Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/302/216. The contractual start date was in December 2007. The draft report began editorial review in June 2011 and was accepted for publication in December 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Sharples et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Lung cancer is the second most common cancer in the UK and is the most common cause of cancer death. Non-small cell lung cancer (NSCLC) accounts for 78% of all cases, with an overall 5-year survival of approximately 8% in the UK. 1 As treatment of lung cancer is influenced by stage, accurate staging is important to optimise treatment.
At present, the mainstay of lung cancer staging involves imaging and biopsy of areas that are suspicious for metastatic spread. The incorporation of positron emission tomography (PET) into staging algorithms has considerably reduced the number of unnecessary thoracotomies performed by identifying locoregional and distant metastases. Positron emission tomography–computerised tomography (PET–CT) is more accurate than computerised tomography (CT) for assessing mediastinal lymph node involvement. Although the negative predictive value (NPV) of PET–CT for mediastinal disease is around 93%, a positive predictive value of 74–90% makes pathological verification of 18-fluorodeoxyglucose (18-FDG)-avid mediastinal nodes necessary in order to determine whether or not they are malignant. 2 Making the assumption that FDG-positive nodes are malignant will potentially deny many patients potentially curative surgery as it is recognised that non-malignant mediastinal nodes can take up FDG in other pathological states, such as infection and inflammation.
Historically, surgical staging of enlarged and/or PET–CT-positive mediastinal lymph nodes has relied on procedures such as mediastinoscopy, mediastinotomy or video-assisted thoracoscopic surgery. These are invasive procedures requiring general anaesthetic and hospitalisation and have low, but well-recognised, morbidity and mortality. The accuracy of these procedures is variable and ranges between 80% and 90%. Although specificity is near 100%, sensitivity ranges between 66% and 90% (see Detterbeck et al. 2 and associated references, Pinto Filho et al. ,3 Anraku et al. 4). Thus, there is room for improvement in terms of the sensitivity of currently available surgical mediastinal staging investigations.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and, more recently, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are two relatively new, less invasive, diagnostic techniques that allow real-time controlled aspiration of mediastinal lymph nodes. These techniques are normally performed in an outpatient setting under moderate sedation. EUS-FNA and EBUS-TBNA are complementary techniques. EUS permits access to mediastinal lymph node groups 2L, 4L, 7, 8L/R, 9L/R, whereas EBUS gives access to mediastinal lymph node stations 2R/L, 4R/L and 7. Using the techniques in combination it is possible to access the majority of mediastinal lymph nodes. EBUS-TBNA also allows access to hilar and intrapulmonary nodal stations 10R/L and 11R/L. In addition, in selected cases, endosonography offers the possibility to assess whether or not a tumour is invading mediastinal structures (T4).
A number of non-randomised prospective studies using EBUS-TBNA have reported sensitivity of around 90% for diagnosis of hilar and/or mediastinal lymph nodes. 5–8 In 2009, two meta-analyses reported pooled sensitivity for EBUS-TBNA of 0.889 [95% confidence interval (CI) 0.79 to 0.94] and 0.9310 (95% CI 0.91 to 0.94). In a systematic review, Varela-Lema et al. 11 reported that sensitivity for the diagnosis of malignancy ranged from 85% to 100%. In all three of these reports the specificity quoted was 1.00, but this figure is artificial, as positive TBNA results were not confirmed by surgical resection in any of these papers. In the case of EUS, reported sensitivity for mediastinal staging varies between 50% and 87%. 2,12–16 The lower figure may be a reflection of the fact that EUS is able to access only the left-sided mediastinal nodes along with the inferior posterior nodes.
To date, there have been three reports documenting a combined approach using both EBUS-TBNA and EUS-FNA for assessing the mediastinum. 17–19 In these series, sensitivity for detection of mediastinal disease ranged from 85% to 100%.
Taken together, it is clear that, although the reported sensitivity of EUS and EBUS for the detection of malignancy in mediastinal lymph nodes is similar to that of mediastinoscopy, there are no reported prospective randomised studies comparing the accuracy of EBUS-TBNA, EUS-FNA and surgical staging for assessment of the mediastinum in lung cancer. Furthermore, to date, no full economic evaluations investigating the cost-effectiveness of EBUS and EUS versus surgical staging have been published.
Therefore, in 2007, this group, led by clinical co-investigators in Ghent, Leiden, Leuven and Cambridge, undertook a prospective randomised controlled trial (RCT) comparing endosonography (combined EBUS-TBNA and EUS-FNA), followed by surgical staging if negative, with surgical staging alone for assessment of the mediastinum in (suspected) NSCLC. In addition to the clinical findings that were published in November 2010,20 data on patient-reported quality of life and the incremental costs and benefits from the perspective of a health-care provider were collected. Here we report resource-use data collection and a cost-effectiveness analysis from a UK NHS perspective.
Chapter 2 Study design
Study objectives
The final protocol is provided in Appendix 1. This RCT was designed to assess whether or not EBUS-TBNA combined with EUS-FNA, followed by surgical staging if these tests were negative, is better than standard surgical staging techniques in the staging of lung cancer in terms of sensitivity, diagnostic accuracy and NPV. The null hypothesis is that there is no difference between the two diagnostic strategies in these outcomes. The health economic study was designed to compare European Quality of Life-5 Dimensions (EQ-5D) utility and cost-effectiveness of the two diagnostic strategies.
Specific study objectives were as follows:
-
The primary research objective of the study was to determine whether or not EBUS-TBNA combined with EUS-FNA, followed by surgical staging if these tests were negative, is better than standard surgical staging techniques in terms of sensitivity for diagnosing and staging the mediastinum in lung cancer. The related NPV of the two diagnostic strategies was also calculated.
-
Determination of the sensitivity and accuracy of EBUS and EUS, followed by surgical staging if these tests were negative, compared with surgical staging for determining mediastinal tumour invasion (T4).
-
A comparative cost-effectiveness analysis of the diagnostic strategies of the two trial arms.
-
Assessment of the complication rates in each arm.
-
An estimation of the saving of surgical staging procedures that might be possible in the future if EBUS-TBNA/EUS-FNA, followed by surgical staging if these tests were negative, is shown to have greater sensitivity and diagnostic accuracy and becomes the new ‘gold standard’ staging approach.
-
Estimation of how many futile thoracotomies can be avoided by performing EBUS-TBNA and EUS-FNA, followed by surgical staging if these tests were negative, rather than surgical staging alone.
-
Assessment of interobserver variability of cytopathological evaluation of EBUS-TBNA and EUS-FNA samples.
Trial design
This was a prospective, international, multicentre, open-label randomised controlled study.
Trial centres
Four centres were involved in the trial: Ghent University Hospital, Belgium; Leuven University Hospitals, Belgium; Leiden University Medical Centre (LUMC), the Netherlands; and Papworth Hospital, UK. Details of the study, the main investigators, trial steering groups and data monitoring committees are provided in the Acknowledgements. In each centre there was a core team comprising a lung cancer physician, a thoracic surgeon and a research nurse. Data collection was completed at each centre using paper-based clinical report forms. All centres followed the same protocol, with the exception of Leuven, where ‘frozen-section’ histopathological analysis was performed in some patients during surgical staging procedures and proceeding directly to thoracotomy was possible if there was no evidence of mediastinal nodal malignancy.
Ethics
The trial was approved by the Cambridge 1 Local Research Ethics Committee in the UK and by the ethical committees of the three participating European hospitals (LUMC in the Netherlands, the University Hospitals of Ghent and Leuven in Belgium). The trial was registered with International Standard Randomised Controlled Trial Number (ISRCTN 97311620) as ASTER (Assessment of Surgical sTaging versus Endosonographic ultrasound in lung cancer: a Randomised clinical trial).
Study population
All consecutive patients referred to the thoracic oncology clinics at the four participating hospitals for staging of lung cancer were considered for the study.
Inclusion criteria for the study were as follows:
-
known or suspected NSCLC and suspected mediastinal lymph node involvement (either N2 or N3), based on available thoracic imaging (CT or CT–PET)
-
pending the results of mediastinal staging, potentially suitable for surgical resection with an intention to cure
-
clinically fit for bronchoscopy, endosonography and diagnostic surgical procedures
-
no evidence of distant metastatic disease after routine clinical work-up
-
able to give informed consent.
Exclusion criteria were as follows:
-
previous treatment (chemotherapy, radiotherapy or surgery) for lung cancer
-
any known clinical reason for not undergoing, or a contraindication to, endosonography or a surgical staging procedure, unsuitability for definitive surgical resection by thoracotomy
-
based on available thoracic imaging, the likelihood that disease cannot be staged accurately by any surgical staging procedure (mediastinoscopy/-otomy, video-assisted thoracoscopic staging)
-
a concurrent malignancy at another site
-
an uncorrected coagulopathy
-
inability to give informed consent.
Study design
Patients who were potentially eligible for participation in the study according to the inclusion and exclusion criteria listed above were identified at the weekly multidisciplinary lung oncology meetings held at each of the centres. The initial diagnostic assessment involved the recording of medical history, a physical examination, full blood count, renal and liver function tests, CT of the chest and upper abdomen and whole-body PET–CT.
Patients were approached in the outpatient clinic by the local principal investigator and those who expressed interest in participating were given a copy of the patient information sheet. All patients were given at least 24 hours to consider participation, and those who indicated that they were willing to participate were consented and then were randomised to either surgical staging alone or endosonography (combined EUS-FNA and EBUS-TBNA) followed by surgical staging (if no nodal metastases were found at endosonography).
Patients were randomly assigned in a 1:1 ratio to either surgical staging alone or endosonography followed by surgical staging. Group allocations were computer generated according to a simple randomisation strategy and were stratified for participating centre. A web-based program was developed, which, on registration of consented patients, provided the next, centre-specific group allocation.
The study design is shown in Figures 1 and 2.
FIGURE 1.
Potential resource use in the first 6 months. CC, complication and comorbidity.
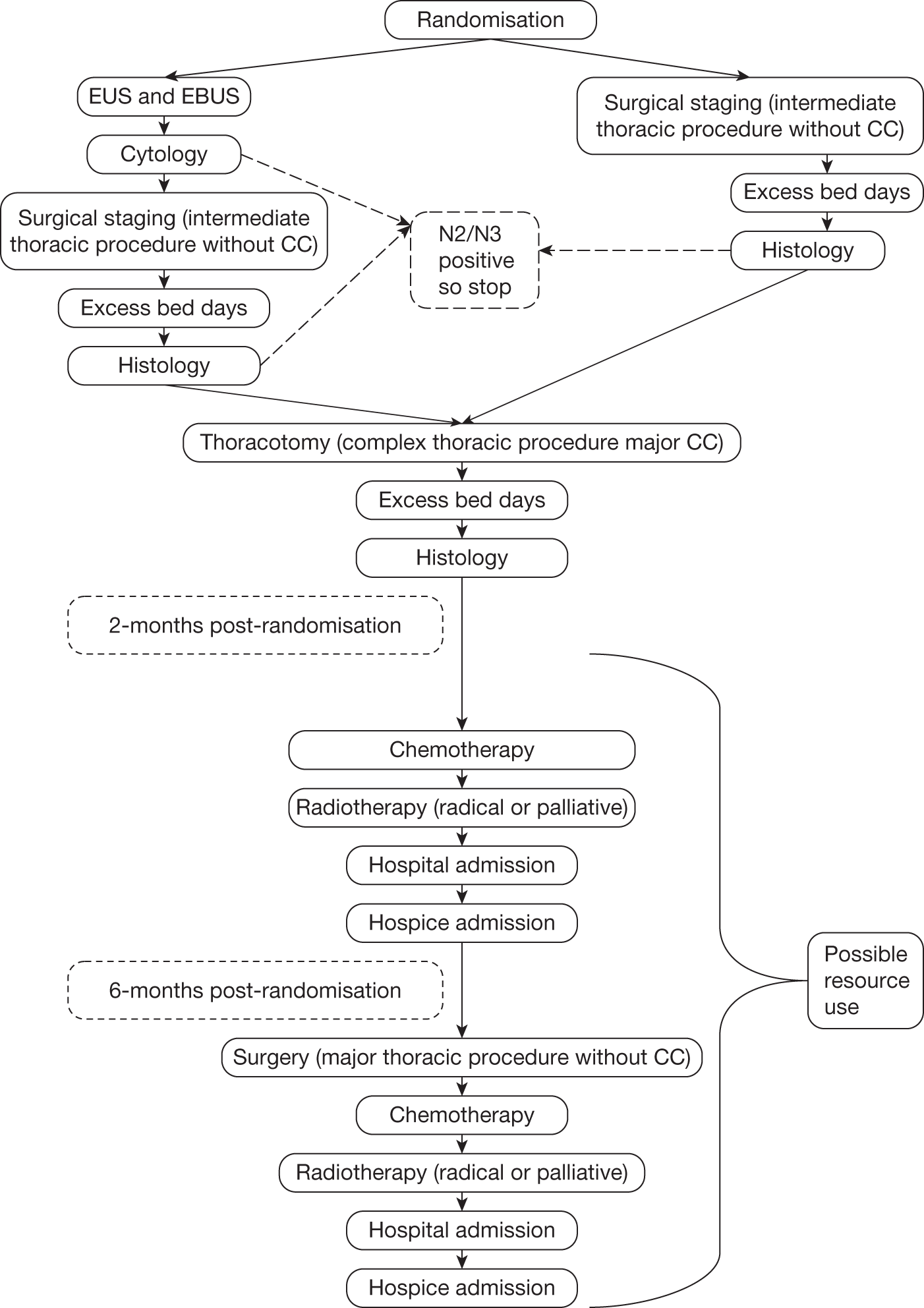
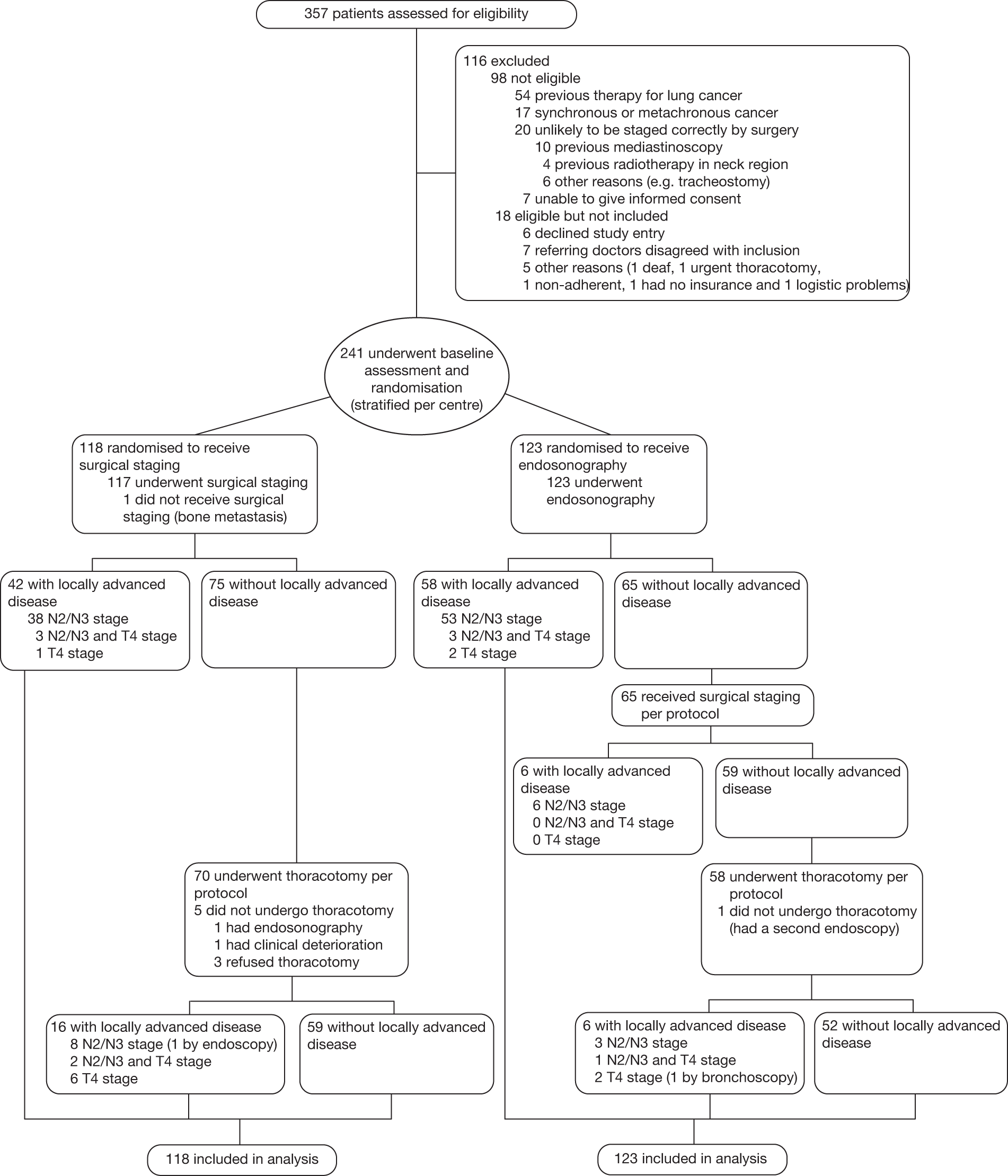
FIGURE 2.
Enrolment and randomisation of study patients.
Patients randomised to endosonography followed by surgical staging if negative
Patients underwent endosonography as detailed below (see Investigation protocols): if there was no evidence of mediastinal nodal metastases, surgical staging was performed as described. In the event of pathological evidence of mediastinal metastases (N2/N3) or mediastinal tumour invasion (T4), either after endosonography or after mediastinoscopy, patients were classified as having locally advanced disease (stage IIIA/B) and were referred for chemoradiotherapy. If after confirmatory surgical staging there was still no evidence of mediastinal nodal disease or direct tumour invasion, a thoracotomy with a systematic lymph node dissection was performed.
Patients randomised to surgical staging
Surgical staging was performed as described below (see Investigation protocols). In the event of pathological evidence of mediastinal metastases (N2/N3) or direct mediastinal tumour invasion (T4), patients were classified as having locally advanced disease (stage IIIA/B) and were referred for chemoradiotherapy. If there was no evidence of mediastinal metastases, a thoracotomy with a systematic lymph node dissection was performed.
Investigation protocols
Endosonography
Endosonography of the mediastinum was performed under moderate sedation. For reasons of convenience and patient comfort, EUS-FNA (Pentax 34UX/38UX, Pentax, Tokyo, Japan or Olympus GF-UCT140-AL5, Olympus, Tokyo, Japan) was performed prior to EBUS-TBNA (Olympus BF-UC160F-OL, Olympus, Tokyo, Japan). A systematic examination of at least left and right paratracheal, subcarinal and paraesophageal mediastinal nodes, as described above, was performed. Nodes that were suspicious on PET–CT or ultrasound imaging were sampled under real-time ultrasound guidance with 22-gauge needles and labelled according to the Mountain–Dresler lymph node classification. 21 When the primary lung tumour was visible by endosonography, the presence or absence of direct mediastinal tumour invasion (T4) was recorded. The cytology preparations were analysed using either May–Grünwald–Giemsa or Papanicolaou stains, dependent on local practice, with additional preparation of cell blocks for histological analysis when appropriate. At completion of the study, all EUS and EBUS samples were re-evaluated by an independent reference pathologist (AGN) to assess interobserver agreement.
Surgical staging
Surgical staging was performed by a (video-) mediastinoscopy according to current guidelines. 2,22 A systematic (five lymph node stations) assessment of left and right higher (2L and 2R) and lower paratracheal (4L and 4R) and subcarinal (7) nodes was performed. If necessary, a left parasternal mediastinotomy or video-assisted thoracoscopy was performed to allow access to nodal stations 5 and 6 or 7–9, respectively. Combinations of the above procedures were permitted. The approach(es) taken were left entirely to the surgeon’s discretion. Nodal samples were labelled and sent for pathological examination. Any evidence of direct mediastinal invasion by the primary tumour (T4) was noted.
Thoracotomy
Thoracotomy with nodal dissection was considered to be the reference in both study arms for patients without N2/N3 nor T4 involvement after mediastinal staging. Thoracotomy was performed when there was no mediastinal nodal metastasis or direct mediastinal tumour invasion following surgical staging in both groups and was carried out according to current guidelines. 23 At the time of lung resection a systematic lymph node dissection (at least three mediastinal stations, including the subcarinal station) was performed according to current guidelines. All hilar and intrapulmonary (N1) lymph nodes were counted as a single station. Histological examination of the resected nodes/resection specimen and pTpN classification were performed according to current guidelines. 24
Histology
Cytology preparations were analysed using either May–Grünwald–Giemsa or Papanicolaou stains, dependent on local practice, with additional preparation of cell blocks for histological analysis where appropriate.
Sample size
The sample size calculation was based on demonstrating a 20% increase in sensitivity [the rate of detecting mediastinal nodal metastases (N2/N3)] from 70% with surgical staging alone to 90% with endosonography followed by surgical staging. Assuming 80% power and a two-tailed α-value of 5%, the required sample size was calculated to be 62 patients per group, total 124. It was further assumed that the prevalence of mediastinal nodal metastases would be 70% and the dropout rate 5%, giving a required sample size of 93 per group, or 186 in total. However, as interim monitoring revealed the prevalence of mediastinal nodal metastases to be 55% rather than 70%, the sample size was increased to 240 patients.
Outcome measures
The primary clinical outcome was the sensitivity of each diagnostic strategy for detection of mediastinal nodal (N2/N3) metastases. The denominator for the calculation of sensitivity was taken to be the number of patients in whom histological examination of nodal tissue biopsied during any procedure was positive for cancer (EUS/EBUS, mediastinoscopy, thoracotomy). The numerator was the number of patients in whom histology was positive during the diagnostic phase (EUS/EBUS and/or mediastinoscopy, depending on group). Patients with tumour-positive nodal findings at EUS, EBUS or surgical staging were regarded as true-positives, as further validation of these findings was judged unethical. The final reference status of the patient was positive if any diagnostic test was positive or if nodal involvement was detected after thoracotomy. The related NPV was also calculated as the number of patients who were free of nodal involvement as a proportion of the number of patients with negative tests during the diagnostic phase. This is interpreted as the probability of a final diagnosis of no metastases given the diagnostic tests were all negative.
Other outcome measures were:
-
determination of the sensitivity of EBUS and EUS (followed by surgical staging) compared with surgical staging alone for determining mediastinal tumour invasion (T4)
-
EQ-5D items and associated utility at end of staging (before thoracotomy) and 2 and 6 months after randomisation
-
cost–utility of the endosonography diagnostic strategy (including surgical staging if negative) relative to surgical staging alone up to 6 months after randomisation
-
complication rates
-
the rate of futile thoracotomies that could be avoided by performing EBUS-TBNA and EUS-FNA rather than surgical staging alone, defined as nodal metastases, tumour invasion, distant metastases, non-malignant disease or death within 30 days of procedure
-
interobserver variability of cytopathological evaluation of EBUS-TBNA and EUS-FNA samples.
Statistical analysis
In the primary analysis, estimation of sensitivity and NPVs was performed on an intention-to-treat basis. Patients in whom diagnostic tests were negative and who did not undergo thoracotomy did not have a reference standard. For these patients, multiple imputation based on the binomial distribution was used for the missing reference standard. An additional worst-case scenario analysis assumed that patients who were staged node negative, but in whom surgical verification was missing, were considered to be false-negatives. A κ-value was calculated to assess the interobserver variability of EUS and EBUS cytology samples. In exploratory analysis, the groups were compared using Fisher’s exact test for binary categorical variables, the chi-squared test for other categorical variables and the independent Student’s t-tests for continuous normally distributed variables. EQ-5D utilities were compared using linear models that included the baseline value as well as the group allocation. Survival rates from randomisation to death or last known survival date were estimated using the Kaplan–Meier method, and compared using a log-rank test. Details of statistical methods used in the cost-effectiveness analysis are given below.
European Quality of Life-5 Dimensions analysis
The EQ-5D questionnaire consists of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. For each dimension, the patient indicates the level of problems experienced by one of three responses: no problems (score 1), some problems (score 2) or extreme problems (score 3).
The EQ-5D questionnaire was completed using standard pro forma at baseline, at the end of staging (after surgical staging but before thoracotomy) and after 2 months and 6 months for all patients recruited at Papworth Hospital. 25 This information was collected for patients in the continental European centres who were recruited after April 2008. For these patients the established Dutch- or Flemish-language versions of the EQ-SD were used. Between February 2007 and April 2008 EQ-5D, data were not available from the continental European centres. As this represented a block of time for which no patient completed the EQ-5D, this information was reasonably assumed to be missing at random.
The social tariff for the EQ-5D, as estimated by Dolan et al.,26 was applied to each patient’s self-reported classification in order to calculate utility values for each patient. 26 Although European tariffs exist for the EQ-5D, this report is from a UK perspective so the UK tariff was applied to all responses. Utilities were scaled so that full health = 1 and death = 0. In the case of patients in whom between one and four dimensions (out of five) of the EQ-5D were missing, a single imputation using an ordinal logistic regression model was used to impute the missing values.
Initially, utilities were summarised according to the nominal times of completion (baseline, end of staging, 2 months, 6 months) of the questionnaires. In order to estimate EQ-5D values at the same times after randomisation for each patient, the exact dates that the questionnaires were completed were used and linear interpolation between the recorded EQ-5D values on these dates gave an estimate at specific days post-randomisation. This allowed estimation of utilities at times 0, 7, 61 and 183 for all patients, and these were summarised. If EQ-5D questionnaire dates were not available, end of staging, 2- and 6-month questionnaires were assumed to have been completed at 7, 61 and 183 days after randomisation, respectively.
