advice only) | Grade 1: 6.5%, grade 2: 80.3%, grade 3: 12.1%, grade 4: 1.1% |
| Proportion of stings that lead to systemic reaction (PhVIT + AAI + HDA) | 0.065 (0.05–0.15) |
| Grade of systemic reaction following sting (PhVIT + AAI + HDA) | Grade 1: 38.5%, grade 2: 54.0%, grade 3: 7.5%, grade 4: 0.0% |
| Proportion of stings that lead to systemic reaction (AAI + HDA) | 0.439 |
| Grade of systemic reaction following sting (AAI + HDA) | Grade 1: 9.8%, grade 2: 83.6%, grade 3: 6.05%, grade 4: 0.55% |
| Probability of death following grade 4 systemic reaction to sting | 0.0125 (0.00625–0.01875) |
| Percentage of people using conventional updosing | 92% |
| Length of maintenance phase | 3 (3–5) years |
| Length of intervals between doses during maintenance | 4 (4–12) weeks |
| Systemic sting reactions with inpatient stay | |
| Grade 1 | 0% |
| Grade 2 | 10% |
| Grade 3 | 30% |
| Grade 4 | 50% |
| QoL decrement due to anxiety of sting and impact on normal activities | 0 |
| QoL decrement in subgroup ‘PhVIT Anxiety QoL Improvement’ | Reduction in QoL due to fear of sting: 0.04 per annum |
| QoL increment due to reduction in anxiety with VIT | 0 |
| QoL increment in subgroup ‘PhVIT Anxiety QoL Improvement’ | Increase in QoL due to VIT: 0.01 per annum (0.004–0.04) |
| Age starting VIT | 37 (5–55) years |
| Discount rate (costs and benefits) | 3.5% (0–5%) |
Several scenario analyses were undertaken and these are summarised in terms of the difference in parameters from the base case (Table 26).
| Scenario | Parameters changed | Value taken (sensitivity analysis) |
|---|---|---|
| 5-, 15-, 20-, 25-year time horizon | Time horizon | 5, 15, 20, 25 years |
| 100% male | Gender | Male 100% |
| 100% female | Gender | Male 0% |
| 100% bee | Percentage of people receiving bee PhVIT only | 100% |
| 100% wasp | Percentage of people receiving wasp PhVIT only | 100% |
| 100% bee/wasp | Percentage of people receiving both bee and wasp PhVIT | 100% |
| 100% conventional updosing | Percentage of people on conventional updosing protocol | 100% |
| PhVIT local ARs | Inclusion of costs for local ARs to PhVIT | Add £0.84 to the cost per PhVIT injection in both phases |
| Equal AR risk updosing/maintenance | Dose risk of systemic reaction during maintenance phase | Risk of systemic AR in maintenance phase 2.0% |
| ARs all grade 1 | Mueller grade of systemic ARs | Grade 1 systemic ARs 100% |
| 25% ARs all grade 4 | Mueller grade of systemic ARs | Grade 4 systemic ARs 25% |
| 50% higher systemic AR cost | Cost of all grades of systemic ARs to PhVIT | Cost of all grades of systemic ARs to PhVIT + 50% |
| 50% lower systemic AR cost | Cost of all grades of systemic ARs to PhVIT | Cost of all grades of systemic ARs to PhVIT – 50% |
| 50% higher systemic sting treatment cost | Cost of all grades of systemic reactions to sting | Cost of all grades of systemic reactions to sting + 50% |
| 50% lower systemic sting treatment cost | Cost of all grades of systemic reactions to sting | Cost of all grades of systemic reactions to sting – 50% |
| No admissions due to systemic ARs to PhVIT | Percentage of grade 4 systemic ARs resulting in admission | 0% |
| Declining PhVIT effectiveness | Risk of systemic reaction from sting with PhVIT | 5% at year 1 following the end of maintenance increasing by 1% per annum to 15% after 10 years following maintenance |
| No systemic reaction inpatient stay | Proportion of people requiring an inpatient stay after systemic sting reaction | 0% |
| 100% grade 4 systemic reaction inpatient stay | Proportion of people requiring an inpatient stay after a grade 4 systemic sting reaction | 100% |
| AAI + HDA no systemic reaction effectiveness | Risk and severity of systemic reaction | Same as advice only intervention |
| PhVIT anxiety QoL improvement only | QoL age-related norms, sting systemic reactions with AAI + HDA | Reduction in QoL due to fear of sting 0.04 per annum, increase in QoL due to PhVIT 0.01 per annum (0.004–0.04). Risk and severity of systemic reaction following sting with AAI + HDA equal to that with PhVIT + AAI + HDA |
| Best case | All parameters varied in base-case sensitivity analysis | Values chosen that make PhVIT the most cost-effective (lowest cost/QALY) |
| Worst case | All parameters varied in base-case sensitivity analysis | Values chosen that make PhVIT the least cost-effective (lowest cost/QALY) |
Results
For the hypothetical cohort of 1000 patients, the total number of systemic ARs to PhVIT, number of stings, severity of systemic reactions to sting, sting-related deaths, total life-years and QALYs over 10 years for each treatment arm for the base case and the two subgroups (‘High Risk of Sting Patients’ and ‘PhVIT Anxiety QoL Improvement’) are shown in Table 27.
| Treatment effect | Treatment arm | Base case | High risk of sting patients | PhVIT anxiety QoL improvement |
|---|---|---|---|---|
| Systemic AR to PhVIT | PhVIT + AAI + HDA | 450 | 450 | 450 |
| Grade 1 | 169 | 169 | 169 | |
| Grade 2 | 169 | 169 | 169 | |
| Grade 3 | 99 | 99 | 99 | |
| Grade 4 | 14 | 14 | 14 | |
| Stings | PhVIT + AAI + HDA | 943 | 49,639 | 943 |
| AAI + HDA | 943 | 49,606 | 943 | |
| Advice only | 943 | 49,554 | 943 | |
| Systemic reaction to sting | PhVIT + AAI + HDA | 61 | 3223 | 61 |
| AAI + HDA | 414 | 21,777 | 414 | |
| Advice only | 528 | 27,750 | 528 | |
| Grade 1 | PhVIT + AAI + HDA | 24 | 1239 | 24 |
| AAI + HDA | 41 | 2134 | 41 | |
| Advice only | 34 | 1804 | 34 | |
| Grade 2 | PhVIT + AAI + HDA | 33 | 1742 | 33 |
| AAI + HDA | 346 | 18,206 | 346 | |
| Advice only | 424 | 22,283 | 424 | |
| Grade 3 | PhVIT + AAI + HDA | 5 | 242 | 5 |
| AAI + HDA | 25 | 1318 | 25 | |
| Advice only | 64 | 3358 | 64 | |
| Grade 4 | PhVIT + AAI + HDA | 0 | 0 | 0 |
| AAI + HDA | 2 | 120 | 2 | |
| Advice only | 6 | 305 | 6 | |
| Sting-related deaths | PhVIT + AAI + HDA | 0.00 | 0.00 | 0.00 |
| AAI + HDA | 0.03 | 1.50 | 0.03 | |
| Advice only | 0.07 | 3.82 | 0.07 | |
| Total life-years | PhVIT + AAI + HDA | 9908.0 | 9908.0 | 9908.0 |
| AAI + HDA | 9907.8 | 9899.8 | 9907.8 | |
| Advice only | 9907.6 | 9887.1 | 9907.6 | |
| Total QALYs | PhVIT + AAI + HDA | 7626.6 | 7626.6 | 7371.9 |
| AAI + HDA | 7626.5 | 7620.7 | 7286.9 | |
| Advice only | 7626.3 | 7611.5 | 7286.7 |
The total costs for the hypothetical 1000-patient cohort in terms of intervention costs, treatment costs for ARs to PhVIT and treatment costs for systemic reactions following sting in the base case and two subgroups are provided in Table 28.
| Cost element | Treatment arm | Base case (£) | High risk of sting patients (£) | PhVIT anxiety QoL improvement (£) |
|---|---|---|---|---|
| Treatment costs | PhVIT + AAI + HDA | 2,299,327 | 2,299,223 | 2,299,327 |
| AAI + HDA | 228,330 | 228,228 | 228,330 | |
| Advice only | 64,000 | 64,000 | 64,000 | |
| Systemic AR | PhVIT + AAI + HDA | 17,637 | 17,637 | 17,637 |
| Systemic reaction to sting | PhVIT + AAI + HDA | 9764 | 513,919 | 9764 |
| AAI + HDA | 69,591 | 3,660,233 | 69,591 | |
| Advice only | 77,285 | 4,060,750 | 77,285 | |
| Total costs | PhVIT + AAI + HDA | 2,326,729 | 2,830,778 | 2,326,729 |
| AAI + HDA | 297,921 | 3,888,461 | 297,921 | |
| Advice only | 141,285 | 4,124,750 | 141,285 |
The incremental cost between the three treatment arms, incremental QALYs and cost per QALY of PhVIT + AAI + HDA compared with the two treatment alternatives for the base case and two subgroups for a 1000-patient cohort are shown in Tables 29–31 respectively.
| AAI + HDA | Avoidance advice only | |
|---|---|---|
| Incremental cost | £2,028,808 | £2,185,444 |
| Incremental QALYs | 0.11 | 0.29 |
| Cost per QALY (ICER) | £18,065,527 | £7,627,835 |
| AAI + HDA | Avoidance advice only | |
|---|---|---|
| Incremental cost | –£1,057,682 | –£1,293,972 |
| Incremental QALYs | 5.91 | 15.06 |
| Cost per QALY (ICER) | –£179,020 | –£85,903 |
| AAI + HDA | Avoidance advice only | |
|---|---|---|
| Incremental cost | £2,028,808 | £2,185,444 |
| Incremental QALYs | 85.00 | 85.17 |
| Cost per QALY (ICER) | £23,868 | £25,661 |
Under the base-case assumptions over 10 years, PhVIT + AAI + HDA generates an additional 0.00011 and 0.00029 QALYs per patient compared with AAI + HDA and avoidance advice respectively. This is at an additional cost of £2029 and £2185 per patient compared with AAI + HDA and avoidance advice respectively. The ICER of PhVIT + AAI + HDA is therefore £18,065,527 per QALY gained compared with AAI + HDA and £7,627,835 per QALY gained compared with avoidance advice.
For the ‘High Risk of Sting Patient’ subgroup, at five stings per year, the reduction in costs from systemic reactions to sting over 10 years because of PhVIT outweighs the VIT treatment costs. As PhVIT also generates additional QALYs by reducing sting deaths for this subgroup, PhVIT + AAI + HDA dominates the alternatives.
The subgroup analysis that allows for QoL changes as a result of sting anxiety and the use of PhVIT estimates that PhVIT + AAI + HDA generates an additional 0.0850 and 0.0852 QALYs per patient compared with AAI + HDA and avoidance advice respectively. The incremental cost per patient is the same as in the base case. The ICER for the subgroup with sting anxiety that is partially alleviated with PhVIT + AAI + HDA is therefore £23,868 per QALY gained compared with AAI + HDA and £25,661 per QALY gained compared with avoidance advice only.
