Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/11/01. The contractual start date was in July 2006. The draft report began editorial review in March 2008 and was accepted for publication in March 2012. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Gillett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Introduction
Type 2 diabetes mellitus (T2DM) accounts for roughly 80–90% of all cases of diabetes. The prevalence of T2DM is rising both in the UK and worldwide as a result of an ageing population, an increasingly sedentary lifestyle across communities, and a rise in the prevalence of obesity. 1 Certain ethnic groups, foremost of which in the UK are South Asians, are at increased risk of developing T2DM. The disease is characterised by hyperglycaemia. It has a slow onset, with few or no symptoms initially, and is sometimes referred to, mistakenly, as ‘mild diabetes’. However, it can lead to complications such as angina, cardiac failure, myocardial infarction (MI), stroke, visual loss and renal failure. The TARDIS-2 report published in 2000 estimated that T2DM costs the NHS approximately £2B per annum, with an additional £36M spent on related social services and private health care. This equates to an annual expenditure of roughly £1738 per patient with T2DM. 2
Prevalence of diabetes, impaired glucose tolerance and impaired fasting glucose in the UK
In 2009 there were estimated to be 2,213,138 people with diabetes in the adult population in England, a prevalence of 5.1%. 3
The Health Profile of England 2009 reports a prevalence of 5.6% of men and 4.2% of women with diabetes in England in 2008. 4 Diabetes was flagged as one of the ‘particular challenges’.
Type 2 diabetes mellitus was estimated to account for 92% of all (diagnosed and undiagnosed) diabetes. The Yorkshire & Humber Public Health Observatory (YHPHO) provides a very useful set of data on diabetes in England. Between 1994 and 2003 the incidence of diabetes doubled from 1.8 to 3.3 per 1000 (age standardised) and the diagnosed prevalence doubled. 5 However, recent data from the Association of Public Health Observatories (APHO) model6 suggest that 26% of men and 22% of women with diabetes are undiagnosed.
The prevalences of doctor-diagnosed T2DM in England in different age and ethnic groups were reported in the Health Survey for England 20047 and are shown in Figures 1 and 2.
FIGURE 1.
Prevalence of doctor-diagnosed T2DM between different ethnic groups in England. Note: figures for the general population were taken from the 2003 survey and represent all ethnic groups in England, which would be predominantly white. Copyright© 2011, re-used with the permission of The Health and Social Care Information Centre. All rights reserved.
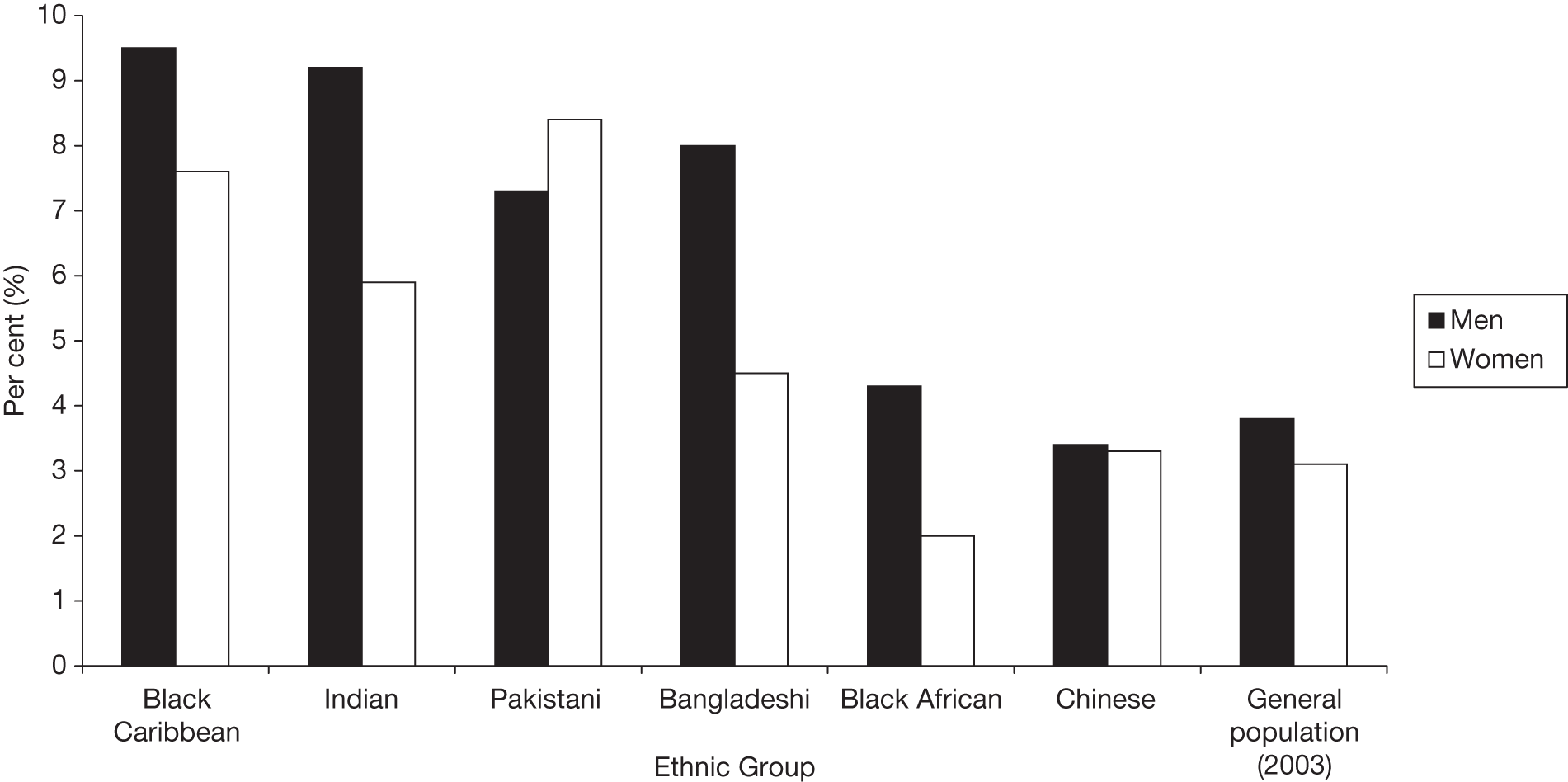
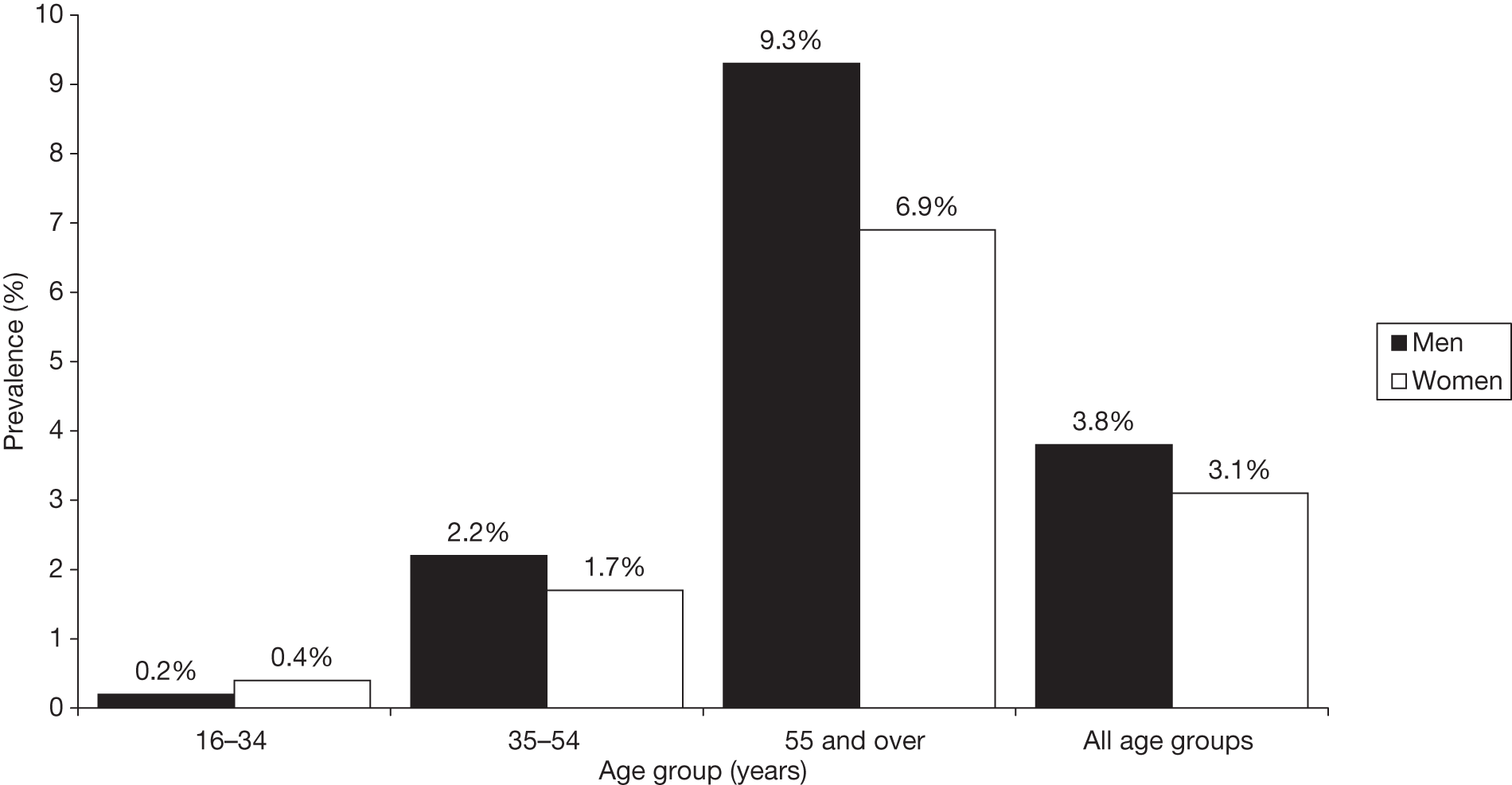
FIGURE 2.
Prevalence of doctor-diagnosed T2DM by age group. Copyright© 2011, re-used with the permission of The Health and Social Care Information Centre. All rights reserved.
A study by Bagust et al. (2002)8 predicts that by 2036 there will be approximately 20% more cases of T2DM in the UK than in 2000, as a result of the population ageing and increased levels of obesity. 8
The consequences and costs of diabetes
Some key consequences are:
-
Life expectancy is reduced. The National Service Framework9 summarised this as being, on average, a loss of up to 10 years of life, but it will vary by age and by gender, with females losing more than males. 5
-
Atherosclerosis – arterial disease, which increases the risk of heart attacks, strokes and amputation. The increase is more marked in women. The relative risk (RR) for fatal coronary heart disease (CHD) is 50% higher in women with diabetes than it is in men. 10
-
Mortality rates from CHD are up to five times higher for people with diabetes, and the risk of stroke is three times higher. 9
-
Nephropathy – disease of the kidneys, which can lead to renal failure requiring dialysis or transplantation. Diabetes is a leading cause of renal failure, and the second commonest cause of lower-limb amputation.
-
Retinopathy – disease of the eye, which can lead to visual loss. Diabetes is the leading cause of blindness in people of working age. 9
-
Neuropathy – damage to the nerves, which cause a range of problems, including loss of sensation, neuropathic pain, muscle wasting, and altered blood flow.
-
Diabetes is estimated to account for 5% of all NHS expenditure and 9% of hospital costs. 5
-
The presence of diabetic complications increases the overall NHS spending per patient more than fivefold and increases by five the chance of a person needing hospital admission. 9 Based on TARDIS-2 data, a person in the UK with T2DM incurs average direct costs of > £2000 per year, accounting for 4.7% of all NHS expenditure. 2
-
One in 20 people with diabetes incurs social services costs, and for these people the average annual cost was £2450 in 1999. 9
Maintenance of blood glucose levels as close to normal as possible slows or prevents the onset and progression of eye, kidney and nerve diseases caused by diabetes. The evidence for the link between glucose control and arterial disease is less strong, but good control reduces the risk. 11–13
The development of type 2 diabetes mellitus
Glucose is the principal energy source for human cells and is derived from three sources: food, de novo synthesis in the body, and breakdown of glycogen (a stored form of glucose). Blood glucose levels are normally kept within a narrow band, with a maximum of 5.5 mmol/l. Insulin and glucagon, hormones secreted by the pancreas, regulate blood glucose levels. Insulin lowers the glucose concentration in the blood, and glucagon raises it, in order to maintain a constant concentration, despite activities such as eating and exercise.
Overweight and obesity are associated with the phenomenon known as insulin resistance, in which higher levels of insulin are required to have the same effects on its target cells. Initially, the beta cells in the pancreas produce more insulin, and the blood glucose level is maintained within the normal range. However, over time the beta cells cannot cope, some die and insulin production falls. By the time people develop T2DM, about half of the beta cell mass has been lost. 14 Insulin resistance is also associated with an increase in cardiovascular disease (CVD). 15
Epidemiology of type 2 diabetes mellitus
Global prevalence of type 2 diabetes mellitus
Based on current estimates, the global prevalence of T2DM will double from 171 million patients in 2000 to 334 million patients in 2025. The increase in the number of patients will be most pronounced in nations that are currently undergoing socioeconomic development, including increasing urbanisation. Global diabetes prevalence estimates from the International Diabetes Federation (IDF) suggest that the region with the highest prevalence of diabetes is North America at 7.9%, followed by Europe at 7.8%, and the Eastern Mediterranean and Middle East at 7%. When absolute numbers of people with diabetes are considered, it is South East Asia and the West Pacific that are expected to experience the highest increases in prevalence over the coming years. 16
Risk factors for developing diabetes
Age, weight and ethnic group are the three factors that most affect prevalence of T2DM in the UK. The Health Survey for England 20047 reported that the prevalence of T2DM increases sharply with age (see Figure 2). In the general population (2003 data), the prevalence of T2DM in men and women increased from 0.2% and 0.4%, respectively, at age 16–34 years, to 2.2% and 1.7%, respectively, at age 35–54 years and to 9.3% and 6.9%, respectively, in those aged ≥ 55 years. The survey reported that in all but the youngest age groups, the prevalence of T2DM was higher in men than in women.
The risk of diabetes is much higher in people of South Asian ethnicity (i.e. from the Indian subcontinent: Pakistan, India, Bangladesh, Nepal) (see Figure 1). We deal with their situation in greater detail later in Chapter 3.
Lifestyle factors are the main determinants of T2DM but there are strong familial influences:
-
In families in which no member has T2DM, the lifetime risk of developing it is approximately 10%.
-
If one parent is affected then the risk increases threefold (i.e. 30% lifetime risk).
-
If a sibling is affected then the risk increases fourfold (i.e. 40% lifetime risk).
-
If both parents are affected then the risk increases sevenfold (i.e. 70% chance).
-
If an identical twin is affected then the risk increases ninefold (i.e. 90% chance). 5
These risks are increased if the affected family member had early onset of T2DM. It is likely that the diabetes is preceded by impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT), and that family history could provide opportunities for targeted screening.
Obesity is a strong risk factor. It has been suggested that it is only obesity [body mass index (BMI of > 30 kg/m2)] that increases the risk and not overweight (BMI of > 26–29 kg/m2). Jarrett (1986),17 reviewing older studies, noted that there was little difference in the risk of diabetes at a range of BMIs of < 31 kg/m2. However, recent work shows the take-off to diabetes at lower BMIs18 [Figure 3, based on data in the study of Ford etal. (1997)18]. It may be that physical activity is less now than in previous decades. Jarrett (1986)17 was reviewing studies from the early 1980s.
FIGURE 3.
Age-adjusted incidence rates of diabetes as a function of baseline BMI in 30- to 55-year-olds (both sexes).
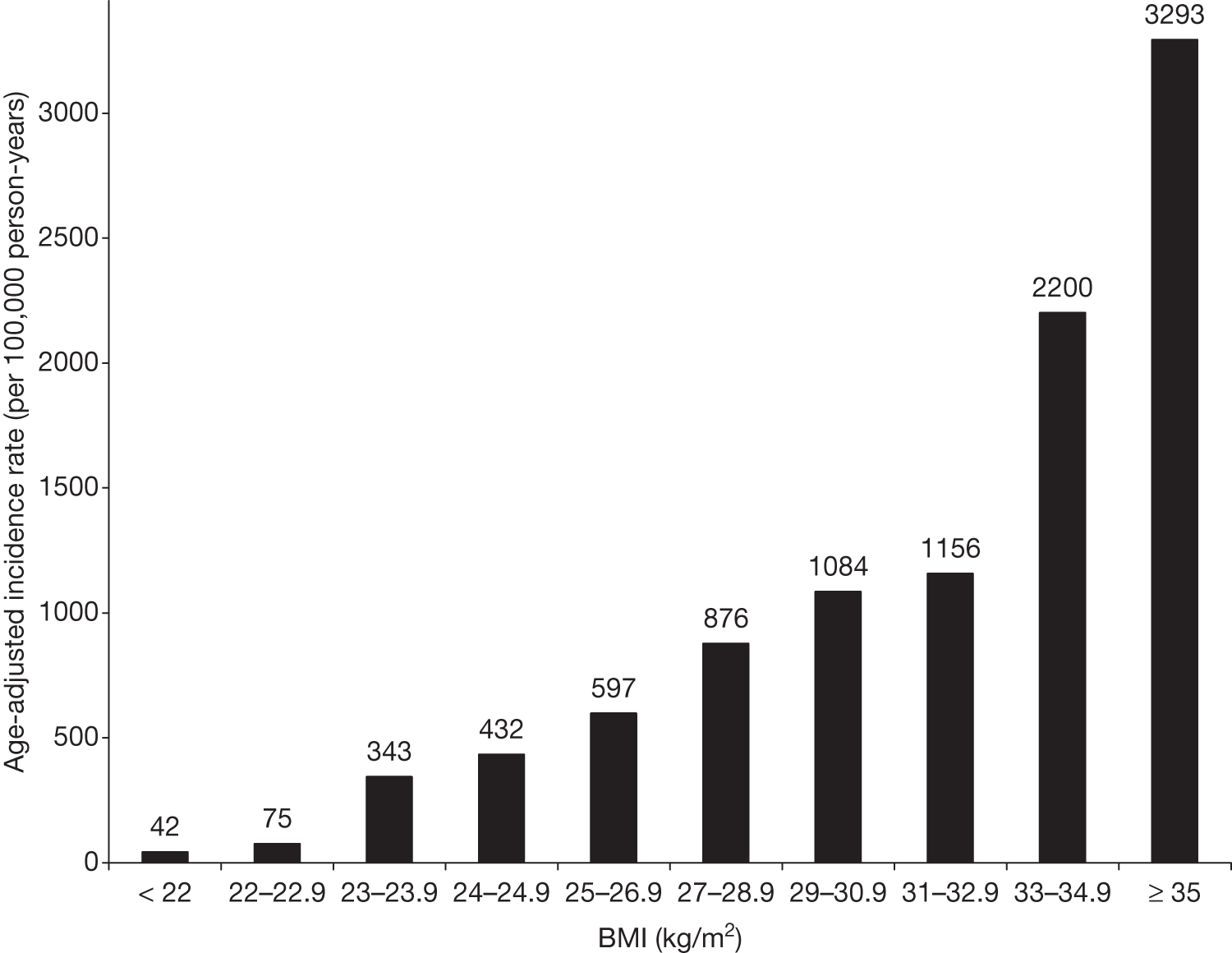
The National Audit Office suggests that 47% of T2DM cases in England can be attributed to obesity. 19 The risk of T2DM is almost 13 times greater in obese women than in women of normal weight. For men the risk is five times greater. 5
Physical activity reduces the risk of developing T2DM. Jeon et al. (2007),20 in a meta-analysis of cohort studies, found that the risk of T2DM was 30% lower in those undertaking regular moderate-intensity activity than in sedentary people. The reduction in risk associated with increased activity levels is independent of BMI. Sui et al. (2007),21 in a study of mortality in older American adults, found that fitness was a predictor of mortality, independent of adiposity; they also reported that when people were grouped into fitness quintiles (based on time tolerated on treadmills), the fittest quintile had a prevalence of diabetes of 12% compared with 25% in the least-fit quintile.
Physical activity is dealt with in greater detail later in Chapter 2.
The National Service Framework for Diabetes noted socioeconomic differences in the risks of developing T2DM. 9 Those in the most deprived fifth of the population are 1.5 times more likely than average to have diabetes at any given age.
Thomas et al. (2007)22 used data from the 1958 British birth cohort to provide an estimate of the national prevalence of diabetes risk in mid-life, using glycated haemoglobin (HbA1c) level of ≥ 5.5% as an indicator for possible subclinical alterations in glucose metabolism, which would detect those with IGT as well. The study consisted of 7799 participants born in England, Scotland and Wales and currently living in the UK.
The majority of the population (79.3%) had an HbA1c level of < 5.5%; 16.7% had an HbA1c level of 5.5–5.9%; 2.0% had an HbA1c level of 6.0–6.9%; and 0.6% had an HbA1c level of ≥ 7.0%. More men than women were found in the higher-HbA1c level categories and a highly significant inverse socioeconomic gradient was apparent for HbA1c levels of ≥ 5.5%. When looking at regional variation in HbA1c levels of ≥ 5.5%, it was found that the east of England had a significantly higher prevalence than the rest of the UK (24.3% vs 19.3%) and Scotland had the second highest prevalence (22%).
On the basis of these data, the authors suggest that a proportion of people are likely to have subclinical elevations in HbA1c level in their mid-40s, and this proportion is greater in some socioeconomic groups and geographical regions than in others. These individuals are likely to represent the population that has IGT, and at risk of developing T2DM.
Children and adolescents with type 2 diabetes mellitus
A recent alarming development is the emergence of T2DM and glucose intolerance in children and adolescents. 23 The year 1979 saw the first childhood cases of T2DM reported in Pima Indians; however, there are now worldwide reports of children with the disorder. This is thought to be associated with the growing problem of childhood obesity over the last 15 years. In particular, those ethnic groups at high risk of diabetes have reported a close association between the development of diabetes and childhood obesity. 24 Other contributing factors should also be considered, including genetic susceptibility as a result of variations in, for example, insulin sensitivity. In the UK, reports of children with T2DM have appeared only recently. 25 Compared with type 1 diabetes mellitus (T1DM), children with T2DM presented later (12.8 years vs 9.3 years) were usually female, overweight or obese (92% vs 28%) and a large proportion were from ethnic minority groups. In common with their adult counterparts, South Asian children have an increased risk of T2DM (RR = 13.7) compared with white UK children. It is therefore becoming apparent that preventative interventions may also need to be targeted at a younger age group than previously thought in order to prevent the progression to diabetes and heart disease at a later age.
Treatment of type 2 diabetes mellitus
Treatments for T2DM aim to lower blood glucose and reduce the development of diabetes-associated secondary complications. 26 However, there is usually a progressive deterioration in blood glucose control in T2DM, necessitating changes in treatment with time. 27 People with T2DM are initially advised on lifestyle changes (weight loss, increased physical activity). If the lifestyle changes fail to control blood glucose, metformin (especially in overweight patients) or sulphonylureas (if metformin is contraindicated or not tolerated, or if the patient is not overweight) are considered. When monotherapy with these drugs no longer provides adequate glycaemic control, combination therapy is the next step (metformin plus sulphonylurea) but it may only be a matter of time before treatment must be intensified (e.g. insulin therapy or thiazolidinediones) to adequately control glucose levels. Other types of glucose-lowering drugs, such as the glucagon-like peptide analogues and the dipeptidyl peptidase 4 inhibitors, are now available.
A full review of the management of T2DM is not relevant here but can be found in the consensus statement from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes,28 in the National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 8729 and the technology assessment report that underpinned the guideline. 30
For our purposes, the key points are that these treatments are far from perfect for controlling T2DM, and have side effects. Some, such as the sulphonylureas and insulins, cause weight gain, which can make glycaemic control more difficult. Others, such as the glitazones, can cause heart failure and fractures. Metformin may not be tolerated because of diarrhoea. Good control may not be achieved despite combination therapy, even with insulin, especially in overweight patients. 31–33 The problems with treatment of T2DM reinforce the need to prevent it as far as possible.
Epidemiology of impaired glucose regulation
Definitions
Two conditions appear to precede T2DM. The first is IGT, in which fasting glucose is normal but there is post-prandial hyperglycaemia. The definition comes from the oral glucose tolerance test (OGTT). The second is IFG, when the fasting level is raised but the post-prandial level does not reach IGT levels. The normal FPG is currently defined as ≤ 5.5mmol/l.
The World Health Organization (WHO) defines diabetes (Table 1) on the basis of a fasting plasma glucose (FPG) level of ≥ 7 mmol/l or a 2-hour level (in an OGTT) of ≥ 11.1 mmol/l. 34
| Category | Fasting | Two-hour OGTT |
|---|---|---|
| Normal | ≤ 6.0 | < 7.8 |
| IFG | 6.1–6.9 | < 7.8 |
| IGT | < 7.0 | 7.8–11.0 |
| Both IFG and IGT | 6.1–6.9 | 7.8–11.0 |
| Diabetes | ≥ 7.0 | ≥ 11.1 |
Impaired glucose tolerance is defined as an FPG level of < 7 mmol/l and a 2-hour level of 7.8–11.0 mmol/l.
Impaired fasting glucose is defined as the range of 6.1–6.9 mmol/l and a 2-hour level of < 7.8 mmol/l.
So those with IFG should exclude IGT, but this is not possible if the 2-hour level has not been measured, whereas those with IGT can have IFG as well.
These criteria differ from those of the ADA, which defined IFG as starting at 5.6 mmol/l. One effect is that a much greater proportion of people with IGT also have IFG. The Danish Inter-99 study35 found that using the WHO criteria, 25% of those with IGT have IFG compared with 60% using the ADA cut-off.
One problem with the WHO criteria is that there is a group of people with FPG levels of 5.6–6.0 mmol/l – above normal but lower than IFG. The ADA definition tidies up this anomaly. 36 There is reluctance in the WHO to do this. First, the prevalence of IFG would greatly increase. In the D.E.S.I.R. study of French men and women aged 30–64 years,37 the prevalence of IFG was 13% in men and 4% in women using the 6.1 mmol/l cut-off, but would rise to 40% and 16%, respectively, using the 5.6 mmol/l cut-off. Second, and more importantly, the risk of progression to diabetes is much higher in people with FPG levels of > 6.1 mmol/l (‘old IFG’) than in those with FPG levels of between 5.6 and 6.0 mmol/l (‘new IFG’).
Progression to type 2 diabetes mellitus from impaired glucose tolerance or impaired fasting glucose
Impaired glucose tolerance and IFG have been called ‘pre-diabetes’ but the term is unsatisfactory because not all people with the two conditions go on to develop diabetes. The proportions of people who progress to diabetes vary among different studies in different populations (Table 2). 38 The 2005 Agency for Healthcare Research and Quality (AHRQ) report on the diagnosis, prognosis and treatment of IGT and IFG concluded that with regard to prognosis of patients following evaluation of a number of trials that reported relevant outcomes:
| IFG status | Incidence of diabetes per 1000 person-years | |||
|---|---|---|---|---|
| Glucose level (mmol/l) | Age (years) | |||
| 30–44 | 45–54 | 55–64 | ||
| Men | ||||
| Normal | < 5.6 | 2.3 | 1.7 | 1.1 |
| New IFG | 5.6–6.0 | 4.9 | 8.5 | 11.5 |
| Old IFG | 6.1–6.9 | 24.7 | 38.9 | 63.9 |
| Women | ||||
| Normal | < 5.6 | 0.4 | 1.4 | 0.7 |
| New IFG | 5.6–6.0 | 5.5 | 7.0 | 5.9 |
| Old IFG | 6.1–6.9 | 35.7 | 52.3 | 66.7 |
There is consistent evidence that IFG and IGT are both risk factors for the development of diabetes mellitus. The pooled RR for new DM is 6.02 [95% confidence interval (CI) 4.66 to 7.38] in people with IGT, 4.70 (95% CI 2.71 to 6.70) in people with IFG, and 12.21 (95% CI 4.32 to 20.10) in people with both disorders.
The D.E.S.I.R. study37,39 examined the risks of progression to diabetes from different baseline levels.
Progression from impaired fasting glucose to diabetes
The effect of different cut-points for IFG on diabetes incidence was examined in the Ely study40 from 1990–2000, a population-based longitudinal study. At baseline there were 1040 non-diabetic adults (defined by OGTT criteria) aged 40–69 years. Baseline glucose status was defined as normoglycaemia < 5.6 mmol/l, IFG-lower 5.6–6.0 mmol/l and IFG-original 6.1–6.9 mmol/l. The all-IFG group included fasting glucose values of 5.6–6.9 mmol/l. Median follow-up time was 10 years. The incidence rate was 7.3 (95% CI 5.7 to 9.4) per 1000 person-years of follow-up. The annual incidence of diabetes was 0.6%.
Forouhi et al. (2007)40 reported the progression rates in the Ely study by baseline FPG category as:
-
from normal glucose tolerance (NGT) (FPG < 5.6 mmol/l): 2.4 per 1000 person-years
-
for those in the 5.6–6.0mmol/l range (the ‘new IFG’ suggested by the ADA): 6.2 per 1000 person-years
-
for original IFG (6.1–6.9 mmol/l): 17.5 per 1000 person-years.
Cumulative incidence also increased by age at baseline:
-
40–49 years: 5.5 per 1000 person-years
-
50–59 years: 7.6 per 1000 person-years
-
60+ years: 9.5 per 1000 person-years.
However, rates by BMI were not reported. The three categories of baseline glucose tolerance had different BMIs: 24.8 kg/m2, 25.8 kg/m2 and 27.8 kg/m2, respectively.
Most people with IFG at baseline regressed to NGT – 83% of those with new IFG and 56% of those with original IFG. The rate of progression was lower in IFG than IGT. Those with both IFG and IGT had higher incidence.
In the Finnish MONICA study,41 the following risks of developing diabetes after 10 years’ follow-up were reported:
-
low NGT (FPG < 4.9 mmol/l): 2.4 per 1000 person-years
-
middle NGT (FPG 4.9–5.3 mmol/l): 2.5 per 1000 person-years
-
high NGT (FPG 5.4–6.0 mmol/l, equivalent to the ADA’s new IFG): 4.5 per 1000 person-years
-
IFG (FPG 6.1–6.9 mmol/l): 15.5 per 1000 person-years.
In the Danish arm of the ADDITION study,42 the progression rate (after 1 year of follow-up) from IFG (FBG 5.6–6.0 mmol/l) was 17.6 per 100 person-years. So there seems to be consistency among these three European studies of a low progression rate from isolated IFG to diabetes of about 17 per 1000 person-years, or about 2% a year.
In the USA, Nichols et al. (2007)43 compared rates of progression with diabetes in people with ‘old IFG’ (FPG 6.1–6.9 mmol/l) and ‘new IFG’ (FPG 5.5–6.0 mmol/l), although they called these ‘original IFG’ and ‘added IFG’. A total of 5.6% of original IFG subjects progressed to diabetes each year, compared with 1.3% of added IFG. During follow-up, ranging among subjects from 2 to 11 years, 8% of those with added IFG and 24% of those with original IFG became diabetic.
Progression from impaired glucose tolerance to diabetes
In the Finnish MONICA study,41 at 10 years of follow-up the progression rate from isolated IGT was 14.7 per 1000 person-years, but for those with both IGT and IFG, it was 50 per 1000 person-years. BMI for the IGT group was about 29 kg/m2 for both sexes, lower than in the Finnish Diabetes Prevention Study (DPS) (see Chapter 4).
In the UK, the Ely study40 of 170 people with IGT reported that after 4.5 years, 10% progressed to diabetes, 33% remained with IGT and 56.5% regressed to NGT, and in Bedford44 only 16% of those with IGT developed diabetes after 10 years.
In the Danish arm of the ADDITION study,42 the progression rate from IGT to diabetes in the first year was 18.9 per 100 person-years. It was 12 per 100 person-years for isolated IGT and 28 per 100 person-years for those with IGT and IFG. Overall, 13% developed diabetes within 1 year. BMI was 29.5 kg/m2. 42
In a study by Little et al. (1994)45 in Pima Indians, 39% progressed from IGT to diabetes after a mean follow-up of 3.3 years, but the proportion developing diabetes was much higher in those with a raised baseline HbA1c level (13 out of 19; 68%) than in those with a normal baseline HbA1c level (13 out of 47; 28%). The upper limit of normal HbA1c level was taken to be 6%.
Edelstein et al. (1997)46 reviewed progression rates from six studies: Baltimore, Rancho Bernardo, San Antonio, Nauru, San Luis Valley and Pima Indians. IGT was defined as FPG of < 7.8 and 2-hour glucose levels of 7.8–11 mmol/l. The old fasting threshold of 7.8 was used for diagnosing diabetes. The overall progression rate to diabetes was 57 per 1000 person-years, but it varied considerably, from 36 per 1000 person-years in the Baltimore study to 87 per 1000 person-years among the Pima Indians. This variation reflected BMI of 26 kg/m2 in the Baltimore study and 33 kg/m2 among the Pima Indians. Predictors of progression included higher baseline fasting (> 6.1 mmol/l) and 2-hour glucose levels, waist circumference (WC), waist–hip ratio and obesity. Family history was not a predictor of progression.
Older studies often state that patients had IGT, and may not give details on proportions that also have IFG. The AHRQ Evidence Report38 reviewed a large number of studies (including some mentioned above, such as Pima Indians and Naurans) and summarised progression rates as follows:
-
isolated IGT: 1.8–34 per 100 person-years
-
isolated IFG: 1.6–23
-
both IGT and IFG: 10–15.
The D.E.S.I.R. study47 also found that progression was commoner in those with both IGT and IFG. That study also found that, among those with IFG, progression correlated strongly with HbA1c level, as did the report from Yoshinaga and Kosaka (1996),48 which found that the proportions of people with IGT progressing to diabetes ranged from 10% in those with HbA1c levels of < 6.4% to 23% in those with HbA1c levels of 6.4–6.7% and 53% in those with HbA1c levels of ≥ 6.8%.
So we have a wide range of progression rates from IGT to diabetes.
Among the studies, several factors increase the risk of progression: older age (although not consistently), IFG and higher baseline FPG, HbA1c level and BMI. Edelstein et al. (1997),46 in the pooled studies, found that every increase of 4 kg/m2 in BMI increased the risk of diabetes by 1.13. So an increase from overweight at BMI 26 kg/m2 to obese at BMI 30 kg/m2 would increase the prevalence of diabetes by 13%. The presence of IFG (defined then as FPG 6.1 to 7.7 mmol/l) increased the progression rate to 55% compared with 35% in those with FPG levels of between 5.6 and 6.0 mmol/l (calculated from figures in table 4 in Edelstein et al. ). 46
Supporting data on the impact of obesity on diabetes come from another source, the results after bariatric surgery. The Swedish Obesity Surgery Study49 reported that large amounts of weight loss were associated with resolution of diabetes. At 10 years, 7% of the surgical and 24% of the control groups had diabetes.
More recently, Dixon et al. (2008)50 from Australia reported that weight loss after gastric banding in people with T2DM led to almost 21% weight loss, with remission of diabetes in 73% of the banded group.
If impaired glucose regulation can be successfully treated and the risk of progression to diabetes prevented, it would also be of interest to know whether the increased cardiovascular risk in those with IGT and, to a lesser extent, IFG51 may also be improved by non-pharmacological intervention. Studies have reported that the prevalence of CVD,52,53 as well as CVD-related deaths and all-cause mortality,54,55 are higher in those with IGT than in those with NGT. Furthermore, an analysis of 6766 subjects from five Finnish cohorts reported that the incidence of CHD and CVD mortality in IGT was similar to that for newly diagnosed diabetes. Clearly, one of the goals of treating people with IGT would not only be to prevent progression to diabetes, but also to improve their cardiovascular risk profile. The 2005 AHRQ report38 on the diagnosis, prognosis and treatment of IGT and IFG concluded that:
They [IGT and IFG] are also both risk factors for fatal and non-fatal cardiovascular outcomes; however, the evidence is less consistent for these outcomes. The pooled RR ranged from 1.48 to 1.66 for CVD mortality and all-cause mortality in people with IGT, and from 1.19 to 1.28 for nonfatal MI, nonfatal CVD, CVD mortality and all-cause mortality in people with IFG.
The AusDiab (Australian Diabetes, Obesity and Lifestyle Study),56 a large national population based cohort, investigated the relationship between different categories of abnormal glucose metabolism and the risk of all-cause and CVD mortality. The glucose tolerance status was determined in 10,428 participants after a median follow-up of 5.2 years. Compared with those with NGT, the adjusted all-cause mortality hazard ratios (HRs) were 1.6 (95% CI 1.0 to 2.4) for those with IFG and 1.5 for those with IGT (95% CI 1.1 to 2.0). For those with known diabetes mellitus, the HR was 2.3 (95% CI 1.6 to 3.2).
When CVD mortality was measured it was found that the HRs were 2.5 (95% CI 1.2 to 5.1) for those with IFG and 2.6 (95% CI 1.4 to 4.7) for those with known diabetes mellitus. However, the risk for those with IGT was not increased.
The authors concluded that there is a strong association between abnormal glucose metabolism and mortality, and suggested that this condition contributes to a large number of CVD deaths in the general population. Hence, in addition to people with known diabetes mellitus, those with milder forms of abnormal glucose metabolism need to be targeted in order to prevent premature mortality, particularly CVD death.
UK prevalence of impaired glucose tolerance and impaired fasting glucose
A number of factors influence the prevalence of IGT. As with T2DM, increasing age, obesity levels and ethnicity are risk factors for the development of IGT. As discussed previously, the use of different diagnostic criteria means prevalence rates may not be standardised across studies. 57 Two studies in London58,59 reported prevalences of IGT in over-40-year-olds of 3% and 4.1%, respectively, whereas a study conducted in a slightly older age group of 59- to 70-year-olds in Hertfordshire reported a dramatically increased prevalence of 18%. 60 The UK prevalence of IFG was reported as being approximately 17% in men and women aged > 60 years from a sample of over 7000. 61
In terms of variation among ethnic groups, a study in Coventry62 has reported higher prevalence of IGT in Asians than in the Caucasian population; they reported prevalences of 11.2% and 8.9% in Asian men and women, respectively, compared with 2.8% and 4.3% in Caucasian men and women.
Global prevalence of impaired glucose tolerance and impaired fasting glucose
The Diabetes Atlas, 2nd edition, 2002, compiled by the IDF, collated global regional estimates of prevalence of IGT in the 20- to 79-year age group using data from 212 countries and territories. 16 In 2003, the South East Asian region had the highest prevalence rate of IGT (13.2%), compared with 10.2% in Europe, 7.3% in both Africa and South/Central America, 7.0% in North America, 6.8% in the Eastern Mediterranean and Middle East and 5.7% in the Western Pacific region. These figures relate to single, broad age categories and did not divide prevalence according to age group and sex. This publication predicts that by 2025 an additional 7 million people in Europe will have IGT.
Studies in other developed countries, such as Australia, support the observation that the rate of IGT varies among studies. Dunstan et al. (2002)63 reported 9% and 12% in men and women, respectively; however, this was much higher than the 3.4% seen in two (smaller) previous Australian studies (Busselton and Victoria, in 1981 and 1992, respectively). 64,65 In the USA, 15.4% of a cross-section sample of adults aged 40–74 years (tested from 1988 to 1994) had IGT. The potential effect of migration on prevalence of IGT may be illustrated by the results from studies on the Chinese population both in the UK and in China. In a population of 375 Chinese people in Newcastle aged between 25 and 64 years, the prevalence of IGT was 8.0% (4.0% to 12.0%) in men and 16.1% (11.0% to 21.2%) in women66 compared with a large study of 92,187 people in the Da Qing province of China,67 which reported prevalence of 0.9% in both sexes. This shows that prevalence rates in source populations may not always be relevant to migrants to the UK.
The prevalence of IFG is not as widely reported as IGT. Dunstan et al. (2002)63 reported that the prevalence of IFG in Australia was 8% in men and 3.4% in women. In the USA, 33.8% of a cross-section sample of adults aged 40–74 years had IFG; however, more recent estimates from 1999 to 2002 indicate that, among US adults aged ≥ 20 years, 26% had IFG, which was similar to the prevalence in 1988–94 (25%). In India, both IFG (8.7%) and IGT (8.1%) show high prevalence with an overlap in one-third.
Quality of life
Compared with the normal population, people with T2DM have increased morbidity and mortality, resulting in reduced quality of life (QoL). 27 The QoL may be reduced even before diabetes is diagnosed, i.e. in those with IGT. The relationship between impaired glucose metabolism and QoL was examined in Ausdiab,68 a population-based study of 11,247 people of whom 610 had IFG and 1264 had IGT. Compared with those with NGT, those with IGT had significantly adjusted odds ratios (ORs) for the physical functioning [1.44 (1.14 to 1.81)] and social functioning [1.46 (1.20 to 1.77)] dimensions on the Short Form questionnaire-36 items (SF-36). In particular, difficulties were reported in performing tasks such as walking, climbing stairs and bending. In contrast, those with IFG showed no evidence of reduced QoL.
Impairments in QoL in patients with IGT or diabetes relate to specific symptoms including psychological distress and well-being, vitality, sleep disturbance, cognitive activities and sexual functioning. 69 A systematic search of the literature up to December 199770 yielded 58 studies examining QoL and T2DM. QoL as measured by SF-36 appeared to be unrelated to glycaemia control or to improvements or deterioration in glycaemic control within individuals; however, scales that measured feelings of distress associated with symptoms and functioning demonstrated responsiveness to changes in glycaemic control.
Current service provision for impaired glucose regulation
In the UK, specific guidelines for non-pharmacological interventions aimed at those with IGT or IFG do not exist; however, lifestyle advice (physical activity and diet) is the mainstay of current care pathways following diagnosis of impaired glucose regulation. Regular monitoring is recommended to assess whether the person’s glucose tolerance is progressing towards a diagnosis of diabetes or regressing towards normal. Not all trusts in England and Wales have specific protocols for management of impaired glucose regulation, partly because people with impaired glucose regulation are detected not through national screening programmes but opportunistically during investigation for other conditions, such as heart disease, or by practice-based screening or case-finding programmes.
A report by the Scottish Public Health Network71 also noted an absence of organised screening for diabetes.
In Chapter 6 we examine the use of data from the General Practice Research Database (GPRD) to indicate current management of intermediate hyperglycaemia.
There have been discussions in the UK National Screening Committee (NSC) and Department of Health (DH) about screening for T2DM. In its early stages, T2DM can display no symptoms but can be causing damage to small and large blood vessels. As reported in a previous technology assessment report,72 if there is screening for diabetes, we would expect, depending on method used and cut-offs chosen, to detect as many, or more, people with IGT and/or IFG, as with diabetes. If there are effective interventions to reduce diabetes, there would be a stronger case for applying them in IGT and IFG compared with the general population. Furthermore, intervention is more likely to be cost-effective than in lower-risk groups.
The conclusions from the NSC and DH discussions are summarised on the NSC website:73
General population screening should not be offered. Whole population screening has been assessed against the UK NSC criteria and does not meet a number of the criteria.
The UK National Screening Committee has identified the need for a Vascular Risk Management Programme, however, which includes diabetes.
The policy on screening for diabetes will be reviewed in 2012.
An integrated approach to cardiovascular risk reduction is advocated, and the health departments in the four nations will devise their own solutions.
Chapter 2 Modifiable risk factors for type 2 diabetes mellitus
Obesity
Obesity is the main risk factor for T2DM,74 but the mechanisms for this association are poorly understood. Manson and Spelsberg (1994)75 reviewed the current epidemiological evidence for potentially modifiable determinants ofT2DM, including obesity, body fat distribution, physical activity, and dietary factors. Achievable reductions in the risk of T2DM were estimated to be 50–75% by reducing obesity and 30–50% by increasing physical activity. Inconsistent results have been observed between specific dietary factors, including saturated fat, sugar and fibre intake.
We know that a number of risk factors are associated with development of T2DM. Being overweight or obese greatly increases the risk. Colditz et al. (1990)76 followed a cohort of 113,861 women aged between 30 and 55 years and reported a positive correlation between BMI and risk of T2DM; over 90% of cases of T2DM could be attributed to BMIs of ≥ 22 kg/m2, with the risk rising progressively with increasing BMI. (Note that the threshold BMI is below that which is defined as overweight.) Meisinger et al. (2006)77 reported that both overall and abdominal obesity were also strongly related to increased risk of T2DM in a German population of 6012 men and women aged between 35 and 74 years, with the highest risk reported in those participants with a high BMI in combination with a high WC and high waist–hip ratio. Hence, a large number of cases of T2DM may theoretically be preventable. In the UK, the prevalence of obesity and overweight are rising. Zaninotto et al. (2007),78 in an obesity forecast for the DH, reported that nearly one-third of men in England will be obese by 2010, with figures increasing from 4.3 million in 2003 to 6.7 million in 2010. The number of overweight men was forecast to increase slightly, from 8.4 million in 2003 to 8.6 million in 2010. Compared with males, a smaller proportional increase in number of obese females was expected, 4.8 million in 2003 to 6.0 million in 2010, with the number of overweight females expected to decrease slightly from 6.7 million in 2003 to 2.5 million in 2010.
A later study by Colditz et al. (1995)79 examined the relation between adult weight change and the risk of diabetes among middle-aged women. The participants were from the US Nurses Study: 114,281 female registered nurses aged 30–55 years followed from 1976 to 1990.
After adjustment for age, BMI was the dominant predictor of risk for diabetes mellitus. The risk increased with greater BMI, and even women with average weight (BMI 24.0 kg/m2) had an elevated risk compared with those with BMIs of < 22 kg/m2 (Figure 4). 79
FIGURE 4.
Attained BMI and RR for T2DM in US women aged 30–55 years in 1976 and followed for 14 years.
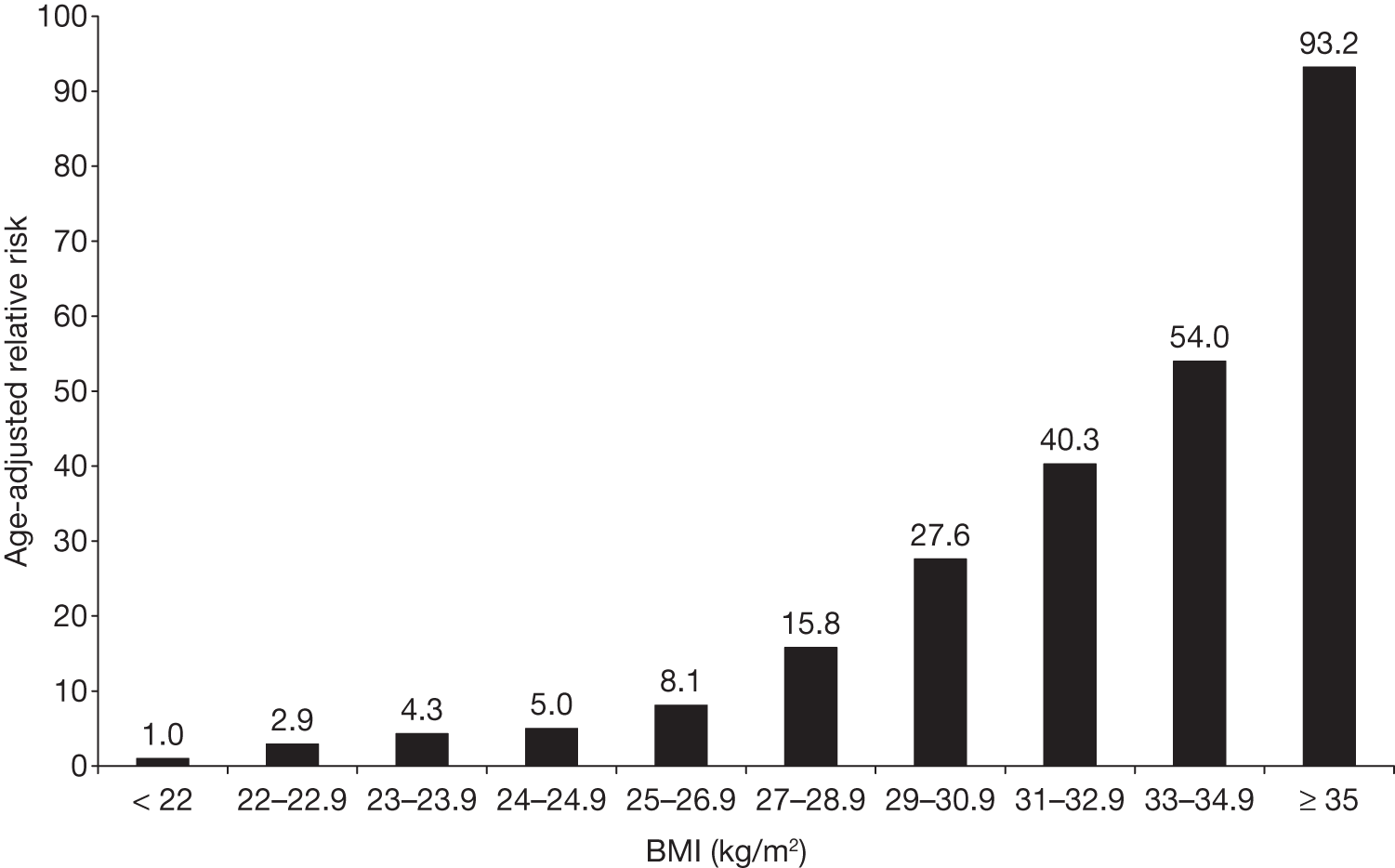
Women of average weight (BMI 24–24.9 kg/m2) had a RR of 5.0 (95% CI 3.6 to 6.6) for T2DM, compared with those with BMIs of < 22 kg/m2. Women with a BMI of ≥ 31.0 kg/m2 had an age-adjusted RR of ≥ 40.0.
Hart et al. (2007),80 from the Renfrew/Paisley study, investigated the relationship between BMI in middle age with development of diabetes mellitus using data from two large prospective studies with around 30 years of follow-up. The participants were 6927 men and 8227 women from the general population study and 3993 men from the collaborative occupational study. Participants were aged 45–64 years and did not have reported diabetes mellitus at the beginning of the study.
Compared with the normal-weight group, the age-adjusted ORs for incident diabetes in the Renfrew/Paisley men were 2.73 (95% CI 2.05 to 3.64) in the overweight and 7.26 (95% CI 5.26 to 10.04) in the obese. The comparable figures for Renfrew/Paisley women were 2.54 (95% CI 1.95 to 3.31) and 5.82 (95% CI 4.41 to 7.68) and for collaborative men were 3.28 (95% CI 2.26 to 4.77) and 9.96 (95% CI 6.29 to 15.77). When the normal, overweight and obese groups were further subdivided, age-adjusted ORs increased as BMI increased. Compared with the lower half of the normal-weight group (BMI 18.5 kg/m2 to < 22.5 kg/m2), even the higher normal-weight group (BMI 22.5 kg/m2 to < 25.0 kg/m2) had between two and three times the risk of developing diabetes.
Assuming a causal relation, the authors estimated that around 60% of cases of diabetes mellitus could be attributed to being above normal weight in these populations.
Similar findings were reported from a large cross-sectional Canadian study by Jiang et al. (2008),81 who reported that in people aged 20–64 years (with an excellent 85% response rate in a national survey) the ORs for diabetes, compared with those with BMIs of < 25 kg/m2, was 2.1 for BMI 25–29 kg/m2 and 4.3 for BMIs of > 30 kg/m2 in men, and 2.6 and 7.7 for the same bands in women. They concluded that > 40% of diabetes in men and > 50% in women could be avoided by keeping to normal weight. The authors note that these proportions are smaller than in some other studies, possibly because of inclusion of people with T1DM, and because of self-reporting biases.
Central adiposity may carry particular risks, even in people with a normal BMI but a large waist. 82 WC, an indicator of central adiposity, is a predictor of risk for developing T2DM. 83
Does ethnicity affect the association between obesity and diabetes?
Diaz et al. (2007)84 used cross-sectional data for eight ethnic groups from the 2003–4 National Health and Nutrition Examination Survey and the 2003–4 Health Survey for England to examine the relationships of BMI, WC and waist–height ratio (WHR) with diabetes risk and across different ethnic groups.
The presence of diabetes was ascertained in 11,624 adults ≥ 20 years old, self-reported as US white, US black, Mexican American, English white, English black, Bangladeshi, Pakistani, Indian or Chinese. Diabetes was defined as self-report of doctor diagnosis or HbA1c level of > 6.1%.The crude prevalence of total diabetes for individuals of < 40 years old did not differ between the ethnic groups, but for individuals ≥ 40 years old there were significant (p < 0.01) differences.
The percentage of individuals with normal BMIs (< 25 kg/m2) who had diabetes was lowest (3.4%) in English white people. Higher prevalences were seen in other ethnic groups (5.0–10.9%). Mexican Americans (10.9%) and Bangladeshis (10.8%) have the highest prevalence of total diabetes, followed by Indians (8.7%) and Pakistanis (8.0%). Receiver operating characteristic curves for the association of total diabetes with BMI, WC and WHR for adults ≥ 40 years old showed that overall, for both genders, WHR and WC have larger areas under the curve than BMI, reflecting a statistically significantly (p < 0.05) higher discriminating ability for these two measures than for BMI. When stratifying by ethnic group it was generally found that WC and WHR generally have larger areas under the curve than BMI. However, there were differences between ethnic groups and between genders. WC and WHR were both significantly better predictors of future diabetes for both men and women in US white people and in women in US black people. In English white people, WHR in men and both WC and WHR were significantly better than BMI. WC and WCR showed no significant difference to BMI in either gender in Bangladeshis and Chinese.
Therefore, it appears that the association of diabetes and BMI may differ among ethnic groups, and different thresholds may be necessary. Adding other anthropomorphic measures, such as WC, may improve risk assessment. An important finding was that diabetes was found in many individuals who were classed as being of normal weight by BMI.
Qualitative aspects of diet
In terms of diet, a high fat intake, rich in saturated fatty acid, and a low intake of dietary fibre, wholegrain cereals and low-glycaemic carbohydrates have been shown to increase the risk of T2DM. 85
The ADA nutritional recommendations86 are shown in Box 1. The letters after each recommendation indicate the level of evidence, with ‘(A)’ the highest and ‘(E)’ the lowest.
-
Among individuals at high risk for developing T2DM, structured programmes that emphasise lifestyle changes that include moderate weight loss (7% body weight) and regular dietary strategies (such as reduced intake of fat) to reduce calories can reduce the risk for developing diabetes and are therefore recommended. (A)
-
Individuals at high risk for T2DM should be encouraged to achieve the US Department of Agriculture (USDA) recommendation for dietary fibre (14 g fibre/1000 kilocalories) and foods containing whole grains (one-half of grain intake). (B)
-
There is not sufficient, consistent information to conclude that low-glycaemic load diets reduce the risk for diabetes. Nevertheless, low-glycaemic index foods that are rich in fibre and other important nutrients are to be encouraged. (E)
-
Observational studies report that moderate alcohol intake may reduce the risk for diabetes, but the data do not support recommending alcohol consumption to individuals at risk of diabetes. (B)
-
Although there are insufficient data at present to warrant any specific recommendations for prevention of T2DM in youth, it is reasonable to apply approaches demonstrated to be effective in adults, as long as nutritional needs for normal growth and development are maintained. (E)
In the technical report that underpinned the ADA recommendations, Franz et al. (2002)87 also considered qualitative aspects of diet. It had been suggested that dietary fat might have an effect independent of calorie intake, perhaps via insulin resistance, but Franz et al. (2002)87 concluded that the main effect was through energy balance, and that if there were any specific metabolic effects of dietary fat then these were minor.
Franz et al. (2002)87 also noted that recent studies have suggested that diets with increased intake of whole grains and fibre may reduce the risk of T2DM, although, again, this might be via an effect on total calorie intake.
Riccardi et al. (2008)88 also reviewed the evidence on low-glycaemic-index foods in a narrative review, and concluded that there had been no intervention studies to assess the value of low-glycaemic-index, high-fibre foods in preventing progression from IGT to diabetes. 88 They did note that some prevention studies included an increase in such foods as part of the intervention package. They also noted that studies of low- glycaemic-index foods suggested benefit in people who already have diabetes.
Carter et al. (2010),89 in a high-quality review of fruit and vegetable intake and incidence of T2DM, found only six studies that were suitable for inclusion. 89 There was considerable heterogeneity and, in meta-analyses of five studies, neither fruit nor vegetables reduced the risk of diabetes. In a further meta-analysis of four of the studies, consumption of green leafy vegetables did appear to reduce the risk, with an OR of 0.86 (0.77 to 0.96) for highest compared with lowest intakes. However, what was meant by green leafy vegetables varied among studies.
Hays et al. (2008),90 as part of a wider review of prevention and treatment of diabetes, provide a useful account of the evidence for 50 food supplements or food types, ranging from alcohol to xanthan gum. 90 They note that, for most, there are insufficient data, with evidence of possible effects in 13 food supplements or food types.
Physical activity
Evidence suggests that people with a physically active lifestyle are less likely to develop insulin resistance, impaired glucose regulation or T2DM. 91,92 Furthermore, physical exercise may prevent progression to T2DM in those with IGT, and reduce the risk of complications in those already diagnosed with T2DM. 93 Potential determinants of exercise-associated prevention of T2DM are the location, frequency, intensity, duration and type (aerobic, resistance or combined) of exercise programme, age of person, dropout rate and the likely adherence to a particular exercise programme.
Description of the ‘FITT principle’:
-
Frequency The frequency refers to the number of times (usually measured per week) that a person exercises.
-
Intensity The intensity refers to the amount of effort required during exercise. Although the metabolic response is influenced by many factors (e.g. nutrition, age, type of exercise and physical condition), work intensity and duration are the most important. 94
-
Time The time refers to how long is spent exercising.
-
Type Exercise is commonly categorised as aerobic or resistance. Aerobic exercise involves the muscular and cardiorespiratory systems (e.g. brisk walking, cycling, swimming, jogging). Resistance exercise involves using muscular strength to move a weight or work against a resistive load (e.g. free weights or weight machines).
Frequency
Evidence suggests that exercise needs to be taken regularly for preventative effect. 95 The effect on insulin sensitivity of a single bout of aerobic exercise lasts between 24 and 72 hours, depending on the duration and intensity. 96 As the duration of increased insulin sensitivity is generally not more than 72 hours, the ADA recommends no more than two consecutive days without aerobic exercise. 94
In men there is a clear dose–response relationship between total energy expenditure in physical activity and the prevention of T2DM. Burchfiel et al. (1995)97 reported that the findings of the Honolulu Heart Program, in a large cohort of over 6800 Japanese American men, indicated a lower risk of diabetes in men who were taking the most exercise. Similarly, in the Physicians’ Health Study, the age-adjusted RR for diabetes gradually fell from 0.77 (CI 0.55 to 1.07) in men who exercised only once a week, when compared with men who did not exercise at all, to 0.58 (CI 0.40 to 0.84) in men who exercised five or more times per week (p = 0.0002 test for trend). 98
The evidence on the frequency of exercise in relation to the risk of diabetes in women is less clear. The Nurses’ Health Study found that regular exercise (at least once a week) was associated with a reduced incidence of T2DM (RR = 0.67, 95% CI 0.6 to 0.75, p < 0.001) but it found no clear trend according to frequency of exercise. 99 Compared with sedentary women (nurses who did not exercise weekly), RRs of T2DM are 0.74 (95% CI 0.6 to 0.91) for those who exercised once a week, 0.55 (95% CI 0.44 to 0.68) for those who exercised twice a week, 0.73 (95% CI 0.59 to 0.90) for those who exercised three times a week, and 0.63 (95% CI 0.53 to 0.75) for those who exercised four or more times a week. 99 In the Nurses’ Health Study, exercise was defined as ‘vigorous’ exercise, i.e. whether participants engaged in ‘any regular activity similar to brisk walking, jogging, bicycling, etc., long enough to work up a sweat’.
Intensity
A Cochrane review100 identified evidence for improvements in glycaemic control over a range of exercise intensities. Analyses conducted using the data from the Nurses’ Health Study cohort found that after controlling for intensity of exercise the RRs of T2DM were 0.67 (95% CI 0.55 to 0.81) for < 1 hour/week and 0.56 (95% CI 0.46 to 0.69) for ≥ 1 hour/week (the test for trend being significant at p < 0.001). 101 In a 14-year cohort of almost 6000 American men, it was found that for every 2.1-megajoule (MJ) (500 calories) increment in weekly energy expenditure, there was a 6% decrease in diabetes risk. 91 The age-adjusted risk of diabetes in American men who played vigorous sport was lower than in men who played moderate sport only. In this study, the age-adjusted risk of diabetes gradually fell from 1.00, 0.90, 0.69 to 0.65, as the levels of sports activity (self-reported at baseline) increased from no sports, moderate sports (energy expenditure 5 kcal/minute), vigorous sports (10 kcal/minute) to a combination of moderate and vigorous sports (p = 0.02 for trend).
Although the majority of evidence suggests that the most vigorous activity is protective, some evidence suggests little additional benefit in exceeding moderate-intensity activity. 95
Moderate- and high-intensity exercise may confer comparable benefits. 102 The Insulin Resistance Atherosclerosis Study reported that increased participation in non-vigorous as well as overall and vigorous physical activity was associated with increased insulin sensitivity. 103 Improvements in insulin sensitivity and glucose tolerance are seen in sedentary individuals who incorporate moderate levels of activity into their lifestyle but the change appears to occur far more slowly and less dramatically compared with higher-intensity activity. 104
In a 10-year cohort study Finnish women who engaged in vigorous activity [a metabolic equivalent of task (MET) value of ≥ 6] less than once a week were found to have a greater age-adjusted risk of diabetes of 2.23 (95% CI 0.95 to 5.23; p = 0.043) than women who engaged in vigorous activity at least once a week. 105 A similar trend was found for men, although the association was weak (age-adjusted RR = 1.63, 95% CI 0.92 to 2.88; p = 0.082). However, the association between intensity of activity and subsequent incidence of diabetes was no longer statistically significant for both genders after adjusting for total amount of activity (total energy expenditure).
In a prospective study of 7577 British men, the risk of diabetes decreases progressively as the intensity of physical activity increased from light to moderate, but the risk was not decreased any further in men who regularly performed vigorous activity. 106 Similar results were reported from a more recent prospective study of 7142 British men. 107 The study reported the age-adjusted risk of developing diabetes was lower in men who engaged in moderate physical activity (RR = 0.53) than in those who engaged in light (RR = 0.66) physical activity. However, no further reduction in risk was seen in men who engaged in moderately vigorous activity (RR = 0.53).
Jeon et al. (2007)20 carried out a systematic review of moderately intense physical activity and the risk of diabetes. ‘Moderately intense’ was defined as requiring a MET task score of 3.0–6.0 but not as high as vigorous (defined as requiring more than six times resting metabolic rate), and studies that involved mixtures of moderate and vigorous were excluded. Using MEDLINE, EMBASE and checking reference lists of retrieved studies, they identified 10 cohort studies involving 310,221 participants, of whom 9367 developed diabetes.
The risk of diabetes was reduced by 31% by moderate physical activity (RR = 0.69; 95% CI 0.58 to 0.83) compared with being sedentary. However, adjusting for BMI reduced the reduction to 17% (RR = 0.83; 95% CI 0.76 to 0.90).
One concern in debates about the prevention of diabetes has been to distinguish between exercise (e.g. an activity taken in addition to activities of daily living) and physical activity (with a broader meaning including all forms of activity). Taking exercise may incur time and financial costs, whereas increasing physical activity could be done as part of daily living, such as simply walking more. Another concern is whether those who take fixed periods of exercise may do less at other times.
Five of the studies in the review by Jeon et al. (2007)20 examined the role of walking, usually defined as at least 2.5 hours a week of brisk walking [e.g. walking at 5.6 km/hour (3.5 miles/hour) on a flat surface requiring 3.8 MET]. 20
Those who walked regularly had a 30% reduction in diabetes (RR = 0.70; 95% CI 0.58 to 0.84), though, again, adjusting for BMI reduced the reduction to 17% (RR = 0.83; 95% CI 0.75 to 0.91). However, this implies that moderate-intensity physical activity can reduce progression to diabetes even in those who do not lose weight. Compared with the minimal amount of walking in the reference category, the highest category was at least 10 MET hours/week, which is equivalent to ∼2.5 hours/week of brisk walking.
Time
The review by Bassuk and Manson (2005)108 identified evidence that shows physical activity sessions lasting as little as 10 minutes can improve the metabolic and cardiovascular risk profile of otherwise sedentary individuals. 102 The US Diabetes Prevention Program (DPP) reported a 58% decrease in the development of diabetes in high-risk individuals who exercised for a minimum of 150 minutes per week (approximately 20 minutes per day).
The same total amount of exercise taken in several instalments appeared to be more effective in a small study by Eriksen et al. (2007). 109 Eighteen patients with T2DM were randomised to either three 10-minute sessions or one 30-minute session daily, of home-based cycle training, for 5 weeks. Both groups got fitter, but the 3 × 10-minute group had lower fasting and post-load glucose levels. The authors speculate that more energy is expended in short bursts.
Type
It is evident that alternative forms of physical activity that produce similar metabolic improvements to aerobic exercise may also be beneficial in the management of T2DM. The review by Eves and Plotnikoff (2006)110 identified evidence to suggest that resistance training may be effective in improving glycaemic control. They reported that results of three randomised controlled trials (RCTs) showed the effectiveness of resistance exercise to improve insulin sensitivity was comparable with that reported for aerobic exercise. These three RCTs compared resistance training with control (either flexibility exercise or no exercise), but the review suggests that the reported effect was comparable with that reported for aerobic exercise in other studies. There is also some evidence that the duration of effect of resistance training on insulin sensitivity is somewhat longer, perhaps as some of its effects are mediated by increases in muscle mass. 111 One RCT randomised 43 individuals with T2DM to either resistance or aerobic training for 4 months. 112 The authors reported that HbA1c level was significantly reduced with resistance training (from 8.3% ± 1.7% to 7.1% ± 0.2%; p < 0.001), but not with aerobic training (from 7.7% ± 0.3% to 7.4% ± 0.3%; p > 0.05). Fasting blood glucose and insulin resistance were also reduced and lipid profile improved with resistance but not aerobic training.
Although both aerobic and resistance exercise increase glucose disposal, resistance exercise tends to increase muscle mass and therefore glucose storage space. 94 The review by Eves and Plotnikoff (2006)110 found that increases in skeletal muscle mass are related to decreases in HbA1c level. 110 This supports the hypothesis that resistance training improves glycaemic control by augmenting the skeletal muscle storage of glucose. In one of the studies cited in the Eves and Plotnikoff review (2006),110 Castaneda et al. (2002)113 reported that resistance training significantly improved glycaemic control, increased fat-free mass and reduced abdominal adiposity, whereas body weight, and total, arm and leg fat mass did not change between intervention and control groups. The review also found ‘no significant relationship between the improvements in insulin sensitivity and the losses in either visceral or subcutaneous fat’ with regard to resistance training. Improvements to functional status and body composition often occur more rapidly with resistance compared with aerobic training, and therefore might be more immediately rewarding. ‘As there is substantial evidence that supports both aerobic and resistance training, it is possible that a combination of both may be the optimal intervention’. 110
A recent meta-analysis of 27 studies to examine the effects of different modes of exercise training on measures of glucose control concluded that all forms of exercise training (aerobic, resistance and combined) produce reductions in HbA1c levels (reductions 0.8% ± 0.3%), a measure of glucose control, with combined training generally being more effective than aerobic or resistance training alone. 114 (Note: These reductions are as great as seen with many glucose-lowering drugs. 30) The authors emphasise that the effects of exercise are similar to those of dietary and drug interventions and the combined effects might be moderate or large. Furthermore, evidence also suggested small beneficial effects on related risk factors for complications of diabetes and those with more severe disease were found to benefit most.
Sigal et al. (2007)115 carried out a RCT in 251 people with T2DM, aged 39–70 years, with four arms:
-
aerobic training
-
resistance training
-
both the above
-
a sedentary control group.
Exercise was carried out three times weekly for 22 weeks, in community facilities. The primary outcome was HbA1c level at 6 months. Other outcomes included plasma lipids and blood pressure. HbA1c level was reduced in the aerobic group by 0.51% (p = 0.007), in the resistance group by 0.38% (p = 0.038) and in the combined group by 0.9% compared with the control subjects. The bigger reductions in the combined group of 0.46% compared with aerobics alone, and 0.59% compared with resistance alone, were also statistically significant (p = 0.014 and 0.001, respectively). This may simply reflect the greater amount of exercise in the combined group. No significant differences were seen in blood pressure and lipids at 6 months.
Some of the conflicting results above may be related to baseline risk. Gill and Cooper (2008)116 carried out a systematic review of both observational studies and prevention trials. In brief, they noted that the marked reduction in diabetes incidence was attenuated once adjusted for BMI but that even after such adjustment physical activity reduced the risk by 20–30%. They also examined the evidence for thresholds, finding that there was uncertainty about whether there was a minimum level to achieve benefit, especially in women, but also that there appeared to be a level above which additional activity conferred no additional benefit. However, perhaps the most useful aspect of their review was the examination of the benefit according to baseline risk. Thus, in lean individuals with very low risk of diabetes, physical activity made little difference, whereas in people at high risk (high BMI, family history), physical activity was clearly beneficial, although especially so if combined with weight loss. They suggest a BMI threshold of 27 kg/m2 (23 kg/m2 in those of Asian descent) for prescribing higher levels of physical activity than the current health guidelines recommend (150 minutes of moderate activity or 60–90 minutes of vigorous activity per week).
Adherence
It is often those who would benefit the most from aerobic exercise that have the greatest difficulty performing it. Adherence with an exercise programme is important for successful outcomes. However, there are considerable problems of adherence with formal exercise programmes. 117 Around 50% of participants commonly drop out of supervised exercise programmes, and as many as 90% of participants drop out after 1 year. 118 Even though the importance of exercise is stressed more to diabetic people than non-diabetic individuals, diabetic individuals are no more likely to exercise than non-diabetic individuals. 117 Those more likely to adhere to exercise programmes appear to be elderly individuals, females, self-referred patients and patients participating in programmes along with a spouse. 117 The type of exercise programme also affects adherence, as described below.
Adherence and frequency
Lack of time is a common barrier to carrying out exercise and maintaining activity levels. As expected, King et al. (1995)119 found better adherence to a programme of exercise on 3–4 days per week compared with 5–7 days per week. However, a study by Perri et al. (2002)120 randomised 379 sedentary, non-diabetic, men and women to walk 30 minutes per day at a frequency of 3–4 or 5–7 days per week and an intensity of 45–55% or 65–75% of the maximum heart rate. The results showed that a prescription of higher frequency walking resulted in a greater amount of exercise (92 minutes per week) compared with moderate frequency (60 minutes per week) over a 6-month period. Similar levels of adherence were observed for exercise prescribed at a moderate (61%) and higher frequency (63%).
Adherence and intensity
A meta-analysis of 127 intervention studies found better adherence to lower-intensity activities than higher-intensity activities. 121 The effect size was found to be far greater for physical activities carried out at a low intensity (r = 0.94; 95% CI 0.91 to 0.97) than with higher intensities (r = 0.24; 95% CI 0.06 to 0.41). Results from a multiple linear regression analysis also showed that interventions emphasising physical activity of low intensity showed better adherence than those using higher-intensity activities.
The greater risk of injury occurrence in high-intensity activity might have a strong negative influence on adherence to high-intensity physical activity compared with lower-intensity activity. In some cases, the higher self-reported injuries during higher-intensity activity may represent ‘excuses’ for not completing an activity that involved maximal effort. 120
Perri et al. (2002)120 found that a prescription for walking at moderate intensity produced both better adherence to the exercise prescription (66% vs 58%) and a greater amount of exercise completed (85 minutes vs 72 minutes per week) compared with a prescription for walking at a higher intensity. McGinnis (2002)122 stressed the importance of incorporating physical activity into one’s lifestyle as it then has the potential to be maintained for years. A 3-year RCT of walking, with a 10-year follow-up, concluded that moderate-intensity activities such as walking are much more likely to be maintained over the years by people of many different ethnic and economic groups than high-intensity sport. 123
Motivating people to exercise is usually a challenge. The Cochrane review by Thomas et al. (2006)100 found that a gradual increase in exercise, starting from low-intensity and increasing to moderate-intensity exercise, performed regularly, may be a more successful approach to incorporate exercise into daily lives on a long-term basis than introducing more intense levels of exercise from the outset, which will be difficult to maintain in the longer term.
