Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 09/90/01. The protocol was agreed in July 2010. The assessment report began editorial review in July 2011 and was accepted for publication in July 2011. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Loveman et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Background to this assessment report
In November 2009, the National Institute for Health and Clinical Excellence (NICE) issued for consultation preliminary recommendations on the use of dasatinib and nilotinib for chronic myeloid leukaemia (CML) in patients whose treatment with imatinib had failed owing to resistance and/or intolerance. This updated Technology Appraisal No. 70 (TA70; 2003). In response to comments received during the consultation period, NICE and the Appraisal Committee agreed that it was preferable to combine an appraisal of the three technologies – high-dose imatinib (600 mg and 800 mg), dasatinib and nilotinib – to establish their comparative incremental clinical effectiveness and cost-effectiveness. Therefore, the following actions were implemented:1
-
The dasatinib and nilotinib multiple technology appraisal was continued for ‘imatinib-intolerant’ people with CML.
-
The dasatinib and nilotinib multiple technology appraisal for ‘imatinib-resistant’ people was rescheduled into the review of TA70, specifically related to high-dose imatinib. An updated draft scope was issued for consultation for the review of TA70, focusing on ‘resistant’ people to include the following interventions: high-dose imatinib, dasatinib and nilotinib.
-
The final appraisal determination (FAD) for imatinib-intolerant patients was planned to be released at the same time as the FAD for imatinib-resistant patients, as the recommendations for the use of dasatinib and nilotinib for the treatment of CML in imatinib-intolerant people could be influenced by the outcome of the appraisal in imatinib-resistant people.
This technology assessment report is of dasatinib, nilotinib and high-dose imatinib within their licensed indications for the treatment of people with CML who are resistant to standard-dose imatinib. The initial 2009 appraisal of people with treatment failure owing to resistance and/or intolerance was informed by a technology assessment report prepared by the Peninsula Technology Assessment Group (PenTAG), University of Exeter, which included much of the evidence relevant to the current appraisal. Therefore, the present assessment report serves as a supplement to the previous PenTAG assessment report (herein referred to as the PenTAG AR2). Reference is made to the PenTAG AR2 where appropriate [this project was funded by the National Institute for Health Research Health Technology Assessment programme (project number 08/31/01) and was published in full in the Health Technology Assessment journal series. The full report is accessible from the project page of the Health Technology Assessment programme website www.hta.ac.uk/1831]. The present assessment was initiated by the West Midlands Health Technology Assessment Collaboration (WMHTAC) and handed over to Southampton Health Technology Assessments Centre (SHTAC) during the early stages. Further details can be found in Chapter 2.
This report describes new evidence on the clinical-effectiveness and cost-effectiveness of dasatinib, high-dose imatinib and nilotinib in imatinib-resistant CML to reflect the current decision problem. For background and epidemiology of CML please refer to the PenTAG AR2 (see pp. 29–44).
Decision problem
This section states the key factors that will be addressed by this assessment, and defines the scope of the assessment in terms of these key factors in line with the definitions provided in the NICE scope.
Three interventions are included within the scope of this assessment. These are dasatinib, nilotinib and high-dose imatinib (600 mg or 800 mg per day) in line with their licensed indications within the different phases of CML (chronic, accelerated and blast-crisis phases; for description of these phases see the PenTAG AR,2 pp. 31–2).
The population of focus in this assessment is people with CML who are resistant to standard-dose imatinib (400–600 mg per day). The definition of imatinib resistance can vary (discussed in detail in the PenTAG AR,2 pp. 40–2). For the present assessment, definitions of imatinib resistance provided in included studies will be used. If sufficient evidence is available, then consideration will be given to the level of previous response to standard-dose imatinib. Additionally, if the evidence allows, consideration will be given to the phase of CML.
In line with the NICE scope, eligible comparators are standard-dose imatinib, interferon alfa, hydroxycarbamide, acute leukaemia-style chemotherapy, allogeneic stem cell transplant, and best supportive care depending on the phase of CML. The scope issued by NICE was updated on 25 October 2010 to also allow the interventions to be compared with one another.
The clinical outcomes of interest are treatment response rates (including haematological, cytogenetic and molecular responses), time to response, duration of response, overall survival, event-free survival, progression-free survival, adverse effects, health-related quality of life (HRQoL), and time to treatment failure.
Objectives
-
To update the systematic review of clinical effectiveness and cost-effectiveness undertaken in the PenTAG AR2 for people with imatinib-resistant disease only.
-
To critique the economic evaluations included in the manufacturers’ submissions to NICE from Bristol-Myers Squibb3 (BMS; dasatinib) and Novartis4 (nilotinib and imatinib) to identify the strengths and weaknesses of the respective submissions.
-
To adapt the economic analysis undertaken in the PenTAG AR2 to run updated cost-effectiveness analyses for the current assessment, reflecting the current scope.
Chapter 2 Methods
This assessment comprises an updated systematic review of clinical effectiveness and cost-effectiveness studies, a review and critique of the economic evaluations included in the manufacturer submissions and an update of the economic analysis undertaken in the previous PenTAG AR2 for chronic-phase CML.
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in the research protocol (see Appendix 1). This assessment was initiated by the WMHTAC. The identification of studies and the initial screening of evidence for clinical effectiveness and cost-effectiveness was undertaken by WMHTAC (as described below), with SHTAC assuming responsibility for the project after this stage.
Identification of studies
A search of the evidence base for published and ongoing studies of clinical effectiveness and safety was undertaken by WMHTAC. Databases were searched from inception to June 2010 by WMHTAC and searches were not limited to the English language. Searches were undertaken using strategies combining text words and index terms relating to the condition (CML) and the interventions (imatinib, dasatinib and nilotinib). Searches were updated by SHTAC in January 2011.
The following databases were searched for published studies and ongoing research: MEDLINE In-Process & Other Non-Indexed Citations (Ovid); MEDLINE (Ovid); EMBASE (Ovid); Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO); Cochrane (Wiley) Central Register of Controlled Trials (CENTRAL) and Cochrane Database of Systematic Reviews (CDSR); Centre for Reviews and Dissemination (CRD) databases; Science Citation Index Expanded (Web of Science); metaRegister of Current Controlled Trials; International Standard Randomised Controlled Trial Number (ISRCTN) database; World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) Portal; and ClinicalTrials.gov for ongoing studies. In addition, specialist abstract and conference proceeding resources were searched and experts in the field consulted. Further details, including an example search strategy, can be found in Appendix 2, and the full search strategies are available from the authors.
Inclusion and exclusion criteria
Population
People with imatinib-resistant CML in the chronic, accelerated or blast-crisis phases were eligible for inclusion.
Interventions
Studies of dasatinib, nilotinib and high-dose imatinib were considered for inclusion.
Comparators
Potential comparators were dasatinib, nilotinib and high-dose imatinib, hydroxycarbamide (hydroxycarbamide), interferon alfa, acute leukaemia-style chemotherapy, allo-stem cell transplantation, standard-dose imatinib and best supportive care, depending on the phase of CML.
Outcomes
Studies reporting one or more of the following outcome measures were eligible for inclusion: treatment response rates [including molecular, cytogenetic and haematological responses (HRs)]; time to, and duration of, response; overall survival; event-free survival; progression-free survival; adverse effects of treatment; HRQoL; time to treatment failure; costs and cost-effectiveness.
Study design
The hierarchy of evidence was used to determine the inclusion of trials and studies into the review. Randomised controlled trials (RCTs) or prospective non-randomised comparative studies, where adequate matching was considered to have been achieved, were eligible for inclusion. Where no such evidence existed, single-arm cohort studies were included.
Studies published as abstracts or conference presentations were eligible to be included only if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
For the systematic review of cost-effectiveness, studies were eligible for inclusion if they reported the results of full economic evaluations, i.e. cost-effectiveness analyses, cost–utility analyses or cost–benefit analyses.
Studies were excluded if participants were aged < 18 years, did not have CML or were imatinib naive or imatinib intolerant. Studies of high-dose imatinib [> 400 mg b.i.d. (twice daily) in chronic phase] as first-line treatment were also excluded.
Inclusion and data extraction process
Studies were selected for inclusion in the systematic reviews of clinical effectiveness and cost-effectiveness through a two-stage process. Literature search results (titles and abstracts) were screened for inclusion to identify all of the citations that might meet the inclusion criteria. Full manuscripts of relevant citations were then retrieved.
Retrieved studies were then assessed by one SHTAC reviewer against the inclusion/exclusion criteria and checked by a second SHTAC reviewer. Discrepancies were resolved by discussion.
Data from included studies were extracted by one reviewer using a standardised data extraction form and each data extraction was checked for accuracy by a second reviewer. Again any discrepancies were resolved by discussion, with involvement of a third reviewer when necessary.
Critical appraisal strategy
The quality of included clinical effectiveness studies was assessed using the criteria used in the previous PenTAG AR2 (see pp. 84–9). Quality criteria were applied by one reviewer and checked by a second reviewer, with any disagreements resolved by consensus or involvement of a third reviewer where necessary. For details of the quality criteria applied to cost-effectiveness studies, see Chapter 4 (Critical appraisal of the economic evaluation).
Method of data synthesis
Data from newly identified clinical effectiveness and cost-effectiveness studies were synthesised through a narrative review with tabulation of the results of included studies. It was considered inappropriate to combine the results of the studies in a meta-analysis owing to methodological shortcomings of the included studies (in terms of study designs, differences in the interventions, and differences in the baseline characteristics of the populations). In cases where data reported by PenTAG have since been updated, both the original data reported by PenTAG2 and the updated data are presented in this report. Relevant sections of the PenTAG AR2 are referred to where appropriate.
Chapter 3 Clinical effectiveness
Quantity and quality of research available
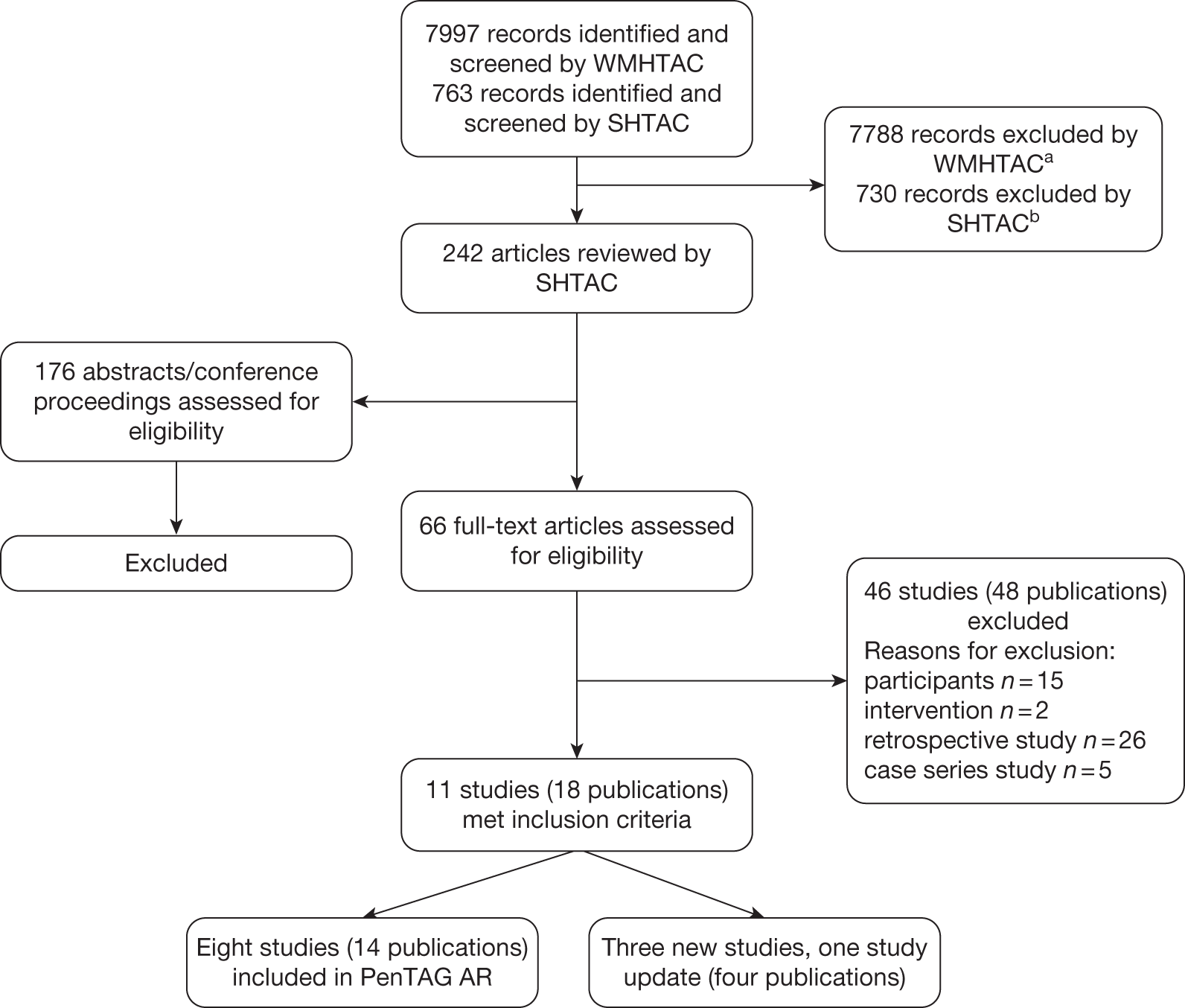
Searching by WMHTAC and SHTAC identified a total of 8760 references after deduplication. After initial screening of titles and abstracts, 242 references were retrieved for further inspection. The total number of published papers included at each stage of the systematic review is shown in the flow chart in Figure 1. In total, 11 studies met the inclusion criteria, four (three new studies, one updated publication) of which included data published since the PenTAG AR. 2 The present report presents data from these four studies in order to supplement and update the PenTAG AR. 2 For data from the eight studies previously reviewed, see PenTAG AR2 (pp. 55–164).
FIGURE 1.
Flow chart of identification of studies for inclusion in the review. aIncludes three foreign-language and three other publications that the British Library was unable to retrieve, but which had initially been included in the WMHTAC first screen. bIncludes four potentially relevant abstracts that could not be obtained.
The studies included in the present report assessed dasatinib and/or high-dose imatinib in chronic-phase CML. No new studies were found by the updated search for accelerated phase or blast phase for any of the interventions. The relevant sections of the PenTAG AR2 for the clinical effectiveness of dasatinib in these subgroups can be found in Table 1. No eligible studies assessing nilotinib were identified. The results for the clinical effectiveness of nilotinib can be found in the PenTAG AR2 (see pp. 138–57).
| Outcome | AP | BP |
|---|---|---|
| CyR | pp. 98–103 | pp. 103–6 |
| HR | pp. 111–14 | pp. 115–16 |
| PFS | pp. 119 | No imatinib-resistant-only data |
| OS | No imatinib-resistant-only data | No imatinib-resistant-only data |
| AEs | pp. 125–9 | pp. 129–31 |
References for the studies retrieved for further inspection, but subsequently excluded can be seen in Appendix 3. The most common reason for exclusion was a retrospective study design. One eligible abstract was identified;5 however, this could not be included owing to insufficient reporting of methods and baseline data. The level of agreement between reviewers assessing study eligibility was generally good, although this was not formally measured.
Design and characteristics of included studies
One published update of a RCT and three new single-arm cohort studies met the inclusion criteria (Figure 1). Data extraction forms for these studies can be seen in Appendix 4. The RCT (Kantarjian and colleagues6) compared high-dose imatinib (600 or 800 mg/day) with dasatinib (140 or 180 mg/day) and was reported in detail in the context of its dasatinib intervention in the PenTAG AR2 (see p. 57, p. 59 and pp. 79–89). The update of this RCT was published in 2009,7 and longer follow-up from the dasatinib arm of this study, as well as data from the high-dose imatinib arm, are included in the present review. However, methodological flaws associated with this RCT render it of limited value as a comparative study (see PenTAG AR,2 section 3.2.4, p. 90), and the PenTAG AR2 presented the dasatinib arm as non-comparative evidence. In line with this, data from the dasatinib and high-dose imatinib arms of the RCT are presented separately and are not compared in the present systematic review (see Chapter 3, Critical appraisal of included evidence)
The single-arm cohort studies each had a single high-dose imatinib arm. In one study, by Rajappa and colleagues,8 all participants received imatinib at 800 mg/day, whereas in the remaining studies the imatinib dose varied from 600 to 800 mg/day according to whether individual participants met criteria for dose escalation or reduction. The interventions in the RCT and observational studies are summarised, respectively, in Tables 2 and 3, and can be viewed in detail in Appendix 4. None of the studies reported whether or not participants received any treatment concurrent with imatinib.
| Study | Arm no. | Drug | Dosage notes | Notes |
|---|---|---|---|---|
| Kantarjian et al. (2009)7 | 1 | HDI |
400 mg b.i.d. Reduction to 600 mg daily was permitted for toxicity in participants who had not previously received 600 mg of imatinib |
Crossover to the alternative treatment was permitted after confirmed progression, lack of MCyR at the week 12 cytogenetic evaluation or intolerance This is Study 017 in the BMS submission3 to NICE |
| 2 | Dasatinib |
70 mg b.i.d. a Escalated to 180 mg for participants with inadequate response at 12 weeks or progression Reduced to 100 or 80 mg for participants experiencing toxicity |
| Study | Arm | Drug | Dosage | Concurrent treatment | Notes |
|---|---|---|---|---|---|
| Breccia et al. (2010)9 | 1 | HDI | Escalated from 400 to 600 mg/day or 800 mg/day if haematological failure, imatinib resistance or suboptimal response | None reported |
600 mg/day: n = 54 800 mg/day: n = 20 |
| Koh et al. (2010)10 | 1 | HDI |
Escalated from 400 to 600 mg/day (CP) or from 400–600 to 600–800 mg/day (AP and BC). High doses were for a minimum of 12 months or until disease progression or intolerable toxicity Reduced from 800 to 600 or 400 mg/day, or from 400 to 300 mg/day in participants with cytopenia and non-haematological toxicity of grade 3 or more. An effort was made to increase dose if participants on reduced dose for 1 month did not experience more than grade 1 toxicity |
None reported | Participants experiencing more than grade 3 toxicity on 300 mg/day were withdrawn |
| Rajappa et al. (2010)8 | 1 | HDI | Escalated from 400 to 800 mg/day for all participants | None reported | Study focuses on kinase domain mutations |
The designs of the RCT and single-arm cohort studies are summarised, respectively, in Tables 4 and 5. The RCT was conducted in 58 centres in 23 countries, including the UK, Europe, the Russian Federation and Asia. The three single-arm cohort studies were conducted in single countries: Republic of Korea, Italy and India. Apart from the Korean study, which involved 19 centres, the number of centres was small or unclear (Table 5). The studies included only participants with chronic-phase CML, except for the single-arm cohort study by Koh and colleagues,10 which also included very small numbers of participants with accelerated phase and blast-crisis phase (Table 6). Inclusion and exclusion criteria were reported in detail for the RCT (Table 4), but only briefly for the single-arm cohort studies (see Table 5). The RCT required participants to be at least 18 years of age and have ‘adequate hepatic and renal function’, and excluded those with BCR–ABL (oncogene fusion protein consisting of BCR and ABL genes) mutations known to be particularly resistant to imatinib. The single-arm cohort study by Koh and colleagues10 required participants to be aged 15–75 years with ‘adequate organ function’. All other inclusion and exclusion criteria reported in the RCT and single-arm cohort studies were based on cytogenetic or molecular aspects of CML or imatinib dosing.
| Study | CP | AP | BC | Countries | No. of centres | Inclusion criteria | Exclusion criteria | Method of allocation | Blinding | Therapy common to all participants |
|---|---|---|---|---|---|---|---|---|---|---|
| Kantarjian et al. (2007),6 (2009)7 (additional references are given in table 6 of PenTAG AR2) | ✓ | Argentina, Australia, Belgium, Brazil, Canada, Estonia, Finland, France, Germany, Israel, Republic of Korea, Norway, Peru, the Philippines, Poland, Puerto Rico, Russian Federation, South Africa, Sweden, Taiwan, Thailand, the UK and the USA | 58 |
Participants with CP-CML and primary or acquired resistance to standard doses of imatinib (400–600 mg), dasatinib naive, at least 18 years of age and had adequate hepatic and renal function. CP was defined by the presence of < 15% blasts, < 20% basophils and < 30% blasts plus promyelocytes in peripheral blood or bone marrow and a platelet count of at least 100,000 per mm3, with no extramedullary involvement Primary resistance to imatinib was defined as a lack of CHR after 3 months of imatinib treatment, a lack of any CyR after 6 months of treatment or a lack of a MCyR (Ph+ cells > 35%) after 12 months of treatment. Relapse after a HR or MCyR was considered as secondary or acquired resistance |
Participants who had received imatinib in the 7 days before the study were ineligible, as were participants who had received imatinib at doses in excess of 600 mg per day. Participants with known specific BCR–ABL mutations (with high resistance to imatinib) before study entry were excluded | 2 : 1 randomisation (no details of methods used) | Open label | Not reported |
| Study | Design | CP | AP | BC | Country | No. of centres | Inclusion criteria | Exclusion criteria | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Breccia et al. (2010)9 | Cohort single arm; judged prospective | ✓ | Italy | 2 | Participants with CML who demonstrated a poor response or relapse after standard imatinib therapy (no other inclusion information given other than a table of baseline characteristics) | Not stated | Investigated the long-term efficacy of dose escalation in participants with CP-CML who demonstrated a poor response or relapse after standard imatinib therapy | ||
| Koh et al. (2010)10 | Prospective cohort single arm | ✓ | ✓ | ✓ | Republic of Korea | 19 | CML participants between 15 and 75 years of age with adequate organ function (not defined). Participants in CP with suboptimal response to 400 mg/day imatinib; participants in AP or BC who failed to achieve CHR after 3 months on 400–600 mg/day imatinib | Participants who experienced more than grade 2 AEs to standard-dose imatinib | Phase IV study to evaluate the efficacy of escalated dose imatinib in participants with suboptimal response to standard-dose imatinib |
| Rajappa et al. (2010)8 | Cohort single arm; judged prospective | ✓ | India | Not stated (all authors from one centre) | CP-CML resistant to imatinib 400 mg/day. No other details reported | Participants with AP or BC | Study focuses on kinase domain mutations |
| Study | Arm | n | Age (years), mean ± SD or median (range) | Sex, male (%) | Imatinib failure (%) | Duration of CML (months), median (range) | BCR–ABL, mutation (%) | MCyR at, baseline (%) | CHRa at, baseline (%) | WBCs × 109/l, median (range) | Platelets × 109/l, median (range) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kantarjian et al. (2009)7 | 1. HDI | 49 |
Median 51 (24 to 80) |
44.9 | Resistance: 100 | 52 (14 to 133) | 22.4 | 0.0 | 55.1 | 7.4 (2 to 133) | 248 (80 to 2318) |
| 2. Dasatinib | 101 |
Median 51 (24 to 85) |
52.5 | Resistance: 100 | 64 (6 to 166) | 40.6 | 5.9 | 50.5 | 7.5 (2 to 153) | 261 (55 to 1903) | |
| Breccia et al. (2010)9 | 1. HDI | 74 |
Median 50 (19 to 85) |
70.3 |
Primary + secondary resistance: 95 Suboptimal response: 5 |
Not reported | Not reported | Not reported | Not reported | 4.5 (3.8 to 6.2)b | 220 (180 to 350)b |
| Koh et al. (2010)10 | 1. HDI |
71: CP = 64 AP = 3 BC = 4 |
Median 49 (20 to 71) |
70.4 |
Treatment failure: 73 Suboptimal response: 27 |
Not reported | Unclearc | Not reported | Not reported | Not reported | Not reported |
| Rajappa et al. (2010)8 | 1. HDI | 90 | Mean 35.7 ± 12 (18 to 65) | 71.1 |
Primary resistance: 33.3 Secondary resistance: 66.7 |
Not reported | 32.2 | Uncleard | Not reportede | 11 (3.7 to 180) | % (range) platelets: 2.7b (0.9 to 11.9) |
Failure on standard-dose imatinib was defined in terms of resistance and suboptimal cytogenetic, haematological and molecular response. None of the studies defined imatinib failure as intolerance (Table 6). The criteria used to define imatinib failure were slightly different in each of the four studies (Table 7).
| Study | HR | CHR | PCyR | CyR | MCyR | CCyR | MMR | BCR–ABL mutations | Other criteria |
|---|---|---|---|---|---|---|---|---|---|
| Primary resistance | |||||||||
| RCT: Kantarjian et al. (2009)7 | Lack at 3 months | Lack at 6 months | Lack at 12 months | ||||||
| Rajappa et al. (2010)8 | Lack at 3 months | Lack at 6 months | Lack at 12 months | Lack at 18 months | |||||
| Imatinib failure | |||||||||
| Breccia et al. (2010)9 | Lack at 3 months or loss at any time | Less than at 12 months | Lack at 6 months | Less than at 18 months or loss at any time | At any time | ||||
| Koh et al. (2010)10 (LeukemiaNET) | Lack at 3 months or loss at any time | Less than at 6 months | Less than at 12 months | Lack at 6 months | Less than at 18 months, or loss at any time | Conferring high insensitivity at any time | |||
| Suboptimal response | |||||||||
| Breccia et al. (2010)9 | Incomplete at 3 months | Less than at 6 months | Less than at 12 months | Less than at 18 months or loss at any time | At any time | Cytogenetic abnormalities in Ph+ cells | |||
| Koh et al. (2010)8 (LeukemiaNET) | Less than at 3 months | Less than at 6 months | Less than at 12 months | Less than at 18 months or loss at any time | Conferring low insensitivity at any time | Additional chromosomal abnormalities in Ph+ cells | |||
| Secondary resistance | |||||||||
| RCT: Kantarjian et al. (2009)7 | Relapse after HR | Relapse after MCyR | |||||||
| Rajappa et al. (2010)8 | Loss at any time | WBCs rise above threshold on two or more occasions > 4 weeks apart, progression to AP or BC | |||||||
Baseline characteristics of the participants in the RCT and cohort studies are summarised in Table 6. For high-dose imatinib, the proportion of male participants in the RCT (45%) was lower than in the three single-arm cohort studies (70–71%). Across the four studies,7–10 the participants ranged in age from 18 to 85 years. The cohort study by Rajappa and colleagues8 included younger participants (mean age 35.7 years) than the three other studies (median age 49–51 years). Duration of CML from diagnosis to imatinib therapy ranged from 14 to 133 months in the RCT, but was not reported in any of the single-arm cohort studies. Baseline genetic and haematological data were not consistently reported across the four studies and are therefore difficult to compare. Only the RCT provided baseline data on the proportion of participants with a major cytogenetic response or a complete haematological response (CHR). The proportion of participants with BCR–ABL mutations at baseline was slightly lower in the RCT high-dose imatinib participant group (22.4%) than in the only single-arm cohort study, by Rajappa and collegues,8 that provided comparable data (32.2%).
The previous imatinib therapy received by participants in each of the four high-dose imatinib studies is summarised in Table 8. The duration of prior imatinib therapy ranged from 0.6 to 70 months (median 18 to 36 months) in the single-arm cohort studies, and from < 1 year to > 3 years in the RCT (not reported more precisely). 7 In two of the single-arm cohort studies all participants had previously received only standard-dose imatinib (400 mg/day). 8,9 In the remaining single-arm cohort study the majority of participants (90%) had received 400 mg/day, although 10% (the accelerated- and blast-phase participants) received 400–600 mg/day. 10 In the RCT the majority of participants (69%) had received 600 mg/day and the remainder (29%) received 400 mg/day (one participant received 500 mg/day). In addition to imatinib, the majority of participants in the RCT had received hydroxycarbamide or anagrelide (93.9%) and/or interferon alfa (67.3%), with some (36.7%) having received chemotherapy or, in a minority of cases (4.1%), stem cell transplantation. Two single-arm cohort studies reported that, in addition to imatinib, participants had previously received interferon alfa (one study, 29.7%) or hydroxycarbamide (one study, % not stated) only.
| Study | Arm | n | Median (range) duration of prior imatinib therapy or n (%) of participants per duration class | Highest prior imatinib dose (mg/day) | Prior therapy (%) | |||
|---|---|---|---|---|---|---|---|---|
| Chemotherapy | HU | IFN-α | SCT | |||||
| Kantarjian et al. (2009)7 | 1. HDI | 49 |
< 1 year: 5 (10%) 1–3 years: 29 (59%) > 3 years: 15 (31%) |
400 (n = 14) (29%) 500 (n = 1) (2%) 600 (n = 34) (69%) |
36.7 | 93.9a | 67.3 | 4.1 |
| 2. Dasatinib | 101 |
< 1 year: 12 (12%) 1–3 years: 44 (44%) > 3 years: 45 (45%) |
400 (n = 36) (36%) 500 (n = 2) (2%) 600 (n = 63) (62%) |
38.6 | 96.0a | 73.3 | 6.9 | |
| Breccia et al. (2010)9 | 1. HDI | 74 | 36 months (21–70) | 400 (n = 74) (100%) | 0 | 0 | 29.7b | 0 |
| Koh et al. (2010)10 | 1. HDI | 71 | 14.6 months (0.6 to 52.8) |
CP: 400 (n = 64) (90%) AP and BC: 400–600 (n = 7) (10%) |
Not reported | Not reported | Not reported | Not reported |
| Rajappa et al. (2010)8 | 1. HDI | 90 | 18 months (3 to 48) | 400 (n = 90) (100%) | 0 | Yes; % not reported | 0 | 0 |
The characteristics of the studies reviewed are shown in the PenTAG AR2 (see pp. 57–75).
Critical appraisal of included evidence
A summary of the critical appraisal of the RCT is provided in the PenTAG AR2 (see section 3.2.3.1, p. 84). As noted in the PenTAG AR,2 the RCT is flawed, which has implications for interpreting effectiveness and safety information. The updated publication by Kantarjian and colleagues7 provides new information on two aspects of the RCT methodology that were not reported in the previous publications and which therefore do not currently appear in the PenTAG AR:2
-
Kantarjian and colleagues7 explained how the sample size was calculated. However, the approach, which is based on arbitrary maximum widths of confidence intervals (CIs) for the primary outcome, was not considered in relation to the statistical power of the trial. This explanation appears to be an attempt to justify the sample size retrospectively.
-
Kantarjian and colleagues7 stated that dasatinib and high-dose imatinib groups were stratified by study site and cytogenetic response (CyR) on previous imatinib.
In the PenTAG AR,2 analyses conducted in the RCT were considered appropriate. Although the statistical methods used were generally appropriate, the way in which they were applied does have serious shortcomings. Specifically, the analyses were not planned a priori and were not adjusted for multiple comparisons. However, data from the individual arms are not compared in this report.
Overall, the new information available from the Kantarjian and colleagues publication7 does not alter the judgement that the RCT was substantially flawed. Of particular relevance to the high-dose imatinib arm of the RCT was that 80% of high-dose imatinib participants with inadequate responses crossed over to the dasatinib arm at a median time of 13 weeks (range 1–68 weeks). Conversely, 20% of participants with inadequate responses to dasatinib crossed over to the high-dose imatinib arm at a median time of 28 weeks (range 1–56 weeks). As a result, outcomes for the high-dose imatinib arm reported at a median of 26 months would include an unknown (not reported) proportion of participants who had predominantly received dasatinib. Interpretation of the outcome data for high-dose imatinib in the RCT is also difficult because follow-up times varied considerably and were reported only as the median and range.
Critical appraisal of the three single-arm cohort studies8–10 of high-dose imatinib is summarised in Table 9. The assessment criteria in Table 9 reflect aspects of study design relevant to interpretation of generalisability and some types of bias, which may help to assess the relative strengths and weaknesses of the individual studies. All three studies have risk of selection bias owing to a lack of any randomised procedures for allocation to study groups, and risk of performance bias owing to a lack of allocation concealment and blinding.
| Indicator | Breccia et al. (2010)9 | Koh et al. (2010)10 | Rajappa et al. (2010)8 |
|---|---|---|---|
| Is the hypothesis/aim/objective of the study clearly described? | Yes | Yes | Yes |
| Were the case series collected at more than one centre? | Yes | Yes | Not reporteda |
| Was the main outcome independently assessed? | Not reported | Not reported | Not reported |
| Are patient characteristics adequately described? | Yes | Yes | Nob |
| Are adequate details provided to assess the generalisability of the results? | Noc | Yesd | Noa |
| Are inclusion and exclusion criteria clearly reported? | No | Yes | Yes (but limited) |
| Were data collected prospectively? | Not reported | Yes | Not reported |
| Were patients recruited consecutively? | Not reported | Not reported | Yes |
| Did all the participants receive the same intervention? | Noe | No e | Yesf |
| Is the use of any concurrent therapies adequately described? | No | No | No |
| Was an ITT analysis performed? | Unclear g | Unclearg | Unclear g |
| Were dropouts from the trial adequately described? | No | Yes | No |
The reporting of these single-arm cohort studies was generally superficial. 8–10 Only the study by Koh and colleagues10 could be clearly identified as a prospective study, although the other two studies were judged to be prospective by reviewers. 8,9 Only the study by Rajappa and colleagues8 reported whether or not participants were recruited consecutively. Breccia and colleagues9 and Rajappa and colleagues8 failed to adequately report the inclusion criteria for their studies, which is a major impediment to interpreting generalisability and selection bias. Although generalisability of the study by Koh and colleagues10 appears to be stronger than for the other two studies, none of the single-arm cohort studies was conducted in the UK. It is, therefore, unclear how relevant the findings from these studies would be to UK patients with chronic-phase CML.
In these single-arm cohort studies the participants within a study did not all receive exactly the same intervention, as dose escalations occurred at different times for individual participants, or subgroups of participants received different dose changes. It is unclear whether or not any participants received concurrent therapies alongside high-dose imatinib, as this was not reported in any of the three studies. None of the studies reported explicitly whether or not all participants allocated to treatment were analysed and whether or not the analyses included attrition [intention-to-treat (ITT) approach]. Only one of the studies, by Koh and colleagues10 adequately reported participant attrition.
Overall, owing to the inherent limitations of a single-arm study design, compounded by generally poor reporting of the methodology, the three single-arm cohort studies of high-dose imatinib appear to be at high risk of bias and limited or unclear relevance to CML patients in a UK setting.
Relationship of identified evidence to research question
The research questions could not be directly addressed by the PenTAG AR2 (see section 3.2.4, p. 90), as there was no comparative evidence available. Similarly, in our update of the PenTAG AR2 for those with imatinib-resistant CML we have not identified any comparative evidence for any of the three interventions of interest. Therefore, caution continues to be recommended in the interpretation of the evidence now presented.
Evidence reported in this review is relevant to patients with chronic-phase CML only. Owing to the paucity of data the review has been unable to consider whether or not the level of previous response to imatinib has any bearing on outcome, and the evidence does not allow the adoption of an early stopping rule to be considered.
Effectiveness of dasatinib: update of Peninsula Technology Assessment Group assessment report
As described earlier, the updated searches identified one study that provided additional data on the effectiveness of dasatinib (Kantarjian and colleagues 2009;7 Appendix 4). A 2007 publication of this RCT6 was described in the PenTAG AR2 (see pp. 91–8), and the following outcomes have been superseded by the 2009 updated publication:7
-
CyR and duration of CyR
-
adverse events (AEs).
In addition, the current report presents data on the following outcomes, which were not reported by the PenTAG AR:2
-
molecular response
-
proportion without treatment failure at 24 months.
There is no difference in the data for the following outcome reported both in 20076 and in 2009:7
-
CHR (see PenTAG AR,2 pp. 106–12).
The PenTAG AR2 presented updated results from the manufacturer’s submission or from conference abstracts for the following outcomes, and the update publication7 does not change the PenTAG AR2 for:
-
CHR rate in participants who had no CHR at baseline (see PenTAG AR,2 p. 109)
-
estimated progression-free survival at 24 months (see PenTAG AR,2 p. 118).