In the case of patients who died within 183 days of randomisation (four EUS/EBUS and seven surgical patients), EQ-5D was assumed to be ‘0’ at the date of death and thereafter. Interpolation between the last recorded EQ-5D and an EQ-5D of ‘0’ at the date of death was carried out to obtain EQ-5Ds for each time point (0, 7, 61 and 183 days).
For patients who died after 183 days but did not have EQ-5Ds recorded for all dates up to day 183, interpolation was again performed between the last known EQ-5D and an EQ-5D of ‘0’ at the date of death (two EUS/EBUS and two surgical patients).
For patients who had monotonic missing EQ-5D values (i.e. had an EQ-5D value up to a certain time point, but all subsequent EQ-5D values missing) and did not die within or after the study, the last recorded EQ-5D value was carried forward. One patient (randomised to the endosonography strategy) had the baseline EQ-5D value carried forward to 6 months. Two patients (one EUS/EBUS and one surgical staging) had the end of staging EQ-5D carried forward. Four patients (one EUS/EBUS and three surgical staging) had the 2-month value carried forward to 6 months. The analysis was repeated using a subset of data that excluded these patients from the quality-adjusted life-year (QALY) calculations and the results were very similar.
For the patients who had an EQ-5D recorded at each time point and did not die within or after the study but the 6-month EQ-5D was earlier than 183 days after randomisation, the last EQ-5D was carried forward. Twelve people from the EUS/EBUS group and 13 from the surgical staging group were included in this group. All had last dates recorded that were within 10 days of the end of the study, except one patient who had the final follow-up at 155 days. This was considered to be a reasonable method to use, as the EQ-5D for these patients would have been unlikely to change dramatically without the investigators knowledge in such a short time period.
Exploratory cost-effectiveness analysis
The 6-month QALY was estimated for each patient using the area under the curve (AUC) method. The maximum QALY achievable was therefore 0.5 years. As the groups were randomised, adjustment for baseline utility was unnecessary in the exploratory analysis.
Costs were estimated from a health-service provider viewpoint using resource use from all of the patients in the trial and, in the base case, costs from a UK NHS perspective. For resource use, a study-specific data collection form was designed (see Appendix 2). Data were recorded prospectively after April 2008 and retrospectively for patients recruited before April 2008. Forms were returned to the Papworth Hospital research and development (R&D) unit for data processing and analysis.
Figure 1 shows the flow diagram of resource use for the ASTER trial.
The following resource-use components were recorded: EBUS/EUS, surgical staging, thoracotomy, surgery other than planned thoracotomy, chemotherapy, radiotherapy, hospital stays and hospice stays.
The final costs assigned to each component of resource use are summarised in Table 1. For standard treatments and procedures the NHS Reference Costs 2008–0927 was used, with specific procedures as shown in Table 1. For EBUS-TBNA and EUS-FNA there were no NHS reference costs, so they were estimated by Papworth Hospital finance department. The costs included staff time, bed occupancy, and hospital costs and equipment, which were assumed to have a 5-year lifetime. Full details of the costing of the endosonography procedures are given in Table 2. Similar unit costs for staging using EBUS/EUS were elicited by the finance departments at the centres in Ghent (€671.8) and Leiden (€1506).
| NHS resource | Mean unit cost (quartiles) (£) | Source |
|---|---|---|
| Hospital/hospice costs | ||
| EUS/EBUS procedure | 1237 | Papworth NHS finance department estimates |
| Surgical staging procedure | 3056 (2360 to 3652) | Code DZ04B27 |
| Surgical staging procedure cost from day 10 | 329 (217 to 424) | Code DZ04B27 |
| Thoracotomy (lobectomy or pneumonectomy) with lymph node dissection | 6525 (5917 to 6903) | DZ02B27 |
| Thoracotomy cost from day 44 | 318 (218 to 458) | Code DZ02B27 |
| Deliver simple parenteral chemotherapy at first attendance1 | 272 (98 to 234) | Code SB12Z27 |
| Deliver subsequent elements of a chemotherapy cyclea | 227 (121 to 236) | Code SB15Z27 |
| Radical radiotherapy (very first fraction) | 274 (123 to 415) | Code SC02Z27 |
| Subsequent radical radiotherapy fractions | 112 (68 to 137) | Code SC22Z27 |
| Palliative radiotherapy (very first fraction) | 274 (123 to 415) | Code SC02Z27 |
| Subsequent palliative radiotherapy fractions | 112 (68 to 137) | Code SC22Z27 |
| Hospital admission | 2126 (1543 to 2475) | Code DZ17B27 |
| Cost of hospital admission per day from day 32 | 224 (168 to 256) | Code DZ17B27 |
| Hospice admission per day | 399 (337 to 406) | Code SD01A27 |
| Surgery | 4120 (3197 to 4677) | Code DZ03B27 |
| Laboratory costs | ||
| Following EUS/EBUS procedure | 17 (9 to 22) | Code DAP83827 |
| Following surgical staging procedure | 26 (7 to 36) | Code DAP82427 |
| Following thoracotomy (lobectomy or pneumonectomy) with lymph node dissection | 26 (7 to 36) | Code DAP 82427 |
| Component | Total cost including VAT (£) | Equipment life (years) | Activity | Unit cost (£) | Notes |
|---|---|---|---|---|---|
| EBUS scope | 61,000 | 5 | 150 | 81 | Assumes 5-year life |
| EUS scope | 92,500 | 5 | 100 | 185 | Assumes 5-year life |
| Ultrasound processor | 58,300 | 5 | 150 | 78 | Assumes 5-year life |
| Two consultants | 300,000 | 3404 | 88 | Assumes 1-hour procedure | |
| Two Band 6 nurses | 60,510 | 3404 | 18 | Assumes 1-hour procedure | |
| One health-care assistant | 15,500 | 1702 | 9 | Assumes 1-hour procedure | |
| Aspiration needle | 155 | 2 | 310 | As per consumables schedule | |
| Balloon | 15 | 1 | 15 | As per consumables schedule | |
| Single-use suction valve | 3 | 1 | 3 | As per consumables schedule | |
| Sterilisation of scopes | 16 | 2 | 32 | ||
| Maintenance contract | 19,000 | 150 | 127 | Assumes maintenance for above equipment only | |
| Day ward bed-day | 150 | 1 | 150 | ||
| Hospital overheads, including capital charges – 15% | 945 | 15% | 142 | Trust overhead included in annual trust costing exercise | |
| Total | 1237 |
The total expected costs from randomisation to 6 months were estimated by summing the resource use multiplied by its unit cost and taking the sample average for each group. The incremental cost-effectiveness ratio (difference in costs divided by difference in effects) was calculated using the sample differences.
A first analysis was restricted to ‘completers’, i.e. individuals for whom both complete cost and QALY information was available. All patients completed a resource-use questionnaire and data surrounding the initial diagnostic strategy was complete. However, because subsequent treatment was often administered in a patient’s local oncology centre (distant from the tertiary diagnostic referral centre), some resource-use information was missing. The number of missing data for each category was:
-
EBUS/EUS (0 missing)
-
surgical staging (0 missing)
-
thoracotomy (0 missing)
-
surgery other than planned thoracotomy (28 missing)
-
chemotherapy (35 missing for 0–2 months, 30 missing for 2–6 months)
-
radiotherapy (22 missing for 0–2 months, 26 missing for 2–6 months)
-
hospital stays (34 missing for 0–2 months, 28 missing for 2–6 months)
-
hospice stays (26 missing for 0–2 months, 29 missing for 2–6 months).
Values were imputed for these missing resource-use items. In this exploratory analysis, a single imputation was performed as follows. Patients were divided by centre, randomisation group and stage (N2-/N3-positive or N2-/N3-negative, as determined at the end of the surgical staging procedure in both groups – i.e. for the EUS/EBUS group, this was the number of people who were found to be N2/N3 positive after endosonography added to the number found to be N2/N3 positive after endosonography followed by surgical staging). Within each of these subgroups, the mean cost for each item was calculated from cases with available information and imputed for those with missing values.
To estimate the standard errors and CIs for the mean cost and QALY, bootstrap samples were generated and the results plotted on the cost-effectiveness plane and as a cost-effectiveness acceptability curve (CEAC).
Full data cost-effectiveness analysis
Bayesian parametric modelling28 was used to estimate expected costs and QALYs. This allowed the estimation of cost-effectiveness to include information from all of the patients rather than just patients for whom complete cost and QALY data were available. The methods are unbiased under the assumption that the missing data were ‘missing at random’; in other words, whether or not an observation is missing depends on other variables for which we adjust, but not on the missing value itself. The QALY can be assumed to be missing ‘completely at random’, as quality-of-life data collected were collected only for patients recruited at later time points.
Overall, QALYs over 6 months were missing for 97 out of 241 patients. The model used for imputation of the missing QALYs was a truncated normal distribution (see Table 3). The mean before truncation was modelled in terms of predictors (randomisation group, centre and baseline EQ-5D) and the resulting posterior distribution for the expected QALY over 6 months was used in the cost-effectiveness estimate.
| Parameter | Imputation model |
|---|---|
| Observed QALYs between randomisation and 6 months | Truncated normal, truncated between the theoretical minimum (–0.297) and maximum (0.5) |
| Day in hospital for EBUS/EUS (0 or 1) | Bernoulli |
| Days in hospital for surgical staging | Beta-binomial |
| Days in hospital for thoracotomy | Beta-binomial |
| Surgery other than planned thoracotomy (0 or 1) | Binomial |
| Chemotherapy cycles | Poisson |
| Radiotherapy fractions | Poisson-gamma |
| Days in hospital | Beta-binomial |
| Days in hospice | Binomial |
As described in the exploratory analysis, some resource-use costs were missing As these costs arise as counts of events (multiplied by a fixed unit cost), a model was defined for each of these event counts. Different parametric models were used to represent the distribution of the event counts for each component.
Although there were no missing data for EUS/EBUS, it was modelled as a binary outcome in order to include it in the model for total mean cost. Surgery other than the planned thoracotomy was modelled as a binary outcome. For the remaining events, owing to the high number of zero counts, a hurdle count-data model was used. 29 In this methodology, a proportion of the patients did not have the event and so were given a value of zero, whereas the remaining patients did have the event and were given a value greater than zero to reflect, for example, the number of days in hospital or the number of fractions of radiotherapy. The non-zero count was assumed to come from a standard count model such as the binomial or Poisson distribution truncated below to be greater than zero. Overdispersed equivalents of these models were used where the counts had a high variance. Binomial or beta-binomial distributions were used for count data with theoretical maximum values. In this case, the number of days spent in hospital over 6 months has a theoretical maximum of 183 days. The beta-binomial is an overdispersed version of the binomial, whereby the outcome probability is allowed to vary according to a beta prior. Poisson distributions were used for theoretically unbounded count data, with a gamma prior assigned to the rate parameter when necessary to allow for overdispersed counts. Specific models for the resource-use components are summarised in Table 3.
Several individuals had a particular cost component observed for 0–2 months, but missing for 2–6 months, and vice versa. For these cases, this component was modelled as right censored at the observed cost value.
The probability of a non-zero cost was modelled in terms of covariates using logistic regression. For hospice admission, the only covariate used in this part of the model was randomisation group. For all other events, randomisation group, centre and stage were included as covariates. Where appropriate, the mean of the Poisson(-gamma) or (beta-)binomial non-zero component is also modelled in terms of randomisation group, centre and stage, except for radiotherapy, for which there was insufficient information to be able to model any covariates. For the Poisson distribution, log-linear regression on the rate was assumed, and, for the beta-binomial distribution, adjustment was based on logistic models.
The total expected cost was calculated as the sum of the component-specific expected costs for each randomisation group, while averaging over the other patient characteristics.
As a Bayesian model was used, uncertainty about the unit costs could be acknowledged. The UK mean unit cost estimates and upper and lower quartiles were available for each resource (see Table 3). These were used to define gamma prior distributions for each unit cost. 30 The point estimates of the unit cost in Table 2 were assigned to the mean, and the variance was estimated as the variance of the normal distribution with the same mean and interquartile range (IQR), so that IQR = 1.35 standard deviation (SD).
The freely available software package WinBUGS31 (MRC Biostatistics Unit, Cambridge, UK) was used to estimate the joint posterior distribution of all unknown parameters involved in the cost and QALY models, hence the posterior distributions of expected total cost and QALY. The ‘WBDev’ add-on to WinBUGS was used to calculate the lower tail probabilities and conditional tail expectations of the binomial distribution, which was required because of the time-dependent change in costs for certain components, for example after 32 days in hospital.
Deterministic sensitivity analysis
One potential alternative diagnostic strategy was identified a priori for investigation. The value of using endosonography as the only diagnostic modality to exclude nodal involvement was assessed by excluding the costs of the confirmatory surgical staging in this group, but adding in costs for additional futile thoracotomies that would have resulted from the lower sensitivity of these tests when used alone. The QALYs were not adjusted, as the difference between groups was very small and the proportion of patients for whom utilities would change is also small.
Chapter 3 Clinical outcomes
Trial progress
Between February 2007 and April 2009, 357 consecutive patients with potentially resectable (confirmed or suspected) NSCLC were assessed for eligibility. Of these, 98 patients did not meet the inclusion criteria owing to previous therapy for lung cancer (n = 54), concurrent cancer at another site (n = 17), improbability of being staged correctly by surgery (n = 20) or inability to give informed consent (n = 7). Eighteen patients were eligible but not included because the patient refused consent (n = 6), referring doctors were unwilling to include the patient (n = 7), the patient was too deaf to complete study requirements (n = 1), urgent thoracotomy was required (n = 1), the patient was known to be non-compliant (n = 1), the patient had no health insurance (one continental European patient) or there were logistic problems (n = 1). The remaining 241 patients were randomised, 118 (49%) to surgical staging and 123 (51%) to endosonography followed by surgery if negative [Figure 2, the CONSORT (CONonsolidated Standards Of Reporting Trials) diagram]. Of the 241 patients recruited, 88 were from Ghent, 81 from Leiden, 44 from Leuven and 28 from Papworth.
Baseline characteristics
The average age of patients was 64.5 years [standard deviation (SD) 8.9 years] and men were in the majority in both groups (74% men in the surgical staging arm and 80% men in the endosonography arm). Further clinical characteristics can be found in Table 4.
| Baseline variable | Surgical staging alone (n = 118) | Endosonography and surgical staging (n = 123) |
|---|---|---|
| Mean (SD) age, years | 64.5 (9.1) | 64.6 (8.7) |
| No. (%) men | 87 (74) | 99 (80) |
| Centre, no. (%) | ||
| LUMC | 39 (33) | 42 (34) |
| Ghent | 43 (36) | 45 (37) |
| Papworth | 14 (12) | 14 (11) |
| Leuven | 22 (19) | 22 (18) |
| Indication for staging, no. (%) | ||
| Quamous cell carcinoma | 44 (37) | 46 (37) |
| Adenocarcinoma | 21 (18) | 28 (23) |
| Adenosquamous | 2 (2) | 3 (2) |
| Large cell carcinoma | 3 (3) | 6 (5) |
| Bronchoalveolar cell carcinoma | 1 (1) | 0 (0) |
| Carcinoma not specified | 18 (15) | 16 (13) |
| Suspected NSCLC | 29 (25) | 24 (20) |
| Tumour location, no. (%) | ||
| Left lower lobe | 17 (14) | 27 (22) |
| Left upper lobe | 18 (15) | 25 (20) |
| Right upper lobe | 30 (25) | 28 (23) |
| Middle lobe | 9 (8) | 10 (8) |
| Right lower lobe | 44 (37) | 33 (27) |
| Tumour stage on PET/CT, no. (%) | ||
| T1 | 26 (22) | 22 (18) |
| T2 | 66 (56) | 80 (65) |
| T3 | 11 (9) | 11 (9) |
| T4 | 15 (13) | 10 (8) |
| Nodal status on PET/CT, no. (%) | ||
| N0 | 15 (13) | 9 (7) |
| N1 | 17 (14) | 20 (16) |
| N2 | 66 (56) | 78 (63) |
| N3 | 20 (17) | 16 (13) |
| Mean (SD) short axis of largest lymph node (mm) | 12.3 (5.1) | 13.2 (4.2) |
| ACCP class, no. (%) | ||
| Massive enlargement (A) | 0 (0) | 0 (0) |
| Discrete enlargement (B) | 73 (62) | 76 (62) |
| Central tumour or hilar node (C) | 35 (30) | 33 (27) |
| Nodes < 10 mm (D) | 10 (8) | 14 (11) |
| Final histopathology grade, no. (%) | ||
| Squamous cell carcinoma | 47 (40) | 51 (41) |
| Adenocarcinoma | 40 (34) | 40 (33) |
| Adenosquamous | 5 (4) | 6 (5) |
| Large cell carcinoma | 6 (5) | 2 (2) |
| Bronchoalveolar carcinoma | 0 (0) | 1 (1) |
| Carcinoma not specified | 12 (10) | 19 (15) |
| Small cell carcinoma | 1 (1) | 4 (3) |
| Benign lesion | 5 (4) | 0 (0) |
| Unknown | 2 (2) | 0 (0) |
Surgical staging alone
Surgical staging was performed in 117 out of the 118 randomised patients. A distant metastasis was found in one patient after randomisation but before the surgical staging procedure could be performed. Cervical mediastinoscopy was performed in 116 of the 117 (99%) which was combined with parasternal mediastinoscopy in three and a thoracoscopy in two. Only one patient underwent a thoracoscopy. Of the 117 who underwent mediastinoscopy, data on mediastinal nodal status were incomplete in seven patients for the following reasons:
-
In one patient, mediastinal invasion (T4) was found during mediastinoscopy without verification of the nodal status.
-
Three patients in whom surgical mediastinoscopy staging was negative, declined verification by thoracotomy.
-
In one patient in whom surgical mediastinoscopy staging was negative, thoracotomy was not possible because of rapid clinical deterioration.
-
One patient underwent an open–close thoracotomy without nodal verification because of haemodynamic instability.
-
In one patient, direct mediastinal invasion was observed at thoracotomy and the surgeon decided to close the thorax without taking nodal biopsies.
In the 110 patients in whom staging was complete, a median of four (range 1–5) mediastinal nodal stations were sampled at surgical staging.
Overall, during staging, mediastinal metastases (N2/N3) were found in 41 out of 118 (35%) patients. In four patients, one without nodal metastases, direct mediastinal invasion of the lung tumour in the mediastinum (T4) was found. Thus, 42 patients had either mediastinal metastases (N2/N3) or mediastinal invasion (T4), or both. Thoracotomy was performed in 70 patients, and two patients underwent further diagnostic tests or had clinical evidence of metastatic disease. Of these patients, 10 had nodal metastases (of whom two also had mediastinal tumour invasion) and a further six had mediastinal invasion alone . Three patients who did not have evidence of mediastinal node involvement after surgical staging refused thoracotomy, so that their final nodal status was not confirmed.
Endosonography followed by surgical staging
Endosonography was performed in 123 patients and detected mediastinal nodal metastases in 56 out of 123 (46%). In five patients it was obvious on endosonographic imaging that the primary lung tumour invaded the mediastinum (T4); two of the five did not have nodal verification. Thus, surgical staging was avoided due to endosonography findings in 47% of patients (58/123). Sixty-five patients without evidence of mediastinal nodal metastases or mediastinal tumour invasion underwent surgical staging showing nodal metastases in six additional patients. These missed mediastinal metastases were located in stations 4R (n = 3), 5 (n = 1), 6 (n = 1) and 7 (n = 1), with those in stations 5 and 6 being out of reach for endosonography.
Fifty-eight (out of 59) patients in whom endosonography or surgical staging revealed no evidence of nodal metastases underwent thoracotomy with nodal dissection. One patient was found to have undeniable mediastinal invasion based on a second endosonography, but did not have nodal verification. In the 58 patients who underwent thoracotomy, nodal metastases were found in four, one of whom also had mediastinal tumour invasion; two others were found to have mediastinal tumour invasion without confirmation of nodal metastases. At endosonography and surgical staging a median of three (range 1–7) different mediastinal nodal stations were sampled. For 121 patients in the endosonography group, the interobserver agreement in relation to cytological diagnosis of samples was assessed by an independent pathologist (Table 5) with κ = 0.97 (95% CI 0.92 to 1.00).
| Local result | Reference observer’s result | Total | |
|---|---|---|---|
| Benign | Malignant | ||
| Benign | 63 | 2 | 65 |
| Malignant | 0 | 56 | 56 |
| Total | 63 | 58 | 121 |
Final diagnosis and false-negative findings
Of the 241 patients, 229 (95%) were diagnosed with NSCLC, five (2%) with small cell lung cancer (SCLC) and five (2%) with other diagnoses, such as sarcoidosis; in two patients (1%), the diagnosis could not be ascertained during the study. Overall, the prevalence of mediastinal nodal metastases (N2/N3) was 49% (117/241). At thoracotomy, a median of five (range 0 to 10) lymph node stations were obtained in both study arms. At pre-operative staging, nodal metastases were missed in 10 patients in the surgical staging arm (stations 4L, 4R, 5 and 7) and in four patients in the endosonography arm (stations 3A, 4L, 4R, 5, 8L and 8R). In eight patients (7%) in the surgical staging arm, negative lymph node findings at staging were not verified by surgery. One of these eight patients had bone metastases before staging and in one surgical staging revealed clear T4 disease and so nodes were not sampled; in a further four of the eight patients, mediastinoscopy was negative and they received no further treatment (three refused thoracotomy and one deteriorated clinically) and in two patients who underwent thoracotomy lymph node confirmation of stage was not performed. There were three patients (2%) in the endosonography arm in whom negative lymph node findings at staging were not verified by surgery: in two of these endosonography clearly demonstrated T4 disease and in one T4 disease was diagnosed by bronchoscopy.
Diagnostic accuracy
Mediastinal nodal (N2/N3) metastases were found in 41 out of 118 patients (35%) by surgery alone compared with 62 out of 123 patients (50%) by endosonography followed by surgical staging if negative (p = 0.02). In the intention-to treat analysis, sensitivity for detecting mediastinal nodal metastases by each of the staging strategies was 79% (41/52, 95% CI 66% to 88%) versus 94% (62/66, 95% CI 85% to 98%) (p = 0.02; Table 6). The corresponding NPVs were 86% (66/77, 95% CI 76% to 92%) and 93% (57/61, 95% CI 84% to 97%) (p = 0.18; Table 6). In the worst-case scenario of treating cases with no surgical verification of negative staging as false-negatives, the sensitivity of surgical staging alone was 68% (41/60, 95% CI 57% to 80%) compared with 90% (62/69, 95% CI 81% to 95%) for endosonography with surgical staging if negative, respectively (p = 0.006), with corresponding NPVs of 75% (58/77, 95% CI 66% to 85%) and 87% (53/61, 95% CI 78% to 94%), respectively (p = 0.08).
| Nodal invasion (N2/N3) | Surgical staging (n = 118) | Endosonography and surgical staging (n = 123) | p-value |
|---|---|---|---|
| No. (%) positive on endosonography | – | 56/123 (46%) | – |
| No. (%) positive on surgical staging | 41/117 (35%) | 6/65 (9%) | – |
| No. (%) positive on thoracotomy | 10/70 (14%) | 4/58 (7%) | |
| Sensitivity of initial strategy | 41/52a (79%) | 62/66 (94%) | 0.02 |
| NPV of initial strategy | 66/77 (86%) | 57/61 (93%) | 0.18 |
Detection of locally advanced disease
In addition to the patients with N2/N3 involvement identified above, the tumour was observed to have invaded the lymph nodes (T4) in one patient in the surgical staging group and in two patients in the endosonography strategy group. Tumour invasion alone was detected by thoracotomy in a further six patients in the surgical staging group and two in the endosonography strategy group. One further patient in the endosonography strategy group was referred for thoracotomy but underwent a second endosonography before planned surgery, which showed clear tumour invasion. Thus, 42 patients (36%) in the surgical staging arm were found to have locally advanced diseases (nodal metastases and/or unforeseen direct mediastinal invasion) during staging, compared with 65 patients (53%) in the endosonography arm (p = 0.009). When the one patient in the endosonography arm who had a second endosonography was removed, the difference remained significant (p = 0.01).
Unnecessary thoracotomies and complications
There were 21 unnecessary thoracotomies among the 118 patients randomised to surgical staging (18%), compared with nine unnecessary thoracotomies in 123 patients randomised to endosonography followed by surgical staging if negative (7%; p = 0.02) (Table 7).
| Reason | Surgical staging (n = 118) | Endosonography and surgical staging (n = 123) |
|---|---|---|
| N2/N3a | 5 | 2 |
| N2/N3/death within 30 days | 1 | 1 |
| N2/N3/M1 | 1 | 0 |
| N2/N3/T4 | 2 | 0 |
| N2/N3/T4/death within 30 days | 0 | 1 |
| T4 | 6 | 1b |
| M1 | 0 | 2 |
| SCLC | 0 | 1 |
| Benign lesions | 2 | 0 |
| Exploratory thoracotomy | 2 | 0 |
| Death within 30 days | 2 | 1 |
| Total | 21 | 9 |
The overall complication rate was 7 out of 118 (6%) in the surgical staging arm compared with 6 out of 123 (5%) in the endosonography arm (p = 0.78; Table 8). There was one pneumothorax that was considered to be directly related to endosonography. This occurred during a EUS-FNA procedure, during which the primary tumour was biopsied. With pleural drainage, full lung expansion was achieved.
| Complication | Surgical staging (n = 118) | Endosonography and surgical staging (n = 123) |
|---|---|---|
| Persistent hoarseness | 2 | 4 |
| Pneumothorax | 1 | 1 |
| Mediastinitis | 0 | 1 |
| Major bleeding | 3 | 0 |
| Conversion to thoracotomy | 1 | 0 |
| Total | 7 | 6 |
The remaining 12 complications were all directly related to the surgical staging procedure. The most common adverse event was persistent hoarseness as a result of recurrent nerve palsy, which was considered to be a severe complication if it lasted at least 6 months and was attributable to the mediastinoscopy. One patient presented with fever 24 hours after mediastinoscopy and mediastinitis was diagnosed; treatment with antibiotics resulted in full recovery.
