Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 09/141/01. The protocol was agreed in September 2010. The assessment report began editorial review in April 2011 and was accepted for publication in December 2011. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Papaioannou et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Epidemiology
Non-Hodgkin’s lymphomas (NHLs) account for approximately 4% of all cancers diagnosed in the UK,1 and are also the fifth most common cancer in the UK for both sexes combined (fifth in males and seventh in females). 2 In 2008, there were 10,319 new cases of NHL registered in England and Wales,3 and 3978 registered deaths in 2008. 4
Follicular lymphoma (FL) is a type of low-grade or indolent NHL, in which the cancer develops slowly, often without symptoms, for many years. FL is the second most common type of NHL within Western Europe and the USA,5 and is reported to account for between 20% and 30% of all NHLs. 6–9 The UK incidence of FL is approximately 3.4 per 100,000 persons (see Table 1), and around 70% of all cases are diagnosed in people aged > 60 years. 10 FL occurs equally in males and females. Most patients with FL present with advanced disease; approximately 50% of patients will present with bone marrow involvement (i.e. stage IV disease; see Staging, later in this chapter).
| NHL/FL incidence | England | Wales | England and Wales |
|---|---|---|---|
| All NHLs: no. of cases (2008) | 9676 | 643 | 10,319 |
| All NHLs: crude rate per 100,000 (2008) | 18.8 | 21.5 | 18.9 |
| FL: no. of cases (2008) | 1757 | 112 | 1869 |
| FL: crude incidence per 100,000 (2008) | 3.4 | 3.7 | 3.4 |
Over 70% of people with FL are still alive 5 years after the diagnosis,11 with the 10-year predicted survival rate for patients in England and Wales in 2007 reported as 50.8%. 2 In the last decade, longer median survival has been reported, with one centre reporting median overall survival (OS) of up to 18 years,12 and the percentage of survival at 20 years as high as 44%. 13 Some have attributed this to novel therapeutic strategies,14,15 including chemoimmunotherapy [i.e. chemotherapy and rituximab (MabThera®, Roche Products)] and radioimmunotherapy. Relevant data on incidence and prevalence are provided in Tables 1 and 2, respectively.
| NHL/FL prevalence | 1-year prevalence | 5-year prevalence | 10-year prevalence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| England | Wales | England and Wales | England | Wales | England and Wales | England | Wales | England and Wales | |
| NHL prevalence (2006) | 6330 | 498b | 6761 | 24,207 | 1516 | 25,723 | 38,227 | 2224 | 40,451 |
| Estimated FL prevalence (based on FLs as 20–30% of NHLs)6–9 | 1266–1899 | 105b | 1371c–2028 | 4841–7262 | 303–455 | 5145–7717 | 7645–11,468 | 445–667 | 8090–12,135 |
The incidence of NHL has been increasing in the UK; rates have increased by more than one-third since the late 1980s, resulting in the incidence in people aged > 75 years being three times higher in 2007 than in 1975. 18 Other countries (Western Europe, USA, Japan, Brazil, India and Singapore) have also noted increasing incidences of NHL. In westernised countries, the annual incidence of FL has increased from 2–3/100,000 during the 1950s to 5–7/100,000 recently (date not specified). 19
It is unclear why the incidences of lymphomas are increasing, although better diagnosis, improved cancer reporting, changes in classification, unknown environmental factors, an increasing elderly population and increases in acquired immunodeficiency syndrome (AIDS)-related lymphomas will contribute to the increase in incidence. However, these factors are estimated to account for about half of the increase in observed incidence. 20
Aetiology
The causes of NHL in general, including FL, are unclear. There are a number of well-established risk factors, such as infectious agents [e.g. human immunodeficiency virus (HIV)],21 immunosuppression (e.g. post organ transplantation),22 genetic susceptibility (e.g. ataxia–telangiectasia)23 and environmental factors (e.g. exposure to agrochemicals). 24 Rare immunodeficiency conditions such as hypogammaglobulinaemia, Wiskott–Aldrich syndrome and ataxia–telangiectasia have been associated with as much as a 25% increased risk of developing lymphoma;25 however, the primary causes of NHLs remain elusive.
Pathology
Background
Non-Hodgkin’s lymphomas are a diverse group of cancers characterised by abnormal growth of tissue in the lymphatic system. The lymphatic system comprises the tissues, organs and vessels that produce, store and deliver cells that fight infection – ‘lymphocytes’. There are two main classes of lymphocytes – T lymphocytes and B lymphocytes – with each having a key role in protecting the body from pathogenic microorganisms. ‘T cells’ are responsible both for cell-mediated immunity and for stimulating ‘B cells’, which, when activated, produce antibody that kills or neutralises antigens. NHL may be classified as a B- or T-cell NHL, depending on whether it is B or T lymphocytes that are proliferating at an abnormal rate. Approximately 85% of all NHLs are of B-cell origin and the remaining 15% of T-cell origin. 26
Follicular lymphoma is classified as a B-cell NHL. It is an indolent (slow-growing) cancer that affects B-cell lymphocytes (centrocytes and centroblasts). Patients with FL typically present with painless, swollen lymph nodes in the neck, armpit or groin. Systemic or ‘B’ symptoms are rare: these include fever, fatigue, night sweats and unexplained weight loss. 5,27 Less frequently, there may be no peripheral lymphadenopathy, or patients develop abdominal or back pain owing to intra-abdominal (often paraortic) lymph node enlargement. 5 Usually disease is disseminated and involves lymph node regions on both sides of the diaphragm (stage III) or possibly extralymphatic organs or tissues (stage IV). 6,28
Despite being treatable, FL is characterised by a relapsing–remitting clinical course over several years, with each successive response becoming more difficult to achieve and of shorter duration. 27 The course and prognosis of FL improved only marginally from 1960 to the early 1990s, with a reported median survival of 8–10 years. 29 However, in the last decade, longer median survival has been reported and has been attributed to novel therapeutic strategies, including chemoimmunotherapy (i.e. chemotherapy and rituximab) and radioimmunotherapy. 14,15
Patients with advanced stage III–IV lymphomas will eventually become resistant to chemotherapy and transform to high-grade or aggressive lymphomas, such as diffuse large B-cell lymphoma (DLBCL). 29,30 Resistant disease or transformation into DLBCL is the usual cause of death for patients with FL. 27 The risk of transformation to aggressive lymphoma is thought to be constant over time;29 the annual risk of transformation has been estimated as 3% per year and the median survival after transformation has been reported as 1.7 years, although this figure comes from the pre-rituximab era. 31 It is not clear whether specific therapies can increase or decrease this risk. 32
Diagnosis and grading
The diagnosis of FL is confirmed by lymph node biopsy, which optimally requires review by an pathologist or haematopathologist (in the UK). 32
Staging
Once FL is identified, it is staged to find out how far the disease has spread. Staging tests determine which areas of the body are affected by FL, the number of lymph nodes affected, and whether or not other organs are affected such as the bone marrow or liver. The Ann Arbor system (see Appendix 2) is a clinical tool that was originally developed for Hodgkin’s disease, but is also used for FL to determine the stage of the lymphoma. It classifies four stages of disease that reflect both the number of sites of involvement and the presence of disease above or below the diaphragm. 34 Each stage of disease is divided into two subsets of patients according to the absence (A) or presence (B) of systemic symptoms. Fever without other cause, night sweats and weight loss of > 10% of body weight are considered to be systemic symptoms. The tests carried out for staging include blood tests, computerised tomography (CT) scan, bone marrow biopsy. Positron emission tomography (PET) scan may also be used, although is not routine in the UK. At most, 10–15% of FLs are detected at the early stage;35 thus the majority present with advanced-stage disease (Ann Arbor stage III–IV).
Grade
Follicular lymphoma is a low-grade or indolent B-cell disease and is diagnosed according to the World Health Organization (WHO) classification. Grade is determined by histology (i.e. by inspecting cells under the microscope), which looks at the number and size of abnormal cells taken from lymph node biopsies. The disease maybe subdivided into grades 1/2 (combined in the latest version of the WHO classification), grade 3a or 3b. These subdivisions of grade 3 are based on the presence of increasing numbers of more aggressive cells termed centroblasts. Grade 3b is treated in the same manner as the common high-grade NHL, DLBCL. Grades 1/2 and 3a are managed as indolent forms. Each disease stage (Ann Arbor stages I–IV) can be assigned a grade (1–3a/b).
Systems of classification
Follicular lymphoma is classified according to its morphology, immune phenotype, genetics, and clinical features of neoplasms. Since the 1970s, various classification systems have been used to differentiate NHLs, which have developed alongside an increasing understanding of the different cellular components of the lymphatic system that the cancer process affects. 36 It is useful to be familiar with previous classification systems in order to interpret the older literature for lymphomas with now outdated names. The third edition of the International Classification of Diseases for Oncology (ICD-O) provides a guide for translation of previous classification systems into the present. 37
The earliest classification systems were based on the cellular morphology of neoplastic cells and their relationship to the lymphoid tissue architecture. The Rappaport Classification, which was used until the 70s, was devised before lymphoid cells were split into T and B cells. 38 In the early 1970s, the Kiel Classification system was proposed, which classified lymphomas according to their cellular morphology and their relationship to cells of the normal peripheral lymphoid system. 39 The Working Formulation devised by the National Cancer Institute in 1982 attempted to translate the recognised classification systems for NHL (it did not include Hodgkin’s lymphomas). The Working Formulation was a purely histological classification and divided lymphomas into four grades (low, intermediate, high and miscellaneous), related to prognosis, and included subdivisions based on the size and shape of affected cells. However, this classification system did not differentiate between T and B cells and is now obsolete.
With the development and application of immunophenotyping and cytogenetic and molecular genetic testing, the Revised European–American Lymphoma Classification (REAL) classification system was devised in the mid-1990s and incorporated immunophenotype and genetic criteria. The WHO classification system, based on the REAL classification, is the latest classification system and the most widely used and accepted. The WHO classification was updated in 2008 and groups lymphomas by cell type and defines phenotypic, molecular and cytogenetic characteristics. There are three large groups of neoplasms: (1) B cell, (2) T cell and (3) natural killer cell neoplasms. FLs are grouped under the B-cell type (ICD-O-3 codes: 9690/3, 9691/3, 9695/3 and 9698/3).
Prognosis
Follicular lymphoma is curable for only a few patients, mainly those with localised or early-stage disease (Ann Arbor stages I and II). 40 Most advanced-stage patients respond to initial drug therapy and their symptoms go into remission. However, despite novel therapies and recent improvements in therapy, advanced FL is not considered curable. Patients with advanced FL undergo multiple relapses with the duration of remissions shortening at each subsequent treatment at recurrence. 30,41
Prognostic factors
Prognostic factors in FL can be categorised as patient-related factors and disease-related factors. By analysing prognostic factors, indices have been developed to predict clinical outcomes such as progression-free survival (PFS) and OS. Two such indices are the International Prognostic Index (IPI) and the Follicular Lymphoma International Prognostic Index (FLIPI).
Patient-related variables
The most important patient-related prognostic factors are performance status and age. 42 Performance status, is defined by the Eastern Cooperative Oncology Group (ECOG)43 and ranges from 0 (fully active) to 4 (completely disabled); thus poorer performance status is associated with a poorer FL prognosis (see Appendix 3 for ECOG performance status in detail). However, only 10–15% of patients with FL present with a poor performance status at the time of diagnosis. 42
Age of > 60 years is a significant factor for prognosis. 44,45 The existence of comorbidities and alterations in immunity with age might limit the drugs that can be used. 46 In addition, alterations in pharmacokinetics and reduction in hepatic and renal function occurs with increasing age. This affects the absorption, distribution, activation, metabolism and clearance of drugs. 46 This impacts on the clinician’s ability to treat elderly patients effectively. Gender has also been shown to be an important prognostic factor; the male sex is associated with a poorer clinical outcome. 28
Disease-related factors
Histological features such as lower degree of follicularity (i.e. greater diffuse areas),47–50 absence of interfollicular fibrosis47 and high content of macrophages in biopsy samples51 are associated with poor prognosis; helper T-cell infiltrates have been associated with a survival benefit. 52,53 Genetic features such as oncogenes or tumour-suppressor genes, chromosomal gains or losses and gene expression profiles have been found to affect prognosis. 42
Factors relating to disease extent are important in predicting prognosis. Patients with limited stage disease (i.e. Ann Arbor stage I or II) are likely to have prolonged survival. 42 However, the majority of patients present with advanced disease (stage III or IV), thus the effect of other clinical parameters has been investigated. A larger number of extranodal sites involved,44,45,54,55 presence of B symptoms,44,54 the presence and greater extent of bone marrow involvement56 and the presence of hepatosplenomgaly30 have all been found to affect adversely prognosis. In addition, tumour burden has been identified as an important prognostic factor; however, it is inconsistently defined according to size of lymph node masses, number of extranodal sites involved, degree of splenomegaly or hepatomegaly and the presence of circulating lymphoma cells. 42
Biological markers such as elevated lactate dehydrogenase (LDH) have been found to predict lower response rates and survival. 28,44,54 A normal haemoglobin level has been found to be a favourable factor for prognosis, whereas a haemoglobin level of < 12 mg/dl is a poor prognostic factor. 30
International Prognostic Index
The IPI was originally designed as a prognostic tool for aggressive NHL (DLBCL), and is based on the presenting features and the extent of disease. The IPI has been reported to discriminate between patients with FL with significantly different survival periods,57 and is now used as a predictive tool for survival in FL (Table 3).
| One point is assigned for each of the following risk factors | The sum of the points allotted correlates with the following risk groups |
|---|---|
|
Age > 60 years Ann Arbor stage III or IV disease Elevated serum LDH ECOG/Zubrod performance status of 2, 3 or 4 More than one extranodal site |
Low risk (zero to one point): 5-year survival of 73% Low-intermediate risk (two points): 5-year survival of 51% High-intermediate risk (three points): 5-year survival of 43% High risk (four to five points): 5-year survival of 26% |
Follicular Lymphoma International Prognostic Index (FLIPI and FLIPI2)
In 2004, the FLIPI was developed specifically for patients with FL. Evaluations of demographic, clinical and biological characteristics from > 4000 patients with FL were used in univariate and multivariate analyses to develop the FLIPI. It provides clinicians and patients with a prognostic index based on five criteria (age > 60 years, Ann Arbor stage III or IV, number of nodal sites of involvement greater than four, elevated serum LDH, and haemoglobin level of < 12 g/dl). The FLIPI assesses OS, i.e. carrying a low (zero to one risk factors), intermediate (two risk factors) or high risk (three to five risk factors). 58 The FLIPI has been further refined to accommodate more recent developments in the collection of biological data and newer treatment modalities such as immunotherapy, resulting in FLIPI2. 59 For example, β2-microglobulin is an independent prognostic marker included in later versions of the FLIPI.
Significance in terms of ill-health (burden of disease)
The nature of NHL in general, and the relapsing–remitting course of FL in particular, suggests that both individually and at a population level it is responsible for a considerable amount of morbidity and mortality (see Epidemiology). In 2009, NHL accounted for 0.8% of all deaths and 2.9% of all cancer deaths in England and Wales (see Appendix 4 for data sources and numbers used), and is the ninth most common cause of cancer mortality in the UK. 2
Current service provision
Objectives of treatment and important health outcomes
Advanced FL is not curable. However, because of the age distribution and presence of comorbidities, patients may remain uncured from FL but may die from other causes unrelated to the disease. The aim of disease management is both to increase patient life expectancy and to increase patient health-related quality of life (HRQoL). First-line treatment aims to produce a maximum initial response by reducing tumour burden,60 to prolong the periods of PFS and OS, to increase the duration between episodes of disease recurrence and to minimise the symptoms associated with relapse and treatment side effects. 61
Therefore, the following outcomes are likely to be of potential importance:
-
absence of disease at given points in time following diagnosis
-
absence of symptoms
-
absence of side effects
-
duration of survival
-
– OS
-
– PFS
-
-
HRQoL
-
patient and carer satisfaction.
Management of disease
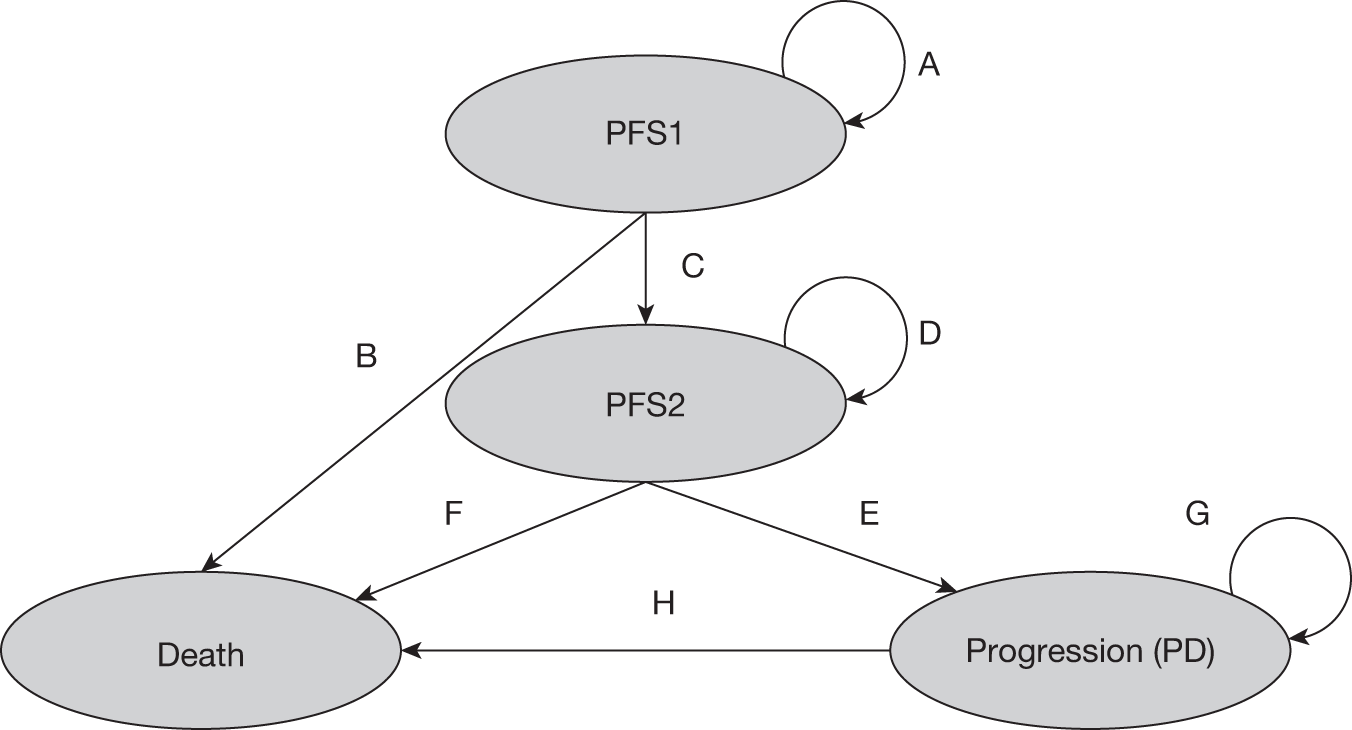
Grading, staging and symptoms determine treatment pathways. Figure 1 gives an overview of the treatment pathway for stage III/IV FL [adapted from the manufacturer’s submission (MS) for this appraisal]. 62 This pathway has been simplified and does not take into account the risk of transformation to DLBCL or the differences in treatment of disease that relapses early compared with later relapse. These are discussed later in this section.
