Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/67/01. The protocol was agreed in October 2010. The assessment report began editorial review in March 2011 and was accepted for publication in July 2011. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by McKenna et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background and definition of the decision problem
Description of the technology under assessment
EOS is a biplane X-ray imaging system manufactured by EOS imaging (formerly Biospace Med, Paris, France). It uses slot-scanning technology to produce a high-quality image with less radiation than standard imaging techniques. EOS has been developed for orthopaedic imaging. The quality and nature of the image is similar to computed radiography (CR) and digital radiography (DR), rather than computerised tomography (CT). CT is significantly more sensitive to gradations of tissue density than conventional X-ray imaging techniques. CT therefore produces more detailed images of different body structures, including bones, soft tissues and blood vessels, which are displayed as a series of cross-sectional images, in a variety of planes.
EOS allows the acquisition of images while the patient is in an upright weight-bearing (or seated or squatting) position, and can image the full length of the body (up to 175 cm), removing the need for digital stitching. The system takes approximately 20 seconds for an adult full-body scan and 4–6 seconds to scan the spine, depending on the patient’s height. As with the widely accepted standard position for all spine radiographs, the patient being scanned is also required to remain motionless, with the arms folded at 45 °, and to hold his/her breath during the scan.
EOS takes posteroanterior (PA) and lateral (LAT) images simultaneously, and the digital image is available immediately on a two-dimensional (2D) workstation. A three-dimensional (3D) image can be reconstructed on the sterEOS workstation using the PA and LAT images and a statistical 3D spine model, generated from a database of scoliotic patients. The reconstruction of a 3D image takes 5–10 minutes for each part of the skeleton (e.g. spine or femur). 1
For EOS to be cost-effective, these benefits relating to the nature of the image need to translate into health benefits for patients. For example, the ability to generate a full-body weight-bearing scan should provide more accurate diagnostic information, which might translate into an improved management strategy for a patient and, consequently, into a health benefit. However, the health gains from developments in diagnostic technologies tend to be relatively small in comparison with those associated with new therapeutic interventions.
The acquisition cost of the EOS system in the UK is in the region of £400,000, with an annual maintenance cost of £32,000. The maintenance contract covers all parts except X-ray tubes, which require replacement every 3–5 years at a cost of £25,000, including fitting. 2 In addition to the cost of purchasing and maintaining the equipment, there may be some building costs to provide a suitable location complying with radiation legislation requirements if existing rooms are not available. EOS requires the same room planning and shielding as a general X-ray room and the same radiation protection protocols apply. EOS is not currently in use in the NHS.
Comparators
Currently available imaging technologies that can be used in an upright weight-bearing position include X-ray film, CR and DR, although film has been replaced by CR and DR in standard UK practice. All of these technologies have higher radiation doses than EOS. X-ray film, CR and DR can take images from only one angle at a time, so simultaneous PA and LAT images are not possible and 3D reconstruction cannot be obtained. When a full-body image is required, these conventional X-ray imaging technologies also require adjustment for distortion or digital stitching from multiple images.
The acquisition cost of CR is approximately £95,000, with an annual maintenance cost of approximately £10,000. CR cassettes require replacement every 3–5 years, at a cost of between £150 and £200 [S MacLachlan, Health Protection Agency (HPA), 10 December 2010, personal communication]. The acquisition cost of DR is between approximately £105,000 and £230,000, with an annual maintenance cost of approximately £18,000. Software upgrades to improve the functionality and performance of DR cost approximately £2000 (S MacLachlan, personal communication).
Condition(s) and aetiology(ies)
The indications in which there may be potential benefit associated with EOS are those that require imaging that is weight-bearing, full-body, simultaneous PA and LAT, 3D, and/or where radiation exposure is a concern because of a need for a large number of X-rays. 3 The National Institute for Health and Clinical Excellence (NICE) scope categorises the indications according to the population affected. In children and adolescents, the relevant indications are spinal deformity (principally scoliosis) and leg length discrepancy and alignment. In adults, the relevant indications are spinal deformity, including degenerative scoliosis, progressive kyphosis and osteoporotic fractures, and conditions involving loss of sagittal and coronal balance, including issues relating to hip and knee where full-body or full-leg-length images are currently requested.
The indications defined in the NICE scope were discussed with clinical experts and a list of relevant indications was developed. Table 1 summarises the indications considered in the economic evaluation and their corresponding International Classification of Diseases, 10th Edition (ICD-10) codes.
| Indications to be considered | ICD-10 code |
|---|---|
| Scoliosis | M41 (except M41.4) |
| Congenital | |
| Early-onset idiopathic | |
| Adolescent (or late-onset) idiopathic | |
| Adult | |
| Kyphosis | |
| Congenital | Q76.4 |
| Scheuermann’s disease | M42 |
| Ankylosing spondylitis | M45 |
| Deforming dorsopathies (umbrella term for spinal deformity) | M43 |
| Congenital deformities | |
| Spine | Q67.5, Q76.3, Q77 |
| Lower limbs | Q68, Q72, Q74 |
| Hips | Q65, Q77, Q78 |
Some conditions that were initially considered relevant for the economic evaluation of EOS were subsequently withdrawn from the analysis. These conditions are lordosis, acquired kyphosis, neurofibromatosis, osteoporotic fracture and issues relating to hip and knee replacement where full-body or full-leg-length images are currently requested. Lordosis was not considered as it is very rare on its own. According to clinical experts, lordosis is associated with scoliosis. Thus, the inclusion of scoliosis should also encompass patients with lordosis secondary to scoliosis. Acquired kyphosis and neurofibromatosis were excluded because of high variability in the patient groups and the relatively small numbers of patients requiring surgery. Osteoporotic fracture was not considered as it is usually associated with minor degrees of spinal deformity. This does not generally require surgical treatment. These fractures heal and long-term imaging is not required. Thus, this is not an important indication in the context of this assessment.
Scoliosis
Scoliosis is a 3D deformity of the spine, characterised by a sideways curve of ≥ 10 °. 4 With this curve there is also a change to the normal front to back curves of the spine and some twisting, which distorts the rib cage and may result in a rib hump. Scoliosis can be broadly categorised as congenital, early-onset idiopathic, late-onset idiopathic, adult (including degenerative scoliosis) and neuromuscular, depending on the conditions causing the scoliotic curve and the age at onset. Congenital scoliosis results from anomalies in the formation of the spine in utero. Idiopathic scoliosis, which accounts for 85% of scoliosis cases,5 refers to a scoliotic curve of unknown origin. Idiopathic scoliosis can be classified according to the age of onset: early onset (< 10 years old) or late onset/adolescent (≥ 10 years or older). 6 Adult scoliosis refers to scoliosis occurring in patients > 20 years old (typically > 50 years old), when skeletal growth has ceased. Neuromuscular scoliosis refers to scoliosis resulting from disorders and impairments of the neurological system, such as cerebral palsy, spina bifida and muscular dystrophies. 6
Neuromuscular scoliosis was not included in the economic evaluation. The great majority of patients suffering from neuromuscular scoliosis are wheelchair bound and require a special chair for X-ray imaging. According to clinical experts, these patients would still be imaged with conventional X-ray, even if EOS was available in the UK centres.
The prevalence of scoliosis in the UK is not well documented. However, it has been estimated that adolescent idiopathic scoliosis occurs in 1–3% of children between 10 and 16 years of age in the USA. 7 A UK-based study of prevalence of idiopathic scoliosis in school children aged 6–14 years reported an overall prevalence of 0.5%: 0.1% among children aged 6–8 years, 0.3% among children aged 9–11 years and 1.2% among children aged 12–14 years. 8
The primary age at onset for idiopathic scoliosis is 10–15 years and the prevalence is equal among boys and girls, but the likelihood of the scoliotic curve progressing to a magnitude that requires treatment is eight times higher in girls than in boys. 5 Progression of scoliosis leads to cosmetic deformity, which, in turn, can lead to poorer body image perception and problems in psychological and social development, loss of flexibility, cardiopulmonary problems and pain.
There is currently no good evidence that either bracing or physiotherapy alter the long-term natural history of back shape in adolescent idiopathic scoliosis. The decision to offer surgical treatment will depend upon many factors, including the degree of curvature of the spine (Cobb angle), rate of progression, cosmetic impact and the patient’s age. Although only approximately 10% of children with adolescent idiopathic scoliosis require surgical intervention,9 nearly 95% of children with early-onset idiopathic scoliosis go on to require surgical treatment. 6 Surgery may be delayed until growth of the skeleton is complete or near complete and therefore monitoring can continue for many years.
Kyphosis
Kyphosis is the term describing a curvature of the spine that causes rounding of the back. Kyphosis can result from congenital malformations, degenerative diseases (such as arthritis), osteoporosis with compression fractures of the vertebra, trauma or simply poor posture or the natural ageing process. Only congenital kyphosis, Scheuermann’s disease and ankylosing spondylitis were considered to be relevant for the economic evaluation of EOS because of the nature of the image and the frequency of imaging required for the monitoring of these patients.
Congenital kyphosis results from anomalies in the formation of the spine in utero. Congenital kyphosis is much less common than congenital scoliosis. 10 The clinical presentation of congenital kyphosis is variable; severe cases may be identified at birth, whereas mild cases may not be identified until adolescence. Congenital kyphosis is a progressive disease, which can cause severe deformity and loss of neurological function if the spinal cord becomes compressed over the kyphotic vertebral region. Progression occurs during rapid periods of spine growth: at ages 1–5 years and during adolescence. 11
Scheuermann’s disease is the most common cause of structural kyphosis in adolescence. It is a rigid thoracic kyphosis, with vertebral wedging and irregular vertebral end plates. The prevalence of Scheuermann’s disease has been estimated at between 0.4% and 8% of the general population. Approximately one-third of patients with Scheuermann’s disease will also have some degree of scoliosis. 12
Ankylosing spondylitis is a progressive rheumatic condition in which some or all of the joints and bones of the spine fuse together, causing pain and stiffness. The prevalence of ankylosing spondylitis is approximately 0.5% among British men and 0.2% among British women; it typically occurs around the late teens or twenties. A small minority of patients with ankylosing spondylitis will require surgery. 13
Deforming dorsopathies
Deforming dorsopathies is an umbrella term for spinal deformities in general; it includes spondylolysis, spondylolisthesis, other fusion of spine and atlantoaxial subluxation. The inclusion of these conditions should ensure that all indications in which patients can potentially benefit from EOS are considered.
Congenital deformities of the spine, hips and lower limbs
Congenital deformities of the spine, hips and lower limbs result from anomalies in the formation of these structures in utero; these conditions include developmental dysplasia of the hip (affecting 1–2 per 1000 live births),14 reduction defects of the lower limb and osteochondrodysplasias. Minor malformations may not be apparent at birth and may be identified only by routine examinations. More severe malformations can be complex, producing severe deformity. Congenital deformities of the spine, hips and lower limbs are particularly significant indications because of the repeated radiation exposure associated with their monitoring. Furthermore, patients suffering from these conditions are typically very young, and hence more sensitive to the adverse effects of radiation exposure.
Care pathways
The management of patients with spinal deformity primarily involves monitoring at intervals to assess disease progression and guide treatment decisions. Progression is measured in terms of the degree of the curvature, which is monitored using serial upright weight-bearing X-ray imaging. The frequency of monitoring depends on the age of the patient, the rate of growth at the time and the nature of the curve. The pattern of monitoring for kyphosis and other deforming dorsopathies is broadly similar to that for scoliosis, which tends to range from every 4 months to almost 2 years. Patients are also monitored using weight-bearing X-rays pre- and postoperatively, for up to 2 years or up to the age of 20 years. Patients with congenital deformities of the lower limbs, hips or spine are likely to undergo surgery at a younger age than patients with acquired scoliosis, kyphosis or other deforming dorsopathies. Patients with spinal deformity may require postsurgical monitoring until skeletal maturity, but for patients with other congenital deformities the period of monitoring may be much shorter.
A weight-bearing image is very important in the evaluation of patients with deformities of the spine because of the effect of gravity. The American College of Radiology Practice guideline for the performance of radiography for scoliosis in children recommends PA and LAT radiography of the spine, obtained in an upright position, for initial or screening examination. 15 Non-weight-bearing images can lead to misinterpretation and misdiagnosis. Full-body images can also help prevent misinterpretation of the spinal curvature by providing information about the position of the pelvis and legs.
Outcomes
Radiation adverse effects
X-rays are a type of ionising radiation. Exposure to radiation can cause cell damage or cell death, depending not only on the amount and type of radiation, but also on the sensitivity of the tissue itself.
The deleterious health effects of radiation exposure depend on the dose received. At high doses, radiation can produce damaging effects that will be evident within a few days of exposure. These effects are termed deterministic or non-random, as there is a clear relationship between the exposure and the effect. Deterministic effects require radiation doses above a certain threshold, which are extremely rare in diagnostic radiology. 16
Exposure to low-dose radiation, such as diagnostic X-rays, results in stochastic (random) effects that are noticeable only years after exposure. A cell exposed to radiation may remain unaffected, may die or may become abnormal. Abnormal cells may become malignant, resulting in cancer or, in the case of reproductive cells, in heritable defects. 16–18 As the dose of radiation increases, so does the probability that a biological effect will occur. However, even at very low doses, there is some, albeit small, probability that a biological effect will occur. In other words, there is no threshold for the deleterious effects of low-dose radiation exposure: the ‘linear-non-threshold’ model. 17 This model is a consensus assumption that is used for radiation protection purposes.
Where patient management involves a number of radiographs, the increased risk has to be considered. This is of particular concern when X-ray monitoring is conducted throughout childhood and puberty, as children are more sensitive to the harmful effects of radiation than adults and are more likely to manifest radiation-induced changes over their lifetime. 19
Measures of radiation exposure/dose
Radiation exposure is quantified using specially developed dosimetric quantities, namely exposure, absorbed dose, entrance skin dose, equivalent dose and effective dose. All are measurable quantities except equivalent dose and effective dose, which are derived from the former. 18 (See Glossary for exact definitions.)
Exposure measures the amount of ionisation produced (in coulombs) by an X-ray beam in 1 kg of air. Exposure is directly related to the strength of the radiation source and is independent of the matter absorbing the radiation itself. 16
The absorbed dose measures the amount of energy deposited in organs and tissues of the human body. Thus, the absorbed dose depends on the type of matter intercepting the X-ray beam. The unit of absorbed dose is the gray (Gy): 1 Gy delivers 1 J of energy per kg of matter. 18
Absorbed dose fails to consider both the variation in biological effect by the different types of radiation and the different sensitivities of the various tissues of the human body. Thus, the concepts of equivalent dose and effective dose were introduced. Measuring radiation exposure using effective or equivalent doses enables the comparison of radiation exposures and the calculation of a cumulative dose following multiple exposures. 18
Equivalent dose takes into account the differential ability of radiation to produce adverse effects in human tissues and organs. Equivalent dose is calculated by taking a weighted sum of the absorbed doses received by a particular tissue or organ, weighted by radiation weighting factors. These weighting factors reflect the radiation’s deleterious effects. Weighting factors are recommended by the International Commission on Radiological Protection (ICRP). The unit of equivalent dose is the sievert (Sv): 1 Sv corresponds to 1 J per kg. 18
Effective dose takes into account the sensitivity to radiation of each of the tissues and organs affected by radiation exposure. This quantity allows the comparison between different exposures. Effective dose is calculated by taking a weighted sum of the equivalent doses of the various tissues affected by the radiation. As with equivalent dose, the weighting factors are recommended by the ICRP. 18 Patient size is also an important factor in determining equivalent dose and effective dose. 16
As exposure depends on a variety of factors relating to the equipment and protocol, so does effective dose. The estimation of an accurate cumulative lifetime dose associated with diagnostic X-rays requires the effective doses per radiograph relevant to clinical practice in the UK.
Measures of radiation-associated risk
The increase in risk of disease in the exposed population is often expressed as the excess relative risk (ERR) per gray or per sievert. ERR is the rate of disease in the exposed population divided by the rate of disease in an unexposed population, minus 1.0. 18
The risk to the exposed population over a lifetime can be expressed in different ways. The excess lifetime risk (ELR) is the difference between the proportion of the exposed population who develop or die from the disease and the corresponding proportion in a similar non-exposed population. The lifetime attributable risk (LAR) describes excess deaths or disease cases over a follow-up period, with population background rates determined by the experience of unexposed individuals. 18
Outcomes included in the assessment
The primary benefit of EOS is to provide radiographic imaging at relatively low-dose radiation. Therefore, the model considers the long-term costs and consequences associated with radiation exposure. The model estimates the total radiation exposure to patients over a lifetime for the diagnosis and long-term monitoring of the indications for both standard radiography (CR and DR imaging) and EOS. The subsequent outcomes from radiation exposure on the risk of cancer and mortality are explicitly modelled to determine the impact on health outcomes and costs to the NHS. Outcomes in the model are expressed in terms of quality-adjusted life-years (QALYs). The model evaluates costs from the perspective of the NHS and Personal Social Services, expressed in UK pounds sterling at a 2011 price base.
The intermediate outcome of image quality is also assessed. Image quality is important because radiographs need to provide the necessary information for accurate diagnosis or monitoring of disease or injury. Radiographic equipment can be used in such a way as to reduce radiation dose, but this reduction in radiation dose results in a reduction in image quality. Radiation dose should be ‘as low as reasonably achievable’ (ALARA); this means obtaining the best image quality necessary for the lowest possible radiation dose. Monitoring of scoliosis in children and adolescents does not require high-quality images because of the nature of the image required; high-contrast bone structure and geometry of the vertebral column, therefore, a low-dose (high-speed) acquisition is appropriate. 19
The quality of radiographic images can be measured using the European guidelines onquality criteria for diagnostic radiographic images20 or, for images of children, the European guidelines on quality criteria for diagnostic radiographic images in paediatrics,21 developed by the European Commission.
A key consideration in the economic modelling is whether or not evidence exists on how any change in the nature and quality of images with EOS, compared with standard X-ray, impacts on patients’ health outcomes. This can be achieved only if such changes result in changes to patients’ pathways of care, i.e. there are changes in patients’ diagnoses and/or therapies that lead directly to gains in quality of life (QoL) and/or life expectancy.
Decision problem
The aim of this project is to determine the clinical effectiveness and cost-effectiveness of the EOS 2D/3D X-ray imaging system for the evaluation and monitoring of scoliosis and other relevant orthopaedic conditions for which there may be a potential benefit associated with EOS, namely kyphosis, deforming dorsopathies and congenital deformities of the spine, hips or lower limbs. The relevant comparator imaging technologies are X-ray film, CR and DR, although film has been replaced by CR and DR in standard UK practice. The primary outcome of interest is radiation-associated risk of cancer.
In order to address this decision problem, systematic reviews of EOS and the adverse effects of diagnostic radiation were required. These are described in Chapter 2 (see Systematic review of the clinical effectiveness of EOS and Adverse effects of diagnostic radiation for patients with orthopaedic conditions, respectively). To inform the economic assessment, a systematic review of previous economic evaluations was conducted, also described in Chapter 2 (see Review of existing economic evaluations). Chapter 2 also presents the de novo model and results (see Description of the decision-analytic model, Model inputs, Analytic methods, Cost-effectiveness results).
Chapter 2 Assessment design and results by condition or aetiology
Systematic review of the clinical effectiveness of EOS
Background
A systematic review was undertaken to assess the clinical effectiveness of EOS for patients with orthopaedic conditions who would benefit from the weight-bearing and full-body imaging aspects of the EOS imaging system.
Methods for reviewing the clinical effectiveness of EOS
A systematic review of the evidence on the clinical effectiveness of EOS, compared with standard X-ray technology, for monitoring or evaluation of any orthopaedic condition was conducted following the general principles recommended in the Centre for Reviews and Dissemination (CRD) guidance22 and the quality of reporting of meta-analyses (QUOROM) statement. 23 The protocol was published on the NICE website prior to study selection and data extraction procedures, and is attached to this report as Appendix 9.
Search strategy
The aim of the literature searches was to systematically identify all the relevant literature on the EOS imaging system, while attempting to remove records in other subject areas that use the same acronym.
The base search strategy was constructed using MEDLINE and then adapted to the other resources searched. The search included the following components: EOS and similar radiography system search terms, not other topics that use the EOS acronym.
Searches of major bibliographic databases were limited by date (1993 to date), as the prototype of the EOS system was purchased by Biospace Med in 1994. No language, study design or other limits were applied. Reference lists of all included studies, relevant editorials and the NICE scope were hand-searched to identify further relevant studies.
The terms for search strategies were identified through discussion between an information specialist and the research team, by scanning the background literature and browsing the MEDLINE medical subject headings (MeSH). The titles and abstracts of bibliographic records were imported into EndNote bibliographic management software (version X1: Thomson Reuters, CA, USA). Details of the search strategies are presented in Appendix 1.
The following databases were searched for relevant clinical effectiveness and cost-effectiveness research on 2 and 3 November 2010, from 1993 to the most recent date available:
-
MEDLINE
-
Allied and Complementary Medicine Database (AMED)
-
BIOSIS Previews
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
The Cochrane Library [including Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Cochrane Central Register of Controlled Trials (CENTRAL), Health Technology Assessment (HTA) Database and NHS Economic Evaluation Database (NHS EED)]
-
EMBASE
-
Health Management Information Consortium (HMIC)
-
Inspec
-
ISI Science Citation Index (SCI)
-
PASCAL [database of INIST (Institut de l’Information Scientifique et Téchnique)].
The following trials registries were searched on 8 November 2010:
-
ClinicalTrials.gov
-
Current Controlled Trials (CCT).
The manufacturer’s website (www.eos-imaging.com) was also searched for potentially relevant studies.
Inclusion and exclusion criteria
Two reviewers independently screened all titles and abstracts. Full-paper manuscripts of any titles/abstracts that appeared to be relevant were obtained where possible and the relevance of each study independently assessed by two reviewers according to the inclusion and exclusion criteria below. Studies that did not meet all of the criteria were excluded and their bibliographic details listed with reasons for exclusion. Any discrepancies were resolved by consensus, or consulting a third reviewer if necessary.
Study design
Comparative studies were included in the evaluation of clinical effectiveness, as this study design allows a comparison to be made between the new technology and current practice, which is essential for the economic model.
Intervention
Studies assessing the EOS system were included in the evaluation of clinical effectiveness.
Comparators
Studies that compared EOS with film, CR or DR were included in the evaluation of clinical effectiveness. Studies comparing EOS with CT were not eligible for inclusion; as CT cannot be performed while the patient is standing, CT was not deemed to be a relevant comparator.
Participants
Studies that included patients with any orthopaedic condition were included in the evaluation of clinical effectiveness. Studies using healthy volunteers, vertebrae from cadavers or the European Spine Phantom were not eligible for inclusion.
Outcomes
Studies reporting any outcome were included in the evaluation of clinical effectiveness. The primary outcome of interest was patient health outcomes. Secondary outcomes of interest were the surrogate outcomes: quality of the image and radiation dose reduction.
Data extraction strategy
Data on study and participant characteristics and outcomes were extracted by one reviewer using a standardised data extraction form and checked for accuracy by a second reviewer. Disagreements were resolved through consensus. The results of data extraction are presented in Appendix 2. Where data were missing, we contacted authors but did not receive a response.
Quality assessment strategy
The quality of the included studies was assessed using the quality assessment tool for diagnostic accuracy studies (QUADAS). 24 Although the included studies were not typical diagnostic cohort studies, they compared two ‘tests’ in a single group of patients, one being standard practice. Therefore, the majority of questions on the QUADAS checklist were applicable to the studies being assessed. An additional six quality items that were specific to the review were also assessed. Dr David Grier, consultant paediatric radiologist, provided assistance in completing questions relating to the appropriateness of the methods used for measuring radiation dose and image quality, and whether or not the execution of the intervention and comparator technologies was as it would be in practice.
A quality assessment tool designed for studies with different treatment groups (such as randomised controlled trials) was not appropriate for the assessment of the studies included in this review, as such checklists primarily focus on the assignment of patients to treatment groups. If the search had identified relevant controlled trials, a quality assessment tool relevant to such a study design would have been used.
The assessment was performed by one reviewer, and checked by a second. Disagreements were resolved through consensus. The results of the quality assessment are presented in Table 2.
| Quality assessment criteria | Study | |||
|---|---|---|---|---|
| Kalifa et al. (1998)25 | Le Bras et al.26 (unpublished) | Deschênes et al. (2010)27 | ||
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | Yes | Yes |
| 2 | Were selection criteria clearly described? | Yes | No | No |
| 3 | Is the reference standard likely to correctly classify the target condition? | N/A | N/A | N/A |
| 4a | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | Yes | Yes |
| 5a | Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis? | Yes | Yes | Yes |
| 6a | Did patients receive the same reference standard regardless of the index test result? | Yes | Yes | Yes |
| 7a | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | Yes | Yes |
| 8a | Was the execution of the index test described in sufficient detail to permit replication of the test? | No | Yes | Yes |
| 9a | Was the execution of the reference standard described in sufficient detail to permit its replication? | No | Yes | Yes |
| 10a | Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | No | Yes |
| 11a | Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | No | Yes |
| 12 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Unclear | Unclear | Unclear |
| 13 | Were uninterpretable/intermediate test results reported? | N/A | N/A | N/A |
| 14 | Were withdrawals from the study explained? | Yes | No | Yes |
| 15 | Was a sample size calculation used? | Yes; but no details were reported. The authors intended to recruit 150 participants; only 140 participants were included in analysis | NR | NR |
| 16 | Was the method for measuring radiation dose appropriate for both the intervention and comparator technologies? | Yes; basic, but appropriate | Yes | Yes; basic, but appropriate |
| 17 | Was the method of measuring image quality appropriate for both the intervention and comparator technologies? | Unclear. Appropriate criteria used; however, it is not clear if results were reported for ‘agreed results’ or if seen by one reader. Not stated how results were categorised as ‘good’ or ‘poor’ – cut-off not defined | Yes | Yes |
| 18 | Was the execution of the intervention technology as it would be in practice? | No; the apparatus used for the paper appears to be a ‘bespoke’ unit (the ‘Charpak system’), but appears to be similar in many ways to EOS. In addition, digital images were viewed on radiographic laser film, rather than on the screen, which is not as it would be in practice | Yes | Yes |
| 19 | Was the execution of the comparator technology as it would be in practice? | Yes | Yes | Yes |
| 20 | Any other comments? |
SDs were not reported for dosimetric results Contradiction in text: ‘All images were analysed separately by two senior radiologists … All discordant results between independent viewers were further reviewed to achieve a consensus verdict’ Comparison between the two systems was made on the frequency with which each radiologist perceived the information as ‘available’ or ‘not available’. There was no attempt to obtain consensus between readers’ |
SDs were not reported for ‘percentage decrease’ for dosimetric results Lots of withdrawals from the analysis: of 62 PA images obtained only 44 were assessed for image quality, 59 for radiation dose using ESAK and 46 for radiation dose using ESD; of 57 LAT images obtained only 41 were assessed for image quality, 52 for radiation dose using ESAK and 36 for radiation dose using ESD |
SDs were not reported for dosimetric results |
Data analysis
In view of the heterogeneity of the included studies, in terms of participant characteristics and comparator technologies, formal meta-analysis was not appropriate. Therefore, the studies were grouped according to the comparator technology used and a narrative synthesis was presented.
Results of the review of clinical effectiveness of EOS
Quantity of research available
A total of 661 records were identified from the clinical effectiveness searches and an additional 22 records were identified via hand-searching (Figure 1). Three studies met the inclusion criteria and were included in the review. Two studies compared EOS with film X-ray imaging25,26 and one study compared EOS with CR. 27 One of the studies used an earlier version of the technology, referred to as ‘the Charpak system’, which used the same slot scanning technology, but only one X-ray tube, so could not take anteroposterior (AP)/PA and LAT images simultaneously. 25 Two studies were published in full, whereas one study was unpublished. 26 The main characteristics of the included studies are presented below (see Table 3). Details of studies excluded at the full publication stage are provided in Appendix 4.
FIGURE 1.
Flow diagram of the study selection process.
| Study characteristics | Kalifa et al. (1998)25 | Le Bras (unpublished)26 | Deschênes et al. (2010)27 |
|---|---|---|---|
| Duration of patient recruitment | December 1994 to January 1996 | NR | NR |
| Patients recruited | 176 | 64 | 50 |
| Patients analysed | 140 | NR | 49 |
| Patient characteristics | Children (aged > 5 years) undergoing follow-up for scoliosis (93) or known hip diseases (47) | Adolescents who required full-spine radiographs for scoliosis detection or follow-up | Children undergoing follow-up for scoliosis |
| Mean age (years) | NR | 14.7 years (SD 4.8) | 14.8 years (SD 3.6) |
| Proportion male (%) | NR | 36% | 22% |
| Intervention | EOS (earlier version, referred to as ‘the Charpak system’) | EOS | EOS |
| Comparator | Film | Film | Fuji FCR 7501S |
| Image quality results | Image quality comparable between EOS and film | Image quality comparable or better with EOS for the majority of quality criteria | Image quality comparable or better with EOS for the vast majority of images |
| Radiation dose results [mean ESD (Gy)] | |||
| Spine PA | EOS 0.07, film 0.92, ratio of means 13.1 | EOS 0.23, film 1.2 (ratio of means: 5.2 calculated by CRD) | |
| Spine LAT | EOS 0.13, film 1.96, ratio of means 15.1 | EOS 0.37, film 2.3 (ratio of means: 6.2 calculated by CRD) | |
| Spine AP | EOS 0.08, film 0.93, ratio of means 11.6 | ||
| Pelvis | EOS 0.06, film 1.13, ratio of means 18.8 | ||
| Centre of back | EOS 0.18, CR 1.04, ratio of means 5.9 | ||
| Proximal LAT point | EOS 0.27, CR 2.38, ratio of means 8.8 | ||
| Outer side of proximal breast | EOS 0.11, CR 0.83, ratio of means 7.6 | ||
| Proximal anterosuperior iliac spine | EOS 0.16, CR 1.47, ratio of means 9.2 | ||
| Proximal iliac crest | EOS 0.30, CR 2.47, ratio of means 8.2 | ||
| Distal iliac crest | EOS 0.11, CR 0.73, ratio of means 6.5 | ||
| Nape of neck | EOS 0.20, CR 0.59, ratio of means 2.9 | ||
Quality of research available
The results of the quality assessment are presented in Table 2.
The study by Kalifa et al. 25 had clearly defined inclusion criteria and included 140 participants. This study reported using a sample size calculation; however, the authors had intended to recruit 150 participants. Some methods were not fully reported, for example the execution of the ‘tests’ was not described in sufficient detail to permit their replication, and the authors did not report whether or not the tube voltage was similar between the two radiographic systems. In addition, this study used an earlier version of the technology ‘the Charpak system’, so the results may not reflect the current EOS machines. Although ranges were reported for the mean dosimetry results, standard deviations (SDs) were not reported. Overall, the quality of this study is considered limited.
A major limitation of the study by Le Bras (unpublished)26 was the high proportion of patient withdrawals. This may have biased the results. In addition, the study report mentioned tables of results that were missing from the report; therefore, the results were taken from the text. Overall, the quality of this study is considered limited.
The study by Deschênes et al. 27 was well reported, with the execution of both ‘tests’ reported in sufficient detail to permit their replication. In addition, the authors attempted to reduce the potential for bias in the interpretation of image results by using blinded assessment of quality outcomes. However, it had a small sample size (only 50 patients) and no SDs were reported for the mean dosimetry results, making it impossible to assess the reliability of the estimates. Overall, the quality of this study is considered limited.
Image quality was assessed using appropriate criteria: the Quality criteria for diagnostic radiographic images28 or the European guidelines on quality criteria for diagnostic radiographic images in paediatrics. 21,29 At least two radiologists assessed each of the images for quality in all studies.
Radiation dose was measured appropriately; entrance surface dose (ESD) was measured using individually calibrated thermoluminescent calcium fluoride pellets placed on the patient’s skin in the centre of the X-ray beam25,26 or optically stimulated luminescence dosimeters (OSLDs) on various locations chosen to assess the main radiosensitive regions of the body. 27 In addition, one study also calculated entrance surface air kerma (ESAK) from output dose rates of the scanners. 26
The patients in the included studies were the same type of patient as would receive the test in practice, primarily children with scoliosis, although one study also included children undergoing follow-up examinations for known hip diseases. 25 The whole sample received both tests within an appropriate time period. However, there was the potential for test review bias and/or diagnostic review bias as the results of the other test may have been known to assessors for two of the studies. 25,26
The execution of EOS and the comparator imaging systems was generally as would be in practice, except that one study used an earlier version of the EOS imaging system (the Charpak system) and viewed images on laser film, rather than on screen. 25 Two of the studies reported that tube voltage was similar between the two radiographic systems. 26,27
Synthesis of the included studies
The main characteristics and results of the included studies are presented in Table 3. Further details are presented in Appendix 2 (data extraction). All three studies included children or adolescents with scoliosis, although one study also included children undergoing follow-up examinations for known hip diseases. 25 Where reported, the mean age of patients was 14 years and the majority of patients were female.
Both studies comparing EOS (or the earlier Charpak system) with film X-ray25,26 found overall image quality to be similar or better with EOS. In the case of both PA and LAT images, the global image quality score was significantly higher for EOS radiographs than for film images. PA images were of significantly better quality with EOS according to four criteria (reproduction of vertebral bodies and pedicles, image blackening and image informative contribution); for other criteria, no significant difference was found between EOS and film images. 27 LAT images were of significantly better quality with EOS for five out of eight criteria assessed. 27 Slightly more images were categorised as ‘good’ quality with the Charpak system than with film for both spine (76 vs 72 images categorised as good) and pelvis (46 vs 45 images categorised as good) images. For spine imaging the Charpak system was associated with improved visibility of some structures, although for pelvis imaging certain criteria were slightly less favourable with the Charpak system, and the Charpak system showed a lack of spatial resolution compared with film. 26
Radiation dose was significantly lower with EOS (or the Charpak system) than with film X-ray for all images: ratio of means for PA spine was 5.226 (13.125); ratio of means for LAT spine was 6.226 (15.125). The mean ESD with EOS (or the Charpak system) for PA spine was 0.23 (0.07) compared with 1.2 (or 0.92) with film. The mean ESD with EOS (or the Charpak system) for LAT spine was 0.37 (0.13) compared with 2.3 (or 1.96) with film. For the Charpak system the mean ESD for the spine AP was 0.08 compared with 0.93 with film and for the pelvis was 0.06 compared with 1.13 with film. The studies did not report confidence intervals (CIs) or SDs. One study reported ranges25 that indicated that they did not overlap for the majority of results.
The study comparing EOS with CR27 found image quality to be comparable or better with EOS for the majority of images. For global image quality EOS was comparable to CR for 50.5% of images and superior for 46.7% of images. In terms of visibility of structures EOS was comparable to CR for 61.9% of images and superior for 32.4% of images.
Radiation dose was considerably lower with EOS than CR for all images; ratio of means for the centre of the back was 5.9 and for the proximal LAT point 8.8. 27 The lowest ratio of means was at the nape of the neck, which was 2.9. 27 The mean ESD with EOS for the centre of the back was 0.18, compared with 1.04 with CR. The mean ESD with EOS for the proximal LAT point was 0.27, compared with 2.38 with CR. The mean ESD with EOS for the nape of the neck was 0.20, compared with 0.59 with CR. This study did not report CIs or SDs.
No other outcomes were reported. There was no evidence from clinical trials that the facilities offered by EOS (such as the ability to scan a full-body image, removing the need for digital stitching, or the ability to take PA and LAT images simultaneously, so that a 3D image can be produced) translated into patient health benefits.
The study comparing EOS with CR27 is the most relevant for current practice, as CR and DR have replaced film X-ray imaging in standard UK practice.
Discussion
This systematic review identified limited quality data suggesting that radiation dose is considerably lower with EOS than with CR or film X-ray imaging, whereas image quality remains comparable or better with EOS. No data were found in relation to the primary outcome of interest: patient health benefits.
The review addressed a clear research question using predefined inclusion criteria. Comprehensive literature searches were performed to locate all relevant published and unpublished studies without any language restrictions, thereby minimising the potential for publication bias and language bias. Hand-searching was also performed in order to identify additional relevant studies. We are therefore confident that we have included all relevant studies. However, only three studies comparing EOS with conventional X-ray imaging were identified; one studied an older version of the EOS system25 and the other two included only a small number of participants. 26,27 There are currently no studies comparing the clinical effectiveness of EOS with DR.
Study selection was undertaken independently by two reviewers and data extraction and quality assessment were checked by a second reviewer to minimise the potential for reviewer bias or error. Validity assessment was undertaken using a validated checklist for diagnostic studies, with additional project-specific quality assessment items added. Clinical expertise was obtained for completing the additional project-specific quality assessment items. However, the included studies were of limited quality. Outcomes assessed in the included studies were image quality and radiation dose. Image quality was assessed by at least two radiologists using appropriate criteria. Radiation dose was measured appropriately.
The studies included children with scoliosis and children undergoing follow-up examinations for known hip diseases, which is representative of children who would be likely to receive EOS in practice. However, no studies assessing EOS in adults were identified. The reduction in radiation dose for adults may not be as substantial as seen in the children included in these studies.
The study by Kalifa et al. 25 reported a much higher ratio of means for radiation dose. The methods used in this study were not fully reported, for example the authors did not report whether tube voltage was similar between the two radiographic systems. In addition, this study used an earlier version of the technology, referred to as ‘the Charpak system’. The Charpak system used the same slot-scanning technology as EOS but only one X-ray tube, so it could not take AP/PA and LAT images simultaneously. This study is also likely to have included younger patients than the other two studies; these differences may help to explain the high ratio of means compared with the other two studies.
Adverse effects of diagnostic radiation for patients with orthopaedic conditions
Background
With the introduction of new imaging techniques, such as digital imaging, there is an increased trend in the annual frequency of medical diagnostic X-ray examinations. 30 As medical diagnostic X-ray radiation exposure continues to grow at a substantial rate, understanding the adverse health effects after exposure is therefore of particular importance. Particular concern has been focused on the relationship between harmful health effects (e.g. cancer risk) of radiation exposure and the cumulative radiation dose.
Through internet searching, and in consultation with experts, we identified four main sources of data for adverse effects of diagnostic X-ray radiation: three international and UK relevant reports [BEIR VII Phase II,17 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR),30 ICRP publication 103 report]18 and personal communication with Paul Shrimpton from the HPA (January to February 2011). These reports produced by the large radiation protection and safety agencies, and personal communication, are the accepted authority on adverse effects of radiation. They are briefly summarised below. The data sources of the reports and personal communication were primarily based on the epidemiological data of the Life Span Study of Japanese atomic bomb survivors.
BEIR VII Phase II
The BEIR VII Phase II report17 (produced by the Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation) is very broad in its scope (covering basic aspects of radiation physics and radiation biology and reviews studies of the adverse effects of radiation exposure, atomic bomb, medical, occupational and environmental) and develops risk estimates for lifetime radiation-induced cancer. Importantly, for the purposes of the present assessment, the report includes a detailed review of medical radiation studies. Medical radiation studies can be divided into studies of radiotherapy used to treat malignant disease, radiotherapy for non-cancerous conditions and the use of radiation for diagnostic purposes.
Cancer risk associated with radiotherapy
Deriving the risk of cancer owing to radiation from studies of cancer radiotherapy is clearly problematic, being subject to confounding and limited follow-up data. Studies in which radiotherapy was used for benign disease in adults and children were also reviewed. Such studies were from a time when radiotherapy was used for the treatment of a number of benign conditions: skin haemangioma, tinea capitis and enlarged thymus in children, and benign breast and gynaecological disease, ankylosing spondylitis and peptic ulcer in adults. This type of radiotherapy typically uses lower doses than those used in malignant disease and survival after treatment is not shortened by the presence of a life-threatening disease. The data from relevant studies of cancer risk associated with radiotherapy for a number of benign diseases showed a wide range for the ERR per gray of various cancers, differing in the type of cancer and between adults and children.
Cancer risk associated with diagnostic radiation
Studies of the cancer risk associated with medical diagnostic radiation are more directly relevant to the current assessment. BEIR VII Phase II17 reported the results of studies using chest fluoroscopy for follow-up of pulmonary tuberculosis and diagnostic radiography in adults, and diagnostic and monitoring radiography in children with scoliosis.
The BEIR VII Phase II report17 reviewed several studies investigating the association between cancer risk and the use of diagnostic radiography in adults. Evidence31 showed significant associations between reported numbers of X-rays and tumours of the parotid gland and chronic myeloid leukaemia. A case–control study32 found that diagnostic radiography in adults had no association with leukaemia but a positive association with multiple myeloma, but no estimate of risk per dose was presented. Another case–control study33 found no association between diagnostic radiography and thyroid cancer.
The BEIR VII Phase II report17 summarised the findings of a pilot study34 and the US Scoliosis Cohort Study. 35 The cohort included only patients diagnosed before the age of 20 years between 1912 and 1965 and the average number of scans per patient was 24.7 (range 0–618) and the average cumulative dose to the breast was 0.11 Gy (range 0–1.7 Gy). Mean age at diagnosis of scoliosis was 10.6 years and mean follow-up was 40.1 years. The ERR for women who had at least one radiographic examination was 2.7 (95% CI –0.2 to 9.3).
Risk estimate models for radiation-induced cancer
The BEIR VII Phase II report17 developed ‘risk models’ to estimate the relationship between exposure to ionising radiation and harmful health effects, primarily based on the cancer incidence data from the Life Span Study for the period 1958–98 and based on DSO2 (Dosimetry System 2002) dosimetry. These risk models supported the hypothesis that harmfulness of ionising radiation was a function of dose, and that there was a linear dose–response relationship between exposure to ionising radiation and the development of radiation-induced solid cancers in humans. 17 Therefore, the BEIR VII Phase II report17 proposed the ‘linear-non-threshold’ model on the basis of the assumption that, in the low dose range, radiation doses greater than zero will increase the risk of excess cancer in a simple proportionate manner.
The BEIR VII Phase II report17 presented the results of cancer risk estimate models for the US population. For example, for an exposure scenario of 0.1 Gy at the age of 10 years, the LAR of solid cancer incidence (per 100,000 exposed persons) was estimated to be 1330 for males and 2530 for females; the LAR of solid cancer mortality was estimated to be 640 for males and 1050 for females. For an exposure scenario of 0.1 Gy at the age of 50 years, the LAR of solid cancer incidence was estimated to be 510 for males and 680 for females; the LAR of solid cancer mortality was estimated to be 290 for males and 420 for females. The estimates showed that females were at higher risk for radiation-induced solid cancer incidence and mortality than males, and that there was a steady decrease in risk with age at exposure for both sexes.
UNSCEAR 2008 (Volume 1)
The UNSCEAR 2008 report (Volume 1)30 (produced by UNSCEAR) presents the estimates of the average annual doses of ionising radiation from all sources, primarily for medical exposures to ionising radiation, and public and occupational exposures to radiation. For medical exposures, the report determines the magnitude of its usage around the globe in the period of 1997–2008 and assesses the trends in radiation exposure from diagnostic radiology, radiation therapy and nuclear medicine. We summarise briefly the data for medical diagnostic radiation and radiation therapy in this section.
Annual frequency of medical diagnostic and therapeutic radiation
The UNSCEAR 2008 report 30 estimates for the annual frequency of diagnostic and therapeutic radiation and the doses of these medical radiation exposures were based on published literature on medical exposures and an analysis of the responses to the UNSCEAR Global survey of medical radiation usage and exposures for the period 1997–2007. During that period, approximately 3.6 billion diagnostic radiology X-ray examinations (including dental radiology) were undertaken annually worldwide. Analyses showed that there were wide variations in the average annual frequency of diagnostic medical and dental radiation examination in the period surveyed, by health-care level (based on the number of physicians per head of population). The annual frequency of medical X-ray examinations was over 65 times higher in countries with the highest level of health care (those that are relatively more developed) than in countries with a lower level of health care.
Trends in radiation exposure from radiation therapy
The estimated annual data on the most common types of radiotherapy during 1997–2007 showed that about 70% of all radiotherapy treatments were administered in countries with the highest level of health care. There was an estimated 5.1 million courses of radiotherapy treatment administered annually during this period, up from an estimated 4.3 million in 1988.
Trends in radiation exposure from diagnostic radiology
There is an increased trend in the use of medical diagnostic radiology and the associated exposures globally. The UNSCEAR 2008 report30 used the collective effective dose to measure the trends. The collective effective dose is calculated as the sum of all individual effective doses over the time period being considered. An increase of approximately 70% of total collective effective dose from medical diagnostic radiation has been observed for the period 1997–2007, with an estimated increased collective effective dose of 1.7 million man-sieverts (rising from approximately 2.3 to 4 million man-sieverts).
Mean effective dose (millisievert) for radiological examinations
Based on the data from the UNSCEAR survey30 of medical radiation usage and exposures, the report estimated the mean effective dose for different radiological examinations in UK practice. The mean effective dose for each relevant orthopaedic exposure was 1.0 millisievert (mSv) for lumbar spine radiograph (AP/PA and LAT combined), 0.7 mSv for thoracic spine radiograph (AP and LAT combined), 0.07 mSv for cervical spine radiograph (AP and LAT combined), 0.00 mSv for limbs/joints radiograph, and 0.50 mSv for pelvis/hip radiograph.
International Commission on Radiological Protection publication 103 report
The ICRP publication 103 report18 provides recommendations and guidance on protection against the risks associated with ionising radiation from artificial sources widely used in medicine, general industry and nuclear enterprises, and from naturally occurring sources. The report updates the radiation and tissue weighting factors in the quantities equivalent and effective dose, updates the estimates of the harmful effect of radiation based on the latest available scientific information of the biology and physics of radiation exposure, and develops risk estimates for lifetime radiation-induced cancer and heritable effects.
Excess cancer and heritable effects associated with radiation
In line with the BEIR VII report,17 the practical system of radiological protection recommended by the ICRP publication 103 report18 was based on the assumption of the ‘linear-non-threshold’ model, i.e. at doses below about 100 mSv a given increment in dose would produce a directly proportionate increment in the risk of cancer and heritable effects attributable to radiation. Assuming a linear response at low doses, the combined detriment due to excess cancer and heritable effects was estimated to be around 5% per sievert.
Risk estimates for radiation-induced cancer
The ICRP publication 103 report 18 developed the risk modelling of radiation-induced cancer using the incidence data from the Life Span Study of Japanese atomic bomb survivors with follow-up from 1958 to 1998 for solid cancers. The risk models for solid cancers involved a linear dose response allowing for modifying the effects of sex, age at exposure and attained age. Based on the cancer incidence-based ERR models, for all solid cancers the ERR per gray at age 70 years for exposure at age 30 years was estimated to be 0.35 for males and 0.58 for females.
Risk estimates for radiation-induced heritable effects
There was no direct evidence from human studies that exposure of parents to radiation led to excess harmful heritable effects in offspring. The follow-up data of mortality and incidence in the offspring of Japanese atomic bomb survivors36,37 did not show convincing evidence of heritable effects because of radiation. However, there was compelling evidence of heritable effects associated with radiation exposure in experimental animals (e.g. mice). The risks of radiation-induced heritable effects were therefore developed by extrapolating data on dose response for germ cell mutations from experimental animals to humans.
Based on the ICRP’s risk estimates for radiation-induced heritable effects, there was a risk coefficient of 0.54% per gray for the reproductive population and 0.22% per gray for the whole population, for the total of three classes of heritable effects (Mendelian diseases, chronic diseases and congenital abnormalities) expressed over two generations.
Personal communication with Paul Shrimpton from the Health Protection Agency
Data were received on risk modelling of radiation-induced lifetime cancer and heritable effects from medical X-ray examinations, including calculation of the organ and effective doses for common X-ray examinations on adult patients in the UK, and the relationship between lifetime cancer risk and effective dose for common X-ray examinations. We briefly summarise the risk estimates of radiation-induced cancer and heritable effects in this section.
Risk of radiation-induced lifetime cancer by organ, age and sex
The lifetime risks of cancer incidence or mortality per unit dose were predicted as a function of organ, age and sex, on the basis of the risk models described in ICPR publication 103 report,18 by incorporating typical organ doses for a range of common X-ray examinations derived by Monte Carlo calculation from patient dose data obtained in recent national surveys of UK practice.
The lifetime risk of cancer incidence for each organ was calculated by averaging over all ages in the whole population and both sexes. The estimates for lifetime risk of cancer incidence predicted by HPA calculations were generally in agreement with the ICRP’s nominal risk coefficients for most cancers such as lung, stomach, colon, bladder, liver, oesophagus and ovary. There were small discrepancies in terms of cancers of breast, leukaemia and thyroid. However, when taking into account all cancers, the total cancer risk predicted by the HPA calculations provided an adequate approximation to the risk estimate predicted by the ICRP models: 6.38% per sievert versus 6.88% per sievert.
When estimating the lifetime risk of all cancer incidences by age and sex for a composite Euro-American population, the HPA’s estimates showed that females were at higher risk than males at all ages, and young children and adolescents were at higher risk than adults of both sexes. For example, young children exposed to radiation at age 0–9 years (lifetime risk for all cancers 9.99% per gray for males and 12.7% per gray for females) were at about twice the risk of adults in their thirties (5.12% per gray for males and 6.46% per gray for females) for both sexes. The estimates showed that the lifetime risk of all radiation-induced cancers was a function of age at exposure and sex (assuming uniform whole-body irradiation), with a steady decrease in the total radiation-induced cancer risk with age at exposure for both sexes and a higher risk in females than males (24–47%) at all ages.
The total radiation-induced cancer risk varied with age at exposure and sex, depending critically on which organs were irradiated. The estimates by individual cancer sites showed a steady decrease in risk with age at exposure for certain cancer sites, but not for others. There was a steady decrease in risk with age at exposure (for both sexes) for cancers of stomach, colon, breast, liver, thyroid and ovary. It should also be noted that there were variations in the rates of decrease between different organs. The rates of decrease in risk with age at exposure over the first four or five age bands (up to the age of 60 years) were noticeably high for breast cancer and thyroid cancer for females.
Risk of radiation-induced heritable effects
The HPA estimated the risk of radiation-induced heritable effects for patients of reproductive potential for complete X-ray examinations involving significant gonad doses. These predictions were based on the assumption that the risks were independent of patient age for patients of reproductive capacity and naturally fall to zero for those beyond their reproductive years. For relevant orthopaedic conditions, for female patients the risks were highest for X-ray examination of lumbar spine (5.0 per million), followed by pelvis (2.6 per million). For male patients the risks were highest for X-ray examination of both hips (11.5 per million), followed by pelvis (11 per million).
Methods for reviewing the adverse effects of diagnostic radiation for patients with orthopaedic conditions
None of these reports focused on medical diagnostic radiation exposure in orthopaedic patients, which is the population of interest in the current assessment. Therefore, to complement the current evidence from the reports we conducted a systematic review of the adverse effects of diagnostic radiation for patients with any orthopaedic condition, following the general principles recommended in the CRD guidance22 and the QUOROM statement. 23
Search strategy
Radiation exposure and cancer risk or adverse reproductive outcomes
Searches were conducted in order to identify references on the link between radiation exposure and cancer risk and radiation exposure and adverse reproductive outcomes. The searches were not intended to be exhaustive, but to supplement the key documents on adverse effects of radiation already identified by the project team.
For both cancer risk and adverse reproductive outcomes, an initial set of searches was conducted for published systematic reviews assessing the association of the adverse event and radiation exposure from radiography. Searches were limited using a systematic reviews/meta-analysis filter designed by the CRD for identification of records for potential inclusion in DARE. A subsequent set of searches then sought to identify evidence from primary studies assessing the association between cancer risk/adverse reproductive outcomes and radiation exposure for each relevant orthopaedic condition included in the review, particularly scoliosis.
The systematic review searches were limited to cancer risk and adverse reproductive outcomes associated with medical radiation for non-malignant conditions, and so excluded all non-medical radiation such as atomic bomb or nuclear accident exposure. Radiation therapy for malignant conditions, such as cancer, was also excluded. The primary study searches were considered sufficiently focused by the orthopaedic condition for this limit to not be required.
The base search strategies were constructed using MEDLINE and then adapted to the other resources searched. The searches included the following components:
-
Systematic review searches Radiography or radiation terms and cancer terms or adverse reproductive outcome terms and systematic review or meta-analysis terms, not non-medical radiation terms and radiotherapy.
-
Primary study searches Radiography or radiation terms and cancer terms or adverse reproductive outcome terms and relevant orthopaedic condition terms.
No language or publication date limits were applied. All databases were searched in December 2010 from the date of inception to the most recent date available. Reference lists of all included studies and relevant editorials were hand-searched to identify further relevant studies.
The terms for search strategies were identified through discussion between an information specialist and the research team, by scanning the background literature and browsing the MEDLINE MeSH. The titles and abstracts of bibliographic records were imported into EndNote bibliographic management software (version X1). Details of the search strategies are presented in Appendix 1.
The following databases were searched for relevant information on 6–21 December 2010 to the most recent date available:
-
MEDLINE
-
The Cochrane Library (including CDSR, DARE, CENTRAL)
-
EMBASE.
Inclusion and exclusion criteria
Two reviewers independently screened all titles and abstracts. Full paper manuscripts of any titles/abstracts that appeared to be relevant were obtained where possible and the relevance of each study was independently assessed by two reviewers according to the inclusion and exclusion criteria below. Studies that did not meet all of the criteria were excluded and their bibliographic details listed with reasons for exclusion. Any discrepancies were resolved by consensus, or consulting a third reviewer if necessary.
Study design
Systematic reviews, cohort studies and case–control studies were included in the evaluation of adverse effects of medical diagnostic X-ray exposure.
Intervention
Studies were included if they investigated exposure to medical X-ray radiation for diagnostic purposes and the association with risk of cancer or adverse reproductive outcomes. Studies investigating prenatal exposure to medical X-ray radiation or exposure to radiation therapy were excluded.
Participants
Studies of patients with any orthopaedic condition were included in the evaluation of adverse effects of medical diagnostic X-ray exposure.
Outcomes
The eligible outcomes for adverse effects of medical diagnostic radiation exposure were incidence of cancer, cancer mortality and any adverse reproductive outcomes.
Data extraction strategy
Data on study and participant characteristics and outcomes were extracted by one reviewer using a standardised data extraction form and checked for accuracy by a second reviewer. Disagreements were resolved through consensus. The results of data extraction are presented in Appendix 3.