Adherence and time
Many people find it easier to conduct fewer longer exercise sessions than five or more weekly sessions. 94 The recommendations from the US Surgeon General’s report state that most people should accumulate > 30 minutes of moderate-intensity activity on most, ideally all, days of the week. 124
A study by Jakicic et al. (1995)125 reported that exercising in multiple short bouts (i.e. 10 minutes) improved adherence compared with single longer daily bouts of exercise.
Adherence and type
The Cochrane review by Thomas et al. (2006)100 reported that varying the type of exercise and having a choice of exercise activity may be an important factor in ensuring adherence with exercise programmes after the intervention period. The easier the exercise is to maintain, the more likely it is that people will do it. As Avenell et al. (2006)126 suggest, the message should be to ‘reduce inactivity’ rather than ‘do more exercise’. The Cochrane review concluded that dedicated exercise regimes should be prescribed in addition to lifestyle-based incidental types of activities for everyday life, such as cycling rather than using the car, using stairs instead of a lift and carrying groceries instead of pushing them in a trolley. 100
The success of exercise programmes is also highly dependent on adherence to a particular regimen. 120,127,128 For example, Perri et al. (2002)120 assessed 379 sedentary adults who were randomly assigned to walk 30 minutes per day at a frequency of either 3–4 or 5–7 days per week, at an intensity of either 45–55% or 65–75% of maximum heart rate reserve. 120 The study reported that prescribing a higher frequency increased the accumulation of exercise without a decline in adherence, whereas prescribing a higher intensity decreased adherence and resulted in the completion of less exercise. Similarly, Sallis et al. (1986)127 reported that, compared with vigorous exercise, rates of exercise adoption were higher and 1-year dropout rates lower for moderate activities.
Supportive evidence was also reported by Dishman and Buckworth (1996),121 who found that, compared with moderate- or high-intensity activities, greater increases in physical activity were seen for low-intensity activities. Surveys also indicate a preference for those activities that can be performed as an individual rather than in more structured settings. 129
In conclusion, easily adoptable exercise regimens of moderate intensity are therefore probably more likely to be successful that those that are of high intensity.
One issue is whether improvement in physical fitness can improve health even if no weight loss occurs. The effects on mortality were examined in the US Nurses’ Health Study by Hu et al. (2004). 130 They defined ‘lean’ as a BMI of < 25 kg/m2 and ‘obese’ as a BMI of ≥ 3 kg/m2, and ‘active’ as spending 3.5 or more hours on exercise each week. Taking lean active women as the reference case, with RR of 1.0, gave the following RRs;
-
lean and active: RR = 1
-
lean but inactive: RR = 1.55
-
obese and active: RR = 1.9
-
obese and inactive: RR = 2.4.
At all levels of BMI, physical activity was beneficial but it could not fully offset the higher risks imposed by obesity.
Summary
Physical activity protects against the development of diabetes. This applies to all forms of exercise. The benefit increases with frequency. However, the evidence on intensity is less clear and there may be an upper threshold above which increased intensity confers no extra benefit in terms of incidence of diabetes.
Adherence is important, and there may be trade-offs between amount and type, and adherence. Greater net benefits may come from a modestly effective form of activity with good compliance, compared with a more vigorous form of exercise with poorer compliance.
Current recommendations
The ADA made the following recommendations on physical activity for prevention of T2DM in June 2006:131
-
In people with IGT, a programme of weight control is recommended, including at least 150 minutes/week of moderate to vigorous physical activity and a healthy diet with modest energy restriction (level of evidence A).
-
To improve glycaemic control, assist with weight maintenance and reduce risk of CVD, at least 150 minutes/week of moderate-intensity aerobic physical activity (40–60% of VO2max max or 50–70% of maximum heart rate) and/or at least 90 minutes/week of vigorous aerobic exercise (> 60% of VO2max or > 70% of maximum heart rate). Physical activity should be distributed over at least 3 days/week and with no more than two consecutive days without physical activity (A).
-
Performing ≥ 4 hours/week of moderate to vigorous aerobic and/or resistance exercise or physical activity is associated with greater CVD risk reduction than lower volumes of activity. (B)
-
For long-term maintenance of major weight loss (> 13.6 kg/30 lb), larger volumes of exercise or (7 hours/week of moderate or vigorous aerobic physical activity may be helpful). (B)
-
In the absence of contraindications, people with T2DM should be encouraged to perform resistance exercise three times a week, targeting all major muscle groups, progressing to three sets of 8–10 repetitions at a weight that cannot be lifted more than 8–10 times. (A)
Physical activity and exercise patterns
It may be useful to distinguish between physical activity, which includes both activity as part of daily life (e.g. walking or cycling to work, occupational activity) and exercise, from the sorts of exercise that require participation in sports or other activities which are not part of everyday life (e.g. hillwalking, going to gyms).
If we try to categorise physical activity patterns over a lifetime, we would need quite a complicated classification, including:
-
always active – active at school and continuing throughout life, albeit with different sports at different ages
-
never active after school, and possibly not even at school
-
active in younger ages, but stopping – girls probably earlier than boys; boys may go on to play sports, such as football or rugby, into their late 20s or longer
-
active in younger age groups then stopping because of work or family commitments, but then starting again for social reasons or after retirement
-
as for (4) but starting again for health reasons
-
mixed stops and starts.
For our purposes, we could think a slow progress towards IGT over many years, with groups (2) and (3) being the most at risk.
Given that the average age at diagnosis of IGT may be in the fifties, we may be identifying a group of people who are not only inactive and overweight, but also have been so for decades. The implication of that is that unhealthy habits may be hard to change. It may be unrealistic to expect them to take up exercise, and the main thrust may have to be to try to encourage an increase in physical activity, such as walking.
This raises two issues. The first is about efficacy compared with effectiveness, with efficacy referring to results in trials (volunteers, perhaps high level of input) and effectiveness referring to results in real life. The second is whether modest increases in physical activity, such as walking, are sufficient to reduce diabetes. Is there a threshold level which must be exceeded to get an effect, or is there a continuum in which all activity has some effect? It may be that strenuous exercise is better metabolically, but modest will achieve greater uptake. A review by the Health Development Agency concluded that132 ‘Interventions that promote moderate-intensity physical activity, particularly walking, and are not facility dependent, are also associated with longer-term changes in behaviour.’
Di Loreto et al. (2003)133 addressed the issues of real-life applicability and achieving adherence in a randomised trial of a carefully designed intervention to promote physical activity in unselected people with T2DM. Having noted the benefits of exercise in diabetes, they go on to comment that133 ‘many physicians do not spend time making an effort to convince type 2 diabetic subjects to exercise, probably because older adults comply poorly with their recommendations.’
They then designed an intervention that was based on a number of factors, including motivation, self-efficacy, family support, removing impediments, enjoyment, and checking on understanding of benefits. They took care not to suggest radical increase in exercise, but used a staged approach so that people were not discouraged. Nevertheless, these small steps achieved, over time, a considerable increase in physical activity. Patients might start with only a 20-minute walk daily but would increase this at weekly intervals. From a baseline activity level of about one MET hour/week, the intervention group increased to 27 METs. The control group were given standard advice, including on exercise, but increased to only four METs/week. The target level of over 10 METs hours/week was achieved by 69% of the intervention group and 18% of the control group. One weakness in the report is that the method of randomisation was not given. Another is that results were given at 24 months, when it appeared that the intervention was continuing. It would be interesting to know if the increase continued after it was stopped.
The authors attribute success partly to their own enthusiasm for exercise – the counselling was given by physically active physicians. They also emphasise the importance of not deterring people: ‘This step-by-step approach intentionally avoided goals that the patient was unable to imagine attaining’.
Ogilvie et al. (2007)134 carried out a systematic review of interventions to promote walking but found a wide range of results, and many very short-term studies. It might have been better to exclude short-term studies. Ogilvy et al. (2007),134 noting the short durations, commented that the review might be showing proof of efficacy rather than effectiveness. But they did conclude that some interventions appeared to increase walking time by 30 to 60 minutes per week. The keys to success seemed to be targeting (usually of people motivated to try to increase activity) and tailoring. However, the authors comment that ‘Few studies in this review found unequivocal improvements in health, risk factors for disease, or even overall levels of physical activity’.
Chapter 3 Ethnicity
Type 2 diabetes mellitus in South Asians
Terminology is important in describing ethnic differences in diabetes. The term ‘South Asian’ is used nowadays to refer to people whose ancestry is in the countries of the Indian subcontinent, including India, Pakistan, Bangladesh and Sri Lanka. 135 It distinguishes these people from those from other parts of Asia. However, South Asian therefore still covers a very wide range of ethnic and cultural groups, and we need to be careful in not extrapolating from, for example, Punjabis to Bangladeshis. Another complicating factor is that there may be considerable intergenerational differences in lifestyles from those born in the subcontinent to their grandchildren born in the UK.
Over 2 million South Asian people (India, Bangladesh and Pakistan) or their descendants have settled in the UK, representing 4% of the total population. Studies have shown a higher prevalence of T2DM in South Asians than in indigenous populations,84,136–138 in addition to higher levels of ischaemic and cardiovascular heart disease, and premature atherosclerosis leading to higher morbidity and mortality rates. 138 Population projections on percentage change in the prevalence of diabetes between 1991 and 2011 among 45- to 74-year-olds from Greater London139 estimated that there would be a slight decline in the numbers affected by diabetes (–5.3% in men and –11.1% in women) in the white European population. However, diabetes is projected to increase in the Indian population (83% in men and 136.8% in women) and in Afro-Caribbeans (33.5% in men and 79.4% in women).
Although it is well established that immigrant ethnic minority populations have higher prevalences of type 2 DM, it is important to identify the risk factors and if generational and regional differences (e.g. different regions within India) existed among them.
Prevalence of type 2 diabetes mellitus in South Asians
We found a number of studies on ethnic differences in prevalence. These are summarised in Appendix 1. Most studies reported higher prevalence of T2DM in South Asians and African immigrants than in Europeans, the exception being that of Davies et al. in 1999140 (30% in Asians and 34% in Caucasians, p < 0.001). The age-adjusted prevalence in South Asians ranged from 4.6% in 1985 [Mather and Keen (1985)141] to 25% in 1997 compared with 1.2% in 1985 and 6.7% in 1997 among Caucasians. 136 A more recent study by Riste et al. (2001)142 reported an age-adjusted prevalence of 33% in Pakistanis compared with 20% in Europeans. Seven times as many Asians as Europeans had been diagnosed between the ages of 30 and 54 years but similar numbers were diagnosed in those aged < 25 years. In a review, Chowdhury and Hitman (2007)143 noted the fourfold risk of T2DM in South Asians compared with white people, and that South Asians had a 1 : 3 risk of developing diabetes in their lifetimes.
South Asians were significantly younger than Europeans at first recorded diagnosis of diabetes (average of 14 years) and had significantly lower BMIs. 144 Median time to referral to hospital clinic is longer for South Asians. 144–146 Differences in fasting glucose concentration, insulin levels and insulin resistance are well advanced by adolescence according to Whincup et al. (2005),147 with a prevalence of IFG markedly higher in 13- to 16-year-old South Asians compared with European young adults (5.6 vs 1.5%; OR 3.9; 95% CI 1.4 to 10.9; p = 0.004).
Diabetes is more common in men than in women in all of the ethnic minorities except Pakistani women. 7,58,62,136,142,148,149 The Southhall study showed that males have high prevalence compared with females despite lower BMIs, which was not observed in the European population. 141 However, the prevalence of IGT seems to be higher in females than males. Simmons et al. (1991)62 reported a 9.8% prevalence of IGT in of males compared with 11.2% in females, and Cruickshank et al. (1991)149 reported a prevalence of IGT of 25% in Gujarati Indian males compared with 32% in Gujarati females.
Within South Asians there is great variation among different subgroups. In a study by Simmons et al. (1992),150 Punjabi Sikh males had a higher prevalence of diabetes than females (89/1000 in males vs 75/1000 in females), but among Hindus (Gujarati and Punjabi) and especially Muslims (Pakistani and Gujarati), prevalence was higher in women. 150 However, these differences were statistically non-significant. Gujarati Muslims had the highest prevalence of diabetes mellitus in this study, despite having a similar diet to Gujarati Hindus and Pakistani Muslims. Simmons et al. (1992)150 wondered if that could be due to either previously diagnosed diabetes or frequent consanguineous marriages in this community.
The prevalence of diabetes may be underestimated, depending on the criteria used to diagnose diabetes in South Asians. A study by Harris et al. (2000)151 assessed the impact of new ADA and WHO diagnosis criteria for diabetes on subjects from three ethnic groups (South Asians, Caucasians and those of African descent) and showed that in South Asians, overall 31/340 (9.1%) qualified for newly diagnosed diabetes mellitus using WHO criteria compared with 17/340(5.0%) by ADA criteria. 151 Overall, the proportion of individuals with impaired glucose homeostasis was 13.7% with WHO criteria (IGT + IFG) compared with 3.8% with ADA criteria (IFG). This difference was greatest for South Asians with 20.3% with impaired glucose regulation under full WHO criteria compared with 4.4% under ADA criteria. Thus, failure to identify people with IGT under the ADA criteria could underestimate the scale of the problem and be detrimental given the greater risk of CHD in people with IGT, especially South Asians.
Diabetes associated risk factors in South Asians
Waist–hip ratio
Waist–hip ratio, reflecting central obesity, is higher in South Asians than Europeans. 58,142,145,149,152,153 Logistic regression analysis of univariate association between glucose tolerance and various anthropometric variables showed a stronger association with waist–hip ratio than BMI. However, the waist–hip ratios were not different among any of the South Asian subgroups. 137,152 Conversely, Yajnik et al. (2008)154 found that once adiposity had been taken into account, waist measurement did not contribute anything further. They studied insulin resistance and adiposity in groups of rural, poor urban and middle-class urban men in or near the city of Pune. The found that adiposity explained two-thirds of the differences in insulin resistance, which was commonest among the prosperous middle-class men. Half of the urban middle-class men were centrally obese. A third were overweight or obese if a BMI threshold of 25 kg/m2 was used. However, that threshold may be too high for South Asians. The mean BMI in the often adipose and centrally obese middle-class men was only 23.6 kg/m2.
Cholesterol
South Asians, in particular females, have lower levels of total cholesterol58,136,145,155 and high-density lipoprotein cholesterol-C (HDL-C)58,153 than Europeans. Cappuccio et al. (1997)136 reported 67% of South Asians having total cholesterol level of > 5.2 mmol/l compared with 78% of the Europeans. However, more recent studies have reported increasing levels of total cholesterol in South Asians and the difference narrowing, so that there was no significant difference in cholesterol between South Asians and Europeans. 144,152,156 Among the subgroups of Indians, McKeigue et al. (1991)58 reported high levels of total cholesterol in Sikhs (6.06 mmol/l) and the lowest levels in Gujarati Hindus (5.45 mmol/l; p < 0.001). However, the HDL-C is high among the Sikhs (1.22 mmol/l) and lowest in Muslim men (1.04 mmol/l; p < 0.001).
Diet
Cultural factors play a part in dietary habits. Grace et al. (2008)157 found that among the Bangladeshi population in Tower Hamlets, it was regarded wrong to serve healthier curries with reduced fat content to guests, because it might be seen as ‘inhospitable’. The same study noted that the community was well aware of diabetes and its risks. 157
Effects of migration on risk factors
Studies have also compared the prevalence of diabetes and its risk factors in populations from the same community who have emigrated to westernised cultures, with those who still live in their own countries. Ramaiya et al. (1995)158 reported higher prevalence of IGT (in both sexes) newly diagnosed diabetes (in women) and hypercholesterolaemia (in men) in the Asian Indian Bhatia community from Gujarat living in Tanzania, and the same community, living in the UK. A more recent study by Patel et al. (2006)159 studied the cardiovascular risk factors among Gujaratis living in Britain and compared it with the non-migrant Gujaratis in India. Although there was no significant difference in prevalence of diabetes, the most striking factor between the migrants and indigenous population was on nutrition. There was increased dietary energy intake in the migrants with significant contribution by fat intake. Serum cholesterol, triglycerides, BMI, and waist–hip ratio were all higher in the Gujarati immigrants to the UK than those in India. This illustrates the risks imposed by migration and cultural adaptation among people from the same cultural, geographic and genetic background.
Physical activity
Physical inactivity is identified as an important risk factor for diabetes and CHD. Physical activity is much lower in South Asians than in Europeans. 142,155,158,160 Fischbacher et al. (2004)160 reviewed 12 studies of levels of physical activity and fitness in South Asians (Indians, Pakistanis and Bangladeshis). Results in adults consistently showed lower levels of physical activity in South Asians than in the general population or white groups (South Asians’ activity levels were ∼ 50–75% of those of Europeans), regardless of the diverse sampling methods, mode of physical activity assessment and criteria for activity levels.
However, there were differences among the Asian groups. Fischbacher et al. (2004)160 reported that Bangladeshis had the lowest physical activity level. Similarly, the Health Survey for England7 reported that Indian, Pakistani and Bangladeshi men were 14%, 30% and 45% less likely than the general population to meet current guidelines for physical activity.
Greater differences were found in activity levels between South Asians groups and the general population in women than in men. Bangladeshi women had very low levels of physical activity compared with the general population, with only 21% achieving recommended levels of physical activity. A lower level of activity was also reported in older respondents in all ethnic groups. This difference was greater among Bangladeshi men and women and Indian women than among corresponding general population groups (13% and 18%, respectively, in Bangladeshi and Indian women aged 16–34 years compared with 1% and 2%, respectively, in the ≥ 55 years age group and 26% and 11%, respectively, in the general population). Fast and brisk walking, and participation in sports and exercise, were less commonly reported in South Asian women.
This reduced level of physical activity is in part due to cultural norms. Grace et al. (2008),157 in a qualitative study among Bangladeshi women in Tower Hamlets, found that exercise (as opposed to physical activity, such as walking) was seen as alien to the culture, and inappropriate behaviour, especially among women and older people. This was less so in later generations. The reasons included views about appropriate dress.
Comparing immigrants from the same community (the Bhatia community in Gujarat) in Tanzania and the UK, diabetes mellitus and cardiovascular risk factors except hypertension were high in people living in Tanzania compared with immigrants to the UK. The most striking difference was the levels of physical activity in the communities despite similar BMIs. Sedentary lifestyle was observed in 63–84% of Gujaratis in Tanzania compared with 26–29% in the UK. High levels of physical activity were observed in 8% of Gujaratis in Tanzania compared with 36% in the UK (p < 0.001). 158
An older study by Samanta et al. (1991)155 from Leicester noted a marked difference in activity between South Asians and white people: 8% active compared with 33% active.
Carroll et al. (2002)161 noted that the barriers to physical activity in South Asian Muslim women were culture, language, religion, age and socioeconomic status. However, a pilot scheme of ‘exercise on prescription’ suggested that these barriers could be overcome.
Smoking and alcohol
Two studies have reported that smoking and alcohol consumption levels are lower in South Asians than in Europeans,58,146 although Bhopal et al. (2004)162 noted that Bangladeshi men have high smoking rates (57%) compared with 32% in Pakistani and 14% in Indian men. A study by Chowdhury et al. (2006)156 reported a non-significant difference in smoking between South Asians and European (23.6% vs 22%; p = 0.46). Among Indians, smoking is more prevalent among Muslims and lower in Hindus and Sikhs because of their religious beliefs. 58,136
Insulin resistance
Many of the features noted above are related to insulin resistance. In his 2007 Bloom Lecture, Felix Burden (2007)163 summarises the effects of the increased insulin resistance in South Asians as:
-
early development of IGT (but not so much IFG)
-
more rapid transition from IGT to diabetes
-
earlier onset of diabetes
-
higher prevalence of diabetes.
Knight et al. (1992)164 compared male manual workers of Asian origin (64% Muslims from Pakistan and the Punjab, 31% mainly Gujarati Hindus) and non-Asian origin in two textile factories in Bradford. Diabetes was observed in 13% of Asian and 4.5% of non-Asians. 164 Insulin resistance was much commoner in the Asians. The serum insulin level at 2 hours after glucose load in Asians was double that in white people. Asians had more central obesity, but lower BMIs (24 kg/m2 vs 25 kg/m2).
Glycaemic control in South Asians
The consequences of diabetes may be worse in South Asians. Mukhopadhya assessed glycaemic control in South Asian diabetic people and reported similar initial levels between South Asians and Europeans. 144 However, after 5 years, HbA1c level deterioration was significantly greater in South Asians than in Europeans (0.23%/year vs 0.16%/year). HbA1c level was 60–113% higher in South Asians, even after adjusting for age, sex, baseline HbA1c level, weight change, time to referral and duration of diabetes. Control of blood pressure and cholesterol levels were also poorer in South Asians. 144,165
The reasons for poor glycaemic control could be problems with compliance and levels of education, language barriers and fatalistic attitudes towards disease status. 166,167 A cross-sectional survey to assess the understanding of diabetes in the South Asian community reported that 28% of patients did not understand the term diabetes and 13% could not provide any description; 22% were unable to suggest any risk factor; and 20% could not give a preventative measure. 168 Among the ethnic groups, the Bangladeshis had lower levels of knowledge and education than Indians and Pakistanis. However, this may be due to differences in educational attainment rather than ethnicity. Thirty per cent of Bangladeshi males had never attended school, compared with 0% of Indians and Pakistanis, and 18% of Bangladeshi females did not attend school compared with 5% of Indians and Pakistanis.
Complications of diabetes mellitus in South Asians
Diabetes-related complications are more common in South Asians than in Europeans. 145,146,153,155,169,170 The age at diagnosis of diabetes is about 10 years lower in South Asians. 143
Diabetic patients from the Southhall survey were followed up after 11 years to ascertain mortality and morbidity data. 169 All-cause mortality rate was higher in South Asians than in Europeans among younger age groups. This study also reported that mortality from circulatory disease and ischaemic heart disease was significantly higher in South Asians than in Europeans in those aged 30–64 years at baseline (risk ratios of 1.8 and 2.0, respectively; p < 0.05). CVD was the commonest cause of death in both groups, but accounted for 77% of all deaths in South Asians. Morbidity due to MI (20% vs 8%, p = 0.001) and retinopathy (36% vs 27%; p = 0.03) was higher in South Asians but no significant differences were found in stroke, hypertension and amputation.
At the time of diagnosis of early-onset T2DM, Chowdhury and Lasker (2002)153 reported that South Asians present with significantly higher prevalences than Europeans of macrovascular disease (15.7% vs 9.4%, p < 0.001), microvascular complications (27.3% vs 16.5%; p < 0.001) and higher absolute 10-year cardiovascular risk (16.9% vs 13.7%; p < 0.001). 153 In contrast, the UKPDS found no significant differences in the prevalence of MI, CVA or retinopathy between South Asians and Europeans. 145
Predictors of diabetes in South Asians
South Asians appear to have a more central distribution of fat than Europeans. 58,142,145,149,152,153 WHR is highly correlated with glucose intolerance in South Asians than any skinfold measurements. 137,142
Diaz et al. (2007)84 compared the optimum cut-off for BMI for predicting diabetes in several ethnic groups, using data from the 2003–4 Health Survey for England. 7 The crude prevalences of diabetes in those aged > 40 years were 9% in English white people, 23% in English Indians, 38% in Pakistanis, 44% in Bangladeshis and 18% in Chinese. The optimum cut-points of BMI for predicting diabetes were 28 kg/m2 for English white people, 29 kg/m2 for English black people, 26.5 kg/m2 for Indians, 25 kg/m2 for Pakistanis and 24 kg/m2 for Bangladeshis.
Cruickshank et al. (1991)149 reported that mean fasting and 2-hour C-peptide concentration, and 2-hour insulin concentration were significantly higher in Gujarati Indians than white people and Afro-Caribbean people (p < 0.001). 149 No significant differences were found between the white and Afro-Caribbean groups.
Progression from impaired glucose tolerance to diabetes
The Newcastle Heart Project followed up South Asian (Indian, Pakistani and Bangladeshi) and European individuals with transient IGT (IGT on one OGTT with a normal repeat OGTT 2–6 weeks later), persistent IGT (IGT on the initial and on the repeat OGTT) and NGT. The individuals were aged 25–76 years and median follow-up was 4.4 years in Europeans and 2.8 years in South Asians. 171,172
South Asians were significantly more likely than Europeans to have persistent IGT (77% vs 48%). Also, in South Asians with transient IGT, the number of new cases of diabetes per 1000 person-years of follow-up was 146.8 (95% CI 40.0 to 375.9), whereas for Europeans it was 29.3 (95% CI 6.1 to 85.7). The equivalent figures for those with persistent IGT were 109.5 (95% CI 54.7 to 195.9) for South Asians and 66.5 (95% CI 24.4 to 144.8) for Europeans.
Therefore, South Asians were more likely than Europeans to have persistent IGT and those with transient IGT also had a high risk of progression to diabetes.
In the Indian Diabetes Prevention study by Ramachandran et al. (2006),173 full details of which are given later, 55% of the control group had developed diabetes by 3 years of follow-up.
Prevalence of diabetes and other risk factors in populations with African origin
In the UK, people of African descent have the second-highest levels of T2DM after South Asians. The age at onset among Afro-Caribbeans is slightly higher than among Europeans (42 vs 39 years), more so for women;174 in contrast, onset of T2DM among Asians typically occurs at a younger age.
The crude prevalence rate of people with West Indian origin but living in the UK is 2.2%, with higher rates of 7.93% in 45- to 65-year-olds. 175 Studies have compared the glucose tolerance in descendants of African origin with those of the same origin living in African countries. Cooper et al. (1997)176 compared the prevalence of diabetes among the African diaspora living in six different countries and reported that among people of West African origin, 2% have diabetes mellitus in Nigeria; within the Caribbean, rates ranged from 3% in men in St Lucia to 11% in women in Jamaica compared with an average of ∼11% in the UK and USA. Another study showed that, in people of West African origin, age-standardised prevalence was 0.8% in rural Cameroon, 2.0% in urban Cameroon, 8.5% in Jamaica and 14.6% in Manchester (UK), with no differences between sexes (p < 0.001). 177
A study that compared Afro-Caribbeans with Europeans found no significant difference in BMI and blood pressure between them. Total cholesterol level was significantly lower in Afro-Caribbean people. Levels of smoking were not different between Afro-Caribbeans and European men but Afro-Caribbean women smoke significantly less. 174
Afro-Caribbeans have a low risk of mortality in a study that followed T2DM Afro-Caribbean patients for 18 person-years. 174 They have a third of the risk of dying from heart disease compared with Europeans [16 vs 59; HR of 0.41 (95% CI 0.23 to 0.75); p = 0.002]. This could be due to the lower levels of cholesterol and higher levels of HDL-C than in Caucasians and Asians. 145,174
Prevalence of risk factors among people of Chinese origin
Groups of people of Chinese descent have been less studied, presumably because they are fewer in number, but several reports of a Newcastle study have included them. Unwin et al. (1997)66 reported that BMIs in Chinese men and women were lower than in Europid men and women (24 vs 26 for men and 23.5 vs 26 for women) but that the prevalence of glucose intolerance was similar to, or higher than, in Europids.
Chapter 4 Systematic review of clinical effectiveness
Research question
Are there effective non-pharmacological interventions that will reduce the progression to diabetes in those with IGT and IFG?
The following interventions, either alone or in combination, are considered:
-
weight loss
-
exercise
-
qualitative changes in diet.
Methods
The review adopted the methodological approach published by the NHS Centre for Reviews and Dissemination. 178
Inclusion and exclusion criteria
Inclusion criteria
Intervention
-
Weight loss.
-
Exercise.
-
Qualitative changes in diet.
Alone or in combination.
Comparators
-
Standard treatment.
-
Non-intensive lifestyle treatment.
Population
-
People with IGT or IFG.
Study design
-
RCTs of at least 2 years’ duration.
-
Systematic reviews of RCTs.
Outcomes
-
Progression to diabetes.
-
Weight loss.
-
Adverse events (AEs).
-
Changes in blood glucose.
-
Changes in diet and physical activity.
-
Changes in blood cholesterol.
Exclusion criteria
Population
-
People with diabetes.
Study design
-
RCTs with < 2 years’ duration.
-
Study designs other than RCTs.
Search strategy
Electronic databases were searched for published systematic reviews, RCTs, economic evaluations and ongoing research up to September 2007. The databases searched were MEDLINE, EMBASE, The Cochrane Library, Science Citation Index, the National Research Register and the UKCRN. Appendix 2 shows the databases searched and the strategy in full. Updating searches were carried out in February 2011, mainly to identify more papers from the main studies. Auto-alerts on MEDLINE were run until September 2011. Selective updating searches in MEDLINE and EMBASE were carried out in January 2012, focusing on new cost-effectiveness analyses and recent reviews.
Abstracts returned by the search strategy were examined independently by two researchers (AS and NW) and screened for inclusion and exclusion. Disagreements were resolved by discussion and consultation with a third researcher (PR). Full texts of the identified studies were obtained. Two researchers (AS and MI) examined these independently.
Data extraction
Two reviewers (AS and MI) extracted data regarding study design and characteristics, details of the intervention, and patient characteristics and outcomes into a specially designed form. Differences in data extraction were resolved by discussion, referring back to the original paper and in consultation with PR and NW.
Quality assessment
To assess the quality of the RCTs, the following criteria were used:
-
method and description of randomisation
-
description of attrition/losses to follow-up
-
specification of eligibility criteria
-
blinding
-
power calculation
-
robustness of outcome measurements
-
similarity of group participants at baseline
-
data analysis.
Overall study quality was rated as follows: A (all quality criteria met), B (one or more of the quality criteria only partially met) or C (one or more criteria not met).
Internal validity:
-
sample size
-
– power calculation at design
-
-
selection bias
-
– explicit eligibility criteria
-
– proper randomisation and allocation concealment
-
– similarity of groups at baseline
-
-
performance bias
-
– similarity of treatment other than the intervention across groups
-
-
attrition bias and intention-to-treat (ITT) analysis
-
– all patients accounted for
-
– number of withdrawals specified and reasons described
-
– analysis undertaken on an ITT basis
-
-
detection bias:
-
– blinding
-
– objective outcome measures
-
– appropriate data analysis
-
– any potential conflict of interest was noted.
-
Results
Systematic reviews
Five good-quality systematic reviews were identified. These were ones that scored highly using the five quality criteria as used for the NHS Centre for Reviews and Dissemination Database of Abstracts of Reviews of Effects (DARE). Four of these met five quality criteria,38,95,179–181 and the other met four and was uncertain on one. 182 These reviews do not include all of the trials now available, nor do they include recent papers from some trials which were available.
The AHRQ published a review of the evidence on the diagnosis, prognosis and treatment of IGT and IFG in 2005, which included six trials of lifestyle interventions. 38 The authors included studies of > 6 months’ duration. As will be reported later, that seems too short because changes are often achieved with lifestyle interventions in the short term which do not persist. We preferred a minimum follow-up of 2 years. However, five of the six trials had a duration of ≥ 2 years. A meta-analysis of four trials of combined diet and exercise gave a RR of 0.54 (95% CI 0.42 to 0.70). The review concluded that there was good evidence that lifestyle interventions could reduce the risk of diabetes in people with IGT.
The Diabetes Australia Guideline covered a wider range of topics, but included consideration of the roles of physical activity and diet in reducing the risk of diabetes. 95 The guideline review concluded that exercise can slow the progression from IGT to T2DM, and that reduction in dietary fat, especially saturated fat, also reduces the risk of diabetes. It also concluded that a combined diet and exercise programme was more effective than either alone.
A review by Gillies et al. (2007),179 published during the preparation of this review, examined the evidence for both pharmacological and lifestyle interventions. 179 They noted that the trials were heterogeneous in the interventions, ethnicity, weight and age, but they carried out meta-analyses that gave the following HRs:
-
diet 0.67 (95% CI 0.49 to 0.92)
-
exercise 0.49 (95% CI 0.32 to 0.74)
-
combined diet and exercise 0.49 (95% CI 0.40 to 0.59)
-
all studies pooled 0.51.
The studies that carried most weight in their meta-analysis were the Finnish DPS183 and the US DPP,108 which will be described in detail later. The longer term results from the Finnish trial were not then available.
The Cochrane review by Norris et al. (2005)180 focused on weight loss or control. In four studies of at least 1 year’s duration, mean weight loss was 2.8 kg, and in the two studies of 2 years’ duration, it was 2.7 kg. The weight loss results from the DPP were larger (4.9 kg at 2 years) but were not included in the meta-analysis because of lack of data on distribution.
Yamaoka et al. (2005)182 reviewed trials on ‘long-term’ (but included studies of ≥ 6 months) non-pharmacological weight loss interventions in pre-diabetes. They concluded that the incidence of T2DM could be reduced by about 50%. They excluded the DPP study,108 but concluded that the meta-analysis of smaller studies matched the results from DPP.
Two Cochrane reviews have looked at separate elements of lifestyle change. Nield et al. (2008)184 set out to assess the effects of dietary advice for preventing T2DM but included only two trials, one being the Da Qing trial described later in this chapter. The other trial did not appear to be restricted to people with IGT. Nield et al. (2008)184 concluded that there was a lack of good data on prevention of T2DM by diet alone.
Orozco et al. (2008)185 assessed the effects of exercise alone or exercise and diet compared with standard advice. They concluded that the combination was effective, but not exercise alone.
Randomised trials
Nine RCTs comparing lifestyle intervention with standard care of IGT were identified: DPP,108 Kosaka et al. (2005),186 Liao et al. (2002),187 Mensink et al. (2003),188 Oldroyd et al. (2006),189 Da Qing,190 Indian DPP,173 Finnish DPS183 and Wein et al. (1999). 191
Some trials gave rise to many papers, only some of which are cited below. Where there are multiple papers from a study, we cite the website from where, in most cases, details of the protocol, copies of the papers and slides can be downloaded.
The Diabetes Prevention Program
The DPP study repository website contains full details of the DPP, including a complete protocol, results, and list of publications. 192
Description and quality of study
The DPP included 3234 participants with IGT, and compared intensive dietary and physical activity advice with standard advice. 108 The sample size necessary to achieve 90% statistical power was estimated to be 2279 participants. This was based on two main assumptions, (1) an expected conversion rate to diabetes of 6.5 per 100 person-years among participants assigned to the standard lifestyle recommendations plus placebo, and (2) that for participants assigned to intensive lifestyle or metformin intervention groups, the diabetes development HR is reduced by ≥ 33%, i.e. to < 4.33 per 100 person-years. The primary outcome was development of diabetes by ADA criteria, using an OGTT.
Participants were recruited using a variety of methods including volunteering in response to advertisements, open screening and referral by health-care providers with the aim of recruiting ≥ 50% women, ≥ 50% ethnic minority and roughly 20% aged ≥ 65 years old. Participants were randomised (stratified by clinical centre) into three treatment groups:
-
intensive lifestyle intervention
-
standard advice plus metformin
-
standard advice plus placebo.
A fourth arm with randomisation to troglitazone was discontinued in 1998 once the toxicity of that drug was realised.
The treatment groups were similar at baseline with respect to age, sex, race, weight and BMI. Baseline physical activity was reported; one of the inclusion criteria for the DPP was being able to walk one-quarter of a mile in 10 minutes. Baseline leisure physical activity levels of the DPP participants using the modifiable activity questionnaire (MAQ) showed that women reported being less active than men (p < 0.0001), and older individuals (> 60 years of age) reported more leisure physical activity (p < 0.0001) than younger age groups.
Baseline diet in terms of total energy intake and fat consumption was reported for only the intensive lifestyle intervention group. 193 Treatment regimens were reported in detail. Staff were provided with ongoing training and provision of intervention materials. Drug administration was double blind; however, if a diagnosis of diabetes was made then participants, investigators and primary care providers were unblinded to the diagnosis and measurements. It was not reported whether any concurrent medication (apart from metformin) was taken; however, participants were excluded at screening if they were using medications known to impair glucose tolerance. Patients were assessed over a mean follow-up of 2.8 years (range 1.8 to 4.6 years) using self-reporting of diet and physical activity and using objective methods for all other measurements. Study attrition was 8% by the end of the study (92.5% had attended a scheduled visit within 5 months of the close of the study). AEs were reported according to treatment group.
Participants
The DPP trial recruited 3234 overweight participants with IGT of ≥ 7.8 to < 11.1 mmol/l (2-hour plasma glucose detected by a 75 g OGTT) and a FPG of between 5.3 and 6.9 mmol/l.
Participants came from a range of ethnic groups: 54.7% were white, 19.9% were African American, 15.7% were Hispanic, 5.3% were American Indian and 4.4% were Asian. In total, 67.7% were female and 69.4% had a family history of diabetes. The minimum age was 25 years. The mean age (± SD) of the 3234 participants was 50.6 ± 10.7 years and the mean weight was 94.2 ± 20.3kg. The minimum BMI was 24 kg/m2. The majority of participants had a BMI of < 40 kg/m2; however, the BMI was ≥ 40 kg/m2 in 8% of men and 21% of women. The overall mean BMI was 34 kg/m2: 30.8% had BMIs of 30 kg/m2 to < 35 kg/m2 and 36.9% had BMIs of ≥ 35 kg/m2, so overall 67.7% had BMIs of ≥ 30 kg/m2. In men, 56.5% had BMIs of ≥ 30 kg/m2, and 73% of women had BMIs of ≥ 30 kg/m2.
A history of and/or treatment for hypertension was present in 27% of participants. More than 37% of men and 33% of women reported a history of and/or treatment for high cholesterol. The authors noted: ‘the DPP cohort includes individuals who are more overweight and hyperinsulinaemic and less hypertensive than the subjects in other studies’. As such, ‘DPP participants may be less susceptible to hypertension-related morbid events that may confound the secondary CVD outcomes attributed to IGT or hyperglycaemia per se’. 192
One issue in interpreting trials is the representativeness of the recruits. Figure 5, below, shows the stages of recruitment to the DPP.
FIGURE 5.
Screening and recruitment for the DPP. Reprinted from Controlled Clinical Trials, volume 23, Rubin et al. , authors, The Diabetes Prevention Program: recruitment methods and numbers. pp. 157–171. Copyright (2002) with permission from Elsevier.
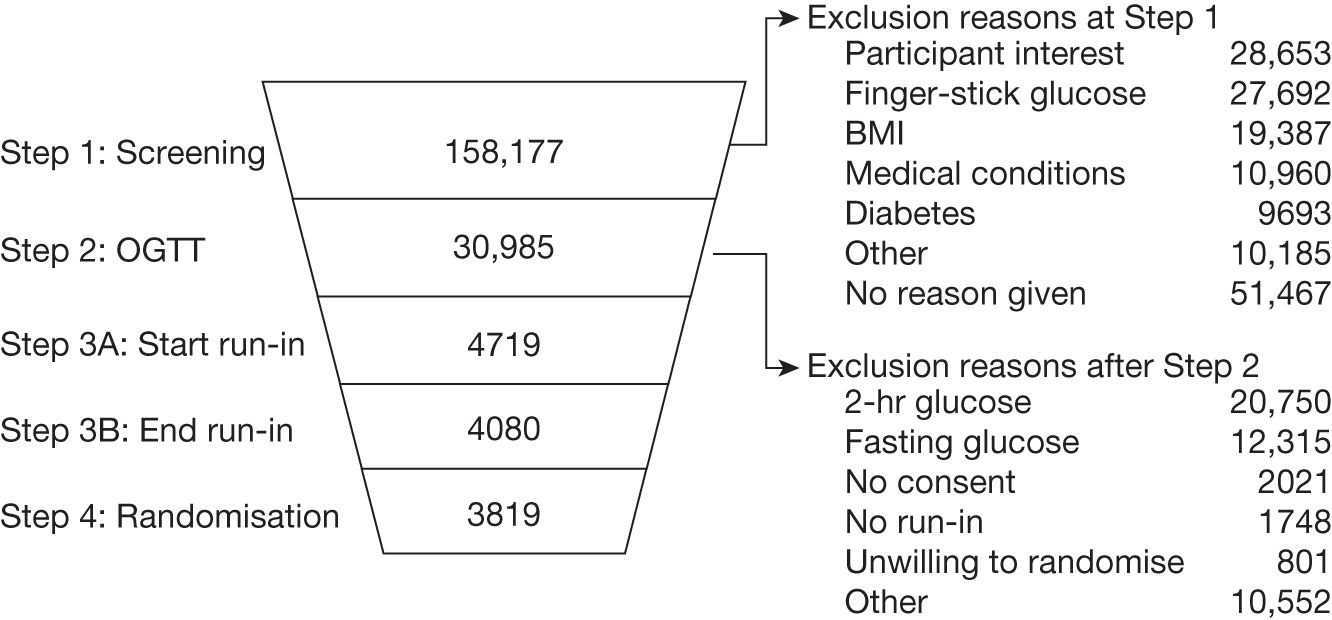
About 80% of the 158,177 potential participants were eliminated between steps 1 and 2. About one-third gave no reason for exclusion, but for those for whom a reason was available it was found that the five primary reasons for stopping after step 1 included (1) choosing not to have an OGTT (18%); (2) being excluded because the finger-stick glucose reading was outwith the entry criteria (17%); (3) BMI (12%); (4) being excluded because taking medications, such as thiazide diuretics, was likely to confound assessment for diabetes (7%); and (5) being given a diagnosis of diabetes (6%).
There was another large reduction (26,266 individuals) at step 2, which involved administration of an OGTT to determine glucose tolerance criteria for eligibility. About two-thirds of these were people whose fasting or 2-hour OGTT results were not in the eligible range. Many people were excluded at this stage for more than one reason. Step 3 was a 3-week run-in period; 81% (3819) who started the run-in were eventually randomised to one of the study arms.
Most of the exclusions of the initial volunteers, therefore, were based on plasma glucose results. However, many people selected themselves out, and this should be borne in mind when considering the generalisability of the results.
Intervention
In the DPP trial, the intensive lifestyle intervention group was assigned a goal of achieving and maintaining at least 7% weight loss through low-calorie, low-fat diet and moderate-intensity physical activity (at least 150 minutes/week). Participants were encouraged to achieve the weight loss (through reduction in dietary fat intake to < 25% of calories) and exercise goals within the first 24 weeks. Sixteen individual (one-to-one) sessions with a case manager (‘lifestyle coach’) within this time covered general information about diet and exercise and behaviour strategies, such as self-monitoring, goal-setting, stimulus control, problem-solving and relapse prevention training (i.e. resource intensive). During maintenance, group courses were offered every 3 months on topics related to exercise, weight loss or behaviour issues. Two comparator groups were randomised to standard lifestyle advice with either placebo (tablets twice daily) or metformin (850 mg twice daily). The standard lifestyle advice (given to all participants including the intensive lifestyle intervention group) consisted of written information and a 20- to 30-minute individual advice session recommending 5–10% weight loss and 30 minutes of physical activity 5 days a week. In addition, participants were advised to avoid excessive alcohol intake and stop smoking. Participants were reviewed annually. The average duration of intervention and follow-up was 2.8 years (range 1.8 to 4.6 years).
Results
Primary outcomes
The DPP assessed the incidence of diabetes by annual OGTT testing or semi-annual FPG testing over 4 years. In addition, testing was prompted if symptoms arose that were suggestive of diabetes. Results were expressed in four ways: (1) the number of cases per 100 person-years; (2) per cent reduction in incidence; (3) estimated cumulative incidence at 3 years; and (4) number needed to treat (NNT) one patient with diabetes.
The number of cases per 100 person-years was significantly lower in the lifestyle intervention and metformin groups than in the placebo group (4.8, 7.8 and 11.0 in the lifestyle, metformin and placebo groups, respectively) equating to 58% (95% CI 48% to 66%) and 31% (95% CI 17% to 43%) lower incidence in the lifestyle and metformin groups, respectively, compared with the placebo group. At 3 years the estimated cumulative incidence of diabetes was 14.4%, 21.7% and 28.9% in the lifestyle, metformin and placebo groups, respectively (so they were quite a high-risk group).
The NNT to prevent one case of diabetes during a 3-year period was estimated to be 6.9 for the lifestyle intervention and 13.9 for metformin. The authors stated:
the incidence of diabetes in the placebo group was higher than expected perhaps owing to greater frequency of glucose testing or selection of persons at higher risk. 108
Subgroup analysis (note: the study had inadequate power for this analysis) found that treatment effects did not differ significantly according to sex, race or ethnic group; however, the effect of the lifestyle intervention was significantly greater among participants with lower baseline glucose concentrations 2 hours after glucose load. Similarly, the effect of the lifestyle intervention over metformin was greater in older participants and those with lower BMIs. The authors stated:
The racial and ethnic-group differences in incidence of diabetes were perhaps reduced by the selection of participants who were overweight, and had elevated fasting and post-load glucose concentrations which are three of the strongest risk factors for diabetes. 108
After adjustment for weight change in the lifestyle group, no independent effects of increased physical activity or decreased per cent fat on diabetes risk were found. 193
Several different measures of body size at baseline were predictive of the subsequent development of diabetes. 83 When analysed at 3.2 years, large WC at baseline was a better predictor of risk for developing diabetes in both sexes than other measures in the placebo and lifestyle groups. The HR for WC was 1.29 (p < 0.01) and 1.53 (p < 0.01) for women in the placebo and lifestyle groups, respectively, and 1.43 and 1.49 for men (adjusted for age and self-reported race/ethnicity) relative to smaller waists. A graded increase in the risk of developing diabetes was seen as the tertile of WC increased in both lifestyle and placebo groups.
The age of participants had an impact on progression to diabetes. 194 Diabetes incidence rates did not vary by age in the placebo group (11, 10.8 and 10.3 cases per 100 person-years in young, middle-aged and older groups, respectively, p = 0.71). In contrast, intensive lifestyle intervention was more effective with increasing age (6.3, 4.9 and 3.3 cases per 100 person-years, in the 25- to 44-year, 45- to 59-year and 60- to 85-year age groups, respectively, p = 0.007). Those in the oldest age group lost more weight and were more physically active.
Diabetic retinopathy was detected in 12.6% of the participants who developed diabetes during the DPP compared with 7.9% of those who did not (p = 0.03). The only characteristics reported to be different between those who developed retinopathy and those who did not were HbA1c level (6.4% ± 0.55% vs 6.2% ± 0.63%; p < 0.05 – as reported in the paper, although the difference of only 0.2% seems trivial) and systolic blood pressure (SBP) (128 mmHg vs 125 mmHg; p < 0.05). 195
The DPP measured FPG and HbA1c levels in all participants every 6 months for up to 4 years. Both FPG and HbA1c levels changed significantly over the time of the study. In the first year, the FPG of the lifestyle and metformin groups decreased; however, in subsequent years the FPG values increased and returned to baseline levels by 2.5 years; further increases were observed thereafter up to 4 years (significant difference between groups; p < 0.001). In the placebo group, FPG values increased at each time point from baseline up to 4 years (significant difference between groups 0.5–3 years; p < 0.001). HbA1c values showed a similar initial decrease in the lifestyle and metformin groups; however, both groups showed an increase thereafter with the metformin group lying between lifestyle and placebo values, as shown in Figure 6. At the start, the HbA1c level was 5.9%. At 4 years, the means were, approximately, placebo 6.1%, lifestyle 6.0% and metformin 6.0%.
FIGURE 6.
Mean change in HbA1c level vs years from randomisation in the DPP. Reprinted with permission from the Massachusetts Medical Society. The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 108
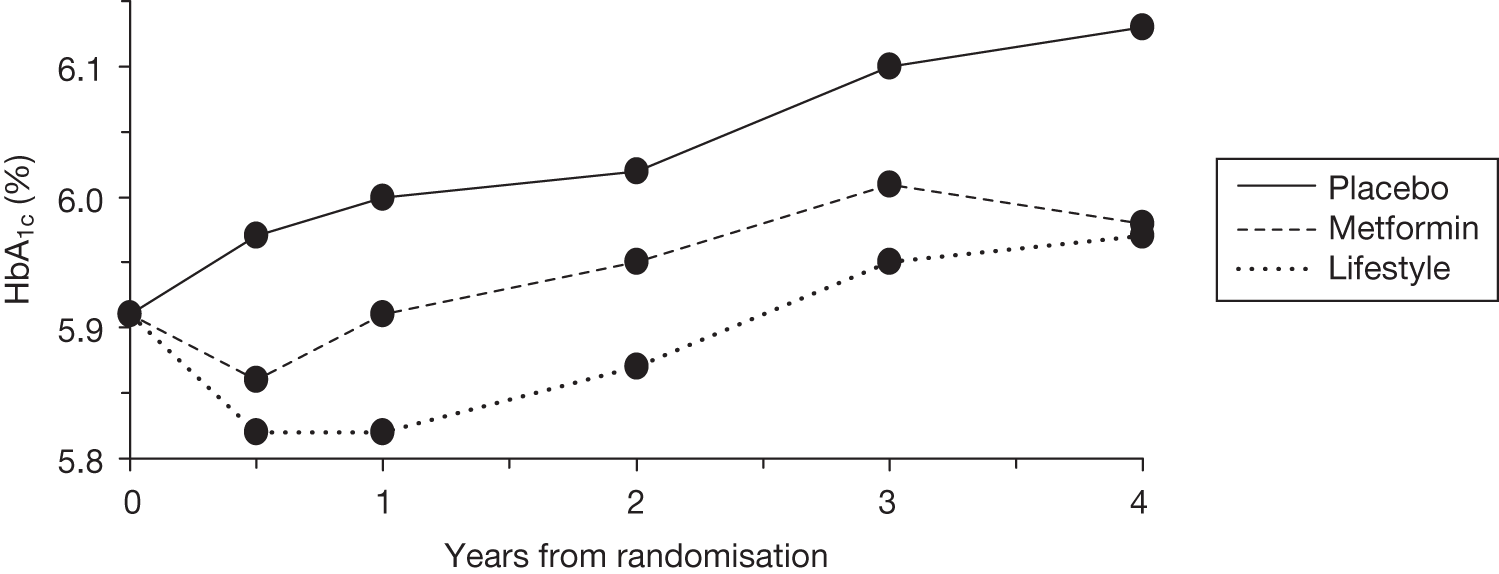
The placebo group saw a constant increase in values from baseline to 4 years. The percentage of participants with normal glucose concentrations was greater at all time points up to 4 years in the lifestyle intervention group compared with both metformin and placebo groups. At 4 years, the percentages with normal fasting glucose were lifestyle 54.1%, metformin 45.1% and placebo 43.8%. The percentages with normal 2-hour glucose were lifestyle 37.8%, metformin 27.9% and placebo 24.2%. The percentages with both normal fasting and 2-hour glucose levels were lifestyle 29.6%, metformin 19.7% and placebo 17.2%. There were no differences in the percentages reverting to NGT among the age groups. 164
The 10-year results showed continuing but reduced benefit, with the cumulative incidence of diabetes reduced by 34% in the lifestyle group (and by 18% in the metformin group). This was despite all groups being offered a modified form of the original intervention (in groups rather than 1 : 1) after the end of the trial, and despite the metformin group being allowed to continue on the drug.
Gastrointestinal symptoms were less frequent in the lifestyle group than in the placebo group (12.9/100 person-years vs 30.7/100 person-years, p < 0.02), whereas the rate of GI symptoms was significantly higher in the metformin group than in the placebo group (77.8/100 person-years vs 30.7/100, p < 0.02). The rate of musculoskeletal symptoms was significantly higher in the lifestyle group (24.1/100 person-years) than in both the metformin and placebo groups (20.0 and 21.1/100 person-years, respectively). No significant differences between groups were seen in hospitalisation or deaths.
Secondary outcomes
Data on weight change were available for 3085 participants at 6 months, 3064 participants at 1 year, 2887 at 2 years and 1510 at 3 years. Participants assigned to the lifestyle intervention had significantly greater weight loss than participants assigned to receive metformin or placebo. Over 4 years the average weight loss was 5.6 kg, 2.1 kg and 0.1 kg in the lifestyle, metformin and placebo groups, respectively (p < 0.001).
Weight loss, reduction in WC and percentage of participants who achieved the 7% weight loss goal all increased with age. 194 The percentages of participants who achieved the weight loss goal (7%) of body weight were 25–44 years = 33%, 45–59 years = 39% and 60–85 years = 55%. Participants aged 60–85 years had the most weight loss.
At the 10-year follow up, the lifestyle group had regained most of the weight, going from a nadir of a mean 7 kg reduction at 1 year back up to a mean weight loss of 2 kg.
One of the papers from the DPP explored the relative contributions of the changes in weight and exercise achieved by studying the intensive lifestyle group. 193 Dietary and exercise data were collected by questionnaires. The number of participants with ≥ 3-year or longer follow-up was only 638 because recruitment went on until May 1999 and the data were collected at end of July 2001.
Hamman et al. (2006)193 reported that the mean weight loss at 3 years was 4.1 kg. Physical activity increased at each year end compared with baseline. The percentage of calorie intake from fat fell from 34% at baseline to 28% at the end of year 1. A total of 153 participants developed diabetes – a rate of 5 per 100 person-years. When all of the changes were examined in a multivariate Cox model, weight loss was the dominant factor. Even small amounts of weight loss helped – on average there was a 16% reduction in the risk of diabetes per kilogram of weight loss. Weight loss reduced diabetes incidence similarly across all race/ethnicity groups, for both sexes, for all ages, and for several levels of physical activity, regardless of the level of initial obesity.
Hamman et al. (2006)193 then looked at the effects of meeting the various goals at year 1, dividing participants into eight subgroups. The subgroup that met all of the goals had the lowest risk of diabetes, with an 89% reduction in risk (HR 0.11, 95% CI 0.05 to 0.24; p < 0.0001) compared with the group meeting none of the goals. Weight loss was responsible for much of the reduction, but there was halving of the incidence of diabetes in those who met the physical activity goal (150 minutes of moderate-intensity activity per week) as shown in the comparison of subgroups 3 and 4 compared with 1 and 2. The reduction was 46% after adjusting for baseline variables and 44% (HR 0.56; 95% CI 0.36 to 0.89; p = 0.012) after adjusting for weight loss over follow-up. There is of course an interaction, in that increased physical activity helped to sustain weight loss. However, among 495 recruits who did not achieve the 12-month weight loss target (7% or 6.6 kg on average), the incidence of diabetes was reduced by 44% in those who achieved the physical activity target. Note that this group did lose some weight (mean 2.6 kg) but adjustment for this had little impact on the effect of physical activity.
The results may also indicate that at least ∼150 minutes per week of moderate activity are required before an effect on diabetes risk is achieved.
Adherence
DPP examined the adherence of participants to intervention. Diet adherence was assessed at 1 year only and showed that the lifestyle group had a significantly greater reduction in daily energy intake over the first year than metformin and placebo groups (–450, –296 and –249 kcal, respectively; p < 0.001). This was associated with a significantly greater change in the average intake of fat from baseline to 1 year (–6.6%, –0.8% and –0.8% of total calories, respectively; p < 0.01). Data for longer time points were not reported. There was no significant difference in reduction of reported caloric intake among different age groups, although older (60–85 years) recruits reduced calorie intake less (by 10% at 12 months) than younger recruits (25–44 years; 18%). 194
Compared with baseline, all study groups had increased their physical activity at 1, 2, 3 and 4 years. However, participants in the lifestyle intervention group had significantly greater change in physical activity at all time points than both the metformin and placebo groups, the physical activity of which increased only slightly over baseline values. 108 The authors stated:
After adjustment for weight change no independent effect of increased physical activity or decreased per cent fat on diabetes risk was found. 193
Paradoxically, those participants who met the physical activity goal of 150 minutes/week of moderate activity had a 44% reduction in diabetes risk, independent of weight loss.
In a representative sample of DPP lifestyle participants (n = 274; 94% of the final 293 lifestyle participants randomised), characteristics that correlated with high levels of baseline, 1-year and end-of-study physical activity were (1) being a man and (2) having lower BMI and lower perceived stress, depression and anxiety scores at baseline. Higher baseline BMI and being a woman correlated with lower baseline, 1-year and end-of-study physical activity levels, with women having significantly higher BMIs and higher levels of depression, anxiety and perceived stress than men. 196
Older age was an independent predictor of achieving the goal of 150 minutes of physical activity at 1 year and 2 years. Lifestyle participants aged > 60 years achieved greater minutes of physical activity and greater per cent weight loss and greater risk reductions for developing diabetes (71% risk reduction compared with 48% risk reduction in persons aged 25–44 years). Higher levels of baseline physical activity correlated with greater readiness to change physical activity levels (p < 0.0001) and lower levels of perceived stress (p = 0.009), depression (p < 0.003), and anxiety (p = 0.03) at baseline, 1-year and end-of study levels. 196
Perhaps time is a factor, with those who are older having more time post retirement. The older age groups had the highest MET-hours of activity and did better with lifestyle change than younger groups, although it should be noted that they started leaner and with better insulin sensitivity. 194
Recruitment to DPP was on the basis of IGT, but a later paper by Orchard et al. (2005)197 reported the prevalence of metabolic syndrome at baseline and the effects of the interventions.
The metabolic syndrome was defined by the criteria from the National Cholesterol Education Program’s Adult Treatment Panel III, with three or more of the following:
-
WC of > 102 cm in men and > 88 cm in women
-
serum triglyceride level of at least 1.7 mmol/l
-
HDL-C level of < 1.03 mmol/l in men and < 1.3 mmol/l in women
-
blood pressure of ≥ 130/85 mmHg
-
FPG level of 6.2 mmol/l.
The metabolic syndrome was present in 53% (n = 1711) of the 3234 participants at baseline, with little variation by age. After 3 years, the prevalence of the metabolic syndrome increased from 55% at baseline to 61% in the placebo group (p = 0.003), did not change (54–55%) in the metformin group (p > 0.2) and decreased from 51% to 43% (p < 0.001) in the lifestyle group. The decrease in the lifestyle group correlated most strongly with decreases in WC and blood pressure.
Fujimoto et al. (2007)198 examined the relationship between changes in body fat and progression to diabetes by 1 year. At baseline, recruits were mainly obese, with mean BMI of 32.1 kg/m2 in men and 33.0 kg/m2 in women. Visceral and subcutaneous fat was measured by CT, as well as by standard measurements such as BMI. The lifestyle group had big reductions in both visceral fat (reduced at L2–3 by 24% in men and 18% in women at 1 year) and subcutaneous fat (again at L2–3, reduced by 16% in men and 11% in women). Progression to diabetes was associated with fat changes differently in the arms of the study. In the metformin group, the reduced diabetes risk was independent of body fat changes. In the placebo group, only the subcutaneous fat changes correlated with diabetes risk, and then only in men. In the lifestyle group, all of the fat variables correlated with diabetes reduction in men. In women, weight, BMI and WC were significant predictors but the association with visceral fat did not quite reach statistical significance.
After the end of the randomised trial, all three groups (lifestyle, metformin and placebo) were offered the lifestyle intervention, albeit in groups rather than the original individualised provision. At the 10-year follow-up, the original lifestyle group had regained about 5 kg of their original (by 12 months) 7 kg weight loss, so that by 10 years their weight was little different from the metformin group, who lost about 2.5 kg. 199 However, the incidence of diabetes by 10 years remained lower in the former lifestyle (by 34%) group and metformin (by 18%) group than in the former placebo group. The onset of T2DM was delayed by about 4 years with lifestyle and by about 2 years by metformin. One feature of note was that not only was the best effect of lifestyle seen in the 60- to 85-year age group, but also that this group had no significant response to metformin. 199
Perreault et al. (2009)200 noted that in some diabetes prevention trials, glucose tolerance regressed to normal, and used DPP data to examine the factors that were associated with this. The factors included baseline evidence indicating a milder condition (lower baseline FPG and 2-hour plasma glucose) and those indicating response to the intervention, especially greater weight loss. As reported by Hamman et al. (2006)193 for every 1 kg weight loss there was a 16% reduction in diabetes risk, and it was the most important predictor of regression. 193 However, Perreault et al. (2009)200 noted that intensive lifestyle interventions appeared to have other components that were independent of weight loss, most probably physical activity.
In another paper, Perreault et al. (2008)201 noted that meeting the goals of the lifestyle intervention was a strong predictor of reduction in progression to diabetes. However, men in the lifestyle arm met more goals than women but had the same progression to diabetes. Perreault et al. (2009)200 explain this on the basis that men were at higher risk from baseline.
The DPP also examined changes in cardiovascular risk factors over time, and found that among those who progressed to diabetes, there were rises in blood pressure and triglycerides, and a fall in HDL-C. 202 These changes were statistically significant, although quite small. Those who regressed from IGT to NGT showed reductions in blood pressure and triglycerides, and improvements in HDL-C levels. These improvements in HDL-C levels were more marked in the lifestyle group than in the metformin one, and LDL cholesterol (LDL-C) also fell in the lifestyle group. Goldberg et al. estimated that in the lifestyle group these changes should bring about a 10–13% reduction in heart disease.
The effect of metformin has been examined in several of the DPP papers. In the main study report it was noted that metformin reduced the risk of diabetes by 31%. 108 However, the effects varied among groups. Metformin had little effect in the oldest age group, whereas lifestyle change was more effective with increasing age. 194 Crandall et al. (2006)194 suggest that this may be related to the oldest age group (60–85 years) being leaner, and having less insulin resistance, at baseline. Metformin may work by reducing insulin resistance and the older group will have less to gain. Another DPP paper reported that metformin was effective mainly in those with BMIs of > 35 kg/m2. In this BMI subgroup, metformin was as effective as lifestyle. 83
Some of the benefits of metformin were the result of weight loss rather than improvements in insulin sensitivity. 203 Lachin et al. (2007)203 estimated that 64% of the metformin benefit was mediated through weight loss. Interestingly, they note that the effect of weight loss achieved with metformin appears to be less than the same weight lost by lifestyle change.
Most DPP papers report prevention of diabetes, but the 10-year follow-up199 also reported that diabetes was delayed, with the time at which 40% of the (high-risk) groups progressed to diabetes being 4 years later with lifestyle and 2 years later with metformin compared with placebo.
Ackermann et al. (2009)204 estimated the direct utility of weight loss, after adjusting for a range of possible confounding factors. They used the SF-6D utility measure, and estimated that there was a gain in utility of 0.007 (on a scale of 0.29–1.00) for every 5 kg of weight loss. Although this fell below the minimum effect size that was deemed to be clinically important (0.04), they considered that the change could improve cost-effectiveness if sustained for years.
In summary, DPP found that intensive diet and exercise intervention in those with IGT significantly reduced the incidence of diabetes by 58% compared with standard lifestyle advice. The reduction was sustained, with a cumulative incidence of diabetes at 6 years of 23% in the intervention group, compared with 38% in the control groups. At 10 years, the incidence of diabetes was 34% lower in the lifestyle group and 18% lower in the metformin group compared with the control group. This was despite the lifestyle group regaining much of the 7 kg weight lost in the first year.
The Finnish Diabetes Prevention Study
Data were obtained from the main study by Tuomilehto et al. (2001),183 with supplementary information cited where appropriate from additional DPS papers. 205–219
Description and quality of trial
This RCT of 523 participants with IGT in Finland compared intensive lifestyle intervention with a control group. Participants were recruited by screening high-risk individuals (e.g. obese subjects or first-degree relatives of those with T2DM), who were identified through previous epidemiological surveys and advertisements. Those with IGT (according to WHO 1985 criteria) on two separate OGTTs were recruited. Additional inclusion criteria were age 40–64 years and BMI of > 25 kg/m2. Power calculation estimated that, in order to detect a 35% reduction in incidence of diabetes with 80% power at 5% significance level, 3252 person-years were required, i.e. 650 subjects to be followed for 5 years or 542 subjects to be followed for 6 years. A total of 523 participants were randomised into two groups: a diet-plus-exercise group and a control group.
Treatment groups were not significantly different at baseline apart from two measurements: SBP and per cent total energy consumed as saturated fat (see Participants, below). Treatment regimens were reported in detail. Use of concurrent medication was reported. Participants were assessed over 6 years (mean follow-up 3.2 years) using subjective self-reporting of diet and physical activity, and using objective measures for all other outcomes. Forty subjects (8%) withdrew from the study: 23 in the intervention group and 17 in the control group. Of these, nine could not be contacted, three withdrew because of severe illness, one died and 27 withdrew for personal reasons. AEs were not reported. No conflict of interest was reported.
Participants
Tuomilehto et al. (2001)183 recruited 523 Finnish men and women with IGT (as defined by WHO 1985 criteria: FPG of < 7.8 mmol/l and/or 75 g OGTT 2-hour ≥ 7.8 mmol/l and < 11.1 mmol/l). Exclusion criteria were: previous diagnosis of diabetes mellitus [other than gestational diabetes mellitus (GDM)]; persons involved regularly in a vigorous exercise programme; subjects receiving treatment to lower blood pressure other than routine dietary and health advice; persons with any chronic disease making a 6-year survival improbable; other medical characteristics that were likely to interfere with participation in the study; and subjects with clinical conditions, such as thyroid and liver diseases, which could interfere with glucose metabolism. The male–female ratio was 33 : 67 and mean age (years ± SD) was 55 ± 7 years. Mean BMI was 31.4 ± 4.6 kg/m2 in the intervention group and 31.0 ± 4.5 kg/m2 in the control group, and mean weight was 86.7 ± 14.0 kg versus 85.5 ± 14.4 kg, respectively. Sixty-six per cent of participants in the intervention group had first-degree relatives with diabetes compared with 61% in the control group. Five per cent of the intervention group were taking cholesterol-lowering drugs compared with 6% of the control group; 30% of the intervention group were taking antihypertensive drugs compared with 31% of the control group. Subjects receiving treatment to lower blood glucose were excluded. Baseline characteristics were similar across groups apart from (1) SBP (± SD) at baseline was slightly, but significantly, higher in the diet-plus-exercise group than in the control group (140 ± 18 vs 136 ± 17; p = 0.03 between groups) and (2) proportion (% ± SD) of total energy consumed as saturated fat was significantly higher in the control group [17 ± 4.3 percentage total energy (E%)] than the intervention group (16.2 ± 4.0 E%; p = 0.019 between groups).
A further paper209 reported the prevalence of the metabolic syndrome, using a modified WHO definition. In the DPS cohort, 78% of the men and 72% of the women had the metabolic syndrome. The mean BMI was higher in women (32 kg/m2) than in men (30 kg/m2) but WHR was higher in men. Men had slightly higher diastolic blood pressure (DBP) and FPG, but lower HDL-C. Obesity was seen in 96.5% of men and 66.3% of women, hypertension in 62.9% of men and 60.9% of women, and dyslipidaemia in 51.2% of men and 48.6% and women.
Intervention
Participants were randomised to two groups: (1) intensive lifestyle (diet-plus-exercise) group and (2) control group. Participants randomised to the diet-plus-exercise intervention group (n = 265) received 15 individual sessions with a nutritionist over 3 years. Participants were advised to consume a diet with: 50% of daily calories from carbohydrates; < 30% fat; < 10% from saturated fat; < 20% from mono- and polyunsaturated fat; < 300 mg/day cholesterol; ≥ 15 g/1000 kcal fibre and approximately 1 g protein/kg ideal body weight/day. Additional topics covered included diabetes risk factors, problem-solving and physical activity. Attendances at further group sessions, expert lectures, low-fat cooking lessons and visits to local supermarkets was encouraged. Participants were also encouraged to lose weight at a rate of 0.5–1 kg/week towards a goal of BMI of < 25 kg/m2 or ≥ 5% weight reduction. (Note: in 48 participants very low-calorie diets were considered after 6 months.) Participants had their level of physical activity assessed individually and were advised to increase their overall level of physical activity making use of supervised (and individualised) exercise sessions (endurance training, resistance training and voluntary group walking). The control group were given general information at baseline about lifestyle and diabetes risk. They were advised to adjust their diet to reduce BMI to < 25 kg/m2, consume < 30% energy as fat, reduce alcohol intake and stop smoking. Counselling was done individually or in one group session (but not individualised, i.e. not tailored to the individual) with annual follow-up visits.
The cost of the intervention diet was, if anything, slightly less than their usual diet had been. 216 Dietary fibre and fat contents were strong predictors of success, even after adjustment for weight loss. 213
Results: primary outcomes
Tuomilehto et al. (2001)183 assessed the incidence of T2DM (WHO 1985 criteria) by annual oral glucose tolerance testing for up to 6 years (mean follow-up 3.2 years). Results were expressed in a number of ways. First, the cumulative incidence of diabetes (years 1–6) was significantly lower (58%) in the intervention group than in the control group [year 1, 5 (2%) in intervention group vs 16 (6%) in control group; year 2, 15 (6%) in intervention group vs 37 (14%) in control group; year 3, 22 (9%) in intervention group vs 51 (20%) in control group; year 4, 27 (11%) in intervention group vs 53 (23%) in control group; year 6, 27 (10%) in intervention group vs 59 (23%) in control group; RR = 0.4; p < 0.001 between groups]. The absolute incidence of diabetes (number of cases per 1000 person-years) was 32 cases per 1000 person-years in the intervention group compared with 78 per 1000 person-years in the control group. Tuomilehto et al. (2001)183 concluded that 22 subjects with IGT must be treated for 1 year (or five subjects for 5 years) to prevent one case of diabetes.
In a later publication, in which mean follow-up was 4.1 years, 114 of the participants (intervention and control group combined) had been diagnosed with diabetes. 214 Those who developed diabetes were more obese at baseline and had higher fasting and 2-hour plasma glucose values. Study authors noted that ‘those subjects who developed diabetes during the first 2 years of the trial did not have a 3 year examination’ [also noted in the 4-year publication by Uusitupa et al. (2003)218]; this may have affected the apparent effectiveness of the intervention.
Results: secondary outcomes
Tuomilehto et al. (2001)183 assessed weight loss annually using standard methods. Results were presented as mean change in body weight (kilograms and per cent) from baseline to year 1 and year 2. The study found that both groups had lost weight at both time points but the intervention group had significantly greater weight loss than the control group (4.2 kg vs 0.8 kg at 1 year; p < 0.0001; and 3.5 vs 0.8 kg gain at 2 years; p < 0.001). By 3 years the weight reductions were 3.5 kg in the intervention group and 0.9 kg in the control groups. 215
BMI was also assessed annually and showed a similar significantly greater improvement in the intervention group (–1.3 kg/m2) compared with the control group (–0.3 kg/m2) (p < 0.0001 for year 1 and year 3 between groups).
At the 7-year follow-up, the control group had lost 0.7 kg since baseline, and the intervention group 3.1 kg, and the modelling showed that weight loss was the main predictor of success. Weight loss did not vary by level of education when level of education was divided into tertiles. 220
Blood pressure levels improved significantly more in the intervention group than in the control group. SBP fell 5 mmHg in the intervention group and did not change in the control group when measured at 2 years (p = 0.0005). DBP fell 5 mmHg in the intervention group and 3 mmHg in the control group, p = 0.0125 (derived from the AHRQ review38).
Adherence
Tuomilehto et al. (2001)183 examined the adherence of participants to intervention. Diet adherence was assessed subjectively over the first year by examining self-reported 3-day food records filled in by participants and completed four times during the year. Data were presented as percentage of groups achieving specific dietary goals. From baseline to year 1, compared with the control group, a significantly greater (p = 0.001) proportion of participants in the intervention group achieved separate goals of fat intake < 30% total energy (47% intervention group vs 26% control group), saturated fat intake < 10% total energy intake (26% vs 11%) and fibre intake > 15 g/1000 kcal (25% vs 12%). Change in dietary intake from baseline to 1 year and 3 years was reported in detail in Lindstrom et al. (2003). 215 Compared with the control group, the intervention group showed a significant decrease in total energy consumed (kcal/day), E% as fat, saturated fat (E%) and monounsaturated fat (E%) from baseline to year 1 and year 3. Similarly, the intervention group showed a significant increase from baseline at 1 year and 3 years in E% of carbohydrates, fibre density (g/1000 kcal) and intake of both water-soluble and insoluble fibre (g/1000 kcal) compared with control groups. No significant differences between groups were seen in alcohol or polyunsaturated fat consumption.
The association between dietary macronutrient composition and change in body weight and WC and diabetes risk was assessed using DPS data. 213
During a mean follow-up of 4.1 years, weight loss was related to an increase in fibre [p-value (p) for trend = 0.001] and decrease in fat (p for trend = 0.018) and energy density (p for trend = 0.001). Reduced diabetes risk was associated with higher fibre density (p for trend = 0.01) and lower fat intake (p for trend = 0.004), after adjusting for group assignment, sex, age, baseline weight, baseline 2-hour glucose, physical activity and baseline intake. This result did not change significantly when further adjusted for weight change.