Endosonography alone versus surgical staging
It is possible to estimate the diagnostic accuracy of endosonography alone using the first part of the endosonography arm strategy (i.e. without additional surgical staging). Of the 66 patients identified with mediastinal nodal involvement, this was observed during endosonography in 56 cases. The sensitivity estimates for surgical staging alone and endosonography alone were 79% (41/52; 95% CI 68% to 89%) and 85% (56/66; 95% CI 74% to 92%), respectively (p = 0.62). The corresponding NPVs were 86% (66/77; 95% CI 75% to 92%) and 85% (57/67; 95% CI 74% to 92%) (p = 1.00), respectively. Complications occurred in 7 out of 118 patients (6%) after surgical staging and in 1 out of 123 patients (1%) following endosonography alone (p = 0.03). Endosonography alone would have resulted in an additional six cases of unnecessary thoracotomy.
Summary
The clinical component of this RCT showed that a strategy of using combined EUS–FNA and EBUS-TBNA (followed by surgical staging only if these tests were negative) had higher sensitivity (94% vs 79%) and negative predicted probability (93% vs 86%) than surgical staging alone, and resulted in a lower rate of unnecessary thoracotomy (7% vs 18%). Other benefits of endosonography include less invasive testing, with no requirement for general anaesthesia or open surgery, and the small number of minor complications (1%).
Chapter 4 Survival and health-related quality of life
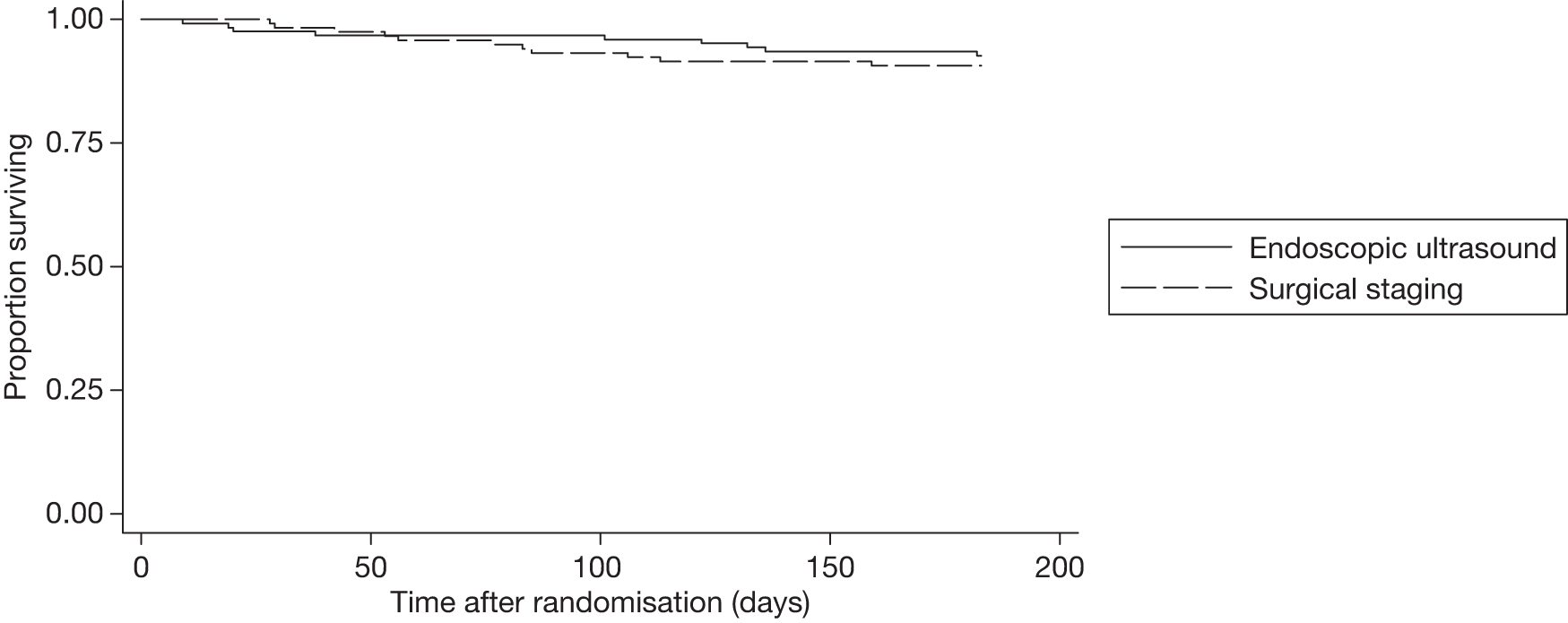
Patients were followed up for survival for 6 months after staging, during which period there were 20 deaths: nine in the endosonography strategy group and 11 in the surgical staging group. Kaplan–Meier estimates in Figure 3 show no difference in survival rates over the 6-month period (log-rank test, p = 0.57).
FIGURE 3.
Kaplan–Meier survival estimates for time (in days) to death.
Compliance
Of the 241 patients randomised into the study, 144 (60%) randomised after April 2008 were asked to complete the EQ-5D questionnaire at baseline, at the end of staging and 2 months and 6 months post-randomisation. All 144 patients completed the questionnaire at baseline. At end of staging and 2 months and 6 months post-randomisation, 139 (97%), 132 (92%) and 124 (86%) patients, respectively, completed the questionnaires. This gave a total of 539 completed questionnaires.
European Quality of Life-5 Dimensions
Of the 539 completed questionnaires, one or more of the dimensions of the EQ-5D was missing in six (1.1%). The missing values were imputed using a single imputation based on an ordinal logistic regression model, including the five dimensions of the EQ-5D.
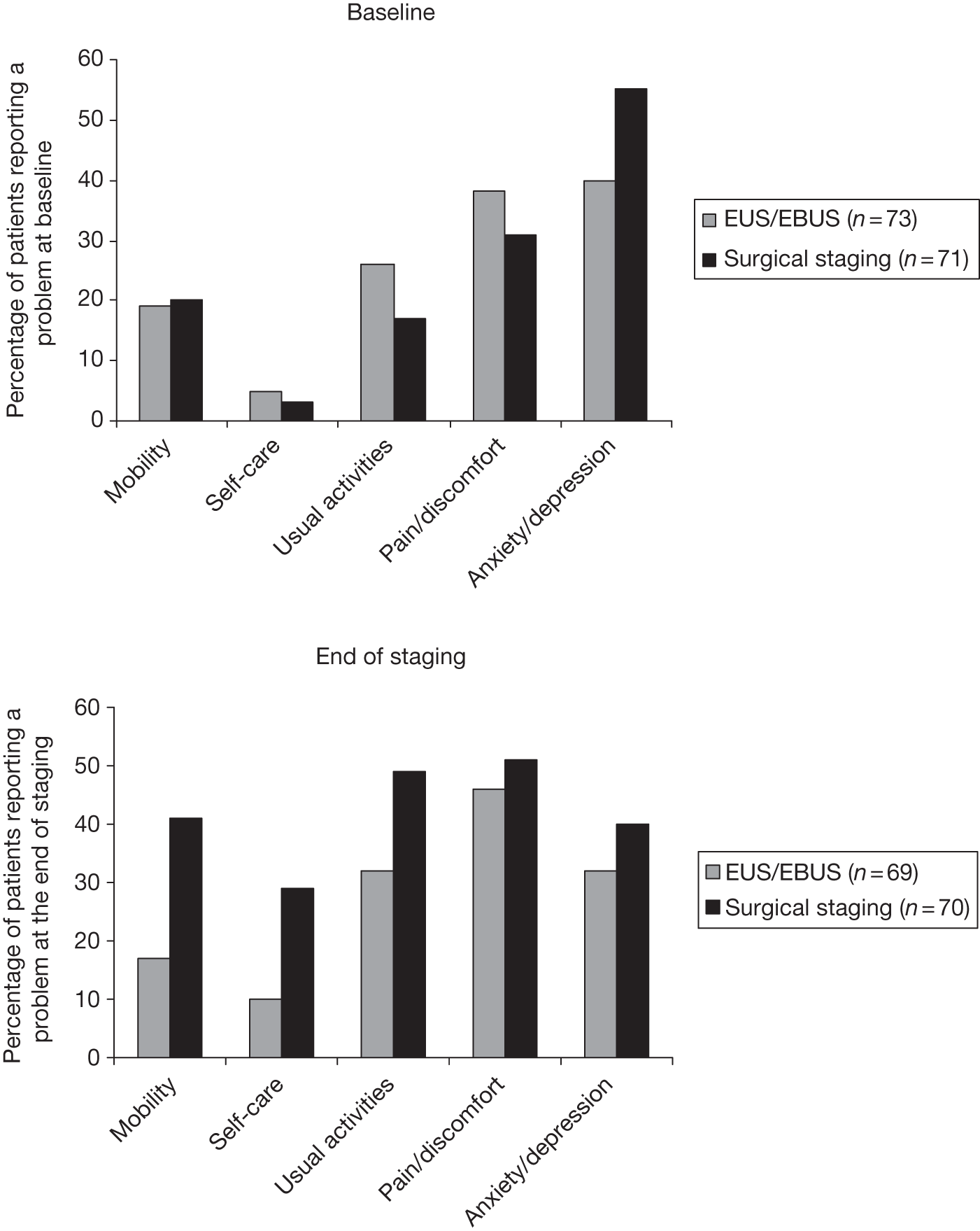
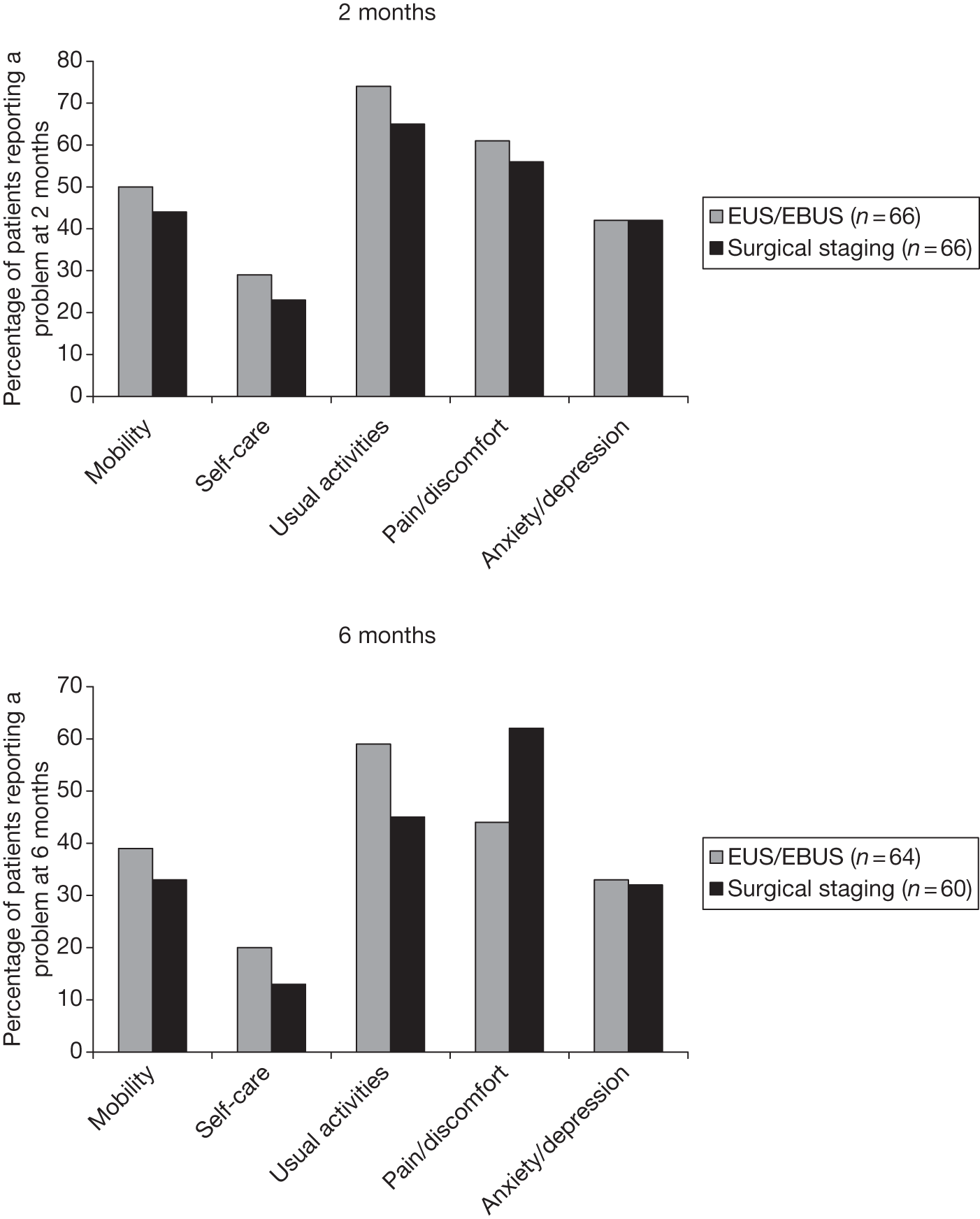
Figure 4 shows the percentage of patients reporting a problem in each dimension (i.e. those patients indicating some/moderate or extreme problems – score 2 or 3). At baseline, anxiety/depression was the most common problem in both groups, and the surgical group had approximately 15% more patients experiencing these symptoms. In all other dimensions, there was a < 10% difference between the groups. At the end of staging (for the endosonography group after surgical staging if the EBUS/EUS was negative or after the EBUS/EUS if it was positive), the surgical group reported more problems in every dimension than the endoscopic group. This was particularly noticeable in the mobility dimension, for which > 20% more patients in the surgical group reported problems. By 2 months the situation was reversed, with those in the endoscopic group faring worse in each dimension, although the differences were < 10%. At 6 months, the endoscopic group reported slightly more problems with usual activities, whereas the surgical group reported more problems with pain/discomfort. The other dimensions were similar between the groups.
FIGURE 4.
Percentage of patients reporting at least some problems on the EQ-5D dimensions. a, Baseline; b, end of staging; c, 2 months; d, 6 months.
European Quality of Life-5 Dimensions utility
The mean (SD) of the EQ-5D utility, by group, at each stage is summarised in Table 9. The values shown are for the nominal time points of baseline, end of staging, and 2 months and 6 months post-randomisation, not the actual dates that the questionnaires were collected. Therefore, there may be some inaccuracies in these estimates if, for example, the surgical staging group completed the end of staging questionnaire at a different time to the EUS/EBUS strategy group. Furthermore, this analysis includes only patients for whom questionnaires were completed. It does not make any attempt to impute missing EQ-5D information. The average age of patients was 65 years (SD 9 years) in both arms, and both groups had a majority of men (74% for the surgical staging arm vs 80% for endosonography and surgical staging). For a population with similar age and sex characteristics to patients in this study, the average EQ-5D score was 0.78. 32
| Time point | EUS/EBUS | Surgical staging |
|---|---|---|
| Baseline (n = 73, n = 71) | 0.81 (0.18) | 0.83 (0.14) |
| End of staging (n = 69, n = 70) | 0.77 (0.26) | 0.63 (0.34) |
| 2 months (n = 66, n = 66) | 0.65 (0.26) | 0.70 (0.24) |
| 6 months (n = 64, n = 60) | 0.74 (0.26) | 0.75 (0.22) |
At baseline, the groups were very similar with values slightly above the population average. However, by the end of staging, the average value for the surgical staging group had decreased by 0.20 compared with only 0.04 for the EUS/EBUS group. By 6 months, both groups were again similar but now slightly lower than the population average values.
European Quality of Life-5 Dimensions index at specified time points
Table 10 and Figure 5 show the estimated mean (SD) EQ-5D utility at days 0, 7, 61 and 183 after randomisation for all patients. This allows for a more accurate comparison between groups. There are 73 and 71 patients in the endosonography (followed by surgical staging if negative) group and surgical staging groups, respectively. All patients who completed a questionnaire at baseline had an EQ-5D calculated at each subsequent time point, with zero utility representing death. When compared with Table 9, the average value for the surgical staging group at the end of staging decreased from baseline to a similar extent. By 6 months, the average value had decreased from baseline by 0.13 and 0.16 for the EUS/EBUS and surgical staging groups, respectively.
| Time point | EUS/EBUS (n = 73) | Surgical staging (n = 71) |
|---|---|---|
| Baseline (day 0) | 0.81 (0.18) | 0.83 (0.14) |
| End of staging (day 7) | 0.78 (0.23) | 0.67 (0.29) |
| 2 months (day 61) | 0.64 (0.27) | 0.65 (0.26) |
| 6 months (day 183) | 0.68 (0.30) | 0.67 (0.31) |
FIGURE 5.
Mean (95% CI) EQ-5D utility at each time point in each group.
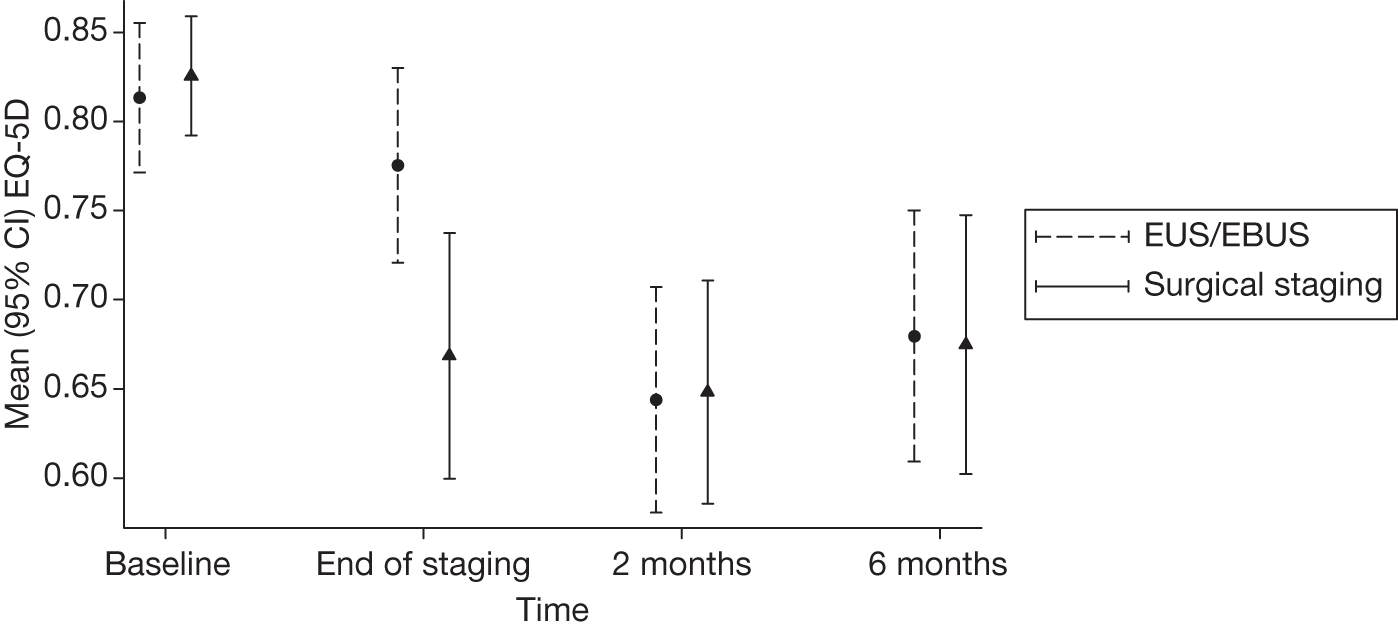
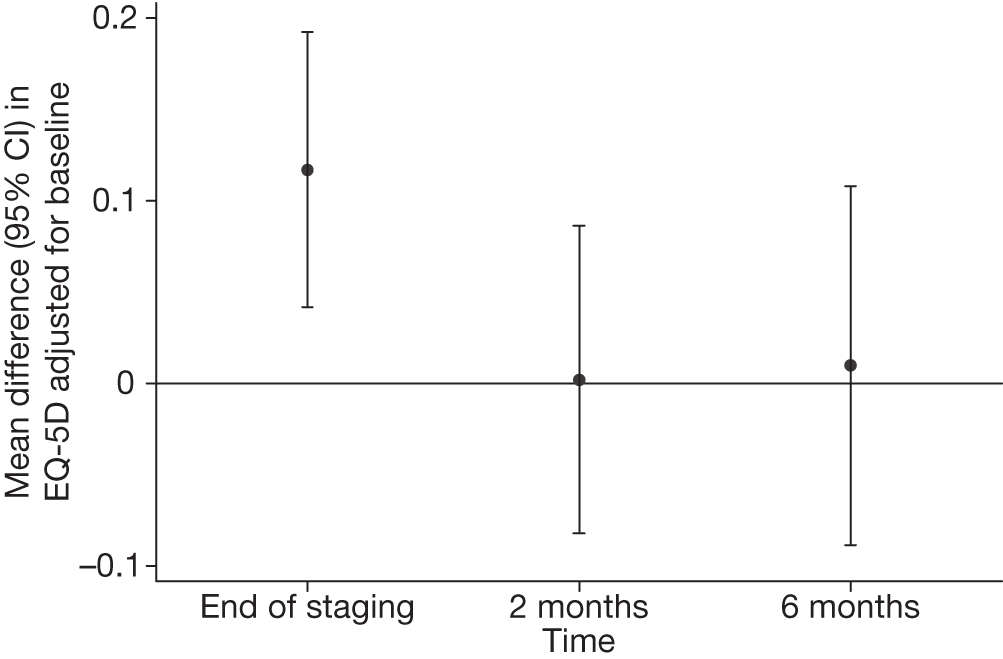
Table 11 shows the difference in EQ-5D between surgical staging and EUS/EBUS groups, both unadjusted and adjusted for baseline. In both analyses, there was a statistically significant difference between the groups at the end of staging, with those patients randomised to EUS/EBUS having a higher utility than those in the surgical staging group. However, at the other time points, there was little difference between the groups (Figure 6).
| Time point | Difference between EUS/EBUS and surgical staging (95% CI) | p-value |
|---|---|---|
| Unadjusted | ||
| End of staging | 0.107 (0.020 to 0.194) | 0.02 |
| 2 months | –0.004 (–0.092 to 0.084) | 0.92 |
| 6 months | 0.005 (–0.096 to 0.105) | 0.92 |
| Adjusted for baseline | ||
| End of staging | 0.117 (0.042 to 0.192) | 0.003 |
| 2 months | 0.002 (–0.082 to 0.086) | 0.96 |
| 6 months | 0.010 (–0.089 to 0.108) | 0.84 |
FIGURE 6.
Mean EQ-5D utility by day after randomisation.
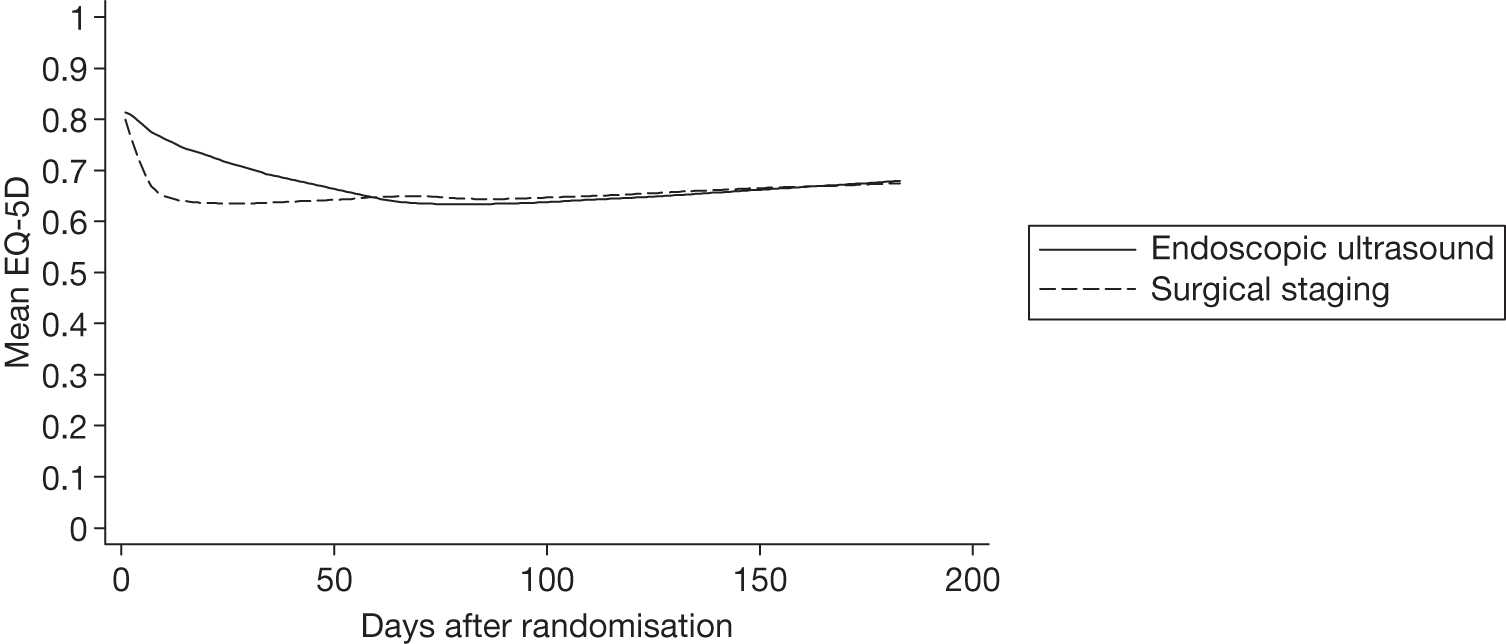
Figure 7 shows the mean difference (95% CI) in EQ-5D values between surgical staging and EUS/EBUS groups adjusted for baseline. This highlights the statistically significant difference between the groups at the end of staging (p = 0.003) when surgical staging patients had a worse quality of life than EUS/EBUS patients.