Cytogenetic response
Complete cytogenetic response improved from 39.6% at median 15 months’ follow-up to 43.6% at median 26 months’ follow-up (Table 10). Major cytogenetic response was similar between the two follow-up periods (52.5% at 15 months6 and 53.5% at 26 months7). Major cytogenetic response was similar between patients with (34/62, 55%) and without (20/39, 51%) a previous CyR on standard-dose imatinib.
| Study | Length of follow-up | Dose (mg) | CCyR (%) | PCyR (%) | MCyR (%) |
|---|---|---|---|---|---|
| Kantarjian et al. (2007)6 | 15a | 70 b.i.d. | 40/101 = 39.6 | 13/101 = 12.9 | 53/101 = 52.5 |
| 24b,c | 70 b.i.d. | 44/101 = 43.6 | |||
| Kantarjian et al. (2009)7 | 26d | 70 b.i.d. | 44/101 = 43.6 | 10/101 = 9.9 | 54/101 = 53.5 (95% CI 43.3 to 63.5) |
Duration of major cytogenetic response
The 2007 publication reported the probability of a maintained response at 1 year as being 0.98. 6 With longer follow-up, the proportion with a maintained major cytogenetic response at 18 months was 90% (95% CI 82% to 98%). 7
Major molecular response
Kantarjian and colleagues7 reported a major molecular response (MMR) in 28.7% of participants (29/101) receiving dasatinib, and in 63.4% (28/44) of those who had a complete cytogenetic response and a molecular response assessment (MMR: generally defined as ≥ 3-log reduction in the level of BCR–ABL transcripts or a BCR–ABL ratio of ≤ 0.05%).
Time to treatment failure and proportion without treatment failure
Median time to treatment failure was not reached with dasatinib in the 2007 publication by Kantarjian and colleagues,6 but was not reported in the 2009 study with longer follow-up. 7 However, the authors reported that the estimated proportion of participants without treatment failure at 24 months was 59%. 7
Adverse events
The PenTAG AR2 (see p. 121) stated that in most of the included evidence, neutropenia and thrombocytopenia each affected in the order of 50% ± 10% of individuals taking dasatinib. The proportion of individuals affected by neutropenia in the RCT is slightly higher with longer follow-up7 (Table 11).
| Event | Kantarjian et al. (2007),6 70 mg b.i.d. | Kantarjian et al. (2009),7 70 mg b.i.d. |
|---|---|---|
| n | 101 | 101 |
| Anaemia | 19.8 | |
| Leucopenia | 23.8 | |
| Neutropenia | 61.4 | 63.4 |
| Thrombocytopenia | 56.4 | 57.4 |
The PenTAG AR2 (see p. 122) described the AEs (of any grade) most commonly reported by its included studies as diarrhoea, dyspnoea, fatigue, headache, nausea, pleural effusion, and rash, at frequencies in the range 10%–40%. The 2009 update7 also reports fluid retention (39%), bleeding (18%), infection (14%) and upper respiratory tract infection or inflammation (11%), and an increase in superficial oedema from 15% to 20% (Table 12).
| Adverse event | Kantarjian et al. (2007),6 70 mg b.i.d. (n = 101) | Kantarjian et al. (2009),7 70 mg b.i.d. (n = 101) |
|---|---|---|
| Abdominal pain | 15 | |
| Anorexia | 12.9 | 17 |
| Asthenia | 12.9 | 15 |
| Bleeding | 18 | |
| Diarrhoea | 34.7 | 37 |
| Dyspnoea | 20.8 | 23 |
| Face oedema | 4.0 | |
| Fatigue | 29.7 | 33 |
| Fluid retention | 39 | |
| Headache | 24.8 | 26 |
| Infection | 14 | |
| Muscle spasms | 2.0 | |
| Musculoskeletal pain | 21 | |
| Nausea | 23.8 | 24 |
| Pain in extremity | 6.9 | |
| Peripheral oedema | 9.9 | |
| Pleural effusion | 16.8 | 25 |
| Pyrexia | 13.9 | 14 |
| Rash | 16.8 | 18 |
| Superficial oedema | 14.9 | 20 |
| Upper respiratory tract infection or inflammation | 11 | |
| Vomiting | 8.9 | 10 |
| Weight increase | 5.0 |
The PenTAG AR2 (see p. 122) reported that grades 3–4 AEs appeared to be fairly rare in the included studies, with only dyspnoea and pleural effusion occurring in more than 5% of participants in any of the included studies. However, grades 3–4 fluid retention occurred in 7% of individuals in the 2009 update publication7 (Table 13).
| Adverse event | Kantarjian et al. (2007),6 70 mg b.i.d. (n = 101) | Kantarjian et al. (2009),7 70 mg b.i.d. (n = 101) |
|---|---|---|
| Abdominal pain | 0 | |
| Anorexia | 0.0 | 0 |
| Asthenia | 0.0 | 0 |
| Bleeding | 1 | |
| Diarrhoea | 2.0 | 3 |
| Dyspnoea | 4.0 | 5 |
| Face oedema | 0.0 | |
| Fatigue | 2.0 | 3 |
| Fluid retention | 7 | |
| Headache | 2.0 | 2 |
| Infection | 4 | |
| Muscle spasms | 0.0 | |
| Musculoskeletal pain | 1 | |
| Nausea | 0.0 | 0 |
| Pain in extremity | 0.0 | |
| Peripheral oedema | 0.0 | |
| Pleural effusion | 4.0 | 5 |
| Pyrexia | 0.0 | 0 |
| Rash | 0.0 | 0 |
| Superficial oedema | 0.0 | 1 |
| Vomiting | 0.0 | 0 |
| Weight increase | 0.0 |
Rates of treatment discontinuation due to AEs in the four studies included in the PenTAG AR2 (see p. 124) that reported this outcome were described as ranging from approximately 5% to 15%. Discontinuations due to AEs increased from 15.8% in the 2007 Kantarjian and colleagues publication6 to 22.8% in the 2009 publication7 (Table 14).
| Study | Length of follow-up (months) | Dose (mg) | Discontinuations |
|---|---|---|---|
| Kantarjian et al. (2007)6 | 15a | 7 b.i.d. | 16/101 = 15.8 % |
| Kantarjian et al. (2009)7 | 26b | 70 b.i.d. | 23/101 = 22.8 % |
Summary of effectiveness of dasatinib
-
No new studies of dasatinib were identified by the updated searches.
-
Additional follow-up data for some outcomes were available for the RCT by Kantarjian and colleagues 6 and included in the PenTAG AR. 2
-
The RCT was methodologically flawed, with a high level of crossovers between treatment arms. As such, the individual treatment arms were considered separately as non-comparative evidence. This is in line with the approach taken by the PenTAG AR. 2
-
Complete cytogenetic response improved slightly from 39.6%6 to 43.6%,7 and major cytogenetic response was similar (52.5%6 to 53.5%7) with longer follow-up.
-
A MMR was reported in 28.7% of participants. 7
-
Additional AEs were reported with longer follow-up (fluid retention, bleeding, infection, upper respiratory tract infection or inflammation), and grades 3–4 fluid retention occurred in 7% of individuals in the update paper. 7
Effectiveness of high-dose imatinib
Cytogenetic response
Table 15 provides a summary of the available data detailing CyR to high-dose imatinib in CML (see Appendix 4 for further details). 6–10 All four studies were in participants with chronic-phase CML, with the exception of one study which also included a small number of participants in accelerated-phase CML (n = 3) and blast-crisis CML (n = 4). 10 Three studies reported complete, partial and major cytogenetic response rates or provided enough information to enable the deduction of each, whereas one study reported only complete cytogenetic response and major cytogenetic response. Minor and minimal responses were not reported by these studies. The definition of response for each category was consistent across studies.
| Study | Length of follow-up (months) | Dose (mg) | CCyR (%) | PCyR (%) | MCyR (%) |
|---|---|---|---|---|---|
| Breccia et al. (2010)9 | 36, median | 600 or 800 | 27/74 = 36.4 | 47/70 = 63.5a | |
| Kantarjian et al. (2007),6 (2009)7 | 15b | 400 b.i.d. | 8/49 = 16.3 | 8/49 = 16.3 | 16/49 = 32.7 |
| 26c | 400 b.i.d. | 9/49 = 18.4 | 7/49 = 14.3 | 16/49 = 32.7 | |
| dKoh et al. (2010)10 | 6 | 600 or 800e | 16/71 = 22.5 | 14/71 = 19.7 | 30/71 = 42.3 |
| 12 | 600 or 800e | 17/71 = 23.9 | 11/71 = 15.5 | 28/71 = 39.4 | |
| Rajappa et al. (2010)8 | 18f | 800 mg | 25/90 = 27.7 | 10/90 = 11.1 | 35/90 = 39.0 |
It should be noted that some participants already had some degree of CyR at baseline. Rajappa and colleagues8 reported that 44.5% of participants had achieved major cytogenetic response (although it is unclear whether this is the proportion at study entry)8 and 43.7% of participants were in partial cytogenetic response in the study by Koh and colleagues. 7 None of the participants in the high-dose imatinib arm of the RCT by Kantarjian and colleagues7 was in major cytogenetic response at baseline. However, 44% (15/34) of participants with a previous CyR on standard-dose imatinib achieved a major cytogenetic response on high-dose imatinib, whereas 7% (1/15) of those without a previous CyR on standard-dose imatinib achieved a major cytogenetic response. CyR was not reported at baseline by Breccia and colleagues. 9
Complete cytogenetic response rates ranged from 18.4%7 to 36.4%. 9 Major cytogenetic response rates ranged from 32.7%7 to 63.5%. 9
Duration of major cytogenetic response
Kantarjian and colleagues7 reported that 74% (95% CI 49% to 100%) of individuals maintained their major cytogenetic response at 18 months. This outcome was not reported by the other three studies. 8–10
Haematological response
Only two of the studies reporting CHR provided a definition,7,9 and there were minor differences between these definitions (Table 16).
| Study | Definition |
|---|---|
| Breccia et al. (2010)9 |
WBC count < 10 × 109/l with no immature cells in the peripheral blood Platelet count < 450 × 109/l Disappearance of all signs and symptoms related to leukaemia |
| Kantarjian et al. (2007),6 (2009)7 |
WBCs ≤ institutional ULN Platelets < 450 × 109/l No blasts or promyelocytes in peripheral blood < 5% myelocytes plus metamyelocytes in peripheral blood No extramedullary involvement (including no hepatomegaly or splenomegaly) |
| Rajappa et al. (2010)8 | Not reported |
Table 17 provides a summary of the available data detailing HR to high-dose imatinib in CML. Three of the included studies reported CHR, with response rates ranging from 55.5% (18-month follow-up)8 to 91.8% (36-month follow-up). 9 It should be noted that 55.1% of participants in the high-dose imatinib arm of the RCT by Kantarjian and colleagues were in CHR at study entry,7 but this was not reported by the other two studies. The CHR in participants without CHR at baseline was 16/22 = 72%. 7
| Study | Length of follow-up (months) | Dose (mg) | Proportion with response |
|---|---|---|---|
| Breccia et al. (2010)9 | 36 median | 600 or 800 | 68/74 = 91.8 % |
| Kantarjian et al. (2007),6 (2009)7 | 15a | 400 b.i.d. | 40/49 = 81.6 % |
| 26b | 400 b.i.d. | 40/49 = 81.6 % | |
| Rajappa et al. (2010)8 | 18c | 800 | 50/90 = 55.5 % |
Duration of major or complete haematological response
These were not reported by the included studies.
Molecular response
Three studies reported molecular response and, as can be seen in Table 18, the definitions of molecular response varied between the studies. A summary of molecular response to high-dose imatinib can be seen in Table 19. Kantarjian and colleagues7 reported a MMR in 12.2% of participants receiving high-dose imatinib, and in 55.6% (5/9) of those who had a complete cytogenetic response and a molecular response assessment. A complete molecular response was found in 13.5% of participants by Breccia and colleagues,9 whereas Koh and colleagues reported that 56.3% of participants achieved a molecular reduction > 50% within 6 months. 10
| Study | Outcome reported | Definition |
|---|---|---|
| Breccia et al. (2010)9 | MMR | BCR–ABL/ABL ratio < 0.1% |
| Complete molecular response | BCR–ABL/ABL ratio < 0.001 | |
| Kantarjian et al. (2007),6 (2009)7 | MMR | BCR–ABL level ≤ 0.1 on the international scale based on standard methodology (references cited) |
| Koh et al. (2010)10 | Early molecular response | A molecular reduction > 50% within 6 months |
| Study | Length of follow-up (months) | Dose (mg) | Complete | Major | Early |
|---|---|---|---|---|---|
| Breccia et al. (2010)9 | 36, median | 600 or 800 | 10/74 = 13.5 % | ||
| Kantarjian et al. (2007),6 (2009)7 | 15b | 400 b.i.d. | 2/49 = 4.1 % | ||
| 26c | 400 b.i.d. | 6/49 = 12.2 % | |||
| aKoh et al. (2010)10 | 6 | 600 or 800d | 40/71 = 56.3 % | ||
| 12 | 600 or 800d | Not reported |
Time to treatment failure
Median time to treatment failure was 18.0 months (range not reported) for all participants (n = 71) in the study by Koh and colleagues. 10 For chronic phase participants (n = 64) it was 27 months, for accelerated-phase participants (n = 3) it was 2.5 months and for blast-crisis-phase participants (n = 4) it was 4.0 months. 10 Median time to treatment failure was 3.5 months (95% CI 3.3 to 3.8 months) with high-dose imatinib in the 2007 publication by Kantarjian and colleagues,6 but was not reported in the 2009 study with longer follow-up. 7 However, the authors reported that the estimated proportion of participants without treatment failure at 24 months was 18%. 7
Progression-free or event-free survival
Three of the included studies reported progression-free survival (progression-free survival) or event-free survival. 6–9 These are reported together, as the definitions, although differing slightly (Table 20), appear to be measuring similar outcomes. Table 21 provides a summary of the available data detailing progression-free survival with high-dose imatinib. Rajappa and colleagues8 reported estimated event-free survival of 34% at 2 years, whereas a higher estimated progression-free survival is reported by the other two studies (65%7 and 87%9).
| Study | Definition |
|---|---|
| Breccia et al. (2010)9 |
PFS Defined from the time of the start of imatinib to progression to an advanced phase of the disease |
| Kantarjian et al. (2007),6 (2009)7 |
PFS Defined as the time from randomisation until: |
| Rajappa et al. (2010)8 |
Event-free survival Time from dose escalation to loss of CHR or CCyR Failure to achieve CHR at 3 months Progression to AP or BC No CyR at 6 months Less than MCyR at 12 months No CCyR at 18 months or death from any cause |
| Study | Follow-up (months) | Dose (mg) | n | 6 months | 12 months | 18 months | 24 months |
|---|---|---|---|---|---|---|---|
| Breccia et al. (2010)9 | 36, median | 600 or 800 | 74 | 0.87 | |||
| Kantarjian et al. (2007),6 (2009)7 | 15a | 400 b.i.d. | 49 | 0.73 | 0.73 | ||
| 26b | 400 b.i.d. | 49 | 0.65 | ||||
| Rajappa et al. (2010)8 | 18 | 800 | 90 | 0.34 |
Overall survival
Two studies reported overall survival (Table 22) of individuals in chronic-phase CML,8,9 although the definitions differ. Breccia and colleagues9 defined overall survival as the time from diagnosis to death or date of last follow-up, and reported an estimated 2-year overall survival of 85%. Rajappa and colleagues8 defined overall survival as time from dose escalation to death owing to any cause, and reported an estimated 2-year overall survival of 93%.
Adverse events
Haematological AEs were reported by all included studies (Table 23). Breccia and colleagues9 reported a low proportion of participants experiencing anaemia and neutropenia (grade not reported). The other three studies reported grades 3–4 haematological AEs, with anaemia occurring in 8%7 to 30%8 of participants, neutropenia in 18%10 to 39%7,8 of participants, leucopenia in 16%7 to 31%8 of participants, and thrombocytopenia in 0%10 to 21%8 of participants.
| AE | All grades | Grades 3 and 4 | ||
|---|---|---|---|---|
| Breccia et al. (2010),9 600 or 800 mg (n = 74) | Kantarjian et al. (2009),7 400 mg b.i.d. (n = 49) | aKoh et al. (2010),10 600 or 800 mg (n = 71) | Rajappa et al. (2010),8 800 mg (n = 90) | |
| Anaemia | 0, 2b | 8 | 16.9 | 30 |
| Leucopenia | 16 | 31 | ||
| Neutropenia | 0, 3b | 39 | 18.3 | 39 |
| Thrombocytopenia | 14 | 0 | 21 | |
The most commonly reported AEs of any grade were anorexia, diarrhoea, fatigue, muscle spasms, musculoskeletal pain, nausea, superficial or peripheral oedema and rash (Table 24); however, the reported proportions varied between the studies. Grades 3 and 4 AEs appeared to be fairly rare, with none occurring in more than 4% of the cohort in the two studies reporting this outcome. 7,10
| AE | All grades | Grades 3 and 4 | ||||
|---|---|---|---|---|---|---|
| Breccia et al. (2010),9 600 or 800 mg (n = 74) | Kantarjian et al. (2009),7 400 mg b.i.d. (n = 49) | aKoh et al. (2010),10 600 or 800 mg (n = 71) | Rajappa et al. (2010),8 800 mg (n = 90) | Kantarjian et al. (2009),7 400 mg b.i.d. (n = 49) | aKoh et al. (2010),10 600 or 800 mg (n = 71) | |
| Abdominal pain | 8 | 2 | ||||
| Anorexia | 8 | 29 or 26b | 0 | |||
| Asthenia | 4 | 0 | ||||
| Bleeding | 8 | 0 | ||||
| Diarrhoea | 29 | 27 | 2 | |||
| Dyspnoea | 4 | 0 | ||||
| Dyspepsia | 14 | |||||
| Fatigue | 22 | 30 | 4 | |||
| Headache | 10 | 2 | ||||
| Infection | 6 | 0 | ||||
| Muscle spasms | 20, 30c | |||||
| Musculoskeletal pain | 12 | 39 | 2 | |||
| Mucositis/oral ulcers | 10 | |||||
| Nausea | 33 | 0 | ||||
| Nausea/vomiting | 11 | |||||
| Oedema | 2.8 | 2.8 | ||||
| Peripheral oedema | 35, 40c | |||||
| Pleural effusion | 0 | 0 | ||||
| Pyrexia | 10 | 0 | ||||
| Rash | 20 | 27 | 0 | |||
| Superficial oedema | 43 | 61 | 0 | |||
| Upper respiratory tract infection or inflammation | 6 | 0 | ||||
| Vomiting | 24 | 0 | ||||
Treatment discontinuation due to AEs was reported by three6–8,10 of the four studies and ranged from 0%10 to 20.4%7 (Table 25).
| Study | Length of follow-up (months) | Dose (mg) | Discontinuation |
|---|---|---|---|
| Kantarjian et al. (2007),6 (2009)7 | 15a | 400 b.i.d. | 9/49 = 18.4 % |
| 26b | 400 b.i.d. | 10/49 = 20.4 % | |
| cKoh et al. (2010)10 | 12 | 600 or 800 | 0/71 = 0 %d |
| Rajappa et al. (2010)8 | 18 | 800 | 3/90 = 3.3 % |
Summary of effectiveness of high-dose imatinib
-
Four studies (one RCT and three single-arm cohort studies) provided data on the effectiveness of high-dose imatinib.
-
The RCT had serious methodological flaws. A high proportion (80%) of participants in the high-dose imatinib arm of the RCT crossed over to the alternative treatment after a median duration of 13 weeks. As such, the individual treatment arms were considered as non-comparative evidence. This is in line with the approach taken in the PenTAG AR. 2
-
The single-arm studies appear to be at high risk of bias and may be of limited relevance to CML patients in a UK setting.
-
Complete cytogenetic response was achieved by 18–36% of individuals.
-
Major cytogenetic response was achieved by 33–64% of individuals.
-
One study reported that around three-quarters of individuals maintained their major cytogenetic response at 18 months.
-
Complete haematological response was achieved by 56–92% of individuals.
-
Event-free survival of 2 years or more occurred in only 34% of individuals in one study. progression-free survival was estimated as 65–87% in two other studies.
-
Overall survival was reported by two studies, which found that 85–93% of people are expected to survive 2 years or more.
-
Haematological AEs (grades 3–4) occurred in up to 40% of individuals.
-
Non-haematological events also occurred, with anorexia, diarrhoea, fatigue, muscle spasms, musculoskeletal pain, superficial oedema and rash reported in varying proportions.
-
Grades 3–4 non-haematological AEs were fairly rare, with none occurring in more than 5% of individuals.
-
Between 0% and 20% of study participants discontinued high-dose imatinib owing to AEs.
-
These results should be interpreted with caution owing to the methodological limitations of the included studies.
Chapter 4 Economic analysis
Systematic review of existing cost-effectiveness evidence
The aim of this section is to assess through a systematic review of the literature the cost-effectiveness of dasatinib, high-dose imatinib and nilotinib compared with each other and with other treatment options in participants with CML resistant to standard-dose imatinib.
Methods of the systematic review
The methods used for the systematic review, including the search strategy, inclusion criteria and data extraction, are shown in Chapter 2. Quality assessment was undertaken using a critical appraisal checklist adapted by the review authors from checklists by Philips and colleagues,12 Drummond and colleagues13 and the NICE reference case requirements. 14
Quantity of existing cost-effectiveness literature
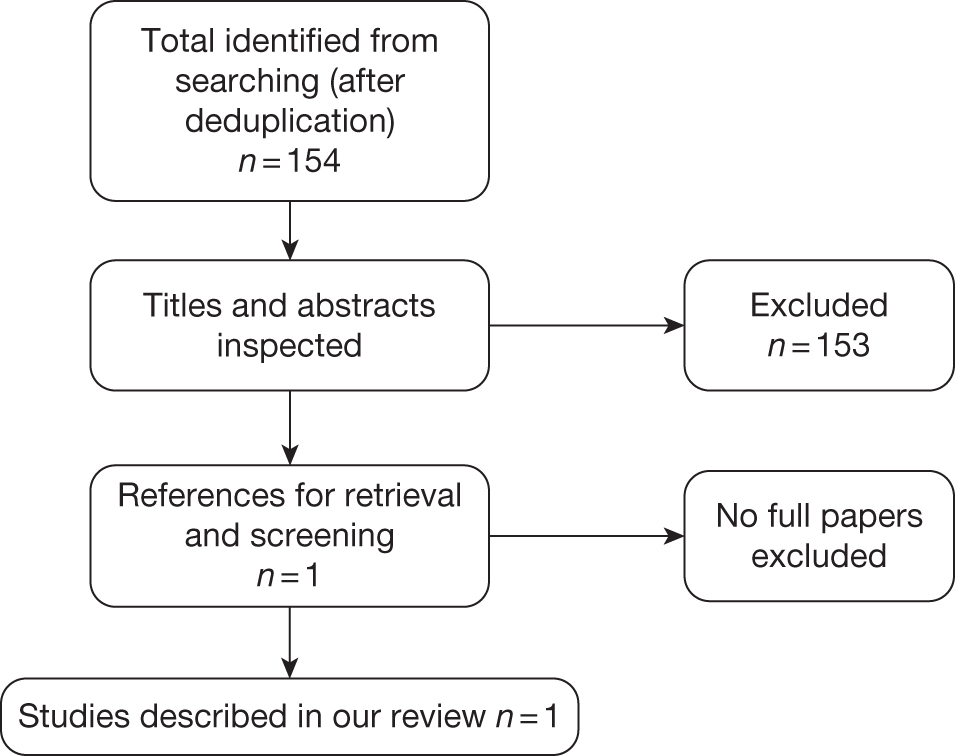
A total of 154 potentially relevant references were identified by the cost-effectiveness searches. Of these, the full text of one paper was retrieved and this study met the a priori inclusion criteria. A summary of the selection process is presented in Figure 2. Full data extraction of the study is given in Appendix 5.
FIGURE 2.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness.
Critical appraisal of the economic evaluation
The included cost-effectiveness study by Ghatnekar and colleagues15 was assessed against the critical appraisal checklist (Table 26).
| Item | Question | Ghatnekar and colleagues (2010)15 | Comments |
|---|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes | |
| 2 | Is the comparator routinely used in UK NHS? | Yes | |
| 3 | Is the patient group in the study similar to those of interest in the UK NHS? | Yes | |
| 4 | Is the health-care system comparable to the UK? | Unclear | Swedish system |
| 5 | Is the setting comparable to the UK? | Unclear | Swedish practice |
| 6 | Is the perspective of the model clearly stated? | Yes | |
| 7 | Is the study type appropriate? | Yes | |
| 8 | Is the modelling methodology appropriate? | Yes | |
| 9 | Is the model structure described and does it reflect the disease process? | Yes | |
| 10 | Are assumptions about the model structure listed and justified? | Unclear | Not listed but some in text |
| 11 | Are the data inputs for the model described and justified? | Yes | |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | No | Phase II trial |
| 13 | Are health benefits measured in QALYs? | Yes | |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | Yes | Reported as EQ-5D |
| 15 | Are the resource costs described and justified? | Yes | |
| 16 | Have the costs and outcomes been discounted? | Yes | |
| 17 | Has uncertainty been assessed? | Yes | |
| 18 | Has the model been validated? | Unclear | No details given |
The cost-effectiveness study appears credible, but its generalisability to the UK is uncertain. The use of a surrogate outcome (response to treatment) is less than ideal, but appears to be accepted practice in the study of CML (see subsequent discussion of models by manufacturers and PenTAG2).
Description and results of the published economic evaluation
Ghatnekar and colleagues15 conducted an economic evaluation on the cost-effectiveness of dasatinib treatment versus high-dose imatinib in chronic-phase CML patients in Sweden who were resistant to standard-dose imatinib. The characteristics of the study, which was funded by BMS,3 are shown in Table 27.
| Author | Ghatnekar and colleagues (2010)15 | ||
|---|---|---|---|
| Publication year | 2010 | ||
| Country | Sweden | ||
| Funding source | BMS | ||
| Study type | Cost–utility analysis | ||
| Perspective | Societal | ||
| Study population | Patients confirmed to be resistant to lower doses of imatinib (≤ 600 mg) | ||
| Intervention(s) |
Dasatinib: 140 mg/day Imatinib: 800 mg/day |
||
| Intervention effect |
Response to treatment No response: dasatinib 7.9% patients, imatinib 18.4% patients CHR: dasatinib 57.4% patients, imatinib 53.1% patients PCyR: dasatinib 13.9% patients, imatinib 20.4% patients CCyR: dasatinib 20.8% patients, imatinib 8.2% patients |
||
| Intervention cost |
Monthly costs (€) Imatinib 800 mg/day 4869 Dasatinib 140 mg/day 4239 |
||
| Currency base | € (2008) | ||
| Model type, health states | Markov model with patients starting in CP, who after 12-week initial treatment period can progress to AP then BC then death; probabilities by response to treatment | ||
| Time horizon | Lifetime | ||
| Baseline cohort | Patients in CP-CML confirmed to be resistant to lower doses of imatinib (≤ 600 mg) aged 60 years | ||
| Base-case results | Dasatinib | HDI | |
| Total direct costs | €350,960 | €346,507 | |
| Total societal costs | €504,532 | €500,281 | |
| LYs | 6.37 | 5.69 | |
| QALYs | 5.19 | 4.57 | |
| ICER (LYs, societal) | €6332 | ||
| ICER (QALYs, societal) | €6880 | ||
| ICER (LYs, direct) | €6645 | ||
| ICER (QALYs, direct) | €7207 | ||
The results from the analysis are expressed in incremental cost per quality-adjusted life-year (QALY) gained. In line with Swedish clinical guidelines, both costs and benefits are presented with a lifetime societal perspective and discounted by 3% per year. Costs were in euros and the price year was 2008. The study used benefits in terms of response to treatment taken from a clinical trial of patients in chronic-phase CML who were resistant to standard doses of imatinib.
Modelling approach of Ghatnekar and colleagues
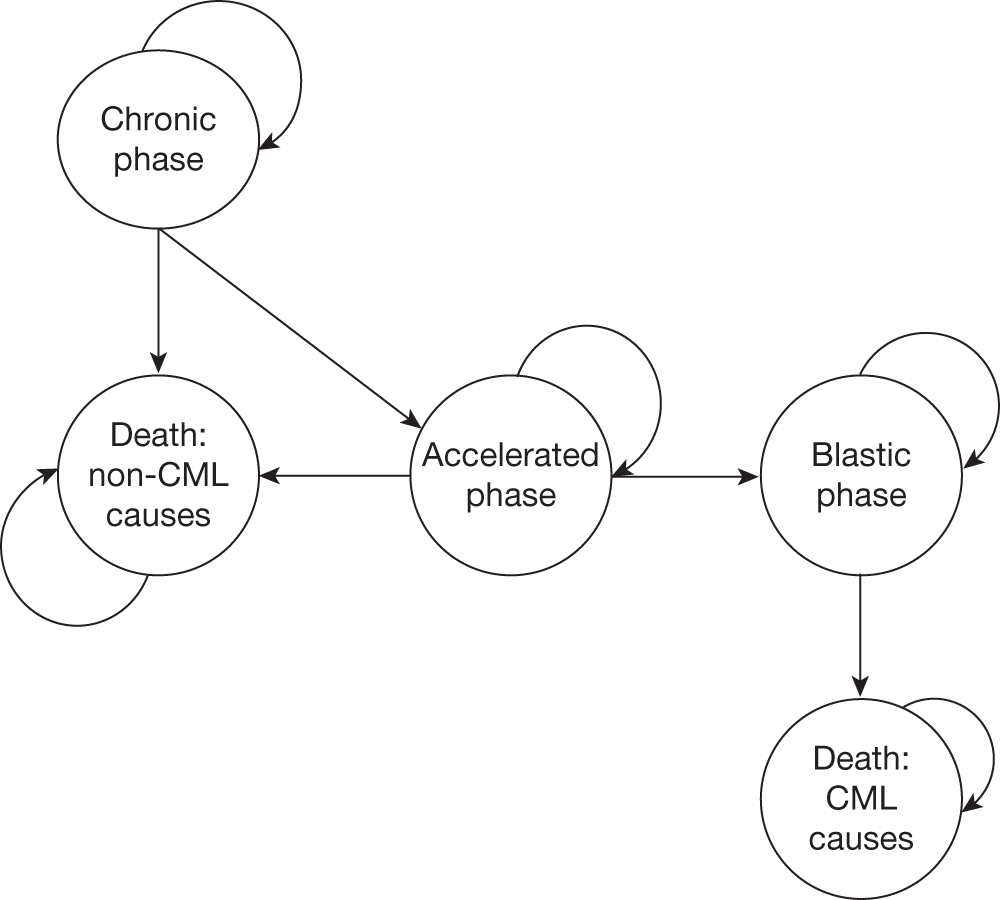
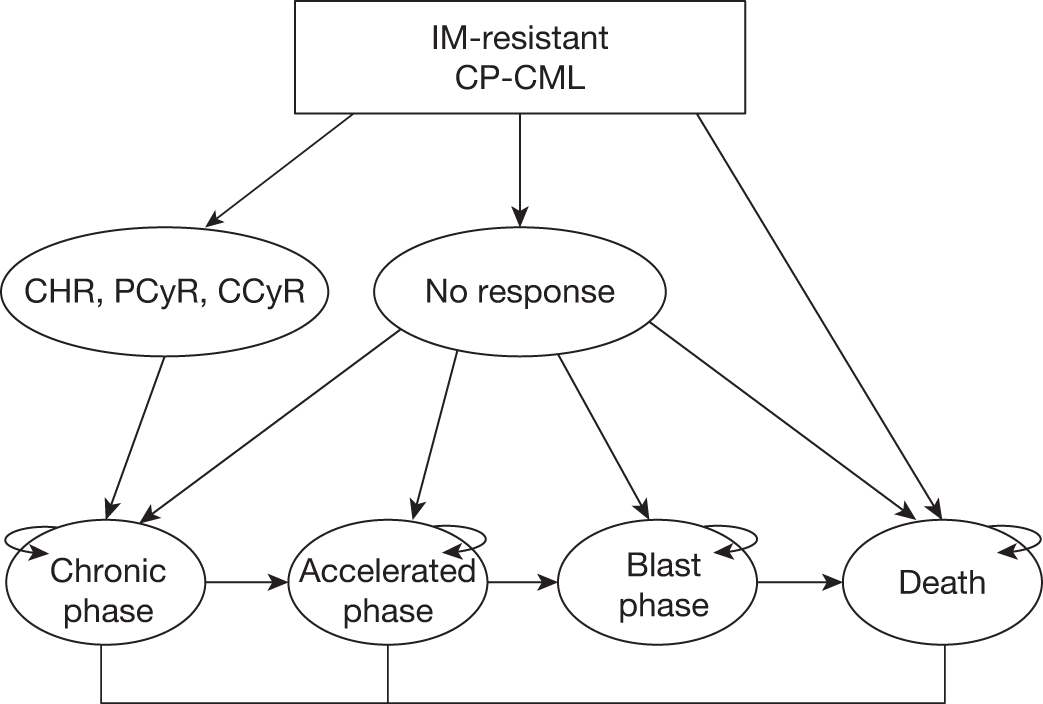
A Markov cost-effectiveness model was developed to calculate the costs and effects associated with dasatinib treatment compared with high-dose imatinib among patients who were confirmed to be resistant to lower doses (≤ 600 mg) of imatinib (Figure 3). The model is an adaptation to Swedish treatment practice of a model developed for the Scottish Medicines Consortia. It uses monthly cycles with probabilities of a health state change, and all patients were assumed to start treatment in chronic phase. The response to treatment after an initial 12-week treatment period determines the disease progression within the four health states: chronic phase, accelerated phase, blast-crisis phase and death (from either CML- or non-CML-related causes). At each monthly cycle the patients face the probability of staying in the same health state or moving to the next. Progression data are taken from several published sources. Patients enter the model at the age of 60 years.
FIGURE 3.
Markov model structure starting with imatinib-resistant chronic-phase CML (adapted from Ghatnekar et al. 15). CCyR, complete cytogenetic response (typically defined as no Ph+ chromosomes in metaphase in bone marrow); CP-CML, chronic-phase chronic myeloid leukaemia; PCyR, partial cytogenetic response (typically defined as between 1% and 35% of Philadelphia-positive (Ph+) chromosomes in metaphase in bone marrow).
Assumptions made by Ghatnekar and colleagues
-
The better the initial response to treatment, the slower the expected cohort disease progression.
-
It is not possible to move from chronic phase to blast-crisis phase directly; the probability of CML-related death is dependent on the health state and the treatment response of the patient.
-
Utilities are assumed to be the same for both study groups.
-
Adverse event rates are limited to the first month only; no disutility weights are used for AEs and patients are assumed to continue with study medication.
-
Patients are treated until disease progression.
Effectiveness of intervention
The effectiveness outcome of the interventions used in the model was best initial response rate, taken from a 12-week head-to-head clinical trial of dasatinib versus high-dose imatinib (Kantarjian and colleagues,6 see Chapter 3, Effectiveness of dasatinib: update of Peninsula Technology Assessment Group assessment report). The proportions of patients in the dasatinib group and the high-dose imatinib group, respectively, were 7.9% and 18.4% for no response; 57.4% and 53.1% for CHR; 13.9% and 20.4% for partial cytogenetic response; and 20.8% and 8.2% for complete cytogenetic response.
Estimation of quality-adjusted life-years
Utility weights for each health state were reported to have been elicited from a time trade-off (TTO) technique using the European Quality of Life-5 Dimensions (EQ-5D) instrument among 100 laypersons in the UK and applied to both the dasatinib and imatinib arms by Levy and colleagues. 16 For the base-case analysis these were 0.90 for chronic phase responder, 0.72 for chronic phase non-responder, 0.53 for accelerated phase and 0.29 for blast-crisis phase.
Estimation of costs
The average number of resources used per patient and month in each health state was elicited from two Swedish clinical haematologists. Direct health care-related costs for drugs and other health-care resources were taken from published Swedish statistics. Costs for study medication are added each month the patient is in chronic phase. Inpatient costs correspond to a bed-day at a haematological clinic plus one haematologist visit per day. The cost of thrombocyte transfusion is based on a regional cost-per-patient study, inflated to year 2008.
Indirect costs in terms of production loss were estimated using the human capital approach, with an average monthly salary for individuals aged 45–64 years including payroll taxes of 41%. The workforce participation was assumed to be 85% among patients with CML patients who were under 65 years, recommended by clinical experts, as not all patients are in the labour market for reasons other than CML diagnosis. The expected increase in public consumption owing to extended survival resulting from either treatment was included in the analysis [increased survival costs equal total consumption less total production during life-years gained (LYGs), according to Swedish guidelines on economic evaluation].
Cost-effectiveness results
The results showed that patients with chronic-phase CML, who are resistant to standard-dose imatinib gain, on average, 0.67 LYs or 0.62 QALYs when treated with dasatinib compared with high-dose imatinib. The incremental societal cost amounts to €4250 during the lifetime period or €6880 per QALY gained. The indirect costs of production losses and increased public consumption almost cancel out.