FIGURE 1.
Treatment pathway for stage III/IV FL (adapted from MS). 62 a, Response can be complete or partial response. b, Note that patients who received chlorambucil in first-line treatment would not be eligible to receive stem cell transplant.
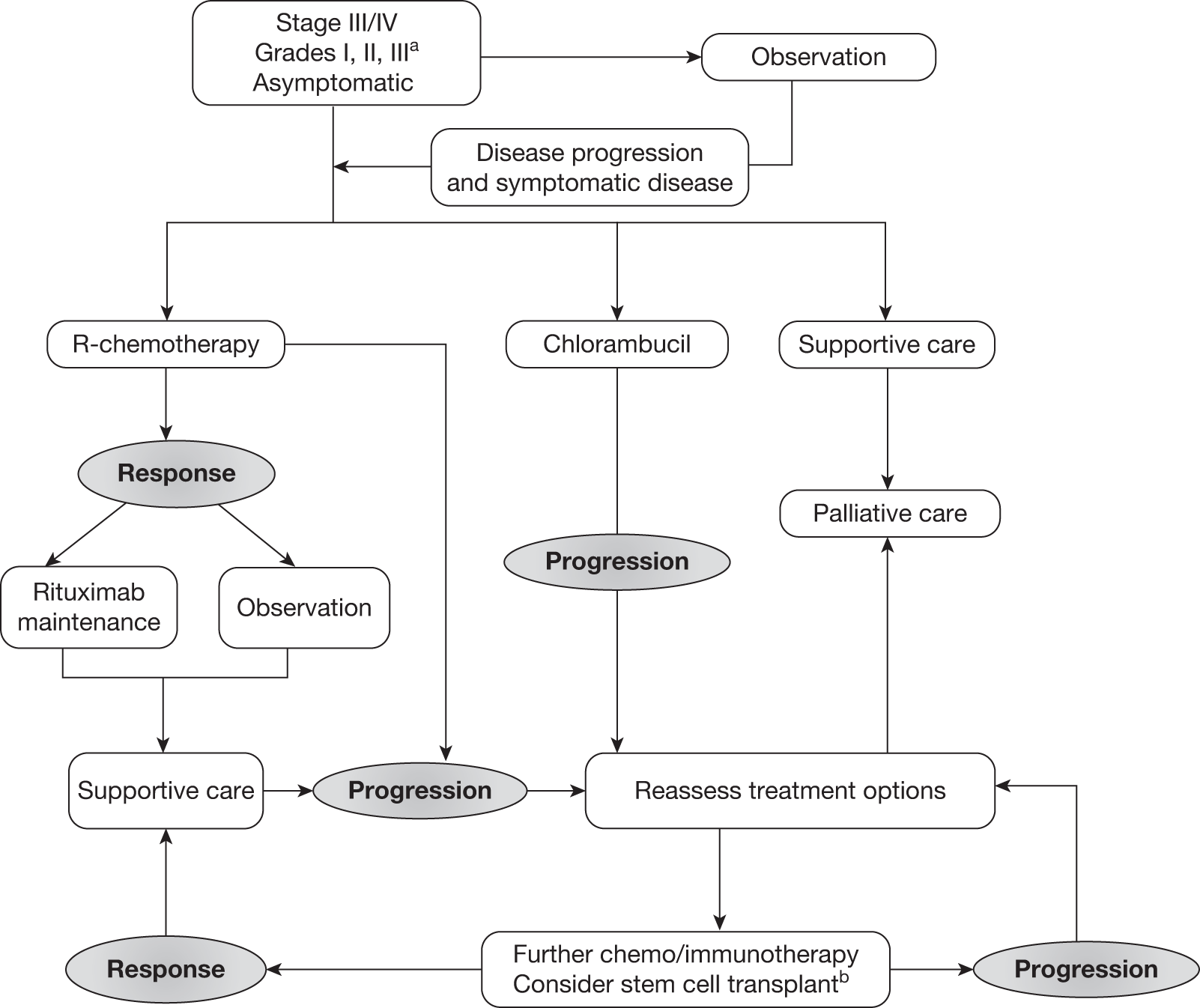
Asymptomatic patients
Most patients are asymptomatic on presentation (painless swelling of one or more lymph nodes) and a ‘watch and wait’ approach is usually adopted. Observational studies63,64 and three randomised controlled trials (RCTs)65–67 have shown that prognosis is not affected by immediate treatment compared with observation until symptomatic disease progression (bulky lymphadenopathy, bone marrow compromise, splenomegaly, etc.). Thus, treatment commences only when the disease becomes symptomatic.
First-line therapy: limited disease (Ann Arbor stages I–II)
Patients diagnosed in the early stages of the disease (stages I–II) usually respond well to radiotherapy and this is the treatment of choice, usually taking the form of extended or involved field form irradiation. This can result in long-term disease-free survival (DFS) and possible cure for between 45% and 80% of patients. 35
First-line therapy: advanced disease (Ann Arbor stages III–IV)
Chemoimmunotherapy [i.e. rituximab and chemotherapy (R-chemotherapy)] is the preferred treatment for first-line therapy in symptomatic advanced FL. The European Society for Medical Oncology (ESMO) clinical practice guidelines recommend that when complete remission and long PFS are the aims of treatment, rituximab in combination with chemotherapy [such as cyclophosphamide, doxorubicin/adriamycin, vincristine and prednisolone (CHOP); cyclophosphamide, vincristine and prednisolone (CVP), fludarabine, cyclophosphamide (FC); fludarabine and mitoxantrone (FM) or bendamustine] should be used. 19 In 2006, National Institute for Health and Clinical Excellence (NICE) guidance stated that rituximab in combination with CVP is indicated for the first-line treatment of symptomatic FL, in line with the licensed indication at the time the guidance was issued. 68 However, in 2008 the licence for rituximab was broadened so that it can be administered with other chemotherapies; there is no consensus, however, on the preferred chemotherapy option. 69 Antibody monotherapy or single-agent alkylating agents [e.g. chlorambucil (Leukeran®, Aspen)] can be considered an alternative in previously untreated patients with FL with particularly low-risk disease or those who are unsuitable for more intensive treatments. 19
Maintenance therapy (first line)
As disease recurrence is inevitable, ways of maintaining or improving the quality of the initial response to treatment are used, such as maintenance therapy. Maintenance treatment is a long-term approach that aims to delay relapse by stabilising the best response to initial therapy, eradicating any residual disease and maintaining remission after successful remission induction therapy. 70
The ESMO clinical practice guidelines acknowledge recent evidence that rituximab maintenance for 2 years can prolong PFS. 71 Guidance issued in June 2011 by NICE recommended rituximab maintenance therapy as an option for the treatment of people with follicular NHL lymphoma who have responded to first-line induction therapy with rituximab in combination with chemotherapy. Prior to this, the UK standard practice has been to closely observe patients during their first remission and retreat only when there is evidence of disease progression.
Aside from rituximab, other agents have been proposed for use as maintenance therapy, such as interferon-alpha (a biological therapy). However, a meta-analysis suggests a limited benefit of interferon-alpha maintenance therapy that has to be balanced against toxicity. 72 Clinical advice is not to use interferon-alpha as patients cannot tolerate the side effects.
Consolidation therapy is another type of treatment that has been proposed following successful induction of first-line remission. Consolidation therapy is delivered immediately after a response to induction therapy; however, it differs from maintenance therapy as it is a short course of treatment that aims to rapidly improve the response to induction therapy. 60 Radioimmunotherapy agents such as ibritumomab tiuxetan (Zevalin®, Spectrum Pharmaceuticals) have been used in consolidation therapy; however, their benefit following a R-chemotherapy combination has not been established. 19
Treatment of relapsed disease
After every relapse, a biopsy should be undertaken to determine if transformation has occurred. 5,19 When transformation does occur, there is usually rapidly increasing lymph node enlargement, elevated LDH levels and development of systemic symptoms. Histological transformation can occur in 20–70% of patients, with the variability in reported incidence reflecting, to a large extent, local practice in terms of whether or not biopsies are performed at each recurrence. 5 Treatments for FL are not effective once transformation has occurred and patients are treated as for high-grade FL or DLBCL. Median survival following transformation has been reported as 18 months, although this figure comes from the pre-rituximab era. 5
When the disease has relapsed, treatment options are reassessed, with the selection of salvage treatment depending on the efficacy of prior regimens. 19 However, there may be some variations between clinical practice in the UK and the ESMO guidelines.
When there is early relapse following first-line R-chemotherapy treatment (< 6 months), the disease is considered as rituximab refractory in the ESMO guidelines, which state that rituximab is not indicated. However, clinical advice to the Assessment Group (AG) indicated that some clinicians may also consider which chemotherapeutic regimen was given in first-line treatment when choosing the second-line treatment. For example, if rituximab, cyclophosphamide, vincristine and prednisolone (R-CVP) had been used in first-line induction therapy and early relapse occurred, rituximab, cyclophosphamide doxorubicin/adriamycin, vincristine and prednisolone (R-CHOP) may be selected for the second-line treatment, with the rationale being that it was the CVP-component rather than the rituximab that was responsible for the early relapse. If, however, R-CHOP had been used in first-line induction therapy, and relapse is early, this is indicative of a poor prognosis (based on clinical advice sought by the AG), making high-dose chemotherapy (with or without rituximab) and stem cell transplant (SCT) an appropriate second-line treatment.
The ESMO guidelines also state that in relapses of < 12 months, a non-cross-resistant scheme should be preferred with regard to the chemotherapy selected (i.e. two differing chemotherapeutic regimens such as fludarabine after CHOP for example). Rituximab monotherapy is also recommended as a treatment option by NICE for people with relapsed or refractory disease when all alternative treatment options have been exhausted. 73
The use of rituximab in retreatment of patients who have received rituximab at first-line treatment has been discussed previously in NICE technology appraisal (TA137), where evidence for clinical effectiveness of rituximab in second-line treatment of FL was from the EORTC 20981 trial,74,75 the population of which were rituximab-naive patients. However, although the Committee considered that ‘it was necessary to be cautious about the assumption that rituximab is as efficacious in patients who had already received it as in patients who are rituximab-naive’; clinical specialists present at the Committee stated that ‘the evidence indicated that follicular NHL could be retreated with rituximab with little or no loss of efficacy’. It was noted by the Committee that although this is as an area of uncertainty, this was biologically plausible given rituximab’s mechanism of action. 73 This is discussed in more detail, see Resistance to rituximab in patients previously exposed to rituximab treatment.
Second-line rituximab maintenance
Following response to second-line induction therapy (with or without rituximab), rituximab monotherapy may also given as second-line maintenance, as recommended by NICE. 73
Stem cell transplant
During the course of treatment, relapses become more frequent with shorter disease-free periods,69 and chemotherapy or chemoimmunotherapy are not able to induce a further stable remission period. SCT is a treatment option for patients with relapsed FL. However, the use of and position of SCT in the treatment pathway of FL has altered since the introduction of rituximab, and the ESMO guidelines state that its use needs to be re-evaluated in the rituximab era. 19 Clinical advice provided to the AG suggests its use has declined in the treatment of FL since the introduction of rituximab in first-line induction and maintenance, and second-line induction and maintenance. In second-line treatment, SCT appears to be reserved for patients with very aggressive disease and short remission periods following first-line induction therapy or patients who have undergone transformation to DLCBL. For patients who do not have aggressive disease and for whom a reasonable remission period has been achieved following first-line treatment, SCT is considered more frequently at the third-line treatment stage. At whichever point SCT is offered in the treatment pathway, it is usually only offered to younger patients (aged < 65 years), although clinical advice suggests that it may be offered to some fit patients up to the age of 70 years.
Relevant national guidelines
A summary of the relevant European Medicines Agency (EMA) licensing and NICE guidelines relating to the use of rituximab in the treatment of FL is presented in Table 4.
| Stage of disease | Treatment | Licensed by EMA | Recommendation by NICE | Conditions of NICE recommendation |
|---|---|---|---|---|
| First-line induction | R-CVP | ✓ | ✓ |
Previously untreated patients Symptomatic patients |
| First-line induction | R-chemotherapya | ✓ |
✗ Considered in this assessment report |
Not applicable |
| First-line maintenance | R-monotherapy | ✓ |
✗ Ongoing technology appraisal |
Being appraised: Only for responders to first-line induction therapy with rituximab in combination with chemotherapy |
| Second-line induction |
R-chemotherapya R-monotherapy |
✓ | ✓ | R-monotherapy only when all alternative treatment options have been exhausted (i.e. if there is resistance to or intolerance of chemotherapy) |
| Second-line maintenance | R-monotherapy | ✓ | ✓ | Only for responders to second-line induction therapy of rituximab or R-chemotherapy |
The ESMO has produced guidelines for the diagnosis, treatment and follow-up of newly diagnosed and relapsed FL19 as discussed above (see Management of disease). The British Committee for Standards in Haematology (BCSH) has produced guidelines on the diagnosis and reporting of NHLs76,77 from the BCSH website). A guideline on the investigation and management of follicular lymphoma is also available from the BCSH website. Archived guidance from the BCSH exists on the diagnosis and therapy for nodal NHL. 27
Variation in services and/or uncertainty about best practice
Although R-chemotherapy is the preferred treatment for first-line therapy in symptomatic advanced FL, there is no consensus on the preferred chemotherapy. 69 No direct trials have been undertaken that compare one R-chemotherapy regimen with another R-chemotherapy regimen; although there are four ongoing Phase III RCTs comparing one or more R-chemotherapy regimens against another R-chemotherapy78–81 (see Chapter 3, Results, for further details of ongoing trials). Siddhartha and Vijay82 conducted a meta-analysis to compare R-CHOP and R-CVP with respect to response rates (two separate analyses were provided for first-line treatment only and first-line plus relapsed treatment) and differences were noted in the quality of the responses achieved. A greater proportion of complete responses (CRs) were observed following R-CVP than R-CHOP. However, overall response rate (ORR) was better following the R-CHOP regimen [owing to more partial responses (PRs)]. It is difficult to know if there is a different effect in quality of response to R-CVP or R-CHOP; however, clinical advice to the AG noted that R-CHOP is more likely given to patients with bulky or more aggressive disease, who are more likely to achieve a PR than a CR.
However, treatment/efficacy outcomes are not the only factors to consider when choosing chemotherapy. Clinical advice suggests that elderly patients or patients with comorbidities, particularly cardiac problems, are less likely to receive CHOP, as it is an anthracycline-based chemotherapy. In addition, where SCT is a potential future treatment, the chemotherapeutic agent selected must not interfere with the potential to harvest stem cells. Thus, in SCT candidates, fludarabine, a purine analogue therapy, is to be avoided as these can compromise the quality of the stem cell harvests.
The manufacturer sought clinical guidance from two clinicians whose responses also reflected the need for an individualised choice of chemotherapeutic agent in patients. 62 The clinicians also highlighted other important factors in treatment selection, including patient choice (e.g. acceptability of alopecia, which is higher after CHOP, and side effects tolerance) and the need to achieve a rapid response if a compression syndrome is present (e.g. deep-vein thrombosis, leg oedema).
Current usage in the NHS
Figures reported in the MS62 from an unpublished survey of UK haemato-oncologists (n = 50) suggest that approximately 92% of all eligible previously untreated stage III–IV patients with FL in the UK currently receive rituximab in combination with chemotherapy as standard treatment (these data were made available by the manufacturer to the AG). 62 The remaining 8% receive single-agent chlorambucil, FM, Bexxar (a radiolabelled monoclonal antibody) or alternative chemotherapy. Of the patients receiving a rituximab-containing regimen, approximately 67% are treated with R-CVP and a further 16% are treated with R-CHOP. The remainder receive rituximab combined with other chemotherapies, which includes R-chlorambucil (R-C), rituximab, fludarabine and cyclophosphamide (R-FC), rituximab, fludarabine, cyclophosphamide and mitoxantrone (R-FCM) and R-fludarabine (R-F). 62 The AG requested access to the survey data from Roche and the results are presented in Table 5.
| Treatment | No. | % |
|---|---|---|
| R-CVP | 80 | 67 |
| CVP | 1 | 1 |
| R-CHOP | 19 | 16 |
| Chlorambucil | 6 | 5 |
| R-C | 4 | 3 |
| R-FC | 5 | 4 |
| FM | 1 | 1 |
| R-FM | 1 | 1 |
| R-F | 1 | 1 |
| Bexxar | 1 | 1 |
| Alternative chemotherapy | 1 | 1 |
Clinical advice sought by the AG suggests that this seems a reasonable estimate, indicating that the great majority of patients receive R-chemotherapy. Chlorambucil as a single-agent chemotherapy regimen is reserved only for patients deemed too unfit or unwell for a R-chemotherapy regimen. The proportions of R-CHOP and R-CVP administered are difficult to quantify according to clinical advice; historically R-CVP has been the first choice chemotherapy arm; however, R-CHOP is the international standard. However, at present R-CHOP is not currently recommended by NICE, which is likely to affect its current uptake within the UK. Clinical advice suggests that the use of other chemotherapy regimens in combination with rituximab such as R-MCP (rituximab, mitoxantrone, chlorambucil and prednisolone), R-CNOP (rituximab, cyclophosphamide, mitoxantrone, vincristine and prednisolone), R-CHVP (rituximab, cyclophosphamide, doxorubicin, etoposide and prednisolone), R-FCM, R-FM, R-F and R-C is very infrequent within the NHS.