FIGURE 7.
Difference in mean EQ-5D (95% CI) between EUS/EBUS (followed by surgical staging if negative) and surgical staging alone, adjusted for baseline. Values > 0 favour the EUS/EBUS group.
Quality-adjusted life-year results by randomisation group
Table 12 shows that the mean (SD) 6-month QALY gain was very similar in the two randomisation groups. Once adjusted for baseline EQ-5D utility, the surgical staging group had a mean QALY gain that was 0.011 less than that of the EUS/EBUS group. The difference was not clinically or statistically significant (p = 0.55; Table 13).
| Measurement | EUS/EBUS (n = 73) | Surgical staging (n = 71) |
|---|---|---|
| 6-month QALY | 0.34 (0.12) | 0.33 (0.12) |
| Analysis | Mean (95% CI) difference in QALY between surgical staging and EUS | p-value |
|---|---|---|
| Unadjusted | 0.008 (–0.032 to 0.047) | 0.70 |
| Adjusted for baseline EQ-5D | 0.011 (–0.026 to 0.048) | 0.55 |
Comparison of European Quality of Life-5 Dimensions and quality-adjusted life-year between centres
There were some differences in the EQ-5D scores between the centres (see Appendix 3). In all centres except Ghent, EQ-5D values at the end of staging were lower in patients undergoing surgical staging than in those who had been allocated to EUS/EBUS. This was particularly apparent at Leuven, where the difference in average values was almost 0.30. By 6 months, EUS/EBUS patients from LUMC and Papworth were doing slightly better, on average, than the surgical patients, whereas the opposite was the case in Ghent and Leuven. However, this was an unadjusted analysis and simply reflects the fact that, at LUMC and Papworth, baseline EQ-5D scores were slightly higher in EUS/EBUS patients than in surgical patients, whereas in Ghent and Leuven baseline EQ-5D scores were slightly better in the surgical staging group than the EUS/EBUS group. The number of patients in each randomisation group, in each centre, was small so that these differences were consistent with between-centre variation. There were no significant differences in QALY gains between the surgical staging and the EUS/EBUS staging strategy in any of the centres. Details for each centre are given in Appendix 3.
Summary
The survival and quality-of-life components of this RCT show that a strategy of using combined EUS–FNA and EBUS-TBNA (followed by surgical staging only if these tests are negative) has no significant impact on survival to 6 months after randomisation and is only slightly more effective, with an increase in QALYs (over 6 months) of 0.011 (95% CI –0.026 to 0.048). However, we found that patients undergoing the endosonography staging strategy (including surgical staging if negative EUS/EBUS) had better quality of life at the end of staging than those who underwent surgical staging alone (mean difference 0.117; 95% CI 0.042 to 0.192). At all other time points, quality of life was similar in the two groups.
Chapter 5 Economic evaluation
A cost–utility analysis of surgical staging alone compared with EUS/EBUS and surgical staging (if the former was negative) from a health service perspective was undertaken. The time horizon was 6 months post-randomisation and all costs were reported in 2008–9 prices (£).
In terms of resource use, all patients followed the protocol for their group with the following exceptions:
-
One patient randomised to surgical staging did not have the procedure because of bone metastases.
-
Three patients randomised to surgical staging had a negative surgical mediastinoscopy staging, but refused thoracotomy.
-
In one patient randomised to surgical staging mediastinoscopy was negative, but because of rapid clinical deterioration the patient did not undergo thoracotomy.
-
One patient randomised to surgical staging who had a negative mediastinoscopy underwent endosonography rather than having a thoracotomy.
-
One patient in the EUS/EBUS group who had negative surgical staging underwent a second endosonography instead of having a thoracotomy.
-
Two patients in the EUS/EBUS group underwent additional surgical staging off protocol.
In addition, where both thoracotomy and surgical resection were recorded within 2 months of randomisation, this was assumed to be the same procedure unless there was evidence to the contrary.
Cost breakdowns for patients who had complete information on each resource item
The number and percentage of patients in each group using each resource item is shown in Table 14. Aside from the initial procedure (EUS/EBUS followed by surgical staging if negative, or surgical staging alone), the main difference in resource use was in the number of thoracotomies, 57 out of 87 (66%) patients in the surgical staging group compared with 45 out of 85 (53%) in the endosonography strategy group. Resource use was similar between the groups in all other items.
| Resource item | No. of patients using each resource item (%) | |
|---|---|---|
| EUS/EBUS (n = 85) | Surgical staging (n = 87) | |
| EUS/EBUS procedure | 85 (100) | 1 (1) |
| Surgical staging procedure | 47 (55) | 86 (99) |
| Thoracotomy (lobectomy or pneumonectomy) with lymph node dissection | 45 (53) | 57 (66) |
| Chemotherapy in the first 2 months | 43 (51) | 39 (45) |
| Radiotherapy in the first 2 months | 10 (12) | 9 (10) |
| Hospital admission in the first 2 months | 18 (21) | 19 (22) |
| Hospice admission in the first 2 months | 0 (0) | 0 (0) |
| Surgery between months 2 and 6 | 7 (8) | 9 (10) |
| Chemotherapy between months 2 and 6 | 40 (47) | 43 (49) |
| Radiotherapy between months 2 and 6 | 32 (38) | 27 (31) |
| Hospital admission between months 2 and 6 | 28 (33) | 25 (29) |
| Hospice admission between months 2 and 6 | 1 (1) | 0 (0) |
The total mean costs for each resource item are presented in Table 15 for those patients who had complete information on all resource items. This includes 85 out of 123 (69%) in the endosonography arm and 87 out of 118 (74%) in the surgical staging arm.
| Resource item | Mean cost per patient (£) | Difference (95% CI) | |
|---|---|---|---|
| EUS/EBUS (n = 85) | Surgical staging (n = 87)a | ||
| EUS/EBUS procedureb | 1254 | 14 | 1240 (1211 to 1268) |
| Surgical staging procedurec | 1712 | 3058 | –1346 (–1682 to –1010) |
| Thoracotomy (lobectomy or pneumonectomy) with lymph node dissectiond | 3543 | 4292 | –749 (–1737 to 239) |
| Total chemotherapy cost in the first 2 months | 528 | 401 | 127 (–35 to 288) |
| Total radiotherapy cost in the first 2 months | 225 | 292 | –68 (–318 to 183) |
| Total hospital admission costs in the first 2 months | 450 | 464 | –14 (–279 to 250) |
| Hospice admission in the first 2 months | 0 | 0 | 0 (0 to 0) |
| Surgery between months 2 and 6 | 339 | 426 | –87 (–449 to 275) |
| Total chemotherapy cost between months 2 and 6 | 493 | 574 | –80 (–289 to 128) |
| Total radiotherapy cost between months 2 and 6 | 1082 | 884 | 198 (–249 to 645) |
| Total hospital admission costs between months 2 and 6 | 766 | 747 | 19 (–436 to 473) |
| Hospice admission between months 2 and 6 | 97 | 0 | 9 (–9 to 28) |
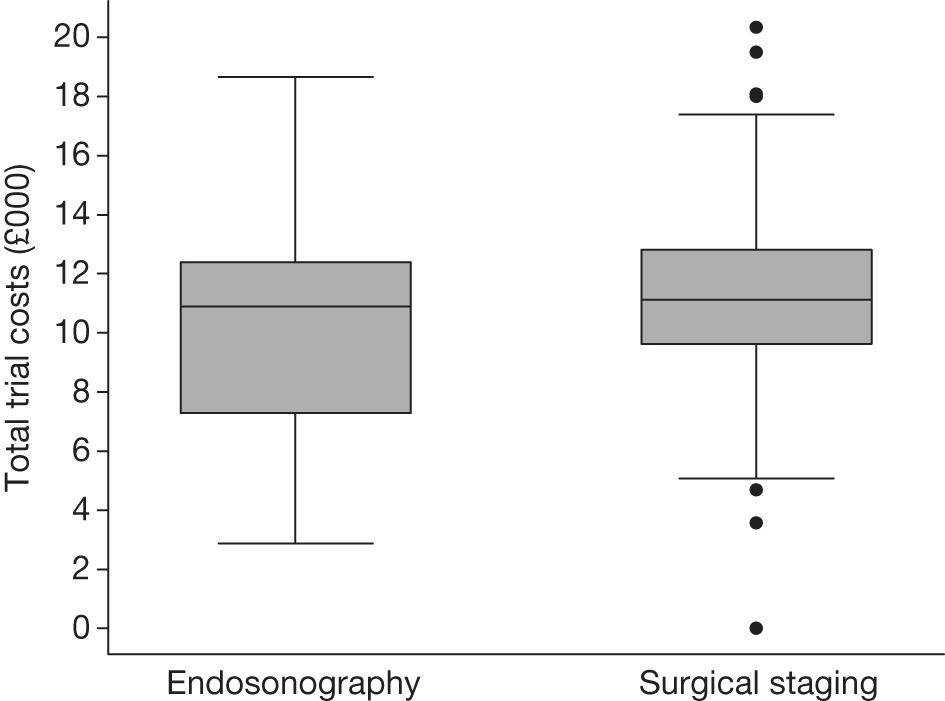
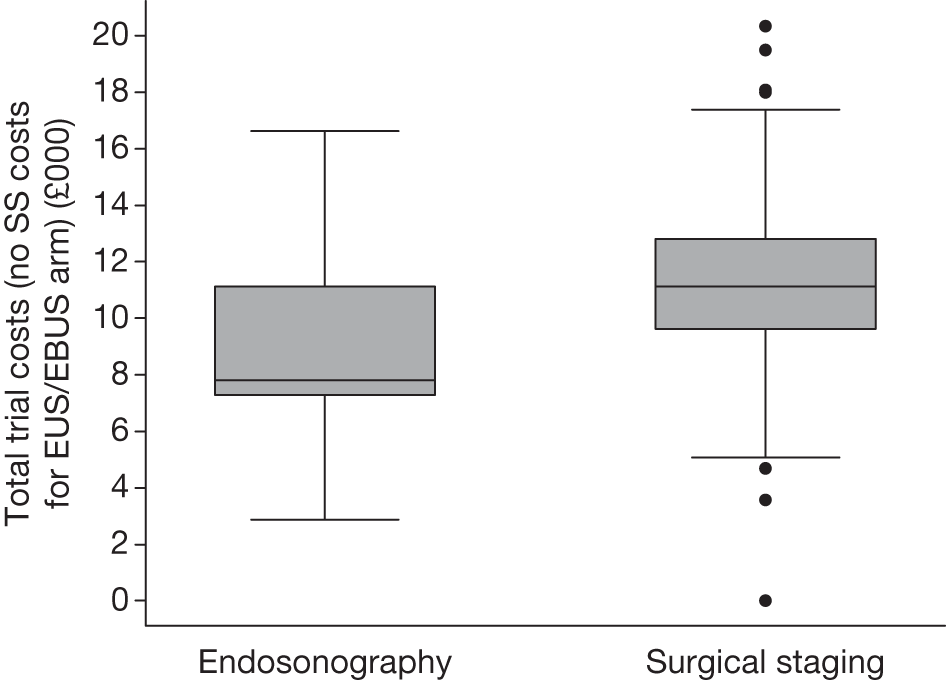
Total trial costs for patients who had complete information on each resource item
The total mean (SD) and median (IQR) trial costs are presented in Table 16 and Figure 8 for those patients who had complete information on all resource items.
| Randomisation group | Mean (SD) total cost (£) | Median (quartiles) total cost (£) |
|---|---|---|
| EUS/EBUS (n = 85) | 10,402 (3639) | 10,887 (7279 to 12,384) |
| Surgical staging (n = 87) | 11,154 (3567) | 11,130 (9633 to 12,801) |
FIGURE 8.
Box plot of total trial costs for patients who had complete information on all resource items: endosonography, n = 85; surgical staging, n = 87.
Cost–utility for completers (patients who have both complete costs and a quality-adjusted life-year estimate)
Both complete cost and QALY information was available for 58 out of 123 (47%) patients in the endosonography (followed by surgical staging if negative) group and 56 out of 118 (47%) in the surgical staging group.
Table 17 and Figure 9 show summaries of total costs for these patients. Figure 10 shows a box plot for the 6-month QALYs by group.
Endosonography (followed by surgical staging if negative) was cheaper overall than surgical staging (Table 18). However, the CI for the mean difference goes from it being cheaper by £2246 to being £394 more expensive. The difference in QALY between the groups is very close to zero. This would make any estimation of the incremental cost-effectiveness ratio (ICER) extremely unreliable.
| Randomisation group | Mean (SD) total trial cost (£) | Median (IQR) total trial cost (£) |
|---|---|---|
| EUS/EBUS (n = 58) | 10,808 (3787) | 10,887 (7778 to 13,013) |
| Surgical staging (n = 56) | 11,735 (3477) | 11,629 (9633 to 13,755) |
| Parameter | Mean | Median | SD | 95% CI for mean |
|---|---|---|---|---|
| Costs (£) | ||||
| Endosonography and surgical staging | 10,808 | 10,887 | 3787 | 9843 to 11,764 |
| Surgical staging | 11,735 | 11,629 | 3477 | 10,843 to 12,674 |
| Cost comparisons (£) | ||||
| Endosonography – surgical staging | –927 | 5141 | –2246 to 394 | |
| QALY | ||||
| Endosonography and surgical staging | 0.348 | 0.361 | 0.103 | 0.321 to 0.373 |
| Surgical staging | 0.342 | 0.350 | 0.100 | 0.316 to 0.367 |
| QALY comparisons | ||||
| Endosonography – surgical staging | 0.00652 | 0.143 | –0.0298 to 0.0418 | |
| Cost per QALY gained | ||||
| Endosonography – surgical staging (from sample) | Dominance | |||
| Bootstrapped mean ICER | Dominance | |||
FIGURE 9.
Total trial costs for patients who had both complete cost and QALY information: endosonography, n = 58; surgical staging, n = 56.
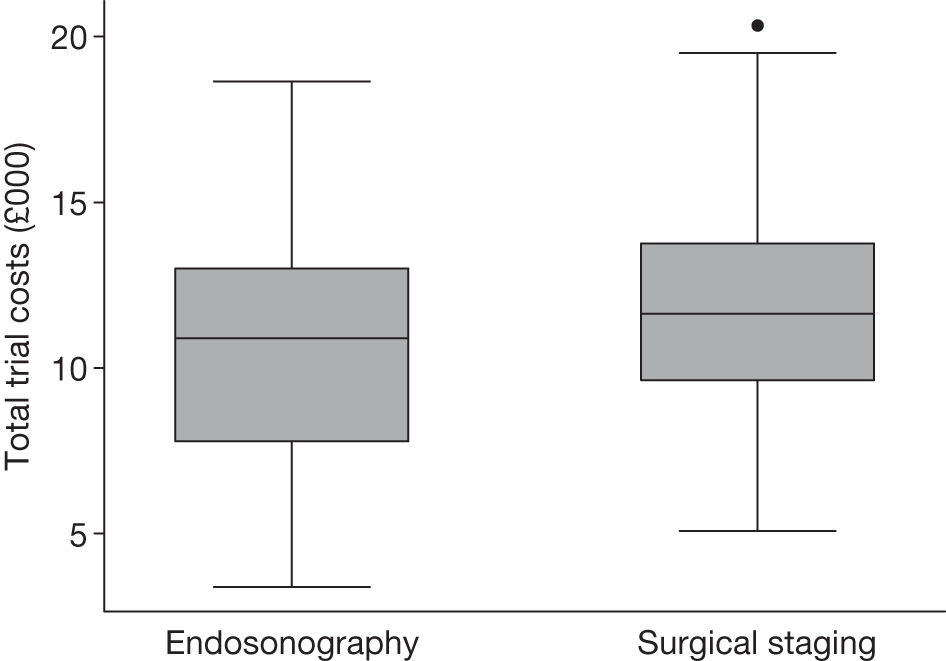
FIGURE 10.
Six-month QALY for patients who had both complete cost and QALY information: endosonography, n = 58; surgical staging, n = 56.
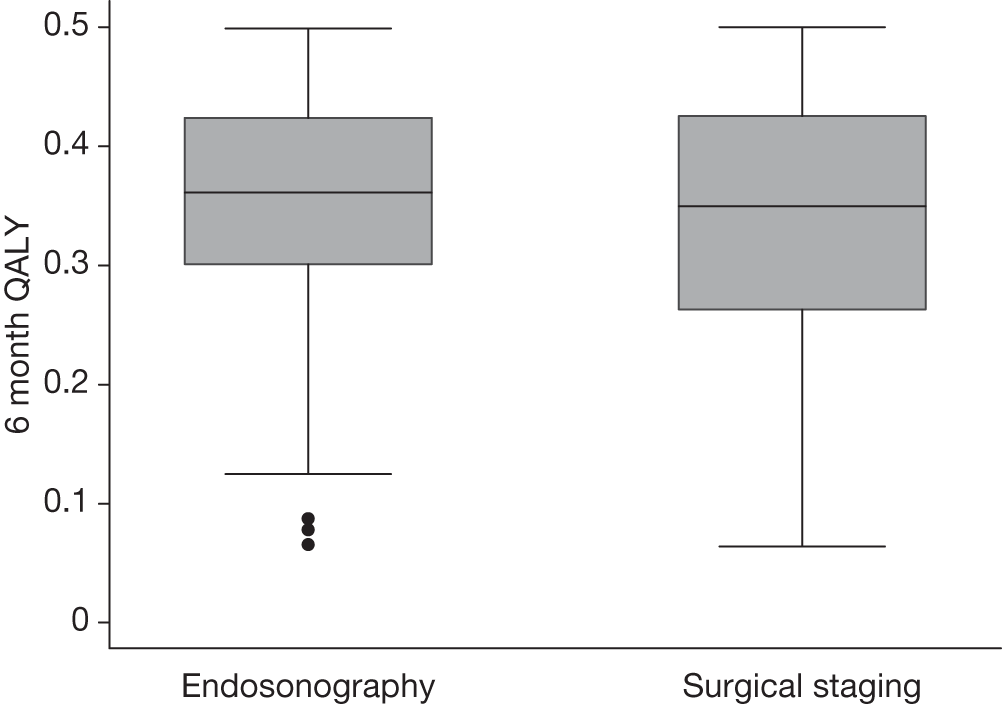
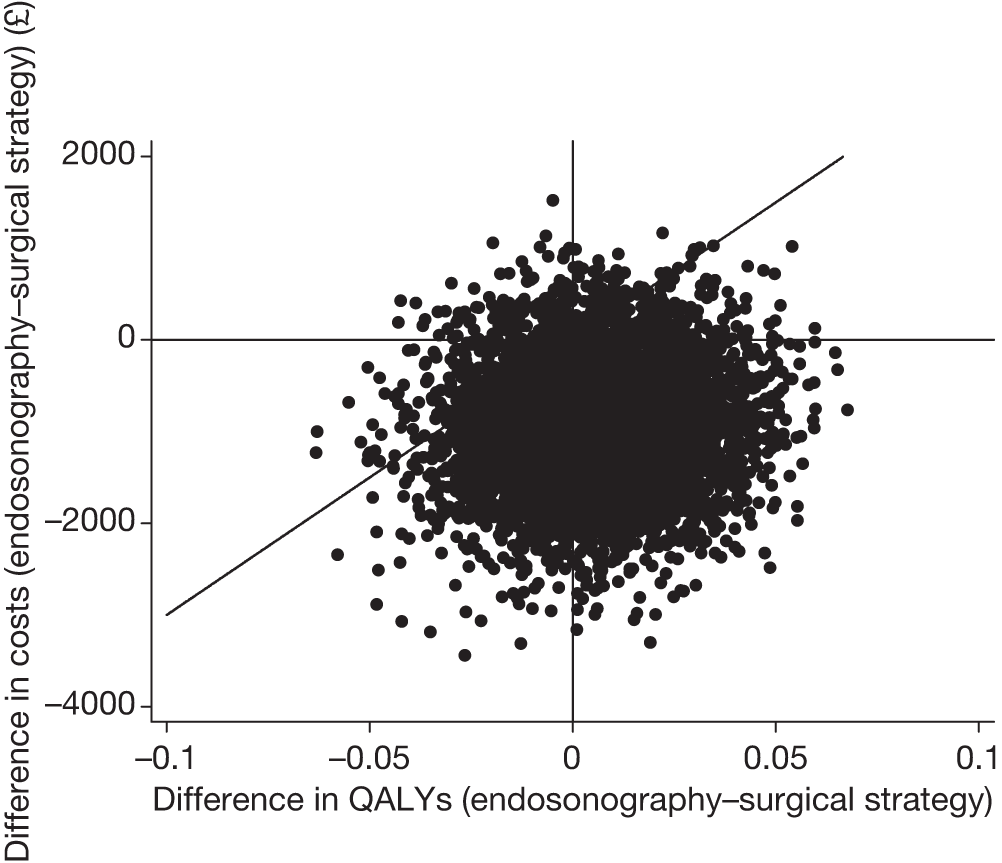
Figure 11 shows 5000 bootstrapped estimates of the cost and QALY differences: 2917 out of 5000 (58%) points show that endosonography, followed by mediastinoscopy if negative, is dominant, i.e. less costly and more effective (bottom right-hand quadrant); 1687 out of 5000 (34%) points show that the endosonography strategy is less costly and less effective (bottom left-hand quadrant); 253 out of 5000 (5%) points show that the endosonography strategy is more costly and more effective (top right-hand quadrant); and 143 out of 5000 (3%) points show that the endosonography strategy is dominated, i.e. more costly and less effective (top left-hand quadrant).
FIGURE 11.
Five thousand bootstrapped samples from the joint distribution of the difference in costs (endosonography strategy – surgical staging) and the difference in QALYs. Positive values indicate that endosonography (followed by surgery if negative) costs more and has greater QALYs. The line shows an ICER of £30,000 per QALY.
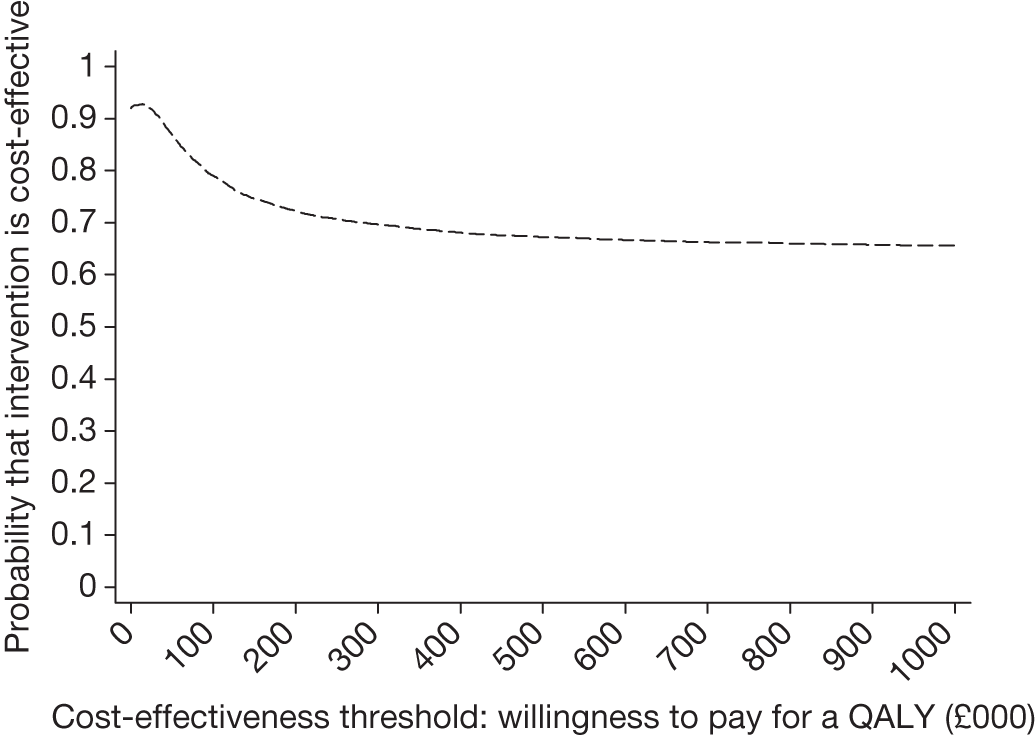
The CEAC for endosonography followed by surgery if negative (Figure 12) crosses the y-axis at 0.92, meaning that 92% of the density involves cost savings. It is a decreasing function of the willingness to pay because not all of the joint density involves health gains, hence the CEAC asymptotes to a value of < 1. At a willingness to pay of £30,000, there is a 91% chance that endosonography strategy compared with surgical staging strategy is cost-effective. The corresponding probability of cost-effectiveness for a willingness to pay of £20,000 is 92%.
FIGURE 12.
Cost-effectiveness acceptability curve for endosonography (with surgical staging if negative) relative to surgical staging alone, based on completers only (endosonography strategy, n = 58; surgical staging, n = 56).
Deterministic sensitivity analysis
The only a priori defined scenario analysis was used to compare surgical staging with endosonography alone, hence assuming that patients randomised to EUS/EBUS did not incur any surgical staging costs, but would be more likely to have a futile thoracotomy. In the case of patients who underwent EUS/EBUS and surgical staging but not thoracotomy, the mean thoracotomy cost was added to the total trial cost to reflect the fact that EUS/EBUS was negative in these patients and they would have then gone on to receive thoracotomy. The total mean (SD) and median (IQR) trial costs, assuming that the EUS/EBUS patients did not undergo surgical staging, are shown in Table 19 and Figure 13.
| Randomisation group | Mean (SD) total cost (£) | Median (IQR) total cost (£) |
|---|---|---|
| EUS/EBUS (n = 85) | 8922 (2785) | 7805 (7279 to 11,123) |
| Surgical staging (n = 87) | 11,154 (3567) | 11,130 (9633 to 12,801) |
FIGURE 13.