In the one-way sensitivity analysis, dasatinib is a dominant treatment option in a 10-year time horizon (both cost saving and generating more benefit). Probabilistic sensitivity analysis results fall below the derived willingness to pay (WTP) for a QALY in Sweden.
Summary of key issues
-
It is unclear how generalisable the model parameters and results are to the UK as the study was conducted in Sweden and takes a societal perspective. In particular, non-medical costs are included, and it is unclear what the results would be if the study were adapted for the UK.
-
Adequate details are provided on the model structure and the methodology used, and results obtained seem credible.
-
There are some methodological limitations and uncertainties, such as the use of a surrogate measure of effectiveness (response rate) from a single Phase II trial and probability data are taken from a range of sources.
-
No base treatment has been used so the study is slightly different from the scope of this appraisal.
-
Patients receive treatment until disease progression.
SHTAC assessment of the manufacturers’ submissions and the PenTAG assessment report evaluation
A structured data extraction form was used to guide the review of the submissions to NICE from Novartis4 (nilotinib) and BMS3 (dasatinib), respectively (see Appendix 5), and also the PenTAG AR2 economic evaluation to aid the interpretation of the subsequent update. Characteristics of the submitted economic evaluations are shown in Table 28, with critical appraisal in Table 29. This is followed by description of the methodology used, results and key issues.
| Author | Novartis 20104 | BMS3 | PenTAG AR2 |
|---|---|---|---|
| Study population | Patients with standard-dose imatinib-resistant CP-CML | Patients with CML who are resistant to imatinib | Patients resistant to imatinib with CP-CML |
| Intervention(s) |
Nilotinib: 800 mg/day HDI: 800 mg/day SCT: Allo-SCT as third-line therapy if appropriate HU: 2 g/day as third-line therapy SCT/HU: second-line exploratory analysis |
Dasatinib: CP 100 mg/day AP/BC 140 mg/day Nilotinib: 800 mg/day Imatinib: doses increased to 800 mg per day in the absence of SAE |
Dasatinib: CP 100 mg/day Nilotinib: 800 mg/day Imatinib: doses increased to 800 mg/day in the absence of SAE IFN-α: 8.65 MU/day |
| Intervention effect |
OS and TTD Nilotinib: 24-month OS 86%; duration of treatment: not stated HDI: 12-month OS 96%; 24-month OS 84%; duration of treatment 14 months SCT: 5-year OS 34% HU: 5-year OS 16%; survival in AP, 9.14 months; survival in BC, 9.89 months |
Dasatinib: 8.1% NR, 33.1% CHR, 15.3% PCyR, 43.5% CCyR Imatinib 400 mg: 100% NR Imatinib 600 mg: 56.4% NR, 15.4% CHR, 28.2% PCyR, 0% CCyR Imatinib 800 mg: 32.1% NR, 13.3% CHR, 14.2% PCyR, 40.5% CCyR Nilotinib: 6.0% NR, 35.0% CHR, 18.0% PCyR, 41.0% CCyR IFN-α: 100% NR. SCT: 100% NR |
MCyR Dasatinib: 58.1% Nilotinib: 52.4% HDI: 44% IFN-α: 22% PFS Dasatinib: 0.77 at 24 months Nilotinib: 0.864, 0.769, and 0.632 at 6, 12 and 18 months HDI: 0.81, 0.57, 0.29 at 12, 24 and 48 months Survival in AP: 9.64 months Survival in BC: 13.12 months |
| Intervention cost |
Quarterly costs Nilotinib: £7928 HDI: £10,490 HU (2 g daily): £38.00 Allo-SCT (first 100 days) £79,380 |
Monthly costs Dasatinib: £2,504.96 Imatinib 400 mg: £1604.08 Imatinib 600 mg: £2406.12 Imatinib 800 mg: £3208.16 Nilotinib: £2613.05 |
Two-month cycle Dasatinib: £5080 Imatinib: £6505 Nilotinib: £5286 IFN-α: £1486 |
| Model type | Markov model to simulate the transition of a hypothetical cohort of 1000 patients in CP who progress to AP, then BC, then death | Markov model to predict the health changes and resulting costs for patients starting dasatinib treatment in each of the three phases of CML: CP, AP and BC | Survival model to predict duration, QALYs and costs, in CP (on or off treatment), AP and BC, and OS in hypothetical cohort of 1000 patients in CP |
| Baseline cohort | An equal number of male and female patients aged 57 years |
Patients starting in CP: age 56 years, 50% male Patients starting in AP: age 56 years, 56% male Patients starting in BC: age 48 years (myeloid), 49 years (lymphoid) |
A cohort of 1000 patients with an assumed age of 56 years |
| Base-case results |
Nilotinib dominates HDI (i.e. is less costly and more effective than HDI) ICER for nilotinib vs SCT/HU is £44,028 |
Dasatinib dominates HDI, nilotinib and SCT |
ICER for nilotinib vs IFN-α is £44,616 Nilotinib dominates HDI ICER for dasatinib vs nilotinib is £277,698 |
| Item | Question | Novartis4 | BMS3 | PenTAG2 |
|---|---|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes | Yes | Yes |
| 2 | Is the comparator routinely used in UK NHS? | Yes | Yes | Unclear |
| 3 | Is the patient group in the study similar to those of interest in the UK NHS? | Yes | Yes | Yes |
| 4 | Is the health-care system comparable to the UK? | Yes | Yes | Yes |
| 5 | Is the setting comparable to the UK? | Yes | Yes | Yes |
| 6 | Is the perspective of the model clearly stated? | Yes | Yes | Yes |
| 7 | Is the study type appropriate? | Yes | Yes | Yes |
| 8 | Is the modelling methodology appropriate? | Yes | Yes | Yes |
| 9 | Is the model structure described and does it reflect the disease process? | Yes | Yes | Yes |
| 10 | Are assumptions about the model structure listed and justified? | Yes | Yes | Yes |
| 11 | Are the data inputs for the model described and justified? | Yes | Yes | Yes |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | Yes | Yes | Yes |
| 13 | Are health benefits measured in QALYs? | Yes | Yes | Yes |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | Yes | Yes | Yes |
| 15 | Are the resource costs described and justified? | Yes | Yes | Yes |
| 16 | Have the costs and outcomes been discounted? | Yes | Yes | Yes |
| 17 | Has uncertainty been assessed? | Yes | Yes | Yes |
| 18 | Has the model been validated? | Unclear | Unclear | Yes |
Characteristics of manufacturers’ models and the PenTAG assessment report model
Novartis
The Novartis submission4 includes an economic evaluation on the cost-effectiveness of nilotinib versus high-dose imatinib for the treatment of adult patients with chronic-phase CML who are resistant to prior standard-dose imatinib therapy. An exploratory analysis versus stem cell transplantation/hydroxycarbamide is also presented in an appendix. Extrapolations within the analysis are based on time to discontinuation (TTD) of treatment and overall survival to predict lifetime costs, QALYs and LYs. All analyses were conducted from a UK NHS and Personal Social Services (PSS) perspective using a lifetime horizon, with costs and benefits discounted at a rate of 3.5%. Costs were in UK pounds and the price year was 2009–10.
Bristol-Myers Squibb
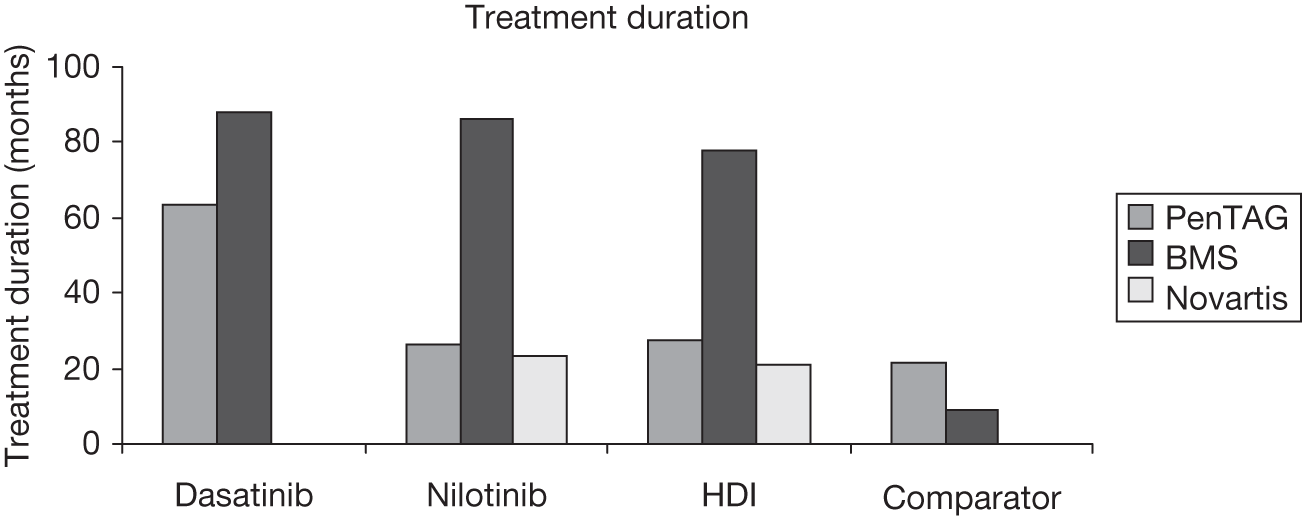
The BMS submission3 includes an economic evaluation on the cost-effectiveness of dasatinib, nilotinib and high-dose imatinib compared with standard-dose imatinib, allo-stem cell transplantation, hydroxycarbamide, interferon alfa, acute leukaemia-style chemotherapy and best supportive care, for patients with CML who are resistant to imatinib. The model uses best initial response to treatment to predict QALYs, progression-free survival and LYs. Analyses are conducted from a UK NHS and PSS perspective, using a 40-year horizon with costs and benefits discounted at 3.5%. Costs are presented in UK pounds for the base year 2009.
PenTAG assessment report
The PenTAG AR2 evaluation includes an economic evaluation on the cost-effectiveness of dasatinib and nilotinib compared with high-dose imatinib for patients with CML who are resistant to imatinib. An appendix considers these treatments compared with interferon alfa. The model uses best initial response to treatment to predict overall survival, and trial data are extrapolated for treatment duration and progression-free survival. Analyses are conducted from a UK NHS and PSS perspective using a lifetime horizon with costs and benefits discounted at 3.5%. Costs are presented in UK pounds for the base year 2009–10.
Critical appraisal of submitted models
In general, all three economic evaluations were appropriately conducted according to the checklist used to assess study quality. However, there are concerns with some of the data used to populate the models and therefore the reliability of the results, which is acknowledged in all three reports. The models are discussed, in turn, below, followed by a comparison of the three approaches and their results.
Description of each of the modelling approaches and results
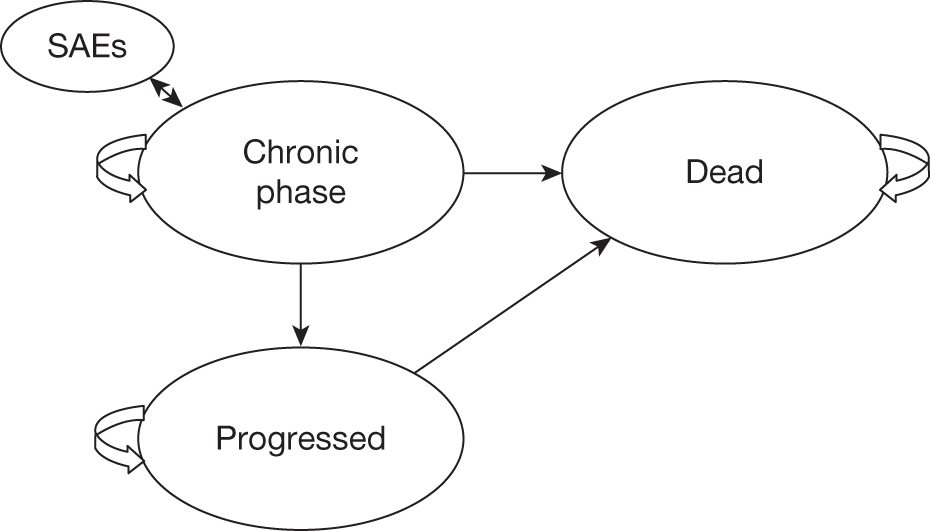
Novartis
Modelling approach
A Markov model was developed to simulate the transition of a hypothetical cohort of 1000 patients resistant to standard-dose imatinib over their lifetime (Figure 4). The cycle length was 1 month for the first six cycles and then 3 months. An equal number of male and female patients in chronic phase, aged 57 years, enter the model. At each cycle, patients have the probability of remaining in chronic phase or progressing to accelerated-phase CML. Patients failing on second-line treatment may remain in chronic phase and receive further treatment (stem cell transplantation if eligible or hydroxycarbamide) before progressing to accelerated phase/blast-crisis phase. Patients can then progress from accelerated phase to blast-crisis phase and, finally, from blast-crisis phase to death. Patients are able to remain in chronic phase, accelerated phase or blast-crisis phase for more than one cycle and they may die from other causes in chronic phase and accelerated phase. Patients may die from CML only in blast-crisis phase. On progression to accelerated phase, patients receive hydroxycarbamide, based on clinical opinion. TTD and overall survival were used to predict lifetime costs, LYG and QALYs.
Assumptions used in the Novartis model4
-
All patients in either the nilotinib arm or high-dose imatinib arm are assumed to receive treatment until treatment failure, when it is assumed they receive allo-stem cell transplantation as a third-line option, if eligible, otherwise hydroxycarbamide; this is assumed to occur before progression to accelerated phase. Patients in both arms who progress to accelerated phase or blast crisis receive hydroxycarbamide.
-
All patients are assumed to have died of CML or other causes by the age of 100 years.
-
Patients may stop taking nilotinib prior to progression to the next phase of treatment, so TTD of treatment is used in the model, rather than progression-free survival, to provide an estimate of time on nilotinib.
-
It was assumed that 10% of patients who discontinued treatment owing to AEs would progress from chronic phase to accelerated phase.
-
Utilities were assumed to be independent of drug therapy and time; also utility values for accelerated phase and blast-crisis phase were assumed to be the same.
Estimation of effectiveness
Overall survival for nilotinib was estimated as 86% from the clinical study (CAMN107A2101) at 24 months’ follow-up. TTD is a Kaplan–Meier estimate of duration of exposure defined as the time difference (days) between the first dose and last dose. Long-term survival was extrapolated from the study data using an exponential curve as this provided a good fit to current data. Overall survival and TTD data were taken from a study by Kantarjian and colleagues17 for high-dose imatinib.
To model stem cell transplantation after failure of nilotinib or high-dose imatinib the outcome of stem cell transplantation was based on a risk score in order to take account of patient characteristics. Thus, the model uses outcomes based on patients with a risk factor score of 4 with a 5-year survival of 34%. 18 Time on hydroxycarbamide following nilotinib failure was estimated based on the time in chronic phase following discontinuation of nilotinib or imatinib, by considering the difference between progression-free survival and TTD curves. An exponential curve was fitted to this difference and provided an estimate of 5-year survival of 16%. The effectiveness of hydroxycarbamide is assumed to be the same regardless of the positioning of hydroxycarbamide in the treatment pathway and the same data are used for the exploratory analysis.
Estimation of quality-adjusted life-years
Utility values were assigned to the different health states derived from a study by Reed and colleagues19,20 [the International randomized study of interferon versus ST1571 (IRIS)] using the EQ-5D. A utility decrement was used for patients experiencing grade 3 or 4 AEs during the first 18 months of treatment in chronic phase, taken from evidence in the nilotinib clinical trial (CAMN107A2101). A decrement was applied to the long-term utility for 52% patients following stem cell transplantation.
The utility values for chronic phase, accelerated phase and blast-crisis phase were 0.854, 0.595 and 0.595, respectively. Disutilities for AEs, taken from various sources, were 0.049 for nilotinib, 0.027 for high-dose imatinib, 0.00 for hydroxycarbamide and 0.079 for stem cell transplantation. The authors assumed the same utilities for both the accelerated-phase/blast-crisis-phase health states.
Estimation of costs
Costs, adjusted to 2009–10 prices, include routine appointments (taken from NHS reference costs 2006/7),21 end-of-life care and treatment for managing AEs. The costs of the drugs were taken from the British National Formulary (BNF) 2010, except for high-dose imatinib, which used a cost that reflected a future cost increase (commercial in confidence at time of review). Where published data were not available, advice was sought from clinical experts. Most of the uncertainty was around the costs for stem cell transplantation; however, this was stated not to affect the overall results given that upon failure of nilotinib or high-dose imatinib patients follow a similar treatment pathway.
The quarterly cost of nilotinib is £7928 and of high-dose imatinib is £10,490.
Cost-effectiveness results
The results for patients with chronic-phase CML who are resistant to imatinib indicate that nilotinib dominates high-dose imatinib, i.e. nilotinib is less costly and more effective than high-dose imatinib. The incremental cost per QALY gained is –£30,513 (Table 30). In the exploratory analysis compared with stem cell transplantation/hydroxycarbamide, the incremental cost per QALY gained for nilotinib is £44,028.
| Intervention | Costs (£) | LYs (£) | QALYs (£) | ICER (LYG) (£) | ICER (QALY) (£) |
|---|---|---|---|---|---|
| HDI | 146,234 | 5.53 | 4.28 | ||
| Nilotinib | 139,216 | 5.80 | 4.51 | –26,006 | –30,513 |
| SCT/HU | 80,933 | 4.21 | 3.18 | 36,748 | 44,028 |
A range of efficacy assumptions, health utilities, costs and other parameters were considered in sensitivity analyses. For the deterministic sensitivity analyses, most incremental cost-effectiveness ratios (ICERs) are close to the base-case result of –£30,000, except for the 5-year time horizon, which gives an ICER of –£82,000 (due to delayed treatment benefit), and for extending high-dose imatinib TTD from 14 months to 19.4 months, which gives an ICER is £201,871 (higher costs of high-dose imatinib treatment with marginal QALY gain for high-dose imatinib vs nilotinib).
Probabilistic sensitivity analysis was undertaken to explore the impact of joint uncertainty in all model parameters on the cost-effectiveness results. The uncertainty around costs was represented based on the interquartile ranges presented within the NHS reference costs where available. Costs were assumed to have a gamma distribution. Quality of life (QoL) of the health states was varied using the uncertainty reported by Reed and colleagues. 19,20 The uncertainty within the TTD curves was based on beta distributions, with alpha and beta parameters representing the number of people who have discontinued and not discontinued, respectively. The uncertainty relating to nilotinib and high-dose imatinib is assumed to be correlated. Results give an ICER of –£86,413 per QALY gained. From cost-effective acceptability curves nilotinib is predicted to be cost-effective at a threshold of over £10,000 per QALY.
Summary of key issues
-
The evaluation compared nilotinib, high-dose imatinib and stem cell transplantation/hydroxycarbamide, but did not include dasatinib.
-
It is not clear if stem cell transplantation/hydroxycarbamide is an appropriate comparator.
-
The derivation of parameters, such as progression to accelerated phase and blast-crisis phase, is poorly explained.
-
Overall survival estimates appear low, but the reasons for this are unclear.
Bristol-Myers Squibb
Modelling approach
A Markov model was developed to predict the health changes and resulting costs for patients starting dasatinib treatment in each of the three phases of CML: chronic phase, accelerated phase and blast-crisis phase (Figure 5). It is not stated whether this is a newly developed model or has been adapted from a previously reported model. The model uses monthly cycles.
The authors state that the Markov process was considered appropriate, as it allows the incorporation of the three disease phases, different response categories and the different rates of disease progression characteristic of CML, and the approach used has been used in previous economic analyses in CML.
For chronic phase and accelerated phase, the model consists of three health states: ‘stable disease’, ‘progressed disease’ and ‘death’. For blast-crisis phase, the model consists of two states: ‘stable disease’ and ‘death’. In each health state, five types of response to treatment are used in the model: no response to treatment (NR); achieve CHR; achieve partial cytogenetic and CHR (partial cytogenetic response); achieve complete cytogenetic and CHR (complete cytogenetic response); and achieve a molecular response (MR).
The modelling incorporates two stages: (1) initial assessment of the patient’s initial best response to treatment and (2) determination of the prognosis of the patient, based on his or her initial best response. Initial best response rates were based on clinical trial data and, in some cases, clinical opinion. As such, once the patients’ initial best response has been determined, they enter a specific ‘submodel’ that links response with long-term prognosis rates (e.g. progression-free survival, overall survival). The prognosis rates for different response groups were based on evidence from the BMS 034 trial. 22
Assumptions used in the Bristol-Myers Squibb3 model
-
Response to treatment is assessed in the initial period; after that, it is assumed to remain at the same level until disease progression.
-
The efficacy of 800 mg imatinib is equivalent to 600 mg in accelerated phase and blast-crisis phases.
-
The efficacy of standard-dose imatinib and interferon alfa is zero.
-
Patients cannot return to the chronic phase from advanced phases of CML.
-
The probability of progressing to the next CML phase and death was estimated from the progression-free survival and overall survival data for patients in a dasatinib trial (i.e. BMS trial 034). 22 The probability of progression or death was (other than by response) independent of treatment.
-
Beyond the trial period, progression rates were assumed to remain constant, at a rate equal to that during the final year of follow-up.
-
After failing imatinib, dasatinib or nilotinib, patients receive post-failure treatment (PFT).
-
Progression rates and other input parameters for patients receiving PFT are assumed equal to those used for non-responders.
-
Patients receiving PFT incur the cost, but not the utility benefits.
-
Utility values do not change over time, as long as the patient remains in the same health state.
-
Where utility estimates for serious adverse events (SAEs) were not available from the non-CML literature a 5% (–0.05) decrement was assumed.
-
Where resource use associated with an AE was not known, a cost of £100 was assumed.
-
Monthly cost of bone marrow stem cell transplantation is based on an aggregate figure to reflect the average costs for different prognoses post stem cell transplantation.
-
Different utility values were used for response and no response groups.
Effectiveness of the interventions
Effectiveness data used in the model are the patient’s initial best response to treatment, which was taken from various studies for the different interventions and is shown in Table 31.
| CP | No response (%) | CHR (%) | PCyR (%) | CCyR (%) | Survive SCT (%) | Mortality (%) | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| Dasatinib22 | 8.1 | 33.1 | 15.3 | 43.5 | 0.0 | 0.0 | 24 |
| Imatinib 400 mga | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA |
| Imatinib 600 mg17 | 56.4 | 15.4 | 28.2 | 0.0 | 0.0 | 0.0 | 12 |
| Imatinib 800 mg23 | 32.1 | 13.3 | 14.1 | 40.5 | 0.0 | 0.0 | 61 |
| Nilotinibb | 6.0 | 35.0 | 18.0 | 41.0 | 0.0 | 0.0 | 19 |
| IFNa | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA |
| Bone marrow SCTb | 0.0 | 0.0 | 0.0 | 0.0 | 65.0 | 35.0 | 25 |
The progression-free survival rates associated with each level of response (as observed in the BMS 034 trial)22 are shown in Table 32.
| Month | No response (%) | CHR (%) | PCyR (%) | CCyR (%) | MCyR (%) |
|---|---|---|---|---|---|
| 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 6 | 30.0 | 94.9. | 100.0 | 100.0 | 99.7 |
| 12 | 30.0 | 84.1 | 94.4 | 98.2 | 98.2 |
| 18 | 30.0 | 77.7 | 83.3 | 98.2 | 97.5 |
| 24 | 30.0 | 63.6 | 83.3 | 94.2 | 94.2 |
| 30 | 30.0 | 55.9 | 83.3 | 94.2 | 94.2 |
| 36 | 30.0 | 38.7 | 77.8 | 94.2 | 94.2 |
| 42 | 25.8 | 25.8 | 71.3 | 94.2 | 94.2 |
| 48 | 24.1 | 25.8 | 59.4 | 94.2 | 93.9 |
Estimation of quality-adjusted life-years
A cross-sectional study was commissioned to calculate utility values for the purpose of the BMS analysis3 (Szabo and colleagues 201024). Ratings for health states and response were elicited from a representative sample of 100 unaffected individuals in the UK using the TTO method and the EQ-5D instrument. The impact of the SAEs on health utility was captured in the model using utility decrements identified in the non-CML literature. Utility values were 0.85 for chronic phase with response, 0.68 for chronic phase no response, 0.79 for accelerated-phase response, 0.50 for accelerated phase no response, 0.50 for blast-crisis-phase response and 0.31 for blast-crisis phase no response. However, there was a mistake in the BMS model3 so that for the accelerated-phase/blast-crisis-phase health states only the value for blast-crisis phase no response was used.
Estimation of costs
Drug costs were estimated based on the recommended doses from their Summary of Product Characteristics and prices were from the BNF (2009). Monthly costs for the interventions were £2504, £3208 and £2613 for dasatinib, high-dose imatinib and nilotinib, respectively (see Table 28), and £863 for interferon alfa and £2400 for stem cell transplantation.
Direct costs were also included for outpatient visits, tests, hospitalisation and other interventions (such as blood transfusion). AE costs were included for treatment-related grades 3–4 SAEs, the most common being neutropenia, thrombocytopenia and leucopenia.
Cost-effectiveness results
The results of the base-case analyses for chronic-phase CML are shown in Table 33. Dasatinib dominates high-dose imatinib, nilotinib and stem cell transplantation. The total cost for dasatinib is £314,413 and the total number of QALYs is 6.425.
| Dasatinib vs: | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|
| Imatinib 400 mg | 179,087 | 4.940 | 36,251 |
| Imatinib 600 mg | 140,707 | 4.031 | 34,907 |
| Imatinib 800 mg | –35,952 | 0.515 | Dasatinib dominant |
| Nilotinib | –4565 | 0.190 | Dasatinib dominant |
| IFN-α | 185,121 | 4.762 | 38,877 |
| SCT | –9821 | 1.687 | Dasatinib dominant |
Parameters used in the model, which were varied in the deterministic sensitivity analysis, were costs, utilities, starting age, time horizon and discounting. The key impact factors were the utility of responders, starting age and the time horizon of the model. However, the sensitivity analyses were not presented in the normal way and are difficult to interpret.
For the probabilistic sensitivity analysis, 1000 iterations were performed, with beta distributions used for probabilities and gamma distributions for costs. For initial response to treatment, the beta distribution was parameterised from the evidence in the selected clinical trials; for survival rates, one single random ‘seed’ was generated and subsequently used to generate the values for the model. The results of the probabilistic sensitivity analysis showed that the probability of dasatinib being cost-effective compared with stem cell transplantation was 81% for a WTP of £30,000. Cost-effectiveness acceptability curves were not presented for all the drugs together and results were not shown for the probability that dasatinib was cost-effective compared with all of its alternatives.
Summary of key issues
-
There is uncertainty around the approach taken with respect to initial best response and extrapolation from this to progression-free survival, with values based on a single trial for surrogate outcomes (using a submodel).
-
A number of assumptions have been made, such as best response rate for imatinib, because of lack of data.
-
Patients are treated until progression, which does not appear to be correct and affects treatment duration and costs.
PenTAG assessment report evaluation
Modelling approach
The PenTAG AR2 cost-effectiveness analysis used a survival model developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The model closely resembles a Markov state-transition approach, using an ‘area under the curve’ method. The number of patients in each state of the model over time is determined by using survival curve data to apportion the overall cohort population between the states at each successive cycle of the model. The structure of the model is shown in Figure 6. The model comprises the following health states: chronic phase (on treatment), chronic phase (following discontinuation of treatment), accelerated phase, blast-crisis phase and death. Patients in chronic-phase CML have either major cytogenetic response or no major cytogenetic response. Patients enter the model in chronic-phase CML and then subsequently progress to accelerated phase, then to blast-crisis phase and then, finally, to death from CML causes. Patients may also die in the chronic phase and accelerated phase states from non-CML causes.
FIGURE 6.
Structure for the PenTAG AR2 CML cost-effectiveness model. Major cytogenetic response (MCyR) typically defined as ≥ 35% Ph+ chromosomes in metaphase in bone marrow (study definitions may vary).
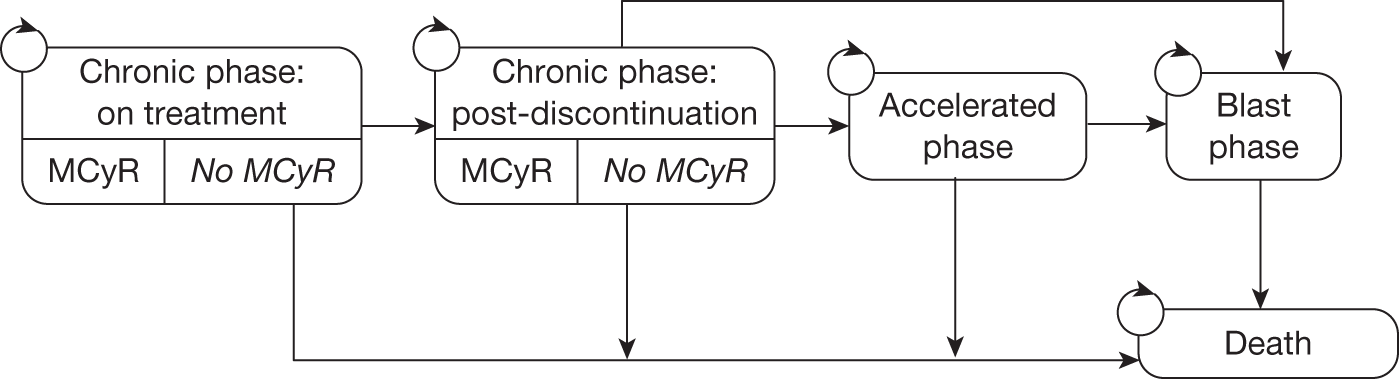
Overall survival is estimated for each of the treatments on the basis of the proportion of major cytogenetic response (responders), with survival duration estimated for major cytogenetic response responders and major cytogenetic response non-responders. A hazard ratio for overall survival of non-responders versus responders was pooled from several studies (hazard ratio = 0.37).
Patients move between the states chronic phase (on treatment) and chronic phase (no treatment) according to estimates for the progression-free survival and the number of individuals who prematurely discontinue treatment. Patients were assumed to have no drug costs in the chronic phase (no treatment) state. Patients spend a predefined time for each of the accelerated-phase and blast-crisis-phase states. The time spent in the chronic phase (no treatment) was adjusted by subtracting the durations for the chronic phase (treatment), accelerated-phase and blast-crisis-phase states from the overall survival duration. Utility values were applied to each of the treatment states to estimate the total QALYs for each of the treatments.
Assumptions in the PenTAG assessment report
-
Overall survival is predicted on the basis of major cytogenetic response and the relationship between major cytogenetic response and overall survival is the same for all treatments, and not affected by the timing, duration and depth of CyR. The hazard ratio for the overall survival for the major cytogenetic response versus non-major cytogenetic response groups is based upon first-line therapy and is still valid for second-line treatments, and is constant over time.
-
Duration of treatment is estimated on the basis of progression-free survival with a deduction to account for premature discontinuations.
-
Times spent in accelerated phase and blast-crisis phases is independent of chronic-phase treatment, i.e. is identical across comparators.
-
Treatment-related AEs incur no utility decrement and no additional costs.
-
Duration of chronic phase (no treatment) is estimated by deducting time spent in chronic-phase (treatment), accelerated-phase and blast-crisis-phase states from overall survival.
Effectiveness of the interventions
A summary of the clinical effectiveness parameter estimates used in the PenTAG AR2 model is shown in Table 34. Parameter estimates were derived through a clinical effectiveness review. Overall survival was estimated for each of the treatments according to the proportion with major cytogenetic response. The data for major cytogenetic response used in the PenTAG AR2 model differed slightly from those reported in the clinical effectiveness sources (which are shown in parentheses). Overall survival rates for responders and non-responders were calibrated using a retrospective study by Jabbour and colleagues23 of the long-term efficacy of high-dose imatinib in a population that had failed standard-dose imatinib, as this source provided the longest available estimates of overall survival for responders and non-responders. For interferon alfa, overall survival was derived using an estimate for the major cytogenetic response rate from the IRIS trial. 19,20
| Parameter | Estimate, PenTAG AR2 (SHTAC) | Sourcea | Justification |
|---|---|---|---|
| MCyR rate: dasatinib | 58.1% (59%) | Shah et al. (2008)a | Only available estimate at currently recommended dose of dasatinib |
| MCyR rate: nilotinib | 52.4% (56%) | Kantarjian et al. (2007)6 | Only available estimate |
| MCyR rate: HDI | 44.0% (54%) | Jabbour et al. (2009)23 | Estimates of OS, PFS and MCyR rate all originate from the same study |
| MCyR rate: IFN-α | 22% | O’Brien et al. (2003)b | Most recent large trial of IFN-α |
| Proportion of patients who discontinue dasatinib prematurely | 10.2% | Shah et al. (2008)a | Treatment discontinuations are included in PFS |
| Proportion of patients who discontinue nilotinib prematurely | 23.2% | Kantarjian et al. (2007)6 | Only available estimated |
| Proportion of patients who discontinue HDI prematurely | 14.8% | Pooled estimate | Pooled from all available data |
| Overall survival hazard ratio between people who achieve a MCyR to imatinib and those who do not | 0.370 | Pooled estimate | Pooling all data sources reduces the uncertainty in the data |
| Mean time spent in AP | 0.80 years | Reed et al. (2004)19 and Cervantes et al. (1996)c | Large, relevant data source |
| Mean time spent in BC | 1.09 years | Reed et al. (2004)19 and Kantarjian et al. (2001)d | Large, relevant data source |
Progression-free survival was derived through fitting survival curves to available progression-free survival data. progression-free survival for dasatinib was estimated from a single data point of 0.77 corresponding to 24-month progression-free survival provided in the submission BMS3 made to NICE at that time. progression-free survival for nilotinib was estimated from the submission Novartis4 made to NICE at that time: 0.864, 0.769 and 0.632 for progression-free survival probability at 6, 12 and 18 months, respectively. progression-free survival for high-dose imatinib was derived from Jabbour and colleagues’23 single-centre retrospective study: 0.865, 0.81, 0.71, and 0.57 for progression-free survival probability at 6, 12, 18 and 24 months, respectively.
Estimation of quality-adjusted life-years
The PenTAG AR2 reviewed QoL studies to use in the economic model. From these data, the authors chose the IRIS study by Reed and colleagues,20 which was also used by Dalziel and colleagues25 in a previous assessment of imatinib for CML, as the most appropriate. These data were drawn from a large sample of patients and reported EQ-5D values. The utility values used for the health states are chronic phase (treatment or no treatment) 0.85; accelerated phase 0.73; and blast crisis 0.52.
Estimation of costs
Cost estimates in the economic evaluation include drug costs, administration costs of interferon alfa and cytarabine, outpatient visits, bone marrow tests, radiography, computerised tomography (CT) scans, blood transfusion and inpatient terminal care. All costs were inflated to 2009–10 values where necessary. The cost of treating patients with AEs has not been modelled. The drug costs are taken from the BNF and are shown above in Table 28. The dosage use differed from that suggested in the BNF, and the usage was taken from the relevant clinical studies. Although the dosage for dasatinib and nilotinib was not affected, dosage for high-dose imatinib (738 mg/day) and interferon alfa (4.8 million units per day) was lower.