Current service cost
Because treatment of FL is part of general haematological or oncology services, the cost of caring for this group of patients is very difficult to derive from the routine financial information available for the NHS. However, consideration of the variety of treatments to which an individual might be exposed during the course of their illness suggests that the costs of caring for FL are likely to be considerable. In this, the support required from both primary and palliative care services in the terminal stages of the disease should not be underestimated.
Significance for the NHS
Rituximab with CVP is currently recommended by NICE for the first-line treatment of FL. 83 Thus, given the number of patients with FL, the introduction of rituximab with other chemotherapies would incur costs. However, neither new equipment nor intensive training would be required.
Description of technology under assessment
Identification of patients and important subgroups
Rituximab in combination with chemotherapy is considered as a possible option for the treatment of symptomatic stage III–IV FL.
Place in treatment pathway
This assessment report is concerned with the use of R-chemotherapy as first-line induction treatment. However, rituximab with or without chemotherapy is recommended by NICE at other points within the treatment pathway, and these impact on the cost-effectiveness of R-chemotherapy in first-line induction therapy (see Table 4 for NICE recommendations of rituximab).
Therapeutic classification
Rituximab is a genetically engineered ‘monoclonal antibody’ that has been designed to recognise an antigen/surface marker on B lymphocytes called CD20. Monoclonal antibodies are produced by fusing single antibody-forming cells (generated in laboratory mice) to tumour cells (grown in culture), producing large quantities of identical antibody molecules from a single, cloned antibody-producing cell, hence the name ‘monoclonal antibodies’. 36
The CD20 antigen/surface marker is present on the surface of B lymphocytes in > 90% of NHLs. 84 When rituximab attaches to the antigen, this causes cell death85 so that cancerous and normal B lymphocytes are destroyed. Although fully developed B lymphoma cells have CD20 on their surface, early B cells do not have the CD20 protein and are not killed.
Brand and generic name
Rituximab is the generic name; Roche’s brand name is MabThera (Genentech Inc.). Rituximab is also known as IDEC-C2B8 and Rituxan®. 86
Dosage form and route
Rituximab is sold as a concentrate for solution for intravenous infusion. A 10-ml single-use vial is available and contains 100 mg of rituximab (sold in packs × two vials). 85 A 50-ml single-use vial is also available (500 mg/50 ml).
Method of administration
Premedication with glucocorticoids should be considered if rituximab is not given in combination with glucocorticoid-containing chemotherapy. Premedication consisting of an antipyretic and an antihistaminic, for example paracetamol and diphenhydramine, should always be administered before each infusion of rituximab. 85
First infusion
The recommended initial rate for infusion is 50 mg/hour; after the first 30 minutes, it can be escalated in 50 mg/hour increments every 30 minutes, to a maximum of 400 mg/hour. 85
Subsequent infusions
Subsequent doses of rituximab can be infused at an initial rate of 100 mg/hour, and increased by 100 mg/hour increments at 30-minute intervals, to a maximum of 400 mg/hour. 84 The prepared rituximab solution should be administered as an intravenous infusion through a dedicated line. It should not be administered as an intravenous push or bolus. 85
Licensed indications
Rituximab is licensed for the treatment of previously untreated patients with stage III–IV FL in combination with chemotherapy. This current licence was issued in January 2008 and does not restrict the type of chemotherapy. The original licence agreement restricted use of rituximab in combination with CVP only and this is reflected in the existing NICE guidance. 83
Rituximab is also licensed for treatment of FL at other stages within the treatment pathway, other types of NHL, and has indications for treatment of chronic lymphocytic leukaemia (CLL) and rheumatoid arthritis. The indications for use in FL and NHL are included below for completeness:
-
Rituximab maintenance therapy is indicated for patients with FL who are responding to induction therapy with chemotherapy with or without rituximab.
-
Rituximab monotherapy is indicated for treatment of patients with stage III–IV FL who are chemoresistant or are in their second or subsequent relapse after chemotherapy.
-
Rituximab is indicated for the treatment of patients with CD20-positive DLBCL in combination with CHOP.
Contraindications
Rituximab is contraindicated for use in NHL in patients who have known hypersensitivity to the active substance or to any of the excipients or to murine proteins, in active severe infections or inpatients in a severely immunocompromised state. 85
Warnings
Infusion reactions
Infusion-related side effects (including cytokine release syndrome) are reported commonly with rituximab and predominantly occur during the first infusion and include symptoms such as fever and chills, nausea and vomiting, allergic reactions (such as rash, pruritus, angioedema, bronchospasm and dyspnoea), flushing and tumour pain. 86 Mild or moderate infusion-related reactions usually respond to a reduction in the rate of infusion, which can be increased on improvement of symptoms. Patients who develop severe reactions, especially severe dyspnoea, bronchospasm or hypoxia should have the infusion interrupted immediately. 85
Before each dose of rituximab, patients should be given an analgesic and an antihistamine to reduce these effects and consideration should be given to premedication with a corticosteroid. In all patients, the infusion should not be restarted until symptoms have resolved and laboratory values and chest radiographs appear normal. Patients who have experienced severe cytokine release syndrome should be closely monitored, as although they may show an improvement in symptoms, this may be followed by deterioration. Thus, such patients must be evaluated for evidence of tumour lysis syndrome and pulmonary infiltrations with chest radiography.
Fatalities following severe cytokine release syndrome (characterised by severe dyspnoea) and associated with features of tumour lysis syndrome have occurred 1–2 hours after infusion of rituximab. Patients with a high tumour burden and those with pulmonary insufficiency or infiltration are at increased risk and should be monitored very closely. 86
Anaphylactic and other hypersensitivity reactions have been reported following the intravenous administration of proteins to patients. Clinical manifestations of anaphylaxis may appear similar to clinical manifestations of the cytokine release syndrome. However, in contrast to cytokine release syndrome, true hypersensitivity reactions typically occurs within minutes after starting infusion. 85
Pregnancy and lactation
Rituximab should be avoided during pregnancy unless the potential benefit to the mother outweighs risk of B-lymphocyte depletion in the fetus. It is also contraindicated in women who are breastfeeding. Effective contraception is required during treatment and for 12 months after treatment. 86
Cardiovascular disease
Rituximab should be used with caution in patients who are receiving cardiotoxic chemotherapy or who have a history of cardiovascular disease because exacerbation of angina, arrhythmia and heart failure have been reported. Transient hypotension occurs frequently during infusion and antihypertensive drugs may need to be withheld for 12 hours before infusion. 86
Infections
Serious infections, including fatalities, can occur during therapy with rituximab. Physicians should exercise caution when considering the use of rituximab in patients with a history of recurring or chronic infections or with underlying conditions that may further predispose patients to serious infection. 86
Personnel involved
Treatment should be undertaken under close supervision of a specialist. 86 The delivery of rituximab requires no additional personnel to the administration of chemotherapy, namely a senior clinician (specialist registrar or above), a specialist nurse and a specialist pharmacist.
Setting
Outpatients would receive intravenous transfusion in the same chemotherapy suite as would be used for the administration of chemotherapy.
Equipment required
Full resuscitation equipment should be at hand. 86 The intervention would require no equipment outside of that normally associated with a chemotherapy suite. Some clinics advise that rituximab is infused while the patient is on a bed, rather than in a chair.
Length of treatment
Each service user would expect to receive one treatment on day one of each cycle, every 3 weeks, for up to eight cycles; in other words, eight intravenous days (4–6 hours each) at the chemotherapy suite, over the course of 24 weeks.
Follow-up required
The ESMO guidelines suggest follow-up treatment both during and after treatment. However, clinical advice to the AG suggests that follow-up differs in UK clinical practice, particularly with regard to the frequency of cross-sectional imaging, which is not undertaken routinely in the absence of clinical suspicion of progression.The BCSH guidelines87 on the investigation and management of follicular lymphoma specifically states that routine scans are not recommended.
During treatment, the ESMO guidelines state that ‘adequate radiological tests should be performed mid-term and after completion of chemotherapy’. Where an insufficient or no response is found, patients should be evaluated for early salvage regimens. The ESMO guidelines19 suggest the following as follow-up after treatment; however, it is noted that clinical advice does not agree with the frequency of imaging:
-
History and physical examination every 3 months for 2 years, every 4–6 months for a further 3 years, and subsequently twice a year with special attention to transformation and secondary malignancies including secondary leukaemia.
-
Blood count and routine chemistry every 6 months for 2 years, then only as needed for evaluation of suspicious symptoms.
-
Evaluation of thyroid function in patients with irradiation of the neck at 1, 2 and 5 years.
-
Minimal adequate radiological or ultrasound examinations every 6 months for 2 years and annually thereafter. (Note that this is not recommended by the clinical advice sought by the AG.)
Anticipated costs associated with intervention
The recommended dose of rituximab is 375 mg/m2; the net price for a 10-ml vial is £174.63 and for a 50-ml vial £873.15. 86
Chapter 2 Definition of the decision problem
Decision problem
Intervention
Rituximab is indicated for the treatment of previously untreated patients with stage III–IV FL in combination with chemotherapy at a recommended dose of 375 mg/m2 of body surface area (BSA) per cycle, for up to eight cycles. This assessment includes interventions where rituximab is given in combination with the following chemotherapy regimens:
-
CVP
-
CHOP
-
CNOP
-
CHVP
-
MCP
-
FCM (fludarabine, cyclophosphamide and mitoxantrone)
-
FM
-
bendamustine
-
fludarabine
-
chlorambucil.
When this appraisal started, bendamustine was not currently licensed as a first-line treatment with rituximab for first-line treatment of FL. However, as the anticipated date of licensing was not known and could occur within the time scales of the appraisal, bendamustine was included as a combination chemotherapy agent (with rituximab). At the time of writing, bendamustine remains unlicensed for use in this population for the first-line treatment indication.
Population including subgroups
The population comprised adults with symptomatic stage III–IV FL (a NHL) who have not received any previous treatment. Indolent FL is considered within this appraisal. Where data are presented for elderly patients with FL (aged ≥ 65 years), these will be examined as a subgroup.
Relevant comparators
Non-rituximab-containing chemotherapies are the relevant comparators, and for this assessment the following comparators are considered:
-
CVP
-
CHOP
-
CNOP
-
CHVP
-
MCP
-
FCM
-
FM
-
bendamustine
-
fludarabine
-
chlorambucil.
Outcomes
The outcomes considered in this appraisal mostly relate to clinical effectiveness and cost-effectiveness and include:
-
OS
-
PFS
-
response rates
-
duration of disease remission/response duration
-
adverse effects of treatment
-
HRQoL.
Overall aims and objectives of assessment
This assessment will address the question ‘What is the clinical effectiveness and cost-effectiveness of rituximab (in its licensed indication) with chemotherapy for the first-line treatment of symptomatic stage III–IV FL?’
The aim of this review is to systematically evaluate and appraise the clinical effectiveness and cost-effectiveness of rituximab (in its licensed indication) in combination with chemotherapy compared with non-rituximab-containing chemotherapy, for the first-line treatment of symptomatic stage III–IV FL. Note that owing to the scope specifying the intervention as rituximab given in combination with chemotherapy, interventions including rituximab in combination with other treatments, such as radioimmunotherapy or bone marrow/SCT, are not considered as an intervention for this appraisal.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
This systematic review was carried out according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 88
Identification of studies
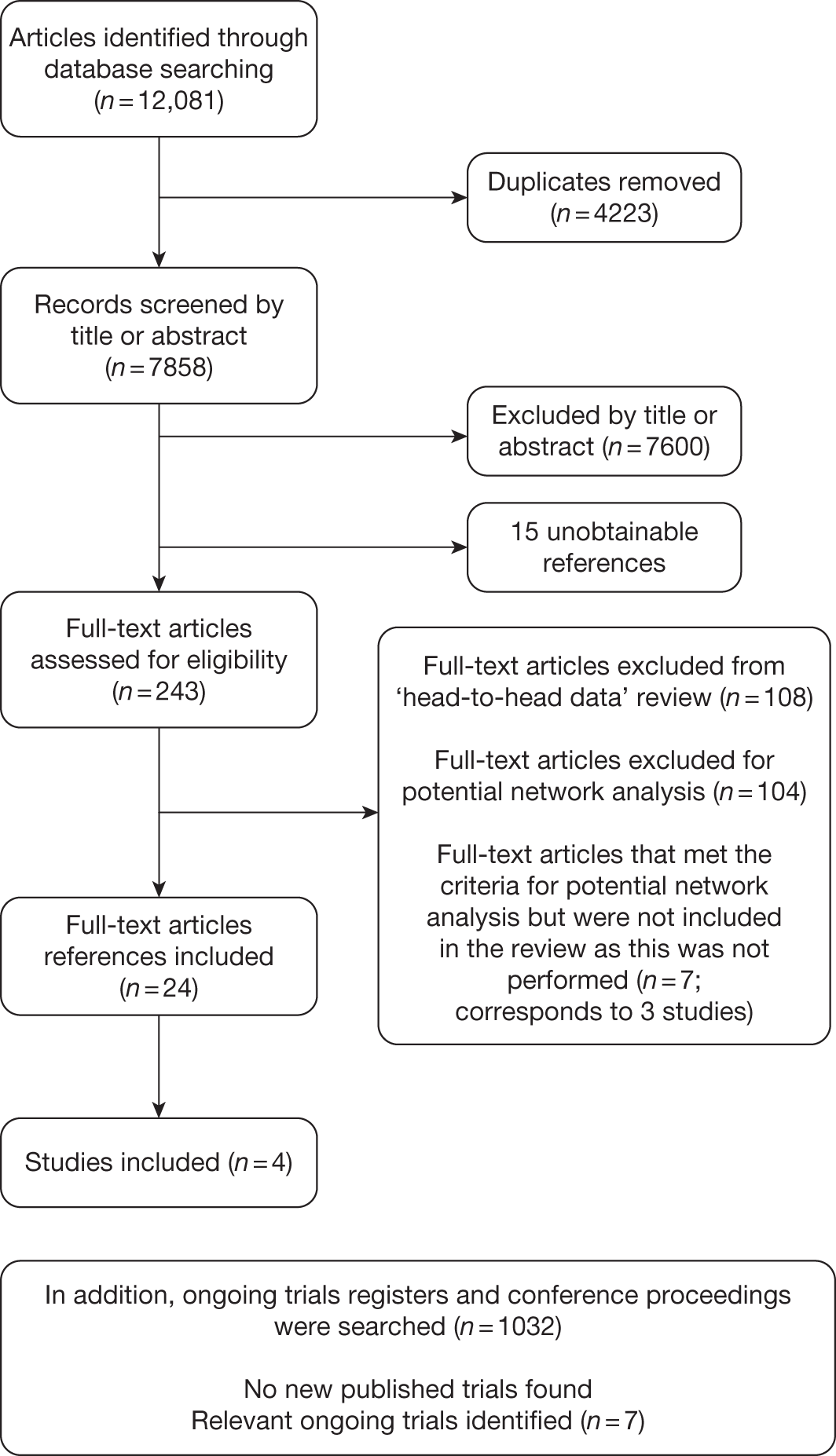
The PRISMA flow diagram shown in Figure 2 provides a summary of the study identification process.
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses-adapted flow diagram.
Search strategy
The search aimed to systematically identify all literature relating to the clinical effectiveness of (1) the intervention: rituximab in combination with chemotherapy or (2) the comparators, i.e. chemotherapy alone for the treatment of FL. The searches were conducted in September and October 2010.
Sources searched
Eleven electronic databases were searched from inception: MEDLINE including MEDLINE In-Process & Other Non-Indexed Citations (Ovid); CINAHL; EMBASE; The Cochrane Library including the Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) databases; Science Citation Index (SCI); and BIOSIS.
Ongoing research was searched using clinical trials databases and registers including NIHR Clinical Research Network Portfolio; National Research Register (NRR) archive 2000–7; Current Controlled Trials (CCT) and ClinicalTrials.gov.
Relevant conference proceedings were searched, including the American Society of Clinical Oncology (ASCO), European Society of Clinical Oncology (ESCO), American Society of Hematology (ASH), the British Society for Haematology (BSH) and the European Hematology Association (EHA).
In addition, the reference list of relevant articles and the MS62 was handsearched. The review team also contacted experts in the field and scrutinised the bibliographies of retrieved papers to identify relevant evidence.
Search terms
A combination of free text and thesaurus terms were used. ‘Intervention’ terms (e.g. rituximab, MabThera, Rituxan) or chemotherapy terms (CHOP, CVP, etc.) were combined with ‘population’ search terms (e.g. lymphoma, non-Hodgkin’s). Copies of the search strategies used in MEDLINE are included in Appendix 5 (these were adapted for use in other databases).
Search restrictions
Searches were not restricted by language or publication date. Where possible, a filter was applied in order to limit search results to systematic reviews/meta-analyses, economic/cost evaluations, quality-of-life studies or RCTs. Examples of the RCT filter, cost-effectiveness filter and quality-of-life filter are provided in Appendix 5.
Inclusion and exclusion criteria
Study design
According to the accepted hierarchy of evidence, RCTs were included for the clinical effectiveness review, as they provide the most authoritative form of evidence. In the event of insufficient data being available from RCTs, it was planned that observational studies or clinical trials would be considered; however, this was not required in this review.
Intervention(s)
Rituximab in combination with any of the following chemotherapy regimens: CVP, CHOP, CNOP, CHVP, MCP, FCM, FM, bendamustine, fludarabine or chlorambucil.
Comparator(s)
The comparator was chemotherapy without rituximab, which for this review was considered to be one of the following: CVP, CHOP, CNOP, CHVP, MCP, FCM, FM, bendamustine, fludarabine or chlorambucil.