Total costs complete case analysis ignoring the costs of surgical staging and increasing the futile thoracotomy rate for the EUS/EBUS group.
The corresponding cost-effectiveness results (Table 20) show that in this scenario endosonography is £2413 cheaper than surgical staging, and there remains very little difference in mean QALY. Because of the very small QALY difference, the ICER cannot be estimated reliably and the analysis reduces to a cost comparison.
| Parameter | Mean | Median | SD | 95% CI for mean |
|---|---|---|---|---|
| Costs (£) | ||||
| Endosonography alone (n = 58) | 9322 | 7822 | 2958 | 8551 to 10,079 |
| Surgical staging (n = 56) | 11,735 | 11,629 | 3477 | 10,843 to 12,674 |
| Cost comparisons (£) | ||||
| Endosonography – surgical staging | –2413 | 4565 | –3605 to –1271 | |
| QALY | ||||
| Endosonography alone | 0.348 | 0.361 | 0.103 | 0.321 to 0.373 |
| Surgical staging | 0.342 | 0.350 | 0.100 | 0.316 to 0.367 |
| QALY comparisons | ||||
| Endosonography – surgical staging | 0.00652 | 0.143 | –0.0298 to 0.0418 | |
| Cost per QALY gained (£) | ||||
| Endosonography – surgical staging | Dominant | |||
| Mean ICER from bootstrapping | 50,688 | |||
Figure 14 shows the bootstrapped estimates plotted on the cost-effectiveness plane and Figure 15 shows the corresponding CEAC. In the cost-effectiveness plane (see Figure 14), 3170 out of 5000 (63%) of the bootstrapped samples show that endosonography alone is less costly and more effective than surgical staging (bottom right-hand quadrant); 1830 out of 5000 (37%) points show that endosonography is less costly and less effective (bottom left-hand quadrant); and 4999 out of 5000 (99.98%) points are below the £30,000 ICER line.
FIGURE 14.
Five thousand bootstrapped samples from the joint distribution of the difference in costs and the difference in QALY, assuming no surgical staging costs but increased thoracotomy rate for EUS/EBUS patients. Positive values indicate that endosonography costs more and has greater QALYs. The line shows an ICER of £30,000 per QALY.
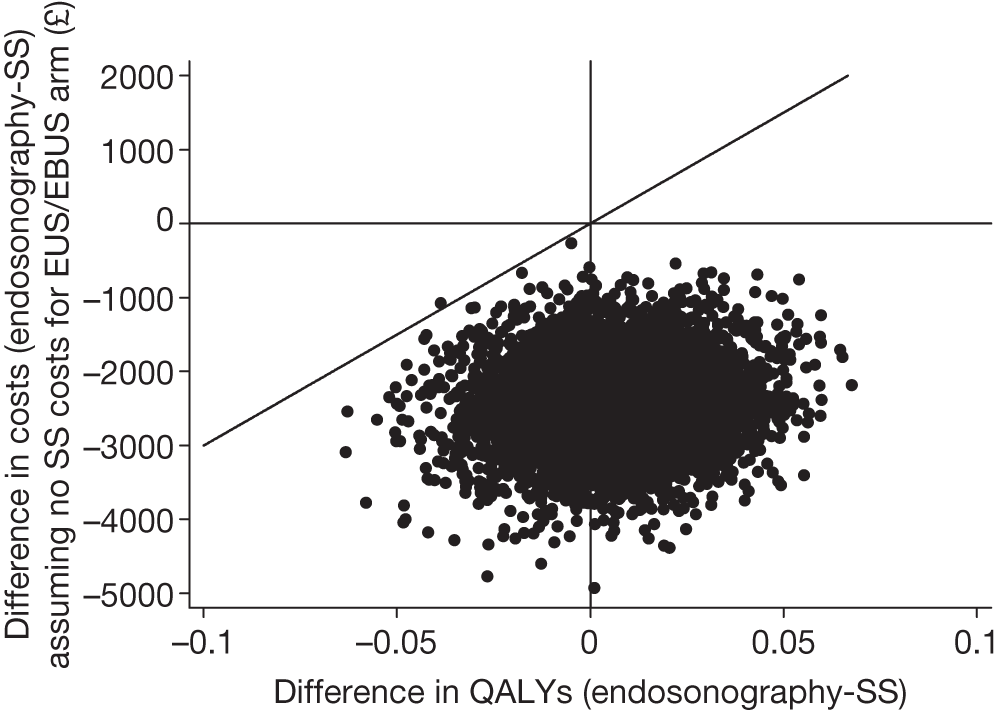
FIGURE 15.
Cost-effectiveness acceptability curve for complete cases (endosonography, n = 58; surgical staging, n = 56) assuming no surgical staging costs, but increased futile thoracotomy rate for the EUS/EBUS group.
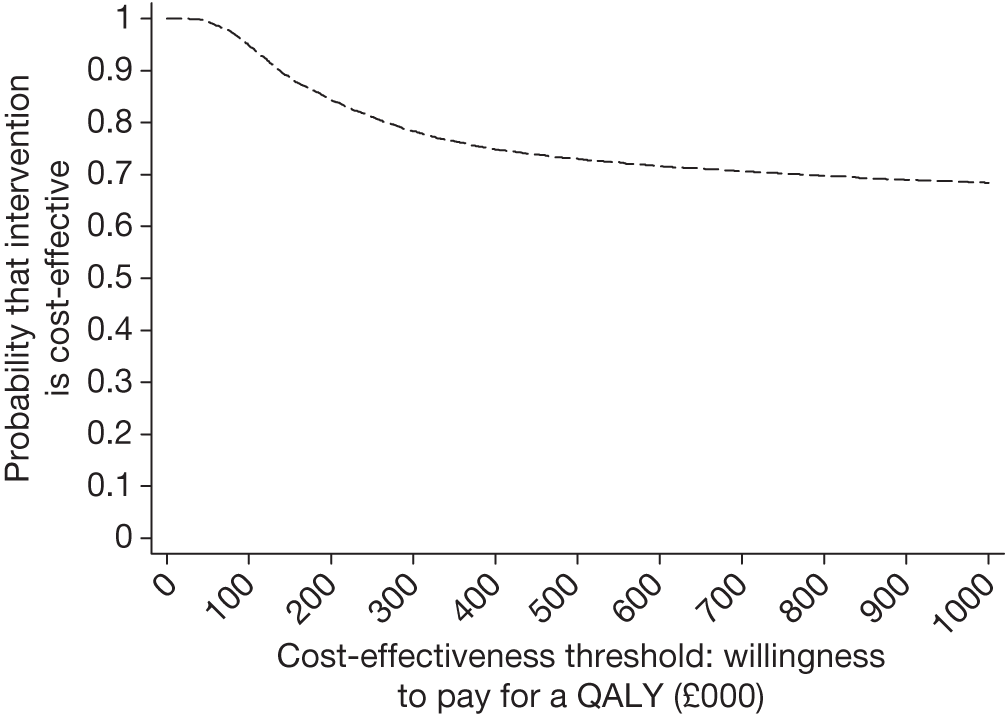
The CEAC (see Figure 15) crosses the y-axis at 1 with a willingness to pay per QALY of £0.00 showing that EUS/EBUS as the sole staging modality will result in cost savings with probability close to 100%.
Comparison of resource use and cost-effectiveness between centres for patients with complete data
There were some differences between the centres in resource use (see Appendix 3). The two Belgian centres were the most different, in that Leuven had the highest percentage (77%) of EUS/EBUS randomised patients going on to have surgical staging, whereas Ghent had the lowest (42%). Leuven also had the highest percentage of EUS/EBUS patients (77%) undergoing thoracotomy compared with Ghent, which had the lowest (39%). The percentage of patients in the surgical staging group undergoing thoracotomy was similar in all centres. Radiotherapy in the first 2 months was used more often in LUMC and Ghent – no patients from Papworth, and only one patient from Leuven, underwent this treatment in the first 2 months. The percentage of patients who required hospital admission was much higher in Leuven (62% in EUS/EBUS and 60% in surgical staging) than in the other centres, where the rate was ≤ 20%. LUMC showed consistently lower rates of chemotherapy, both in the first 2 months and between 2 and 6 months, compared with the other centres. These differences represent the diversity of practice in tertiary centres and the number of patients in each randomisation group, in each centre, was too small to reliably estimate centre-specific results.
Full Bayesian economic analysis
Base-case analysis
Table 21 presents cost-effectiveness of the two strategies using the fully Bayesian model that combines information from all 241 patients, including those with complete data alongside those for whom some or all resource usage or health-related quality of life data were missing. The expected 6-month costs under both strategies were around £1000 less than those that were calculated using the 114 patients with complete data only. Again there was no significant difference in expected cost between the two strategies – the posterior mean expected cost under endosonography (followed by surgical staging if negative) was £746 less than under surgical staging alone, but the 95% credible interval (CrI) for the difference spanned zero. Similarly, expected QALYs were substantively the same between the two strategies, with only a very small increase for the endosonography arm, therefore an ICER for endosonography (followed by surgical staging if positive) would not be meaningful.
| Parameter | Posterior mean | Posterior 95% CrI |
|---|---|---|
| Expected costs (£) | ||
| Endosonography and surgical staging | 9713 | 7209 to 13,307 |
| Surgical staging | 10,459 | 7732 to 13,890 |
| Expected cost comparisons (£) | ||
| Endosonography – surgical staging | –746 | –2494 to 756 |
| Expected QALY | ||
| Endosonography and surgical staging | 0.344 | 0.292 to 0.383 |
| Surgical staging | 0.329 | 0.274 to 0.371 |
| Expected QALY comparisons | ||
| Endosonography – surgical staging | 0.015 | –0.023 to 0.052 |
Figure 16 represents the uncertainty about the expected costs and effects as a posterior distribution on the cost-effectiveness plane. The associated CEAC for endosonography (followed by surgical staging if positive) is given in Figure 17. At any cost-effectiveness threshold, about 80% of the posterior distribution lies in a region where endosonography (followed by surgical staging if positive) is cost-effective, i.e. has a positive expected net benefit.
FIGURE 16.
Posterior distribution of incremental cost and QALY under base-case full Bayesian model.
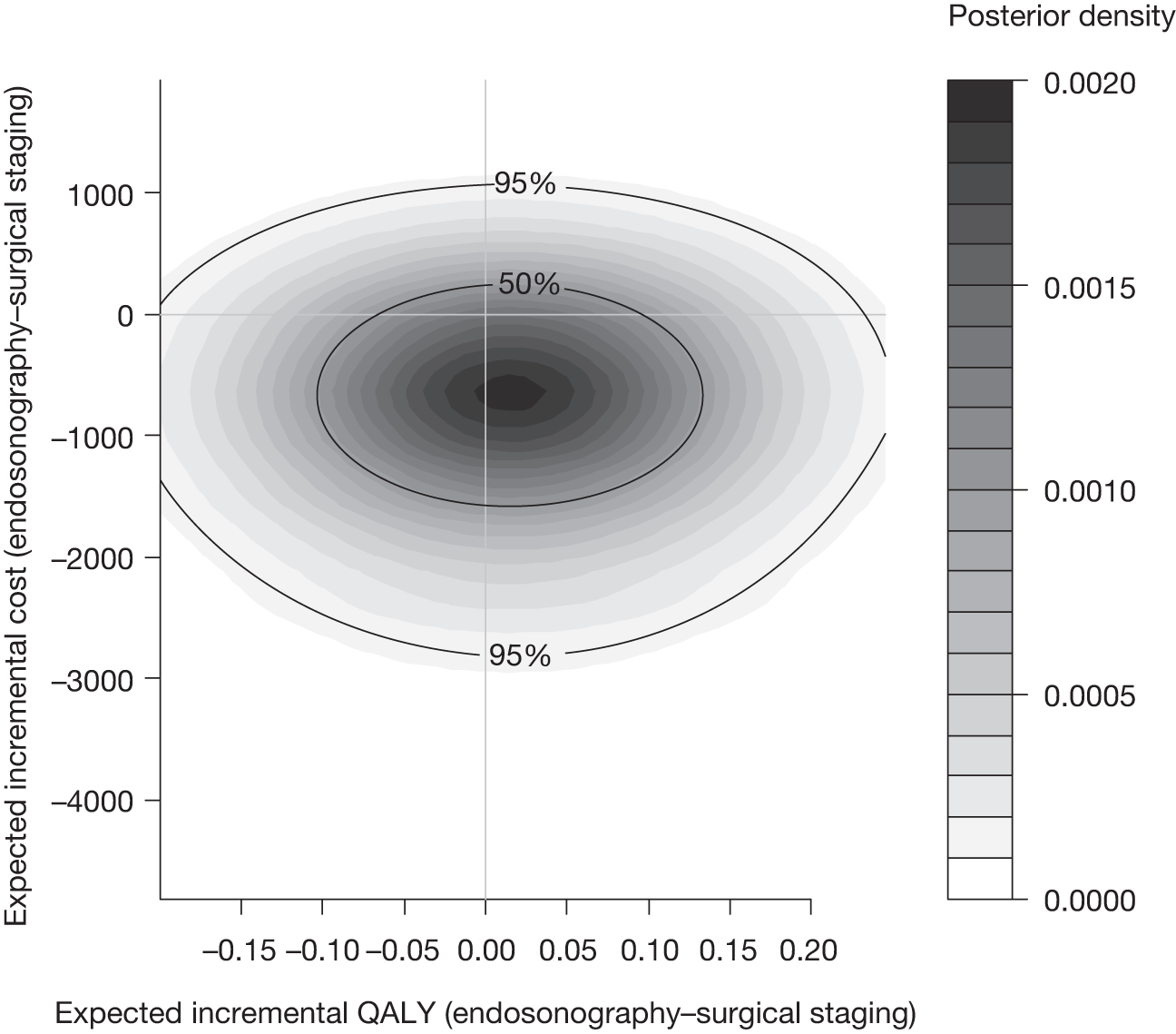
FIGURE 17.
Cost-effectiveness acceptability curve under base-case full Bayesian model.
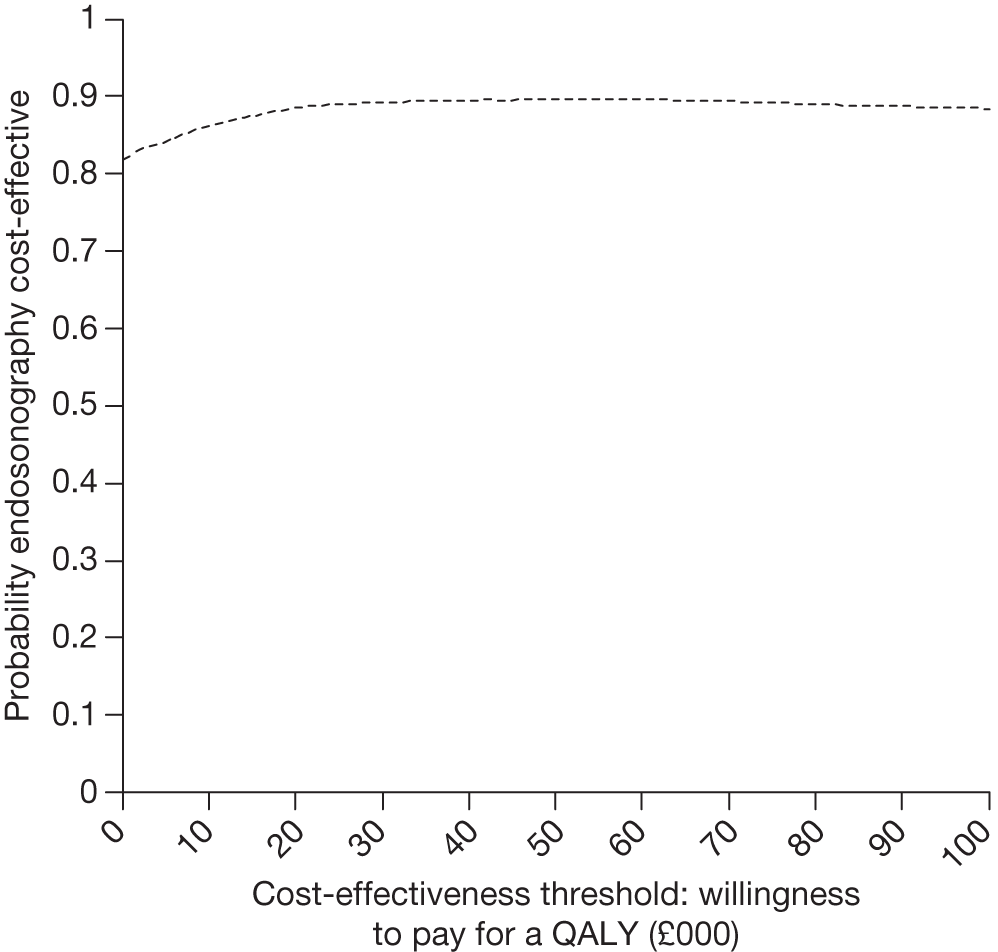
Results by centre are summarised in Appendix 4 and are similar to those for the completers analysis.
Deterministic sensitivity analyses
The Bayesian model was adapted to assume that patients randomised to endosonography do not undergo surgical staging after negative tests, and that their chance of receiving a futile thoracotomy is slightly increased [by 6/123, i.e. in 6 out of the 123 patients randomised to endosonography (followed by surgical staging if positive) tests were negative but subsequent surgical staging revealed locally advanced disease]. The expected cost for endosonography alone is reduced from £9713 to £8335 (Table 22), significantly less than under surgical staging, for which the cost is unchanged. The QALY for either strategy is unchanged. The distribution of cost-effectiveness (Figure 18) under this assumption is shifted in favour of endosonography, so that the probability that endosonography is cost-effective (Figure 19) is about 90%.
| Parameter | Posterior mean | Posterior 95% CrI |
|---|---|---|
| Expected costs (£) | ||
| Endosonography alone | 8335 | 6270 to 11,343 |
| Surgical staging | 10,459 | 7732 to 13,890 |
| Expected cost comparisons (£) | ||
| Endosonography – surgical staging | –2124 | –4560 to –167 |
| Expected QALY | ||
| Endosonography alone | 0.344 | 0.292 to 0.383 |
| Surgical staging | 0.329 | 0.274 to 0.371 |
| Expected QALY comparisons | ||
| Endosonography – surgical staging | 0.015 | –0.023 to 0.052 |
FIGURE 18.
Posterior distribution of incremental cost and QALY under Bayesian model: sensitivity analysis assuming no surgical staging after negative endosonography.
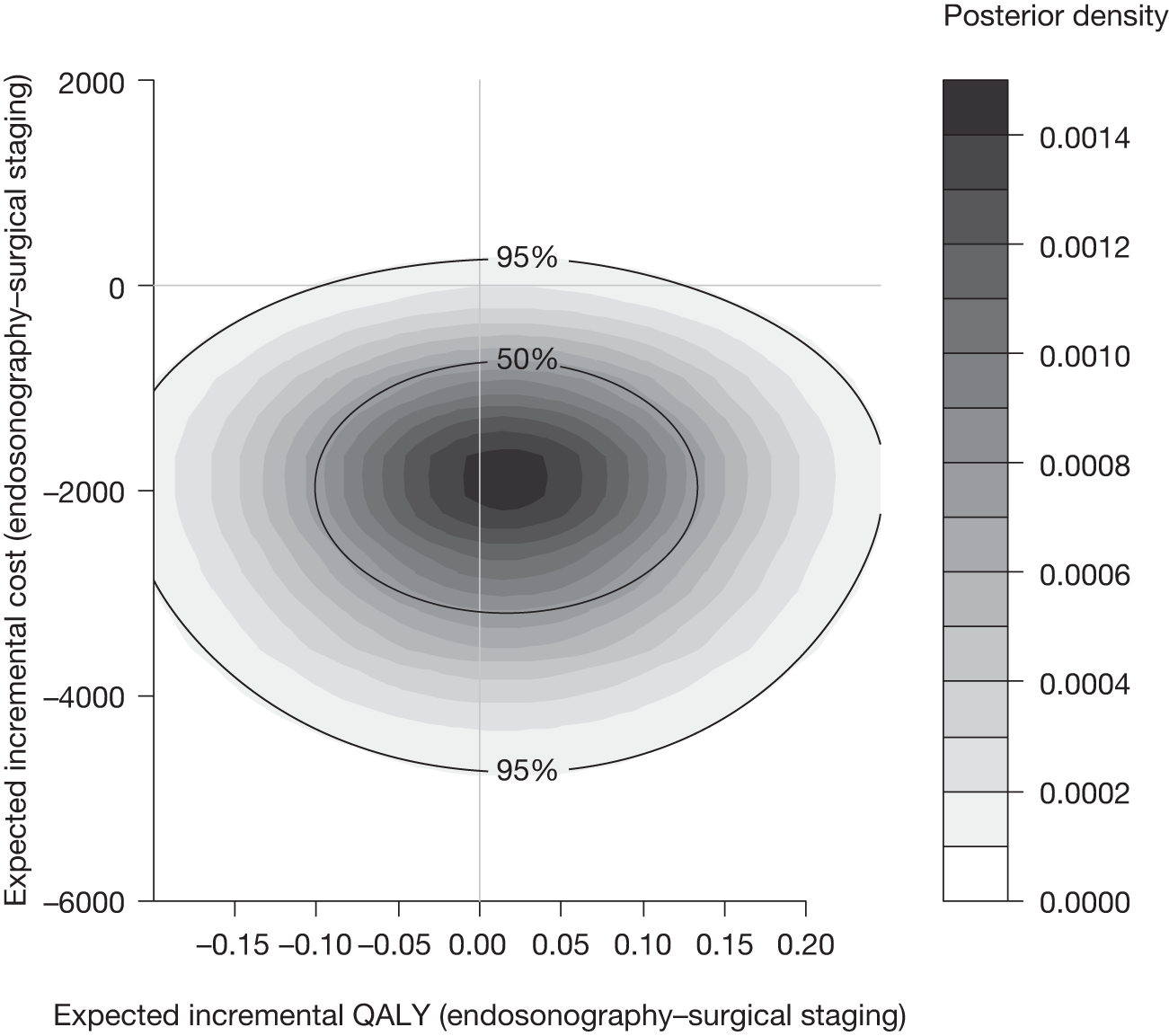
FIGURE 19.
Cost-effectiveness acceptability curve under Bayesian model: sensitivity analysis assuming no surgical staging after negative endosonography.
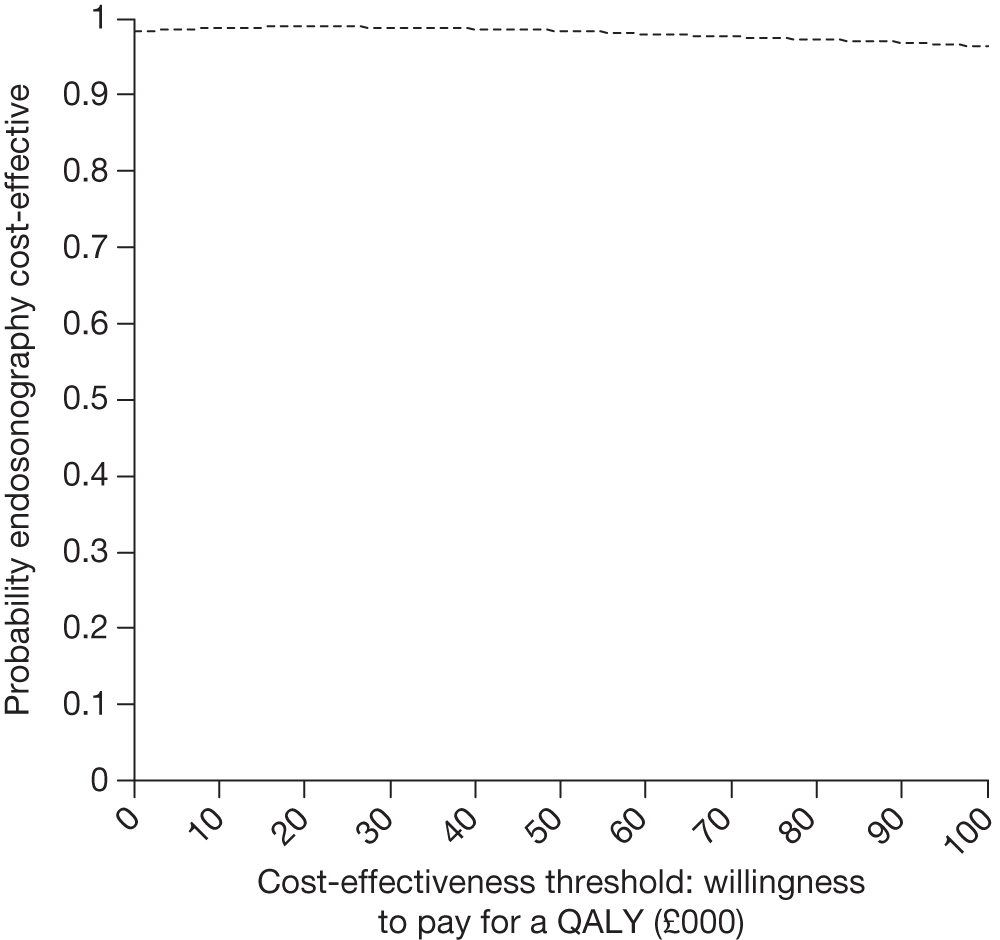
Alternative Bayesian models were explored in further sensitivity analyses. Different sets of predictors (age, sex and cancer stage, as well as randomisation group and study centre) were used to infer the missing resource usage components from those with partially observed data. Under none of these alternatives did expected costs and QALYs differ significantly between randomisation groups or differ substantially from the base-case model.
