Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/67/01. The contractual start date was in June 2010. The draft report began editorial review in May 2011 and was accepted for publication in December 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Rodgers et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
The term ‘depression’ can refer to a range of mental health problems primarily characterised by persistent depressed mood and loss of interest in activities, among other associated emotional, cognitive, physical and behavioural symptoms. 1
Depression is the most common mental disorder in community settings, and a major cause of disability across the world. A World Health Organization cross-sectional survey revealed the global 1-year prevalence of a depressive episode to be 3.2%. 2 The prevalence is greater still in people with other medical conditions (e.g. 10–14% of patients receiving general hospital care). 3 Neuropsychiatric disorders account for one-third of all years lost to disability (YLDs), with unipolar major depressive disorder alone accounting for 11% of global YLDs. 3
The Psychiatric Morbidity Survey (PMS) of UK adults aged 16–74 years in 2000 reported a overall prevalence rate for depression of 26 per 1000 people, with slightly higher rates for women than for men. 4 This survey also suggested that having a depressive episode was associated with unemployment, belonging to social classes 4 and below, having no formal educational qualifications, living in local authority or housing association accommodation, moving three or more times in the last 2 years, and living in an urban environment. 4
Various theories for the causation of depression have derived from research on the impact of physical and endocrine processes,5 brain structure and function,6 and cognitive and emotional processes. 7 All of these factors are likely to influence an individual’s vulnerability to depression, alongside factors such as gender, genetic and family factors, adverse childhood experiences, personality factors and social circumstances. 8 In terms of depression, vulnerability factors (e.g. genetic factors) interact with social or physical triggers, such as stressful life events or physical illness, to result in a depressive episode. The stress–vulnerability model suggests that the probability of a mental health problem occurring is based on an interaction between a person’s vulnerability to developing that problem and that person’s exposure to particular stressors or risk factors for that problem. 9 However, some episodes of depression occur in the absence of a stressful event, and, conversely, many such events are not followed by a depressive disorder in those with vulnerabilities. 8
Even after successful treatment, the risk of relapse after remission is significant, and has been reported as 50% among patients having experienced one episode of major depression, and 70% and 90% after two and three episodes, respectively. 10 In many of these individuals this pattern becomes worse with subsequent repeated depressive episodes, with an increase in severity and frequency and a lack of responsiveness to treatments. 11,12 Research has shown that the long-term outcome for those individuals who experience multiple episodes has altered little in the last 20 years. 13 At least 10% of patients have persistent or chronic depression. 14
Current guidance from the National Institute for Health and Clinical Excellence (NICE) cites a review by the King’s Fund, which estimated that there were 1.24 million people with depression in England in 2006, and this was projected to rise to 1.45 million by 2026. Based on these figures, the total costs for depression in 2007 (including prescribed drugs, inpatient care, other NHS services, supported accommodation, social services and lost employment in terms of workplace absenteeism) were estimated to be £1.7B, with lost employment increasing this total to £7.5B. These figures were projected to be £3B and £12.2B, respectively, by 2026. 15
Diagnosis
Depression is typically diagnosed according to criteria set out in either the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV),1 or the International Statistical Classification of Diseases and Related Health Problems, 10th Edition (ICD-10). 16 DSM-IV was developed by the American Psychiatric Association, whereas the ICD-10 is the comparable European guide for diagnosis of mental disorders. Although similar, the two systems are not identical, having slightly differing thresholds for the number of symptoms required for a depressive episode (as termed in ICD-10; ‘major depressive episode’ in DSM-IV).
The 2010 NICE guideline8 on depression states that a diagnosis of a depression requires assessment of three linked but separate factors: (1) severity, (2) duration and (3) course. Diagnosis requires a minimum of 2 weeks’ duration of symptoms and including at least one key symptom (low mood, loss of interest or pleasure). Individual symptoms should be assessed for severity and impact on function and be present for most of every day. The following categories adapted from DSM-IV were outlined:
-
subthreshold depressive symptoms (fewer than five out of nine symptoms of depression)
-
mild depression (few, if any, symptoms in excess of the five required to make the diagnosis, and the symptoms result in only minor functional impairment)
-
moderate depression (symptoms or functional impairment are between ‘mild’ and ‘severe’)
-
severe depression (most symptoms, and the symptoms markedly interfere with functioning).
Treatment
The objective of treatment is to achieve remission or at least adequate control of depressive symptoms. For some, depression can become a gateway to a lifetime of disability and impairment, so it is important to develop interventions and services to not only reduce depressive symptoms and restore functioning, but also to enable people to self-manage their problems and prevent relapse and recurrence of episodes of major depression. This acknowledgement of the nature of recurrent depression and the high potential of recurrence has therefore led to a greater emphasis on long-term management approaches. 17
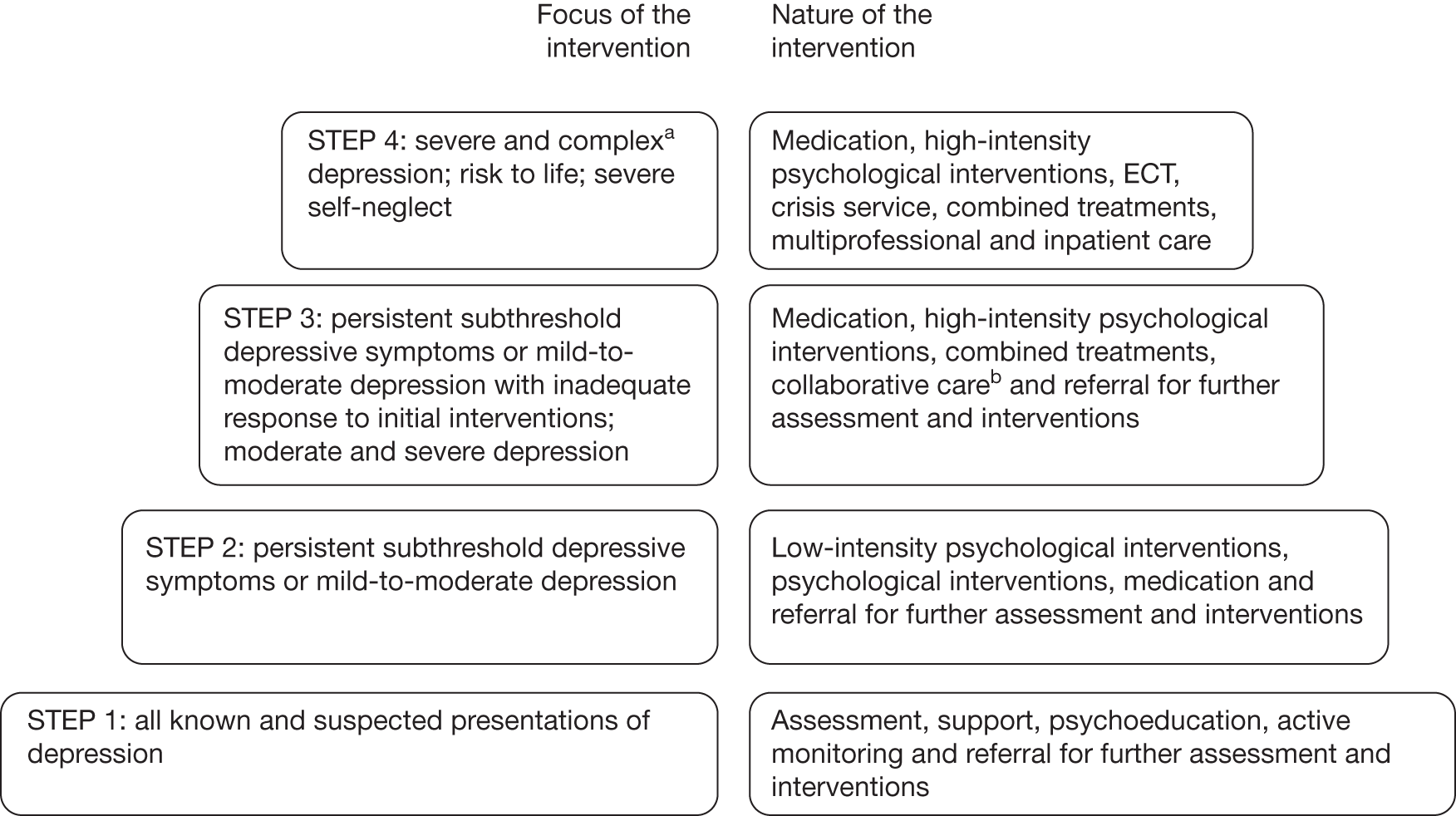
Many people are unwilling to seek help for depression and there is a failure to recognise depression, especially in primary care; of those patients diagnosed with depression, the majority will receive psychological, pharmacological or combined treatment in primary care. 8 Pharmacological treatments typically include antidepressant agents such as tricyclic antidepressants (TCAs) or, more commonly, specific serotonin reuptake inhibitors (SSRIs). Other drugs used either alone or in combination with antidepressants include lithium salts and antipsychotics, although these are usually reserved for people with severe, psychotic or chronic depression, or as prophylactics. 8 Psychological treatments for depression reviewed in the most recent NICE depression guidelines include cognitive behavioural therapy (CBT), behaviour therapy (BT), interpersonal therapy (IPT), problem-solving therapy (PST), counselling, short-term psychodynamic psychotherapy and couple-focused therapies. Owing to the different needs of individuals with depression, the NICE clinical guidelines advocate a ‘stepped-care’ treatment model (Figure 1), which aims to provide a framework to organise the provision of services supporting patients, carers and health-care professionals in identifying and accessing the most effective interventions. 8
FIGURE 1.
The stepped-care model. 8 a, Complex depression includes depression that shows an inadequate response to multiple treatments, is complicated by psychotic symptoms and/or is associated with significant psychiatric comorbidity or psychosocial factors. b, Only for depression in someone who also has a chronic physical health problem and associated functional impairment (see ‘Depression in adults with a chronic physical health problem: treatment and management’, NICE clinical guideline 918). ECT, electroconvulsive therapy.
Reproduced with permission from the National Collaborating Centre for Mental Health. Depression. The NICE guideline on the treatment and management of depression in adults (updated edition). National clinical practice guideline 90. London: National Institute for Health and Clinical Excellence; 2010.
Improving access to psychological therapies
The Improving Access to Psychological Therapies (IAPT) programme was launched by the UK government in October 2007. The programme aimed to invest an additional £173M per annum from 2008 to 2011 in evidence-based psychological therapies for the treatment of depression or anxiety disorders recommended by NICE, and to promote a more person-centred approach to therapy. 18 Both ‘low-intensity’ (e.g. guided self-help, computerised CBT) and ‘high-intensity’ interventions (e.g. CBT, IPT, counselling) were considered within NICE’s proposed stepped-care model, within which low-intensity approaches would initially be considered for the treatment of mild-to-moderate depression. 8 Much of the IAPT investment is for the training of new psychological therapists to deliver such low-intensity interventions. 18 These ‘psychological well-being practitioners’ (PWPs; previously termed ‘low-intensity therapy workers’) typically provide high-volume, low-intensity cognitive behaviour-based interventions to patients with less severe depression and/or anxiety disorders.
A key argument initially put forward for increasing access to psychological services was the potential for a reduction in public costs (e.g. welfare benefits, medical costs) and increase in revenues (e.g. taxes from return to employment, increased productivity). 19,20 Although this argument was put forward on the basis of many people being unable to access appropriate mental health services, the notion of improving access to low-intensity interventions in order to prevent relapse of depression even among treated patients might also be considered an investment on similar grounds.
Low-intensity interventions for depression
In general, people with depression tend to prefer psychological and psychosocial interventions to pharmacological interventions. 21 However, high-intensity psychological and psychosocial therapies (e.g. CBT, problem-solving, counselling) that involve one-to-one therapy with a mental health professional over extended periods of time are resource intensive. Consequently, less intensive therapies and innovative delivery formats such as group-based work have been developed. Less resource-intensive therapies include a variety of psychological treatments in which there is no or only a low level of therapist involvement, including computer-delivered treatment and bibliotherapy among other intervention technologies. The 2010 NICE guideline8 on depression refers to such approaches as ‘low-intensity psychosocial interventions’ and provides clinical evidence on three main forms of low-intensity therapy:
-
Computerised cognitive behavioural therapy (CCBT) provides a structured programme of care based on the principles of standard therapist-delivered CBT, but is delivered via a CD-ROM/DVD or the internet. Where CCBT is delivered as a primary intervention with minimal therapist involvement, it is considered a low-intensity intervention.
-
Guided self-help involves the use of evidence-based self-help books or manuals aimed specifically at depression. Guided self-help is distinct from ‘pure’ self-help in that a health-care professional (or para-professional) facilitates the use of the material by introducing, monitoring and assessing the outcome of the intervention.
-
Physical activity programmes have been defined as any structured physical activity with a recommended frequency, intensity and duration when used for depression. This could be aerobic (e.g. running/jogging, dancing) or anaerobic (e.g. resistance training), and be supervised or unsupervised, and undertaken in a group or individually.
The NICE clinical practice guidelines recommend that CCBT, individual-guided self-help and structured group physical activity programmes be considered for people with persistent subthreshold depressive symptoms or mild-to-moderate depression. The recommended duration of CCBT and guided self-help is 9–12 weeks including follow-up. Group physical activity with practitioner support is recommended for three sessions per week over 10–14 weeks. 8
Although the NICE guidance covers low-intensity psychological interventions, it does not provide a clear definition of what constitutes ‘low-intensity’ treatment more broadly. However, recent good practice guidance produced by the IAPT programme states22 that ‘A low-intensity intervention … may use simple or “single strand” approaches that are less complex to undertake than formal psychotherapy; contact with people is generally briefer than in other forms of therapy and can be delivered by para-professionals or peer supporters using non-traditional methods such as telephone or the internet’. Low intensity, therefore, is defined on the basis of four characteristics: the complexity of the intervention, the duration of contact, the level of training and the mode of delivery. In IAPT, a particular emphasis is on interventions delivered by PWPs without formal health-care professional or CBT therapist qualifications. 23 Although the IAPT guidance states that there is no arbitrary session limit, evidence from the IAPT demonstration site showed that the mean number of low-intensity CBT-based interventions was around five per person, although there was considerable variability around this figure. 22
A similar definition of low intensity is offered by Bennett-Levy et al,24 who identified the ability of an intervention to offer high-volume access to treatment as the defining feature of a low-intensity intervention, which can be achieved through strategies such as reduced practitioner–patient contact and the use of practitioners who do not have formal professional or high-intensity therapy qualifications. They also pointed out that the definition of low intensity remains contested. For example, they chose to include mindfulness-based cognitive therapy (MBCT) as a low-intensity treatment. This is a complex intervention that is typically delivered by a qualified mental health professional with specific expertise in its delivery but which limits patient contact time per therapist because it is delivered in a group format. They recognised that not everyone would agree with its inclusion.
Relapse and recurrence of depression
Given the high risk of repeated depressive episodes for some individuals with depression, it is important to aim not only to reduce depressive symptoms and restore functioning, but also to enable people to self-manage their problems and prevent relapse and recurrence of episodes of major depression.
Definitions
In an effort to standardise terms and facilitate communication, several conceptual definitions of improvement and subsequent return of depressive symptoms exist. 25–26 In terms of improvement, a distinction is made between response, remission and recovery. Response is defined as a clinically meaningful improvement in depressive symptoms that has continued for a sufficient length of time (3 consecutive weeks) to protect against misclassification owing to symptom variation or measurement error. 26 Response is typically operationalised as an improvement of ≥ 50% over pre-treatment scores. However, problems have been noted with this approach; for example, such a definition may be too stringent for patients with highly treatment-resistant depression. 26
Remission relies on a definition of an asymptomatic range, defined as the presence of no or very few symptoms. A person can be judged to be in the asymptomatic range only if neither of the two essential features of depression (sad mood and loss of interest or pleasure) is present and fewer than three of the additional core symptoms of depression are present. 26 Remission requires that the person remains in this range for at least 3 weeks, again to protect against factors such as natural symptom variation. After this point, remission status is still ascribed if the person’s symptoms fall above the asymptomatic range but fall short of meeting diagnostic criteria for a major depressive episode. Recovery is defined as an extended length of time in remission, which has been operationalised as at least 4 months. 26
The definitions of relapse and recurrence are linked to these definitions of improvement. Relapse occurs when a person in remission experiences a return to full symptoms of a major depressive episode. Relapse, therefore, occurs after achieving remission but before the recovery phase. Recurrence indicates the return to the full symptoms of depression after a person has achieved recovery status.
The definitions of relapse and recurrence are also linked to the differentiation of treatment phases. Acute-phase treatment is defined as treatment during an episode of depression, the aim of which is to achieve remission. Continuation-phase treatment occurs during the remission phase with the aim of continuing remission and ultimately achieving recovery. Maintenance treatment occurs during the recovery stage with the aim of maintaining this state.
Despite these definitions, there is still inconsistent use of the terminology within the literature, particularly in terms of the distinction made between relapse and recurrence. In the current project, ‘relapse and recurrence’ will be used as phrase throughout to refer to the return of full depressive symptoms. When the results of particular studies are described the terms used in that study are retained, even when their use is different to the definitions given above.
Interventions to reduce depressive relapse or recurrence
Pharmacological interventions
Although the preventative effects of antidepressant medication do not extend beyond the end of treatment,27 there is evidence that their continued use after an acute treatment phase can reduce the risk of relapse. A systematic review identified 31 randomised controlled trials (RCTs: total n = 44,210) that compared continued treatment with a range of antidepressants (predominantly TCAs and SSRIs) against placebo in people who had responded to treatment with antidepressants during an acute phase. 28 Continued treatment ranged from under 6 months to 36 months, with most studies having approximately 12 months of follow-up. Relapse rates were 41% in the placebo group and 19% for those continuing active medication [pooled odds ratio for relapse = 0.30, 95% confidence interval (CI) 0.22 to 0.38]. The different classes of antidepressant performed comparably and there appeared to be no substantial differences in the proportional risk reduction according to length of initial treatment or continued treatment. The majority of the studies were conducted in secondary care settings, so caution is needed in generalising these results to primary care settings, in which the risk of relapse may be lower. 28
High-intensity psychological interventions
Unlike pharmacological interventions, psychological treatment during an acute phase does have relapse-preventative effects that continue beyond the end of treatment. A meta-analysis of the effect of CBT on reducing relapse and recurrence in depression identified seven trials that compared relapse rates after acute-phase treatment with CBT or antidepressant medication in which no continuation phase was offered for either treatment. CBT significantly reduced relapse compared with medication. 29 Over a mean of 68 weeks’ follow-up, relapse–recurrence rates were 39% for CBT and 61% for medication. 29
Meta-analyses of behavioural activation, an intervention that shares some similarities with CBT, suggest no significant differences between acute-phase behavioural activation and CBT in terms of depressive symptoms at follow-up. 30–32 For example, one meta-analysis found effect sizes that were small and non-significant at a range of follow-up time points (1–3, 4–6, 7–12 and 13–24 months),32 although for the longest phase of follow-up findings relied on a small number of studies. 33–34 Evidence for the effects of other psychological treatments relative to CBT is small, but there are indications of no significant differences between acute-phase CBT and other treatments, such as IPT, in terms of relapse rates. 35
Although there is evidence that psychological interventions have preventative effects that continue after the end of treatment, it is of note that subsequent rates of relapse remain high; for example, one review reports a 1-year relapse–recurrence rate of 29% and 2-year rate of 54% for those who had responded to acute-phase CBT. 29 Acute-phase psychological treatment, although it reduces relapse relative to acute-phase antidepressant medication, may also be insufficient for the reduction of relapse risk. In recognition of this, a number of continuation-phase psychological interventions have been developed.
Vittengl et al. 29 identified four trials that compared continuation- or maintenance-phase CBT with a non-active control treatment. In these studies, continuation treatment significantly reduced relapse–recurrence relative to control. Over a mean of 41 weeks of follow-up, relapse–recurrence rates were 12% in the CBT, whereas in the control arms the rates were 38%. Vittengl et al. 29 also compared the preventative effects of continuation-phase CBT with those of other active treatments. This comparison identified five studies. Although there were no significant differences, there was a trend towards significance favouring CBT (p < 0.06). Over a mean of 27 weeks, relapse–recurrence rates were 10% for CBT and 22% for the other active treatments. 29
A small number of studies have also compared continuation and maintenance IPT with non-active control subjects and active treatments. One study randomised currently remitted patients with recurrent depression to one of five arms: (1) IPT alone; (2) IPT and antidepressant medication (imipramine) at acute dosage; (3) IPT with a drug placebo; (4) antidepressant medication (imipramine) at acute dosage with medication clinical visits; and (5) drug placebo with medication clinical visits. Survival analysis suggested that the addition of IPT to antidepressant did not lower recurrence rates compared with antidepressant treatment alone. IPT without active medication had a prophylactic effect between antidepressant medication and placebo. 36 A study by the same research group in adults aged > 60 years found that the combination of maintenance antidepressant medication (nortriptyline) and IPT showed a trend towards significance relative to antidepressant treatment alone in reducing recurrence. 37
Low-intensity psychological interventions
Although the effectiveness of low-intensity interventions has been extensively evaluated to treat primary symptoms of psychological difficulties,38–40 there has been substantially less research examining the use of these interventions as a relapse prevention strategy.
As discussed earlier, the definition of a low-intensity psychological intervention is not agreed on, which can make it difficult to distinguish low-intensity interventions from high-intensity interventions. The Vittengl et al. 29 meta-analysis, for instance, combined studies that would clearly be classified as high intensity with those that under some definitions could be classified as low intensity, such as MBCT. 29 We were unable to identify any previous reviews that focused exclusively on low-intensity interventions, however defined. This is the aim of the current review.
Chapter 2 Definition of decision problem
Decision problem
The decision problem concerns the clinical effectiveness and cost-effectiveness of low-intensity psychological interventions to prevent relapse or recurrence in patients who have received and responded to treatment for depression.
As discussed above, the terms relapse and recurrence are not consistently used in the literature. Therefore, we have considered both relapse and recurrence; we will refer to relapse or recurrence in our discussions unless a clear distinction has been made between the terms, but when reporting the findings of identified studies we will use the terminology as defined by individual study authors.
There is a lack of a clear, generally agreed on definition of low-intensity psychological interventions. We chose to emphasise the characteristic of the practitioner delivering the treatment as the main defining feature because of the current policy and practice context in the UK. Low-intensity psychological interventions are predominantly used in IAPT services, and in these services they are delivered by PWPs, who do not have formal health-care professional or CBT therapist qualifications.
However, in recognition that the definition of low intensity remains unclear, we also considered a broader definition of brief interventions typically delivered by clinical psychologists, CBT therapists, and other qualified mental health professionals involving limited patient contact time (delivered in a group setting or involving brief individual encounters). An inclusion threshold of 6 hours of contact per patient was used to select these intervention studies. We did not distinguish between the types of group intervention, although there is a very wide range; some interventions, such as psychoeducational groups and large community-based interventions, are low intensity, whereas others are high intensity and require high-level group therapy skills. For group interventions, the total contact time of the mental health professional(s) was divided by the number of patients in the group to create an average duration per patient. Although of less direct relevance to the decision problem, these interventions may be of interest to decision-makers concerned with improving access to psychological therapies, and so the literature in this area is briefly described and classified in a scoping review. Thus, the review was conducted in two parts as described below.
Overall aims and objectives
The main aims of this project are to determine the clinical effectiveness and cost-effectiveness of low-intensity psychological or psychosocial interventions to prevent relapse or recurrence in patients with depression.
As the broader definition of ‘low-intensity’ psychological intervention is somewhat contested, and the resources of the review were limited, the review was conducted in two parts:
-
A systematic review of all evaluations of ‘low-intensity’ interventions that were delivered by para-professionals, peer supporters or PWPs as defined by the IAPT programme. Such evaluations were not restricted by length of treatment or number of sessions.
-
A scoping review of all relevant evaluations of interventions involving qualified mental health professionals (e.g. psychiatrists, clinical psychologists, CBT therapists) involving < 6 hours of contact per patient.
Chapter 3 Assessment of clinical effectiveness
The review of the evidence for clinical effectiveness was undertaken systematically following the general principles recommended in the Centre for Reviews and Dissemination (CRD)’s guidance for undertaking reviews in health care41 and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 42
Methods for reviewing clinical effectiveness
Search strategy
Literature searches were developed to systematically identify studies on the effectiveness of low-intensity psychological interventions to prevent relapse or recurrence after depression. The base search strategy was constructed using MEDLINE and then adapted for other resources searched. The search strategy included the following components:
-
depression terms, and
-
relapse terms, and
-
low-intensity psychological intervention-related terms.
The searches were restricted to studies published after 1950. No language restrictions or study design filters were applied.
Search terms were identified by scanning key papers identified at the beginning of the project, through discussion with the review team and clinical experts, and the use of database thesauri.
Sources of information were identified by an information specialist with input from the project team. The following databases were searched during September 2010:
-
MEDLINE (via OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations (via OvidSP)
-
PsycINFO (via OvidSP)
-
EMBASE (via OvidSP)
-
The Cochrane Library (via Wiley)
-
– CDSR (Cochrane Database of Systematic Reviews)
-
– DARE (Database of Abstracts of Reviews of Effects)
-
– CENTRAL (Cochrane Central Register of Controlled Trials)
-
– HTA (Health Technology Assessment Database)
-
-
Science Citation Index (via ISI Web of Knowledge)
-
Social Science Citation Index (via ISI Web of Knowledge)
-
BIOSIS Previews (via ISI Web of Knowledge and Dialog).
In addition, a range of resources were searched or browsed to identify guidelines on the treatment of depression. The bibliographies of relevant reviews and guidelines and included studies were checked for further potentially relevant studies.
Records were managed within an EndNote library, version X3 (Thomson Reuters, CA, USA). After de-duplication, 9112 records in total were identified.
The full search strategies and results for each database can be found in Appendix 1.
Inclusion and exclusion criteria
Two reviewers independently examined titles and abstracts for relevance; all potentially relevant papers meeting the inclusion criteria were ordered. All full papers were then independently screened by two reviewers, with disagreements resolved by consensus.
Population
Studies of participants who have received treatment for depression were included. Studies establishing a diagnosis using a gold-standard structured interview for DSM or ICD criteria, such as the Structure Clinical Interview for DSM Disorders (SCID)43 were included, as were studies defining depression on the basis of a score above a cut-off point on a recognised psychometric measure or on the basis of unaided clinical diagnosis. The decision problem is concerned with the prevention of relapse or recurrence in patients who have received and responded to treatment. Consequently, studies of patients who were treated for an acute episode and then subsequently measured for relapse or recurrence were excluded; studies where patients had ‘recovered’ from their acute episode (responding to treatment or asymptomatic) and the aim was to prevent subsequent relapse or recurrence were included. Studies of participants with bipolar disorder were excluded, as were studies of children.
Interventions
For part A (systematic review of efficacy), all evaluations of ‘low-intensity’ interventions as defined by the IAPT programme22,23 were considered relevant. Specifically, this incorporated any unsupported psychological/psychosocial interventions or any supported interventions that did not involve highly qualified mental health professionals. ‘Highly qualified professionals’ includes clinicians, who, in most instances, will have a core professional qualification (e.g. psychiatrist, clinical psychologist, mental health nurse) and have received formal, specialist training in the delivery of complex psychological interventions (e.g. 16+ session CBT, psychodynamic psychotherapy, systematic therapy, etc.).
Any interventions involving support from para-professionals, peer supporters, PWPs, physical trainers, case managers (as in collaborative care models) or no personal support at all (e.g. entirely computerised interventions) were included. ‘Para-professionals’ includes people who do not have a core professional qualification and do not have specialist training in complex psychological interventions, although may have some training in less complex interventions. Inclusion was not restricted by length of treatment, number of sessions or mode of delivery.
For part B (scoping review), all relevant evaluations of interventions involving qualified mental health professionals (e.g. clinician, CBT therapist) were included if they involved < 6 hours of contact per patient. For group treatment, contact estimates per patient were calculated by dividing treatment duration by the mean number of patients per group (with adjustments as necessary if there is > 1 therapist). Where the amount of contact time was unclear, study authors were contacted to obtain additional details. If authors could not be contacted or did not respond, clinical experts (ML, DM) were consulted as to whether or not the intervention was likely to be brief (i.e. < 6 hours per patient).
High-intensity psychological interventions requiring ongoing interaction with a mental health professional (e.g. CBT, behavioural activation, problem-solving therapy and couples therapy) were excluded. Studies evaluating interventions for the acute phase of treatment of an acute episode of depression were also excluded.
Studies evaluating pharmacotherapy alone [including TCAs, SSRIs, serotonin–norepinephrine reuptake inhibitors (SNRIs), anxiolytic medication, mood stabilisers and others] were excluded from the review of clinical effectiveness, as were studies of alternative and complementary treatment methods.
Comparators
Study inclusion was not restricted by type of comparator treatment and could include no treatment (including waiting list control), placebo, psychological or pharmacological interventions.
Outcomes
Studies reporting outcomes related to relapse or recurrence (e.g. relapse rate, time to relapse, and severity of relapse episode) after initial treatment success were included. Other relevant outcomes such as social function and quality-of-life (QoL) measures were recorded where reported.
Study designs
Randomised, quasi-randomised and non-randomised studies with concurrent control patients were considered for inclusion. Animal models, preclinical and biological studies, reviews, editorials and opinions were excluded.
Translations of non-English-language papers and additional details of studies published only as meeting abstracts were obtained where time and budget constraints allowed.
Data extraction strategy
Data were extracted independently by one reviewer using a standardised data extraction form and checked by another. Discrepancies were resolved by discussion, with involvement of a third reviewer when necessary. Authors were contacted for any missing data or for clarification where necessary. Data from multiple publications of the same study were extracted as a single study. Extraction included data on patient characteristics, interventions, comparators, study design and outcomes.
Critical appraisal strategy
The quality appraisal checklist for quantitative intervention studies described in NICE’s guide to methods for developing guidance in public health was obtained for assessing the internal and external validity of studies included in the systematic review of low-intensity interventions (part A). 44 For the scoping review of brief therapy interventions (part B), formal critical appraisal of the included studies was not planned or conducted, with the exception of one study,45 in which the necessity of health professionals to deliver the intervention was unclear (see Assessment of effectiveness, Part B: brief therapy interventions for the prevention of relapse of depression, below).
Methods of data synthesis
Given the limited number of included studies and their clinical and methodological heterogeneity, a meta-analysis was not appropriate. Therefore, extracted data have been tabulated and discussed in a narrative synthesis.
Results of review of clinical effectiveness
Quantity and quality of research available
A total of 9112 unique records were identified from the searches and 129 articles were ordered for assessment. Figure 2 shows the flow of records through the review process, and the numbers included and excluded at each stage. Details of studies excluded at the full publication stage are presented in Appendix 2 (excluded studies).
FIGURE 2.
Study selection process for clinical effectiveness.
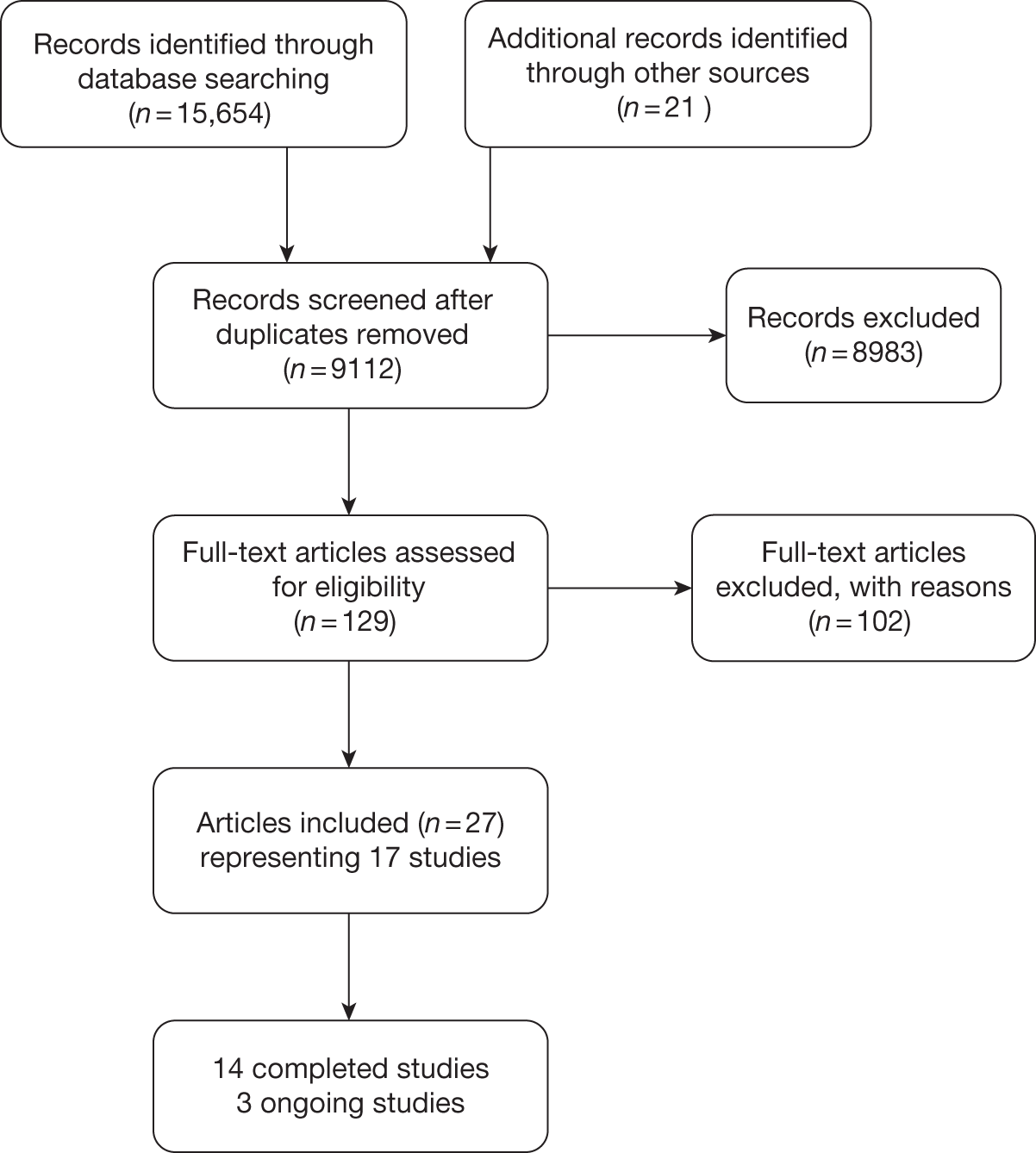
Part A: low-intensity interventions for the prevention of relapse of depression
No studies met the main part A inclusion criteria for ‘low-intensity’ interventions that were delivered by para-professionals, peer supporters or PWPs as defined by the IAPT programme, without any restriction on length of treatment.