Cost-effectiveness results
The results presented in the main PenTAG AR2 are supplemented with additional analyses in appendices, which include the comparator interferon alfa. Table 35 presents the aggregated totals for the base-case model results for the four treatments. Outputs are shown for total LYs (undiscounted), and total discounted QALYs and costs for each treatment over the time horizon of the model.
| Dasatinib | Nilotinib | HDI | IFN | |
|---|---|---|---|---|
| LYs: mean, undiscounted | ||||
| CP treated | 6.50 | 2.44 | 2.68 | 2.04 |
| CP not treated | 5.00 | 8.65 | 7.79 | 6.82 |
| AP | 0.80 | 0.80 | 0.80 | 0.80 |
| BC | 1.09 | 1.09 | 1.09 | 1.09 |
| Total (mean) | 13.40 | 12.98 | 12.37 | 10.75 |
| Total (median) | 10.76 | 10.21 | 9.45 | 7.75 |
| QALYs: mean, discounted | ||||
| CP treated | 4.50 | 1.89 | 2.10 | 1.27 |
| CP not treated | 2.62 | 5.00 | 4.46 | 4.16 |
| AP | 0.37 | 0.38 | 0.38 | 0.41 |
| BC | 0.36 | 0.36 | 0.37 | 0.39 |
| Total | 7.846 | 7.630 | 7.311 | 6.229 |
| Costs (£): mean, discounted | ||||
| Drug costs | 161,432 | 70,143 | 88,883 | 15,936 |
| Drug administration | 0 | 0 | 0 | 4390 |
| Monitoring outpatient appointment | 6818 | 6728 | 6597 | 6259 |
| Bone marrow tests | 6518 | 2732 | 3038 | 2199 |
| Radiography | 726 | 736 | 752 | 795 |
| CT scans | 428 | 434 | 444 | 469 |
| Blood transfusions | 4058 | 4117 | 4205 | 4445 |
| Inpatient palliative care | 41,346 | 76,439 | 68,496 | 64,325 |
| Total | 221,325 | 161,330 | 172,415 | 98,818 |
The incremental cost–utility of dasatinib, nilotinib, high-dose imatinib and interferon alfa, as estimated in the PenTAG AR2 model, is shown in Table 36. The PenTAG AR2 appendix results use interferon alfa as the base case, as it is the least costly of the alternatives. The ICER for nilotinib compared with interferon alfa is more than £30,000 per QALY gained. high-dose imatinib is dominated by nilotinib, i.e. nilotinib is predicted to be both cheaper and more effective, so the PenTAG AR2 model suggests that high-dose imatinib would not be considered a viable option. Compared with nilotinib, dasatinib provides only a small additional QALY at substantial extra cost and thus the ICER is more than £250,000 per QALY gained.
| Treatment | Cost (£) | Utility (QALY) | Incremental cost (£) | Incremental utility (QALY) | Incremental £/QALY (ICER) |
|---|---|---|---|---|---|
| IFN-α | 98,800 | 6.229 | |||
| Nilotinib | 161,300 | 7.630 | 62,500 | 1.401 | 44,600 |
| HDI | 172,400 | 7.311 | 11,100 | –0.318 | Dominated |
| Dasatinib | 221,300 | 7.846 | 60,000 | 0.216 | 277,700a |
One-way deterministic sensitivity analyses for nilotinib and dasatinib versus high-dose imatinib were performed by varying single parameters. Deterministic sensitivity analyses were not performed that included interferon alfa. In the majority of sensitivity analyses, nilotinib dominated high-dose imatinib. In only one case was nilotinib not cost-effective compared with high-dose imatinib, where progression-free survival for nilotinib was assumed to be identical to that with high-dose imatinib, i.e. treatment duration was approximately equal. The base-case conclusion for dasatinib is not affected by changes to the parameter values except for changing the treatment duration to be the same as for either high-dose imatinib or nilotinib. In these cases, dasatinib becomes cost-effective compared with these treatments.
Probabilistic sensitivity analyses were run with 1000 Monte Carlo simulations. A cost-effectiveness acceptability curve was constructed to show the probability that each treatment would be considered to most cost-effective, for a range of WTPs thresholds. At a conventional WTP threshold of £30,000 per QALY, the PenTAG AR2 model estimates the probability of interferon alfa providing optimal cost–utility at 97%, with corresponding likelihoods for nilotinib, high-dose imatinib and dasatinib of 3%, 0% and 0%, respectively. At a WTP threshold of around £45,000 per QALY, nilotinib is predicted to be the optimal choice. The model predicts that it is unlikely that dasatinib would be considered the best option; even when WTP approaches £150,000 per QALY, the probability of dasatinib being most cost-effective is < 20%.
Summary of key issues
-
Overall survival is based on the surrogate outcome of major cytogenetic response. However, data used for major cytogenetic response do not appear to match data in trials.
-
Although overall survival is based on major cytogenetic response, progression-free survival is not and so there is no link between overall survival and progression-free survival.
-
Survival for interferon is likely to be over-optimistic (according to comments from clinical advisers).
Comparison of the economic models
In the preceding sections, we have presented a summary of the cost-effectiveness studies by the manufacturers of dasatinib and nilotinib and by the PenTAG AR. 2 In this section, differences in the results produced by these three analyses for people starting in chronic-phase CML are described and explored.
Summary results are shown in Table 37 for each of the models compared with the base treatment, i.e. using a conventional treatment. The PenTAG AR2 and BMS3 models compared dasatinib, nilotinib and high-dose imatinib with interferon alfa. Novartis4 compared nilotinib and high-dose imatinib with hydroxycarbamide/stem cell transplantation. No single analysis has provided full disaggregated results in the format required for comparison and so some of the results shown here have been derived from the economic models. These comparative results are analysed in more detail below.
| Analysis | Base treatment | Interventions | ||||
|---|---|---|---|---|---|---|
| HU-SCT | IFN | Nilotinib | HDI | Dasatinib | ||
| Total cost (£) | PenTAG2 | 98,900 | 161,300 | 172,400 | 221,300 | |
| BMS3 | 129,292 | 318,978 | 350,365 | 314,413 | ||
| Novartis4 | 80,933 | 139,216 | 146,234 | |||
| Total QALY | PenTAG2 | 6.229 | 7.63 | 7.311 | 7.846 | |
| BMS3 | 1.664 | 6.235 | 5.91 | 6.425 | ||
| Novartis4 | 3.18 | 4.51 | 4.28 | |||
| Incremental cost vs base treatment (£) | PenTAG2 | – | 62,400 | 73,500 | 122,400 | |
| BMS3 | – | 189,686 | 221,073 | 185,121 | ||
| Novartis4 | – | 58,283 | 65,301 | |||
| Incremental QALY vs base treatment | PenTAG2 | – | 1.401 | 1.082 | 1.617 | |
| BMS3 | – | 4.571 | 4.246 | 4.761 | ||
| Novartis4 | – | 1.33 | 1.10 | |||
| ICER vs base treatment (£ per QALY) | PenTAG2 | – | 44,540 | 67,930 | 75,696 | |
| BMS3 | – | 41,498 | 52,066 | 38,883 | ||
| Novartis4 | – | 43,822 | 59,364 | |||
Table 37 and Figure 7 show the cost-effectiveness estimates of each of the treatments compared with the base treatment. Each of the treatments has an ICER of greater than £30,000 per QALY gained for all analyses. Generally, of the three treatments, nilotinib has the lower ICER and for each analysis this is around £45,000 per QALY gained. The ICER for high-dose imatinib is between £50,000 and £70,000 per QALY gained. There is a difference in the results between the PenTAG AR2 and BMS3 models for dasatinib, with ICERs ranging from £38,000 to £75,000 per QALY gained. The reasons for this difference are explored below in more detail.
FIGURE 7.
Cost-effectiveness vs base treatment. HDI, high-dose imatinib.
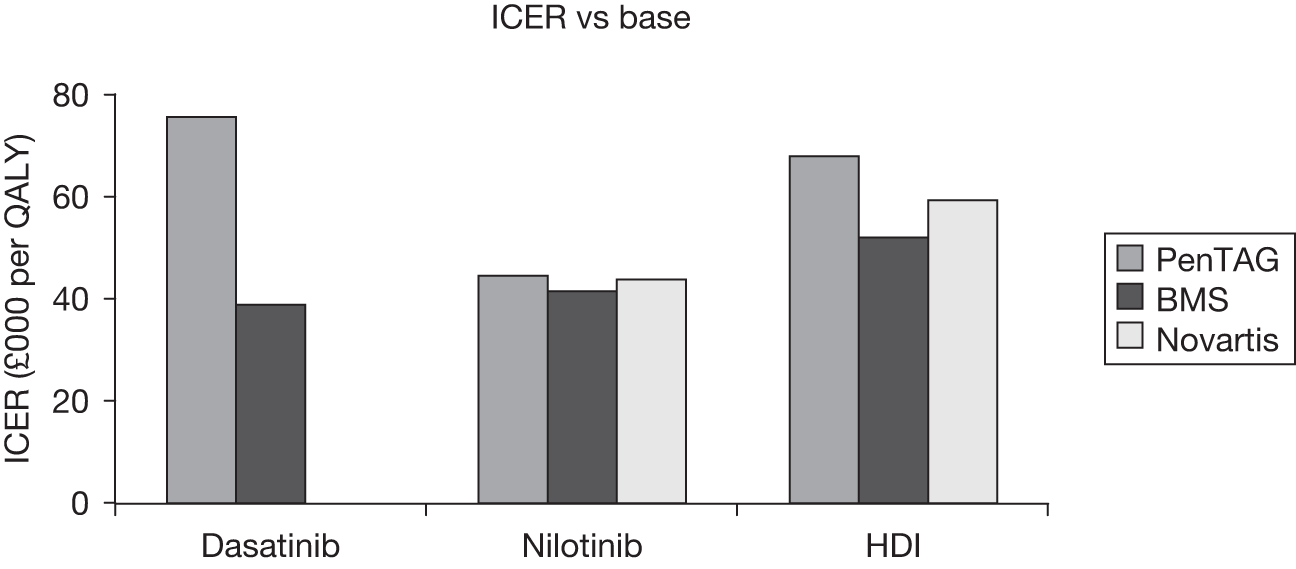
The costs for the interventions for each of the models are shown in Table 38 and Figures 8 and 9. These show that the total costs for nilotinib and high-dose imatinib for the BMS model3 are more than double those from the other analyses. This is due to higher treatment costs because of longer treatment duration in this analysis. The treatment duration for the BMS model3 is almost three times greater than for the other analyses except for dasatinib (Figure 10). The treatment duration and drug cost are similar for nilotinib and high-dose imatinib for both the PenTAG AR2 and Novartis4 analyses. For the PenTAG AR2 analysis, the treatment duration and cost are much greater for dasatinib than for the other interventions. Finally, the treatment duration is much longer for interferon alfa for the PenTAG AR2 analysis than for the BMS analysis. 3
| Model | Dasatinib | Nilotinib | HDI | Base treatmenta | |
|---|---|---|---|---|---|
| Drug costs (£) (discounted) | PenTAG2 | 161,432 | 70,143 | 88,883 | 15,936 |
| BMS3 | 224,268 | 228,576 | 254,018 | 6764 | |
| Novartis4 | 62,363 | 67,947 | 74,418 | ||
| Other costs (£) (discounted) | PenTAG2 | 59,893 | 91,187 | 83,532 | 82,882 |
| BMS3 | 90,145 | 90,402 | 96,347 | 122,528 | |
| Novartis4 | 76,853 | 78,287 | 6515 | ||
| Treatment duration (months) (undiscounted) | PenTAG2 | 64 | 27 | 27 | 21 |
| BMS3 | 88 | 86 | 78 | 9 | |
| Novartis4 | 24 | 21 |
The QALYs and LYs for each of the interventions are shown in Table 39 and Figures 11–14. These show that the LYs and QALYs for each of the interventions are similar for each economic model. The QALYs and LYs in the Novartis4 analysis are about half those in the PenTAG AR2 and BMS3 analyses. The reason for the lower life expectancy in the Novartis4 model is in the assumed high mortality associated with stem cell transplantation. The LYs and total QALYs for the interventions in the PenTAG AR2 analysis are higher than for BMS. 3 The reason for this appears to be an error for the utility value used in the BMS analysis3 for the accelerated-phase + blast-crisis-phase state. The number of LYs for interferon alfa is much higher in the PenTAG AR2 analysis than in the BMS analysis. 3 A clinical expert has indicated that the overall survival for interferon alfa would be considerably less than 6.5 years, and possibly as low as 1–2 years.
| Outcome | Model | Dasatinib | Nilotinib | HDI (800 mg) | Base treatmenta |
|---|---|---|---|---|---|
| Total LYs | PenTAG2 | 9.57 | 9.32 | 8.95 | 7.99 |
| BMS3 | 8.16 | 7.95 | 7.67 | 3.17 | |
| Novartis4 | 5.80 | 5.53 | 4.21 | ||
| CP (LYs) | PenTAG2 | 8.38 | 8.11 | 7.72 | 6.39 |
| BMS3 | 7.30 | 7.10 | 6.80 | 1.87 | |
| Novartis4 | 5.40 | 5.13 | 3.81 | ||
| BC+AP (LYs) | PenTAG2 | 1.20 | 1.21 | 1.24 | 1.31 |
| BMS3 | 0.86 | 0.85 | 0.87 | 1.30 | |
| Novartis4 | 0.40 | 0.40 | 0.40 | ||
| Total QALYS | PenTAG2 | 7.85 | 7.63 | 7.31 | 6.23 |
| BMS3 | 6.43 | 6.24 | 5.91 | 1.66 | |
| Novartis4 | 4.51 | 4.28 | 3.18 | ||
| CP QALYs | PenTAG2 | 7.12 | 6.89 | 6.56 | 5.43 |
| BMS3 | 6.17 | 5.97 | 5.64 | 1.26 | |
| Novartis4 | 4.27 | 4.04 | 2.94 | ||
| BC + AP QALYs | PenTAG2 | 0.73 | 0.74 | 0.75 | 0.80 |
| BMS3 | 0.26 | 0.27 | 0.27 | 0.40 | |
| Novartis4 | 0.24 | 0.24 | 0.24 |
FIGURE 11.
Total LYs of the interventions. HDI, high-dose imatinib.
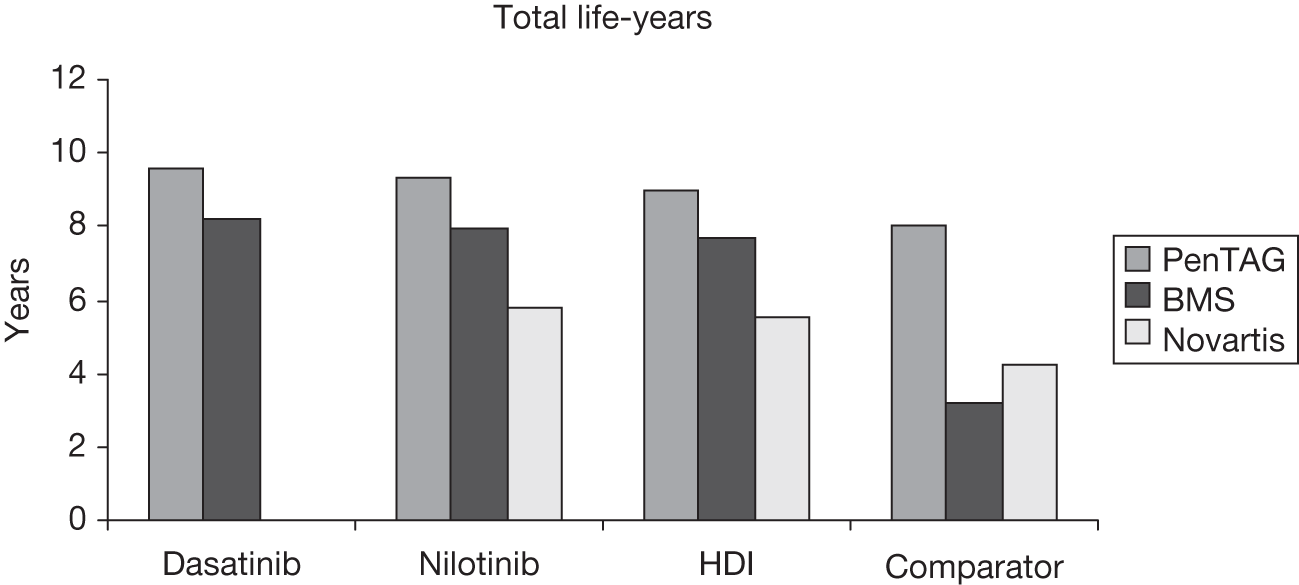
FIGURE 12.
Total QALYs of the treatments. HDI, high-dose imatinib.
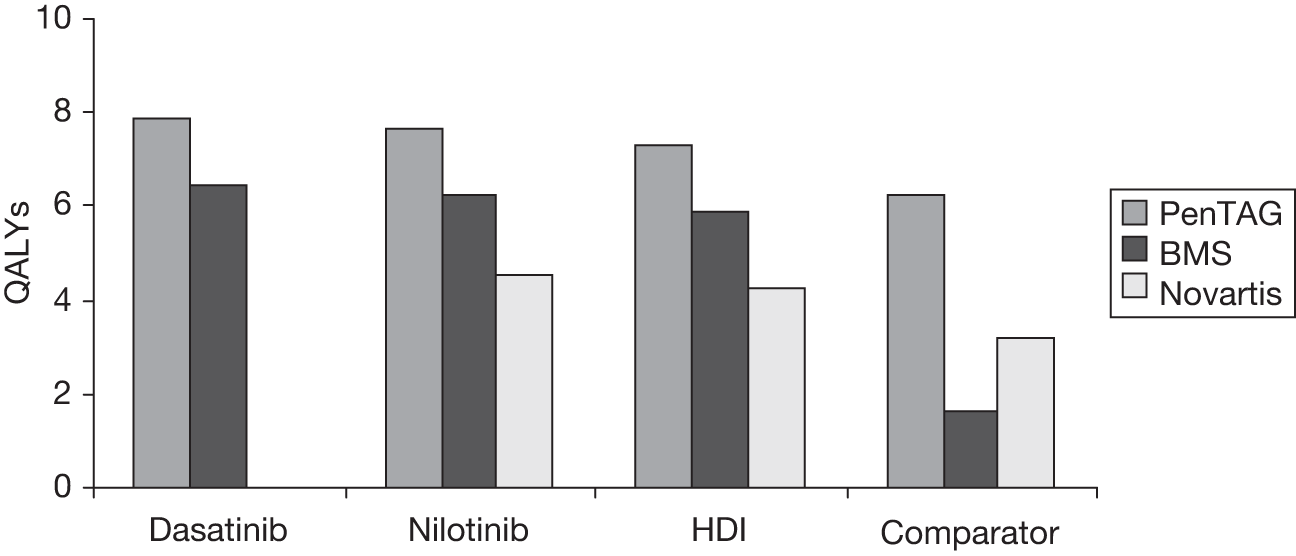
FIGURE 13.
Duration of chronic phase for treatments. CP, chronic phase; HDI, high-dose imatinib.
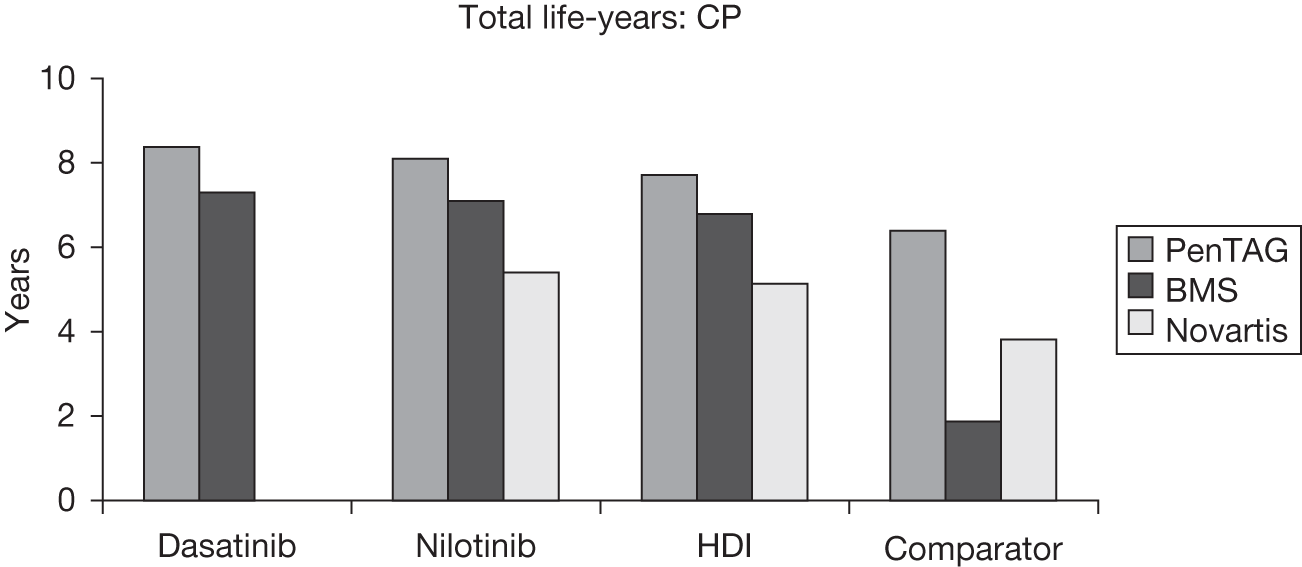
FIGURE 14.
Duration of accelerated phase and blast crisis for treatments. AP, accelerated phase; BC, blast crisis; HDI, high-dose imatinib.
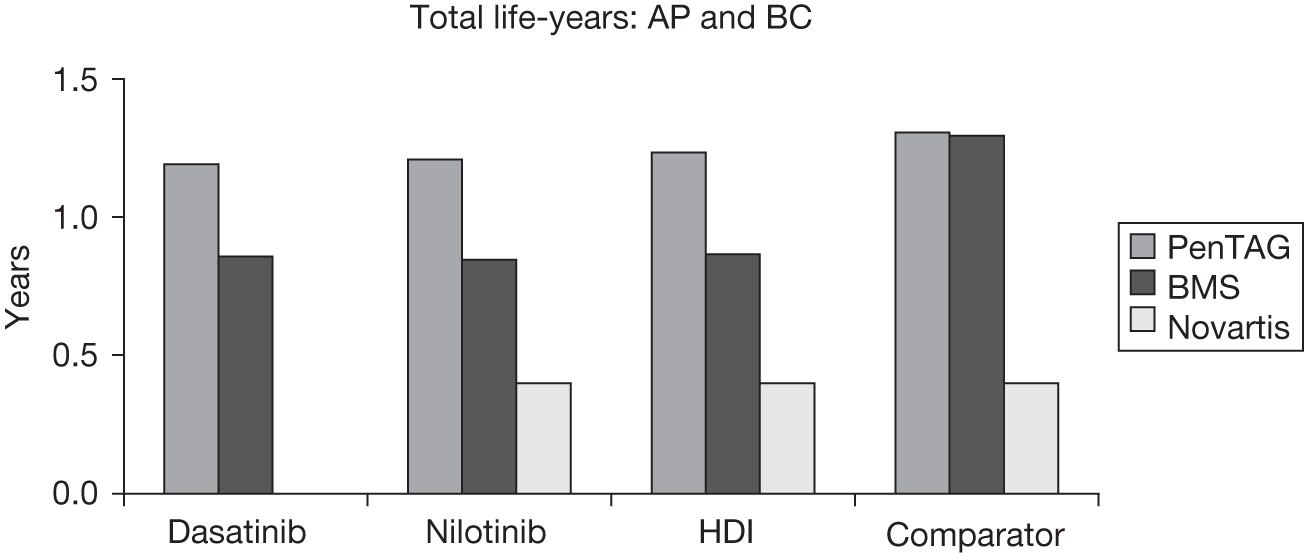
Survival estimates in chronic-phase CML by PenTAG AR2 and BMS3 for the interventions are similar and about double the estimates from Novartis. 4 Survival estimates in the accelerated-phase plus blast-crisis-phase stages for the interventions vary in the analyses from around 0.4 years for Novartis,4 0.8 years for BMS,3 to 1.7 years for the PenTAG AR. 2
Summary
-
There are some similarities and differences between the model results.
-
There are concerns with the PenTAG AR2 model with respect to the overall survival for interferon alfa and the length of time on treatment with dasatinib.
-
There are concerns with the BMS model3 with respect to the time spent on treatment, and the utility values used for accelerated phase and blast-crisis phase.
-
There are concerns with the Novartis4 model with respect to survival estimates, which are much lower than for the other two models owing to the assumed high mortality associated with stem cell transplantation, which is used with hydroxycarbamide as a comparator.
SHTAC analyses
We have conducted new analyses using the PenTAG AR2 model with minor modifications to take into account the issues raised in previous sections of the report. The PenTAG AR2 analyses were limited to the chronic phase of CML only because of the lack of clinical effectiveness data for comparator treatments in the accelerated and blast-crisis phases. As our update systematic review did not find any suitable data to analyse the cost-effectiveness of these phases of CML, our analysis is also limited to those patients who started in the chronic phase of CML.
Our analyses are presented for each of the interventions and comparators in the appraisal scope. For the chronic phase the interventions are dasatinib, nilotinib and high-dose imatinib, and the comparators are interferon alfa, standard-dose imatinib (400 mg), stem cell transplantation and hydroxycarbamide. Although there are different views over which is the most appropriate comparator and there is a lack of reliable data for the key parameters for these comparators, for completeness all comparators have been included in the analyses. However, it must be stressed that because of the concerns relating to data for the comparators, the results should be treated with due caution.
We have conducted base-case analysis and deterministic, scenario and threshold analyses around some of the model input parameters and probabilistic sensitivity analyses to explore the uncertainty around model results.
Model structure and approach
The PenTAG AR2 model is described in detail above (see Description and results of the published economic evaluation). No structural changes have been made for the SHTAC analyses. It is a lifetime survival model with five health states: chronic phase (on treatment), chronic phase (following discontinuation of treatment), accelerated phase, blast-crisis phase and death. Patients enter the model in chronic-phase CML and progress to accelerated phase, then to blast-crisis phase and then, finally, death from CML causes. The model uses best initial response to treatment to predict overall survival, with survival duration estimated for major cytogenetic response responders and major cytogenetic response non-responders. The relationship between major cytogenetic response and overall survival is assumed to be the same for all treatments. Patients move between the states chronic phase (on treatment) and chronic phase (no treatment) according to estimates for progression-free survival and the number of patients who prematurely discontinue treatment. Trial data are extrapolated for treatment duration and progression-free survival.
Data inputs for the interventions
In our update systematic review we have not identified any new clinical effectiveness data that provide better estimates than the data used in the PenTAG AR2 [described above (see Description and results of the published economic evaluation) and shown in Tables 28, 34, 40 and 41]. The parameters therefore used for the interventions are mostly as for the PenTAG AR,2 with the exception of parameter values for progression-free survival and treatment duration for dasatinib. The costs of the drugs were taken from the BNF (2010). The cost of high-dose imatinib is due to increase in a subsequent edition of the BNF (unpublished at the time of the review). The effect of this cost increase for high-dose imatinib is presented in Appendix 6.
| Outcome | Dasatinib | Nilotinib | HDI |
|---|---|---|---|
| MCyR rate (%) | 58.1 | 52.4 | 44.0 |
| PFS (months) | 0.864, 0.769, 0.632 at 6, 12, 18a | 0.864, 0.769, 0.632 at 6, 12, and 18 | 0.81, 0.57, 0.29 at 12, 24, 48 |
| Intervention | Monthly treatment cost (£) | OS (years) | Treatment duration (years) | Health-state utility (CP on treatment) |
|---|---|---|---|---|
| Dasatinib | 2540 | 13.4 | 3.1 | 0.85 |
| Nilotinib | 2643 | 12.98 | 2.4 | 0.85 |
| HDI | 3253 | 12.4 | 2.7 | 0.85 |
| IFN-α | 743 | 3.6 | 0.5 | 0.71 |
| Standard-dose imatinib (400 mg) | 1604 | 3.6 | 0.7 | 0.85 |
| SCTa | 2400 | 13 | 9.5 | 0.71 |
| HU | 13 | 3.5 | 1.5 | 0.85 |
There were concerns over the parameter values chosen in the PenTAG AR,2 which gave treatment duration of dasatinib as double that for nilotinib (data taken from poor-quality studies). One of our clinical experts advised us that no difference in efficacy has been shown between nilotinib and dasatinib and, hence, no difference would be expected between these drugs in progression-free survival and treatment duration. During the consultation process for the PenTAG AR,2 BMS asserted that: ‘patients treated with dasatinib would not be expected to remain on treatment for (on average) 4 years longer than those receiving nilotinib, instead the duration of treatment for the two drugs should be considerably more similar.’ PenTAG defended their choice of treatment duration as evidence based. They pointed out that the treatment duration was based upon progression-free survival, which was clearly shorter with nilotinib than with dasatinib (0.63 at 18 months compared with 0.77 at 24 months, respectively). However, PenTAG conceded that differences between populations and outcome definitions make comparisons between these data precarious.
In our base-case analyses we assume that progression-free survival for dasatinib is the same as that for nilotinib (Table 40), based on the view of our clinical expert. An additional scenario analysis is presented subsequently, which uses the progression-free survival parameter values for dasatinib from the PenTAG AR2 model.
Although our review of clinical effectiveness identified a range of new data for high-dose imatinib (reported in Chapter 3), we have used the PenTAG AR2 as the data source for high-dose imatinib because the values chosen by PenTAG are mid-way in this range.
Data inputs for the comparators
Interferon alfa, stem cell transplantation, hydroxycarbamide and standard-dose imatinib are examined through exploratory analyses using data available from the PenTAG AR2 or the manufacturers’ models where no suitable data are available in the PenTAG AR. 2 Therefore, data for hydroxycarbamide are taken from the Novartis4 model and data for standard-dose imatinib and stem cell transplantation from the BMS model. 3 For these analyses we have derived our best estimates of the following parameters: monthly treatment cost, treatment duration, overall survival and health-state utility for the chronic-phase treatment period (see Table 41). The PenTAG AR2 estimated overall survival by extrapolating from the surrogate outcome major cytogenetic response. However, owing to the lack of data for overall survival and major cytogenetic response for these comparators, we were unable to derive survival curves in this way and so have instead taken a simple pragmatic approach and selected an estimate for overall survival (see below). In the model, an overall survival curve is derived from this overall survival estimate, by assuming a negative exponential distribution for mortality. The PenTAG AR2 estimated progression-free survival and treatment duration by fitting distributions to the clinical trial data. In the absence of any reliable data for the comparators, we were unable to provide estimates of the clinical data and so instead use plausible estimates for treatment duration for each of the parameters.
Interferon alfa
An appendix to the PenTAG AR2 and the BMS submission3 included interferon alfa as a comparator. PenTAG2 derived overall survival using an estimate for the major cytogenetic response rate for interferon alfa from the IRIS trial19,20 (22%). Results from the PenTAG2 model give overall survival for interferon alfa as almost 11 years. Results from the BMS submission3 for the interferon alfa analysis had an overall survival for interferon alfa of 3.6 years. The BMS submission3 assumed that there were 0% major cytogenetic response for those treated with interferon alfa and the progression-free survival and overall survival is estimated according to the progression-free survival rates for this cohort (Table 32). However, there is no discussion of the data or justification for their selection in the submission.
Therefore, in the absence of any clinical data on the overall survival and progression-free survival for interferon alfa, and our clinical advice which suggests that the overall survival for interferon alfa could be as low as 1–2 years, we have set overall survival as a model parameter and assumed overall survival of 3.6 years (Table 41) with the range 3.6 to 11 years considered in scenario analyses. The treatment duration was assumed to be about one-third of the whole time in the chronic phase, as seen for the interventions nilotinib and high-dose imatinib (see Table 35). The monthly cost varies between £743 (PenTAG2) and £863 (BMS3), as the dosage was based upon different trials. In the absence of more reliable data, we have used the treatment cost for interferon alfa from the PenTAG AR,2 as this is an independent model (£743 per month). Similarly, the utility value we have used for interferon alfa (0.71) is also taken from the PenTAG AR2 (taken from the IRIS trial19,20).
Standard-dose imatinib (400 mg)
The PenTAG AR2 did not include standard-dose imatinib as a comparator. Standard-dose imatinib (400 mg) was included as a comparator in the BMS submission3 for the current appraisal. However, BMS3 point out that, ‘it is illogical and unethical to treat patients with standard-dose imatinib when patients have already failed such treatments’ (BMS,3 p. 44). Therefore, their analysis is illustrative only. In the absence of more reliable data, we have adopted some of the BMS3 assumptions in our analysis.
In the BMS3 economic modelling an assumption was made that the treatment efficacy (major cytogenetic response) with maintenance standard-dose imatinib is zero. No data were identified for treatment duration, overall survival or progression-free survival. The BMS3 submission results showed a treatment duration of 7.9 months and an overall survival for standard-dose imatinib equal to that for interferon alfa. We therefore have used this treatment duration, together with an equivalent overall survival of 3.6 years, as for interferon alfa (Table 41). The monthly cost for standard-dose imatinib (400 mg) is £1604.08 (BNF 60). In the absence of any evidence to the contrary, the utility value during the treatment phase has been assumed to be the same as for high-dose imatinib (i.e. 0.85).
Stem cell transplantation
Bristol-Myers Squibb3 and Novartis4 included stem cell transplantation as a comparator in their analyses; however, Novartis4 used a combination of stem cell transplantation and hydroxycarbamide. As stem cell transplantation combined with hydroxycarbamide is not part of the scope, we have used some of the assumptions from the BMS3 analysis. The BMS3 submission states that the cost of stem cell transplantation varies between £80,000 and £140,000 per person plus the monthly cost of £2400; we have used the lower figure of £80,000 for the transplant plus the monthly cost of £2400 in the SHTAC analysis. Post-transplant treatment costs include costs associated with graft-versus-host disease, treatment of comorbidities, management of relapse and treatment of symptoms (chemotherapy, palliative regimens and lymphocyte infusions).
The utility value for those receiving stem cell transplantation varied between 0.6 (BMS3) and 0.81 (Novartis4). We have therefore used the same utility for treated patients as used for interferon alfa (i.e. 0.71; Table 41), as this is mid-way between the manufacturers’ utility estimates.
The BMS3 submission modelled patient prognosis using Kaplan–Meier curves published in Gratwohl and colleagues18 and the model results for overall survival for these patients was similar to dasatinib, nilotinib and high-dose imatinib with patients treated for 9.5 years. In the absence of any more reliable data, we have assumed that overall survival for stem cell transplantation is similar to that for the interventions, i.e. overall survival of 13 years and that patients were treated for 9.5 years (Table 41).
Hydroxycarbamide
Novartis4 included hydroxycarbamide as a comparator in its exploratory analyses. It used a combination of stem cell transplantation and hydroxycarbamide, where those individuals who did not receive stem cell transplantation received hydroxycarbamide instead. Novartis4 estimated progression-free survival and overall survival for patients on hydroxycarbamide, by analysing clinical trial data26 for imatinib-resistant patients who were re-treated with nilotinib and then treated with hydroxycarbamide upon nilotinib failure. It should be noted that this is a different patient group from those treated with hydroxycarbamide as second line after imatinib resistance.
In the absence of any more reliable data, we have used the data and assumptions from the Novartis4 submission model in our analyses. Thus, the cost of hydroxycarbamide was £38 per quarter, utility while on treatment in the chronic phase was 0.85, overall survival was 3.5 years and treatment duration was 1.5 years (Table 41).
Results of SHTAC analyses
The base-case deterministic results are shown in Table 42. As the evidence for each of the comparators and interventions is poor, and many assumptions have had to be made to model long-term outcomes, the results should be treated with caution. The results are shown in Table 42 for nilotinib, dasatinib, high-dose imatinib, and each comparator against hydroxycarbamide, as hydroxycarbamide is the cheapest comparator, with the treatments ordered by increasing effectiveness. The comparators are also compared against the previous best option, i.e. a treatment that is more clinically effective and cost-effective compared with a preceding one (i.e. not dominated or extendedly dominated). At a cost-effectiveness threshold of £30,000, interferon alfa, standard-dose imatinib (400 mg) and stem cell transplantation are not cost-effective compared with hydroxycarbamide. This can be seen in Figure 15 by considering the cost-effectiveness frontier. The cost-effectiveness frontier is a line connecting the most cost-effective treatments. Treatments above this line are not cost-effective, as they are either dominated or extendedly dominated by the treatments on the frontier.
| Intervention | QALY | Cost (£) | Inc QALY vs HU | Inc cost vs HU (£) | ICER vs HU (£) | ICER vs next-best optiona (£) |
|---|---|---|---|---|---|---|
| HU | 2.20 | 18,128 | ||||
| IFN | 2.20 | 34,403 | 0.00 | 16,275 | 242,448,508 | Extendedly dominated |
| Standard-dose imatinib | 2.27 | 39,400 | 0.07 | 21,272 | 306,331 | Extendedly dominated |
| SCT | 6.35 | 305,846 | 4.15 | 287,718 | 69,279 | Dominated |
| HDI | 7.31 | 172,647 | 5.11 | 154,519 | 30,229 | Dominated |
| Nilotinib | 7.63 | 161,667 | 5.43 | 143,539 | 26,434 | 26,434b |
| Dasatinib | 7.85 | 172,473 | 5.65 | 154,345 | 27,336 | 50,016c |
FIGURE 15.
Cost-effectiveness plane for treatments for CML. HDI, high-dose imatinib; HU, hydroxycarbamide.
