Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/142/01. The contractual start date was in January 2011. The draft report began editorial review in July 2011 and was accepted for publication in November 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Tappenden et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
In old age, reduction in physical function can lead to loss of independence, the need for hospital and long-term nursing home care, and premature death. The importance of physical, functional, psychological and social factors in realising a healthy old age is recognised by older people,1 health-care professionals,2 policy advisors3 and decision-makers.
As the number of older people increases, the needs of older people are expected to become an increasingly important health issue. It has been estimated that by the year 2025, around 20% of the population in industrial countries will be aged 65 years and over as a consequence of people living longer. Changing family structures and greater mobility in the working population mean that many more older people will be living alone, and social isolation and loneliness are likely to become increasingly widespread. It has been suggested that the number of older people with mental health problems will also grow; estimates suggest that, by the year 2021, more than 1 in every 15 people will be an older person experiencing a mental health problem. 4
The objective of enabling older people to remain in their own homes has been a cornerstone of government policy for several decades. In recent years, considerable emphasis has been placed on health promotion and other preventative measures as a means of delaying the onset of illness and dependency that eventually lead older people to need long-term care. 5
Home-based health promotion programmes for older people, carried out by nurses and other health-care professionals (such as occupational therapists and physiotherapists), have the potential to positively affect health and functional status, and may promote independent functioning of older people. Such programmes may also aim to reduce hospital and nursing/residential home admissions. A substantial number of studies have examined the effects of preventative home-visiting programmes on older people living in the community. Since 2000, 10 systematic reviews of the clinical effectiveness of home- or community-based programmes have been published. 6–15 However, these reviews have reported inconsistent and conflicting results. Subgroup analyses of the largest published meta-analysis suggested that effective home-visiting programmes include multidimensional assessment and numerous follow-up visits and were targeted at individuals who were at lower risk of death. 8 However, none of the existing reviews included an assessment of the cost-effectiveness of home-visiting programmes nor did they limit the analysis to the UK context. This assessment seeks to address these gaps and to explore what is known about the factors that may contribute to the effectiveness of this type of complex intervention.
Current service provision
Older people potentially have a great deal to gain from effective preventative programmes and from health promotion. Prevention services may lead to better health outcomes and a more efficient use of resources over the long term, with decreased demand on costly acute and social care services. However, there is evidence of an uneven uptake of health-promoting services such as immunisation and screening programmes in older people. 16 Furthermore, general practitioners (GPs) may be less likely to discuss lifestyle changes such as weight reduction, smoking, alcohol and safe drinking with older people than with younger people. 17
Nurses may play an important role in promoting health and preventing ill health in older people, who may experience a range of health and social care problems. The NHS Improvement Plan18 described a new clinical role for nurses. Known as community matrons, these experienced skilled nurses use case management techniques with patients who meet criteria denoting very high-intensity use of health care. With special intensive help, these patients are able to remain at home longer and to have more choice about their health care. Community nurses, including practice nurses, health visitors (public health nurses) and district nurses, are also well placed to promote health in older people. A recent survey of community nurses suggested that they recognise health promotion as part of their role but may be limited by a range of factors including organisational constraints, the absence of specific training, variable knowledge and the unplanned approach to this area of work, suggesting that nurses working in primary care may currently be ill equipped to enable older people to increase or maintain their levels of physical activity and function. 19
Description of the intervention under assessment
The World Health Organization defines health promotion as ‘the process of enabling people to increase control over, and to improve, their health. It moves beyond a focus on individual behaviour towards a wide range of social and environmental interventions’ (www.who.int/topics/health_promotion/en/). Health promotion can take a variety of forms including provision of advice and education for improving health and avoiding ill health, the implementation of service improvements and policy agenda-setting. Hubley and Copeman20 have put forward a framework for describing the range of activities that may be encompassed within health promotion programmes. This is comprised of three main types of activity: (1) health education, which involves communication directed at individuals, families and communities to influence; (2) service improvement, which involves quality and quantity of service; and (3) advocacy, which involves agenda-setting for healthy public policy.
Given the range of possible ways of implementing a home-based, nurse-led health promotion programme, the intervention under consideration within this assessment would be best described as a complex intervention, in that it that may comprise multiple, potentially interacting components. The focus within this assessment is principally on nurse-led health promotion activities undertaken within the subject’s home. It should be noted, however, that within several of the studies included within this assessment, the home-based intervention did not consist solely of health promotion activities for the prevention of illness, but also extended to treatment and other care-related elements of nursing activity.
Chapter 2 Description of decision problem
Research question
The commissioning brief for the assessment sought to address the following questions:
-
Do home-based, nurse-led interventions work, and if so what do they prevent or promote?
-
If these interventions work effectively, what features of the intervention are crucial to their effectiveness and how much will the beneficial effects cost?
Aims and objectives of this assessment
The main research question addressed by this study is ‘What is the clinical effectiveness and cost-effectiveness of nurse-led health promotion intervention delivered at home for older people at risk of admission to hospital, residential or nursing care in the UK?’ The specific objectives of this assessment are to:
-
evaluate the clinical effectiveness of home-based, nurse-led health promotion programmes in the UK
-
review existing health economic evaluations of home-based, nurse-led health promotion programmes from the perspective of the NHS and Personal Social Services (PSS)
-
explore, as far as existing evidence allows, those elements of this form of complex intervention that may contribute to its clinical effectiveness and
-
identify key gaps in current evidence and to identify areas in which future research may be warranted.
The main facets of the decision problem addressed by the review are detailed below:
-
Intervention Structured home-based, nurse-led health promotion.
-
Population Older people > 75 years of age with long-term medical or social needs at risk of admission to hospital, residential or nursing care.
-
Setting Interventions delivered at home, relating to a UK context.
-
Comparator Standard care including joint health and social assessment. Health promotion delivered in a different setting or not delivered by a nurse.
-
Design This assessment report includes two related systematic reviews: (1) a systematic review of clinical effectiveness studies (see Chapter 3) and (2) a systematic review of cost-effectiveness studies (see Chapter 4). A de novo cost-effectiveness model was not developed as part of this study.
Chapter 3 Assessment of clinical effectiveness
Introduction
This chapter presents the methods and results of a systematic review of the clinical effectiveness of home-based, nurse-led health promotion programmes.
Methods for reviewing clinical effectiveness
Identification of studies
A comprehensive literature search was undertaken across 12 different databases and research registers between February and March 2011. Information on the provider and coverage dates of the sources are detailed in Table 1.
| Database | Provider/interface | Coverage |
|---|---|---|
| MEDLINE and MEDLINE in Process & Other Non-Indexed Citations | Ovid | 1948–present |
| EMBASE | Ovid | 1980–present |
| Science Citation Index Expanded (SCIE) | Web of Science | 1899–present |
| Cochrane Database of Systematic Reviews (CDSR) | Wiley InterScience | 1996–present |
| Cochrane Central Register of Controlled Trials (CCRCT) | Wiley InterScience | 1898–present |
| NHS Health Economic Evaluation Database (NHS EED) | Wiley InterScience | 1995–present |
| Health Technology Assessment database (HTA) | Wiley InterScience | 1995–present |
| Database of Abstracts of Reviews of Effects (DARE) | Wiley InterScience | 1995–present |
| Cumulative Index to Nursing and Allied Health Literature (CINAHL) | EBSCO | 1982–present |
| UK Clinical Research Network (CRN) Portfolio Databasea | National Institute for Health Research (NIHR) | 2001–present |
| ClinicalTrials.gov | United States-National Institutes of Health (US-NIH) | 2000–present |
| Health Economics Evaluations Database (HEED) | OHE-IFPMA database | 1967–present |
Where applicable, sensitive search filters were applied to identify three study designs: (1) randomised controlled trials (RCTs), (2) systematic reviews and (3) economic evaluations (Table 2; see also Appendix 1). MEDLINE and MEDLINE in Process & Other Non-Indexed Citations, EMBASE and the Web of Science were searched for all three study designs. Completed and unpublished studies were identified through searches in the Health Technology Assessment (HTA) database and two web-based research registers, including the UK Clinical Research Network (CRN) Portfolio Database and ClinicalTrials.gov. Searches for economic evaluations were supplemented by searching MEDLINE and EMBASE, HTA database, NHS Health Economic Evaluation Database (NHS EED), Database of Abstracts of Reviews of Effects (DARE) and Cumulative Index to Nursing and Allied Health Literature (CINAHL).
| Database | Study design | ||
|---|---|---|---|
| RCTs | Systematic reviews | Economic evaluations | |
| MEDLINE and MEDLINE in Process & Other Non-Indexed citations | ✓ | ✓ | ✓ |
| EMBASE | ✓ | ✓ | ✓ |
| SCIE | ✓ | ✓ | ✓ |
| CDSR | ✗ | ✓ | ✗ |
| HTA and DARE | ✓ | – | ✓ |
| CCRCT | ✓ | – | ✗ |
| NHS EED | ✗ | ✗ | ✓ |
| CINAHL | ✓ | ✓ | ✓ |
| UK CRN | ✓ | ✗ | ✗ |
| ClinicalTrials.gov | ✓ | ✗ | ✗ |
| HEED | ✗ | ✗ | ✓ |
It was agreed among the research team that the searches would be limited by date from 2001 onwards and that an English-language limit would also be applied as only UK-specific studies were relevant to the scope of the assessment. Other studies published prior to this date were identified by hand-searching existing systematic reviews. RCT filters were not applied to searches in The Cochrane Library [HTA and Cochrane controlled trials reports (CCTR)] and research registers (UK CRN and ClinicalTrials.gov), as these are trial-based sources. Similarly, the economic evaluation filter was not applied to the NHS EED and the Health Economic Evaluations Database (HEED) as these constitute the largest collection of economic evaluations. Given that the largest number of records was retrieved from the RCT searches compared with the systematic reviews and economic evaluation searches, a geographic filter was applied to identify studies that were related to the UK setting.
All citations were imported into Reference Manager, version 12 (Thomson Reuters, Philadelphia, PA, USA) software and duplicates were removed. Titles and abstracts of all unique citations were then screened by one reviewer (FC) using the inclusion criteria outlined in Chapter 3 (see Inclusion/exclusion criteria). Any uncertainty regarding possible inclusion of studies was resolved by discussion between the members of the research team, or through retrieval and subsequent examination of the full study publication. The full papers of all potentially relevant citations were retrieved to enable an in-depth assessment concerning study inclusion in the review. In the event that published papers did not report potentially relevant data, corresponding authors were contacted by e-mail; where further relevant data were made available through this route, they were included in the analysis.
Inclusion/exclusion criteria
The inclusion criteria for the systematic review of clinical effectiveness were as follows:
-
Population Older people (> 75 years or > 70 years when considered a vulnerable population on the basis of age) with long-term medical or social needs at risk of admission to hospital, residential or nursing care.
-
Interventions Structured home-based, nurse-led health promotion.
-
Comparators Standard care including joint health and social assessment. Health promotion delivered in a different setting or not delivered by a nurse.
-
Setting Interventions delivered in the home setting, undertaken in the UK.
-
Outcomes Admission to hospital, residential or nursing care, mortality, morbidity including depression, falls, accidents, deteriorating health status, patient satisfaction.
-
Study design RCTs.
Studies were excluded from the review if the effectiveness of the intervention was not assessed within a UK setting, if the intervention was not predominantly delivered by nurses, if the population did not include a substantial proportion of individuals aged over 75 years, or if the intervention did not include any discernible elements of health promotion. In instances whereby all inclusion criteria were met except for the age-restriction criterion, this was sometimes relaxed based on subjective judgement and discussions among the research team. Non-randomised studies were also excluded from the review.
Data extraction strategy
Data were extracted independently by one reviewer using a standardised data extraction form.
Quality assessment strategy
The methodological quality of studies included in the review was assessed using the Cochrane Risk of Bias tool (available from www.cochrane.org/). In particular, consideration of study quality included the following factors:
-
timing, duration and length of follow-up of the study
-
method of randomisation
-
method of allocation concealment
-
blinding
-
numbers of participants randomised, excluded and lost to follow-up (LTFU)
-
whether or not intention-to-treat (ITT) analysis has been performed.
Methods of analysis and evidence synthesis
Data from included studies were tabulated and discussed in a narrative review. Where appropriate, statistical meta-analysis was undertaken to estimate a summary measure of effect on relevant outcomes based on ITT analyses. Meta-analysis was undertaken using random-effects models using Review Manager (RevMan) software, version 5.0 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). The results of these analyses were reported as odds ratios (ORs). Heterogeneity was explored through consideration of the study populations, methods and interventions, by visualisation of analysis results and through consideration of the I2-statistic.
Results
Quantity and quality of research available
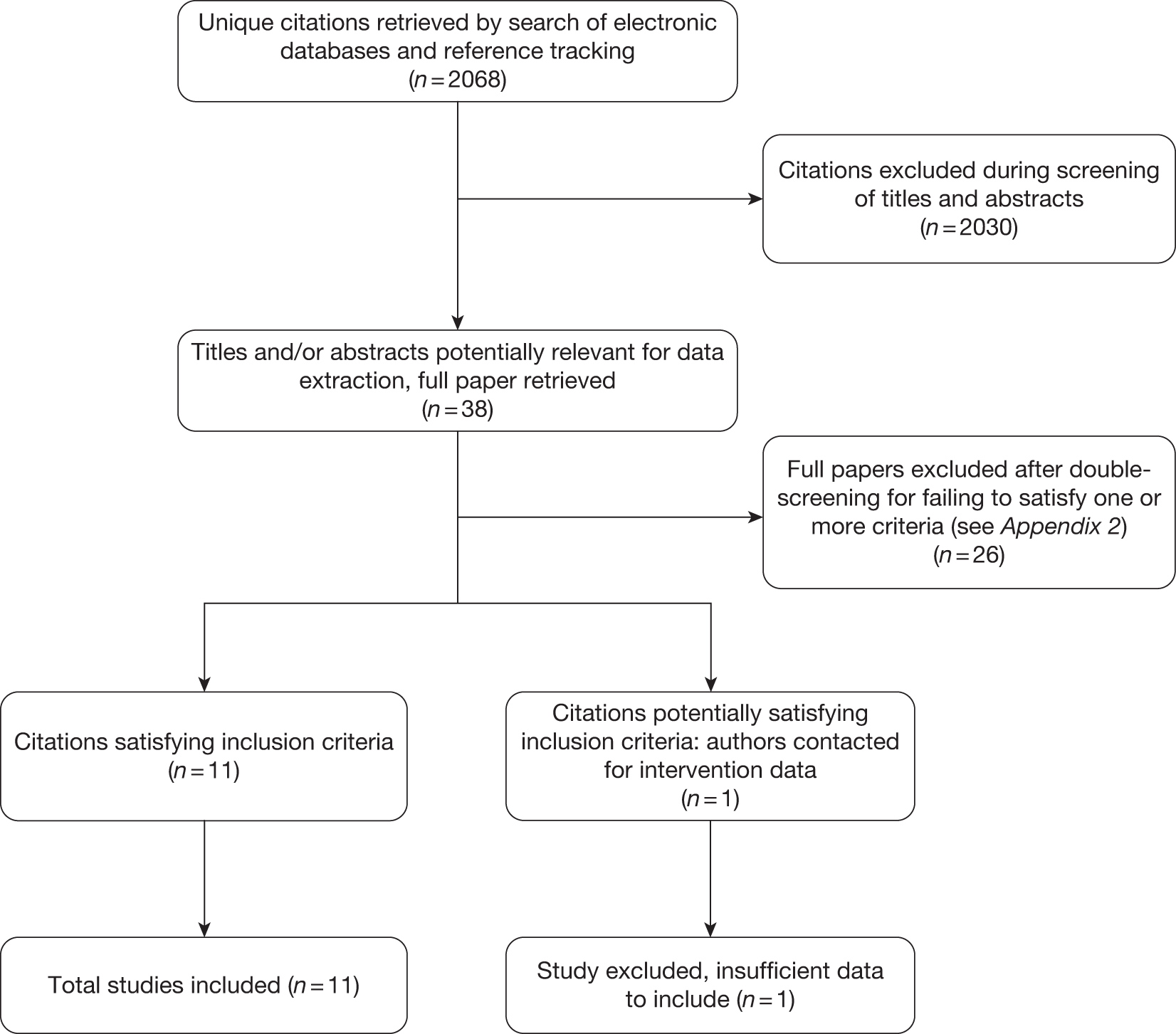
Following the removal of duplicate citations, the systematic searches for RCTs and systematic reviews identified 2068 potentially relevant records. Of these, 38 were retrieved for a more detailed inspection. Of these, 26 studies were excluded from the review. In total, 11 studies were included in the final review of clinical effectiveness. This information is summarised in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram presented in Figure 1.
FIGURE 1.
The PRISMA flow diagram.