Quality assessment strategy
The quality of studies of cancer risk was assessed using the quality assessment tool for cohort studies, adapted from the Newcastle Ottawa Scale. 38 The quality of all of the included studies was assessed based on criteria described in CRD’s guidance for undertaking systematic reviews. 22 The assessment was performed by one reviewer, and checked by a second. Disagreements were resolved through consensus. The results of the quality assessment are presented in the data extraction table (see Appendix 3).
Data analysis
The levels of clinical and methodological heterogeneity were investigated. Given the high degree of clinical heterogeneity between the included studies (e.g. different outcome measures and length of follow-up), pooling studies using standard meta-analytic methods was not appropriate. A narrative synthesis was therefore performed.
Results of the systematic review of adverse effects of diagnostic radiation for patients with orthopaedic conditions
Quantity of research available
A total of 1005 records were identified from the diagnostic radiation adverse effect searches (Figure 2). The initial set of searches identified 32 systematic reviews or non-systematic overviews assessing the adverse effects of diagnostic radiation exposure. Thirty-one reviews/overviews were excluded, because they discussed the cancer risk estimates associated with radiation exposure or estimated the radiation-associated cancer mortality risk based on data sources that were not from a diagnostic radiation population, such as the BEIR VII report17 and the ICRP publication 103 report. 18
FIGURE 2.
Flow diagram of the study selection process.
Only one potentially relevant systematic review of cancer risk associated with diagnostic X-ray exposure39 was identified. This review assessed the risk of childhood cancer associated with pre- or postnatal diagnostic X-rays by including 19 case–control studies and six cohort studies published between 1990 and 2006. However, it should be noted that this review primarily focused on prenatal radiation exposure for patients with non-orthopaedic conditions; only one included study was of postnatal exposure for those with an orthopaedic condition (scoliosis). Therefore, the review by Schulze-Rath et al. 39 was excluded because of insufficient relevant evidence for the harmful adverse effects associated with diagnostic X-ray exposure for patients with orthopaedic conditions.
Six primary studies met the inclusion criteria and were included in our review. Four studies investigated the association between cancer risk and diagnostic X-ray exposure,34,35,40,41 whereas two studies assessed the association between the risk of adverse reproductive outcomes and diagnostic X-ray exposure. 42,43 Full data extraction is presented in Appendix 3 and details of studies excluded at the full publication stage are provided in Appendix 5.
Cancer risk associated with diagnostic radiation
Quality of research available
The four included studies assessing cancer risk associated with diagnostic X-ray radiation were large prospective cohort studies. 34,35,40,41 The four studies34,35,40,41 were based on the same cohort of US patients with scoliosis and they were conducted by the same group of investigators. The study by Hoffman et al. 34 was a pilot study, which recruited only 1030 female patients with scoliosis, diagnosed between 1922 and1965. The following three studies comprised 5573 female patients with spinal curvature, diagnosed between 1912 and 1965. 35,40,41 The results of quality assessment for these studies are presented in Table 4. All studies were available as journal publications.
| Quality criteria | US Scoliosis Cohort Study | |||
|---|---|---|---|---|
| (Pilot) 198934 | 200035 | 200840 | 201041 | |
| Representativeness of the exposed cohort (yes/no) | Yes | Yes | Yes | Yes |
Ascertainment of exposure
|
A | A | A | A |
| Analyses control for the important confounding factor(s) (yes/no) | No | No | Yes | Yes |
Assessment of outcome
|
C | B | C | B |
| Was follow-up long enough for outcomes to occur? (yes/no) | No | Yes | Yes | Yes |
Adequacy of follow-up of cohorts
|
B | B | B | B |
In all four studies,34,35,40,41 the exposed cohort was representative of the patient population with orthopaedic conditions of interest. All the studies applied reliable methods using secure medical records in ascertaining the medical exposure being investigated. Two studies appropriately adjusted for important confounding factors in their analyses. 40,41 However, there was a failure to control for some important confounding factors (e.g. family history of breast cancer and reproductive history) in the studies by Hoffman et al. 34 and Doody et al. ,35 which may have compromised the validity of study results.
In terms of assessment of outcomes, two studies35,41 appropriately used reliable methods in assessing outcomes, as both studies used formal records of death certificate to evaluate the outcome of mortality. However, there was potential recall bias in the other two studies,34,40 as the authors relied on self-report for breast cancer incidence and family history of breast cancer in their studies.
In all of the four studies34,35,40,41 more than 80% of patients were included in the follow-up assessment. The relatively low numbers of loss to follow-up in these studies were unlikely to introduce bias to the analyses. Apart from the pilot study by Hoffman et al. ,34 the majority of included studies35,40,41 had adequate duration of follow-up for outcomes to occur, with the mean length of follow-up ranging from 39.5 to 46.9 years. Additionally, the estimate of cumulative radiation dose was unlikely to be reliable in all four studies,34,35,40,41 as the authors acknowledged that it may be subject to error.
Synthesis of the included studies
The main characteristics and results of the included studies are presented in Table 5. All four studies (based on the same US Scoliosis Cohort) included children or adolescents with scoliosis and other spinal curvatures. In the included studies, the mean age of patients at follow-up ranged from 41.4 to 58 years. All included patients were female. The vast majority of patients had scoliosis and the proportion of patients with idiopathic scoliosis ranged from 49.2% to 60%. Where reported, the mean age of patients at scoliosis or curvature diagnosis was about 11 years old.
| Study characteristics | US Scoliosis Cohort Study | |||
|---|---|---|---|---|
| (Pilot) 198934 | 200035 | 200840 | 201041 | |
| Mean age at follow-up; male (%) | 41.4 years; 0 | 51 years (range 2 to 89); 0 | 51 years (range 30 to 84); 0 | 58 years (range 2.1–96.5); 0 |
| Dates of recruitment | From 1935 to 1965 (year of diagnosis 1922–65) | Not stated (year of diagnosis 1912–65) | Not stated (year of diagnosis 1912–65) | Not stated (year of diagnosis 1912–65) |
| No. of patients recruited/analysed | 1030, of whom 856 responded to the questionnaire/telephone interview [either in person (818), or a surrogate response was received for deceased patients (38)]; 973 patients were included in the analyses, as 51 patients could not be located, and dates of radiography were missing for six patients |
5573, of whom vital status was determinable in 4971 5466 patients were included in the subgroup analyses, as 34 patients contributed no woman-years of follow-up, 18 patients had missing exit dates and 55 were known to have died, but the cause of death was unknown |
3010 female patients with scoliosis (analysis cohort) |
5573, of whom vital status was determinable in 5513 Risk of dying from cancer was assessed for the subgroup of 3121 women who completed the health survey in the previous study40 |
| Disease characteristics | 60% of participants had idiopathic scoliosis | The vast majority of patients had scoliosis (92.7%). Around half of patients (49.2%) had idiopathic disease. Most patients were diagnosed at the age of 10 years or above (62.7%) | 59% patients had idiopathic scoliosis. Mean age at scoliosis diagnosis was 11 years (range 0–19 years) | The mean age at curvature diagnosis was 10.6 years (range 0–19.9 years) |
| Length of follow-up | The average length of follow-up for the 973 patients with usable follow-up information was 25.6 years | The average length of follow-up was 40.5 years | The mean length of follow-up was 39.5 years (range 13–68 years) | The mean length of follow-up was 46.9 years |
| Primary analyses |
Incidence of breast cancer Observed breast cancers vs expected breast cancers: 11 vs 6 SIR:a 1.82, 90% CI 1.0 to 3.0 |
All-cause mortality: SMR:b 1.71, 95% CI 1.6 to 1.8 Breast cancer mortality: SMR 1.69, 95% CI 1.3 to 2.1 Leukaemia mortality: SMR 1.21, 95% CI 0.6 to 2.3 Lung cancer mortality: SMR 0.73, 95% CI 0.5 to 1.1 |
Radiation dose response (during 118,905 woman-years of follow-up with median 35.5 years based on 78 cases of invasive breast cancer): ERR/Gy = 2.86, 95% CI –0.07 to 8.62; p = 0.058 Radiation dose response (for women who reported a family history of breast cancer in first- or second-degree relatives): ERR/Gy = 8.37, 95% CI 1.50 to 28.16 Effect of modification for radiation dose response for breast cancer by any family history of breast cancer (p = 0.03) |
All-cause mortality: SMRc 1.46, 95% CI 1.39 to 1.54 Cancer mortality: SMR 1.08, 95% CI 0.97 to 1.20 Breast cancer mortality: SMR 1.68, 95% CI 1.38 to 2.02 Liver cancer mortality: SMR 0.17, 95% CI 0.00 to 0.94 Cervical cancer mortality: SMR 0.31, 95% CI 0.06 to 0.92 Lung cancer mortality: SMR 0.77, 95% CI 0.59 to 1.00 |
| Secondary/subgroup analyses |
Breast cancer risk Patients aged ≥ 15 years at the time of their first radiograph: SIR 3.1, 90% CI 1.4 to 6.2 Patients for whom time since first radiograph was ≥ 30 years: SIR 2.4, 90% CI 0.9 to 5.0; trend for increased risk with time (p = 0.02) Patients who received a total of ≥ 30 radiographs: SIR 2.0, 90% CI 0.07 to 4.7 Patients who received a total of ≥ 60 radiographs: SIR 3.1, 90% CI 1.1 to 7.1 Patients who had a radiation dose to the breast of 20 rad or more: SIR 3.4, 90% CI 1.2 to 7.8; trend for increased risk with increased dose (p = 0.08) |
Breast cancer mortality Patients aged ≥ 10 years at the time of diagnosis: SMR 2.01, 95% CI 1.5 to 2.6 Patients with neuromuscular scoliosis: SMR 2.09, 95% CI 1.4 to 3.1 Patients with unknown aetiology: SMR 2.61, 95% CI 1.1 to 5.1 Patients with a maximum curve magnitude of 30–59 °: SMR 2.29, 95% CI 1.3 to 3.8 Patients who had surgery: SMR 2.52, 95% CI 1.7 to 3.6 Patients receiving ≥ 50 radiographs: SMR 3.86, 95% CI 1.9 to 6.9 Patients with a cumulative dose of ≥ 20 cGy to the breast: SMR 3.36, 95% CI 2.0 to 5.3 Patients aged 10–13 years at the time of their first radiograph: age 10–11 years, SMR 3.36, 95% CI 2.1 to 5.1; age 12–13 years, SMR 1.85, 95% CI 1.2 to 2.8 Patients with a longer time since their first radiograph: 30–39 years, SMR 2.43, 95% CI 1.6 to 3.6; ≥ 40 years, SMR 2.07, 95% CI 1.5 to 2.8 Test for trend: Trend for increased risk of breast cancer as the number of radiograph exposures increased (p = 0.0006) Trend for increased risk of breast cancer with increased cumulative radiation dose (p = 0.001) |
Breast cancer risk Patients receiving ≥ 60 radiographs with mean total dose 33.5 cGy vs patients receiving 1–9 radiographs with mean total dose 3 cGy: RR 3.14, 95% CI 1.33 to 7.44 Test for trend for total number of radiographs (p = 0.12) Patients with a second-degree relative affected by breast cancer vs patients with no known family history of breast cancer: RR 2.71, 95% CI 1.57 to 4.66 Test for trend for family history of breast cancer (p = 0.008) Patients with three to five relatives or one to two relatives with breast cancer vs patients with no known relatives with breast cancer: RR 5.65, 95% CI 1.73 to 18.5 and RR 2.12, 95% CI 1.32 to 3.41, respectively Test for trend for number of relatives with breast cancer (p = 0.0003) Patients with a family history of early-onset breast cancer (diagnosed before age of 50 years) vs patients with no known family history of early-onset breast cancer: RR 2.84, 95% CI 1.10 to 6.03 Test for trend for family history of early-onset breast cancer (p = 0.03) |
Breast cancer mortality Patients who received ≥ 50 radiographs (involving exposure to the breasts) vs those receiving < 25 radiographs: RR 2.7, 95% CI 1.3 to 5.5 Patients with a cumulative breast dose of ≥ 30 cGy vs those with a cumulative dose of 0–9 cGy: RR 2.4, 95% CI 1.2 to 4.8. p-value for trend = 0.001 ERR/Gy = 3.9, 95% CI 1.0 to 9.3 |
Cancer mortality
There was a non-significant difference in the risk of dying from cancer in female spinal curvature patients compared with the general population [standardised mortality ratio (SMR) 1.08, 95% CI 0.97 to 1.20]. 41 The data did not show significant increases in the risk of dying from cancers other than breast cancer, such as leukaemia or liver, cervical and lung cancer (see Table 5).
Breast cancer mortality
Two of the studies reported a significant increase in the risk of dying from breast cancer in spinal curvature patients compared with the general population, with SMR 1.69 (95% CI 1.3 to 2.1)35 and SMR 1.68 (95% CI 1.38 to 2.02). 41
There was a highly significant trend for increased risk of breast cancer mortality with increased cumulative radiation dose (p = 0.001). 41 Compared with patients with a cumulative dose of 0–9 cGy, patients with a cumulative breast dose of ≥ 30 cGy were significantly associated with a higher risk of dying from breast cancer [relative risk (RR) 2.4, 95% CI 1.2 to 4.8]. 41
Compared with patients receiving < 25 radiographs, a significant increase in the risk of dying from breast cancer was observed in patients who received ≥ 50 radiographs (involving exposure to the breasts) (RR 2.7, 95% CI 1.3 to 5.5). 41 The ERR for breast cancer mortality increased significantly as the radiation dose to the breast increased (ERR/Gy = 3.9, 95% CI 1.0 to 9.3). 41
The study by Doody et al. 35 assessed the relationship between breast cancer mortality risk and age at radiation exposure. The female patients with scoliosis, aged ≥ 10 years at the time of diagnosis were significantly associated with an increased risk of dying of breast cancer compared with the general population (SMR 2.01, 95% CI 1.5 to 2.6). Stratification analyses showed that there was a higher risk of dying from breast cancer in female scoliosis patients aged 10–11 years at the time of their first X-ray exposure (SMR 3.36, 95% CI 2.1 to 5.1) compared with the risk in those aged 12–13 years at the time of their first X-ray exposure (SMR 1.85, 95% CI 1.2 to 2.8). 35 However, this analysis was not adjusted for family history of breast cancer or reproductive history.
Breast cancer risk
There was a significant trend for increased risk of breast cancer with increased number of radiograph exposures (p = 0.0006) and with increased cumulative radiation dose (p = 0.001). 35 This finding was not adjusted for family history of breast cancer or reproductive history.
A later study (based on the radiation dose response during 118,905 woman-years of follow-up with median 35.5 years based on 78 cases of invasive breast cancer) reported a marginal significance of radiation dose response for breast cancer risk among female patients with scoliosis: the ERR/Gy was 2.86 (95% CI –0.07 to 8.62; p = 0.058). 40 A subgroup analysis showed a significant effect of modification for radiation dose response for breast cancer by any family history of breast cancer (p = 0.03). Among women who reported a family history of breast cancer in first- or second-degree relatives, a highly significant radiation dose response was observed: the ERR/Gy was 8.37 (95% CI 1.50 to 28.16). 40 However, these analyses were susceptible to recall bias, as the authors relied on self-report for breast cancer incidence and family history of breast cancer.
Summary of evidence
Evidence for the cancer risk associated with diagnostic X-ray radiation exposure in patients with orthopaedic conditions is limited to that from four studies all based on the same US Scoliosis Cohort. Based on the data from the study with the longest follow-up and largest sample size,41 there was good evidence of an increase in the risk of breast cancer mortality in female spinal curvature patients compared with the general population and a significant radiation dose response was observed. There was a highly significant trend for increased risk of breast cancer mortality with increased cumulative radiation dose.
An earlier analysis40 revealed a marginal significance of radiation dose response for breast cancer risk among female scoliosis patients. It was noteworthy that this radiation dose response was significant in patients with a family history of breast cancer. 40 However, these findings may have been subject to the possibility of recall bias.
The data did not show significant increases in the risk of dying from other cancers such as leukaemia or liver, cervical and lung cancer.
Risk of adverse reproductive outcomes associated with diagnostic radiation
Quality of research available
The two included studies43,44 of assessing the risk of adverse reproductive outcomes associated with diagnostic X-ray radiation were controlled retrospective cohort studies, one of which43 had a large sample size. The exposed cohort in both studies was representative of the patient population with orthopaedic conditions of interest. In both studies, the details of pregnancies and offspring were obtained by personal interview or postal questionnaire, thereby introducing the potential for recall bias. In particular, the information on spontaneous abortion in both studies was unlikely to be accurate, as early miscarriage may have been forgotten or unrecognised.
In terms of the assessment of other reproductive outcomes, all causes of stillbirths and neonatal deaths and diagnosis of abnormalities requiring hospitalisation were confirmed objectively in the study by Cox. 42 However, none of the responses on reproductive outcomes from the study by Goldberg et al. 43 were validated objectively.
It should be noted that some other factors (e.g. family history, maternal health during pregnancy and exposure to X-ray radiation during pregnancy) may have influenced the reproductive outcomes in both studies. The failure to adjust for these confounding factors in the analyses may have threatened the validity of the study findings.
Synthesis of the included studies
The main characteristics and results of the included studies are presented in Table 6. Full data extraction is presented in Appendix 3. Both studies included cases who were females exposed to multiple X-rays for an orthopaedic condition during childhood or adolescence.
| Outcomes | Goldberg et al. (1998)43 (exposed group vs non-exposed group) | Cox (1964)42 (exposed group vs non-exposed group) |
|---|---|---|
| Exposed group, n = 1292 (adolescent patients with idiopathic scoliosis); non-exposed group, n = 1134 | Exposed group, n = 91 (congenital dislocation of the hip + 36% of X-ray examinations performed during pregnancy); non-exposed group, n = 157 (77 males) | |
| Unsuccessful attempts at pregnancy | Adjusted ORa 1.33, 95% CI 0.84 to 2.13 | NR |
| Stillbirths | Adjusted ORb 0.38, 95% CI 0.15 to 0.97 |
2% (4/200) vs 0.8% (3/375); p = 0.34 ORc 2.53, 95% CI 0.56 to 11.42 |
| Neonatal deaths | NR |
0% vs 1.9%; p = 0.10 NC |
| Spontaneous abortions | Adjusted ORd 1.35, 95% CI 1.06 to 1.73 |
10.3% (23/223) vs 8.6% (38/442); p = 0.58 ORc 1.22, 95% CI 0.71 to 2.11 |
| Abnormalities in offspring | NR |
12.9% (26/202) vs 5.7% (23/404); p = 0.004 ORc 2.45, 95% CI 1.36 to 4.41 |
The results of the small study42 indicated an association between radiation exposure and increased stillbirths, spontaneous abortion and abnormalities in offspring, this last result being highly statistically significant (p = 0.004).
The larger study in a sample of adolescent patients with idiopathic scoliosis found a statistically significant association between radiation exposure and a reduction in stillbirths but an increase in spontaneous abortion. 43 It found a non-significant association with unsuccessful attempts at pregnancy and did not report on neonatal deaths or abnormalities in offspring.
Overall, the limited data did not show evidence of an increased risk of stillbirths associated with diagnostic X-ray exposure during childhood and adolescence for patients with orthopaedic conditions, but indicated an increased risk of spontaneous abortions.
Discussion
This systematic review identified a limited number of relevant studies assessing the association between the risk of cancer or adverse reproductive outcomes and diagnostic X-ray exposure. Based on the quality assessment using the prespecified criteria, the majority of included studies evaluating cancer risk associated with diagnostic radiation were of reasonable quality. All of the data from the four included studies were derived from the same large US Scoliosis Cohort, differing only in terms of the outcome measures, methods of analysis and length of follow-up. It should also be noted that the findings from most studies were based on patient samples exposed to X-rays before 1965. Therefore, these findings may not be generalisable to the current patients with scoliosis, as radiation dose of modern machines has been reduced and other methods are now used to minimise organ dose.
The quality of two studies42,43 assessing the risk of adverse reproductive outcomes associated with diagnostic X-rays was poor, owing to the potential for substantial recall bias (particularly for spontaneous abortion) and failure to adjust for important confounding factors in the analyses (such as age of mother, smoking status and alcohol consumption). In addition, the results of the small study42 are subject to strong confounding factors, in particular the exposure to radiation during pregnancy. The results of this study cannot be interpreted as reliable, nor are they generalisable to a population exposed at times other than pregnancy.
The US Scoliosis Cohort studies provided evidence of increased breast cancer mortality risk in female patients with spinal curvature who were exposed to multiple X-rays. The data demonstrated a significant trend for increased risk of breast cancer mortality as the cumulative radiation dose to the breast increased. The data showed a marginally significant radiation dose–response relationship for breast cancer risk among female patients with scoliosis, which was statistically significant in those reporting a family history of breast cancer.
The data did not show significant increases in the risk of dying from other cancers, such as leukaemia or liver, cervical and lung cancer.
There were only sparse poor-quality data available assessing the risk of abnormal reproductive outcomes in adulthood associated with medical diagnostic X-ray radiation exposure received in childhood and adolescence for orthopaedic conditions. The limited and poor-quality data did not show an increased risk of stillbirths for patients exposed to diagnostic X-rays, but indicated an increased risk of spontaneous abortion.
Conclusions
The evidence relating to the risks of radiation exposure has been reviewed in the reports of international and UK radiation authorities. Our systematic review contributes an evaluation of the risk of cancer and adverse reproductive outcomes associated with diagnostic X-ray radiation exposure specifically for patients with orthopaedic conditions. Despite the limited data, the findings from our review showed that, when compared with the general female population, there was a clear association between increased risk of breast cancer mortality and diagnostic X-ray exposures among female patients with scoliosis or spinal curvature, with a significant radiation dose–response relationship. There was a highly significant trend for increased risk of breast cancer with increased cumulative radiation dose, particularly in patients with a family history of breast cancer. Only limited poor-quality data were available regarding the risk of adverse reproductive outcomes in orthopaedic patients.
Review of existing economic evaluations
Methods
Systematic searches of the literature were conducted to identify potentially relevant studies for inclusion in the assessment of cost-effectiveness. Three separate searches were undertaken to identify:
-
Full economic evaluations of EOS against any comparators A broad range of study designs was considered, including economic evaluations conducted alongside randomised or non-randomised comparator trials, modelling studies, cost analyses, and analyses of administrative databases. Searches for economic evaluations were conducted as part of the EOS systematic review literature searches, as described earlier (see Methods for reviewing the clinical effectiveness of EOS, Search strategy, above). The following electronic sources were searched for relevant published literature:
-
MEDLINE
-
EMBASE
-
CINAHL
-
HMIC
-
ISI SCI
-
The Cochrane Library (including CDSR, DARE, HTA Database, NHS EED and the CENTRAL).
Full details of the search strategies are presented in Appendix 1.
-
-
Economic evaluations in the indications of interest, where standard X-ray was assessed against other comparators These searches were conducted with a view to gaining insights into the modelling methods, structural assumptions and sources of data (including costs) that might be used in the development of a new decision-analytic model for EOS. These studies were not subject to a formal review unless they complemented the evaluation of EOS. The searches did not specifically search for cost data on EOS, as this would have been retrieved by the generic searches conducted for EOS. They were not intended to be exhaustive but rather to identify the most relevant publications in the subject area. The following electronic databases were searched on 15 November 2010 from 2000 to the most recent date available. Full details of the search strategies are presented in Appendix 1.
-
EconLit
-
EMBASE
-
MEDLINE
-
NHS EED.
-
-
Quality of life and cost data for the relevant indications The searches were conducted to provide potential sources of data, highlight areas of uncertainty and provide benchmark values on which to compare QoL and cost estimates used in the de novo economic evaluation. Again, the searches were not intended to be exhaustive, but aimed to identify the most relevant publications in the subject area.
The following electronic databases were searched on 22 November 2010 from 2000 to the most recent date available:
-
The Cochrane Library (including CENTRAL and the NHS EED)
-
EconLit
-
EMBASE
-
MEDLINE.
Full details of the search strategies are presented in Appendix 1.
The assessment of all retrieved titles and abstracts for inclusion was undertaken independently by two reviewers, and discrepancies resolved by consensus. The quality of any cost-effectiveness studies identified would be assessed according to the methods guidance for economic evaluations developed by NICE. 44
The manufacturer of EOS imaging system was requested to provide any information and relevant literature on the costs and potential benefits of EOS, including economic evaluation studies. Economic evaluations received from the manufacturer are discussed below.
Results
The systematic literature search identified no economic evaluation studies of EOS that met the inclusion criteria for the review. The searches for economic evaluations in relevant indications did not identify any studies that would complement the evaluation of EOS.
The manufacturer provided four electronic files relating to economics of the EOS system: the recommendation of CEDIT (Comité d’Évaluation et de Diffusion des Innovations Technologique – Committee for the Evaluation and Diffusion of Innovative Technologies),45 and three costing analyses, one for the French setting,46 and two focusing on the US setting. 47,48
None of the files provided by the manufacturer was a full economic evaluation that compared two or more options and considered both costs and consequences (including cost-effectiveness, cost–utility or cost–benefit analyses). CEDIT45 compared the costs and throughput of EOS with conventional X-ray (CR and DR). Potential health benefits of the intervention were not considered in the analysis. CEDIT45 estimated the average real cost of an EOS examination to be €74, assuming an activity level of 5000 examinations per year. It concluded that the acquisition of an EOS system is justified for (1) centres undertaking a minimum of 4000 whole-spine radiographs per year, assuming a fixed reimbursement price of approximately €108 per procedure and (2) centres undertaking a minimum of 5000 examinations per year (composed of 50% whole spine radiographs, 25% of lower limbs radiographs and 25% of pelvis radiographs). CEDIT’s full report was not made available to the external assessment group (EAG). Therefore, the EAG was unable to review the analysis and relate its validity to the UK setting.
The costing analysis for the French setting consisted of a financial analysis of the potential revenue that could be achieved through the acquisition of EOS, based on tariff prices for different types of radiographs. 46 This analysis is not considered relevant to the perspective of the UK NHS, which operates a tariff based on health-care resource groups and not individual procedures.
The two costing analyses for the US setting were based on projected Medicare and private fees for each X-ray scan and projected activity for the EOS system. 47,48 The increase in revenue from the use of EOS compared with conventional X-ray was because of a projected increase in the quantity of scans undertaken through the acquisition of EOS. Similarly to the analyses for the French setting, these studies are not considered relevant to the perspective of the UK NHS. Neither study compared EOS with an alternative technology, nor considered the potential health benefits to patients. Consequently, these costing studies are not considered further in the assessment of the cost-effectiveness of EOS.
The following section presents a new decision-analytic model that has been developed to provide an assessment of the cost-effectiveness of EOS in the context of the UK NHS.
Description of decision-analytic model
Overview
A decision-analytic model was developed to formally assess the cost-effectiveness of EOS for monitoring the indications listed in Table 1 from the perspective of the UK NHS. The model provides a framework for the synthesis of data from the review of clinical effectiveness of EOS (see Systematic review of the clinical effectiveness of EOS) and other relevant parameters, such as the risk of cancer from radiation exposure, in order to evaluate the potential long-term cost-effectiveness of EOS. The relevant comparators to EOS are standard X-ray CR and DR.
The primary benefit of EOS is to provide radiographic imaging at relatively low-dose radiation. Therefore, the model considers the long-term costs and consequences associated with radiation exposure. The model estimates the total radiation exposure to patients over a lifetime for the diagnosis and long-term monitoring of the indications for both standard X-ray (CR and DR imaging) and EOS. The subsequent outcomes from radiation exposure on the risk of cancer and mortality are explicitly modelled to determine the impact on health outcomes and costs to the NHS and Personal Social Services.
In addition, threshold analysis is undertaken to assess the magnitude of health benefit over and above that associated with reduction in radiation which EOS would need to achieve to be considered cost-effective. This would relate to any changes in the pathway of care for patients resulting from the use of EOS rather than standard X-ray, i.e. changes in diagnosis and/or therapy that ultimately have a positive impact on patients’ life expectancy or QoL. Outcomes in the model are expressed in terms of QALYs. The model evaluates costs from the perspective of the NHS and Personal Social Services, expressed in UK pounds sterling at a 2011 price base. Both costs and outcomes are discounted using a 3.5% annual discount rate, in line with current guidelines. 44 All stages of the work were informed by discussion with our clinical advisors to provide feedback on specific aspects of the analysis such as the modelling approach, data inputs and assumptions. The internal validity of the model was undertaken by two reviewers, who independently checked the inputs and the calculations of the model to ensure that all data and calculations were accurate.
The following sections outline the structure of the model and provide an overview of the key assumptions and data sources used to populate the model in detail.
Modelling approach
The model estimates the total radiation exposure over the monitoring period for the various indications. In order to estimate the lifetime radiation dose owing to diagnostic imaging, the model requires the following inputs for each of the indications considered:
-
the average patient age at diagnosis
-
the frequency of monitoring over a lifetime
-
differences in monitoring for patients where surgery is indicated
-
type of radiographs used for diagnosis and monitoring
-
radiation dose associated with each type of radiograph.
The frequency of monitoring over a patient’s lifetime depends on age at diagnosis, pattern of monitoring, child and adolescent growth and whether or not surgery is indicated. The radiation dose for each type of radiograph used during diagnosis and monitoring is estimated. The lifetime risk of cancer attributable to radiation exposure (LAR) is then calculated. Subsequent health effects from cancer in terms of reductions in life expectancy and QoL, as well as an increase in costs, are modelled using previously developed cancer screening models. Figure 3 shows the modelling approach.
FIGURE 3.
The modelling approach.
As well as a potential reduction in radiation dose, and hence cancer risk, the use of EOS may have implications for the quality and nature of the image. This may have knock-on effects on medical or surgical care, with consequent implications for patients’ health outcomes. Threshold analyses are undertaken to explore the necessary size of these effects, in addition to the impact of cancer risks, in order for EOS to be considered cost-effective.
The cost-effectiveness of EOS is evaluated by comparing the costs and health outcomes associated with EOS with those from standard X-ray. The model will ascertain whether or not the additional costs of EOS are offset by the reduction in cancer risk achieved through reduced lifetime radiation exposure. Resource utilisation and costs were estimated for EOS and its comparators, with particular attention given to patient throughput. Patient throughput is likely to be a major determinant of the cost-effectiveness of EOS. The average cost per procedure of EOS or standard X-ray decreases with utilisation: the greater the number of procedures undertaken, the lower the average cost. Estimates of likely throughput with EOS are both uncertain (there is little reliable evidence to use for this purpose) and variable (they depend on how many EOS scanners are introduced in the NHS and the relevant patient throughput in each centre). The same applies for standard X-ray. A range of scenarios is considered regarding throughput with EOS and standard X-ray, as well as threshold analysis to explore the critical throughput levels to be achieved for EOS to be considered cost-effective.
The following sections provide a detailed overview of the model inputs and the main assumptions. Appendix 8 also provides a summary of the model inputs. A base-case analysis is then undertaken using a particular set of assumptions. A series of detailed scenario analyses follow, exploring the impact of a range of alternative assumptions on the overall cost-effectiveness results. Threshold analyses are used to explore the parameter values required to generate a cost-effectiveness ratio acceptable to the UK NHS.
Model inputs
Types of radiograph
In order to estimate the cumulative radiation dose of EOS and standard X-ray, it is necessary to identify the types and numbers of radiographs used for the monitoring of each indication. Different indications require specific types of radiographs for diagnosis and monitoring. In the absence of published literature, expert advice was used to establish the type of radiograph required for monitoring each of the indications. Table 7 summarises the type of radiograph required for monitoring each indication.
| Indication | Type of radiograph | |
|---|---|---|
| Children | Adolescents and adults | |
| Scoliosis | ||
| Congenital kyphosis | Spine PA or AP | Thoracic spine PA or AP |
| Ankylosing spondylitis | Spine LAT | Thoracic spine LAT |
| Scheuermann’s disease | Lumbar spine PA or AP | |
| Other deforming dorsopathies | Lumbar spine LAT | |
| Congenital deformities of spine | ||
| Congenital deformities of lower limbs and hips | Frontal femur | Frontal femur |
| Frontal lower legs | Frontal lower legs | |
| Pelvis PA | Pelvis PA | |
Frontal spine radiographs are usually performed in the PA position in order to reduce irradiation of the sensitive organs. However, in some cases AP views are taken, either to reduce image distortion or in patients who have difficulty in standing without support. 49
Monitoring pattern
The monitoring pattern for each indication relates to how often patients are scanned throughout their lifetime. The frequency of monitoring depends on the age at diagnosis, the pattern of monitoring, child and adolescent growth, whether or not surgery is indicated and the age at which patients have surgery.
Given the limited evidence in the published literature, expert advice was sought to establish for the average patient and for each indication, the monitoring pattern, the age at diagnosis, age at surgery and the proportion of patients undergoing surgery. Inevitably, there will be considerable variability around this average.
In the absence of formal evidence, where surgery is indicated, it is assumed to take place 2 years post diagnosis for scoliosis, congenital kyphosis and Scheuermann’s disease. For ankylosing spondylitis and congenital deformities of spine, hips and limbs, surgery is assumed to take place at the same age as the first scan for spinal deformity. Details of the monitoring pattern assumptions made for each indication are summarised and briefly described below (see Figures 4–7).
FIGURE 4.
Monitoring pattern for scoliosis.
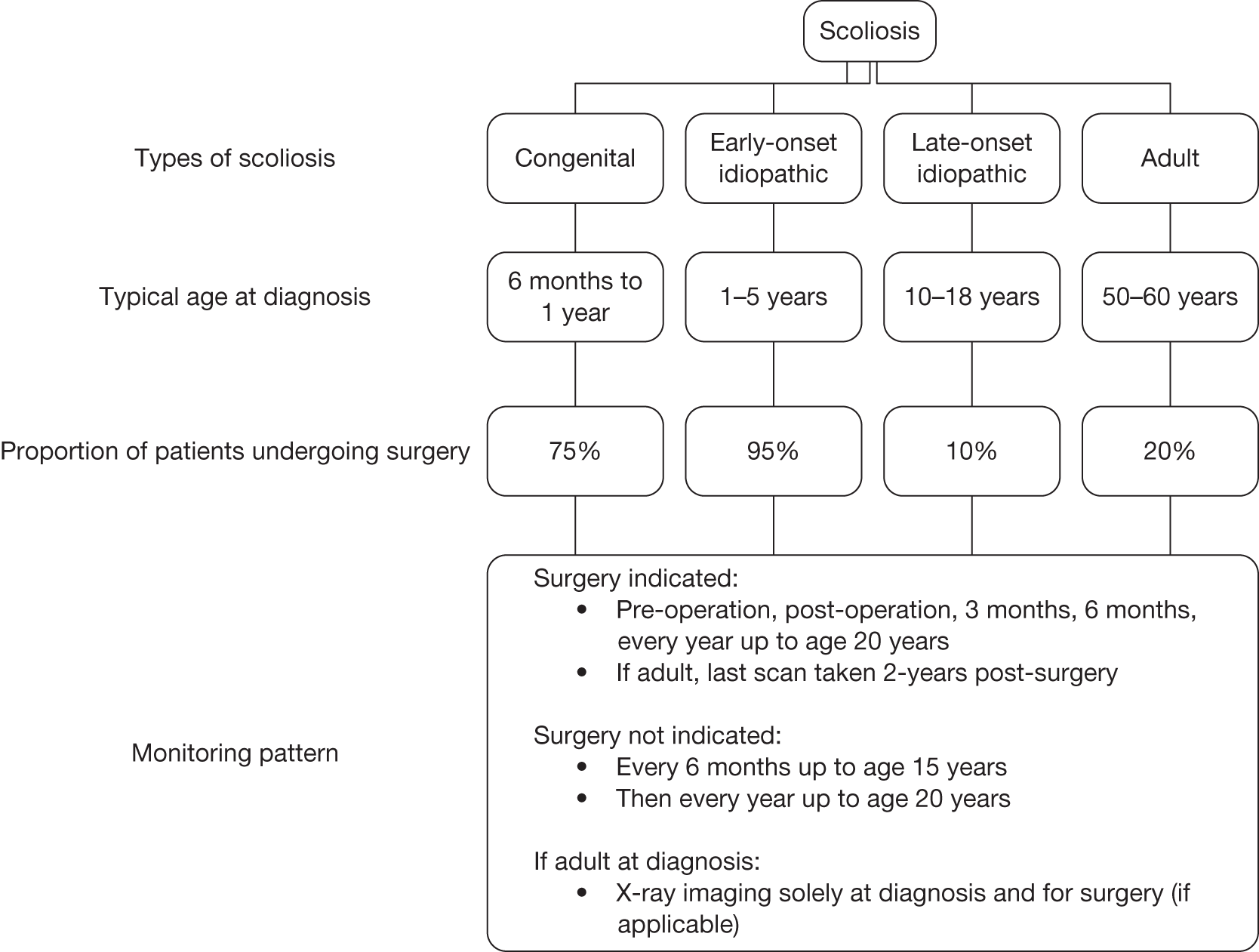
FIGURE 5.
Monitoring pattern for kyphosis.
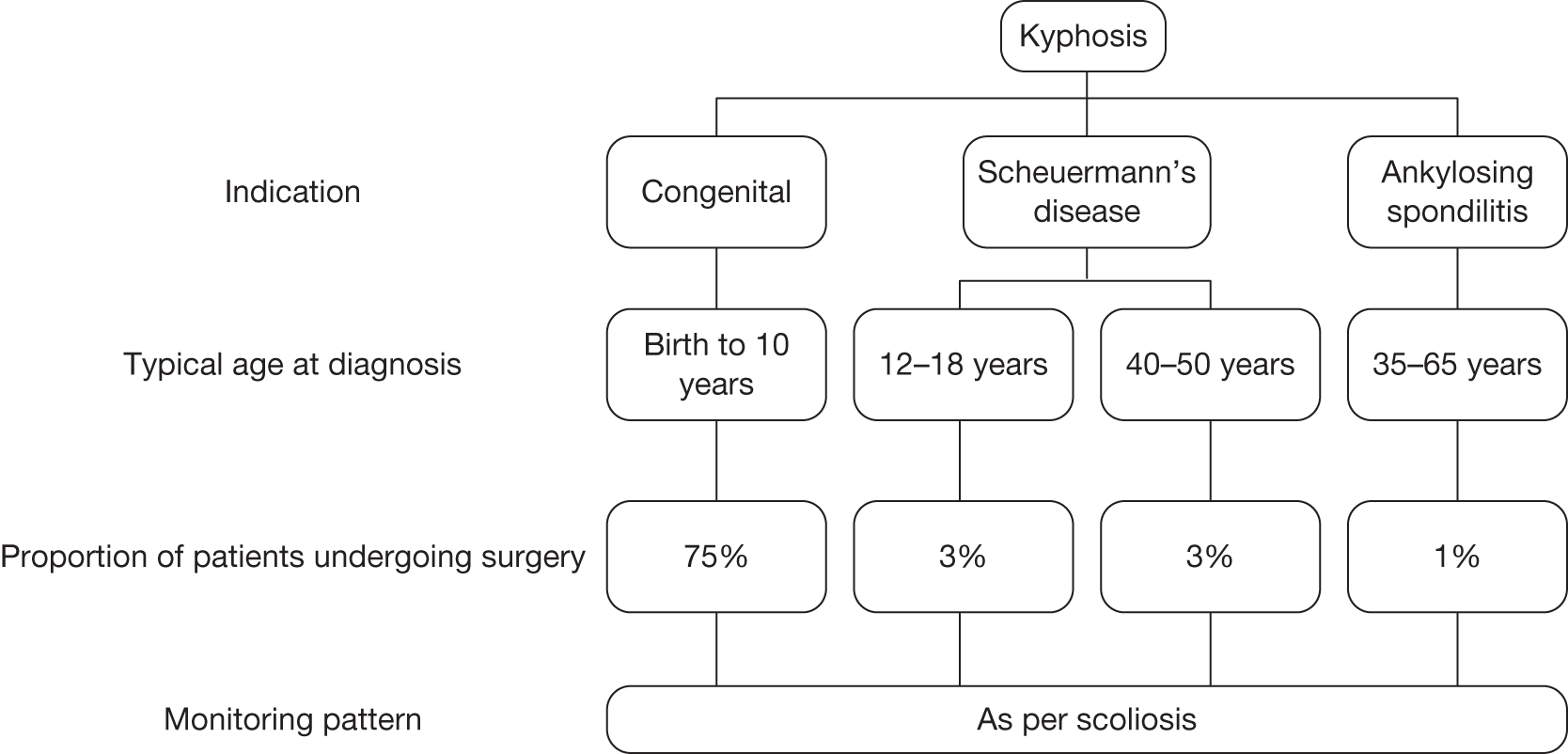
FIGURE 6.
Monitoring pattern for deforming dorsopathies.
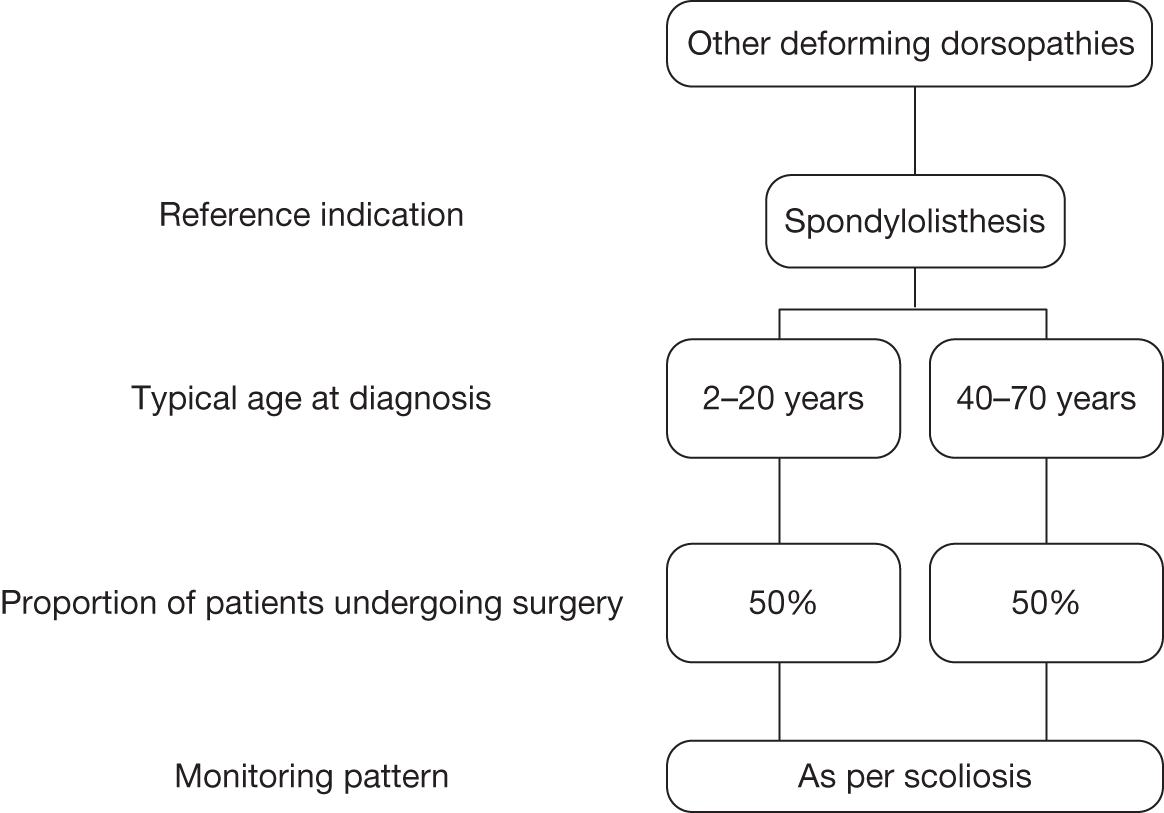
FIGURE 7.
Monitoring pattern for congenital deformities.
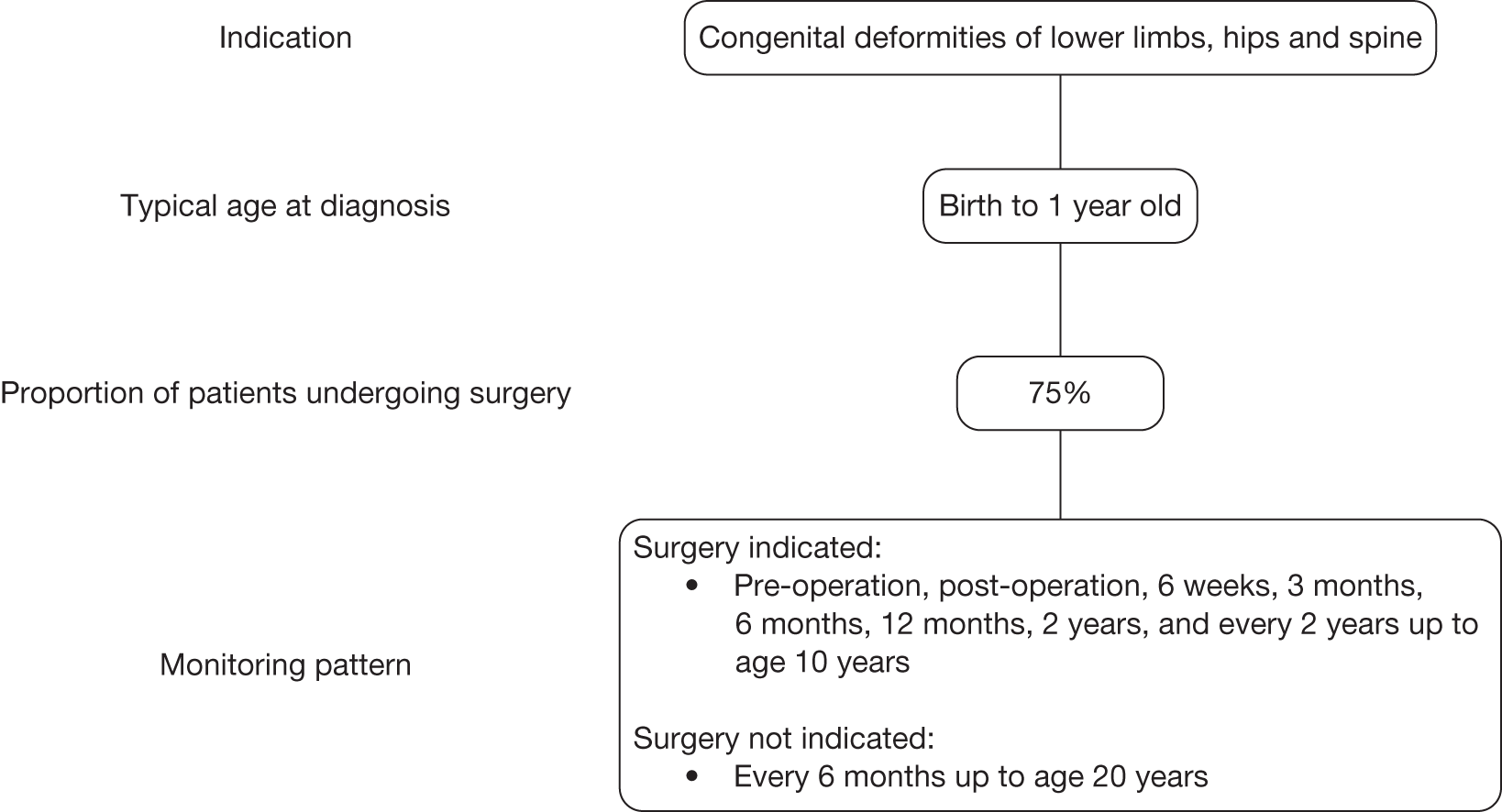
Scoliosis
For the four scoliosis indications (congenital, early-onset idiopathic, late-onset idiopathic and adult), when spine surgery is indicated, the patient has to be scanned preoperatively. Following surgery, a patient is assumed to be scanned postoperatively, at 3 months, 6 months, and then every year up to the age of 20 years. The last scan for an adult patient (> 18 years of age) is assumed to occur 2 years after surgery. If surgery is not indicated, the average patient with scoliosis is assumed to be scanned every 6 months up to the age of 15 years then every year thereafter up to cessation of skeletal growth. Cessation of skeletal growth varies between individuals, but it is assumed that the average point of cessation is at the age of 20 years. Figure 4 summarises the age at diagnosis, the proportion of patients undergoing surgery and the monitoring pattern for scoliosis assumed in the model.
Kyphosis
Kyphosis can be subdivided into congenital and acquired. Acquired kyphosis can be caused by a variety of indications. However, for the evaluation of EOS, only Scheuermann’s disease and ankylosing spondylitis were considered within the scope, in addition to congenital kyphosis (see Chapter 1, Descriptions of the technology under assessment).
Patients with congenital kyphosis may be diagnosed between birth and 10 years old. X-ray imaging is usually taken every 6 months to 1 year up to cessation of skeletal growth. Depending on the location of the kyphotic curve, patients may develop compensatory lordosis, which can be associated with secondary scoliosis. X-ray imaging for congenital kyphosis is assumed to follow the same monitoring pattern as congenital scoliosis.
Patients with ankylosing spondylitis typically present with spinal deformity between the ages of 35 and 65 years. X-ray imaging is usually taken at diagnosis but it is assumed that regular monitoring is not required. A small proportion of patients with ankylosing spondylitis may undergo spine surgery. The monitoring of these patients is assumed to follow the same pattern as spine surgery in adult scoliosis.
Scheuermann’s disease can be diagnosed during adolescence or adulthood. It is assumed that patients in their mid-teens, largely male, are managed in the same way as those with adolescent scoliosis. Adult patients typically present between the ages of 40 and 50 years. X-ray imaging is taken at diagnosis but it is assumed that regular monitoring is not required. From discussions with clinical experts, it is also assumed that around 3% of patients with Scheuermann’s disease require spine surgery. The monitoring of these patients is assumed to follow the same pattern as spine surgery in scoliosis.
Figure 5 summarises the age at diagnosis, the proportion of patients undergoing surgery and the monitoring pattern for the kyphotic indications assumed in the model.
Other deforming dorsopathies
For other deforming dorsopathies that do not fall under the indications of scoliosis or kyphosis, spondylolisthesis was used as a reference indication. Children and adolescents under the age of 20 years are assumed to be scanned every year up to cessation of skeletal growth. X-ray imaging for adults, who typically present after the age of 40 years, is assumed to follow the same monitoring pattern as above for scoliosis. From discussions with clinical experts, it is assumed that 50% of patients with deforming dorsopathies require spine surgery. Figure 6 summarises the age at diagnosis, the proportion of patients undergoing surgery and the monitoring pattern for deforming dorsopathies assumed in the model.
Congenital deformities of lower limbs, hips and spine
Congenital deformities encompass a number of indications, which makes it difficult to define precisely an average pattern of monitoring. The model assumes that a patient is diagnosed at birth and undergoes surgery at 1 year if surgery is indicated. X-ray imaging is assumed to take place preoperatively, postoperatively, 6 weeks post surgery, and then 12 weeks, 6 months, 12 months, 2 years post surgery and every 2 years up to the age of 10 years. Inevitably there will be considerable variability around this average. In the case of patients who do not undergo surgery, X-ray imaging is assumed to be every 6 months up to age 20 years. Figure 7 summarises the age at diagnosis, the proportion of patients undergoing surgery and the monitoring pattern for congenital deformities assumed in the model.
Radiation dose
Radiation dose associated with standard X-ray
The Centre for Radiation, Chemical and Environmental Hazards (CRCE), formerly the National Radiological Protection Board (NRPB), of the HPA, collects information on patients undergoing medical and dental X-ray examinations and interventional procedures in the UK NHS and independent sector, and stores it in the National Patient Dose Database (NPDD). The purpose of the NPDD is to monitor trends in patient doses and provide national reference doses. 50 Every 5 years, the HPA reports these national measures of dose. In a personal communication with Paul Shrimpton from HPA (January to February 2011), typical organ doses and effective doses were estimated for a range of diagnostic X-ray examinations from UK data for 2005. Effective doses were calculated using tissue weighting factors recommended by the ICRP publication 103 report18 and ICRP publication 60 report. 51
Table 8 provides a summary of the effective doses for adult patients for the radiographs of interest above (see Types of radiograph). The effective dose ranges from 0.14 to 0.39 mSv for the thoracic and lumbar spine. The estimates are considered to represent the best available evidence of radiation dose associated with diagnostic radiographs in the UK. However, the estimates are based on data collected between 2001 and 2006 and fewer than one-quarter of the total rooms recorded information on the type of imaging equipment for the radiographic examinations. Of the rooms where this detail was recorded, 55% used a film–screen combination, 40% used CR and 5% used DR. 50 Generally, doses were reported to be similar between the three types of system, with a few exceptions in which significant reductions were achieved with CR. 50 In the absence of formal evidence, the model assumes equivalent effective doses for CR and DR. An alternative scenario examines a reduction of two-thirds in effective dose for DR compared with CR (S MacLachlan, HPA, 16 February 2011, personal communication).
| Radiograph | Effective dose (mSv) |
|---|---|
| Thoracic spine AP | 0.24 |
| Thoracic spine LAT | 0.14 |
| Lumbar spine AP | 0.39 |
| Lumbar spine LAT | 0.21 |
| Pelvis AP | 0.28 |
| Femur AP | 0.011 |
| Knee AP | 0.0001 |
Organ and effective doses for children might be expected to be lower than for adults if full optimisation of the exposure conditions to the size of the patient is practised during radiographic examinations. Data obtained for adults included examples of estimated effective doses to children for three radiographic examinations of the chest, abdomen and pelvis/hips when following guidelines of best practice52 and compared these doses with those of adults. Of these examinations, only the pelvis/hips are of interest for the indications described in Chapter 1 [see Condition(s) and aetiology(ies)]. The effective dose for children and adolescents aged between 1 and 15 years ranged from 0.01 to 0.11 mSv for pelvis/hips AP. This was comparable to 0.42 mSv for the same radiograph in adults.