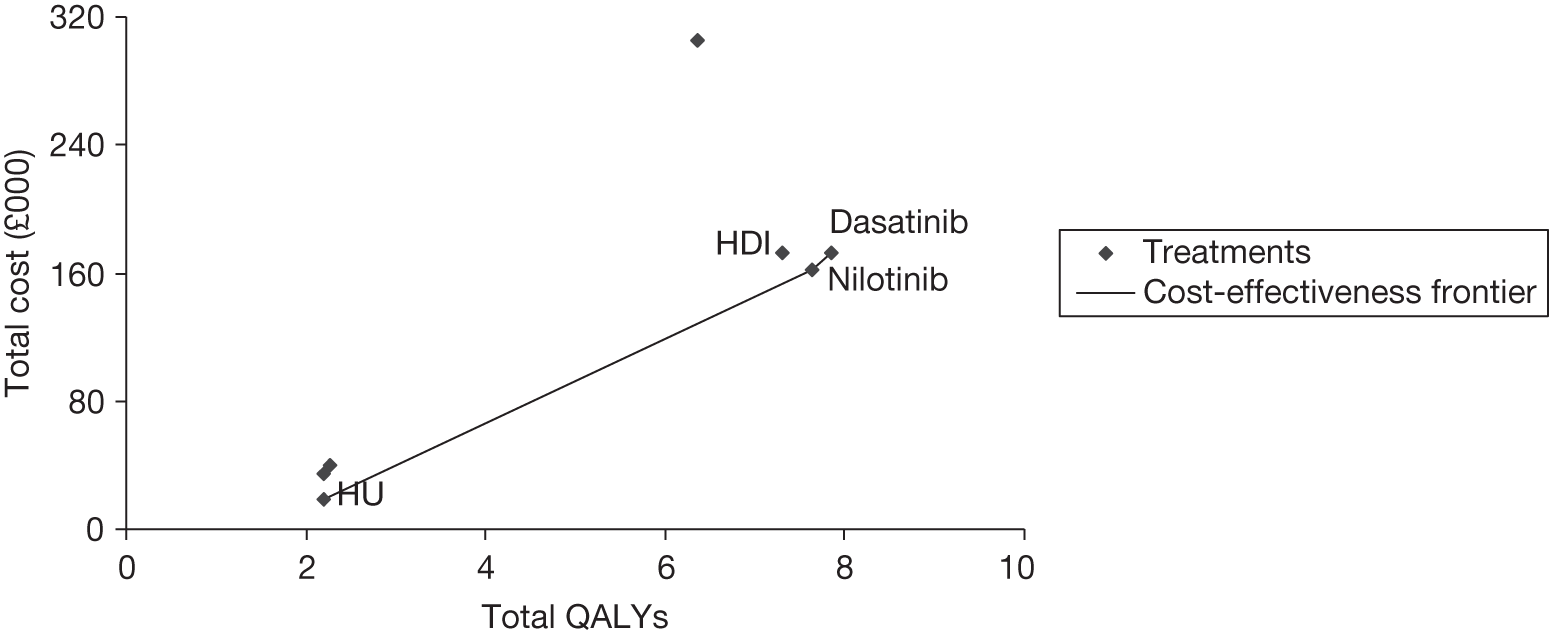
These results show that each intervention (dasatinib, nilotinib and high-dose imatinib) has a similar total cost and QALY and each intervention has an ICER around £30,000 per QALY compared with hydroxycarbamide. In this analysis nilotinib and dasatinib were marginally more cost-effective compared with hydroxycarbamide than high-dose imatinib owing to lower costs and better efficacy (major cytogenetic response) (see Table 34). Owing to the uncertainty around the clinical data, it is unclear which of the interventions – dasatinib, high-dose imatinib or nilotinib – would be the most cost-effective. This is shown in Figure 15, in which high-dose imatinib is dominated by nilotinib. The cost-effectiveness of dasatinib versus nilotinib is £50,000 per QALY gained.
Sensitivity analyses
The uncertainty around the model results was explored using deterministic sensitivity analyses, threshold analyses and probabilistic sensitivity analyses. The deterministic sensitivity analyses were performed around some of the model input parameters that had been shown to have the greatest effect on the model results in the PenTAG AR. 2 The interventions dasatinib, nilotinib and high-dose imatinib are compared with hydroxycarbamide. The other comparator treatments were not included in the sensitivity analyses as they were dominated by hydroxycarbamide in base-case analyses; however, we have examined the other comparator treatments in threshold analyses.
A deterministic sensitivity analysis was performed for changes to the overall survival for hydroxycarbamide and treatment efficacy for the interventions dasatinib, nilotinib and high-dose imatinib. There are no data for the likely range in overall survival for hydroxycarbamide so a plausible range was chosen (2–6.5 years). Treatment efficacy (major cytogenetic response) has been varied between 30% and 70% for the interventions. This reflects the range of efficacy found in the clinical review (see Chapter 3, Effectiveness of high-dose imatinib) for high-dose imatinib.
We have compared the results for each intervention versus hydroxycarbamide (Table 43). Changes in the length of overall survival for hydroxycarbamide have only a small impact on the cost-effectiveness of any of the interventions. Similarly, changes to major cytogenetic response also have little impact on the results.
| Sensitivity analysis | Base value | High value | ICER (£/QALY) | Low value | ICER (£/QALY) | Range (£/QALY) |
|---|---|---|---|---|---|---|
| Nilotinib | ||||||
| HU OS | 3.6 years | 6.5 years | 32,500 | 2 years | 24,300 | 8200 |
| MCyR, nilotinib | 52.4% | 70% | 25,200 | 30% | 28,500 | 3300 |
| Dasatinib | ||||||
| HU OS | 3.6 | 6.5 | 33,500 | 2 | 25,200 | 8300 |
| MCyR, dasatinib | 58.1% | 70% | 26,400 | 30% | 30,100 | 3700 |
| HDI | ||||||
| HU OS | 3.6 years | 6.5 years | 39,200 | 2 years | 27,300 | 11,900 |
| MCyR, HDI | 44.0% | 70% | 27,800 | 30% | 32,000 | 4200 |
A two-way sensitivity analysis was performed for the effect of different overall survival times and treatment durations for nilotinib versus hydroxycarbamide (Table 44). These results show that most of the cost-effectiveness estimates are below £40,000 per QALY gained, except if the overall survival for nilotinib was much lower or the treatment duration was much longer than chosen for the base case. Similar results are obtained for dasatinib versus hydroxycarbamide and for high-dose imatinib versus hydroxycarbamide (not shown here).
| OS (years) | Treatment duration (years) | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |
| 7 | NA | ||||
| 9 | |||||
| 11 | |||||
| 13 | x | ||||
| 15 | |||||
In our critique of the PenTAG AR,2 we raise concerns for the values chosen for major cytogenetic response for each of the interventions, as these do not match the data cited. We ran analyses using the data values found in the studies cited, i.e. major cytogenetic response – dasatinib 59%, nilotinib 56% – and high-dose imatinib 54%: however, these changes did not impact.
Threshold analysis for interventions
Threshold sensitivity analysis was performed for high-dose imatinib versus nilotinib and for dasatinib versus nilotinib (Table 45), as nilotinib was the most cost-effective treatment in the base results. The analysis varied the most influential parameters (i.e. overall survival and treatment duration). These parameters were varied for nilotinib, whereas the parameters for dasatinib and high-dose imatinib were unchanged.
| Parameters varied | OS (years), (MCyR) nilotinib | |||
|---|---|---|---|---|
| 13 (52.4%) | 12.4 (45%) | 12.1 (40%) | 11.3 (30%) | |
| Comparison: ICER (£/QALY gained) | ||||
| Dasatinib vs nilotinib | 50,000 | 30,400 | 26,200 | 22,300 |
| Nilotinib vs HDI | HDI dominated | HDI dominated | – | – |
| HDI vs nilotinib | – | – | 120,000 | 45,000 |
| Treatment duration (years), nilotinib | ||||
| 2.5 | 2.65 | 2.8 | 2.95 | |
| Comparison: ICER (£/QALY gained) | ||||
| Dasatinib vs nilotinib | 50,000 | 35,500 | 24,900 | 13,600 |
| Nilotinib vs HDI | HDI dominated | HDI dominated | HDI dominated | HDI dominated |
The results are fairly robust for changes in overall survival and treatment duration for nilotinib versus high-dose imatinib and dasatinib versus nilotinib. However, as there was a lack of reliable data for the appropriate value for treatment duration for dasatinib, there is large uncertainty around the results for dasatinib.
Probabilistic sensitivity analysis
The uncertainty around the model results was explored using probabilistic sensitivity analysis. This was run comparing the interventions dasatinib, nilotinib, high-dose imatinib and hydroxycarbamide using the PenTAG AR2 model. We were unable to run a probabilistic sensitivity analysis for all the comparator treatments, as the PenTAG AR2 model did not include all of the comparator treatments, and there is no evidence available for the distributions around the parameter values for many of the treatments. The probabilistic sensitivity analysis was run with 1000 iterations. For each iteration parameter values are sampled at random from their probability distributions. The parameter values and distributions used in the probabilistic sensitivity analysis are described in the PenTAG AR. 2 However, we have used hydroxycarbamide as a comparator instead of interferon alfa for consistency with our base case.
The results for the probabilistic sensitivity analysis are shown graphically in a cost-effectiveness acceptability curve (Figure 16), which shows the probability that each treatment is the most cost-effective at different WTP values. For a WTP threshold of £20,000 per QALY, hydroxycarbamide is the most cost-effective treatment (probability = 100%). For a WTP threshold of £30,000 per QALY, nilotinib, dasatinib, hydroxycarbamide and high-dose imatinib have a probability of being cost-effective of 60%, 28%, 12% and 0%, respectively. These results reflect those in the deterministic analyses, in which high-dose imatinib is dominated by nilotinib (see Table 42) and high-dose imatinib does not become the optimal treatment for any of the probabilistic sensitivity analysis iterations.
FIGURE 16.
Cost-effectiveness acceptability curve for nilotinib, dasatinib, high-dose imatinib and hydroxycarbamide.
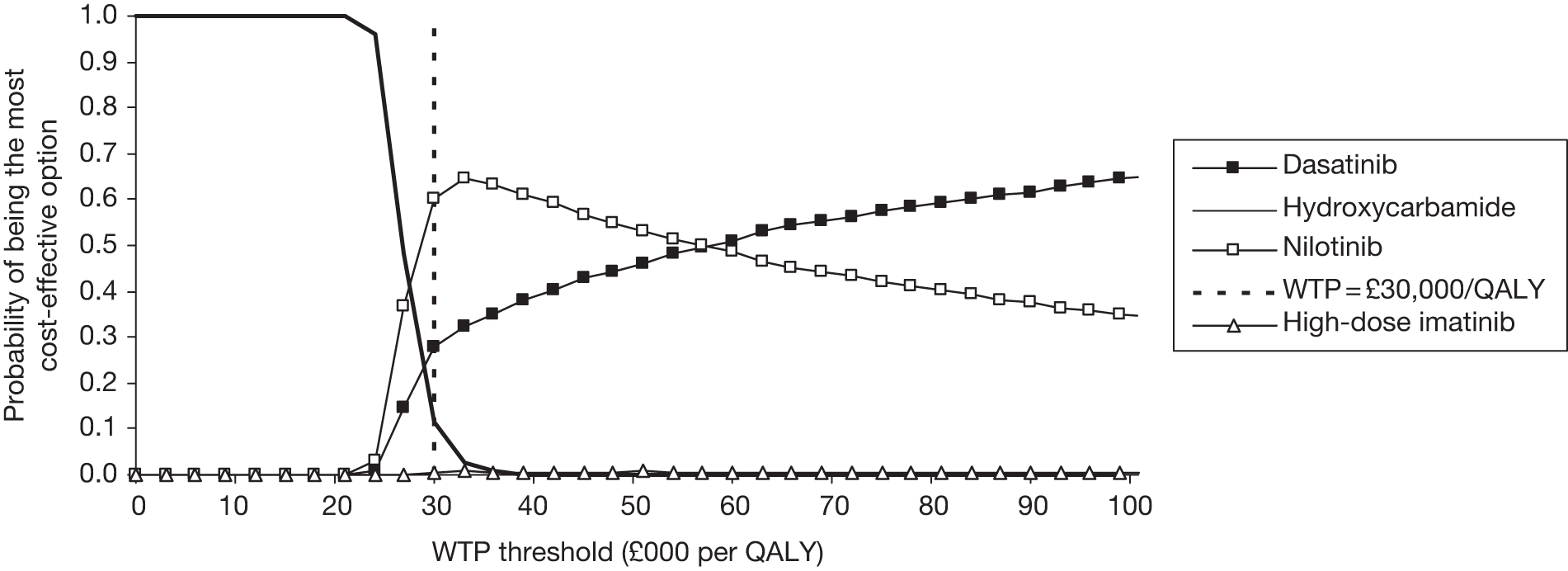
A scatterplot of the probabilistic sensitivity analysis runs (Figure 17) shows that there is considerable overlap between the results for the interventions dasatinib, nilotinib and high-dose imatinib, but all three have a cost-effectiveness of around £30,000 per QALY versus hydroxycarbamide.
FIGURE 17.
Scatterplot for probabilistic sensitivity analysis for nilotinib, dasatinib, high-dose imatinib and hydroxycarbamide. HDI, high-dose imatinib; HU, hydroxycarbamide.
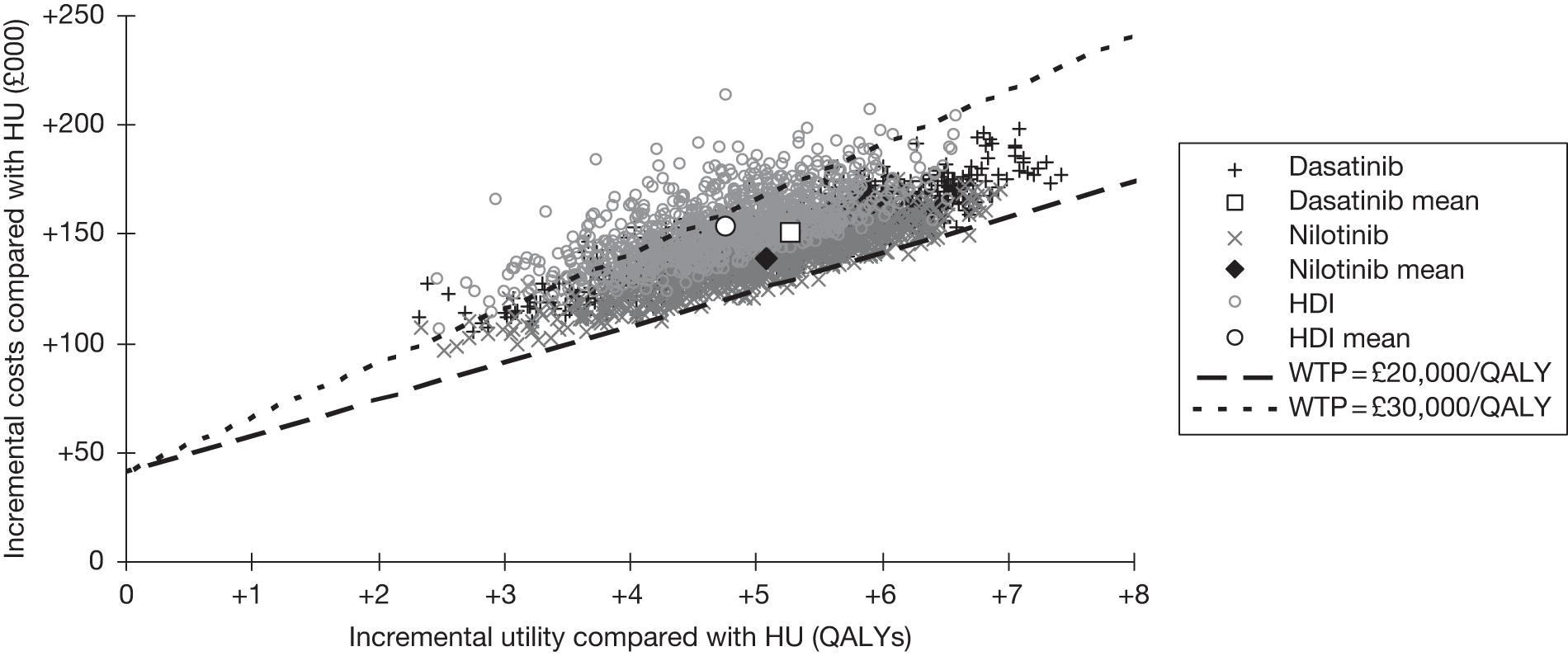
Scenario analyses
To further explore uncertainty in parameter values, we have conducted scenario analyses relating to length of progression-free survival and treatment duration, and overall survival for interferon alfa, and threshold analyses for the comparators.
Progression-free survival and treatment duration
For the base analyses, we have assumed that progression-free survival for dasatinib was the same as for nilotinib. However, in the PenTAG AR,2 progression-free survival was based upon trial data, albeit of poor quality, which gave a longer treatment duration for dasatinib that was double the treatment duration of nilotinib. We explored the case where dasatinib has a longer treatment duration than high-dose imatinib and nilotinib by varying the progression-free survival for dasatinib while keeping progression-free survival for high-dose imatinib and nilotinib constant. The scenario analysis results are shown in Table 46. Extending the progression-free survival and treatment duration of dasatinib results in higher costs for dasatinib with no change in QALY, thus the longer the treatment duration, the less favourable the results compared with the other interventions. For additional scenario analyses see also Appendix 7.
| Results | Treatment duration of dasatinib (years) | ||||
|---|---|---|---|---|---|
| 3.1 | 3.7 | 4.5 | 5.4 | 6.5 | |
| Cost (£) | 172,473 | 182,401 | 194,350 | 206,229 | 221,325 |
| ICER vs nilotinib (£/QALY) | 50,000 | 96,000 | 151,300 | 206,300 | 276,100 |
| ICER vs HDI (£/QALY) | Dominates HDI | 18,200 | 40,600 | 62,800 | 91,100 |
| ICER vs HU (£/QALY) | 27,300 | 29,100 | 31,200 | 33,300 | 36,000 |
Overall survival for interferon alfa
For the base analyses, we have assumed that overall survival for interferon alfa was 3.6 years. However, in the PenTAG AR,2 overall survival was based upon trial data, albeit of poor quality, which gave a longer overall survival of almost 11 years. We explored alternative overall survival for interferon alfa of between 3.6 years and 11 years. The results for the interventions versus interferon alfa are shown in Table 47. These indicate that cost-effectiveness for nilotinib, high-dose imatinib and dasatinib versus interferon alfa varies between £23,000 and about £73,000 per QALY, depending on the length of survival. The interventions have ICERs of around £30,000 per QALY if the overall survival for interferon alfa is < 7 years.
| Results | OS for IFN (years) | ||||
|---|---|---|---|---|---|
| 3.6 | 5 | 7 | 9 | 10.8 | |
| Cost, IFN (£) | 34,403 | 48,604 | 67,011 | 83,379 | 98,900 |
| QALY, IFN | 2.2 | 3.1 | 4.2 | 5.3 | 6.2 |
| ICER nilotinib vs IFN (3) | 23,400 | 25,100 | 28,700 | 35,200 | 44,800 |
| ICER HDI vs IFN (£) | 27,000 | 29,700 | 35,500 | 46,800 | 72,700 |
| ICER dasatinib vs IFN (£) | 24,500 | 26,300 | 30,000 | 36,500 | 48,100 |
During the consultation process for the PenTAG AR,2 BMS3 disputed the overall survival of patients treated with interferon alfa. It stated that: ‘the mean overall survival seen in clinical practice for first line clinical use of interferon alfa is 5.08–5.5 years (Helhmann et al. 1994, Allan et al. 1995).’ PenTAG2 defended its choice by stating that those who had interferon alfa would receive life-prolonging third-line treatment in chronic phase.
We have not shown results for analyses of interferon alfa overall survival of < 3.6 years because of the structure of the PenTAG2 model, which assumes that patients spend 1.9 years in accelerated-phase and blast-crisis-phase CML. For short survival times modelled, this is unlikely to be the case.
Threshold analyses for comparators
Because of the uncertainty around the data and assumptions for the comparators, we conducted threshold analyses to indicate how much the parameter values would need to change in order for these comparators to become cost-effective. In our base analyses, interferon alfa, standard-dose imatinib and stem cell transplantation were all either dominated or extendedly dominated, and so the interventions were compared with hydroxycarbamide. Therefore, in the threshold analyses we have compared the comparators with hydroxycarbamide. In order to obtain an ICER of < £30,000 per QALY gained, overall survival and treatment duration need to be varied simultaneously for the comparators interferon alfa and standard-dose imatinib and the monthly cost and utility value need to be varied for stem cell transplantation.
The results (Table 48) show that overall survival for interferon alfa and standard-dose imatinib would have to rise to more than 5.6 years and 6 years, respectively, and treatment duration would have to rise to more than 0.8 and 1.0 years, respectively, for them to be cost-effective compared with hydroxycarbamide (i.e. < £30,000 per QALY gained). For stem cell transplantation, the monthly cost would have to decrease to < £1350 and the utility would need to be at least 0.85 for it to be cost-effective compared with hydroxycarbamide. The parameter values in Table 48 show the effects of choosing one combination of parameters to achieve the threshold results; there are other combinations which could also have been chosen that would have achieved the threshold results.
| Treatment | OS (years) | Treatment duration (years) | Monthly treatment cost (£) | Health-state utility | QALY | Cost (£) |
|---|---|---|---|---|---|---|
| IFN-α | 5.6 (3.6) | 0.8 (0.5) | UC | UC | 3.5 | 54,360 |
| Standard-dose imatinib | 6 (3.6) | 1.0 (0.67) | UC | UC | 3.8 | 65,843 |
| SCT | UC | UC | 1350 (4800) | 0.85 (0.71) | 7.3 | 170,044 |
Summary and conclusions to the cost-effectiveness section
-
The systematic review identified one cost-effectiveness study15 that compared dasatinib with high-dose imatinib (800 mg/day). The results showed that chronic-phase CML patients resistant to standard-dose imatinib gain 0.62 QALYs when treated with dasatinib compared with high-dose imatinib and the incremental societal cost is €4250 during the lifetime period or €6880 per QALY gained. It is unclear how generalisable these results are to the UK NHS, as the study was conducted in Sweden and takes a societal perspective.
-
The Novartis4 submission compared nilotinib with high-dose imatinib and also had an exploratory analysis versus stem cell transplantation/hydroxycarbamide. The results showed that nilotinib dominates high-dose imatinib (i.e. is more effective and less costly). Exploratory analysis gives an ICER of about £44,000 for nilotinib versus stem cell transplantation/hydroxycarbamide.
-
The BMS3 submission compared dasatinib, nilotinib and high-dose imatinib with standard-dose imatinib, stem cell transplantation, hydroxycarbamide, interferon alfa, acute leukaemia-style chemotherapy and best supportive care. The results showed that dasatinib dominates high-dose imatinib, nilotinib and stem cell transplantation.
-
The PenTAG AR2 economic evaluation compared dasatinib and nilotinib to high-dose imatinib. An appendix compared these treatments with interferon alfa. The results showed that nilotinib dominates high-dose imatinib and the ICER for nilotinib versus interferon alfa is about £44,600. The ICER for dasatinib versus nilotinib is > £277,000. Concerns relate to the fact that there is no link between overall survival and progression-free survival, as overall survival is based on major cytogenetic response but progression-free survival is not, and also the estimate for survival on interferon alfa appears unrealistically high.
-
There are two main differences between the industry models.
-
These key assumptions drive the differences between the models. Therefore, we felt justified in using the PenTAG AR2 model to explore further analyses using some different data inputs.
-
SHTAC conducted these analyses for the interventions dasatinib, nilotinib and high-dose imatinib, and the comparators interferon alfa, standard-dose imatinib (400 mg), stem cell transplantation and hydroxycarbamide.
-
The results suggest that the three interventions – dasatinib, nilotinib and high-dose imatinib – have similar costs and cost-effectiveness.
-
Nilotinib, dasatinib and high-dose imatinib are all cost-effective when compared with hydroxycarbamide, for a WTP of about £30,000 per QALY.
-
Nilotinib and dasatinib are slightly more cost-effective than high-dose imatinib because of slightly lower costs and better effectiveness than high-dose imatinib.
-
It is not possible to derive firm conclusions about the relative cost-effectiveness of the three interventions owing to the great uncertainty around data inputs.
-
The parameters that had the most impact on the model results were overall survival, treatment efficacy and treatment durations. The results were fairly robust to changes in overall survival and treatment duration for nilotinib versus high-dose imatinib and dasatinib.
-
A probabilistic sensitivity analysis was run comparing the interventions dasatinib, nilotinib, high-dose imatinib and hydroxycarbamide. For a WTP threshold of £20,000 per QALY, hydroxycarbamide is the most cost-effective treatment. For a WTP threshold of £30,000 per QALY, nilotinib, dasatinib, hydroxycarbamide and high-dose imatinib have a probabilities of being cost-effective of 60%, 28%, 12% and 0%, respectively.
Chapter 5 Discussion
Statement of principal findings
This report is a supplement to the PenTAG AR2 and, as such, the results reported herein must be considered in conjunction with the PenTAG AR. 2 No new evidence was identified for accelerated-phase or blast-phase CML, therefore this systematic review and economic evaluation refer only to chronic-phase CML. For a discussion of findings for people with imatinib-resistant CML in accelerated phase, see PenTAG AR2 (see pp. 357–63). For a discussion of blast crisis-CML see PenTAG AR (see pp. 363–6).
Clinical effectiveness
The updated searches identified one RCT of dasatinib versus high-dose imatinib and three single-arm cohort studies of high-dose imatinib in people with imatinib-resistant CML. No new studies of nilotinib were identified. Earlier publications of the RCT had been included in the PenTAG AR;2 however, owing to major limitations in the design of the RCT, only the dasatinib arm of the trial was reported and no comparative evidence was discussed. In line with this, outcomes with longer follow-up from the dasatinib and high-dose imatinib arms of the RCT were dealt with as non-comparative evidence in the present systematic review. Key limitations of the RCT include a high crossover rate of 80% from the high-dose imatinib arm to the dasatinib arm at a median of 13 weeks and a 20% crossover from dasatinib to high-dose imatinib at a median of 28 weeks. The criteria used to define imatinib failure were slightly different in each of the four included studies. All participants had chronic-phase CML, except in one of the single-arm cohort studies, which also included very small numbers with accelerated phase and blast crisis (three and four patients, respectively). The three single-arm cohort studies appeared to have a high risk of bias. The following results should be interpreted with caution owing to the methodological limitations of the included studies.
Dasatinib
Earlier publications of the RCT included in the PenTAG AR2 reported outcomes at median 15 months’ follow-up, although some data with longer follow-up had been obtained from abstracts and the manufacturer’s submission to that appraisal. The 2009 publication of the RCT reported a median of 26 months’ follow-up. There was no change to the data reported in the PenTAG AR2 for the following outcomes:
-
complete haematological response
-
complete haematological response in participants who had no CHR at baseline
-
estimated progression-free survival at 24 months.
Complete cytogenetic response improved slightly from 39.6% at a median of 15 months to 43.6% at median 26 months. Major cytogenetic response was similar between patients with (55%) and without (51%) a previous CyR on standard-dose imatinib. At 18 months, 90% of participants maintained a major cytogenetic response. A MMR was achieved in 28.7% of participants. The estimated proportion of participants without treatment failure at 24 months was 59%. Additional AEs of any grade reported in the 2009 publication included fluid retention (39%), bleeding (18%), infection (14%) and upper respiratory tract infection or inflammation (11%). An increase in superficial oedema from 15% to 20% also occurred with longer follow-up. Grades 3–4 fluid retention occurred in 7% of participants.
High-dose imatinib
Data from the RCT described above and from three single-arm cohort studies were included. Despite the RCT having a median follow-up of 26 months, median treatment duration for the high-dose imatinib arm was only 3 months (range 0.16 to 26.3 months). This was due to the high crossover rate.
Complete cytogenetic response ranged from 18.4% to 36.4%, and major cytogenetic response ranged from 32.7% to 63.5%. Major cytogenetic response differed between those with (44%) and without (7%) a previous CyR on standard-dose imatinib. Three-quarters of individuals maintained their CyR at 18 months in the one study reporting this outcome. HR ranged between 55.5% and 91.8% in three studies, but duration of HR was not reported by the studies. A complete molecular response was found in 13.5% of participants in one study, and a MMR was found in 12.2% of participants in another study. In one single-arm cohort study, median time to treatment failure was 27 months for chronic phase (n = 64), 2.5 months for accelerated phase (n = 3) and 4 months for blast-crisis phase (n = 4). In the RCT, the median time to treatment failure was 3.5 months (95% CI 3.3 to 3.8 months) and the estimated proportion of participants without treatment failure at 24 months was 18%. Event-free survival at 2 years was estimated to be 34% by one study, whereas progression-free survival was estimated at 65% to 87%. Overall survival was estimated at 85% in one study and 93% in another, but the studies used different definitions so the outcomes are not directly comparable.
Grades 3–4 haematological AEs occurred in up to 40% if individuals, but grades 3–4 non-haematological events were fairly rare, with none occurring in more than 5% of individuals. AEs of any grade included anorexia, diarrhoea, fatigue, muscle spasms, musculoskeletal pain, superficial oedema and rash.
Cost-effectiveness
One cost-effectiveness study was identified for this update. This compared dasatinib with high-dose imatinib. The results showed that chronic-phase CML patients resistant to standard-dose imatinib gain 0.62 QALYs when treated with dasatinib compared with high-dose imatinib and the incremental societal cost is €4250 during the lifetime period or €6880 per QALY gained. It is unclear how generalisable these results are to the UK NHS as the study was conducted in Sweden and takes a societal perspective.
In addition, economic evaluations from two manufacturer submissions were reviewed and critiqued and the economic evaluation presented in the PenTAG AR2 is also summarised and critiqued.
The Novartis4 economic evaluation compared nilotinib with high-dose imatinib for those with chronic-phase CML who are imatinib resistant. An exploratory analysis versus stem cell transplantation/hydroxycarbamide was also undertaken. The results showed that nilotinib dominates high-dose imatinib (i.e. is more effective and less costly). Exploratory analysis gives an ICER of about £44,000 for nilotinib versus stem cell transplantation/hydroxycarbamide.
The BMS3 economic evaluation compared dasatinib, nilotinib and high-dose imatinib with standard-dose imatinib, stem cell transplantation, hydroxycarbamide, interferon alfa, acute leukaemia-style chemotherapy and best supportive care for patients with CML who are resistant to imatinib. The results showed that dasatinib dominates high-dose imatinib, nilotinib and stem cell transplantation.
The PenTAG AR2 economic evaluation compared dasatinib and nilotinib with high-dose imatinib, and also compared the three treatments with interferon alfa, for patients with CML who are resistant to imatinib. The results showed that nilotinib dominates high-dose imatinib and the ICER for nilotinib versus interferon alfa is about £44,600. The ICER for dasatinib versus nilotinib is > £277,000.
All three economic evaluations were assessed in terms of their study quality and were deemed to be appropriately conducted. There are two main differences in the assumptions of the two industry models, which drive the differences between the models:
-
In the BMS3 model, patients are treated until progression, which incurs greater costs.
-
In the Novartis4 model, the assumed third-line treatment is stem cell transplantation/hydroxycarbamide, which has associated high mortality and reduced overall survival.
We therefore used the PenTAG AR2 economic model to explore further analyses using some different data inputs and to include the three interventions, dasatinib, nilotinib and high-dose imatinib, and the comparators interferon alfa, standard-dose imatinib, stem cell transplantation and hydroxycarbamide.
The SHTAC analysis
An exploratory analysis, using the PenTAG AR2 economic model, suggested that dasatinib, nilotinib and high-dose imatinib have similar costs and effectiveness. Nilotinib, dasatinib and high-dose imatinib are all cost-effective when compared with hydroxycarbamide, for a WTP of about £30,000 per QALY. Nilotinib and dasatinib are slightly more cost-effective than high-dose imatinib because of slightly lower costs and better effectiveness than high-dose imatinib. However, there is great uncertainty around the data inputs and, as such, it is not possible to derive firm conclusions about the relative cost-effectiveness of the three interventions. Where possible, exploration of these uncertainties was undertaken using sensitivity analyses. Deterministic sensitivity analyses showed that changes in overall survival for hydroxycarbamide and changes in treatment efficacy of the interventions had little impact on results. A probabilistic sensitivity analysis was run comparing the interventions dasatinib, nilotinib, high-dose imatinib and hydroxycarbamide. For a WTP threshold of £20,000 per QALY, hydroxycarbamide would be the most cost-effective treatment. For a WTP threshold of £30,000 per QALY, nilotinib, dasatinib, hydroxycarbamide and high-dose imatinib have a probability of being cost-effective of 60%, 28%, 12% and 0%, respectively.
Issues
There are several important issues of relevance to this update report:
-
As already described, the lack of comparative clinical effectiveness data has hindered the assessment of dasatinib, nilotinib and high-dose imatinib for people with imatinib-resistant CML.
-
In addition, the included studies were heterogeneous in terms of the definitions of imatinib resistance and other patient characteristics. The lengths of follow-up of studies varied, and many studies had small sample sizes and other methodological issues that increase the risk of bias.
-
In most cases the outcomes reported are surrogates for assessing the effects of the treatments, with few reliable estimates of final outcomes, such as survival. In addition, the definitions used for these outcomes varied.
-
We were not able to identify any new evidence on the effects of these treatments in those with accelerated-phase or blast-crisis-phase CML and, as such, are unable to fully inform the scope of this appraisal.
-
This report has reviewed the three economic models submitted by BMS,3 Novartis4 and PenTAG. 2 Although all three models were of reasonable quality, and used similar model structures, there were some notable differences between them in assumptions and parameter estimates, which produced large differences between model results.
-
The inadequacies in the evidence base are such that all cost-effectiveness analyses must be regarded as an exploration of uncertainty. Although this is not satisfactory, no other approach can be justified in the absence of so many data needed to populate a model.
-
Of the three economic models submitted, the one from PenTAG2 appeared to be the most structurally robust, and we have used this model for our analyses. We have some concerns over some of the assumptions and parameter values used and these have been discussed and explored with sensitivity analyses.
-
Concerns have been expressed by one of our advisors that the models do not reflect clinical practice, in particular that patients treated with one of the interventions who do not respond are likely to then receive an alternative (nilotinib may be followed by dasatinib, for example). It has not been possible to capture this third-line therapy in the model owing to the paucity of data available. The PenTAG AR2 model is limited to second-line treatment for the chronic phase of CML in imatinib-resistant patients and does not consider the whole clinical pathway, although an average cost has been included for third-line and subsequent treatment. PenTAG2 defended this approach by asserting that this would not make a significant difference to marginal costs and benefits. This seems a reasonable assumption given the lack of data to indicate otherwise.
-
There are concerns over the comparators used in the economic analyses, as these do not reflect current clinical practice.
-
The PenTAG AR2 model is based on response to treatment (major cytogenetic response) and is therefore a reflection of the treatment approach even if it is limited to one part of the clinical pathway and one phase of the disease. However, there are large concerns over the use of surrogates to predict final outcomes such as overall survival.
-
Results are presented using the principles of incremental analysis, where each technology is compared with the next cheapest non-dominated alternative. Our analyses are presented for each of the interventions and comparators in the appraisal scope, i.e. high-dose imatinib, dasatinib and nilotinib have been compared against hydroxycarbamide, stem cell transplantation, standard-dose imatinib and interferon alfa. According to this analysis, stem cell transplantation, standard-dose imatinib and interferon alfa are all dominated by other treatments and therefore the base option is considered to be hydroxycarbamide as it is the least costly of the available alternatives. Although there may be issues with this assumption, it is was considered the most appropriate option.
-
In keeping with the PenTAG AR,2 the Jabbour and colleagues23 study has been used as a source of efficacy data for high-dose imatinib in the exploratory economic analysis. However, this is a retrospective study, and sensitivity analyses have been conducted to explore the effect of alternative assumptions based on the range of data shown in the review of clinical effectiveness.
Strengths and limitations of the assessment
This report has a number of strengths:
-
It is independent of any vested interest and has been undertaken following the principles for conducting a systematic review. The methods were set out in an a priori research protocol (see Appendix 1), which defined the research question, inclusion criteria, quality criteria, data extraction process and methods to be used at different stages of the review.
-
The review updates a previous assessment and brings together new evidence on the clinical effectiveness and cost-effectiveness of dasatinib, nilotinib and high-dose imatinib for those with imatinib-resistant CML. This evidence has been critically appraised and presented in a consistent and transparent manner.
-
In addition, the review was informed by comments received from an expert advisory group and the advisory group has reviewed and commented on the final report.
