Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 02/11/04. The contractual start date was in October 2004. The draft report began editorial review in January 2011 and was accepted for publication in May 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
PT received funding from the pharmaceutical industry to deliver a lecture on stroke and from Boehringer Ingelheim to attend the 2010 European Stroke Conference. AV, LD, AH and PT receive expenses for attending meetings of the National Institute for Health Research Health Technology Assessment panels. Several of the NHS Trusts participating in the study received funding from The Stroke Association towards their start up (year 1) excess treatment costs. During the study, GP and MW (retired 2005) were employed by charities representing people with stroke, Speakeasy and The Stroke Association. AY receives annual royalties from Authors’ Licensing and Collecting Society for academic publications.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Bowen et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In 2002 the UK’s National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme identified the need to evaluate the effectiveness and cost-effectiveness of speech and language (SL) therapy for aphasia and dysarthria following stroke, using therapy strategies likely to be effective yet feasible for routine NHS delivery and comparing these with an attention control (AC) involving patient contact but not specific therapy. This report describes the work commissioned to address these issues and to elicit the views of service users and carers.
Stroke
‘A stroke occurs when blood flow to part of the brain is interrupted causing damage to the brain tissue’. 1 The 2010 National Audit Office (NAO) report concluded that 110,000 people have a stroke each year in England alone, around 300,000 people are living with moderate to severe disabilities as a result and stroke care costs the NHS £3B per year.
Aphasia and dysarthria after stroke
Stroke can disrupt communication in different ways, through impaired motor speech production (dysarthria) or use of language (aphasia). Dysarthria is an output problem, resulting from impaired movements of the speech musculature including lips, tongue, palate, larynx and respiration. This limits intelligibility for the listener and may cause frustration and distress for the person with stroke. Aphasic problems can be receptive or expressive, affecting spoken, written or gestural forms of communication and are associated with emotional distress up to 6 months post stroke and beyond. 2–4 Restricted activity and social participation are common consequences of aphasia and dysarthria, as are psychological effects, vulnerability and adverse effects on families and informal caregivers. 5 Apraxia of speech also occurs6 but is outside the remit of this study.
Epidemiology of communication problems
Data on the frequency and recovery rates of dysarthria and aphasia vary between studies, depending on methodological factors such as sampling methods, timing of screening/assessment, method of assessment, expertise of the assessor and length of follow-up.
The prospective Lausanne Stroke Registry study7 of 1000 consecutive hospital admissions with first-ever stroke suggested that 46% had impaired communication (34% with aphasia) in the acute phase. A prospective, population-based study8 found that 30% of those with first-ever ischaemic stroke (FEIS) had aphasia and that the overall incidence rate attributable to FEIS from multiple overlapping sources of information was 43 per 100,000 inhabitants [95% confidence interval (CI) 33 to 52 per 100,000 inhabitants].
Despite some degree of spontaneous recovery, the prevalence of persisting speech and language difficulties (6 months post stroke) is 30–50 per 100,000 population. 9 The Royal College of Speech and Language Therapists (RCSLT) draws on Department of Health (DoH) data to conclude overall that around one-third of people are left with communication disability after stroke. 5
It is generally accepted that aphasia occurs more commonly than dysarthria, although they may co-occur. Research interest, both epidemiological and concerning effectiveness, has disproportionately focused on aphasia rather than dysarthria.
Speech and language therapy
Speech and language therapists in the NHS work with people with aphasia and dysarthria and their families in different settings across the stroke pathway, for example as part of a multidisciplinary team (MDT) on a specialist stroke unit, in outpatient clinics and in the community, although most provision is probably in the first few months after stroke. This system mostly works by referrals from MDT members. One of the most recent guidelines to summarise the specialist role of SL therapists is the 2010 Scottish guideline. 10 This outlines six key elements of the intervention for aphasia or dysarthria:
-
diagnosis
-
information (to patients, carers and health-care staff)
-
detailed assessment (to include impact on the individual and family and psychosocial situation and general well-being)
-
individualised care programme (including goal achievement and compensatory strategies)
-
access (to coping strategies, support groups, etc.)
-
facilitating referral.
Several authorities promote the main aim of rehabilitation as being about maximising the person’s ability to participate successfully in everyday communicative interactions. 5,11 Although the professional body is keen to stress that there is ‘no universally accepted treatment’ that can be applied to every person with aphasia, the reader is referred to the RCSLT’s detailed resource manual5 for commissioning and planning services for aphasia, which provides specific description of interventions for this client group, including how these address all three levels of the International Classification of Functioning, Disability and Health (ICF) framework: impairment, activity, participation. 12 The overall approach is summarised by RCSLT as use of multiple strategies to:
-
help the person to use remaining abilities
-
restore language abilities as much as possible by developing strategies
-
compensate for remaining problems
-
learn other methods of communication
-
coach others (family, health and social care staff) to learn effective communication skills to maximise the aphasic person’s competence.
Evidence for speech and language therapy for people with dysarthria
The Cochrane systematic review of SL therapy for dysarthria, published prior to the start of the ACT NoW (Assessing the effectiveness of Communication Therapy in the North West) study, was updated in 200513 following further literature searches. The authors initially identified 16 potential studies but subsequently excluded them all. Most (13) were not randomised controlled trials (RCTs), and nine of these were before-and-after group studies. The three RCTs were excluded because the intervention was not SL therapy14,15 or the participants had communication problems other than dysarthria and had a progressive condition. 16 This review highlighted a lack of RCTs of SL therapy for dysarthria after stroke or other non-progressive brain injury, and identified an urgent need for good-quality research in this area. 13
The Scottish Intercollegiate Guidelines Network (SIGN) 201010 guideline confirmed that there have been no further completed trials or systematic reviews since 2005. SIGN concluded that evidence for the effectiveness of SL therapy for dysarthria continues to be restricted to small group studies, single case studies or expert opinion, and that expert opinion is firmly in favour of the value of SL therapy intervention. They recommend referral to SL therapy but do not specify how early this should happen or at what intensity or duration.
The current National Clinical Guideline for Stroke17 observes that dysarthria has been poorly researched. They recommend (under certain circumstances) assessment, direct intervention and training communication partners. There is no dysarthria-specific intensity/duration recommendation beyond the generic rehabilitation recommendation of 45 minutes per day if needed and tolerated, and if the patient is willing.
Evidence for speech and language therapy for people with aphasia
Current clinical guidelines for the rehabilitation of aphasia post stroke10,17 and the SL therapist profession’s recent commissioning and planning manual5 are heavily influenced by two reviews. 18,19 The RCSLT draws attention to variations in the validity of the available reviews and distinguishes between the high quality of the Cochrane review11,18 and the serious methodological flaws of others. 19 These reviews reach opposing conclusions about effectiveness, unsurprising, perhaps, given their methodological differences.
Robey’s19 review of 55 group quasi-experiments including non-stroke populations (an update and expansion of his 21-study review20) generates 75 estimates of treatment effects. Most (60) are from the studies that made pre- and post-therapy comparisons, with the remaining 15 derived from non-randomised comparisons of treatment versus no treatment. Robey19 concludes that ‘on average, treatment for aphasic persons is effective’ and he argues, in this heavily cited paper, that further studies ‘to reinforce this general conclusion’ would waste resources.
However, Robey19 concludes that including RCTs (and homogeneous treatments) would reduce the number of effects to zero. This is supported by the findings from a systematic review by Greener et al. ,18 conducted using internationally accepted methods21 to exclude the sources of bias likely to have influenced Robey’s findings. 19 Greener et al. 18 considered the 12 existing RCTs of aphasia and concluded that because of their ‘poor or unassessable methodological quality’ they cannot be used to determine effectiveness.
The aphasia Cochrane review18 has recently been extensively modified and updated. 11 The revised protocol included a restructuring of the research questions, revision of the eligibility criteria and information on how to pool extracted data. It now comprises 30 RCTs, incorporating 10 of the original 12 included studies. This is a broad review with 41 subcomparisons, making it difficult to draw an overall conclusion. Some studies provided intervention in the acute phase post stroke, whereas others recruited people months and years later, with concerns raised by the review authors about the clinical relevance of this data set for a typical SL therapy clinical population.
The review’s summary of results covers three topics:
-
speech and language therapy versus no SL therapy
-
speech and language therapy versus social support and stimulation
-
one SL therapy approach versus another SL therapy approach.
A receptive language subtest was reported as favouring SL therapy. The review authors also observed a consistency in the direction of effect across most of the subcomparisons (85%), which favoured the provision of SL therapy. As a result, they concluded that there was ‘some indication’ of the effectiveness of SL therapy for people with aphasia following stroke. In the other two topics they concluded that SL therapy by a trained supervised volunteer was as effective as by a professional SL therapist, and that there was insufficient evidence to establish the effectiveness of one SL therapy approach over another.
In contrast to dysarthria, there is vibrant research activity into aphasia, much of which comes from the UK. This has mainly focused on theoretical development of the underlying impairment and differences in the range of aphasic presentations. The body of studies evaluating the effectiveness of therapy is growing but is mostly concentrated at the ‘theory/modelling’ stages (pre-clinical and phase I) of the Medical Research Council (MRC) framework for trials of complex interventions, consisting largely of single case experiments and case series. 22 Interventions that show promise in phase I studies are not typically taken forward into phase II and III trials. Although trials have been conducted (and systematically reviewed), they do not appear to have followed the progressive development suggested by the MRC framework. Even though methodological failings and insufficiently detailed therapy protocols within some RCTs have been rightly criticised, misunderstandings about RCTs per se (e.g. heterogeneity, efficacy vs effectiveness) are perpetuated, which presents barriers to building a robust evidence base. A prime example is the paper titled Ask a silly question: two decades of troublesome trials23 which, despite including several valid pro-RCT comments, tends to be wrongly interpreted as proof that RCTs are inappropriate for SL therapy.
Limitations of previous studies
There are no RCTs of specific SL therapy interventions for people with dysarthria after stroke nor of SL therapy service delivery for this client group. The limitations of previous research into SL therapy for aphasia after stroke include the following:
-
Observational studies and experimental single case designs or series contribute to the evidence base but cannot provide robust, reliable evidence of clinical effectiveness that generalises to the target clinical population.
-
Randomised controlled trials have not included an economic evaluation.
-
Randomised controlled trials have either not standardised the therapy, or the amount and content provided was not described in sufficient detail for replication or implementation into clinical services.
-
Randomised controlled trials have either not used or not reported methods to reduce bias (e.g. allocation concealment, blinding outcome assessors) as per the CONSORT (Consolidated Standards of Reporting Trials) guidelines. 24
-
Randomised controlled trials have not included service user and carer opinions in the design and conduct of the study, for example to insure the inclusion of adequate patient-reported outcome measures (PROMs) or aphasia-friendly (accessible) qualitative study methods.
-
Several RCTs have not included outcome measurement at the ICF activity and participation levels, focusing instead on impairment-level measures, which, on their own, do not provide a meaningful evaluation of whether an intervention is effective.
-
Some RCTs have not measured outcomes at a time point appropriate to determine whether there is a meaningful maintenance of any immediate post-therapy benefits, for example to at least 6 months.
Justification for the current study
Communication problems affect the lives of a considerable proportion of those who survive a stroke. They can persist for years, resulting in lifelong activity-level restrictions and decreased opportunity for social participation. Service user surveys repeatedly find high levels of dissatisfaction with current service level provision. 25 National audits highlight low levels of provision of SL therapy, although these figures perhaps attract less attention than they should, given concerns about the even worse provision of clinical psychology. 26 In 2010 the NAO found that the actual staffing levels [0.4 whole-time equivalent (WTE) per 10 beds] of SL therapists on stroke units in April 2009 were about half of that expected by the 2007 DoH strategy staffing assumptions (0.8 WTE), and were far lower than occupational therapy and physiotherapy 2009 levels (1.1 and 1.3 WTE, respectively) and that swallowing therapy may have taken precedence over communication therapy.
Although many stakeholders consider that the amount of SL therapy currently provided within the NHS is less than desirable, it is nevertheless an intervention that is routinely commissioned within the NHS, in hospital and community services. Given the cost to the public purse, and in the wider context of improvements to stroke service provision, an examination of the effectiveness and cost-effectiveness of SL therapy for aphasia and dysarthria is justified and indeed essential. The ACT NoW study was designed to evaluate the effectiveness, cost-effectiveness and service users’ views of an early, well-resourced intervention by SL therapists for people with aphasia or dysarthria admitted to hospital with stroke.
Structure of the Health Technology Assessment monograph
The ACT NoW study consisted of two stages, each containing several study types. Chapter 2 summarises the aims, methods and findings of the component parts of stage 1, the feasibility study. Stage 2, the main study, consisted of a RCT (Chapters 3 and 4), economic evaluation (Chapters 5 and 6) and qualitative study (Chapter 7). The design and delivery of the SL therapy data and that from the control arm are described in Chapters 2–4. The studies are combined into an overall discussion and conclusion in Chapters 8 and 9, respectively. The appendices contain detailed additional information.
Chapter 2 Feasibility study
The ACT NoW study consisted of two stages. This chapter summarises stage 1, the feasibility study and its components. The feasibility study was only a means to an end, and was reported on in detail to the funder as it progressed, and so will be reported in brief here. At the time of writing this monograph, four papers have been published27–30 and others are in preparation for submission in 2012. [Young A, Gomersall T, Bowen A. Trial participants’ experiences of early, enhanced speech and language therapy after stroke compared with employed visitor support: a qualitative study nested within a RCT. Clin Rehabil 2012;in press; Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Does early, enhanced speech and language therapy for people with aphasia or dysarthria after stroke add more benefit than providing support alone? The ACT NoW randomised controlled trial (Assessing Communication Therapy in the North West). BMJ 2012;submitted.] The reader is referred to these for detailed descriptions of our development of novel outcome measures27,28 and our validation of a novel use of an existing outcome measure. 29,30
Aims and objectives of the feasibility study
The overall aim of the feasibility study was to develop the methods and materials needed for each part of the subsequent main study (the RCT, economic evaluation and qualitative study), to pilot these and determine whether a main study would be feasible.
There were eight specific objectives. These were to:
-
define the patient population of interest and develop a pragmatic screening procedure
-
describe the intervention in a written manual, so it could be replicated and generalised
-
test the feasibility of recruiting and training volunteers, to provide the AC
-
develop a method of maximising recruitment, given difficulties recruiting to previous RCTs in this area
-
select, and if necessary develop, outcome measures for the main RCT, based on user/carer preferences and adequate for statistical comparisons
-
develop qualitative methods for the main study, to engage service users and carers in driving the research process, and to elicit their views of NHS services
-
develop cost data collection methods, tailored for these specific clinical conditions and services
-
test the feasibility of recruiting and retaining participants in a RCT (implementing the methods and materials developed in objective 4) and quantify the patient population.
Perceived challenges
At the outset we perceived many challenges to the successful running of the study (of the RCT in particular), such as whether ethics and research governance approval would be granted (given the initial plan to deliver the AC through volunteers) and whether the essential involvement of NHS SL therapists could be obtained.
These challenges were overcome and helped plan the timetable for the main study. The feasibility study received research ethics approval (04/MRE03/30 ‘The ACT NoW pilot study’) and research governance approval (including, eventually, honorary contracts for the volunteers) for a pilot RCT, health economics and qualitative study to recruit people with stroke admitted to four NHS acute stroke services and follow them through into the community 6 months later. This work began in October 2004. These approvals were amended to include a larger outcomes validation study that completed data collection in August 2006 and has since been published. 27–30
Research partnerships
NHS speech and language therapists and service users
We formed two key partnerships. The first was with the NHS SL therapists who would design and implement the screening and intervention approaches. The SL therapist group’s role and achievements were essential for achieving objectives 1 and 2 (see Aims and objectives of the feasibility study) and are described below (see Partnership with NHS SL therapists: screening and intervention).
Research user group
The second partnership was with stroke service users and their carers, who named themselves ‘the RUG’ (research user group). The RUG’s role began during the feasibility study and continued through to its support in the dissemination of the main study. A detailed paper has been prepared for publication, describing the methods used to work in partnership with service users to maximise recruitment to the RCT. A second paper is in preparation, cowritten with the RUG members, and focuses on the impact on them of their long-term involvement in this research study. In addition, we produced a paper in January 2007 at the request of the Stroke Research Network entitled ‘ACT NoW: involving service users in research’. This was a detailed guide for researchers intending to work collaboratively with service users with stroke, includes materials used in the ACT NoW study and is downloadable from www.uksrn.ac.uk (under PCPI, links).
In summary the RUG’s main tasks were to:
-
advise on the interview panel for the selection of research staff
-
train research staff in how to facilitate successful communication with research participants with aphasia
-
develop accessible, aphasia-friendly information and consent materials in a variety of media (objective 4: Figure 1 and Chapter 3)
-
advise on the content and accessibility of the outcome measures including a newly developed and validated patient-centred outcome measure [objective 5; see COAST (Communication Outcomes After Stroke scale), etc., below, under Outcomes validation study]
-
advise on the choice of accessible qualitative study interview questions (objective 6; see below and Chapter 7).
FIGURE 1.
Information and consent materials developed in collaboration with the RUG. Readers can view and download the written recruitment materials from www.psych-sci.manchester.ac.uk/actnow/patients/needtoknow/

All of these tasks were achieved through a model of facilitated regular meetings at a convenient location in a meeting hall in the community. During the feasibility stage, eight service users with aphasia and/or dysarthria and two carers collaborated with researchers. There were a few early changes to membership, which otherwise remained remarkably steady throughout the subsequent 4 years of the main study.
Outcomes validation study
Following a comprehensive consideration of 10 possible outcome measures and decision that none were suitable in their current form, a 20-item, patient-centred COAST was developed and validated with a sample of 102 people following a stroke and 68 carers (objective 5). 28 The RUG worked with the project leads AL and AH to provide input on the content (items) and the presentation (aphasia-friendly visual aids and layout). The COAST measures participants’ perception of their communication effectiveness and the impact this has on their quality of life (Figure 2). A parallel version, the Carer COAST (Communication Outcomes After Stroke scale, carer version), was validated to provide the carers’ perspective on the patients’ communication effectiveness and its effect on the carers’ own related quality of life. 27 The COAST and Carer COAST were included as secondary outcome measures in the main RCT (these and other resources developed are available at www.psych-sci.manchester.ac.uk/actnow/outputs/resources/).
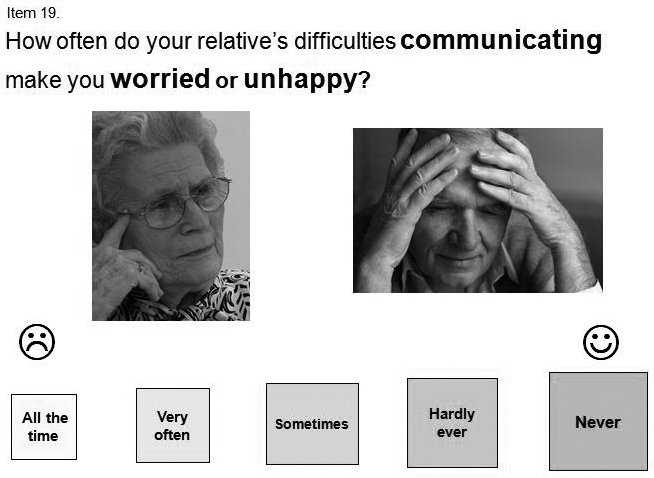
FIGURE 2.
Items from the COAST (left) and Carer COAST (right). Images for the example from the Carer COAST are reproduced with permission from http://en.fotolia.com/ (©Fotolia).

A clinically feasible procedure for collecting a video-recorded, semistructured conversation sample between the service user and an unfamiliar conversation partner was developed. Such a conversation sample provided data for the main RCT’s primary outcome measure. We tested its rating by expert SL therapists, using the existing Therapy Outcome Measure activity subscale (TOM). The agreement between service user, carer and therapist perspectives on the person’s functional communication has also been explored. 30 As we discuss in one of our papers,29 ‘Our findings support the use of the TOM by an unfamiliar observer to rate a short conversation as part of outcome measurement. The use of independent expert SL therapists to provide TOM activity-level ratings on structured conversation samples with an unfamiliar partner reduced the variability known to affect judgements of conversation, and was shown to have promise as a clinically feasible, socially relevant and reliable measure (p. 971)’.
Findings
The outcomes validation study resulted in the successful production of novel, patient-centred outcome measures, with demonstrated reliability, indicative evidence on convergent validity and practical to use in research, and a standardised method of collecting blinded professional ratings, suitable for making valid and reliable statistical comparisons in the RCT.
Partnership with NHS speech and language therapists: screening and intervention
A working party of NHS SL therapists and university-based researchers was established to develop a pragmatic screening procedure (objective 1) and define the intervention (objective 2) so that both could be standardised across the four sites participating in the pilot RCT. The latter is described below (see Intervention).
All admissions with stroke to the four sites participating in the feasibility study’s pilot RCT were screened. This was considered necessary as therapists were initially concerned that potential participants, especially those people with milder or more subtle language problems, would be missed if solely dependent on referrals to SL therapy from other NHS staff. A multistep screening method was used:
-
A therapy assistant (TA) reviewed the medical notes of everyone admitted with stroke to identify those with a possible communication problem and to search for documentation of any of the study’s exclusion criteria, for example some people had died or were receiving end-of-life care, whereas others lived out of the area covered by the NHS SL therapy service or were not fluent in English.
-
Those who may have communication problems and appeared eligible progressed to the second screening stage, at which they were seen by an SL therapist who used the Frenchay Aphasia Screening Test (FAST)31 and TOM32 to confirm the presence/absence and persistence of communication problems, provide a differential diagnosis (of aphasia, dysarthria or aphasia and dysarthria), determine the severity of the communication problem at the level of impairment and restricted activity (disability), and rule out all exclusion criteria. Dysarthria was diagnosed by a TOM rating of the FAST’s picture description task.
-
Anyone referred to SL therapy automatically progressed to the second screening stage (i.e. without note review by an assistant).
-
Those who appeared eligible but were not yet able to engage in stage 2 were rescreened by assistants during the following 2 weeks to avoid excluding people who needed a bit more time before they were ready to participate.
-
Those with no documented communication problems in their notes (and no obvious exclusion criteria) were also seen by the SL therapist to identify people with subtle communication problems who were not identified in the standard clerking or referral procedures. Up to four specific methods were used: a brief conversation with the therapist, the Graded Naming Test,33 rapid lip and tongue movements [diadochokinetic rates (DDK)] and a graded oral spelling test. 34 Those failing any of the four were screened as described above (see step 2).
The numbers admitted and screened are considered below (see Findings from the pilot randomised controlled trial). In brief, 103 of the 265 admissions were initially considered as eligible for participation. Following SL therapists’ screening, 34 of the 66 who continued to have communication problems were considered eligible. The other 32 were excluded for a variety of reasons, as several of the exclusion criteria were not apparent at note screening, for example pre-existing dementia or learning difficulties, severe cognitive deficits or other medical problems rendering the SL therapy intervention unsuitable.
The study found that although the screening procedure was feasible the additional elements (conversation, DDK rates, naming and spelling) did not identify any further eligible patients. Therefore, the feasibility study concluded that only those referred to SL therapy or with communication problems documented in their notes needed to be screened by SL therapists in the main RCT. (Apraxia of speech was outside the remit of this study. If present in the participants it was not treated as a unitary disorder and was assessed only as part of the speech or language disorder.)
Intervention
During the feasibility study the NHS SL therapists and academic colleagues worked together across a series of intensive week-long workshops to agree and describe an intervention suitable for piloting in a RCT (objective 2). The methods used were to review the literature, including the grey literature around clinical guidelines, and to reach a consensus on what was considered to be best practice for the early treatment of aphasia and dysarthria. This included both the specific components of interventions and service delivery issues. This can be described as a set of best practice guidelines and a compendium of resources. The therapists also established an agreed coding system to record the type and amount of therapy received by each participant. As well as providing these quantitative measures, the participating therapists attended bimonthly aphasia meetings at the University of Manchester (which are open to all aphasia therapists in the area), at which each site presented one case to illustrate to other therapists how they had implemented the guidelines. The feasibility study allowed the therapists to try out the intervention and recording methods, highlight any practical difficulties and make changes prior to the main RCT.
Six key components were identified ranging from assessment (summarised in Appendix 16) and direct one-to-one intervention with patients through to the SL therapist’s role within the MDT and the SL therapists’ support for informal carers. These are listed briefly in Table 1.
| 1. Assessment | Initial and ongoing, standardised, functional, case history, goal setting |
| 2. Information provision | Communication problem, strategies/equipment to assist communication, intervention plan, therapist opinion of progress, available information resources and support networks |
| 3. Provision of communication materials | Communication book for recording activities; an AAC device if required |
| 4. Carer contact | Discussion and information giving, observation and participation in therapy, conversation partner training, preparation for the end of the research intervention |
| 5. Indirect contact | Written descriptions of needs, abilities and strengths, discussions with clinical teams, MDT goal planning |
| 6. Direct contact | Therapy to improve language skills at all levels of the World Health Organization ICF model:12 impairment (improving language skills), activity (compensatory strategies), participation (developing confidence, accessible information) |
Key features
The intervention was designed for those admitted to hospital with stroke and timed to begin during the acute phase of the stroke pathway. As such, it was specifically designed to evaluate early intervention, a period when most therapy is currently provided aimed at channelling recovery and adjustment to disability. However, it was to be practical for NHS delivery and one aspect of this meant that the intervention start date was flexible, depending on what was most appropriate for each individual, as determined by the SL therapist’s clinical judgement. Intensity and duration were to be available up to three times a week, for up to 3 months but flexibly and not of a one-size-fits-all prescribed dose. Intensity and duration varied both between and within participants, as determined by the SL therapist’s clinical judgement and agreement with the patient about what was appropriate. Another important feature of the intervention was that it was to be capable of being provided in whatever setting the patient was in, for example to provide continuity into the community for those discharged from inpatient care.
Findings
Seventeen of the 34 participants eligible for the pilot RCT consented (or provided proxy consent from an informal carer) to take part. Nine of these were randomised to the intervention arm, of whom five had aphasia and dysarthria, two had aphasia only and two had dysarthria only. The full range of severity (mild to severe) on the TOM scores was represented in this small group.
The intervention data recorded suggested that information giving, carer contact, indirect contact (MDT) and direct contact (face to face) with a SL therapist were common to all participants. The most popular therapy types were impairment, functional and conversation based. Counselling and support for both participant and carers was also a common feature. Five participants required therapy for the full 3 months. Of the other four, one died and the other three all had mild TOM scores.
Some intervention techniques were not tested in the feasibility study owing to the small numbers of participants and the rolling admission into the study over 3 months, for example group work with participants or carers, high-technology alternative or augmentative communication (AAC) devices.
The best practice guidelines were modified as the feasibility study progressed. A key change was the increase in the maximum duration from 3 to 4 months, based on a consensus among therapists that more time was likely to be needed by some participants. At the end of the feasibility study a summary of the revised intervention for evaluation in the main RCT was produced (see Appendix 1) along with a detailed 103-page manual and compendium of resources (available on request). Two descriptive case studies were written to illustrate the range of activities and the time course for these over the intervention period (available on request).
Attention control
The feasibility study aimed to develop an AC and test the feasibility of delivering this through the use of volunteers (objective 3). The intention was to provide participants allocated to the AC arm with an equivalent amount of contact time (attention) as those in the intervention arm (up to three times a week, for up to 3 months) but without providing SL therapy. The AC acted as a comparator to determine if between-group differences were truly due to the early communication input provided by SL therapists as opposed to the potential psychosocial benefits of regular, frequent one-to-one contact.
Attention control was to be delivered by a volunteer rather than an SL therapist because of the likelihood of contamination between arms if both were provided by the latter. It was also clear that therapists would not feel comfortable providing the AC, nor would they have sufficient resource to see both groups as early and as frequently as might be necessary.
Volunteer recruitment and training
Seventy-four people responded to advertisements for volunteers for the feasibility study using posters and leaflets in sports, health and jobs centres, shops, university and college campuses across the north-west of England. Volunteering agencies were also used, and university students were targeted at fairs and by e-mail. Volunteers had to be ≥ 18 years and have no prior experience or specific training in stroke rehabilitation. Informal information sessions were delivered across the four pilot RCT sites, aimed at providing potential volunteers with more detailed information about the research. Fifty-one people completed the next step, a 2-day training session, and 26 of these became operational volunteers in the pilot RCT. The training programme was a modified version bought in from a voluntary sector organisation.
A volunteer coordinator was used. The coordinator managed the administration around research governance requirements of using volunteers within a clinical research study [e.g. applications for enhanced disclosures from the Criminal Records Bureau (CRB) and securing honorary NHS contracts for volunteers to visit research participants in hospital and later in the stroke pathway in the participants’ homes]. The coordinator also monitored the quality and quantity of each contact between volunteer and participant by accompanying volunteers on initial visits and reviewing the volunteers’ data collection sheets.
Findings
The feasibility study proved invaluable in highlighting limitations with the use of volunteers to provide AC to acute stroke patients in the feasibility study:
-
It was difficult to recruit and retain the type of volunteer considered best suited to this clinical research study. Twenty of the 26 operational volunteers were students. Five others were professionals and one person was retired. All but two of the 26 were aged 18–29 years. The large time commitment placed on volunteers (visiting up to three times a week, up to 3 months) was the main reason for dropout or failure to deliver all the required visits.
-
The training package was difficult to adapt for research purposes and, with hindsight, did not prepare the volunteers adequately for the difficult task of visiting the eight people with aphasia or dysarthria who had been allocated to the AC in the pilot RCT. Data collection forms did not adequately record the content of each visit, essential to ensure that volunteers were providing only attention (e.g. conversation, companionship) rather than offering communication strategies.
-
A lack of clarity in research governance guidelines at the time on how to register non-employed volunteers operating with a clinical research study meant that there were long delays that jeopardised research deadlines (e.g. NHS honorary contracts were difficult to obtain) and in fact reduced the pilot RCT recruitment phase to 12 weeks.
-
Volunteers were unable to offer the flexibility of time and travel essential to cover all sites and fit in with participants’ requirements. These restrictions on their availability meant that the employed coordinator had to conduct 42% of the 86 AC visits. Furthermore, the average number of sessions for participants allocated to the pilot RCT’s AC arm was 1.1 per week compared with 1.8 per week for those allocated to SL therapy.
In conclusion, use of an AC group with this clinical population was feasible but several difficulties needed to be overcome for the main study. Participants did agree to the concept of randomisation and 86 visits were successfully carried out with the eight people randomised. As described below, see Findings from the pilot qualitative study, AC participants reported their experiences positively. However, the feasibility study showed that the use of volunteers would be, counterintuitively, too costly and time-consuming for delivering AC in the main RCT. This model also risked failing in its main objective – to offer equivalent amounts of attention to both arms. This finding led to extensive redesign of the AC arm by employing paid part-time visitors, designing a new training and monitoring package and employing an experienced person to monitor the visitors for the duration of the main RCT. A summary of the main study’s AC is provided in Appendix 2 and a paper is in preparation for publication comparing AC in the feasibility and main study.
Pilot studies
This chapter has so far presented examples of materials and methods developed in collaboration with the RUG, for example accessible, aphasia-friendly, information and consent materials to maximise recruitment (objective 4, also described below) and RCT outcome measures, based on user/carer preferences and adequate for statistical comparisons (objective 5). We have also described the pragmatic screening procedure and defined intervention (objectives 1 and 2) that were developed by NHS SL therapists. To test the feasibility of recruiting and retaining participants in a RCT and to quantitate the patient population (objective 8) we tested these materials/methods with pilot studies. The RUG also contributed through a series of facilitated and communication supported group discussions to the development of qualitative methods to elicit communication-impaired RCT participants’ views of the intervention and control arms (objective 6). The final set of materials and methods developed during the feasibility study were the service use data collection forms for the economic evaluation, tailored for these specific clinical conditions and services (objective 7).
Findings from the pilot randomised controlled trial
The intention of the pilot RCT was not to compare outcomes but to determine the feasibility of conducting the main Phase III trial. We decided, for practical reasons, to recruit from four sites (both large urban hospitals and smaller more rural hospitals) for 4 months. This was reduced to 3 months because of delays obtaining the necessary approvals for volunteers and resulted in 17 participants who were allocated to a maximum of 3 months’ intervention or AC and were followed up at 6 months. This provided data to estimate the recruitment rate for the main RCT.
As discussed above, 265 people with stroke were admitted to hospital. All were screened to ensure that subtle communication problems would not be missed if depending on referrals alone. One hundred and three people had possible communication problems at note screening. This was reduced to 34 people following the SL therapists’ face-to-face screen, usually because mild communication problems had resolved or other exclusion criteria, not obvious from the early medical notes, were identified at interview. Exclusion criteria could be identified at various steps in the initially complicated screening process, making it difficult to obtain definitive rates for specific eligibility criteria. Once one exclusion criterion was identified the screening ended and so the actual incidence of each individual criterion was not known. Changes to data coding and simplification of the screening process itself were introduced by the therapists for the main study on the basis of lessons learned.
Ten of the eligible 34 refused any information but the recruitment materials were well received by the 24 who expressed an interest in receiving them, and resulted in a consent/proxy consent rate of 17/24. The procedure, which was developed with service user input from the RUG, was for a researcher to meet the potential participant to present the information in a manner most suited to the particular needs of that patient. Three versions of the information booklet were produced and used:
-
standard information (17)
-
aphasia friendly (5)
-
simplified/pictorial (2).
Audio and audio-visual formats (see Figure 1) were offered on three occasions in place of or to supplement the paper versions. Different levels of consent materials were also used. Five people were recruited by witnessed consent and one by proxy consent.
Attrition and breach of protocol were low and outcome assessments progressed without difficulty. One person died in the control arm and one refused their allocation to AC to obtain NHS SL therapy but agreed to take part in outcome measures. One person in the intervention arm breached protocol after the 3-month maximum had been reached, in order to obtain extra NHS SL therapy before the 6-month outcome assessment.
Fifteen of the 24 provided with information about the study had aphasia and some (8) had both aphasia and dysarthria. Of the seven who refused consent, three had dysarthria alone.
Findings from the pilot economic evaluation
Data collection tools and methods were developed by the ACT NoW team, based on available literature and knowledge of the services used by people following stroke. Two forms were produced for measuring resource use. A ‘Use of Hospital Services’ form was completed by research assistants (RAs), largely through hand-searching medical records retrospectively at the 6-month outcome assessment. This focused on use of hospital-based services. A ‘Support from Others’ form was completed by, and from the perspective of, the main informal carer at the 6-month assessment and also included use of primary- and community-based services. Both forms were tested and modified within the pilot RCT. The final forms developed for the main RCT are described in Chapters 5 and 6. The process of the pilot study and the data collected, combined with discussion with the study team, indicated that it was not feasible to collect reliable and consistent information from carers about the time they spent providing care and support to the study participant. Accordingly, these data were not collected in the main trial. This meant that the perspective of the economic study was changed from a societal viewpoint to that of the NHS, providers/funders of non-hospital care facilities and of patients and families. These actors are expected to incur the key costs and benefits of services for SL therapy for communication difficulties due to aphasia/dysarthria following stroke.
Findings from the pilot qualitative study
Four participants were sampled for qualitative analysis, two from each arm of the pilot RCT, purposively chosen to ensure interview materials were piloted across the range of severity of communication difficulties following stroke. The qualitative interview had three distinct parts to:
-
elicit participants’ perception of what had occurred during their intervention or control sessions
-
discuss what they had found ‘good’ and ‘difficult’
-
explore respondents’ views on how the sessions had impacted upon their life, either through improvements in their communication or through any other factors salient to the respondent.
Interviews were transcribed and coded thematically. The interviews worked well and revealed interesting insights. All four respondents valued their experience with either the volunteer or the SL therapist, and felt that their communicative abilities had improved, including gaining support from the volunteer/therapist and the amiable characteristics of the volunteer/therapists, which contributed to the respondents’ overall experience.
Conclusion of the feasibility study
The feasibility study, which began in 2004, had eight specific objectives, all of which were achieved by the end of 2006:
-
define the patient population and develop a pragmatic screening procedure
-
define the intervention, so that it can be replicated and generalised
-
test the feasibility of recruiting and training volunteers to provide the AC
-
develop a method of maximising recruitment, given known difficulties recruiting to previous RCTs
-
select RCT outcome measures and develop and validate a patient-centred measure of communication difficulties (COAST and Carer COAST) and a standardised method of collecting blinded professional ratings
-
develop qualitative methods for the main study, to engage service users and carers in driving the research process, and to elicit their views of NHS services
-
develop service use data collection forms for the health economic evaluation, tailored for these specific clinical conditions and services
-
test the feasibility of recruiting and retaining participants in a RCT, and quantitate the patient population.
The following chapters describe the methods and results used in the main RCT (Chapters 3 and 4), stated preference survey and economic evaluation (Chapters 5 and 6) and qualitative study (Chapter 7). Data on the actual intervention and AC provided to the main RCT participants, and issues around concordance, are described within Chapter 4.
Chapter 3 Randomised controlled trial methods
This chapter describes the design and conduct of the RCT. This was used to meet the study’s primary objective and the first three secondary objectives below. The remaining objectives, relating to the health economics components and the embedded qualitative study, are listed here to give an overall picture of the ACT NoW study but are then not addressed until Chapters 5–7.
Methods
Study design
The ACT NoW study was a pragmatic, multicentre, parallel-group RCT comparing clinical effectiveness and cost-effectiveness for two groups allocated in a 1 : 1 ratio. An embedded qualitative study was used to explore users’ and carers’ views on the process and effects of therapy and of AC. (A PDF of the protocol, version 4, dated 1 December 2008, is available on the NIHR HTA site at ‘www.hta.ac.uk/project/1390.asp#outputs’ and in Appendix 3.)
Primary objective
To evaluate the clinical effectiveness of an early well-resourced SL therapy intervention for people with aphasia or dysarthria after stroke when delivered by NHS therapists, compared with AC, as measured by blinded experts’ TOM ratings of videos of service users’ functional communication after 6 months.
Secondary objectives
Six months after entry to the study, to:
-
evaluate the clinical effectiveness of SL therapy compared with AC, as measured by service users’ self-reported functional communication and quality of life
-
evaluate the clinical effectiveness of SL therapy compared with AC, as measured by carers’ perceptions of service users’ functional communication
-
evaluate the clinical effectiveness of SL therapy compared with AC, as measured by carers’ own well-being and quality of life
-
evaluate the relative resource use, costs and cost-effectiveness of SL therapy compared with AC, including a societal perspective of valuations
-
elicit users’ and carers’ views on the process and outcomes of therapy compared with the views of those who received the AC
-
construct a ‘service user’s checklist’ of indicators of satisfaction and quality, which can inform and monitor the future implementation of the evaluated technology.
Summary of design of randomised controlled trial
Figure 3 summarises the study method.
FIGURE 3.
Study method flow chart. SRN, Stroke Research Network.
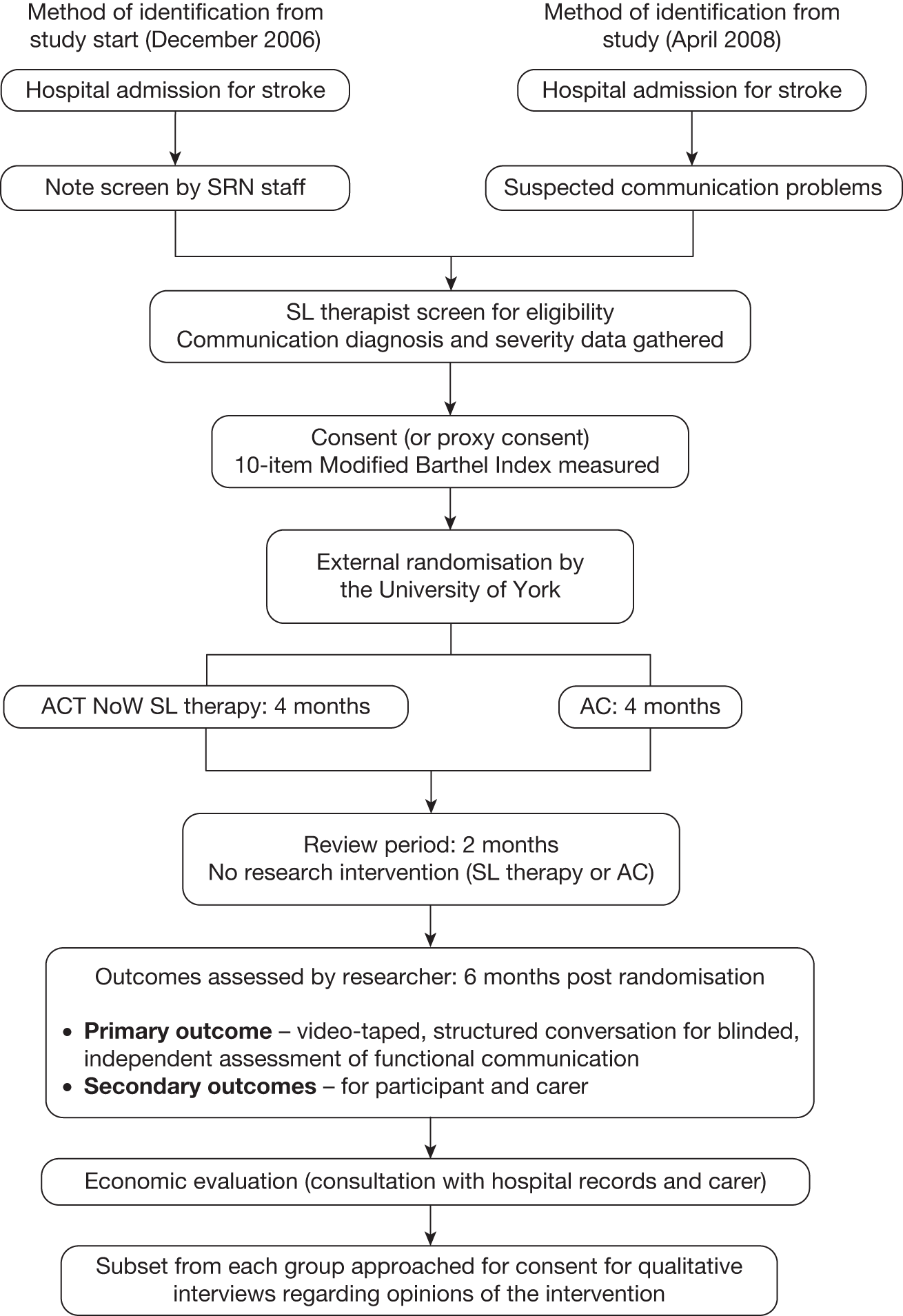
Adults admitted to hospital with stroke and communication problems were screened as soon as possible by SL therapists to determine eligibility. (As described in Chapter 2, the screening method was revised until it was, from April 2008, reduced to SL therapist screening of only those with suspected communication problems.) Participants had to be able to engage in and likely to benefit from SL therapy (as assessed by SL therapists themselves). Participants who consented to join the trial were externally randomised (see Randomisation) to either SL therapy or AC with concealment of allocation in a 1 : 1 ratio. Randomisation was stratified by:
-
severity of communication impairment assessed on the TOM for the worse of aphasia and dysarthria (two levels: 0 to 2, 2.5 to 5)
-
recruiting site.