Sensitivity analysis
The results of the sensitivity analysis for the base case and two subgroups are presented in Tables 32–34, respectively, and show the impact on the ICER when parameters are varied; PhVIT + AAI + HDA is compared with the two treatment alternatives.
| Parameter | Cost per QALY vs AAI + HDA (£) | Cost per QALY vs advice only (£) |
|---|---|---|
| No. of stings per year (0.014–0.183) | 9,122,183 to 125,668,803 | 3,846,558 to 53,122,895 |
| Pre-injection health check (nurse specialist time) | 15,724,577 to 20,406,478 | 6,710,254 to 8,545,415 |
| Venom injection preparation (nurse specialist time) | 17,115,470 to 19,015,585 | 7,255,442 to 8,000,228 |
| Post-injection observation (nurse specialist time) | 17,590,498 to 18,540,556 | 7,441,638 to 7,814,031 |
| Proportion of PhVIT doses leading to systemic AR (updosing phase) | 17,979,460 to 19,098,328 | 7,594,099 to 8,032,661 |
| Proportion of PhVIT doses leading to systemic AR (maintenance phase) | 17,994,545 to 18,267,553 | 7,600,012 to 7,707,023 |
| Proportion of stings that lead to systemic reaction (PhVIT + AAI + HDA) | 18,045,462 to 18,179,228 | 7,619,970 to 7,672,402 |
| Probability of death following grade 4 systemic reaction to a sting | 36,130,899 to 12,043,736 | 15,255,509 to 5,085,277 |
| Length of maintenance phase | 18,065,527 to 27,331,818 | 7,627,835 to 11,259,936 |
| Length of intervals between injections during maintenance phase | 18,065,527 to 7,903,259 | 7,627,835 to 3,644,538 |
| Age starting PhVIT | 16,924,162 to 21,390,991 | 7,148,354 to 9,015,238 |
| Discount rate (costs and benefits) | 15,129,011 to 19,487,889 | 6,452,674 to 8,196,637 |
| Parameter | Cost per QALY vs AAI + HDA | Cost per QALY vs advice only |
|---|---|---|
| No. of stings per year (1–10) | £1,234,283 to –£355,512 | £511,546 to –£160,398 |
| Pre-injection health check (nurse specialist time) | Dominates | Dominates |
| Venom injection preparation (nurse specialist time) | Dominates | Dominates |
| Post-injection observation (nurse specialist time) | Dominates | Dominates |
| Proportion of PhVIT doses leading to systemic AR (updosing phase) | Dominates | Dominates |
| Proportion of PhVIT doses leading to systemic AR (maintenance phase) | Dominates | Dominates |
| Proportion of stings that lead to systemic reaction (PhVIT + AAI + HDA) | Dominates | Dominates |
| Probability of death following grade 4 systemic reaction to a sting | Dominates | Dominates |
| Length of maintenance phase | Dominates | Dominates |
| Length of intervals between injections during maintenance phase | Dominates | Dominates |
| Age starting PhVIT | Dominates | Dominates |
| Discount rate (costs and benefits) | Dominates | Dominates |
| Parameter | Cost per QALY vs AAI + HDA (£) | Cost per QALY vs advice only (£) |
|---|---|---|
| No. of stings per year (0.014–0.183) | 23,189 to 24,495 | 24,853 to 26,409 |
| Pre-injection health check (nurse specialist time) | 20,775 to 26,961 | 22,574 to 28,748 |
| Venom injection preparation (nurse specialist time) | 22,613 to 25,124 | 24,408 to 26,914 |
| Post-injection observation (nurse specialist time) | 23,241 to 24,496 | 25,035 to 26,287 |
| Proportion of PhVIT doses leading to systemic AR (updosing phase) | 23,755 to 25,233 | 25,547 to 27,023 |
| Proportion of PhVIT doses leading to systemic AR (maintenance phase) | 23,775 to 24,135 | 25,567 to 25,927 |
| Proportion of stings that lead to systemic reaction (PhVIT + AAI + HDA) | 23,842 to 24,019 | 25,634 to 25,811 |
| Probability of death following grade 4 systemic reaction to sting | 23,883 to 23,853 | 25,702 to 25,620 |
| Length of maintenance phase | 23,868 to 36,111 | 25,661 to 37,880 |
| Length of intervals between injections during maintenance phase | 23,868 to 10,442 | 25,661 to 12,261 |
| Age starting PhVIT | 23,711 to 24,697 | 25,497 to 26,510 |
| Discount rate (costs and benefits) | 21,065 to 25,140 | 22,875 to 26,925 |
| Age-related QoL norm (decreases by 0.04), QoL norm with PhVIT (increases by 0.004 to 0.04) | 5973 to 59,558 | 6431 to 63,845 |
Scenario analysis
The impact of changes on the ICERs for PhVIT + AAI + HDA, under the different scenarios presented, compared with the alternative treatments is provided in Tables 35–37 for the base case, ‘High Risk of Sting Patients’ subgroup and ‘PhVIT Anxiety QoL Improvement’ subgroup respectively.
| Scenario | Cost per QALY vs AAI + HDAa (£) | Cost per QALY vs advice onlya (£) |
|---|---|---|
| 5-year time horizon | 58,112,401 (+ 321.68) | 23,728,992 (+ 311.08) |
| 15-year time horizon | 9,475,380 (–47.55) | 4,112,030 (–46.09) |
| 20-year time horizon | 6,158,390 (–65.91) | 2,733,478 (–64.16) |
| 25-year time horizon | 4,549,884 (–74.81) | 2,056,752 (–73.04 |
| 100% male | 18,094,419 (+ 0.16) | 7,639,773 (+ 0.16) |
| 100% female | 17,950,948 (–0.63) | 7,580,491 (–0.62) |
| 100% bee venom | 16,391,486 (–9.27) | 6,971,662 (–8.60) |
| 100% wasp venom | 18,034,830 (–0.17) | 7,615,802 (–0.16) |
| 100% bee/wasp venom | 23,872,923 (+ 32.15) | 9,904,156 (+ 29.84) |
| 100% conventional updosing protocol | 18,095,990 (+ 0.17) | 7,639,775 (+ 0.16) |
| 100% modified rush updosing protocol | 17,715,201 (–1.94) | 7,490,518 (–1.80) |
| PhVIT local ARs | 18,439,612 (+ 2.07) | 7,774,465 (+ 1.92) |
| Equal AR risk updosing/maintenance | 18,540,562 (+ 2.63) | 7,814,034 (+ 2.44) |
| ARs all grade 1 | 18,039,836 (–0.14) | 7,617,765 (–0.13) |
| 25% ARs grade 4 | 18,247,022 (+ 1.00) | 7,698,975 (+ 0.93) |
| 50% higher systemic AR cost | 18,144,052 (+ 0.43) | 7,658,614 (+ 0.40) |
| 50% lower systemic AR cost | 17,987,003 (–0.43) | 7,597,056 (–0.40) |
| 50% higher systemic sting treatment cost | 17,799,166 (–1.47) | 7,510,002 (–1.54) |
| 50% lower systemic sting treatment cost | 18,331,888 (+ 1.47) | 7,745,668 (+ 1.54) |
| No admissions due to systemic ARs | 18,043,808 (–0.12) | 7,619,321 (–0.11) |
| Declining PhVIT effectiveness | 18,087,837 (+ 0.12) | 7,636,580 (+ 0.11) |
| No systemic reaction inpatient stay | 18,185,034 (+ 0.66) | 7,700,600 (+ 0.95) |
| 100% grade 4 systemic reaction inpatient stay | 18,062,700 (–0.02) | 7,624,569 (–0.04) |
| AAI + HDA no systemic reaction effectiveness | 7,002,582 (–61.24) | NA |
| Best-case scenario | 1,449,007 (–91.98) | 731,302 (–90.41) |
| Worst-case scenario | 570,668,032 (+ 3058.88) | 232,820,521 (+ 2952.25) |
| Scenario | Cost per QALY vs AAI + HDAa (£) | Cost per QALY vs advice onlya (£) |
|---|---|---|
| 5-year time horizon | 274,556 | 84,006 |
| 15-year time horizon | Dominates | Dominates |
| 20-year time horizon | Dominates | Dominates |
| 25-year time horizon | Dominates | Dominates |
| 100% male | Dominates | Dominates |
| 100% female | Dominates | Dominates |
| 100% bee venom | Dominates | Dominates |
| 100% wasp venom | Dominates | Dominates |
| 100% bee/wasp venom | Dominates | Dominates |
| 100% conventional updosing protocol | Dominates | Dominates |
| 100% modified rush updosing protocol | Dominates | Dominates |
| PhVIT local ARs | Dominates | Dominates |
| Equal AR risk updosing/maintenance | Dominates | Dominates |
| ARs all grade 1 | Dominates | Dominates |
| 25% ARs grade 4 | Dominates | Dominates |
| 50% higher systemic AR cost | Dominates | Dominates |
| 50% lower systemic AR cost | Dominates | Dominates |
| 50% higher systemic sting treatment cost | Dominates | Dominates |
| 50% lower systemic sting treatment cost | 87,248 | 31,829 |
| No admissions due to systemic ARs | Dominates | Dominates |
| Declining PhVIT effectiveness | Dominates | Dominates |
| No systemic reaction inpatient stay | Dominates | Dominates |
| 100% grade 4 systemic reaction inpatient stay | Dominates | Dominates |
| AAI + HDA no systemic reaction effectiveness | Dominates | NA |
| Best-case scenario (fixed at five stings per annum) | Dominates | Dominates |
| Worst-case scenario (fixed at five stings per annum) | 547,263 | 172,930 |
| Scenario | Cost per QALY vs AAI + HDAa (£) | Cost per QALY vs advice onlya (£) |
|---|---|---|
| 5-year time horizon | 44,328 (+ 85.72) | 46,126 (+ 79.75) |
| 15-year time horizon | 17,128 (–28.24) | 18,912 (–26.30) |
| 20-year time horizon | 13,806 (–42.16) | 15,582 (–39.28) |
| 25-year time horizon | 11,879 (–50.23) | 13,647 (–53.18) |
| 100% male | 23,884 (+ 0.07) | 25,677 (+ 0.06) |
| 100% female | 23,807 (–0.26) | 25,598 (–0.25) |
| 100% bee venom | 21,657 (–9.27) | 23,453 (–8.60) |
| 100% wasp venom | 23,828 (–0.17) | 25,620 (–0.16) |
| 100% bee/wasp venom | 31,541 (+ 32.15) | 33,319 (+ 29.84) |
| 100% conventional updosing protocol | 23,909 (+ 0.17) | 25,701 (+ 0.16) |
| 100% modified rush updosing protocol | 23,406 (–1.94) | 25,199 (–1.80) |
| PhVIT local ARs | 24,363 (+ 2.07) | 26,154 (+ 1.92) |
| Equal AR risk updosing/maintenance | 24,496 (+ 2.63) | 26,287 (+ 2.44) |
| ARs all grade 1 | 23,834 (–0.14) | 25,627 (–0.13) |
| 25% ARs grade 4 | 24,108 (+ 1.01) | 25,900 (+ 0.93) |
| 50% higher systemic AR cost | 23,972 (+ 0.44) | 25,764 (+ 0.40) |
| 50% lower systemic AR cost | 23,765 (–0.43) | 25,557 (–0.40) |
| 50% higher systemic sting treatment cost | 23,516 (–1.47) | 25,265 (–1.55) |
| 50% lower systemic sting treatment cost | 24,220 (+ 1.48) | 26,057 (+ 1.54) |
| No admissions due to systemic ARs | 23.840 (–0.12) | 25,632 (–0.11) |
| Declining PhVIT effectiveness | 23,898 (+ 0.12) | 25,690 (+ 0.11) |
| No systemic reaction inpatient stay | 24,026 (+ 0.66) | 25,906 (+ 0.95) |
| 100% grade 4 systemic reaction inpatient stay | 23,865 (–0.01) | 25,650 (–0.04) |
| AAI + HDA no systemic reaction effectiveness | 23,557 (–1.30) | NA |
| PhVIT anxiety QoL improvement only | 24,605 (+ 3.09) | NA |
| Best-case scenario (fixed at 0.01 per annum PhVIT QoL improvement) | 6179 (–74.11) | 7906 (–69.19) |
| Worst-case scenario (fixed at 0.01 per annum PhVIT Qol improvement) | 47,390 (+ 98.55) | 49,320 (+ 92.20) |
Summary of economics evidence
-
No published economic evaluations relevant to the decision problem were identified by the systematic review of cost-effectiveness studies.