Part B: brief therapy interventions for the prevention of relapse of depression
Seventeen studies, reported in 27 publications, met the part B inclusion criteria for brief therapy interventions delivered by mental health professionals involving < 6 hours of contact per patient. 45–71 Fourteen of the studies were completed and published;45–68 three are ongoing. 69–71
Table 1 provides details of the related publications for each of the included studies. In the following sections, reference will be made to the primary study only; the other linked publications provided additional information or results that are included in the data extraction tables (see Appendix 3).
| Primary publication | Linked publications |
|---|---|
| Completed studies | |
| Bockting 200546 | Bockting 2004,47 2006,48 2008,49 200950 |
| Bondolfi 201051 | None |
| Fava 199852 | Fava 200452 |
| Godfrin 201054 | None |
| Hepburn 200955 | None |
| Howell 200856 | None |
| Katon 200145 | Lin 2003,57 Ludman 2003,59 Ludman 2000,58 Simon 200260 |
| Kuhner 199661 | Kuhner 199462 |
| Kuyken 200863 | None |
| Ma 200464 | None |
| Rohde 200865 | None |
| Takanashi 200266 | None |
| Teasdale 200067 | None |
| Wilkinson 200968 | None |
| Ongoing studies | |
| Kuyken 201069 | None |
| Watkins 201070 | None |
| Williams 201071 | None |
Completed studies
Of the 14 completed studies,45–68 12 were parallel-group RCTs;45,46,51,52,54–56,63–65,67,68 the remaining two studies61,66 were non-randomised with concurrent control patients. Eight of the RCTs recruited participants from multiple centres,45,46,51,56,63,65,68,72 one of which used cluster randomisation. 56
Assessment of effectiveness
Part A: low-intensity interventions for the prevention of relapse of depression
No studies evaluating ‘low-intensity’ interventions that could be delivered by para-professionals, peer supporters, or PWPs as defined by the IAPT programme were identified.
Part B: brief therapy interventions for the prevention of relapse of depression
The following section provides a classification and description of studies identified which were identified as meeting the ‘part B’ inclusion criteria (i.e. they evaluated brief therapy interventions in which participants had up to 6 hours’ contact with mental health professionals, such as clinicians or CBT therapists). As these studies fall outside the primary focus of this review, they are briefly described in an overview below, with key study characteristics presented in Appendix 3. In one study (Katon et al. 45), the intervention could potentially be delivered by PWPs or equivalent practitioners, although in the retrieved evaluations it was delivered by mental health professionals. Given the potential relevance of this study to our part A question, it is discussed in greater detail below, and has been assessed for internal and external validity (see Appendix 4).
Completed studies
Ten of the completed studies evaluated interventions delivered in a group setting. 46,51,54,55,61,63,64,66–68 Of these, six specifically evaluated some form of MBCT. 51,54,55,63,64,67 Three MBCT studies were based on an identical protocol that involved eight weekly sessions of 2 hours’ duration, in which up to 12 participants met with experienced cognitive therapists to receive a programme based on the principles of CBT and mindfulness-based stress reduction. 51,64,67 Participants in this programme attended two further meetings during the subsequent 52 weeks of follow-up. Other MBCT programmes were of a similar duration (typically 2 hours to 2 hours and 45 minutes, weekly for 8 weeks), although with larger groups of up to 15 or 17 participants. 54,55,63 Three studies46,66,68 evaluated brief group CBT of a similar intensity to the MBCT interventions, but without any explicit mindfulness content. The one remaining group-based intervention was a brief 12-week ‘Coping with Depression’ (CWD) course, which was based on a multimodal psychoeducational approach, delivered by clinical psychologists and psychiatrists. 61
Four studies evaluated brief therapy interventions delivered by mental health professionals to participants on an individual basis. 45,52,56,65 One such intervention51 provided individuals with a brief CBT-based intervention (30 minutes every other week for 20 weeks) alongside ongoing pharmacotherapy. A second intervention56 incorporated a multimodal skills-based approach, providing support materials and general practitioner (GP) training to allow tailoring of evidence-based psychosocial strategies to individual patients in Australian primary care (‘Keeping The Blues Away’); this is a small pilot study for which it may be that the intervention could potentially be delivered by PWPs or equivalent but it is unclear from the detail provided what level of training is required. One study65 evaluated the effects of ‘continuation CBT’ (around 6 hours per patient) following initial CBT treatment in adolescents with depression. Another study45 evaluated a ‘multifaceted relapse prevention programme’ for patients who were at high risk of relapse, which is described in more detail below.
Eight of the 14 studies formally established the occurrence of relapse or recurrence using gold standard criteria, specifically Diagnostic and Statistical Manual of Mental Disorders – Third Edition-Revised (DSM-III-R) or DSM-IV criteria. 45,46,51,54,61,63,64,67 Of these, seven explicitly stated that they established this outcome using SCID. 45,46,51,54,63,64,67 Elsewhere, relapse was established using other criteria [Research Diagnostic Criteria (RDC)]52 or a variety of self-report and clinician-administered symptom scales [Beck Depression Inventory (BDI),55,66 Montgomery–Åsberg Depression Rating Scale (MADRS),68 Clinical Global Impression Improvement scale (CGI-I),65 Depression Anxiety Stress Scales (DASS)56].
The results of this diverse group of interventions in terms of preventing relapse or recurrence of depression are mixed. Even among MBCT studies following the same protocol, findings were inconsistent: two studies64,67 reported a statistically significant benefit for MBCT over treatment as usual (TAU) in patients with three or more previous episodes of depression at 14 months, but a third trial restricting inclusion to this subgroup of patients reported no overall difference in relapse between treatment groups over the same period. 51 Other studies reported results that clearly favoured MBCT over TAU54 were of borderline significance63 or showed no difference between groups. 55 One study68 suggested no significant benefit of brief CBT over TAU for preventing relapse, whereas another suggested any such benefit was restricted to participants with at least five previous depressive episodes. 46 One observational study did not report relapse rates and found no significant difference in scores 1 year after the intervention. 66 One study reported a statistically significant benefit of a multimodal psychoeducational approach over no intervention in terms of relapse prevention over 6 months, although this small observational study had several methodological limitations. 61
The study evaluating a brief CBT-based intervention (alongside ongoing pharmacotherapy51) reported a statistically significant impact on relapse after 2 years, an effect that remained at 6 years’ follow-up. Relapse rates were similar for the ‘Keeping The Blues Away’ programme and usual care in Australian primary care. 56 The study of ‘continuation CBT’ in adolescents reported significant benefits of the intervention alone over both antidepressant medication treatment and combined continuation CBT/medication. 65
Katon et al.
Five articles reported the findings of just one study (Katon et al. ). 45,57–60 Although the practitioners in this study were predominantly mental health professionals, and therefore did not strictly meet our part A inclusion criteria, it was unclear whether or not delivery by a mental health professional was mandatory for the implementation of the intervention. Therefore, this study was critically appraised and is summarised in further detail below.
This study was a RCT that evaluated the effectiveness of a ‘multifaceted relapse prevention programme’ in a US primary care setting (see Appendix 3). 45 This programme was provided to adult patients who had recovered from depression but who were at high risk of relapse and were encouraged to continue with antidepressant medication. The relapse prevention programme included aspects of patient education/self-help (patients were provided with a book and videotape developed by the trial investigators) alongside ongoing support from ‘depression specialists’. Each participant was scheduled two face-to-face sessions with a depression specialist (an initial 90-minute session and a 60-minute follow-up session), which were followed by three ‘telephone visits’ scheduled at 1, 4 and 8.5 months after the second face-to-face session. In addition, participants received ‘personalised mailings’ (at 2, 6, 10 and 12 months), containing a graph of participant BDI score over time and checklists on symptoms and medication adherence. The depression specialist alerted the primary care physician if the participant appeared to be symptomatic or had discontinued medication, based on data from participant feedback or from a monthly review of automated pharmacy data on antidepressant refills. Each depression specialist met with a supervising psychiatrist for 15–30 minutes each week to review cases and adjust treatment recommendations.
The focus of the relapse prevention intervention appeared to be largely on maintaining adherence to antidepressant medication. Meetings between patients and intervention ‘depression specialists’ integrated cognitive–behavioural and motivational interviewing approaches and provided information on the prevalence, course and efficacious treatment of depression. The depression specialist explained why each patient was at high risk of relapse, while acknowledging the individual’s attitudes, beliefs and treatment choices. Depression specialists and patients discussed evidence illustrating the efficacy of pharmacotherapy for preventing relapse and recurrence, the perceived risks and benefits of long-term pharmacotherapy, approaches to manage specific medication side-effects and concerns of the patient. In addition, the depression specialist attempted to improve self-efficacy for preventing relapse and recurrence of depression through self-management behaviours such as monitoring depressive symptoms and scheduling pleasant activities.
In this trial, three different depression specialists were provided for 194 patients receiving the relapse prevention intervention programme. One depression specialist was a psychologist, one was a nurse practitioner with a master’s degree in psychosocial nursing and the third was a social worker. Each of these had received a 60-page training manual and attended two half-day training sessions with a psychiatrist, a psychologist and a primary care physician before the start of the trial.
A total of 191 participants in the comparison group received ‘usual care’, which typically consisted of prescription for antidepressant medication (as in the intervention group), plus between two and four visits with a family physician over the first 6 months of treatment, with the option to refer to health maintenance organisation (HMO)-provided mental health services.
Relapse/recurrence was defined as either a current episode of depression according to the SCID (at 3, 6, 9 or 12 months) or incidence of an episode within each 3-month period according to the Longitudinal Interval Follow-up Evaluation. 73 Other outcomes included depressive symptoms [measured by the 20-item Hopkins Symptom Checklist (SCL-20)], medication adherence, and number of primary care visits for reasons other than depression.
The authors reported significantly greater adherence to antidepressant medication in the relapse prevention intervention group than the usual-care group (adjusted odds ratio 1.91, 95% CI 1.37 to 2.65; p < 0.001). Depressive symptoms (as measured by the SCL-20) improved in both groups over time, with a small but significant greater reduction for the intervention group (p = 0.04). However, the rates of relapse/recurrence for the intervention and usual-care groups (35% vs 34.6%) are almost identical, suggesting that the intervention did not prevent relapse relative to usual care over 12 months’ follow-up. The authors suggested that a more intensive programme might be needed to reduce relapse rates.
The internal and external validity of this study were assessed using the quality appraisal checklist for quantitative intervention studies described in NICE’s guide to methods for developing guidance in public health (see Appendix 4). 44 This appeared to be a reasonably well-conducted RCT, although with some important limitations. Given the nature of the interventions, blinding of participants and clinicians was not possible, although the authors did not state whether or not the outcome assessors were blinded to allocation (which may have led to bias). Other concerns raised by the assessment were the lack of a power calculation and the methods use to adjust findings to account for missing data. However, given other strengths of the study, the reported lack of benefit for the relapse-prevention programme is unlikely to be due to a type II error (i.e. a ‘false-negative’ finding), but is likely to be a reasonably valid finding for the studied population. However, as with any such study comparing an intervention against ‘usual care’, it is difficult to separate benefits of the treatment programme per se from benefits of the attendant increase in support, engagement and monitoring that the intervention involves. In terms of external validity, the study population was drawn from four primary care clinics of one HMO in western Washington, USA. Participants were predominantly female, white, college educated and in paid employment. The findings of this study may not therefore be directly generalisable to more socially or ethnically diverse populations or to a UK primary care setting.
Ongoing studies
Three of the identified studies are ongoing RCTs. 69–71 Two of these studies are evaluating MBCT approaches,69,71 one alongside cognitive psychoeducation without any mindfulness content. 71 The third trial is evaluating the impact of cognitive training self-help in addition to TAU. 70 The available details of these studies are presented in Appendix 3.
Chapter 4 Assessment of cost-effectiveness evidence
Methods for reviewing cost-effectiveness
The purpose of this review was to examine the existing cost-effectiveness literature on low-intensity psychological interventions for the secondary prevention of relapse after depression in detail, with the aim of identifying important structural assumptions, highlighting key areas of uncertainty and outlining the potential issues of generalising the results of the existing body of work. This review was used to identify the central issues associated with adapting existing work to address the specific research question posed and, if the evidence allowed, to assist in the development of a de novo economic model drawing on the issues identified in the clinical effectiveness and cost-effectiveness review.
Search strategy
The literature search strategy for the identification of cost-effectiveness studies was developed from the base search strategy usedfor the clinical effectiveness searches (see Chapter 3, Search strategy). Economic terms were added to the strategy to limit retrieval to economic studies. The additional economic terms were from the search strategy used for identifying studies for the NHS Economic Evaluations Database (NHS EED).
The following databases were searched in October 2011:
-
MEDLINE (via OvidSP)
-
EMBASE (via OvidSP)
-
EconLit (via OvidSP)
-
The Cochrane Library (via Wiley)
-
– CENTRAL (Cochrane Central Register of Controlled Trials)
-
– NHS EED
-
-
IDEAS [via Research Papers in Economics (RePEc)].
After de-duplication in EndNote X3, 466 records were identified. The full search strategies and results for each database can be found in Appendix 1.
Inclusion and exclusion criteria
Two reviewers independently examined titles and abstracts for relevance; all potentially relevant papers meeting the inclusion criteria were ordered. All full papers were then independently screened by two reviewers, with disagreements resolved by consensus.
In addition to the criteria used to screen for the clinical papers (see Chapter 3, Data extraction strategy) a set of cost-effectiveness criteria were also applied to screen for the papers on cost-effectiveness. Only full economic evaluations that compared two or more treatment options and considered both costs and consequences (including cost-effectiveness, cost–utility and cost–benefit analyses) were included in the review of economic literature. Economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases were all considered for inclusion. As with the clinical review, the review of cost-effectiveness evidence was also conducted in two parts: A and B (see Chapter 2, Overall aims and objectives).
Critical appraisal strategy
The quality of the cost-effectiveness studies was assessed according to a checklist updated from that developed by Drummond and Jefferson. 74 This information is summarised within the text of the report, alongside a detailed critique of included studies and the relevance to the NHS.
Methods of data synthesis
Drawing on the findings from the systematic reviews of both clinical effectiveness and cost-effectiveness, the intention was to develop a de novo economic model to assess the cost-effectiveness of low-intensity interventions to prevent relapse in patients with depression, and to further use this economic model to estimate the expected value of perfect information (EVPI) in order to help determine future research priorities in this area. However, given the lack of any studies meeting the main part A inclusion criteria in either the clinical effectiveness or cost-effectiveness review, the development of a de novo model was not considered feasible. Instead, through our review of the existing literature we have highlighted key issues that we think should be addressed as part of any future modelling work in this area.
Results of review of cost-effectiveness
Quantity and quality of research available
A total of 466 unique records were identified from the searches and 23 articles were ordered for assessment. Figure 3 shows the flow of records through the review process and the numbers included and excluded at each stage.
FIGURE 3.
Study selection process for cost-effectiveness.
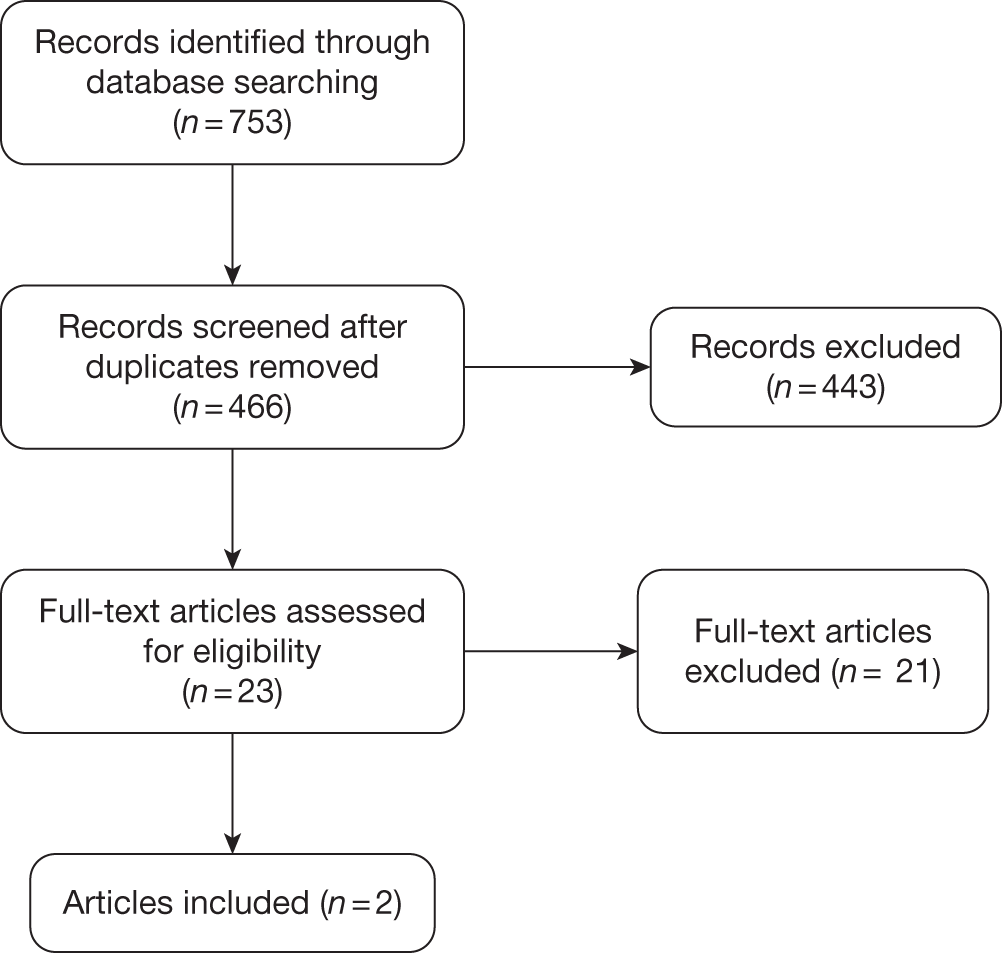
Part A: low-intensity interventions for the prevention of relapse of depression
No papers met the main part A inclusion criteria for ‘low-intensity’ interventions that were delivered by para-professionals, peer supporters or PWPs as defined by the IAPT programme, without any restriction on length of treatment.
Part B: brief therapy interventions for the prevention of relapse of depression
Two papers met the part B inclusion criteria for brief therapy interventions delivered by mental health professionals involving < 6 hours of contact per patient. One of these papers, by Simon et al. ,75 is the economic evaluation based on the Katon et al. 45 study discussed in detail in Chapter 3 (see Assessment of effectiveness). The other paper, by Kuyken et al. ,63 is a cost-effectiveness analysis of a trial of MBCT in a UK primary care setting.
Assessment of cost-effectiveness
The following sections provide a detailed critique of the cost-effectiveness evidence from the included studies and an assessment of the quality and relevance of the data from the perspective of the NHS. A quality assessment checklist is provided in Appendix 5.
Part A: low-intensity interventions for the prevention of relapse of depression
No studies evaluating ‘low-intensity’ interventions that could be delivered by para-professionals, peer supporters or PWPs, as defined by the IAPT programme, were identified.
Part B: brief therapy interventions for the prevention of relapse of depression
Two papers were identified as potentially meeting the ‘part B’ inclusion criteria (i.e. they evaluated brief therapy interventions in which participants had up to 6 hours of contact with mental health professionals, such as clinicians or CBT therapists). These two papers are critically appraised and are summarised in further detail below.
Review of Simon et al.
This study is an economic evaluation based on the Katon et al. 45 study discussed in the clinical review (see Chapter 3, Assessment of effectiveness, above, and Appendix 3). The study is a trial-based evaluation of the cost-effectiveness of a multifaceted low-intensity relapse prevention programme (patient education, two visits with a depression specialist, telephone monitoring and follow-up) in addition to usual care (antidepressant medication and visits to a physician) compared with usual care alone for the prevention of relapse in patients with either long-term depression or a history of recent depression. The economic evaluation had a 12-month time horizon and was conducted from a strict health insurer perspective. Costs were expressed in 1997–8 US dollars (USDs). No discounting was applied to either costs or effects, given the short time horizon used. The primary outcome measure was the incremental cost per depression-free day, with a secondary outcome of the incremental cost per quality-adjusted life-year (QALY).
Effectiveness was measured by means of a SCL-20 score assessed at baseline, 3, 6, 9 and 12 months. Days with SCL depression scores of ≤ 0.5 were considered depression free. Days with SCL depression scores of ≥ 2.0 were considered fully symptomatic. Days with intermediate severity scores were assigned a value between depression free and fully symptomatic by linear interpolation, between the two cut-off points (0.5 and 2.0 on the SCL-20). Depression severity data from the consecutive outcome assessments were used to estimate depression severity for each day during the intervals between assessments, again using linear interpolation. Using this approach, the number of depression-free days was calculated as the sum of the depression-free proportion of each day in the study period. This was achieved by computing the area under the interpolated line joining the five measured points. The mean number of depression-free days during the 12-month period was 253.2 (95% CI 241.7 to 264.7 depression-free days) in the relapse prevention group and 239.4 (95% CI 227.3 to 251.4 depression-free days) in the usual-care group. After adjusting for patient age, sex, baseline SCL depression score and chronic disease score, the incremental number of depression-free days in the relapse prevention group was calculated as 13.9 (95% CI –1.5 to 29.3 depression-free days); this difference was not statistically significant at the 5% significance level. CIs for depression-free days were estimated by bootstrap resampling with 1000 draws using bias correction. The difference in health-related quality of life (HRQoL) between fully symptomatic depression and fully recovered was reported as being between 0.2 [derived from intermediate health status measures, such as the European Quality of Life-5 Dimensions (EQ-5D76)] and 0.4 (derived from direct assessment methods, such as the standard gamble or time trade-off techniques77). These values were used in conjunction with the incremental cost per depression-free day to calculate incremental costs per QALY.
The resource use and costs evaluated included the direct costs of the intervention itself, as well as other health service utilisation directly related to depression treatment over the 12-month period. Health plan computerised data were used to identify all of the health services provided or paid for by the HMO during the 12 months after randomisation. These costs were split across the 15 different components captured by the HMO’s accounting system. The costs of intervention visits were estimated based on costs of similar services provided by the HMO. Costs of other intervention services were estimated using actual input costs. Estimated direct costs of the intervention programme were $256 (95% CI $249 to $264). The costs of antidepressant prescriptions were approximately $100 higher for the participants in the relapse prevention programme than for those receiving usual care, but these were offset by the costs of the other speciality mental health care, which were approximately $100 lower for those on the relapse prevention programme. Adjusted mean total costs were estimated to be $273 (95% CI $102 to $418) higher in the relapse prevention programme than the usual-care arm (details of the adjustments made were not provided). CIs around these cost values were estimated by bootstrap resampling with 1000 draws using bias correction.
The cost per additional depression-free day was reported as $24 (95% CI –$59 to $496). This was used to estimate the cost per QALY gained as $21,650 per QALY using an increment of 0.4 as the difference in HRQoL of depression-free year over a fully symptomatic year and as $43,800 per QALY using a QALY increment of 0.2. No attempt at characterising the uncertainty around these estimates of incremental costs per QALY was reported. The cost-effectiveness analysis was based on a 12-month time horizon over which the intervention appeared to be marginally more costly and marginally (although not statistically significantly at the 5% significance level) more effective than usual care.
The quality assessment highlighted several important issues that potentially limit the generalisability of the findings from this study to UK clinical practice. Key issues influencing the internal and external validity of these findings are discussed below, together with a more general discussion of the potential difficulties of generalising from the results of this study to inform UK practice.
The cost-effectiveness analysis did not directly address relapse prevention as suggested by the title; rather it assessed differences in levels of depressive symptoms between the two treatment options. Linear interpolation was used extensively in the calculation of the intermediate outcome measure, number of depression-free days, with the result being based on the five assessments. The point assessments themselves were calculated by interpolating SCL scores; this assumed a linear relationship between SCL scores and the proportion of the day that can be classed as depression free. Limited sensitivity analysis was reported to have been conducted on the conversion rates between SCL scores and depression-free days, but the results of this analysis were not reported. The analysis used complete case analysis rather than intention to treat (ITT) and missing data were not balanced across trial arms. The results indicated that the dropout rate was lower in the intervention arm than in the control arm, suggesting possible bias. Uncertainty around the cost per depression-free day was reported as a CI. This is potentially misleading and ambiguous, especially as in this case negative values were reported, which can reflect a treatment either being dominated (it is more costly and less effective than its comparator) or dominating (it is less costly and more effective than its comparator). 77 No substantial attempt was made in the study to quantify the HRQoL differences between the treatments. Furthermore, no formal method was used to derive the QALY calculations or assess the uncertainty around them.
The cost-effectiveness analysis was conducted in the USA and costs were measured from a strict health insurer perspective, omitting any out-of-service costs not covered by the patient’s health plan. Unit costs and resource-usage levels were reported for only a subset of the total costs. We updated these estimates to current UK values by converting the 1997–8 USD results to UK prices using purchasing power parity (PPP) exchange rates for that year and then inflating the costs to 2009–10 UK prices using health-care-specific inflation indices. 78–79 This gives a cost per depression-free day of £24 (95% CI –£59 to £493) and cost per QALY estimates of £21,511 and £43,519 based on the two HRQoL impacts of depression from the paper (0.4 and 0.2, respectively). However, there are significant differences in the way the US and UK health-care systems are structured, resulting in different models of care, as well as widely differing health-care costs. 80 In addition, the dated nature of the cost data and the various limitations noted previously makes generalising the results to the UK difficult.
The cost-effectiveness analysis had a short-term time horizon looking at cost-effectiveness over a 12-month period in a group of patients with a high risk of relapse. The main outcome measure was the number of depression-free days, a measure not directly related to relapse. The effectiveness results were highly uncertain, and it was not clear from the results that the intervention was either clinically effective or cost-effective compared with usual care. The evaluation did not attempt to measure the HRQoL scores of the different depression states observed; instead it informally assigns a QALY value to the value of depression-free days. Uncertainty around the cost-effectiveness estimates was not adequately addressed and where sensitivity analyses had been conducted the results of these were not presented. The evaluation was conducted from a health insurer’s perspective in a US primary care setting and did not detail the breakdown of the costs incurred. The combination of the issues identified made it difficult to generalise these results to a NHS setting.
Review of Kuyken et al.
This trial based cost-effectiveness analysis compared MBCT with maintenance antidepressant medication (m-ADM) in depressive relapse prevention for patients with recurrent depression. The evaluation took a societal perspective and had a 15-month time horizon. No discounting was applied to either costs or effects. The primary outcome of the cost-effectiveness analysis was the incremental cost per relapse prevented with a secondary outcome of the incremental cost per depression-free day. The analysis was based on a RCT conducted within a primary care setting in England. Patients were followed up at 3-month intervals over the 15-month time horizon of the evaluation. Patients in the MBCT arm of the trial took part in 8-weekly (2-hour) MBCT group sessions and were supported in tapering and discontinuing their antidepressant medication (ADM).
Clinical effectiveness was measured in terms of time to relapse/recurrence using the depression module of the Structured Clinical Interview for DSM-IV to assess retrospectively the 3-month period between assessments. Relapse/recurrence was defined as an episode meeting the DSM-IV criteria for major depressive disorder. Cox regression was used to compare the relative reduction in hazard of relapse/recurrence of MBCT compared with m-ADM. The results indicated that there was borderline evidence of MBCT having a greater hazard reduction effect: ITT analysis gave a hazard ratio of 0.63 (95% CI 0.39 to 1.04).
A societal perspective was taken in measuring costs and resource usage. All hospital (inpatient, outpatient, emergency department), community health, social services and productivity losses resulting from time off work owing to illness were accounted for. Economic data were collected at baseline and then in 3-month intervals up to 15-months post randomisation using the Adult Service Use Schedule (AD-SUS), an instrument also used in other studies of adult mental health populations. All unit costs were for the financial year 2005–6 and no discounting was applied. National UK unit costs were applied where appropriate and productivity losses were calculated using the human capital approach. 80 Costs were converted to 2006 international dollars (Int$) using World Bank PPP indices. The mean per-person cost for MBCT over the 15 months was higher than that for m-ADM by Int$427 (95% CI –Int$853 to Int$1705), but this difference in costs was not statistically significant.
Cost-effectiveness estimates of Int$962 per relapse/recurrence prevented and Int$50 per depression-free day were reported. Uncertainty around the cost-effectiveness of the intervention, based on willingness to pay per relapse prevented, was characterised in the form of a cost-effectiveness acceptability curve (CEAC).
The quality assessment highlighted important issues that potentially limit the generalisability of the findings from this study to UK clinical practice. Key issues influencing the internal and external validity of these findings are discussed below, together with a more general discussion of the potential difficulties of generalising from the results of this study to inform UK practice.
The cost-effectiveness analysis did not detail the data and methods used to calculate the estimates of the two cost-effectiveness outcome measures reported. Details of the data and methods used to characterise the uncertainty around these estimates were also omitted. Given the lack of detail it was difficult to assess how appropriate these cost-effectiveness estimates were.
The cost-effectiveness analysis did not attempt to measure utility. Cost-effectiveness estimates were instead reported using the measures of incremental cost per relapse prevented, and incremental cost per depression-free day. The use of disease-specific measures for cost-effectiveness made it difficult to generalise the results and compare them with other health-care interventions. We updated these estimates to current UK values by converting the 2005–6 USD results to UK prices using PPP exchange rates for that year and then inflating the costs to 2009–10 UK prices using health-care-specific inflation indices. 78–79 This gives a cost of £680 per relapse/recurrence prevented and £35 per depression-free day. The societal perspective taken for the analysis is also not in keeping with standard UK practice, which recommends limiting the perspective to the NHS and Personal Social Services (PSS) only. 81
It is unclear whether or not MBCT was more cost-effective than m-ADM in terms of preventing depression relapse. Similarly, all the results presented were highly uncertain. Methods and data used in conducting the analysis were not reported, making it difficult to judge the appropriateness of the results. The evaluation was conducted in the UK and measures all of the relevant costs from a societal perspective; however, its use of disease-specific measures in reporting cost-effectiveness made it difficult to generalise the results.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Although there is a substantial volume of literature on the effectiveness of low-intensity,38,82,83 high-intensity84–86 and mixed-intensity31,87,88 psychological treatments for the initial treatment of depression, this review has shown that there is currently very little intervention research specifically focused on the effectiveness of low-intensity interventions for relapse prevention.
No studies met the main review inclusion criteria (part A); a total of 17 completed and ongoing studies evaluating brief (≤ 6 hours of contact per patient) high-intensity therapy interventions (e.g. therapist-delivered continuation CBT, group MBCT) were identified and described (part B). These studies were clinically and methodologically diverse, and reported differing degrees of efficacy for the evaluated interventions. Of these, one study45 was felt to be of particular potential relevance to the main focus of the project, if the intervention could be delivered by PWP or equivalent practitioners. This was a RCT that evaluated a collaborative care-type programme which was specifically aimed at prevention of depressive relapse in high-risk patients in a US primary care setting. This study, which involved providing patients with face-to-face, telephone and postal contact with trained ‘depression specialists’, reported no difference between patients receiving the intervention and those receiving usual care in terms of relapse of depression over 12 months.
Cost-effectiveness
In the review of cost-effectiveness evidence, no studies met the main review inclusion criteria (part A); two studies that met the criteria for brief interventions (part B) were identified. 63,75 One of these was an economic evaluation of the same study identified as being potentially relevant to the main focus of the project in the clinical effectiveness review. This study demonstrated that the low-intensity intervention evaluated (providing patients with face-to-face, telephone and postal contact with trained ‘depression specialists’ in addition to usual care) may be a cost-effective use of NHS resources when compared with usual care. 75 However, the reported incremental cost-effectiveness ratios (ICERs) when converted into sterling and inflated to 2010 prices of £21,511 per QALY to £43,519 per QALY ranged from borderline cost-effective to not cost-effective under accepted thresholds for cost-effectiveness. 81 It was also unclear how valid these estimates were for the NHS. The other study (regarding the use of MBCT to prevent relapse) was inconclusive; furthermore, its use of disease-specific measures in reporting cost-effectiveness made it difficult to generalise the results. 63
Strengths and limitations
Clinical effectiveness
This is currently the only systematic review of the literature on the effectiveness of low-intensity interventions for the prevention of relapse or recurrence of depression. This review involved a comprehensive search for relevant evidence; over 9000 records identified from searches of electronic databases, online resources, clinical guidelines and other sources were independently screened by two or more reviewers, with primary study authors contacted where necessary.
The effectiveness of high-intensity interventions for the prevention of relapse or recurrence in depression have been reviewed elsewhere. 29,89 However, given the dearth of evidence on the effectiveness of low-intensity interventions, this review also incorporated a scoping exercise covering evaluations of brief high-intensity therapies for the prevention of relapse or recurrence typically delivered by clinical psychologists, CBT therapists, and other qualified mental health professionals. Inclusion was restricted to interventions involving limited patient contact time (delivered in a group setting or involving very brief individual encounters), as these approaches may be of interest to decision-makers who are concerned with improving access to psychological therapies and/or maximising available resources. Although any definition of ‘brief’ is likely to be somewhat arbitrary, an inclusion threshold of 6 hours of contact per patient was used to select these brief high-intensity intervention studies. The majority of studies excluded on this basis evaluated clearly resource-intensive interventions, although occasionally studies with similar treatment protocols to those included in the scoping review had to be excluded on the basis of having only slightly more than 6 hours of contact per patient (e.g. Fava et al. 90,91). A full list of excluded studies with reasons for exclusion is available in Appendix 2.
Cost-effectiveness
The review of cost-effectiveness evidence found minimal evidence supporting the use of low-intensity interventions for the prevention of relapse or recurrence in depression, with the one study that could potentially be relevant to the main focus of the project (administered by PWPs or equivalent practitioners) suggesting that the evaluated intervention may have been borderline cost-effective, although the results were highly uncertain and their validity to the NHS is questionable. 75 It should also be noted that the study compared its intervention in addition to usual care with usual care alone; other systematic reviews have considered other interventions for the prevention of relapse or recurrence. 29,89 The review has made it apparent that there are many low-intensity interventions, such as CCBT, which have not been evaluated for the prevention of relapse or recurrence in depression.
Both studies identified as relevant for part B had relatively short time horizons (12 and 15 months)63,75 and, given the chronic nature of depression, it is unclear if these time horizons would capture all the possible differences in costs and effects between the treatment arms, as would be considered good practice in economic evaluation. 77 The cost-effectiveness studies also made no attempt to explore any heterogeneity in terms of patient characteristics, with no subgroup analyses conducted in either study.
Uncertainties
Given the lack of relevant evidence identified, many uncertainties remain. The existing evidence does not provide a robust evaluation of the clinical effectiveness or cost-effectiveness of low-intensity interventions for the prevention of relapse or recurrence of depression. Further research is needed to address this issue.
This review only considered evaluations of interventions preventing the relapse or recurrence of depression; evaluations typically focus on specific stages of an illness, for example, with depression, the initial treatment of a depressive episode or, as in this case, the prevention of relapse or recurrence. Such an approach may ignore important factors, for example possible interactions between treatment for the initial episode and subsequent treatment for relapse prevention. Instead a more comprehensive approach could be taken, with the whole-patient pathway being considered: optimising the use of particular interventions within a broader range of alternatives considering the entire treatment pathway of patients.
Below, specific issues relevant to the evaluation of relapse and recurrence prevention are discussed in detail; such issues would also need to be considered in any wider evaluation of whole-patient pathways as well.
Definition of low-intensity interventions
There are likely to be a range of alternative low-intensity interventions that could be feasible in the NHS. In the 2010 NICE guideline92 on depression three forms of low-intensity intervention were distinguished: CCBT, guided self-help and physical activity programmes. However, this list is not exhaustive and other interventions could be described as low intensity; for example, the intervention described in Katon et al. 45 could conceivably be delivered by para-professionals or PWPs and therefore be classed as low intensity. The same point could be made about a number of other interventions, some of which are currently classified as high intensity. It is not clear what level of training is required to adequately deliver such interventions effectively. Similarly, group work (as found in most of the interventions identified in part B studies) is ‘high-intensity’ in the sense that it is typically delivered by a mental health professional, although delivering interventions in a group setting could potentially provide more efficient use of resources and increased throughput. The further evaluation of such group interventions may be of use. There are also likely to be pragmatic considerations that need to be taken into account when deciding on which low-intensity interventions should be evaluated; for example, some interventions may be more feasible for widespread introduction in the NHS than others.