A review of the literature for effective doses for children identified a study by Hansen et al. ,49 which examined spine radiographs in children and adolescents. Examinations were undertaken in a small sample of 49 children using plain film and 21 using CR. 49 These doses were used to provide estimates for radiographs of the spine PA, spine AP and spine LAT in children and adolescents. For the pelvis, the doses from the NPDD were used. In the absence of evidence for the femur and lower legs in children, the dose ratio between adult and children for pelvis AP was applied to the adult doses in Table 8 to obtain an estimate of effective dose in children. Table 9a and b provides a summary of the effective doses for children for the radiographs of interest.
| Type of radiograph | Age range (years) | |||
|---|---|---|---|---|
| 1–2 | 3–6 | 7–12 | 13–18 | |
| Spine AP | 0.0600 | 0.0490 | 0.0290a | 0.0300a |
| Spine PA | 0.0600a | 0.0490 | 0.0290 | 0.0300 |
| Spine LAT | 0.0780b | 0.0780 | 0.0580 | 0.0480 |
| Type of radiograph | Age range (years) | |||
|---|---|---|---|---|
| 1–4 | 5–9 | 10–14 | > 15 | |
| Pelvis AP | 0.01 | 0.06 | 0.08 | 0.11 |
| Femur APa | 0.00022 | 0.00154 | 0.00209 | 0.00286 |
| Knee APa | 0.000002 | 0.000014 | 0.000019 | 0.000026 |
| Ratio of doses: children–adults | 0.02 | 0.14 | 0.19 | 0.26 |
Radiation dose associated with EOS
The systematic review of the clinical effectiveness of EOS described above (see Systematic review of the clinical effectiveness of EOS) identified three relevant studies comparing the radiation dose associated with EOS to standard X-ray:
-
Kalifa et al. 25 compared EOS with film radiography in 140 children aged > 5 years.
-
Le Bras et al. 26 (unpublished) compared EOS with film radiography in adolescents.
-
Dechênes et al. 27 compared EOS with CR in 49 children.
In summary, Kalifa et al. 25 and Le Bras et al. 26 (unpublished) report ESD for different types of radiographs for both EOS and film X-ray. Deschênes et al. 27 reports ESD to specific locations irradiated in the body. 27 The ratio of mean ESD between standard X-ray and EOS varies largely depending on the study and type of X-ray examination (see Table 3). Kalifa et al. 25 reported ratios of between 11.6 and 18.8 for spine AP, PA, LAT and pelvis, whereas ratios of 5.2 for spine PA and 6.2 for spine LAT can be estimated from Le Bras et al. 26 The ratio of mean ESD in the more recent study by Deschênes et al. 27 varies between 2.9 and 9.2, depending on the body site. 27
As discussed above (see Systematic review of the clinical effectiveness of EOS), there is considerable uncertainty regarding the reduction of radiation dose achieved with EOS, both within and between studies. The ratios of mean ESD reported in Dechênes et al. 27 are approximately in line with the ratios reported in Le Bras et al. 26 In contrast, the dose reduction reported in Kalifa et al. 25 is much higher. The reason behind this discordance in results is not clear but may be because of the older technology used in Kalifa et al. 25 in comparison to the more recent studies. As none of the studies reported SDs or CIs, the full extent of uncertainty in these estimates is unknown.
In order to reflect the uncertainty and heterogeneity, no formal synthesis of these studies was attempted. The model assumes a mean dose reduction of 6.73, which corresponds to the average of the values reported in Dechênes et al. 27 and Le Bras et al. 26 The sensitivity of the results to this assumption will be explored by examining an extreme scenario of a high-dose reduction with ratio of means equal to 18.83, corresponding to the highest dose reduction observed across the three studies.
It is worth noting that effective dose was not used as the comparative measure of radiation exposure in these studies. All three studies reported ESD. ESD does not account for the variation in radiosensitivity of the different organs of the body, the thickness of the patient’s body and the distribution of absorbed dose. Following the advice of experts, it was considered appropriate to use the ratio of mean ESD applied to effective dose as a first approximation for the reduction in radiation exposure achieved with EOS, on the assumption that X-ray beam sizes, anatomical positions and radiation qualities are similar.
Cancer risk because of radiation exposure
As discussed in Chapter 1 (see Outcomes), radiation from diagnostic X-rays can result in stochastic (random) effects that are noticeable only years after exposure. Diagnostic X-rays are the largest man-made source of radiation exposure to the general population, contributing to around 14% of total annual exposure worldwide from all sources. 30 A review of the literature above (see Adverse effects of diagnostic radiation for patients with orthopaedic conditions) identified four sources of data on the effects of low levels of ionising radiation on health:
-
BEIR VII Phase II report Health risks from exposure to low levels of ionising radiation by the National Academy of Sciences, published in 2006. 17
-
UNSCEAR 2008 Sources and effects of ionising radiation by UNSCEAR. 30
-
ICRP publication 103 report The 2007 Recommendations of the ICRP. 18
-
Personal communication with Paul Shrimpton from the HPA (January to February 2011).
Each of these sources estimated the risk of cancer based on epidemiological data from (1) the Japanese atomic bomb survivors; (2) medical radiation studies; (3) occupational radiation studies; and (4) environmental radiation studies. The majority of what is known about the effects of low level ionising radiation is from the epidemiological data of the Life Span Study of atomic bomb survivors. The latest report by the Life Span Study is based on data of over 80,000 atomic bomb survivors who were within 10 km of the hypocentre, as well as around 25,000 individuals who were not in the cities at the time of the bombing, and were followed for over 40 years. 17
Epidemiological data on radiation-induced cancers has been historically analysed using dose–response models of excess absolute risk and ERR. The simplest model, and the one most favoured, assumes that the risk caused by the exposure is proportional to the baseline risk as well as to the exposure. 17,18,30 These models follow a linear non-threshold approach, which implies that the risk of cancer is proportional to exposure in a linear way and that there is no safe exposure dose. 17,18,30,53,54 Therefore, the total cumulative lifetime cancer risk can be obtained by adding the cancer risk associated with each radiographic examination.
In a personal communication with Paul Shrimpton from the HPA (discussed in previous section: see Radiation dose), lifetime risk of radiation-induced cancer was calculated as a function of age at exposure and sex according to the risk models in ICRP publication 103 report. 18 Table 10 provides a summary of the lifetime cancer risk per unit dose for all cancers by age and sex at exposure. Similar risk estimates are available in the BEIR VII report17 for the US population, and these were used as part of a sensitivity analysis.
| Age at exposure (years) | Risk of all cancers (per Gya) | |
|---|---|---|
| Males | Females | |
| 0–9 | 0.0999 | 0.1270 |
| 10–19 | 0.0800 | 0.0994 |
| 20–29 | 0.0623 | 0.0795 |
| 30–39 | 0.0512 | 0.0646 |
| 40–49 | 0.0422 | 0.0562 |
| 50–59 | 0.0327 | 0.0441 |
| 60–69 | 0.0223 | 0.0320 |
| 70–79 | 0.0132 | 0.0194 |
| 80–89 | 0.0055 | 0.0075 |
| 90–99 | 0.0004 | 0.0002 |
The lifetime risks of radiation-induced cancer in Table 10 were applied to the effective dose estimates for each type of radiograph (see Radiation dose, previous section) used during diagnosis and monitoring of the indications to estimate a total risk of cancer attributable to radiation exposure for standard X-ray. The ratio of reduction in radiation associated with EOS was then applied to obtain a reduced risk of radiation-induced cancer for EOS. Table 11 summarises the lifetime cancer risk for EOS compared with standard X-ray for the indications, taking account of the frequency of monitoring.
| Indication | Lifetime cancer riska | |
|---|---|---|
| Standard X-ray | EOS | |
| Congenital scoliosis | 0.0009949 | 0.0001478 |
| Early-onset idiopathic scoliosis | 0.0009139 | 0.0001358 |
| Adolescent or late-onset scoliosis | 0.0008079 | 0.0001200 |
| Adult scoliosis | 0.0000903 | 0.0000134 |
| Congenital kyphosis | 0.0009043 | 0.0001343 |
| Congenital deformities | 0.0003750 | 0.0000557 |
| Scheuermann’s disease: adolescent | 0.0006101 | 0.0000906 |
| Scheuermann’s disease: adult | 0.0000583 | 0.0000087 |
| Ankylosing spondylitis | 0.0000403 | 0.0000060 |
| Deforming dorsopathies: adolescent | 0.0009954 | 0.0001479 |
| Deforming dorsopathies: adult | 0.0001693 | 0.0000252 |
Consequences of cancer
The effects of radiation exposure on the risk of cancer are related to final health outcomes from cancer, expressed in QALYs. This is necessary in order to provide an indication of the net health effect of EOS, relative to its additional cost and the effects of standard X-ray, in units that permit comparison with other uses of health service resources.
Cancer results in a decrease in life expectancy and QoL, as well as an increase in costs. In order to estimate the costs and QALYs associated with cancer, previously developed cancer models were sought. The School of Health and Related Research (ScHARR) at the University of Sheffield has undertaken comprehensive assessments of the economic burden of treating colorectal and prostate cancer. 55,56 In collaboration with Paul Tappenden (ScHARR, 25 January 2011, personal communication), costs and outcomes for colorectal and prostate cancer were obtained. 55,56 These cancer models were able to provide an estimate of the number of life-years and QALYs lost from the point of cancer diagnosis to death for an average age at diagnosis compared with the general population. In addition, total costs from the point of clinically confirmed cancer diagnosis to death were obtained for both colorectal and prostate cancer, based on treatments used in current practice.
Similar models were also obtained for breast and lung cancer. 57,58 These models provided an average age at diagnosis, average costs, life-years and QALYs from the point of cancer diagnosis to death. With the exception of prostate cancer, all models were run probabilistically, in that each input in the model was entered as an uncertain rather than a fixed parameter. The results in the form of a set of probabilistic sensitivity analysis simulations were read directly into our model to allow exploration of the sensitivity of the cost-effectiveness results to uncertainty in the cancer estimates. Table 12 provides a summary of the total costs and QALYs lost because of cancer for the four cancers where access to an economic model was available.
| Cancer | Age at diagnosis (years) | Costs of cancer (£) | QALYs lost due to cancer |
|---|---|---|---|
| Breast | 40 | 14,990 | 5.6988 |
| 60 | 13,927 | 3.4219 | |
| Lung | 72 | 22,712 | 6.8011 |
| Colorectal | 74 | 14,075 | 3.4493 |
| Prostate | 74 | 12,389 | 4.6226 |
In the absence of cancer models for all types of cancer, a weighted average of costs and QALYs for the four cancers was used to provide an estimate of costs and QALYs associated with all cancer. This weighting was based on the incidence of radiation-induced cancer reported by type of cancer in BEIR VII:17 for males, the weights were approximately 46% colorectal, 42% lung and 12% prostate, whereas for females the weights were 16% colorectal, 50% lung and 34% breast.
An underlying assumption of the model is that radiation exposure results in a higher risk of cancer incidence, but it is unclear whether or not the age of cancer diagnosis would differ from that of the general population. In the absence of formal evidence, the model assumes the same age of cancer diagnosis as the average patient in the general population with such a diagnosis, although this assumption is explored using scenario analysis. This assumption could have a marked impact on the cost-effectiveness results because of the effects of discounting. Future costs and QALYs are discounted back to their present value to reflect a positive rate of time preference; i.e. benefits obtained today are preferred to benefits accrued in the future. 59 For children and adolescents, this means that the effects of cancer, which is assumed to occur at a much later age in life, are considerably reduced. For adults, the age at diagnosis for spinal deformities is closer to the age of cancer occurrence than in children and adolescents. Hence, the present value of the consequences of cancer is greater for adults than for children and adolescents.
Figure 8 illustrates the effect of discounting on the valuation of the consequences of cancer for the costs and QALYs lost associated with colorectal cancer. At the average age of colorectal cancer diagnosis, costs and QALYs of colorectal cancer are valued at £14,075 and 3.4493, respectively (see Table 12). However, patients enter the model at the age at diagnosis of the relevant orthopaedic indication. Consequently, the costs and QALYs of the cancer occurring in the future are discounted back to that age. In congenital scoliosis, for example, patients are assumed to be diagnosed at 1 year old. Therefore, the future costs accrued and QALYs lost because of cancer are discounted back to 1 year old and are valued at £1153 and 0.2827, respectively. Conversely, in adult scoliosis, patients undergo their first scan at 55 years old. Therefore, costs and QALYs of cancer are discounted back to 55 years old, and are valued at £7392 and 1.8115.
FIGURE 8.
Discounting costs and QALYs lost from colorectal cancer to age at diagnosis.
Costs of EOS and standard X-ray
The cost-effectiveness of EOS is evaluated by comparing the additional costs of EOS to the reduction in consequences achieved through reduced radiation exposure compared with standard X-ray. Therefore, an estimate of the average cost per procedure of EOS, CR and DR is required.
The average cost of an examination is determined by the set-up cost, annual recurring costs and per patient costs. The set-up costs consist of the fitting out of a suitable room, the capital cost of the machine, and the installation costs of the technology (if not included with the capital cost of the machine). The recurring costs consist of the annual maintenance costs, the costs involved in replacing equipment and overheads. Per patient costs consist of the consumables utilised for each procedure and of the staff required. Table 13 summarises the costs included in the average cost per procedure.
| Set-up costs | |
|---|---|
| Fitting out a suitable room | Fitting out a suitable location complying with radiation legislation requirements |
| Capital cost of machine | Capital cost to include all aspects of workstation and software |
| Installation costs | Installation including workstation and software |
| Recurring costs | |
| Annual maintenance costs | Service contract |
| Equipment replacement costs | Replacement parts as required |
| Overheads | For example, electricity, heating |
| Per-patient costs | |
| Consumables | Consumables required per patient visit |
| Staffing costs | Number and type of staff involved and grade |
| Useful life of technology | |
| Technology lifetime | Lifetime of a new system until requiring replacement |
In estimating the costs of EOS and standard X-ray, it is assumed that some categories of cost are equivalent for the two modalities. This assumed equivalence applies to the costs of fitting out a suitable room for the equipment, installation costs, overheads and staff costs. All other costs potentially differ by the type of procedure and are described below.
Costs of EOS
The systematic review of EOS did not retrieve any published information on its costs (see Systematic review of the clinical effectiveness of EOS). In the absence of published literature, the information provided by the manufacturer was used to estimate the costs of EOS (Table 14).
| Costs | Contract 1 | Contract 2 |
|---|---|---|
| Set-up costs (£)b | 400,000 | 400,000 |
| Recurring costs (£)c | ||
| Maintenance | 32,000 per year | 48,000 per year |
| Other | 25,000 (X-ray tube) | |
| Useful life of EOS (years) | 10d | |
Costs of computed radiography and digital radiography
The systematic review of the literature on costs relating to standard X-ray did not identify any studies providing costs of CR or DR in the UK setting. In the absence of formal literature, expert advice was sought from manufacturers and hospital accounting systems to provide information on the costs of CR and DR. Table 15 provides a summary of the estimated costs for CR and DR.
| Costs | CR | DR |
|---|---|---|
| Set-up costs (£) | 95,000 | 167,500b |
| Recurring costs (£) | ||
| Maintenance | 10,000 per year | 18,000 per year |
| Others | 150–200 (cassette)c | 2000 (software upgrades)d |
| Useful life of technology (years) | 10e | |
Patient throughput
Patient throughput is likely to be a major determinant of the cost-effectiveness of EOS. The average cost per procedure of EOS or standard X-ray decreases with utilisation: the greater the number of procedures undertaken, the lower the average cost.
An estimate of patient throughput is needed in order to allocate the fixed costs of providing diagnostic services (e.g. capital costs, maintenance) to the level of the individual procedure and hence to the average patient based on the number of diagnostic procedures they are assumed to require. For EOS this throughput needs to focus on the types of patient numbers expected for the indications for which EOS has a potential benefit. In principle, this throughput can be defined at a national level (e.g. England) – the number of centres for which EOS is purchased then determines how this national throughput is allocated to particular equipment and hence the average cost per procedure. For standard X-ray, the throughput of patients is not tied to the particular indications for which EOS is potentially of value because the equipment can be routinely used for a much wider set of indications.
As a first approximation of throughput for EOS, Hospital Episode Statistics (HES) data were explored. The objective was to provide an estimate of the number of examinations per year performed for each of the indications considered potentially relevant for EOS. HES data consists of three data sets containing details of all admissions to NHS hospitals in England: admitted patients, which includes inpatients and day cases; outpatients; and accident and emergency patients. The HES data are based on financial years, and it has been collected since 1989–90. The most recent collection available at the time of this analysis was for 2008–9. 60
The inpatient data set for 2008–9 was the source used for the estimates on number of procedures undertaken for each relevant indication. These estimates rely on the assumption that each patient episode is associated with a radiography examination. Table 16 summarises the number of episodes per indication in 2008–9 obtained from the HES inpatient data set. These episodes represent an estimate of the total expected patient throughput across England in 1 year. Appendix 6 provides a more detailed breakdown of the number of episodes and patients per four-digit ICD-10 code.
| Indication | Episodes |
|---|---|
| Congenital scoliosis | 153 |
| Early-onset idiopathic scoliosis | 292 |
| Late-onset scoliosis | 1827 |
| Adult scoliosis | 1841 |
| Congenital kyphosis | 167 |
| Scheuermann’s disease: adolescent | 52 |
| Scheuermann’s disease: adult | 27 |
| Ankylosing spondylitis | 1109a |
| Deforming dorsopathies: adolescent | 132 |
| Deforming dorsopathies: adult | 5323 |
| Congenital deformities of spine, hip and lower limbs | 5959 |
| Total | 16,882 |
It is recognised that these figures are likely to underestimate the current X-ray utilisation by patients with the relevant indications being assessed for EOS. This is because many patients are outpatients and, therefore, their visits will not appear as inpatient episodes. However, the outpatient HES data set could not be used to quantify patient throughput owing to very low numbers of episodes recorded in the outpatient database for the indications of interest. Appendix 7 summarises the outpatient attendance for the relevant diagnosis codes during 2008–9. Hence, HES data are used as one of three alternative assumptions on patient throughput. A second assumption uses the same patient throughput as that assumed for standard X-ray (30 patients per working day; see below), and a third assumption uses a higher utilisation for EOS than for standard X-ray, i.e. 48 patients per working day.
As described above, CR and DR systems are routinely used for indications other than those specified in the NICE scope for EOS. Estimates on the throughput of CR and DR should reflect current practice in the NHS. The literature searches on the costs of standard X-ray did not identify any relevant publications to guide estimates of throughput. Owing to the lack of published literature, expert advice was sought to provide estimates of throughput for radiography rooms in NHS hospitals.
Patient throughput depends on the type of examination and on patient characteristics. Some examinations, such as chest radiography, may require shorter appointments and therefore daily throughput could be higher. On the other hand, some patients with mobility difficulties may require a longer appointment slot, reducing daily throughput. In order to reflect the variation in current practice, and based on expert advice, the base case assumed a standard X-ray throughput of 30 patients per working day, assuming 251 working days per year.
Table 17 provides a summary of the assumptions used in the model for the costs of EOS, CR and DR.
| Element of cost | Assumption |
|---|---|
| Costs not considered in the economic evaluation | The following costs were assumed equivalent across EOS, CR and DR:
|
| Costs considered in the economic evaluation | The following costs are considered in the economic evaluation:
|
| Patient throughput for EOS | Inpatient HES data for 2008–9 is assumed representative of the average yearly utilisation |
| No. of scans per year estimated for EOS assumes that every hospital visit is associated with a radiography examination | |
| Patient throughput for standard X-ray | 30 patients per day over 251 working days per year |
| Annual equivalent costs | A discount rate of 3.5% per annum and a useful lifetime of the equipment of 10 years are assumed to translate capital costs into annual equivalent costs59 |
Average cost per scan for EOS
An acquisition cost of £400,000 for EOS results in an annual cost of £48,097, annuitised over 10 years at a rate of 3.5% per annum. The additional costs of the service contract and equipment replacement give a total cost of £86,347 per year without replacement of X-ray tubes (contract 1), or a total cost of £96,097 with the replacement of X-ray tubes (contract 2). The model assumes the cheaper contract (contract 1) would be selected by the NHS.
Applying the estimates of annual patient throughput (see Table 16) to one centre with a single EOS machine gives a cost per scan for each indication, as shown in Table 18. For indications where the patient throughput is low, the cost per scan for that indication is high. In order to give EOS a conservative or optimistic estimate, the cost per scan was obtained by grouping the patient throughput by indication. For example, the cost per scan for each of the four scoliosis indications was based on the total throughput for scoliosis, i.e. the sum of 153, 292, 1827 and 1841 for congenital, early-onset idiopathic, late-onset idiopathic and adult scoliosis, respectively. These estimates (in the last column of Table 16) were used in the base-case analysis as one throughput assumption.
| Indication | Patient throughput by indication | Patient throughput by grouped indications | Cost per scan by indication (£) | Cost per scan by grouped indications (£) |
|---|---|---|---|---|
| Congenital scoliosis | 153 | 4113 | 677.23 | 25.19 |
| Early-onset idiopathic scoliosis | 292 | 4113 | 354.85 | 25.19 |
| Adolescent or late-onset scoliosis | 1827 | 4113 | 56.71 | 25.19 |
| Adult scoliosis | 1841 | 4113 | 56.28 | 25.19 |
| Congenital kyphosis | 167 | 6126 | 620.45 | 16.91 |
| Congenital deformities | 5959 | 6126 | 17.39 | 16.91 |
| Scheuermann’s disease: adolescent | 52 | 79 | 1992.61 | 1311.59 |
| Scheuermann’s disease: adult | 27 | 79 | 3837.62 | 1311.59 |
| Ankylosing spondylitis | 1109 | 1109 | 93.43 | 93.43 |
| Deforming dorsopathies: adolescent | 132 | 5455 | 84.97 | 18.99 |
| Deforming dorsopathies: adult | 5323 | 5455 | 19.47 | 18.99 |
| All indications | 16,882 | 16,882 | 6.14 | 6.14 |
It is important to note that the underlying assumption in the cost estimates presented in Table 18 is that there is only one centre in the UK with a single EOS machine. Increasing the number of centres in the UK with EOS (i.e. dividing the throughput for the relevant indications between more machines), increases the average cost per scan. For example, if there are two EOS machines in the UK, the cost per scan doubles, as the throughput represents the expected patient numbers per annum at national level for the indications for which EOS has a potential benefit. However, there may be indications other than those formally modelled here for which EOS could be used. Adding these additional patients to the throughput for EOS would reduce the average cost per scan. The implications of adding such patients to the EOS throughput for health outcomes are unknown. The analysis considers the implication of adding these other patients to EOS throughput for the cost-effectiveness of the system by examining a scenario where EOS is used at ‘full capacity’ (i.e. 48 patients per working day). Throughput based on full capacity corresponds to a cost per scan of £8.60.
Average cost per scan for computed radiograph and digital radiography
The acquisition cost of standard X-ray is estimated as £95,000 for CR and £167,500 for DR. These capital costs result in an annual cost of £11,423 for CR and £20,140 for DR, annuitised over a useful life of the equipment of 10 years at a rate of 3.5% per annum. The additional costs of the service contract and equipment replacement, including VAT at 20%, give a total cost of £25,760 and £46,369 per year for CR and DR, respectively.
For standard X-ray, the throughput of patients is not tied to the particular indications for which EOS is potentially of value because the equipment is routinely used for a much wider set of uses. As discussed above, the base case assumed a standard X-ray throughput of 30 patients per working day, assuming 251 working days per year. Therefore, the average cost per scan is based on the average activity per patient visit. Table 19 summarises the cost per scan for CR and DR with 100% utilisation of a machine.
| Patient throughput | Throughput per year | Cost per scan for CR (£) | Cost per scan for DR (£) |
|---|---|---|---|
| 30 patients per working daya | 7530 | 3.42 | 6.16 |
Analytic methods
Base-case analysis
The model results are presented according to a particular set of assumptions used as part of the base-case analysis. The impact of using alternative assumptions is then explored using different scenarios. The cost-effectiveness of EOS, in each of the indications, is evaluated by comparing the additional costs of EOS to the reduction in consequences achieved through reduced lifetime radiation exposure compared with standard X-ray. Mean costs and QALYs for EOS, CR and DR are calculated and their cost-effectiveness compared using conventional decision rules, estimating incremental cost-effectiveness ratios (ICERs) as appropriate. 61 The ICER presents the additional costs that one intervention incurs over another and compares this with the additional benefits. To provide a reference point, NICE uses a threshold cost per QALY of around £20,000–£30,000 to determine whether or not an intervention represents good value for money in the NHS. 44 Consequently, if the ICER is < £20,000 then EOS should be considered potentially cost-effective. ICERs within the range (i.e. between £20,000 and £30,000 per QALY) are considered borderline and an ICER > £30,000 is not typically considered cost-effective. When more than two interventions are being compared, the ICERs are calculated using the following process:
-
The interventions are ranked in terms of cost (least expensive to most costly).
-
If an intervention is more expensive and less effective than any other intervention, then the intervention is said to be dominated and is excluded from the calculation of the ICERs.
-
The ICERs are calculated for each successive alternative, from the cheapest to the most costly. If the ICER for a given intervention is higher than that of any more effective intervention, then this intervention is ruled out on the basis of extended dominance.
The base-case analysis assumes that the radiation dose associated with DR is equivalent to the radiation dose of CR. Therefore, there is no differential effect on health outcomes for CR and DR. Given that DR is more expensive than CR and is assumed to produce the same outcomes, DR is ruled out on the basis that it is dominated by CR. Thus, the base-case analysis simplifies to a comparison of the total costs and QALYs of EOS and CR. CR also represents the majority of standard X-ray imaging equipment in current use in the NHS. An alternative scenario compares EOS to DR assuming a lower radiation dose for DR.
Patient throughput is likely to be a major determinant of the cost-effectiveness of EOS, as the average cost per procedure of EOS decreases with utilisation. However, throughput is highly uncertain (there are no reliable data available to provide estimates) and potentially variable between centres. Furthermore, in principle, the use of EOS in the NHS could be centrally planned in such a way that the throughput of patients using the technology could be determined, for example by locating EOS in one specialist or a small number of specialist centres to which patients with particular indications could be sent. Therefore, EOS throughput can be seen as a matter of policy choice in its own right rather than an uncertain parameter to estimate.
Although throughput estimates were obtained from HES, as discussed above (see Model inputs, Costs of EOS and standard X-ray), these are likely to underestimate the true utilisation of X-rays for the relevant indications. Consequently, the base-case results are presented using three alternative throughput assumptions for EOS:
-
Throughput assumption 1, known as TA1. Under this assumption, patient throughput is based on HES data and grouped by indications. For example, the cost-effectiveness of EOS in congenital scoliosis is based on the total throughput for scoliosis as a whole from the HES data (i.e. includes congenital, early-onset idiopathic, late-onset idiopathic and adult scoliosis).
-
Throughput assumption 2, known as TA2. Patient throughput is based on a capacity of 30 patients per working day, corresponding to a total throughput of 7530 per year. This is equivalent to the throughput assumed for CR.
-
Throughput assumption 3, known as TA3. Patient throughput is based on ‘full capacity’ of 48 patients per working day for EOS, corresponding to a total throughput of 12,048 per year. Under this assumption, the throughput for CR remains at 30 patients per working day.
Threshold analysis is also used to establish what patient throughput would be required to achieve ICERs of £20,000 and £30,000 per QALY.
On the benefits side, the model formally assesses the potential reduction in radiation dose, and hence cancer risk, from EOS compared with standard X-ray. Although there is no evidence to confirm this, the use of EOS may have implications for the quality and nature of the image, which, in turn, could have beneficial effects on medical or surgical management with consequent positive implications for patients’ health outcomes. Owing to a lack of formal evidence and insufficient time formally to elicit estimates from clinical experts, the model was unable to explore these implications explicitly. Instead, threshold analyses are undertaken to explore the necessary size of these health effects, in addition to the impact of cancer risk, in order for EOS to be considered cost-effective. These are reported as the additional QALY gains that EOS would need to generate, over and above those associated with reduced radiation, for the technology to be cost-effective assuming a threshold of £20,000.
Scenario analysis
A number of alternative scenarios are considered in which the assumptions used as part of the base-case analysis are varied. These analyses are undertaken to assess the robustness of the base-case results to variation in (1) the sources of data used to populate the model and (2) alternative assumptions relating to the model.
Table 20 summarises the alternative scenarios considered. For each element, the position in the base-case analysis is outlined, alongside the alternative assumption applied. The cost-effectiveness of EOS is considered under each of the scenarios for each of the indications. The same throughput assumptions and threshold analyses outlined above are also undertaken for each of the scenarios.
| Scenario | Element | Position in base-case analysis | Variation in scenario analysis |
|---|---|---|---|
| 1 | Age of cancer diagnosis | Radiation exposure results in a higher risk of cancer incidence but the age of cancer diagnosis is the same as the general population |
For children and adolescents, the age of cancer diagnosis is earlier than the general population: Breast cancer: age of onset from 60 years to 40 years in children and adolescents Lung cancer: from 72 years to 55 years in all populations Colorectal and prostate cancer: from 74 years to 55 years in all populations |
| 2 | Discount rate | 3.5% applied to both costs and outcomes | 0% applied to both costs and outcomes |
| 3 | Effect of EOS on radiation dose | Mean dose reduction of 6.73 (ratio of means comparing EOS to standard X-ray) | High dose reduction with ratio of means 18.83, corresponding to the highest dose reduction in the study by Kalifa et al. (1998)25 |
| 4 | Uncertainty in the costs and QALYs lost due to cancer | Deterministic estimates of mean costs and QALYs lost from cancer models | To explore uncertainty in estimates, probabilistic sensitivity analysis of costs and QALYs lost as a result of cancer |
| 5 | Lifetime risk of radiation-induced cancer | Recent estimates by the HPA based on risk models in ICRP publication 103 report18 | Risk estimates reported in BEIR VII17 for a 1999 US population17 |
| 6 | Radiation dose for DR | Radiation dose for DR is equivalent to dose for CR. CR dominates DR | Radiation dose for DR is reduced to two-thirds of the dose for CR. EOS is compared with DR |
Cost-effectiveness results
Results of the base-case analysis
Table 21 reports the total costs and QALYs for EOS compared with CR in each indication, under TA1 (throughput based on HES data). The ICER for EOS is well above conventional thresholds of £20,000 and £30,000 per additional QALY in all indications. The incremental costs of EOS relative to CR range from £49 to £8702 across the indications, while the incremental QALYs range from 0.000086 to 0.000869. The marked variation in the ICERs across the indications is largely owing to different throughput for the grouped indications of scoliosis (4113 patients per year), congenital kyphosis and deformities (6126 patients per year), Scheuermann’s disease (79 patients per year), ankylosing spondylitis (1109 patients per year) and deforming dorsopathies (5455 patients per year). Owing to small patient numbers at national level for Scheuermann’s disease, it is unlikely that EOS could ever be considered cost-effective in this indication alone under these assumptions regarding throughput.
| Indication | Total QALYs | Incremental QALYs: EOS vs CR | Throughput based on HES TA1 | Total costs (£) | Incremental costs (£): EOS vs CR | ICER (£): EOS vs CR | ||
|---|---|---|---|---|---|---|---|---|
| CR | EOS | CR | EOS | |||||
| Congenital scoliosis | 24.6962 | 24.6969 | 0.000655 | 4113 | 77.19 | 551.90 | 474.72 | 724,903 |
| Early-onset idiopathic scoliosis | 24.6207 | 24.6213 | 0.000623 | 4113 | 70.87 | 506.19 | 435.32 | 699,162 |
| Adolescent or late-onset scoliosis | 23.4768 | 23.4776 | 0.000810 | 4113 | 32.47 | 218.55 | 186.09 | 229,855 |
| Adult scoliosis | 14.9069 | 14.9071 | 0.000230 | 4113 | 8.74 | 57.47 | 48.74 | 212,030 |
| Congenital kyphosis | 24.3772 | 24.3778 | 0.000674 | 6126 | 67.53 | 322.58 | 255.04 | 378,388 |
| Congenital deformities | 24.6967 | 24.6969 | 0.000247 | 6126 | 58.64 | 285.79 | 227.15 | 918,618 |
| Scheuermann’s disease: adolescent | 23.3582 | 23.3588 | 0.000624 | 79 | 24.96 | 8726.64 | 8701.68 | 13,938,864 |
| Scheuermann’s disease: adult | 17.5999 | 17.6000 | 0.000104 | 79 | 4.51 | 1562.65 | 1558.14 | 15,018,084 |
| Ankylosing spondylitis | 16.3470 | 16.3471 | 0.000086 | 1109 | 4.00 | 99.44 | 95.45 | 1,106,210 |
| Deforming dorsopathies: non-adult | 23.9112 | 23.9120 | 0.000869 | 5455 | 53.95 | 283.04 | 229.09 | 263,576 |
| Deforming dorsopathies: adult | 14.9067 | 14.9071 | 0.000431 | 5455 | 16.12 | 79.84 | 63.73 | 147,863 |
Table 22 examines alternative assumptions regarding patient throughput. Under TA2, patient throughput is based on the capacity of EOS at 30 patients per working day (equivalent to CR). This throughput corresponds to a much higher utilisation of EOS compared with the estimates from HES. For example, the throughput from HES varies between 79 and 6126 patients per year across the indications (see Table 21), while 30 patients per working day corresponds to an utilisation of 7530 per year. This higher utilisation assumes that the NHS can find enough patients for each indication to use the machine at a workload of 30 patients per working day. If, to satisfy this level of throughput, patients with indications other than that formally evaluated are included, the estimated ICERs assume that EOS generates the same clinical benefit for those other indications as the one formally modelled. Despite the higher utilisation, the ICERs under TA2 are well above conventional thresholds of £20,000 and £30,000 per additional QALY in all indications. The lowest ICER is for deforming dorsopathies in adults at £96,983 per QALY.
| Indication | ICER (£) for alternative throughput | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 724,903 | 342,703 | 170,185 | 25,200 | 23,500 |
| Early-onset idiopathic scoliosis | 699,162 | 330,479 | 164,061 | 25,000 | 23,300 |
| Adolescent or late-onset scoliosis | 229,855 | 107,590 | 52,401 | 18,600 | 15,900 |
| Adult scoliosis | 212,030 | 98,846 | 47,756 | 17,900 | 15,200 |
| Congenital kyphosis | 378,388 | 289,252 | 143,405 | 24,400 | 22,600 |
| Congenital deformities | 918,618 | 703,218 | 350,776 | 27,600 | 26,500 |
| Scheuermann’s disease: adolescent | 13,938,864 | 107,191 | 52,196 | 18,600 | 15,900 |
| Scheuermann’s disease: adult | 15,018,084 | 115,158 | 55,904 | 18,900 | 16,300 |
| Ankylosing spondylitis | 1,106,210 | 123,951 | 60,332 | 19,400 | 16,900 |
| Deforming dorsopathies: non-adult | 263,576 | 173,983 | 85,659 | 21,700 | 19,400 |
| Deforming dorsopathies: adult | 147,863 | 96,983 | 46,823 | 17,700 | 15,100 |
Table 22 also considers an even higher utilisation for EOS than for CR. Under TA3, it is assumed that EOS can work at a full capacity of 48 patients per working day, which corresponds to 12,048 scans per year, an increase of 60% in utilisation compared with CR. In this case, it is assumed that the machine is used intensively and enough patients are available to achieve this workload. Again, if there are not enough patients with the indications of interest, achieving the estimated ICERs would require the assumption that the equipment is also used for other indications with the same health benefits as the indication of interest. The resulting ICERs in Table 22 under TA3 are all > £30,000 per QALY. The results of the base-case analysis therefore suggest that EOS is not cost-effective for any indication under the three alternative throughput assumptions.
A threshold analysis for patient throughput is also shown in Table 22 to establish what patient throughput would be required to achieve ICERs of £20,000 and £30,000 per QALY for each indication. For a threshold of £20,000, the throughput ranges from 17,700 to 27,600 scans per year, which corresponds to a workload of 71–110 patient appointments per working day. For the threshold of £30,000, the throughput ranges from 15,100 to 26,500, corresponding to a workload of 60–106 patients per day. Therefore, EOS would have to be used much more intensively than conventional X-ray imaging in order to be cost-effective under base case assumptions. Under TA3, one EOS machine at full capacity could perform 12,048 scans per year, corresponding to 48 patient appointments per day. In order for EOS to be considered cost-effective, utilisation would have to increase by at least 25% from 12,048 to 15,100 scans per year. It is also worth noting that these throughput estimates are based on the assumption that utilisation of CR is 7530 scans per year, corresponding to just 30 appointments per day. If patient throughout for CR is higher in practice, EOS utilisation would have to increase yet further in order for EOS to become cost-effective.
Figure 9 illustrates the cost-effectiveness of EOS based on the relationship between throughput for EOS and CR for the four indications that are closest to being potentially cost-effective. In each of the figures, the throughput for CR (x-axis) and EOS (y-axis) is varied from 0 to 20,000 scans per year to determine what throughput is required for EOS to be considered cost-effective. The lines create two ‘borders’ of cost-effectiveness at the thresholds of £20,000 and £30,000 per QALY, respectively. The area to the left of the second line represents the region where the ICER for EOS is < £30,000 per QALY; the area between the lines represents the region where the ICER is between £20,000 and £30,000 per QALY; and the area to the left of the first line represents the region where the ICER is < £20,000 per QALY. Figure 9 shows that EOS can only be considered cost-effective if it is used much more intensively than CR. For example, if utilisation of CR is in the region of 7530 scans per year (corresponding to 30 patients per working day), EOS would need to be used at a capacity of 18,600 scans per year (corresponding to a workload of 74 patients per working day) in order to be considered more cost-effective than CR at a threshold of £30,000 per QALY. Alternatively, if full capacity for EOS is considered to be at 12,048 scans per year, the utilisation for CR would need to be < 4000 scans per year (or < 15 patients per working day) in order for EOS to be cost-effective at conventional thresholds. In summary, EOS can only be shown to be cost-effective when patient throughput for EOS is around double the throughput for CR.
FIGURE 9.
Two-way threshold analysis for the throughput of EOS and CR. (a) Late-onset scoliosis; (b) deforming dorsopathies in adults; (c) adult scoliosis; and (d) Scheuermann’s disease in adolescents.
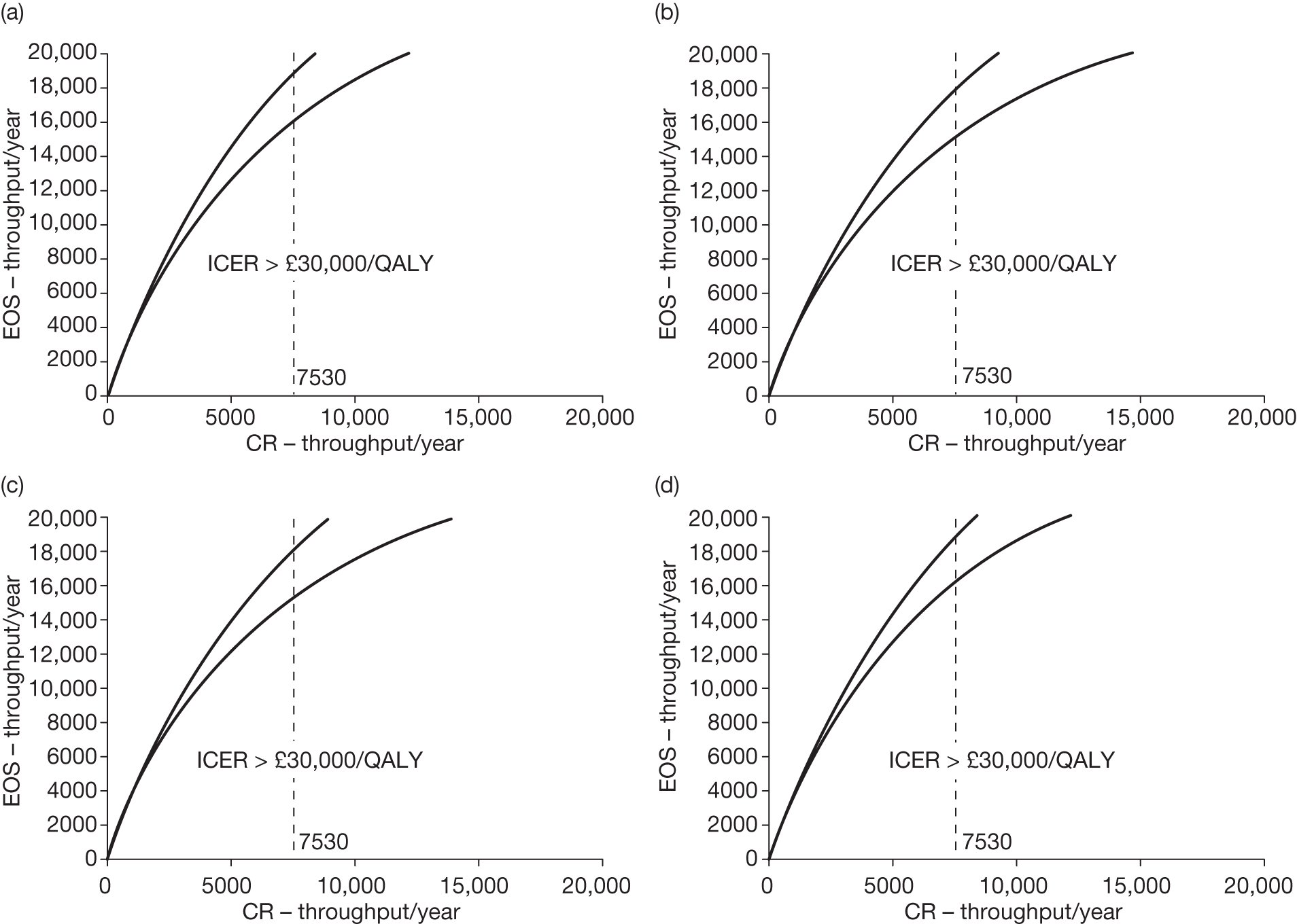
The base-case analysis has established that EOS requires a minimum of 15,100 scans per year in order to be considered cost-effective under conventional cost-effectiveness thresholds. HES data suggest that there are at least 16,882 scans per year at national level across all indications. Therefore, in order for EOS to be considered cost-effective, it must be assumed that the minimum throughput of 15,100 scans per year can be achieved in one centre with a single EOS machine at a workload of 60 patients per working day or, if EOS is used in more than one centre, additional patients can be identified to achieve that throughput with other types of indications for which EOS can achieve the same health benefit.
The estimated ICERs in Tables 21 and 22 rely on the underlying assumption that the only health benefit from EOS is reduced radiation exposure and, therefore, reduced risk of cancer compared with conventional X-ray. Although there is no evidence to confirm this, the use of EOS may have implications for the quality and nature of the image, which, in turn, could have beneficial health effects. Table 23 presents threshold analysis to show the necessary size of these health effects, in addition to the impact of cancer risk, in order for EOS to be considered cost-effective. The table reports the number of additional QALYs, over and above those associated with reduced radiation, required to achieve an ICER of £20,000/QALY under TA1, TA2 and TA3. Under TA1, health outcomes would need to increase by between 0.003 and 0.435 QALYs (factors of between 7 and 749 relative to the health benefits estimated from reduced radiation dose) to generate an ICER within acceptable thresholds. Similarly, under TA2, health benefits would need to increase by between 0.001 and 0.003 QALYs (factors of between 4 and 35 compared with radiation only). Under the most optimistic assumption of throughput, TA3, health benefits would need to increase by between 0.0002 and 0.002 QALYs (factors between 2.3 and 17.5).
| Indication | Incremental QALYs: EOS vs X-ray (base case) | QALYs for threshold of £20,000 for TA1 | Ratio: TA1–base case | QALYs for threshold of £20,000 for TA2 | Ratio: TA2–base case | QALYs for threshold of £20,000 for TA3 | Ratio: TA3–base case |
|---|---|---|---|---|---|---|---|
| Congenital scoliosis | 0.000655 | 0.02374 | 36 | 0.01122 | 17.1 | 0.00557 | 8.5 |
| Early-onset idiopathic scoliosis | 0.000623 | 0.02177 | 35 | 0.01029 | 16.5 | 0.00511 | 8.2 |
| Adolescent or late-onset scoliosis | 0.000810 | 0.00930 | 11 | 0.00436 | 5.4 | 0.00212 | 2.6 |
| Adult scoliosis | 0.000230 | 0.00244 | 11 | 0.00114 | 4.9 | 0.00055 | 2.4 |
| Congenital kyphosis | 0.000674 | 0.01275 | 19 | 0.00975 | 14.5 | 0.00483 | 7.2 |
| Congenital deformities | 0.000247 | 0.01136 | 46 | 0.00869 | 35.2 | 0.00434 | 17.5 |
| Scheuermann’s disease: adolescent | 0.000624 | 0.43508 | 697 | 0.00335 | 5.4 | 0.00163 | 2.6 |
| Scheuermann’s disease: adult | 0.000104 | 0.07791 | 749 | 0.00060 | 5.8 | 0.00029 | 2.8 |
| Ankylosing spondylitis | 0.000086 | 0.00477 | 55 | 0.00053 | 6.2 | 0.00026 | 3.0 |
| Deforming dorsopathies: non-adults | 0.000869 | 0.01145 | 13 | 0.00756 | 8.7 | 0.00372 | 4.3 |
| Deforming dorsopathies: adult | 0.000431 | 0.00319 | 7 | 0.00209 | 4.8 | 0.00101 | 2.3 |
Results of the scenario analysis
Tables 22–28 detail the results of the alternative scenarios for each indication under the same throughput assumptions analysed in the base case. The results of the threshold analysis for health benefits (expressed in QALYs) and yearly throughput (expressed in number of scans per year) required in order to achieve ICERs of £20,000 and £30,000 per QALY are also presented. The results across the alternative scenarios draw similar conclusions to those from the base-case analysis. The results support the view that the main driver of cost-effectiveness is patient throughput for EOS compared with standard X-ray.
| Indication | ICER (£) for alternative throughput | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 332,424 (0.02370, 16.6)a | 156,867 (0.01118, 7.8) | 77,623 (0.00553, 3.9) | 21,400 | 18,800 |
| Early-onset idiopathic scoliosis | 320,601 (0.02173, 16.0) | 151,252 (0.01025, 7.6) | 74,811 (0.00507, 3.7) | 21,100 | 18,600 |
| Adolescent or late-onset scoliosis | 101,970 (0.00925, 5.1) | 47,431 (0.00430, 2.4) | 22,813 (0.00207, 1.1) | 12,900 | 10,200 |
| Adult scoliosis | 212,030 (0.00244, 10.6) | 98,846 (0.00114, 4.9) | 47,756 (0.00055, 2.4) | 17,900 | 15,000 |
| Congenital kyphosis | 170,762 (0.01271, 8.5) | 130,406 (0.00971, 6.5) | 64,373 (0.00479, 3.2) | 20,200 | 17,500 |
| Congenital deformities | 422,201 (0.01134, 21.1) | 323,074 (0.00868, 16.2) | 160,880 (0.00432, 8.0) | 25,100 | 23,300 |
| Scheuermann’s disease: adolescent | 6,125,407 (0.43504, 306.3) | 46,542 (0.00331, 2.3) | 22,372 (0.00159, 1.1) | 12,800 | 10,100 |
| Scheuermann’s disease: adult | 15,018,084 (0.07791, 750.9) | 115,158 (0.00060, 5.8) | 55,904 (0.00029, 2.8) | 18,900 | 16,300 |
| Ankylosing spondylitis | 1,106,210 (0.00477, 55.3) | 123,951 (0.00053, 6.2) | 60,332 (0.00026, 3.0) | 19,400 | 16,900 |
| Deforming dorsopathies: non-adult | 117,001 (0.01140, 5.9) | 77,040 (0.00751, 3.9) | 37,645 (0.00367, 1.9) | 16,500 | 13,600 |
| Deforming dorsopathies: adult | 147,863 (0.00319, 7.4) | 96,983 (0.00209, 4.8) | 46,823 (0.00101, 2.3) | 17,700 | 15,100 |
| Indication | ICER (£) for alternative throughput | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 129,386 (0.03001, 6.5)a | 59,765 (0.01386,3.0) | 28,339 (0.00657, 1.4) | 14,300 | 11,700 |
| Early-onset idiopathic scoliosis | 126,571 (0.02697, 6.3) | 58,428 (0.01245, 2.9) | 27,670 (0.00590, 1.4) | 14,200 | 11,500 |
| Adolescent or late-onset scoliosis | 51,270 (0.00963, 2.6) | 22,658 (0.00425, 1.1) | 9742 (0.00183, 0.5) | 8200 | 6200 |
| Adult scoliosis | 125,099 (0.00254, 6.3) | 57,570 (0.00117, 2.9) | 27,088 (0.00055, 1.4) | 14,000 | 11,400 |
| Congenital kyphosis | 71,310 (0.01501, 3.6) | 53,892 (0.01134, 2.7) | 25,393 (0.00534, 1.3) | 13,600 | 11,000 |
| Congenital deformities | 154,951 (0.01355, 7.7) | 117,986 (0.01032, 5.9) | 57,504 (0.00503, 2.9) | 19,200 | 16,500 |
| Scheuermann’s disease: adolescent | 3,298,534 (0.46721, 164.9) | 22,867 (0.00324, 1.1) | 9843 (0.00139, 0.5) | 8200 | 6200 |
| Scheuermann’s disease: adult | 6,264,843 (0.07910, 313.2) | 46,002 (0.00058, 2.3) | 21,276 (0.00027, 1.1) | 12,400 | 9900 |
| Ankylosing spondylitis | 528,197 (0.00478, 26.4) | 57,570 (0.00052, 2.9) | 27,088 (0.00025, 1.4) | 14,000 | 11,400 |
| Deforming dorsopathies: non-adult | 53,997 (0.01249, 2.7) | 34,766 (0.00804, 1.7) | 15,808 (0.00366, 0.8) | 10,600 | 8300 |
| Deforming dorsopathies: adult | 88,489 (0.00337, 4.4) | 57,570 (0.00219, 2.9) | 27,088 (0.00103, 1.4) | 14,000 | 11,400 |
| Indication | ICER (£) for alternative throughput | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 651,517 (0.02372, 32.6)a | 307,851 (0.01121, 15.4) | 152,727 (0.00556, 7.6) | 24,700 | 22,900 |
| Early-onset idiopathic scoliosis | 628,371 (0.02176, 31.4) | 296,859 (0.01028, 14.8) | 147,220 (0.00510, 7.4) | 24,600 | 22,700 |
| Adolescent or late-onset scoliosis | 206,379 (0.00929, 10.3) | 96,441 (0.00434, 4.8) | 46,816 (0.00211, 2.3) | 17,800 | 15,100 |
| Adult scoliosis | 190,298 (0.00243, 9.5) | 88,525 (0.00113, 4.4) | 42,586 (0.00054, 2.1) | 17,100 | 14,400 |
| Congenital kyphosis | 339,938 (0.01274, 17.0) | 259,788 (0.00974, 13.0) | 128,646 (0.00482, 6.4) | 23,900 | 22,000 |
| Congenital deformities | 825,701 (0.01135, 41.3) | 632,018 (0.00869, 31.6) | 315,110 (0.00433, 15.8) | 27,300 | 26,200 |
| Scheuermann’s disease: adolescent | 12,533,206 (0.43507, 626.7) | 96,081 (0.00334, 4.8) | 46,631 (0.00162, 2.3) | 17,800 | 15,100 |
| Scheuermann’s disease: adult | 13,503,559 (0.07791, 675.2) | 103,187 (0.00060, 5.2) | 49,908 (0.00029, 2.5) | 18,200 | 15,500 |
| Ankylosing spondylitis | 994,323 (0.00477, 49.7) | 111,099 (0.00053, 5.6) | 53,894 (0.00026, 2.7) | 18,700 | 16,100 |
| Deforming dorsopathies: non-adult | 236,700 (0.01144, 11.8) | 156,140 (0.00755, 7.8) | 76,721 (0.00371, 3.8) | 21,100 | 18,600 |
| Deforming dorsopathies: adult | 132,600 (0.00318, 6.6) | 86,850 (0.00208, 4.3) | 41,747 (0.00100, 2.1) | 16,900 | 14,300 |
| Indication | ICER (£) for alternative throughput | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 726,300 (0.02374, 35.2)a | 344,018 (0.01122, 16.4) | 170,750 (0.00557, 8.7) | 25,300 | 23,600 |
| Early-onset idiopathic scoliosis | 700,510 (0.02177, 34.0) | 331,747 (0.01029, 15.8) | 164,606 (0.00511, 8.4) | 25,000 | 23,400 |
| Adolescent or late-onset scoliosis | 230,264 (0.00930, 11.2) | 107,978 (0.00436, 5.1) | 52,562 (0.00212, 2.7) | 18,700 | 15,900 |
| Adult scoliosis | 212,068 (0.00244, 10.6) | 98,825 (0.00114, 4.9) | 47,727 (0.00055, 2.3) | 17,900 | 15,200 |
| Congenital kyphosis | 379,090 (0.01275, 18.4) | 290,331 (0.00975, 13.8) | 143,866 (0.00483, 7.4) | 24,500 | 22,700 |
| Congenital deformities | 920,397 (0.01136, 44.7) | 705,929 (0.00869, 33.6) | 351,950 (0.00434, 18.0) | 23,700 | 26,600 |
| Scheuermann’s disease: adolescent | 13,962,807 (0.43508, 678.4) | 107,566 (0.00335, 5.1) | 52,350 (0.00163, 2.7) | 18,800 | 16,000 |
| Scheuermann’s disease: adult | 15,022,108 (0.07791, 747.9) | 115,125 (0.00060, 5.7) | 55,873 (0.00029, 2.7) | 19,100 | 16,400 |
| Ankylosing spondylitis | 1,106,403 (0.00477, 55.1) | 123,925 (0.00053, 6.1) | 60,295 (0.00026, 2.9) | 19,500 | 16,900 |
| Deforming dorsopathies: non-adult | 264,045 (0.01145, 12.8) | 174,613 (0.00756, 8.3) | 85,923 (0.00372, 4.4) | 21,700 | 19,500 |
| Deforming dorsopathies: adult | 147,890 (0.00319, 7.4) | 96,963 (0.00209, 4.8) | 46,794 (0.00101, 2.3) | 17,800 | 15,200 |
| Indication | ICER (£) for alternative throughput | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 347,698 (0.02363, 17.4)a | 163,585 (0.01112, 8.2) | 80,480 (0.00547, 4.0) | 21,400 | 18,900 |
| Early-onset idiopathic scoliosis | 341,733 (0.02167, 17.1) | 160,753 (0.01019, 8.0) | 79,061 (0.00501, 4.0) | 21,300 | 18,800 |
| Adolescent or late-onset scoliosis | 122,084 (0.00920, 6.1) | 56,428 (0.00425, 2.8) | 26,792 (0.00202, 1.3) | 14,000 | 11,300 |
| Adult scoliosis | 139,505 (0.00242, 7.0) | 64,404 (0.00112, 3.2) | 30,504 (0.00053, 1.5) | 14,800 | 12,200 |
| Congenital kyphosis | 192,523 (0.01266, 9.6) | 146,837 (0.00966, 7.3) | 72,086 (0.00474, 3.6) | 20,700 | 18,200 |
| Congenital deformities | 374,116 (0.01131, 18.7) | 285,990 (0.00864, 14.3) | 141,797 (0.00429, 7.1) | 24,400 | 22,500 |
| Scheuermann’s disease: adolescent | 7,679,190 (0.43501, 384.0) | 57,753 (0.00327, 2.9) | 27,450 (0.00155, 1.4) | 14,100 | 11,500 |
| Scheuermann’s disease: adult | 10,392,181 (0.07790, 519.6) | 78,597 (0.00059, 3.9) | 37,590 (0.00028, 1.9) | 16,200 | 13,500 |
| Ankylosing spondylitis | 641,063 (0.00476, 32.1) | 70,521 (0.00052, 3.5) | 33,568 (0.00025, 1.7) | 15,500 | 12,800 |
| Deforming dorsopathies: non-adult | 134,040 (0.01133, 6.7) | 87,997 (0.00744, 4.4) | 42,606 (0.00360, 2.1) | 17,200 | 14,500 |
| Deforming dorsopathies: adult | 98,121 (0.00315, 4.9) | 63,959 (0.00205, 3.2) | 30,281 (0.00097, 1.5) | 14,700 | 12,100 |
Under TA1 and TA2, the ICERs are well above conventional thresholds of cost-effectiveness irrespective of the scenario in all indications. For TA3, in all bar one scenario – which was a reduction in the discount rate from 3.5% to 0% per annum (see Table 25) – the ICER was > £20,000 per QALY across the various indications. This scenario demonstrates the effects of discounting future costs and benefits from cancer developed later in life back to present values to explain the results of the modelling but it does not illuminate any specific policy option.
