Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/27/06. The contractual start date was in May 2010. The draft report began editorial review in June 2011 and was accepted for publication in November 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Thangaratinam et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Aim
The aim of this health technology assessment (HTA) project was to evaluate the effectiveness and harm of dietary and lifestyle interventions in pregnancy for reducing or preventing obesity and on obstetric, fetal and neonatal outcomes, through a systematic review of literature.
Background
Obesity in pregnancy
In total, 50% of women of childbearing age are either overweight [body mass index (BMI) 24.9–29.9 kg/m2] or obese (BMI ≥ 30 kg/m2), with 18% starting pregnancy as obese. 1 Currently, in the USA and Europe, 20–40% of women are found to gain more than the recommended weight during pregnancy,2 resulting in an increased risk of maternal and fetal complications. 3 More than half of women who die during pregnancy, childbirth or the puerperium are either obese or overweight. The Confidential Enquiry into Maternal and Child Health (CEMACH) report identified maternal obesity as a growing overall threat to the childbearing population in the UK. 4 The maternal risks of obesity include maternal death or severe morbidity, cardiac disease, spontaneous first-trimester and recurrent miscarriage, pre-eclampsia, gestational diabetes, thromboembolism, post-caesarean wound infection, infection from other causes such as urinary and respiratory infections, post-partum haemorrhage and low breastfeeding rates. 4,5 There is also an identified, although poorly studied, adverse psychological impact on obese pregnant women. The fetal risks include stillbirth and neonatal death, macrosomia, neonatal unit admission, preterm birth, congenital abnormalities and childhood obesity with associated long-term risks. 5,6
Excessive weight gain in pregnancy is associated with persistent retention of the weight beyond pregnancy in the mother. 7–10 Interpregnancy weight gain increases the risk of adverse maternal and fetal outcomes in subsequent pregnancies. 11 An increase in BMI of ≥ 3 units between pregnancies doubles the risk of pre-eclampsia, gestational diabetes, stillbirth and large-for-gestational-age (LGA) birth in subsequent pregnancies. Maternal obesity is also a major risk factor for childhood obesity. The obesity rate is doubled in 2- and 4-year-old children born to obese mothers. Excess weight gain during pregnancy is predictive of offspring obesity, independent of other factors. 12 This link is primarily associated with the mother’s ability to breastfeed, poor dietary and exercise habits of the mother before and during pregnancy, the parenting practices of overweight and obese mothers and the exposure of the child to poor dietary behaviours and a sedentary lifestyle once they are born.
The joint Royal College of Obstetricians and Gynaecologists (RCOG) and Centre for Maternal and Child Enquiries (CMACE, formerly CEMACH) guidelines13 and the National Institute for Health and Clinical Excellence (NICE) guidance14 recommend that women with a BMI of ≥ 30 kg/m2 should have consultant care rather than midwifery-led care, which places a massive burden on maternity unit resources. Obese women spend an average of 4.83 more days in hospital, resulting in a fivefold increase in the cost of antenatal care. 15 The costs associated with newborns are also increased, as babies born to obese mothers have a 3.5-fold increased risk of admission to the neonatal intensive care unit (NICU). 4 Obesity now costs the NHS around £1B a year and the UK economy a further £2.3B of indirect costs. Reducing maternal and childhood obesity, through effective obesity treatment programmes, could result in significant advantages for the NHS and society.
The RCOG has identified weight management interventions targeting mothers as an important long-term challenge that needs research. 16 The antenatal period is an ideal time to provide dietary and physical activity interventions to manage weight. Pregnant women are highly motivated to make changes and they have opportunities for regular contact with health professionals. 17 Weight management in pregnancy plays a crucial role not only in reducing women’s future risk of obesity but also in reducing their children’s behavioural risk factors for obesity. Even a modest fall in BMI of > 1 unit (equivalent to 2.5 kg) between pregnancies reduces the risks of pre-eclampsia, gestational diabetes and LGA birth. 11 There is a need to identify the optimal interventions that can be delivered in pregnancy and which are effective, acceptable and safe in improving the short- and long-term outcomes for the mother and the baby.
Existing guidelines and reviews
Current recommendations from NICE,14 RCOG18 and the American Congress of Obstetricians and Gynaecologists (ACOG)19 for the management of obesity include healthy diet and exercise in pregnancy with referral to a nutritionist if required. The target weights for weight gain in pregnancy are based on the recommendations provided by the Institute of Medicine (IOM),20 ACOG19 and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). 21 The recent NICE guidance has recommended a ‘life course approach’ by focusing on pregnancy and 1 year after childbirth as the crucial periods to target weight management interventions based on behavioural change and dietary and physical activity. 14
A recent review in this area found insufficient evidence to recommend specific dietary and/or physical activity interventions to moderate gestational weight gain in pregnant women. 22 The latest CMACE/RCOG guideline on the management of obese women in pregnancy provides recommendations on the antenatal, intrapartum and postnatal care of this group of high-risk women;13 however, gestational weight gain and the role of dietary and lifestyle interventions in pregnancy were prespecified to be outside the scope of the guideline.
Systematic reviews help clinicians, patients and policy-makers make decisions by summarising evidence. The details of the existing reviews evaluating the effect of weight management interventions on maternal and fetal outcomes are provided in Appendix 1. Existing reviews of the effectiveness and adverse effects of weight management interventions in pregnancy show deficiencies in quality and evidence when assessed against a validated tool and reporting checklists: PRISMA23 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta analysis of Observational Studies in Epidemiology). 24 This is one of the main reasons for their limitations in the role of informing practice. An accurate and reliable summary of the evidence with clear and transparent reporting is needed to maximise their usefulness to clinicians, patients and policy-makers. 3
Objectives of the project
This HTA project was undertaken to meet the following objectives:
-
to determine, primarily, the effectiveness of dietary and lifestyle interventions in pregnant obese and normal-weight women for:
-
– maternal weight change
-
– fetal and neonatal weight
-
-
to determine, secondarily, the effectiveness of dietary and lifestyle interventions in pregnant obese and normal weight women for:
-
– obstetric and medical complications in pregnancy
-
– fetal and neonatal morbidity and mortality
-
-
to evaluate the potential short- and long-term adverse effects in mother and baby resulting from the type of intervention in pregnancy.
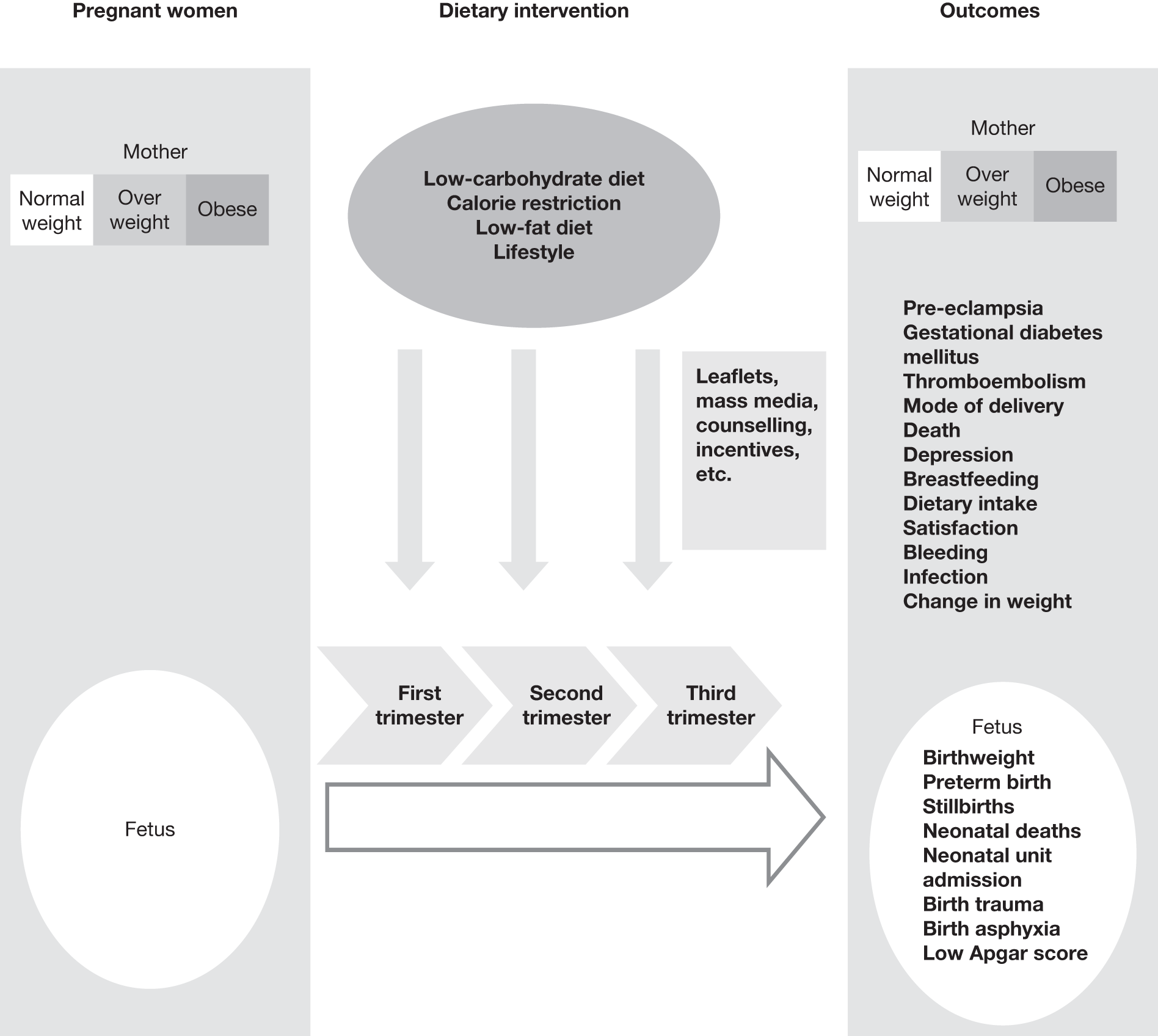
Figure 1 shows our proposed framework for the work undertaken.
FIGURE 1.
A framework to study the effectiveness of dietary and lifestyle interventions for maternal and fetal outcomes.
Chapter 2 Systematic review methods
Protocol development
Systematic reviews of the effectiveness of and harm caused by interventions were carried out using methodology25–27 in line with the recommendations of the NHS Centre for Reviews and Dissemination and the Cochrane Collaboration, including the Cochrane Adverse Methods Subgroup. 25–33 The systematic reviews of effectiveness and of adverse effects were carried out simultaneously .
The protocol for this review included the following: a detailed literature search to identify all relevant citations, prioritisation of outcomes relevant to clinical practice by Delphi survey, assessment of the risk of bias for the individual studies and evaluation of the strength of evidence for individual outcomes using GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology.
Research question
The structured question addressed by the project is given in Table 1.
| Question components | Details |
|---|---|
| Population | Pregnant women who are obese (BMI ≥ 30 kg/m2) or overweight (BMI 25–29.9 kg/m2) and pregnant women of normal weight (BMI 18.5–24.9 kg/m2) |
| Intervention | Dietary intervention, physical activity-based intervention and mixed approach (see Table 2) |
| Outcomes |
Primary outcome: weight-related outcomes Secondary outcomes: obstetric outcomes, fetal and neonatal morbidity and mortality (see Table 3) |
| Study design | Systematic review |
Methods for effectiveness review
Search strategy
A detailed search of the relevant published and unpublished literature was conducted by constructing a comprehensive search strategy for the effectiveness of dietary and lifestyle interventions in pregnancy. The following databases were searched: MEDLINE, EMBASE, BIOSIS, Latin American and Caribbean Health Sciences Literature (LILACS), Science Citation Index, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), HTA database and PsycINFO. In addition, information on studies in progress and unpublished research or research reported in the grey literature were sought by searching a range of relevant databases including Inside Conferences, Systems for Information in Grey Literature (SIGLE), Dissertation Abstracts and ClinicalTrials.gov. Internet searches were also carried out using specialist search gateways (such as OMNI: www.omni.ac.uk/), general search engines (such as Google: www.google.co.uk/) and meta-search engines (such as Copernic: www.copernic.com/). The aim was to identify all studies evaluating the effectiveness of interventions for weight management in pregnancy.
The search strategy was designed in a multistep process by combining search terms related to pregnancy and weight. The search was limited by including search filters for ‘human studies’ and ‘study type’ (randomised clinical trials and observational trials without case series and case studies). Existing search strategies or filters, such as the InterTASC Information Specialists’ Sub-Group Search Filter Resource, were used to develop the search strategy with some modifications as needed. No further limitations were applied. The detailed search strategy for effectiveness is provided in Appendix 2. MEDLINE and EMBASE were searched from inception to May 2010. Other databases were searched from inception to June 2010. The search was repeated and updated until March 2011. A comprehensive master database of articles was constructed using Reference Manager 12.0® software (Thomson Reuters, New York, NY, USA).
Inclusion criteria
The criteria for inclusion of studies in the effectiveness review are described in the following sections.
Population
Pregnant women expecting one or more than one baby (i.e. twins or triplets) were included. We included women who were of normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2). We excluded pregnant women who were underweight (BMI < 18.5 kg/m2).
Setting
Any setting including primary care or secondary and tertiary units.
Interventions
We included any dietary, physical activity and behavioural change intervention that has the potential to influence weight change in pregnancy. Studies that evaluated interventions mainly based on dietary advice were classified in the dietary interventions group. Interventions primarily based on physical activities such as swimming, running and aerobic exercise were classified in the physical activity group. The mixed approach interventions group included studies that employed diet and physical activity components that may, or may not, be underpinned by behavioural theory. Table 2 lists the various interventions reviewed.
| Interventions and intervention delivery | Details |
|---|---|
| Dietary intervention | Energy and intake of total diet and specific food (e.g. low-carbohydrate diet, low-fat diet, high-fibre diet, low-protein diet, balanced diet, Atkins diet, Slimming World diet); dietary patterns, frequency of eating; and meal composition |
| Physical activity-based intervention | Walking, swimming, aerobic dancing, low-intensity resistance exercise, aqua aerobics and exercise regimes of various intensity |
| Mixed approach intervention | Intensive counselling regarding diet and physical activity in pregnancy and stepped-care advice. Behavioural change model (e.g. transtheoretical model, theory of planned behaviour, self-determination theory) predominantly underpinning the intervention |
| Intervention delivery |
One-to-one counselling, motivational talk, dietary consultation, group exercise, supermarket tours, cooking demonstration, parentcraft classes, walking group, benefits/incentives, slimming club and mass media (TV, radio, DVD, social websites, NHS websites) BMI chart, diet self-monitoring tools, self-weight check, postal questionnaires, IOM weight gain grid; Bassett obstetric chart |
Comparison
The control group consisted of women with no intervention or routine antenatal care. In women with obstetric or medical complications the care provided was appropriate to the condition (e.g. insulin in diabetic women).
Outcomes
The maternal and fetal outcomes included in the review are provided in Table 3.
| Outcomes | Components |
|---|---|
| Weight-related outcomes (primary) | |
| Maternal | Change in maternal weight (absolute gain or loss in weight; percentage of weight gained or reduced in comparison with pre-intervention weight), fat content measurement (BMI, skinfold thickness, ponderal index, fat-free mass) and fat distribution measures (waist-to-hip ratio, waist size) in pregnancy |
| Fetal | Birthweight related to gestational age and sex, fetal fat mass and ponderal index (weight/length3) |
| Obstetric and pregnancy-related outcomes | |
| Fetal and neonatal complications | Pre-eclampsia, gestational diabetes mellitus, gestational hypertension, premature rupture of membranes, caesarean section, post-partum haemorrhage, sepsis, maternal death, preterm labour, abruption, complications of labour and delivery, instrumental delivery, perineal trauma, induction of labour, need for hospitalisation, day-care unit visits in pregnancy and the puerperium, use of intensive care in pregnancy or the puerperium, thromboembolism, stillbirth, perinatal and neonatal death, congenital abnormalities, prematurity, abnormal Apgar score, neonatal respiratory distress, shoulder dystocia, abnormal cord pH at birth, hypoxic–ischaemic encephalopathy, long-term neurological sequelae, need for NICU admission, mechanical ventilation and duration of hospital stay |
| Childhood and adult outcomes in offspring | Childhood obesity, adult obesity, diabetes mellitus, coronary heart disease, hypertension, stroke, depression and death |
| Other relevant outcomes | Maternal: cardiac arrest, stroke, psychiatric problems, depression, self-esteem, low back pain, and change in diet and exercise |
Study design
We included randomised controlled trials (RCTs) evaluating the effectiveness of dietary and lifestyle weight management interventions in pregnancy for maternal and fetal outcomes. Non-randomised studies (NRSs) and observational studies (cohort and case–control) were included in the analysis only when the evidence from RCTs was insufficient. Studies that did not provide data to estimate effectiveness measures such as relative risk (RR) or mean difference (MD) were excluded.
Subgroups
The following subgroups were specified a priori and reported in the review:
-
intervention: dietary, physical activity and mixed approach interventions
-
BMI: obese only, obese and overweight and mixed-group populations
-
setting: studies in developed countries and developing countries
-
year of publication: studies published before 1990 and since 1990
-
diabetes in pregnancy
-
responders to the intervention with significant reduction in gestational weight gain.
Study selection
Study selection was conducted in two stages: an initial screening of titles and abstracts against the inclusion criteria to identify potentially relevant papers followed by screening of the full papers of the identified citations without language restrictions. Two reviewers independently assessed each citation (ER and SG) for inclusion in the review. Any differences in opinion were resolved by discussion and by involving a third reviewer. Further information was sought from the study authors if required. The process of study identification and selection is presented in Figure 2, consistent with the PRISMA guidelines.
Study quality assessment
The studies were classified by study design according to the NICE guidelines algorithm for classifying quantitative study designs. 34 Quality assessment was carried out separately for the different study designs (RCTs, NRSs and observational studies).
Randomised controlled trials
We assessed the risk of bias – selection bias, performance bias, measurement bias and attrition bias – in line with the recommendations made in the Cochrane handbook for systematic reviews of interventions. 35 Study quality was assessed in six domains: sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias.
Sequence generation
An adequate sequence generation should describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether or not it should produce comparable groups. The use of a random component was considered to be adequate sequence generation. Systematic methods, such as alternation or assignment based on date of birth, case record number or date of presentation, were considered to be inadequate.
Allocation concealment
A study was categorised as being at low risk of bias for allocation concealment if it described the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
The quality of allocation concealment was chosen using the following criteria:
-
adequate concealment of allocation, such as telephone randomisation, consecutively numbered sealed opaque envelopes
-
unclear whether adequate concealment of allocation
-
inadequate concealment of allocation such as random number tables, sealed envelopes that are not numbered or opaque.
Where the method of allocation concealment was unclear, whenever possible attempts were made to contact authors to provide further details.
Blinding
Adequate blinding described all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. It should also provide any information relating to whether or not the intended blinding was effective. In assessing the risk of bias from blinding, we specifically assessed who was and who was not blinded. Furthermore, we also assessed separately the risk of bias for subjective and objective outcomes.
Incomplete outcome data
We evaluated the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We assessed whether attrition and exclusions were reported, the numbers in each intervention group (compared with the total number of randomised participants), reasons for attrition or exclusions where reported and any reinclusions in the analyses.
A study was considered to be at low risk of bias for missing outcome data when we were confident that the participants included in the analysis were exactly those who were randomised into the trial. The risk of bias was considered to be unclear if the numbers randomised into each intervention group were not clearly reported. A study was labelled as having a high risk of bias for missing outcome data when there was a difference in the proportion of incomplete outcome data across groups and the availability of outcome data was determined by the participants’ true outcomes.
Selective outcome reporting
We compared the outcomes reported in the individual studies with the rest of the studies to assess the possibility of selective outcome reporting. The risk of this bias was assessed at the study level.
Other sources of bias
Any other important concerns about bias not addressed in the above domains were highlighted as other sources of bias. The proportions of studies with various risks of bias are shown in Appendix 4. The entries for each domain were marked as ‘Yes’, ‘No’ or ‘Unclear’ as appropriate.
Non-randomised studies
Quality assessment of NRSs was performed using a methodology checklist presented in Appendix 5. The Newcastle–Ottawa scale (NOS) was used to assess the quality of the observational comparative studies with cohort and case–control designs. 25 The cohort studies were assessed for the following risks of bias:
-
selection of cohorts regarding the representativeness and selection of the exposed cohort, ascertainment of exposure and that the outcome of interest was not present at the start of study
-
comparability of the cohorts based on methods or analysis
-
assessment of outcome by evaluating the details of outcome assessment, adequacy of length of follow-up for the outcomes to appear and adequacy of follow-up of the cohorts.
The case–control studies were evaluated for the following risks of bias:
-
selection of cases and controls, assessing representativeness and adequate definition of the cases and adequate selection and definition of the controls
-
comparability of the cases and controls
-
ascertainment of exposure, method of ascertaining exposure of the cases and controls and rates of non-response in the groups.
The studies are allocated stars according to the rating. A study can be awarded a maximum of four stars for selection, two for comparability and three for ascertainment of exposure. 36
Data extraction
Study clinical characteristics and findings were extracted in duplicate by independent reviewers using predesigned and piloted data extraction forms. Any disagreements were resolved by consensus and/or arbitration involving a third reviewer. Missing information was obtained from investigators if it was crucial to the subsequent analysis. To avoid introducing bias, unpublished information was treated in the same way as published information. In addition to using multiple reviewers to ensure the reproducibility of the overview, sensitivity analyses around important or questionable judgements regarding the inclusion or exclusion of studies, the validity assessments and data extraction were performed. A copy of the data extraction form for the effectiveness review is provided in Appendix 18.
Data synthesis
We calculated pooled RRs with 95% confidence intervals (CIs) for dichotomous data. Continuous data were summarised as MD with standard deviation or median change in relation to the baseline. In the case of missing standard deviations, imputation techniques were used based on Cochrane recommendations. 35 Separate analyses were performed on randomised and non-randomised data. Non-randomised data were used for outcomes for which there were no RCTs or a very small number of poor-quality RCTs. The I2 statistic was used to assess statistical heterogeneity between trials. In the absence of significant heterogeneity, results were pooled using a fixed-effect model. If substantial heterogeneity was detected (I2 > 50%), possible causes were explored and subgroup analyses for the main outcomes performed. Subgroups defined a priori were BMI of the women, type of intervention, responders, publication year (before and after 1980), study quality and setting. Heterogeneity that was not explained by subgroup analyses was modelled using random-effects analysis where appropriate. For outcomes for which meta-analysis was not appropriate, the RCT and NRS results were presented, where possible, on a forest plot but without summary scores, allowing a visual presentation of the effects of each included trial. For observational studies, a narrative summary of the findings was given. Statistical analysis was performed when sufficient data were presented. RevMan, version 5.0, (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used in the statistical analyses.
Methods for adverse effects review
The review of harm of interventions was undertaken based on recommended methods for systematic reviews, particularly those of observational studies and adverse events, including those of the Cochrane Adverse Effects Subgroup. 30,37–39
Search strategy
The scope of the review of adverse effects of any dietary intervention on pregnant women and their children was purposefully kept broad. This was to identify a variety of adverse effects that were previously not known or recognised. In addition to the search for relevant reviews and primary studies on the effectiveness of interventions, including those that were excluded from the analysis of benefit, we evaluated studies that specifically provided details of adverse effects resulting from the dietary and lifestyle interventions and weight loss in pregnancy. We designed a separate search strategy to identify studies on harm by including adverse effects text words and indexing terms in the databases previously described in the section on the effectiveness review. Existing search strategies or filters, such as the InterTASC Information Specialist Sub-Group Search Filter Resource, were used to develop the search strategy for this review, with some modifications if needed. The search was limited by including search filters for ‘adverse events’ , ‘human studies’ and ‘study type’ (exclusion of editorials and letters). The detailed search strategy for adverse effects can be found in Appendix 2. MEDLINE and EMBASE were searched from inception to June 2010. Other databases were searched from inception to July 2010. The search was updated until March 2011.
Inclusion criteria
The criteria for inclusion of studies in the adverse effects review are described in the following sections.
Population
Pregnant women expecting one or more than one baby (i.e. twins or triplets) were included. We included women who were of normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2). We excluded pregnant women who were underweight (BMI < 18.5 kg/m2).
Setting
We included studies carried out in any setting including primary care or secondary and tertiary units.
Interventions
Any dietary and physical activity intervention or exposure that has the potential to cause harm to the mother or baby.
Outcomes
We included any clinically significant adverse outcomes in the mother and the child resulting from (1) a dietary intervention or (2) weight change in pregnancy. We also evaluated the most common adverse effects that led to pregnant women discontinuing an intervention.
Study design
Both comparative (RCTs, NRSs and observational studies) and non-comparative studies including case series and case reports were included. This encompassed any publication as an abstract or full text without any language restrictions.
Study selection and quality assessment
Criteria used to assess the quality of studies for the evaluation of adverse effects followed the same concepts as for assessing study quality for effectiveness: assessing risk of bias, inconsistency of results, indirectness of the evidence, imprecision and publication bias. For assessing the risk of bias in estimating adverse event rates associated with weight management interventions in pregnancy24 we took into account existing checklists for the evaluation of randomised and non-randomised studies,39,40 including study design and other features associated with outcome [e.g. small for gestational age (SGA), preterm delivery]. Quality assessment and presentation of results were carried out separately for RCTs, NRSs and observational studies with a control group and for observational studies without a control group (case series, case reports). Additionally, information on weight change per se in mother and baby were also extracted as these could be associated with adverse event rates or severity. The methodological quality of all eligible data sets (‘risk of bias’) was assessed to investigate internal validity (the extent to which the information is probably free of bias) using the following attributes:41
-
reporting of adverse maternal and fetal outcome definitions to reduce bias in ascertainment of denominator data in the series (any published definition reported vs no definition)
-
adequacy of data source to ascertain a capture of denominator data that is as complete as possible (use of multiple data sources, special surveys or clinical studies vs routine registration enrolment in weight loss programmes, in which adequate attribution of cause of harm has been shown to be questionable for maternal and fetal outcomes, leading to substantial under-reporting)
-
use of a robust approach to ascertain that the cause of harm is a representation of the underlying condition that is as true as possible (confidential enquiries, use of multiple sources of outcome vs no special efforts to confirm cause)
-
a sufficiently high proportion of cases with an attributable cause of harm established (< 5% unclassified).
Data extraction
Methods for study selection and data extraction for the adverse event review were similar to those for the effectiveness review. Study clinical characteristics and findings were extracted in duplicate by independent reviewers using a predesigned and piloted data extraction form (see Appendix 19). Any disagreements were resolved by consensus and/or arbitration involving a third reviewer. Missing information was obtained from investigators if it was crucial to subsequent analysis. To avoid introducing bias, unpublished information was treated in the same way as published information. In addition to using multiple reviewers to ensure the reproducibility of the overview, sensitivity analyses around important or questionable judgements regarding the inclusion or exclusion of studies, the validity assessments and data extraction were performed.
Data synthesis
The number of adverse events reported in pregnant women and children was obtained for each intervention to compute a percentage of the total number of women and children in whom the occurrence of a particular adverse event or confirmation of its absence was reported. 41 It is inappropriate to calculate adverse event rates from case studies; thus, a qualitative summary was undertaken. Quantitative adverse event rate calculations were restricted to series of women undergoing weight management interventions and weight change as identified from RCTs and observational studies, with and without controls (case series). The adverse events were quantified as RRs and 95% CIs. The point estimates of proportions and their 95% CIs are represented in forest plots to explore heterogeneity, and the possibility of the differences being due to chance was assessed statistically using Cochran’s Q test.
Grading of evidence
The quality of the evidence was assessed and reported separately for each outcome following the GRADE methodology. This is because even within one review the quality of the evidence can vary between the outcomes. We defined quality of evidence as ‘the extent of confidence that an estimate of effect is correct’. 42 The GRADE system classifies quality of evidence into one of four levels: high, moderate, low and very low (Table 4).
| High quality | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate quality | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| Low quality | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very-low quality | Any estimate of effect is very uncertain |
To assess the quality, we considered, first of all, the risk of bias (internal validity), that is, the extent to which the design, methods, execution and analysis were not controlled for bias in the assessment of effectiveness. 30 Furthermore, we explored the (in)consistency of results (heterogeneity), (in)directness of the evidence (with respect to the question under consideration, including surrogate parameters), (im)precision of the results and publication bias. We assigned all evidence a ‘high’ level of quality when it was based on RCTs. If any of the reasons below applied to the body of evidence, for each comparison–outcome pair the quality level was downgraded by one level (if the reason was classified as serious) or two levels (if the reason was classified as very serious):
-
Risk of bias may arise from limitations in the study design and implementation. We downgraded evidence quality if there was lack of allocation concealment (selection bias), lack of blinding (performance bias), incomplete accounting of patients and outcome events (attrition bias), and other limitations affecting outcome assessment (detection bias).
-
Inconsistency referred to heterogeneity in results, which could arise from differences in populations, interventions or outcomes. Widely differing estimates of the effects across studies suggests that there might be true differences in underlying effect. When heterogeneity existed, but investigators failed to identify a plausible explanation, the quality of evidence was downgraded by one or two levels, depending on the magnitude of the inconsistency in the results.
-
Indirectness referred to broader or more restricted assessment of the review question components including population, intervention, comparator and outcomes.
-
Imprecision of results referred to wide 95% CIs as a result of few participants or few events. We downgraded the quality of evidence because of imprecision if there was a non-significant result or wide CIs.
We tabulated these features and assigned an overall quality grade to the evidence for each comparison–outcome pair. The footnotes in each table (e.g. Table 10) provide an explanation as to how we downgraded evidence in light of various deficiencies (Table 5).
| Bias | No downgrading | Downgrading by one (possibly two) levels | Downgrading by two or three levels |
|---|---|---|---|
| 1. Selection bias | Studies with randomisation, allocation concealment, similarity of groups at baseline | RCTs with some deficiencies in randomisation e.g. lack of allocation concealment, or NRSs with either similarities at baseline or use of statistical methods to adjust for any baseline differences | Non-randomised, with obvious differences at baseline, and without analytical adjustment for these differences |
| 2. Performance bias | Differed only in intervention, which was adhered to without contamination; groups were similar for cointerventions or statistical adjustment was made for any differences | Confounding was possible, but some adjustment was made in the analysis | Intervention was not easily ascertained or groups were treated unequally other than for intervention or there was non-adherence, contamination or dissimilarities in groups and no adjustments made |
| 3. Measurement bias | Outcome measured equally in both groups, with adequate length of follow-up (i.e. at least 2 years after delivery); direct verification of outcome, with data to allow calculation of precision estimates | Inadequate length of follow-up or length not given | Inadequate reporting or verification of maternal mortality or differences in measurement in both groups |
| 4. Attrition bias | No systematic differences in withdrawals between groups and with appropriate imputation for missing values | Incomplete follow-up data, not intention-to-treat analysis or lacking reporting on attrition |
The secondary maternal and fetal outcomes critical to clinical care of the patient were prioritised by a two-round Delphi survey of clinicians. The Delphi panel of clinicians was chosen for their interest in the field. A structured list of these outcomes (Box 1) was sent to 20 clinicians along with a covering letter explaining the purpose of this survey. The questionnaire was sent by e-mail and anonymity was maintained between panellists. In the first round, the experts were asked to rank the outcomes for their importance on a 1–9 scale (1–3 not important; 4–6 important, but not critical; 7–9 critical). They were given the opportunity to add outcomes that were considered to be relevant but not included in the list. Summary statistics such as medians and interquartile ranges (IQRs) were generated for each outcome. The median was used to identify the location on the appropriateness scale and an IQR (i.e. a measure of dispersion generated by taking the difference between the 75th and the 25th percentiles) of ≤ 2 was predefined to indicate consensus. In the second round the experts were asked to reconsider their previous ratings in view of the panel score. The new median scores and IQRs were recalculated. The top 10 outcomes were identified for inclusion in the GRADE evidence profile in addition to the primary weight-related outcomes.
Gestational diabetes mellitus
Pre-eclampsia/pregnancy-induced hypertension
Post-partum haemorrhage
Prolonged labour
Preterm delivery
Induction of labour
Prelabour rupture of membranes
Caesarean section
Instrumental delivery
Perineal trauma
Puerperal pyrexia (≥ 38°C)
Miscarriage
Need for resuscitation at delivery
Antepartum haemorrhage
Thromboembolism
Admission to the high-dependency unit/intensive care unit
Anaemia
Back pain
Infections
Postnatal incontinence
Postnatal depression
Anxiety
Quality of life
Physical activity
Dietary behaviour
Body fat (%)
Breastfeeding
Threatened miscarriage
Failed instrumental delivery
Coronary artery disease
Non-infective respiratory distress
Fetal, neonatal and childhood outcomesSmall for gestational age
Large for gestational age
Skinfold thickness (mm)
Fetal fat mass (%)
Abdominal circumference
Head circumference
Ponderal index (g/cm3 × 100)
Neonate length/crown–heel length
Head-to-abdomen ratio
Birthweight-related outcomes such as BMI
Hypoglycaemia
Hyperbilirubinaemia
Intrauterine death
Respiratory distress syndrome
Admission to NICU
Shoulder dystocia
One or more perinatal complications
Birth trauma
Neural tube defect
Cleft lip or palate or both
Other congenital abnormalities
Abnormal Apgar score
Cardiotocographic abnormalities
Cord pH abnormal
Long-term neurological sequelae
Cord abnormalities
Long-term metabolic sequelae
The strength of evidence for each outcome was assessed. The main maternal and fetal weight-related outcomes and those prioritised by the Delphi panel were assessed by GRADE methodology using GRADEpro software version 3.2.2 [GRADEpro (computer program), version 3.2 for Windows; Jan Brozek, Andrew Oxman and Holger Schürmann, 2008]. Two reviewers independently assessed the quality of each study; disagreements were resolved by consensus or arbitration involving a third reviewer. For each comparison–outcome pair we deployed a two-dimensional chart plotting five variables represented on equiangular spokes starting from the same point, each spoke representing one of the domains used in evidence grading. 43 These included study design, risk of bias, inconsistency, indirectness and imprecision. The data length of a spoke was proportional to the magnitude of the quality, ranging from high to moderate to low to very low. A line connected the data values for each spoke generating a pentagon. Consistent use of the same position and angle of the spokes in all comparison–outcome pairs was used for easy visual interpretation in a multiplot format.
Chapter 3 Effectiveness of the interventions
Study selection
At the final update on 31 March 2011, 19,563 potentially relevant citations were identified from the major electronic databases to evaluate the effectiveness of weight management interventions in pregnancy for maternal and fetal outcomes. A further 23 studies were identified from the reference lists of the identified studies. In total, 88 articles were included in the review. Figure 2 shows the flow diagram of study identification, selection and exclusion.
FIGURE 2.
Flow chart of study identification and selection in the effectiveness review.
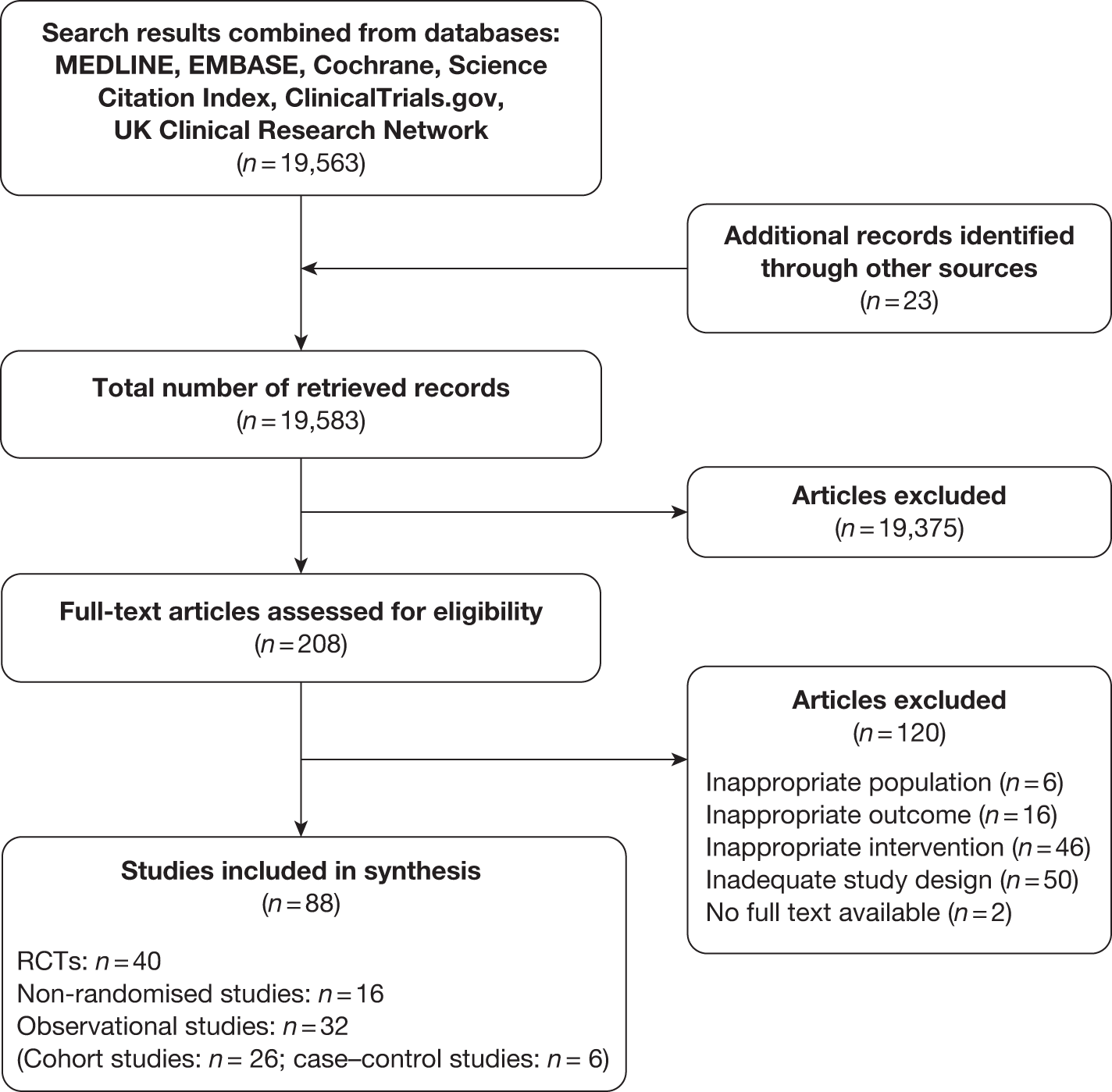
A total of 56 experimental studies (40 randomised and 16 non-randomised controlled studies;44–59 involving 8842 women) and 32 observational studies (26 cohort60–85 and six case–control studies;86–91 involving 173,297 women) evaluated the effectiveness of dietary, physical activity and other lifestyle interventions in pregnancy for maternal and fetal outcomes. The 40 RCTs included 12 trials on dietary interventions,92–103 20 on physical activity104–123 and eight on mixed approach124–130 in pregnancy for the prevention or reduction of obesity. Appendix 3 provides details of the included RCTs.
Quality of included studies
Randomised controlled trials
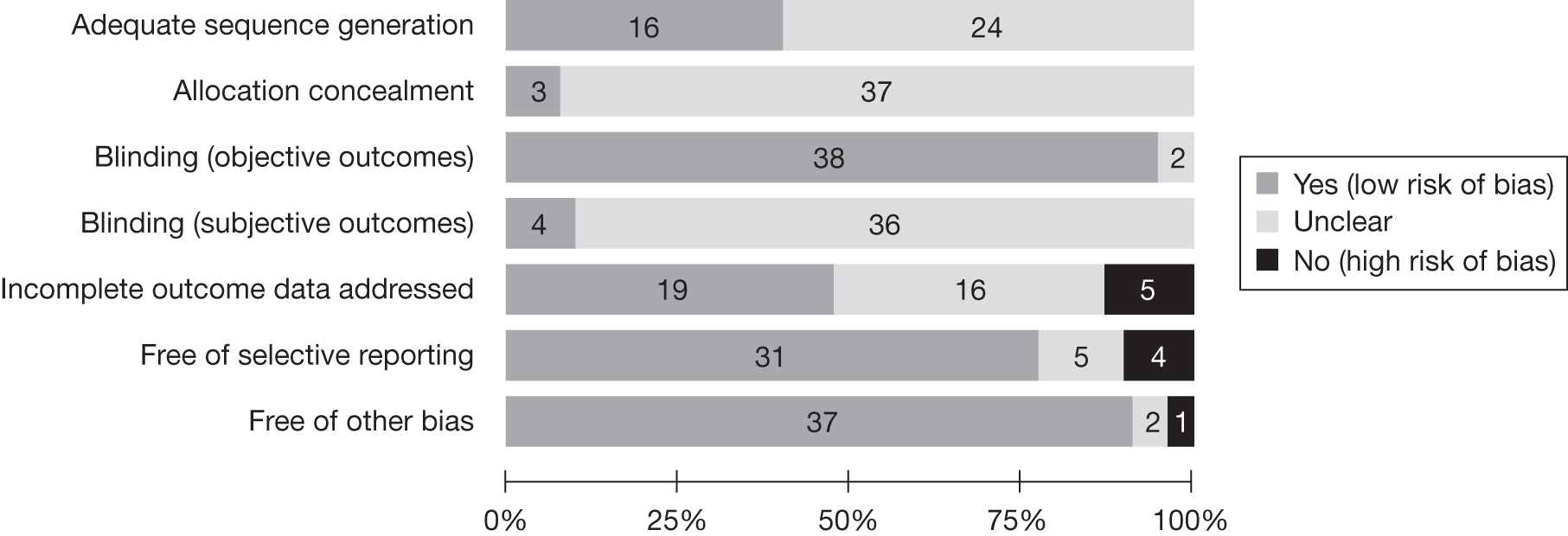
Figure 3 demonstrates the risk of bias of the included RCTs in the seven domains. Two-thirds of studies scored a low risk of bias for selective reporting of outcomes and blinding for objective outcomes. Although there was no obvious evidence of a high risk of bias for sequence generation, allocation concealment and blinding for subjective outcomes, a large proportion of the studies were unclear in their reporting in these domains. Appendix 4 provides a detailed quality assessment of the individual RCTs.
FIGURE 3.
Quality assessment of the included RCTs.
Non-randomised studies and observational studies
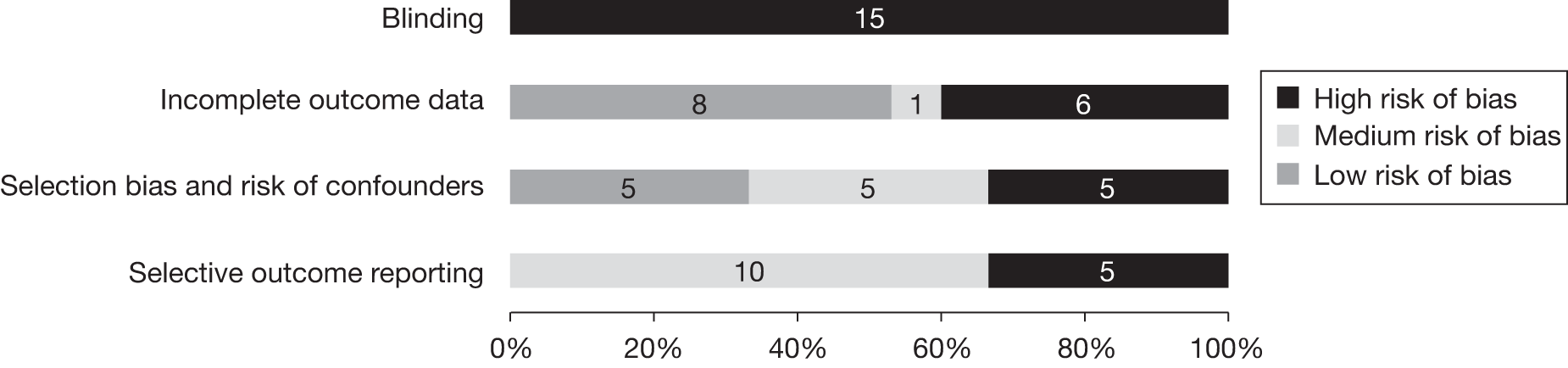
The internal validity of NRSs has been assessed in line with the NICE checklist. 34 Figure 4 presents the quality of the included NRSs. Further details of the individual study quality for non-randomised and observational studies are provided in Appendices 5 and 6. The observational studies were evaluated using the NOS and could score a maximum of nine stars, with four stars for selection, two for comparison and three for outcome assessment. In total, 7/26 (26.9%) cohort studies had a low risk of bias and scored seven or more stars, 18/26 (69.2%) had a medium risk of bias and scored between four and six stars and one study (3.8%) had a high risk of bias (see Appendix 6). All six case–control studies had a medium risk of bias.
FIGURE 4.
Quality assessment of the included NRSs.
Effect of the interventions on weight-related outcomes
Maternal weight-related outcomes
Maternal weight gain in pregnancy
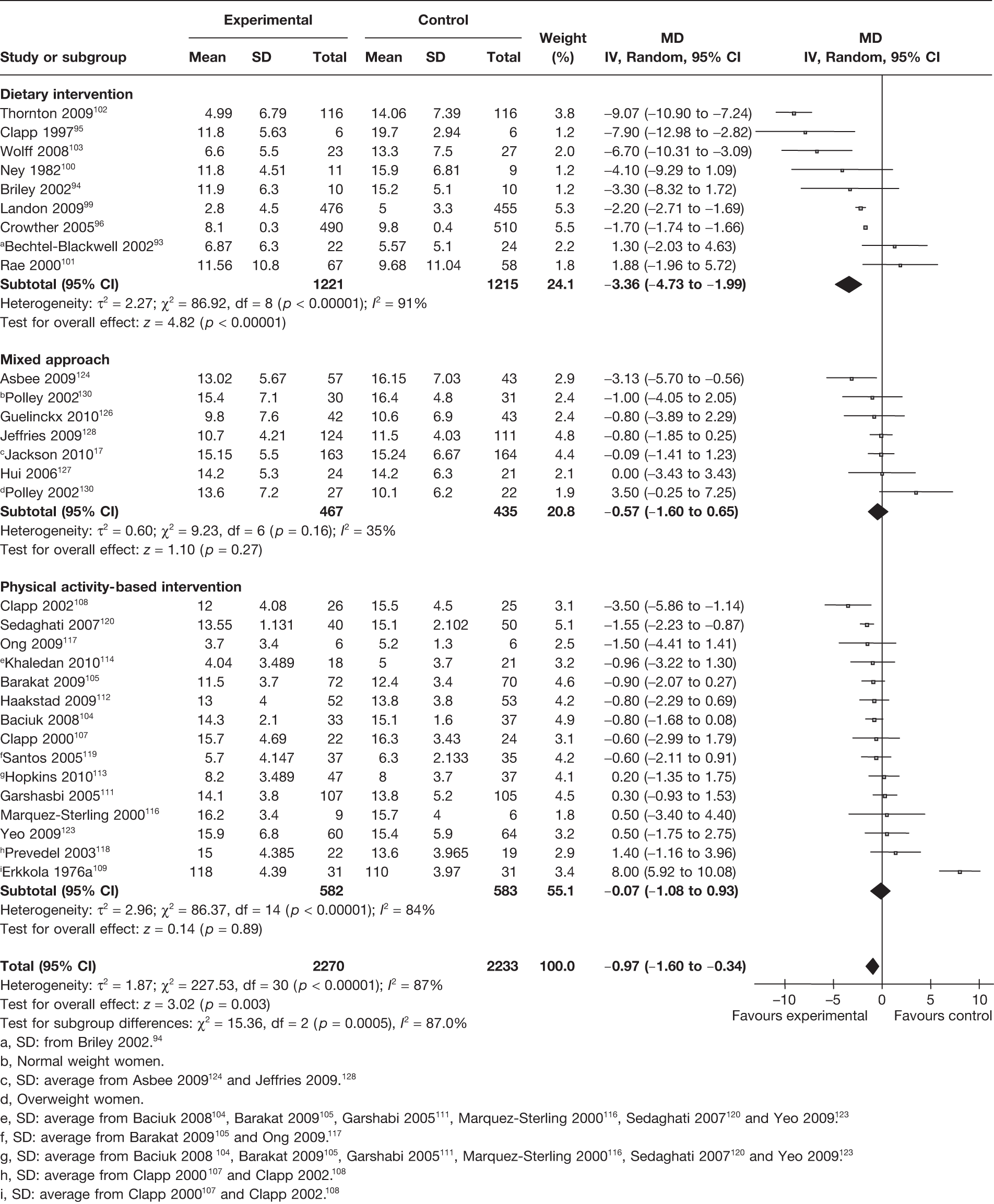
A total of 30 RCTs17,93–96,99–105,107–109,111–114,116–120,123,124,126–128,130 including 4503 women evaluated the effect of interventions on maternal weight gain in pregnancy. This included nine93–96,99–103 trials on dietary interventions, six17,124,126–128,130 on mixed approach and 15104,105,107–109,111–114,116–120,123 on physical activity interventions. There was a significant decrease in weight gain in pregnancy with interventions of 0.97 kg (95% CI –1.60 kg to –0.34 kg; p = 0.003; I2 = 87%). The largest reduction in weight gain was observed in the dietary intervention studies, with a MD of –3.36 kg (95% CI –4.73 kg to –1.99 kg; p < 0.00001; I2 = 91%), followed by mixed approach, with a MD of –0.57 kg (95% CI –1.60 kg to 0.65 kg; p = 0.27; I2 = 35%). The studies were heterogeneous with an I2 of 87%. There was a statistically significant difference between the intervention groups (p = 0.0005) (Figure 5).
FIGURE 5.
Effect of weight management interventions on maternal weight gain in pregnancy. SD, standard deviation. a, SD: from Briley 2002. 94 b, Normal weight women. c, SD: average from Asbee 2009124 and Jeffries 2009. 128 d, Overweight women. e, SD: average from Baciuk 2008104, Barakat 2009105, Garshabi 2005111, Marquez-Sterling 2000116, Sedaghati 2007120 and Yeo 2009. 123 f, SD: average from Barakat 2009105 and Ong 2009. 117 g, SD: average from Baciuk 2008104, Barakat 2009105, Garshabi 2005111, Marquez-Sterling 2000116, Sedaghati 2007120 and Yeo 2009. 123 h, SD: average from Clapp 2000107 and Clapp 2002. 108 i, SD: average from Clapp 2000107 and Clapp 2002. 108
Maternal body mass index at delivery
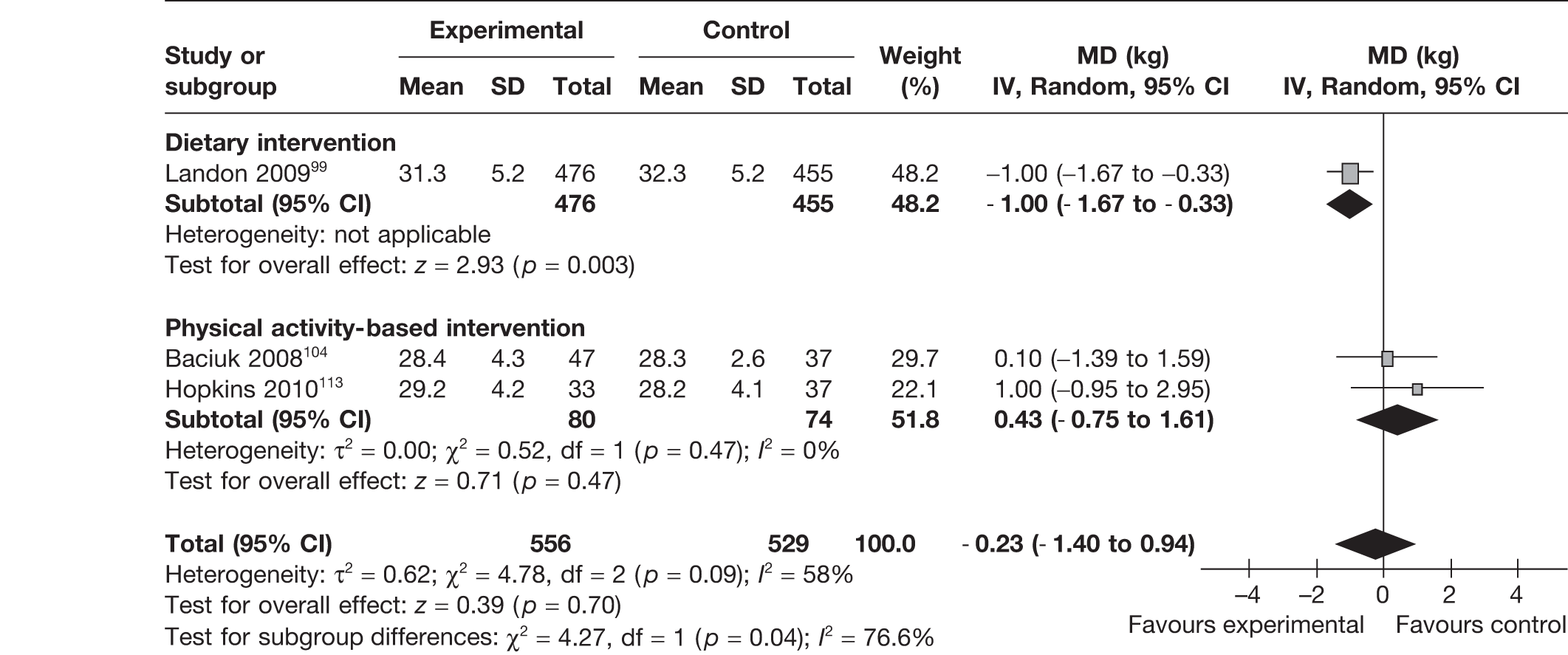
Three RCTs99,104,113 reported on the effect of interventions on the mother’s BMI at delivery. There was a significant reduction in BMI with dietary intervention, with a MD of –1.00 kg/m2 (95% CI –1.67 kg/m2 to –0.33 kg/m2; p = 0.003). This effect was not observed with interventions based on physical activity. The overall pooled estimate showed a MD of –0.23 kg/m2 (95% CI –1.4 kg/m2 to 0.94 kg/m2; p = 0.70) with a heterogeneity of I2 = 58%. There was a significant difference between the subgroups (p = 0.04) (Figure 6).