Therefore, it would seem that a high-fibre, low-fat diet can result in sustained weight reduction, and it can significantly decrease the risk of progression to diabetes, even independently of weight loss.
The authors doubted the accuracy of some of the self-reported dietary changes:
-
The energy intakes calculated from the food records revealed that under-reporting had taken place.
-
Overweight and obese people are known to be even more prone to dietary under-reporting than normal-weight people.
-
Individuals who succeeded in weight reduction were possibly more likely to report consuming the recommended diet.
Adherence
Adherence to physical activity guidance was assessed at baseline and at every annual visit using the Kuopio Ischaemic Heart Disease Risk Factor Study 12-month LTPA quantitative questionnaire. The duration (minutes per week) of total physical activity and moderate- and high-intensity LTPA were calculated. Lindstrom et al. (2003)215 reported changes from baseline to year 1 and year 3: 86% of the intervention group compared with 71% of the control group (p = 0.001) achieved the exercise goal at year 1. 215 These showed that compared with the control group (n = 250), the intervention group (n = 256) showed no significant difference in total LTPA at either time point but a significant increase in the minute/week spent doing moderate-to-vigorous LTPA: baseline to year 1, +49 minutes in intervention group compared with +14 minutes in control group (p = 0.0073); baseline to year 3, +61 minutes in intervention group compared with +6 minutes in control group (p < 0.0057).
Compared with the control group, significantly fewer individuals in the intervention group were classified as sedentary at year 1 (14% intervention group vs 30% control group; p < 0.001) and year 3 (17% intervention group vs 29% control group; p = 0.028). The study authors noted that:
Frequency, duration, and intensity of leisure time and lifestyle physical activity during the preceding 12 months was estimated by the participants at each annual visit. It was not straightforward and may have been incomplete due to difficulty recollecting. 215
Laaksonen et al. (2005)212 provide further analysis of physical activity data from an extended follow-up to a mean of 4.1 years, compared with the original trial end of 3.2 years. During this time, the randomised allocation and intervention was maintained. 210
Questionnaires were completed each year. Moderate to vigorous exercise was defined as ≥ 3.5 METs. The intervention group reported an increase of about 48 minutes per week in this level of exercise; the control group reported little change. The main difference was in what was classed as ‘strenuous structured’ physical activity (other than walking).
There was a dose–response relationship. Those who increased exercise the most were > 60% less likely to develop diabetes, although this difference was slightly reduced after adjusting for weight loss – the RR in the highest tertile of activity then became 0.51 compared with the lowest tertile. Participants whose increase in walking for exercise was in the upper third were 59% less likely to develop diabetes than those whose change was in the lower third, independent of other factors.
The most common form of LTPA was walking and the second most common was cycling. Non-leisure activity included gardening and shovelling snow. The conclusion is that increasing physical activity may substantially reduce the incidence of T2DM in high-risk individuals. A key message from a public health standpoint would be that at least 2.5 hours/week of walking for exercise during follow-up seemed to decrease the risk of diabetes by 63–69%, largely independent of dietary factors and BMI.
One question for this review is whether or not interventions to reduce progression to diabetes would also reduce the increased risk of CVD in people with IGT. A substudy of the Finnish DPS183 examined changes in fibrinolytic activity. A reduction in fibrinolytic activity is thought to increase CVD. Hamalainen et al. (2005)206 reported that in five centres improvement in fibrinolytic activity was seen by 1 year [31% reduction in plasminogen activator inhibitor-1 (PAI-1)]. 206 In one centre (Turku) it was measured again at 3 years and was shown to persist. The factor that most explained the improvement was weight loss. Hence, it appears that cardiovascular risk is also reduced by lifestyle change.
After a median of 4 years of active intervention period, participants who were still free of diabetes were further followed up for a median of 3 years, with median total follow-up of 7 years. No further intervention was provided but the participants were seen annually by the study nurse (which could be regarded as an intervention). 214
Participants were divided into six groups according to how many lifestyle goals were achieved, so that group 5 achieved all and group 0 achieved none. Table 3 shows the incidence of diabetes for each group, expressed as a ratio to group 0.
| Success score | HR |
|---|---|
| 0 | 1.00 |
| 1 | 0.85 (0.57 to 1.28) |
| 2 | 0.66 (0.40 to 1.09) |
| 3 | 0.69 (0.38 to 1.26) |
| 4–5 | 0.23 (0.10 to 0.52) |
In terms of incidence per 100 person-years, group 0 had a rate of 8.4 and group 4/5 a rate of 2.0.
Weight loss from baseline was strongly associated with the other goals, as is shown by the weight loss in each group (Table 4).
| Success score | Three-year weight reduction (%) |
|---|---|
| 0 | 0.50 |
| 1 | 2.10 |
| 2 | 4.30 |
| 3 | 4.70 |
| 4–5 | 8.70 |
Lindstrom et al. (2006)214 also examined the incidence of diabetes in the post-intervention years, and showed that it remained 36% lower because most of the intervention group maintained their lifestyle changes. However, the difference between the incidence in the intervention and control groups narrowed with longer follow-up, from a RR reduction of 58% at the study end at 3 years to a 43% reduction at 7 years.
The results vary according to whether ITT analysis is used, because not all of the intervention group complied. If only those who achieved high success scores are considered then reduction in diabetes is much greater. Weight reduction appeared to be the most important factor.
A further study212 examined whether baseline factors predicted response. This could be important if there was scope for selecting those most likely to respond. They looked at the effect of gender, age, BMI, 2-hour plasma glucose and the Finnish Diabetes Risk Score (FINDRISC). HRs for diabetes in the intervention group compared with the control group were calculated. The effect of the intervention increased with age (HRs for tertiles of age were 0.78, 0.49 and 0.36; p for interaction = 0.013) and also for tertiles of baseline FINDRISC (HRs 1.19, 0.77 and 0.25). Gender, BMI and 2-hour plasma glucose were not significant predictors.
These results may imply that intervention would be more cost-effective in older people, and those with a high baseline risk.
A follow-up paper208 on the metabolic syndrome reported a significant reduction in the prevalence of the metabolic syndrome in the intervention group, compared with the control subjects, with ORs of 0.62 for metabolic syndrome and 0.48 for the prevalence of abdominal obesity. The authors comment that these changes should reduce the risk of CVD as well as diabetes.
The cost-effectiveness of the DPS intervention was addressed by Lindgren et al. (2007). 221 They applied the findings to a population-based cohort from Stockholm, using a Markov model in which people start in IGT and are at risk of developing diabetes or vascular disease. Therefore the model can assess the effects of the intervention on cardiovascular risk, not just diabetes. The cost assumptions reflected the intensity of the DPS, with, for example, seven meetings with the dietitian in year 1, and four times per year later, plus group circuit training and an annual physician visit. The high cost of the intervention is offset by reduced costs, but it is not clear from the paper where these come from or what proportions of the savings are from reducing diabetes, or reducing CVD. Intervention is said to yield a gain of 0.2 quality-adjusted life-years (QALYs), in a cohort of 397 people.
One way of improving the cost-effectiveness of the intervention would be to target it at people at higher risk. In the DPS,212 the NNT to prevent one case of diabetes was 7.7. However, among those with a baseline FINDRISC score of < 15, the NNT was 25, whereas among those with a baseline FINDRISC score of ≥ 15 the NNT was only 3.6.
Disappointingly, the 10-year follow-up did not show any difference in CVD between the groups, nor any change in overall mortality. However, as Uusitupa et al. (2009)222 report, this may be as a result of low power, arising from very low event rates in the DPS volunteers. A comparison with a population-based cohort, the FINDRISC group, showed that the DPS groups (combined) had a relative mortality risk of only 0.3 (95% CI 0.17 to 0.54), probably related to lower cardiovascular risk scores at baseline.
In summary, lifestyle intervention significantly reduced the incidence of diabetes in Finnish subjects with IGT, with the 7-year follow-up showing a fall in the incidence of diabetes from 7.4% to 4.3%, a reduction of 42%.
The Da Qing Study
Description and study quality
This trial was conducted in the Chinese industrial city of Da Qing, and compared three lifestyle interventions and a control group. 52,190,223 Five hundred and seventy-seven participants with IGT were recruited; no power calculation was reported. Participants were recruited from health-care clinic patients screened for T2DM and IGT. Clinics (rather than participants) were randomised (authors performed analysis that showed overall outcomes would probably not be altered as a result) to one of four groups: diet only, exercise only, diet plus exercise or control. Of the 577 participants randomised, 530 completed the study and had baseline values reported. Treatment groups were similar at baseline and treatment regimens were described in detail. All physicians, nurses and technicians involved in the study attended annual 2-day training sessions to receive standardised instructions on diet and exercise interventions. Exclusion criteria were not specified.
Participants were assessed over 6 years using subjective self-reporting of diet and physical activity and using objective measures for all other outcomes. The 6-year analysis included 530/577 (92%) participants: seven refused follow-up, 29 left Da Qing and 11 died during the course of the study. There were no deaths in the exercise-only group, three deaths in the control group (pneumonia, n = 1; cirrhosis, n = 2), three deaths in the diet-only group (cancer, n = 2; septicaemia, n = 1) and five deaths in the diet-plus-exercise group (stroke, n = 1; cancer, n = 2; accident, n = 1; Crohn’s disease, n = 1). AEs were not reported. No conflict of interest was reported.
Participants
Pan et al. (1993)52 recruited 577 men and women with IGT (as defined by WHO 1985 criteria; FPG of < 7.8 mmol/l and 75 g OGTT 2-hour ≥ 7.8 mmol/l and < 11.1 mmol/l). Participants with IGT were recruited from a total of 110,660 patients screened (87% of target population) for diabetes and IGT at 33 health clinics across the city. Participants were aged > 25 years. Baseline characteristics were not reported for the randomised population (n = 577) but were reported for those participants (n = 530) who completed 6 years of follow-up. The mean age (± SD) of the participants who completed 6 years of follow-up was 45.0 ± 9.1. Fifty-three per cent of participants were male; the male–female ratio varied between groups, although not significantly, with the diet-only group having slightly more women and with a male predominance in the other three groups. 190 Mean BMI was 25.8 ± 3.8 kg/m2.
Intervention
Participants allocated to the diet-alone group (n = 130 at 6 years) received individual instruction (frequency not reported) and small group counselling from physicians weekly for the first month, monthly for 3 months and quarterly every year for the remainder of the study. Participants were given advice according to their baseline BMI. Those with a BMI of < 25 kg/m2 were prescribed a diet containing 25–30 kcal/kg body weight, 55–56% carbohydrate, 10–15% protein and 25–30% fat. They were also encouraged to eat vegetables, control alcohol intake and reduce intake of simple sugars. Those with BMI ≥ 25 kg/m2 were advised to reduce their calorie intake with the aim of losing weight at a rate of 0.5–1.0 kg/month until they had achieved a BMI of 23 kg/m2.
Participants allocated to the exercise-alone group (n = 141 at 6 years) received counselling sessions weekly for the first month, monthly for the next two months and quarterly for the remainder of the study. They were advised to increase the amount of physical activity undertaken by at least one unit/day (two units/day if possible for those < 50 years of age with no evidence of CVD or arthritis); one unit equated to mild exercise for 30 minutes, moderate exercise for 20 minutes, strenuous exercise for 10 minutes or very strenuous exercise for 5 minutes. Participants in the diet-plus-exercise group (n = 126 at 6 years) received instructions and counselling for both diet and exercise interventions similar to both regimens described above. Participants in the control group (n = 133 at 6 years) received general information and instructions about diabetes and IGT, diet and increased physical activity. No individual instruction or group counselling was offered.
Results: primary outcomes
Pan et al. (1997)190 assessed the incidence of diabetes (WHO 1985 criteria: FPG of ≥ 7.8 mmol and/or 2-hour glucose ≥ 11.1 mmol/l) by oral glucose tolerance testing at 2 years, 4 years and 6 years. Results were expressed as the cumulative number (%) of participants with diabetes at 6 years and incidence per 100 person-years. They found that at 6 years the incidence of diabetes was significantly lower (p < 0.05) in each of the three intervention groups than in the control group (68%). The lowest incidence was reported in the exercise-alone group (41%) followed by the diet-alone group (44%) and the diet-plus-exercise group (46%).
Incidence was 10.0 (95% CI 7.5 to 12.5) in the diet-alone group compared with 8.3 (95% CI 6.4 to 10.3) in the exercise-alone group vs 9.6 (95% CI 7.2 to 12.0) in the diet-plus-exercise group compared with 15.7 (95% CI 12.7 to 18.7) in the control group. The influence of type of intervention and baseline characteristics on development of diabetes was assessed using a proportional hazards model; overall reduction in incidence of diabetes of 33% in the diet-only group (p < 0.03), 47% in the exercise-only group (p < 0.0005) and 38% in the diet-plus-exercise group (p < 0.005). Only modest changes in incidence were seen after adjustment for baseline factors.
Subgroup analysis of those participants with BMIs of < 25 kg/m2 (n = 208) compared with those with BMIs of > 25 kg/m2 (n = 322) showed that the incidence rates of diabetes in the control group of overweight participants were higher than those in the control group of lean participants (17.2 vs 13.3/100 person-years, p < 0.05). Li et al. (2002)223 stratified groups according to insulin resistance and β-cell insulin secretion levels at baseline and analysis showed that both were significantly associated (p < 0.05 and p = 0.01, respectively) with development of diabetes at 6-year follow-up; increasing insulin resistance and decreasing β-cell insulin secretion levels at baseline were associated with greater incidence of diabetes. Similarly, BMI and 2-hour plasma glucose levels at baseline were also significantly positively related to the development of diabetes (p < 0.01 and p = 0.01, respectively).
The 20-year results showed mixed results. 224 The follow-up 14 years after the 6-year intervention showed a 43% reduction in progression to diabetes, but no difference in cardiovascular events or all-cause mortality. Li et al. (2002)223 comment that the Da Qing study did not have statistical power for such events, but the RR for first CVD events was 0.98, suggesting that there was no difference.
Most people progressed to diabetes: 80% in the intervention group, 93% in the control groups.
Results: secondary outcomes
Pan et al. (1997)190 assessed weight loss at 3-month intervals using standard methods. Results are presented as weight change (kg) from baseline to 6 years in those with and without diabetes. 190 It found that in those participants without diabetes only those in the diet-plus-exercise group had mean weight loss (–1.77 kg) compared with a mean gain of 0.93 kg, 0.71 kg and 0.27 kg in the diet-alone, exercise-alone and control groups, respectively. In those who had developed diabetes, participants in each group had mean weight loss of 2.43 kg in the diet-alone group, 1.93 kg in the exercise-alone group, 3.33 kg in diet-plus-exercise group and 1.55 kg in the control group. No further analysis was shown.
Gong et al. (2011)225 reported retinopathy rates at 20-year follow-up. There was a 47% reduction in severe retinopathy (9% in the intervention group, 16% in the control group). No reduction in renal failure was seen, but numbers of end points were very small.
Adherence
Pan et al. (1997)190 examined the adherence of participants to diet intake. It was not entirely clear from the publications how diet adherence was assessed (unlike other studies no mention was made of food diaries); however, compliance with the intervention regimen was discussed with nurses and clinic staff at 3-month intervals using interviews and forms and at 2-year intervals physicians in Beijing recorded diet changes. These showed that there was no significant difference between groups in estimated total calorie intake, per cent carbohydrates, per cent protein, per cent fat or amount of alcohol consumed (g/day). Study authors noted that dietary changes and assessments were carried out by interviewers who were not masked as to the intervention.
As with assessment of dietary adherence, it is not clear how physical activity was recorded; however, compliance with the intervention regimen was discussed with nurses and clinic staff at 3-month intervals using interviews and forms and at 2-year intervals physicians in Beijing exercise changes. Change in physical activity (units/day) from baseline to 6 years was reported and showed that, compared with the control group, average units per day of exercise were slightly increased at 6 years in the diet-plus-exercise group, whereas little change or a decrease occurred in other groups; however, it should be noted that average units per day of exercise were significantly higher at baseline in the exercise and in the diet-plus-exercise groups.
In summary, lifestyle interventions (either diet, exercise or both) in a population with IGT led to a significant decrease in the incidence of diabetes at 6 years’ follow-up from 68% to 41–46%. Both increased insulin resistance and decreased β-cell function at baseline were predictors of greater incidence of diabetes. Longer-term follow-up showed continuing reduction of progression to diabetes, but no benefit in terms of CVD or all-cause mortality.
The Indian Diabetes Prevention Programme
Description and quality of trial
This trial in Chennai, India, compared three intervention groups (lifestyle interventions plus or minus pharmacological intervention and pharmacological only) with a control group. 173,226–228
Participants were recruited by screening a middle-class population (n = 10,839) working in service organisations and their families, who were identified by workplace announcements and circulars. Those with IGT (according to WHO 1999 criteria) on two separate OGTTs were recruited. Power calculation was not reported. A total of 531 participants were randomised into four groups: (1) diet plus exercise; (2) metformin; (3) diet plus exercise plus metformin; and (4) control group. Treatment groups were not significantly different at baseline apart from slightly greater prevalence of family history of diabetes in the diet-plus-exercise-plus-metformin group (p = 0.031). Treatment regimens were reported in detail. Use of concurrent medication was not reported and no exclusion criteria were reported. Participants were assessed over 3 years (median follow-up 30 months) using subjective self-reporting of diet and physical activity and using objective measures for all other outcomes. Twenty-nine participants (5%) did not complete the study; three in the control group (one death and two lost to follow-up), 13 in the diet-plus-exercise group (one death, five not willing and seven lost to follow-up), five in the metformin group (two not willing and three lost to follow-up) and eight in the diet-plus-exercise-plus-metformin group (one death, two not willing and five lost to follow-up). AEs (cardiovascular, gastrointestinal and deaths) were reported. No conflict of interest was reported.
Participants
Ramachandran et al. (2006)173 recruited 531 Indian men and women with IGT (as defined by WHO 1999 criteria: FPG of < 7.0 mmol/l and/or 75 g OGTT 2-hour ≥ 7.8 mmol/l and < 11.0 mmol/l). Participants with IGT were recruited from a total population of 10,839 working in service organisations and their families. Participants were to have no diabetes, no major illness, aged 35–55 years. The age distribution of participants was as follows: 35–39 years (15.4%), 40–44 years (24.5%), 45–49 years (29.2%) and 50–55 years (30.9%). Seventy-nine per cent (429/531) of participants were male, 49.5% had a family history of diabetes, 21.7% were smokers and 31.8% had hypertension. Mean BMI was between 25.6 ± 3.7 kg/m2 and 26.3 ± 3.7 kg/m2 in the four groups. The authors noted:
The Indian population have a young age of onset of diabetes, relatively lower BMI, with high rates of insulin resistance and lower thresholds for risk factors for diabetes. 173
In addition:
The Indian study cohort consisted of a middle-class working population, many of whom were already physically active and were on a diet similar to that prescribed. 173
Intervention
Participants were randomised to four groups: (1) diet plus exercise [lifestyle modification (LSM)]; (2) metformin; (3) diet plus exercise plus metformin (LSM + metformin); and (4) control. Participants randomised to LSM intervention group (n = 133) and the group randomised to LSM + metformin (n = 129) received monthly telephone calls and individual sessions every 6 months for lifestyle advice. Participants were encouraged to improve their diet (reduce total calories, refined carbohydrates and fats, avoidance of sugar and inclusion of fibre-rich food). Participants had their level of physical activity assessed and advice given accordingly; those who were involved in physical labour or who had to walk or cycle > 30 minutes/day or were performing exercises regularly were asked to continue their activity. Those participants engaged in sedentary or light physical activity were advised to walk briskly for at least 30 minutes per day. Participants randomised to metformin (n = 133) or LSM + metformin (n = 129) were given 250 mg twice daily. (Note: first 50 patients received doses titrated up to 500 mg twice daily but the dose was lowered owing to high incidence of symptoms suggestive of hypoglycaemia.) The control group regimen was not described.
Results: Primary outcomes
Ramachandran et al. (2006)173 assessed the incidence of T2DM (WHO criteria) by oral glucose tolerance testing at 6, 12, 18, 24, 30 and 36 months. Results were expressed in a number of ways: as the cumulative incidence of diabetes at 3 years, absolute and RR reduction and the NNT for 3 years. At 3 years, compared with the control group, the incidence of diabetes was significantly lower (p < 0.03) in each of the three intervention groups (55% control vs 39.3% LSM vs 39.5% LSM + metformin vs 40.5% metformin). Absolute risk reductions for the three intervention groups were 15.7%, 14.5% and 15.5% for the LSM, metformin and LSM + metformin groups, respectively. Similarly, the RR reductions (NNT) were 28.5% (6.4), 26.4% (6.9) and 28.2% (6.5), respectively.
The relationship between development of diabetes and other variables was examined using a Cox’s proportional hazards model. This found that, in addition to the three intervention regimens, the following variables also significantly influenced the development of diabetes: baseline 2-hour plasma glucose, fasting insulin and 2-hour insulin. Factors shown to have no influence on the development of diabetes were age, sex, family history of diabetes, BMI, WC, FPG, hypertension and smoking. Presumably these factors became non-contributory after the glucose and insulin levels were factored in.
In all of the groups, progression to diabetes was commoner in the subgroups that had both IGT and IFG than in those with only IGT, but these difference were not statistically significant, perhaps because of the relatively small numbers in the IFG + IGT groups. 229
Eleven cardiovascular events were reported: two in the control group (one person died following surgery for CVA), four in the LSM group and five in the LSM-plus-metformin group.
Results: secondary outcomes
Weight loss was assessed annually using standard methods. Results were presented as mean change in body weight (kg) from baseline to 12, 24 and 30 or 36 months. The study found that, of the four groups, only the weight of the control group increased significantly at each time point compared with baseline (p < 0.01). Weight change was not significant in either the metformin group or the metformin + LSM group; however, in the LSM group a significant increase in weight was seen at 24 months relative to baseline values (p = 0.035). BMI did not change much in the intervention group (25.5 kg/m2 at baseline, 25.7 kg/m2 at follow-up) but increased a little more in the control group (26.0–26.4 kg/m2). So the reduction in progression to diabetes occurred without weight loss. 230
Adherence
Ramachandran et al. (2006)173 examined the adherence of participants to intervention. Diet adherence was assessed subjectively every 6 months up to 3 years by examining self-reported weekly food records filled in by participants and calculating average ‘adherence scores’ every 6 months. These showed that both the LSM group and the LSM-plus-metformin group improved their adherence over the study. The diet adherence of the LSM group improved from 62.5% to 81.6%, whereas the LSM + metformin group improved from 62% to 81.9%.
Adherence to physical activity guidance was assessed subjectively by weekly self-report physical activity and by averaging the ‘adherence scores’ from 6 months to 3 years. There was an increase in the percentage physical activity adherence from 41.7% to an average of 58.8% in the LSM group and an increase from 45.9% to 62.9% in the LSM-plus-metformin group.
Ramachandran et al. (2006)173 also determined the prevalence of the metabolic syndrome, as defined by the WHO criteria, in their trial participants. 227 It was found in 46% (95% CI 42% to 52%) overall, but was commoner in women (62%; 95% CI 52% to 71%) than men (42%; 95% CI 37% to 47%). The presence of metabolic syndrome did not increase the incidence of diabetes.
In summary, lifestyle intervention (either alone or in combination with metformin) significantly reduced the incidence of diabetes in Asian Indians with IGT, from 55% to 40%. No added benefit was seen when combining them.
Kosaka et al.186
Description and quality of study
This RCT compared a diet and exercise intervention with standard advice. 186
Japanese male subjects with IGT were randomised (number not reported; however, n = 458 at 1 year and 4.7–5.5% dropped out during first year) and assessed over a 4-year period using glucose, anthropometry, blood pressure and lipid measurements. Inclusion and exclusion criteria were explicit; however, a priori sample size calculation and randomisation method was not reported. Subjects were randomised into two treatment groups: in the first group (diet and exercise n = 102 at 1 year) participants were recommended to reduce their weight at a rate of 0.5–1 kg/month to achieve their target BMI (22 kg/m2) through a combination of intensive individually-tailored dietary and physical activity advice. In the second group (control n = 356 at 1 year) participants received standard advice to improve diet, increase physical activity and lose weight.
Lifestyle and control groups were similar at baseline in terms of age, sex, BMI, blood pressure and cholesterol levels. Baseline physical activity was not reported; however, 15% of subjects in the lifestyle intervention group were already performing the required amount of exercise and were recommended to maintain this level. The percentage of subjects in the control group already performing exercise was not reported. Concurrent medication and mean follow-up were not reported.
Participants
Kosaka et al. (2005)186 recruited Japanese males with IGT (as defined by WHO 1980 criteria; FPG of < 7.8 mmol/l and/or 100 g OGTT 2-hour of ≥ 8.8 mmol/l and < 13.27 mmol/l) randomly selected from population of government workers undergoing health screening. Men were excluded if they had a previous history of diabetes, diagnosed or suspected malignant neoplasms, diagnosed or suspected disease of the liver, pancreas, endocrine organs, or kidney, ischaemic heart disease or cerebrovascular disease or history of such disease. Baseline characteristics were not reported for the overall population but were given separately for the two groups who completed 1 year. The mean age of the participants was not reported; however, approximately 87% of participants in both groups were between the ages of 40 and 60 years. All participants were Japanese males and approximately 42% in both groups had first-degree relatives with diabetes. Mean BMI was approximately 24 mg/kg2 in both groups (so participants in Japan were leaner than those in the American and Finnish studies) and mean weight was not reported. Baseline characteristics were not significantly different between groups.
Intervention
Participants allocated to the intensive lifestyle intervention group (n = 102 at 1 year) were given advice according to their baseline BMI. Those with BMI of ≥ 22 kg/m2 were informed of their desirable body weight to achieve a BMI of 22 kg/m2. They were advised to reduce their weight at a rate of 0.5–1 kg per month until their target weight was achieved. Those with a BMI of < 22 kg/m2 were advised to maintain their present weight. To achieve the body weight objective participants were given individually tailored advice every 3–4 months at each hospital visit. They were advised to reduce amount of food, increase consumption of vegetables, reduce intake of fat and alcohol, and take 30–40 minutes of moderate exercise per day. Participants in the control group (n = 356 at 1 year) were also given advice based on their baseline BMI but this was given every 6 months; those with a BMI of > 24 kg/m2 were advised to lose weight by eating smaller meals and increasing their physical activity, whereas those with a BMI of < 24 kg/m2 were told to avoid weight gain by dieting and exercise.
Results: primary outcomes
The progression to diabetes (FPG of ≥ 7.8 mmol/l in consecutive tests within 2-week time period) was assessed by testing FPG every 2–3 months over 4 years. Results were expressed as the cumulative incidence of diabetes during the 4-year follow-up. The study found that the cumulative incidence of diabetes was significantly lower in the intensive lifestyle intervention compared with the control group (3.0% vs 9.3% respectively; p < 0.043) equating to a reduction of 67.4% in the intensive lifestyle group.
The relationship between progression to diabetes and weight change was evaluated in the control group alone. It was found that when participants in the control group were subdivided according to change in weight, the progression to diabetes was significantly less in those who had lost weight than in those whose weight had increased. Cumulative incidence of diabetes was 4.3% in those whose weight had decreased by > 1.0 kg (n = 126) compared with 10.6% [p = not significant (NS)] in those with no weight change (< ± 1 kg) (n = 173) and 14.7% (p = 0.006) in those who had gained ≥ 1.0 kg (n = 57).
Kosaka et al. (2005)186 also assessed regression of participants from IGT to NGT (FPG of < 7.8 mmol/l and/or 100 g OGTT 2-hour cut-off ≤ 8.8 mmol/l). After 4 years, 53.8% in the intervention group compared with 33.9% in the control group had improved from IGT to non-IGT. When the control group was stratified according to weight change, it was seen that the rate of improvement was significantly greater in those whose weight had decreased relative to those whose weight was unchanged or increased: 47.6% in those whose weight had decreased > 1 kg (n = 126) compared with 12.5% in those whose weight had increased by ≥ 1.0 kg (n = 57) and 30.1% in those whose weight was unchanged (< ± 1kg) (n = 173 kg).
Results: secondary outcomes
Participants assigned to the lifestyle intervention had significantly greater weight loss than did those in the control group. Over 4 years the average weight loss was 2.18 ± 1.63 kg and 0.39 ± 1.42 kg in the intervention and control groups, respectively (p < 0.001). In the intervention group, mean body weight had decreased by 2.5 kg at 1 year and tended to increase slightly thereafter; however, it remained significantly lower than baseline at the end of 4 years. In contrast, the control group had mean weight loss of 0.57 kg (measured from graph) at 1 year with weight increasing slightly every year thereafter.
In summary, Kosaka et al. (2005)186 found that intensive diet and exercise intervention in those with IGT significantly reduced the incidence of diabetes by 67.4% compared with standard lifestyle intervention (3.0% vs 9.3%; p = 0.043) and increased the rate of regression from IGT to NGT by 58.7% compared with standard lifestyle intervention (53.8% vs 33.9%; p < 0.001).
Liao et al.187
Description and quality of study
The RCT undertaken by Liao et al. (2002)187 in 74 Japanese American participants with IGT compared an intensive diet and aerobic exercise intervention with a standard diet and stretching exercise intervention. 187
Participants were recruited after telephone screening of 340 Japanese individuals; of 102 diagnosed with IGT, 74 were randomised (28 were not randomised because of medical information or declining to participate) and baseline data were presented for 64 who completed 6 months. No power calculation was reported. Participants were randomised into two treatment groups: an intensive-diet-and-aerobic-exercise group compared with a standard-diet-and-stretching-exercise control group. Treatment groups were not significantly different at baseline when sex was taken into consideration (see Participants, below). Treatment regimens were reported in detail. It was not reported whether any concurrent medication was taken; however, participants were excluded at screening if they were using lipid-lowering drugs.
Patients were assessed over 24 months using subjective self-reporting of diet and physical activity and using objective methods for all other measurements. Fifty-eight participants completed the study (n = 29 in both groups); three participants did not take part because of poor venous access; one had an abnormal treadmill test; nine dropped out; two developed diabetes at 6 months; and one developed diabetes at 12 months. Sixty-four participants completed 6 months and their results were included from baseline to 24 months. (Note: it is not clear why an additional six patients have contributed data to the end of the study.) AEs were not reported. No conflict of interest was reported.
Participants
Liao et al. (2002)187 recruited 74 Japanese American participants with IGT (as defined by WHO 1998 criteria; FPG of < 7.0 mmol/l and/or 75 g OGTT 2-hour ≥ 7.8 mmol/l and < 11.1 mmol/l) selected after telephone screening of 340 individuals of full Japanese ancestry. Subjects were excluded if they had a history of significant coronary artery disease; valvular heart disease; hypertension (blood pressure > 160/90 mmHg); arthritis; pulmonary, neurological/psychiatric disease or dementia, which hindered ability to participate; unusual dietary restrictions (e.g. strict vegetarians); current use of lipid-lowering drugs; or tobacco use. Participants were also excluded if laboratory tests showed evidence of liver or kidney disease or anaemia (haematocrit: < 38% for men, < 36% for women) or if triglyceride levels were > 300 mg/dl.
Baseline characteristics were not reported for the overall population but were given separately for the two groups that completed 6 months. The mean age of the participants in the intervention and control groups was 55.8 ± 1.8 years and 52.2 ± 1.8 years, respectively. Mean BMI was 25.6 ± 0.8 kg/m2 and 26.6 ± 0.8 kg/m2 in the intervention and control groups, respectively, and mean weight was 66.1 ± 2.9 kg and 69.7 ± 2.6 kg. Baseline characteristics were not significantly different between groups; the intervention group had more women and hence a significantly lower amount of intra-abdominal fat (p = 0.038) and waist girth (p = 0.04). The differences were not significant after adjusting for sex. It was noted in the discussion that many of the study participants were already consuming an American Heart Association (AHA) step 1 diet at baseline (low in saturated fat) and that in this respect they may not be representative of all Japanese Americans.
Intervention
Participants randomised to the intensive lifestyle intervention group (n = 36) received 6 months’ supervised endurance exercise on a treadmill for 1 hour three times a week with a goal to be exercising at 70% of heart rate reserve by 3 months. In addition, they were prescribed an isocaloric AHA step 2 diet comprising < 30% of total calories as fat (< 7% as saturated fat), 55% as carbohydrate, the balance as protein, and < 200 mg of cholesterol daily. Participants randomised to the control group (n = 38) received supervised stretching exercises for 1 hour three times per week and were prescribed an isocaloric AHA step 1 diet comprising 30% of total calories as fat (10% as saturated fat), 50% as carbohydrate, 20% as protein, and < 300 mg cholesterol daily. All participants were instructed to continue their diet and exercise unsupervised for an additional 18 months and were reminded of this at 12 months.
Results: primary outcomes
Liao et al. (2002)187 assessed the incidence of diabetes by oral glucose tolerance testing at 6, 12 and 24 months. It should be noted that this study was not designed to demonstrate prevention of diabetes. Its objective was to determine whether a lifestyle intervention would improve adiposity and body fat distribution in Japanese Americans with IGT. Results were expressed as the incidence of diabetes at 12 months, and found that one participant in the intervention group had developed diabetes at 12 months compared with two participants in the control group. No analysis of these results was undertaken.
Liao et al. (2002)187 also assessed regression of participants from IGT to NGT. Results were expressed as the proportion of participants showing NGT at least once during their 24 months of follow-up and they found that this was significantly greater in the intervention group than in the control group (67% vs 30%, respectively; p = 0.01).
Results: Secondary outcomes
Participants assigned to the intensive lifestyle intervention had significantly greater weight loss than participants assigned to the control group. Weight was measured at 6 months and 24 months using a standard method. At 6 months the intervention group had mean weight change from baseline (± SD) of –2.7 ± 0.4 kg compared with –0.9 ± 0.3 kg in the control group (p = 0.0003 adjusted for sex and baseline values). At 24 months, the difference between groups, although significant, was not as marked; intervention group weight change –1.8 ± 0.5 kg compared with 0.7 ± 0.6 kg in the control group (p = 0.0043).
BMI was also measured at 6 months and 24 months. Results showed significant differences between groups at both time points; at 6 months change in mean BMI (± SD) from baseline in the intervention group was –1.1 ± 0.2 kg/m2 compared with –0.4 ± 0.1 kg/m2 in the control group (p = 0.0003 adjusted for sex and baseline values). Similarly, at 24 months the intervention group maintained the decrease in BMI below baseline values at –0.7 ± 0.2 kg/m2 compared with an increase from baseline of 0.2 ± 0.2 kg/m2 in the control group (p = 0.0022).
Adherence
Liao et al. (2002)187 examined the adherence of participants to intervention. Diet adherence was assessed at 3, 6, 9, 12 and 24 months using 3-day food records submitted by participants (subjective outcome) and these were compared with the recommended intake according to allocated diet (either AHA step 2 for intervention group or AHA step 1 for control group). Results showed that, on average, at the five time points both the intensive lifestyle intervention group and the control group met their dietary goals.
The intervention group were assigned dietary goals of < 30% calories from fat and 7% from saturated fat. On average over the five time points they consumed 22–23.3% of calories from fat and 5.8–6.6% of calories from saturated fat. However, not all participants in this group achieved the dietary goals; between 79% and 88% of participants consumed < 30% of calories from fat, 55–70% consumed < 7% of calories as saturated fat and 66–97% consumed < 200 mg of cholesterol. Similarly, the control group achieved their respective dietary goals of approximately 30% of calories as fat (24.6 to 29.7% over the five time points) and < 10% of calories as saturated fat (7.1% to 8.5% over the five time points). Between 59 and 79% of participants consumed < 30% of calories from fat, 77–88% consumed < 10% calories as saturated fat and 74–89% consumed < 300 mg of cholesterol.
Physical fitness was assessed at 6 months (end of supervised exercise) and 24 months by measuring the change in VO2max. At the end of the 6-month supervised period, the intervention group achieved a 3.3 ± 0.8 ml/kg/minute improvement in VO2max (p < 0.0001) compared with the control group (–0.6 ± 0.6 ml/kg/minute). At 24 months, the intervention group still had a significantly greater improvement over baseline (2.6 ± 0.7 ml/ kg/minute) compared with the control group (–0.7 ± 0.5 ml/kg/minute, p = 0.0002). The percentage of participants showing a change in VO2max of ≥ 1.5 ml/kg/minute (selected arbitrarily as indicative of physical fitness) was 51.6% in the intervention group compared with 15.6% in the control group at the end of the 6-month supervision period (p = 0.006), and 59.3% and 13.8%, respectively, at 24 months (p = 0.001).
In summary, Liao et al. (2002)187 found that AHA step 2 diet and endurance exercise intervention in those with IGT significantly improved BMI and weight loss up to 24 months compared with AHA step 1 diet and stretching exercises intervention. A significantly greater percentage of patients in the intensive lifestyle intervention (AHA step 2 and endurance exercise) (67%) achieved NGT at least once during 24 months than in the control group (30%).
Maastricht (SLIM) Study: Mensink et al.188
Description and quality of study
This trial188,231–234 compared intensive diet and exercise intervention with standard advice about lifestyle.Participants were recruited from an existing cohort in the Maastricht area; subjects with high risk of glucose intolerance were invited to undergo OGTT. The authors stated:
Participation rate was low, approximately 50%, as subjects were selected from an ongoing monitoring project for health and disease and therefore may be suffering from ‘research-fatigue’ possibly leading to selection bias. 233
It should be noted that classification was based on single OGTT only (the risk of diabetes is higher in those with IGT classified using two OGTTs). Power calculations indicated that two groups of 50–60 participants would be sufficient to detect a 1-mmol/l difference in 2-hour glucose between groups following 2 years of intervention. A total of 114 participants were randomised into two groups: intensive lifestyle intervention compared with control (standard lifestyle advice). Treatment groups were not significantly different at baseline. Treatment regimens were reported in detail. Use of concurrent medication was not reported; however, subjects taking medication known to interfere with glucose intolerance (e.g. steroids) were excluded. Patients were assessed over 2 years using subjective self-reporting of diet and physical activity and using objective measures for all other outcomes. One hundred and two participants were included in the 1-year analysis and 88 at 2 years. Twenty-six participants dropped out of the study: 12 at 1 year and another 14 at 2 years (no differences in baseline values between participants and dropouts). AEs were not reported. No conflict of interest was reported.
Participants
Mensink et al. (2003)188 recruited 114 participants with IGT (as defined by WHO 1999 criteria; FPG of < 7.8 mmol/l and/or 75 g OGTT 2-hour ≥ 7.8 mmol/l and < 12.5 mmol/l) selected from a cohort at high risk of glucose intolerance. Subjects were excluded if they had previously diagnosed diabetes mellitus (other than GDM); mean 2-hour blood glucose > 12.5 mmol/l; medication known to interfere with glucose tolerance (e.g. chronic steroid use); participation in regular vigorous exercise or an intensive weight reduction programme during the year before the start of the study; any chronic disease that made participation in a lifestyle intervention programme impossible, or had an improbable 5-year survival. Baseline characteristics were not reported for the overall population but were given separately for the two groups following randomisation. The mean ages [± standard error (SE)] of the participants in the intervention and control groups were 55.6 ± 0.9 years and 57.8 ± 1.0 years, respectively. Mean BMIs were 29.8 ± 0.5 kg/m2 and 29.3 ± 0.4 kg/m2 in the intervention and control groups, respectively, and mean weights were 86 ± 1.9 kg and 83.7 ± 1.5 kg. In the intervention group, 25.5% had a family history of diabetes compared with 35.5% of those in the control group. Details of ethnicity were not reported.
Intervention
Participants randomised to intensive lifestyle intervention group (n = 55) were encouraged to undertake ≥ 30 minutes of moderate physical activity every day for at least 5 days/week. A supervised exercise programme (including aerobic exercise and resistance training) was encouraged. In addition, specific dietary recommendations were issued (based on Dutch Guidelines): carbohydrate intake ≥ 55% total energy; fat 30–35% total energy; saturated fatty acids < 10%; cholesterol < 33 mg/MJ; protein 10–15% total energy; dietary fibre 3 g/MJ. Participants were given the goal of losing 5–7% of their body weight. Follow-up visits took place at 4–6 weeks, 3 months and every 3 months thereafter. Participants in the control group were given oral and written information about healthy diet, weight loss and increased physical activity. No individual advice, programmes or follow-up visits were offered to the control group.
Results: primary outcomes
The number of participants with regression to NGT was assessed by annual OGTT. At 2 years significantly more participants in the intervention group (50%) than in the control group (29%) had NGT (p < 0.05).
At 3 years, the cumulative incidence of diabetes among those who completed the study was 18% in the intervention group and 38% in the control group, a RR of 0.42 (0.18 to 0.96). 235 However, an ITT analysis gave an incidence of only 32% in the control group, a RR of 0.52 (0.25 to 1.10).
Results: secondary outcomes
Weight loss was assessed using standard methods at 1 year (n = 102) and 2 years (n = 88 participants who completed the study). Participants assigned to the intensive lifestyle intervention had significantly greater weight loss than participants assigned to the control group. At 1 year the intervention group had mean weight change from baseline (± SE) of –2.7 ± 0.5 kg compared with –0.2 ± 0.5 kg in the control group (p < 0.01). At 2 years, a significant difference between groups was maintained; intervention group weight change –2.4 ± 0.7 kg compared with +0.1 ± 0.5 kg in the control group (p < 0.01).
The paper by Roumen et al. (2008)235 that reported the 3-year results gives slightly different figures for weight loss, reporting that in the intervention group, weight loss at 12 months was 2.77 kg. The 3-year weight loss was only 1.08 kg, so the gap between intervention and control groups had narrowed, although it was still just statistically significant, partly because the control group had gained a little more.
Change in mean BMI from baseline was significantly different between groups at both 1 year (n = 102) and 2 years (n = 88). At 1 year in the intervention group mean BMI changed by –0.9 ± 0.2 kg/m2 compared with 0.0 ± 0.2 kg/m2 in the control group (p < 0.001). This difference was maintained at 2 years; intervention group mean BMI decreased by 0.8 ± 0.2 kg/m2 compared with 0.0 ± 0.2 kg/m2 in the control group (p < 0.01).
No significant difference in LDL or cholesterol levels between groups was reported.
Adherence
Diet adherence was assessed subjectively at 1 year (n = 102) and 2 years (n = 88) by examining 3-day food records filled in annually by participants and checked by a dietitian. These showed that the lifestyle group had a significantly greater reduction in daily energy intake over the first year, but not the second year, compared with the control group (baseline to year 1: –1.2 MJ/day with intervention vs –0.3 MJ/day with control; p = 0.02; baseline to year 2: –0.9 MJ/day vs –0.3 MJ/day; p = NS); this was associated with a significantly greater change in the carbohydrate intake as percentage of total calories from baseline to both 1 year and 2 years (baseline to year 1: +4.7% with intervention vs +0.7% with control; p < 0.02; baseline to year 2: +5.5% vs +0.8%; p < 0.01). Similarly, the intervention group had significantly reduced their intake of fat as percentage of total calories from baseline to both 1 year and 2 years (baseline to year 1: –5.0% with intervention vs –1.0% with control; p = 0.01; baseline to year 2: –4.8% vs –0.3%; p < 0.01).
Physical fitness was assessed objectively at 1 year (n = 102) and 2 years (n = 88) by measuring VO2max. The lifestyle group had significantly improved their VO2max compared with the control group from baseline to both 1 year and 2 years. Change in VO2max (± SE) from baseline to year 1 was +0.10 l/min ± 0.03 in the intervention group compared with +0.00 l/min ± 0.03 in the control group (p < 0.05). Similarly, from baseline to year 2 change in VO2max (± SE) was +0.09 l/min ± 0.04 in the intervention group compared with –0.03 ± 0.04 l/min in the control group (p < 0.05).
In summary, Mensink et al. (2003)188 found that intensive lifestyle intervention in those with IGT significantly improved BMI and weight loss up to 24 months compared with standard advice about lifestyle. A significantly greater percentage (50% vs 29%) of patients in the intensive lifestyle intervention group achieved NGT during 24 months.
The Newcastle trials
Description and quality of study
The first study by Oldroyd et al. (2001,236 2006189) was a relatively small trial in 78 men and women with IGT. It compared an intensive diet and physical activity counselling intervention with a control group who were offered no dietary or physical activity advice. Power calculation found that a sample size of 100 participants was required to have 90% probability of detecting a 0.6-mmol/l difference in mean FPG and a 20% difference in the proportion with glucose intolerance; therefore, the study was underpowered with only 78 participants recruited, and results for only 69 participants at 6 months, 62 at 12 months and 54 at 2 years. Participants were selected from existing studies, hospital databases and general practitioner (GP) surgeries. Participants were selected from individuals diagnosed with IGT on two consecutive OGTTs, and randomised using a random number table to two treatment groups; intensive lifestyle intervention and usual care (no lifestyle advice). Treatment groups were not similar at baseline; the intervention group had twice as many females as the control group, mean resting pulse was lower in the control group compared with intervention group (p = 0.011) and significantly more control participants reported engaging in regular physical activity (p = 0.017) compared with the intervention group. The interventions were described in detail. It was not reported whether any concurrent medication was taken. Participants were assessed over 2 years using subjective self-reporting of diet and physical activity and using objective methods for all other measurements. Sixty-nine per cent (54/78) of participants completed the study (n = 24 in the control group and n = 30 in the intervention group); 24 patients were lost to follow-up. Of these, 14 participants (intervention, n = 5; control, n = 9) withdrew from the study owing to family problems, work commitments or ill health. Nine participants (intervention, n = 3; control, n = 6) failed to attend 2-year follow-up and one participant died after a stroke between 12 months and 2 years. AEs were not reported. No conflict of interest was reported.
Participants
Oldroyd et al. (2006,189 2001236) recruited 78 men and women with IGT. Inclusion criteria were age between 24 and 75 years old and European origin. Participants were excluded if they were pregnant, on therapeutic diets or unable to undertake moderate physical activity. Baseline characteristics were not reported for the randomised population (n = 78) but were reported for those participants (n = 69) who completed 6 months of follow-up. The mean age of the participants who completed 6 months of follow-up in the intervention and control group was 58.2 years (range 41–75 years) and 57.5 years (range 41–73 years). In the intervention group, 10/32 (31%) participants were females compared with 20/37 (54%) in the control group (p = NS). Mean weight ± SD was 85.5 ± 14.2 kg and 85.3 ± 17.9 kg, respectively.
Intervention
Participants randomised to the intensive lifestyle intervention group (n = 39) received 12 appointments over 24 months for regular motivational counselling and written information from a dietitian and physiotherapist. Participants were encouraged to improve their diet (regular meals, more fruit and vegetables, reduce fat and sugar intake and eat adequate fibre) with a goal of reducing their BMI to < 25 kg/m2 in those who were overweight or obese. Specific dietary goals included fat intake ≤ 30% of total energy; polyunsaturated to saturated fat ratio of ≥ 1.0; 50% of energy from carbohydrate and dietary fibre intake of ≥ 20 g per 4.2 MJ. In addition, participants were given an individually tailored physical activity plan designed to enable 20–30 minutes of aerobic exercise at least once a week. Participants in the control group received no dietary or physical activity advice for the duration of the study.
Results: primary outcomes
Oldroyd et al. (2001,236 2006,189) assessed the incidence of diabetes by oral glucose tolerance testing at 6, 12 and 24 months. At 24 months there was no difference in the percentage of participants in the intervention group 7/32 (22%) and control group 8/37 (22%) who developed diabetes over the 24-month trial.
More participants from the intervention than the control group reverted to NGT (FPG < 7.8 mmol/l) at 12 months’ and 24 months’ follow-up (22% vs 17% at 12 months; 20% vs 13% at 24 months). Significance level was not reported.
Results: secondary outcomes
Weight loss was assessed at 6 months (n = 69), 12 months (n = 62) and 2 years (n = 54 participants who completed the study). At all time points, participants assigned to the intensive lifestyle intervention had significantly greater weight loss than the control group. Mean weight loss from baseline (± SD) at 6 months was –1.1 kg in the intervention group compared with +0.54 kg in the control group (p = 0.010), at 12 months it was –1.1 kg vs +1.5 kg (p = 0.001) and at 24 months it was –1.8 kg vs +1.5 kg (p = 0.008).
There were no significant differences in LDL or cholesterol levels between groups at 6, 12 or 24 months.
Oldroyd et al. (2001,236 2006189) found changes in systolic BP and diastolic BP of borderline significance. Change in systolic BP at 6 months ± SD was –7.9 ± 16.7 mmHg in the intervention group vs –0.27 ± 14.3 mmHg in the control group (p = 0.05). Change in diastolic BP at 6 months was –2.9 ± 9.9 mmHg vs +1.9 ± 10.0 mmHg (p = 0.052). The authors noted:
We cannot exclude the possibility that greater familiarisation of intervention participants with the intervention team, which occurred during review appointments, compared with controls, contributed to the decrease we observed in blood pressure. 236
Adherence
Diet adherence was assessed subjectively at 6 months (n = 69), 12 months (n = 62) and 2 years (n = 54) by examining 4-day food records filled in by participants and checked by a dietitian. These showed that, compared with the control group, the lifestyle group had a significantly greater reduction in total fat intake (g/day) at all time points (6 months: –13.6 ± 35.3 vs +3.7 ± 30.4; p = 0.037; 12 months –16.7 ± 26.5 vs –0.43 ± 33.5, p = 0.044; 24 months: –24.4 ± 24.5 vs –6.5 ± 30.9; p = 0.027). Changes in polyunsaturated/saturated fat (P/S) ratio and dietary fibre were not significantly different between groups at any time point. The number (%) of participants achieving dietary targets was assessed at baseline and 24 months. This showed that the number of participants in the lifestyle intervention group, but not the control group, who achieved the following nutritional targets was significantly increased from baseline to 24 months: ≤ 30% energy as fat; ≥ 50% total energy as carbohydrate and dietary fibre ≥ 20 g per 4.2 MJ energy (p < 0.02 for each outcome).
Physical fitness was assessed at 6 months (n = 69), 12 months (n = 62) and 2 years (n = 54) by objective measurement of distance covered in shuttle walking test and change in resting pulse and subjective self-reported measures of participation in regular activity. Oldroyd et al. (2001,236 2006189) observed a significant increase in the percentage of participants at 6, 12 and 24 months who reported undertaking regular physical activity once a week sufficient to get their heart thumping: change from baseline in intervention and control group (6 months: +33.3% 95% CI 13 to 50 with intervention vs –3.1% with control 95% CI –14 to 8.5; p = 0.03; 12 months: +34.3 95% CI 16 to 49 vs +7.1 95% CI –8 to 21; p = 0.02; 2 years: +32.1 95% CI 12 to 48 vs –4.2 95% CI –23 to 14; p = 0.03). No change in resting pulse rate was observed at 6 and 12 months, but a small but significant decline from baseline in resting pulse rate was reported at 24 months (–4.4 ± 8.5 in the intervention group vs 1.2 ± 8.5 in the control group; p = 0.023); however, there was no significant difference at any time during follow-up for the mean distance walked in the shuttle test (data were not shown). It was noted by the authors that caution should be exercised in interpreting the results, as (1) medication that may affect pulse rate was not recorded and (2) self-reported physical activity and physical fitness are generally poorly correlated because of over-reporting of physical activity.
Adherence was also assessed by percentage attendance at review appointments (diet and exercise) of intervention participants. Twenty-four out of 39 (62%) participants attended all of the review appointments up to 6 months, and this increased to 28/39 (72%) at 12 months; however, by 24 months attendance lay at 36% with only 14/39 of the randomised participants attending the review appointments [average in the first 6 months was 80% (range 67–95%)].
In summary, Oldroyd et al. (2001,236 2006189) found that regular counselling on lifestyle over a 24-month period from a dietitian and physiotherapist resulted in significant improvements in weight. Cardiovascular risk factors were unchanged.
A later study from the same group also reported a reduction in diabetes incidence, by about half, from 33 per 1000 person-years in the intervention group, to 67 in the control group. 237 This study came from the Newcastle centre of the European Diabetes Prevention Study (EDIPS), and had only 102 participants. The full EDIPS will have 750 participants.
Wein et al.191
Description and quality of study
This RCT compared an intensive lifestyle guidance intervention with initial lifestyle guidance in pregnant women with IGT over a median 4.25-year period (range 11.7–81.1 months) in an intervention group compared with median 4.0 years in a control group; p = 0.021. 191 Inclusion criteria were IGT, English and non-English speakers, and no exclusion criteria were specified in the publication. A priori sample size calculation and randomisation method were not reported. Subjects were randomised into two treatment groups: intensive lifestyle group and control group. Both groups were similar at baseline. Concurrent medication was not reported. Seven patients were lost to follow-up (three in the intervention group and four in the control group). Adverse effects were not reported.
Participants
Wein et al. (1999)191 recruited 200 pregnant Australian females with IGT (as defined by WHO 1985 criteria; FPG < 7.8 mmol/l and 75 g OGTT 2-hour ≥ 7.8 mmol/l and < 11.1 mmol/l) selected from a long-term follow-up of women with GDM at a single hospital. Participants were regular attendees for follow-up testing. Baseline characteristics were similar at baseline. The mean age of the participants was 39.5 years in the intervention group and 37.8 years in the control group. Mean weight was 64.9 kg and 66.4 kg, respectively, and BMI was 25.2 kg/m2 and 25.6 kg/m2, respectively. Participant’s country of birth was recorded and no significant difference between groups was found. In the intervention and control groups, 48% and 45%, respectively, were born in Australia or New Zealand, with smaller percentages from the Mediterranean/Middle East (20% and 21%, northern Europe (7% and 6%), South East Asia (20% and 23%) and the Indian subcontinent (5% and 5%). Parity was not significantly different between groups. The study authors noted the following: (1) participants may be a self-selected compliant group in that they elected to attend follow-up and (2) participants had already been exposed to counselling with respect to diet and exercise during their pregnancy.
Intervention
Participants allocated to the intervention group (intensive lifestyle guidance, n = 100) were given a standard diet advice sheet (‘Target on Healthy eating’ recommended by the state Health Department) and reminded of the need for regular exercise (brisk walking for 30 minutes, three times a week). Every 3 months, a dietitian contacted the participants by telephone. The dietitian’s role was to answer questions regarding diet and encourage compliance with the diet and exercise recommendation. In comparison, the control group (n = 100) received the same standard advice sheet and exercise advice but had initial advice only and no additional contact with the dietitian.
Results: primary outcomes
The progression to diabetes was assessed by oral glucose tolerance testing annually. Results were expressed as the prevalence of diabetes, annual incidence of diabetes, the cumulative rates of decay to diabetes up to 5 years and the RR of diabetes.
The study found that the prevalence of diabetes over median follow-up of 51 months (longer follow-up in the intervention group) was not significantly different between groups [26/97 (26.8%) in intervention group vs 27/96 (28.1%) in the control group; p = 0.957]. (Note: percentage based on participants who completed study.) The annual incidence of diabetes was not significantly different between groups. Cox regression analysis of all participants showed that baseline BMI (p = 0.0007), fasting (p = 0.04) and 2-hour plasma glucose (p < 0.0001) levels were associated with an increased risk of diabetes. No significant association was found with change in BMI and age at start of study.
Wein et al. (1999)191 also assessed regression of participants from IGT to NGT (no specific definition in publication). Results were expressed as n (%) participants with NGT status after a median 51 months’ follow-up and showed that there was no significant difference between groups in the progression to NGT [43/97 (44.3%) in the intervention group vs 43/96 (44.8) in the control group]. (Note: percentage based on participants who completed study.)
Results: secondary outcomes
No significant difference between groups was seen in BMI. Presumably that explains why there was no difference in diabetes.
Adherence
The adherence of participants to intervention was examined. Diet adherence was assessed using a questionnaire to record diet history at baseline and annually until final assessment. The fat, residue and sugar content of the diet were scored from ‘1’ to ‘3’ (representing ‘poor’ to ‘good’) and the total score calculated. Baseline and final assessment data were presented and both intervention and control groups showed an improvement in diet score (+0.64 and +0.56 points, respectively; p = 0.32); however, no significant difference between groups was found. Study authors noted that dietary advice may not have an impact without the addition of stronger reinforcement, such as periodic weighing.
Adherence to physical activity guidance was assessed at baseline and annually to final assessment using a questionnaire to record exercise history. The amount of exercise was scored on a scale of 0–7, where ‘0’ was totally sedentary, ‘1’ mildly active, ‘2’ active without formal exercise, ‘3’ physical work or walking once a week, to ‘7’ athletic training. Results showed that there was no significant difference in exercise scores between groups at the initial visit (3.1 vs 2.9; p = 0.26) or at follow-up (3.2 vs 3.1; p = 0.43).
In summary, Wein et al. (1999)191 found that there were no significant differences, in progression to diabetes or regression to NGT, between intensive diet and lifestyle advice given 3-monthly compared with the same diet and lifestyle advice given once. However, no differences in weight and exercise were achieved between the groups. Progression to diabetes was associated with increased fasting and 2-hour plasma glucose at baseline at high baseline BMI levels.
Wing et al.238
This trial recruited individuals who were overweight or obese (30–100% of ideal body weight; mean weight at baseline about 98 kg, BMI 36 kg/m2) and had a family history of T2DM. 238 About half had IGT, but most results are not given separately for that subgroup. However, the trial provides a useful illustration of the main problem with lifestyle interventions.
There were four arms of the trial: diet, exercise, both diet and exercise and control group. The intervention groups had weekly group meetings for 6 months then reduced to fortnightly for a further 6 months. The diet group started with a strict low-calorie diet aiming at 800–1000 calories daily, relaxed to 1200–1500 after 8 weeks. They also had weekly education sessions. The exercise group had the same frequency of meetings, with supervised weekly walks for about an hour. They were asked to take exercise such as brisk walking at least 5 days each week. The combined group had both interventions. The control group were given educational materials but had no meetings. In the second year, there were only two refresher courses, which were poorly attended: 36% of the diet group, 15% of the exercise group and 29% of the combined group participated. However, attendance at the final assessment visits at 2 years was good at 84%.
At 6 months, the diet group had lost 9 kg, the exercise group 2 kg, the combined group 10 kg and the control group 1.5 kg. Results for the IGT group were not given separately. Unfortunately, by 2 years, most of the weight had been regained in all groups, although the diet and combined group were still about 2 kg lighter than at baseline.
This illustrates a common problem: even among volunteers, improvements are not sustained once the intervention is stopped or reduced. Attendance dropped from 61% at meetings in the first 6 months to 27% thereafter.
Progression to diabetes is reported in the text of the article, but not separately for the arms. The presence of IGT at baseline increased the risk of diabetes, with 25% of those with IGT developing diabetes compared with 6% of those with NGT. Weight loss of 4.5 kg reduced the development of diabetes by 26% in those with IGT compared with those with NGT and no weight loss.
Similar findings were reported by Page et al. (1992)239 in a pilot study in 31 people with IGT. After a 6-month diet and exercise intervention, there were improvements in various parameters in the intervention group but no change in the eight control subjects. For example, total cholesterol fell from a mean of 5.2 to 4.5 mmol/l, and mean SBP by 6 mmHg. However, the gains did not persist once the intervention was stopped.
There is a good body of evidence that some T2DM can be prevented, with the best evidence coming from the larger longer-term trials such as DPS,183 DPP108 and Da Qing. 190
Table 5 summarises the results. Combining studies into a meta-analysis was not done because of the differences in intervention, duration and recruits, which also means that the results in the table should not be compared between studies.
| Study (no. of recruits) | Progression to diabetes, % (95% CI) | Regression to NGT, % | ||
|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | |
| DPP (3234) 10-year annual incidence | 4.8 (4.1 to 5.7) | 11 (9.3 to 13.3) | 54 | 44 |
| Kosaka (500+) | 3 (1.2 to 4.8)a | 9.3 (3.6 to 15) | 54 | 34 |
| Da Qing (577) incidence per 100 patient-years | 7.9 (6.8 to 9.1) | 11.3 (9.3 to 13.3) | – | – |
| Ramachandran (531) 3-year prevalence | 39 (30.4 to 48.5) | 55 (46 to 63.5) | – | – |
| DPS (523) annual incidence | 4.3 (3.4 to 5.4) | 7.4 (6.1 to 8.9) | – | – |
Most of the studies show that progression to diabetes can be reduced, and regression to NGT increased.
Adherence to the lifestyle measures was clearly a problem for some. The results come from groups among which compliance varied. Figure 2 from the DPS183 shows a strong inverse correlation between the proportions progressing to diabetes and the success scores in achieving the targets.
There was also a tendency for the benefits to be lost not long after the intervention ended, with, for example, regain of weight. The exception was the DPS,183 where benefit was largely maintained for 3 years after intervention ended. Perhaps a 4-year intervention can permanently improve lifestyle change, whereas short intervention does not.
However, as noted above, studies with the longest follow-up show disappointing results in terms of CVD.
The benefits of the lifestyle intervention were greatest in those with the highest compliance and who achieved more of the targets (such as weight loss and dietary change). For example, in the Finnish study,183 those who achieved four or five of the five targets had a risk of developing diabetes which was only 23% of those who achieved none.
However, even among the volunteers in the trials, many did not succeed and others succeeded in the short term (such as the first 6 months) but not in the longer term. The key to success is sustained lifestyle change, especially weight loss.
In conclusion, lifestyle measures can be highly effective in reducing progression to diabetes but adherence to lifestyle change is the most important factor.
Chapter 5 Review of economic models assessing the cost-effectiveness of lifestyle interventions for people with impaired glucose tolerance and/or impaired fasting glucose
Introduction
The aim of this chapter is to assess the existing evidence relating to the long-term cost-effectiveness of lifestyle interventions for the prevention of diabetes in high-risk groups. As part of a previous literature review on screening for diabetes,72 we previously appraised all published studies relevant to this question (up to the end of June 2005) using the British Medical Journal (BMJ) guidelines for reviewers of economic evaluations240 in conjunction with a checklist for good practice in decision-analytic modelling in Health Technology Assessment (HTA). 241 The main objective of this chapter is to update the previous review with any relevant studies published up until August 2006. First of all, the findings of the previous review are summarised. Following this, new studies are appraised and then discussed in the context of the previously reviewed papers.
Summary of findings from previous review
Our previous review appraised five modelling studies that assessed the long-term costs and health outcomes associated with delaying or preventing diabetes in high-risk groups. Three of the studies assessed the long-term costs and benefits that would be expected with lifestyle or pharmacological interventions for people with IGT or IFG. 242–244 Another study considered a broad range of interventions for diverse populations, from a media campaign for the general public to surgery for morbidly obese people. 245 The final study only assessed the impact that delaying the onset of diabetes would have on future morbidity, mortality and health service costs. 246 It did not assess the costs-effectiveness of alternative interventions for preventing or delaying the onset of diabetes.
The four studies that assessed the long-term costs and consequences of delivering interventions to prevent or delay the onset of diabetes all used Markov-type models (using cohort or microsimulation) to simulate progression from a state of IGT, or IFG, through the onset of diabetes to the development of clinical diabetes and its complications. The reader should consult Waugh et al. (2007)72 (see Chapter 7) for detailed appraisal and discussion of these models. Although the models were of variable quality and varied in their structure and assumptions, all predicted that diabetes prevention interventions would provide good value for money. 241 In particular, two models, that were judged to be of reasonable quality according to published guidelines for good practice in decision-analytic modelling, reported favourable cost per life-year or cost per QALY ratios for the lifestyle intervention of the DPP. 108 This is a resource-intensive intervention consisting of 16 one-on-one lessons on diet and exercise, followed by monthly maintenance visits that included both group and individual sessions with a trained case manager. Herman et al. (2005)244 estimated that this intervention would cost US$1124 per QALY compared with placebo from a single payer perspective. Palmer et al. (2004)243 estimated that, in a UK setting, the same intervention would cost 6381 euros (€) per life-year compared with standard advice on diet and exercise from the health service perspective. The fifth study included in our review assessed only the impact that delaying diabetes would have on future health outcomes and medical costs. 246 However, it also produced findings that were broadly consistent with those of the other models, i.e. that delaying the onset of diabetes by even modest periods would substantially reduce the incidence of vascular complications (microvascular and cardiovascular), improve QoL and avoid future medical costs.
Based on the appraisal of these studies, we previously concluded that lifestyle interventions, similar to those reported in the DPP trial, would probably be cost-effective in the UK setting. 72 However, some uncertainty remained as to how effective alternative options might prove in routine practice, and how this might affect cost-effectiveness. One of the appraised studies243 also suggested that the characteristics of the cohort would have some bearing on the option considered to offer best value. Palmer et al. (2004)243 estimated that the metformin intervention would have a better impact on costs and life expectancy than the lifestyle intervention in younger more obese patients, but would have less benefit and higher costs in older cohorts (aged 65 years at baseline) and those with a BMI of < 30 kg/m2.
Finally, uncertainty remained as to how such interventions should be targeted. The prevalence of IGT will be highest in groups who have other CVD risk factors. The question here is, if interventions are to be offered to people found to have IGT or IFG, is it more cost-effective to do this by identifying these people using systematic screening or case finding? Or would it be better to provide such interventions to everyone with a certain CVD risk profile? These questions require a more thorough exploration of the cost-effectiveness of different screening strategies, where the benefits of treating those identified with both IGT and undiagnosed diabetes are incorporated. It is also important that added benefits from reducing CVD in people with IGT are incorporated in cost-effectiveness models, as interventions to prevent progression to diabetes should also reduce risk of CVD.
Methods
A systematic literature search was undertaken to identify any further economic assessments of lifestyle interventions for prevention of diabetes in high-risk groups. Databases searched were MEDLINE, EMBASE, the NHS Economic Evaluation Database (NHS EED), and the Science Citation Index from May 2005 to the end of August 2006. The start date was chosen because our previous review of screening covered earlier economic studies. Abstracts were reviewed and studies that were potentially relevant were retrieved.
Inclusion and exclusion criteria
To be included, studies had to assess the long-term costs and consequences of treating people with lifestyle interventions to prevent progression to diabetes. Studies that only assessed short-term costs and consequences were excluded because many of the benefits and cost savings that may be associated with diabetes prevention efforts are likely to occur over a long time horizon. Studies reported in languages other than English were not included.
Data extraction
Data were extracted from the long-term modelling studies using headings that were consistent with those used in the previous HTA review on screening for diabetes:72
-
author and year
-
decision problem (comparators, population, setting, objectives)
-
cohort information (characteristics and numbers)
-
model structure, perspective and scope (basic structure and assumptions)
-
modelling of disease progression (details of structure and assumptions used for modelling diabetes progression)
-
modelling of diabetes complications (details of structure and assumptions used to model progression of diabetes complications)
-
mortality (details of how mortality was modelled)
-
costs (details of costs considered in the model)
-
outcomes (outcomes reported and methods for calculating life-years or QALYs)
-
findings (reported results of the base-case analysis)
-
sensitivity analysis (details and results of any sensitivity analysis conducted).
Quality assessment of included studies
One reviewer critically appraised identified studies using the BMJ guidelines for reviewers of economic evaluations240 in conjunction with a checklist for good practice in decision-analytic modelling in HTA. 241 When the individual reviewer was uncertain about the appropriateness of methods or assumptions used in any included study, the opinions of two further reviewers were sought.
Results
The updated literature searches revealed two further modelling studies, published since the previous review, which met the inclusion criteria. 247,248 The first of these studies was essentially a repeat of the previous reviewed analysis by Herman et al. (2005). 244 The difference was that in this analysis the authors assessed the potential for private insurance companies, Medicare, and individuals/employers to share the cost of providing the interventions. 247 The study has limited relevance to the UK setting where the NHS covers the cost of care for everybody regardless of age. The second study identified was a new analysis conducted using a previously developed model called the Archimedes Diabetes Model. 249 Eddy et al. (2005)248 used this model to assess the cost-effectiveness of treating high-risk individuals with the DPP intensive lifestyle intervention or metformin for prevention of diabetes. The model is discussed in detail below under the identified data extraction headings. Searches revealed two other potentially relevant articles, which were obtained and examined, but these were excluded because they were found to be reviews. 250,251
Decision problem
As in previous studies,243,244 Eddy et al. (2005)248 assessed the cost-effectiveness of providing either the DPP lifestyle or metformin intervention for people with IGT compared with a baseline strategy of no treatment other than dietary advice. They assumed individuals would enter into an intensive management programme if their HbA1c levels rose to > 7%. However, Eddy et al. (2005)248 also included an alternative strategy in which the lifestyle intervention described in the DPP trial commenced at time of onset of diabetes rather than prior to onset. This strategy is a feasible option for people who have been identified as having IGT/IFG, as their progression to diabetes would be closely monitored.
Cohort information
As in previous studies, Eddy et al. (2005)248 analysed the costs and effects of the alternative management strategies for a cohort with the characteristics of those enrolled in DPP trial – 10,000 people who would meet the inclusion criteria for this trial were simulated to receive each alternative and followed over a period of 30 years.
Model structure, perspective and scope
The type of model developed and used by Eddy et al. (2005,248 2003249) is very different to the Markov models previously used to address this issue. It is very complex and not widely used in health-care decision modelling. It was therefore difficult to appraise the model using the published guidelines for good practice in decision-analytic modelling that were used in the previous review. 241 However, a number of validations have been performed in which the model has been used to simulate existing randomised trials. 252 The results of these provide a high degree of confidence in the model’s ability to predict clinical outcomes over the short to medium term. Very briefly, the model uses object-oriented programming and relies on a large number of differential equations to model the physiological mechanisms and interactions that underlie the development and progression of diabetes and its complications. There are no discrete disease states or time cycles in the model, rather the underlying biological variables are continually interacting in a very large number of possible combinations, and these variables can give rise to clinical events at any point in time. The authors provide a detailed description of how some variables and equations were derived from empirical sources in an appendix and separate technical paper. 249,253
In their analysis relating to the prevention of diabetes, Eddy et al. (2005)248 present annualised rates of change in key biological variables and the cumulative incidence of important clinical events. As mentioned above, the costs and effects of the alternative treatment strategies were tracked over a time horizon of 30 years, somewhat shorter than the 70-year time horizon used by Herman et al. (2005)244 in a previous analysis. Like Herman et al. (2005),244 Eddy et al. (2005)248 apply utility decrements to events that impact upon QoL, and estimate the cost per QALY for the alternative strategies. Eddy et al. (2005)248 also assess the problem from three different perspectives: the individual patient (in terms of individual risk), a 100,000-member health plan, and society as a whole (i.e. for the entire US IGT/IFG population).
Modelling diabetes progression
As already mentioned, Eddy et al. ’s model (2005)248 differs substantially from the Markov models used in previous analyses. Markov models consist of a limited number of discrete disease states. Individual patients or cohorts are simulated to transit between these based on annual transition probabilities calculated from epidemiological or randomised studies. The more complex of the Markov models used in previous analyses of diabetes prevention allow key biological variables, such as HbA1c level, to mirror annual changes observed over time in several diabetes trials. The modelled HbA1c levels in turn influence the probabilities of developing micro- and macrovascular complications as represented by several discrete state submodels. The model of Eddy et al. (2005)248 on the other hand is much more complex allowing multiple interactions between many continuous biological variables and clinical events. For example, the development of diabetes is influenced by age, sex, race/ethnicity, BMI, and a factor that registers the effect of glucose intolerance. The authors explain how a host of variables – representing FPG in people without diabetes, hepatic glucose production, the effect of insulin resistance, the amount of insulin produced, the efficiency with which the body uses insulin, and patient characteristics (age, sex, race/ethnicity, BMI) are used to model the physiological mechanisms underlying rising FPG levels and the development of diabetes. 249
Diabetes complications
Eddy et al. (2005)248 include coronary artery disease (MI and congestive heart failure), stroke, nephropathy, neuropathy and retinopathy in their model. Coronary artery disease is modelled through the occurrence of coronary artery occlusions that occur as a result of atherosclerotic plaque formation and rupture, and/or the development of occlusive thrombi. Many underlying biological variables are used to determine the progression and timing of these events through sets of differential equations. The authors state that stroke is modelled in a similar way. The progression of nephropathy is modelled as a function of the person’s FPG, blood pressure, and glycaemic load (a variable that represents the degree of elevation in FPG combined with the duration of time it has been elevated to different degrees). Retinopathy is modelled in a similar way to nephropathy. Here, it is the clinical manifestations of retinopathy (microaneurysms, haemorrhages and exudates) that are modelled as a function of a person’s FPG, blood pressure, and glycaemic load. Finally, diabetic neuropathy (loss of sensation) is modelled as a function of a person’s FPG, blood pressure and glycaemic load. Diabetic foot ulcers and amputations are further functions of the neuropathy feature.