In two other scenarios under TA3, the ICERs fall between £20,000 and £30,000 per additional QALY for two of the indications (late-onset scoliosis and Scheuermann’s disease in adolescents). These two scenarios are:
-
An earlier age of cancer diagnosis compared with the general population (see Table 24). The age at diagnosis of radiation-induced cancer is assumed to be 55 years for lung, prostate and colorectal cancer compared with an average age at diagnosis of these cancers among the general population of 72–74 years.
-
An alternative source for the estimate of LAR of radiation-induced cancer (see Table 28). The BEIR VII Phase II17 report, which estimates the risk of cancer incidence for a 1999 US population, was used instead of the data from the personal communication with Paul Shrimpton from HPA.
Discussion
Whether or not EOS is considered a cost-effective use of NHS resources hinges on two key issues. The first is the number of patients using the equipment on an annual basis. This measure of throughput determines the number of patients over which the fixed capital costs of EOS are allocated – the greater the throughput the lower the average cost per scan. There are no reliable data on the current number of scans undertaken in the NHS for the indications that have the greatest potential benefit for EOS. Although numbers have been derived from HES, these are likely to be significant underestimates. Furthermore, even if accurate data were available on numbers of scans undertaken in the NHS for the indications of interest, the throughput of EOS, if it were to be introduced, would depend on the number of centres in which it was installed and how intensively it was used during the average working day, both of which are, in principle, policy decisions.
The cost-effectiveness modelling has, therefore, not sought to use a single set of patient throughput estimates for EOS. Rather, it has looked at three alternative assumptions of throughput: (1) that based on HES data (TA1); (2) that similar to the throughput assumed with CR – 30 patients per working day or 7530 per year (TA2); and (3) more intensive use of EOS – 48 patients per working day or 12,048 scans per year, an increase of 60% in utilisation compared with CR (TA3). Under base-case assumptions, the ICERs of EOS for all indications are well above £30,000 per QALY whatever the throughput scenario assumed (see Table 22).
Hence, the levels of annual throughput with EOS that would generate ICERs of £20,000 and £30,000 per QALY are reported (see Table 22). In order for EOS to be considered cost-effective, utilisation would have to increase by at least 25% from above the highly intensive TA3 to 15,100 scans per annum. If an insufficient number of patients with the relevant indications can be identified to achieve this level of utilisation, it would have to be assumed that any other patients identified with other indications to increase utilisation would experience the same health benefits as for the indications of interest. Furthermore, these throughput ‘thresholds’ are based on the assumption that utilisation of CR is 7530 scans per year, corresponding to just 30 appointments per day. If CR were to be used more intensively then the throughput of EOS would need to increase yet further to be cost-effective. These conclusions are not greatly influenced by the alternative assumptions explored in further scenario analyses (see Tables 24–29). Only one alternative assumption – that cancer incidence due to X-ray radiation occurs earlier in life than in other patients with cancer diagnosis – generates ICERs < £30,000 per QALY gained: for adolescent or late-onset scoliosis and adolescent Scheuermann’s disease.
| Indication | ICER (£ for alternative throughput) | Throughput required for threshold of | |||
|---|---|---|---|---|---|
| Throughput based on HES data (TA1) | Throughput based on capacity at 30 patients per day – same as CR (TA2) | Throughput based on full capacity at 48 patients per day (TA3) | £20,000/QALY | £30,000/QALY | |
| Congenital scoliosis | 951,590 (0.02077, 47.6)a | 378,290 (0.00826, 18.9) | 119,513 (0.00261, 6.0) | 15,700 | 15,200 |
| Early-onset idiopathic scoliosis | 917,832 (0.01905, 45.9) | 364,807 (0.00757, 18.2) | 115,181 (0.00239, 5.8) | 15,600 | 15,100 |
| Adolescent or late-onset scoliosis | 302,372 (0.00816, 15.1) | 118,974 (0.00321, 5.9) | 36,191 (0.00098, 1.8) | 13,600 | 12,600 |
| Adult scoliosis | 279,161 (0.00214, 14.0) | 109,384 (0.00084, 5.5) | 32,750 (0.00025, 1.6) | 13,400 | 12,300 |
| Congenital kyphosis | 453,042 (0.01018, 22.7) | 319,337 (0.00717, 16.0) | 100,567 (0.00226, 5.0) | 15,400 | 14,900 |
| Congenital deformities | 1,099,020 (0.00906, 55.0) | 775,919 (0.00640, 38.8) | 247,256 (0.00204, 12.4) | 16,000 | 15,900 |
| Scheuermann’s disease: adolescent | 20,866,045 (0.43420, 1043.3) | 118,535 (0.00247, 5.9) | 36,043 (0.00075, 1.8) | 13,600 | 12,600 |
| Scheuermann’s disease: adult | 22,481,769 (0.07775, 1124.1) | 127,381 (0.00044, 6.4) | 38,500 (0.00013, 1.9) | 13,700 | 12,700 |
| Ankylosing spondylitis | 1,610,462 (0.00463, 80.5) | 137,074 (0.00039, 6.9) | 41,646 (0.00012, 2.1) | 13,900 | 13,000 |
| Deforming dorsopathies: non-adult | 326,591 (0.00946, 16.3) | 192,203 (0.00557, 9.6) | 59,717 (0.00173, 3.0) | 14,700 | 14,000 |
| Deforming dorsopathies: adult | 183,651 (0.00264, 9.2) | 107,330 (0.00154, 5.4) | 32,090 (0.00046, 1.6) | 13,300 | 12,300 |
The other key issue on which the cost-effectiveness of EOS hinges is the source of the health benefits assumed for the technology. The base-case assumption is that health benefit is derived solely from reduced radiation dose and hence lower incidence of cancer. Although no evidence has been identified to sustain it, there may be health benefits from EOS as a result of the nature and quality of the image which prompts therapeutic changes and hence better outcomes. Given an absence of any evidence on such outcomes, the gain in QALYs with EOS from this source that would be necessary for EOS to achieve cost-effectiveness is reported, using the different throughput scenarios (see Table 23). In order to assess how plausible these QALY gains are, it may be helpful to think about the factor increase they represent over and above the health improvement from reduced radiation dose alone: between 7 and 749 times under TA1; between 4 and 35 under TA2; and between 2.3 and 17.5 under TA3. In other words, the health gains from any therapeutic changes to the EOS image would need to be significantly greater than those from reduced radiation dose alone.
Another way of assessing the plausibility of the necessary QALY gains is to compare them with the QALY gains estimated for other diagnostic tests based on firmer evidence. In many situations the health gains from changes in diagnostic technologies tend to be relatively small, as only a proportion of patients have their diagnoses altered as a result, a smaller proportion still experience a therapeutic change and a yet smaller group actually has a change in outcomes. For example, in evaluating different diagnostic strategies for coronary artery disease in patients of 55 years of age, Garber and Solomon62 found differences in lifetime QALYs of between 0.001 and 0.025 across six diagnostic strategies.
Only the cancer effects of radiation were considered for the estimation of the costs accrued and QALYs lost from radiation exposure. Other potential consequences of radiation, such as heritable or fertility effects, were not explored. Nevertheless, the threshold analysis on the number of additional QALYs required for EOS to be considered cost-effective provides some insight into the necessary magnitude of the QALYs. Furthermore, only costs and QALYs associated with the four cancers (lung, prostate, breast and colorectal cancer) were incorporated in the analysis. As cancers can be heterogeneous in nature, the costs and QALYs used may not fully reflect the costs and QALYs associated with all cancers.
Conclusions
There are major uncertainties in the evidence necessary to assess the cost-effectiveness of EOS in the NHS. Even under extreme assumptions about the intensity with which EOS could be used in routine practice, the ICERs for EOS generally do not fall below £30,000 per QALY. The conclusion that EOS has potential to be cost-effective, therefore, is likely to rest on the plausibility of the additional QALY gains that might be expected as a result of any therapeutic responses to the nature of the quality of the EOS image compared with standard X-ray.
Chapter 3 Discussion
Statement of principal findings
The systematic review of the clinical effectiveness of EOS found three limited-quality studies suggesting that radiation dose is considerably lower with EOS than with CR or film X-ray imaging, although image quality remains comparable or better with EOS. No evidence was found on the impact of EOS on patients’ pathways of care or ultimate health outcomes.
The evidence relating to the risks of radiation exposure have been reviewed in the reports of international and UK radiation authorities. Our systematic review contributes an evaluation of the risk of cancer and adverse reproductive outcomes associated with diagnostic X-ray radiation exposure specifically for patients with orthopaedic conditions. Despite the limited data, the findings from our review showed that, when compared with the general female population, there was a clear association between increased risk of breast cancer mortality and diagnostic X-ray exposures for female patients with scoliosis or spinal curvature, with a significant radiation dose response. There was a highly significant trend for increased risk of breast cancer with increased cumulative radiation dose, particularly in patients with a family history of breast cancer. Only limited poor-quality data were available regarding the risk of adverse reproductive outcomes in orthopaedic patients.
A decision-analytic model was developed to assess the cost-effectiveness of EOS in the relevant indications compared with standard X-ray (CR and DR imaging). The model provided a framework for the synthesis of data from the review of clinical effectiveness of EOS and adverse effects of diagnostic radiation exposure, such as the risk of cancer, in order to evaluate the potential long-term cost-effectiveness of EOS. The model incorporated a lifetime horizon to estimate outcomes in terms of QALYs and costs from the perspective of the NHS. Cost-effectiveness was assessed using ICERs for each indication. This was complemented by threshold analysis to determine the sensitivity of the cost-effectiveness threshold to uncertainty in the assumed base-case parameters.
The ICERs for EOS, for the various indications considered, were well above conventional thresholds of £20,000 and £30,000 per additional QALY in all indications. Patient throughput was a major determinant of the cost-effectiveness of EOS. A range of scenarios was considered regarding throughput with EOS and standard X-ray, as well as threshold analysis to explore the critical throughput levels to be achieved for EOS to be considered cost-effective. Three alternative assumptions regarding patient throughput were used to examine whether or not EOS could be shown to be cost-effective:
-
TA1 used patient throughput based on HES data, which provided an estimate of the number of examinations per year for each of the various indications at national level.
-
In recognition that HES may underestimate current X-ray utilisation, TA2 was based on the capacity that a machine could utilise in a working day. TA2 assumed equivalent throughput for EOS and standard X-ray at 30 patients per working day, corresponding to an annual throughput of 7530 scans per year (assuming 251 working days per year).
-
TA3 was based on a higher utilisation for EOS compared with standard X-ray at 48 patients per working day, corresponding to an annual throughput of 12,048 scans per year (assuming 251 working days per year).
Under none of the alternative throughput assumptions did EOS appear to be cost-effective at thresholds of £20,000 and £30,000 per QALY under base-case assumptions.
The threshold analysis on patient throughput showed that 17,700–27,600 scans per year (corresponding to a workload of 71–110 patient appointments per working day) were needed to achieve an ICER of £20,000 per QALY or between 15,100 and 26,500 (corresponding to a workload of 60–106 patient appointments per working day) for an ICER of £30,000 per QALY. These estimates were based on the assumption that the throughput for CR was 7530 scans per year (30 patient appointments per working day). Two-way threshold analysis examining the relationship between the cost-effectiveness of EOS and the throughput of CR and EOS suggested that EOS would not be cost-effective unless its utilisation can be assumed to be markedly greater than CR. For example, in deforming dorsopathies of adults, which is the closest indication to being potentially cost-effective, the minimum throughput for EOS to generate an ICER of < £30,000 per QALY would be 15,100 scans per year, as long as the throughput for CR is 7530 scans per year.
The base-case analysis assumed that any health benefit from EOS would come through reduced radiation doses. Although no evidence exists to confirm it, EOS may confer health benefits through the nature and quality of its images influencing the results of therapeutic interventions such as surgery. To address this issue and in the absence of formal evidence, threshold analysis was used to calculate the necessary health gain from the EOS image, over and above benefit from reduction in radiation dose, to achieve acceptable ICERs. This analysis suggested that the necessary absolute QALY gains from non-radiation sources varied by the throughput scenario. For the lowest throughput scenario (TA1), the necessary gains ranged from 0.003 to 0.4 (an increase in the order of magnitude of 7–697); for the scenario TA2 from 0.002 to 0.003 (an increase in the order of magnitude of 4.8–35); and for TA3 from 0.0002 to 0.002 (an increase in the order of magnitude of 2.3–17).
Judgements regarding the plausibility of these necessary QALY gains may be aided by a comparison with the QALY gains estimated for other diagnostic tests based on firmer evidence. In many situations the health gains from changes in diagnostic technologies tend to be relatively small, as only a proportion of patients have their diagnoses altered as a result, and a smaller proportion still experience a therapeutic change. For example, in evaluating different diagnostic strategies for coronary artery disease in patients of 55 years of age, Garber and Solomon62 found differences in lifetime QALYs of between 0.001 and 0.025 across six strategies.
A number of alternative scenarios were considered, which varied the assumptions used as part of the base-case analysis. Under TA1 and TA2, EOS was not cost-effective across any of the scenarios considered when reduced radiation dose is assumed to be the only source of health benefit. For TA3, in all bar one scenario, which was a reduction in the discount rate from 3.5% to 0% per annum, the ICER was > £20,000 per QALY across the various indications. This scenario demonstrated the effects of discounting future costs and benefits from cancer developed later in life back to present values to explain the results of the modelling but it does not illuminate any specific policy option.
In two other scenarios, the ICERs fell between £20,000 and £30,000 per additional QALY for two of the indications (late-onset scoliosis and Scheuermann’s disease in adolescents): (1) a scenario that considered an earlier age of cancer diagnosis compared with the general population and (2) a scenario that used an alternative source (BEIR VII report17 instead of the personal communication with Paul Shrimpton from HPA) for the estimate of LAR of radiation-induced cancer. However, for EOS to be cost-effective in these indications, the throughput must be twice that for CR.
Strengths and limitations of the assessment
Strengths
We conducted a rigorous systematic review of the clinical effectiveness of EOS, which addressed a clear research question using predefined inclusion criteria. Comprehensive literature searches were performed to locate all relevant published and unpublished studies without any language restrictions, thereby minimising the potential for publication bias and language bias. Hand-searching was also performed in order to identify additional relevant studies. We are therefore confident that we have included all relevant studies.
Study selection was undertaken independently by two reviewers and data extraction and quality assessment were checked by a second reviewer to minimise the potential for reviewer bias or error. Validity assessment was undertaken using a validated checklist for diagnostic studies, with additional project-specific quality assessment items added. Clinical expertise was obtained for completing the additional project-specific quality assessment items.
Owing to the high degree of clinical heterogeneity identified between included studies, a narrative synthesis was appropriate.
Similarly to the systematic review of the clinical effectiveness of EOS, a comprehensive search was conducted to identify potentially relevant studies for inclusion in the assessment of cost-effectiveness, including full economic evaluations of EOS against any comparators and economic evaluations in the indications of interest where standard X-ray was assessed against any comparator. No studies were found, so a new decision-analytic model was developed to provide an assessment of the cost-effectiveness of EOS from the perspective of the NHS and Personal Social Services. This model is the first to fully quantify the long-term costs and health consequences associated with diagnostic imaging using EOS.
The model provided a framework for the synthesis of data from the review of clinical effectiveness of EOS and adverse effects of diagnostic radiation exposure. Radiation exposure increases the risk of cancer, which, in turn, is associated with an increase in health-care costs and loss of life-years and QoL. These costs and health consequences were combined with the costs of monitoring patients for the various indications to provide an estimation of total costs and health outcomes from diagnostic imaging with EOS compared with standard X-ray. Cost-effectiveness was assessed using ICERs for each indication. This was complemented with threshold analysis in order to determine the critical throughput levels and additional QALYs (from sources other than reduced radiation) needed to demonstrate the cost-effectiveness of EOS under conventional thresholds.
The estimation of lifetime cancer risk attributable to radiation exposure from diagnostic X-ray imaging was based on the most up-to-date evidence on the effects of ionising radiation. Four key sources of information, namely, BEIR VII Phase II,17 UNSCEAR,30 ICRP publication 103 report18 and personal communication with Paul Shrimpton from the HPA (January to February 2011) were identified. These include comprehensive reports, produced by large international radiation protection and safety agencies, examining the risk of radiation-induced cancer. For the base-case analysis, we used the most recent data from a personal communication with Paul Shrimpton from the HPA (January to February 2011), which calculated organ and effective doses for common X-ray examinations on adult patients in the UK and investigated the relationship between lifetime cancer risk and effective dose for common X-ray examinations. The estimates are based on the most recent models by the ICRP. 18 For the scenario analysis, the BEIR VII Phase II17 report was used as an alternative source to estimate cancer risk owing to radiation exposure. Both data sources take account of long-term evidence on the adverse effects of radiation exposure based on epidemiological data from the atomic bomb survivor studies, medical radiation studies, occupational and environmental studies.
Limitations
The main limitation of the systematic review of the clinical effectiveness of EOS was the limited amount and quality of the data available. Only three studies comparing EOS with conventional X-ray imaging were identified,25–27 and two of the studies included only a small number of participants. There were no studies comparing EOS with DR, and no studies assessing the clinical effectiveness of EOS in adults. There were also no studies to confirm or refute the fact that EOS may confer health benefits over and above those associated with reduced radiation exposure through the nature and quality of its images, which could influence the results of therapeutic interventions such as surgery.
The major determinant of the cost-effectiveness of EOS is patient throughput. Throughput is highly uncertain and potentially variable between centres. There are no reliable data available to provide estimates of throughput at national level. The HES data for inpatient episodes during 2008–9 is likely to underestimate current X-ray utilisation by patients with the various indications of interest. This is because many patients will receive X-ray imaging as outpatients, but the outpatient HES data record very low numbers of patient visits for these indications. This uncertainty is a key limitation of the economic model.
Given this uncertainty about likely throughput with EOS, three alternative assumptions were used in the analysis: throughput estimates based on (1) the number of inpatient episodes as recorded in HES; (2) a capacity of 30 patients per working day for EOS and CR; and (3) a capacity of 48 patients per working day for EOS and 30 patients per day for CR. Threshold analyses were also used to determine the levels of throughput required to achieve an ICER within an acceptable range of cost-effectiveness. These critical levels provide an estimate of the throughput needed but judgement is required on the feasibility of achieving these levels. In principle, the use of EOS in the NHS could be centrally planned in such a way that the throughput of patients using the technology could be determined, for example by locating EOS in one or a small number of specialist centres to which patients with particular indications could be sent. Therefore, EOS throughput can be seen as a matter of policy choice in its own right rather than an uncertain parameter to estimate.
Uncertainty in the model inputs was not fully explored owing to a lack of SDs or CIs reported in the published literature for most of the parameters. The model was constructed to be run probabilistically but only the outcomes from cancer (costs and QALYs associated with cancer) were entered as an uncertain rather than a fixed parameter, as the uncertainty in all other parameters was unknown. As a result, uncertainty in the cost-effectiveness results was not presented.
Uncertainties
Despite comprehensive searches of the literature for available research evidence a number of uncertainties remain. Firstly, it is uncertain how generalisable to the UK context the findings of the clinical evaluation of EOS are. EOS is currently not available in the UK. Only three studies, which were undertaken outside the UK, have compared EOS with conventional X-ray imaging of film and CR,25–27 and two of these studies have only included a small number of participants. There have been no studies comparing EOS with DR and no studies assessing the clinical effectiveness of EOS in adults. Therefore, it is unclear how representative these studies are to the practice of diagnostic imaging in the UK.
The model evaluates the cost-effectiveness of EOS through reducing the amount of radiation exposure to patients over the monitoring period for the various indications. The estimates for radiation dose associated with each type of radiograph used during diagnosis and monitoring may not accurately represent the radiation exposure to patients. The best available evidence was used, based on the doses recorded in the UK NPDD, but no estimate of uncertainty was presented on the average values.
In addition, the data were collected between January 2001 and February 2006, hence radiation dose may be out-dated from doses used in current practice. Data were collected in 316 hospitals and clinics, which represent only 23% of the institutions with diagnostic X-ray facilities in the UK. Therefore, the data may not represent the majority of radiographs taken in the NHS. Furthermore, information on the type of equipment used was only provided for 24% of the rooms. The majority of these used a film–screen combination, 40% used CR and 5% used DR. At present, film–screen radiography is no longer used in the NHS and expert advice suggested that CR represents the majority of equipment used in current practice.
The model formally assesses the potential reduction in radiation dose, and hence cancer risk, from EOS compared with standard X-ray. However, there remains uncertainty whether or not EOS has implications for the quality and nature of the image, which in turn could have beneficial effects on medical or surgical management with consequent positive implications for patients’ health outcomes. Owing to a lack of formal evidence and insufficient time formally to elicit estimates from clinical experts, the model was unable to explore these implications explicitly.
Other relevant factors
A wider set of patients, with indications other than those explicitly considered here, could have their scans with EOS to help achieve these ‘target’ throughput levels. However, the use of such patients would only be cost-effective if the incremental benefits they experience from EOS are similar to those estimated for patients with the indications that have been modelled.
The evidence base for NHS investment in EOS is, therefore, highly uncertain. The upfront capital cost of the machine may represent an irreversible cost to the NHS if research or other information emerging in the future suggests it is not as cost-effective as existing X-ray and if there is limited resale value for the equipment. 63 For this reason, if the NHS decides to invest in EOS, there may be a case for the use of rental agreements rather than outright purchase.
Chapter 4 Conclusions
No evidence was found on the impact of EOS on patients’ pathways of care or ultimate health outcomes. Radiation dose is considerably lower with EOS than CR or film X-ray imaging, whereas image quality remains comparable or better with EOS.
Patient throughput is the major determinant of the cost-effectiveness of EOS. The average cost per procedure of EOS decreases with utilisation. Therefore, the greater the number of procedures that can be demonstrated compared with those for standard X-ray, the greater the likelihood of cost-effectiveness. Using the estimates of patient throughput at national level from the HES data suggests that EOS is not cost-effective for any of the indications considered. Patient throughput in the region of 15,100–26,500 (corresponding to a workload of 60–106 patient appointments per working day) for EOS compared with a throughput of only 7530 for CR (corresponding to a workload of 30 patient appointments per working day) is needed to achieve an ICER of £30,000 per QALY. EOS can be shown to be cost-effective only when compared with CR if the utilisation for EOS is about double the utilisation of CR. As the throughput for CR is not tied to the particular indications for which EOS is potentially of value, as the equipment can be used for a much wider set of uses, it is unlikely that the throughput for CR would be considerably lower than for EOS. The conclusion that EOS has potential to be cost-effective is, therefore, likely to rest on the plausibility of the additional QALY gains that might be expected as a result of any therapeutic responses to the nature of the quality of the EOS image compared with standard X-ray imaging.
Implications for service provision
The cost-effectiveness of EOS depends on the feasibility of achieving the critical patient throughput levels. The economic analysis has demonstrated that the ICERs for EOS for the various indications are consistently above conventional thresholds of cost-effectiveness unless a minimum throughput of 15,100 scans per year can be achieved. This has implications for service provision – the NHS would need to reconfigure services. Clinics using EOS would have to be organised in such a manner to ensure that this minimum utilisation is achieved for each centre using EOS. A throughput of 15,100 scans per year is equivalent to 60 patient appointments per working day, over 251 working days per year.
A key question is whether or not such throughput is achievable with current patient numbers and, if so, how many EOS systems would be required. As the minimum throughput is in the order of 15,000 scans per year, this would require that each centre with an EOS machine would serve enough patients to ensure such utilisation. It may be possible to identify patients with conditions other than those formally assessed here for whom EOS could reasonably be used instead of standard X-ray and hence increase throughput to the necessary thresholds. However, this would only be a cost-effective option if the health benefits experienced by those patients were comparable to the main indications considered in the modelling.
There is also an impact on patients or carers. The acquisition of one EOS system would require patients and carers to travel to the facility, further compromising the achievement of utilisation required for the technology to become cost-effective. Whether or not the acquisition of the EOS system depends on the specific type of hospital (e.g. orthopaedic or children’s hospitals) should be considered.
The evidence base for NHS investment in EOS is, therefore, highly uncertain. The upfront capital cost of the machine may represent an irreversible cost to the NHS if research or other information emerging in the future suggests it is not as cost-effective as existing X-ray and if there is limited resale value for the equipment. 63 For this reason, if the NHS decides to invest in EOS, there may be a case for the use of rental agreements rather than outright purchase.
Suggested research priorities
-
The benefits to patients from reduced radiation with EOS appear minimal. Further research is required in order to establish the nature and extent of any additional benefits to the patient, for example benefits arising from the 3D nature of the image. There is a need to formally assess the implications of any changes in the quality and nature of the image with EOS compared with standard X-ray for patient health outcomes, over and above the reduction in radiation. This will require research to establish, for relevant indications, the proportion of patients for whom use of EOS changes diagnosis and/or therapy, and whether or not any therapeutic changes result in improved quality-adjusted life expectancy.
-
Estimates of likely throughput with EOS are both uncertain (there is little evidence to use for this purpose) and variable (they depend on how many EOS machines are introduced in the NHS and the relevant patient throughput in each centre). For EOS this throughput needs to be based on the patient numbers expected for the indications for which EOS has a potential benefit. This throughput should be defined at national level based on numbers of patients requiring scans and number of centres throughout the UK.
Acknowledgements
We would like to thank the following for providing information and advice: Mr Peter Millner, Consultant Orthopaedic and Spinal Surgeon, Leeds Teaching Hospitals NHS Trust; Dr Paul Shrimpton, Leader of Medical Dosimetry Group, CRCE, HPA; Dr Erika Denton, Consultant Radiologist, National Clinical Director for Imaging; Sally MacLachlan, Senior Clinical Officer, Medical Exposure Department, HPA; Maxine Clarke, PACS and Radiology IT Manager, Norfolk and Norwich University Foundation Hospital Trust; and Peter Howells, Head of Radiation Protection, Leeds General Infirmary; Rita Santos and Rossella Verzulli for assistance with the analysis of the HES data; Paul Tappenden, Jim Chilcott, Helen Campbell, Nathalie Kulin and Deborah Marshall for providing results from their cancer models; and Simon Walker for advice on the modelling of the effects of radiation exposure.
We would also like to thank the following members of the Diagnostic Advisory Committee for additional information and advice: Dr James Rankine, Consultant Radiologist, Leeds Teaching Hospitals NHS Trust; Dr David Grier, Consultant Paediatric Radiologist, Bristol Royal Hospital for Children; and Professor Jeremy Fairbank, Consultant Orthopaedic Surgeon, Nuffield Orthopaedic Centre NHS Trust.
Contributions of authors
Claire McKenna (Research Fellow) was responsible for the cost-effectiveness section; writing the protocol, study selection, data extraction, development of the economic model and writing the final report.
Ros Wade (Research Fellow) was responsible for the clinical effectiveness section; writing the protocol, study selection, data extraction, validity assessment and writing the final report.
Rita Faria (Research Fellow) was involved in the cost-effectiveness section; study selection, data extraction, development of the economic model and writing the final report.
Huiqin Yang (Research Fellow) was involved in the clinical effectiveness section; writing the protocol, study selection, data extraction, validity assessment and writing the final report.
Lisa Stirk (Information Specialist) devised the search strategy, carried out the literature searches and wrote the search methodology sections of the final report.
Nigel Gummerson (Consultant Orthopaedic Trauma and Spinal Surgeon) provided clinical advice and commented on drafts of the protocol and final report.
Mark Sculpher (Professor of Health Economics) provided input at all stages, was involved in the development of the economic model, commented on drafts of the report and had overall responsibility for the cost-effectiveness section of the report.
Nerys Woolacott (Senior Research Fellow) provided input at all stages, commented on drafts of the report and had overall responsibility for the clinical effectiveness section of the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Mitton D, De Guise JA, Dubousset J, Skalli W, Gonzalez Y, Cerrolaza M. Bioengineering modeling and computer simulation. Barcelona: International Centre for Numerical Methods in Engineering; 2007.
- National Institute for Health and Clinical Excellence (NICE) . Diagnostics Assessment Programme: EOS Ultra Low Dose 2D 3D X-Ray Imaging System for Postural Assessment. Request for Information on Topic 2010.
- National Institute for Health and Clinical Excellence (NICE) . EOS 2D 3D X-Ray Imaging System: Final Scope 2010.
- Gummerson NW, Millner PA. Spinal fusion for scoliosis, clinical decision-making and choice of approach and devices. Skeletal Radiol 2010;39:939-42.
- National Scoliosis Foundation Website n.d. www.scoliosis.org/info.php (accessed 6 February 2011).
- Scoliosis Association UK n.d. www.sauk.org.uk/ (accessed 6 February 2011).
- Weinstein SL, Dolan LA, Cheng JCY, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet 2008;371:1527-37.
- Stirling AJ, Howel D, Millner PA, Sadiq S, Sharples D, Dickson RA. Late-onset idiopathic scoliosis in children six to fourteen years old. A cross-sectional prevalence study. J Bone Joint Surg Am 1996;78:1330-6.
- Lenssinck MLB, Frijilink AC, Berger MY, Bierma-Zeinstra SMA, Verkerk K, Verhargen AP. Effect of bracing and other conservative interventions in the treatment of idiopathic scoliosis in adolescents: a systematic review of clinical trials. Phys Ther 2005;85:1329-39.
- McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis. A study of one hundred and twelve patients. J Bone Joint Surg Am 1999;81:1367-83.
- Hedequist DJ, Kim DH, Betz RR, Huhn SL, Newton PO. Surgery of the pediatric spine. New York, NY: Thieme Medical Publishers; 2007.
- Lowe TG. Surgery of the pediatric spine. New York, NY: Thieme Medical Publishers; 2007.
- The National Ankylosing Spondylitis Society . Guidebook for Patients – a Positive Response to Ankylosing Spondylitis: Answers and Practical Advice 2008.
- Clarke NMP, Sakthivel K. The diagnosis and management of congenital dislocation of the hip. Paediatr Child Health 2008;18:268-71.
- American College of Radiology . Practice Guideline for the Performance of Radiography for Scoliosis in Children 2009:1-6.
- Huda W. Assessment of the problem: pediatric doses in screen-film and digital radiography. Pediatr Radiol 2004;34:S173-82.
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, Board on Radiation Effects Research, Division on Earth and Life Studies, National Research Council of the National Academies . Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2 2006. www.nap.edu/catalog.php?record_id=11340 (accessed 27 October 2010).
- International Commission on Radiological Protection (ICRP) . The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1-332.
- Seibert JA. Tradeoffs between image quality and dose. Pediatr Radiol 2004;34:S183-95.
- European Commission . European Guidelines on Quality Criteria for Diagnostic Radiographic Images 1996.
- European Commission . European Guidelines on Quality Criteria for Diagnostic Radiographic Images in Paediatrics 1996.
- Centre for Reviews and Dissemination (CRD) . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Moher D, Cook D, Eastwood S, Olkin I, Rennie D, Stroup D. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUORUM statement. Lancet 1999;354:1896-900.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Kalifa G, Charpak Y, Maccia C, Fery-Lemonnier E, Bloch J, Boussard JM, et al. Evaluation of a new low-dose digital x-ray device: first dosimetric and clinical results in children. Pediatr Radiol 1998;28:557-61.
- Le Bras A, Dorion I, Ferey S, Maccia C, Parent S, Kalifa G. Low Dose 2D-3D X-Ray Scanning Imaging for Osteoarticular Pathologies: Initial Results on Scoliotic Children n.d.
- Deschênes S, Charron G, Beaudoin G, Labelle H, Dubois J, Miron M-C, et al. Diagnostic imaging of spinal deformities: reducing patients radiation dose with a new slot-scanning X-ray imager. Spine 2010;35:989-94.
- Commission of the European Communities (CEC) . Quality Criteria for Diagnostic Radiographic Images. Working Document XII 173 90 1990.
- European Commission . European Guidelines on Quality Criteria for Diagnostic Radiographic Images in Pediatrics 1964.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) . Sources and Effects of Ionizing Radiation. UNSCEAR 2008 – Report to the General Assembly With Scientific Annexes. Volume I 2010. www.unscear.org/unscear/en/publications/2008_1.html (accessed 27 October 2010).
- Preston-Martin S, Thomas DC, White SC, Cohen D. Prior exposure to medical and dental x-rays related to tumors of the parotid gland. J Natl Cancer Inst 1988;80:943-9.
- Boice JD, Morin MM, Glass AG, Friedman GD, Stovall M, Hoover RN, et al. Diagnostic x-ray procedures and risk of leukemia, lymphoma, and multiple myeloma. JAMA 1991;265:1290-4.
- Inskip PD, Ekbom A, Galanti MR, Grimelius L, Boice JD. Medical diagnostic x rays and thyroid cancer. J Natl Cancer Inst 1995;87:1613-21.
- Hoffman DA, Lonstein JE, Morin MM, Visscher W, Harris BS, Boice JD. Breast cancer in women with scoliosis exposed to multiple diagnostic x-rays. J Natl Cancer Inst 1989;81:1307-12.
- Doody MM, Lonstein JE, Stovall M, Hacker DG, Luckyanov N, Land CE. Breast cancer mortality after diagnostic radiography: findings from the US. Scoliosis Cohort Study. Spine 2000;25:2052-63.
- Izumi S, Suyama A, Koyama K. Radiation-related mortality among offspring of atomic bomb survivors: a half-century of follow-up. Int J Cancer 2003;107:292-7.
- Izumi S, Koyama K, Soda M, Suyama A. Cancer incidence in children and young adults did not increase relative to parental exposure to atomic bombs. Br J Cancer 2003;89:1709-13.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; n.d.
- Schulze-Rath R, Hammer GP, Blettner M. Are pre- or postnatal diagnostic X-rays a risk factor for childhood cancer? A systematic review. Radiat Environ Biophys 2008;47:301-12.
- Ronckers CM, Doody MM, Lonstein JE, Stovall M, Land CE. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2008;17:605-13.
- Ronckers CM, Land CE, Miller JS, Stovall M, Lonstein JE, Doody MM. Cancer mortality among women frequently exposed to radiographic examinations for spinal disorders. Radiat Res 2010;174:83-90.
- Cox DW. An investigation of possible genetic damage in the offspring of women receiving multiple diagnostic pelvic x-rays. Am J Hum Genet 1964;16:214-30.
- Goldberg MS, Mayo NE, Levy AR, Scott SC, Poitras B. Adverse reproductive outcomes among women exposed to low levels of ionizing radiation from diagnostic radiography for adolescent idiopathic scoliosis. Epidemiology 1998;9:271-8.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 27 October 2010).
- Comité d’Évaluation et de Diffusion des Innovations Technologiques (CEDIT) . EOS Low Dose 2D 3D X-Ray Imager 2007. http://ancien-cedit.aphp.fr/servlet/siteCeditGB?Destination=reco%26numArticle=07.03/Re1/07 (accessed 27 October 2010).
- Biospace Med . Calcul Rentabilité Etablissement 190110 n.d.
- Integrity Medical Capital . Enhancement Cost Analysis For: Prospective Biospace Med Integrity Medical Capital Lessee 2009.
- ROI Proforma Input Sheet US n.d.
- Hansen J, Grethe Jurik A, Fiirgaard B, Egund N. Optimisation of scoliosis examinations in children. Pediatr Radiol 2003;33:752-65.
- Hart D, Hillier MC, Wall BF. Doses to patients from radiographic and fluorographic x-ray imaging procedures in the UK – 2005 review. Didcot, Oxfordshire: Health Protection Agency; 2007.
- International Commission on Radiological Protection (ICRP) . ICRP publication 60: 1990 recommendations of the International Commission on Radiological Protection. Ann ICRP 1991;21.
- Cook JV, Shah K, Pablot S, Kyriou J, Pettett A. Guidelines on best practice in the X-ray imaging of children. London: Queen Mary’s Hospital for Children and The Radiological Protection Centre, St George’s Healthcare NHS Trust; 1998.
- Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing cancer risks of low-dose radiation. Nat Rev Cancer 2009;9:596-604.
- Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzales A. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 2009;251:6-12.
- Chilcott JB, Hummel S, Mildred M. Option appraisal: screening for prostate cancer. Sheffield: School of Health and Related Research, University of Sheffield; 2010.
- Tappenden P. A Methodological Framework for Developing Whole Disease Models to Inform Resource Allocation Decisions: An Application in Colorectal Cancer 2010.
- Campbell HE, Epstein D, Bloomfield D, Griffin S, Manca A, Yarnold J, et al. The cost-effectiveness of adjuvant chemotherapy for early breast cancer: a comparison of no chemotherapy and first, second, and third generation regimens for patients with differing prognoses. Eur J Cancer Care 2011. 10.1016/j.ejca.2011.06.019.
- Fenwick E, Kulin NA, Marshall D, Hall Long K. A probabilistic decision model to guide optimal health policy decisions for lung cancer screening. Presentation HSR-51. Med Decis Making 2011;31.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Health and Social Care Information Centre . Hospital Episode Statistics: HES User Guide 2010. www.hesonline.nhs.uk (accessed 27 October 2010).
- Johannesson M, Weinstein S. On the decision rules of cost-effectiveness analysis. J Health Econ 1993;12:459-67.
- Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med 1999;130:719-28.
- Palmer S, Smith PC. Incorporating option values into the economic evaluation of health care technologies. J Health Econ 2000;19:755-66.
- Clinical Trials.gov . Postural Balance of the Adult n.d. http://clinicaltrials.gov/show/NCT00926432.
- Clinical Trials.gov . Bracing During Infantile Scoliosis: Airways Study n.d. http://clinicaltrials.gov/show/NCT01087034.
- Clinical sites activity. Paris: Biospace Med; n.d.
- Biospace Med’s EOS . Marketing Authorisation 2007. www.accessdata.fda.gov/cdrh_docs/pdf7/K071546.pdf.
- Anonymous . EOS – a new 2D|3D, low dose musculoskeletal imaging system. EHM: Executive Healthcare 2008;3.
- Alison M, Azoulay R, Tilea B, Grandjean S, Lefevre T, Achour I, et al. Evaluation of workflow in a pediatric radiology department using ultra low dose digital imaging system. Pediatr Radiol 2009;39.
- Assi A, Presedo A, Baudoin A, Mitton D, Ghanem I, Skalli W. Specific 3D reconstruction for children lower limbs using a low dose biplanar X-ray system. Reproducibility of clinical parameters for cerebral palsy patients. Comput Methods Biomech Biomed Engin 2007;10:27-8.
- Aubin CE, Dansereau J, Parent F, Labelle H, de Guise JA. Morphometric evaluations of personalised 3D reconstructions and geometric models of the human spine. Med Biol Eng Comput 1997;35:611-18.
- Azmy C, Guerard S, Bonnet X, Gabrielli F, Skalli W. EOS(R) orthopaedic imaging system to study patellofemoral kinematics: assessment of uncertainty. Orthop Traumatol Surg Res 2010;96:28-36.
- Barthe N, Chatti K, Coulon P, Maitrejean S, Basse-Cathalinat B. Recent technologic developments on high-resolution beta imaging systems for quantitative autoradiography and double labeling applications. Nucl Instrum Methods Phys Res A 2004;527:41-4.
- Baru SE, Khabakhpashev AG, Shekhtman LI. A low-dose X-ray imaging device. Eur J Phys 1998;19:475-83.
- Benameur S, Mignotte M, Destrempes F, De Guise JA. Three-dimensional biplanar reconstruction of scoliotic rib cage using the estimation of a mixture of probabilistic prior models. IEEE Trans Biomed Eng 2005;52:1713-28.
- Benameur S, Mignotte M, Labelle H, De Guise JA. A hierarchical statistical modeling approach for the unsupervised 3-D biplanar reconstruction of the scoliotic spine. IEEE Trans Biomed Eng 2005;52:2041-57.
- Benameur S, Mignotte M, Parent S, Labelle H, Skalli W, De Guise JA. 3D Biplanar Reconstruction of Scoliotic Vertebrae Using Statistical Models n.d.:577-82.
- Benameur S, Mignotte M, Parent S, Labelle H, Skalli W, De Guise JA. A Hierarchical Statistical Modeling Approach for the Unsupervised 3D Reconstruction of the Scoliotic Spine n.d.:I561-I8.
- Bertrand S, Laporte S, Parent S, Skalli W, Mitton D. Reproducibility evaluation of rib cage 3D reconstruction from stereoradiography. Comput Methods Biomech Biomed Engin 2005;8:37-8.
- Bertrand S, Laporte S, Parent S, Skalli W, Mitton D. Three-dimensional reconstruction of the rib cage from biplanar radiography. IRBM 2008;29:278-86.
- Billuart F, Devun L, Skalli W, Mitton D, Gagey O. Role of deltoid and passives elements in stabilization during abduction motion (0 °–40 °): an ex vivo study. Surg Radiol Anat 2008;30:563-8.
- Breton S, Stindel E, Genu A, Auffret M, Senecail B, Forlodou P. Limb Length Measurement With the EOS System: Comparison With Conventional Systems n.d.
- Chaibi Y, Cresson T, Aubert B, Hausselle J, Neyret P, Hauger O, et al. Fast 3D reconstruction of the lower limb using a parametric model and statistical inferences and clinical measurements calculation from biplanar x-rays. Comput Methods Biomech Biomed Engin 2012. 10.1080/10255842.2010.540758.
- Chaibi Y. Adaptation Des méthodes De Reconstruction 3D Rapides Par stéréoradiographie: Modélisation Du Membre inférieur Et Calcul Des Indices Cliniques En présence De déformation Structurale 2010.
- Charpak MG, Chouard MCH, Picard MJ, Menkes MCJ, Paolaggi MJB, Sraer MJD, et al. A new 2D and 3D imaging approach to musculo-skeletal physiology and pathology with low-dose radiation and the standing position: the EOS system – discussion. Bull Acad Natl Med 2005;189:297-300.
- Chateil JF. From Charpak’s experimental wire chamber to clinical applications. J Radiol 2005;86.
- Cheriet F, Laporte C, Kadoury S, Labelle H, Dansereau J. A novel system for the 3D reconstruction of the human spine and rib cage from biplanar X-ray images. IEEE Trans Biomed Eng 2007;54:1356-8.
- Comité d’Évaluation et de Diffusion des Innovations Technologiques (CEDIT) . Radiological Application of Charpak’s Multiwire Chamber (III) 1996. http://cedit.aphp.fr/servlet/siteCeditGB?Destination=reco%26numArticle=93.10.3 (accessed 27 October 2010).
- Cresson T, Branchaud D, Chav R, Godbout B, de Guise JA. 3D shape reconstruction of bone from two x-ray images using 2D/3D non-rigid registration based on moving least-squares deformation. Proc SPIE 2010;7623.
- Cresson T, Chav R, Branchaud D, Humbert L, Godbout B, Aubert B, et al. Coupling 2D 3D Registration Method and Statistical Model to Perform 3D Reconstruction from Partial X-Rays Images Data 2009:1008-11.
- de la Simone M, Gomes C, Nizard R, Laredo JD, Wybier M, Bellaiche L, et al. Know-how in osteoarticular radiology. Paris: Sauramps Medical; 2010.
- Deschênes S. Clinical Studies With EOS in CHU Sainte-Justine and CHUM n.d.
- Deschênes S, Charron G, Beaudoin G, Miron MC, Parent S, Labelle H, et al. Dose reduction in scoliosis follow-up using a new slot-scanner radiographical system. Pediatr Radiol 2009;39:S538-9.
- Deschênes S, Godbout B, Branchaud D, Mitton D, Pomero V, Bleau A, et al. 3D reconstruction of the human spine from bi-planar radiographs: using multi-scale wavelets analysis and splines interpolators for semi-automation. Proc SPIE 2003;5032:754-61.
- Despres P, Beaudoin G, Gravel P. Evaluation of a full-scale gas microstrip detector for low-dose X-ray imaging. Nucl Instrum Methods Phys Res A 2005;536:52-60.
- Douglas TS, Sanders V, Pitcher R, van As AB. Early detection of fractures with low-dose digital X-ray images in a pediatric trauma unit. J Trauma 2008;65:E4-7.
- Douglas TS, Vaughan CL, Wynne SM. Three-dimensional point localisation in low-dose X-ray images using stereo-photogrammetry. Med Biol Eng Comput 2004;42:37-43.
- Dubousset J. Clinical case: degenerative scoliosis in the elderly. Paris: Biospace Med; n.d.
- Dubousset J, Charpak G, Dorion I, Skalli W, Lavaste F, Deguise J, et al. A new 2D and 3D imaging approach for musculo-skeletal physiology and pathology with low radiation dose and standing position: the EOS system. Bull Acad Natl Med 2005;189:287-300.
- Dubousset J, Charpak G, Dorion I, Skalli W, Lavaste F, Deguise J, et al. A new imaging 2D and 3D for musculo-skeletal physiology and pathology with low radiation dose and standing position: the EOS system. Radioprotection 2005;40:245-55.
- Dubousset J, Charpak G, Skalli W, de Guise J, Kalifa G, Wicart P. Skeletal and spinal imaging with EOS system. Arch Pediatr 2008;15:665-6.
- Dubousset J, Charpak G, Skalli W, Deguise J, Kalifa G. EOS: A new imaging system with low dose radiation in standing position for spine and bone and joint disorders. J Musculoskelet Res 2010;13:1-12.
- Dubousset J, Charpak G, Skalli W, Kalifa G, Lazennec JY. EOS stereo-radiography system: whole-body simultaneous anteroposterior and lateral radiographs with very low radiation dose. Rev Chir Orthop Reparatrice Appar Mot 2007;93:141-3.
- Dumas R, Blanchard B, Carlier R, de Loubresse CG, Le Huec J-C, Marty C, et al. A semi-automated method using interpolation and optimisation for the 3D reconstruction of the spine from bi-planar radiography: a precision and accuracy study. Med Biol Eng Comput 2008;46:85-92.
- Dumas R, Le Bras A, Champain N, Savidan M, Mitton D, Kalifa G, et al. Validation of the relative 3D orientation of vertebrae reconstructed by bi-planar radiography. Med Eng Phys 2004;26:415-22.
- Dumas R, Mitton D, Laporte S, Dubousset J, Steib JP, Lavaste F, et al. Explicit calibration method and specific device designed for stereoradiography. J Biomech 2003;36:827-34.
- Dumas R, Mitton D, Steib JP, de Guise JA, Skalli W. Pre and post 3D modeling of scoliotic patients operated with in situ contouring technique. Stud Health Technol Inform 2002;91:291-5.
- Dumas R, Steib J-P, Mitton D, Lavaste F, Skalli W. Three-dimensional quantitative segmental analysis of scoliosis corrected by the in situ contouring technique. Spine 2003;28:1158-62.
- Dumas R, Aissaoui R, Mitton D, Skalli W, de Guise JA. Personalized body segment parameters from biplanar low-dose radiography. IEEE Trans Biomed Eng 2005;52:1756-63.
- Gangnet N, Dumas R, Pomero V, Mitulescu A, Skalli W, Vital J-M. Three-dimensional spinal and pelvic alignment in an asymptomatic population. Spine 2006;31:E507-12.
- Gangnet N, Pomero V, Dumas R, Skalli W, Vital JM. Variability of the spine and pelvis location with respect to the gravity line: a three-dimensional stereoradiographic study using a force platform. Surg Radiol Anat 2003;25:424-33.
- Gille O, Champain N, Benchikh-El-Fegoun A, Vital JM, Skalli W. Reliability of 3D reconstruction of the spine of mild scoliotic patients. Spine 2007;32:568-73.
- Glard Y, Pomero V, Collignon P, Skalli W, Jouve J-L, Bollini G. Sagittal balance in scoliosis associated with Marfan syndrome: a stereoradiographic three-dimensional analysis. J Child Orthop 2008;2:113-18.
- Glard Y, Pomero V, Collignon P, Skalli W, Jouve J-L, Bollini G. Three-dimensional analysis of the vertebral rotation associated with the lateral deviation in Marfan syndrome spinal deformity. J Pediatr Orthop, Part B 2009;18:51-6.
- Guenoun B, Zadegan F, Aim F, Hannouche D, Nizard R. Interest of the EOS(R) three dimensional reconstructions for the measurement of lower limb clinical parameters. EFORT, Madrid; 2010.
- Hascall T, Yee EF, Gomez AG. EOS, EOS . . . oh!. J Gen Intern Med 2002;17.
- Humbert L, Carlioz H, Baudoin A, Skalli W, Mitton D. 3D Evaluation of the acetabular coverage assessed by biplanar X-rays or single anteroposterior X-ray compared with CT-scan. Comput Methods Biomech Biomed Engin 2008;11:257-62.
- Humbert L, De Guise JA, Aubert B, Godbout B, Skalli W. 3D reconstruction of the spine from biplanar X-rays using parametric models based on transversal and longitudinal inferences. Med Eng Phys 2009;31:681-7.
- Humbert L, De Guiset JA, Godbou B, Parent S, Dubousset J, Skalli W. Comparative study of scoliotic spines using a fast 3D reconstruction method from biplanar X-rays. Comput Methods Biomech Biomed Engin 2008;11:117-18.
- Illes T. Clinical Case: Correction of a Major Grade Secondary Scoliosis in Cerebral Palsy n.d.
- Illes T, Tunyogi-Csapo M, Somoskeoy S. Three-Dimensional Visualization and Complete 3D Characterization of the Spine in Scoliosis: A Preliminary Clinical Study Based on Vertebrae Vectors n.d.
- Illes T, Tunyogi-Csapo M, Somoskeoy S. Breakthrough in three-dimensional scoliosis diagnosis – significance of horizontal plane view and vertebra vectors. EuroSpine J 2011;20:135-43.
- Janssen MMA, Drevelle X, Humbert L, Skalli W, Castelein RM. Differences in male and female spino-pelvic alignment in asymptomatic young adults: a three-dimensional analysis using upright low-dose digital biplanar X-rays. Spine 2009;34:E826-32.
- Jolivet E, Sandoz B, Laporte S, Mitton D, Skalli W. Fast 3D reconstruction of the rib cage from biplanar radiographs. Med Biol Eng Comput 2010;48:821-8.
- Journe A, Belicourt C, Sautet CA, Dousounian L. New method of analysing acetabular cup position with the low dose irradiation EOS System. Evaluation of reproducibility and repeatability. Rev Chir Orthop Traumatol 2010;96.
- Kadoury S, Cheriet F, Labelle H. A Statistical Image-Based Approach for the 3D Reconstruction of the Scoliotic Spine from Biplanar Radiographs n.d.
- Kadoury S, Cheriet F, Labelle H. Personalized x-ray 3-D reconstruction of the scoliotic spine from hybrid statistical and image-based models. IEEE Trans Med Imag 2009;28:1422-35.
- Kalifa G, Boussard JM. Digital radiography: Charpak’s device. J Radiol 1996;77.
- Lafage V, Dubousset J, Lavaste F, Skalli W. Finite element simulation of various strategies for CD correction. Stud Health Tech Informat 2002;91:428-32.
- Laporte S, Skalli W, Aubert B, Bertrand S, Mitton D. Rib cage tridimensional reconstruction from stereoradiography. Arch Physiol Biochem 2004;112.
- Laporte S, Skalli W, De Guise J, Mitton D. A 3D reconstruction method from biplanar X-rays based on 2D contours. Arch Physiol Biochem 2002;110.
- Laville A, Laporte S, Skalli W. Parametric and subject-specific finite element modelling of the lower cervical spine. Influence of geometrical parameters on the motion patterns. J Biomech 2009;42:1409-15.
- Lazennec JY, Rangel a, Baudoin A, Skalli W, Catonne Y, Rousseau MA. Case report: patellofemoral syndrome as unusual complication following THP. Radiological 3D Analysis With the EOS(R) System n.d.
- Le Bras A, Laporte S, Bousson V, Mitton D, De Guise JA, Laredo JD, et al. Personalised 3D reconstruction of proximal femur from low-dose digital biplanar radiographs. Int Congr Ser 2003;1256:214-19.
- Le Bras A, Laporte S, Bousson V, Mitton D, De Guise JA, Laredo JD, et al. 3D reconstruction of the proximal femur with low-dose digital stereoradiography. Comput Aided Surg 2004;9:51-7.
- Le Bras A, Laporte S, Mitton D, de Guise JA, Skalli W. 3D detailed reconstruction of vertebrae with low dose digital stereoradiography. Stud Health Technol Inform 2002;91:286-90.
- Le Bras A, Laporte S, Mitton D, De Guise JA, Skalli W. Three-dimensional (3D) detailed reconstruction of human vertebrae from low-dose digital stereoradiography. Eur J Orthop Surg Traumatol 2003;13:57-62.
- Mitton D, Deschenes S, Laporte S, Godbout B, Bertrand S, de Guise JA, et al. 3D reconstruction of the pelvis from bi-planar radiography. Comput Methods Biomech Biomed Engin 2006;9:1-5.