However, this review also has certain limitations:
-
Although searches were not limited to the English language, time and resource constraints meant that we were unable to retrieve some foreign-language papers and therefore we may have omitted non-English-language, but otherwise eligible, studies from our review.
-
Synthesis of the included studies was through narrative review, as differences in the included studies precluded any statistical pooling of the data.
-
There are a number of uncertainties around the data inputs used in the economic modelling and therefore caution is required in the interpretation of our results in terms of cost-effectiveness.
Chapter 6 Conclusions
Chronic-phase chronic myeloid leukaemia
The PenTAG AR2 (see p. 368) concluded that effectiveness data were limited, but dasatinib and nilotinib appeared efficacious in terms of obtaining CyRs and HRs in the imatinib-resistant population. The extent to which greater frequency and/or degrees of response would impact on long-term outcomes was more difficult to conclude given the limited nature of the evidence base. In particular, only one study had compared either agent (dasatinib) with high-dose imatinib. The findings of this open-label study, that higher proportions of patients experienced positive responses to dasatinib than to high-dose imatinib, were importantly confounded by substantial crossover at an early point in follow-up.
The findings of the updated systematic review do not alter the conclusions of the PenTAG AR. 2 Additional data on the clinical effectiveness of high-dose imatinib have been identified, which suggest that CyRs and HRs can be obtained in a proportion of imatinib-resistant people. However, there remains an absence of evidence with which to assess the relative effectiveness of dasatinib, nilotinib and high-dose imatinib in imatinib-resistant CML.
The uncertainties in the data mean that our exploratory cost-effectiveness analysis should be interpreted with caution. Although we have attempted to address the key areas of uncertainty in this update analysis, we do not feel able to make firm conclusions regarding the use of these technologies in chronic-phase CML patients.
Accelerated- and blast-phase chronic myeloid leukaemia
The PenTAG AR2 did not produce a de novo economic evaluation for accelerated- and blast-phase CML owing to the lack of relevant clinical effectiveness data for comparator treatments. No new evidence on dasatinib, nilotinib and high-dose imatinib was identified in accelerated or blast phase imatinib-resistant CML.
Suggested research priorities
The lack of comparative evidence hindered the assessment of the clinical effectiveness and cost-effectiveness of dasatinib, nilotinib and high-dose imatinib for people with imatinib-resistant CML. The PenTAG AR2 recommended that a three-way, double-blind, RCT of dasatinib, nilotinib and high-dose imatinib should be undertaken. It is our view that it remains a research priority to undertake a comparative study, and where feasible this should be randomised; however, we note that it is unlikely that a double-blind study could be undertaken owing to the different dosing schedules of the treatments.
Acknowledgements
We would like to thank members of our advisory group who provided expert advice and comments on the draft of this report: Professor J Apperley, Department of Haematology, Imperial College London; Professor S Bryan, School of Population and Public Health, University of British Columbia; and Professor J Goldman, Emeritus Professor of Haematology, Imperial College London.
We are grateful to WMHTAC, which developed the original protocol, undertook the searches of the literature databases, and screened titles and abstracts for inclusion in the review of clinical effectiveness and cost-effectiveness.
We are also grateful to Jonathan Shepherd, Principal Research Fellow, SHTAC, and Jeremy Jones, Principal Research Fellow in Health Economics, SHTAC, for reviewing a draft of this report, and Karen Welch, Information Specialist, SHTAC, and Angela Vincent, Research Programme Administrator, SHTAC, for running the updated searches and the retrieval of references, respectively.
Contribution of authors
E Loveman (Senior Research Fellow) developed the research protocol, assessed studies for inclusion, drafted and edited the final report, and project managed the study.
K Cooper (Senior Research Fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, undertook the economic analyses, and drafted the report.
J Bryant (Principal Research Fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, undertook the economic analysis and drafted the report.
JL Colquitt (Senior Research Fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted the final report.
GK Frampton (Research Fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, and drafted the final report.
A Clegg (Professor/Director of SHTAC) developed the research protocol, and drafted/edited the final report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- National Institute for Health and Clinical Excellence (NICE) . Imatinib-Intolerant Chronic Myeloid Leukemia: Dasatinib and Nilotinib. Project History n.d. http://guidance.nice.org.uk/TA/Wave17/18#history (accessed 27 January 2011).
- Rogers G, Hoyle M, Thompson Coon J, Moxham T, Liu Z, Pitt M, et al. Dasatinib and nilotinib for imatinib-resistant or -intolerant chronic myeloid leukaemia: a systematic review and economic evaluation. Health Technol Assess 2012;16.
- Bristol-Myers Squibb Pharmaceuticals . Dasatinib, High-Dose Imatinib and Nilotinib for the Treatment of Imatinib-Resistant Chronic Myeloid Leukaemia (CML) (Part Review of TA70) 2010.
- Novartis Pharmaceuticals UK . Nilotinib, Within Its Licensed Indications, for the Treatment of Adults With Chronic Myeloid Leukaemia Who Are Resistant to Standard-Dose Imatinib 2010.
- Dybko J, Medras E, Haus O, Jazwiec J, Duszenko E, Jaskowiec A, et al. Dasatinib is a potent agent in second line treatment for chronic phase chronic myeloid leukemia patients: single center experience. Haematologica 2010;95:542-3.
- Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood 2007;109:5143-50.
- Kantarjian H, Pasquini R, Levy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily. Cancer 2009;115:4136-47.
- Rajappa S, Mallavarapu KM, Gundeti S, Roshnipaul T, Jacob RT, Digumarti R. Kinase domain mutations and responses to dose escalation in chronic myeloid leukemia resistant to standard dose imatinib mesylate. Leuk Lymphoma 2010;51:79-84.
- Breccia M, Stagno F, Vigneri P, Latagliata R, Cannella L, Del Fabro V, et al. Imatinib dose escalation in 74 failure or suboptimal response chronic myeloid leukaemia patients at 3-year follow-up. Am J Hematol 2010;85:375-7.
- Koh Y, Kim I, Yoon SS, Kim BK, Kim DY, Lee JH, et al. Phase IV study evaluating efficacy of escalated dose of imatinib in chronic myeloid leukemia patients showing suboptimal response to standard dose imatinib. Ann Hematol 2010;89:725-31.
- Baccarani M, Rosti G, Saglio G, Cortes J, Stone R, Niederwieser DW, et al. Dasatinib time to and Durability of major and complete cytogenetic response (MCyR and CCyR) in patients with chronic myeloid leukemia in chronic phase (CML-CP). ASH Annual Meeting Abstracts 2008;112.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technology Assessment 2004;8.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Ghatnekar O, Hjalte F, Taylor M. Cost-effectiveness of dasatinib versus high-dose imatinib in patients with chronic myeloid leukemia (CML), resistant to standard dose imatinib: a Swedish model application. Acta Oncol 2010;49:851-8.
- Levy AR, Szabo SM, Tabberer M, Davis C. Utility values for health states for chronic myelogenous leukaemia (CML): estimates from laypersons in Australia, The United Kingdom (UK) and Canada. Value Health 2007;10.
- Kantarjian HM, Larson RA, Guilhot F, O’Brien SG, Mone M, Rudoltz M, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer 2009;115:551-60.
- Gratwohl A, Brand R, Apperley J, Crawley C, Ruutu T, Corradini P, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: Transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2006;91:513-21.
- Reed SD, Anstrom KJ, Ludmer JA, Glendenning GA, Schulman KA. Cost-effectiveness of imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 2004;101:2574-83.
- Reed SD, Anstrom KJ, Li Y, Schulman KA. Updated estimates of survival and cost effectiveness for imatinib versus interferon-alpha plus low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukaemia. Pharmacoeconomics 2008;26:435-46.
- Department of Health (DOH) . NHS Reference Costs 2006 7 2008.
- Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010;95:232-40.
- Jabbour E, Kantarjian HM, Jones D, Shan J, O’Brien S, Reddy N, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood 2009;113:2154-60.
- Szabo SM, Levy AR, Davis C, Holyoake TL, Cortes J. A multinational study of health state preference values associated with chronic myelogenous leukemia. Value Health 2010;13:103-11.
- Dalziel K, Round A, Stein K, Garside R, Price A. Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukemia in chronic phase: a systematic review and economic analysis. Health Technology Assess 2004;8.
- Allan NC, Richards SM, Shepherd PC. Medical Research Council randomised, multicentre trial of interferon-alpha n1 for chronic myeloid leukaemia: improved survival irrespective of cytogenetic response. The UK Medical Research Council’s Working Parties for Therapeutic Trials in Adult Leukaemia. Lancet 1995;345:1392-7.
- Centre for Reviews and Dissemination (CRD) . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
Appendix 1 Report methods for the synthesis of evidence of clinical effectiveness and cost-effectiveness
The systematic review of clinical effectiveness and cost effectiveness undertaken in the previous report of dasatinib and nilotinib for people with imatinib-resistant and imatinib-intolerant CML2 will be updated for those with imatinib-resistant disease only.
Search strategy
A systematic review will be conducted to obtain all relevant studies investigating dasatinib, nilotinib or high-dose imatinib in patients with imatinib-resistant CML.
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles outlined in CRD report entitled ‘Undertaking Systematic Reviews of Research on Effectiveness’. 27
Searches will be undertaken from database inception to 2010 inclusive and search strategies will include a combination of text words and index terms relating to chronic myeloid leukaemia and the interventions (imatinib, dasatinib and nilotinib). Separate searches will be conducted to identify studies of clinical effectiveness, cost-effectiveness, HRQoL. The following resources will be used with no language restrictions:
-
Bibliographic databases – MEDLINE (Ovid) 1950–present, EMBASE (Ovid) 1980-present, CINAHL (EBSCO) 1982–present, Cochrane (Wiley) CENTRAL current issue, the NHS CRD HTA database and the Science Citation Index 1981–present for clinical effectiveness studies.
-
Current controlled trials metaRegister, ISRCTN database, WHO ICTRP Portal and ClinicalTrials.gov for ongoing studies.
-
Subject specific internet sites.
-
Specialist abstract and conference proceeding resources (British Library’s Electronic Table of Contents – ZETOC – and ISI Proceedings).
-
Consultation with experts in the field.
-
Checking of reference lists of included studies and relevant reviews.
Selection of studies
Title/abstract screening
Titles/abstracts will be screened and studies will be selected for full-paper retrieval according to the following criteria:
-
patients have CML
-
patients have experienced disease progression while being treated with imatinib
-
dasatinib, nilotinib or high-dose imatinib are used.
Where this information is not available, and it is not fully clear that the study does not fit the inclusion criteria, full-paper copies will be retrieved for further assessment.
Study inclusion/exclusion criteria
Full papers will be assessed for inclusion with reference to the patients, interventions, comparators and outcomes described in the decision problem above. Questions for inclusion/exclusion are designed to be less specific than details stated in the decision problem so that all trials and studies that can potentially contribute relevant data will be included.
Population
Patients with imatinib-resistant CML in the chronic, accelerated or blast phase. Included studies will be those where:
-
patients have undergone previous treatment with imatinib
-
patients experience imatinib resistance, as classified by study authors.
Studies of imatinib-intolerant or imatinib-naive patients will be excluded from the review.
Interventions
Studies of dasatinib, nilotinib and high-dose imatinib at any dosage will be considered for inclusion in the review.
Comparators
Potential comparators are dasatinib, nilotinib and high-dose imatinib, hydroxycarbamide, interferon alfa, acute leukaemia-style chemotherapy, allogeneic stem cell transport and standard-dose imatinib.
Studies with these treatments at any dosage levels will be considered for inclusion in the review.
Outcomes
Studies must include one or more of the following outcome measures for inclusion in this review:
-
treatment response rates (including molecular, cytogenetic and haematological responses)
-
time to and duration of response
-
overall survival
-
event-free survival
-
progression-free survival
-
adverse effects of treatment
-
health-related quality of life
-
time to treatment failure
-
cost per QALY.
Study design
The hierarchy of evidence will be used to determine the inclusion of trials and studies into the review. RCTs or prospective non-randomised comparative studies, where adequate matching is considered to have been achieved, will be included in the review. Depending on the volume of relevant literature identified, prospective non-comparative studies, such as case series, will be obtained and identified in the review, but it is anticipated that these may not contribute to the discussion of clinical effectiveness findings.
Studies published as abstracts or conference presentations will be included only if sufficient details are presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
For the systematic review of cost-effectiveness, studies will be included only if they report the results of full economic evaluations, i.e. cost-effectiveness analyses, cost-utility analyses or cost–benefit analyses.
Quality assessment and data extraction
The quality assessment of studies will be conducted using the quality criteria outlined in the previous report. 2 Study quality will be assessed by one reviewer and checked by a second reviewer. Any disagreements will be resolved by consensus with reference to a third reviewer where necessary. Data extraction of pre-specified information on features of the trial/study design, population, intervention, comparators, outcomes and results will be conducted by one reviewer and checked by a second reviewer to ensure accuracy.
Methods of synthesis and analysis
Studies in patients with chronic-, accelerated- and blast-phase CML will be reviewed separately where data permit. Where data are reported as pooled results for different types of patients, where it is not possible to disaggregate results, pooled results will be presented in the review. It is also anticipated that, in trials and studies, there may not always be clarity around whether patients are in chronic, accelerated or blast phases and whether they move between phases during the course of trials or studies. It is therefore anticipated that some judgement may be required to assign data to different phases.
Studies using different comparators will be grouped separately. Additional comparisons may be undertaken, as guided by the available evidence, industry submissions and clinical input. Where data allows, studies will be combined using meta-analysis. Where results for different treatment doses are combined, this will be highlighted. However, it is anticipated that study heterogeneity may limit this type of analysis. In this case, presentation of individual study results and qualitative combination of the data will be used. The use of indirect comparisons of treatments used across different studies will be considered subject to the quantity and quality of available evidence.
Sub-group analysis
Patients in different phases of CML will be considered separately in the main review and these are therefore not considered to be sub-groups. If evidence allows, patients with different levels of previous imatinib response will be considered in a sub-group analysis.
Report methods for synthesising evidence of cost-effectiveness
Cost-effectiveness review
The sources outlined above (see Search strategy) will be used to identify studies of the cost-effectiveness of dasatinib, nilotinib or high-dose imatinib in patients with imatinib-resistant CML. The inclusion and exclusion criteria for the systematic review of published cost-effectiveness studies will be identical to that applied in the review of clinical effectiveness, with the exception of study design (see Study inclusion/exclusion criteria). The quality of the included economic evaluations will be assessed using a critical appraisal checklist based upon that proposed by Drummond and colleagues13 and Philips and colleagues. 12 The data from these studies will be tabulated and discussed in a narrative review.
Economic evaluation
An assessment of dasatinib and nilotinib as the intervention treatments compared with high-dose imatinib has recently been conducted. 2 In this previous assessment, an economic model was developed in which high-dose imatinib and interferon alfa were used as comparators in the modelling approach. We will critically appraise this existing model using the same checklist as specified above (see Cost-effectiveness review). In addition we will adapt this existing model to run updated analyses for the current assessment to reflect the current scope. That is, we will compare each intervention with one another and against all comparators specified in Study inclusion/exclusion criteria, if feasible and appropriate. Additional searches will be undertaken if required to inform specific parameters; these will be determined during the review of the model.
Handling the company submission(s)
It is anticipated that separate manufacturer submissions will be received for dasatinib, nilotinib and imatinib. Company submissions by the manufacturers/sponsors will be considered if received by the Technology Assessment Report team with adequate time in which to incorporate data. These dates are subject to the timelines for this review.
If the clinical information in company submissions meets the inclusion criteria for the review, it will be extracted and quality assessed in accordance with the procedures outlined in this protocol. Any economic evaluations included in the company submission, provided they comply with NICE’s advice on presentation, will be assessed for clinical validity, reasonableness of assumptions and appropriateness of the data used in the economic model. Economic models will be appraised and an assessment of the reliability of the cost-effectiveness estimates will be provided. Sensitivity analysis of the manufacturer’s model will be undertaken where possible and appropriate to test the robustness of the model results.
Any ‘commercial-in-confidence’ data taken from a company submission, and specified as confidential in the check list, will be highlighted in blue and underlined in the assessment report (followed by an indication of the relevant company name e.g. in brackets).
Appendix 2 Search strategy
The MEDLINE search strategy for the clinical effectiveness section (presented below) was adjusted as necessary for cost-effectiveness searches and other electronic database searches. Search strategies for the systematic review are available from the authors on request.
Database: MEDLINE (Ovid) 1950 to July week 1 2010
Search strategy:
-
imatinib.mp.
-
glivec.mp.
-
gleevec.mp.
-
sti571.mp.
-
sti 571.mp.
-
sti-571.mp.
-
STI 571.mp.
-
STI571.mp.
-
STI-571.mp.
-
nilotinib.mp.
-
tasigna.mp.
-
AMN107.mp.
-
AMN 107.mp.
-
AMN-107.mp.
-
dasatinib.mp.
-
sprycel.mp.
-
BMS354825.mp.
-
BMS-354825.mp.
-
BMS 354825.mp.
-
or/1-19
-
chronic myeloid leuk?emia.mp. or exp Leukemia, Myelogenous, Chronic, BCR-ABL Positive/
-
(chronic myel$ adj2 leuk?emia).mp.
-
exp Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative/
-
cml.mp.
-
or/21-24
-
20 and 25.
Appendix 3 List of excluded studies
-
Baldazzi C, Luatti S, Marzocchi G, Stacchini M, Gamberini C, Castagnetti F, et al. Emergence of clonal chromosomal abnormalities in Philadelphia negative hematopoiesis in chronic myeloid leukemia patients treated with nilotinib after failure of imatinib therapy. Leuk Res 2009;33:e218–20.
-
Bergeron A, Rea D, Levy V, Picard C, Meignin V, Tamburini J, et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am J Respir Crit Care Med 2007;176:814–18.
-
Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome: positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res 2008;14:352–9.
-
Breccia M, Palandri F, Iori AP, Colaci E, Latagliata R, Castagnetti F, et al. Second-generation tyrosine kinase inhibitors before allogeneic stem cell transplantation in patients with chronic myeloid leukemia resistant to imatinib. Leuk Res 2010;34:143–7.
-
Cervantes F, Lopez-Garrido P, Montero MI, Jonte F, Martinez J, Hernandez-Boluda JC, et al. Early intervention during imatinib therapy in patients with newly diagnosed chronic-phase chronic myeloid leukemia: a study of the Spanish PETHEMA group. Haematologica 2010;95:1317–24.
-
Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O’Brien S, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007;110:4005–11.
-
Cortes J, Rousselot P, Kim D-W, Ritchie E, Hamerschlak N, Coutre S, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood 2007;109:3207–13.
-
Cortes J, Kim D-W, Raffoux E, Martinelli G, Ritchie E, Roy L, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia 2008;22:2176–83.
-
Fabarius A, Haferlach C, Muller MC, Erben P, Lahaye T, Giehl M, et al. Dynamics of cytogenetic aberrations in Philadelphia chromosome positive and negative hematopoiesis during dasatinib therapy of chronic myeloid leukemia patients after imatinib failure. Haematologica 2007;92:834–7.
-
Faber E, Friedecky D, Micova K, Divoka M, Katrincsakova B, Rozmanova S, et al. Imatinib dose escalation in two patients with chronic myeloid leukemia, with low trough imatinib plasma levels measured at various intervals from the beginning of therapy and with suboptimal treatment response, leads to the achievement of higher plasma levels and MMR. Int J Hematol 2010;91:897–902.
-
Fava C, Kantarjian HM, Jabbour E, O’Brien S, Jain N, Rios MB, et al. Failure to achieve a complete hematologic response at the time of a major cytogenetic response with second-generation tyrosine kinase inhibitors is associated with a poor prognosis among patients with chronic myeloid leukemia in accelerated or blast phase. Blood 2009;113:5058–63.
-
Garg RJ, Kantarjian H, O’Brien S, Quintas-Cardama A, Faderl S, Estrov Z, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-up. Blood 2009;114:4361–8.
-
Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia 2010;24:1299–301.
-
Hazarika M, Jiang X, Liu Q, Lee S-L, Ramchandani R, Garnett C, et al. Tasigna for chronic and accelerated phase Philadelphia chromosome-positive chronic myelogenous leukemia resistant to or intolerant of imatinib. Clin Caner Res 2008;14:5325–31.
-
Hochhaus A, Muller MC, Radich J, Branford S, Kantarjian HM, Hanfstein B, et al. Dasatinib-associated major molecular responses in patients with chronic myeloid leukemia in chronic phase following imatinib failure: response dynamics and predictive value. Leukemia 2009;23:1628–33.
-
Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood 2008;112:3965–73.
-
Jabbour E, Cortes J, Kantarjian HM, Giralt S, Jones D, Jones R, et al. Allogeneic stem cell transplantation for patients with chronic myeloid leukemia and acute lymphocytic leukemia after Bcr-Abl kinase mutation-related imatinib failure. Blood 2006;108:1421–3.
-
Jabbour E, Cortes J, Kantarjian H, Giralt S, Andersson BS, Giles F, et al. Novel tyrosine kinase inhibitor therapy before allogeneic stem cell transplantation in patients with chronic myeloid leukemia: No evidence for increased transplant-related toxicity. Cancer 2007;110:340–4.
-
Jabbour E, Jones D, Kantarjian HM, O’Brien S, Tam C, Koller C, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood 2009;114:2037–43.
-
Jabbour E, Kantarjian HM, Jones D, Shan J, O’Brien S, Reddy N, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood 2009;113:2154–60.
-
Kantarjian HM, Talpaz M, O’Brien S, Giles F, Garcia-Manero G, Faded S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 2003;101:473–5.
-
Kantarjian H, O’Brien S, Talpaz M, Borthakur G, Ravandi F, Faderl S, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer 2007;109:1556–60.
-
Kantarjian HM, Larson RA, Guilhot F, O’Brien SG, Mone M, Rudoltz M, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer 2009;115:551–60.
-
Kim D-W, Goh Y-T, Hsiao H-H, Caguioa PB, Kim D, Kim W-S, et al. Clinical profile of dasatinib in Asian and non-Asian patients with chronic myeloid leukemia. Int J Hematol 2009;89:664–72.
-
Kim DH, Kamel-Reid S, Chang H, Sutherland R, Jung CW, Kim H-J, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica 2009;94:135–9.
-
Klamova H, Faber E, Zackova D, Markova M, Voglova J, Cmunt E, et al. Dasatinib in imatinib-resistant or -intolerant CML patients: data from the clinical practice of 6 hematological centers in the Czech Republic. Neoplasma 2010;57:355–9.
-
Le CP, Ottmann OG, Giles F, Kim D-W, Cortes J, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood 2008;111:1834-9.
-
Milojkovic D, Nicholson E, Apperley JF, Holyoake TL, Shepherd P, Drummond MW, et al. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica 2010;95:224–31.
-
Piazza RG, Magistroni V, Andreoni F, Franceschino A, Tornaghi L, Varella-Garcia M, et al. Imatinib dose increase up to 1200 mg daily can induce new durable complete cytogenetic remissions in relapsed Ph+ chronic myeloid leukemia patients. Leukemia 2005;19:1985–7.
-
Poire X, Artz A, Larson RA, Kline J, Odenike O, Rich E, et al. Allogeneic stem cell transplantation with alemtuzumab-based conditioning for patients with advanced chronic myelogenous leukemia. Leuk Lymphoma 2009;50:85–91.
-
Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 2008;112:1005–12.
-
Quintas-Cardama A, Kantarjian H, O’Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol 2007;25:3908–14.
-
Quintas-Cardama A, Kantarjian H, Jones D, Nicaise C, O’Brien S, Giles F, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood 2007;109:497–9.
-
Quintas-Cardama A, Cortes JE, O’Brien S, Ravandi F, Borthakur G, Liu D, et al. Dasatinib early intervention after cytogenetic or hematologic resistance to imatinib in patients with chronic myeloid leukemia. Cancer 2009;115:2912–21.
-
Rea D, Etienne G, Corm S, Cony-Makhoul P, Gardembas M, Legros L, et al. Imatinib dose escalation for chronic phase-chronic myelogenous leukaemia patients in primary suboptimal response to imatinib 400 mg daily standard therapy. Leukemia 2009;23:1193–6.
-
Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer 2010;116:3852–61.
-
Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: Evaluation of its impact within a subgroup of the randomized German CML study IV. Blood 2010;115:1880–5.
-
Saydam G, Haznedaroglu IC, Temiz Y, Soysal T, Sucak G, Tombuloglu M, et al. Retrospective evaluation of patients treated with dasatinib for Philadelphia-positive leukemias: Turkish experience of 16 months. UHOD Int J Hematol Oncol 2009;19:195–204.
-
Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Bohm A, et al. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest 2009;39:1098–109.
-
Sohn SK, Moon JH, Cho YY, Chae YS, Kim JG, Lee KS, et al. Efficacy of dose escalation of imatinib mesylate in patients with cytogenetic or hematologic resistance. Leuk Lymphoma 2007;48:1659–61.
-
Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006;354:2531–41.
-
Tojo A, Usuki K, Urabe A, Maeda Y, Kobayashi Y, Jinnai I, et al. A Phase I/II study of nilotinib in Japanese patients with imatinib-resistant or -intolerant Ph+ CML or relapsed/refractory Ph+ ALL. Int J Hematol 2009;89:679–88.
-
Verma D, Kantarjian H, Shan J, O’Brien S, Estrov Z, Garcia-Manero G, et al. Survival outcomes for clonal evolution in chronic myeloid leukemia patients on second generation tyrosine kinase inhibitor therapy. Cancer 2010;116:2673–81.
-
Yavuz AS, Elcioglu OC, Akpinar T, Cosan F, Ucur A, Bayrak A, et al. Nilotinib efficacy in 21 imatinib-resistant or-intolerant T (9;22) positive chronic myeloid leukemia patients with and without additional chromosomal changes. Nobel Med 2010;6:57–62.
-
Zhou L, Meng F, Yin O, Wang J, Wang Y, Wei Y, et al. Nilotinib for imatinib-resistant or -intolerant chronic myeloid leukemia in chronic phase, accelerated phase, or blast crisis: A single- and multiple-dose, open-label pharmacokinetic study in Chinese patients. Clin Ther 2009;31:1568–75.
-
Zonder JA, Pemberton P, Brandt H, Mohamed AN, Schiffer CA. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate haematologic or cytogenetic response to initial treatment. Clin Cancer Res 2003;9:2092–7.