Characteristics of included studies
Eleven studies21–31 were included in this review, with the number of participants ranging from 51 to 1286. The total number of participants was 5761. All of the included studies were conducted in the UK. The characteristics of the included studies in terms of study subjects and interventions are reported in Tables 3 and 4, respectively. The 11 studies included RCTs which differed in terms of the target population and the purpose of the health promotion intervention. Four studies were designed to evaluate home-based, nurse-led interventions for particular groups of older people with existing morbidities; these included patients populations with chronic heart failure,21 Parkinson’s disease22 or venous leg ulcers,23 and individuals who had suffered a stroke. 24 The focus of health promotion was to slow or prevent further deterioration or complications of the conditions. Four studies25–28 focused on preventing falls in older people by providing home-based nursing assessment and health promotion. Two studies29,30 evaluated programmes that provided home-based screening and health promotion by nurses to older populations. One study31 assessed the effectiveness of a home-based rehabilitation programme.
| Author | n | Mean age (years) | Living alone | Health condition | No. (%) male |
|---|---|---|---|---|---|
| Blue et al.21 | 165 | I, 74.4 (8.6); C, 75.6 (7.9) | 76/165 (46.1%) | Patients with heart failure | 95/165 (57.6%) |
| Brooks et al.23 | 51 | 80 | NR | Patients had suffered from venous leg ulcers | 22/51 (43.1%) |
| Burton and Gibbon24 | 176 | 75.3 | NR | Patients discharged from hospital following a stroke | 92/176 (52.3%) |
| Jarman et al.22 | 1859 | NR but 577/1836 (31.4%) aged > 77, 649/1836 (35.3%) aged 70–77 and 610/1836 (33.2%) aged < 70 | NR | Patient’s with Parkinson’s disease | 1044/1859 (56.2%) |
| Lightbody et al.28 | 348 | Median 75 (IQR range 70–81) | 153/348 (44.0%) | Patients discharged from A&E, Barthel Index (SD): I, 19 (2.0)/171; C, 19 (2.3)/177 | 89/348 (25.6%) |
| Kingston et al.27 | 109 | 71.9 | NR | Patients who had attended an A&E department following a fall | NR |
| Vetter et al.25 | 674 | Patients > 70 recruited | NR | 41% no disability | NR |
| Vetter et al.30 | 1286 | Patients > 70 recruited | NR | General elderly | NR |
| Spice et al.26 | 516 | C, 83 (6.6)/159; I1, 83 (6.7)/136; I2: 81 (6.6)/210 | NR | Median Barthel Index (IQR): I1, 18 (11 to 20); C, 18 (5 to 20) | 133/516 (25.8%) |
| McEwan et al.29 | 296 | NR | NR | Nottingham Health Profile: Mobility: I, 17.5 (SD)/132; C, 21.8 (SD)/130 | NR |
| Cunliffe et al.31 | 370 | Median (IQR) 80 (73–85) | 123/370 (33.2%) | Median Barthel Index (IQR):18 (17–20) | 114/370 (30.1%) |
The mean age of participants, where reported in the paper, ranged from 71.9 years to 83 years across the included studies. The health status of participants at baseline was not directly comparable between studies. Three studies26,28,31 recorded Barthel Index scores (a tool designed to assess independence with a 0 to 20 score range) at baseline; these studies reported average scores of 19,26 1828 and 1831 (see Glossary). Three studies21,28,31 reported the number of older people living alone. These results also suggested fairly similar populations, with the proportion of older people living alone ranging from 33.2% to 46%. The number of male participants ranged from 25.8% to 58%, with greater proportions of men in the groups with a pre-existing morbidity. 21–24
Description of the interventions
The interventions were delivered by nurses, although the background experience and additional training requirements required for the practitioners was not consistently described in the included studies. In three RCTs,25,27,30 the intervention was delivered by health visitors; these are public health nurses, working in the community, whose role concerns the protection and promotion of health. In two studies26,29 community nurses were given additional training before the study commenced. In one study,24 a specialist stroke nurse was given additional training to provide continuity of care in the community following the study subjects’ discharge from hospital. In five studies, the authors simply state that nurses were given additional training but do not provide further information with respect to their grade or level of qualification. 21–23,28,31 In one study,31 the nurse worked within a multidisciplinary team including physiotherapists and occupational therapists (not doctors). In the other 10 studies,21–30 the nurses worked independently, referring to other health- and social-care professionals as necessary.
The number of home visits made by the nurses also varied between the studies; this quantity was not consistently reported within the study publications. Those home-based interventions delivered to older people discharged from hospital with an existing morbidity received the most visits. In the study reported by Cunliffe et al. ,31 up to four visits were made per day, 7 days per week, for up to 4 weeks. Burton and Gibbon24 reported an average of three visits per patient. Blue et al. 21 did not report how many visits were made to each patient, but these were of decreasing frequency over time and were supplemented by telephone contact as judged necessary. In other studies26,28–30 single visits were made, with additional visits as judged necessary, but follow-up continued over 12 months. In four studies,22,23,25,27 the number of visits was not reported.
Control interventions were consistently described as usual care. In all of the 11 studies21–30 this was care managed by the GP once the patient was discharged from hospital and did not involve a home visit from a nurse. The nature of ‘usual care’ may have differed considerably between studies, but there is insufficient information to evaluate the heterogeneity of care in the control groups between studies.
The nature of the health promotion intervention itself also varied between the included studies (Table 4). For those home-based interventions delivered to patients with existing morbidities, the focus of the intervention was related to managing and monitoring their condition to prevent exacerbation of their disease. The intervention also focused on improving recovery and therefore regaining health following discharge from hospital. Education about medications, recognising symptoms, ensuring appropriate follow-up, encouraging concordance with medications, and health advice and providing advice about healthy lifestyle were features of the intervention in those studies in which the subjects had existing morbidities;21–24,31 in these instances the focus of the intervention was concerned with promoting recovery. Information was delivered verbally but also supported by written information21,23,29 and contact by telephone. 22 The nurses’ roles also included supporting the carers, and where necessary, instigating respite and day hospital care. 28 The nurses’ roles could also involve other health-promoting activities, such as assessing entitlement to social security benefit. 22 Those interventions targeting older people who had experienced falls were designed to reduce risk of future falls and involved in-depth assessments of health state and environmental hazards with appropriate referral to other services. 25–29 This might include working with local councils to raise awareness of local hazards for older people.
| Author | n | Study purpose | Intervention | Nurse | No. of visits | Control | Duration of intervention |
|---|---|---|---|---|---|---|---|
| Blue et al.21 | 165 | To determine whether or not specialist nurse intervention improves outcomes in patients with chronic heart failure | Nurses provided education about heart failure and its treatment, optimisation of drug treatments, diet, exercise, monitoring electrolyte concentrations, teaching self-monitoring and management. Nurses liaised with other health-care and social workers as required and provided psychological support. They also provided booklets containing an explanation of heart failure and its treatment, dietary advice, contact details for the heart failure nurses, a list of their drugs, weights, blood test results and details of planned visits (dates and times) | Training for nurses in role before start of the study | Planned home visits of decreasing frequency, supplemented by telephone contact as needed | Usual care, managed by the admitting physician and subsequently the GP | 12 months |
| Burton and Gibbon24 | 87 | The study aimed to test the hypothesis that expanding the stroke nurse role to provide continuity in care to stroke survivors and carers after discharge from hospital would improve recovery from stroke | Experimental groups received a follow-up visit from the stroke nurse at the place of discharge within 2 days of discharge. A holistic assessment was undertaken, with the stroke nurse specifically reviewing ‘health promotion’. Subsequent input was flexible, determined by the stroke nurse in consultation with the patient and carer | A bespoke training programme was provided for the stroke nurse prior to the study | The average number of contacts between the patient and stoke nurse was three (range 0–28). Contact was typically maintained every 2 months (range 0–12 months) | Control group members received usual care on discharge from the rehabilitation unity. Those in the control group did not receive home visit or any further intervention from the stroke nurse | 12 months |
| Brooks et al.23 | 51 | The study evaluated the effects of a structured nurse-led education programme that aimed to improve patient concordance and prevent venous leg ulcer recurrence | Qualified nurses and nursing auxiliaries in this group attended a 3-hour education session that focused on enhancing patient concordance on leg ulcer prevention. Patients were given information leaflets on prevention and exercise leaflets developed by the researcher. These explained seven key ways that they could prevent ulcer recurrence. The nurses emphasised the importance of the prevention strategies described in the leaflets. This was reinforced every 3 months. Where possible, relatives and carers were also encouraged to reinforce concordant behaviour | Qualified nurses and nursing auxiliaries in this group attended a 3-hour education session that focused on enhancing patient concordance with leg ulcer prevention | NR | Patients received ‘usual’ care. Qualified nurses caring for this group received mandatory 1-day training for leg ulcer management. Compression hosiery used in both groups was changed weekly and replaced every 3 months | Data were collected weekly for 52 weeks |
| Jarman et al.22 | 1859 | The study aimed to determine the effects of community-based nurses specialising in Parkinson’s disease on health outcomes and health-care costs | Nine nurses who were trained in meeting the special needs of people with Parkinson’s disease and their carers. Nurses were advisory to the GP. Each nurse was supplied with a leased car and a mobile telephone, and assumed areas of responsibility under the guidance of a nurse manager. Nurses’ roles included counselling and educating patients and carers about Parkinson’s disease in their homes, at health centres, and GP clinics, in hospital outpatients departments, and via telephone; providing information on drugs; monitoring clinical well-being and response to treatment; instigating respite/day hospital care where appropriate; assessing entitlement to social security benefit; and liaising with local multidisciplinary primary care teams for ongoing assessment and therapy | Nurses were given additional training | NR | Patients in the control group were not provided with additional services until the end of the 2-year intervention. They were subsequently offered one assessment by a nurse specialist | 2 years |
| Lightbody et al.28 | 348 | The study aimed to assess a nurse-led intervention for older people discharged from the A&E department, requiring a single visit, through which action on falls, risk factor modification could be taken through usual channels | The intervention group was assessed for risk factors for falls at home by the falls nurse 2–4 weeks after the index fall. Medication, ECG, blood pressure, cognition, visual acuity, hearing, vestibular dysfunction, balance, mobility, feet and footwear were assessed using adapted versions of the falls checklist. The environmental assessment identified inadequate lighting, tripping hazards and education about safety in the home, and simple modifications were made with consent. Risk factors requiring further action were referred to relatives, community therapy services, social services and/or the primary care team. Direct referrals were not made to hospital outpatients or day hospital | Nurses were given criteria for initial assessment and onward referral developed in consultation with therapists and clinicians | Single visit | Usual care | 6 month follow-up |
| Kingston et al.27 | 109 | The study aimed to test the hypothesis that a health-visiting intervention delivered within 5 working days of attending an A&E department with a fall would improve the medium-term self-reported functional status of older women who had fallen | The health visiting intervention included pain control and medication, including advice on appropriate analgesia. Advice from the health visitor also included the type of analgesics to use and the correct times at which they should be taken; how to get up after a fall; individuals were also educated about risk factors for falls both in terms of environmental risks and risk factors related to drugs, alcohol, etc. Patients were also given advice on diet and exercise. The intervention group received a rapid health visiting intervention within 5 working days of the index fall. All individuals within the intervention group were care managed on an individual requirement basis for 12 months post fall | Health visitor | NR | The control group received standard post-fall treatment administered in the A&E. This consisted of a letter from the A&E department to their GP detailing the clinical event, and any follow-up | 12 months |
| Spice et al.26 | 516 | The study examined two interventions in community-dwelling older recurrent fallers, who had not attended an A&E department for their most recent fall, comparing effectiveness in preventing falls against usual care within a cluster RCT design |
Group 1: Primary care intervention group participants received an assessment by a designated trained nurse to identify risk factors for falls. If problems were identified referrals to appropriate professionals in primary or secondary care were made Group 2: Secondary care intervention group participants attended a one-stop multidisciplinary clinic with referral for investigations, interventions (including home check) and follow-up if necessary Intervention assessments in the primary and secondary groups were standardised: further management of each participant was then individualised |
Designated trained nurse working in the community, using a risk factor review and subsequent targeted referral to other professionals | Not described – appears to be one-off assessment | The usual care group received a baseline assessment but were managed by their primary care team without specific guidance: referral to routine services made was at the discretion of the primary care clinicians | Unclear |
| Vetter et al.25 | 674 | RCT. Households, rather than individuals were randomised | A health visitor was employed in the practice with the task of reducing the incidence of fractures within the intervention group. This was to be achieved by visiting the households at least once per year for those not presenting any problems, assessing patients risk of falls or fractures and intervening in those who had obvious risk characteristics or who had a history of such problems. Those older people who had problems were visited as often as deemed necessary by the health visitor. The health visitor also referred people with problems to other professionals. The health visitor first obtained a history of illness and then concentrated on four factors:
|
Health visitor | Older people who had problems were visited as often as was thought necessary by the health visitor | Usual care | 4 years |
| Vetter et al.30 | 1286 | The study assessed the effectiveness of using health visitors to visit and monitor of a caseload of older individuals within their respective general practices | Health visitors were instructed to interview patients and to keep notes according to usual health visiting practice. In addition, a problem sheet and procedure form had to be completed at each interview. These were copied on to a card which was placed in the patients practice notes and this acted as a means of communication between the practitioners and the health visitor. No major changes in either the membership of the general practices or of their policies with regard to older patients occurred during the study | Health visitors, already working with older people | Health visitors made one unsolicited visit a year. They followed up patients who were in trouble at that visit and they were also alerted by the other professionals in the practice if one of their patients had any difficulties | Usual care | 2 years |
| McEwan et al.29 | 296 | This study evaluated the effectiveness of a primary care-linked screening programme to resolve health and related problems and to improve the quality of life of older people | Home visit from one of the care plan nurses. An assessment lasting about 45 minutes was undertaken, which included the following: activities of daily living, social functioning, sensory functions, mental and emotional assessment, current medical problems, measurement of blood pressure, urinalysis and haemoglobin level, and apparent compliance with medication. The requirements for care were decided on the basis of the findings at this consultation and appropriate referrals were made. The intervention consisted of a special screening assessment and referrals and/or advice based on the results. A booklet which described the health social and voluntary services available locally for older people was left with each test group participant | Community nurse trained in interviewing techniques | Single visit | The control group received the usual pattern of care from the primary care team | Not described. Follow-up at 20 months |
| Cunliffe et al.31 | 370 | This study examined the effect of an EDRS in Nottingham, UK | The ERDS was staffed by two occupational therapists, two physiotherapists, three nurses, a community care officer (liaising with social services), seven rehabilitation assistants, and secretarial support. There were no doctors in the EDRS: medical care was provided by the hospital team while in hospital and by the GP when at home. The EDRS aimed to assess the patient and arrange discharge as soon as possible. Up to four visits per day could be provided, up to 7 days per week, between the hours of 8 am and 10 pm. The package of care could last up to 4 weeks and was tailored to individual needs. Some patients when assessed in hospital by the EDRS were deemed not to require any further input. All standard after-care services were available, if required for those allocated to the EDRS | Part of team with occupational therapists, rehabilitation assistants and physiotherapists | Up to four visits per day, 7 days per week | Usual care – patients were managed in hospital until fit for home, using existing after-care services as required. After-care services comprised hospital outpatient rehabilitation, geriatric day hospitals and usual social services | Up to 4 weeks |
Quality of the included studies
Quality assessment of the included studies is presented in Table 5. Seven studies21,22,24–26,30,31 were judged to be at low risk of bias. These studies adopted appropriate methods of randomisation, described the numbers of participants lost to follow-up, reported ITT analyses and reported well-balanced patient groups at study baseline. Two studies24,31 attempted to overcome the challenges of blinding by ensuring that outcome assessors were blinded to the allocation groups of the participants. Two studies27,28 that did not adopt an ITT analysis were judged to be at medium risk of bias. Only one study, by Brooks et al. ,23 was judged to be at high risk of bias, as it failed to use a randomisation process; in particular, this introduces the possibility of selection biases that may influence the observed effectiveness of the intervention. It appears in this study that subjects within the experimental group were in a better health state at baseline; however, the potential impact of this imbalance was not examined statistically.