FIGURE 6.
Effect of weight management interventions on maternal BMI at delivery. SD, standard deviation.
Exceeding the Institute of Medicine’s recommendations on weight gain in pregnancy
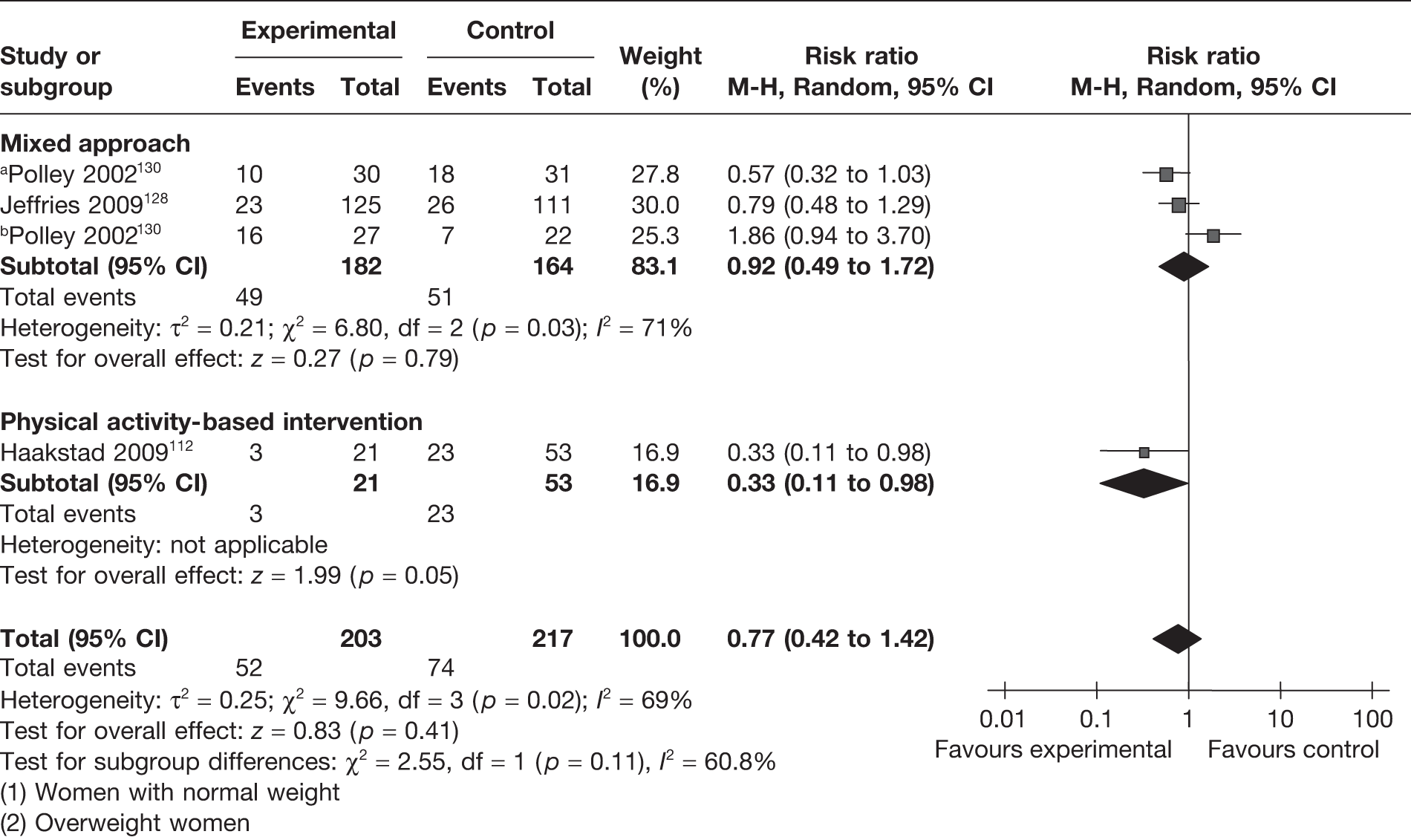
The IOM guidelines131 recommend the optimum weight gain in pregnancy for American women based on their BMI. The recommended gestational weight gain is 11.5–16.0 kg in women with normal BMI (BMI 18.5–24.9 kg/m2), 7.0–11.5 kg in overweight women (BMI 25–29.9 kg/m2) and 5.0–9.0 kg in obese women (BMI ≥ 30 kg/m2). Two RCTs128,130 reported a reduction in the number of women exceeding IOM recommendations with a dietary and physical activity intervention, which was not statistically significant (Figure 7).
FIGURE 7.
Effect of weight management interventions on IOM recommendations. a, Women with normal weight. b, Overweight women.
Fetal and neonatal weight-related outcomes
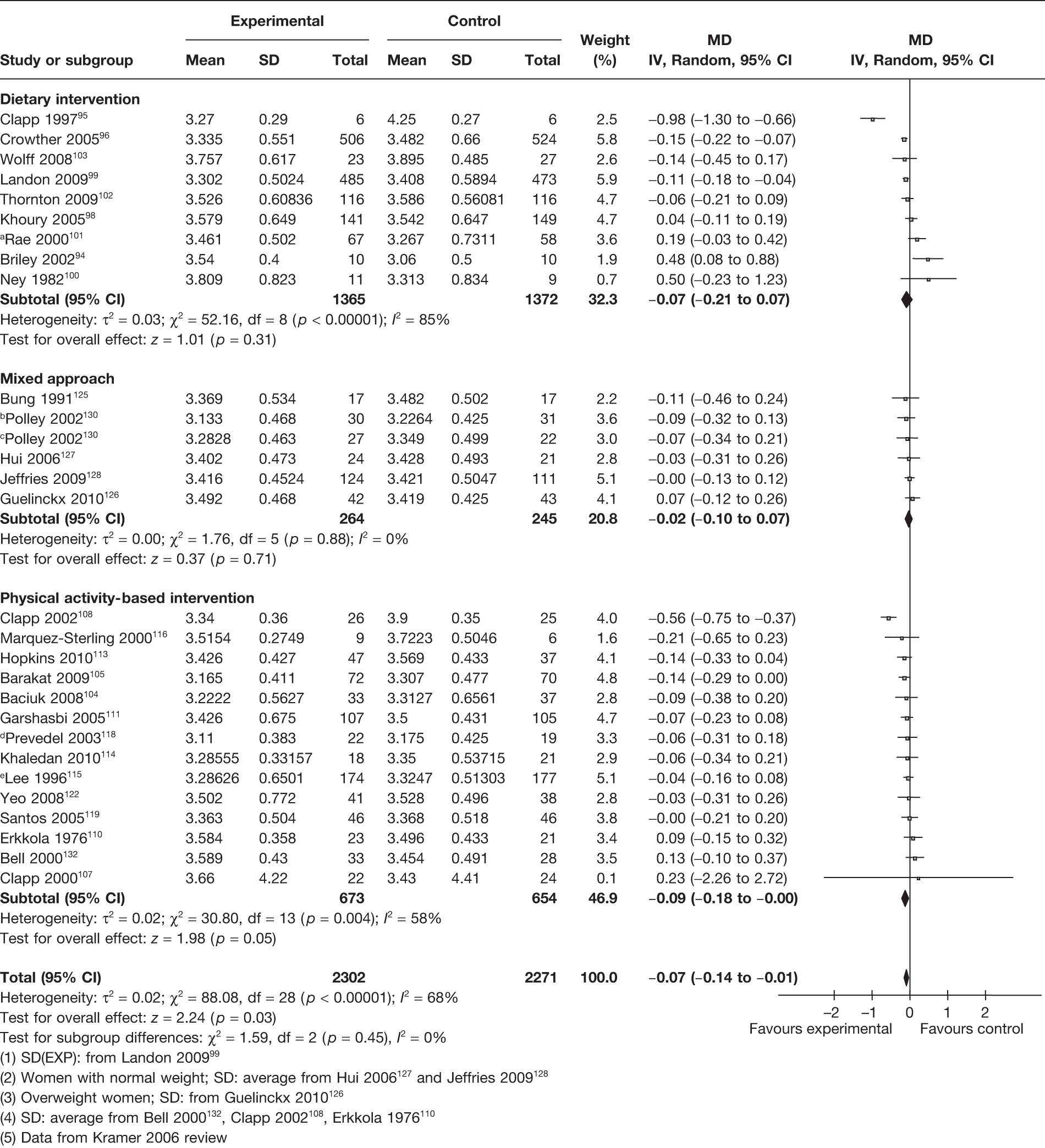
Birthweight
A total of 28 RCTs (4573 newborns) evaluated the effect of the interventions on the birthweight of the newborn. This included nine RCTs on dietary interventions,94–96,98–103 five on a mixed approach intervention125–128,130 and 14 on physical activity-based interventions. 104,105,107,108,110,113–116,118,119,122,132 Overall, there was a small, but statistically significant, reduction in the mean birthweight of 0.07 kg (95% CI –0.14 kg to –0.01 kg; p = 0.03). There was heterogeneity observed among the groups (I2 = 68%), with no large birthweight reduction in the three intervention subgroups (Figure 8).
FIGURE 8.
Effect of weight management interventions on birthweight. SD, standard deviation. a, SD(EXP): from Landon 2009. 99 b, Women with normal weight; SD: average from Hui 2006167 and Jeffries 2009. 128 c, Overweight women; SD: from Guelinckx 2010. 126 d, SD: average from Bell 2000132, Clapp 2002108, Erkkola 1976. 110 e, Data from Kramer 2006 review.
Large for gestational age at birth
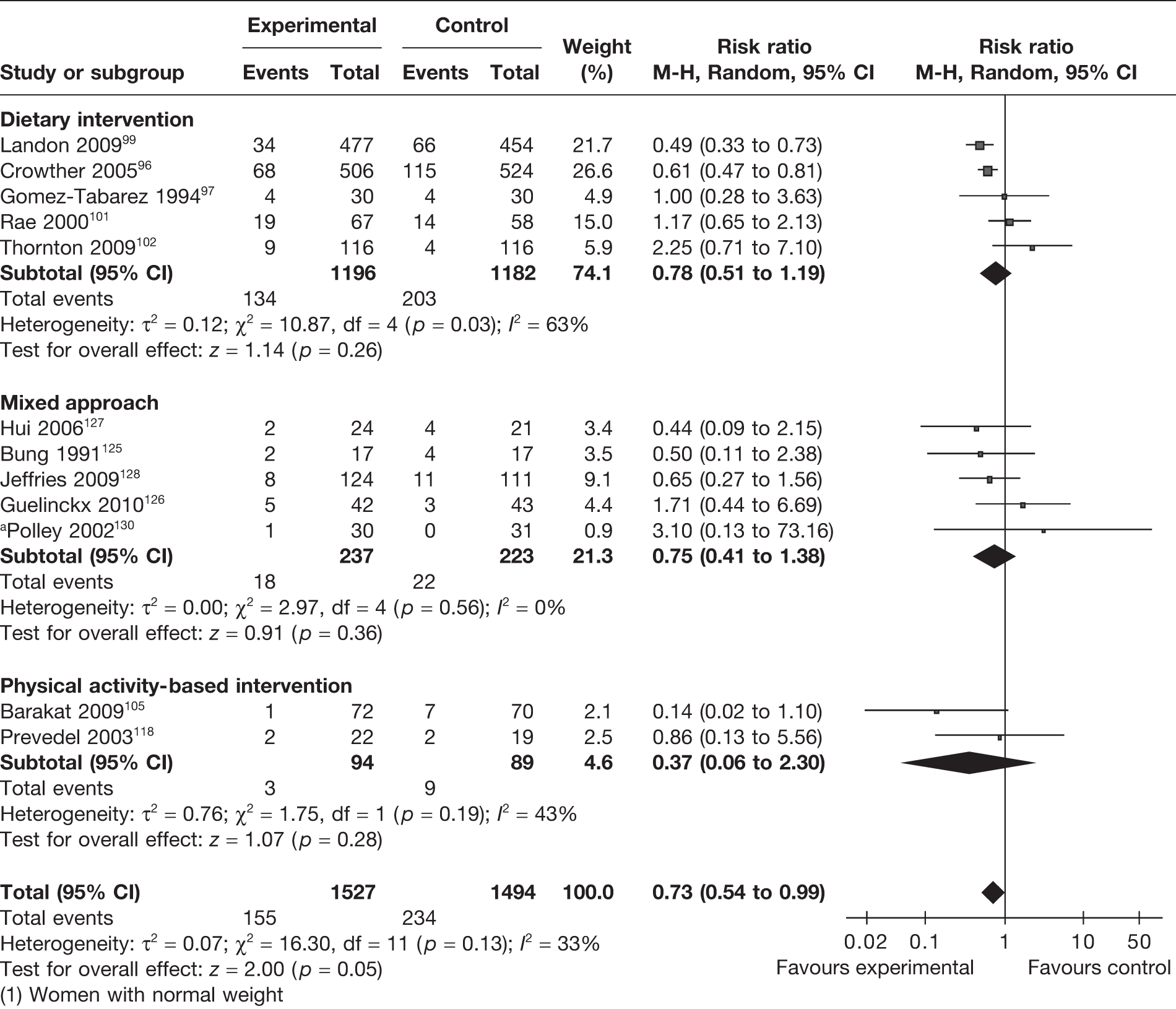
We defined LGA infants as those above the 90th centile or with a birthweight > 4 kg. Twelve RCTs96,97,99,101,102,105,118,125–128,130 evaluated this outcome in 3021 newborns. There was a 27% reduction (RR 0.73, 95% CI 0.54 to 0.99; p = 0.05) in the risk of having a LGA newborn. The results were not heterogeneous, with an I2 of 33% (p = 0.13). This reduction in the incidence of LGA infants was observed with all interventions in pregnancy (Figure 9). Five RCTs reported the effects of the interventions on obese and overweight women. There was no significant difference in the incidence of LGA infants between the experimental and control groups of obese and overweight women (RR 1.32, 95% CI 0.55 to 3.16; p = 0.54; I2 = 78%).
FIGURE 9.
Effect of weight management interventions on the incidence of LGA infants. a, Women with normal weight.
Small for gestational age at birth
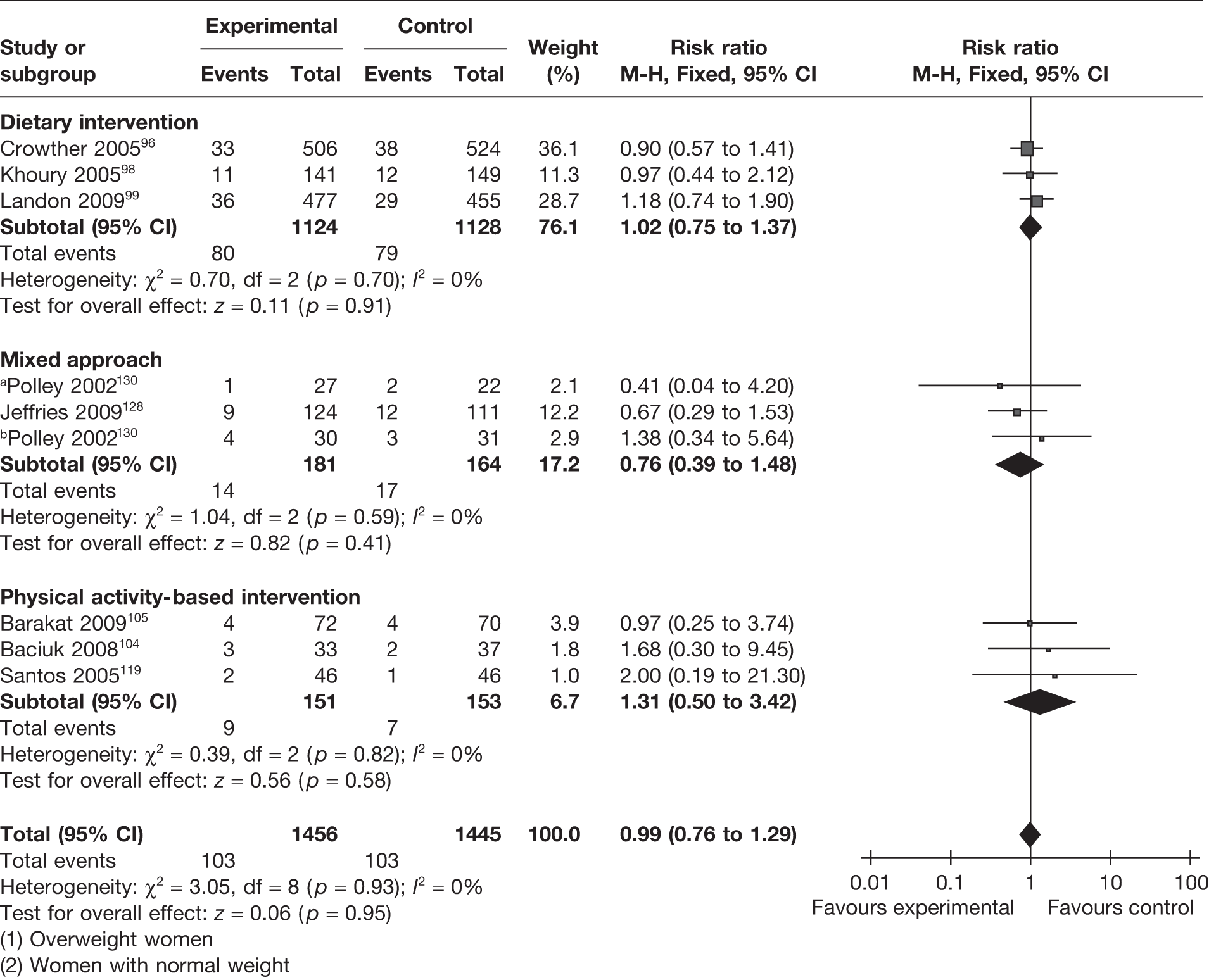
Small-for-gestational-age newborns were defined as those with a birthweight below the 10th centile or < 2.5 kg. This outcome served the dual purpose of assessment of the beneficial effect of the intervention and assessment of any adverse effect of the intervention on fetal weight. Eight RCTs96,98,99,104,105,119,128,130 (2901 newborns) evaluated the effectiveness of the weight management interventions for this outcome. The summary estimate of the RCTs showed no difference in the incidence of SGA infants with a RR of 0.99 (95% CI 0.76 to 1.29). The studies were homogeneous. The effect was consistently observed with all three interventions (Figure 10).
FIGURE 10.
Effect of weight management interventions on the incidence of SGA infants. a, Overweight women. b, Women with normal weight.
Ponderal index
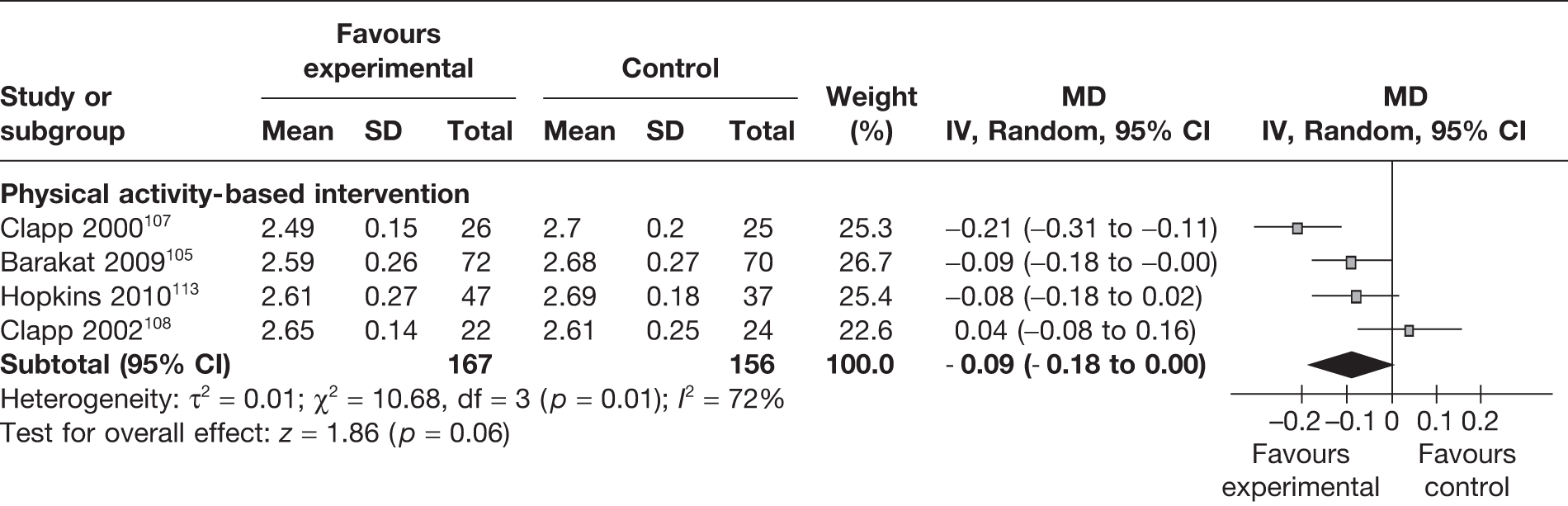
The ponderal index for newborns assesses the relationship between the weight of the newborn and its length (kg/m3). Four RCTs105,107,108,113 (333 newborns) evaluated the effect of the weight management interventions on the ponderal index. The summary estimate of the trials showed no significant difference in ponderal index of the newborns between the intervention and the control groups, with a MD of –0.09 kg/m3 (95% CI –0.18 to 0.00 kg/m3, I2 = 72%) (Figure 11).
FIGURE 11.
Effect of weight management interventions on ponderal index. SD, standard deviation.
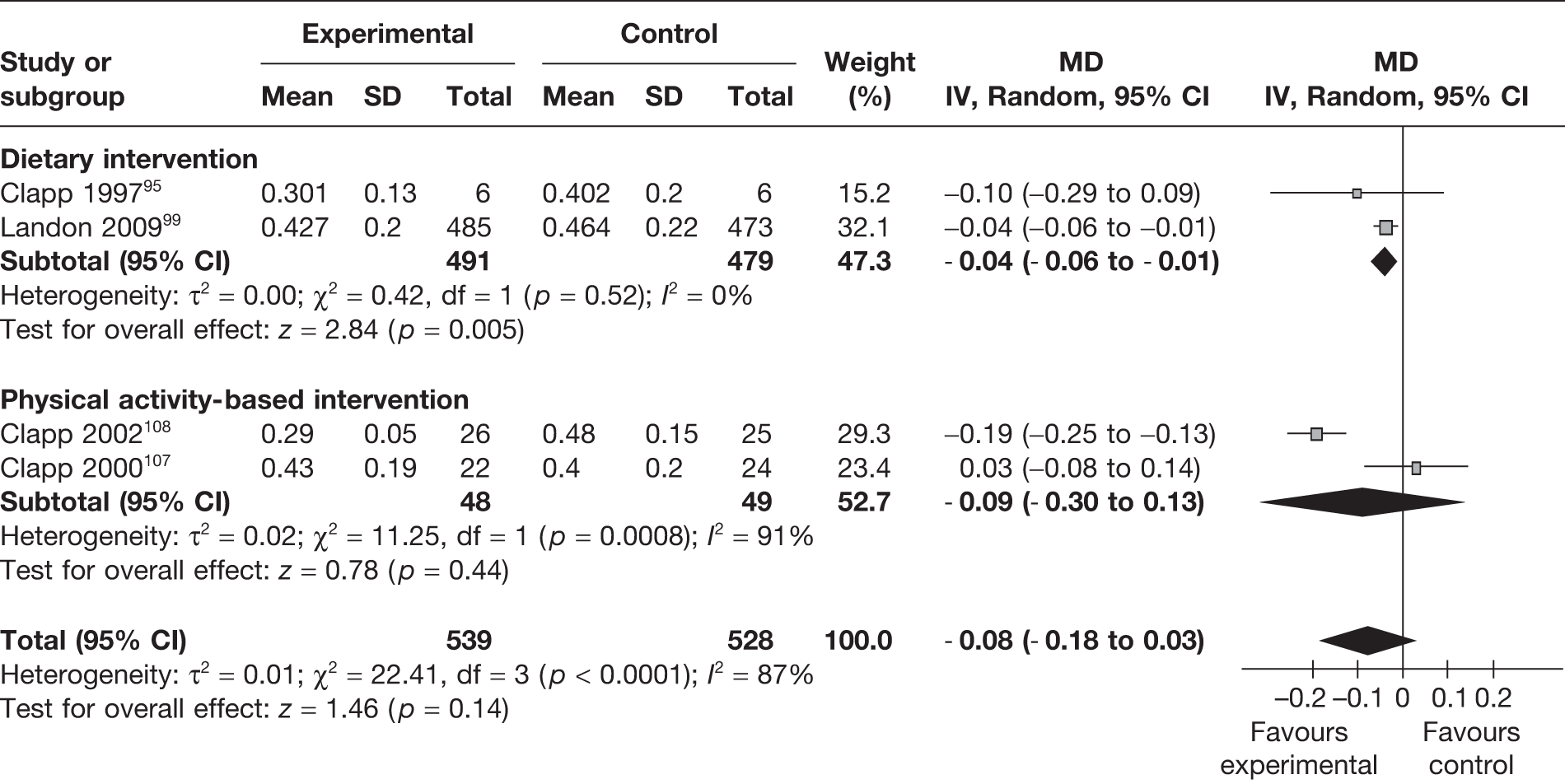
Fetal fat mass
Fetal fat mass in kilograms was reported in four trials. 95,99,107,108 Dietary interventions resulted in a significant reduction in fetal fat mass in the intervention group, with a MD of –0.04 kg (95% CI –0.06 kg to –0.01 kg; p = 0.005; I2 = 0%) (Figure 12).
FIGURE 12.
Effect of weight management interventions on fetal fat mass. SD, standard deviation.
Effect of the interventions on obstetric maternal outcomes
Gestational diabetes mellitus
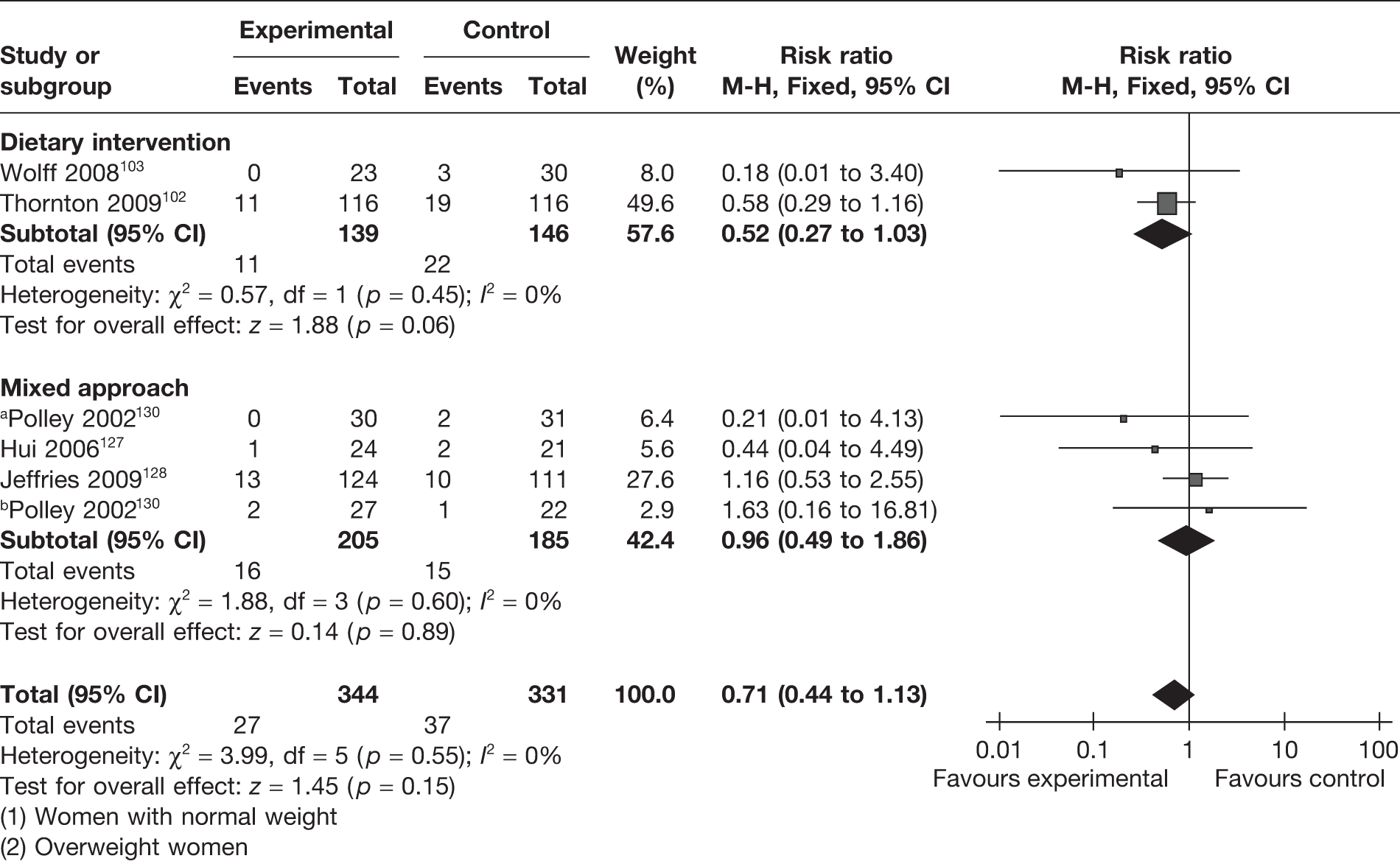
Five RCTs (involving 675 women) reported on the effect of weight management interventions on gestational diabetes mellitus (GDM). Three studies included only obese or overweight pregnant women for the evaluation of a dietary intervention (two RCTs102,103) and a mixed approach-based intervention (one RCT130). There was an overall reduction in the incidence of GDM of 29% (RR 0.71, 95% CI 0.44 to 1.13; p = 0.15), which was not statistically significant (Figure 13). Weight management interventions in obese and overweight women showed a reduction of 42% (RR 0.58, 95% CI 0.30 to 1.09; p = 0.09). The findings were homogeneous (I2 =0) across studies and did not reach statistical significance.
FIGURE 13.
Effect of weight management interventions on GDM. a, Women with normal weight. b, Overweight women.
Pre-eclampsia
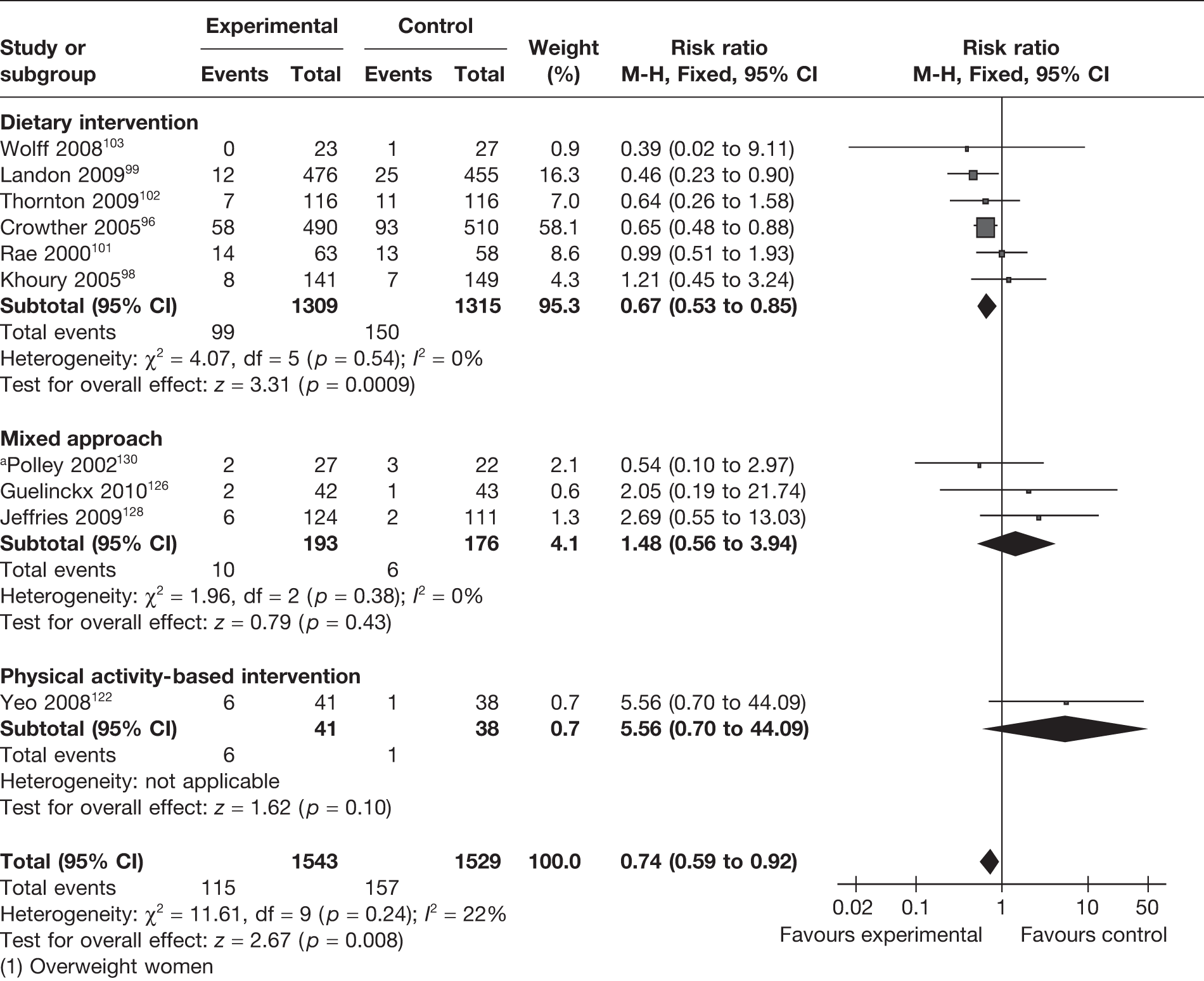
Ten studies96,98,99,101–103,122,126,128,130 (involving 3072 women) reported the effect of weight management interventions on the incidence of pre-eclampsia. There was an overall statistically significant reduction in pre-eclampsia of 26% (RR 0.74, 95% CI 0.59 to 0.92; p = 0.008; I2 = 22%). The largest reduction in pre-eclampsia (33%) was observed with dietary intervention (RR 0.67, 95% CI 0.53 to 0.85; p = 0.0009) with no heterogeneity (I2 = 0). A similar effect was not observed with physical activity-based intervention or a mixed approach (Figure 14). Six studies included only obese and overweight women and showed a significant reduction in pre-eclampsia with the interventions (RR 0.65, 95% CI 0.44 to 0.97; p = 0.04; I2 = 0).
FIGURE 14.
Effect of weight management interventions on the incidence of pre-eclampsia. a, Overweight women.
Gestational hypertension
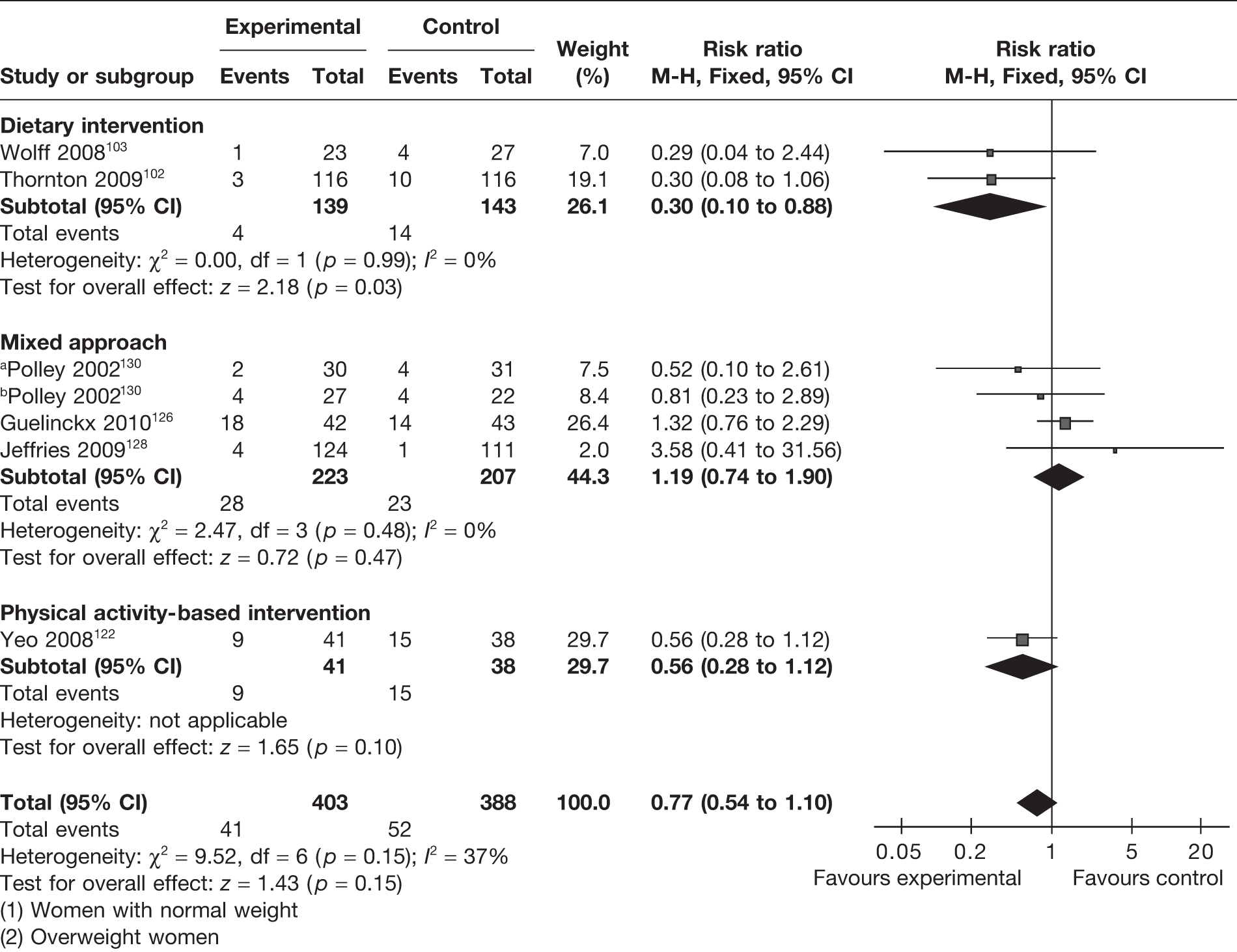
Gestational hypertension was evaluated as an outcome in six RCTs. 102,103,122,126,128,130 There was a reduction in gestational hypertension with interventions, which was not statistically significant (RR 0.77, 95% CI 0.54 to 1.1; I2 = 37%) (Figure 15). Dietary intervention (two RCTs)102,103 in pregnancy showed the greatest benefit by reducing gestational hypertension by 70% (RR 0.30, 95% CI 0.10 to 0.88; p = 0.03), with homogeneity between the studies (I2 = 0). Both of the studies on dietary intervention were undertaken in obese and overweight women. The four studies on obese and overweight women102,103,126,130 showed a reduction in gestational hypertension incidence that was not significant (RR 0.70, 95% 0.30 to 1.16; p = 0.4).
FIGURE 15.
Effect of weight management interventions on the incidence of gestational hypertension. a, Women with normal weight. b, Overweight women.
Preterm delivery
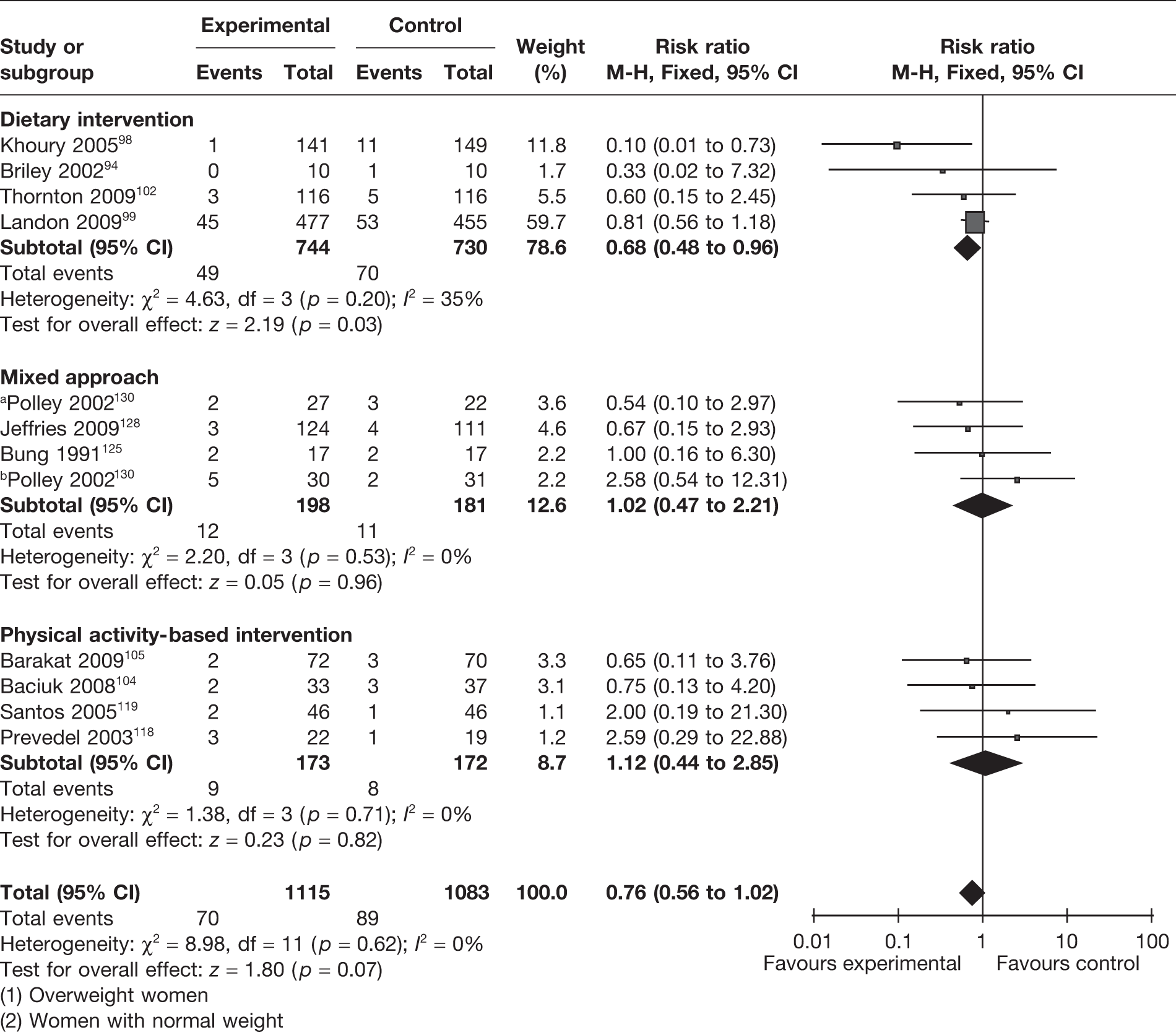
Eleven RCTs (involving 2198 women)94,98,99,102,104,105,118,119,125,128,130 evaluated the effectiveness of weight management interventions in pregnancy on preterm delivery before 37 weeks of gestation. There was no overall difference in the rates of preterm births between the two groups, with a RR of 0.76 (95% CI 0.56 to 1.02) (Figure 16). The studies were homogeneous (I2 = 0%). The four RCTs94,98,99,102 that evaluated a dietary intervention (n = 1474) showed a significant reduction in preterm births of 32% (RR 0.68, 95% CI 0.48 to 0.96; p = 0.03; I2 = 35%). Four RCTs99,102,119,130 (involving 1305 women) including obese and overweight women showed a reduction in preterm births that was not statistically significant (RR 0.80, 95% CI 0.53 to 1.13; p = 0.21, I2 = 0%).
FIGURE 16.
Effect of weight management interventions on preterm delivery before 37 weeks of gestation. a, Overweight women. b, Women with normal weight.
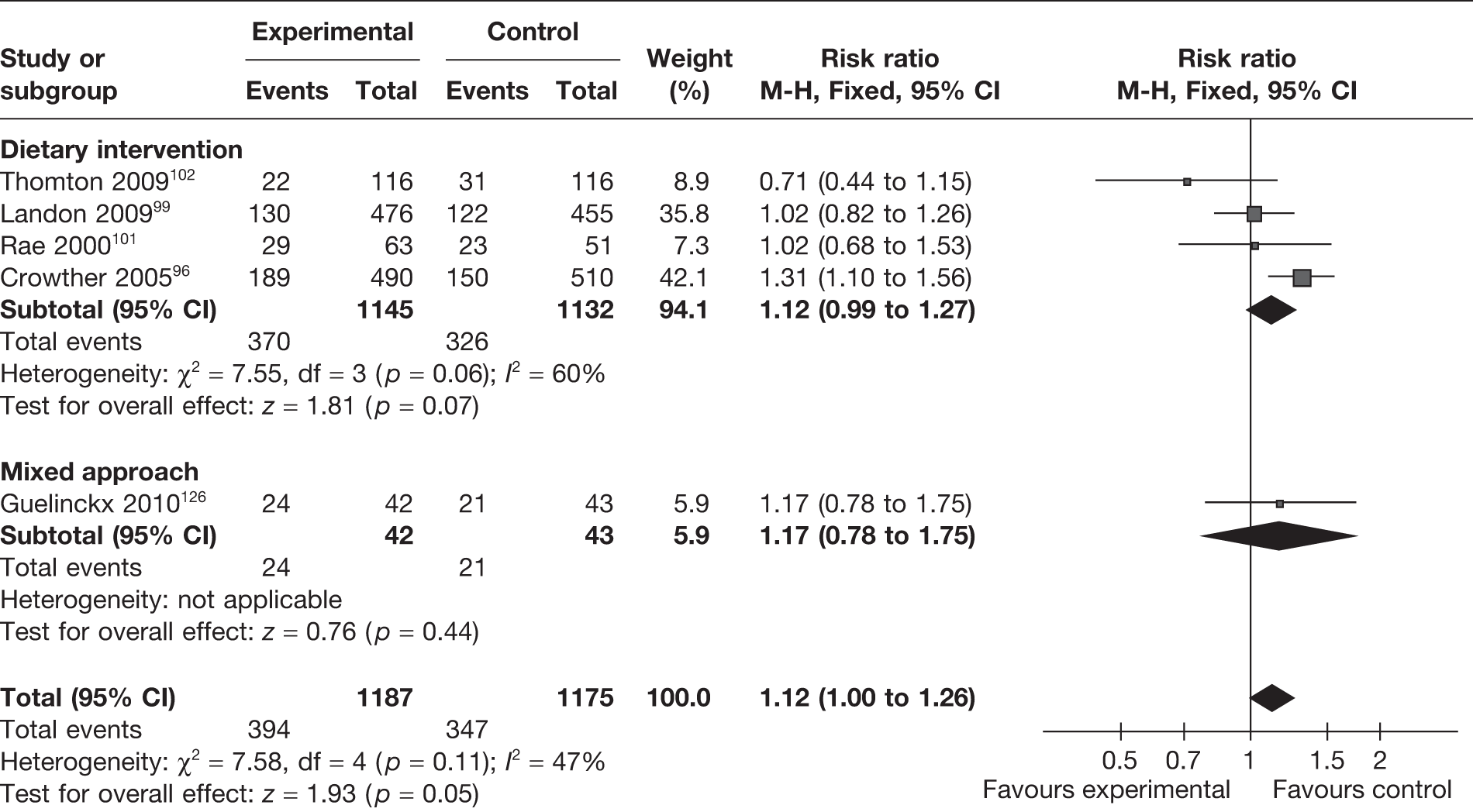
Gestational age at delivery
A total of 20 RCTs96,98–105,107,108,110,111,113–116,120,125–127 (4028 women) evaluated the effect of the interventions on the gestational age at delivery. There were no significant differences in the gestational age at delivery between the intervention and control groups, with a MD of 0.03 weeks (95% CI –0.13 weeks to 0.07 weeks; I2 = 33%) (Figure 17). There was low heterogeneity between studies (I2 = 33%). Dietary intervention (six RCTs, involving 2625 women) resulted in a MD in the gestational age at delivery of 0.05 weeks (95% CI –0.18 weeks to 0.08 weeks; p = 0.42; I2 = 71%).
FIGURE 17.
Effect of weight management interventions on gestational age at delivery. SD, standard deviation. a, Women with normal weight. b, Overweight women. c, Data from Kramer 2006 review.