Potential for a network meta-analysis
The literature search was undertaken to allow identification of trials involving either an intervention or comparator defined in the decision problem, as it was anticipated that the work may require a network meta-analysis to be undertaken to determine efficacy. It was planned to populate such an analysis with all identified trials involving either an intervention or a comparator. Although it is noted that the network meta-analysis could potentially be strengthened by the inclusion of RCTs involving two pharmaceuticals that were neither interventions nor comparators (provided there were RCTs comparing these pharmaceuticals with an intervention or a comparator), literature searches for all RCTs from these pharmaceuticals were not conducted, as they are likely to have little impact on the results of interest and would have significant resource implications. In addition, where the evidence allowed, interventions were planned to be compared with each other.
Population
The population comprised adults with symptomatic stage III–IV FL who had not received any previous treatment.
Outcomes
The primary outcome of interest for this appraisal in relation to clinical effectiveness was OS. Secondary outcomes were PFS, response rates (CR, PR and ORR), duration of disease remission/response duration, and adverse/toxic effects of treatment.
Overall survival was defined and calculated as the time from randomisation to the date of death by any cause. PFS was defined and calculated as the time from randomisation to disease progression or death. Response rate was defined in the terms laid down by Cheson et al. 87 (see Appendix 6). ORR combined CRs and PRs. Unconfirmed complete responses (CRus) were considered as PRs so that the CR and PR rates were comparable between studies. However, it is noted this may result in an underestimation of CR, as clinical advice suggests that CRus are more likely to follow a similar clinical course to CRs. Duration of disease remission/response duration was taken as the time from response achieved (CR or PR) to disease progression or death. Adverse events (AEs) were defined as any adverse change from the patient’s baseline condition, including intercurrent illness that occurred during the course of the clinical trial after the start of treatment, whether or not considered related to trial treatment. HRQoL was also considered as a secondary outcome.
Exclusion criteria
Reviews of primary studies were not included in the analysis, but were retained for discussion and identification of additional trials. Studies that were considered methodologically unsound were excluded from the review as well as the following publication types: non-randomised studies; animal models; preclinical and biological studies; narrative reviews, editorials, opinions; non-English-language papers and reports in which insufficient methodological details are reported to allow critical appraisal of study quality. In addition, although not stated in the protocol, studies that included populations other than those described above or studies that included NHL populations but did not provide outcome data separately for patients with FL who were excluded.
Study selection
Studies were selected for inclusion through a two-stage process according to the above inclusion/exclusion criteria. Titles and abstracts were examined for inclusion by one reviewer. Screening was checked by a second reviewer on 10% of citations. The kappa coefficient (range 0–1) calculated to measure inter-rater reliability was good, approaching ‘very good’ at 0.79. Discrepancies were resolved by discussion between the two reviewers when necessary, and did not require involvement of a third reviewer. Full manuscripts of selected citations were retrieved and assessed by one reviewer against the inclusion/exclusion criteria.
Data extraction strategy
Data were extracted by one reviewer using a standardised data extraction form and checked by a second reviewer. Discrepancies were resolved by discussion and did not require input from a third reviewer. Where multiple publications of the same study were identified, data were extracted and reported as a single study.
Critical appraisal strategy
The methodological quality of each included study was assessed by one reviewer and checked by a second reviewer, according to criteria based on those proposed by the NHS Centre for Reviews and Dissemination (CRD) for RCTs. 90
The following factors were considered: method of randomisation, allocation concealment, blinding of patients, outcome assessors and data analysts, numbers of participants randomised, baseline comparability between groups, specification of eligibility criteria, whether or not intention-to-treat (ITT) analysis was performed, completeness of follow-up and whether or not study power calculations were performed and reported.
Methods of data synthesis
Data were tabulated and discussed in a narrative review. Exploratory meta-analyses were performed to estimate a summary measure of the effect of response rates (ORR, CR and PR) based on ITT analyses. CRus were considered as PRs in the meta-analyses so that the CR and PR rates were comparable between studies. However, it is noted this may result in an underestimation of CR, as clinical advice suggests that CRus are more likely to follow a similar clinical course to CRs. Heterogeneity in these analyses was explored through consideration of the study populations, methods and interventions, by visualisation of results and, in statistical terms, by the chi-squared test for homogeneity and the I2-statistic. Meta-analysis was carried out using random-effects models, using the Cochrane Collaboration Review Manager© (RevMan) software, version 5.0 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
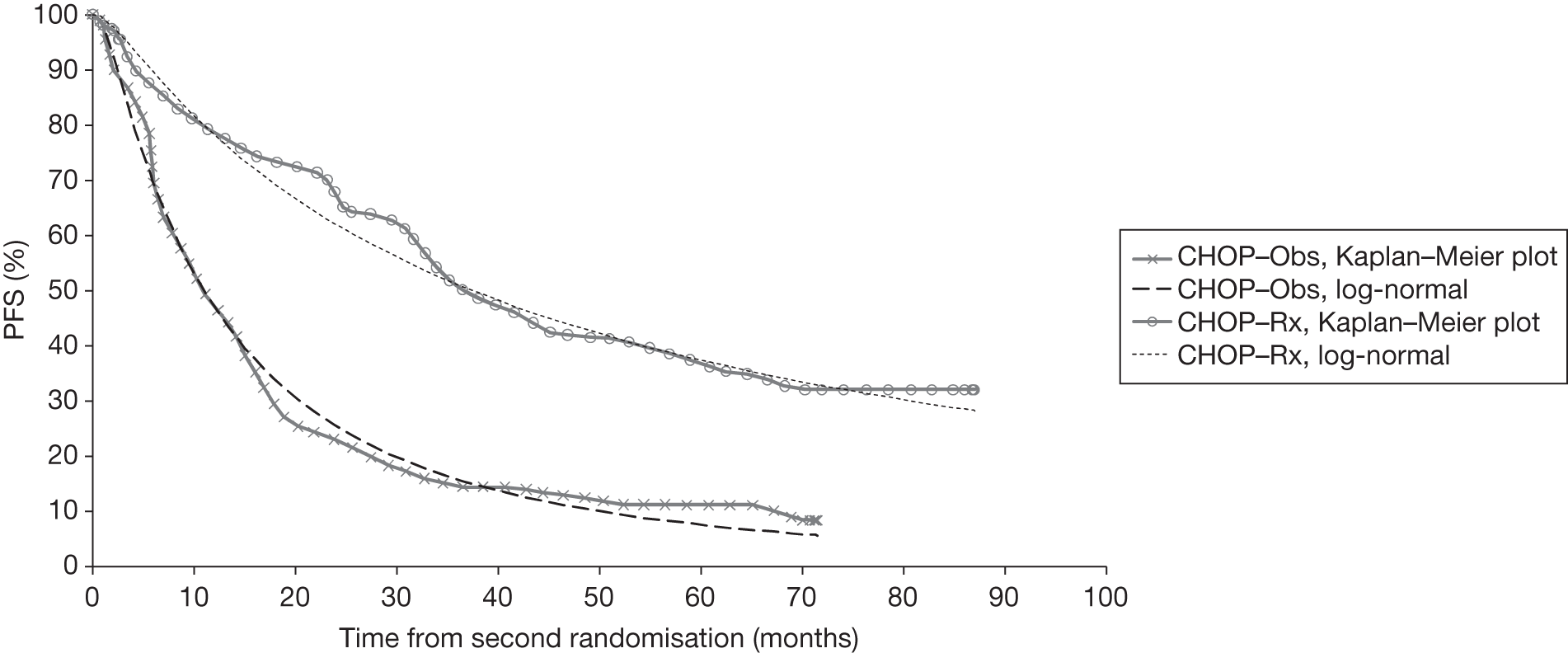
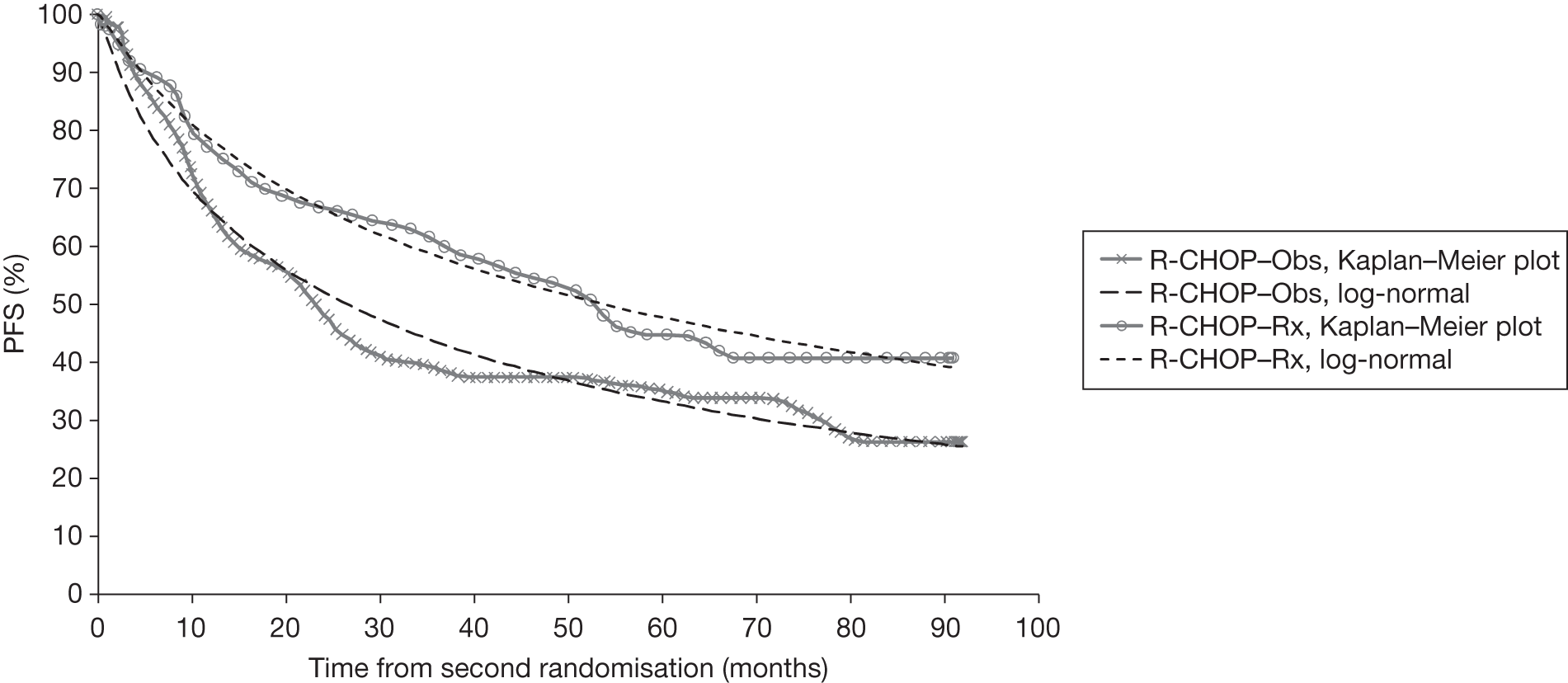
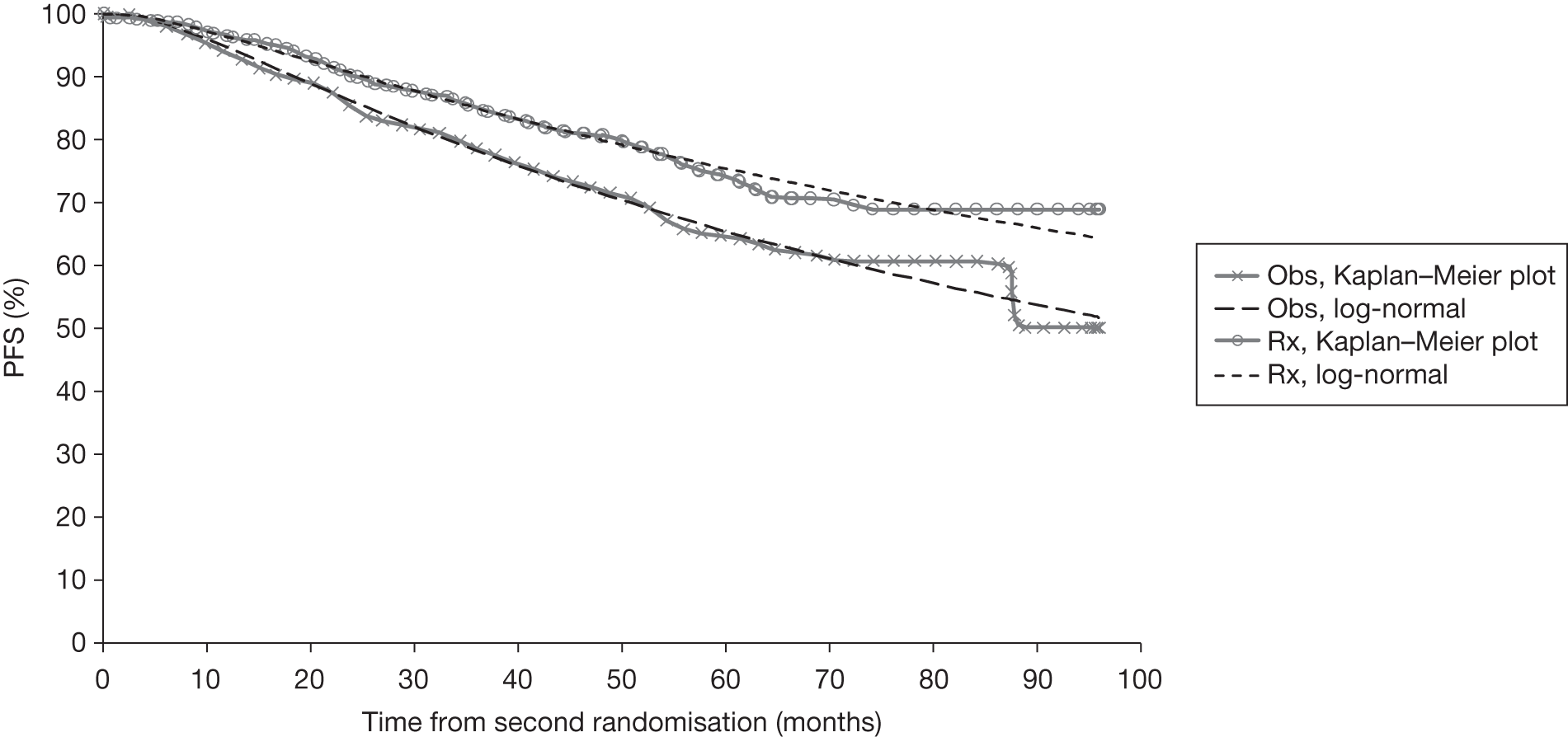
Meta-analysis was not performed for the outcome of PFS as only one study was identified measuring this outcome. Meta-analysis was not performed for the outcome of OS because of problems with the data in three of the trials. The population in two studies were given subsequent treatment as part of the study intervention. The German Low Grade Lymphoma Study-2000 (GLSG-2000) trial91,92 randomised responders who were aged < 60 years old to receive either interferon maintenance or dose-escalation chemotherapy and SCT; responders aged > 60 years old were given interferon maintenance therapy. Responders in the trial93 East German Society of Haematology and Oncology (OSHO-39; R-MCP vs MCP) were all given interferon maintenance therapy. Thus, the subsequent maintenance therapy confounds the OS data. The population in the (follicular lymphoma-2000 FL2000) trial94 included 10% patients with stage II FL and included the biological therapy interferon as part of the 6-month induction treatment phase and as a consolidation treatment for a further 12 months.
Other time-to-event data were presented in the included studies such as event-free survival (EFS), DFS, time to progression (TTP). No meta-analyses were performed on these additional time-to-event outcomes owing to inconsistencies in the way the outcomes were defined. These issues are discussed in more detail below (see Results, below). A network meta-analysis was not carried out. The reasons for this are discussed below (see Quantity and quality of research available).
Results
Quantity and quality of research available
Number of studies identified
The search retrieved 7858 unique citations relating to clinical effectiveness (4223 duplicates were removed). Of these, 7600 articles were excluded at title/abstract stage, 243 articles were examined at full-text level, and 15 articles were unobtainable from the interlibrary loans service (see Appendix 7). In addition, 1032 articles were examined from ongoing trials registers and conference proceedings.
Number and type of studies included
Four RCTs were included: M39021 trial by Marcus et al. ,95,96 GLSG-2000 by Hiddemann et al. ,91,92 OSHO-39 trial by Herold et al. 93 and the FL2000 trial by Salles et al. 94 Overall, 24 published reports were identified which related to the four included studies, and these are listed in Appendix 8. The principal source/sources for each study are listed in Table 6.
Number and type of studies excluded
In total, 212 citations were excluded from the full text selection (see Appendix 9). Studies that could potentially have provided head-to-head data for the interventions and comparators accounted for 108 excluded articles; 44 were excluded because they were not RCTs, i.e. case reports, literature reviews, commentaries and single-arm interventions; 29 studies were excluded because the interventions used were not relevant; 13 studies were excluded because the patient group was clinically heterogeneous and data for patients with FL were not reported separately; nine studies were excluded because patients did not have FL (e.g. Hodgkin’s disease or NHL unspecified) or had aggressive disease; six studies did not provide first-line treatment; five non-English-language studies were excluded; two were study protocols; and one did not provide relevant outcome data.
One hundred and four citations that were potential candidates to inform a network meta-analysis were excluded. Fifty-four were excluded because the participants did not have FL (e.g. NHL not specified) or the disease was not indolent; 21 were excluded because the population was heterogeneous and data relating to FL were not reported separately; 15 were excluded because the interventions were not relevant; eight were excluded because they were not RCTs; four were excluded because they were non-English-language reports; one was excluded as outcome data were not relevant; and one study was not included as it did not report on first-line treatment.
Studies identified for a potential network meta-analysis
Three additional studies (corresponding to seven references – see Appendix 10 for list) met the criteria for providing evidence within a network meta-analysis, i.e. the population included FL (with analysis for FL presented separately), the therapy being investigated was either a relevant intervention or comparator (as stated in the decision problem – see Chapter 2) and appropriate outcomes were reported (as stated in the decision problem – see Chapter 2) (Figure 3).
FIGURE 3.
Network of evidence. F, fludarabine; R-B, rituximab and bendamustine; R-CHVP, rituximab, cyclophosphamide, doxorubicin, etoposide, prednisolone and interferon-alpha.