| Author | Randomisation procedure | Allocation concealment | Blinding | ITT/LTFU | Baseline comparability | Risk of bias |
|---|---|---|---|---|---|---|
| Blue et al.21 | Central computerised randomisation | Yes | No |
Yes Withdrawals Details given. Only one withdrawal from the intervention group C: Six died before discharge I: One died before discharge and one died after discharge to a hospice |
Yes | Low |
| Burton and Gibbon24 | Randomisation was stratified by admitting hospital, first or subsequent stroke, destination on discharge and levels of functional dependence on discharge. Randomisation used a computer database | Yes | Yes, of outcome assessors |
Yes ITT: 12 months Withdrawals I, 6/87; C, 5/89 Died I, 7/87; C, 8/89 LTFU I, 10/87; C, 14/89 |
Yes | Low |
| Brooks et al.23 | Allowed manipulation and some controls, but not random assignment of individual subjects to treatment conditions. Patients in the two arms were from six regions in Oxfordshire, which were divided to produce two demographically similar groups | No | Staff were unaware the trial had two arms | Experimental group appear in a better health state at baseline. No randomisation. No test of similarity | High | |
| Lightbody et al.28 | Consecutive block randomisation | No | NR |
No Four patients LTFU |
There were no differences between intervention and usual care groups in baseline characteristics except total number of medications | Medium |
| Jarman et al.22 | 438 general practices in nine randomly selected English health authority areas. Health authorities were stratified by three factors that influence service organisation and accessibility: size, population density and area deprivation score. Randomisation was performed centrally by an independent organisation. Patients were randomised within practice using block randomisation lists that reflected the randomisation ratio of the health authority area | NR | NR |
Yes LTFU I, n = 163; C, n = 116 |
No differences observed between treatment groups for age, sex, accommodation, social class, disease duration, disease severity or drugs | Low |
| Kingston et al.27 | NR | NR | NR |
No 17 patients LTFU |
Both intervention and the control group had the same mean age (71.9 years) and could undertake the same activities of daily living before the fall. However, the control group reported significantly greater levels of treatment for depression (p = 0.04) and angina (p = 0.04) in the 12 months prior to the fall | Medium |
| Vetter et al.25 | RCT randomisation by household. A group practice of five GPs took part in the study. Randomisation was undertaken using random number tables with subjects study numbers and without direct contact with the subjects | NR | NR | Yes | Similar age and gender distributions. Greater degree of disability in the intervention group was different from that in the control group: 159 (45%) of the intervention group and 117 (36%) of control subjects had no initial disability | Low |
| Vetter et al.30 | Method not described | Method not described | NR | NR | No significant differences in physical disability, scores for anxiety | Unclear |
| Spice et al.26 | Cluster RCT. Practices were stratified into urban and rural and randomly allocated to the three arms, in blocks of three, using a random number generator | NR | Blinding to the intervention group of those collecting and analysing data were impractical, but all data collected were entered without alteration | Yes | Groups were very similar but more participants were recruited to the secondary care arm owing to differences in the underlying demography of participating practices | Low |
| McEwan et al.29 | 296 people were stratified into the age–sex groups 75–84 years and ≥ 95 years, then randomly allocated to the test (151 patients) and control groups (145 patients) | NR | NR |
No I, LTFU n = 17; C, LTFU n = 11 |
No significant differences in mental test scores, in the proportion living alone, in sheltered housing or residential care and the proportion consulting a general practitioner in the last 6 months | Medium |
| Cunliffe et al.31 | Telephone randomisation services were used for allocation using computer-generated balanced randomisation within strata. Stratification was by diagnostic group and by Barthel Index at randomisation | NR | Outcomes were assessed blind | Yes | Well matched at baseline | Low |
Assessment of clinical effectiveness
Mortality
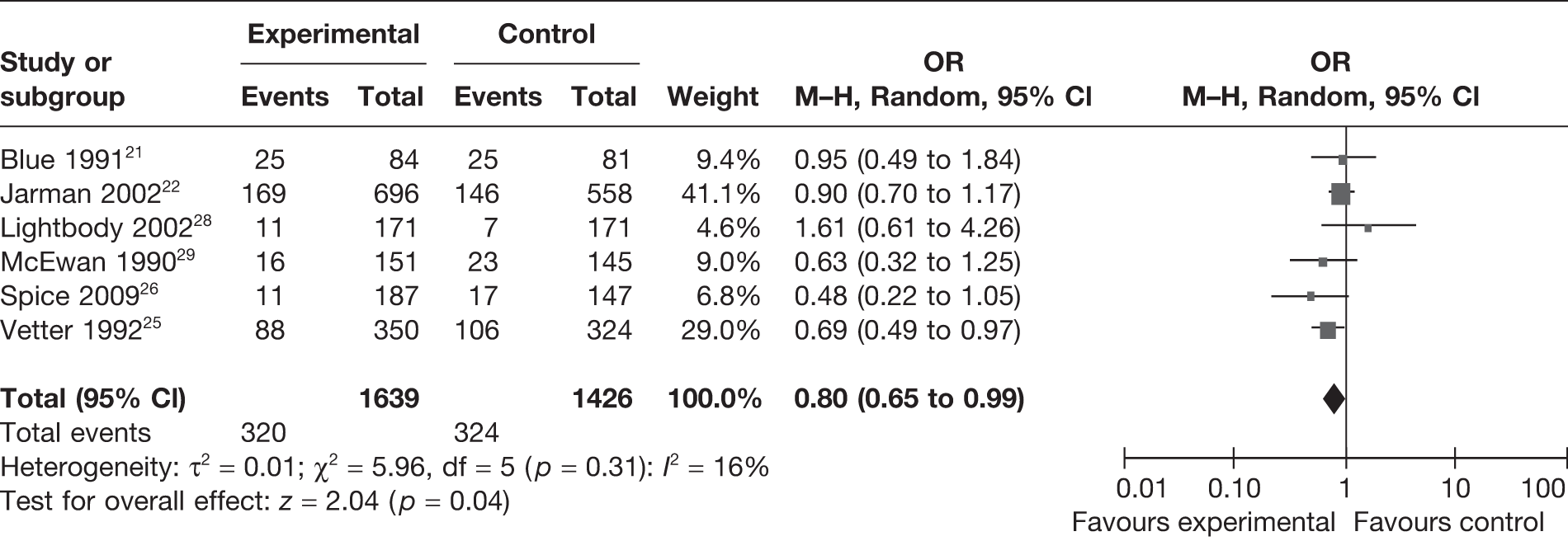
Eight studies21,22,25–31 reported mortality rates, with a total of 4583 participants included in the analysis. Random-effects meta-analysis (Figure 2) suggests that the intervention significantly reduced the risk of death [odds ratio (OR) = 0.80, 95% confidence interval (CI) 0.68 to 0.95]. There was little heterogeneity present in this analysis (I2 = 9%).
FIGURE 2.
Random-effects meta-analysis results for mortality.
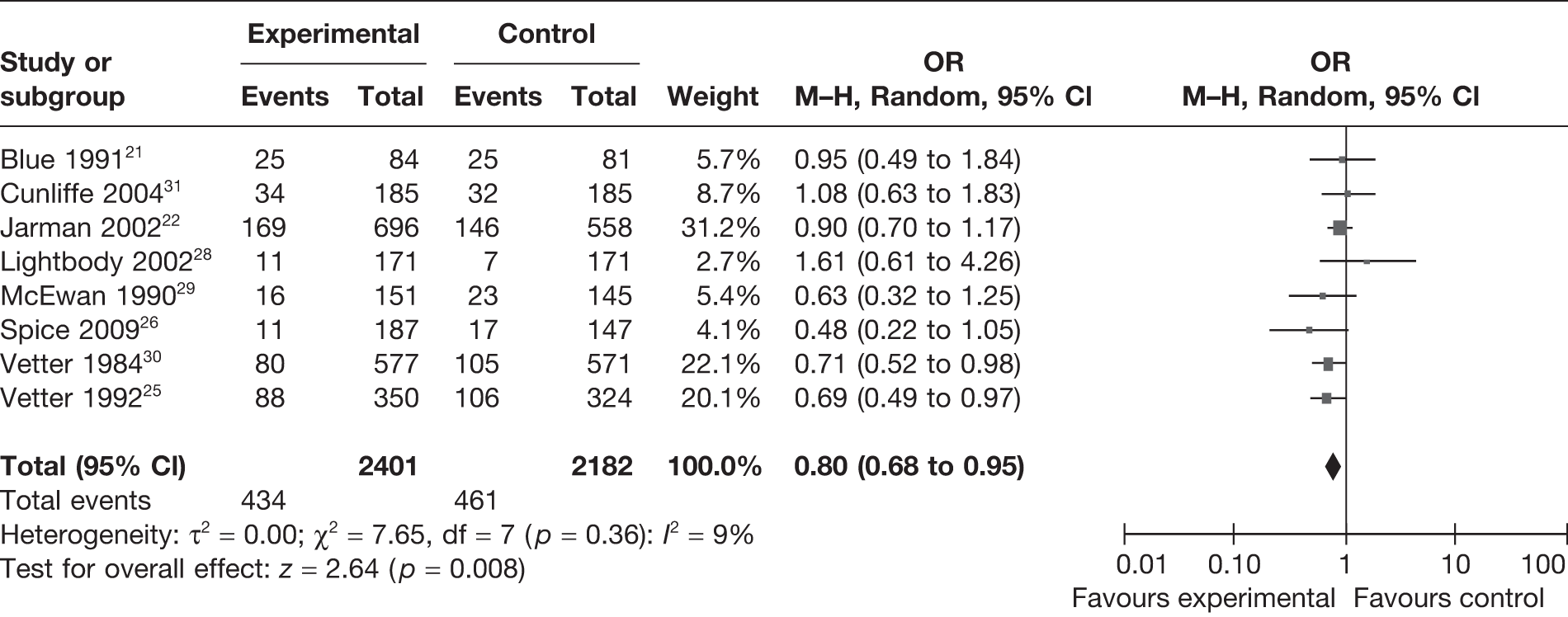
Exclusion of the two studies – Cunliffe et al. 31 and Vetter et al. 30 – from the above random-effects meta-analysis (Figure 3) did not differ significantly in reducing the overall risk of death (OR = 0.80, 95% CI 0.65 to 0.99). However, the degree of heterogeneity increased in this analysis (I2 = 16%).
Falls
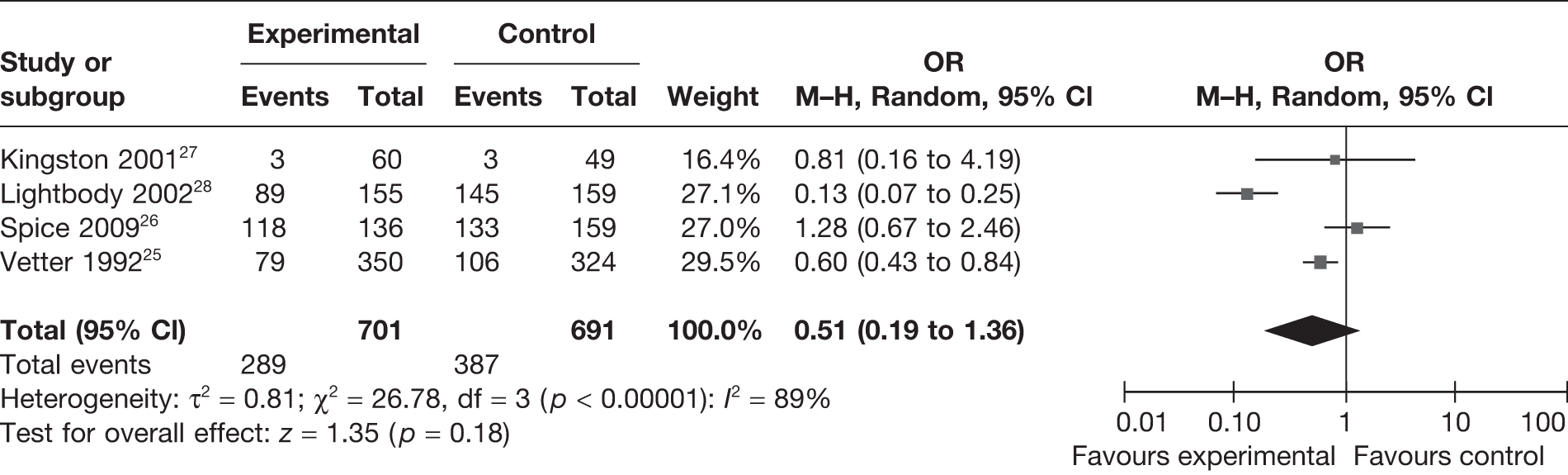
Four studies25–28 reported the number of falls experienced within the intervention and control groups. Assessment of risk and health promotion activities designed to reduce future falls were objectives of these studies. A total of 1392 participants were included in this analysis (Figure 4). Although there appears to be a trend favouring the intervention, with fewer falls occurring in the intervention group compared with usual care, this difference was not statistically significant at the 95% level (OR = 0.51, 95% CI 0.19 to 1.36). There was evidence of considerable heterogeneity in this analysis (I2 = 89%).
FIGURE 4.
Random-effects meta-analysis results for incidence of falls.
Measures of independence
Four studies24,26,28,31 reported outcomes using the Barthel Index (Table 6). The results were not presented in sufficient detail across the trials to enable meta-analysis to be performed. Two studies24,28 reported a significant difference, with those participating in the intervention group demonstrating greater independence than those in the control group. Spice et al. 26 and Cunliffe et al. 31 did not report a significant difference between the intervention and control groups. The differences in these findings are not attributable to the baseline conditions of the participants or the frequency of contact with the nurse during the intervention period.
| Study | Time of measurement (months) | Intervention, mean (SD) | Control, mean (SD) | Significance |
|---|---|---|---|---|
| Burton and Gibbon24 | 12 | Median 17, IQR 10 (n = 63); change score from 3–12 months 0.0 (2.0) (n = 63) | 13 (7.25) (n = 62); change score from 3–12 months: 0.0 (1.0) (n = 62) | NS (p = 0.049)a |
| Lightbody et al.28 | 6 | 18.5 (2.37) (n = 155) | 17.8 (3.6) (n = 159) | p < 0.04 |
| Spice et al.26 | 12 | Difference from the control group at 12 months: 0.07 (–0.54 to –0.67) | NR | p = 0.824 |
| Cunliffe et al.31 | 12 | Mean difference at 12 months 0.2 (–0.7 to 1.1) | NR | NS |
Other outcomes
A number of other outcomes were measured and recorded in the included studies (Table 7). These included admission to hospital, moving to residential care, leg ulcer recurrence, the Nottingham Health Profile, the Beck Depression Inventory, Caregiver Strain Index, the General Health Questionnaire (GHQ) and Short Form questionnaire-36 items (SF-36). Brooks et al. 23 found a significant reduction in leg ulcer recurrence in participants in the intervention group (4% vs 36%, p = 0.004). During the intervention, participants were encouraged to perform leg exercises and to keep his or her legs elevated for a prescribed period during the day. Interventions were also successful in improving Nottingham Health Profile scores,24 reducing caregiver strain,24 improving health and well-being as measured by the GHQ,31 and using a global health question. 22
| Study | Time of measurement | Intervention | Control | Significance |
|---|---|---|---|---|
| Admission to hospital | ||||
| Blue et al.21 | 12 months | 47/84 (56%) | 49/81 (60%) | p = 0.27 |
| No. moving to residential care | ||||
| Spice et al.26 | 12 months | 3/113 (3%) | 7/133 (5%) | p = 0.39 |
| Leg ulcer recurrence | ||||
| Brooks et al.23 | 12 months | 1/25 (4%) | 15/42 (36%) | p = 0.004 |
| Nottingham Health Profile (higher scores reflect greater difficulty) | ||||
| Burton and Gibbon24 | 12 months | Median (IQR): 134.9 (133.47)/63 | Median (IQR): 177.51 (184.05) | p = 0.012 |
| McEwan et al.29 | 20 months | 97.4 (SD)/101 | 130 (SD)/92 | NR |
| Beck Depression Inventory | ||||
| Burton and Gibbon24 | 12 months | Median (IQR): 8(6)/61 | Median (IQR): 10 (7)/56 | p = NS |
| Caregiver Strain Index | ||||
| Burton and Gibbon24 | Median (IQR): 4 (3.5)/37 | Median (IQR): 5.5 (3.8)/36 | Significant when measured as change from 3 to 12 months | |
| Global health question | ||||
| Jarman et al.22 | 24 months | Mean (SD) 4.79 (1.50)/696 | Mean (SD) 5.02 (1.38)/558 | p = 0.008 |
| GHQ (high score unfavourable) | ||||
| Cunliffe et al.31 | 3 months | Mean difference at 2.4 (–4.1 to 0.7) favouring intervention | ||
| SF-36 (36–0) | ||||
| Kingston et al.27 | 12 weeks | 1.6 (SD) | 3.1 (SD) | p = 0.81 |
Statement of principal findings
Eleven studies21–31 with a total of 5761 participants were included in the clinical effectiveness review. The studies varied in the nature of the interventions: four21–24 targeted participants with pre-existing morbidities (heart disease, Parkinson’s disease, stroke, venous leg ulcers), one31 provided care at home for patients recently discharged from hospital, two29,30 undertook assessment visits of older people and four25–28 delivered interventions to older people with the purpose of preventing falls. The nature of the interventions varied, with some delivered by nurses visiting more frequently over a limited period of time, whereas others included one visit, with future visits as deemed necessary, and patients being followed up for a longer period of time. The background training and experience of the nurses also varied between studies. Some interventions were delivered by health visitors, stroke nurse specialists or nurses who had been given training specific to the role required for delivering the intervention. Interventions comprised information provision, reinforcement of prescribed treatment and health behaviour, healthy lifestyle information, support for carers, psychological support and referral to other health- and social-care professionals.
Ten21,22,24–31 of the studies were judged to be of medium or low risk of bias. The consistency of high methodological quality in the studies facilitated meta-analysis using a random-effects model.
Eight studies21,22,25,26,28–31 reported mortality rates. These results were pooled in the meta-analysis, using a random-effects model owing to the heterogeneous nature of the intervention and participants. Home-based nursing significantly reduced the risk of death (OR = 0.80, 95% CI 0.68 to 0.95). There was little heterogeneity present in this analysis (I2 = 9%). Four studies25–28 reported the number of falls experienced by participants; a random-effects meta-analysis found a non-significant trend to improved outcomes in the intervention group, but the results were not statistically significant (OR = 0.51, 95% CI 0.19 to 1.36). There was evidence of considerable heterogeneity in this analysis (I2 = 89%). Other outcomes were measured and reported differently between studies preventing meta-analysis. Barthel Index scores were reported in four studies. 24,26,28,31 Two26,28 of these reported a statistically significant effect favouring the intervention, whereas the other two24,31 found no evidence of beneficial effect. Other outcomes measured showing a statistically significant effect favouring the intervention included leg ulcer recurrence,23 Nottingham Health Profile,24,29 Caregiver Strain Index,24 the GHQ31 and a global health question. 22 The following outcomes failed to demonstrate a statistically significant difference: admission to hospital,21 number of individuals moving into residential care,26 the SF-3627 and the Beck Depression Inventory. 24
Four existing systematic reviews8,10,14,32 incorporated meta-analysis. These were reviews of home- or community-based interventions to support older people. The reviews were not limited to nurse-led interventions and were not focused on the UK context. Three of these reviews8,14,32 did not find a significant reduction in mortality. However, the results from the review by Elkan et al. 10 concur with the findings of the meta-analyses presented here. They found a significant reduction in mortality (OR = 0.76, 95% CI 0.64 to 0.97). Stuck et al. 8 and Beswick et al. 14,32 both reported statistically significant benefits for the intervention group in terms of reduced nursing home admission, risk of hospital admissions, falls and functional decline. Stuck et al. 8 found, however, that the effect on functional decline was dependent on the number of home visits performed during follow-up. The positive effects seen in these reviews are mirrored in our review, supporting the conclusion that home visits to older people can reduce mortality and appear to improve the health and well-being in older people.
Chapter 4 Assessment of cost-effectiveness
Introduction
This chapter presents the methods and results of a systematic review of existing UK-based economic evaluations of home-based, nurse-led health promotion programmes.