Mode of delivery
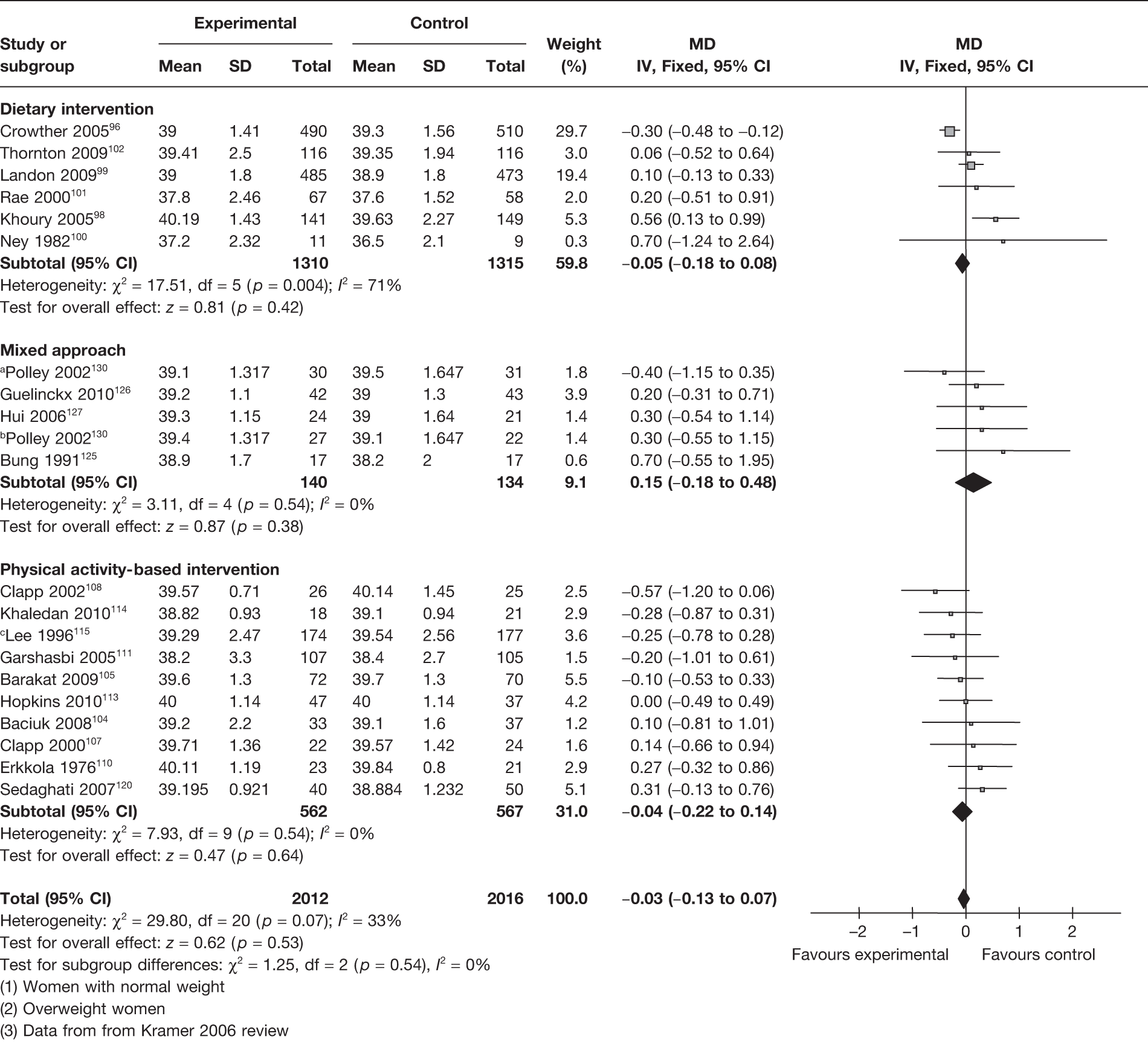
The rate of caesarean section was evaluated as an outcome in 14 RCTs96,97,99,102–104,114–116,124–126,128,130 involving 3312 women. This included five trials96,97,99,102,103 on dietary interventions, four104,114–116 on physical activity-based interventions and five124–126,128,130 on a mixed approach. There were no differences between the experimental and the control groups with any intervention. The summary estimate for caesarean section was a RR of 0.93 (95% CI 0.85 to 1.03; p = 0.15) (Figure 18). There was no significant heterogeneity between the groups (p = 0.22, I2 = 21%). A total of 6 of the 14 RCTs involved obese and overweight women and showed no change in the rate of caesarean section (RR 0.97, 95% CI 0.73 to 1.28; I2 = 61%).
FIGURE 18.
Effect of weight management interventions on rate of caesarean section. a, Overweight women. b, Women with normal weight.
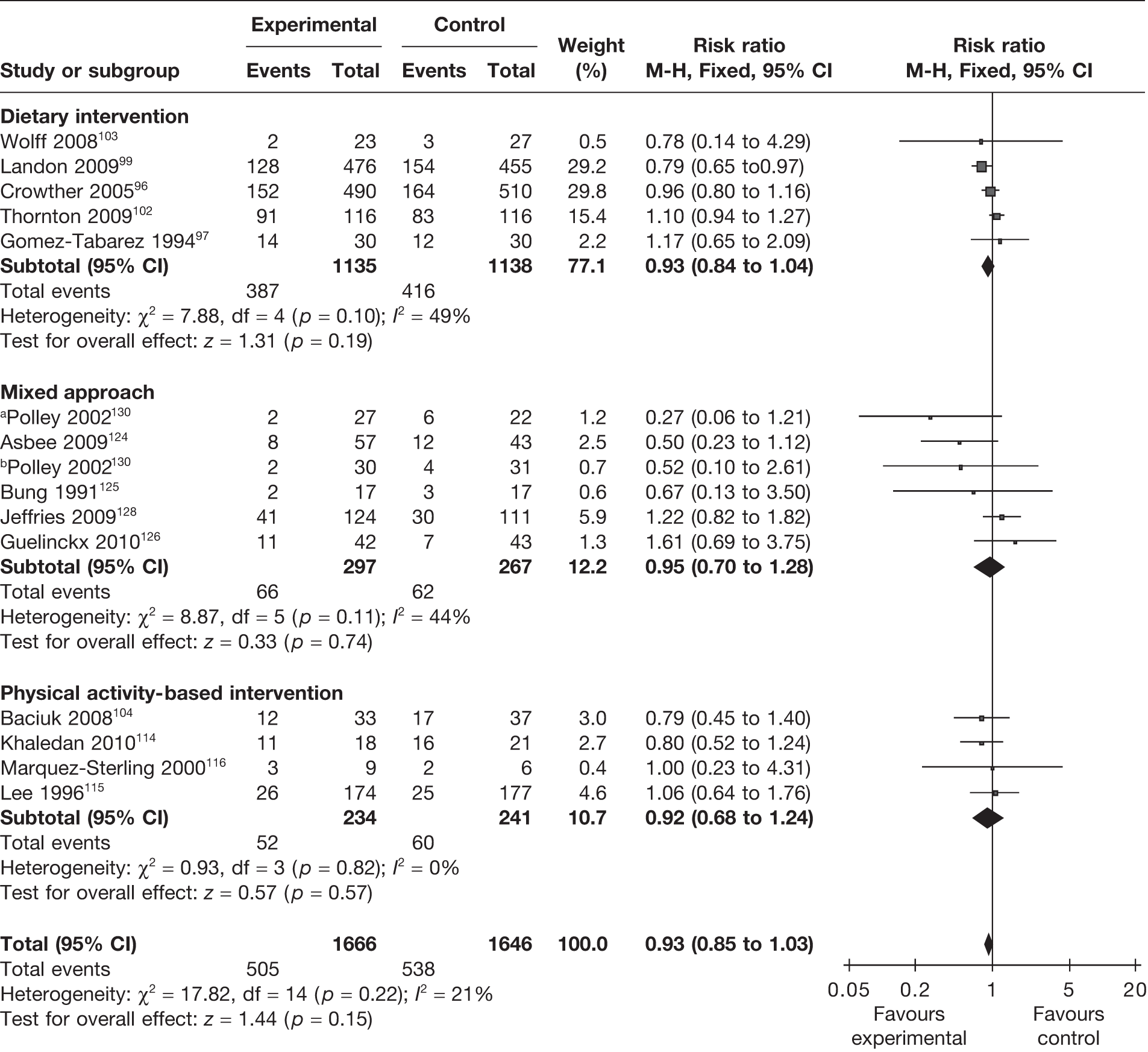
The rate of vaginal delivery was evaluated in five RCTs. 99,101,104,115,125 There was no difference in the rate of vaginal delivery with any intervention. The pooled estimate showed a RR of 1.00 (95% CI 0.94 to 1.07; p = 1). The studies were homogeneous (Figure 19). The effect of dietary intervention on vaginal delivery in obese and overweight mothers was studied in two RCTs. 99,101 The rate of vaginal delivery did not change with the intervention, with a RR of 0.97 (95% CI 0.89 to 1.07; I2 = 0).
FIGURE 19.
Effect of weight management interventions on rate of vaginal delivery.
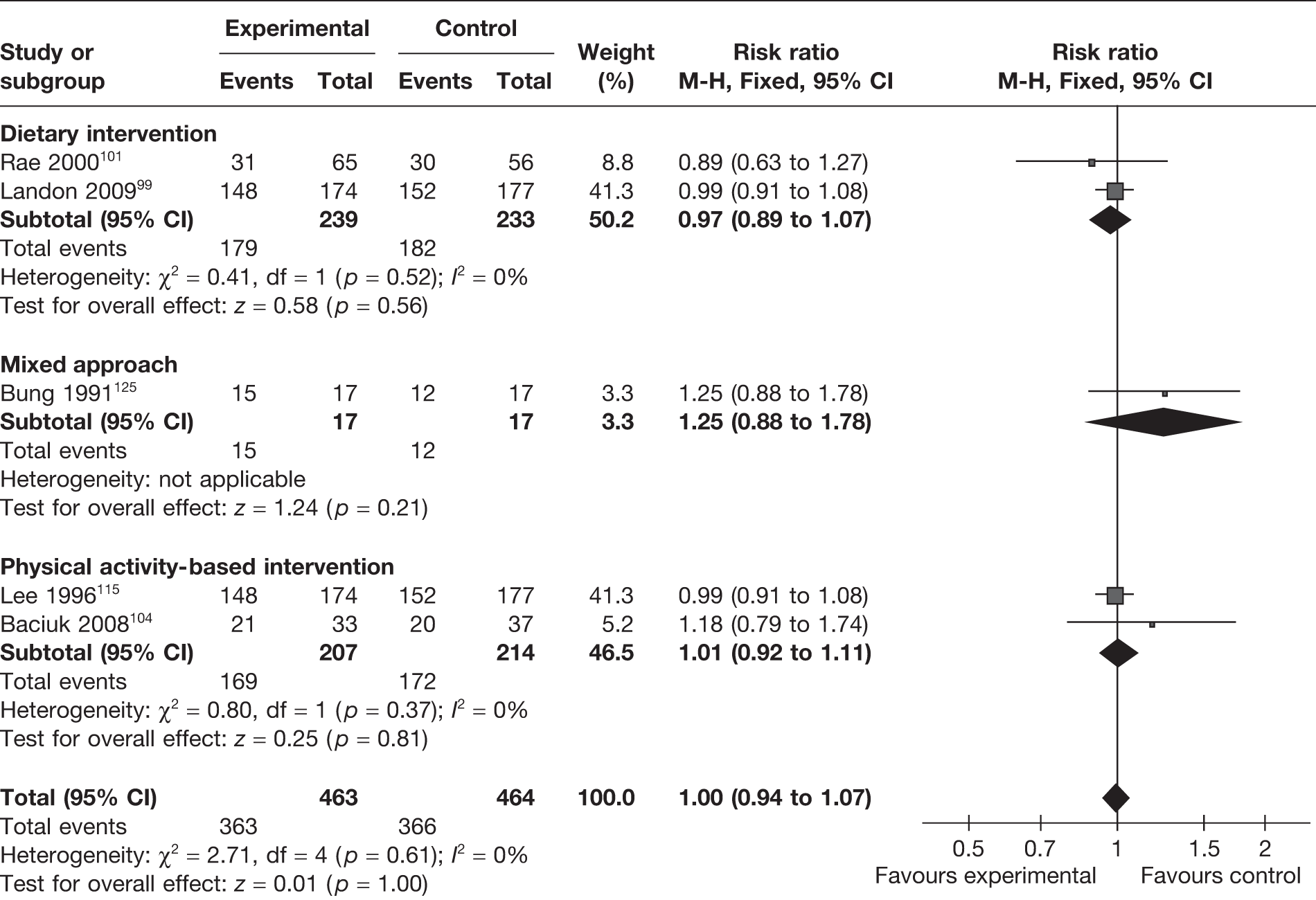
Induction of labour
The effect of weight management interventions in pregnancy on induction of labour was studied in five RCTs (involving 2362 women). 96,99,101,102,126 There was a slight increase in induction of labour in the intervention arm that was not significantly different from that of the control arm (RR 1.12, 95% CI 1.00 to 1.26; p = 0.05; I2 = 47%) (Figure 20). Obese and overweight women only were included in four RCTs99,101,102,126 (involving 1362 women); in these studies there was no difference in the rate of induction of labour between the intervention and control groups (RR 0.99, 95% CI 0.84 to 1.16; I2 = 0%).
FIGURE 20.
Effect of weight management interventions on induction of labour.
Post-partum haemorrhage
Two RCTs96,102 (n = 1232) compared the rates of post-partum haemorrhage between the weight management intervention group and the control group. The pooled estimate of the studies did not show any significant differences between the groups (RR 0.90, 95% CI 0.57 to 1.42; I2 = 0%) (Figure 21).
FIGURE 21.
Effect of weight management interventions on post-partum haemorrhage.
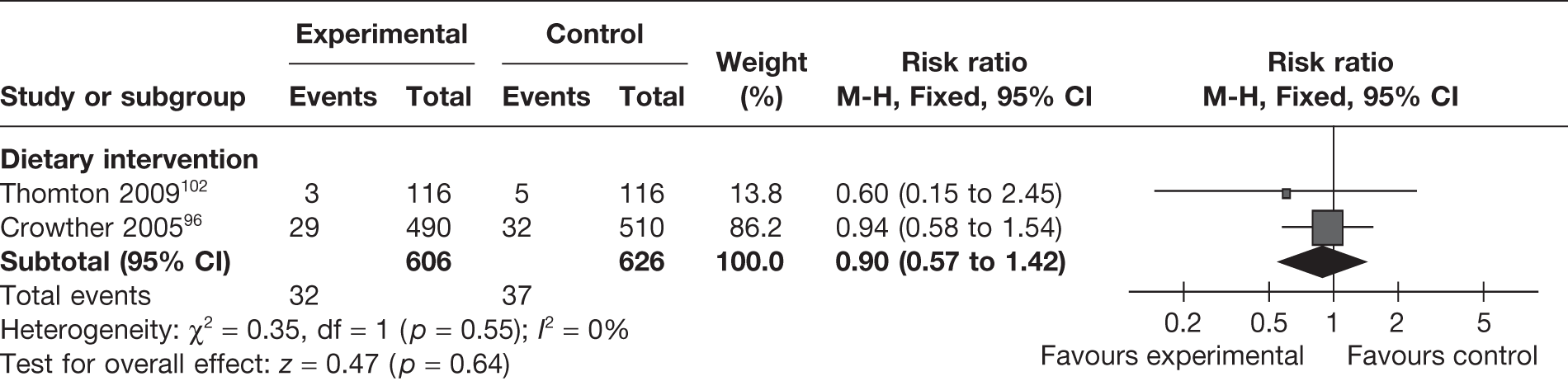
Two observational case–control studies77,78 studied the effect of physical activity-based interventions on post-partum haemorrhage and found no difference between the intervention and control groups.
Low back pain
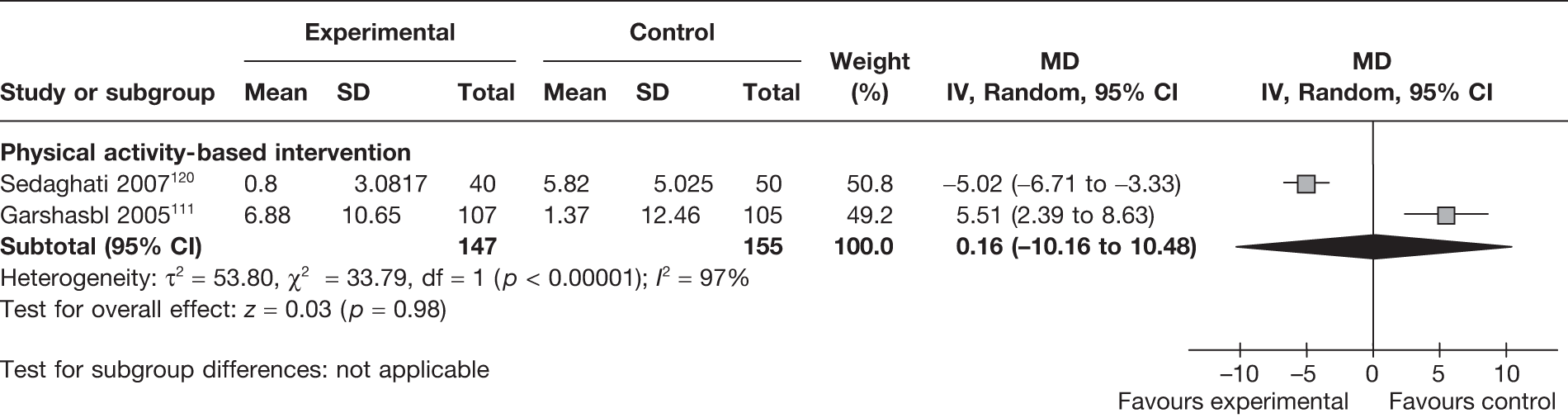
Low back pain was reported as an outcome in two RCTs111,126 (involving 302 women) evaluating physical activity-based interventions. The severity of low back pain was increased in one study111 and decreased in the other study. 120 The pooled estimate did not show any differences in back pain between the two groups (MD 0.16, 95% CI –10.16 to 10.48; I2 = 97%) (Figure 22).
FIGURE 22.
Effect of weight management interventions on low back pain in pregnancy. SD, standard deviation.
Effect of the interventions on fetal and neonatal morbidity and mortality
Shoulder dystocia
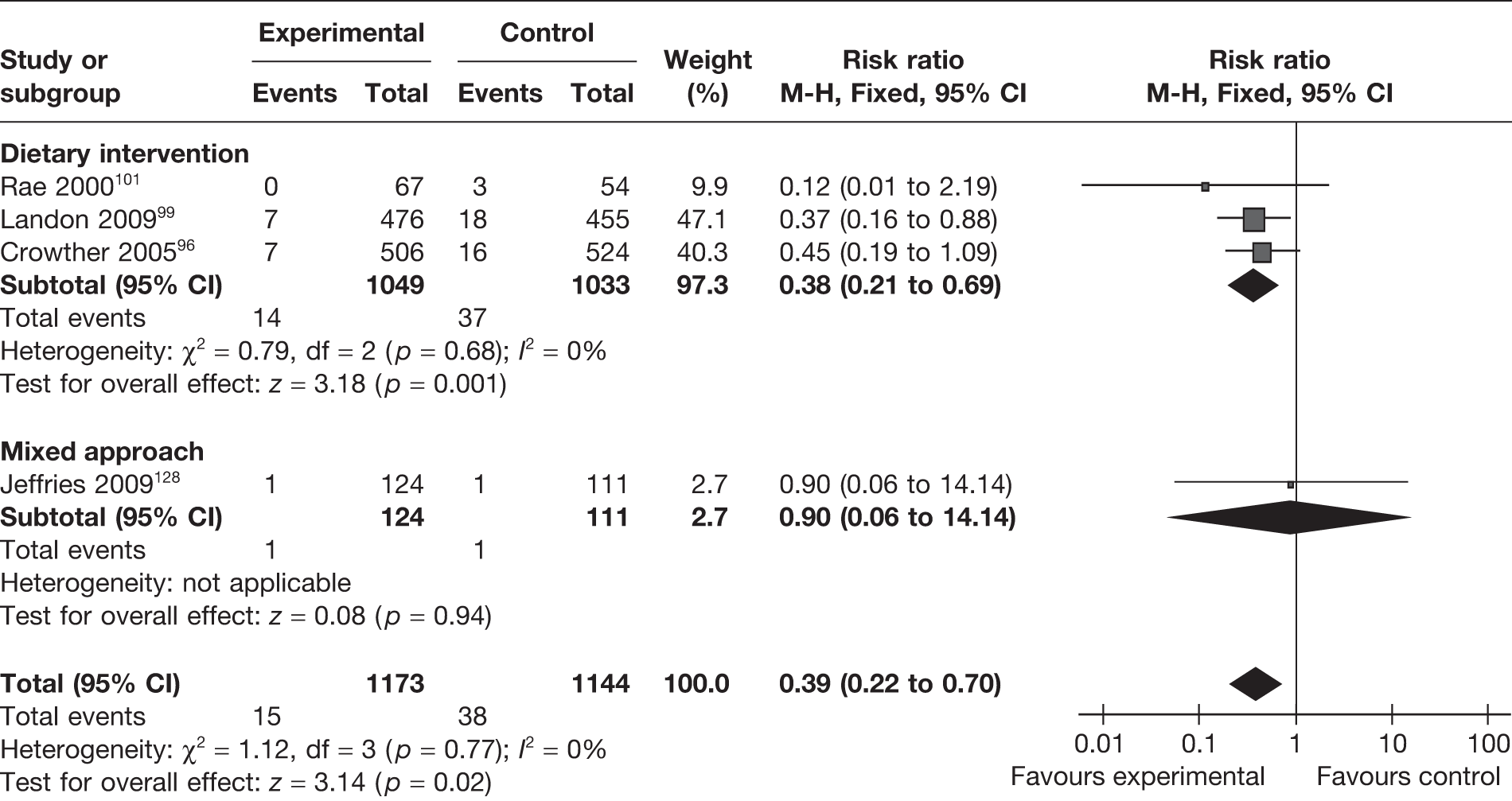
Four RCTs96,99,101,128 (2317 newborns) evaluated the effect of interventions (three dietary96,99,101 and one mixed128 approach) on the incidence of shoulder dystocia. Overall, there was a 61% reduction in the incidence of shoulder dystocia (RR 0.39, 95% CI 0.22 to 0.70; p = 0.02). The studies were homogeneous (I2 = 0%). The largest proportion of women in the analysis were in the dietary intervention group, which showed a similar effect (Figure 23). This beneficial effect was increased in the population of obese and overweight women (RR 0.33, 95% CI 0.14 to 0.74; p = 0.008).
FIGURE 23.
Effect of weight management interventions on shoulder dystocia.
Intrauterine death
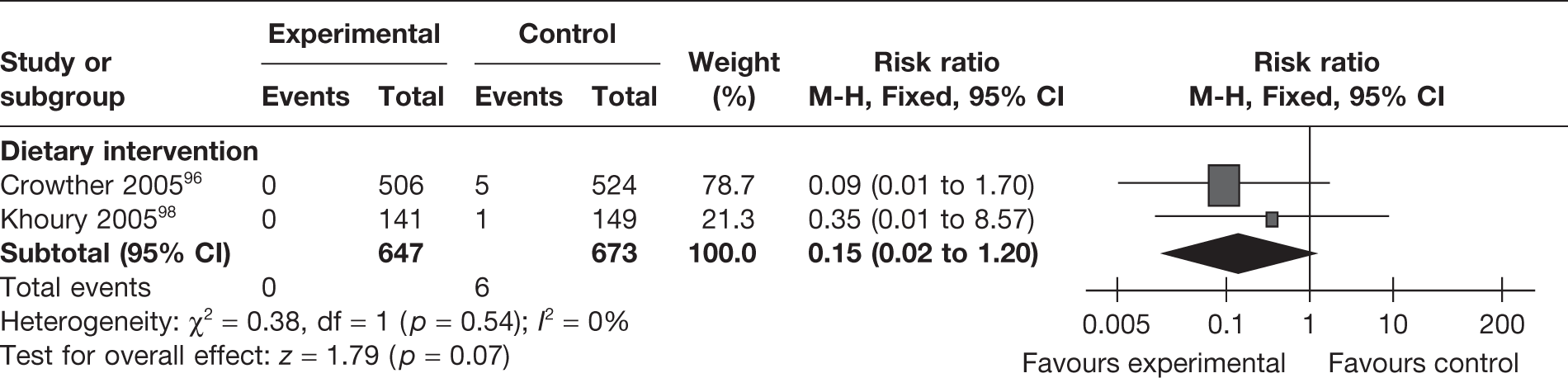
Two RCTs96,98 (involving 1320 women) evaluated the effect of dietary intervention on stillbirths. There was a reduction in the incidence of intrauterine death, which was not statistically significant (RR 0.15, 95% CI 0.02 to 1.20; p = 0.07; I2 = 0%) (Figure 24).
FIGURE 24.
Effect of weight management interventions on intrauterine death.
One observational cohort study by Perichart et al. 82 evaluated the effect of a dietary intervention compared with no intervention on intrauterine death. There were no significant differences between the groups. This effect was consistent for women with type 2 diabetes [unadjusted odds ratio (OR) 0.96, 95% CI 0.12 to 1.09] or GDM (unadjusted OR 1.00, 95% CI 0.06 to 16.57).
Respiratory distress syndrome
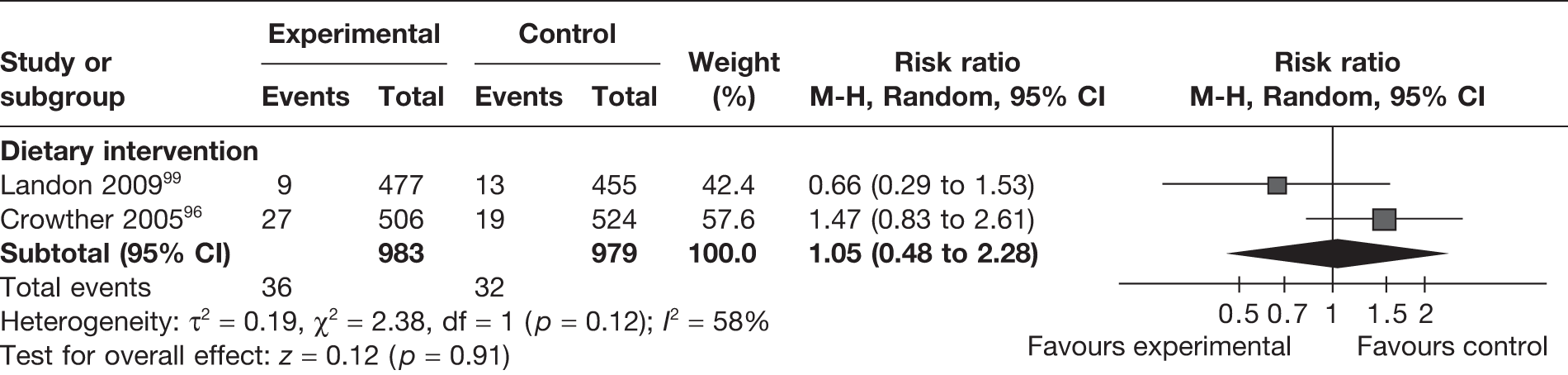
Two RCTs96,99 (involving 1962 women) evaluated respiratory distress syndrome with the newborn in mothers undergoing a weight management intervention in pregnancy. The two studies were on dietary interventions and the pooled estimate did not show a difference between the intervention and control groups (RR 1.05, 95% CI 0.48 to 2.28; I2 = 58%) (Figure 25).
FIGURE 25.
Effect of weight management interventions on respiratory distress syndrome.
Admission to the neonatal intensive care unit
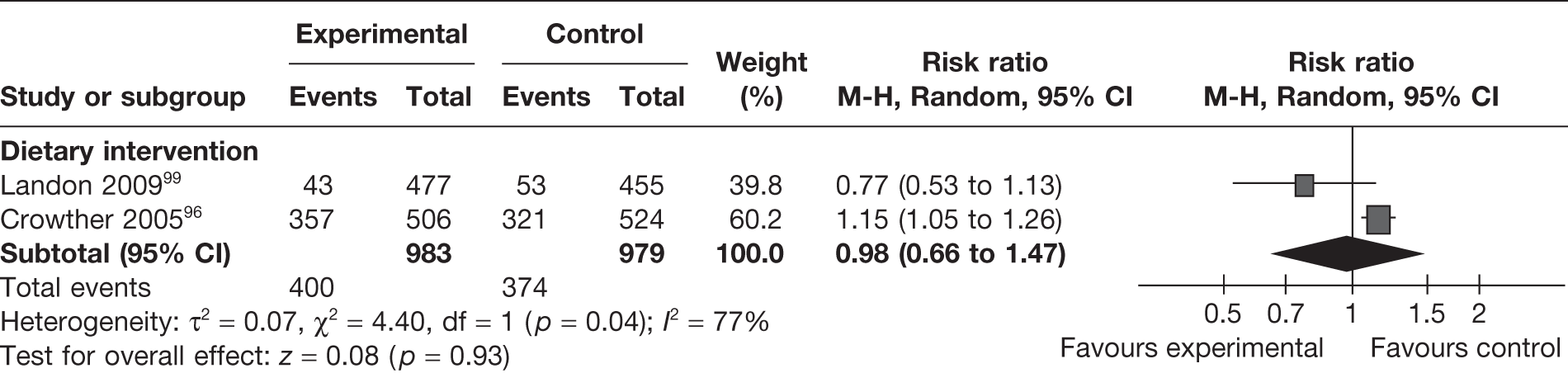
Admission to NICU was reported as an outcome in two RCTs96,99 (involving 1962 women) evaluating dietary interventions. The studies were heterogeneous (I2 = 77%) and the pooled estimate did not show any difference between the groups (RR 0.98, 95% CI 0.66 to 1.47) (Figure 26). One observational study82 evaluating a dietary intervention in pregnancy reported on NICU admission in two groups: women with type 2 diabetes and those with GDM. The reported unadjusted OR was significant only in the case of women with type 2 diabetes (OR 0.21, 95% CI 0.03 to 0.51).
FIGURE 26.
Effect of weight management interventions on admission to NICU.
Apgar scores
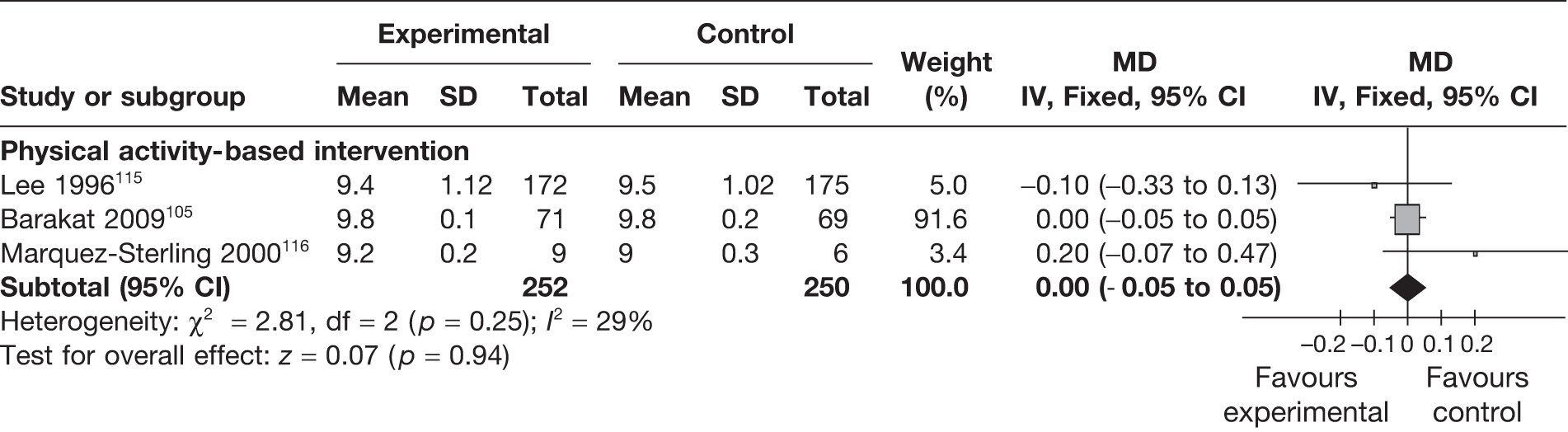
Apgar scores were evaluated as an outcome in six RCTs96,102,105,115,116,128 studying the effect of weight management interventions in pregnancy. Three studies96,102,128 reported scores of < 7 at 5 minutes and three studies105,115,116 provided the scores at 5 minutes for comparison. There were no differences in the abnormal scores (< 7 at 5 minutes) (RR 0.64, 95% CI 0.27 to 1.49; p = 0.3, I2 = 0%; Figure 27) or in the mean scores (MD 0.0, 95% CI –0.05 to 0.05; p = 0.94; Figure 28) between the two groups.
FIGURE 27.
Effect of weight management interventions on abnormal Apgar scores (< 7 at 5 minutes).
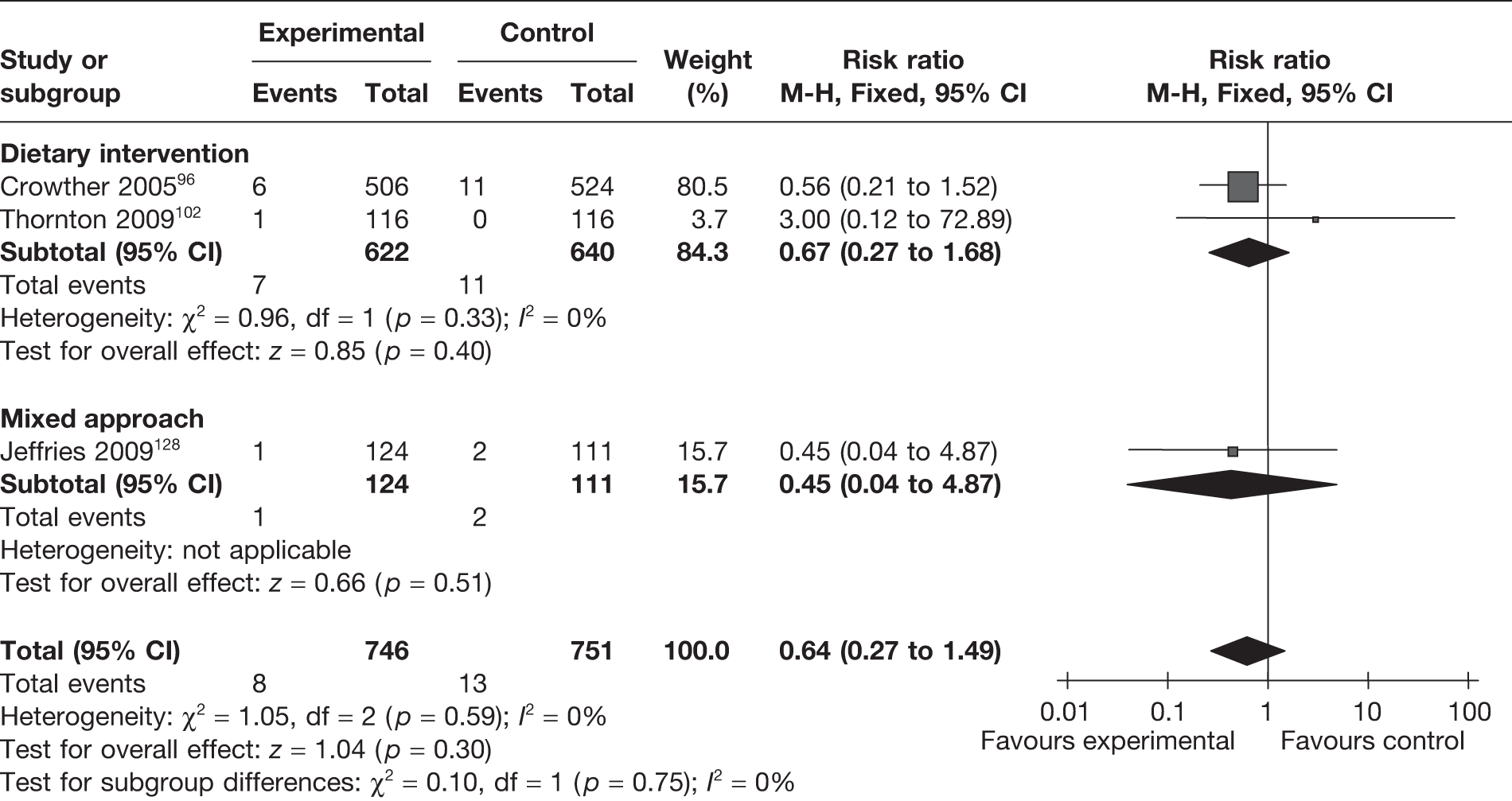
FIGURE 28.
Effect of weight management interventions on Apgar scores at 5 minutes. SD, standard deviation.
Infant hypoglycaemia
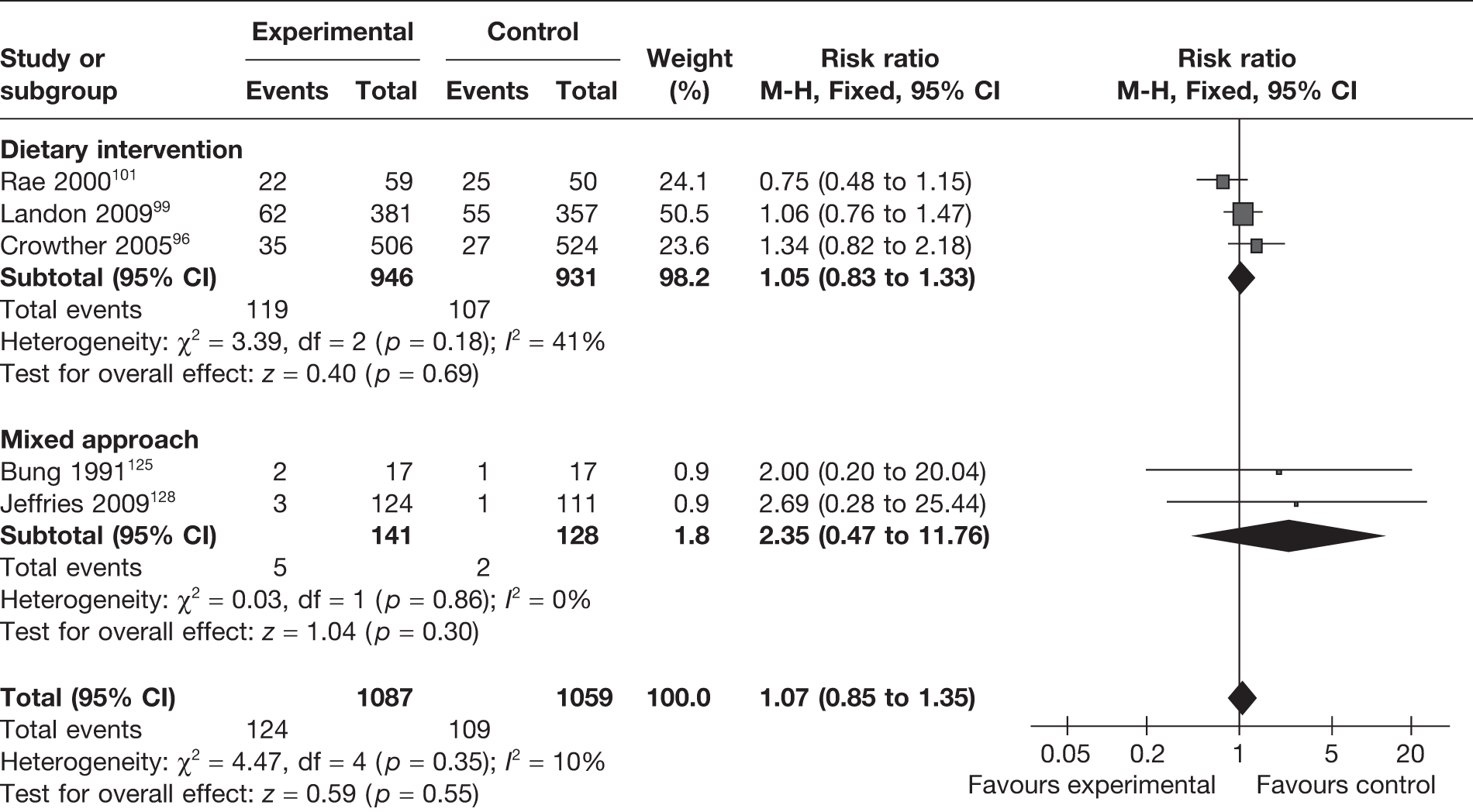
Hypoglycaemia in the first few days after birth is defined as blood glucose < 40 mg/dl. In preterm infants, repeated blood glucose levels of < 50 mg/dl may be associated with neurodevelopmental delay. Five RCTs96,99,101,125,128 reported the rate of hypoglycaemia among the children of studied mothers. Neither a comprehensive approach nor dietary interventions had any significant influence on hypoglycaemia rate (Figure 29).
FIGURE 29.
Effect of weight management interventions on infant hypoglycaemia.
Infant hyperbilirubinaemia
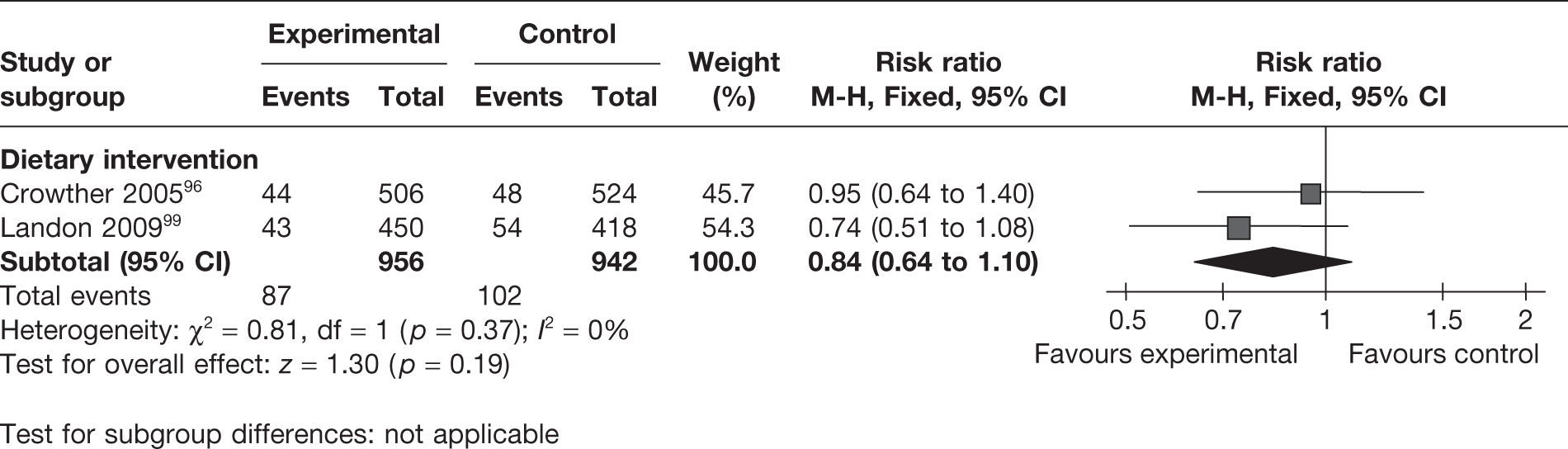
Two RCTs96,99 evaluated the effect of dietary interventions on the rates of hyperbilirubinaemia in 1898 newborns. The studies were homogeneous. There was a trend towards a reduction in hyperbilirubinaemia with the interventions, which was not significant (Figure 30).
FIGURE 30.
Effect of weight management interventions on infant hyperbilirubinaemia.
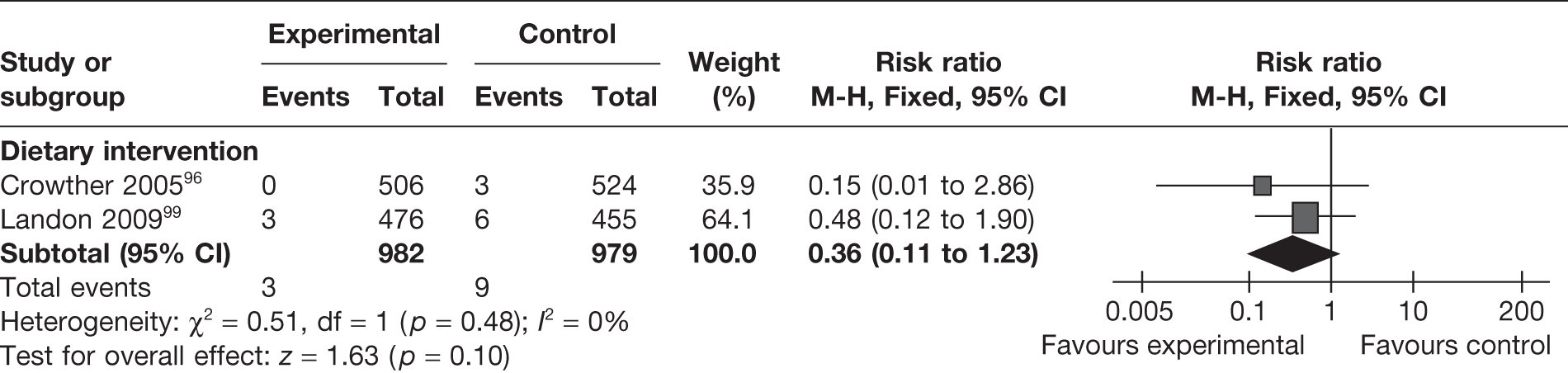
Birth trauma
Two RCTs96,99 evaluated the effect of dietary interventions on the risk of birth trauma. The studies showed a reduction in the risk of birth trauma (RR 0.36, 95% CI 0.11 to 1.23; I2 = 0%), which was not statistically significant (Figure 31).
FIGURE 31.
Effect of weight management interventions on birth trauma.
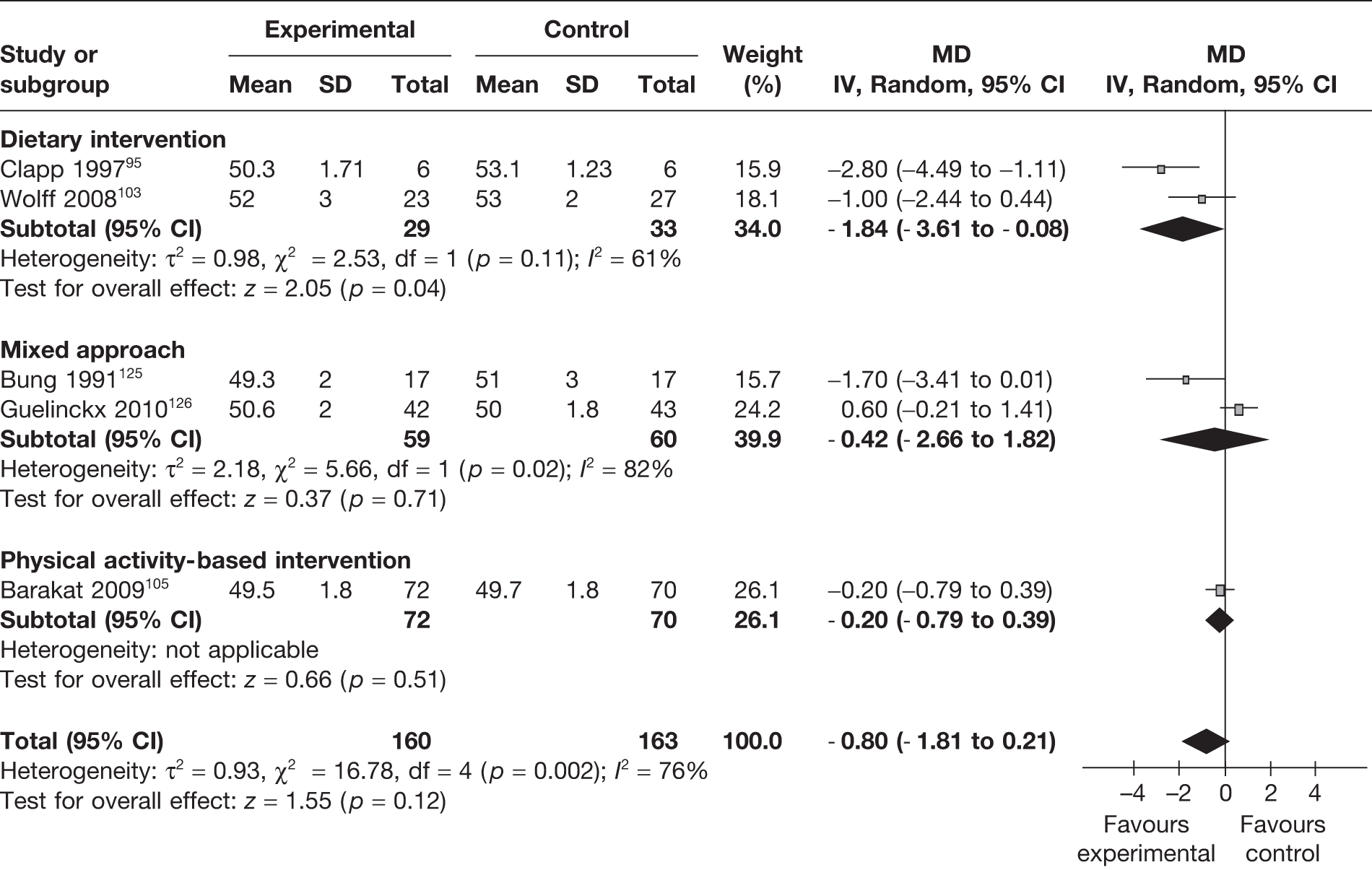
Effect of interventions on neonatal anthropometric measurements at birth
Child’s birth length
Five RCTs95,103,105,125,126 (323 newborns) evaluated the birth length of the newborn. The birth length of the newborn was reduced with the interventions, but the difference was not statistically significant (Figure 32).
FIGURE 32.
Effect of weight management interventions on birth length. SD, standard deviation.
Abdominal circumference of the newborn
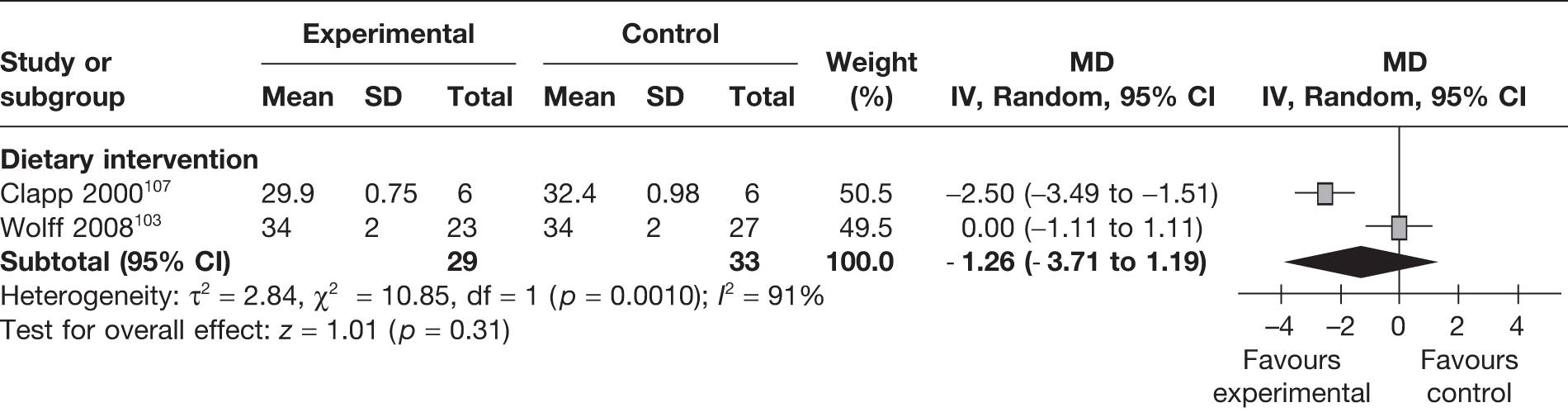
Two RCTs103,107 evaluated the effect of dietary weight management interventions on abdominal circumference in 62 newborns. The studies were heterogeneous and overall there was no significant change in the intervention group in comparison with the control group (MD –1.26 cm, 95% CI –3.71 cm to 1.19 cm; p = 0.31; I2 = 91%) (Figure 33).
FIGURE 33.
Effect of weight management interventions on abdominal circumference. SD, standard deviation.