Relevant comparators
It is important to consider carefully what the relevant comparators should be in any future evaluation. ‘Psychological placebo’ or sham psychological treatments may have limited use in the evaluation of psychological treatments. One of the factors that a placebo condition should control for is patient expectancy, which may in part be related to a practitioner’s expectancy of whether or not a treatment is likely to be effective. There are obvious difficulties in ensuring that a practitioner is unaware of whether a psychological intervention is designed to be a genuine intervention or a control condition. It is also possible that the patient will be able to discern whether or not an intervention is intended to be therapeutic. Therefore, TAU may be an appropriate comparator; however, studies must report what participants in the TAU group actually received, including medication (NICE recommends that patients receive maintenance antidepressant therapy), any additional psychosocial support and previous treatments (e.g. medication and/or IAPT interventions).
There are many other interventions, including both high-intensity psychological interventions and pharmacological therapies, which are used to prevent relapse or recurrence of depression. 93 However, there may be constraints which limit the relevance of particular interventions as comparators; for example, there may be a limited number of qualified health-care professionals so more intensive psychological interventions are not feasible or long-term antidepressant use may not be acceptable for patients. Many studies examining the use of psychotherapy for the treatment of depression involve psychotherapy provided in addition to usual care by a GP, which will often include an antidepressant. 93 Similarly, collaborative care and case management strategies have also been used in the treatment of depression, and have been found to be cost-effective in the treatment of depressive episodes. 93 This suggests that any future evaluation should also consider relevant combinations of interventions/therapies as comparators.
Furthermore, it may be appropriate to consider evaluating the whole treatment pathway for a patient, to incorporate any possible treatment interactions. Treatment interactions may exist between combinations of interventions or between interventions received for the initial depressive episode and subsequent interventions. Such interactions may alter the patient population, which will be at risk of relapse in the future. Evaluating interventions separately without recourse to this may result in contradictory results. Adopting a more comprehensive evaluative approach to the whole-patient pathway being considered would allow the use of particular interventions to be optimised within a broader range of alternatives considering the entire treatment pathway of patients.
Outcomes
Any future evaluation also needs to consider carefully which outcomes are of importance when evaluating interventions preventing relapse or recurrence in depression. Clearly, relapse or recurrence is a key outcome; however, QoL measures, such as depression-free days, etc., are also important but were rarely measured in the identified studies. Good practice in cost-effectiveness analysis demands the use of generic (non-disease-specific) outcome measures so as to allow decisions on resource allocation across disease areas, not simply within them. 77 In the UK, the recommended outcome measure is the QALY, which takes into account both quantity of life and HRQoL. 81 Although depression has been shown to affect mortality,94 it also has a major impact on QoL; it is therefore important to consider by what process an intervention may affect QoL.
Previous economic models that have considered the treatment of depression have often modelled patients as either in a depressive episode or not, with no in-between and no consideration of severity. 82,95 However, depression is not a dichotomous disorder and instead there is a scale of severity, such that individuals considered to be in remission may at times have depressive symptoms that affect their HRQoL, and those individuals who are considered to be depressed may be so to varying severities (see Chapter 1, Diagnosis, for details on different categories of depression). The study by Simon et al. 75 identified in the review of cost-effectiveness, described itself as addressing depression relapse; however, the primary measure of effectiveness used is the number of depression-free days, not the prevention of relapse, and even depression-free days are measured as continuous rather than dichotomous, with intermediate severity scores being treated as between depression free and fully symptomatic. Although prevention of relapse is clearly an important factor in the effectiveness of treatment, and something that needs to be included in any future evaluation, there may be other effects of treatment that cannot be captured by focusing solely on relapse prevention. For example, it may be important to capture the effects of treatment on HRQoL during periods of remission or depressive episodes, as well as the impact on risk of relapse or recurrence.
Heterogeneity of patients
As we have shown, there is currently minimal evidence to support the use of low-intensity psychological interventions for the prevention of relapse or recurrence. However, this patient group is not homogeneous, and within this group there are many sources of explorable heterogeneity that could be considered. Therefore, before any future evaluation it is important to consider which subgroups should be examined.
Risk of relapse within depression is heavily dependent on the number of previous episodes; for example, it has been reported that risk of relapse is 50% among patients having experienced one episode of major depression, rising to 70% among patients who have experienced two episodes and 90% among patients who have experienced three episodes. 14 Previous models examining treatments for depression have taken account of the number of previous depressive episodes. 95 Any future evaluation needs to take account of the differing baseline risk of relapse by number of previous episodes, as this will impact on the benefits of treatment. Similarly, it may also be worth examining if treatment effects differ by number of previous episodes.
As discussed previously, depression is not a dichotomous disorder and patients experiencing depressive episodes may suffer from different severities of the condition, with the associated differences in HRQoL. If the severity of prior depressive episodes is associated with the future severity of depression following relapse, then it may also be appropriate to consider this as a source of identifiable heterogeneity.
Comorbidity is also likely to be important in considering response to treatment and risk of relapse. For example, comorbid anxiety and depression are particularly common96 and are associated with poorer compliance with, and response to, treatment. 97
Another possible source of heterogeneity is the treatment received for the previous depressive episode (or episodes). Both low- and high-intensity psychological interventions are widely used in the treatment of depressive episodes; however, not all patients receive the same intervention, or a psychological intervention at all, with many patients receiving only pharmacological therapy. 98 It could be expected that there would be treatment interactions between the therapy received for the initial depressive episode and that received to prevent relapse or recurrence; for example, psychological therapies for depressive episodes have been found to have a relapse-preventative effect beyond the end of treatment,29 and, as such, this should be considered in any future evaluation. As discussed previously, this supports focusing on the whole-patient pathway rather than a selected part of it.
Societal costs
In the UK, guidelines for the economic evaluation of health-care technologies recommend that the perspective taken on costs should be that of the NHS and PSS; in exceptional circumstances when a substantial proportion of the costs fall outside of the NHS and PSS, costs to other government bodies may be considered as well when they are not reflected in HRQoL measures. 81 However, there is a large literature showing the substantial wider societal costs of depression; for example, one study99 found the annual cost of depression to England was over £9B, of which only £370M was in direct treatment costs. Key to whether or not these costs should be considered in an economic evaluation is whether or not the estimates of HRQoL capture the financial impact on the patient (and possibly any carer). If they do then capturing this impact in costs as well as in the HRQoL measure will result in double counting. Although many have argued that the measure of HRQoL should not capture the financial impact,100 the EQ-5D, the preferred measure within the UK,81 includes in its description of health states the ability to perform a ‘usual social role’, which will include participation in the labour market and its financial implications.
Any future evaluation also needs to consider what health-care resource should be collected to enable comparison between interventions. Although the inclusion of resources related directly to the treatment of depression is evident, depression has also been shown to increase the use of other health-care resources by patients even after controlling for comorbidities. 101 Good practice in economic evaluation involves including any differences between treatments in terms of resource use; therefore, a broad view of health-care resource use should be considered. 77
Chapter 6 Conclusions
There is inadequate evidence to determine the clinical effectiveness or cost-effectiveness of low-intensity interventions for the prevention of relapse or recurrence of depression, either unsupported psychological interventions or the type of supported interventions that might be delivered by PWPs (as defined by IAPT) or by similar para-professionals.
A scoping review of interventions using a broader definition of brief high-intensity therapies indicates that some approaches (e.g. MBCT in a group setting) have shown promise in some studies, but findings have not been consistent.
Careful consideration should be given to the scope of future research to inform this issue; it is important to evaluate the broader patient pathway accounting for the entire treatment pathway and consider the wide range of heterogeneous patient groups within those patients in remission or who have recovered from a depressive episode.
Suggested research priorities
Given the lack of relevant evidence identified, many uncertainties remain. The existing evidence does not provide a robust evaluation of the clinical effectiveness or cost-effectiveness of low-intensity interventions for the prevention of relapse or recurrence of depression. Further research is needed to address this issue.
For many individuals, depression must be seen as a relapsing or recurrent condition that requires long-term management to minimise the impact on people’s QoL. Approaches to the evaluation of low-intensity therapies and other interventions for depression should therefore consider the management of an individual episode within the broader context of managing the entire course of the condition. Future research should also consider a number of key issues relevant to the evaluation of relapse and recurrence prevention, such as defining the interventions and comparators, outcomes and populations of interest. It is also important that any research question is set in the context of the entire patient pathway accounting, where possible, for the impact of important factors such as initial treatment alternatives and patient characteristics. Treatment interactions may exist between combinations of interventions or between interventions received for the initial depressive episode and subsequent interventions. Adopting a more comprehensive evaluative approach to the whole-patient pathway being considered will allow the use of particular interventions to be optimised within a broader range of alternatives considering the entire treatment pathway of patients.
The definition of low-intensity is unclear and there is likely to be a number of alternative interventions that could be feasible, including unsupported interventions, interventions delivered by those without formal health-care qualifications, or group work (as found in most of the interventions identified in part B studies) delivered by a mental health professional, but potentially providing more efficient use of resources and increased throughput. Some interventions may be more feasible for widespread introduction in the NHS than others. In defining the intervention it is important to provide clarity on the type of practitioner providing the training, including their training and supervision; this will help clarify the extent to which interventions can be considered as low intensity and inform cost-effectiveness evaluations.
It is important to consider carefully what the relevant comparators should be in any future evaluation. There are numerous interventions, including both high-intensity psychological interventions and pharmacological therapies or a combination of interventions, which are used to prevent relapse or recurrence of depression, but any constraints that limit the relevance of interventions will need to be considered, for example the availability of clinicians or the acceptability of long-term medication. TAU may be an appropriate comparator but it is important to detail precisely what this entails.
Relapse or recurrence is a key outcome; however, QoL measures, such as depression-free days, etc., are also important. Other effects, such as the effects of treatment on HRQoL during periods of remission or depressive episodes, should also be considered.
The patient group is not homogeneous and consideration should be given to which subgroups are important in terms of both the clinical effectiveness and cost-effectiveness of low-intensity psychological interventions for the secondary prevention of relapse after depression. Significant factors include the severity of depression, comorbidities and the number of previous episodes. Future evaluations should take account of the differing baseline risk of relapse, and it may also be worth examining if relative treatment effects differ by the number of previous episodes. Patients experiencing depressive episodes may suffer from different severities of the condition, with the associated differences in HRQoL; if the severity of prior depressive episodes is associated with the future severity of depression following relapse then this should be accounted for.
Recent clinical guidelines published by the Scottish Intercollegiate Guidelines Network (SIGN) suggest that MBCT in a group setting may be considered as a treatment option to reduce relapse in patients with depression who have had three or more episodes. 102 This recommendation was based on a systematic review performed in 2007. 103 The current scoping review identified three further RCTs of group-based MBCT which were not included in the 2007 review, two69,71 of which are UK-based and currently ongoing. An updated systematic review of group-based MBCT on completion of these trials may be of value. Any such systematic review should investigate any potential impact of the duration and intensity of the intervention on the relapse and recurrence of depression.
Acknowledgements
We would like to thank all primary study authors who responded to our requests for further information. We would also like to thank Tony Danso Appiah for contributing to study selection, data extraction and validity assessment in the clinical effectiveness sections of the report.
Contribution of authors
Mark Rodgers was responsible for study selection, data extraction, validity assessment, data analysis and writing the report.
Miqdad Asaria and Simon Walker were responsible for the review of cost-effectiveness evidence and contributed to writing the report.
Dean McMillan and Mike Lucock provided clinical advice throughout the project and commented on drafts of the report.
Melissa Harden devised the search strategy, carried out the literature searches, maintained the library of references and wrote the search methodology sections of the report.
Stephen Palmer contributed to all aspects of the economic sections.
Alison Eastwood contributed to all aspects of the clinical effectiveness sections and has overall responsibility for the project.
All authors contributed to, and commented on, the report.
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- American Psychiatric Association (APA) . Diagnostic and Statistical Manual of Mental Disorders 2000.
- World Health Organization (WHO) . The Global Burden of Disease: 2004 Update 2008.
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry 2003;54:216-26.
- Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H. Psychiatric morbidity among adults living in private households, 2000. London: The Stationery Office; 2001.
- Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res 2002;53:849-57.
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008;213:93-118.
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry 2008;165:969-77.
- National Collaborating Centre for Mental Health . Depression. The NICE Guideline on the Treatment and Management of Depression in Adults (updated edition). National Clinical Practice Guideline 90 2010.
- Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull 1984;10:300-12.
- Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry 1991;52:28-34.
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the ‘kindling’ hypothesis. Am J Psychiatry 2000;157:1243-51.
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
- Kennedy N, Abbott R, Paykel ES. Remission and recurrence of depression in the maintenance era: long-term outcome in a Cambridge cohort. Psychol Med 2003;33:827-38.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105.
- McCrone P, Dhanasiri S, Patel A, Knapp A, Lawton-Smith M. Paying the price: the cost of mental health care in England to 2026. London: King’s Fund; 2008.
- World Health Organization (WHO) . The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research 1993.
- Andrews G. Should depression be managed as a chronic disease?. BMJ 2001;322:419-21.
- Clark DM, Layard R, Smithies R, Richards DA, Suckling R, Wright B. Improving access to psychological therapy: initial evaluation of two UK demonstration sites. Behav Res Ther 2009;47:910-20.
- Layard R, Bell S, Clark D, Knapp M, Meacher M, Priebe S, et al. The Depression Report: A New Deal for Depression and Anxiety Disorders 2006.
- Layard R, Clark D, Knapp M, Mayraz G. Cost–benefit analysis of psychological therapy. Natl Inst Econ Rev 2007;202:90-8.
- Prins MA, Verhaak PFM, Bensing JM, Van der Meek K. Health beliefs and perceived need for mental health care of anxiety and depression: the patients’ perspective explored. Clin Psychol Rev 2008;28:1038-58.
- NHS Improving Access to Psychological Therapies . Good Practice Guidance on the Use of Self-Help Materials Within IAPT Services 2010.
- Richards D, Chellingsworth M, Hope R, Turpin T, Whyte M. Reach Out: national programme supervisor materials to support the delivery of training for psychological wellbeing practitioners delivering low intensity interventions. London: Rethink; 2010.
- Bennett-Levy J, Richards DA, Farrand P, Bennett-Levy J, Richards DA, Farrand P, et al. Oxford guide to low intensity CBT interventions. Oxford: Oxford University Press; 2010.
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 1991;48:851-5.
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006;31:1841-53.
- Thase ME. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr 2006;11:12-21.
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361:653-61.
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol 2007;75:475-88.
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev 2007;27:318-26.
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611-23.
- Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: a meta-analysis and review. Clin Psychol 2009;16:383-411.
- Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol 2008;76:468-77.
- Gortner ET, Gollan JK, Dobson KS, Jacobson NS. Cognitive-behavioral treatment for depression: relapse prevention. J Consult Clin Psychol 1998;66:377-84.
- Shea MT, Elkin I, Imber SD, Sotsky SM, Watkins JT, Collins JF, et al. Course of depressive symptoms over follow-up. Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry 1992;49:782-7.
- Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990;47:1093-9.
- Reynolds CF, Frank E, Perel JM, Imber SD, Cornes C, Miller MD, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA 1999;281:39-45.
- Mead G, Morley W, Campbell P, . Exercise for depression. Cochrane Database Syst Rev 2008;4.
- National Institute for Health and Clinical Excellence (NICE) . Computerised Cognitive Behaviour Therapy for Depression and Anxiety. Review of Technology Appraisal 51 2006.
- Richardson R, Richards DA, Barkham M. Self-help books for people with depression: a scoping review. J Ment Health 2008;17:543-52.
- Centre for Reviews and Dissemination (CRD) . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;151:W65-94.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
- National Institute for Health and Clinical Excellence (NICE) . Methods for the Development of NICE Public Health Guidance 2009.
- Katon W, Rutter C, Ludman EJ, Von Korff M, Lin E, Simon G, et al. A randomized trial of relapse prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241-7.
- Bockting CLH, Schene AH, Spinhoven P, Koeter MWJ, Wouters LF, Huyser J, et al. Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. J Consult Clin Psychol 2005;73:647-57.
- Bockting CLH, Schene AH, Spinhoven P, Koeter MWJ, Wouters LF, Huyser J. Preventing new episodes in recurrent depression by an eight session cognitive group therapy: a randomized controlled trial. J Affect Disord 2004;78:92-3.
- Bockting CLH, Spinhoven P, Koeter MWJ, Wouters LF, Visser I, Schene AH, et al. Differential predictors of response to preventive cognitive therapy in recurrent depression: a 2-year prospective study. Psychother Psychosom 2006;75:229-36.
- Bockting CLH, Spinhoven P, Schene AH. Preventing relapse in recurrent depression using new forms of cognitive therapy: a randomized controlled trial with up to 6 years follow-up. J Affect Disord 2008;107:97-8.
- Bockting CLH, Spinhoven P, Wouters LF, Koeter MWJ, Schene AH. DELTA Study Group . Long-term effects of preventive cognitive therapy in recurrent depression: a 5.5-year follow-up study. J Clin Psychiatry 2009;70:1621-8.
- Bondolfi G, Jermann F, der Linden MV, Gex-Fabry M, Bizzini L, Rouget BW, et al. Depression relapse prophylaxis with mindfulness-based cognitive therapy: replication and extension in the Swiss health care system. J Affect Disord 2010;122:224-31.
- Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 1998;55:816-20.
- Fava GA, Ruini C, Rafanelli C, Finos L, Conti S, Grandi S. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry 2004;161:1872-6.
- Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: a randomized controlled study. Behav Res Ther 2010;48:738-46.
- Hepburn SR, Crane C, Barnhofer T, Duggan DS, Fennell MJV, Williams JMG. Mindfulness-based cognitive therapy may reduce thought suppression in previously suicidal participants: findings from a preliminary study. Br J Clin Psychol 2009;48:209-15.
- Howell CA, Turnbull DA, Beilby JJ, Marshall CA, Briggs N, Newbury WL. Preventing relapse of depression in primary care: a pilot study of the ‘Keeping the blues away’ program. Med J Aust 2008;188:138-41.
- Lin EHB, Von Korff M, Ludman EJ, Rutter C, Bush TM, Simon GE, et al. Enhancing adherence to prevent depression relapse in primary care. Gen Hosp Psychiatry 2003;25:303-10.
- Ludman E, Von Korff M, Katon W, Lin E, Simon G, Walker E, et al. The design, implementation, and acceptance of a primary care-based intervention to prevent depression relapse. Int J Psychiatry Med 2000;30:229-45.
- Ludman E, Katon W, Bush T, Rutter C, Lin E, Simon G, et al. Behavioural factors associated with symptom outcomes in a primary care-based depression prevention intervention trial. Psychol Med 2003;33:1061-70.
- Simon GE, Von Korff M, Ludman EJ, Katon WJ, Rutter C, Unutzer J, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Med Care 2002;40:941-50.
- Kuhner C, Angermeyer MC, Veiel HOF. Cognitive-behavioral group intervention as a means of tertiary prevention in depressed patients: acceptance and short-term efficacy. Cognit Ther Res 1996;20:391-409.
- Kuhner C, Angermayer MC, Veiel HOF. The efficacy of a cognitive-behavioral group intervention in preventing depressive relapses. Verhaltenstherapie 1994;4:4-12.
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol 2008;76:966-78.
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol 2004;72:31-40.
- Rohde P, Silva SG, Tonev ST, Kennard BD, Vitiello B, Kratochvil CJ, et al. Achievement and maintenance of sustained response during the treatment for adolescents with depression study continuation and maintenance therapy. Arch Gen Psychiatry 2008;65:447-55.
- Takanashi Y. A study of the prevention for periodic depression with cognitive behavioral therapy. Tokyo Jikeikai Ika Daigaku Zasshi 2002;117:405-17.
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000;68:615-23.
- Wilkinson P, Alder N, Juszczak E, Matthews H, Merritt C, Montgomery H, et al. A pilot randomised controlled trial of a brief cognitive behavioural group intervention to reduce recurrence rates in late life depression. Int J Geriatr Psychiatry 2009;24:68-75.
- Kuyken W. Preventing depressive relapse in NHS practice through mindfulness-based cognitive therapy (MBCT). The National Institute for Health Research Health Technology Assessment Programme; 2010.
- Watkins E. Cognitive training as a facilitated self-help relapse prevention for depression [ISRCTN44812125]. Current Controlled Trials Limited; 2010.
- Williams JMG, Russell IT, Crane C, Russell D, Whitaker CJ, Duggan DS, et al. Staying well after depression: trial design and protocol. BMC Psychiatry 2010;10.
- Teasdale J. A randomised controlled trial comparing the effectiveness of two versions of Mindfulness Based Cognitive Therapy (MBCT) in preventing relapse/recurrence in recovered depressed patients. National Research Register Archive, National Institute for Health Research; 2002.
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The longitudinal interval follow-up evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 1987;44:540-8.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Simon GE, Katon WJ, VonKorff M, Unutzer J, Lin EHB, Walker EA, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry 2001;158:1638-44.
- EuroQol Group . What Is EQ-5D? n.d. www.euroqol.org/eq-5d/what-is-eq-5d.html (accessed 1 February 2011).
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Organisation for Economic Co-operation and Development (OECD) . PPPs and Exchange Rates n.d. http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4 (accessed 1 February 2011).
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit; 2009.
- Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess 2004;8.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006;10.
- Donke T, Griffiths KM, Cuijpers P, Christensen H. Psychoeducation for depression, anxiety and psychological distress: a meta-analysis. BMC Med 2009;7.
- Leichsenring F, Rabung S, Leibing E. The efficacy of short-term psychodynamic psychotherapy in specific psychiatric disorders. A meta-analysis. Arch Gen Psychiatry 2004;61:1208-16.
- Leichsenring F, Rabung S. Effectiveness of long-term psychodynamic psychotherapy: a meta-analysis. JAMA 2008;300:1551-65.
- Abbass AA. Intensive short-term dynamic psychotherapy of treatment-resistant depression: a pilot study. Depress Anxiety 2006;23:449-52.
- Cuijpers P, Van Straten A, Van Oppen P, Andersson G. Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta-analysis of comparative studies. J Clin Psychiatry 2008;69:1675-85.
- Cuijpers P, van Straten A, Warmerdam L, Andersson G. Psychological treatmeant of depression: a meta-analytic database of randomized studies. BMC Psychiatry 2008;8.
- Fava G, Tomba E. New modalities of assessment and treatment planning in depression: the sequential approach. CNS Drugs 2010;24:1-13.
- Fava GA, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry 1998;155:1443-5.
- Fava GA, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am J Psychiatry 1994;151:1295-9.
- National Institute for Health and Clinical Excellence (NICE) . Common Mental Health Disorders: Identification and Pathways to Care (guideline in Development) 2010. http://guidance.nice.org.uk/CG/WaveR/83 (accessed 4 October 2010).
- Barrett B, Byford S, Knapp M. Evidence of cost-effective treatments for depression: a systematic review. J Affect Disord 2005;84:1-13.
- Seymour J, Benning TB. Depression, cardiac mortality and all-cause mortality. Adv Psychiatr Treat 2009;15:107-13.
- Sobocki P, Ekman M, Agren H, Jonsson B, Rehnberg C. Model to assess the cost-effectiveness of new treatments for depression. Int J Technol Assess Health Care 2006;22:469-77.
- Roy-Byrne P, Katon W, Broadhead WE, Lepine JP, Richards J, Brantley PJ, et al. Subsyndromal (‘mixed’) anxiety-depression in primary care. J Gen Intern Med 1994;9:507-12.
- Lecrubier Y. The impact of comorbidity on the treatment of panic disorder. J Clin Psychiatry 1998;59:11-4.
- Kendrick T, Dowrick C, McBride A, Howe A, Clarke P, Maisey S, et al. Management of depression in UK general practice in relation to scores on depression severity questionnaires: analysis of medical record data. BMJ 2009;338.
- Thomas CM, Morris S. Cost of depression among adults in England in 2000. Br J Psychiatry 2003;183:514-19.
- Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 1996.
- Luber MP, Meyers BS, Williams-Russo PG, Hollenberg JP, DiDomenico TN, Charlson ME, et al. Depression and service utilization in elderly primary care patients. Am J Geriatr Psychiatry 2001;9:169-76.
- Scottish Intercollegiate Guidelines Network (SIGN) . Non-Pharmaceutical Management of Depression in Adults. A National Clinical Guideline 2010.
- Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psychol 2007;75:1000-5.
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York, NY: Guilford Press; 1979.
- Munoz RF, Ying YW. The Prevention of Depression: Research and Practice. Baltimore, MD: The Johns Hopkins University Press; 2002.
- Strunk DR, Stewart MO, Hollon SD, DeRubeis RJ, Fawcett J, Amsterdam JD, et al. Can pharmacotherapists be too supportive? A process study of active medication and placebo in the treatment of depression. Psychol Med 2010;40:1379-87.
Appendix 1 Literature search strategies
Clinical effectiveness
MEDLINE
OvidSP http://ovidsp.ovid.com/
1950 to week 4 August 2010.
Searched on 6 September 2010.
2455 records were retrieved.
-
Beating the Blues.ti,ab. (11)
-
Depression Relief.ti,ab. (5)
-
Overcoming Depression.ti,ab. (9)
-
(BluePages or Blue Pages).ti,ab. (5)
-
(MoodGYM or Mood GYM).ti,ab. (15)
-
Keeping the Blues Away.ti,ab. (1)
-
Sadness Program.ti,ab. (0)
-
Stressbusters.ti,ab. (2)
-
Think feel do.ti,ab. (0)
-
Wellbeing Program.ti,ab. (3)
-
Living Life to the Full.ti,ab. (3)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (49)
-
exp Depressive Disorder/ (66,517)
-
Depression/ (57,156)
-
(depression or depressive or depressed).ti,ab. (224,153)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (6848)
-
13 or 14 or 15 or 16 (259,354)
-
Recurrence/ (136,231)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (376,377)
-
Secondary Prevention/ (499)
-
(secondary adj3 prevent$).ti,ab. (12,026)
-
(prophylaxis or prophylactic$).ti,ab. (96,242)
-
Remission Induction/ (25,968)
-
(remission or remitted).ti,ab. (67,244)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (2704)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (967)
-
18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (584,508)
-
17 and 27 (16,144)
-
Cognitive Therapy/ (10,799)
-
exp Behavior Therapy/ (41,600)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (11,263)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (31,770)
-
CBT.ti,ab. (2696)
-
cognitive restructuring.ti,ab. (404)
-
(cCBT or iCBT).ti,ab. (93)
-
Telemedicine/ (7621)
-
Therapy, Computer-Assisted/ (3887)
-
Computer-Assisted Instruction/ (7483)
-
(telepsychology or teletherapy or telemedicine or telehealth).ti,ab. (6043)
-
(Interactive Voice Response or IVR).ti,ab. (507)
-
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 (87,903)
-
41 and 28 (998)
-
counseling/or directive counseling/ (24,264)
-
counsel$.ti,ab. (51,434)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (1728)
-
(cybercounsel$ or cyber counsel$).ti,ab. (2)
-
43 or 44 or 45 or 46 (64,489)
-
47 and 28 (191)
-
mindfulness.ti,ab. (594)
-
49 and 28 (41)
-
exp Self Care/ (31,830)
-
Self-Help Groups/ (6870)
-
(selfcare or self care).ti,ab. (7272)
-
(selfmanage$ or self manage$).ti,ab. (4803)
-
(selfmonitor$ or self monitor$).ti,ab. (3133)
-
(selfhelp or self help).ti,ab. (3641)
-
(selftreat$ or self treat$).ti,ab. (911)
-
(selfadminister$ or self administer$).ti,ab. (16,624)
-
Bibliotherapy/ (277)
-
Manuals as Topic/ (3199)
-
Books/ (1941)
-
bibliotherap$.ti,ab. (208)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (8770)
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 (75,989)
-
64 and 28 (278)
-
exp Exercise/ (52,567)
-
exp Exercise Therapy/ (21,709)
-
exp Exercise Movement Techniques/ (3903)
-
exp Sports/ (86,678)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (180,715)
-
((resistance or strength$ or weight) adj training).ti,ab. (4258)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (1120)
-
(bicycl$ or cycle$ or cycling).ti,ab. (321,463)
-
(run$ or jog$ or treadmill$).ti,ab. (105,892)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (498)
-
(yoga or yogic or pilates or danc$).ti,ab. (3789)
-
66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 (642,671)
-
77 and 28 (1028)
-
Patient Education as Topic/ (59,612)
-
(psychoeducation$ or psycho education$).ti,ab. (2157)
-
79 or 80 (61,166)
-
81 and 28 (254)
-
Allied Health Personnel/ (9265)
-
Case Management/ (6903)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (700)
-
peer support$.ti,ab. (901)
-
((patient$ or client$) adj2 support group$).ti,ab. (257)
-
mental health peer$.ti,ab. (4)
-
graduate mental health worker$.ti,ab. (9)
-
low intensity worker$.ti,ab. (0)
-
health care assistant$.ti,ab. (133)
-
(case adj (worker$ or management)).ti,ab. (5798)
-
stepped care.ti,ab. (515)
-
(collaborative adj (care or management)).ti,ab. (600)
-
83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 (22,348)
-
96 and 28 (70)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (12)
-
Increasing Access to Psychological Therap$.ti,ab. (1)
-
Improving Access to Psychological Therap$.ti,ab. (5)
-
IAPT.ti,ab. (6)
-
98 or 99 or 100 or 101 (22)
-
12 or 42 or 48 or 50 or 65 or 78 or 82 or 97 or 102 (2540)
-
Animals/ (4,635,961)
-
Humans/ (11,394,975)
-
104 not (104 and 105) (3,440,150)
-
103 not 106 (2484)
-
letter.pt. (688,191)
-
editorial.pt. (261,694)
-
comment.pt. (418,836)
-
108 or 109 or 110 (1,019,325)
-
107 not 111 (2455)
Key
-
/ = indexing term (MeSH heading)
-
exp = exploded MeSH heading
-
$ = truncation
-
? = embedded truncation
-
pt = publication type
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order).
MEDLINE In-Process & Other Non-Indexed Citations
OvidSP http://ovidsp.ovid.com/
3 September 2010.
Searched on 6 September 2010.
107 records were retrieved.
-
Beating the Blues.ti,ab. (1)
-
Depression Relief.ti,ab. (0)
-
Overcoming Depression.ti,ab. (0)
-
(BluePages or Blue Pages).ti,ab. (0)
-
(MoodGYM or Mood GYM).ti,ab. (2)
-
Keeping the Blues Away.ti,ab. (0)
-
Sadness Program.ti,ab. (0)
-
Stressbusters.ti,ab. (0)
-
Think feel do.ti,ab. (0)
-
Wellbeing Program.ti,ab. (0)
-
Living Life to the Full.ti,ab. (0)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (2)
-
exp Depressive Disorder/ (0)
-
Depression/ (0)
-
(depression or depressive or depressed).ti,ab. (7827)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (204)
-
13 or 14 or 15 or 16 (7908)
-
Recurrence/ (3)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (13,341)
-
Secondary Prevention/ (1)
-
(secondary adj3 prevent$).ti,ab. (495)
-
(prophylaxis or prophylactic$).ti,ab. (2953)
-
Remission Induction/ (0)
-
(remission or remitted).ti,ab. (1770)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (148)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (49)
-
18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (17,891)
-
17 and 27 (572)
-
Cognitive Therapy/ (0)
-
exp Behavior Therapy/ (0)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (729)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (1558)
-
CBT.ti,ab. (228)
-
cognitive restructuring.ti,ab. (25)
-
(cCBT or iCBT).ti,ab. (11)
-
Telemedicine/ (1)
-
Therapy, Computer-Assisted/ (0)
-
Computer-Assisted Instruction/ (0)
-
(telepsychology or teletherapy or telemedicine or telehealth).ti,ab. (274)
-
(Interactive Voice Response or IVR).ti,ab. (115)
-
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 (2291)
-
41 and 28 (51)
-
counseling/or directive counseling/ (0)
-
counsel$.ti,ab. (1889)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (133)
-
(cybercounsel$ or cyber counsel$).ti,ab. (0)
-
43 or 44 or 45 or 46 (2003)
-
47 and 28 (9)
-
mindfulness.ti,ab. (59)
-
49 and 28 (9)
-
exp Self Care/ (0)
-
Self-Help Groups/ (0)
-
(selfcare or self care).ti,ab. (278)
-
(selfmanage$ or self manage$).ti,ab. (351)
-
(selfmonitor$ or self monitor$).ti,ab. (176)
-
(selfhelp or self help).ti,ab. (124)
-
(selftreat$ or self treat$).ti,ab. (35)
-
(selfadminister$ or self administer$).ti,ab. (648)
-
Bibliotherapy/ (0)
-
Manuals as Topic/ (0)
-
Books/ (0)
-
bibliotherap$.ti,ab. (9)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (471)
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 (1996)
-
64 and 28 (13)
-
exp Exercise/ (2)
-
exp Exercise Therapy/ (0)
-
exp Exercise Movement Techniques/ (0)
-
exp Sports/ (0)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (7289)
-
((resistance or strength$ or weight) adj training).ti,ab. (314)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (72)
-
(bicycl$ or cycle$ or cycling).ti,ab. (15,967)
-
(run$ or jog$ or treadmill$).ti,ab. (5926)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (36)
-
(yoga or yogic or pilates or danc$).ti,ab. (286)
-
66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 (28,416)
-
77 and 28 (34)
-
Patient Education as Topic/ (1)
-
(psychoeducation$ or psycho education$).ti,ab. (113)
-
79 or 80 (114)
-
81 and 28 (6)
-
Allied Health Personnel/ (0)
-
Case Management/ (0)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (8)
-
peer support$.ti,ab. (64)
-
((patient$ or client$) adj2 support group$).ti,ab. (15)
-
mental health peer$.ti,ab. (0)
-
graduate mental health worker$.ti,ab. (1)
-
low intensity worker$.ti,ab. (0)
-
health care assistant$.ti,ab. (5)
-
(case adj (worker$ or management)).ti,ab. (198)
-
stepped care.ti,ab. (18)
-
(collaborative adj (care or management)).ti,ab. (67)
-
83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 (373)
-
96 and 28 (4)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (1)
-
Increasing Access to Psychological Therap$.ti,ab. (1)
-
Improving Access to Psychological Therap$.ti,ab. (1)
-
IAPT.ti,ab. (3)
-
98 or 99 or 100 or 101 (4)
-
12 or 42 or 48 or 50 or 65 or 78 or 82 or 97 or 102 (110)
-
Animals/ (27)
-
Humans/ (134)
-
104 not (104 and 105) (13)
-
103 not 106 (110)
-
letter.pt. (15,007)
-
editorial.pt. (9160)
-
comment.pt. (23,331)
-
108 or 109 or 110 (40,683)
-
107 not 111 (107)
Key
-
/ = indexing term (MeSH heading)
-
exp = exploded MeSH heading
-
$ = truncation
-
? = embedded truncation
-
pt = publication type
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order).
PsycINFO
OvidSP http://ovidsp.ovid.com/
1806 to week 5 August 2010.
Searched on 6 September 2010.
1891 records were retrieved.