-
The manufacturer of PhVIT did not submit any supporting clinical effectiveness or cost-effectiveness evidence to NICE.
-
The AG developed a de novo economic model to compare PhVIT with currently available NHS treatments in patients with a history of type 1 IgE-mediated systemic allergic reaction to bee and/or wasp venom.
-
In the AG’s base case, PhVIT + HDA + AAI reached an ICER of £18,065,527 per QALY gained compared with AAI + HDA.
-
In the AG’s base case, PhVIT + HDA + AAI reached an ICER of £7,627,835 per QALY gained compared with avoidance advice only.
-
In the AG’s base case the results of the sensitivity analyses and scenario analyses showed that the results of the economic evaluation were robust for every plausible change in parameter made.
-
The AG’s ‘High Risk of Sting Patients’ subgroup analysis showed that the PhVIT + HDA + AAI dominates both AAI + HDA and avoidance advice only.
-
The AG’s ‘VIT Anxiety QoL Improvement’ subgroup analysis showed that PhVIT + HDA + AAI vs HDA + AAI had an ICER of £23,868 per QALY gained.
-
The AG’s ‘VIT Anxiety QoL Improvement’ subgroup analysis showed that PhVIT + HDA + AAI vs avoidance advice only had an ICER of £25,661 per QALY gained.
Discussion of economics results and key issues
No relevant economic evaluations of PhVIT versus any comparator were identified from the systematic review of cost-effectiveness literature. The manufacturer did not submit any clinical effectiveness or cost-effectiveness evidence to NICE, which means that the AG did not have any additional data from the manufacturer. The AG developed a de novo economic model to answer the decision problem set by NICE.
Under the base case the incremental cost per QALY gained for PhVIT + AAI + HDA compared with an emergency kit of AAI + HDA is never < £1M under any scenario or any plausible values for parameters within the model. The ICER falls below £1M only when PhVIT + AAI + HDA is compared with avoidance advice and the most optimistic scenario for PhVIT + AAI + HDA is considered; however, this ICER still exceeds £700,000 per QALY gained.
As scenario analysis explored extreme values when assumptions had to be made – such as in the costs associated with treating a systemic reaction following sting – this finding can be considered robust and unlikely to change if additional information were available to provide more accurate values for these assumptions. The underlying driver for this ICER is that, although PhVIT can achieve savings through reduced systemic reaction treatment costs and generate QALYs through saving lives, the likelihood of being stung and then dying from that sting is very low – even for individuals allergic to sting. The ability of PhVIT to generate QALY gains and reduce demand on NHS resources is therefore low.
The findings are considerably different for the two subgroups that are considered in our analysis. First, considering allergic individuals at high risk of sting, subgroup analysis suggests that, under all other base-case values, at a rate of five stings per year, PhVIT + AAI + HDA reduces the number of systemic reactions to stings, and therefore total costs of systemic sting reaction, to a point that it actually costs less than the other treatment arms. Although even at this level of sting the number of deaths averted and therefore QALYs generated is low with PhVIT + AAI + HDA, as it still generates some QALYs compared with the other treatment arms its lower cost means that it dominates the other arms as a treatment option. This finding is invariant to the changes made to almost all parameters in scenario analysis and sensitivity analysis. The exceptions are if a time horizon of only 5 years is considered, treatment costs for systemic reaction are 50% lower than in the base case and the most pessimistic plausible values for all parameters in the model are chosen.
Our survey found that allergy clinics advise all people that, if stung and having signs of systemic reaction, they should attend A&E. It is therefore not plausible that this cost should be lower than we have considered. The only other cost of treatment considered that could significantly inflate the cost of treatment is inpatient care. Under the scenario of no inpatient care following sting, PhVIT + AAI + HDA still dominated.
Assuming that all other parameters for the base case hold, the number of stings at which PhVIT + AAI + HDA would no longer dominate and incremental costs per QALY would be generated would be 3.3 stings per year compared with AAI + HDA and 3.2 stings per year compared with avoidance advice only. The number of stings per year for which PhVIT + AAI + HDA would generate an ICER of £30,000 per QALY gained is 3.1 compared with AAI + HDA and 2.8 compared with avoidance advice only. We considered a third subgroup that would combine an improvement in utility from reduction in anxiety in a population with a high risk of sting. As PhVIT + AAI + HDA dominates, assuming no improvement in QoL from receiving PhVIT, this subgroup analysis was considered unnecessary.
For people with the base-case risk of sting or lower risk of sting, keeping in mind that the base-case risk will potentially include people at significantly higher risk of sting than others and that the sting risk is a combined wasp and bee sting risk and people may not have an allergic response to both, the cost-effectiveness of PhVIT improves substantially if QALYs are generated not only by stopping sting deaths, but also through reductions in sting anxiety. The evidence on improvement in QoL is limited but suggests that PhVIT does effectively reduce sting anxiety. Although the actual effect of this on utility as measured by a recognised survey is absent, the research by the University of York previously discussed suggests that QoL can be substantially influenced both by the individual’s inability to undertake usual activities and because of anxiety.
Our analysis explored how the cost-effectiveness of PhVIT varies if fear of sting has only a small negative impact on QoL compared with the potential impact identified by the University of York research. It also assumed that PhVIT + AAI + HDA has only a small impact in negating this loss in utility. If fear of sting reduces utility by 0.04 of a QALY per annum and PhVIT improves utility by 25% of this value (0.01 of a QALY per annum), the ICERs for PhVIT + AAI + HDA are < £30,000 per QALY gained compared with AAI + HDA and avoidance advice only if all other base-case values hold. This result holds across a range of scenarios and potential plausible parameter values, even if PhVIT is assumed to be no more effective than an emergency kit of AAI + HDA at stopping and alleviating systemic reactions to sting.
The finding is somewhat sensitive to PhVIT treatment costs, most notably the length of the maintenance phase. With a maintenance phase of 5 years the ICER rises to just under £40,000 per QALY gained compared with the alternative treatments. For people requiring both bee and wasp PhVIT the ICER also rises to between £33,440 per QALY gained and £35,163 per QALY gained compared with AAI + HDA and avoidance advice only respectively.
If the reduction in utility from sting anxiety is 0.04 per annum, then for PhVIT + AAI + HDA to generate an ICER of £30,000 per QALY gained it has to negate this reduction by 0.008 per annum compared with AAI + HDA and 0.009 compared with avoidance advice only. For people receiving both bee and wasp PhVIT, the incremental increase in QoL per annum has to rise from 0.01 to 0.011 to achieve an ICER of £30,000 per QALY gained compared with both AAI + HDA and avoidance advice only.
As the treatment costs are all incurred within the first 5 years of the analysis but benefits continue to accrue past this point, the ICERs at 5 years are higher than the base-case ICERs at 10 years and continue to fall up to 25 years. As the available evidence suggests that PhVIT continues to be effective up to at least 10 years but is limited beyond this, the choice of a 10-year time horizon is in our opinion justified.
Although we consider the findings robust, there are some key weaknesses of our analysis:
-
the lack of data on effectiveness of PhVIT from RCTs
-
the lack of any published evidence on PhVIT + AAI + HDA versus AAI + HDA or avoidance advice only
-
the absence of direct data on the number of stings in PhVIT people in the UK and the number of stings that are from bees or wasps
-
the absence of direct data on the likelihood of death following sting for sting-allergic people
-
the absence of robust data on the improvement in utility because of sting anxiety in allergic people.
To counter this lack of evidence and potential criticism of simplifying assumptions, substantial sensitivity and scenario analyses were used to highlight those parameters that are key to the cost-effectiveness analysis and explore the impact on the cost-effectiveness results of the intervention in question across ranges of plausible values. The final weakness is shown to be irrelevant if increases in utility from reduced sting anxiety arise through PhVIT as the findings hold even if PhVIT has no effectiveness on systemic reactions to sting.
Chapter 5 Conclusions
The current use of PhVIT in clinical practice in the NHS appears to be based on limited and poor-quality clinical effectiveness research.
The AG did not identify any studies of PhVIT that directly addressed the original decision problem set for this appraisal, i.e. to compare the use of PhVIT with the alternative treatment options of advice on the avoidance of bee and wasp venom, HDA and/or AAIs.
This lack of evidence and the need to identify data to inform the development of an economic model prompted the AG to broaden the search criteria for the systematic review in order to compare PhVIT with other PhVIT and PhVIT with non-PhVIT, to consider data from non-comparative studies of PhVIT, and to examine studies reporting the clinical effectiveness of non-PhVIT.
In general, research in the area is limited to small-scale studies that do not appear to have been carried out using robust methods, and none of the studies reported on the use of PhVIT within the UK. There is also heterogeneity in the published evidence related to the methods of PhVIT administration and length of treatment described in the trials. Therefore, conclusions regarding the clinical effectiveness of PhVIT to reduce the rate of future systemic reactions in patients with a history of bee and/or wasp allergic reaction cannot be drawn with any confidence. Available evidence indicates that sting reactions following the use of PhVIT are low and that the ARs related to treatment are minor and easily treatable.
Anxiety related to the possibility of future stings is an issue for debate, and data from studies of VIT indicate a small improvement in QoL as a result of a decrease in sting-related anxiety after VIT.