During the intervention phase, participants were seen up to three times per week for a maximum of 4 months. After 4 months, support was stopped for a ‘review’ period of 2 months. At the end of this period (6 months post randomisation) outcomes were assessed and service use data collated. Participants and carers from each arm of the trial were sampled after outcome assessment and approached for consent to take part in qualitative interviews (see Chapter 7).
Setting and locations
The study was coordinated by the University of Manchester and involved multiple sites across the north-west of England. Eight sites began the study but during the course of the recruitment period four other sites were set up to boost patient recruitment.
Participants were always identified initially after an inpatient hospital stay but were often discharged from inpatient care after the first few weeks when the trial intervention continued in other settings, for example outpatients, domiciliary visits.
The sites involved in the study were:
-
Bolton patients admitted to Royal Bolton Infirmary
-
Burnley patients admitted to Burnley General Hospital. Part-way through the study, stroke services at Burnley were reorganised and patients were then identified after admission to Royal Blackburn hospital. Participants were sometimes transferred to Pendle Community Hospital for pre-discharge rehabilitation
-
Central Manchester patients admitted to Manchester Royal Infirmary
-
North Manchester patients admitted to North Manchester General Hospital
-
Salford patients admitted to Salford Royal (Hope Hospital)
-
Trafford patients admitted to Trafford General Hospital
-
Warrington patients admitted to Warrington General Hospital
-
Wigan patients admitted to Royal Albert Edward Infirmary and often transferred to Leigh Infirmary for pre-discharge stroke rehabilitation.
The above sites were involved in ACT NoW from the start of the study in December 2006 until the end (last patients identified January 2010 and completed outcome assessments in July 2010). The four additional sites were:
-
Blackpool patients admitted to Royal Victoria Hospital; joined the study in July 2008 and recruited participants for 1 year until July 2009, after which they were unable to continue due to clinical staffing problems
-
Crewe patients admitted to Leighton Hospital; joined the study in April 2009 and remained recruiting until the end of the study
-
Lancaster patients admitted to Royal Lancaster Infirmary but could be discharged to a range of secondary care hospitals including Westmorelands and Kendall; joined the study in April 2009 and remained recruiting until the end of the study
-
Stoke patients admitted to University Hospital of North Staffordshire and North Staffordshire Royal Infirmary with a number of secondary care facilities, including Haywood Hospital; joined the study in September 2009, with only 4 months to recruit patients.
Inclusion criteria
Adults with a stroke who were admitted to hospital were eligible for inclusion if they met the following criteria:
-
communication impaired due to aphasia or dysarthria
-
considered, by the SL therapist, able to engage in therapy
-
considered, by the SL therapist, likely to benefit from communication therapy
-
informed consent or proxy consent provided by carers.
Exclusion criteria
Screening, as described below, was used to identify the following exclusion criteria:
-
subarachnoid haemorrhage
-
dementia
-
pre-existing learning disabilities likely to prevent benefits from therapy
-
unable to communicate in the English language (provision of bilingual SL therapists was considered to be beyond the scope of this study)
-
other serious concomitant medical conditions (such as newly diagnosed terminal disease)
-
the patient being unable to complete eligibility screening, even after three attempts over a 2-week period
-
family or carer objections
-
(rare) cases when a SL therapist was asked to contribute to an urgent assessment of a person’s mental capacity to consent to an NHS treatment, before the therapist had time to complete screening to determine eligibility for the trial.
Screening and baseline assessment
A standardised procedure for communication screen was used across all sites. This determined eligibility and baseline assessment information for stratification and was always completed by an SL therapist.
The baseline communication screen consisted of two sections:
-
Language screen All patients were assessed using the FAST. The FAST provides health-care professionals working with patients who might have aphasia with a quick and simple method to identify the presence of a language deficit. It takes approximately 3–10 minutes to administer and has four subscales – comprehension, verbal expression, reading and writing – and is scored out of a total of 30 points. The verbal expression subscale involves a picture description that is also used as a speech sample to rate the severity of the language difficulties. If patients had severe difficulties and were unable to cope with the FAST then they were assessed informally using a selection of 10 object pictures or everyday objects, for example simple word to picture matching tasks. All patients were then rated on the impairment subscale of the aphasia TOM rating scale. This is an 11-point scale (0–5, with half points in between) that allows patients to be rated from ‘0’ (unable to communicate in any way) to ‘5’ (effective communication at all times).
-
Dysarthria screen The speech sample gained from the FAST and during conversation also led to a severity rating on the impairment subscale of the dysarthria TOM rating scale.
After these two screens, participants were also given a score on the aphasia/dysarthria activity subscale of the TOM and diagnosed for presence or absence of dysphagia (swallowing problems). (Apraxia of speech was outside the remit of this study. If apraxia of speech was present in the participants it was not treated as a unitary disorder and assessed only as part of the speech or language disorder.)
Prior to randomisation, the SL therapist provided all participants with an accessible leaflet about their diagnosis. Stickers were placed in the medical notes, meaning that the members of the MDT were aware that the patient had been diagnosed with aphasia or dysarthria. SL therapists did not provide support for communication to the patient, family or MDT until after they had had been informed about the study and, if consent was gained, until they had been randomised to the therapy arm.
Prior to randomisation, RAs also rated the patient on the 10-item Modified Barthel Index (BI)35 with the help of the hospital MDT. The BI gives a score out of 20 and indicates severity of overall disability (beyond communication) by rating the patient across 10 activities of daily living (ADLs), such as continence, personal care, feeding mobility and dressing.
Recruitment process
Once NHS SL therapists determined eligibility they telephoned the RAs, who met potential participants within the next day or two to provide information on the study and take consent. Participants were given a minimum of 24 hours between receiving first information about the study and joining the trial. This was to ensure that sufficient time for consideration and questions had been given and that participants had the opportunity to discuss joining the trial with family without a RA being present.
The use of aphasia-friendly information and consent materials (see Figure 1) ensured that RAs could provide an accessible and flexible approach to giving information to communication-impaired participants. Information materials were available in three versions: standard, aphasia-friendly and simplified/pictorial versions. All versions were in booklet format, printed on coloured A5 card and spiral bound to facilitate easy handling by patients with hemiplegia. Each of the written information materials had an associated audiotape that could be left with patients (as well as a personal tape player). These read out the information on each page to participants who had trouble reading. There was also a DVD/video of the standard-level information materials available, designed to be useful for families to take home and watch.
Consent materials were available in standard format (one-page consent form) or aphasia-friendly versions (with one statement per page, picture/icon support for the concept, broken down to facilitate understanding and large, easy-to-tick agreements).
If, because of their communication difficulties, the potential participant could not provide informed consent at that time, proxy consent could be requested from a carer/relative or Independent Mental Capacity Advocate/Consultee. Where proxy consent was gained, RAs later gave regular opportunities for participants to directly provide or withdraw their consent.
Randomisation
Once patients had consented to join the trial, their details were passed to the coordinating centre at the University of Manchester.
Randomisation was by an external, independent, web-based randomisation service from the York Trials Unit (University of York) to ensure concealment of allocation. Randomisation was stratified by severity of communication impairment assessed on the TOM for the worse of aphasia and dysarthria (two levels: 0 to 2, 2.5 to 5) and recruiting site. It was intended to stratify randomisation by a third factor, diagnosis (three levels: aphasia only, dysarthria only, both). During data checks after study completion it became clear that this had not occurred. Diagnosis and impairment were assessed by SL therapists at initial screening. Participants were randomised using a 1 : 1 allocation ratio and block sizes of two, four and six with differing combinations depending on the site. Variable block sizes were used to reduce the likelihood of selection bias through predictable allocation sequences. North Manchester, Trafford and Wigan all used strings generated using block sizes of two and four. All the other sites used blocks of four and six. No sites were aware of their block sizes.
Sites were able to temporarily suspend recruitment if staffing levels dipped below a point at which they would be able to take participants into the SL therapy arm, for example because of difficulty providing maternity leave cover. However, to prevent suspension from biasing selection it was permitted only if staffing problems were anticipated to last at least 3 months. This cut-off was necessary as staffing problems were a frequent occurrence and affected most, if not all, sites. Services had difficulty arguing for replacement posts because of the NHS research funding regulation that ‘excess treatment costs’ must be funded by normal commissioning36 and because the 80% of costs recoverable through a DoH central subvention was recoverable only retrospectively per person recruited to the SL therapy arm.
Intervention and control conditions
Participants were randomly allocated to the SL therapy intervention or an AC delivered by part-time employed visitors. See Chapter 2 for the development of these and Appendices 1 and 2 for summaries of content including minimum standards set for the intervention arm. The following sections describe the processes used to monitor delivery of both arms ensuring fidelity to the manuals.
Were minimum standards met, as set out in the therapy manual?
The SL therapy coordinator monitored and supported the SL therapists involved in the study. The aim of the monitoring process was to check that therapists were adhering to the therapy process described in the therapy manual and meeting the minimum standards for therapy provision described. Monitoring of therapy involved direct monitoring and bimonthly meetings at which the SL therapists presented to their peers details of a participant with whom they were working.
The SL therapy coordinator regularly visited all of the sites that were delivering therapy during the study to carry out 48 sessions of direct monitoring. During a monitoring visit, the following format was used: audit of case notes to check that the process of assessment (see Appendices 1 and 16) had been completed and goals for therapy had been clearly stated. As described in the minimum standards, this meant that impairments and functional limitations had been considered and therapy goals were based on sound theoretical rationale and the needs expressed by the participant. There would always be a discussion with the therapist about the rationale, and this also gave the therapists an opportunity to ask questions or for advice about therapy plans. In addition, checks were made to ensure that information had been given to participants about their difficulties, and that all were considered for AAC where appropriate. In addition, monitoring and support also took place by telephone and via e-mail, ensuring that therapists had someone who was more or less ‘on call’ to help with issues/queries as they arose.
There were a total of 22 therapy support meetings; at each meeting two sites presented a case study describing the therapy delivered to a participant in some detail, which was followed by peer discussion to promote consistency across sites.
Throughout the study, the therapists completed therapy record sheets that used codes to record interventions with participants in the study. Once completed, these were sent to the therapy monitor, and used as an additional check to ensure treatment fidelity. The monitor and peer group confirmed that standards were met during the trial. Therapy delivery at one site raised concern. This was closely monitored and extra support provided until the monitor was satisfied that the intervention was being provided as intended.
Visitor monitoring
Appointment of an AC monitor to provide appropriate training, close monitoring and supervision of the visitors was a major element of change and learning from the feasibility study. The primary purpose of this post was to ensure protocol adherence in guaranteeing that activities did not involve any form of communication strategy.
The monitor carried out the following duties and responsibilities:
-
prepared all training materials for visitors
-
selected and trained all visitors prior to visiting and ad hoc training as and when required when new visitors were appointed
-
ensured that visitors adhered to all relevant policies and procedures and to all health-and-safety-related guidelines
-
ensured that every visitor was monitored at least once at each of the three stages of the AC (rapport-building, core sessions and winding-down sessions)
-
ensured that monitoring visits covered both hospital and community sessions
-
highlighted any potential problems and/or training issues with the trial manager
-
planned and chaired bimonthly supervision/information sessions for all visitors
-
recruited replacement visitors, together with the trial manager, at the end of 1-year contracts (necessary to avoid a build-up of strategies for dealing with people with aphasia and dysarthria).
In the main this was a good framework, but it required some flexibility to enable the AC monitor to support and supervise lay visitors in an appropriate way, and to ensure that the highest level of adherence to research protocols was achieved.
Briefing programme
An initial 2-day briefing programme was delivered by the AC monitor, as described in the Visitors Manual, which prepared visitors for delivering appropriate social attention to AC participants.
More time than expected was required during the briefing programme for the visitors to fully comprehend and accept the strict protocols about not employing any form of communication strategy. As lay visitors, it really challenged their inherent instincts, as all thought that as a visitor they would be ‘helping’ people with their communication problems. Adjusting the programme and adding further experiential exercises helped, in part, to address this concern. It was only in practice that this was fully understood and only then that visitors found their own different strategies, with support, to avoid their natural instincts.
Feedback from visitors provided us with a clear message that the training was hard and emotionally challenging, but essential to do the job. And, had they not had such thorough preparation for the role, some would have dropped out, as they felt it took a great deal of courage and confidence to engage with someone with complex communication difficulties while complying with the AC protocols in consciously avoiding any form of communication strategy. Training and support throughout proved essential for their high performance.
Team meetings
Visitors were recruited in groups and team meetings were held every month for the first 3 months of a visitor’s contract then every 2 months for the remaining term. The meetings gave the chance to share experiences and common problems, discuss ideas for activities and gave the AC monitor the opportunity to informally monitor practices. It proved to be very important for the visitors, who all worked from a home base and did not have the benefit of peer support on a daily basis, although the AC monitor and trial manager were always available by telephone.
Monitoring and supervision
The early visits proved stressful for most visitors as every patient presented differently and visitors were testing out coping strategies they had only practised through role play. In the early weeks, one-to-one supervision was well utilised – with some visitors needing to talk on the telephone at length to the AC monitor after each visit. This was time-consuming for the monitor, but proved to be time well spent as this support system provided knowledge, advice and emotional underpinning, which helped to build visitors’ confidence fairly quickly.
Through close and prompt scrutiny of the weekly log forms, which reported the content, timings and any problems, the AC monitor was able to rectify activities, or anything that seemed inappropriate or needed clarification immediately, by telephone or by e-mail.
Outcome assessments
All outcomes were assessed at 6 months post randomisation. RAs visited patients and carers in their own homes or wherever they were living at that time point.
For the primary outcome, a semistructured conversation between the participant and a relatively unfamiliar communication partner (study RA) was videotaped (see Chapter 2). RAs were not SL therapists but all had gained experience of interacting with people with communication difficulties post stroke and had been specifically trained in communication-supportive techniques. Supportive techniques used a range of linguistic, paralinguistic and augmentative ramps to communication but RAs did not take specific communication aids with them. Only pens and paper were used, with participants encouraged to utilise any communication aids with which they had been provided as part of the research.
A framework script for the conversation was developed, which involved several starter and follow-up questions, to be used as necessary. Starter questions were open ended. The follow-up questions were more specific or offered more conceptual or linguistic support in order to facilitate a response. The same standard question was used to open (‘Can you tell me about your family and friends?’). If a natural conversation flowed from this initial starter there would be no necessity to return to the script. Further starter questions were brought in if conversation dried up on a particular topic and follow-up questions were used to enable responses within each topic as necessary. The aim was 10 minutes of interaction, although if all questions had been used, or at the RA’s discretion (e.g. if a participant became distressed), the conversation could be ended earlier.
The videotape of the conversation was stored and tapes were then sent in batches to an independent expert SL therapist, blinded to treatment allocation and not involved in treating study participants, who rated functional communication on the communication activity scale of the TOM (aphasia and dysarthria assessments share the same activity scale). Overall, three raters were trained in the use of this scale and the reliability of their ratings was confirmed by a quality monitoring check. We previously confirmed the reliability of using the TOM in this way in a published study involving 12 therapist raters and 102 videotaped conversations involving unfamiliar conversation partners. 29 The semistructured conversation script along with TOM rating sheet used is shown in Appendix 4. 29 The TOM activity scale ranges from 0 to 5 and includes half-points, resulting in a 11-point scale. The higher the score, the better the outcome (level of communication activity).
Secondary outcomes were as follows:
-
Participants’ perception of their functional communication and quality of life was assessed using the COAST. 28 As described in Chapter 2, its content, accompanying illustrations and layout were informed by items from the Stroke and Aphasia Quality of Life Scale (SAQOL)37 and input from the RUG and NHS therapists to facilitate its use with patients with communication difficulties. It covers both understanding and expression in a range of communication situations and functional activities, including five items measuring quality of life. The overall score is converted to a percentage (but see Statistical analysis), with higher scores indicating better outcome. (See Figure 2 for visual presentations of items and Appendix 5 for the wording of the 20 items.)
-
Carers’ perception of the participants’ functional communication was measured using the first 15 questions on the Carer COAST. 27 An example of carer adaptation is that ‘… how well could you read …’ is changed to ‘… how well could your relative/friend read …’. The overall score is converted to a percentage, with higher scores indicating better outcome.
-
Carer ‘well-being’ was measured using the Carers of Older People in Europe (COPE) Index. This is a validated 15-item self-completed measure comprising three subscales: negative impact (a high score on this subscale is a poor outcome as it indicates stress), positive impact and quality of support (high scores on both of these subscales are a good outcome as they indicate satisfaction from carer role and that the carer feels supported). Carers’ own quality of life was also assessed with the relevant five questions from the Carer COAST. As for the parallel COAST, the overall score is converted to a percentage, with higher scores indicating better outcome.
-
Adverse events – second subsequent stroke: events leading to increased hospital stay or readmission to hospital, and death.
-
Participants also completed a European Quality of Life-5 Dimensions (EQ-5D). This is a validated, widely used short measure of health status. The resulting score will be somewhere between ‘1’ (the person is in full health) and ‘0’ (the person has died). It is also possible for scores to be < 0, representing states that are considered by some to be worse than death. Service use data were also collected by RAs. These data were used in the economic evaluation and are described in Chapter 6.
Blinding
It would have been impossible to blind participants and those delivering intervention to the allocation group. Attempts were made to ensure that RAs who collected data on outcome assessments were blinded to the randomisation group. Certain precautions were taken within the study team (such as password-protected data related to allocation) and participants were asked not to mention group allocation at outcomes in a letter and by RAs on arrival. However, it was anticipated that RAs could become unblinded (e.g. by reference to name of therapist or visitor, or by seeing communication aids in use and knowing these would not have been provided to the control group). The primary outcome was based on the videotaped structured conversation, which was assessed by expert SL therapists who did not know the patients and were blinded as to allocation.
Safety evaluation
Both trial interventions were non-medicinal with no anticipated serious adverse reactions. It was hypothesised that SL therapy could have an impact on adverse events in the sense that improving communication could ensure better adherence to other therapy and secondary prevention activities. Therefore, serious adverse events (SAEs) that would be anticipated in a large group of stroke patients were recorded:
-
death (whether or not from stroke)
-
prolonged hospital stay or readmission to hospital (whether or not from stroke)
-
second stroke (during first admission or after discharge).
Statistical analysis
The primary analysis used regression methods to estimate group differences in outcomes after adjustment for the intended stratification criteria – site, diagnostic group and baseline severity of communication impairment on the TOM. The adverse event rates were compared without adjustment as they were not anticipated to be sufficiently common to allow multifactorial analysis.
Analyses included all participants assigned to their allocation group regardless of protocol adherence: a complete case analysis under the intention-to-treat approach. Participants who were lost to follow-up or declined assessment were excluded. Those known to have died were included as having the worst possible outcome: no functional communication on the primary outcome. No other imputation was undertaken.
For COAST and Carer COAST outcomes were compared for those providing valid assessments. Validity was defined as < 10% of applicable items being unanswered. No adjustment took place when responses between participant and carer might be construed as incompatible as the instruments were designed to reflect the individual’s self-perception.
Secondary analyses fell broadly into two categories: sensitivity analyses and data-driven analyses. The former reanalysed the primary outcome data in a number of ways to assess how robust conclusions were to the choice of approach. Non-adjustment for intended stratification criteria, allowance for possible therapist effects, omission of people who have died and per-protocol analyses were all considered. The exact choice of such sensitivity analyses was inevitably data-driven to some extent. For example, if primary analysis suggested a group difference, the robustness of this conclusion needed to be examined (e.g. allowance for possible therapist effect). Conversely, if primary analysis did not suggest a group difference, the sensitivity analysis would focus on approaches that may identify possible explanations (e.g. per-protocol analysis).
Subsidiary analyses
There were concerns at one site that SL therapy intervention was not being delivered in accordance with the manual. Integrity of the site’s data was assessed by comparison of its participants’ outcomes with those of other sites and, further, by comparison of the estimated treatment effect at this site with that at other sites. This was achieved by addition to the primary analysis of a two-level site covariate (site X, not site X) and its interaction with the group covariate. This site’s data would have been omitted from final analyses if there was evidence that was supportive of the concerns.
How sample size was determined
The original protocol proposed a total sample size of 300 participants for 90% power to detect a difference of 0.5 points on the primary outcome of TOM. The target effect size of 0.5 on TOM was decided upon as it is the smallest measurable difference on the scale. This calculation allowed for differential clustering between the two arms due to therapist effects.
Recruitment was slower than anticipated, leading to revision of the target. The observed standard deviation (SD) of the primary outcome for the first 43 recruited participants, adjusted as for primary analysis, was 1.1 points. The initial plan to incorporate therapist effects in the primary analysis was dropped as there was insufficient power to examine these potential effects. This led to recalculation of a target sample size of 170 participants to give 80% power at the 5% significance level to detect a difference of 0.5 points, allowing for approximately 10% loss to follow-up.
Interim analyses and stopping guidelines
No formal stopping rule was applied to interim analyses reported to the Data Monitoring and Ethics Committee (DMEC). The videotapes for assessment of the primary outcome data were stored for distribution in batches. This precluded early stopping as the primary outcome remained largely unmeasured at the time of DMEC meetings. Consequently, no adjustments were made to the significance levels or CIs in presented analyses.
Participant withdrawal criteria
No specific withdrawal criteria were defined for the study. Participants were of course able to withdraw their consent and discontinue their allocated intervention prematurely. In these cases they could choose to enter standard services for SL therapy at their site. Regardless of this potential protocol deviation, RAs attempted to collect outcome data for all participants.
Given that participants were assessed for eligibility soon after stroke admission, specific exclusion criteria might come to light after randomisation. In these cases, the independent DMEC would be consulted as to the appropriateness of the participant continuing in the trial.
Ethical arrangements and research governance
Multicentre Research Ethics Committee (MREC) approval was granted (06/MRE03/42). For each individual centre, a site-specific approval was obtained from the appropriate local research ethics committee. Research and development approval was obtained from each participating trust. The trial was conducted in accordance with the legislation, the International Conference on Harmonisation–Good Clinical Practice (ICHGCP)38 and the Research Governance Framework for Health and Social Care. 39
A Trial Steering Committee (TSC) and DMEC were established. The TSC comprised an independent chairperson, four independent researchers (all of whom had expertise in rehabilitation research, clinical trials, stroke or all of the above) and five members of the study team: chief investigator (AB), trial manager (EP succeeded Mihaylov), trial statistician (AV), trial health economics lead (LD) and qualitative lead (AY). AH was acting chief investigator twice for maternity leave cover, during the feasibility study and a second time during the main trial.
The DMEC was chaired by the director of a clinical trials unit, and included an expert SL therapist and a consultant neurologist, both with experience in research trials. Data reports were prepared by the trial statistician in confidence from the study team.
Amendments to the study following commencement
All amendments were carried out following consultation with MREC.
Objectives
The lengthy aims and objectives from the grant application and protocol have been summarised in the abstract to ‘The effectiveness, cost-effectiveness and service users’ views of enhanced early intervention by SL therapists compared with attention control’. We have also made the nature of the AC ‘contacts by employed visitors’ and the settings more explicit ‘Setting: Twelve English NHS, hospital and community stroke services’.
Sponsor
The trial was funded by the NIHR HTA programme. NIHR HTA initially acted as research sponsor until, at its request, the University of Manchester (the coordinating centre) took over.
Recruitment materials
Improvements and adjustments to materials were made after experience identified some commonly asked questions. The recruitment materials were always meant to be used with a RA present so that these questions were always addressed, even before changes were made. However, the amendment to materials ensured that some of these questions (such as ‘Where will the SL therapist/visitor see me?’) could easily be answered by independent perusal of the recruitment materials.
We also designed a summary information sheet to be used with those who may be asked to give proxy consent for a patient into the trial. This asked carers to consider the prior known wishes and attitudes of the patient before agreeing to enter them into a trial to ensure that the study met the research provisions of the Mental Capacity Act 2005.
Outcome assessment materials
Pictures were used in the COAST and Carer COAST. We wanted to ensure that pictures were representative and clear and, after some feedback from participants and carers, we made minor improvements to the pictures in conjunction with the RUG.
Case ascertainment
Initially (from December 2006) all stroke admissions to participating hospitals had a preliminary note screen for eligibility by Stroke Research Network staff or TAs working under the direction of NHS SL therapists.
After a review of note-screening support it was found that this system did not identify people with (non-temporary) communication problems who would otherwise have been missed. Therefore, note screening, which used significant resource, was discontinued from April 2008 and patients with suspected communication problems were screened for eligibility by SL therapists.
Recruitment rate issues
The recruitment start date was delayed. After lower than anticipated recruitment, several changes took place. As described above, the recruitment target and planned analysis were altered, additional sites were added and an 8-month extension was granted by the funder following agreement from all participating sites to continue in the trial.
Qualitative study
There was not time to achieve the objective to construct a ‘service user’s checklist’ of indicators of satisfaction and quality that could inform and monitor the future implementation of the evaluated technology.
A smaller than intended sample size was recruited owing to the time available but the number of participants was considered appropriate for a meaningful qualitative study.
Cost-effectiveness
The process of the pilot study and the data collected, combined with discussion with the study team, indicated that it was not feasible to collect reliable and consistent information from carers about the time they spent providing care and support to the study participant. Accordingly, these data were not collected in the main trial. This meant that the perspective of the economic study was changed from a societal viewpoint to that of the NHS, providers/funders of non-hospital care facilities and of patients and families.
Chapter 4 Randomised controlled trial results: delivery and clinical effectiveness
This chapter reports on the process of recruiting and conducting 6-month outcome assessments with participants in the main RCT. It also contains the data on delivery of the intervention and control before presenting the 6-month outcomes data relating to clinical effectiveness.
Randomised controlled trial process
Study recruitment
Between December 2006 and January 2010, 170 participants were recruited to the ACT NoW study from 12 sites (Figure 4). Four of these sites did not join until 2008 and 2009. The proportion consenting represented almost half of those eligible. Eighty-five were randomised to each group, with 72 from the AC and 81 from the SL therapy group included in primary analysis.
FIGURE 4.
Study flow chart.
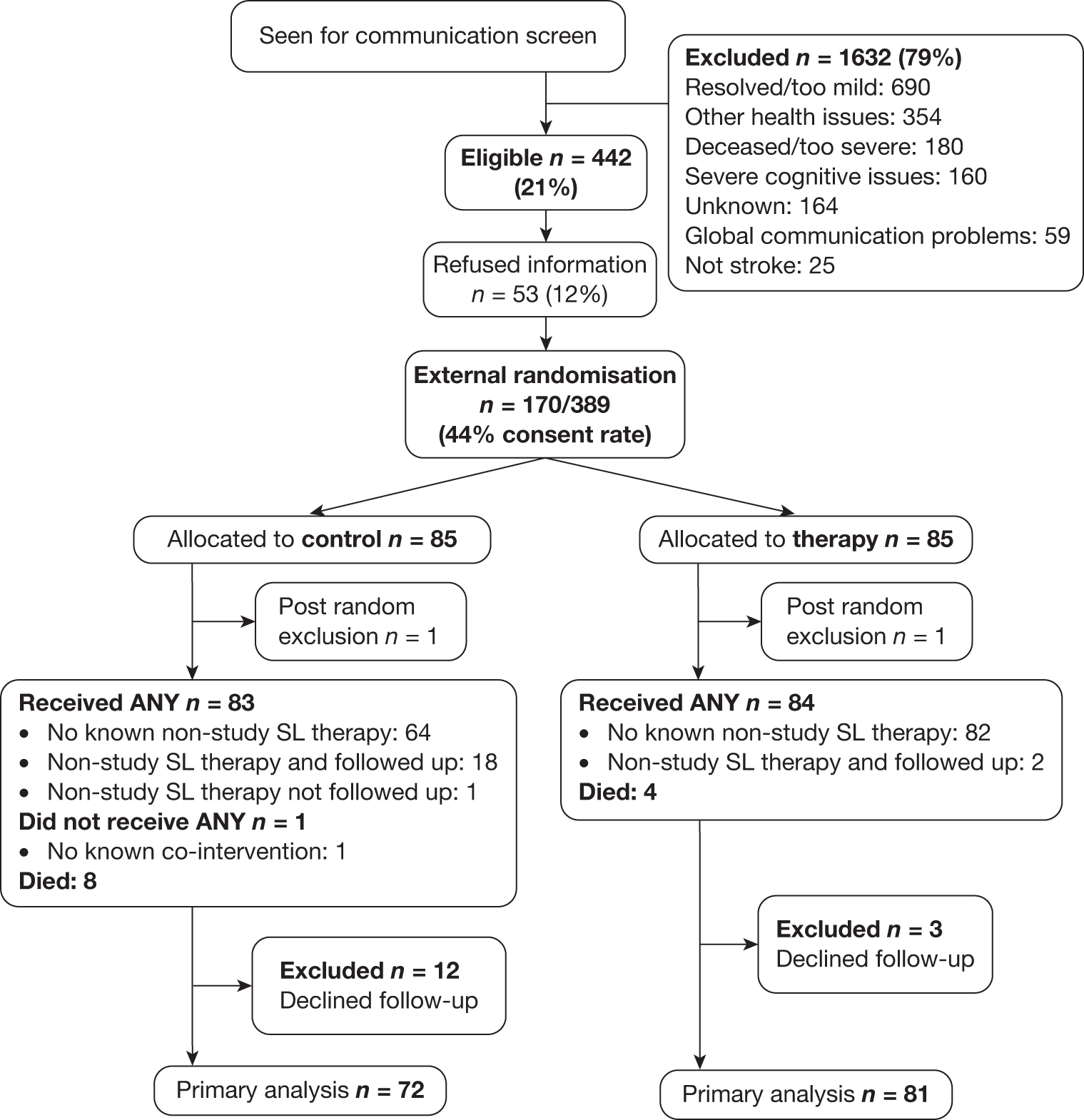
To set this in the context of usual NHS stroke care, around one-fifth of those with a hyperacute communication problem (referred to SL therapy or detected through note screening) and living within the areas served by the participating NHS services were considered eligible for inclusion by NHS SL therapists after face-to-face screening. The main reason (43%) for exclusion post screening was that communication had resolved or was considered too mild to require intervention. In contrast, < 4% were excluded because their communication problem was too severe. Patients’ general health problems, including severe cognitive impairments, accounted for another 43% of those excluded, with the reason unknown in 10% of excluded cases.
External validity (generalisability)
Once patients were identified as eligible and agreed to receive information about the study, they were approached by a RA for consent. People who declined consent after agreeing to see the study RA were asked if they would like to give a reason. The most common reason was the desire to receive SL therapy intervention (Table 2).
| Reason | n (%) |
|---|---|
| Do not want any communication therapy | 14 (6) |
| Do want SL therapy | 130 (59) |
| Do not want to be in research | 55 (25) |
| Do not want to be filmed for outcomes | 1 (0) |
| Unknown | 19 (9) |
Participants were not asked to specify a reason for participation. Anecdotal reports from RAs identified the following reasons:
-
take part in a research project
-
help people in the future
-
have more ‘contact’ than from standard SL therapy service
-
potentially get more SL therapy*
-
have someone to spend time with them outside of their family/friends*
-
put communication ‘on hold’ for 6 months as more concerned about other aspects of disability.
*Although RAs asked them not to join if their preference for either allocation was so strong that they would drop out if allocated to the other arm.
Those who consented (either directly or by proxy) were similar in their measured characteristics to those who declined (Table 3). The latter appeared to have slightly more severe communication disability (restrictions at the activity level of measurement), although there are some missing data (37) from those who declined.
| Characteristic | Participants (n = 170) | Eligible but declined (n = 272) |
|---|---|---|
| Male | 95 (56%) | 145/270 (54%) |
| Mean age, years (range) | 70 (32–97) | 72 (31–95) |
| Aphasiaa | 153 (90%), of whom 64% severe | 238/247 (88%), of whom 55% severe |
| Dysarthriaa | 66 (39%), of whom 53% severe | 104/239 (44%), of whom 52% severe |
| Overall communication (activity level) | 51% severe | 59% severe |
| Dysphagia | 87 (51%) | 135/252 (54%) |
Recruitment process measurements
The manualised therapy of ACT NoW was intended to be delivered from a time post stroke ‘as soon as clinically indicated’. Table 4 summarises the times taken for each stage of the recruitment process. Date of stroke onset can be difficult to determine accurately and so time from admission to hospital with stroke is used throughout. The success of randomising and delivering intervention/control within the postacute phase is discussed further later in this chapter (see Participant baseline characteristics and Delivery of the interventions).
| Time period | Median (IQR) days |
|---|---|
| Stroke admission to referral for screening | 5 (3–9) |
| Referral to consent | 5 (3–7) |
| Stroke admission to randomisation | 12 (9–16) |
| Randomisation to first contact | 3 (1–5) |
The written materials were used flexibly and interchangeably with patients and carers, and proved extremely useful (Table 5). The simplified/pictorial version was used with more than half of the participants. When patients or carers had difficulty with a certain aspect of the study, the researcher could switch to an alternative booklet to aid comprehension (see Chapters 2 and 3).
| Materials used | n (%) |
|---|---|
| Standard only | 16 (9) |
| Standard and aphasia friendly | 3 (2) |
| Standard and simplified/pictorial | 19 (11) |
| Aphasia friendly only | 52 (31) |
| Aphasia friendly and simplified/pictorial | 10 (6) |
| Simplified/pictorial only | 69 (41) |
| All three | 1 (< 1) |
Additional supplementary materials such as audiotapes and DVDs were used to aid recruitment with 10 participants who agreed to take part. Pragmatic issues related to leaving patients with Walkmans that they may not be able to utilise independently, as well as difficulties finding suitable ways to play the DVD/video, contributed to the low use of these additional materials. DVDs and videos were more useful with carers and typically used with individuals who could not read. For the most part, patients and carers preferred to have written information and our easy-to-read booklets (printed on coloured card and spiral bound to facilitate easy handling) were well received.
Participant baseline characteristics
Randomisation achieved balance between groups on the stratification factors and other demographic measures (Table 6). Ethnicity was undisclosed in two cases but otherwise did not differ between groups, with 98% of the overall sample described as white. Socioeconomic status was not collected. On average, the AC group was slightly more severely affected in terms of communication disability, dysphagia and ADLs.
Not all participants (79%) had an identified carer who was willing to complete outcome measures. Most carers who took part were female family members in the same household, not in paid employment and were younger than the stroke participants (Table 7).
| Characteristic | AC (n = 85) | SL therapy (n = 85) |
|---|---|---|
| Mean age, years (range) | 70 (40–92) | 70 (32–97) |
| Male | 46 (54%) | 49 (58%) |
| Diagnosis | ||
| Aphasia only | 53 (62%) | 51 (60%) |
| Dysarthria only | 8 (9%) | 9 (11%) |
| Both | 24 (28%) | 25 (29%) |
| Aphasia impairment, mean (SD) | 1.9 (1.1), n = 77 | 1.9 (1.2), n = 76 |
| Dysarthria impairment, mean (SD) | 2.5 (1.1), n = 32 | 2.2 (1.2), n = 34 |
| Either impairmenta severe (0–2) | 58 (68%) | 58 (68%) |
| Communication activity | ||
| Mean (SD) | 2.2 (1.2) | 2.3 (1.3) |
| Severe | 47 (55%) | 40 (47%) |
| Dysphagia present | 47 (55%) | 41 (48%) |
| BI | ||
| Mean (SD) | 10.7 (7.3) | 12.7 (7.2) |
| Mild (18–20) | 22 (26%) | 36 (42%) |
| Moderate (11–17) | 22 (26%) | 17 (20%) |
| Severe (0–10) | 41 (48%) | 32 (38%) |
| Characteristic | AC (n = 62) | SL therapy (n = 73) | Overall (n = 135) |
|---|---|---|---|
| Mean age, years (range) | 56 (23–79) | 56 (21–80) | 56 (21–80) |
| Male | 21 (34%) | 20 (27%) | 41 (30%) |
| Family | 60 (97%) | 70 (96%) | 130 (96%) |
| Employmenta | |||
| Full-time | 13 (21%) | 19 (28%) | 32 (25%) |
| Part-time | 11 (18%) | 12 (17%) | 23 (18%) |
| Retired/unemployed | 37 (61%) | 38 (55%) | 75 (56%) |
| Distancea | |||
| Same house | 34 (56%) | 38 (54%) | 72 (55%) |
| Walking distance | 7 (11%) | 6 (9%) | 13 (10%) |
| Driving (> 10 minutes) | 20 (33%) | 26 (37%) | 46 (35%) |
Treatment fidelity and participant follow-up
One participant from each group was withdrawn post randomisation on the advice of DMEC. For a participant in the AC group information came to light post randomisation regarding mental capacity that called into question the validity of the patient’s consent. The participant was withdrawn when proxy consent was declined (from the participant’s social worker, as the participant had no known family or friends). One participant in the SL therapy group was ineligible as she lived out of area and could not be treated once discharged.
Detailed data on the amount and content of SL therapy or AC delivered to each arm are presented later in this chapter. In summary, participants within the SL therapy group were deemed to have received at least the minimum standards described within the manual and those in the AC group were offered a comparable amount of time.
Protocol violation was more common in the AC group. Of the 72 participants who completed primary outcome assessment, 18 (25%) received some NHS SL therapy before assessment. In the SL therapy group the corresponding figures were 2 of 81 (2%). Analysis of factors associated with study completion without protocol violation (that is, predictive of inclusion within per-protocol analysis) suggested that younger participants with more disabled communication were more likely to breach protocol (Table 8). Sensitivity analyses therefore accounted for these factors as well as the baseline difference in stroke severity (see Table 6) when comparing the per-protocol groups.
| Baseline factor | Measure | OR (95% CI) | p-value |
|---|---|---|---|
| Allocated group | SL therapy | 8.8 (3.3 to 24) | < 0.001 |
| Age | Per decade | 1.5 (1.1 to 2.2) | 0.02 |
| Communication activity | Per TOM point | 1.8 (1.2 to 2.7) | 0.002 |
Eight participants died and a further 12 declined follow-up in the AC group. In the SL therapy group, four participants died and three declined follow-up. Median [interquartile range (IQR)] time to outcome assessment for remaining participants was around the intended 6-month point, 180 (169–182) days.
Delivery of the interventions
This section provides detailed descriptive and quantitative descriptions of the intervention and control provided to the 153 participants included in the outcomes analyses (81 SL therapy and 72 AC), essentially comparing the actual with the intended delivery. It starts with the SL therapy, examining the amount delivered, when, where and by whom, before looking at more clinical detail of the components of the intervention. Data are provided first on the whole group allocated to SL therapy as well as exploring the two diagnostic subgroups separately: aphasia and dysarthria. A further subgroup description, which may prove useful for commissioning services, is the breakdown by dichotomised level of severity. This section ends with the data delivered to the AC group.
Speech and language therapy: how much and when?
The 81 participants allocated to the intervention arm received an average of 22 ACT NoW SL therapy contacts, for 18 hours (mean). The maximum scheduled by the research protocol was 16 weeks of up to three times a week, i.e. 48 contacts. The maximum received by any individual was 43 contacts (42 hours) and the least amount was three contacts. Additionally, two participants refused their allocation and received a small amount (mean 5 hours) of additional NHS therapy (e.g. beyond the scheduled maximum), although this made little difference to the intervention group total contact time.
These data were used to examine how early was the intended early intervention. On average, the first SL therapy intervention session began around 16 days after admission to hospital, comprising 13 days for screening, consent and randomisation and 3 days from that point to intervention commencing. These data show that SL therapists managed to provide early intervention within a couple of weeks post admission, a stage in the stroke pathway sometimes referred to as postacute.