Mortality
Eddy et al. (2005)248 state that they model death from diabetes and its complications based on the extent of failure in the underlying physiological systems:
A person in the model will die if a coronary artery is occluded and the subsequent infarction reduces their myocardial function to the point that cardiac output and blood pressure cannot be maintained. 248
Deaths from other causes are also included in the model.
Resource use and costs
The direct and non-direct medical costs associated with the interventions to prevent progression to diabetes, were taken as those collected alongside the DPP trial. 108 Eddy et al. (2005)248 also state that they included a set of algorithms in their model that describe the treatment processes providers follow for patients with a complete range of clinical circumstances. The model also includes system resources, and use of these resources is triggered whenever a patient encounters the health system as a result of a clinical event or treatment process being implemented. The model calculates cost by tracking all resource use and adding up the cost for each use. The itemised costs used in the model come from a detailed micro-costing survey of an integrated managed care organisation that provides comprehensive care for people with diabetes with no deductibles or co-payments. All costs reported in the analysis are in US dollars (US$) for the year 2000. This additive costing approach used by Eddy et al. (2005)248 is somewhat different to Herman et al. ’s (2005)244 approach of using a multiplicative costing model. It may be more accurate, as Herman et al. (2005),244 to some extent, rely on assumptions to estimate the multipliers for their model.
Outcomes
In terms of outcomes used to establish cost-effectiveness, Eddy et al. (2005)248 estimated QALYs using utility weights reported in the DPP trial108 for people with IGT. For people with diabetes they used weights from another published survey. Both surveys used the Quality of Well-Being Index to measure utility decrements associated with IGT, diabetes and complications. These are the same surveys used by Herman et al. (2005)244 to estimate QALYs in their model of diabetes prevention. The QoL decrements were assumed to be additive for people with more than one complication.
Findings
Eddy et al. (2005)248 estimated that, from an individual perspective, the intensive lifestyle intervention of the DPP108 would reduce the 30-year probability of a person with DPP characteristics (see Table 7) developing diabetes by 11 percentage points compared with the baseline strategy (from 72% to 61%). From the perspective of a 100,000-member health plan, with 4% of its population at high risk of diabetes, the model predicted that the intensive lifestyle intervention would reduce the number of people developing diabetes by 434 compared with the baseline strategy (2887–2453). The expected value of the cost per QALY came to US$143,000. The cost per QALY was higher over shorter time horizons. From the societal perspective (i.e. providing a national DPP lifestyle programme to all high risk people in the USA), the lifestyle intervention cost about US$62,000 per QALY compared with the baseline strategy. However, when the authors assessed the cost-effectiveness of implementing the intensive lifestyle intervention only when individuals actually developed diabetes, they predicted this strategy would result in a lower 30-year cost per QALY of US$24,500 compared with the control group. Using this as the reference strategy, the incremental cost per QALY for the DPP strategy increased to US$202,000. But as people with IGT are at about 1.8 times the risk of heart disease, this approach would allow some to die – those who had MIs before being diagnosed as diabetic. It is unclear whether or not this increased risk is captured by Eddy et al. ’s model. Using this as the reference strategy, the incremental cost per QALY for the DPP strategy increased to US$202,000. Thus, Eddy et al. ’s243,244 findings are much less favourable than those of previous analyses that assessed the cost-effectiveness of the DPP interventions.
Sensitivity analysis
Two approaches have been used to assess the validity and robustness of the Archimedes Diabetes Model249 and its findings relating to the diabetes prevention interventions. First of all, the model has been validated by simulating 19 clinical trials, including the DPP,108 and comparing the results with those found in the actual trials. This provides a high degree of confidence in the validity of the model over short to medium time horizons. However, uncertainty remains over the ability of the model to accurately predict outcomes beyond the follow-up period of available clinical trials. To further address uncertainty relating to the values of variables used in the model, the authors state that distributions were assigned to variables and individuals were created by simultaneously drawing a value for each variable from each distribution. To explore the uncertainty relating to the effectiveness of the DPP intervention, the authors incorporated a distribution for this parameter based on the 95% CI reported in the original trial. They observed that this source of uncertainty substantially increased the width of the CI for the 30-year chance of developing diabetes. However, the assumption of a linear increase in diabetes prevalence does not fit with the data from the GPRD (presented later) and it is more likely that most of those who will progress to diabetes will do so within 10 years, and so the Eddy model243,244 will underestimate person-years of diabetes. Based on their simulations, the authors present a probability distribution of the cost per QALY for the lifestyle intervention and conclude that it is extremely unlikely (< 0.1% chance) that the cost per QALY would fall below the threshold of US$50,000 for a 100,000-member health plan. The incremental cost per QALY was also found to be sensitive to the discount rate, and most notably, the cost of the lifestyle intervention. If, for example, the cost of delivering the DPP lifestyle intervention could be reduced to US$217 per person per year, without reducing its effectiveness, then the cost per QALY ratio would be much more favourable.
Discussion
The findings of Eddy et al. 248,249 differ from those obtained from the other previous studies that have modelled the cost-effectiveness of the DPP intervention. The studies by Herman et al. (2005)244 and Palmer et al. (2004)243, reported that the DPP lifestyle intervention would cost US$1124 per QALY (single-payer perspective) and €6381 per life-year (UK health service perspective) compared with placebo, respectively. 243,244 Eddy et al. (2005)248 report much less favourable incremental cost-effectiveness ratio (ICER) of US$143,000 and US$62,000 per QALY from a health-plan and societal perspective, respectively. 248 Eddy et al. ’s (2005)248 findings also differ dramatically from a cost-effectiveness analysis based on patient-level data collected alongside the DPP trial. 254 This within-trial cost-effectiveness analysis reported an ICER of US$31,550 per QALY for the DPP lifestyle intervention over the 3-year follow-up period (health-system perspective). The modelling study by Eddy et al. (2005)248 reports an ICER of US$2.7M per QALY over a 5-year horizon from the perspective of a 100,000-member health plan. Possible explanations for these differences in findings are discussed below (Tables 6 and 7) and further recommendations are suggested.
| Authors | Cohort demographic characteristics | Source of cohort information | Cohort age range (years) | Number of patients in cohort |
|---|---|---|---|---|
| Palmer et al. 2004243 | Mean age 50.6 years, mean body weight 92 kg, and mean BMI 34 kg/m2; 32% men and 45% from minority groups | Patients enrolled in the DPP trial | ≥ 25 | Not reported |
| Herman et al. 2005244 | Mean age 50.6 years, mean body weight 92 kg, and mean BMI 34 kg/m2; 32% men and 45% from minority groups | Patients enrolled in the DPP trial | ≥ 25 | Not reported |
| Eddy et al. 2005248 | Individuals at high risk of developing diabetes as defined by the entry criteria for the DPP trial (IGT ≥ 25 years; BMI ≥ 24 kg/m2) | Published prevalence projections based on the NHANES III survey255 | Not explicitly reported |
4000 individuals (4%) from a 100,000-member health plan 10,000 individuals for analysis from societal perspective |
| Author | Lifestyle interventions compared | Model type | Complications modelled | Benefits modelled | Results (ICERs) | External validations |
|---|---|---|---|---|---|---|
| Palmer et al. 2004243 |
|
Markov model | Not modelled explicitly | Reduced mortality due to prevention of diabetes and future cost savings |
€6381 per life-year (1 vs 3) UK health service perspective |
No specific external validations reported |
| Herman et al. 2005244 |
|
Markov model (cohort analysis) | Retinopathy, nephropathy, neuropathy, CHD (angina and MI/CA), stroke | Reduced mortality due to prevention of diabetes, QALY gains from reduced diabetes complications, and future cost savings |
US$1124 per QALY (1 vs 3) Single-payer perspective |
No specific external validations reported |
| Eddy et al. 2005248 |
|
Simulation model using object-orientated programming (relies on a large number of differential equations to model the physiological mechanisms and interactions underlying the development and progression of diabetes and its complications in individual people) | Retinopathy, nephropathy, neuropathy, MI and CHF, and stroke | Reduced mortality due to prevention of diabetes, QALY gains from reduced diabetes complications, and future cost savings |
|
Independently predicted the annualised rate of progression from pre-diabetes to diabetes as observed in the DPP trial Predicted rates of complication development, for individuals with clinically diagnosed diabetes, validated against various epidemiological and clinical studies344 Rate of disease progression (FPG) validated for people with pre-diabetes and clinical diabetes Uncertainty remains over validity of predicted rates of complication development in people with pre-diabetes and preclinical diabetes |
The large discrepancy between the trial-based cost-effectiveness analysis254 and the 5-year ICER predicted by the Archimedes Diabetic Model248 is probably due to differences in the way the two studies estimate QALY gains associated with the lifestyle intervention. In the trial, patient-level utility scores and cost data were collected prospectively over a 3-year period, allowing mean costs and QALYs to be estimated for each arm of the trial. The authors of the trial report that the lifestyle intervention resulted in a mean QALY gain of 0.072 compared with placebo over the 3-year follow-up period. 254 This gain may have been due to effects of the lifestyle intervention on general well-being (or weight loss) rather than its impact on the progression of diabetes. Such an effect may be consistent with the earlier reported finding that changes in QoL in people with IGT, as measured by SF-36, were independent of glycaemia control or changes in glycaemia control (see Chapter 1, Quality of life). However, it is unclear whether this QALY gain represents a real improvement in health-related QoL. This is because the authors did not present baseline scores for the individual arms of the trial and the differences between the arms do not appear to be statistically significant. It would be useful if future studies could clarify the impact that lifestyle interventions have on health-related QoL independently of their effect on the progression of diabetes, for example via a reduction in weight. In Eddy et al. ’s model (2005),248 it appears that the lifestyle intervention affects QoL only through its impact on the progression of diabetes and its complications. As very few people progress to diabetic complications that significantly impact on QoL during the first 5 years of the simulation, the model predicts very low QALY gains over this period and high costs associated with implementing the intervention. This accounts for the very high 5-year ICER reported by Eddy et al. (2005). 248 However, it is not clear whether Eddy et al. (2005)248 takes account of associated morbidity and mortality, most notably from CVD, before the development of diabetes. If not, the model may underestimate the beneficial effects of lifestyle interventions.
The above issue may also partly account for the different findings predicted by the different modelling studies. Herman et al. (2005)244 appear to have assigned a slightly higher utility weight to people with IGT receiving the lifestyle intervention compared with those in the control group. It is difficult to establish from their report, but Eddy et al. (2005)248 may have assigned the same utility weights to everyone with IGT regardless of which intervention they were receiving. This could account for some of the difference in QALY gains observed between the two analyses.
The use of different time horizons may also partially explain the different cost per QALY ratios predicted by the two models. Eddy et al. (2005)248 used a 30-year time horizon, whereas Herman et al. (2005)244 assessed costs and outcomes over the lifetime of patients. If preventing or delaying the onset of diabetes prevents or delays complications far in the future then a 30-year time horizon may miss some of these events. However, Eddy et al. (2005)248 argue that it is cost-effectiveness over shorter time horizons that is most important because modelling outcomes and costs far into the future rely on the assumption that the programme will be in place for decades without change and that no new technologies will become available for the management of diabetes. Despite the difference in time horizons, it has been noted that the models project similar life expectancy. 256 Therefore, it is likely that the difference in cost-per-QALY estimates is due to the Archimedes Diabetes Model249 reporting lower complication rates than the Herman et al. model (2005). 244 As Engelgau (2005)256 points out in an editorial accompanying the publication of Eddy et al. ’s (2005)248 analysis, the model by Herman et al. (2005)244 predicts higher cumulative incidences for all the major micro- and macrovascular complications, despite predicting similar survival times. To give an example, the lifestyle intervention reduced the cumulative 30-year incidence of retinopathy blindness in the at-risk population (those with IGT) from 0.03 to 0.016 in the model by Eddy et al. (2005). 248 The lifetime risk reduction in the model by Herman et al. (2005)244 is from 0.056 to 0.034. As indicated in Table 2, Eddy et al. (2005)248 have undertaken a series of external validations, showing that their model accurately predicts rates of complication development observed for people with clinical diabetes in epidemiological and clinical studies. However, uncertainty still exists in relation to the rate of complication development beyond the follow-up period of existing clinical trials and the rate of complication development in people with pre-diabetes and preclinical diabetes.
The reason why Eddy et al. (2005)248 project lower complication rates than Herman et al. (2005)244 is very difficult to ascertain given the complexity of the models and the many differences between them. However, as Engelgau (2005)256 suggests, it probably has something to do with differences in the way glycaemia progression is modelled.
The speed of progression of hyperglycaemia from onset of diabetes to clinical diabetes and the progression of micro- and macrovascular complications during this period are subject to debate. Herman et al. (2005)244 assume this progression takes 10 years. A much slower rate of progression predicted by Eddy et al. ’s model (2005)248 is consistent with the lower complication rates reported. The assumption of a linear progression257 has been challenged by Ferrannini et al. (2004),258 who postulate an initial slow progression followed by a rapid onset of clinical diabetes. More evidence is available relating to the progression of HbA1c level and the development of complications (and response to treatment) for people with clinically diagnosed diabetes. There has been debate between the authors as to which model provides the most accurate and reliable prediction of cost-effectiveness, but the debate seems to be inconclusive, with Eddy et al. (2005)248 sticking by their findings and Herman (2005)244 and Palmer et al. (2004)243 in agreement.
Despite disagreement in terms of the overall cost-effectiveness, the different models do agree on several qualitative points. First of all they agree that, if maintained, lifestyle changes and weight loss have a significant impact on the risk of developing diabetes and micro- and macrovascular complications. The cost-effectiveness estimates predicted by the different decision-analytic models are also sensitive to changes in the same parameters, particularly the cost of delivering interventions, likely adherence and, thus, maintenance of effectiveness.
Affordability is also a major concern relating to the implementation of resource-intensive lifestyle interventions, even if such interventions are shown to be cost-effective over a lifetime. To address this issue, Johnson et al. (2006)259 recently conducted a discrete choice experiment in the USA to estimate high-risk individuals’ willingness to pay for risk-reduction programmes. This was to assess whether a potential cost-sharing scheme could be used to finance implementation. This study did find that individuals at high-risk were hypothetically willing to pay approximately 65% of the monthly cost of delivering a lifestyle intervention similar to that of the DPP trial. 108 However, the study also found, not surprisingly, that individuals valued hypothetical programmes with large benefits (weight loss and risk reduction) and low sacrifices most highly. This finding suggests that a trade-off may exist between the intensity of the intervention (e.g. amount of exercise and dietary restriction) and likely engagement and adherence. The effectiveness of programmes that involve high levels of exercise and dietary restriction, which could in theory have large benefits in terms of reducing the risk of diabetes, might be undermined by poor uptake and adherence. On the other hand, if high uptake and adherence can be achieved by encouraging moderate lifestyle changes, then the overall benefits may be greater. This is an important point to consider when designing future intervention strategies. It may also be possible to consider flexible interventions that can be tailored to suit individual patients’ needs, rather than thinking of interventions in terms of one fits all.
Studies published since August 2006
As this chapter was completed early in the life of the project, in order to inform further economic modelling, it only included studies published up to August 2006. Since then, several relevant studies have been published. We carried out updating searches in January 2012. These studies are summarised briefly below. Although they have not been fully critically appraised, they all use similar modelling approaches to those of Herman et al. (2005)244 and Palmer et al. (2004),243 and come to similar conclusions. Thus the above discussion on the differences in findings between Eddy et al. (2005)248, Palmer et al. (2004)243 and Herman et al. (2005)244 is also relevant to these more recent studies.
A study by Jacobs-van der Brugen et al. (2007)260 from the Netherlands is partly relevant. It compared the cost-effectiveness of a community-based programme focusing on nutrition and exercise, targeted at the low-risk general population,261 with an intensive lifestyle intervention targeted at obese adults, over a 20-year time horizon. The intensive intervention delivered in a health-care setting is that used in the lifestyle arm of the RCT by Mensink et al. 188,234 The authors estimated that the community-based intervention would be more cost-effective. However, they also concluded that the intensive lifestyle intervention would provide a cost-effective use of resources from a health service perspective (£5000–£21,000 per QALY).
Hoerger et al. (2007)262 carried out a study of the economics of screening, using the lifestyle intervention from the DPP for treating those found to have pre-diabetes. In effect it was an extension of the previous studies by Herman et al. (2005)244 mentioned above (see Introduction).
The main thrust of the recent study was to consider two strategies for intervention, one more selective than the other. The first would apply the DPP lifestyle only to those who had both IGT and IFG; the second would provide it to those with either IGT or IFG (or both). The first group are at higher risk of progression and so intervention might be expected to be more cost-effective. This was shown to be the case, but intervention was cost-effective in both groups, with costs per QALY of US$8181 and US$9511, respectively.
Icks et al. (2007)263 applied the results of the DPP to the German population in Augsberg, modelling no intervention, lifestyle and metformin, with a 3-year timescale. 263 However, they tried to model a ‘real-life’ scenario with pessimistic estimates of participation. They assumed from previous experience in Germany, and elsewhere, that participation in screening would be low (30–35%), adherence to lifestyle would be poor (in a German disease management programme only 40% of patients with diabetes followed lifestyle training), and obese patients would have even poorer adherence (only 20% participated in a lifestyle programme to lose weight). They concluded that few cases of diabetes would be prevented by a complete screening and intervention programme and that, in Germany, lifestyle would be more cost-effective than metformin.
Lindgren et al. (2007)221 assessed the cost-effectiveness of the Finnish DPS intervention,183 applied to a Swedish population. They used a Markov model, starting with a cohort of people aged 60 years with IGT, who could progress to diabetes or a cardiovascular event such as a stroke or MI in the first year. Those who did not would stay in IGT, and repeat the process. No mention is made of regression to NGT, as some would have done. The risk of diabetes was taken from the placebo arm of the DPS, and the risks of vascular events from the UKPDS model (which may overestimate the risk a little as that applied to diagnosed diabetes). For their base case, they assumed that the lifestyle intervention would continue for at least 6 years longer than in the DPS, and hence be more costly. However, their results indicated that the cost of the intervention was offset by savings, so that the QALY gain (of 0.20 at probably 6 years – the duration is unclear) is achieved at lower cost than the no-prevention arm; prevention dominates in ICER terms.
Ramachandran et al. (2007)226 from the Indian Diabetes Prevention Programme have published their costs and cost-effectiveness estimates. Their figures are not applicable to a UK context. They conclude that lifestyle was the most cost-effective, closely followed by metformin, with combined lifestyle and metformin some way behind, when compared with the control arm. The cost of the lifestyle intervention was only slightly more than that of metformin, as a result of the very low labour costs in India.
Saha et al. (2010)264 examined reasons why different models give differing results. These reasons included:
-
The timescale of the modelling. Some studies examined cost-effectiveness only during the duration of a trial, and these give far higher ICERs than studies that adopt a 20-year or lifetime approach. The underlying problem here is the need to extrapolate from short trials to lifetimes.
-
Different costings, and in particular whether the cost of screening was included in the cost of prevention of diabetes. Icks et al. (2007)263 estimated that 36% of the cost came from the screening.
-
Different assumptions on duration of benefit, with pessimists assuming that the benefit would end when the intervention did and optimists assuming that they would last for life. Modelling based on the DPS might assume some prevention of diabetes, modelling based on the DPP might assume just a delay.
-
Different assumptions about adherence in ‘real-life’ settings. Icks et al. (2007)263 assumed that the cost-effectiveness of a DPP-style intervention would be less in routine care because adherence to lifestyle measures would be poorer and shorter.
-
Assumptions about costs of interventions. For example, delivering the DPP intervention in groups considerably reduced the ICER. Some studies used costs based on those in the trial, whereas others based costs on national health-care cost databases.
-
Different timings of studies. For example, those studies that were carried out before generic statins became available produced higher ICERs.
-
Different methods for estimating QALYs.
Gillies 2008
In a very thorough analysis, Gillies et al. (2008)265 assessed four screening and prevention strategies:
-
no screening
-
screening for T2DM
-
screening for T2DM and IGT, with lifestyle intervention
-
screening for diabetes and IGT, with pharmacological intervention.
Hence, their decision problem was broader than that in this review, which starts with people already diagnosed. Gillies et al. (2008)265 studied the whole pathway from screening to death. Their main aim was to examine whether screening should be for T2DM alone, or for diabetes and IGT. However, the latter pathway will be more effective than screening only for diabetes alone, if intervening in those with IGT is cost-effective.
The base-case scenario was one-off screening at the age of 45 years, in people with above-average risk of diabetes. Risk was based on known history of ischaemic heart disease, hypertension, dyslipidaemia, family history of T2DM, and BMI score of > 25kg/m2. They base their effectiveness estimates for the prevention of diabetes on a previous review of pharmacological and non-pharmacological interventions of their own. 179
The modelling was done using a mixed decision tree and Markov modelling approach. Because their costs per QALY are for the whole pathway they are not comparable with ones in this review, but the key finding for our purposes was that screening for both diabetes and IGT, with lifestyle intervention in the latter, was more cost-effective than screening for diabetes alone, with costs per QALY of £6242 and £14,150, respectively, both compared with no screening. They assumed that lifestyle intervention would cost about £400 in the first year, and £280 per year thereafter. These costs were derived from a review of interventions in obesity266 rather than from the diabetes trials.
Irvine et al. (2011)267 carried out a cost-effectiveness analysis based on the University of East Anglia Impaired Fasting Glucose programme. This programme screened almost 4000 high-risk individuals and identified 209 with IFG. Of these, 85% entered the lifestyle intervention trial, in which they were randomised to the intervention (dietary advice and physiotherapist-led exercise groups, the aim being to achieve 7% weight loss over 6 months) or to the control group, which received a 2-hour session of diet and exercise advice. Costs were assessed for the intervention and control arms. Recruits completed European Quality of Life-5 Dimensions (EQ-5D) questionnaires and ICERs were calculated.
However, there were major weaknesses in this study. First, follow-up was short, a mean of around 7 months. Second, and more importantly, benefits were assessed only during the trial, and ICERs were therefore very high because the duration of benefit was short. The intervention was assessed as having an ICER of £67,163 per QALY. When a subgroup of participants (number not given) with longer follow-up was analysed, the cost per QALY fell to £17,075. No modelling of the long-term effects of weight loss was carried out.
Attempts are being made to adapt the DPP for delivery by community organisations. Ackermann and Marrero (2007)268 are collaborating with the YMCA of Greater Indianapolis to evaluate the effectiveness, adoption, implementation, and maintenance of a modified DPP lifestyle intervention for delivery in YMCA branch facilities. The intervention is called the Group-Organized YMCA Diabetes Prevention Program (GO-YDPP). The GO-YDPP core curriculum involves the same 16-lesson approach as the original DPP, but lessons are delivered in groups of 10 to 12 participants and are held over just 16 weeks. GO-YDPP has retained the same physical activity and weight loss goals of the original DPP lifestyle intervention. The YMCA has estimated the total costs during the first year to be US$275 to US$325 per participant. This compares favourably with the original DPP intervention, estimated to cost more than US$1400 per participant.
Ackermann et al. (2009)204 used data from the DPP to assess the utility benefit from weight loss. Data on QoL were collected using SF-36, from the start of the trial, in 3206 subjects. From this, the DPP investigators derived the SF-6D, and assessed the effects of weight loss on that, at 12 and 24 months, adjusted for other variables. SF-6D gives a range of values from 0.29 to 1.0, with 1.0 being best possible. Mean BMI among DPP patients was 34 kg/m2 and mean weight was 94 kg. The lifestyle group lost an average 6.7 kg by 1 year. Their SF-6D scores rose (improved) by 0.010 for every 5 kg of weight lost. Meaningful improvements in health-related QoL appeared only once subjects had lost at least 5–10 kg. The DPP investigators noted that, if weight loss is sustained for years, the small increase in utility would become significant in cost-effectiveness analysis.
Bertram et al. (2010)269 populated a microsimulation model with data from several sources, including AusDiab (Australian Diabetes Obesity and Lifestyle Study). In AusDiab, people were screened at the age of > 55 years, or at age > 45 years if they had risk factors such as hypertension, family history of diabetes of a high BMI, or if they were deemed to be high risk, such as having had previous GDM. The model had three lifestyle interventions: diet alone, exercise alone or both. It also had three pharmacological interventions, including metformin. The effectiveness of interventions was based on the meta-analysis by Gillies et al. (2007)179 from the UK. The modelling showed that the most cost-effective interventions were diet and exercise combination, and metformin. Cost-effectiveness was taken to be an ICER of < AU$50,000 (AU$, Australian dollars) per disability-adjusted life-year (DALY). Adding metformin to diet and exercise was not cost-effective because the cost per DALY rose to around AU$81,000, though with very wide CI (14,000 to 130,000). Exercise alone and diet alone had ICERs of AU$30,000 and AU$38,000 but the upper confidence limits were well above affordable levels, whereas the upper confidence limit for the combination was AU$35,000. A number of assumptions were made, including that results in general population would be as good as in trials such as DPP and DPS.
Another Australian study by Colagiuri and Walker (2008)270 used a different model – the Diabetes Cost–benefit Model – to assess the cost–utility of screening for undiagnosed diabetes. However, although the main analysis was on diabetes, some results are provided for detecting and intervening in IGT. The model uses AusDiab data for two groups: all of those aged 55–74 years, and those aged 45–54 years but with one or more risk factors, such as obesity, family history of diabetes, or hypertension. Those with IGT would be offered a lifestyle programme estimated to cost AU$500 (but not specified, so technically its effectiveness is unproven) but they also assessed the effect of using a more costly AU$1000 intervention based on the DPS lifestyle arm. They used the DPP and DPS results of a 58% reduction in progression to diabetes but rounded it to 60%. They estimated savings in health-care costs based on the UK Prospective Diabetes Study (UKPDS) 61 paper271 (which showed that diagnosis at lower PG had better outcomes and hence lower costs – Colaguiri was first author of that paper). They pessimistically assumed that only about half of those offered the lifestyle intervention would accept it, and that about one-third of those who did would thereby avoid progression to diabetes. The cost per DALY avoided is around AU$50,000, which is considered borderline acceptable. Limiting screening and intervention to the over-55s would make it more cost-effective (ICER AU$48,000).
Johansson et al. (2009)272 assessed the cost-effectiveness of the Stockholm Diabetes Prevention Program, but this was not restricted to people with IGT and so not relevant to this review. The results were mixed: in some groups the programme appeared cost-effective, in others not.
Li et al. (2010)273 reviewed cost-effectiveness studies of prevention of diabetes. Most were in people with IGT, and most have been included in this review. Some were not in people with IGT. Li et al. (2010)273 conclude that intensive lifestyle interventions to prevent diabetes in people with IGT are ‘very cost-effective’. Most of the included studies were based on the DPP results.
Zhuo et al. (2012)274 reported that in the USA, a nationwide community-based lifestyle programme could break even within 14 years. However, the programme they used was a hypothetical one, based on the small pilot study by Ackermann et al. (2008),275 whereas the reduction in diabetes used in their modelling was based on the results from DPP and DPS.
Conclusions/recommendations
The current literature is divided on the cost-effectiveness of lifestyle interventions for the prevention of diabetes, making it difficult to reach a definitive conclusion. The majority of studies conclude that it is cost-effective, with the study by Eddy et al. (2005)248 being an outlier. This may be because of similarities in the approaches taken by models by authors other than Eddy et al. (2005),248 the key difference being assumptions on rates of progression to diabetes and complications. The Archimedes Diabetes Model249 is unusual, in that rather than using the traditional Markov approach it is based on human physiology; it is much more complex, but, as stated above, has been extensively validated, and may well be the best model. Hence, although it comes to different conclusions from most others, it could be that it provides more reliable estimates. Other factors preventing a definitive conclusion on the cost-effectiveness of lifestyle interventions being reached include a lack of available evidence on the cost-effectiveness of other less-intensive interventions, and uncertainty relating to adherence and maintained effectiveness of such interventions in routine practice.
It is very difficult to ascertain which model predicts the cost-effectiveness of the DPP intervention most accurately and reliably. This is because no prospective studies have assessed the impact of such interventions on long-term morbidity and mortality. However, the model by Eddy et al. (2005)248 has been extensively validated in terms of its ability to accurately predict short-term outcomes for people with IGT and medium-term outcomes for people with clinical diabetes. The greatest uncertainty relates to the duration of the asymptomatic period between onset of diabetes and the development of clinical diabetes, and the rate of progression of glycaemia and complications during this period. This is something that future epidemiological studies might be able to address. The second and third points can be addressed through further modelling, drawing on effectiveness data from trials of less resource-intensive interventions, and data on the relationship between intervention intensity and patient adherence.
Another issue throwing doubt on the cost-effectiveness of lifestyle interventions for those with IGT is the finding that it may be more cost-effective to postpone implementation of such interventions until people actually develop diabetes. When this is used as the comparator, Eddy et al. (2005)248 report that the ICER for implementing the DPP intervention becomes substantially less favourable. However, it is not clear whether Eddy et al. ’s model (2005)248 takes account of the cardiovascular risk of IGT, nor the development of complications before diagnosis.
In summary, the analysis by Eddy et al. (2005)248 generates uncertainty. Given this, further modelling work using resource use and cost data relevant to the UK context is advisable. Models should include at least three alternatives: (1) do nothing other than provide basic advice for those with IGT until they develop symptomatic diabetes (e.g. HbA1c level of 7%) and then implement an intensive treatment management regime; (2) do nothing other than provide basic advice for those with IGT until the onset of preclinical diabetes (detected by regular screening) then implement an intensive lifestyle intervention followed by an intensive diabetes management strategy for those whose HbA1c level rises above 7%; and (3) provide an intensive lifestyle intervention for everyone with IGT and implement an intensive diabetes management strategy for those whose HbA1c level rises above 7%. On top of this, future models should evaluate less resource-intensive strategies and consider potential trade-offs between intensity and adherence. Given that the costs and benefits of treating IGT will arise as wider costs and benefits of any screening programme for diabetes, models should also incorporate the screening process, and include costs and benefits of treating both IGT and undiagnosed diabetes.
Finally, given uncertainty surrounding the rate of progression of glycaemia and vascular complications in people with IGT and preclinical diabetes, estimates of cost-effectiveness will need to be shown to be robust to large changes in these parameters. This will probably require good evidence to show that substantially less-costly interventions can be as effective as the DPP lifestyle intervention.
Chapter 6 Impaired glucose tolerance and impaired fasting glucose: a descriptive study of current practice in primary care
Introduction
In the UK there is currently no national screening programme for diabetes mellitus or IGT/IFG. As described earlier, the prevalence of IGT/IFG in the UK is poorly characterised, with estimates ranging from 3% to 18%. The recognition and diagnosis of IGT/IFT in primary care in the UK has not, to the best of our knowledge, been reported previously. The current state of practice is important when it comes to considering the costs of any recommendations, because those costs will depend on how much is being done at present.
In order to estimate the extent to which people with IGT/IFG are being diagnosed in primary care, and to determine how they were being managed, we conducted a descriptive study of clinical practice using the UK GPRD.
Methods
Data source
The GPRD is one of the largest longitudinal primary care databases, and provides anonymised data for research. It was established in the late 1980s and, since then, more than four million residents of the UK have registered with GP practices contributing data. General practices from across England, Wales, Northern Ireland and, to a lesser extent, Scotland voluntarily contribute anonymised patient data to the research database. GPRD covers approximately 6% of the UK population. Age and sex structures have been shown to closely reflect the UK population as a whole. 276–282 The database is currently held and maintained by the UK DH.
Details of patient characteristics, prescribed treatments and clinical diagnoses are recorded in the database. Clinical diagnoses are recorded using two disease coding systems. Until the late 1990s (with a gradual replacement), diagnoses were recorded using a modified version of the Oxford Medical Information System (OXMIS). Later, this was replaced by the READ coding system, now in widespread use in the UK.
The GPRD has been used extensively for research in drug therapy and outcomes and people with diabetes mellitus have been studied previously. The estimated prevalence of diabetes has been reported to be in keeping with other studies and national surveys. There was evidence of over recording of codes related to diabetes in children and young adults but, in older age groups, the recording was similar to national survey data. 283
Study population
For this study, we used data from approximately 300 GP practices contributing to the GPRD that have been well validated and used in research previously. 284 We identified a study population of all patients who were registered with a GPRD practice at any time between 1 January 2000 and April 2005, and with a minimum of 6 months of follow-up in the database. People with < 6 months’ follow-up were excluded.
Identifying people with impaired glucose tolerance/impaired fasting glucose
To identify people recorded as having a diagnosis of IGT/IFG (cases), we searched the computer records of each person in the study population for any of the codes in Table 8. IGT/IFG diagnosis was based on the reported measurement of glucose in blood or urine following glucose loading or from random samples, or specific codes that suggested IGT/IFG. The date of the first recording of one of these codes was taken as the ‘index date’. People with a diagnosis of diabetes mellitus, or a prescription for diabetes mellitus treatment recorded before the index date, were excluded.
| Category | OXMIS | Condition or finding | READ code | Condition or finding |
|---|---|---|---|---|
| Blood based | 250 RG | Hyperglycaemia | R102.00 | GTT abnormal |
| 250 K | Diabetes chemical (abnormal) | R102.12 | IGT test | |
| 250 RR | Blood sugar raised | 44V2.00 | GTT impaired | |
| L1300RB | Random blood sugar raised | 44V3.00 | GTT abnormal | |
| L1300RE | Random blood sugar raised (capillary) | 44U5.00 | Blood glucose 7–9.9 mmol/l | |
| L1300RX | Random blood sugar raised (venous) | 44U6.00 | Blood glucose 10–13.9 mmol/l | |
| 44UZ.00 | Blood glucose 14+ mmol/l | |||
| 44U9.00 | Blood glucose abnormal | |||
| 44Uz.00 | Blood glucose raised | |||
| 44Uz.11 | Hyperglycaemia | |||
| R105700 | Blood glucose abnormal | |||
| 44T1200 | Random blood sugar raised | |||
| Urine based | 7895 | Glycosuria | 4663 | Urine glucose test = trace |
| 7895A | Sugar in urine | 4664 | Urine glucose test = + | |
| 7895PV | Glycosuria positive | 4665 | Urine glucose test = ++ | |
| L2200AA | Urine glucose + | 4666 | Urine glucose test = +++ | |
| L2200BB | Urine glucose ++ | 4667 | Urine glucose test = ++++ | |
| L2200CC | Urine glucose +++ | 4668 | Glycosuria | |
| L2200PV | Glucose urine positive | |||
| L2400GP | Urine positive glucose | |||
| Other | 2500AH | Latent diabetes | R102.11 | Pre-diabetes |
| 212 6300 | Diabetes mellitus resolved | |||
| 212H.00 | Diabetes resolved |
To validate the appropriateness of the codes selected, we undertook a pilot study where we reviewed a random sample of 20 complete computer patient records from among those with one of the IGT/IFG codes provided in Table 8. From this, it was apparent that GPs often used these codes as initial consultation codes and that they were soon upgraded to a diagnosis of diabetes mellitus after appropriate diagnostic tests. We therefore classified potential cases of IGT/IFG as follows:
-
IGT/IFG cases Those with no subsequent diabetes mellitus diagnosis for at least 1 month after the index date.
-
Excluded Those with a subsequent diabetes mellitus diagnosis < 1 month after the index date.
For IGT/IFG cases, we examined their complete computerised records to obtain details of characteristics: age, sex and BMI. For estimation of BMI, we used the last recording of weight before the index date. We searched patients’ records for evidence of hypertension, CVD, stroke or obesity – present either before or after the diagnosis of IGT/IFG. We followed the IGT/IFG patients’ records prospectively, from the index date, to ascertain if they subsequently developed diabetes mellitus. Finally, we sought evidence of prescribing of metformin or glitazones to treat IGT/IFG and whether any cases were already receiving medicines important in reducing risk of vascular disease (i.e. statins or aspirin). This was done because it is likely that the diagnosis of IGT or IFG, both of which are associated with an increased risk of heart disease, might trigger the use of statins, and we wanted to know the extent to which such patients were already on a statin. If a high proportion was already being treated because of other risk factors, the benefits of screening for and treatment of IGT would be reduced.
Using a further random sample of 50 full computerised records, two researchers checked the data extraction for the above variables against the original record. This was found to be satisfactory with no errors in the data extracted.
Analysis
A descriptive analysis of those diagnosed with IGT/IFG was undertaken, reporting the proportion by sex, age and BMI. Prevalence rates for IGT/IFG were reported per 100,000 persons. The proportion and timing of progression to diabetes mellitus was estimated. Analysis was conducted using Stata version 9.2 (StataCorp LP, College Station, TX, USA).
Results
From a study population of approximately 2.8 million people registered with a GP practice between 1 January 2000 and April 2005, we identified 12,214 people with at least one code for IGT or IFG.
We excluded 3118 people from the analysis. The most common reason for exclusion was because their code for IGT/IFG occurred < 1 month before a code for diabetes mellitus (n = 2862). Other reasons for exclusion included data quality issues, such as invalid weight or height, and where the codes for IGT/IFG occurred in children or young adults for whom the diagnosis was thought to be clinically unlikely.
A total of 9096 people with codes suggesting IGT/IFG were included in the analysis. We used codes for three clinical indicators of a diagnosis: raised blood glucose, glycosuria or an ‘other’ diagnostic code. Table 9 shows the number of people who had a first (index) code that was a blood, urine or ‘other’ code.
| Code | No. | % |
|---|---|---|
| Blood | 5839 | 64.19 |
| Urine | 3091 | 33.98 |
| Other | 166 | 1.82 |
| Total | 9096 |
Prevalence of recorded impaired glucose tolerance/impaired fasting glucose
The trend in IGT/IFG prevalence increased during the 5-year study period from 17 per 100,000 in 2000 to 31 per 100,000 in 2004 (Figure 7).
FIGURE 7.
Annual prevalence of IGT/IFG.
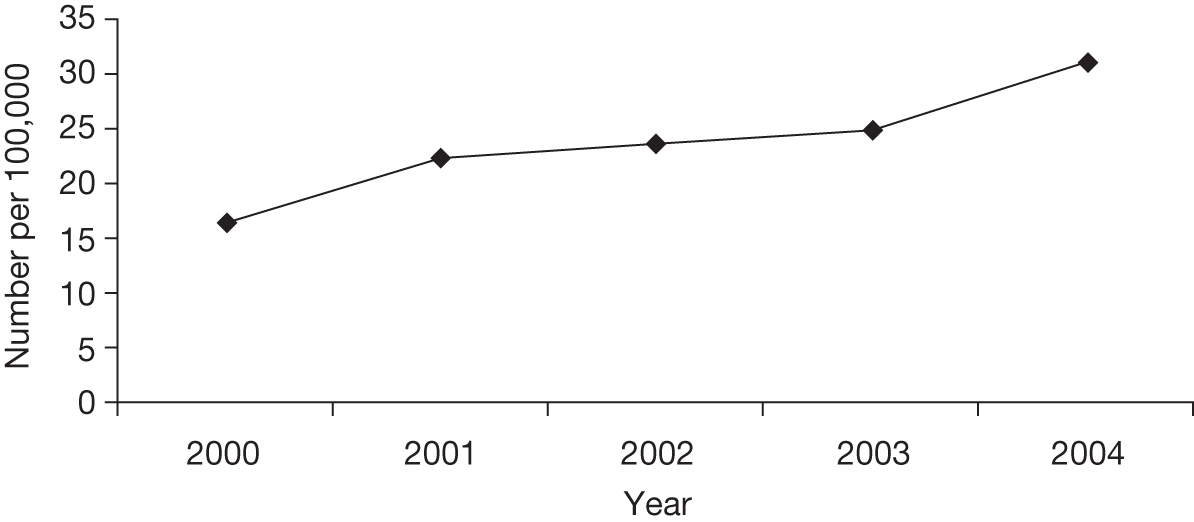
Characteristics of people with impaired fasting glucose/impaired glucose tolerance
Of the people with IGT/IFG, 4298 (47.3%) were male and 4798 (52.7%) were female. The median age was 60.5 years [interquartile range (IQR) 22.8 years]. Table 10 provides the distribution by age. The BMI, based on the last recorded weight before the index date (a median of 3 years before the index date), is summarised in Table 10. Only 225, 2.5%, had been given a coded diagnosis of ‘obesity’. The high percentage with BMI scores of < 25 kg/m2 is somewhat surprising. Metformin and glitazones have been suggested as potential treatments for IGT/IFG, but in this group only 253 (2.8%) of those with IGT/IFG were prescribed metformin or glitazones.
| Sex | Total: n = 9096 (100%) | % | ||||
|---|---|---|---|---|---|---|
| Male: n = 4298 (47.3%) | % | Female: n = 4798 (52.7%) | % | |||
| Age band (years) | ||||||
| 20–39 | 942 | 21.9 | 421 | 8.8 | 1363 | 15 |
| 40–59 | 1220 | 28.4 | 1874 | 39.1 | 3094 | 34 |
| 60–79 | 1723 | 40.1 | 2211 | 46.1 | 3934 | 43.2 |
| ≥ 80 | 413 | 9.6 | 292 | 6.1 | 705 | 7.8 |
| BMI ( kg/m2)a | ||||||
| < 25 | 1075 | 35.3 | 905 | 26.7 | 1980 | 30.8 |
| 25–29 | 943 | 30.9 | 1484 | 43.8 | 2427 | 37.7 |
| 30–34 | 574 | 18.8 | 728 | 21.5 | 1302 | 20.2 |
| 35–39 | 267 | 8.8 | 188 | 5.5 | 455 | 7.1 |
| ≥ 40 | 188 | 6.2 | 87 | 2.6 | 275 | 4.3 |
Cardiovascular risk
Prior to diagnosis of IGT/IFG 3.7% of people had experienced a previous cardiovascular event and 12.5% had a history of hyperlipidaemia (Table 11). A total of 1364 people (15%) were already receiving a prescription for aspirin at the time of their diagnosis and 990 (10%) were already receiving a prescription for statins, whereas 582 (6%) were prescribed both aspirin and statins.
| Risk factor | Before index date | % |
|---|---|---|
| CVDb | 334 | 3.7 |
| HBP | 525 | 5.8 |
| Hyperlipidaemia | 1134 | 12.5 |
Progression to diabetes mellitus
We followed each person with IGT/IFG from their index date to establish if they went on to develop diabetes. In total, 2662 (29.3%) developed diabetes mellitus during their follow-up. Of the 6434 people who did not develop diabetes mellitus, 506 died. The remaining 5928 had not developed diabetes mellitus at the time of their last follow-up. The median duration of follow-up was 2.9 years (minimum 0.9 years, maximum 53.6 years).
Among those who progressed to develop diabetes mellitus, 778 (29.2%) had a weight and height recording that indicated they had been obese (BMI ≥ 30 kg/m2). Table 12 summarises the distribution of BMI, gender and age among those who progressed to develop diabetes mellitus.
| No. | % | |
|---|---|---|
| Sex | ||
| Male | 1147 | 43.1 |
| Female | 1515 | 56.9 |
| Age band (years) | ||
| 20–39 | 214 | 8 |
| 40–59 | 1047 | 39.3 |
| 60–79 | 1262 | 9.8 |
| ≥ 80 | 139 | 5.2 |
| BMI (kg/m2) | ||
| < 25 | 327 | 12.3 |
| 25–29 | 665 | 25 |
| 30–34 | 483 | 18.1 |
| 35–39 | 178 | 6.7 |
| ≥ 40 | 117 | 4.4 |
| Missing | 892 | 33.5 |
| Total | 2662 | 100 |
Of the 2662 who went on to develop diabetes, 799 (30.0%) progressed within 6 months after first diagnosis of IGT/IFG. A further 323 (12.1%) progressed within 12 months. By 5 years, 2256 (85% of those who went on to get diabetes mellitus) had progressed to develop diabetes mellitus (Table 13).
| Progression time (months) | No. who developed diabetes mellitus | No. with follow-up | Percentage of those with follow-up |
|---|---|---|---|
| 1–5.9 | 799 | 9096 | 8.8 |
| 6–11.9 | 323 | 8283 | 3.9 |
| 12–23.9 | 467 | 7485 | 6.2 |
| 24–35.9 | 322 | 5910 | 5.4 |
| 36–47.9 | 210 | 4472 | 4.7 |
| 48–59.9 | 135 | 3385 | 4 |
| 60–71.9 | 90 | 2694 | 3.3 |
| 72–83.9 | 72 | 2160 | 3.3 |
| 84–95.9 | 49 | 1769 | 2.8 |
| 96–107.9 | 52 | 1449 | 3.6 |
| 108–119.9 | 40 | 1191 | 3.4 |
| ≥ 120 | 97 | 958 | 10.1 |
| Unknown | 6 | ||
| Total | 2662 |
Discussion
The primary aim of this study was to identify whether people with IGT/IFG were being diagnosed in clinical practice. We used data for the period up to April 2005, and the situation may have changed since then. This is one limitation of this analysis.
Using the GPRD, we were able to identify more than 9000 people who appeared to have IGT/IFG. GPs were identifying people with abnormal blood or urine test results, who they did not label with a diagnosis of diabetes mellitus. The GPs did not regularly use specific codes for IGT/IFG at this initial identification, although this may reflect the inadequacies of the coding systems, the lack of a specific code for IGT/IFG in OXMIS, and the use of the term ‘pre-diabetes’ in READ. Our prevalence estimates for IGT/IFG, based on cases identified in general practice, were substantially lower than those reported in population-based studies where participants were screened, which is not surprising as screening is not routine practice.
However, we found that coding of abnormal test results consistent with IGT/IFG was increasing and the estimated prevalence of IGT/IFG had nearly doubled over the 5 years of our study. A number of factors may explain this rise. Testing for diabetes may be increasing, leading to more diagnosis of IGT/IFG. There may also be greater recording of abnormal results. Practice nurses, who frequently undertake routine health check-ups, are increasingly recording data electronically and coding of glucose values may have thus increased. Lastly, with the increasing prevalence of obesity, the true prevalence of IGT/IFG would also be expected to be increasing.
Using the definition of a relevant abnormal blood or urine code in the absence of a diagnosis of diabetes mellitus, we identified a population of patients who we considered to have IGT/IFG. A higher proportion of cases were female (52.7%). The age at first recording of the diagnosis differed between men and women, with a higher proportion of men (21.9%) compared with women (8.8%) in the 20- to 39-year-old age band. A total of 31.6% of people with IGT/IFG were obese.
We found evidence that, even where abnormal glucose results were identified, management of cardiovascular risk and weight were not maximised. Prescribing of aspirin and statins was low; 10% were prescribed aspirin and 15% were prescribed statins. Furthermore, only 70.6% had a meaningful weight recorded prior to their diagnosis with IGT/IFG and, even when a weight was recorded, the median time between most recent weight and diagnosis was long. This may reflect the fact that weight monitoring was being undertaken by practice nurses who, at least in the past, may not have recorded clinical data electronically. Medications to treat IGT/IFG, such as metformin or glitazones, were not frequently used.
Approximately 30% of people with IGT/IFG progressed to diabetes mellitus during their follow-up, with 42% of those who progressed having done so within 1 year and 85% within 5 years. Those progressing to diabetes mellitus tended to be younger than the overall IGT/IFG population, and were more likely to be obese, with 44% having a BMI of ≥ 30 kg/m2 compared with 31.6% in the general IGT/IFG population.
Conclusions
Impaired glucose tolerance/IFG appears to be underdiagnosed in UK primary care, and where people have been noted to have test results consistent with IGT/IFG, the diagnosis does not appear to trigger medications to reduce progression to diabetes, or for reduction of CVD. This provides a baseline for consideration of future changes.
Chapter 7 Modelling the cost-effectiveness of non-pharmacological interventions for impaired glucose tolerance
Introduction
The modelling that follows assumes that IGT is detected by an organised screening programme for diabetes. Our assessment addresses the cost-effectiveness of a lifestyle intervention compared with routine ‘basic advice’ in patients who have already been diagnosed with IGT; the economics of screening for IGT are outside the scope of this project.
Although aiming to assess the expected cost-effectiveness in a UK setting, in the absence of a UK diabetes prevention trial, it was necessary to rely on some data from another country. We chose to source the effectiveness data from the Finnish DPS,183 as the DPS was considered to be most similar to what could happen in the UK, as well as Finland being a European country with a state health-care system.
Conceptually, the modelling is relatively straightforward, but the evidence base underpinning the numerous assumptions and modelling methods is often complex. In particular, the prediction of long-term diabetes incidence and adjustment of cardiovascular risk scores according to state of glucose tolerance are subject to considerable complexity and uncertainty. We have undertaken a number of sensitivity analyses to demonstrate the importance of key assumptions and parameters.
Summary of the Sheffield Type 2 Diabetes Model
The Sheffield Type 2 Diabetes Model is an integrated health-state simulation model of the natural history of diabetes and the lifetime cost-effectiveness of different treatments for T2DM. The model replicates patients’ risk of progression through five comorbidities: retinopathy, nephropathy, neuropathy, CHD and cerebrovascular disease. The intensity of management and monitoring can be varied by altering targets, such as those for glycaemic control, requirement for insulin, blood pressure control, and intensity of lipid-lowering therapy. For microvascular complications, the model is largely based on the Eastman models,285 using results from the Diabetes Control and Complications Trial (DCCT). For macrovascular complications, the model uses equations from the UKPDS. 286–288 The time spent by patients in each state for each comorbidity is recorded, for example years spent on dialysis, severe vision loss, etc., together with transitions between states. The effects of treatments on complications are modelled either via a RR (e.g. for the effect of photocoagulation on risk of severe vision loss) or via the effect on underlying risk factors (e.g. the effect of antiglycaemic medication on HbA1c level). Complications are driven by individual demographic and modifiable characteristics at each time period, and the model includes diabetes and other-cause mortality. Total costs are obtained by adding the costs of therapy, the costs of one-off treatments (e.g. cost of amputation), and ongoing treatment of complications (e.g. treatment following stroke). The health benefit, the incremental QALYs, is obtained by applying QoL measures to the time spent in the various diabetic health states. Cost-effectiveness estimates for potential interventions are obtained by dividing the total costs by the incremental QALYs.
Methods
Model structure
The structure of the model used to assess the cost-effectiveness of lifestyle interventions to prevent diabetes is shown in Figure 8. ‘Control parameters’ include HbA1c level switching thresholds for oral hypoglycaemic agents (OHAs) and insulin, targets for blood pressure, and assumptions regarding lipid-related therapy.
FIGURE 8.
Model structure.
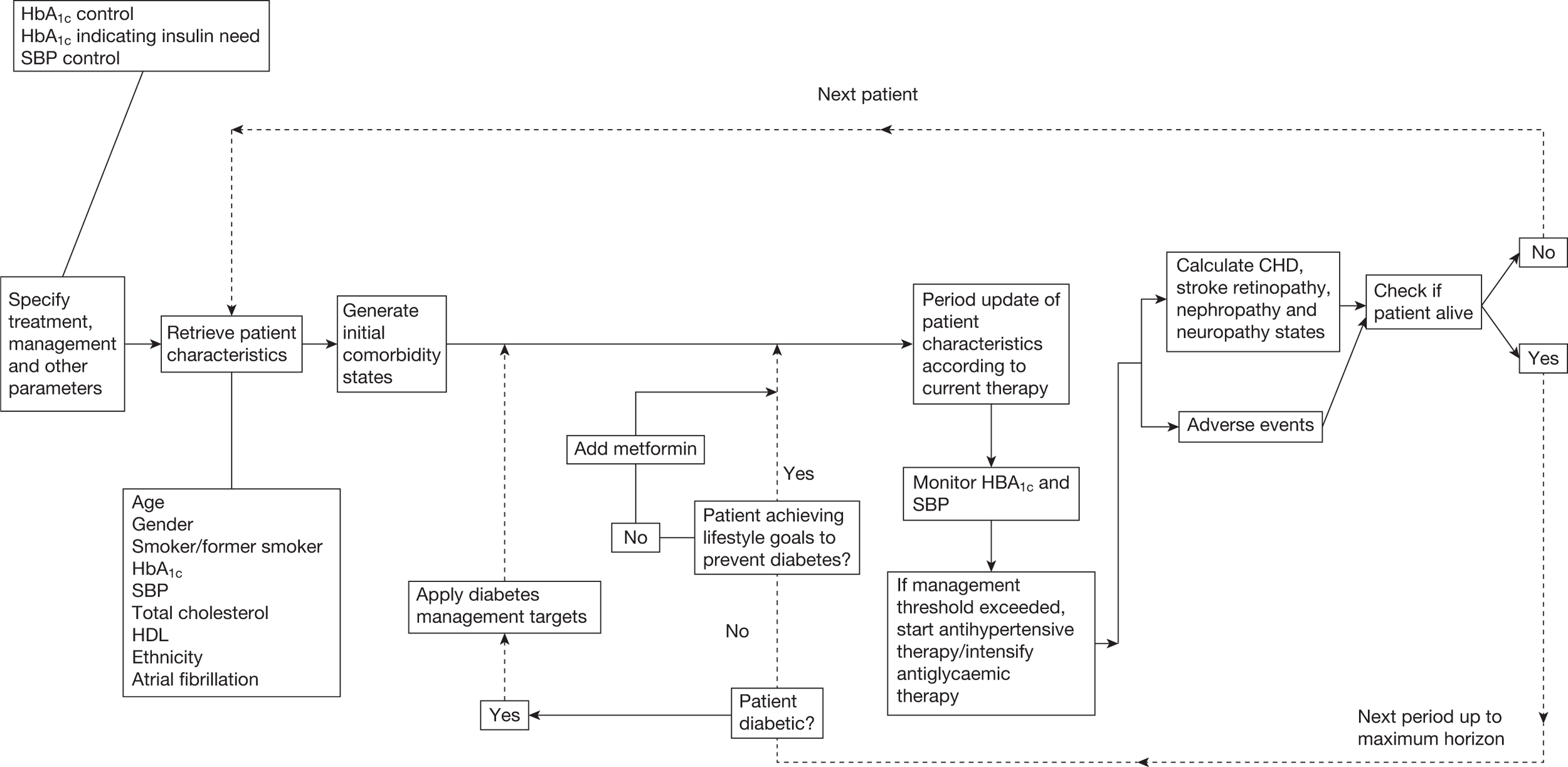
Inputs and parameters specific to this assessment of interventions for preventing diabetes include:
-
incidence curves over time for diabetes in patients with IGT
-
the RR for diabetes of lifestyle interventions
-
the cost of lifestyle interventions
-
the effect of lifestyle interventions on other measures, such as weight and blood pressure.
Baseline characteristics
Table 14 below shows the baseline characteristics, some of which are discussed further below.
Characteristics were generated for 40,000 patients who progressed to diabetes. Matching between the two arms was undertaken (using ranking of duration to progression to diabetes) so that the same patient could not progress to diabetes faster in the intervention arm than in the control group.
| Characteristic | Value: mean (SD) or % | Source/assumption |
|---|---|---|
| Age | 55 (5.8) |
Mean per Finnish DPS183 SD calculateda |
| Gender | 50% | Assumption (only 33% were male in DPS183) |
| Weight (kg) | 86.1 | DPS |
| Two-hour glucose (mmol/l) |
8.82 (1.47) Maximum set to 10.75, otherwise borderline diabetic |
DPS |
| HbA1c (%) | 5.80 (0.08) | Derivationb from Woerle et al.291 |
| Total cholesterol (mmol/l) – pre-statin level | 5.57 (0.93) | DPS |
| LDL-C (mmol/l) | 3.56 | Estimated using Friedewald formula |
| HDL-C (mmol/l) | 1.20 (0.30) | DPS |
| SBP (mmHg) | 138 (17.5) | DPS |
| Smoking | 15%c | QOF 2004–55 |
| Ethnicity | 86.3% white people, 4.4% Afro-Caribbean, 9.3% Asian | Derived from YHPHO Diabetes Prevalence Model (Phase I) 6 |
| Atrial fibrillation | 1% | UKPDS 60286 |
| History of MI | 6% | Saydah et al.292 (IGT patients) |
| History of stroke | 2% | See below |
Age
In the Finnish DPS183 the baseline age was 55 years. Analysis of GPRD data gave an age of around 60 years at diagnosis of IGT, as reported in Chapter 6. Given that the age might be lower in the context of an organised screening testing programme, we chose 55 years as the average baseline age and sampled age characteristics used the standard deviation (SD) of 13.5 from the GPRD analysis. Baseline age was limited to a range of 45–65 years because the value of treating older patients with IGT may be limited, as it has been suggested that diabetes is not an additional risk factor for CHD for men of > 65 years of age. 289 The range of 45–65 years is also in line with the range in DPS. Applying this criteria to age range led to a reduced SD of 5.8.
Stroke prevalence
The EPIC (European Prospective Investigation into Cancer) study290 reported a prevalence of heart attack or stroke of 5.5% in the 5–5.4% HbA1c group and 8.8% in the 5.5–6.9% group. Based on this we estimate the prevalence in a group with baseline HbA1c level of 5.80% to be 7.5%. Based on previous analyses, we split this in the ratio 7 : 3 between CHD and stroke, i.e. approximately 2% for stroke.
Microvascular complications
We assume that patients with IGT have been detected sufficiently early for the incidence of complications to be negligible.
Mix of impaired glucose tolerance/impaired fasting glucose and risk profile of cohort in the model
As discussed in Chapter 1 (see Progression to type 2 diabetes mellitus from impaired glucose tolerance or impaired fasting glucose), the co-existence of IFG alongside IGT has implications for risk of progression to diabetes. The purpose of the modelling is to assess the cost-effectiveness of treating the IGT population in the UK. The generalisability of the DPS183 incidence rates to the UK IGT population therefore needs to be considered. The DPS183 was a group of individuals at high risk of diabetes. Progression among a group representing the full range of IGT would be expected to be lower, as shown in several studies, such as the study in Bedford44 (see Chapter 1, Progression to type 2 diabetes mellitus from impaired glucose tolerance or impaired fasting glucose). Whether the DPS183 was a higher-risk group than would be identified via a UK vascular risk programme depends on the choice of methods for identifying and screening patients, and the cut-offs used within these methods.
The 4-year incidence of 29% from the GPRD analysis (see Table 13) is similar, however, to the 4-year incidence in the control arm of the DPS,183 so this suggests that the risk profiles of the DPS183 and patients currently identified as having IGT in the UK may be reasonably similar. We have also undertaken a sensitivity analysis assuming a lower effectiveness for the intervention relative to our ‘basic advice’ arm.
In the DPS, the mean baseline FPG was 109.5 mg/dl with a SD of 14. Based on the ADA criteria for IFG of 110 mg/dl,293 this suggests that a considerable proportion of the DPS participants183 had both IFG and IGT. Hoerger et al. (2007)262 reported that, in the National Health and Nutritional Examination Survey III (NHANES III), only 43% of patients with pre-diabetes had isolated IGT, and 26% had both IGT and IFG, so the DPS183 might have had more patients with both IGT and IFG than in a population setting. Valensi et al. (2005)294 reports that in all prevalence studies to date, up to half of subjects with IFG have IGT, whereas a lower proportion (20–30%) with IGT also have IFG.
Two considerations follow from this:
-
The baseline diabetes incidence rate in the DPS might have been higher than observed in a population setting The Hoorn study [de Vegt et al. (2001)295] reported that patients with both IGT and IFT were nearly four times as likely to develop diabetes than those with isolated IFG or isolated IGT. Nathan et al. (2007)296 report that individuals with both IFG and IGT develop diabetes approximately twice as often as individuals with just one of the two conditions.
-
The effectiveness of the intervention may depend on the mix of IGT and IFG IFG may be less amenable to treatment with interventions which primarily target insulin resistance.
These issues highlight the fact that cost-effectiveness may vary across different subgroups of the IGT population (e.g. the most obese, the highest age bracket, etc.).
Importantly, it should not be assumed that the clinical effectiveness of the DPS intervention (and hence our cost-effectiveness results) are applicable to patients with isolated IFG because:
-
The incidence of diabetes and the effectiveness of any intervention may differ.
-
The excess risk of CVD (compared with the general population) is less with IFG than IGT. 55
Description and cost of intervention
Modelling a ‘real-life’ scenario
Modelling based on what happens in trials may not reflect what happens in routine care. Trials are protocol driven, and patients are expected to adhere to the treatment to which they are randomised. However, in routine care, clinical judgements are applied throughout, and if a treatment is not working, it should be stopped promptly. This can make treatments much more cost-effective, because only those people who are responding will continue to incur the cost.
The effect of this was illustrated in the review of new drugs for lung cancer for NICE. 297 In the trials, patients continued the drugs for the full series of chemotherapy courses, if they could tolerate them. In routine care, the effectiveness could be assessed after one or two courses, and the drug was stopped if there was no response. This considerably reduced the cost per QALY.
Hence, while modelling the results, as seen in the trials, has the merit of being based on observed data, it may be misleading as a guide to cost-effectiveness in practice.
Lifestyle measures are effective in preventing or delaying diabetes if adhered to but not all patients will adhere. This will apply even when those starting lifestyle interventions have been self-selected by, first, volunteering to be screened knowing what intervention would follow, and, second, coming back for intervention after being found screen positive.
There will probably be a range of adherence, rather than a dichotomy into full adherence and none. But, broadly speaking, some people will succeed and others will not. There is little point in pursuing lifestyle measures in those who do not adhere sufficiently, especially when we have an effective and inexpensive alternative – metformin. (However, lifestyle will always be the first choice, as it is more effective. In the DPP, lifestyle changes reduced the development of diabetes by 55%, and metformin by 30%, although the advantage of lifestyle changes over metformin was most evident in the > 45-year age groups.)
If we abandon lifestyle interventions in the non-adherent group, those left in the intervention group will be those doing best, and their results will be better than the average in the lifestyle groups in the trials. We will also reduce the cost, because the numbers left in the lifestyle group will be smaller (so we will need fewer groups, assuming that the intervention was group based). Simultaneously, the non-adherent group will be doing better on metformin (on the assumption that most will take tablets but not exercise and diet: in the DPP adherence to metformin was 71%, defined as taking 80% or more of the prescribed dose. 298
The question for modelling is how we define failure and at what interval. Various studies have shown that the effect of interventions wear off after they are stopped, or reduced in intensity.
Figure 9 shows the effect of discontinuation [based on data from Swinburn et al. (2001)299], and Figure 10 shows the effect of a reduction in intensity [based on data from Wing et al. (1998)238]. In the former, Swinburn et al. (2001)299 provided the reduced-fat diet intervention for 12 months, and the effect lasted for another 12 months. Weight loss was 3 kg at 6 months, 3.3 kg at 12 months and 3.2 kg at 2 years. However, weight gain resumed at this point, and the intervention group weight curve meets the control subjects after 5 years.
FIGURE 9.
Change in weight (kg) over time: reduced-fat diet intervention.
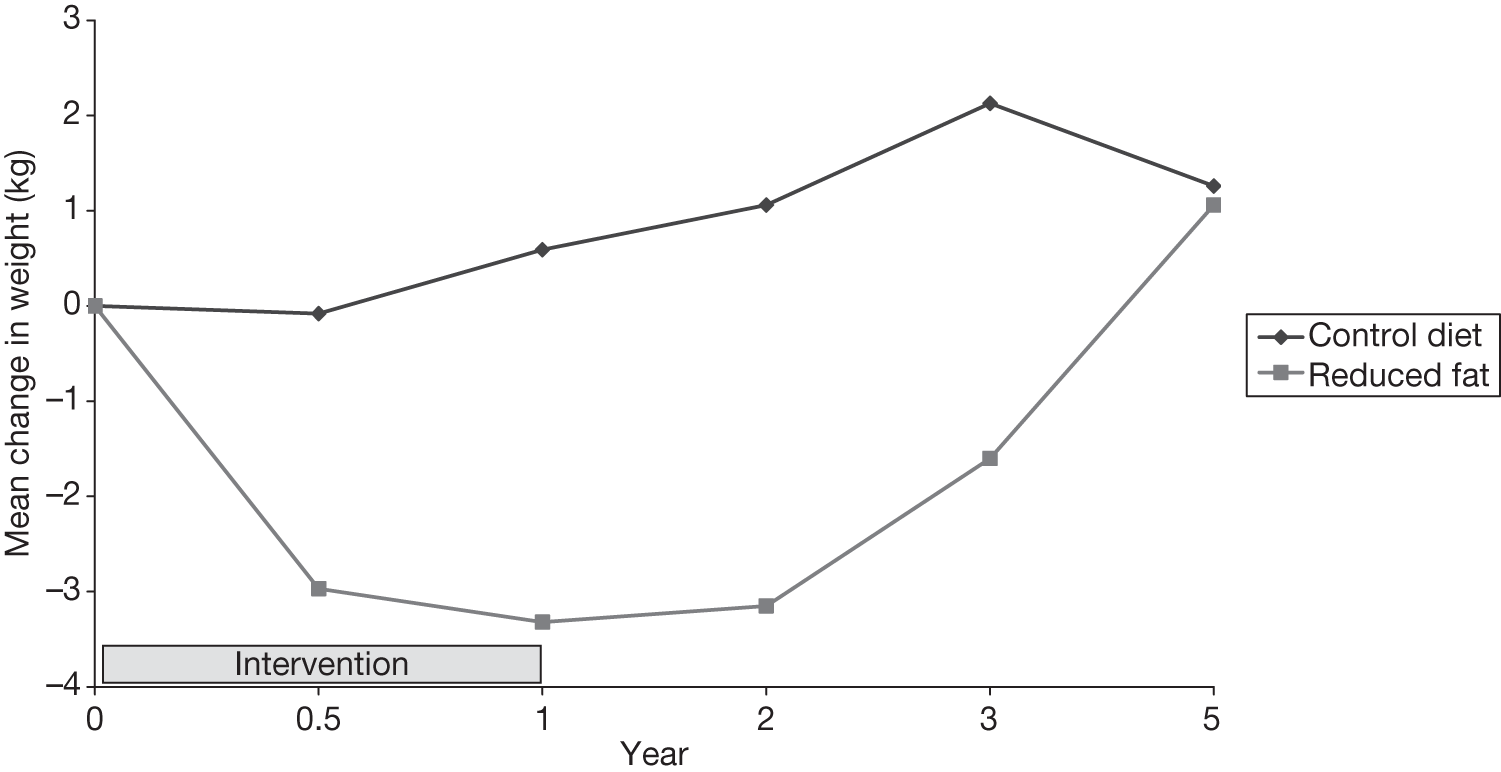
FIGURE 10.
Change in weight (kg) vs time: 2-year lifestyle interventions in overweight individuals. LI, lifestyle intervention; BA, basic advice.
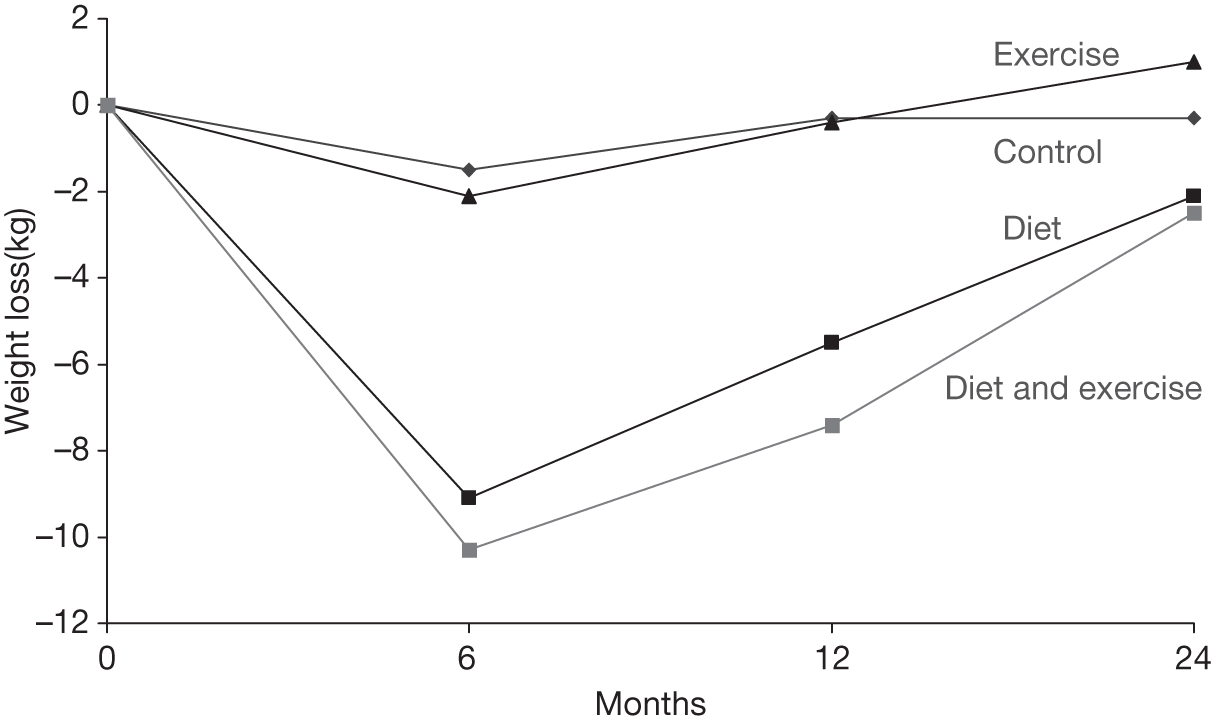
In the latter, Wing et al. (1998)238 provided weekly meetings for the diet groups for the first 6 months, reducing to fortnightly for the second 6 months. As the graph shows, reduction in intensity was accompanied by an increase in weight. By 6 months, 30–40% of recruits had ceased attending. By 12 months, most had ceased to attend.
However, in this case the non-adherers probably adhered for a short period and then relapsed. It seems best therefore to base discontinuation on the results from the larger trials, such as DPS, where there was initial improvement (weight loss and physical activity) in the first 6 months, maintained to 12 months, followed by a loss of some of that gain by 18 months and beyond. In most trials,108,238,299 results are good for 6 months, after which they tend to plateau for a while before the subject starts to regain weight. These results reflect the mix of adherers and non-adherers. We will use that interval as the point at which non-adherers will leave the lifestyle arm and switch to metformin.
Trials such as DPP and DPS did not provide scatterplots of results at time intervals so we do not have distributions showing how many succeeded. However, the DPS reported progression to diabetes by ‘success score’ based on how many of the five lifestyle goals were met. Diabetes did not develop (at the 12-month visit) in any of those who achieved all five goals. If we take a success score of three or more as the indicator of adherence then only 37% of the intervention group adhered. 13% of the control group also achieved this level of success, so standard care works for some: the marginal effect of intervention was 24%.
Therefore, assumption 1 is that only about 40% will do well enough to justify continued intervention.
In addition to the interval, we also need to decide how to define failure. The key result in the trials is weight loss, except in the Indian trial,173 in which the intervention groups did not show significant changes in weight from baseline (although the control group gained weight). The key indicator of failure, therefore, should be not losing weight or not losing enough weight. That raises the question of defining ‘enough’, which has to be applied at the time point of switching treatment: 12 months. In the DPS, the average amount of weight lost was modest (3.5 kg at 2 years, vs 0.8 kg in the control group – but with a large SD of 5.5 kg, indicating that some had lost a lot of weight), but was clearly enough to reduce progression to diabetes. The target weight loss in the DPS was ≥ 5 kg and this was achieved by 43% at 12 months and 42% at 2 years. The group that lost most weight showed the greatest gain in insulin sensitivity after four years. 218 At the 7-year follow-up, weight loss was the strongest predictor of success, and in multivariate analysis, weight loss was the only factor that remained significant. 213,214
In the DPP, the intervention group lost on average 7 kg by 6 months. In the DPP, the target was weight loss of ≥ 7%, and that was achieved by 50% at the 24-week follow-up point (when the 16-lesson curriculum ended). Weight loss was the main determinant of diabetes prevention in the DPP. 193
With good compliance, patients should be losing weight slowly but steadily, about 2 kg per month, or about 1 lb a week. That would suggest a loss of about 12 kg at 6 months. The DPS and DPP results show much less than that in the lifestyle groups, but their means reflect the full range of compliance, so those who adhere best will achieve more. In the DPS, those in the highest tertile of weight loss achieved losses of 8–17%, equivalent to 7–15 kg. Those who lost less had very little improvement in insulin sensitivity.
The definition of failure might therefore be taken as weight loss, being liberal, of < 5% at 6 months, which would exclude 60% of those in the DPS, namely those who did not achieve three or more of the targets. (See Projected incidence of diabetes: intervention arm. )
Duration and cost of intervention
The intensive intervention is assumed to continue for 4 years as per the median active intervention period in the DPS. 214 After that, an annual appointment with a nurse is assumed each year to check glycaemic status and re-enforce advice. We chose not to assume a longer period of intensive intervention because:
-
there are no data to show the effect of a longer treatment duration
-
the DPS214 showed sustained reduction in risk of diabetes beyond the 4-year trial and intervention duration. It can be argued, therefore, that in many patients who achieve targets, the lifestyle changes become engrained beyond 4 years.