No published research on the cost-effectiveness of PhVIT or non-PhVIT was identified by the literature searches. The results of the AG’s de novo base-case economic evaluation demonstrate that PhVIT + AAI + HDA compared with AAI + HDA and with avoidance advice only yield ICERs in the range of £8–18M per QALY gained. The results of extensive sensitivity and scenario analyses demonstrate that the base-case results are robust. Two subgroups were considered in the economic evaluation, and the AG concludes that use of PhVIT + AAI + HDA may be cost-effective in both groups. In the subgroup of patients at high risk of future stings (five stings per year), PhVIT + AAI + HDA dominates the alternatives. In the subgroup of patients whose QoL improves because PhVIT reduces anxiety, when PhVIT + AAI + HDA is compared with the alternatives the ICERs are in the range of £23,868–25,661 per QALY gained.
Future research
Use of PhVIT in clinical practice in the UK NHS is commonplace and it is therefore highly unlikely that placebo-controlled studies will ever be carried out. The findings of this review indicate, however, that it is necessary to identify more clearly the groups of patients most likely to benefit from treatment and ensure that clinical practice is focused on these groups. Second, given the paucity of UK data in this area it would be informative if data could be collected routinely when VIT is administered in the NHS (e.g. rates of systemic ARs to VIT, rates of systemic reactions to bee/wasp stings).
Acknowledgements
The authors are pleased to acknowledge Dr Tina Dixon (Royal Liverpool University Hospital), who provided clinical feedback on the AG report, and Dr David Luyt (Leicester Royal Infirmary), who provided clinical opinions to aid in the economic model assumptions.
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme. Any errors are the responsibility of the authors.
Contributions of authors
Juliet Hockenhull: project lead, review of clinical evidence.
Mariam Elremeli: contribution of data from ongoing Cochrane review of venom immunotherapy.
M Gemma Cherry: support of review process (clinical).
James Mahon: development of de novo economic model.
Monica Lai: development of economic model, input into all aspects of the economic review.
Jim Darroch: clinical advisor.
James Oyee: statistical analysis.
Angela Boland: support of review process (clinical and economic).
Rumona Dickson: support of review process.
Yenal Dundar: development of search strategies.
Robert Boyle: contribution of data from ongoing Cochrane review of venom immunotherapy.
All authors read and commented on draft versions of the Evidence Review Group (ERG) report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- King T, Lu G, Gonzalez M, Qian N, Soldatova L. Yellow jacket venom allergens, hyaluronidase and phospholipase: sequence similarity and antigenic cross-reactivity with their hornet and wasp homologs and possible implications for clinical allergy. J Allergy Clin Immunol 1996;98:588-600.
- Lu G, Villalba M, Coscia M, Hoffman D, King T. Sequence analysis and antigenic cross-reactivity of a venom allergen, antigen 5, from hornets, wasps, and yellow jackets. J Immunol 1993;150:2823-30.
- Müller U. New developments in the diagnosis and treatment of hymenoptera venom allergy. Int Arch Allergy Immunol 2001;124:447-53.
- Fitzgerald K, Flood A. Hymenoptera stings. Clin Tech Small Anim Pract 2006;21:194-20.
- Bilo MB, Bonifazi F. The natural history and epidemiology of insect venom allergy: clinical implications. Clin Exp Allergy 2009;39:1467-76.
- Demain J, Minaei A, Tracy J. Anaphylaxis and insect allergy. Curr Opin Allergy Clin Immunol 2010;10:318-22.
- Freeman T. Hypersensitivity to hymenoptera stings. N Engl J Med 2004;351:1978-84.
- Mueller UR, Mueller UR. Insect sting allergy. Stuttgart: Gustav Fischer; 1990.
- Pumphrey R, Stanworth S. The clinical spectrum of anaphylaxis in north-west England. Clin Exp Allergy 1996;26:1364-70.
- Bilo BM, Bonifazi F. Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol 2008;8:330-7.
- Golden DB. Epidemiology of allergy to insect venoms and stings. Allergy Proc 1989;10:103-7.
- Bilo B, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink J. the EAACI Interest Group on Insect Venom Hypersensitivity . Diagnosis of Hymenoptera venom allergy. Allergy 2005;60:1339-49.
- Settipane G, Newstead G, Boyd G. Frequency of hymenoptera allergy in an atopic and normal population. J Allergy 1972;50:146-50.
- Diwakar L, Noorani S, Huissoon AP, Frew AJ, Krishna MT. Practice of venom immunotherapy in the United Kingdom: a national audit and review of literature. Clin Exp Allergy 2008;38:1651-8.
- The Anaphylaxis Campaign . Allergy to Bee and Wasp Stings 2005. www.anaphylaxis.org.uk (accessed June 2011).
- Pumphrey R, Roberts I. Postmortem findings after fatal anaphylactic reactions. J Clin Pathol 2000;53:273-6.
- Pumphrey R. Anaphylaxis. Chichester: Wiley; 2004.
- Golden D, Moffitt J, Nicklas R, Freeman T, Graft D, Reisman R, et al. Stinging insect hypersensitivity: a practice parameter update 2011. J Allergy Clin Immunol 2011;127:852-4.
- Bilo BM, Bonifazi F. Advances in hymenoptera venom immunotherapy. Curr Opin Allergy Clin Immunol 2007;7:567-73.
- Adkis C, Blesken T, Akdis M. Role of interleukin 10 in specific immunotherapy. J Clin Invest 1998;102.
- O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med 2004;10:801-5.
- Bellinghausen I, Knop J, Saloga J. Role of interleukin 10-producing T cells in specific (allergen) immunotherapy. J Allergy Clin Immunol 2000;12:20-5.
- Working Group of the Resuscitation Council (UK) . Emergency Treatment of Anaphylactic Reactions: Guidelines for Healthcare Providers 2008 n.d. www.resus.org.uk/pages/reaction.pdf (accessed June 2011).
- Müller U, Mosbech H, Aberer W, Dreborg S, Ewan P, Kunkel G, et al. EAACI Position Paper. Adrenaline for emergency kits. Allergy 1995;50:783-7.
- Soar J, Pumphrey R, Cant A, Clarke S, Corbett A, Dawson P, et al. Emergency treatment of anaphylactic reactions – guidelines for healthcare providers. Resuscitation 2008;77:157-69.
- Joint Task Force on Practice Parameters, American Academy of Allergy Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunology . Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol 2007;120:25-8.
- British Society for Allergy and Clinical Immunology n.d. www.bsaci.org/index.php?option=com_clinics%26Itemid=26 (accessed June 2011).
- Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med 1978;299:157-61.
- Portnoy J, Moffitt J, Golden D, Bernstein W, Dykewicz M, Fineman S, et al. Stinging insect hypersensitivity: a practice parameter. J Allergy Clin Immunol 1999;103:963-80.
- Moffitt JE, Golden DB, Reisman RE, Lee R, Nicklas R, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol 2004;114:869-86.
- ALK Abelló . Pharmalgen Summary of Product Characteristics n.d. www.alk-abello.com/UK/products/pharma/Lists/Pharmalgen/Pharmalgen%20Wasp%20Venom%20SmPC.pdf (accessed June 2011).
- Patriarca G, Nucera E, Roncallo C, Aruanno A, Lombardo C, Decinti M, et al. Sublingual desensitization in patients with wasp venom allergy: preliminary results. Int J Immunopathol Pharmacol 2008;21:669-77.
- Elremeli M, Bulsara Max K, Daniels M, Boyle RJ. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev 2012.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare n.d. www.york.ac.uk/inst/crd/darefaq.htm (accessed June 2011).
- Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Multi-parameter Evidence Synthesis Research Group . Formal Methods for Multi-Parameter Evidence Synthesis and Their Role in Epidemiology and Economic Evaluation n.d. www.bristol.ac.uk/cobm/research/mpes (accessed June 2011).
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55.
- Cadario G, Marengo F, Ranghino E, Rossi R, Gatti B, Cantone R, et al. Higher frequency of early local side effects with aqueous versus depot immunotherapy for Hymenoptera venom allergy. J Investig Allergol Clin Immunol 2004;14:127-33.
- Golden D, Valentine MD, Sobotka AK, Lichtenstein LM. Regimens of hymenoptera venom immunotherapy. J Allergy Clin Immunol 1979;63.
- Golden DBK, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Prolonged maintenance interval in hymenoptera venom immunotherapy. J Allergy Clin Immunol 1981;67:482-4.
- Golden DBK, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Dose dependence of hymenoptera venom immunotherapy. J Allergy Clin Immunol 1981;67:370-4.
- Golden DBK, Valentine MD, Kagey-Sobotka A, Lichtenstein LM. Regimens of hymenoptera venom immunotherapy. Ann Intern Med 1980;92:620-4.
- Mosbech H, Malling HJ, Biering I. Immunotherapy with yellow jacket venom. A comparative study including three different extracts, one adsorbed to aluminium hydroxide and two unmodified. Allergy 1986;41:95-103.
- Müller U, Lanner A, Schmid P, Bischof M, Dreborg S, Hoigné R. A double blind study on immunotherapy with chemically modified honey bee venom: monomethoxy polyethylene glycol-coupled versus crude honey bee venom. Int Arch Allergy Appl Immunol 1985;77:201-3.
- Müller U, Rabson AR, Bischof M, Lomnitzer R, Dreborg S, Lanner A. A double-blind study comparing monomethoxy polyethylene glycol-modified honeybee venom and unmodified honeybee venom for immunotherapy. I. Clinical results. J Allergy Clin Immunol 1987;80:252-61.
- Quercia O, Rafanelli S, Puccinelli P, Stefanini GF. The safety of cluster immunotherapy with aluminium hydroxide-adsorbed honey bee venom extract. J Investig Allergol Clin Immunol 2001;11:27-33.
- Thurnheer U, Müller U, Stoller R. Venom immunotherapy in hymenoptera sting allergy. Comparison of rush and conventional hyposensitization and observations during long-term treatment. Allergy 1983;38:465-75.
- Mueller HL. Diagnosis and treatment of insect sensitivity. J Asthma Res 1966;3:331-3.
- Wuthrich B. Classification of sting insect sensitivity. J Investig Allergol Clin Immunol 2001;11.
- Huber PJ. Atopie Und Hymenopterenstichallergie 1981.
- Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM, Baird-Warren IA, Bukantz SC. The Hymenoptera venom study. III: safety of venom immunotherapy. J Allergy Clin Immunol 1990;86:775-80.
- Ramirez DA, Londono S, Evans IRD. Adverse reactions to venom immunotherapy. Ann Allergy 1981;47:435-9.
- Carballada Gonzalez FJ, Crehuet Almirall M, Manjon Herrero A, De la Torre F, Boquete Paris M. Hymenoptera venom allergy: characteristics, tolerance and efficacy of immunotherapy in the paediatric population. Allergol Immunopathol 2009;37:111-15.
- Graft DF, Schuberth KC, Kagey-Sobotka A. Assessment of prolonged venom immunotherapy in children. J Allergy Clin Immunol 1987;80:162-9.
- Urbanek R, Forster J, Kuhn W, Ziupa J. Discontinuation of bee venom immunotherapy in children and adolescents. J Pediatr 1985;107:367-71.