- Mitton D, Landry C, Veron S, Skalli W, Lavaste F, De Guise JA. 3D reconstruction method from biplanar radiography using non-stereocorresponding points and elastic deformable meshes. Med Biol Eng Comput 2000;38:133-9.
- Mitulescu A, Laporte S, Boulay C, De Guise JA, Skalli W. 3D reconstruction of the pelvis using the NSCP technique. Stud Health Technol Inform 2002;88:177-81.
- Ngoc Hoan N, Majewski S, Charpak G, Policarpo AJPL. An efficient, gaseous detector with good low-energy resolution for (less than or equal to 50 keV) imaging. J Nucl Med 1979;20:335-40.
- Node-Langlois L, Laporte S, Badet R, Neyret P, Mitton D, Lavaste F, et al. Three-dimensional reconstruction of the knee from biplanar X-rays clinical evaluation: a review of seven pathological cases. Int Congr Ser 2003;1256.
- Novosad J, Eng B, Cheriet F, Delorme S, Poirier S, Beau S, et al. Self-calibration of biplanar radiographs for a retrospective comparative study of the 3D correction of adolescent idiopathic scoliosis. Stud Health Technol Inform 2002;91:272-5.
- Obeid I. Clinical case: 3D evaluation of surgical correction of idiopathic scoliosis by vertebral column manipulation. Paris: Biospace Med; n.d.
- Ohl X, Stanchina C, Billuart F, Skalli W. Shoulder bony landmarks location using the EOS(R) low-dose stereoradiography system: a reproducibility study. Surg Radiol Anat 2010;32:153-8.
- Pomero V, Deschenes S, Branchaud D, Mitton D, Laporte S, Godbout B, et al. Fast semiautomatic stereoradiographic reconstruction of scoliotic spines using multi-scale image processing and statistical geometric models. Int Congr Ser 2003;1256:207-13.
- Pomero V, Mitton D, Laporte S, de Guise JA, Skalli W. Fast accurate stereoradiographic 3D-reconstruction of the spine using a combined geometric and statistic model. Clin Biomech 2004;19:240-7.
- Rillardon L, Campana S, Mitton D, Skalli W, Feydy A. Evaluation of the intervertebral disc spaces with a low dose radiographic system. J Radiol 2005;86:311-19.
- Rousseau M-A, Laporte S, Chavary-Bernier E, Lazennec J-Y, Skalli W. Reproducibility of measuring the shape and three-dimensional position of cervical vertebrae in upright position using the EOS stereoradiography system. Spine 2007;32:2569-72.
- Sabourin M, Jolivet E, Miladi L, Wicart P, Rampal V, Skalli W. Three-dimensional stereoradiographic modeling of rib cage before and after spinal growing rod procedures in early-onset scoliosis. Clin Biomech 2010;25:284-91.
- Sandoz B, Laporte S, Skalli W, Mitton D. Subject-specific mass and 3D localisation of the mass centre of child body segments using biplanar X-rays. Comput Methods Biomech Biomed Engin 2008;11:203-4.
- Sapin De Brosses E, Briot K, Kolta S, Skalli W, Roux C, Mitton D. Subject-specific mechanical properties of vertebral cancellous bone assessed using a low-dose X-ray device. IRBM 2010;31:148-53.
- Sapin E, Briot K, Kolta S, Gravel P, Skalli W, Roux C, et al. Bone mineral density assessment using the EOS low-dose X-ray device: a feasibility study. Proc Inst Mech Eng H 2008;222:1263-71.
- Sapin E, Gravel P, Skalli W, Mitton D. Bone mineral density (BMD) assessment using the EOS (R) low-dose X-ray device: a feasibility study. Comput Methods Biomech Biomed Engin 2007;10:49-50.
- Sato T, Koga Y, Omori G. Three-dimensional lower extremity alignment assessment system: application to evaluation of component position after total knee arthroplasty. J Arthroplasty 2004;19:620-8.
- Sauli F. Applications of Gaseous Particle Detectors in Physics and Medicine n.d.:335-44.
- Schlatterer B, Suedhoff I, Bonnet X, Catonne Y, Maestro M, Skalli W. Skeletal landmarks for TKR implantations: evaluation of their accuracy using EOS imaging acquisition system. Orthop Traumatol Surg Res 2009;95:2-11.
- Sebag G, Dubois J. Ultra low dose radiology in children. Paris: Biospace Med; n.d.
- Situ W, Li Y, Li Z, Hu Z, He J. SLOT Scan imaging in teenagers with scoliosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2009;34:675-9.
- Steffen JS, Humbert L, Dubousset J, Vialle R, Skalli W. Patient specific modelling of the spine affected with congenital malformations: a preliminary study. Comput Methods Biomech Biomed Engin 2008;11:205-7.
- Steffen J-S, Obeid I, Aurouer N, Hauger O, Vital J-M, Dubousset J, et al. 3D postural balance with regard to gravity line: an evaluation in the transversal plane on 93 patients and 23 asymptomatic volunteers. Eur Spine J 2010;19:760-7.
- Sudhoff I, Van Driessche S, Laporte S, de Guise JA, Skalli W. Comparing three attachment systems used to determine knee kinematics during gait. Gait Posture 2007;25:533-43.
- Sushkov AV, Andreev VF, Kravtsov DE. Duplex multiwire proportional x-ray detector for multichord time-resolved soft x-ray and electron temperature measurements on T-10 tokamak. Rev Sci Instrum 2008;79.
- Vital JM, Dubousset J, Gille O, Hauger O, Aurouer N, Obeid I. Clinical applications of the EOS system in diseases of locomotor apparatus. ArgoSpine News J 2008;20:117-21.
- Wahrburg J, Kerschbaumer F. Thoughts on the use of mechatronic implantation aids in minimal approaches in hip prostheses. Orthopade 2000;29:650-7.
- Zheng G. Reconstruction of Patient-Specific 3D Bone Model from Biplanar X-Ray Images and Point Distribution Models n.d.
- Zheng G, Schumann S. 3-D reconstruction of a surface model of the proximal femur from digital biplanar radiographs n.d.:66-9.
- Aguilar Naranjo JJ, Santos Andres JF, Usabiaga Bernal T, Boren A, Escalada Recta F, Arguelles Bravo C, et al. Risk of radiation in the treatment of scoliosis. Need of reducing the radiographic exposure. Rehabilitacion 1987;21:43-8.
- Anasti JN. Premature ovarian failure: an update. Fertil Steril 1998;70:1-15.
- Ashley WW, McKinstry RC, Leonard JR, Smyth MD, Lee BC, Park TS. Use of rapid-sequence magnetic resonance imaging for evaluation of hydrocephalus in children. J Neurosurg 2005;103:124-30.
- Atkinson L, Pratt S, Slevin T, Fritschi L, Catchpole B, Mendelson R, et al. Towards appropriate use of diagnostic imaging in general practice. J Med Imaging Radiat Oncol 2009;53.
- Bailey HD, Armstrong BK, de Klerk NH, Fritschi L, Attia J, Lockwood L, et al. Exposure to diagnostic radiological procedures and the risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev 2010;19:2897-909.
- Baker SR, Bhatti WA. The thyroid cancer epidemic: is it the dark side of the CT revolution?. Eur J Radiol 2006;60:67-9.
- Barcellos-Hoff MH, Nguyen DH. Radiation carcinogenesis in context: how do irradiated tissues become tumors?. Health Phys 2009;97:446-57.
- Berrington de Gonzales A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 2004;363:345-51.
- Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009;169:2071-7.
- Berrington de Gonzalez A, Kim KP, Yee J. CT colonography: perforation rates and potential radiation risks. Gastrointest Endosc Clin N Am 2010;20:279-91.
- Bone C, Hseih G. The risk of carcinogenesis from radiographs to pediatric orthopaedic patients. J Pediatr Orthop 2000;20:251-4.
- Brenner DJ, Elliston CD, Hall EJ, Berdon WE. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289-96.
- Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology 2004;232:735-8.
- Butler PF, Thomas AW, Thompson WE, Wollerton MA, Rachlin JA. Simple methods to reduce patient exposure during scoliosis radiography. Radiol Technol 1986;57:411-17.
- Campbell H. A review of studies of breast cancer in human populations excluding endocrine studies. Proc R Soc Med 1972;65:641-5.
- Chawla SC, Federman N, Zhang D, Nagata K, Nuthakki S, McNitt-Gray M, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol 2010;40:681-6.
- Chew FS, Weissleder R. Radiation-induced osteochondroma. AJR Am J Roentgenol 1991;157.
- Colditz GA. Hormone replacement therapy increases the risk of breast cancer. Ann N Y Acad Sci 1997;833:129-36.
- Cozen L. Breast cancer and scoliosis. Am J Orthop 1999;28.
- De Smet AA, Fritz SL, Asher MA. A method for minimizing the radiation exposure from scoliosis radiographs. J Bone Joint Surg Am 1981;63:156-61.
- Don S. Radiosensitivity of children: potential for overexposure in CR and DR and magnitude of doses in ordinary radiographic examinations. Pediatr Radiol 2004;34:S167-72.
- Don S. Radiosensitivity of children: potential for overexposure in CR and DR and magnitude of doses in ordinary radiographic examinations. Pediatr Radiol 2004;34:S234-41.
- Dreyer NA, Friedlander E. Identifying the health risks from very low-dose sparsely ionizing radiation. Am J Public Health 1982;72:585-8.
- Dutkowsky JP, Shearer D, Schepps B, Orton C, Scola F. Radiation exposure to patients receiving routine scoliosis radiography measured at depth in an anthropomorphic phantom. J Pediatr Orthop 1990;10:532-4.
- Einstein A, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317-23.
- Friedler G. Paternal exposures: impact on reproductive and developmental outcome. An overview. Pharmacol Biochem Behav 1996;55:691-700.
- Frik W. Mass screening of the hip joints in infants, and radiation protection (an expertise of the German Roentgen Society). RoFo 1972;116:453-5.
- Gerber TC, Gibbons RJ. Weighing the risks and benefits of cardiac imaging with ionizing radiation. JACC Cardiovasc Imaging 2010;3:528-35.
- Goss PE, Sierra S. Current perspectives on radiation-induced breast cancer. J Clin Oncol 1998;16:338-47.
- Hallen S, Martling K, Mattsson S, Gustafsson M, Thilander A, Thornberg L. Dosimetry at X ray examinations of scoliosis. Radiat Prot Dosimetry 1992;43:49-54.
- Hart D, Hillier MC, Wall BF. Doses to patients from medical x-ray examinations in the UK – 2000 review. Didcot: National Radiological Protection Board; 2002.
- Hart D, Wall BF. Radiation exposure of the UK population from medical and dental x-ray examinations. Didcot: National Radiological Protection Board; 2002.
- Hart D, Wall BF. UK population dose from medical X-ray examinations. Eur J Radiol 2004;50:285-91.
- Hart D, Hillier MC, Wall BF. National reference doses for common radiographic, fluoroscopic and dental X-ray examinations in the UK. Br J Radiol 2009;82:1-12.
- Hendry JH. Response of human organs to single (or fractionated equivalent) doses of irradiation. Int J Radiat Biol 1989;56:691-700.
- Hrabovszky Z, Nikl I. Roentgenological examinations and a review of their indications with special reference to gonadal hazard. I. Determination of the gonadal dose. Magy Radiol 1964;16:65-88.
- Hughes JS, Watson SJ, Jones AL, Oatway WB. Review of the radiation exposure of the UK population. J Radiol Prot 2005;25:493-6.
- Huncharek M. Non-asbestos related diffuse malignant mesothelioma. Tumori 2002;88:1-9.
- Huppmann MV, Johnson WB, Javitt MC. Radiation risks from exposure to chest computed tomography. Semin Ultrasound CT MR 2010;31:14-28.
- Infante-Rivard C, Mathonnet G, Sinnett D. Risk of childhood leukemia associated with diagnostic irradiation and polymorphisms in DNA repair genes. Environ Health Perspect 2000;108:495-8.
- Jansen-van der Weide MC, Greuter MJW, Jansen L, Oosterwijk JC, Pijnappel RM, Oudkerk M, et al. Mammography screening and radiation-induced breast cancer among women with a familial or genetic predisposition: a meta-analysis. Eur J Cancer 2010;8.
- Kelsey JL. A review of the epidemiology of human breast cancer. Epidemiol Rev 1979;1:74-109.
- Kline J. Does maternal exposure to radiation before conception affect reproduction?. Epidemiology 1998;9:231-2.
- Kratzsch E. Gonade exposure in roentgen diagnosis of congenital hip dislocation. Dtsch Gesundheitsw 1972;27:1810-14.
- Leone A, Aulisa A, Perisano C, Re T, Galli M. Advantages of a two-step procedure for school-based scoliosis screening. Radiol Med 2010;115:238-45.
- Levy AR, Goldberg MS, Hanley JA, Mayo NE, Poitras B. Projecting the lifetime risk of cancer from exposure to diagnostic ionizing radiation for adolescent idiopathic scoliosis. Health Phys 1994;66:621-33.
- Levy AR, Goldberg MS, Mayo NE, Hanley JA, Poitras B. Reducing the lifetime risk of cancer from spinal radiographs among people with adolescent idiopathic scoliosis. Spine 1996;21:1540-8.
- LiVolsi VA, LoGerfo P, Feind CR. Coexistent parathyroid adenomas and thyroid carcinoma. Can radiation be blamed?. Arch Surg 1978;113:285-6.
- Mahmoud MS, Merhi ZO, Yelian FD. Mechanisms of premature ovarian failure: reappraisal and overview. J Reprod Med 2007;52:623-9.
- Mills DM, Tsai S, Meyer DR, Belden C. Pediatric ophthalmic computed tomographic scanning and associated cancer risk. Am J Ophthalmol 2006;142:1046-53.
- Muirhead CR. Projecting radiation-induced cancer risks across time and populations. Soz Praventivmed 1991;36:249-54.
- Muirhead CR. Projection of radiation-induced cancer risks across time and populations. Radiat Prot Dosimetry 1991;36:321-5.
- Nash CL, Gregg EC, Brown RH, Pillai K. Risks of exposure to x-rays in patients undergoing long-term treatment for scoliosis. J Bone Joint Surg Am 1979;61:371-4.
- Neta R. The promise of molecular epidemiology in defining the association between radiation and cancer. Health Phys 2000;79:77-84.
- Nussbaum RH, Kohnlein W. Inconsistencies and open questions regarding low-dose health effects of ionizing radiation. Environ Health Perspect 1994;102:656-67.
- Pape R, Harasta A. On the genetic radiation hazards of children in radiograms of the hip. Wien Med Wochenschr 1963;113:791-3.
- Preston RJ. Radiation biology: concepts for radiation protection. Health Phys 2004;87:3-14.
- Rao PS, Gregg EC. A revised estimate of the risk of carcinogenesis from x-rays to scoliosis patients. Invest Radiol 1984;19:58-60.
- Rice HE, Frush DP, Farmer D, Waldhausen JH. APSA Education Committee . Review of radiation risks from computed tomography: essentials for the pediatric surgeon. J Pediatr Surg 2007;42:603-7.
- Richter W, Kruger HR. Pelvic radiography in 4-month-old infants and its diagnostic dangers. Beitr Orthop Traumatol 1979;26:555-8.
- Rohrer R. Diagnostic radiation exposures among children diagnosed with leukemia vs. solid tumours in US, UK and Germany, 1995–2005. Eur J Cancer 2010;8.
- Ron E. Cancer risks from medical radiation. Health Phys 2003;85:47-59.
- Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res 2005;7:21-32.
- Royal HD. Effects of low level radiation: what’s new?. Semin Nucl Med 2008;38:392-40.
- Sadetzki S, Mandelzweig L. Childhood exposure to external ionising radiation and solid cancer risk. Br J Cancer 2009;100:1021-5.
- Samet JM. Epidemiologic studies of ionizing radiation and cancer: past successes and future challenges. Environ Health Perspect 1997;105:883-9.
- Sankaranarayanan K, Chakraborty R. Cancer predisposition, radiosensitivity and the risk of radiation-induced cancers. I. Background. Radiat Res 1995;143:121-43.
- Semelka RC, Armao DM, Elias J, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007;25:900-9.
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg 2003;237:474-82.
- Smith PG. The 1957 MRC report on leukaemia and aplastic anaemia in patients irradiated for ankylosing spondylitis. J Radiol Prot 2007;27:B3-14.
- Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078-86.
- Soffer D, Gomori JM, Pomeranz S, Siegal T. Gliomas following low-dose irradiation to the head report of three cases. J Neurooncol 1990;8:67-72.
- Solheim OP. Bone sarcomas following external irradiation. Acta Radiol Ther Phys Biol 1967;6:197-201.
- Stein SC, Hurst RW, Sonnad SS. Meta-analysis of cranial CT scans in children. A mathematical model to predict radiation-induced tumors. Pediatr Neurosurg 2008;44:448-57.
- Theocharopoulos N, Chatzakis G, Damilakis J. Is radiography justified for the evaluation of patients presenting with cervical spine trauma?. Med Phys 2009;36:4461-70.
- Woods DR. FDA seeks help to reduce adolescent x-ray exposure. J Sch Health 1987;57.
Appendix 1 Literature search strategies
Searches for review of clinical effectiveness and cost-effectiveness of EOS
Date searches conducted: 2–8 November 2010
-
Limits: 1993–date
-
Records found (after deduplication and hand-sifting for relevance): 661
-
Records found (before deduplication): 1811.
Databases searched
-
MEDLINE
-
AMED
-
Biosis Previews
-
CINAHL
-
The Cochrane Library
-
CDSR
-
DARE
-
CENTRAL
-
HTA Database
-
NHS EED
-
-
EMBASE
-
HMIC
-
INSPEC
-
ISI SCI
-
PASCAL.
Trials registries searched
-
ClinicalTrials.gov
-
CCT.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (OvidSP)
-
1993 – October week 3 2010
-
Date searched: 2 November 2010
-
Records found: 388.
-
((low dose or ultralow dose) adj (digital x-ray$ or digital xray$ or digital radiograph$ system$ or x-ray imag$ or xray imag$ or 2d 3d x-ray$ or 2d 3d xray$)).ti,ab. (14)
-
charpak.ti,ab. (3)
-
(multiwire chamber$ or multi wire chamber$).ti,ab. (7)
-
slot scan$.ti,ab. (41)
-
((biplanar or bi planar) adj2 (radiograph$ or x-ray$ or xray$)).ti,ab. (160)
-
stereoradiograph$.ti,ab. (110)
-
eos.ti,ab. (980)
Lines 1–7 capture terms for EOS and general terms for this radiography system
-
(eosinophil$ or schizophreni$ or end of synthesis or essential oil$ or protein$ or (ethanolamine adj2 sulphate) or endogenous opioid system$ or (early onset adj2 (sepsis or septic?emia)) or early onset sarcoidois or ceramide$ or ikaros or genome$ or (equation$adj2 state$) or composite or zinc or sodium).ti,ab. (2,362,051)
-
7 not 8 (276)
Line 9 excludes records where EOS is commonly used as an acronym in other subject areas
-
1 or 2 or 3 or 4 or 5 or 6 or 9 (593)
-
exp animals/ not humans/ (3,586,189)
-
10 not 11 (502)
Line 12 excludes animal-only studies
-
limit 12 to yr=“1993 -Current” (388)
Line 13 limits the search results to 1993–date
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
Allied and Complementary Medicine Database (OvidSP)
-
1993 – October 2010
-
Date searched: 2 November 2010
-
Records found: 28
-
((low dose or ultralow dose) adj (digital x-ray$ or digital xray$ or digital radiograph$system$ or x-ray imag$ or xray imag$ or 2d 3d x-ray$ or 2d 3d xray$)).ti,ab.
-
charpak.ti,ab.
-
(multiwire chamber$ or multi wire chamber$).ti,ab.
-
slot scan$.ti,ab.
-
((biplanar or bi planar) adj2 (radiograph$ or x-ray$ or xray$)).ti,ab.
-
stereoradiograph$.ti,ab.
-
eos.ti,ab.
-
(eosinophil$ or schizophreni$ or end of synthesis or essential oil$ or protein$ or (ethanolamine adj2 sulphate) or endogenous opioid system$ or (early onset adj2 (sepsis or septic?emia)) or early onset sarcoidois or ceramide$ or ikaros or genome$ or (equation$adj2 state$) or composite or zinc or sodium).ti,ab.
-
7 not 8
-
1 or 2 or 3 or 4 or 5 or 6 or 9
-
limit 10 to yr=“1993 -Current”
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
BIOSIS Previews (ISI Web of Knowledge)
-
1993–2008
-
Date searched: 3 November 2010
-
Records found: 193.
-
# 9 193 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
Refined by: Concept Codes=(06504, RADIATION BIOLOGY – RADIATION AND ISOTOPE TECHNIQUES OR 24004, NEOPLASMS – PATHOLOGY, CLINICAL ASPECTS AND SYSTEMIC EFFECTS OR 18002, BONES, JOINTS, FASCIAE, CONNECTIVE AND ADIPOSE TISSUE – ANATOMY OR 18004, BONES, JOINTS, FASCIAE, CONNECTIVE AND ADIPOSE TISSUE – PHYSIOLOGY AND BIOCHEMISTRY OR 18006, BONES, JOINTS, FASCIAE, CONNECTIVE AND ADIPOSE TISSUE – PATHOLOGY OR 12504, PATHOLOGY – DIAGNOSTIC OR 25000, PEDIATRICS OR 11310, CHORDATE BODY REGIONS – BACK AND BUTTOCKS OR 06502, RADIATION BIOLOGY – GENERAL OR 11102, ANATOMY AND HISTOLOGY – GROSS ANATOMY OR 00530, GENERAL BIOLOGY – INFORMATION, DOCUMENTATION, RETRIEVAL AND COMPUTER APPLICATIONS OR 01012, METHODS – PHOTOGRAPHY OR 11106, ANATOMY AND HISTOLOGY – RADIOLOGIC ANATOMY OR 18001, BONES, JOINTS, FASCIAE, CONNECTIVE AND ADIPOSE TISSUE – GENERAL AND METHODS)
-
Databases=PREVIEWS Timespan=1993–2008
-
# 8 425 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
Databases=PREVIEWS Timespan=1993–2008
-
# 7 322 Topic=(eos not (composite or zinc or sodium or “equation* state*” or ceramide* or ikaros or genome or “early onset sarcoidois” or (“early onset” same (sepsis or septicemia or septicaemia)) or “endogenous opioid system*” or (ethanolamine same sulphate) or “essential oil*” or protein* or eosinophil* or schizophreni*))
-
Databases=PREVIEWS Timespan=1993–2008
-
# 6 35 Topic=(stereoradiograph*)
-
Databases=PREVIEWS Timespan=1993–2008
-
# 5 61 Topic=((biplanar or “bi-planar”) same (radiograph* or x-ray* or xray*))
-
Databases=PREVIEWS Timespan=1993–2008
-
# 4 7 Topic=(“slot scan*”)
-
Databases=PREVIEWS Timespan=1993–2008
-
# 3 0 Topic=(“multiwire chamber*” or “multi wire chamber*”)
-
Databases=PREVIEWS Timespan=1993–2008
-
# 2 1 Topic=(charpak)
-
Databases=PREVIEWS Timespan=1993–2008
-
# 1 7 Topic=((“low dose” or “ultralow dose”) same (“digital x-ray*” or “digital xray*” or “digital radiograph* system*” or “x-ray imag*” or “xray imag*” or “2d 3d x-ray*” or “2d 3d xray*”))
Key:
-
Topic = searches terms in Title, Abstract, Author Keywords and Keywords Plus fields
-
* = truncation
-
“ ” = phrase search
-
same = terms within same sentence
BIOSIS Previews (Dialog)
-
2008–10
-
Date searched: 3 November 2010
-
Records found: 47.
-
S (low(w)dose or ultralow(w)dose)(n)(digital(w)x(w)ray? or digital(w)xray? or digital(w)radiograph?(w)system? or x(w)ray(w)imag? or xray(w)imag? or 2d(w)3d(w)x(w)ray? or 2d(w)3d(w)xray)
-
S charpak
-
S multiwire(w)chamber? or multi(w)wire(w)chamber?
-
S slot(w)scan?
-
S (biplanar or bi(w)planar)(2n)(radiograph? or x(w)ray? or xray?)
-
S stereoradiograph?
-
S eos
-
Ss s1:s7
-
S composite or zinc or sodium or (equation?(w)state) or ceramide? or ikaros or genome or (early(w)onset(w)sarcoidois) or ((early(w)onset)(2n)(sepsis or septicemia or septicaemia)) or (endogenous(w)opioid(w)system?) or (ethanolamine(2n)sulphate) or essential(w)oil? or protein? or eosinophil? or schizophreni?
-
S s8 not s9
-
S s10/2008:2010
-
S s11/HUMAN
-
S CC=(06504 OR 24004 OR 18002 OR 18004 OR 18006 OR 12504 OR 25000 OR 11310 OR 06502 OR 11102 OR 00530 OR 01012 OR 11106 OR 18001)
-
S s12 and s13
Key:
-
? = truncation
-
(w) = terms adjacent to each other (same order)
-
(n) = terms adjacent to each other (any order)
-
(2n) = terms within two words of each other (any order)
-
CC = Concept code (for subject area limitation)
-
S s10/2008:2010 – limits set 10 to records published between 2008 and 2010 (inclusive)
Cumulative Index to Nursing and Allied Health Literature (EBSCO)
-
1993 – date
-
Date searched: 2 November 2010
-
Records found: 25
-
S22 S21 Limiters – Published Date from: 19930101–20101231
-
S21 S7 not s20 (26)
-
S20 S8 or S9 or or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 (67,105)
-
S19 composite or zinc or sodium (14,583)
-
S18 equation* n2 state* (11)
-
S17 ceramide* or ikaros or genome* (3192)
-
S16 “early onset sarcoidois” (0)
-
S15 “early onset” n2 (sepsis or septicemia or septicaemia) (0)
-
S14 “endogenous opioid system*” (26)
-
S13 ethanolamine n2 sulphate (0)
-
S12 protein* (41,211)
-
S11 “essential oil*” (773)
-
S10 “end of synthesis” (0)
-
S9 schizophreni* (8346)
-
S8 eosinophil* (1630)
-
S7 eos (51)
-
S6 stereoradiograph* (7)
-
S5 (biplanar or “bi planar”) n2 (radiograph* or x-ray* or xray*) (0)
-
S4 “slot scan*” (3)
-
S3 “multiwire chamber*” or “multi wire chamber*” (0)
-
S2 charpak (1)
-
S1 (“low dose” or “ultralow dose”) n1 (“digital x-ray*” or “digital xray*” or “digital radiograph* system*” or “x-ray imag*” or “xray imag*” or “2d 3d x-ray*” or “2d 3d xray*”) (0)
Key:
-
* = truncation
-
“ ” = phrase search
-
n1 = terms within one word of each other (any order)
-
n2 = terms within two words of each other (any order)
The Cochrane Library
-
1993–2010 Issue 10
-
Date searched: 3 November 2010
-
Records found: CDSR (0) DARE (0) CENTRAL (14) HTA (2) NHS EED (1).
-
#1 (“low dose” next (“digital x ray*” or “digital xray*” or “digital radiograph* system*” or “x ray imag*” or “xray imag*” or “2d 3d x ray*” or “2d 3d xray*”)):ti,ab (0)
-
#2 (“ultralow dose” next (“digital x ray*” or “digital xray*” or “digital radiograph* system*” or “x ray imag*” or “xray imag*” or “2d 3d x ray*” or “2d 3d xray*”)):ti,ab (0)
-
#3 charpak:ti,ab (2)
-
#4 (“multiwire chamber*” or “multi wire chamber”):ti,ab (0)
-
#5 “slot scan*”:ti,ab (0)
-
#6 (biplanar near/2 (radiograph* or “x ray” or xray*)):ti,ab (2)
-
#7 (“bi planar” near/2 (radiograph* or “x ray*” or xray*)):ti,ab (0)
-
#8 stereoradiograph*:ti,ab (4)
-
#9 eos:ti,ab (40)
-
#10 (eosinophil* or schizophreni* or “end of synthesis” or “essential oil*” or protein*):ti,ab (29,905)
-
#11 (ethanolamine near/2 sulphate):ti,ab (5)
-
#12 (“endogenous opioid system*”):ti,ab (76)
-
#13 (“early onset” near/2 (sepsis or septicemia or septicaemia)):ti,ab (17)
-
#14 “early onset sarcoidois”:ti,ab (0)
-
#15 (ceramide* or ikaros or genome*):ti,ab (228)
-
#16 (equation* near/2 state*):ti,ab (0)
-
#17 (composite or zinc or sodium):ti,ab (19,086)
-
#18 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) (48,219)
-
#19 (#9 AND NOT #18) (13)
-
#20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #19) (21)
-
#21 (#20), from 1993 to 2010 (17)
Key:
-
* = truncation
-
“ ” = phrase search
-
:ti,ab = terms in either title or abstract fields
-
near/2 = terms within two words of each other (any order)
-
next = terms are next to each other
EMBASE (OvidSP)
-
1993 – week 43 2010
-
Date searched: 2 November 2010
-
Records found: 463.
-
((low dose or ultralow dose) adj (digital x-ray$ or digital xray$ or digital radiograph$ system$ or x-ray imag$ or xray imag$ or 2d 3d x-ray$ or 2d 3d xray$)).ti,ab. (14)
-
charpak.ti,ab. (3)
-
(multiwire chamber$ or multi wire chamber$).ti,ab. (4)
-
slot scan$.ti,ab. (41)
-
((biplanar or bi planar) adj2 (radiograph$or x-ray$or xray$)).ti,ab. (169)
-
stereoradiograph$.ti,ab. (114)
-
eos.ti,ab. (1180)
-
(eosinophil$ or schizophreni$ or end of synthesis or essential oil$ or protein$or (ethanolamine adj2 sulphate) or endogenous opioid system$ or (early onset adj2 (sepsis or septic?emia)) or early onset sarcoidois or ceramide$ or ikaros or genome$ or (equation$adj2 state$) or composite or zinc or sodium).ti,ab. (2,434,795)
-
7 not 8 (378)
-
1 or 2 or 3 or 4 or 5 or 6 or 9 (701)
-
animal/ or nonhuman/ (5,139,514)
-
exp human/ (12,060,870)
-
11 not (11 and 12) (4,127,271)
-
10 not 13 (594)
-
limit 14 to yr=“1993 -Current” (463)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Health Management Information Consortium (OvidSP)
-
1993 – September 2010
-
Date searched: 2 November 2010
-
Records found: 0.
-
((low dose or ultralow dose) adj (digital x-ray$ or digital xray$ or digital radiograph$ system$ or x-ray imag$ or xray imag$ or 2d 3d x-ray$ or 2d 3d xray$)).ti,ab.
-
charpak.ti,ab.
-
(multiwire chamber$ or multi wire chamber$).ti,ab.
-
slot scan$.ti,ab.
-
((biplanar or bi planar) adj2 (radiograph$ or x-ray$ or xray$)).ti,ab.
-
stereoradiograph$.ti,ab.
-
eos.ti,ab.
-
(eosinophil$ or schizophreni$or end of synthesis or essential oil$ or protein$ or (ethanolamine adj2 sulphate) or endogenous opioid system$ or (early onset adj2 (sepsis or septic?emia)) or early onset sarcoidois or ceramide$ or ikaros or genome$ or (equation$adj2 state$) or composite or zinc or sodium).ti,ab.
-
7 not 8
-
1 or 2 or 3 or 4 or 5 or 6 or 9
-
limit 10 to yr=“1993 -Current”
Key:
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Inspec (OvidSP)
-
1993 – week 42 2010
-
Date searched: 2 November 2010
-
Records found: 138.
-
((low dose or ultralow dose) adj (digital x-ray$ or digital xray$ or digital radiograph$ system$ or x-ray imag$ or xray imag$ or 2d 3d x-ray$ or 2d 3d xray$)).ti,ab.
-
charpak.ti,ab.
-
(multiwire chamber$ or multi wire chamber$).ti,ab.
-
slot scan$.ti,ab.
-
((biplanar or bi planar) adj2 (radiograph$ or x-ray$ or xray$)).ti,ab.
-
stereoradiograph$.ti,ab.
-
eos.ti,ab.
-
(eosinophil$ or schizophreni$ or end of synthesis or essential oil$ or protein$ or (ethanolamine adj2 sulphate) or endogenous opioid system$ or (early onset adj2 (sepsis or septic?emia)) or early onset sarcoidois or ceramide$ or ikaros or genome$ or (equation$ adj2 state$) or composite or zinc or sodium).ti,ab.
-
7 not 8
-
1 or 2 or 3 or 4 or 5 or 6 or 9
-
“X-rays and particle beams (medical uses) “.cc.
-
“Patient diagnostic methods and instrumentation “.cc.
-
“X-ray techniques: radiography and computed tomography (biomedical imaging/measurement) “.cc.
-
biomedical imaging/ or diagnostic radiography/ or medical image processing/ or x-ray imaging/
-
radiography/
-
or/11–15
-
10 and 16
-
limit 17 to yr=“1993 -Current”
Key:
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
-
.cc. = classification code
PASCAL (Dialog)
-
1993–2010
-
Date searched: 3 November 2010
-
Records found: 27.
-
S ((low(w)dose or ultralow(w)dose)(n)(digital(w)x(w)ray? or digital(w)xray? or digital(w)radiograph?(w)system? or x(w)ray(w)imag? or xray(w)imag? or 2d(w)3d(w)x(w)ray? or 2d(w)3d(w)xray))/ET
-
S charpak
-
S (multiwire(w)chamber? or multi(w)wire(w)chamber?)/ET
-
S (slot(w)scan?)/ET
-
S ((biplanar or bi(w)planar)(2n)(radiograph? or x(w)ray? or xray?))/ET
-
S (stereoradiograph?)/ET
-
S eos/ET
-
Ss s1:s7
-
S composite or zinc or sodium or (equation?(w)state) or ceramide? or ikaros or genome or (early(w)onset(w)sarcoidois) or ((early(w)onset)(2n)(sepsis or septicemia or septicaemia)) or (endogenous(w)opioid(w)system?) or (ethanolamine(2n)sulphate) or essential(w)oil? or protein? or eosinophil? or schizophreni? or earth(w)observing
-
S s8 not s9
-
S s10/1993:2010
-
S (Radiography or Radiology or Image reconstruction or Image processing or Image quality or Scanning or X ray or EOS system or Tridimensional image or X ray Radiography or Digital radiography or Medical imagery or Spinal cord disease or Vertebral canal or Cervical spine)/DE
-
S s11 and s12
Key:
-
? = truncation
-
(w) = terms adjacent to each other (same order)
-
(n) = terms adjacent to each other (any order)
-
(2n) = terms within 2 words of each other (any order)
-
/ET = English title
-
/DE = Descriptor field
-
S s10/1993:2010 – limits set 10 to records published between 1993 and 2010 (inclusive)
Science Citation Index (ISI Web of Knowledge)
-
1993 – date
-
Date searched: 2 November 2010
-
Records found: 482.
-
#9 482 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
Refined by: Subject Areas=(MEDICAL INFORMATICS OR SURGERY OR IMAGING SCIENCE & PHOTOGRAPHIC TECHNOLOGY OR RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING OR PEDIATRICS OR ORTHOPEDICS OR MEDICINE, GENERAL & INTERNAL OR ENGINEERING, BIOMEDICAL)
-
# 8 4,854 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
# 7 4,533 Topic=(eos not (composite or zinc or sodium or “equation* state*” or ceramide* or ikaros or genome or “early onset sarcoidois” or (“early onset” same (sepsis or septicemia or septicaemia)) or “endogenous opioid system*” or (ethanolamine same sulphate) or “essential oil*” or protein* or eosinophil* or schizophreni*))
-
# 6 51 Topic=(stereoradiograph*)
-
# 5 132 Topic=((biplanar or “bi-planar”) same (radiograph* or x-ray* or xray*))
-
# 4 63 Topic=(“slot scan*”)
-
# 3 52 Topic=(“multiwire chamber*” or “multi wire chamber*”)
-
# 2 21 Topic=(charpak)
-
# 1 25 Topic=((“low dose” or “ultralow dose”) same (“digital x-ray*” or “digital xray*” or “digital radiograph* system*” or “x-ray imag*” or “xray imag*” or “2d 3d x-ray*” or “2d 3d xray*”))
Key:
-
Topic = searches terms in Title, Abstract, Author Keywords and Keywords Plus fields
-
Subject Areas = subject category
-
* = truncation
-
“ ” = phrase search
-
same = terms within same sentence
ClinicalTrials.gov
-
Date searched: 8 November 2010
-
Records found: 24.
-
eos NOT (schizophrenia OR protein OR sepsis OR eosinophil OR sarcoidosis OR genome OR copd OR septicemia)
-
“ultra low dose digital x-ray” OR “ultralow dose digital x-ray” OR “ultra low dose digital xray” OR “ultralow dose digital xray”
-
“digital radiography system” OR “3d x-ray” OR “3d xray”
-
charpak OR “multiwire chamber” OR “multi wire chamber” OR “slot scanner” OR “slot scanning” OR stereoradiography
-
“biplanar radiography” OR “bi planar radiography” OR “biplanar xray” OR “bi planar xray” OR “biplanar x-ray” OR “bi planar x-ray”
Key:
-
“ ” = phrase search
Current Controlled Trials
-
Date searched: 8 November 2010
-
Records found: 28.
-
eos NOT (schizophrenia OR protein OR sepsis OR eosinophil OR sarcoidosis OR genome OR copd OR septicemia)
-
ultra low dose digital x-ray OR ultralow dose digital x-ray OR ultra low dose digital xray OR ultralow dose digital xray
-
digital radiography system OR x-ray imaging OR xray imaging OR 3d x-ray OR 3d xray
-
charpak OR multiwire chamber OR multi wire chamber OR slot scanner OR slot scanning OR stereoradiography
-
biplanar radiography OR bi planar radiography OR biplanar xray OR bi planar xray OR biplanar x-ray OR bi planar x-ray
Searches for costs of digital and computerised radiography
-
Date searches conducted: 15 November 2010
-
Limits: 2000 – most recent date available
-
Records found (after deduplication): 545
-
Records found (before deduplication): 394.
Searches use an economic search filter based on that which is used for identification of economic evaluations and other cost studies for inclusion in the NHS EED.
Databases searched
-
MEDLINE
-
EconLit
-
EMBASE
-
NHS EED.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
-
2000 – November week 1 2010
-
Date searched: 15 November 2010.
Records found: 215
-
(digital adj (radiograph$ or xray$ or x-ray$)).ti,ab. (2143)
-
(computed adj (radiograph$ or xray$ or x-ray$)).ti,ab. (1012)
-
(computer adj2 (radiograph$ or xray$ or x-ray$)).ti,ab. (381)
-
or/1–3 (3356)
Lines 4 combines X-ray terms
-
economics/ (26,019)
-
exp “costs and cost analysis”/ or Cost Allocation/ or Cost-Benefit Analysis/ or Cost Control/ or Cost of Illness/ or Cost Sharing/ or Health Care Costs/ or Health Expenditures/ (154,720)
-
exp “economics, hospital”/ or Hospital Charges/ or Hospital Costs/ (16,995)
-
economics, medical/ (8336)
-
economics, nursing/ (3827)
-
(economic$ or cost$ or price or prices or pricing).tw. (368,815)
-
(expenditure$ not energy).tw. (14,503)
-
(value adj1 money).tw. (20)
-
budget$.tw. (15,317)
-
(utili?ation or throughput or through put).ti,ab. (131,597)
-
or/5–14 (593,004)
Line 15 combines economic evaluation terms
-
((energy or oxygen) adj cost).ti,ab. (2375)
-
(metabolic adj cost).ti,ab. (618)
-
((energy or oxygen) adj expenditure).ti,ab. (13,328)
-
or/16–18 (15,702)
-
15 not 19 (588,726)
Line 20 excludes irrelevant records referring to energy expenditure
-
4 and 20 (241)
Line 21 combines X-ray terms and economic evaluation terms
-
Radiographic Image Interpretation, Computer-Assisted/ec (1)
-
Radiographic Image Interpretation/ec (1)
-
*Radiology Department, Hospital/ec (373)
-
*Technology, Radiologic/ec (70)
-
radiographic image enhancement/ec (94)
-
or/22–26 (522)
Line 27 combines relevant MeSH subject heading limited by the ‘economics’ subheading
-
21 or 27 (732)
Line 28 combines lines 21 and 27
-
exp animals/ not humans/ (3,598,672)
-
28 not 29 (724)
Line 30 excludes animal-only studies
-
limit 30 to yr=“2000 – 2010” (253)
Line 31 limits to references published between 2000 and 2010
-
(mammography or mammogram$ or dental or dentist$ or lung or lungs or tuberculosis).ti,ab. (670,019)
-
31 not 32 (215)
Line 33 excludes mammography, dental, lung and tuberculosis X-rays
Key:
-
/ = indexing term (MeSH heading)
-
/ec = indexing term (MeSH heading) limited to ‘economic’ subheading
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
EconLit (Ovid)
-
2000 – October 2010
-
Date searched: 15 November 2010
-
Records found: 1.
-
(digital adj (radiograph$ or xray$ or x-ray$)).ti,ab. (1)
-
(computed adj (radiograph$ or xray$ or x-ray$)).ti,ab. (0)
-
(computer adj2 (radiograph$ or xray$ or x-ray$)).ti,ab. (0)
-
or/1–3 (1)
-
limit 4 to yr=“2000 -Current” (1)
Key:
-
$ = truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
EMBASE (Ovid)
-
2000–10 week 44
-
Date searched: 15 November 2010
-
Records found: 279.
-
(digital adj (radiograph$ or xray$ or x-ray$)).ti,ab,ot. (2351)
-
(computed adj (radiograph$ or xray$ or x-ray$)).ti,ab,ot. (1087)
-
(computer adj2 (radiograph$ or xray$ or x-ray$)).ti,ab,ot. (421)
-
*digital radiography/ (1742)
-
*computer assisted radiography/ (254)
-
*radiology department/ (2603)
-
or/1–6 (7374)
-
Health Economics/ (29,673)
-
exp Economic Evaluation/ or Cost Benefit Analysis/ or Cost Control/ or Cost Effectiveness Analysis/ or Cost Minimization Analysis/ or Cost of Illness/ or Cost Utility Analysis/ (160,878)
-
exp Health Care Cost/ or Health Care Financing/ or Nursing Cost/ or Hospital Cost/ (154,337)
-
(econom$ or cost$ or price or prices or pricing).ti,ab,ot. (434,362)
-
(expenditure$ not energy).ti,ab,ot. (16,470)
-
(value adj2 money).ti,ab,ot. (859)
-
budget$.ti,ab,ot. (17,570)
-
(utili?ation or throughput or through put).ti,ab,ot. (148,570)
-
or/8–15 (726,240)
-
(metabolic adj cost).ti,ab,ot. (623)
-
((energy or oxygen) adj cost).ti,ab,ot. (2460)
-
((energy or oxygen) adj expenditure).ti,ab,ot. (14,500)
-
or/17–19 (16,937)
-
16 not 20 (721,679)
-
7 and 21 (959)
-
exp ANIMAL/ (1,635,235)
-
exp animal experiment/ (1,400,150)
-
Nonhuman/ (3,525,296)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh,ot. (3,961,093)
-
or/23–26 (5,691,873)
-
exp human/ (12,073,160)
-
exp human experiment/ (283,292)
-
28 or 29 (12,074,541)
-
27 not (27 and 30) (4,506,199)
-
22 not 31 (934)
-
limit 32 to yr=“2000 -Current” (327)
-
(mammography or mammogram$ or dental or dentist$ or lung or lungs or tuberculosis).ti,ab. (712,037)
-
33 not 34 (279)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab,ot = terms in either title, original title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
NHS Economic Evaluation Database (The Cochrane Library)
-
2000–10 Issue 11
-
Date searched: 15 November 2010
-
Records found: 50.
-
#1 (“digital radiograph*” or “digital xray*” or “digital x-ray*”):ti,ab (9)
-
#2 (“computed radiograph*” or “computed xray*” or “computed x-ray*”):ti,ab (7)
-
#3 ((computer near/2 radiograph*) or (computer near/2 xray*) or (computer near/2 x-ray*)):ti,ab (9)
-
#4 MeSH descriptor Radiographic Image Interpretation, Computer-Assisted, this term only (157)
-
#5 MeSH descriptor Radiographic Image Enhancement, this term only (328)
-
#6 MeSH descriptor Radiology Department, Hospital, this term only (86)
-
#7 MeSH descriptor Technology, Radiologic, this term only (50)
-
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) (588)
-
#9 (mammography or mammogram* or dental or dentist* or lung or lungs or tuberculosis):ti,ab (23,847)
-
#10 (#8 AND NOT #9) (486)
-
#11 (#10), from 2000 to 2010 (265)
Key:
-
* = truncation
-
“ ” = phrase search
-
:ti,ab = terms in either title or abstract fields
-
near/2 = terms within two words of each other (any order)
Searches for quality-of-life data
-
Date searches conducted: 22 November 2010
-
Limits: 2000 – most recent date available
-
Records found (after deduplication): 1226
-
Records found (before deduplication): 644.
Searches use a QoL search filter that was adapted for the purpose of this study to be of high precision and lower sensitivity.
Databases searched
-
MEDLINE
-
EconLit
-
EMBASE
-
NHS EED
-
CENTRAL.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid) 2000 – November week 2 2010
-
Date searched: 22 November 2010
-
Records found: 541.
-
*spinal curvatures/ or *kyphosis/ or *scheuermann disease/ or *lordosis/ or *scoliosis/ or *spinal osteochondrosis/ or *spondylolysis/ or *spondylolisthesis/ (14,857)
-
*Spondylitis, Ankylosing/ (7860)
-
*Leg Length Inequality/ (1512)
-
*Enchondromatosis/ (330)
-
*neurofibromatoses/ or *neurofibromatosis 1/ or *neurofibromatosis 2/ (7176)
-
*Hypophosphatemic Rickets, X-Linked Dominant/ (122)
-
scoliosis.ti,ab. (11,652)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (6509)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (3737)
-
deforming dorsopath$.ti,ab. (1)
-
(valgus deformit$ or flexion deformit$).ti,ab. (1717)
-
((limb$length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (1512)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (163)
-
scheuermann$ disease.ti,ab. (314)
-
(ollier$ disease or enchondromatosis).ti,ab. (339)
-
neurofibromatosis.ti,ab. (8940)
-
hypophosphat?emic rickets.ti,ab. (688)
-
proximal focal femoral deficiency.ti,ab. (24)
-
fibular hemimelia.ti,ab. (75)
-
hemi hypertrophy.ti,ab. (26)
-
skeletal dysplasia$.ti,ab. (1492)
-
short stature.ti,ab. (6495)
-
tumo?r reconstruction.ti,ab. (42)
-
blount$ disease.ti,ab. (209)
-
*Hip Dislocation, Congenital/ (5316)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (27,385)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (459,952)
-
26 and 27 (3947)
-
arthrogryposis multiplex congenita.ti,ab. (465)
-
*Spina Bifida Occulta/ (1273)
-
spina bifida occulta.ti,ab. (398)
-
*Klippel-Feil Syndrome/ (561)
-
klippel feil syndrome.ti,ab. (507)
-
congenital spondylolisthesis.ti,ab. (12)
-
exp *Osteochondrodysplasias/ (18,120)
-
short rib syndrome.ti,ab. (16)
-
chondrodysplasia punctata.ti,ab. (531)
-
achondroplasia.ti,ab. (1132)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (1165)
-
osteogenesis imperfecta.ti,ab. (3114)
-
osteopetrosis.ti,ab. (1787)
-
enchondromatosis.ti,ab. (164)
-
multiple congenital exostoses.ti,ab. (2)
-
osteoporotic fracture$.ti,ab. (3466)
-
or/1–25 (59,426)
-
or/28–44 (30,011)
-
45 or 46 (84,956)
Line 47 combines terms for the relevant orthopaedic conditions
-
(utilit$ approach$ or health gain or hui or hui2 or hui3).ti,ab. (1095)
-
(health measurement$ scale$ or health measurement$ questionnaire$).ti,ab. (30)
-
health related quality of life.ti,ab. (14,187)
-
(utility weight$ or utility value$ or preference weight$ or quality weight$).ti,ab. (710)
-
(standard gamble$ or categor$ scal$ or linear scal$ or linear analog$ or visual scal$or magnitude estimat$).ti,ab. (3738)
-
(time trade off$ or rosser$ classif$ or rosser$ matrix or rosser$ distress$ or hrqol).ti,ab. (4961)
-
(index of wellbeing or index of well being or quality of wellbeing or quality of well being or qwb).ti,ab. (362)
-
(multiattribute$ health ind$ or multi attribute$ health ind$).ti,ab. (2)
-
(health utilit$ index or health utilit$ indices).ti,ab. (472)
-
(health utilit$ scale$ or classification of illness state$).ti,ab. (8)
-
health state$ utilit$.ti,ab. (170)
-
(multiattribute$ utilit$ or multi attribute$ utilit$).ti,ab. (150)
-
health utilit$scale$.ti,ab. (7)
-
(euro qual or euro qol or eq-5d or eq5d or eq 5d or euroqual or euroqol).ti,ab. (2200)
-
(qualy or qaly or qualys or qalys or quality adjusted life year$).ti,ab. (4528)
-
(sf36 or sf 36).ti,ab. (9563)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab. (4447)
-
(sf 6d or sf6d or short form 6d or shortform 6d or sf six$ or shortform six$ or short form six$).ti,ab. (231)
-
*”Quality of Life”/ (38,142)
-
*”Quality-Adjusted Life Years”/ (1079)
-
or/48–67 (58,367)
Line 69 combines QoL terms
-
exp animals/ not humans/ (3,599,786)
-
68 not 69 (58,124)
Line 70 excludes animal-only studies
-
limit 70 to yr=“2000 -Current” (44,103)
-
47 and 71 (541)
Line 72 limits to references published between 2000 and 2010
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
The Cochrane Library 2000 – Issue 11 2010
-
Date searched: 23 November 2010.
Cochrane Central Register of Controlled Trials
-
Records found: 88.
NHS Economic Evaluation Database (The Cochrane Library)
-
Records found: 14.
-
#1 MeSH descriptor Spinal Curvatures, this term only (6)
-
#2 MeSH descriptor Kyphosis, this term only (67)
-
#3 MeSH descriptor Scheuermann Disease, this term only (1)
-
#4 MeSH descriptor Lordosis, this term only (25)
-
#5 MeSH descriptor Scoliosis, this term only (179)
-
#6 MeSH descriptor Spinal Osteochondrosis, this term only (0)
-
#7 MeSH descriptor Spondylolysis, this term only (8)
-
#8 MeSH descriptor Spondylolisthesis, this term only (62)
-
#9 MeSH descriptor Spondylitis, Ankylosing, this term only (362)
-
#10 MeSH descriptor Leg Length Inequality, this term only (36)
-
#11 MeSH descriptor Enchondromatosis, this term only (0)
-
#12 MeSH descriptor Neurofibromatoses, this term only (0)
-
#13 MeSH descriptor Neurofibromatosis 1, this term only (10)
-
#14 MeSH descriptor Neurofibromatosis 2, this term only (2)
-
#15 MeSH descriptor Hypophosphatemic Rickets, X-Linked Dominant, this term only (1)
-
#16 scoliosis:ti,ab (255)
-
#17 (kyphosis or lordosis or “flatback syndrome”):ti,ab (152)
-
#18 (spondylolysis or spondylolisthesis or “atlantoaxial subluxation” or “vertebral subluxation”):ti,ab (119)
-
#19 “deforming dorsopath*”:ti,ab (0)
-
#20 (“valgus deformit*” or “flexion deformit*”):ti,ab (0)
-
#21 “unequal limb* length*”:ti,ab (0)
-
#22 “unequal leg* length*”:ti,ab (0)
-
#23 “leg length* inequalit*”:ti,ab (0)
-
#24 “limb* length* inequalit*”:ti,ab (0)
-
#25 “leg length* discrepancy*”:ti,ab (8)
-
#26 “limb* length* discrepancy*”:ti,ab (9)
-
#27 “leg length* misalignment*”:ti,ab (0)
-
#28 “limb* length* misalignment*”:ti,ab (0)
-
#29 (spine near/2 osteochondrosis):ti,ab (0)
-
#30 (spinal near/2 osteochondrosis):ti,ab (2)
-
#31 (“lower limb*” near/3 “congenital* deform*”):ti,ab (0)
-
#32 (leg near/3 “congenital* deform*”):ti,ab (0)
-
#33 (legs near/3 “congenital* deform*”):ti,ab (0)
-
#34 “scheuermann* disease”:ti,ab (0)
-
#35 (“ollier* disease” or enchondromatosis):ti,ab (0)
-
#36 neurofibromatosis:ti,ab (15)
-
#37 (“hypophosphatemic rickets” or “hypophosphataemic rickets”):ti,ab (9)
-
#38 “proximal focal femoral deficiency”:ti,ab (0)
-
#39 “fibular hemimelia”:ti,ab (0)
-
#40 “hemi hypertrophy”:ti,ab (0)
-
#41 “skeletal dysplasia*”:ti,ab (2)
-
#42 “short stature”:ti,ab (228)
-
#43 (“tumor reconstruction” or “tumour reconstruction “):ti,ab (0)
-
#44 “blount* disease”:ti,ab (0)
-
#45 MeSH descriptor Hip Dislocation, Congenital, this term only (60)
-
#46 (congenital and (subluxation or deformity or deformities or dislocation or malformation* or bowing or defect or defects)):ti,ab (305)
-
#47 (hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or “lower limb”* or “pelvic girdle” or “bony thorax” or “cervical rib*”):ti,ab (27,305)
-
#48 (#46 AND #47) (15)
-
#49 “arthrogryposis multiplex congenita”:ti,ab (0)
-
#50 MeSH descriptor Spina Bifida Occulta, this term only (3)
-
#51 “spina bifida occulta”:ti,ab (1)
-
#52 MeSH descriptor Klippel-Feil Syndrome, this term only (1)
-
#53 “klippel feil syndrome”:ti,ab (1)
-
#54 “congenital spondylolisthesis”:ti,ab (0)
-
#55 MeSH descriptor Osteochondrodysplasias explode all trees (47)
-
#56 “short rib syndrome”:ti,ab (0)
-
#57 “chondrodysplasia punctata”:ti,ab (0)
-
#58 achondroplasia:ti,ab (6)
-
#59 ((dystrophic or chondroectodermal or spondyloepiphyseal or “polyostotic fibrous” or “progressive diaphyseal” or metaphyseal) and dysplasia*):ti,ab (0)
-
#60 “osteogenesis imperfecta”:ti,ab (35)
-
#61 osteopetrosis:ti,ab (1)
-
#62 enchondromatosis:ti,ab (0)
-
#63 “multiple congenital exostoses”:ti,ab (0)
-
#64 “osteoporotic fracture*”:ti,ab (63)
-
#65 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) (986)
-
#66 (#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39) (42)
-
#67 (#40 OR #41 OR #42 OR #43 OR #44 OR #45) (289)
-
#68 (#48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64) (134)
-
#69 (#65 OR #66 OR #67 OR #68) (1422)
-
#70 MeSH descriptor Quality of Life, this term only (11,382)
-
#71 MeSH descriptor Quality-Adjusted Life Years, this term only (2759)
-
#72 (“utilit* approach*” or “health gain” or hui or hui2 or hui3):ti,ab (109)
-
#73 (“health measurement* scale*” or “health measurement* questionnaire”):ti,ab (4)
-
#74 “health related quality of life”:ti,ab (2640)
-
#75 (“utility weight*” or “utility value*” or “preference weight*” or “quality weight*”):ti,ab (13)
-
#76 (“standard gamble*” or “categor* scal*” or “linear scal*” or “linear analog*” or “visual scal*” or “magnitude estimat*”):ti,ab (153)
-
#77 (“time trade off*” or “rosser* classif*” or “rosser* matrix” or “rosser* distress*” or hrqol):ti,ab (695)
-
#78 (“index of wellbeing” or “index of well being” or “quality of wellbeing” or “quality of well being” or qwb):ti,ab (66)
-
#79 (“multiattribute* health ind*” or “multi attribute* health ind*”):ti,ab (0)
-
#80 (“health utilit* index” or “health utilit* indices”):ti,ab (0)
-
#81 (“health utilit* scale*” or “classification of illness state*”):ti,ab (0)
-
#82 “health state* utilit*”:ti,ab (0)
-
#83 (“multiattribute* utilit*” or “multi attribute* utilit*”):ti,ab (0)
-
#84 “health utilit* scale*”:ti,ab (0)
-
#85 (“euro qual” or “euro qol” or “eq-5d” or eq5d or “eq 5d” or euroqual or euroqol):ti,ab (586)
-
#86 (qualy or qaly or qualys or qalys or “quality adjusted life year*”):ti,ab (559)
-
#87 (sf36 or “sf 36”):ti,ab (1864)
-
#88 (“short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirtysix” or “short form thirty six”):ti,ab (793)
-
#89 (“sf 6d” or sf6d or “short form 6d” or “shortform 6d” or “sf six*” or “shortform six*” or “short form six*”):ti,ab (71)
-
#90 (#70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89) (16,224)
-
#91 (#69 AND #90) (113)
-
#92 (#91), from 2000 to 2010 (107)
Key:
-
* = truncation
-
“ ” = phrase search
-
:ti,ab = terms in either title or abstract fields
-
near/2 = terms within two words of each other (any order)
EconLit (Ovid) 2000 to October 2010
-
Date searched: 22 November 2010
-
Records found: 1.