Appendix 4 Data extraction tables: studies of clinical effectiveness
Kantarjian et al.6,7
Study details
| Study details | Population | Arms | Outcomes |
|---|---|---|---|
|
Author(s): Kantarjian et al. Title: Dasatinib or HDI for CP-CML after failure of first-line imatinib: a randomised Phase II trial Study: Kantarjian et al. (2007),6 (2009)7 Secondary publications: See PenTAG AR,2 appendix 3, for list of secondary publications Trial code: START-R CP: Yes AP: No BC: No Countries: Argentina, Australia, Belgium, Brazil, Canada, Estonia, Finland, France, Germany, Israel, Republic of Korea, Norway, Peru, the Philippines, Poland, Puerto Rico, Russian Federation, South Africa, Sweden, Taiwan, Thailand, UK, USA No. of centres: 58 Notes: NCT00103844 |
Inclusion criteria Patients with CP-CML with primary or acquired resistance to conventional doses of imatinib (400–600 mg), dastinib naive, at least 18 years of age, and have adequate hepatic and renal function. CP was defined by the presence of < 15% blasts, < 20% basophils and < 30% blasts plus promyelocytes in peripheral blood or bone marrow and a platelet count of at least 100,000 per cubic millimetre, with no extramedullary involvement. Primary resistance to imatinib was defined as a lack of complete haematological response after 3 months of imatinib treatment, a lack of any CyR after 6 months of treatment or a lack of a MCyR (Ph+ cells > 35%) after 12 months of treatment. Relapse after a haematological response or MCyR was considered as secondary or acquired resistance Exclusion criteria Patients who had received imatinib in the 7 days before the study were ineligible, as were patients who had received imatinb at doses in excess of 600 mg per day. Patients with known specific BCR–ABL mutations (with high resistance to imatinib) before study entry were exclued Method of allocation Randomised 2 : 1 to receive dasatinib or HDI; randomisation was stratified by study site and CyR on imatinib (any response vs no response) Blinding Open label Therapy common to all participants Not reported |
Arm 1: Dasatinib n: 101 Drug: Dasatinib Starting daily dose (mg): 140 Dosage details 70 mg b.i.d., escalated to 180 mg for participants with inadequate response at 12 weeks or progression Reduced to 100 or 80 mg for participants experiencing toxicity Notes Crossover to the alternative treatment was permitted after confirmed progression, lack of MCyR at the week 12 cytogenetic evaluation or intolerance Arm 2: HDI n: 49 Drug: Imatinib Starting daily dose (mg): 800 Dosage details: 400 mg b.i.d. Reduction to 600 mg was permitted for toxicity in participants who had not previously received 600 mg ofimatinib Notes: Crossover to the alternative treatment was permitted after confirmed progression, lack of MCyR at the week 12 cytogenetic evaluation or intolerance |
CyR Evaluated through bone marrow aspirates every 12 weeks CCyR (0% Ph+) PCyR (1% and 35% Ph+) MCyR (complete + partial) Duration of MCyR Haematological response Weekly blood counts for the first 12 weeks of treatment and every 2 weeks thereafter CHR (WBCs ≤ institutional ULN; platelets < 450 × 109/l; no blasts or promyelocytes in peripheral blood; < 5% myelocytes plus metamyelocytes in peripheral blood; < 20% basophils in peripheral blood; no extramedullary involvement (including no hepatomegaly or splenomegaly) Molecular response MMR (not defined in paper or in study referenced as providing definitions of response; usually defined as a reduction in BCR–ABL transcript levels of at least 3 log) Survival Time to treatment failure [time from randomisation to progression (see PFS) or end of treatment (lack of response, study drug intolerance, or off treatment for any reason); subjects still on treatment were censored as of their last day of dosing] Progression-free survival (time from randomisation until disease progression (accelerated-phase disease, BC, loss of CHR or MCyR, or increasing WBC count), death, or discontinuation of treatment because of progression prior to crossover) Participant disposition Withdrawal owing to AEs AEs: grades 1–4 Assessed continuously and graded according to the NCI-CTC 3.0. Specific focus was given to cases of myelosuppression and fluid retention |
Baseline characteristics
| Dasatinib | HDI | p-value | |||||
|---|---|---|---|---|---|---|---|
| n | k | Mean | n | k | Mean | ||
| Demographics | |||||||
| Age (median) | 101 | 51 (range 24 to 85) | 49 | 51 (range 24 to 80) | |||
| Sex (n male) | 101 | 53 | (52.5%) | 49 | 22 | (44.9%) | 0.486a |
| Imatinib failure | |||||||
| Resistance: loss of MCyR | 101 | 21 | (20.8%) | 49 | 14 | (28.6%) | 0.395a |
| Resistance: loss of CHR | 101 | 24 | (23.8%) | 49 | 15 | (30.6%) | 0.485a |
| Resistance: increasing WBC count | 101 | 4 | (4.0%) | 49 | 2 | (4.1%) | 0.683a |
| Resistance: no CHR after 3 months | 101 | 3 | (3.0%) | 49 | 2 | (4.1%) | 0.897a |
| Resistance: no CyR after 6 months | 101 | 39 | (38.6%) | 49 | 16 | (32.7%) | 0.596a |
| Resistance: no MCyR after 12 months | 101 | 39 | (38.6%) | 49 | 24 | (49.0%) | 0.303a |
| Prior therapy | |||||||
| Best response to imatinib: CHR | 101 | 93 | (92.1%) | 49 | 47 | (95.9%) | 0.593a |
| Best response to imatinib: CCyR | 101 | 15 | (14.9%) | 49 | 4 | (8.2%) | 0.372a |
| Best response to imatinib: PCyR | 101 | 13 | (12.9%) | 49 | 10 | (20.4%) | 0.337a |
| Time on imatinib: < 1 year | 101 | 12 | (11.9%) | 49 | 5 | (10.2%) | 0.977a |
| Time on imatinib: 1–3 year | 101 | 44 | (43.6%) | 49 | 29 | (59.2%) | 0.105a |
| Time on imatinib: > 3 years | 101 | 45 | (44.6%) | 49 | 15 | (30.6%) | 0.145a |
| Highest imatinib dose: > 400 mg/day | 101 | 65 | (64.4%) | 49 | 35 | (71.4%) | 0.498a |
| Prior hydroxycarbamideb | 101 | 97 | (96.0%) | 49 | 46 | (93.9%) | 0.860a |
| Prior chemotherapy | 101 | 39 | (38.6%) | 49 | 18 | (36.7%) | 0.966a |
| Prior interferon | 101 | 74 | (73.3%) | 49 | 33 | (67.3%) | 0.576a |
| Prior transplantation | 101 | 7 | (6.9%) | 49 | 2 | (4.1%) | 0.747a |
| Disease history | |||||||
| Duration of CML (months) (median) | 101 | 64 (range 6 to 166) | 49 | 52 (range 14 to 133) | |||
| CHR at study entry | 101 | 51 | (50.5%) | 49 | 27 | (55.1%) | 0.722a |
| Baseline status | |||||||
| CHR at study entry | 101 | 51 | (50.5%) | 49 | 27 | (55.1%) | 0.722a |
| Disease history | |||||||
| MCyR at study entry | 101 | 6 | (5.9%) | 49 | 0 | (0.0%) | 0.283a |
| Baseline status | |||||||
| MCyR at study entry | 101 | 6 | (5.9%) | 49 | 0 | (0.0%) | 0.283a |
| Imatinib failure | |||||||
| BCR–ABL mutation | 101 | 41 | (40.6%) | 46c | 11 | (22.4%) | 0.045a |
| Baseline status | |||||||
| BCR–ABL mutation | 101 | 41 | (40.6%) | 49 | 11 | (22.4%) | 0.045a |
| Laboratory parameters | |||||||
| WBCs × 109/l (median) | 100c | 7.5 (range 2 to 153) | 48c | 7.4 (range 2 to 133) | |||
| WBCs: 20 × 109/l or more | 101 | 11 | (10.9%) | 49 | 7 | (14.3%) | 0.740a |
| Platelets × 109/l (median) | 101 | 261c (range 55 to 1903) | 49 | 248 (range 80 to 2318) | |||
Results
| Dasatinib | HDI | p-value | |||||
|---|---|---|---|---|---|---|---|
| n | k | Mean | n | k | Mean | ||
| Kantarjian et al. (2007)6 | |||||||
| At median follow-up, 15 months (range 1 to 21 months)a | |||||||
| CyR | |||||||
| CCyR | 101 | 40 | (39.6%) | 49 | 8 | (16.3%) | 0.007b |
| PCyR | 101 | 13 | (12.9%) | 49 | 8 | (16.3%) | 0.748b |
| MCyR | 101 | 53 | (52.5%) | 49 | 16 | (32.7%) | 0.035b |
| Duration of MCyR: 0 months | 53 | 1 | 16 | 1 | |||
| Duration of MCyR: 12 months | 7 | 0.98 | 0 | 0.425 | |||
| Duration of MCyR: 4 months | 44 | 0.98 | 9 | 0.835 | |||
| Duration of MCyR: 8 months | 37 | 0.98 | 6 | 0.835 | |||
| Haematological response | |||||||
| CHR | 101 | 94 | (93.1%) | 49 | 40 | (81.6%) | 0.065b |
| Molecular response | |||||||
| MMR | 101 | 16 | (15.8%) | 49 | 2c | (4.1%) | 0.070b |
| Study medication | |||||||
| Duration of study therapy (months) (median) | 101 | 13.7 (range 0.2 to 19.3) | 49 | 3.1 (range 0.2 to 15.6) | |||
| Average daily dose (mg/day) (median) | 101 | 103 (range 38 to 175) | 49 | 796 (range 358 to 800) | |||
| Survival | |||||||
| Time to treatment failure: 0 months | 101 | 1 | 49 | 1 | |||
| Time to treatment failure: 12 months | 66 | 0.74 | 9 | 0.205 | |||
| Time to treatment failure: 15 months | 17 | 0.715 | 0 | 0.155 | |||
| Time to treatment failure: 18 months | 4 | 0.715 | |||||
| Time to treatment failure: 3 months | 95 | 0.935 | 36 | 0.735 | |||
| Time to treatment failure: 6 months | 86 | 0.845 | 10 | 0.205 | |||
| Time to treatment failure: 9 months | 79 | 0.78 | 10 | 0.205 | |||
| PFS: 0 months | 101 | 1 | 49 | 1 | |||
| PFS: 12 months | 66 | 0.925 | 9 | 0.73 | |||
| PFS: 15 months | 17 | 0.925 | 0 | 0.545 | |||
| PFS: 18 months | 4 | 0.925 | |||||
| PFS: 3 months | 95 | 0.99 | 36 | 0.87 | |||
| PFS: 6 months | 86 | 0.975 | 10 | 0.73 | |||
| PFS: 9 months | 79 | 0.94 | 10 | 0.73 | |||
| Median time to treatment failure (months) | Not reached | 3.5 | (95% CI 3.3 to 3.8) | ||||
| Participant disposition | |||||||
| Withdrawal owing to AEsd | 101 | 16 | (15.8%) | 49 | 9 | (18.4%) | |
| AEs grades 1–4 | |||||||
| Anorexia | 101 | 13 | (12.9%) | 49 | 4 | (8.2%) | 0.748b |
| Asthenia | 101 | 13 | (12.9%) | 49 | 2 | (4.1%) | 0.563b |
| Diarrhoea | 101 | 35 | (34.7%) | 49 | 14 | (28.6%) | 0.008b |
| Dyspnoea | 101 | 21 | (20.8%) | 49 | 2 | (4.1%) | 0.087b |
| Face oedema | 101 | 4 | (4.0%) | 49 | 5 | (10.2%) | 0.003b |
| Fatigue | 101 | 30 | (29.7%) | 49 | 11 | (22.4%) | 0.099b |
| Headache | 101 | 25 | (24.8%) | 49 | 5 | (10.2%) | 0.701b |
| Muscle spasms | 101 | 2 | (2.0%) | 49 | 6 | (12.2%) | < 0.001b |
| Nausea | 101 | 24 | (23.8%) | 49 | 16 | (32.7%) | < 0.001b |
| Pain in extremity | 101 | 7 | (6.9%) | 49 | 5 | (10.2%) | 0.030b |
| Peripheral oedema | 101 | 10 | (9.9%) | 49 | 10 | (20.4%) | < 0.001b |
| Pleural effusion | 101 | 17 | (16.8%) | 49 | 0 | (0.0%) | 0.009b |
| Pyrexia | 101 | 14 | (13.9%) | 49 | 5 | (10.2%) | 0.431b |
| Rash | 101 | 17 | (16.8%) | 49 | 7 | (14.3%) | 0.147b |
| Superficial oedema | 101 | 15 | (14.9%) | 49 | 21 | (42.9%) | < 0.001b |
| Vomiting | 101 | 9 | (8.9%) | 49 | 12 | (24.5%) | < 0.001b |
| Weight increase | 101 | 5 | (5.0%) | 49 | 5 | (10.2%) | 0.008b |
| Haematological AEs grades 3–4 | |||||||
| Thrombocytopenia | 101 | 57 | (56.4%) | 49 | 7 | (14.3%) | |
| Neutropenia | 101 | 62 | (61.4%) | 49 | 19 | (38.8%) | |
| AEs grades 3–4 | |||||||
| Anorexia | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Asthenia | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Diarrhoea | 101 | 2 | (2.0%) | 49 | 1 | (2.0%) | 0.835b |
| Dyspnoea | 101 | 4 | (4.0%) | 49 | 0 | (0.0%) | 0.533b |
| Face oedema | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Fatigue | 101 | 2 | (2.0%) | 49 | 2 | (4.1%) | 0.171b |
| Headache | 101 | 2 | (2.0%) | 49 | 1 | (2.0%) | 0.835b |
| Muscle spasms | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Nausea | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Pain in extremity | 101 | 0 | (0.0%) | 49 | 1 | (2.0%) | 0.209b |
| Peripheral oedema | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Pleural effusion | 101 | 4 | (4.0%) | 49 | 0 | (0.0%) | 0.533b |
| Pyrexia | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Rash | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Superficial oedema | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Vomiting | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Weight increase | 101 | 0 | (0.0%) | 49 | 0 | (0.0%) | 0.482b |
| Kantarjian et al. (2009)7 | |||||||
| At 12 weeks | |||||||
| CyR | |||||||
| CCyR | 101 | 22 | 22% | 49 | 4 | 8% | 0.041 |
| MCyR | 101 | 36 | 36% (26.4% to 45.8%) | 49 | 14 | 29% (16.6% to 43.3%) | 0.402 |
| At median follow-up 26 months (range 6.9 to 32.7 months)a | |||||||
| Estimated proportion without treatment failure at 24 months | 101 | 59% | 49 | 18% | |||
| Estimated progression-free survival at 24 months | 101 | 86% | 49 | 65% | 0.0012 | ||
| Estimated progression | 101 | 13 | 49 | 10 | |||
| 600 mg/day subgroup | 63 | 11 | 34 | 8 | 0.0033 | ||
| 400 mg/day subgroup | 36 | 2 | 14 | 2 | 0.0562 | ||
| Primary resistance subgroup | 53 | 4 | 24 | 2 | 0.2110 | ||
| Acquired resistance subgroup | 43 | 9 | 24 | 8 | 0.0069 | ||
| Before crossovere | |||||||
| CyR | |||||||
| CCyR | 101 | 44 | 44% | 49 | 9 | 18% | 0.0025 |
| PCyR | 101 | 10 | 10% | 49 | 7 | 14% | |
| MCyR (95% CI) | 101 | 54 | 53% (43.3% to 63.5%) | 49 | 16 | 33% (19.9% to 47.5%) | 0.017 |
| Previous imatinib 600 mg/day | 63 | 32 | 51% | 34 | 8 | 24% | |
| Previous imatinib 400 mg/day | 36 | 22 | 61% | 14 | 7 | 50% | |
| % (95% CI) without loss of a MCyR at 18 months | 54 | 90% (82% to 98%) | 16 | 74% (49% to 100%) | |||
| MCyR in patients with a previous CyR on imatinib | |||||||
| All | 62 | 34 | 55% | 34 | 15 | 44% | |
| Previous imatinib 600 mg/day | 40 | 20 | 50% | 23 | 8 | 35% | |
| Previous imatinib 400 mg/day | 20 | 14 | 70% | 10 | 6 | 60% | |
| MCyR in patients without a previous CyR on imatinib | |||||||
| All | 39 | 20 | 51% | 15 | 1 | 7% | |
| Previous imatinib 600 mg/day | 23 | 12 | 52% | 11 | 0 | 0% | |
| Previous imatinib 400 mg/day | 16 | 8 | 50% | 4 | 1 | 25% | |
| CyR by imatinib resistancef | |||||||
| Primary resistance | 53 | 24 | |||||
| MCyR | 53 | 30 | 57% | 24 | 7 | 29% | |
| CCyR | 53 | 22 | 42% | 24 | 3 | 13% | |
| Acquired resistance | 43 | 24 | |||||
| MCyR | 43 | 21 | 49% | 24 | 8 | 33% | |
| CCyR | 43 | 19 | 44% | 24 | 5 | 21% | |
| With protocol-specified mutations | 17 | 2 | |||||
| MCyR | 17 | 7 | 41% | 2 | 0 | 0% | |
| CCyR | 17 | 4 | 24% | 2 | 0 | 0% | |
| Haematological response | |||||||
| CHR (95% CI) | 101 | 94 | 93.1% (86.25% to 97.2%) | 49 | 40 | 81.6% (68% to 91.2%) | 0.0341 |
| Without loss of CHR at 24 months,% (95% CI) | 84% (76% to 93%) | 73% (49% to 96%) | |||||
| Timing not specified | |||||||
| CCyR in patients without a baseline CCyR | 97 | 41 | 42% | 49 | 9 | 18% | |
| MCyR in patients without a baseline MCyR | 95 | 49 | 52% | 49 | 16 | 33% | |
| MMR | 101 | 29 | 29% | 49 | 6 | 12% | 0.028 |
| MMR in patients who had a CCyR and a molecular response assessment | 44 | 28 | 64% | 9 | 5 | 56% | |
| CHR in patients without CHR at baseline | 50 | 43 | 86% | 22 | 16 | 72% | |
| Treatment | |||||||
| Median (range) treatment duration (months) | 101 | 23 (0.16 to 29.4) | 49 | 3 (0.16 to 26.3) | |||
| Median (range) dose (mg/day) | 101 | 105 (42 to 177) | 49 | 796 (358 to 800) | |||
| Dose interruptions | 101 | 86 | 85% | 49 | 17 | 35% | |
| For haematological toxicity | 101 | 62 | 61% | 49 | 8 | 16% | |
| For non-haematological toxicity | 101 | 18 | 18% | 49 | 4 | 8% | |
| Dose reductions | 101 | 71 | 70% | 49 | 6 | 12% | |
| For haematological toxicity | 101 | 47 | 47% | 49 | 2 | 4% | |
| For non-haematological toxicity | 101 | 14 | 14% | 49 | 2 | 4% | |
| Withdrawalsd | |||||||
| From initial therapy | 101 | 50 | 50% | 49 | 40 | 82% | |
| Due to AEs | 101 | 23 | 23% | 49 | 10 | 20% | |
| Due to haematological AEs | 101 | 10 | 10% | 49 | 4 | 8% | |
| Due to non-haematological AEs | 101 | 13 | 13% | 49 | 6 | 12% | |
| Among patients who achieved a MCyR | |||||||
| Due to loss of major haematological response | 5% | 6% | |||||
| Due to intolerance | 3% | 4% | |||||
| Due to other reasons | 1% | 4% | |||||
| AEs grades 1–4g,h | |||||||
| Treatment related | 101 | 94 | 93% | 49 | 44 | 90% | |
| Abdominal pain | 101 | 15 | 15% | 49 | 4 | 8% | |
| Anorexia | 101 | 17 | 17% | 49 | 4 | 8% | |
| Asthenia | 101 | 15 | 15% | 49 | 2 | 4% | |
| Bleeding | 101 | 18 | 18% | 49 | 4 | 8% | |
| Diarrhoea | 101 | 37 | 37% | 49 | 14 | 29% | |
| Dyspnoea | 101 | 23 | 23% | 49 | 2 | 4% | |
| Face oedema | Not reported | Not reported | |||||
| Fatigue | 101 | 33 | 33% | 49 | 11 | 22% | |
| Fluid retention | 101 | 39 | 39% | 49 | 21 | 43% | |
| Headache | 101 | 26 | 26% | 49 | 5 | 10% | |
| Infection | 101 | 14 | 14% | 49 | 3 | 6% | |
| Muscle spasms | Not reported | Not reported | |||||
| Musculoskeletal pain | 101 | 21 | 21% | 49 | 6 | 12% | |
| Nausea | 101 | 24 | 24% | 49 | 16 | 33% | |
| Pain in extremity | Not reported | Not reported | |||||
| Peripheral oedema | Not reported | Not reported | |||||
| Pleural effusion | 101 | 25 | 25% | 49 | 0 | 0% | |
| Pyrexia | 101 | 14 | 14% | 49 | 5 | 10% | |
| Rash | 101 | 18 | 18% | 49 | 10 | 20% | |
| Superficial oedema | 101 | 20 | 20% | 49 | 21 | 43% | |
| Upper respiratory tract infection or inflammation | 101 | 11 | 11% | 49 | 3 | 6% | |
| Vomiting | 101 | 10 | 10% | 49 | 12 | 24% | |
| Weight increase | Not reported | Not reported | |||||
| AEs grades 3–4 | |||||||
| Treatment related | 101 | 62 | 61% | 49 | 19 | 39% | |
| Abdominal pain | 101 | 0 | 0% | 49 | 1 | 2% | |
| Anorexia | 101 | 0 | 0% | 49 | 0 | 0% | |
| Asthenia | 101 | 0 | 0% | 49 | 0 | 0% | |
| Bleeding | 101 | 1 | 1% | 49 | 0 | 0% | |
| Diarrhoea | 101 | 3 | 3% | 49 | 1 | 2% | |
| Dyspnoea | 101 | 5 | 5% | 49 | 0 | 0% | |
| Face oedema | Not reported | Not reported | |||||
| Fatigue | 101 | 3 | 3% | 49 | 2 | 4% | |
| Fluid retention | 101 | 7 | 7% | 49 | 0 | 0% | |
| Headache | 101 | 2 | 2% | 49 | 1 | 2% | |
| Infection | 101 | 4 | 4% | 49 | 0 | 0% | |
| Muscle spasms | Not reported | Not reported | |||||
| Musculoskeletal pain | 101 | 1 | 1% | 49 | 1 | 2% | |
| Nausea | 101 | 0 | 0% | 49 | 0 | 0% | |
| Pain in extremity | Not reported | Not reported | |||||
| Peripheral oedema | Not reported | Not reported | |||||
| Pleural effusion | 101 | 5 | 5% | 49 | 0 | 0% | |
| Pyrexia | 101 | 0 | 0% | 49 | 0 | 0% | |
| Rash | 101 | 0 | 0% | 49 | 0 | 0% | |
| Superficial oedema | 101 | 1 | 1% | 49 | 0 | 0% | |
| Upper respiratory tract infection or inflammation | 101 | 1 | 1% | 49 | 0 | 0% | |
| Vomiting | 101 | 0 | 0% | 49 | 0 | 0% | |
| Weight increase | Not reported | Not reported | |||||
| Haematological AEs grades 3–4 | |||||||
| Anaemia | 101 | 20 | 20% | 49 | 4 | 8% | |
| Leucopenia | 101 | 24 | 24% | 49 | 8 | 16% | |
| Neutropenia | 101 | 64 | 63% | 49 | 19 | 39% | |
| Thrombocytopenia | 101 | 58 | 57% | 49 | 7 | 14% | |
| Death | 101 | 2 | 49 | 0 | |||
Quality appraisal
|
Breccia et al.9
Study details
| Study details | Population | Arms | OUTCOMES |
|---|---|---|---|
|
Study: Breccia et al. (2010)9 Design: Cohort single arm; judged to be prospective although unclear reporting Chronic phase: Yes AP: No BC: No Country: Italy No. of centres: 2 Notes Investigated the long-term efficacy of dose escalation in patients with CP-CML who demonstrated a poor response or relapse after standard imatinib therapy |
Inclusion criteria CML patients who demonstrated a poor response or relapse after standard imatinib therapy; no other inclusion information given other than the table of baseline characteristics (below) Exclusion criteria Not reported |
Arm 1 n: 74 Drug: HDI Starting daily dose (mg): 600 or 800 Dose details Dose escalation to 600 or 800 mg/day if haematological failure, imatinib resistancea or suboptimal responseb Concurrent treatment None reported |
CyR Haematological response Molecular response Stated that CyR was assessed before dose escalation and thereafter at 3 and 6 months of therapy then every 6 months Survival Defined as the time from diagnosis to death or date of last follow-up Progression-free survival Defined from the time of the start of imatinib to progression to an advanced phase of disease AEs (Not explicitly specified as an outcome) |
Baseline characteristics
| Intervention | |||
|---|---|---|---|
| n | K | Median (range), percentage or score | |
| Demographics | |||
| Age, years: median (range) | 74 | 50 (19 to 85) | |
| Sex (n male) | 74 | 52 | 70.3%a |
| Disease status | |||
| WBC count (× 109/l) | 74 | 4.5 (3.8 to 6.2)b | |
| Platelet count (× 109/l) | 74 | 220 (180 to 350)b | |
| Haemoglobin concentration (g/dl) | 74 | 13 (11.8 to 15)b | |
| Sokal score | |||
| Low | 74 | 41b | |
| Intermediate | 74 | 24b | |
| High | 74 | 9b | |
| Treatment history | |||
| IFN-α (late CP-CML) | 74 | 22 | 29.7%a |
| Standard-dose imatinib only (early chronic phase) | 74 | 52 | 70.3%a |
| Cause of imatinib dose escalation | |||
| Primary resistancec | 74 | 34 | 45.9%a |
| Haematological | 74 | 10 | 13.5%a |
| Cytogenetic | 74 | 24 | 32.4%a |
| Secondary resistancec | 74 | 36 | 48.6%a |
| Haematological | 74 | 3 | 4.1%a |
| Cytogenetic | 74 | 33 | 44.5%a |
| Suboptimal responsec | 74 | 4 | 5.4%a |
| Cytogenetic | 3 | ||
| Molecular | 1 | ||
| Median (range) time from diagnosis to therapy (months) | 74 | 3 (1 to 13) | |
| Median (range) duration of imatinib therapy (months) | 74 | 36 (21 to 70) | |
| Dose escalation | |||
| From 400 to 600 mg/day | 74 | 54 | 73.0%c |
| From 400 to 800 mg/day | 74 | 20 | 27.0%c |
Results
| Intervention | |||
|---|---|---|---|
| n | K | Median, percentage or p-value | |
| At 36 months’ median follow-up | |||
| Maintained or achieved a CCyRa | 74 | 27 | 36.4%b |
| Haematological failure patients | 13 | 5 | 38% |
| Cytogenetic resistant patients | 57 | 22 | 39%c |
| Difference between subgroups | p = 0.345 | ||
| Primary cytogenetic resistance subgroup | 24 | 27% | |
| Acquired cytogenetic resistance subgroup | 33 | 50% | |
| Difference between subgroups | p = 0.02 | ||
| Achieved a MCyRa | |||
| Cytogenetic failure | 57 | 41 | 72% |
| Haematological failure | 13 | 6 | 46% |
| Difference between subgroups | p = 0.002 | ||
| Achieved a CyRa | |||
| 600-mg/day dose escalation subgroup | 53d | 40 | |
| 800-mg/day dose escalation subgroup | 20 | 10 | |
| Difference between subgroups | p = 0.234 | ||
| Median time to a CyR (months) | 3.5 | ||
| Maintained or achieved a CHRe | 74 | 68 | 91.8% |
| Achieved a complete molecular responsef | 74 | 10 | |
| Cytogenetic failure | 10 | 7 | |
| Escalated dose for suboptimal CyR | 10 | 3 | |
| Estimated 2-year outcomes | |||
| PFS | 87% | ||
| OS | 85% | ||
| AEsg | |||
| Muscle cramps | |||
| 600-mg/day imatinib subgroup | 20% | ||
| 800-mg/day subgroup | 30% | ||
| Difference between subgroups | NS (p ≥ 0.05) | ||
| Peripheral oedema | |||
| 600-mg/day imatinib subgroup | 35% | ||
| 800-mg/day subgroup | 40% | ||
| Difference between subgroups | NS (p ≥ 0.05) | ||
| Haematological toxicity | |||
| Anaemia | |||
| 600-mg/day imatinib subgroup | 0% | ||
| 800-mg/day subgroup | 2% | ||
| Difference between subgroups | Not reported | ||
| Neutropenia | |||
| 600-mg/day imatinib subgroup | 0% | ||
| 800-mg/day subgroup | 3% | ||
| Difference between subgroups | Not reported | ||
Quality appraisal
| 1. General |
| 1.1. Is the hypothesis/aim/objective of the study clearly described? YES |
| 1.2. Were the case series collected at more than one centre? YES |
| 1.3. Was the main outcome independently assessed? NOT REPORTED |
| 1.4. Are patient characteristics adequately described? YES (baseline characteristics reported) |
| 1.5. How easy is it to assess generalisability of the results? LOW (Italian population, but inclusion and exclusion criteria not stated) |
| 2. Assessment of selection bias |
| 2.1. Are inclusion and exclusion criteria clearly reported? NO |
| 2.2. Were data collected prospectively? NOT REPORTED |
| 2.3. Were patients recruited consecutively? NOT REPORTED (a date range for recruitment is given) |
| 3. Assessment of performance bias |
| 3.1. Did all of the participants receive the same intervention? NO (subgroups received different dose escalations) |
| 3.2. Is the use of any concurrent therapies adequately described? NO (not reported whether there were any concurrent therapies) |
| 4. Assessment of attrition bias |
| 4.1. Was an ITT analysis performed? UNCLEAR (not explicitly reported; dropouts not reported) |
| 4.2. Were dropouts from the study adequately described? NO |
Koh et al.10
Study details
| Study details | Population | Arms | OUTCOMES |
|---|---|---|---|
|
Study: Koh et al. (2010)10 Design: Prospective cohort single arm CP: Yes (n = 64) AP: Yes (n = 3) BC: Yes (n = 4) Country: Korea No. of centres: 19 Notes Phase IV study to evaluate the efficacy of escalated dose imatinib in patients with suboptimal response to standard-dose imatinib |
Inclusion criteria CML patients between 15 and 75 years of age with adequate organ function (not defined). Patients in CP with suboptimal response to 400 mg/day of imatinib; patients in AP or BC who failed to achieve CHR after 3 months on 400–600 mg/day of imatinib Suboptimal response and treatment failure were defined according to European LeukemiaNET (reference cited) Exclusion criteria Patients who experienced more than grade 2 AEs to standard-dose imatinib |
Arm 1 n: 71 Drug: HDI Starting daily dose (mg): 600 Dose details Dose escalation to 800 mg/day permitted in patients with AP or BC. Escalated doses were for a minimum of 12 months or until disease progression or intolerable toxicity. Patients with cytopenia and non-haematological toxicity of grade 3 or more received dose reduction from 800 to 600 mg/day then 400 mg/day; or from 400 to 300 mg/day. Patients experiencing more than grade 3 toxicity on 300 mg/day were withdrawn. An effort was made to increase dose if patients on reduced dose for 1 month did not experience more than grade 1 toxicity Concurrent treatment None reported |
CyR Assessed every 6 months Molecular response Assessed every 3 months Time to treatment failure Defined according to LeukemiaNET (reference cited), based on cytogenetic evaluation; refers to the time from dose escalation to the time of treatment failure or drug discontinuation due to intolerable toxicity AEs (Not explicitly specified as an outcome) |
Baseline characteristics
| Intervention | |||
|---|---|---|---|
| n | K | Median (range) or percentage | |
| Demographics | |||
| Age, years: median (range) | 71 | 49 (20 to 71) | |
| Sex (n male) | 71 | 50 | 70.4% |
| Disease status | |||
| CP | 71 | 64 | 90.1% |
| AP | 71 | 3 | 4.2% |
| BC | 71 | 4 | 5.6% |
| CyRa | |||
| Partial | 71 | 31 | 43.7 |
| Less than partial | 71 | 40 | 56.3 |
| Median (range) duration (months) of standard-dose imatinib | 14.6 (0.6 to 52.8) | ||
| Treatment outcome on standard-dose imatinib | |||
| Suboptimal response | 71 | 19 | 26.8 |
| Treatment failure | 71 | 52 | 73.2 |
| Dose escalation | |||
| To 600 mg/day | 71 | 65 | 91.5 |
| To 800 mg/day | 71 | 6 | 8.5 |
Results
| Intervention | |||
|---|---|---|---|
| n | K | Median, percentage or p-value | |
| At 6 months’ follow-up | |||
| Evaluable for CyR | 71 | 52 | 73.2%a |
| Unevaluable owing to early disease progression | 71 | 4 | 5.6%a |
| Unevaluable owing to refusal or loss to follow-up | 71 | 15 | 21.1%a |
| Achieved a CyRb | |||
| Complete | 71 | 16 | 22.5% |
| Partial | 71 | 14 | 19.7% |
| Less than partial | 71 | 22 | 31.0% |
| Overall rate of achieving a CCyR for evaluable patients | 52 | 16 | 30.8% |
| Frequency achieving a CCyR, by subgroups | |||
| A. Partial responders at baseline | Not reportedc | ||
| B. Less than partial responders at baseline | Not reportedc | ||
| Difference, subgroups A vs B | p = 0.034 | ||
| C. Suboptimal response on standard dose at baseline | Not reported | ||
| D. Treatment failure on standard dose at baseline | Not reported | ||
| Difference, subgroups C vs D | p = 0.076 | ||
| E. Early molecular respondersd | Not reportede | ||
| F. Non-early molecular responders | Not reportede | ||
| Difference, subgroups E vs F | p = 0.010 | ||
| Evaluable for molecular response | 71 | 61 | 85.9%a |
| Unevaluable owing to early disease progression | 71 | 4 | 5.6%a |
| Unevaluable owing to loss to follow-up | 71 | 6 | 8.5%a |
| Achieved a molecular response | |||
| Early molecular responsed | 71 | 40 | 56.3% |
| AP patients achieving | 3 | 2 | |
| BC patients achieving | 4 | 0 | |
| Non-early molecular response | 71 | 21 | 29.6% |
| At 12 months’ follow-up | |||
| Evaluable for CyR | 71 | 43 | 60.6%a |
| Unevaluable owing to early disease progression | 71 | 12 | 16.9%a |
| Unevaluable owing to refusal or loss to follow-up | 71 | 16 | 22.5%a |
| Achieved a CyRb | |||
| Complete | 71 | 17 | 23.9% |
| Partial | 71 | 11 | 15.5% |
| Less than partial | 71 | 14 | 19.7% |
| Overall rate of achieving a CCyR for evaluable patients | 43 | 17 | 40.5% |
| Frequency achieving a CCyR, by baseline subgroups | |||
| A. Partial responders at baseline | Not reportedc | ||
| B. Less than partial responders at baseline | Not reportedc | ||
| Difference, subgroups A vs B | p = 0.012 | ||
| C. Suboptimal response on standard dose at baseline | Not reported | ||
| D. Treatment failure on standard dose at baseline | Not reported | ||
| Difference, subgroups C vs D | p = 0.206 | ||
| E. Early molecular respondersd | Not reportede | ||
| F. Non-early molecular responders | Not reportede | ||
| Difference, subgroups E vs F | p < 0.001 | ||
| Median time to treatment failure (months) | 71 | 18 | |
| CP patients | 27.0 | ||
| AP patients | 2.5 | ||
| BC patients | 4.0 | ||
| Difference between CP and AP/BCf | p < 0.001 | ||
| Comparisons by subgroups: | |||
| A. Partial cytogenetic responders at baseline | Not reached | ||
| B. Less than partial cytogenetic responders at baseline | 12.0 | ||
| Difference, subgroups A vs B | p < 0.001 | ||
| C. Suboptimal response on standard dose at baseline | Not reached | ||
| D. Treatment failure on standard dose at baseline | 12.3 | ||
| Difference, subgroups C vs D | p = 0.009 | ||
| E. Early molecular respondersd | Not reached | ||
| F. Non-early molecular responders | 11.0 | ||
| Difference, subgroups E vs F | p < 0.001 | ||
| AEs | |||
| Haematological AEs above grade 2 | |||
| Anaemia | 71 | 12 | 16.9%a |
| Neutropenia | 71 | 13 | 18.3%a |
| Thrombocytopenia | 71 | 0 | 0% |
| Non-haematological AEs above grade 2 | |||
| Grade 3 oedema | 71 | 2 | 2.8%a |
| Other AEs above grade 2 | 71 | 0 | 0% |
| Other AEsg | |||
| Stopped imatinib owing to intolerable toxicity | 71 | 0 | 0% |
Quality appraisal
| 1. General |
| 1.1. Is the hypothesis/aim/objective of the study clearly described? YES |
| 1.2. Were the case series collected at more than one centre? YES |
| 1.3. Was the main outcome independently assessed? NOT REPORTED |
| 1.4. Are patient characteristics adequately described? YES (limited to ethnicity, age, sex and CML characteristics) |
| 1.5. How easy is it to assess generalisability of the results? HIGH (specifically a Korean population of known age range and CML status, although potential prognostic factors such as weight not reported) |
| 2. Assessment of selection bias |
| 2.1. Are inclusion and exclusion criteria clearly reported? YES |
| 2.2. Were data collected prospectively? YES |
| 2.3. Were patients recruited consecutively? NOT REPORTED (a date range for recruitment is given) |
| 3. Assessment of performance bias |
| 3.1. Did all the participants receive the same intervention? NO (subgroups received different dose escalations or reductions if warranted) |
| 3.2. Is the use of any concurrent therapies adequately described? NO (not reported whether or not there were any concurrent therapies) |
| 4. Assessment of attrition bias |
| 4.1. Was an ITT analysis performed? UNCLEAR (not explicitly reported; however, data for CyRs and molecular responses were conservatively presented as percentages of all patients rather than as percentages of those available and eligible for assessment) |
| 4.2. Were dropouts from the study adequately described? YES |
Rajappa et al.8
Study details
| Study details | Population | Arms | OUTCOMES |
|---|---|---|---|
|
Study: Rajappa et al. (2010)8 Design: Cohort single arm judged to be prospective, although unclear reporting Chronic phase: Yes AP: No BC: No Country: India No. of centres: Assumed one (not reported; all authors from one centre) Notes: Study focuses on kinase domain mutations |
Inclusion criteria CP-CML resistant to imatinib 400 mg/day. No other details reported Primary resistance defined as treatment failure to 400 mg/day of imatinib, i.e. failure to achieve CHR after 3 months, failure to achieve any CyR after 6 months, MCyR after 12 months, and CCyR after 18 months of therapy Secondary resistance defined as loss of CCyR or rising WBC counts to > 10 × 109/l on two occasions more than 4 weeks apart, progression to AP or BC Exclusion criteria Patients with AP or BC |
Arm 1 n: 90 Drug: HDI Starting daily dose (mg): 800 Dose details Dose escalated from 400 to 800 mg/day for all participants Concurrent treatment None reported |
Event-free survival Defined as: ‘time from dose escalation to loss of CHR or CCyR, failure to achieve CHR at 3 months, progression to AP or BC, no CyR at 6 months, less than MCyR at 12 months and no CCR at 18 months or death from any cause’ Transformation-free survival Defined as time from dose escalation until transformation to AP or BC, or death due to any cause OS Defined as time from dose escalation to death due to any cause AEs |
Baseline characteristics
| Intervention | |||
|---|---|---|---|
| n | K | Mean, median or percentage | |
| Demographics | |||
| Age, years: mean ± SD (range) | 90 | 35.7 ± 12 (18 to 65) | |
| Sex (n male) | 90 | 64 | 71.1% |
| Imatinib failure | |||
| Intolerancea | |||
| Primary resistance | 90 | 30 | |
| Secondary resistance | 90 | 60 | |
| Disease groupb | |||
| Primary haematological resistance | 90 | 10 | 11.1% |
| Primary cytogenetic resistance | 90 | 20 | 22.2% |
| Loss of haematological response | 90 | 55 | 61.1% |
| Loss of CyR | 90 | 5 | 5.5% |
| Prior therapy (stated that participants received only imatinib 400 mg/day and HU; no other prior therapy) | |||
| Median (range) time (months) from diagnosis to start of imatinib 400 mg/day | 90 | 5 (0.5 to 20) | |
| < 6 months | 75 | ||
| > 6 months | 15 | ||
| Median (range) time (months) on imatinib 400 mg/day before resistance | 90 | 18 (3 to 48) | |
| ≤ 18 months | 65 | ||
| > 18 months | 25 | ||
| Best response to imatinib 400 mg/day | |||
| CCyR | 90 | 10 | 11.1% |
| PCyR | 90 | 21 | 23.3%c |
| Minor CyR | 90 | 58 | 64.4%d |
| CHR | 90 | 80 | 88.8% |
| MCyR to imatinib 400 mg/day | 90 | 41 | 45.5% e |
| Laboratory and other clinical parameters | |||
| Median (range) haemoglobin concentration (g/dl) | 90 | 11.2 (7.9 to 15.1) | |
| Median (range) total leucocyte count (109/l) | 90 | 11 (3.7 to 180) | |
| Median (range)% blasts | 90 | 2 (0 to 9) | |
| Median (range)% basophils | 90 | 4 (0 to 12) | |
| Median (range)% platelets | 90 | 2.7 (0.9 to 11.9) | |
| Sokal score low and intermediate | 90 | 60 | |
| Sokal score high | 90 | 30 | |
| BCR–ABL mutations | 90 | 29 | 32.2% |
Results
| Intervention | |||
|---|---|---|---|
| N | K | Percentage, median or p-value | |
| At | |||
| Event-free survival at follow-up | 90 | 35 | 39% |
| OS at follow-up | 90 | 84 | 93% |
| Follow-up time unclear | |||
| CCyR | |||
| All participants | 90 | 25 | 27.7% |
| Cytogenetic failure subgroup | 26 | 13 | 50% |
| Haematological failure subgroup | 64 | 12 | 18.75% |
| Difference between subgroups | p = 0.004 | ||
| PCyR | |||
| All participants | 90 | 10 | 11.1%a |
| Cytogenetic failure subgroup | 26 | 6 | 23% |
| Haematological failure subgroup | 64 | 4 | 6.25% |
| Difference between subgroups | p = 0.03 | ||
| MCyR | 90 | 35 | 39% |
| Median (range) time to CyR (months) | 11 (6 to 18) | ||
| CHR | 90 | 50 | 55.5% |
| Estimated 2-year outcomes | |||
| Event-free survival | |||
| All participants | 90 | 34% | |
| Cytogenetic failure subgroup | 26 | 73% | |
| Haematological failure subgroup | 64 | 22% | |
| Difference between subgroups | p=0.0018 | ||
| Event-free survival for patients achieving a MCyR to dose escalation | |||
| All participants | 35 | 67% | |
| Cytogenetic failure subgroup | 90% | ||
| Haematological failure subgroup | 51% | ||
| Difference between subgroups | p=0.0006 | ||
| OS | |||
| All participants | 90 | 93% | |
| Cytogenetic failure subgroup | 26 | 100% | |
| Haematological failure subgroup | 64 | 93% | |
| Difference between subgroups | Stated NS | ||
| Transformation-free survival | |||
| All participants | 90 | 86% | |
| Cytogenetic failure subgroup | 26 | 95% | |
| Haematological failure subgroup | 64 | 74% | |
| Difference between subgroups | Not reported | ||
| Events at median follow-up of 18 (range 3–40) months | 90 | 55 | 61% |
| Failure to achieve CHR | 55 | 37 | 67.3%b |
| Loss of haematological response | 55 | 2 | 3.6% |
| Failure to achieve CyR | 55 | 7 | 12.7% |
| Progression to AP or BC | 55 | 3 | 5.5%b |
| Death | 55 | 6 | 10.9%b |
| Consequences of haematological toxicity | |||
| Dose decrease | 90 | 16 | 18% |
| Dose interruption | 90 | 31 | 34% |
| Discontinuation due to AEs | 90 | 3 | 3% |
| Able to continue imatinib dose > 600 mg/day | 90 | 60 | 67%c |
| AEs | |||
| Grades 3–4 haematological AEs | |||
| Anaemia | 90 | 27 | 30% |
| Leucopenia | 90 | 28 | 31% |
| Neutropenia | 90 | 35 | 39%d |
| Thrombocytopenia | 90 | 19 | 21% |
| Non-haematological AEs | |||
| Superficial oedema | 90 | 55 | 61% |
| Musculoskeletal pain | 90 | 35 | 39% |
| Fatigue | 90 | 27 | 30% |
| Anorexia | 90 | 26 or 23e | 29% or 25.55%e |
| Rash | 90 | 24 | 27% |
| Diarrhoea | 90 | 24 | 27% |
| Dyspepsia | 90 | 13 | 14% |
| Nausea/vomiting | 90 | 10 | 11% |
| Mucositis/oral ulcers | 90 | 9 | 10% |
Quality appraisal
| 1. General |
| 1.1. Is the hypothesis/aim/objective of the study clearly described? YES |
| 1.2. Were the case series collected at more than one centre? NOT REPORTED (but all authors based at one centre) |
| 1.3. Was the main outcome independently assessed? NOT REPORTED |
| 1.4. Are patient characteristics adequately described? NO (this study appears to be on an Indian population but ethnicity and socioeconomic status are not reported) |
| 1.5. How easy is it to assess generalisability of the results? LOW (appears to be single-centre study) |
| 2. Assessment of selection bias |
| 2.1. Are inclusion and exclusion criteria clearly reported? YES (although limited) |
| 2.2. Were data collected prospectively? NOT REPORTED (cannot ascertain from description of methods) |
| 2.3. Were patients recruited consecutively? YES (stated in methods) |
| 3. Assessment of performance bias |
| 3.1. Did all of the participants receive the same intervention? YES (but timing of intervention varied among patients and is not reported, hence giving a broad range of imprecise follow-up times) |
| 3.2. Is the use of any concurrent therapies adequately described? NO (not reported whether there were any concurrent therapies) |
| 4. Assessment of attrition bias |
| 4.1. Was an ITT analysis performed? UNCLEAR (not explicitly reported; stated that only patients with at least one cytogenetic evaluation after 6 months of dose escalation were analysed, but not whether or not all 90 participants analysed met this criterion; also, some percentages incorrect – unclear whether or not a different denominator used) |
| 4.2. Were dropouts from the study described? NO |
Note: this paper gives only a vague indication of follow-up time (median and wide range). This is a major limitation that is not captured by the quality-appraisal criteria above.