-
Beating the Blues.ti,ab. (10)
-
Depression Relief.ti,ab. (8)
-
Overcoming Depression.ti,ab. (32)
-
(BluePages or Blue Pages).ti,ab. (2)
-
(MoodGYM or Mood GYM).ti,ab. (10)
-
Keeping the Blues Away.ti,ab. (0)
-
Sadness Program.ti,ab. (1)
-
Stressbusters.ti,ab. (1)
-
Think feel do.ti,ab. (3)
-
Wellbeing Program.ti,ab. (0)
-
Living Life to the Full.ti,ab. (2)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (67)
-
exp major depression/ (70,891)
-
“depression (emotion)”/ (19,720)
-
atypical depression/ (129)
-
seasonal affective disorder/ (810)
-
(depression or depressive or depressed).ti,ab. (160,866)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (9393)
-
13 or 14 or 15 or 16 or 17 or 18 (168,930)
-
“relapse (disorders)”/ (4222)
-
relapse prevention/ (1519)
-
exp “remission (disorders)”/ (1959)
-
maintenance therapy/ (675)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (32,060)
-
(secondary adj3 prevent$).ti,ab. (1795)
-
(prophylaxis or prophylactic$).ti,ab. (3829)
-
(remission or remitted).ti,ab. (7924)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (1054)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (1115)
-
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 (46,330)
-
19 and 30 (12,129)
-
exp cognitive techniques/ (12,609)
-
exp cognitive behavior therapy/ (6703)
-
exp behavior modification/ (34,126)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (21,958)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (49,995)
-
CBT.ti,ab. (4577)
-
cognitive restructuring.ti,ab. (1576)
-
computer assisted therapy/ (234)
-
computer assisted instruction/ (10,048)
-
online therapy/ (623)
-
telemedicine/ (1260)
-
(telehealth or telemedicine or teletherapy or telepsychology).ti,ab. (840)
-
Interactive Voice Response.ti,ab. (139)
-
IVR.ti,ab. (115)
-
(cCBT or iCBT).ti,ab. (59)
-
32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 (96,113)
-
47 and 31 (1082)
-
exp counseling/ (57,787)
-
counsel$.ti,ab. (70,051)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (2543)
-
(cybercounsel$ or cyber counsel$).ti,ab. (24)
-
49 or 50 or 51 or 52 (100,086)
-
53 and 31 (183)
-
mindfulness/ (1174)
-
mindfulness$.ti,ab. (1901)
-
55 or 56 (1959)
-
57 and 31 (101)
-
self care skills/ (2754)
-
exp self help techniques/ (6420)
-
(selfcare or self care).ti,ab. (3985)
-
(selfmanage$ or self manage$).ti,ab. (3625)
-
(selfmonitor$ or self monitor$).ti,ab. (3801)
-
(selfhelp or self help).ti,ab. (5425)
-
(selftreat$ or self treat$).ti,ab. (236)
-
(selfadminister$ or self administer$).ti,ab. (6963)
-
bibliotherapy/ (507)
-
exp books/ (3974)
-
reading materials/ (1454)
-
bibliotherap$.ti,ab. (714)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (1782)
-
59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 (34,914)
-
72 and 31 (145)
-
exp exercise/ (11,910)
-
movement therapy/ (505)
-
exp sports/ (11,533)
-
dance therapy/ (570)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (42,784)
-
((resistance or strength$ or weight) adj training).ti,ab. (528)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (353)
-
(bicycl$ or cycle$ or cycling).ti,ab. (27,764)
-
(run$ or jog$ or treadmill$).ti,ab. (26,735)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (205)
-
(yoga or yogic or pilates or danc$).ti,ab. (5067)
-
74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 (109,028)
-
85 and 31 (521)
-
psychoeducation/ (2414)
-
client education/ (2519)
-
(psychoeducation$ or psycho education$).ti,ab. (5693)
-
87 or 88 or 89 (8441)
-
90 and 31 (147)
-
allied health personnel/ (498)
-
paraprofessional personnel/ (1297)
-
case management/ (2140)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (1648)
-
peer support$.ti,ab. (1461)
-
((patient$ or client$) adj2 support group$).ti,ab. (70)
-
mental health peer$.ti,ab. (9)
-
graduate mental health worker$.ti,ab. (6)
-
health care assistant$.ti,ab. (25)
-
low intensity worker$.ti,ab. (3)
-
(case adj (worker$ or management)).ti,ab. (3379)
-
stepped care.ti,ab. (248)
-
(collaborative adj (care or management)).ti,ab. (393)
-
92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 (9135)
-
106 and 31 (49)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (13)
-
Increasing Access to Psychological Therap$.ti,ab. (3)
-
Improving Access to Psychological Therap$.ti,ab. (15)
-
IAPT.ti,ab. (20)
-
108 or 109 or 110 or 111 (38)
-
12 or 48 or 54 or 58 or 73 or 86 or 91 or 107 or 112 (1955)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (202,139)
-
113 not 114 (1934)
-
(editorial or letter).dt. (27,226)
-
115 not 116 (1901)
-
limit 117 to yr = “1950 -Current” (1891)
Key
-
/ = indexing term
-
exp = exploded indexing term
-
$ = truncation
-
? = embedded truncation
-
dt. = document type
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
-
sh = subject heading field.
EMBASE
OvidSP http://ovidsp.ovid.com/
1980 to week 38 2010.
Searched on 29 September 2010.
2905 records were retrieved.
-
Beating the Blues.ti,ab. (16)
-
Depression Relief.ti,ab. (8)
-
Overcoming Depression.ti,ab. (14)
-
(BluePages or Blue Pages).ti,ab. (7)
-
(MoodGYM or Mood GYM).ti,ab. (19)
-
Keeping the Blues Away.ti,ab. (1)
-
Sadness Program.ti,ab. (0)
-
Stressbusters.ti,ab. (2)
-
Think feel do.ti,ab. (1)
-
Wellbeing Program.ti,ab. (4)
-
Living Life to the Full.ti,ab. (3)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (67)
-
exp *depression/ (125,235)
-
(depression or depressive or depressed).ti,ab. (264,422)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (8650)
-
13 or 14 or 15 (299,144)
-
*recurrent disease/ (5836)
-
*relapse/ (2425)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (450,289)
-
*secondary prevention/ (1035)
-
(secondary adj3 prevent$).ti,ab. (16,107)
-
*prophylaxis/ (4975)
-
(prophylaxis or prophylactic$).ti,ab. (117,902)
-
*remission/ (1457)
-
(remission or remitted).ti,ab. (79,158)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (3160)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (1183)
-
17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 (628,701)
-
16 and 28 (19,094)
-
*cognitive therapy/ (7474)
-
*behavior therapy/ (13,293)
-
*behavior modification/ (1031)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (18,961)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (48,358)
-
CBT.ti,ab. (4164)
-
cognitive restructuring.ti,ab. (677)
-
(cCBT or iCBT).ti,ab. (129)
-
exp *telehealth/ (7617)
-
*computer assisted therapy/ (1548)
-
(telepsychology or teletherapy or telemedicine or telehealth).ti,ab. (6906)
-
*interactive voice response system/ (39)
-
(Interactive Voice Response or IVR).ti,ab. (676)
-
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 (77,933)
-
43 and 29 (1110)
-
exp *counseling/ (22,273)
-
counsel$.ti,ab. (61,285)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (2415)
-
(cybercounsel$ or cyber counsel$).ti,ab. (2)
-
45 or 46 or 47 or 48 (73,648)
-
49 and 29 (236)
-
mindfulness.ti,ab. (924)
-
51 and 29 (68)
-
exp *self care/ (14,330)
-
(selfcare or self care).ti,ab. (8667)
-
(selfmanage$ or self manage$).ti,ab. (6339)
-
(selfmonitor$ or self monitor$).ti,ab. (4162)
-
(selfhelp or self help).ti,ab. (4653)
-
(selftreat$ or self treat$).ti,ab. (1176)
-
(selfadminister$ or self administer$).ti,ab. (19,015)
-
*book/ (3872)
-
bibliotherap$.ti,ab. (299)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (11,170)
-
53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 (66,094)
-
63 and 29 (268)
-
exp *exercise/ (71,270)
-
exp *sport/ (37,531)
-
exp *physical activity/ (53,097)
-
exp *kinesiotherapy/ (16,054)
-
*music therapy/ (2050)
-
*treadmill/or *treadmill exercise/ (2173)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (215,071)
-
((resistance or strength$ or weight) adj training).ti,ab. (5135)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (1384)
-
(bicycl$ or cycle$ or cycling).ti,ab. (365,808)
-
(run$ or jog$ or treadmill$).ti,ab. (126,820)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (681)
-
(yoga or yogic or pilates or danc$).ti,ab. (5196)
-
65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 (739,132)
-
78 and 29 (1264)
-
*psychoeducation/ (333)
-
*patient education/ (20,510)
-
(psychoeducation$ or psycho education$).ti,ab. (3197)
-
80 or 81 or 82 (23,553)
-
83 and 29 (255)
-
*paramedical personnel/ (5290)
-
*case management/or *case manager/ (3110)
-
*support group/ (508)
-
*peer group/ (2658)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (677)
-
peer support$.ti,ab. (1155)
-
((patient$ or client$) adj2 support group$).ti,ab. (345)
-
mental health peer$.ti,ab. (6)
-
graduate mental health worker$.ti,ab. (14)
-
low intensity worker$.ti,ab. (1)
-
health care assistant$.ti,ab. (151)
-
(case adj (worker$ or management)).ti,ab. (6627)
-
stepped care.ti,ab. (631)
-
(collaborative adj (care or management)).ti,ab. (770)
-
85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 (20,104)
-
100 and 29 (80)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (12)
-
Increasing Access to Psychological Therap$.ti,ab. (3)
-
Improving Access to Psychological Therap$.ti,ab. (21)
-
IAPT.ti,ab. (21)
-
102 or 103 or 104 or 105 (46)
-
12 or 44 or 50 or 52 or 64 or 79 or 84 or 101 or 106 (2972)
-
editorial.pt. (355,119)
-
letter.pt. (702,108)
-
108 or 109 (1,057,227)
-
107 not 110 (2962)
-
exp animal/ (1,629,045)
-
exp nonhuman/ (3,502,467)
-
exp animal experiment/ (1,392,148)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (3,938,570)
-
112 or 113 or 114 or 115 (5,657,477)
-
exp human/ (11,999,569)
-
exp human experiment/ (282,161)
-
117 or 118 (12,000,950)
-
116 not (116 and 119) (4,482,506)
-
111 not 120 (2905)
Key
-
/ = indexing term (EMTREE heading)
-
* = focused EMTREE heading
-
exp = exploded EMTREE heading
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
-
sh = subject heading field.
The Cochrane Library
Wiley http://onlinelibrary.wiley.com/
Cochrane Database of Systematic Reviews (CDSR), Issue 9, September 2010.
Database of Abstracts of Reviews of Effects (DARE), Issue 3, 2010.
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 3, 2010.
Health Technology Assessment database (HTA), Issue 3, 2010.
Searched on 17 September 2010.
702 records were retrieved – 20 from CDSR, six from DARE, 674 from CENTRAL, two from HTA database.
#1 “Beating the Blues”:ti,ab (4)
#2 “Depression Relief”:ti,ab (2)
#3 “Overcoming Depression”:ti,ab (6)
#4 (“BluePages” or “Blue Pages”):ti,ab (3)
#5 (“MoodGYM” or “Mood GYM”):ti,ab (9)
#6 “Keeping the Blues Away”:ti,ab (1)
#7 “Sadness Program”:ti,ab (0)
#8 “Stressbusters”:ti,ab (0)
#9 “Think feel do”:ti,ab (0)
#10 “Wellbeing Program”:ti,ab (0)
#11 “Living Life to the Full”:ti,ab (0)
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) (23)
#13 MeSH descriptor Depressive Disorder explode all trees (6039)
#14 MeSH descriptor Depression, this term only (3924)
#15 (depression or depressive or depressed):ti,ab (24,924)
#16 (melancholi* or dysphori* or dysthymi*):ti,ab (1130)
#17 (#13 OR #14 OR #15 OR #16) (26,859)
#18 MeSH descriptor Recurrence, this term only (10,144)
#19 (recur* or reoccur* or (re NEXT occur*) or relaps*):ti,ab (26,763)
#20 MeSH descriptor Secondary Prevention, this term only (39)
#21 (secondary NEAR/3 prevent*):ti,ab (1375)
#22 (prophylaxis or prophylactic*):ti,ab (16,307)
#23 MeSH descriptor Remission Induction, this term only (2326)
#24 (remission or remitted):ti,ab (7994)
#25 (maintain* NEAR/3 (health or wellbeing or (well NEXT being))):ti,ab (134)
#26 ((another or further or second or repeat* or previous or initial or subsequent) NEAR/4 (episode* or bout* or instance* or symptom* or occurrence*) NEAR/4 depress*):ti,ab (137)
#27 (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) (52,009)
#28 (#17 AND #27) (2833)
#29 MeSH descriptor Cognitive Therapy, this term only (3139)
#30 MeSH descriptor Behavior Therapy explode all trees (7327)
#31 (cognitive NEAR/3 (therap* or treatment* or intervention* or program* or package* or training or group*)):ti,ab (5233)
#32 ((behavior* or behaviour*) NEAR/3 (therap* or treatment* or intervention* or program* or package* or training or activat* or modif* or group*)):ti,ab (7966)
#33 CBT:ti,ab (1147)
#34 (cognitive NEXT restructuring):ti,ab (208)
#35 (cCBT or iCBT):ti,ab (27)
#36 MeSH descriptor Telemedicine, this term only (595)
#37 MeSH descriptor Therapy, Computer-Assisted, this term only (418)
#38 MeSH descriptor Computer-Assisted Instruction, this term only (598)
#39 (telepsychology or teletherapy or telemedicine or telehealth):ti,ab (456)
#40 (“Interactive Voice Response” or IVR):ti,ab (104)
#41 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40) (15,592)
#42 (#28 AND #41) (417)
#43 MeSH descriptor Counseling, this term only (2088)
#44 MeSH descriptor Directive Counseling, this term only (121)
#45 counsel*:ti,ab (4665)
#46 (motivation* NEAR/2 (interview* or enhance* or intervention* or therap*)):ti,ab (707)
#47 (cybercounsel* or (cyber NEXT counsel*)):ti,ab (0)
#48 (#43 OR #44 OR #45 OR #46 OR #47) (5885)
#49 (#28 AND #48) (51)
#50 mindfulness:ti,ab (178)
#51 (#28 AND #50) (26)
#52 MeSH descriptor Self Care explode all trees (2741)
#53 MeSH descriptor Self-Help Groups, this term only (458)
#54 (selfcare or (self NEXT care)):ti,ab (733)
#55 (selfmanage* or (self NEXT manage*) or selfmonitor* or (self NEXT monitor*)):ti,ab (1728)
#56 (selfhelp or (self NEXT help)):ti,ab (768)
#57 (selftreat* or (self NEXT treat*)):ti,ab (96)
#58 (selfadminister* or (self NEXT administer*)):ti,ab (1631)
#59 MeSH descriptor Bibliotherapy, this term only (66)
#60 MeSH descriptor Manuals as Topic, this term only (107)
#61 MeSH descriptor Books, this term only (25)
#62 bibliotherap*:ti,ab (103)
#63 ((patient* or client* or user*) NEAR/3 (manual* or handbook* or workbook* or guide*)):ti,ab (773)
#64 (#52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63) (7409)
#65 (#64 AND #28) (71)
#66 MeSH descriptor Exercise explode all trees (7313)
#67 MeSH descriptor Exercise Therapy explode all trees (4063)
#68 MeSH descriptor Exercise Movement Techniques explode all trees (725)
#69 MeSH descriptor Sports explode all trees (6128)
#70 (exercise* or workout* or (work NEXT out*) or (physical* NEXT activ*)):ti,ab (26,568)
#71 ((resistance or strength* or weight) NEAR training):ti,ab (2529)
#72 (walk* NEAR/3 (fitness or aerobic or program* or intervention* or session* or regime*)):ti,ab (445)
#73 (bicycl* or cycle* or cycling):ti,ab (15,471)
#74 (run* or jog* or treadmill*):ti,ab (9264)
#75 ((tai NEXT ji) or taiji or taijiquan or (tai NEXT chi) or (t NEXT ai NEXT chi) or taichi or (shadow NEXT boxing)):ti,ab (194)
#76 (yoga or yogic or pilates or danc*):ti,ab (444)
#77 (#66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76) (48,050)
#78 (#77 AND #28) (177)
#79 MeSH descriptor Patient Education as Topic, this term only (4958)
#80 (psychoeducation* or (psycho NEXT education*)):ti,ab (758)
#81 (#79 OR #80) (5513)
#82 (#81 AND #28) (78)
#83 MeSH descriptor Allied Health Personnel, this term only (131)
#84 MeSH descriptor Case Management, this term only (606)
#85 (psychological NEXT wellbeing NEXT practitioner*):ti,ab (0)
#86 (psychological NEXT (well NEXT being) NEXT practitioner*):ti,ab (0)
#87 (personal NEXT wellbeing NEXT practitioner*):ti,ab (0)
#88 (personal NEXT (well NEXT being) NEXT practitioner*):ti,ab (0)
#89 ((para NEXT professional*) or paraprofessional*):ti,ab (89)
#90 (peer NEXT support*):ti,ab (122)
#91 ((patient* or client*) NEAR/2 (support NEXT group*)):ti,ab (10)
#92 (mental NEXT health NEXT peer*):ti,ab (1)
#93 (graduate NEXT mental NEXT health NEXT worker*):ti,ab (1)
#94 (low NEXT intensity NEXT worker*):ti,ab (0)
#95 (health NEXT care NEXT assistant*):ti,ab (5)
#96 (case NEXT (worker* or management)):ti,ab (771)
#97 (stepped NEXT care):ti,ab (168)
#98 (collaborative NEXT (care or management)):ti,ab (153)
#99 (#83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98) (1682)
#100 (#99 AND #28) (29)
#101 ((low NEXT intensity) NEAR/5 (psychological or psychosocial)):ti,ab (2)
#102 “Increasing Access to Psychological Therapy”:ti,ab (0)
#103 “Increasing Access to Psychological Therapies”:ti,ab (0)
#104 “Improving Access to Psychological Therapy”:ti,ab (0)
#105 “Improving Access to Psychological Therapies”:ti,ab (0)
#106 IAPT:ti,ab (0)
#107 (#101 OR #102 OR #103 OR #104 OR #105 OR #106) (2)
#108 (#12 OR #42 OR #49 OR #51 OR #65 OR #78 OR #82 OR #100 OR #107), from 1950 to 2010 (707)
Key
-
MeSH descriptor = indexing term (MeSH heading)
-
* = truncation
-
“ “ = phrase search
-
:ti,ab = terms in either title or abstract fields
-
NEAR/2 = terms within two words of each other (any order)
-
NEXT = terms are next to each other.
Science Citation Index (SCI), Social Science Citation Index (SSCI)
ISI Web of Knowledge www.isinet.com/
SSCI 1956–present, SCI 1899–present.
Searched on 16 September 2010.
3656 records were retrieved.
| # 79 | 3656 | #78 Databases = SCI-EXPANDED, SSCI Timespan = 1945–2010 |
| # 78 | 3656 | #76 NOT #77 |
| # 77 | > 100,000 | TS = (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep) |
| # 76 | 3849 | #75 OR #70 OR #57 OR #55 OR #46 OR #41 OR #36 OR #34 OR #11 |
| # 75 | 81 | #74 OR #73 OR #72 OR #71 |
| # 74 | 50 | TS = IAPT |
| # 73 | 8 | TS = “Improving Access to Psychological Therap*” |
| # 72 | 2 | TS = “Increasing Access to Psychological Therap*” |
| # 71 | 27 | TS = (“low-intensity” SAME (psychological or psychosocial)) |
| # 70 | 137 | #69 AND #23 |
| # 69 | 9222 | #68 OR #67 OR #66 OR #65 OR #64 OR #63 OR #62 OR #61 OR #60 OR #59 OR #58 |
| # 68 | 1014 | TS = (“collaborative care” or “collaborative management”) |
| # 67 | 425 | TS = “stepped care” |
| # 66 | 5009 | TS = (“case worker*” or “case management”) |
| # 65 | 55 | TS = “health care assistant*” |
| # 64 | 1 | TS = “low-intensity worker*” |
| # 63 | 6 | TS = “graduate mental health worker*” |
| # 62 | 4 | TS = “mental health peer*” |
| # 61 | 742 | TS = ((patient* or client*) SAME “support group*”) |
| # 60 | 1060 | TS = “peer support*” |
| # 59 | 1055 | TS = (“para-professional*” or paraprofessional*) |
| # 58 | 0 | TS = (“psychological well-being practitioner*” or “personal well-being practitioner*”) |
| # 57 | 185 | #56 AND #23 |
| # 56 | 3303 | TS = (psychoeducation* or “psycho-education*”) |
| # 55 | 1140 | #54 AND #23 |
| # 54 | > 100,000 | #53 OR #52 OR #51 OR #50 OR #49 OR #48 OR #47 |
| # 53 | 9365 | TS = (yoga or yogic or pilates or danc*) |
| # 52 | 791 | TS = (“tai-ji” or taiji or taijiquan or “tai-chi” or “t-ai-chi” or taichi or “shadow boxing”) |
| # 51 | > 100,000 | TS = (run* or jog* or treadmill*) |
| # 50 | > 100,000 | TS = (bicycl* or cycle* or cycling) |
| # 49 | 3248 | TS = (walk* SAME (fitness or aerobic or program* or intervention* or session* or regime*)) |
| # 48 | 10,705 | TS = ((resistance or strength* or weight) SAME training) |
| # 47 | > 100,000 | TS = (exercise* or workout* or “work-out*” or “physical* activ*”) |
| # 46 | 518 | #45 AND #23 |
| # 45 | 79,054 | #44 OR #43 OR #42 |
| # 44 | 43,581 | TS = ((patient* or client* or user*) SAME (manual* or handbook* or workbook* or guide*)) |
| # 43 | 469 | TS = bibliotherap* |
| # 42 | 35,847 | TS = (selfcare or “self-care” or selfmanage* or “self-manage*” or selfmonitor* or “self-monitor*” or selfhelp or “self-help” or selftreat* or “self-treat*” or selfadminister* or “self-administer*”) |
| # 41 | 232 | #40 AND #23 |
| # 40 | 61,995 | #39 OR #38 OR #37 |
| # 39 | 3 | TS = (cybercounsel* or “cyber-counsel*”) |
| # 38 | 4742 | TS = (motivation* SAME (interview* or enhance* or intervention* or therap*)) |
| # 37 | 57,750 | TS = (counsel*) |
| # 36 | 111 | #35 AND #23 |
| # 35 | 1308 | TS = mindfulness |
| # 34 | 2018 | #33 AND #23 |
| # 33 | > 100,000 | #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 |
| # 32 | 1200 | TS = (“Interactive Voice Response” or IVR) |
| # 31 | 6254 | TS = (telepsychology or teletherapy or telemedicine or telehealth) |
| # 30 | 2742 | TS = ((“computer-assisted” or online) SAME (therap* or instruction*)) |
| # 29 | 102 | TS = (cCBT or iCBT) |
| # 28 | 542 | TS = “cognitive restructuring” |
| # 27 | 122 | TS = (“cognitive techniques”) |
| # 26 | 3694 | TS = CBT |
| # 25 | > 100,000 | TS = ((behavior* or behaviour*) SAME (therap* or treatment* or intervention* program* or package* or training or activat* or modif* or group*)) |
| # 24 | 37,852 | TS = (cognitive SAME (therap* or treatment* or intervention* or program* or package* or training or group*)) |
| # 23 | 17,019 | #22 AND #14 |
| # 22 | > 100,000 | #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 |
| # 21 | 2273 |
TS = ((second or repeat* or previous or initial or subsequent) SAME (episode* or bout* or instance* or symptom* or occurrence*) SAME (depress*)) |
| # 20 | 6200 | TS = (“maintenance therapy”) |
| # 19 | 14,545 | TS = (maintain* SAME (health or wellbeing or “well-being”)) |
| # 18 | 52,360 | TS = (remission or remitted) |
| # 17 | 86,889 | TS = (prophylaxis or prophylactic*) |
| # 16 | 14,461 | TS = (secondary SAME prevent*) |
| # 15 | > 100,000 | TS = (recur* or reoccur* or “re-occur*” or relaps*) |
| # 14 | > 100,000 | #13 OR #12 |
| # 13 | 7608 | TS = (melancholi* or dysphori* or dysthymi*) |
| # 12 | > 100,000 | TS = (depression or depressive or depressed) |
| # 11 | 82 | #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| # 10 | 3 | TS = “Living Life to the Full” |
| # 9 | 2 | TS = “Wellbeing Program” |
| # 8 | 1 | TS = “Stressbusters” |
| # 7 | 0 | TS = “Sadness Program” |
| # 6 | 1 | TS = “Keeping the Blues Away” |
| # 5 | 15 | TS = (“MoodGYM” or “Mood GYM”) |
| # 4 | 18 | TS = (“BluePages” or “Blue Pages”) |
| # 3 | 30 | TS = “Overcoming Depression” |
| # 2 | 4 | TS = “Depression Relief” |
| # 1 | 15 | TS = “Beating the Blues” |
Key
-
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields
-
* = truncation
-
“ “ = phrase search
-
SAME = terms within same sentence.
BIOSIS Previews
ISI Web of Knowledge www.isinet.com/
1969–2008.
Searched on 17 September 2010.
3121 records were retrieved.
| # 83 | 3121 | #76 NOT #82 |
| # 82 | > 100,000 | #81 OR #80 OR #79 OR #78 OR #77 |
| # 81 | 82,651 | TI = (cow or cattle or livestock or swine or poultry) |
| # 80 | > 100,000 | TI = (rabbit or rabbits or moss or mosses or fungus or fungi) |
| # 79 | > 100,000 | TI = (bat or bats or bee or bees or grass or grasses or bird or birds or avian) |
| # 78 | > 100,000 | TI = (fly or flies or fish or fishes or fisheries or horse or horses or equine) |
| # 77 | > 100,000 | TI = (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or canine or cat or cats or feline or bovine or sheep) |
| # 76 | 3202 | #75 OR #70 OR #57 OR #55 OR #46 OR #41 OR #36 OR #34 OR #11 |
| # 75 | 24 | #74 OR #73 OR #72 OR #71 |
| # 74 | 13 | TS = IAPT |
| # 73 | 0 | TS = “Improving Access to Psychological Therap*” |
| # 72 | 0 | TS = “Increasing Access to Psychological Therap*” |
| # 71 | 11 | TS = (“low-intensity” SAME (psychological or psychosocial)) |
| # 70 | 49 | #69 AND #23 |
| # 69 | 2309 | #68 OR #67 OR #66 OR #65 OR #64 OR #63 OR #62 OR #61 OR #60 OR #59 OR #58 |
| # 68 | 154 | TS = (“collaborative care” or “collaborative management”) |
| # 67 | 231 | TS = “stepped care” |
| # 66 | 1324 | TS = (“case worker*” or “case management”) |
| # 65 | 6 | TS = “health care assistant*” |
| # 64 | 0 | TS = “low-intensity worker*” |
| # 63 | 0 | TS = “graduate mental health worker*” |
| # 62 | 0 | TS = “mental health peer*” |
| # 61 | 288 | TS = ((patient* or client*) SAME “support group*”) |
| # 60 | 171 | TS = “peer support*” |
| # 59 | 161 | TS = (“para-professional*” or paraprofessional*) |
| # 58 | 0 | TS = (“psychological well-being practitioner*” or “personal well-being practitioner*”) |
| # 57 | 81 | #56 AND #23 |
| # 56 | 888 | TS = (psychoeducation* or “psycho-education*”) |
| # 55 | 1351 | #54 AND #23 |
| # 54 | > 100,000 | #53 OR #52 OR #51 OR #50 OR #49 OR #48 OR #47 |
| # 53 | 2,966 | TS = (yoga or yogic or pilates or danc*) |
| # 52 | 227 | TS = (“tai-ji” or taiji or taijiquan or “tai-chi” or “t-ai-chi” or taichi or “shadow boxing”) |
| # 51 | > 100,000 | TS = (run* or jog* or treadmill*) |
| # 50 | > 100,000 | TS = (bicycl* or cycle* or cycling) |
| # 49 | 1647 | TS = (walk* SAME (fitness or aerobic or program* or intervention* or session* or regime*)) |
| # 48 | 6863 | TS = ((resistance or strength* or weight) SAME training) |
| # 47 | > 100,000 | TS = (exercise* or workout* or “work-out*” or “physical* activ*”) |
| # 46 | 357 | #45 AND #23 |
| # 45 | 36,610 | #44 OR #43 OR #42 |
| # 44 | 20,819 | TS = ((patient* or client* or user*) SAME (manual* or handbook* or workbook* or guide*)) |
| # 43 | 63 | TS = bibliotherap* |
| # 42 | 15,951 | TS = (selfcare or “self-care” or selfmanage* or “self-manage*” or selfmonitor* or “self-monitor*” or selfhelp or “self-help” or selftreat* or “self-treat*” or selfadminister* or “self-administer*”) |
| # 41 | 207 | #40 AND #23 |
| # 40 | 26,670 | #39 OR #38 OR #37 |
| # 39 | 0 | TS = (cybercounsel* or “cyber-counsel*”) |
| # 38 | 1763 | TS = (motivation* SAME (interview* or enhance* or intervention* or therap*)) |
| # 37 | 25,085 | TS = (counsel*) |
| # 36 | 34 | #35 AND #23 |
| # 35 | 157 | TS = mindfulness |
| # 34 | 1460 | #33 AND #23 |
| # 33 | 84,588 | #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 |
| # 32 | 313 | TS = (“Interactive Voice Response” or IVR) |
| # 31 | 1527 | TS = (telepsychology or teletherapy or telemedicine or telehealth) |
| # 30 | 600 | TS = ((“computer-assisted” or online) SAME (therap* or instruction*)) |
| # 29 | 44 | TS = (cCBT or iCBT) |
| # 28 | 263 | TS = “cognitive restructuring” |
| # 27 | 52 | TS = (“cognitive techniques”) |
| # 26 | 1370 | TS = CBT |
| # 25 | 70,488 | TS = ((behavior* or behaviour*) SAME (therap* or treatment* or intervention* program* or package* or training or activat* or modif* or group*)) |
| # 24 | 16,609 | TS = (cognitive SAME (therap* or treatment* or intervention* or program* or package* or training or group*)) |
| # 23 | 16,005 | #22 AND #14 |
| # 22 | > 100,000 | #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 |
| # 21 | 1244 | TS = ((second or repeat* or previous or initial or subsequent) SAME (episode* or bout* or instance* or symptom* or occurrence*) SAME (depress*)) |
| # 20 | 5114 | TS = (“maintenance therapy”) |
| # 19 | 9079 | TS = (maintain* SAME (health or wellbeing or “well-being”)) |
| # 18 | 52,528 | TS = (remission or remitted) |
| # 17 | > 100,000 | TS = (prophylaxis or prophylactic*) |
| # 16 | 7255 | TS = (secondary SAME prevent*) |
| # 15 | > 100,000 | TS = (recur* or reoccur* or “re-occur*” or relaps*) |
| # 14 | > 100,000 | #13 OR #12 |
| # 13 | 5152 | TS = (melancholi* or dysphori* or dysthymi*) |
| # 12 | > 100,000 | TS = (depression or depressive or depressed) |
| # 11 | 14 | #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| # 10 | 0 | TS = “Living Life to the Full” |
| # 9 | 0 | TS = “Wellbeing Program” |
| # 8 | 0 | TS = “Stressbusters” |
| # 7 | 0 | TS = “Sadness Program” |
| # 6 | 0 | TS = “Keeping the Blues Away” |
| # 5 | 6 | TS = (“MoodGYM” or “Mood GYM”) |
| # 4 | 5 | TS = (“BluePages” or “Blue Pages”) |
| # 3 | 1 | TS = “Overcoming Depression” |
| # 2 | 3 | TS = “Depression Relief” |
| # 1 | 2 | TS = “Beating the Blues” |
Key
-
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields
-
* = truncation
-
“ “ = phrase search
-
SAME = terms within same sentence
-
TI = title field.
BIOSIS Previews
Dialog www.dialog.com/
1993 to week 2 September 2010.
Searched on 20 September 2010.