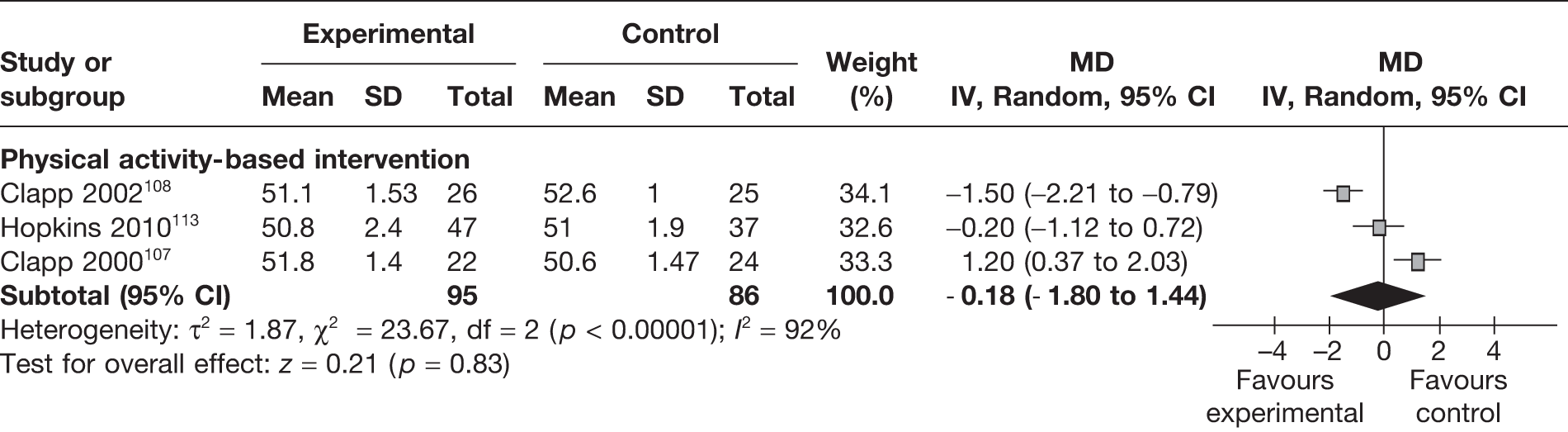
Crown–heel length
Three RCTs107,108,113 evaluated the effect of physical activity based weight management interventions on crown–heel length in 181 newborns. The studies were heterogeneous and overall there was no significant change in the intervention group in comparison with the control group (MD –0.18 cm, 95% CI –1.80 cm to 1.44 cm; p = 0.83; I2 = 92%) (Figure 34).
FIGURE 34.
Effect of weight management interventions on crown–heel length. SD, standard deviation.
Subgroup analyses
Subgroup analyses on the basis of period of publication, country of study (developed vs developing), GDM status and risk of bias from allocation concealment showed no differences in the summary estimates of gestational weight gain, birthweight and incidence of LGA and SGA infants. The type of intervention resulted in significant differences (p = 0.003) between the groups for weight gain in pregnancy, with the maximum reduction in gestational weight gain seen in the dietary intervention group (MD –3.36 kg, 95% CI –4.73 kg to –1.99 kg). Women with diabetes in pregnancy showed a significant reduction in the incidence of pre-eclampsia with weight management interventions (RR 0.65, 95% CI 0.50 to 0.84) compared with women without diabetes (RR 1.16, 95% CI 0.70 to 1.93), and the difference in the summary estimates between the groups was statistically significant (p = 0.04). There was a significant reduction in pre-eclampsia in the responders – women with significantly reduced gestational weight gain with intervention (RR 0.61, 95% CI 0.47 to 0.79) – compared with the group with no significant change in weight (RR 1.33, 95% CI 0.84 to 2.11) (p = 0.004). There was a significant difference between the responders (MD –0.29 kg, 95% CI –0.46 kg to –0.12 kg) and non-responders (MD –0.02 kg, 95% CI –0.06 kg to –0.03 kg) for birthweight (p = 0.002). Subgroup analysis of the summary estimates of birthweight and incidence of LGA and SGA infants did not show a statistically significant difference according to the type of intervention (Table 6).
| Subgroup | Gestational weight gain (kg) | Pre-eclampsia | Birthweight (kg) | LGA infants | SGA infants | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | MD (95% CI) | p-value for interaction | No. of studies | RR (95% CI | p-value for interaction | No. of studies | MD (95% CI) | p-value for interaction | No. of studies | RR (95% CI) | p-value for interaction | No. of studies | RR (95% CI | p-value for interaction | |
| Publication year | |||||||||||||||
| After 1990 | 28 | –1.22 (–1.77 to –0.66) | 0.57 | – | – | – | 26 | –0.08 (–0.15 to –0.02) | 0.11 | – | – | – | – | – | – |
| Before 1990 | 2 | 2.19 (–9.66 to 14.04) | – | – | 2 | 0.14 (–0.13 to 0.41) | – | – | – | – | |||||
| Country status | |||||||||||||||
| Developed countries | 24 | –1.09 (–1.92 to –0.27) | 0.42 | – | – | – | 23 | –0.08 (–0.15 to 0.00) | 0.77 | 10 | 0.72 (0.51 to 1.03) | 0.63 | 6 | 0.97 (0.74 to 1.27) | 0.40 |
| Developing countries | 6 | –0.64 (–1.39 to 0.12) | – | – | 5 | –0.06 (–0.16 to 0.04) | 2 | 0.95 (0.33 to 2.75) | 2 | 1.79 (0.44 to 7.23) | |||||
| Intervention type | |||||||||||||||
| Diet | 9 | –3.36 (–4.73 to –1.99) | 0.003 | 6 | 0.67 (0.53 to 0.85) | 0.05 | 9 | –0.07 (–0.21 to 0.07) | 0.45 | 5 | 0.78 (0.51 to 1.19) | 0.73 | 3 | 1.02 (0.75 to 1.37) | 0.61 |
| Mixed | 6 | –0.36 (–1.40 to 0.68) | 1 | 5.56 (0.70 to 44.09) | 14 | –0.02 (–0.10 to 0.07) | 5 | 0.75 (0.41 to 1.38) | 2 | 0.76 (0.39 to 1.48) | |||||
| Physical activity | 15 | –0.07 (–1.08 to 0.93) | 3 | 1.48 (0.56 to 3.94) | 5 | –0.09 (–0.18 to 0.00) | 2 | 0.37 (0.06 to 2.30) | 3 | 1.31 (0.50 to 3.42) | |||||
| Diabetic status | |||||||||||||||
| Women with diabetes | 5 | –1.84 (–2.36 to –1.32) | 0.09 | 3 | 0.65 (0.50 to 0.84) | 0.04 | 5 | –0.06 (–0.17 to 0.05) | 0.75 | 4 | 0.65 (0.46 to 0.92) | 0.30 | 2 | 1.03 (0.74 to 1.42) | 0.73 |
| Normal women | 25 | –0.86 (–1.85 to 0.13) | 7 | 1.16 (0.70 to 1.93) | 23 | –0.08 (–0.16 to 0.00) | 8 | 0.91 (0.53 to 1.59) | 6 | 0.93 (0.59 to 1.46) | |||||
| Risk of bias – allocation concealment | |||||||||||||||
| High risk | 27 | –0.81 (–1.60 to –0.01) | 0.18 | 8 | 0.77 (0.60 to 0.98) | 0.48 | 25 | –0.08 (–0.15 to 0.00) | 0.85 | 11 | 0.82 (0.57 to 1.16) | 0.06 | 5 | 0.88 (0.62 to 1.26) | 0.33 |
| Low risk | 3 | –1.79 (–2.98 to –0.60) | 2 | 0.62 (0.36 to 1.06) | 3 | –0.06 (–0.16 to 0.03) | 1 | 0.49 (0.33 to 0.73) | 3 | 1.15 (0.77 to 1.70) | |||||
| Maternal weight change with intervention | |||||||||||||||
| Significantly reduced gestational weight gain | 4 | 0.61 (0.47 to 0.79) | 0.004 | 6 | –0.29 (–0.46 to –0.12) | 0.002 | 3 | 0.67 (0.41 to 1.07) | 0.36 | 2 | 1.03 (0.74 to 1.42) | 0.73 | |||
| No significant change in gestational weight gain | 6 | 1.33 (0.84 to 2.11) | 22 | –0.02 (–0.06 to –0.03) | 9 | 0.88 (0.60 to 1.30) | 7 | 0.93 (0.59 to 1.46) | |||||||
Sensitivity analysis that excluded studies on women with diabetes in pregnancy consistently showed a overall reduction in gestational weight gain with interventions (MD –0.88 kg, 95% CI –1.85 kg to 0.09 kg; p = 0.001), including diet (MD –5.18 kg, 95% CI –9.44 kg to –0.91 kg; p < 0.00001) and physical activity (MD –0.07 kg, 95% CI –1.08 kg to 0.93 kg; p < 0.00001). The reduction in birthweight with intervention persisted (MD –0.08 kg, 95% CI –0.16 kg to 0.0 kg; p = 0.04) with no differences in the incidence of SGA and LGA infants or shoulder dystocia between the groups. The estimates of other studies for the effect of diet on the incidence of gestational hypertension, preterm birth, vaginal delivery, caesarean section and SGA infants were similar after excluding studies on women with diabetes. There was a trend towards a reduction in the incidence of pre-eclampsia with diet in these studies.
Summary
This review on the effectiveness of weight management interventions has identified a large number of RCTs, especially for the primary weight-related outcomes in the mother and the fetus. Two-thirds of the included studies showed a low risk of bias for addressing incomplete outcome data, selective reporting and blinding for objective outcomes. Fewer than one-sixth of the studies showed a high risk of bias for addressing incomplete outcome data and selective reporting. The commonly reported outcomes were maternal weight gain in pregnancy and birthweight of the newborn.
Weight management interventions in pregnancy resulted in a statistically significant reduction in weight-related outcomes such as maternal weight gain in pregnancy, and birthweight of the newborn. However, there were no differences between the intervention and control groups for incidence of SGA fetuses. Although we did not observe a beneficial effect of reduction in growth restriction in the babies with intervention, it was a reassuring finding because there have been concerns over fetal weight reduction with weight management interventions.
There was a significant decrease in the rates of key obstetric outcomes such as pre-eclampsia and shoulder dystocia in the analysis of outcomes for all interventions. It is likely that this reduction in shoulder dystocia will be of greatest benefit in women with GDM or pre-existing diabetes. There was a trend towards a reduction in the rates of obstetric complications such as GDM, gestational hypertension and preterm birth before 37 weeks with weight management interventions.
Of the three interventions, dietary intervention showed the most beneficial effect by significantly reducing rates of obstetric complications such as gestational hypertension, preterm births, pre-eclampsia and shoulder dystocia. The significant reduction in the rate of preterm births with dietary interventions is likely to be reflected in the finding of increased gestational age with dietary interventions. For fetal outcomes the evidence was limited to dietary interventions only and showed a trend towards a reduction in rates of intrauterine deaths, birth trauma and hyperbilirubinaemia.
The dietary components of the interventions evaluated a balanced diet of carbohydrates, fat and protein, moderate energy and caloric restriction based on individual requirements, low-fat and -cholesterol diets and the use of a food diary for monitoring. The physical activity-based interventions included weight-bearing sessions, walking for 30 minutes a day and low-intensity resistance training. The mixed approach group included dietary and physical activity interventions with associated in-depth behavioural risk assessments and tailored counselling.
The main strengths of the effectiveness review were the peer-reviewed protocol, the comprehensive search strategy without any language restrictions and the use of randomised data to draw inferences. Non-randomised data were included only when there was a paucity of evidence. This review has identified the largest body of evidence on this topic, for both weight-related outcomes and clinically relevant obstetric and fetal outcomes. Dietary interventions in pregnancy have consistently shown a beneficial effect on weight-related, obstetric and fetal and neonatal outcomes compared with other interventions. The review findings are limited by the lack of detail about the components of the intervention in some of the included studies, gestational age at which the intervention was commenced, its frequency and the method of delivery. Furthermore, there are very few studies for important clinical outcomes such as intrauterine death, maternal admission to the high-dependency unit (HDU) and neonatal admissions to NICU. There are no data available to assess the long-term effects of these outcomes on the mother and the fetus.
[Note: The results of this systematic review for effectiveness of weight management interventions in pregnancy includes only studies published before March 2011. The findings with the updated search (until January 2012) can be accessed at BMJ 2012;344:e2088 doi10.1136/bmj.e2088.]
Chapter 4 Adverse effects of interventions
Study selection
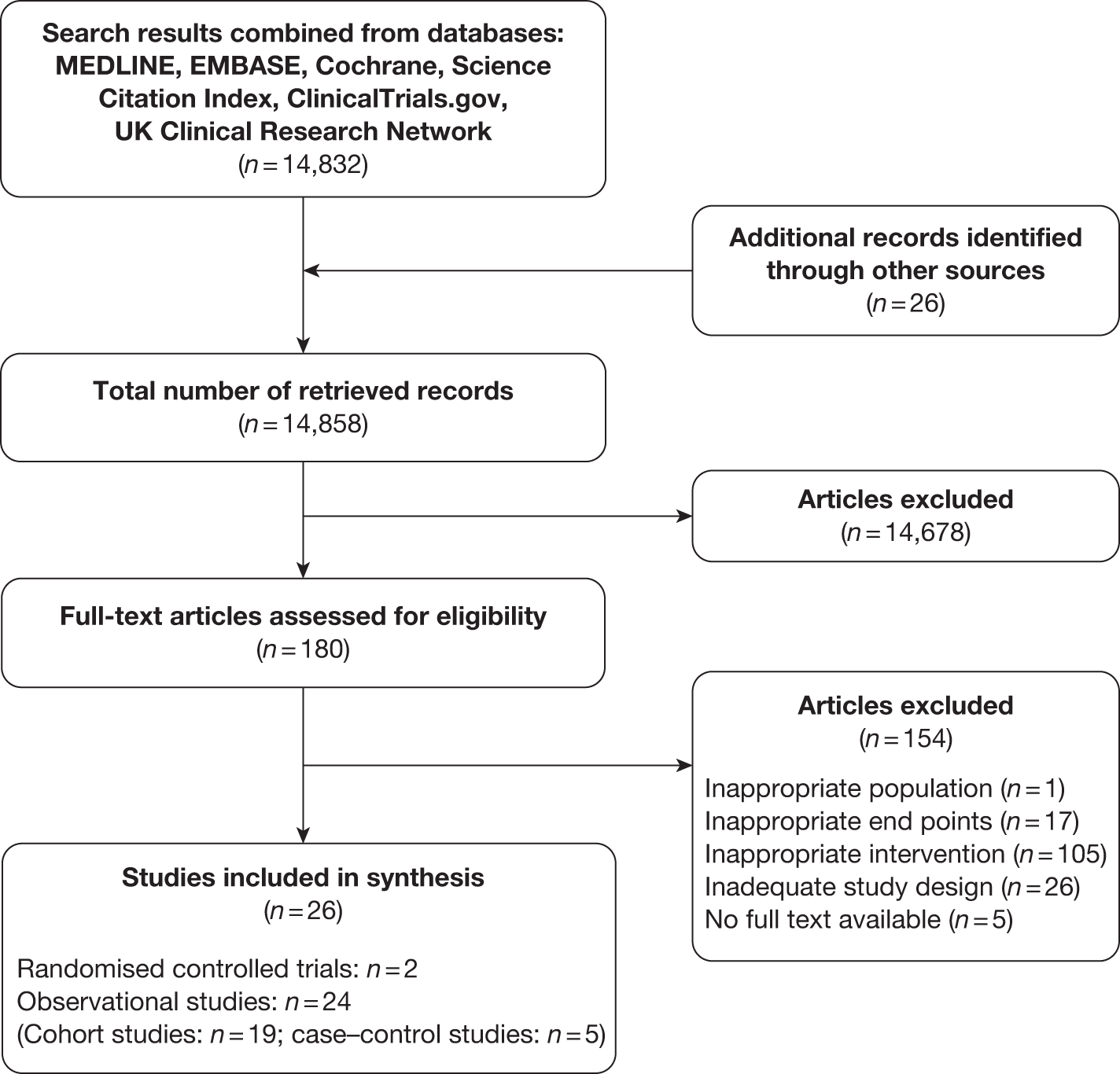
From a systematic search of the literature to identify the maternal and fetal adverse effects of weight management interventions in pregnancy, 14,832 potentially relevant records were obtained (up to 31 March 2011). A search of the reference lists of the relevant articles led to the identification of 26 further citations. After reviewing the abstracts, the full texts of 180 papers were obtained for detailed assessment. After exclusion of 154 publications, 26 papers were included in the review. Figure 35 provides details of the process of study selection.
FIGURE 35.
Flow chart of study identification and selection for the evaluation of adverse maternal and fetal outcomes.
Of the included studies, two were RCTs (involving 277 women)129,132 and 24 were observational studies (19 cohort studies and five case–control studies, involving 468,581 women). 63,64,67,68,70,73–77,80,85,89,133–143 The studies evaluated the effect of dietary, physical activity and other lifestyle interventions in pregnancy on maternal and fetal outcomes. Appendices 7 and 10 provide details of the included RCTs and observational studies, respectively, that assessed the adverse effects of outcomes.
Quality of the included studies
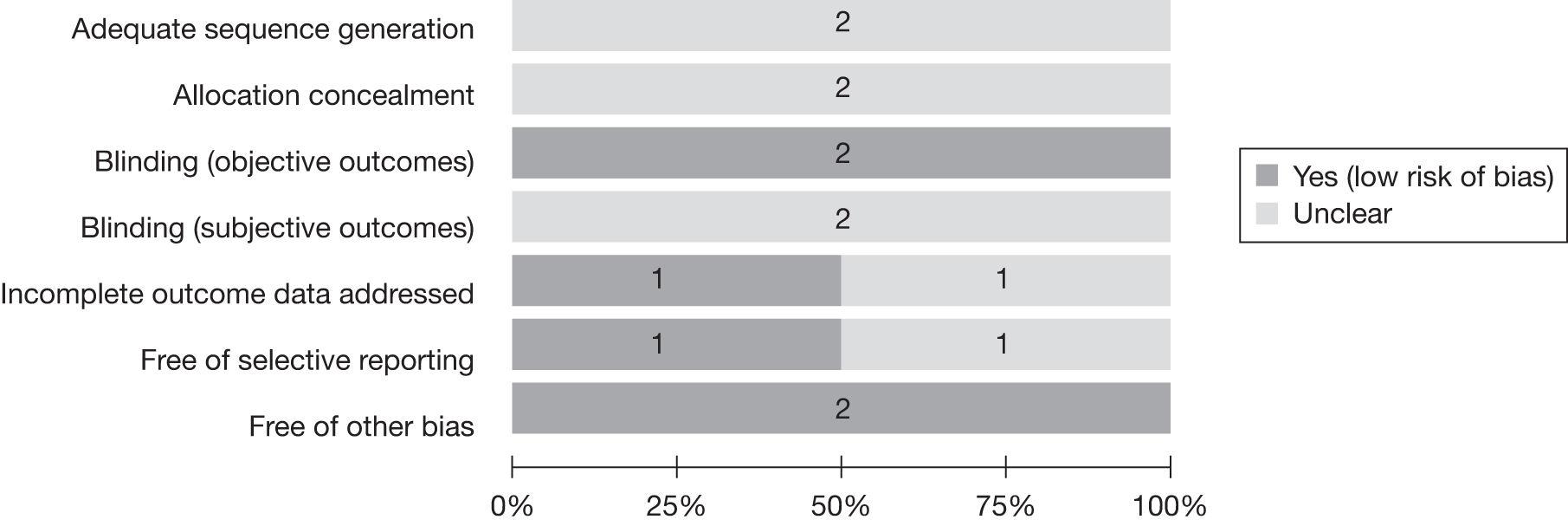
Randomised controlled trials
The quality of the two included RCTs129,132 is shown in Figure 36. The details regarding sequence generation, allocation concealment and blinding for subjective outcomes were unclear in both studies. A detailed quality assessment of the included RCTs is provided in Appendix 8.
FIGURE 36.
Quality of the included RCTs for the adverse effects review.
Observational studies
The 24 observational studies included 19 cohort studies and five case–control studies. 63,64,67,68,70,73–77,80,85,89,133–143 The quality assessment of the cohort and case–control studies is summarised in Appendix 9. The studies, evaluated using NOS, could score a maximum of nine stars, with four stars for selection, two for comparison and three for outcome assessment. In total, 3/19 (15.8%) cohort studies had a low risk of bias and scored seven or more stars; 16/19 (84.2%) had a medium risk of bias and scored between four and six stars.
Results
The adverse outcomes included in the review were defined as those that occurred unintentionally with potential harm to the mother or baby. We also included those outcomes that may have been the direct result of the intervention itself, for example risk of preterm delivery due to strenuous physical exercise.
Randomised clinical trials
The two RCTs129,132 were conducted in women already planning to exercise in pregnancy and pregnant athletes. Kulpa et al. 129 reported on the outcomes of meconium-stained amniotic fluid, uterine atony and chorioamnionitis. Estimated RRs for the above outcomes were 0.62 (95% CI 0.20 to 1.90; p = 0.40), 0.93 (95% CI 0.22 to 3.89; p = 0.92) and 3.69 (95% CI 0.15 to 88.13; p = 0.42) respectively. Bell and Palma132 evaluated the effect of vigorous exercise in pregnancy (exercising five or more times per week) on the risk of reduction in birthweight. There was no difference in birthweight between the vigorous exercise group and the control group.
Observational studies
A total of 18 studies68,73–76,80,85,89,133–139,141–143 observed the effect of diet on maternal and fetal outcomes. The majority of the included studies produced data on the effects of a severe reduction in caloric intake in extreme conditions such as war or famine (Table 7). The studies on physical activity included women undergoing exercises of various intensities or other recreational physical activity in pregnancy. The rates of congenital abnormalities such as neural tube defects (NTDs) were observed in those following dietary interventions that aimed to significantly reduce weight133 or in those intaking food with a very high- or a very low-glycaemic index. 143 The risks of coronary artery disease, metabolic syndrome, breast cancer and diabetes were studied in infants born to mothers who were severely diet restricted owing to famine. 68,135,139
| Outcome | Study | Characteristics of intervention or exposure | Types of intervention | Total N | Intervention or exposure (n/N) | Control (n/N) | OR or HR (95% CI) |
|---|---|---|---|---|---|---|---|
| NTD | Carmichael 2003133 | Diet to lose weight | Diet | 1077 | 29/538 | 14/539 | OR 2.1 (1.1 to 4.1)a |
| Fasting diet | 17/538 | 3/539 | OR 5.8 (1.7 to 20.0)a | ||||
| Other special diet | 17/538 | 3/539 | OR 1.0 (0.3 to 3.1)a | ||||
| Eating disorder | 18/538 | 11/539 | OR 1.7 (0.8 to 3.6)a | ||||
| Any special diet or eating disorder | 61/538 | 31/539 | OR 2.1 (1.3 to 3.3)a | ||||
| Binge eating (self-reported dieting behaviour for any time during 3 months before pregnancy or during pregnancy) | 36/538 | 44/539 | OR 0.8 (0.5 to 1.3)a | ||||
| NTD | Yazdy 2010143 | Glycaemic index low < 60 | Diet | 1394 | 522/698 | 594/696 | OR 2.0 (1.5 to 2.6)a |
| Glycaemic index high ≥ 60 | 176/698 | 102/696 | OR 1.5 (1.1 to 2.0)b | ||||
| Glycaemic load low < 205 | 668/698 | 683/696 | OR 2.4 (1.2 to 4.6)a | ||||
| Glycaemic load high ≥ 205 | 30/698 | 13/696 | OR 1.8 (0.8 to 4.0)b | ||||
| Glycaemic index low < 60 | Subgroup BMI ≥ 30 kg/m2 (100) | 23/36 | 53/64 | OR 2.7 (1.1 to 7.0)a | |||
| Glycaemic index high ≥ 60 | 13/36 | 11/64 | OR 2.0 (0.6 to 7.3)b | ||||
| Glycaemic load low < 205 | 32/36 | 59/64 | OR 1.5 (0.4 to 5.9)a | ||||
| Glycaemic load high ≥ 205 | 4/36 | 5/64 | OR 0.9 (0.2 to 4.7)b | ||||
| Glycaemic index low < 60 | Subgroup BMI < 30 kg/m2 (816) | 138/185 | 540/631 | OR 2.0 (1.4 to 3.0)a | |||
| Glycaemic index high ≥ 60 | 47/185 | 91/631 | OR 1.7 (1.1 to 2.7)b | ||||
| Glycaemic load low < 205 | 177/185 | 623/631 | OR 3.8 (1.4 to 10.5)a | ||||
| Glycaemic load high ≥ 205 | 8/185 | 8/631 | OR 3.3 (1.0 to 10.6)b | ||||
| Cord abnormalities | Magann 200277 | Exercise: various intensities | Physical activity | 750 | |||
| Light | 15/222 | 18/217 | OR 0.80 (0.39 to 1.63)a | ||||
| Moderate | 7/73 | OR 1.17 (0.47 to 2.93)a | |||||
| Heavy | 9/238 | OR 0.43 (0.19 to 0.99)a | |||||
| Coronary heart disease (adult) | Roseboom 2000139 | Diet: famine | Diet | 736 | |||
| Exposed in late gestation | 3/120 | 6/232 | Exposed in late gestation vs not exposed prenatally: OR 0.8 (0.2 to 2.8) | ||||
| Exposed in mid-gestation | 1/108 | 8/208 | Exposed in mid-gestation: OR 3.0 (0.0 to 2.2) | ||||
| Exposed in early gestation | 6/68 | Exposed early gestation: OR 3.0 (1.1 to 8.0) | |||||
| de Rooij 2006134 | Diet: famine | Diet | 694 | OR 0.79 (0.42 to 1.49)a | |||
| Metabolic syndrome (adult) | de Rooij 200768 | Diet: famine | Diet | 783 | OR 1.2 (0.9 to 1.7) | ||
| de Rooij 2006134 | Diet: famine | Diet | 694 | OR 1.09 (0.78 to 1.51)a | |||
| Hypertension (adult) | Lumey 200976 | Diet: famine | Diet | 638 | 224/344 | 168/294 | OR 1.40 (1.02 to 1.93)a |
| Breast cancer | Painter 2008135 | Diet: famine | Diet | 475 | HR (all exposed) 2.6 (0.9 to 7.7)a | ||
| Exposed in late gestation | 3/82 | 1/126 | HR 2.6 (0.9 to 7.7)b | ||||
| Exposed in mid-gestation | 3/77 | 4/144 | HR 2.5 (0.8 to 7.4)b | ||||
| Exposed in early gestation | 4/46 | HR 4.0 (1.1 to 14.5)b | |||||
| Cleft lip, cleft palate or both | Vujkovic 2007142 |
Diet: Western vs prudent Western (by tertile) |
Diet | 381 | |||
| T1 (127) | 58/203 | 69/178 |
T1: ref. T2: OR 1.3 (0.8 to 2.2)a T3: OR 1.9 (1.2 to 3.1)a |
||||
| T2 (127) | 67/203 | 60/178 |
T2: OR 1.2 (0.7 to 2.1)b T3: OR 1.7 (1.0 to 3.0)b |
||||
| T3 (127) | 78/203 | 49/178 |
T2: OR 1.2 (0.8 to 2.1)b T3: OR 1.8 (1.0 to 2.9)b |
||||
| Prudent (by tertile) | |||||||
| T1 (127) | 68/203 | 59/178 |
T1: ref. T2: OR 0.9 (0.5 to 1.4)a T3: OR 1.1 (0.7 to 1.8)a |
||||
| T2 (127) | 64/203 | 63/178 |
T2: OR 0.8 (0.5 to 1.4)b T3: OR 1.3 (0.8 to 1.8)b |
||||
| T3 (127) | 71/203 | 56/178 |
T2: OR 0.7 (0.5 to 1.2)b T3: OR 1.0 (0.6 to 1.7)b |
||||
| Antisocial personality disorder | Neugebauer 199980 |
Diet: famine, western Holland By trimester |
Diet | 76,630 | |||
| First, second or third | 26/14,310 | 50/45,007 |
OR 1.6 (1.02 to 2.6) OR 2.0 (1.2 to 3.3)b |
||||
| First and/or second | 20/9252 |
OR 2.0 (1.2 to 3.5) OR 2.5 (1.5 to 4.2)b |
|||||
| First only | 6/2443 |
OR 2.2 (0.95 to 5.0) OR 2.9 (1.2 to 6.7)b |
|||||
| First and second only | 6/2223 |
OR 2.4 (1.04 to 5.7) OR 3.0 (1.3 to 7.0)b |
|||||
| Second only | 9/4586 |
OR 1.8 (0.9 to 3.6) OR 2.1 (1.03 to 4.4)b |
|||||
| Thirrd only | 5/5058 |
OR 0.9 (0.4 to 2.2) OR 1.1 (0.4 to 2.7)b |
|||||
| By severity | |||||||
| Severely exposed | 26/14,310 | 50/45,007 |
OR 1.9 (1.02 to 2.6) OR 2.0 (1.2 to 3.3)b |
||||
| Moderately exposed | 10/9615 |
OR 0.9 (0.6 to 1.9) OR 0.7 (0.3 to 1.6)b |
|||||
| Dyslipidaemia (adult) | Lumey 200976 | Diet: famine | Diet | 638 | 96/344 | 85/294 | OR 0.95 (0.61 to 1.34)a |
| Obesity (adult) | Ravelli 1976136 | Diet: famine (by trimester) | Diet | 307,700 | |||
| Third | 51/6200 | 148/11,200 | OR 0.62 (0.45 to 0.85)a | ||||
| Second and third | 126/7500 | 286/17,600 | OR 1.03 (0.84 to 1.28)a | ||||
| First and second | 119/4300 | 230/15,900 | OR 1.94 (1.55 to 2.43)a | ||||
| First | 41/2500 | 162/10,500 | OR 1.06 (0.75 to 1.50)a | ||||
| IGT or type 2 diabetes (adult) | Stanner 1997141 | Diet: famine | Diet | 357 | |||
| Known diabetes | 4/169 | 7/188 | OR 0.63 (0.18 to 2.18)a | ||||
| Newly diagnosed diabetes | 3/169 | 5/188 | OR 0.66 (0.16 to 2.81)a | ||||
| IGT | 16/169 | 16/188 | OR 1.12 (0.54 to 2.32)a | ||||
| Ravelli 1998137 | Diet: famine | Diet | 702 | ||||
| Exposed in late gestation | 24/116 | 33/221 | General: OR 1.19 (0.79 to 1.79)a | ||||
| Exposed in mid-gestation | 14/100 | 30/202 | |||||
| Exposed in early gestation | 10/63 | ||||||
| Meconium in fluid | Clapp 199064 | Physical activity: exercise regularly or at > 50% of their preconceptional level throughout pregnancy | Physical activity | 131 | 12/87 | 11/44 | OR 0.48 (0.19 to 1.20)a |
| Abnormal heart rate | Clapp 199064 | Physical activity: exercise regularly or at > 50% of their preconceptional level throughout pregnancy | Physical activity | 131 | 12/87 | 11/44 | OR 0.48 (0.19 to 1.20)a |
| Nuchal cord | Clapp 199064 | Physical activity: exercise regularly or at > 50% of their preconceptional level throughout pregnancy | Physical activity | 131 | 23/87 | 24/44 | OR 0.30 (0.14 to 0.64)a |
| Threatened abortion | Dale 198267 | Physical activity: running | Physical activity | 33 | 1/21 | 1/11 | OR 0.50 (0.03 to 8.85)a |
| Chorioamnionitis secondary to prolonged rupture of membranes | Dale 198267 | Physical activity: running | Physical activity | 33 | 0/21 | 1/11 | OR 0.16 (0.01 to 4.35)a |
| Asphyxia/meconium staining/fetal distress | Dale 198267 | Physical activity: running | Physical activity | 33 | 0/21 | 4/11 | OR 0.04 (0.00 to 0.81) |
| Sepsis | Dale 198267 | Physical activity: running | Physical activity | 33 | 0/21 | 1/11 | OR 0.16 (0.01 to 4.35)a |
The observational studies on physical activity in pregnancy did not show any significant adverse maternal or fetal outcomes. This was consistently observed for different activities of varying severity.
The detailed clinical characteristics of the included studies for the evaluation of adverse effects are provided in Appendix 10.
Summary
The review of adverse effects identified two RCTs and a relatively large number of observational studies. The data from the observational studies showed a possible association between extremes of diet (exposure to famine) and adverse outcomes; however, there was no evidence to suggest that dietary interventions evaluated in the review or currently offered in clinical practice could be associated with adverse maternal or fetal outcomes. Physical activity in pregnancy and maternal and fetal outcomes were studied in the randomised trials and observational studies. Various forms of physical activity such as structured exercises, running and recreational activities of differing intensities were not associated with adverse maternal and fetal outcomes.
The strength of the review is the systematic search for evidence using a broad search strategy. The inclusion of both randomised and non-randomised data including case series has ensured that the review identifies the evidence for all potential adverse effects of interventions. The review was limited by the RCTs being of poor quality. A large proportion of the evidence from the observational studies was devoted to extremes of diet rather than the components of a balanced healthy diet. There was insufficient evidence on popular diets such as the Atkins diet, the Slimming World diet and ‘high-protein’ diets. The studies on physical activity in pregnancy were mainly concerned with cord abnormalities and abnormal fetal heart rate patterns. The data from RCTs on women undergoing physical activity in pregnancy show no effect on gestational age at delivery or preterm delivery provide reassuring evidence on the safety of these interventions for these outcomes.
Chapter 5 Grading of Recommendations Assessment, Development and Evaluation (GRADE) findings
Prioritisation of outcomes
The primary outcomes were weight-related outcomes. There were numerous secondary outcomes. These were ranked through a two-iteration Delphi survey.
First iteration
A total of 19 clinicians (19/20, 95%) completed the questionnaire. Five maternal outcomes – GDM, pre-eclampsia and pregnancy-induced hypertension, caesarean section, thromboembolism and admission to the HDU/intensive therapy unit (ITU) – had a median score of ≥ 8 with an IQR of ≤ 2. The six fetal outcomes that were scored in a similar fashion were SGA infants, intrauterine death, admission to NICU, shoulder dystocia, birth trauma and long-term neurological sequelae. In addition to the outcomes provided, the panel considered breastfeeding, back pain, threatened miscarriage, failed instrumental delivery, maternal coronary artery disease, maternal non-infective respiratory distress, cord abnormalities and long-term metabolic sequelae in the infant to be relevant to the question posed. These outcomes were added to the initial outcomes and sent for scoring for importance in the second round.
Second iteration
A total of 16 panellists (16/19, 84%) participated in the second round of the survey. For maternal outcomes there was evidence of consensus for GDM, thromboembolism and admission to HDU/ITU, as reflected in the median scores of 8 and a fall in IQR from the first round score. Pre-eclampsia continued to be considered as a critically important outcome, with a median score of > 8, although there was an increase in the IQR from 1.5 to 2. Induction of labour scored a median of 8 and was included in the final list of outcomes. Caesarean section as an outcome scored lower (median 7) than in the first round.
For fetal outcomes there was consistency in the ranking, with median scores of > 8 and IQRs of ≤ 1.25 for birth trauma, intrauterine death, admission to NICU and shoulder dystocia. All of the selected fetal outcomes consistently demonstrated a narrowing of the IQR scores in the second round, demonstrating consensus between the participants. The ten outcomes considered to be critical to patient care are provided in Box 2. The scores for the outcomes in the two rounds of the Delphi survey are provided in Appendix 11.
GDM
Pre-eclampsia/gestational hypertension
Admission to HDU/ITU
Thromboembolism
Induction of labour
SGA infants
Shoulder dystocia
Birth trauma
Admission to NICU
Long-term neurological sequelae
Grading of evidence for the effectiveness and adverse effects of interventions
The grading of the evidence for the primary outcomes related to maternal and fetal weight commissioned by the HTA programme and the outcomes considered to be critically important for patient management are summarised graphically in Figure 37. This two-dimensional chart plots five variables represented by equiangular spokes, which represent the quality domains used in evidence grading for each comparison–outcome pair. For each of the spokes, the length represents the magnitude of the quality, ranging from very low at the centre of the plot to high at its maximum length.
FIGURE 37.
Graphic display of the evidence quality for the effect of various interventions on weight-related and clinically important outcomes.
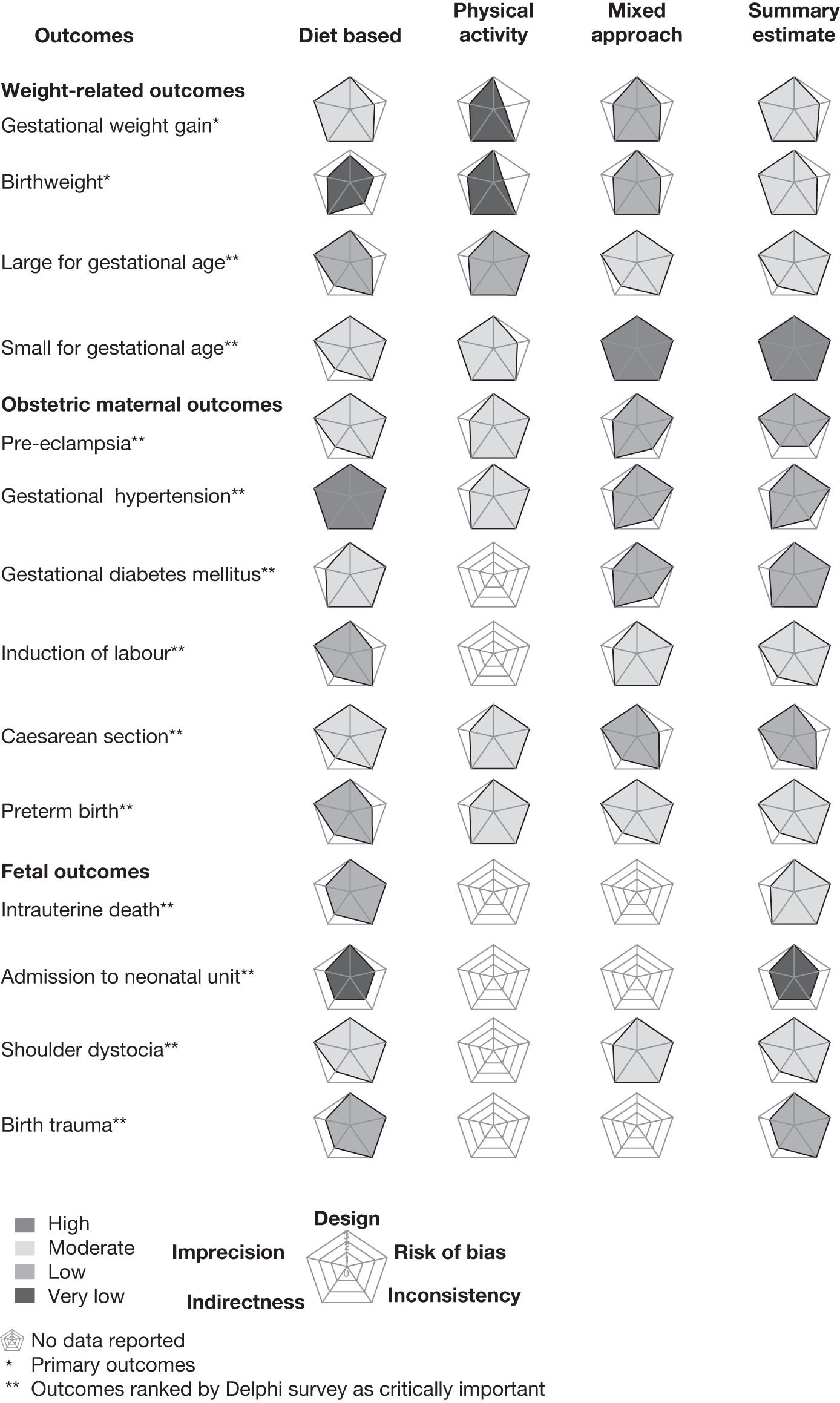
Details of the quality assessment are provided in Appendix 12. The overall strength of evidence for weight gain in pregnancy and birthweight was moderate for all interventions considered together. The strength of evidence for all interventions together was moderate for shoulder dystocia and high for SGA infants. The quality of the pooled evidence for all interventions was moderate for gestational hypertension in obese and overweight women and intrauterine death, and low for reduction in pre-eclampsia and birth trauma. The trend in reduction of GDM was graded low (Table 8). Although thromboembolism, maternal admission to HDU/ITU and long-term neurological sequelae to the fetus were considered to be critically important to the clinicians, we did not identify relevant evidence for these outcomes. Dietary interventions in pregnancy were graded moderate to high for the important outcomes more often than the other interventions (see Appendix 13).
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE)b | |
|---|---|---|---|---|---|
| Assumed risk, control | Corresponding risk, all weight management interventions | ||||
| Gestational weight gain (kg) | The mean gestational weight gain (kg) in the intervention groups was 0.94 kg lower (1.57 kg to 0.3 kg lower) | 4595 (30) |
⊕⊕⊕⊝ |
||
| Birthweight (kg) | The mean birthweight (kg) in the intervention groups was 0.07 kg lower (0.14 kg to 0.01 kg lower) | 4573 (28) |
⊕⊕⊕⊝ |
||
| LGA | 157 per 1000 | 115 per 1000 (85 to 155) | RR 0.73 (0.54 to 0.99) | 3021 (12) |
⊕⊕⊕⊝ |
| SGA | 71 per 1000 | 70 per 1000 (54 to 92) | RR 0.99 (0.76 to 1.29) | 2901 (8) |
⊕⊕⊕⊕ Highh |
| Pre-eclampsia | 103 per 1000 | 76 per 1000 (61 to 95) | RR 0.74 (0.59 to 0.92) | 3072 (10) |
⊕⊕⊝⊝ |
| Gestational hypertension | 134 per 1000 | 103 per 1000 (72 to 147) | RR 0.77 (0.54 to 1.1) | 791 (6) |
⊕⊕⊝⊝ Lowk |
| GDM | 112 per 1000 | 80 per 1000 (49 to 127) | RR 0.71 (0.44 to 1.13) | 675 (5) |
⊕⊕⊝⊝ |
| Preterm birth | 82 per 1000 | 62 per 1000 (46 to 84) | RR 0.76 (0.56 to 1.02) | 2198 (11) |
⊕⊕⊕⊝ |
| Caesarean section | 327 per 1000 | 304 per 1000 (278 to 337) | RR 0.93 (0.85 to 1.03) | 3312 (14) |
⊕⊕⊝⊝ |
| Induction of labour | 295 per 1000 | 330 per 1000 (295 to 372) | RR 1.12 (1.0 to 1.26) | 2362 (5) |
⊕⊕⊕⊝ |
| Post-partum haemorrhage | 59 per 1000 | 53 per 1000 (24 to 84) | RR 0.90 (0.57 to 1.43) | 1232 (2) |
⊕⊕⊝⊝ Lowg,n |
| Intrauterine death | 9 per 1000 | 1 per 1000 (0 to 11) | RR 0.15 (0.02 to 1.2) | 1320 (2) |
⊕⊕⊕⊝ Moderateh |
| Admission to NICU | 382 per 1000 | 374 per 1000 (252 to 562) | RR 0.98 (0.66 to 1.47) | 1962 (2) |
⊕⊝⊝⊝ |
| Shoulder dystocia | 33 per 1000 | 13 per 1000 (7 to 23) | RR 0.39 (0.22 to 0.7) | 2317 (4) |
⊕⊕⊕⊝ Moderateg |
| Birth trauma | 9 per 1000 | 3 per 1000 (1 to 11) | RR 0.36 (0.11 to 1.23) | 1961 (2) |
⊕⊕⊝⊝ |
| Neonatal hypoglycaemia | 103 per 1000 | 110 per 1000 (88 to 139) | RR 1.07 (0.85 to 1.35) | 2146 (5) |
⊕⊝⊝⊝ |
The quality of the evidence for adverse outcomes for studies reporting diet and physical activity in pregnancy is provided in Table 9. The strength of evidence was very low for all of the outcomes evaluated for dietary intervention. Poor quality of evidence was also observed for physical activity interventions in pregnancy.
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE)b | |
|---|---|---|---|---|---|
| Assumed risk, control | Corresponding risk, diet | ||||
| NTD | See comment o | See comment o | Not estimable | 0 (2) |
⊕⊝⊝⊝ |
| Coronary heart disease: long-term outcome in children | 32 per 1000 | 90 per 1000 (35 to 209)f | OR 3 (1.1 to 8.0) | 508 (1) |
⊕⊝⊝⊝ |
| Metabolic syndrome: long-term outcome in children | 1 per 1000 | 1 per 1000 (1 to 2)i | OR 1.2 (0.9 to 1.7) | 59,317 (1) |
⊕⊝⊝⊝ |
| Hypertension: long-term outcome in children | 571 per 1000 | 651 per 1000 (576 to 720) | OR 1.4 (1.02 to 1.93) | 638 (1) |
⊕⊝⊝⊝ |
| Antisocial personality disorder: long-term outcome in children | 1 per 1000 | 2 per 1000 (1 to 3)i | OR 2.0 (1.2 to 3.3) | 59,317 (1) |
⊕⊝⊝⊝ |
| Dyslipidaemia | 289 per 1000 | 279 per 1000 (199 to 353) | OR 0.95 (0.61 to 1.34) | 638 (1) |
⊕⊝⊝⊝ |
| Obesity: in adulthood: long-term outcome in children | 13 per 1000l | 8 per 1000 (6 to 11)l | OR 0.62 (0.45 to 0.85) | 17,400 (1) |
⊕⊝⊝⊝ |
| Obesity: in adulthood: long-term outcome in children | 14 per 1000n | 27 per 1000 (22 to 33)n | OR 1.94 (1.55 to 2.43) | 20,200 (1) |
⊕⊝⊝⊝ |
| IGT: long-term outcome in children | 85 per 1000 | 94 per 1000 | OR 1.12 (0.54 to 2.32) | 357 (1) |
⊕⊝⊝⊝ |
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE)b | |
|---|---|---|---|---|---|
| Assumed risk, control | Corresponding risk, physical activity | ||||
| Cord abnormalities | 83 per 1000 | 37 per 1000 (17 to 82)c | OR 0.43 (0.19 to 0.99) | 455 (3) |
⊕⊝⊝⊝ Very lowd |
| Stimulation for abnormal labour pattern | 205 per 1000 | 115 per 1000 (43 to 303) | RR 0.56 (0.21 to 1.48) | 131 (1) |
⊕⊝⊝⊝ |
| Meconium in amniotic fluid | 170 per 1000 | 105 per 1000 (34 to 323) | RR 0.62 (0.2 to 1.9) | 85 (1) |
⊕⊕⊝⊝ |
| Abnormal fetal heart rate | 250 per 1000 | 138 per 1000 (60 to 286) | OR 0.48 (0.19 to 1.2) | 131 (1) |
⊕⊝⊝⊝ |
| Nuchal cord | 545 per 1000 | 264 per 1000 (144 to 434) | OR 0.3 (0.14 to 0.64) | 131 (1) |
⊕⊝⊝⊝ Very lowd |
| Threatened abortion | 91 per 1000 | 48 per 1000 (3 to 470) | OR 0.5 (0.03 to 8.85) | 32 (1) |
⊕⊝⊝⊝ |
| Failure to progress with oxytocin augmentation | 273 per 1000 | 142 per 1000 (26 to 503) | OR 0.44 (0.07 to 2.7) | 32 (1) |
⊕⊝⊝⊝ |
| Chorioamnionitis | 26 per 1000 | 0 per 1000 | OR 3.69 (0.15 to 88.13) | 85 (1) |
⊕⊕⊝⊝ |
| Maternal anaemia | 182 per 1000 | 143 per 1000 (24 to 541) | OR 0.75 (0.11 to 5.3) | 32 (1) |
⊕⊝⊝⊝ |
| Maternal sepsis | 91 per 1000 | 16 per 1000 (1 to 303) | OR 0.16 (0.01 to 4.35) | 32 (1) |
⊕⊝⊝⊝ |
| Uterine atony | 85 per 1000 | 79 per 1000 (19 to 331) | RR 0.93 (0.22 to 3.89) | 85 (1) |
⊕⊕⊝⊝ |
Summary
The Delphi survey prioritised outcomes that were considered to be critical in the management of women in pregnancy. The evidence quality on the primary outcomes related to weight, maternal weight gain in pregnancy and birthweight was graded as moderate. The strength of evidence was low for secondary outcomes such as pre-eclampsia, GDM, gestational hypertension and caesarean section and low to high for preterm birth, induction of labour, shoulder dystocia, birth trauma, incidence of SGA and LGA infants and intrauterine death for all interventions. The strength of evidence for adverse outcomes due to diet and physical activity was mostly very low reflecting the paucity of evidence in this area.
The weight-related outcomes were regarded as critical in the HTA commissioning brief (HTA No. 09/27/06) for an evaluation of the reduction or prevention of obesity in pregnancy. In addition to the large benefits observed with dietary intervention, the strength of evidence for this intervention was also rated better than that for the other interventions. The evidence for gestational weight gain was of moderate quality for dietary interventions and low for the physical activity and mixed approach interventions. For subgroups of overweight women and obese women the strength of evidence was low to very low for all three interventions. This was a result of the imprecision in the estimates and incomplete reporting of the outcome data. The quality of evidence for the incidence of SGA infants, which showed no significant differences between the intervention and control groups, was moderate to high for all of the interventions. This finding is reassuring to an extent as it negates the perceived risks of interventions for the growth of the fetus.
The evidence quality for reduction in the rate of pre-eclampsia was moderate for dietary intervention, which showed the largest reduction in risk. In the subgroups of obese and overweight women the beneficial effect of dietary intervention in reducing pre-eclampsia scored a moderate-to-high grade for the quality of evidence. Overall, there was moderate-quality evidence that weight management interventions reduce the risks of shoulder dystocia, with the potential to reduce associated morbidity and mortality. The strength of evidence was low for the trend towards a reduction in the incidence of GDM. It is possible that a different panel may have identified a different group of clinically important outcomes.
The graphic display has captured the quality of the evidence for many comparisons and outcomes simultaneously in one diagram making it possible to comprehend large numbers of data in one glance. The diagram, once understood, allows for appraisal of key issues concerning risk of bias, heterogeneity, directness of evidence in relation to the question, and precision of results. This critical appraisal alters the trust that we can place in the evidence collated for decision-making.
The GRADE profile findings are limited because of the paucity of evidence for some important outcomes such as thromboembolism, maternal admission to HDU/ITU, long-term neurological sequelae and more than one perinatal complication. Further research is likely to have an important impact on the confidence of our estimate and is likely to change the estimate. We have refrained from assessing the quality of evidence across outcomes as it is in the domain of the guideline developers. As systematic reviewers we have limited ourselves to the GRADE profiling of the important outcomes.
Chapter 6 Discussion
Introduction
This review evaluated the effects of dietary and lifestyle interventions, including physical activity, on the prevention and reduction of obesity in pregnancy, an important area of public health given the increasing prevalence of obesity. We undertook three distinct but related pieces of work:
-
a systematic review of the evidence to evaluate the effect of dietary and lifestyle interventions on maternal and fetal weight (primary outcome), obstetric outcomes and fetal and neonatal morbidity and mortality
-
a systematic review of the evidence to evaluate the risks of adverse effects in the mother or fetus as a result of interventions in pregnancy
-
grading of the quality of evidence for critical and important outcomes.
This work has been described in detail in the previous sections. This chapter summarises the key findings and limitations of the work undertaken. It draws conclusions and makes recommendations for research.
Main findings
-
Interventions to manage weight in pregnancy were effective at reducing weight gain in pregnancy, with dietary interventions being the most effective.
-
The commonest diet evaluated in the studies was a balanced calorie regime with low fat or cholesterol and high fibre. Interventions were delivered in both primary and secondary care. Physical activity involved moderate exercise with low-intensity resistance training.
-
The small reduction in birthweight appeared to be of benefit by reducing the risk of LGA fetuses. This reduction in birthweight did not show as an increase in the incidence of SGA fetuses.
-
Dietary intervention showed benefit in reducing obstetric complications such as pre-eclampsia, gestational hypertension and preterm delivery compared with other interventions. Dietary intervention also reduced the risks of shoulder dystocia of the fetus. There was no effect on any other fetal and neonatal morbidity and mortality outcomes with any intervention.