Data were collected concerning the ending of therapy (duration and reason for discharge) as shown in Figure 5. On average, SL therapy-allocated participants received therapy for 13 weeks of the scheduled maximum of 16 (minimum 4, maximum 18). Fourteen of the 81 participants had no more than 10 contacts with an SL therapist, and, at the other extreme, around half (54%) had more than 20 contacts and 25% had more than 30. The data show that, despite challenges staffing their services, SL therapists managed to deliver intervention at a high intensity for a considerable proportion of participants. Furthermore, the breadth of the range of intervention durations suggests that, as expected, therapists provided a flexible intervention tailored to individual patients’ need and readiness for engagement.
FIGURE 5.
The SL therapy delivered to the 81 people involved in primary analysis.
Therapists recorded the reasons for ending therapy (n = 81):
-
10% chose to self-discharge
-
5% died or became too ill for therapy
-
36% completed the required therapy before the scheduled end (16 weeks)
-
43% did not complete by the scheduled end and were referred for NHS therapy to commence after the 6-month outcomes were collected.
Commissioners and providers tasked with predicting longer-term demand for SL therapy may find it helpful to note that 43% required intervention beyond 6 months post stroke.
Speech and language therapy: where and by whom?
Participants allocated to the intervention arm moved through a range of inpatient, outpatient and community services as part of usual care in the NHS. The ACT NoW SL therapy intervention aimed to provide continuity for stroke service users and their families by following the participant along their stroke care pathway. This included the important transition period following discharge/transfer from inpatient care, a notoriously high-risk moment for lack of continuity and service user dissatisfaction. Indeed, when securing the additional resources (treatment and excess treatment costs) required to lift NHS provision to the level necessary for ACT NoW most of the 12 participating sites required extra staff for the community phase.
Therapists collected data that enable us to examine two aspects that may contribute to good continuity of care: the location of therapy and the number of SL therapists working with any one individual. Calculations of outpatient versus community visits were informed by cross-referencing with date and location of discharge. Of the 22 (average) contacts provided to participants, 9.5 were in a hospital setting and 12.5 were in the participant’s home (median 6 and 11, respectively). Most of the hospital contacts were on an inpatient basis, as outpatient contacts were very rarely provided (mean 0.65, median 0). Most people (65%) allocated to SL therapy intervention worked with one or two therapists across their stroke pathway. However, 11 participants (14%) saw four or more SL therapists (although most of their sessions, 69%, were carried out by two therapists). This was in contrast with the attention arm, for which participants typically saw the same visitor throughout their involvement in the study.
Intervention was intended to be led by a qualified SL therapist of Band 6 or above. Leadership meant having oversight of the treatment plan and providing clinical support for the therapist(s) delivering the intervention. This was achieved, and in practice the vast majority of participants (87%) had intervention led by a therapist of Band 7 or above. In terms of delivery of the intervention, almost all contacts were by Band 5 or above (Table 9), with only 1% delivered by Band 3/4. Lower-banded therapists acted under the guidance of higher-band therapists to ensure that the quality and rationale of therapy was of a sufficiently high standard and consistent with the best practice guidelines (as described in Chapters 2 and 3).
| Group/subgroup (n) | Mean contacts | Mean hours | % Contacts by Band 7/8 | % Contacts by Band 5/6 |
|---|---|---|---|---|
| All (81) | 22 | 18 | 42 | 57 |
| Any aphasia (72) | 23 | 18 | 42 | 57 |
| Any dysarthria (33) | 20 | 15 | 43 | 54 |
| Mild/moderate (44) | 19 | 15 | 47 | 51 |
| Severe (37) | 26 | 21 | 37 | 62 |
The severely impaired subgroup was more likely to see a Band 5/6 therapist, which may have been because they also received more contacts than the mild/moderate subgroup. There are no striking differences in number of contacts or hours between those with any aphasia or any dysarthria (although comparisons should be made with caution as these are mutually overlapping groups).
Components of the intervention
The SL therapy intervention had six key components (see Chapter 2 and Appendix 1): assessment, information provision, provision of communication materials, carer contact, indirect contact (e.g. MDT), direct contact. These can be summarised in two ways to describe the therapy delivered:
-
The proportion of participants receiving each of the six components. This is calculated from a simple absence/presence of a component (e.g. assessment) per person. It provides a useful overview of whether or not each component was used for all participants. However, it does not tell us how many contacts with therapists were devoted to each component. As it would be helpful to know whether carer contact typically occurred in 1% rather than 50% of the 1863 contacts delivered to all participants, the data are also summarised a second way.
-
The relative delivery of each component. This is a count of the occurrences of each component (e.g. direct therapy) within contacts, summarised as a percentage of the total number of activities provided (4860) during the 1863 contacts by therapists. It allows for the clinically pragmatic occurrence of multiple components within any one contact, i.e. during a 45-minute home visit a therapist might carry out three activities such as assessment, direct therapy and carer contact. To avoid overly burdensome data collection distracting therapists from delivering the intervention, and to keep therapy as natural as possible within any research project, we asked therapists to record duration and contents of each contact but not the minutes devoted to each component. Therefore, these data do not provide duration of each component, although it is reasonable to assume that more frequently occurring components were likely to have consumed more of the total time spent (average 18 hours per person) than less frequently occurring components. [Note that throughout this monograph we use the term ‘contacts’ rather than ‘sessions’ (with a therapist) because the latter is commonly understood within the NHS to mean half a day, whereas each contact in ACT NoW typically lasted < 1 hour.]
Subgroups: diagnosis and severity
The data on delivery of the therapy components are described in Tables 10–13. The first row of Tables 10, 12 and 13 describes the 81 people allocated to the intervention arm who were subsequently included in the final analyses. In Table 11, the figures refer to the 73 cases with participating partners. Additional rows have been added for ease of exploring within clinical subgroups. There were 72 people with any aphasia and 33 with any dysarthria (not mutually exclusive groups and so comparisons should be made with caution). Service providers and commissioners may also find it helpful to review the final two rows comparing the actual therapy delivered to the 37/81 with severe communication problems and the 44 classified as having mild/moderate communication problems. Where these labels ‘severe’ and ‘mild/moderate’ are used below they always refer to communication severity rather than stroke severity.
| Group/subgroup (n) | Assessment | Goal-setting | Information | Communication aid |
|---|---|---|---|---|
| All (81) | 100 | 91 | 78 | 54 |
| Aphasia (72) | 100 | 90 | 79 | 57 |
| Dysarthria (33) | 100 | 91 | 78 | 39 |
| Severe (37) | 100 | 86 | 86 | 65 |
| Mild/moderate (44) | 100 | 95 | 70 | 45 |
| Group/subgroup (n) | Overall | Specific activities | ||
|---|---|---|---|---|
| Any type | Observation/participation in therapy, case conferences, etc. | Specific goals set with carer | SPPARC or conversation partner training | |
| All (73) | 96 | 95 | 41 | 11 |
| Aphasia (64) | 97 | 95 | 41 | 13 |
| Dysarthria (29) | 93 | 93 | 38 | 7 |
| Severe (34) | 97 | 94 | 38 | 18 |
| Mild/moderate (39) | 95 | 95 | 44 | 5 |
| Group/subgroup (n) | Any type of MDT contact as per above (including goal setting) | Goal-setting with MDT |
|---|---|---|
| All (81) | 84 | 17 |
| Aphasia (72) | 83 | 18 |
| Dysarthria (33) | 85 | 12 |
| Severe (37) | 81 | 27 |
| Mild/moderate (44) | 86 | 9 |
| Group/subgroup (n) | Specific activities | ||||||
|---|---|---|---|---|---|---|---|
| Any | Impairment | Functional | Conversation practice | Goal set | Work set for review period | Other | |
| All (81) | 100 | 93 | 67 | 60 | 86 | 30 | 85 |
| Aphasia (72) | 100 | 92 | 65 | 60 | 85 | 32 | 85 |
| Dysarthria (33) | 100 | 97 | 61 | 61 | 85 | 27 | 85 |
| Severe (37) | 100 | 92 | 70 | 65 | 81 | 24 | 84 |
| Mild/moderate (44) | 100 | 93 | 64 | 57 | 91 | 34 | 86 |
Proportion receiving each therapy component
Assessment and goal-setting
Assessments were intended to be diagnosis specific, initial and ongoing, and standardised. As shown in Table 10, everyone was assessed, regardless of diagnosis or severity. Goal-setting (with patient, carer and MDT) is an important part of assessment and so is shown separately. As shown, goals were set for almost everyone. Some participants had only a brief period of therapy ending before goals were agreed. Those with severe communication were least likely to have goals set; however, this was still accomplished for the vast majority of this subgroup.
Information provision
The guidelines intended that information would be provided on strategies, progress, available support networks, etc. Some of the information topics summarised in Table 10 include:
-
introductory information/what to expect from SL therapy/services available
-
information from/to MDT regarding progress in therapy
-
information about end of therapy, ensuring closure achieved.
This component can overlap with others and so was difficult to isolate and should be interpreted with caution. The best estimate we have is that the majority of participants received information as intended. Information provision appears to be higher for those with severe communication problems than for those with mild/moderate communication problems, although the quality of the data on this one component is questionable.
Provision of communication materials
The manual stipulated that participants who could utilise communication materials (low to high technology) should be provided with these materials. These communication aids could range from simple notebooks to electronic talkers:
-
communication charts/booklets
-
personalised advice booklet for aphasia (PABA)
-
link notebook (to record sessions and key pieces of information to utilise)
-
patient lifebook.
Some sites already had alternative AAC or access to AAC as part of their normal NHS provision. Under NHS research funding rules, AAC had to be funded as a treatment cost or excess treatment cost rather than a research cost. However, because of delays obtaining NHS trusts’ agreement to cover their treatment costs, sites were awarded start-up equipment funded by the Stroke Association, including a 4 × 1 talker communication aid.
Around half of the participants received a communication aid (see Table 10) and those who did were more likely to be those with severe communication problems at baseline.
Carer contact
The Best Practice Guideline stated that carers should be involved in intervention with the agreement of the client. Not all participants had carers. As shown in Table 11, in almost all cases where carers were available they were involved in therapy. This was similar across the subgroups. Therapists engaged carers in different ways. The most common was direct involvement in a therapy session or therapy planning. Conversation partner training was rarely deemed appropriate, probably because of the earliness of this research intervention.
Indirect contact
The main form of intended indirect contact was for SL therapists to work with members of the MDT to share information on the participants’ needs, abilities and strengths, and to achieve goals (see Table 12). Referral on to other services followed normal NHS stroke service protocols for that site.
As expected, this was a commonly occurring activity for most participants. Data collected specifically on joint goal setting with the MDT showed that this was an infrequent means of MDT working, but was more likely for those with severe communication problems, plausibly because they had more complex difficulties beyond their communication problems.
Direct contact
The best practice guidelines specified minimum standards for direct contact for all those with dysarthria and aphasia of one-to-one contact with a qualified SL therapist to improve their ability to express themselves clearly (dysarthria) or language skills (aphasia).
All participants received direct contact (see Table 13). Therapy focused at addressing the underlying impairment was almost always provided. Functional-level activity and conversation practice were provided for the majority. Around one-third of people were provided with work to carry out independently beyond the scheduled end of the research intervention. Many of this subgroup were among those referred for NHS SL therapy after the 6-month outcomes had been collected (see above). However, independent work was more likely to be set for those with mild/moderate than severe problems, suggesting clinical decision making among therapists, weighing up participants’ need for communication practice against their ability and perhaps motivation to work independently.
There were no striking subgroup differences or patterns in the type of direct contact carried out. The severely impaired subgroup did slightly more functional and conversation work and less goal-setting than the mild/moderate group.
When therapy was devised, it was anticipated that group therapy would be provided. However, this was never feasible owing to the numbers recruited and probably the early timing of the research intervention.
The relative delivery of each component
As described above, the second way of describing the intervention provided is to count the occurrences of each of the six core components of the intervention, summarised as a percentage of the total number of activities provided. Several activities could be provided per contact and there were 4860 activities provided during 1863 contacts between 81 people with stroke and their SL therapists. The following percentages describe the proportion of activities attributed to each of the six components, giving an indication of what was occurring most frequently:
-
assessment 14%
-
information provision 8%
-
provision of communication materials 3%
-
carer contact 15%
-
indirect contact (e.g. MDT) 11%
-
direct contact 53%.
The sum of components slightly exceeds 100% either because of rounding up or related to the difficulty, mentioned previously, of coding the information provision component. Direct contact activities (face to face with the participant) accounted for half of all activities conducted by therapists. Carer contact and assessment of participants were the next most frequent components. When direct contact is broken down into specific therapy approaches, impairment-focused therapy accounted for half of the direct contact activity (and almost one-quarter of activity overall).
Attention control
This final section describes the amount and content delivered to the 72 participants allocated to the control arm and included in the primary analysis.
Nine part-time visitors were employed by the university throughout the study on short-term contracts but with honorary NHS contracts. There were seven women and two men, aged from 26 to 61 (mean 48) years. None had professional experience of stroke or SL therapy. They had a high level of educational attainment: five had degrees, two had teaching qualifications, one had completed an access course and another had achieved A levels. Five had vocational experience within health, social care or education at varying levels of seniority, ranging from an NHS receptionist to a retired head teacher. What they had in common was an ability to put aside knowledge from their past professions, an ability to work in challenging circumstances, good time management and a capacity for lone working. All had well-developed social skills, and were natural communicators capable of expressing warmth and empathy appropriately.
Control partipants received an average of 19 ACT NoW visitor contacts, for a mean of 15 hours. The maximum scheduled by the research protocol was 16 weeks of up to three times a week, i.e. 48 contacts. The maximum received by any individual was 45 contacts (41 hours) and the lowest number was one contact. Additionally, 18 participants refused their allocation and received additional NHS SL therapy (mean 3 hours). This meant that control participants received an average total (visitor + NHS SL therapist) of 23 contacts and 18 hours, an almost identical amount to the total received by the intervention arm (22 contacts, 18 hours). We also found that the first visitor contact usually began 1 day later (day 17) than the first SL therapy contact and that slightly more visits were made at home than in hospital (it was not appropriate for visitors to provide outpatient contacts).
In contrast with the SL therapy’s six components, the AC simply consisted of three phases (see summary in Appendix 2) and a list of everyday activities carried out during the visits. In all cases the right sequence of codes was used to denote phases of activity, i.e. ‘introductory sessions’ at the beginning, ‘regular contact sessions’ in the middle and ‘winding-down sessions’ at the end. On average, there were five of the introductory sessions, 11 regular sessions and four winding-down sessions.
Most visitors prepared a rough plan for each visit, based on what they picked up about a participant’s interests/family/job, but generally let the sessions be patient led, which often resulted in general conversation. It was often difficult to then bring in activities without it seeming contrived. However, there could be more than one activity carried out per session. The activity that occurred most frequently was, not surprisingly, conversation. The average count was 19, implying it occurred during each of the 19 visits. Other activities (counts) occurred but far less often: reading to the participant (four), games (four), TV/radio/music (three) and ‘other’, which included jigsaws, looking at photos, picture books, going out and making coffee (four).
Acceptability of attention control for participants
Of the 72 participants allocated to AC that could be included in the primary analysis, 50% continued to accept visits, eight (11%) died and the remainder stopped the visits sooner than the maximum possible of 16 weeks. Twenty of these wanted to see an NHS SL therapist instead of a visitor.
Clinical effectiveness
Primary outcome
Before conducting the between-group comparison on the primary outcome measure (TOM) at 6 months, observational data of change scores on the TOM are described. For the whole sample (n = 153 for whom we have 6-month follow-up data) there was an overall improvement of 0.8 on the activity-level scale of the TOM from pre-randomisation baseline scores taken by an NHS SL therapist. This suggests a clinically meaningful gain in functional communication, from a baseline mean of 2.4 (‘limited communication’, ‘relies on cues and context to make basic needs understood’) to a mean of 3.2 6 months later. As shown in the selected descriptors in Figure 6 (see Appendix 4 for full wording), scores > 3 indicate that participants have progressed to ‘consistently make their needs known’.
FIGURE 6.
Therapy outcome measure activity-level scale.
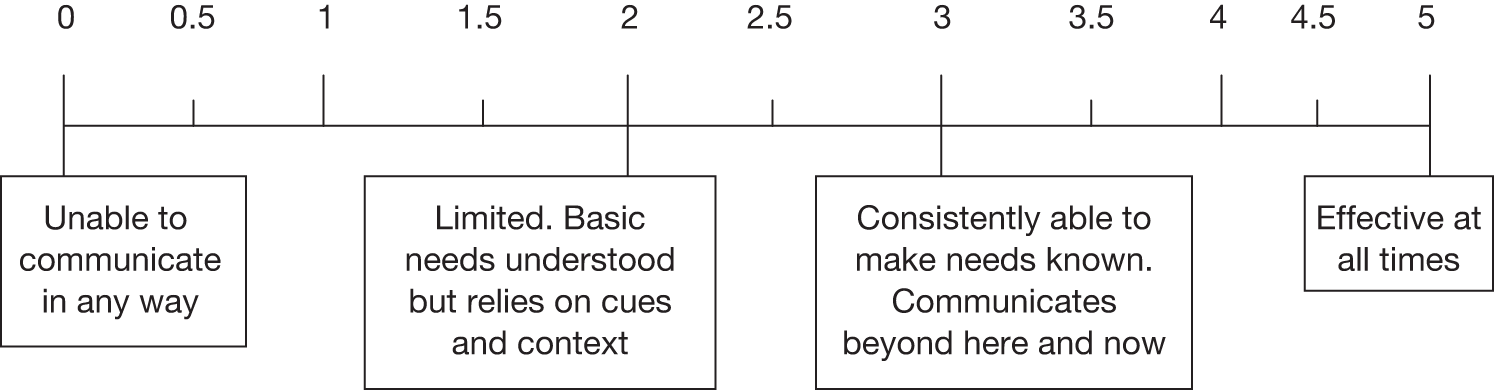
As shown in Table 14, a similar magnitude of improvement was seen for both arms, with the AC participants starting and completing the study with slightly lower scores.
| Group/subgroup (n) | Baseline | Outcome | Mean difference (95% CI) |
|---|---|---|---|
| All (n = 153) | 2.4 | 3.2 | 0.8 (0.6 to 1.0) |
| AC (n = 72) | 2.3 | 3.0 | 0.7 (0.4 to 1.1) |
| SL therapy (n = 81) | 2.4 | 3.3 | 0.9 (0.6 to 1.2) |
Primary outcome analysis was conducted by comparing the between-group difference in TOM activity scores at 6 months. The distribution of scores for the two groups is shown in Figure 7. The mean was slightly higher in the SL therapy group, which was a little less variable: mean (SD) of 3.0 (1.6) for AC and 3.3 (1.4) for SL therapy participants.
FIGURE 7.
Comparison of primary outcome by allocation group.
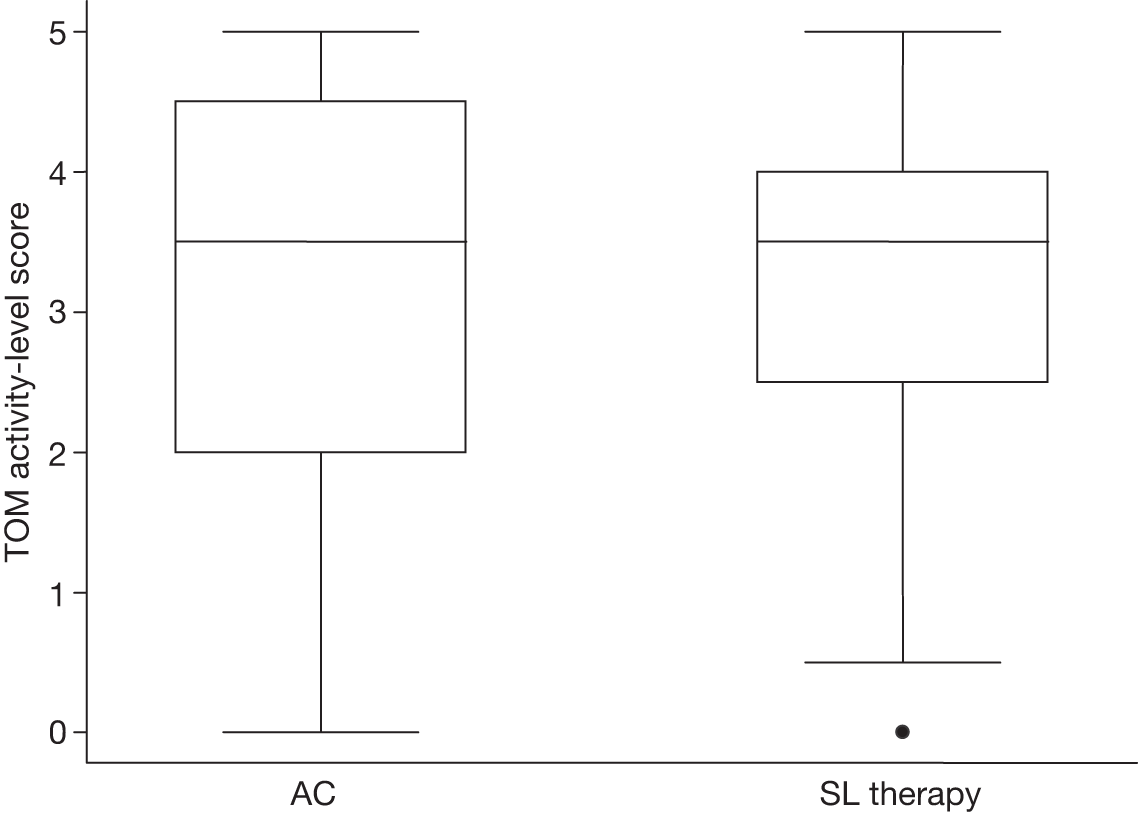
The planned primary analysis, adjusting for intended stratification factors and including deaths, gave an estimated difference of 0.25 (95% CI –0.19 to 0.69) points in favour of SL therapy (p = 0.27). The CI included the 0.5-point difference the study was powered to detect but also included zero. This suggests an absence of evidence that there is any added benefit of SL therapy intervention over and above the observed benefits from temporal factors or the early, well-resourced AC.
Sensitivity analyses
The primary outcome measure was further explored using various sensitivity analyses:
-
exclude deaths
-
add adjustment for baseline differences in TOM activity and BI
-
restrict to per-protocol groups with adjustment for baseline age (identified as predictive of follow-up), TOM activity and BI
-
restrict to per-protocol groups with adjustment for baseline age, TOM activity and BI, and excluding deaths.
As shown in Table 15 and Figure 7, the findings are robust. However the primary analyses are adjusted, there is no suggestion of an added benefit of SL therapy intervention. In particular, either exclusion of deaths or adjustment for baseline differences moves the estimated treatment difference to near zero and removes the continued possibility of the targeted 0.5-point difference between groups. This suggests evidence of absence of a treatment effect rather than simply absence of evidence. The two per-protocol analyses give similar conclusions to their intention-to-treat counterparts. This removal from analyses of people who refused their allocation and received NHS SL therapy at some point (including the 18 control participants allocated to see only a visitor) suggests that the protocol deviation did not cause a dilution of therapy effectiveness.
| Analysis | AC: mean (SD), n | SL therapy: mean (SD), n | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| Excluding deaths | 3.4 (1.3), 64 | 3.4 (1.2), 77 | 0.05 (–0.33 to 0.43) | 0.80 |
| Baseline adjustment | 3.0 (1.6), 72 | 3.3 (1.4), 81 | 0.04 (–0.34 to 0.43) | 0.83 |
| Per protocol | 3.0 (1.8), 54 | 3.3 (1.3), 79 | 0.17 (–0.28 to 0.62) | 0.46 |
| Per protocol, excluding deaths | 3.5 (1.3), 46 | 3.5 (1.1), 75 | –0.04 (–0.41 to 0.33) | 0.83 |
Subgroup analyses
There was no suggestion within the data that the treatment effect differed between subgroups of ‘diagnosis’ and ‘baseline severity of communication impairment’. However, it is recognised that future researchers and in particular systematic reviewers may require separate data for these categories. Table 16 presents results for these subgroups. Note that the diagnostic categories are not exclusive – participants with both aphasia and dysarthria contribute data to both diagnostic analyses as presenting these as ‘any aphasia’ (i.e. may include dysarthria too) is more relevant to future service delivery than ‘only aphasia’. Presented analyses are calculated for subgroups using the primary analysis method (inclusion of deaths and adjustment for intended stratification factors but not for observed baseline imbalances). The conclusions are similar as for the overall cohort, with wider CIs resulting from reduced sample sizes. For completeness and visual comparison these results are included in Figure 8.
| Subgroup | AC: mean (SD), n | SL therapy: mean (SD), n | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| Aphasia | 3.0 (1.6), 64 | 3.2 (1.4), 72 | 0.20 (–0.28 to 0.69) | 0.41 |
| Dysarthria | 3.1 (1.7), 27 | 3.1 (1.4), 33 | 0.07 (–0.69 to 0.83) | 0.85 |
| Severe | 2.6 (1.7), 47 | 2.9 (1.3), 55 | 0.28 (–0.34 to 0.89) | 0.38 |
| Mild/moderate | 3.9 (1.1), 25 | 4.0 (1.1), 26 | 0.17 (–0.50 to 0.83) | 0.62 |
FIGURE 8.
Sensitivity and subgroup analyses for the primary outcome.
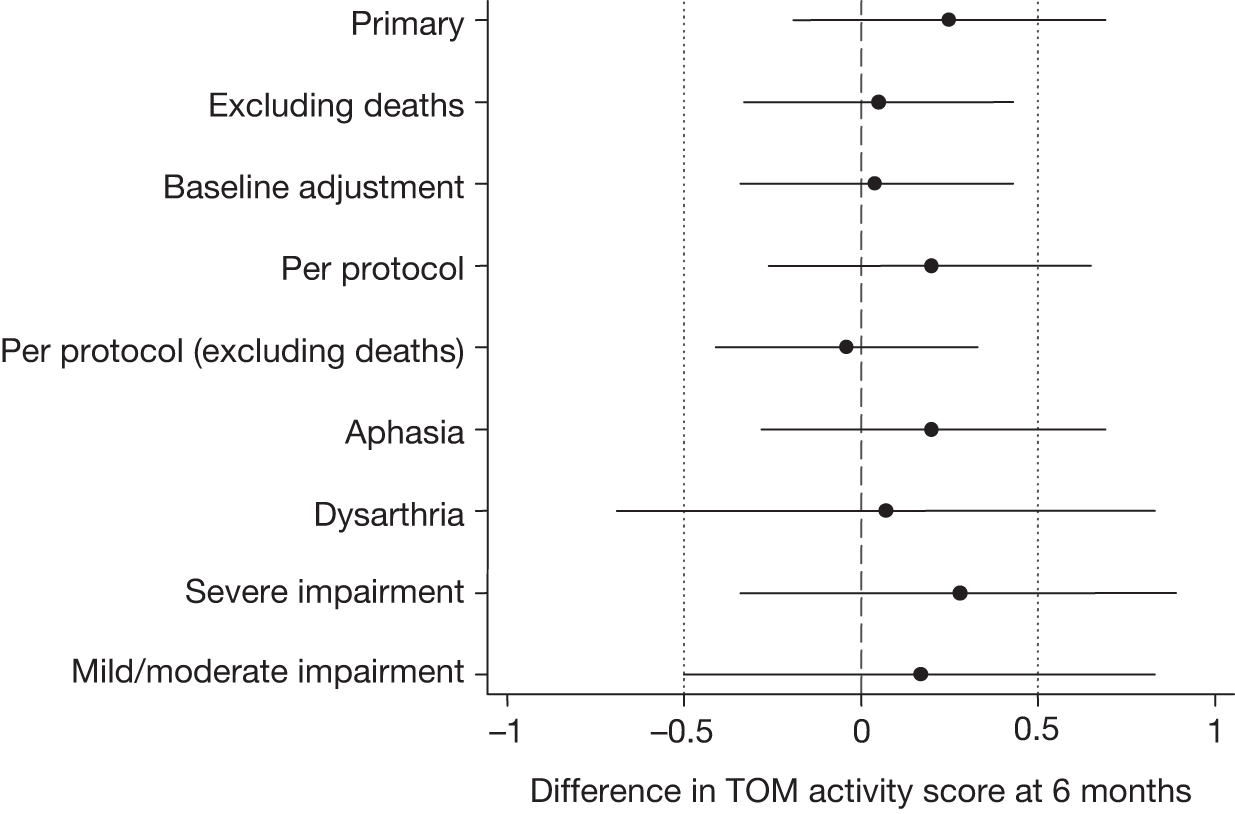
Secondary outcomes
Table 17 presents the summary statistics and analyses of the secondary outcomes adjusting for the intended stratification criteria. All outcome measures present a consistent pattern from the perspectives of different stakeholders, i.e. participants and carers. Groups were similar for all scales of the COAST, Carer COAST and COPE measured at 6 months, and for all subscales (not shown). This means there was no evidence of added benefit of the SL therapy intervention over and above the AC on patient or carer perceptions of the patients’ communication, or on carer perceptions of impact on themselves in terms of their own quality of life or carer well-being.
| Scale | AC: mean (SD), n | SL therapy: mean (SD), n | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| COAST | 73 (18), 50 | 71 (18), 67 | –1 (–7 to 6) | 0.85 |
| Carer COAST | 62 (18), 59 | 62 (21), 70 | 0 (–7 to 7) | 0.91 |
| COPE | ||||
| Negative | 23 (3.2), 58 | 24 (3.5), 67 | 0.6 (–0.6 to 1.9) | 0.34 |
| Positive | 13 (2.4), 57 | 13 (2.5), 68 | –0.0 (–0.9 to 0.9) | 0.96 |
| Support | 11 (3.2), 57 | 12 (3.3), 65 | 0.4 (–0.7 to 1.6) | 0.47 |
Serious adverse events
There were no suspected adverse reactions and no unexpected SAEs during the trial. Overall, 12 participants died, six survived further strokes and four others required extended or repeat hospitalisation (Table 18). Numbers of each were higher in the AC group. There was no statistically significant difference in either overall SAE or death rates between the groups but also low power to detect such differences. Given the similarity in outcome between the groups it would be challenging to hypothesise a mechanism for increased adverse events in either group.
| Worst SAE | AC (n = 85) | SL therapy (n = 85) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Hospitalisation | 3 (4%) | 1 (1%) | ||
| Stroke | 4 (5%) | 2 (2%) | ||
| Death | 8 (9%) | 4 (5%) | 0.48 (0.14 to 1.6) | 0.24 |
| Any | 15 (18%) | 7 (8%) | 0.42 (0.16 to 1.1) | 0.07 |
Summary
-
The trial reached its target recruitment and retention rates.
-
Communication activity improves in both groups.
-
No evidence of SL therapy benefit over and above AC and, arguably, evidence of no benefit.
-
Lack of observed benefit robust to method of analysis and consistent across subgroups of participants and all measured outcomes.
Lack of benefit over and above AC should not be confused with lack of benefit per se. There were elements common to each group including early diagnosis by SL therapist and treatment by MDTs who had experience of working with SL therapists. Detailed discussion follows in Chapter 9.
Chapter 5 Preferences for communication outcomes and waiting time
Background
An important secondary outcome of the ACT NoW study is the participants’ own perception of their functional communication and quality of life. This was assessed using COAST, a specially developed and tested self-rated scale (see Chapter 2).
The (COAST) rating scale was developed from a review of the literature and existing measures and from consultations with the ACT NoW RUG, specialist SL therapists and researcher colleagues. The RUG comprised people who had communication problems (aphasia and/or dysarthria) following stroke and carers, Everyone in the group had experience of living with the consequences of communication impairment and emphasised its wide-ranging impact on everyday activities and social participation. 28
The cost-effectiveness analysis (described in Chapter 6) explores the impact of different measures of outcome on the results of the economic evaluation. These included the primary outcome of the trial, participants’ functional communication and health-related quality of life (HRQoL), or utility, measured by the EQ-5D health status index and associated utility weights. However, it is clear that communication therapy comprises a complex intervention with a correspondingly complex set of outcomes. The impact of communication on social and family life or interests and hobbies may be as important information for decision-makers as traditionally measured health status and associated measures of health-related utility. 40
This means that standard measures of clinical outcome, health status, and the value of gains in HRQoL may be insufficient for the rigorous evaluation of communication therapy. In addition, constraints on NHS resources mean that there are waiting lists of up to 6 months for standard communication therapy. This is reflected in the design of the ACT NoW randomised clinical trial. If enhanced communication therapy is proven to be effective, waiting times for standard or enhanced communication therapy may increase; therefore, the relative importance of waiting times for therapy needs to be assessed.
Aims and research questions
The aims of this part of the ACT NoW study are to:
-
assess whether participants make trade-offs between the different attributes of outcome and between these attributes and waiting time for therapy
-
estimate the willingness of participants to wait for therapy aimed at improving communication ability.
Methods
A discrete choice experiment (DCE) was designed to assess preferences for outcomes and waiting time for therapy.
Discrete choice experiments41 are a stated preference method that is firmly rooted in economic theory, specifically random utility theory, which closely reflects how we make decisions about which products to choose and use every day. The theory assumes that the total value (utility) a consumer attaches to an intervention or service is described by the sum of individual attributes (factors or characteristics). These attributes can relate to the clinical outcome of the service (such as functional communication ability), the ability of a person to participate in aspects of daily life (such as social and family or interests and hobbies) or the process of providing the service (such as waiting times for therapy). The ability to incorporate both outcome and process attributes is one of the key advantages of this method.
The DCE used a binary choice design. In each choice set, the participant was asked to choose between two alternative treatments, each of which described a different outcome and waiting time. The DCE did not include an opt-out/neither or indifferent option. This meant that participant preferences were conditional on them choosing one of the options. The rationale for the forced choice was that a key question to be addressed was: how long are participants willing to wait for treatment to gain an improvement in communication outcomes? In addition, the communication outcomes described could occur with or without treatment and process attributes of the therapy itself were not included. This meant that the options in each choice set were ones that respondents would hypothetically have to choose between in real life (i.e. good or bad outcomes).
Questionnaire design
The attributes included in this DCE were selected from the items included in the 20-item COAST rating scale developed specifically for the ACT NoW study (Chapter 2). 28 Each item has a five-level response from worst possible outcome to as good as before the stroke. The COAST rating scale items and levels were developed with the ACT NoW RUG and incorporated the views of patients and health professionals about what constitutes important outcomes for communication problems following stroke. By using items from the rating scale, the estimated valuations from the DCE can be related to the outcomes of patients in the ACT NoW study who complete those items on the rating scale.
In order to ensure that the DCE was feasible for participants to complete, a reduced set of items from the full COAST was used. The selection of these items was informed by the subscales of the COAST and an additional factor analysis of the pilot data collected to test the rating scale. These subscales were interactive communication, overview of communication and impact of communication on quality of life.
An additional factor analysis conducted specifically to identify items for the DCE indicated a set of four items that represented the subscales and different aspects of the impact of communication problems and improvement in communication on daily life. These are the impact of:
-
ability to communicate on social and family life (interactive communication)
-
ability to communicate on involvement in interests and hobbies (quality of life)
-
confidence about communicating on daily activities (overview of communication)
-
communication on levels of worry and unhappiness (quality of life).
The description of these attributes and levels that was used in the DCE was developed from the rating scale and was kept as close as possible to the phrasing used in the rating scale. However, some changes to the phrasing were made to ensure grammatical consistency and ease of interpretation in the context of the DCE. In addition, a fifth attribute – waiting time for treatment – was included.
Waiting time was used as an alternative to price for a number of reasons. First, it is an important issue for the NHS in providing SL therapy for communication problems following stroke. Second, discussion with the ACT NoW study team and comments of participants from an initial pilot study suggested that the acceptability of including price of treatment was unclear. The five attributes are shown in the example question in Table 19. Table 20 gives the levels and descriptions used for each attribute.
| Please read the five statements on the left and choose the treatment you would prefer by ticking the box under Treatment A or Treatment B | ||
|---|---|---|
| Statement | Treatment A | Treatment B |
| After therapy your ability to communicate with family and friends is: | Quite poor | Fair |
| After therapy your ability to communicate means your involvement in interests and hobbies is: | Fair | Quite poor |
| After therapy your confidence in communicating affects what you do: | Very often | Sometimes |
| After therapy your ability to communicate means you are worried or unhappy: | Sometimes | Hardly ever |
| Waiting list for treatment is: | 1 month | 6 months |
|
Which treatment would you prefer? (Tick one) |
☐ | ☐ |
| Outcome | Bad → | Good | |||
|---|---|---|---|---|---|
| Abilities are: | The worst possible | Quite poor | Fair | Quite good | As good as before the stroke |
| Negative occurrences happen: | All the time | Very often | Sometimes | Hardly ever | Never |
| Waiting lists: | 1 year | 6 months | 3 months | 1 month | 1 week |
Participants were given background information about the communication problems following stroke and instructions to help them complete the survey. (A full copy of the DCE questionnaire can be provided on request from Linda Davies.)
The DCE survey comprised a number of choice questions that ask the respondent to choose between one of two treatments, described in terms of five attributes, each with five levels. The full factorial or combination of all attributes and levels is estimated as 55 or 3125. A survey that included all possible combinations would not be feasible within the constraints of the ACT NoW study. For this reason, a fractional factorial design was used. This is a smaller set of combinations that contain sufficient information to allow analysis of the data.
An objective of the analysis is to estimate the main effect of each of attribute (independent variable) on the choice of treatment (dependent variable). The number of choice questions included in the DCE was estimated from the number needed to estimate the main effects in the regression analysis. It is important that the fractional factorial selected is unbiased and allows estimation of the appropriate indirect utility function. 42 A design that considers only main effects, as used in this DCE survey, may be biased if the indirect utility functions to be estimated are not additive. However, the need for a design that was sufficient to estimate main effects and interactions needed to be balanced against the practicalities of a questionnaire that was acceptable to respondents and a survey that was within the resource constraints of the ACT NoW study.
Published design catalogues were used to generate the design (Sloane: www2.research.att.com/∼njas/oadir/) and determine which combination of attributes and levels to select.
The design comprised 25 treatment scenarios. These were used as treatment option A for each choice set. A recent randomised trial compared the response to two DCEs with different numbers of choice questions (8 vs 16 choice questions). This indicated that respondents can answer at least 16 choice questions. 43 A recently published DCE to develop preference weights for a glaucoma scale indicated that respondents could complete 32 choice questions, with six attributes in each choice. 44 This suggests that 25 choice sets for the DCE for the ACT NoW study is potentially feasible for respondents to complete. However, the feasibility of including additional choices in each of the choice sets and/or additional choice sets to increase efficiency or estimate main effects plus interactions was unclear.
To determine the levels for each attribute in treatment option B, the levels for each attribute in treatment option A were systematically changed, using modulo arithmetic. 45 This ensured that, for each attribute in each choice set, the levels in treatment options A and B were different. The design of the choice questions met published criteria so that each level appears with equal frequency (level balance), there is no overlap between attribute levels in each choice set, there is efficiency and near orthogonality (i.e. the attributes are statistically independent and uncorrelated). 46
The design was unblocked and each respondent was asked to compare the same 25 questions. The efficiency of the design was assessed using the online computer software developed by Burgess. 47 This indicated that the main effects were uncorrelated and that the efficiency of the design compared with an optimal design for choice set size of two was 89%; the efficiency compared with an optimal design for a choice set of five treatment options was 56%.
Questions about the socioeconomic and demographic characteristics of the respondent were included to assess whether or not participants’ preferences differed between different subgroups of the study sample. There were also questions to identify members of the public who may have experience of communication problems and/or SL therapy. At the end of the survey, participants were asked to rank the attributes in order of importance.
The DCE survey was piloted using electronic survey techniques to assess the feasibility and acceptability of the questionnaire, background information and instructions for completion. The sample for the electronic survey was staff and students at Manchester University using a centrally controlled electronic distribution list.
As with many DCE surveys in health care,48 there was no evidence of participants making irrational choices in the pilot study. In addition, there is evidence that the underlying random utility theory is sufficiently robust to choices that apparently do not conform to consumer theory. 42 Lancsar and Louviere42 also suggest that excluding participant choices that do not appear to conform to theory may result in excluding valid responses (which could bias the analysis) and reduce the statistical efficiency of the survey. For these reasons additional choice sets to test for rational or non-rational choices were not included in the main DCE survey. It was also assumed that the process of developing the COAST measure and the process of selecting the attributes and levels from the COAST ensured they adequately covered relevant attributes and levels.
Study population
The study population comprised members of the public. It was assumed that the preferences and values of the study population approximate the values and preferences of society that are relevant to those responsible for NHS policy and resource allocation decisions.
Study sample and recruitment
Members of the general public were recruited by postal invitation to participate in the discrete choice survey. The participants for the postal survey were randomly selected from Postcode Address Files of the north-west. 49 Invitees to the study were sent an information sheet, instructions and a paper copy of the DCE survey. The invitation letter and information sheet also included a URL and password for those participants who preferred to complete an electronic, online version of the survey. Members of the public indicated their consent to take part in the study by returning a completed survey. No reminders were sent. No incentives were used to increase response rates.
Ethical approval for the DCE survey was obtained as a substantial amendment to the main study protocol.
Inclusion and exclusion criteria
The survey included members of the public who are able to complete the postal or online survey. No exclusion criteria were applied.
Survey sample size
There are no clear guidelines to estimate the sample size required for DCE surveys. It was assumed (based on the design of the questionnaire) that the true probability of choosing treatment A is 50% and the probability of choosing treatment B is also 50%. It was also assumed that an acceptable error for this probability is ± 2.5% in the sampled population. With a survey of 25 choice sets, and significance level of 5%, a sample of 246 fully completed questionnaires was required. 50 A random sample, comprising 4000 members of the general public, was invited to participate.