- Carballada F, Boquete M, Nunez R, Lombardero M, de la Torre F. Follow-up of venom immunotherapy (VIT) based on conventional techniques and monitoring of immunoglobulin E to individual venom allergens. J Investig Allergol Clin Immunol 2010;20:506-13.
- Haeberli G, Bronnimann M, Hunziker T, Müller U. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin Exp Allergy 2003;33:1216-20.
- Kochuyt AM, Stevens EAM. Safety and efficacy of a 12-week maintenance interval in patients treated with hymenoptera venom immunotherapy. Clin Exp Allergy 1994;24:35-41.
- Lerch E, Müller UR. Long-term protection after stopping venom immunotherapy: results of re-stings in 200 patients. J Allergy Clin Immunol 1998;101:606-12.
- Müller UR, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol 1992;89:529-35.
- Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy 1989;44:412-8.
- Sanchez-Machin I, Moreno C, Gonzalez R, Iglesias-Souto J, Perez E, Matheu V. Safety of a 2-visit cluster schedule of venom immunotherapy in outpatients at risk of life-threatening anaphylaxis. J Investig Allergol Clin Immunol 2010;20:91-2.
- Kalogeromitros D, Makris M, Koti I, Chliva C, Mellios A, Avgerinou G, et al. A simple 3-day ‘rush’ venom immunotherapy protocol: documentation of safety. Allergol Immunopathol 2010;38:69-73.
- Fricker M, Helbling A, Schwartz L, Müller U. Hymenoptera sting anaphylaxis and urticaria pigmentosa: clinical findings and results of venom immunotherapy in ten patients. J Allergy Clin Immunol 1997;100:11-5.
- Haugaard L, Norregaard OF, Dahl R. In-hospital sting challenge in insect venom-allergic patients after stopping venom immunotherapy. J Allergy Clin Immunol 1991;87:699-702.
- Schiavino D, Nucera E, Pollastrini E, De Pasquale T, Buonomo A, Bartolozzi F, et al. Specific ultrarush desensitization in hymenoptera venom-allergic patients. Ann Allergy Asthma Immunol 2004;92:409-13.
- Szymanski W, Chyrek-Borowska S. Humoral immunological response in patients with venom allergy during specific immunotherapy. Rocz Akad Med Bialymst 1995;40:376-82.
- Carballada F, Martin S, Boquete M. High efficacy and absence of severe systemic reactions after venom immunotherapy. J Investig Allergol Clin Immunol 2003;13:43-9.
- Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of hymenoptera venom hypersensitivity: a meta-analysis. Clin Ther 2000;22:351-8.
- Watanabe AS, Fonseca LA, Galvao CE, Kalil J, Castro FF. Specific immunotherapy using Hymenoptera venom: systematic review. Sao Paulo Med J 2010;128:30-7.
- Graft DF, Schuberth KC, Kagey-Sobotka A, Kwiterovich KA, Niv Y, Lichtenstein LM, et al. The development of negative skin tests in children treated with venom immunotherapy. J Allergy Clin Immunol 1984;73:61-8.
- Müller U, Thurnheer U, Patrizzi R, Spiess J, Hoigne R. Immunotherapy in bee sting hypersensitivity. Bee venom versus wholebody extract. Allergy 1979;34:369-78.
- Schuberth KC, Lichtenstein LM, Kagey-Sobotka A, Szklo M, Kwiterovich KA, Valentine MD. Epidemiologic study of insect allergy in children. II. Effect of accidental stings in allergic children. J Pediatr 1983;102:361-5.
- Tsicopoulos A, Tonnel AB, Wallaert B, Joseph M, Ameisen JC, Ramon P, et al. Decrease of IgE-dependent platelet activation in Hymenoptera hypersensitivity after specific rush desensitization. Clin Exp Immunol 1988;71:433-8.
- Wyss M, Scheitlin T, Stadler BM, Wuthrich B. Immunotherapy with aluminum hydroxide adsorbed insect venom extracts (Alutard SQ): immunologic and clinical results of a prospective study over 3 years. Allergy 1993;48:81-6.
- Yunginger JW, Paull BR, Jones RT, Santrach PJ. Rush venom immunotherapy program for honeybee sting sensitivity. J Allergy Clin Immunol 1979;63:340-7.
- Brown SG, Wiese MD, Blackman KE, Heddle RJ. Ant venom immunotherapy: a double-blind, placebo-controlled, crossover trial. Lancet 2003;361:1001-6.
- Valentine MD, Schuberth KC, Kagey-Sobotka A, Graft DF, Kwiterovich KA, Szklo M, et al. The value of immunotherapy with venom in children with allergy to insect stings. N Engl J Med 1990;323:1601-3.
- Golden DBK, Kelly D, Hamilton RG, Craig TJ. Venom immunotherapy reduces large local reactions to insect stings. J Allergy Clin Immunol 2009;123:1371-5.
- Elberink HO, Monchy JD, Guyatt G, Dubois A. Venom immunotherapy (VIT) improves health-related quality of life (HROL) in patients with allergic reactions following yellow-jacket stings – extended observations. J Allergy Clin Immunol 2001;107.
- Oude Elberink JNG, De Monchy JGR, Van Der Heide S, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol 2002;110:174-82.
- Oude Elberink JNG, van der Heide S, Guyatt GH, Dubois AEJ. Analysis of the burden of treatment in patients receiving an EpiPen for yellow jacket anaphylaxis. J Allergy Clin Immunol 2006;118:699-704.
- Oude Elberink H. Efficacy of yellow jacket immunotherapy during and after stopping venom immunotherapy (VIT) after 1–24 years: followup of 499 patients. Allergy 2009;64.
- Severino MG, Cortellini G, Bonadonna P, Francescato E, Panzini I, Macchia D, et al. Sublingual immunotherapy for large local reactions caused by honeybee sting: a double-blind, placebo-controlled trial. J Allergy Clin Immunol 2008;122:44-8.
- Oude Elberink JNG, Van Der Heide S, Guyatt GH, Dubois AEJ. Immunotherapy improves health-related quality of life of adult patients with dermal reactions following yellow jacket stings. Clin Exp Allergy 2009;39:883-9.
- Song F. Checklist for quality assessment of published reviews. York: NHS Centre for Reviews and Dissemination, University of York; 1994.
- Oude Elberink J, de Monchy J, Golden D, Brouwer J, Guyatt G, Dubois A. Development and validation of a health-related quality-of-life questionnaire in patients with yellow jacket allergy. J Allergy Clin Immunol 2002;109:162-70.
- Bernstein JA, Kagen SL, Bernstein DI, Bernstein IL. Rapid venom immunotherapy is safe for routine use in the treatment of patients with Hymenoptera anaphylaxis. Ann Allergy 1994;73:423-8.
- Shaker MS. An economic evaluation of prophylactic self-injectable epinephrine to prevent fatalities in children with mild venom anaphylaxis. Ann Allergy Asthma Immunol 2007;99:424-8.
- Brown KF, Shaker MS, Jenkins PC, Verdi MS. A cost-effectiveness analysis of venom desensitization in children treated for cure and risk-reduction. J Allergy Clinical Immunol 2006;117.
- Department of Health . NHS Reference Costs (2009–2010) 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed June 2011).
- Curtis L. Unit costs of health and social care 2009. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2007. www.bnf.org/bnf/ (accessed June 2011).
- Office for National Statistics . Age-Specific Death Rates: By Sex, 2001. Regional Trends 2001. www.statistics.gov.uk/STATBASE/ssdataset.asp?vlnk=7673 (accessed June 2011).
- Bonifazi F, Jutel M, Bilo BM, Birnbaum J, Müller U. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy 2005;60:1459-70.
- Haye R, Dosen LK. Insect sting allergy. A study from 1980 to 2003 of patients who started treatment with venom immunotherapy between 1980 and 1998. Clin Mol Allergy 2005;3.
- Roesch A, Boerzsoenyi J, Babilas P, Landthaler M, Szeimies RM. Outcome survey of insect venom allergic patients with venom immunotherapy in a rural population. J Dtsch Dermatol Ges 2008;6:292-7.
- Reisman RE. Natural history of insect sting allergy: relationship of severity of symptoms of initial sting anaphylaxis to re-sting reactions. J Allergy Clin Immunol 1992;90:335-9.
- Sheikh A, Shehata YA, Brown SGA, Simons FER. Adrenaline for the Treatment of Anaphylaxis: Cochrane Systematic Review. Denmark: Cochrane Collaboration 2009. http://ovidsp.ovid.com/ovidweb.cgi?T=JS%26PAGE=reference%26D=medl%26NEWS=N%26AN=19178399 (accessed June 2011).
- Sheikh A, Ten Broek V, Brown SGA, Simons FER. H1-Antihistamines for the Treatment of Anaphylaxis: Cochrane Systematic Review. Denmark: Cochrane Collaboration 2007. http://ovidsp.ovid.com/ovidweb.cgi?T=JS%26PAGE=reference%26D=medl%26NEWS=N%26AN=17620060 (accessed June 2011).
- Pumphrey R, Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction?. Curr Opin Allergy Clin Immunol 2004;4:285-90.
- National Health Service . Hospital Episode Statistics 2010. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937%26categoryID=245 (accessed June 2011).
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Alessandrini AE, Berra D, Rizzini FL, Mauro M, Melchiorre A, Rossi F, et al. Flexible approaches in the design of subcutaneous immunotherapy protocols for Hymenoptera venom allergy. Ann Allergy Asthma Immunol 2006;97:92-7.
- Bilo B, Severino M, Cilia M, Pio A, Casino G, Campodonico P, et al. Safety and tolerability of venom immunotherapy with purified extracts in comparison with nonpurified products. A randomised controlled multicentre trial in 94 patients. Allergy 2009;64:341-2.
- Bilo MB, Severino M, Cilia M, Pio A, Casino G, Ferrarini E, et al. The VISYT trial: venom immunotherapy safety and tolerability with purified vs nonpurified extracts. Ann Allergy Asthma Immunol 2009;103:57-61.
- Birnbaum J, Charpin D, Vervloet D. Rapid hymenoptera venom immunotherapy: comparative safety of three protocols. Clin Exp Allergy 1993;23:226-30.
- Bousquet J, Fontez A, Aznar R. Combination of passive and active immunization in honeybee venom immunotherapy. J Allergy Clin Immunol 1987;79:947-54.
- Brehler R, Wolf H, Kutting B, Schnitker J, Luger T. Safety of a two-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injections. J Allergy Clin Immunol 2000;105:1231-5.
- Clayton WF, Reisman RE, Mueller U, Arbesman CE. Modified rapid venom desensitization. Clin Allergy 1983;13:123-9.
- Glerant JC, Martinez P, Guillaume C, Jounieaux V. Comparison of 2 maintenance doses (100 mug vs 200 mug) in Hymenoptera venom immunotherapy: influence of the maintenance close on the immunologic response. Ann Allergy Asthma Immunol 2005;94:451-6.
- Hafner T, DuBuske L, Kosnik M. Long-term efficacy of venom immunotherapy. Ann Allergy Asthma Immunol 2008;100:162-5.