Costs per annum are based on resource use reported in an economic analysis of the DPS intervention in a Swedish setting. 221 These are separate for the first and subsequent years and are shown below in Table 15.
| Component of intervention | Unit cost – Sweden (€) | First year | Subsequent year | ||
|---|---|---|---|---|---|
| Resource | Cost (€) | Resource | Cost (€) | ||
| Visits to the physician | 73 | 1 | 73 | 1 | 73 |
| Visits to the nutritionists | 39 | 7 | 273 | 4 | 156 |
| Participation in two circuit-type resistance training sessions per week, each estimated to cost €818 (per year) for a group of 15 persons – assumes a mean participation rate of 67.5% | 37 | 2 | 74 | 2 | 74 |
| Costs associated with time and travel to physicians | 38 | 8 | 304 | 5 | 190 |
| Total cost | 724 | 493 | |||
These bottom-up costs reconcile well with the total costs of €730 and €498 reported in Lindgren et al. (2007). 221
Although UK unit costs are available from Curtis (2007),300 we did not use these to build up a total UK cost because the duration of contact time for each resource of the DPS was not reported.
The costs associated with time and travel to physicians were excluded from our analysis, as these are indirect costs.
For the unit cost of the intervention, we have costed in all seven dietitian visits offered per protocol as though adherence is 100%. The actual costs incurred are adjusted for non-adherence as described below (see Adherence to interventions). There may be some difference between the cost of circuit-type resistance training reported in the DPS221 and the cost in real life. The average participation rate among those who are successful in achieving lifestyle goals would be higher than the 67.5% assumed in the DPS. 221 However, in real life, the number of participants would initially be sufficient to generate work for several classes, so that if attendance dropped, classes would probably be able to merge, rather than being less efficient for fewer people. Overall, the results are likely to be robust to any small variation in the cost of circuit training.
This gives a total direct intervention cost of €420 (724 minus 304) and €303 (493 minus 190). We have converted these direct costs to pounds sterling [GBP (£)], as we do not have data on the durations of time involved with each individual component. This gives first-year and subsequent annual UK costs of £294 and £211, respectively, using a conversion rate of €1.43 = 1 GBP. 301 This compares with £324 and £178, respectively, reported by Avenell et al. (2004)266 costing the DPS lifestyle intervention using UK unit costs. After uplifting costs for inflation from 2003 levels to 2008 levels using the Hospital and Community Health Services Pay and Price Inflation Index provided in Curtis (2007),300 the first and subsequent year costs used in the model are £360 and £260, respectively. 300
In addition, we assume an annual HbA1c test costing £5 and 50% of patients requiring a diagnostic OGTT (because HbA1c tests are not 100% sensitive for diagnosing diabetes) costing £20, giving an annual average test cost of £15.
For the control arm, we have assumed that basic advice is given as a one-off at the initial GP visit (at diagnosis of IGT). Thereafter, we assume an annual appointment with a clinical nurse specialist for re-enforcement of basic advice at a cost of £14. Test costs are assumed to be the same for the lifestyle intervention group. We assume 100% adherence to these and that this achieves the clinical outcomes in line with the control arm of the DPS trial221 (these were not insignificant). This may be underestimating the resources needed to achieve the DPS221 control arms results because the protocol included annual visits (and possibly was influenced by the ‘research effect’) and by the fact that recruits were volunteers.
Modelling transitions
In reality, transition to diabetes or regression to NGT is not a linear process. Equally, for patients who remain in the IGT state, there may be intermittent periods of increasing and decreasing glycaemia. It would be too complex for our purposes to model such variability. We do not have data to model the variation (across individuals) in average HbA1c levels and 2-hour glucose change over the 20-year horizon that we have used to project incidence of diabetes. For simplicity, to obtain the distribution of time to progression, we have randomly assigned individuals states at year 20, based on actual rates of progression in the DPS study and statistical modelling to 20 years. For individuals who progress to diabetes, we have also assigned a year of progression based on a distribution of time to progression obtained.
Progression rates in the Diabetes Prevention Study
Our analyses use data from the Finnish DPS study183 because the American DPP study108 had a high proportion of patients with a family history of diabetes that may lead to an unrepresentatively high rate of progression in that study. Actual rates observed in Finnish DPS183 are shown below in Table 16.
| Treatment arm | Cumulative incidence at: | |
|---|---|---|
| 4 years | 8 years | |
| Intervention | 11% (95% CI 6% to 15%) | 23% (CIs not reported) |
| Control | 23% (95% CI 17% to 29%) | 38% (CIs not reported) |
| RR at 4 years | 0.42 | 0.57 |
| (Integrated) HR | 0.4 (95% CI 0.3 to 0.7) | 0.57 (95% CI 0.43 to 0.76) |
The integrated HR takes account of the relative cumulative incidence over the duration of the treatments rather than at a single end point.
In the DPS,183 the median duration of treatment was four years but median follow-up was 7 years with longest reported RRs at 8 years. 214 Although the 43% risk reduction at year 8 was less than the 58% at year 4, the difference in the absolute proportion of patients who progressed continued to increase beyond year 4, i.e. there was a sustained reduction in risk even after the intervention had finished. A possible explanation for this is that the continuing research follow-up in DPS183 may have been inadvertently therapeutic through motivating patients to continue adopted lifestyle changes, which were presumably still more intensive in the intervention arm than in control patients. Further data from the DPS183 report a 40% risk reduction at year 10. 302
Projected incidence of diabetes: control arm
As a base case, we assume that adherence to the intervention was in line with the DPS achieving the same effectiveness over 8 years.
Long-term incidence of diabetes
It is difficult to extrapolate with precise accuracy the transition rates much beyond the duration of the DPS trial. 183 One source estimates that, over the course of a lifetime, as many as 83% of persons with pre-diabetes (IGT), who neither lose weight nor engage in moderate physical activity, will develop diabetes. This compares with approximately 65% of persons with pre-diabetes who lose weight and engage in moderate physical activity who will go on to develop diabetes. 244,295
The available evidence suggests that incidence begins to decline: many/most who are going to develop diabetes will do so within 10 years, so we have assumed that the cumulative incidence begins to plateau (see GPRD data in Chapter 6) and adopted an exponential (declining) curve for incidence in the control arm. We have projected incidence to year 20 with the incidence slowing beyond the 8-year duration of the DPS183 as shown below. The cumulative incidence is assumed to reach 70% after 20 years. This may be a little low but, in order not to overestimate the benefits of intervention, a conservative estimate was chosen. Also, the basic advice given in the control arm may reduce incidence to lower levels than observed in some studies.
Projected incidence of diabetes: intervention arm
In the DPS,183 each subject was (1) given advice on physical activity, (2) offered circuit-type training and (3) offered seven sessions with a nutritionist during the first year of the study and one session every 3 months thereafter.
We assume that, after the initial 4-year course, those in the lifestyle intervention arm benefit from some sustained reduction in diabetes risk as observed in the DPS183 follow-up, although it is unclear how much is prevention compared with delay. It may be too optimistic to assume that the benefit of having prevented some patients progressing to diabetes is sustained indefinitely. The 20-year results224 from the Chinese Da Qing study also add weight to an assumed mix of prevention and delay.
Intervention arm strategy: assessment of success at 12 months
In the DPS,183 no subjects who achieved four or five of the targets progressed to diabetes during the trial, and at 10 years only 2% had developed diabetes. This suggests that the intervention was highly effective, if complied with. But many patients either do not respond well to lifestyle interventions or do not adhere to them. This means that expensive interventions may not be worthwhile in these patients and affordability could be improved by linking continuation of the intervention to attainment of targets.
Based on the proportion in the DPS183 who failed to achieve a goal success score of ≥ 3, we assume that 60% of patients switch away from the lifestyle intervention arm after 12 months. This is same proportion who also failed to achieve the 5% weight loss target at 12 months. We will refer to the subgroups that did and did not achieve a goal success score of ≥ 3 as ‘responders’ and ‘non-responders’ from this point. In the main analysis, patients who are switched off the intensive lifestyle intervention are assumed to subsequently receive the same intervention as control subjects.
Projecting incidence of diabetes for responders and non-responders
Responders
For the overall lifestyle group, the RR for having developed diabetes compared with the overall control group increased over time from 0.48 at year 4 to 0.65 at year 8 (although the absolute difference in incidence remained about the same, suggesting that there is some sustained protective effect after 4 years). Fitting curves to the data for the intervention period and follow-up period suggests a logarithmic form that is consistent with the concept of an increasing RR but one that increases at an ever slower rate (because of some sustained preventative effect).
Among those in the lifestyle intervention group of the DPS achieving a success score of ≥ 3 at the end of year 1, the incidence of diabetes during the intervention period was 2.2% (2/87) compared with 24% (56/233) in the overall control group, giving a RR of 0.10 (95% CI 0.02 to 0.38). The RR was 0.67 for non-responders in the lifestyle arm compared with the overall control group.
In order to estimate how the RR in the responders changes between year 4 and year 8, we explored alternative scenarios for how the RR in responders and non-responders might change, while the combined RR changes from 0.48 to 0.65. A conservative but plausible scenario is for the RR in the non-responders to change from 0.67 at year 4 to 1.0 at 20 years. The corresponding change in the responders’ RR is to increase from 0.1 after 4 years to 0.46 at year 20 as shown below (Figure 11).
FIGURE 11.
Change in RR for having developed diabetes: responders vs control group.
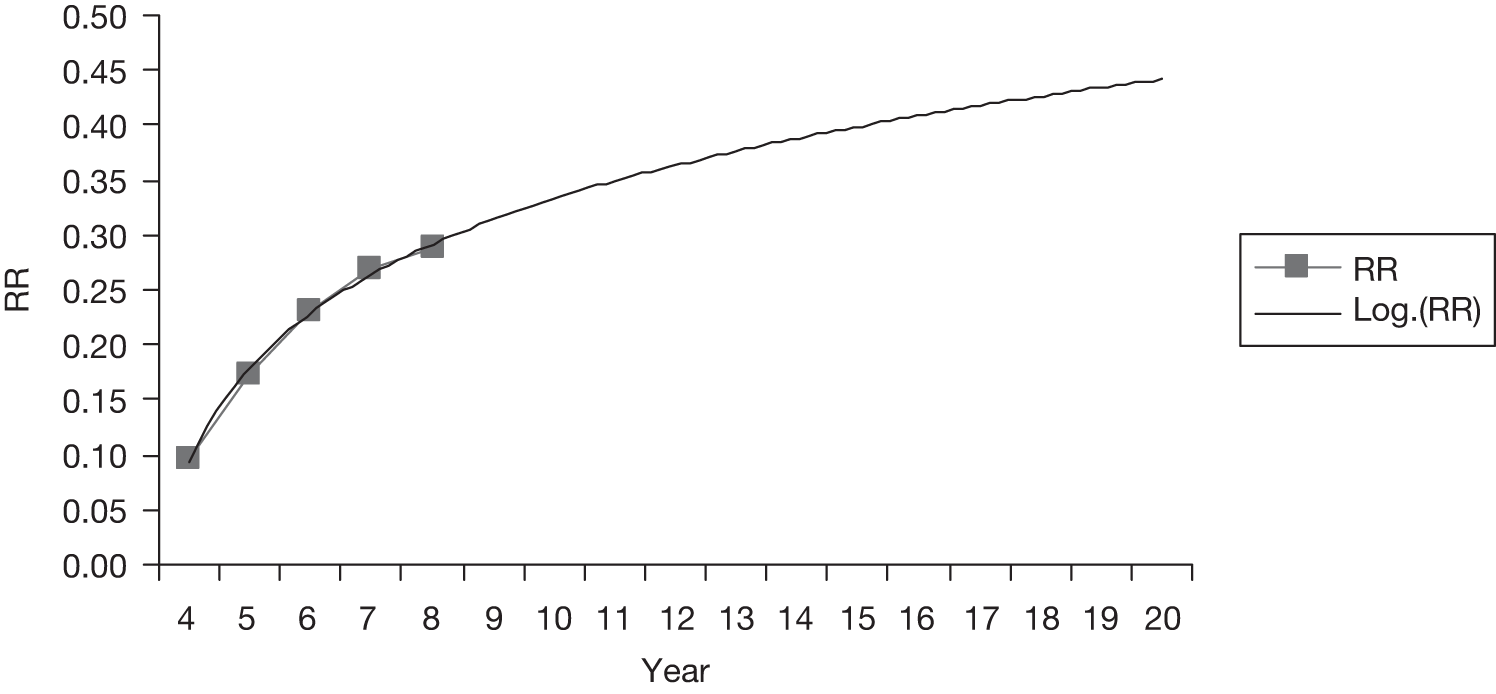
Non-responders
For non-responders, after switching to basic advice we assume that these patients are at the same risk as the corresponding least successful group in the control arm of the DPS, i.e. we are assuming that non-response is partly a result of non-adherence to the intervention and advice. Based on the risk of those achieving a goal success score of 0 or 1 in the control arm of the DPS183 (55% of patients), we obtained a RR of 1.07 for the non-responders, after switching to basic advice, compared with all control subjects.
Combined incidence of diabetes with a switching strategy for non-responders
The estimated and projected cumulative incidence of diabetes obtained, under our base-case strategy involving switching those who do not achieve targets, is shown in Table 17 and Figure 12. Actual values are estimated from the incidence curves. We assume no further incidence of diabetes in either arm after year 20.
| Year | Lifestyle intervention (%) | Control (%) |
|---|---|---|
| 0 | 0.0 | 0 |
| 1 | 2.1 | 7 |
| 2 | 10.1 | 15 |
| 3 | 14.5 | 22 |
| 4 | 18.4 | 28 |
| 5 | 24.0 | 34 |
| 6 | 29.0 | 40 |
| 7 | 32.0 | 43 |
| 8 | 36.5 | 49 |
| 9 | 40.2 | 53 |
| 10 | 43.4 | 56 |
| 11 | 46.0 | 59 |
| 12 | 48.2 | 62 |
| 13 | 50.1 | 64 |
| 14 | 51.7 | 65 |
| 15 | 53.0 | 67 |
| 16 | 54.1 | 68 |
| 17 | 55.1 | 69 |
| 18 | 55.9 | 69 |
| 19 | 56.6 | 70 |
| 20 | 57.2 | 70 |
FIGURE 12.
Calculated and projected (dashed) cumulative incidence of diabetes. BA, basic advice; LI, lifestyle intervention.
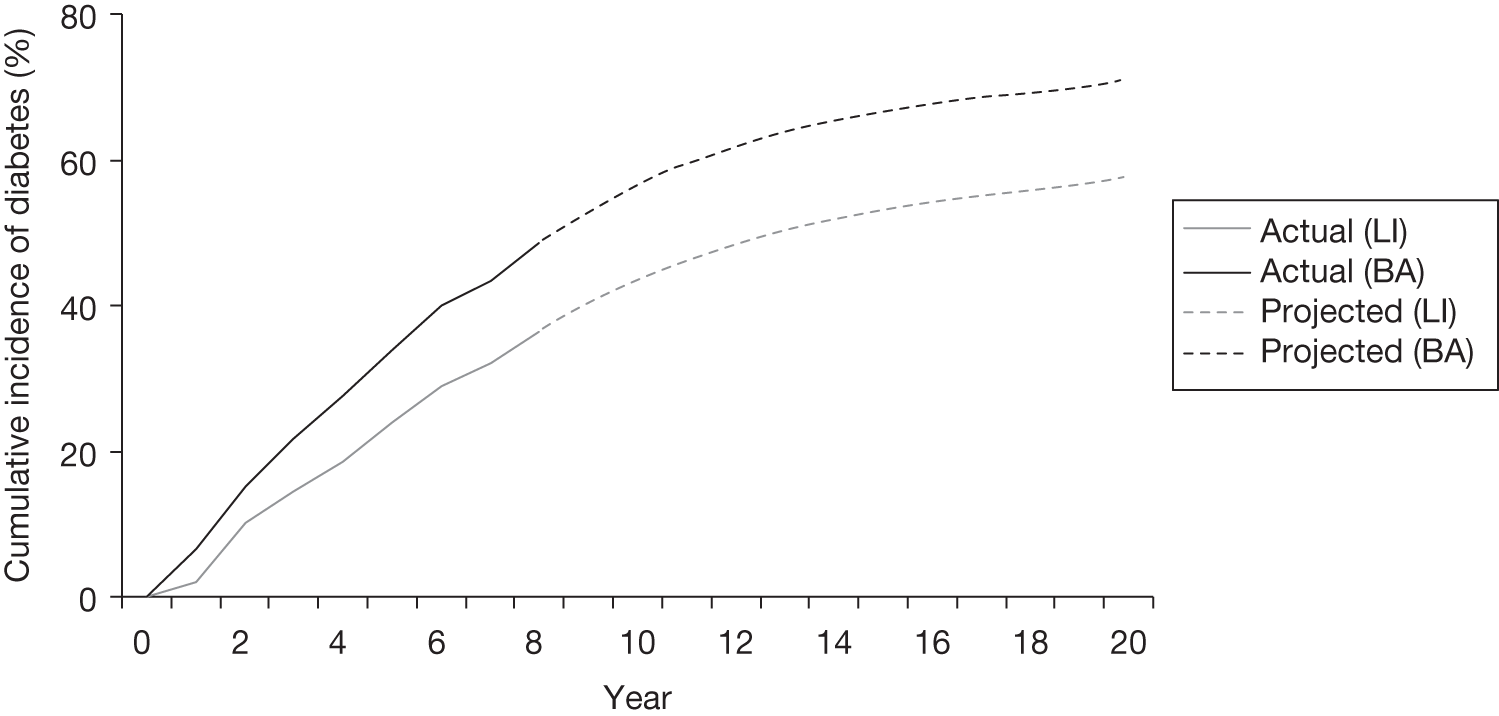
Under these assumptions, for the 57% of patients in the control arm who are projected to progress to diabetes over the 20 years, the average time to diabetes is approximately 4.9 years compared with 7.4 years for the first 57% of patients to progress in the intervention arm, a delay of 2.5 years.
At year 20, there is a difference between treatment arms in the proportion of patients who have progressed to diabetes. Although it is not known whether, as we have assumed, these patients have been prevented from developing diabetes indefinitely, this assumption is less important for the overall results because:
-
patients will be aged 75 years on average by then so that life expectancy beyond is much reduced
-
any convergence of the incidence curves beyond year 20 is likely to be gradual
-
costs and benefits beyond the 20-year horizon will be heavily discounted.
Alternative scenarios for projected cumulative incidence of diabetes
Although we believe that the above assumptions are reasonable for the base-case analysis, the incidence projections involve considerable extrapolation and assumptions. Therefore, we have adopted two alternative scenarios to be tested within the sensitivity analyses:
-
a more conservative scenario in which the cumulative incidence curves gradually close until they converge after 20 years
-
a more optimistic scenario in which the sustained benefit in those who achieve lifestyle targets is greater – in this case the RR of having developed diabetes in responders is assumed to increase from 0.1 at year 4 to 0.18 at year 20 (this is obtained by adopting an alternative assumption for how RR of diabetes changes after year 4 for non-responders compared with control subjects, this assumption being that it increases to 1.0 by year 10).
Alternative treatment pathway for non-responders
In a sensitivity analysis, non-responders are switched to metformin rather than basic lifestyle advice, in line with IDF guidance. 303 The DPP showed that the RR for progression to diabetes with metformin increased with age at baseline, although there was some benefit even in the oldest subgroup. 194 For our population starting with an average age of 55 years, we estimated the RR compared with placebo to be 0.75. Although we assumed that metformin would be given indefinitely (given its very low cost), over an intervention period spanning 20 years, the RR is likely to be higher than that observed in the DPP, with an average follow-up of only 3.2 years. For the purposes of the modelling, we considered it more conservative to use the RR for a 65-year-old at baseline, which we estimated to be of 0.83.
Adherence to interventions
Lifestyle intervention
In generating the above incidence curves, we have assumed that adherence to our intervention mirrors that in the DPS. 183 The absence in the real world of financial incentives (e.g. reimbursement of travel expenses) is not considered as being likely to affect adherence as any prevention programme would be readily accessible to most patients using local GP surgeries and/or pharmacies. In the DPS,183 the average reported change in dietary and exercise habits during the first year was 58%, suggesting suboptimal adherence. As with many lifestyle interventions, it is also likely that adherence levels in the DPS fell after the first year. However, patients who adhere least are most likely to be the ones who do not achieve the lifestyle goals and are switched (either to control therapy or to metformin) after 12 months.
These levels of adherence are assumed:
-
58% average adherence across all patients during year 1
-
100% adherence in the 40% of patients who remain on the intervention until year 4, and 100% adherence to the ‘re-enforcement intervention’ thereafter.
The intervention costs each year are adjusted according to the adherence level.
Metformin
In the DPP in USA,108 adherence in the metformin arm was 72%. 108 We assume the same level is achievable in the real world. Some studies have reported lower rates of adherence to metformin so, during interim analyses, we tested out the effect of only 40% adherence to metformin when prescribed to prevent diabetes (assuming a linear relationship between level of adherence and reduction in risk of progression to diabetes). The results were not very sensitive to this assumption so we did not include this sensitivity analysis in the final analyses.
Non-progressors and regressors to normal glucose tolerance
In patients who do not progress to diabetes, there may still be some benefit from lifestyle intervention through improved cardiovascular risk profile, especially reduced SBP. Lower glucose levels, better lipid profile (e.g. HDL-C improvement through exercise) and the effect of weight loss or increased physical activity on CVD (independent of the above risk factors) may also confer benefits but appear to be less significant or unknown. Weight loss is also known to improve QoL.
However, in the absence of any evidence on differential CVD risk profiles between treatment arms for those who remain with IGT, we have not included any such effects.
Similarly, we have not modelled rates of regression to NGT because there is a lack of evidence to accurately quantify:
-
timing of regression
-
the benefits of relatively small differences in glycaemia at relatively low levels (i.e. below the threshold for IGT but above the normal level).
Not including such effects has a conservative effect on the cost-effectiveness of lifestyle interventions.
Other treatment and monitoring assumptions for impaired glucose tolerance patients
Some patients with IGT have a high enough risk of a CVD event that they should be taking statin therapy. This is because of a combination of their age, presence of features of the metabolic syndrome, or presence of established CVD. We estimated that approximately 20% of patients should already have a prescription for a statin at the point of diagnosis of IGT.
For this assessment, assumptions in respect of the benefit from statin therapy are important in setting a baseline level of CVD risk. We assume an average dose of 40 mg generic simvastatin at a cost of £3.80 per 28 [British National Formulary (BNF) 54]. 304 Only 5.5% of patients in DPS were treated at baseline with lipid-lowering therapy, so the baseline LDL-C level of 3.56 mmol/l as per Table 14 is effectively the pre-statin level. With an underlying adherence rate of 86% in statin trials,305 an LDL-C fall of 37% with 40 mg of simvastatin is expected based on the analysis of Law et al. (2003),306 i.e. 1.32 mmol/l. This reduction would lead to a RR of CHD with statins of 0.50 taking the rate for 60-year-olds to be most appropriate for this purpose. It is assumed that primary and secondary prevention RRs are similar307 and that these are similar in diabetic people and non-diabetic people. 308. Given a 10% fall in stroke per 1.0 mmol/l fall in LDL-C, a 1.32 mmol/l fall in LDL-C is assumed to give a RR of stroke of 0.91.32/1 = 0.87. Adjustment to CVD risk is made for the difference between the real-world statin adherence level [which we assumed to be 60% guided by Kopjar et al. (2003)305 and Penning-van Beest et al. (2007)309] and that in the trials (on which the above RRs were based).
In both arms, we assume an additional appointment with a GP nurse each year to monitor IGT status.
No testing for microvascular complications is assumed until a patient becomes diabetic (even though some retinopathy and albuminuria develops in patients with IGT).
Glycaemic progression during impaired glucose tolerance
Studies such as that by Ferranini et al. (2004)258 report that there is a relatively rapid increase during the latter stage of progression when the pancreas is no longer able to adequately compensate fully for the effect of insulin resistance. Lifestyle intervention in the US DPP108 reduced HbA1c levels from approximately 5.9% to 5.8%.
Subsequently, a linear rise in glucose, especially where transition to diabetes is slow, might overestimate the raised cardiovascular risk during the earlier period of progression. For this reason we have assumed an exponential rate of increase in HbA1c level from the baseline level to that at diagnosis of diabetes.
The HbA1c level at the time of clinical diagnosis of diabetes will be determined by the sensitivity of test to diabetes. If an OGTT was carried out annually as in the DPS,183 the HbA1c level at diagnosis would be relatively close to the diagnostic level shown above (5.85%). In the NHS, however, an FPG or HbA1c test might be preferred as the initial test as it is more practical – this might result in a higher HbA1c level at diagnosis. Our previous work modelling screening for diabetes estimated 6.4% to be level at diagnosis in a cohort without annual testing. In CDC and Eddy models, UKPDS levels were used but quite clearly HbA1c would be much lower with annual monitoring.
Participants in the DPP had a mean HbA1c level of 6.4% at the onset of diabetes with 6-monthly testing (from baseline of 5.9%). 244
We have assumed 6.2% to be a reasonable estimate of HbA1c level at diagnosis (Table 18).
| Stage | HbA1c level (%) |
|---|---|
| HbA1c at diagnosis of diabetes in practice among annually monitored patients IGT | 6.2 |
| Onset of diabetes | 5.872 |
| IGT | 5.6 |
| NGT | 5.3 (assumption) |
Two-hour glucose adjustment
We were unable to establish the significance of changes in 2-hour glucose levels arising through the lifestyle intervention. The DECODE study group demonstrated an association between 2-hour glucose and CVD mortality suggesting this might be a parameter worthy of inclusion in the model, especially as 2-hour glucose changes proportionally more than HbA1c level during progression from IGT to diabetes. 51 However, we decided that there is too much uncertainty and complexity to attempt to take account of this because:
-
whether the relationship between 2-hour glucose and CVD events is causative has not been firmly established
-
HbA1c level is a parameter in the UKPDS risk engines and is correlated to 2-hour glucose (although the correlation itself changes as HbA1c increases)
-
the effect of treatment after diagnosis of diabetes is less clear (it was not recorded in UKPDS).
Effect of intervention on weight and systolic blood pressure
Weight loss is not only an important determinant of the differences in diabetes risk already discussed, but also of changes in QoL and CVD risk factors. The reported and expected changes in weight over the long term can guide assumptions about differences between groups in QoL and CVD risk factors.
Intervention arm
Weight loss reported in the DPS183 over the first year was 4.2 kg in the intervention arm. In the intervention arm, this fell to 3.49 kg after 2 years, and 2.09 kg after 5 years on average. However, for success scores from 0, 1, 2, 3, to 4–5, the 3-year weight reductions were 0.5%, 2.1%, 4.3%, 4.7%, and 8.7%, respectively. Combining this with the number of patients with each success score gives an average weight loss of 7% (or 6 kg) in the subgroup which continues with the intervention after the first year. We assume that this weight loss was fairly stable throughout the intervention period.
In the UKPDS, the mean weight loss was 5 kg over the initial 3 months of dietary advice and monthly visits but, without a sustained intervention, weight subsequently increased again. 27 Similarly, beyond the active intervention period of the DPS, there was noticeable regain of weight, on average, in the intervention group. Although this is likely to be greater in those patients that had not established improved lifestyle habits during the intervention period, some longer-term regain might still be expected in the group that adheres to the intervention for 4 years. We assume that the weight loss of 6 kg, in those that remain on the intervention, is sustained until year 4, but is on average only 3 kg between years 5–8, and is lost completely beyond year 8. We consider these to be fairly conservative assumptions.
Control arm
Weight loss reported in the DPS183 over the first year was 0.8 kg in the control arm. This figure gradually declined over the 4-year study period, so we have assumed an average weight loss of 0.4 kg over the 4 years.
Switchers to metformin (sensitivity analysis)
For patients who switch to metformin after the first year, weight loss would have been 2.6 kg in the first year but is assumed to cease after stopping the intervention. After switching, metformin would be expected to induce some weight loss. Based on the 0.3 kg weight loss per annum (p.a.) in the ADOPT study,310 we assume an approximate 1.1 kg weight loss on average during treatment.
Weight changes due to oral hypoglycaemic agent and insulin
For patients starting on metformin, we assume a 1.1 kg weight loss as outlined in the previous paragraph. Addition of sulphonylurea to metformin is assumed to add 2.6 kg to weight. We have assumed an initial rise in weight of 3.5 kg in the first year following insulin therapy, followed by a trend of +0.3 p.a. The assumptions behind these are shown in Appendix 5.
It should be noted that the effect of insulin on QoL may be greater than the effect of weight change. This is a subject of debate as it has been suggested that many patients adapt to the demands of regular injections and the discomfort of self-monitoring. Two QoL studies [CODE-2,311 Coffey et al. (2002)312] that measure the effect of insulin do suggest, however, a significant effect of around –0.03, which seems greater than that explained by weight change alone.
Effect of weight loss on cardiovascular risk
We are unaware of any clear evidence of whether weight is an independent risk factor for CVD in diabetic people after taking account of the risk factors in the UKPDS equations.
Effect of intervention on blood pressure
In the DPS, patients’ blood pressure (and cholesterol) was checked at annual visits, and they were advised to contact their physician for treatment and follow-up if levels were abnormal. However, use of antihypertensive drugs was unchanged from baseline to year 1 in each arm, so the difference between treatments in the reduction in SBP at the end of year 1 (5 mmHg vs 1 mmHg; p = 0.007) can be related to the intervention effect that probably arises from the effect of weight loss on blood pressure. Similarly, a meta-analysis undertaken by the Cochrane group (which included the DPS results) reported a mean difference in blood pressure of 4.4 mmHg between groups. 180
For the 40% of patients who achieve the goals of the lifestyle intervention, the fall in blood pressure would be expected to be more than 5 mmHg. Taking the reported 14 mmHg SD in the fall in the DPS, applying a truncated normal distribution and assuming that the best achievers obtain the greatest falls, gives a mean fall in SBP of 8.5 mmHg in this subgroup. However, the correlation between weight loss and SBP would not be perfect, so we have adopted a more conservative estimate of 6.5 mmHg for the average SBP fall in the lifestyle achievers. We assume that this reduction is maintained throughout the intervention period. Beyond this, partial regain of weight may lead to loss of some of this benefit, so we conservatively assumed that this is lost between years 5–8.
For the 60% of patients who switch away from the lifestyle intervention after 12 months, the average fall in this subgroup at year 1 would be 4 mmHg to agree with the average fall of 5 mmHg of the group. It is assumed that this is lost by year 4, as it is assumed that without active treatment there would be no sustained weight loss to maintain the effect on SBP.
The net effect of the above in the overall lifestyle arm (responders and switchers) is that the initial fall in SBP of 5 mmHg is lost at an assumed approximate constant rate of 0.63 mmHg per year.
For controls, we assume that the 1 mmHg fall in SBP at year one is gradually lost by the end of year four given that weight was regained. For patients in the control arm that become diabetic, we assume that negligible HbA1c reductions will be achieved through increased diet and exercise, given that they are likely to be asymptomatic with a low baseline HbA1c level of 6.2%. We also assume that they will not achieve sufficient diet & exercise changes to derive any SBP change at diagnosis. Alternative assumptions are tested out in a sensitivity analysis.
Effect of intervention on lipids
Although the Cochrane review by Norris et al. (2005)180 reported a slightly greater fall in total cholesterol with lifestyle interventions (0.18), it is unclear that this would be replicated in a setting of routine statin therapy, so we have assumed no reduction in cholesterol. Reported HDL-C effects are negligible. We assume that lipid levels do not change significantly from diagnosis of IGT to diagnosis of diabetes. This is reasonable given our assumptions on statin use.
Effect of intervention on quality of life
Weight loss has been shown in several studies to improve QoL as shown in Table 19.
| Trial | Intervention | Patients | Utility gain per kilogram lost |
|---|---|---|---|
| SAT as reported in Warren et al.313 | Sibutramine | 362 in total | 0.00297 |
| Placebo | 0.00472 | ||
| HTA sibutramine assessment for NICE314 | Sibutramine | 308 | 0.00185 (95% CI 0.00048 to 0.00322) |
| Placebo | 216 | 0.00142 (95% CI 0.00058 to 0.00341) |
A simple weighted average of the above gives a utility change of 0.0025 per kilogram change.
The incorporation of weight-based utility effects in the model is further supported by a recent review by Dennett et al. (2008). 315
In the DPP,108 the average overall QoL over 3 years in the lifestyle group was 0.02 higher than in the placebo group (0.70 vs 0.68). This is more than might be predicted based on weight changes alone, so lifestyle interventions may impact QoL in other ways, for example a feeling of well-being from exercise.
Some aspects of the DPP108 were more intensive than the DPS183 (e.g. monthly follow-up, target weight loss of > 7% rather than 5%), so we have based our QoL assumptions on weight differences. This gives the effects shown in Table 20.
| Treatment group | Period | Weight change (kg) | Cumulative QoL effect over period |
|---|---|---|---|
| Lifestyle group | Year 1 | –4.2 | +0.0105 |
| Lifestyle group: achievers | Years 2–4 of intervention | –6 | +0.0150 |
| Years 5–8 (follow-up of intervention) | –3 | +0.0075 | |
| Years 9+ | 0 | 0 | |
| Lifestyle group: non-achievers (if switch to control) | Years 2+ | 0 | 0 |
| Lifestyle group: non-achievers (if switch to metformin) | Years 2+ | –1.1 | +0.0028 |
| Control group | Years 1–4 | –0.4 | +0.0010 |
| Years 5+ | 0 | 0 |
These effects apply only while patients are pre-diabetic.
A sensitivity analysis assuming a more conservative effect of weight on QoL, 0.001 per kg, was undertaken.
Relationship between glycaemia and cardiovascular disease risk
UK Prospective Diabetes Study equations and glycated haemoglobin
The relationship between glycaemia and CVD risk should be modelled as a continuous one between the periods before and after diagnosis of diabetes, i.e. diagnosis does not confer any stepped change in risk. To achieve such a continuous relationship, we decided to calculate CVD risks using the UKPDS CVD risk equations286–288 to predict events and mortality.
This might slightly overestimate CVD risk. Although it is reassuring that the age and duration of diabetes coefficients in the CVD risk equations are fairly similar, the difference in risks between patients with IGT and those with diabetes is not fully accounted for by differences in HbA1c, SBP and lipids. The greatest assumption is in using the UKPDS equations to model long-term CVD risk in patients that remain in the IGT state.
We considered an alternative approach of using the Framingham risk equations for patients with IGT. We judged that this would not be appropriate because there is no way of making the change in risk from IGT to diabetes a continuous one, and because the Framingham equations seemed to give a higher risk than the UKPDS equations anyway.
We have carried out a sensitivity analysis assuming that CVD risks in patients with IGT are 30% lower than predicted by the UKPDS diabetes risk equations.
Microvascular complications during impaired glucose tolerance period
Studies have reported incidence of retinopathy and albuminuria in the pre-diabetic period, albeit low rates. 316 We have used the same risk equations as used in the full diabetes model, as these will generate low rates of incidence for patients with IGT because of the exponential relationship between HbA1c level and events.
Management of diabetes: parameters and assumptions
-
HbA1c threshold for switching (to OHAs): 7.4%. 317
-
HbA1c threshold for switching (to insulin): 8.5%.
[Although earlier use of insulin might be advocated, response to insulin is known to be relatively poor in many overweight patients, who may therefore be more reluctant to start. Rubino et al. (2007)318 suggest that, in practice, many wait 5 years or more with an HbA1c level of > 8% before starting, and many above 9% insulin, which suggests switching at a higher threshold. 318]
-
assumed HbA1c level cap under insulin therapy: 9.2%.
-
blood pressure control target (SBP, mmHg): 140 mmHg for IGT, 135 mmHg for diabetes
-
OHA treatment strategy for diabetes: metformin, then metformin + sulphonylurea, then metformin + insulin.
Effect of treatment following diagnosis of diabetes
Initial effect on glycated haemoglobin
For patients who do not receive intensive treatment for IGT but progress to diabetes, it is assumed that these are generally poor adherers to even basic lifestyle changes, who are unlikely to achieve the falls in HbA1c level and weight from diet and exercise reported after the diagnosis of diabetes in the UKPDS. We therefore assume no cost of intensive diet and exercise therapy and no benefit. This may be a pessimistic assumption – perhaps the shock of being told they now had diabetes (rather than just IGT) would have motivated them to make lifestyle changes.
For patients who have progressed despite intensive treatment, we assume that patients do not obtain a further fall in HbA1c level through diet and exercise at diagnosis of diabetes (because they have effectively already consumed the benefit available from this ‘therapy’).
For IGT patients monitored annually, any diagnosis of diabetes would be at a subclinical level in most cases. We assume that diabetes is treated intensively at diagnosis, i.e. metformin being added to diet and exercise, rather than waiting until HbA1c level reaches a higher level, such as 7%.
Long-term glycaemic progression
Based on the UKPDS, HbA1c level increases at an estimated rate of 0.2% p.a. until treatment is intensified. 11
There is, however, some evidence to suggest that glycaemic progression is slower during the early stages of diabetes. Our base-case assumption is a rate of change in HbA1c level of 0.15% p.a. from diagnosis of diabetes until a level of 7% is reached. As this is an area of uncertainty, we have added a sensitivity analysis to test out the effect of a more conservative assumption, with an HbA1c trend of 0.1% p.a. during this ‘preclinical’ phase.
Insulin dose requirements
Typically, the dose of insulin needed by an individual increases over time (Table 21). For this type of assessment in which there are significant differences between treatment arms in the duration to insulin initiation, it is important to use a time-dependent dose of insulin rather than a constant average one. We used the following time-dependent ideal doses dependent on duration of insulin use, assuming an average weight based on the DPS plus the effect of metformin, i.e. 85 kg, and an insulin dose of 0.64 units/kg (when combined with metformin) in the first year. 319 In the UKPDS, average insulin doses increased from 0.27 units/kg per day at 3 years to 0.42 units/kg per day at 12 years,320 but this 60% increase is probably partly due to the low baseline. We have conservatively assumed that the titration rate in practice is at half of that reported in the UKPDS, i.e. equivalent to an increase in the daily dose of 0.02 international units (IU)/kg over the course of a year.
| Years with insulin | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15+ | |
| IU/kg per day | 0.64 | 0.66 | 0.68 | 0.70 | 0.73 | 0.75 | 0.77 | 0.79 | 0.81 | 0.83 | 0.85 | 0.87 | 0.90 | 0.92 | 0.94 |
| Dose required per day (IU) | 57 | 59 | 61 | 63 | 65 | 67 | 69 | 72 | 74 | 76 | 78 | 80 | 83 | 85 | 87 |
Adherence to these doses is assumed to be 71% based on Donnelly et al. (2007). 321
Costs of commonly used insulins are £17.27 per 1000 IU for insulin aspart and £26 per 1000 IU for glargine. The corresponding costs using pens are £29.43 for insulin aspart and £39 per 1500 IU for glargine. 304 The use of glargine has increased considerably recently, as, to a lesser extent, has insulin detemir. The cost of glargine is considered to be a reasonable estimate of the average cost of insulins currently used for T2DM in the UK.
Other model parameters and assumptions
Unit costs
Costs are based on, or uplifted to, 2007 (Table 22).
| Unit costs | Value (£)a | Source |
|---|---|---|
| Non-fatal MI | 7355 | UKPDS 65322 |
| Fatal MI | 1660 | UKPDS 65322 |
| Annual cost following MI | 669 | UKPDS 65322 |
| Non-fatal stroke | 3411 | UKPDS 65322 |
| Fatal stroke | 4875 | UKPDS 65322 |
| Annual cost following stroke | 5729 | Chambers et al.323 |
| CHF incidence | 3426 | UKPDS 65322 |
| CHF state cost | 909 | UKPDS 65322 |
| Haemodialysis p.a. | 35,000 | UK Transplant324 |
| Peritoneal dialysis p.a. | 17,500 | UK Transplant324 |
| Transplant – first year | 17,000 | UK Transplant324 |
| Cost of immunosuppression p.a. | 5000 | UK Transplant324 |
| Annual cost of neuropathy | 210 | Gordois et al.325 |
| Amputation | 12,194 | UKPDS 65322 |
| Post-amputation costs p.a. | 405 | Palmer et al.326 |
| Major hypoglycaemic episode | 994 | bHeaton et al.327 |
| Retinal photocoagulation | 1050 | UK NSC328 |
| Severe vision loss p.a. | 405 | UKPDS 65322 |
| Cost of management/monitoring – clinic visits, glucose tests, and proteinuria and eye screening | 142 |
The cost of monitoring for patients that have diabetes is a significant factor in the economics of preventing diabetes. The additional annual cost arising through monitoring diabetes is estimated to be £142, as shown in Table 23. The cost of antiglycaemic medication is show in Table 24.
| Visits | Average visits | Unit cost (£) | Cost source | Inflation uplift factor | Total cost (£) |
|---|---|---|---|---|---|
| Nurse at general practice (to do bloods, pulse check, feet, flu jab) | 2 | 8.00 | Curtis300 | 1.00 | 16 |
| GP clinic | 2 | 30 | Curtis300 | 1.00 | 60 |
| Dietitian | 0.5 | 37 | Curtis300 | 1.00 | 19 |
| HbA1c test | 2 | 6.50 | UKPDS 63329 | 1.46 | 19 |
| Eye screening (annual) | 21.00 | James et al.330 | 1.34 | 28 | |
| Total cost | 142 |
| Drug | Dose | Unit cost (£) | Cost per day (£) | Source |
|---|---|---|---|---|
| Metformin | 3 × 850 mg per day (high dose assumed as first-line failure) | 2.23 per 56 | 0.12 | BNF 53331 |
| Sulphonylurea | 4 mg glimepiride daily | 13.62 for 30 | 0.45 | BNF 53331 |
| 320 mg gliclazide daily | 1.91 per 60 × 80 mg | 0.13 | ||
| Average | 0.29 | |||
| Insulin (cost in first year of use) | Based on 0.64 IU/kg/day, average weight of 88.5 kg at end of first year, average cost of aspart/lispro and glargine | 1.35/day for insulin | 1.59 | BNF 53331 and calculation including £0.32/day for needles/consumables/education |
Utility decrements
The decrements relating to the reduction in QoL due to the presence of comorbidities are shown in Table 25.
| Comorbidity | Substate | Value | Reference |
|---|---|---|---|
| Diabetes with no complications (average of male and female) | – | 0.7850 | UKPDS 62332 |
| CHD | Post MI | –0.0550 | UKPDS 62332 |
| CHF | – | –0.1080 | UKPDS 62332 |
| Stroke | –0.1640 | UKPDS 62332 | |
| Nephropathy | Microalbuminuria | –0.0110 | Coffey et al.312 |
| Gross proteinuria | –0.0110 | ||
| Post transplant | –0.0520 | Mount Hood 4 Conference data333 | |
| Dialysis | –0.0780 | Coffey et al.312 | |
| Neuropathy | Peripheral neuropathy | –0.0650 | Coffey et al.312 |
| Amputation | –0.2800 | UKPDS 62332 | |
| Retinopathy | Proliferative retinopathy | –0.0200 | Mount Hood 4 Conference data333 |
| Macular oedema | –0.0200 | ||
| Severe vision loss | –0.0800 | ||
| Weight (per kg) | – | –0.0025 | See Effect of intervention on quality of life |
Perspective and indirect costs
An NHS perspective is taken to inclusion of costs. In line with NICE guidance, indirect costs are excluded, for example the ongoing cost of caring for patients who have suffered a stroke includes only NHS costs and excludes any patient carer costs. Similarly, the indirect cost to patients of time and travel expenses involved in participating in a programme of lifestyle changes is not included in the main analysis but is worthy of inclusion in a sensitivity analysis.
We also do not account for non-diabetes-related costs [in terms of the difference between production and consumption as discussed in Lindgren et al. (2007)221] accruing from any increased life expectancy derived from the intervention).
Horizon
Diabetes incidence is modelled over 20 years but the overall health consequences of having IGT or diabetes are modelled over a lifetime.
Sensitivity analyses
We have undertaken some sensitivity analyses around key uncertainties in the evidence base, as shown in Table 26. Given that the intervention is highly cost-effective in the base-case analysis, we have mostly focused on sensitivity analyses to demonstrate the effect that more conservative assumptions would make on the cost-effectiveness of the intervention.
| Sensitivity analysis | Rationale |
|---|---|
| Treatment pathways | |
| Non-responders (goal score < 3) in lifestyle arm switched to metformin rather than basic advice (control) intervention | For patients with IGT in the lifestyle arm who do not adhere or respond (i.e. a goal success score of ≥ 3), switching to metformin is an option. This strategy can be compared with the base-case intervention arm to see which is the most cost-effective strategy compared with the control intervention |
| Intensive treatment beyond the 4-year DPS duration |
People may regress in their lifestyle habits without continued re-enforcement, and some other cost-effectiveness studies (e.g. Hoerger et al. 262) assumed continued treatment while patients had IGT Table 2 of the DPS follow-up paper suggests some weight regain in those free from diabetes at start of follow-up period, although we do not have data on whether this occurred in both study arms A sensitivity analysis is undertaken to explore the effect of requiring three annual visits, each twice as long as assumed for the base-case cost of re-enforcement in the lifestyle arm post year 4 |
| Treatment benefit | |
| Uncertainty around long-term prevention with the intervention |
The 4-year RR for diabetes vs all control subjects, if non-responding lifestyle participants with success score ≥ 2 are switched to control treatment, is 0.09. The CI around this is 0.02 to 0.38, so a sensitivity analysis is needed. Also, it is unclear to what extent the benefit is sustained in the long term We undertook a sensitivity analysis assuming that the incidence curves for the two arms converge at year 20 (see Alternative scenarios for projected cumulative incidence of diabetes) |
| More optimistic scenario – greater prevention/delay of diabetes | Assumes that the RR of having developed diabetes in responders increases from 0.1 at year 4 to 0.18 at year 20 (see Alternative scenarios for projected cumulative incidence of diabetes) |
| Greater benefit achievable with treatment at diagnosis in control arm | Although these patients were unable to achieve adequate lifestyle changes to prevent diabetes, some lifestyle-related improvements may be achievable at diagnosis of diabetes: assumed 2 kg weight loss, 2 mmHg reduction in SBP, 0.69% initial HbA1c level fall |
| Diabetes progression | |
| HbA1c progression during early diabetes | The rate of glycaemic progression after onset of diabetes is likely to be an important factor in the extent of the burden of complications arising from failure to prevent diabetes. The rate may be lower than during later stages of the disease so this sensitivity analysis tests out the effect of a slower rate of progression, 0.1% p.a., from onset of diabetes until an HbA1c level of 7% is reached |
| Cardiovascular risk | |
| Effect of metformin on CHD risk | The UKPDS recently reported a 33% fall in CHD risk in the metformin arm compared with placebo.334 The degree of risk reduction is uncertain, so we tested out the effect of a lower reduction in risk – 16% |
| CVD risk in patients with IGT | We used the UKPDS risk engines to predict CVD risks in patients with IGT. This could lead to some overestimation of CVD risk, although this is uncertain. A sensitivity analysis was carried out to assess the effect of risks in patients with IGT being 30% lower than predicted by these equations |
| Other | |
| Lower utility gain per kilogram of weight loss | Test out the effect of 0.0010/kg instead of 0.0025/kg |
| Daily cost of statins | Daily cost £0.30 instead of £0.17, based on 2007 prescribing volumes (with a minority prescribed the more expensive atorvastatin) |
| Pessimistic scenario | Based on incidence curves for the two arms converging at year 20, 80th percentile (i.e. lower) reduction in SBP and weight in the lifestyle arm compared with control arm (i.e. intervention arm relatively less effective). Also, assumes only 0.001 utility loss per kilogram of weight gained and that three annual visits, each twice as long as assumed for the base case, are needed for re-enforcement of lifestyle changes in the intervention arm post year 4 |
Results
Base-case results
Table 27 provides a summary of the base-case results.
| Scenario | Total costs (£) | Incremental costs (£) | Total QALYs | Incremental QALYs | Net benefita (£) | ICER (£) | ||
|---|---|---|---|---|---|---|---|---|
| Lifestyle | Control | Lifestyle | Control | |||||
| Base case | 14,224 | 14,104 | 121 | 11.2649 | 11.1986 | 0.0663 | 1205 | 1819 |
Table 28 gives a detailed breakdown of the base-case results.
| Treatment | Total for 40,000 patients | Per patient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidences of all events | ‘State years’ (non-fatal events) | Total costs (£) | Utility/(utility loss): QALYs | Incremental results | ||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | Costs | QALYs | |
| Utility of those alive (unpenalised for complications) – of which ‘complication free’ | 11.6136 | 11.5827 | 0.0309 | |||||||
| States | ||||||||||
| No retinopathy | 879,057 | 868,609 | ||||||||
| Non-proliferative retinopathy | 4057 | 4658 | 28,645 | 32,740 | ||||||
| Proliferative retinopathy | 159 | 191 | 1017 | 1293 | 2 | 2 | –0.0002 | –0.0002 | 0 | 0.0001 |
| Significant macular oedema | 1737 | 1994 | 14,088 | 16,149 | 22 | 25 | –0.0030 | –0.0034 | –3 | 0.0004 |
| Severe vision loss | 406 | 477 | 3330 | 3861 | 12 | 14 | –0.0024 | –0.0028 | –2 | 0.0004 |
| No history of heart disease | 799,668 | 796,833 | ||||||||
| Non-fatal MI | 8586 | 8477 | 105,864 | 104,834 | 2345 | 2336 | –0.0840 | –0.0832 | 9 | –0.0007 |
| CHF | 4367 | 4422 | 24,003 | 24,329 | 469 | 475 | –7 | |||
| Death (from MI only) | 11,921 | 12,003 | ||||||||
| Death (from CHF only) | – | – | ||||||||
| No cerebrovascular history | 810,689 | 807,962 | ||||||||
| Stroke or transient ischaemic attack | 13,403 | 13,270 | 115,448 | 114,690 | 7410 | 7383 | –0.2191 | –0.2182 | 27 | –0.0010 |
| Death due to stroke only | 3162 | 3305 | ||||||||
| No nephropathy | 827,699 | 821,001 | ||||||||
| Microalbuminuria | 6981 | 7282 | 91,565 | 93,670 | –0.0130 | –0.0133 | 0.0003 | |||
| Gross proteinuria | 800 | 941 | 6564 | 7661 | –0.0007 | –0.0008 | 0.0001 | |||
| Dialysis (haemodialysis or peritoneal dialysis) | 66 | 68 | 217 | 217 | 57 | 57 | –0.0001 | –0.0001 | 1 | 0.0000 |
| No nephropathy post transplant | 15 | 17 | 92 | 103 | 5 | 6 | 0.0000 | 0.0000 | –1 | 0.0000 |
| Death due to nephropathy only | 16 | 15 | ||||||||
| No neuropathy | 909,010 | 904,455 | ||||||||
| Neuropathy | 1345 | 1452 | 16,031 | 16,933 | 41 | 43 | –0.0127 | –0.0133 | –2 | 0.0006 |
| Amputation (A1 or A2) | 166 | 187 | 1096 | 1264 | 24 | 27 | –0.0026 | –0.0029 | –3 | 0.0003 |
| Death – involving other causes | 24,901 | 24,677 | ||||||||
| Managing diabetes including therapy | ||||||||||
| Drugs | ||||||||||
| Preventative/diabetes related | ||||||||||
| First-line prevention | 585 | 337 | 248 | |||||||
| Second-line prevention (switchers) | 201 | 201 | ||||||||
| First-line diabetes (metformin) | 59 | 67 | –8 | |||||||
| Second-line diabetes (metformin + sulphonylurea) | 151 | 173 | –21 | |||||||
| Third-line diabetes (insulin + metformin) | 1253 | 1473 | –220 | |||||||
| Antihypertensive | 429 | 438 | –9 | |||||||
| Lipid related | 591 | 597 | –6 | |||||||
| Total drug costs | 3269 | 3085 | 184 | |||||||
| Monitoring | 514 | 592 | –78 | |||||||
| Hypoglycaemic episodes | 2529 | 2789 | 42 | 48 | –6 | |||||
| Temporary heart failure | – | – | ||||||||
| Oedema | 16,948 | 14,614 | 12 | 11 | 1 | |||||
| Total AEs | 19,477 | 17,403 | 54 | 58 | –4 | |||||
| Effect of weight on QoL | –0.0109 | –0.0458 | 0.0348 | |||||||
| Totals | 101,565 | 100,839 | 4,634,083 | 4,616,604 | 14,224 | 14,104 | 11.2649 | 11.1986 | 121 | 0.0663 |
The total incremental cost (per patient with IGT) of the intervention amounts to £121. This can be attributed largely as follows:
-
an additional £449 cost of the intervention (although this is partly a result of longer duration in the pre-diabetic state, on average, hence longer duration of both active intervention and follow-up treatment)
-
£220 saving: a mix of preventing and delaying the need for initiating expensive insulin therapy (a slightly lower but similar effect would have been observed if triple OHA therapy had been an option within the treatment pathway before insulin)
-
£78 saving: avoiding the excess monitoring costs (including retinal screening) that arise once diabetes has been diagnosed.
Savings relating to treatment of complications were only £19.
The QALY benefits of the lifestyle intervention can be attributed largely as follows:
-
0.0309: improved survival driven partly by lower average blood pressure and HbA1c levels
-
0.0348: QoL impact of the significant differences between treatment arms, achieved through both
-
– weight loss during the 4-year period of the intervention for IGT
-
– preventing or delaying the weight gains that arise with sulphonylureas and especially insulin.
-
Although the incremental QALY gain seems small, in our experience this is expected for diabetes-related interventions that target a fairly broad patient group that is more at risk of future adverse health consequences than imminent ones. There is nevertheless a tangible and cost-effective population benefit.
The table above shows that, although estimated cardiovascular mortality is reduced in the intervention arm, the total number of CHD and stroke events is almost the same. This seems counterintuitive but can be explained as follows:
-
The lower blood pressure levels, and to a lesser extent HbA1c levels, during the earlier years reduces the case fatality rate so that the proportion of events that are fatal is lower in the intervention arm.
-
A delayed diagnosis of diabetes results in delayed initiation of metformin therapy, which is likely to be cardioprotective – this has an upwards effect on the total number of CHD events in the intervention arm relative to the control arm.
-
Lower blood pressure levels in the intervention arm during the earlier years result in lower CVD risk and fewer patients crossing the threshold for starting statin therapy – this also has an upward effect on the relative number of events in the intervention arm compared with the control arm.
-
The reduced mortality in the intervention arm leads to greater secondary non-fatal events (because more patients are alive and at risk of recurrent events).
Even though total CHD and stroke events may not be reduced as expected, there is a reduction in CVD mortality and less heart failure, as well as other significant benefits – largely a delay in the need for insulin (or other expensive antiglycaemic therapy) and improved QoL due to weight loss.
In the sensitivity analysis (see Sensitivity analysis results, below) in which those not achieving lifestyle goals with the intervention subsequently switch to metformin rather than basic lifestyle advice, both non-fatal and fatal CHD events were lower in the intervention arm. This is because of the expected effect of metformin on CHD risk and is one of the factors that needs to be considered in evaluating the merits of using lifestyle or metformin therapy for prevention of diabetes.
Affordability
Even if the intervention is cost-effective in the long run, an equally important outcome is how affordable the intervention is initially, as often cost savings are generated in the longer term. For a lifestyle intervention the net 1-, 3-, 5- and 10-year cumulative outlay per patient diagnosed with IGT is shown in Table 29. Ten-year cumulative outlay is estimated at approximately £346 per patient, i.e. significant investment is needed in the early years, even though the intervention is likely to result in small annual cost savings in the long run through reduced costs of monitoring diabetes, and lower therapy costs (especially insulin).
| Time horizon (years) | Cumulative net financial investment (£) |
|---|---|
| 1 | 177 |
| 3 | 338 |
| 5 | 397 |
| 10 | 346 |
| 15 | 279 |
| 20 | 195 |
Figure 13 shows than annual net investment over the first 10 years. Within 5 years, annual cash flows are predicted to be positive.
FIGURE 13.
Annual net investment over time.
Compared with drug therapies, lifestyle intervention can be relatively labour intensive and the staff resourcing needs of a national programme, whether through the NHS or external contract arrangements, would need to be carefully assessed.
Sensitivity analysis results
The following key sensitivity analyses (Table 30) have been undertaken (as per Sensitivity analyses, above).
| Sensitivity analyses | Incremental costs (£) | Incremental QALYs | ICER (£) | Effect on cost-effectiveness |
|---|---|---|---|---|
| Treatment pathways | ||||
| Assuming that patients who do not achieve a goal score of ‘3’ with the intensive lifestyle intervention switch to metformin rather than control intervention | –45 | 0.1322 | Intervention dominates | Strategy with non-responders switching to metformin is dominating (more efficacious and cost saving) and more cost-effective than switching to basic advice |
| Treatment in the intervention arm beyond 4-year DPS intervention: assume three annual visits needed, each twice as long as assumed for the base-case cost | 283 | 0.0663 | 4277 | Intervention still cost-effective |
| Treatment benefit | ||||
| Uncertainty around long-term prevention with the intervention (assume that the incidence curves for the two arms converge at year 20) | 453 | 0.0537 | 8437 | Intervention still cost-effective |
| More optimistic scenario – greater prevention/delay of diabetes (see Alternative scenarios for projected cumulative incidence of diabetes) | –28 | 0.0685 | Intervention dominates | |
| Greater benefit achievable with treatment at diagnosis in control arm (2 kg weight loss, 2 mmHg reduction in SBP, 0.69% HbA1c level fall) | 176 | 0.0482 | 3658 | Intervention still cost-effective |
| Diabetes progression | ||||
| Slower HbA1c level progression during early diabetes (0.1% p.a.) | 129 | 0.0666 | 1943 | Intervention still cost-effective |
| Cardiovascular risk | ||||
| Lower effect of metformin on CHD risk | 158 | 0.0755 | 2097 | Intervention still cost-effective |
| CVD risk while IGT 30% lower than obtained from UKPDS diabetes risk equations | 36 | 0.0848 | 419 | Intervention still cost-effective |
| Other | ||||
| Lower utility gain per kilogram of weight loss: 0.0010/kg instead of 0.0025/kg | 121 | 0.0454 | 2657 | Intervention still cost-effective |
| Daily cost of statins £0.30 instead of £0.17, based on 2007 prescribing volumes (with a minority prescribed the more expensive atorvastatin) | 116 | 0.0663 | 1752 | Intervention still cost-effective |
| Pessimistic scenario | ||||
| Based on incidence curves for the two arms converging at year 20, 80th percentile for the change in SBP and weight in the lifestyle arm compared with control arm (i.e. intervention arm relatively less effective). Also, assumes only 0.001 utility loss per kilogram of weight gained and that three annual visits, each twice as long as assumed for the base case, are needed for re-enforcement of lifestyle changes in the intervention arm post year 4 | 587 | 0.0351 | 16,720 | Intervention still cost-effective |
Discussion
Our analysis suggests that a lifestyle intervention, when continued in those who respond during the first year, is highly cost-effective. This is still the case across a range of one-way sensitivity analyses, including a very cautious scenario that assumes the incidence of diabetes curves converge. A pessimistic scenario combining several conservative assumptions still yields cost-effective results. This is not particularly surprising given that:
-
Non-responders do not continue to participate with the intervention.
-
Even if, in the long-term, the incidence curves almost converge (which seems unlikely), there would still be delays in progression to diabetes which delay the need for expensive antiglycaemic therapies.
-
Weight loss achieved, even if not sustained in the long term, leads to short- to medium-term QoL benefits.
Critical success factors of the Diabetes Prevention Study
To our knowledge, it is not known which components of the DPS were critical in achieving the required weight loss that seems to be a threshold for reducing risk of diabetes. Replicating the effectiveness of the DPS in a real-world UK setting may be dependent on specific goals, perhaps significant uptake of exercise classes, as weight loss is known to be frequently less effective when pursued through dietary approaches alone.
It might be possible to design a cheaper intervention than that used in the DPS but with the same effectiveness, perhaps taking into account any advances, since the DPS, in knowledge on optimal diets for reducing diabetes risk, or using counselling to re-enforce behavioural changes.
Maintaining lifestyle changes in the real world
Ability to comply with, and respond to, lifestyle changes varies from patient to patient, particularly the ability to lose weight. Even in the DPS, only 43% achieved the weight reduction goal, and 36% of subjects increased their physical activity. Furthermore, the Good Ageing in Lahti Region (GOAL) Lifestyle Implementation Trial demonstrated that replicating, in the real world, the same degree of physical activity and weight reduction as that observed in trials may be difficult. 335 Re-enforcement of lifestyle changes through effective counselling strategies might be possible nevertheless. 133
Durability of benefit in responders
In the DPS, the HR for diabetes in the intervention arm increased steadily between years 4 and 8. This is probably owing to a mix of participants not sustaining the intensity of lifestyle changes, a wearing-off of the protective effect of the intervention, and age-related beta cell loss. In those that successfully sustain weight loss and other lifestyle changes, the extent to which the benefit of preventative interventions is sustained in the long term will determine whether diabetes can be truly prevented rather than just delayed.
Affordability of the lifestyle intervention
Well-supported lifestyle interventions are typically not cheap, more than the cost of first-line drug treatment for diabetes including the monitoring cost. If lifestyle interventions are not affordable because of the initial investment needed, or are beyond available manpower resources, then metformin, although less effective in trials, might be a cheap and convenient alternative (metformin costs only £44 per year). This is particularly relevant to non-responders to a lifestyle intervention. A sensitivity analysis with a strategy that switches non-responders to metformin significantly increased the cost-effectiveness of intervening to prevent diabetes.
Probabilistic sensitivity analysis
We did not undertake a probabilistic sensitivity analysis (PSA) owing to time constraints and the emerging conclusions concerning the cost-effectiveness of the intervention (i.e. the intervention remaining cost saving across a range of one-way sensitivity analyses and a couple of multiway pessimistic scenarios). Given this, the probability of the intervention not being cost-effective is unlikely to reach a level that would influence decision-making.
Threshold for discontinuing the lifestyle intervention
We have examined the incremental cost-effectiveness of switching those who do not achieve lifestyle targets away from the intervention, to either routine ‘basic advice’ or to metformin. The exact threshold at which to apply this switching, and the subsequent treatment of those who are switched, would have to be further evaluated. The intervention strategy may still be cost-effective if only those failing to achieve a DPS goals success score of at least two were switched. It would be worth undertaking further research to examine the costs and benefits of continuing the lifestyle intervention in those with a goal success score of exactly 1 and exactly 2. The weight loss and reduction in diabetes risk from the lifestyle intervention in these subgroups might still be sufficient to be cost-effective.
Using a DPS score of at least 2 after 12 months as the criteria for continuing the lifestyle intervention would mean approximately 25% more patients continuing with the lifestyle intervention beyond the first year than if the criteria was at least 3 (possibly meaning 25% fewer patients requiring medical treatment with metformin to reduce the risk of diabetes).
Perverse consequence of delaying diabetes
There may be some adverse consequences of delaying a diagnosis of diabetes and thereby the intensification of treatment that follows as a result of it. In particular:
-
The initiation of metformin is delayed, thereby delaying the potentially substantial reduction in CVD risk with metformin (see Base-case results, above).
-
More intensive but cost-effective treatment of blood pressure and lipids may be delayed – this depends on whether lipids are initially treated with a ‘fire-and-forget’ policy or treated to targets or based on CVD risk. Intensification may lead to what seem like relatively small improvements in risk factors but some analyses undertaken suggest these can significantly influence the results.
-
Two-hour glucose may slowly drift upwards towards 11 mmol/l, leaving patients exposed to increased CVD risk over a long period.
However, given how cost-effective the results show the intervention to be, these factors are unlikely to outweigh the benefits.
Other benefits of treating obesity in patients with impaired glucose tolerance
There are likely to be some other benefits from lifestyle improvements that are not included in the model, such as a reduction in obesity-related cancer and other problems (e.g. osteoarthritis in knees and hips). This together with the weight-related QoL benefit may be enough to justify lifestyle intervention in lower risk populations in which a significant proportion may never progress to diabetes. Furthermore, CVD risk equations may not fully capture the benefits of lifestyle changes, especially the benefits of exercise and weight loss. In non-diabetic people, at least, the QRISK equations suggested an association between weight and CVD risk, independent of blood pressure and lipids. 336
Comparison with other cost-effectiveness assessments of interventions for impaired glucose tolerance
It is difficult to compare our results directly with previous economic assessments because:
-
Our treatment pathway involves patients who do not achieve lifestyle goals switching off the intensive treatment. This greatly improves the cost-effectiveness.
-
We have assumed that lifestyle changes become habitual in those who achieve targets within the 4-year intervention period, so that ongoing intensive intervention is not needed after 4 years.
The fact that our results are cost-effective is nevertheless consistent with an assessment of the DPS in Swedish setting, and assessments by Palmer et al. (2004)243 and Herman et al. (2005). 244 Our decision rule, by which those not achieving weight loss after 12 months no longer continue with the intervention, is a key factor in the intervention being cost-effective. An assessment by Eddy et al. (2005)248 did, however, produce less favourable results. Possible reasons for this are multiple and were discussed in detail in Chapter 5.
Implications for further research and modelling
As discussed above (see Mix of impaired glucose tolerance/impaired fasting glucose and risk profile of cohort in the model), these results apply to patients that have IGT (either with or without IFG). Further research may be needed in order to determine the effectiveness of lifestyle interventions in patients without isolated IFG, particularly in respect of:
-
the reduction in risk of progression to diabetes
-
the relationship between IFG and risk of CVD.
The exact threshold for determining treatment failure with lifestyle intervention and pursuing alternative means of prevention (probably with metformin) requires further modelling and analysis. It is also often asserted, based on the Indian DPP,173 that there is no added benefit from combining metformin with lifestyle intervention above lifestyle or metformin alone. There was considerable overlap between the CI around the results for each treatment arm in the Indian DPP – a more accurate interpretation is that is was not possible to establish whether there was any added benefit, and further research results are needed.
More evidence is required on the extent to which lifestyle intervention and metformin lead to sustained benefits in the long term. It is also unknown whether it would be worthwhile continuing with some form of active intervention over a longer period that that used in prevention trials to date (typically 4–6 years).
The cost-effectiveness of any preventative or screening-related diabetes intervention is affected by the large uncertainty in the CVD risk reduction of metformin. Future economic assessments and clinical risk assessment in practice would benefit from a more precise estimate of metformin’s effect. The DPP Outcomes study (follow-up to the main DPP study) may provide some evidence for this, or it could be incorporated into the design of a future trial of interventions for IGT.
The cost-effectiveness of lifestyle interventions is determined by various cost and QoL impacts, often underpinned by an uncertain evidence base, so there is uncertainty in the results and conclusions. However, we have carried out a range of sensitivity analyses, each suggesting that lifestyle intervention (sustained only in those achieving weight loss/lifestyle targets) is cost-effective. Some further (but probably less important) sensitivity analyses that could be undertaken are summarised in Appendix 6.
Chapter 8 Discussion
Statement of principal findings
-
There is consistent evidence from the trials, such as the DPP108 and DPS,183 that lifestyle measures – weight loss and physical activity – can reduce the development of diabetes in those with IGT. The results are best in those who achieve more of the goals. Lifestyle intervention in those who adhere is also highly cost-effective.
-
It may be worth distinguishing between physical activity and exercise, with the former term referring to activities, such as walking, that can be incorporated into daily life and the latter referring to activities that require, for example, going to gyms or participating in sports.
-
The main problem is adherence. (Adherence is now preferred to the older term ‘compliance’ because it is supposed to have connotations of partnership and concordance, rather than ‘following doctor’s orders’. 337)
-
Some ethnic groups, such as South Asians, are more at risk of diabetes, and may get it earlier in life and at lower BMI levels. Cultural influences may make lifestyle changes more difficult, especially among women.
-
A review of previous economic modelling of prevention of diabetes showed that most studies conclude that is it cost-effective, with one prominent outlier. Uncertainties include the duration of the asymptomatic period between onset of diabetes and development of clinical diabetes, the rate of progression and whether it is linear, and whether the risk is constant over lifetime, or whether those who are going to become diabetic do so within 10 years or so. It is worth noting that fewer than half of people with ‘pre-diabetes’ go on to develop diabetes.
-
Analysis of GPRD data showed that there appears to be little current activity in detection of, and intervention in, IGT, so any national programme would have to start from a low baseline.
-
Our modelling suggested that lifestyle intervention, when continued in those who respond during the first year, is highly cost-effective. This remains the case under a range of sensitivity analyses.
-
In those who do not lose weight and increase physical activity, a strategy of switching to metformin after 12 months is cost-effective.
-
A common finding in most lifestyle intervention studies is that good initial effects are not sustained over the long term, especially after the intervention ends. However, the Finnish DPS183 has produced 7-year results, 3 years after the end of the intervention period, showing persisting benefit. Perhaps an intervention that lasts for several years is required to produce a permanent change in lifestyle.
Clinical effectiveness evidence issues
A number of issues should be considered when assessing the validity of the trials and their outcomes, particularly in terms of generalisability to the UK population. 338–343
-
Trials were conducted in populations across the world (China, Finland, India, USA, Japan, the Netherlands, UK); as such, genetic and cultural variation may potentially confound the results. Progression rates varied considerably, with 80% of the intervention group in the Chinese trial (Da Qing190) progressing to diabetes.
-
Trials recruited participants using different criteria for IGT, different age ranges, sex and BMIs. Self-selection (and therefore an increased likelihood of compliance) may have occurred with some recruitment methods.
-
Not all studies were powered or designed to look at progression to diabetes.
-
Duration of intervention and duration of follow-up varied between trials.
-
Some lifestyle interventions were individualised whereas others were conducted in groups and the number of intervention contacts, for example with dietitian, varied between trials.
-
Physical activity advice varied from recommended participation in light exercise once a day to several supervised sessions of moderate activity every week.
-
Dietary intervention ranged from recommendations to eat more fruit and vegetables to specific guidelines on recommended daily amounts of nutrients.
-
Subjective self-reported measurements of dietary intake and physical activity adherence are known to be unreliable.
-
Analysis was not on an ITT basis and because more subjects in the control groups developed diabetes and were withdrawn from study for treatment this may confound the results.
Cost-effectiveness
Previous models have used data from the trials. We have chosen to apply a ‘real-life’ scenario wherein people who do not comply with intensive lifestyle interventions, after a reasonable chance of 12 months, are switched to cheaper metformin. Adherence with metformin was 72% in the DPP – much better than to lifestyle changes.
Our model demonstrates that it is cost-effective to switch those who do not adhere to, or succeed with, lifestyle changes, aimed at reducing BMI and increasing activity, to metformin after 12 months.
Another way of improving cost-effectiveness would be by more selective targeting. The Finnish risk scoring system [Finnish Diabetes Risk Score (FINDRISC)] has eight items, giving a score from 0 to 24, and could be used to target intervention at those with the highest risk, who have more to gain. 212 Alternatively, rather than selective targeting, it could be used for prioritising cases in order to manage the workload more smoothly.
An important emerging issue is uncertainty about whether intervention not only reduces progression to diabetes, but also reduces cardiovascular risk. Hopper et al. (2011)344 carried out a meta-analysis of 10 RCTs, and concluded that interventions did not result in reductions in all causes of cardiovascular mortality or MI, except, possibly, stroke. 344 The RCTs included both drug interventions and four lifestyle trials (DPP,108 DPS,183 Indian DDP,226 Da Qing190), with the drug interventions including trials with pioglitazone, rosiglitazone, ramipril, metformin, nateglinide, valsartan and acarbose. However, the overall results, as shown in the forest plots, showed little difference in directions of effect. One of the drug trials contributed 62% of the weight in the all-cause mortality plot. The authors cite the UKPDS study345 as showing support for their conclusion on lack of reduction in CVD complications, but not the long-term follow-up study which showed that the trend towards reduction in CVD became significant only after longer follow-up. 334 It may be, as Hopper et al. (2011)344 comment, that the lack of effect in their meta-analysis is because most trials were too short, or had too few events, to have the power to show a reduction. They do report that there was ‘a non-significant trend towards reduced risk of fatal and non-fatal MI’.