Data analysis
The analysis of the quantitative data from the DCE aimed to identify the weight attached to each attribute included in the questionnaire. A basic linear additive model comprising all five attributes was used. The main effects model assumes that preferences for a level of one attribute are statistically independent of the levels taken by one or more other attributes. A multinomial logistic regression model was used. Further details about the analysis are given in Appendix 6.
Results
A total of 278 people responded to the survey, a response rate of 7% (278/4000 invitations). Of these, 259 returned a paper questionnaire that was either partially or fully completed and 19 submitted an online version that was either partially or fully completed. Overall, 213/278 (77%) people completed all of the choice questions and 40/278 (14%) people answered one or more of the 24 choice questions. Of the 40 participants who answered some but not all of the choice questions, five (13%) answered four or fewer questions and 26 (65%) answered 20 or more of the 25 choice questions. The remainder answered one or more of the background questions about themselves. Table 21 reports the characteristics of those respondents who completed all of the choice questions. The detailed data for those who completed some and those who completed all the choice questions are reported in Appendix 7, Table 36.
| Characteristic | All N (%) |
|---|---|
| Retirement age and above (%)a | 62 (30) |
| Gender | |
| Male | 98 (47) |
| Female | 112 (53) |
| Ethnic group | |
| White British | 199 (96) |
| Not white British | 9 (4) |
| Experience of SL therapy or stroke | |
| Participant ever had SL therapy | 6 (3) |
| Participant family/close friends ever had SL therapy | 43 (20) |
| Participant ever had a stroke | 10 (5) |
| Participant family/close friends had a stroke | 111 (53) |
| Participant employment status | |
| In employmentb | 127 (60) |
| Not in employment | 83 (40) |
The characteristics of those who completed the choice questions and those who did not were similar in many respects. However, there appeared to be differences in the age of those who completed none of the choice questions (mean age 66 years, SD 16 years) compared with those who completed some (mean age 55 years, SD 16 years) or all (mean age 55 years, SD 15 years) of the choice questions.
There were differences between the survey sample of respondents and the adult populations of England and the north-west of England in terms of proportion of people of retirement age, gender, ethnicity and employment (Table 22). This means that the choices of the respondents may not reflect those that the general population would make. It is not clear whether or not the response rate and representativeness of the survey sample are typical of those found in other DCE surveys of the general population. Response rates, where reported, are varied. However, the response rate of the DCE survey reported here appears low.
| Characteristic | England | North-west of England |
|---|---|---|
| Above retirement age (%)a | 19 | 20 |
| Gender | ||
| % Female | 51 | 51 |
| % Male | 49 | 49 |
| Ethnicity | ||
| % White British | 84 | 89 |
| % Not white British | 16 | 11 |
| Employment | ||
| % In employmentb | 73 | 71 |
| % Not in employment | 27 | 29 |
Logistic regression indicated that age, gender and whether or not family or friends had ever had SL therapy for communication problems were associated with the choice of treatment option in the choice sets (p < 0.05) (estimated as Cij = βXi1 + βXi2 + ... + βXin + εij, n = 1, ..., N, where Cij is the choice made in choice set j by individual i, and Xin is the characteristic n for individual i). These variables were interacted with each of the attributes to test the impact of these characteristics on the preferences for each of the attribute levels.
Participants rank order of attributes
At the end of the DCE survey, participants were asked to rank the attributes in order of importance, from one (most important) to five (least important). The data in Table 23 indicate that participants felt that the most important attribute was that of ‘ability to communicate affects social or family life’, which was ranked as most important by 64% of people who responded and least important by 5%. The ranks for the other attributes were more widely distributed. Full details of these data are given in Appendix 7, Table 37.
| Attribute | Most important, n (%) | Least important, n (%) | Average rank, mean (SD) |
|---|---|---|---|
| Ability to communicate affects social or family life | 165 (64) | 12 (5) | 1.65 (1.09) |
| Ability to communicate affects involvement in interests and hobbies | 25 (10) | 71 (28) | 3.41 (1.29) |
| Confidence in communicating affects what you do | 28 (11) | 23 (9) | 2.98 (1.14) |
| Ability to communicate affects whether worried or unhappy | 33 (13) | 44 (17) | 3.06 (1.29) |
| Waiting time for treatment | 50 (19) | 91 (35) | 3.35 (1.55) |
Choice questions
Over all the choice sets, participants selected option treatment A in 43% of the questions and selected treatment option B in 57%. More details about the choices made are given in Appendix 7, Table 38.
The discrete choice data were analysed using a multinomial logistic regression model. The results of this analysis are shown in Table 24. In this model, each level is compared with the lowest level or reference case:
-
reference case for ability to communicate affects social or family life is worst possible
-
reference case for ability to communicate affects involvement in interests and hobbies is worst possible
-
reference case for confidence in communicating affects what you do is all the time
-
reference case for ability to communicate affects whether worried or unhappy is all the time
-
reference case for waiting time for treatment is 1 week.
| Choice | Coefficient (∆x) | SE | p-value | 95% CI |
|---|---|---|---|---|
| Ability to communicate with family or friends is | ||||
| Quite poor | 1.12 | 0.08 | 0.000 | 0.97 to 1.27 |
| Fair | 1.84 | 0.10 | 0.000 | 1.66 to 2.03 |
| Quite good | 2.11 | 0.10 | 0.000 | 1.91 to 2.31 |
| As good as before the stroke | 2.80 | 0.10 | 0.000 | 2.60 to 3.00 |
| After therapy your ability to communicate means your involvement in interests and hobbies is | ||||
| Quite poor | 0.70 | 0.08 | 0.000 | 0.53 to 0.86 |
| Fair | 0.79 | 0.10 | 0.000 | 0.58 to 0.99 |
| Quite good | 0.92 | 0.10 | 0.000 | 0.72 to 1.12 |
| As good as before the stroke | 1.33 | 0.07 | 0.000 | 1.20 to 1.47 |
| After therapy your confidence in communicating affects what you do | ||||
| Very often | 0.20 | 0.09 | 0.024 | 0.03 to 0.38 |
| Sometimes | 0.86 | 0.09 | 0.000 | 0.67 to 1.04 |
| Hardly ever | 0.70 | 0.10 | 0.000 | 0.50 to 0.90 |
| Never | 0.93 | 0.07 | 0.000 | 0.80 to 1.07 |
| After therapy your ability to communicate means you are worried or unhappy | ||||
| Very often | 0.83 | 0.07 | 0.000 | 0.69 to 0.97 |
| Sometimes | 1.54 | 0.10 | 0.000 | 1.35 to 1.74 |
| Hardly ever | 1.63 | 0.11 | 0.000 | 1.42 to 1.85 |
| Never | 1.34 | 0.09 | 0.000 | 1.16 to 1.52 |
| Waiting list for treatment is | ||||
| 1 month | 0.43 | 0.09 | 0.000 | 0.26 to 0.61 |
| 3 months | –0.15 | 0.10 | 0.128 | –0.34 to 0.04 |
| 6 months | –0.43 | 0.09 | 0.000 | –0.61 to –0.24 |
| 1 year | –0.87 | 0.08 | 0.000 | –1.03 to –0.71 |
| Overall | –0.07 | 0.00 | 0.000 | –0.08 to –0.06 |
The data in Table 24 indicate that, overall, the coefficients for the communication outcome attributes increase as the level of communication outcome improves from worst possible to best possible. This suggests that participants preferred good to poor outcomes and conforms to what would be predicted by random utility theory. Combined with method of selecting the attributes for the DCE this provides evidence of the internal validity of the attributes and levels included in the survey.
The coefficients for the attributes of confidence and feeling worried or unhappy generally increase as outcome improves, but one or more levels of improved outcome has a lower coefficient than the preceding levels. This suggests that preferences for these variables are non-monotonic. For example, in the case of confidence in ability to communicate affecting what you do, participants apparently prefer an outcome of sometimes (coefficient = 0.86) to one of hardly ever (coefficient = 0.70) of ability to communicate rather than a quite good outcome. There are a number of possible reasons for this result. First, participants may have found it hard to distinguish between two or more levels (e.g. sometimes or hardly ever) or, second, felt that one or more of the levels was unrealistic (e.g. never feeling worried or unhappy). A third possibility is that there may be an optimum level of outcome for each of these variables, above which the utility of the participant decreases and the person is made worse off.
The negative coefficients for the attribute for waiting time indicate that shorter waiting times are preferred to the reference case of 1 week. The attribute is non-linear in that the coefficient 1 month compared with 1 week is positive. This implies that a 1-month wait for treatment may be preferred to a 1-week wait. This may reflect the severity of the underlying cause of the communication problems – stroke. Participants may have considered the first week after stroke to be too soon to initiate therapy for the communication difficulties.
The coefficients in Table 24 suggest that the most important attribute is ability to communicate with family and friends, which has the highest coefficient of all the attributes at each level. This is similar to the results of the ranking exercise in the survey, in which participants were asked to rank the attributes in order of importance.
However, the importance of the attributes implied by the discrete choice questions differs from that suggested by the rank data for the attributes for interests and hobbies, confidence and waiting time.
Table 25 reports the willingness to wait (WTW) for an improvement in outcome, for the main analysis. The WTW for an improvement was estimated as the marginal rate of substitution. This is calculated by dividing the coefficient for each attribute by the overall coefficient for waiting time. For example, the WTW value for moving from the worst possible to quite poor ability to communicate with family and friends is calculated by dividing 1.12 (the coefficient for the quite poor level in Table 24) by –0.07 (the coefficient for overall waiting time in Table 24).
| Attribute and level | WTW value (months) | Pseudo-95% CI |
|---|---|---|
| Ability to communicate with family or friends is | ||
| Quite poor | 14 | 10 to 19 |
| Fair | 25 | 17 to 33 |
| Quite good | 28 | 19 to 37 |
| As good as before the stroke | 37 | 26 to 48 |
| After therapy your ability to communicate means your involvement in interests and hobbies is | ||
| Quite poor | 10 | 7 to 14 |
| Fair | 12 | 5 to 18 |
| Quite good | 14 | 7 to 21 |
| As good as before the stroke | 19 | 12 to 26 |
| After therapy your confidence in communicating affects what you do | ||
| Very often | 3 | –1 to 7 |
| Sometimes | 10 | 5 to 16 |
| Hardly ever | 8 | 3 to 13 |
| Never | 12 | 7 to 16 |
| After therapy your ability to communicate means you are worried or unhappy | ||
| Very often | 10 | 6 to 14 |
| Sometimes | 20 | 14 to 26 |
| Hardly ever | 20 | 15 to 26 |
| Never | 17 | 12 to 22 |
The WTW values in Table 25 again illustrate that ability to communicate with family and friends and impact of communication ability on whether worried or unhappy were considered the most important outcome attributes. This corresponds with the proportion of participants who ranked these outcomes as most important.
The impact of communication on involvement in interests and hobbies and on confidence had lower WTW values, suggesting that they were less important outcomes. Again this is similar to the results of the ranking exercise.
Subgroup analyses
The results shown in Tables 24 and 25 assume that individual characteristics of the participants have no impact on the choices they made in the DCE. The analysis was repeated, including interaction terms between each attribute level and the participant characteristics of age (< or ≥ 60 years), whether or not the participants or their family/friends had used SL therapy for communication problems, whether or not the participants or their family/friends had ever had a stroke, gender and ethnic group (white British, not white British). The analysis indicated that age may be associated with the choices participants made in the DCE. Subgroup analyses for these two characteristics were conducted. The results of the regression analyses by age group are given in Appendix 7, Table 39. Table 26 shows the WTW values for the two age groups. The results of this analysis suggest that the younger survey participants were willing to wait longer for treatment than the older participants. This may be related to expectancies about future survival and the number of years left to live. Participants with a lower life expectancy may be less willing to wait for treatment, as any benefits they get from therapy will be gained for a fewer number of years.
| Attribute and level | WTW, months (95% CI) | |
|---|---|---|
| Age < 60 years (n = 123) | Age ≥ 60 years or above (n = 93) | |
| Ability to communicate with family or friends is | ||
| Quite poor | 22 (6 to 38) | 10 (5 to 15) |
| Fair | 36 (10 to 63) | 18 (11 to 25) |
| Quite good | 40 (11 to 69) | 21 (13 to 29) |
| As good as before the stroke | 53 (18 to 89) | 27 (17 to 37) |
| After therapy your ability to communicate means your involvement in interests and hobbies is | ||
| Quite poor | 17 (5 to 29) | 7 (3 to 11) |
| Fair | 22 (1 to 43) | 4 (–3 to 11) |
| Quite good | 24 (4 to 45) | 6 (–2 to 13) |
| As good as before the stroke | 29 (6 to 52) | 12 (5 to 19) |
| After therapy your confidence in communicating affects what you do | ||
| Very often | 9 (–1 to 19) | –3 (–7 to 2) |
| Sometimes | 21 (4 to 37) | 3 (–3 to 8) |
| Hardly ever | 16 (3 to 30) | 1 (–4 to 6) |
| Never | 17 (4 to 30) | 8 (3 to 12) |
| After therapy your ability to communicate means you are worried or unhappy | ||
| Very often | 14 (2 to 26) | 7 (2 to 12) |
| Sometimes | 26 (8 to 44) | 16 (10 to 21) |
| Hardly ever | 25 (9 to 41) | 17 (11 to 22) |
| Never | 21 (7 to 35) | 14 (9 to 18) |
Summary
Overall, 213 out of 278 (77%) people completed all of the choice questions and 40 out of 278 (14%) people answered one or more of the choice questions. The analysis indicated that all of the attributes had statistically significant coefficients for each level, which suggests that all the outcome and waiting time attributes were important contributors to the preferences of participants. Overall, participants identified ability to communicate with family and friends as the most important attribute. Participants were willing to wait longer for treatment to achieve an improvement in this outcome compared with the other communication outcomes included in the DCE. The importance of ability to communicate demonstrated by the analysis of the choice questions was supported by the results of a separate ranking exercise. Overall, 64% of participants who completed the ranking exercise at the end of the survey ranked ability to communicate with family and friends as the most important communication outcome.
Overall, the results of the survey suggest that participants are willing to wait longer than 1 year for treatment that improves their ability to communicate and the impact that this has on their lives. This is longer than the maximum waiting time included in the survey, which reflected national policy and practice at the time of the survey. Younger people are willing to wait for longer for therapy than older people. However, a number of assumptions were made in the design and conduct of the DCE survey. When combined with the low response rate (6%) to the survey, these could affect the validity and robustness of the results. The strengths and limitations of the survey are discussed in more detail in Chapter 8.
Chapter 6 Economic evaluation: methods and results
This chapter presents the economic analysis of the relative resource use, costs and cost-effectiveness of SL therapy compared with AC.
Methods
Aims and objectives
The aim of the economic analysis was to evaluate the relative resource use, costs and cost-effectiveness of SL therapy compared with AC, at 6 months, for patients with communication difficulties because of aphasia/dysarthria following stroke. Within the context of the particular interventions used in this trial, the specific objectives of the economic evaluation were to:
-
identify, measure and value key services and resources used by all participants and assess whether or not the direct costs of care differed between the SL therapy and AC groups
-
measure and value the health status of participants at 6-month follow-up and assess whether or not health status differed between the SL therapy and AC groups
-
assess whether or not SL therapy was a cost-effective intervention compared with AC.
Approach
Cost-effectiveness, cost-effectiveness acceptability analysis (CEAA) and net benefit analyses were used to relate costs and outcomes and explore the value for money of SL therapy.
The economic evaluation used the combined perspectives of the NHS, providers/funders of non-hospital care facilities and of patients and families. These actors are expected to incur the key costs and benefits of services for SL therapy for communication difficulties because of aphasia/dysarthria following stroke. This is for two reasons. First, it is unlikely that other health and social care providers and funders would experience differences in costs associated with the trial interventions within the 6-month time horizon of the economic evaluation. This is discussed in more detail in Costs. Second, the pilot study indicated that it was not feasible to collect robust data on these services. Therefore, the perspectives of these groups were not sought.
The range of costs is described below. The economic evaluation used resource use, cost and outcome data collected for all of the participants enrolled in the RCT described in previous chapters.
The time horizon of the evaluation was the 6-month follow-up period used for the trial. The short time horizon means that discounting of costs and outcomes is not relevant and was not conducted.
The trial interventions were conducted in both an inpatient setting and following discharge of the patient to home or community-based care facilities. This means that the setting for the economic evaluation covers both inpatient and community/primary care-based settings in the north-west of England.
The resource use and outcome data were collected between 2006 and 2010. Unit cost data are for the financial year 2008–9.
Costs
Data were collected on a range of health-care and social care resources used as inputs to produce and provide SL therapy, AC and associated care of the patient. These data were combined with unit cost information to estimate the 6-month costs associated with each intervention.
The range of services measured was informed by the feasibility study and a review of the existing literature, and included the following:
-
Length of stay in inpatient care. From admission to hospital for the index stroke to randomisation; from randomisation to discharge and for any subsequent admissions to hospital up to the end of scheduled follow-up at 6 months, including stays in specialist inpatient rehabilitation units. Detailed information on the type of wards used was collected, as the unit cost per day of hospital stay can vary substantially by type of ward.
-
Length of stay in community-based rehabilitation and care facilities during the 6-month follow-up period.
-
Time taken by SL therapists to provide the trial specific SL therapy intervention and the time (including training) of personnel providing the AC intervention. In addition, data were collected on the use of non-trial SL therapy by participants in both the SL therapy and AC groups, following discharge from hospital. (It was assumed that the costs of non-trial SL therapy provided while the participant was an inpatient would be included in the cost per day of hospital stay.)
-
Use of hospital-based outpatient and day patient clinics and services. Detailed information was collected on the type of outpatient and day patient service used, as the unit cost per visit can vary substantially according to the purpose and nature of the clinic or service.
Information on the use of invasive procedures, tests and assessments and use of therapy services while the participant was an inpatient was also collected. However, because the unit costs used to estimate the direct costs of inpatient care included the costs of all services provided as part of the inpatient episode, these data were not separately costed.
At the end of follow-up, data were also collected from carers on whether or not the trial participants had used other community and primary care services. It was not considered feasible to collect more detailed information on the frequency and intensity of service use within this trial; therefore, these services are not included in the direct costs reported here.
However, the use of the data is reported in order to provide a check on whether or not the proportion of people using these services differs between the SL therapy and AC groups.
The key service use data collection forms are available on request. Data on the use of trial-specific SL therapy and AC staff were obtained from data collected for monitoring and trial management purposes.
National unit cost data were collected as follows:
-
for inpatient stay, by ward
-
for stay in other rehabilitation and care home facilities
-
for outpatient and day patient services, by type
-
for SL therapy services.
These data were collected from two main published sources: the reference cost database for NHS Trusts and primary care trusts combined (NSRC4), published by the DoH,52 and the Unit Costs of Health and Social Care 2009. 53
Unit costs were adjusted for inflation where necessary,53 and the price year was 2008–9. The unit costs used and source are detailed below (see Table 27) and in Appendix 9.
| Item of resource use | Average unit cost | Sources |
|---|---|---|
| Inpatient stay, per day | 160–1194 | DoH Reference Costs 2008–952 |
| Community-based care facilities, per week | ||
| Nursing home | 678 | PSSRU 200953 |
| Residential home | 467 | PSSRU 200953 |
| Sheltered care | 271 | PSSRU 200953 |
| Day patient attendances, per visit | 104–531 | DoH Reference Costs 2008–9,52 CIPFA 2005,62 inflated to 2008–9 price year,53 PSSRU 2006,63 inflated to 2008–9 price year53 |
| Outpatient attendances, per visit | ||
| Hospital | 36–228 | DoH Reference Costs 2008–9,52 PSSRU 2009,53 CIPFA 2005,62 inflated to 2008–9 price year53 |
| Home | 21–128 | DoH Reference Costs 2008–9,52 PSSRU 2009,53 CIPFA 2005,62 inflated to 2008–9 price year53 |
| Primary care services, per visit | ||
| GP, surgery visit | 36 | PSSRU 200953 |
| GP, home visit | 58 | PSSRU 200953 |
| Nurse, surgery visit | 10 | PSSRU 200953 |
| Nurse, home visit | 24 | PSSRU 200953 |
Details of the process used to estimate the costs of SL therapy and AC time are provided in Appendix 8. For the primary economic analysis, the national unit cost published by Curtis53 was used. The national costs were used to reflect the NHS staff and other costs that would be incurred if the SL therapy and AC interventions provided in this trial were implemented in routine care.
Differences in use of resources and services and in direct costs are presented descriptively, but no formal tests to identify statistically significant differences were conducted (see Analysis, below, for further discussion).
Outcomes
Three alternative outcomes were identified as relevant and of interest to policy-makers, providers and funders of care and patients. These were:
-
the primary measure of effectiveness of communication, used for the clinical component of the RCT
-
a summary measure of health-related utility, derived from the overall health status of the participant
-
a measure of preferences for communication outcomes.
The data from the pilot study indicated that, for this group of patients, it was feasible to measure health status, using the EQ-5D54 to use to estimate utility values. The EQ-5D is described below and is recommended by the National Institute for Health and Clinical Excellence (NICE). In addition, the outcome measures for the main trial individually cover a range of outcomes relevant to health status and quality-adjusted life-years (QALYs). The study team felt that using the main effectiveness measure would not give an indication of the value of the outcome of care to participants in the trial, or to society more generally, and may present a partial view of the relative cost-effectiveness of the intervention. In addition, NICE now clearly recommends QALYs as the most relevant measure of benefit or outcome for an economic evaluation. For all these reasons, although it was not possible to estimate QALYs (as explained below), it was felt that summary measure of health-related utility, derived from the overall health status of the participant, was the most relevant outcome measure for the economic analysis. This was decided before data collection was completed and the data made available for analysis.
However, it is important to assess whether or not using alternative outcome measures to estimate the relative cost-effectiveness of the SL therapy intervention changes the results and conclusions of the analysis. This is particularly important if the relevance of an outcome measure varies according to the viewpoint of the decision-maker. For this reason, sensitivity analysis was used to estimate incremental cost-effectiveness ratios (ICERs) and net benefit statistics for each of these measures.
Value-based measures of outcome of care
Health status and health-related utility
The health status of participants was measured at the 6-month follow-up assessment using the EQ-5D. 54
This is a simple generic measure of health, which has been extensively tested and validated both in the UK and internationally. EQ-5D consists of five areas or domains: mobility; self-care; usual activities; anxiety and depression; and pain and distress. Each domain is rated by the participant on a three-point scale: no problems; some problems; extreme problems. These domains are also relevant to the health states that may follow stroke.
A set of utility values or weights that reflect preferences (of a general population sample) for different health states has been derived to provide a single index for the EQ-5D. The EQ-5D and these utility weights were used to estimate a value-based utility measure of outcome for participants in the trial.
Differences in utility between the SL therapy and AC group at the end of follow-up were estimated, but no formal tests to identify statistically significant differences were conducted (see Analysis, below, for further discussion). The utility measure was used to estimate the net cost per utility point gained by SL therapy for the cost-effectiveness and CEAAs.
Given the severity of illness of participants and potential distress of participants and informal carers when they were assessed and entered into the trial, the trial management group felt that it was not feasible to collect EQ-5D data at baseline from either patients or informal carers. Proxy versions of the EQ-5D are designed to be completed by someone who knew the participant well, so may not be reliable if filled in by a person (e.g. member of hospital staff) who does not know the participant well.
Initial analysis of the baseline data from the trial indicated that there were potential differences in stroke severity between the participants randomised to the SL therapy group and those randomised to the AC group. The measure of stroke severity at baseline was statistically significantly associated with the EQ-5D utility score at follow up, independent of allocation group (see Statistical analysis). This meant that it was not possible to assume an equal utility value between allocation groups at baseline.
Mapping of utility values using clinical measures is complex and leads to high levels of uncertainty. For these reasons we felt that using the available data to estimate QALYs was not feasible and would not provide added value. It also means that the utility values used in the analysis cannot be interpreted as implicit QALY values. This would require the assumptions that baseline utility values and length of survival were equivalent between the SL therapy and AC groups. These factors mean that the utility value (when used as the outcome measure in the economic evaluation) is an effectiveness measure that indicates differences in the health status of participants at the end of follow-up.
Stated preference for communication outcomes
A DCE was used to value preferences for key attributes of the outcomes of care measured by the COAST, the communication outcomes scale developed and validated for the ACT NoW trial. The DCE is described in detail in Chapter 5.
Willingness-to-wait values for the COAST attribute were presented in Chapter 5. For this analysis, a set of preference weights that reflect participant preferences for the four communication outcomes were estimated as the Coast Quality Weights (CQW). These weights combine the four outcome attributes from the COAST into a single index, the CQW. This is estimated by adding the coefficients of the best levels, and rescaling between ‘0’ (the worst outcome) and ‘1’ (the best outcome). CQW = 1/(∑ΔBi), where CQW is the index score and Bi = best outcome of attribute i.
These preference weights were applied to the relevant attributes of the COAST to derive a single measure of communication outcomes that reflected the survey participants’ preferences for the four attributes. The weighted communication measure was used to estimate the net cost per point gained by SL therapy for the cost-effectiveness and CEAAs.
Incremental cost-effectiveness ratio and net benefit
The primary economic analysis is of ICERs. ICERs were estimated using cost and the three outcome measures outlined above.
Regression models were used to estimate incremental or net costs and net outcomes for the ICER (see Analysis, below, for further details). The estimates of incremental costs and outcomes from the regression were bootstrapped55 to simulate 10,000 pairs of net cost and net outcomes of SL therapy.
These simulated data were used for a CEAA, to estimate the probability that allocation to SL therapy was cost-effective compared with allocation to AC. This is an approach recommended by NICE for health technology appraisals. 56 The approach revalues effects or outcomes in monetary terms. However, in the UK there is no universally agreed monetary value for the types of outcome measures used in cost-effectiveness analyses. An approach used in health care is to ask the question: what is the maximum amount decision-makers are willing to pay to gain one unit of outcome? An analysis of decisions made by NICE suggests a range of implicit values between £15,000 and £30,000 for the amount a decision-maker is prepared to pay to gain one QALY. 57
For this analysis, the outcomes were revalued using a range of maximum willingness-to-pay values from £1 to £30,000 to gain one unit of outcome. These reflect a range of hypothetical willingness-to-pay thresholds (WTPTs) from decision-makers being willing to pay £1 to gain a one-unit increase in outcome to them being willing to pay £30,000 to gain a one-unit increase in outcome. Decision-makers may not be willing to pay as much to gain one unit of outcome measured by an effectiveness measure, such as utility, as they would to gain one QALY. This is because the QALY combines both survival and the utility of health over a period of time. In contrast, the utility and communication measures used in this analysis reflect the value of a health state, or ability to communicate at a single point in time. The minimum WTPT of £1 reflects the lower implicit values decision-makers may have for these outcomes compared with QALYs. The higher WTPT of £30,000 reflects the maximum amount decision-makers are likely to be willing to pay to gain one unit of utility or improvement in communication. However, it is important to note that decision-makers may not be willing to pay up to this amount for these outcomes.
The data for the cost-effectiveness acceptability curve are derived by first revaluing each of the 10,000 net outcome scores from the bootstrap simulation by a single WTPT. This is repeated for each WTPT. A net benefit statistic (NB) for each pair of simulated net costs and net outcomes for each WTPT can then be calculated as NB = (O × WTPT) – C, where O = net outcome score and C = net cost.
This calculation was repeated for each WTPT. Cost-effectiveness acceptability curves plot the proportion of bootstrapped simulations where the net benefit of an intervention is > 0 for each WTPT. 55,58–60
All analyses were run in Stata/IC version 11 (StataCorp LP, College Station, TX, USA).
Statistical analysis
The primary measure of interest for the economic analysis is the ICER, which is a joint measure of costs and outcomes, rather than the individual cost and outcome variables that are used to estimate the ICER. Accordingly, no formal statistical tests of differences in mean costs or outcomes are reported. The mean costs and outcomes, with SDs, are presented in a descriptive analysis.
Regression models were used to estimate the net costs and outcomes, controlling for baseline covariates. The starting model was that used for the main analysis of effectiveness, which included diagnosis, communication severity and study site.
For the economic evaluation, the need for additional baseline covariates was assessed by defining potential determinants of costs and utility values from previous literature. These were evaluated for use as covariates in this study with correlation (continuous) and analysis of variance (ANOVA) (categorical) analyses on the participant sample independent of allocation to identify associations between baseline variables and costs and utility values.
Variables with a p-value of ≤ 0.05 were included as covariates to estimate adjusted means for total cost per person and total outcome per person and to conduct the CEAA.
The baseline covariates were used to adjust the primary and all sensitivity analyses. In a sensitivity analysis, the regression model used for the main analysis of effectiveness was used.
Sensitivity analysis
Sensitivity analysis was used to assess the robustness of the results to the methods used for analysis. This approach indicates whether the results or conclusions of the analysis are likely to change if different key assumptions, analytical methods, or methods of measuring outcomes are used. Sensitivity analysis was used to explore the impact of the following:
-
using a trial-specific cost rather than national unit cost for the SL therapy and AC interventions
-
using available case data rather than imputing missing data; the available case approach requires excluding participants with one or more missing cost or outcome observations, even if they completed scheduled follow-up
-
using an alternative regression model to estimate the incremental costs, outcomes and ICER of SL therapy. In particular, this sensitivity analysis explored whether restricting the covariates to those used in the clinical analysis and excluding a measure of baseline severity of stroke changed the results
-
using alternative outcome measures rather than the measure of utility included in the primary economic analysis.
The statistical analyses and bootstrap simulations for the CEAA were conducted in Stata/IC version 11.
Missing observations
There were relatively high levels of missing data in the total costs (34% SL therapy and 47% AC) and utility scores (13% SL therapy and 22% AC). In addition, the level of missing data was higher in the AC group than in the SL therapy group. However, the level of missing data for the key costs, of inpatient stay and the use of the AC and SL therapy interventions was low, with 12% and 20% of missing inpatient stay data for the SL therapy and AC groups, respectively. There were complete data for the use of the trial interventions.
Using available case data potentially biases the results if some or all of the missing data are missing systematically, rather than at random. 61
One reason for low available cases for total costs appeared to be low numbers of complete observations for outpatient data. These data were obtained from patient case notes. Reports from the staff responsible for collecting these data suggest that a common problem was that the case notes indicated a referral or attendance at an outpatient clinic, but no further information about the number of visits or type of clinic was available.
Multiple imputation was used to impute values to the missing total cost and utility score observations. The imputation model for the total cost variable included the variables for use of services, plus covariates. The model for the utility score included the scores on each of the domains of the EQ-5D plus covariates. This assumed that the data were missing at random or that any systematic missing observations were missing as a result of observed covariates.
The multiple imputation was conducted in Stata/IC version 11.
Results
Correlations and baseline covariates for costs and utility scores
Appendix 10 shows the results of the correlation analysis to assess variables for inclusion as covariates in the analyses of incremental (net) costs and utility scores and for use in the models for multiple imputation of missing data. This indicated that severity of stroke (measured by the BI) at baseline is likely to be associated with both costs and utility scores. Intuitively, it makes sense that the costs and outcomes of the participants in this trial would also be affected by the severity of stroke at baseline. Accordingly, this was included as a covariate in the primary analysis. Diagnosis, severity of communication difficulties at baseline and site were identified as covariates for the clinical effectiveness analysis presented previously and so were included as covariates for all the economic analyses.
Costs
Table 27 summarises the unit costs used to estimate the direct costs of resources and services used in the analysis (the detailed unit costs are presented in Appendix 9, Table 43).
Table 28 shows the unit costs of SL therapy and AC interventions. The national average costs per minute were estimated and used for the main analysis, to represent what the costs of the SL therapy and AC interventions would be if implemented in routine practice. The cost of SL therapy and AC interventions for each session were estimated as the cost per minute multiplied by the length of that session.
| Item of resource use | Average unit cost | Sources |
|---|---|---|
| National average cost, for primary analysisa | ||
| SL therapist, AfC Band 5, full cost per hour of client contact | 44.00b | PSSRU 2009c |
| AC worker, AfC Band 3, full cost per hour of client contact | 21.0053 | PSSRU 2009c |
| Actual cost incurred in trial,d for sensitivity analysis | ||
| SL therapist, full cost per client contact, average length of client contact = 0.78 hours | 163.14 | PSSRU 2009,c trial data on SL therapy activity |
| AC worker, full cost per client contact, average length of client contact = 0.65 hours | 129.10 | Trial data on activity and expenditure |
However, both the SL therapy and AC interventions incurred high levels of training and supervision to ensure adherence to the intervention protocols. The potential impact of this was explored in the sensitivity analysis. The costs of SL therapy and AC visitors actually incurred in the trial includes these additional costs, so represents the costs that may occur in the early stages of implementing a service to provide the interventions assessed in this trial.
Table 29 presents a summary of the use of services by the trial participants and Table 30 summarises the average costs of trial participants. These data are for available cases and have not been adjusted for baseline covariates. Appendix 11 gives detailed information about the length of stay by type of ward and Appendix 12 presents the proportion of participants using primary and community care services. There were no statistically significant differences between the AC and SL therapy groups in use of these services after adjusting for baseline covariates [available cases, multivariate analysis of variance (MANOVA), p = 0.78].
| Item of service use | AC | SL therapy | ||||
|---|---|---|---|---|---|---|
| n | Mean use | SD | n | Mean use | SD | |
| No. of inpatient hospital admissions | 71 | 1.87 | 1.04 | 80 | 1.65 | 0.93 |
| Inpatient hospital stay (days) | ||||||
| Pre randomisation | 71 | 13.63 | 5.92 | 78 | 12.27 | 7.04 |
| Post randomisation | 68 | 40.52 | 47.54 | 75 | 30.22 | 34.37 |
| No. of admissions to care facilities | 71 | 0.18 | 0.49 | 79 | 0.20 | 0.43 |
| Length of stay in care facilities (days) | 71 | 13.31 | 36.23 | 79 | 18.57 | 44.24 |
| Outpatient visits | 54 | 10.33 | 32.32 | 64 | 6.27 | 9.25 |
| Day patient visits | 70 | 0.14 | 0.77 | 79 | 0.37 | 2.49 |
| Non-trial NHS SL therapy | ||||||
| Visits | 73 | 3.96 | 9.51 | 81 | 0.16 | 1.16 |
| Minutes | 73 | 195.55 | 474.58 | 81 | 6.98 | 52.28 |
| Trial intervention | ||||||
| Visits | 85 | 17.46 | 12.65 | 85 | 21.92 | 11.12 |
| Minutes | 85 | 38.73 | 14.11 | 85 | 46.59 | 11.24 |
| Item of service use | AC | SL therapy | ||||
|---|---|---|---|---|---|---|
| n | Mean cost | SD | n | Mean cost | SD | |
| Inpatient hospital stay (days) | ||||||
| Pre randomisation | 69 | 3684 | 1755 | 78 | 3327 | 2078 |
| Post randomisation | 68 | 10,954 | 12,740 | 75 | 8020 | 8936 |
| Stay in care facilities | 71 | 1560 | 4726 | 79 | 1708 | 4086 |
| Outpatient visits | 49 | 458 | 934 | 62 | 413 | 510 |
| Day patient visits | 70 | 36 | 187 | 78 | 85 | 575 |
| Non-trial NHS SL therapy | 73 | 75 | 182 | 81 | 5 | 38 |
| Trial intervention | 85 | 295 | 254 | 85 | 776 | 454 |
| Total costs, post randomisation | 45 | 13,522 | 14714 | 56 | 11,020 | 11,758 |
Total costs could be estimated for only 53% (45/85) of participants in the AC group and 66% (56/85) of participants in the SL therapy group, in the baseline sample. Using available case data potentially biases the results if some or all of the missing data are missing systematically, rather than at random. To address this, multiple imputation was used to impute values to the missing total cost observations for participants who completed the scheduled follow-up for at least one of the outcome measures. This meant that people who were completely lost to follow-up and not known to have died were excluded from the analysis. This assumed that the data were missing at random or that any systematic missing observations were missing as a result of observed covariates. The imputation model included age, gender, ethnicity, severity of stroke, allocation arm and site as covariates.
The data including imputed cost values are presented in Incremental cost-effectiveness analysis: main analysis.
Health status and utility scores
Table 31 summarises the health status of participants at 6-month follow-up and Table 32 shows the estimated utility score. Detailed health status data are given in Appendix 13. Overall, the data suggest that the health and HRQoL (as measured by the utility score) of participants is similar. However, there are differences in the number of participants completing one or more domains of the EQ-5D and for whom data are available.
| Health domain | AC (n = 85) | SL therapy (n = 85) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Participant completed one or more items on EQ-5D | 63 | 74 | 74 | 87 |
| Participant died before assessment | 8 | 9 | 4 | 5 |
| No problem with mobility | 22/63 | 35 | 24/74 | 32 |
| No problem with self-care | 34/62 | 55 | 45/74 | 61 |
| No problem with usual activities | 22/62 | 35 | 31/73 | 42 |
| No problem with pain | 27/59 | 46 | 40/73 | 55 |
| No problem with anxiety and depression | 31/60 | 52 | 26/72 | 36 |
| AC | SL therapy | ||||
|---|---|---|---|---|---|
| n | Mean utility | SD | n | Mean utility | SD |
| 66 | 0.47 | 0.38 | 74 | 0.51 | 0.42 |
As with the cost data, missing observations meant that there is the potential for bias in the data, so multiple imputation was used to impute missing utility values for the main analysis and reduce the impact of missing observations.
The data, including imputed utility values, are presented in the following section (see Table 33).
| Analysis | Coefficient net effect | 95% CI/percentile |
|---|---|---|
| A. Non-bootstrapped data | ||
| Net utility of SL therapy | 0.00 | –0.12 to 0.12 |
| Net cost of SL therapy | 135 | –2539 to 2810 |
| B. Bootstrapped data | ||
| Net utility of SL therapy | 0.01 | –0.03 to 0.04 |
| Net cost of SL therapy | 110 | –640 to 861 |
Incremental cost-effectiveness analysis: main analysis
The primary outcome measure for the economic analysis is the ICER or net cost per additional unit of health gain.
The ICER for the SL therapy intervention is calculated as the net cost divided by the net health gain of SL therapy compared with AC. Table 33 reports the incremental costs and utility scores for SL therapy.
The net costs and utility were estimated using imputed values for missing observations (estimated using multiple imputation) and the analyses were adjusted for baseline covariates (utility = arm allocated, 10-item Modified BI score, site, communication diagnosis severity; total cost = arm allocated, 10-item Modified BI score, site communication diagnosis severity). The detailed results of the analysis are shown in Appendix 14.
The net utility and cost results from the regression analysis (reported in section A of Table 33) were simulated using a bootstrap procedure to produce 10,000 replicates or estimates of pairs of net costs and net utility scores. These are summarised in section B of Table 33.
The data in Table 33 indicate that, independently, the costs and utility scores of the SL therapy and AC groups appear similar, as the 95% CIs and percentiles cross zero. However, it is the joint distribution of incremental costs and utility scores that is important for the economic analysis.
Figure 9 presents a scatterplot that demonstrates the distribution of the 10,000 replicates from the bootstrap procedure. Each dot in Figure 9 represents a pair of simulated net cost and net utility gained by SL therapy when compared with AC. The distribution suggests that slightly more than half of the simulations are associated with a net gain in utility (that is, more than half of the points lie to the right of the vertical line). The distribution of dots also indicates that more than half of the simulated costs are net costs for SL therapy.
FIGURE 9.
Scatterplot of bootstrapped net cost and utility scores of SL therapy compared with AC.