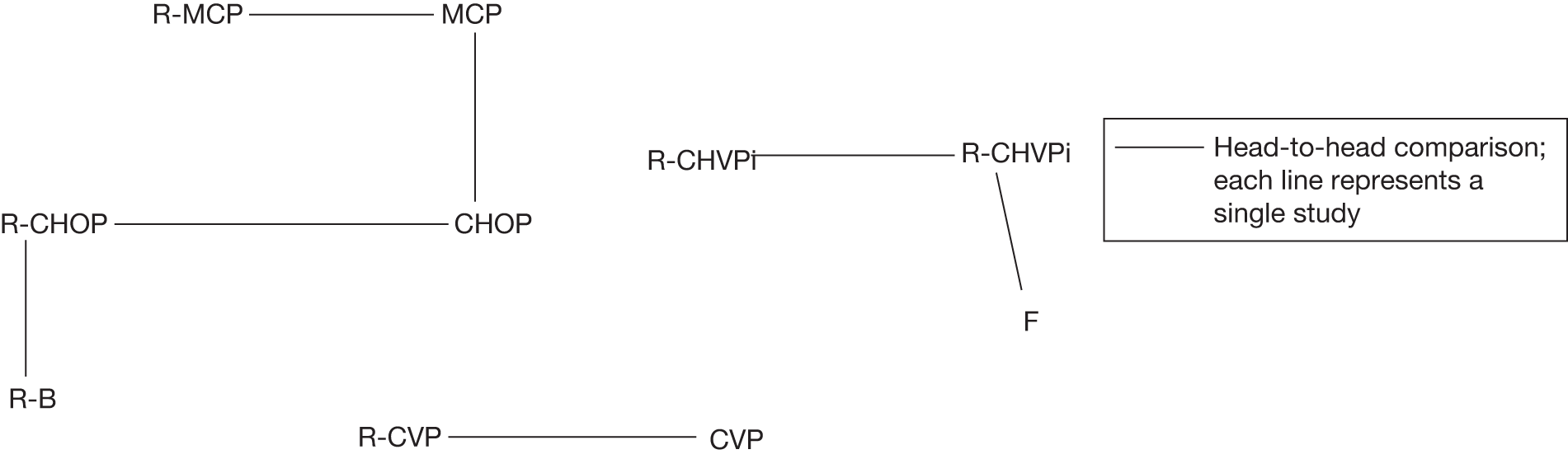
Incorporating these three studies into a network of evidence would facilitate the comparison of interventions when a direct head-to-head trial was not available (as depicted in Figure 3). However, the network meta-analysis was not undertaken, as it was not deemed appropriate given that treatment efficacy is not the only factor in terms of choice of chemotherapy selection (see Chapter 1 for discussion of other factors). Additionally, head-to-head data were available to inform a comparison between a chemotherapy regimen and that regimen with the addition of rituximab. It is noted that NICE has a strong preference for evidence from head-to-head RCTs that directly compare the technology with the appropriate comparator in the relevant patient groups as stated in the NICE methods guide (p. 15). 97
Ongoing trials
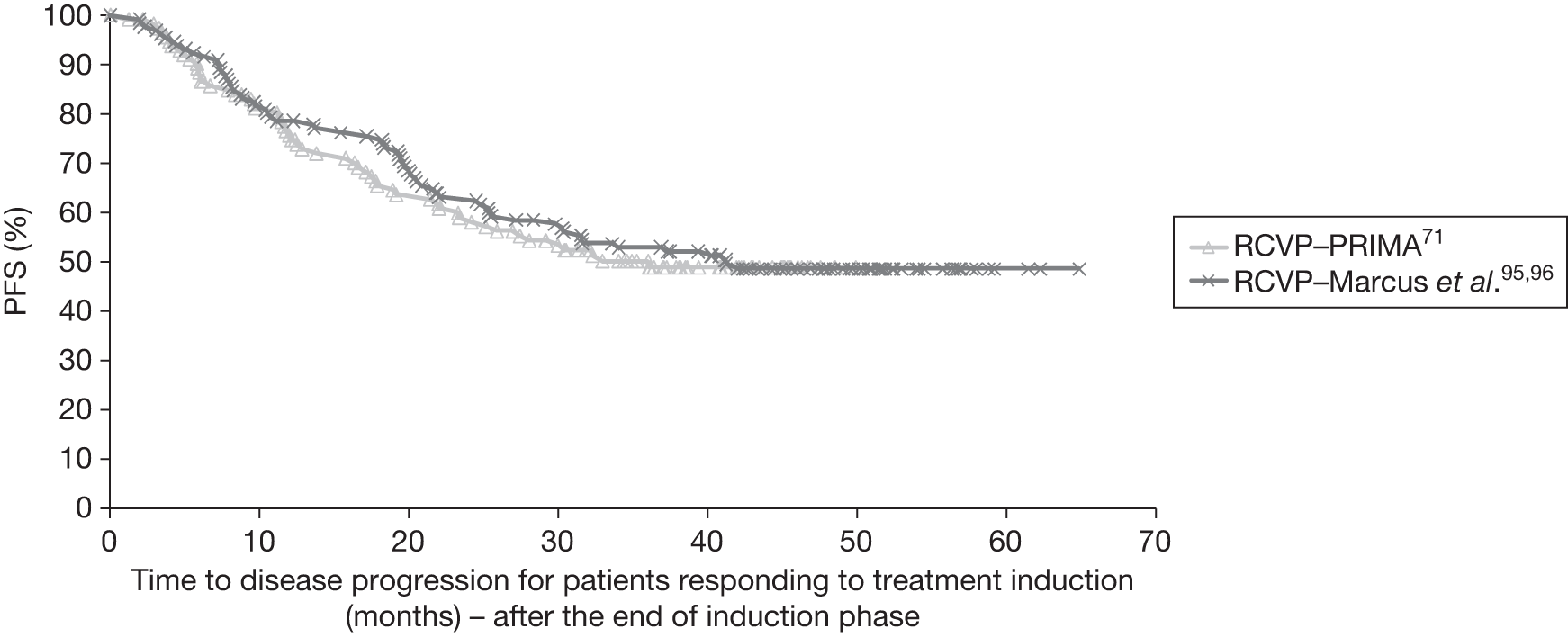
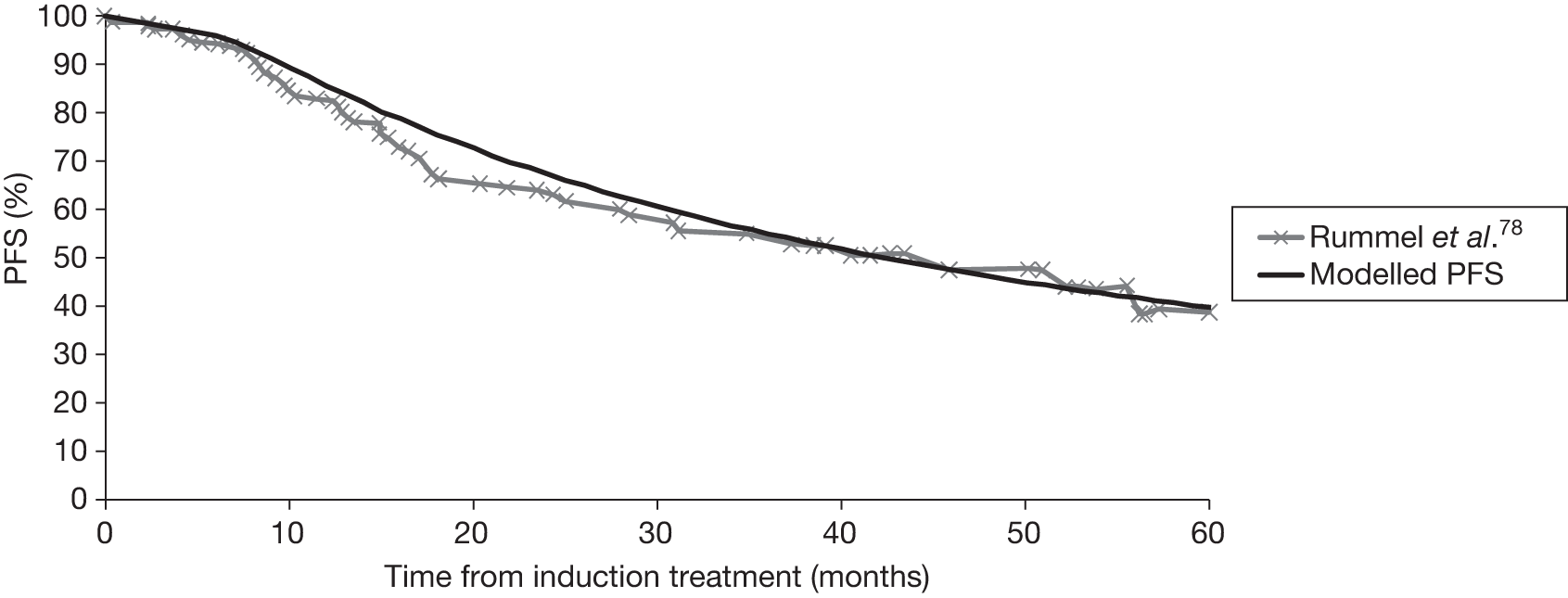
Seven ongoing studies were identified (Table 7). 70,77–80,98,99 Four studies are investigating one R-chemotherapy against another R-chemotherapy; one study is closed [a randomised phase III study of the STiL (Study Group Indolent Lymphomas)] with study follow-up complete and initial results reported as a conference abstract;78 one study is ongoing but not recruiting (ML17638)98 and two studies are ongoing and still recruiting [Purine-Alkylator Combination In Follicular lymphoma Immuno-Chemotherapy for Older patients (PACIFICO80) and Polish Lymphoma Research Group 479 (PLRG4)]. The study population in the PACIFICO trial81 is patients with FL aged > 60 years or aged < 60 years but with an anthracycline-based therapy contraindicated. Two ongoing studies are investigating the use of rituximab in maintenance following first-line induction therapy; one study is closed with follow-up completed [Primary Rituximab and Maintenance (PRIMA) study71], whereas the other study (ML17638)98 is ongoing but not recruiting. One study99 is investigating one chemotherapy compared with another chemotherapy regimen [British Lymphoma Investigation Group (BNLI) MCD vs FMD].
| Study characteristics | Study | ||||||
|---|---|---|---|---|---|---|---|
| PRIMA study71 | STiL trial (Rummel et al.78) | BNLI MCD vs FMD99 | R-CVP vs R-CHOP vs R-FM79 | ML1763899 | PACIFICO81 | PLRG480 | |
| Study identifier | UKCRN ID 2249 | ClinicalTrials.gov ID NCT00991211 | UKCRN ID 908 | ClinicalTrials.gov ID NCT00774826 | ClinicalTrials.gov ID NCT01144364 | UKCRN ID 6898 | ClinicalTrials.gov ID NCT00801281 |
| Participants | FL | FL and MCL | FL | FL (including stage II) | FL | FL | FL |
| n = 1200 | n = 549 | n = 400 | n = 431 | Target sample size 100–500 | n = 680 | n = 250 | |
| Age: > 18 years | Age: ≥ 18 years | Age: 18–70 years | Age: 18–75 years | Age: 60–75 years | Age: ≥ 60 years, or < 60 years but anthracycline-based therapy contraindicated | Age: ≥ 18 years | |
| Treatment | After induction of response with rituximab and chemotherapy:
|
|
|
|
After brief induction with chemotherapy (FMD) plus rituximab:
|
|
|
| Status | Closed: follow-up complete | Closed: follow-up complete | Closed: follow-up complete | Ongoing treatment phase: not recruiting | Ongoing treatment phase: not recruiting | Ongoing treatment phase: recruiting | Ongoing treatment phase: recruiting |
Summary of trials
Four multicentre, open-label trials were included, which randomised between 322 and 630 participants. The GLSG-200091,92 and OSHO-39 trials93 were undertaken in Germany; the M39021 trial95,96 was undertaken in centres across 11 countries including the UK, and FL2000 trial94 was undertaken in centres within France and Belgium. Three trials compared a R-chemotherapy regimen with a chemotherapy-alone regimen; the FL2000 trial compared a R-chemotherapy biological regimen with a chemotherapy biological regimen alone. The median follow-up ranged from 47 to 60 months (Table 8).
| Trial | Study type, country | Numbers randomised | Intervention | Comparator | Follow-up |
|---|---|---|---|---|---|
| M3902195,96 |
Multicentre, open-label RCT 47 centres in Australia, Belgium, Brazil, Canada, France, Israel, Poland, Portugal, Spain, Switzerland and the UK |
n = 322a Stage III–IV FL |
R-CVP (n = 162) | CVP (n = 159) | Median 53 months (no range reported) |
| GLSG-200091,92 |
Multicentre, open-label RCT 200 institutions in Germany |
n = 630b Stage III–IV FL |
R-CHOP (n = 279) | CHOP (n = 278) | Median 56 months (no range reported) |
| OSHO-3993 |
Multicentre, open-label RCT 34 centres in Germany |
n = 376 (including MCL) n = 201/376 were FL Stage III–IV FL |
R-MCP (n = 105) | MCP (n = 96) | Median 47 months (49 months for R-MCP and 42 months for MCP) (no range reported) |
| FL200094 |
Multicentre, open-label RCT 54 centres in France and Belgium |
n = 360c Stage II–IV |
R-CHVPi (n = 175) | CHVPi (n = 183) | Median 60 months (range 0.2–6.4 years) |
Population
Baseline demographic data are provided in Table 9. The target population were advanced-stage patients with FL who were symptomatic and requiring treatment (detailed eligibility criteria for each study are presented in the data extraction tables in Appendix 11). The M3902195,96 and GLSG-2000 trials91,92 recruited patients with stage III–IV FL, whereas the FL2000 trial94 recruited patients with stage II-IV FL. The OSHO-39 trial93 included CD20-positive patients with indolent NHL, which included lymphoplasmacytic lymphoma or mantle cell lymphoma (MCL); however, the primary analysis population was defined as the population of patients with FL. The OSHO-3993 and GLSG-200092 trials limited to grade 1 or 2 FL (WHO classification); the M39021 trial95,96 included grade 1–3 FL; and the FL2000 trial94 included grades 1, 2 and 3a FL.
| Demographics | M3902195,96 | GLSG-200091,92 | OSHO-3993 | FL200094 | ||||
|---|---|---|---|---|---|---|---|---|
| R-CVP (n = 162) | CVP (n = 159) | R-CHOP (n = 279) | CHOP (n = 278) | R-MCP (n = 105) | MCP (n = 96) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| Age and gender | ||||||||
| Median age in years (range) | 52 | 53 | 57 (27–90) | 57 (21–81) | 60 (33–78) | 57 (31–75) | 61 (25–75) | |
| Aged > 60 years: no. (%) | 41 (25) | 44 (28) | NR | NR | NR | NR | 89 (51) | 96 (52) |
| Male: no. (%) | 88 (54) | 85 (53) | 120 (43) | 146 (53) | 53 (50) | 36 (37) | 96 (55) | 82 (45) |
| Female: no. (%) | 74 (46) | 74 (47) | 159 (57) | 132 (47) | 52 (50) | 60 (63) | 79 (45) | 101 (55) |
| Ann Arbor stage, no. (%) | ||||||||
| II | 2 (1) | 2 (1) | 0 | 0 | 0 | 0 | 23 (13) | 18 (10) |
| III | 45 (28) | 45 (28) | NR | NR | 30 (29) | 22 (23) | 152 (87) | 165 (90) |
| IV | 114 (70) | 112 (70) | 194 (70) | 191 (69) | 75 (71) | 74 (77) | ||
| Not evaluable/missing | 1 (1) | 0 (0) | NR | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Performance status (ECOG), no. (%) | ||||||||
| 0 | 93 (57) | 90 (57) | 97 (35) | 88 (32) | 68 (65) | 54 (56) | 164 (94) | 167 (91) |
| 1 | 65 (40) | 60 (38) | 155 (56) | 167 (60) | 29 (28) | 36 (38) | ||
| > 1 | 4 (2) | 8 (5) | 18 (6) | 19 (7) | 7 (7) | 6 (6) | 11 (6) | 16 (9) |
| Not evaluable/missing | 0 (0) | 1 (0.6) | 9 (3) | 4 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| IPI, no. (%) | ||||||||
| 0 | 1 (1) | 1 (1) | NR | NR | NR | NR | ||
| 1 | 72 (44) | 69 (43) | NR | NR | NR | NR | ||
| 2 | 57 (35) | 57 (36) | NR | NR | NR | NR | ||
| 3 | 19 (12) | 21 (13) | NR | NR | 60 (34) | 71 (39) | ||
| 4 | 2 (1) | 3 (2) | NR | NR | ||||
| Not evaluable/missing | 11 (7) | 8 (5) | NR | NR | NR | NR | ||
| FLIPI, no. (%) | ||||||||
| Low (0–1) | 80 (49) | 75 (47) | 39 (14) | 31 (11) | 8 (8) | 6 (6) | 28 (16) | 37 (20) |
| Intermediate (2) | 114 (41) | 119 (43) | 38 (36) | 37 (39) | 63 (36) | 59 (32) | ||
| High (3–5) | 71 (44) | 75 (47) | 123 (44) | 123 (44) | 59 (56) | 53 (55) | 79 (45) | 83 (45) |
| Not evaluable/missing | 11 (7) | 9 (6) | 3 (1) | 5 (2) | 0 (0) | 0 (0) | 5 (3) | 4 (2) |
| Other factors, no. (%) | ||||||||
| B symptoms presence | 65 (40) | 51 (32) | 108 (39) | 113 (41) | ≥ 46 (44) | ≥ 34 (35) | 38 (22) | 52 (28) |
| Bone marrow involvement | 103 (64) | 102 (64) | 180 (65) | 179 (64) | 73 (70) | 71 (74) | 108 (62) | 121 (66) |
| More than extranodal site | 28 (17) | 27 (17) | NR | NR | NR | NR | 60 (34) | 73 (40) |
| Elevated LDHb | 39 (26) | 39 (26) | 73 (26) | 66 (24) | 31 (30) | 30 (31) | 64 (37) | 66 (36) |
| β2-Microglobulin > 3 mg/lc | 146 (99) | 141 (100) | NR | NR | NR | NR | 62 (35) | 56 (31) |
| Haemoglobin < 12 g/dl | NR | NR | 54 (19) | 56 (20) | NR | NR | 37 (21) | 30 (16) |
The median age of patients randomised across the trials ranged from 52 to 61 years. Two trials presented the percentage of participants aged over 60 years: 26% in the M39021 trial95,96 and 52% in FL2000 trial. 94 The majority of patients had stage IV FL (69–77% in the three studies that reported these data). Most participants had an ECOG performance status of 0–1, ranging from 91% to 97%. Bone marrow involvement was present in 62–74% of patients, and 22–44% presented with one or more B symptoms (defined as fever, weight loss or night sweating). Elevated LDH levels (a marker of aggressive disease) were recorded in 26–37% of patients.