- Kranzelbinder B, Schuster C, Aberer W, Sturm G. Hymenoptera venom immunotherapy: comparison of different updosing regimes regarding side effects and efficacy. Allergy 2009;64.
- Malling HJ, Djurup R, Sondergaard I, Weeke B. Clustered immunotherapy with yellow jacket venom. Evaluation of the influence of time interval on in vivo and in vitro parameters. Allergy 1985;40:373-83.
- Quercia O, Emiliani F, Pecora S, Burastero SE, Stefanini GF. Efficacy, safety, and modulation of immunologic markers by immunotherapy with honeybee venom: comparison of standardized quality depot versus aqueous extract. Allergy Asthma Proc 2006;27:151-8.
- Reisman RE, Lantner R. Further observations of stopping venom immunotherapy: comparison of patients stopped because of a fall in serum venom-specific IgE to insignificant levels with patients stopped prematurely by self-choice. J Allergy Clin Immunol 1989;83:1049-54.
- Reisman RE, Dvorin DJ, Randolph CC, Georgitis JW. Stinging insect allergy: natural history and modification with venom immunotherapy. J Allergy Clin Immunol 1985;75:735-40.
- Rerinck HC, Przybilla B, Ruff F. Venom immunotherapy (VIT) in patients with systemic mastocytosis (SM) and Hymenoptera venom anaphylaxis (HVA): safety and efficacy of different maintenance doses. J Allergy Clin Immunol 2009;123.
- Rueff F, Wolf H, Schnitker J, Ring J, Przybilla B. Specific immunotherapy in honeybee venom allergy: a comparative study using aqueous and aluminium hydroxide adsorbed preparations. Allergy 2004;59:589-95.
- Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. N Engl J Med 2004;351:668-74.
- Lui CL, Heddle RJ, Kupa A, Coates T, Roberts-Thomas PJ. Bee venom hypersensitivity and its management: patients perception of venom desensitisation. Asian Pac J Allergy 1995;13:95-100.
- Oude Elberink J. Venom immunotherapy (VIT): clinical efficacy and improvement in quality of life. Drugs Today 2008;44:43-5.
- Smith PL, Kagey-Sobotka A, Bleecker ER, Traystman R, Kaplan AP, Gralnick H, et al. Physiologic manifestations of human anaphylaxis. J Clin Invest 1980;66:1072-80.
- Cichocka-Jarosz E, Tobiasz-Adamczyk B, Brzyski P, Lis G, Jedynak U, Pietrzyk J, et al. Health related quality of life (HRQoL) in Polish children treated with specific venom immunotherapy (VIT): a multicenter study. Allergy 2009;64.
- Confino-Cohen R, Melamed S, Goldberg A. Debilitating beliefs, emotional distress and quality of life in patients given immunotherapy for insect sting allergy. Clin Exp Allergy 1999;29:1626-31.
- Confino-Cohen R, Melamed S, Goldberg A. Debilitating beliefs and emotional distress in patients given immunotherapy for insect sting allergy: a prospective study. Allergy Asthma Proc 2009;30:546-51.
- Kahan E, Ben-Moshe R, Derazne E, Tamir R. The impact of Hymenoptera venom allergy on occupational activities. Occup Med 1997;47:273-6.
- Koutsostathis N, Vovolis V, Poulios G, Sifnaios E, Keratsas S, Mikos N. Factors associated to proper technique and carrying compliance with self-injectable adrenaline in insect venom allergic patients and its effect on patients’ quality of life. Allergy 2009;64:34-5.
- Roberts-Thomson PJ, Harvey P, Sperber S, Kupa A, Heddle RJ. Bee sting anaphylaxis in an urban population of South Australia. Asian Pac J Allergy 1985;3:161-4.
Appendix 1 Literature search strategies
| Searches | Results | |
|---|---|---|
| 1 | exp wasp/or exp bee/or exp hymenoptera/or exp bumblebee/or exp honeybee/or exp orchid bee/or exp stingless bee/ | 13,498 |
| 2 | (wasp$or bees or honeybee$or bumblebee$or orchid bee$or yellow hornet$or yellow jacket$or white hornet$or poliste$).tw. | 9959 |
| 3 | exp hymenoptera venom/or exp bee sting/or exp bee venom/or exp wasp venom/ | 3382 |
| 4 | ((wasp$or bees) adj (venom$or sting$or hypersensitivit$or allerg$or anaphyla$or systemic reaction$)).tw. | 818 |
| 5 | (pharmalgen or venom immunotherapy).af. | 692 |
| 6 | exp pharmalgen/ | 84 |
| 7 | or/1–4 | 19,103 |
| 8 | or/5–6 | 692 |
| 9 | 7 and 8 | 518 |
| 10 | limit 9 to english language | 435 |
| Searches | Results | |
|---|---|---|
| 1 | exp Wasps/or exp Bees/or exp Hymenoptera/ | 12,580 |
| 2 | (wasp$or bees or honeybee$or bumblebee$or orchid bee$or yellow hornet$or yellow jacket$or white hornet$or poliste$).tw. | 8437 |
| 3 | exp Wasp Venoms/or exp Bee Venoms/ | 5214 |
| 4 | ((wasp$or bees) adj (venom$or sting$or hypersensitivit$or allerg$or anaphyla$or systemic reaction$)).tw. | 662 |
| 5 | exp “Insect Bites and Stings”/ | 4448 |
| 6 | or/1–5 | 22,197 |
| 7 | (pharmalgen or immunotherapy).af. | 52,392 |
| 8 | exp Desensitization, Immunologic/or *Immunotherapy/or Anaphylaxis/th | 19,439 |
| 9 | 7 or 8 | 57,963 |
| 10 | 6 and 9 | 1130 |
| 11 | limit 10 to english language | 906 |
| Searches | Results | |
|---|---|---|
| 1 | MeSH descriptor Wasps explode all trees | 7 |
| 2 | MeSH descriptor Bees explode all trees | 13 |
| 3 | MeSH descriptor Wasp Venoms explode all trees | 11 |
| 4 | MeSH descriptor Bee Venoms explode all trees | 28 |
| 5 | wasp* or bees | 231 |
| 6 | (#1 OR #2 OR #3 OR #4 OR #5) | 231 |
Appendix 2 Excluded studies
| Comparing active treatments, none of which were Pharmalgen |
|---|
| Alessandrini AE, Berra D, Rizzini FL, Mauro M, Melchiorre A, Rossi F, et al. Flexible approaches in the design of subcutaneous immunotherapy protocols for Hymenoptera venom allergy. Ann Allergy Asthma Immunol 2006;97:92–7106 |
| Bilo B, Severino M, Cilia M, Pio A, Casino G, Campodonico P, et al. Safety and tolerability of venom immunotherapy with purified extracts in comparison with nonpurified products. A randomised controlled multicentre trial in 94 patients. Allergy 2009;64:341–2107 |
| Bilo MB, Severino M, Cilia M, Pio A, Casino G, Ferrarini E, et al. The VISYT trial: venom immunotherapy safety and tolerability with purified vs nonpurified extracts. Ann Allergy Asthma Immunol 2009;103:57–61108 |
| Birnbaum J, Charpin D, Vervloet D. Rapid hymenoptera venom immunotherapy: comparative safety of three protocols. Clin Exp Allergy 1993;23:226–30109 |
| Bousquet J, Fontez A, Aznar R. Combination of passive and active immunization in honeybee venom immunotherapy. J Allergy Clin Immunol 1987;79:947–54110 |
| Brehler R, Wolf H, Kutting B, Schnitker J, Luger T. Safety of a two-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injections. J Allergy Clin Immunol 2000;105:1231–5111 |
| Clayton WF, Reisman RE, Mueller U, Arbesman CE. Modified rapid venom desensitization. Clin Allergy 1983;13:123–9112 |
| Elberink HO, Monchy JD, Guyatt G, Dubois A. Venom immunotherapy (VIT) improves health-related quality of life (HROL) in patients with allergic reactions following yellow-jacket stings – extended observations. J Allergy Clin Immunol 2001;107:S22282 |
| Glerant JC, Martinez P, Guillaume C, Jounieaux V. Comparison of 2 maintenance doses (100 mug vs 200 mug) in Hymenoptera venom immunotherapy: influence of the maintenance close on the immunologic response. Ann Allergy Asthma Immunol 2005;94:451–6113 |
| Hafner T, DuBuske L, Kosnik M. Long-term efficacy of venom immunotherapy. Ann Allergy Asthma Immunol 2008;100:162–5114 |
| Kranzelbinder B, Schuster C, Aberer W, Sturm G. Hymenoptera venom immunotherapy: comparison of different updosing regimes regarding side effects and efficacy. Allergy 2009;64:457115 |
| Malling HJ, Djurup R, Sondergaard I, Weeke B. Clustered immunotherapy with yellow jacket venom. Evaluation of the influence of time interval on in vivo and in vitro parameters. Allergy 1985;40:373–83116 |
| Quercia O, Emiliani F, Pecora S, Burastero SE, Stefanini GF. Efficacy, safety, and modulation of immunologic markers by immunotherapy with honeybee venom: comparison of standardized quality depot versus aqueous extract. Allergy Asthma Proc 2006;27:151–8117 |
| Reisman RE, Lantner R. Further observations of stopping venom immunotherapy: comparison of patients stopped because of a fall in serum venom-specific IgE to insignificant levels with patients stopped prematurely by self-choice. J Allergy Clin Immunol 1989;83:1049–54118 |
| Reisman RE, Dvorin DJ, Randolph CC, Georgitis JW. Stinging insect allergy: natural history and modification with venom immunotherapy. J Allergy Clin Immunol 1985;75:735–40119 |
| Rerinck HC, Przybilla B, Ruff F. Venom immunotherapy (VIT) in patients with systemic mastocytosis (SM) and Hymenoptera venom anaphylaxis (HVA): safety and efficacy of different maintenance doses. J Allergy Clin Immunol 2009;123(Suppl. 2):242120 |
| Rueff F, Wolf H, Schnitker J, Ring J, Przybilla B. Specific immunotherapy in honeybee venom allergy: a comparative study using aqueous and aluminium hydroxide adsorbed preparations. Allergy 2004;59:589–95121 |
| Comparing VIT with placebo, WBE or no treatment but not Pharmalgen |
| Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. N Engl J Med 2004;351:668–74122 |
| Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med 1978;299:157–6128 |
| Lui CL, Heddle RJ, Kupa A, Coates T, Roberts-Thomas PJ. Bee venom hypersensitivity and its management: patients perception of venom desensitisation. Asian Pac J Allergy 1995;13:95–100123 |
| Müller U, Thurnheer U, Patrizzi R, Spiess J, Hoigne R. Immunotherapy in bee sting hypersensitivity. Bee venom versus wholebody extract. Allergy 1979;34:369–7874 |
| Oude Elberink J. Venom immunotherapy (VIT): clinical efficacy and improvement in quality of life. Drugs Today 2008;44:43–5124 |