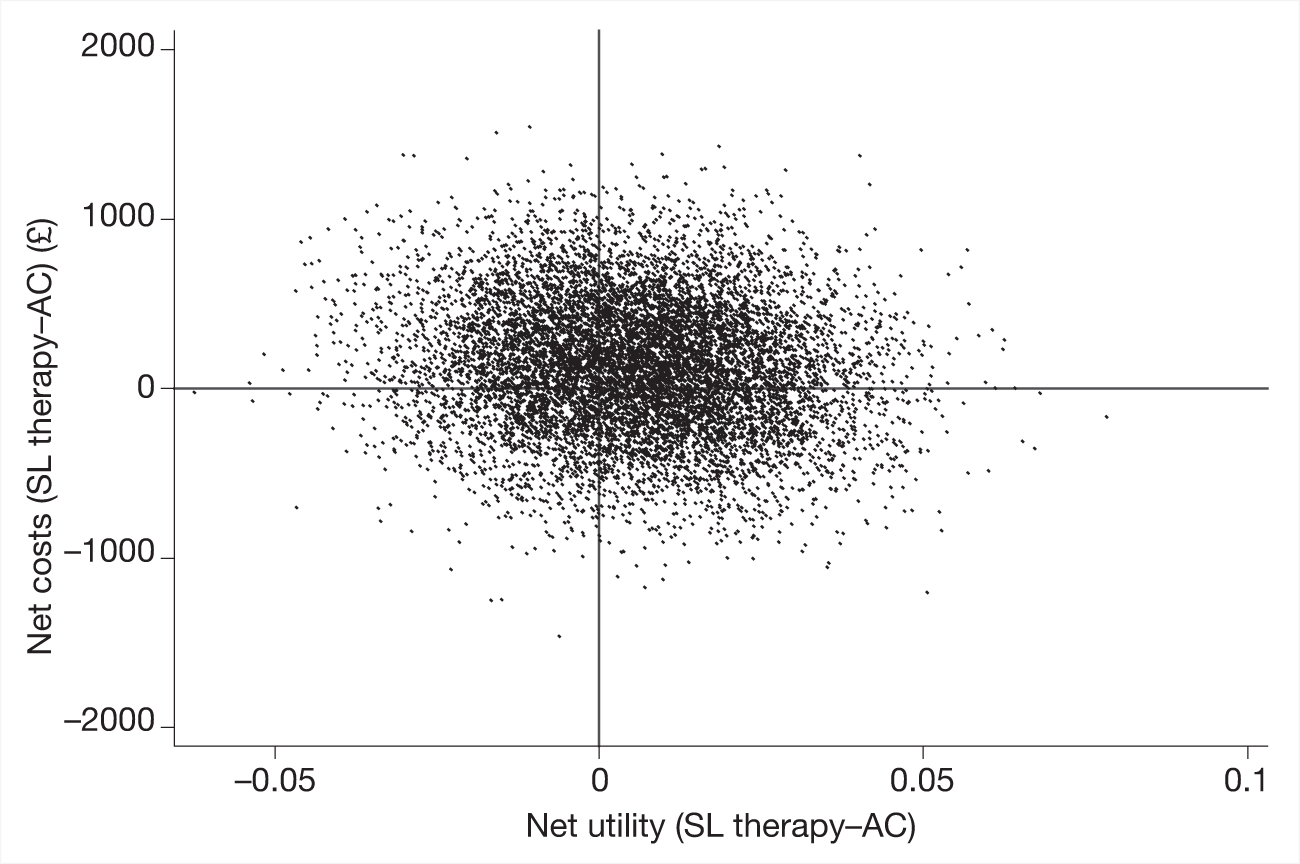
This analysis is extended in Figure 10, which shows a cost-effectiveness acceptability curve. This curve represents the probability that the simulated net costs and utility scores are cost-effective. This analysis uses hypothetical amounts that decision-makers might be willing to pay to gain improvements in health status that result in a one-point increase in utility. The hypothetical levels of willingness to pay represent possible thresholds of willingness-to-pay thresholds (WTPTs).
FIGURE 10.
Probability that SL therapy is cost-effective compared with AC, by WTPT.
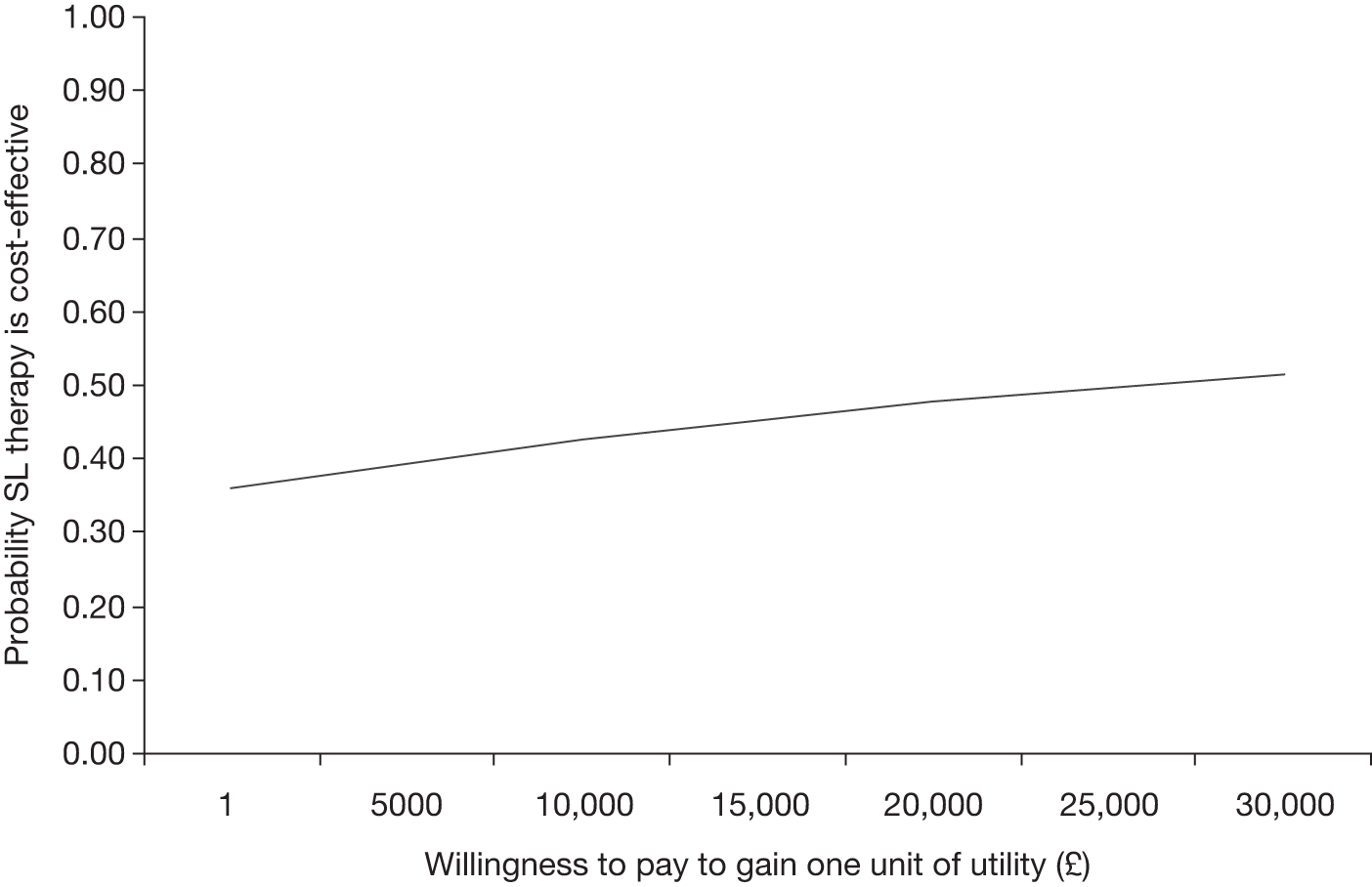
The data used to produce Figure 10 are summarised in terms of net benefit and probability estimates in Table 34 for each level of WTPT.
| WTPT (£) | Net benefit | Probability that SL therapy is | |
|---|---|---|---|
| Cost-effective | Not cost-effective | ||
| 1 | –136 | 0.36 | 0.64 |
| 5000 | –108 | 0.39 | 0.61 |
| 10,000 | –80 | 0.43 | 0.57 |
| 15,000 | –53 | 0.45 | 0.55 |
| 20,000 | –25 | 0.48 | 0.52 |
| 25,000 | 3 | 0.50 | 0.50 |
| 30,000 | 31 | 0.51 | 0.49 |
The probability that SL therapy is cost-effective increases as decisions-makers’ willingness to pay increases, but reaches 50% only if decision-makers are prepared to pay £25,000 for a one-point gain in utility.
Sensitivity analysis
Sensitivity analysis was used to assess the robustness of the results to the methods used for analysis. This indicates whether the results or conclusions of the analysis are likely to change if different key assumptions, analytical methods or methods of measuring outcomes are used. The impact of the following on the results were explored:
-
using trial-specific rather than national unit cost for the SL therapy and AC interventions, which may represent early start-up costs of implementing the trial interventions in routine practice
-
using available case rather than imputed missing data
-
using an alternative regression model to estimate the incremental costs, outcomes and ICER of SL therapy; in particular, this sensitivity analysis explored whether or not restricting the covariates to those used in the clinical analysis and excluding a measure of baseline severity of stroke changed the results
-
using alternative outcome measures rather than the measure of utility included in the primary economic analysis.
Figure 11 below summarises the sensitivity analysis and presents the cost-effectiveness acceptability curves for each sensitivity analysis.
FIGURE 11.
Probability that SL therapy is cost-effective compared with AC, by WTPT, sensitivity analysis. MI, multiple imputation.
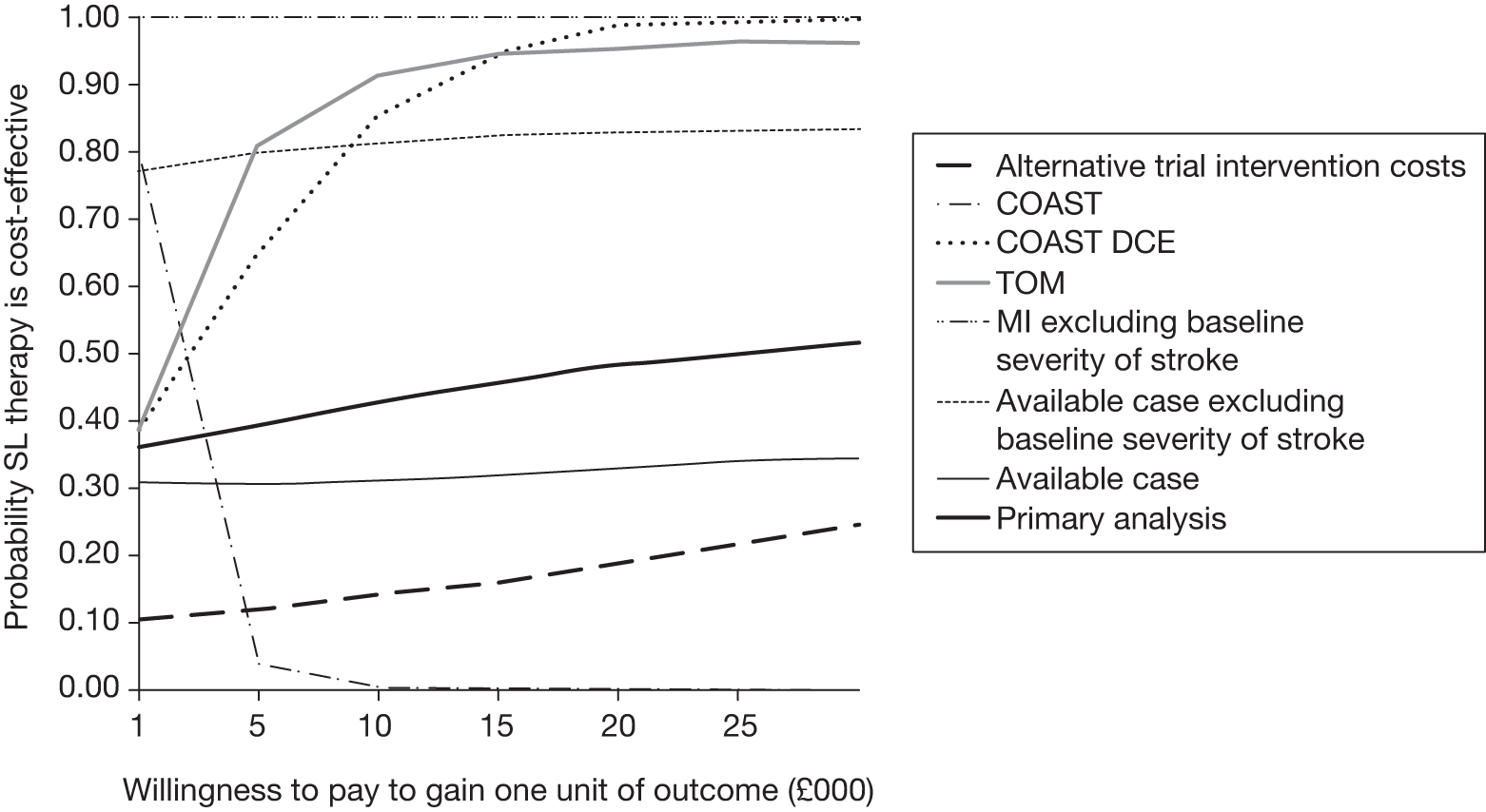
The analyses indicate that AC is more cost-effective than SL therapy at any WTPT < £25,000 for the following primary and sensitivity analyses:
-
primary analysis
-
analysis using the COAST measure of communication outcomes (adjusted for baseline severity of stroke)
-
analysis using available case rather than imputed data
-
analysis using the costs incurred in the trial for the trial SL therapy and AC interventions.
This suggests that the results may not be sensitive to the presence of missing data or the approach used to impute missing data, or the unit costs used to estimate the costs of the trial interventions.
However, the analyses indicate that SL therapy is more cost-effective than AC at any WTPT for the following sensitivity analyses:
-
analysis excluding severity of stroke at baseline as a covariate in the regression model
-
analysis using the TOM measure of communication outcomes measure (adjusted for baseline severity of stroke)
-
analysis using the five items from the COAST combined using the DCE weights (COAST DCE).
This suggests that the results are sensitive to the inclusion of severity of stroke at baseline as a covariate.
Summary
Overall, the bootstrapped analyses suggest that the SL therapy intervention is associated with an additional cost of £110 per person when compared with the AC group. This additional cost is associated with wide 95% percentiles, which indicate that the cost lies between a net saving of £640 and a net cost of £861. There is a slight net gain of 0.01 (95% percentiles –0.03 to 0.04) in utility for the intervention group compared with the AC group. However, the 95% percentiles for this net utility gain cross zero, suggesting that there may be a net loss or net gain in utility for SL therapy compared with the AC group. Also, it is not clear what value a net gain of 0.01 utility has for clinicians or patients. Neither is it clear whether or not such a small difference in utility is important for patient or clinician decisions.
The primary CEAA indicates that the SL therapy intervention is likely to be cost-effective only if decision-makers are willing to pay ≥ £25,000 to gain a one-point increase in utility (p = 0.50, net monetary benefit = £3). This is at the upper threshold of the acceptable WTPTs to gain one QALY implied by NICE decisions,57 where the QALY combines length of survival as well improvements in health. Decision- and policy-makers need to assess whether or not they would be willing to pay £25,000 for a small gain in utility, measured at one point in time. Decision-makers may not be willing to pay as much to gain one unit of utility as they would to gain one QALY. This is because the QALY combines survival and the utility of health over a period of time. In contrast, the utility measure used in this analysis reflects the value of a health state or ability to communicate at a single point in time.
The sensitivity analyses indicate that whether or not SL therapy is cost-effective depends on the outcome measure used and the baseline severity of stroke. The sensitivity analysis suggests that SL therapy is cost-effective when the following measures are used: utility, TOM measure of communication, the four COAST attributes weighted by the preference values derived from the DCE, or when severity of stroke is not used as a baseline covariate for the analysis. The sensitivity analysis also indicates that SL therapy is not cost-effective when the COAST measure of communication outcomes (adjusted for baseline severity of stroke) is used, when the analysis uses available case rather than imputed data, or when the actual costs incurred in the trial for the trial SL therapy and AC interventions are used, rather than national average unit costs. The uncertainty about the results, shown by the sensitivity analysis of different outcomes measures, reflects the fact that the different outcomes represent different dimensions of well-being. These can change in different directions or to different extents. This can mean that the broader the evaluation, the less clear the findings are unless the intervention is clearly superior on many of the different dimensions or attributes of health and well-being.
Therefore, the results of the primary analysis indicate that it is unclear whether SL therapy is more or less cost-effective than AC. The relative cost-effectiveness of SL therapy depends upon the amount a decision-maker is willing to pay to gain one unit of improvement in outcome, the measure used to assess outcome for the economic analysis and the severity and impact of the initial stroke of the patient.
Chapter 7 Qualitative study: methods and results
Introduction
This chapter presents the aims and methods of the qualitative study and describes the principal findings. Please note that in consultation with the ACT NoW research user group we chose to reproduce participants’ quotes verbatim in order to make visible the communication of people with aphasia and dysarthria, which is rarely represented in print.
Research aims
-
To explore participants’/carers’ experiences of SL therapy intervention or visitor support.
-
To evaluate from participants’/carers’ perspectives the effectiveness of SL therapy intervention or visitor support, in terms of both process and outcome.
-
To compare the perceived impact on participant and carer well-being of SL therapy intervention or visitor support.
Data from the qualitative study, although standing on their own right, were also designed to assist in the interpretation of the results of the trial and their potential implications.
Method
Sampling strategy and recruitment
All participants were drawn from the broader ACT NoW sample. At the time of data collection, June 2008 to mid-April 2009, there was only a potential pool of 36 participants who had completed their post-outcome assessment. All were approached. Participants were required to give a separate consent for involvement in the qualitative study. This was (1) to ensure that any concerns about having to be interviewed did not unduly influence recruitment to the main trial and (2) to distinguish the aims of the qualitative study from other aspects of the trial so that it would be clear what might be expected of those who agreed to be interviewed. Twenty-two participants consented out of a potential pool of 36. Of those who declined, six did not want further research involvement, two declined the qualitative study information, one participant was readmitted to hospital and five withdrew from the ACT NoW trial. Carers of the 22 participants in the qualitative study were separately approached to consent to a carer interview and could decline independently of the participant’s involvement. A carer was defined as a relative or friend identified by the participant as fulfilling a caring role. Ten participated and reasons for declining were: five carers lived too far away, two had had strokes themselves and did not want to take part and five gave no reason.
Participant and carer sample characteristics
The sample was drawn from all ACT NoW trial sites and included people with both good and poor communication outcomes as determined by the self-report COAST measure. 28,29 Data on participants’ socioeconomic status were unavailable, but participants mentioned both ‘blue-collar’ and ‘white-collar’ occupations in the course of participant–researcher interactions. There were 13 men and nine women in the sample, with a median age of 73 (range 53–98) years. Five had a diagnosis of dysarthria, 12 of aphasia and five of both aphasia and dysarthria. The median age of carers in the sample was 56 (range 38–77) years. Eight were women and two men. All but one were live-in carers, with 8 out of the 10 being the participant’s spouse. Seven were in full-time employment.
Data collection: interviewing approach and instrument design
Previous qualitative research has often excluded people with severe communication difficulties, or used proxies instead, based on the assumption that it would be impossible to gather data suitable for qualitative data analysis from this group. 64–67 Basic assumptions of qualitative interviewing such as an expectation of sustained narrative engagement, ability to express oneself in ‘own terms’, referential meaning and particularisation of context and experience are all potentially subverted by the communication challenges faced by those with aphasia/dysarthria. Also, communication strengths might lie in modalities other than the fluent, spoken word, which is generally expected for qualitative data. The study took up the challenge of seeking to include within a qualitative interview process those with severe communication difficulties, as well as those with less impairment, by means of interviewer training, the design of the interview itself and the approach to analysis.
The interviewer was provided with training in how to communicate effectively with people with aphasia/dysarthria from SL therapists. The use of supported communication techniques68 and attention to meta-communication strategies were emphasised. 69 The ACT NoW research user group also supplied training through means of role play and feedback on mock interviews in which they both participated and critically observed.
An interview approach was developed, which was at once highly structured (to enable participation from people with more severe impairment) yet highly flexible (to accommodate the variety of communication strengths and weaknesses and to allow participants to introduce their own ideas). It was based around a pictorial interview schedule, which was structured in three parts:
-
what happened in the sessions
-
what participants felt was good/difficult about the sessions
-
evaluation of overall impact.
These corresponded to the three levels of perception with which we wished all participants to engage: description, appraisal and evaluation.
Part 1 consisted of pictorial representations of nine potential activities engaged in with the SL therapist or visitor. These could be ignored by those participants able to extemporise on the content of sessions, used as a structured means of reference to different potential answers that could then be elaborated on, used as an aide-memoire should participants experience memory difficulties as their answer progressed, or used as a means of pointing to assist non-verbal communication. Any and all uses were encouraged.
In part 2, participants were asked ‘what were the good things?’, ‘what was difficult?’ and ‘what did you want more of?’. For those for whom sustaining conversation about these issues in the abstract was a challenge, prompt cards were available, which could either stand in for an answer or be used as a communication ramp to support an answer if full expression was proving difficult. For the most challenged, a picture was available as a consistent referent for a gesture or other means of non-verbal communication.
In part 3, a visual analogue scale was made available to indicate evaluation of overall impact, which could be elaborated upon by those able to communicate in that way but would stand as a common denominator for all regardless of any extended communication.
All interviews were both audio- and video-recorded to capture the holistic nature of a participant’s communication strategies.
Carers were viewed as an important but distinct part of the rehabilitation process and as people who experience different yet related challenges from those with aphasia and dysarthria. The semistructured carer interview was designed to explore how carers perceived the SL therapist/visitor, and the support he or she provided; views on the friend’s/relative’s communication improvements since participation; and the impact on the carer’s life of participation in either the SL therapy intervention or visitor support. These interviews were audio-recorded and transcribed verbatim for purposes of analysis.
Data management
The data generated varied considerably in terms of its style of expression, elaboration, intelligibility and medium. With conventionally generated spoken language data the first stage of the analysis process is usually a written transcription with a range of additional markers to preserve features of the expression that would not be immediately perceived from the text (such as irony). The vast majority of the interview data were amenable to this form of data processing. In addition, gestural communication that clearly supported spoken meaning was relatively straightforward to mark within a transcribed text, for example if a gesture replaced an adjectival expression that could not be found by the participant. However, in around 10% of interviews there was so little spoken language expression and/or non-verbal communication that the context and intent was not understood with a high degree of certainty. Therefore, a conventional form of transcription was not possible. In between these two extremes, in around 10% of interviews some content was ambiguous to the researcher.
Consequently, a data transformation protocol was developed to manage data that could not be conventionally transcribed. It was guided by three principles: (1) a respect for participants’ efforts to ensure that their opinions were recorded, by whatever media of communication they could use; (2) a concern not to over-interpret data whose the meaning was not clear; and (3) to develop a process that had the potential to address the three levels of meaning sought in the data collection, i.e. what happened, participants’ perceptions of what occurred and participants’ views of the impact of what occurred. The data transformation involved the re-presentation of data in a prose form amenable to conventional data processing. We were not concerned with the capturing of meaning at the level of narrative, i.e. how and why someone expresses themselves as a legitimate focus of analysis, therefore transformed data were not rendered in the first person. We sought instead to summarise semantic intent (in third person), where discernible, for example, ‘he indicated positive feelings about the visits’ rather than ‘I felt positive about those visits’.
The protocol for data transformation
-
All tapes were watched and conventional verbatim transcription was applied when there was clarity – this included the marking in written form of any gestural communication where meaning was straightforward.
-
All tapes were re-watched and where there were gaps in the verbatim text transcription the researcher added notes using the NVivo 2.0 (QSR International, Melbourne, Australia) tool ‘data-bites’. These notes addressed the possible meaning of the data and degree of certainty of interpretation.
-
Where the researcher was less confident about the interpretations, an experienced SL therapist watched the relevant sections of video and independently interpreted the meaning. If there was disagreement, the SL therapist and researcher discussed their interpretations until a collaborative meaning was reached. Where agreement was still not possible, the data section was not used.
-
A new document was created in NVivo 2.0 consisting of a prose summary made up of content statements derived from the data-bite notes. A doc-link was then created between the re-presentation and the original transcript to insert the prose in the appropriate section of the original interview.
Analysis
Raw data (interview transcripts and transformed interview summaries) were entered into the NVivo 2.0 software package and read iteratively by the researcher who had carried out the data collection interviews. A thematic analysis approach was taken, initially using an open coding approach in which the codes were informed by the three levels of consumer perceptions outlined in the interview; the overall study aims; and openness to unexpected themes in the data. Initially 40 codes were generated, which eventually became nine thematic categories collapsed into seven categories. The nine thematic categories and their working definitions are available in Appendix 15. Separate codes were developed for carers; however, there were many points of overlap with the study participants.
Presentation of findings
Introduction
Data analysis has allowed us to show the points of intersection and difference in how participants experienced both arms of the trial. It has also enabled us to demonstrate the priorities of the participants, regardless of their intervention experiences. In what follows we will concentrate mainly on the findings from the trial participants but connections will be made with data generated by the carer interviews. Seven out of the nine thematic categories are presented; two were collapsed into others in the final analysis.
Emotional well-being
A recurring theme for all participants, regardless of SL therapist or visitor experience, was the impact of those experiences on their emotional well-being. The affective impact of stroke is well documented, including emotional lability and depression as well as the secondary consequences for the individual of adjusting to differences in communication, understanding and physical abilities. As one participant described the consequences of his stroke:
Because I, I this article in The Times to me, this fellow felt he was trapped in his brain and I now understand, I now understand having had the feedback from what people heard I was saying, and what I thought I was saying, and obviously to get what, what really badly affected people, is literally being locked, up there, unable to communicate. And you suddenly think, um, y’know it’s just horrible and I feel so lucky, that I’ve been able to er … come out of the cell to which I had been put, temporarily. Unfortunately for some people, they’re still in their cells and I feel very sorry for them, because, it must be terrible, thinking but not actually communicating, or communicative as in a way that people actually understand.
(SL therapy arm)
The impact therefore on mood of encounters with visitor or SL therapist became a central concern in the perceived effectiveness of that contact. Participants drew attention to the importance of knowing that a friendly and supportive person was there for them, particularly when they were feeling ‘low’:
Well, I knew there’d be someone there when I was on, when I was very down, I knew she was there.
(SL therapy arm)
This effect was clearly observable in both arms of the trial. For carers too, the mood-lifting effects of regular contact with someone, be they a visitor or a SL therapist, were valued:
… on the actual, the specifics of what she was trying to do I don’t know I wouldn’t be able to say … I think that worked or it didn’t but the most important thing for me was what it did for his mood … whenever she’d been to see him, he was always on an upbeat, always on a real like upswing, um, and I know he used to look forward to the visits.
(Son, visitor arm)
In generating these positive effects, the professional identity or role remit of the individual was of far less importance than their personal qualities including:
-
ability to put the participant at ease
-
ability to make the participant feel individually of importance
-
the visitor/SL therapist displaying a positive mood themselves (being ‘jolly’, ‘a right infectious laugh’)
-
being empathic
-
a good communicator.
Interpersonal qualities such as these were not the only driver for a positive effect on well-being. Some participants explained how having contact with the SL therapist or visitor could distract them from the day-to-day emotional difficulties of living with the consequences of stroke by giving them something new and enjoyable to focus on:
Um … was it useful? It was useful in the fact that it took your mind off what was going on. Um … if you’ve had a stroke, which is a dreadful thing to have it’s on your mind the whole time and I thought well, at least I’m doing that, I’m starting to have these conversations. Which I did … it stopped me thinking, it put me in someone else’s spot for an hour, didn’t it? I stopped thinking about number one and started thinking about somebody else … It’s very easy to get involved in your own little thing isn’t it? where he would tell me different things. Yeah, I think it was good.
(Visitor arm)
Another aspect of emotional well-being concerned learning to cope with how one was feeling and finding strategies to deal with changes in mood. Visits from the SL therapist or visitor were also helpful in this respect. This participant describes the importance of learning to deal with her emotional responses in the course of doing ordinary things with the visitor, which, in her view, acted as a preparation for being able to tackle a more structured approach to therapy later.
… I said that was nice y’know, er we have to laugh y’know and yeah (nods) no we did things, allsorts … Oh yeah she used to, yeah, yeah, yeah come come here, she knows I’m a bit (clenches fists) frustrated y’know but I’m not there for long because she’ll say ‘well we’ll do this now c’mon y’know have a drink and we’ll start’, y’know she’s right, yeah she just, came with me y’know looked after and, yeah yes it was goo it was a good idea was that def- y’know whoever comes, y’know it was great that sort of thing but not ash- actually las- but next week where I’m starting now wi’ this I’ll be (points to mouth with both index fingers) doing more of that sort of thing not (touches top of chest with both sets of fingers, fingers slightly curled) aren’t I, trying a bit of, yeah now to what I did with, but, I wouldn’t’ve be, I wasn’t ready for that sort of thing ee in y’know, it, y’know we had to (holds both hands out, palms facing down and fingers extended, moves hands downwards) calm it, I couldn’t cope with that.
(Visitor arm)
Confidence
Participants identified the importance of their experiences of visitor or SL therapist for their confidence. Many discussed the devastating effects of their stroke on their personal confidence, whether that meant their perceived abilities to carry out tasks, social engagement or just their sense of self-worth. Carers too recognised the central importance of confidence and how contact with a visitor or a SL therapist could boost damaged confidence:
I think first of all, just the mere fact that um, he was getting all this help was really good for his confidence and that was so important I mean it was partly the fact that I think I, it led him to think that … he was going to get better do you know what I mean there was definitely a psychological effect …
(Wife, SL therapy arm)
Although participants in both arms of the trial talked about the positive effects of the contact they had received on their confidence, there were differences in how the processes associated with confidence enhancement were discussed, depending on whether the individual had experience of a visitor or a SL therapist. Those with an experience of the visitor tended to talk about how the normalising effects of regular contact with a stranger boosted their confidence. For example, they had to engage in everyday social interactions and keep the conversation going. They had to face their concerns about communicating with someone who did not know them well. They had to practise everyday tasks like getting up to answer the door or making a cup of tea, which they might no longer feel confident to do outside the immediate circle of family and friends.
Did it help me? Um … yes I suppose it did really because it was someone coming to my house that I didn’t know and actually speaking to people that you don’t know is sometimes a bit … (grits teeth and rocks head from side to side) sometimes I can go in a shop, know what I want and I can’t say it. It’s like somebody knocking at your door that you don’t know but you have to say ‘hi, I’m (name) and I’m …’ y’know … I would say it did me good, yeah.
(Visitor arm)
There was, however, an important caveat. Such perceived benefits were only possible because of how skilled interpersonally participants felt the visitors were. Again, it was not contact per se, but contact with a person who had particular qualities that meant such benefits as improvement in confidence could be realised.
Those participants who had the SL therapy tended to view improvements in confidence as direct consequence of specific tasks and explicit agreed courses of action, rather than indirect benefits resulting from the encounter.
Well it’s given me confidence yeah, by giving little exercises I’ve gone to the veg shop, I’ve gone to [indecipherable word], going out talking to people on the street for the first time in months. It just gave me the confidence to speak to people again outside.
(SL therapy arm)
The same participant explained that he was a keen cook, so the tasks were made relevant to him through a goal-setting strategy that reinforced an ability that had been important to him before his stroke. Other examples concerned the giving of specific strategies that could be deployed in conversations should communication become difficult:
Very um … helpful she’d point out where you were going wrong and, and finding you … how to get it right … just build your confidence up so where, where you think ‘oh, I can’t do that word’, just, just try a different way or … work out what you could say instead, take out words you couldn’t say y’know so y’know like when they say, oh, I use three words instead of one it’s because you can’t do the one (laughs) so use three, it’s easier.
(SL therapy arm)
Observing progress
The third issue that participants emphasised regardless of whether they had visitor or SL therapist experience concerned the importance to them of being able to observe progress. In some respects the extent of improvement was less important than a sense of moving forward, rather than feeling stuck.
He’s done a good job, I was talking to everybody and I don’t know, maybe I’m going back, but everybody says, me sister says ‘you can talk a lot better, I can understand you now’.
(Visitor arm)
It doesn’t seem that much but it is a big thing doing things like that for you and one of the girls on the… me, meat thing [in the supermarket], she were good ’cause I just had to point to what I wanted, but I saw her last week, she said ‘ooh, yes, we know what you can say now can’t you?’ Y’know.
(SL therapy arm)
For many participants there was an acknowledgement of spontaneous recovery or improvement, for example in speech or mobility. Consequently, the extent to which the visitor or SL therapist contact was seen to be a contributory factor also varied. Nonetheless, the sense of being able to observe progress was of over-riding importance. How this was perceived to be achieved was different depending on whether the participant had the experience of the visitor or the SL therapist.
Those with SL therapy experience described how the SL therapist might deliberately point out their areas of weakness or skills they needed to develop/re-learn in a targeted way. It was then possible to learn new strategies for overcoming these difficulties, in some cases through specific techniques, described by one participant as ‘embedding something in my thinking’. Before and after measures of how well they were doing were also built in.
How do I put it? You think you’re better than you actually are until you actually test yourself, and what they will provide you is the test function then say, those aren’t working, why? How best to … help you build up your … and they did it very very well for me they did an exceptional job and er … they gave me that incentive to work at it, which, if they hadn’t been there, I would’ve imagined I was better than I actually was, and the … test with the newspaper for example I thought sorted that, in reality, I hadn’t the brain was making the, was reading a few words, the word it didn’t know, ’cause it readily understood the word that came after in the general context, my brain was explaining ‘this is what the article’s about’ but it wasn’t actually reading, wasn’t understanding the word and so … and then they went through that and I started realising how many words I was some of them I knew, and couldn’t say, I couldn’t pronounce them, but then with help and practice, that reinforced that.
(SL therapy arm)
Sometimes the extent of the deliberate strategy of the SL therapist was only appreciated with hindsight. For carers too, being able tangibly to recognise progress and make comparisons between then and now was identified as vitally important:
I think on the final one where they actually took a recording of his voice and played it back and they give him the recording from the beginning and played it back and me husband turned round and said ‘who’s that?!’ So they said (laughing) ‘well that’s actually you’ [and] he says ‘Now I can understand what you were turning round and saying, you were getting frustrated, you were crying, you were screaming and shouting it caused a lot of arguments at the beginning’.
(Carer, SL therapy arm)
For those with experience of the visitor, the emphasis was much more on self-perceived differences and reflections, rather than specific tests of functional improvements. The simple fact of having to communicate socially in a sustained way with the same person was seen as a good basis for judgements of improvement. For some people, the fact that the conversation partner was not someone who knew them well was important because they had to make additional efforts to understand and be understood. By contrast, close family and friends might assume understanding through familiarity, leaving little incentive to make communication improvements. Observations of progress required critical enough feedback to know whether progress was real or not. Some participants described checking out with the visitor whether the positive feedback on progress received from their family was correct or not. Others described how the visitor had encouraged them to try things again, which their family were dissuading them to do out of protectiveness. Without contact with an outsider, some participants doubted they would have pushed themselves in the same way and seen the progress they could make in these additional domains.
Like I said before it’s not the same for somebody coming from outside is it y’know y’know when friends say ‘oh you shouldn’t do this or that’ but, they, ’cause (the visitor) come here … they know what to do y’know. That were er, that were good, but I don’t think I’d’ve gone, no, I couldn’t’ve done, well I don’t know, no I couldn’t, because I felt so … down I didn’t know what to do they helped me through all of ‘em’.
(Visitor arm)
For some participants who lived alone or had very limited contact with family and friends, something as basic as an assured, regular social encounter was a prerequisite for testing out whether or not they were getting better. Without it they might not talk regularly with anyone.
Guidance and support
Unsurprisingly, participants gave very different descriptions of the activities they had done with the SL therapist and visitor and how they perceived the nature of the guidance and support they were offered. The training of the visitors clearly emphasised that they should not engage in strategies of deliberate therapeutic activities. The fact that the participants did not perceive them to be doing so is important for evidencing the fidelity of the contact in terms of the parameters of AC within the overall trial design. However, beyond this observation, how participants described the effectiveness of the support of visitors and SL therapists gives another window onto what it might be about early and sustained contact that is important to them.
Speech and language therapy support was highly valued for the perceived professional expertise it brought to an individual’s situation. Participants expressed this in terms of being given ‘knowledge’ and strategies to overcome specific difficulties that had been jointly identified.
Interviewer: OK so the second one, how much did therapy help with your communication? Again this is from the lowest to the most possible.
Participant (points to top score): Top one.
Interviewer: OK, can you tell me a bit about why you’ve chosen that one?
Participant: Because they were, they were telling me things that I didn’t really know, how, how things work, that’s it … ’Cause when [SL therapist] told me when I went to the shop, she said to me [indecipherable speech, approx. 1 second] ‘go to the shop and ask for something, anything, think about what you’re saying.’ And she told me I had to slow down (laughs).
(SL therapy arm)
There was also a strong perception of purposefulness in how participants described the support from SL therapists, with multiple references to ‘building blocks’ and the importance of focusing on difficulties and ‘gaps’ that were relevant to the individual’s personal and social context.
In contrast, visitors tended not to approach specific difficulties in such a structured way. In the following example it is the visitor’s sensitivity that is valued, giving the participant a positive experience of overcoming a difficulty:
Interviewer: OK, so um … was there anything you did in the visits that you think helped you um … to get out of this verbal cul-de-sac?
Participant: Inasmuch that um … the, when I did she was patient and waited, which was much better than suggesting words, er in my view, because it’s a very facile thing to start prompting, er… it doesn’t work with me maybe it works with others but er, she didn’t prompt, she just waited until I collected my thoughts and continued the thread of the er … discussion.
(Visitor arm)
When later the interviewer mistakenly suggests that the visitor might have helped the participant identify specific difficulties, the participant refutes the suggestion and suggests that the visitor can lead to a different kind of knowledge about one’s problems than that of directly knowing where specific difficulties lie, and how best to negotiate these:
Interviewer: I’m interested in something you said in there, was it to … pinpoint what was going wrong did you say?
Participant: That was not, in a sense, part of the um, situation with the visitor, um … I wasn’t looking for pinpointing. Pinpointing might happen, but I didn’t recognise it, um, that was not what I was going for. I was taking and assuming and working towards um, um, a conversation er more than anything else. Not even a totally an exchange of views, but giving me an opportunity to babble away and er, that’s quite therapeutic in itself er you become aware of the difficulties that you have.
(Visitor arm)
There was one feature that was unique to descriptions of the visitor experience, namely what the encounter with the visitor enabled the participants to give to the visitor in terms of social interaction. There were numerous examples of when participants described how they had offered their own knowledge to the visitor in connection with a topic and identified a therapeutic effect in offering their own knowledge in social interaction with the visitor.
I gave her the name of one or two greenfly sprays that I found useful. Now it could well have been that she knew all that, but she accepted it in the sense that it was new to her, so it made me feel as though I was achieving something imparting information.
(Visitor arm)
We did not observe data like this in the SL therapy arm, which, perhaps, suggests greater perceived reciprocity within the visitor arm. Conversely, the recognition of a more systematised and structured approach to functional improvements was apparent only in the SL therapy arm.
Meeting individual needs
Underpinning participants’ various descriptions of what the SL therapy or visitor had done and their perceptions of its value was an additional marker of effectiveness – the extent to which a participant felt that his or her individual needs were being recognised and met. The meeting of individual needs encompassed recognition not just of the complexity and range of severity with which aphasia and dysarthria might be manifest, but also of the individual’s psychosocial context. Whether or not the structured intervention of the SL therapist or the informal discourse of the visitor, perceiving that it had been made relevant to the individual and their circumstances was a key marker of effectiveness from both carers’ and participants’ point of view.
I’m … football fanatic so most of the things she got me to read and do was over football and that’s where … the letter ‘M’ came into it. I found I struggled saying [inaudible]. As soon as you’d made the vowel sounds and the normal sounds, [Manchester] United, she did football teams to make it interesting for me. She’d pick my interests out and put it into a way of teaching me that I enjoyed. I think that’s why I enjoyed the speech therapy so much.
(SL therapy arm)
Well she liked baking cakes and ’cause y’know, that was something that she needed er … she had to work at so she had to learn how to weigh things and … all the y’know the ins and outs whereas the other parts were just straight reading or y’know nothing, she didn’t have to think whereas on this, on the baking she did have to think and concentrate for er … a fair amount of time really y’know against nothing at all really, two or three minutes whereas baking was … half an hour to an hour.
(Carer, visitor arm)
On the rare occasion when a participant expressed dissatisfaction with the contact he or she had received, failure to recognise individual need was one component of the problem.
From carers’ accounts a further aspect of meeting individual needs also emerged, namely the flexibility displayed by both SL therapists and visitors in ensuring that their contact did not disrupt anything else that might be of importance to the participant at the time:
But if it was Tuesday they did it or Thursday or whatever, and (the Visitor) was very … er cooperative in … if we had to change for whatever reason so y’know like er, have to go to a doctor’s appointment occasionally or something like that y’know and er … she changed it so very adaptable in that er … in that respect … we didn’t er sacrifice anything.
(Carer, visitor arm)
Amount and intensity
A distinguishing feature of the ACT NoW trial was the intensive, as well as early, nature of SL therapy (and therefore of contact with a visitor too). Participants were aware that they were receiving a very different experience of contact than might be the case if they were not in the trial. Discussions about the amount and the intensity of that contact were therefore of considerable interest.
Participants valued a high amount of contact, whether that be with SL therapists or visitors. High amount of contact was defined by frequency, number and length of visits and/or amount of time spent with them. Furthermore, the amount of support was perceived to be closely connected with the benefit. More contact felt like more benefit in quite a straightforward equation for the majority of participants. In this example, a participant discusses the perceived relationship between amount of support and impact on facilitating a return to work, with an awareness of the enhanced amount of support he had received because of involvement with ACT NoW:
And what surprises me is … from what I understand is that if I hadn’t been on the scheme, I wouldn’t’ve not had anything level, I would not have had anything like the level of, support, and I think for people who like me aren’t as … I thought might be affected ’cause I’m not as badly affected as a lot of people there’s a lot that the speech therapists can do to return, people like me into, back into any working environment, in a way that also boosts my confidence that things are getting better somehow which helps and reinforces the improvements that you need to, you need to have things ha evolving and changing, make you think you are making progress and I think that’s something that for me in my situation, I thought was extremely beneficial and without it I wouldn’t be as well advanced as I am now and I certainly wouldn’t’ve started back at work which I think from the po for my personal … well-being, my return to usefulness and the loss of my skills to the company would’ve been totally lost.
(SL therapy arm)
Amount of contact was not the only issue. Some participants also discussed the importance of quantity of contact being tempered with a sensitivity to meeting the particular needs that participants were experiencing at any given time. Part of this sensitivity was about flexibility and awareness of how easy it might be to feel overloaded, which could undermine the benefits of a large amount of contact. This was true both among those who had SL therapist contact and among those who had visitor contact.