Methods for reviewing cost-effectiveness
The systematic review was undertaken to identify existing economic analyses of the use of home-based, nurse-led health promotion interventions specifically from the perspective of the UK NHS and PSS. The purpose of this review was to identify, appraise and summarise existing evidence concerning the cost-effectiveness of home-based, nurse-led health promotion in order to determine whether or not, and under what circumstances, and for whom, such a programme may represent good value for money for the NHS and associated sectors. A de novo health economic model was not developed as part of this review.
Identification of studies
A comprehensive systematic search of key health and medical databases was undertaken, as detailed in Chapter 3. Additional searching using Google Scholar was also undertaken to attempt to identify any relevant unpublished literature not identified by the systematic searches. The full economic search strategy is presented in Appendix 1.
The inclusion and exclusion criteria for the review of economic analyses are detailed below.
Study inclusion/exclusion criteria for review of economic evaluations
Inclusion criteria
The following inclusion criteria (additional to those presented in Chapter 3) were applied:
-
Full comparative economic evaluations that present results in terms of both costs and health outcomes (cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost–consequence analyses). Cost minimisation studies were included, although, strictly speaking, these are not full economic evaluations.
-
Studies undertaken from the perspective of the UK NHS and PSS.
Exclusion criteria
The following types of studies were excluded:
-
studies that report only costs or outcomes
-
studies that evaluate interventions delivered in any other setting than the subjects’ home (e.g. institutional, residential or nursing home care)
-
studies in which a substantial proportion of patients were < 75 years of age
-
non-comparative studies
-
studies in which a substantive element of the intervention was not delivered by nurses
-
studies in which the intervention was not specifically related to health promotion
-
studies that were undertaken within a non-UK setting
-
studies referred to only in editorials, commentaries or letters were also excluded.
No exclusion criteria were applied with respect to the targeted nature of the intervention, i.e. the review does not discriminate between interventions that are intended to improve outcomes within the general older population whereby their capacity to benefit is assumed solely on the criterion of age, or those interventions that are applied on the basis of increased risk owing to a history of a specific medical condition (e.g. stroke, dementia, history of falls). Studies undertaken within a non-UK setting were excluded from the review; these were retained, however, to examine the availability of economic evidence within a non-UK setting.
Identification of relevant studies
All citations were imported into Reference Manager version 12 and duplicates were removed. UK-specific citations were identified; the abstracts of these were then sifted to identify any potentially relevant economic evaluation studies for inclusion in the review. In addition, the studies included in the review of clinical effectiveness (see Chapter 3) were also scrutinised to identify any potentially relevant economic studies missed by the economic searches. Full papers of potentially relevant studies were retrieved and scrutinised in greater detail by two reviewers (PT and AR). Subjective judgement on the part of the reviewers was required with respect to the application of certain inclusion criteria, in particular the age distribution of study subjects (the proportion of subjects ≥ 75 years and < 75 years, and the extent to which this is reported), the extent to which the intervention involves health promotion rather than care, and the extent of nurse involvement in the delivery of the intervention. Studies that included a slightly younger patient population were given additional consideration (substantial proportion subjects ≥ 70 years of age) if all of the other inclusion criteria were met. All sifting was undertaken by two reviewers (PT and AR) and disagreements were resolved through discussion among the research team.
Critical appraisal methods
Included studies were critically appraised using the checklist for economic evaluations reported by Drummond et al. 33
Results of the cost-effectiveness review
Number and type of included studies
The systematic searches for economic evaluations identified 1988 potentially relevant citations, excluding duplicated records. Following an initial sift of abstracts and titles, full papers of 49 studies were retrieved for more detailed inspection. Forty-five of these studies failed to meet the inclusion criteria and were hence excluded from the review. The most common reasons for study exclusion were (1) the inclusion of younger age groups; (2) the absence of any substantive nursing element within the description of the intervention; (3) the absence of any form of health promotion in the definition of the intervention; or (4) the failure to undertake a comparative economic evaluation. In many instances, studies were excluded for more than one reason. In total, only three studies, reported across four papers, met the inclusion criteria for the review. Further hand-searching of included studies and web-based searching did not result in the retrieval of any additional relevant studies. An abridged PRISMA diagram is shown in Figure 5.
Table 8 presents a summary of the characteristics of the economic studies included in the review. Table 9 summarises the main resource components included within each study. Table 10 presents the results of the critical appraisal.
FIGURE 5.
PRISMA diagram for systematic review of cost-effectiveness.
| Study | Form of evaluation | Population | Intervention | Comparator | Primary economic outcome measure | Perspective | Time horizon |
|---|---|---|---|---|---|---|---|
| Bakerly et al.34 | Case-matched cost minimisation analysis | COPD | Integrated care model including nurse-led education and advice (n = 130) | Usual care (n = 95) | Cost difference | NHS | 60 days |
| Hurwitz et al.,35 Jarman et al.22 | EEACT (presented as a cost–consequence analysis) | Parkinson’s disease | Parkinson’s disease nurse specialist service (n = 1041) (including counselling and education-based roles) | Usual care (n = 818) | EQ-5D, cost difference | Appears to be NHS and local authority | 2 years |
| Miller et al.36 | EEACT | Older patients on discharge from acute hospital inpatient stay | EDRS (n = 185) | Usual care (n = 185) | Incremental cost per QALY gained | NHS/PSS | 1 year |
| Resource groups/components | Bakerly et al.34 | Hurwitz et al.,35 Jarman et al.22 | Miller et al.36 |
|---|---|---|---|
| Primary care | |||
| Nurse home visits | ✓ | ✓ | ✓ |
| GP/community care | ✓ | ✓ | ✓ |
| Occupational therapist home visit/home adaptations | ✓ | ? | ✗ |
| Ambulance transfers | ✓ | ✗ | |
| Pharmacological and non-pharmacological treatments | |||
| Drugs/other therapies | × | ✓ | ✗ |
| Secondary care | |||
| Hospital outpatient visits | ✓ | ✓ | ✓ |
| A&E department admissions | ✓ | ✓ | ? |
| Inpatient costs | ✓ | ✓ | ✓ |
| Institutional/residential care | |||
| Institutional/residential/respite care | ✗ | ✓ | ✓ |
| Day care/home help | ✗ | ✓ | ✓ |
| Community and GP care | ✗ | ✓ | ✓ |
| Other | |||
| Social security benefits | ✗ | ✓ | ✗ |
| Question | Bakerly et al.34 | Hurwitz et al.,35 Jarman et al.22 | Miller et al.36 |
|---|---|---|---|
| Was a well-defined question posed in an answerable form? | Yes | Yes | Yes |
| Was a comprehensive description of the competing alternative given? | Yes | Yes | Yes |
| Was there evidence that the programme’s effectiveness had been established? | Questionable | Not in terms of QALYs | Not in terms of QALYs |
| Were all the important and relevant cost and consequences for each alternative identified? | Yes for costs | Yes for costs | Yes |
| No outcomes included | No outcomes included | ||
| Were costs and consequences measured accurately in appropriate physical units? | Yes for costs | Yes for costs | Yes for costs |
| No outcomes included | No outcomes included | Unclear how/if QALYs were measured at baseline | |
| Were costs and consequences valued credibly? | Yes for costs | Yes for costs | Yes |
| No outcomes included | No outcomes included | ||
| Were costs and consequences adjusted for differential timing? | No | No | No |
| Was an incremental analysis of costs and consequences of alternatives performed? | Yes for costs | Yes for costs | Not for expected ICER |
| No outcomes included | No outcomes included | ||
| Was allowance made for uncertainty in the estimates of costs and consequences? | No | No | Yes |
| Did the presentation and discussion of results include all issues of concern to users? | Yes | Yes | Yes |
Critical assessment of included studies
This section presents a critical appraisal of the three included studies22,34–36 in the systematic review.
Bakerly et al.
The study reported by Bakerly et al. 34 presents the methods and results of a cost minimisation analysis based on the results of a non-randomised prospective study of an early discharge and integrated care protocol for patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease (COPD). This study was not included in the review of clinical effectiveness (see Chapter 3) owing to its non-randomised design. Although the authors purport to have adopted a NHS perspective, PSS costs were also included within the analysis. Costs were valued at year 2007 prices.
The population within the intervention group included 130 out of 546 patients who were admitted to hospital with acute exacerbations of COPD and who consented to the integrated care intervention during the period August 2003 to August 2004. The comparator group for the economic analysis comprised 95 out of 662 patients who were admitted to hospital with acute exacerbations of COPD between August 2002 and August 2003, and who stayed in hospital for the full duration of his or her treatment. Members of the control population were selected to match members of the intervention population in terms of age, gender and postcode.
The average age of patients who were allocated to the intervention group was 70 years [standard deviation (SD) = 8 years] and the average age of patients within the retrospective comparator group was 68 years (SD = 11 years). The intervention assessed within this study was an early discharge and integrated care protocol in which patients discharged from hospital early were visited at home by specialist respiratory nurses until they were totally discharged. The Acute COPD Assessment Service (ACAS) was able to provide short-term nebulisers and oxygen to patients. The ACAS team was staffed by three full-time specialist respiratory nurses and a middle-grade physician, who dedicated 40% of his time to the programme. During the last visit, patients and their carers were educated regarding COPD and its causes, how to prevent ill health as a result of the disease and how to manage suspected COPD exacerbations, and were given advice on exercise, healthy living and smoking cessation. In addition, patients were also given a written self-management plan (in conjunction with their GPs), and were given steroids and antibiotics to initiate at home when required. Patients were assessed in clinic 60 days after the index episode and a comprehensive management plan was agreed with the patient and communicated to their GP. Patients who were deemed unfit for early discharge were followed up daily in the hospital by programme nurses until they were well enough to be discharged with or without integrated care support. The comparator group received inpatient hospital care until the patients were considered well enough for discharge.
The objective of the study was to determine any cost savings that could be achieved by the introduction of an early discharge and integrated care protocol. Within the analysis, outcomes for the ACAS programme were assumed to be equivalent between the intervention and control groups. Although the authors did refer to a previous systematic review reported by Ram et al. 37 as evidence that the intervention was safe, the study did not involve the prospective collection or analysis of evidence regarding health-related quality of life (HRQoL), survival or other intermediate clinical outcomes.
Resource use was measured for patients receiving the early discharge and integrated care protocol and for patients within the retrospective control group. Resource components included the original hospital admission and length of stay, emergency ambulance transfers, and accident and emergency visits prior to their original hospital admission. Additional resource-use components recorded within the intervention group included visits by specialist respiratory nurses, emergency home visits, contact with various health-care professionals, accident and emergency visits following discharge, hospital readmissions, and outpatient clinic visits. Unit costs used to value resource use were obtained from appropriate reference sources, including NHS Reference Costs 2007/0838 and Curtis et al. 39
The authors did not report the results of any sensitivity analysis to examine the impact of costing assumptions on the likely cost savings of the protocol. The use of discounting was not reported; although this may be considered appropriate given the short time horizon for resource measurement (60 days), the potential resource and cost differences beyond this time point remain subject to considerable uncertainty.
The results of the analysis reported by Bakerly et al. 34 are summarised in Table 11.
| Treatment group | Mean cost per patient (£) | 95% CI (£) |
|---|---|---|
| Early intervention and discharge (intervention) | 1653 | 1521 to 1802 |
| Inpatient care (control) | 2256 | 2126 to 2407 |
| Cost difference | 603 | – |
The results of the economic analysis suggest that the early discharge and integrated care protocol for patients admitted to hospital with acute exacerbations of COPD may generate substantial cost savings compared with usual inpatient care. However, it should be noted that the study design adopted by Bakerly et al. 34 used a non-randomised design without any form of blinded allocation to the groups under assessment. In particular, although the prospective intervention and historical control groups were selected for inclusion in the study using a case-matching approach, subjects were matched only on the basis of age, gender and postcode. Prognostic factors and other baseline characteristics were not included as part of this process. Consequently, the study may be at risk of selection bias.
Hurwitz et al./Jarman et al.
The study reported by Hurwitz et al. 35 and Jarman et al. 22 presents the methods and results of an economic analysis of a RCT of community-based nursing for patients with Parkinson’s disease. The authors describe the economic analysis as a cost minimisation analysis and report primary economic outcomes in terms of the cost difference between the intervention and control arms of the trial. However, the study would be more accurately described as a cost–consequence analysis, as disease-specific clinical outcomes and HRQoL outcomes are also reported within both papers. 22,35 The perspective adopted for the analysis was not clearly reported in either paper; however, the types of resource components included within the analysis include those that would typically fall on the NHS (although some local authority costs were also included such as institutional and respite care). Costs were valued at year 1996 prices.
Within the RCT, patients were randomly assigned either to a community-based nursing intervention (n = 1041) or standard care (n = 818). The study population consisted of patients identified as suffering from Parkinson’s disease by 438 general practices in nine randomly selected health authorities in England. The intervention was delivered by nurses who had no previous experience of nursing patients with Parkinson’s disease in the community. However, some of the nurses did have experience of nursing patients with Parkinson’s disease in a hospital setting. All of the nurses attended a course on meeting the needs of people with Parkinson’s disease and their carers. The clinical position of the nurses during the trial was not clinically autonomous; rather they worked in an advisory role to GPs and consultants. The nurses counselled and educated patients and carers about Parkinson’s disease, monitored the clinical well-being of patients and their response to treatment at least twice a year, and reported the results back to GPs or consultants as appropriate. The nurses also investigated options for respite or day hospital care, visited patients in hospital and liaised with hospital staff on discharge, assessed social security benefit entitlement, and, where appropriate, liaised with members of local multidisciplinary primary care teams regarding ongoing assessment and therapy. The nurses also provided drug information to patients under the auspices of GPs and consultants. Although the nurses were not empowered to change patient medication, they could make suggestions to GPs about altering a patient’s dose regimen. The comparator intervention was defined as standard care; however, patients allocated to the control arm were offered a single assessment by a Parkinson’s disease nurse specialist at the end of the 2-year intervention period. Further details of this trial are included in Chapter 3 of this report.
A number of primary and secondary clinical and health-related quality-of-life outcomes were measured. The primary outcomes include the results from the stand-up test and the dot-in-square test, the proportion of patients sustaining fracture and HRQoL as measured using the European Quality of Life-5 Dimensions (EQ-5D) questionnaire. Patient well-being was also measured using the PDQ-39 (Parkinson’s disease-specific measure of health status questionnaire) and a self-perceived global health question asking patients to rate their change in general health over the previous year on a scale from 0 (much better) to 4 (much worse). Outcomes assessments were undertaken during interviews between patients and non-professional interviewers employed by the National Centre for Social Research. Interviewers had received prior training. Other secondary outcomes included the median dose of l-dopa in each group, the proportion of patients on l-dopa controlled-release medication, the proportion of patients on a combination of pharmaceuticals, the proportion of patients referred to ancillary therapy, and the proportion of patients referred to a Parkinson’s disease specialist. Again, these secondary outcomes were measured during interviews. Patient mortality for each group was obtained from the NHS Central Registry.
All of the patients in the study were interviewed to estimate NHS resources used. Resource components included institutional, respite, hospital and day care, community and general practitioner care, social security benefits, home aids, adaptations and pharmaceuticals. Unit cost estimates were obtained from appropriate reference sources including the Monthly Index of Medical Specialities (MIMS)40 and Netten et al. 41 The authors calculated that, including administrative costs and car hire, the intervention would cost approximately £200 per patient per year.
The authors did not report the results of any sensitivity analysis. Although the authors used non-parametric bootstrapping to check the assumptions of their mean estimates, a comprehensive analysis of decision uncertainty was not reported. Even though the intervention period was 2 years, there is no evidence that discounting of future costs was undertaken. The headline economic results for the study are summarised in Table 12. Although the economic study design adopted here is reported to be that of a cost minimisation analysis, EQ-5D scores were actually reported to be non-significantly lower in the intervention arm (mean EQ-5D difference = –0.02). On the basis of the total direct NHS costs for each group in those completing the study, and absolute EQ-5D differences between the groups, the nurse-led intervention appears to be dominated by standard care (less effective and more expensive). However, this is subject to considerable uncertainty.
| Treatment group | Cost (£) | ||
|---|---|---|---|
| EQ-5D utility | Year 1 | Year 2 | |
| Nurse group | 4055 | 5860 | 0.37 |
| Control group | 3480 | 5630 | 0.39 |
| Difference | 575 | 230 | –0.02 |
Miller et al.
Miller et al. 36 present the methods and results of an economic evaluation conducted alongside a RCT to estimate the cost-effectiveness of an early discharge and rehabilitation service for older patients admitted to hospital. The analysis adopted a NHS and PSS perspective. The formal price year was unspecified within the paper; however, it appears from the cost sources used that costs were valued at year 2000 prices.
Within the RCT, patients were randomly assigned to one of two groups: (1) an early discharge and rehabilitation service (n = 185) or (2) standard social services home care and outpatient rehabilitation (n = 185). The study population consisted of patients who had been admitted to hospital for acute care; the most frequent reasons for admission were fracture (28%), neurological conditions including stroke (26%) and cardiorespiratory illness (14%). The median age of patients was 80 years, although the trial was open to any patient aged ≥ 65 years who was medically ready for discharge, had rehabilitation needs that could be met at home and did not need 24-hour care. Of the patients recruited to the trial, 246 were female (67%) and 247 lived alone (67%). The median hospital length of stay was 13.5 days. The intervention comprised a home care and rehabilitation service that was delivered by a team of nurses, physiotherapists, occupational therapists and rehabilitation assistants during up to four visits per day for up to 4 weeks. Patients who were allocated to the comparator arm received standard care, which included social services home care and rehabilitation delivered through an outpatient department.
European Quality of Life-5 Dimensions HRQoL estimates were elicited at 12-month follow-up using postal questionnaires. Sixty-six patients died before follow-up and were assigned a zero score. The remaining 32 patients withdrew consent or declined follow up. EQ-5D estimates were obtained for 272 patients of the recruited who were still alive at 12 months. Importantly, the authors do not report whether or not EQ-5D assessments were undertaken at baseline; hence, the methods used to estimate incremental QALYs between the groups are not entirely clear. This may affect the credibility of the results of the economic analysis.