-
There was no evidence of maternal or fetal harm resulting from the diet and physical activity interventions recommended in current clinical practice.
-
Evidence quality for effectiveness outcomes was more often graded moderate or high compared with evidence quality for adverse effects. The quality of evidence for adverse effects for both diet and physical activity was very low.
Strengths of the report
This systematic review comprehensively addressed the benefits and harms of the various weight management interventions in pregnancy. In doing so, compared with other reviews, it identified the largest quantity of evidence, especially RCTs. A Delphi survey of clinicians was the first attempt to rank the outcomes according to their importance. The grading of the strength of evidence for the outcomes prioritised provides the much-needed clarity to make judgements about effects and generate recommendations.
Limitations of the report
-
It was not possible to provide effectiveness data for all of the outcomes and subgroups; however, the critical and important outcomes are well covered.
-
The interpretation of the findings is limited by the paucity of descriptive information on the intensity and duration of intervention, means of provision, patient compliance and any management that can potentially facilitate or hinder implementation. The estimate of reduced gestational weight gain with diet was associated with significant heterogeneity.
-
No studies performed a face-to-face comparison of various interventions, thereby restricting the ranking of interventions based on effectiveness.
-
The grading of evidence was often limited by the poverty of reporting. The poor quality of evidence on adverse effects was a particular problem.
-
There was no evidence on popular diets such as the ‘high-protein, low-carbohydrate’, ‘no carbohydrate’, Slimming World and Atkins diets.
-
There were no relevant data on the quality of life of the participants.
Overall conclusion
Despite the above limitations some clear conclusions can be made. There is benefit from weight management interventions, especially dietary intervention, in reducing weight gain in pregnancy (evidence quality moderate). Interventions reduced the risk of pre-eclampsia and shoulder dystocia (evidence quality low to high). Interventions based on diet are effective in reducing the main obstetric complications such as pre-eclampsia, gestational hypertension and shoulder dystocia (evidence quality moderate to moderate). Weight management interventions reduce the risk of having large babies. There is no evidence of harm to the mother or fetus from the diet or physical activity components of the interventions currently used.
Recommendations for research
These recommendations are guided by gaps identified and the evidence grading:
-
If RCTs are undertaken they should focus on clinically relevant outcomes.
-
Individual patient data meta-analysis can improve the interpretation of current data.
-
The long-term effects of the interventions on the mother and fetus and the safety of the interventions needs further evaluation.
-
Engagement with pregnant women can identify the outcomes that they consider relevant to themselves and their babies.
-
Cost-effectiveness can be assessed by undertaking a model-based health economic evaluation.
-
If weight management interventions are implemented based on current evidence and ongoing studies, service evaluation should include an assessment of uptake, compliance and adverse effects.
Acknowledgements
Ann Daly, Clinical Librarian at the Birmingham Women’s Hospital, Birmingham, UK, provided expert input into the literature search. Mario Merialdi, World Health Organization, Geneva, Switzerland, provided input into the development of outcomes.
Contribution of authors
ST developed the protocol, conducted the review, drafted the manuscript and led the project. ER and SG undertook the literature searches, study selection, data extraction and data analysis. WD, JW and EB provided input into the review conduct and the drafting of the initial manuscript. JT and KJ provided input into the protocol development and the drafting of the manuscript. TR was involved in the review of adverse effects of interventions. RK provided input into the use of GRADE. AC and BWM were involved in project development and provided input at all stages. KSK provided input into the development of the protocol, the conduct of the review and the final version of the manuscript.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Kanagalingam MG, Forouhi NG, Greer IA, Sattar N. Changes in booking body mass over a decade: retrospective analysis from a Glasgow Maternity Hospital. BJOG 2005;112:1431-3.
- Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet 2006;93:269-74.
- Thangaratinam S, Jolly K. Obesity in pregnancy: a review of reviews on the effectiveness of interventions. BJOG 2010;117:1309-12.
- Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer – 2003–2005. The Seventh Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom 2007.
- Ramachenderan J, Bradford J, McLean J. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol 2008;48:228-45.
- Dodd JM, Crowther CA, Robinson JS. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: a systematic review. Acta Obstet Gynecol 2008;87:702-6.
- Okens K, Taveras EM, Kleinman KP, Rich Edwards JW, Gillman MW. Maternal weight gain during pregnancy and child adiposity at age 3 years. Am J Obstet Gynecol 2007;322:e1-8.
- Sharma AJ, Cogswell ME, Grummer Strawn LM. The association between pregnancy weight gain and childhood overweight is modified by mother’s pre pregnancy BMI. Pediatr Res 2005;58.
- Linne Y, Dye L, Barkeling B, Rossner S. Long term weight development in women: a 15 year follow up of the effects of pregnancy. Obes Res 2004;12:1166-78.
- Rooney BL, Scahuberger CW. Excess pregnancy weight gain and long term obesity: one decade later. Obstet Gynecol 2002;100.
- Villamar E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population based study. Lancet 2006;368:1164-70.
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140:387-98.
- Centre for Maternal and Child Enquires and the Royal College of Obstetricians and Gynaecologists . Joint Guideline – Management of Women With Obesity in Pregnancy 2010.
- National Institute for Health and Clinical Excellence (NICE) . Dietary Interventions and Physical Activity Interventions for Weight Management Before, During and After Pregnancy 2010.
- Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr 2000;71:1242-8.
- Royal College of Obstetricians and Gynaecologists 2010. www.rcog.org.uk/news/cmace-release-cmace-publishes-information-obesity-pregnancy (accessed 18 April 2012).
- Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns 2011;83:203-9.
- Royal College of Obstetricians and Gynaecologists . Obesity and Reproductive Health – Study Group Statement. Consensus Views Arising from the 53rd Study Group: Obesity and Reproductive Health 2007.
- American College of Obstetricians and Gynaecologists . ACOG committee opinion number 315, September 2005: obesity in pregnancy. Obstet Gynecol 2005;106:671-5.
- Rasmussen KM, Yaktine AL. Weight gain during pregnancy: re-examining the guidelines. Washington, DC: National Academies Press; 2009.
- US Department of Health and Human Services . Healthy Eating and Physical Activity across Your Life Span: Fit for Two: Tips for Pregnancy: NIDDK Weight Control Information Network 2002.
- Campbell F, Messina J, Johnson M, Guillaume L, Madan J, Goyder E. Systematic review of dietary and/or physical activity interventions for weight management in pregnancy. Sheffield: NICE Centre for Public Health Excellence; 2009.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson D, Rennie D, et al. Meta analysis of observational studies in epidemiology: a proposal for reporting. Meta analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12.
- Wells G. The Newcastle Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analysis n.d.
- Latthe PM, Foon R, Khan KS. Non surgical treatment of stress urinary continence (SUI): grading of evidence in systematic reviews. BJOG 2008;115:435-44.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.
- Cochrane Adverse Effects Methods Group n.d. http//aemg.cochrane.org/resources-review-authors (accessed 18 April 2012).
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Khan KS, ter Riet G, Glanville J, Sowden AJ, Kleijnen J. Undertaking Systematic Reviews of Research on Effectiveness. CRD’s Guidance for Carrying Out or Commissioning Reviews 2001.
- Khan KS, Kunz R, Kleijnen J, Antes G. Systematic reviews to support evidence-based medicine: how to review and apply findings of systematic reviews. London: Royal Society of Medicine; 2003.
- Loke YK, Price D, Herxheimer A. for the Cochrane Adverse Effects Methods Group . Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol 2007;7.
- Wilson A, Lissauer D, Thangaratinam S, Khan KS, MacArthur C, Coomarasamy A. Clinical officers versus medical doctors for Caesarean surgery in the developing world: a meta-analysis of controlled studies. BMJ 2011;342. 10.1136/bmj.d2600.
- National Institute for Health and Clinical Excellence (NICE) . Methods for the Development of NICE Public Health Guidance (second Edition) 2009.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottowa Hospital Research Institute; n.d.
- Ross SD. Drug-related adverse events – a readers’ guide to assessing literature reviews and meta-analyses. Arch Intern Med 2001;161:1041-6.
- Bagnall A-M, Jones L, Ginnelly L, Lewis R, Glanville J, Gilbody S, et al. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess 2003;7.
- Royal Society of Medicine . Systematic Reviews to Support Evidence-Based Medicine: How to Review and Apply Findings of Systematic Reviews 2003.
- Downs S, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84.
- Glasziou P, Irwig L, Bain C, Colditz G. Systematic reviews in healthcare: a practical guide. Cambridge, Cambridge University Press; 2001.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schunemenn HJ. GRADE: what is ‘quality of evidence’ and why is it important to clinicians?. BMJ 2008;336:995-8.
- Khan KS, Borowiack E, Roos C, Kowalska M, Zapalska A, Mol BW, et al. for the EBM-CONNECT Collaboration . Making GRADE accessible: a proposal for graphic display of evidence quality assessments. Evid Based Med 2011;16:65-9.
- Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Appl Physiol Nutr Metab 2007;32:596-601.
- Borberg C, Gillmer MD, Brunner EJ, Gunn PJ, Oakley NW, Beard RW. Obesity in pregnancy: the effect of dietary advice. Diabetes Care 1980;3:476-81.
- Campbell DM, MacGillivray I. The effect of a low calorie diet or a thiazide diuretic on the incidence of pre-eclampsia and on birth weight. Br J Obstet Gynaecol 1975;82:572-7.
- Campbell DM, Campbell DM, Gillmer MDG. Nutrition in Pregnancy. Proceedings of the 10th Study Group of the RCOG 1983:243-50.
- Casanueva E, Legarreta D, Diaz-Barriga M, Soberanis Y, Cardenas T, Iturriaga A, et al. Weight gain during pregnancy in adolescents: evaluation of a non-nutritional intervention. Rev Invest Clin 1994;46:157-61.
- Claesson I-M, Sydsjo G, Brynhildsen J, Cedergren M, Jeppsson A, Nystrom F, et al. Weight gain restriction for obese pregnant women: a case–control intervention study. BJOG 2008;115:44-50.
- Claesson I-M, Brynhildsen J, Cedergren M, Jeppsson A, Sydsjo A, Josefsson A. Weight gain restriction during pregnancy is safe for both the mother and neonate. Acta Obstet Gynecol Scand 2009;88:1158-62.
- Clapp JF, Little KD. Effect of recreational exercise on pregnancy weight gain and subcutaneous fat deposition. Med Sci Sports Exerc 1995;27:170-7.
- Collings CA, Curet LB, Mullin JP. Maternal and fetal responses to a maternal aerobic exercise programme. Am J Obstet Gynecol 1983;145:702-7.
- El Hiday MM, Zumrawi FY. The effect of a nutrition education programme on pregnant women attending Khartoum model clinic. Ahfad J 1992;9:23-36.
- Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. Can Med Assoc J 2000;163:1247-51.
- Hall DC, Kaufmann DA. Effects of aerobic and strength conditioning on pregnancy outcomes. Am J Obstet Gynecol 1987;157:1199-203.
- Kardel KR, Kase T. Training in pregnant women: effects on fetal development and birth. Am J Obstet Gynecol 1998;178:280-6.
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, et al. Preventing excessive weight gain during pregnancy – a controlled trial in primary health care. Eur J Clin Nutr 2007;61:884-91.
- Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P, et al. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am J Clin Nutr 2006;84:807-12.
- Narendran S, Nagarathna R, Narendran V, Gunasheela S, Nagendra HR. Efficacy of yoga on pregnancy outcome. J Altern Complement Med 2005;11:237-44.
- Bell RJ, Palma SM, Lumley JM. The effect of vigorous exercise during pregnancy on birth-weight. Aust N Z J Obstet Gynaecol 1995;35:46-51.
- Bungum TJ, Peaslee DL, Jackson AW, Perez MA. Exercise during pregnancy and type of delivery in nulliparae. J Obstet Gynecol Neonatal Nurs 2000;29:258-64.
- Clapp JF, Dickstein S. Endurance exercise and pregnancy outcome. Med Sci Sports Exerc 1984;16:556-62.
- Clapp JF, Capeless EL. Neonatal morphometrics after endurance exercise during pregnancy. Am J Obstet Gynecol 1990;163:1805-11.
- Clapp JF. The course of labor after endurance exercise during pregnancy. Am J Obstet Gynecol 1990;163:1799-805.
- Cogswell ME, Scanlon KS, Fein SB, Schieve LA. Medically advised, mother’s personal target, and actual weight gain during pregnancy. Obstet Gynecol 1999;94:616-22.
- Conway R, Reddy S, Davies J. Dietary restraint and weight gain during pregnancy. Eur J Clin Nutr 1999;53:849-53.
- Dale E, Mullinax KM, Bryan DH. Exercise during pregnancy: effects on the fetus. Can J Appl Sport Sci 1982;7:98-103.
- de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr 2007;86:1219-24.
- Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol 2004;159:663-70.
- Hatch MC, Shu XO, McLean DE, Levin B, Begg M, Reuss L, et al. Maternal exercise during pregnancy, physical fitness, and fetal growth. Am J Epidemiol 1993;137:1105-14.
- Horns PN, Ratcliffe LP, Leggett JC, Swanson MS. Pregnancy outcomes among active and sedentary primiparous women. J Obstet Gynecol Neonatal Nurs 1996;25:49-54.
- Jackson MR, Gott P, Lye SJ, Ritchie JW, Clapp JF. The effects of maternal aerobic exercise on human placental development: placental volumetric composition and surface areas. Placenta 1995;16:179-91.
- Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr 2008;62:463-70.
- Lenders CM, Hediger ML, Scholl TO, Khoo CS, Slap GB, Stallings VA. Effect of high-sugar intake by low-income pregnant adolescents on infant birth weight. J Adolesc Health 1994;15:596-602.
- Lenders CM, Hediger ML, Scholl TO, Khoo CS, Slap GB, Stallings VA. Gestational age and infant size at birth are associated with dietary sugar intake among pregnant adolescents. J Nutr 1997;127:1113-17.
- Lumey LH, Stein AD, Kahn HS, Romijn JA. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am J Clin Nutr 2009;89:1737-43.
- Magann EF, Evans SF, Weitz B, Newnham J. Antepartum, intrapartum, and neonatal significance of exercise on healthy low-risk pregnant working women. Obstet Gynecol 2002;99:466-72.
- Melzer K, Schutz Y, Soehnchen N, Othenin-Girard V, Martinez de TB, Irion O, et al. Effects of recommended levels of physical activity on pregnancy outcomes. Am J Obstet Gynecol 2010;202:266-6.
- Mottola MF, Giroux I, Gratton R, Hammond JA, Hanley A, Harris S, et al. Nutrition and exercise prevent excess weight gain in overweight pregnant women. Med Sci Sports Exerc 2010;42:265-72.
- Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA 1999;282:455-62.
- Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol 2004;191:530-6.
- Perichart PO, Balas NM, Parra CA, Rodriguez CA, Ramirez TA, Ortega GC, et al. A medical nutrition therapy programme improves perinatal outcomes in Mexican pregnant women with gestational diabetes and type 2 diabetes mellitus. Diabetes Educ 2009;35:1004-13.
- Piravej K, Saksirinukul R. Survey of patterns, attitudes, and the general effects of exercise during pregnancy in 203 Thai pregnant women at King Chulalongkorn Memorial Hospital. J Med Assoc Thailand 2001;84:S276-82.
- Shirazian T, Monteith S, Friedman F, Rebarber A. Lifestyle modification programme decreases pregnancy weight gain in obese women. Am J Perinatol 2010;27:411-14.
- Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr 2007;85:869-76.
- Berkowitz GS, Kelsey JL, Holford TR, Berkowitz RL. Physical activity and the risk of spontaneous preterm delivery. J Reprod Med 1983;28:581-8.
- Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, et al. A case–control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2004;66:203-15.
- Dye TD, Knox KL, Artal R, Aubry RH, Wojtowycz MA. Physical activity, obesity, and diabetes in pregnancy. Am J Epidemiol 1997;146:961-5.
- Gregory PB, Rush D. Iatrogenic caloric restriction in pregnancy and birthweight. Am J Perinatol 1987;4:365-71.
- Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol 2006;108:1200-7.
- Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension 2003;41:1273-80.
- Badrawi H, Hassanein MK, Badraoui MHH, Wafa YA, Shawky HA, Badrawi N. Pregnancy outcome in obese pregnant mothers. J Perinat Med 1992;20.
- Bechtel-Blackwell DA. Computer-assisted self-interview and nutrition education in pregnant teens. Clin Nurs Res 2002;11:450-62.
- Briley C, Flanagan NL, Lewis N. In-home prenatal nutrition intervention increased dietary iron intakes and reduced low birthweight in low-income African-American women. J Am Diet Assoc 2002;102:984-7.
- Clapp JF. Diet, exercise, and feto–placental growth. Arch Gynecol Obstet 1997;260:101-8.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-86.
- Gomez TG, Delgado JG, Agudelo AA, Hurtado H. Diet effects on the perinatal result of obese pregnant patient. [Spanish]. Rev Colomb Obstet Ginecol 1994;45:313-6.
- Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomised clinical trial. Am J Obstet Gynecol 2005;193:1292-301.
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomised trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339-48.
- Ney D, Hollingsworth DR, Cousins L. Decreased insulin requirement and improved control of diabetes in pregnant women given a high-carbohydrate, high-fiber, low-fat diet. Diabetes Care 1982;5:529-33.
- Rae A, Bond D, Evans S, North F, Roberman B, Walters B. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Aust N Z J Obstet Gynaecol 2000;40:416-22.
- Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomised clinical trial. J Natl Med Assoc 2009;101:569-77.
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomised trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes 2008;32:495-501.
- Baciuk EP, Pereira RI, Cecatti JG, Braga AF, Cavalcante SR. Water aerobics in pregnancy: cardiovascular response, labor and neonatal outcomes. Reprod Health 2008;5. 10.1186/1742-4765-5-10.
- Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn’s birth size: a randomised controlled trial. Int J Obes 2009;33:1048-57.
- Cavalcante SR, Cecatti JG, Pereira RI, Baciuk EP, Bernardo AL, Silveira C. Water aerobics II: maternal body composition and perinatal outcomes after a programme for low risk pregnant women. Reprod Health 2009;6.
- Clapp JF, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol 2000;183:1484-8.
- Clapp JF, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol 2002;186:142-7.
- Erkkola R. The influence of physical exercise during pregnancy upon physical work capacity and circulatory parameters. Scand J Clin Lab Invest 1976;36:747-59.
- Erkkola R, Makela M. Heart volume and physical fitness of parturients. Ann Clin Res 1976;8:15-21.
- Garshasbi A, Faghih ZS. The effect of exercise on the intensity of low back pain in pregnant women. Int J Gynaecol Obstet 2005;88:271-5.
- Haakstad L, Bo K. Effect of supervised aerobic dance exercise in prevention of excessive weight gain in pregnancy: a single blind randomised controlled trial. Int J Gynaecol Obstet 2009;107.
- Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab 2010;95:2080-8.
- Khaledan A, Mirdar Sh, Motahari Tabari NS, Ahmad Shirvani M. Effect of an aerobic exercise programme on fetal growth in pregnant women. HAYAT 2010;16.
- Lee G, Challenger S, McNabb M, Sheridan M. Exercise in pregnancy. Mod Midwife 1996;6:28-33.
- Marquez-Sterling S, Perry AC, Kaplan TA, Halberstein RA, Signorile JF. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med Sci Sports Exerc 2000;32:58-62.
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009;35:418-21.
- Prevedel T, Calderon I, De Conti M, Consonni E, Rudge M. Maternal and perinatal effects of hydrotherapy in pregnancy. Rev Bras Ginecol Obstet 2003;25:53-9.
- Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, et al. Aerobic exercise and submaximal functional capacity in overweight pregnant women: a randomised trial. Obstet Gynecol 2005;106:243-9.
- Sedaghati P, Ziaee V, Ardjmand A. The effect of an ergometric training programme on pregnants weight gain and low back pain. Gazz Med Ital Arch Sci Med 2007;166:209-13.
- Yeo S, Steele NM, Chang MC, Leclaire SM, Ronis DL, Hayashi R. Effect of exercise on blood pressure in pregnant women with a high risk of gestational hypertensive disorders. J Reprod Med 2000;45:293-8.
- Yeo S, Davidge S, Ronis DL, Antonakos CL, Hayashi R, O’Leary S. A comparison of walking versus stretching exercises to reduce the incidence of preeclampsia: a randomised clinical trial. Hypertens Pregnancy 2008;27:113-30.
- Yeo S. Adherence to walking or stretching, and risk of preeclampsia in sedentary pregnant women. Res Nurs Health 2009;32:379-90.
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomised controlled trial. Obstet Gynecol 2009;113:305-12.
- Bung P, Artal R, Khodiguian N, Kjos S. Exercise in gestational diabetes. An optional therapeutic approach?. Diabetes 1991;40:182-5.
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomised controlled trial. Am J Clin Nutr 2010;91:373-80.
- Hui AL, Ludwig SM, Gardiner P, Sevenhuysen G, Murray R, Morris M, et al. Community-based exercise and dietary intervention during pregnancy: a pilot study. Can J Diabetes 2006;30:169-75.
- Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust 2009;191:429-33.
- Kulpa PJ, White BM, Visscher R. Aerobic exercise in pregnancy. Am J Obstet Gynecol 1987;156:1395-403.
- Polley BA, Wing RR, Sims CJ. Randomised controlled trial to prevent excessive weight gain in pregnant women. Int J Obes 2002;26:1494-502.
- Institute of Medicine Subcommittee on Nutritional Status and Weight Gain in Pregnancy . Nutrition During Pregnancy 1990.
- Bell R, Palma S. Antenatal exercise and birthweight. Aust N Z J Obstet Gynaecol 2000;40:70-3.
- Carmichael SL, Shaw GM, Schaffer DM, Laurent C, Selvin S. Dieting behaviors and risk of neural tube defects. Am J Epidemiol 2003;158:1127-31.
- de Rooij SR, Painter RC, Phillips DI, Osmond C, Tanck MW, Bossuyt PM, et al. Cortisol responses to psychological stress in adults after prenatal exposure to the Dutch famine. Psychoneuroendocrinology 2006;31:1257-65.
- Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DIW, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 2008;115:1243-9.
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349-53.
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998;351:173-7.
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr 2000;72:1101-6.
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 2000;84:595-8.
- Schramm WF, Stockbauer JW, Hoffman HJ. Exercise, employment, other daily activities, and adverse pregnancy outcomes. Am J Epidemiol 1996;143:211-18.
- Stanner SA, Bulmer K, Andrès C, Lantseva OE, Borodina V, Poteen VV, et al. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. BMJ 1997;315:1342-8.
- Vujkovic M, Ocke MC, Van Der Spek PJ, Yazdanpanah N, Steegers EA, Steegers-Theunissen RP. Maternal western dietary patterns and the risk of developing a cleft lip with or without a cleft palate. Obstet Gynecol 2007;110:378-84.
- Yazdy MM, Liu S, Mitchell AA, Werler MM. Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol 2010;171:407-14.
- Dodd JM, Crowther CA, Robinson JS. Dietry and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: a systematic review. Acta Obstet Gynecol Scand 2008;87:702-6.
- Dodd J, Grivell R, Crowther C, Robinson J. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010;117:1316-26.
- Kuhlmann AKS, Dietz PM, Galavotti C, England LJ. Weight-management interventions for pregnant or postpartum women. Am J Prev Med 2008;34:523-8.
- Leet T, Flick L. Effect of exercise on birthweight. Clin Obstet Gynecol 2003;46:423-31.
- Liu L, Mirza M, Thomas H. Effectiveness of interventions to prevent excessive weight gain during pregnancy. Effective Public Health Practice Project (EPHPP), Epidemiology and Evaluation. City of Hamilton, Public Health Services; n.d.
- Ronnberg AK, Nilsson K. Interventionsduring pregnancy to reduce excessive gestational weight gain: a systematic review assessing current clinical evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. BJOG 2010;117:1327-34.
- Schlüssel MM, Bicalho de Souza E, Reichenheim ME, Kac G. Physical activity during pregnancy and maternal-child health outcomes: a systematic literature review. Cad Saude Publica 2008;24:S531-S544.
- Skouteris H, Hartley-Clark L, McCabe M, Milgrom J, Kent B, Herring SJ, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obes Rev 2010;11:757-68.
- Widga AC, Lewis NM. Defined, in-home prenatal nutrition intervention for low-income women. J Am Diet Assoc 1999;99:1058-62.
Appendix 1 List of reviews evaluating the effect of weight management interventions on maternal and fetal outcomes
| Review | Question | Search criteria | Studies included |
|---|---|---|---|
| Dodd 2008144 |
Population: overweight and obese women during pregnancy Intervention: dietary and lifestyle interventions (alone or in combination) to limit weight gain with the intention of improving maternal, fetal and infant health outcomes Outcomes: weight gain, maternal, fetal and infant health outcomes Design of included studies: RCTs |
Databases searched: MEDLINE, The Cochrane Library, Australian (ACTR) and International (ICTN) Clinical Trials Registry Hand searching: not stated Search restrictions: none stated |
RCTs: Polley 2002,130 Rae 2000101 |
| Dodd 2010145 |
Population: pregnant women who are overweight or obese Intervention: antenatal dietary or lifestyle interventions Outcomes: LGA infants, mean gestational weight gain, hypertension, pre-eclampsia or eclampsia, GDM, preterm birth before 37 weeks of gestation, infection, need for induction of labour, caesarean section, post-partum haemorrhage requiring blood transfusion, perinatal death (stillbirth and neonatal death), congenital anomalies, infant birthweight of > 4500 g, infant birthweight of < 2500 g, Apgar score of < 7 at 5 minutes of age, hypoglycaemia requiring intravenous treatment, hyperbilirubinaemia requiring treatment, admission to NICU and birth trauma. Childhood outcomes of relevance relate to body size (including height, weight, and BMI) and body composition Study design: RCTs |
Databases searched: PubMed, CENTRAL, ACTR, ICTN Hand searching: yes Search restrictions: no |
RCTs: Asbee 2008, Brankston 2004, Guelinckx 2008, Magee 1990, Polley 2002,130 Rae 2000,101 Santos 2005,119 Thornton 2009,102 Wolff 2008103 |
| Kuhlmann 2008146 |
Population: pregnant or post-partum women Intervention: exercise Outcomes: pregnancy weight gain in excess of the IOM recommendations or post-partum weight retention Design of included studies: RCTs |
Databases searched: MEDLINE, EMBASE, PsycINFO, Sociological Abstracts, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Hand searching: yes Search restrictions: studies published January 1985 to August 2007, English language |
RCTs: Leermakers 1998, O’Toole 2003, Polley 2002,130 |
| Leet 2003147 |
Population: pregnant women Intervention: exercise Outcomes: infant birthweight Design of included studies: RCTs, non-randomised controlled studies, observational studies |
Databases searched: MEDLINE, Doctor Dissertation Abstracts Online Hand searching: yes Search restrictions: English language |
Experimental: Bell 2000,132 Carr 1992, Clapp 2000,107 Clapp 2002,108 Collings 1983,52 Erkkola 1976, Lee 1996,115 Marquez-Sterling 2000116 Quasi-experimental: Brenner 1995, Lewis 1998, Webb 1988 Observational: Bell 1995,60 Botkin 1991, Burger 1988, Clapp 1984,62 Clapp 1990, Clapp 1992, Clapp 1995,51 Clapp 1998, Dale 1982,67 Hatch 1993,70 Horns 1996,71 Jackson 1995,72 Johson 1994, Madison 1989, Melgar 1997, Piravej 2001,83 Rice 1991, Sternfeld 1995 |
| Liu 2005148 |
Population: pregnant women Intervention: an intervention applicable to public health practice consistent with Ontario’s Mandatory Health Programs and Services Guidelines; primary prevention and not designed specifically for pregnant women who are obese or diabetic (pregnant or obese women can be included in the study population) Outcomes: proportion of women exceeding the upper limit of the IOM recommended gestational weight gain range Design of included studies: RCTs, non-randomised controlled studies, prospective studies with control group |
Databases searched: MEDLINE, EMBASE, CINAHL, PsycINFO, Sociological Abstracts, SPORTDiscus Hand searching: yes Search restrictions: studies published 1980 to 2005, English language |
RCTs: Clapp 1995,51 Olson 2004,81 Polley 2002130 |
| Ronnberg 2010149 |
Population: pregnant women Exclusion: women with diabetes mellitus Intervention: intervention studies specifically designed to prevent excessive gestational weight gain Outcomes: weight gain in pregnancy Study design: RCTs, NRSs, observational studies |
Databases searched: PubMed, The Cochrane Library, CINAHL, Physiotherapy Evidence Database (PEDro) Hand searching: yes Search restrictions: limited to English and Scandinavian languages |
RCTs: Asbee 2008, Bechtel-Blackwell 2002,93 Polley 2002,130 Wolff 2008103 NRSs and observational: Claesson 2008,49 Gray-Donald 2000,54 Kinnunen 2007,57 Olson 200481 |
| Scharr 201022 |
Population: pregnant women expecting a single baby, women seeking preconception advice, women actively planning a pregnancy Intervention: dietary and/or physical activity advice, personal one-to-one and group counselling, physical activity groups or classes, educational and informative literature given to pregnant women, monitoring by health professionals or self-assessment, tracking of progress and tailoring programmes to meet current needs of pregnant women Outcomes: weight-related outcomes, dietary and physical activity outcomes, other mother-related outcomes, outcomes relating to the infant Design of included studies: RCTs, NRSs, observational studies |
Databases searched: MEDLINE, EMBASE, The Cochrane Library, Science Citation Index, ClinicalTrials.com, UK Clinical Research Network Portfolio, other: Applied Social Sciences Index and Abstracts (ASSIA) via CSA, British Nursing Index via OVID SP, CINAHL via OVID SP, EconLit via OVID SP, Maternity and Infant Care via OVID SP, PyscINFO via OVID SP, Social Science Citation Index via Web of Science Hand searching: yes Search restrictions: searches were limited by year (1990–2008) and to human studies (where this option was available) |
RCTs: Asbee 2008, Guelinckx 2008, Hui 2002, Polley 2002,130 Wolff 2008103 NRSs: Claesson 2008,49 Gray-Donald 2000,54 Kardel 1998,56 Kinnunen 2007,57 Olson 200481 Case series: Galletly 1996, Mendelson 1991 Observational: Bergmann 1997, Bungum 1999, Cambell 2001, Cogswell 1996, Conway 1999,66 Gunderson 2004, Horns 1996,71 Keppel 1993, Lof 2008, Mumford 2008, Olson 2003, Sternfeld 1995, Symons Downs 2007, Taffel 1993 |
| Schlüssel 2008150 |
Population: pregnant women Intervention: physical activity for pregnant women: (1) occupational physical activities and (2) leisure-time physical activities Outcomes: pre-eclampsia, gestational arterial hypertension, GDM, gestational weight gain, miscarriage, mode of delivery, fetal growth or development, birthweight, length at birth or prematurity Design of included studies: cross-sectional, case–control or follow-up (cohort) epidemiological studies |
Databases searched: MEDLINE, LILACS Hand searching: yes Search restrictions: published between 1980 and 2005, Portuguese, English, or Spanish language |
Cohort: Begun 2000, Bell 1995, Clapp 1989, Clapp and Little 1995, Dempsey 2004, Florack 1993, Florack 1995, Hatch 1993, Hatch 1998, Henriksen 1995, Horns 1996, Jarrett and Sppelday 1983, Klebanoff 1990, Koemeester 1995, Magann 2002, Misra 1998, Rabkin 1990, Rao 2003, Rose 1991, Saftlas 2004, Stamfeld 1995, Takito 2005 Case–control: Alderman 1998, Berkowitz 1983, Campbell and Mottola 2001, Carmichael 2002, Dempsey 2004, El Metwall 2001, Letke 1999, Marcoux 1989, Schramm 1996, Sorensen 2003, Spinillo 1995, Spinillo 1996 Cross-sectional: Dye 1997, Leiferman and Evenson 2003 |
| Skouteris 2010151 |
Population: pregnant women Intervention: intervention studies specifically designed to prevent excessive gestational weight gain; interventions specifically targeting diabetes mellitus and/or designed for adolescents or post-partum women were excluded Outcomes: excessive weight gain in pregnancy Study design: RCTs, NRSs, observational studies |
Databases searched: CINAHL, Global Health, MEDLINE, PsycINFO, Academic Search Premier Hand searching: not stated Search restrictions: limited to English papers published between January 2000 and April 2010 |
RCTs: Asbee 2008, Guelinckx 2008, Hui 2002, Jeffries 2009,128 Polley 2002,130 Wolff 2008103 NRSs: Claesson 2008,49 Gray-Donald 2000,54 Kinnunen 2007,57 Olson 200481 |
Appendix 2 Search strategies
Search strategy in MEDLINE for the effect of dietary and lifestyle interventions in pregnancy on maternal and fetal outcomes
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1950 to present.
| # | Searches | Results |
|---|---|---|
| 1 | Pregnancy/ | 605,292 |
| 2 | pregnan*.tw. | 299,525 |
| 3 | Gravidity/ | 495 |
| 4 | gravid*.tw. | 8201 |
| 5 | gestation*.tw. | 116,230 |
| 6 | Pregnant Women/ | 4361 |
| 7 | pregnant wom#n.tw. | 47,172 |
| 8 | (child adj3 bearing).tw. | 1653 |
| 9 | childbearing.tw. | 6924 |
| 10 | matern*.tw. | 141,495 |
| 11 | or/1-10 | 746,528 |
| 12 | Weight Gain/ph [Physiology] | 2614 |
| 13 | weight gain*.tw. | 32,374 |
| 14 | Weight Loss/ph [Physiology] | 2846 |
| 15 | weight loss*.tw. | 38,743 |
| 16 | weight change*.tw. | 5183 |
| 17 | Obesity/dh, me, ph, pc, px, th [Diet Therapy, Metabolism, Physiology, Prevention & Control, Psychology, Therapy] | 33,441 |
| 18 | obes*.tw. | 111,828 |
| 19 | Adiposity/ph [Physiology] | 609 |
| 20 | adipos*.tw. | 43,101 |
| 21 | Overweight/dh, me, ph, pc, px, th [Diet Therapy, Metabolism, Physiology, Prevention & Control, Psychology, Therapy] | 1397 |
| 22 | overweight*.tw. | 21,881 |
| 23 | Body Mass Index/ | 50,740 |
| 24 | bmi.tw. | 41,380 |
| 25 | or/12-24 | 249,023 |
| 26 | exp Randomised Controlled Trial/ | 289,035 |
| 27 | “randomised controlled trial”.pt. | 289,035 |
| 28 | “controlled clinical trial”.pt. | 81,125 |
| 29 | (random$ or placebo$).tw,sh. | 695,701 |
| 30 | ((singl$ or double$ or triple$ or treble$) and (blind$ or mask$)).tw,sh. | 119,769 |
| 31 | single-blind method/ | 13,834 |
| 32 | double-blind method/ | 105,956 |
| 33 | exp Case-Control Studies/ | 460,490 |
| 34 | (case$ and control$).tw. | 239,150 |
| 35 | exp Cohort Studies/ | 757,527 |
| 36 | cohort$.tw. | 157,621 |
| 37 | observational study.tw. | 17,760 |
| 38 | non-randomised study.tw. | 577 |
| 39 | Evaluation Studies/ | 132,483 |
| 40 | Comparative Study/ | 1,477,175 |
| 41 | or/26-40 | 3,133,968 |
| 42 | 11 and 25 and 41 | 6878 |
| 43 | exp Animals/ | 14,612,094 |
| 44 | (rat$ or mouse or mice or hamster$ or animal$ or dog$ or cat$ or bovine or sheep or lamb$).af. | 7,246,173 |
| 45 | 43 or 44 | 15,284,475 |
| 46 | Humans/ | 11,152,314 |
| 47 | human$.tw,ot,kf. | 1,568,770 |
| 48 | 46 or 47 | 11,413,435 |
| 49 | 45 not (45 and 48) | 3,949,418 |
| 50 | 42 not 49 | 5941 |
Search strategy in MEDLINE for the adverse effects of dietary and lifestyle interventions in pregnancy on maternal and fetal outcomes
Ovid MEDLINE(R) 1950 to May week 4 2010.
| # | Searches | Results |
|---|---|---|
| 1 | Pregnancy/ | 608,934 |
| 2 | pregnan*.tw. | 294,859 |
| 3 | Gravidity/ | 502 |
| 4 | gravid*.tw. | 8054 |
| 5 | gestation*.tw. | 11,4581 |
| 6 | Pregnant Women/ | 4376 |
| 7 | pregnant wom#n.tw. | 46,264 |
| 8 | (child adj3 bearing).tw. | 1621 |
| 9 | childbearing.tw. | 6805 |
| 10 | matern*.tw. | 139,237 |
| 11 | or/1-10 | 741,261 |
| 12 | (ae or to).fs. | 1,363,123 |
| 13 | exp safety/ | 40,253 |
| 14 | (safe or safety).tw. | 296,532 |
| 15 | side effect$.tw. | 136,451 |
| 16 | (adverse and (reaction$ or event$ or response$)).tw. | 98,046 |
| 17 | ((adverse or undesirable or harms$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).tw. | 204,126 |
| 18 | exp Clinical Trials, Phase IV as Topic/ | 150 |
| 19 | (toxicity or complication$ or noxious or tolerability).tw. | 649,502 |
| 20 | harm$.tw,hw. | 60,216 |
| 21 | ((undesired or undesirable) and (result$ or effect$)).tw. | 9837 |
| 22 | or/12-21 | 2,131,088 |
| 23 | exp diet/ | 155,881 |
| 24 | diet$.tw. | 290,808 |
| 25 | energy intake/ | 25,172 |
| 26 | energy intake.tw. | 10,074 |
| 27 | calor$.tw. | 42,201 |
| 28 | nutrition$.tw. | 131,024 |
| 29 | (food adj3 intake).tw. | 27,605 |
| 30 | Fasting/ | 24,834 |
| 31 | fast$.tw,kf. | 246,556 |
| 32 | Starvation/co, dh, me, ph [Complications, Diet Therapy, Metabolism, Physiology] | 2421 |
| 33 | starvation.tw,kf. | 16,448 |
| 34 | or/23-33 | 720,466 |
| 35 | exp EXERCISE/ | 51,394 |
| 36 | exp Exercise Therapy/ | 21,162 |
| 37 | exercis$.af. | 205,665 |
| 38 | (aerobics or physical therapy or physical activity or physical inactivity).af. | 71,067 |
| 39 | (fitness adj (class$ or regime$ or program$)).af. | 526 |
| 40 | (aerobics or physical therapy or physical training or physical education).af. | 55,042 |
| 41 | dance therapy.af. | 161 |
| 42 | Yoga.tw. | 911 |
| 43 | pilates.tw. | 43 |
| 44 | swimming.tw. | 12,793 |
| 45 | aerobic$.tw. | 41,405 |
| 46 | aquarobic$.tw. | 1 |
| 47 | (aqua adj3 aerobic$).tw. | 7 |
| 48 | fitness.tw. | 24,492 |
| 49 | (Body adj3 ball).tw. | 31 |
| 50 | (Aqua adj3 fitness).tw. | 2 |
| 51 | (Nordic adj3 walking).tw. | 26 |
| 52 | (Recreational adj3 activit*).tw. | 1633 |
| 53 | (brisk adj3 walking).tw. | 230 |
| 54 | walking.tw. | 28,317 |
| 55 | cycling.tw. | 24,848 |
| 56 | bicycle.tw. | 8940 |
| 57 | treadmill.tw. | 18,047 |
| 58 | jogging.tw. | 921 |
| 59 | (training adj3 exercise$).tw. | 9097 |
| 60 | (upper adj3 extremity adj3 exercise$).tw. | 119 |
| 61 | Stretching.tw. | 10,794 |
| 62 | Dancing.tw. | 656 |
| 63 | (Tai adj3 chi).tw. | 449 |
| 64 | (tai adj3 ji).tw. | 7 |
| 65 | (belly adj3 dancing).tw. | 4 |
| 66 | (motor adj3 activit*).tw. | 13,891 |
| 67 | (Occupational adj3 activit*).tw. | 1528 |
| 68 | (household adj3 activit*).tw. | 461 |
| 69 | (locomot* adj3 activit*).tw. | 13,405 |
| 70 | (daily adj3 physic* adj3 activit*).tw. | 1092 |
| 71 | or/35-70 | 398,556 |
| 72 | 34 or 71 | 1,072,658 |
| 73 | 11 and 22 and 72 | 9858 |
| 74 | exp Animals/ | 14,729,014 |
| 75 | (rat$ or mouse or mice or hamster$ or animal$ or dog$ or cat$ or bovine or sheep or lamb$).af. | 7,120,771 |
| 76 | 74 or 75 | 15,216,122 |
| 77 | Humans/ | 11,246,110 |
| 78 | human$.tw,ot,kf. | 1,550,517 |
| 79 | 77 or 78 | 11,474,007 |
| 80 | 76 not (76 and 79) | 3,800,283 |
| 81 | letter.pt. | 680,151 |
| 82 | comment.pt. | 411,317 |
| 83 | editorial.pt. | 256,472 |
| 84 | 81 or 82 or 83 | 1,004,073 |
| 85 | 73 not 80 | 6997 |
| 86 | 73 not (80 or 84) | 6883 |
Appendix 3 Clinical characteristics of the randomised controlled trials evaluating the effect of diet, physical activity and a mixed approach for weight management in pregnancy on maternal and fetal outcomes
| Study, year, language | Methods | Participants | Interventions | Control | Outcomes |
|---|---|---|---|---|---|
|
Asbee 2009124 English |
Method of randomisation: randomisation was performed using computer-generated random allocation. Randomisation occurred in consecutive order at the time of the antenatal visit Allocation concealment: study randomisation was numbered and sealed in an opaque envelope Blinding: no blinding used |
Inclusion criteria: antenatal care established at 6–16 weeks of gestation; age 18–49 years; all antenatal care received at the resident obstetrics clinic; English speaking, Spanish speaking or both; singleton pregnancy Exclusion criteria: antenatal care established at more than 16 weeks of gestation; non-English speaking or non-Spanish speaking; multiple pregnancy; BMI > 40 kg/m2; pre-existing diabetes, untreated thyroid disease or hypertension requiring medication or other medical conditions that might affect body weight; delivery at institution other than Carolinas Medical Centre-Main; pregnancy ending in premature delivery (< 37 weeks); limited prenatal care (fewer than four visits) Number of participants: experimental 57, control 43 |
Programme of intensive counselling regarding diet and lifestyle during pregnancy. The intervention provided education and feedback about weight gain, appropriate exercise in pregnancy and pregnancy-specific dietary counselling At the initial visit the study group met with a registered dietitian to receive a standardised counselling session, including information on pregnancy-specific dietary and lifestyle choices. The counselling consisted of recommendations for a patient-focused caloric value divided in a 40% carbohydrate, 30% protein and 30% fat ratio. Patients were instructed to engage in moderate-intensity exercise at least three times per week and preferably five times per week. They also received information on the appropriate weight gain during pregnancy using the IOM guidelines. Each participant met with the dietitian only at the time of enrolment At each routine obstetric appointment the participant’s weight was measured using a balance beam scale and charted on an IOM Gestational Weight Gain Grid in front of the participant. The health-care provider (physician or nurse practitioner) informed the participant whether or not her weight gain was at the appropriate level. If her weight gain was within the IOM guidelines, the patient was praised and encouraged to continue her current diet and exercise regimen. If her weight gain was not within the IOM guidelines, the participant’s diet and exercise regimen was reviewed and she was advised on increasing or decreasing her food intake and increasing or decreasing exercise |
No intervention | IOM adherence, caesarean delivery rate, weight gain from prepregnancy to delivery |
|
Baciuk 2008104 English |
Method of randomisation: computer-generated randomisation list of numbers; volunteers were enrolled sequentially and randomised to one of the two study groups Allocation concealment: each sequential number corresponded to a sealed opaque envelope containing the information on the randomisation group Blinding: outcome assessors |
Inclusion criteria: pregnant women of < 20 weeks of gestation; singleton pregnancy; no gestational risk factors; receiving prenatal care at the research institution and intending to give birth there Exclusion criteria: practising regular physical exercise; two or more caesarean sections; clinical and/or laboratory diagnoses of neurological, cardiovascular, pulmonary, musculoskeletal or endocrine disorders; any disorder that could represent a risk to the woman’s health, such as morbid obesity, severe anaemia or vaginal bleeding during pregnancy Number of participants: experimental 34, control 37 |
Physical activity: water aerobics The intervention was the regular, moderate practise of water aerobics for 50 minutes three times a week in an indoor swimming pool with water warmed at 28–30°C. Water aerobics was initiated following the first physical evaluation and continued up to delivery. The moderate intensity of exercises during the sessions was assured by monitoring the patient’s heart rate using a heart rate monitor and keeping the rate at around 70% of their predicted maximum heart rate |
No intervention | Request for analgesia, caesarean section, Apgar score at 1 minute ≥ 7, vaginal delivery, preterm birth (< 37 weeks), low birthweight (< 2500 g), adequacy of neonatal weight to gestational age, length of labour (minutes), birthweight, gestational age, weight gain, body fat (%), fat-free mass (%), BMI |
|
Badrawi 199292 English |
Method of randomisation: participants were divided ‘randomly’ into two groups Allocation concealment: not reported Blinding: no blinding used |
Obese pregnant mothers, aged between 25 and 35 years Number of participants: 100 |
Balanced calorie diet 1500–2000 kcal/day | No intervention | Pregnancy-induced hypertension |
|
Barakat 2009105 English |
Method of randomisation: not reported Allocation concealment: the researcher in charge of randomly assigning participants did not know in advance which treatment the next person would receive and did not participate in assessment Blinding: outcome assessors |
Inclusion criteria: gravida with singleton and uncomplicated gestation; not at high risk for preterm delivery (no history of recurrent spontaneous preterm birth, i.e. number of previous preterm deliveries ≤ 1); 25–35 years of age; being sedentary before gestation (exercising < 20 minutes on < 3 days/week); being under medical follow-up throughout the entire pregnancy period (and planning to give birth) in the same obstetrics hospital department (Hospital Severo Ochoa, Madrid, Spain); having no absolute or relative contraindication to exercise participation during pregnancy [such as, among others, haemodynamically significant heart disease, restrictive lung disease, pregnancy-induced hypertension, severe anaemia, maternal cardiac arrhythmia, chronic bronchitis, type 1 diabetes or extreme morbid obesity (BMI 40 kg/m2)] Exclusion criteria: women not planning to give birth in the same obstetrics hospital department (Hospital Severo Ochoa, Madrid, Spain); women not under medical follow-up throughout the entire pregnancy period; women with any serious medical condition preventing them from exercising safely Number of participants: experimental 80, control 80 |
Light-intensity resistance exercise training performed during the second and third trimesters The training intensity was carefully and individually controlled and was kept to light to moderate with relatively low cardiovascular stress (i.e. heart rate 80% of age-predicted maximum heart rate value, calculated as 220 minus age) Three sessions per week for about 26 weeks (originally planned an average of 80 training sessions for each participant in the event of no preterm delivery) Each session consisted of 35–40 minutes of exercise divided into a low-intensity (60% of maximal heart rate) warm-up period (8 minutes), followed by toning and very light resistance exercises (20 minutes) and finishing with a low-intensity cool-down (8 minutes) period The core portion consisted of toning and joint mobilisation exercises involving major muscle and joint groups. Exercises included shoulder shrugs and rotations, arm elevations, leg lateral elevations, pelvic tilts, and rocks. Resistance exercises included one set of 10–12 repetitions of abdominal curls, biceps curls, arm extensions, arm side lifts, shoulder elevations, seated bench press, seated lateral row, lateral leg elevations, leg circles, knee extensions, knee (hamstring) curls, and ankle flexion and extensions. The women used barbells (3 kg per exercise) or low- to medium-resistance bands (Therabands) All participants wore a heart rate monitor (Accurex Plus, Polar Electro OY, Finland) during the training sessions, so heart rate was continuously monitored. To further minimise cardiovascular stress, the researchers specifically instructed participants to avoid the Valsalva manoeuvre All resistance exercise training sessions were performed under observation and supervision in an exercise room. Exercise training facilities from the primary care medical centre in which the participants were monitored throughout the pregnancy were used To reduce participant drop-out and to maintain adherence to the training programme, all sessions were accompanied by music and were performed in an airy, well-lit exercise room. A qualified fitness specialist worked with groups of 10–12 women The exercise training programme started in the second trimester (weeks 12–13) and was continued until the end of pregnancy (weeks 38–39) |
No intervention | Birthweight, preterm delivery, weight gain from prepregnancy to delivery, birth length, ponderal index, head circumference, Apgar score at 1 minute, Apgar score at 5 minutes, gestational age |
|
Bechtel-Blackwell 200293 English |
Method of randomisation: not reported Allocation concealment: unclear Blinding: patients |
Inclusion criteria: African-American adolescent primigravidas, age 13–18 years; receiving prenatal care from an adolescent prenatal clinic Number of participants: experimental 30, control 30 |
Nutritional education intervention The nutrition assessment using CASI (computer-assisted self-interviewing) and GWDCF (Gestational Weight Data Collection Form) was administered to all participants at four separate times: on admission to the study in the first trimester, at 24–26 weeks’ gestation (second trimester), at 32–34 weeks’ gestation (third trimester) and 6 weeks post partum. The nutrition education intervention consisted of three 20-minute group sessions that addressed the nutritional needs specific to the women’s stage of pregnancy |
No intervention | Gestational weight, post-partum weight retention |
| Briley 200294 English |
Method of randomisation: randomly assigned to either an intervention or a control group Allocation concealment: none reported Blinding: no blinding used |
Inclusion criteria: African-American women with representative rates of low birthweight similar to those of the USA Number of participants: experimental 15, control 12 |
Prenatal nutrition intervention: counselling The intervention protocol was adapted from Widga and Lewis152 Included a minimum of six individualised in-home nutrition assessment and counselling visits. Visits were scheduled weekly for the first 4 weeks and then monthly for two more visits |
No intervention | Preterm birth, weight gain, birthweight |
|
Bung 1991125 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Women with gestational diabetes diagnosed by 3-hour glucose tolerance test Inclusion criteria: persistent fasting plasma glucose > 5.88 mmol but < 7.22 mmol, which would then require insulin by standard clinical protocol; up to 33 weeks gestational age (to allow minimum exercise training programme of 4 weeks) Exclusion criteria: other medical or obstetric complications of pregnancy; patients at risk for premature labour Number of participants: experimental 21, control 20 |
Physical activity and diet (30 kcal/kg diet) (EXE – EXercise) At enrolment and then every 4 weeks subjects in the EXE study underwent a symptom-limited VO2max test on a bicycle ergometer. The result of this test determined a standardised exercise prescription for all subjects at 50% of VO2max and reflected in heart rates identified at this workload. This exercise routine assured a comparable exercise prescription for all subjects All EXE subjects were instructed to conduct a non-sedentary lifestyle and return to the exercise laboratory three times a week to exercise under medical supervision. In the laboratory, the subjects exercised on a recumbent bicycle at 50% of their last determined maximum aerobic capacity. The total duration of the exercise was 45 minutes, divided into three periods of 15 minutes, interspersed with two 5-minute rest periods to facilitate fetal monitoring. This exercise routine was judged to be moderate and to generate an approximate energy use 5–7.5 times the resting metabolic rate Each exercise session was preceded by a 10-minute rest-monitoring period. Before and immediately after the exercise sessions, the subjects’ plasma glucose concentrations and blood pressures were obtained and recorded. Throughout the exercise sessions maternal heart rate and uterine activity were continuously monitored |