Another aspect of amount and intensity concerned what happened between contacts with their SL therapist or visitor. Many of those with SL therapy experience were, as part of their therapy, given ‘homework’ between sessions with perhaps inevitable differences of opinion about whether or not this was helpful. For some it contributed to a feeling of overload. For others it was a vital component of that sense of a tempered approach to their needs:
I did a lot (laughs) [of homework] because they told me do it twice a day, I’d do it three or four times a day (laughs) but I’d nothing else to do, y’know you stay in the house for six months, what can I do? Ah! I’ll get to go through these (laughs) it … passed the time it helped.
(SL therapy arm)
Those who had experience of the visitor were not left ‘homework’ in any structured or deliberate way. That lay outside the protocol of what visitors should be doing with participants. Nonetheless a few talked about creating their own homework and so self-regulating the intensity of the consequences of their contact:
‘(laughs) Um, it’s like anything else, you go round the house and … I used to talk to the radio, I used to talk to everything, everybody in the house I spoke to. The more I spoke, the better I got.
(Visitor arm)
Closure
Participants were specifically asked about their experiences of the end of therapy/contact with the visitor and frequently reflected on this aspect in some depth. The ACT NoW protocol withdrew support, whether from a SL therapist or visitor, after a 4-month period. How participants regarded the termination of well-resourced SL therapy or visitor contact was therefore of interest.
A small number of participants in both arms of the trial saw the end of the intervention as premature. This perception seemed to tie in with where on the journey to recovery participants perceived themselves to be; they saw therapy/contact with the visitor as unfinished work and 4 months as too brief a contact. The impact of termination for many participants was experienced emotionally, entailing a sense of loss or bereavement. While some saw this as inevitable, others did not and regretted they could not continue the regular contact. For a minority the end of contact was experienced as relief rather than a source of regret because the intensity or the work involved with visitor or SL therapist was perceived as too difficult to sustain. For a few, despite how difficult it felt, the termination of contact was seen as a positive marker of progress:
Yeah, I really thought ‘oh, that’s, y’know, good. I’m on my way back now’ … I had come to the end and I thought ‘yes, that’s exactly what I want to do, I want to move on’ and I certainly did move on y’know I didn’t … once they’d gone I didn’t kind’ve sit back and think ‘I can’t do anything else now, there’s nobody coming’, I didn’t feel like that yeah, got dressed up and went out …
(Visitor arm)
Summary
-
For all participants regardless of whether they had SL therapist or visitor contact, there were three key priorities that emerged as markers of effectiveness from their perspective: emotional well-being, impact on confidence and the extent to which contact enabled observation and review of personal progress.
-
Certain personal qualities of the individual SL therapists or visitors were prerequisites for effective engagement and realisation of these priorities. These included being able to put the participant at ease; the ability to make the participant feel individually of importance; the visitor/SL therapist displaying a positive mood themselves; being empathetic; and being a good communicator.
-
Those with experience of SL therapy identified explicit strategies that helped them build confidence. Those with visitor experience identified indirect benefits more grounded in the everyday ‘practice’ of social engagement that visitor contact required.
-
The input of ‘outsiders’, whether SL therapists or visitors, was highly valued in providing opportunities to mark and observe personal progress in recovery. For those with SL therapist contact this was facilitated through targeted and structured work. For those with visitor contact, self-observations of progress occurred as resultant effects of interaction on which participants reflected and through the opportunity to discuss others’ (e.g. family’s) observations of progress with an outsider.
-
Guidance and support from SL therapists included explicitly pointing out problems, specific targeting of areas of weakness and the formulation of strategies and gaining of new knowledge to overcome these. Guidance and support from visitors was less deliberate. Participants’ growing awareness of difficulties was an indirect effect of interaction.
-
An important dimension of perceptions of effectiveness involved considerations about the extent to which the SL therapist or visitor had met the individual needs of participants and whether contact and activities with SL therapist or visitor were made relevant to participants’ past interests/current priorities.
-
Amount of contact was directly equated to amount of perceived benefit. More intense levels of contact as a result of ACT NoW involvement were positively appreciated. It was important to participants and carers to temper the amount of contact with flexibility and to remain sensitive to feelings of overload.
-
There were no significant ways in which participants’ descriptions of the termination of contact could be associated with whether they had visitor or SL therapist experience. Variations in response were more a result of individual attitude.
Chapter 8 Discussion
Key findings
Feasibility study
Prior to commencing the main trial, a feasibility study was conducted. This proved an extremely valuable learning experience and led to several methodological developments and the production and validation of a range of materials for the RCT and qualitative study, such as aphasia-friendly consent materials* and the qualitative research techniques described in Chapter 7. Achievements included publication of new patient-centred measures of functional communication, the COAST28 and the Carer COAST. 27 In addition to their use in this trial as secondary outcome measures they are being used by clinicians outside the north-west of England in their clinical practice with adults. Clinical academics from several other countries have begun translating them into other languages and reapplying them into practice, and interest has been expressed for modifying them for use with paediatric and adolescent populations with acquired neurological conditions. (*Readers can view and download the written recruitment materials from ‘www.psych-sci.manchester.ac.uk/actnow/patients/needtoknow/’.)
Of particular significance was the feasibility study finding that the AC could not be provided by volunteers, leading to a change in the design, duration and costing of the main trial whereby part-time non-therapist staff (ACT NoW visitors) were employed instead of volunteers. A strong research collaboration was also established with two key stakeholder groups, members of the RUG (service users and carers who maintained long-term involvement in the ACT NoW study through to the final dissemination) and NHS SL therapists. The latter produced a manualised Best Practice Guideline for screening and intervention, suitable for early post stroke, standardised for consistency among the 12 participating clinical sites yet flexible enough for individualised patient care using therapists’ discretion, and a short summary document (see Appendix 1).
Delivery of the intervention and control
Delivery of the SL therapy and AC began as intended during the early, postacute phase, around 16 days after admission to hospital with stroke. Despite difficulties funding and staffing the extra SL therapy time needed, the ACT NoW SL therapy was provided at an intensity likely to effect meaningful recovery, an average of 22 contacts, with a total duration of 18 hours, over 13 weeks. As intended, the amount delivered covered a wide range, from 3 to 43 contacts, suggesting individual tailoring to patient’s needs and readiness. Just over half of the contacts were delivered in the community, almost always at the patient’s place of residence. Many people (65%) allocated to SL therapy saw no more than two therapists over the duration, a factor considered important for continuity of care. Qualified SL therapists (Band 5 or above) delivered 99% of the contacts.
Exploration of the contents of contacts showed that around half of the activities coded during contacts with therapists were ‘direct intervention’, almost always including impairment-focused therapy. Prospective monitoring by a seconded therapist, regular peer support meetings and review of the therapy data confirmed that minimum standards were met as intended.
Delivery of the ACT NoW AC by employed visitors was offered at a similar intensity, although with slightly less uptake (19 contacts, 15 hours), reflecting patient choice. However, this uptake rate remains high, reflecting the positive value users placed on visits at this early phase of their adjustment to stroke and ensuring that methodological requirements for an adequate amount of AC were met. Although more control subjects refused their allocation and requested NHS SL therapy, the actual amount provided averaged out to be quite low (3 hours) and per-protocol analyses (see below) suggested that it had negligible impact on outcomes.
Primary outcome measure
Patients with aphasia or dysarthria after stroke who received an early, well-resourced but individually tailored Best Practice SL therapy intervention demonstrated similar levels of functional communication ability at 6 months to those who received a similar amount of visiting from a non-therapist employed to provide an AC consisting largely of informal conversation but no specific communication training. Mean scores on the TOM activity-level scale at 6 months were comparable in the SL therapy (3.3) and control (3.0) groups. Planned primary analysis, adjusting for recruiting site, communication diagnosis and severity of baseline communication impairments estimated a difference of 0.25 (95% CI –0.19 to 0.69) in favour of SL therapy. This includes the possibility of the 0.5 clinically meaningful difference that the study was designed to detect.
However, thorough sensitivity analyses (excluding deaths, adjusting for baseline differences at the activity/disability level, per protocol, and per protocol excluding deaths) suggested that this difference was because of the imbalance in baseline severity and the imputation of values for deaths. Per-protocol analyses were conducted to explore and subsequently reject a possible dilution of therapy from the control participants mentioned above who rejected their allocation at some point and received some NHS SL therapy. These findings are robust and exclude the possibility of a clinically significant difference in either direction.
An observational comparison of TOM activity-level measures taken by recruiting NHS SL therapists at baseline and blinded research SL therapists at 6 months suggests a clinically meaningful level of improvement in functional communication of 0.8 points (95% CI 0.6 to 1.0) regardless of group allocation. To give clinical meaning to these numbers, this took someone from typically ‘limited communication, relies on cues and context to make basic needs understood’ to ‘communicates beyond here and now, consistently able to make needs known’. One possibility that cannot be excluded in the absence of a no-contact control group is that all improvement was because of spontaneous recovery (either biological or environmental or a combination of the two). Although this is theoretically possible, the more clinically plausible explanation for improvement of this size seems to be that both groups benefited from the early, well-resourced contacts regardless of whether or not it was with a therapist or visitor. Our data on the high uptake of these contacts and positive user perceptions support the suggestion that repeated practice of functional communication and the opportunity to develop awareness of difficulties and observe progress is at least a contributory factor and probably fills a known gap in current NHS practice. The rehabilitation technique of repeated task practice showed great promise in a recent high-quality systematic review. 70
Secondary outcome measures
Patients’ and carers’ perceptions of patients’ recovery
Patient-reported outcomes (overall functional communication, and subscales including communication-related quality of life) assessed on the COAST at 6 months were similar in the SL therapy and control groups, for example overall difference –1 (95% CI –7 to 6). Carers’ perceptions of their relatives’ functional communication showed no difference overall 0 (95% CI –7 to 7), consistent with the patients’ own reports. Although there were missing data, many participants (and slightly more carers) were able to complete these measures, which were developed specifically for this trial, validated and published as part of the feasibility study.
Outcomes for carers
Most, but not all, participants had an identified informal carer and the study found that SL therapists, but not visitors, almost always engaged carers (96%). The most common method of carer working was direct involvement in therapy planning or delivery. In contrast, conversation partner training methods were appropriate for only 1 in 10 cases, most likely because of the earliness of this intervention. Outcomes measuring carers’ own well-being and quality of life were similar for SL therapy and control groups on the relevant subscale of the Carer COAST and on the three COPE subscales: negative impact, positive impact and quality of support (all CIs included zero).
The lack of effect on carers is surprising given that only those in the therapy group received any support. However, as SL therapy itself in the first 4 months after stroke gave no added benefit over and above visiting, perhaps involving carers in SL therapy is not useful at this early stage. That is not to say that excluding carers is advisable. On the contrary, accurate interpretation of all these findings must bear in mind that this was not a ‘no-contact’ comparison. Although AC was not directly intended for carers it may have had indirect benefits, for example from seeing how positively their family members responded to visits. In addition, it may have provided opportunity for informal respite as, once the visitor and participant had developed a good rapport, carers often took the opportunity to leave them alone and take time for themselves.
Serious adverse events
Overall, 12 of the 170 randomised participants died, six survived further strokes and four others required extended or repeat hospitalisation. There was no statistically significant difference in either overall SAEs or specifically in death rates between the groups, although numbers of each were higher in the control group and the study had low power to detect such differences. Given that the control group had higher levels of disability at baseline (although similar impairment) and the finding of similarities between the groups in all outcome measures for patients and carers it would be challenging to hypothesise a mechanism for increased adverse events in either group.
Subgroup analyses
The participants for whom we achieved follow-up on the primary outcomes had aphasia (136), dysarthria (60) or sometimes both (29% of those randomised). Although the ACT NoW study was a pragmatic evaluation of the communication problems typically experienced early after stroke, Cochrane reviews in this area have been impairment specific and so we conducted planned subgroup analyses. For aphasia, the between-group difference on the primary outcome was 0.20 (95% CI –0.28 to 0.69). Similarly, for dysarthria, there was no suggestion of a differential group effect (0.07), with wider CIs (95% CI –0.69 to 0.83) from the reduced numbers in the analysis.
Further subgroup analyses by severity found no differential group effect, providing no evidence of added benefit from SL therapy compared with AC, regardless of severity of baseline communication impairment. It should be noted that the study was not powered to detect differential subgroup effects.
The data on the actual SL therapy delivered suggest possible patterns specific to severity, although conclusions based on diagnosis should be made cautiously as the data in Chapter 4 are not presented in mutually exclusive categories. We chose not to as it is more meaningful to clinical service delivery to report subgroups with any aphasia (i.e. even if they also have dysarthria) or any dysarthria rather than the less clinically typical presentations of only aphasia or only dysarthria. Although only half of the SL therapy arm received a communication aid, this was more likely for those with severe (65%) rather than mild/moderate (45%) communication problems at baseline (aphasia 57%, dysarthria 39%). Those with severe communication impairment at baseline were less likely to have goals set; however, this was still accomplished for the vast majority (86%). There were no striking subgroup differences or patterns in the type of direct contact carried out. The severely impaired subgroup did slightly more functional (70%) and conversation work (65%) and less goal setting (81%) than the mild/moderate group. Conversation partner training was rarely appropriate, probably because of the earliness of this research intervention, but was possibly carried out more often with carers of people with aphasia (13%) than with carers of those with dysarthria (7%), with the caveat above about overlapping categories.
Preferences for communication outcomes and waiting time
Overall, 213 out of 278 (77%) people completed all of the choice questions and 40 out of 278 (14%) people answered one or more of the choice questions. The analysis indicated that all of the attributes had statistically significant coefficients for each level, which suggests that all the outcome and waiting time attributes were important contributors to the preferences of participants. The direction of the coefficients from the analysis of the choice responses indicated that overall participants preferred good to poor outcomes and lower waiting times for treatment to longer waiting times. This conforms to what would be predicted by consumer theory. This, combined with method of selecting the attributes for the DCE, provides evidence of the internal validity of the attributes and levels included in the survey.
The results indicated that participants identified ability to communicate with family and friends as the most important attribute. This was the attribute with the highest coefficient at each level. Participants were willing to wait longer for treatment to achieve an improvement in this outcome compared with the other communication outcomes included in the DCE. The importance of ability to communicate demonstrated by the analysis of the choice questions was supported by the results of a separate ranking exercise. Overall, 64% of participants who completed the ranking exercise at the end of the survey ranked ability to communicate with family and friends as the most important communication outcome.
The results of the survey suggest that participants are willing to wait longer than 1 year for treatment that improves their ability to communicate and the impact that this has on their lives. This is longer than the maximum waiting time included in the survey, which reflected national policy and practice at the time of the survey. Younger people (aged < 60 years) were willing to wait longer than older people (aged ≥ 60 years) for therapy.
Economic evaluation
The bootstrapped analyses suggest that the SL intervention is associated with an additional cost of £110 per person when compared with the AC group. This additional cost is associated with wide 95% percentiles, which indicate that the cost lies between a net saving of £640 and a net cost of £861. There is a slight net gain of 0.01 (95% percentiles –0.03 to 0.04) in utility for the intervention group compared with the AC group.
The primary CEAA indicates that the SL intervention is only likely to be cost-effective if decision-makers are willing to pay > £25,000 to gain a one-point increase in utility (p = 0.50, net monetary benefit = £3). This is at the upper threshold of the acceptable WTPTs to gain one QALY implied by NICE decisions,57 where the QALY combines length of survival as well improvements in health. Decision- and policy-makers need to assess whether or not they would be willing to pay £25,000 for a small gain in utility, measured at one point in time.
The sensitivity analyses indicate that whether SL therapy is cost-effective depends on the outcome measure used and the baseline severity of stroke. Therefore, the results of the primary analysis indicate that it is unclear whether SL therapy is more or less cost-effective than AC. The relative cost-effectiveness of SL therapy depends upon the amount a decision-maker is willing to pay to gain one unit of improvement in outcome, the measure used to assess outcome for the economic analysis and the severity and impact of the initial stroke of the patient.
Qualitative study
Twenty-two RCT participants contributed to a qualitative study conducted after the 6-month RCT outcome measures were completed and reflecting a wide range of outcomes. Ten of the participants had been allocated to the AC arm. A further 10 carers were interviewed.
The interviews provide additional insight into the experiences of the different SL therapy/AC arms of the trial from the participant and carer perspectives. Regardless of whether they saw a visitor or an SL therapist, participants reported a highly constructive experience that made a positive impact on their lives in the early months after a stroke. The differences reported in the two processes support the conclusion that the therapy and AC were delivered as intended. Those who saw a visitor reported the process of social interaction outside the family circle, having to do ‘normal’ things, practising daily life and being stretched by this. Therapy participants reported these plus carrying out specific tasks, exercises and strategies. However, differences in process (and experience) did not translate to differences in perceived impact or effectiveness (outcomes).
When combined with quantitative data of high uptake of the services offered the qualitative data help distinguish the potential, active ingredients of early, frequent contacts with an employed person from those components of therapy that do not add any benefit if delivered during the first 4 months of stroke. Both therapy and control participants valued the frequent sustained contact with a person outside their circle of family and friends. The amount of contact and the interpersonal skills/personal qualities of the person providing it (visitor of SL therapist) were identified as important drivers for recovery in the first 6 months, which resulted in building confidence and developing a positive affect (mood). These psychological factors were identified by users as necessary for engagement in intervention. Opportunity for observing one’s own progress over time was also experienced and was prioritised by users. Both groups believed that an important mechanism for recovery of communicative ability and growing awareness of residual disability was having repeated practice of daily communicative activities with a professional person who showed empathy and interest in their individual needs.
Methodological issues
Comparison with other studies
There were several methodological features to this study, which means that there is no other study similar enough for a comparison of results. However, there has been recent active interest in this area, with several trials in progress or recently completed, and these will be reviewed for the next update of the Cochrane reviews.
Although there are 30 trials included in the current Cochrane systematic review of aphasia11 (none in the review of dysarthria13) and five were included in the subcomparison addressing use of ‘social support or stimulation’, this is the first pragmatic approach to evaluate an intervention for people with aphasia or dysarthria or both. In clinical practice, services are not set up to be aphasia or dysarthria specific.
The current trial was also the first designed to disentangle therapy from attention by using an employed (not volunteer) AC comparator for the clinical population of people with acute stroke. The first few months after stroke are an important period both theoretically (potential for intervention to shape and augment plasticity) and from the service delivery perspective that this is when most of the usual care SL therapy is provided. It is also an important time when rehabilitation aims to promote awareness of disabilities and adjustment to change.
The strengths and limitations of other methodological features of ACT NoW, which make a unique contribution to the evidence base for service development, are described further below, including active service user and provider collaboration, low risk of bias, outcome measurement at the functional communication level and using standardised patient-reported outcome measures, use of mixed methods including a qualitative study, and economic evaluation.
Research partnerships: service users
The coordinated involvement of service users and carers was a huge asset to this study. The RUG met regularly. In addition to completing specific tasks such as developing the accessible range of consent materials in various media and training RAs how to support communication with people with aphasia, they were consulted on various aspects throughout the 6-year duration of the feasibility and main study. Although the direct costs (paying users their expenses, subsistence and an honorarium for each meeting) were a relatively small part of the overall research budget, the essential indirect costs of facilitating their involvement were high, both in the extra time required at each stage and paying staff to support RUG meetings and to represent users’ views at management meetings.
Research partnerships: service providers
Collaborative working between researchers and NHS SL therapists was essential for the successful completion of this study. Therapists developed the therapy to be evaluated and the screening process for identifying eligible participants. Principal investigators (PIs) from each site met regularly and a lead PI represented their views on the management group. A therapist from the feasibility study was seconded part-time from the NHS for the paid role of SL therapy monitor. The Trial Manager and Chief Investigator had regular contact with PIs and were physically based in the same department as the lead PI and therapy monitor. This was a challenging trial and looked unlikely to continue at several points. Had the management been bought in from an external trials unit, as is often recommended, it may well not have completed recruitment without the opportunity for frequent open personal contact offered by in-house networking. Future trials of complex interventions should consider this carefully and resource their trials appropriately.
One of the strengths of giving so much control over screening and intervention (both choice and delivery of) to NHS therapists was the ensured pragmatic nature of the outputs and generalisability of the findings to clinical practice. Inclusion/exclusion criteria were kept simple to invite anyone who a therapist felt might need and benefit from communication therapy, within service-level limitations of areas served and lack of bilingual therapists. Although screening records were generally well kept (reason for exclusion recorded in around 90% of the 2074 people screened), the eligibility rate was lower than expected. Exclusions were investigated in detail and were justified by therapists who repeatedly confirmed that only 21% of those screened were eligible. This rate varied between the 12 sites, but that was expected given the different populations served and services offered (e.g. ranging from hyperacute to rural general hospitals). Resolving problems or, at the other extreme, patients who were too poorly for therapy were the main reasons for exclusion. Those eligible and consenting were very similar to those who declined. Furthermore, the baseline communication impairment scores in those who consented extended across the full range from mild to severe. ‘Global communication problems’ was the reason for exclusion on < 4% of occasions.
Generalisability of findings
Partnership with NHS therapists has led to generalisable results in terms both of the target clinical population and defining a therapy that was feasible for early NHS services. To repeat a key message, the findings should not be overgeneralised to therapy delivery later post stroke or to specific therapy interventions such as conversation partner training at any time. Nor should they be used for conclusions about comparisons with ‘no contact’ given that both groups in ACT NoW were allocated to early more frequent visiting than currently provided in the NHS. ACT NoW does not provide evidence to support the removal of current service provision as without a replacement this would be the equivalent of the unknown quantity, a no-contact situation. Depending solely on natural recovery to achieve the improvements in functional communication seen here after early regular contacts is a high-risk strategy and ill advised.
Dysphagia
In terms of clinical implications, the study did not evaluate the early role of SL therapists after stroke. A large proportion of SL therapists’ early workload is for the assessment and treatment of dysphagia and, in usual practice, communication and swallowing are managed together. We evaluated one aspect – communication therapy – not swallowing therapy. Additionally, the evaluation of the communication therapy began after a diagnosis had been made and provided to the user, family and MDT and therefore precludes conclusions about the value of diagnosis by an SL therapist.
Multidisciplinary team working
Following on from the previous point, SL therapists work as members of MDTs. As our data show, they spend considerable time consulting with other health and social care staff to maintain high levels of awareness around aphasia and dysarthria and how best to support communication with this client group (MDT working in 84% of all cases seen and accounted for 11% of all recorded contacts). Although SL therapists did not liaise directly with MDTs over the care of people in the control group the cumulative effects of prior MDT collaborations could not be deleted overnight through use of our AC group. If SL therapists were permanently removed from early stroke services, in time with staff turnover, this is likely to affect MDTs’ understanding, awareness and interaction with people with communication problems.
Low risk of bias
Randomisation was carried out post baseline assessment and by an external trials unit (all other trial management was performed in-house), ensuring that therapists and researchers were unable to predict allocation. Randomisation was blocked and sites were unaware of the randomly permuted block sizes. An independent DMEC was appointed by the funder and was fully involved throughout. Participants were clearly not blinded and, although efforts were made to blind RAs, they are likely to be unblinded by comments made by participants. This was not considered a limitation as the raters completing the primary outcome measure (who saw only a video tape) were blinded. Postrandomisation exclusions were minimal and approved by DMEC. Loss to follow-up was always a withdrawal of consent for outcomes assessment and never an inability to locate someone. Participants were sent reminders that there was an intentional period of no contact after the end of intervention before outcome assessments took place, and were sent study newsletters to increase involvement. Intention-to-treat analysis was performed as well as planned per-protocol analysis, given that more people in the control arm refused their allocation or outcome assessment. The trial recruited the minimum number required by the power calculation for the primary outcome (despite stopping on a prespecified date rather than when that number was reached) and attrition did not exceed the predicted rate.
Baseline factors
One limitation was that stratification was intended to include diagnosis (aphasia only, dysarthria only, both) and analyses pre-planned to adjust for these. It was discovered when writing this monograph that, because of misunderstanding, the trials unit running the randomisation did not include diagnosis as a stratifying factor. Good balance was nevertheless achieved and the analyses proceeded as planned, adjusting for the intended stratification factors.
A further possible limitation was that we stratified for baseline communication at the level of impairment but not disability. Stratification factors were kept to a minimum and we hoped that balancing for impairment would balance for disability. It transpired that there was slight imbalance on both communication disability and overall disability (ADLs), with greater disability in the control group. We adjusted for the imbalance in sensitivity analyses but would recommend stratification in future trials. Had the results suggested the clinical effectiveness of SL therapy, the imbalance in favour of the therapy group may have raised doubts over the findings. As therapy was not more effective than AC this does not limit our results.
Heterogeneity
The heterogeneity of the included participants may appear a limitation to therapists more used to the highly selective single case and case series methods that predominate aphasia research. This has been a frequently asked question throughout this study and so it is worth reminding policy-makers that the fact that the participants are heterogeneous (in terms of type and severity of communication impairment and presence of other effects of stroke) is a strength not a limitation. This clinical heterogeneity reflects presentation in clinical practice and this pragmatic study’s findings have external validity. The reality of heterogeneity is the reason why a RCT is necessary, rather than the misconception that heterogeneity is a flaw in trials.
The one important limitation was that the sample recruited was almost exclusively white and this does not reflect the multicultural stroke population served by the UK’s NHS. One possible reason for this is that eligibility was restricted to people who were able to communicate in the English language. Aphasia varies between different languages and the provision of bilingual NHS SL therapists was considered to be beyond the scope of this already challenging study. However, it was never the intention to exclude people with English as a second language and although assessment tools and some of the impairment-focused aspects of therapy may not have been appropriate many core rehabilitation principles could have been applied. Future aphasia trials should monitor ethnicity rates throughout recruitment to avoid unintentional exclusion.
Outcome measurement
Outcomes were measured at the functional communication (disability) level rather than the impairment level and used standardised patient-reported outcome measures because these are of most relevance to service users and to policy-makers. Rehabilitation research across many clinical populations including stroke has moved away from impairment-level measures. Some aphasia researchers still advocate for impairment measures to evaluate clinical effectiveness. This study intentionally did not include impairment-level outcomes (although baseline impairment was assessed and adjusted for in the analyses) and this is seen as a strength rather than a limitation.
Many of the collaborating therapists would have preferred that we used an impairment-based measure. Any concern about the TOM disability subscale’s ability to detect meaningful change in functional communication can be resolved by our findings. As described above, an observational comparison of TOM activity-level measures taken by recruiting NHS SL therapists at baseline and blinded research SL therapists at 6 months found a clinically meaningful level of improvement in functional communication (suggesting that the measure performed well in detecting improvements regardless of group allocation). However, there was no between-group difference in 6-month TOM (the primary outcome suggesting no added benefit of therapy over AC).
A possible limitation is that we measured outcomes only at one time point, 6 months post entry to the trial, and 2 months after the end of a possible maximum intervention duration of 4 months. A longer-term follow-up may be desirable but would have meant participants agreeing to wait even longer for referral to NHS SL therapy should they express an interest on exiting the study. A 6-month outcome was considered sufficient to show recovery and ensure maintenance of benefits beyond the end of intervention. Any adverse impact on consent rates would reduce the validity of the findings.
Overall, outcome measurement elicited the views of key stakeholders, using appropriate measures at a time point relevant to clinical practice without sacrificing methodological requirements:
-
people with stroke (through the COAST and qualitative study and EQ-5D)
-
carers’ perceptions of impact on participants (Carer COAST)
-
carers on their own well-being (COPE and Carer COAST)
-
and service providers’ ratings of communication effectiveness (SL therapists on the TOM).
Attention control
The AC visiting was not planned as an alternative intervention for implementation in the NHS. It was delivered by part-time university staff, with no training in aphasia or dysarthria, who were carefully monitored by a person with extensive experience of stroke care and aphasia. Although monitoring ensured that the visitors adhered to the protocol and did not offer communication strategies of their own devising, the complementary evidence from the qualitative study, the high uptake of visitor contacts and the observational improvements in functional communication suggest that there are therapeutically active components of early, regular contacts with a person having the status of an employee. These require further research exploration as suggested in our conclusions. Future studies should not underestimate the level of management required to prepare, monitor and supervise lay visitors. Visiting people with aphasia and dysarthria proved to be a difficult and stressful job for lay people, and without the high level of support available for visitors it would probably have led to protocol violations and visitor retention issues. It was initially thought that non-professional people would be ideally suited to the visitor role. In practice, it was professional people from various (unrelated to medicine) backgrounds who proved to be more successful at the job. This may be due to the fact that in their profession they had always worked with clear professional and emotional boundaries.
Further issues around the use of an AC comparator in this study are discussed in the conclusions, including possible impact of alternatives such as a no-contact or a usual care control.
Qualitative study
The inclusion of an integrated qualitative study, analysed prior to the results of the RCT being known, makes an important contribution to the overall results while serving to reinforce the main findings. Key strengths include the successful engagement of people with severe communication difficulties within a qualitative data generation process.
This group has been routinely excluded in qualitative studies in the past of people with acquired communication impairments such as aphasia/dysarthria. 67,71 In addition, the study developed a new protocol for the transformation of non-standard qualitative data in such a way as to make it amenable to conventional data analysis.
Key limitations include the small number of participants; however, this sample size is not unusual for qualitative studies and the data demonstrate that an acceptable degree of saturation of themes had been achieved. It was not possible to engage in theoretical sampling because of the small pool of potential participants at the time of data collection. If it had been possible, the explanatory power of some of the findings would have been further enhanced. Participants were not re-engaged in a process of validation of key interpretations and findings; however, main findings were shared with the research user group for comment and question.
Preferences for communication outcomes and waiting time
It is important to remember that the outcome attributes, levels and descriptions for the DCE were derived from COAST. 28 This meant that service users directly contributed to the content and the presentation of the measure and indirectly contributed to the content of the DCE. These factors help to ensure that the preferences and WTW values estimated from the survey are relevant and important aspects of outcome and process to both service users and policy-makers.
However, there are some limitations to the survey that could affect the validity and robustness of the results. First, only a small proportion of those invited to participate did so (6%), although a low response rate of around 10% was anticipated. This means that it is not possible to assess whether or not the choices expressed by those who completed the survey reflect the views of the general population. There were also differences between the survey sample and the general population in terms of age, gender, ethnicity and employment.
Second, the sample size of those who completed all of the discrete choice questions is slightly lower than that indicated by the estimates of sample size (86%). However, it was possible to estimate coefficients for all the attribute levels, and the coefficient for each attribute level was statistically significant. These factors suggest that there were sufficient completed responses to the survey.
Third, the results of the DCE suggest that the treatment process (waiting time to access care) is important to participants. One potential limitation is that participants were not given the choice to opt out, so were forced to choose between treatments, even if there was no gain in communication outcomes that was important to them.
Fourth, the DCE only included 4 of the 20 items from the COAST measure, so may not fully capture the impact of participants’ preferences for different communication outcomes and WTW for treatment. However, the four items that were included were selected to be representative of the full measure and the subscales, and were supported by the additional factor analyses conducted for the DCE.
In addition, the WTW values derived from the DCE give the relative weight of different attributes and levels of selected communication outcomes rather than value health outcomes per se.
Fifth, the DCE used a fractional factorial design to estimate main effects on each of the attributes. However, the design was not sufficient to explore possible interactions between attributes, within the constraints of the ACT NoW study. Interactions between attributes would mean that the utility or benefit derived from one attribute depends in part on the utility gained from another attribute.
Finally, an issue for many DCEs is distinguishing the importance of an attribute from the underlying utility scale values associated with each of the attribute levels. One reason for this is that the attributes used have different scales. In this DCE survey, the outcome attributes all used a common scale, with common anchor points (worst possible/all the time and as good as before the stroke/never). This supports the assumption that the distance between each level on each outcome attribute had the same meaning and underlying utility scale.
Economic evaluation
There are a number of limitations in the methods and data used for the economic evaluation. First, the baseline health state of participants was judged likely to be too severe to measure with the EQ-5D and mapping utility values using clinical measures leads to high levels of uncertainty. It was not feasible, nor did it add value, to use the available data to estimate QALYs (the outcome preferred by NICE). However measures that estimated the value of the outcome of care to participants in the trial, or to society more generally, were included.
Second, the sample size for the economic evaluation is small. Post hoc power calculations suggest that if decision-makers consider important differences in costs and utility to be £110 and 0.01, respectively, and are prepared to pay £30,000 to gain one unit of utility then there was 2–3% power to detect statistically significant differences in net monetary benefit. Using a Bayesian framework, the CEAAs and cost-effectiveness acceptability curves directly address the question of whether or not an intervention is likely to be cost-effective, without hypothesis testing, and without the risk of a type 2 error. 55,72 The primary analysis using the observed data from this trial indicated that SL therapy was likely to be cost-effective (probability > 50%) only if decision-makers were prepared to pay ≥ £25,000 to gain one unit of utility.
Third, the cost data were skewed with high variance and the utility data followed a bimodal distribution. Bootstrap simulations reduced, but did not eliminate, the impact of this.
Fourth, there was a high level of missing observations for the total cost measures and differences between the groups in the proportions who had complete data. In contrast, there was a high level of complete data for key cost items, for example inpatient stay and the costs of the trial interventions. To address potential bias, organisational and baseline patient characteristics and treatment allocation were included in the regression models for multiple imputations, along with reasons for dropout. Analyses using available case and imputed data gave similar conclusions.
There is no linkage between patient-level data on use of primary, community and secondary care services in the NHS. Many of the centres did not have electronic patient records. This meant that key data on the use of secondary care services had to be collected by trial researchers directly from patient notes and data on the use of primary and community care services had to be collected separately.
The feasibility study indicated that key items of service use were likely to be for hospital inpatient and outpatient care in the first 6 months following the stroke. Further research is needed to explore the use of community and primary care services following stroke, and whether either therapy or control types of interventions have the potential to alter the use of these services.
Overall, the likely cost-effectiveness of therapy was at the upper end of NICE’s acceptable WTPTs but analyses indicated a high level of uncertainty, suggesting that it is not possible to conclude whether therapy is more or less cost-effective than AC. However, uncertainty over the cost-effectiveness is of no practical concern given the lack of evidence of clinical effectiveness.
Chapter 9 Conclusions
What the study found
The ACT NoW study provides robust evidence and definitive answers to clinically important questions, with good generalisability of its findings. Functional communicative ability at 6 months had improved by a clinically meaningful amount for people in both groups. However, there was no evidence of an added benefit of SL therapy in the first 4 months of stroke for people with communication disability or their carers over and above that from the AC and from natural recovery, when both were provided at a higher level than in typical standard practice.
Service users highly valued the opportunity for early and frequent visits (not currently provided in the NHS) from a professional outside of their family and friends (therapist or visitor) who showed empathy and interest in their individual needs. They felt that these impacted positively on their confidence and mood by providing opportunity for repeated practice of everyday communication that stretched them and made them aware of their limitations and progress.
Less definitive were the results on adverse events and the economic evaluation. There remains the possibility that therapy reduced the rate of deaths, further strokes and rehospitalisation. This may have warranted further research had a mechanism been established, but it had not (i.e. had therapy improved communication).
Primary and sensitivity economic analyses indicated a high level of uncertainty, suggesting that it is not possible to conclude whether therapy is more or less cost-effective than AC. However, the evidence for absence of clinical effectiveness, and the fact that the comparator was AC rather than an alternative intervention, diminishes the role of economic evaluation.
Implications for clinical practice
Given the lack of a between-group difference one may question whether AC was the most suitable choice for the ACT NoW study and how this informs clinical practice. However, the results would have been no different with a usual care control. Assuming an effect along a continuum for therapy per se as opposed to attention, with usual care theoretically in between the two ACT NoW groups, our finding of no difference between the two extremes would be replicated when comparing the extreme and midpoint. We can therefore conclude that there is no evidence to recommend enhancing the provision of early communication therapy by a qualified SL therapist over and above usual care, and instead the evidence suggests that current service provision should be reorganised.
When faced with negative findings, NHS policy-makers should draw careful conclusions for clinical practice and avoid misinterpretation. The reaction to stop referrals to SL therapy following the 1984 trial’s73 negative findings arguably set back service development, as RCTs were largely avoided by aphasia researchers until recently. ACT NoW answers several questions very well but should not be used to address others outside its remit. It tells us that functional communication can be improved, that this is not due to the SL therapy specifically and suggests that the benefits may be due to highly valued, early regular contact with a therapist or visitor (but this needs testing in further research).
It may help to outline the clinical questions outside of ACT NoW’s remit, such as whether or not the ACT NoW SL therapy intervention might be effective if delivered later to the many people with chronic communication problems. Second, it remains unknown whether or not any SL therapy for people with acute stroke (usual care or ACT NoW therapy) is more effective than no contact at all (although the feasibility of recruiting to a no-contact control trial is very unlikely). Third, the study did not evaluate the whole early role of SL therapists after stroke. A large proportion of SL therapists’ early workload is for the assessment and treatment of dysphagia and in usual practice communication and swallowing are managed together. We evaluated one aspect, communication therapy, not swallowing therapy. Fourth, the evaluation of the communication therapy began after a diagnosis was provided to the user, family and MDT. This precludes conclusions about the value of diagnosis by an SL therapist and the impact on MDTs if they lost their SL therapist input around communication problems. Given these unknowns there is no evidence to end early SL therapy for communication problems.
Future commissioning of SL therapy from the acute phase and across the stroke pathway can be informed by several strands of evidence from ACT NoW and elsewhere. All of the audits and user surveys highlight considerable gaps in service provision and user dissatisfaction with low or no provision to meet psychosocial needs, including those resulting from impaired communication. ACT NoW has shown that an early well-resourced intervention is feasible for delivery in the NHS (by therapists or highly monitored employed visitors), has user and carer acceptability and high uptake and may be associated with, although we do not have evidence to say it is causally related to, meaningful improvements on a blinded rating of functional communication at 6 months. Reorganisation of SL therapy services should consider ways to use skill mix to provide early, regular opportunities for frequent supported practice of everyday communication, perhaps within a stepped care model as recently recommended by the Stroke Improvement Programme for psychological support. 74
Implications for research
Research should assess a reorganised SL therapy communication service that uses a stepped care model of intervention with careful consideration of skill mix and timing. SL therapists’ early role could be around diagnosis, communicating this to the user and MDT and supervising assistants for regular visits similar to those provided for the AC group in ACT NoW. Intervention would later step up to direct SL therapist contact for those with persisting need. Usual care by NHS SL therapists would be an appropriate comparator.
Further research should investigate whether or not the ACT NoW SL therapy was delivered too soon in the stroke pathway by evaluating its effectiveness with a more chronic clinical population, those with persisting communication problems months and years post stroke. There is huge unmet need in this large population yet considerable uncertainty about service delivery and an understandable tendency to assign scarce resources to those in the first 6 months of their recovery. A future study would challenge the unlikely but sometimes cited suggestion that recovery is possible only in the short to medium term.
In addition to evaluating broad approaches to therapy, the ACT NoW methods of collaborating with clinicians and users to develop interventions and research tools could be used to evaluate specific promising but unproven therapeutic strategies, such as conversation partner training later in the stroke pathway.
Future economic evaluation needs to find ways of capturing valid baseline EQ-5D data from acutely ill communication-impaired participants. An alternative is to ensure that measures are included at baseline and follow-up that would facilitate mapping from these measures to impute baseline EQ-5D scores. It is important to allow sufficient resources to extract missing data from incomplete and difficult to access NHS records. Further research is also required to understand patient and family preferences for treatment and outcomes. This is needed to inform the choice of outcome measure for future economic evaluations and assessment of cost-effectiveness, as well as understand the implications of changes in policy and practice.
Productive research partnerships with service users could be replicated in future research such as for our development and validation of patient-centred outcome measures, for example COAST and Carer COAST. There is already international interest in translating these for other countries and the possibility of developing a paediatric/adolescent version has been suggested by therapists, conscious of a lack of appropriate measures for these clinical populations with acquired neurological communication disability.
Therapists’ morale is likely to be knocked by some of the ACT NoW findings but there are also several positive messages. The future of clinical services does looks brighter now that RCTs of aphasia are emerging, with several interesting trials reporting around the time of ACT NoW. The authors of the Cochrane aphasia review are tracking the emerging evidence which focuses on topics such as intensity of therapy. SL therapists’ collaboration with future trials is essential for service development. Most importantly, there continues to be a pressing need for research and service development for people with dysarthria, and aphasia therapy needs to include those populations without English as a first language.
Acknowledgements
The ACT NoW study research costs were funded by the NIHR HTA programme. The excess treatment costs were funded by an NHS subvention, by the participating NHS trusts and initially by The Stroke Association. Coordinating the funding was a challenge and we are extremely grateful to those who helped us, especially Stephen Kennedy, Trudi Simmons, Peter Fentem, Julie Saunders and Richard Macey. The North West Stroke Research Network provided staff to assist with the identification of eligible participants, organised most efficiently by Judy Ford. We are grateful to Simon Bevan, Tom Walley and others at the NIHR HTA for their open and supportive communications throughout the study and to Bill Hackett for helpful research ethics advice and administration.
The ACT NoW grantholders were: Audrey Bowen,* Linda Davies,* Anne Hesketh,* Matt Lambon Ralph,* Andrew Long, Gill Pearl, Karen Sage,* Pippa Tyrrell,* Caroline Watkins, Andy Vail* and Alys Young.* [*Several grantholders are employed by member organisations of the Manchester Academic Health Science Centre (MAHSC).]