| Oude Elberink JNG, De Monchy JGR, Van Der Heide S, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol 2002;110:174–8283 |
| Oude Elberink JNG, van der Heide S, Guyatt GH, Dubois AEJ. Analysis of the burden of treatment in patients receiving an EpiPen for yellow jacket anaphylaxis. J Allergy Clin Immunol 2006;118:699–70484 |
| Oude Elberink JNG, Van Der Heide S, Guyatt GH, Dubois AEJ. Immunotherapy improves health-related quality of life of adult patients with dermal reactions following yellow jacket stings. Clin Exp Allergy 2009;39:883–987 |
| Schuberth KC, Lichtenstein LM, Kagey-Sobotka A, Szklo M, Kwiterovich KA, Valentine MD. Epidemiologic study of insect allergy in children. II. Effect of accidental stings in allergic children. J Pediatr 1983;102:361–575 |
| Smith PL, Kagey-Sobotka A, Bleecker ER, Traystman R, Kaplan AP, Gralnick H, et al. Physiologic manifestations of human anaphylaxis. J Clin Invest 1980;66:1072–80125 |
| Valentine MD, Schuberth KC, Kagey-Sobotka A, Graft DF, Kwiterovich KA, Szklo M, et al. The value of immunotherapy with venom in children with allergy to insect stings. N Engl J Med 1990;323:1601–380 |
| Economic papers but not Pharmalgen |
| Bernstein JA, Kagen SL, Bernstein DI, Bernstein IL. Rapid venom immunotherapy is safe for routine use in the treatment of patients with Hymenoptera anaphylaxis. Ann Allergy 1994;73:423–890 |
| Brown KF, Shaker MS, Jenkins PC, Verdi MS. A cost-effectiveness analysis of venom desensitization in children treated for cure and risk-reduction. J Allergy Clinical Immunol 2006;117:S30992 |
| Shaker MS. An economic evaluation of prophylactic self-injectable epinephrine to prevent fatalities in children with mild venom anaphylaxis. Ann Allergy Asthma Immunol 2007;99:424–891 |
| Details of QoL but not RCTs |
| Cichocka-Jarosz E, Tobiasz-Adamczyk B, Brzyski P, Lis G, Jedynak U, Pietrzyk J, et al. Health related quality of life (HRQoL) in Polish children treated with specific venom immunotherapy (VIT): a multicenter study. Allergy 2009;64:343126 |
| Confino-Cohen R, Melamed S, Goldberg A. Debilitating beliefs, emotional distress and quality of life in patients given immunotherapy for insect sting allergy. Clin Exp Allergy 1999;29:1626–31127 |
| Confino-Cohen R, Melamed S, Goldberg A. Debilitating beliefs and emotional distress in patients given immunotherapy for insect sting allergy: a prospective study. Allergy Asthma Proc 2009;30:546–51128 |
| Kahan E, Ben-Moshe R, Derazne E, Tamir R. The impact of Hymenoptera venom allergy on occupational activities. Occup Med 1997;47:273–6129 |
| Koutsostathis N, Vovolis V, Poulios G, Sifnaios E, Keratsas S, Mikos N. Factors associated to proper technique and carrying compliance with self-injectable adrenaline in insect venom allergic patients and its effect on patients’ quality of life. Allergy 2009;64:34–5130 |
| Roberts-Thomson PJ, Harvey P, Sperber S, Kupa A, Heddle RJ. Bee sting anaphylaxis in an urban population of South Australia. Asian Pac J Allergy 1985;3:161–4131 |
| Roesch A, Boerzsoenyi J, Babilas P, Landthaler M, Szeimies RM. [Outcome survey of insect venom allergic patients with venom immunotherapy in a rural population.] J Dtsch Dermatol Ges 2008;6:292–799 |
Appendix 3 Included studies
| RCTs | |
|---|---|
| 1 | Golden DBK, Valentine MD, Kagey-Sobotka A, Lichtenstein LM. Regimens of hymenoptera venom immunotherapy. Ann Intern Med 1980;92:620–444 |
| 2 | Golden D, Valentine MD, Sobotka AK, Lichtenstein LM. Regimens of hymenoptera venom immunotherapy. J Allergy Clin Immunol 1979;63:18041 |
| 3 | Mosbech H, Malling HJ, Biering I. Immunotherapy with yellow jacket venom. A comparative study including three different extracts, one adsorbed to aluminium hydroxide and two unmodified. Allergy 1986;41:95–10345 |
| 4 | Müller U, Rabson AR, Bischof M, Lomnitzer R, Dreborg S, Lanner A. A double-blind study comparing monomethoxy polyethylene glycol-modified honeybee venom and unmodified honeybee venom for immunotherapy. I. Clinical results. J Allergy Clin Immunol 1987;80:252–6147 |
| 5 | Müller U, Lanner A, Schmid P, Bischof M, Dreborg S, Hoigné R. A double blind study on immunotherapy with chemically modified honey bee venom: monomethoxy polyethylene glycol-coupled versus crude honey bee venom. Int Arch Allergy Appl Immunol 1985;77:201–346 |
| 6 | Quercia O, Rafanelli S, Puccinelli P, Stefanini GF. The safety of cluster immunotherapy with aluminium hydroxide-adsorbed honey bee venom extract. J Investig Allergol Clin Immunol 2001;11:27–3348 |
| Non-RCTs | |
| 7 | Cadario G, Marengo F, Ranghino E, Rossi R, Gatti B, Cantone R, et al. Higher frequency of early local side effects with aqueous versus depot immunotherapy for Hymenoptera venom allergy. J Investig Allergol Clin Immunol 2004;14:127–3340 |
| 8 | Golden DBK, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Prolonged maintenance interval in hymenoptera venom immunotherapy. J Allergy Clin Immunol 1981;67:482–442 |
| 9 | Golden DBK, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Dose dependence of hymenoptera venom immunotherapy. J Allergy Clin Immunol 1981;67:370–443 |
| 10 | Patriarca G, Nucera E, Roncallo C, Aruanno A, Lombardo C, Decinti M, et al. Sublingual desensitization in patients with wasp venom allergy: preliminary results. Int J Immunopathol Pharmacol 2008;21:669–7732 |
| 11 | Thurnheer U, Müller U, Stoller R. Venom immunotherapy in hymenoptera sting allergy. Comparison of rush and conventional hyposensitization and observations during long-term treatment. Allergy 1983;38:465–7549 |
Appendix 4 Quality assessment
| Checklist item | Cadario 200440 | Golden 198044 | Golden 198143 | Golden 198142 | Mosbech 198645 | M✓ller 198747 | Patriarca 200832 | Quercia 200148 | Thurnheer 198349 |
|---|---|---|---|---|---|---|---|---|---|
| Randomisation | |||||||||
| Was the randomisation method adequate? | NA | NS | NA | NA | NS | NS | NA | NS | NA |
| Was the allocation of treatment adequately concealed? | NA | NS | NA | NA | NS | NS | NA | NS | NA |
| Was the number of participants randomised stated? | NA | ✓ | NA | NA | ✓ | ✓ | NA | ✓ | NA |
| Baseline comparability | |||||||||
| Were details of baseline comparability presented?a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were the groups similar for prognostic factors? | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Eligibility criteria and co-interventions | |||||||||
| Were the eligibility criteria for study entry specified? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were any co-interventions identified? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blinding | |||||||||
| Were outcome assessors blinded to the treatment allocation? | ✗ | ✗ | ✗ | ✗ | ✗ | NSa | ✗ | ✗ | ✗ |
| Were administrators blinded to the treatment allocation? | ✗ | ✗ | ✗ | ✗ | ✗ | NSa | ✗ | ✗ | ✗ |
| Were people blinded to the treatment allocation? | ✗ | ✗ | ✗ | ✗ | ✗ | NSa | ✗ | ✗ | ✗ |
| Was the blinding procedure assessed? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Withdrawals | |||||||||
| Any unexpected imbalances in dropouts between groups? Were they explained or adjusted for? | ✗, NA | ✓, ✗ | ✓, | NS, NS | ✓, ✗ | ✓, ✗ | ✓ | ✗, NA | ✗, NA |
| Were ≥ 80% of people included in the final analysis? | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Were reasons for withdrawals stated? | NA | ✓ | ✓ | ✗/✓ | ✓ | ✓ | ✓ | NA | ✓ |
| Was an ITT analysis included? Was this appropriate? Were appropriate methods used to account for missing data? | NA | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | NA | ✗ |
| Outcomes | |||||||||
| Evidence of more outcomes measured than reported? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
Appendix 5 Economic survey results
| Questions | Response |
|---|---|
| Type of clinical unit |
14 from a unit in an acute hospital 1 from a unit in a community hospital 1 unit in a specialist hospital, no acute service |
| Type of individual receiving VIT in unit |
12 units provide VIT only to adults 2 units provide VIT only to children 2 units provide VIT to children and adults |
| No. of new venom-allergic individuals in a typical year |
Wasp venom: 9.37 Bee venom: 3 Both wasp and bee venom: 0.87 Note that these are simple averages from 15 responses (one clinician did not fill in this question). No weighting was taken into account because we did not ask for the total number of individuals in each clinical unit. One provided a range of 5 to 10, and the median 7.5 was used for the average calculation |
| Age proportions of new individuals with severe systemic reaction to bee/wasp venom in a typical year |
Under 20 years: 15% 20–39 years: 30% 40+ years: 54% These are simple averages without weighting |
| Treatment options prescribed to new patients with severe bee/wasp bee venom allergy | The majority of clinics provide VIT + HDA + AAI; four clinics provide VIT + AAI and 1 clinic uses VIT monotherapy only. For individuals not able to receive VIT, 10 clinics use HDA + AAI as an alternative treatment option. Very small numbers of clinics prescribe either HDA only or AAI only |
| Antihistamines prescribed (dosage) | Acrivastine (16 mg), acrivastine (8 mg), cetirizine (10–20 mg), fexofenadine (180 mg), piriton, loratadine (10–20 mg), chlorphenamine (8 mg) |
| VIT for individuals with both bee and wasp allergy |
5 clinics provide VIT for the more severe allergy 3 clinics provide VIT for both bee and wasp allergy |