Summary
In the exploratory analysis of resource use, the strategy of EUS–FNA and EBUS-TBNA (followed by surgical staging only if these tests were negative) resulted in fewer staging mediastinoscopies (55% vs 99%) and fewer thoracotomies (53% vs 66%) than surgical staging alone, which must be weighed up against the number of EUS–FNA and EBUS-TBNA investigations required (100% vs 1%). In the full Bayesian analysis, which simultaneously estimates cost-effectiveness outcomes and missing data, the strategy of EUS–FNA and EBUS-TBNA (followed by surgical staging only if these tests were negative) cost £746 (95% CrI –£756 to £2494) less than surgical staging alone, with the diagnostic work-up and thoracotomy accounting for the majority of the difference. There was a small but non-significant gain in QALYs for endosonography followed by surgical staging if positive (0.015, 95% CrI –0.023 to 0.052). Thus, the endosonographic strategy is said to dominate (is cheaper and more effective), although there remains some uncertainty in the decision (CrIs cross zero and the probability that endosonography, followed by surgical staging if positive, is cost-effective of approximately 80%). A simple sensitivity analysis suggested that omitting the confirmatory surgical staging in the event of a negative EUS–FNA and EBUS-TBNA investigation might result in greater cost-effectiveness, but this is based on a number of uncertain assumptions.
Chapter 6 Discussion and recommendations
In this RCT, a strategy of using combined non-invasive endosonography (EUS–FNA and EBUS-TBNA), followed by surgical staging if these tests were negative, had higher sensitivity (94% vs 79%) and NPV (93% vs 86%), resulted in a lower rate of unnecessary thoracotomy (7% vs 18%) and better quality of life during staging (difference in utility 0.117), and was slightly more effective (difference in QALY 0.015) and less expensive (difference in costs £746) than the current practice of lung cancer staging using surgical methods alone. Although the endosonography strategy dominated in this study (was cheaper and more effective), CrIs for both the difference in costs and the difference in QALYs included zero. The CEACs, both for completers and in the full Bayesian analysis, suggested that endosonography (followed by surgical staging if positive) has a probability of at least 65% of being cost-effective, but there remains considerable uncertainty about the cost-effectiveness decision. Further benefits of endosonography include less invasive testing, with no requirement for general anaesthesia or open surgery, and the small number of minor complications in this study.
Based on this study, initial endosonographic staging, followed by surgical staging in the event that endosonography is negative for malignancy, provides an accurate strategy for mediastinal staging. The estimate of sensitivity of 94% for the combined EUS–FNA and EBUS-TBNA procedure was higher than in many studies that used either test alone, with reported sensitivity ranging from 50% to 84% for EUS-FNA alone and from 46% to 94% for EBUS-TBNA, when used in practice. 33 Although we did not formally assess specificity in this study, the rate of unnecessary thoracotomy gives an indication of the false-negative rate and at 93% the false-negative rate was similar to specificities reported in the literature. 33 Similarly, the diagnostic accuracy of surgical staging observed in this study is consistent with published reports2 giving support for the generalisability of our results.
Accurate staging of the lymph nodes is important, as subsequent patient management will depend on whether or not there has been metastatic spread of the disease. The most useful measurements of diagnostic accuracy are the sensitivity (true-positive rate) and the related NPV (disease-free rate in those with negative tests). In this study of staging strategies, as in other diagnostic studies in this area, not all patients underwent the gold standard assessment of nodal involvement, as it was considered unethical for patients to undergo thoracotomy if there was already evidence of metastatic disease. Thus, our results rely on the assumption that histology of cells from sampling of lymph nodes during these tests has a false-positive rate of zero. That is, we assume that if we see cancerous cells in the lymph nodes then metastasis has occurred. We consider this to be a realistic assumption in this context, as the diagnosis was made on the basis of histology and our validation study suggested that the staging from these samples was robust. The rate of N2/N3 involvement was higher in the endosonography group (54% vs 44%) but the difference was not significant (p = 0.137) and the randomised trial design suggests that any difference in the rate could have arisen by chance.
Although RCTs provide robust estimates of treatment effects, they are limited in the number of possible treatment pathways that can be included. In this study we chose to concentrate on the (pre-trial) current standard practice in our centres (mediastinal surgical staging) and a likely alternative pathway of combined EUS-FNA/EBUS-TBNA followed by surgical staging if endosonography was negative for malignancy. In addition to a cost-effectiveness analysis, this design also allowed us to assess sensitivity of endosonography against a composite gold standard of surgical staging and thoracotomy. A potential alternative pathway that was analysed in the clinical study was to exclude the confirmatory surgical staging step following a negative-for-malignancy endosonography, and to proceed straight to thoracotomy. Using the trial data, we re-estimated the difference in costs in a deterministic sensitivity analysis based on this proposed pathway and found that EUS-FNA/EBUS-TBNA alone was significantly cheaper than surgical staging alone, although the effect on QALYs was difficult to assess without making further assumptions about utilities in patients who were treated by thoracotomy inappropriately. Based on the assumption that QALYs would not change relative to the trial strategies, endosonography alone was judged to be cost-effective with high probability. However, this analysis is speculative, based on untestable assumptions, and should be interpreted cautiously.
This is the first RCT of surgical staging versus endosonography (followed by surgical staging if positive) to be reported. 20 The recently published 2011 National Institute for Health and Clinical Excellence (NICE) guideline33 for lung cancer diagnosis and treatment includes an economic model for a number of potential diagnostic pathways, with patients split into three groups according to the findings on CT imaging. However, the model was limited by the lack of empirical evidence on endosonography, as well as other competing modalities, and was largely based on expert judgement. Probabilistic sensitivity analysis was also not possible, with only point estimates presented. Despite this, the differences in costs and QALY between the surgical staging and endosonography strategies were consistent with this study, strengthening the external validity of our trial. The cost of endosonography used in the NICE guideline33 was provided by the University Hospital in Leicester, and at £1365 for EBUS alone was slightly higher than our unit cost of £1237.
The diagnosis and staging of lung cancer is complicated, and there are other modalities that can provide information on diagnosis and stage. The 2011 NICE guideline33 also provided cost-effectiveness estimates for PET–CT, non-ultrasound-guided TBNA and ultrasound of the neck, together with combination strategies and varying time order of the different tests. 33 The analysis found that PET–CT alone was the best strategy for patients with no enlarged nodes (short axis < 10 mm) on CT. PET–CT followed by conventional non-ultrasound-guided TBNA was most cost-effective for patients with more than one small-volume (short axis 10–20 mm) node(s) on CT, and for patients with any node of short axis > 20 mm the preferred strategy was neck ultrasound followed by non-ultrasound-guided TBNA, followed by PET–CT. It should be noted that these results were based on expert judgement and some strong methodological assumptions, without any probabilistic sensitivity analysis, so interpretation should be cautious. This is reflected in the fact that a degree of flexibility was incorporated into the management algorithms. For example, in the intermediate category (node short axis 10–20 mm) ultrasound-guided or non-ultrasound-guided TBNA was recommended to reflect the fact that ultrasound-guided tests have greater accuracy, yet are still well below the cost threshold.
In line with the findings of this study, the updated NICE lung cancer guidelines33 state that mediastinal staging can begin with combined endobronchial and endoscopic ultrasound in place of surgical staging. However, in the event of a negative endosonographic examination, surgical staging is recommended if clinical suspicion of malignancy remains high.
This multicentre study was adequately powered for the clinical outcomes and carried out in a well-defined population, with few exclusions, and was based on intention-to-treat analysis, so that results should be generalisable to other lung cancer centres. However, it is noted that all the centres involved in this trial were able to perform combined EUS-FNA and EBUS-TBNA, and all EUS and EBUS operators were highly experienced practitioners. Although EUS and EBUS are now available at many centres in the UK, there is only a handful of groups that can currently offer both techniques combined in the same session. For the most part, EBUS is performed by respiratory physicians in the UK and few are trained in EUS. In practice, this means that a combined EBUS and EUS may require two operators, a chest physician for EBUS and a gastroenterologist or radiologist for EUS. However, Annema et al. 14 have recently reported on an EUS implementation study in which respiratory physicians in several centres in the Netherlands underwent a structured training programme in EUS and were shown to achieve similar levels of diagnostic sensitivity and accuracy to the expert centre after 50 cases. A similar implementation strategy could be used in the UK. Recently, two groups have reported their initial experience of using the linear endobronchial ultrasound bronchoscope to perform EBUS and EUS in a single session. 34,35 Although this approach was not addressed in the current study, it offers a potential future strategy for the complete assessment of the mediastinum. Such an approach would potentially be less costly, as only an endobronchial ultrasound bronchoscope would be required and both procedures could be performed by a single operator. The estimated cost saving for endosonography would be of the order of £355 per case (no separate EUS, single operator, single needle and lower sterilisation costs), so that the cost difference between surgical staging and endosonography (followed by surgical staging if negative) would be £1101 per case (95% CrI –£401 to £2849). Further research is required to establish the diagnostic accuracy of this single-operator approach.
The multicentre, multinational nature of this trial did introduce some difficulties. Varying times of entry into the cost-effectiveness component of the study for the different centres meant that the EQ-5D questionnaire was not administered in the first half of the trial. Resource-use data collection was simple and covered only 6 months after randomisation, and the majority of the data could be retrieved retrospectively, but EQ-5D scores could not. Although the full Bayesian analysis allowed simultaneous estimation of missing data and cost-effectiveness outcomes, the results were estimated with less precision than expected. Nevertheless, the analysis based on patients for whom data were complete and the full Bayesian analysis gave similar point estimates.
Although there were only 20 deaths during the 6-month follow-up period, 144 patients contributed an EQ-5D utility curve from which to estimate mean QALYs. This number of patients, coupled with the measurement properties of the EQ-5D (continuous coverage over the measurement space, bounded, sensitivity to within- and between-patient changes), suggests that effectiveness results were robust and measured reasonably precisely. A further limitation of the EQ-5D is that it is a generic quality-of-life measure that is unlikely to illustrate changes in quality of life that are specific to the disease course. The addition of detailed quality-of-life studies would have been useful in understanding the impact that the diagnostic process and subsequent management had on patients, but was not considered feasible in the current multinational study. Furthermore, the EQ-5D utility was able to pick up small changes in quality of life occurring during the initial staging and management.
The cost-effectiveness study was trial based and restricted to the first 6 months after randomisation. Beyond this time we expect costs and effects to be determined by the course of the lung cancer and the success of the initial treatments, and these should not be affected to a large extent by the initial diagnostic strategy taken. This is supported by the utility curves in Figure 6, which are very similar beyond the initial period in which staging and thoracotomy is undertaken. Thus, we believe that a long-term economic model is not necessary, as it is unlikely to change the cost-effectiveness decision and it may require a complicated model, involving many assumptions.
Implications for practice
Taking the clinical, quality-of-life and health resource data together, evidence from this study suggests that lung cancer staging could commence with a combined EUS/EBUS examination, followed by surgical staging if these tests are negative. If there is no evidence for mediastinal nodal disease in either test, then patients could proceed directly to thoracotomy with lymph node dissection. All the centres involved in this trial were able to perform combined EUS-FNA and EBUS-TBNA, and all EUS and EBUS operators were experienced practitioners. The number of centres in the UK where both EBUS and EUS can be performed in a single session is very low (probably 5–10). A structured training programme in EBUS and EUS could support chest physicians and thoracic surgeons who are involved in lung cancer staging in the UK.
Recommendations for research
This RCT considered standard surgical staging and a single alternative for patients with lung cancer who were potential candidates for surgery, and in whom mediastinal nodal involvement had to be ruled out. Other possibilities for staging include PET–CT, non-ultrasound-guided TBNA and ultrasound of the neck, together with combination strategies, and these alternative methods should be subject to the same rigorous evaluation used in ASTER. The cost–utility analysis was trial based and did not model the long-term effects of the diagnostic strategies. Given the short-lived effect on utility observed in ASTER, we do not consider development of a long-term model to be a useful extension of this work.
Further research could consider the following:
-
Is mediastinoscopy following negative EBUS/EUS really needed? Further work is required before we can confidently recommend omitting confirmatory surgical staging in the event of negative endosonographic examination.
-
Can chest physicians be trained to perform both EBUS and EUS effectively? In the ASTER trial EBUS was performed by a chest physician and EUS by a gastrointestinal endoscopist.
-
Does combined EBUS/EUS using a single EBUS scope provide equivalent diagnostic accuracy to using separate EBUS and EUS scopes? In the ASTER study we used separate EBUS and EUS scopes, but recently a licence has been given for the EBUS scope to be used in the oesophagus.
Acknowledgements
The clinical study was conceived and designed by Jouke Annema (Consultant Physician), Klaus Rabe (Professor of Respiratory Medicine), Jerry Braun (Consultant Thoracic Surgeon) and Michel Versteegh (Consultant Thoracic Surgeon), LUMC, the Netherlands; Kurt Tournoy (Consultant Physician) and Jan van Meerbeeck (Professor of Respiratory Medicine), Ghent University Hospitals, Belgium; Robert Rintoul (Consultant Physician), Papworth Hospital, UK; and Christophe Dooms (Professor of Respiratory Medicine), Department of Pulmonology, Leuven University Hospitals, Leuven, Belgium.
We are grateful to the ASTER trial steering committee members who oversaw the running of the trial and met regularly to discuss study progress. The international group comprised Jouke Annema, Kurt Tournoy, Christophe Dooms, Robert Rintoul, Klaus Rabe, Ellen Deschepper and Olaf Dekkers. The Papworth ASTER study group comprised Robert Rintoul, Linda Sharples, Robert Buttery, Martin Buxton, Alistair Grant, Gethin Griffith, Victoria Hughes, Cliff Choong, Nick Carroll, Lavinia Magee and Jane Elliott. We are also grateful for the advice and support from the following independent data monitoring committee members: Mick Peake (chairperson), Edwin Chilvers, John Edwards and Sarah Barry (independent statistician). Donna Dickens provided valuable administrative support in preparing the report.
This UK cost-effectiveness study was funded by a grant from the UK National Health Service R&D Health Technology Assessment programme (project no. 06/302/216). Ella Wheaton was funded by the National Institute for Health Research (NIHR) as a Medical Research Council (MRC) Clinical Trials Training Fellow, and Linda Sharples and Chris Jackson were funded by the MRC. Gethin Griffith was employed by the University of Brunel during the study. Robert Rintoul was supported in part by the NIHR Cambridge Biomedical Research Centre and Cambridge Experimental Cancer Medicine Centre funding. Local support for data collection at Ghent University Hospital was provided by the Zorg-programma Oncologie Gent (ZOG). We are grateful to the ASTER trial management team, trial steering and data management committees, all of the staff at the participating centres, and the referring chest physicians and the patients for their participation.
The clinical results from ASTER were first published in the Journal of the American Medical Association. (Annema JT, van Meerbeeck P, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer. JAMA 2010;304:2245–52.)
Contribution of authors
Linda D Sharples conceived and designed the cost-effectiveness study, contributed to trial monitoring in the UK, supervised the cost-effectiveness analysis and, jointly with RCR, produced the final report.
Chris Jackson designed and implemented the final Bayesian cost-effectiveness model, and drafted the sections of the report relating to this analysis.
Ella Wheaton conducted the initial exploratory analysis of the resource-use and quality-of-life data, and drafted the sections of the report relating to this analysis.
Gethin Griffith designed the resource-use data collection, provided appropriate unit costs and drafted the sections of the report relating to this analysis.
Jouke T Annema conceived and designed the clinical part of the study, took responsibility for trial conduct in Leiden, and analysed and reported on the clinical results.
Christophe Dooms took responsibility for trial conduct in Leuven, and analysed and reported on the clinical results.
Kurt G Tournoy conceived and designed the clinical part of the study, took responsibility for trial conduct in Ghent, and analysed and reported on the clinical results.
Ellen Deschepper conducted the statistical analysis, and contributed to the reporting of the clinical results.
Vikki Hughes contributed to the design of the cost-effectiveness study and managed the UK arm of the trial.
Lavinia Magee contributed to the design of the cost-effectiveness data collection and was responsible for data collection in the UK.
Martin Buxton conceived and designed the cost-effectiveness study, and supervised the costing study.
Robert C Rintoul contributed to the design of the cost-effectiveness study, took responsibility for trial conduct in Papworth, analysed and reported on the clinical results, contributed to the cost-effectiveness analysis and, jointly with LDS, produced the final report.
All authors contributed to the production and editing of the final report. Linda Sharples and Robert Rintoul contributed equally to the report.
Publication
Annema JT, van Meerbeeck P, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer. JAMA 2010;304:2245–52.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- National Lung Cancer Audit 2009. www.ic.nhs.uk/webfiles/Services/NCASP/audits%20and%20reports/NHS%20IC%20Lung%20Cancer%20AUDIT%202009%20FINAL.pdf.
- Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edn). Chest 2007;132:202-20.
- Pinto Filho DR, Avino AJ, Brandao SL, Spiandorello WP. Joint use of cervical mediastinoscopy and video-assisted thoracoscopy for the evaluation of mediastinal lymph nodes in patients with non-small cell lung cancer. J Bras Pneumol 2009;35:1068-74.
- Anraku M, Miyata R, Compeau C, Shargall Y. Video-assisted mediastinoscopy compared with conventional mediastinoscopy: are we doing better?. Ann Thorac Surg 2010;89:1577-81.
- Szlubowski A, Kuzdzal J, Kolodziej M, Soja J, Pankowski J, Obrochta A, et al. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg 2009;35:332-5.
- Ernst A, Eberhardt R, Krasnik M, Herth FJ. Efficacy of endobronchial ultrasound-guided transbronchial needle aspiration of hilar lymph nodes for diagnosing and staging cancer. J Thorac Oncol 2009;4:947-50.
- Hwangbo B, Kim SK, Lee HS, Kim MS, Lee JM, Kim HY, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009;135:1280-7.
- Fielding D, Windsor M. Endobronchial ultrasound convex-probe transbronchial needle aspiration as the first diagnostic test in patients with pulmonary masses and associated hilar or mediastinal nodes. Intern Med J 2009;39:435-40.
- Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62.
- Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96.
- Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64.
- Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: a systematic review and meta-analysis. Chest 2007;131:539-48.
- Sawhney MS, Kratzke RA, Lederle FA, Holmstrom AM, Nelson DB, Kelly RF. EUS-guided FNA for the diagnosis of advanced lung cancer. Gastrointest Endosc 2006;63:959-65.
- Annema JT, Bohoslavsky R, Burgers S, Smits M, Taal B, Venmans B, et al. Implementation of endoscopic ultrasound for lung cancer staging. Gastrointest Endosc 2010;71:64-70.
- Szlubowski A, Zielinski M, Soja J, Annema JT, Sosnicki W, Jakubiak M, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging: a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9.
- Yasuda I, Kato T, Asano F, Okubo K, Omar S, Kako N, et al. Mediastinal Lymph node staging in potentially resectable non-small cell lung cancer: a prospective comparison of CT and EUS/EUS-FNA. Respiration 2009;78:423-31.
- Rintoul RC, Skwarski KM, Murchison JT, Wallace WA, Walker WS, Penman ID. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J 2005;25:416-21.
- Vilmann P, Krasnik M, Larsen SS, Jacobsen GK, Clementsen P. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9.
- Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6.
- Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomised trial. JAMA 2010;304:2245-52.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23.
- De Leyn P, Lardinois D, Van Schil PE, Rami-Porta R, Passlick B, Zielinski M, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8.
- Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92.
- Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33.
- Kind P. The EuroQol instrument: an index of health-related quality of life. Philadelphia, PA: Lippincott-Raven; 1996.
- Dolan P GC, Kind P. A social tariff for EuroQoL: results from a UK general population survey. York: University of York; 1995.
- Department of Health (DoH) . NHS Reference Costs 2008–09 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591.
- Nixon RM, Thompson SG. Parametric modelling of cost data in medical studies. Stat Med 2004;23:1311-31.
- Long SJ. Regression models for categorical and limited dependent variables. Thousand Oaks, CA: Sage Publications; 1997.
- Briggs A. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Lunn D TA, Best N, Spiegelhalter D. WinBUGS: a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Kind P HG, Macran S. UK population norms for EQ-5D. York: University of York; 1999.
- National Institute for Health and Clinical Excellence (NICE) . The Diagnosis and Treatment of Lung Cancer 2011.
- Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4.
- Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802.
Appendix 1 Final protocol
Assessment of Surgical sTaging versus Endoscopic ultrasound in lung cancer: a Randomised controlled trial (ASTER)
Principal Investigators:
-
Dr Jouke Annema Leiden University Medical Centre, Leiden, Holland
-
Dr Kurt Tournoy Ghent University Hospital, Ghent, Belgium
-
Dr Robert Rintoul Papworth Hospital, Cambridge, UK
-
Statistician: Professor Georges Van Maele, Ghent University Hospital
-
Dr Linda Sharples: Papworth Hospital, Cambridge, UK
-
Date of Registration: 8/2/2007 (http://clinicaltrials.gov/)
-
Registration Nr: NCT00432640
-
Protocol version 3.0 Dated 27th March 2008
1. Summary
Background:
Lung cancer is the second most common cause of cancer in the UK and has a very high mortality rate. Both treatment and prognosis depend upon stage at presentation. Mediastinal staging is a field that is rapidly developing. Staging by FDG-PET has dramatically reduced the rate of futile thoracotomies. EUS-FNA and EBUS-TBNA are two complementary ultrasound-guided biopsy techniques which together allow access to almost all mediastinal lymph nodes (LN): for EUS-FNA: 4L, 7, 8L/R, 9L/R and for EBUS-TBNA: 2R/L, 4R/L, 7. This means that the combination of both techniques allows a comprehensive (bilateral N2 and N3) mediastinal examination (with the exception of the para-aortic stations 5 and 6). Non-randomised case series have indicated the potential of EUS-FNA and EBUS-TBNA for mediastinal staging. However, these techniques have not been validated against the current ‘gold standard’ of care which is surgical staging in a prospective randomised controlled fashion.
Hypothesis:
The null hypothesis is that there is no difference between sensitivity, diagnostic accuracy and negative predictive value of endobronchial with endoscopic ultrasound-guided biopsy of lymph nodes and surgical staging.
Patients:
Patients with (suspected) NSCLC who are judged to be candidates for surgical resection but in whom malignant N2/N3 lymph node involvement is suspected based on clinical staging (including chest X-ray, CT thorax, FDG-PET or integrated FDG-PET/CT) are eligible for this study. A cytological or histological diagnosis of lung cancer is not required at the time of randomisation. Patients with proven distant metastases (M1) are excluded from this study.
Study design:
A prospective randomised controlled multi-centre double arm diagnostic phase III trial in which patients are randomly assigned to either surgical staging (arm B) or echo-endoscopic staging with both EUS-FNA and EBUS-TBNA (arm A). EUS-FNA and EBUS-TBNA are performed in one session. Surgical staging is defined as cervical mediastinoscopy, anterior (parasternal) mediastinotomy, thoracoscopic mediastinal exploration or any combination.
EUS-FNA/EBUS-TBNA will be considered positive if one or both of the diagnostic procedures yield tissue proof of mediastinal metastases (N2/N3).
In arm A (study arm), if no N2 or N3 lymph node metastases are found by either EUS-FNA or EBUS-TBNA patients will subsequently be offered a confirmatory surgical staging procedure prior to proceeding to a thoracotomy with systematic lymph node dissection.
Objectives:
-
Primary objective:
-
– The primary research objective of the study is to determine whether EBUS-TBNA combined with EUS-FNA is better than standard surgical staging techniques in terms of sensitivity, diagnostic accuracy and negative predictive value for diagnosing and staging the mediastinum in lung cancer.
-
-
Secondary objectives are:
-
– Determination of the sensitivity and accuracy of EBUS and EUS compared with surgical staging for determining mediastinal tumour invasion (T4).
-
– A comparative cost analysis of the diagnostic strategies of the two trial arms.
-
– Assessment of the complication rates in each arm
-
– An estimation of the saving of surgical staging procedures that might be possible in the future if EBUS-TBNA/EUS-FNA is shown to have greater sensitivity and diagnostic accuracy and becomes the new ‘gold standard’ staging procedure.
-
– Estimation of how many futile thoracotomies can be avoided by performing EBUS-TBNA and EUS-FNA rather than surgical staging procedures.
-
– Assessment of inter-observer variability of cytopathological evaluation of EBUS-TBNA and EUS-FNA samples.
-
Statistical analysis:
In the sample size calculation the following assumptions were made:
-
The prevalence of mediastinal nodal disease in patients with lung cancer is 70%. The sensitivity of mediastinoscopy to detect mediastinal nodal involvement is 70%. The sensitivity of EUS-FNA and EBUS-TBNA for detection of mediastinal nodal involvement is 90%.
-
Using standard calculation techniques, the sample size required is (2 × 71 in each arm) with a power of 1-β = 0.8, type 1 error α = 0.05 and two sided testing. Assuming 5% incomplete CRFs and assuming that only 70% of patients will have mediastinal disease the total sample size becomes 214 patients.
2. Introduction
2.1 Background
Lung cancer is the second most common cancer in England and Wales and is the most common cause of cancer death. Non small cell lung cancer (NSCLC) accounts for 80% of all cases. The overall five-year survival is approximately 10%1. Treatment of lung cancer is influenced by stage. Accurate staging is therefore important in order to optimise treatment regimens. The incorporation of positron emission tomography (PET) into staging algorithms has considerably reduced the number of futile thoracotomies2. PET/CT is more accurate than computerised tomography (CT) in detecting mediastinal lymph node metastases, with a negative predictive value of 93–95%. However, a positive predictive value of 74–90% makes pathological verification of mediastinal hotspots necessary in order to avoid patients being denied possible curative surgery3–5.