The authors would like to express their thanks to the following:
-
People with stroke and carers who participated in the study.
-
Speech and language therapists/principal investigators: Siân Davies, Sandra Wilson, Helen Mee, Helen Turner, Jennifer Bee, Lorraine Wackrill, Rachel Hemingway, Sarah Nelsson, Satty Boyes, Sue McCormick, Sue Roberts, Tessa Pemberton, Veronica Southern and TAs.
-
The supportive stroke teams at all 12 participating NHS sites.
-
Research staff and TAs over the 6 years, especially Jenny Freed, Kate Webster, Tim Gomersall, Gwen Alvey, Angela Webster, Louise Dooks, Svet Mihaylov, Karen Schafheutle, and the feasibility study team: Mel, Gemma, Aneela, Jane and Angela.
-
Trial Steering Committee and DMEC external members: Peter Langhorne, Pam Enderby, Jenny Donovan, David Howard, Julia Brown, John Bamford and Jane Marshall.
-
Members of the RUG: Sean Crosby, Jean Wright, Victor Wright, Liz Royle, Steve Hall, Harry Price, Pat Gallagher, and all of those involved in the feasibility study, and members of Speakeasy.
Contribution of authors
Audrey Bowen (Senior Lecturer in Psychology) was Chief Investigator responsible for the design and conduct of the study and drafting the report.
Anne Hesketh (Senior Clinical Lecturer in SL therapy) was acting Chief Investigator during two maternity leaves and, with Andrew Long (Professor of Health Systems Research), led on the choice of the outcome measures including developing novel, now published, measures.
Emma Patchick (Trial Manager) was responsible for day-to-day management through most of the main study, RA prior to that and wrote early drafts of much of the final report.
Alys Young (Professor of Social Work Education and Research) and Linda Davies (Professor of Health Economics) led the qualitative study and economic evaluation, respectively, wrote Chapters 5–7 and were members of the TSC.
Andy Vail was the study statistician, who designed and analysed the quantitative results, oversaw their reporting and interpretation and sat on the TSC and DMEC.
Caroline Watkins (Professor of Stroke and Older People’s care) and Mo Wilkinson (AC Monitor) led on the development, piloting, redesign and conduct of the AC in the main study and contributed to the final report.
Gill Pearl (SL therapist, Speakeasy) facilitated the RUG and represented their views at trial management meetings.
Matt Lambon Ralph (Professor of Cognitive Neuroscience) led the development of the SL therapy screening and intervention, its piloting and implementation in the main study (with Karen Sage and Siân Davies, SL therapists).
Pippa Tyrrell (Consultant Stroke Physician) was lead clinician at the lead NHS site, led the North West Stroke Research Network, which adopted and facilitated the study, and supported Anne as joint acting chief investigator in 2008.
All authors have contributed to analyses or interpretation of results and drafts of the report.
Publication
Young A, Gomersall T, Bowen A. Trial participants’ experiences of early, enhanced speech and language therapy after stroke compared with employed visitor support: a qualitative study nested within a RCT. Clin Rehabil 2012;in press.
Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Does early, enhanced speech and language therapy for people with aphasia or dysarthria after stroke add more benefit than providing support alone? The ACT NoW randomised controlled trial (Assessing Communication Therapy in the North West). BMJ 2012;submitted.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- National Audit Office . Progress in Improving Stroke Care 2010.
- Brumfitt S. The measurement of psychological well-being in the person with aphasia. Int J Lang Commun Disord 1998;33:S116-20.
- Hilari K, Northcott S, Roy P, Marshall J, Wiggins RD, Chataway J, et al. Psychological distress after stroke and aphasia: the first six months. Clin Rehabil 2010;24:181-90.
- Thomas SA, Lincoln NB. Factors relating to depression after stroke. Br J Clin Psychol 2006;45:49-61.
- Royal College of Speech and Language therapists (RCSLT) . RCSLT Resource Manual for Commissioning and Planning Services for SLCN 2009.
- West C, Hesketh A, Vail A, Bowen A. Interventions for apraxia of speech following stroke. Interventions for apraxia of speech following stroke. Cochrane Database Syst Rev 2005;4. 101002/14651858CD004298pub2.
- Bogousslavsky J, Vanmelle G, Regli F. The Lausanne stroke registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988;19:1083-92.
- Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37:1379-84.
- Enderby P, Davies P. Communication disorders: planning a service to meet the needs. Br J Disord Commun 1989;24:301-31.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Patients With Stroke: Rehabilitation, Prevention and Management of Complications, and Discharge Planning. A National Clinical Guideline 2010.
- Kelly H, Brady MC, Enderby P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 2010;5. 10.1002/14651858.CD000425.pub2.
- World Health Organization (WHO) . International Classification of Functioning, Disability and Health (ICF). Resolution WHA 54.21 n.d. http://www.who.int/classifications/icf/en/ (accessed February 2012).
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non-progressive brain damage. Cochrane Database Syst Rev 2005;3. 10.1002/14651858.CD002088.pub2.
- Cohen NS, Masse R. The application of singing and rhythmic instruction as a therapeutic intervention for persons with neurogenic communication disorders. J Music Ther 1993;30:81-99.
- Braverman SE, Spector J, Warden DL, Wilson BC, Ellis TE, Bamdad MJ, et al. A multidisciplinary TBI inpatient rehabilitation programme for active duty service members as part of a randomized clinical trial. Brain Injury 1999;13:405-15.
- Main A, Kelly S, Manley G. The Use of Palatography in Acquired Dysarthria n.d.
- Intercollegiate Stroke Working Party . National Clinical Guidelines for Stroke 2008.
- Greener J, Enderby P, Whurr R. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 1999;4. 10.1002/14651858.CD000425.
- Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res 1998;41:172-87.
- Robey RR. The efficacy of treatment for aphasic persons: a meta analysis. Brain Lang 1994;47:582-608.
- Higgins JPT, Green SE. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; n.d.
- Medical Research Council (MRC) . A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health 2000.
- Pring T. Ask a silly question: two decades of troublesome trials. Int J Lang Commun Disord 2004;39:285-302.
- CONSORT group . CONSORT Statement 2007 n.d. www.consort-statement.org/?o=1004 (accessed March 2012).
- Commission for Healthcare Audit and Inspection . Survey of Patients 2005: Stroke 2006.
- Intercollegiate Stroke Working Party . National Sentinel Stroke Audit (phase 1) Organisational Audit 2008 2008.
- Long A, Hesketh A, Bowen A. Communication outcome after stroke: a new measure of the carer’s perspective. Clin Rehabil 2009;23:846-56.
- Long AF, Hesketh A, Paszek G, Booth M, Bowen A, Study ACR. Development of a reliable self-report outcome measure for pragmatic trials of communication therapy following stroke: the Communication Outcome after Stroke (COAST) scale. Clin Rehabil 2008;22:1083-94.
- Hesketh A, Long A, Patchick E, Lee J, Bowen A. The reliability of rating conversation as a measure of functional communication following stroke. Aphasiology 2008;22:970-84.
- Hesketh A, Long A, Bowen A. on behalf of the ACT NoW study team . Agreement on outcome: speaker, carer and therapist perspectives on functional communication after stroke. Aphasiology 2011;25:291-308.
- Enderby PM, Wood VA, Wade DT, Hewer RL. The Frenchay Aphasia Screening Test: a short, simple test for aphasia appropriate for non-specialists. Int Rehabil Med 1987;8:166-70.
- Enderby P, John A. Therapy Outcome Measure: speech-language pathology technical manual. London: Singular; 1997.
- Warrington EK. The graded naming test: a restandardisation. Neuropsychol Rehabil 1997;7:143-6.
- Baxter DM, Warrington EK. Measuring dysgraphia: a graded-difficulty spelling test. Behav Neurol 1994;7:107-16.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3.
- Department of Health (DoH) . Attributing Revenue Costs of Externally-Funded Non-Commercial Research in the NHS (ARCO) 2005.
- Hilari K, Byng S, Lamping DL, Smith SC. Stroke and aphasia quality of life scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke 2003;34:1944-50.
- The International Conference on Harmonisation (ICH) Efficacy Guidelines 1996 . Good Clinical Practice (GCP) n.d. http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed 15 February 2012).
- Department of Health . Research Governance Framework for Health and Social Care 2001. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4008777 (accessed 15 February 2012).
- Cruice M, Worrall L, Hickson L, Murison R. Finding a focus for quality of life with aphasia: social and emotional health, and psychological well-being. Aphasiology 2003;17:333-53.
- Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Pol 2003;2:55-64.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics 2008;26:661-77.
- Coast J, Flynn TN, Salisbury C, Louviere J, Peters TJ. Maximising responses to discrete choice experiments: a randomised trial. Appl Health Econ Health Pol 2006;5:249-60.
- Burr J, Kilonzo M, Vale L, Ryan M. Developing a preference based glaucoma utility index using a discrete choice experiment. Optom Vis Sci 2007;84:797-808.
- Burgess L, Street DJ. Optimal designs for choice experiments with asymmetric attributes. J Stat Plann Infer 2005;134:288-301.
- Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Market Res 1996;33:307-17.
- Burgess L. Discrete choice experiments (computer software). Department of Mathematical Sciences, University of Technology, Sydney; n.d.
- Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. New York, NY: Springer; 2008.
- Royal Mail . Postcode Address File (PAF®) n.d. http://www.royalmail.com/marketing-services/address-management-unit/address-data-products/postcode-address-file-paf (accessed March 2012).
- Hensher DA, Rose JM, Greene WH. Applied choice analysis. Cambridge: Cambridge University Press; 2005.
- UK National Statistics . Regional Trends n.d. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-254239 (accessed September 2010).
- Department of Health (DoH) . NHS n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed March 2012).
- Curtis L. Unit costs of health and social care. PSSRU, University of Kent at Canterbury; 2009.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for Euroqol: results from a UK general population survey. Centre for Health Economics discussion paper 138. York: Centre for Health Economics, University of York; 1995.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401.
- National Institute for Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Sendi PP, Briggs AH. Affordability and cost-effectiveness: Decision-making on the cost-effectiveness plane. Health Econ 2001;10:675-80.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing … presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92.
- Chartered Institute of Public Finance Accountants (CIPFA) . The CIPFA Database 2005 2005.
- Curtis L, Netten A. Unit costs of health and social care. PSSRU, University of Kent at Canterbury; 2006.
- Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors’ perspective. Disabil Rehabil 2005;27:1213-23.
- Perry L, McLaren S. Coping and adaptation at six months after stroke: experiences with eating disabilities. Int J Nurs Stud 2003;40:185-95.
- Proot IM, Crebolder HF, Abu-Saad HH, Macor TH, ter Meulen RH. Facilitating and constraining factors on autonomy: the views of stroke patients on admission into nursing homes. Clin Nurs Res 2000;9:460-78.
- Roding J, Lindstrom B, Malm J, Ohman A. Frustrated and invisible: younger stroke patients’ experiences of the rehabilitation process. Disabil Rehabil 2003;25:867-74.
- Kagan A. Supported conversation for adults with aphasia: methods and resources for training conversation partners. Aphasiology 1998;12:816-30.
- Gillespie A, Murphy J, Page M. Reconstructing Intersubjectivity: Adaptation and Identity in Informal Care Relationships Which Have Been Disrupted by Aphasia: Full Research Report, ESRC End of Award Report, RES-000-22-2473 2009.
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2007;4. 10.1002/14651858.CD006073.pub2.
- Turner B, Fleming J, Cornwell P, Worrall L, Ownsworth T, Haines T, et al. A qualitative study of the transition from hospital to home for individuals with acquired brain injury and their family caregivers. Brain Injury 2007;21:1119-30.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6:1-8.
- Lincoln NB, Mulley GP, Jones AC, McGuirk E, Lendrem W, Mitchell JRA. Effectiveness of speech-therapy for aphasic stroke patients: a randomized controlled trial. Lancet 1984;1:1197-200.
- Gillham S, Clark L. Psychological care after stroke. Leicester: NHS Improvement; 2011.
- Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and application. Cambridge: Cambridge University Press; 2000.
Appendix 1 Summary of speech and language therapy intervention
Purpose of this document
This document aims to provide a non-specialist summary of the intervention to be evaluated, providing core information on when, where, how often and by whom the intervention is delivered to those participants randomised to this arm of the trial. It also summarises the core activities of the intervention, emphasising a ‘minimum standard’ to indicate the included and excluded activities. Full details for the participating specialist SL therapists are provided in the ‘Best Practice Standards’.
Aims of the intervention
Intervention aims to facilitate the rehabilitation of communication skills in people with speech (dysarthria) or language (aphasia) problems after stroke. This includes direct remediation of speech/language, promoting alternative means of communication, supporting adjustment to communication disability and improving communication environments (e.g. training carers and professionals to be effective communication partners). Carers also receive support to promote their own psychosocial well-being.
When, where and how often
-
Intervention starts about 2 weeks after admission to hospital.
-
Lasts a maximum of 16 weeks and three contacts per week, but variable number of contacts.
-
Early sessions take place in inpatient facilities, often a specialist stroke unit.
-
Many people will be discharged from inpatient care after the first few weeks when intervention will continue in other settings, for example outpatients, domiciliary visits.
By whom
The intervention is designed, implemented and monitored by qualified SL therapists registered with the Health Professionals Council and employed by NHS trusts. Participating SL therapists will be trained in the intervention. SL therapists deliver most of the one-to-one contacts with clients, but some specific activities are delivered by supervised assistants.
Contents of the intervention
The intervention is multifaceted and must be tailored to individual needs and abilities. However, there are six core components.
1. Assessment
Minimum standards are that participants will receive initial and ongoing assessment:
-
from a qualified SL therapist
-
with standardised tools (e.g. the full Robertson Dysarthria Assessment)
-
of functional communication using the agreed protocol and through observation
-
taking a case history, interviewing carers, interviewing health professionals
-
initiated within 3 weeks of randomisation
-
resulting in short- and long-term goal-planning.
2. Information provision
At minimum participants will be provided with information:
-
on the nature of their aphasia or dysarthria and likely impact on their lifestyle
-
on strategies and equipment to assist communication
-
on the intervention plan to achieve agreed goals
-
on the therapist’s opinion of progress
-
on available information resources and support organisations
-
provided to both participants and their carers
-
in accessible formats and through both spoken and written media.
3. Provision of communication materials
As a minimum each participant is given and trained in the use of:
-
a structured folder for retaining the written information described above
-
a communication book for recording interventions and activities
-
an appropriate AAC device (provided on loan to each person with AAC needs).
4. Carer contact
As a minimum, all carers are offered at least one contact with a qualified SL therapist for:
-
discussion and information giving
-
observation and participation in therapy
-
conversation partner training
-
preparation for the end of the research intervention.
5. Indirect contact
The minimum standards for indirect contact are that a qualified SL therapist will offer multidisciplinary colleagues at least:
-
two written descriptions of the participants’ needs, abilities and strategies
-
two spoken discussions with key members of the clinical team.
6a. Direct contact: speech (dysarthria)
As a minimum standard, all of those with dysarthria will be offered one-to-one contact with a qualified SL therapist to improve their ability to express themselves clearly using:
-
if range and strength of movement are impaired oral muscle exercises
-
if motor speech processes (e.g. respiration) are impaired targeted speech exercises (e.g. breath control)
-
if intelligibility is poor low- and high-tech AAC, for example alphabet charts, electronic communicators
-
if psychosocial barriers are present specific activities, for example role play to develop confidence
-
if problems adjusting to communication disability information and counselling.
6b. Direct contact: language (aphasia)
All of those with aphasia will be offered one-to-one contact with a qualified SL therapist to improve their language skills at the following minimum standards:
-
at all three levels of the World Health Organization model of Impairment, Activity and Participation:
-
– Impairment the cognitive neuropsychological model will be used to determine a hypothesis-driven approach, for example exercises for spoken word sound and meaning, written word spelling and visual structure, grammatical exercises for sentence comprehension and production
-
– Activity compensatory strategies (drawing, gesture, communication book), and conversational skills training (turn-taking, eye contact, topic maintenance)
-
– Participation specific exercises, for example role play to develop confidence, information and counselling, supported conversation approach.
-
Excluded interventions
-
Interventions restricted to one of the World Health Organization levels, for example activity/functional only or impairment only.
-
Interventions for long-term adjustment, for example ‘living with aphasia’ therapies, identity and self-advocacy.
-
General stimulation–facilitation activities without clearly stated aims.
-
Speech and language therapist combined with psychotherapeutic, pharmacological or surgical interventions.
Appendix 2 Summary of the ACT NoW attention control
Purpose of this document
This document provides a summary of the AC provided as part of the ACT NoW RCT. It provides core information on when, where, how often and by whom the AC is delivered to those participants randomised to this arm of the trial. It also summarises the core activities of the AC, emphasising a ‘minimum standard’ to indicate the included and excluded activities.
Aims of the attention control
To determine if between-group differences are truly due to the early enhanced communication therapy, persons admitted to hospital with stroke and who suffer communication problems as a result (aphasia, dysarthria or both) are randomised into either an enhanced therapy intervention or an AC of similar duration and dose.
When, where and how often
Attention control starts about 2 weeks after admission to hospital. AC lasts a maximum of 16 weeks and three contacts per week, but the number of contacts is variable. Early sessions take place in inpatient facilities, often a specialist stroke unit. Many people will be discharged from inpatient care after the first few weeks when contact with the visitor will continue in other settings, for example domiciliary visits.
By whom
Sessions are planned and implemented by paid part-time staff, known as ACT NoW visitors. ACT NoW visitors will be persons aged ≥ 18 years who have no prior experience or specific training in stroke rehabilitation. They will be selected on the basis of their interpersonal skills and ability to tailor their conversation to the specific needs of our research participants. ACT NoW visitors are trained to deliver a manualised AC and are regularly supervised and monitored to ensure adherence to the research protocol.
Contents of the attention control
The AC sessions are of 60 minutes maximum duration, are multifaceted and must be tailored to individual needs and abilities. Activities will be participant led in so far as they are confined to pastimes that do not involve any form of communication strategy, number/money, reading or writing. The AC follows three distinct stages, as shown below.
Building rapport (two to four sessions)
Initial sessions may be of short duration (10–30 minutes) and should, where possible, involve carer(s) or family members.
The role of the visitor as well as the limitation of his or her input will be discussed with the participant as will be the fact that the AC is limited to a maximum of 16 weeks. Visitors provide the opportunity to get to know a new person, to provide someone to listen and to talk to.
Initial sessions will be aimed at getting to know each other, finding common ground and/or finding out about each other’s lives, likes, dislikes and experiences.
Activities of interest to the participant can be agreed from a ‘menu’, for example films, games, etc., but this will need to be reassessed on a regular basis.
Regular contact sessions
Visitors will vary sessions according to the participant’s ability, general state of health and general interests. Sessions will aim to be participant led but the visitors will have access to some materials/items (see below) to suggest activities.
ACT NoW visitor sessions will include, wherever possible:
-
General conversation, suggested topics:
-
– activities since last visit and/or plans for later (as two-way exchange, i.e. the visitor sharing their activities/plans as well)
-
– current affairs, hobbies and interests, friends and family
-
– progress made/achievements since the stroke.
-
-
Activities such as:
-
– the visitor reading from books, magazines, newspapers to entertain the participant and create topics for conversation
-
– watching television or videos, listening to the radio, music
-
– playing selection of games of tactics and strategy (i.e. not communication), for example puzzles, Connect 4, chess, draughts
-
– other games if suggested by participant, for example cards, dominoes
-
– creative activities, for example arts and crafts (making greetings cards, photo albums)
-
– gardening (where mobility and general health allow).
-
ACT NoW visitors will have access to selected board games supplied by the ACT NoW study, but can supplement these by using public libraries to obtain books, tapes, CDs, videos or DVDs. Session content can be agreed in advance and, if requested by the participant, the visitor may agree to bring a magazine or book to share, film to watch or a music tape to listen to (borrowed from library to avoid expense) or to find a radio programme or TV programme to watch together.
Winding-down sessions (two to four sessions)
Participants will be made aware of, and be prepared for, the visitor’s time with them coming to an end. Participants will be given written information on a provisional outcomes assessment date with a RA.
What is not included in the attention control
ACT NoW visitors are not trained to deliver any specific support or therapy. They are not trained to assess the condition and/or needs of a participant following stroke and shall not:
-
carry out activities aimed at improving communication
-
provide any equipment aimed at facilitating communication
-
help with feeding or give participants food or drink because of potential dysphagia (swallowing problems) after stroke
-
advise on or handle medical equipment (e.g. hoist, percutaneous endoscopic gastrostomy (PEG) feeding tube or similar) or medicines
-
advise on medical needs – visitors will suggest the participant contacts their ward staff or general practitioner
-
run errands or handle the participant’s money or valuables.
Appendix 3 ACT NoW protocol
Version 4, dated 1 December 2008
Assessing the effectiveness of Communication Therapy in the North West: the ACT NoW study
-
Funded by: The Department of Health, Health Technology Assessment Programme
-
Sponsor: The University of Manchester
-
HTA ref. 02/11/04
-
ISRCN ref. ISRCTN78617680
-
MREC: 06/MRE03/42 (The ACT NoW main Study),
-
Chief investigator: Dr Audrey Bowen, University of Manchester, audrey.bowen@manchester.ac.uk
-
Acting Chief Investigators (during maternity leave):
-
– Dr Anne Hesketh, University of Manchester, anne.hesketh@manchester.ac.uk
-
– Dr Pippa Tyrrell, Salford Royal Hospitals Trust, Ptyrrell@hope.man.ac.uk
-
Note: The appendices mentioned were sent to MREC but are not included in this monograph as much of this information is already in the monograph.
Summary
-
This HTA commissioned project will provide robust evidence about the effectiveness and cost effectiveness of speech and language therapy (SLT) for people with aphasia or dysarthria following stroke. Service users and providers of SLT services will participate in all stages of the design and implementation of the trial to ensure that the evidence is relevant to the needs of decision makers and the target population. A multicentre, stratified randomised controlled trial (RCT) will ensure accurate comparison of effects and costs. The trial protocol, intervention and setting were defined during the feasibility study (04/MRE03/30), in conjunction with SL therapists currently employed in the North West Region. These therapists will also provide the intervention in their own care setting. This design will ensure that results are directly generalisable and practicable within the NHS. The trial will include quantitative and qualitative assessment of effects and patient outcomes.
Background
-
Stroke can affect communication in different ways. The person may have impairments of motor speech production (dysarthria) or of language skills (aphasia). A person with aphasia (also referred to as dysphasia) may have difficulty understanding or expressing the spoken or written word. SL therapists work with people with aphasia and dysarthria in different settings, as part of a multidisciplinary team on a specialist stroke unit, in out-patient clinics and in the community. There is good evidence that people managed by a specialist team in a stroke unit have lower rates of mortality and morbidity1. The NHS now needs to determine the specific contribution of the components of the stroke unit e.g. the provision of an SLT service for communication difficulties.
-
Aphasia research is vibrant and of high quality. This is reflected in a large number of international journals that include or are dedicated to aphasia (e.g., Aphasiology, Brain and Language, Cognitive Neuropsychology) and has led to advances in assessment so that therapy can be targeted. The range of therapeutic approaches has also increased to include those aimed at the levels of ‘activity’ and ‘participation’. In contrast, the research into evaluating the effectiveness of therapy is disappointing. Advances in single case experiments have not been generalised to the wider clinical population. High quality RCTs are required. These are noticeably lacking.
-
The Cochrane systematic review of SLT for dysarthria rejected all 12 identified studies on the grounds of poor quality and likely bias2. The review of aphasia therapy had similar findings3. Inadequate statistical power was a common failing, and none of 12 identified trials had sufficient detail for a complete description and analysis. Two other reviews4,5 are widely cited. Neither was systematic. Though well-intentioned, these reviews are prone to bias and do not provide the level of evidence required.
-
The first UK RCT of aphasia was published 20 years ago. The SLT profession objected strongly to this trial and, in rejecting the findings as invalid, many went so far as to reject the value of RCTs for therapy per se6. There is consequently a need to educate SL therapists about the true strengths and limitations of RCTs, and dispel prevailing myths.
-
The Greener et al aphasia review3 made several recommendations with which we agree. They suggested that ‘treatment’ by non-therapists should also be evaluated. They advised against use of a design where the SL therapist provides active therapy in one arm but not in the other as it is not feasible to expect to avoid contamination. RCTs to date have not addressed outcome at the level of functional communication on a day-to-day basis in the person’s own social context. Our project will do this.
-
Greener also highlighted the exclusion of users’ and carers’ perspectives in previous trials. Our project is designed to enable these key stakeholders to participate throughout. One of our key strategies is to nest a qualitative study within the framework of the RCT. This approach has been successfully used in previous studies7. Our project will also make use of the synergy between quantitative and qualitative methods in adopting Donovan et al’s8 recommended strategies for providing information and obtaining informed consent.
-
We conducted a large feasibility study (04/MRE03/30 The ACT NoW pilot study) which is currently being completed and reported. This provided us with the opportunity to develop the necessary methods and materials for phase two (the main study) and to see if a main study was feasible. The current protocol relates to the main ACT NoW study and is submitted with the REC application.
Aims
-
The main study aims to determine the relative effectiveness and cost effectiveness of an SLT intervention for people with aphasia or dysarthria following stroke, when delivered by NHS therapists in a usual care setting. There are six specific objectives:
-
Evaluate the relative effectiveness of SLT compared with attention control, as measured by service users’ functional communication after six months;
-
Evaluate the relative effectiveness of SLT compared with attention control, as measured by users’ quality of life after six months;
-
Evaluate the relative effectiveness of SLT compared with attention control, as measured by carers’ well-being after six months;
-
Evaluate the relative resource use, costs and cost effectiveness of SLT compared with attention control, at six months from a societal perspective;
-
Determine, using qualitative research, users’ and carers’ views on the process and effects of therapy compared with the views of those who received the attention control;
-
Construct a ‘service user’s checklist’ of indicators of satisfaction and quality, that can inform and monitor the future implementation of the evaluated technology.
-
Design
-
SLT is a complex intervention. It consists not only of direct communication therapy with service users but also includes: direct contact that is not communication therapy (e.g. self-perceived changes in identity, confidence building); training carers; joint therapy sessions with other health professionals; contributing to multidisciplinary team planning. Complex healthcare interventions cannot be adequately evaluated using a single methodological approach9.
-
We will evaluate this complex intervention using:
-
– A. a pragmatic, multicentred, randomised controlled trial (RCT), stratified by diagnosis/severity and therapist/centre, using an ‘intention to treat’ approach.
-
– B. a health economics evaluation;
-
– C. a qualitative study based on interviews with 30 patients and 10 carers purposively sampled from each arm of the trial.
-
The following pages detail the procedure, sample sizes, measures etc of the RCT, health economics and qualitative methods.
Study population
Adults admitted to hospital with a stroke will be eligible for inclusion as soon as they meet the following criteria:
-
Communication impaired due to aphasia and/or dysarthria
-
Considered, by the SL therapist, able to engage in therapy
-
Considered, by the SL therapist, likely to benefit from communication therapy
-
Given informed consent or carers’ assent.
Exclusion criteria Subarachnoid haemorrhage, progressive dementia, pre-existing learning disabilities likely to prevent benefits from therapy, unable to communicate in the language of English. (The provision of bilingual SL therapists is considered to be beyond the scope of this project). Experiences from the feasibility study have further highlighted the need to heed other reasons for exclusions, including: other serious concomitant medical conditions (such as newly-diagnosed terminal disease), the patient being unable to complete eligibility screening even after three attempts over a two-week period, family or carer objections, as well as (rare) cases, where crisis intervention by SLT becomes necessary before eligibility screening is completed.
Recruitment
During the feasibility study and for around the first year of the main study all stroke admissions to the participating hospitals were note-screened for eligibility by staff working under the direction of NHS Speech and Language Therapists. This note screening was to identify those patients with communication difficulties who needed to be communication screened by SL therapists. However, after a review of note screening support, it was found that this system did not identify people with (non-temporary) communication problems who would otherwise have been missed – therefore, it has been discontinued.
As of June 07, patients are now being identified at the point of referral to SLT through standard local procedures and then screened for eligibility by SLTs. As previously, a standardised procedure for communication screen will be used across all sites, which was developed during the feasibility study. This will determine eligibility and provide information for stratification (e.g. diagnosis and severity). Those who appear eligible but are not yet able to engage will be re-screened during the following two weeks. We do not wish to exclude people who may need a bit more time before they are ready to participate.
Once eligibility is determined research assistants (RA) will meet potential participants to provide information on the study. During the feasibility study (04/MRE03/30), we developed and tested a range of aphasia-friendly information and consent materials (e.g. booklets, DVDs etc). At least 24 hours later RAs will return to give more information if required, answer questions and seek consent. If, because of their communication difficulties, the potential participant cannot provide informed consent at that time, proxy consent can be requested from a carer/relative or Independent Mental Capacity Advocate. For detailed information on the recruitment procedure including appropriate consultees for proxy consent see Appendix XX (sent to MREC but not included in monograph). Where proxy consent is gained RAs will later give opportunities for participants to directly provide or withdraw their consent.
Randomisation
Research staff will contact an independent randomisation service by telephone or via their secure internet-based service to ensure allocation concealment. Identifying data are recorded before participants are allocated in a stratified 1:1 ratio. Half will receive the SLT research therapy immediately while the others will receive the attention control, each for a maximum period of four months. Six months after randomisation, outcomes are assessed for both groups. Participants exit the study following this comparison and are eligible for standard NHS SL therapy, if the service user or provider deems this necessary. There is no funding to provide the research therapy beyond this point, nor is it considered ethical since the risks and benefits will not be known until data from all participants have been analysed. A flow chart summarising the study and describing the paths from screening to outcome assessment is provided [Appendix XX (sent to MREC but not included in monograph)].
Proposed interventions
The research therapy (health technology being assessed) is a defined and reproducible package of care delivered by NHS SL therapists. It contains a consensus-based assessment and treatment approach, flexible to individual needs and feasible for routine NHS delivery (if adequately resourced). It is provided earlier, more intensively, more systematically, in different settings and for longer than current typical NHS SLT. It is manualised to ensure consistency between sites and generalisability of findings. Those allocated to the control arm will receive attention control only for the first six months i.e. the same time, intensity and duration of contact in the same settings. However, contact is not by a therapist but by a ‘visitor’. The use of an attention control is required to investigate whether potential benefits from the research therapy are due to the actual therapy provided by a qualified therapist and are not general benefits due to the intensive ‘attention’ provided by a regular visitor. Attached are summaries of the intervention and control [see Appendix XX (sent to MREC but not included in monograph)].
Setting
The interventions will be provided in hospital and community settings.
Proposed sample size
The original protocol proposed a sample size of 300 participants for 90% power to detect a difference of 0.5 standard deviations (SD) on the primary outcome. Slower than anticipated recruitment has led the sponsor to request revision and clarification based on the observed SD of the primary outcome of recruited participants. Importantly, interim analysis and revision of the sample size estimate is not based on the observed group difference to date.
The revised target is to recruit a minimum of 170 participants. This is based on the observation that the minimally important clinical effect of a 0.5 point difference in the TOM scale will constitute a standardised effect size of 0.45 SD. Assuming approximately 10% loss to follow-up (as observed to date) and a standard analysis without clustering in either arm, this will provide 80% power at the 5% significance level.
From recruitment experience to date we know we can recruit an average of around 0.55 participants per month per participating site. At this recruitment rate, and with 12 participating sites from March 2009, it should take around 38 months to complete recruitment. We therefore plan to continue recruitment through to the end of January 2010 to ensure reaching the minimum target of 170 participants.
Statistical analysis
The statistical analyses will determine whether the differences in six-month outcome between the two arms of the trial are greater than those expected by chance. Analysis of covariance will take into account variability between individuals at baseline. Stratified randomisation will balance groups in terms of centre and baseline communication disability. We will endeavour to collect outcome data for those who violate the protocol, including giving participants the option to undergo outcome assessment even if they choose to withdraw from therapy/control. This intention to treat approach will reduce bias due to non-compliance and provide evidence on effectiveness, not just efficacy. Deaths will be recorded and included in outcomes analyses.
Proposed outcome measures
Measurement will take place six months after randomisation. All outcome measure data collection will be completed by trained research assistants (RAs) who will offer to visit participants and carers in their own homes. The videos used for determining the primary outcome will then be assessed by independent raters (qualified SLTs blinded to group allocation). Following assessment, the raters will document their belief as to which treatment has been received, and their degree of confidence in this belief.
Rationale for choosing outcomes
The key issues we considered when choosing and developing our measures were:
-
Coverage of areas of communicative importance for people with aphasia and/or dysarthria (face validity from the perspective of users, carers and SLT practitioners)
-
Validity – disease-specific, appropriate for post-stroke communication disorders, measured at the functional activity level rather than the impairment level, appropriate for non-verbal as well as verbal communication
-
Reliability – consistent measurement
-
Practicality – ability to be completed in appropriate length of time (maximum of half an hour for RA administration), clarity of stimuli and ability to be completed on the basis of an isolated visit by a RA
-
Acceptability – to patient, carer and SLT practitioner
The primary outcome will be participants’ functional communication. This will be measured by a ‘semi-structured conversation’ with the RA (10-minute) task. It will be videoed and subsequently rated blindly by independent expert (S<) assessors using the Therapy Outcomes Measure10 (TOM) 11 point disability scale. [The semi-structured conversation script along with TOM rating sheet used is attached in Appendix XX (sent to MREC but not included in monograph).]
Secondary outcomes take the following form:
-
Participant’s perception of their functional communication and quality of life will be assessed using a 27-item self-rated, RA-administered scale of functional communication [ACT NoW rating scale – see Appendix XX (sent to MREC but not included in monograph)]. Its content, accompanying illustrations and layout were designed to facilitate its use with patients with communication difficulties. It covers both understanding and expression in a range of communication situations and functional activities, including seven items measuring quality of life. Five of the items, covering difficulties in communicating, are taken from the Stroke and Aphasia Quality of Life Scale (SAQOL-39)11.
-
Carer’s perception of the participant’s functional communication: This will be measured using a carer-adapted version of the ACT NoW rating scale which mirrors the patient rating scale. An example of carer adaptation is that “…how well could you read…” is changed to “…how well could your relative/friend read…”.
-
Participants will also complete a EuroQol (EQ-5D)12. This is a validated, widely used 5-item practical measure of the outcomes of health care; it is attached in Appendix XX (sent to MREC but not included in monograph).
-
Carer ‘well-being’: This will be measured using the Carers of Older People in Europe (COPE) Index13. This is a validated 15-item self-completed measure and is included in Appendix XX (sent to MREC but not included in monograph).
-
In addition, the costs of communication therapy versus attention control will be compared. Carers will self-complete the health economics form [see Appendix XX (sent to MREC but not included in monograph)]. Participants’ health economics data will be gathered by RAs through patient hospital records [see Appendix XX (sent to MREC but not included in monograph)].
Valuations of resource use will be taken at the end of the trial from a societal perspective. Therefore University rather than NHS ethics approval will be sought for this.
Qualitative study
Aims
To explore the experiences and effectiveness of Speech and Language therapy, the perspectives of both users and carers will be collected from both arms of the trial:
-
To evaluate, from the perspectives of service users themselves, the effectiveness of both the processes and outcomes of SLT for people with aphasia or dysarthria.
-
To compare, using the perceptions and constructions of the users, the experience of therapy between the two arms of the trial.
-
To understand from the perspective of the carer, the impact of SLT and the therapy experience on the well being of both carer and patient.
-
To construct, from users’/carers’ accounts in this study, a users’ checklist of indicators of satisfaction and quality to inform future delivery of therapy.
Background
This study is designed to complement the RCT by providing elaborated data that both stand in their own right as a study of users’/carers’ experience of therapy and work to further interpret and illuminate the findings from the trial itself. The study seeks to capture the experience of both the processes and the outcomes of therapy by inviting users to define, in their own terms, effects, quality markers of practice, concerns, and the wider personal/social impact of their encounter with therapy. As such, users are cast as evaluators, whose stories and understandings contain significant insights into a range of factors that will inevitably mediate between therapeutic intent and outcome. The qualitative study will, therefore, explore personal psycho-social influences as well as the effects of particular therapeutic practice and setting over and above the clinical issues involved in successful or unsuccessful use of therapy.
By comparing data generated by each group of users, it will be possible at a meta level to make meaningful comparisons of, for example, self perceived effects, degree of involvement in process, impact on perceptions of quality of life, concerns, suggested improvements in therapeutic processes and delivery, satisfaction with practice and professional intent/behaviour. Such comparisons will serve to enhance and qualify the results of the RCT as well as primarily giving users a ‘voice’ in the evaluation of a health technology of which they will be the recipients. In addition to the data collected from users, principal carers will also be involved to provide a detailed narrative evaluation of the processes and outcomes of their encounter with the therapy/attention control.
Sampling
Thirty users (15 per arm) and 10 carers (five per arm) will be chosen to include differences both in degree and type of communication disability, and spread of setting in which therapy is delivered, socio- demographic factors (such as age, sex, socio-economic circumstance and home/care context). By sampling after the six month quantitative assessment has been conducted we can also choose individuals with ‘good’ quantitative outcomes and those without for an in-depth exploration of users’ experiences. The participants in the attention control will not be ‘matched’ in a formal sense, but will be chosen to be similar in terms of the key variables.
Methods
The key challenge in engaging users will be the effects of the impairments they experience in both expression and processing of language at different points through the therapeutic process. This challenge is particularly difficult because of the exploratory nature of this study that is seeking to tap perception, conceptualisation and description in ‘own terms’. An approach will be taken, therefore, that provides participants with a menu of different means of participating in the data generation depending on their preferences and particular abilities at the time. In essence, the same kinds of information are sought but in a variety of ways.
The means of engaging participants in an exploration of these issues will involve: responding to direct questions through choice on visual scales; use of pictures and words corresponding to emotions/adjectives from which choices can be made; use of short video scenarios from which ‘best fit’ to agree/disagree of point of view can be made; joint interview with carer who is able to ‘interpret’ expressions/responses; and direct interview with participant (if possible). Through our present and previous contacts with the user organisations Speakeasy, and The Stroke Association, we involved previous users in the design, production and testing of the different elements of this menu of engagement. This work was completed as part of our feasibility study (04/MRE03/30).
Outcomes
The main focus of enquiry will be the appraisal of the effectiveness of the four months of SLT (or attention control) in terms of view of the actions, practices and attitudes of those delivering the therapy/attention; judgments of its impact on self and functioning; identifying what made it successful/not successful; explanation of ease/difficulty in participation; and view of what could be done differently. Carers will also be interviewed using a semi structured approach to capture their narrative of the impact of the therapy received, the evaluation of its delivery, their role in contributing to the effectiveness of the process, and features outside the therapy that have enabled success or been barriers to improvement.
Data will be stored and sorted using the NVIVO computerised sort and retrieve programme. From the wealth of data generated, it will be possible to construct a simple ‘users’ checklist’ of features of therapeutic practice and process that are considered facilitative. These may be highly practical issues to do with setting, or attitudinal to do with information, communication and interaction for example. Its aim is to inform the commissioners and providers of SLT to contribute to the maximisation of quality and uptake. The draft will be circulated to the original participants and their carers for comment prior to the final version.
Detailed documentation on the qualitative materials and interview schedule can be found in Appendix XX (sent to MREC but not included in monograph).
- Stroke Unit Trialists' Collaboration . The Cochrane Library 2003.
- Sellars C, Hughes T, Langhorne P. The Cochrane Library. Oxford; 2003.
- Greener J, Enderby P, Whurr R. The Cochrane Library 2003.
- Robey RR. The efficacy of treatment for aphasic persons: a meta-analysis. Brain and Language 1994;47:582-608.
- Whurr R, Lorch M, Nye CA. (1992). Meta-analysis of studies carried out between 1946 and 1988 concerned with the efficacy of speech and language therapy treatment for aphasic patients. European Journal of Disorders of Communication n.d.;27:1-18.
- Code C. (2000). The problem with RCTs. Bulletin of the RCSLT n.d.;575:14-5.
- Dowswell G, Lawler J, Young J, Forster A, Hearn J. (1997). A qualitative study of specialist nurse support for stroke patients and care-givers at home. Clinical Rehabilitation n.d.;11:293-301.
- Donovan J, Mills N, Smith M, . Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT study. BMJ 2002;325:766-70.
- MRC Health Services and Public Health Research Board . A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health 2000.
- John A, Enderby P. (2000). Reliability of speech and language therapists using therapy outcome measures. International Journal of Language &Amp; Communication Disorders n.d.;35:287-302.
- Hilari K, Byng S. 2001 Measuring quality of life in people with aphasia: the stroke specific quality of life scale. International Journal of Language and Communication Disorders n.d.;36:86-91.
- EuroQoL Group (1990) . EuroQoL: a new facility for the measurement of health-related quality of life. Health Policy n.d.;16:199-208.
- McKee KJ, Philp I, Lamura G, Prouskas C, Őberg B, . (2003) The COPE Index – a first stage assessment of negative impact, positive value and support of caregiving in informal carers of older people. Aging &Amp; Mental Health n.d.;7:39-52.
- Netton A, Dennett J. Unit costs of health and social care. PSSRU, University of Kent at Canterbury; 1997.