Within the individual studies, the treatment groups were well balanced with respect to demographic and disease characteristics, with the exception of gender in OSHO-39 trial92 (more males in the R-MCP group; no p-value reported) and the GLSG-2000 trial (higher proportion of males in the CHOP arm; p = 0.027). The populations were reasonably similar when compared across the four studies, although there were some differences, including younger median age (52–53 years) in the M39021 trial,95,96 and larger proportion of patients aged > 60 years and inclusion of stage II participants in the FL2000 trial. 93 The study populations included were generally reflective of the general FL population, with the exception of age – the median age of participants in the trials being younger than seen in clinical practice (70% are aged > 60 years when diagnosed). 10 The younger median age of trial participants meant that ECOG performance status was better than that seen in clinical practice. In addition, the M3902195,96 and OSHO-3993 trials excluded patients with an ECOG performance status of > 2.
Interventions and comparators
The interventions in each of the four studies were a R-chemotherapy combination; each trial used a different chemotherapy agent. The comparator within each trial was the chemotherapy regimen minus rituximab. These are described in Table 10. Two studies provided subsequent treatment following response to first-line treatment. The OSHO-39 trial93 planned to provide all responders with interferon-alpha maintenance [3 × million international units (MIU)/week] until disease progression. The GLSG-2000 trial92,93 randomised responding patients who were aged < 60 years to a high-dose chemotherapy regimen followed by autologous stem cell transplant (ASCT) or interferon-alpha maintenance treatment (3 × 5 MIU/week until disease progression of intolerable AEs). Patients aged ≥ 60 years received interferon-alpha maintenance.
| Author/study | Treatment regimens | Cycles | Response assessment | Amendment to dose or cycles |
|---|---|---|---|---|
| M3902195,96 |
CVP: 750 mg/m2 cyclophosphamide i.v. on day 1; 1.4 mg/m2 of vincristine, up to a maximal dose of 2 mg i.v. on day 1; and 40 mg/m2 of prednisone per day p.o. on days 1–5 Rituximab: 375 mg/m2 infusion on day 1 |
Every 21 days for a maximum of eight cycles | Assessed after cycle 4 and at the end of treatment | Insufficient therapeutic response, i.e. disease progression or stable disease after cycle 4 were withdrawn from study treatment. Those achieving at least a PR continued to eight cycles |
| GLSG-200091,92 |
CHOP: 750 mg/m2 cyclophosphamide; 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine: all given i.v. on day 1. Prednisolone given 100 mg/m2 daily on days 1–5 p.o. Rituximab: 375 mg/m2 infusion on the day before the respective CHOP course |
Every 21 days for a total of six to eight cycles | Assessed every two cycles and 4 weeks after completion of last course |
Patients, in either study arm, with disease progression at any time during the study were taken off the study Patients achieving CR after four cycles were treated with a total of six cycles; all other patients received eight cycles |
| OSHO-3993 |
MCP: 8 mg/m2 mitoxantrone i.v. on days 1 and 2; 3 × 3 mg/m2 chlorambucil and 25 mg/m2 prednisolone p.o. on days 1–5 Rituximab: 375 mg/m2 i.v. infusion on day 1 (8 mg/m2 mitoxantrone i.v. on days 3 and 4; 3 × 3 mg/m2 chlorambucil and 25mg/m2 prednisolone p.o. on days 3–7) |
Every 28 days for a maximum of eight cycles | After completion of induction treatment, patients were observed every 8 weeks during the first year, at 3-month intervals during the second year, and then every 6 months from the third year onwards |
Patients with disease progression after two cycles of therapy or who had not reached a PR or CR after six cycles of therapy were prematurely withdrawn from study CR or a PR after six cycles of treatment received a further two cycles of treatment |
| FL200094 |
CHVPi: 600 mg/m2 cyclophosphamide i.v. on day 1 and 25 mg/m2 i.v. doxorubicin on day 1 and 100 mg/m2 etoposide, all administered i.v. on day 1; 40 mg/m2 prednisolone p.o. from days 1–5 Interferon-alpha s.c. 3 × 4.5* MIU/week (*3 MIU for patients > 70 years) Rituximab: 375 mg/m2 infusion on days 1 and 8 of cycles 3 and 4, and day 1 of cycles 5 and 6 (thus, CHVP only in cycles 1 and 2) |
CHVPi: Six monthly cycles followed by six bimonthly cycles) and 18 months of interferon-alpha R-CHVPi: Six monthly cycles CHVP or R-CHVP (see column to left) and 18 months concurrent interferon-alpha |
Evaluation of response performed after six chemotherapy courses (6 months) and at the end of the whole treatment (18 months) | No dose reduction of chemotherapy was planned or allowed (but could be delayed for 7 days if the absolute neutrophil count was < 1.5 g/l or the platelet count was < 100 g/l) |
Outcomes
The clinical efficacy outcomes reported in the four studies91–96 are shown in Table 11; primary outcomes are highlighted in grey. All four studies91–96 included the appropriate outcome measure of OS; defined as the time from randomisation to the date of death by any cause. The OSHO-39 trial93 was the only trial to report PFS, defined as randomisation to disease progression or death from NHL. All four studies91–96 appropriately reported response rates (according to the International Workshop criteria described by Cheson et al. 89). Two studies91–93 did not use the category of ‘unconfirmed complete responder’ (CRu), instead counting such patients within the PR category. The FL2000 trial94 and M39021 trial95,96 used the category of CRus and presented the numbers separately from CRs and PRs. No studies reported the duration of disease remission, although the studies did report a number of time-to-event outcomes which approximated disease remission, for example all four studies reported response duration as an outcome.
| Study | PFS | OS | ORR | CR | PR | RD | EFS | TTF | TTNT | DFS | TTP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M3902195,96 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| GLSG-200091,92 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| OSHO-3993 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| FL200094 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Other time-to-event outcomes reported by one or more of the studies were EFS, time to treatment failure (TTF), time to next antilymphoma treatment (TTNT), DFS and TTP. However, these outcomes were inconsistently defined by the four studies91–96 and thus not directly comparable across the four studies. For example, the M3902195,96 and GLSG-2000 trials91,92 measured TTF and both studies considered a treatment failure as disease progression. However, the M39021 trial95,96 additionally considered death by any cause, relapse after response, new antilymphoma treatment or stable disease after cycle 4 as treatment failures, whereas the GLSG-2000 trial91,92 also considered resistance to initial therapy and death not specified as treatment failures. In addition, when the definitions for each time-to-event outcome were cross-referenced against each other, no outcomes were directly comparable (e.g. we examined whether or not PFS as measured in the OSHO-39 trial93 may have matched the definition used for EFS as measured by FL2000 trial;94 however, this was not the case). Appendix 12 provides the definitions for each outcome described in the four studies. 91–96
All four studies reported data on AEs. The M39021, OSHO-39 and FL2000 trials93–96 graded AEs in accordance with the National Cancer Institute Common Toxicity Criteria (NCI-CTC) grading system,100 and the GLSG-2000 trial91,92 used the WHO toxicity criteria101 to record AEs. The GLSG-2000 and OSHO-39 trials93 reported data for grade 1, 2, 3 and 4 AEs separately,92,93 and the M39021 and FL2000 trials94–96 reported AEs for grades 3 and 4 combined. None of the studies reported HRQoL as an outcome.
Quality assessment
All four included studies91–96 were randomised and allocation was concealed using centralised allocation to treatment. Numbers randomised were stated in all four studies. None of the studies were blinded; all were open label and none of the studies reported attempting to conceal treatment allocation from the outcome assessors. Power calculations were undertaken by all four included studies. 91–96 At least 80% of patients were followed up in all four studies. All four studies reported baseline characteristics and were mostly balanced between treatment groups; with the exception of gender in OSHO-39 trial93 (greater number of males in the R-MCP group; no p-value reported) and the GLSG-2000 trial (higher proportion of males in the CHOP arm; p = 0.027). The M3902195,96 and FL-2000 trials94 reported no significant differences in baseline data. All studies specified eligibility criteria.
Co-interventions were used in three studies. 91–94 Interferon maintenance therapy was given to patients in the OSHO-39 trial93 achieving a partial or complete remission; this was initiated within 4–8 weeks after treatment completion. In the GLSG-2000 trial,91,92 patients < 60 years who had achieved either CR or PR were offered a second randomisation of Dexa-BEAM regime (salvage chemotherapy) followed by stem cell harvest and radiochemotherapy, or long-term interferon maintenance, whereas patients aged > 60 years were given interferon maintenance. Patients in both arms in the FL2000 trial94 were given interferon-alpha as part of initial treatment (6 months) and then as a consolidation treatment for a further 12 months. In addition, 11% of patients in the FL2000 trial94 had stage II FL. Reasons for withdrawals were unclear in the four studies. 91–96 Most withdrawals were stated as being a result of disease progression; however, withdrawals relating to AEs were not explicitly stated. All four studies91–96 reported using ITT analyses. See Figure 4 for overview of the quality assessment.
FIGURE 4.
Quality assessment of the included trials (+, yes; – , no; ?, unclear).

Assessment of effectiveness
Response to treatment
Response to treatment is reported in Table 12. ORR was significantly improved for patients receiving R-chemotherapy than those who received chemotherapy alone in three studies91–93,95,96 (the FL2000 trial94 did not report a p-value). The ORR in the four studies ranged from 81% to 97% for the R-chemotherapy arm and from 57% to 91% for the chemotherapy-only arm. The difference in ORR between the treatment and comparator arms in each of the four studies ranged between 5% and 24%; the greatest difference was between the R-CVP and CVP arm. R-CHOP, R-CHVPi and R-MCP were the regimens that provided the highest ORR of 96%, 94% and 92%, respectively. CHOP alone provided a high ORR of 91%.
| Median follow-up | M3902195,96 | GLSG-200091,92 | OSHO-3993 | FL200094 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6-month follow-up data | 18-month follow-up data | |||||||||
| 53 months | 56 months | 47 months | 60 months | 60 months | ||||||
| R-CVP (n = 162) | CVP (n = 159) | R-CHOP (n = 279) | CHOP (n = 278) | R-MCP (n = 105) | MCP (n = 96) | R-CHVPi (n = 175) | CHVPi (n = 183) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| OR: n (%) | 131 (81) (95% CI 74% to 87%) |
90 (57) (95% CI 49% to 64%) |
268 (96) (no CI reported) | 253 (91) (no CI reported) | 97 (92) (no CI reported) | 72 (75) (no CI reported) | 164 (94) (no CI reported) | 156 (85) (no CI reported) | 142 (81) (no CI reported) | 131 (72) (no CI reported) |
| p-value reported in study | < 0.0001 | 0.0046 | 0.0009 | NR | NR | |||||
| RRb (95% CI) | 1.43 (1.22 to 1.67) | 1.06 (1.01 to 1.10) | 1.23 (1.08 to 1.40) | 1.10 (1.02 to 1.18) | 1.13 (1.01 to 1.27) | |||||
| CR: n (%) | 49 (30) | 12 (8) | 53 (19) | 47 (17) | 52 (50) | 24 (25) | 63 (36) | 29 (16) | 90 (51) | 71 (39) |
| p-value reported in study | < 0.001 | No p-value reported | 0.0004 | NRc | NRd | |||||
| RRb (95% CI) | 4.01 (2.22 to 7.25) | 1.12 (0.79 to 1.60) | 1.98 (1.33 to 2.95) | 2.27 (1.54 to 3.35) | 1.33 (1.05 to 1.67) | |||||
| PR: n (%) | 82 (51) | 78 (49) | 215 (77) | 206 (74) | 45(43) | 48 (50) | 101 (58) | 127 (69) | 52 (30) | 60 (33) |
| No p-value reported | No p-value reported | No p-value reported | NRc | NRd | ||||||
| RRb (95% CI) | 1.03 (0.83 to1.29) | 1.04 (0.95 to 1.14) | 0.86 (0.64 to 1.15) | 0.83 (0.71 to 0.98) | 0.91 (0.67 to 1.23) | |||||
| Stable disease: n (%) | 12 (7) | 33 (21) | 6 (2)e | 17 (6)e | NRf | NRf | 2 (1) | 9 (5) | 1 (1) | 3 (2) |
| p-value reported in study | No p-value reported | No p-value reported | No p-value reported | NRc | NRd | |||||
| Progressive disease: n (%) | 17 (10) | 31 (19) | 3 (1) | 6 (2) |
3 (3) (After two cycles) |
10 (10) (After two cycles) |
8 (5) | 18 (10) | 31 (18) | 47 (26) |
| p-value reported in study | No p-value reported | No p-value reported | No p-value reported | NRc | NRd | |||||
Difference in the CR rates between treatment and comparator arms in the four studies91–96 ranged from 2% to 25%, and was reported as significant in two studies. 93,95,96 The regimens providing the highest CR rates were R-CHVPi and R-MCP (51% and 50%, respectively). The number of CRs in the GLSG-2000 trial91,92 for both R-CHOP and CHOP (19% and 17%, respectively) were notably lower than those reported in the other studies. The greatest difference in CR between treatment and comparator arms was reported in the OSHO-39 trial93 between R-MCP and MCP (25%).
The difference in PR rate ranged from 2% to 11%. None of the four studies reported a p-value for the difference between treatment and comparator arms.
The GLSG-200091,92 and FL2000 trials94 reported low numbers of patients within the stable disease category. However, the M39021 trial95,96 reported greater numbers of patients with stable disease (7% in R-CVP and 21% in CVP). Meta-analysis of response rates in the four trials has been explored (see Meta-analysis, for further discussion).
Chi-squared test for response rates
The AG performed a chi-squared test on the response rate data to compare the numbers within each category of response between the two trial arms for each of the four trials. The results showed that there was a statistically significant difference in the numbers in the response categories for the R-chemotherapy arm compared with the chemotherapy-alone arm for R-CVP compared with CVP, R-MCP compared with MCP and R-CHVPi compared with CHVPi 6-month response rate (p < 0.001 for all comparisons). The difference between the categories of response was not statistically significant for R-CHOP compared with CHOP (p = 0.15) and for the R-CHVPi compared with CHVPi 18-month response rate (p = 0.12).
A second analysis was performed for each trial, which combined relevant categories of response (e.g. progressive disease or death) where necessary so that the number of observations within each category was greater than five per cell. Where grouping was performed, death was categorised with progressive disease. In one analysis, stable disease was categorised with disease progression and death, as clinical advice to the AG indicates that patients with stable disease are treated as patients with disease progression and not responders. In terms of statistical significance at the 5% level, the effects of grouping altered only on comparison of R-CHVPi and CHVPi at 18 months, which became statistically significant. Analyses are presented in full in Appendix 13.
Overall survival
The OS rate in the four studies91–96 ranged from 83% to 90% in the R-chemotherapy arms and from 77% to 84% in the chemotherapy-alone arms (Table 13). The difference in OS rate was significantly improved in three trials when R-chemotherapy was compared with chemotherapy alone; the exception being the FL2000 trial94 (p = 0.1552). The median OS was reported as not reached in three studies and was not reported in the FL2000 trial. 94 The OS data from the GLSG-200091,92 and OSHO-3993 trials were confounded owing to the effects of subsequent therapy provided to all responders to first-line treatment. The FL2000 trial94 also provided additional treatment (interferon-alpha) to both treatment arms during the 6-month remission induction phase. In addition, the FL2000 trial94 provided a further 12-month treatment phase in which the chemotherapy-alone arm received bimonthly CHVP and both treatment arms received interferon-alpha.
| M3902195 | GLSG-200091,92 | OSHO-3993 | FL200094 | |||||
|---|---|---|---|---|---|---|---|---|
| R-CVP (n = 162) | CVP (n = 159) | R-CHOP (n = 279) | CHOP (n = 278) | R-MCP (n = 105) | MCP (n = 96) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| Median follow-up | 53 months | 56 months | 47 months | 60 months | ||||
| OS rate (%) | 83 (95% CI 77 to 89a) | 7 (95% CI 70 to 83a) | 90 (CI NRb) | 84 (CI NRb) | 87 (CI NRc) | 74 (CI NRc) | 84 (95% CI 78 to 84d) | 79 (95% CI 72 to 84d) |
| p-value reported in trial | < 0.0290 | 0.0493 | 0.0096 | 0.1552 | ||||
| Median OS | Not reached | Not reached | Not reached | Not reached | Not reached | Not reached | NR | NR |
| No. of deaths | 23e | 35e | 6f | 17f | 15g | 25g | NR | NR |
| p-value reported in trial | No p-value reported | 0.016 | No p-value reported | No p-value reported | ||||
| HRsh | 0.64 | 0.58 | 0.40 | 0.69 | ||||
Overall survival: hazard ratios
The hazard ratios (HRs) for OS were not available in the manuscripts for each of the individual trials. The AG used Kaplan–Meier plot data provided in the health economic model in the MS,61 which provided a series of survival probability estimates at monthly time points for two of the four trials: M39021 and OSHO-39. 93,95,96 Visual inspection of these probability estimates alongside the Kaplan–Meier data provided in the publications for each trial indicated that these data were reasonable. Kaplan–Meier data for OS for the FL2000 trial94 and the most-up-to-date data for the GLSG-2000 trial91 were digitised by the AG using TechDig© software to estimate survival probability estimates at time points along the Kaplan–Meier curve.
The corresponding HRs were calculated by taking the ratio of the cumulative hazard from the R-chemotherapy and chemotherapy arms from the OS Kaplan–Meier curves. The cumulative hazard was calculated by summing the negative log of the survival probabilities {H(t) = –Slog[S(t)]} for each treatment arm, restricted to the clinical follow-up reported in the publications. 102 There are limitations with this method of calculating HRs, namely that it relies on the data from the trial publications rather than patient-level data and that estimating survival probabilities from digitised curves are subject to inaccuracies. As such, these estimates provide an indication of the HR for OS rather than definitive values. Given resource constraints and data limitations, it was not possible to calculate the standard errors (SEs) and confidence intervals (CIs) to give an indication of the uncertainty in the data.