Resource costs were measured for all participants in both the intervention arm and the control arm; these included the costs of the intervention, the costs of the acute hospital stay following randomisation, the costs of any readmissions to hospital or outpatient visits, and the costs of any nursing home admissions or any contact with GPs, community health services or social services within the 12-month follow-up period. Total resource use for each patient was estimated using data collected from service providers over the follow-up period. The cost of the intervention was based on recorded client contact time with members of the early discharge rehabilitation service. Hospital inpatient admissions were costed according to the length of stay and clinical specialty. Outpatient attendances were also costed according to clinical specialty. The cost of contact with GPs was based on the recorded number of face-to-face and telephone consultations. The cost of contact with community health service professionals was based on recorded contact time. Unit costs were obtained from standard references sources including the NHS Reference Costs 2000/0142 and Netten et al. 43 The cost of nursing and residential home admission was based on duration of stay multiplied by the average cost obtained from Netten et al. 43 The cost of referrals to social services professionals was based on the assumption of 1 hour of contact time per visit, with the hourly rate being obtained from Netten et al. 43 The costs of local authority funded social services were based on recorded contact time.
Uncertainty surrounding costs and health outcomes was explored using a paired bootstrapping technique developed by Barber and Thompson. 44 The patient-level data set was resampled 2000 times to generate estimates of variance. Uncertainty surrounding unit cost estimates does not appear to have been considered within the analysis. The results of the uncertainty analysis were presented as cost-effectiveness planes and cost-effectiveness acceptability curves. However, the authors do not actually report mean (expected) incremental quality-adjusted life-years (QALYs) gained or an expected incremental cost-effectiveness ratio (ICER; i.e. the incremental cost per QALY gained).
The paper does not mention the use of discounting to adjust for time preferences in the accrual of future costs and health benefits. Given the short time horizon of the RCT, this may be considered methodologically appropriate, but does raise questions concerning potential longer-term differences in costs and outcomes between the groups.
The main results presented by Miller et al. 36 are summarised in Table 13 and Figures 6 and 7.
| Treatment group | Mean cost (£) | 95% CI |
|---|---|---|
| Intervention cohort | 8361 | 7302 to 9420 |
| Control cohort | 10,088 | 8690 to 11,486 |
| Difference | 1727 | –754 to 4208 |
FIGURE 6.
Cost-effectiveness plane for early discharge and rehabilitation vs standard care. (Reproduced with permission from Miller et al. 36)
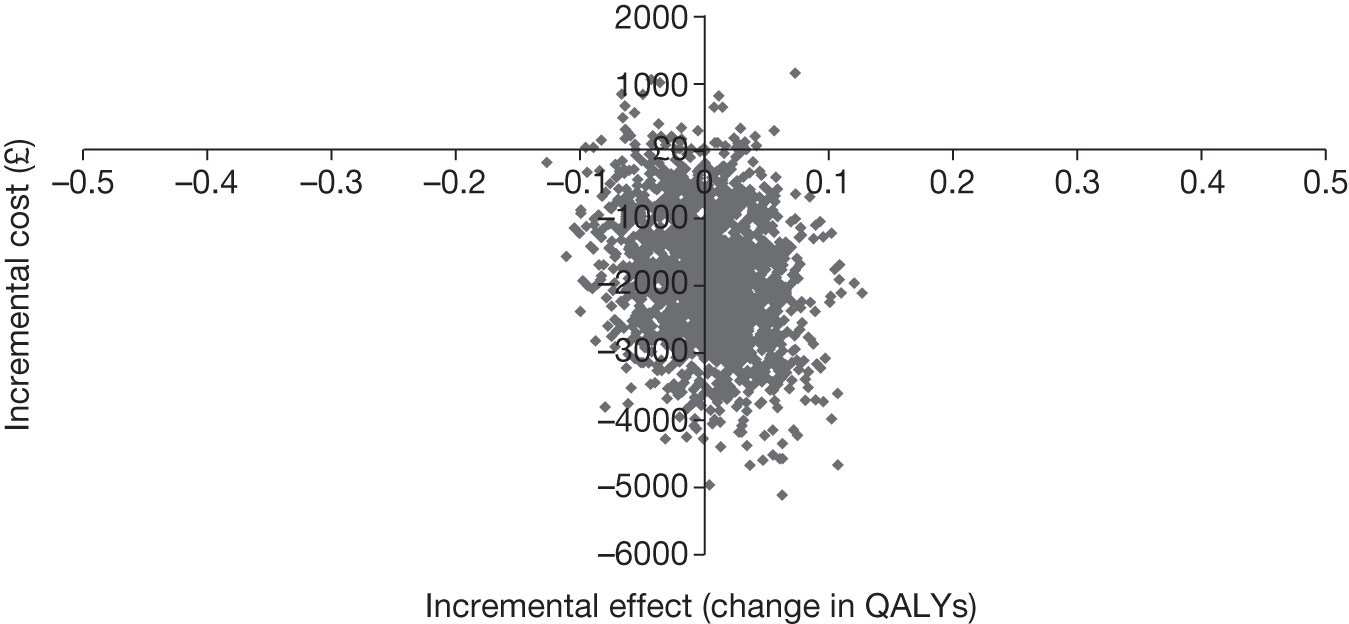
FIGURE 7.
Cost-effectiveness acceptability curve for early discharge and rehabilitation vs standard care. (Reproduced with permission from Miller et al. 36) EDRS, Early Discharge and Rehabilitation Service.
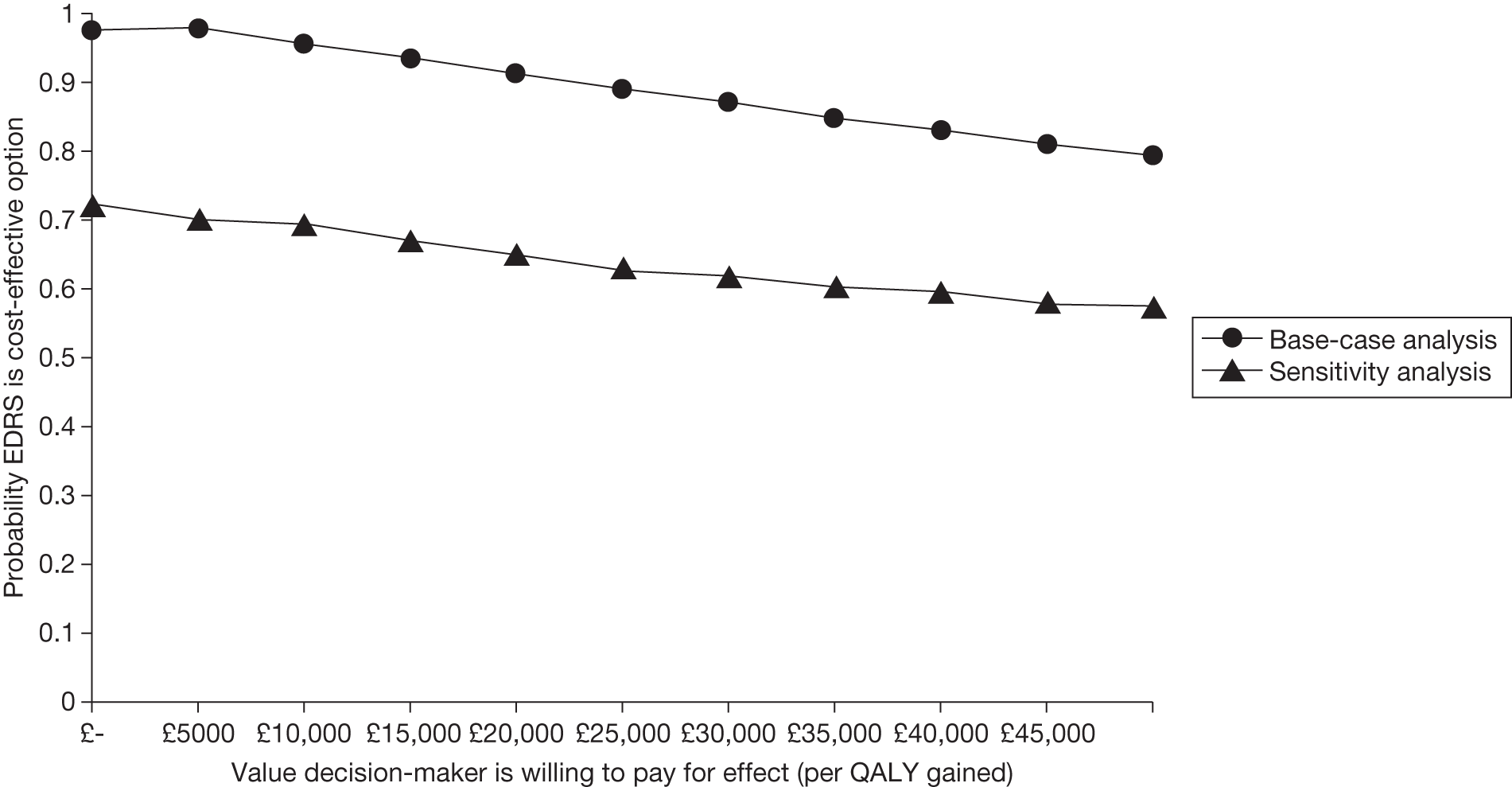
The authors used three different alternative estimators of the population mean to control for the skewed nature of the cost data and found that in each case this technique yielded an increase in the cost savings that was attributable to the intervention. The results presented on the cost-effectiveness plane demonstrate that the majority of the sample points fell below the horizontal axis, which indicates that the intervention was likely to result in cost savings. However, the QALY estimates appear to be fairly evenly distributed around the vertical axis; this indicates considerable uncertainty around whether or not the service offered any incremental health gain. As such, there appears to be a marked possibility that the intervention was less effective than standard care. Overall, there remains uncertainty, in both the short and longer term, about the actual benefit of the intervention.
Statement of principal findings
The systematic review presented within this chapter highlights a dearth of relevant evidence concerning the cost-effectiveness of home-based, nurse-led health promotion in older people. Of the substantial number of potentially relevant studies identified by the systematic searches, only three studies met the criteria for inclusion in the review. The majority of the trials included in the clinical effectiveness review (see Chapter 3) did not include a formal economic evaluation or did they provide sufficient resource-use information to allow such an analysis to be undertaken post hoc.
The relevant evidence base included within this health economic review comprises one cost minimisation analysis based on non-randomised case matching34 and two economic evaluations22,35,36 undertaken alongside RCTs. One of these trial-based analyses also adopted a cost minimisation design. Strictly speaking, cost minimisation analyses are not full economic evaluations. Two of the included studies34,35 involved an intervention targeted specifically at patients with a known underlying incurable disease (COPD and Parkinson’s disease), whereas the third study36 examined the clinical effectiveness and cost-effectiveness of early discharge in patients with a range of conditions including fractures, neurological conditions and cardiorespiratory conditions.
Summary of main findings
The main findings of the three included studies are summarised below:
-
One cost minimisation study, by Bakerly et al. ,34 reported cost savings of approximately £600 per patient, associated with an early discharge and integrated care protocol for patients admitted to hospital with acute exacerbations of COPD. This analysis was based on a case-matching exercise involving historical control subjects and prospectively identified subjects within the intervention arm. This study may be open to potential bias as a result of the case-matching exercise, as this did not include any baseline prognostic factors. Furthermore, the economic analysis assumes equivalent effectiveness between the intervention and usual care groups and adopts a very short time horizon for resource-use data collection (60 days).
-
The second study22,35 reports a cost–consequence analysis of community-based nursing versus standard care for patients with Parkinson’s disease. This study reported increased costs in the intervention arm compared with standard care; however, the mean increase in costs over 2 years was £266 lower for the intervention group than the standard care group. Although the economic study design adopted here is reported to be that of a cost minimisation analysis, EQ-5D scores were actually reported to be non-significantly lower in the intervention arm (mean EQ-5D difference = –0.02). On the basis of the total direct NHS costs for each group in those completing the study, and absolute EQ-5D differences between the groups, the nurse-led intervention appears to be dominated by standard care (less effective and more expensive). Of particular concern are the short time horizon adopted within this study (2 years) and the potentially perverse assumption of equivalence between the intervention and comparator groups.
-
The third study36 reported a cost–utility analysis of an early discharge and rehabilitation service compared with usual care. The authors reported a high probability that the Early Discharge and Rehabilitation Service (EDRS) would be cost-effective. However, reporting within this study was problematic and the authors did not report expected ICERs. Judging from the presentation of probabilistic sensitivity analysis, although there is a high likelihood that the intervention offers cost savings, there also appears to be a strong possibility that the EDRS offered little or no incremental QALY gain over usual care. As with the other two included studies, the time horizon for this economic analysis is very short (1 year).
Other non-UK economic analyses of home-based, nurse-led health promotion
During the process of preparing the economic review, a number of other non-UK economic analyses of home-based, nurse-led health promotion were identified. Although these studies did not meet the inclusion criteria for this review, as they do not relate to home-based, nurse-led programmes implemented within the UK context, they do provide some notion of the alternative types of programme that have been assessed elsewhere. Generally speaking, these studies fall into one of three groups – studies in which the home-based, nurse-led programme is:
-
widely implemented solely on the basis of older age (e.g. Sahlen et al. 45 and Kronborg et al. 46) – there is no equivalent evidence from the UK perspective
-
targeted at individuals with some non-comorbid increased risk factor (e.g. history of falls47,48)
-
targeted at individuals with increased risk owing to the presence of an incurable disease.
It is difficult, however, to draw any firm conclusions from these studies, as the clinical effectiveness and patterns of resource use for the home-based, nurse-led intervention and standard care may differ markedly by geographical location.
Conclusions of the health economic review
There remains considerable uncertainty surrounding the expected cost-effectiveness of home-based, nurse-led health promotion in the UK. The existing evidence base is very limited and, for methodological reasons, should be interpreted with caution. In particular, the effectiveness of interventions as measured using preference-based HRQoL measures remains a key area of uncertainty. It is also noteworthy that a number of RCTs included within the clinical effectiveness review (see Chapter 3) did not collect or report any resource-use information; this should be an essential element of any future RCT of home-based, nurse-led health promotion. Furthermore, the use of cost minimisation analyses within the included studies, in which effectiveness is assumed to be equivalent between competing programmes of care, is not just unhelpful but actually misleading, as it masks the uncertainty surrounding estimates of incremental benefit between competing alternatives. The goal of any health economic analysis should be concerned with fully reflecting the impact of this uncertainty on the likelihood of making the correct decision given available evidence. Two of the three included studies fall short in this respect.
Given the available evidence there is at best a weak suggestion that the cost-effectiveness of home-based, nurse-led health promotion programmes may be dependent on the population at whom the programme is targeted. However, on balance, the current economic evidence base does not provide a sufficient basis for informing policy decisions.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness findings
The systematic review of clinical effectiveness included 11 studies, comprising a total of 5761 participants. There was considerable heterogeneity between the studies in terms of the study populations and the nature, purpose and composition of the interventions. There were also marked differences between studies in terms of the level of training of the nurses delivering the interventions. All but one of the included studies were judged to be at medium or low risk of bias.
Random-effects meta-analysis of eight studies suggested a statistically significant mortality benefit for the home-based nursing groups, whereas a meta-analysis of four studies suggested non-significant benefits in terms of improvement in falls. Positive outcomes for home-based nursing interventions were also reported within individual studies: these outcomes included the Barthel Index (although this finding was not consistent across all studies), leg ulcer recurrence, the Nottingham Health Profile, the Caregiver Strain Index, the GHQ and a global health question. The following outcomes failed to demonstrate a statistically significant difference: admission to hospital, the number of subjects moving into residential care, SF-36 quality of life and the Beck Depression Inventory scores.
Four existing systematic reviews8,10,14,32 reported a meta-analysis of included studies. These were reviews of home- or community-based interventions to support in older people. The reviews were not limited to nurse-led interventions and were not focused solely on the UK context. Three reviews8,14,32 did not find a significant reduction in mortality. However, the results reported by Elkan et al. 10 concur with the findings of this review, as the authors also reported a significant reduction in mortality (OR = 0.76, 95% CI 0.64 to 0.97). 8,14 Both of these reviews reported statistically significant benefits for the intervention group in terms of reduced nursing home admissions, risk of hospital admissions, falls and functional decline. However, Stuck et al. 8 found that the effect on functional decline depended on the number of home visits performed during follow-up. The positive effects seen in these reviews are mirrored in our review, supporting the conclusion that home visits to older people can reduce mortality and appears to improve the health and well-being of those in the intervention group compared with the control group.
On the basis of the evidence included in this systematic review, home-based, nurse-led health promotion may offer clinical benefits across a number of important health dimensions. However, it is generally unclear from the available studies that components of this type of complex intervention contribute towards individual aspects of benefit. This is particularly so when nurses are working within a multidisciplinary team; determining the effect of the nursing intervention distinct from that of other professionals or trained non-professionals is difficult to elicit.
Owing to time and resource constraints for this short report, data from the included clinical studies were extracted by a single reviewer. This should be noted as a possible limitation of the systematic review.
Cost-effectiveness findings
The systematic review of existing economic evaluation studies highlights a dearth of relevant economic evidence supporting the use of home-based, nurse-led health promotion for older people. A total of three studies were included in the cost-effectiveness review. This evidence base is comprised of one cost minimisation analysis based on non-randomised case matching, and two economic evaluations undertaken alongside RCTs. Two of the included studies involved an intervention targeted specifically at patients with a known underlying incurable disease (COPD and Parkinson’s disease), whereas the third study examined the clinical effectiveness and cost-effectiveness of early discharge in patients with a range of conditions including fractures, neurological conditions and cardiorespiratory conditions.