Appendix 4 Semistructured conversation script and TOM activity rating sheet
Outline structure for conversation
If the participant was able to give little or no response to the open questions (italic text), follow-up questions were provided which gave more structure and required shorter, more specific responses before ending the topic on another more open stimulus. Questions were used only as necessary to continue the interaction; conversational partners were free to develop any topic further on the basis of participants’ responses and did not have to cover all questions.
-
Can you tell me about your family and friends?
-
– Where do your family live?
-
– Do they live near?
-
– Who do you see over a week?
-
– What about friends and neighbours?
-
-
Can you tell me about things you do or things you’re interested in?
-
– What do you like to do in your spare time?
-
– Do you like to watch sport?
-
– Do you watch TV?
-
– What kinds of things do you enjoy?
-
-
What kind of work did you do before you were ill/before you retired?
-
– Did you use to work?
-
– Were you still working when you had your stroke?
-
– Can you tell me more about it?
-
-
Tell me about some places you’ve been on holiday
-
– Is there anywhere you’ve been to a lot?
-
– Is there somewhere special you really enjoyed?
-
– What did you like about it?
-
-
Can you describe a typical day for me?
-
– What time do you get up?
-
– What would you have for breakfast?
-
– What do you do in the afternoon?
-
-
Can you tell me what you remember about your stroke?
-
– Did you have to go to hospital?
-
– Which hospital were you in?
-
– About how long did you stay in?
-
– Can you remember much about that?
-
| 0 | Unable to communicate in any way. No effective communication. No interaction |
| 1 | Occasionally able to make basic needs known with familiar persons or trained listeners in familiar contexts. Minimal communication with maximal assistance |
| 2 | Limited functional communication. Consistently able to make basic needs/conversation understood but is heavily dependent on cues and context. Communicates better with trained listener or family members or in familiar settings. Frequent repetition required. Maintains meaningful interaction related to here and now |
| 3 | Consistently able to make needs known but can sometimes convey more information than this. Some inconsistency in unfamiliar settings. Is less dependent for intelligibility on cues and context. Occasional repetition required. Communicates beyond here/now with familiar persons; needs cues and prompting |
| 4 | Can be understood most of the time by any listener despite communication irregularities. Holds conversation; requires occasional prompts particularly with a wider range of people |
| 5 | Communicates effectively in all situations |
Appendix 5 The 20-item COAST scale
The actual COAST scale, the COAST script, guidance on recording and scoring answers and references to the published papers are available for download (as is the Carer COAST) at www.psych-sci.manchester.ac.uk/actnow/outputs/resources/.
For brevity the wording of the 20 COAST items is below:
-
In the past week, how well could you show that you mean ‘yes’ or ‘no’?
-
Nowadays, how well can you use other ways to help you communicate (e.g. pointing or writing)?
-
In the past week or so how well could you have a chat with someone you know well?
-
In the past week or so how well could you have a short conversation with an unfamiliar person?
-
In the past week or so how well could you join in a conversation with a group of people?
-
Nowadays, how well can you make yourself understood in longer sentences?
-
In the past week or so how well could you understand simple spoken information?
-
Nowadays, how well can you show that you don’t understand?
-
In the past week or so how well could you follow a change of subject in a conversation?
-
In the past week or so how well could you read?
-
In the past week or so how well could you write?
-
Nowadays, how well can you deal with money?
-
How much has your communication changed since just after your stroke?
-
What do you think about your communication now?
-
How often does confidence about communicating affect what you do?
-
Nowadays, what effect do your speech or language problems have on your family life?
-
Nowadays, what effect do your speech or language problems have on your social life?
-
Nowadays, what effect do your speech or language problems have on your interests or hobbies?
-
How often do difficulties communicating make you worried or unhappy?
-
How do you rate your overall quality of life?
Appendix 6 Discrete choice experiment data analysis
A regression model was used to analyse the data and estimate the weights. The probability of each respondent in the sample choosing A or B for each choice is dependent on the five attributes included:
Choose A or B = 1social and family life + Δ2interests and hobbies + Δ3 confidence in daily activities + Δ4 worry and unhappiness + Δ5waiting time +e + u, where Δ1 … Δ6 are the beta coefficients that describe the effect of each attribute.
The outputs of the analysis describe the significance and the relative importance of each attribute. The model provides information about the direction of the influence of each attribute, for example a positive sign for the social and family life attribute indicates that an intervention with lower detrimental impact on this attribute will be preferred to an intervention with a higher detrimental impact. Including an attribute that measures waiting time for treatment provides a method of indirect estimation of the amount of time respondents are willing to trade off against changes in the other attributes. For example, analysis of the marginal rate of substitution (amount of one attribute an individual is prepared to trade off against another) between waiting time and impact on social and family life gives an estimate of the WTW to get an improvement in social and family life. The marginal rate of substitution for impact on family social life and waiting time is estimated as: Δ1social and family life/Δwaiting time.
A multinomial logit (MNL) model (conditional logit model) was used. This is a fixed-effects model which is widely used in applications of this nature48 and is the statistical technique used to fit McFadden’s choice model. 75 The MNL is a procedure to allow estimation of the choice model implied by random utility theory and underpins the rationale for the DCE survey. The MNL is a relatively simple technique but it does rely on some restrictive assumptions (although it is often robust to violation of these restrictions75). These include, first, the independence of irrelevant alternatives. A second restriction is that utility is determined by the combinations of attributes and levels in each choice set, not the influence of individual characteristics (fixed effects). This means that for each participant preferences will vary between choice sets, reflecting the differences in attribute levels, but (un)observed variables or factors such as demographic characteristics will not affect the choices made and therefore utility of the attributes and levels. For this binary choice data set, a key restriction is the assumption of fixed (homogeneous) rather than random (heterogeneous) effects. Behaviourally, it may be expected that individual characteristics such as age will affect utility. For example, older people may have different preferences for each of the attributes to those of younger people. The presence of systematic heterogeneity owing to individual characteristics that were observed in the survey was controlled for by including interaction terms between each attribute and each sociodemographic characteristic. Subgroup analysis was used to explore the impact of any sociodemographic characteristics that were found to have a statistically significant impact on the model. However, this approach does not take account of any random variation in individual preferences owing to unobserved characteristics. Random-effect model specifications can help to reduce the impact of heterogeneity on the model, but the additional complexity of the models may reduce the consistency of the estimates and lead to confounding of the model parameters. 42 As a sensitivity analysis, the model for the main analysis was re-estimated using a random effects logistic regression.
Appendix 7 Results of discrete choice experiment survey
| Characteristic | Participant answered any choice questions | |||
|---|---|---|---|---|
| None: n (%) | Some: n (%) | All: n (%) | Total: n (%) | |
| Retirement age and abovea | 16 (67) | 17 (44) | 62 (30) | 95 (34) |
| Gender | ||||
| Male | 10 (42) | 14 (36) | 98 (47) | 122 (45) |
| Female | 14 (58) | 25 (64) | 112 (53) | 151 (55) |
| Ethnic group | ||||
| White British | 24 (100) | 38 (97) | 199 (96) | 261 (96) |
| Not white British | 0 (0) | 1 (3) | 9 (4) | 10 (4) |
| Experience of SL therapy or stroke | ||||
| Participant ever had SL therapy | 0 (0) | 2 (5) | 6 (3) | 8 (3) |
| Participant family/close friends ever had SL therapy | 2 (8) | 9 (23) | 43 (20) | 54 (20) |
| Participant ever had a stroke | 3 (12) | 0 (0) | 10 (5) | 13 (5) |
| Participant family/close friends had a stroke | 12 (48) | 29 (74) | 111 (53) | 152 (55) |
| Participant employment status | ||||
| In employmentb | 5 (22) | 25 (64) | 127 (60) | 157 (58) |
| Not in employment | 18 (78) | 14 (36) | 83 (40) | 115 (42) |
| Attribute | Most important n (%) |
2 n (%) |
3 n (%) |
4 n (%) |
Least important n (%) |
Average rank Mean (SD) |
|---|---|---|---|---|---|---|
| Ability to communicate affects social or family life | 165 (64) | 53 (21) | 15 (6) | 12 (5) | 12 (5) | 1.65 (1.09) |
| Ability to communicate affects involvement in interests and hobbies | 25 (10) | 37 (14) | 74 (29) | 50 (19) | 71 (28) | 3.41 (1.29) |
| Confidence in communicating affects what you do | 28 (11) | 63 (25) | 75 (29) | 68 (26) | 23 (9) | 2.98 (1.14) |
| Ability to communicate affects whether worried or unhappy | 33 (13) | 63 (25) | 60 (23) | 57 (22) | 44 (17) | 3.06 (1.29) |
| Waiting time for treatment | 50 (19) | 37 (14) | 33 (13) | 46 (18) | 91 (35) | 3.35 (1.55) |
| Choice set | Treament option | |||
|---|---|---|---|---|
| A | B | |||
| Frequency | % | Frequency | % | |
| 1 | 163 | 59 | 85 | 31 |
| 2 | 161 | 58 | 81 | 29 |
| 3 | 38 | 14 | 209 | 75 |
| 4 | 203 | 73 | 46 | 17 |
| 5 | 15 | 5 | 234 | 84 |
| 6 | 4 | 1 | 243 | 87 |
| 7 | 234 | 84 | 11 | 4 |
| 8 | 18 | 6 | 228 | 82 |
| 9 | 109 | 39 | 129 | 46 |
| 10 | 10 | 4 | 232 | 83 |
| 11 | 75 | 27 | 164 | 59 |
| 12 | 216 | 78 | 23 | 8 |
| 13 | 43 | 15 | 197 | 71 |
| 14 | 213 | 77 | 30 | 11 |
| 15 | 185 | 67 | 56 | 20 |
| 16 | 68 | 24 | 170 | 61 |
| 17 | 189 | 68 | 45 | 16 |
| 18 | 209 | 75 | 33 | 12 |
| 19 | 55 | 20 | 185 | 67 |
| 20 | 105 | 38 | 137 | 49 |
| 21 | 4 | 1 | 238 | 86 |
| 22 | 76 | 27 | 163 | 59 |
| 23 | 152 | 55 | 82 | 29 |
| 24 | 47 | 17 | 192 | 69 |
| 25 | 18 | 6 | 225 | 81 |
| Choice | Coefficient (∆x) | SE | p-value | 95% CI |
|---|---|---|---|---|
| Participant aged < 60 years | ||||
| Ability to communicate with family or friends is | ||||
| Quite poor | 1.24 | 0.12 | 0.000 | 1.01 to 1.47 |
| Fair | 1.94 | 0.14 | 0.000 | 1.67 to 2.20 |
| Quite good | 2.17 | 0.14 | 0.000 | 1.89 to 2.46 |
| As good as before the stroke | 2.89 | 0.15 | 0.000 | 2.60 to 3.17 |
| After therapy your ability to communicate means your involvement in interests and hobbies is | ||||
| Quite poor | 0.83 | 0.13 | 0.000 | 0.58 to 1.09 |
| Fair | 1.09 | 0.14 | 0.000 | 0.81 to 1.37 |
| Quite good | 1.16 | 0.14 | 0.000 | 0.88 to 1.43 |
| As good as before the stroke | 1.47 | 0.10 | 0.000 | 1.27 to 1.67 |
| After therapy your confidence in communicating affects what you do | ||||
| Very often | 0.48 | 0.12 | 0.000 | 0.24 to 0.72 |
| Sometimes | 1.23 | 0.13 | 0.000 | 0.97 to 1.49 |
| Hardly ever | 1.02 | 0.14 | 0.000 | 0.74 to 1.30 |
| Never | 0.99 | 0.10 | 0.000 | 0.79 to 1.19 |
| After therapy your ability to communicate means you are worried or unhappy | ||||
| Very often | 0.86 | 0.11 | 0.000 | 0.66 to 1.07 |
| Sometimes | 1.49 | 0.14 | 0.000 | 1.23 to 1.76 |
| Hardly ever | 1.50 | 0.15 | 0.000 | 1.21 to 1.80 |
| Never | 1.23 | 0.12 | 0.000 | 1.00 to 1.47 |
| Waiting list for treatment is | ||||
| 1 month | 0.50 | 0.14 | 0.000 | 0.23 to 0.76 |
| 3 months | –0.05 | 0.15 | 0.710 | –0.34 to 0.23 |
| 6 months | –0.31 | 0.14 | 0.024 | –0.58 to –0.04 |
| 1 year | –0.63 | 0.11 | 0.000 | –0.83 to –0.42 |
| Overall | –0.05 | 0.01 | 0.000 | –0.07 to –0.04 |
| Participant aged ≥ 60 years or above | ||||
| Ability to communicate with family or friends is | ||||
| Quite poor | 1.12 | 0.13 | 0.000 | 0.88 to 1.37 |
| Fair | 2.06 | 0.24 | 0.000 | 1.59 to 2.53 |
| Quite good | 2.39 | 0.25 | 0.000 | 1.90 to 2.88 |
| As good as before the stroke | 3.08 | 0.25 | 0.000 | 2.58 to 3.58 |
| After therapy your ability to communicate means your involvement in interests and hobbies is | ||||
| Quite poor | 0.73 | 0.14 | 0.000 | 0.46 to 1.00 |
| Fair | 0.22 | 0.28 | 0.425 | –0.32 to 0.77 |
| Quite good | 0.37 | 0.27 | 0.167 | –0.16 to 0.90 |
| As good as before the stroke | 1.19 | 0.11 | 0.000 | 0.98 to 1.39 |
| After therapy your confidence in communicating affects what you do | ||||
| Very often | –0.39 | 0.23 | 0.100 | –0.84 to 0.07 |
| Sometimes | 0.23 | 0.23 | 0.323 | –0.23 to 0.68 |
| Hardly ever | 0.12 | 0.23 | 0.617 | –0.34 to 0.57 |
| Never | 0.91 | 0.12 | 0.000 | 0.69 to 1.14 |
| After therapy your ability to communicate means you are worried or unhappy | ||||
| Very often | 0.83 | 0.11 | 0.000 | 0.61 to 1.05 |
| Sometimes | 1.88 | 0.25 | 0.000 | 1.39 to 2.36 |
| Hardly ever | 2.10 | 0.29 | 0.000 | 1.54 to 2.67 |
| Never | 1.69 | 0.26 | 0.000 | 1.18 to 2.19 |
| Waiting list for treatment is | ||||
| 1 month | 0.47 | 0.14 | 0.001 | 0.19 to 0.74 |
| 3 months | –0.12 | 0.15 | 0.424 | –0.42 to 0.18 |
| 6 months | –0.48 | 0.15 | 0.002 | –0.79 to –0.18 |
| 1 year | –1.38 | 0.24 | 0.000 | –1.84 to –0.91 |
| Overall | –0.11 | 0.02 | 0.000 | –0.14 to –0.08 |
Appendix 8 Costing method to estimate unit costs of speech and language therapy and attention control
The cost per minute of SL therapist time was calculated from the reported cost per hour. 53 The cost per hour was reported for a median full-time equivalent basic salary for Agenda for Change (AfC) Band 5 of the January–March 2009 NHS staff earnings estimates. The cost included basic salary plus hours-related pay, overtime, occupation payments, location payments and other payments, including redundancy pay or payment of notice periods, salary on costs, revenue and capital overheads, travel and indirect SL therapist time spent on travel and non-clinical activity. 53
For the AC intervention, the job description and role was mapped on to the AfC bands for NHS staff by a local NHS human resources manager. The nearest match was for a higher-grade care worker, AfC Band 3. The nearest match to national published unit costs was for a hospital-based clinical support worker, which is a higher-grade AfC Band 3 role (note that this is not meant to imply that the AC could, or should be, provided by hospital-based clinical support workers if the intervention were to be implemented into routine care). The cost per hour was reported for a median full-time equivalent basic salary for AfC Band 3 of the January–March 2009 NHS staff earnings estimates for unqualified nurses. The cost included basic salary plus hours-related pay, overtime, occupation payments, location payments and other payments including redundancy pay or payment of notice periods, salary on costs, revenue and capital overheads, travel and indirect care worker time spent on travel and non-clinical activity. 53 The proportion of indirect care worker was based on that used for SL therapy, to reflect the higher amount of time the care worker would spend outside the hospital to deliver the AC intervention, compared with a clinical support worker. In a sensitivity analysis, the actual grades of SL therapy staff used in the trial were costed and the actual costs incurred for the AC staff were used.
Appendix 9 Detailed unit costs of resources
| Hospital inpatient stay, per day | Average unit cost | Sources |
|---|---|---|
| A&E, < 1-day stay | 160 | Average of accident and emergency < 1-day stay, reported in DoH reference costs 2008–952 |
| A&E > 1-day stay | 298 | Average of accident and emergency for > 1-day stay, reported in DoH reference costs 2008–952 |
| Acute stroke, combined acute stroke and rehabilitation, discharge lounge | 266 | Average of non-elective stroke admissions, reported in DoH reference costs 2008–952 |
| Stroke rehabilitation unit | 260 | Average of stroke rehabilitation (without treatment episode) admissions, reported in DoH reference costs 2008–952 |
| General medical | 233 | Average of rehabilitation medicine and other medicine, reported in DoH reference costs 2008–952 |
| General surgical | 392 | CIPFA 2005,62 inflated to 2008–9 price year53 |
| Geriatric | 174 | CIPFA 2005,62 inflated to 2008–9 price year53 |
| Other | 312 | Average of general medical and general surgery costs |
| MAU or clinical decisions unit | 204 | Average of rehabilitation assessment and other medicine, reported in DoH reference costs 2008–952 |
| ICU | 1149 | Average of adult critical care, reported in DoH reference costs 2008–952 |
| Emergency observation, emergency assessment unit, HDU | 560 | Assumed to be similar to critical care, no organs supported, reported in DoH reference costs 2008–952 |
| Cardiology | 554 | CIPFA 2005,62 inflated to 2008–9 price year53 |
| Hospital inpatient stay, per week | Average unit cost | Sources |
|---|---|---|
| Nursing home | 678 | PSSRU 200953 |
| Residential home | 467 | PSSRU 200953 |
| Sheltered care | 271 | PSSRU 200953 |
| Day hospital per visit | Average unit cost | Sources |
|---|---|---|
| Rehabilitation | 228 | Average of rehabilitation outpatient visits, reported in DoH reference costs 2008–952 |
| Physiotherapy | 457 | Chest physiotherapy day-cases reported in DoH reference costs 2008–952 |
| Ophthalmology | 240 | Assumed to equal other medical, CIPFA 2005,62 inflated to 2008–9 price year63 |
| Gastroenterology, chest | 200 | CIPFA 2005,62 inflated to 2008–9 price year53 |
| Endoscopy | 531 | Average of endoscopy day-case visits, reported in DoH reference costs 2008–952 |
| Community day hospital | 104 | PSSRU 2006,63 inflated to 2008–9 price year53 |
| Circulation laboratory | 206 | Assumed equal to cardiology outpatient visit (two outpatient sessions = 1-day attendance) |
| Outpatient clinic per visit | Average unit cost | Sources | |
|---|---|---|---|
| Hospital/clinic | Home | ||
| NHS (non-trial) SL therapy | 54 | 47 | Average of SL therapy hospital outpatient visits, reported in DoH reference costs 2008–9,52 home visit, PSSRU 200953 |
| Physiotherapy | 228 | 47 | Average of physiotherapy hospital outpatient visits, reported in DoH reference costs 2008–9,52 home visit, PSSRU 200953 |
| Occupational therapy | 50 | 46 | Average of occupational therapy hospital outpatient visits, reported in DoH reference costs 2008–9,52 home visit, PSSRU 200953 |
| Stroke consultant, stroke team, community stroke team, community neurological rehabilitation team | 83 | 128 | Clinic visit DoH reference costs 2008–9,52 home visit, PSSRU 200953 |
| Cardiology | 108 | NA | Average of cardiology outpatient visits, reported in DoH reference costs 2008–952 |
| Anticoagulant clinic | 36 | NA | Assumes equal to GP surgery visit, PSSRU 200953 |
| Dermatology | |||
| Elderly medicine/care, geriatrics | 158 | NA | Average of geriatrics outpatient visits, reported in DoH reference costs 2008–952 |
| ENT | |||
| Neurology | 131 | NA | Average of neurology outpatient visits, reported in DoH reference costs 2008–952 |
| Clinical neurosciences | 135 | NA | Average of imaging outpatient visits, reported in DoH reference costs 2008–952 |
| Ophthalmology | 73 | NA | Average of ophthalmology outpatient visits, reported in DoH reference costs 2008–952 |
| Podiatry | 11 | 21 | PSSRU 200953 |
| Urology | 87 | NA | Average of urology outpatient visits, reported in DoH reference costs 2008–952 |
| Vascular surgery | 100 | NA | Average of vascular surgery outpatient visits, reported in DoH reference costs 2008–952 |
| District nurse/nurse/continence advisor nurse | 10 | 24 | PSSRU 200953 |
| Medical specialties | |||
| Orthopaedics | 86 | NA | Average of orthopaedics outpatient visits, reported in DoH reference costs 2008–952 |
| GP/doctor | 36 | 58 | PSSRU 200953 |
| Dietitian | NA | 87 | PSSRU 200953 |
Appendix 10 Correlation between economic variables and clinical and demographic variables
| EQ-5D utility scoresb | Total costb | BI scorec | Agec | Genderc | Ethnicityc | TOM | COAST | |
|---|---|---|---|---|---|---|---|---|
| EQ-5D utility scores | ||||||||
| Correlation | 1.00 | –0.46 | 0.51 | 0.08 | –0.06 | –0.12 | 0.36 | 0.31 |
| p-value | 0.00 | 0.00 | 0.38 | 0.38 | 0.09 | 0.00 | 0.00 | |
| n | 140 | 90 | 140 | 140 | 140 | 140 | 128 | 120 |
| Total cost | ||||||||
| Correlation | –0.46 | 1.00 | –0.76 | 0.14 | 0.08 | –0.14 | –0.72 | –0.46 |
| p-value | 0.00 | 0.00 | 0.15 | 0.34 | 0.09 | 0.00 | 0.00 | |
| n | 90 | 101 | 101 | 101 | 101 | 101 | 90 | 80 |
| BI score | ||||||||
| Correlation | 0.51 | –0.76 | 1.00 | –0.12 | –0.05 | –0.03 | 0.59 | 0.29 |
| p-value | 0.00 | 0.00 | 0.11 | 0.45 | 0.65 | 0.00 | 0.00 | |
| n | 140 | 101 | 170 | 170 | 170 | 170 | 141 | 126 |
| Age | ||||||||
| Correlation | 0.08 | 0.14 | –0.12 | 1.00 | 0.15 | 0.04 | –0.17 | –0.04 |
| p-value | 0.38 | 0.15 | 0.11 | 0.02 | 0.50 | 0.05 | 0.64 | |
| n | 140 | 101 | 170 | 170 | 170 | 170 | 141 | 126 |
| Gender | ||||||||
| Correlation | –0.06 | 0.08 | –0.05 | 0.15 | 1.00 | –0.06 | 0.00 | –0.08 |
| p-value | 0.38 | 0.34 | 0.45 | 0.02 | 0.43 | 0.97 | 0.27 | |
| n | 140 | 101 | 170 | 170 | 170 | 170 | 141 | 126 |
| Ethnicity | ||||||||
| Correlation | –0.12 | –0.14 | –0.03 | 0.04 | –0.06 | 1.00 | –0.11 | –0.12 |
| p-value | 0.09 | 0.09 | 0.65 | 0.50 | 0.43 | 0.12 | 0.11 | |
| n | 140 | 101 | 170 | 170 | 170 | 170 | 126 | 141 |
Appendix 11 Detailed use of inpatient services, by ward type, available cases, unadjusted for baseline covariates
| Ward type | AC | SL therapy | ||||
|---|---|---|---|---|---|---|
| n | Mean use | SD | n | Mean use | SD | |
| Pre randomisation length of stay (from index stroke to day of randomisation) | ||||||
| A&E | 71 | 0.30 | 0.63 | 79 | 0.22 | 0.47 |
| Acute stroke | 69 | 6.15 | 5.65 | 77 | 5.00 | 5.24 |
| Stroke rehabilitation | 69 | 3.19 | 5.65 | 77 | 2.58 | 4.87 |
| General medical | 69 | 1.32 | 4.51 | 76 | 1.10 | 3.69 |
| General surgical | 68 | 0.11 | 0.74 | 75 | 0.16 | 0.97 |
| Geriatric | 68 | 0.29 | 2.38 | 76 | 0.17 | 0.82 |
| Other | 68 | 1.02 | 2.81 | 75 | 0.65 | 2.49 |
| Combined stroke and rehabilitation | 69 | 1.23 | 3.87 | 76 | 1.95 | 5.34 |
| MAU | 71 | 0.26 | 0.74 | 76 | 0.36 | 0.81 |
| Emergency assessment unit | 68 | 0.07 | 0.30 | 75 | 0.24 | 1.50 |
| Discharge lounge | 68 | 0.00 | 0.00 | 75 | 0.00 | 0.00 |
| ICU | 68 | 0.09 | 0.73 | 75 | 0.00 | 0.00 |
| Emergency observation | 68 | 0.02 | 0.17 | 75 | 0.05 | 0.31 |
| Clinical decisions unit | 68 | 0.12 | 0.53 | 76 | 0.07 | 0.23 |
| Cardiology | 68 | 0.00 | 0.00 | 75 | 0.19 | 1.50 |
| Missing or ward type not known | 69 | 0.04 | 0.27 | 77 | 0.00 | 0.00 |
| Post randomisation length of stay | ||||||
| A&E | 71 | 0.13 | 0.34 | 79 | 0.14 | 0.75 |
| Acute stroke | 69 | 7.45 | 13.81 | 77 | 3.27 | 6.77 |
| Stroke rehabilitation | 69 | 27.62 | 41.95 | 77 | 21.08 | 31.24 |
| General medical | 69 | 1.31 | 5.78 | 76 | 1.13 | 4.83 |
| General surgical | 68 | 0.09 | 0.54 | 75 | 0.78 | 3.25 |
| Geriatric | 68 | 0.18 | 1.46 | 76 | 0.28 | 1.71 |
| Other | 68 | 1.95 | 5.62 | 75 | 0.65 | 2.76 |
| Combined stroke and rehabilitation | 69 | 0.96 | 5.66 | 76 | 3.64 | 13.82 |
| MAU | 71 | 0.10 | 0.38 | 76 | 0.05 | 0.28 |
| Emergency assessment unit | 68 | 0.06 | 0.39 | 75 | 0.04 | 0.27 |
| Discharge lounge | 68 | 0.06 | 0.47 | 75 | 0.03 | 0.23 |
| ICU | 68 | 0.25 | 2.04 | 75 | 0.00 | 0.00 |
| Emergency observation | 68 | 0.00 | 0.00 | 75 | 0.00 | 0.00 |
| Clinical decisions unit | 68 | 0.08 | 0.42 | 76 | 0.02 | 0.12 |
| Cardiology | 68 | 0.69 | 5.70 | 75 | 0.11 | 0.92 |
| Missing or ward type not known | 68 | 0.00 | 0.00 | 75 | 0.00 | 0.00 |
Appendix 12 Use of primary and community care services by participant, at 6-month scheduled follow-up, available cases, unadjusted for baseline covariates
| Services | AC (N = 85) | SL therapy (N = 85) | ||
|---|---|---|---|---|
| n | % | n | % | |
| GP (surgery visit) | 41 | 68 | 48 | 70 |
| GP (home visit) | 28 | 47 | 24 | 36 |
| District nurse, health visitor or community health nurse | 39 | 67 | 44 | 66 |
| Social worker | 30 | 49 | 29 | 41 |
| Therapist | 45 | 75 | 48 | 70 |
| Counsellor | 3 | 5 | 2 | 3 |
| Home help/care worker | 22 | 37 | 25 | 35 |
| Citizen Advice/Welfare Rights | 8 | 13 | 7 | 10 |
| Psychiatrist/psychologist | 4 | 7 | 1 | 1 |
| Day centre | 5 | 9 | 11 | 16 |
| Social club | 4 | 7 | 4 | 6 |
| Delivery of food, medication or laundry | 18 | 31 | 25 | 36 |
| Family or patient self-help group | 11 | 18 | 21 | 30 |
Appendix 13 Health status measured by the EQ-5D at 6-month scheduled follow-up, available cases, unadjusted for baseline covariates
| Health domain | AC (N = 85) | SL therapy (N = 85) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Participant completed one or more items on EQ-5D | 63 | 74 | 74 | 87 |
| Participant died before assessment | 8 | 9 | 4 | 5 |
| Mobility | 63 | 74 | ||
| No problem | 22 | 35 | 24 | 32 |
| Some problem | 35 | 56 | 42 | 57 |
| Extreme problem | 6 | 10 | 8 | 11 |
| Self-care | 62 | 74 | ||
| No problem | 34 | 55 | 45 | 61 |
| Some problem | 23 | 37 | 15 | 20 |
| Extreme problem | 5 | 8 | 14 | 19 |
| Usual activities | 62 | 73 | ||
| No problem | 22 | 35 | 31 | 42 |
| Some problem | 30 | 48 | 28 | 38 |
| Extreme problem | 10 | 16 | 14 | 19 |
| Pain | 59 | 73 | ||
| No problem | 27 | 46 | 40 | 55 |
| Some problem | 23 | 39 | 24 | 33 |
| Extreme problem | 9 | 15 | 9 | 12 |
| Anxiety and depression | 60 | 72 | ||
| No problem | 31 | 52 | 26 | 36 |
| Some problem | 21 | 35 | 34 | 47 |
| Extreme problem | 8 | 13 | 12 | 17 |
Appendix 14 Incremental costs and outcomes, main analysis
| Covariate | Coefficient net effect | SE | 95% CI |
|---|---|---|---|
| Trial SL therapist | 0.00 | 0.06 | –0.12 to 0.12 |
| Total baseline 10-item Modified BI score | 0.03 | 0.01 | 0.02 to 0.04 |
| Trial site 2 | –0.08 | 0.12 | –0.32 to 0.17 |
| Trial site 3 | –0.19 | 0.14 | –0.48 to 0.09 |
| Trial site 4 | –0.04 | 0.17 | –0.38 to 0.29 |
| Trial site 5 | –0.30 | 0.17 | –0.64 to 0.04 |
| Trial site 6 | –0.01 | 0.18 | –0.37 to 0.35 |
| Trial site 7 | –0.03 | 0.13 | –0.29 to 0.23 |
| Trial site 8 | –0.04 | 0.13 | –0.31 to 0.22 |
| Trial site 9 | –0.04 | 0.14 | –0.32 to 0.24 |
| Trial site 10 | –0.36 | 0.33 | –1.01 to 0.30 |
| Trial site 11 | –0.18 | 0.19 | –0.54 to 0.19 |
| Trial site 12 | –0.02 | 0.24 | –0.49 to 0.45 |
| Dysarthria | –0.02 | 0.10 | –0.23 to 0.19 |
| Both dysarthria and aphasia | –0.04 | 0.08 | –0.20 to 0.13 |
| Severity of communication problem | –0.11 | 0.07 | –0.25 to 0.04 |
| Constant | 0.31 | 0.13 | 0.06 to 0.56 |
| Covariate | Coefficient net effect | SE | 95% CI |
|---|---|---|---|
| Trial SL therapist | 135 | 1353 | –2539 to 2810 |
| Total baseline 10-item Modified BI score | –1141 | 117 | –1373 to –908 |
| Trial site 2 | 2829 | 2760 | –2640 to 8298 |
| Trial site 3 | 4573 | 3142 | –1646 to 10,792 |
| Trial site 4 | 3732 | 3612 | –3447 to 10,910 |
| Trial site 5 | 1844 | 3737 | –5563 to 9251 |
| Trial site 6 | –440 | 3617 | –7594 to 6714 |
| Trial site 7 | –560 | 3281 | –7085 to 5966 |
| Trial site 8 | 1371 | 2746 | –4084 to 6827 |
| Trial site 9 | –1138 | 3125 | –7339 to 5064 |
| Trial site 10 | –9536 | 6602 | –22,590 to 3519 |
| Trial site 11 | 7461 | 4297 | –1050 to 15,972 |
| Trial site 12 | 4002 | 5424 | –6720 to 14,725 |
| Dysarthria | –758 | 2452 | –5610 to 4094 |
| Both dysarthria and aphasia | 1001 | 1856 | –2699 to 4700 |
| Severity of communication problem | 2306 | 1636 | –934 to 5546 |
| Constant | 21,929 | 2871 | 16,240 to 27,619 |
Appendix 15 Participant thematic categories and their working definitions
| Code name | Description |
|---|---|
| Emotional well-being | Any reference by participants to affective responses they experienced following the stroke and the impact of emotional aspects of support from SL therapists/visitors. Mention of specific emotions and how they were used also included (e.g. humour) |
| Amount and intensity | Any reference by participants to amount and/or intensity of intervention by either SL therapist or visitor including actual description and perceived consequences as well as meaning attributed to amount/intensity effects |
| Closure | Any reference by participants to feelings and thoughts associated with the end of contact with SL therapist/visitor, including the initiation and timing of ending, reflections on closure process |
| Confidence | Any reference by participants to the impact of SL therapy/visits on their perceived level of confidence, including impact on independence, experienced self-worth and confidence in talking to others |
| Guidance and support | Any reference by participants to the guidance and support offered by the SL therapist/visitor, ranging from informal social support to more structured guidance with, for example, speech problems |
| Interpersonal factors | Any reference by participants to their experiences of the interpersonal relationship between themselves and the SL therapist/visitor, including perceived impact of the interpersonal relationship on efficacy of the intervention |
| Meeting individual needs | Any reference by participants to how the SL therapist/visits met or failed to meet their perceived needs, including work, social and communication needs |
| Observing progress | Any reference by participants to how they have been able to perceive their progress, for instance through relatives noticing an improvement, by noticing changes in daily linguistic functioning or by comparing before/after samples of own voice |
| Social factors | Any reference by participants to the way in which their social situation impacted on the perceived efficacy of the intervention, particularly with reference to the support network offered locally by friends/family |
Appendix 16 Extracts from the 102-page ACT NoW Therapy Manual*
(*Developed by NHS SL therapists, Sage and Lambon Ralph.)
Assessment (from pp. 16–19 of manual)
As a minimum standard, all participants will be assessed at the start of ACT NoW intervention. The assessment will be as follows:
-
All participants will complete the ACT NoW Functional Assessment
-
All dysarthric participants will complete the Robertson Dysarthria profile
-
All aphasic participants will be assessed with the ACT NoW Aphasia Assessment (AANA).
The ACT NoW aphasia assessment
All participants who have aphasia will complete the ANAA aphasia battery. The ANAA is on p. 40 of the Resources section, and details of how to administer and score individual sections are available in the test manuals for the corresponding tests.
The ACT NoW functional assessment
The ACT NoW functional assessment is on p. 57 of the Resources section.
To complete the functional assessment, a variety of assessment techniques will be used. These will include observation and informal conversation with the participant, as well as some informal assessment, for example reading from the hospital menu sheet. The observations of other members of the MDT and family/visitors may also be gathered.
The assessment is divided into five sections: verbal, reading, writing, non-verbal and augmentative communication. For each item, the SL therapist will note whether the participant needs prompts to communicate their message to complete the task.
Verbal communication skills
Many of the items in this section can be completed through informal conversation.
Participants will be allocated a score of 2–0:
-
2 indicates that no prompts were needed: the participant successfully communicated the item without any help
-
1 indicates that some prompts were needed
-
0 indicates that even with prompts the participant was unable to complete the message.
The score is purely a measure of the success of the communication, and it does not measure the language skills used to convey the message. A score of ‘2’ will be given even if there are obvious word finding difficulties, spelling errors or syntactical errors.
For the final two items, the participants’ awareness of their errors and attempts to use repair strategies are scored. A score of:
-
2 indicates that a high level of awareness of errors and when they have not been understood
-
1 indicates some awareness of errors recognition they have not been understood
-
0 indicates no awareness of errors or communication breakdown.
Reading skills
The reading section will be observed using items that are readily available in a ward environment, such as greetings cards and hospital menu cards. If a newspaper is available, the participant will be asked to briefly give some details of one story. Questions will be used to determine whether the participant has understood the written content of the other items.
A score of 2–0 will be given:
-
2 indicates accurate understanding of what has been read
-
1 indicates partial understanding of what has been read
-
0 indicates no understanding of what has been read.
Writing skills
The participant will be asked to write their name and address; they will only be asked to fill in forms or attempt crosswords if they are available and of relevance to the participant.
-
2 indicates that writing is accurate enough to convey information
-
1 indicates that writing is attempted but is not accurate enough to convey the message without prompts
-
0 indicates that writing is either not attempted, or is not accurate enough to convey any information even with prompts.
Non-verbal skills
The participants’ use of gesture and pointing is scored.
-
2 indicates that gesture and pointing are used successfully without the need for prompts
-
1 indicates that gesture and pointing are used with partial success; some prompting may also be needed
-
0 indicates that either gesture and pointing are not used, or that they are not used successfully.
Awareness/insight
Consider the participants’ awareness of their condition and implications it may have.
Augmentative communication
In this section, any initial observations about the participants’ likely ability to be able to use either a picture or alphabet communication chart are noted.
The scores for each section should be considered, and the information gathered from the functional assessment will be used when providing written information for the family/carers and the multi-disciplinary team.
Robertson dysarthria profile
All participants who have dysarthria will complete the full Robertson Dysarthria Profile as directed in the profile manual.
Additional assessments
During ACT NoW intervention, other assessments may be administered to support the planning and delivery of the intervention. The details of other speech and language assessments are on p. 71 in the Resources section.
Communication history form
For all participants with moderate to severe communication difficulties, a communicative history form will be given to relatives and carers. The purpose of this form is to gather background social information about the interests of the participant. This information will be incorporated into making therapy functional and relevant to the participant, and it will also be used if making a communication folder for participants. The Communication History form is on p. 61 in the Resources section.
Alternative or augmentative communication
All participants with severe dysarthria and/or aphasia will be considered as potential AAC users and assessed using the AAC assessment form. This will be completed in the initial stage of therapy. The AAC assessment forms are on p. 69 in the Resources section.
ACT NoW Aphasia Assessment (from p. 40 of manual)
| SUMMARY SHEET | |||||
|---|---|---|---|---|---|
| Name: | Date: | ||||
| Test name | Norm score | Score | Pass/fail | Comments | |
| 1 | PALPA 47 | Mean = 19.65, SD = 0.55, range = 17 to 20 | |||
| 2 | Pyramid and Palm Trees Test | Mean = 24 to 26 | |||
| 3 | Synonym Judgement Test | Mean = 15.9, SD = 0.31 | |||
| 4 | Boston Naming Test & Picture to Word Matching |
Mean = 55.8, SD = 3.8 Mean = 56.8, SD = 3.0 Mean = 55.2, SD = 4.0 Mean = 53.3, SD = 4.6 Mean = 48.9, SD = 6.3 |
|||
| 5 | Spoken Cookie Theft Description | Complexity Index: Mean = 1.8, SD = 0.42 | |||
| 6 | PALPA 9 |
LI-LF: Mean = 19.67, SD = 0.58 Non-words: Mean = 19.99, SD = 1.68 |
|||
| 7 | PALPA 31 | LI-LF: Mean = 19.52 ,SD = 0.68 | |||
| 8 | PALPA 36 |
Three-letter: Mean = 5.77, SD = 0.71 Four-letter: Mean = 5.89, SD = 0.43 Five-letter: Mean = 5.57, SD = 0.90 Six-letter: Mean = 5.65, SD = 0.85 |
|||
| 9 |
Comprehension of orally read sentences (BDAE) Repetition performance |
Mean = 4.87, SD = 0.35 | |||
| 10 | Writing screen | ||||
| 11 | Written Cookie Theft description | Complexity Index: Mean = 1.8, SD = 0.42 | |||
List of abbreviations
- AAC
- alternative or augmentative communication
- AANA
- ACT NoW Aphasia Assessment
- AC
- attention control
- ACT NoW
- Assessing the effectiveness of Communication Therapy in the North West
- ADLs
- activities of daily living
- AfC
- Agenda for Change
- BDAE
- Boston Diagnostic Aphasia Examination
- BI
- Barthel Index
- CEAA
- cost-effectiveness acceptability analysis
- CI
- confidence interval
- COAST and Carer COAST
- Communication Outcomes After Stroke scale and associated carer version
- COPE
- Carers of Older People in Europe index
- CQW
- Coast Quality Weights
- DCE
- discrete choice experiment
- DDK
- diadochokinetic
- DoH
- Department of Health
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- Euroqol – European Quality of Life-5 Dimensions
- FAST
- Frenchay Aphasia Screening Test
- HRQoL
- health-related quality of life
- HTA
- health technology assessment
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning, Disability and Health
- IQR
- interquartile range
- LI-LF
- low imageability, low frequency
- MDT
- multidisciplinary team
- MREC
- Multicentre Research Ethics Committee
- NAO
- National Audit Office
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- PALPA
- Psycholinguistic Assessment of Language Processing in Aphasia
- PI
- principal investigator
- PROM
- patient-related outcome measure
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RCSLT
- Royal College of Speech and Language Therapists
- RCT
- randomised controlled trial
- RUG
- research user group (service users and carers as research partners)
- SAE
- serious adverse event
- SAQOL
- Stroke and Aphasia Quality of Life Scale
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- SL
- speech and language
- SLT
- speech and language therapy
- SRN
- Stroke Research Network
- TA
- therapy assistant
- TOM
- Therapy Outcome Measure activity subscale
- TSC
- Trial Steering Committee
- WTE
- whole-time equivalent
- WTPT
- willingness-to-pay threshold
- WTW
- willingness to wait
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health