The current standard of care requires surgical staging of enlarged and/or FDG-PET/CT avid mediastinal lymph nodes by a surgical staging procedure such as mediastinoscopy, mediastinotomy or thoracoscopic mediastinal exploration6. However, these techniques are invasive and require general anaesthesia and hospitalisation. In addition, the accuracy of these procedures is variable and ranges between 80–90%6;7. Although the specificity is 100%, the sensitivity is lower and ranges between 66%8 and 75–90%6. The accuracy of mediastinoscopy to stage lung cancer is therefore mainly determined by the high specificity while there is room to improve the sensitivity and the negative predictive value.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and more recently endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are two minimally invasive diagnostic techniques that allow real-time controlled punctures of mediastinal lymph nodes8–16. These techniques are performed in an outpatient setting under conscious sedation. Non-randomised trials in selected patient populations have suggested that these techniques can obviate the need for surgical staging procedures in up to 70% of the cases9;17. EUS-FNA and EBUS-TBNA are complementary techniques with EUS allowing access to mediastinal lymph node groups 4L, 7, 8L/R, 9L/R and EBUS giving access to mediastinal lymph node stations 2R/L, 4R/L, 7,18;13. This means that the combination of both techniques enables a complete (bilateral) mediastinal examination. With EBUS-TBNA hilar and intrapulmonary nodal stations 10R/L, 11R/L can also be assessed. In addition, in selected cases echoendoscopy offers the possibility to assess whether a tumour is invading the mediastinum (T4)8. In previous studies we have reported the value of adding EUS-FNA to mediastinoscopy regarding mediastinal staging8 and the impact of EUS-FNA on the prevention of surgical staging9;12.
Data regarding combined echo-endoscopic staging (EUS-FNA combined with EBUS-TBNA) compared with surgical staging for evaluation of mediastinal lymph nodes are currently not available.
2.2 Rationale for this study
Current international guidelines for the staging of NSCLC advocate staging by mediastinoscopy when locally advanced disease is suspected6;19;7;20. Locally advanced disease is defined as either N2 or N3 or T4. Mediastinoscopy has limitations in its diagnostic reach to access some mediastinal nodes, is expensive and requires an in-patient stay. Recent reports suggest that complete accurate loco-regional staging can be assessed by the combination of EUS-FNA and EBUS-TBNA in an ambulatory setting. (Villman ref 18, Wallace, World EUS/DDW 2006). If this holds true, improved, less invasive and more cost-effective care can be provided for this large group of patients.
3. Study Objectives
3.1 Primary objectives
The primary research objective of the study is to determine whether EBUS-TBNA combined with EUS-FNA is better than standard surgical staging techniques in terms of sensitivity, diagnostic accuracy and negative predictive value for diagnosing and staging the mediastinum in lung cancer.
The null hypothesis is that there is no difference between sensitivity, diagnostic accuracy and negative predictive value of EBUS-TBNA combined with EUS-FNA and surgical staging.
3.2 Secondary objectives
-
Determination of the sensitivity and accuracy of EBUS and EUS compared with surgical staging for determining mediastinal tumour invasion (T4).
-
A comparative cost analysis of the diagnostic strategies of the two trial arms.
-
Assessment of the complication rates in each arm.
-
An estimation of the saving of surgical staging procedures that might be possible in the future if EBUS-TBNA/EUS-FNA is shown to have greater sensitivity and diagnostic accuracy and becomes the new ‘gold standard’ staging procedure.
-
Estimation of how many futile thoracotomies can be avoided by performing EBUS-TBNA and EUS-FNA rather than surgical staging procedures.
-
Assessment of inter-observer variability of cytopathological evaluation of EBUS-TBNA and EUS-FNA samples.
4. Study Plan and Procedures
4.1 Overall study design
This is a prospective international multi-centre open randomised controlled phase III study.
4.2 Clinical work-up (CWU)
Patients are evaluated by history, physical examination, full blood count, renal and liver function tests, chest X-ray, bronchoscopy, CT of the chest and upper abdomen and whole body FDG-PET or integrated whole body FDG-PET/CT. If clinical suspicion exists, a brain scan (CT or MRI) or a bone scan can be performed.
4.3 Randomisation
Recruitment and randomisation will occur when clinical work-up identifies a patient with (suspected) lung cancer in whom further loco-regional staging is indicated. Randomisation will be performed in a 1:1 ratio. Randomisation will occur using a web based program and will be stratified for each participating institution.
4.4 Detailed study design: flow chart
4.5 Inclusion criteria
Consecutive patients with known or suspected NSCLC and in whom mediastinal lymph node involvement (either N2 or N3) is suspected based on the available thoracic imaging (CT or CT-PET).
Pending the results of mediastinal staging the patient must be considered to be a candidate for surgical resection with an intention to cure.
The patient is clinically fit for bronchoscopy, endoscopy and diagnostic surgical procedures.
There is no evidence of distant metastatic disease after routine clinical work up.
The patient is able to give informed consent.
4.6 Exclusion criteria
-
Previous treatment (chemotherapy or radiotherapy or surgery) for lung cancer
-
Any clinical reason why it is thought that the patient is unable to undergo or has a contra-indication to a bronchoscopy, endoscopy, a surgical staging procedure or who is not suitable for definitive surgical resection by thoracotomy.
-
Patients who, based on available thoracic imaging, are unlikely to be staged accurately by any surgical staging procedure (mediastinoscopy/-otomy, VATS).
-
A Concurrent malignancy.
-
An uncorrected coagulopathy.
-
Inability to give informed consent.
-
Patients who are eligible for this study but who are not included (no informed consent obtained, logistical reasons) will be recorded with the reason why study participation did not occur.
4.7 EUS-FNA and EBUS-TBNA (Arm A)
Systematic evaluation of all mediastinal lymph node stations will be undertaken by either EUS-FNA or EBUS-TBNA. Aspirates will be taken of nodes suspected for malignant involvement. It is not in the scope of this study to compare EUS-FNA and EBUS-TBNA, and thus, it is not necessary to double evaluate those lymph node stations that can be reached by either endoscope (for example LN 7). It is also not in the scope of this study to evaluate the additional value of EBUS-TBNA after EUS-FNA precluding split-echoendoscopy sessions. For reasons of convenience and patient-comfort EUS-FNA will be performed before EBUS-TBNA.
EUS-FNA is performed in a fasting patient as described21. Pharyngeal anaesthesia and intravenous conscious sedation will be administered according to local practice. If necessary, prophylaxis for endocarditis will be given according to local institutional practice. If the patient takes oral anticoagulation (warfarin and derivatives or clopidogrel), then this medication should be stopped before the procedure, and proof of normalisation of coagulation tests should be available pre-procedure. It is not necessary to stop aspirin or NSAID before this procedure, unless the investigator feels that this is necessary. During the procedure monitoring of pulse rate and oxygen saturation will be performed. EUS will be performed with a linear scanning ultrasound endoscope with Doppler flow imaging for the detection of blood vessels. The EUS endoscope will be introduced into the distal oesophagus, and the investigator will evaluate the mediastinal lymph nodes by scanning 360° transaxially at 1- to 2-cm intervals upwards up to level 2 lymph nodes.
Lymph nodes will be assessed using ultrasonographic criteria for malignancy (short axis diameter, echo-texture, shape, margins, vascular pattern) and suspicious nodes will be biopsied using a 22-guage needle (Echotip®, Wilson-Cook Medical Inc.; Hancke–Vilmann, Winston-Salem, NC or EUS needle, Olympus). Lymph node selection is at the discretion of the operator – it is not within the scope of this study to puncture all lymph nodes. In each patient, lymph nodes suspected of harbouring N3 disease will be sampled first. The presence or absence of direct mediastinal tumour invasion (T4) will also be recorded. If rapid on-site cytopathological evaluation is available it will be utilised although it is not essential within the study. If ROSE is not available suspicious lymph nodes will be sampled a minimum of four times. The number of biopsies per node will be recorded. If necessary, several lymph nodes can be sampled. Samples will be categorised as positive (tumour cells present), negative (lymphocytes present but no tumour cells), or inconclusive (poor cellularity, or unable to perform adequate biopsy).
EBUS will be performed immediately following EUS. EBUS will be performed with a linear scanning ultrasound bronchoscope (BF-UC160F-OL8, Olympus Ltd) connected to a processor unit (Olympus EU C2000) with Doppler flow imaging for the detection of blood vessels. The bronchoscope will be introduced via the mouth with the patient lying supine and the operator standing behind the patient. Blood vessels will be confirmed using the Doppler mode. Lymph nodes will be evaluated by scanning transaxially at 1- to 2-cm intervals from the peripheral regions of interest (lymph node stations 10–11) upwards to station 2. Lymph nodes will be assessed using ultrasonographic criteria for malignancy (short axis diameter, echo-texture, shape, margins, vascular pattern) and suspicious nodes will be biopsied using a 22-guage needle (EBUS needle NA-201SX-4022, Olympus, Ltd) with a 10-mL syringe for suction. The presence or absence of mediastinal invasion of the primary tumours (T4 or not) will be assessed.
In the event of a patient who is randomised to the test arm being unable to tolerate EBUS/EUS then they will be offered a surgical staging procedure under general anaesthesia. This is in keeping with standard clinical practice. Data will be interpreted on an ‘intention to treat’ basis for those patients who are randomised.
The mediastinal lymph node map of the American Joint Committee on Cancer will be used to localise abnormalities at CT, FDG-PET or integrated FDG-PET/CT, EUS-FNA, EBUS-TBNA and for mediastinal dissection22.
4.8 Surgical intervention procedures (Arm B)
These include cervical mediastinoscopy, left anterior mediastinotomy or thoracoscopic mediastinal exploration. Surgeons will perform these procedures according to their local institutional practice. However, for cervical mediastinoscopy, the standard of practice requires a systematic sampling of the following lymph node stations: 2R/L, 4R/L and 76.
In the event of mediastinal lymph node evaluation in the EBUS-TBNA/EUS-FNA arm being negative, the patient will proceed to a confirmatory surgical staging procedure prior to a thoracotomy with surgical resection.
At thoracotomy with intra-operative staging, the IASLC guidelines will be followed23. This means that a ‘systematic lymph node dissection’ will be performed for each patient who progresses to a thoracotomy (lobectomy or pneumonectomy). Systematic lymph node dissection is the technique of choice for accurate intraoperative mediastinal staging24. It is not mandatory that all mediastinal tissue is removed during intra-operative staging23.
The following LN stations should be considered:
-
Right upper lobe: 2R, 4R and 7
-
Right middle lobe: 2R, 4R and 7
-
Right lower lobe: 4R, 7, 8 and 9
-
Left upper lobe: 4, 5, 6 and 7
-
Left lower lobe: 7, 8 and 9.
4.9 Assessment of lymph node cytology
Lymph node biopsies will be collected and processed by the pathology department according to local protocols. Papanicolau and Giemsa stains will be performed. If sufficient cellular material is available a cell block will be made aiming to complete the cytological analysis of the tumour cells by immunocytochemistry (IHC). The outcome of the cytological analysis will be the presence or absence of malignant cells. The presence of lymphocytes will be regarded as proof of a representative lymph node puncture. A sample of fine needle aspirates obtained by EUS-FNA and EBUS-TBNA will be evaluated by an independent reference cytopathologist in order to assess inter-observer variability. However, the findings of the initial cytopathologist will be used for patient management and the primary analysis. In the event of any dubiety on the part of the pathologist reporting a lymph node biopsy specimen a confirmatory surgical staging procedure or thoracotomy will be undertaken to ensure that the patient is not in any way disadvantaged by a possible false positive result.
4.10 Safety measures and variables
Continuous clinical monitoring and oxygen saturation monitoring during EUS-FNA and EBUS-TBNA procedures will be performed. For all other procedures, routine safety precautions according to local institutional practice will be followed. Any complications of either the EUS-FNA and EBUS-TBNA procedures as well as the surgical procedures will be recorded.
5. Data Collection and Management
Data collection will be performed in all participating centres. Electronic patient record forms (CRF forms) will be provided in ACCESS. Patient demographics will be recorded. Further recording includes randomisation arm (0 = ARM A and 1 = ARM B) and randomisation date. All imaging techniques will be recorded (X-ray chest, CT-scan Thorax, CT-scan abdomen, bone scan, FDG-PET/CT scan, brain scan; 0 = not done, 1 = performed). Following intrathoracic lymph node staging either by EUS-FNA/EBUS-TBNA or surgical staging or both, a cTNM will be noted. Of highest importance, a pTNM will be recorded following each thoracotomy.
Specific EUS/EBUS variables will be recorded. For each lymph node station the presence (1) or absence (0) of enlarged lymph nodes and whether the LN was punctured (0 = no, 1 = yes), the ultrasonographic characteristics of each LN, the presence of complications (0 = no, 1 = yes).
Data collection and analysis will be monitored according to good clinical practice. Clinical monitoring will be organised in a cross-over fashion where CRF files will undergo a quality check. Members of each centre will assess some of the files of each other centre. An independent data monitoring and ethics committee and a trial steering group will meet regularly to review the progress of the study and to evaluate the implications of any adverse clinical incidents.
6. Health Economics
A cost-utility analysis from a health service perspective will be undertaken up to 6 months post-randomisation. Resource use and cost data to be collected prospectively during the study will include resource use associated with the staging and surgical procedures; inpatient length of stay; any adverse events requiring hospital re-admission; and any concomitant oncology treatment (radiotherapy/chemotherapy) that the patients may receive. The outcome measure of interest in the economic evaluation is quality-adjusted survival, measured by QALYs (Quality-adjusted life-years). In order to calculate QALYs, patient utilities will be derived from the EQ-5D questionnaire and combined with patient-specific survival. The EQ-5D questionnaire will be administered to patients at the following points: a) baseline (at time of randomisation); b) immediately post-staging (EUS/EBUS for group A or surgical staging for group B); c) 2 months post-randomisation; and d) 6-months post-randomisation. The 6-month total mean costs and QALYs will be combined in order to calculate the ICER (incremental cost-effectiveness ratio).
7. Statistical Methods
7.1 Sample size and outcomes
In the sample size calculation the following assumptions were made:
-
The prevalence of mediastinal nodal disease in patients with lung cancer is 70%. The sensitivity of mediastinoscopy to detect mediastinal nodal involvement is 70%. The sensitivity of EUS and EBUS for detection of mediastinal nodal involvement is 90%.
-
Therefore, using standard calculation techniques, the sample size required is (2 x 71 in each arm) with a power of 1-β = 0.8, type 1 error α = 0.05 and two sided testing. Assuming 5% incomplete CRFs and assuming that only 70% of patients will have mediastinal disease the total sample size becomes 214 patients.
-
For the purposes of statistical analysis, a case of mediastinal disease is defined as a patient with tumour in lymph nodes detected by any of the following: EBUS, EUS, surgical staging techniques or histology following thoracotomy. Thus the ‘gold standard’ definition is based on a series of tests. Since this definition does not allow for false positive test results, both the specificity and the positive predictive value are necessarily one. Therefore, analysis will focus on the estimation of sensitivity (probability of a positive test in those who have mediastinal disease) and the negative predictive value (probability of no mediastinal disease in those with a negative test). The negative predictive value does depend upon the prevalence of mediastinal disease and as discussed above the a priori rate of 70% has been assumed for the study population. Formal comparison of the sensitivities will be performed using Fisher’s Exact test.
8. Publication and Authorship
Investigators who significantly contribute to the conduct, analysis and publication of the study will be eligible to be a co-author. The study will be registered in the international RCT trial registry.
9. References
- Tyczynski JE, Bray F, Parkin DM. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol 2003;4:45-5.
- van Tinteren H, Hoekstra OS, Smit EF, . Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388-93.
- Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg 2003;238:180-8.
- LeBlanc JK, Espada R, Ergun G. Non-small cell lung cancer staging techniques and endoscopic ultrasound: tissue is still the issue. Chest 2003;123:1718-25.
- Tournoy KG, Maddens S, Gosselin R, . Integrated FDG-PET/CT does not make invasive staging of the intrathoracic lymph nodes in non-small cell lung cancer redundant. Thorax n.d.
- Detterbeck FC, DeCamp MM, Kohman LJ, . Lung cancer. Invasive staging: the guidelines. Chest 2003;123:167S-175S.
- Toloza EM, Harpole L, Detterbeck F, . Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:157S-166S.
- Annema JT, Versteegh MI, Veselic M, . Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA 2005;294:931-6.
- Annema JT, Versteegh MI, Veselic M, . Endoscopic Ultrasound-Guided Fine-Needle Aspiration in the Diagnosis and Staging of Lung Cancer and Its Impact on Surgical Staging. J Clin Oncol 2005;23:8357-61.
- Eloubeidi MA, Cerfolio RJ, Chen VK, . Endoscopic ultrasound-guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann Thorac Surg 2005;79:263-8.
- Kramer H, van Putten JW, Post WJ, . Oesophageal endoscopic ultrasound with fine needle aspiration improves and simplifies the staging of lung cancer. Thorax 2004;59:596-601.
- Tournoy KG, Praet MM, Van Maele G, . Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: high accuracy for the diagnosis of mediastinal lymphadenopathy. Chest 2005;128:3004-9.
- Herth FJ, Lunn W, Eberhardt R, . Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med 2005;171:1164-7.
- Herth FJ, Eberhardt R, Vilmann P, . Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8.
- Yasufuku K, Chiyo M, Koh E, . Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005.
- Yasufuku K, Chiyo M, Sekine Y, . Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8.
- Larsen SS, Krasnik M, Vilmann P, . Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax 2002;57:98-103.
- Vilmann P, Krasnik M, Larsen SS, . Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9.
- Silvestri GA, Tanoue LT, Margolis ML, . The noninvasive staging of non-small cell lung cancer: the guidelines. Chest 2003;123:147S-156S.
- Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-146S.
- Kramer H, van Putten JW, Douma WR, . Technical description of endoscopic ultrasonography with fine-needle aspiration for the staging of lung cancer. Respir Med 2005;99:179-85.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23.
- Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33.
- Semik M, Netz B, Schmidt C, . Surgical exploration of the mediastinum: mediastinoscopy and intraoperative staging. Lung Cancer 2004;45:S55-S61.
Appendix 2 Resource-use collection pro forma
ASTER
Case report form (CRF) – Health Economics
PART 1: IDENTIFIERS (so that this CRF data can be tied up with main CRF)
-
Patient initials __ (first letter first name) __ __ (first two letters surname)
-
Date of birth (DD–MM–YYYY)
-
Sex: ○ M ○ F
-
Study centre:
-
– ○ LUMC
-
– ○ Ghent
-
– ○ Papworth
-
– ○ Leuven
-
-
Study number (e.g. LUMC 001, etc.)
-
Randomisation group:
-
○ A (endoscopic ultrasound)
-
○ B (surgical staging)
-
PART 2: ADMISSION/DISCHARGE DATES (please complete relevant sections)
-
What date was the patient admitted for the EUS/EBUS procedure?
(DD–MM–YYYY)
-
What date was patient discharged following EUS/EBUS procedure?
(DD–MM–YYYY)
-
Date of admission for surgical staging procedure
(DD–MM–YYYY)
-
Date of discharge following surgical staging procedure
(DD–MM–YYYY)
-
Date of admission for thoracotomy?
(DD–MM–YYYY)
-
Date of discharge following thoracotomy
(DD–MM–YYYY)
PART 3: HEALTH RESOURCE DATA
-
Did the patient complete the EQ-5D questionnaire at baseline?
○ yes, ○ no
-
If yes, date of completion
(DD–MM–YYYY)
-
Did the patient complete the EQ-5D questionnaire after:
EBUS/EUS procedure for patients in arm A
Surgical staging procedure for patients in arm B
○ yes, ○ no
-
If yes, date of completion
(DD–MM–YYYY)
-
Did the patient complete the EQ-5D questionnaire at 2 months post-randomisation?
○ yes, ○ no
-
If yes, date of completion
(DD–MM–YYYY)
-
Did the patient complete the EQ-5D questionnaire at 6 months post-randomisation?
○ yes, ○ no
-
If yes, date of completion
(DD–MM–YYYY)
PART 4: 2 MONTHS POST RANDOMISATION (AT CLINIC OR VIA TELEPHONE/POST)
-
Has the patient undergone surgical resection in the last 2 months?
○ yes, ○ no
-
Has the patient undergone chemotherapy treatment in the last 2 months?
○ yes, ○ no
-
If yes, how many cycles? (circle) 1 2 3 4
-
Has the patient undergone any radiotherapy treatment in the last 2 months?
○ yes, ○ no
-
If yes, was it:
○ radical
○ palliative
-
If yes, how many fractions did the patient receive? ____
-
Has the patient been admitted to hospital in the last 2 months for any reason OTHER than for a trial procedure?
○ yes, ○ no
-
If yes, what was their length of stay in hospital? ___ days
-
Has the patient been admitted to a hospice in the last 2 months?
○ yes, ○ no
-
If yes, what was their length of stay in the hospice? ___ days
PART 5: 6 MONTHS POST RANDOMISATION (AT CLINIC OR VIA TELEPHONE/POST)
-
Has the patient undergone surgery in the last 4 months (i.e. since last questionnaire)?
○ yes, ○ no
-
Has the patient undergone chemotherapy treatment in the last 4 months (ie since last questionnaire)?
○ yes, ○ no
-
If yes, how many cycles? (circle) 1 2 3 4
-
Has the patient undergone any radiotherapy treatment in the last 4 months (since last questionnaire)?
○ yes, ○ no
-
If yes, was it:
○ radical
○ palliative
-
If yes, how many fractions did the patient receive? ___
-
Has the patient been admitted to hospital in the last 4 months OTHER than for a trial procedure?
○ yes, ○ no
-
If yes, what was their length of stay in hospital? ___ days
-
Has the patient been admitted to a hospice in the last 4 months?
○ yes, ○ no
-
If yes, what was their length of stay in the hospice? ___ days
PART 6
-
DATE OF DEATH (IF APPLICABLE) DD–MM–YYYY
Appendix 3 Quality of life and resource use by centre
European Quality of Life-5 Dimensions analysis by centre
Table 23 shows the mean (SD) EQ-5D utility by centre and Figure 20 shows the mean (95% CI) EQ-5D utility by centre.
| Centre, time point | EUS/EBUS | Surgical staging |
|---|---|---|
| LUMC (n = 22 and n = 19) | ||
| Baseline | 0.83 (0.13) | 0.82 (0.15) |
| End of staging | 0.80 (0.26) | 0.65 (0.32) |
| 2 months | 0.73 (0.25) | 0.69 (0.27) |
| 6 months | 0.77 (0.27) | 0.66 (0.30) |
| Ghent (n = 21 and n = 17) | ||
| Baseline | 0.80 (0.21) | 0.90 (0.09) |
| End of staging | 0.74 (0.26) | 0.87 (0.10) |
| 2 months | 0.60 (0.32) | 0.72 (0.25) |
| 6 months | 0.62 (0.35) | 0.75 (0.34) |
| Papworth (n = 14 and n = 14) | ||
| Baseline | 0.83 (0.11) | 0.78 (0.12) |
| End of staging | 0.77 (0.09) | 0.71 (0.14) |
| 2 months | 0.68 (0.14) | 0.61 (0.22) |
| 6 months | 0.66 (0.14) | 0.58 (0.27) |
| Leuven (n = 16 and n = 21) | ||
| Baseline | 0.78 (0.24) | 0.80 (0.16) |
| End of staging | 0.78 (0.26) | 0.49 (0.35) |
| 2 months | 0.56 (0.30) | 0.57 (0.29) |
| 6 months | 0.66 (0.37) | 0.69 (0.31) |
FIGURE 20.
Mean (95% CI) EQ-5D by centre.