Insulin and diet (30 kcal/kg diet) | Spontaneous vaginal delivery, vacuum or forceps delivery, caesarean section, macrosomia, neonatal hypoglycaemia, premature labour, gestational age at delivery, birthweight, birth length |
|
Clapp 199795 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
12 healthy women, physically active [training regime throughout pregnancy – supervised exercise consisting of 20 minutes of weight-bearing exercise three times a week at an intensity equal to 55% of each individual’s maximum capacity (VO2max)] Number of participants: experimental 6, control 6 |
Aboriginal carbohydrate diet, diet containing carbohydrates derived from low-glycaemic sources Diet containing carbohydrates derived from low-glycaemic sources included carbohydrate products made from unprocessed wholegrain, fruits, beans, vegetables and many dairy products. The so-called ‘aboriginal’-type carbohydrate diet included most dense wholegrain and multigrain breads, bran cereals, pastas, fresh fruits and vegetables, yogurt, ice cream and nuts. Both diets were designed to contain 17–19% protein, 20–25% fat and 55–60% carbohydrate. Total caloric content was based on fat-free mass and weight stability in the non-pregnant state (35–45 kcal/kg lean body mass/day). During pregnancy all women were allowed to increase caloric intake according to appetite with advancing gestation Dietary compliance was assessed by 24-hour dietary recalls obtained at random times twice each week. Caloric intake, diet composition, the glycaemic index of the carbohydrate portion of the diet and the overall dietary glycaemic index were calculated using a standardised approach |
Cafeteria carbohydrate diet: isocaloric diet containing similar quantities of protein, fat and carbohydrate whose carbohydrates were derived from high-glycaemic sources Included carbohydrate products that came from highly processed grains, root vegetables and simple sugars. Included many highly refined breads, potatoes, instant rice, most breakfast cereals, deserts and snack-type foods (so-called ‘cafeteria’ type carbohydrate) |
Birthweight, length, head circumference, abdominal circumference, body fat (%), fat mass, lean body mass, weight gain from 8 weeks to delivery, skinfold thickness at five sites |
|
Clapp 2000107 English |
Method of randomisation: randomly assigned by envelope draw to a no-exercise control group or an exercise group Allocation concealment: not reported Blinding: no blinding used |
Low-risk pregnant women Inclusion criteria: non-substance abusing; viable singleton pregnancy Number of participants: experimental 25, control 25 |
Physical activity: one of three forms of weight-bearing exercise (treadmill, step aerobics or stair-stepper) Exercise carried out for 20 minutes three to five times each week for the remainder of pregnancy at an intensity between 55% and 60% of the preconception maximum aerobic capacity. No attempt was made to assess the physical activity associated with everyday life or to challenge the veracity of the women about additional unmonitored recreational physical activity Exercise sessions were monitored and exercise intensity was checked every 2 weeks by means of respiratory calorimetry |
No intervention | Birthweight, crown–heel length, ponderal index, head circumference, head–abdomen ratio, percentage body fat, fat mass, lean body mass, weight gain from 8 weeks to delivery, gestational age at delivery |
|
Clapp 2002108 English |
Method of randomisation: randomly assigned by envelope draw Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: healthy; regularly exercising (three or more times each week); non-substance abusing; viable singleton pregnancy Number of participants: overall randomised 80; completed treatment: Lo-Hi group 26, Mod-Mod group 24, Hi-Lo group 25 |
Physical activity: weight-bearing (treadmill, step aerobics or stair-stepper) exercise regimens, each of which was conducted at a standard intensity (oxygen consumption, 55–60% of prepregnancy VO2max) This design provided between-group variation in weekly exercise volume in both early and late pregnancy that was quantitated with the use of the duration–intensity index (the product of exercise intensity and exercise time) in both early and late pregnancy The three regimens were: (1) 20 minutes 5 days a week through week 20, gradually increasing to 60 minutes 5 days a week by week 24 and maintaining that regimen until delivery (Lo-Hi); (2) 40 minutes 5 days a week from week 8 until delivery (Mod-Mod); (3) 60 minutes 5 days a week through week 20, gradually decreasing to 20 minutes 5 days a week by week 24 and maintaining that regimen until delivery (Hi-Lo) Women in the Lo-Hi group exercised for 1100 units/week in early pregnancy, increasing to 3300 units/week in late pregnancy; the women in the Mod-Mod group exercised for 2200 units/week throughout; and the women in the Hi-Lo group exercised for 3300 units/week in early pregnancy, decreasing to 1100 units/week in late pregnancy. Exercise sessions were monitored, and exercise intensity was checked every 2 weeks with the use of respiratory calorimetry |
Women with gradually decreasing exercise by 24 weeks until delivery | Weight gain from 8 weeks to delivery, fat retention, gestational age at delivery, birthweight, crown–heel length, ponderal index, head circumference, head/abdomen ratio, body fat, fat mass, lean body mass |
| Crowther 200596 |
Method of randomisation: stratification was according to centre and singleton or twin gestation. Randomisation was performed centrally with the use of numbers generated by computer with variable block sizes of 6, 8 and 10 Allocation concealment: not reported Blinding: patients and investigators/clinicians |
Inclusion criteria: singleton or twin pregnancy; between 16 and 30 weeks’ gestation; attended antenatal clinics at the collaborating hospitals; had one or more risk factors for GDM on selective screening or a positive 50 g oral glucose challenge test [glucose level 1 hour after glucose challenge at least 7.8 mmol/l (140 mg/dl)]; had a 75 g oral glucose tolerance test at 24–34 weeks’ gestation in which the venous plasma glucose level was < 7.8 mmol /l after an overnight fast and was 7.8–11.0 mmol/l (198 mg/dl) at 2 hours Exclusion criteria: previously treated GDM or active chronic systemic disease (except essential hypertension); severe glucose impairment Number of participants: experimental 490, control 510 |
Diet: dietary advice. The care of the women in the intervention group replicated clinical care in which universal screening and treatment for gestational diabetes are available Interventions included individualised dietary advice from a qualified dietitian, which took into consideration a woman’s prepregnancy weight, activity level, dietary intake and weight gain; instructions on how to self-monitor glucose levels, which the woman was then asked to do four times daily until the levels had been in the recommended range for 2 weeks [fasting glucose levels of at least 3.5 mmol/l (63 mg/dl) and no more than 5.5 mmol/l (99 mg/dl), preprandial levels of no more than 5.5 mmol/l, and levels 2 hours postprandially that were no more than 7.0 mmol/l (126 mg/dl)], followed by daily monitoring at rotating times during the day; and insulin therapy, with the dose adjusted on the basis of glucose levels if there were two capillary-blood glucose results during the 2-week period in which the fasting level was at least 5.5 mmol/l or the postprandial level was at least 7.0 mmol/l at ≤ 35 weeks’ gestation or at least 8.0 mmol/l (144 mg/dl) at > 35 weeks’ gestation, or if there was one capillary-blood glucose result during the 2-week period of at least 9.0 mmol/l (162 mg/dl) |
No intervention (the care of the women in the routine care group replicated clinical care in which screening for gestational diabetes is not available) | Perinatal complications (stillbirth, neonatal death, shoulder dystocia, bone fracture, nerve palsy, admission to neonatal nursery, jaundice requiring phototherapy), induction of labour, caesarean delivery, neonatal convulsions, respiratory distress syndrome, LGA infants, macrosomia, SGA infants, 5-minute Apgar score < 7, hypoglycaemia, antenatal admission, antenatal pre-eclampsia, any perineal trauma, post-partum haemorrhage (≥ 600 ml), puerperal pyrexia (≥ 38°C), EPDS (Edinburgh Postnatal Depression Scale) score > 12, birthweight, weight gain (from first prenatal visit to last visit), gestational age at birth, length of postnatal stay, quality of life during pregnancy [SF-36 (Short Form questionnaire-36 items) (questionnaire: emotional role, mental health, overall physical component, overall mental component, health-state utility, anxiety)] |
|
Erkkola 1976109 English |
Method of randomisation: ‘randomly’ divided into the training group and the control group Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: healthy primigravidae, aged 20–26 years; regular menstruation Exclusion criteria: miscarriage, threatened miscarriage, labour before 38th week of gestation, legal abortion Number of participants: experimental 38, control 38 |
Physical activity Training group received both written and oral instructions for training. They were instructed to perform strenuous exercise for 1 hour a day three times a week throughout pregnancy. All subjects exceeded 60 hours in total of training; over half performed more than 80 hours of training. The women themselves controlled the intensity of the training by measuring their pulse, which was supposed to be 140 beats/minute. During first and second trimesters all types of exercise were recommended but during the third trimester exercises with any bumping and compressing effects on the uterus were disallowed Types of exercise: walking, running, climbing stairs, cycling, swimming, gymnastics, skiing, training school, ball playing, rowing |
No intervention | Weight change from week 26 to week 38 of pregnancy |
|
Garshasbi 2005111 English |
Method of randomisation: not reported Allocation concealment: sealed envelopes; not clear if they were opaque and numbered Blinding: outcome assessors |
Inclusion criteria: healthy primigravidae; between 20 and 28 years; between 17 and 22 weeks of gestation; housewives; high school educated Exclusion criteria: any absolute and relative contraindications to aerobic exercise during pregnancy according to 2002 ACOG guidelines; history of exercise before pregnancy; history of orthopaedic disease or surgery Number of participants: experimental 161, control 105 |
Physical activity: exercise programme during second half of pregnancy This programme was designed to strengthen the abdominal muscles and hamstrings muscles and increase traction of the iliopsoas and paravertebral muscles. The exercise programme included 15 movements in 60 minutes: 5 minutes of slow walking, 5 minutes of extension movements and 10 minutes of general warming up, 15 minutes of anaerobic exercise, 20 minutes of specific exercise and 5 minutes return to the first position. The exercises were recommended by the Tarbiat Modares Faculty of Sport and tested for pregnant women by physiotherapists. Women exercised three times a week, supervised by a midwife. The intensity of the exercise was controlled by maternal pulse rate. If the pulse rate exceeded 140 beats/minute the exercise was stopped |
No intervention | Experience of any kind of low back pain, weight gain from prepregnancy to 38 weeks, pregnancy length, weight of the neonate |
|
Gomez-Tabarez 199497 Spanish/English (abstract) |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: negative for glucose tolerance test in 28th week of gestation; obesity: weight at least 20% above ideal weight Exclusion criteria: abnormities in glucose level; pre-eclampsia; gestation-induced hypertension Number of participants: experimental 30, control 30 |
Diet: diet for gestational diabetes; 30 kcal/kg ideal weight: 50% carbohydrates, 30% fat, 20% proteins. The total energy capacity could not be < 1600 kcal and > 2200 kcal | No intervention | Macrosomia, caesarean section because of LGA infant, Apgar score ≥ 7 at 5 minutes |
|
Guelinckx 2010126 English |
Method of randomisation: patients randomly assigned by using block randomisation Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: obese (BMI > 29.0 kg/m2 according to IOM criteria); white women consecutively attending the prenatal clinic before 15 weeks of gestation Exclusion criteria: pre-existing diabetes or developing GDM; multiple pregnancy; recruitment after 15 weeks of gestation; premature labour (delivery before 37 weeks of gestation); primary need for nutritional advice because of a metabolic disorder, kidney problems, Crohn’s disease, allergic conditions; inadequate knowledge of the Dutch language Number of participants: experimental (active) 65, experimental (passive) 65, control 65 |
Lifestyle intervention based on a brochure or on active education: passive group given a brochure during the first prenatal consultation; active group received the same brochure and actively counselled by a trained nutritionist in three group sessions. A maximum of five women were brought together in these 1-hour sessions, which were scheduled at 15, 20 and 32 weeks of pregnancy The sessions provided subjects with recommendations on a balanced, healthy diet, based on the official National Dietary Recommendations (9–11% of the energy should come from proteins, 30–35% from fat and 50–55% from carbohydrates). The dietary intervention aimed to limit the intake of energy-dense foods (e.g. fast food and sweets) by substituting them with healthier alternatives (e.g. fruit), increasing consumption of low-fat dairy products and wholewheat grains and reducing consumption of saturated fatty acids. Moreover, more general topics such as energy balance, body composition, food labels and how to increase physical activity were discussed. Techniques of behavioural modification were used to give the women insight into controlling periods of emotional eating, preventing binge eating sessions, etc. Brochure was specifically designed for the study and provided advice on nutrition and physical activity and tips to limit pregnancy-related weight gain Energy intake was never restricted in any group; however, by reducing consumption of energy-dense foods, the intervention indirectly aimed to reduce total energy intake. In case of weight gain above IOM recommendations, patients were advised to limit the intake of energy-dense foods Nutritional data were obtained from 7-day dietary records A physical activity score was calculated for each trimester of the pregnancy using the Baecke questionnaire |
No intervention | Pregnancy-induced hypertension, gestational weight gain in accordance with IOM, gestational weight gain > 11.2 kg, weight gain from prepregnancy to 38 weeks, chronic hypertension, pre-eclampsia, induction of labour, caesarean section, birthweight > 4000 g, total physical activity score, gestational weight gain, gestational age, birthweight, infant length |
|
Haakstad 2009112 English (abstract) |
Method of randomisation: not reported Allocation concealment: not reported Blinding: not reported |
Inclusion criteria: sedentary, primiparous women; mean age 30.7 (± 4.0) years Number of participants: experimental 52, control 53 |
Physical activity: 12-week aerobic dance exercise programme during pregnancy The exercise programme followed the ACOG exercise prescription and consisted of supervised aerobic dance and strength training for 60 minutes, performed at least twice a week for a minimum of 12 weeks |
No intervention | Exceeding IOM recommendations, weight gain |
|
Hopkins 2010113 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: healthy nulliparous women; between 20 and 40 years of age; singleton pregnancy; < 20 weeks of gestation Exclusion criteria: alcohol consumption or tobacco use at recruitment; a personal or family history of type 2 diabetes mellitus; development of any medical condition for which participation in an exercise programme was contraindicated by ACOG (e.g. pre-eclampsia, fetal growth restriction, preterm birth) Number of participants: experimental 49, control 49 |
Physical activity: aerobic exercise training in the second half of pregnancy The aerobic exercise programme was home based, using stationary cycling, and was individually prescribed for a maximum of five sessions of 40 minutes a week. Exercise programmes aimed to achieve a moderate exercise intensity of approximately 65% of predicted aerobic capacity (VO2max) The study protocol recommended that regular exercise was maintained until at least 36 weeks of gestation. After this time participants were encouraged to maintain as close to their prescribed exercise programme as possible until delivery (subject to capacity). During a fortnightly supervised exercise session, maternal heart rate and blood pressure responses were monitored, and exercise prescription was updated to maintain the prescribed exercise intensity. Compliance with the exercise programme was assessed by self-reported exercise diaries and downloadable heart rate monitors (Polar S625, Polar, Kempele, Finland). The required workload was estimated using linear regression of oxygen uptake and workload obtained from aerobic fitness testing, with standard equations used to calculate energy expenditure for all exercise sessions. Weekly energy expenditure, exercise duration (minutes) and exercise intensity (in metabolic equivalents) were averaged for each phase of the exercise programme: familiarisation (20–27 weeks), maintenance (28–35 weeks) and subject to capacity (36–40 weeks). Compliance was reported as the percentage of prescribed weekly exercise duration completed |
No intervention | Body weight at baseline, 19 weeks and 35 weeks, BMI, gestational age, crown–heel length, head circumference, neonatal BMI, ponderal index, birthweight |
|
Hui 2006127 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: < 26 weeks of gestation; no pre-existing diabetes Exclusion criteria: medical, obstetric, skeletal or muscular disorders that could contraindicate physical exercise during pregnancy Number of participants: overall 52; completed treatment: experimental 24, control 21 |
Lifestyle, diet and physical activity: community-based exercise/dietary intervention programme; group exercise sessions and home-based exercise Participants in the AI (additional information) group were instructed in group exercise sessions and home-based exercises. Recommended activities included walking, swimming, mild aerobics, stretching and strength exercises (e.g. lifting a 500-g food can with each hand).Weekly group sessions were held in an air-conditioned gymnasium in a community centre in the urban core provided by the government of the city of Winnipeg. Floor aerobics, stretching and strength exercises in group sessions (~ 45 minutes/session) were led by professional trainers. Student assistants taught participants to correctly use a pedometer, self-monitor their heart rate and record daily physical activities in a diary before or after the sessions. Exercise three to five times a week for 30–45 minutes per session was recommended for participants in the AI group. Video exercise instruction was produced in both VHS and DVD formats and provided to participants to assist with home-based exercise. Information about daily physical activity, including a self-recorded activity diary, were collected and analysed by student research assistants Dieticians provided a personalised plan for participants, including recommended changes in food choice, frequency, portion size and pattern of intake, if required (after assessment of normal 1-week food intake) |
No intervention (standard care, SC): physical activity was recommended for participants in the SC group, but they were not instructed in the group exercise sessions or home-based exercises. An information package of materials from Health Canada was provided containing dietary recommendations for a healthy pregnancy | Excessive weight gain, GDM, need for birthweight-related procedures, macrosomia, weight gain from 26 weeks to delivery, weight of newborn, pregnancy duration, physical activity level |
|
Jackson 201017 English |
Method of randomisation: computer-generated randomisation Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: English-speaking women; ≥ 18 years; < 26 weeks of gestation Number of participants: experimental 158, control 163 |
Lifestyle: Video Doctor teaching and counselling session about nutrition, exercise and weight gain Video Doctor is a computer program delivered on laptop computers in the clinic setting. It conducts in-depth behavioural risk assessments, delivers tailored counselling messages and produces printed output for both the patient and the clinician. An actor-portrayed Video Doctor appears and offers education on exercise, nutrition and weight gain based on principles of motivational interviewing. The Video Doctor engages subjects in a confidential, ‘face-to-face’ discussion in which the Video Doctor actor expresses reflexive understanding of the subject’s concerns, shows compassion for the subject and provides non-judgemental counselling. The Video Doctor simulates an ideal conversation with a health-care provider and has been highly acceptable to diverse samples of patients. Using a library of digital video clips, extensive branching logic and participant input the computer programme matches counselling video clips to the participant’s BMI, eating and exercise habits, and readiness to change. At the conclusion of each session the programme prints a cueing sheet for the clinician that offers a summary of the patient’s risk profile and suggests counselling statements. an educational worksheet that contains information presented by the Video Doctor and includes questions for self-reflection is printed for the patient to keep. In summary, the intervention consists of three parts: Video Doctor counselling session, cueing sheet for the clinician and educational worksheet for the patient The intervention group received dietary counselling focused on increasing intake of fruits and vegetables and whole grains, increasing consumption of healthful versus unhealthful fats and decreasing consumption of sugary foods. The Video Doctor emphasised dietary and exercise behaviour changes over weight gain. The Video Doctor portion required 10–15 minutes to complete. The participant then proceeded to her prenatal care appointment and returned briefly to the research assistant to report whether nutrition, exercise or weight had been discussed and to obtain the computer-generated educational worksheet specific to the patient’s risk profile Both intervention and control participants were invited back for a follow-up assessment at least 4 weeks after the baseline session consisting of the same diet and exercise questions. Intervention participants received a brief ‘booster’ Video Doctor counselling session after the follow-up questionnaire had been completed, including feedback reflecting changes made since baseline and an updated cueing sheet and educational worksheet |
No intervention | Weight gain from before 26 weeks to delivery |
|
Jeffries 2009128 English |
Method of randomisation: randomisation sequence obtained using a computer random number generator Allocation concealment: number cards allocating women to either the intervention or control group were placed in opaque, sequentially numbered envelopes Blinding: patients |
Inclusion criteria: women from a tertiary obstetric hospital in Melbourne, Australia Exclusion criteria: > 14 weeks’ gestation at first appointment; non-English speaking; < 18 or > 45 years of age; multiple pregnancy; type 1 or 2 diabetes mellitus Number of participants: experimental 148, control 138 |
Advisory: women advised of their optimal gestational weight gain Women allocated to the intervention group were given personalised weight measurement card, advised of their optimal gestational weight gain (based on their BMI at the time of recruitment and the IOM guidelines) and instructed to record their weight at 16, 20, 24, 28, 30, 32 and 34 weeks’ gestation Weight measurements during pregnancy were carried out on either the participants’ own scales at home or the scales at the hospital, according to patient preference The control group was weighed at recruitment and at 36 weeks’ gestation, but was not given instructions about regular weight measurement |
No intervention | Gaining more weight than in IOM guidelines, birthweight < 10th percentile, birthweight > 90th percentile, preterm delivery, instrumental delivery, caesarean delivery, pre-eclampsia, pregnancy-induced hypertension, GDM, Apgar score < 7 at 5 minutes, hypoglycaemia, shoulder dystocia, weeks’ gestation at delivery, birthweight, weight gain per week, total weight gain from 11 weeks to delivery |
|
Khaledan 2010114 Persian/English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: single pregnancy; intact amniotic membranes Exclusion criteria: any contraindications for exercise, heart disease associated with significant haemodynamic changes, chronic pulmonary disease, cervical insufficiency or correction of cervical insufficiency; multiple pregnancy; permanent vaginal bleeding in second and third trimester of pregnancy; placenta praevia after 26 weeks of pregnancy; risk of preterm delivery in the current pregnancy; rupture of fetal membranes; presence of hypertension during pregnancy; severe anaemia; unchecked arrhythmia in the mother; chronic inflammation of the airways; type 1 diabetes mellitus with poor control; extreme morbid obesity; very low maternal weight; history of completely sedentary lifestyle; fetal growth restriction in current pregnancy; skeletal and structural limitations; seizure disorders; uncontrolled hyperthyroidism; heavy smoking Number of participants: experimental 20, control 24 |
Physical activity: specific aerobic exercise Three sessions of 30–45 minutes a week for 8 weeks. The first 15 minutes of stretching was carried out to make muscles and joints soft and flexible. The aerobic stage rally was performed to continue with the rhythm so that the person takes a walk so slowly in a second leg on the ground. This step lasted 5 minutes in the first session and then in each session 1 minute was added to the time and in eighteenth session the time was reached to 15 minutes; it then remained constant for the rest of the sessions. The intensity of exercise was based on 60% of the maximal heart rate, calculated by 220 – age × 60/100 All participants received diet information based on food pyramid guidelines recommended by the American Agricultural Department plus iron and folic acid tablets |
No intervention | Caesarean section, failure of labour, mother’s weight after 2 months of receiving the intervention from 28 to 36 weeks of pregnancy, neonatal weight, gestational age at delivery |
|
Khoury 200598 English |
Method of randomisation: the randomisation list was generated from a table of random numbers drawn up by one of the investigators (who had no contact with the pregnant women) Allocation concealment: sealed, consecutively numbered opaque envelopes Blinding: investigators/clinicians and outcome assessors |
Inclusion criteria: non-smoking (previous smokers had to have quit ≥ 5 years before inclusion); white; single healthy fetus; age 21–38 years; BMI of 19–32 kg/m2; no previous pregnancy complications; first, second or third pregnancy; not vegetarian or following a Mediterranean-type diet or immigrants to Norway from non-Western countries Exclusion criteria: high-risk pregnancy caused by diabetes mellitus, endocrine disease, chronic hypertension, drug abuse, history of thromboembolic disease or significant gastrointestinal, cardiac, pulmonary or haematological disease; women with complications during a previous pregnancy including neonatal death, stillbirth or preterm delivery, or with a history of habitual abortion (more than three previous spontaneous abortions); women who experienced ongoing hyperemesis gravidarum or bleeding after gestational week 12 in the current pregnancy Number of participants: experimental 141, control 149 |
Diet/dietary advice: cholesterol-lowering diet from gestational week 17–20 to birth | No intervention: control group was asked to consume their usual diet based on Norwegian foodstuffs and not to introduce more oils or low-fat meat and dairy products than usual; energy intake aimed at a weight gain of 8–14 kg, as in the intervention group | Preterm delivery, preterm stillbirth, intrauterine growth restriction, hypertensive complications, fetal distress, pre-eclampsia, birthweight, gestational age at delivery |
|
Kulpa 1987129 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: age 18–34 years; non-smoker; ≤ 15% of their ideal body weight; ≥ 10 metabolic equivalents of 3.5 ml/kg/minute of oxygen consumption performance on the treadmill; no known medical problems; no high-risk obstetric complications according to criteria of Williams Obstetrics; interest in recreational sports Exclusion criteria: spontaneous aborters; non-complying subjects; dropouts Number of participants: overall 141; completed treatment: experimental 38, control 47 |
Exercise (no particular aerobic exercise) and nutritional counselling | No intervention | Premature rupture of membranes, post-dates pregnancy, chorioamnionitis, meconium-stained amniotic fluid, oxytocin induction or augmentation of labour, use of forceps, uterine atony, total weight gain from prepregnancy to delivery |
|
Landon 200999 English |
Method of randomisation: women were randomly assigned by the co-ordinating centre with the use of the simple urn method, stratified by clinical centre Allocation concealment: not reported. Blinding: no blinding used |
Inclusion criteria: between 24 weeks 0 days and 30 weeks 6 days of gestation; blood glucose concentration between 135 and 200 mg/dl (between 7.5 and 11.1 mmol/l) 1 hour after a 50 g glucose loading test; mild GDM defined as a fasting glucose level of < 95 mg/dl (5.3 mmol/l) and two or three timed glucose measurements that exceeded established thresholds: 1-hour, 180 mg/dl (10.0 mmol/l); 2-hour, 155 mg/dl (8.6 mmol/l); and 3-hour, 140 mg/dl (7.8 mmol/l) Exclusion criteria: pre-existing diabetes mellitus; fasting glucose level of ≥ 95 mg/dl on the diagnostic oral glucose tolerance test; abnormal result on a glucose screening test before 24 weeks of gestation; previous GDM; history of stillbirth; multifetal gestation; asthma or chronic hypertension; taking corticosteroids; known fetal anomaly; if imminent or preterm delivery was likely because of maternal disease or fetal condition Number of participants: experimental 485, control 473 |
Diet: formal nutritional counselling and diet therapy along with insulin if required | No intervention | Hypoglycaemia, hyperbilirubinaemia, birth trauma, birthweight > 4000 g, LGA infants, preterm delivery, SGA infants, admission to NICU, intravenous glucose treatment, respiratory distress syndrome, induction of labour, caesarean delivery, shoulder dystocia, pre-eclampsia, gestational age at birth, birthweight (g), fat mass (g), BMI at delivery, weight gain (kg) from 29 weeks to delivery |
|
Lee 1996115 English |
Method of randomisation: random number table Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: nulliparous; singleton pregnancy; booked at St Thomas’ Hospital, London, UK Exclusion criteria: pregnancy exclusion factors: diabetes, weight of < 50 kg or > 90 kg, history of more than one spontaneous abortion, spinal or leg injuries, cervical suture in situ, use of drugs of addiction (including cigarettes but excluding alcohol in moderation); exclusion factors developing during pregnancy: insulin-dependent GDM, abruptio placentae, pregnancy-included hypertension, anaemia of < 9 g/dl of haemoglobin, discovery of multiple pregnancy, threatened abortion, placenta praevia, intrauterine growth retardation, preterm labour, breech presentation at 40 weeks; smoking Number of participants: experimental 182, control 188 |
Physical activity: planned programme of aerobic exercise for 1 hour three times a week Exercises were designed to allow women to perform at moderate intensity (about 60–70% of age-related maximum heart rate). Classes were run by aerobic teachers trained in exercise during pregnancy. Resting and exercise pulse rates were recorded manually and with electronic pulse watches Local venues, travel expenses and exercise shoes were provided |
No intervention | Caesarean section, vaginal delivery, postnatal incontinence, postnatal physical pain and discomfort, perceived positive physical outcome related to exercise, requests for postnatal exercise classes, perceived positive social outcome related to exercise, requests for maternity services, miscellaneous comments |
|
Marquez-Sterling 2000116 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: completed a medical questionnaire; provided a sonogram of the fetus; classified as low risk by physician; sedentary; not exercised on a regular basis for at least 1 year before conception Number of participants: experimental 10, control 10 |
Physical activity: aggressive exercise programme The training programme consisted of a series of 1-hour sessions held three times a week for 15 weeks. Subjects were taught to use their heart rate monitors so that they could adhere to their target heart rates during each training session. Each session started with a 5-minute warm-up on the stationary bicycle ergonometer or treadmill after which subjects were introduced to a combination of rowing, stationary cycling and walk-jogging as part of the aerobic portion of their training After the acclimation period a rhythmic calisthenics class, which was a modification of the Fitness Canada programme, and a step class were added to the aerobic workout. After 6 weeks the StairMaster was included as part of the aerobic workout and alternated with other equipment. On brisk nights the aerobic programme was modified and brisk walks were performed instead to add diversity to the aerobic programme. These were carried out using quick marching steps, long deliberate strides, leg kicks and knee kicks. All exercise sessions ended with standing and floor-supported stretches and were conducted by certified personnel |
No intervention | Caesarean section, weight gain from prepregnancy to delivery, skinfold thickness, infant birthweight, Apgar score at 5 minutes |
|
Ney 1982100 English |
Method of randomisation: not reported Allocation method: not reported Blinding: no blinding used |
Inclusion criteria: type 1 or type 2 diabetes mellitus Number of participants: experimental 11, control 9 |
Diet: high-carbohydrate, high-fibre, low-fat (HCF) diet All patients were hospitalised in the University of California San Diego School of Medicine General Clinical Research Centre at 10–30 weeks’ gestation for an 8-day baseline evaluation and for metabolic studies and intensive dietary education During the initial 24-hour study each patient received her usual dose of insulin and a 2000-kcal control meal pattern with a standardised nutrient distribution, including three meals at 8:00, 12:00 and 17:00) and three between-meal snacks (at 10:00, 15:00 and 22:00) |
No intervention (diet commonly prescribed for pregnancy) | Weight gain from prepregnancy to delivery, gestational age, birthweight |
|
Ong 2009117 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: singleton pregnancy; normal 18-week anatomy scan; no evidence of cardiovascular disease or pre-existent diabetes Number of participants: experimental 6, control 6 |
Physical activity: home-based supervised exercise programme consisting of three sessions a week of stationary cycling beginning at week 18 of gestation Exercise training was performed on an upright stationary cycle ergometer (Marquee Series, Healthstream) that each participant kept in her home for the duration of the intervention. Each session involved a 10-minute warm-up followed by one or two 15-minute bouts of cycling (with rest periods if necessary) at an intensity of 50–60% of maximum heart rate. As the weeks progressed the exercise intensity was increased to 60–70% of maximum heart rate and the duration was increased to 40–45 minutes. Sessions ended with a 10-minute cool-down period of easy pedalling |
No intervention | Weight gain in kg from 18 to 28 weeks |
|
Polley 2002130 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: pregnancy before 20 weeks’ gestation Exclusion criteria: underweight women (BMI < 19.8 kg/m2 based on self-reported weight and height at the last menstrual period); women younger than 18 years; first prenatal visit > 12 weeks’ gestation; high-risk pregnancy (i.e. drug abuse, chronic health problems, previous complications during pregnancy or current multiple gestation) Number of participants: experimental 61, control 59 |
Stepped care behavioural intervention: education and feedback about weight gain during pregnancy, stressing modest exercise and healthy, low-fat eating | No intervention | Exceeded, within or below IOM recommendations at some point during pregnancy, low birthweight (< 2500 g), macrosomia, preterm delivery, caesarean delivery, pre-eclampsia, maternal hypertension, GDM, total weight gain from prepregnancy to last prenatal visit before delivery, post-partum weight loss at 8 weeks, net weight retention, birthweight, weeks’ gestation at delivery |
|
Prevedel 2003118 Portuguese (Brazilian) |
Method of randomisation: women were randomly selected (model randomised) Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: primiparous or adolescents, with singleton pregnancy; absence of medical or obstetric disease; gestational age 16–20 weeks Exclusion criteria: more than three absences a month at hydrotherapy sessions were considered as withdrawal; prenatal care and childbirth out of service; development of medical or obstetric complications Number of participants: experimental 29, control 31 |
Physical activity: moderate-intensity hydrotherapy programme The hydrotherapy programme was delivered by the physiotherapist in the company of the obstetrician in subgroups of up to 10 pregnant women. The programme was carried out with moderate intensity for 1 hour three times a week in a covered and heated swimming pool (between 28°C and 32°C). The sessions comprised five phases of aquatic exercise, taking into consideration the recommendations of ACOG: stretching, heating, resistance, localised exercises and relaxation with breathing exercises During the sessions of hydrotherapy heart rate was monitored by frequency-grip to control the intensity of the exercise |
No intervention | Preterm birth, adequate weight, LGA, body weight at baseline, 16–20 weeks and close to delivery (36–40 weeks), lean mass, total fat, relative fat (%), birthweight |
|
Rae 2000101 English |
Method of randomisation: women were allocated at random by draw of opaque numbered envelopes Blinding: patients and investigators/clinicians |
GDM women only Inclusion criteria: gestation ≤ 35 weeks and 6 days; > 110% of ideal body weight for height (adjusted for expected pregnancy weight gain and using a BMI of 25 kg/m2 as equal to 100% of ideal body weight); oral glucose tolerance test with fasting plasma glucose > 5.4 mmol/l and/or 2-hour plasma glucose > 7.9 mmol/l Number of participants: experimental 67, control 58 |
Diet: energy restriction (30% – moderate) The intervention comprised instruction in a moderately energy-restricted diabetic diet providing between 6800 and 7600 kJ (1590–1776 kcal). This represents 70% of the recommended dietary intake for pregnant women (National Health and Medical Research Council of Australia) To monitor diet compliance, 3-day food diaries were kept by participants at three time periods after recruitment, and were later analysed using System for Online Dietary Analysis (soda version 5B, 1991, developed by Computer Models, Cottesloe, Western Australia) The decision to commence insulin therapy was made by medical staff who were blinded to the group allocation of each participant All women were seen by the research dietitian at each antenatal visit |
No intervention [diabetic diet that was not energy restricted, providing approximately 8600–9500 kJ (2010–2220 kcal) a day] | Pre-eclampsia, induction of labour, vaginal delivery, assisted delivery, elective lower uterine segment caesarean section, non-elective lower uterine segment caesarean section, shoulder dystocia, infants ≥ 4000 g, infants ≥ 90th centile (birthweight), hypoglycaemia, weight change from treatment to delivery, weight lost from treatment to delivery, weight change from prepregnancy to delivery, gestation at delivery, mean birthweight, estimated birthweight ratio, skinfold thickness (neonatal) |
|
Santos 2005119 English |
Method of randomisation: randomised following a blocked sequence generated from a random number table by a statistician not participating in other aspects of the study Allocation concealment: numbered, opaque envelopes Blinding: outcome assessors |
Inclusion criteria: healthy, non-smoking pregnant women; age ≥ 20 years; gestational age < 20 weeks; BMI between 26 and 31 kg/m2 (corresponding to a prepregnancy BMI of 25–30 kg/m2) Exclusion criteria: compliance to the run-in period protocol; hypertension; diabetes mellitus; conditions considered to contraindicate exercise such as preterm labour, an incompetent cervix, high-order multiple gestation (more than three) and uncontrolled thyroid disease Number of participants: experimental 46, control 46 |
Physical activity: supervised, gymnasium-style physical activity programme of aerobic exercise The intervention consisted of an unblinded programme of supervised physical exercise of 60 minutes’ duration performed three times a week. Each session consisted of 5–10 minutes of warm-up, 30 minutes of heart rate-monitored aerobic activity, 10–15 minutes of exercise involving upper and lower limbs and 10 minutes of stretching and relaxation. Aerobic activities were always performed at between 50% and 60% of the maximum predicted heart rate, never exceeding 140 beats/minute. The exercises followed the recommendations concerning physical activity practice during pregnancy of the American College of Sports Medicine and ACOG. Aerobic exercises included walking, pedalling a bicycle ergometer and aerobic gymnastics. Upper extremity resistance exercises were performed with hand-held dumbbells (up to 1 kg), rods and tennis balls. For the legs, body weight resistance exercises such as squats and lunges were performed |
The control group participated in once-weekly sessions that included relaxation (respiratory exercises and light stretching but no aerobic or weight-resistance exercises) and focus group discussions concerning maternity. Control participants were neither encouraged to exercise nor discouraged from exercising | Low birthweight, prematurity delivery, weight gain of mother from 18 to 30 weeks, birthweight, Apgar score |
|
Sedaghati 2007120 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria: attendance at prenatal clinics in Qom province, Islamic Republic of Iran Exclusion criteria: any absolute and relative contraindications to aerobic exercise during pregnancy; history of exercise before pregnancy; history of orthopaedic disease or surgery; missing three sessions of the exercise programme Number of participants: experimental 50, control 50 |
Physical exercise: special pregnancy exercise in preventing or reducing low back pain Exercise programmes included a 15-minute warm-up and cool down plus 30 minutes cycling in the range of 55–65% of maximal heart rate with respect to age The warm-up consisted of 5 minutes of extension movements and 5 minutes slow cycling and the cool down (return to the first condition) consisted of 5 minutes of extension movements (nonsense, probably mistake in publication). The cycling exercise was defined as 30 minutes of cycling (three sessions a week at moderate intensity). The exercises were prescribed by a physical training specialist and were offered to the pregnant women after evaluation of the required criteria During the running of the whole programme supervision was carried out by a midwife |
No intervention | Weight gain from 20–22 weeks to delivery, pregnancy length, low back pain |
|
Thornton 2009102 English |
Method of randomisation: envelopes were prepared and sequentially numbered. A card indicating the assigned group was placed in the envelope and the envelope was sealed. A random number table was used to assign each consecutively numbered envelope to either the study or the control group in blocks of 10 Allocation concealment: numbered and sealed envelopes; not known if they were opaque Blinding: no blinding used |
Obese women Inclusion criteria: pregnant with a single fetus; between 12 and 28 weeks of gestation; BMI ≥ 30 kg/m2 Exclusion criteria: pre-existing diabetes; hypertension; chronic renal disease Number of participants: experimental 124, control 133 |
Diet intervention based on a balanced nutritional regiment The study group was placed on an 18–24 kcal/kg balanced nutritional regimen consisting of 40% carbohydrates, 30% protein and 30% fat. No patient received a diet of < 2000 calories. All women in the study group were asked to record in a diary all of the foods and beverages consumed each day. Participants in both groups were encouraged to engage in 30 minutes of walking a day |
No intervention | GDM, pre-eclampsia, gestational hypertension, post-partum haemorrhage, preterm delivery, labour induction, caesarean delivery, macrosomic infant, Apgar score (< 7 at 5 minutes), infant birthweight, gestational age at delivery, weight gain from 20–22 weeks to delivery, weight loss difference post partum |
|
Wolff 2008103 English |
Method of randomisation: computerised randomisation Allocation concealment: not reported Blinding: investigators/clinicians |
Inclusion criteria: non-diabetic; Caucasian; BMI ≥ 30 kg/m2; early pregnancy (15 ± 3 weeks of gestation) Exclusion criteria: smoking; age < 18 or > 45 years; multiple pregnancy; medical complications known to affect fetal growth adversely; contraindication to limitation of weight gain Number of participants: experimental 28, control 38 |
Dietary consultations (healthy diet, restriction of energy intake) The intervention group received 10 consultations of 1 hour each with a trained dietitian during the pregnancy. Women were instructed to eat a healthy diet according to the official Danish dietary recommendations [fat intake: maximum 30 energy per cent (E%); protein intake: 15–20 E%; carbohydrate intake: 50–55 E%] Energy intake was restricted based on individually estimated energy requirements and estimated energetic cost of fetal growth [energy requirement = basal metabolic rate × 1.4 (physical activity level factor of 1.2 + 0.2 added to cover energetic cost of fetal growth)] |
No intervention | GDM, pregnancy-induced hypertension, pre-eclampsia, caesarean delivery, gain in body mass from 15 to 36 weeks, birthweight, infant length, head circumference, abdominal circumference |
|
Yeo 2000121 English |
Method of randomisation: not reported Allocation concealment: not reported Blinding: not reported |
Inclusion criteria: at least 18 years old; high risk of gestational hypertensive disorders Exclusion criteria: diabetes mellitus; renal disease; multiple pregnancies; extremely vigorous exercisers [more than three times a week at a level above Rating of Perceived Exertion (RPE; a widely accepted subjective measure of exercise intensity) 14 for > 30 minutes per session] Number of participants: experimental 8, control 8 |
Exercise at RPE level 13 The exercise group visited the laboratory three times a week to perform 30 minutes of exercise at RPE level 13, considered a moderate level of exercise A motorised treadmill and bicycle ergometer were alternated. Exercise started with a 5-minute warm-up using the branching protocol, followed by 30 minutes steady state (RPE 13), finishing with a 10-minute cool-down |
No intervention | Mean per cent body fat of mother |
|
Yeo 2008122 English |
Method of randomisation: simple randomisation Allocation concealment: not reported Blinding: outcomes assessors |
Inclusion criteria: pre-eclampsia during a previous pregnancy; lower than average cardiovascular fitness level (i.e. peak oxygen consumption ≤ 50th percentile); sedentary lifestyle Exclusion criteria: chronic hypertension; pregestational diabetes mellitus; medical or physical condition that prohibits daily regular exercise; recommendation of a primary care provider not to participate; inability to communicate reasonably with research staff (language; mental state) Number of participants: experimental (stretching) 41, control (walking) 38 |
The stretching exercise programme consisted of 40 minutes of stretching exercises five times a week without increasing the heart rate > 10% of the resting heart rate. The stretch movements consisted of slow muscle movements that had neither aerobic nor muscle resistance components. Movements were selected from maternity nursing textbooks and the maternity guidebooks distributed at the data collection clinics. A videotape of the movements was developed for the study. Subjects followed the videotaped movement at each session to control the movement and the duration. Subjects wore a portable heart rate monitor to keep the heart rate within the specified range | Walking exercise was defined as 40 minutes of walking five times a week at moderate intensity. This programme was consistent with the recommendations of the Surgeon General for healthy people and ACOG for healthy pregnant women. Moderate-intensity cardiovascular exercise was defined by: (1) heart rate between 55% and 69% of maximum heart rate (HRmax); (2) oxygen uptake (VO2) between 50% and 74% of peak VO2; and (3) RPE of either 12 or 13 | Pre-eclampsia, gestational hypertension, birthweight |
|
Yeo 2009123 English |
Method of randomisation: women were randomised to two groups using a pre-generated allocation schedule Allocation concealment: sealed envelopes to withhold knowledge of future assignments from both the women and the researchers Blinding: no blinding used |
Inclusion criteria: < 14 weeks’ gestation; lower than average cardiovascular fitness level or peak oxygen consumption ≤ 50th percentile of women in same age group; sedentary lifestyle/estimated energy expenditure for daily physical activity during the index pregnancy of < 840 kcal/week Exclusion criteria: chronic hypertension; pregestational diabetes (at the time of recruitment); medical/physical condition prohibiting daily regular exercise; recommendation of primary care provider not to participate; inability to communicate (language, mental status) Number of participants: experimental (stretching) 60, control (walking) 64 |
The stretching programme consisted of slow muscle movements that had neither aerobic nor muscle resistance components. A 40-minute videotape of the stretching movements was given to each stretching participant so that she could follow movement sequences at a prespecified pace Once randomised, participants individually visited the exercise laboratory three times in the 18th week of gestation. During these visits a staff exercise specialist trained and supervised participants in their assigned exercises Stretchers were trained in stretching manoeuvres and were also taught the warning signs indicating that they should either stop or not start exercise to ensure maternal and fetal safety. Participants were instructed to exercise two more times on their own at home for the required five times a week. In the 19th week of gestation participants exercised twice at the exercise laboratory under the supervision of an exercise specialist and three times on their own at home. From then on they visited the exercise laboratory once a week for supervised exercise by a trained staff member and completed the other four exercise sessions on their own at home Participants received a weekly exercise log; they were asked to check off the date and time after each exercise session. At the end of each week they submitted the form filled out for the previous week and received a new form for the next week. Stretchers recorded the number of completed stretching sessions. The total number of sessions for each week was entered as the frequency of exercise performed |