For all four trials,91–96 there was an increased likelihood of survival if receiving R-chemotherapy. For R-CVP compared with CVP, there was 36% increased survival benefit, for R-CHOP compared with CHOP there was a 42% increased survival benefit, for R-MCP compared with MCP a 60% increased survival benefit and for RCHVPi compared with CHVPi there was a 31% survival benefit. However, it is noted that the treatment effect on OS is confounded in the latter three trials owing to additional trial treatments administered after response to first-line treatment.
Progression-free survival
The median PFS was significantly prolonged in OSHO-39 trial93 for the R-chemotherapy arm (R-MCP) (28.8 months MCP vs median not reached R-MCP; p < 0.0001). PFS was not reported in the other three trials.
Other time-to-event data
Several other efficacy outcomes, namely time-to-event data, were reported in the four studies. 91–96 As stated above (see Summary of trials), these outcomes were inconsistently defined between the four studies and thus not directly comparable (see Appendix 12). In addition, the time-to-event data were confounded in GLSG-200091,92 and OSHO-3993 trials owing to the effects of subsequent treatment provided to responders to first-line treatment in these trials. However, we present a summary of the findings in Table 14.
| M3902195,96 | GLSG-200091,92 | OSHO-3993 | FL200094 | |||||
|---|---|---|---|---|---|---|---|---|
| R-CVP (n = 162) | CVP (n = 159) | R-CHOP (n = 279) | CHOP (n = 278) | R-MCP (n = 105) | MCP (n = 96) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| Median follow-up, months | 53 | 56 | 47 | 60 | ||||
| Median PFS, months | – | – | – | – | Not reached | 28.8 | – | – |
| p-value | – | – | < 0.0001 | – | ||||
| No. of events (%) | – | – | 30 (29) | 50 (52) | – | |||
| % PFS at 4 years | – | – | 71 | 40 | – | |||
| Median TTF, months | 27 (95% CI 25 to 37) | 7 (95% CI 6 to 9) | Not reached | 35 | – | – | – | – |
| p-value | < 0.0001 | < 0.0001 | – | – | ||||
| Median EFS, months | – | – | – | – | Not reached | 26 | Not reached | 35 |
| p-value | < 0.0001 | 0.0004 | ||||||
| Five-year EFS | – | – | – | – | – | – | 53% (95% CI 45% to 60%) | 37% (95% CI 29% to 44%) |
| p-value | 0.001 | |||||||
| Median response duration, months | 38, (95% CI 28 to NE) | 14, (95% CI 9 to 18) | – | – | Not reached | 35 | – | – |
| p-value | < 0.0001 | – | < 0.0001 | – | ||||
| Duration of response at x years | – | 66%a | 35%a | – | 64%b (95% CI 55% to 72%) | 44%b (95% CI 32% to 54%) | ||
| p-value | < 0.0001a | 0.012b | ||||||
| Median TTNT, months | 49 (95% CI 32 to NE) | 12 (95% CI 10 to 18) | – | Not reached | 29.4 | – | – | |
| p-value | < 0.0001 | 0.001c | 0.0002 | – | ||||
| Median DFS, months | Not reached (95% CI 35 to NE) | 21 (95% CI 14 to 38) | – | – | – | – | – | – |
| p-value | 0.0001 | – | – | – | ||||
| Median TTP, months | 34 (95% CI 27 to 48) | 15 (95% CI 12 to 18) | – | – | – | – | – | – |
| p-value | < 0.0001 | – | – | – | ||||
The median response duration was significantly prolonged for the R-chemotherapy arm compared with the chemotherapy-alone arms (p < 0.001) in the M39021 and OSHO-39 trials. 93,95,96 Two studies reported the duration of response, which differed significantly between treatment and comparator arms; at 5 years in the GLSG-2000 trial91,92 (p < 0.0001) and the 4-year estimates presented in the FL2000 trial94 (p = 0.012). Significantly prolonged (p < 0.0001) median TTF was reported for the R-chemotherapy arm compared with chemotherapy-alone arm in the M39021 and GLSG-2000 trials. 91,92,95,96 Similarly, median EFS was significantly improved in the R-chemotherapy arms in two studies compared with the chemotherapy-alone arms [median EFS MCP 26 months, not reached in R-MCP (p < 0.0001); median EFS 35 months in CHVPi, not reached in R-CHVPi (p = 0.0004)]. 93,94 The M39021, GLSG-2000 and OSHO-39 trials reported a statistically significant difference in TTNT. 91–93,95,96 The M39021 trial95,96 reported significantly improved DFS and TTP for R-CVP compared with CVP.
Clinical efficacy in subpopulations
Overall, R-chemotherapy compared with chemotherapy alone improved treatment outcomes for all subgroups (including FLIPI score, IPI score, age, quality of response to induction therapy and other prognostic factors). It is noted that the univariate analyses presented may be misleading owing to interaction between variables.
Follicular Lymphoma International Prognostic Index score
All four studies91–96 presented analysis of treatment outcomes according to FLIPI score subgroups. The M39021 trial95 found after undertaking univariate analyses that median TTP was significantly improved in the R-CVP group at 53-month follow-up for all FLIPI groups (low, intermediate and high risk) (Table 15). Similarly, the GLSG-2000 study91 found significantly prolonged 5-year TTF associated with the addition of rituximab in all FLIPI subgroups [84% vs 46% for low risk (p = 0.0021); 73% vs 37% for intermediate risk (p < 0.0001) and 49% vs 23% for high risk (p < 0.0001)].
| Subgroup | R-CVP | CVP | p-value |
|---|---|---|---|
| FLIPI 0–1 (low risk) | Not reached (95% CI 38 months to NE) | 22 months (95% CI 16 to 40 months) | 0.0085 |
| FLIPI 2 (intermediate risk) | 37 months (95% CI 28 months to NE) | 17 months (95% CI 13 to 35 months) | 0.0003 |
| FLIPI 3–5 (high risk) | 26 months (95% CI 16 to 34 months) | 11 months (95% CI 10 to 15 months) | 0.0004 |
Marcus et al. 95 conducted a multivariate analysis (which included the FLIPI score as a composite along with other prognostic factors that are not incorporated in the FLIPI), which found that only the FLIPI low-risk and intermediate groups combined (0–2) compared with high-risk groups (3–5) was a significant prognostic parameter for TTP in addition to trial treatment.
The MS62 presented data on the OSHO-39 trial,93 which demonstrated that treatment with R-MCP significantly increased the 4-year PFS rate, as well as prolonging the median TTP or death in patients with intermediate (p = 0.0016), as well as high-risk (p = 0.0011) FLIPI subgroups. Among patients with high-risk disease, a significant improvement in OS was also seen among those treated with R-MCP compared with MCP (p = 0.0096). 104 No such significant improvement between treatment arms was noted for median OS for the FLIPI intermediate subgroup (p = 0.8607). These data are presented in the MS61 and are reproduced in Table 16; FLIPI 0–1 data were not presented.
| Subgroup | Parameter | MCP | R-MCP | p-value |
|---|---|---|---|---|
| FLIPI 2 (intermediate risk) | Median PFS | 37 months | Not reached | 0.0016 |
| Four-year PFS | 43% | 82% | – | |
| FLIPI 3–5 (high risk) | Median PFS | 26.5 months | Not reached | 0.0011 |
| Four-year PFS | 36% | 61% | – | |
| FLIPI 2 (intermediate risk) | Median OS | Not reached | Not reached | 0.8607 |
| Four-year OS | 90% | 92% | – | |
| FLIPI 3–5 (high risk) | Median OS | 54 months | Not reached | 0.0096 |
| Four-year OS | 63% | 81% | – |
In the FL2000 trial,94 when patients with either a low (n = 65) or an intermediate (n = 122) FLIPI score were grouped together, no significant difference in EFS or OS was seen between the treatment arms. However, significant improvements in 5 years’ EFS (p < 0.001) and OS (p = 0.025) were seen between the treatment arms in the high-risk FLIPI subgroup. Cox regression analysis, which included the FLIPI score (low and intermediate vs high) and the treatment arm, confirmed the impact of both parameters on EFS [FLIPI, HR = 2.08 (95% CI 1.6 to 2.8); R-CHVPi treatment, HR = 0.59 (95% CI 0.44 to 0.78)] and OS [FLIPI, HR = 4.11 (95% CI 2.34 to 7.23); R-CHVPi treatment, HR = 0.67 (95% CI 0.41 to 1.11)].
International Prognostic Index
Marcus et al. 95 conducted a univariate analysis of the M39021 trial data, which found significantly prolonged median TTP for all IPI risk groups (Table 17). Similarly analysis of the GLSG-2000 trial data91 found significantly prolonged TTF at 18-month follow-up by IPI risk group (Table 18).
| Subgroup | Parameter | R-CVP | CVP | p-value |
|---|---|---|---|---|
| IPI 0–1 (low risk) | Median TTP | 44 months (95% CI 30 months to NE) | 20 months (95% CI 13 to 26 months) | < 0.0001 |
| IPI 2 (intermediate risk) | Median TTP | 27 months (95% CI 20 to 39 months) | 14 months (95% CI 10 to 17 months) | 0.0003 |
| IPI 3–4 (high risk) | Median TTP | 40 months (95% CI 11 months to NE) | 12 months (95% CI 8 to 25 months) | 0.0333 |
| Subgroup | Estimated median TTF for CHOP | p-value for Cox regression | Estimated RR for treatment failure for R-CHOP (95% CI) |
|---|---|---|---|
| IPI 1–2 | Not reached | 0.001 | 0.412 (0.242 to 0.701) |
| IPI 3–5 | 29 months | 0.009 | 0.331 (0.144 to 0.761) |
Age
Eighteen-month follow-up data in the GLSG-2000 trial92 found that TTF was prolonged in the R-CHOP arm for patients of any age (Table 19). The relative risk (RR) of treatment failure in the R-CHOP arm compared with the CHOP arm was 0.417 (95% CI 0.233 to 0.747) for patients aged < 60 years and was 0.354 (95% CI 0.175 to 0.715) for patients aged ≥ 60 years.
| Age (years) | Estimated median TTF for CHOPa | p-value for Cox regression | Estimated RR for treatment failure for R-CHOP (95% CI) |
|---|---|---|---|
| < 60 | Not reached | 0.003 | 0.417 (0.233 to 0.747) |
| ≥ 60 | 29 months | 0.004 | 0.354 (0.175 to 0.715) |
Quality of response
Salles et al. 94 analysed the response duration for the subgroup of patients who were in CR/CRu at 18 months of treatment in the FL2000 trial. The response duration was significantly different between the two treatment arms, with 4-year estimates of 44% (95% CI 32% to 54%) compared with 64% (95% CI 55% to 72%) in the CHVPi and R-CHVPi arms, respectively (p = 0.012). Therefore, as well as rituximab and chemotherapy increasing the number of CR/CRus, patients are also more likely to have a longer response duration.
Other prognostic factors
Marcus et al. 95 conducted several univariate analyses for a number of prognostic factors (Table 20) in the M39021 trial. The R-CVP treatment arm was associated with a significant prolonged TTP when compared with CVP alone for all subgroups investigated including baseline histology, presence or absence of B symptoms, and presence or absence of bulky disease. A significant improvement in TTP was seen in patients with baseline-only haemoglobin of at least 12 g/dl; however, no difference in TTP was observed between the R-CVP and CVP arms in patients with baseline haemoglobin of < 12g/dl (p = 0.3941).
| Subgroup | R-CVP | CVP | p-value |
|---|---|---|---|
| Histology at central review (IWF) | |||
| Class B | 34 months (95% CI 27 months to NE) | 17 months (95% CI 11 to 24 months) | 0.0037 |
| Class C | |||
| Class D | |||
| Histology at central review (IWF) | |||
| Class C | 35 months (95% CI 26 months to NE) | 15 months (95% CI 10 to 21 months) | < 0.0001 |
| Class D | Not reached (95% CI 30 months to NE) | 14 months (95% CI 7 to 24 months) | 0.0046 |
| B symptomsa ≥ 1 | 32 months (95% CI 22 months to NE) | 17 months (95% CI 12 to 23 months) | 0.0014 |
| No B symptomsa | 37 months (95% CI 26 months to 48) | 14 months (95% CI 11 to 20 months) | < 0.0001 |
| Bulky disease | |||
| Yes | 38 months (95% CI 25 months to 48) | 13 months (95% CI 11 to 21 months) | < 0.0001 |
| No | 32 months (95% CI 26 months to NE) | 16 months (95% CI 13 to 21 months) | < 0.0001 |
| Haemoglobin | |||
| ≥ 12 g/dl | 39 months (95% CI 31 months to NE) | 17 months (95% CI 13 to 22 months) | < 0.0001 |
| < 12 g/dl | 11 months (95% CI 9 to 28 months) | 12 months (95% CI 10 to 16 months) | 0.3941 |
Marcus et al. 95 also undertook two multivariate analyses: one that included the IPI as a composite along with other prognostic factors not incorporated in the IPI and one that included the individual factors which make up the FLIPI and IPI, together with other prognostic factors. These analyses found that only haemoglobin level (< 12 g/dl) and number of nodal areas involved (> 1) were statistically significant predictors of TTP in addition to trial treatment.
Buske and Hoster105 conducted a multivariate analysis on the GLSG-2000 trial92 data at 20-month follow-up including the individual FLIPI risk factors. This found that a serum LDH level higher than the upper normal limit (RR 2.6, 95% CI 1.5 to 4.5) and a haemoglobin level of < 12 g/dl (RR 2.5, 95% CI 1.4 to 4.3) were independently associated with a shorter TTF in addition to trial treatment. However, age (≥ 60 years vs < 60 years; RR 0.9, 95% CI 0.5 to 1.5) and the number of nodal areas (> 4 vs ≤ 4; RR 1.5, 95% CI 0.8 to 2.6) did not significantly influence the TTF.
Meta-analysis
Three exploratory meta-analyses were conducted to explore the results of synthesising the ORR, CR and PR from the four trials.
There were several problems with the validity of these analyses. First, the level of statistical heterogeneity calculated in RevMan using the I2-statistic was very high (range I2 = 56–88%). The I2-statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance),106 and an I2-value > 50% is considered to be a high enough level of heterogeneity to suggest meta-analysis is not appropriate. Ideally, this high level of heterogeneity would be explored further and explained by estimating the predictive distribution of a new study. This was not undertaken owing to resource constraints.
Reasons for the high level of heterogeneity could be because of differences in treatment effects in the four trials. 91–96 Examination of the CIs for the results from the individual trials showed that there was little overlap in the meta-analyses for CR, and to a lesser extent for PR, indicating evidence for heterogeneity of intervention effects. Indeed, the GLSG-2000 trial91,92 observed much higher ORR (a combination of CR and PR) for both the R-chemotherapy and chemotherapy-alone arms in comparison with the other studies. This was mostly accounted for by an increase in the numbers of PR (20% CR and 77% PR in the R-CHOP arm), whereas in the OSHO-39 trial93 there was a more even split between the CR/PR categories (R-MCP CR = 50% and PR = 43%). As well as evidence for different intervention effects in the four trials, there are other possible explanations for the high level of heterogeneity. First, each study administered a different therapeutic intervention with respect to the chemotherapy regimen used; this included different chemotherapeutic agents (CVP, CHOP, MCP and CHVPi) and different regimens of treatment (treatment every 3 weeks vs every 4 weeks; six cycles of treatment vs eight cycles of treatment). Second, there was a difference in the sample sizes of the studies; for example, the GLSG-2000 trial91,92 was the largest trial with an ITT population of n = 557 patients, whereas the OSHO-39 trial93 was substantially smaller (n = 201).
The AG also notes that the choice of chemotherapeutic regimen is not solely determined by clinical efficacy. For example, R-CHOP is less likely to be given to patients who are elderly or unfit, but more likely to be given to treat aggressive or bulky disease, which may impact on the perceived efficacy. Additionally, the analyses assume that rituximab has no synergistic interaction with the chemotherapeutic component of a regimen for the treatment effect. The AG also comment that the analyses of ORR, CR and PR are not independent analyses given that the same patients are counted in more than one analysis.
The AG therefore believes the response rates from the individual trials to be a more robust estimator of the efficacy of the specific R-chemotherapy regimens. These are subsequently used in the decision model (see Chapter 4) rather than meta-analysed response rates. The results from the meta-analyses are presented in Appendix 14 for completeness, but the use of these are strongly cautioned against.
Safety data
The evaluation of the safety of R-chemotherapy is mainly derived from data reported from the four included trials,91–96 which are described above (see Summary of trials). AE data were extracted from the four trials (see Appendix 11 for completed data extraction forms). In addition, postmarketing surveillance data from the MS are presented. 62
The M39021, OSHO-39 and FL2000 trials93–96 graded AEs in accordance with the NCI-CTC grading system,100 but the GLSG-2000 trial91,92 used the WHO toxicity criteria101 to record AEs. However, there are no substantial differences between these two scales. 107
Treatment completion and withdrawals
The M39021, OSHO-39 and FL2000 trials93–96 reported data on the number of treatment cycles that were completed. No data were presented on the planned cycle completion, doses of study drugs administered and withdrawal numbers or reasons in the GLSG-2000 trial. 91,92
Overall, a greater proportion of patients in the R-chemotherapy arms received the planned number of cycles when compared with the chemotherapy-alone arm (Table 21). No differences in dose of chemotherapy received were noted between the R-chemotherapy and chemotherapy-alone arms, with the exception of cyclophosphamide in the M39021 trial. 95,96 Reasons for withdrawal from treatment appeared to be mostly owing to disease progression or treatment failure (e.g. failing to achieve a response to treatment after a defined number of cycles). However, there was a lack of transparency in the studies regarding withdrawals for other reasons such as AEs/reactions. This is considered in more detail by trial.