777 records were retrieved.
| 1. | 129,826 | DEPRESSION OR DEPRESSIVE OR DEPRESSED/TI,AB,DE |
| 2. | 3814 | MELANCHOLI? OR DYSPHORI? OR DYSTHYMI?/TI,AB,DE |
| 3. | 131,070 | S1:S2 |
| 4. | 191,054 | RECUR? OR REOCCUR? OR RE OCCUR? OR RELAPS?/TI,AB,DE |
| 5. | 4702 | SECONDARY (3W) PREVENT?/TI,AB,DE |
| 6. | 591,974 | PROPHYLAXIS OR PROPHYLACTIC?/TI,AB,DE |
| 7. | 40,324 | REMISSION OR REMITTED/TI,AB,DE |
| 8. | 1048 | MAINTAIN? (3W) (HEALTH OR WELLBEING OR WELL (W) BEING)/TI,AB,DE |
| 9. | 4485 | MAINTENANCE (W) THERAP?/TI,AB,DE |
| 10. | 143 | (ANOTHER OR FURTHER OR SECOND OR REPEAT? OR PREVIOUS OR INITIAL OR SUBSEQUENT) (4W) (EPISODE? OR BOUT? OR INSTANCE? OR SYMPTOM? OR OCCURRENCE?)(4W) DEPRESS?/TI,AB,DE |
| 11. | 760,034 | S4:S10 |
| 12. | 15,541 | S3 AND S11 |
| 13. | 6418 | COGNITIVE (3W) (THERAP? OR TREATMENT? OR INTERVENTION? OR PROGRAM? OR PACKAGE? OR TRAINING OR GROUP?)/TI,AB,DE |
| 14. | 4118 | BEHAVIO?R? (3W) (THERAP? OR TREATMENT? OR INTERVENTION? OR PROGRAM? OR PACKAGE? OR TRAINING OR ACTIVAT? OR MODIF? OR GROUP?)/TI,AB,DE |
| 15. | 1696 | CBT/TI,AB,DE |
| 16. | 197 | COGNITIVE(W) (RESTRUCTURING OR TECHNIQUE?)/TI,AB,DE |
| 17. | 66 | (CCBT OR ICBT)/TI,AB,DE |
| 18. | 84 | (COMPUTER-ASSISTED OR ONLINE (W)THERAPY OR COMPUTER (W) ASSISTED (W) THERAPY)/TI,AB,DE |
| 19. | 201 | (COMPUTER-ASSISTED OR ONLINE (W) INSTRUCTION OR COMPUTER(W) ASSISTED (W) INSTRUCTION)/TI,AB,DE |
| 20. | 1461 | (TELEPSYCHOLOGY OR TELETHERAPYOR TELEMEDICINE OR TELEHEALTH)/TI,AB,DE |
| 21. | 240 | (INTERACTIVE VOICE RESPONSE OR IVR)/TI,AB,DE |
| 22. | 12,269 | S13:S21 |
| 23. | 1095 | S12 AND S22 |
| 24. | 20,163 | COUNSEL?/TI,AB,DE |
| 25. | 868 | MOTIVATION? (2W) (INTERVIEW? OR ENHANCE? OR INTERVENTION? OR THERAP?)/TI,AB,DE |
| 26. | 0 | (CYBERCOUNSEL? OR CYBER (W) COUNSEL?)/TI,AB,DE |
| 27. | 20,888 | S24:S26 |
| 28. | 241 | S12 AND S27 |
| 29. | 240 | MINDFULNESS/TI,AB,DE |
| 30. | 54 | S12 AND S29 |
| 31. | 1587 | (SELFCARE OR SELF (W) CARE)/TI,AB,DE |
| 32. | 1711 | (SELFMANAG? OR SELF (W) MANAG?)/TI,AB,DE |
| 33. | 1340 | (SELFMONITOR? OR SELF (W) MONITOR?)/TI,AB,DE |
| 34. | 1013 | (SELFHELP OR SELF (W) HELP)/TI,AB,DE |
| 35. | 327 | (SELFTREAT? OR SELF (W) TREAT?)/TI,AB,DE |
| 36. | 8586 | (SELFADMINISTER? OR SELF (W) ADMINISTER?)/TI,AB,DE |
| 37. | 46 | BIBLIOTHERAP?/TI,AB,DE |
| 38. | 2388 | (PATIENT? OR CLIENT? OR USER?)(3W) (MANUAL? OR HANDBOOK? OR WORKBOOK? OR GUIDE?)/TI,AB,DE |
| 39. | 16,652 | S31:S38 |
| 40. | 230 | S12 AND S39 |
| 41. | 111,105 | (EXERCISE? OR WORKOUT? OR WORK (W) OUT? OR PHYSICAL? (W) ACTIV?)/TI,AB,DE |
| 42. | 3454 | (RESISTANCE OR STRENGTH? OR WEIGHT) (W) TRAINING/TI,AB,DE |
| 43. | 374 | WALK? (3W) (FITNESS OR AEROBIC OR PROGRAM? OR INTERVENTION? OR SESSION? OR REGIME?)/TI,AB,DE |
| 44. | 308,119 | (BICYCL? OR CYCLE? OR CYCLING)/TI,AB,DE |
| 45. | 88,249 | (RUN? OR JOG? OR TREADMILL?)/TI,AB,DE |
| 46. | 242 | (TAI (W) JI OR TAIJI OR TAIJIQUAN OR TAI (W) CHI OR T (W) AI (W) CHI OR TAICHI OR SHADOW (W) BOXING)/TI,AB,DE |
| 47. | 2126 | (YOGA OR YOGIC OR PILATES OR DANC?)/TI,AB,DE |
| 48. | 483,154 | S41:S47 |
| 49. | 1023 | S12 AND S48 |
| 50. | 848 | (PSYCHOEDUCATION? OR PSYCHO (W) EDUCATION?)/TI,AB,DE |
| 51. | 95 | S12 AND S50 |
| 52. | 0 | (PSYCHOLOGICAL OR PERSONAL) (W) (WELLBEING (W) PRACTITIONER? OR WELL (W) BEING (W) PRACTITIONER?)/TI,AB,DE |
| 53. | 72 | (PARA (W) PROFESSIONAL? OR PARAPROFESSIONAL?)/TI,AB,DE |
| 54. | 149 | PEER (W) SUPPORT?/TI,AB,DE |
| 55. | 103 | (PATIENT? OR CLIENT?) (2W) SUPPORT (W) GROUP?/TI,AB,DE |
| 56. | 1 | MENTAL (W) HEALTH (W) PEER?/TI,AB,DE |
| 57. | 0 | GRADUATE (W) MENTAL (W) HEALTH (W) WORKER?/TI,AB,DE |
| 58. | 0 | LOW (W) INTENSITY (W) WORKER?/TI,AB,DE |
| 59. | 10 | HEALTH(W) CARE (W) ASSISTANT?/TI,AB,DE |
| 60. | 1310 | CASE (W) (WORKER? OR MANAGEMENT)/TI,AB,DE |
| 61. | 154 | STEPPED (W) CARE/TI,AB,DE |
| 62. | 195 | COLLABORATIVE (W) (CARE OR MANAGEMENT)/TI,AB,DE |
| 63. | 1975 | S52:S62 |
| 64. | 59 | S12 AND S63 |
| 65. | 1 | LOW (W) INTENSITY (5W) (PSYCHOLOGICAL OR PSYCHOSOCIAL)/TI,AB,DE |
| 66. | 0 | INCREASING (W) ACCESS (2W) PSYCHOLOGICAL (W) THERAP?/TI,AB,DE |
| 67. | 1 | IMPROVING (W) ACCESS(2W) PSYCHOLOGICAL (W) THERAP?/TI,AB,DE |
| 68. | 14 | IAPT/TI,AB,DE |
| 69. | 15 | S65:S68 |
| 70. | 2502 | S23 OR S28 OR S30 OR S40 OR S49 OR S51 OR S64 OR S69 |
| 71. | 867,148 | (RAT OR RATS OR MOUSE OR MICE OR HAMSTER OR HAMSTERS OR ANIMAL OR ANIMALS OR DOG OR DOGS OR CANINE OR CAT OR CATS OR FELINE OR BOVINE OR SHEEP)/TI |
| 72. | 88,548 | (FLY OR FLIES OR FISH OR FISHES OR FISHERIES OR HORSE OR HORSES OR EQUINE)/TI |
| 73. | 54,830 | (BAT OR BATS OR BEE OR BEES OR GRASS OR GRASSES OR BIRD OR BIRDSOR AVIAN)/TI |
| 74. | 72,372 | (RABBIT OR RABBITS OR MOSS OR MOSSES OR FUNGUS OR FUNGI)/TI |
| 75. | 44,920 | (COW OR CATTLE OR LIVESTOCK OR SWINE OR POULTRY)/TI |
| 76. | 1,116,322 | S71:S75 |
| 77. | 2474 | S70 NOT S76 |
| 78. | 777 | S77/2008:2010 |
Key
-
? = truncation
-
/TI,AB,DE = terms in title, abstract, or descriptor fields
-
(W) = terms adjacent to each other (same order)
-
(2W) = terms within three words of each other (same order)
-
PY = publication year
-
: = range e.g. PY = 2008:2011 means year = 2008 OR 2009 OR 2010 OR 2011
-
S77/2008:2010 – limits set 77 to records published between 2008 and 2010 (inclusive).
Guideline searches
A range of resources were searched or browsed for guidelines on the treatment of depression.
Clinical Evidence
http://clinicalevidence.bmj.com/ceweb/index.jsp
Searched on 20 September 2010.
Four relevant reviews found.
National Institute for Health and Clinical Excellence (NICE)
http://guidance.nice.org.uk/Topic/MentalHealthBehavioural
Searched on 4 October 2010.
Five relevant guidelines found.
NHS Evidence – Guidelines Finder
www.library.nhs.uk/guidelinesFinder/
Searched on 5 October 2010.
Seven relevant guidelines found.
National Guidelines Clearing House
Searched on 5 October 2010.
Fourteen relevant guidelines found.
New Zealand Guidelines Group
Searched on 5 October 2010.
Two relevant guidelines found.
Australian National Health and Medical Research Council: clinical practice guidelines
www.nhmrc.gov.au/publications/subjects/clinical.htm
Searched on 5 October 2010.
No relevant guidelines found.
Canadian Medical Association – Infobase: clinical practice guidelines
www.cma.ca/index.php/ci_id/54316/la_id/1.htm
Searched on 5 October 2010.
Six relevant guidelines found.
Health Canada: guidelines
www.hc-sc.gc.ca/ahc-asc/legislation/guide-ld/index-eng.php
Searched on 5 October 2010.
No relevant guidelines found.
Public Health Agency of Canada: guidelines
www.phac-aspc.gc.ca/dpg-eng.php
Searched on 5 October 2010.
One relevant guideline found.
Cost-effectiveness
The Cochrane Library
http://onlinelibrary.wiley.com/
NHS Economic Evaluation Database (NHS EED), Issue 4, 2010.
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 4, 2010.
Searched 22 October 2010.
62 records were retrieved – three from NHS EED and 43 from CENTRAL.
#1 “Beating the Blues”:ti,ab (4)
#2 “Depression Relief”:ti,ab (2)
#3 “Overcoming Depression”:ti,ab (6)
#4 (“BluePages” or “Blue Pages”):ti,ab (3)
#5 (“MoodGYM” or “Mood GYM”):ti,ab (10)
#6 “Keeping the Blues Away”:ti,ab (1)
#7 “Sadness Program”:ti,ab (0)
#8 “Stressbusters”:ti,ab (0)
#9 “Think feel do”:ti,ab (0)
#10 “Wellbeing Program”:ti,ab (0)
#11 “Living Life to the Full”:ti,ab (0)
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) (24)
#13 MeSH descriptor Depressive Disorder explode all trees (6154)
#14 MeSH descriptor Depression, this term only (4010)
#15 (depression or depressive or depressed):ti,ab (25,249)
#16 (melancholi* or dysphori* or dysthymi*):ti,ab (1136)
#17 (#13 OR #14 OR #15 OR #16) (27,205)
#18 MeSH descriptor Recurrence, this term only (10,301)
#19 (recur* or reoccur* or (re NEXT occur*) or relaps*):ti,ab (27,197)
#20 MeSH descriptor Secondary Prevention, this term only (47)
#21 (secondary NEAR/3 prevent*):ti,ab (1404)
#22 (prophylaxis or prophylactic*):ti,ab (16,468)
#23 MeSH descriptor Remission Induction, this term only (2357)
#24 (remission or remitted):ti,ab (8105)
#25 (maintain* NEAR/3 (health or wellbeing or (well NEXT being))):ti,ab (135)
#26 ((another or further or second or repeat* or previous or initial or subsequent) NEAR/4 (episode* or bout* or instance* or symptom* or occurrence*) NEAR/4 depress*):ti,ab (141)
#27 (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) (52,739)
#28 (#17 AND #27) (2884)
#29 MeSH descriptor Cognitive Therapy, this term only (3258)
#30 MeSH descriptor Behavior Therapy explode all trees (7539)
#31 (cognitive NEAR/3 (therap* or treatment* or intervention* or program* or package* or training or group*)):ti,ab (5313)
#32 ((behavior* or behaviour*) NEAR/3 (therap* or treatment* or intervention* or program* or package* or training or activat* or modif* or group*)):ti,ab (8142)
#33 CBT:ti,ab (1196)
#34 (cognitive NEXT restructuring):ti,ab (213)
#35 (cCBT or iCBT):ti,ab (30)
#36 MeSH descriptor Telemedicine, this term only (616)
#37 MeSH descriptor Therapy, Computer-Assisted, this term only (433)
#38 MeSH descriptor Computer-Assisted Instruction, this term only (621)
#39 (telepsychology or teletherapy or telemedicine or telehealth):ti,ab (476)
#40 (“Interactive Voice Response” or IVR):ti,ab (110)
#41 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40) (15,916)
#42 (#28 AND #41) (427)
#43 MeSH descriptor Counseling, this term only (2146)
#44 MeSH descriptor Directive Counseling, this term only (133)
#45 counsel*:ti,ab (4780)
#46 (motivation* NEAR/2 (interview* or enhance* or intervention* or therap*)):ti,ab (747)
#47 (cybercounsel* or (cyber NEXT counsel*)):ti,ab (0)
#48 (#43 OR #44 OR #45 OR #46 OR #47) (6054)
#49 (#28 AND #48) (53)
#50 mindfulness:ti,ab (191)
#51 (#28 AND #50) (28)
#52 MeSH descriptor Self Care explode all trees (2817)
#53 MeSH descriptor Self-Help Groups, this term only (465)
#54 (selfcare or (self NEXT care)):ti,ab (741)
#55 (selfmanage* or (self NEXT manage*) or selfmonitor* or (self NEXT monitor*)):ti,ab (1788)
#56 (selfhelp or (self NEXT help)):ti,ab (780)
#57 (selftreat* or (self NEXT treat*)):ti,ab (98)
#58 (selfadminister* or (self NEXT administer*)):ti,ab (1652)
#59 MeSH descriptor Bibliotherapy, this term only (67)
#60 MeSH descriptor Manuals as Topic, this term only (112)
#61 MeSH descriptor Books, this term only (27)
#62 bibliotherap*:ti,ab (104)
#63 ((patient* or client* or user*) NEAR/3 (manual* or handbook* or workbook* or guide*)):ti,ab (793)
#64 (#52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63) (7557)
#65 (#64 AND #28) (73)
#66 MeSH descriptor Exercise explode all trees (7550)
#67 MeSH descriptor Exercise Therapy explode all trees (4256)
#68 MeSH descriptor Exercise Movement Techniques explode all trees (746)
#69 MeSH descriptor Sports explode all trees (6317)
#70 (exercise* or workout* or (work NEXT out*) or (physical* NEXT activ*)):ti,ab (27,147)
#71 ((resistance or strength* or weight) NEAR training):ti,ab (2601)
#72 (walk* NEAR/3 (fitness or aerobic or program* or intervention* or session* or regime*)):ti,ab (460)
#73 (bicycl* or cycle* or cycling):ti,ab (15,694)
#74 (run* or jog* or treadmill*):ti,ab (9350)
#75 ((tai NEXT ji) or taiji or taijiquan or (tai NEXT chi) or (t NEXT ai NEXT chi) or taichi or (shadow NEXT boxing)):ti,ab (202)
#76 (yoga or yogic or pilates or danc*):ti,ab (458)
#77 (#66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76) (48,971)
#78 (#77 AND #28) (179)
#79 MeSH descriptor Patient Education as Topic, this term only (5087)
#80 (psychoeducation* or (psycho NEXT education*)):ti,ab (776)
#81 (#79 OR #80) (5656)
#82 (#81 AND #28) (79)
#83 MeSH descriptor Allied Health Personnel, this term only (134)
#84 MeSH descriptor Case Management, this term only (612)
#85 (psychological NEXT wellbeing NEXT practitioner*):ti,ab (0)
#86 (psychological NEXT (well NEXT being) NEXT practitioner*):ti,ab (0)
#87 (personal NEXT wellbeing NEXT practitioner*):ti,ab (0)
#88 (personal NEXT (well NEXT being) NEXT practitioner*):ti,ab (0)
#89 ((para NEXT professional*) or paraprofessional*):ti,ab (93)
#90 (peer NEXT support*):ti,ab (128)
#91 ((patient* or client*) NEAR/2 (support NEXT group*)):ti,ab (10)
#92 (mental NEXT health NEXT peer*):ti,ab (2)
#93 (graduate NEXT mental NEXT health NEXT worker*):ti,ab (1)
#94 (low NEXT intensity NEXT worker*):ti,ab (0)
#95 (health NEXT care NEXT assistant*):ti,ab (5)
#96 (case NEXT (worker* or management)):ti,ab (781)
#97 (stepped NEXT care):ti,ab (178)
#98 (collaborative NEXT (care or management)):ti,ab (160)
#99 (#83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98) (1720)
#100 (#99 AND #28) (31)
#101 ((low NEXT intensity) NEAR/5 (psychological or psychosocial)):ti,ab (2)
#102 “Increasing Access to Psychological Therapy”:ti,ab (0)
#103 “Increasing Access to Psychological Therapies”:ti,ab (0)
#104 “Improving Access to Psychological Therapy”:ti,ab (0)
#105 “Improving Access to Psychological Therapies”:ti,ab (0)
#106 IAPT:ti,ab (0)
#107 (#101 OR #102 OR #103 OR #104 OR #105 OR #106) (2)
#108 (#12 OR #42 OR #49 OR #51 OR #65 OR #78 OR #82 OR #100 OR #107) (723)
#109 MeSH descriptor Economics, this term only (76)
#110 MeSH descriptor Costs and Cost Analysis explode all trees (30,817)
#111 MeSH descriptor Economics, Dental, this term only (7)
#112 MeSH descriptor Economics, Hospital explode all trees (3247)
#113 MeSH descriptor Economics, Medical, this term only (143)
#114 MeSH descriptor Economics, Nursing, this term only (29)
#115 MeSH descriptor Economics, Pharmaceutical, this term only (690)
#116 (econom* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*) (58,512)
#117 (expenditure* not energy) (2134)
#118 (value NEAR/1 money) (4)
#119 budget* (835)
#120 (#109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119) (58,779)
#121 ((energy or oxygen) NEAR/1 cost) (242)
#122 (metabolic NEAR/1 cost) (51)
#123 ((energy or oxygen) NEAR/1 expenditure) (1485)
#124 (#121 OR #112 OR #123) (4906)
#125 (#120 AND NOT #124) (55,202)
#126 (#108 AND #125) (62)
Key
-
MeSH descriptor = indexing term (MeSH heading)
-
* = truncation
-
“ “ = phrase search
-
:ti,ab = terms in either title or abstract fields
-
NEAR/2 = terms within two words of each other (any order)
-
NEXT = terms are next to each other.
MEDLINE
OvidSP http://ovidSP.ovid.com
1950 to week 2 October 2010.
Searched on 22 October 2010.
158 records were retrieved.
-
Beating the Blues.ti,ab. (11)
-
Depression Relief.ti,ab. (5)
-
Overcoming Depression.ti,ab. (9)
-
(BluePages or Blue Pages).ti,ab. (5)
-
(MoodGYM or Mood GYM).ti,ab. (16)
-
Keeping the Blues Away.ti,ab. (1)
-
Sadness Program.ti,ab. (0)
-
Stressbusters.ti,ab. (2)
-
Think feel do.ti,ab. (0)
-
Wellbeing Program.ti,ab. (3)
-
Living Life to the Full.ti,ab. (3)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (50)
-
exp Depressive Disorder/ (67,684)
-
Depression/ (58,231)
-
(depression or depressive or depressed).ti,ab. (228,568)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (6964)
-
13 or 14 or 15 or 16 (264,178)
-
Recurrence/ (137,770)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (381,860)
-
Secondary Prevention/ (556)
-
(secondary adj3 prevent$).ti,ab. (12,217)
-
(prophylaxis or prophylactic$).ti,ab. (97,325)
-
Remission Induction/ (26,272)
-
(remission or remitted).ti,ab. (68,089)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (2761)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (1009)
-
18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (592,441)
-
17 and 27 (16,518)
-
Cognitive Therapy/ (11,080)
-
exp Behavior Therapy/ (42,681)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (11,630)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (32,564)
-
CBT.ti,ab. (2786)
-
cognitive restructuring.ti,ab. (411)
-
(cCBT or iCBT).ti,ab. (97)
-
Telemedicine/ (7759)
-
Therapy, Computer-Assisted/ (3969)
-
Computer-Assisted Instruction/ (7640)
-
(telepsychology or teletherapy or telemedicine or telehealth).ti,ab. (6110)
-
(Interactive Voice Response or IVR).ti,ab. (531)
-
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 (90,094)
-
41 and 28 (1033)
-
counseling/or directive counseling/ (24,542)
-
counsel$.ti,ab. (52,258)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (1813)
-
(cybercounsel$ or cyber counsel$).ti,ab. (2)
-
43 or 44 or 45 or 46 (65,484)
-
47 and 28 (199)
-
mindfulness.ti,ab. (621)
-
49 and 28 (44)
-
exp Self Care/ (32,543)
-
Self-Help Groups/ (6922)
-
(selfcare or self care).ti,ab. (7406)
-
(selfmanage$ or self manage$).ti,ab. (4946)
-
(selfmonitor$ or self monitor$).ti,ab. (3226)
-
(selfhelp or self help).ti,ab. (3684)
-
(selftreat$ or self treat$).ti,ab. (925)
-
(selfadminister$ or self administer$).ti,ab. (17,074)
-
Bibliotherapy/ (283)
-
Manuals as Topic/ (3229)
-
Books/ (1970)
-
bibliotherap$.ti,ab. (213)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (8973)
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 (77,546)
-
64 and 28 (291)
-
exp Exercise/ (53,519)
-
exp Exercise Therapy/ (22,146)
-
exp Exercise Movement Techniques/ (3963)
-
exp Sports/ (88,172)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (183,654)
-
((resistance or strength$ or weight) adj training).ti,ab. (4351)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (1150)
-
(bicycl$ or cycle$ or cycling).ti,ab. (330,414)
-
(run$ or jog$ or treadmill$).ti,ab. (108,076)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (509)
-
(yoga or yogic or pilates or danc$).ti,ab. (3884)
-
66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 (657,412)
-
77 and 28 (1052)
-
Patient Education as Topic/ (60,271)
-
(psychoeducation$ or psycho education$).ti,ab. (2208)
-
79 or 80 (61,864)
-
81 and 28 (260)
-
Allied Health Personnel/ (9346)
-
Case Management/ (7023)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (704)
-
peer support$.ti,ab. (923)
-
((patient$ or client$) adj2 support group$).ti,ab. (263)
-
mental health peer$.ti,ab. (5)
-
graduate mental health worker$.ti,ab. (10)
-
low intensity worker$.ti,ab. (0)
-
health care assistant$.ti,ab. (134)
-
(case adj (worker$ or management)).ti,ab. (5885)
-
stepped care.ti,ab. (520)
-
(collaborative adj (care or management)).ti,ab. (624)
-
83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 (22,658)
-
96 and 28 (72)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (12)
-
Increasing Access to Psychological Therap$.ti,ab. (1)
-
Improving Access to Psychological Therap$.ti,ab. (6)
-
IAPT.ti,ab. (7)
-
98 or 99 or 100 or 101 (24)
-
12 or 42 or 48 or 50 or 65 or 78 or 82 or 97 or 102 (2617)
-
Animals/ (4,722,671)
-
Humans/ (11,541,120)
-
104 not (104 and 105) (3,501,448)
-
103 not 106 (2559)
-
letter.pt. (696,237)
-
editorial.pt. (265,801)
-
comment.pt. (425,820)
-
108 or 109 or 110 (1,033,359)
-
107 not 111 (2529)
-
economics/ (25,987)
-
exp “costs and cost analysis”/ (153,908)
-
economics, dental/ (1835)
-
exp “economics, hospital”/ (16,898)
-
economics, medical/ (8323)
-
economics, nursing/ (3826)
-
economics, pharmaceutical/ (2155)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (334,038)
-
(expenditure$ not energy).ti,ab. (13,844)
-
(value adj1 money).ti,ab. (18)
-
budget$.ti,ab. (14,021)
-
or/113–123 (443,197)
-
((energy or oxygen) adj cost).ti,ab. (2244)
-
(metabolic adj cost).ti,ab. (583)
-
((energy or oxygen) adj expenditure).ti,ab. (12,761)
-
or/125–127 (14,993)
-
124 not 128 (439,696)
-
letter.pt. (696,237)
-
editorial.pt. (265,801)
-
historical-article.pt. (270,029)
-
or/130–132 (1,219,838)
-
129 not 133 (415,600)
-
animals/ (4,722,671)
-
human/ (11,541,120)
-
135 not (135 and 136) (3,501,448)
-
134 not 137 (391,795)
-
112 and 138 (158)
Key
-
/ = indexing term (MeSH heading)
-
exp = exploded MeSH heading
-
$ =truncation
-
? = embedded truncation
-
pt = publication type
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order).
EMBASE
OvidSP http://ovidSP.ovid.com/
1980 to week 41 2010.
Searched on 22 October 2010.
423 records were retrieved.
-
Beating the Blues.ti,ab. (16)
-
Depression Relief.ti,ab. (8)
-
Overcoming Depression.ti,ab. (14)
-
(BluePages or Blue Pages).ti,ab. (7)
-
(MoodGYM or Mood GYM).ti,ab. (19)
-
Keeping the Blues Away.ti,ab. (1)
-
Sadness Program.ti,ab. (0)
-
Stressbusters.ti,ab. (2)
-
Think feel do.ti,ab. (1)
-
10 Wellbeing Program.ti,ab. (4)
-
Living Life to the Full.ti,ab. (3)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (67)
-
exp depression/ (230,624)
-
(depression or depressive or depressed).ti,ab. (265,744)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (8685)
-
13 or 14 or 15 (356,576)
-
recurrent disease/ (104,082)
-
relapse/ (33,031)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (452,614)
-
Secondary Prevention/ (9386)
-
(secondary adj3 prevent$).ti,ab. (16,204)
-
prophylaxis/ (44,266)
-
(prophylaxis or prophylactic$).ti,ab. (118,378)
-
remission/ (48,652)
-
(remission or remitted).ti,ab. (79,463)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (3185)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (1187)
-
17 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 (705,789)
-
16 and 28 (24,581)
-
Cognitive Therapy/ (22,766)
-
behavior therapy/ (32,526)
-
behavior modification/ (5677)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (17,204)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (41,007)
-
CBT.ti,ab. (4196)
-
cognitive restructuring.ti,ab. (679)
-
(cCBT or iCBT).ti,ab. (130)
-
exp telehealth/ (10,779)
-
computer assisted therapy/ (2607)
-
(telepsychology or teletherapy or telemedicine or telehealth).ti,ab. (6927)
-
interactive voice response system/ (131)
-
(Interactive Voice Response or IVR).ti,ab. (682)
-
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 (96,338)
-
43 and 29 (1917)
-
exp counseling/ (77,508)
-
counsel$.ti,ab. (61,636)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (2440)
-
(cybercounsel$ or cyber counsel$).ti,ab. (2)
-
45 or 46 or 47 or 48 (106,226)
-
49 and 29 (466)
-
mindfulness.ti,ab. (937)
-
51 and 29 (75)
-
exp Self Care/ (35,497)
-
(selfcare or self care).ti,ab. (8715)
-
(selfmanage$ or self manage$).ti,ab. (6402)
-
(selfmonitor$ or self monitor$).ti,ab. (4188)
-
(selfhelp or self help).ti,ab. (4669)
-
(selftreat$ or self treat$).ti,ab. (1180)
-
(selfadminister$ or self administer$).ti,ab. (19,130)
-
book/ (12,945)
-
bibliotherap$.ti,ab. (300)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (11,266)
-
53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 (89,419)
-
63 and 29 (405)
-
exp Exercise/ (146,682)
-
exp sport/ (70,614)
-
exp physical activity/ (153,076)
-
exp kinesiotherapy/ (34,433)
-
music therapy/ (3057)
-
treadmill/or treadmill exercise/ (12,465)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (216,208)
-
((resistance or strength$ or weight) adj training).ti,ab. (5173)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (1400)
-
(bicycl$ or cycle$ or cycling).ti,ab. (368,044)
-
(run$ or jog$ or treadmill$).ti,ab. (127,399)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (689)
-
(yoga or yogic or pilates or danc$).ti,ab. (5228)
-
65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 (855,163)
-
78 and 29 (1796)
-
psychoeducation/ (1832)
-
patient education/ (70,747)
-
(psychoeducation$ or psycho education$).ti,ab. (3207)
-
80 or 81 or 82 (74,268)
-
83 and 29 (628)
-
paramedical personnel/ (9745)
-
case management/or case manager/ (5489)
-
support group/ (4954)
-
peer group/ (8195)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (677)
-
peer support$.ti,ab. (1165)
-
((patient$ or client$) adj2 support group$).ti,ab. (348)
-
mental health peer$.ti,ab. (7)
-
graduate mental health worker$.ti,ab. (15)
-
low intensity worker$.ti,ab. (1)
-
health care assistant$.ti,ab. (151)
-
(case adj (worker$ or management)).ti,ab. (6668)
-
stepped care.ti,ab. (632)
-
(collaborative adj (care or management)).ti,ab. (779)
-
85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 (35,819)
-
100 and 29 (156)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (12)
-
Increasing Access to Psychological Therap$.ti,ab. (3)
-
Improving Access to Psychological Therap$.ti,ab. (21)
-
IAPT.ti,ab. (21)
-
102 or 103 or 104 or 105 (46)
-
12 or 44 or 50 or 52 or 64 or 79 or 84 or 101 or 106 (4592)
-
editorial.pt. (356,865)
-
letter.pt. (704,364)
-
108 or 109 (1,061,229)
-
107 not 110 (4485)
-
exp animal/ (1,632,799)
-
exp nonhuman/ (3,514,218)
-
exp animal experiment/ (1,396,171)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (3,950,443)
-
112 or 113 or 114 or 115 (5,675,551)
-
exp human/ (12,039,117)
-
exp human experiment/ (282,706)
-
117 or 118 (12,040,498)
-
116 not (116 and 119) (4,494,950)
-
111 not 120 (4418)
-
health-economics/ (29,603)
-
exp economic-evaluation/ (160,071)
-
exp health-care-cost/ (153,513)
-
exp pharmacoeconomics/ (132,321)
-
122 or 123 or 124 or 125 (369,725)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (407,190)
-
(expenditure$ not energy).ti,ab. (16,385)
-
(value adj2 money).ti,ab. (854)
-
budget$.ti,ab. (17,453)
-
127 or 128 or 129 or 130 (424,985)
-
126 or 131 (645,743)
-
letter.pt. (704,364)
-
editorial.pt. (356,865)
-
note.pt. (424,000)
-
133 or 134 or 135 (1,485,229)
-
132 not 136 (578,462)
-
(metabolic adj cost).ti,ab. (622)
-
((energy or oxygen) adj cost).ti,ab. (2457)
-
((energy or oxygen) adj expenditure).ti,ab. (14,419)
-
138 or 139 or 140 (16,852)
-
137 not 141 (574,620)
-
exp animal/ (1,632,799)
-
exp animal-experiment/ (1,396,171)
-
nonhuman/ (3,514,218)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (3,950,443)
-
143 or 144 or 145 or 146 (5,675,551)
-
exp human/ (12,039,117)
-
exp human-experiment/ (282,706)
-
148 or 149 (12,040,498)
-
147 not (147 and 150) (4,494,950)
-
142 not 151 (534,924)
-
121 and 152 (423)
Key
-
/ = indexing term (EMTREE heading)
-
* = focused EMTREE heading
-
exp = exploded EMTREE heading
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order)
-
sh = subject heading field.
EconLit
OvidSP http://ovidSP.ovid.com/
1969 to September 2010.
Searched on 22 October 2010.
12 records were retrieved.
-
Beating the Blues.ti,ab. (0)
-
Depression Relief.ti,ab. (1)
-
Overcoming Depression.ti,ab. (0)
-
(BluePages or Blue Pages).ti,ab. (0)
-
(MoodGYM or Mood GYM).ti,ab. (0)
-
Keeping the Blues Away.ti,ab. (0)
-
Sadness Program.ti,ab. (0)
-
Stressbusters.ti,ab. (0)
-
Think feel do.ti,ab. (0)
-
10 Wellbeing Program.ti,ab. (0)
-
Living Life to the Full.ti,ab. (0)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (1)
-
(depression or depressive or depressed).ti,ab. (2745)
-
(melancholi$ or dysphori$ or dysthymi$).ti,ab. (4)
-
13 or 14 (2746)
-
(recur$ or reoccur$ or re occur$ or relaps$).ti,ab. (3173)
-
(secondary adj3 prevent$).ti,ab. (15)
-
(prophylaxis or prophylactic$).ti,ab. (51)
-
(remission or remitted).ti,ab. (40)
-
(maintain$ adj3 (health or wellbeing or well being)).ti,ab. (46)
-
((another or further or second or repeat$ or previous or initial or subsequent) adj4 (episode$ or bout$ or instance$ or symptom$ or occurrence$) adj4 depress$).ti,ab. (1)
-
16 or 17 or 18 or 19 or 20 or 21 (3321)
-
15 and 22 (27)
-
(cognitive adj3 (therap$ or treatment$ or intervention$ or program$ or package$ or training or group$)).ti,ab. (21)
-
(behavio?r$ adj3 (therap$ or treatment$ or intervention$program$ or package$ or training or activat$ or modif$ or group$)).ti,ab. (525)
-
CBT.ti,ab. (18)
-
cognitive restructuring.ti,ab. (0)
-
(cCBT or iCBT).ti,ab. (1)
-
(telepsychology or teletherapy or telemedicine or telehealth).ti,ab. (16)
-
(Interactive Voice Response or IVR).ti,ab. (5)
-
24 or 25 or 26 or 27 or 28 or 29 or 30 (579)
-
31 and 23 (1)
-
counsel$.ti,ab. (483)
-
(motivation$ adj2 (interview$ or enhance$ or intervention$ or therap$)).ti,ab. (18)
-
(cybercounsel$ or cyber counsel$).ti,ab. (0)
-
33 or 34 or 35 (501)
-
36 and 23 (0)
-
mindfulness.ti,ab. (6)
-
38 and 23 (0)
-
(selfcare or self care).ti,ab. (21)
-
(selfmanage$ or self manage$).ti,ab. (332)
-
(selfmonitor$ or self monitor$).ti,ab. (24)
-
(selfhelp or self help).ti,ab. (241)
-
(selftreat$ or self treat$).ti,ab. (4)
-
(selfadminister$ or self administer$).ti,ab. (62)
-
bibliotherap$.ti,ab. (0)
-
((patient$ or client$ or user$) adj3 (manual$ or handbook$ or workbook$ or guide$)).ti,ab. (151)
-
40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 (832)
-
48 and 23 (0)
-
(exercise$ or workout$ or work out$ or physical$activ$).ti,ab. (6161)
-
((resistance or strength$ or weight) adj training).ti,ab. (2)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (8)
-
(bicycl$ or cycle$ or cycling).ti,ab. (18,680)
-
(run$ or jog$ or treadmill$).ti,ab. (26,985)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (4)
-
(yoga or yogic or pilates or danc$).ti,ab. (156)
-
50 or 51 or 52 or 53 or 54 or 55 or 56 (50,234)
-
57 and 23 (4)
-
(psychoeducation$ or psycho education$).ti,ab. (3)
-
59 and 23 (1)
-
((psychological or personal) adj (wellbeing practitioner$ or well being practitioner$)).ti,ab. (0)
-
(para professional$ or paraprofessional$).ti,ab. (10)
-
peer support$.ti,ab. (12)
-
((patient$ or client$) adj2 support group$).ti,ab. (0)
-
mental health peer$.ti,ab. (0)
-
graduate mental health worker$.ti,ab. (0)
-
low intensity worker$.ti,ab. (0)
-
health care assistant$.ti,ab. (0)
-
(case adj (worker$ or management)).ti,ab. (80)
-
stepped care.ti,ab. (0)
-
(collaborative adj (care or management)).ti,ab. (17)
-
61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 (119)
-
72 and 23 (1)
-
(low intensity adj5 (psychological or psychosocial)).ti,ab. (0)
-
Increasing Access to Psychological Therap$.ti,ab. (0)
-
Improving Access to Psychological Therap$.ti,ab. (1)
-
IAPT.ti,ab. (5)
-
74 or 75 or 76 or 77 (5)
-
12 or 32 or 37 or 39 or 49 or 58 or 60 or 73 or 78 (12).
Key
-
$ = truncation
-
? = embedded truncation
-
.ti,ab. = terms in either title or abstract fields
-
adj = terms adjacent to each other (same order)
-
adj2 = terms within two words of each other (any order).
IDEAS database
http://ideas.repec.org/search.html
Searched on 3 November 2010.
114 records retrieved.
((recur|recurs|recurrence|recurred|relapse|relapses|relapsed|reoccur|reoccurs|reoccurrence|reoccurred|repeat|subsequent)+(depression|depressive|depressed|melancholia|melancholic|melancholy|dysphoria|dysphoric|dysthymia|dysthymic))
Match: Change to Boolean
Word forms:Change to Exact
Use Synonyms: Change to No
Key
-
| = OR
-
+ = AND.