Each of the three studies indicated some likelihood that home-based, nurse-led health promotion may offer cost savings to the NHS (and potentially associated sectors such as social services). However, one study did not report any health outcomes and simply assumed equivalence between the intervention and comparator, whereas the other two studies suggested at best a negligible benefit in terms of preference-based HRQoL measures. Within these last two studies, there is a marked possibility that the intervention offers cost savings but no discernable positive health benefits. The level of uncertainty surrounding measured quality-of-life outcomes suggests that there is also a possibility that the interventions assessed result in a lower aggregate level of health gain compared with standard care. Clearly, these findings are inconsistent with those arising from the clinical review. The critical appraisal of available economic studies highlighted a number of methodological concerns associated with the available studies, which, to some degree, may explain the conflicting findings of the clinical and economic reviews presented here.
Recommendations for future research
Health promotion can be viewed as an umbrella concept that covers a wide range of activities from different disciplines – physical, psychological, social and environmental as well as spiritual – all of which aim to improve the health of the population. 49 Given the limitations of the existing evidence base, there remains a substantial degree of uncertainty surrounding how home-based, nurse-led health promotion should be targeted, implemented and evaluated. This gives rise to a number of potentially relevant policy questions. For instance, would it be more effective to target such a programme at all older people or to limit the intervention to specific disease groups? Would it be better to focus on prevention of disease events (e.g. COPD exacerbations or falls) or focus on the healthy population? Should the intervention be led solely by nurses or within multidisciplinary teams? It is also not possible to determine from the existing research whether another health professional or non-professional trained volunteer could have the same benefits. Clearly, there is considerable scope for future research surrounding the value of health promotion programmes in older people. Rather than suggest one particular research design, Figure 8 attempts to draw out the main domains in which choices exist for future empirical health promotion studies.
FIGURE 8.
Choice domains for the design of future home-based health promotion studies. Adapted from a general conceptual framework reported by Kimani et al. 50
Key issues and considerations relating to each domain are detailed below:
Nature of the programme
-
Who will represent the target population? Will the programme include specific at-risk groups?
-
Will the programme be implemented as a separate initiative or will it be integrated within existing general health and social services?
-
Will the programme be delivered throughout the country or will it be implemented within one or more pilot regions?
Population criteria
-
Should home-based, nurse-led interventions be restricted to certain sections of the population on the basis of demographics such as age, gender, education or ethnicity? If so, what restrictions will be applied and why?
-
Should the intervention be targeted at individuals who are in need of emotional and psychological support and care? Or should the intervention include only those individuals who are primarily in need of physical support and care?
-
Should the level of income of the individual or their families concerned be taken into consideration while assessing the eligibility criteria for inclusion? How should these restrictions be assessed and applied?
Health complications
-
What are the relevant health complications covered under such a programme?
-
Will the programme target older people with pre-existing morbidity or will it focus on healthy living in general, targeting the healthy older population? Or will it focus on both?
Promotional activities
-
What activities will the health promotion programme comprise? For example, will it include educational aspects to raise awareness of healthy lifestyles, will it focus on prevention of particular events or will it focus on the early identification and management of problems? Will the programme attempt to achieve more than one of these objectives?
Programme implementation
-
Who should operate the programme? Will it be led by the NHS alone or will it be funded and implemented in conjunction with other sectors?
-
Will patient views be captured within the quality assurance of the delivery of the service?
-
What levels of disability among older people will determine the coverage of the intervention?
Recommendations for the evaluation of home-based, nurse-led health promotion programmes
The evaluation of health promotion programmes requires consideration not only of health outcomes accrued by the recipient, and the costs of generating these, but also whether or not the intervention has wider indirect impacts on other individuals (e.g. carers) and other resources incurred outside of the health service. As a consequence, the full range of opportunity costs may be difficult to identify, measure and value. Although the available UK economic evidence base reviewed in Chapter 4 is sparse, there is some evidence of variability concerning the inclusion of relevant resource costs (see Table 9). It has previously been argued that an intersectoral approach is required to identify the broad range of costs and benefits of public health interventions;51 as such, this goes far beyond the standard methods recommended by existing economic reference cases for cost–utility analysis. 52 For instance, Weatherly et al. 51 suggest that the social care and/or health service sector may pay for the social care services and any of the sectors including social care, health service, voluntary or private may provide such services. These issues create an additional layer of complexity to the evaluation of health promotion programmes. The design of future health promotion studies should include prospective consideration of the following issues:
-
The definition of the comparator for evaluation may be subject to geographical heterogeneity and may differ according to the particular population risk group under evaluation. Future research should take in ensuring that the comparisons assessed are meaningful from a policy perspective.
-
Standard reference cases for economic analysis in the UK typically recommend the adoption of a NHS and PSS perspective, whereby relevant health outcomes are defined as those accrued by NHS patients and relevant costs are those borne by the NHS. Consideration should be given to wider societal costs and benefits. 51,53
-
As health promotion is a complex intervention, it is difficult to associate changes in any particular set of disease events as a direct or indirect result of the intervention on health outcomes. Future research should ensure that preference-based HRQoL instruments (e.g. the EQ-5D) are used as a matter of course and that any potential mortality impacts are also captured. It should also be noted that the QALY may fail to capture other multidimensional aspects of health promotion interventions; Weatherly et al. 51 suggest the development of sector-specific generic outcomes outside of health (e.g. a carer QALY).
-
Future studies should ensure that the duration of the study follow-up period is sufficient to capture all relevant costs and outcomes between intervention and comparator groups.
-
Future studies should also seek to characterise the full range of uncertainty relevant to the policy decision; hence, the use of cost minimisation should be avoided.
Chapter 6 Other factors relevant to the NHS
The implementation of home-based nurse-led health promotion within the UK gives rise to a number of implications for the NHS and associated sectors (e.g. social services). These relate to three elements that may have a marked impact on the clinical effectiveness and cost-effectiveness of such programmes.
The appropriate level of nurse training
Within the studies identified by this review, there were notable variations in terms of the appropriate level of training for nurses delivering this service. Appropriate training of nurses, and potentially other elements of a multidisciplinary team, may have considerable implications in terms of costs of training, supervision and staffing capacity within trusts. This may also have implications for which types of nurses should deliver health promotion. For example, the training of health visitors and district nurses is focused more on community care, whereas that for other groups may not be focused on home visiting to the same extent. It should also be noted that within multidisciplinary and single profession settings, staff often have generic skills that extend beyond traditional views of their role. For example, physiotherapists may take an individual’s blood pressure or nurses may assess equipment required for individual patients.
Composition and frequency of home-based nursing visits
There is considerable uncertainty concerning which elements of home-based nursing visits contribute to positive health outcomes. Given the current evidence base, the most appropriate design of this type of complex intervention remains generally unclear. There exists some evidence to suggest that increased numbers of visits may contribute to positive outcomes, although the actual beneficial components remain unclear. Clearly, the intended designs of such programmes will have a significant bearing on both the costs and health gains arising from them. There may be a role for qualitative research in identifying which components of the intervention patients value or derive benefit from; however, such studies do not provide a suitable comparative basis for evaluating alternative programmes.
Targeting of population groups who have the capacity to benefit
The way in which home-based visiting programmes are designed and delivered, and the nature and size of the target population (e.g. all older individuals, history of stroke, and so on), will likely have substantial implications for staffing capacity and programme costs.
Acknowledgements
Gill Rooney provided administrative support in preparing and formatting the report. We would also like to thank Gill Agar, Jon Tosh, Eva Kaltenthaler and Gail Mountain for their useful comments on the draft report.
Contributions of authors
Paul Tappenden acted as principal investigator for the assessment. Fiona Campbell undertook the review of clinical effectiveness. Andrew Rawdin and Paul Tappenden undertook the review of existing health economic evaluations. Ruth Wong undertook the systematic searches of clinical effectiveness and cost-effectiveness. Paul Tappenden and Neelam Kalita prepared the discussion of future research priorities.
About the School of Health and Related Research
The School of Health and Related Research (ScHARR) is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the National Institute for Health Research (NIHR) Health Technology Assessment programme on behalf of a range of policy-makers, including the National Institute for Health and Clinical Excellence (NICE). ScHARR-TAG is part of a wider collaboration of a number of units from other regions, including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsula Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Group; and Kleijnen Systematic Reviews.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Bowling A, Banister D, Sutton S. Adding quality to quantity: older people’s views on quality of life and its enhancement. London: Economic and Social Research Council; 2003.
- Phelan EA, Anderson LA, LaCroix AZ, Larson EB. Older adults’ views of ‘successful aging’: how do they compare with researchers’ definitions?. J Am Geriatr Soc 2004;52:211-16.
- World Health Organization (WHO) . Active Ageing: A Policy Framework 2002.
- Lee M. Improving Services and Support for Older People With Mental Health Problems: The Second Report from the UK Inquiry into Mental Health and Wellbeing in Later Life 2007.
- Sutherland S. With Respect to Old Age: Long Term Care: Rights and Responsibilities. A Report by the Royal Commission on Long Term Care 1999.
- Bouman A, van Rossum E, Nelemans P, Kempen GI, Knipschild P. Effects of intensive home visiting programs for older people with poor health status: a systematic review. BMC Health Serv Res 2008;8.
- van Haastregt JCM, Diederiks JPM, van Rossum E, de Witte LP, Crebolder HFJM. Effects of preventive home visits to elderly people living in the community: systematic review. BMJ 2000;320:754-8.
- Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA 2002;287:1022-8.
- Meinck M, Lauterberg J, Robra BP. Preventive home visits to the elderly: systematic review of available evidence. Gesundheitswesen 2004;66:732-8.
- Elkan R, Kendrick D, Dewey M, Hewitt M, Robinson J, Blair M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ 2001;323:719-24B.
- Daniels R, van Rossum E, de Witte L, Kempen G, van den Heuvel W. Interventions to prevent disability in frail community-dwelling elders: a systematic review. BMC Health Serv Res 2008;8.
- Gustafsson S, Edberg AK, Johansson B, Dahlin-Ivanoff S. Multi-component health promotion and disease prevention for community-dwelling frail elderly persons: a systematic review. Eur J Ageing 2009;6:315-29.
- Kennedy C, Christie J, Harbison J, Maxton F, Rutherford I, Moss D. Establishing the contribution of nursing in the community to the health of the people of Scotland: integrative literature review. J Adv Nurs 2008;64:416-39.
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008;371:725-35.
- Markle-Reid M, Browne G, Weir R, Gafni A, Roberts J, Henderson SR. The effectiveness and efficiency of home-based nursing health promotion for older people: a review of the literature. Med Care Res Rev 2006;63:531-69.
- Age Concern . Just Ageing? Fairness Equality and Human Rights Commission and Health the Aged 2009.
- Centre for Policy on Ageing (CPA) . Ageism and Age Discrimination in Primary and Community Health Care in the United Kingdom. A Review from the Literature 2009.
- HM Government . The NHS Improvement Plan: Putting People At The Heart of Public Services 2004.
- Goodman C, Davies SL, Dinan S, Tai SS, Lliffe S. Activity promotion for community dwelling older people: a survey of the contribution of primary care nurses. Br J Community Nurs 2011;16:12-7.
- Hubley J, Copeman J. Practical Health Promotion. Cambridge: Polity Press; 2008.
- Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001;323:715-18.
- Jarman B, Hurwitz B, Cook A, Bajekal M, Lee. A Effects of community based nurses specialising in Parkinson’s disease on health outcome and costs: randomised controlled trial. BMJ 2002;324:1072-5.
- Brooks J, Ersser SJ, Lloyd A, Ryan TJ. Nurse-led education sets out to improve patient concordance and prevent recurrence of leg ulcers. J Wound Care 2004;13:111-16.
- Burton C, Gibbon B. Expanding the role of the stroke nurse: a pragmatic clinical trial. J Adv Nurs 2005;52:640-50.
- Vetter NJ, Lewis PA, Ford D. Can health visitors prevent fractures in elderly people. BMJ 1992;304:888-90.
- Spice CL, Morotti W, George S, Dent THS, Rose J, Harris S, et al. The Winchester falls project: a randomised controlled trial of secondary prevention of falls in older people. Age Ageing 2009;38:33-40.
- Kingston P, Jones M, Lally F, Crome P. Older people and falls: a randomized controlled trial of a health visitor (HV) intervention. Rev Clin Gerontol 2001;11:209-14.
- Lightbody E, Watkins C, Leathley M, Sharma A, Lye M. Evaluation of a nurse-led falls prevention programme versus usual care: a randomized controlled trial. Age Ageing 2002;31:203-10.
- McEwan RT, Davison N, Forster DP, Pearson P, Stirling E. Screening elderly people in primary care: a randomised controlled trial. Br J Gen Pract 1990;40:94-7.
- Vetter NJ, Jones DA, Victor CR. Effect of health visitors working with elderly patients in general practice: A randomised controlled trial. BMJ 1984;288:369-72.
- Cunliffe AL, Gladman JR, Husbands SL, Miller P, Dewey ME, Harwood RH. Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age Ageing 2004;33:246-52.
- Beswick AD, Gooberman-Hill R, Smith A, Wylde V, Ebrahim S. Maintaining independence in older people. Rev Clin Gerontol 2010;20:128-53.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997.
- Bakerly ND, Davies C, Dyer M, Dhillon P. Cost analysis of an integrated care model in the management of acute exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis 2009;6:201-8.
- Hurwitz B, Jarman B, Cook A, Bajekal M. Scientific evaluation of community-based Parkinson’s disease nurse specialists on patient outcomes and health care costs. J Eval Clin Pract 2005;11:97-110.
- Miller P, Gladman JR, Cunliffe AL, Husbands SL, Dewey ME, Harwood RH. Economic analysis of an early discharge rehabilitation service for older people. Age Ageing 2005;34:274-80.
- Ram FSF, Wedzicha JA, Wright J. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease: systematic review of evidence. BMJ 2004;329:315-18.
- Department of Health (DoH) . NHS Reference Costs 2008.
- Curtis L, Netten A. Unit costs of health and social care 2006. Canterbury: Personal Social Service Research Unit, University of Kent; 2006.
- MIMS, The Monthly Index of Medical Specialities . Net Ingredient Costs 1996.
- Netten A, Dennett J. Unit costs of health and social care 1996. Canterbury: Personal Social Services Research Unit, University of Kent; 1996.
- Department of Health (DoH) . NHS Reference Costs 2000–01 2001.
- Netten A, Curtis L. Unit Costs of Health and Social Care 2000. Canterbury: Personal Social Services Research Unit, University of Kent; 2000.
- Barber J, Thompson S. Analysis and interpretation of cost data in randomised controlled trials: review of published studies. BMJ 1998;317:1195-200.
- Sahlen KG, Lofgren C, Mari HB, Lindholm L. Preventive home visits to older people are cost-effective. Scand J Publ Health 2008;36:265-71.
- Kronborg C, Vass M, Lauridsen J, Avlund K. Cost effectiveness of preventive home visits to the elderly: economic evaluation alongside randomized controlled study. Eur J Health Econ 2006;7:238-46.
- Hendriks MR, van Haastregt JC, Diederiks JP, Evers SM, Crebolder HF, van Eijk JT. Effectiveness and cost-effectiveness of a multidisciplinary intervention programme to prevent new falls and functional decline among elderly persons at risk: design of a replicated randomised controlled trial [ISRCTN64716113]. BMC Publ Health 2005;5.
- Robertson MC, Devlin N, Gardner MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. BMJ 2001;322:697-701.
- Tones K, Green J. Health promotion: planning and strategies. London: SAGE Publications; 2006.
- Kimani V, Mwanthi M, Olenja J. Community home based care: action research in Kenya. Geneva: World Health Organization; 2001.
- Weatherly H, Drummond M, Claxton K, Cookson R, Ferguson B, Godfrey C, et al. Methods for assessing the cost-effectiveness of public health interventions: key challenges and recommendations. Health Policy 2009;93:85-92.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guidetothemethodsoftechnologyappraisal.jsp.
- Kelly MP. Economic appraisal of public health interventions. London: Health Development Agency; 2005.
- Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al. A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic. Health Technology Assess 2002;6.
- Campbell A.J, Robertson M.C, La Grow S.J, Kerse N.M, Sanderson G.F, Jacobs R.J., et al. Randomised controlled trial of prevention of falls in people aged ≥ 75 with severe visual impairment: the VIP trial. BMJ 2005;331.
- Carpenter I, Gambassi G, Topinkova E, Schroll M, Finne-Soveri H, Henrard JC, et al. Community care in Europe. The aged in HOme Care project (AdHOC). Aging Clin Exp Res 2004;16:259-69.
- Clarke M, Clarke SJ, Jagger C. Social intervention and the elderly: a randomized controlled trial. Am J Epidemiol 1992;136:1517-23.
- Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The effectiveness of a community-based program for reducing the incidence of falls in the elderly: a randomized trial. J Am Geriatr Soc 2004;52:1487-94.
- Dalal HM, Evans PH, Campbell JL, Taylor RS, Watt A, Read KLQ, et al. Home-based versus hospital-based rehabilitation after myocardial infarction: A randomized trial with preference arms: Cornwall Heart Attack Rehabilitation Management Study (CHARMS). Int J Cardiol 2007;119:202-11.
- Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen SD, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail 2009;11:319-25.
- Davies JA, Bull RH, Farrelly IJ, Wakelin MJ. A home-based exercise programme improves ankle range of motion in long-term venous ulcer patients. Phlebology 2007;22:86-9.
- Degischer S, Labs K-H, Hochstrasser J, Aschwanden M, Tschoepl M, Jaeger KA. Physical training for intermittent claudication: a comparison of structured rehabilitation versus home-based training. Vasc Med 2002;7:109-15.
- Drennan V, Iliffe S, Haworth D, Tai SS, Lenihan P, Deave T. The feasibility and acceptability of a specialist health and social care team for the promotion of health and independence in ‘at risk’ older adults. Health Soc Care Community 2005;13:136-44.