-
scoliosis.ti,ab. (0)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (0)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (0)
-
deforming dorsopath$.ti,ab. (0)
-
(valgus deformit$ or flexion deformit$).ti,ab. (0)
-
((limb$ length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (0)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (0)
-
((lower limb$ or leg or legs) adj3 congenital$ deform$).ti,ab. (0)
-
scheuermann$ disease.ti,ab. (0)
-
(ollier$ disease or enchondromatosis).ti,ab. (0)
-
neurofibromatosis.ti,ab. (0)
-
hypophosphat?emic rickets.ti,ab. (0)
-
proximal focal femoral deficiency.ti,ab. (0)
-
fibular hemimelia.ti,ab. (0)
-
hemi hypertrophy.ti,ab. (0)
-
skeletal dysplasia$.ti,ab. (0)
-
short stature.ti,ab. (6)
-
tumo?r reconstruction.ti,ab. (0)
-
blount$ disease.ti,ab. (0)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (4)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (288)
-
20 and 21 (0)
-
arthrogryposis multiplex congenita.ti,ab. (0)
-
spina bifida occulta.ti,ab. (0)
-
klippel feil syndrome.ti,ab. (0)
-
congenital spondylolisthesis.ti,ab. (0)
-
short rib syndrome.ti,ab. (0)
-
chondrodysplasia punctata.ti,ab. (0)
-
chondrodysplasia punctata.ti,ab. (0)
-
achondroplasia.ti,ab. (0)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (0)
-
osteogenesis imperfecta.ti,ab. (0)
-
osteopetrosis.ti,ab. (0)
-
enchondromatosis.ti,ab. (0)
-
multiple congenital exostoses.ti,ab. (0)
-
osteoporotic fracture$.ti,ab. (6)
-
or/1–19 (6)
-
or/22–36 (6)
-
37 or 38 (12)
-
(utilit$ approach$ or health gain or hui or hui2 or hui3).ti,ab. (192)
-
(health measurement$ scale$ or health measurement$ questionnaire$).ti,ab. (0)
-
health related quality of life.ti,ab. (92)
-
(utility weight$ or utility value$ or preference weight$ or quality weight$).ti,ab. (111)
-
(standard gamble$ or categor$ scal$ or linear scal$ or linear analog$ or visual scal$or magnitude estimat$).ti,ab. (91)
-
(time trade off$ or rosser$ classif$ or rosser$ matrix or rosser$ distress$ or hrqol).ti,ab. (72)
-
(index of wellbeing or index of well being or quality of wellbeing or quality of well being or qwb).ti,ab. (12)
-
(multiattribute$ health ind$ or multi attribute$ health ind$).ti,ab. (0)
-
(health utilit$ index or health utilit$ indices).ti,ab. (31)
-
(health utilit$ scale$ or classification of illness state$).ti,ab. (1)
-
health state$ utilit$.ti,ab. (23)
-
(multiattribute$ utilit$ or multi attribute$ utilit$).ti,ab. (81)
-
health utilit$ scale$.ti,ab. (1)
-
(euro qual or euro qol or eq-5d or eq5d or eq 5d or euroqual or euroqol).ti,ab. (68)
-
(qualy or qaly or qualys or qalys or quality adjusted life year$).ti,ab. (278)
-
(sf36 or sf 36).ti,ab. (24)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab. (8)
-
(sf 6d or sf6d or short form 6d or shortform 6d or sf six$ or shortform six$ or short form six$).ti,ab. (21)
-
or/40–57 (861)
-
39 and 58 (1)
-
limit 59 to yr=“2000 -Current” (1)
Key:
-
$ = truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
EMBASE (Ovid) 2000–10 week 46
-
Date searched: 22 November 2010
-
Records found: 582.
-
*scoliosis/ or *idiopathic scoliosis/ or *kyphoscoliosis/ or *kyphosis/ (12,000)
-
*lordosis/ or *scheuermann disease/ or *spondylolisthesis/ or *spondylolysis/ (4397)
-
*atlantoaxial subluxation/ (399)
-
*ankylosing spondylitis/ (9024)
-
*valgus deformity/ (384)
-
*leg length inequality/ (1161)
-
*enchondromatosis/ (251)
-
*neurofibromatosis/ (8764)
-
*familial hypophosphatemic rickets/ or *hypophosphatemic rickets/ or *autosomal dominant hypophosphatemic rickets/ or *x linked hypophosphatemic rickets/ or *hereditary hypophosphatemic rickets with hypercalciuria/ (156)
-
scoliosis.ti,ab. (12,272)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (6857)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (4032)
-
deforming dorsopath$.ti,ab. (1)
-
(valgus deformit$ or flexion deformit$).ti,ab. (1783)
-
((limb$ length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (1507)
-
((lower limb$ or leg or legs) adj2 congenital$ deform$).ti,ab. (0)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (164)
-
scheuermann$ disease.ti,ab. (333)
-
(ollier$ disease or enchondromatosis).ti,ab. (335)
-
neurofibromatosis.ti,ab. (9835)
-
hypophosphat?emic rickets.ti,ab. (753)
-
proximal focal femoral deficiency.ti,ab. (31)
-
fibular hemimelia.ti,ab. (70)
-
hemi hypertrophy.ti,ab. (20)
-
*bone dysplasia/ (3136)
-
skeletal dysplasia$.ti,ab. (1621)
-
*short stature/ (2636)
-
short stature.ti,ab. (7177)
-
tumo?r reconstruction.ti,ab. (47)
-
*Blount disease/ (78)
-
blount$ disease.ti,ab. (203)
-
*congenital hip dislocation/ (4080)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (29,479)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (506,364)
-
33 and 34 (4106)
-
arthrogryposis multiplex congenita.ti,ab. (475)
-
*occult spinal dysraphism/ (56)
-
spina bifida occulta.ti,ab. (398)
-
*Klippel Feil syndrome/ (584)
-
klippel feil syndrome.ti,ab. (522)
-
congenital spondylolisthesis.ti,ab. (10)
-
exp *chondrodysplasia/ (2310)
-
short rib syndrome.ti,ab. (18)
-
*chondrodysplasia punctata/ (602)
-
chondrodysplasia punctata.ti,ab. (559)
-
*achondroplasia/ (1261)
-
achondroplasia.ti,ab. (1177)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (1170)
-
*osteogenesis imperfecta/ (3178)
-
osteogenesis imperfecta.ti,ab. (3277)
-
osteopetrosis.ti,ab. (1838)
-
*enchondromatosis/ (251)
-
enchondromatosis.ti,ab. (167)
-
multiple congenital exostoses.ti,ab. (2)
-
*SPONDYLOEPIPHYSEAL DYSPLASIA/ (304)
-
*fragility fracture/ (1656)
-
osteoporotic fracture$.ti,ab. (4181)
-
or/1–32 (66,204)
-
or/35–57 (22,326)
-
58 or 59 (84,393)
-
*”quality of life”/ or *quality adjusted life year/ or *”quality of life index”/ or *short form 12/ or *short form 20/ or *short form 36/ or *short form 8/ (34,848)
-
(utilit$ approach$ or health gain or hui or hui2 or hui3).ti,ab,ot. (1965)
-
(health measurement$ scale$ or health measurement$ questionnaire$).ti,ab,ot. (43)
-
health related quality of life.ti,ab,ot. (16,562)
-
(utility weight$ or utility value$ or preference weight$ or quality weight$).ti,ab,ot. (883)
-
(standard gamble$ or categor$ scal$ or linear scal$ or linear analog$ or visual scal$ or magnitude estimat$).ti,ab,ot. (3915)
-
(time trade off$ or rosser$ classif$ or rosser$ matrix or rosser$ distress$ or hrqol).ti,ab,ot. (5875)
-
(index of wellbeing or index of well being or quality of wellbeing or quality of well being or qwb).ti,ab,ot. (393)
-
(multiattribute$ health ind$ or multi attribute$ health ind$).ti,ab,ot. (2)
-
(health utilit$ index or health utilit$ indices).ti,ab,ot. (536)
-
(health utilit$ scale$ or classification of illness state$).ti,ab,ot. (6)
-
health state$ utilit$.ti,ab,ot. (215)
-
(multiattribute$ utilit$ or multi attribute$ utilit$).ti,ab,ot. (181)
-
health utilit$ scale$ .ti,ab,ot. (5)
-
(euro qual or euro qol or eq-5d or eq5d or eq 5d or euroqual or euroqol).ti,ab,ot. (2828)
-
(qualy or qaly or qualys or qalys or quality adjusted life year$).ti,ab,ot. (5392)
-
(sf36 or sf 36).ti,ab,ot. (11,417)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (4801)
-
(sf 6d or sf6d or short form 6d or shortform 6d or sf six$ or shortform six$ or short form six$).ti,ab,ot. (269)
-
or/61–79 (61,502)
-
exp ANIMAL/ (1,638,643)
-
exp animal experiment/ (1,402,804)
-
Nonhuman/ (3,534,351)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh,ot. (3,970,200)
-
or/81–84 (5,705,932)
-
exp human/ (12,100,407)
-
exp human experiment/ (283,763)
-
86 or 87 (12,101,788)
-
85 not (85 and 88) (4,515,714)
-
80 not 89 (61,086)
-
limit 90 to yr=“2000 -Current” (46,540)
-
60 and 91 (582)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab,ot = terms in either title, original title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Searches for radiation exposure and cancer risk
-
Date searches conducted: 6 December 2010
-
Limits: no date limits applied.
Systematic reviews
-
Records found (after deduplication): 191
-
Records found (before deduplication): 207.
Databases searched
-
MEDLINE
-
The Cochrane Library
-
CDSR
-
DARE
-
-
EMBASE.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid) 1950 – November week 3 2010
-
Date searched: 7 December 2010
-
Records found: 120.
-
exp Neoplasms, Radiation-Induced/ (15,568)
-
radiography/ or radiographic image enhancement/ or radiographic image interpretation, computer-assisted/ or exp radiography, thoracic/ or exp tomography, x-ray/ or Tomography, X-Ray Computed/ (299,342)
-
exp neoplasms/ (2,199,022)
-
2 and 3 (99,908)
-
radiation.ti,ab. and 4 (5936)
-
radiography/ae or radiographic image enhancement/ae or radiographic image interpretation, computer-assisted/ae or exp radiography, thoracic/ae or exp tomography, x-ray/ae or Tomography, X-Ray Computed/ae (2121)
-
3 and 6 (705)
-
((radiography or xray$ or x-ray$) and (cancer$ or neoplasm$ or malignan$ or tumor$ or tumour$)).ti,ab. (20,440)
-
radiation.ti,ab. (201,125)
-
8 and 9 (3255)
-
1 or 5 or 7 or 10 (23,741)
Line 11 combines all of the terms in sets 1, 5, 7 and 10 that relate to the cancer adverse effects of radiation
-
systematic$ review$.ti,ab. (28,073)
-
meta-analysis as topic/ (11,028)
-
meta-analytic$.ti,ab. (2429)
-
meta-analysis.ti,ab,pt. (39,637)
-
meta-analysis.ti,ab. (100)
-
metaanalysis.ti,ab. (825)
-
meta analysis.ti,ab. (28,152)
-
meta-synthesis.ti,ab. (97)
-
metasynthesis.ti,ab. (70)
-
meta synthesis.ti,ab. (97)
-
meta-regression.ti,ab. (1026)
-
metaregression.ti,ab. (148)
-
meta regression.ti,ab. (1026)
-
(synthes$ adj3 literature).ti,ab. (1008)
-
(synthes$ adj3 evidence).ti,ab. (2478)
-
integrative review.ti,ab. (414)
-
data synthesis.ti,ab. (5909)
-
(research synthesis or narrative synthesis).ti,ab. (329)
-
(systematic study or systematic studies).ti,ab. (5616)
-
(systematic comparison$ or systematic overview$).ti,ab. (1353)
-
evidence based review.ti,ab. (825)
-
comprehensive review.ti,ab. (4303)
-
critical review.ti,ab. (8691)
-
quantitative review.ti,ab. (355)
-
structured review.ti,ab. (319)
-
realist review.ti,ab. (12)
-
realist synthesis.ti,ab. (7)
-
or/12–38 (92,622)
-
review.pt. (1,587,483)
-
medline.ab. (37,708)
-
pubmed.ab. (12,244)
-
cochrane.ab. (16,961)
-
embase.ab. (14,312)
-
cinahl.ab. (5378)
-
psyc?lit.ab. (839)
-
psyc?info.ab. (4549)
-
(literature adj3 search$).ab. (15,124)
-
(database$ adj3 search$).ab. (14,297)
-
(bibliographic adj3 search$).ab. (814)
-
(electronic adj3 search$).ab. (4493)
-
(electronic adj3 database$).ab. (5251)
-
(computeri?ed adj3 search$).ab. (2159)
-
(internet adj3 search$).ab. (1092)
-
included studies.ab. (3019)
-
(inclusion adj3 studies).ab. (3278)
-
inclusion criteria.ab. (19,917)
-
selection criteria.ab. (14,376)
-
predefined criteria.ab. (710)
-
predetermined criteria.ab. (571)
-
(assess$ adj3 (quality or validity)).ab. (27,853)
-
(select$ adj3 (study or studies)).ab. (28,090)
-
(data adj3 extract$).ab. (18,583)
-
extracted data.ab. (3997)
-
(data adj2 abstracted).ab. (2353)
-
(data adj3 abstraction).ab. (546)
-
published intervention$.ab. (77)
-
((study or studies) adj2 evaluat$).ab. (74,611)
-
(intervention$ adj2 evaluat$).ab. (4341)
-
confidence interval$.ab. (149,317)
-
heterogeneity.ab. (69,784)
-
pooled.ab. (30,954)
-
pooling.ab. (5787)
-
odds ratio$.ab. (98,780)
-
(Jadad or coding).ab. (99,087)
-
or/41–75 (573,641)
-
40 and 76 (74,609)
-
review.ti. (195,610)
-
78 and 76 (23,543)
-
(review$ adj4 (papers or trials or studies or evidence or intervention$or evaluation$)).ti,ab. (72,323)
-
39 or 77 or 79 or 80 (190,532)
Line 81 combines sets 39, 77, 79 and 80 containing terms for systematic reviews or meta-analyses
-
11 and 81 (417)
Line 82 combines radiation and cancer with systematic review/meta-analysis terms
-
animals/ not (animals/ and humans/) (3,521,885)
-
82 not 83 (409)
Line 84 excludes animal-only studies
-
exp Radiotherapy/ (115,830)
-
Nuclear Power Plants/ or Nuclear Reactors/ or Radioactive Hazard Release/ or Nuclear Warfare/ or chernobyl nuclear accident/ (11,283)
-
Occupational Diseases/ or Occupational Exposure/ or Environmental Exposure/ (137,244)
-
“Ultraviolet Rays”/or sunlight/ (64,697)
-
Cellular Phone/ (2097)
-
(radiotherapy or radiation therapy).ti,ab. (124,381)
-
or/85–90 (399,744)
-
84 not 91 (120)
Line 92 excludes non-diagnostic radiation
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
The Cochrane Library
-
All years – 2010 Issue 11
-
Date searched: 6 December 2010
-
Records found: CDSR (two – hand-sifted for relevance: no relevant records found), DARE (six – hand-sifted for relevance: one relevant record found).
-
#1 MeSH descriptor Neoplasms, Radiation-Induced explode all trees (80)
-
#2 MeSH descriptor Radiography, this term only with qualifier: AE (11)
-
#3 MeSH descriptor Radiographic Image Enhancement explode all trees with qualifier: AE (23)
-
#4 MeSH descriptor Radiographic Image Interpretation, Computer-Assisted, this term only with qualifier: AE (0)
-
#5 MeSH descriptor Radiography, Thoracic explode all trees with qualifier: AE (3)
-
#6 MeSH descriptor Tomography, X-Ray explode all trees with qualifier: AE (16)
-
#7 MeSH descriptor Tomography, X-Ray Computed, this term only with qualifier: AE (15)
-
#8 (#2 OR #3 OR #4 OR #5 OR #6 OR #7) (36)
-
#9 MeSH descriptor Neoplasms explode all trees (41,225)
-
#10 (#8 AND #9) (10)
-
#11 ((radiography or xray* or x-ray*) and (cancer* or neoplasm* or malignan* or tumor* or tumour*)):ti,ab (378)
-
#12 radiation:ti,ab (5866)
-
#13 (#11 AND #12) (56)
-
#14 (#1 OR #10 OR #13) (140)
Key:
-
* = truncation
-
:ti,ab = terms in either title or abstract fields
-
Qualifier: AE = applies ‘Adverse Effects’ limit to MeSH headings
EMBASE (Ovid) 1980 – 2010 week 48
-
Date searched: 7 December 2010
-
Records found: 86.
-
radiation induced neoplasm/ (199)
-
radiography/ (249,960)
-
thorax radiography/ (79,329)
-
tomography/ (12,797)
-
computer assisted tomography/ (364,509)
-
digital radiography/ (2953)
-
computer assisted radiography/ (621)
-
or/2–7 (630,912)
-
exp neoplasm/ (2,509,506)
-
8 and 9 (195,807)
-
radiation.ti,ab. and 10 (9305)
-
((radiography or xray$ or x-ray$) and (cancer$ or neoplasm$ or malignan$ or tumor$ or tumour$)).ti,ab. (22,901)
-
radiation.ti,ab. (217,761)
-
12 and 13 (3619)
-
1 or 11 or 14 (12,487)
-
exp meta analysis/ or “systematic review”/ (70,957)
-
meta-analys$.ti,ab. (38,595)
-
metaanalys$.ti,ab. (1838)
-
meta analys$.ti,ab. (38,595)
-
review$.ti. (222,383)
-
overview$.ti. (27,758)
-
(synthes$ adj3 (literature$ or research$ or studies or data)).ti,ab. (17,777)
-
pooled analys$.ti,ab. (2875)
-
((data adj2 pool$) and studies).mp. (3165)
-
(medline or medlars or embase or cinahl or scisearch or psychinfo or psycinfo or psychlit or psyclit).ti,ab. (45,869)
-
((hand or manual or database$ or computer$) adj2 search$).ti,ab. (20,057)
-
((electronic or bibliographic$) adj2 (database$ or data base$)).ti,ab. (7122)
-
((review$ or overview$) adj10 (systematic$ or methodologic$ or quantitativ$ or research$or literature$ or studies or trial$ or effective$)).ab. (257,499)
-
16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (542,709)
-
(retrospective$ adj2 review$).ti,ab,sh. (80,224)
-
(case$ adj2 review$).ti,ab,sh. (80,768)
-
(record$ adj2 review$).ti,ab,sh. (17,192)
-
(patient$ adj2 review$).ti,ab,sh. (128,790)
-
(patient$ adj2 chart$).ti,ab,sh. (5601)
-
(chart$ adj2 review$).ti,ab,sh. (23,716)
-
(case$ adj2 report$).ti,ab,sh. (364,721)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (3,976,038)
-
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 (4,600,047)
-
29 not 38 (392,620)
-
editorial.pt. (360,486)
-
letter.pt. (710,085)
-
40 or 41 (1,070,571)
-
39 not 42 (380,560)
-
exp animal/ (1,640,039)
-
exp nonhuman/ (3,542,502)
-
44 or 45 (5,166,244)
-
exp human/ (12,122,948)
-
46 not (46 and 47) (4,145,607)
-
43 not 48 (370,621)
-
15 and 49 (467)
-
exp radiotherapy/ (264,670)
-
exp “nuclear energy and related phenomena”/ (21,573)
-
atomic warfare/ (3194)
-
occupational disease/ (48,382)
-
occupational exposure/ (52,954)
-
environmental exposure/ (58,300)
-
exp ultraviolet radiation/ (70,076)
-
sunlight/ (8169)
-
mobile phone/ (3187)
-
(radiotherapy or radiation therapy).ti,ab. (141,833)
-
or/51–60 (552,391)
-
50 not 61 (86)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Primary studies
-
Records found (after deduplication): 255
-
Records found (before deduplication): 323.
Databases searched
-
MEDLINE
-
CENTRAL
-
EMBASE.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid) 1950 – November week 3 2010
-
Date searched: 7 December 2010
-
Records found: 198.
-
exp Neoplasms, Radiation-Induced/ (15,568)
-
radiography/ or radiographic image enhancement/ or radiographic image interpretation, computer-assisted/ or exp radiography, thoracic/ or exp tomography, x-ray/ or Tomography, X-Ray Computed/ (299,342)
-
exp neoplasms/ (2,199,022)
-
2 and 3 (99,908)
-
radiation.ti,ab. and 4 (5936)
-
radiography/ae or radiographic image enhancement/ae or radiographic image interpretation, computer-assisted/ae or exp radiography, thoracic/ae or exp tomography, x-ray/ae or Tomography, X-Ray Computed/ae (2121)
-
3 and 6 (705)
-
((radiography or xray$ or x-ray$) and (cancer$ or neoplasm$ or malignan$ or tumor$ or tumour$)).ti,ab. (20,440)
-
radiation.ti,ab. (201,125)
-
8 and 9 (3255)
-
1 or 5 or 7 or 10 (23,741)
Line 11 combines all of the terms in sets 1, 5, 7 and 10 that relate to the cancer adverse effects of radiation
-
*spinal curvatures/ or *kyphosis/ or *scheuermann disease/ or *lordosis/ or *scoliosis/ or *spinal osteochondrosis/ or *spondylolysis/ or *spondylolisthesis/ (15,153)
-
*Spondylitis, Ankylosing/ (7888)
-
*Leg Length Inequality/ (1579)
-
*Enchondromatosis/ (333)
-
*neurofibromatoses/ or *neurofibromatosis 1/ or *neurofibromatosis 2/ (7199)
-
*Hypophosphatemic Rickets, X-Linked Dominant/ (125)
-
scoliosis.ti,ab. (11,882)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (6610)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (3831)
-
deforming dorsopath$.ti,ab. (1)
-
(valgus deformit$ or flexion deformit$).ti,ab. (1791)
-
((limb$ length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (1577)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (165)
-
scheuermann$ disease.ti,ab. (319)
-
(ollier$ disease or enchondromatosis).ti,ab. (347)
-
neurofibromatosis.ti,ab. (8998)
-
hypophosphat?emic rickets.ti,ab. (694)
-
proximal focal femoral deficiency.ti,ab. (25)
-
fibular hemimelia.ti,ab. (81)
-
hemi hypertrophy.ti,ab. (26)
-
skeletal dysplasia$.ti,ab. (1507)
-
short stature.ti,ab. (6527)
-
tumo?r reconstruction.ti,ab. (42)
-
blount$ disease.ti,ab. (215)
-
*Hip Dislocation, Congenital/ (5559)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (27,671)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (466,155)
-
37 and 38 (4105)
-
arthrogryposis multiplex congenita.ti,ab. (477)
-
*Spina Bifida Occulta/ (1281)
-
spina bifida occulta.ti,ab. (401)
-
*Klippel-Feil Syndrome/ (564)
-
klippel feil syndrome.ti,ab. (513)
-
congenital spondylolisthesis.ti,ab. (12)
-
exp *Osteochondrodysplasias/ (18,249)
-
short rib syndrome.ti,ab. (16)
-
chondrodysplasia punctata.ti,ab. (531)
-
achondroplasia.ti,ab. (1143)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (1169)
-
osteogenesis imperfecta.ti,ab. (3150)
-
osteopetrosis.ti,ab. (1803)
-
enchondromatosis.ti,ab. (168)
-
multiple congenital exostoses.ti,ab. (2)
-
osteoporotic fracture$.ti,ab. (3485)
-
or/12–36 (60,396)
-
or/39–55 (30,360)
-
56 or 57 (86,126)
Line 58 combines all of the terms in sets 12 to 36 and 39 to 55 relating to the orthopaedic conditions of interest
-
11 and 58 (198)
Line 59 combines radiation adverse effect terms and the orthopaedic terms
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
Cochrane Central Register of Controlled Trials (The Cochrane Library)
-
All years – 2010 Issue 11
-
Date searched: 8 December 2010
-
Records found: 27.
-
#1 MeSH descriptor Neoplasms, Radiation-Induced explode all trees (80)
-
#2 MeSH descriptor Radiography, this term only with qualifier: AE (11)
-
#3 MeSH descriptor Radiographic Image Enhancement explode all trees with qualifier: AE (23)
-
#4 MeSH descriptor Radiographic Image Interpretation, Computer-Assisted, this term only with qualifier: AE (0)
-
#5 MeSH descriptor Radiography, Thoracic explode all trees with qualifier: AE (3)
-
#6 MeSH descriptor Tomography, X-Ray explode all trees with qualifier: AE (16)
-
#7 MeSH descriptor Tomography, X-Ray Computed, this term only with qualifier: AE (15)
-
#8 (#2 OR #3 OR #4 OR #5 OR #6 OR #7) (36)
-
#9 MeSH descriptor Neoplasms explode all trees (41,225)
-
#10 (#8 AND #9) (10)
-
#11 ((radiography or xray* or x-ray*) and (cancer* or neoplasm* or malignan* or tumor* or tumour*)):ti,ab (378)
-
#12 radiation:ti,ab (5866)
-
#13 (#11 AND #12) (56)
-
#14 (#1 OR #10 OR #13) (140)
-
#15 MeSH descriptor Radiotherapy explode all trees (4243)
-
#16 MeSH descriptor Nuclear Power Plants, this term only (0)
-
#17 MeSH descriptor Nuclear Reactors, this term only (8)
-
#18 MeSH descriptor Radioactive Hazard Release, this term only (14)
-
#19 MeSH descriptor Nuclear Warfare, this term only (5)
-
#20 MeSH descriptor Chernobyl Nuclear Accident, this term only (3)
-
#21 MeSH descriptor Occupational Diseases, this term only (706)
-
#22 MeSH descriptor Occupational Exposure, this term only (374)
-
#23 MeSH descriptor Environmental Exposure, this term only (370)
-
#24 MeSH descriptor Ultraviolet Rays, this term only (417)
-
#25 MeSH descriptor Sunlight, this term only (199)
-
#26 MeSH descriptor Cellular Phone, this term only (133)
-
#27 (radiotherapy or “radiation therapy”):ti,ab (8906)
-
#28 (#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) (12,577)
-
#29 (#14 AND NOT #28)
Key:
-
* = truncation
-
“ ” = phrase search
-
:ti,ab = terms in either title or abstract fields
-
Qualifier: AE = applies ‘Adverse Effects’ limit to MeSH headings
EMBASE (Ovid) 1980–2010 week 48
-
Date searched: 7 December 2010
-
Records found: 98.
-
radiation induced neoplasm/ (199)
-
radiography/ (249,960)
-
thorax radiography/ (79,329)
-
tomography/ (12,797)
-
computer assisted tomography/ (364,509)
-
digital radiography/ (2953)
-
computer assisted radiography/ (621)
-
or/2–7 (630,912)
-
exp neoplasm/ (2,509,506)
-
8 and 9 (195,807)
-
radiation.ti,ab. and 10 (9305)
-
((radiography or xray$ or x-ray$) and (cancer$ or neoplasm$ or malignan$ or tumor$ or tumour$)).ti,ab. (22,901)
-
radiation.ti,ab. (217,761)
-
12 and 13 (3619)
-
1 or 11 or 14 (12,487)
-
*scoliosis/ or *idiopathic scoliosis/ or *kyphoscoliosis/ or *kyphosis/ (12,010)
-
*lordosis/ or *scheuermann disease/ or *spondylolisthesis/ or *spondylolysis/ (4404)
-
*atlantoaxial subluxation/ (400)
-
*ankylosing spondylitis/ (9051)
-
*valgus deformity/ (386)
-
*leg length inequality/ (1163)
-
*enchondromatosis/ (252)
-
*neurofibromatosis/ (8770)
-
*familial hypophosphatemic rickets/ or *hypophosphatemic rickets/ or *autosomal dominant hypophosphatemic rickets/ or *x linked hypophosphatemic rickets/ or *hereditary hypophosphatemic rickets with hypercalciuria/ (156)
-
scoliosis.ti,ab. (12,287)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (6879)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (4040)
-
deforming dorsopath$.ti,ab. (1)
-
(valgus deformit$ or flexion deformit$).ti,ab. (1788)
-
((limb$ length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (1510)
-
((lower limb$ or leg or legs) adj2 congenital$ deform$).ti,ab. (1)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (164)
-
scheuermann$ disease.ti,ab. (333)
-
(ollier$ disease or enchondromatosis).ti,ab. (336)
-
neurofibromatosis.ti,ab. (9848)
-
hypophosphat?emic rickets.ti,ab. (754)
-
proximal focal femoral deficiency.ti,ab. (31)
-
fibular hemimelia.ti,ab. (70)
-
hemi hypertrophy.ti,ab. (20)
-
*bone dysplasia/ (3140)
-
skeletal dysplasia$.ti,ab. (1627)
-
*short stature/ (2642)
-
short stature.ti,ab. (7194)
-
tumo?r reconstruction.ti,ab. (47)
-
*Blount disease/ (78)
-
blount$ disease.ti,ab. (203)
-
*congenital hip dislocation/ (4081)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (29,521)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (507,710)
-
48 and 49 (4111)
-
arthrogryposis multiplex congenita.ti,ab. (475)
-
*occult spinal dysraphism/ (56)
-
spina bifida occulta.ti,ab. (398)
-
*Klippel Feil syndrome/ (584)
-
klippel feil syndrome.ti,ab. (523)
-
congenital spondylolisthesis.ti,ab. (10)
-
exp *chondrodysplasia/ (2310)
-
short rib syndrome.ti,ab. (18)
-
*chondrodysplasia punctata/ (602)
-
chondrodysplasia punctata.ti,ab. (559)
-
*achondroplasia/ (1263)
-
achondroplasia.ti,ab. (1179)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (1172)
-
*osteogenesis imperfecta/ (3181)
-
osteogenesis imperfecta.ti,ab. (3283)
-
osteopetrosis.ti,ab. (1842)
-
*enchondromatosis/ (252)
-
enchondromatosis.ti,ab. (168)
-
multiple congenital exostoses.ti,ab. (2)
-
*SPONDYLOEPIPHYSEAL DYSPLASIA/ (305)
-
*fragility fracture/ (1676)
-
osteoporotic fracture$.ti,ab. (4212)
-
or/16–47 (66,329)
-
or/50–72 (22,393)
-
73 or 74 (84,577)
-
15 and 75 (98)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Searches for radiation exposure and adverse reproductive outcome risk
-
Date searches conducted: 21 December 2010
-
Limits: no date limits applied.
Systematic reviews
-
Records found (after deduplication): 353
-
Records found (before deduplication): 318.
Databases searched
-
MEDLINE
-
The Cochrane Library
-
CDSR
-
DARE
-
-
EMBASE.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid) 1950 – November week 3 2010
-
Date searched: 21 December 2010
-
Records found: 93.
-
radiography/ or radiographic image enhancement/ or radiographic image interpretation, computer-assisted/ or exp radiography, thoracic/ or exp tomography, x-ray/ or Tomography, X-Ray Computed/ (299,620)
-
radiation dosage/ or radiation injuries/ (50,902)
-
radiation.ti,ab. (201,611)
-
(radiography or xray$ or x-ray$).ti,ab. (207,809)
-
or/1–4 (677,940)
Line 5 combines radiation terms in lines 1–4
-
exp Infertility/ or Fertility/ (73,130)
-
(infertility or infertile or subfertility or subfertile or fertility or fertile).ti,ab. (82,964)
-
“Abortion, Spontaneous”/ (12,909)
-
“Fetal Death”/ (21,935)
-
*”Pregnancy Complications”/ (47,506)
-
*”Pregnancy Outcome”/ (10,741)
-
((fetal or foetal) adj1 death).ti,ab. (4573)
-
(human reproduction or reproductive system).ti,ab. (5699)
-
exp Urogenital System/co, in, re [Complications, Injuries, Radiation Effects] (19,839)
-
((pregnant or pregnancy) adj2 (complicat$ or difficult$ or problem$ or unsuccessful$)).ti,ab. (10,490)
-
adverse reproductive outcome$.ti,ab. (254)
-
stillbirth$.ti,ab. (6024)
-
or/6–17 (228,167)
Line 18 combines infertility and reproductive problem terms in lines 6–17
-
5 and 18 (7933)
Line 19 combines radiation terms and infertility/reproductive problem terms
-
systematic$ review$.ti,ab. (28,245)
-
meta-analysis as topic/ (11,048)
-
meta-analytic$.ti,ab. (2444)
-
meta-analysis.ti,ab,pt. (39,834)
-
meta-analysis.ti,ab. (100)
-
metaanalysis.ti,ab. (824)
-
meta analysis.ti,ab. (28,338)
-
meta-synthesis.ti,ab. (98)
-
metasynthesis.ti,ab. (72)
-
meta synthesis.ti,ab. (98)
-
meta-regression.ti,ab. (1033)
-
metaregression.ti,ab. (149)
-
meta regression.ti,ab. (1033)
-
(synthes$ adj3 literature).ti,ab. (1016)
-
(synthes$ adj3 evidence).ti,ab. (2489)
-
integrative review.ti,ab. (416)
-
data synthesis.ti,ab. (5916)
-
(research synthesis or narrative synthesis).ti,ab. (332)
-
(systematic study or systematic studies).ti,ab. (5639)
-
(systematic comparison$or systematic overview$).ti,ab. (1358)
-
evidence based review.ti,ab. (827)
-
comprehensive review.ti,ab. (4327)
-
critical review.ti,ab. (8720)
-
quantitative review.ti,ab. (357)
-
structured review.ti,ab. (323)
-
realist review.ti,ab. (12)
-
realist synthesis.ti,ab. (7)
-
or/20–46 (93,071)
-
review.pt. (1,589,337)
-
medline.ab. (37,901)
-
pubmed.ab. (12,371)
-
cochrane.ab. (17,095)
-
embase.ab. (14,429)
-
cinahl.ab. (5428)
-
psyc?lit.ab. (839)
-
psyc?info.ab. (4678)
-
(literature adj3 search$).ab. (15,204)
-
(database$ adj3 search$).ab. (14,377)
-
(bibliographic adj3 search$).ab. (822)
-
(electronic adj3 search$).ab. (4518)
-
(electronic adj3 database$).ab. (5276)
-
(computeri?ed adj3 search$).ab. (2163)
-
(internet adj3 search$).ab. (1096)
-
included studies.ab. (3044)
-
(inclusion adj3 studies).ab. (3299)
-
inclusion criteria.ab. (20,025)
-
selection criteria.ab. (14,415)
-
predefined criteria.ab. (717)
-
predetermined criteria.ab. (572)
-
(assess$ adj3 (quality or validity)).ab. (27,970)
-
(select$ adj3 (study or studies)).ab. (28,195)
-
(data adj3 extract$).ab. (18,679)
-
extracted data.ab. (4031)
-
(data adj2 abstracted).ab. (2359)
-
(data adj3 abstraction).ab. (548)
-
published intervention$.ab. (77)
-
((study or studies) adj2 evaluat$).ab. (74,888)
-
(intervention$ adj2 evaluat$).ab. (4365)
-
confidence interval$.ab. (150,097)
-
heterogeneity.ab. (70,008)
-
pooled.ab. (31,066)
-
pooling.ab. (5803)
-
odds ratio$.ab. (99,301)
-
(Jadad or coding).ab. (99,338)
-
or/49–83 (576,005)
-
48 and 84 (747,77)
-
review.ti. (196,122)
-
86 and 84 (23,679)
-
(review$ adj4 (papers or trials or studies or evidence or intervention$ or evaluation$)).ti,ab. (72,618)
-
47 or 85 or 87 or 88 (191,304)
Line 89 combines sets 47, 85, 87 and 88 containing terms for systematic reviews or meta-analyses
-
19 and 89 (100)
Line 90 combines radiation and infertility problems with systematic review/meta-analysis terms
-
exp animals/ not humans/ (3,607,750)
-
90 not 91 (93)
Line 92 excludes animal-only studies
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
The Cochrane Library
-
All years – 2010 Issue 12
-
Date searched: 21 December 2010
-
Records found: CDSR (two – hand-sifted for relevance: no relevant records found), DARE (two – hand-sifted for relevance: no relevant records found).
-
#1 MeSH descriptor Radiography, this term only (154)
-
#2 MeSH descriptor Radiographic Image Enhancement, this term only (328)
-
#3 MeSH descriptor Radiographic Image Interpretation, Computer-Assisted, this term only (157)
-
#4 MeSH descriptor Radiography, Thoracic explode all trees (303)
-
#5 MeSH descriptor Tomography, X-Ray explode all trees (2867)
-
#6 MeSH descriptor Tomography, X-Ray Computed, this term only (2596)
-
#7 MeSH descriptor Radiation Dosage, this term only (382)
-
#8 MeSH descriptor Radiation Injuries, this term only (617)
-
#9 (radiography or xray* or x-ray*):ti,ab (3880)
-
#10 radiation:ti,ab (5867)
-
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) (12,938)
-
#12 MeSH descriptor Infertility explode all trees (1593)
-
#13 MeSH descriptor Fertility, this term only (123)
-
#14 (infertility or infertile or subfertility or subfertile or fertility or fertile):ti,ab (2535)
-
#15 MeSH descriptor Abortion, Spontaneous, this term only (251)
-
#16 MeSH descriptor Fetal Death, this term only (188)
-
#17 MeSH descriptor Pregnancy Complications explode all trees (6279)
-
#18 MeSH descriptor Pregnancy Outcome, this term only (2157)
-
#19 (“fetal death” or “foetal death”):ti,ab (101)
-
#20 (“human reproduction” or “reproductive system”):ti,ab (143)
-
#21 MeSH descriptor Urogenital System explode all trees with qualifiers: CO,IN,RE (167)
-
#22 ((pregnan* near/2 complicat*) or (pregnan* near/2 difficult*) or (pregnan* near/2 problem*) or (pregnan* near/2 unsuccessful*)):ti,ab (353)
-
#23 “adverse reproductive outcome*”:ti,ab (0)
-
#24 stillbirth*:ti,ab (143)
-
#25 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) (10,438)
-
#26 (#11 AND #25) (98)
Key:
-
* = truncation
-
:ti,ab = terms in either title or abstract fields
-
Qualifier: CO = applies ‘Complications’ limit to MeSH headings
-
Qualifier: IN = applies ‘Injuries’ limit to MeSH headings
-
Qualifier: RE = applies ‘Radiation effects’ limit to MeSH headings
-
“ ” = phrase search
-
:ti,ab = terms in either title or abstract fields
-
near/2 = terms within two words of each other (any order)
EMBASE (Ovid) 1980–2010 week 50
-
Date searched: 21 December 2010
-
Records found: 260.
-
radiography/ (250,023)
-
thorax radiography/ (79,635)
-
tomography/ (12,834)
-
computer assisted tomography/ (365,687)
-
digital radiography/ (2955)
-
computer assisted radiography/ (622)
-
radiation dose/ (78,208)
-
radiation injury/ (36,833)
-
radiation.ti,ab. (218,310)
-
(radiography or xray$ or x-ray$).ti,ab. (217,251)
-
or/1–10 (1,032,688)
-
exp infertility/ (72,065)
-
exp fertility/ (41,798)
-
(infertility or infertile or subfertility or subfertile or fertility or fertile).ti,ab. (87,517)
-
spontaneous abortion/ (18,553)
-
fetus death/ (17,751)
-
*pregnancy complication/ (43,020)
-
*pregnancy outcome/ (4808)
-
((fetal or foetal) adj1 death).ti,ab. (4843)
-
(human reproduction or reproductive system).ti,ab. (8600)
-
((pregnant or pregnancy) adj2 (complicat$ or difficult$ or problem$ or unsuccessful$)).ti,ab. (11,528)
-
adverse reproductive outcome$.ti,ab. (253)
-
stillbirth$.ti,ab. (6100)
-
exp *genital system/ (201,259)
-
or/12–24 (406,357)
-
11 and 25 (9507)
-
exp meta analysis/ or “systematic review”/ (71,246)
-
meta-analys$.ti,ab. (38,842)
-
metaanalys$.ti,ab. (1854)
-
meta analys$.ti,ab. (38,842)
-
review$.ti. (222,916)
-
overview$.ti. (27,800)
-
(synthes$ adj3 (literature$ or research$ or studies or data)).ti,ab. (17,811)
-
pooled analys$.ti,ab. (2896)
-
((data adj2 pool$) and studies).mp. (3174)
-
(medline or medlars or embase or cinahl or scisearch or psychinfo or psycinfo or psychlit or psyclit).ti,ab. (46,068)
-
((hand or manual or database$ or computer$) adj2 search$).ti,ab. (20,126)
-
((electronic or bibliographic$) adj2 (database$ or data base$)).ti,ab. (7156)
-
((review$ or overview$) adj10 (systematic$ or methodologic$ or quantitativ$ or research$ or literature$ or studies or trial$ or effective$)).ab. (258,419)
-
27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 (544,295)
-
(retrospective$ adj2 review$).ti,ab,sh. (80,558)
-
(case$ adj2 review$).ti,ab,sh. (80,894)
-
(record$ adj2 review$).ti,ab,sh. (17,255)
-
(patient$ adj2 review$).ti,ab,sh. (129,259)
-
(patient$ adj2 chart$).ti,ab,sh. (5622)
-
(chart$ adj2 review$).ti,ab,sh. (23,812)
-
(case$ adj2 report$).ti,ab,sh. (365,713)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (3,981,033)
-
41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 (4,606,912)
-
40 not 49 (393,807)
-
editorial.pt. (361,349)
-
letter.pt. (711,270)
-
51 or 52 (1,072,619)
-
50 not 53 (381,711)
-
exp animal/ (1,640,043)
-
exp nonhuman/ (3,549,954)
-
55 or 56 (5,173,697)
-
exp human/ (12,141,885)
-
57 not (57 and 58) (4,150,708)
-
54 not 59 (371,742)
-
26 and 60 (260)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Primary studies
-
Records found (after deduplication): 237
-
Records found (before deduplication): 88.
Databases searched
-
MEDLINE
-
CENTRAL
-
EMBASE.
Search strategies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid) 1950 – November week 3 2010
-
Date searched: 21 December 2010
-
Records found: 56
-
radiography/ or radiographic image enhancement/ or radiographic image interpretation, computer-assisted/ or exp radiography, thoracic/ or exp tomography, x-ray/ or Tomography, X-Ray Computed/ (299,620)
-
radiation dosage/ or radiation injuries/ (50,902)
-
radiation.ti,ab. (201,611)
-
(radiography or xray$ or x-ray$).ti,ab. (207,809)
-
or/1–4 (677,940)
Line 5 combines radiation terms in lines 1–4
-
exp Infertility/ or Fertility/ (73,130)
-
(infertility or infertile or subfertility or subfertile or fertility or fertile).ti,ab. (82,964)
-
“Abortion, Spontaneous”/ (12,909)
-
“Fetal Death”/ (21,935)
-
*”Pregnancy Complications”/ (47,506)
-
*”Pregnancy Outcome”/ (10,741)
-
((fetal or foetal) adj1 death).ti,ab. (4573)
-
(human reproduction or reproductive system).ti,ab. (5699)
-
exp Urogenital System/co, in, re [Complications, Injuries, Radiation Effects] (19,839)
-
((pregnant or pregnancy) adj2 (complicat$ or difficult$ or problem$ or unsuccessful$)).ti,ab. (10,490)
-
adverse reproductive outcome$.ti,ab. (254)
-
stillbirth$.ti,ab. (6024)
-
or/6–17 (228,167)
Line 18 combines infertility and reproductive problem terms in lines 6–17
-
5 and 18 (7933)
Line 19 combines radiation terms and infertility/reproductive problem terms
-
*spinal curvatures/ or *kyphosis/ or *scheuermann disease/ or *lordosis/ or *scoliosis/ or *spinal osteochondrosis/ or *spondylolysis/ or *spondylolisthesis/ (15,159)
-
*Spondylitis, Ankylosing/ (7894)
-
*Leg Length Inequality/ (1579)
-
*Enchondromatosis/ (333)
-
*neurofibromatoses/ or *neurofibromatosis 1/ or *neurofibromatosis 2/ (7204)
-
*Hypophosphatemic Rickets, X-Linked Dominant/ (126)
-
scoliosis.ti,ab. (11,906)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (6638)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (3836)
-
deforming dorsopath$.ti,ab. (1)
-
(valgus deformit$ or flexion deformit$).ti,ab. (1793)
-
((limb$ length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (1585)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (166)
-
scheuermann$ disease.ti,ab. (319)
-
(ollier$ disease or enchondromatosis).ti,ab. (347)
-
neurofibromatosis.ti,ab. (9017)
-
hypophosphat?emic rickets.ti,ab. (694)
-
proximal focal femoral deficiency.ti,ab. (25)
-
fibular hemimelia.ti,ab. (81)
-
hemi hypertrophy.ti,ab. (26)
-
skeletal dysplasia$.ti,ab. (1512)
-
short stature.ti,ab. (6542)
-
tumo?r reconstruction.ti,ab. (42)
-
blount$ disease.ti,ab. (215)
-
*Hip Dislocation, Congenital/ (5560)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (27,716)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (467,120)
-
45 and 46 (4110)
-
arthrogryposis multiplex congenita.ti,ab. (478)
-
*Spina Bifida Occulta/ (1281)
-
spina bifida occulta.ti,ab. (403)
-
*Klippel-Feil Syndrome/ (564)
-
klippel feil syndrome.ti,ab. (513)
-
congenital spondylolisthesis.ti,ab. (12)
-
exp *Osteochondrodysplasias/ (18,261)
-
short rib syndrome.ti,ab. (16)
-
chondrodysplasia punctata.ti,ab. (533)
-
achondroplasia.ti,ab. (1144)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (1170)
-
osteogenesis imperfecta.ti,ab. (3152)
-
osteopetrosis.ti,ab. (1803)
-
enchondromatosis.ti,ab. (168)
-
multiple congenital exostoses.ti,ab. (2)
-
osteoporotic fracture$.ti,ab. (3497)
-
or/20–44 (60,504)
-
or/47–63 (30,395)
-
64 or 65 (86,264)
Line 66 combines all of the terms in sets 20–44 and 47–63 relating to the orthopaedic conditions of interest
-
19 and 66 (59)
Line 66 combines radiation, infertility and the orthopaedic conditions of interest
-
exp animals/ not humans/ (3,607,750)
-
67 not 68 (56)
Line 69 excludes animal-only records
Key:
-
/ = indexing term (MeSH heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj1 = terms within one word of each other (any order)
-
adj2 = terms within two words of each other (any order)
Cochrane Central Register of Controlled Trials (The Cochrane Library)
-
All years – 2010 Issue 12
-
Date searched: 21 December 2010
-
Records found: 93.
-
#1 MeSH descriptor Radiography, this term only (154)
-
#2 MeSH descriptor Radiographic Image Enhancement, this term only (328)
-
#3 MeSH descriptor Radiographic Image Interpretation, Computer-Assisted, this term only (157)
-
#4 MeSH descriptor Radiography, Thoracic explode all trees (303)
-
#5 MeSH descriptor Tomography, X-Ray explode all trees (2867)
-
#6 MeSH descriptor Tomography, X-Ray Computed, this term only (2596)
-
#7 MeSH descriptor Radiation Dosage, this term only (382)
-
#8 MeSH descriptor Radiation Injuries, this term only (617)
-
#9 (radiography or xray* or x-ray*):ti,ab (3880)
-
#10 radiation:ti,ab (5867)
-
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) (12,938)
-
#12 MeSH descriptor Infertility explode all trees (1593)
-
#13 MeSH descriptor Fertility, this term only (123)
-
#14 (infertility or infertile or subfertility or subfertile or fertility or fertile):ti,ab (2535)
-
#15 MeSH descriptor Abortion, Spontaneous, this term only (251)
-
#16 MeSH descriptor Fetal Death, this term only (188)
-
#17 MeSH descriptor Pregnancy Complications explode all trees (6279)
-
#18 MeSH descriptor Pregnancy Outcome, this term only (2157)
-
#19 (“fetal death” or “foetal death”):ti,ab (101)
-
#20 (“human reproduction” or “reproductive system”):ti,ab (143)
-
#21 MeSH descriptor Urogenital System explode all trees with qualifiers: CO,IN,RE (167)
-
#22 ((pregnan* near/2 complicat*) or (pregnan* near/2 difficult*) or (pregnan* near/2 problem*) or (pregnan* near/2 unsuccessful*)):ti,ab (353)
-
#23 “adverse reproductive outcome*”:ti,ab (0)
-
#24 stillbirth*:ti,ab (143)
-
#25 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) (10,438)
-
#26 (#11 AND #25) (98)
Key:
-
* = truncation
-
:ti,ab = terms in either title or abstract fields
-
Qualifier: CO = applies ‘Complications’ limit to MeSH headings
-
Qualifier: IN = applies ‘Injuries’ limit to MeSH headings
-
Qualifier: RE = applies ‘Radiation effects’ limit to MeSH headings
-
“ ” = phrase search
-
:ti,ab = terms in either title or abstract fields
-
near/2 = terms within two words of each other (any order)
EMBASE (Ovid) 1980–2010 week 50
-
Date searched: 21 December 2010
-
Records found: 88.