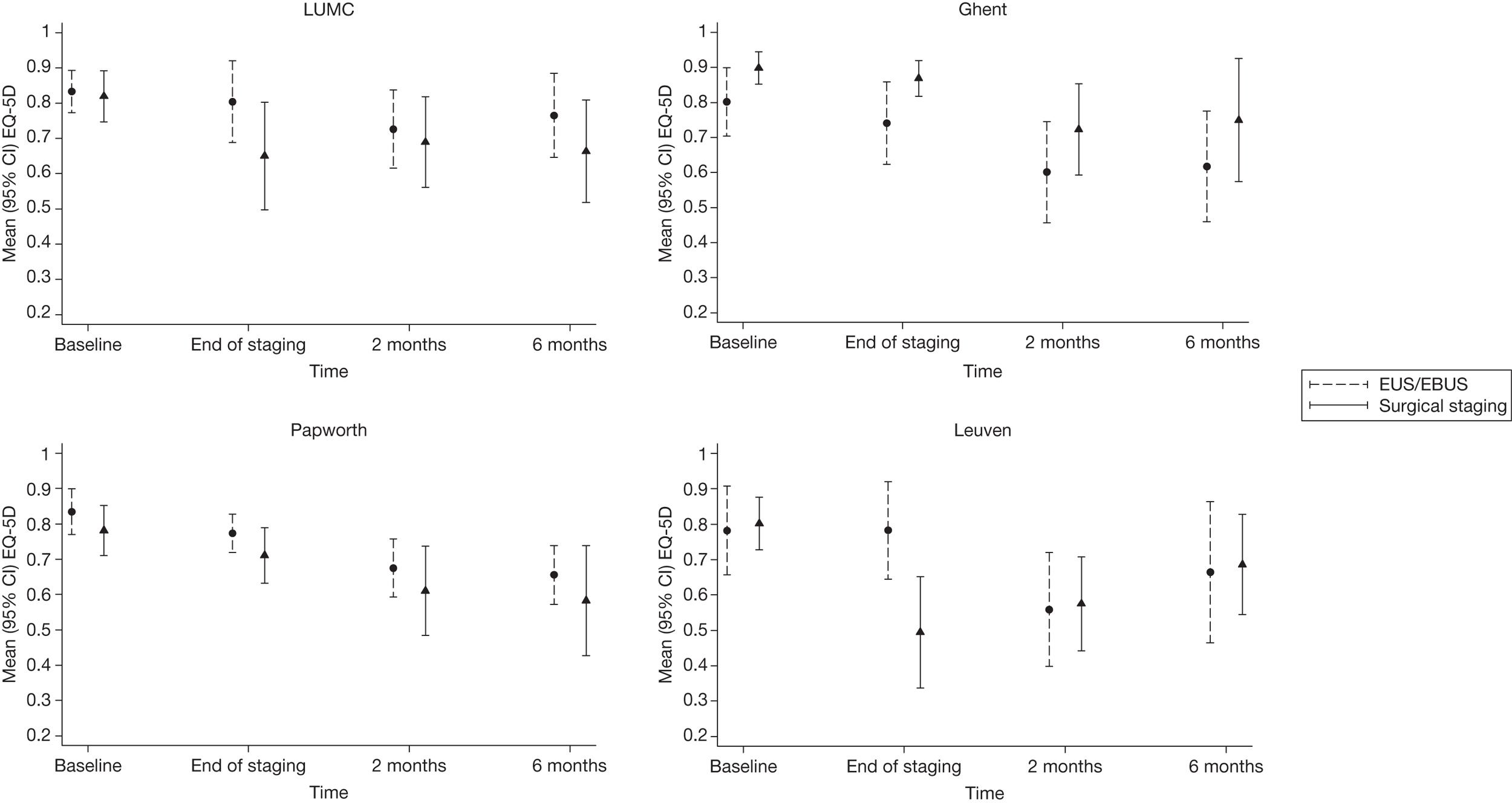
Table 24 shows the differences between the endosonography (followed by surgical staging if positive) and surgical staging groups, by centre, both unadjusted and adjusted for baseline. Figure 21 shows adjusted differences. At the end of staging, only Leuven showed a statistically significant difference between the groups, favouring the endosonography strategy (p = 0.009) in the unadjusted and adjusted analyses. Ghent showed a borderline statistically significant difference in the unadjusted analyses, favouring surgical staging, but when adjusted for baseline this was no longer true. LUMC showed a borderline statistically significant difference between the groups at end of staging, favouring EUS/EBUS. At no other stages in either analysis were statistically significant differences seen.
| Analysis, time point | LUMC | Ghent | Papworth | Leuven | ||||
|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) | p-value | Difference (95% CI) | p-value | Difference (95% CI) | p-value | Difference (95% CI) | p-value | |
| Unadjusted | ||||||||
| End of staging | 0.154 (–0.029 to 0.336) | 0.10 | –0.128 (–0.262 to 0.007) | 0.06 | 0.063 (–0.028 to 0.154) | 0.17 | 0.288 (0.078 to 0.498) | 0.009 |
| 2 months | 0.037 (–0.127 to 0.200) | 0.65 | –0.122 (–0.314 to 0.070) | 0.21 | 0.065 (–0.079 to 0.208) | 0.36 | –0.016 (–0.216 to 0.184) | 0.87 |
| 6 months | 0.102 (–0.078 to 0.282) | 0.26 | –0.132 (–0.360 to 0.096) | 0.25 | 0.072 (–0.096 to 0.241) | 0.38 | –0.022 (–0.250 to 0.207) | 0.85 |
| Adjusted for baseline | ||||||||
| End of staging | 0.138 (–0.017 to 0.294) | 0.08 | –0.043 (–0.140 to 0.053) | 0.37 | 0.035 (–0.047 to 0.117) | 0.39 | 0.299 (0.099 to 0.499) | 0.005 |
| 2 months | 0.027 (–0.126 to 0.181) | 0.72 | –0.053 (–0.237 to 0.131) | 0.56 | 0.048 (–0.010 to 0.196) | 0.51 | –0.012 (–0.214 to 0.189) | 0.90 |
| 6 months | 0.095 (–0.082 to 0.271) | 0.28 | –0.076 (–0.307 to 0.155) | 0.51 | 0.030 (–0.130 to 0.190) | 0.70 | –0.021 (–0.254 to 0.211) | 0.85 |
FIGURE 21.
Difference in mean EQ-5D (95% CI) between endosonography (followed by surgical staging if negative) and surgical staging groups, by centre, adjusted for baseline. Values > 0 favour the endosonography strategy.
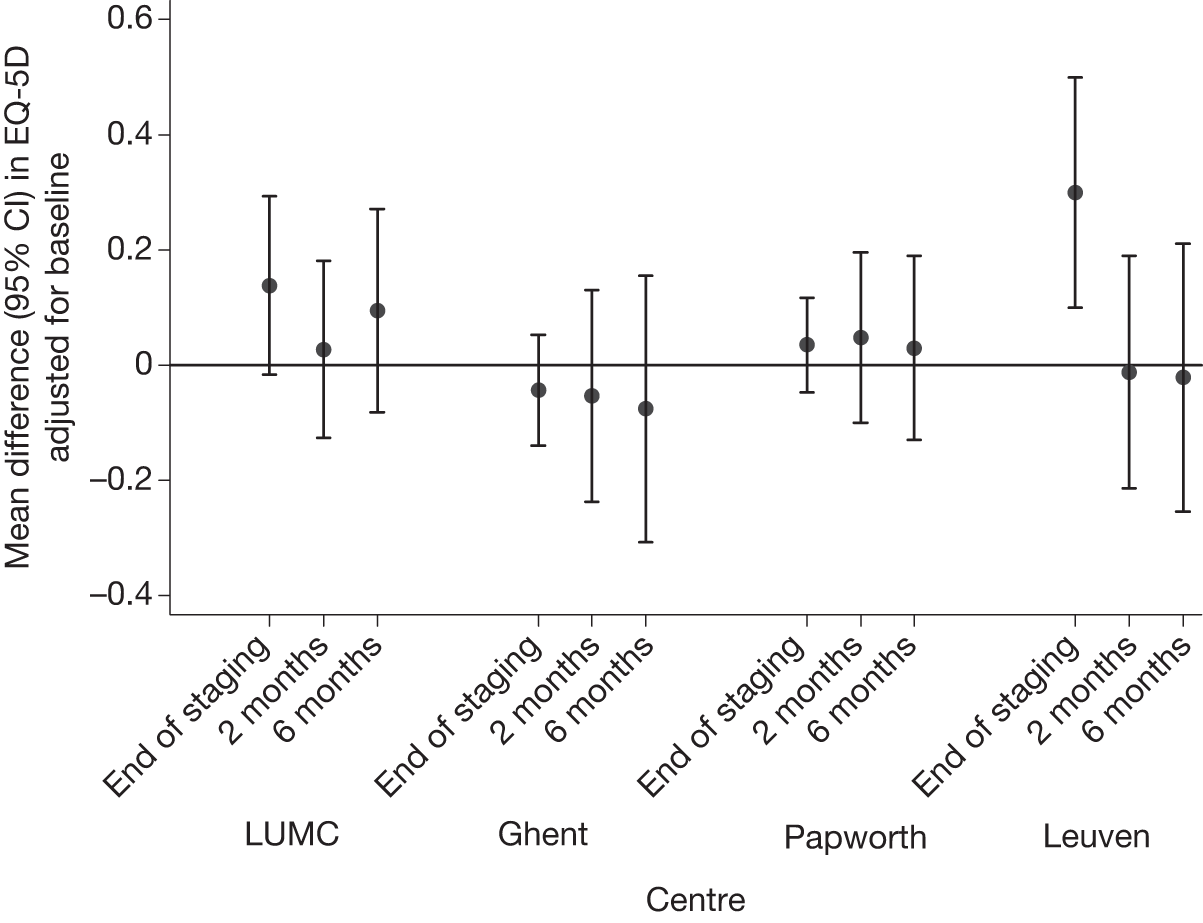
Quality-adjusted life-year results by centre
Table 25 and Figure 22 show the average 6-month QALYs by centre. The biggest difference between groups was seen in Ghent, where the average QALY value was 0.07 higher in the surgical staging group than in the EUS/EBUS group. However, once adjusted for baseline this difference reduced to 0.03. Table 25 also shows the mean (95% CI) difference in QALYs adjusted for baseline. There were small differences between the groups; no difference was statistically significant.
| Centre | EUS/EBUS | Surgical staging | Difference adjusting for baseline EQ-5D (EUS/EBUS – surgical staging) |
|---|---|---|---|
| LUMC (n = 22, n = 19) | 0.38 (0.12) | 0.34 (0.12) | 0.034 (–0.036 to 0.10) |
| Ghent (n = 21, n = 17) | 0.31 (0.13) | 0.38 (0.11) | –0.030 (–0.10 to 0.043) |
| Papworth (n = 14, n = 14) | 0.34 (0.06) | 0.30 (0.10) | 0.021 (–0.043 to 0.085) |
| Leuven (n = 16, n = 21) | 0.31 (0.14) | 0.29 (0.13) | 0.015 (–0.076 to 0.11) |
FIGURE 22.
Six-month QALY by centre (unadjusted for baseline utility).
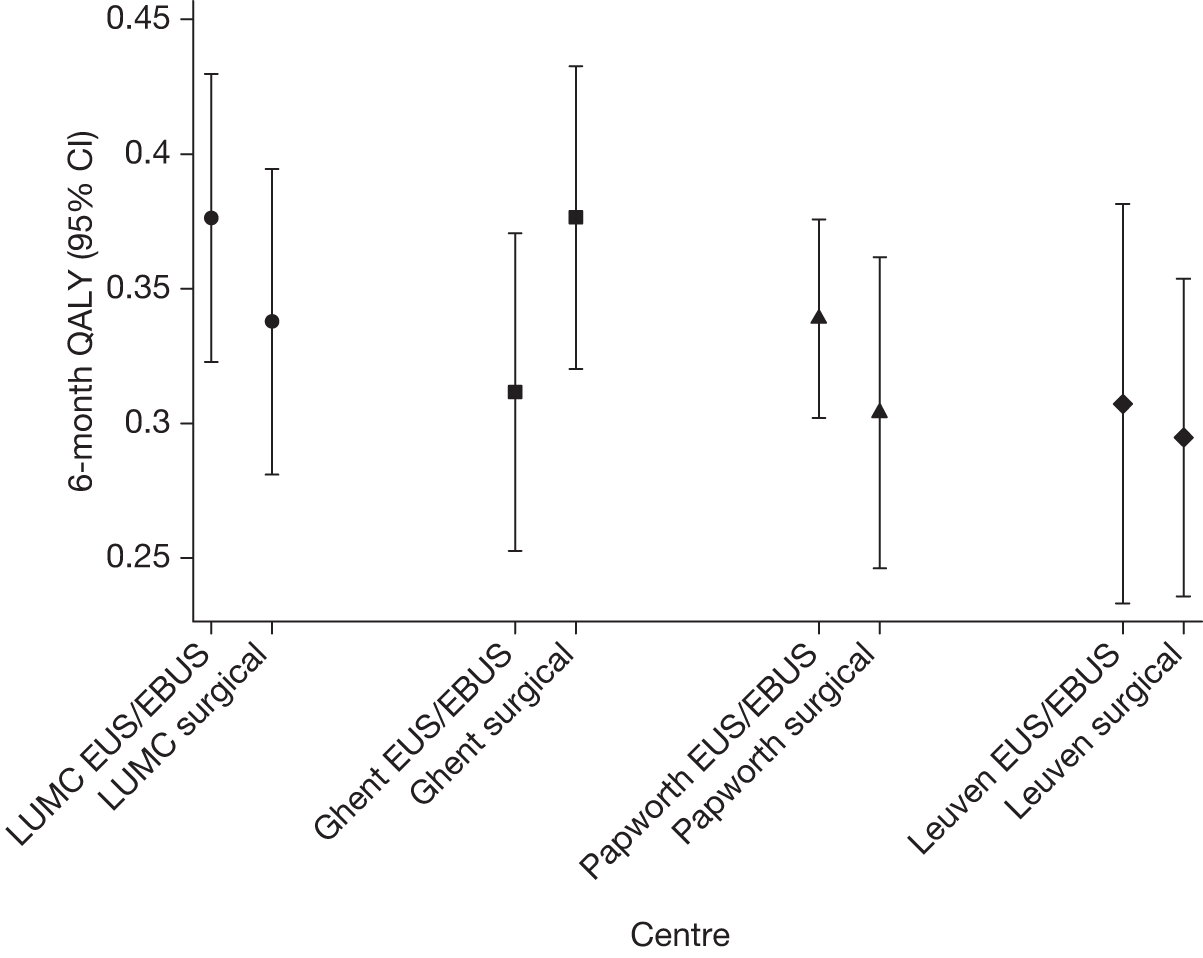
Resource-use analysis by centre for patients who had complete information on each resource item
Table 26 shows resource use by centre.
| Resource item | No. of people using each resource item (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| LUMC | Ghent | Papworth | Leuven | |||||
| EUS/EBUS (n = 30) | Surgical staging (n = 29) | EUS/EBUS (n = 31) | Surgical staging (n = 28) | EUS/EBUS (n = 11) | Surgical staging (n = 10) | EUS/EBUS (n = 13) | Surgical staging (n = 20) | |
| EUS/EBUS procedure | 30 (100) | 0 (0) | 32 (101) | 1 (4) | 11 (100) | 0 (0) | 13 (100) | 0 (0) |
| Surgical staging procedure | 19 (63) | 28 (97) | 13 (42) | 28 (100) | 5 (45) | 10 (100) | 10 (77) | 20 (100) |
| Thoracotomy (lobectomy or pneumonectomy) with lymph node dissection | 17 (57) | 18 (62) | 12 (39) | 17 (61) | 6 (55) | 7 (70) | 10 (77) | 15 (75) |
| Chemotherapy in the first 2 months | 13 (43) | 9 (31) | 19 (61) | 14 (50) | 4 (36) | 5 (50) | 7 (54) | 11 (55) |
| Radiotherapy in the first 2 months | 5 (17) | 4 (14) | 5 (16) | 4 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (5) |
| Hospital admission in the first 2 months | 2 (7) | 2 (7) | 6 (19) | 3 (11) | 2 (18) | 2 (20) | 8 (62) | 12 (60) |
| Hospice admission in the first 2 months | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Surgery between months 2 and 6 | 4 (13) | 3 (10) | 4 (13) | 3 (10) | 1 (9) | 1 (10) | 2 (15) | 2 (10) |
| Chemotherapy between months 2 and 6 | 10 (33) | 9 (31) | 17 (55) | 16 (57) | 6 (55) | 6 (60) | 7 (54) | 12 (60) |
| Radiotherapy between months 2 and 6 | 6 (20) | 10 (34) | 18 (58) | 12 (43) | 6 (55) | 3 (30) | 2 (15) | 2 (10) |
| Hospital admission between months 2 and 6 | 5 (17) | 6 (21) | 9 (29) | 4 (14) | 5 (45) | 2 (20) | 9 (69) | 13 (65) |
| Hospice admission between months 2 and 6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) |
Total trial costs by centre, for patients who had complete information on each resource item
The total mean (SD) and median (IQR) trial costs by centre are presented in Table 27 and Figure 23.
| Centre | EUS/EBUS (n = 85) | Surgical staging (n = 87) | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) cost (£) | Median (IQR) total cost (£) | n | Mean (SD) cost (£) | Median (IQR) total cost (£) | |
| LUMC | 30 | 9762 (3488) | 10,887 (7279 to 10,887) | 29 | 10,213 (3422) | 9882 (9633 to 12,322) |
| Ghent | 31 | 9609 (2931) | 8897 (6833 to 10,887) | 28 | 11,064 (3609) | 10,427 (8661 to 12,758) |
| Papworth | 11 | 10,461 (4498) | 12,011 (5652 to 15,009) | 10 | 10,848 (3388) | 11,380 (7480 to 12,035) |
| Leuven | 13 | 13,723 (3217) | 11,123 (10,887 to 17,135) | 20 | 12,797 (3484) | 12,291 (9633 to 15,632) |
FIGURE 23.
Box plots by centre of the total trial costs for patients who had complete information on all resource items.
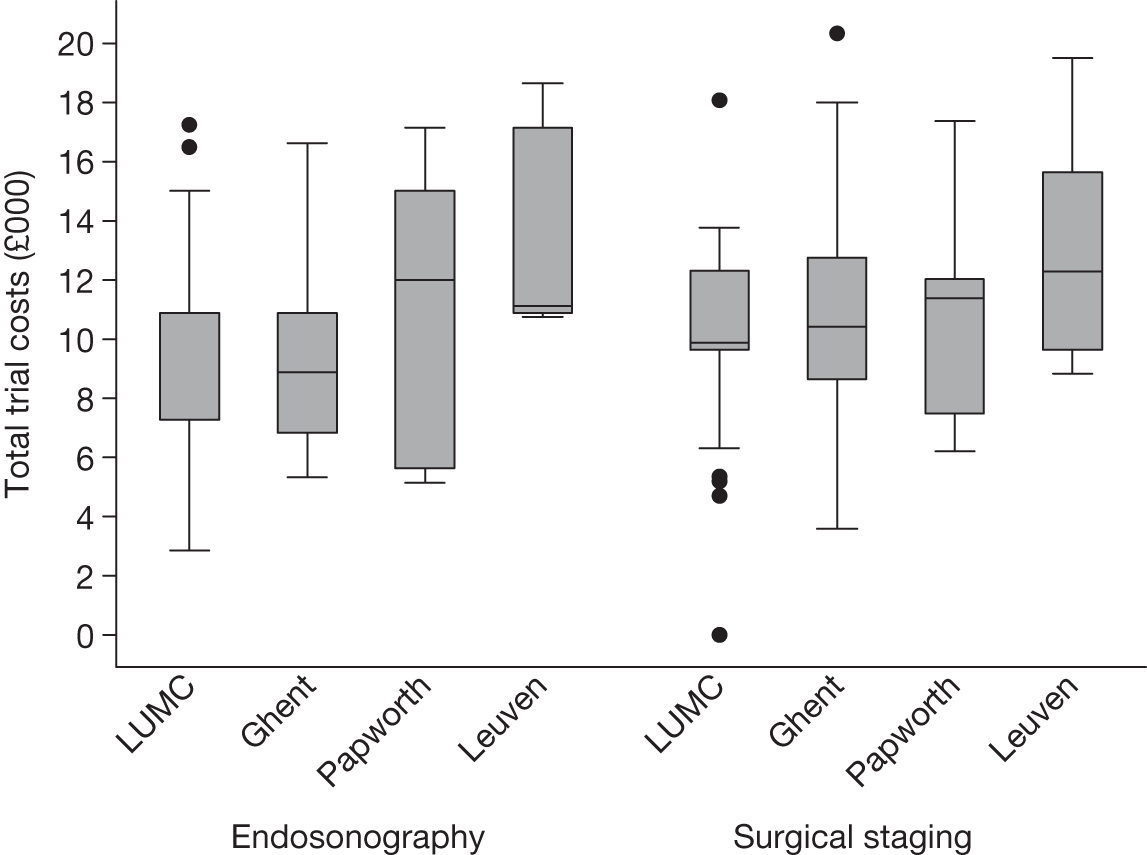
Total trial costs by centre for patients who had complete information on each resource item, assuming that there are no surgical staging costs for the EUS/EBUS group
Table 28 and Figure 24 show the total mean (SD) and median (IQR) trial costs by centre for completers, ignoring the cost of surgical staging for the EUS/EBUS group.
| Centre | EUS/EBUS | Surgical staging | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) cost (£) | Median (IQR) total cost (£) | n | Mean (SD) cost (£) | Median (IQR) total cost (£) | |
| LUMC | 30 | 8247 (2673) | 7805 (7279 to 9423) | 29 | 10,213 (3422) | 9882 (9633 to 12,322) |
| Ghent | 31 | 8527 (2277) | 7805 (6833 to 9302) | 28 | 11,064 (3609) | 10,427 (8661 to 12,758) |
| Papworth | 11 | 9060 (3184) | 9647 (5652 to 11,927) | 10 | 10,848 (3388) | 11,380 (7480 to 12,035) |
| Leuven | 13 | 11,301 (2817) | 11,123 (7805 to 14,053) | 20 | 12,797 (3484) | 12,291 (9633 to 15,632) |
FIGURE 24.
Box plots by centre of the total trial costs for completers, ignoring the costs of surgical staging and increasing the futile thoracotomy rate for the EUS/EBUS group.
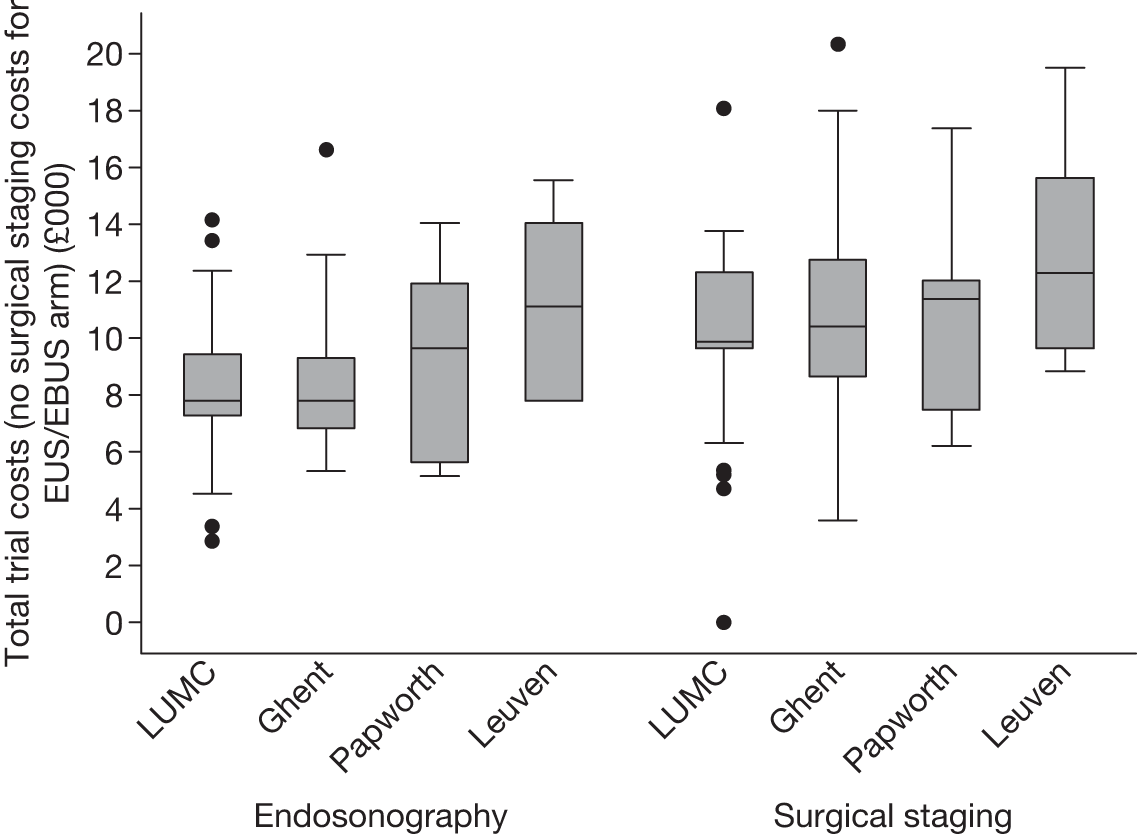
Appendix 4 Full Bayesian analysis by centre
The Bayesian model was also used to estimate expected costs and QALYs by centre (Table 29). Expected 6-month costs under either strategy are estimated to be around £2000–3000 higher in Leuven (the highest) than in Ghent (the lowest). In Ghent, the endosonography strategy is expected to be about £1100 cheaper than surgical staging alone, whereas in Leuven it is around £200 cheaper than surgical staging. Expected QALYs over 6 months (which are adjusted for baseline EQ-5D) differ between centres by about 0.03. All CrIs for the difference between the two diagnostic strategies span zero reflecting the lack of precision when considering each centre individually.
| Centre | Endosonography and surgical staging | Surgical staging | Incremental (endosonography – surgical staging) | |||
|---|---|---|---|---|---|---|
| Posterior mean | Posterior 95% CrI | Posterior mean | Posterior 95% CrI | Posterior mean | Posterior 95% CrI | |
| Expected costs (£) | ||||||
| LUMC | 9401 | 7494 to 11,652 | 10,023 | 7711 to 12,737 | –621 | –2136 to 740 |
| Ghent | 8694 | 6845 to 10,872 | 9818 | 7455 to 12,590 | –1125 | –2884 to 365 |
| Papworth | 10,651 | 8268 to 13,334 | 11,368 | 8674 to 14,387 | –718 | –2506 to 759 |
| Leuven | 11,748 | 9433 to 14,424 | 11,983 | 9433 to 14,937 | –235 | –1679 to 995 |
| Expected QALYs | ||||||
| LUMC | 0.357 | 0.32 to 0.387 | 0.344 | 0.304 to 0.375 | 0.013 | –0.02 to 0.046 |
| Ghent | 0.346 | 0.306 to 0.381 | 0.331 | 0.282 to 0.371 | 0.015 | –0.022 to 0.052 |
| Papworth | 0.333 | 0.284 to 0.375 | 0.317 | 0.263 to 0.362 | 0.016 | –0.024 to 0.056 |
| Leuven | 0.322 | 0.274 to 0.365 | 0.305 | 0.257 to 0.347 | 0.017 | –0.027 to 0.058 |
List of abbreviations
- ASTER
- Assessment of Surgical sTaging versus Endosonographic ultrasound in lung cancer: a Randomised clinical trial
- AUC
- area under the curve
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CrI
- credible interval from a Bayesian posterior distribution
- CONSORT
- CONonsolidated Standards Of Reporting Trials
- CT
- computerised tomography
- EBUS-TBNA
- endobronchial ultrasound-guided transbronchial needle aspiration
- EQ-5D
- European Quality of Life-5 Dimensions
- EUS-FNA
- endoscopic ultrasound-guided fine-needle aspiration
- FNA
- fine-needle aspiration
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LUMC
- Leiden University Medical Centre
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- NSCLC
- non-small cell lung cancer
- PET
- positron emission tomography
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- SCLC
- small cell lung cancer
- SD
- standard deviation
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health