Appendix 2 Table of excluded studies with rationale
Key
| Reason for exclusion | Inclusion criteria | No. of studies excluded |
|---|---|---|
| 1 | Children (aged < 12 years) | 0 |
| 2 | Not previously treated for depression | 27 |
| 3 | Not currently asymptomatic or responding to treatment | 18 |
| 4 | Not unipolar depression | 2 |
| 5 | Study design | 20 |
| 6 | Not part A or part B intervention | 29 |
| 7 | Insufficient information available or paper unobtainable | 6 |
Excluded studies
| Study | Reason |
|---|---|
| 1. Allen M, Bromley A, Kuyken W, Sonnenberg SJ. Participants’ experiences of mindfulness-based cognitive therapy: ‘It changed me in just about every way possible’. Behav Cogn Psychother 2009;37:413–30 | 5 |
| 2. Andersson G, Bergstrom J, Hollandare F, Ekselius L, Carlbring P. Delivering cognitive behavioral therapy for mild to moderate depression via the Internet: predicting outcome at 6-month follow-up. Verhaltenstherapie 2004;14:185–9 | 2 |
| 3. Aubert RE, Fulop G, Xia F, Thiel M, Maldonato D, Woo C. Evaluation of a depression health management program to improve outcomes in first or recurrent episode depression. Am J Manag Care 2003;9:374–80 | 3 |
| 4. Baker AL, Wilson PH. Cognitive-behavior therapy for depression: the effects of booster sessions on relapse. Behav Ther 1985;16:335–44 | 3 |
| 5. Barrera AZ, Torres LD, Munoz RF. Prevention of depression: the state of the science at the beginning of the 21st Century. Int Rev Psychiatry 2007;19:655–70 | 2 |
| 6. Bennett K, Reynolds J, Christensen H, Griffiths KM. e-hub: an online self-help mental health service in the community. Med J Aust 2010;192(Suppl.):48–52 | 2 |
| 7. Berlin S. Maintaining reduced levels of self-criticism through relapse-prevention treatment. Soc Work Res Abstr 1985;21:21–33 | 2 |
| 8. Bertschy GB, Jermann F, Bizzini L, Weber-Rouget B, Myers-Arrazola M, van der Linden M. Mindfulness based cognitive therapy: a randomized controlled study on its efficiency to reduce depressive relapse/recurrence. J Affect Disord 2008;107(Suppl. 1):59–60 | 6 |
| 9. Bondolfi G, Jermann F, der Linden MV, Gex-Fabry M, Bizzini L, Weber RB, et al. Depression relapse prophylaxis with mindfulness-based cognitive therapy: a replication randomized controlled study. World Psychiatry 2009;8(Suppl. 1):198 | 7 |
| 10. Britton WB, Haynes PL, Fridel KW, Bootzin RR. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med 2010;72:539–48 | 6 |
| 11. Brown RA, Lewinsohn PM. A psychoeducational approach to the treatment of depression: comparison of group, individual, and minimal contact procedures. J Consult Clin Psychol 1984;52:774–83 | 2 |
| 12. Carreira K, Miller MD, Frank E, Houck PR, Morse JQ, Dew MA, et al. A controlled evaluation of monthly maintenance interpersonal psychotherapy in late-life depression with varying levels of cognitive function. Int J Geriatr Psychiatry 2008;23:1110–13 | 6 |
| 13. Carvalho M, Estevens D, Guete-Tur O. Efficacy of cognitive-behavioral therapy for the treatment of recurrent depression in adults. Eur Psychiatry 2010;25(Suppl. 1):1042 | 6 |
| 14. Checkley S. The efficacy of cognitive therapy when added to drug therapy for recurrent and pharmacotherapy-resistant depression – a pilot study. National Research Register Archive, National Institute for Health Research; 1999. URL: www.nihr.ac.uk/Profiles/NRR.aspx?Publication_ID=N0042002867 (cited 3 November 2010) | 7 |
| 15. Clark DM, Layard R, Smithies R, Richards DA, Suckling R, Wright B. Improving access to psychological therapy: initial evaluation of two UK demonstration sites. Behav Res Ther 2009;47:910–20 | 2 |
| 16. Clarke G, Eubanks D, Reid E, Kelleher C, O’Connor E, DeBar LL, et al. Overcoming Depression on the Internet (ODIN) (2): a randomized trial of a self-help depression skills program with reminders. J Med Internet Res 2005;7:e16 | 3 |
| 17. Clarke G, Reid E, Eubanks D, O’Connor E, DeBar LL, Kelleher C, et al. Overcoming Depression on the Internet (ODIN): a randomized controlled trial of an Internet depression skills intervention program. J Med Internet Res 2002;4:e14 | 3 |
| 18. Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive-behavioral treatment of adolescent depression: efficacy of acute group treatment and booster sessions. J Am Acad Child Adolesc Psychiatry 1999;38:272–9 | 3 |
| 19. Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psychol 2007;75:1000–5 | 5 |
| 20. College voor zorgverzekeringen. Cognitive self therapy in patients with chronic-repeating depressive or panic disorders. Diemen: College voor zorgverzekeringen; 2005 | 7 |
| 21. Conradi HJ, de Jonge P, Ormel J. Cognitive-behavioural therapy v. usual care in recurrent depression. Br J Psychiatry 2008;193:505–6 | 3 |
| 22. Cuijpers P, van Lammeren P. Secondary prevention of depressive symptoms in elderly inhabitants of residential homes. Int J Geriatr Psychiatry 2001;16:702–8 | 2 |
| 23. D’Ambrosio A, Quartucci R, Morrone G, Vacca L. [Cognitive therapy as prophylaxis of depressive relapse]. Neurol Psichiatr Sci Um 1990;10:643–9 | 5 |
| 24. Dimidjian S, Davis KJ. Newer variations of cognitive-behavioral therapy: behavioral activation and mindfulness-based cognitive therapy. Curr Psychiatry Rep 2009;11:453–8 | 5 |
| 25. Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol 2008;76:468–77 | 5 |
| 26. Dombrovski AY, Lenze EJ, Dew MA, Mulsant BH, Pollock BG, Houck PR, et al. Maintenance treatment for old-age depression preserves health-related quality of life: a randomized, controlled trial of paroxetine and interpersonal psychotherapy. J Am Geriatr Soc 2007;55:1325–32 | 6 |
| 27. Fava GA, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am J Psychiatry 1994;151:1295–9 | 6 |
| 28. Fava GA, Grandi S, Zielezny M, Rafanelli C, Canestrari R. Four-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry 1996;153:945–7 | 6 |
| 29. Fava GA, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry 1998;155:1443–5 | 6 |
| 30. Fava GA, Ruini C, Fabbri S. Well-being therapy and modified cognitive approaches for relapse prevention in depression. J Affect Disord 2002;68:91 | 5 |
| 31. Fava M, Kaji J. Continuation and maintenance treatments of major depressive disorder. Psychiatr Ann 1994;24:281–90 | 2 |
| 32. Foley D, Baille A, Renner P. CBT plus mindfulness for depression and anxiety outside model programs: increased treatment gains? Decreased relapse rates? 29th Australian Association for Cognitive and Behaviour Therapy Annual Conference, Manly, Sydney, 18–23 October 2006. p. 43 | 7 |
| 33. Frank E, Kupfer DJ. Maintenance treatment of recurrent unipolar depression: pharmacology and psychotherapy. Adv Biochem Psychopharmacol 1985;40:139–51 | 4 |
| 34. Friedberg MW, Mindfulness-based cognitive therapy: a potential new alternative to medication for recurrent depression. J Clin Outcomes Manag 2009;16:63–64 | 5 |
| 35. Gellatly J, Bower P, Hennessy S, Richards D, Gilbody S, Lovell K. What makes self-help interventions effective in the management of depressive symptoms? Meta-analysis and meta-regression. Psychol Med 2007;37:1217–28 | 2 |
| 36. Gervasoni N, Legendre-Simon P, Aubry J-M, Gex-Fabry M, Bertschy G, Bondolfi G. Early telephone intervention for psychiatric outpatients starting antidepressant treatment. Nord J Psychiatry 2010;64:265–7 | 2 |
| 37. Glasman D, Finlay WML, Brock D. Becoming a self-therapist: using cognitive-behavioural therapy for recurrent depression and/or dysthymia after completing therapy. Psychol Psychother 2004;77:335–51 | 5 |
| 38. Golkaramnay V, Bauer S, Haug S, Wolf M, Kordy H. The exploration of the effectiveness of group therapy through an Internet chat as aftercare: a controlled naturalistic study. Psychother Psychosom 2007;76:219–25 | 7 |
| 39. Gonzalez Gonzalez S, Fernandez Rodriguez C, Perez Rodriguez J, Amigo I. [Depression secondary prevention in primary care.] Psicothema 2006;18:471–7 | 3 |
| 40. Gonzalez Gonzalez S, Fernandez Rodriguez C, Perez Rodriguez J, Amigo I. Secondary prevention of depression in primary care. Psychol Spain 2007;11:24–32 | 2 |
| 41. Gould RA, Clum GA. A meta-analysis of self-help treatment approaches. Clin Psychol Rev 1993;13:169–86 | 2 |
| 42. Griffiths KM, Christensen H. Internet-based mental health programs: a powerful tool in the rural medical kit. Aust J Rural Health 2007;15:81–7 | 2 |
| 43. Hautzinger M. Relapse prevention in recurrent depression. J Affect Disord 2010;122(Suppl. 1):35 | 6 |
| 44. Hick SF, Chan L. Mindfulness-based cognitive therapy for depression: effectiveness and limitations. Soc Work Ment Health 2010;8:225–37 | 2 |
| 45. Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry 2005;62:417–22 | 6 |
| 46. Hotopf M. Cognitive behaviour therapy reduced relapses in recurrent major depressive disorder. Evid Based Med 2005;10:82 | 5 |
| 47. Jamison C, Scogin F. The outcome of cognitive bibliotherapy with depressed adults. J Consult Clin Psychol 1995;63:644–50 | 3 |
| 48. Jarrett RB, Basco MR, Risser R, Ramanan J, Marwill M, Kraft D, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients? J Consult Clin Psychol 1998;66:1036–40 | 5 |
| 49. Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Arch Gen Psychiatry 2001;58:381–8 | 6 |
| 50. Jarrett RB, Kraft D, Schaffer M, Witt-Browder A, Risser R, Atkins DH, et al. Reducing relapse in depressed outpatients with atypical features: a pilot study. Psychother Psychosom 2000;69:232–9 | 6 |
| 51. Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemp Clin Trials 2010;31:355–77 | 6 |
| 52. Jarrett RB, Vittengl JR, Clark LA. How much cognitive therapy, for which patients, will prevent depressive relapse? J Affect Disord 2008;111:185–92 | 6 |
| 53. Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006;10(33) | 2 |
| 54. Kashner TM, Henley SS, Golden RM, Rush AJ, Jarrett RB. Assessing the preventive effects of cognitive therapy following relief of depression: a methodological innovation. J Affect Disord 2007;104:251–61 | 6 |
| 55. Kennard BD, Emslie GJ, Mayes TL, Nightingale-Teresi J, Nakonezny PA, Hughes JL, et al. Cognitive-behavioral therapy to prevent relapse in pediatric responders to pharmacotherapy for major depressive disorder. J Am Acad Child Adolesc Psychiatry 2008;47:1395–404 | 6 |
| 56. Kingston T, Dooley B, Bates A, Lawlor E, Malone K. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother 2007;80:193–203 | 3 |
| 57. Klein DN, Santiago NJ, Vivian D, Blalock JA, Kocsis JH, Markowitz JC, et al. Cognitive-behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. J Consult Clin Psychol 2004;72:681–8 | 6 |
| 58. Kocsis JH, Gelenberg AJ, Rothbaum BO, Klein DN, Trivedi MH, Manber R, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP trial. Arch Gen Psychiatry 2009;66:1178–88 | 3 |
| 59. Kroll L, Harrington R, Jayson D, Fraser J, Gowers S. Pilot study of continuation cognitive-behavioral therapy for major depression in adolescent psychiatric patients. J Am Acad Child Adolesc Psychiatry 1996;35:1156–61 | 5 |
| 60. Kuehner C. An evaluation of the ‘Coping with Depression Course’ for relapse prevention with unipolar depressed patients. Psychother Psychosom 2005;74:254–9 | 5 |
| 61. Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol 2010;13:595–602 | 2 |
| 62. Learmonth D, Trosh J, Rai S, Sewell J, Cavanagh K. The role of computer-aided psychotherapy within an NHS CBT specialist service. Counsell Psychother Res J 2008;8:117–23 | 2 |
| 63. Lenz G. Cognitive-psychoeducative group-therapy vs TAU with additional information group: a randomized controlled study. Eur Psychiatry 2010;25(Suppl. 1):19 | 4 |
| 64. Lin EHB, Katon WJ, Simon GE, Von Korff M, Bush TM, Walker EA, et al. Low-intensity treatment of depression in primary care: is it problematic? Gen Hosp Psychiatry 2000;22:78–83 | 5 |
| 65. Mahalik JR, Kivlighan DM. Self-help treatment for depression: who succeeds? J Couns Psychol 1988;35:237–42 | 2 |
| 66. Maneeton N, Thongkam A, Maneeton B. Cognitive-behavioral therapy added to fluoxetine in major depressive disorder after 4 weeks of fluoxetine-treatment: 16-week open label study. J Med Assoc Thai 2010;93:337–42 | 3 |
| 67. Marrs RW. A meta-analysis of bibliotherapy studies. Am J Community Psychol 1995;23:843–70 | 5 |
| 68. Mathew KL, Whitford HS, Kenny MA, Denson LA. The long-term effects of mindfulness-based cognitive therapy as a relapse prevention treatment for major depressive disorder. Behav Cogn Psychother 2010;38:561–76 | 2 |
| 69. Michalak J, Heidenreich T, Meibert P, Schulte D. Mindfulness predicts relapse/recurrence in major depressive disorder after mindfulness-based cognitive therapy. J Nerv Ment Dis 2008;196:630–3 | 5 |
| 70. Mirabel-Sarron C. [The choice between care and prevention in cognitive and behaviour therapies for depression]. Ann Med Psychol 2007;165:593–7 | 5 |
| 71. Munoz RF, Le HN, Clarke GN, Barrera AZ, Torres LD. Preventing first onset and recurrence of major depressive episodes. In Hammen CL, Gotlib IH, editors. Handbook of depression. 2nd edn. New York, NY: Guilford Press; 2009. pp. 533–53 | 5 |
| 72. O’Hara MW, Schiller CE, Stuart S. Interpersonal psychotherapy and relapse prevention for depression. In Richards CS, Perri MG, editors. Relapse prevention for depression. Washington, DC: American Psychological Association; 2010. pp. 77–97 | 5 |
| 73. Paykel E. Cognitive therapy in relapse prevention in unipolar depression. Eur Psychiatry 2002;17(Suppl. 1):55 | 6 |
| 74. Paykel ES, Scott J, Teasdale J. Cognitive therapy prevents relapse in residual depression. 39th Annual Meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, 10–14 December 2000. p. 36 | 6 |
| 75. Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry 1999;56:829–35 | 6 |
| 76. Perlis RH, Nierenberg AA, Alpert JE, Pava J, Matthews JD, Buchin J, et al. Effects of adding cognitive therapy to fluoxetine dose increase on risk of relapse and residual depressive symptoms in continuation treatment of major depressive disorder. J Clin Psychopharmacol 2002;22:474–80 | 6 |
| 77. Petersen T, Harley R, Papakostas GI, Montoya HD, Fava M, Alpert JE. Continuation cognitive-behavioural therapy maintains attributional style improvement in depressed patients responding acutely to fluoxetine. Psychol Med 2004;34:555–61 | 6 |
| 78. Petersen TJ, Pava JA, Buchin J, Matthews JD, Papakostas GI, Nierenberg AA, et al. The role of cognitive-behavioral therapy and fluoxetine in prevention of recurrence of major depressive disorder. Cognit Ther Res 2010;34:13–23 | 6 |
| 79. Rafanelli C, Park SK, Fava GA. New psychotherapeutic approaches to residual symptoms and relapse prevention in unipolar depression. Clin Psychol Psychother 1999;6:194–201 | 5 |
| 80. Risch AK, Stangier U. New cognitive-behavioural approaches for relapse prevention of recurrent depression. Verhaltenstherapie 2006;16:275–81 | 6 |
| 81. Rosenberg NK, Licht R, Rasmussen NA. Cognitive therapy and relapse prevention of depression: an effect study. Nord J Psychiatry 2000;54(Suppl. 43):6 | 2 |
| 82. Rosso G, Crespi C, Martini B, Maina G. Combining brief dynamic therapy with antidepressants in major depressive disorder. Clin Neuropsychiatry 2009;6:56–62 | 2 |
| 83. Schlogelhofer M, Eder H, Itzlinger U, Wiesegger G, Bailer U, Leisch F, et al. [Bibliotherapie: Kognitive therapie in buchform als selbsthilfe bei patienten mit teilremittierter depression.] J Neurol Neurochir Psychiatr 2003;4:33–5 | 2 |
| 84. Schlogelhofer M, Wiesegger G, Bailer U, Eder H, Itzlinger U, Jorgl G, et al. The effectiveness of bibliotherapy – cognitive-behavioural selfhelp – in patients with partially remitted depression. Eur Psychiatry 2004;19(Suppl. 1):213 | 3 |
| 85. Scogin F, Hamblin D, Beutler L. Bibliotherapy for depressed older adults: a self-help alternative. Gerontologist 1987;27:383–7 | 2 |
| 86. Scogin F, Jamison C, Davis N. Two-year follow-up of bibliotherapy for depression in older adults. J Consult Clin Psychol 1990;58:665–7 | 3 |
| 87. Scott J, Palmer S, Paykel E, Teasdale J, Hayhurst H. Use of cognitive therapy for relapse prevention in chronic depression. Cost-effectiveness study. Br J Psychiatry 2003;182:221–7 | 6 |
| 88. Simon GE, Katon WJ, VonKorff M, Unutzer J, Lin EHB, Walker EA, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry 2001;158:1638–44 | 3 |
| 89. Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment. A randomized controlled trial. JAMA 2004;292:935–42 | 2 |
| 90. Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ 2000;320:550–54 | 2 |
| 91. Stant AD, Ten Vergert EM, den Boer PCAM, Wiersma D. Cost-effectiveness of cognitive self-therapy in patients with depression and anxiety disorders. Acta Psychiatr Scand 2008;117:57–66 | 3 |
| 92. Stant AD, TenVergert EM, Kluiter H, Conradi HJ, Smit A, Ormel J. Cost-effectiveness of a psychoeducational relapse prevention program for depression in primary care. J Ment Health Policy Econ 2009;12:195–204 | 2 |
| 93. Taylor DJ, Walters HM, Vittengl JR, Krebaum S, Jarrett RB. Which depressive symptoms remain after response to cognitive therapy of depression and predict relapse and recurrence? J Affect Disord 2010;123:181–7 | 6 |
| 94. Teasdale J. A randomised controlled trial comparing the effectiveness of two versions of Mindfulness Based Cognitive Therapy (MBCT) in preventing relapse/recurrence in recovered depressed patients. National Research Register Archive, National Institute for Health Research; 2002. URL: www.nihr.ac.uk/Profiles/NRR.aspx?Publication_ID=N0287052536 (cited 3 November 2010) | 7 |
| 95. Teasdale JD, Segal Z, Williams JMG. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav Res Ther 1995;33:25–39 | 5 |
| 96. Titov N, Andrews G, Davies M, McIntyre K, Robinson E, Solley K. Internet treatment for depression: a randomized controlled trial comparing clinician vs technician assistance. PLoS One 2010;5:e10939 | 3 |
| 97. Van Voorhees BW. A randomized controlled trial of a primary care Internet based depression prevention intervention for adolescents (CATCH-IT): 12-month outcomes. J Investig Med 2010;58:654 | 2 |
| 98. Vittengl JR, Clark LA, Jarrett RB. Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychol Med 2004;34:643–58 | 6 |
| 99. Vittengl JR, Clark LA, Jarrett RB. Continuation-phase cognitive therapy’s effects on remission and recovery from depression. J Consult Clin Psychol 2009;77:367–71 | 6 |
| 100. Vittengl JR, Clark LA, Jarrett RB. Moderators of continuation phase cognitive therapy’s effects on relapse, recurrence, remission, and recovery from depression. Behav Res Ther 2010;48:449–58 | 6 |
| 101. Wells A, Fisher P, Myers S, Wheatley J, Patel T, Brewin CR. Metacognitive therapy in recurrent and persistent depression: a multiple-baseline study of a new treatment. Cognit Ther Res 2009;33:291–300 | 3 |
| 102. Wollersheim JP, Wilson GL. Group treatment of unipolar depression: a comparison of coping, supportive, bibliotherapy, and delayed treatment groups. Prof Psychol Res Pr 1991;22:496–502 | 3 |
Appendix 3 Data extraction tables
Completed studies
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Bockting46 Year 2005 Country Netherlands Full publication? Yes Study design RCT Linked publications |
Inclusion criteria Patients who have suffered at least two major depressive episodes (DSM-IV; SCID) in last 5 years; currently in remission between 10 weeks to 2 years; and having < 10 current score on HRSD Exclusion criteria Current mania or hypomania; history of bipolar of psychotic disorder; organic brain damage; alcohol or drug misuse; predominant anxiety disorder; recent ECT; recent or current cognitive therapy; recent or current psychotherapy more than twice per month Previous treatment(s) received Not stated Setting 69% participants recruited through media announcements, 31% from psychiatric centres No. included at ba s eline 187 No. lost to follow-up 22/187 ITT analysis? Partial. All patients who started treatment (172/187) were accounted for in ‘ITT’. Dropouts prior to first treatment were not |
Name of intervention Brief cognitive therapy + TAU Structured content? Treatment manual, homework and review of homework with regular supervision. Sessions were audiotaped and any adherence/competence issues raised before the next session Delivered by? Specifically trained (16 hours) cognitive behavioural therapists (> 5 years prior training) Group intervention? Yes No. of patients per group (if applicable) 7–12 (mean 8) No. of sessions 8 Session duration and frequency 2 hours weekly Total intervention duration 8 weeks Concurrent treatments ~50% participants in each group received concurrent antidepressant medication |
Comparator name TAU Comparator details Standard treatment (including no treatment) as typically provided by the referring agencies. There were no restrictions on the use of pharmacotherapy throughout the study or follow-up periods Concurrent treatments ~50% participants in each group received concurrent antidepressant medication |
Definition of relapse/recurrence Kaplan–Meier cumulative relapse/recurrence rates. Assessed using SCID-I at 3, 12, 24 and 66 months. Severity of relapse scored as low (< 6 symptoms), medium (6–7) or high (8–9) Other outcomes Severity of depressive residual symptoms (measured using HRSD); dysfunctional attitudes (measured using DAS); stress – daily hassles (measured using EPCL); Stress – Life events, (measured using Negative Life Events Questionnaire) – medication and other psychological treatment |
Intervention relapse rate Cumulative rate at 24 months: Patients with ≥ 5 previous episodes 46% (53% in abstract) Patients with < 5 previous episodes 63% (69% in abstract) Cumulative rate at 66 months: Patients with ≥ 4 previous episodes 75% (99% CI 61 to 86) Patients with < 4 previous episodes 82% (99% CI 67 to 93) Comparator relapse rate Cumulative rate at 24 months: Patients with ≥ 5 previous episodes 72% (80% in abstract) Patients with < 5 previous episodes 59% (64% in abstract) Cumulative rate at 66 months: Patients with ≥ 4 previous episodes 95% (99% CI 83 to 100) Patients with < 4 previous episodes 79% (99% CI 64 to 90) p-value for difference between rates ‘Significant’ for ≥ 5 previous episodes group, but not < 5 episodes group at 24 months |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Bondolfi51 Year 2010 Country Switzerland Full publication? Yes Study design RCT |
Inclusion criteria Patients aged 18–65 years with a history of recurrent major depression (DSM-IV; assessed with SCID); three or more past depressive episodes (two episodes in the past 5 years and at least one episode in the past 2 years); remission for ≥ 3 months at time of enrolment. MADRS ≤ 13. History of antidepressant treatment, but currently off medication for ≥ 3 months before enrolment Exclusion criteria History of schizophrenia or schizoaffective disorder, current substance abuse, eating disorder, or obsessive compulsive disorder, organic mental disorder, pervasive developmental disorder or borderline personality disorder; dysthymia with onset before age 20 years; more than four sessions of CBT ever; current psychotherapy or counselling more than once per month; current practice of meditation > once per week or yoga > twice per week Previous treatment(s) received Antidepressants (no details given) Setting Patients recruited through media announcements and mailings to psychiatrists and GPs in the French-speaking region of Switzerland. Study conducted at Geneva and Lausanne University Hospitals No. included at baseline 60 (31 MBCT; 29 TAU) No. lost to follow-up Five (55 patients with complete data at 14-month relapse or recurrence) ITT analysis? ITT and PP presented |
Name of intervention MBCT Structured content? French translation of MBCT manual used. Participants given two CDs containing the standard practices proposed in the programme (body scan, sitting meditation, mindful movements, and 3-minute breathing space). Trial groups monitored for adherence to the MBCT protocol by audiotaping sessions Delivered by? Three senior CBT psychologists and a senior CBT psychiatrist. All had undergone at least one training by one of the developers of MBCT; two instructors attended 9-day professional training in mindfulness-based stress reduction. They had all led three specialised MBCT groups prior to this study Group intervention? Yes No. of patients per group (if applicable) Not stated No. of sessions Eight, plus four booster sessions ≥ 4 MBCT sessions considered minimal dose Session duration and frequency Weekly 2-hour sessions. Booster sessions every 3 months (duration not stated) Total intervention duration 8 weeks (excluding booster sessions) plus 52 weeks’ follow-up Concurrent treatments Not stated |
Comparator name Treatment at usual Comparator details TAU participants were told to seek help from their family doctor or other sources as they normally would if they had worsening symptoms or other difficulties Concurrent treatments Not stated |
Definition of relapse/recurrence Occurrence of relapse or recurrence meeting DSM-IV criteria for major depressive episode. Patients interviewed using SCID at baseline, end of interventions (2 months) and follow-up (5, 8, 11 and 14 months) Other outcomes Time to relapse (no. days from enrolment to relapse). Severity of depressive symptoms measured with the MADRS. Frequency of mindfulness practices measured with an ad hoc questionnaire (details provided) |
Intervention relapse rate 14 months ITT 9/31 (29%), PP 9/27 (33%), median time to relapse 204 days Comparator relapse rate 14 months ITT 10/29 (34%), PP 10/28 (36%), median time to relapse 69 days (range 15–191 days) p-value for difference between rates 14 months p = 0.78 (ITT), p = 1.0 (PP), p = 0.06 (time to relapse) Relative risk/odds ratio/hazard ratio Cox regression suggested no significant difference between the intervention and control groups (hazard ratio not reported) p = 0.58 (ITT), p = 0.60 (PP) |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Fava52 Year 1998 Country Italy Full publication? Yes Study design RCT Linked publication Fava 200453 |
Inclusion criteria Diagnoses were established using the Schedule for Affective Disorders and Schizophrenia. Participants with a current diagnosis of primary major depressive disorder according to the RDC for a Selected Group of Functional Disorders; three or more previous episodes of depression, with the immediately preceding episode being no more than 2.5 years before the onset of the present episode; minimum 10-week remission according to the RDC between the index episode and immediately preceding episode; minimum global severity score of 7 for the current episode of depression. Only patients rated as ‘better’ or ‘much better’ after antidepressant drug treatment according to a global scale of improvement and as being in full remission were included. Also no evidence of depressed mood according to a modified version of Paykel’s CID Exclusion criteria Participants with history of manic, hypomanic or cyclothymic features; history of drug or alcohol abuse or personality disorder (DSM-IV) or antecedent dysthymia; active medical illness Previous treatment(s) received All participants received 3–5 months of full-dose antidepressants Setting Patients referred to the Affective Disorders Programme of the University of Bologna No. included at baseline 45 No. lost to follow-up Five ITT analysis? 40/45 patients randomised were analysed. The remaining three patients were excluded because their antidepressant drugs could not be tapered |
Name of intervention Pharmacotherapy and CBT Structured content? CBT conducted as described by Beck et al. 104 Main components included: CBT of residual symptoms of major depression; lifestyle modification; well-being therapy. Four sessions were taped to check integrity Delivered by? Psychiatrist, experienced in CBT Group intervention? No No. of sessions 10 Session duration and frequency 30 minutes every other week Total intervention duration 20 weeks Concurrent treatments Pharmacotherapy, taped over time |
Comparator name Pharmacotherapy and clinical management Comparator details Clinical management consisted of monitoring medication tapering, reviewing patient’s clinical status, provide advice and support if necessary Concurrent treatments Pharmacotherapy, tapered over time |
Definition of relapse/recurrence The occurrence of an RDC-defined episode of major depression at 3, 6, 9, 12, 15, 18, 21 and 24 months Other outcomes Time until relapse Symptoms of depression as measured by CID at 3, 6, 9, 12, 15, 18, 21 and 24 months |
Intervention relapse rate 2 years: 5/20 (25%) 6 years: 8/20 (40%) Comparator relapse rate 2 years: 16/20 (80%) 6 years: 18/20 (90%) p-value for difference between rates 6 years: p = 0.001 |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Godfrin54 Year 2010 Country Belgium Full publication? Yes Study design RCT |
Inclusion criteria Patients aged ≥ 18 years with a history of at least three previous episodes of depression according to DSM-IV-R criteria, the end of the last episode being at least 8 weeks prior to recruitment. Participants should not suffer from a current depressive episode according to the DSM-IV-R criteria, scored < 14 on the HRSD, and from a well-defined geographic area Exclusion criteria Current depression or dysthymia according to DSM-IV-R criteria; substance use disorder; obsessive–compulsive disorder; bipolar disorder; acute psychosis; schizophrenia/schizoaffective disorder; cognitive disorder; organic mental disorder; pervasive developmental disorder; mental retardation; primary diagnosis of axis-II disorder/risk of suicide; extended experience of Zen or Vipassana (mindfulness) meditation; physical problems hampering participation; or concurrent psychiatric consultation (> 1 consultation per 3–4 weeks), intensive psychotherapy, or other forms of meditation Previous treatment(s) received: 87/106 (82.1%) received psychotherapy/counselling 81/106 (76.4%) received antidepressant medication 23/106 (21.7%) were hospitalised 88/106 (83%) visited GP Rates were similar between intervention and control groups Setting Outpatients recruited through advertisement, word of mouth or referral No. included at baseline 106 (52 MBCT; 54 TAU) No. lost to follow-up 30 (18 MBCT; 12 TAU) ITT analysis? Yes |
Name of intervention MBCT Structured content? Manualised class-based skills training programme, based on mindfulness-based stress reduction and CBT. The intervention aims at increasing the capacity to attend, non-judgementally and moment by moment to patterns of thoughts, bodily sensations and feelings. Participants were asked to complete daily homework exercises (including meditation practices and exercises to integrate the awareness skills into daily life) for at least 45 minutes per day, 6 days per week. The treatment protocol was checked to ensure it delivered the essential elements of MBCT Delivered by? Medical practitioner with extensive experience of MBCT Group intervention? Yes No. of patients per group (if applicable) 12–15 No. of sessions Eight Session duration and frequency 2 hours and 45 minutes per week Total intervention duration 8 weeks Concurrent treatments Baseline: 29/52 (58.0%); depression-related visits to GP 28/52 (53.8%); treatment by psychiatrist 14/52 (26.9%); treatment by psychologist 38/52 (73.1%); antidepressant medication 2/52 (4.2%); hospitalisation because of psychic complaints At 14 months: 24/52 (63.2%); depression-related visits to GP 20/52 (51.3%); treatment by psychiatrist 11/52 (28.2); treatment by psychologist 25/52 (64.1%); antidepressant medication 1/52 (2.6%); hospitalisation because of psychic complaints. Some data were missed so different denominators were used for calculating concurrent treatment at 14 months |
Comparator name TAU Comparator details Waiting list control Concurrent treatments Baseline: 37/54 (69.8%); depression-related visits to GP 16/54 (30.2%); treatment by psychiatrist 7/54 (13.2%); treatment by psychologist 33/54 (61.1%); antidepressant medication At 14 months: 35/54 (77.8%); depression-related visits to GP 12/54 (26.7%); treatment by psychiatrist 6/54 (13.3%); treatment by psychologist 28/54 (62.2%); antidepressant medication Some data were missed so different denominators were used for calculating concurrent treatment at 14 months |
Definition of relapse/recurrence Relapse/recurrence (DSM-IV-R criteria for major depressive episode, using SCID-I interview) Time to relapse/recurrence since recruitment Reported at baseline, 2, 8 and 14 months Other outcomes Level of depressive symptoms (HRSD and BDI) Current mood states Self-reported QoL (QLDS) Reported at baseline, 2, 8 and 14 months |
Intervention relapse rate 14 months Relapse rate 12/40 (30%) Mean time to first relapse 53.7 weeks Comparator relapse rate 14 months Relapse rate 32/47 (68%) Mean time to first relapse 39.5 weeks p-value for difference between rates p < 0.0005 relapse rate, p < 0.001 mean time to first relapse Relative risk/odds ratio/hazard ratio Significant reduction in hazard of relapse/recurrence for intervention group Hazard ratio = 0.23 (95% CI 0.09 to 0.63), p < 0.01 |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Hepburn55 Year 2009 Country UK Full publication? Yes Study design RCT |
Inclusion criteria Patients aged 18–65 years; experienced both depression (minimum of one episode) and suicidality (attempt or severe ideation); and in remission or recovery. No participant had current depression according to diagnostic interview at first assessment. Participants were interviewed with the mini international neuropsychiatric interview Exclusion criteria Not fluent in English; receiving CBT without subsequent depressive relapse; symptom of substance misuse; psychosis or mania in the last 6 months prior to the study Previous treatment(s) received Not stated; groups did not differ significantly in rates of past hospitalisation or psychotherapy Setting Clinician referrals or self-referrals from community advertisements No. included at baseline 68 patients (33 MBCT; 35 control) No. lost to follow-up 25 (13 MBCT; 12 control) ITT analysis? Not stated |
Name of intervention MBCT Structured content? MBCT group received the new programme for suicidality consisting of weekly classes and daily homework. No details of class content were given. Daily homework (maximum 1 hour) included formal audio-guided meditation, and informal practices integrating mindfulness into everyday life Delivered by? Experienced CBT and mindfulness-based therapists Group intervention? Yes No. of patients per group (if applicable) Up to 17 per class No. of sessions Eight classes and one all-day session. Attending fewer than four classes considered non-completers Session duration and frequency 2-hour classes every week and one all-day session (6 hours) Total intervention duration Not stated Concurrent treatments Not stated |
Comparator name Waiting list control Comparator details Participants continued with TAU (including medication), seeking help from GPs or other sources if participants encountered difficulties. MBCT was offered when the study was complete Concurrent treatments Not stated |
Definition of relapse/recurrence Depression symptoms measured using BDI Other outcomes Self-reported thought suppression measured by the suppression subscale of the WBSI, with an additional question about short-term attempts at thought suppression in the last week |
Intervention relapse rate Pre-intervention BDI = 15.62 (SD 13.84) and post-intervention BDI = 8.67 (SD 12.00) Pre-intervention thought suppression: Past week thought suppression = 3.70 (SD 1.30) and post-intervention past week thought suppression = 2.60 (SD 11.42) Comparator relapse rate Pre-intervention BDI = 12.83 (SD 9.59) and post-intervention BDI = 12.25 (SD 11.14) Pre-intervention thought suppression: Past week thought suppression= 3.58 (SD 1.59) and post-intervention past week thought suppression= 4.12 (SD 1.42) p-value for difference between rates For BDI: MBCT; p < 0.01 (post minus pre-pre-effect size) TAU; p = 0.75 (post minus pre-pre-effect size) For WBSI: MBCT; no statistically significant difference (post minus pre-intervention WBSI) TAU; no statistically significant difference (post minus pre-intervention WBSI) Relative risk/odds ratio/hazard ratio Not reported |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Howell56 Year 2008 Country Australia Full publication? Yes Study design Cluster RCT; randomisation by practice |
Inclusion criteria Patients aged 18 years or older; met diagnostic criteria for a depressive disorder according to the DSM-IV; availability to be followed up for 12 months; and ability to give informed consent. Not all patients were in remission, as patients were assessed on severity rather than using a cut-off) Exclusion criteria Undergoing a separate treatment programme; suffering from psychoses; unable to complete the English-language questionnaires or interview Setting GP setting (both urban and rural) No. included at baseline 110 patients (62 KBA; 48 usual care) 23 practices (45 GPs)No. lost to follow-up 16 (15 KBA; 1 usual care) ITT analysis? Yes |
Name of intervention KBA Structured content? The KBA consisted of GP training manual or patient manual and relaxation CD; 20 hours of training on depression, the study protocol, assessment tools and skills. KBA involves a multimodal, skilled-based approach and utilises a range of evidence-based psychosocial strategies, such as problem-solving, which can be tailored to the individual patient. The programme incorporates 10 steps (details given) and started once the patient’s depression has been stabilised by initial treatment Delivered by? GPs who completed 20 hours of training conducted by GP and a psychologist. The GPs’ training kit contained information on depression, the study protocol, assessment tools, and skill training related to the KBA programme. GPs in the control group completed a 3-hour training session on the study protocol. A psychology graduate was trained to review case notes using an audit record, and was blinded to patients’ group allocation Group intervention? No No. of sessions Mean no. of visits = 7 Session duration and frequency Typical visit around 30 minutes Total intervention duration Not stated Concurrent treatments Usual medication as clinically indicated |
Comparator name Usual care Comparator details Usual medication Concurrent treatments |
Definition of relapse/recurrence A 50% relative reduction of depression relapse, assessed with DASS. Relapse was assessed retrospectively through blinded case note review. Evidence of depression relapse sought from the notes included increased symptoms of depression, medication changes, hospital admissions, new symptoms or suicidality Other outcomes |
Intervention relapse rate 13/62 (46.4%) at 12 months Comparator relapse rate 15/48 (53.6%) at 12 months p-value for difference between rates p = 0.23 Relative risk/odds ratio/hazard ratio Relative risk = 0.77 (95% CI 0.50 to 2.05) |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Katon45 Year 2001 Country USA Full publication? Yes Study design RCT Linked publications |
Inclusion criteria Patients aged 18–80 years who had recovered from an episode of depression or anxiety following a new antidepressant prescription; at high risk of relapse as determined by SCID for DSM-III-R; fewer than four major depressive symptoms and a history of three or more episodes of major depression or dysthymia, or four residual depressive symptoms but with a mean SCL-20 depression score of < 1.0 and a history of major depression/dysthymia. No prior prescriptions 120 days prior to baseline assessment Exclusion criteria Score of 2 or more on CAGE alcohol screening questionnaire; pregnant or nursing; planning to disenrol from HMO in next 12 months; currently seeing a psychiatrist; limited command of the English language; recent use of lithium or antipsychotic medication Previous treatment(s) received Not stated Setting Four primary care clinics in western Washington (with 88,000 patients and 73 family physicians) No. included at baseline 386 No. lost to follow-up 155/194 (79.9%) of intervention patients completed all three telephone follow-ups. Details not available for usual-care arm ITT analysis? Unclear |
Name of intervention Multifaceted relapse prevention programme Structured content? Patient education, face-to-face and telephone follow-up sessions at 2, 6, 10 and 12 months and personalised mailings (showing BDI scores over time, plus symptom and medication adherence checklists). Self-treatment intervention was designed to build on principles of motivational interviewing and cognitive behavioural theories of relapse prevention. Aim to improve adherence to medication, increase awareness of prodromal symptoms and develop proactive steps, increase daily use of depression treatment techniques. Ultimate aim was to have each patient completed a personal relapse prevention plan Delivered by? Depression specialists (psychologist, nurse practitioner with Master’s degree in psychosocial nursing, social worker). All received a manual and training with the trial investigators Group intervention? No, individualised No. of patients per group (if applicable) No. randomised 386 (194 intervention; 192 usual care). At 12 months’ follow-up: 10.3% of intervention group and 20.8% of usual-care group missed interviews. 315 (82%) completed all follow-up assessments and 377 (98%) remained enrolled throughout the follow-up period No. of sessions Two face-to-face visits, three telephone visits, four personalised mailings Session duration and frequency Two face-to-face sessions with depression specialists were 90 minutes (first session) and 60 minutes (follow-up). Telephone visits were scheduled at 1, 4 and 8.5 months after second face-to-face session (duration not reported). Personalised mailings were scheduled at 2, 6, 10 and 12 months. Specialist received pharmacy data and alerted physician and telephoned patients when feedback indicated they were symptomatic and/or had discontinued medication Total intervention duration 12 months Concurrent treatments Participants were encouraged to adhere to their antidepressant medication plan. Patients could also self-refer to mental health services |