-
radiography/ (250,023)
-
thorax radiography/ (79,635)
-
tomography/ (12,834)
-
computer assisted tomography/ (365,687)
-
digital radiography/ (2955)
-
computer assisted radiography/ (622)
-
radiation dose/ (78,208)
-
radiation injury/ (36,833)
-
radiation.ti,ab. (218,310)
-
(radiography or xray$ or x-ray$).ti,ab. (217,251)
-
or/1–10 (1,032,688)
-
exp infertility/ (72,065)
-
exp fertility/ (41,798)
-
(infertility or infertile or subfertility or subfertile or fertility or fertile).ti,ab. (87,517)
-
spontaneous abortion/ (18,553)
-
fetus death/ (17,751)
-
*pregnancy complication/ (43,020)
-
*pregnancy outcome/ (4808)
-
((fetal or foetal) adj1 death).ti,ab. (4843)
-
(human reproduction or reproductive system).ti,ab. (8600)
-
((pregnant or pregnancy) adj2 (complicat$ or difficult$ or problem$ or unsuccessful$)).ti,ab. (11,528)
-
adverse reproductive outcome$.ti,ab. (253)
-
stillbirth$.ti,ab. (6100)
-
exp *genital system/ (201,259)
-
or/12–24 (406,357)
-
11 and 25 (9507)
-
*scoliosis/ or *idiopathic scoliosis/ or *kyphoscoliosis/ or *kyphosis/ (12,027)
-
*lordosis/ or *scheuermann disease/ or *spondylolisthesis/ or *spondylolysis/ (4407)
-
*atlantoaxial subluxation/ (400)
-
*ankylosing spondylitis/ (9077)
-
*valgus deformity/ (387)
-
*leg length inequality/ (1165)
-
*enchondromatosis/ (253)
-
*neurofibromatosis/ (8786)
-
*familial hypophosphatemic rickets/ or *hypophosphatemic rickets/ or *autosomal dominant hypophosphatemic rickets/ or *x linked hypophosphatemic rickets/ or *hereditary hypophosphatemic rickets with hypercalciuria/ (156)
-
scoliosis.ti,ab. (12,315)
-
(kyphosis or lordosis or flatback syndrome).ti,ab. (6896)
-
(spondylolysis or spondylolisthesis or atlantoaxial subluxation or vertebral subluxation).ti,ab. (4050)
-
deforming dorsopath$.ti,ab. (1)
-
(valgus deformit$ or flexion deformit$).ti,ab. (1795)
-
((limb$length$ or leg length$) adj1 (unequal or inequalit$ or discrepancy or misalignment)).ti,ab. (1514)
-
((lower limb$ or leg or legs) adj2 congenital$ deform$).ti,ab. (1)
-
((spine or spinal) adj2 osteochondrosis).ti,ab. (164)
-
scheuermann$ disease.ti,ab. (333)
-
(ollier$ disease or enchondromatosis).ti,ab. (337)
-
neurofibromatosis.ti,ab. (9869)
-
hypophosphat?emic rickets.ti,ab. (754)
-
proximal focal femoral deficiency.ti,ab. (31)
-
fibular hemimelia.ti,ab. (70)
-
hemi hypertrophy.ti,ab. (20)
-
*bone dysplasia/ (3142)
-
skeletal dysplasia$.ti,ab. (1633)
-
*short stature/ (2653)
-
short stature.ti,ab. (7234)
-
tumo?r reconstruction.ti,ab. (47)
-
*Blount disease/ (78)
-
blount$ disease.ti,ab. (203)
-
*congenital hip dislocation/ (4084)
-
(congenital adj3 (subluxation or deformity or deformities or dislocation or malformation$ or bowing or defect or defects)).ti,ab. (29,597)
-
(hip or hips or spine or spinal or knee or knees or femur or femurs or tibia or tibias or fibula or fibulas or leg or legs or musculoskeletal or lower limb$ or pelvic girdle or bony thorax or cervical rib$).ti,ab. (509,423)
-
59 and 60 (4119)
-
arthrogryposis multiplex congenita.ti,ab. (475)
-
*occult spinal dysraphism/ (56)
-
spina bifida occulta.ti,ab. (398)
-
*Klippel Feil syndrome/ (584)
-
klippel feil syndrome.ti,ab. (524)
-
congenital spondylolisthesis.ti,ab. (10)
-
exp *chondrodysplasia/ (2312)
-
short rib syndrome.ti,ab. (18)
-
*chondrodysplasia punctata/ (602)
-
chondrodysplasia punctata.ti,ab. (559)
-
*achondroplasia/ (1263)
-
achondroplasia.ti,ab. (1180)
-
((dystrophic or chondroectodermal or spondyloepiphyseal or polyostotic fibrous or progressive diaphyseal or metaphyseal) adj1 dysplasia$).ti,ab. (1175)
-
*osteogenesis imperfecta/ (3186)
-
osteogenesis imperfecta.ti,ab. (3288)
-
osteopetrosis.ti,ab. (1845)
-
*enchondromatosis/ (253)
-
enchondromatosis.ti,ab. (169)
-
multiple congenital exostoses.ti,ab. (2)
-
*SPONDYLOEPIPHYSEAL DYSPLASIA/ (305)
-
*fragility fracture/ (1694)
-
osteoporotic fracture$.ti,ab. (4222)
-
or/27–58 (66,487)
-
or/61–83 (22,442)
-
84 or 85 (84,779)
-
26 and 86 (88)
Key:
-
/ = indexing term (EMTREE heading)
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
Appendix 2 Data extraction table: systematic review of the clinical effectiveness of EOS
| Study details and design | Participant details | Intervention/comparators | Outcomes/analyses | Results | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Kalifa et al. 1998, 25 France Type of publication: Full publication Funding: CEDIT, French Ministry of Health, Agence Nationale pour la Valorisation de la Recherche, Biocrit, Baxter SA and Cogema Study design: Comparative study of a diagnostic technology Setting: Outpatient Duration of recruitment: From December 1994 to January 1996 |
Inclusion/exclusion criteria: Patients at potentially higher risk because of X-ray exposures (paediatric patients with repeated examinations). Children of at least the age of 5 years undergoing follow-up examinations for known hip diseases (e.g. congenital dislocation and osteonecrosis) Children undergoing follow-up radiography for scoliosis No. recruited: 176 (the number of patients with known hip diseases or scoliosis NR) No. analysed: 140 (93 with scoliosis and 47 with known hip disease). Thirty-six patients were excluded from analysis because examination was inadequate, film was given to patient without a duplicate being retained or because of double counting Mean age (SD): NR Male (%): NR Disease history: Mean (SD) duration NR The authors reported that a similar study was conducted on chest films in adults |
Intervention: The Charpak system. Digital images were analysed on radiographic laser film and not on the screen Comparator: Film X-ray imaging No. of patients Intervention: Charpak X-ray imaging system No. recruited: 176 No. analysed: 140 (93 with scoliosis and 47 with known hip disease) Comparator: Film X-ray imaging No. recruited: 176 No. analysed: 140 (93 with scoliosis and 47 with known hip disease) No. of images Intervention: Charpak X-ray imaging system No. obtained: NR No. analysed: 93 spinal images and 47 pelvis images Comparator: Film X-ray imaging No. obtained: NR No. analysed: 93 spinal images and 47 pelvis images |
Outcome measuresNo. of assessors: All images assessed by two senior radiologists; bone detail reviewed by a senior orthopaedic surgeon Analysis of image quality: Two approaches were used:Statistical analyses: The interobserver agreement of image quality was assessed using the kappa coefficient. The potential for unbalancing of agreement in favour of one imaging system was analysed by McNemar’s test or Bowder’s test of symmetry |
ESD (mGy): mean (range) Spine AP Charpak system vs film: 0.08 (0.02–0.19) vs 0.93 (0.47–2.15) Ratio of means: 11.6 Spine PA Charpak system vs film: 0.07 (0.01–0.2) vs 0.92 (0.44–2.14) Ratio of means: 13.1 Spine LAT Charpak system vs film: 0.13 (0.03–0.84) vs 1.96 (0.46–3.43) Ratio of means: 15.1 Pelvis Charpak system vs film: 0.06 (0.01–0.21) vs 1.13 (0.47–7.48) Ratio of means: 18.8 Quality of image Image quality comparison between Charpak system and film Charpak systemFilmGoodPoorNo agreement between assessorsSpineGood6129Poor511No agreement1004PelvisGood4410Poor200No agreement000 For the pelvis imaging, certain criteria were slightly less favourable with the Charpak system, especially for the details of cancellous bone, fine analysis of the cortex and visibility of periarticular fat lines For the spine imaging, there was no significant difference in terms of the quality criteria between the Charpak system and film images. However, the Charpak system images were associated with improved visibility of iliac crests and vertebral pedicles compared with conventional films The Charpak system showed a lack of spatial resolution compared with film Interobserver agreement on image quality Significant disagreements between readers were observed for both X-ray systems |
Charpak system | Film | Good | Poor | No agreement between assessors | Spine | Good | 61 | 2 | 9 | Poor | 5 | 1 | 1 | No agreement | 10 | 0 | 4 | Pelvis | Good | 44 | 1 | 0 | Poor | 2 | 0 | 0 | No agreement | 0 | 0 | 0 | |||||||||||||||||
| Charpak system | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Film | Good | Poor | No agreement between assessors | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spine | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Good | 61 | 2 | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Poor | 5 | 1 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||
| No agreement | 10 | 0 | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Pelvis | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Good | 44 | 1 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Poor | 2 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||
| No agreement | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||
|
Le Bras et al. , 26 Brussels, Belgium and France Type of publication: Unpublished study Funding: European Union through the ‘GROWTH’ programme. Lead author was employed by Biospace Med, manufacturer of EOS device Study design: Comparative study of a diagnostic technology Setting: Outpatient Duration of recruitment: NR |
Inclusion/exclusion criteria: Adolescents who required full spine radiographs for scoliosis detection or follow-up. (No further details were reported) No. recruited: 64 No. analysed: NR Mean age (SD): 14.7 (4.8) years Male (%): 23 (35.9) Disease history: Mean (SD) duration NR |
Intervention: EOS X-ray imaging system. Tube voltage similar to that of the film X-ray. High-contrast spatial resolution was set to 2 lp/mm. Images were viewed on a CRT-screen with a standard DICOM viewer Comparator: Film X-ray imaging. Large screen-film cassette (30 × 90 cm, 5 lp/mm, 400 speed class) with an antiscatter grid (ratio 8 : 1). Films were observed directly on a viewing box No. of patients Intervention: EOS X-ray imaging system No. recruited: 64 No. analysed: Not stated Comparator: Film X-ray imaging No. recruited: 64 No. analysed: Not stated No. of images Intervention: EOS X-ray imaging system No. obtained: 62 PA images and 57 LAT images No. analysed: 44 PA images and 41 LAT images for image quality; 59 PA images and 52 LAT images for radiation dose using ESAK; 46 PA images and 36 LAT images for radiation dose using ESD Comparator: Film X-ray imaging No. obtained: 62 PA images and 57 LAT images No. analysed: 44 PA images and 41 LAT images for image quality; 59 PA images and 52 LAT images for radiation dose using ESAK; 46 PA images and 36 LAT images for radiation dose using ESD |
Outcome measuresNo. of assessors: All images assessed by two radiologists Statistical analyses: The comparative paired scores of image quality were assessed using the non-parametric Wilcoxon test. The interobserver agreement of image quality was assessed using the kappa coefficient |
ESAK (mGy): mean (SD) PA full-spine views EOS vs film: 0.12 (0.03) vs 0.81 (0.24) (p < 0.001) Average dose reduction of 85% LAT full-spine views EOS vs film: 0.19 (0.04) vs 1.67 (0.65) (p < 0.001) Average dose reduction of 89% ESD (mGy): mean (SD) PA full-spine views EOS vs film: 0.23 (0.10) without correction factor applied/0.18 (0.07) with correction factor applied vs 1.2 (0.32) (p < 0.001) Average dose reduction of 85% (with correction factor applied) LAT full-spine views EOS vs film: 0.37 (0.14) without correction factor applied/0.27 (0.10) with correction factor applied vs 2.3 (1.1) (p < 0.001) Average dose reduction of 89% (with correction factor applied) Quality of image Image quality comparison between EOS and film PA images EOS images were significantly better for four criteria (reproduction of vertebral bodies and pedicles, image blackening and image informative contribution) than film images (p < 0.05). For the other three inclusion criteria and the diagnostic contribution criterion, no significant difference was found between EOS and film images (p > 0.05) In terms of reproduction of articular, spinous and transverse processes, one outcome assessor found a significant difference in favour of EOS (p < 0.01), but the second found a non-significant difference (p > 0.05) LAT images EOS images were significantly better on five out of eight quality criteria than film images (p < 0.001). For the diagnostic contribution of coccyx inclusion, no significant difference was found between EOS and film images (p > 0.05) In terms of inclusion of the skull base, one outcome assessor found a significant difference in favour of film (p = 0.05), but the second found a non-significant difference (p > 0.05) Global image quality EOS images had a significantly higher global image quality score for PA and LAT radiographs than film images (p < 0.001) Interobserver agreement on image quality: Film PA images: Kappa coefficient 0.76 < κ < 1 for all criteria (very good agreement) LAT images: Kappa coefficient 0.70 < κ < 1 (good or very good agreement) for all criteria except for lumbar vertebrae reproduction (κ = 0.55; moderate agreement) Interobserver agreement on image quality: EOS PA images: Kappa coefficient 0.60 < κ < 0.70 for seven criteria (good agreement). Kappa values cannot be calculated for two criteria (iliac crests inclusion and diagnostic contribution). κ < 0.1 (very poor agreement) for the criterion of coccyx inclusion and image blackening. The authors state that this is because of the sensitivity of the kappa method when scores are grouped at only one level; agreement was actually > 95% LAT images: Informative contribution criterion (κ = 0.55; moderate agreement); skull base inclusion (κ = 0.27; poor agreement); thoracic spine reproduction (κ = 0.30; poor agreement); for the other four criteria, the kappa values could not be calculated or showed a very poor agreement (kappa coefficients not reported). Again, the authors state that this is due to the sensitivity of the kappa method when scores are grouped at only one level; agreement was actually ≥ 95% All results are taken from the text of the results section, several tables (providing more detailed results) were mentioned in the text, but were missing from the report. Therefore, these figures have not been checked against the tables. Missing tables were 4a and 4b – ESAK values; 5a and 5b – ESD values; 6 and 7 – quality criteria scores; and figure 3 – box plots of global image quality scores. The authors were contacted for these missing data, but did not respond |
||||||||||||||||||||||||||||||||||||||||||||||||
|
Deschênes et al. 2010, 27 Canada Type of publication: Full publication Funding: Biospace Med Study design: Comparative study of a diagnostic technology Setting: Outpatient Duration of recruitment: NR |
Inclusion/exclusion criteria: Patients who required spine radiographs were recruited. The background of the paper states that the study was of patients followed up for scoliosis No. recruited: 50 No. analysed: 49. One patient’s radiographs had to be rejected due to a technical problem during image acquisition Mean age (SD): 14.8 (3.6) years Male (%): 11 (22) Disease history: Mean (SD) duration NR |
Intervention: EOS X-ray imaging system. Distance between sources and detectors is 1.3 m, with patient standing at approximately 1 m from both sources Comparator: Fuji FCR 7501S. Distance between source and imaging plates is 1.83 m, with patient standing approximately 30 cm from the plate PA and sagittal views of the spine were taken, including at least the last cervical vertebra and the pelvis Comparable quality of images was obtained using the same radiograph tube voltage on both systems, while tube currents were selected to match signal–noise ratios on a phantom. On CR, dose was increased with respect to patient’s thickness of the iliac crests (full details were reported) No. of patients Intervention: EOS X-ray imaging system No. recruited: 50 No. analysed: 49 Comparator: CR (Fuji FCR 7501S) No. recruited: 50 No. analysed: 49 No. of images Total no. of images No. obtained: NR No. analysed: n = 196 Intervention: EOS X-ray imaging system No. obtained: NR No. analysed: 98 Comparator: CR (Fuji FCR 7501S) No. obtained: NR No. analysed: 98 |
Outcome measuresNo. of assessors: All images assessed by two orthopaedists and two radiologists. Images were anonymised Statistical analyses: A simple assessment of the comparative paired scores of image quality between EOS and CR was performed. The comparative paired visibility scores were assessed using the non-parametric Wilcoxon test. Interobserver agreement was assessed using an analysis of variance test |
ESD (mGy): mean (range) Nape of the neck EOS vs CR: 0.20 vs 0.59 Ratio of means: 2.9 Centre of the back EOS vs CR: 0.18 vs 1.04 Ratio of means: 5.9 Proximal LAT point EOS vs CR: 0.27 vs 2.38 Ratio of means: 8.8 Outer side of the proximal breast EOS vs CR: 0.11 vs 0.83 Ratio of means: 7.6 Proximal anterosuperior iliac spine EOS vs CR: 0.16 vs 1.47 Ratio of means: 9.2 Proximal iliac crest EOS vs CR: 0.30 vs 2.47 Ratio of means: 8.2 Distal iliac crest EOS vs CR: 0.11 vs 0.73 Ratio of means: 6.5 Quality of image Image quality comparison between EOS and CR system EOS = CREOS > CRCR > EOSGlobal image quality50.5%46.7%2.8%Structures visibility61.9%32.4%5.7% Comparison of visibility scores Compared with CR, the visibility on EOS images is significantly better for all structures in the PA view (p < 0.006) and for all structures in sagittal view (p < 0.037) except for the lumbar spinous process, for which CR has better visibility (p < 0.003) Interobserver agreement on the visibility of structures For PA views, all outcome assessors agreed on the visibility of all structures, except that one assessor disagreed on the lumbar transverse process For sagittal views, all outcome assessors agreed on all structures above the lumbar region. However, results are less consistent for the lumbar region |
EOS = CR | EOS > CR | CR > EOS | Global image quality | 50.5% | 46.7% | 2.8% | Structures visibility | 61.9% | 32.4% | 5.7% | |||||||||||||||||||||||||||||||||||||
| EOS = CR | EOS > CR | CR > EOS | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Global image quality | 50.5% | 46.7% | 2.8% | |||||||||||||||||||||||||||||||||||||||||||||||||
| Structures visibility | 61.9% | 32.4% | 5.7% | |||||||||||||||||||||||||||||||||||||||||||||||||
Appendix 3 Data extraction table: systematic review of the adverse effects of diagnostic radiation for patients with orthopaedic conditions
| Study details | Participant details | Outcomes measured | Results | Comments on quality |
|---|---|---|---|---|
|
Cox 1964 42 Type of publication: Journal article Country of origin: Canada Source of funding: Supported by a research grant allocated by the Province of Ontario under the National Grants Programme Study design: Controlled cohort study Aim of study: To detect any indications of genetic damage from radiation in the offspring of women treated during childhood for congenital dislocation of the hip |
Inclusion/exclusion criteria Cases: Married women who were at least 20 years of age at the onset of the study, who had been patients at The Hospital for Sick Children for congenital dislocation of the hip in 1925 or later were eligible for inclusion. Patients who lived more than 200 miles away from Toronto, or who could not be located or personally consulted were excluded Control subjects: Married male and female siblings of the cases Dates of recruitment: 1925 – NR (although participants had to be at least 20 years of age at the onset of the study) No. recruited: 91 cases and 157 control subjects Age: All cases were aged 20–40 years at the onset of the study (none were aged > 40 years at follow-up) Male (%): 0 cases and 77 (49) control subjects Disease characteristics: Cases: Childhood congenital dislocation of the hip |
Outcome measuresMethods used for collecting dataCases: Personal histories were obtained by interview, usually in the participant’s own home. If the participant had died as an adult, a member of their immediate family was interviewed. Participants were asked similar information about their married siblings (the control group) Control subjects: The control group were interviewed (n = 57) or sent a questionnaire (n = 96) in order to verify and add information about themselves and their families. If the participant had died as an adult, a member of their immediate family responded. Four control group participants were not surveyed Cases and control subjects: Causes of stillbirths and neonatal deaths were confirmed by the office of the Registrar General of the Province of Ontario. Each diagnosis of abnormalities requiring hospitalisation was confirmed by the hospital at which treatment was carried out Statistical analyses: Chi-squared tests were used Length of follow-up: NR |
Cases Mean no. of pelvic X-ray exposures during the course of treatment and follow-up: 37.4 (at age 0–2 years: 8.7, at age 3–7 years: 13.9, at age 8–11 years: 8.1, at age 12–16 years: 6.7) Mean no. of exposures while patient in plaster: 11.4 Mean X-ray dose measured on phantoms: age 6 months AP: 108 mrads, age 4 years AP: 140 mrads, age 12 years AP: 180 mrads Total mean X-ray dose per child: 6.1 rads [5.58 rads (at age 0–2 years: 0.96 rads, at age 3–7 years: 1.95 rads, at age 8–11 years: 1.46 rads, at age 12–16 years: 1.21 rads) + 0.51 rads owing to increase in exposure of average 45 mrads for each exposure through a plaster cast]. The authors state that this estimate is subject to a number of errors and is probably considerably lower than the actual mean dose received No. and type of X-rays received since childhood: 56 cases had received pelvic X-rays prior to conception of their last child; 33 exposures during pregnancy and 58 exposures when not pregnant. The authors estimated the mean adult ovarian radiation dose as 1.4 rads per woman, making the total estimated mean ovarian dose per patient a minimum of 7.5 rads (up to a maximum of 20) Cases and control subjects: Details of pregnancies and offspring: There was no significant difference between cases and control subjects in the number of offspring (201 vs 402) or the proportion of male offspring (49% vs 53%) Stillbirths (at least 28 weeks’ gestation) and neonatal deaths (within 28 days after birth): There was no significant difference between cases and control subjects in the number of stillborn offspring [4 (2%) vs 3 (0.8%); p = 0.34] or neonatal deaths [0 vs 8 (1.9%); p = 0.10] Spontaneous abortions (earlier than 28 weeks’ gestation): There was no significant difference between cases and controls in the number of spontaneous abortions [23 (10.3% of pregnancies) vs 38 (8.6% of pregnancies); p = 0.58] Frequency of abnormal offspring (including stillborn offspring): There was a statistically significant difference in the proportion of offspring with abnormalities between cases and control subjects [26 (12.9%) vs 23 (5.7%); p = 0.004]. There was a statistically significant difference in the proportion of offspring with more severe abnormalities (i.e. those requiring hospitalisation, and excluding hernia) [15 (7.5%) vs 10 (2.5%); p = 0.008] The congenital abnormalities requiring hospitalisation for offspring of cases were: anencephalus, hydrocephalus, Down syndrome, intestinal atresia, harelip and cleft palate, haemangioma of scrotum, facial pigmented naevus, cavernous plantar haemangioma, shoulder and abdominal haemangiomata, Duchenne muscular dystrophy, torticollis, undescended testes, bilateral clubfoot, bilateral nerve deafness (in two siblings). Abnormalities not requiring hospitalisation for offspring of cases were: inguinal hernia (n = 4), umbilical hernia, inguinal hernia and umbilical hernia, strabismus, flexion deformity of toe, overlapping toes, haemangioma (n = 2) The congenital abnormalities requiring hospitalisation for offspring of control subjects were: anencephalus (n = 2), hydrocephalus (n = 2), spina bifida, pyloric stenosis (n = 2), dermoid cyst of orbit, congenital heart disease, tracheo-oesophageal fistula with immaturity. Abnormalities not requiring hospitalisation for offspring of control subjects were: inguinal hernia (n = 3), umbilical hernia, epigastric hernia, hernia unspecified, strabismus (n = 4), shortening of leg, bilateral tibial torsion, metatarsus varus Birthweight: Mean birthweight for male offspring was lower for cases than control subjects (3175 g vs 3320 g; p > 0.025). However, when birthweights were compared within birth orders, there were no significant differences. Mean birthweight for male offspring was lower than the Ontario population mean birthweight using birth data from 1960 (3385 g; p < 0.001) There was no significant difference in mean birthweight for female offspring between cases and control subjects (3149 g vs 3212 g), or the Ontario population mean birthweight (3255 g) Authors’ conclusions: The frequencies of stillbirths, infant deaths and spontaneous abortions were similar for irradiated mothers and control subjects. The proportion of males tended to be lower among the offspring of cases than among offspring of control subjects. The frequency of abnormal offspring was significantly higher among the exposed mothers. The mean birthweights of offspring, particularly males, appeared to be lower for the offspring of exposed mothers than of control subjects |
The estimate of total mean X-ray dose is unlikely to be a reliable estimate; the authors acknowledge that it is subject to a number of errors The majority of cases had received pelvic X-rays prior to conception of their last child; including 33 exposures during pregnancy, which may have had an impact on pregnancies and offspring Details of pregnancies and offspring were obtained by personal interview/questionnaire, which may be subject to recall bias. The authors acknowledge that information on spontaneous abortion is unlikely to be accurate; early miscarriage may have been forgotten or unrecognised. However, causes of stillbirths and neonatal deaths and diagnosis of abnormalities requiring hospitalisation were confirmed objectively Using siblings as control subjects appears to be appropriate, as they share a greater similarity in social, economic and genetic background than unrelated controls subjects Other factors that may have influenced birthweight and congenital abnormalities were not reported, such as illness/injury during pregnancy, pre-term birth, family history, etc. The authors do not report the reasons for the 33 X-ray exposures amongst the cases during pregnancy |
|
Goldberg et al. 1998 43 Type of publication: Journal article Country of origin: Canada Sources of funding: Atomic Energy Control Board of Canada, Université de Montréal, and Le Fonds de la recherché en santé du Québec (FRSQ) Study design: Controlled retrospective cohort study Aim of study: To assess the association between exposure to low-dose ionising radiation from diagnostic radiography received in adolescence and subsequent adverse reproductive outcomes in adulthood |
Inclusion/exclusion criteria Cases: Female patients included in the Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study were eligible for inclusion. The Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study included 2092 children and young adults referred to Ste-Justine Hospital, Montreal, for the diagnosis and management of adolescent idiopathic scoliosis. Of the 1793 females included, the authors were able to trace 88.8%, of which 80.3% returned their questionnaires (1292) Control subjects: 1134 women selected randomly from the general population, identified using residential, non-confidential telephone numbers. Control subjects were approximately frequency-matched to cases according to age and general area of residence Dates of recruitment: Cases were recruited to the Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study from 1960 to 1979. Dates of recruitment of the control group are not stated No. recruited: 1292 cases and 1134 control subjects Age: 15 to > 45 years. The majority of patients were aged 25–39 years Male (%): 0 Disease characteristics: Cases: adolescent idiopathic scoliosis |
Outcome measures Methods used for collecting data Statistical analyses: The authors used logistic regression to analyse unsuccessful attempts at pregnancy. Other binary pregnancy outcomes were analysed using logistic regression that accounted for the clustered nature of data through the Generalised Estimating Equations GEE framework, as clustering can occur from women having multiple pregnancies, with the consequence that multiple adverse outcomes are positively correlated Analyses were conducted using cumulative ovarian dose as a continuous linear variable, and according to quartiles. Analyses were conducted using the control group as a baseline category (no radiation exposure in adolescence from scoliosis), and excluding the control group, but comparing within the cases between levels of dose. GEE was used to analyse birthweight as a continuous variable, assuming a Gaussian error structure. Sensitivity analyses were conducted to verify assumptions about cut-points and linearity Sixteen pairs of twins were excluded from the analyses (because of their lower birthweight) Covariates included in the final models were those variables found from univariate logistic regression analysis to be associated with each of the outcomes under consideration (e.g. education, alcohol consumption, smoking status, body mass index and occupation) Length of follow-up: NR |
Cases and control subjects were fairly evenly matched in terms of education, marital status, alcohol consumption, self-perception of health, body mass index and physical recreational activity. A higher proportion of cases lived in Montreal, fewer cases were aged 15–24 years, more cases were aged 30–34 years, and there was a higher proportion of ‘never smokers’ among the cases Organ-specific radiation dose: The mean dose to the ovaries was 0.925 (SD 0.760) cGy Reproductive outcomes: Difference between cases and controls in the number of: Unsuccessful attempts at pregnancy 49 (3.8%) vs 32 (2.8%) adjusted OR 1.33, 95% CI 0.84 to 2.13 Stillbirths 6 (0.5%) vs 19 (1.5%) adjusted OR 0.38, 95% CI 0.15 to 0.97 Low-birthweight infants 74 (6.4%) vs 94 (7.6%) adjusted OR 0.84, 95% CI 0.59 to 1.21 Infants with congenital malformations 47 (4.0%) vs 36 (2.9%) adjusted OR 1.20, 95% CI 0.78 to 1.84 Spontaneous abortions 209 (12.8%) vs 158 (9.7%) adjusted OR 1.35, 95% CI 1.06 to 1.73 Subgroup analysis (quartiles of dose, cGy): When comparing adolescent idiopathic scoliosis patients at higher organ-specific doses to those in the lowest dose group (0–0.312 cGy), none of the reproductive outcomes was significantly different between groups. However, the outcome low birthweight (< 2500 g) almost reached statistical significance when the highest-dose group (≥ 1.444 cGy) was compared with the lowest-dose group: 33 (8.5%) vs 9 (3.6%); adjusted OR 2.34, 95% CI 1.0 to 5.6 Authors’ conclusions: Associations between adverse reproductive outcomes and radiotherapy have been observed previously, but this is the first study in which an association with birthweight has been found with diagnostic radiography |
This was a large cohort study; however, some of the events were rare (such as stillbirth) Details of pregnancies and offspring were obtained by postal questionnaire, which may be subject to recall bias. None of the responses on reproductive outcomes was validated objectively. The authors acknowledge that this study is open to errors in recall, in particular information on spontaneous abortion is unlikely to be accurate; early miscarriage may have been forgotten or unrecognised The authors presented the results as adjusted ORs, with no indication of which results were statistically significant, and which were likely to be because of chance The authors also acknowledge that other factors may have affected reproductive outcomes, such as age of the mother and smoking during pregnancy The authors acknowledge that chance or some undetected bias in selecting persons into the control group may account for the fact that they observed a dose–response relationship in the adolescent idiopathic scoliosis group for low birthweight, but there was a lower proportion of low-birthweight infants in this group than in the control group. Other factors may have been involved, such as medical conditions during pregnancy, gestational age or sex of the infant |
|
US Scoliosis Cohort Study (pilot) 1989 34 Type of publication: Journal article Country of origin: USA Sources of funding: Public Health Service contracts from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; the Scoliosis Research Society; the Twin Cities Scoliosis Research Fund; and the Medical Education and Research Foundation of Gillette Children’s Hospital, St Paul, MN Study design: Uncontrolled retrospective cohort study Aim of study: To determine whether or not X-ray exposures during scoliosis treatment in the past might be associated with a detectable risk of breast cancer |
Inclusion/exclusion criteria: Women with a confirmed diagnosis of scoliosis or kyphosis who were seen at one of four medical facilities in the Minneapolis-St Paul, MN area. Patients were excluded if they were diagnosed after 1965, were aged > 19 years at diagnosis, survived < 3 years after diagnosis, or had a history of cancer or radiotherapy Dates of recruitment: 1935–65 (year of diagnosis 1922–65) No. recruited: 1030, of which 856 responded to the questionnaire/telephone interview (either in person (818), or a surrogate response was received for deceased patients (38); 973 patients were included in the analyses, as 51 patients could not be located, and dates of radiographs were missing for six patients Age: Mean age at follow-up was 41.4 years Male (%): 0 Disease characteristics: 60% of participants had idiopathic scoliosis |
Outcome measuresMethods used for collecting dataStatistical analyses: Person-years of follow-up began 3 years after the date of the first X-ray exposure or scoliosis diagnosis and ended at the date of breast cancer diagnosis, death or date of last known vital status. Expected numbers of breast cancers were calculated by multiplying age-, sex- and calendar time-specific breast cancer incidence rates from the Connecticut Tumor Registry by the appropriate person-years of follow-up. The SIR (the ratio of observed cases to expected cases) was calculated, with 90% CIs. Tests of trend of increasing SIR with time and dose were performed by applying the multiplicative models of Breslow et al. a Tests were one-sided Length of follow-up: The average length of follow-up for the 973 patients with usable follow-up information was 25.6 years |
Radiation dose estimation: The average number of radiographs taken per patient was 41.5 (range 0–618) and were given over an average of 8.7 years. Among the 951 women for whom a dose of radiation to the breast could be estimated, the average dose was 12.8 rad (range 0–159 rad). Average doses to the thyroid and active bone marrow were 6.9 rad and 3.3 rad, respectively Observed and expected breast cancers: The proportion of patients who had a history of breast cancer was higher than the number expected (11 vs 6 expected cases; SIR 1.82, 90% CI 1.0 to 3.0) Subgroup analyses: When examining the number of cases of breast cancer by age, time since first radiograph, and radiation exposure, there was a higher incidence of breast cancer than the expected incidence, for patients aged ≥ 15 years at the time of their first radiograph (SIR 3.1, 90% CI 1.4 to 6.2), patients for whom time since first radiograph was 30 years or more [SIR 2.4 (90% CI 0.9 to 5.0) trend for increased risk with time p = 0.02], patients who received a total of 30 or 60 radiographs or more (SIRs 2.0, 90% CI 0.07 to 4.7 and 3.1, 90% CI 1.1 to 7.1 respectively) and patients who had a radiation dose to the breast of 20 rad or more (SIR 3.4, 90% CI 1.2 to 7.8) (trend for increased risk with increased dose p = 0.08) No patients were diagnosed with breast cancer within 15 years of their first radiograph, which was expected. Risk of breast cancer increased with increasing radiation dose to the breast within both the group of women who had had a full-term pregnancy, and the group of women who had not. Patients with more severe scoliosis were less likely to have had a full-term pregnancy Authors’ conclusions: These data suggest that frequent exposure to low-level diagnostic radiation during childhood or adolescence may increase the risk of breast cancer |
This was a large cohort study; however, there were only 11 cases of breast cancer. The authors acknowledge that their findings require confirmation by larger studies The authors acknowledge that the radiation dose estimation may be subject to error Other factors that may have influenced breast cancer incidence were not adjusted for, such as age at menarche, history of benign breast disease and family history of breast cancer The authors acknowledge that factors associated with severe scoliosis, such as inability to carry a pregnancy to term, might influence the results, since nulliparous women are at higher risk for breast cancer. Therefore, the observed association between higher number of radiographs (more common for more severe scoliosis) and breast cancer may have been influenced by this risk factor |
|
US Scoliosis Cohort Study 2000 35 Type of publication: Journal article Country of origin: USA Source of funding: The National Cancer Institute, US Public Health Service, Bethesda, MD Study design: Uncontrolled retrospective cohort study Aim of study: To evaluate patterns in breast cancer mortality among women with scoliosis, with special emphasis on risk associated with diagnostic radiograph exposures |
Inclusion/exclusion criteria: Women with a confirmed diagnosis of scoliosis, kyphosis, lordosis or kyphoscoliosis, who were seen at any of 14 large orthopaedic medical centres in the USA (including those patients enrolled in the pilot study). 34 161 patients with congenital scoliosis were included in the pilot study; however, no additional patients with congenital scoliosis were enrolled in this study. Exclusion criteria included patients who were diagnosed after 1965, were > 19 years of age at diagnosis, or had a history of cancer or radiotherapy or other characteristics that could have been associated with multiple radiograph exposures at other institutions Dates of recruitment: Not stated (year of diagnosis 1912–65) No. recruited: 5573, of which vital status was determinable for 4971 patients. 5466 patients were included in the subgroup analyses, as 34 patients contributed no woman-years of follow-up, 18 patients had missing exit dates and 55 were known to have died but the cause of death was unknown Age: Mean age at follow-up was 51 (range 2–89) years Male (%): 0 Disease characteristics: The vast majority of patients had scoliosis (92.7%). Around half of patients (49.2%) had idiopathic disease. Most patients were diagnosed at the age of 10 years or above (62.7%) |
Outcome measuresMethods used for collecting dataStatistical analyses: Person-years of follow-up began at the date of scoliosis diagnosis for patients from the 10 expanded study centres and 3 years after scoliosis diagnosis for the pilot study patients. Follow-up ended at the date of death, date of last known vital status or 1 January 1997 Expected numbers of deaths, by cause, were calculated by multiplying the age- and calendar-specific woman-years at risk, in 5-year intervals, by the corresponding mortality rates in the general population. SMRs were calculated by dividing the number of observed deaths by the number of deaths expected Exact and asymptotic methods were used to calculate 95% CIs and statistical significance levels for SMRs, RRs, and tests for non-homogeneity and trend among different levels of factor Length of follow-up: The average length of follow-up was 40.5 years |
Radiation dose estimation: The total number of radiographs recorded was 137,711. Most X-rays (77.3%) were of the spine and approximately 64% were AP. The average number of radiographs taken per patient was 24.7 (range 0–618). The average estimated cumulative dose to the breast per patient was 10.8 (range 0–170) cGy Mortality rates: 985/4971 patients (20%) were confirmed deceased with death certificate, 61 (1%) were presumed deceased with cause of death unknown There was a statistically significant increase in the risk of dying of all causes for patients with scoliosis, compared with the general population (SMR 1.71, 95% CI 1.6 to 1.8), primarily of infectious, circulatory, respiratory and musculoskeletal conditions There was a statistically significant increase in the risk of dying of breast cancer for patients with scoliosis, compared with the general population (77 vs 45.6 expected deaths; SMR 1.69, 95% CI 1.3 to 2.1) The risk of dying of leukaemia or lung cancer were not significantly different between patients with scoliosis and the general population (SMR 1.21, 95% CI 0.6 to 2.3; nine cases and SMR 0.73, 95% CI 0.5 to 1.1; 29 cases, respectively) Significant dose response relationships were observed for deaths from infectious, circulatory, respiratory, digestive and musculoskeletal conditions Subgroup analyses: Breast cancer deaths by scoliosis characteristics: There was a statistically significantly higher risk of dying of breast cancer, compared with the expected number of deaths, for patients aged ≥ 10 years at the time of diagnosis (SMR 2.01, 95% CI 1.5 to 2.6), patients diagnosed between 1940 and 1959 (SMR 2.35, 95% CI 1.6 to 3.3), patients with neuromuscular scoliosis (SMR 2.09, 95% CI 1.4 to 3.1) or unknown aetiology (SMR 2.61, 95% CI 1.1 to 5.1), patients with a maximum curve magnitude of 30–59 °(SMR 2.29, 95% CI 1.3 to 3.8) or unknown magnitude (SMR 1.55, 95% CI 1.2 to 2.0), patients who had surgery (SMR 2.52, 95% CI 1.7 to 3.6) and patients who had a higher number of surgeries (two surgeries: SMR 2.79, 95% CI 1.4 to 5.0, three surgeries: SMR 3.83, 95% CI 1.7 to 7.5) Statistical tests for trend when adjusted for radiation dose were only statistically significant for age at scoliosis diagnosis, p = 0.02 Breast cancer deaths by radiation exposure characteristics: There was a statistically significantly higher risk of dying of breast cancer, compared with the expected number of deaths, for patients with a higher number of radiographs, particularly patients receiving ≥ 50 radiographs (SMR 3.86, 95% CI 1.9 to 6.9), patients with a higher cumulative radiation dose to the breast, particularly patients with a cumulative dose of ≥ 20 cGy (SMR 3.36, 95% CI 2.0 to 5.3), those aged 10–13 years at the time of their first radiograph (age 10–11 years, SMR 3.36, 95% CI 2.1 to 5.1; age 12–13 years, SMR 1.85, 95% CI 1.2 to 2.8), those with a longer time since their first radiograph (30–39 years, SMR 2.43, 95% CI 1.6 to 3.6; ≥ 40 years, SMR 2.07, 95% CI 1.5 to 2.8) and those who were older at study exit (45–49 years, SMR 2.19, 95% CI 1.2 to 3.6; ≥ 50 years, SMR 1.74, 95% CI 1.3 to 2.3) Statistical tests for trend when adjusted for radiation dose were only statistically significant for age at first radiographic examination (p = 0.01) Authors’ conclusions: These data suggest that exposure to multiple diagnostic radiographic examinations during childhood and adolescence may increase the risk of breast cancer among women with scoliosis; however, potential confounding between radiation dose and severity of disease and thus with reproductive history may explain some of the increased risk observed |
|
|
US Scoliosis Cohort Study 2008 40 Type of publication: Journal article Country of origin: USA Source of funding: Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics Study design: Uncontrolled retrospective cohort study Aim of study: To quantify the radiation dose–response relationship for fractionated exposures at a vulnerable age, assess whether or not known breast cancer risk factors modify dose response, and explore possible developmental intervals of increased radiation sensitivity |
Inclusion/exclusion criteria: US Scoliosis Cohort Study patients (see above)35 Of the 5573 eligible patients, 19% were lost to follow-up and 16% were deceased. The authors contacted 3620 (65%) patients; 3121 patients (86%) participated in the health survey; 6% refused, 4% were unable to participate owing to illness, language problems or other reasons and 4% did not respond. An additional 111 patients were excluded because they had congenital scoliosis, so were likely to have had radiographic examinations for concomitant medical conditions in other hospitals Dates of recruitment: Not stated (year of diagnosis 1912–65) No. recruited: 3010 female patients with scoliosis (analysis cohort) Age: Mean age at follow-up was 51 (range 30–84) years Male (%): 0 Disease characteristics: 59% patients had idiopathic scoliosis. Mean age at scoliosis diagnosis was 11 (range 0–19) years |
Outcome measuresMethods used for collecting dataStatistical analyses: Woman-years of follow-up began at the date of scoliosis diagnosis until the date of first breast cancer diagnosis or survey completion. All woman-years were cross-classified by time-dependent variables for age, total breast dose, and by breast cancer risk factors and scoliosis characteristics. ERR per unit dose was calculated. Subgroup analyses were used to assess whether or not the dose response differed according to specific epidemiological characteristics (breast cancer risk factors). Enhanced sensitivity to radiation according to breast development stage (before breast budding, between breast budding and menarche, between menarche and birth of a first child and after birth of a first child) was also assessed. Results were presented as RRs with 95% CIs Length of follow-up: The mean length of follow-up was 39.5 (range 13–68) years |
Radiation dose estimation: The mean number of breast-exposed radiographs taken per patient was 26.8 (range 0–332). The mean estimated cumulative dose to the breast per patient was 12.1 (range 0–111) cGy Breast cancer: 88 women reported breast cancer and one woman reported a non-defined cancer; invasive breast cancer was confirmed for 68 women. Eleven women had a confirmed diagnosis of in situ breast cancer, which was not included in most of the analyses; 78 confirmed or non-denied invasive breast cancers were included in the analyses Compared with patients who received 1–9 radiographs (mean total dose 3 cGy), patients who received ≥ 60 radiographs (mean total dose 33.5 cGy) had a statistically significantly higher risk of breast cancer (RR 3.14, 95% CI 1.33 to 7.44). p-value for trend for total number of radiographs = 0.12 Compared with patients who had a baby when aged < 25 years, patients who had no children, or had children aged ≥ 35 years had a statistically significantly higher risk of breast cancer (RR 2.13, 95% CI 1.21 to 3.75 and RR 3.02, 95% CI 1.03 to 8.87, respectively). p-value for trend for age at first live birth = 0.03 Postmenopausal women were at a significantly higher risk than pre-menopausal women (RR 3.13, 95% CI 1.38 to 7.09). p-value for trend = 0.004 Women with an annual household income of ≥ US$60,000 were at a significantly higher risk than women with a household income < US$30,000 (RR 2.84, 95% CI 1.52 to 5.30). p-value for trend for household income = 0.003 Women with a second-degree relative affected by breast cancer were at a significantly higher risk than women with no known family history of breast cancer (RR 2.71, 95% CI 1.57 to 4.66). p-value for trend for family history of breast cancer = 0.008 Women with three to five relatives with breast cancer were at the highest risk (RR 5.65, 95% CI 1.73 to 18.5), while women with one or two relatives with breast cancer were also at a significantly higher risk than those with no known relatives with breast cancer (RR 2.12, 95% CI 1.32 to 3.41). p-value for trend for number of relatives with breast cancer = 0.0003 Women with a family history of early-onset breast cancer (diagnosed before the age of 50 years) were at a significantly higher risk than women with no known family history of early-onset breast cancer (RR 2.84, 95% CI 1.10 to 6.03). p-value for trend for family history of early-onset breast cancer = 0.03 There were no statistically significant differences associated with curve magnitude, parity, education level or reported alcohol use or smoking status. The authors report that risk was not related to age at menarche, oral contraceptive use or hormone replacement therapy (data not shown) Adjustment for age at birth of first child, menopausal status at questionnaire completion, household income and family history of breast cancer significantly improved the statistical fit of the model; therefore, these factors were included as additional baseline term covariates in all subsequent analyses Compared with patients with breast doses of < 10 cGy, those with doses of 20–29 or ≥ 30 cGy had a statistically significant double risk of breast cancer. The radiation dose response for breast cancer was statistically significantly modified by any family history of breast cancer (p = 0.03): ERR/Gy = 8.37 (95% CI 1.50 to 28.16) There was no evidence of variation in the risk of breast cancer when assessing subgroups according to breast development stage |
This was a very large cohort study; although there were still only 78 confirmed or non-denied cases of breast cancer The authors acknowledge that the estimate of cumulative radiation dose to the breast may be subject to error The authors also acknowledge that breast cancer rates among patients with scoliosis may be higher than the general population, owing to risk factors other than radiation exposure (such as reproductive characteristics) The authors also acknowledge the potential for bias, when relying on self-report for breast cancer and family history of breast cancer. They state that the risks associated with family history of breast cancer may be overestimated in the study, as patients with breast cancer are more likely to report complete family histories of breast cancer, than women without breast cancer |
|
US Scoliosis Cohort Study 2010 41 Type of publication: Journal article Country of origin: USA Source of funding: Intramural Research Program of the National Institutes for Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics Study design: Uncontrolled retrospective cohort study Aim of study: To describe the spectrum of cancer mortality after an average follow-up of 47 years, 8 years longer than the earlier report. 35 In addition, to evaluate risks for all cancers and assess potential confounding |
Inclusion/exclusion criteria: US Scoliosis Cohort Study patients (see above)35 Of the 5573 eligible patients, the authors were able to determine vital status for 5513 (99%) Dates of recruitment: Not stated (year of diagnosis 1912–65) No. recruited: 5573, of which vital status was determinable for 5513 patients Risk of dying from cancer was assessed for the subgroup of 3121 women who completed the health survey in the previous study40 Age: Mean age at follow-up was 58 (range 2.1–96.5) years Male (%): 0 Disease characteristics: The mean age at curvature diagnosis was 10.6 (range 0–19.9) years |
Outcome measuresMethods used for collecting data:Statistical analyses: Woman-years of follow-up began at the date of curvature diagnosis and ended at the date of death, date of last known vital status or 31 December 2004 Expected numbers of deaths, by cause, were calculated by multiplying the age- and calendar year-specific woman-years at risk, in 5-year intervals, by the corresponding mortality rates in the general population. SMRs were calculated by dividing the number of observed deaths by the number of deaths expected. Breast doses were lagged 10 years before cancer diagnosis for cases and study exit for non-cases to allow for latency RRs for breast cancer mortality and lung cancer mortality according to spinal curvature history were estimated using a Cox proportional hazards model with age as the time scale. ERR per unit dose was calculated Length of follow-up: The mean length of follow-up was 46.9 years |
Radiation dose estimation: The total number of radiographs recorded was 137,711. The average number of radiographs taken per patient, that included exposure to the breast was 22.9 (range 0–553). The average estimated cumulative dose to the breast per patient was 10.9 cGy (maximum 170 cGy). The average estimated cumulative dose to the lung per patient was 4.1 cGy (maximum 67.6 cGy). The average estimated cumulative dose to the active bone marrow per patient was 1.0 cGy (maximum 16 cGy). The average estimated cumulative dose to the thyroid gland per patient was 7.4 cGy (maximum 137 cGy). The average estimated cumulative dose to the ovary per patient was 2.7 cGy (maximum 33.7 cGy) Cancer mortality rates: 1527/5513 patients (28%) were dead, 3614 (66%) were alive and 372 (7%) were lost to follow-up There was a statistically significant increase in the risk of dying of all causes for patients with curvature, compared with the general population (SMR 1.46, 95% CI 1.39 to 1.54) There were a total of 355 cancer deaths amongst the curvature patients, which was not significantly different to that of the general population (SMR 1.08, 95% CI 0.97 to 1.20) Breast cancer was the only cancer where there was a statistically significant increase in risk amongst curvature patients, compared with the general population (SMR 1.68, 95% CI 1.38 to 2.02). There were 112 deaths from breast cancer Other cancer sites where risk was increased (though not statistically significantly) were:Bladder SMR 1.34, 95% CI 0.36 to 3.42 Brain and CNS SMR 1.48, 95% CI 0.81 to 2.48 There were significantly fewer deaths from liver and cervical cancer among the curvature patients, compared with the general population (SMR 0.17, 95% CI 0.00 to 0.94 and SMR 0.31, 95% CI 0.06 to 0.92, respectively); however, these were based on very small numbers of deaths of these cancers (one and three, respectively). The number of patients dying of lung cancer was lower than the general population (57 patients, SMR 0.77, 95% CI 0.59 to 1.00), although this result was not statistically significant. The authors state that these types of cancer are smoking related Subgroup analyses: Risk of death from breast cancer did not vary significantly by age at curvature diagnosis, type of curvature, aetiology, maximum curve magnitude or number of spinal surgeries. However, there was an increase in risk of dying from breast cancer amongst patients who received ≥ 50 radiographs (involving exposure to the breasts), compared with those receiving < 25 radiographs (RR 2.7, 95% CI 1.3 to 5.5). Patients with a cumulative breast dose of ≥ 30 cGy had a statistically significantly higher risk of dying of breast cancer than those with a cumulative dose of 0–9 cGy (RR 2.4, 95% CI 1.2 to 4.8). p-value for trend = 0.001. ERR/Gy = 3.9 (95% CI 1.0 to 9.3) Among the subgroup of 3121 patients who responded to the health survey in the previous study,40 30 patients died of breast cancer and 17 patients died of lung cancer between 1993 and 2004. Results of subgroup analyses were broadly similar to results for the entire cohort. Risk of lung cancer was strongly associated with cigarette smoking and alcohol use, but not with scoliosis characteristics or with category of estimated lung dose Authors’ conclusions: Women who were diagnosed with scoliosis before 1965 have increased risk of breast cancer, clearly related to radiation exposure from diagnostic radiographs during the period 1920–80, when doses were much higher than they are today. Mortality rates from cancers other than breast cancer were lower than expected |
This was a very large cohort study, although numbers of patients dying from many of the cancers assessed were very low The estimate of cumulative radiation dose to the breast may be subject to error The authors acknowledge that breast cancer rates among scoliosis patients may be higher than the general population, owing to risk factors other than radiation exposure (such as reproductive characteristics) This study only assessed cancer mortality rates, not cancer incidence rates; other characteristics of curvature patients may affect their eligibility for/response to treatment, which may impact on survival rates. The authors acknowledge that by relying on cancer mortality data, it was not feasible to study cancers with low lethality, such as thyroid cancer |
Appendix 4 Table of excluded studies with rationale: systematic review of the clinical effectiveness of EOS
| Study details | Reason for exclusion | Further detail |
|---|---|---|
| ClinicalTrials.gov: NCT0092643264 | Not controlled study | Ongoing study – currently recruiting participants |
| ClinicalTrials.gov: NCT0108703465 | Not conventional X-ray control | Ongoing study – currently recruiting participants |
| Biospace Med66 | Not controlled study | PowerPoint slides on number of EOS examinations undertaken |
| Food and Drug Administration (2007)67 | Not controlled study | FDA Marketing Authorisation – not a study |
| Biospace Med68 | Not controlled study | Overview of EOS – not a study |
| Alison (2009)69 | Not controlled study | Presentation on examination time for EOS |
| Assi (2007)70 | Not conventional X-ray control | Feasibility study for 3D X-ray reconstruction in patients with cerebral palsy |
| Aubin (1997)71 | Not EOS | Not EOS |
| Azmy (2010)72 | Not orthopaedic patients | Cadaver specimens. Assessing 3D reconstruction |
| Barthe (2004)73 | Not orthopaedic patients | Rats |
| Baru (1998)74 | Not EOS | Not EOS |
| Benameur (2005)75 | Not EOS | Not EOS |
| Benameur (2005)76 | Not conventional X-ray control | Assessment of 3D modelling, rather than EOS |
| Benameur (2001)77 | Not conventional X-ray control | Assessment of 3D modelling, rather than EOS |
| Benameur (2003)78 | Not conventional X-ray control | Assessment of 3D modelling, rather than EOS |
| Bertrand (2005)79 | Not orthopaedic patients | Asymptomatic volunteers. Assessing intra- and interobserver agreement for 3D reconstruction of rib cage |
| Bertrand (2008)80 | Not orthopaedic patients | Duplicate report of the above study |
| Billuart (2008)81 | Not orthopaedic patients | Cadaveric specimens. Not a controlled study |
| Breton (2010)82 | Not orthopaedic patients | Dry femurs. Assessing accuracy of femur length measurement, interobserver agreement and radiation dose |
| Chaibi (2010)83 | Not conventional X-ray control | Healthy volunteers and cadavers. Comparing 3D EOS models with CT |
| Chaibi (2010)84 | Not conventional X-ray control | French PhD thesis – above study is part of this |
| Charpak (2005)85 | Not controlled study | Discussion – not a study |
| Chateil (2005)86 | Not controlled study | Discussion – not a study |
| Cheriet (2007)87 | Not EOS | Not EOS |
| Comité d’Évaluation et de Diffusion des Innovations Technologiques (CEDIT) (1996)88 | Not controlled study | CEDIT recommendations – not a study |
| Comité d’Évaluation et de Diffusion des Innovations Technologiques (CEDIT) (2007)45 | Not controlled study | CEDIT recommendations – not a study |
| Cresson (2010)89 | Not orthopaedic patients | Assessment of 3D reconstruction (EOS vs CT). Dry bones – six femurs |
| Cresson (2009)90 | Not conventional X-ray control | Assessment of 3D reconstruction using CT as control |
| de la Simone (2010)91 | Not controlled study | Overview of EOS – not a study |
| Deschênes92 | Not controlled study | PowerPoint slides discussing studies we had already identified |
| Deschênes (2009)93 | Duplicate publication (abstract for included study) | Duplicate publication |
| Deschênes (2003)94 | Not conventional X-ray control | Assessment of 3D reconstruction. Not a controlled study |
| Despres (2005)95 | Not conventional X-ray control | Not a controlled study |
| Douglas (2008)96 | Not EOS | Not EOS |
| Douglas (2004)97 | Not EOS | Not EOS |
| Dubousset98 | Not conventional X-ray control | Case study |
| Dubousset (2005)99 | Not controlled study | Overview discussing patients from studies we had already identified |
| Dubousset (2005)100 | Not controlled study | Duplicate report of above study |
| Dubousset (2008)101 | Not controlled study | Description of the technology – not a study |
| Dubousset (2010)102 | Not controlled study | Overview – discusses patients from studies we had already identified |
| Dubousset (2007)103 | Not controlled study | Description of the technology |
| Dumas (2008)104 | Not EOS | Not EOS |
| Dumas (2004)105 | Not orthopaedic patients | Dried vertebrae |
| Dumas (2003)106 | Not EOS | Not EOS |
| Dumas (2002)107 | Not conventional X-ray control | Assessing 3D reconstruction – not clear if EOS |