Probabilistic sensitivity analysis
We did not undertake a PSA owing to time constraints and the emerging conclusions concerning the cost-effectiveness of the intervention (i.e. the intervention remaining cost saving across a range of one-way sensitivity analyses and a couple of multiway pessimistic scenarios). Given this, the probability of the intervention not being cost-effective is very unlikely to reach a level that would influence decision-making.
Threshold for discontinuing the lifestyle intervention
The incremental cost-effectiveness of those who achieved a goal success score of ‘2’ in the DPS is worth further examination. In our model, these patients were deemed not to have met treatment targets and were switched to the control intervention (or metformin in the sensitivity analysis). The weight loss and reduction in diabetes risk from the lifestyle intervention in this subgroup might still be sufficient to be cost-effective. If so, this would mean approximately 70% of patients continuing with the lifestyle intervention beyond the first year rather than 40% (possibly meaning 30% fewer patients requiring medical treatment with metformin to reduce the risk of diabetes).
Adherence to lifestyle recommendations
The trials evaluated in this review recruited volunteers who were probably not typical of the general population. Kriska et al. (2006)346 reported that participants were more physically active than the general population, as found from the NHANES.
They had frequent follow-up, and more intensive care than would be expected in routine care. Despite that, attrition rates were quite high. High rates of adherence were often seen initially, but gradually decreased with time.
Dishman et al. (1990,347 1996121) reported that approximately 50% of individuals who begin an exercise program will drop out within the first 6 months.
Ruge et al. (2007)348 studied the recruitment rate of high-risk individuals to a RCT, the aim of which was to reduce the incidence of diabetes in high-risk individuals. The intervention consisted of physical activity and dietary information, whereas the control group received information about lifestyle change. They consecutively recruited 40-, 50- and 60-year-old participants with IGT and/or impaired IFG. Of the 50 (of 404) subjects who were eligible and informed about the intervention study, only eight agreed to participate. Eleven (of 42) subjects filled in the dropout questionnaire, and the majority gave lack of time as the main reason for non-participation.
A number of factors influence the likelihood of someone adhering to an exercise programme. Age appears to be a determinant of adherence. In the USA, only 30% of older men and 15% of older women report participating in regular sustained physical activity,124 but, in the DPP adherence and results were better in the over-60s. Is that because retired people have more time for physical activity?
A Danish study by Berentzen et al. (2007)349 found that the people who were most susceptible to developing IGT, because of obesity, were unfortunately less likely to exercise. Yet even among obese people, physical activity can reduce the risk. Borodulin et al. (2006)350 in Finland found that even in the highest tertile of waist–hip ratio, those who were physically active had a lower risk of IGT.
South Asians have higher prevalence of T2DM, have earlier onset of diabetes, and many do not have good glycaemic control after diagnosis. Their higher risk may make them a high priority for lifestyle intervention, but cultural (less physical activity and fatalistic attitudes), language and religious barriers need to be identified and addressed. According to Rankin and Bhopal (2001),168 providing translated leaflets was the most common suggestion for ways of informing the South Asian communities. There was a lack of understanding of the role of obesity as an important risk factor.
Should intervention be earlier than at impaired glucose tolerance stage?
The remit of this review was to examine the evidence for intervention in people with IGT. However, there may be a case for earlier intervention to prevent people developing IGT. We know from the US Nurses’ Health Study that BMI is the dominant risk factor for the development of diabetes in women. 79 Even weight gain of 5–8 kg almost doubles the risk. Similar findings have been reported in men. 18 Hence, if the population could be persuaded not to gain weight, the prevalence of diabetes would be greatly reduced.
Implementation
Would trial interventions work in ‘real life’?
Seidel et al. (2008)351 did not think that the DPP intervention could easily be replicated in community settings. Therefore, they set out to test a group-based lifestyle balance intervention in urban areas of Pittsburgh, described as ‘medically underserved’, and being a socioeconomically depressed area following the decline of the steel industry, with an ageing population owing to out-migration. A community approach was taken, with notices in sites such as churches, shops, local newspapers and radio. Only short-term (6-month) results are as yet available, but look promising, with about half of the recruits losing at least 5% of body weight. Only 88 people joined but most of these completed the course. It would be useful to see longer-term follow-up with larger numbers.
The Greater Green Triangle Diabetes Prevention Project352 aimed to evaluate whether a structured group programme of lifestyle intervention, set in an Australian primary health-care setting, would give similar reductions in risk factors to that found in the RCTs.
The 12-month study used before and after testing. Patients from general practices, who were at high risk of developing T2DM, were screened opportunistically using The Diabetes Risk Score tool. 352 The intervention, based on the Finnish GOAL study353 consisted of six structured 90-minute group sessions over 8 months.
Participants were aged 40–75 years with moderate or high risk of developing T2DM. Only 76% attended both the baseline and 12-month clinical tests and at least one group session.
After 12 months statistically significant improvements were observed in participants’ mean weight, WC, fasting and 2-hour glucose lipids, DBP, and most psychological measures. However, follow-up is still short, and only time will tell if the benefits are sustained.
From this, it would appear that the results of the trial can be replicated in routine primary care.
In Finland, the national diabetes programme (DEHKO) includes a subprogramme to prevent diabetes (the FIN-D2D project),354 involving three strategies:
-
a population-based approach to prevent obesity and diabetes
-
the high-risk strategy – identification and screening of people at high risk, followed by lifestyle measures
-
early diagnosis of people with T2DM and prompt treatment to prevent complications.
The 1-year follow-up reported that the risk of diabetes was 0.31 (95% CI 0.16 to 0.59) in those who lost 5% or more of weight relative to those who maintained weight. 355 This was in a group identified by a high (≥ 15) FINDRISC score.
Good results were reported from a DPP-style intervention in Montana, with 39% of respondents reporting that they had maintained or achieved 7% weight loss. 356 However, there was considerable attrition in this study. Of 591 initial recruits, only 79% (466) completed the programme, and only 40% of these (188) responded to the follow-up questionnaire. So only 12% of the initial cohort had lost 7% of weight at about 18 months after entry. It should also be noted that the data were self-reported.
The primary goal of the IDF Taskforce on Prevention and Epidemiology consensus workshop in 2006 was the prevention of T2DM in both the developed and developing world. 303 The IDF plan is aimed at simultaneously controlling modifiable risk factors with lifestyle modification in two target groups: (1) people at high risk of developing T2DM and (2) the entire population.
An editorial by Simmons et al. (2007)357 on the IDF consensus statement questioned whether individualistic approaches to diabetes prevention would be effective, and felt that the emphasis should be more on population-based approaches. They made the point that the rising prevalence of diabetes seen in increasingly obese populations is a result of a shift in the entire distribution of glucose in that population, rather than simply an increase in the number of people at the tail of the distribution. Therefore, the most effective strategy for lowering the mean glucose level of the population, thus lowering the population burden of CVD attributable to hyperglycaemia, is to attempt to increase average levels of activity and reduce obesity in the large number of people with moderately raised levels of glucose.
The evidence base for individual approaches to diabetes prevention is stronger than for population-based approaches, as the former are much easier to test in RCTs. However, Simmons et al. (2007)357 feel that in order to correct this imbalance and to be able to evaluate population-based approaches, researchers need to be more open to other study designs, such as natural experiments in local communities using quasi-experimental designs.
The GOAL Lifestyle Implementation Trial335 aimed to test whether the findings achieved in the DPS trial183 could be replicated in a ‘real world’ setting. 335 The study used a longitudinal pre-test and post-test study design, and focused on the five key lifestyle changes derived from the DPS. 183
The 352 participants, mean BMI > 32 kg/m2 and aged between 50 and 65 years, were recruited from primary health-care centres in Finland. Risk status for T2DM was determined using a standardised risk questionnaire. The inclusion criterion was set at risk score of ≥ 17% 10-year risk; 25% of the participants had IGT at baseline. The intervention included six group counselling sessions given over 12 months.
Only 57% of the participants attended all six counselling sessions, and 33 participants dropped out of the study. The number of participants in this study who attained four or five of the lifestyle objectives was 20%, which was similar to the DPS (18%). However, the > 5% weight loss goal was significantly less frequently achieved in this study (12%) than in the DPS (43%). The physical activity goal was significantly less frequently achieved in this study (66% in GOAL335 vs 86% in DPS183), whereas the fibre objective was significantly higher in this study. After 1 year, several clinical risk factors decreased significantly, including DBP, weight and BMI (only men), and WC (both sexes). The results at 36 months showed that the weight loss was maintained. 358
Therefore it would seem that this lower-intensity (and hence lower-cost) 12-month intervention was successful in decreasing diabetes risk in a ‘real world’ setting. However, it is not known whether these results will be sustained over the longer term.
One general practice in Glasgow tried to implement healthy eating and exercise over a 3-year period, with group sessions, an exercise scheme, and dietetic and medical time. However, maintenance of gains proved difficult unless the intervention was continued. As Guthrie comments:359
In the end all of these patients required a continuous personal input to maintain their weight loss, regular exercise, or healthy eating, and it simply became unsustainable.
Perhaps we need to look at interventions that are less ‘medical’ in nature. Truby et al. (2006)360 carried out a randomised trial of four commercial weight loss programmes, including WeightWatchers. All four diet programmes had good results, with an average weight loss of almost 6 kg at 6 months. We need much longer follow-up to see if weight loss continues or is sustained.
Can exercise alone reduce progression to diabetes?
Yates et al. (2007)361 conducted a systematic review of controlled trials to establish whether increasing physical activity, independent of changes in diet or weight loss, can reduce the risk of T2DM in people with pre-diabetes (IGT and/or IFG).
The review included eight trials (seven randomised and one non-randomised) in individuals with IGT. Seven of the studies used a multicomponent lifestyle intervention and one used a structured gym-based exercise training intervention. Four studies included the incidence of diabetes as the main outcome and found that diabetes incidence was reduced by 42–64% compared with the control group. These studies reported only small changes in physical activity. The other four studies used 2-hour plasma glucose levels as the primary indicator of glucose control, and only one reported a significant improvement. Three of these studies reported small to moderate increases in maximal oxygen uptake, suggesting adherence. All but one of the studies included in the review reported significant weight loss among participants.
The conclusion of the review was that the role of physical activity independent of other lifestyle changes in the treatment of pre-diabetes remains uncertain. Given the relatively modest increases in physical activity, the success of the interventions is probably due to the weight loss.
Laaksonen et al. (2007),362 in response to the Yates review, did not agree that the 9 minutes/day increase in moderate to vigorous physical activity reported in the intervention group of the Finnish DPS183 was insubstantial. They found that the percentage of sedentary individuals (< 1 hour/week of moderate to vigorous physical activity) in the intervention group of the DPS183 decreased from 37% to 15%, and those engaging in at least 2.5 hours/week increased from 41% to 62%, and felt that this was likely to result in health benefits.
They also reported that individuals who engaged in at least 2.5 hours/week of brisk walking or other forms of moderate to vigorous physical activity were 44–69% less likely to develop diabetes than individuals engaging in < 1 hour/week. These findings were based on post hoc analyses from the intervention arm.
Burns et al. (2007)363 examined the effects of a 3-month aerobic exercise training programme in young obese insulin-resistant subjects with and without T2DM. They recruited 13 subjects with T2DM and 18 non-diabetic control subjects for the baseline study. The two groups were matched for age, BMI, body fat and physical fitness. An exercise intervention (involving 1 hour of exercise training four times per week for 12 weeks) was completed by seven of the subjects with T2DM and 14 of the obese control subjects. The overall mean age of the completers was 26 years and mean BMI was 34 kg/m2.
The authors had hypothesised that exercise alone, while maintaining a stable diet, should improve insulin sensitivity in these severely insulin-resistant subjects, but found to their surprise that neither group showed metabolic improvements after the aerobic exercise intervention. The authors comment that this result raises interesting new questions about the pathogenesis and treatment of early-onset T2DM in obese young people.
Carnethon (2007)364 (in an editorial on the Yates 2007361 review) comments that it is important to understand whether it is the diet or physical activity component of the lifestyle intervention that is the key to its success. Physiological responses to, and compliance with, the diet and exercise components of the lifestyle intervention vary greatly between individuals. By focusing the interventions on the most effective component, or the component that is easiest to adopt, we might be able to improve compliance with the intervention.
A Cochrane review by Orozco et al. (2008)185 examined the role of exercise and diet in preventing T2DM. 185 The eight studies they included differed from our inclusions. They included Bo et al. (2007),365 which recruited patients with metabolic syndrome. They did not include three studies that were included in this review. One was Wein et al. (1999),191 which appears to have been suitable for inclusion according to their criteria because it focused on women with previous GDM. It is not listed as an exclusion, or indeed anywhere in the Cochrane review. It may have been missed. They listed Mensink et al. (2003)188,233,234 as awaiting assessment because the final 3-year results had not been published – they were published a few months later and have been included in this review. They also excluded Liao et al. (2002)187 because ‘the control group received an intervention that differed from standard recommendation’. It is not clear what was meant by this.
The final conclusion of the Cochrane review was that more evidence was required on the effects of exercise alone.
Would UK populations comply with increased activity?
In Finland, a combined strategy of general population and targeting of high-risk individuals is being used. For this to work in the UK, we would need to encourage the population to be more physically active. Current statistics suggest that this might be difficult.
Data from the Information Centre ‘Statistics on Obesity, Physical Activity and Diet: England 2006’366 show that:
-
In 2004, only 35% of men and 24% of women reported achieving the physical activity targets of at least 30 minutes of moderate activity five times a week.
-
The main reasons given were that health was not good enough (50%, which seems implausible), lack of time (18%) and lack of interest (15%).
The lowest levels of activity were seen in those with the highest BMI. The proportions who were physically active fell from 44% of men with a good BMI, to 31% in the obese (BMI 30–40 kg/m2 and to 16% in the morbidly obese (BMI > 40 kg/m2). The same trend was seen in women: 30% with high activity levels in those with normal BMI to 18% in the obese. Figure 14 shows the proportions achieving the target level of at least 30 minutes of physical activity 5 days per week.
FIGURE 14.
Percentage participation in physical activity vs BMI.
In the Norfolk cohort of the EPIC,290 only 20% of the participants met three or more of the diabetes prevention goals (similar to those in the Finnish DPS183), and only 1% achieved all five; 10% achieved none. 367
The results of the PREPARE (Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement) trial368–370 give some grounds for optimism. The aim of the PREPARE trial was to see if structured education could increase physical activity and improve glucose tolerance. (It was not a trial of preventing T2DM and so was not eligible for inclusion in Chapter 4). It recruited individuals with IGT who were randomised to usual care, or to the PREPARE education programme, with or without pedometer use. The trial was quite small with initially 103 recruits, falling to 73 by the 2-year follow-up. 368–370 Only 32% of those invited to take part did so. The 12-month follow-up showed no difference in weight, but did show a significant decrease in fasting and 2-hour blood glucose in the pedometer group compared with the control group. The education-only group did not show any difference. The 2-year results showed a continuing benefit in terms of blood glucose with a reduction of 1.6 mmol/l in the pedometer group, although by this time there were only 22 patients in this arm. It would be worth repeating this trial with larger numbers and longer-term follow-up. The intervention was inexpensive.
Could genetic studies help?
Genotyping participants might help to target interventions to those who are at high risk and who will best respond to lifestyle interventions.
Laaksonen et al. (2007)211 examined the interactions of the physical activity, dietary, and weight loss components of the intervention with the 12Glu9 polymorphism of the ADRA2B gene in the development of T2DM in the combined intervention and control groups of the Finnish DPS. 183 Average follow-up time was 4.1 years.
Increased LTPA decreased the likelihood of diabetes more in those with the 12Glu allele of the ADRA2B gene. The RRs of upper compared with lower tertiles for increased LTPA were Glu12/12 = 0.12 (0.03–0.53); Glu12/9 = 0.30 (0.11–0.79) and Glu9/9 = 1.05 (0.32–3.47).
Favourable dietary changes reduced the risk of diabetes more in those who were homozygous for the 9Glu allele. The RRs of upper compared with lower tertiles for dietary changes were Glu12/12 = 0.65 (0.23–1.84); Glu12/9 = 0.85 (0.28–2.27) and Glu9/9 = 0.21 (0.06–0.75).
Weight reduction seemed to decrease the risk of diabetes more in 12Glu9 heterozygotes, but the interaction was not significant. The RRs of upper compared with lower tertiles were, for decrease in BMI, Glu12/12 = 0.48 (0.14–1.60); Glu12/9 = 0.11 (0.05–0.28) and Glu9/9 = 0.81 (0.26–2.48).
A better understanding of the genetic factors responsible for the different responses to various components of lifestyle interventions might allow us to predict who will respond to lifestyle interventions, and to tailor the intervention according to the individual’s genotype.
Ongoing research
Given the success of the big trials, some of the research now is around ‘real-life’ delivery.
The Norfolk DPS371 is an ambitious project, which will screen 10,000 people at risk of diabetes over 5 years, and randomise 950 people with ‘pre-diabetes’ into a 36-month RCT of a novel diet and lifestyle intervention. The intervention contains three arms, and will be delivered by health-care professionals in group settings. One arm will be part delivered by lay mentors who have existing T2DM.
To take part, participants must be > 40 years old, live in the county of Norfolk or be registered with a GP in the county of Norfolk, and meet at least one of the following inclusion criteria: BMI of ≥ 30 kg/m2, family history of T2DM, history of CHD or previous GDM, or previous IGT or IFG.
Ambitious although that may be, it is dwarfed by the Qingdao Diabetes Prevention Project. 372,373 This study, sponsored by Helsinki University, aims to translate the trial experience to real-life settings with goals to (1) raise the public awareness of diabetes and diabetes risk factors, and promote healthy diet and physical activity; (2) reduce the number of people at high-risk of developing diabetes through lifestyle counselling; (3) attain early diagnosis of diabetes; and (4) evaluate the clinical effectiveness, cost-effectiveness, feasibility, acceptability and sustainability of the programmes. The project involves community-based targeting of the entire population of 1.94 million people living in four administration districts of the city of Qingdao in China.
The project applies both a population approach and a high-risk approach. In the first phase of the project (2005–8) the work emphasis was on health promotion. In the second phase (2008–12) lifestyle counselling sessions will be provided to about 242,112 high-risk individuals identified, and the efficacy and the cost of the project will be evaluated at the end of the project in 2012.
In Bournemouth, an 8-month intensive lifestyle programme is being tested in obese people without diabetes but with a family history of the condition. The study size is quite small, 66 patients, but the main interest is in levels of glucagon-like peptide 1. 374 The aim is to investigate whether gradual weight loss achieved with healthy lifestyle changes influences hormonal factors affecting appetite and blood glucose control in obese people without the presence of diabetes.
In Canada, a study called PREPARE (Prediabetes Research and Education Promoting Activity and Responsible Eating)375 is being run from Brescia University College in London, Ontario. PREPARE375 is a 6-month community-based pre-diabetes lifestyle and behavioural change programme for adults aged ≥ 30 years with pre-diabetes. It includes a series of six interactive education sessions, of 2 hours each, on healthy eating and physical activity. Individuals self-selecting the control arm receive the current standard of care for pre-diabetes, which is a one-time 2-hour group education session. The primary outcome measure is the average number of vegetable and fruit servings consumed per day, measured at 6 months and 12 months after the baseline assessment.
In the USA, a pilot RCT will compare the clinical effectiveness and cost-effectiveness of two programmes – the DPP system and a community-based Health Living Program – delivered in primary care. 376 The primary outcome measure is 7% reduction in participant weight at 22 weeks, and the estimated enrolment is 200 people. Participants must be aged ≥ 18 years and diagnosed with pre-diabetes.
In Colorado, a RCT called Adaptation of the Diabetes Prevention Program for Primary Care377 aims to assess the efficacy of adding in-person visits to the use of portion-controlled foods for long-term weight loss, and to assess the use of trained lay counsellors for the maintenance of weight loss. Participants will be recruited primarily from primary care practices at the University of Colorado. Up to 200 patients will be provided with 6 months of high-intensity weight loss counselling. Those participants remaining after the first 6 months will be randomly assigned to either standard maintenance or intensified maintenance during months 7–18. The standard maintenance group will receive information handouts regarding weight maintenance, whereas those in the intensified maintenance group will continue to have monthly in-person visits with the weight loss counsellor (‘weight coach’). The primary outcome is weight change.
In East Harlem, in New York, a community-based peer-led programme, HEED (Help Educate to Eliminate Diabetes),378 will randomise overweight adults with pre-diabetes to intervention or usual care, with weight loss as the primary outcome. HEED is a 10-week course. 378 The intervention group will participate in an eight-session course held over a 10-week period. The comparator group will receive a delayed intervention, i.e. they will be offered the chance to participate in the course 1 year after enrolment into the trial. The estimated enrolment is 400 participants.
Also in the USA, the Diabetes Prevention Program Outcomes Study (DPPOS)379 is an observational extension to the DPP. The primary outcome is the development of diabetes, and secondary outcomes include composite microvascular and macrovascular measures. It aims to have 3250 participants.
A small feasibility study from Emory University380 is looking at developing a culturally appropriate lifestyle intervention in South Asians (people with origins in India, Pakistan, Bangladesh, Nepal, Sri Lanka, etc.) living in or near Atlanta, GA. This study will test the acceptability of a culturally appropriate lifestyle intervention for the prevention of diabetes in the South Asian community. The intervention will be based on the DPP but tailored to the needs of the community, based on feedback gathered in focus groups. Participants will be required to attend one group exercise class per week, based on traditional Indian dances and other culturally appropriate activities.
In Catalonia, Spain, a two-stage study, Diabetes in Europe – Prevention Using Lifestyle, Physical Activity and Nutritional Intervention in Catalonia (DE-PLAN-CAT),381 is combining screening methods (FINDRISC vs OGTT) with a later intervention study comparing usual care with individual- or group-based education. A total of 2082 people have been screened, and one-third are expected to present high-risk criteria. They will choose one of three possible interventions to modify their lifestyle (informative approach, one-to-one or group training). Final results are expected in 2016.
The DE-PLAN approach is also being studied in a cluster RCT, conducted by Osakidetza382 in the Basque Country in high-risk (FINDRISC > 14) populations seen in 14 primary care centres. The plan is to recruit over 2500 subjects. The intervention group will receive a structured educational intervention on healthy lifestyles (diet and physical activity) and the control group will receive standard care for the prevention and treatment of T2DM. The primary outcome is the incidence of diabetes at 24 months, and results are expected at the end of 2013.
The Prevention of Diabetes and Obesity in South Asians (PODOSA) study383,384 has screened 1300 people of Indian and Pakistani origin for hyperglycaemia, and has recruited 170 at high risk to an intervention study of healthy eating and increasing physical activity. It is due to report in 2013 (see progress at www.podosa.org/progress.html). Some findings have been published. Gill et al. (2011)383 reported that there was an association between time spent sitting and the 2-hour PG, but not with fasting PG, among all people who were screened for possible inclusions. Douglas et al. (2011)384 reported on the difficulties of recruitment of this group through the usual channels such as GPs and diabetes registers, but noted that there was much greater success using community associations and encouraging recruits to bring in others (‘snowballing’).
Research needs
The highest priority appears to be research into ways of improving adherence to lifestyle measures.
However, given that at least some people will adhere, the next priority is to refine the interventions, addressing questions such as:
-
What level of provision or contact is necessary? Could the gains seen in the DPS and DPP be achieved at lower cost?
-
How long do interventions need to be continued for?
-
What type (or types) of physical activity – in terms of frequency and intensity – will give the best balance between efficacy and adherence?
-
How can physical activity be increased?
-
How can adherence be improved?
-
Does genetic testing have a role to play in determining which intervention should be used?
-
What benefits are accrued by those on lifestyle interventions who return to NGT, or who remain in IGT, other than avoiding diabetes?
-
Would an intervention, such as the Finnish DPS,183 achieve the same benefits in the UK? Are progression rates similar? We know that at least one UK group, the South Asians, do worse.
-
What interventions would be most effective in the highest-risk groups?
Conclusion
The prevalence of diabetes is increasing owing to the sedentary lifestyle and unhealthy diet favoured by developed societies. The pressure to introduce screening for undiagnosed T2DM is growing. However, were we to screen for diabetes, we would, depending on choice of test and cut-off levels used, identify more, or far more, people with IGT than with diabetes. This review was commissioned in response to the identification of that problem in our previous review of screening for diabetes. Our remit was limited to non-pharmacological interventions.
There is a strong body of evidence that there are effective ways of reducing progression to diabetes in people with IGT by lifestyle interventions, and these are likely to be considered cost-effective. Progression to diabetes could be reduced by about half, if the results in the volunteers in trials such as DPP and DPS can be reproduced in routine care.
However, adherence tends to be poor. The benefits of the lifestyle intervention were greatest in those with the highest compliance and who achieved more of the targets (such as weight loss and dietary change). For example, in the Finnish study,183 those who achieved four or five of the five targets had a risk of developing diabetes which was only 23% of those who achieved none. Weight loss is the most important goal.
Furthermore, even among the volunteers in the trials, many did not succeed, and others succeeded in the short term (such as the first 6 months) but not in the longer term. The key to success is sustained lifestyle change, especially weight loss. We know what people need to do to reduce their risk of progression to diabetes, but not how to motivate them to do so.
Acknowledgements
We thank Professor Raj Bhopal of the University of Edinburgh for useful comments on a draft report.
Contribution of authors
Michael Gillett Conducted the economic modelling (Chapter 7).
Pamela Royle Undertook literature searches; extracted data, co-wrote Chapter 4, contributed to other chapters, edited final report.
Ailsa Snaith Extracted data. Contributed to writing the report (background, systematic review and discussion).
Graham Scotland Reviewed the economic literature (Chapter 5).
Amudha Poobalan Contributed to writing the report (Chapter 3).
Mari Imamura Extracted data and contributed to writing the report (Chapter 4).
Corri Black Analysed GPRD data and wrote Chapter 6.
Massoud Boroujerdi Analysed GPRD data (Chapter 6).
Sue Jick Extracted GPRD data.
Laura Wyness Contributed to writing the report (exercise section in Chapter 2).
Paul McNamee Assisted with the review of economic literature (Chapter 5).
Alan Brennan Assisted with the economic modelling (Chapter 7).
Norman Waugh Contributed to writing the report (all sections) and final editing.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-53.
- Moore P. Type 2 diabetes is a major drain on resources. BMJ 2000;320.
- Diabetes UK. Diabetes in the UK 2010: Key Statistics on Diabetes 2010. www.diabetes.org.uk/Documents/Reports/Diabetes_in_the_UK_2010.pdf (accessed 22 March 2012).
- Department of Health (DH) . Health Profile of England 2009 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsStatistics/DH_114561 (accessed 22 March 2012).
- Yorkshire & Humber Public Health Observatory . Diabetes Key Facts 2006. www.yhpho.org.uk/resource/item.aspx?RID=8872 (accessed 1 February 2012).
- Yorkshire & Humber Public Health Observatory . APHO Diabetes Prevalence Model 2011. www.yhpho.org.uk/default.aspx?RID=81090 (accessed 1 February 2012).
- National Statistics . Health Survey for England 2004: The Health of Minority Ethnic Groups 2005. www.ic.nhs.uk/webfiles/publications/hlthsvyeng2004ethnic/HealthSurveyForEngland161205_PDF%20.pdf (accessed 22 March 2012).
- Bagust A, Hopkinson PK, Maslove L, Currie CJ. The projected health care burden of type 2 diabetes in the UK from 2000 to 2060. Diabet Med 2002;19:1-5.
- Department of Health (DH) . National Service Framework for Diabetes 2001. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/Browsable/DH_4096591 (accessed 22 March 2012).
- Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73-8.
- Turner RC, Holman RR, Cull CA, Stratton IM, Matthews DR, Frighi V, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421-31.
- Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J 2006;152:27-38.
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007;28:187-218.
- Lebovitz HE. Insulin resistance: a common link between type 2 diabetes and cardiovascular disease. Diabetes Obes Metab 2006;8:237-49.
- International Diabetes Federation . Diabetes Atlas: Second Edition 2002;URL. www.idf.org/sites/default/files/IDF_Diabetes_Atlas_2ndEd.pdf (accessed 22 March 2012).
- Jarrett RJ. Is there an ideal body weight?. Br Med J 1986;293:493-5.
- Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214-22.
- National Audit Office . Tackling Obesity in England 2001. www.nao.org.uk/publications/0001/tackling_obesity_in_England.aspx (accessed 22 March 2012).
- Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744-52.
- Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 2007;298:2507-16.
- Thomas C, Hypponen E, Power C. Diabetes risk in British adults in mid life: a national prevalence study of glycated haemoglobin. Diabet Med 2007;24:317-21.
- Invitti C, Guzzaloni G, Gilardini L, Morabito F, Viberti G. Prevalence and concomitants of glucose intolerance in European obese children and adolescents. Diabetes Care 2003;26:118-24.
- Balakrishnan R, Webster P, Sinclair D. Trends in overweight and obesity among 5–7-year-old White and South Asian children born between 1991 and 1999. J Public Health 2008;30:139-44.
- Ehtisham S, Hattersley AT, Dunger DB, Barrett TG. First UK survey of paediatric type 2 diabetes and MODY. Arch Dis Child 2004;89:526-9.
- Turner R, Cull C, Holman R. United Kingdom prospective diabetes study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med 1996;124:136-45.
- UKPDS Study Group . UKPDS 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 1995;44:1249-58.
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2006;49:1711-21.
- National Institute for Health and Clinical Excellence (NICE) . Type 2 Diabetes: Newer Agents (partial Update of CG66) 2009. www.nice.org.uk/CG87 (accessed 1 February 2012).
- Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14.
- Yki-Jarvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1999;130:389-96.
- Hayward RA, Manning WG, Kaplan SH, Wagner EH, Greenfield S. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA 1997;278:1663-9.
- Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med 2006;23:736-42.
- World Health Organization (WHO) . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO IDF Consultation 2006. www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf (accessed 22 March 2012).
- Glumer C, Jorgensen T, Borch-Johnsen K. Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 2003;26:2335-40.
- American Diabetes Association (ADA) . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26:5-20.
- Balkau B, Hillier T, Vierron E, D’Hour A, Lepinay P, Royer B, et al. (2004) Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia n.d.;47:1396-402.
- Agency for Healthcare Research and Quality (AHRQ) . Diagnosis, Prognosis, and Treatment of Impaired Glucose Tolerance and Impaired Fasting Glucose 2005. www.ahrq.gov/clinic/tp/impglutp.htm (accessed 22 March 2012).
- Balkau B, Eschwege E, Tichet J, Marre M. Proposed criteria for the diagnosis of diabetes: evidence from a French epidemiological study (D.E.S.I.R.). Diabetes Metab 1997;23:428-34.
- Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabet Med 2007;24:200-7.
- Qiao Q, Lindström J, Valle TT, Tuomilehto J. Progression to clinically diagnosed and treated diabetes from impaired glucose tolerance and impaired fasting glycaemia. Diabet Med 2003;20:1027-33.
- Rasmussen SS, Glumer C, Sandbaek A, Lauritzen T, Borch-Johnsen K. Progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high-risk screening programme in general practice: the ADDITION Study, Denmark. Diabetologia 2007;50:293-7.
- Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care 2007;30:228-33.
- Keen H, Jarrett RJ, McCartney P. The ten-year follow-up of the Bedford survey (1962–1972): glucose tolerance and diabetes. Diabetologia 1982;22:73-8.
- Little RR, England JD, Wiedmeyer HM, Madsen RW, Pettitt DJ, Knowler WC, et al. Glycated haemoglobin predicts progression to diabetes mellitus in Pima Indians with impaired glucose tolerance. Diabetologia 1994;37:252-6.
- Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701-10.
- Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, et al. Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006;29:1619-25.
- Yoshinaga H, Kosaka K. High glycosylated hemoglobin levels increase the risk of progression to diabetes mellitus in subjects with glucose intolerance. Diabetes Res Clin Pract 1996;31:71-9.
- Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93.
- Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-23.
- The DECODE study group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999;354:617-21.
- Pan XR, Hu YH, Li GW, Liu PA, Bennett PH, Howard BV. Impaired glucose tolerance and its relationship to ECG-indicated coronary heart disease and risk factors among Chinese. Da Qing IGT and diabetes study. Diabetes Care 1993;16:150-6.
- Rewers M, Shetterly SM, Baxter J, Marshall JA, Hamman RF. Prevalence of coronary heart disease in subjects with normal and impaired glucose tolerance and non-insulin-dependent diabetes mellitus in a biethnic Colorado population. The San Luis Valley Diabetes Study. Am J Epidemiol 1992;135:1321-30.
- Jarrett RJ. The cardiovascular risk associated with impaired glucose tolerance. Diabet Med 1996;13:S15-19.
- Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999;22:920-4.
- Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151-7.
- Davies MJ, Muehlbayer S, Garrick P, McNally PG. Potential impact of a change in the diagnostic criteria for diabetes mellitus on the prevalence of abnormal glucose tolerance in a local community at risk of diabetes: impact of new diagnostic criteria for diabetes mellitus. Diabet Med 1999;16:343-6.
- McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991;337:382-6.
- Forrest RD, Jackson CA, Yudkin JS. Glucose intolerance and hypertension in north London: the Islington Diabetes Survey. Diabet Med 1986;3:338-42.
- Phillips DI, Goulden P, Syddall HE, Aihie SA, Dennison EM, Martin H, et al. Fetal and infant growth and glucose tolerance in the Hertfordshire Cohort Study: a study of men and women born between 1931 and 1939. Diabetes 2005;54:145-50.
- Thomas MC, Walker MK, Emberson JR, Thomson AG, Lawlor DA, Ebrahim S, et al. Prevalence of undiagnosed type 2 diabetes and impaired fasting glucose in older British men and women. Diabet Med 2005;22:789-93.
- Simmons D, Williams DR, Powell MJ. The Coventry Diabetes Study: prevalence of diabetes and impaired glucose tolerance in Europids and Asians. Q J Med 1991;81:1021-30.
- Dunstan DW, Zimmet PZ, Welborn TA, De Court, Cameron AJ, Sicree RA, . The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002;25:829-34.
- Glatthaar C, Welborn TA, Stenhouse NS, Garcia-Webb P. Diabetes and impaired glucose tolerance. A prevalence estimate based on the Busselton 1981 survey. Med J Aust 1985;143:436-40.
- Guest CS, O’Dea K, Hopper JL, Nankervis AJ, Larkins RG. The prevalence of glucose intolerance in aborigines and Europids of south-eastern Australia. Diabetes Res Clin Pract 1992;15:227-35.
- Unwin N, Harland J, White M, Bhopal R, Winocour P, Stephenson P, et al. Body mass index, waist circumference, waist–hip ratio, and glucose intolerance in Chinese and Europid adults in Newcastle, UK. J Epidemiol Community Health 1997;51:160-6.
- King H, Rewers M. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes Care 1993;16:157-77.
- Tapp RJ, Dunstan DW, Phillips P, Tonkin A, Zimmet PZ, Shaw JE. Association between impaired glucose metabolism and quality of life: results from the Australian diabetes obesity and lifestyle study. Diabetes Res Clin Pract 2006;74:154-61.
- Testa MA, Simonson DC. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA 1998;280:1490-6.
- Testa MA, Simonson DC, Turner RR. Valuing quality of life and improvements in glycemic control in people with type 2 diabetes. Diabetes Care 1998;21:C44-52.
- Waugh N, Millard A, Robertson L, Royle P. Research Review for the Scottish Public Health Network. Screening for and Prevention of Type 2 Diabetes 2011. www.scotphn.net/projects/previous_projects/type_2_diabetes_needs_assessment (accessed 22 March 2012).
- Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11.
- UK National Screening Committee (NSC) . The UK NSC Policy on Diabetes Screening in Adults 2012. www.screening.nhs.uk/diabetes (accessed 22 March 2012).
- Holbrook TL, Barrett-Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes 1989;13:723-9.
- Manson JE, Spelsberg A. Primary prevention of non-insulin-dependent diabetes mellitus. Am Prev Med 1994;10:172-84.
- Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132:501-13.
- Meisinger C, Doring A, Thorand B, Heier M, Lowel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr 2006;84:483-9.
- Zaninotto P, Mindell J, Hirani V. Prevalence of cardiovascular risk factors among ethnic groups: results from the Health Surveys for England. Atherosclerosis 2007;195:e48-57.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481-6.
- Hart CL, Hole DJ, Lawlor DA, Davey SG. How many cases of type 2 diabetes mellitus are due to being overweight in middle age? Evidence from the Midspan prospective cohort studies using mention of diabetes mellitus on hospital discharge or death records. Diabet Med 2007;24:73-80.
- Jiang Y, Chen Y, Mao Y. The contribution of excess weight to prevalent diabetes in Canadian adults. Public Health 2008;122:271-6.
- Han TS, Sattar N, Lean M. ABC of obesity. Assessment of obesity and its clinical implications. BMJ 2006;333:695-8.
- Diabetes Prevention Program Research Group . Relationship of body size and shape to the development of diabetes in the diabetes prevention program. Obesity 2006;14:2107-17.
- Diaz VA, Mainous AG, Baker R, Carnemolla M, Majeed A. How does ethnicity affect the association between obesity and diabetes?. Diabet Med 2007;24:1199-204.
- Hu FB, van Dam RM, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805-17.
- American Diabetes Association . Nutrition Recommendations and Interventions for Diabetes: a position statement of the American Diabetes Association. Diabetes Care 2007;30:48-65.
- Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002;25:148-98.
- Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr 2008;87:S269-74.
- Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 2010;341.
- Hays NP, Galassetti PR, Coker RH. Prevention and treatment of type 2 diabetes: Current role of lifestyle, natural product, and pharmacological interventions. Pharmacol Ther 2008;118:181-91.
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991;325:147-52.
- Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia 1991;34:891-8.
- Sherwin RS, Anderson RM, Buse JB, Chin MH, Eddy D, Fradkin J, et al. Prevention or delay of type 2 diabetes. Diabetes Care 2004;27:47-54.
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care 2004;27:2518-39.
- Diabetes Australia . Evidence Based Guideline for the Primary Prevention of Type 2 Diabetes 2008. www.diabetesaustralia.com.au/PageFiles/763/Primarypreventionguideline.pdf (accessed 22 March 2012).
- Wallberg-Henriksson H, Rincon J, Zierath JR. Exercise in the management of non-insulin-dependent diabetes mellitus. Sports Med 1998;25:25-3.
- Burchfiel CM. Physical activity and incidence of diabetes: The Honolulu Heart Program. Am J Epidemiol 1995;141:360-8.
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 1992;268:63-7.
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774-8.
- Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006;3.
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282:1433-9.
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 2005;99:1193-204.
- Mayer-Davis EJ, D’Antonio A, Martin M, Wandersman A, Parra-Medina D, Schulz R. Pilot study of strategies for effective weight management in type 2 diabetes: Pounds Off with Empowerment (POWER). Fam Community Health 2001;24:27-35.
- Kriska A. Physical activity and the prevention of type 2 diabetes mellitus: how much for how long?. Sports Med 2000;29:147-51.
- Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Association between leisure time physical activity and 10-year body mass change among working-aged men and women. Int J Obes Relat Metab Disord 1997;21:288-96.
- Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 1995;310:560-4.
- Wannamethee SG, Shaper AG, Walker M, Ebrahim S. Lifestyle and 15-year survival free of heart attack, stroke, and diabetes in middle-aged British men. Arch Intern Med 1998;158:2433-40.
- Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40.
- Eriksen L, Dahl-Petersen I, Haugaard SB, Dela F. Comparison of the effect of multiple short-duration with single long-duration exercise sessions on glucose homeostasis in type 2 diabetes mellitus. Diabetologia 2007;50:2245-53.
- Eves ND, Plotnikoff RC. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care 2006;29:1933-41.
- Zachwieja JJ, Toffolo G, Cobelli C, Bier DM, Yarasheski KE. Resistance exercise and growth hormone administration in older men: effects on insulin sensitivity and secretion during a stable-label intravenous glucose tolerance test. Metabolism 1996;45:254-60.
- Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 2005;86:1527-33.
- Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002;25:2335-41.
- Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 2006;29:2518-27.
- Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:357-69.
- Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med 2008;38:807-24.
- Eriksson JG. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Med 1999;27:381-91.
- Schneider SH, Khachadurian AK, Amorosa LF, Clemow L, Ruderman NB. Ten-year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. Diabetes Care 1992;15:1800-10.
- King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation 1995;91:2596-604.
- Perri MG, Anton SD, Durning PE, Ketterson TU, Sydeman SJ, Berlant NE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol 2002;21:452-8.
- Dishman RK, Buckworth J. Increasing physical activity: a quantitative synthesis. Med Sci Sports Exerc 1996;28:706-19.
- McGinnis JM. Diabetes and physical activity: Translating evidence into action. Am J Prev Med 2002;22:1-2.
- Pereira MA, Kriska AM, Day RD, Cauley JA, LaPorte RE, Kuller LH. A randomized walking trial in postmenopausal women: effects on physical activity and health 10 years later. Arch Intern Med 1998;158:1695-701.
- National Center for Chronic Disease Prevention and Health Promotion . Physical Activity and Health: A Report of the Surgeon General 1999. www.cdc.gov/nccdphp/sgr/sgr.htm (accessed 22 March 2012).
- Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obes Relat Metab Disord 1995;19:893-901.
- Avenell A, Sattar N, Lean M. ABC of obesity. Management: Part I – behaviour change, diet, and activity. BMJ 2006;333:740-3.
- Sallis JF, Haskell WL, Fortmann SP, Vranizan KM, Taylor CB, Solomon DS. Predictors of adoption and maintenance of physical activity in a community sample. Prev Med 1986;15:331-41.
- Pollock ML, Carroll JF, Graves JE, Leggett SH, Braith RW, Limacher M, et al. Injuries and adherence to walk/jog and resistance training programs in the elderly. Med Sci Sports Exerc 1991;23:1194-200.
- Iverson DC, Fielding JE, Crow RS, Christenson GM. The promotion of physical activity in the United States population: the status of programs in medical, worksite, community, and school settings. Public Health Rep 1985;100:212-24.
- Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694-703.
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:1433-8.
- Hillsdon M, Foster C, Cavill N, Crombie H, Naidoo B. Health Development Agency . Effectiveness of Public Health Interventions for Increasing Physical Activity Among Adults: A Review of Reviews (evidence Briefing) 2005. www.nice.org.uk/niceMedia/pdf/physical_activity_adults_eb.pdf (accessed 22 March 2012).
- Di LC, Fanelli C, Lucidi P, Murdolo G, De CA, Parlanti N, et al. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care 2003;26:404-8.
- Ogilvie D, Foster CE, Rothnie H, Cavill N, Hamilton V, Fitzsimons CF, et al. Interventions to promote walking: systematic review. BMJ 2007;334.
- Bhopal R. Glossary of terms relating to ethnicity and race: for reflection and debate. J Epidemiol Community Health 2004;58:441-5.
- Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart 1997;78:555-63.
- McKeigue PM, Pierpoint T, Ferrie JE, Marmot MG. Relationship of glucose intolerance and hyperinsulinaemia to body fat pattern in south Asians and Europeans. Diabetologia 1992;35:785-91.
- Barnett AH, Dixon AN, Bellary S, Hanif MW, O’hare JP, Raymond NT, et al. Type 2 diabetes and cardiovascular risk in the UK south Asian community. Diabetologia 2006;49:2234-46.
- Gulliford MC, Mejia A. Trends in diabetes mellitus in Greater London 1991–2011: associations with ethnicity. Diabet Med 1999;16:174-5.
- Davies MJ, Ammari F, Sheriff C, Burden ML, Gujral J, Burden AC. Screening for type 2 diabetes mellitus in the UK Indo-Asian population. Diabet Med 1999;16:131-7.
- Mather HM, Keen H. The Southall Diabetes Survey: prevalence of known diabetes in Asians and Europeans. Br Med J 1985;291:1081-4.
- Riste L, Khan F, Cruickshank K. High prevalence of type 2 diabetes in all ethnic groups, including Europeans, in a British inner city: relative poverty, history, inactivity, or 21st century Europe?. Diabetes Care 2001;24:1377-83.
- Chowdhury TA, Hitman GA. Type 2 diabetes in people of South Asian origin: Potential strategies for prevention. Br J Diabetes Vasc Dis 2007;7:279-82.
- Mukhopadhyay B. Diabetes and cardiac disease in South Asians. Br J Diabetes Vasc Dis 2005;5:253-9.
- UKPDS Study Group . UKPDS 12: Differences between Asian, Afro-Caribbean and white Caucasian type 2 diabetic patients at diagnosis of diabetes. UK Prospective Diabetes Study Group. Diabet Med 1994;11:670-7.
- Nicholl CG, Levy JC, Mohan V, Rao PV, Mather HM. Asian diabetes in Britain: a clinical profile. Diabet Med 1986;3:257-60.
- Whincup PH, Gilg JA, Owen CG, Odoki K, Alberti KG, Cook DG. British South Asians aged 13–16 years have higher fasting glucose and insulin levels than Europeans. Diabet Med 2005;22:1275-7.
- Simmons D, Williams DR, Powell MJ. Prevalence of diabetes in a predominantly Asian community: preliminary findings of the Coventry diabetes study. BMJ 1989;298:18-21.
- Cruickshank JK, Cooper J, Burnett M, MacDuff J, Drubra U. Ethnic differences in fasting plasma C-peptide and insulin in relation to glucose tolerance and blood pressure. Lancet 1991;338:842-7.
- Simmons D, Williams DR, Powell MJ. Prevalence of diabetes in different regional and religious south Asian communities in Coventry. Diabet Med 1992;9:428-31.
- Harris TJ, Cook DG, Wicks PD, Cappuccio FP. Impact of the new American Diabetes Association and World Health Organisation diagnostic criteria for diabetes on subjects from three ethnic groups living in the UK. Nutr Metab Cardiovasc Dis 2000;10:305-9.
- Potts J, Simmons D. Sex and ethnic group differences in fat distribution in young United Kingdom South Asians and Europids. J Clin Epidemiol 1994;47:837-41.
- Chowdhury TA, Lasker SS. Complications and cardiovascular risk factors in South Asians and Europeans with early-onset type 2 diabetes. QJM 2002;95:241-6.
- Yajnik CS, Joglekar CV, Lubree HG, Rege SS, Naik SS, Bhat DS, et al. Adiposity, inflammation and hyperglycaemia in rural and urban Indian men: Coronary Risk of Insulin Sensitivity in Indian Subjects (CRISIS) Study. Diabetologia 2008;51:39-46.
- Samanta A, Burden AC, Jagger C. A comparison of the clinical features and vascular complications of diabetes between migrant Asians and Caucasians in Leicester, UK. Diabetes Res Clin Pract 1991;14:205-13.
- Chowdhury TA, Lasker SS, Mahfuz R. Ethnic differences in control of cardiovascular risk factors in patients with type 2 diabetes attending an Inner London diabetes clinic. Postgrad Med J 2006;82:211-15.
- Grace C, Begum R, Subhani S, Kopelman P, Greenhalgh T. Prevention of type 2 diabetes in British Bangladeshis: qualitative study of community, religious, and professional perspectives. BMJ 2008;337.
- Ramaiya KL, Denver E, Yudkin JS. Diabetes, impaired glucose tolerance and cardiovascular disease risk factors in the Asian Indian Bhatia community living in Tanzania and in the United Kingdom. Diabet Med 1995;12:904-10.
- Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS, et al. Impact of migration on coronary heart disease risk factors: comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis 2006;185:297-306.
- Fischbacher CM, Hunt S, Alexander L. How physically active are South Asians in the United Kingdom? A literature review. J Public Health 2004;26:250-8.
- Carroll R, Ali N, Azam N. Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’. Health Technol Assess 2002;6.
- Bhopal R, Vettini A, Hunt S, Wiebe S, Hanna L, Amos A. Review of prevalence data in, and evaluation of methods for cross cultural adaptation of, UK surveys on tobacco and alcohol in ethnic minority groups. BMJ 2004;328.
- Burden AC. State of the Art Lecture. Rolling stone. The 2007 Arnold Bloom Lecture. Practical Diabetes Int 2007;24:377-83.
- Knight TM, Smith Z, Whittles A, Sahota P, Lockton JA, Hogg G, et al. Insulin resistance, diabetes, and risk markers for ischaemic heart disease in Asian men and non-Asian in Bradford. Heart 1992;67:343-50.
- Mukhopadhyay B, Forouhi NG, Fisher BM, Kesson CM, Sattar N. A comparison of glycaemic and metabolic control over time among South Asian and European patients with type 2 diabetes: results from follow-up in a routine diabetes clinic. Diabet Med 2006;23:94-8.
- Lawton J, Ahmad N, Hanna L, Douglas M, Hallowell N. Diabetes service provision: a qualitative study of the experiences and views of Pakistani and Indian patients with type 2 diabetes. Diabet Med 2006;23:1003-7.
- Mather HM. Diabetes in elderly Asians. J R Soc Med 1994;87:615-16.
- Rankin J, Bhopal R. Understanding of heart disease and diabetes in a South Asian community: cross-sectional study testing the ‘snowball’ sample method. Public Health 2001;115:253-60.
- Mather HM, Chaturvedi N, Fuller JH. Mortality and morbidity from diabetes in South Asians and Europeans: 11-year follow-up of the Southall Diabetes Survey, London, UK. Diabet Med 1998;15:53-9.
- Mather HM, Chaturvedi N, Kehely AM. Comparison of prevalence and risk factors for microalbuminuria in South Asians and Europeans with type 2 diabetes mellitus. Diabet Med 1998;15:672-7.
- Bhopal R, Unwin N, White M, Yallop J, Walker L, Alberti KG, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ 1999;319:215-20.
- Oldroyd JC, Yallop J, Fischbacher C, Bhopal R, Chamley J, Ayis S, et al. Transient and persistent impaired glucose tolerance and progression to diabetes in South Asians and Europeans: new, large studies are a priority. Diabet Med 2007;24:98-9.
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289-97.
- Chaturvedi N, Jarrett J, Morrish N, Keen H, Fuller JH. Differences in mortality and morbidity in African Caribbean and European people with non-insulin dependent diabetes mellitus: results of 20 year follow up of a London cohort of a multinational study. BMJ 1996;313:848-52.
- Odugbesan O, Rowe B, Fletcher J, Walford S, Barnett AH. Diabetes in the UK West indian community: the Wolverhampton survey. Diabet Med 1989;6:48-52.
- Cooper RS, Rotimi CN, Kaufman JS, Owoaje EE, Fraser H, Forrester T, et al. Prevalence of NIDDM among populations of the African diaspora. Diabetes Care 1997;20:343-8.
- Mbanya JC, Cruickshank JK, Forrester T, Balkau B, Ngogang JY, Riste L, et al. Standardized comparison of glucose intolerance in west African-origin populations of rural and urban Cameroon, Jamaica, and Caribbean migrants to Britain. Diabetes Care 1999;22:434-40.
- Centre for Reviews and Dissemination . CRD’s Guidance for Undertaking Reviews in Health Care 2008. www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf (accessed 22 March 2012).
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334.
- Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst Rev 2005;2.
- Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Schmid CH, et al. Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. Am J Prev Med 2005;28:126-39.
- Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2005;28:2780-6.
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50.
- Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H. Dietary advice for the prevention of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev 2008;3.
- Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roque IF, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev 2008;3.
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152-62.
- Liao D, Asberry PJ, Shofer JB, Callahan H, Matthys C, Boyko EJ, et al. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care 2002;25:1504-10.
- Mensink M, Blaak EE, Corpeleijn E, Saris WH, de Bruin TW, Feskens EJ. Lifestyle intervention according to general recommendations improves glucose tolerance. Obes Res 2003;11:1588-96.
- Oldroyd JC, Unwin NC, White M, Mathers JC, Alberti KG. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Res Clin Pract 2006;72:117-27.
- Pan X-R, Li G-W, Hu Y-H, Wang J-X, Yang W-Y, An Z-X, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and diabetes study. Diabetes Care 1997;20:537-44.
- Wein P, Beischer N, Harris C, Permezel M. A trial of simple versus intensified dietary modification for prevention of progression to diabetes mellitus in women with impaired glucose tolerance. Aus N Z J Obstet Gynaecol 1999;39:162-6.
- Diabetes Prevention Program Research Group . Diabetes Prevention Program Study Documents and Website 2012. www.bsc.gwu.edu/dpp/index.htmlvdoc (accessed 22 March 2012).
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102-7.
- Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 2006;61:1075-81.
- Diabetes Prevention Program Research Group . The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137-44.
- Delahanty LM, Conroy MB, Nathan DM. Diabetes Prevention Program Research Group . Psychological predictors of physical activity in the diabetes prevention program. J Am Diet Assoc 2006;106:698-705.
- Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142:611-19.
- Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, et al. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes 2007;56:1680-5.
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, . Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677-86.
- Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF. Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583-8.
- Perreault L, Ma Y, Dagogo-Jack S, Horton E, Marrero D, Crandall J, et al. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care 2008;31:1416-21.
- Goldberg RB, Temprosa M, Haffner S, Orchard TJ, Ratner RE, Fowler SE, et al. Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care 2009;32:726-32.
- Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes 2007;56:1153-9.
- Ackermann RT, Edelstein SL, Narayan KMV, Zhang P, Engelgau MM, Herman WH, et al. Changes in Health State Utilities With Changes in Body Mass in the Diabetes Prevention Program. Obesity 2009;17:2176-81.
- Eriksson J, Lindström J, Valle T, Aunola S, Hamalainen H, Ilanne-Parikka P, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999;42:793-801.
- Hamalainen H, Ronnemaa T, Virtanen A, Lindström J, Eriksson JG, Valle TT, et al. Improved fibrinolysis by an intensive lifestyle intervention in subjects with impaired glucose tolerance. The Finnish Diabetes Prevention Study. Diabetologia 2005;48:2248-53.
- Herder C, Peltonen M, Koenig W, Kraft I, Muller-Scholze S, Martin S, et al. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes 2006;55:2340-6.
- Ilanne-Parikka P, Eriksson JG, Lindström J, Peltonen M, Aunola S, Hamalainen H, et al. Effect of Lifestyle Intervention on the Occurrence of Metabolic Syndrome and its Components in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:805-7.
- Ilanne-Parikka P, Eriksson JG, Lindström J, Hamalainen H, Keinanen-Kiukaanniemi S, Laakso M, et al. Prevalence of the metabolic syndrome and its components: findings from a Finnish general population sample and the Diabetes Prevention Study cohort. Diabetes Care 2004;27:2135-40.
- Laaksonen DE, Lindström J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 2005;54:158-65.
- Laaksonen DE, Siitonen N, Lindström J, Eriksson JG, Reunanen P, Tuomilehto J, et al. Physical activity, diet, and incident diabetes in relation to an ADRA2B polymorphism. Med Sci Sports Exerc 2007;39:227-32.
- Lindström J, Peltonen M, Eriksson JG, Aunola S, Hamalainen H, Ilanne-Parikka P, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008.
- Lindström J, Peltonen M, Eriksson JG, Louheranta A, Fogelholm M, Uusitupa M, et al. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia 2006;49:912-20.
- Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673-9.
- Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230-6.
- Ottelin AM, Lindström J, Peltonen M, Martikainen J, Uusitupa M, Gylling H, et al. Costs of a self-selected, health-promoting diet among the participants of the Finnish Diabetes Prevention Study. Diabetes Care 2007;30:1275-7.
- Siitonen N, Lindström J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Association between a deletion/insertion polymorphism in the alpha2B-adrenergic receptor gene and insulin secretion and type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia 2004;47:1416-24.
- Uusitupa M, Lindi V, Louheranta A, Salopuro T, Lindström J, Tuomilehto J, et al. Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes 2003;52:2532-8.
- Uusitupa M, Louheranta A, Lindström J, Valle T, Sundvall J, Eriksson J, et al. The Finnish Diabetes Prevention Study. Br J Nutr 2000;83:S137-42.
- Wikstrom K, Peltonen M, Eriksson JG, Aunola S, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, et al. Educational attainment and effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Res Clin Pract 2009;86:e1-5.
- Lindgren P, Lindström J, Tuomilehto J, Uusitupa M, Peltonen M, Jonsson B, et al. Lifestyle intervention to prevent diabetes in men and women with impaired glucose tolerance is cost-effective. Int J Technol Assess Health Care 2007;23:177-83.
- Uusitupa M, Peltonen M, Lindström J, Aunola S, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, et al. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study: secondary analysis of the randomized trial. PLoS ONE 2009;4.
- Li G, Hu Y, Yang W, Jiang Y, Wang J, Xiao J, et al. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the DA Qing IGT and Diabetes Study. Diabetes Res Clin Pract 2002;58:193-200.
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783-9.
- Gong Q, Gregg EW, Wang J, An Y, Zhang P, Yang W, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300-7.
- Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost Effectiveness of the Interventions in the Primary Prevention of Diabetes among Asian Indians: Within trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care 2007;30:2548-52.
- Ramachandran A. Metabolic syndrome does not increase the risk of conversion of impaired glucose tolerance to diabetes in Asian Indians–Result of Indian diabetes prevention programme. Diabetes Res Clin Pract 2007;76:215-18.
- Ramachandran A, Snehalatha C, Mukesh B, Simon M, Kumar CK, Vijay V. Persistent impaired glucose tolerance has similar rate of risk factors as for diabetes–results of Indian diabetes prevention programme (IDPP). Diabetes Res Clin Pract 2006;73:100-3.
- Ramachandran A, Arun N, Shetty AS, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP-1 and IDPP-2). Diabetes Care 2010;33:2164-8.
- Snehalatha C, Mary S, Selvam S, Sathish Kumar CK, Shetty SB, Nanditha A, et al. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme-1 (IDPP-1). Diabetes Care 2009;32:1796-801.
- Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, de Bruin TW, et al. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia 2006;49:2312-409.
- Corpeleijn E, Feskens EJ, Jansen EH, Mensink M, Saris WH, Blaak EE. Lifestyle intervention and adipokine levels in subjects at high risk for type 2 diabetes: the Study on Lifestyle intervention and Impaired glucose tolerance Maastricht (SLIM). Diabetes Care 2007;30:3125-7.
- Mensink M, Corpeleijn E, Feskens EJ, Kruijshoop M, Saris WH, de Bruin TW, et al. Study on lifestyle-intervention and impaired glucose tolerance Maastricht (SLIM): design and screening results. Diabetes Res Clin Pract 2003;61:49-58.
- Mensink M, Feskens EJ, Saris WH, de Bruin TW, Blaak EE. Study on Lifestyle Intervention and Impaired Glucose Tolerance Maastricht (SLIM): preliminary results after one year. Int J Obesity 2003;27:377-84.
- Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med 2008;25:597-605.
- Oldroyd JC, Unwin NC, White M, Imrie K, Mathers JC, Alberti KG. Randomised controlled trial evaluating the effectiveness of behavioural interventions to modify cardiovascular risk factors in men and women with impaired glucose tolerance: outcomes at 6 months. Diabetes Res Clin Pract 2001;52:29-43.
- Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9.
- Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21:350-9.
- Page RC, Harnden KE, Cook JT, Turner RC. Can life-styles of subjects with impaired glucose tolerance be changed? A feasibility study. Diabet Med 1992;9:562-6.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Caro JJ, Getsios D, Caro I, Klittich WS, O’Brien JA. Economic evaluation of therapeutic interventions to prevent type 2 diabetes in Canada. Diabet Med 2004;21:1229-36.
- Palmer AJ, Roze S, Valentine WJ, Spinas GA, Shaw JE, Zimmet PZ. Intensive lifestyle changes or metformin in patients with impaired glucose tolerance: modeling the long-term health economic implications of the diabetes prevention program in Australia, France, Germany, Switzerland, and the United Kingdom. Clin Ther 2004;26:304-21.
- Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323-32.
- Segal L, Dalton AC, Richardson J. Cost-effectiveness of the primary prevention of non-insulin dependent diabetes mellitus. Health Promot Int 1998;13:197-209.
- McEwan P, Peters JR, Currie CJ. Evaluation of the clinical outcome and financial costs of delaying the onset of frank type 2 diabetes. Diabetes 2005;54.
- Ackermann RT, Marrero DG, Hicks KA, Hoerger TJ, Sorensen S, Zhang P, et al. An evaluation of cost sharing to finance a diet and physical activity intervention to prevent diabetes. Diabetes Care 2006;29:1237-41.
- Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251-64.
- Eddy DM, Schlessinger L. Archimedes: a trial-validated model of diabetes. Diabetes Care 2003;26:3093-101.
- Vijgen SMC, Hoogendoorn M, Baan CA, De Wit GA, Limburg W, Feenstra TL. Cost effectiveness of preventive interventions in type 2 diabetes mellitus: a systematic literature review. Pharmacoeconomics 2006;24:425-41.
- Wylie-Rosett J, Herman WH, Goldberg RB. Lifestyle intervention to prevent diabetes: intensive and cost effective. Curr Opin Lipidol 2006;17:37-44.
- Eddy DM, Schlessinger L. Validation of the archimedes diabetes model. Diabetes Care 2003;26:3102-10.
- Schlessinger L, Eddy DM. Archimedes: a new model for simulating health care systems: the mathematical formulation. J Biomed Inform 2002;35:37-50.
- Diabetes Prevention Program Research Group . Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518-23.
- Benjamin SM, Valdez R, Geiss LS, Rolka DB, Narayan KM. Estimated number of adults with prediabetes in the US in 2000: opportunities for prevention. Diabetes Care 2003;26:645-9.
- Engelgau MM. Trying to predict the future for people with diabetes: a tough but important task. Ann Intern Med 2005;143:301-2.
- Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20–74 yr. Diabetes 1987;36:523-34.
- Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes 2004;53:160-5.
- Johnson FR, Clayton LJ, Manjunath R, Hoerger TJ, Mansfield CA, Zhang P. High risk individuals willingness to pay for diabetes risk reduction programs. Diabetes Care 2006;29:1351-6.
- Jacobs-van der Bruggen MA, Bos G, Bemelmans WJ, Hoogenveen RT, Vijgen SM, Baan CA. Lifestyle interventions are cost-effective in people with different levels of diabetes risk: results from a modeling study. Diabetes Care 2007;30:128-34.
- Schuit AJ, Wendel-Vos GC, Verschuren WM, Ronckers ET, Ament A, Van AP, et al. Effect of 5-year community intervention Hartslag Limburg on cardiovascular risk factors. Am J Prev Med 2006;30:237-42.
- Hoerger TJ, Hicks KA, Sorensen SW, Herman WH, Ratner RE, Ackermann RT, et al. Cost-effectiveness of screening for pre-diabetes among overweight and obese US adults. Diabetes Care 2007;30:2874-9.
- Icks A, Rathmann W, Haastert B, Gandjour A, Holle R, John J, et al. Clinical and cost-effectiveness of primary prevention of type 2 diabetes in a ‘real world’ routine healthcare setting: model based on the KORA Survey 2000. Diabet Med 2007;24:473-80.
- Saha S, Gerdtham UG, Johansson P. Economic evaluation of lifestyle interventions for preventing diabetes and cardiovascular diseases. Int J Environ Res Public Health 2010;7:3150-95.
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8.
- Irvine L, Barton GR, Gasper AV, Murray N, Clark A, Scarpello T, et al. Cost-effectiveness of a lifestyle intervention in preventing type 2 diabetes. Int J Technol Assess Health Care 2011;27:275-82.
- Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ 2007;33:69-.
- Bertram MY, Lim SS, Barendregt JJ, Vos T. Assessing the cost-effectiveness of drug and lifestyle intervention following opportunistic screening for pre-diabetes in primary care. Diabetologia 2010;53:875-81.
- Colagiuri S, Walker AE. Using an economic model of diabetes to evaluate prevention and care strategies in Australia. Health Affairs 2008;27:256-68.
- Colagiuri S, Cull CA, Holman RR. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: UK Prospective Diabetes Study 61. Diabetes Care 2002;25:1410-17.
- Johansson P, Ostenson CG, Hilding AM, Andersson C, Rehnberg C, Tillgren P. A cost-effectiveness analysis of a community-based diabetes prevention program in Sweden. Int J Technol Assess Health Care 2009;25:350-8.
- Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010;33:1872-94.
- Zhuo X, Zhang P, Gregg EW, Barker L, Hoerger TJ, Pearson-Clarke T, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save $5.7 billion in 25 years. Health Affairs 2012;31:50-6.
- Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35:357-63.
- General Practice Research Database (GPRD) . Observational Data Representative of the UK Population 2007. www.gprd.com/academia/primarycare.asp (accessed 22 March 2012).
- Jick SS, Bradbury BD. Statins and newly diagnosed diabetes. Br J Clin Pharmacol 2004;58:303-9.
- Wood SL, Jick H, Sauve R. The risk of stillbirth in pregnancies before and after the onset of diabetes. Diabet Med 2003;20:703-7.
- Kornegay CJ, Vasilakis-Scaramozza C, Jick H. Incident diabetes associated with antipsychotic use in the United Kingdom general practice research database. J Clin Psychiatry 2002;63:758-62.
- Jick SS, Stender M, Myers MW. Frequency of liver disease in type 2 diabetic patients treated with oral antidiabetic agents. Diabetes Care 1999;22:2067-71.
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia 2006;49:660-6.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006;23:516-21.
- Gulliford MC, Charlton J, Latinovic R. Increased utilization of primary care 5 years before diagnosis of type 2 diabetes: a matched cohort study. Diabetes Care 2005;28:47-52.
- Jick SS, Kaye JA, Vasilakis-Scaramozza C, Garcia Rodriguez LA, Ruigomez A, Meier CR, et al. Validity of the general practice research database. Pharmacotherapy 2003;23:686-9.
- Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Copley-Merriman C, Maier W, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997;20:735-44.
- Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, et al. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke 2002;33:1776-81.
- Stevens RJ, Kothari V, Adler AI, Stratton IM. United Kingdom Prospective Diabetes Study (UKPDS) Group . The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci 2001;101:671-9.
- Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care 2004;27:201-7.
- Tan HH, McAlpine RR, James P, Thompson P, McMurdo ME, Morris AD, et al. Diagnosis of type 2 diabetes at an older age: effect on mortality in men and women. Diabetes Care 2004;27:2797-9.
- Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and nutrition (EPIC-Norfolk). BMJ 2001;322:15-8.
- Woerle HJ, Pimenta WP, Meyer C, Gosmanov NR, Szoke E, Szombathy T, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med 2004;164:1627-32.
- Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 2001;24:447-53.
- American Diabetes Association . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-97.
- Valensi P, Schwarz EH, Hall M, Felton AM, Maldonato A, Mathieu C. Pre-diabetes essential action: a European perspective. Diabetes Metab 2005;31:606-20.
- de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001;285:2109-13.
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753-9.
- Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer. Health Technol Assess 2001;5.
- Walker EA, Molitch M, Kramer MK, Kahn S, Ma Y, Edelstein S, et al. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care 2006;29:1997-2002.
- Swinburn BA, Metcalf PA, Ley SJ. Long-term (5-year) effects of a reduced-fat diet intervention in individuals with glucose intolerance. Diabetes Care 2001;24:619-24.
- Curtis L. Unit Costs of Health and Social Care 2007. www.pssru.ac.uk/project-pages/unit-costs/2007/index.php (accessed 22 March 2012).
- On-line currency conversion 2008. http://currencyconverter.uk.com/ (accessed 22 March 2012).
- Nichols G. Preventing and Predicting Diabetes. Medscape Report on 2007 American Diabetes Association (ADA) 67th Scientific Sessions 2007. www.medscape.com/viewarticle/560724_2 (accessed 22 March 2012).
- Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451-63.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2007. www.bnf.org/bnf/ (accessed 22 March 2012).
- Kopjar B, Sales AE, Pineros SL, Sun H, Li YF, Hedeen AN. Adherence with statin therapy in secondary prevention of coronary heart disease in veterans administration male population. Am J Cardiol 2003;92:1106-8.
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326.
- Ward S, Lloyd JM, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11.
- Costa J, Borges M, David C, Vaz CA. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ 2006;332:1115-24.
- Penning-van Beest FJ, Termorshuizen F, Goettsch WG, Klungel OH, Kastelein JJ, Herings RM. Adherence to evidence-based statin guidelines reduces the risk of hospitalizations for acute myocardial infarction by 40%: a cohort study. Eur Heart J 2007;28:154-9.
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427-43.
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217-30.
- Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238-43.
- Warren E, Brennan A, Akehurst R. Cost-effectiveness of sibutramine in the treatment of obesity. Med Decis Making 2004;24:9-19.
- O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G. The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment. Health Technol Assess 2002;6.
- Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health 2008;11:478-86.
- Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet 2008;371:736-43.
- Quality framework at GPcontract.info . National Quality and Outcomes Framework Incorporated into 2004 GP Contract 2004. www.gpcontract.info/quality/qualitydiabetes.htm (accessed 22 March 2012).
- Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with type 2 diabetes: a population-based analysis in the UK. Diabet Med 2007;24:1412-18.
- Douek IF, Allen SE, Ewings P, Gale EA, Bingley PJ. the Metformin Trial Group . Continuing metformin when starting insulin in patients with type 2 diabetes: a double-blind randomized placebo-controlled trial. Diabet Med 2005;22:634-40.
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160-7.
- Donnelly LA, Morris AD, Evans JM. Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM 2007;100:345-50.
- Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS 65). Diabet Med 2003;20:442-50.
- Chambers M, Hutton J, Gladman J. Cost-effectiveness analysis of antiplatelet therapy in the prevention of recurrent stroke in the UK. Aspirin, dipyridamole and aspirin-dipyridamole. Pharmacoeconomics 1999;16:577-93.
- NHS Blood and Transplant . Cost-Effectiveness of Transplantation 2009. www.uktransplant.org.uk/ukt/newsroom/fact_sheets/cost_effectiveness_of_transplantation.jsp (accessed 1 February 2012).
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003;26:1790-5.
- Palmer AJ, Weiss C, Sendi PP, Neeser K, Brandt A, Singh G, et al. The cost-effectiveness of different management strategies for type I diabetes: a Swiss perspective. Diabetologia 2000;43:13-26.
- Heaton A, Martin S, Brelje T. The economic effect of hypoglycemia in a health plan. Manag Care Interface 2003;16:23-7.
- UK National Screening Committee (NSC) . The UK NSC Policy on Diabetic Retinopathy Screening in Adults 2012. www.screening.nhs.uk/diabeticretinopathy (accessed 1 February 2012).
- Gray A, Clarke P, Farmer A, Holman R. Implementing intensive control of blood glucose concentration and blood pressure in type 2 diabetes in England: cost analysis (UKPDS 63). BMJ 2002;325.
- James M, Turner DA, Broadbent DM, Vora J, Harding SP. Cost effectiveness analysis of screening for sight threatening diabetic eye disease. BMJ 2000;320:1627-31.
- British National Formulary 2007. www.bnf.org/bnf/index.htm (accessed 1 February 2012).
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9.
- Mount Hood 4 Modeling Group . Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638-46.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89.
- Absetz P, Valve R, Oldenburg B, Heinonen H, Nissinen A, Fogelholm M, et al. Type 2 diabetes prevention in the ‘real world’: one-year results of the GOAL Implementation Trial. Diabetes Care 2007;30:2465-70.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335.
- Vermeire E, Wens J, Van RP, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2.
- Davies MJ, Tringham JR, Troughton J, Khunti KK. Prevention of type 2 diabetes mellitus. A review of the evidence and its application in a UK setting. Diabet Med 2004;21:403-14.
- Hussain A, Claussen B, Ramachandran A, Williams R. Prevention of type 2 diabetes: a review. Diabetes Res Clin Pract 2007;76:317-26.
- Inzucchi SE, Sherwin RS. The prevention of type 2 diabetes mellitus. Endocrinol Metab Clin North Am 2005;34:199-21.
- Curtis J, Wilson C. Preventing type 2 diabetes mellitus. J Am Board Fam Med 2005;18:37-43.
- Simpson RW, Shaw JE, Zimmet PZ. The prevention of type 2 diabetes: lifestyle change or pharmacotherapy? A challenge for the 21st century. Diabetes Res Clin Pract 2003;59:165-80.
- Zimmet P, Shaw J, Alberti KG. Preventing type 2 diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabet Med 2003;20:693-702.
- Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil 2011;18:813-23.
- Turner RC, Holman RR, Stratton IM, Cull CA, Matthews DR, Manley SE, et al. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65.
- Kriska AM, Edelstein SL, Hamman RF, Otto A, Bray GA, Mayer-Davis EJ, et al. Physical activity in individuals at risk for diabetes: diabetes prevention program. Med Sci Sports Exercise 2006;38:826-32.
- Dishman RK, Steinhardt M. Health locus of control predicts free-living, but not supervised, physical activity: a test of exercise-specific control and outcome-expectancy hypotheses. Res Q Exerc Sport 1990;61:383-94.
- Ruge T, Nystrom L, Lindahl B, Hallmans G, Norberg M, Weinehall L, et al. Recruiting high-risk individuals to a diabetes prevention program: how hard can it be?. Diabetes Care 2007;30.