Comparator name Usual care Comparator details Typically prescription for antidepressant medication, two to four visits with family physician over first 6 months of treatment and option to refer to mental health services |
Definition of relapse/recurrence Episode of depression defined by SCID at 3, 6, 9 or 12 months; or had an interval episode based on the Longitudinal Interval Follow-up Evaluation Other outcomes No. of primary care visits Medication adherence (% antidepressant refills; antidepressant dose adequacy). Depressive symptoms (average SCL-20 score) |
Intervention relapse rate 35% Comparator relapse rate 34.6% p-value for difference between rates Not statistically significant Relative risk/odds ratio/hazard ratio Not reported |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Kuhner61 Year 1996 Country Germany Full publication? Yes Study design Non-RCT with concurrent control group Linked publication Kuhner 199462 |
Inclusion criteria 18- to 60-year-olds formerly depressed inpatients 1 or 7 months after discharge and patients recruited directly through the psychiatric outpatient department; ICD-9 diagnosis of endogenous, neurotic or reactive depression, major depressive episode according to DSM-III, or score ≥ 25 on IDD Exclusion criteria Current or past organic, schizophrenic, paranoid or schizoaffective disorders, bipolar disorders, primary substance abuse; mental retardation; patients undergoing individual therapy; living too far; shift workers Previous treatment(s) received 69% received antidepressants Setting Inpatients at the psychiatric clinic of the Central Institute of Mental Health at the University of Mannheim No. included at baseline 42 not depressed at baseline No. lost to follow-up Unclear ITT analysis? Unclear |
Name of intervention CWD course Structured content? Same as cognitive behavioural group intervention: highly structured, based on a multimodal psychoeducational approach. Addresses specific target behaviours assumed to counteract the development and maintenance of depression. Weekly instructor-supervised meetings to assure standardisation of procedures Delivered by? Clinical psychologists and psychiatrists Group intervention? Yes No. of patients per group (if applicable) 4–8 No. of sessions 16 Session duration and frequency 90 minutes to 2 hours weekly, with four additional sessions after the fourth, sixth, eighth and 12th weeks Total intervention duration 12 weeks Concurrent treatments Unclear |
Comparator name No CWD intervention Comparator details Participants who refused CWD, who were > 7 months after discharge or did not meet the inclusion criteria for CWD Concurrent treatments Unclear |
Definition of relapse/recurrence Relapse (MDE according to DSM-III-R) Other outcomes None |
Intervention relapse rate 6 months: 3/21 (14%) Comparator relapse rate 6 months: 9/21 (43%) p-value for difference between rates p < 0.05 |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Kuyken63 Year 2008 Country UK Full publication? Yes Study design Parallel two-group RCT, stratified by symptomatic status (HRSD ≥ 8) |
Inclusion criteria Patients aged 18 years or older; history of three or more previous depression episodes meeting the DSM criteria-IV; treated with therapeutic dose of antidepressants over the last 6 months; and currently in full or partial remission Exclusion criteria Comorbid diagnosis of current substance dependence; organic brain damage; current/past psychosis; bipolar disorder; persistent antisocial behaviour; persistent self-injury requiring clinical management/therapy; unable to engage with MBCT for physical, practical or other reasons (e.g. very disabling physical problem, unable to comprehend materials); and formal concurrent psychotherapy Previous treatment(s) received 30/123 patients received previous psychiatric treatment, specific therapies not stated Setting Primary care settings across a range of urban and rural locations in Devon, UK. Patients were identified from computerised practice databases No. included at baseline 123 (MBCT = 61; m-ADM = 62) No. lost to follow-up 8 (2 MBCT; 6 m-ADM) 19 (9 MBCT; 10 m-ADM) fell outside protocol (attended less than four sessions of MBCT, discontinued medication m-ADM) ITT analysis? Yes (PP analysis also undertaken) |
Name of intervention MBCT and antidepressant tapering/discontinuation Structured content? Manualised MBCT, grouped-based skill training programme: session content included guided mindfulness practice; inquiry into patients’ experience of these practices; review of weekly homework; and teaching and discussion of cognitive behavioural skills. Sessions were videotaped to monitor therapist competence and treatment adherence. Antidepressant tapering/discontinuation regimes determined by primary care physician and patients, with guideline information provided by study team Delivered by? Clinical psychologist or occupational therapist. Both had undergone training by one of the developers of MBCT, had run at least two supervised pilot groups and had an ongoing personal mindfulness practice. Therapist competence was adequately assessed Group intervention? Yes No. of patients per group (if applicable) 9–15 patients No. of sessions Eight sessions, plus four follow-up sessions (four or more sessions considered adequate dose) Session duration and frequency Weekly 2-hour sessions. Follow-up sessions (no details provided) Total intervention duration 8 weeks with follow-up sessions in the following 12 months Concurrent treatments Not stated |
Comparator name Maintenance m-ADM Comparator details Patients managed according to standard clinical practice and the British National Formulary; therapeutic dose was required; physician required to meet patient regularly to review their medication treatment. Medication adherence monitored through self-report every 3 months, practice databases and MMAS Concurrent treatments Not stated |
Definition of relapse/recurrence Time to relapse/recurrence, using SCID for retrospective assessment of previous 3 months. Relapse/recurrence defined as an episode meeting DSM-IV criteria for major depressive disorder; if considered marginal, a conservative position of no relapse was recorded. Once a judgement about relapse was made, the onset of relapse was dated from randomisation to the point at which criteria were met Other outcomes Severity of relapse/recurrence, assessed using DSM-IV every 3 months over 15 months. Duration of any relapse/recurrence (defined as period of time in months that a person met SCID criteria), and associated distress (rated by patients on a 1- to 100-point scale ranging from 0 (least distressing episode of depression ever experienced) to 100 (most distressing episode of depression ever experienced). Residual depressive symptoms, assessed by the observer-rated interviewer-administered 17-item version of the HRSD and 21-item self-reported BDI (BDI-II). QoL, assessed using the 26-item, self-report, short version of the WHOQOL-BREF. QoL was subjective and was assessed in four domains: physical, psychological, social and environmental. Economic evaluation looked at all hospital (inpatient, outpatient, emergency department) and community health and social services (primary care, social work, complementary therapies), plus productivity losses resulting from time off work due to illness. Economic data were collected at baseline and at 3-month intervals for up to 15 months post randomisation using the AD-SUS, with missing items added |
Intervention relapse rate 29/61 (47%) in MBCT over the 15-month follow-up period 24/52 (46%) in the PP analysis Comparator relapse rate 37/62 (60%) over 15-month period (ITT analysis) 31/52 (60%) over 15-month period (PP analysis) p-value for difference between rates p = 0.21 for the ITT analysis p = 0.07 for the PP analysis Relative risk/odds ratio/hazard ratio Hazard ratio = 0.63 (95% CI 0.39 to 1.04) for the ITT analysis Hazard ratio = 0.59 (95% CI 0.34 to 1.00) for the PPT analysis |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Ma64 Year 2004 Country UK Full publication? Yes Study design RCT |
Inclusion criteria Patients aged 18–65 years; a history of major recurrent depression (at least two episodes of major depressions within the past 5 years, one of which should have occurred within the past 2 years) in the absence of a history of mania and hypomania; meeting enhanced DSM-III-R; a history of treatment by a recognised antidepressant medication, but being off antidepressant medication and in recovery/remission at the time of baseline assessment and for at least the preceding 12 weeks; and scored < 10 on the 17-item HRSD at baseline Exclusion criteria History of schizophrenia or schizoaffective disorder; current substance abuse; current eating disorder or obsessive compulsive disorder; organic mental disorder, pervasive developmental delay, or borderline personality disorder; dysthymia before age 20 years; more than four lifetime sessions of CBT; and current psychotherapy or counselling more frequently than once per month Previous treatment(s) received 3% MBCT and 10% TAU had been hospitalised for depression 68% MBCT and 74% TAU received psychotherapy/counselling Setting Participants were recruited through GPs and advertisements in local newspapers. Note: the setting was a replica of Teasdale 2000 study, except that this was a single-centre study, whereas the Teasdale’s study involved three centres No. included at baseline 75 (37 MBCT; 38 TAU) No. lost to follow-up 6/75 (8%) all MBCT; three failed to attend any training session and three dropped out after attending fewer than four sessions that were considered to be satisfactory in order to be included in the PP analysis ITT analysis? Yes; also PP analysis |
Name of intervention MBCT Structured content? Manualised group-based MBCT training programme, including daily homework exercises (guided and unguided) during the treatment phase. This MBCT programme is based on integration of aspects of CBT for depression, with components of MBSR programme designed to teach patients skills that allow individual to disengage from habitual dysfunctional cognitive routines. MBCT sessions were videotaped or audiotaped with the patient’s permission to allow monitoring of treatment Delivered by? Two experienced cognitive therapists; both had previously led at least two groups of recovered depressed patients through the MBCT programme Group intervention? Yes No. of patients per group (if applicable) Up to 12 patients No. of sessions Eight sessions (plus one initial individualised orientation session) Session duration and frequency 2 hours every week for 8 weeks Total intervention duration 8 weeks, 2 follow-up meetings at 1 and 6 months Concurrent treatments For patients who had reported a lifetime two episodes of depression For patients who had reported three or more episodes of depression |
Comparator name TAU Comparator details Patients received their usual treatment and were instructed to seek help from their family doctor, or other sources, as they normally would, if they encountered symptomatic deterioration or other difficulties over the course of the study. Assessment was conducted every 3 months Concurrent treatments For patients who had reported two episodes of depression prior to the study ∼36% had one or more depression-related visit to GP ∼30% sought counselling, psychotherapy, or professional mental health support |
Definition of relapse/recurrence An episode meeting DSM-IV criteria for major depressive disorder, assessed on the modelled Structured Clinical Interview for DSM-III-R. The assessment was done by a clinical psychologist blind to the patient’s treatment condition Other outcomes Time to onset of relapse or recurrence of depression. The occurrence of a significant life event was evaluated for those patients experiencing relapse/recurrence |
Intervention relapse rate Patients with a history of three or more episodes of depression: 10/28 (36%) Patients with a history of two episodes of depression: 4/8 (50%) from ITT and 1/4 (20%) from PP analysis Comparator relapse rate Patients with a history of three or more episodes of depression: 21/27 (78%) Patients with a history of two episodes of depression: 2/10 (20%) from ITT and PP p-value for difference between rates p = 0.001 (for three or more episodes of depression) Relative risk/odds ratio/hazard ratio Hazard ratio 0.278 (95% CI 0.130 to 0.597) for patients with a history of three or more episodes of depression. No significant difference in HR for patients with previous episodes |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Rohde65 Year 2008 Country USA Full publication? Yes Study design RCT |
Inclusion criteria 12–17 years of age; current DSM-IV MDD; CDRS-R score of 45 or higher; responder status on 7-point CGI scale; stable mood symptoms for at least 6 weeks; impairment in at least two settings Exclusion criteria Psychiatric disorders requiring out-of-protocol treatments; one failed CBT trial or two failed SSRI trials for depression; current psychiatric treatment (other than stable dose stimulant medication for ADHD); non-English speaking; confounding medical condition; previous intolerance to fluoxetine; pregnant or sexually active while refusing acceptable birth control; or danger to self or others Previous treatment(s) received After randomisation, participants received acute treatment (12 weeks), followed by continuation treatment (6 weeks) then maintenance treatment (18 weeks) Setting 13 US sites No. included at baseline 147 had achieved a sustained response to acute treatment (week 12) and went on to have continuation therapy No. lost to follow-up 40 ITT analysis? Yes |
Name of intervention Structured content? Acute treatment (first 12 weeks) consisted of tailored CBT, including psychoeducation about depression and its causes, goal-setting, mood monitoring, increasing pleasant activities, social problem-solving, cognitive restructuring and addressing social skill deficits. Continuation treatment (6 weeks of further CBT) varied in intensity, based on the patient’s response to acute treatment Maintenance treatment (18 weeks) – participants met their clinicians every 6 weeks for three CBT booster sessions and, if applicable, continued taking medication. CBT treatment was manualised Delivered by? Not stated Group intervention? No, individualised No. of sessions Acute treatment: 15 Continuation treatment: 6 Maintenance treatment: 3 Session duration and frequency Acute treatment: 50–60 minutes, weekly Continuation treatment: 50–60 minutes, weekly Maintenance treatment: 50–60 minutes, every 3 weeks Total intervention duration Acute treatment: 10–12 hours Continuation treatment: 5–6 hours Maintenance treatment: 2.5–3 hours Concurrent treatments Combination treatment group received fluoxetine alongside CBT |
Comparator name Clinical management with placebo (for 12 weeks of acute treatment only) Comparator details Not stated Concurrent treatments Not stated |
Definition of relapse/recurrence ‘Sustained response’ was defined as two consecutive ratings of ‘full response’ according to the CGI-I (score 1–2) during acute treatment. Maintenance of sustained response was classified as ‘failed to maintain’ (i.e. relapse/recurrence, CGI-I score of 3–7) ‘Maintained sustained response’ – given continued full responder status (CGI-I score 1–2) or ‘unknown’ (independent evaluation data unavailable) |
Intervention relapse rate 1/76 (3.1%) for CBT 14/80 (25.9%) for fluoxetine 7/86 (11.5%) for combination CBT/fluoxetine |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Takanashi66 Year 2002 Country Japan Full publication? Yes (Japanese, with English abstract) Study design Non-RCT with concurrent control group |
Inclusion criteria Patients currently being treated for depression, diagnosed according to ICD-10 F32 (depressive episode) or F33 (recurrent depressive episode) were recruited to the study group. The control group were currently being treated for depression, but considered to be in remission (no details given) Exclusion criteria Suicidal thoughts or mood disorder; drug or alcohol dependence; dementia or other brain conditions Previous treatment(s) received Not reported Setting University hospital outpatient department No. included at baseline 53 (31 intervention group; 22 control group) No. lost to follow-up Not reported ITT analysis? Not reported |
Name of intervention CBT; programme described by Munoz and Ying105 adapted to Japanese setting Structured content? Manualised content, classes covering influence of thoughts on emotions; learning how to change thoughts; effect of behaviour on feelings; increasing enjoyable activities; effects of interpersonal interactions on feelings; increasing interpersonal activities; and prevention of depression Delivered by? Psychiatrists (no. not given). Training or experience not reported Group intervention? Yes No. of patients per group (if applicable) Four or five No. of sessions Eight Session duration and frequency 60–90 minutes per weekly session Total intervention duration 2 months Concurrent treatments Not reported |
Comparator name Control group Comparator details Outpatients attending hospital for maintenance treatment, considered to be in remission (no details reported) Concurrent treatments Not reported |
Definition of relapse/recurrence Recurrence defined as an increase in medication or a worsening of social adjustment or a decline in ability to undertake work/housework measured at baseline and post intervention (for the intervention group only) and at 12-month follow-up by the following: BDI, HRSD, CES-D, SCL. Remission not defined Other outcomes |
Intervention relapse rate Not reported Comparator relapse rate Not reported p-value for difference between rates Not reported Relative risk/odds ratio/hazard ratio Kaplan–Meier curves of the proportion of patients remaining in remission up to 5 years suggest that the intervention may have an affect |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Teasdale67 Year 2000 Country UK and Canada Full publication? Yes Study design Multicentre RCT |
Inclusion criteria Patients aged 18–65 years; a history of major recurrent depression (at least two episodes of major depressions within the past 5 years and that one of the episodes was within the past 2 years) in the absence of a history of mania and hypomania; meeting enhanced DSM-III-R; a history of treatment by a recognised antidepressant medication and off treatment for at least 12 weeks preceding the study; currently in remission or recovery and scored < 10 on the 17-item HRSD at baseline Exclusion criteria History of schizophrenia or schizoaffective disorder; current substance abuse; eating disorder or obsessive–compulsive disorder); organic mental disorder, pervasive developmental delay, or borderline personality disorder; dysthymia before age 20 years; more than four sessions of cognitive-behavioural therapy ever; current psychotherapy or counselling more frequently than once per month; and current practice of meditation more than once per week or yoga more than twice per week Previous treatment(s) received 100% for MBCT and TAU took antidepressant medication; 11% MBCT and 17% TAU hospitalised for depression; and 73% MBCT and 68% TAU received psychotherapy/counselling Setting Patients were recruited from community health-care facilities and by media announcements at three different sites: north Wales around Bangor (a predominantly rural Welsh-speaking area); Cambridge (UK) and surrounding area; and Toronto, ON (Canada) a metropolis. No academic staff or students in the site in Cambridge were included No. included at baseline 145 patients (MBCTn = 76 and TAU n = 69) No. lost to follow-up 13/145 (8.97%); 95% of ITT, 97% of PP ITT analysis? Both ITT and PP |
Name of intervention MBCT Structured content? Manualised group-based MBCT training programme; daily homework exercises. The programme includes daily homework exercises that includes some form of guided (taped) or unguided awareness exercises, directed at increasing moment-by-moment non-judgemental awareness of bodily sensation, thoughts, and feelings, together with exercises designed to integrate application of awareness skills into daily life. This MBCT programme is based on integration of aspects of CBT for depression with components of MBSR programme designed to teach patients skills that allow individual to disengage from habitual (manic) dysfunctional cognitive routines. MBCT sessions were videotaped or audiotaped with the patient’s permission to allow monitoring of treatment Delivered by? Three experienced cognitive therapists who jointly developed the MBCT programme and previously led at least one cohort of recovered depressed patients through the MBCT programme Group intervention? Yes No. of patients per group (if applicable) Up to 12 No. of sessions Eight group sessions plus one individualised orientation session before the start of treatment, and four follow-up sessions Session duration and frequency 2-hour group session every week, followed by four follow-up sessions (duration not stated) at 1, 2, 3 and 4 months after the initial session, or bimonthly Total intervention duration 6 months Concurrent treatments ∼58% had one or more depression-related visit to GP ∼49% received counselling/psychotherapy/professional mental health support ∼45% received medication for depression ∼17% received other mental health contact ∼10% received psychiatric outpatient treatment |
Comparator name TAU Comparator details Patients received their usual treatment and were instructed to seek help from their family doctor, or other sources, as they normally would, if they encountered symptomatic deterioration or other difficulties over the course of the study Concurrent treatments ∼52% had one or more depression-related visit to GP ∼40% received medication for depression ∼34% received counselling/psychotherapy/professional mental health support ∼21% had other mental health contact ∼8% received psychiatric treatment as outpatients ∼2% received psychiatric treatment as day patients ∼2% received psychiatric treatment as inpatients |
Definition of relapse/recurrence Depression episode meeting DSM-III-R criteria, assessed by DSM-III-R (SCID). The assessment was undertaken by a clinical psychologist blind to the patient’s treatment condition. Intervention audiotaped and all who met criteria for major depression were evaluated by an independent blind assessor Other outcomes Medication use for depression Severity: Patients completed BDI at baseline and each follow-assessment HRSD measured at baseline and each follow-up assessment |
Intervention relapse rate For participants with three or more previous episodes: 22/55 (40%) at the end of 60 weeks of follow-up (ITT analysis). 18/49 (37%) at the end of 60 weeks of follow-up (PP analysis) Comparator relapse rate 33/50 (66%) in TAU at the end of 60 weeks of follow-up (ITT analysis) 33/50 (66%) at the end of 60 weeks of follow-up (PP analysis) p-value for difference between rates p < 0.01 Relative risk/odds ratio/hazard ratio Hazard ratio 0.473 (CI 0.267 to 0.836) (for the ITT analysis) Hazard ratio 0.419 (CI 0.229 to 0.766) (for the PP analysis). For participants with two previous episodes, there was no significant difference in relapse/recurrence between the two groups |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Wilkinson68 Year 2009 Country UK Full publication? Full Study design RCT |
Inclusion criteria Patients aged 60 years and over who had experienced an episode of major depression (ICD-10 criteria) within the last year that had remitted for at least 2 months on antidepressant medication; still taking antidepressants and scored < 10 on the MADRS Exclusion criteria MMSE < 24, current severe alcohol problems, bipolar disorder Previous treatment(s) received All patients had received antidepressant treatment during their index illness: 11/45 had received neuroleptics, 4/45 ECT and 9/45 other (not specified). Rates appear similar between groups Setting Patients recruited from GP surgeries and psychiatric services in Oxford and Southampton No. included at baseline 45 No. lost to follow-up 9/45 (20%) ITT analysis? Yes |
Name of intervention Brief group cognitive behaviour therapy (CBT-G) Structured content? CBT-G manual written for the study. Structured CBT-G course with full details of session content provided. All sessions supported by reading the therapy manual. Adherence assessed by videotaping sessions Delivered by? Clinical psychologist with diploma in cognitive therapy Group intervention? Yes No. of patients per group (if applicable) 4–6 No. of sessions Eight Session duration and frequency 90-minute weekly sessions (except weeks 7 and 9) Total intervention duration 10 weeks Concurrent treatments TAU; all participants were being treated with antidepressants equivalent to fluoxetine 20 mg or amitriptyline 150 mg |
Comparator name TAU Comparator details TAU (follow-up by GP or community mental health team and monitoring of antidepressant medication). Participants did not receive any psychological treatment Concurrent treatments All participants were being treated with antidepressants equivalent to fluoxetine 20 mg or amitriptyline 150 mg |
Definition of relapse/recurrence Rate of recurrence (MADRS ≥ 10) at 6 and 12 months after starting CBT-G Other outcomes Proportion of patients with BDI ≥ 12 patient satisfaction at the end of CBT-G treatment (CBT-G arm only) assessed by questionnaire |
Intervention relapse rate 1/18 (6%) at 6 months 5/18 (28%) at 12 months Comparator relapse rate 4/19 (21%) at 6 months 8/18 (44%) at 12 months p-value for difference between rates ‘Non-significant’ Relative risk/odds ratio/hazard ratio Relative risk = 0.34 (95% CI 0.03 to 3.35) at 6 months Relative risk = 0.70 (95% CI 0.26 to 1.94) at 12 months |
Ongoing studies
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Kuyken69 Year 2010 Country UK Full publication? Protocol only, study ongoing Study design RCT |
Inclusion criteria Diagnosis of recurrent major depressive disorder in full or partial remission according to the DSM-IV, with three or more previous major depressive episodes; aged 18 years or older; and on a therapeutic dose of antidepressant medication in line with the British National Formulary and NICE guidance; must have experienced three previous episodes when depression is the primary disorder and not secondary to substance abuse or bereavement Exclusion criteria Currently depressed, comorbid diagnoses of current substance abuse; organic brain damage; current/past psychosis, including bipolar disorder; persistent antisocial behaviour; persistent self-injury requiring clinical management/therapy; and formal concurrent psychotherapy Previous treatment(s) received Antidepressant medication Setting Participants recruited through primary care in south-west England No. included at baseline 420 (planned) |
Name of intervention MBCT Structured content? Fully manualised psychosocial intervention with the treatment rationale for each session outlined in full. Derived both from mindfulness-based stress reduction and from CBT. Session content includes psycho-education, teaching/discussion of key cognitive behavioural skills, guided mindfulness practices, review of weekly homework (40 minutes of mindfulness practice per day and generalisation of cognitive behavioural skills). Competence/adherence independently assessed using videotaped sessions Delivered by? Mental health professionals with extensive training in MBCT Group intervention? Yes No. of patients per group (if applicable) 12–15 No. of sessions Eight, plus four follow-up sessions Session duration and frequency Weekly for initial 8 weeks. Four follow-up sessions over 2 years (first at 3–5 weeks’ follow-up) Total intervention duration Unclear Concurrent treatments Initial antidepressant medication, tapered with GP support |
Comparator name Maintenance antidepressants (m-ADMs) Comparator details Patients encouraged to continue to take a therapeutic level of antidepressants for the 2-year duration of the trial. Medication adherence monitored through patients’ self-report and through manual checks of GP practice databases at 12- and 24-month follow-ups Concurrent treatments None |
Definition of relapse/recurrence Rate and time to relapse/recurrence, assessed using the SCID designed for longitudinal studies of depression. Relapse/recurrence defined as having a major depressive episode (a score of 5 for two consecutive weeks) at any time during the 24-month follow-up period Other outcomes Residual depressive symptoms (HRSD and BDI); psychiatric comorbidity (SCID); medical comorbidities; depression-free days (SCID); QoL (EQ-5D) |
Protocol only – no results currently available |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Watkins106 Year 2010 Country UK Full publication? No – protocol only, study ongoing Study design RCT |
Inclusion criteria History of at least two previous episodes of major depression; not currently depressed; aged 18 years or over Exclusion criteria Current psychotherapy; psychosis; current substance/alcohol use No. included at baseline 70 (planned sample size) |
Name of intervention Cognitive training self-help in addition to TAU Structured content? Initial meeting lasting approx 1.5 hours, during which the researcher will explain the rationale for why cognitive training is helpful and then practice relaxation or the cognitive training paradigm Total intervention duration 6 months |
Comparator name Relaxation training self-help in addition to TAU |
Definition of relapse/recurrence HRSD measured at baseline plus 2, 5 and 8 months post baseline Other outcomes BDI-II measured at baseline plus 2, 5 and 8 months post baseline |
Protocol only – no results currently available |
| Study details | Participant details | Intervention details | Comparator details | Outcomes measured | Results |
|---|---|---|---|---|---|
|
Author Williams71 Year 2010 Country UK Full publication? Protocol only, study ongoing Study design Multicentre RCT (Oxford and Bangor) |
Inclusion criteria Age 18–70 years; meeting DSM-IV criteria for history of recurrent major depression; meeting NIMH guidelines for recovery or remission at the time of baseline assessment; informed consent; consent received from participant’s GP Exclusion criteria History of schizophrenia, schizoaffective disorder, bipolar disorder, current severe substance abuse, organic mental disorder, pervasive developmental delay, a primary diagnosis of obsessive–compulsive disorder or eating disorder, or regularly self-harm; positive continuing response to CBT; receiving psychotherapy or counselling more than once per month; cannot complete baseline assessment (e.g. difficulties with English, visual impairment or cognitive difficulties) Setting Participants to be recruited through advertisements in the community, in clinics and GP surgeries, as well as through referrals from GPs and mental health clinicians No. included at baseline Aim to recruit 375 participants, with final sample 300 after accounting for attrition ITT analysis? Yes |
Name of intervention Structured content? MBCT and CPE, both consist of 8 weekly classes of 2 hours’ duration. MBCT is a manualised treatment programme that combines training in mindfulness meditation with cognitive therapy techniques. As well as sessions, advise participants to spend about an hour per day on home-based practice which includes regular meditation practice and smaller tasks aimed at cultivating mindfulness in everyday life. CPE includes all of the elements of the MBCT programme except those that are intended to support participants in experientially cultivating mindfulness Delivered by? Four therapists, each led six classes Group intervention? Yes No. of patients per group (if applicable) 12 No. of sessions 10 Session duration and frequency Two-hour sessions, weekly for 8 weeks, then one additional session at 6–8 weeks and one 6 months after treatment Total intervention duration Eight weeks, plus 6-month follow-up session |
Comparator name TAU |
Definition of relapse/recurrence Time to relapse or recurrence meeting DSM-IV criteria for major depression, assessed by SCID at 3, 6, 9 and 12 months. ‘Return to treatment’ considered a relapse or recurrence if the participant experienced exacerbation of symptoms that would have met criteria for major depression in the absence of immediate treatment Other outcomes Severity of depression and hopelessness (HSRD, BDI, Beck Hopelessness Scale). Cognitive measures relevant to risk of relapse or recurrence (mindfulness, self-compassion, rumination, self-discrepancy, autobiographical memory and executive capacity before and immediately after treatment and at the end of the follow-up) |
Protocol only – no results currently available |
Appendix 4 Quality assessment of effectiveness evaluations
| Study identification: | Katon et al.45,57–60 | |
|---|---|---|
| Study design: | Individual RCT | |
| Guidance topic: | Low-intensity interventions for the prevention of relapse of depression | |
| Assessed by: | MR (checked ADA) | |
| Section 1: Population | ||
|
1.1 Is the source population or source area well described? Was the country (e.g. developed or non-developed, type of health-care system), setting (primary schools, community centres, etc.), location (urban, rural), population demographics, etc. adequately described? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Four primary care clinics of one HMO in western Washington, DC, USA Predominantly female (> 70%), white (∼90%), college educated (> 85%), employed (∼78%) |
|
1.2 Is the eligible population or area representative of the source population or area? Was the recruitment of individuals/clusters/areas well defined (e.g. advertisement, birth register)? Was the eligible population representative of the source? Were important groups under-represented? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments May not be generalisable to more diverse racial and ethnic groups, patients from lower socioeconomic status, other types of primary care setting |
|
1.3 Do the selected participants or areas represent the eligible population or area? Was the method of selection of participants from the eligible population well described? What percentage of selected individuals/clusters agreed to participate? Were there any sources of bias? Were the inclusion/exclusion criteria explicit and appropriate? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments Patients with potential high risk for relapse were assessed for eligibility using SCID: 12.4% refused to enrol. 8% of eligible patients refused baseline interview |
| Section 2: Method of allocation to intervention (or comparison) | ||
|
2.1 Allocation to intervention (or comparison). How was selection bias minimised? Was allocation to exposure and comparison randomised? Was it truly random (++) or pseudo-randomised (+) (e.g. consecutive admissions)? If not randomised, was significant confounding likely (−) or not (+)? If a crossover, was order of intervention randomised? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Computer-generated randomisation sequence in blocks of eight |
|
2.2 Were interventions (and comparisons) well described and appropriate? Were intervention/s and comparison/s described in sufficient detail (i.e. enough for study to be replicated)? Was comparison/s appropriate (e.g. usual practice rather than no intervention)? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments Multifaceted relapse prevention programme including patient education, visits with a depression specialist, telephone monitoring and follow-up Focus on medication maintenance and increased self-efficacy Compared with usual care |
|
2.3 Was the allocation concealed? Could the person(s) determining allocation of participants/clusters to intervention or comparison groups have influenced the allocation? Adequate allocation concealment (++) would include centralised allocation or computerised allocation systems |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Computerised allocation |
|
2.4 Were participants and/or investigators blind to exposure and comparison? Were participants and investigators – those delivering and/or assessing the intervention – kept blind to intervention allocation? (triple or double blinding score [++]) If lack of blinding is likely to cause important bias, score (−) |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments Unblinded comparison against usual care. There was no ‘sham’ relapse prevention, so not possible to separate the effects of additional attention from programme content. Telephone interviewer was blinded to randomisation status |
|
2.5 Was the exposure to the intervention and comparison adequate? Is reduced exposure to intervention or control related to the intervention (e.g. adverse effects leading to reduced compliance) or fidelity of implementation (e.g. reduced adherence to protocol)? Was lack of exposure sufficient to cause important bias? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments 93.3% of patients attended both face-to-face visits; 79.9% completed all three telephone follow-ups |
|
2.6 Was contamination acceptably low? Did any in the comparison group receive the intervention or vice versa? If so, was it sufficient to cause important bias? If a crossover trial, was there a sufficient washout period between interventions? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments No crossovers reported |
|
2.7 Were other interventions similar in both groups? Did either group receive additional interventions or have services provided in a different manner? Were the groups treated equally by researchers or other professionals? Was this sufficient to cause important bias? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Both groups encouraged to maintain medication and had the option to self-refer to a mental health provider |
|
2.8 Were all participants accounted for at study conclusion? Were those lost-to-follow-up (i.e. dropped or lost pre-/during/post intervention) acceptably low (i.e. typically < 20%)? Did the proportion dropped differ by group? For example, were dropouts related to the adverse effects of the intervention? |
[ ] ++ [ ] + [•] – [ ] NR [ ] NA |
Comments Over 12 months, 10.3% of intervention and 20.8% of control patients missed follow-up interviews |
|
2.9 Did the setting reflect usual UK practice? Did the setting in which the intervention or comparison was delivered differ significantly from usual practice in the UK? For example, did participants receive intervention (or comparison) condition in a hospital rather than a community-based setting? |
[ ] ++ [ ] + [•] – [ ] NR [ ] NA |
Comments US primary care HMO setting |
|
2.10 Did the intervention or control comparison reflect usual UK practice? Did the intervention or comparison differ significantly from usual practice in the UK? For example, did participants receive intervention (or comparison) delivered by specialists rather than GPs? Were participants monitored more closely? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments Usual care as described similar to UK primary care, except option to self-refer to mental health specialist. Intervention could conceivably be implemented in UK primary care |
| Section 3: Outcomes | ||
|
3.1 Were outcome measures reliable? Were outcome measures subjective or objective (e.g. biochemically validated nicotine levels [++] vs self-reported smoking [−])? How reliable were outcome measures (e.g. inter- or intra-rater reliability scores)? Was there any indication that measures had been validated (e.g. validated against a gold standard measure or assessed for content validity)? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Relapse/recurrence assessed with SCID and Longitudinal Interval Follow-up Evaluation. Symptoms using SCL-20. Medication adherence using automated pharmacy data |
|
3.2 Were all outcome measurements complete? Were all/most study participants who met the defined study outcome definitions likely to have been identified? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments |
|
3.3 Were all important outcomes assessed? Were all important benefits and harms assessed? Was it possible to determine the overall balance of benefits and harms of the intervention vs comparison? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments |
|
3.4 Were outcomes relevant? Where surrogate outcome measures were used, did they measure what they set out to measure? (e.g. a study to assess impact on physical activity assesses gym membership – a potentially objective outcome measure – but is it a reliable predictor of physical activity?) |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Measured attitudes about antidepressants, side effect management, self-management practices and medication use alongside relapse |
|
3.5 Were there similar follow-up times in exposure and comparison groups? If groups are followed for different lengths of time then more events are likely to occur in the group followed up for longer, distorting the comparison Analyses can be adjusted to allow for differences in length of follow-up (e.g. using person-years) |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments Planned length of follow-up same for both groups, but greater loss to follow-up in usual-care group. Used imputation models to adjust for missing data |