Appendix 5 Data extraction of cost-effectiveness studies
Ghatnekar and colleagues 2010
Study characteristics
Reference (lead author, year, refid)
| Ghatnekar et al. 201015 |
Health technology
| Dasatinib |
Interventions and comparators
What interventions/strategies were included?
| HDI |
Was a no-treatment/supportive care strategy included?
| No |
Describe interventions/strategies:
| Dasatinib: 140 mg/day |
| Imatinib: 800 mg/day |
Research question
What are the stated objectives of the evaluation?
| To evaluate the cost-effectiveness of dasatinib treatment vs HDI in patients with CP-CML who are resistant to standard-dose imatinib in Sweden |
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
| Cost-effectiveness and cost–utility study |
Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
|
Patients confirmed to be resistant to lower doses of imatinib (≤ 600 mg). Resistance defined as any of:The median age was 51 years and approximately 50% of dasatinib group and 20% of the imatinib group were male. Medium duration of disease was 64 and 52 months for dasatinib and imatinib, respectively Full details of patient characteristics given in supplied reference (Kantarjian et al. 2007)6 |
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
| Not stated (outpatient setting for CP, inpatient for AP and BP?) |
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in, and does the publication give the base year to which those costs relate?
| Country is Sweden. Currency is euros. Base year 2008 |
Funding source
| Bristol-Myers Squibb AB, Sweden |
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and PSS, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)?
| Societal perspective adopted |
Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation:
|
Response to treatment was taken from a 12-week head-to-head Phase II clinical trial This was defined as best initial response (no response, CHR, PCyR, CCyR) to treatment with the percentage of patients for each treatment (only applicable to CP): |
Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate), as well as sources for unit costs used:
| Resource item | Resource use per montha | Unit cost (SD) | Source | |||
|---|---|---|---|---|---|---|
| CP responder | CP non-responder | AP | BC | |||
| Haematologist visit | 0.33 | 1.00 | 2.00 | 2.00 | 184 (75) | SRHCC |
| Inpatient stay | 0.00 | 0.00 | 0.17 | 0.42 | 541 (222) | SRHCC |
| Chest radiography | 0.00 | 0.00 | 0.33 | 0.50 | 56 (8) | SRHCC |
| CT scan | 0.00 | 0.00 | 0.08 | 0.08 | 220 (31) | SRHCC |
| Bone marrow test | 0.33 | 0.33 | 0.33 | 0.33 | 784 (110) | SRHCC |
| Cytogenetic testing | 0.33 | 0.33 | 0.33 | 0.33 | 365 (51) | SRHCC |
| PCR test | 0.33 | 0.08 | 0.00 | 0.00 | 655 (92) | SRHCC |
| Other laboratory tests | 1.50 | 2.00 | 2.00 | 4.00 | 14 (2) | SRHCC |
| Thrombocyte transfusion | 0.00 | 0.00 | 0.63 | 2.50 | 914 (375) | SRHCC |
| Imatinib 800 mg/day (monthly cost) | 4869 | FASS 2008 | ||||
| Dasatinib 140 mg/day (monthly cost) | 4239 | FASS 2008 | ||||
AEs costs (€ 2008) are included in sensitivity analysis: diarrhoea 184 (CP), 2865 (AP or BC); headache 184; dyspnoea (dasatinib) 240; neutropenia (dasatinib) 3530; pleural effusion (dasatinib) 885.
Cost data are derived from two Swedish official price lists [FASS 2008 (Pharmaceutical Specialties in Sweden), Southern Regional Health Care Committee 2008 (SRHCC)].
Indirect costs: costs due to lost productivity, unpaid inputs to patient care
Were indirect costs included?
| Item | Unit cost (€ 2008) | Source |
|---|---|---|
| Monthly production loss (85% activity) | 3830 | Income Distribution Survey 2003, Statistics Sweden |
| Public consumption age 50–64 years | 1461 | Ekman. Stockholm School of Economics 2002 |
| Public consumption age 65–74 years | 1465 | Ekman. Stockholm School of Economics 2002 |
| Public consumption age 75–84 years | 1678 | Ekman. Stockholm School of Economics 2002 |
| Public consumption age 85+ years | 2514 | Ekman. Stockholm School of Economics 2002 |
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
| Utility weights for each health state were elicited with a TTO technique using the EQ-5D instrument among 100 laypersons in the UK and applied to both the dasatinib and imatinib arms (Levy et al. 200716). Variation around the mean is used for PSA. In the sensitivity analysis, weights provided for the NICE appraisal of imatinib were used (Dalziel et al. 200425). |
List the utility values used in the evaluation:
| CP responder | CP non-responder | AP | BC | Source | |
|---|---|---|---|---|---|
| Utility weights | |||||
| Base case | 0.90 | 0.72 | 0.53 | 0.29 | Levy et al. 200716 |
| CI 0.87 to 0.93 | CI 0.67 to 0.77 | CI 0.48 to 0.57 | CI 0.24 to 0.34 | ||
| One-way sensitivity analysis | 0.85 | 0.85 | 0.73 | 0.52 | Dalziel et al. 200425 |
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? List them if reported:
| Initial response rate | Probability of being in the health state months 4–12 | Probability of being in the health state months 13+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CP | AP | BC | Death | CP | AP | BC | Death | ||
| No response | CP | 0.831a | 0.169b | 0.000c | 0.000c | 0.831 | 0.169b | 0.000c | 0.000c |
| AP | 0.000c | 0.826a | 0.124d | 0.050d | 0.000c | 0.833a | 0.124d | 0.043d | |
| BP | 0.000c | 0.000c | 0.826a | 0.174d | 0.000c | 0.000c | 0.926a | 0.074d | |
| CHR | CP | 0.993a | 0.007e | 0.000c | 0.000c | 0.980 | 0.020f | 0.000b | 0.000c |
| AP | 0.000c | 0.977a | 0.006d | 0.017d | 0.000c | 0.943a | 0.038d | 0.018d | |
| BP | 0.000c | 0.000c | 0.941a | 0.059d | 0.000c | 0.000c | 0.977a | 0.023d | |
| PCyR | CP | 0.997a | 0.003e | 0.000c | 0.000c | 0.994 | 0.006f | 0.000c | 0.000c |
| AP | 0.000c | 1.000a | 0.000d | 0.000d | 0.000c | 0.962a | 0.022d | 0.016d | |
| BP | 0.000c | 0.000c | 0.941a | 0.059d | 0.000c | 0.000c | 0.964a | 0.036d | |
| CCyR | CP | 0.997a | 0.003e | 0.000c | 0.000c | 0.995 | 0.005f | 0.000c | 0.000c |
| AP | 0.000c | 1.000a | 0.000d | 0.000d | 0.000c | 0.995a | 0.002d | 0.003d | |
| BP | 0.000c | 0.000c | 0.985a | 0.015d | 0.000c | 0.000c | 0.989a | 0.011d | |
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text):
| The probability of remaining in CP the next month was 0.831 or moving to AP was 0.169 |
| In the next month, patients remaining in CP have the same probabilities. Patients in AP have the probability of remaining in AP of 0.826 or moving to BC of 0.124 or death 0.05 |
| Progression data taken from several sources |
What is the model time horizon?
| Lifetime horizon |
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
| Costs and benefits discounted by 3% per year |
Results/analysis
What measure(s) of benefit were reported in the evaluation?
| Life-years and QALYs |
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation:
| Base-case results | |||
| Dasatinib 140 mg/day | Imatinib 800 mg/day | Difference | |
| Life-years | 6.37 | 5.69 | 0.67 |
| QALYs | 5.19 | 4.57 | 0.62 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation.
| Cost item | Dasatinib (€) | Imatinib (€) | Difference (€) |
|---|---|---|---|
| Treatment (drug) | 277,778 | 278,210 | −432 |
| Specialist visits | 8063 | 7734 | 329 |
| Inpatient stay | 1916 | 1963 | −48 |
| Imaging and blood tests | 2254 | 2113 | 40 |
| Bone marrow tests | 19,791 | 17,703 | 2088 |
| Cytogenetic tests | 9219 | 8247 | 972 |
| PCR test | 14,133 | 12,320 | 1813 |
| Thrombocyte transfusions | 17,806 | 18,217 | −411 |
| Total direct cost | 350,960 | 346,507 | 4452 |
| Production losses | 41,834 | 53,826 | −11,991 |
| Increased public consumption | 111,738 | 99,948 | 11,789 |
| Total societal cost | 504,532 | 500,281 | 4250 |
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results:
| Incremental societal cost/LY €6332 |
| Incremental societal cost/QALY €6880 |
Give results of any statistical analysis of the results of the evaluation:
| Medication accounts for almost 80% of direct health costs in both treatment arms |
| Direct costs only ICER is €7207 |
| After 10 years 22% dasatinib patients estimated to be in CP (3.4 percentage points more than imatinib arm); 4 percentage points more patients are alive compared with imatinib arm (and consuming health care and other resources) |
Was any sensitivity analysis performed? If yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]?
| One-way sensitivity analysis and PSA performed |
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, QoL or disease progression rates)?
|
One-way sensitivity analyses conducted: Probabilistic sensitivity analyses conducted: variation of initial response, utilities, direct costs (not medication), using beta and gamma distributions for probabilities and costs, respectively |
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base case analysis? If so, what were the suggested causes?
Deterministic sensitivity analysis:
| Parameter change | Incremental cost (€) | QALY gained | ICER (€) |
|---|---|---|---|
| Base case | 4250 | 0.62 | 6880 |
| 1. 10-year perspective | −4212 | 0.45 | Dominant |
| 2. Discount rate: 0% | 11 075 | 0.79 | 13,981 |
| 3. Including costs for AE | 4296 | 0.62 | 6955 |
| 5. Utility weights from imatinib study | 4250 | 0.58 | 7322 |
The ICER is €6869/QALY. Results from 1000 cohort iterations are presented as a scatterplot in the incremental cost-effectiveness plane. Incremental direct costs are scattered on both sides of the x-axis, indicating that dasatinib can generate cost-savings. Two per cent of the observations are to the left of the y-axis, indicating observations where the QALY gain is higher for imatinib. Results suggest that dasatinib could be expected to generate more health gain in terms of QALYs gained, but it is uncertain whether this health benefit comes at extra cost or if it generates cost-savings. There is a clear relationship between incremental survival and incremental costs, as greater life expectancy carries a health-care burden due to greater resource utilisation. All observations fall below the derived WTP threshold for a QALY in Sweden (based on avoiding a traffic fatality) indicating that dasatinib treatment would be cost-effectiveness if this threshold is the same for the health-care sector.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis:
| The authors conclude that dasatinib is a cost-effective treatment among imatinib-resistant patients with CML in Sweden compared with imatinib 800 mg/day |
What are the implications of the evaluation for practice?
| Dasatinib is expected to generate greater health benefits at a cost per QALY of about €6880 with a life-long societal perspective |
SHTAC commentary
Selection of comparators:
| Appropriate |
Validity of estimate of measure of benefit:
| Reasonable, but long-term benefits estimated through surrogate measures |
Validity of estimate of costs:
| Reasonable although includes indirect costs in terms of production losses |
Bristol-Myers Squibb Pharmaceuticals
Study characteristics
Reference (lead author, year, refid)
| Bristol-Myers Squibb Pharmaceuticals Ltd |
Health technology
| Dasatinib |
Interventions and comparators
What interventions/strategies were included?
| Dasatinib, nilotinib and HDI |
Was a no-treatment/supportive care strategy included?
| No. Bone marrow/SCT, IFN-α |
Describe interventions/strategies:
|
Dasatinib: 100 mg daily oral dose for CP and 140 mg/day AP and BP. Treatment continues until disease progression or until intolerant toxicity Nilotinib: Oral dose of 800 mg daily. Treatment continues until disease progression or intolerable toxicity Imatinib: Doses increased to 800mg per day in the absence of severe adverse drug reaction. Treatment in the model is assumed to continue until disease progression or intolerable toxicity |
Research question
What are the stated objectives of the evaluation?
| To appraise the clinical effectiveness and cost-effectiveness of dasatinib, nilotinib and HDI compared with standard-dose imatinib, allo-SCT, HU, IFN-α, acute leukaemia-style chemotherapy and best supportive care, for patients with CML who are resistant to imatinib |
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
| Cost–utility |
Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
|
Three separate scenarios were explored: Initiating dasatinib treatment in the CP, AC and BC of CML |
| Value | Source | |
|---|---|---|
| Patients starting treatment in CP | ||
| 100 mg q.d. (n = 167) | ||
| Age (years) median | 56 | 034 trial |
| Sex (% male) | 50 | 034 trial |
| Time since diagnosis (months) | 55 | 034 trial |
| Prior treatments failed at baseline | 61/167 > 600 mg/day imatiniba | 034 trial |
| Patients starting treatment in AP | ||
| 140 mg q.d. (n = 158) | ||
| Age (years) median | 56 | 035 trial |
| Sex (% male) | 56.5 | 035 trial |
| Median CML duration (months) | 74 | 035 trial |
| Prior imatinib > 600mg/day (%) | 43 | 035 trial |
| Prior imatinib > 3 years (%) | 53 | 035 trial |
| Patients starting treatment in BP: myeloid | ||
| 140 mg q.d. (n = 75) | ||
| Age (years) median | 48 | 035 trial |
| Median CML duration (months) | 41 | 035 trial |
| Prior imatinib > 600 mg/day (%) | 44 | 035 trial |
| Prior imatinib > 3 years (%) | 29 | 035 trial |
| Patients starting treatment in BP: lymphoid | ||
| 140 mg q.d. (n = 33) | 035 trial | |
| Age (years) median | 49 | 035 trial |
| Median CML duration (months) | 46 | 035 trial |
| Prior imatinib > 600 mg/day (%) | 46 | 035 trial |
| Prior imatinib > 3 years (%) | 21 | 035 trial |
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
| Not stated, but likely outpatient setting |
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
| Setting not discussed. Costs presented in £ UK for a base year 2009 |
Funding source
| Bristol-Myers Squibb |
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and PSS, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)?
| NHS/PSS |
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
| Not stated how effectiveness data was derived. Data were from several clinical trials. Disease prognosis was based solely upon the patient’s initial best response to treatment |
| CP | NR (%) | CHR (%) | PCyR (%) | CCyR (%) | Survive bone marrow SCT (%) | (%) | Follow-up |
|---|---|---|---|---|---|---|---|
| Dasatinib22 | 8.1 | 33.1 | 15.3 | 43.5 | 0.0 | 0.0 | 24 months |
| Imatinib 400 mga | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA |
| Imatinib 600 mg17 | 56.4 | 15.4 | 28.2 | 0.0 | 0.0 | 0.0 | 12 months |
| Imatinib 800 mg23 | 32.1 | 13.3 | 14.1 | 40.5 | 0.0 | 0.0 | 61 months |
| Nilotinibb | 6.0 | 35.0 | 18.0 | 41.0 | 0.0 | 0.0 | 19 months |
| IFN-αa | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NR |
| Bone marrow SCTb | 00 | 0.0 | 0.0 | 0.0 | 5. | 35.0 | 250 months |
Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used:
| Drug costs were estimated based on the recommended doses from their ‘Summary of Product Characteristics’. Prices were from the BNF |
| One-off cost (£) | Monthly cost (£) | |
|---|---|---|
| Dasatinib | 0.00 | 2504.96 |
| Imatinib 400 mg | 0.00 | 1604.08 |
| Imatinib 600 mg | 0.00 | 2406.12 |
| Imatinib 800 mg | 0.00 | 3208.16 |
| Nilotinib | 0.00 | 2613.05 |
| IFN-α | 0.00 | 863.51 |
| Bone marrow SCT | 80,000 | 2400.00 |
Direct costs also included for outpatient visits, tests and hospitalisation (table 24) and other interventions (e.g. transfusion).
AE costs were included for treatment-related grades 3–4 SAEs (table 25). Most common SAEs were neutropenia, thrombocytopenia and leucopenia.
Indirect costs: costs due to lost productivity, unpaid inputs to patient care
Were indirect costs included?
| None |
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
|
A cross-sectional study was commissioned to calculate utility values for the purpose of the BMS analysis3 (Szabo et al. 2010). 24 Ratings for health states and response were elicited from a representative sample of 100 unaffected individuals in the UK using the TTO method and the EuroQoL EQ-5D instrument The impact of the SAEs on health utility was captured in the model using utility decrements identified in the non-CML literature |
List the utility values used in the evaluation:
| Health state | Utilities |
|---|---|
| Cured (following bone marrow SCT) | 0.60a |
| Chronic (no response) | 0.68b |
| Chronic (with response) | 0.85b |
| Accelerated (no response) | 0.50b |
| Accelerated (with response) | 0.79b |
| Blast (no response) | 0.31b |
| Blast (with response) | 0.50b |
| Dead | 0.00b |
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? List them if reported.
|
A Markov model was developed to predict the health changes and resulting costs for patients starting dasatinib treatment in each of the three phases of CML: CP, AP and BC. It is not stated whether this is a newly developed model or has been adapted from a previously reported model. The model uses monthly cycles The authors state that the Markov process was considered appropriate as it allows the incorporation of the three disease phases, different response categories and the different rates of disease progression characteristic of CML, and the approach used has been used in previous economic analyses in CML For the CP and AP models, the model consists of three health states: ‘stable disease’, ‘progressed disease’ and ‘death’; for the BC model, the model consists of two states: ‘stable disease’ and ‘death’. In each health state, five types of response to treatment are used in the model: ‘no response to treatment’ (NR); ’achieve CHR’; ‘achieve partial cytogenetic and CHR (PCyR)’; ‘achieve complete cytogenetic and CHR (CCyR)’; and ‘achieve a molecular response’ The modelling incorporates two stages: (1) initial assessment of the patient’s initial best response to treatment and (2) determination of prognosis of the patient, based on their initial best response. Initial best response rates were based on clinical trial data and, in some cases, based on clinical opinion. As such, once the patients’ initial best response has been determined, they enter a specific ‘submodel’ that links response with long-term prognosis rates (e.g. PFS, OS). The prognosis rates for different response groups were based on evidence from the BMS 034 trial22 A list of assumptions is shown in table 20 |
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text).
| The PFS rates associated with each level of response (as observed in the 034 trial) are shown in Table 57 |
| Month | NR (%) | CHR (%) | PCyR (%) | CCyR (%) | MR (%) |
|---|---|---|---|---|---|
| 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 6 | 30.0 | 94.9 | 100.0 | 100.0 | 99.7 |
| 12 | 30.0 | 84.1 | 94.4 | 98.2 | 98.2 |
| 18 | 30.0 | 77.7 | 83.3 | 98.2 | 97.5 |
| 24 | 30.0 | 63.6 | 83.3 | 94.2 | 94.2 |
| 30 | 30.0 | 55.9 | 83.3 | 94.2 | 94.2 |
| 36 | 30.0 | 38.7 | 77.8 | 94.2 | 94.2 |
| 42 | 25.8 | 25.8 | 71.3 | 94.2 | 94.2 |
| 48 | 24.1 | 25.8 | 59.4 | 94.2 | 93.9 |
Initial best response rate for the CP is shown in table 49 (see treatment effectiveness). Initial best response rates for AP and BC are shown in tables 18 and 19.
What is the model time horizon?
| 40 years |
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
| 3.5% for costs and benefits |
Results/analysis
What measure(s) of benefit were reported in the evaluation?
| QALYs, PFS, LYs |
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation:
| QALYs | PFS | LYs | |
|---|---|---|---|
| Dasatinib | 6.425 | 10.720 | 11.764 |
| Imatinib 400 mg | 1.485 | 2.094 | 3.557 |
| Imatinib 600 mg | 2.394 | 4.606 | 3.155 |
| Imatinib 800 mg | 5.910 | 11.013 | 9.938 |
| Nilotinib | 6.235 | 10.368 | 11.435 |
| IFN-α | 1.664 | 2.094 | 3.557 |
| Bone marrow SCT | 4.738 | 11.563 | 11.982 |
| Results for AP and BC shown in tables 30–33 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation:
| Treatment cost (£) | SAE cost (£) | PFT cost (£) | Other cost (£) | Total cost (£) | |
|---|---|---|---|---|---|
| Dasatinib | 224,268 | 342 | 11,654 | 78,149 | 314,413 |
| Imatinib 400 mg | 12,565 | 282 | 31,699 | 90,780 | 135,326 |
| Imatinib 600 mg | 61,171 | 282 | 24,249 | 88,002 | 173,705 |
| Imatinib 800 mg | 254,018 | 282 | 15,909 | 80,155 | 350,365 |
| Nilotinib | 228,576 | 414 | 11,573 | 78,415 | 318,978 |
| IFN-α | 6764 | 49 | 31,699 | 90,780 | 129,292 |
| Bone marrow SCT | 302,937 | 0 | 3664 | 17,633 | 324,234 |
| Results for AP and BC shown in tables 30 and 32 |
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results:
| Dasatinib vs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|
| Imatinib 400 mg | 179,087 | 4.940 | 36,251 |
| Imatinib 600 mg | 140,707 | 4.031 | 34,907 |
| Imatinib 800 mg | –35,952 | 0.515 | Dasatinib dominant |
| Nilotinib | –4565 | 0.190 | Dasatinib dominant |
| IFN-α | 185,121 | 4.762 | 38,877 |
| Bone marrow SCT | –9821 | 1.687 | Dasatinib dominant |
| Results for AP and BC shown in tables 31 and 33 |
Give results of any statistical analysis of the results of the evaluation:
| None |
Was any sensitivity analysis performed – if yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]:
| Deterministic and PSAs |
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, QoL or disease progression rates)?
| Parameters used in the model were varied in the deterministic sensitivity analysis, including costs, utilities, starting age, time horizon and discounting |
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base case analysis? If so, what were the suggested causes?
|
The key impact factors include the utility of responders, starting age, and time horizon of the model. The sensitivity analyses were not presented in the normal way and are difficult to interpret The PSA showed the probability of dasatinib being cost-effective compared with bone marrow SCT of 81%. Cost-effectiveness acceptability curves were not presented for all possible drugs together, and results were not shown for the probability that dasatinib was cost-effective compared with its alternatives |
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis:
| Dasatinib is more clinically effective than HDI and cost-effective compared with HDI which BMS3 considers the appropriate comparator |
What are the implications of the evaluation for practice?
| Potential savings of different scenarios are shown in table 40. In summary, if all eligible patients switch to dasatinib from HDI, the average annual savings are £6,591,445; if all eligible patients switch to dasatinib from nilotinib, the average annual savings are £953,037 |
SHTAC commentary
Selection of comparators:
| Appear to be valid and reasonable |
Validity of estimate of measure of benefit:
| Concerns over the approach taken with regard to initial best response and extrapolation from this to PFS (using a submodel) |
Validity of estimate of costs:
| Concerns over length of the treatment durations used to estimate total treatment costs |
Novartis
Study Characteristics
Reference (lead author, year, refid)
| Novartis manufacturer’s submission 20104 |
Health technology
| Nilotinib |
Interventions and comparators
What interventions/strategies were included?
|
Nilotinib compared with: |
Was a no treatment/supportive care strategy included?
| No |
Describe interventions/strategies:
|
Nilotinib: 400 mg twice/day orally HDI: 800 mg/day SCT: allo-SCT as third-line therapy if appropriate HU: 2 g/day as third-line therapy |
Research question
What are the stated objectives of the evaluation?
| To evaluate the cost-effectiveness of nilotinib for the treatment of adult patients with CML who are resistant to prior standard-dose imatinib therapy in the CP |
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
| Cost-effectiveness and cost utility |
Study population
What definition was used for (condition)? What are the characteristics of the baseline cohort for the evaluation?
|
Patients with standard dose imatinib-resistant CML in CP An equal number of male and female patients aged 57 years entered the model No further characteristics presented |
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
| Not specified – presumably outpatient for CP/AP and some inpatient setting for BC |
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
| UK £, 2009–10 |
Funding source
| Novartis |
Analytical perspective
What is the perspective adopted for the evaluation [health service, health and PSS, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)]?
| UK NHS and PSS perspective |
Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
|
Effectiveness data derived from a systematic review TTD of treatment, which is a Kaplan–Meier estimate of duration of exposure defined as the time difference (days) between the first dose and last dose (and included censored observations over time); OS rate Nilotinib: 24-month OS 86%; duration of treatment NR (study CAMN107A2101E2) HDI: OS at 12 months 96%, at 24 months 84%; duration of treatment 14 months (Kantarjian et al. 20097) SCT: 5-year OS 34% (Gratwohl et al. 200918) HU: 5-year OS 16% (Allan et al. 199526); survival in AP 9.14 months, survival in BC 9.89 months Probability of non-CML death taken from age-specific mortality rates (Office for National Statistics 2008) Discontinuation rate nilotinib: 18% (data on file) Discontinuation rate HDI: 2% (Kantarjian et al. 20097) Discontinuation rate HU: 0% (assumption) |
Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate), as well as sources for unit costs used.
| Quarterly cost (£) of nilotinib (400 mg b.i.d.) | 7928 | BNF 60, 2010 |
| Quarterly cost (£) of HDI (400 mg b.i.d.) | 10,490 | Novartis, personal communication |
| Quarterly cost (£) of HU (2 g daily) | 38.00 | BNF 60, 2010 |
| Cost (£) of routine appointment (outpatient visit) | 103 | NHS reference costs 2006/07 |
| Cost (£) of inpatient visits | 300 | NHS reference costs 2006/07 |
| Quarterly cost (£) of AEs (nilotinib) | 135 | Details given in appendix 2 |
| Quarterly cost (£) of AEs (HDI) | 125 | Details given in appendix 2 |
| Quarterly cost (£) of AEs (HU) | 0 | Assumption |
| Cost (£) of allo-SCT: first 100 days | 79,380 | NHS reference costs 2006/07 |
| Cost of allo-SCT: adjustment for long-term costs | 25% | Saito 2007 |
Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
| No |
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
|
Utility values were assigned to the different health states derived from Reed et al. 2004 from the IRIS study using EQ-5D. Utilities were assumed to be independent of drug therapy and time A utility decrement was used for patients experiencing grades 3 or 4 AEs during first 18 months of treatment in CP, except those receiving HU (assumption) A decrement was applied to the long-term utility for 52% patients following SCT (assumption) |
List the utility values used in the evaluation.
|
CP 0.854; AP 0.595; BC 0.595 Disutility for AEs: 0.049 nilotinib; 0.027 HDI; 0.00 HU (various sources) SCT decrement: 0.079 |
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? List them if reported:
|
Markov model was developed to simulate the transition of a hypothetical cohort of 1000 patients over a lifetime of patients resistant to standard-dose imatinib Cycle length was 3 months with a monthly cycle for the first six cycles All patients assumed to have died of CML or other causes by age 100 years At each cycle patients have the probability of remaining in CP or progressing to AP. Patients failing on second-line treatment may remain in CP and receive further treatment (SCT if eligible or HU) before progressing to AP/BC. Patients can then progress from AP to BC and finally from BC to death. Patients are able to remain in CP, AP or BC for more than one cycle and they may die from other causes in CP and AP. Patients may only die from CML in BC. On progression to AP patients receive HU TTD and OS used to predict lifetime costs, LYG and QALYs |
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text):
| Not stated (uses data shown above for effectiveness) |
What is the model time horizon?
| Lifetime horizon |
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
| Costs and benefits discounted at 3.5% |
Results/analysis
What measure(s) of benefit were reported in the evaluation?
| LYG and QALYs gained |
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation:
| LYs | QALYs | |
|---|---|---|
| HDI | 5.53 | 4.28 |
| Nilotinib | 5.80 | 4.51 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation:
| Costs (£) | |
|---|---|
| HDI | 146,234 |
| Nilotinib | 139,216 |
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results:
| ICER [cost (£)/LYG] | ICER [cost (£)/QALY] | |
|---|---|---|
| HDI | ||
| Nilotinib | –26,006 | –30,513 |
| Nilotinib dominates HDI (Nilotinib is less costly and more effective than HDI) | ||
Give results of any statistical analysis of the results of the evaluation:
| Not applicable |
Was any sensitivity analysis performed? If yes, what type(s) (i.e. deterministic (one-way, two-way, etc.) or probabilistic)?
| One-way sensitivity analyses and PSAs |
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, QoL or disease progression rates)?
|
Range of efficacy assumptions, health utilities, costs and other parameters were considered DSA Efficacy outcomes HU 5-year OS 29% (Allan et al. 1995) HDI higher time on treatment (median 19.4 months) (Kantarjian et al. 2009) Outcome of SCT in third line based on risk factors 3 and 5 (base-case 4) Health utility CP upper 95% CI 0.862, lower 95% CI 0.846 Decrement with allo-SCT long-term 0.1 and no utility decrement Disutility associated with AEs increased to upper 95% CI Costs Cost of allo-SCT assuming 50% long-term cost and excluding 25% long-term costs Cost of AE treatment doubled Other parameters Upper age limit for allo-SCT = 70 years (base-case 60 years) Proportion of patients receiving allo-SCT in both arms reduced by 30% Age of cohort increased to 70 years (no patients eligible for SCT so all receive HU as third line) Age of cohort decreased to 50 years (majority of patients with a donor will have SCT as third line) Five-year time horizon PSA Costs: interquartile ranges presented in NHS reference costs using gamma distribution QoL: uncertainty reported by Reed et al. 2004, using beta distribution TTD: widest range around these parameters was adopted so that the minimum TTD does not become negative, using normal distribution |
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base-case analysis? If so, what were the suggested causes?
|
Results of one-way sensitivity analyses are reported in table 16 (and table 15 of Appendix 2) For the DSA, most ICERs are close to the base-case result of –£30,000 except for the 5-year time horizon when the ICER is –£82,000 (owing to reduced treatment costs) and for extending HDI TTD from 14 months to 19.4 months when the ICER is £201,871 (higher costs of HDI treatment with marginal QALY gain for HDI vs nilotinib) |
| PSA results (reported in separate document): |
| Costs (£) | LYs | QALYs | ICER (LYG) (£) | ICER (QALY gained) (£) | |
|---|---|---|---|---|---|
| HDI | 157,729 | 5.81 | 4.52 | – | – |
| Nilotinib | 144,344 | 5.99 | 4.68 | –73,813 | –86,413 |
From cost-effectiveness acceptability curves, nilotinib is predicted to be cost-effective at a threshold of over around £10,000.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis:
|
Nilotinib represents a clinically effective and cost-effective treatment option for patients with CP-CML, who are resistant to standard-dose imatinib (From exploratory analyses reported only in an appendix, when compared with SCT/HU, the cost per QALY gained for nilotinib in CP is £44,028) |
What are the implications of the evaluation for practice?
|
It is estimated that the annual incidence of patients becoming eligible for treatment with nilotinib is 41. It is assumed that uptake of nilotinib will be 52% over a 5-year period. Some patients will discontinue treatment The net impact of treating CP-resistant patients with nilotinib rather than HDI will be cost saving (–£1,651,241 by year 5) |
SHTAC commentary
Selection of comparators:
| Appropriate (meets scope and current practice) |
Validity of estimate of measure of benefit:
| Reasonable although concerns over the duration of OS |
Validity of estimate of costs:
| Reasonable |
Appendix 6 Base-case analysis reflecting updated cost of high-dose imatinib
For the SHTAC analysis, the cost of the interventions dasatinib, nilotinib and high-dose imatinib were taken from the BNF 60 (2010). The cost of high-dose imatinib is due to increase in a subsequent edition of the BNF. This cost was commercial-in-confidence at the time of the SHTAC analysis but has subsequently been made public by the manufacturer, although this remains unpublished. The effect of this cost increase on the SHTAC analysis is presented here. The effect of this price change increases the ICER for high-dose imatinib versus hydroxycarbamide from £30,200 to £31,500 and does not alter the bottom-line results.
| Intervention | QALY | Cost (£) | ICER vs HU (£/QALY) | ICER vs next-best optiona (£/QALY) |
|---|---|---|---|---|
| HU | 2.20 | 18,128 | ||
| HDI | 7.31 | 179,338 | 31,538 | Dominated |
| Nilotinib | 7.63 | 161,667 | 26,434 | 26,434b |
| Dasatinib | 7.85 | 172,473 | 27,336 | 50,016c |
Appendix 7 Additional scenario analyses
The SHTAC assessment report conducted analyses using the PenTAG2 model with minor modifications to take into account a number of issues, including the treatment duration of the interventions.
In the PenTAG AR,2 progression-free survival was based upon trial data, albeit of poor quality, which gave a longer treatment duration for dasatinib that was more than double the treatment duration of nilotinib. For the base analyses, SHTAC assumed that progression-free survival for dasatinib was the same as for nilotinib, based on clinical evidence from nilotinib and high-dose imatinib studies.
The SHTAC base-case results differ from other models for a number of reasons, including:
-
different comparators were used
-
different survival estimates for the chosen comparators were used
-
different treatment schedules were used
-
different assumptions regarding how long patients remain on treatment were used.
There is uncertainty around the correct treatment duration for the interventions. The clinical evidence suggests that treatment duration is shorter than the time to disease progression to accelerated phase. However, to test the effect of different treatment durations, SHTAC was requested to undertake two additional scenario analyses, which are presented here. The SHTAC analyses included each of the interventions and comparators in the appraisal scope. However, in Table 42 the comparators interferon alfa, standard-dose imatinib and stem cell transplantation were dominated or extendedly dominated in the base case, and therefore in the subsequent scenario analyses these data have not been presented for ease of reference. It is important to note that in the PenTAG2 model, the time between the end of treatment and disease progression is labelled as ‘chronic phase no treatment’. During this time, the model assumes that third-line treatments are given to patients.
Scenario analysis one
A scenario analysis was undertaken to use the progression-free survival and treatment duration estimates from the PenTAG AR2 for dasatinib (treatment duration of 6.5 years) for nilotinib and high-dose imatinib. The result of this scenario analysis can be seen in Table 64.
| Intervention | QALY | Cost (£) | ICER vs HU (£/QALY) | ICER vs next-best optiona (£/QALY) |
|---|---|---|---|---|
| HU | 2.2 | 18,128 | ||
| HDI | 7.31 | 238,594 | 43,151 | Dominated |
| Nilotinib | 7.63 | 222,093 | 37,562 | Dominated |
| Dasatinib | 7.85 | 221,325 | 36,007 | 36,007b |
There is no change in QALY because the PenTAG2 model predicts overall survival independently of progression-free survival and time to treatment discontinuation. Time in chronic phase is calculated by subtracting time in accelerated phase and blast crisis from overall survival. As the time in accelerated phase and blast crisis are constant, the time in chronic phase is also constant unless overall survival is changed. As utility values on and off treatment in the chronic phase is the same, changes to treatment duration have no effect on the QALYs.
Scenario analysis two
A scenario analysis was also undertaken to test treatment duration of approximately 10 years for all interventions. The result of this scenario analysis can be seen in Table 65.
| Intervention | QALY | Cost (£) | ICER vs HU (£/QALY) | ICER vs next-best optiona (£/QALY) |
|---|---|---|---|---|
| HU | 2.2 | 18,128 | ||
| HDI | 7.31 | 300,182 | 55,179 | Dominated |
| Nilotinib | 7.63 | 266,204 | 45,685 | Dominated |
| Dasatinib | 7.85 | 265,521 | 43,816 | 43,816b |
List of abbreviations
- allo-SCT
- allogeneic stem cell transplantation/transplant
- AE
- adverse event
- AR
- assessment report
- BCR–ABL
- oncogene fusion protein consisting of BCR and ABL genes
- b.i.d.
- twice daily
- BMS
- Bristol-Myers Squibb
- BNF
- British National Formulary
- CENTRAL
- Central Register of Controlled Trials
- CHR
- complete haematological response
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CML
- chronic myeloid leukaemia
- CRD
- Centre for Reviews and Dissemination
- CT
- computerised tomography
- CyR
- cytogenetic response
- EQ-5D
- European Quality of Life-5 Dimensions
- FAD
- final appraisal determination
- HDI
- high-dose imatinib
- HR
- haematological response
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- IRIS
- International randomized study of interferon versus ST1571
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- LY
- life-year
- LYG
- life-year gained
- MMR
- major molecular response
- NICE
- National Institute for Health and Clinical Excellence
- PenTAG
- Peninsula Technology Assessment Group
- PenTAG AR
- Peninsula Technology Assessment Group assessment report
- PFT
- post-failure treatment
- Ph+
- Philadelphia chromosome-positive
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SHTAC
- Southampton Health Technology Assessments Centre
- TTD
- time to discontinuation
- TTO
- time trade-off
- WHO
- World Health Organization
- WMHTAC
- West Midlands Health Technology Assessment Collaboration
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health