- Berentzen T, Petersen L, Pedersen O, Black E, Astrup A, Sorensen TI. Long-term effects of leisure time physical activity on risk of insulin resistance and impaired glucose tolerance, allowing for body weight history, in Danish men. Diabet Med 2007;24:63-72.
- Borodulin K, Tuomilehto J, Peltonen M, Lakka TA, Sundvall J, Jousilahti P. Association of leisure time physical activity and abdominal obesity with fasting serum insulin and 2-h postchallenge plasma glucose levels. Diabet Med 2006;23:1025-8.
- Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an Urban Medically Underserved Community: A Non-Randomized Prospective Intervention Study. Diabetes Care 2008;31:684-9.
- Kilkkinen A, Heistaro S, Laatikainen T, Janus E, Chapman A, Absetz P, et al. Prevention of type 2 diabetes in a primary health care setting. Interim results from the Greater Green Triangle (GGT) Diabetes Prevention Project. Diabetes Res Clin Pract 2007;76:460-2.
- Uutela A, Absetz P, Nissinen A, Valve R, Talja M, Fogelholm M. Health psychological theory in promoting population health in Paijat-Hame, Finland: first steps toward a type 2 diabetes prevention study. J Health Psychol 2004;9:73-84.
- Finnish Diabetes Association . Dehko’s FIN-D2D Project 2012. www.diabetes.fi/en/finnish_diabetes_association/diabetes_programme_dehko/fin-d2d (accessed 22 March 2012).
- Saaristo T, Moilanen L, Korpi-Hyovalti E, Vanhala M, Saltevo J, Niskanen L, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146-51.
- Vanderwood KK, Hall TO, Harwell TS, Butcher MK, Helgerson SD. Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup . Implementing a state-based cardiovascular disease and diabetes prevention program. Diabetes Care 2010;33:2543-5.
- Simmons RK, Griffin SJ, Wareham NJ. Researching how to realize the potential of diabetes prevention. Diabet Med 2007;24:1055-7.
- Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care 2009;32:1418-20.
- Guthrie C. Prevention of type 2 diabetes. Health promotion helps no one. BMJ 2001;323.
- Truby H, Baic S, deLooy A, Fox KR, Livingstone MB, Logan CM, et al. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC ‘diet trials’. BMJ 2006;332:1309-14.
- Yates T, Khunti K, Bull F, Gorely T, Davies MJ. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia 2007;50:1116-26.
- Laaksonen DE, Lindström J, Tuomilehto J, Uusitupa M. Increased physical activity is a cornerstone in the prevention of type 2 diabetes in high-risk individuals. Diabetologia 2007;50:2607-8.
- Burns N, Finucane FM, Hatunic M, Gilman M, Murphy M, Gasparro D, et al. Early-onset type 2 diabetes in obese white subjects is characterised by a marked defect in beta cell insulin secretion, severe insulin resistance and a lack of response to aerobic exercise training. Diabetologia 2007;50:1500-8.
- Carnethon MR. Can we out-run the diabetes epidemic?. Diabetologia 2007;50:1113-15.
- Bo S, Ciccone G, Baldi C, Benini L, Dusio F, Forastiere G, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J Gen Intern Med 2007;22:1695-703.
- Information Centre . Statistics on Obesity, Physical Activity and Diet: England 2006. www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles/obesity/statistics-on-obesity-physical-activity-and-diet-england-2006 (accessed 22 March 2012).
- Simmons RK, Harding A-H, Jakes RW, Welch A, Wareham NJ, Griffin SJ. How much might achievement of diabetes prevention behaviour goals reduce the incidence of diabetes if implemented at the population level?. Diabetologia 2006;49:905-11.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Rationale, design and baseline data from the Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: a randomized controlled trial. Patient Educ Couns 2008;73:264-71.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 2009;32:1404-10.
- Yates T, Davies MJ, Sehmi S, Gorely T, Khunti K. The Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: are improvements in glucose regulation sustained at 2 years?. Diabet Med 2011;28:1268-71.
- Murray NJ, Abadi S, Blair A, Dunk M, Sampson MJ. The importance of type 2 diabetes prevention: The Norfolk Diabetes Prevention Study. Br J Diabetes Vasc Dis 2011;11:308-13.
- Helsinki University, The World Diabetes Foundation, Qingdao Municipal Center For Disease Control & Prevention . Qingdao Diabetes Prevention Project 2010. www.ClinicalTrials.gov/show/NCT01053195 (accessed 1 February 2012).
- Qiao Q, Pang Z, Gao W, Wang S, Dong Y, Zhang L, et al. A large-scale diabetes prevention program in real-life settings in Qingdao of China (2006–2012). Prim Care Diabetes 2010;4:99-103.
- The Royal Bournemouth Hospital . Lifestyle Intervention in Obese Non-Diabetic Adults With a Family History of Diabetes 2011. www.ClinicalTrials.gov/show/NCT00993603 (accessed 1 February 2012).
- Brescia University College . PREPARE: ‘Prediabetes Research and Education Promoting Activity and Responsible Eating’ 2011. www.ClinicalTrials.gov/show/NCT01396772 (accessed 1 February 2012).
- University of Rochester . Comparing Diabetes Prevention Programs 2011. www.ClinicalTrials.gov/show/NCT01409889 (accessed 1 February 2012).
- American Heart Association (AHA) . Adaptation of the Diabetes Prevention Program for Primary Care n.d. www.ClinicalTrials.gov/show/NCT01220089 (accessed 1 February 2012).
- Mount Sinai School of Medicine, North General Hospital NY, Albert Einstein College of Medicine of Yeshiva University . Effectiveness Study of Community-Based, Peer-Led Education on Weight Loss and Diabetes 2011. www.ClinicalTrials.gov/show/NCT01004848 (accessed 1 February 2012).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute on Aging (NIA), The Office of Research on Minority Health and Health Disparities, Office of Women’s Health, Indian Health Service . Diabetes Prevention Program Outcomes Study 2011. www.ClinicalTrials.gov/show/NCT00038727 (accessed 1 February 2012).
- Emory University, American Diabetes Association (ADA), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) . A Culturally Tailored Lifestyle Intervention to Prevent Diabetes in South Asians 2011. www.ClinicalTrials.gov/show/NCT01084928 (accessed 1 February 2012).
- Jordi G. Instituto de Salud Carlos III, Departament de Salut de la General . Diabetes in Europe – Prevention Using Lifestyle, Physical Activity and Nutritional Intervention in Catalonia 2012. www.ClinicalTrials.gov/show/NCT01519505 (accessed 1 February 2012).
- Basque Health Service . Effectiveness of the DE-PLAN Program for the Primary Prevention of Type 2 Diabetes in Routine Primary Care 2011. www.ClinicalTrials.gov/show/NCT01365013 (accessed 1 February 2012).
- Gill JM, Bhopal R, Douglas A, Wallia S, Bhopal R, Sheikh A, et al. Sitting time and waist circumference are associated with glycemia in U.K. South Asians: data from 1,228 adults screened for the PODOSA trial. Diabetes Care 2011;34:1214-18.
- Douglas A, Bhopal RS, Bhopal R, Forbes JF, Gill JM, Lawton J, et al. Recruiting South Asians to a lifestyle intervention trial: experiences and lessons from PODOSA (Prevention of Diabetes & Obesity in South Asians). Trials 2011;12.
- Samanta A, Burden AC, Fent B. Comparative prevalence of non-insulin-dependent diabetes mellitus in Asian and white Caucasian adults. Diabetes Res Clin Pract 1987;4:1-6.
- Matthews DR, Charbonnel BH, Hanefeld M, Brunetti P, Schernthaner G. Long-term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with type 2 diabetes: a randomized, comparative study. Diabetes Metab Res Rev 2005;21:167-74.
- Feinglos M, Dailey G, Cefalu W, Osei K, Tayek J, Canovatchel W, et al. Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM. Diabetes Res Clin Pract 2005;68:167-75.
- Charpentier G, Fleury F, Kabir M, Vaur L, Halimi S. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med 2001;18:828-34.
- Schernthaner G, Grimaldi A, Di MU, Drzewoski J, Kempler P, Kvapil M, et al. GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest 2004;34:535-42.
- Taylor R, Davies R, Fox C, Sampson M, Weaver JU, Wood L. Appropriate insulin regimes for type 2 diabetes: a multicenter randomized crossover study. Diabetes Care 2000;23:1612-18.
- Peacock I, Tattersall RB. The difficult choice of treatment for poorly controlled maturity onset diabetes: tablets or insulin?. Br Med J 1984;288:1956-9.
- Chandalia HB. Weight gain in type 2 diabetics with different treatment modalities. Metab Syndr Relat Dis 2005;3:130-6.
- Aas AM, Bergstad I, Thorsby PM, Johannesen O, Solberg M, Birkeland KI. An intensified lifestyle intervention programme may be superior to insulin treatment in poorly controlled type 2 diabetic patients on oral hypoglycaemic agents: results of a feasibility study. Diabet Med 2005;22:316-22.
- Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Jarvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care 2005;28:254-9.
- Riddle MC, Rosenstock J, Gerich J, Insulin G. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-6.
- Larger E, Rufat P, Dubois-Laforgue D, Ledoux S. Insulin therapy does not itself induce weight gain in patients with type 2 diabetes. Diabetes Care 2001;24:1849-50.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999;281:2005-12.
Appendix 1 Prevalence of type 2 diabetes mellitus in ethnic minorities
| Author and year | Mode of assessment | Results | Reasons for differences and other relevant data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mather et al. 1985141 | Self-reported questionnaires and ascertained from local diabetic clinics | Asians | Europeans | Afro-Caribbeans |
Seven times as many Asians as Europeans had been diagnosed between the ages of 30 and 54 years but similar numbers were diagnosed at < 25 years BMI were the same in Asian and European populations. Significant differences in BMI between men and women in Asians but not in Europeans Mortality from circulatory disease and ischaemic heart disease was 1.80 and 2.02 times higher, respectively, in South Asians compared with Europeans in those aged 30–64 years at baseline (p < 0.05) MI requiring hospital admissions and history of laser therapy for retinopathy are higher in South Asians than Europeans No difference was found between Asians and Europeans in stroke, hypertension and amputation |
||||
| Sample size | 34,230 | 27,075 | 3780 | ||||||
| No. of diabetics | 761 | 324 | 44 | ||||||
| Males/females | 453/308 | 162/162 | |||||||
| Overall prevalence (1981 Census) (%) | 6.4 | 1.2 (5.3 times higher in Asians) | |||||||
| 3 years’ Census adjustment (%) | 4.6 | 1.2 (3.8 times higher in Asians) | |||||||
| Men/women (%) | 2.6/1.9 | 1.2 | |||||||
| Age at diabetes mellitus diagnosis | < 25 years | 22 | 27 | ||||||
| 30–54 years | 523 | 76 | |||||||
| BMI (kg/m2) (n) | 25.7 ± 3.9 (n = 424) | 25.6 ± 4.3 (n = 232) | |||||||
| Men/women | 25.2 ± 3.7/26.7 ± 4.3 (p < 0.001) | 25.5 ± 3.9/25.7 ± 4.8 (NS) | |||||||
| Mather et al. 1998169 (11-year follow-up study) | Mortality ascertained by death certificates and questionnaire survey for morbidity | Asians | Europeans | Rate ratio (95% CI) | |||||
| Sample size (diagnosed with diabetes mellitus in 1984) | 730 | 304 | |||||||
| Mean age in 1984 (years) | 55 | 67 | |||||||
| Deaths | 242 (33%) | 172 (57%) | |||||||
| All-cause mortality rate (number and rate) | 30–54 years | 72/386 (18.4) | 8/57 (13.5) | 150 (0.72 to 3.12) | |||||
| 55–64 years | 95/220 (49.4) | 22/63 (39.3) | 120 (0.75 to 0.91) | ||||||
| 65–74 years | 56/97 (75.4) | 61/94 (87.3) | 0.90 (0.62 to 1.31) | ||||||
| 75+ years | 19/27 (116.3) | 81/90 (200.3) | 0.53 (0.31 to 0.89) | ||||||
| Morbidity ( n /%) | OR | p -value | |||||||
| Asians | Europeans | ||||||||
| Sample size | 461 | 129 | |||||||
| MI | 82 (20) | 9 (8) | 3.8 (1.8 to 8.0) | 0.001 | |||||
| Stroke | 53 (13) | 11 (9) | 1.9 (0.9 to 3.9) | 0.1 | |||||
| Renal disease | 10 (2) | 2 (2) | 1.8 (0.3 to 9.3) | 0.5 | |||||
| Laser treatment for retinopathy | 149 (36) | 32 (27) | 1.7 (1.1to 2.8) | 0.03 | |||||
| Hypertension | 189 (45) | 47 (40) | 1.3 (0.8 to 2.0) | 0.2 | |||||
| Amputation of leg or foot | 12 (3) | 3 (3) | 1.0 (0.3 to 3.9) | 0.9 | |||||
| Samanta et al. 1987385 | Symptoms and doctor diagnosis | Asians | White Caucasians | Prevalence of T2DM is significantly higher in Asians in all age bands, approximately twice as high as in white Caucasians | |||||
| Sample size | 20,053 | 18,068 | |||||||
| Asians | Caucasians | RR (95% CI) | |||||||
| 16- to 29-year-olds | |||||||||
| No. with diabetes | 12/9438 | 0/4619 | |||||||
| Prevalence (%) | 0.1 | – | > 2 | ||||||
| 30- to 44-year-olds | |||||||||
| No. with diabetes | 77/5569 | 22/3023 | |||||||
| Prevalence (%) | 1.4 | 0.7 | 1.9 (1.1 to 2.9) | ||||||
| 45- to 64-year-olds | |||||||||
| No. with diabetes | 418/3925 | 241/4102 | |||||||
| Prevalence (%) | 10.6 | 5.9 | 1.8 (1.5 to 2.2) | ||||||
| ≥ 65 years | |||||||||
| No. with diabetes | 228/1121 | 516/6324 | |||||||
| Prevalence (%) | 20.3 | 8.2 | 2.5 (2.1 to 2.9) | ||||||
| Simmons et al. 1989148 (preliminary results) | Whole blood glucose by glucose oxidase analyser. After overnight fast OGTT was done in screen positives and 10% of screen negatives. Diabetes mellitus ascertained by 1985 WHO criteria | Asians | Whites | Prevalence of diabetes mellitus was four times higher in Asian men than white men, and twice as high in Asian women as in white women | |||||
| Sample size | 2283 | 1710 | |||||||
| No. screened | 2130 | 1242 | |||||||
| No. who had OGTT | 281 | 161 | |||||||
| Males | Females | Males | Females | ||||||
| No. of known diabetics | 63 | 47 | 21 | 24 | |||||
| No. of new diabetics | 29 | 19 | 4 | 12 | |||||
| No. with IGT | 12 | 18 | 16 | 14 | |||||
| Unadjusted prevalence of diabetes (%) | 8.8 | 5.7 | 4 | 4.6 | |||||
| Age-adjusted prevalence (%) | 11.2 | 8.9 | 2.8 | 4.3 | |||||
| Undiagnosed diabetes mellitus (%) | 30 | 26 | |||||||
| Simmons et al. 199162 (final results) | Same as above | Asians | Europids |
Undiagnosed diabetes mellitus is higher in Europids than Asians (64.9% vs 40.2%) Asians have higher prevalence of diabetes mellitus and IGT than Europids, more so in males Higher ratio of T2DM to IGT in Asians suggests that greater proportion of Asians with IGT become diabetic |
|||||
| Sample size for screening | 3692 | 3529 | |||||||
| Known diabetics | 223 | 104 | |||||||
| Sample size for OGTT | 780 | 719 | |||||||
| New diabetics | 98 | 69 | |||||||
| IGT | 87 | 104 | |||||||
| Males | Females | Males | Females | ||||||
| Overall crude prevalence of diabetes mellitus (%) | 9.7 | 7.5 | 3.6 | 6.4 | |||||
| Age-adjusted prevalence with 95% CI | |||||||||
| T2DM (all) | 12.4 (11.0 to 13.8) | 11.2 (10.0 to 12.5) | 3.2 (2.6 to 4.0) | 4.7 (4.0 to 5.5) | |||||
| T2DM (known) | 7.2 (6.1 to 8.3) | 6.8 (5.8 to 7.9) | 1.4 (1.0 to 1.9) | 1.5 (1.2 to 2.1) | |||||
| T2DM (new) | 5.2 (4.4 to 6.3) | 4.3 (3.6 to 5.3) | 1.8 (1.4 to 2.4) | 3.1 (2.5 to 3.8) | |||||
| IGT | 9.8 (8.7 to 11.2) | 11.2 (10.0 to 12.6) | 5.7 (4.8 to 6.6) | 6.8 (5.9 to 7.8) | |||||
| Prevalence ratio with 95% CI | |||||||||
| T2DM/IGT | 1.27 (1.17 to 1.51) | 0.97 (0.83 to 1.14) | 0.56 (0.43 to 0.73) | 0.69 (0.58 to 0.82) | |||||
| Europid vs Asians: males | Europid vs Asians: females | ||||||||
| T2DM | 3.9 (3.1 to 5.0) | 2.4 (2.0 to 2.9) | |||||||
| IGT | 1.7 (1.4 to 2.1) | 1.6 (1.4 to 1.9) | |||||||
| Cruickshank et al. 1991149 | Oral GTT with 75 g of glucose after fasting blood samples and followed by 2-hour blood sample. Diabetes mellitus diagnosed by WHO 1985 criteria | Gujarati Indians | Whites | Afro-Caribbeans |
Mean fasting and 2-hour C-peptide concentration in Gujarati Indians were significantly higher than the other two groups (p < 0.001). No significant differences between the White and Afro-Caribbean groups Mean Insulin response levels were higher only at 2-hour values in Indians during GTT compared with the other two groups Logistic regression to examine association of C-peptide or insulin concentration with T2DM showed a powerful effect of C-peptide (p = 0.005) and insulin concentration (p = 0.009) on T2DM. Once these two were added age, sex and WHR were no longer related to T2DM and the ethnic group was of borderline significance or non-significant |
||||
| Males | Females | Males | Females | Males | Females | ||||
| Sample size | 47 | 60 | 49 | 52 | 53 | 53 | |||
| Mean (SD) | |||||||||
| BMI (kg/m2) | 25.2 (3.0) | 26.8 (5.0) | 26.2 (4.0) | 26.3 (5.0) | 26.0 (4.0) | 29.1 (5.0) | |||
| WHR | 0.946 (0.06) | 0.888 (0.09) | 0.914 (0.06) | 0.835 (0.07) | 0.918 (0.06) | 0.882 (0.08) | |||
| SBP (mmHg) | 137 (20) | 123 (25) | 129 (20) | 128 (18) | 138 (18) | 132 (19) | |||
| DBP(mmHg) | 77 (13) | 66 (14) | 77 (14) | 75 (12) | 84 (12) | 81 (11) | |||
| IGT | |||||||||
| % (95%CI) | 25 (12 to 38) | 32 (20 to 44) | 25 (12 to 37) | 14 (4 to 23) | 4 (0 to 9) | 17 (7 to 27) | |||
| No. | 12 | 19 | 12 | 7 | 2 | 9 | |||
| New diabetics | |||||||||
| % (95% CI) | 8 (0 to 16) | 13 (4 to 22) | 4 (0 to 10) | 0 13 (4 to 22) | 4 (0 to 9) | ||||
| No. | 4 | 8 | 2 | 7 | 2 | ||||
| Known diabetics | |||||||||
| % (95% CI) | 23 (11 to 36) | 15 (6 to 24) | 0 | 2 (0 to 6) | 28 (16 to 40) | 13 (4 to 22) | |||
| No. | 11 | 9 | 1 | 15 | 7 | ||||
| Change in C-peptide and insulin during GTT | |||||||||
| Gujarati Indians | Whites | Afro-Caribbeans | |||||||
| C-peptide (pmol/l): mean (SD) | |||||||||
| Fasting | 924 (589)a | 801 (311) | 780 (392) | ||||||
| 0.5hr | 2058 (845) | 2131 (704) | 2073 (937) | ||||||
| 2 h | 3705 (2059)a | 2692 (1056) | 2508 (1403) | ||||||
| Insulin (pmol/l): mean (SD) | |||||||||
| Fasting | 99.3 (53.0) | 68.7 (47.0) | 85.4 (56.0) | ||||||
| 0.5 hour | 460 (308) | 334 (164) | 447 (306) | ||||||
| 2 hour | 626 (453)a | 312 (215) | 390 (322) | ||||||
|
Notes: a Significantly greater than in other two groups (p < 0.001 ANOVA) Regression analysis for indicators of T2DM show significant association between T2DM and fasting C-peptide [OR 2.1 (1.4 to 3.0), p = 0.0003] and insulin concentration [1.77 (1.20 to 2.70), p = 0.004] Effect of ethnic group is displaced by inclusion of these factors in the model |
|||||||||
| Samanta et al. 1991155 | Plasma glucose at diagnosis and age at diagnosis ascertained by case notes. Complication measured by investigations | Asians | Caucasians | p -value |
Asians are older at diagnosis, have higher rates of diabetes mellitus in the first-degree relative, and less ketonuria at presentation Peripheral vascular disease and retinopathy were less but renal disease was more in Asians than in Caucasians. Ischaemic heart rate was similar between the two ethnic groups |
||||
| Sample size | 456 | 451 | |||||||
| All data number (%) or mean with SD | |||||||||
| Age at diagnosis (years) | 46.5 (12.8) | 40.6 (17.5) | < 0.01 | ||||||
| Plasma glucose at diagnosis | 18.2 (4.5) | 19.3 (4.7) | < 0.01 | ||||||
| No ketonuria at diagnosis | 362 (85.3) | 202 (47.8) | |||||||
| Severe ketonuria | 21 (4.9) | 99 (23.4) | < 0.01 for both status | ||||||
| HbA1c | 9.4 (2.5) | 9.1 (2.3) | < 0.05 | ||||||
| Total cholesterol | 5.98 (1.7) | 6.01 (1.5) | NS | ||||||
| Triglycerides | 2.03 (0.5) | 2.02 (0.7) | NS | ||||||
| BMI (kg/m2) | 26 (0.04) | 25 (0.04) | 0.05 | ||||||
| Hypertension | 86 (18.5) | 85 (19.5) | NS | ||||||
| Physical exercise | < 0.01 | ||||||||
| Sedentary | 122 (26.9) | 90 (20.1) | |||||||
| Moderate | 294 (64.8) | 210 (46.9) | |||||||
| Active or fit | 318 (8.3) | 148 (33.1) | |||||||
| Complications of diabetes | |||||||||
| Asians | Caucasians | p -value | RR (95% CI) | ||||||
| Males | Females | Males | Females | ||||||
| Sample size | 283 | 173 | 266 | 185 | |||||
| Prevalence of large vessel disease (%) | |||||||||
| Heart vascular disease | 66 (23.8) | 48 (27.7) | 65 (24.4) | 36 (19.5) | NS | 1.15 (0.84 to 1.57) | |||
| Perivascular disease | 11 (3.9) | 6 (3.5) | 31 (11.7) | 11 (5.9) | < 0.05 | 0.51 (0.27 to 0.96) | |||
| Cerebrovascular disease | 8 (2.8) | 2 (1.2) | 11 (4.1) | 5 (2.7) | NS | 0.61 (0.27 to 1.37) | |||
| Prevalence of small vessel disease (%) | |||||||||
| Eye disease | 30 (10.6) | 23 (13.3) | 84 (31.6) | 62 (33.5) | < 0.01 | 0.31 (0.19 to 0.51) | |||
| Cataracts | 30 (10.6) | 15 (8.7) | 17 (6.4) | 6 (3.2) | Not reported | 6.35 (1.43 to 28.16) | |||
| Kidney disease | 69 (24.4) | 33 (19.1) | 35 (13.2) | 22 (11.9) | < 0.01 | 3.36 (1.88 to 5.99) | |||
| Raised serum creatinine | 27 (9.5) | 5 (2.9) | 13 (4.9) | 12 (6.5) | Not reported | 0.65 (0.25 to 1.70) | |||
| Note: The p-value is the difference between Asians and Caucasians and not for the RR | |||||||||
| McKeigue et al. 199158 |
Serum insulin by radioimmunoassay based on double-antibody solid-phase technique, cholesterol and triglycerides by enzymatic calorimetric technique Skinfold thicknesses were measured with callipers (Holtain, Dafyd, UK) |
South Asians | Europeans |
Prevalence of diabetes was 4.3 times higher, mean serum insulin levels 1.4 times higher in fasting state and 2.1 times higher after glucose in South Asians than in Europeans Total cholesterol was lower in South Asians than Europeans Insulin resistance syndrome in South Asians is due to high dietary energy intake, decreased physical activity, and increased central obesity |
|||||
| Males | Females | Males | Females | ||||||
| Sample size | 1421 | 291 | 1515 | 246 | |||||
| Note: All results are age-adjusted mean with 95% CI | |||||||||
| Diabetes | |||||||||
| Prevalence (%) | 19.6 (17.5 to 21.7) | 16.1 (11.7 to 20.5) | 4.8 (3.7 to 5.8) | 2.3 (only six cases) | |||||
| Serum insulin ( μL ) a | |||||||||
| Fasting | 9.8 (9.5 to 10.2) | 7.5 (7.0 to 8.0) | 7.2 (7.0 to 7.4) | 4.8 (4.5 to 5.2) | |||||
| 2 hours after glucose | 41 (39 to 43) | 44 (40 to 48) | 19 (19 to 20) | 21 (19 to 23) | |||||
| BMI (kg/m2) | 25.7 (25.5 to 25.8) | 27.0 (26.5 to 27.5) | 25.9 (25.7 to 26.1) | 25.2 (24.7 to 25.7) | |||||
| WHR | 0.98 (0.97 to 0.98) | 0.85 (0.84 to 0.86) | 0.94 (0.93 to 0.94) | 0.76 (0.75 to 0.77) | |||||
| Total cholesterol (mmol/l) | 5.98 (5.91 to 6.04) | 5.96 (5.82 to 6.09) | 6.11 (6.06 to 6.17) | 6.29 (6.16 to 6.43) | |||||
| HDL-C | 1.16 (1.15 to 1.18) | 1.38 (1.34 to 1.42) | 1.25 (1.23 to 1.27) | 1.58 (1.53 to 1.62) | |||||
| Fasting triglyceridea | 1.73 (1.68 to 1.79) | 1.38 (1.31 to 1.46) | 1.48 (1.44 to 1.52) | 1.21 (1.14 to 1.28) | |||||
| % fall in triglyceride | |||||||||
| Fasting to 2 hoursa | 1 (0 to 2) | 8 (6 to 10) | 6 (6 to 7) | 16 (15 to 18) | |||||
|
a Excluding diabetics Afro-Caribbeans (only 209 men) had a high prevalence of diabetes [14.6% (9.6 to 19.5)] but serum insulin levels, BMI and WHR were no higher in this group than in European men. Afro-Caribbeans had lower cholesterol than Europeans (5.87 vs 6.11 mmol/l) CHD risk factors in South Asians, by subgroup, compared with native British men |
|||||||||
| Native British | Sikh | Punjabi Hindu | Gujarati Hindu | Muslim | p -value a | ||||
| Sample size | 1268 | 731 | 159 | 127 | 211 | ||||
| Diabetes prevalence (%) | 5 | 20 | 19 | 22 | 19 | NS | |||
| Smokers (%) | 30 | 4 | 21 | 33 | 30 | < 0.001 | |||
| Total cholesterol (mmol/l) | 6.12 (0.03) | 6.06 (0.04) | 5.94 (0.09) | 5.45 (0.10) | 5.95 (0.08) | < 0.001 | |||
| HDL-C | 1.24 | 1.22 | 1.17 | 1.14 | 1.04 | < 0.001 | |||
| WHR | 0.932 | 0.979 | 0.981 | 0.972 | 0.977 | NS | |||
| Median SBP (mmHg) | 121 | 128 | 126 | 122 | 120 | < 0.001 | |||
| a Differences among South Asian subgroups | |||||||||
| McKeigue et al. 1992137 (same study as above but reported relationship of glucose intolerance to body fat pattern) |
Serum insulin by radioimmunoassay based on double-antibody solid-phase technique, cholesterol and triglycerides by enzymatic calorimetric technique Skinfold thicknesses were measured with callipers (Holtain, Dafyd, UK) |
Men | Women |
Age-standardised prevalence of glucose tolerance (IGT and diabetes) was 26% in South Asians and 7% in Europeans The strongest univariate relationship with glucose tolerance were with waist girth, abdominal diameter and subscapular skinfold Association was stronger with WHR and subscapular–anterior thigh skinfold ratio than the BMI No significant relationship between physical activity and glucose tolerance, although most men in both groups were physically inactive |
|||||
| South Asians | Europeans | South Asians | Europeans | ||||||
| Age-standardised prevalence | |||||||||
| IGT (%) | 7 | 3 | 8 | 3 | |||||
| Known diabetic (%) | 13 | 3 | 9 | 2 | |||||
| New diabetic (%) | 6 | 2 | 6 | 0 | |||||
| Age-adjusted means for anthropometric measurements | |||||||||
| Waist girth | 92.6 | 91.1a | 83.1 | 75.7a | |||||
| Abdominal diameter | 21.9 | 21.3a | 20.4 | 17.9a | |||||
| Subscapular–triceps skinfold ratio | 2.01 | 1.65a | 1.08 | 0.88a | |||||
| Subscapular–anterior thigh skinfold ratio | 1.72 | 1.42a | 0.78 | 0.57a | |||||
|
a p < 0.001 for differences between Europeans and South Asians within each sex Logistic regression analysis of univariate associations between glucose intolerance and anthropometric variables (controlled for age) |
|||||||||
| Standardised logistic regression coefficients | |||||||||
| South Asian men | European men | South Asian women | |||||||
| Waist girth | 1.54a | 2.00a | 2.16a | ||||||
| Abdominal diameter | 1.59a | 1.99a | 2.17a | ||||||
| Hip girth | 1.17b | 1.53a | 0.85 | ||||||
| Thigh girth | 1.12c | 1.32b | 0.96 | ||||||
| Skinfolds | |||||||||
| Triceps | 1.15d | 1.53a | 1.16 | ||||||
| Subscapular | 1.56a | 1.94a | 1.89a | ||||||
| Anterior thigh | 1.06 | 1.10 | 0.76d | ||||||
| Composite indices | |||||||||
| BMI (kg/m2) | 1.43a | 1.97a | 1.62b | ||||||
| WHR | 1.81a | 2.27a | 3.41a | ||||||
| Subscapular–triceps skinfold ratio | 1.37a | 1.40a | 1.51b | ||||||
| Subscapular–anterior thigh skinfold ratio | 1.45a | 1.76a | 2.26a | ||||||
|
Notes: a p < 0.001, b p < 0.01, c p < 0.1, d p < 0.05, for association between glucose tolerance and anthropometric variable European women excluded because of small numbers |
|||||||||
| Simmons D et al. 1992150 | Capillary whole blood glucose measurement followed by OGTT with 75 g if ≥ 6mmol/l within 2 hours of meal or ≥ 5mmol/l 2 hours or more post prandial and random of 10% of others | Punjabi Sikhs | Pakistani Muslims | Gujaratis | Punjabi Hindus |
T2DM high in Gujarati Muslims compared with others owing to excess of previously diagnosed diabetes They have similar diet to Gujarati Hindus and same meat consumption as Pakistanis Kin marriages are common in Gujarati Muslims and 60% of Gujarati Muslims had married their first cousins |
|||
| Muslims | Hindus | ||||||||
| Sample size | 1930 | 928 | 276 | 664 | 348 | ||||
| Age-adjusted prevalence of T2DM/1000 (95% CI) | |||||||||
| Males | 89 (72 to 110) | 91 (67 to 120) | 160 (107 to 228) | 84 (57 to 120) | 113 (74 to 171) | ||||
| Females | 75 (60 to 94) | 103 (78 to 133) | 204 (144 to 283) | 88 (62 to 122) | 116 (77 to 174) | ||||
| Mean BMI (kg/m 2 ) (95% CI) | |||||||||
| Males | 26 (25.7 to 26.3) | 25.1 (24.7 to 25.6) | 24.5 (23.6 to 25.3) | 24.4 (24 to 24.9) | 25.3 (24.7 to 25.8) | ||||
| Females | 25.8 (25.4 to 26.1) | 26 (25.5 to 26.5) | 26.1 (25.3 to 27.1) | 25.1 (24.5 to 25.6) | 25.9 (25.2 to 26.9) | ||||
| Percentage of undiagnosed T2DM | |||||||||
| Males | 49 | 42 | 20 | 42 | 29 | ||||
| Females | 32 | 53 | 18 | 42 | 45 | ||||
| UKPDS Group 1994145 | FPG by venous sample. Ophthalmic assessment by ophthalmoscope, retinal colour photography; cholesterol by precipitation methods | Asians | Caucasians | Afro-Caribbeans |
Age at diagnosis is lower in Asians than in Caucasians and Afro-Caribbeans Blood pressure is lower in Asians than other groups and Afro-Caribbeans have significantly higher DBP than Caucasians Asians mostly had a first-degree relative with diabetes mellitus Although the clinical and biochemical differences exist between the three ethnic groups, there were no significant differences in prevalence of complications at diagnosis of diabetes mellitus |
||||
| Males | Females | Males | Females | Males | Females | ||||
| Sample size | 362 | 172 | 2425 | 1752 | 219 | 168 | |||
| Biophysical characteristics (mean ± SD) | |||||||||
| Age at diagnosis | 46.8 ± 8.6b | 47.6 ± 8.8b | 51.8 ± 8.8 | 52.9 ± 8.7 | 51.6 ± 7.4 | 50.2 ± 7.2b | |||
| BMI (kg/m2) | 25.9 ± 3.8b | 28.4 ± 4.8b | 28.2 ± 4.8 | 30.7 ± 6.6 | 26.6 ± 3.4 | 29.5 ± 4.8a | |||
| WHR | 0.95 ± 0.05b | 0.88 ± 0.08a | 0.94 ± 0.06 | 0.86 ± 0.08 | 0.93 ± 0.05 | 0.86 ± 0.07 | |||
| SBP (mmHg) | 123 ± 16b | 129 ± 19b | 134 ± 19 | 140 ± 21 | 133 ± 18 | 139 ± 20 | |||
| DBP (mmHg) | 79 ± 10a | 81 ± 9 | 82 ± 10 | 81 ± 9 | 84 ± 10a | 86 ± 11b | |||
| Biochemical characteristics (mean ± SD) | |||||||||
| Fasting plasma | |||||||||
| Glucose | 11.0 ± 3.5 | 11.9 ± 3.4 | 11.6 ± 3.6 | 12.4 ± 3.8 | 12.3 ± 3.7a | 12.8 ± 3.7 | |||
| HbA1c (%) | 9 ± 2.2 | 9.0 ± 2.1 | 9.1 ± 2.2 | 9.3 ± 2.2 | 9.9 ± 2.5b | 10.0 ± 2.6b | |||
| Insulin | |||||||||
| Sensitivity (%) | 21.5b | 19.6a | 24.3 | 20.8 | 30.4b | 24.8b | |||
| Total cholesterol | 5.3 ± 1.0 | 5.3 ± 1.0b | 5.5 ± 1.1 | 5.9 ± 1.2 | 5.3 ± 1.2 | 5.6 ± 1.3 | |||
| HDL-C | 1.0 ± 0.24 | 1.08 ± 0.24 | 1.01 ± 0.24 | 1.09 ± 0.25 | 1.13 ± 0.26b | 1.23 ± 0.28b | |||
| Asians | Caucasians | Afro-Caribbeans | |||||||
| Males | Females | Males | Females | Males | Females | ||||
| Sample size | 362 | 172 | 2425 | 1752 | 219 | 168 | |||
| Prevalence of hypertension, macrovascular and microvascular disease | |||||||||
| Percentage hypertensive | 19a | 30b | 34 | 46 | 33 | 54 | |||
| Prevalence of MI (%) | 1 | 0 | 2 | 1 | 0 | 1 | |||
| Retinopathy (%) | 18 | 11 | 22 | 16 | 26 | 23 | |||
| Cerebrovascular accident (%) | 1 | 0 | 1 | 2 | 1 | 2 | |||
|
a p < 0.001 b p < 0.0 The p-values are Asians and Afro-Caribbeans vs Caucasians after adjustment for age |
|||||||||
| Potts et al. 1994152 | Whole blood glucose and plasma cholesterol measured by oxidase methods on an LM3 Microstat analyser | Asians | Europids |
Asians had higher FPG and cholesterol levels, as well as higher WHR, WTR and subscapular–triceps skinfold ratio Glucose and cholesterol concentrations correlated positively in all groups with WHR in both ethnic groups |
|||||
| Males | Females | Males | Females | ||||||
| Sample size | 40 | 40 | 40 | 40 | |||||
| All results are mean with 95% CI | |||||||||
| Glucose (mmol/l) | 5.1 (4.9 to 5.2) | 5.1 (4.9 to 5.2) | 4.6 (4.5 to 4.7) | 4.5 (4.4 to 4.7) | |||||
| Cholesterol (mmol/l) | 5.5 (5.4 to 5.6) | 5.2 (5.0 to 5.3) | 5.2 (5.1 to 5.3) | 4.6 (4.3 to 4.8) | |||||
| BMI (kg/m2) | 21.3 (20.4 to 22.1) | 22.0 (21.2 to 22.7) | 22.5 (21.7 to 23.2) | 23.1 (21.9 to 24.2) | |||||
| WHR | 0.87 (0.85 to 0.89) | 0.79 (0.78 to 0.81) | 0.80 (0.79 to 0.81) | 0.73 (0.71 to 0.75) | |||||
| WTR | 1.46 (1.44 to 1.48) | 1.23 (1.19 to 1.27) | 1.40 (1.37 to 1.42) | 1.17 (1.15 to 1.19) | |||||
| Subscapular–triceps skinfold ratio | 1.44 (1.32 to 1.55) | 1.38 (1.31 to 1.45) | 0.99 (0.93 to 1.05) | 0.93 (0.87 to 1.00) | |||||
| Ramaiya et al. 1995158 | Glucose analysed using Yellow Springs instruments in Tanzania and glucose oxidase method in UK. DM diagnosed using WHO criteria | Gujarati Asians in Tanzania | Gujarati Asians in UK | p -value |
Mean fasting and 2-hour glucose levels are significantly higher in South Asians from Bhatia community in Gujarat living in Tanzania than in the same community living in the UK High prevalence of IGT (both sexes), newly diagnosed diabetes (women) and hypercholesterolaemia (men) among Asians in Tanzania, whereas newly diagnosed HT is much more frequently found in women in the UK In the UK, levels of physical activity did not show significant relation with 2-hour glucose compared with Tanzania, where it had significant negative association (r = –0.43; p < 0.001) |
||||
| Males | Females | Males | Females | ||||||
| Sample size | 111 | 111 | 92 | 88 | |||||
| Level of physical activity (%) | |||||||||
| Sedentary | 63.5 | 84.6 | 26.4 | 29.5 | |||||
| Light to moderate | 28.4 | 15.4 | 37.4 | 34.1 | |||||
| Heavy | 8.1 | 0.0 | 36.2 | 36.4a | < 0.001;b < 0.001 | ||||
| Smokers (%) | 24.3 | 0.0 | 7.7 | 0.0a | < 0.001 | ||||
| Alcohol (%) | 19.8 | 0.0 | 48.4 | 5.7a | < 0.001;b < 0.01 | ||||
|
Between Gujarati Asians (Bhatia community) living in Tanzania and in the UK: a Difference in males b Difference in females |
|||||||||
| Gujarati Asians in Tanzania | Gujarati Asians in the UK | p -value | |||||||
| Sample size | 222 | 180 | |||||||
| Biochemical variables adjusted for age, sex and BMI (mean) | |||||||||
| Fasting glucose (mmol/l) | 5.5 | 5.1 | < 0.001 | ||||||
| 2 h glucose (mmol/l) | 6.8 | 6.0 | < 0.001 | ||||||
| Serum cholesterol (mmol/l) | 4.8 | 4.8 | 0.7 | ||||||
| Serum triglycerides (mmol/l) | 1.3 | 1.5 | < 0.03 | ||||||
| SBP (mmHg) | 127 | 135 | < 0.01 | ||||||
| DBP (mmHg) | 80 | 82 | 0.20 | ||||||
| Age- and sex-adjusted prevalence rates (%) | |||||||||
| IGT | 28.4 | 11.45 | 0.001 | ||||||
| Newly diagnosed diabetes mellitus | 8.6 | 1.5 | < 0.01 | ||||||
| Known diabetes mellitus | 7.0 | 5.9 | 0.40 | ||||||
| Hypercholesterolaemia | 8.9 | 1.7 | 0.002 | ||||||
| Hypertryglyceridaemia | 22.7 | 21.7 | 0.45 | ||||||
| Newly diagnosed hypertension | 7.8 | 12.4 | 0.08 | ||||||
| Known hypertension | 12.5 | 8.5 | 0.13 | ||||||
| Overweight | 24.3 | 26.8 | 0.32 | ||||||
| Obesity | 19.0 | 15.6 | 0.22 | ||||||
| Cappuccio et al. 1997136 | Diabetes mellitus diagnosed by either glycosuria checked by urine diastix or by oral GTT (diagnosis by WHO Criteria) | South Asians | Whites | African decent |
Age-adjusted prevalence rates between Hindus and Muslims differed only for obesity and smoking (low among Hindus and high in Muslims) Prevalence rates did not differ between Caribbeans and West Africans except smoking (low among West Africans) One-third of diabetics were unaware of status in South Asians |
||||
| Sample size | 505 | 524 | 549 | ||||||
| Age-adjusted prevalence rates of risk factors for CVD:% (95% CI) | |||||||||
| Men | Women | Men | Women | Men | Women | ||||
| Diabetes | 25.4 (20 to 32) | 20.5 (15 to 27) | 6.7 (4 to 11) | 5.2 (3 to 9) | 17.9 (12 to 25) | 14.9 (11 to 20) | |||
| HT | 27.9 (23 to 34) | 26.1 (21 to 32) | 17.8 (13 to 24) | 12.7 (9 to 17) | 36.8 (30 to 44) | 39.8 (35 to 45) | |||
| BMI > 30 kg/m2 | 8.4 (5 to 12) | 19.7 (15 to 25) | 14.8 (11 to 20) | 18.8 (15 to 24) | 14.8 (11 to 21) | 39.8 (35 to 45) | |||
| Cholesterol > 5.2 mmol/l | 67.9 (62 to 74) | 67.6 (62 to 74) | 77.8 (73 to 83) | 78.1 (73 to 83) | 58.0 (51 to 65) | 60.6 (55 to 66) | |||
| Smoking | 25.2 (20–31) | 2.9 (1–6) | 39.6 (33 to 46) | 33.3 (28 to 39) | 18.6 (14 to 25) | 9.2 (7 to 13) | |||
| Both men and women of South Asian and African decent have three- to fourfold higher prevalence of diabetes than the white people, with a prevalence ratio of 3.8 (95% CI 2.6 to 5.6) in South Asians and 2.7 (95% CI 1.8 to 4.0) in Africans | |||||||||
|
Mather et al. |
Microalbuminuria by in-house immunoturbidimetric method, HbA1c by high-performance liquid chromatography method; cholesterol by automated enzymatic method, nitrites and leucocytes by Labstix and creatinine by blanked Jaffe method | South Asians | Europeans | p -value |
Prevalence of microalbuminuria in South Asians in men and women is increased by 1.2- and 1.7-fold, respectively, compared with the Europeans There was no evidence of any interaction between risk factors and ethnicity on risk of albuminuria |
||||
| Sample size (M/F) | 542/347 | 347/236 | |||||||
| Microalbuminuria (% ± SE) | |||||||||
| Men | 40 ± 2.1 | 33 ± 2.6 | 0.003 | ||||||
| Women | 33 ± 2.8 | 19 ± 2.6 | < 0.0001 | ||||||
| Albumin–creatinine ratio (95% CI) | |||||||||
| Men | 2.40 (2.14 to 2.71) | 1.95 (1.95 to 2.29) | 0.04 | ||||||
| Women | 2.89 (2.47 to 3.30) | 2.05 (1.69 to 2.50) | 0.01 | ||||||
| Age-adjusted as there were significant differences in age between the South Asians and Europeans. | |||||||||
| HbA1c (95% CI) | 1.64 (1.43 to 1.89) | 1.43 (1.19 to 1.72) | |||||||
| Relationship between risk factors (age, sex, duration of diabetes mellitus, age of diagnosis, HbA1c, blood pressure, cholesterol, triglycerides, smoking, previous MI, type of treatment and retinopathy) and microalbuminuria was examined by standardised regression and found SBP, HbA1c, triglycerides and retinopathy were all identified as risk factors for albumin–creatinine ratio, of which retinopathy was strongest variable | |||||||||
| Davies et al. 1999140 |
Self-testing for glucosuria 1 hour after main meal by post Oral GTT (75 g) after 10-hour fast in people with glucosuria. Repeat OGTT after 2 weeks |
Asians | Caucasians | p -value |
A total of 123 new cases were identified or diagnosed as a result of screening programme (80 in Asians and 43 in Caucasians) Additional 63 newly diagnosed cases of diabetes (46 in Asians and 17 in Caucasians) Cost/person screened for whole programme was approximately £1.10, and £72 for each case of diabetes diagnosed Asians had low response for screening for glucosuria |
||||
| Sample size | 2134 | 1991 | |||||||
| Glucosuria (self-testing) | |||||||||
| No. (%) (95% CI) | 175 (8.2) [7.4 to 9.0] | 64 (3.2) [2.6 to 3.8] | < 0.001 | ||||||
| Sample size | 152 | 50 | |||||||
| Diabetes (OGTT) | |||||||||
| No. (%) [95% CI] | 46 (30.3) [27.4 to 37.2] | 17 (34) [27.5 to 40.5] | < 0.001 | ||||||
| IGT | 24 (15.8)[10.7–20.9] | 5 (10) [5.9 to 14.1] | |||||||
| Prevalence of diabetes before and after screening in screened and total populations | |||||||||
| Before screening | |||||||||
| 369/6640 (5.6 ± 0.3) | 88/3856 (2.3 ± 0.2) | ||||||||
| After screening | |||||||||
| In screened population | 449/2503 (17.9 ± 0.8) | 131/2075 (6.3 ± 0.5) | |||||||
| In total population | 449/6566 (6.8 ± 0.3) | 131/3787 (3.6 ± 0.3) | |||||||
| Harris et al. 2000151 | Comparing diabetes prevalence using full WHO criteria (i.e. fasting and 2 hour post-load sample) and ADA or new partial WHO criteria (using fasting glucose alone) | South Asians | Caucasians | African descent |
Overall 61/1067 (5.7%) qualified for newly diagnosed diabetes mellitus using WHO criteria compared with 35/1067 (3.3%) by ADA criteria No. of individuals with impaired glucose homeostasis was 13.7% with WHO criteria compared with 3.8% by ADA criteria |
||||
| Sample size | 340 | 380 | 347 | ||||||
| Based on new full WHO criteria: based on fasting and post-load glucose samples: n (%) | |||||||||
| Diabetes prevalence | 31 (9.1) | 10 (2.6) | 20 (5.8) | ||||||
| IGT prevalence | 62 (18.2) | 30 (7.9) | 35 (10.1) | ||||||
| IFG prevalence | 7 (2.1) | 6 (1.6) | 6 (1.7) | ||||||
| Based on ADA and new partial WHO criteria: based on FPG alone: n (%) | |||||||||
| Diabetes prevalence | 17 (5.0) | 4 (1.1) | 14 (4.0) | ||||||
| IFG prevalence | 15 (4.4) | 11 (2.9) | 15 (4.3) | ||||||
| Riste et al. 2001142 | Plasma glucose assessed by standardised glucose oxidase analyser and ascertained by WHO 1999 criteria | Pakistanis | Europeans | Afro-Caribbeans |
Pakistan men and women have largest waist-hip ratio, most obese with high BMI and physically inactive compared with Afro-Caribbeans and Europeans Multiple regression shows independent association with both fasting and 2-hour plasma glucose with WC (0.39, p < 0.001) |
||||
| Males | Females | Males | Females | Males | Females | ||||
| Sample size | 67 | 65 | 219 | 252 | 131 | 185 | |||
| Characteristics: mean (95% CI) | |||||||||
| Physically active (%) | 6.8 (0 to 130 | 5.2 (0 to 11) | 37.8 (25 to 53) | 29 (13 to 46) | 25 (14 to 37) | 34 (23 to 45) | |||
| BMI (kg/m2) | 27.5 (27 to 29) | 29.6 (28 to 31) | 27.4 (27 to 28) | 27.2 (27 to 28) | 26.9 (26 to 27) | 30.2 (29 to 31) | |||
| WHR | 0.96 | 0.88 | 0.92 | 0.81 | 0.92 | 0.84 | |||
| Age-adjusted prevalence of known and newly detected T2DM: % (95% CI) | |||||||||
| Glucose tested | 52 | 52 | 66 | 83 | 76 | 95 | |||
| New diabetics | 18 (7.6 to 28.5) | 21.9 (10.7 to 33.2) | 14.1 (5.7 to 22.5) | 16.8 (8.8 to 24.9) | 9.2 (2.7 to 15.7) | 6.0 (1.2 to 10.7) | |||
| Known and new | 29.9 (19 to 40.9) | 35.7 (24 to 47.3) | 20.8 (15.5 to 26.1) | 19.9 (15 to 24.8) | 23.4 (17 to 29.6) | 20.8 (15 to 26.6) | |||
| Known and new diabetes, with male and females combined | 33 (25 to 41) | 20 (17 to 24) | 22 (18 to 26) | ||||||
| Chowdhury et al. 2002153 | Diabetes diagnosed on at least two FPG levels of > 7 mmol/l or random plasma glucose. Neuropathy using Semmes–Weinstein filament, insulin sensitivity by HOMA | South Asians | Europeans | p -value |
South Asians have higher prevalence of diabetic complication and increased absolute risk of CVD at the time of diagnosis At diagnosis, one-quarter of all patients had evidence of at least one diabetic complication South Asians have higher WHR, decreased HDL-C with no difference in total cholesterol levels Analysis of South Asian cohort (Indian, Pakistani, Bangladeshi and Sri Lankan) separately showed no significant difference from the combined South Asian cohort in all clinical parameters |
||||
| Sample size | 165 | 127 | |||||||
| Demographic characteristics at diagnosis (data as mean ± SD unless otherwise stated) | |||||||||
| Family history of diabetes mellitus: n (%) | 86 (52.1) | 41 (32.3) | < 0.001 | ||||||
| Family history of early vascular disease: n (%) | 68 (41.2) | 27 (21.2) | < 0.001 | ||||||
| FPG (mmol/l) | 9.4 ± 2.4 | 9.5 ± 2.6 | 0.45 | ||||||
| HbA1c (%) | 8.6 ± 1.4 | 8.4 ± 1.9 | 0.17 | ||||||
| Beta-cell function (%) | 46.2 ± 9.6 | 41.7 ± 10.2 | < 0.001 | ||||||
| Insulin sensitivity (%) | 28.7 ± 6.7 | 32.8 ± 7.5 | 0.05 | ||||||
| Evidence of macrovascular disease at diagnosis: n (%) | 26 (15.7) | 12 (9.4) | < 0.001 | ||||||
| Prevalence of microvascular complications at diagnosis: n (%) | |||||||||
| Neuropathy | 5 (3) | 8 (6.3) | 0.08 | ||||||
| Microalbuminuria | 22 (13.3) | 7 (5.5) | < 0.001 | ||||||
| Macroalbuminuria | 8 (4.8) | 3 (2.3) | < 0.001 | ||||||
| Background retinopathy | 23 (13.9) | 8 (6.3) | < 0.001 | ||||||
| Sight-threatening retinopathy | 6 (3.6) | 2 (1.6) | < 0.001 | ||||||
| Overall | 45 (27.3) | 21 (16.5) | < 0.001 | ||||||
| Cardiovascular risk factors at diagnosis (data as mean ± SD unless otherwise stated) | |||||||||
| Current smokers: n (%) | 39 (23.6) | 28 (22) | 0.46 | ||||||
| BMI (kg/m2) | 26.0 ± 5.4 | 27.2 ± 5.3 | 0.07 | ||||||
| WHR | 0.95 ± 0.2 | 0.90 ± 0.2 | 0.05 | ||||||
| Total cholesterol (mmol/l) | 5.2 ± 1.3 | 5.4 ± 1.2 | 0.38 | ||||||
| LDL-C (mmol/l) | 3.3 ± 0.8 | 3.5 ± 0.7 | 0.19 | ||||||
| HDL-C (mmol/l) | 1.0 ± 0.3 | 1.3 ± 0.2 | < 0.001 | ||||||
| Absolute 10-year CHD risk (%) | 16.9 ± 5.4 | 13.7 ± 4.6 | < 0.001 | ||||||
| National Statistics health report for England – 20047 | Health survey | Prevalence of doctor-diagnosed diabetes weighted for non-response: n (%) |
Diabetes more common in men than in women in all ethnic minorities except Pakistani women Diabetes mellitus is higher among Indians, followed by Black Caribbeans and Bangladeshis |
||||||
| Men | Women | ||||||||
| Indian | 903 (9.2) | 1067 (5.9) | |||||||
| Pakistani | 423 (7.3) | 499 (8.4) | |||||||
| Bangladeshi | 178 (8) | 208 (4.5) | |||||||
| Black Caribbean | 480 (9.5) | 676 (7.6) | |||||||
| Black African | 377 (4.3) | 476 (2.0) | |||||||
| Chinese | 151 (3.4) | 163 (3.3) | |||||||
| General population | 7202 (3.8) | 7634 (3.1) | |||||||
| Mukhopadhyay et al. 2005144 | Retrospective analysis | South Asians | Europeans | p -value |
South Asians had lower BMI and blood pressure (both SBP and DBP) but there was no significant difference in cholesterol level between the groups Reasons for deterioration could be owing to lower compliance with medication in South Asians, cultural or language barrier presenting difficulty in strict adherence to glycaemic control |
||||
| At baseline (either as %, mean with SD, median with IQR) | |||||||||
| Age at diagnosis | 45.9 (11.0) | 57.3 (11.6) | < 0.001 | ||||||
| Time to referral (years) | 3.2 (1.6 to 7.4) | 1.9 (1.4 to 5.8) | < 0.001 | ||||||
| BMI (kg/m2) | 28.7 (4.9) | 29.9 (5.6) | 0.003 | ||||||
| Total cholesterol | 5.57 (1.08) | 5.71 (1.28) | 0.136 | ||||||
| Triglycerides | 2.27 (1.60 to 3.05) | 2.23 (1.58 to 3.28) | 0.857 | ||||||
| HDL-C | 1.13 (0.30) | 1.16 (0.31) | 0.234 | ||||||
| SBP (mmHg) | 139.5 (21.9) | 150.9 (22.3) | < 0.001 | ||||||
| DBP (mmHg) | 83.5 (12.1) | 86.7 (12.5) | < 0.001 | ||||||
| HbA1c (%) | 7.46 (2.26) | 7.27 (2.040 | 0.221 | ||||||
| At follow-up (after mean of 5.3 years) | |||||||||
| Current smokers (%) | 13.4 | 26.6 | < 0.001 | ||||||
| ΔCholesterol | –0.60 (–1.7 to 0.10) | –0.90 (–1.9 to 0.05) | 0.044 | ||||||
| ΔSBP (mmHg) | 4.23 (21.27) | –1.85 (23.38) | < 0.001 | ||||||
| ΔDBP (mmHg) | –6.23 (13.87) | –11.15 (14.41) | < 0.001 | ||||||
| ΔHbA1c | 1.31 (2.31) | 0.82 (2.2) | < 0.003 | ||||||
| HbA1c | 8.09% | 8.74% | < 0.001 | ||||||
| Whincup et al. 2005147 | FPG by Falcor 600 automated analysis; serum insulin using specific enzyme-linked immunosorbent assays and insulin resistance by HOMA | South Asian | European | Difference (95% CI) | p -value | Differences in fasting glucose concentration as well as insulin level, and insulin resistance is well advanced by adolescence | |||
| Sample size | 90 | 1248 | |||||||
| BMI (kg/m2)a | 20.4 | 20.7 | –1.7% (–6.0% to 2.4%) | 0.42 | |||||
| Percentage body fat | 27.9 | 26.2 | 1.7 (0.2 to 3.2) | 0.02 | |||||
| WHRa | 0.76 | 0.75 | 0.55% (–1.01% to 1.98%) | 0.47 | |||||
| Subscapular–triceps skinfold ratio | 0.857 | 0.782 | 0.074 (0.019 to 0.129) | 0.008 | |||||
| Glucose (mmol/l) | 5.22 | 5.04 | 0.19 (0.08 to 0.29) | 0.0005 | |||||
| Insulin (μl)a | 10.81 | 8.96 | 17.2% (7.2% to 26.1%) | 0.001 | |||||
| Insulin resistance (HOMA)a | 2.50 | 1.99 | 20.2% (9.9% to 29.4%) | 0.003 | |||||
|
a Geometric means and percentage differences All means and differences adjusted for sex, age and time of day IFG was markedly higher in the South Asians (5.6 vs 1.5%, OR 3.9, 95% CI 1.4 to 10.9, p = < 0.0001) |
|||||||||
| Patel et al. 2006159 | Venous blood samples and diabetes mellitus diagnosed by WHO criteria. Serum cholesterol, triglycerides, HDL calculated by Mira autoanalyser. LDL by Friedewald formula | Gujarati Indians |
Most striking factor between the migrants and indigenous population was on nutrition. There was increased dietary energy intake in the migrants with significant contribution by fat intake Serum cholesterol, triglycerides, BMI, WHR were all higher in the Gujarati immigrants compared with those in India |
||||||
| Sandwell, UK | Navsari, India | ||||||||
| Men | Women | Men | Women | ||||||
| Sample size | 119 | 123 | 139 | 155 | |||||
| Characteristics and nutritional factors: % or mean (95% CI) | |||||||||
| Known diabetics (%) | 14.5 (8.1 to 21.00) | 7.7 (2.9 to 12.5) | 9.1 (4.3 to 13.8) | 3.9 (0.9 to 7.0) | |||||
| New diabetics (%) | 5.1 (0.7 to 9.4) | 8.9 (3.4 to 14.5) | 9.1 (4.0 to 14.2) | 7.1 (2.9 to 11.4) | |||||
| Non-diabetic IGT (%) | 3.3 (0.5 to 6.1) | 7.5 (2.8 to 12.3) | 17.7 (11.3 to 24.0) | 16.3 (9.5 to 23.0) | |||||
| BMI (kg/m2) (mean) | 25.9 (25.1 to 26.7)a | 26.6 (25.7 to 27.3)a | 21.0 (20.3 to 21.7) | 20.8 (20.3 to 21.6) | |||||
| WHR | 0.92 (0.90 to 0.94)a | 0.82 (0.81 to 0.84)b | 0.87 (0.86 to 0.88) | 0.79 (0.78 to 0.80) | |||||
| Current smokers (%) | 10.2 (4.5 to 15.0)a | 0 | 39.7 (31.6 to 47.8) | 3.2 (0 to 6.4) | |||||
| Alcohol (%) | 75.8 (69.1 to 82.6)b | 29.6 (21.4 to 37.8)b | 60.0 (51.9 to 68.2) | 0.7 (0 to 2.2) | |||||
| Physical activity (kcal/day) | 2350 (2200 to 2490)a | 1750 (1640 to 1870) | 1820 (1630 to 2000) | 1680 (1540 to 1810) | |||||
| Energy intake (kcal/day) | 2330 (2160 to 2510)a | 1690 (1580 to 1790)a | 1440 (1390 to 1590) | 1210 (1090 to 1330) | |||||
| Dietary energy from fat | 38.8 (37.4 to 40.00a | 39.5 (38.5 to 40.4)a | 31.2 (28.6 to 33.9) | 31.7 (29.5 to 33.9) | |||||
| Biochemical characteristics: mean with 95% CI | |||||||||
| Sample size | 103 | 108 | 116 | 144 | |||||
| Serum cholesterol | 5.36 (5.17 to 5.56)a | 5.28 (5.10 to 5.47)a | 4.82 (4.62 to 5.02) | 4.84 (4.68 to 5.01) | |||||
| Triglycerides | 1.22 (1.12 to 1.33)a | 1.05 (0.96 to 1.14)a | 0.91 (0.84 to 0.99) | 0.84 (0.78 to 0.90) | |||||
| Plasma insulin (mU/l) | 10.6 (9.4 to 11.9)a | 10.3 (9.2 to 11.6)a | 7.4 (6.6 to 8.4) | 8.6 (7.7 to 9.6) | |||||
| Statistical significance between Navsari and Sandwell: ap < 0.001; bp < 0.05 | |||||||||
| Odugbesan et al. 1989175 | From patient records. Complication identified by physical signs at presentation at regular clinic review | Age-specific prevalence (%) of known diabetics in West Indians |
A total of 95% were diagnosed after the age of 30 years The reasons reported by authors for the higher prevalence compared with native West Indians were dietary changes and rapid transition in way of life, obesity, decreased physical activity and psychological stress |
||||||
| Age (years) | No. of diabetic patients | Unadjusted prevalence | Adjusted prevalence | ||||||
| 0–19 | 1/5343 | 0.02 | 0.02 | ||||||
| 20–29 | 4/1400 | 0.28 | 0.18 | ||||||
| 30–44 | 7/1605 | 0.43 | 0.38 | ||||||
| 45–64 | 191/2520 | 7.57 | 7.93 | ||||||
| > 65 | 48/198 | 24.24 | 5.03 | ||||||
|
Notes: Crude prevalence rate was 2.2% (males = 2.3%; females = 2.2%) Adjusted age-specific prevalence rate was calculated assuming that the population had aged by 6 years between previous census (1981) and survey |
|||||||||
| Diabetic micro- and macrovascular complications in 251 West Indian diabetic patients | |||||||||
| Complications | n | % | |||||||
| Hypertension | 99 | 40 | |||||||
| Proteinuria | 14 | 6 | |||||||
| Retinopathy | 53 | 21 | |||||||
| Cataracts | 43 | 17 | |||||||
| Neuropathy | 28 | 11 | |||||||
| Peripheral vascular diseases | 22 | 9 | |||||||
| Ischaemic heart disease | 10 | 4 | |||||||
| Author and year | Mode of assessment | Results | Reasons for differences and other relevant data | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chaturvedi et al. 1996174 |
Diabetes mellitus measured by non-fasting blood samples. Questionnaires were used to get medical history details of diagnosis, height and weight Proteinuria measured by salicylic sulphuric acid |
At baseline | Afro-Caribbeans | Europeans | p -value |
Age of onset higher in Afro-Caribbeans, more so in women Afro-Caribbeans were treated with more oral drugs than with insulin compared with Europeans Afro-Caribbeans with diabetes have one-third of the risk of dying from heart disease compared with Europeans |
|||||
| Men | Women | Men | Women | ||||||||
| Sample size | 27 | 50 | 95 | 55 | |||||||
| Age at onset | 42 | 42 | 41 | 39 | 0.6,a 0.06b | ||||||
| BMI (kg/m2) | 25.1 | 27.9 | 26.3 | 28.2 | 0.1,a 0.8b | ||||||
| Median SBP (mmHg) | 128 | 138 | 136 | 138 | 0.8,a 0.9b | ||||||
| Median DBP (mmHg) | 86 | 90 | 88 | 88 | 0.2,a 0.1b | ||||||
| Mean cholesterol (mmol/l) | 5.4 | 5.3 | 5.9 | 6.1 | 0.07,a 0.001b | ||||||
| Current smokers (%) | 43 | 22 | 35 | 42 | 0.05,a 0.02b | ||||||
| Creatinine (mmol/l) | 1.10 | 0.87 | 1.00 | 0.80 | 0.01,a 0.03b | ||||||
| Heart disease (%) | 4 | 2 | 13 | 4 | 0.2,a 0.6b | ||||||
| Retinopathy (%) | 22 | 30 | 27 | 35 | 0.6,a 0.5b | ||||||
| Proteinuria (%) | 8 | 16 | 22 | 24 | 0.1,a 0.3b | ||||||
|
a Between men within each ethnic group b Between women within each ethnic group |
|||||||||||
| At follow-up after 18 person-years (range 0–20) | |||||||||||
| Afro-Caribbeans | Europeans | HR (95% CI) | p -value | ||||||||
| Sample size | 77 | 150 | Unadjusted | ||||||||
| No. of deaths | |||||||||||
| Due to all causes | 16 | 59 | 0.41 (0.23 to 0.73) | 0.002 | |||||||
| Due to circulatory diseases | 9 | 39 | 0.33 (0.15 to 0.70) | 0.004 | |||||||
| Due to heart disease | 8 | 31 | 0.37 (0.16 to 0.85) | 0.02 | |||||||
|
Note: Ethnic differences in mortality risk ratio after adjusting for sex, BMI, proteinuria and smoking became non-significant |
|||||||||||
| Cooper et al. 1997176 | Diabetes ascertained by self-report or physician’s diagnosis, except in Nigeria, where it was obtained by fasting blood plasma blood glucose. Diabetes mellitus diagnosed by WHO criteria | West Africa (Nigeria) | Caribbean | UK | USA |
Among people of West African origin, 2% have diabetes mellitus in Nigeria. Within the Caribbean, rates varied from 3% in men in St Lucia to 11% in women in Jamaica. In the UK and USA it was at an average of ~11% Across geographical locations, BMIs were highly related to prevalence of diabetes. Prevalence increases with increase in BMI and WHR, more so with WHR using multiple logistic regression analysis |
|||||
| Jamaica | St Lucia | Barbados | Manchester | Maywood, IL | |||||||
| Sample size | 247 | 820 | 1089 | 813 | 336 | 1518 | |||||
| Prevalence of diabetes | |||||||||||
| Total crude | |||||||||||
| Prevalence (%) | 2.8 | 8.6 | 6.1 | 8.9 | 14.4 | 10.5 | |||||
| Age adjusted | |||||||||||
| Prevalence (%) | 2.0 | 8.1 | 6.2 | 8.2 | 10.8 | 10.6 | |||||
| Anthropometric measures | |||||||||||
| BMI (kg/m 2 ) | |||||||||||
| Men | 21.7 ± 3.6 | 23.4 ± 4.0 | 24.3 ± 3.7 | 25.9 ± 4.3 | 26.6 ± 3.6 | 27.1 ± 5.5 | |||||
| Women | 22.6 ± 4.7 | 27.4 ± 6.5 | 27.3 ± 6.2 | 29.4 ± 6.4 | 28.6 ± 5.9 | 30.8 ± 7.7 | |||||
| WHR | |||||||||||
| Men | 0.88 ± 0.06 | 0.84 ± 0.07 | 0.87 ± 0.06 | 0.88 ± 0.07 | 0.90 ± 0.07 | 0.89 ± 0.07 | |||||
| Women | 0.79 ± 0.06 | 0.80 ± 0.07 | 0.82 ± 0.07 | 0.82 ± 0.07 | 0.81 ± 0.08 | 0.82 ± 0.08 | |||||
| ORs with 95% CI and PAR% comparing people with BMI < 25 kg/m 2 to those BMI ≥ 25 kg/m 2 | |||||||||||
| % BMI ≥ 25 kg/m2 | 18.6 | 48.6 | 51.9 | 65.7 | 67.4 | 69.1 | |||||
| OR (95% CI) | 1.8 (0.3 to 9.7) | 1.5 (0.9 to 2.6) | 2.5 (1.4 to 4.4) | 1.6 (0.9 to 2.8) | 1.6 (0.8 to 3.2) | 1.8 (1.2 to 2.7) | |||||
| PAR (%) | 13.0 | 20.0 | 43.8 | 28.3 | 28.8 | 35.6 | |||||
| Mbanya et al. 1999177 | Plasma glucose by glucose oxidate method by either spectrometer of automated glucose oxidase analyser. Diabetes mellitus diagnosed according to WHO criteria | Cameroon | Jamaica | Manchester | p -value |
In people of African origin, after age standardisation, highest prevalence of diabetes was observed for men and women in the UK and Jamaica compared with rural/urban Cameroon Jamaica had highest prevalence of IGT in both men and women Study of prevalence of glucose tolerance in similar West African genetic background show that higher levels are found in migrants to Britain |
|||||
| Rural | Urban | ||||||||||
| In men: data are median (quartiles) or percentages | |||||||||||
| Sample size | 188 | 138 | 199 | 181 | |||||||
| BMI (kg/m2) | 21.3 (20.2 to 23.4) | 25.2 (22.5 to 28.2) | 22.5 (20.5 to 25) | 26.8 (24.4 to 28.9) | 0.0001 | ||||||
| % BMI ≥ 25 kg/m2 | 10 | 51 | 29 | 68 | 0.0001 | ||||||
| Fast glucose (mmol/l) | 3.9 (3.6 to 4.4) | 4.2 (3.7 to 4.6) | 4.9 (4.6 to 5.5) | 5.1 (4.7 to 5.5) | 0.0001 | ||||||
| 2-hour glucose (mmol/l) | 5.6 (4.3 to 5.8) | 4.9 (4.2 to 5.6) | 5.7 (4.6 to 7.2) | 6.0 (5.1 to 7.1) | 0.0001 | ||||||
| Age standardised prevalence (95% CI) | |||||||||||
| Diabetes | 1.1 (0.1 to 4.0) | 1.0 (0.1 to 3.6) | 6.5 (3.3 to 11.3) | 15.3 (7.7 to 25.9) | < 0.001 | ||||||
| IGT | 6.4 (3.3 to 11.3) | 1.6 (0.3 to 4.6) | 16.3 (11.1 to 22.9) | 11.1 (3.2 to 23.9) | 0.2 | ||||||
| Diabetes or IGT | 7.6 (4.1 to 12.7) | 2.1 (0.6 to 5.3) | 22.8 (16.6 to 30.5) | 26.3 (14.8 to 41.6) | < 0.001 | ||||||
| In women: data are median (quartiles) or percentages | |||||||||||
| Sample size | 196 | 157 | 198 | 224 | |||||||
| BMI (kg/m2) | 21.9 (20.1 to 23.7) | 26.8 (24.3 to 30.4) | 26.9 (22.7 to 30.6) | 28.2 (24.0 to 32.1) | 0.0001 | ||||||
| % BMI ≥ 25 kg/m2 | 16 | 67 | 62 | 70 | 0.0001 | ||||||
| Fasting glucose (mmol/l) | 4.1 (3.7 to 4.4) | 4.2 (3.7 to 4.6) | 5.0 (4.5 to 5.6) | 5.0 (4.6 to 5.4) | 0.0001 | ||||||
| 2-hour glucose (mmol/l) | 5.0 (4.3 to 5.8) | 4.9 (4.2 to 5.4) | 6.5 (5.3 to 7.9) | 5.5 (4.3 to 7.1) | 0.0001 | ||||||
| Age standardised prevalence (95% CI) | |||||||||||
| Diabetes | 0.5 (0 to 3.0) | 2.8 (0.9 to 6.6) | 10.6 (6.5 to 16.1) | 14.0 (7.7 to 23.4) | < 0.001 | ||||||
| IGT | 3.1 (1.1 to 6.8) | 4.6 (0.8 to 13.0) | 19.6 (13.9 to 26.8) | 14.4 (6.0 to 27.5) | < 0.001 | ||||||
| Diabetes or IGT | 3.6 (1.4 to 7.6) | 7.5 (2.6 to 15.5) | 30.1 (23.0 to 38.8) | 28.5 (17.4 to 43.3) | < 0.001 | ||||||
|
Note: Kruskal–Wallis test for quantitative variables and chi-squared test for qualitative variables |
|||||||||||
Appendix 2 Search strategies
Clinical effectiveness searches
MEDLINE 1966 to October 2007
-
exp Prediabetic State/
-
exp Glucose Intolerance/
-
exp Diabetes Mellitus, Type 2/pc [Prevention & Control]
-
(pre-diabet$ or prediabet$ or fasting glucose or impaired glucose tolerance or (elevated adj3 glucose) or glucose intolerance or hyperglycemia or hyperglycaemia or metabolic syndrome or insulin resistance or (risk$ adj2 diabet$)).tw.
-
1 or 2 or 3 or 4 or 5
-
exp Diet Therapy/
-
Exercise/
-
exp Life Style/
-
((prevent$ adj3 diabet$) or non-pharmacological or non-drug or (diet$ adj3 weight loss) or exercise or life-style or life style or physical activity).tw.
-
7 or 8 or 9 or 10
-
randomized controlled trial.pt. or controlled clinical trial.pt.