|
3.6 Was follow-up time meaningful? Was follow-up long enough to assess long-term benefits/harms? Was it too long, for example participants lost to follow-up? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments 12 months |
| Section 4: Analysis | ||
|
4.1 Were exposure and comparison groups similar at baseline? If not, were these adjusted? Were there any differences between groups in important confounders at baseline? If so, were these adjusted for in the analyses (e.g. multivariate analyses or stratification)? Were there likely to be any residual differences of relevance? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Similar in terms of age, sex, level of education, race, employment status, SCL-depression, recurrent depression and antidepressant use. Slight difference in major depression within last 2 years (intervention 78.5%; control 87.5%) |
|
4.2 Was ITT analysis conducted? Were all participants (including those that dropped out or did not fully complete the intervention course) analysed in the groups (i.e. intervention or comparison) to which they were originally allocated? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments Missing response data were imputed using baseline values. No sensitivity analysis |
|
4.3 Was the study sufficiently powered to detect an intervention effect (if one exists)? A power of 0.8 (i.e. it is likely to see an effect of a given size if one exists, 80% of the time) is the conventionally accepted standard Is a power calculation presented? If not, what is the expected effect size? Is the sample size adequate? |
[ ] ++ [ ] + [ ] – [•] NR [ ] NA |
Comments |
|
4.4 Were the estimates of effect size given or calculable? Were effect estimates (e.g. relative risks, absolute risks) given or possible to calculate? |
[ ] ++ [ ] + [ ] – [•] NR [ ] NA |
Comments |
|
4.5 Were the analytical methods appropriate? Were important differences in follow-up time and likely confounders adjusted for? If a cluster design, were analyses of sample size (and power), and effect size performed on clusters (and not individuals)? Were subgroup analyses prespecified? |
[•] ++ [ ] + [ ] – [ ] NR [ ] NA |
Comments Where necessary, generalised models were used to account for repeated measures |
|
4.6 Was the precision of intervention effects given or calculable? Were they meaningful? Were CIs and/or p-values for effect estimates given or possible to calculate? Were CIs wide or were they sufficiently precise to aid decision-making? If precision is lacking, is this because the study is underpowered? |
[ ] ++ [•] + [ ] – [ ] NR [ ] NA |
Comments CIs and/or p-values reported for some outcomes, although not for relapse/recurrence |
| Section 5: Summary | ||
|
5.1 Are the study results internally valid (i.e. unbiased)? How well did the study minimise sources of bias (i.e. adjusting for potential confounders)? Were there significant flaws in the study design? |
[ ] ++ [•] + [ ] – |
Comments Not possible to separate the effects of attention from programme content. Validity of imputed data uncertain, although similarity of relapse outcomes would indicate bias favouring intervention is unlikely |
|
5.2 Are the findings generalisable to the source population (i.e. externally valid)? Are there sufficient details given about the study to determine if the findings are generalisable to the source population? Consider: participants, interventions and comparisons, outcomes, resource and policy implications |
[ ] ++ [•] + [ ] – |
Comments May not be generalisable to more diverse racial and ethnic groups, patients from lower socioeconomic status, UK primary care setting |
Appendix 5 Quality assessment of economic evaluations
Simon et al. (2002)60
| Study question | Grade | Comments |
|---|---|---|
| Costs and effects examined | Yes | |
| Alternatives compared | Yes | |
| The viewpoint(s)/perspective of the analysis is clearly stated (e.g. NHS, society) | Yes | Strict health insurer perspective used |
| Selection of alternatives | ||
| All relevant alternatives are compared (including do nothing if applicable) | Yes | Multifaceted intervention compared with standard care |
| The alternatives being compared are clearly described (who did what, to whom, where and how often) | Yes | |
| The rationale for choosing the alternative programmes or interventions compared is stated | Yes | |
| Form of evaluation | ||
| The choice of economic evaluation is justified in relation to the questions addressed | Yes | |
| If a cost minimisation design is chosen, have equivalent outcomes been adequately demonstrated? | NA | |
| Effectiveness data | ||
| The source(s) of effectiveness estimates used are stated (e.g. single study, selection of studies, systematic review, expert opinion) | Yes | Single study used |
| Effectiveness data from RCT or review of RCTs | Yes | From RCT |
| Potential biases identified (especially if data not from RCTs) | Yes | |
| Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of studies) | NA | Single RCT used |
| Costs | ||
| All the important and relevant resource use included | No | Costs of out of plan services not measured |
| All the important and relevant resource use measured accurately (with methodology) | Yes | Methodology given, but results not always reported |
| Appropriate unit costs estimated (with methodology) | Yes | |
| Unit costs reported separately from resource use | Yes | |
| Productivity costs treated separately from other costs | NA | |
| The year and country to which unit costs apply is stated with appropriate adjustments for inflation and/or currency conversion | Yes | USD 1997–8 |
| Benefit measurement and valuation | ||
| The primary outcome measure(s) for the economic evaluation is clearly stated | Yes | Cost per depression-free day |
| Methods to value health states and other benefits are stated | No | Value of depression-free year assumed to be 0.2 to 0.4 QALYs higher than fully symptomatically depressed year |
| Details of the individuals from whom valuations were obtained are given | Yes | |
| Decision modelling | ||
| Details of any decision model used are given (e.g. decision tree, Markov model) | NA | Not model based |
| The choice of model used and the key input parameters on which it is based are adequately detailed and justified | NA | |
| All model outputs described adequately | NA | |
| Discounting | ||
| Discount rate used for both costs and benefits | No | Discount rates not applied to either costs or benefits. Very short time horizon of study (12 months) |
| Do discount rates accord with NHS guidance? | NA | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
| Details of statistical tests and CIs are given for stochastic data | Yes | Bootstrapping used to estimate CIs for costs and number of depression-free days |
| Uncertainty around cost-effectiveness expressed (e.g. CI around ICER CEACs) | No | |
| Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | No | |
| Stochastic analysis of decision models | ||
| Are all appropriate input parameters included with uncertainty? | NA | Not model based |
| Is second-order uncertainty (uncertainty in means) included rather than first order (uncertainty between patients)? | NA | |
| Are the probability distributions adequately detailed and appropriate? | NA | |
| Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | NA | |
| Deterministic analysis | ||
| The approach to sensitivity analysis is given (e.g. univariate, threshold analysis, etc.) | No | |
| The choice of variables for sensitivity analysis is justified | No | |
| The ranges over which the variables are varied are stated | No | |
| Presentation of results | ||
| Incremental analysis is reported using decision rules | Yes | Results converted to QALYs and compared with other approved interventions |
| Major outcomes are presented in a disaggregated as well as aggregated form | Yes | |
| Applicable to the NHS setting | No | Not all relevant cost captured |
Kuyken et al. (2008)63
| Study question | Grade | Comments |
|---|---|---|
| Costs and effects examined | Yes | |
| Alternatives compared | Yes | |
| The viewpoint(s)/perspective of the analysis is clearly stated (e.g. NHS, society) | Yes | Broad perspective including all hospital, community health and social services, as well as productivity losses due to time off work |
| Selection of alternatives | ||
| All relevant alternatives are compared (including do nothing if applicable) | Yes | Usual care (maintenance antidepressant medication) vs mindfulness-based CBT |
| The alternatives being compared are clearly described (who did what, to whom, where and how often) | Yes | |
| The rationale for choosing the alternative programmes or interventions compared is stated | Yes | |
| Form of evaluation | ||
| The choice of economic evaluation is justified in relation to the questions addressed | Yes | |
| If a cost minimisation design is chosen, have equivalent outcomes been adequately demonstrated? | NA | |
| Effectiveness data | ||
| The source(s) of effectiveness estimates used are stated (e.g. single study, selection of studies, systematic review, expert opinion) | Yes | Single study |
| Effectiveness data from RCT or review of RCTs | Yes | Single RCT |
| Potential biases identified (especially if data not from RCTs) | Yes | |
| Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of studies) | NA | Based on single study |
| Costs | ||
| All the important and relevant resource use included | Yes | Patient’s and family expenses and costs of informal care excluded |
| All the important and relevant resource use measured accurately (with methodology) | Yes | |
| Appropriate unit costs estimated (with methodology) | Yes | |
| Unit costs reported separately from resource use | No | |
| Productivity costs treated separately from other costs | Yes | |
| The year and country to which unit costs apply is stated with appropriate adjustments for inflation and/or currency conversion | Yes | UK 2005–6 converted to international dollars using PPP exchange rate of 0.6 from World Bank |
| Benefit measurement and valuation | ||
| The primary outcome measure(s) for the economic evaluation is clearly stated | Yes | Cost per depressive relapse/recurrence prevented |
| Methods to value health states and other benefits are stated | NA | Health states not valued |
| Details of the individuals from whom valuations were obtained are given | NA | |
| Decision modelling | ||
| Details of any decision model used are given (e.g. decision tree, Markov model) | NA | Not model based |
| The choice of model used and the key input parameters on which it is based are adequately detailed and justified | NA | |
| All model outputs described adequately | NA | |
| Discounting | ||
| Discount rate used for both costs and benefits | No | No discounting of costs or benefits |
| Do discount rates accord with NHS guidance? | NA | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
| Details of statistical tests and CIs are given for stochastic data | Yes | |
| Uncertainty around cost-effectiveness expressed (e.g. CI around ICER CEACs) | Yes | |
| Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | No | |
| Stochastic analysis of decision models | ||
| Are all appropriate input parameters included with uncertainty? | NA | Not model based |
| Is second-order uncertainty (uncertainty in means) included rather than first order (uncertainty between patients)? | NA | |
| Are the probability distributions adequately detailed and appropriate? | NA | |
| Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | NA | |
| Deterministic analysis | ||
| The approach to sensitivity analysis is given (e.g. univariate, threshold analysis, etc.) | NA | No sensitivity analysis conducted |
| The choice of variables for sensitivity analysis is justified | NA | |
| The ranges over which the variables are varied are stated | NA | |
| Presentation of results | ||
| Incremental analysis is reported using decision rules | Yes | CEAC used |
| Major outcomes are presented in a disaggregated as well as aggregated form | No | |
| Applicable to the NHS setting | No | Results reported using disease-specific outcome measures |
Appendix 6 Review protocol
Title of the project
The clinical effectiveness and cost-effectiveness of low-intensity psychological interventions for the secondary prevention of relapse after depression: a systematic review and decision analytical model.
Name of TAR team and ‘lead’
CRD/CHE Technology Assessment Group (Centre for Reviews and Dissemination/Centre for Health Economics), University of York.
Mark Rodgers
Research Fellow Centre for Reviews and Dissemination, University of York, Heslington, York YO10 5DD Tel: (01904) 321086 Fax: (01904) 321041
Email: mr14@york.ac.uk
Stephen Palmer Senior Research Fellow Centre for Health Economics University of York, Heslington, York YO10 5DD Tel: (01904) 321434 Fax: (01904) 321402
Email: sjp21@york.ac.uk
Plain English summary
Depression is a common condition defined by persistent depressed mood and loss of interest in activities. Even after successful treatment, a high proportion of people will go on to have a relapse of their depression. People with depression may be treated with medicines, psychological interventions or both. Psychological interventions can be classed as ‘high-intensity’ or ‘low-intensity’ depending on the amount of direct contact between the patient and a health professional. Low-intensity psychological interventions can include approaches such as computer-delivered treatments and self-help books, for which people may or may not receive personal support. The aim of this project is specifically to determine the clinical effectiveness and cost-effectiveness of low-intensity psychological interventions in preventing relapse of depression.
Decision problem
Background
Depression
Depression can refer to a range of mental health problems primarily characterised by persistent depressed mood and loss of interest in activities, among other potential symptoms. 1 A World Health Organization cross-sectional survey revealed the global one year prevalence of a depressive episode to be 3.2%. 2 The prevalence is greater still in people with other medical conditions (10–14% of patients receiving general hospital care). 3 Neuropsychiatric disorders account for a third of all years lost to disability (YLD), with unipolar major depressive disorder alone accounting for 11% of global YLDs. 2
Initial treatment and relapse of depression
Over 80% of patients diagnosed with depression receive psychological, pharmacological or combined treatment in primary care. 4 The objective of treatment is to achieve remission of depressive symptoms. However, the risk of relapse after remission is significant, and has been reported as 50% among patients having experienced one episode of major depression and 70% and 90% after two and three episodes respectively. 5 At least 10% of patients have persistent or chronic depression. 6
Low-intensity interventions
In general, people with depression tend to prefer psychological and psychosocial interventions to pharmacological interventions. 7 However, high-intensity psychological and psychosocial therapies (e.g. cognitive behavioural therapy, problem solving, counselling) that involve one-to-one therapy with a health professional over extended periods of time, are resource intensive. Consequently, less intensive therapies and innovative delivery formats such as group-based work have been developed. ‘Self-directed interventions’ may refer to a variety of psychological treatments in which there is no or only a low level of therapist involvement, and include computer-delivered treatment and bibliotherapy among other intervention technologies. The 2009 NICE guideline on depression4 refers to these as ‘low-intensity psychosocial interventions’, and this is the term that will be used throughout this protocol.
The NICE guideline provides clinical evidence on three main forms of low-intensity therapy:
-
Computerised cognitive behavioural therapy (CCBT) provides a structured programme of care based on the principles of standard therapist-delivered CBT but is delivered via a CD-ROM/DVD or the internet. Where CCBT is delivered as a primary intervention with minimal therapist involvement, it is considered a low-intensity intervention.
-
Guided self-help involves the use of evidence-based self-help books or manuals aimed specifically at depression. Guided self-help is distinct from ‘pure’ self-help in that a healthcare professional (or para-professional) facilitates the use of the material by introducing, monitoring and assessing the outcome of the intervention.
-
Physical activity programmes have been defined as any structured physical activity with a recommended frequency, intensity and duration when used for depression. This could be aerobic (e.g. running/jogging, dancing) or anaerobic (e.g. resistance training), and be supervised or unsupervised, and undertaken in a group or individually.
The NICE clinical practice guidelines recommend that CCBT, individual guided self-help and structured group physical activity programmes be considered for people with persistent subthreshold depressive symptoms or mild to moderate depression. Recommended duration of CCBT and guided self-help is 9 to 12 weeks including follow-up. Group physical activity with practitioner support is recommended for three sessions per week over 10 to 14 weeks. 4
Though the NICE guidance covers low-intensity psychological interventions, it does not provide a clear definition of what constitutes ‘low-intensity’ treatment. However, recent guidance produced by the NHS Improving Access to Psychological Therapies (IAPT) programme states that “A low-intensity intervention. . .may use simple or ‘single strand’ approaches that are less complex to undertake than formal psychotherapy; contact with people is generally briefer than in other forms of therapy and can be delivered by paraprofessionals or peer supporters using non-traditional methods such as telephone or the internet”. 8 Emphasis is on interventions delivered by ‘psychological well-being practitioners’ without formal healthcare professional or CBT therapist qualifications. 9 Though the IAPT guidance states that there is no arbitrary session limit, evidence from the IAPT demonstration site showed that the mean number of low-intensity CBT-based interventions was around five per person, though there was considerable variability around this figure. 8
Although the effectiveness of low-intensity interventions has been extensively evaluated to treat primary symptoms of psychological difficulties,10–12 there has been substantially less research examining the use of these interventions as a relapse prevention strategy.
Objective
The main aims of this project are to determine the clinical effectiveness and cost-effectiveness of low-intensity psychological or psychosocial interventions to prevent relapse in patient with depression. Where possible the relative efficacy of different types of intervention will be determined, as will the cost-effectiveness of these alternatives to current standard care.
As the definition of ‘low-intensity’ psychological intervention is somewhat contested, and the resources of the review are limited, the review will be conducted in two parts:
-
A: All evaluations of ‘low-intensity’ interventions that can be delivered by paraprofessionals or peer supporters as defined by the IAPT programme will be indentified and reviewed. These will not be restricted by length of treatment or number of sessions. These will be synthesised in a full systematic review of clinical effects.
-
B: All relevant evaluations of interventions involving qualified health professionals (e.g. clinicians, CBT therapists) will be included if they involve less than six hours of contact per patient. As a minimum, the literature in this area will be described and classified in a scoping review. However, should resources allow, these studies will also be extracted and synthesised as part of the full systematic review.
Report methods for synthesis of evidence of clinical effectiveness
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles recommended in CRD’s guidance for undertaking reviews in health care13 and the PRISMA statement. 14
Search strategy
The search will comprise the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of reviews and retrieved papers.
For clinical effects, the following databases will be searched: BIOSIS, CENTRAL, EMBASE, MEDLINE, MEDLINE in process, PsycINFO, Science Citation Index (SCI) and Social Science Citation Index.
In addition, guidelines and reviews will be identified using: Clinical Evidence, Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA), National Institute for Health and Clinical Evidence (NICE) website, National Library for Health (NLH) Guidelines Finder, and SIGN Guidelines. These reviews and guidelines will be used to identify primary studies.
No language or date of publication restrictions will be placed on the search. Details of an example search strategy are presented in Appendix 1.
The bibliographies of all relevant reviews and guidelines and all included studies will be checked for further potentially relevant studies. In addition, citation searching will be undertaken for selected papers.
Inclusion and exclusion criteria
Titles and abstracts will be examined for relevance by two reviewers independently; all potentially relevant papers meeting the inclusion criteria below will be ordered. All full papers will be screened by two reviewers independently, relevance to the review and the decision to include studies or not will be made according to the inclusion criteria detailed below. Disagreements will be resolved by consensus.
Population
Patients who have received treatment for depression will be included. Our initial scoping of the literature identified only a small number of studies meeting criteria. We will not, therefore, restrict inclusion to studies in which depression was established using a gold-standard structured clinical interview. Studies defining depression on the basis of scores above a cut-off point on a recognised psychometric measure or on the basis of unaided clinical diagnosis will also be considered. The effects of the inclusion of these studies will be examined. Trials of participants with bipolar disorder will be excluded, as will studies of children.
Interventions
In part A of the clinical efficacy review, all relevant evaluations of ‘low-intensity’ interventions as defined by the IAPT programme8,9 will be indentified and reviewed. This will incorporate any unsupported psychological/psychosocial interventions or any supported interventions that do not involve highly qualified health professionals such as clinicians or CBT therapists. Such interventions may involve support from paraprofessionals, peer supporters, physical trainers, case managers (as in collaborative care models), or no personal support at all (e.g. entirely computerised interventions). ‘Highly qualified professionals’ would include clinicians who in most instances will have a core professional qualification (e.g., psychiatrist, clinical psychologist, mental health nurse) and has received formal, specialist training in the delivery of complex psychological interventions (e.g., 16+ session CBT, psychodynamic psychotherapy, systematic therapy etc.) ‘Paraprofessionals’ would include people who do not have a core profession and do not have specialist training in complex psychological interventions, though may have some training in less complex interventions. Inclusion will not be restricted by length of treatment or number of sessions. We expect that studies assessed in part A will include various methods of delivering the intervention (e.g., face-to-face, telephone, email, computer, web-based forums etc.); no exclusions will be made on the basis of the mode of delivery.
In part B of the review, all relevant evaluations of interventions involving qualified health professionals (e.g. clinician, CBT therapist) will be included if they involve less than six hours of contact per patient. For group treatment, contact estimates per patient will be calculated by the mean number of patients per group (with adjustments as necessary if there is more than one therapist). As a minimum, the characteristics of the literature in this area will be described and classified in a scoping review. This is likely to include interventions such as mindfulness-based cognitive therapy delivered in a group format for depressive relapse, computerised CBT supported by a clinician, and brief maintenance or booster sessions of CBT taking place during a remission phase. Should resources allow, these studies will be extracted and synthesised alongside studies identified in part A of the systematic review.
High-intensity psychological interventions requiring ongoing interaction with a mental health professional (e.g. CBT, behavioural activation, problem solving therapy and couples therapy) will be excluded.
Studies evaluating pharmacotherapy alone (including tricyclic antidepressants, SSRIs, SNRIs, anxiolytic medication, mood stabilizers and others) will be excluded from the review of clinical effectiveness, as will studies of alternative and complementary treatment methods.
Comparators
Study inclusion will not be restricted by type of comparator treatment and can include no treatment (including waiting list control), placebo, psychological or pharmacological interventions.
Outcomes
Studies reporting outcomes related to relapse or recurrence (e.g. relapse rate, time to relapse, and severity of relapse episode) after initial treatment success will be included. Other relevant outcomes such as social function and quality of life measures will be recorded where reported.
Study designs
Randomised, quasi-randomised and non-randomised studies with concurrent controls will be considered for inclusion. Animal models, preclinical and biological studies, reviews, editorials, and opinions will be excluded.
Translations of non-English-language papers and additional details of studies published only as meeting abstracts will be obtained where time and budget constraints allow.
Data extraction strategy
Data will be extracted independently by one reviewer using a standardised data extraction form and checked by another. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary. If time constraints allow, attempts will be made to contact authors for any missing data. Data from multiple publications of the same study will be extracted as a single study. Extraction will include data on: patient characteristics (e.g. age, gender, length of treated and untreated depression, number of previous episodes, age of onset, baseline severity of depression, previous treatment, comorbid conditions, concomitant treatment use), intervention (e.g. intervention type, length of treatment, whether it is structured/manualised, who delivers it and for how long, level of support provided, and the number of sessions, if any, attended), comparison (e.g. frequency of follow-up, additional interventions), study quality, and reported outcomes pertinent to the review (e.g. relapse, social function, adherence, quality of life).
Quality assessment strategy
The internal and external validity of all included studies will be assessed according to the quality appraisal checklist for quantitative intervention studies described in NICE’s guide to methods for developing guidance in public health. 15 Study quality will be incorporated into the synthesis by comparing quality scores across studies and where possible focusing on the findings from evidence with less potential for bias (e.g. studies with low attrition rates, using randomisation, blinding, etc).
Methods of analysis/synthesis
Given the expected clinical and methodological heterogeneity of included studies, in the first instance data will be tabulated and discussed in a narrative synthesis. Studies may be grouped according to participant (e.g. comorbid conditions) or intervention (e.g. level of support/guidance) characteristics. As well as the main effects of each type of low-intensity intervention, the impact of factors such as previous treatment(s) for depression and duration of preventative treatment/length of contact time will be investigated.
If appropriate for any subgroups of studies, meta-analysis will be employed to estimate a summary measure of effect on relevant outcomes based on intention to treat analyses. Meta-analysis will be carried out using fixed or random effects models, using appropriate software. Heterogeneity will be explored through consideration of the study populations, methods and interventions, by visualisation of results and, in statistical terms, by the chi-squared test for homogeneity and the I2 statistic. Analyses will be conducted using the stand-alone software package META-ANALYST.
Report methods for synthesising evidence of cost-effectiveness
Identifying and systematically reviewing published cost-effectiveness studies
Systematic searches will be undertaken to identify existing published studies reporting the cost-effectiveness of low intensive psychological interventions for the secondary prevention of relapse after depression. The following databases will be searched: MEDLINE, EMBASE, CENTRAL and EconLit. In addition, searches of NHS EED will be carried out, along with a search of the Economics Working Papers archive (IDEAS).
A broad range of studies will be considered in the assessment of cost-effectiveness including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compare two or more options and consider both costs and consequences (including cost-effectiveness, cost-utility and cost–benefit analyses) will be included in the review of economic literature.
Evaluation of costs and cost-effectiveness
The quality of the cost-effectiveness studies will be assessed according to a checklist updated from that developed by Drummond et al (2005)16 and Philips et al. (2002). 17 This checklist will reflect the criteria for economic evaluation detailed in the methodological guidance developed by the National Institute for Clinical for Health and Excellence (NICE). This information will be tabulated and summarised within the text of the report. In particular information will be extracted on the comparators, study population, main analytic approaches (e.g. patient-level analysis/decision-analytic modelling), primary outcome specified for the economic analysis, details of adjustment for quality-of life, direct costs (medical and non-medical) and productivity costs, estimates of incremental cost-effectiveness and approaches to quantifying decision uncertainty (e.g. deterministic/probabilistic sensitivity analysis).
The review will examine existing decision-analytic models in detail, with the aim of identifying important structural assumptions, highlighting key areas of uncertainty and outlining the potential issues of generalising from the results of existing models. This review will be used to identify the central issues associated with adapting existing decision models to address the specific research question posed and to assist in the development of a new decision model drawing on the issues identified in the clinical effectiveness and cost-effectiveness review. The presence of any data gaps (e.g. resource use data) that may need to be filled during the development of the model will be identified and used to inform additional searches where required.
Subject to the availability of suitable data, a new decision-analytic model will be developed to estimate the cost-effectiveness of self-directed interventions.
Development of a new decision-analytic model
Subject to the availability of appropriate data, a decision-analytic model will be developed to estimate the cost-effectiveness of alternative low-intensity psychological interventions for the prevention of relapse in adults treated for depression. The interventions evaluated will be informed by the results of the clinical effectiveness review.
The specific objectives of the cost-effectiveness analysis are:
-
To structure an appropriate decision model to evaluate the long-term cost-effectiveness of relapse prevention in adults treated for depression.
-
To populate this model using the most appropriate data identified systematically from published literature and routine data sources.
-
To relate intermediate outcomes from the clinical effectiveness review (e.g. relapse rates, time to relapse and severity of relapse) to final health outcomes, expressed in terms of quality-adjusted life years (QALYs). This is necessary in order to provide decision makers with an indication of the health gain achieved by each intervention, relative to its additional cost, in units which permit comparison with other uses of health service resources.
-
To estimate the mean cost-effectiveness of alternative low-intensity psychological interventions based on an assessment of NHS and Personal Social Service costs and QALYs.
-
To explore heterogeneity in the cost-effectiveness estimates using subgroup analysis where appropriate.
-
To characterise the uncertainty in the data used to populate the model and to present the uncertainty in these results to decision makers. A probabilistic model will be developed which requires that each input in the model is entered as an uncertain, rather than a fixed, parameter. Using Monte Carlo simulation, this parameter uncertainty, is translated into uncertainty in the overall results. This ultimately helps decision makers understand the probability that, in choosing to fund an intervention, they are making the wrong decision – that is, decision uncertainty. This is presented using cost-effectiveness acceptability curves which show the probability that each intervention is cost-effective conditional on a range of possible threshold values which NHS decision makers attach to an additional QALY.
The model structure will be developed during the review period. However, it is anticipated that the model will take the form of a Markov model to capture the longer-term impact of periods of relapse and remission in terms of associated resource utilisation and quality of life.
It is anticipated that additional systematic searches will be necessary to populate specific parameter inputs and assumptions applied in the longer-term Markov model. In order to estimate QALYs required for the cost-effectiveness analysis, it will be necessary to systematically search for appropriate published utility or preference scores related to depression (and remission from depression). Additional evidence may also be needed to supplement the proposed clinical effectiveness review to consider the potential cost-effectiveness of low-intensity psychological interventions compare to other potentially relevant strategies (e.g. pharmacological management). Should this additional evidence be required then this will be sought from previously published meta-analyses and the results presented as a separate scenario.
Resource utilisation will reflect the inputs associated with the psychological interventions themselves, medication and depression-related events. Resource use data will be informed from the clinical effectiveness and cost-effectiveness reviews and expert clinical opinion where necessary. These data will be combined with national sources of cost data (e.g. NHS Reference Costs, British National Formulary etc.) in order to estimate the total costs associated with each strategy considered.
To consider future research priorities in the NHS, the model will also be used to undertake analyses of the expected value of information. The expected value of perfect information (EVPI) will be estimated for the overall decision problem and for key parameters. EVPI represents the expected costs of decision uncertainty as perfect information would eliminate the possibility of making the wrong decision. Hence, EVPI for the overall decision problem represents the value of eliminating all uncertainty and EVPI for key parameters (termed partial EVPI) represents the value of eliminating uncertainties in particular subsets of parameters. Separate analyses will be undertaken to reflect the variability considered in the decision model itself. Per patient EVPI estimates will be scaled up to reflect the relevant UK population size and will adopt an appropriate time-horizon.
EVPI also represents the maximum amount that a decision-maker should be willing to pay for additional evidence to inform this decision in the future. EVPI provides an upper bound on the value of additional research. This valuation provides an initial hurdle, acting as a necessary requirement for determining the potential efficiency of further primary research. Applying this decision rule, additional research should only be considered if the EVPI exceeds the expected cost of the research. In addition to providing a global estimate of the total cost of uncertainty related to all inputs in the model, EVPI can also be estimated for individual parameters (and groups of parameters) contained in the model. The objective of this analysis (termed partial EVPI) is to identify the model parameters where it would be most worthwhile obtaining more precise estimates.
The results from the clinical effectiveness review and the EVPI results will be used to identify future research recommendations.
References
- American Psychiatric Association, editor . Diagnostic and Statistical Manual of Mental Disorders 2000.
- World Health Organization . The Global Burden of Disease: 2004 Update 2008.
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry 2003;54:216-26.
- National Institute for Clinical Excellence . Depression in Adults (update). Depression: Management of Depression in Primary and Secondary Care 2009.
- Kupfer DJ. Long-term treatment of depression. Long-term treatment of depression. J Clin Psychiatry n.d.;52:28-34.
- Kessler RC, Berglund P, Demler O, . The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105.
- Prins MA, Verhaak PFM, Bensing JM, . Health beliefs and perceived need for mental health care of anxiety and depression: the patients’ perspective explored. Clin Psychol Rev 2008;28:1038-58.
- NHS Improving Access to Psychological Therapies . Good Practice Guidance on the Use of Self-Help Materials Within IAPT Services: NHS IAPT 2010.
- Richards D, Chellingsworth M, Hope R, Turpin T, Whyte M. Reach Out: National Programme Supervisor Materials to Support the Delivery of Training for Psychological Wellbeing Practitioners Delivering Low Intensity Interventions. London: Rethink; 2010.
- Mead GE, Morley W, Campbell P, . Exercise for depression. Cochrane Database Syst Rev 2008;4.
- National Institute for Clinical Excellence . Computerised Cognitive Behaviour Therapy for the Treatment of Depression and Anxiety. Review of Technology Appraisal 51 2006.
- Richardson R, Richards D.A, Barkham M. Self-help books for people with depression: a scoping review. J Ment Health 2008;17:543-52.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Liberati A AD, Tetzlaff J, Mulrow C, Gøtzsche PC, . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6.
- National Institute for Clinical Excellence . Methods for the Development of NICE Public Health Guidance 2009.
- Drummond M SM, Torrence G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 3rd edn. Oxford: Oxford University Press; 2005.
- Philips Z GL, Sculpher M, Claxton K, Golder S, Riemsma R, . Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2002;8:1-158.
List of abbreviations
- AD-SUS
- Adult Service Use Schedule
- ADM
- antidepressant medication
- BDI
- Beck Depression Inventory
- BT
- behaviour therapy
- CBT
- cognitive behavioural therapy
- CCBT
- computerised cognitive behavioural therapy
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CGI-I
- Clinical Global Impression Improvement Scale
- CHE
- Centre for Health Economics
- CI
- confidence interval
- CID
- Clinical Interview for Depression
- CRD
- Centre for Reviews and Dissemination
- CWD
- Coping with Depression course
- DARE
- Database of Abstracts of Reviews of Effects
- DASS
- Depression Anxiety Stress Scale
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders – Third Edition-Revised
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition
- EQ-5D
- European Quality of Life-5 Dimensions (EQ-5D™ is a trade mark of the EuroQol Group; it is a standardised measure of health-related quality of life)
- GP
- general practitioner
- HMO
- health maintenance organisation
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies programme
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, 10th Edition
- ICER
- incremental cost-effectiveness ratio
- IPT
- interpersonal therapy
- ITT
- intention to treat
- m-ADM
- maintenance antidepressant medication
- MADRS
- Montgomery–Åsberg Depression Rating Scale
- MBCT
- mindfulness-based cognitive therapy
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- PMS
- Psychiatric Morbidity Survey
- PPP
- purchasing power parity
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- PST
- problem-solving therapy
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RDC
- Research Diagnostic Criteria
- SCID
- Structured Clinical Interview for DSM Disorders
- SCL-20
- 20-item Hopkins Symptom Checklist
- SIGN
- Scottish Intercollegiate Guidelines Network
- SNRI
- serotonin–norepinephrine reuptake inhibitor
- SSRI
- specific serotonin reuptake inhibitor
- TAU
- treatment as usual
- TCA
- tricyclic antidepressant
- USD
- US dollar
- YLD
- year lost to disability
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health