| Advice given to people undergoing VIT should they experience re-sting |
3 clinics advise use of HDA followed by AAI (if systemic reaction occurs); also advise visit to A&E 4 clinics advise use of HDA and administration of AAI if individual has difficulty breathing or feels faint 1 clinic advises use of HDA + steroid + AAI if systemic reaction occurs 1 clinic advises HDA only 1 clinic advises removal of sting and use of HDA + AAI |
| Most common ARs during VIT |
Local reactions (mainly swelling and itching) stated by all 15 clinics Other common ARs include urticaria and fatigue. Less common reactions include pain, wheezing, local redness, Arthus-type reaction, anxiety tachycardia, headache, anaphylaxis and reduction in peak expiratory flow rate |
Appendix 6 Data abstraction tables
| Study ID | Intervention | Updosing: doses and frequency |
|---|---|---|
| Cadario 200440 | Aqueous induction and aqueous maintenance |
12 doses in 8 visits (weekly), total 8 weeks Week 1: 0.01 μg, 0.1 μg (30 minutes between); week 2: 1 μg, 2 μg (30 minutes between); week 3: 4 μg, 8 μg (60 minutes between); week 4: 10 μg, 20 μg (60 minutes between); week 5: 40 μg; week 6: 60 μg; week 7: 80 μg; week 8: 100 μg |
| Depot induction and depot maintenance |
15 doses in 15 visits (weekly), total 15 weeks Week 1: 0.02 μg; week 2: 0.04 μg; week 3: 0.08 μg; week 4: 0.2 μg; week 5: 0.4 μg; week 6: 0.8 μg; week 7: 2 μg; week 8: 4 μg; week 9: 8 μg; week 10: 10 μg; week 11: 20 μg; week 12: 40 μg; week 13: 60 μg; week 14: 80 μg; week 15: 100 μg |
|
| Golden 198041,44 | Slow therapy |
14 doses in 14 visits (weekly), total 14 weeks Week 1: 0.01 μg; week 2: 0.03 μg; week 3: 0.1 μg; week 4: 0.25 μg; week 5: 1.0 μg; week 6: 2.5 μg; week 7: 5.0 μg; week 8: 10.0 μg; week 9: 20.0 μg; week 10: 30.0 μg; week 11: 40.0 μg; week 12: 60.0 μg; week 13: 80.0 μg; week 14: 100.0 μg |
| Step therapy |
10 doses in 8 visits, total 11 weeks Initial: 1, 5, 10 μg (every 30 minutes); week 1: 25 μg; week 3: 25 μg; week 5: 25 μg; week 6: 50 μg; week 8: 50 μg; week 10: 50 μg; week 11: 100 μg |
|
| Rush therapy |
6 doses in 4 visits (every 2 weeks), total 6 weeks Initial: 1, 5, 10 μg (every 30 minutes); week 2: 30 μg; week 4: 60 μg; week 6: 100 μg |
|
| Golden 198143 | 50 μg maintenance |
6 doses in 6 visits (weekly), total 6 weeks 1 μg on first day and achieving 50-μg dose after 6 weeks |
| 100 μg maintenance44 |
6 doses in 4 visits every 2 weeks, total 6 weeks Designed to achieve 100-μg dose within 6 weeks |
|
| 100 μg maintenance28 |
12? doses in 9? visits, total 4 weeks Designed to achieve 100-μg dose within 4 weeks |
|
| Golden 198142 | 4-weekly maintenance a | NA |
| 6-weekly maintenance | NA | |
| 4-weekly maintenance b | NA | |
| Müller 198746,47 | HBV |
9 doses in 7 visits (weekly), total 6 weeks Week 0: 0.1, 1.0, 3.0 μg; week 1: 5 μg; week 2: 10 μg; week 3: 20 μg; week 4: 40 μg; week 5: 65 μg; week 6: 100 μg |
| Monomethoxy polyethylene glycol-coupled HBV |
7 doses in 5 visits (weekly), total 4 weeks Week 0: 0.5, 5.0, 10.0 μg; week 1: 30 μg; week 2: 60 μg; week 3: 100 μg; week 4: 200 μg |
|
| Mosbech 198645 | Pharmalgen |
25 doses in 13 visits (twice weekly), total 13 weeks > 1 injection per visit initially until local swelling exceeded 5 cm in diameter 0.2, 0.4, 0.8 ml at 0.001 μg/ml concentration; 0.2, 0.4, 0.8 ml at 0.01 μg/ml concentration; 0.2, 0.4, 0.8 ml at 0.1 μg/ml concentration; 0.2, 0.4, 0.8 ml at 1 μg/ml concentration; 0.2, 0.3, 0.4, 0.6, 0.8 ml at 10 μg/ml concentration; 0.1, 0.15, 0.2, 0.3, 0.4, 0.6, 0.8, 1 ml at 100 μg/ml |
| Alutard |
19 doses in 19 visits (weekly), total 19 weeks Once a week: 0.02, 0.04, 0.08, 0.2, 0.4, 0.8, 2.0, 3.0, 4.0, 6.0, 8, 10, 15, 20, 30, 40, 60, 80, 100 μg |
|
| Aquagen |
25 doses in 13 visits (twice weekly), total 13 weeks > 1 injection per visit initially until local swelling exceeded 5 cm in diameter 0.2, 0.4, 0.8 ml at 0.001 μg/ml concentration; 0.2, 0.4, 0.8 ml at 0.01 μg/ml concentration; 0.2, 0.4, 0.8 ml at 0.1 μg/ml concentration; 0.2, 0.4, 0.8 ml at 1 μg/ml concentration; 0.2, 0.3, 0.4, 0.6, 0.8 ml at 10 μg/ml concentration; 0.1, 0.15, 0.2, 0.3, 0.4, 0.6, 0.8, 1 ml at 100 μg/ml |
|
| Patriarca 200832 | Ultra-rush SCIT |
6 doses in 1 visit (every 30 minutes), total 3 hours Day 1: 0.1, 1, 10, 20, 30, 40 μg |
| Ultra-rush SLIT |
10 doses in 1 visit (every 20 minutes), total 3 hours Dilution 1 : 10,000, 1 drop; dilution 1 : 1000, 1 drop; dilution 1 : 100, 1 drop; dilution 1 : 10, 1 drop; pure, 1 drop; pure, 2 drops; pure, 4 drops; pure, 6 drops; pure, 7 drops; pure, 10 drops |
|
| Quercia 200148 | Pharmalgen: cluster |
12 doses in 6 visits (every week), total 6 weeks Week 1: 5 doses 0.01, 0.1, 1.0, 3.0, 6.0 μg (hourly); week 2: 1 dose 20.0 μg; week 3: 1 dose 40.0 μg; week 4: 1 dose 60.0 μg; week 5: 2 doses 40.0, 40.0 μg; week 6: 2 doses 50.0, 50.0 μg |
| Pharmalgen: rush |
13 doses in 4 visits (every day), total 4 days Day 1: 4 doses 0.01, 0.1, 1.0, 2.0 μg (hourly); day 2: 4 doses 4.0, 6.0, 10.0, 20.0 μg (hourly then fourth 30 minutes); day 3: 2 doses 40.0, 40.0 μg (hourly); day 4: 3 doses 60.0, 50.0, 50.0 μg (hourly) |
|
| Depot cluster |
12 doses in 5 visits (weekly), total 5 weeks Week 1: 4 doses 0.03, 0.1, 0.3, 1.0 μg (hourly); week 2: 2 doses 2.0, 4.0 μg (hourly); week 3: 2 doses 10.0, 20.0 μg (hourly); week 4: 2 doses 40.0, 40.0 μg (hourly); week 5: 2 doses 50.0, 50.0 μg (hourly) |
|
| Thurnheer 198349 | Conventional |
24 doses in 10 visits (weekly), total 10 weeks Day 1: 0.1 ml (0.0001 μg/ml), 0.1 ml (0.001 μg/ml), 0.1 ml (0.01 μg/ml); day 8: 0.1 ml (0.1 μg/ml), 0.1 ml (1 μg/ml), 0.2 ml (1 μg/ml); day 15: 0.4 ml, 0.8 ml (1 μg/ml); day 22: 0.1 ml, 0.2 ml (10 μg/ml); day 29: 0.4 ml, 0.8 ml (10 μg/ml); day 36: 0.1 ml, 0.2 ml (100 μg/ml); day 43: 0.3 ml, 0.4 ml (100 μg/ml); day 50: 0.5 ml, 0.6 ml (100 μg/ml); day 57: 0.7 ml, 0.8 ml (100 μg/ml); day 64: 0.9 ml, 1.0 ml (100 μg/ml) |
| Rush |
35 doses in 10 visits (daily), total 10 days Day 1: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (0.0001 μg/ml); day 2: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (0.001 μg/ml); day 3: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (0.01 μg/ml); day 4: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (0.1 μg/ml); day 5: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (1 μg/ml); day 6: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (10 μg/ml); day 7: 0.1 ml, 0.2 ml, 0.4 ml, 0.8 ml (100 μg/ml); day 8: 0.4 ml, 0.5 ml, 0.6 ml (100 μg/ml); day 9: 0.7 ml, 0.8 ml (100 μg/ml); day 10: 0.9 ml, 1.0 ml (100 μg/ml) |
Appendix 7 Project protocol
Glossary
Technical terms and abbreviations are used throughout this report. The meaning is usually clear from the context, but a glossary is provided for the non-specialist reader.
- Anaphylaxis
- A severe type 1 hypersensitivity allergic reaction.
- Aqueous solution
- A solution in which water is the solvent.
- Cost-effectiveness
- Cost-effectiveness has numerous meanings; however, for practical purposes it is usually given to mean that the cost per quality-adjusted life-year gained is below a notional willingness-to-pay threshold.
- Depot
- An injection of a pharmacological agent that releases its active compound in a consistent way over a long period of time.
- Field sting
- A sting occurring accidentally.
- Hymenoptera
- An order of stinging insects that includes bees, wasps and ants.
- Immunoglobulin E
- Class of antibody that plays an important role in allergy.
- Local reactions
- Reactions mediated by allergic mechanisms but that involve only the part of the body in contact with the sting site.
- Sting challenge
- A sting purposefully inflicted in a controlled environment.
- Systemic allergic reactions
- Reactions mediated by allergic mechanisms that spread to other organs in the body.
- Venom immunotherapy
- A type of allergic desensitisation therapy for people who are highly susceptible to Hymenoptera venom.
List of abbreviations
- AAAAI
- American Academy of Allergy, Asthma and Immunology
- AAI
- adrenaline auto-injector
- AG
- Assessment Group
- AR
- adverse reaction
- BOT
- burden of treatment
- CRD
- Centre for Reviews and Dissemination
- EAACI
- European Academy of Allergy and Clinical Immunology
- EQ-5D
- European Quality of Life-5 Dimensions
- FS
- field sting
- HBV
- honey bee venom
- HDA
- high-dose antihistamine
- HES
- hospital episode statistics
- ICER
- incremental cost-effectiveness ratio
- IDT
- intradermal skin testing
- IgE
- immunoglobulin E
- IgG
- immunoglobulin G
- ITT
- intention to treat
- LLR
- large local reaction
- MCMC
- Markov chain Monte Carlo
- MTC
- mixed-treatment comparison
- N/A
- not available
- NA
- not applicable
- NICE
- National Institute for Health and Clinical Excellence
- non-PhVIT
- venom immunotherapy using non-Pharmalgen® products
- NR
- not reported
- PhVIT
- venom immunotherapy using Pharmalgen® products
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RAST
- radioallergosorbent testing
- RCT
- randomised controlled trial
- SC
- sting challenge
- SCIT
- subcutaneous immunotherapy
- SLIT
- sublingual immunotherapy
- SmPC
- summary of product characteristics
- SPT
- skin prick testing
- STAI
- State-Trait Anxiety Inventory
- VIT
- venom immunotherapy
- VQLQ
- Vespid Allergy Quality of Life Questionnaire
- WBE
- whole bee extract
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health