Walkers were trained to walk at the prescribed target heart rate and at RPE 12 or 13 and were taught the warning signs indicating that they should either stop or not start exercise to ensure maternal and fetal safety The walkers brought in their heart rate monitors to weekly laboratory visits and heart rate data during each exercise session were downloaded to determine the length of exercise and the proportion of exercise within the target heart rate. In order to monitor their daily physical activity during the intervention all participants wore a pedometer daily from when they woke up until bedtime |
Weight gain from prepregnancy to 37 weeks |
Appendix 4 Risk of bias in randomised controlled trials included in the effectiveness review
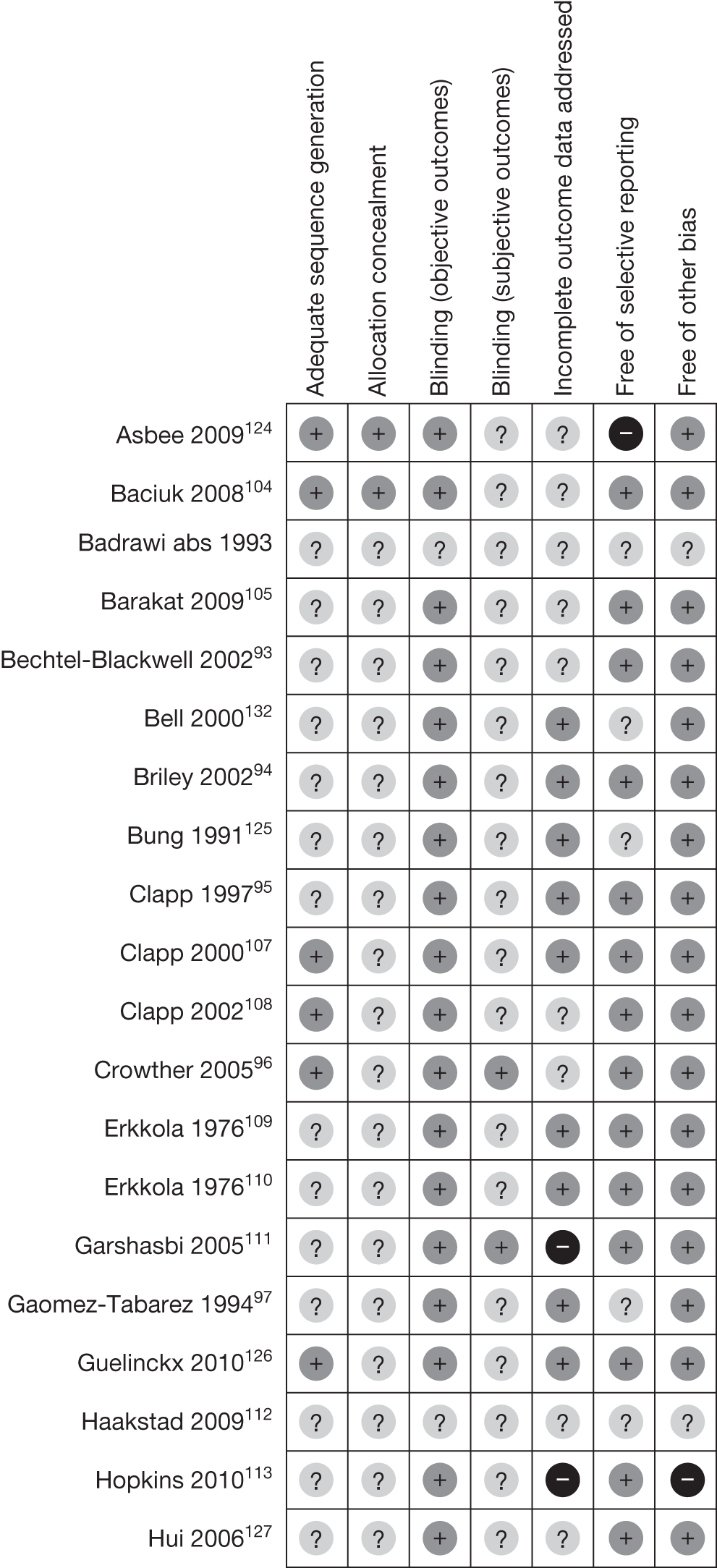
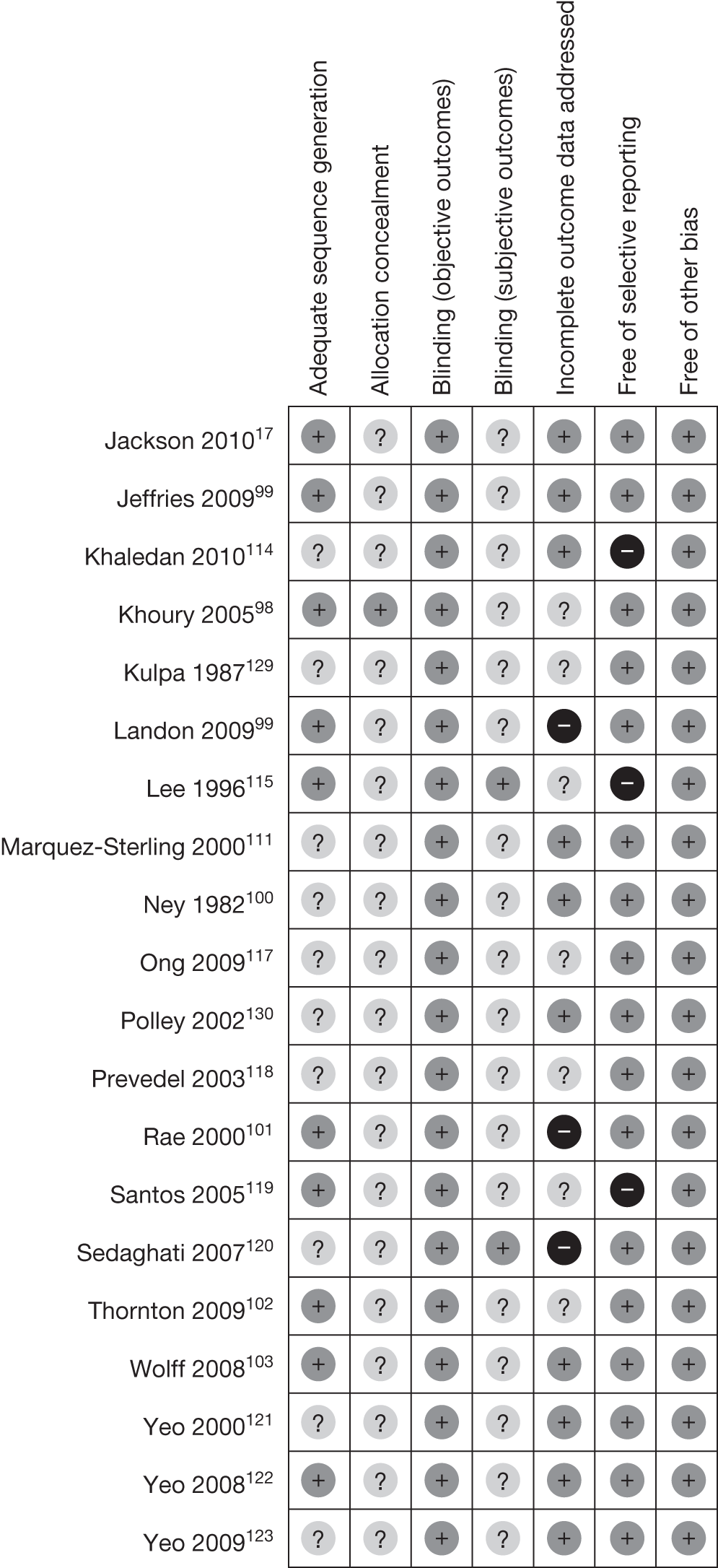
Appendix 5 Quality assessment of individual non-randomised studies evaluating the effectiveness of weight management interventions in pregnancy
Intervention based on a mixed approach
| Study | Blinding | Incomplete outcome data | Selective outcome reporting | Selection bias and risk of confounders |
|---|---|---|---|---|
| Casanueva 199448 | Not used (–) | No loss to follow-up (++) | Unclear | Baseline differences (–) |
| Claesson 200849 | Not used (–) | No (–) | Unclear | No differences (++) |
| Gray-Donald 200054 | Not used (–) | No (–) | Yes (+) | No differences (++) |
| Kinnunen 200757 | Not used (–) | Yes (28/132 lost to follow-up, intention-to-treat analysis not performed) (+) | Unclear | Baseline differences, adjustment made in the analysis (++) |
Intervention based mainly on dietary intervention
| Study | Blinding | Incomplete outcome data | Selective outcome reporting | Selection bias and risk of confounders |
|---|---|---|---|---|
| Borberg 198045 | Not used (–) | No loss to follow-up (++) | Unclear | No differences (++) |
| Campbell 197546 | Not used (–) | No (–) | Yes (+) | No differences, patients matched (++) |
| Campbell 198347 | Not used (–) | No (–) | Yes (+) | No differences, patients matched (++) |
| El Hiday 199253 | Not used (–) | No loss to follow-up (++) | No (–) | No differences (++) |
| Moses 200658 | Not used (–) | 8/62 lost to follow-up, intention-to-treat analysis performed (++) | Yes (+) | Baseline differences, adjustment made in the analysis (++) |
Physical activity-based intervention
| Study | Blinding | Incomplete outcome data | Selective outcome reporting | Selection bias and risk of confounders |
|---|---|---|---|---|
| Artal 200744 | Not used (–) | No (–) | Yes (+) | Baseline differences (–) |
| Clapp 199551 | Not used (–) | No loss to follow-up (++) | Yes (+) | Baseline differences (–) |
| Collings 198352 | Not used (–) | No (–) | Yes (+) | No differences (++) |
| Hall 198755 | Not used (–) | No loss to follow-up (++) | Yes (+) | Unclear |
| Kardel 199856 | Not used (–) | No loss to follow-up (++) | Yes (+) | Baseline differences (–) |
| Narendran 200559 | Not used (–) | No loss to follow-up (++) | Yes (+) | No differences, patients matched (++) |
Appendix 6 Quality assessment of the observational studies evaluating the effectiveness of weight management interventions in pregnancy
Cohort studies
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | Overall score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|
| Bell 199560 | + | + | – | + | – | – | + | – | ++++ |
| Bungum 200061 | – | + | – | – | + | – | + | – | +++ |
| Clapp 198462 | + | + | + | – | ++ | + | + | – | +++++++ |
| Clapp 199063 | + | – | + | + | – | + | + | – | +++++ |
| Clapp 199064 | + | + | + | + | – | + | + | – | ++++++ |
| Cogswell 199965 | + | + | – | – | ++ | – | + | – | +++++ |
| Conway 199966 | + | + | + | – | – | – | + | + | +++++ |
| Dale 198267 | – | – | + | + | – | + | + | – | ++++ |
| Dempsey 200469 | + | + | + | + | ++ | + | + | + | +++++++++ |
| de Rooij 200768 | + | + | – | – | + | + | – | + | +++++ |
| Hatch 199370 | + | + | + | – | – | + | – | – | ++++ |
| Horns 199671 | + | + | + | + | – | + | + | + | +++++++ |
| Jackson 199572 | + | + | – | – | – | + | + | + | +++++ |
| Knudsen 200873 | + | + | – | + | ++ | + | + | + | ++++++++ |
| Lenders 199474 | + | + | + | + | + | + | + | + | ++++++++ |
| Lenders 199775 | + | + | + | + | + | + | + | + | ++++++++ |
| Lumey 200976 | + | – | – | – | ++ | + | + | + | ++++++ |
| Magann 200277 | – | + | + | + | + | – | + | + | ++++++ |
| Melzer 201078 | + | + | + | – | – | + | – | – | ++++ |
| Mottola 201079 | + | – | + | – | – | – | + | + | ++++ |
| Neugebauer 199980 | – | + | – | – | + | + | + | + | +++++ |
| Olson 200481 | + | – | + | – | + | – | – | + | ++++ |
| Perichart 200982 | – | – | – | – | – | + | + | + | +++ |
| Piravej 200183 | + | + | + | – | – | + | + | – | +++++ |
| Shirazian 201084 | + | + | + | + | + | + | + | – | +++++++ |
| Stein 200785 | + | – | – | – | ++ | + | + | + | ++++++ |
Case–control studies
| Study | Is case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Overall score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|
| Berkowitz 198386 | + | + | + | – | + | – | + | + | ++++++ |
| Dempsey 200487 | – | + | + | + | + | – | + | – | +++++ |
| Dye 199788 | + | + | + | – | + | – | + | – | +++++ |
| Gregory 198789 | + | + | + | – | + | + | + | – | ++++++ |
| Oken 200690 | + | + | + | + | ++ | – | + | – | +++++++ |
| Sorensen 200391 | + | – | + | + | + | + | + | – | ++++++ |
Appendix 7 Clinical characteristics of the randomised controlled trials included in the review of adverse effects
| Study | Methods | No. of patients | Population | Intervention/ Comparator |
|---|---|---|---|---|
| Bell 2000132 |
Randomisation: not reported Allocation concealment: not reported Blinding: not used |
61 | Women already intending to exercise during pregnancy |
Intervention: physical exercise more than five times a week Comparator: exercise three or less times a week |
| Kulpa 1987129 |
Randomisation: not reported Allocation concealment: not reported Blinding: not used |
141 | Pregnant recreational athletes aged 18–49 years |
Intervention: exercise (no particular aerobic exercise) and nutritional counselling Comparator: no intervention |
Appendix 8 Risk of bias summary of the randomised controlled trials included in the review of adverse effects
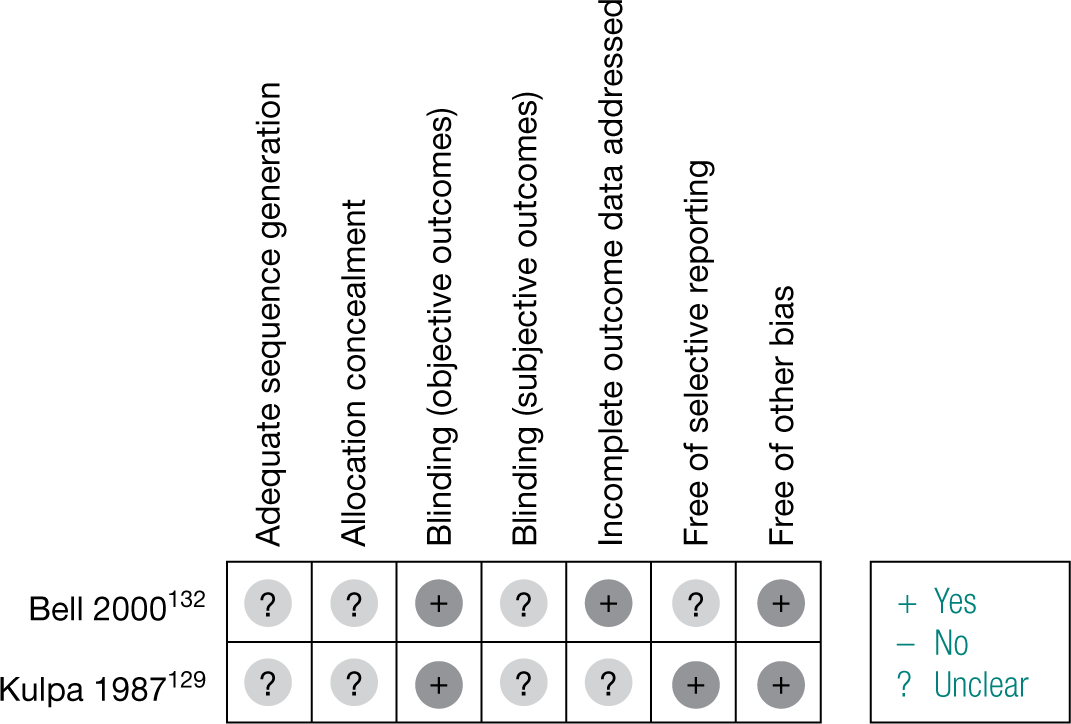
Appendix 9 Quality assessment of the observational studies evaluating the adverse effects of weight management interventions in pregnancy
Cohort studies
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | Overall score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|
| Clapp 199063 | + | – | + | + | – | + | + | – | +++++ |
| Clapp 199064 | + | + | + | + | – | + | + | – | ++++++ |
| Dale 198267 | – | – | + | + | – | + | + | – | ++++ |
| de Rooij 2006134 | + | + | – | – | + | + | – | + | +++++ |
| de Rooij 200768 | + | + | – | – | + | + | – | + | +++++ |
| Hatch 199370 | + | + | + | – | – | + | – | – | ++++ |
| Knudsen 200873 | + | + | – | + | + | + | + | + | ++++++++ |
| Lenders 199474 | + | + | + | + | + | + | + | + | ++++++++ |
| Lenders 199775 | + | + | + | + | + | + | + | + | ++++++++ |
| Lumey 200976 | + | – | – | – | ++ | + | + | + | ++++++ |
| Magann 200277 | – | + | + | + | + | – | + | + | ++++++ |
| Neugebauer 199980 | – | + | – | – | + | + | + | + | +++++ |
| Painter 2008135 | + | + | – | – | + | – | – | + | ++++ |
| Ravelli 1976136 | + | – | – | – | – | + | + | + | ++++ |
| Ravelli 1998137 | + | + | – | – | + | + | – | + | +++++ |
| Roseboom 2000138 | + | + | – | – | + | + | + | + | ++++++ |
| Roseboom 2000139 | + | + | – | – | + | + | – | + | +++++ |
| Stanner 1997141 | + | – | – | – | + | + | + | + | +++++ |
| Stein 200785 | + | – | – | – | ++ | + | + | + | ++++++ |
Case–control studies
| Study | Is case definition adequate | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls | Ascertainment of exposure | Same Method of ascertainment for cases and controls | Non-response rate | Overall score (max. 9) |
|---|---|---|---|---|---|---|---|---|---|
| Carmichael 2003133 | + | + | + | + | + | – | + | + | +++++++ |
| Gregory 198789 | + | + | + | – | + | + | + | – | ++++++ |
| Schramm 1996140 | + | + | + | – | ++ | – | + | – | ++++++ |
| Vujkovic 2007142 | + | + | + | – | + | – | + | – | +++++ |
| Yazdy 2010143 | + | + | + | – | + | – | + | – | +++++ |
Appendix 10 Clinical characteristics and findings of the observational studies evaluating the adverse effects of weight management interventions in pregnancy
| Outcome | Study | N | Intervention/exposure | n/N | Control group | n/N | OR or HR (95% CI) | Comments |
|---|---|---|---|---|---|---|---|---|
| NTD |
Carmichael 2003133 Case–control study |
1077 | Infants/fetuses diagnosed with NTD | Infants/fetuses with no defects | Diet: different types (during first trimester of pregnancy) | |||
| Diet to lose weight | 29/538 | Diet to lose weight | 14/539 | OR 2.1 (1.1 to 4.1)a | ||||
| Fasting diet | 17/538 | Fasting diet | 3/539 | OR 5.8 (1.7 to 20.0)a | ||||
| Other special diet | 17/538 | Other special diet | 3/539 | OR 1.0 (0.3 to 3.1)a | ||||
| Eating disorder | 18/538 | Eating disorder | 11/539 | OR 1.7 (0.8 to 3.6)a | ||||
| Any special diet or eating disorder | 61/538 | Any special diet or eating disorder | 31/539 | OR 2.1 (1.3 to 3.3)a | ||||
| Binge eating (self-reported dieting behaviour for any time during 3 months before pregnancy or during pregnancy) | 36/538 | Binge eating (self-reported dieting behaviour for any time during 3 months before pregnancy or during pregnancy) | 44/539 | OR 0.8 (0.5 to 1.3)a | ||||
| NTD |
Yazdy 2010147 Case–control study |
1394 | Infants with NTD | Infants with no major congenital anomalies | ||||
| Glycemic index low < 60 | 522/698 | Glycemic index low < 60 | 594/696 | OR 2.0 (1.5 to 2.6)a | ||||
| Glycemic index high ≥ 60 | 176/698 | Glycemic index high ≥ 60 | 102/696 | OR 1.5 (1.1 to 2.0)b | ||||
| Glycemic load low < 205 | 668/698 | Glycemic load low < 205 | 683/696 | OR 2.4 (1.2 to 4.6)a | ||||
| Glycemic load high ≥ 205 | 30/698 | Glycemic load high ≥ 205 | 13/696 | OR 1.8 (0.8 to 4.0)b | ||||
| Subgroup BMI ≥ 30 kg/m2 (100) | Glycemic index low < 60 | 23/36 | Glycemic index low < 60 | 53/64 | OR 2.7 (1.1 to 7.0)a | |||
| Glycemic index high ≥ 60 | 13/36 | Glycemic index high ≥ 60 | 11/64 | OR 2.0 (0.6 to 7.3)b | ||||
| Glycemic load low < 205 | 32/36 | Glycemic load high ≥ 205 | 59/64 | OR 1.5 (0.4 to 5.9)a | ||||
| Glycemic load high ≥ 205 | 4/36 | Glycemic load high ≥ 205 | 5/64 | OR 0.9 (0.2 to 4.7)b | ||||
| Subgroup BMI < 30kg/m2 (816) | Glycemic index low < 60 | 138/185 | Glycemic index low < 60 | 540/631 | OR 2.0 (1.4 to 3.0)a | |||
| Glycemic index high ≥ 60 | 47/185 | Glycemic index high ≥ 60 | 91/631 | OR 1.7 (1.1 to 2.7)b | ||||
| Glycemic load low < 205 | 177/185 | Glycemic load low < 205 | 623/631 | OR 3.8 (1.4 to 10.5)a | ||||
| Glycemic load high ≥ 205 | 8/185 | Glycemic load high ≥ 205 | 8/631 | OR 3.3 (1.0 to 10.6)b | ||||
| Cord abnormalities |
Magann 200277 Cohort study |
750 | Exercise: different levels | No exercise | 18/217 | p = 0.051 | ||
| Light | 15/222 | OR 0.80 (0.39 to 1.63)a | ||||||
| Moderate | 7/73 | OR 1.17 (0.47 to 2.93)a | ||||||
| Heavy | 9/238 | OR 0.43 (0.19 to 0.99)a | ||||||
| Coronary heart disease (adult) |
Roseboom 2000138 Cohort study |
736 | Diet: famine | Unexposed to famine: | ||||
| Exposed in late gestation | 3/120 | Conceived after | 6/232 | Exposed late gestation vs not exposed prenatally: OR 0.8 (0.2 to 2.8) | ||||
| Exposed in mid-gestation | 1/108 | Born before | 8/208 | Exposed mid-gestation: OR 3.0 (0.0 to 2.2) | ||||
| Exposed in early gestation | 6/68 | Exposed early: OR 3.0 (1.1 to 8.0) | ||||||
|
de Rooij 2006134 Cohort study |
694 | Diet: famine | Unexposed to famine: | Exposed generally vs not exposed prenatally: OR 0.79 (0.42 to 1.49)a | ||||
| Exposed in late gestation | 7/120 | Conceived after | 14/197 | Exposed late: OR 0.82 (0.35 to 1.92)a | ||||
| Exposed in mid-gestation | 4/100 | Born before | 15/215 | Exposed mid: OR 0.55 (0.19 to 1.60)a | ||||
| Exposed in early gestation | 5/62 | Exposed early: OR 1.16 (0.43 to 3.11)a | ||||||
| Metabolic syndrome (adult) |
de Rooij 200768 Cohort study |
783 | Diet: famine | Unexposed to famine: | General: OR 1.2 (0.9 to 1.7) | |||
| Exposed in late gestation | 54/141 | Conceived after | 64/214 | Exposed late: OR 1.4 (0.9 to 2.1) | ||||
| Exposed in mid-gestation | 34/116 | Born before | 71/238 | Exposed mid: OR not available | ||||
| Exposed in early gestation | 28/74 | Exposed early: OR 1.4 (0.6 to 1.5)a | ||||||
|
de Rooij 2006134 Cohort study |
694 | Diet: famine | Unexposed to famine: | Exposed generally vs not exposed prenatally: OR 1.09 (0.78 to 1.51)a | Metabolic syndrome definition according to NCEP (National Cholesterol Educational Programme) | |||
| Exposed in late gestation | 40/120 | Conceived after | 59/197 | Exposed late: OR 1.16 (0.75 to 1.79)a | ||||
| Exposed in mid-gestation | 28/100 | Born before | 65/215 | Exposed mid: OR 0.90 (0.56 to 1.47)a | ||||
| Exposed in early gestation | 22/62 | Exposed early: OR 1.28 (0.73 to 2.24)a | ||||||
| Hypertension (adult) |
Lumey 200976 Cohort study |
638 | Diet: famine | 224/344 | Unexposed to famine (hospital control subjects) | 168/294 | OR 1.40 (1.02 to 1.93)a |
p = 0.03 Systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or prior diagnosis with medication |
| Breast cancer |
Painter 2006135 Cohort study |
475 | Diet: famine | Unexposed to famine: | HR (all exposed) 2.6 (0.9 to 7.7)a | p < 0.005 (Cox regression) | ||
| Exposed in late gestation | 3/82 | Conceived after | 1/126 | HR 2.6 (0.9 to 7.7)b | Adjusted for maternal cancer status | |||
| Exposed in mid-gestation | 3/77 | Born before | 4/144 | HR 2.5 (0.8 to 7.4)b | Adjusted for birthweight | |||
| Exposed in early gestation | 4/46 | HR 4.0 (1.1 to 14.5)b | Adjusted for BMI | |||||
| Cleft lip or palate or both |
Vujkovic 2007142 Case–control study |
381 | Diet: Western vs prudent | Diet: Western vs prudent |
Adjusted for periconception maternal folic acid intake and/or multivitamin intake The Western diet case group consisted of 12 cleft palate-only mothers in T1, 7 mothers in T2 and 13 mothers in T3 In the prudent dietary pattern, 6 cases were present in T1, 12 cases in T2 and 14 cases in T3 Tertiles were calculated by summing of intake food groups weighted by their factor loadings. The factor score for each pattern was calculated by adding up the intakes of the food groups weighted by the factor loadings T1: lowest tertile of the daily pattern scores; T2: middle tertile of the daily pattern scores; T3: highest tertile of the daily pattern scores |
|||
| Western (by tertile) | Western (by tertile) |
T1: ref. T2: OR 1.3 (0.8 to 2.2)a T3: OR 1.9 (1.2 to 3.1)a |
||||||
| T1 (127) | 58/203 | T1 (127) | 69/178 |
T2: OR 1.2 (0.7 to 2.1)b T3: OR 1.7 (1.0 to 3.0)b |
||||
| T2 (127) | 67/203 | T2 (127) | 60/178 |
T2: OR 1.2 (0.8 to 2.1)b T3: OR 1.8 (1.0 to 2.9)b |
||||
| T3 (127) | 78/203 | T3 (127) | 49/178 | |||||
| Prudent (by tertile) | Prudent (by tertile) |
T1: ref. T2: OR 0.9 (0.5 to 1.4)a T3: OR 1.1 (0.7 to 1.8)a |
||||||
| T1 (127) | 68/203 | T1 (127) | 59/178 |
T2: OR 0.8 (0.5 to 1.4)b T3: OR 1.3 (0.8 to 1.8)b |
||||
| T2 (127) | 64/203 | T2 (127) | 63/178 |
T2: OR 0.7 (0.5 to 1.2)b T3: OR 1.0 (0.6 to 1.7)b |
||||
| T3 (127) | 71/203 | T3 (127) | 56/178 |
T1: ref. T2: OR 1.3 (0.8 to 2.2)a T3: OR 1.9 (1.2 to 3.1)a |
||||
| Antisocial personality disorder |
Neugebauer 199980 Cohort study |
76,630 | Diet: famine western Holland |
Adjusted for social class (manual laborers including farmers and non-manual laborers The comparison between the odds of antisocial personality disorder associated with moderate vs severe exposure is statistically significant; the comparison between the moderately exposed and unexposed is not |
||||
| By trimester | Unexposed to famine | 50/45,007 | ||||||
| First, second or third | 26/14,310 |
OR 1.6 (1.02 to 2.6) OR 2.0 (1.2 to 3.3)b |
||||||
| First and/or second | 20/9252 |
OR 2.0 (1.2 to 3.5) OR 2.5 (1.5 to 4.2)b |
||||||
| First only | 6/2443 |
OR 2.2 (0.95 to 5.0) OR 2.9 (1.2 to 6.7)b |
||||||
| First and second only | 6/2223 |
OR 2.4 (1.04 to 5.7) OR 3.0 (1.3 to 7.0)b |
||||||
| Second only | 9/4586 |
OR 1.8 (0.9 to 3.6) OR 2.1 (1.03 to 4.4)b |
||||||
| Third only | 5/5058 |
OR 0.9 (0.4 to 2.2) OR 1.1 (0.4 to 2.7)b |
||||||
| By severity | Unexposed to famine | 50/45,007 | ||||||
| Severely exposed | 26/14,310 |
OR 1.9 (1.02 to 2.6) OR 2.0 (1.2 to 3.3)b |
||||||
| Moderately exposed | 10/9615 |
OR 0.9 (0.6 to 1.9) OR 0.7 (0.3 to 1.6)b |
||||||
| Dyslipidaemia (adult) |
Lumey 200976 Cohort study |
638 | Diet: famine | 96/344 | Unexposed to famine (hospital control subjects) | 85/294 | OR 0.95 (0.61 to 1.34)a |
p = 0.39 Ratio of total cholesterol to high-density lipoprotein cholesterol > 5.0 or use of cholesterol-lowering medication |
| Obesity (adults) |
Ravelli 1976136 Cohort study |
307,700 | Diet: famine (by trimester) | Unexposed to famine (by trimester) | Obesity was defined as a value of weight for height ≥ 120% of the standard | |||
| Third | 51/6200 | Third | 148/11,200 | OR 0.62 (0.45 to 0.85)a | p < 0.005 | |||
| Second and third | 126/7500 | Second and third | 286/17,600 | OR 1.03 (0.84 to 1.28)a | Not significant | |||
| First and second | 119/4300 | First and second | 230/15,900 | OR 1.94 (1.55 to 2.43)a | p < 0.0005 | |||
| First | 41/2500 | First | 162/10,500 | OR 1.06 (0.75 to 1.50)a | Not significant | |||
| IGT or type 2 diabetes mellitus (adults) |
Stanner 1997141 Cohort study |
357 | Diet: famine | Unexposed to famine | Diabetes mellitus and IGT were classified according to World Health Organization criteria | |||
| Known diabetes | 4/169 | Known diabetes | 7/188 | OR 0.63 (0.18 to 2.18)a | ||||
| Newly diagnosed diabetes | 3/169 | Newly diagnosed diabetes | 5/188 | OR 0.66 (0.16 to 2.81)a | ||||
| IGT | 16/169 | IGT | 16/188 | OR 1.12 (0.54 to 2.32)a | ||||
|
Ravelli 1998137 Cohort study |
702 | Diet: famine | Unexposed to famine | General OR 1.19 (0.79 to 1.79)a | ||||
| Exposed in late gestation | 24/116 | Conceived after | 33/221 | |||||
| Exposed in mid-gestation | 14/100 | Born before | 30/202 | |||||
| Exposed in early gestation | 10/63 | |||||||
| Artificial rupture of membranes |
Clapp 199064 Cohort study |
131 | Physical activity: exercise regularly at or above 50% of preconceptional level throughout pregnancy | 20/87 | Discontinued regular exercise regimen before the end of the first trimester | 22/44 | OR 0.33 (0.14 to 0.65)a | p = 0.01 |
| Stimulation for abnormal labour pattern |
Clapp 199064 Cohort study |
131 | Physical activity: exercise regularly at or above 50% of preconceptional level throughout pregnancy | 11/87 | Discontinued regular exercise regimen before the end of the first trimester | 9/44 | OR 0.56 (0.21 to 1.48)a | |
| Meconium in fluid |
Clapp 199064 Cohort study |
131 | Physical activity: exercise regularly at or above 50% of preconceptional level throughout pregnancy | 12/87 | Discontinued regular exercise regimen before the end of the first trimester | 11/44 | OR 0.48 (0.19 to 1.20)a | p = 0.01 |
| Abnormal heart rate |
Clapp 199064 Cohort study |
131 | Physical activity: exercise regularly at or above 50% of preconceptional level throughout pregnancy | 12/87 | Discontinued regular exercise regimen before the end of the first trimester | 11/44 | OR 0.48 (0.19 to 1.20)a | |
| Nuchal cord |
Clapp 199064 Cohort study |
131 | Physical activity: exercise regularly at or above 50% of preconceptional level throughout pregnancy | 23/87 | Discontinued regular exercise regimen before the end of the first trimester | 24/44 | OR 0.30 (0.14 to 0.64)a | p = 0.01 |
| Threatened abortion |
Dale 198267 Cohort study |
33 | Physical activity: running | 1/21 | Not active women: not participating in any type of exercise programme | 1/11 | OR 0.50 (0.03 to 8.85)a | |
| Chorioamnionitis secondary to prolonged rupture of membranes |
Dale 198267 Cohort study |
33 | Physical activity: running | 0/21 | Not active women: not participating in any type of exercise programme | 1/11 | OR 0.16 (0.01 to 4.35)a | |
| Failure to progress with oxytocin augmentation |
Dale 198267 Cohort study |
33 | Physical activity: running | 3/21 | Not active women: not participating in any type of exercise programme | 3/11 | OR 0.44 (0.07 to 2.70)a | |
| Anaemia (mother) |
Dale 198267 Cohort study |
33 | Physical activity: running | 3/21 | Not active women: not participating in any type of exercise programme | 2/11 | OR 0.75 (0.11 to 5.30)a | |
| Anaemia |
Magann 200277 Cohort study |
750 | Exercise: different levels | No exercise |
n = 217 12.91 ± 0.87 |
p = 0.442 | ||
| Light |
n = 222 12.78 ± 0.94 |
OR –0.13 (–0.30 to 0.04)a | ||||||
| Moderate |
n = 73 12.98 ± 0.79 |
OR 0.07 (–0.15 to 0.29)a | ||||||
| Heavy |
n = 238 13.0 ± 2.19 |
OR 0.09 (–0.21 to 0.39)a | ||||||
| Asphyxia/meconium staining/fetal distress |
Dale 198267 Cohort study |
33 | Physical activity: running | 0/21 | Not active women: not participating in any type of exercise programme | 4/11 | OR 0.04 (0.00 to 0.81)a | |
| Sepsis |
Dale 198267 Cohort study |
33 | Physical activity: running | 0/21 | Not active women: not participating in any type of exercise programme | 1/11 | OR 0.16 (0.01 to 4.35)a |
Appendix 11 Delphi ranking of maternal and fetal weight management outcomes according to their importance in the management of maternal weight in pregnancy
| Outcomes | First round | Second round | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Maternal outcomes | ||||
| Weight gain in pregnancy | 6 | 3 | 6 | 1.25 |
| Post-partum weight retention | 6 | 2.5 | 6 | 1.25 |
| Interpregnancy weight gain | 7 | 3 | 7 | 1.25 |
| GDMa | 8 | 1 | 8 | 0.25 |
| Pre-eclampsia/pregnancy-induced hypertensiona | 8 | 1.5 | 8 | 2 |
| Post-partum haemorrhage | 7 | 2 | 7 | 0.25 |
| Prolonged labour | 7 | 2 | 6 | 1 |
| Preterm delivery | 7 | 2.5 | 7 | 2 |
| Induction of laboura | 7 | 1.5 | 8 | 1.25 |
| Prelabour rupture of membranes | 6 | 3.5 | 6 | 1.25 |
| Caesarean section | 8 | 1 | 7 | 1 |
| Instrumental delivery | 7 | 1 | 7 | 1.25 |
| Perineal trauma | 7 | 2.5 | 6.5 | 1 |
| Puerperal pyrexia (≥ 38°C) | 6 | 2 | 5 | 1 |
| Miscarriage | 5 | 2 | 6 | 1.5 |
| Need for resuscitation at delivery | 7 | 2 | 7 | 0.25 |
| Antepartum haemorrhage | 6 | 2.5 | 6 | 1 |
| Thromboembolisma | 8 | 2 | 8 | 1.25 |
| Admission to HDU/ITUa | 8 | 2 | 8 | 1 |
| Anaemia | 6 | 4 | 5 | 3 |
| Infections | 6 | 2.5 | 6 | 2 |
| Postnatal infections | 6 | 2.5 | 6 | 2.25 |
| Postnatal depression | 6 | 2 | 6 | 2.25 |
| Anxiety | 5 | 1.5 | 5 | 0.5 |
| Quality of life | 6 | 2 | 6 | 1.25 |
| Physical activity | 6 | 2 | 6 | 0.25 |
| Dietary behaviour | 7 | 3 | 7 | 0.25 |
| Body fat (%) | 6 | 2 | 6 | 2.25 |
| Back painb | 6 | 2 | ||
| Breast feedingb | 5 | 2.25 | ||
| Threatened abortionb | 3.5 | 2 | ||
| Failed instrumental deliveryb | 7 | 2 | ||
| Coronary artery diseaseb | 6 | 3.25 | ||
| Non-infective respiratory distressb | 5.5 | 2.25 | ||
| Fetal outcomes | ||||
| SGAa | 8 | 2 | 8 | 1.25 |
| LGA | 7 | 2 | 7 | 1.25 |
| Skinfold thickness | 6 | 2 | 6 | 1 |
| Fetal fat mass (%) | 6 | 0.5 | 6 | 1.25 |
| Abdominal circumference | 6 | 0.5 | 6 | 1.25 |
| Head circumference | 5 | 1.5 | 5 | 0.25 |
| Ponderal index (g/cm3 × 100) | 6 | 1.5 | 6 | 2 |
| Neonate length/crown–heel length | 5 | 1.5 | 5 | 0.25 |
| Head-to-abdomen ratio | 5 | 2 | 5 | 1 |
| Birthweight-related outcomes, e.g. BMI | 6 | 2 | 6 | 2 |
| Hypoglycaemia | 7 | 1 | 7 | 1 |
| Hyperbilirubinaemia | 6 | 1 | 6 | 2 |
| Intrauterine deatha | 8 | 2 | 8.5 | 1 |
| Respiratory distress syndrome | 7 | 1.5 | 7 | 1 |
| Admission to NICUa | 8 | 1 | 8 | 1 |
| Shoulder dystociaa | 8 | 1 | 8 | 1 |
| One or more perinatal complicationa | 7 | 2 | 8 | 1 |
| Birth traumaa | 8 | 2 | 8 | 0.5 |
| NTD | 6 | 2 | 6 | 2 |
| Cleft lip or palate or both | 6 | 2.5 | 6 | 1.25 |
| Other congenital abnormalities | 7 | 2 | 6.5 | 1.25 |
| Apgar score | 6 | 2 | 6 | 1 |
| CTG abnormalities | 6 | 2 | 5.5 | 1.25 |
| Abnormal cord pH | 7 | 2 | 7 | 2 |
| Long-term neurological sequelae | 8 | 3 | 8 | 2.25 |
| Cord abnormalitiesb | 5 | 2.25 | ||
| Long-term metabolic sequelaeb | 7.5 | 1.25 | ||
Appendix 12 Grading the quality of randomised evidence for the primary and clinically important outcomes for the effectiveness of weight management interventions in pregnancy
| Quality assessment | Summary of findings | Importance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Effect | Quality | ||||||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Dietary and lifestyle interventions | Control | Relative (95% CI) | Absolute | ||
| Gestational weight gain (kg) (better indicated by lower values) | ||||||||||||
| 30 | Randomised trials | Seriousa,b,c | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2309 | 2286 | – | MD 0.94 lower (1.57 to 0.3 lower) |
⊕⊕⊕⊝ Moderate |
Important |
| Birthweight (kg) (better indicated by lower values) | ||||||||||||
| 28 | Randomised trials | Seriousa,b,d | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2302 | 2271 | – | MD 0.07 lower (0.14 to 0.01 lower) |
⊕⊕⊕⊝ Moderate |
Important |
| LGA | ||||||||||||
| 12 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | None | 155/1527 (10.2%) | 234/1494 (15.7%) | RR 0.73 (0.54 to 0.99) | 42 fewer per 1000 (from 2 fewer to 72 fewer) |
⊕⊕⊕⊝ Moderate |
Critical |
| SGA | ||||||||||||
| 8 | Randomised trials | No serious limitationsf | No serious inconsistency | No serious indirectness | No serious imprecision | None | 103/1456 (7.1%) | 103/1445 (7.1%) | RR 0.99 (0.76 to 1.29) | 1 fewer per 1000 (from 17 fewer to 21 more) |
⊕⊕⊕⊕ High |
Critical |
| Pre-eclampsia | ||||||||||||
| 10 | Randomised trials | No serious limitationsf | Seriousg | Serioush | No serious imprecision | None | 115/1543 (7.5%) | 157/1529 (10.3%) | RR 0.74 (0.59 to 0.92) | 27 fewer per 1000 (from 8 fewer to 42 fewer) |
⊕⊕⊝⊝ Low |
Critical |
| Gestational hypertension | ||||||||||||
| 6 | Randomised trials | No serious limitations | Seriousi | No serious indirectness | Serious | None | 41/403 (10.2%) | 52/388 (13.4%) | RR 0.77 (0.54 to 1.1) | 31 fewer per 1000 (from 62 fewer to 13 more) |
⊕⊕⊝⊝ Low |
Critical |
| GDM | ||||||||||||
| 5 | Randomised trials | No serious limitationsf | No serious inconsistency | No serious indirectness | Seriousj | Reporting biask | 27/344 (7.8%) | 37/331 (11.2%) | RR 0.71 (0.44 to 1.13) | 32 fewer per 1000 (from 63 fewer to 15 more) |
⊕⊕⊝⊝ Low |
Critical |
| Preterm birth | ||||||||||||
| 11 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | None | 70/1115 (6.3%) | 89/1083 (8.2%) | RR 0.76 (0.56 to 1.02) | 20 fewer per 1000 (from 36 fewer to 2 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Caesarean section | ||||||||||||
| 14 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | Reporting biask | 505/1666 (30.3%) | 538/1646 (32.7%) | RR 0.93 (85 to 1.03) | 23 fewer per 1000 (from 49 fewer to 10 more) |
⊕⊕⊝⊝ Low |
Critical |
| Induction of labour | ||||||||||||
| 5 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | None | 394/1187 (33.2%) | 347/1175 (29.5%) | RR 1.12 (1 to 1.26) | 35 more per 1000 (from 0 more to 77 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Post-partum haemorrhage | ||||||||||||
| 2 | Randomised trials | No serious limitations | No serious inconsistency | Seriouse | No serious imprecision | Reporting biasl | 32/606 (5.3%) | 37/626 (5.9%) | RR 0.80 (0.57 to 1.42) | 6 fewer per 1000 (from 25 fewer to 25 more) |
⊕⊕⊝⊝ Low |
Critical |
| Intrauterine death | ||||||||||||
| 2 | Randomised trials | No serious limitationsf | No serious inconsistency | No serious indirectness | Serious | None | 0/647 (0%) | 6/673 (0.9%) | RR 0.15 (0.02 to 1.2) | 8 fewer per 1000 (from 9 fewer to 2 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Admission to NICU | ||||||||||||
| 2m | Randomised trials | No serious limitationsa,b | Seriousg | Very seriouse | Seriousj | None | 400/983 (40.7%) | 374/979 (38.2%) | RR 0.98 (0.66 to 1.47) | 8 fewer per 1000 (from 130 fewer to 180 more) |
⊕⊝⊝⊝ Very low |
Critical |
| Shoulder dystocia | ||||||||||||
| 4 | Randomised trials | No serious limitations | No serious inconsistency | Seriouse | No serious imprecision | None | 15/1173 (1.3%) | 38/1144 (3.3%) | RR 0.39 (0.22 to 0.7) | 20 fewer per 1000 (from 10 fewer to 26 fewer) |
⊕⊕⊕⊝ Moderate |
Critical |
| Birth trauma | ||||||||||||
| 2m | Randomised trials | No serious limitationsf | No serious inconsistency | Seriouse | Serious | None | 3/982 (0.3%) | 9/979 (0.9%) | RR 0.36 (0.11 to 1.23) | 6 fewer per 1000 (from 8 fewer to 2 more) |
⊕⊕⊝⊝ Low |
Critical |
| Neonatal hypoglycaemia | ||||||||||||
| 5 | Randomised trials | No serious limitations | No serious inconsistency | Seriouse | Seriousj | Reporting biask | 124/1087 (11.4%) | 109/1059 (10.3%) | RR 1.07 (0.85 to 1.35) | 7 more per 1000 (from 36 fewer to 36 fewer) |
⊕⊝⊝⊝ Very low |
Critical |
Appendix 13 Grading the quality of evidence for the primary and clinically important outcomes for the effectiveness of dietary interventions in pregnancy
| Quality assessment | Summary of findings | Importance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Effect | Quality | ||||||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Diet and nutrition counselling | Control | Relative (95% CI) | Absolute | ||
| Gestational weight gain (kg) (better indicated by lower values) | ||||||||||||
| 9 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Serious | No serious imprecision | None | 1221 | 1215 | – | MD 3.36 lower (4.73 to 1.99 lower) |
⊕⊕⊕⊝ Moderate |
Important |
| Birthweight (kg) (better indicated by lower values) | ||||||||||||
| 9 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriousc | Seriousd | Reporting biase | 1365 | 1372 | – | MD 0.07 lower (0.21 lower to 0.07 higher) |
⊕⊝⊝⊝ Very low |
Important |
| LGA | ||||||||||||
| 5 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriousc | No serious imprecision | Reporting biase | 134/1196 (11.2%) | 203/1182 (17.2%) | RR 0.78 (0.51 to 1.19) | 38 fewer per 1000 (from 84 fewer to 33 more) |
⊕⊕⊝⊝ Low |
Critical |
| SGA | ||||||||||||
| 3 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriousc | No serious imprecisiond | None | 80/1124 (7.1%) | 79/1128 (7%) | RR 1.02 (0.75 to 1.37) | 1 more per 1000 (from 18 fewer to 26 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Pre-eclampsia | ||||||||||||
| 6 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriousc | No serious imprecision | None | 99/1309 (7.6%) | 150/1315 (11.4%) | RR 0.67 (0.53 to 0.85) | 38 fewer per 1000 (from 17 fewer to 54 fewer) |
⊕⊕⊕⊝ Moderate |
Critical |
| Gestational hypertension | ||||||||||||
| 2 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | No serious imprecision | None | 4/139 (2.9%) | 14/143 (9.8%) | RR 0.3 (0.1 to 0.88) | 69 fewer per 1000 (from 12 fewer to 88 fewer) |
⊕⊕⊕⊕ High |
Critical |
| GDM | ||||||||||||
| 2 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | Seriousf | Reporting biasg | 11/139 (7.9%) | 22/146 (15.1%) | RR 0.52 (0.27 to 1.03) | 72 fewer per 1000 (from 110 fewer to 5 more) |
⊕⊕⊝⊝ Low |
Critical |
| Preterm delivery | ||||||||||||
| 4 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriousc | No serious imprecision | Reporting biasg | 49/744 (6.6%) | 70/730 (9.6%) | RR 0.68 (0.48 to 96) | 31 fewer per 1000 (from 50 fewer to 9110 more) |
⊕⊕⊝⊝ Low |
Critical |
| Caesarean section | ||||||||||||
| 5 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriousc | No serious imprecision | Nonee | 387/1135 (34.1%) | 416/1138 (36.6%) | RR 0.93 (0.84 to 1.04) | 26 fewer per 1000 (from 58 fewer to 15 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Induction of labour | ||||||||||||
| 4 | Randomised trials | No serious limitationsa,b | No serious inconsistencyh | Seriousc | No serious imprecision | Reporting biasg | 370/1145 (32.3%) | 326/1132 (28.8%) | RR 1.12 (0.99 to 1.27) | 35 more per 1000 (from 3 fewer to 78 more) |
⊕⊕⊝⊝ Low |
Critical |
| Post-partum haemorrhage | ||||||||||||
| 2 | Randomised trials | No serious limitations | No serious inconsistency | Seriousc | No serious imprecision | Reporting biasi | 32/606 (5.3%) | 37/626 (5.9%) | RR 0.80 (0.57 to 1.42) | 6 fewer per 1000 (from 26 fewer to 25 more) |
⊕⊕⊝⊝ Low |
Critical |
| Intrauterine death | ||||||||||||
| 2 | Randomised trials | No serious limitationsa | No serious inconsistency | Serious | Seriousd | None | 0/647 (0%) | 6/673 (0.9%) | RR 0.15 (0.02 to 1.2) | 8 fewer per 1000 (from 9 fewer to 2 more) |
⊕⊕⊝⊝ Low |
Critical |
| Admission to NICU | ||||||||||||
| 2 | Randomised trials | No serious limitationsa,b | Seriousj | Seriousc | Seriousd | None | 400/983 (40.7%) | 374/979 (38.2%) | RR 0.98 (0.66 to 1.47) | 8 fewer per 1000 (from 130 fewer to 180 more) |
⊕⊝⊝⊝ Very low |
Critical |
| Shoulder dystocia | ||||||||||||
| 3 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Serious | No serious imprecision | None | 14/1049 (1.3%) | 37/1033 (3.6%) | RR 0.38 (0.21 to 0.69) | 22 fewer per 1000 (from 11 fewer to 28 fewer) |
⊕⊕⊕⊝ Moderate |
Critical |
| Birth trauma | ||||||||||||
| 2 | Randomised trials | No serious limitationsa | No serious inconsistency | Serious | Seriousd | None | 3/982 (0.3%) | 9/979 (0.9%) | RR 0.36 (0.11 to 1.23) | 6 fewer per 1000 (from 8 fewer to 2 more) |
⊕⊕⊝⊝ Low |
Critical |
| Neonatal hypoglycaemia | ||||||||||||
| 3 | Randomised trials | No serious limitations | No serious inconsistency | Seriousc | No serious imprecision | Reporting biasi | 119/946 (12.6%) | 107/931 (11.5%) | RR 1.05 (0.83 to 1.33) | 12 more per 1000 (from 38 more to 20 fewer) |
⊕⊕⊝⊝ Low |
Critical |
Appendix 14 Grading the quality of evidence for the primary and clinically important outcomes for the effectiveness of physical activity interventions in pregnancy
| Quality assessment | Summary of findings | Importance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Effect | Quality | ||||||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Physical activity and counselling about physical activity | Control | Relative (95% CI) | Absolute | ||
| Gestational weight gain (kg) (better indicated by lower values) | ||||||||||||
| 15 | Randomised trials | Seriousa,b,c | No serious inconsistency | No serious indirectness | No serious imprecision | Reporting biasd | 582 | 583 | – | MD 0.07 lower (1.08 lower to 0.93 higher) |
⊕⊕⊝⊝ Low |
Important |
| Birthweight (kg) (better indicated by lower values) | ||||||||||||
| 15 | Randomised trials | Very seriousa,b,e | No serious inconsistency | No serious indirectness | Seriousf | None | 673 | 654 | – | MD 0.09 lower (0.18 lower to 0 higher) |
⊕⊝⊝⊝ Very low |
Important |
| LGA | ||||||||||||
| 2 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | Very seriousf | None | 3/94 (3.2%) | 9/89 (10.1%) | RR 0.37 (0.06 to 2.3) | 64 fewer per 1000 (from 95 fewer to 131 more) |
⊕⊕⊝⊝ Low |
Critical |
| SGA | ||||||||||||
| 3 | Randomised trials | Seriousa,b,g,h | No serious inconsistency | No serious indirectness | No serious imprecisionf | None | 9/151 (6.0%) | 7/153 (4.6%) | RR 1.31 (0.5 to 3.42) | 14 more per 1000 (from 23 fewer to 111 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Pre-eclampsia | ||||||||||||
| 1 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | Seriousi | None | 6/41 (14.6%) | 1/38 (2.6%) | RR 5.56 (0.7 to 44.09) | 120 more per 1000 (from 8 fewer to 1134 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Gestational hypertension | ||||||||||||
| 1 | Randomised trials | No serious limitationsa,b,g | No serious inconsistency | No serious indirectness | Seriousf | None | 9/41 (22.0%) | 15/38 (39.5%) | RR 0.56 (0.28 to 1.12) | 174 fewer per 1000 (from 284 fewer to 47 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| GDM | ||||||||||||
| 1 | Observational studiesj | No serious limitations | No serious inconsistency | No serious indirectness | Very seriousf | None | 23/615 (3.7%) | 19/294 (6.5%) | OR 0.58 (0.32 to 1.06) | 26 fewer per 1000 (from 43 fewer to 4 more) |
⊕⊝⊝⊝ Very low |
Critical |
| Preterm delivery | ||||||||||||
| 4 | Randomised trials | a,b | No serious inconsistency | No serious indirectness | Seriousf | None | 9/173 (5.2%) | 8/172 (4.7%) | RR 1.12 (0.44 to 2.85) | 6 more per 1000 (from 26 fewer to 86 more) | Critical | |
| Caesarean section | ||||||||||||
| 4 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | f | None | 52/234 (22.2%) | 60/241 (24.9%) | RR 0.92 (0.68 to 1.24) | 20 fewer per 1000 (from 80 fewer to 60 more) | Critical | |
| Induction of labour: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | 0 fewer per 1000 (from 0 fewer to 0 fewer) | Critical | |
| Post-partum haemorrhage: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
| Intrauterine death | ||||||||||||
| 1 | Observational studiesk | No serious limitationsl | No serious inconsistency | No serious indirectness | Seriousf | None | 2/169 (1.2%) | 3/166 (1.8%) | RR 0.65 (0.11 to 3.68) | 6 fewer per 1000 (from 16 fewer to 48 more) |
⊕⊝⊝⊝ Very low |
Critical |
| Admission to NICU: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
| Shoulder dystocia: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
| Birth trauma: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
| Neonatal hypoglycaemia: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
Appendix 15 Grading the quality of evidence for the primary and clinically important outcomes for the effectiveness of mixed approach interventions in pregnancy
| Quality assessment | Summary of findings | Importance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Effect | Quality | ||||||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Mixed approach | Control | Relative (95% CI) | Absolute | ||
| Gestational weight gain (kg) (better indicated by lower values) | ||||||||||||
| 6 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | Seriousc | Reporting biasd | 506 | 488 | – | MD 0.36 lower (1.4 lower to 0.68 higher) |
⊕⊕⊝⊝ Low |
Important |
| Birthweight (kg) (better indicated by lower values) | ||||||||||||
| 5 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | Seriousc | Reporting biasd | 264 | 245 | – | MD 0.02 lower (0.1 lower to 0.07 higher) |
⊕⊕⊝⊝ Low |
Important |
| LGA | ||||||||||||
| 5 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | None | 18/237 (7.6%) | 22/223 (9.9%) | RR 0.75 (0.41 to 1.38) | 25 fewer per 1000 (from 58 fewer to 37 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| SGA | ||||||||||||
| 2 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | No serious imprecision | None | 14/181 (7.7%) | 17/164 (10.4%) | RR 0.76 (0.39 to 1.48) | 25 fewer per 1000 (from 63 fewer to 50 more) |
⊕⊕⊕⊕ High |
Critical |
| Pre-eclampisa | ||||||||||||
| 3 | Randomised trials | No serious limitationsa,b | Seriousf | No serious indirectness | Seriousc | None | 10/193 (5.2%) | 6/176 (3.4%) | RR 1.48 (0.56 to 3.94) | 16 more per 1000 (from 15 fewer to 100 more) |
⊕⊕⊝⊝ Low |
Critical |
| Gestational hypertension | ||||||||||||
| 3 | Randomised trials | No serious limitationsa,b | Seriousf | No serious indirectness | Seriousc | None | 28/223 (12.6%) | 23/207 (11.1%) | RR 1.19 (0.74 to 1.9) | 21 more per 1000 (from 29 fewer to 100 more) |
⊕⊕⊝⊝ Low |
Critical |
| GDM | ||||||||||||
| 3 | Randomised trials | No serious limitationsa,b | No serious indirectness | Seriousc | None | 16/205 (7.8%) | 15/185 (8.1%) | RR 0.96 (0.49 to 1.86) | 3 fewer per 1000 (from 41 fewer to 70 more) |
⊕⊕⊕⊝ Moderate |
Critical | |
| Preterm delivery | ||||||||||||
| 3 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | Noned | 12/198 (6.1%) | 11/181 (6.1%) | RR 1.02 (0.47 to 2.21) | 1 more per 1000 (from 32 fewer to 74 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Caesarean section | ||||||||||||
| 5 | Randomised trials | No serious limitationsa,b | No serious inconsistency | Seriouse | No serious imprecision | Reporting biasg | 66/297 (22.2%) | 62/267 (23.2%) | RR 0.95 (0.7 to 1.28) | 12 fewer per 1000 (from 70 fewer to 65 more) |
⊕⊕⊝⊝ Low |
Critical |
| Induction of labour | ||||||||||||
| 1 | Randomised trials | No serious limitations | No serious inconsistencyh | No serious indirectnessi | Seriousc | None | 24/42 (57.1%) | 21/43 (48.8%) | RR 1.17 (0.78 to 1.75) | 83 more per 1000 (from 107 fewer to 366 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Post-partum haemorrhage: not reported | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
| Intrauterine death: not reported | ||||||||||||
| 1k | Observational studies | Very serious1 | No serious inconsistency | Seriouse | No serious impression | None | 3/88 (3.4%) | 3/86 (3.5%) | OR 098 (0.19 to 2.56) | 1 fewer per 1000 (from 28 fewer to 50 more) |
⊕⊝⊝⊝ Very low |
Critical |
| Admission to NICU: not reported | ||||||||||||
| 1k | Observational studies | Very serious1 | No serious inconsistency | Seriouse | No serious impression | None | 21/88 (23.9%) | 42/86 (48.8%) | OR 0.33 (0.17 to 0.63) | 249 fewer per 1000 (from 113 fewer to 349 fewer) |
⊕⊝⊝⊝ Very low |
Critical |
| Shoulder dystocia | ||||||||||||
| 1 | Randomised trials | No serious limitationsa,b | No serious inconsistency | No serious indirectness | Seriousc | None | 1/124 (0.8%) | 1/111 (0.9%) | RR 0.9 (0.06 to 14.14) | 1 fewer per 1000 (from 8 fewer to 118 more) |
⊕⊕⊕⊝ Moderate |
Critical |
| Birth trauma: not measured | ||||||||||||
| 0 | – | – | – | – | – | None | 0/0 (0%) | 0/0 (0%) | – | – | Critical | |
| Neonatal hypoglycaemia | ||||||||||||
| 2 | Randomised trials | No serious limitations | No serious inconsistency | No serious indirectness | Seriousc | Reporting biasj | 5/141 (3.5%) | 2/128 (1.6%) | RR 2.35 (0.47 to 11.76) | 19 more per 1000 (from 172 more to 9 fewer) |
⊕⊕⊝⊝ Low |
Critical |
Appendix 16 Grading the quality of evidence for the adverse outcomes of diet in pregnancy
| Quality assessment | Summary of findings | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Effect | Quality | |||||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Diet | Control | OR (95% CI) | ||
| NTD | |||||||||||
| 2 | Observational studies | Very seriousa | No serious inconsistency | Seriousb | Serious |
Reporting biasb Strong associationc |
0/0 (0%) | 0/0 (0%) | Not pooled |
⊕⊝⊝⊝ Very low |
Important |
| Coronary heart disease: long term in children as adults | |||||||||||
| 1 | Observational studies | Very seriousd | No serious inconsistency | Seriouse | No serious imprecision | Strong associationc | 6/68 (8.8%)f | 14/440 (3.2%) | OR 3 (1.1 to 8) |
⊕⊝⊝⊝ Very low |
N/A |
| Metabolic syndrome: long term in children as adults | |||||||||||
| 1 | Observational studies | Very seriousd | No serious inconsistency | Seriouse | No serious imprecision | None | 26/14,310 (0.2%)g | 50/45,007 (0.1%) | OR 1.2 (0.9 to 1.7) |
⊕⊝⊝⊝ Very low |
Critical |
| Hypertension: long term in children as adults | |||||||||||
| 1 | Observational studies | Very serioush | No serious inconsistency | Seriouse | No serious imprecision | None | 224/344 (65.1%) | 168/294 (57.1%) | OR 1.4 (1.02 to 1.93) |
⊕⊝⊝⊝ Very low |
N/A |
| Cleft lip or palate or both: child | |||||||||||
| 1 | Observational studies | Very seriousd | No serious inconsistency | No serious indirectness | No serious imprecision | None | 0/0 (0%) | 0/0 (0%) | Not estimable |
⊕⊝⊝⊝ Very low |
Important |
| Antisocial personality disorder: long term in children as adults | |||||||||||
| 1 | Observational studies | Very seriousd | No serious inconsistency | Seriouse | No serious imprecision | Nonec | 26/14,310 (0.2%)g | 50/45,007 (0.1%) | OR 2.0 (1.2 to 3.3) |
⊕⊝⊝⊝ Very low |
Critical |
| Dyslipidaemia: long term in children as adults | |||||||||||
| 1 | Observational studies | Very serioush | No serious inconsistency | Seriouse | Very seriousi | None | 96/344 (27.9%) | 85/294 (28.9%) | OR 0.95 (0.61 to 1.34) |
⊕⊝⊝⊝ Very low |
N/A |
| Obesity: long term in children as adults | |||||||||||
| 1 | Observational studies | Very seriousj | No serious inconsistency | Seriouse | No serious imprecision | None | 51/6200 (0.8%)k | 148/11,200 (1.3%)k | OR 0.62 (0.45 to 0.85) |
⊕⊝⊝⊝ Very low |
Critical |
| Adult obesity: long-term outcome in children | |||||||||||
| 1 | Observational studies | Very seriousj | No serious inconsistency | Seriouse | No serious imprecision | None | 119/4300 (2.8%)l | 230/15,900 (1.4%)l | OR 1.94 (1.55 to 2.43) |
⊕⊝⊝⊝ Very low |
Critical |
| IGT or type 2 diabetes mellitus: long term in children as adults | |||||||||||
| 1 | Observational studies | Very seriousd | No serious inconsistency | Seriouse | No serious imprecision | None | 0/0 (0%) | 0/0 (0%) | Not estimable |
⊕⊝⊝⊝ Very low |
Critical |
Appendix 17 Grading the quality of evidence for the adverse outcomes of physical activity in pregnancy
| Quality assessment | Summary of findings | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Effect | Quality | |||||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Physical activity | Control | OR/RR (95% CI) | ||
| Cord abnormalities | |||||||||||
| 3 | Observational studies | Very seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 9/238 (3.8%)b | 18/217 (8.3%) | OR 0.43 (0.19 to 0.99) |
⊕⊝⊝⊝ Very low |
Important |
| Stimulation for abnormal labour pattern | |||||||||||
| 1 | Observational studies | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousc | None | 11/87 (12.6%) | 9/44 (20.5%) | RR 0.56 (0.21 to 1.48) |
⊕⊝⊝⊝ Very low |
N/A |
| Meconium-stained liquor | |||||||||||
| 1 | Randomised trials | No serious limitationsd,e,f | No serious inconsistency | No serious indirectness | Very seriousc | None | 4/38 (10.5%) | 8/47 (17.0%) | RR 0.62 (0.2 to 1.9) |
⊕⊕⊝⊝ Low |
N/A |
| Abnormal fetal heart rate | |||||||||||
| 1 | Observational studies | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousc | None | 12/87 (13.8%) | 11/44 (25.0%) | OR 0.48 (0.19 to 1.2) |
⊕⊝⊝⊝ Very low |
N/A |
| Nuchal cord | |||||||||||
| 1 | Observational studies | Very seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 23/87 (26.4%) | 24/44 (54.5%) | OR 0.3 (0.14 to 0.64) |
⊕⊝⊝⊝ Very low |
N/A |
| Threatened abortion | |||||||||||
| 1 | Observational studies | Very seriousg | No serious inconsistency | No serious indirectness | Very seriousc | None | 1/21 (4.8%) | 1/11 (9.1%) | OR 0.5 (0.03 to 8.85) |
⊕⊝⊝⊝ Very low |
Important |
| Failure to progress with oxytocin augmentation: mother | |||||||||||
| 1 | Observational studies | Very seriousg | No serious inconsistency | No serious indirectness | Very seriousc | None | 3/21 (14.3%) | 3/11 (27.3%) | OR 0.44 (0.07 to 2.7) |
⊕⊝⊝⊝ Very low |
N/A |
| Chorioamnionitis | |||||||||||
| 1 | Randomised trials | No serious limitations | No serious inconsistency | No serious indirectness | Serious imprecision | None | 1/38 (2.6%) | 0/47 (0%) | OR 3.69 (0.15 to 88.13) |
⊕⊕⊕⊕ Low |
Important |
| Maternal anaemia | |||||||||||
| 1 | Observational studies | Very seriousg | No serious inconsistency | No serious indirectness | Very seriousc | None | 3/21 (14.3%) | 2/11 (18.2%) | OR 0.75 (0.11 to 5.3) |
⊕⊝⊝⊝ Very low |
Important |
| Maternal sepsis | |||||||||||
| 1 | Observational studies | Very seriousg | No serious inconsistency | No serious indirectness | Very seriousc | None | 0/21 (0%) | 1/11 (9.1%) | OR 0.16 (0.01 to 4.35) |
⊕⊝⊝⊝ Very low |
N/A |
| Uterine atony | |||||||||||
| 1 | Randomised trials | No serious limitationsd,e,f | No serious inconsistency | No serious indirectness | Serious imprecision | None | 3/38 (7.9%) | 4/47 (8.5%) | RR 0.93 (0.22 to 3.89) |
⊕⊕⊕⊕ Low |
N/A |
Appendix 18 Data extraction form for effectiveness of interventions for weight management in pregnancy
Part I: General
Date (dd/mm/yy )
Reviewer ID Study ID
| Study title | ||
| First author | ||
| Publication year | ||
|
Source of publication Journal yy;vol.(issue):pp |
||
| Language | ||
| Publication type | □ Journal Abstract | □ Other (specify): |
| If included study is a comparative experimental study (randomised or non-randomised controlled trial), then go to point A in Part II |
| If included study is a comparative observational study (case–control, cohort), then go to point B in Part II |
Part II
A) Comparative experimental studies
1. Study characteristics
| Methods/methodological quality | ||
|---|---|---|
| Study design | □ RCT □ NRS | |
| RCT | ||
| Method of randomisation |
Specify and assess the method: ....................................................... □ Adequate □ Inadequate □ Unclear □ Not reported |
|
| Allocation concealment |
□ Adequate □ Inadequate □ Unclear □ Not reported Describe........................................ |
|
| Blinding | Select blinded subjects: | |
|
□ Patients □ Investigators/clinicians □ Outcome assessors □ No blinding used |
||
|
Assess the method: □ Adequate □ Inadequate □ Unclear □ Not reported |
||
| Information about drop-outs |
□ Precise information (number of patients and reasons) □ Inaccurate information □ Lack of information |
|
| Statistical technique used | ||
|
Intention-to-treat analysis What was the definition of ITT in the study? |
□ Implemented □ Not implemented ............................................................................... ............................................................................... |
|
| Sample size calculation | ||
| Was sensitivity analysis performed? | □ Yes □ No □ Not applicable | |
| How problem with missing data was resolved? | ||
| Were missing data accounted for in the analyses? | □ Yes □ No | |
| Post hoc analysis | ||
| Funding source | ||
| NRS | ||
| Control group selection |
Specify and assess the method: ........................................................................................................ □ Adequate □ Inadequate □ Unclear □ Not reported |
|
| Allocation concealment |
□ Adequate □ Inadequate □ Unclear □ Not reported Describe......................................................................................... |
|
| Blinding | Select blinded subjects: | |
|
□ Patients □ Investigators/clinicians □ Outcome assessors □ No blinding used |
||
| Assess the method: | ||
| □ Adequate □ Inadequate □ Unclear □ Not reported | ||
| Information about drop-outs |
□ Precise information (number of patients and reasons) □ Inaccurate information □ Lack of information |
|
| Statistical technique used | ||
|
Intention-to-treat analysis What was the definition of ITT in the study? |
□ Implemented □ Not implemented ............................................................................... ............................................................................... |
|
| Sample size calculation | ||
| Was sensitivity analysis performed? | □ Yes □ No □ Not applicable | |
| How problem with missing data was resolved? | ||
| Were missing data accounted for in the analyses? | □ Yes □ No | |
| Post hoc analysis | ||
| Funding source | ||
| Population | ||
| Trial inclusion criteria |
■ ■ ■ ■ |
|
| Trial exclusion criteria |
■ ■ ■ ■ |
|
| Intervention group | Control group | |
| Number of enrolled patients | ||
|
Number of patients randomised, NR (RCT) Number of patients included, N(NRS) |
||
| Number of patients who completed treatment, n (%) | ||
| Number of patients available for follow-up, n (%) | ||
|
Age in years Specify the measure: ............................................................. |
||
| Ethnicity, n (%) | ||
| BMI at baseline (mean, SD) | ||
| ■ Normal (25–29.9 kg/m2) | □ Normal ................ | □ Normal .................... |