- Finucane FM, Horton J, Purslow LR, Savage DB, Brage S, Besson H, et al. Randomized controlled trial of the efficacy of aerobic exercise in reducing metabolic risk in healthy older people: The Hertfordshire Physical Activity Trial. BMC Endocr Disord 2009.
- Fletcher K, Mant J. A before and after study of the impact of Specialist Workers for Older People. J Eval Clin Pract 2009;15:335-40.
- Fletcher PAE, Price GM, Ng ESW, Stirling SL, Bulpitt PCJ, Breeze E, et al. Population-based multidimensional assessment of older people in UK general practice: a cluster-randomised factorial trial. Lancet 2004;364:1667-77.
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002;347:1068-74.
- Harari D, Iliffe S, Kharicha K, Egger M, Gillmann G, von Renteln-Kruse W, et al. Promotion of health in older people: a randomised controlled trial of health risk appraisal in British general practice. Age Ageing 2008;37:565-71.
- Hendriksen C, Lund E, Stromgard E. Consequences of assessment and intervention among elderly people: a three year randomised controlled trial. Br Med J 1984;289:1522-4.
- Huffman KM, Sloane R, Peterson MJ, Bosworth HB, Ekelund C, Pearson M, et al. The impact of self-reported arthritis and diabetes on response to a home-based physical activity counselling intervention. Scand J Rheumatol 2010;39:233-9.
- Jolly K, Lip GY, Sandercock J, Greenfield SM, Raftery JP, Mant J, et al. Home-based versus hospital-based cardiac rehabilitation after myocardial infarction or revascularisation: design and rationale of the Birmingham Rehabilitation Uptake Maximisation Study (BRUM): a randomised controlled trial [ISRCTN72884263]. BMC Cardiovasc Dis 2003;3.
- Jolly K, Taylor R, Lip GY, Greenfield S, Raftery J, Mant J, et al. The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Home-based compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence. Health Technol Assess 2007;11.
- Jolly K, Taylor RS, Lip GY, Davies M, Davis R, Mant J, et al. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM-CHF) study. Eur J Heart Fail 2009;11:205-13.
- Jones MI, Greenfield S, Jolly K. BRUM Trial Steering Committee. Patients’ experience of home and hospital based cardiac rehabilitation: a focus group study. Eur J Cardiovasc Nurs 2009;8:9-17.
- Khunti K, Stone M, Paul S, Baines J, Gisborne L, Farooqi A, et al. Disease management programme for secondary prevention of coronary heart disease and heart failure in primary care: a cluster randomised controlled trial. Heart 2007;93:1398-405.
- Perry M, Melis RJ, Teerenstra S, Draskovic I, van Achterberg T, van Eijken MI, et al. An in-home geriatric programme for vulnerable community-dwelling older people improves the detection of dementia in primary care. Int J Geriatr Psychiatr 2008;23:1312-19.
- Ramsbottom R, Ambler A, Potter J, Jordan B, Nevill A, Williams C. The effect of 6 months training on leg power, balance, and functional mobility of independently living adults over 70 years old. J Aging Phys Activ 2004;12:497-510.
- Roderick P, Low J, Day R, Peasgood T, Mullee MA, Turnbull JC, et al. Stroke rehabilitation after hospital discharge: a randomized trial comparing domiciliary and day-hospital care. Age Ageing 2001;30:303-10.
- Strachan G, Wright GD, Hancock E. An evaluation of a community health intervention programme aimed at improving health and wellbeing. Health Educ J 2007;66:277-85.
- Yeom HA, Keller C, Fleury J. Interventions for promoting mobility in community-dwelling older adults. JAANP 2009;21:95-100.
Appendix 1 Search strategies
Terms for the population for people over 65 years were identified (statements 1–11) and combined with broad terms for home-based, nurse-led or community interventions (statements 13–45). The search strategy was translated across various databases.
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R): Ovid 1950–present
-
aged/
-
“aged, 80 and over”/
-
frail elderly/
-
aged.tw.
-
aging.tw.
-
geriatric.tw.
-
elder$.tw.
-
senior$.tw.
-
pensioner$.tw.
-
(over 65 or over sixty-five$ or over sixty five$).tw.
-
(old$ adj20 (adult$ or person or people)).tw.
-
or/1–11
-
Health Education/
-
health education.tw.
-
Health Promotion/
-
(health adj (promotion$ or campaign$ or prevention$ or protection)).tw.
-
wellness program$.tw.
-
primary prevention.tw.
-
or/13–18
-
(nurse led or nurse-led or home or community based or community-based).tw.
-
19 and 20
-
((home-based or home based or home) adj nursing).tw.
-
Home Care Services/
-
home care service$.tw.
-
Home Nursing/
-
Health Services for the Aged/
-
House Calls/
-
house call$.tw.
-
(home visit$ or house visit$).tw.
-
Geriatric Nursing/
-
geriatric health service$.tw.
-
Community Health Nursing/
-
(community adj (health or nursing)).tw.
-
Public Health Nursing/
-
public health nursing.tw.
-
Specialties, Nursing/
-
specialist nurse$.tw.
-
district nurs$.tw.
-
visiting nurse$.tw.
-
health visitor$.tw.
-
advanced practitioner$.tw.
-
Nurse Practitioners/
-
nurse practitioner$.tw.
-
Nurse Clinicians/
-
clinical nurse specialist$.tw.
-
or/22–42
-
12 and (21 or 46)
EMBASE: Ovid 1980–present
-
aged/
-
FRAIL ELDERLY/
-
aged.tw.
-
aging.tw.
-
geriatric.tw.
-
elder$.tw.
-
senior$.tw.
-
pensioner$.tw.
-
(over 65 or over sixty-five$ or over sixty five$).tw.
-
(old$ adj20 (adult$ or person or people)).tw.
-
or/1–10
-
health education/
-
health education.tw.
-
health promotion/
-
(health adj (promotion$ or campaign$ or prevention$ or protection)).tw.
-
wellness program$.tw.
-
primary prevention/
-
primary prevention.tw.
-
or/12–18
-
(nurse led or nurse-led or home or community based or community-based).tw.
-
19 and 20
-
((home-based or home based or home) adj nursing).tw.
-
home care/
-
home care service$.tw.
-
elderly care/
-
house call$.tw.
-
(home visit$ or house visit$).tw.
-
geriatric nursing/
-
geriatric health service$.tw.
-
community health nursing/
-
(community adj (health or nursing)).tw.
-
public health nursing.tw.
-
nursing discipline/
-
specialist nurse$.tw.
-
district nurs$.tw.
-
visiting nurse$.tw.
-
health visitor$.tw.
-
nurse practitioner/
-
nurse practitioner$.tw.
-
nurse clinician$.tw.
-
clinical nurse specialist$.tw.
-
or/22–41
-
11 and (21 or 42)
Science Citation Index: Web of Science 1899–present
-
# 10 #8 AND #7
Refined by: Publication Years =thinsp;(2009 OR 2008 OR 2002 OR 2006 OR 2001 OR 2004 OR 2005 OR 2010 OR 2003 OR 2007)
-
# 9 #8 AND #7
-
# 8 Topic =thinsp;(“cost–benefit analysis” or “economic value of life” or “quality-adjusted life years” or “economic model*” or “cost utilit*” or “cost benefit*” or “cost minim*” or “cost effect*” or “economic evaluation*”)
-
# 7 #6 AND #1
-
# 6 #5 OR #4
-
# 5 Topic =thinsp;(“home-based nursing” or “home based nursing” or “home nursing”) OR Topic =thinsp;(“home care service*” or “health services for the aged” or “house call*” or “home visit*” or “house visit*” or “geriatric nursing” or “geriatric health service*” or “community health” or “community nursing” or “public health nursing” or “specialities nursing”) OR Topic =thinsp;(“specialist nurse*” or “district nurs*” or “visiting nurse*” or “health visitor*” or “advanced practitioner*” or “nurse practitioner*” or “nurse clinician*” or “clinical nurse specialist*”)
-
# 4 #3 AND #2
-
# 3 Topic =thinsp;(“nurse led” or nurse-led or home or “community based” or community-based)
-
# 2 Topic =thinsp;(“health education” or “health promotion*” or “health campaign*” or “health prevention*” or “health protection” or “wellness program*” or “primary prevention”)
-
# 1 Topic =thinsp;(aged or aging or geriatric or elder* or senior* or pensioner*) OR Topic =thinsp;(“over 65” or “over sixty-five*” or “over sixty five*”) OR Topic =thinsp;(old* SAME20 (adult* or person or people))
Cochrane Database of Systematic Reviews: Wiley InterScience 1996–present; Cochrane Central Register of Controlled Trials: Wiley InterScience 1898–present; NHS Economic Evaluation Database: Wiley InterScience 1995–present; Health Technology Assessment Database: Wiley InterScience 1995–present; Database of Abstracts of Reviews of Effects: Wiley InterScience 1995–present
-
#1 MeSH descriptor Aged explode all trees
-
#2 (aged):ti,ab
-
#3 (aging):ti,ab
-
#4 (geriatric):ti,ab
-
#5 (elder*):ti,ab
-
#6 (senior*):ti,ab
-
#7 (pensioner*):ti,ab
-
#8 (over 65 or over sixty-five* or over sixty five*):ti,ab
-
#9 (old* NEAR/20 (adult* or person or people)):ti,ab
-
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
-
#11 MeSH descriptor Health Education, this term only
-
#12 (health education):ti,ab
-
#13 MeSH descriptor Health Promotion explode all trees
-
#14 (health NEXT (promotion* or campaign* or prevention* or protection)):ti,ab
-
#15 (wellness program*):ti,ab
-
#16 (primary prevention):ti,ab
-
#17 (#11 OR #12 OR #13 OR #14 OR #15 OR #16)
-
#18 (nurse led or nurse-led or home or community based or community-based):ti,ab
-
#19 (#17 AND #18)
-
#20 (home-based or home based or home) NEXT nursing:ti,ab
-
#21 MeSH descriptor Home Care Services, this term only
-
#22 (home care service*):ti,ab
-
#23 MeSH descriptor Home Nursing explode all trees
-
#24 MeSH descriptor Health Services for the Aged, this term only
-
#25 MeSH descriptor House Calls, this term only
-
#26 (house call*):ti,ab
-
#27 (home visit* or house visit*):ti,ab
-
#28 MeSH descriptor Geriatric Nursing, this term only
-
#29 (geriatric health service*):ti,ab
-
#30 MeSH descriptor Community Health Nursing, this term only
-
#31 (community NEXT (health or nursing)):ti,ab
-
#32 MeSH descriptor Public Health Nursing, this term only
-
#33 (public health nursing):ti,ab
-
#34 MeSH descriptor Specialties, Nursing, this term only
-
#35 (specialist nurse*):ti,ab
-
#36 (district nurs*):ti,ab
-
#37 (visiting nurse*):ti,ab
-
#38 (health visitor*):ti,ab
-
#39 (advanced practitioner*):ti,ab
-
#40 MeSH descriptor Nurse Practitioners, this term only
-
#41 (nurse practitioner*):ti,ab
-
#42 MeSH descriptor Nurse Clinicians, this term only
-
#43 (clinical nurse specialist*):ti,ab
-
#44 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43)
-
#45 (#19 OR #44)
-
#46 (#10 AND #45)
-
#47 (#46), from 2001 to 2011
CINAHL: EBSCO 1982–present
-
S39 S5 and S12 and S37 Limiters – Published Date from: 20020101–20111231
-
S38 S5 and S12 and S37
-
S37 S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36
-
S36 TI “clinical nurse specialist*” or AB “clinical nurse specialist*”
-
S35 (MH “Clinical Nurse Specialists”)
-
S34 TI “nurse practitioner*” or AB “nurse practitioner*”
-
S33 (MH “Nurse Practitioners”)
-
S32 TI “advanced practitioner*” or AB “advanced practitioner*”
-
S31 TI “health visitor*” or AB “health visitor*”
-
S30 TI “visiting nurse*” or AB “visiting nurse*”
-
S29 TI “district nurs*” or AB “district nurs*”
-
S28 TI “specialist nurse*” or AB “specialist nurse*”
-
S27 (MH “Specialties, Nursing”)
-
S26 TI public health nursing or AB public health nursing
-
S25 TI ((community N2 health) or (community N2 nursing)) or AB ((community N2 health) or (community N2 nursing))
-
S24 (MH “Community Health Nursing”)
-
S23 TI geriatric health service* or AB geriatric health service*
-
S22 (MH “Gerontologic Nursing”)
-
S21 TI (“house call*” or “home visit*” or “house visit*”) or AB (“house call*” or “home visit*” or “house visit*”)
-
S20 (MH “Home Visits”)
-
S19 (MH “Health Services for the Aged”)
-
S18 (MH “Home Nursing”)
-
S17 TI “home care service*” or AB “home care service*”
-
S16 (MH “Home Health Care”)
-
S15 TI (“home-based nursing” or “home based nursing” or “home nursing”) or AB (“home-based nursing” or “home based nursing” or “home nursing”)
-
S14 S12 and S13
-
S13 TI (“nurse led” or nurse-led or home or “community based” or community-based) or AB (“nurse led” or nurse-led or home or “community based” or community-based)
-
S12 S6 or S7 or S8 or S9 or S10 or S11
-
S11 TI primary prevention or AB primary prevention
-
S10 TI wellness program* or AB wellness program*
-
S9 TI ((health N2 promotion*) or (health N2 campaign*) or (health N2 prevention*) or (health N2 protection)) or AB ((health N2 promotion*) or (health N2 campaign*) or (health N2 prevention*) or (health N2 protection))
-
S8 (MH “Health Promotion”)
-
S7 TI health education or AB health education
-
S6 (MH “Health Education”)
-
S5 S1 or S2 or S3 or S4
-
S4 TI ((old* N20 adult*) or (old* N20 person) or (old* N20 people)) or AB ((old* N20 adult*) or (old*N20 person) or (old* N20 people))
-
S3 TI (“over 65” or “over sixty-five*” or “over sixty five*”) or AB (“over 65” or “over sixty-five*” or “over sixty five*”)
-
S2 TI (aged or aged or geriatric or elder* or senior* or pensioner*) or AB (aged or aged or geriatric or elder* or senior* or pensioner*)
-
S1 (MH “Aged”) OR (MH “Aged, 80 and Over”) OR (MH “Frail Elderly”)
UK Clinical Research Network: NIHR 2001–present
http://public.ukcrn.org.uk/search/
-
Home based nursing
-
Home-based nursing
-
Home nursing
-
Nursing.
Clinical Trials.gov: US-National Institutes of Health (US-NIH) 2000–present
http://clinicaltrials.gov/ct2/search
-
Found 92 studies with search of: home based nursing | Senior | received from 1 January 2001 to 1 March 2011.
Health Economic Evaluations Database: OHE-IFPHA 1967–present
-
AX =thinsp;(aged) or (aging) or (geriatric) or (elder*) or (senior*) or (pensioner*) or (old people)
-
AX =thinsp;(nurse led) or (nurse-led) or (home) or (community based) or (community-based)
-
AX =thinsp;(primary prevention) or (health education) or (health protection)
-
CS =thinsp;2 and 3
-
AX =thinsp;(home-based nursing) or (home based nursing) or (home nursing)
-
AX =thinsp;(geriatric nursing) or (community health) or (community nursing) or (public health nursing)
-
CS =thinsp;4 or 5 or 6
-
CS =thinsp;1 and 7
-
JD> =thinsp;2001
-
CS =thinsp;8 and 9
Methodological filters
Randomised controlled trial including UK filter, e.g. in MEDLINE
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
or/1–9
-
(clinic$adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
(allocated adj2 random).tw.
-
or/11–15
-
10 or 16
-
Case report.tw.
-
Letter/
-
Historical article/
-
or/18–20
-
exp Animals/
-
Humans/
-
22 not 23
-
21 or 24
-
17 not 25
-
26 and 47 (last statement of MEDLINE strategy)
-
exp Great Britain/
-
(britain or england or uk or united kingdom or scotland or wales or british or northern ireland or gb).af.
-
28 or 29
-
27 and 30
Systematic reviews filter, e.g. in MEDLINE
-
meta-analysis as topic/
-
(meta analy$ or metaanaly$).tw.
-
Meta-Analysis/
-
(systematic adj (review$1 or overview$1)).tw.
-
“Review Literature as Topic”/
-
or/1–5
-
(cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or bids or cancerlit).ab.
-
((reference adj list$) or bibliograph$ or hand-search$ or (relevant adj journals) or (manual adj search$)).ab.
-
((selection adj criteria) or (data adj extraction)).ab.
-
“review”/
-
9 and 10
-
comment/or editorial/or letter/
-
Animals/
-
Humans/
-
13 and 14
-
13 not 15
-
12 or 16
-
6 or 7 or 8 or 11
-
18 not 17
-
19 and 47 (last statement of MEDLINE strategy)
Economic filter, e.g. in MEDLINE
-
Cost–benefit analysis/
-
Economic value of life/
-
Quality-adjusted life years/
-
exp models, economic/
-
cost utilit$.tw.
-
cost benefit$.tw.
-
cost minim$.tw.
-
cost effect$.tw.
-
economic evaluation$.tw.