-
random$.tw.
-
meta-analysis.pt.
-
(systematic review or systematic overview).tw.
-
12 or 13 or 14 or 15
-
6 and 11 and 16
-
limit 17 to english language
EMBASE 1980 to October 2007
-
exp Impaired Glucose Tolerance/
-
exp Glucose Intolerance/
-
exp Non Insulin Dependent Diabetes Mellitus/pc [Prevention]
-
exp Metabolic Syndrome X/
-
(pre-diabet$ or prediabet$ or fasting glucose or impaired glucose tolerance or (elevated adj3 glucose) or glucose intolerance or hyperglycemia or hyperglycaemia or metabolic syndrome or insulin resistance or (risk$ adj2 diabet$)).tw.
-
1 or 2 or 3 or 4 or 5
-
exp diet therapy/
-
exp exercise/
-
exp Physical Activity/
-
exp lifestyle/
-
((prevent$ adj3 diabet$) or non-pharmacological or non-drug or (diet$ adj3 weight loss) or exercise or life-style or life style or physical activity).tw.
-
7 or 8 or 9 or 10 or 11
-
Randomized Controlled Trial/
-
exp meta analysis/or exp “systematic review”/
-
(random$ or systematic review or meta-analysis).tw.
-
13 or 14 or 15
-
6 and 12 and 16
-
limit 17 to english language
The Cochrane Library: 2007 issue 3
(pre-diabet* or prediabet* or fasting glucose or impaired glucose tolerance or glucose intolerance) and ((prevent* near diabet*) or non-pharmacological or non-drug or (diet* near weight loss) or exercise or life-style or life style or physical activity))
Science Citation Index: 1980–2007 October
Topic = ((pre-diabet* or prediabet* or fasting glucose or impaired glucose tolerance or glucose intolerance) and (non-pharmacological or non-drug or diet* or weight loss or exercise or life-style or life style or physical activity) and (prevent* same diabet*))
Cost-effectiveness searches
MEDLINE 1966 to August 2007
-
exp Prediabetic State/
-
exp Glucose Intolerance/
-
(pre-diabet$ or prediabet$ or fasting glucose or impaired glucose tolerance or (elevated adj5 glucose) or glucose intolerance or hyperglycemia or hyperglycaemia or metabolic syndrome or insulin resistance).mp. [mp = title, original title, abstract, name of substance word, subject heading word]
-
1 or 2 or 3
-
(reduc$ adj3 (risk or progress$ or develop$ or incidence) adj3 (diabetes or heart or cardiovascular)).tw.
-
((prevent$ or delay$) adj3 (diabetes or heart or cardiovascular)).tw.
-
diabetes prevention program$.tw.
-
5 or 6 or 7
-
exp Economics/
-
exp “Quality of Life”/
-
(cost$or economic$or (quality adj3 life)).mp. [mp = title, original title, abstract, name of substance word, subject heading word]
-
9 or 10 or 11
-
4 and 8 and 12
-
limit 13 to english language
EMBASE 1980 to August 2007
-
exp Glucose Intolerance/
-
exp Impaired Glucose Tolerance/
-
(pre-diabet$or prediabet$or fasting glucose or impaired glucose tolerance or (elevated adj5 glucose) glucose intolerance or hyperglycemia or hyperglycaemia or metabolic syndrome or insulin resistance).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
1 or 2 or 3
-
(reduc$adj3 (risk or progress$or develop$or incidence) adj3 (diabetes or heart or cardiovascular)).tw.
-
((prevent$or delay$) adj3 (diabetes or heart or cardiovascular)).tw.
-
5 or 6
-
(non-pharmacological or non-drug or weight or diet$or exercis$or life-style$or lifestyle$or life style$or behavio$or psychological or smoking or sport$or physical activity).tw.
-
random$.af.
-
(controlled adj2 trial$).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
9 or 10
-
exp ECONOMICS/
-
exp Health Economics/
-
exp “Quality of Life”/
-
(quality adj3 life).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
12 or 13 or 14 or 15
-
4 and 7 and 11
-
4 and 7 and 16
-
4 and 8 and 11
-
4 and 8 and 16
-
17 or 18 or 19 or 20
-
diabetes prevention program.mp.
-
21 or 22
Also searches above adapted as appropriate and run on: NHS EED, and the Science Citation Index from May 2005 to August 2007.
Epidemiology of pre-diabetes or diabetes searches
MEDLINE 1966 to August 2007
-
incidence/or prevalence/
-
Epidemiology/
-
Glucose Intolerance/ep [Epidemiology]
-
Prediabetic State/ep [Epidemiology]
-
exp *Glucose Intolerance/
-
exp *Prediabetic State/
-
(1 or 2) and (5 or 6)
-
(impaired glucose intolerance or impaired fasting glucose or prediabet$or pre-diabet$).tw.
-
8 and (1 or 2)
-
3 or 4 or 7 or 9
-
limit 10 to english language
Also ran second search for epidemiology T2DM
-
*Diabetes Mellitus, Type 2/
-
incidence/or prevalence/
-
Epidemiology/
-
1 and (2 or 3)
-
*Diabetes Mellitus, Type 2/ep [Epidemiology]
-
4 and 5
-
limit 7 to english language
EMBASE 1980 to August 2007
-
exp epidemiology/
-
exp Impaired Glucose Tolerance/ep [Epidemiology]
-
exp *Impaired Glucose Tolerance/
-
1 and 3
-
(impaired glucose intolerance or impaired fasting glucose or prediabet$or pre-diabet$).tw.
-
1 and 5
-
2 or 4 or 6
Prevalence of diabetes or pre-diabetes in UK Asians searches
MEDLINE 1966 to week 3 August 2006
-
Glucose Intolerance/ep [Epidemiology]
-
Prediabetic State/ep [Epidemiology]
-
exp *Glucose Intolerance/
-
exp *Prediabetic State/
-
(impaired glucose intolerance or impaired fasting glucose or prediabet$ or pre-diabet$).tw.
-
exp Diabetes Mellitus/
-
1 or 2 or 3 or 4 or 5 or 6
-
exp Incidence/
-
exp Prevalence/
-
exp Epidemiology/
-
(incidence or prevalence or epidemiology).tw.
-
8 or 9 or 10 or 11
-
7 and 12
-
(asian$ or indian$).mp. [mp = title, original title, abstract, name of substance word, subject heading word]
-
13 and 14
-
exp Great Britain/
-
(britain or united kingdom or england or UK).mp. [mp = title, original title, abstract, name of substance word, subject heading word]
-
16 or 17
-
15 and 18
-
7 and 12 and 18
-
exp Ethnic Groups/
-
ethnic$.tw.
-
21 or 22
-
20 and 23
EMBASE 1980 to 2006 week 34
-
exp Impaired Glucose Tolerance/ep [Epidemiology]
-
exp *Impaired Glucose Tolerance/
-
(impaired glucose intolerance or impaired fasting glucose or prediabet$or pre-diabet$).tw.
-
exp Diabetes Mellitus/
-
1 or 2 or 3 or 4
-
exp incidence/or exp prevalence/
-
exp epidemiology/
-
(incidence or prevalence).tw.
-
6 or 7 or 8
-
exp United Kingdom/
-
(britain or england or UK or united kingdom).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
10 or 11
-
(asia$or india$).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
5 and 9 and 12 and 13
-
limit 14 to english language
-
exp “ethnic, racial and religious groups”/
-
ethnic$.tw.
-
16 or 17
-
5 and 9 and 12 and 18
Type 2 diabetes mellitus and physical activity/exercise searches
MEDLINE 1996 to week 3 July 2006
-
*diabetes mellitus/or *diabetes mellitus, type 2/
-
exp Weight Loss/
-
exp Diet Therapy/
-
exp Life Style/
-
exp Exercise/
-
((exercise or physical activity or weight loss or life style or life-style or lifestyle) and diabetes).m_titl.
-
2 or 3 or 4 or 5
-
1 and 7
-
6 or 8
-
review.pt.
-
meta-analysis.pt.
-
systematic review.mp.
-
10 or 11 or 12
-
9 and 13
-
limit 14 to (english language and yr = “1990 – 2006”)
Adherence to exercise searches
MEDLINE 1996 to week 3 July 2006
-
exp Prediabetic State/
-
exp Glucose Intolerance/
-
diabetes mellitus/or exp diabetes mellitus, type 2/
-
exp Metabolic Syndrome X/
-
(pre-diabet$ or prediabet$ or fasting glucose or impaired glucose tolerance or (elevated adj3 glucose) or glucose intolerance or hyperglycemia or hyperglycaemia or metabolic syndrome or insulin resistance or diabet$).tw.
-
1 or 2 or 3 or 4 or 5
-
exp Diet Therapy/
-
exp Exercise/
-
exp Life Style/
-
((diet$ adj3 weight loss) or exercise or life-style or life style or physical activity).tw.
-
7 or 8 or 9 or 10
-
exp Patient Compliance/
-
exp Treatment Refusal/
-
(adhere$or complian$ or non-complian$ or noncomplian$ or refusal or drop-out$ or dropout or concordan$).tw.
-
12 or 13 or 14
-
6 and 11 and 15
-
limit 16 to english language
-
limit 17 to yr = “1990 – 2006”
The Cochrane Library: 2006 issue 3
“diabetes and (weight or diet or exercise or physical activity or life-style or lifestyle) in Title, Abstract or Keywords in The Cochrane Database of Systematic Reviews”
Research in progress
-
National Research Register.
-
UK Clinical Research Network.
Appendix 3 Studies excluded from the systematic review of clinical effectiveness
-
Bourn DM, Mann JI, McSkimming BJ, Waldron MA, Wishart JD. Impaired glucose tolerance and NIDDM: does a lifestyle intervention program have an effect? Diabetes Care 1994;17:1311–19. Reason for exclusion: Not a RCT.
-
Brekke HK, Sunesson A, Axelsen M, Lenner RA. Attitudes and barriers to dietary advice aimed at reducing risk of T2DM in first-degree relatives of patients with T2DM. J Hum Nutr Diet 2004;17:513–21. Reason for exclusion: Participants did not have IGT.
-
Brekke HK, Lenner RA, Taskinen MR, Mansson JE, Funahashi T, Matsuzawa Y, et al. Lifestyle modification improves risk factors in T2DM relatives. Diabetes Res Clin Pract 2005;68:18–28. Reason for exclusion: Participants did not have IGT.
-
Brekke HK, Jansson PA, Lenner RA. Long-term (1- and 2-year) effects of lifestyle intervention in T2DM relatives. Diabetes Res Clin Pract 2005;70:225–34. Reason for exclusion: Participants did not have IGT.
-
Dyson PA, Hammersley MS, Morris RJ, Holman RR, Turner RC. The Fasting Hyperglycaemia Study: II. Randomized controlled trial of reinforced healthy-living advice in subjects with increased but not diabetic fasting plasma glucose. Metabolism 1997;46:50–5. Reason for exclusion: Only 1-year follow-up.
-
Eriksson K-F, Lindgarde F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmo Preventive Trial with diet and exercise. Diabetologia 1998;41:1010–16. Reason for exclusion: Participants were not randomised.
-
Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia 1991;34:891–8. Reason for exclusion: Participants were not randomised.
-
Jarrett RJ, Keen H, McCartney P. The Whitehall Study: ten year follow-up report on men with impaired glucose tolerance with reference to worsening to diabetes and predictors of death. Diabet Med 1984;1:279–83. Reason for exclusion: Compared diet compared with phenformin.
-
Jarrett RJ, Keen H, Murrells T. Changes in blood pressure and body weight over ten years in men selected for glucose intolerance. J Epidemiol Community Health 1987;41:145–51. Reason for exclusion: No data on progression to diabetes.
-
Ley SJ, Metcalf PA, Scragg RKR, Swinburn BA. Long-term effects of a reduced fat diet intervention on cardiovascular disease risk factors in individuals with glucose intolerance. Diabetes Res Clin Pract 2004;63:103–12. Reason for exclusion: Only 1-year follow-up.
-
Lindahl B, Nilsson TK, Jansson JH, Asplund K, Hallmans G. Improved fibrinolysis by intense lifestyle intervention. A randomized trial in subjects with impaired glucose tolerance. J Intern Med 1999;246:105–12. Reason for exclusion: Only 1-year follow-up; only some participants had IGT; few details of randomisation.
-
Maji D, Roy RU, Das S. Prevention of T2DM in the prediabetic population. J Indian Med Assoc 2005;103:609–11. Reason for exclusion: No control group (lifestyle vs three drug groups).
-
Page RC, Harnden KE, Cook JT, Turner RC. Can life-styles of subjects with impaired glucose tolerance be changed? A feasibility study. Diabet Med 1992;9:562–6. Reason for exclusion: No progression to diabetes; small study.
-
Swinburn BA, Woollard GA, Chang EC, Wilson MR. Effects of reduced-fat diets consumed ad libitum on intake of nutrients, particularly antioxidant vitamins. J Am Diet Assoc 1999;99:1400–5. Reason for exclusion: Only 1-year follow-up.
-
Swinburn BA, Metcalf PA, Ley SJ. Long-term (5-year) effects of a reduced-fat diet intervention in individuals with glucose intolerance. Diabetes Care 2001;24:619–24. Reason for exclusion: Only 1-year follow-up.
-
Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care 1997;20:26–31. Reason for exclusion: Only 1-year follow-up; mixed group – some IGT, some not.
-
Watanabe M, Yamaoka K, Yokotsuka M, Tango T. Randomized controlled trial of a new dietary education program to prevent T2DM in a high-risk group of Japanese male workers. [Erratum published in Diabetes Care 2004;27:856.] Diabetes Care 2003;26:3209–14. Reason for exclusion: Only 1-year follow up.
-
Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21:350–9. Reason for exclusion: Mixed group of overweight and IGT, most results not split by glucose status.
Appendix 4 Clinical effectiveness: data from the trials
| Reference | Intervention | Recruitment method | Inclusion criteria | Dropout/attrition | Duration of intervention | Duration of follow-up |
|---|---|---|---|---|---|---|
| DPP 2002, USA108 |
Three groups: Lifestyle (diet and exercise), Metformin, Placebo |
Mass media, direct mail and telephone contacts, and also through employment or social groups or health-care systems Goal: ≥ 50% women, ≥ 50% from minority racial/ethnic group, ~20% ≥ 65 years old |
n = 3234 Age ≥ 25 years BMI ≥ 24 kg/m2 (≥ 22 kg/m2) among Asian Americans) |
8% | Throughout the trial | Average 2.8 years (range 1.8–4.6 years) |
| Tuomilehto 2001, Finland183 |
Two groups: Diet and physical activity, Control |
Opportunistic population screening of high-risk groups who responded to local advertisements, or epidemiological surveys |
n = 523 IGT Age 40–64 years BMI >25 (kg/m2) |
8% (n = 40) | Throughout the trial | 6 years (mean 3.2 years); 3 years (Lindstrom 2003215); 4 years (Uusitupa 2003218) |
| Pan 1997, Canada190 |
Four groups: Diet and physical activity, Diet, Physical activity, Control |
87% of target population (aged > 25 years, an industrial city) screened |
n = 577 IGT |
8.1% (n = 47) (our calculation) |
Throughout the trial | 6 years |
| Wein 1999, Australia191 |
Two groups: Diet, Control |
Selected from a long-term follow-up of women with GDM at a hospital |
n = 200 IGT Able to communicate directly or through interpreter |
3.5% (n = 7) | Throughout the trial |
Median 4.25 years (range 11.7 months to 6.8 years) Note: 4.9 years (intervention) vs 4.0 years (control) |
| Oldroyd 2006, UK189 |
Two groups: Diet and physical activity, Control |
Selected from existing studies, hospital databases and GP surgeries |
n = 78 IGT European origin Age 24–75 years |
17.9% (n = 14 withdrawal) | Throughout the trial | 2 years |
| Ramachandran 2006, India173 |
Four groups: Lifestyle (diet and physical activity), Metformin, Lifestyle and metformin, Control |
Screening of middle-class population working in service organisations, and their families identified by workplace advertisement |
n = 531 IGT Age 35–55 years No major illness Compare with 421 men, 110 women |
5.5% (502/531 followed up) | Throughout the trial | 3 years (median 2.5 years) |
| Liao 2002, USA187 |
Two groups: Diet and physical activity, Control |
Telephone screening of individuals with Japanese ancestry, and medical examination |
n = 74 IGT Americans with full Japanese ancestry |
21% (58 completed study) | Supervised for 6 months then unsupervised | 2 years |
| Kosaka 2005, Japan186 |
Two groups: Diet and physical activity, Control |
General health screening of mainly government employees |
n = 458 at 1 years IGT Male |
4.7% (intervention) and 5.5% (control) during 1 year; 6.9% (n = 7) (intervention) and 9% (n = 32) (control) at 4 years | Throughout the trial | 4 years |
| Mensink 2003, the Netherlands188 |
Two groups: Diet and physical activity, Control |
Selected from existing community cohort at high risk of diabetes |
n = 114 IGT Age 40–70 years Caucasian Family history of diabetes or BMI ≥ 25 kg/m2 |
10% (n = 12) at 1 year 23% (n = 26) at 2 year |
Throughout the trial | 2 years |
| Reference | Comparison group care | Intervention group care | |||
|---|---|---|---|---|---|
| Group/individual | Facilitator | Dietary intervention | Physical activity intervention | ||
| DPP 2002, USA108 | Oral and written information on healthy lifestyle at baseline and annually |
Individual lessons with some group sessions ‘The curriculum … was flexible, culturally sensitive and individualised’ (DPP 2002: 3)108 |
Case manager (‘lifestyle coach’), usually a dietitian |
Goal 7% weight loss; low calorie, low fat Sixteen sessions on nutrition, exercise and behaviour modification in first 24 weeks, then in-person contact every 2 months, and telephone contact at least once between visits |
Moderate-intensity exercise (e.g. brisk walking) for 150 minutes/week Supervised group sessions twice a week (voluntary), with supplemental group courses, throughout the trial |
| Tuomilehto 2001, Finland183 | General oral and written information about diet and exercise at baseline and annually | Individual advice with voluntary group sessions | Nutritionist, physician |
Goal ≥ 5% weight reduction (or BMI < 25 kg/m2 or 5–10 kg weight loss) and low fat, high-fibre diet (< 30% fat; < 10% saturated fat, ≥ 15 g/1000 kcal fibre); also advised > 50% carbohydrate, < 300 mg/day cholesterol Use of very-low-calorie diet considered after 6 months (n = 48) Seven sessions with nutritionist in first year then every 3 months |
Moderate-intensity physical activity ≥ 30 minutes/day Individual counselling; supervised and individualised resistance training; exercise programmes differ between study centres |
| Pan 1997, Canada190 | General information about diabetes and IGT, and general written instructions on diet and/or physical activity | Both | Physician |
If BMI < 25 kg/m2, 25–30 kcal/kg body weight, 50–65% carbohydrate, 10–15% protein, 25–30% fat If BMI ≥25 kg/m2, lose 0.5–1.0 kg/month until BMI 23 kg/m2 Individual counselling with physician; small group sessions weekly for 1 month, monthly for 3 months then every 3 months |
Advice to increase physical exercise by 1 unit/day, or 2 units/day if age < 50 years; duration dependent on intensity (e.g. 30 minutes if ‘mild’, 5 minutes if ‘very strenuous’) Frequency of counselling as in the diet group |
| Wein 1999, Australia191 | Written information about standard diet advice, dietary questionnaire; reminded of the need for regular exercise | Individual | Dietitian |
Written information about standard diet advice Telephone contact with dietitian every 3 months who answered questions regarding diet and encouraged compliance with diet and exercise |
Reminded of the need for regular exercise at baseline Compliance encouraged by dietitian |
| Oldroyd 2006, UK189 | Usual care | Individual | Dietitian, physiotherapist |
Goal BMI < 25 kg/m2, ≤ 30% fat, ≥ 1.0 polyunsaturated–saturated ratio, 50% carbohydrate, ≥ 20 g/4.2 MJ (1000 kcal) fibre; regular meals, reduce sugar Written information on nutrition and 12 appointments (each 15–20 minutes long) with dietitian and physiotherapist (three at 2 weekly intervals, three at monthly intervals, one after 9 months then every 2 months between 12 and 24 months) |
Individualised plan to enable aerobic exercise 20–30 minutes at least once a week; encouraged walking, cycling, swimming, dancing and playing golf Information leaflet and up to 80% discount on use of public leisure facilities Regular counselling with physiotherapist (see diet section) |
| Ramachandran 2006, India173 | No details provided | Individual |
Unclear Compare with: ‘The team members included a physician, three laboratory technicians, a dietitian, a social worker and a helper’ (p. 290) |
Reduce total calories, fat and refined carbohydrates, avoid sugar and increase fibre Individual advice at baseline, and again by telephone 2 weeks later or by letter, followed by monthly telephone calls and personal sessions every 6 months |
If involved in physical labour, walking or cycling > 30 minutes/day or performing regular exercise, continue routine activities If engaged in sedentary or light physical activity, walk briskly > 30 minutes/day Individual advice given (see diet section) |
| Liao 2002, USA187 |
AHA step 1 diet (30% fat, 50% carbohydrate, < 300 mg cholesterol) with individual prescription from dietitian Stretching exercises in a group three times a week Diet and exercise supervised for 6 months, then unsupervised; reminded at 12 months |
Individual | Dietitian, exercise physiologist |
AHA step 2 diet (< 30% fat, 55% carbohydrate; < 200 mg cholesterol) Individual prescription and counselling from dietitian for first 6 months then continued unsupervised for next 18 months; reminded at 12 months Note: The study was not designed to demonstrate prevention of diabetes. Weight loss was not an intended goal |
Supervised treadmill (walk/jog) for 1 hour three times a week for 6 months; initial goal 50% maximum heart rate reserve, then increase to 70% Continued unsupervised for next 18 months, reminded at 12 months |
| Kosaka 2005, Japan186 |
General oral information about diabetes and healthy lifestyle; if BMI ≥ 24 kg/m2, take 5–10% smaller meals than normal, advice to increase physical activity and lose weight; if BMI < 24 kg/m2, avoid weight gain by diet and exercise Repeatedly explained at hospital visits every 6 months |
Individual | Unclear |
If BMI ≥ 22 kg/m2, lose 0.5–1.0 kg/months to BMI 22 kg/m2; If BMI < 22 kg/m2, maintain present weight Instructions to reduce food intake by 10%, increase vegetables, < 50 g fat, reduce or stop alcohol consumption, eat out no more than once a week Instructions repeated at hospital visits every 3–4 months |
Moderate-intensity exercise (e.g. walking) 30–40 minutes/day Note: ~15% of participants already performing this level of exercise told to maintain same level Instructions repeated at hospital visits every 3–4 months |
| Mensink 2003, the Netherlands188 | General oral and written information about healthy diet, weight loss and physical activity | Individual (and group sessions for exercise?) | Dietitian, exercise physiologist |
Goal 5–7% weight loss; 30–35% fat, 55% carbohydrate, < 300 mg/day cholesterol, > 3 g/MJ fibre (based on Dutch guidelines); stop smoking, reduce alcohol Mild energy restriction prescribed if no weight loss during first year Individual counselling by skilled dietitian at 4–6 weeks, 3 months then every 3 months |
Moderate activity (walking, cycling, swimming) ≥ 30 minutes for ≥ 5 days a week; training (aerobic/resistance) sessions with trainer ≥ 1 hour/week (free access) |
| Study | n | Duration of follow-up (years) | Intervention: % becoming diabetic (mean ± SD unless stated otherwise) | Control: % becoming diabetic | Difference between groups | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPP 2002, USA108 |
Initial total: 3234 (1079/1073/1082) In analysis: ( ) |
3 | Diet and physical activity | Metformin | Placebo | Estimated cumulative incidence at 3 years; no. of cases/100 person-years is 4.8 for Diet and Physical Activity, 7.8 for Metformin, and 11.0 for Placebo | ||||
| 14.4 | 21.7 | 28.9 | < 0.001 | |||||||
| Compare with 4-year data estimated from graph | 20.5 | 29.4 | 36.8 | ≤ 0.001 | ||||||
| Tuomilehto 2001, Finland183 |
Initial total: 522 (265/257) In analysis: |
6, mean 3.2 | Diet and physical activity | No. of cases/1000 person-years is 32 for intervention group, 78 for control group | ||||||
| n = 27 | n = 59 | <0.001 | ||||||||
|
Pan 1997, Canada190 Da Qing |
Initial total: 577 In analysis: 530 (126/130/141/133) |
6 | Diet and physical activity | Diet | PA | No. of cases/100 (p < 0.05 compared with control) | ||||
| n = 58 | n = 57 | n = 58 | n = 90 | |||||||
| 9.6/100 person-years (7.2 to 12) |
10.6/100 person-years (7.2 to 12.5) |
8.3/100 person-years (6.4 to 10.3 |
15.7/100 person-years (12.7 to 18.7) |
|||||||
| Wein 1999, Australia191 |
Initial total: 200 (100/100) In analysis: 193 (97/96) |
Median 4.25 Note: Diet group has a significantly longer median length of follow-up than control: 4.9 years vs 4.0 years, p = 0.021 |
Diet | < 0.957 | Annual incidence rates = 6.1% for diet group, 7.3% for control group; annual incidence ratio = 0.83 (0.47 to 1.48), p = 0.5 | |||||
| n = 26 | n = 27 | |||||||||
| Oldroyd 2006, UK189 |
Initial total: 78 (39/39) In analysis: 69 (37/32) |
2 | Diet and physical activity | |||||||
| 15/69 at 24 months | n = 8 | |||||||||
| Ramachandran 2006, India173 |
Initial total: 531 (133/133/129/136) In analysis: 502 (120/128/121/133) |
3, median 2.5 | Lifestyle (Diet and Physical Activity) | Metformin | Lifestyle and metformin | Calculated using the Cox proportional hazards model; p = 0.018 (Lifestyle), 0.029 (Metformin), 0.022 (Lifestyle and Metformin) vs control | ||||
| 39.3% (30.4 to 48.5%) | 40.5% (32.0 to 49.7%) | 39.5% (30.9 to 48.9%) | 55% (46.0 to 63.5%) | |||||||
| Liao 2002, USA187 |
Initial total: 74 (36/38) In analysis: 58 (29/29) |
2 | Diet and physical activity | |||||||
| n = 1 | n = 2 | |||||||||
| Kosaka 2005, Japan186 |
Initial total: ( ) In analysis: 458 (102/356) at 1 year |
4 | Diet and physical activity | |||||||
| n = 3 | n = 32 | < 0.043 | ||||||||
| Study | n | Duration of follow-up (years) | Intervention (mean ± SD unless stated otherwise): % | Control: % | Difference between groups | Comment | |
|---|---|---|---|---|---|---|---|
| DPP 2002, USA108 | Initial total: 3234 (1079/1073/1082) | 4 (data estimated from graph) | Lifestyle | Metformin | Placebo | Note: Based on fasting and post load; fasting-only and post-load-only data available | |
| 29.4 | 19.4 | 17.7 | |||||
| Wein 1999, Australia191 |
Initial total: 200 (100/100) In analysis: 193 (97/96) |
4.25 (median) Note: Diet group has a significantly longer median length of follow-up than control: 4.9 years vs 4.0 years, p = 0.021 |
44.3 (n = 43) | 44.8 (n = 43) | NS (0.957) | ||
| Oldroyd 2006, UK189 |
Initial analysis: 78 (39/39) In analysis: 69 (37/32) at 6 months or 54 (30/24) at 24 months |
2 | 20 | 13 | |||
| Liao 2002, USA187 |
Initial total: 74 (36/38) In analysis: 58 (29/29) |
2 |
67 Compare with baseline values presented for 64 (32/32) participants who completed 6 months |
30 | 0.010 | Percentage of participants with NGT at least once during 24 months | |
| Kosaka 2005, Japan186 | In analysis: 458 (102/356) at 1 year | 4 | 53.8 | 33.9 | < 0.001 | ||
| Mensink 2003, the Netherlands188 |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
2 | 50 (n = 20) | 29 (n = 16) | < 0.05 | ||
| Study | Outcome | n | Time point | Intervention (mean ± SD unless stated otherwise) | Control | Difference between groups | |||
|---|---|---|---|---|---|---|---|---|---|
| DPP 2002, USA108 | FPG (mg/dl) | Initial total: 3234 (1079/1073/1082) | Lifestyle | Metformin | |||||
| Baseline | 106.3 (8.1) | 106.5 (8.5) | 106.7 (8.4) | < 0.001 | |||||
| 4 years (4-year data estimated from graph) | 107.4 | 107.9 | 114.3 | ||||||
| HbA1c (%) | Initial total: 3234 (1079/1073/1082) | Lifestyle | Metformin | < 0.001 (0.5–3 years) | |||||
| Baseline | 5.91 (0.51) | 5.91 (0.50) | 5.91 (0.50) | ||||||
| 4 years (4-year data estimated from graph) | 5.97 | 5.98 | 6.13 | ||||||
|
Tuomilehto 2001, Finland183 (Lindström 2003215) |
FPG (mmol/l) |
Initial total: 522 (265/257) In analysis: 434 (231/203) |
Baseline | 6.1 (0.8) | 6.2 (0.7) | 0.0664 | |||
| 3 years | –0.0 (0.7) | 0.1 (0.7) | |||||||
| 2-hour plasma glucose (mmol/l) |
Initial total: 523 (265/257) In analysis: 434 (231/203) |
Baseline | 8.9 (1.5) | 8.9 (1.5) | 0.0664 | ||||
| 3 years | –0.5 (2.4) | –0.1 (2.2) | |||||||
| HbA1c (%) |
Initial total: 523 (265/257) In analysis: 434 (231/203) |
Baseline | 5.7 (0.6) | 5.6 (0.6) | |||||
| 3 years | –0.2 (0.6) | 0.0 (0.6) | 0.002 | ||||||
| Pan 1997, Canada190 | FPG (mmol/l) |
Initial total: 577 In analysis: 530 (126/130/141/133) |
Diet plus exercise | Diet | Exercise | ||||
| Baseline | 5.67 (0.80) | 5.56 (0.81) | 5.56 (0.83) | 5.52 (0.82) | |||||
| 6 years | 7.15 (2.72) | 6.94 (4.49) | 6.83 (2.24) | 7.59 (2.59) | |||||
| 2-hour plasma glucose (mmol/l) |
Initial total: 577 In analysis: 530 (126/130/141/133) |
Diet plus exercise | Diet | Exercise | |||||
| Baseline | 9.11 (0.93) | 9.03 (0.94) | 8.83 (0.79) | 9.03 (0.89) | |||||
| 6 years | 10.76 (4.37) | 10.51 (4.89) | 10.51 (3.93) | 12.99 (4.19) | |||||
| Wein 1999, Australia191 | FPG (mmol/l) |
Initial total: 200 (100/100) In analysis: 193 (97/96) |
Baseline | 5.5 (95% CI 5.4 to 5.7) | 5.6 (95% CI 5.5 to 5.8) | 0.13 | |||
| 4.25 years (median) | 5.7 (95% CI 5.6 to 5.9) | 6.1 (95% CI 5.8 to 6.3) | |||||||
| 2-hour plasma glucose (mmol/l) |
Initial total: 200 (100/100) In analysis: 193 (97/96) |
Baseline | 9.9 (95% CI 9.7 to 10.0) | 9.8 (95% CI 9.7 to 10.0) | 0.71 | ||||
| 4.25 years (median) | 9.8 (95% CI 9.3 to 10.3) | 9.9 (95% CI 9.5 to 10.4) | |||||||
| Oldroyd 2006, UK189 | FPG (mmol/l) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Baseline | 6.05 (0.89) | 6.16 (0.89) | 0.593 | |||
| 2 years | Change 0.25 (0.77) | Change 0.12 (1.0) | |||||||
| Baseline values are presented for 69 (37/32) who completed 6 months. | |||||||||
| 2-hour plasma glucose (mmol/l) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Baseline | 9.15 (0.89) | 9.22 (0.92) | 0.564 | ||||
| 2 years | Change 0.23 (1.6) | Change –0.52 (1.9) | |||||||
| Mensink 2003, the Netherlands188 | FPG (mM) – mean (SE) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 5.9 (SE 0.1) | 5.8 (SE 0.1) | NS | |||
| 2 years | Change 0.2 (SE 0.1) 95% CI (0.0 to 0.4) |
Change 0.5 (SE 0.1) 95% CI (0.2 to 0.7) |
|||||||
| 2-hour plasma glucose (mM) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 8.9 (SE 0.3) | 8.6 (SE 0.2) | < 0.01 | ||||
| 2 years |
Change –0.6 (SE 0.3) 95% CI (–1.3 to 0.0) |
Change 0.8 (SE 0.4), 95% CI (0.0 to 1.6) | |||||||
| HbA1c (%) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 5.9 (SE 0.1) | 5.8 (SE 0.1) | NS | ||||
| 2 years |
Change 0.0 (SE 0.1) 95% CI (–0.2 to 0.2) |
Change –0.1 (SE 0.1), 95% CI (–0.2 to 0.0) | |||||||
| Study | Outcome | n | Time point | Intervention (mean ± SD unless stated otherwise) | Control | Difference between groups | |||
|---|---|---|---|---|---|---|---|---|---|
| DPP 2002, USA108 | Weight (kg) |
Initial total: 3234 (1079/1073/1082) In analysis: ( ) |
Lifestyle | Metformin | |||||
| Baseline | 94.1 (20.8) | 94.3 (19.9) | 94.3 (20.2) | < 0.001 | |||||
| 4 year (4 year-data estimated from graph) | Change –3.5 | Change –1.3 | Change –0.2 | ||||||
|
Tuomilehto 2001, Finland183 (Lindström 2003215) |
Weight (kg) |
Initial total: 523 (265/257) In analysis: 434 (231/203) |
Baseline | 86.7 (14.0) | 85.5 (14.4) | < 0.0001 | |||
| 3 year | Change –3.5 (5.1) = –4% (5.8) | Change –0.9 (5.4) = –1.1% (6.2) | |||||||
| BMI (kg/m2) |
Initial total: 522 (265/257) In analysis: 434 (231/203) |
Baseline | 31.4 (4.5) | 31.1 (4.5) | < 0.0001 | ||||
| 3 year | Change –1.3 (1.9) | Change –0.3 (2.0) | |||||||
| Pan 1997, Canada190 | Weight (kg) |
Initial total: 577 In analysis: 530 (126/130/141/133) |
Diet plus exercise | Diet | Exercise | ||||
| Values are change from baseline to 6 years | No diabetes 1.77 | No diabetes 0.93 | No diabetes 0.71 | No diabetes 0.27 | |||||
| With diabetes –3.33 | With diabetes –2.43 | With diabetes –1.93 | With diabetes –1.55 | ||||||
| BMI (kg/m2) |
Initial total: 577 In analysis: 530 (126/130/141/133) |
Diet plus exercise | Diet | Exercise | |||||
| Values are change from baseline to 6 years | Lean 0.4 (2.2) | Lean 0.8 (1.5) | Lean 0.2 (1.5) | Lean 0.6 (1.8) | |||||
| Overweight –1.6 (1.6) | Overweight –1.1 (2.0) | Overweight –0.9 (1.6) | Overweight –0.9 (2.9) | ||||||
| Wein 1999, Australia191 | BMI (kg/m2) |
Initial total: 200 (100/100) In analysis: 193 (97/96) |
Baseline | 25.2 (95% CI 24.1 to 26.4) | 25.6 (95% CI 24.5 to 26.8) | 0.76 | |||
| 4.25 years (median) | 26.0 (95% CI 24.8 to 27.2) | 26.2 (95% CI 25.0 to 27.5) | |||||||
| Oldroyd 2006, UK189 | Weight (kg) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Values are change from baseline to 2 years | 85.3 (17.9) | 85.5 (14.2) | 0.008 | |||
| Change –1.8 (5.9) | Change 1.5 (2.6) | ||||||||
| Note: Baseline values are presented for 69 (37/32) who completed 6 months | |||||||||
| Ramachandran 2006, India173 | Weight (kg) |
Initial total: 531 (133/133/129/136) In analysis: 502 (120/128/121/133) |
Lifestyle | Metformin | Lifestyle plus metformin | ||||
| Values are change from baseline to 3 years (estimated from graph) | 0.54 | 0.46 | 0.60 | 0.86 | |||||
| p < 0.035 at 2 years (vs baseline) | p < 0.01 (vs baseline) | ||||||||
| Liao 2002, USA187 | Weight (kg) |
Initial total: 74 (36/38) In analysis: 58 (29/29) |
Baseline | 66.1 (2.9) | 69.7 (2.6) | 0.0022 | |||
| 2 years | Change –1.8 (0.5) | Change 0.7 (0.6) | |||||||
| BMI (kg/m2) |
Initial total: 74 (36/38) In analysis: 58 (29/29) |
Baseline | 25.6 (0.8) | 26.6 (0.8) | 0.0023 | ||||
| 2 years | Change –0.7 (0.2) | Change 0.2 (0.2) | |||||||
| Kosaka 2005, Japan186 | Weight (kg) |
Initial total: In analysis: 458 (102/356) after 1 year of intervention |
Values are change from baseline to 4 years | –2.18 (1.63) | –0.39 (1.42) | < 0.001 | |||
| Mensink 2003, the Netherlands188 | Weight (kg) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 86.0 (SE 1.9) | 83.7 (SE 1.5) | < 0.01 | |||
| 2 years | Change –2.4 (SE 0.7); 95% CI (–3.7 to –1.0) | –0.1 (SE 0.5); 95% CI (–1.0 to 0.9 | |||||||
| BMI (kg/m2) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 29.8 (SE 0.5) | 29.3 (SE 0.4) | < 0.01 | ||||
| 2 years | –0.8 (SE 0.2); 95% CI (–1.2 to –0.3) | 0.0 (SE 0.2) 95% CI (–0.3 to 0.4) | |||||||
| Study | Outcome | n | Time point | Intervention (mean ± SD unless stated otherwise) | Control | Difference between groups | ||
|---|---|---|---|---|---|---|---|---|
| Tuomilehto 2001, Finland183 (Lindström 2003215) | WC (cm) | Initial total: 522 (265/257) In analysis: 434 (231/203) | Baseline | 102.0 (11.0) | 100.5 (10.9) | 0.0005 | ||
| 3 year | –3.3 (5.7) | –1.2 (5.9) | ||||||
| Oldroyd 2006, UK189 | WC (cm) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Values are change from baseline to 2 years | 99.0 (12.0) (n = 36) | 99.6 (11.3) | 0.073 | ||
| –0.35 (6.9) (n = 29) | 2.5 (4.5) | |||||||
| Ramachandran 2006, India173 | WC (cm) |
Initial total: 531 (133/133/129/136) In analysis: 502 (120/128/121/133) |
Lifestyle (lifestyle) | Metformin | Lifestyle plus metformin | |||
| Values are change from baseline to 3 years (estimated from graph) | 0.84 | 0.40 | 0.37 | 0.89 | NS compared with baseline | |||
| Liao 2002, USA187 | WC (cm) |
Initial total: 74 (36/38) In analysis: 58 (29/29) |
Baseline | 80.9 (2.0) | 87.2 (2.2) | 0.04 (significant) | ||
| 2 years | –1.2 (0.9) | 0.9 (0.9) | 0.096 | |||||
| Body fat (%) |
Initial total: 74 (36/38) In analysis: 58 (29/29) |
Baseline | 30.1 (1.5) | 31.2 (1.4) | 0.014 | |||
| 2 years | Change –0.8 (0.3) | Change 0.7 (0.5) | ||||||
| Mensink 2003, the Netherlands188 | WC (cm) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 102.4 (SE 1.5) | 102.3 (SE 1.1) | NS | ||
| 2 year | –1.9 (SE 0.7); 95% CI (–3.4 to –0.5) | –0.6 (SE 0.6) 95% CI (–1.8 to 0.6) | ||||||
| Body fat (%) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Values are change from baseline to 2 year | –1.0 (SE 0.3), 95% CI (–1.6 to –0.3) | –0.5 (SE 0.3), 95% CI (–1.1 to 0.0) | NS | |||
| Study | Outcome | n | Time point | Intervention (mean ± SD unless stated otherwise) | Control | Difference between groups |
|---|---|---|---|---|---|---|
| Tuomilehto 2001, Finland183 | SBP (mmHg) |
Initial total: 522 (265/257) In analysis: 506 (256/250) |
Baseline | 140 (18) | 136 (17) | 0.03 (significant) |
| 1 year | Change –5 (14), (95% CI –7 to –3) | Change –1 (15), (95% CI –3 to 1) | 0.007 | |||
| DBP (mmHg) | Initial total: 523 (265/257) In analysis: 506 (256/250) | Baseline | 86 (9) | 86 (10) | 0.02 | |
| 1 year | Change –5 (9) (95% CI –6 to –4) | Change –3 (9) (95% CI –4 to –2) | ||||
|
Oldroyd 2006, UK189 (Oldroyd 2001236) |
SBP (mmHg) |
Initial total: 78 (39/39) In analysis: 67 (35/32) |
Baseline | 137.2 (19.9) | 132.8 (16.4) | 0.050 |
| 6 months | 129.3 (19.5); change –7.9 (16.7) | 132.6 (14.4); change –0.27 (14.3) | ||||
| DBP (mmHg) |
Initial total: 78 (39/39) In analysis: 67 (35/32) |
Baseline | 77.0 (12.6) | 75.5 (9.8) | 0.052 | |
| 6 months |
74.1 (10.0) Change –2.9 (9.9) |
77.4 (9.2) Change 1.9 (10.0) |
| Study | Outcome | n | Time point | Intervention (mean ± SD unless stated otherwise) | Control | Difference between groups |
|---|---|---|---|---|---|---|
|
Tuomilehto 2001, Finland183 (Lindström 2003215) |
Total cholesterol (mmol/l) |
Initial total: 523 (265/257) In analysis: 434 (231/203) |
Baseline | 5.6 (1.0) | 5.6 (0.9) | 0.0712 |
| 3 years | Change –0.1 (0.9) | Change 0.1 (0.8) | ||||
| HDL |
Initial total: 523 (265/257) In analysis: 434 (231/203) |
Baseline | 1.2 (0.3) | 1.2 (0.3) | 0.1354 | |
| 3 years | Change 0.14 (0.20) | Change 0.11 (0.19) | ||||
| Triglycerides (mM) |
Initial total: 523 (265/257) In analysis: 434 (231/203) |
Baseline | 1.7 (0.8) | 1.7 (0.8) | 0.024 | |
| 3 years | Change –0.1 (0.6) | Change 0.0 (0.8) | ||||
| Oldroyd 2006, UK189 | Total cholesterol (mmol/l) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Baseline | 5.6 (1.1) (n = 34) | 5.7 (1.0) (n = 31) | 0.587 |
| 24 months | Change 0.04 (0.79) (n = 29) | Change –0.06 (0.59) | ||||
| LDL (mM) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Baseline | 3.6 (1.1) (n = 32) | 3.6 (1.0) (n = 30) | 0.768 | |
| 24 months | Change –0.09 (0.71) (n = 27) | Change –0.14 (0.56) | ||||
| Mensink 2003, the Netherlands188 | Cholesterol (mM) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 5.1 (SE 0.1) | 5.2 (SE 0.1) | NS |
| 2 years | 0.3 (SE 0.1), 95% CI (0.1 to 0.5) | 0.4 (SE 0.1), 95% CI (0.2 to 0.6) | ||||
| HDL (mM) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 1.16 (SE 0.04) | 1.10 (SE 0.03) | NS | |
| 2 years | 0.06 (SE 0.03), 95% CI (0.01 to 0.11) | 0.05 (SE 0.02), 95% CI (0.00 to 0.09) | ||||
| LDL (mM) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 3.30 (SE 0.10) | 3.44 (SE 0.10) | NS | |
| 2 years | 0.32 (SE 0.11), 95% CI (0.11 to 0.54) | 0.32 (SE 0.09), 95% CI (0.15 to 0.49) | ||||
| Triglycerides (mM) |
Initial total: 114 (55/59) In analysis: 88 (40/48) |
Baseline | 1.59 (SE 0.18) | 1.46 (SE 0.11) | < 0.01 | |
| 2 years | –0.30 (SE 0.12), 95% CI (–0.53 to –0.06) | 0.25 (SE 0.11), 95% CI (0.03 to 0.47) |
| Study | Outcome | n | Time point | Intervention (mean ± SD unless stated otherwise) | Control | Difference between groups |
|---|---|---|---|---|---|---|
| Oldroyd 2006, UK189 | Fasting serum insulin, change in KITT – median (IQR) |
Initial total: 78 (39/39) In analysis: 54 (30/24) |
Baseline | Baseline values are presented for 69 (37/32) who completed 6 months | ||
| 2 years | ||||||
| Mensink 2003, the Netherlands188 | Fasting insulin, HOMA – mean (SE) |
Initial total: 114 (55/59) In analysis: 88 (40/48) at 2 years |
Baseline | |||
| 2 years |
Appendix 5 Weight changes with antiglycaemic therapies
Metformin monotherapy
For patients starting metformin monotherapy, we assume a 1.1 kg weight loss on average maintained during treatment based on the ADOPT study. 310 This assumes 6 years for HbA1c level to progress from 6.2% to 7.5%.
Addition of sulphonylurea to metformin
This is estimated to add an average 2.6 kg to weight over the duration of treatment, based on reported changes in the UKPDS11 and four other studies. 386–389
Use of insulin with metformin after failure with combined oral hypoglycaemic agent therapy
In the large UKPDS study,11 there was an initial weight gain of 3.5 kg followed by a trend, post year 2, of 0.3 p.a. [(7.6–3.5)/13] for 15 years (the duration reported in the UKPDS). In Taylor et al. (2000),390 weight began to level out at around 6 kg after 1 year; Peacock and Tattersall (1984)391 reported 4.2 kg/6 months and Chandalia (2005)392 reports 1.8 kg per year. In the study by Aas et al. (2005)393 in patients failing on OHAs, those who started insulin put on 3.5 kg in weight by 12 months. Janka et al. (2005)394 reported that in people failing on OHAs, those who went on to glargine plus continued OHAs put on 1.4 kg by 24 weeks, whereas those who went on to premixed regular/neutral protamine Hagedorn (NPH) (without OHAs) put on 2.1 kg. In Riddle et al. (2003),395 patients were randomised to addition of glargine or NPH after failing on OHAs and, after 24 weeks, both groups had gained about 3 kg.
Although in some studies, weight gain when insulin is initiated may be a reversal of prior glycaemia-induced weight loss,396 this is less likely to be the case in studies such as the UKPDS with tighter control. UKPDS 49397 reported that asymptomatic patients had a baseline HbA1c level of 8.1% compared with 9.6 for those with symptoms.
Other factors to consider are:
-
the use of metformin in combination with insulin may reduce the degree of weight gain319
-
the dose is also an important factor (doses in UKPDS were relatively low) – a relatively obese population with IGT might require larger than average doses with larger than average weight gain.
Based on the large UKPDS study, we have assumed an initial rise in weight of 3.5 kg in the first year following insulin therapy, followed by a trend of +0.3 p.a.
It is assumed that weight gains with insulin replace rather than add to those on prior sulphonylureas, which are assumed to be lost once that therapy has been withdrawn.
Appendix 6 Potential further sensitivity analyses
| Sensitivity analysis | Rationale |
|---|---|
| Threshold for switching away from lifestyle intervention | The incremental cost-effectiveness of those who achieved a goal success score of 2 in the DPS is worth further examination to see if treating these patients is still cost-effective (if so, this would mean another 20% continuing with the lifestyle intervention) |
| SBP effect of the lifestyle intervention | Assume either SBP:
|
| Lower burden of onset of diabetes |
Assume that either a lower cost and/or weight gain with antiglycaemic therapy after treatment failure with combined metformin-plus-sulphonylurea therapy The effect of an assumed lower HbA1c level trend (0.1% instead of 0.2%) with one of the newer OHAs could also be tested |
| Insulin-induced weight gain | Assume that the 3.5 kg rise in the first year as per UKPDS is just a reversal of prior glycaemia-induced weight loss |
| Re-enforcement of lifestyle changes after year 4 | What additional costs beyond year 4 would have to be incurred to make the intervention only just cost-effective? |
| Lower baseline incidence rate for diabetes | DPSa probably had a higher proportion of patients with both IGT and IFG than in a population setting (only 26% in NHANES III). The DPS diabetes incidence rate may therefore have been higher than in real world (in both arms) |
| Sustainability of weight loss with lifestyle intervention | Assumed weight loss not sustained as long |
| Sensitivity analyses re weight loss on metformin plus sulphonylurea | Assume alternative to 2.6 kg weight gain |
| Intensification of SBP/lipid management at diagnosis of diabetes |
We assumed that any patient taking a statin is prescribed the same dose. A diagnosis of diabetes may lead, however, to titration of statin therapy to more intensively manage CVD risk. This would reduce the cost-effectiveness of the lifestyle intervention Can also assume a tighter target for blood pressure |
| Increasing the risk of diabetes beyond the 4-year DPS intervention |
People may regress in their lifestyle habits without continued re-enforcement. Table 2 of the DPS follow-up paper suggests some weight regain in those free from diabetes at start of follow-up period, although we do not have data on whether this occurred in both study arms A sensitivity analysis could test out the effect of a reduced longer-term risk reduction than assumed in the main analysis Note: conversely, it could be argued that participants in the DPS control arm might have responded better than a real-world control group because of the effect of being in a trial, with follow-up to 8 years. |
| Higher alternative QoL benefit with lifestyle intervention |
There may be QoL benefits beyond those related to weight. The QoL effect of the DPP intervention was 0.02, which was greater than that predicted by weight changes alone A more pessimistic sensitivity analysis around the mean weight change at year 1 could be done – the CIs around the mean 3.4 kg difference between treatments was 2.6–4.2 kg |
| Effect of alternative adherence assumptions |
Outcomes would be much improved if adherence could be improved. Analyses have shown that those who meet nearly all of the lifestyle change targets, or lose 5% body weight, appear to have very low risk of diabetes. But it is unclear how much non-achievement of targets is due to non-adherence vs non-response We could assess what the impact would be of an alternative adherence rate |
| Cost-effectiveness and affordability in different subgroups |
The analysis by Gillies et al. (2007)179 showed that interventions are more cost-effective in patients with a higher BMI. Success of the intervention is also likely to be influenced by baseline insulin secretory capacity. Payback may also be shorter in these subgroups The identification of high-risk subgroups would allow treatment to be targeted in a more cost-effective and affordable manner. Specific subgroups include: |
| Costs during IGT have been reported to rise and may not be fully accounted for by rising incidence of CVD (e.g. might be more GP visits) |
Could use year-on-year cost trend while IGT in line with evidence sourced by Palmer et al. (2004)243 There is also a study from the UK of resource use in primary care in the 5 years before diabetes is diagnosed282 |
| Insulin and QoL | Apply a utility decrement of –0.03 for insulin users rather than that based purely on weight gain |
| Remove microvascular benefits predicted during IGT period | The risks of complications at relatively low levels of glycaemia are not well quantified and may be overstated using risk equations derived from data sets that included only patients with diabetes |
Appendix 7 Protocol
Technology assessment report commissioned by the NHS R&D Programme on behalf of the HTA programme.
Title
Non-pharmacological interventions for adults with impaired glucose tolerance.
TAR team
-
Lead and contact person:
Prof. Norman WaughDept of Public HealthMedical School BuildingsForesterhillAberdeen AB25 2ZD
Corri Black, lecturer in public health
Massoud Bourejerdi, statistician
Michael Gillett, ScHARR
Mari Imamura, systematic reviewer
Paul McNamee, SRF, HERU
Amudha Poobalan, research fellow
Pam Royle, senior research fellow (information specialist and systematic reviewer)
Graham Scotland, health economist, Health Economics Research Unit
Ailsa Snaith, systematic reviewer
Sue Jick from Boston will assist with the survey of current practice using GPRD data.
Plain English summary
Diabetes is characterised by elevated blood glucose levels, and there is international agreement on how high the level has to be before diabetes is diagnosed – a good bit above normal. So some people have blood glucose levels that are not normal, but not diabetic. Some of these people have high glucose levels only after meals, or after glucose tolerance tests (when the body’s reaction to a glucose drink is tested). They are said to have impaired glucose tolerance or IGT. Others have high levels while fasting but their glucose level after a meal may be normal. They are said to have impaired fasting glucose, IFG.
IGT and IFG are important for three reasons. Firstly, they may both progress to diabetes. Secondly, both, though more so IGT, are associated with an increased risk of heart disease.
Thirdly, if we were to screen for type 2 diabetes, we would find more people with IGT and IFG, depending on which screening test was used, than with diabetes. Having found them, we need to be able to advise on management.
This review will examine non-pharmacological ways of reducing the risk of IGT and IFG progressing to diabetes, and will also consider ways of reducing the risk of heart disease in people diagnosed with the conditions.
Many will be diagnosed not by screening, but by their own doctors, for example if they are being checked for heart disease risk, or because of a family history of diabetes.
Background
The prevalence of diabetes is increasing. In type 1 diabetes, we do not know the cause or causes and therefore it cannot be prevented. However, in type 2 diabetes, which makes up about 80% of all diabetes, we know that being overweight or obese greatly increases the risk, and so much T2DM is theoretically preventable. Exercise may also play a role independently of weight, as well as associated with it.
The prevalence of obesity is also rising. It is likely that if we could prevent some obesity, we would prevent or at least delay, a corresponding amount of T2DM.
Two conditions (which may co-exist) appear to precede T2DM. The first is impaired glucose tolerance, in which fasting glucose is normal but there is post-prandial hyperglycaemia. The definition comes from the oral glucose tolerance test (OGTT). The second is impaired fasting glucose, when the fasting level is raised but the post-prandial level does not reach IGT levels.
| Fasting | 2-hour OGTT | |
|---|---|---|
| Normal | 6.0 or under | < 7.8 |
| IFG | 6.1 to 6.9 | < 7.8 |
| IGT | < 7.0 | 7.8 to 11.0 |
| Diabetes | 7.0 or over | 11.1 or over |
IFG and IGT have been called ‘pre-diabetes” but the term is unsatisfactory because not all people with the two conditions go on to develop diabetes. However, about half (REFs) do. So they represent a group in whom intervention may be able to prevent or delay the onset of diabetes.
There are currently discussions in the National Screening Committee and Department of Health about screening for type 2 diabetes. In its early stages, T2DM can cause no symptoms, but can be causing damage to small and large blood vessels.
If there is screening for diabetes, we would expect, depending on method used, to detect as many people with IGT and/or IFG, as with diabetes. Hence, before any screening programme starts, we need to decide what to do with those with IGT and IFG.
Possible interventions include drugs such as metformin, but the main focus of this review will be on non-pharmacological interventions. Metformin has been used as an arm in trials of prevention and therefore will be a comparator for some purposes.
Decision problem
Key question: Are there clinically and cost-effective interventions which will reduce the development of diabetes in those with IGT and IFG?
Interventions to be examined
Weight loss in those who are overweight (BMI 26–29) or obese (BMI 30 and over), by calorie restriction, alone or combined with exercise.
Exercise therapies. Does exercise alone, without weight loss, lead to reduction in risk?
Qualitative changes in diet – i.e. without calorie restriction and weight loss.
All of the above depend on compliance, so we will also look for evidence on ways in which adherence to diet and exercise can be improved.
Ethnic differences. The risk of diabetes is higher in people of South Asian ancestry, and there is some evidence that their exercise habits may differ from indigenous Britons. We will therefore look specifically for trials in this population.
Comparators
The comparator will be standard care. In primary care, this is changing because of the new contract, but in brief it will be taken as no organised screening; the usual lifestyle advice given opportunistically; and care of diabetes when it becomes symptomatic. However, we will carry out a survey of primary care using the GPRD database, to see if there are data on recent practice.
Population and subgroups
The risk of IGT and diabetes increases steeply with age, and it could be argued that only, say, the over-45s should be included. However, it is likely that in addition to diabetes increasing in prevalence, there is also a reduction in age at onset. True T2DM is being seen in children. A counter-argument might be that intervention should therefore be much earlier, in the hope of establishing healthier habits at a younger age that would then persist.
Subgroups of interest will be influenced by the debate on screening, but will include;
-
the South Asian population
-
those who are overweight as children and young adults
-
older age groups, because of the rising prevalence with age
-
possibly, those with other features of the metabolic syndrome such as hypertension, central obesity and high lipids.
The remit for the review starts with the fact of IGT and IFG, and is concerned with reduction of progression to diabetes. However, inevitably, the costs and benefits of treating IGT and IFG will affect the wider economics of screening, and this is considered in the economics section.
We will note and briefly report on any evidence for prevention of IGT and IFG. Strictly speaking that is outwith the remit, but measures to prevent IGT and IFG are probably similar to those for treating them. Similarly if we retrieve trials dealing with people with metabolic syndrome (however defined) but who do not have IGT or IFG, we will note them in passing, since potentially the interventions could reduce later IGT.
Methods: clinical effectiveness
The patient group is defined by the remit – those diagnosed with IGT and IFG.
Search strategy
Some of the topics that need to be considered have been covered by other reviews. Our first step will be to search for reviews, and to identify good quality ones. Their findings will then be summarised.
They will include:
-
recent Cochrane reviews, including that by Norris and colleagues on ‘Long-term non-pharmacological with loss interventions for adults with pre-diabetes’
-
the Australian Evidence-based guideline for the primary prevention of type 2 diabetes
-
the guide to community preventive services: diabetes and physical activity: Task Force on Community Preventative Services 2002 (USA)
-
the New Zealand Health Technology Assessment Centre report on dose, intensity and type of physical activity required to affect risk factors for cardiovascular disease.
We will then search for primary evidence from more recent studies, not included in previous reviews.
Several key-note studies will have been covered in other reviews, but will be summarised in this review for convenience, and their applicability to the UK considered.
We will search MEDLINE, EMBASE, and all sections of The Cochrane Library. The last two years of the Science Citation Index – for meeting abstracts only – will also be searched.
Our general approach to literature searching will be as follows, looking in sequence at:
-
The epidemiology and natural history of IGT and IFG, to give baseline data against which to judge the interventions. Population-based epidemiological studies will be sought.
-
Effectiveness and cost-effectiveness of lifestyle interventions for ‘pre-diabetes’, IGT and IFG, and ‘metabolic syndrome’ (however defined). We will look for trials of interventions such as physical activity and weight loss, back to 1990. Preliminary investigation indicates that these can be successful in trials. The outcomes here will be progression to diabetes, side-effects, quality of life, cost-effectiveness.
-
Adherence to lifestyle interventions for the above conditions, plus diabetes. Success in routine care may be less than in trials, and so we will look also for reviews and RCTs, back to 1990, which provide evidence on ways of increasing motivation to participate in and persist with such lifestyle changes. Given the problem of volunteer bias, only RCTs will be used for conclusions on effectiveness, but other studies may be included if they help to explain adherence or non-adherence. The outcomes here will be adherence or its converse, dropout rate.
-
Type 2 diabetes and physical activity. Some evidence from people who already have diabetes may be useful. We will include only reviews from 2000 onwards in this section.
The search strategy below will be run in MEDLINE for studies on the epidemiology and natural history of IGT and IFG:
-
incidence/or prevalence/
-
Epidemiology/
-
Glucose Intolerance/ep [Epidemiology]
-
Prediabetic State/ep [Epidemiology]
-
(impaired glucose intolerance or impaired fasting glucose or prediabet$or pre-diabet$).tw.
-
(1 or 2) and 5
-
3 or 4 or 6.
The search strategy below will initially be run in MEDLINE (back to 1966) for reviews, RCTs and economic evaluations:
-
exp Prediabetic State/
-
exp Glucose Intolerance/
-
exp Diabetes Mellitus, Type 2/pc [Prevention & Control]
-
Metabolic Syndrome X/
-
(pre-diabet$or prediabet$or fasting glucose or impaired glucose tolerance or (elevated adj3 glucose) or glucose intolerance or hyperglycemia or hyperglycaemia or metabolic syndrome or insulin resistance or (risk$adj2 diabet$)).tw.
-
1 or 2 or 3 or 4 or 5
-
exp Diet Therapy/
-
Exercise/
-
exp Life Style/
-
((prevent$adj3 diabet$) or non-pharmacological or non-drug or (diet$adj3 weight loss) or exercise or life-style or life style or physical activity).tw.
-
7 or 8 or 9 or 10
-
randomized controlled trial.pt.
-
random$.tw.
-
meta-analysis.pt.
-
(systematic review or systematic overview).tw.
-
12 or 13 or 14 or 15
-
6 and 11 and 16
-
limit 17 to english language.
This strategy will then be combined with appropriate search filters for systematic reviews, RCTs, and economic evaluations. The search strategy will then be adapted as appropriate and run in the other databases mentioned above.
The search will be limited to English language only.
If further relevant search terms or interventions become apparent during the course of review then the above strategy may be modified.
In addition, the National Research Register will be checked for ongoing studies and contact may be made with key authors for unpublished data.
Inclusions and exclusions
The focus will be on lifestyle interventions such as diet and physical activity. Gastric surgery for morbid obesity will not be included (but we will summarise and refer to previous reviews such as the HTA monograph).
Because the interventions relevant to this review are lifestyle ones, only RCTs will be included because of the risk of bias in non-randomised studies, such as volunteer bias (people willing to take part, and to persist with, trials of weight loss or exercise, may have been going to do better without the intervention, so randomisation to intervention or control groups is essential).
Trials of less than 2 years’ duration will be excluded. Ideally, we would like follow-up of 10 years or more.
We will prefer UK-based studies for prevalence and natural history but will use with caution studies from countries with a similar ethnic and socio-economic mix (Australia, New Zealand).
Studies in the general population may not be applicable to people diagnosed with IGT, partly because of the effect of the diagnosis, partly because of associated factors such as overweight.
Study selection will be made independently by two reviewers. Discrepancies will be resolved by discussion, involving a third reviewer if necessary.
Outcomes of interest
Primary outcomes
-
Prevention of diabetes in those with IGT and IFG.
-
Regression from IGT and IFG to normal blood glucose levels.
-
Cardiovascular mortality and morbidity.
Secondary outcomes (mainly affecting cardiovascular risk)
-
Weight loss of 5kg or more if sustained for more than 2 years.
-
Significant reduction in plasma cholesterol (% with TC under 5.2 mmol/l, or with drops of 1 mmol/l or more).
-
Reduction in blood pressure.
-
Costs of health care.
Compliance with interventions will not of itself be used as an outcome.
Data extraction strategy
Data will be extracted independently by two reviewers using pre-defined data extraction forms. Discrepancies will be resolved by discussion, with involvement of a third reviewer if necessary.
Quality assessment of reviews and trials
The quality of each study will be assessed by one reviewer. Uncertainties will be discussed with a second reviewer. Criteria used will be those from CRD report number 4, amended if necessary.
Analysis and reporting
The results of good quality reviews will be reported in a narrative form. If there are differing conclusions among these reviews, the reasons will be explored.
The results of trials will be reported, summarised in table form, and may be presented in a meta-analysis if appropriate.
Information on prevalence and natural history will be reported in narrative form.
Cost-effectiveness
Existing economic studies will be reviewed. Evidence on the increased lifetime costs of diabetes, compared with being non-diabetic and pre-diabetic, will be sought from published literature and models.
Assuming that there is evidence of clinical effectiveness – that intervention can prevent or delay progression to diabetes – the interventions will be costed from the perspective of the NHS. Intervention could be double, in the sense of there being a compliance intervention to improve adherence to a lifestyle one.
As a first step, the cost per case of diabetes prevented, or of at least two-year delay in onset, will be calculated. The two year period is really too short but we are pessimistic about finding evidence from long-term (e.g. 10 or more years).
Secondly, the monetary savings over a life-time from prevention or delay will be estimated.
Thirdly, the disutility from being diabetic will be derived from published literature and the impact on quality of life estimated; the benefits of prevention can then be expressed in QALY gains.
Fourthly, cost per QALY will be estimated. Costs and QALYs will be discounted by 3.5%.
We will consider patient costs at such a time, and any costs of diet or exercise. We will also consider, if data appear, any benefits to other family members.
We will not develop a long-term diabetes economic model. Diabetes models are complex, and several tried and tested ones already exist. We will renew a previous collaboration with ScHARR, who have a well-developed model of type 2 diabetes.
An outline of the draft model structure is shown below. The three main components are:
-
an annual Markov model representing transitions from normoglycaemic and pre-diabetic states to one of these or to diabetes
-
a diabetes progression model that predicts risk such cardiovascular events and mortality and other-cause mortality
-
a risk model for cardiovascular events in normoglycaemic and pre-diabetic states.
Possible progression pathways include:
IGT > diabetes and later > cardiovascular disease
IGT > cardiovascular disease but without diabetes
IGT > both diabetes and CVD.
Intervention to reduce the risk of progression to diabetes, would probably increase the rate of regression from IGT to normality. That would not affect diabetic outcomes, but would affect cardiovascular ones. The Sheffield model will be expanded to add an IGT locus but also a normality one. We will need to do some literature reviewing specifically to populate the economic model.
Cardiovascular risk in the normoglycaemic and pre-diabetic states will need to incorporate traditional risk factors such as blood pressure and cholesterol, and in particular, a relationship between glucose (and possibly weight?) and CHD risk. This is important as pre-diabetic patients have a significantly elevated CHD risk compared with the general population. The best mechanism for this needs further consideration but might involve using data from the DECODE study to modify risks obtained from Framingham or the UKPDS risk equations.
Sensitivity analyses will undertaken to identify which variables contribute most to uncertainty in the results, and a restricted probabilistic sensitivity analysis (PSA) will be undertaken (unless cost-effectiveness is demonstrated across all sensitivity assumptions). Colleagues at Sheffield have developed methods for substantially reducing the computational burden of using PSA in models.
Survey of current practice
A survey of current recorded prevalence, regression, persistence and progression, of IGT and IFG, and of treatments given, will be undertaken using the UK General Practice Research Database (GPRD). The GPRD is one of the largest longitudinal primary care records database, anonymised and used for research. Since 1988, over 4 million residents of the United Kingdom have registered with more than 300 GP practices that provide data for the GPRD. Details of patient characteristics, treatments prescribed and clinical diagnoses are available. GPRD has been used extensively for research in drug therapy and outcomes in people with diabetes mellitus. It has not, to the best of our knowledge, been used to identify people with pre-diabetic states.
The aims of this survey will include:
-
Establishing if people with IGT and IFG are identified by their GPs and that information is recorded in the GP records. The fitness of GPRD data for this purpose is not known at present. This survey will provide useful insight into how well this condition is currently recognised and recorded by GPs; providing relevant information to inform policy regarding approaches to the management of potentially pre-diabetic states.
-
Estimate the prevalence of IGT and IFG recorded in UK general practice and trends in recording over time
-
Describe the characteristics of people reported to have the conditions including evidence of other components of a metabolic syndrome, clinical management, and disease progression (or regression).
We would focus on GPRD data from 2000 to 2005 in order to be able to describe current clinical practice but will also look at historical data to describe trends and if possible follow people with IGT and IFG diagnoses to describe disease progression.
The coding system available to GPs using the GPRD does include a code for impaired glucose tolerance, and also for ‘pre-diabetes’ (which we have tended to avoid because not all people with IGT or IFG progress to diabetes). We propose to use this code, as well as looking for combinations of codes such as abnormal glucose in the absence of a prior diagnosis of diabetes mellitus (Table 2). Once potential cases have been identified using this screening approach, we will review patients’ computer records to classify people as:
-
probable IGT or IFG
-
possible IGT or IFG
-
IGT or IFG excluded.
| Code | Terms |
|---|---|
| 1408.00 | At risk of DM |
| 212 6300 | DM resolved |
| R102.11 | Pre-diabetes |
| R102.00 | GTT abnormal |
| R102.12 | Impaired GTT |
| 44U5.00 | Blood glucose 7–9.9 |
| 44U6.00 | Blood glucose 10–13.9 |
| 44UZ.00 | Blood glucose 14+ |
| 44U9.00 | Blood glucose abnormal |
| 44Uz.00 | Blood glucose raised |
| 44Uz.11 | Hyperglycaemia |
| 44V2.00 | GTT impaired |
| 44V3.00 | GTT abnormal |
| R105700 | Blood glucose abnormal |
For each ‘probable’ or ‘possible’ case, details of follow up, treatment, and outcomes would be recorded. Other metabolic syndrome risk factors will be noted.
Total GPRD population counts will also be obtained in order to allow us to estimate age specific rates of IGT and IFG.
Collaborations and costs
A group in the Aberdeen Medical School has secured a contract for a review of interventions in obesity. Following discussion, the timescale for that review will be compatible with our one. This should reduce the work involved.
The Scottish Evidence-based Child Health Unit has carried out a review of prevention of obesity in childhood. IGT and IFG are probably rare in children, but obesity is becoming more common, so this assumption may not be justified. Data on prevalence will be sought. Key points from the SEBCHU review will be summarised in our review, either in the main text or as an appendix, depending on perception of relevance.
The recent review of screening for type 2 diabetes covered some issues that relate to this review, and this will also offset the time costs.
Several other reviews are likely to be useful, and as already mentioned, will be summarised. This ‘review of reviews’ may reduce the number of primary studies requiring to be data-extracted, hence offset the cost of this TAR, and enable us to transfer some funds to the GPRD survey.
As regards the modelling, colleagues in ScHARR will extend their model, run it to provide data, and we will write it up in collaboration.
Timelines
Final protocol sent to NCCHTA on 28 August.
Literature searches by 3 August.
Clinical effectiveness review August to October.
Survey of current practice using GPRD, by mid-October.
Cost-effectiveness review and modelling September to October.
Draft sent out for peer review by end of November.
Comments back by late December.
Final draft to NCCHTA by end of January.
Glossary
- Adherence
- The level of participation achieved in a behavioural regimen once the individual has agreed to undertake it.
- Aerobic exercise
- Rhythmic, repeated and continuous movements of the same large muscle groups for at least 10 minutes at a time, for example walking, cycling, jogging, swimming.
- Attrition
- The decline in numbers due to dropouts and other losses to follow-up as trials go on.
- Intermediate hyperglycaemia
- A condition in which the level of blood glucose is above normal but below the levels at which diabetes is diagnosed.
- Resistance exercise
- Activities that use muscular strength to move a weight against a resistive load, for example weightlifting and exercise using weight machines.
List of abbreviations
- ADA
- American Diabetes Association
- AE
- adverse event
- AHA
- American Heart Association
- AHRQ
- Agency for Healthcare Research and Quality
- AusDiab
- Australian Diabetes, Obesity and Lifestyle Study
- BMI
- body mass index
- CHD
- coronary heart disease
- CHF
- coronary heart failure
- CI
- confidence interval
- CVD
- cardiovascular disease
- DALY
- disability-adjusted life-year
- DBP
- diastolic blood pressure
- DH
- Department of Health
- DPP
- Diabetes Prevention Program
- DPS
- Diabetes Prevention Study
- E%
- percentage total energy
- EDIPS
- European Diabetes Prevention Study
- EPIC
- European Prospective Investigation into Cancer
- EQ-5D
- European Quality of Life-5 Dimensions
- FINDRISC
- Finnish Diabetes Risk Score
- FPG
- fasting plasma glucose
- GDM
- gestational diabetes mellitus
- GOAL
- Good Ageing in Lahti Region
- GO-YDPP
- Group-Organized YMCA Diabetes Prevention Program
- GP
- general practitioner
- GPRD
- General Practice Research Database
- GTT
- glucose tolerance test
- HbA1c
- glycated haemoglobin
- HDL-C
- high-density lipoprotein cholesterol
- HEED
- Help Educate to Eliminate Diabetes
- HOMA
- homeostasis model assessment.
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDF
- International Diabetes Federation
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- IQR
- interquartile range
- ITT
- intention to treat
- LDL-C
- low-density lipoprotein cholesterol
- LSM
- lifestyle modification
- LTPA
- Leisure Time Physical Activity
- MET
- metabolic equivalent
- mET
- metformin
- MI
- myocardial infarction
- NGT
- normal glucose tolerance
- NICE
- National Institute for Health and Clinical Excellence
- NNT
- number needed to treat
- NPH
- neutral protein Hagedorn
- NS
- not significant
- NSC
- National Screening Committee
- OGTT
- oral glucose tolerance test
- OHA
- oral hypoglycaemic agent
- OXMIS
- Oxford Medical Information System
- p.a.
- per annum
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SBP
- systolic blood pressure
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- UKPDS
- UK Prospective Diabetes Study
- WC
- waist circumference
- WHO
- World Health Organization
- WHR
- waist–height ratio
- YHPHO
- York and Humber Public Health Observatory
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health