| ■ Overweight (30–34.9 kg/m2) | □ Overweight .......... | □ Overweight ............... |
| ■ Obese (≥ 35 kg/m2) | □ Obese ................... | □ Obese ....................... |
| Weight at baseline (mean, SD) | ||
| Singleton pregnancy only (if no give percentage) | Yes/no/unclear (......) | Yes/no/unclear (......) |
| Primiparas only (if no give percentage) | Yes/no/unclear (......) | Yes/no/unclear (......) |
| Gestational age (week; SD; SE) | ||
| Other baseline characteristics | ||
| Are the treatment groups comparable at baseline? |
□ Yes □ No If ‘no’ please specify the reasons: ................................................................................... .................................................................................. .................................................................................. |
|
| Intervention | ||
| Type and specifics of intervention(s) used (diet, physical activity, behavioural change, lifestyle) | ||
| How was intervention delivered | ||
| Intervention duration | ||
| Intervention provider(s) | ||
| Duration of follow-up | ||
| Comparator | ||
| Comparator |
□ No intervention □ Other intervention (specify) .................... |
|
| Outcomes | ||
| Maternal outcomes related with (more than one possible) |
□ Safety *Outcome assessment.................. □ Delivery *Outcome assessment.................. □ Pregnancy-related diseases *Outcome assessment.................. □ Mental state *Outcome assessment................. □ Weight change *Outcome assessment................. □ Others *Outcome assessment………… |
|
| Fetal outcomes related with (more than one possible) |
□ Safety *Outcome assessment................... □ Others *Outcome assessment................... |
|
| Childhood and adult outcomes in offspring (more than one possible) |
□ Childhood obesity *Outcome assessment.................... □ Adult obesity *Outcome assessment.................... □ Diabetes mellitus *Outcome assessment.................... □ Coronary heart disease *Outcome assessment................... □ Hypertension *Outcome assessment................... □ Stroke *Outcome assessment.................. □ Depression *Outcome assessment................... □ Death *Outcome assessment.................. □ Other (specify) *Outcome assessment.................... □ Not stated in study |
|
*Outcome assessment:
-
Self-reported
-
Hospital records
-
Trained assessor
-
Other
-
Blinded
-
Unblinded
2. Results
Dichotomous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | n (%) | N’ | n (%) |
|---|---|---|---|
|
Effect estimate □ RR □ OR (95% CI □ SE □ p) |
|||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
|
Assess the method: □ Adequate □ Inadequate □ Unclear □ Not reported |
|||
| Incomplete outcome data addressed | |||
Time-to-event data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | Median | N’ | Median |
|
Effect estimate □ RR □ OR (95% CI □ SE □ p) |
|||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Continuous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||||||
|
Intervention group NR/N = |
Control group NR/N = |
||||||
| N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
| Blinding | Select blinded subjects: | ||||||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||||||
| Assess the method: | |||||||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||||||
| Incomplete outcome data addressed | |||||||
Reviewers’ comments
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
B) Comparative observational studies
1. Study characteristics
| Methods/methodological quality | ||
|---|---|---|
| Study design | □ Case–control □ Cohort | |
| Case–control | ||
| Is case definition adequate? | □ Independent validation Record linkage Self-reported None | |
| Are the cases representative? |
□ All cases arising from same population or group □ Not known |
|
| Selection of controls | □ Same population as cases Not known or no | |
| Definition of controls |
□ Outcome of interest not present in history □ No mention of history of outcome |
|
| Comparability of cases and controls | □ Yes No Unclear | |
| Ascertainment of exposure to intervention |
□ Secure record □ Structured interview where blind to case/control status □ Interview not blinded to case/control status □ Written self-report of medical record only □ No description |
|
| Was the method of ascertainment of exposure for cases and controls the same? | □ Yes No Unclear | |
| Non-response rate |
□ Same for both groups □ Non-respondents described □ Rate different and no designation |
|
| Cohort | ||
| Is the cohort representative | □ Yes No Unclear | |
| Selection of non-exposed cohort | □ Same population as exposed cohort not known or no | |
| Ascertainment of exposure |
□ Secure record □ Structured interview □ Written self-report □ No description |
|
| Demonstration that outcome of interest was not present at start of study? | □ Yes No Unclear | |
| Comparability of cohorts on the basis of the design or analysis | □ Yes No Unclear | |
| Assessment of outcome | □ Independent or blind assessment Record linkage Self-report No description | |
| Was follow-up long enough for outcomes to occur? |
□ Yes No Unclear If ‘yes’, specify........................................................ |
|
| Was follow-up of cohorts adequate? |
□ Complete follow-up □ Subjects lost to follow-up unlikely to introduce bias, small number lost (.....%) □ Follow-up rate ....%, and no description of this lost □ No statement |
|
| Were the objectives or the hypothesis of the study stated? | □ Yes No Unclear | |
| Method of allocation to groups | ||
| For patients who were not eligible for study, are the reasons why stated? | □ Yes No | |
| Information about drop-outs |
□ Precise information (number of patients and reasons) □ Inaccurate information □ Lack of information |
|
| Statistical technique used | ||
|
Intention-to-treat analysis What was the definition of ITT in the study? |
□ Implemented Not implemented ............................................................................... ............................................................................... |
|
| Sample size calculation | ||
| Was loss to follow-up taken into account in the analysis? | □ Yes □ No | |
| Comparability of groups established | □ Yes □ No | |
| Were any confounders mentioned? | □ Yes, please describe............................................ No | |
| Were confounders accounted for in analyses? | □ Yes □ No | |
| How problem with missing data was resolved? | ||
| Were missing data accounted for in the analyses? | □ Yes □ No | |
| Was the impact of biases assessed? | □ Yes □ No□ Not clearly assessed | |
| Funding source | ||
| Population | ||
| Trial inclusion criteria |
■ ■ ■ ■ |
|
| Trial exclusion criteria |
■ ■ ■ ■ |
|
| Is target population defined? | □ Yes □ No | |
| Intervention group | Control group | |
| Number of eligible patients | ||
| Number of included patients, N | ||
| Number of patients who completed treatment, n (%) | ||
|
Age in years Specify the measure: ............................................................. |
||
| Ethnicity, n (%) | ||
| BMI at baseline (mean, SD) | □ Normal ................ | _Normal .................... |
| Normal (25–29.9 kg/m2) | □ Overweight .......... | _Overweight ............... |
| Overweight (30–34.9 kg/m2) | □ Obese ................... | _Obese ....................... |
| Obese (≥ 35 kg/m2 | ||
| Weight at baseline (mean, SD) | ||
| Singleton pregnancy only (if no give percentage) | Yes/no/unclear (......) | Yes/no/unclear (......) |
| Primiparas only (if no give percentage) | Yes/no/unclear (......) | Yes/no/unclear (......) |
| Gestational age (week; SD; SE) | ||
| Other baseline characteristics | ||
| Are the treatment groups comparable at baseline? |
□ Yes □ No If ‘no’ please specify the reasons: ................................................................................... .................................................................................. .................................................................................. |
|
| Intervention | ||
| Type and specifics of intervention(s) used (diet, physical activity, behavioural change, lifestyle) | ||
| How was intervention delivered | ||
| Intervention duration | ||
| Intervention provider(s) | ||
| Duration of follow-up | ||
| Comparator | ||
| Comparator |
□ No intervention □ Other intervention (specify) .................... |
|
| Outcomes | ||
| Maternal outcomes related with (more than one possible) |
□ Safety *Outcome assessment.................. □ Delivery *Outcome assessment.................. □ Pregnancy-related diseases *Outcome assessment.................. □ Mental state *Outcome assessment................. □ Weight change *Outcome assessment................. □ Others *Outcome assessment............ |
|
| Fetal outcomes related with (more than one possible) |
□ Safety *Outcome assessment................... □ Others *Outcome assessment................... |
|
| Childhood and adult outcomes in offspring (more than one possible) |
□ Childhood obesity *Outcome assessment.................... □ Adult obesity *Outcome assessment.................... □ Diabetes mellitus *Outcome assessment.................... □ Coronary heart disease *Outcome assessment................... □ Hypertension *Outcome assessment................... □ Stroke *Outcome assessment.................. □ Depression *Outcome assessment................... □ Death *Outcome assessment.................. □ Other (specify) *Outcome assessment.................... □ Not stated in study |
|
Outcome assessment:
-
Self-reported
-
Hospital records
-
Trained assessor
-
Other
-
Blinded
-
Unblinded
2. Results
Dichotomous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | n (%) | N’ | n (%) |
|---|---|---|---|
| Effect estimate □ RR □ OR (95% CI □ SE □ p) | |||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Time-to-event data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | Median | N’ | Median |
| Effect estimate □ RR □ OR (95% CI □ SE □ p) | |||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Continuous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||||||
|
Intervention group NR/N = |
Control group NR/N = |
||||||
| N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
| Blinding | Select blinded subjects: | ||||||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||||||
| Assess the method: | |||||||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||||||
| Incomplete outcome data addressed | |||||||
Reviewers’ comments
.......................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
Appendix 19 Data extraction form for adverse effects of weight management interventions in pregnancy
Part I: General
Date (dd/mm/yy )
Reviewer ID Study ID
| Study title | ||
| First author | ||
| Publication year | ||
|
Source of publication Journal yy;vol.(issue):pp |
||
| Language | ||
| Publication type | □ Journal Abstract | □ Other (specify): |
| If included study is a comparative experimental study (randomised or non-randomised controlled trial), then go to point A in Part II |
| If included study is a comparative observational study (case–control or cohort), then go to point B in Part II |
| If included study is a non-comparative study, then go to point C in Part II |
Part II
A) Comparative experimental studies
1. Study characteristics
| Methods/methodological quality | ||
|---|---|---|
| Study design | □ RCT □ NRS | |
| RCT | ||
| Population indirectness | □ Very □ Serious □ Not serious □ Difficult to assess | |
| Was the eligible population representative of the source? Were important groups under-represented? |
Describe ................................................................................................................ ............................................................................................................... ............................................................................................................... |
|
| Method of randomisation |
Specify and assess the method: ....................................................... |
|
| □ Adequate □ Inadequate □ Unclear □ Not reported | ||
| Allocation concealment |
□ Adequate □ Inadequate □ Unclear □ Not reported Describe........................................ |
|
| Blinding | Select blinded subjects: | |
|
□ Patients □ Investigators/clinicians □ Outcome assessors □ No blinding used |
||
| assess the method: | ||
| □ Adequate □ Inadequate □ Unclear □ Not reported | ||
| Information about drop-outs |
□ Precise information (number of patients and reasons) □ Inaccurate information □ Lack of information |
|
| Rate of loss to follow-up | ||
| Patients lost to follow-up analysed for adverse events | ||
| Was the follow-up adequate to ascertain adverse effects? |
□ Yes □ No □ Unclear If ‘yes’, specify.......................................................... |
|
| Statistical technique used | ||
| Was adequate statistical analysis of potential confounders performed? | □ Yes □ No □ Unclear | |
|
Intention-to-treat analysis What was the definition of ITT in the study? |
□ Implemented □ Not implemented ......................................................................................... ..................................................................... |
|
| Sample size calculation | ||
| Was sensitivity analysis performed? | □ Yes □ No □ Not applicable | |
| How problem with missing data was resolved? | ||
| Were missing data accounted for in the analyses? | □ Yes □ No | |
| Post hoc analysis | ||
| Funding source | ||
| NRS | ||
|
Population indirectness Was the eligible population representative of the source? Were important groups under-represented? |
□ Very □ Serious □ Not serious □ Difficult to assess Describe ................................................................................................ .............................................................................................................. .............................................................................................................. |
|
| Control group selection |
Specify and assess the method: ........................................................................................................ □ Adequate □ Inadequate □ Unclear □ Not reported |
|
| Allocation concealment |
□ Adequate □ Inadequate □ Unclear □ Not reported Describe......................................................................................... |
|
| Blinding | Select blinded subjects: | |
|
□ Patients □ Investigators/clinicians □ Outcome assessors □ No blinding used |
||
| Assess the method: | ||
| □ Adequate □ Inadequate □ Unclear □ Not reported | ||
| Information about drop-outs |
□ Precise information (number of patients and reasons) □ Inaccurate information □ Lack of information |
|
| Rate of loss to follow-up | ||
| Patients lost to follow-up analysed for adverse events | ||
| Was the follow-up adequate to ascertain adverse effects? |
□ Yes □ No □ Unclear If ‘yes’, specify.......................................................... |
|
| Statistical technique used | ||
| Was adequate statistical analysis of potential confounders performed? | □ Yes □ No □ Unclear | |
|
Intention-to-treat analysis What was the definition of ITT in the study? |
□ Implemented □ Not implemented ............................................................................... ............................................................................... |
|
| Sample size calculation | ||
| Was sensitivity analysis performed? | □ Yes □ No □ Not applicable | |
| How problem with missing data was resolved? | ||
| Were missing data accounted for in the analyses? | □ Yes □ No | |
| Post hoc analysis | ||
| Funding source | ||
| Population | ||
| Trial inclusion criteria |
■ ■ ■ ■ |
|
| Trial exclusion criteria |
■ ■ ■ ■ |
|
| Intervention group | Control group | |
| Number of enrolled patients | ||
|
Number of patients randomised, NR (RCT) Number of patients included, N (NRS) |
||
| Number of patients who completed treatment, n (%) | ||
| Number of patients available for follow-up, n (%) | ||
|
Age in years Specify the measure: ............................................................. |
||
| Ethnicity, n (%) | ||
| BMI at baseline (mean, SD) | ||
| Normal (18.5–24.9 kg/m2) | □ Normal ................ | □ Normal .................... |
| Overweight (25–29.9 kg/m2) | □ Overweight .......... | □ Overweight ............... |
| Obese (≥ 30 kg/m2) | □ Obese ................... | □ Obese ....................... |
| Weight at baseline (mean, SD) | ||
| Singleton pregnancy only (if no give percentage) | Yes/no/unclear (......) | Yes/no/unclear (......) |
| Primiparas only (if no give percentage) | Yes/no/unclear (......) | Yes/no/unclear (......) |
| Gestational age (week; SD; SE) | ||
| Other baseline characteristics | ||
| Are the treatment groups comparable at baseline? |
□ Yes □ No If ‘no’ please specify the reasons: ................................................................................... .................................................................................. .................................................................................. |
|
| Intervention | ||
| Type of dietary or lifestyle intervention with description | ||
| How was intervention delivered | ||
| Intervention duration | ||
| Intervention provider | ||
| Duration of follow-up | ||
| Comparator | ||
| Comparator |
□ No intervention □ Other intervention (specify) .................... |
|
| Outcomes (harms) | ||
| Definition of outcomes |
□ Any published definition □ No definition |
|
| Adequacy of data source |
□ Reliable □ Non-reliable |
|
| Approach to ascertain the cause of harm |
□ Adequate □ Non-adequate |
|
| Proportion of cases with attributable cause of harm established |
□ ........(%) □ Unclassified |
|
| Adverse effects occurred in |
□ Mother □ Fetus/baby/child □ Both |
|
| Outcomes (adverse effects) related with |
□ Weight change in pregnancy □ Dietary intervention type □ Not clear □ Others (specify)............ |
|
| Maternal outcomes (adverse effects) |
■ *Outcome assessment............ ■ *Outcome assessment............ ■ *Outcome assessment............ |
|
| Child outcomes (adverse effects) |
■ *Outcome assessment............ ■ *Outcome assessment............ ■ *Outcome assessment............ |
|
*Outcome assessment:
-
Self-reported
-
Hospital records
-
Trained assessor
-
Other
-
Blinded
-
Unblinded
2. Results
Dichotomous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | n (%) | N’ | n (%) |
|---|---|---|---|
| Effect estimate □ RR □ OR (95% CI □ SE □ p) | |||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Time-to-event data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | Median | N’ | Median |
| Effect estimate □ RR □ OR (95% CI □ SE □ p) | |||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Continuous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||||||
|
Intervention group NR/N = |
Control group NR/N = |
||||||
| N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
| Blinding | Select blinded subjects: | ||||||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||||||
| Assess the method: | |||||||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||||||
| Incomplete outcome data addressed | |||||||
Reviewers’ comments
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
B) Comparative observational studies
1. Study characteristics
| Methods/methodological quality | ||
|---|---|---|
| Study design | □ Case–control □ Cohort | |
| Case–control | ||
|
Population indirectness Was the eligible population representative of the source? Were important groups under-represented? |
□ Very □ Serious □ Not serious □ Difficult to assess Describe ……………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… |
|
| Is case definition adequate? | □ Independent validation □ Record linkage □ Self-reported □ None | |
| Are the cases representative? | □ All cases arising from same population or group □ Not known | |
| Selection of controls | □ Same population as cases □ Not known or no | |
| Definition of controls |
□ Outcome of interest not present in history □ No mention of history of outcome |
|
| Comparability of cases and controls | □ Yes □ No □ Unclear | |
| Ascertainment of exposure to intervention |
□ Secure record □ Structured interview where blind to case/control status □ Interview not blinded to case/control status □ Written self-report of medical record only □ No description |
|
| Was the method of ascertainment of exposure for cases and controls the same? | □ Yes □ No □ Unclear | |
| Non-response rate |
□ Same for both groups □ Non-respondents described □ Rate different and no designation |
|
| Cohort | ||
|
Population indirectness Was the eligible population representative of the source? Were important groups under-represented? |
□ Very □ Serious □ Not serious □ Difficult to assess Describe ……………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… |
|
| Is the cohort representative | □ Yes □ No □ Unclear | |
| Selection of non-exposed cohort | □ Same population as exposed cohort □ Not known or no | |
| Ascertainment of exposure |
□ Secure record □ Structured interview □ Written self-report □ No description |
|
| Demonstration that outcome of interest wasn’t present at start of study? | □ Yes □ No □ Unclear | |
| Assessment of outcome | Independent or blind assessment Record linkage Self-report No description | |
| Was follow-up long enough for outcomes to occur? |
□ Yes □ No □ Unclear If ‘yes’, specify........................................................ |
|
| Was follow-up of cohorts adequate? |
□ Complete follow-up □ Subjects lost to follow-up unlikely to introduce bias, small number lost (…..%) □ Follow-up rate ….%, and no description of this lost □ No statement |
|
| Are the objectives or the hypothesis of the study stated? | □ Yes □ No □ Unclear | |
| Method of allocation to groups | ||
| For patients who were not eligible for study, are the reasons why stated? | □ Yes □ No | |
| Information about drop-outs |
□ Precise information (number of patients and reasons) □ Inaccurate information □ Lack of information |
|
| Statistical technique used | ||
| Sample size calculation | ||
| Was loss to follow-up taken into account in the analysis? | □ Yes □ No | |
| Were any confounders mentioned? | □ Yes, please describe.......................................... □ No | |
| Were confounders accounted for in analyses? | □ Yes □ No | |
| Were missing data accounted for in the analyses? | □ Yes □ No | |
| Was the impact of biases assessed? | □ Yes □ No □ Not clearly assessed | |
| Funding source | ||
| Population | ||
| Trial inclusion criteria |
■ ■ ■ ■ |
|
| Trial exclusion criteria |
■ ■ ■ ■ |
|
| Is target population defined? | □ Yes □ No | |
| Intervention group | Control group | |
| Number of eligible patients | ||
| Number of included patients, N | ||
| Number of patients who completed treatment, n (%) | ||
|
Age in years Specify the measure: ............................................................. |
||
| Ethnicity, n (%) | ||
| BMI at baseline (mean, SD) | ||
| ■Normal (18.5–24.9 kg/m2) | □ Normal ................ | □ Normal ................ |
| Overweight (25–29.9 kg/m2) | □ Overweight .......... | □ Overweight .......... |
| Obese (≥ 30 kg/m2) | □ Obese ................... | □ Obese ................... |
| Weight at baseline (mean, SD) | ||
| Singleton pregnancy only (if no give percentage) | Yes/no/unclear (…..) | Yes/no/unclear (…..) |
| Primiparas only (if no give percentage) | Yes/no/unclear (…..) | Yes/no/unclear (…..) |
| Gestational age (week; SD; SE) | ||
| Other baseline characteristics | ||
| Are the treatment groups comparable at baseline? |
□ Yes□ No If ‘no’ please specify the reasons: ................................................................................... .................................................................................. .................................................................................. |
|
| Intervention | ||
| Type of dietary intervention with description | ||
| How was intervention delivered | ||
| Intervention duration | ||
| Intervention provider | ||
| Duration of follow-up | ||
| Comparator | ||
| Comparator |
□ No intervention □ Other intervention (specify) .................... |
|
| Outcomes (harms) | ||
| Adverse effects occurred in |
□ Mother □ Fetus/baby/child □ Both |
|
| Outcomes (adverse effects) related with |
□ Weight change in pregnancy □ Dietary intervention type □ Not clear □ Others (specify)………… |
|
| Maternal outcomes (adverse effects) |
■ *Outcome assessment………… ■ *Outcome assessment………… ■ *Outcome assessment………… |
|
| Child outcomes (adverse effects) |
■ *Outcome assessment………… ■ *Outcome assessment………… ■ *Outcome assessment………… |
|
| Definition of outcomes |
□ Any published definition □ No definition |
|
| Adequacy of data source |
□ Reliable □ Non-reliable |
|
| Approach to ascertain the cause of harm |
□ Adequate □ Non-adequate |
|
| Proportion of cases with attributable cause of harm established |
□ ……..(%) □ Unclassified |
|
*Outcome assessment:
-
Self-reported
-
Hospital records
-
Trained assessor
-
Other
-
Blinded
-
Unblinded
2. Results
Dichotomous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | n (%) | N’ | n (%) |
|---|---|---|---|
| Effect estimate □ RR □ OR (95% CI □ SE □ p) | |||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Time-to-event data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||
|
Intervention group NR/N = |
Control group NR/N = |
||
| N’ | Median | N’ | Median |
| Effect estimate □ RR □ OR (95% CI □ SE □ p) | |||
| Blinding | Select blinded subjects: | ||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||
| Assess the method: | |||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||
| Incomplete outcome data addressed | |||
Continuous data
| Outcome:............................................................ Category:........................................................ Follow up:................................................... | |||||||
|
Intervention group NR/N = |
Control group NR/N = |
||||||
| N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
N’ |
Mean value at baseline (□ SD/ □ SE/ □ other) |
Mean end-point value (□ SD/ □ SE/ □ other) |
Mean change from baseline (□ SD/ □ SE/ □ other) |
| Blinding | Select blinded subjects: | ||||||
|
□ Patients □ Outcome assessors |
□ Investigators/clinicians □ No blinding used |
||||||
| Assess the method: | |||||||
| □ Adequate □ Inadequate □ Unclear □ Not reported | |||||||
| Incomplete outcome data addressed | |||||||
Reviewers’ comments
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
C) Non-comparative studies
Quality assessment according to checklist from Methods for the Development of NICE Public Health Guidance (second edition)
Type of study, methodology description
.......................................................................................................
.......................................................................................................
.......................................................................................................
| Population |
|---|
| Trial inclusion criteria |
| Trial exclusion criteria |
| Number of enrolled patients |
| Number of patients who completed treatment, n (%) |
| Number of patients available for follow-up, n (%) |
|
Age in years Specify the measure: ................................ |
| Other baseline characteristics |
| Treatment |
| Type of treatment used (technique, no. of sessions) |
| Treatment duration |
| Duration of follow-up |
| Outcomes |
| Definition and unit of measurement |
Reviewers’ comments
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
........................................................................................................
Appendix 20 Review protocol
1. Existing reviews
In preparing this proposal, we have conducted a scoping search in the major electronic databases MEDLINE, EMBASE and The Cochrane library to collate citations of individual research studies and systematic reviews on effectiveness and harm of various dietary interventions on weight change in pregnancy. Although there are 3 reviews in this area they have not included all the relevant studies on effectiveness and harm of dietary interventions. The existing Cochrane review on the adverse effect of weight loss or dietary intervention on mother and fetus provides some data but has not included all relevant studies. The review needs updating and quality assessment of included studies to generate firm inferences. This scoping exercise has identified the following reviews in Table 1 which are not up to date or have limitations in quality. Furthermore the reviews on harm are infrequent. Thus there is a need for new reviews.
| Review | Last updated | Primary studies included | Population | Type of intervention | Method of delivery of intervention |
|---|---|---|---|---|---|
| Dodd | 2008 | Polley (RCT) | Overweight and obese | Dietary and lifestyle | Stepped care behavioural intervention |
| Rae (RCT) | Obese women with gestational diabetes | Diet with energy restriction | Provision of dietary information | ||
| Gray-Donald | Normal weight, overweight and obese | Dietary and lifestyle |
Nutritionist counselling Modelling Skill training Self monitoring Leaflets Radio Supermarket tours Cooking demonstration Individual counselling Exercise or walking group |
||
| Birdsall | 2008 | Claesson | Obese | Diet |
Weekly motivational talk Aquarobics |
| Bechtel-Blackwell | Adolescent pregnancy | Healthy diet | 20 minute talk by health worker | ||
| Polley (RCT) | Normal weight, overweight and obese | Healthy diet and exercise | Stepped care behavioural intervention | ||
| Olson | Normal weight, overweight and obese | Healthy diet |
Health check book Newsletters Incentives |
||
| Kinnunen | Normal weight, overweight and obese |
Regular meals 5 portions fruit and vegetables High fibre Restricting high sugar snacks |
Advice by public health nurse | ||
| Cochrane | 2003 | Campbell | Increased weight gain and obese | Low energy diet | |
| Campbell | Obese | Low energy diet | |||
| Badrawi | Obese | Balanced low energy diet |
2. Objectives:
Our project will follow the key steps involved in health technology assessment of treatment and will meet the commissioned brief by fulfilling the following objectives:
-
Effectiveness of dietary interventions on maternal and fetal outcomes: To determine the effectiveness of various dietary interventions that prevent or treat obesity on
-
– maternal outcomes in pregnancy, puerperium and long term
-
– fetal, neonatal and long term outcome in children
-
-
Effectiveness of dietary interventions in pregnancy on maternal weight: To determine the effectiveness of various dietary interventions in pregnant women on
-
– weight change in pregnancy and afterwards in obese (BMI 30 or more) and overweight (BMI 25 to 29.9) pregnant women
-
– prevention of excessive weight gain in pregnancy and afterwards in women with normal weight (BMI 18.5 to 24.9)
-
-
Harm of dietary interventions in pregnancy: To evaluate the potential short term and long term adverse effects in mother and baby due to
-
– weight change in pregnancy in a) obese and overweight women b) normal weight women
-
– the type of dietary intervention in a) obese and overweight women b) normal weight women.
-
3. Research Methods
Systematic reviews of effectiveness and harm of interventions will be carried out using review methodology that has been used by the applicants in their previous systematic reviews. It is in line with the recommendations of the NHS Centre for Reviews and Dissemination and the Cochrane Collaboration including those of the Cochrane Adverse Methods Subgroup. The investigation will be carried out simultaneously executing the systematic reviews of effectiveness and harm. Our strategy for these will be based on a prospective protocol, which is briefly outlined below. We will carry out: review of existing reviews; update of out-of-date review; and reviews of topics not reviewed in the literature.
The GRADE methodology will guide us when assessing the quality of the evidence and summarising the results. We have previously used the GRADE methodology in our reviews. The mission of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) group is to help resolve the confusion among the different systems of rating evidence and recommendations and increase transparency within individual evidence syntheses. While the GRADE system has originally been developed for making recommendations, it is now also used for only assessing the quality of the evidence and the outcomes for patients. In that sense, the Cochrane collaboration has now adopted the GRADE-methodology by adding summary of finding tables to its Cochrane reviews.
We plan to explore the need for a health economic evaluation, including decision analytical modelling, of the various dietary and lifestyle interventions on various clinically relevant outcomes. The outputs of our reviews would help us populate a decision-tree, which may be necessary to examine the competitive merits of various strategies.
We will address the following structured question in our project defining population, interventions and comparison and study designs as shown in Table 2.
| Outcome | Maternal outcomes |
| Pregnancy related outcomes (standardised through GLOBE project): pre eclampsia; gestational diabetes mellitus; gestational hypertension; premature rupture of membranes; caesarean section, postpartum haemorrhage; sepsis; maternal death | |
| Other relevant outcomes: cardiac arrest; abruption; stroke; psychiatric problems; complications of labour and delivery; instrumental delivery; induction of labour; need for hospitalisation, day care unit visits, use of intensive care; depression; self esteem, change in diet and exercise | |
| Maternal weight gain/change: Change in maternal weight (absolute gain/loss in weight, percentage of weight gained/reduced in comparison to pre intervention weight); fat content measurement (body mass index, skin fold thickness, ponderal index, fat free mass); fat distribution measures (waist hip ratio, waist size) in pregnancy | |
| Fetal outcomes | |
| Fetal outcomes (standardised through GLOBE project): Macrosomia stillbirths; fetal abnormalities including neural tube defects, congenital heart disease; perinatal death; intrauterine growth restriction; prematurity; abnormal Apgar; neonatal respiratory distress; shoulder dystocia | |
| Other relevant fetal outcomes: abnormal pH at birth or antenatal; hypoxic ischemic encephalopathy; long term effect, learning disabilities, developmental and special needs after discharge; need for neonatal intensive care admission, mechanical ventilation and duration of hospital stay | |
| Childhood and adult outcomes in offspring | |
| Childhood obesity; adult obesity, diabetes mellitus; coronary heart disease; hypertension; stroke; depression; death | |
| Adverse events | |
| Clinically significant adverse outcomes in mother and child due to a) dietary intervention b) weight change in pregnancy | |
| Most common adverse effects that lead to pregnant women discontinuing the intervention |
The major maternal and fetal outcomes to be reviewed have been standardised through the GLOBE project. We shall identify evidence on additional relevant outcomes for mother and fetus /child and rank them according to their importance for decision making: critical for decision making, important (but not critical) for decision making and not important for decision making. The ranking will be done by Delphi methodology.This step is crucial in order to potentially identify knowledge gaps on critical / important outcomes that have not been investigated so far.
4. Systematic review of effectiveness of interventions
Study identification and selection
For this HTA project, a database of published and unpublished literature will be assembled from searches using a comprehensive search strategy, as well as hand searching, contacting commercial weight management organisations and consultation with experts in the area. We will communicate with major centres of obesity research and the first author of each selected study published in the last five years, with enquiry for any published or unpublished relevant studies not included on our list. Language restrictions will not be applied to electronic searches.
The following databases will be searched: MEDLINE, EMBASE, BIOSIS, LILACS, Pascal, Science Citation Index, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment Database (HTA). In addition, information on studies in progress, from commercial providers like Weight Watchers, Slimming world and unpublished research or research reported in the grey literature will be sought by searching a range of relevant databases including the Inside Conferences, Systems for Information in Grey Literature (SIGLE), Dissertation Abstracts and Clinical Trials.gov. Internet searches will also be carried out using specialist search gateways (such as OMNI: http://www.omni.ac.uk/), general search engines (such as Google: http://www.google.co.uk/) and meta-search engines (such as Copernic: http://www.copernic.com/). Citations identified by the search will be selected for inclusion in the review in a two-stage process using predefined and explicit criteria regarding populations, interventions, outcomes and study design. First, a master database of the literature searches will be constructed by amalgamation of all the citations from various database sources. The citation will be scrutinised by two reviewers. Copies of full manuscripts of all citations that are likely to meet the selection criteria will be obtained. Two reviewers will then independently select the studies, which meet the predefined criteria. These criteria will be pilot tested using a sample of papers and agreement between reviewers will be measured. Disagreements will be resolved by consensus and/or arbitration involving a third reviewer.
Study quality assessment and data extraction
The quality of the selected primary randomised controlled trials (RCT’s) and observational studies will be assessed based on accepted contemporary standard. Following the GRADE methodology, the quality assessment and reporting of results will be done separately for each outcome, since even within one review the quality of the evidence can vary between outcomes. We define quality of evidence as ‘the extent of confidence that an estimate of effect is correct’. The GRADE system classifies quality of evidence into one of four levels: high, moderate, low and very low.
To assess the quality, we consider first of all risk of bias (internal validity), i.e. the extent to which design, methods, execution and analysis did not control for bias in assessment of effectiveness (Table 4). Furthermore, we explore the (in-) consistency of results (heterogeneity), (in-) directness of the evidence (to the question under consideration, including surrogate parameters), (im-) precision of the results and publication bias. Deficiencies on those criteria in the body evidence from RCTs will lower the quality of the evidence from high to moderate or low, perhaps even very low. Deficiencies in the body of evidence from non-RCTs will lower the quality of evidence from low to very low.
| High quality | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate quality | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| Low quality | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low quality | Any estimate of effect is very uncertain |
| No downgrading | Downgrading by one (possibly two) levels | Downgrading by two or three levels | |
|---|---|---|---|
| 1. Selection bias: | Studies with randomisation, allocation concealment, similarity of groups at baseline | RCTs with some deficiencies in randomisation e.g. lack of allocation concealment, or nonrandomised studies with either similarities at baseline or use of statistical methods to adjust for any baseline differences | Non randomised, with obvious differences at baseline, and without analytical adjustment for these differences |
| 2. Performance bias: | Differed only in intervention, which was adhered to without contamination, groups were similar for cointerventions or statistical adjustment was made for any differences | Confounding was possible but some adjustment was made in the analysis | Intervention was not easily ascertained or groups were treated unequally other than for intervention or there was non-adherence, contamination or dissimilarities in groups and no adjustments made |
| 3. Measurement bias: | Outcome measured equally in both groups, with adequate length of followup (i.e. at least 2 years after delivery), direct verification of outcome, with data to allow calculation of precision estimates | Inadequate length of follow up or length not given | Inadequate reporting or verification of maternal mortality or differences in measurement in both groups |
| 4. Attrition bias: | No systematic differences in withdrawals between groups and with appropriate imputation for missing values | Incomplete follow-up data, not intention-to-treat analysis or lacking reporting on attrition |
Individual studies will be described by study type, intervention, numbers taking part, population denominator (eg pregnant women or fetuses) and study quality. In addition to using study quality as possible explanations for differences in results (heterogeneity), the extent to which primary research met methodological standards is important per se for assessing the strength of any conclusions that are reached. Studies’ findings will be extracted in duplicate using pre-designed and piloted data extraction forms, which we have already developed and used in our previously completed reviews. Any disagreements will be resolved by consensus and/or arbitration involving a third reviewer. Missing information will be obtained from investigators if it is crucial to subsequent analysis. To avoid introducing bias, unpublished information will be coded in the same fashion as published information. In addition to using multiple coders to insure the reproducibility of the overview, sensitivity analyses around important or questionable judgements regarding the inclusion or exclusion of studies, the validity assessments and data extraction will be performed.
Data synthesis
We will use RevMan and Stata softwares to conduct analyses. The former will allow uniformity with Cochrane reviews and the latter will allow the data analytic flexibility that we will need to examine issues not included in the RevMan software. Separate analyses will be performed on randomised and non-randomised data. Any heterogeneity of results between studies will be statistically and graphically assessed, including use of funnel plots. We will explore causes of the heterogeneity and proceed to perform meta-analysis if appropriate. To explore causes of heterogeneity subgroup analyses will be planned a priori to see whether variations in clinical factors e.g. populations, interventions, outcomes or study quality affect the estimation of effects. Individual factors explaining heterogeneity will also be analysed using meta-regression to determine their unique contribution to the heterogeneity. Conclusions regarding the typical estimate of an effect size of the intervention will be interpreted cautiously if there is significant heterogeneity.
5. Review of adverse effect of interventions
In the proposed project addition to the search for relevant reviews and primary studies on effectiveness of interventions including those that were excluded from analysis of benefit, we will evaluate studies that specifically provide details of adverse effects due to the dietary interventions. We will conduct review of harm of interventions based on recommended methods for systematic reviews, particularly those of observational studies and adverse events including those of Cochrane adverse effects subgroup.
Study identification and selection for adverse events
We have purposefully kept the scope of the question of adverse effects of any dietary intervention on pregnant women and their children broad. This will enable us to identify a variety of adverse effects that were previously not known or recognised. The adverse outcomes to be evaluated will be in 3 groups and similar to the outcomes in the effectiveness review, they will be ranked according to their importance: critical for decision-making, important for decision making and not important.
-
clinically significant adverse maternal outcomes in pregnancy and later due dietary interventions in (i) overweight or obese women and (ii) women with normal weight
-
clinically significant adverse fetal, neonatal, childhood and adult outcomes in the offspring of pregnant women undergoing dietary interventions
-
Most common adverse effects that lead to pregnant women discontinuing the intervention
We will design a separate search strategy to identify studies on harm by including adverse effects text words and indexing terms to ensure that they are not missed in the databases previously described. We will use datasets providing counts or proportions attributed to specific interventions or weight change in pregnancy leading to maternal and fetal adverse outcomes, from direct counting or from special surveys. We use the term dataset because some sources are research studies but others are direct counts or other forms of routine data collection (such as vital registration; membership of weight reduction club, web table). We will include only those datasets that represent the target population in the final analysis. In cases of partial data duplication with overlapping datasets, we will select the most recent and largest dataset.
Study quality assessment and data extraction for adverse events
Criteria used to assess study quality will follow the same concept as for assessing study quality for effectiveness: assessing risk of bias, inconsistency of results, indirectness of the evidence, imprecision and publication bias. For assessing the risk of bias in estimating adverse event rates associated with dietary intervention in pregnancy, we will take into account existing checklists for evaluation of randomised and non-randomised studies, including study design and other features associated with outcome (e.g. small for gestational age, pre term delivery etc). For the three possible designs (RCTs, observational studies with a control group, and observational studies without controls (case series)) quality assessment and presentation of results will be done separately. Additionally, information on weight change per se on mother and baby will also be extracted as these could be associated with adverse event rates or severity. The methodological quality of all eligible datasets (‘risk of bias’) will be assessed to investigate internal validity (the extent to which the information is probably free of bias) with the following attributes:
-
reporting of adverse maternal and fetal outcome definition to reduce bias in ascertainment of denominator data in the series (any published definition reported Vs no definition)
-
adequacy of data source to ascertain a capture of denominator data that is as complete as possible (use of multiple data sources, special surveys, or clinical studies vs routine registration enrolment in weight loss programmes, in which adequate attribution of cause of harm has been shown to be questionable for maternal and fetal outcomes, leading to substantial underreporting)
-
use of a robust approach to ascertain that the cause of harm is a representation of the underlying condition that is as true as possible (confidential enquiries, use of multiple sources of outcome vs no special efforts to confirm cause)
-
sufficiently high proportion of cases with attributable cause of harm established (< 5% unclassified).
Quality assessment will be done for each outcome. Randomised studies will start as high quality, observational studies with controls will start as low quality, and uncontrolled studies will start as very low quality. The evidence will be downgraded in the presence of methodological weaknesses and uncertainty; it can be upgraded in the presence of large effects, dose–response gradient and remaining plausible confounding which would reduce a demonstrated effect. Based on these criteria, the datasets will be classified into different quality groups.
Data synthesis for adverse events
The number of adverse events reported in pregnant women and children will be obtained for each intervention to compute a percentage of the total number of women and children in whom the occurrence of that particular adverse event or confirmation of its absence was reported. It is inappropriate to calculate adverse events rates from case studies, thus a qualitative summary will be undertaken. Quantitative adverse events rates calculations will be restricted to series of women undergoing dietary interventions and weight change as identified from RCTs and observational studies, with and without controls (case series). We shall quantify the adverse events as relative risks and 95% confidence intervals. The point estimates of proportions and their 95% CIs will be represented in forest plots to explore heterogeneity and the possibility of the differences being due to chance assessed statistically by Cochran Q test. To explore the presence of heterogeneity and its causes, regression models will be adjusted to the proportions attributed to every individual cause of maternal and fetal complications. The proportions will be transformed with the logit transformation. Explanatory variables considered in these models are: type of intervention and dataset methodological quality items.
6. Evidence Synthesis using the GRADE methodology
Once the systematic reviews for effectiveness and harm of dietary interventions have been undertaken, we shall prepare standardised evidence profiles using the GRADE profiling software GRADEPro. Profiles will be done for both groups (obese or overweight women and normal weight women at risk of excessive weight gain), with a separate quality assessment and summary of findings for each critical and important outcome that will allow a quick and informative summary of the evidence.
The following steps will be undertaken to come to an overall judgement: having assessed the quality of evidence for each maternal and fetal outcome, and having decided on the relative importance of the outcomes (critical or important to a decision), we will come up with a judgement on the overall quality of evidence across the most important outcomes, balancing net benefits and harms.
7. Project timetable
Figure shows the project timetable and milestones for the accuracy and effectiveness reviews and economic modelling.
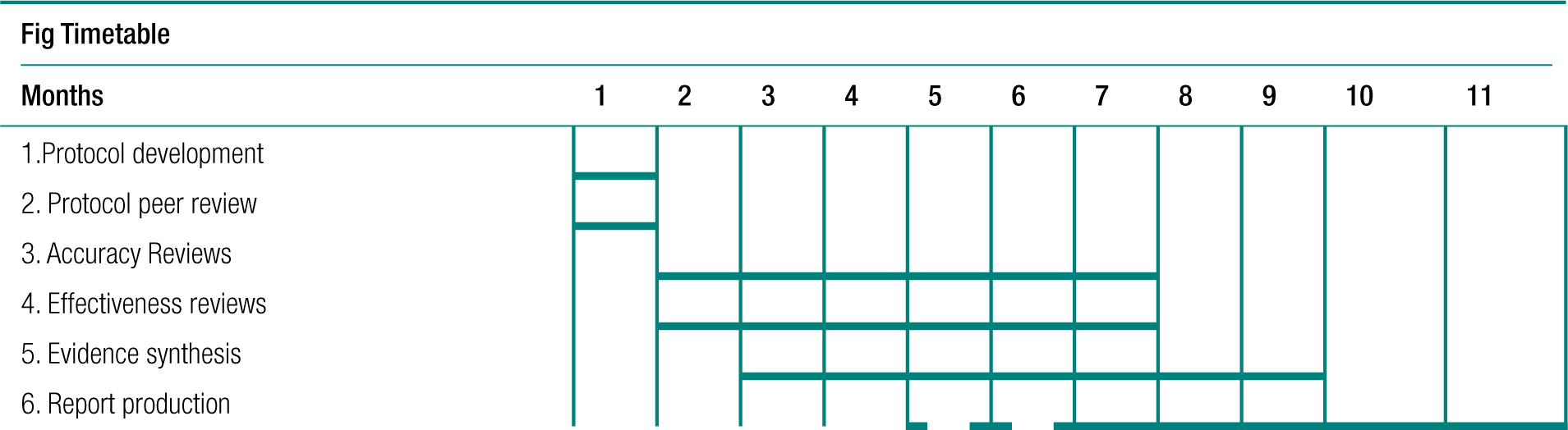
List of abbreviations
- ACOG
- American Congress of Obstetricians and Gynaecologists
- BMI
- body mass index
- CEMACH
- Confidential Enquiry into Maternal and Child Health
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CMACE
- Centre for Maternal and Child Enquiries
- GDM
- gestational diabetes mellitus
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HDU
- high-dependency unit
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- IGT
- impaired glucose tolerance
- IOM
- Institute of Medicine
- IQR
- interquartile range
- ITU
- intensive therapy unit
- LGA
- large for gestational age
- LILACS
- Latin American and Caribbean Health Sciences Literature
- MD
- mean difference
- NICE
- National Institute for Health and Clinical Excellence
- NICU
- neonatal intensive care unit
- NOS
- Newcastle–Ottawa Scale
- NRS
- non-randomised study
- NTD
- neural tube defect
- OR
- odds ratio
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RR
- relative risk
- SGA
- small for gestational age
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health