| Dumas (2003)108 | Not conventional X-ray control | Assessing 3D reconstruction – not clear if EOS |
| Dumas (2005)109 | Not orthopaedic patients | Healthy volunteers. Assessment of 3D reconstruction using EOS, rather than assessment of EOS |
| Gangnet (2006)110 | Not orthopaedic patients | Not EOS. Assessing 3D reconstruction using healthy volunteers |
| Gangnet (2003)111 | Not EOS | Not EOS |
| Gille (2007)112 | Not EOS | Not EOS |
| Glard (2008)113 | Not EOS | Not EOS |
| Glard (2009)114 | Not EOS | Not EOS |
| Guenoun (2010)115 | Not conventional X-ray control | PowerPoint slides. Describes study of EOS vs pangonogram in preoperative assessment of total hip arthroplasty |
| Hascall (2002)116 | Not EOS | Not EOS |
| Humbert (2008)117 | Not orthopaedic patients | EOS vs CT in three sets of bones from cadavers |
| Humbert (2009)118 | Not conventional X-ray control | Controlled part of the study used CT scan |
| Humbert (2008)119 | Not conventional X-ray control | Controlled part of the study used CT scan |
| Illes (2010)120 | Not controlled study | Case study |
| Illes (2010)121 | Not conventional X-ray control | Before and after X-rays, no control |
| Illes (2011)122 | Not conventional X-ray control | Before and after X-rays, no control. Case study |
| Janssen (2009)123 | Not orthopaedic patients | Healthy volunteers. No control |
| Jolivet (2010)124 | Not conventional X-ray control | CT control. Healthy volunteers |
| Journe (2010)125 | Not orthopaedic patients | Dry bones. CT control |
| Kadoury (2008)126 | Not EOS | Not EOS |
| Kadoury (2009)127 | Not EOS | Not EOS |
| Kalifa (1996)128 | Not controlled study | Editorial – not a study |
| Lafage (2002)129 | Not EOS | Not EOS |
| Laporte (2004)130 | Not EOS | Not EOS |
| Laporte (2002)131 | Not EOS | Not EOS |
| Laville (2009)132 | Not orthopaedic patients | Cadavers. No control |
| Lazennec133 | Not conventional X-ray control | CT control. Case study |
| Le Bras (2003)134 | Not orthopaedic patients | EOS vs CT in dry bones |
| Le Bras (2004)135 | Not orthopaedic patients | EOS vs CT in dry bones. Includes most of same patients as above |
| Le Bras (2002)136 | Not orthopaedic patients | EOS vs CT in dry bones |
| Le Bras (2003)137 | Not orthopaedic patients | EOS vs CT in dry bones |
| Mitton (2007)1 | Not orthopaedic patients | EOS vs CT in dry bones |
| Mitton (2006)138 | Not orthopaedic patients | EOS vs CT in dry bones |
| Mitton (2000)139 | Not EOS | Not EOS |
| Mitulescu (2002)140 | Not EOS | Not EOS |
| NICE2 | Not controlled study | Information from manufacturer – not a study |
| Ngoc Hoan (1979)141 | Not EOS | Not EOS |
| Node-Langlois (2003)142 | Not EOS | Not EOS |
| Novosad (2002)143 | Not EOS | Not EOS |
| Obeid144 | Not conventional X-ray control | Case study |
| Ohl (2010)145 | Not orthopaedic patients | Healthy volunteers. No control |
| Pomero (2003)146 | Not conventional X-ray control | Not EOS. Dry bones. No control |
| Pomero (2004)147 | Not conventional X-ray control | Not sure if EOS. CT control |
| Rillardon (2005)148 | Not conventional X-ray control | EOS vs MRI on discs (not live patients) |
| Rousseau (2007)149 | Not orthopaedic patients | Healthy volunteers. No control |
| Sabourin (2010)150 | Not conventional X-ray control | EOS vs CT |
| Sandoz (2008)151 | Not orthopaedic patients | Healthy volunteers. No control |
| Sapin De Brosses (2010)152 | Not orthopaedic patients | Dry bones. Not a controlled study. Assessing bone mineral density |
| Sapin (2008)153 | Not conventional X-ray control | European spine phantom. Assessing bone mineral density |
| Sapin (2007)154 | Not orthopaedic patients | European spine phantom. Assessing bone mineral density |
| Sato (2004)155 | Not EOS | Not EOS |
| Sauli (1994)156 | Not controlled study | Overview – not a study |
| Schlatterer (2009)157 | Not conventional X-ray control | Healthy volunteers + two knee surgery patients. Not a controlled study |
| Sebag158 | Not controlled study | PowerPoint slides on examination time |
| Situ (2009)159 | Not EOS | Not EOS |
| Steffen (2008)160 | Not conventional X-ray control | Case study. CT control |
| Steffen (2010)161 | Not conventional X-ray control | Control was asymptomatic patients |
| Sudhoff (2007)162 | Not conventional X-ray control | Assessment of knee attachment systems. No control |
| Sushkov (2008)163 | Not EOS | Not EOS |
| Vital (2008)164 | Not controlled study | Overview – not a study |
| Wahrburg (2000)165 | Not EOS | Not EOS |
| Zheng (2006)166 | Not orthopaedic patients | Dry bones. Not clear if EOS |
| Zheng (2008)167 | Not orthopaedic patients | Dry bones. Assessment of 3D reconstruction technique, not clear if EOS. Not standard X-ray control |
Appendix 5 Table of excluded studies with rationale: systematic review of the adverse effects of diagnostic radiation for patients with orthopaedic conditions
| Study details | Reason for exclusion |
|---|---|
| Aguilar Naranjo (1987)168 | Not a study or systematic review |
| Anasti (1998)169 | Not medical diagnostic radiation |
| Ashley (2005)170 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Atkinson (2009)171 | Not a study or systematic review |
| Bailey (2010)172 | Not orthopaedic patients |
| Baker (2006)173 | Not a study or systematic review |
| Barcellos-Hoff (2009)174 | Not orthopaedic patients |
| Berrington de Gonzalez (2004)175 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Berrington de Gonzalez (2009)176 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Berrington de Gonzalez (2010)177 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Bone (2000)178 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Brenner (2001)179 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Brenner (2004)180 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Butler (1986)181 | Not an assessment of adverse effects |
| Campbell (1972)182 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Chawla (2010)183 | Not an assessment of adverse effects |
| Chew (1991)184 | Case study |
| Colditz (1997)185 | Not medical diagnostic radiation |
| Committee to Assess Health Risks from Exposure to Low Levels of Ionising Radiation (2006)17 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Cozen (1999)186 | Not a study or systematic review |
| De Smet (1981)187 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Don (2004)188 | Not a study or systematic review |
| Don (2004)189 | Not a study or systematic review |
| Dreyer (1982)190 | Not medical diagnostic radiation |
| Dutkowsky (1990)191 | Not an assessment of adverse effects |
| Einstein (2007)192 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Friedler (1996)193 | Not medical diagnostic radiation |
| Frik (1972)194 | Not a study or systematic review |
| Gerber (2010)195 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Goss (1998)196 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Hallen (1992)197 | Not an assessment of adverse effects |
| Hart (2002)198 | Not an assessment of adverse effects |
| Hart (2002)199 | Not an assessment of adverse effects |
| Hart (2004)200 | Not an assessment of adverse effects |
| Hart (2007)50 | Not an assessment of adverse effects |
| Hart (2009)201 | Not an assessment of adverse effects |
| Hendry (1989)202 | Not medical diagnostic radiation |
| Hrabovszky (1964)203 | Not a study or systematic review |
| Hughes (2005)204 | Not an assessment of adverse effects |
| Huncharek (2002)205 | Not medical diagnostic radiation |
| Huppmann (2010)206 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Infante-Rivard (2000)207 | Not orthopaedic patients |
| Jansen-van der Weide (2010)208 | Not orthopaedic patients |
| Kelsey (1979)209 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Kline (1998)210 | Not a study or systematic review |
| Kratzsch (1972)211 | Not an assessment of adverse effects |
| Leone (2010)212 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Levy (1994)213 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Levy (1996)214 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| LiVolsi (1978)215 | Not medical diagnostic radiation |
| Mahmoud (2007)216 | Not medical diagnostic radiation |
| Mills (2006)217 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Muirhead (1991)218 | Not medical diagnostic radiation |
| Muirhead (1991)219 | Not medical diagnostic radiation |
| Nash (1979)220 | Not an assessment of adverse effects |
| Neta (2000)221 | Not a study or systematic review |
| Nussbaum (1994)222 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Pape (1963)223 | Not an assessment of adverse effects |
| Preston (2004)224 | Not a study or systematic review |
| Rao (1984)225 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Rice (2007)226 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Richter (1979)227 | Not an assessment of adverse effects |
| Rohrer (2010)228 | Not orthopaedic patients |
| Ron (2003)229 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Ronckers (2005)230 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Royal (2008)231 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Sadetzki (2009)232 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Samet (1997)233 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Sankaranarayanan (1995)234 | Not medical diagnostic radiation |
| Schulze-Rath (2008)39 | Systematic review of pre/post-natal diagnostic X-ray, not primarily orthopaedic patients |
| Semelka (2007)235 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Singletary (2003)236 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Smith (2007)237 | Not medical diagnostic radiation |
| Smith-Bindman (2009)238 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Soffer (1990)239 | Not medical diagnostic radiation |
| Solheim (1967)240 | Not medical diagnostic radiation |
| Stein (2008)241 | Not medical diagnostic radiation |
| Theocharopoulos (2009)242 | Review primarily based on data from atomic bomb survivors or radiation therapy, not diagnostic radiation |
| Woods (1987)243 | Not a study or systematic review |
Appendix 6 Number of episodes and number of patients per ICD-10 code during 2008–9
| Indication per four-digit ICD-10 code | Episodes | Patients | |
|---|---|---|---|
| M41.0 | Infantile idiopathic scoliosis | 270 | 150 |
| M41.1 | Juvenile idiopathic scoliosis | 803 | 564 |
| M41.2 | Other idiopathic scoliosis | 265 | 195 |
| M41.3 | Thoracogenic scoliosis | 26 | 19 |
| M41.5 | Other secondary scoliosis | 96 | 69 |
| M41.8 | Other forms of scoliosis | 192 | 149 |
| M41.9 | Scoliosis, unspecified | 2308 | 1667 |
| M42.0 | Juvenile osteochondrosis of spine | 52 | 42 |
| M42.1 | Adult osteochondrosis of spine | 6 | 6 |
| M42.9 | Spinal osteochondrosis, unspecified | 21 | 15 |
| M43.0 | Spondylolysis | 499 | 431 |
| M43.1 | Spondylolisthesis | 4674 | 3885 |
| M43.2 | Other fusion of spine | 82 | 77 |
| M43.3 | Recurrent atlantoaxial subluxation with myelopathy | 17 | 13 |
| M43.4 | Other recurrent atlantoaxial subluxation | 32 | 24 |
| M43.5 | Other recurrent vertebral subluxation | 25 | 23 |
| M43.8 | Other specified deforming dorsopathies | 60 | 51 |
| M43.9 | Deforming dorsopathies, unspecified | 66 | 44 |
| M45. | Ankylosing spondylitis (between 35 and 65 years old) | 3445 | 1109 |
| Q65.0 | Congenital dislocation of hip, unilateral | 1079 | 681 |
| Q65.1 | Congenital dislocation of hip, bilateral | 437 | 253 |
| Q65.2 | Congenital dislocation of hip, unspecified | 182 | 137 |
| Q65.3 | Congenital subluxation of hip, unilateral | 136 | 98 |
| Q65.4 | Congenital subluxation of hip, bilateral | 55 | 38 |
| Q65.5 | Congenital subluxation of hip, unspecified | 39 | 28 |
| Q65.6 | Unstable hip | 183 | 172 |
| Q65.8 | Other congenital deformities of hip | 1842 | 1423 |
| Q65.9 | Congenital deformity of hip, unspecified | 116 | 100 |
| Q67.5 | Congenital deformity of spine | 170 | 116 |
| Q68.2 | Congenital deformity of knee | 65 | 53 |
| Q68.3 | Congenital bowing of femur | 1 | 1 |
| Q68.4 | Congenital bowing of tibia and fibula | 17 | 13 |
| Q68.5 | Congenital bowing of long bones of leg, unspecified | 9 | 8 |
| Q68.8 | Other specified congenital musculoskeletal deformities | 209 | 184 |
| Q72.0 | Congenital complete absence of lower limb(s) | 2 | 2 |
| Q72.1 | Congenital absence of thigh and lower leg with foot present | 2 | 2 |
| Q72.2 | Congenital absence of both lower leg and foot | 1 | 1 |
| Q72.3 | Congenital absence of foot and toe(s) | 8 | 8 |
| Q72.4 | Longitudinal reduction defect of femur | 46 | 37 |
| Q72.5 | Longitudinal reduction defect of tibia | 9 | 8 |
| Q72.6 | Longitudinal reduction defect of fibula | 17 | 12 |
| Q72.7 | Split foot | 1 | 1 |
| Q72.8 | Other reduction defects of lower limb(s) | 124 | 94 |
| Q72.9 | Reduction defect of lower limb, unspecified | 47 | 43 |
| Q74.1 | Congenital malformation of knee | 129 | 127 |
| Q74.2 | Other congenital malformation of lower limb(s) including pelvic girdle | 363 | 342 |
| Q74.3 | Arthrogryposis multiplex congenita | 79 | 45 |
| Q74.8 | Other specified congenital malformations of limb(s) | 69 | 57 |
| Q74.9 | Unspecified congenital malformation of limb(s) | 16 | 15 |
| Q76.2 | Congenital spondylolisthesis | 16 | 15 |
| Q77.1 | Thanatophoric short stature | 2 | 2 |
| Q77.2 | Short rib syndrome | 2 | 2 |
| Q77.3 | Chondrodysplasia punctata | 44 | 31 |
| Q77.4 | Achondroplasia | 126 | 86 |
| Q77.5 | Dystrophic dysplasia | 2 | 2 |
| Q77.6 | Chondroectodermal dysplasia | 1 | 1 |
| Q77.7 | Spondyloepiphyseal dysplasia | 39 | 23 |
| Q77.8 | Other osteochondrodysplasias with defects of growth of tubular bones and spine | 25 | 10 |
| Q78.1 | Polyostotic fibrous dysplasia | 87 | 50 |
| Q78.3 | Progressive diaphyseal dysplasia | 5 | 5 |
| Q78.5 | Metaphyseal dysplasia | 11 | 8 |
| Q78.8 | Other specified osteochondrodysplasias | 127 | 99 |
| Q78.9 | Osteochondrodysplasia, unspecified | 19 | 12 |
| Q76.3 | Congenital scoliosis due to congenital bony malformation | 153 | 104 |
| Q76.4 | Other congenital malformations of spine, not associated with scoliosis | 167 | 142 |
Appendix 7 Number of outpatient appointments per ICD-10 code during 2008–9
| Indication per four-digit ICD-10 code | Outpatient appointments | |
|---|---|---|
| R69.X | Unknown and unspecified causes of morbidity | 58,768,712 |
| M40.0 | Postural kyphosis | 3 |
| M40.2 | Other and unspecified kyphosis | 16 |
| M40.5 | Lordosis, unspecified | 4 |
| M41.1 | Juvenile idiopathic scoliosis | 1 |
| M41.2 | Other idiopathic scoliosis | 5 |
| M41.4 | Neuromuscular scoliosis | 0 |
| M41.9 | Scoliosis, unspecified | 67 |
| M42.0 | Juvenile osteochondrosis of spine | 4 |
| M42.9 | Spinal osteochondrosis, unspecified | 3 |
| M43.0 | Spondylolysis | 6 |
| M43.1 | Spondylolisthesis | 10 |
| M43.2 | Other fusion of spine | 5 |
| M43.8 | Other specified deforming dorsopathies | 1 |
| M43.9 | Deforming dorsopathy, unspecified | 4 |
| M45.X | Ankylosing spondylitis | 1338 |
| Q65.2 | Congenital dislocation of hip, unspecified | 1 |
| Q65.6 | Unstable hip | 1 |
| Q65.8 | Other congenital deformities of hip | 34 |
| Q67.3 | Plagiocephaly | 3 |
| Q67.5 | Congenital deformity of spine | 5 |
| Q67.6 | Pectus excavatum | 2 |
| Q68.0 | Congenital deformity of sternocleidomastoid muscle | 1 |
| Q68.2 | Congenital deformity of knee | 18 |
| Q68.8 | Other specified congenital musculoskeletal deformities | 2 |
| Q74.0 | Other congenital malformation of upper limb(s) including shoulder girdle | 9 |
| Q74.1 | Congenital malformation of knee | 5 |
| Q74.8 | Other specified congenital malformations of limb(s) | 1 |
| Q75.9 | Congenital malformation of skull and face bones, unspecified | 1 |
| Q76.1 | Klippel–Feil syndrome | 4 |
| Q76.4 | Other congenital malformation of spine not associated with scoliosis | 2 |
| Q76.5 | Cervical rib | 9 |
| Q76.6 | Other congenital malformations of ribs | 1 |
| Q77.3 | Chondrodysplasia punctata | 2 |
| Q77.4 | Achondroplasia | 1 |
| Q78.0 | Osteogenesis imperfecta | 20 |
| Q78.1 | Polyostotic fibrous dysplasia | 3 |
| Q78.4 | Enchondromatosis | 1 |
| Q78.8 | Other specified osteochondrodysplasias | 2 |
| Q85.0 | Neurofibromatosis (non-malignant) | 87 |
Appendix 8 Model inputs
| Input | Section | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of radiograph | 4.5.1 | ||||||||||||||
| Indication | Children | Adolescents and adults | |||||||||||||
| Scoliosis | Spine PA or AP | Thoracic spine PA or AP | |||||||||||||
| Congenital kyphosis | Spine LAT | Thoracic spine LAT | |||||||||||||
| Ankylosing spondylitis | Lumbar spine PA or AP | ||||||||||||||
| Scheuermann’s disease | Lumbar spine LAT | ||||||||||||||
| Other deforming dorsopathies | |||||||||||||||
| Congenital deformities of spine | |||||||||||||||
| Congenital deformities of lower limbs and hips | Frontal femur | Frontal femur | |||||||||||||
| Frontal lower legs | Frontal lower legs | ||||||||||||||
| Pelvis PA | Pelvis PA | ||||||||||||||
| Monitoring pattern | 4.5.2 | ||||||||||||||
| Indication | Age at diagnosis (years) | Monitoring pattern | % surgery | ||||||||||||
| Surgery indicated | Surgery not indicated | ||||||||||||||
| Congenital scoliosis | 1 | Two scans per year up to age 15 years | Two scans per year up to age 3 years | 45 | |||||||||||
| One scan per year up to age 20 years | Four scans per year up to age 4 years | ||||||||||||||
| One scan per year up to age 20 years | |||||||||||||||
| Early-onset idiopathic scoliosis | 2 | Two scans per year up to age 15 years | Two scans per year up to age 4 years | 95 | |||||||||||
| One scan per year up to age 20 years | Four scans per year up to age 5 years | ||||||||||||||
| One scan per year up to age 20 years | |||||||||||||||
| Late-onset idiopathic scoliosis | 14 | Two scans per year up to age 15 years | Two scans per year up to age 16 years | 10 | |||||||||||
| One scan per year up to age 20 years | Four scans per year up to age 17 years | ||||||||||||||
| One scan per year up to age 20 years | |||||||||||||||
| Adult scoliosis | 55 | One scan per year up to age 55 years | One scan per year up to age 57 years | 20 | |||||||||||
| Four scan per year up to age 58 years | |||||||||||||||
| One scan per year up to age 59 years | |||||||||||||||
| Congenital kyphosis | 5 | Two scans per year up to age 15 years | Two scans per year up to age 7 years | 75 | |||||||||||
| One scan per year up to age 20 years | Four scans per year up to age 8 years | ||||||||||||||
| One scan per year up to age 20 years | |||||||||||||||
| Congenital deformities | 1 | Two scans per year up to age 20 years | Two scans per year up to age 3 years | 75 | |||||||||||
| Five scans per year up to age 4 years | |||||||||||||||
| 0.5 scan per year up to age 10 years | |||||||||||||||
| Scheuermann’s disease (adolescents) | 15 | Two scans per year up to age 15 years | Two scans per year up to age 17 years | 3 | |||||||||||
| One scan per year up to age 20 years | Four scans per year up to age 18 years | ||||||||||||||
| One scan per year up to age 20 years | |||||||||||||||
| Scheuermann’s disease (adults) | 45 | One scan per year up to age 45 years | One scans per year up to age 47 years | 3 | |||||||||||
| Four scans per year up to age 48 years | |||||||||||||||
| One scan per year up to age 49 years | |||||||||||||||
| Ankylosing spondylitis | 50 | One scan per year up to age 50 years | One scans per year up to age 52 years | 1 | |||||||||||
| Four scans per year up to age 53 years | |||||||||||||||
| One scan per year up to age 54 years | |||||||||||||||
| Deforming dorsopathies (adolescents) | 10 | Two scans per year up to age 15 years | Two scans per year up to age 12 years | 50 | |||||||||||
| One scan per year up to age 20 years | Four scans per year up to age 13 years | ||||||||||||||
| One scan per year up to age 20 years | |||||||||||||||
| Deforming dorsopathies (adults) | 55 | One scan per year up to age 55 years | One scan per year up to age 57 years | 50 | |||||||||||
| Four scan per year up to age 58 years | |||||||||||||||
| One scan per year up to age 59 years | |||||||||||||||
| Radiation dose | 4.5.3. | ||||||||||||||
| Adults | Children and adolescents | ||||||||||||||
| Radiograph | Effective dose (mSv) | Type of radiograph | Age range (years) | ||||||||||||
| 1–2 | 3–6 | 7–12 | 13–18 | ||||||||||||
| Thoracic spine AP | 0.24 | Spine AP | 0.0600 | 0.0490 | 0.0290a | 0.0300a | |||||||||
| Thoracic spine LAT | 0.14 | Spine PA | 0.0600a | 0.0490 | 0.0290 | 0.0300 | |||||||||
| Lumbar spine AP | 0.39 | Spine LAT | 0.0780b | 0.0780 | 0.0580 | 0.0480 | |||||||||
| Lumbar spine LAT | 0.21 | 1–4 | 5–9 | 10–14 | > 15 | ||||||||||
| Pelvis AP | 0.28 | Pelvis AP | 0.01 | 0.06 | 0.08 | 0.11 | |||||||||
| Femur AP | 0.011 | Femur AP | 0.00022 | 0.00154 | 0.00209 | 0.00286 | |||||||||
| Knee AP | 0.0001 | Knee AP | 0.000002 | 0.000014 | 0.000019 | 0.000026 | |||||||||
| Cancer risk due to radiation exposure | 4.5.4 | ||||||||||||||
| Age at exposure (years) | Risk of all cancers (per unit Gy) | ||||||||||||||
| Males | Females | ||||||||||||||
| 0–9 | 0.0999 | 0.1270 | |||||||||||||
| 10–19 | 0.0800 | 0.0994 | |||||||||||||
| 20–29 | 0.0623 | 0.0795 | |||||||||||||
| 30–39 | 0.0512 | 0.0646 | |||||||||||||
| 40–49 | 0.0422 | 0.0562 | |||||||||||||
| 50–59 | 0.0327 | 0.0441 | |||||||||||||
| 60–69 | 0.0223 | 0.0320 | |||||||||||||
| 70–79 | 0.0132 | 0.0194 | |||||||||||||
| 80–89 | 0.0055 | 0.0075 | |||||||||||||
| 90–99 | 0.0004 | 0.0002 | |||||||||||||
| Consequences of cancer | 4.5.5. | ||||||||||||||
| Cancer | Age at diagnosis (years) | Costs of cancer (£) | QALYs lost due to cancer | ||||||||||||
| Breast | 40 | 14,990 | 5.6988 | ||||||||||||
| Breast | 60 | 13,927 | 3.4219 | ||||||||||||
| Lung | 72 | 22,712 | 6.8011 | ||||||||||||
| Colorectal | 74 | 14,075 | 3.4493 | ||||||||||||
| Prostate | 74 | 12,389 | 4.6226 | ||||||||||||
| Costs of EOS and standard X-rays | 4.5.6. | ||||||||||||||
| Costs of standard X-rays | Throughput assumption | Costs of EOS (£) | |||||||||||||
| Type of X-ray | Cost (£) | TA1 (based on HES data) | Indication | Cost (£) per scan by grouped indications | |||||||||||
| CR | 3.42 | Congenital scoliosis | 25.19 | ||||||||||||
| Early-onset idiopathic scoliosis | 25.19 | ||||||||||||||
| DR | 6.16 | Adolescent or late-onset scoliosis | 25.19 | ||||||||||||
| Adult scoliosis | 25.19 | ||||||||||||||
| Congenital kyphosis | 16.91 | ||||||||||||||
| Congenital deformities | 16.91 | ||||||||||||||
| Scheuermann’s disease: adolescent | 1311.59 | ||||||||||||||
| Scheuermann’s disease: adult | 1311.59 | ||||||||||||||
| Ankylosing spondylitis | 93.43 | ||||||||||||||
| Deforming dorsopathies: adolescent | 18.99 | ||||||||||||||
| Deforming dorsopathies: adult | 18.99 | ||||||||||||||
| TA2 | 13.76 (30 patients per day) | ||||||||||||||
| TA3 | 8.60 (48 patients per day) | ||||||||||||||
Appendix 9 Protocol (submitted 28 October 2010)
Evidence Assessment and Analysis Report commissioned by the NIHR HTA Programme on behalf of the National Institute for Health and Clinical Excellence – Protocol
1. Title of the project
EOS 2D/3D X-ray Imaging System
2. Name of External Assessment Group (EAG) and project lead
CRD/CHE Technology Assessment Group (Centre for Reviews and Dissemination/Centre for Health Economics), University of York.
Ros Wade Research Fellow Centre for Reviews and Dissemination University of York, Heslington, York YO10 5DD Tel: (01904) 321051 Fax: (01904) 321041
Email: ros.wade@york.ac.uk
Claire McKenna Research Fellow Centre for Health Economics University of York, Heslington, York YO10 5DD Tel: (01904) 321457 Fax: (01904) 321402
Email: claire.mckenna@york.ac.uk
3. Plain English summary
The taking of images such as X-rays is very important to help guide the treatment of many orthopaedic conditions. There are some conditions where it can be beneficial to take an image that is weight-bearing, full body, or three-dimensional (3D). One example of such a condition is scoliosis.
Scoliosis is a 3D deformity of the spine. It is characterised by a curve from side to side. With this curve there is also a change in the normal front to back curves of the spine and some twisting. This distorts the rib cage and may give the patient a rib hump. The size, stiffness and cosmetic consequences of the curve change over time. Scoliosis usually develops during childhood and adolescence. When the condition has no clear underlying cause, it is referred to as ‘idiopathic’, which is the most common type of scoliosis. It has been estimated that adolescent idiopathic scoliosis occurs in 1–3% of children between 10 and 16 years of age. Scoliosis is also seen in adults.
Medical management aims to prevent the scoliosis from worsening or to straighten the spine in more severe cases. The treatment plan is often determined by the severity of the curvature and the patient’s age. This necessitates periodic monitoring of curve progression. The repeated monitoring results in a high dose of radiation exposure with conventional X-ray imaging devices.
An alternative imaging device which can be used in conditions like scoliosis is the EOS 2D/3D X-ray imaging system, which is a new digital radiography system, capable of providing uninterrupted full-body, weight-bearing digital 2D and 3D imaging in a single scan with a low radiation dose.
The main purpose of this project is to assess the benefits, adverse effects and cost-effectiveness of the EOS 2D/3D X-ray imaging system compared with conventional X-ray devices for monitoring and evaluation of scoliosis and other relevant orthopaedic conditions.
4. Decision problem
Objectives
The aim of the project is to determine the clinical effectiveness and cost-effectiveness of the EOS 2D/3D X-ray imaging system for the evaluation and monitoring of scoliosis and other relevant orthopaedic conditions where there are potential benefits associated with imaging that is weight-bearing, full body, simultaneously posteroranterior (PA) and lateral, and/or 3D, and where radiation exposure is a concern. The relevant comparator imaging technologies are X-ray film, computed radiography (CR), and digital radiography (DR). The clinical outcomes to be considered will be the radiation-associated risk of cancer and other patient health benefits.
Background
The management of many orthopaedic conditions involves the use of imaging for diagnosis, treatment planning and assessment and monitoring. For certain conditions and/or stages of management, certain features of the imaging are important, for example being weight-bearing, uninterrupted full body, the ability to scan PA and laterally simultaneously, or to produce a 3D image. One example of such a condition is scoliosis.
Scoliosis
Scoliosis is a 3D deformity of the spine. It is characterised by a curve from side to side. With this curve there is also a change in the normal front to back curves of the spine and some twisting. This distorts the rib cage and may give the patient a rib hump. The size, stiffness and cosmetic consequences of the curve change over time. 1 Progression of scoliosis leads to cosmetic deformity, which in turn can lead to poorer body image perception and problems in psychological and social development, loss of flexibility, cardiopulmonary problems and pain.
The causes of scoliosis include problems of nerve or muscle, infection, tumours, injuries or problems during development in the womb. However, the majority of spinal curves have no clear underlying cause and are therefore described as ‘idiopathic’. 1 Most of these are of late onset (appearing during adolescence), which may occur due to an imbalance in the growth of the spine. It has been estimated that adolescent idiopathic scoliosis occurs in 1–3% of children between 10 and 16 years of age. 2 For the majority of patients, their back shape will change with growth and then stabilise when they are fully grown.
The management of children and adolescents with scoliosis primarily involves monitoring at intervals to assess disease progression and guide treatment decisions. Progression is measured in terms of the degree of the curvature, which is monitored using serial upright weight-bearing X-rays. The interval chosen between X-rays will be determined by the age of the patient, their rate of growth at the time and the nature of their curve. However, the interval between X-ray monitoring tends to range from four months to almost two years. Other techniques, such as body surface scans may also be used in conjunction with the weight-bearing X-rays to assess other characteristics of the patient’s deformity.
There is currently no good evidence that either bracing or physiotherapy alter the long-term natural history of back shape in adolescent idiopathic scoliosis. The decision to offer surgical treatment will depend upon many factors including the degree of curvature of the spine (Cobb angle), rate of progression, cosmetic impact and the patient’s age. Whilst only approximately 10% of children with adolescent scoliosis require surgical intervention,3 nearly 95% of children with early onset scoliosis go on to require surgical treatment. 4 Surgery is often not performed until growth of the skeleton is complete or near complete and therefore monitoring can continue for many years. Patients are also monitored using weight-bearing X-ray post-operatively, for up to two years.
Scoliosis is also seen in adults, some of whom may go on to have surgical treatment. These may be patients who develop a new curve due to wear-and-tear changes in the spine. Alternatively, it may be patients who developed a curve as a child and then go on to develop additional wear-and-tear problems which cause some changes in the shape of the back.
A weight-bearing image is very important in the evaluation of patients with scoliosis due to the effect of gravity. The American College of Radiology Practice Guideline for the Performance of Radiography for Scoliosis in Children recommends PA and lateral radiography of the spine obtained in an upright position for initial or screening examination. 5 Non-weight-bearing images can lead to misinterpretation and misdiagnosis. Full body images can also help prevent misinterpretation of the spinal curvature by providing information about the position of the pelvis and legs.
Other potentially relevant orthopaedic conditions
Other orthopaedic conditions that may similarly benefit from the availability of reduced radiation dose, weight-bearing, full body, simultaneous PA and lateral imaging, and/or 3D imaging include: other spinal deformities in children and adolescents; leg length discrepancy and misalignment in children and adolescents; adult spinal deformities including degenerative scoliosis, progressive kyphosis and osteoporotic fractures; and loss of sagittal and coronal balance in adults, including hip and knee problems where a full body or full leg length image is required for treatment planning (e.g. joint replacement surgery).
Imaging technologies and the risks associated with radiation exposure
All exposure to radiation carries an increased risk of cancer. Where patient management involves a number of X-rays the increased risk has to be considered. This is of particular concern when X-ray monitoring is conducted throughout childhood and puberty. 6 Children are more sensitive to the harmful effects of radiation than adults. Studies have linked radiation exposure from the evaluation of scoliosis progression with harmful outcomes, such as breast cancer. 6,7 Therefore radiation exposure, and subsequent detrimental health outcomes, is an important consideration in the selection of an imaging technology.
EOS 2D/3D X-ray imaging system
EOS is a biplane X-ray imaging system manufactured by Biospace Med, Paris, France. It uses slot-scanning technology to produce a high quality image with less irradiation than standard imaging techniques. EOS allows the acquisition of images while the patient is in an upright weight-bearing (or seated or squatting) position, and can image the full length of the body (up to 175 cm), removing the need for digital stitching. The system takes approximately 20 seconds for an adult full body scan and 4–6 seconds to scan the spine, depending on the patient’s height. As with the widely accepted standard position for all spine radiographs, the patient being scanned is also required to remain motionless, with their arms folded at 45°, and hold their breath during the scan.
EOS takes PA and lateral images simultaneously, and the digital image is available immediately on a 2D workstation. A 3D image can be reconstructed on the sterEOS workstation using the PA and lateral images and a statistical 3D spine model, generated from a database of scoliotic patients. The reconstruction of a 3D image takes 5 to 10 minutes for each part of the skeleton (e.g. spine or femur). 8
The acquisition cost of the EOS system in the UK is in the region of £400,000, with an annual maintenance cost of £32,000. The maintenance contract covers all parts except X-ray tubes, which cost £25,000 to replace, including fitting. 9 In addition to the cost of purchasing and maintaining the equipment, there may be some building costs to provide a suitable location complying with radiation legislation requirements, if existing rooms are not available. EOS requires the same room planning and shielding as a general X-ray room and the same radiation protection protocols apply.
Comparator imaging technologies
Currently available imaging technologies that can be used in an upright weight-bearing position include X-ray film, CR and DR. All of these technologies have higher radiation doses than EOS. X-ray film, CR and DR can only take images from one angle at a time, so simultaneous PA and lateral images are not possible and 3D reconstruction cannot be obtained. When a full body image is required, these conventional X-ray imaging technologies also require adjustment for distortion or digital stitching from multiple images.
5. Report methods for assessing the outcomes arising from the use of the interventions
To evaluate the clinical benefits of EOS 2D/3D X-ray imaging system relative to standard X-ray, a review of the evidence will be conducted. It is anticipated that much of the information required for this assessment will not be available in the published literature nor be retrievable using standard systematic review methods. However, the review will be conducted as far as possible following the general principles recommended in CRD’s guidance10 and the PRISMA statement11 although not all searches will be exhaustive. In addition, where clinical study evidence is lacking for key parameters, formal elicitation of expert opinion may be undertaken.
Inclusion and exclusion criteria
The titles and abstracts of records identified by the search strategy will be examined for relevance by two reviewers independently. Full papers of any potentially relevant records will be obtained where possible and screened by two reviewers independently. The relevance of each study to the review and the decision to include/exclude studies will be made according to the inclusion criteria detailed below. Any disagreements will be resolved by consensus.
Participants
Primarily, adolescents and children undergoing monitoring and evaluation of scoliosis will be eligible for inclusion.
The eligible patients will also include those with other relevant orthopaedic conditions where the benefits of reduced radiation dose, weight-bearing imaging, full body imaging, simultaneous PA and lateral imaging, and/or 3D imaging are likely to be clinically important for patient management. These additional conditions will include:
-
children and adolescents with leg length discrepancy and misalignment;
-
adults with spinal deformities (e.g. degenerative scoliosis, progressive kyphosis, and osteoporotic fractures);
-
adults with loss of sagittal and coronal balance, including hip and knee problems where a full body or full leg length image is required for treatment planning (e.g. joint replacement surgery).
Interventions/comparators
The EOS 2D/3D X-ray imaging system will be reviewed. The comparators will be conventional 2D PA/anteroposterior (AP) and lateral radiographs from X-ray film, CR or DR imaging.
Outcomes
The primary outcome will be cumulative radiation dose and its impact on the risk of cancer. Other outcomes will be condition specific, reflecting any beneficial effect on patient health, adverse effects and quality of life. For example, in scoliosis or other spinal deformity, outcomes may include improvement in patient health associated with the use of EOS, and for patients undergoing joint replacement surgery, outcomes may include the likelihood of success of the replacement.
Study designs
To evaluate the risk of cancer from the radiation exposure associated with the relevant interventions, controlled or uncontrolled studies that provide information relevant to current UK practice will be sought. This will include studies of radiation dose and cancer risk where available. Additionally, guidelines, studies or reviews that provide data on the number of images required for the clinical management of each relevant orthopaedic condition will be sought.
To evaluate the other outcomes (clinical benefits) of EOS studies that compare EOS with conventional 2D PA/AP and lateral radiographs will be included in the review, where available.
Literature searching
Searches of the literature will be conducted in order to identify studies and other relevant information in the following key areas:
-
Extensive searches of the EOS literature
-
Standard practice and treatment pathways for scoliosis and other relevant orthopaedic conditions
-
Information on radiation dose for all relevant indications
-
Evidence on adverse effects of diagnostic x-ray radiation, such as cancer and infertility.
Additional supplementary searches will be carried out as necessary. Searches for studies for cost and quality of life data will also be included, as outlined in Section 6.
Electronic sources will be searched for primary and secondary studies. These sources will include MEDLINE, EMBASE, CINAHL, HMIC, ISI Science Citation Index and The Cochrane Library (including the Cochrane Database of Systematic Reviews (CDSR), the Database of Abstracts of Reviews of Effects (DARE), the Health Technology Assessment (HTA) Database, the NHS Economic Evaluation Database (NHS EED) and the Cochrane Central Register of Controlled Trials).
In addition, relevant reviews and guidelines will be identified through the following resources: Clinical Evidence, National Institute for Health and Clinical Evidence (NICE) website, NHS Evidence – National Library of Guidelines, SIGN Guidelines, the Guidelines International Network website and the Medicines and Healthcare products Regulatory Agency.
Search terms will be identified by scanning key papers identified during the review, through discussion with the review team and clinical experts, and by using database thesauri. Reference lists of included papers will be assessed for additional relevant studies and where necessary, authors of eligible studies will be contacted for further information. No limits relating to language, date of publication or study design will be applied to the searches.
Data extraction strategy
Data relating to both study design and results will be extracted by one reviewer using a standardised data extraction form and independently checked by another. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary. If time constraints allow, attempts will be made to contact authors for any missing data. Data from multiple publications of the same study will be extracted as a single study.
Quality assessment strategy
The quality of included studies will be assessed using standard checklists10 adapted as necessary to incorporate topic-specific quality issues. The assessment will be performed by one reviewer, and independently checked by another. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
Methods of analysis/synthesis
In the initial analysis/synthesis of data, the results of data extraction will be presented in structured tables and as a narrative summary, grouped by participant and intervention characteristics. Where sufficient clinically and statistically homogenous data are available, data will be pooled using appropriate meta-analytic techniques. Clinical, methodological and statistical heterogeneity will be investigated. If necessary, sensitivity analyses will be performed when permitted by sufficient data.
6. Report methods for synthesising evidence of cost-effectiveness
Identifying and systematically reviewing published cost-effectiveness studies
Searches for economic evaluations, as well as quality of life and cost data will be undertaken in the databases listed in Section 5. These sources will be used to identify any studies of the cost-effectiveness of EOS against its relevant comparators. A broad range of study designs will be considered in the assessment of cost-effectiveness including economic evaluations conducted alongside randomised or non-randomised comparator trials, modelling studies and analyses of administrative databases. The focus for the review will be full economic evaluations that compare two or more options and consider both costs and consequences (including cost-effectiveness, cost–utility and cost–benefit analyses). With a view to gaining insights into the modelling methods we might employ, we will also consider modelling studies for scoliosis monitoring and the other orthopaedic conditions where the interventions and comparators listed in Section 5 are assessed for cost-effectiveness; and cost analyses of EOS. These studies will not be subject to a formal review (unless they complement the evaluation of the EOS 2D/3D X-ray imaging system) but will be used to assist in the overall development of a new decision-analytic model, with the aim of identifying important structural assumptions, parameter estimates (including costs) and highlighting key areas of uncertainty.
The quality of the cost-effectiveness studies will be assessed according to the criteria for economic evaluation detailed in the methodological guidance developed by NICE. 12 This information will be tabulated and summarised within the report. In particular, information will be extracted on the comparators, study population, main analytic approaches, primary outcomes, quality of life estimates, costs, estimates of incremental cost-effectiveness and approaches to quantifying decision uncertainty (e.g. deterministic/probabilistic sensitivity analysis).
Evaluation of costs, quality of life and cost-effectiveness
A decision-analytic model will be developed to estimate the cost-effectiveness of EOS and standard X-rays (film, CR, DR) for monitoring spinal deformity (principally scoliosis) and the other relevant orthopaedic conditions listed in Section 5 where full body or full leg length images are currently requested. The perspective will be that of the National Health Service and Personal Social Services, health benefits will be expressed in terms of quality-adjusted life-years (QALYs) and both costs and quality-adjusted life-years discounted at a rate of 3.5% per annum.
Since the primary benefit of EOS is to provide imaging at low dose radiation, the model will focus on evaluating the cost-effectiveness of EOS through reducing the amount of radiation exposure to patients, particularly to children and adolescents, over the monitoring period for scoliosis and the various conditions. The subsequent outcomes from radiation exposure on the risk of cancer, mortality and any other adverse effects will be explicitly modelled to determine the impact of radiation doses on quality-adjusted life-years (QALYs). The robustness of the analysis will depend on the availability of evidence linking radiation exposure to cancer risk, as well as the effect of cancer on quality-adjusted life expectancy.
Establishing a direct link between diagnostic test accuracy, or the quality of the imaging, and final health outcomes is unlikely to be possible due to limited or no formal evidence. Should the review of clinical effectiveness allow us to establish the impact of the alternative interventions on image quality/accuracy, the longer-term impact (including any therapeutic implications) and subsequent prognosis of patients for the various conditions will be included. In the likely absence of formal evidence linking image quality/accuracy with patients’ health outcomes, formal elicitation of clinical opinions13,14 on these parameters may be undertaken.
Resource utilisation and costs will be estimated for EOS and standard X-rays. For EOS, these costs will include the capital cost of the equipment, including installation of workstation and software, consumables, annual maintenance costs and patient throughput. Consideration will also be given to building costs where a suitable location complying with radiation legislation requirements may be required if existing rooms are not available. Similar cost considerations apply to standard film, CR and DR imaging but these systems are probably in place and will not require special implementation. Particular attention will be paid to how per patient costs vary with total patient throughput for EOS, standard X-rays and the indications listed in Section 5. The implication of this variation is likely to be explored using sensitivity and threshold analysis. Data for the cost analysis will be drawn from routine NHS sources, discussions with individual hospitals and with the EOS and comparator manufacturers.
Further details of the model structure and data to be used to populate the model will have to await the findings from the systematic searches of the literature. However, we expect to give particular consideration to the following key variables:
-
Amount of radiation dose exposed to the body and possibly to specific organs/parts of the body from the different types of imaging.
-
The frequency of follow-up and monitoring for the various conditions.
-
The link between radiation exposure and cancer risk and mortality.
-
The duration of examination assessment time.
-
Therapeutic implications and change in quality of life resulting from the alternative interventions.
-
Resource utilisation and costs for EOS and standard X-rays.
-
Patient throughput for the various conditions.
The specific objectives of the cost-effectiveness analysis are:
-
To use an economic model to estimate the amount of radiation received over the entire monitoring period for the evaluation of scoliosis and the other conditions and use it to establish the impact of that radiation on overall QALYs by examining cancer risk and mortality.
-
Subject to the availability of suitable data on image quality/accuracy and with the potential of using formal elicitation of expert judgements, to use the model to characterise patients’ subsequent prognosis for the various conditions and alternative interventions in a way that is clinically appropriate.
-
To populate the model using the most appropriate data identified systematically from published literature and routine sources. If feasible, formal methods of expert elicitation of clinical opinion will be used to help inform key model parameters.
-
To relate intermediate outcomes to final health outcomes, expressed in terms of QALYs. This is necessary in order to provide decision makers with an indication of the health gain achieved by each intervention, relative to its additional cost, in units which permit comparison with other uses of health service resources.
-
To estimate the mean cost-effectiveness of EOS and standard X-rays based on an assessment of long-term NHS costs and quality-adjusted survival.
-
To use threshold analysis in the absence of formal evidence on specific parameters to establish the threshold of benefit required to achieve good value for money within the NHS.
-
To characterise the uncertainty in the data used to populate the model and to present the uncertainty in these results to decision makers. A probabilistic model will be developed which requires that each input in the model is entered as an uncertain, rather than a fixed parameter. Using Monte Carlo simulation, this parameter uncertainty will be translated into uncertainty in the overall results. This will be presented graphically using cost-effectiveness acceptability curves which show the probability that each intervention is cost-effective conditional on a range of possible threshold values which NHS decision makers attach to an additional QALY.
-
To use sensitivity analysis to examine alternative assumptions in the data and to see how sensitive the cost-effectiveness threshold is to uncertainty in the assumed base case parameters.
7. Handling information from the companies
Any ‘commercial in confidence’ data provided by the manufacturer (BioSpace Med) and specified as such will be highlighted in blue and underlined in the assessment report. Any ‘academic in confidence’ data provided by the manufacturer will be highlighted in yellow and underlined in the assessment report.
8. Competing interests of authors
None of the authors has any conflicts of interest.
9. Timetable/milestones
| Milestone | Date to be completed |
|---|---|
| Submission of final protocol | 28/10/10 |
| Diagnostics Assessment Report (DAR) due (protocol sign off + 20 weeks) | 09/03/11 |
References
- Gummerson NW, Millner PA. Spinal fusion for scoliosis, clinical decision-making and choice of approach and devices. Skeletal Radiol 2010;39:939-42.
- Weinstein SL, Dolan LA, Cheng JCY, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet 2008;371:1527-37.
- Lenssinck MLB, Frijilink AC, Berger MY, Bierma-Zeinstra SMA, Verkerk K, Verhargen AP. Effect of bracing and other conservative interventions in the treatment of idiopathic scoliosis in adolescents: a systematic review of clinical trials. Phys Ther 2005;85:1329-39.
- Scoliosis Association UK [Internet]. London: Scoliosis Association (UK); n.d.
- American College of Radiology . Practice guideline for the performance of radiography for scoliosis in children 2009:1-6.
- Hoffman DA, Lonstein JE, Morin MM, Visscher W, Harris BSH, Boice JD. Breast cancer in women with scoliosis exposed to multiple diagnostic x rays. J Natl Cancer Inst 1989;81:1307-12.
- Bone C, Hseih G. The risk of carcinogenesis from radiographs to pediatric orthopaedic patients. J Pediatr Orthop 2000;20:251-54.
- Mitton D, De Guise JA, Dubousset J, Skalli W, Gonzalez Y, Cerrolaza M. Bioengineering Modeling and Computer Simulation. Barcelona: CIMNE; 2007.
- National Institute for Health and Clinical Excellence . Diagnostics Assessment Programme: EOS Ultra Low Dose 2D 3D X-Ray Imaging System for Postural Assessment. Request for Information on Topic n.d.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. URL: www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf.
- O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, et al. Uncertain judgements: eliciting experts’ probabilities. Chichester: John Wiley and Sons Ltd; 2006.
- Bojke L, Claxton K, Bravo Vergel Y, Sculpher M, Palmer S, Abrams K. Eliciting distributions to populate decision analytic models. Value Health 2010;13:557-64.
Glossary
- Absorbed dose, D
- The fundamental dose quantity given by:D=dε¯dmwhere dε¯ is the mean energy imparted to the matter of mass dm by ionising radiation. The unit of absorbed dose is joule per kilogram (J/kg) and its special name is gray (Gy).
- Adverse effect
- An abnormal or harmful effect caused by, and attributable to exposure to, a medication or other intervention (e.g. diagnostic X-ray) that is indicated by some result such as death, a physical symptom or visible illness. An effect may be classed as adverse if it causes functional or anatomical damage, causes irreversible change in the homeostasis of the organism or increases the susceptibility of the organism to other chemical or biological stress.
- Ankylosing spondylitis
- A progressive rheumatic condition in which some or all of the joints and bones of the spine fuse together.
- Atlantoaxial subluxation
- A condition in which the vertebrae of the cervical spine are misaligned, usually as a result of major neck trauma. In severe cases the subluxed spine may compress the spinal cord, leading to irreversible neurological damage. Atlantoaxial subluxation is also known as ‘atlantoaxial instability’.
- Centigray (cGy)
- Measurement unit of absorbed dose of ionising radiation (e.g. X-rays). One centigray is 0.01 of a gray, and the gray is defined as the absorption of 1 J of energy from ionising radiation by 1 kg of matter, for example human tissue.
- Cobb angle
- The radiographic measurement of scoliotic curve severity obtained from a radiograph. A measurement of < 10 ° is regarded as ‘normal’, between 10 ° and 30 ° is classed as ‘mild’, and anything > 60 ° is classed as ‘severe’.
- Computed radiography (CR)
- A type of X-ray imaging used to visualise internal body structures (such as bones) to diagnose and monitor disease or injury. CR uses similar equipment to conventional radiography, except that, in place of a film to create the image, an imaging plate is used.
- Deforming dorsopathies
- Umbrella term for spinal deformity.
- Digital radiography (DR)
- A type of X-ray imaging used to visualise internal body structures (such as bones) to diagnose and monitor disease or injury. DR uses a digital image capture device to record the image.
- Effective dose, E
- The tissue-weighted sum of the equivalent doses in all specified tissues and organs of the body, given by the expression: E=∑TWT∑RWRDT,R or E=∑TWTHTwhere HT or wR DT,R is the equivalent dose in a tissue or organ T, and wT is the tissue weighting factor. The unit of effective dose is the same as for absorbed dose, J/kg, and its special name is sievert (Sv).
- Entrance surface dose (ESD)
- A method of measuring radiation dose to the body. ESD can be measured using thermoluminescent dosimeters (e.g. calcium fluoride pellets) or optically stimulated luminescence dosimeters placed on the patient’s skin.
- Equivalent dose, HT
- The dose in a tissue or organ T given by: HT=∑RWRDT,Rwhere DT,R is the mean absorbed dose from radiation R in a tissue or organ T, and wR is the radiation weighting factor. As wR is dimensionless, the unit of equivalent dose is the same as for absorbed dose, J/kg, and its special name is sievert (Sv).
- Excess lifetime risk (ELR)
- A measure of radiation-associated risk. The ELR is the difference between the proportion of the exposed population who develop or die from the disease and the corresponding proportion in a similar non-exposed population.
- Excess relative risk (ERR)
- A measure of radiation-associated risk. The ERR is the rate of disease in the exposed population divided by the rate of disease in an unexposed population, minus 1.0.
- Exposure
- Exposure measures the amount of ionisation produced (in coulombs) by an X-ray beam in 1 kg of air. Exposure is directly related to the strength of the radiation source, and is independent of the matter absorbing the radiation itself.
- Gray (Gy)
- Measurement unit for absorbed dose of ionising radiation (e.g. X-rays). A gray is defined as the absorption of 1 J of energy from ionising radiation by 1 kg of matter, for example human tissue.
- International Classification of Diseases, 10th Edition (ICD-10) codes
- The ICD is the international standard diagnostic classification for clinical use and health management purposes. It is used to classify diseases and health problems recorded on many types of record, such as death certificates and medical records. ICD-10 is the latest in the series and was endorsed by the 43rd World Health Assembly in May 1990 and came into use in World Health Organization member states from 1994.
- Kyphosis
- A curving of the spine that causes rounding of the back, leading to a hunchback posture. Kyphosis can be seen with scoliosis.
- Lifetime attributable risk (LAR)
- A measure of radiation-associated risk. The LAR describes excess deaths or disease cases over a follow-up period with population background rates determined by the experience of unexposed individuals.
- Lordosis
- An excessive inward curvature of the spine, usually in the lumbar region, giving a ‘swayback’ appearance.
- Milligray (mGy)
- Measurement unit of absorbed dose of ionising radiation (e.g. X-rays). One mGy is 0.001 of a gray, and the gray is defined as the absorption of 1 J of energy from ionising radiation by 1 kg of matter, for example human tissue.
- Millisievert (mSv)
- Measurement unit of equivalent dose and effective dose of ionising radiation (e.g. X-rays). One mSv is 0.001 of a sievert, and the sievert is defined as the absorption of 1 J of energy from ionising radiation by 1 kg of matter, for example human tissue.
- Neurofibromatosis
- A genetic disorder affecting the nervous system and skin, causing benign tumours to grow on nerves throughout the body.
- Quality-adjusted life-year (QALY)
- An index of health gain by which survival duration is weighted or adjusted by the patient’s quality of life during the survival period. QALYs have the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- Quality of life (QoL)
- A concept incorporating all the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors which might affect their physical, mental and social well-being.
- Scheuermann’s disease/Scheuermann’s kyphosis
- Adolescent kyphosis, caused by the wedging together of several vertebrae in a row.
- Scoliosis
- A three-dimensional deformity of the spine characterised by a sideways curve of ≥ 10 °. This curve causes the spine to twist, which distorts the rib cage and may result in a rib hump.
- Sievert (Sv)
- Measurement unit of equivalent dose and effective dose of ionising radiation (e.g. X-rays). A sievert is defined as the absorption of 1 J of energy from ionising radiation by 1 kg of matter, for example human tissue.
- Spondylolisthesis
- A spinal condition in which one vertebra in the lower part of the spine slips out of position on to the vertebra immediately below it.
- Spondylolysis
- A stress fracture in the posterior part of the spine known as the pars interarticularis. It is most commonly seen in the fifth lumbar vertebra.
- Statistical significance
- An estimate of the probability of an association (effect) as large or larger than what is observed in a study occurring by chance, usually expressed as a p-value.
- Threshold analysis
- Amount of variance needed in parameter values to achieve a specified value. In the context of cost-effectiveness analysis in the UK NHS, this specified value is the cost-effectiveness threshold of £20,000–30,000 per additional quality-adjusted life-year gained.
- X-ray
- X-ray imaging is used to visualise internal body structures (such as bones) to diagnose and monitor disease or injury. Currently available imaging technologies that can be used in an upright weight-bearing position include X-ray film, computed radiography and digital radiography.
List of abbreviations
- 2D
- two-dimensional
- 3D
- three-dimensional
- ALARA
- as low as reasonably achievable
- AP
- anteroposterior
- CEDIT
- Comité d’Évaluation et de Diffusion des Innovations Technologique (Committee for the Evaluation and Diffusion of Innovative Technologies)
- CI
- confidence interval
- cGy
- centigray
- CR
- computed radiography
- CRCE
- Centre for Radiation, Chemical and Environmental Hazard
- CRD
- Centre for Reviews and Dissemination
- CT
- computed tomography
- DR
- digital radiography
- EAG
- external assessment group
- ELR
- excess lifetime risk
- ERR
- excess relative risk
- ESAK
- entrance surface air kerma
- ESD
- entrance surface dose
- Gy
- gray
- HES
- Hospital Episode Statistics
- HPA
- Health Protection Agency
- ICD-10
- International Classification of Diseases, 10th Edition
- ICER
- incremental cost-effectiveness ratio
- ICRP
- International Commission on Radiological Protection
- LAR
- lifetime attributable risk
- LAT
- lateral
- mGy
- milligray
- mSv
- millisievert
- NICE
- National Institute for Health and Clinical Excellence
- NPDD
- National Patient Dose Database
- OSLD
- optically stimulated luminescence dosimeter
- PA
- posteroanterior
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RR
- relative risk
- SD
- standard deviation
- Sv
- sievert
- SMR
- standardised mortality ratio
- TA1
- throughput assumption 1
- TA2
- throughput assumption 2
- TA3
- throughput assumption 3
- UNSCEAR
- United Nations Scientific Committee on the Effects of Atomic Radiation
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health