-
or/1–9
-
10 and 47 (last statement of MEDLINE strategy)
Appendix 2 Excluded papers
Table of excluded papers
| Paper | Reason for exclusion |
|---|---|
| Caine 200254 | Not a home-based intervention |
| Campbell 200555 | New Zealand-based study |
| Carpenter 200456 | Survey design |
| Clarke 199257 | Intervention not delivered by nurses |
| Clemson 200458 | Study based in Australia |
| Dalal 200759 | Mean age < 70 years |
| Dar 200960 | Telemonitoring intervention delivered by medical specialists and a nurse specialist |
| Davies 200761 | The type of professional delivering the intervention was not described |
| Degischer 200262 | Not conducted in the UK or delivered by nurses |
| Drennan 200563 | Intervention delivered by multiagency team |
| Finucane 200964 | Not home based |
| Fletcher 200465 | Intervention was different approaches to assessment |
| Fletcher 200966 | Before-and-after study |
| Gill 200267 | US-based study |
| Harari 200868 | Intervention was assessment |
| Hendriksen 198469 | Study based in Denmark |
| Huffman 200270 | Unable to extract data for the very elderly population |
| Jolly 200371 | Study protocol |
| Jolly 200772 | Study protocol |
| Jolly 200973 | Mean age < 70 years |
| Jones 200974 | Qualitative study |
| Khunti 200775 | Mean age < 75 years. Interventions delivered not delivered at home |
| Perry 200876 | Based in the Netherlands |
| Ramsbottom 200477 | Intervention not delivered at home |
| Roderick 200178 | Intervention not delivered by nurses |
| Strachan 200779 | Quantitative survey |
| Yeom 200980 | US-based study |
Appendix 3 Protocol
The clinical and cost-effectiveness of home-based health promotion for older people
HTA 09/142
Draft Protocol
1 December 2010
Title of the project
The clinical and cost-effectiveness of home-based health promotion for older people in the United Kingdom.
Name of TAR team and project ‘lead’
-
Paul Tappenden
ScHARR
University of Sheffield
Regent Court
30 Regent Street
Sheffield, S1 4DA
Tel: 0114 2222 0855
Fax: 0114 272 4095
Plain English Summary
Older age is associated with numerous health risks. Physical health may decline and frailty increases, bringing with it additional risks such as falls. Social isolation may become more common due to reduced physical mobility and changing family structures and working patterns. Social isolation can lead to deterioration in emotional and psychological health. Older peoples’ needs may become an increasingly important health issue as the number of older people increases. Changing family structures and greater mobility in the working population means that more older people will be living alone, and social isolation and loneliness may become increasingly widespread. By 2021 it has been estimated that more than one in every 15 people will be an older person experiencing a mental health problem.
In older age, reduction in physical function can lead to loss of independence, the need for hospital and long-term nursing-home care and premature death. The importance of physical, functional, psychological and social factors in realising a healthy old age is recognised by elderly people,1 health care professionals2 and policymakers. 3 Physical and psychological health promotion for the elderly may have many important benefits for individuals, families and society as a whole.
Enabling older people to remain in their own homes has been a relevant government objective for several decades. In recent years, emphasis has been placed on health promotion and other preventative measures to delay the onset of illness and dependency that lead to long-term care needs. 4 In the UK, annual assessments of physical and cognitive health for individuals aged over 75 became a necessity in primary care in 1989. In 2005, a targeted approach to assessment and care was developed with community nurse-led case management of elderly people with medical conditions. Home-visiting programmes for older people may positively affect health and functional status, promote independent functioning and reduce hospital and nursing home admissions.
Since 2000, nine systematic reviews5–10;11–13 have been published. These reported conflicting results regarding the benefits of home-visiting programmes; five found beneficial effects, three found no evidence of benefit and two were inconclusive. A subgroup analysis within one review suggested that effective home-visiting programmes include multidimensional assessment, many follow-up visits and targeted people at a lower risk of death. 7 These reviews did not include consider cost-effectiveness concerns and none were UK-specific.
This assessment will seek to address these gaps to identify the factors which contribute to the effectiveness of these interventions and to examine whether such programmes represent value for money.
Decision Problem
Research Question
What is the clinical and cost-effectiveness of nurse-led health promotion intervention delivered at home for older people at risk of admission to hospital, residential or nursing care in the UK?
Intervention
Structured home-based nurse led health promotion.
Patient population
Frail older people (>75 years) with long-term medical or social needs at risk of admission to hospital, residential or nursing care.
Setting
In the home or community.
Relevant comparators
Standard care including joint health and social assessment. Health promotion delivered in a different setting or not delivered by a nurse.
Design
An evidence synthesis in the form of a systematic review of studies undertaken in the UK, including older people with longer-term medical or social needs and at risk of admission to hospital, residential or nursing care. A decision analytic model will be developed to investigate the cost-effectiveness of nurse-led, home- or community-based health promotion.
Outcomes
The systematic review will summarise the evidence for home-based nurse-led interventions designed to promote health and prevent the deterioration of health. The review will look at the components of the review and seek to identify factors that contribute to the clinical effectiveness of particular programmes.
Key factors to be addressed
Do home-based nurse-led interventions work, and if so what do they prevent or promote? If these interventions work effectively, what features of the interventions are crucial to their effectiveness and do these represent good value for money for the NHS?
Report methods for synthesis of evidence of clinical effectiveness
A systematic review of the evidence for clinical effectiveness will be undertaken following the general principles recommended in the QUOROM statement. The review will assess the effectiveness of nurse-led, home-based health promotion interventions for frail older people. It will also seek to identify the effective components of the intervention.
Population
Frail older people (>75 years) with long-term medical or social needs at risk of admission to hospital, residential or nursing care.
Interventions
Structured home based nurse-led health promotion.
Comparators
Standard care including joint health and social assessment. Health promotion delivered in a different setting or not delivered by a nurse.
Outcomes
Admission to hospital, residential or nursing care, mortality, morbidity including depression, falls, accidents, deteriorating health status, patient satisfaction.
Search Strategy
The search will be limited by date from 2001 to 2010. The Stuck et al (2002) review will be used as a source for identifying studies publishing earlier prior to 2002 (their search was conducted from January 1985 to November 2001). Bibliographies of previous systematic reviews, review articles and included studies will be handsearched to identify any other relevant studies.
The search strategy will comprise the following elements:
-
Searching of electronic databases
-
Handsearching of bibliographies of retrieved papers
-
Contact with experts in the field.
Databases to be searched include the following:
-
MEDLINE
-
MEDLINE in Process (last 12 months)
-
EMBASE
-
CINAHL
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, DARE, NHS EED and HTA databases
-
Science Citation Index (via Web of Science)
-
National Research Register
-
www.clinicaltrials.gov.
Inclusion Criteria
Studies will be included if they were conducted in the UK. They will be included if they evaluated a nurse-led health promoting intervention delivered in a home or community setting. Studies will only be included if they adopted an RCT design.
Exclusion Criteria
Non-randomised studies, non-English-language papers and reports published as meeting abstracts only where insufficient methodological details are reported to allow critical appraisal of study quality. Non-UK studies and interventions led by professionals other than nurses.
Data Extraction Strategy
Data will be extracted by one reviewer (FC).
Quality Assessment Strategy
Quality will be assessed using the Cochrane Risk of Bias tool. In particular, consideration of study quality will include the following factors:
Trial characteristics
-
Timing, duration and length of follow-up of the study
-
Method of randomisation
-
Method of allocation concealment
-
Blinding
-
Numbers of participants randomised, excluded and lost to follow-up
-
Whether intent-to-treat analysis is performed
-
Methods for handling missing data.
Methods of analysis/synthesis
Data will be tabulated and discussed in a narrative review. Where appropriate, meta-analysis will be employed to estimate a summary measure of effect on relevant outcomes based on intention to treat analyses. Meta-analysis will be undertaken using fixed and random effects models, using Revman software. Heterogeneity will be explored through consideration of the study populations, methods and interventions, by visualisation of results and by the I2 statistic.
Where available data is sufficient, subgroup analysis will be conducted to explore factors identified in earlier work as being significant in influencing intervention effectiveness including risk factors associated with the elderly person, the number of visits and the nature of the initial assessment. Sensitivity analysis will be used to explore the impact of study design on measures of effectiveness.
Methods for estimating quality of life
Studies describing relevant health-related quality of life outcomes will be identified from published sources as deemed appropriate from the definition of the decision problem.
Report methods for synthesising evidence of cost-effectiveness
The cost-effectiveness of alternative NHS-based home nursing interventions will be assessed against standard care from the perspective of the NHS and Personal Social Services. Published trials and economic studies will be examined to identify existing comparative evidence concerning the cost-effectiveness of such interventions. If appropriate/required, a de novo health economic model will be developed. Relevant events, costs and outcomes for inclusion in the model, and the relationship between these, will be elicited from the literature and from the views of clinical experts through a formal and transparent problem structuring process using cognitive mapping. Cost-effectiveness will most likely be assessed in terms of the incremental cost per quality adjusted life year (QALY) gained. Discounting will be undertaken using standard methods. The precise structure of the model will be determined upon consideration of relevant issues arising from the problem structuring process.
Expertise in this TAR team
TAR Centre
The ScHARR Technology Assessment Group (ScHARR-TAG) undertakes reviews of the effectiveness and cost-effectiveness of healthcare interventions for the NHS R&D Health Technology Assessment Programme on behalf of a range of policymakers in a short timescale, including the National Institute for Health and Clinical Excellence. The group has extensive expertise in information retrieval, systematic reviewing and health economic modelling.
Contributions of team members:
Paul Tappenden, Senior Research Fellow
Paul will be the lead on this TAR project. Paul will manage the day-to-day progress of the assessment and will design and undertake the economic analysis.
Fiona Campbell, Research Fellow, ScHARR
Fiona will be the main reviewer on this project. Fiona will undertake the study selection, data extraction and do the meta-analyses.
Ruth Wong, Information Specialist, ScHARR
Ruth will undertake the systematic searches for the review.
Gill Rooney, Project Administrator, ScHARR
Gill will assist in the retrieval of papers and in preparing and formatting the report.
Expert advisors
Two expert advisors will be provide advice for the assessment: Margaret Osborne, who is a heart failure nurse specialist, and Gill Agar, who is a physiotherapist coordinating home based health promotion to prevent falls amongst the elderly. Both are health professionals currently involved in delivering home based health promotion to the elderly in their homes.
Competing interests of authors
None.
Timetable/milestones
The project is expected to run from 1 December 2010 to 3 May 2011.
| Milestone | Deadline |
|---|---|
| Draft protocol | 1 December 2010 |
| Final protocol | 15 December 2010 |
| Start review | 1 March 2011 |
| Progress report | 5 April 2011 |
| Assessment report | 3 May 2011 |
Appendices
Appendix 1– MEDLINE search strategy
-
aged/
-
“aged, 80 and over”/
-
frail elderly/
-
aged.tw.
-
aging.tw.
-
geriatric.tw.
-
elder$.tw.
-
senior$.tw.
-
pensioner$.tw.
-
(over 65 or over sixty-five$ or over sixty five$).tw.
-
(old$ adj20 (adult$ or person or people)).tw.
-
or/1–11
-
Health Education/
-
health education.tw.
-
Health Promotion/
-
(health adj (promotion$ or campaign$ or prevention$ or protection)).tw.
-
wellness program$.tw.
-
primary prevention.tw.
-
or/13–18
-
(nurse led or nurse-led or home or community based or community-based).tw.
-
19 and 20
-
((home-based or home based or home) adj nursing).tw.
-
Home Care Services/
-
home care service$.tw.
-
Home Nursing/
-
Health Services for the Aged/
-
House Calls/
-
house call$.tw.
-
(home visit$ or house visit$).tw.
-
Geriatric Nursing/
-
geriatric health service$.tw.
-
Community Health Nursing/
-
(community adj (health or nursing)).tw.
-
Public Health Nursing/
-
public health nursing.tw.
-
Specialties, Nursing/
-
specialist nurse$.tw.
-
district nurs$.tw.
-
visiting nurse$.tw.
-
health visitor$.tw.
-
advanced practitioner$.tw.
-
Nurse Practitioners/
-
nurse practitioner$.tw.
-
Nurse Clinicians/
-
clinical nurse specialist$.tw.
-
or/22–42
-
12 and (21 or 46)
Searches will be limited by year from 2001 to present. A highly sensitive filter will be applied to limit searches by publication (reviews, RCTs and economic studies).
Appendix 2 – Sample data extraction form
| STUDY | Baseline characteristics | Description of Intervention | Outcomes | Study Design |
|---|---|---|---|---|
|
Author: Date: Setting: |
Total number: Mean Age: Indicator of Health Status: % Male: Ethnic group: Indicator of provision of social support: Indicator of provision of existing social and/or health care support |
Provider details (training, work load) Nature of intervention (purpose, frequency, duration of intervention and duration of follow-up) |
Mortality during intervention and follow-up: Hospital or nursing home admission: Indicator of deterioration in health status: Patient satisfaction: |
Baseline comparability: RCT or Cluster RCT: Method of allocation concealment: Method of randomisation: Blinding of outcome assessors: Loss to follow-up: Participant withdrawals: Other potential bias: |
References
- Age Concern . Adding Quality to Quantity; Older people’s Views on Quality of Life and Its Enhancement 2003.
- Phelan EA, Anderson LA, LaCroix AZ, Larson EB. Older adults’ views of “successful aging” – how do they compare with researchers’ definitions?. J Am Geriatr Soc 2004;52:211-6.
- WHO . Active Ageing: A Policy Framework 2002.
- Sutherland S. With Respect to Old Age: Long Term Care – Rights and Responsibilities. A Report by the Royal Commission on Long Term Care 1999.
- van Haastregt JC, Diederiks JP, van RE, de Witte LP, Crebolder HF. Effects of preventive home visits to elderly people living in the community: systematic review. BMJ 2000;320:754-8.
- Elkan R, Kendrick D, Dewey M, Hewitt M, Robinson J, Blair M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ 2001;323:719-25.
- Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA 2002;287:1022-8.
- Meinck M, Lubke N, Lauterberg J, Robra BP. [Preventive home visits to the elderly: systematic review of available evidence]. Gesundheitswesen 2004;66:732-8.
- Markle-Reid M, Browne G, Weir R, Gafni A, Roberts J, Henderson SR. The effectiveness and efficiency of home-based nursing health promotion for older people: a review of the literature. Med Care Res Rev 2006;63:531-69.
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008;371:725-3.
- Bouman A, van RE, Nelemans P, Kempen GI, Knipschild P. Effects of intensive home visiting programs for older people with poor health status: a systematic review. BMC Health Serv Res 2008;8.
- Gustafsson S, Edberg AK, Johansson B, Dahlin-Ivanoff S. Multi-component health promotion and disease prevention for community-dwelling frail elderly persons: a systematic review. European Journal of Ageing 2009;6:315-29.
- Daniels R, van Rossum E, de Witte L, Kempen G, van den Heuvel W. Interventions to prevent disability in frail community-dwelling elderly: a systematic review. BMC Health Services Research 2008;8.
Glossary
- Barthel Index
- A tool to measure an individual’s level of daily functioning, specifically relating to the activities of daily living and mobility. The instrument includes 10 items, such as feeding, bathing, mobility, dressing and toilet use. Total scores are calculated as the simple sum of scores across all dimensions. Using the modified index, scores range from 0 to 20, with lower scores indicating lower functioning.
- Beck Depression Inventory
- An instrument used to measure patient depression. The inventory consists of 21 items associated with psychological and physical symptoms of depression, such as sadness, agitation, concentration, loss of pleasure, self-dislike, tiredness and fatigue, and changes in appetite. Total scores range from 0 to 63, with higher total scores indicating more severe depressive symptoms.
- Caregiver Strain Index
- An instrument used to measure perceptions of strain in carers. The instrument consists of 13 questions across domains including employment, financial, physical, social and time. Total scores range from 0 to 13, with higher scores indicating a greater level of stress.
- European Quality of Life-5 Dimensions (EQ-5D)
- A five-dimension preference-based health status measure used to estimate health utility. A score of 1 represents a notional state of ‘perfect health’, whereas a score of 0 represents a notional state of ‘death’. Scores < 0 (as low as –0.594) represent states worse than death.
- General Health Questionnaire (GHQ)
- An instrument for identifying psychiatric illness specifically in general practice. The questionnaire covers recent physical and psychiatric symptoms experienced by patients. The original version of this measure included 60 items, but modified versions include fewer items. Each item includes four possible outcomes. Total scores depend on whether the adopted scoring method is bimodal (0–0–1–1) or adopts a Likert-type scoring scale (1–2–3–4). Higher scores indicate a greater severity of symptoms.
- Health promotion
- The process of enabling people to increase control over, and to improve, their health.
- I2-statistic
- A measure of statistical heterogeneity between studies.
- Meta-analysis
- A statistical method by which the results of a number of studies are pooled to give a combined summary statistic.
- Nottingham Health Profile
- An instrument used to measure patient perceptions of general health, including emotional health, social isolation, pain, mobility, energy and sleep. The tool includes six main dimensions with subquestions for each. Scores range from 0 to 100 for each section, with higher scores indicating a worse level of general health.
- Short-form 36 (SF-36) questionnaire
- A general short-form questionnaire with 36 items consisting of eight scaled scores. These dimensions include vitality, physical functioning, bodily pain, general health perceptions, physical/emotional/social role functioning, and mental health. Each scale is transformed to a score from 0 to 100 and is given equal weight, with the total score also ranging from 0 to 100. Lower scores indicate a lower level of quality of life.
List of abbreviations
- ACAS
- Acute COPD Assessment Service
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COPD
- chronic obstructive pulmonary disease
- CRN
- UK Clinical Research Network Portfolio Database
- DARE
- Database of Abstracts of Reviews of Effects
- EDRS
- Early Discharge and Rehabilitation Service
- EQ-5D
- European Quality of Life-5 Dimensions
- GHQ
- General Health Questionnaire
- GP
- general practitioner
- HEED
- Health Economic Evaluations Database
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LTFU
- lost to follow-up
- MIMS
- Monthly Index of Medical Specialities
- NHS EED
- National Health Service Economic Evaluation Database
- OR
- odds ratio
- PDQ-39
- Parkinson’s Disease Questionnaire
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- US-NIH
- United States-National Institutes of Health
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health