M39021 trial
A Consolidated Standards of Reporting Trials diagram108 was reported for the M39021 trial,95,96 which showed the flow of patients through the trial. This showed that 137/162 (85%) of patients in the R-CVP arm and 108/159 (68%) patients in the CVP arm completed eight cycles. 95,96 The MS62 provided further details on cycle completion, with 6/162 (4%) of patients in the R-CVP arm withdrawn before cycle 4 compared with 13/159 (8%) in the CVP arm. Thus, 19/162 (12%) patients in the R-CVP arm were withdrawn after cycle 4 compared with 38/159 (24%) in the CVP arm. The majority of patients appear to have been withdrawn owing to an insufficient treatment response (defined as disease progression or stable disease after cycle 4). However, a number of patients were withdrawn before cycle 4 for which the reasons are not made explicit. The authors note that two patients were withdrawn as a result of grade 3 or 4 rituximab infusion-related reactions and one patient withdrew consent and thus withdrew from the trial; however, this does not account for all patients.
Marcus et al. 96 report the proportion of patients in the M39031 trial who received the planned doses of chemotherapy. The proportion of patients that received > 90% of the planned dose of prednisolone and vincristine at each administered cycle was comparable between the R-CVP and CVP arms. However, the proportion of patients who received > 90% of cyclophosphamide was higher in the CVP group (> 94%) than the R-CVP group (> 85%). The authors state that this was ‘mainly due to dose modifications in the R-CVP group for NCI–CTC grades 3 and 4 neutropenia’. Clinical advice suggests this is now less of a problem as granulocyte-stimulating factor is routinely used to treat neutropenia. Ninety-six per cent of patients received > 90% of the planned dose of rituximab at each administered cycle. 96
OSHO-39 trial
In the OSHO-39 trial,93 88% of patients in the R-MCP arm and 67% in the MCP arm completed all eight cycles of treatment. Treatment failure owing to disease progression after two cycles occurred in three patients in the R-MCP arm and in 10 patients in the MCP arm. Failure to achieve at least a PR after six cycles occurred in seven patients in the R-MCP arm and 22 patients in the MCP arm. Numbers of patient withdrawals (n = 16) prior to the study drug administration and the associated reasons were reported; however, this includes patients with MCL as well as FL. The authors state that all other withdrawals were because of non-response/treatment failure during therapy (which was defined as disease progression after two cycles of therapy or failure to reach a PR or CR after six cycles of therapy). The authors do not state if there were any withdrawals because of AEs or reactions.
The mean dose of study drugs administered in the OHSO-39 trial92 were rituximab, 660−680 mg/cycle; mitoxantrone, 24−28 mg/cycle; chlorambucil, 68−81 mg/cycle and prednisolone, 226−231 mg/cycle. The authors stated that the dose intensity of the chemotherapy did not differ between treatment arms. 93 Interferon-alpha maintenance treatment (3 × 4.5 MIU/week until disease progression) was initiated in 97% and 92% of responding patients in the R-MCP and MCP arms, respectively.
FL2000 trial
In the FL2000 study,94 the MS62 noted that 95% of patients in the R-CHVPi arm and 94% of patients in the CHVPi arm received the initial six cycles of treatment. Among patients who did not progress during therapy, 161 (98%) and 153 (98%) of the patients received the planned chemotherapy courses during the first 6 months in the R-CHVPi and CHVPi arms, respectively. In the CHVPi arm, 116 (87%) of 134 patients without death or progression received the six planned cycles of chemotherapy consolidation; the R-CHVPi arm did not receive this chemotherapy consolidation. Two hundred and thirty-seven (66%) patients followed the interferon treatment according to the protocol, with dose adaptation (45 patients) or short (< 4 weeks) interruptions (55 patients), without significant differences in adaptation between the two study arms. In addition, interferon treatment was stopped in 50 patients resulting from disease progression (R-CHVPi arm, 19 cases, and CHVPi arm, 31 cases, respectively) and was interrupted either for > 1 month (16 cases) or definitively (72 cases) resulting from toxicity. These major interruptions were observed in 41 patients in the RCHVPi arm and 47 patients in the CHVPi arm. One patient withdrew consent after registration, and one patient had a major inclusion violation (registered at relapse) and thus were withdrawn from the treatment in the FL2000 trial. 94 No further details are provided on withdrawals in the FL2000 trial94 during treatment; although not all patients received the planned six cycles of initial treatment.
Adverse events of any grade
Adverse events of any grade were reported as more frequent in the R-MCP arm than in the MCP arm in the OSHO-39 trial93 (99% vs 86% of patients, respectively). However, the M39021 trial95,96 reported that the proportion of patients that reported at least one AE was comparable between the CVP (95%) and R-CVP (97%) groups. Marcus et al. 96 report that AEs associated with the gastrointestinal and nervous systems as well as general disorders and administration site reactions were the most commonly occurring types of events in both treatment groups in the M39021 trial. 95,96 Fatigue, neutropenia and back pain were the most common severe AEs and occurred at a slightly higher frequency in patients receiving R-CVP. These data were not available within the manuscripts95,96 reporting on the M39201 trial but appear to be confirmed by data presented in the MS,62 which reports on all grades of AEs in the M39201 trial.
Grade 1 and 2
The OSHO-3993 and GLSG-200092 trials reported grade 1 and 2 AEs. The authors in each trial reported that there were no significant differences between the treatment arms. The most common grade 1/2 AE in the OSHO-39 trial92 study was infection, which affected 42% of patients receiving R-MCP, and 35% receiving MCP. In the GLSG-2000 trial,92 the most commonly reported grade 1/2 AE was low haemoglobin level, with 50% of R-CHOP and 49% of CHOP patients affected. Neurotoxicity was another frequent grade 1 or 2 AE reported in the GLSG-2000 trial92 (R-CHOP 34%, CHOP 42%). Reduced platelet count was also a common AE, especially in the OSHO-39 trial93 (R-MCP 30%, MCP 33%), whereas the GLSG-2000 trial92 reported lower incidences for patients receiving the CHOP-based treatments (R-CHOP 17%, CHOP 16%). Nausea and vomiting was another frequent grade 1 or 2 AE in both trials (R-CHOP 45%, CHOP 44% in the GLSG-2000 trial and R-MCP 24%, MCP 15). 93 For a detailed list of grade 1 and 2 AEs see Table 22.
| AEs: n (%) | bGLSG-200092 | cOSHO-393 | ||
|---|---|---|---|---|
| R-CHOP (n = 223) | CHOP (n = 205) | R-MCP (n = 105) | MCP (n = 96) | |
| Low haemoglobin level | 112 (50) | 100 (49) | 18 (17) | 18 (19) |
| Leucocytopenia | 54 (24) | 57 (28) | 3 (3) | 8 (8) |
| Granulocytopenia | 42 (19) | 41 (20) | – | – |
| Reduced platelet count | 38 (17) | 33 (16) | 31 (30) | 32 (33) |
| Infection | 74 (33) | 59 (29) | 44 (42) | 34 (35) |
| Bleeding | 9 (4) | 6 (3) | – | – |
| Nausea/vomiting | 100 (45) | 90 (44) | 25 (24) | 14 (14) |
| Stomatitis | 58 (26) | 59 (29) | 11 (10) | 7 (7) |
| Obstipation (severe constipation) | 33 (15) | 27 (13) | – | – |
| Diarrhoea | 25(11) | 23 (11) | 11 (10) | 4 (4) |
| Fever | 65 (29) | 45 (22) | NR | NR |
| Cardiac dysfunction | 7 (3) | 8 (4) | NR | NR |
| Alopecia | 42 (19) | 51 (25) | NR | NR |
| Cardiac arrhythmia | 13 (6) | 8 (4) | NR | NR |
| Neurotoxicity | 76 (34) | 86 (42) | NR | NR |
| CNS toxicity | 4 (2) | 4 (2) | NR | NR |
| Allergy | 13 (6) | 0 (0) | NR | NR |
| Rash | NR | NR | 16 (15) | 1 (1) |
| Heartburn | NR | NR | 15 (14) | 3 (3) |
| Insomnia | NR | NR | 15 (14) | 7 (7) |
| Bone pain | NR | NR | 10 (10) | 10 (10) |
| Gastrointestinal | NR | NR | 9 (9) | 5 (5) |
| Other (not specified) | NR | NR | 11 (10) | 8 (8) |
Grade 3 and 4 adverse events
All fours studies reported grade 3 and 4 AEs; the GSLG-200092 and OSHO-3993 trials reported grade 3 and 4 AEs separately, whereas the M3902195,96 and FL200094 trials combined the numbers of grade 3 or 4 AEs. The most common AEs observed in the four trials were related to the blood and bone marrow, including leucocytopenia, neutropenia and granulocytopenia. For two trials, the most common grade 3 and 4 AEs were reduced leucocyte (white blood cell) levels; this was observed in 69% of R-CHOP and 61% CHOP patients in the GLSG-2000 trial92 and 72% R-MCP and 58% MCP patients in the OSHO-39 trial. 93 The statistical significance of the difference in grade 3/4 leucopenia between the treatment arms in the OSHO-39 trial93 was not reported by the authors, whereas the difference between the R-CHOP and CHOP treatment arms in the GLSG-2000 trial92 was reported as not significant.
The most common AE in the M39021 trial95,96 was neutropenia (24% in R-CVP and 14% in CVP arms); however, the authors do not state if this was a statistically significant difference between treatment arms. In the FL2000 trial,94 the most common grade 3/4 AE was neutrophil toxicity (59% R-CHVPi and 62% in CHVP arms). However, the FL2000 trial94 only noted a significant difference in grade 3 or 4 AEs for neutrophil toxicity during the 12-month consolidation period, which was more frequent in the chemotherapy-alone arm than the rituximab-containing arm (p < 0.001) (results presented in the data extraction form for the FL2000 trial94 in Appendix 11).
There were a number of patients who had a low granulocyte count of grade 3 or 4 severity in the GSLG-2000 trial92 and the difference between the treatment arms was statistically significant (R-CHOP 63%, CHOP 53%; p = < 0.01). In addition, grade 3 or 4 alopecia was a frequently observed AE in both arms of the GLSG-2000 trial92 (R-CHOP 67%, CHOP 61%).
Blood or bone marrow AEs may be associated with infection. However, the difference in frequency of blood or bone marrow AEs between treatment arms is of minor clinical significance as they did not translate into a difference in infection rates between the treatment arms for all three studies. Infections of grade 3 or 4 were observed in 8% of the MCP group and 7% of the R-MCP group; 5% R-CHOP arm and 7% CHOP arm and 2% of the R-CHVPi arm and 0% CHVPI arm. 92–94 The MS61 reports all grades of infections for three trials, and follows a similar pattern (33% R-CVP and 32% CVP, 38% R-CHOP and 36% CHOP, 49% R-MCP and 43% MCP).
More detail on grade 3/4 AEs combined for the four trials and grade 3 or 4 AEs reported separately (only for the GSLG-200092 and OSHO-3993 trials) are reported in Tables 23 and 24, respectively.
| AEs: n (%) | M3902195,96 | GLSG-200092 | OSHO-3993 | bFL200094 | ||||
|---|---|---|---|---|---|---|---|---|
| R-CVP (n = 162) | CVP (n = 159) | R-CHOP (n = 223) | CHOP (n = 205) | R-MCP (n = 105) | MCP (n = 96) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| Low haemoglobin level | – | – | 20 (9) | 21 (10) | 3 (3) | 4 (4) | 6 (3) | 9 (5) |
| Leucocytopeniac | 19 (12) | 14 (9) | 154 (69) | 125 (61) | 75 (72) | 56 (58) | – | – |
| Neutropenia | 39 (24) | 22 (14) | – | – | – | – | 103 (59) | 114 (62) |
| Granulocytopenia | – | – | 140 (63) | 109 (53) | – | – | – | – |
| Reduced platelet count | – | – | 13 (6) | 16 (8) | 4 (4) | 7 (7) | 5 (3) | 6 (3) |
| Bleeding | – | – | 0 (0) | 0 (0) | – | – | – | – |
| Nausea/vomiting | – | – | 9 (4) | 12 (6) | 1 (1) | 6 (6) | – | – |
| Stomatitis | – | – | 2 (1) | 4 (2) | 1 (1) | 1 (1) | – | – |
| Obstipation (severe constipation) | – | – | 4 (2) | 2 (1) | – | – | – | – |
| Diarrhoea | – | – | 4 (2) | 6 (3) | 2 (2) | 2 (2) | – | – |
| Fever | – | – | 0 (0) | 2 (1) | – | – | 2 (1) | 2 (1) |
| Alopecia | – | – | 149 (67) | 125 (61) | – | – | – | – |
| Infection | – | – | 11 (5) | 14 (7) | 7 (7) | 8 (8) | 4 (2) | 0 (0) |
| Cardiac dysfunction | – | – | 7 (3) | 2 (1) | – | – | 2 (1) | 3 (2) |
| Cardiac arrhythmia | – | – | 4 (2) | 0 (0) | – | – | – | – |
| Neurotoxicity | – | – | 2 (1) | 4 (2) | – | – | – | – |
| CNS toxicity | – | – | 2 (1) | 0 (0) | – | – | – | – |
| Allergy | – | – | 2 (1) | 0 (0) | – | – | – | – |
| Rash | – | – | – | – | 0 | 2 (2) | – | – |
| Heartburn | – | – | – | – | 1 (1) | 0 (0) | – | – |
| Insomnia | – | – | – | – | 0 (0) | 0 (0) | – | – |
| Bone pain | – | – | – | – | 2 (2) | 0 (0) | – | – |
| Gastrointestinal | – | – | – | – | 2 (2) | 2 (2) | – | – |
| Other | – | – | – | – | 0 (0) | 2 (2) | – | – |
| AEs: n (%) | GLSG-200092 | OSHO-3993 | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |||||
| R-CHOP (n = 223) | CHOP (n = 205) | R-CHOP (n = 223) | CHOP (n = 205) | R-MCP (n = 105) | MCP (n = 96) | R-MCP (n = 105) | MCP (n = 96) | |
| Haemoglobin level | 18 (8) | 18 (9) | 2 (1) | 2 (1) | 2 (2) | 3 (3) | 1 (1) | 1 (1) |
| Leucocyte/white blood cells | 96 (43) | 78 (38) | 58 (26) | 47 (23) | 25 (24) | 21 (22) | 50 (48) | 35 (36) |
| Granulocyte count | 49 (22) | 47 (23) | 91 (41) | 62 (30) | – | – | – | – |
| Platelet count | 9(4) | 10 (5) | 4 (2) | 6 (3) | 4 (4) | 6 (6) | 0 (0) | 1 (1) |
| Bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – | – | – | – |
| Nausea/vomiting | 9 (4) | 12(6) | 0 (0) | 0(0) | 1 (1) | 6 (6) | 0 (0) | 0 (0) |
| Stomatitis | 2 (1) | 4 (2) | 0 (0) | 0(0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Obstipation (severe constipation) | 4(2) | 2 (1) | 0 (0) | 0(0) | – | – | 0 (0) | 0 (0) |
| Diarrhoea | 4 (2) | 6 (3) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 2 (2) |
| Fever | 0 (0) | 2 (1) | 0 (0) | 0 (0) | – | – | – | – |
| Alopecia | 140 (63) | 115 (56) | 9 (4) | 10 (5) | – | – | – | – |
| Infection | 11 (5) | 12 (6) | 0 (0) | 2 (1) | 6 (6) | 7 (7) | 1 (1) | 1 (1) |
| Cardiac dysfunction | 4 (2) | 2(1) | 2 (1) | 0 (0) | – | – | – | – |
| Cardiac arrhythmia | 2 (1) | 0 (0) | 2 (1) | 0 (0) | – | – | – | – |
| Neurotoxicity | 2 (1) | 4 (2) | 0 (0) | 0 (0) | – | – | – | – |
| CNS toxicity | 2 (1) | 0 (0) | 0 (0) | 0 (0) | – | – | – | – |
| Allergy | 2 (1) | 0 (0) | 0 (0) | 0 (0) | – | – | – | – |
| Rash | – | – | – | – | 0 (0) | 2 (2) | 0 (0) | 0 (0) |
| Heartburn | – | – | – | – | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Insomnia | – | – | – | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bone pain | – | – | – | – | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal | – | – | – | – | 2 (2) | 1 (1) | 0 (0) | 1 (1) |
| Other | – | – | – | – | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
Infusion-related reactions
Infusion-related reactions were observed in 7% of courses during the first infusion in the GSLG-2000 trial92 and early cessation of rituximab therapy was required in two patients. Fourteen (9%) patients in the M39201 trial95,96 had a grade 3 or 4 rituximab infusion-related reaction, and two of these patients were withdrawn from study treatment. More patients in the R-CVP group than in the CVP group experienced an AE within 24 hours of an infusion (71% vs 51%, respectively). One grade 3 infusion-related reaction was reported in the OSHO-39 trial93 in the MS62 and related to the full study population of FL and MCL.
Death and life-threatening adverse events
Overall, there were very few AEs reported as life-threatening or leading to death within the trials. The M39201 trial95,96 reported that five patients experienced a total of six life-threatening events following R-CVP; however, no treatment-related deaths occurred. The remaining three studies did not report whether or not AEs were either life-threatening or led to death.
The number of deaths reported for the chemotherapy-alone arms were consistently higher compared with the R-chemotherapy arms in all four trials. A total of 49 deaths were reported in the M39201 trial96 from 30-month follow-up96 (21 in the R-CVP arm and 28 in the CVP arm; patients may have received second-line therapy at this stage). Twenty-three deaths (17 CHOP and six R-CHOP) and 40 deaths (25 MCP and 15 R-MCP) occurred in study GLSG-200091 and study OSHO-39,93 respectively. In the FL2000 trial,94 a total of 45 patients had died at the time of the analysis at 42 months (16 R-CHVPi and 29 CHVPi). The majority of deaths were attributed to lymphoma progression. The GLSG-2000 study92 reported the additional reasons for death in detail (Table 25); however, the other three trials did not report this information.
| Death and reasons for death | aM3902196 | bGLSG-200092 | OSHO-3993 | cFL200094 | ||||
|---|---|---|---|---|---|---|---|---|
| R-CVP (n = 162) | CVP (n = 159) | R-CHOP (n = 223) | CHOP (n = 205) | R-MCP (n = 105) | MCP (n = 96) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| Total nos. (%) of deaths | 21 (13) | 28( 18) | 6 | 17 | 15 (14) | 25 (26) | 16 | 29 |
| Reasons for death | ||||||||
| Lymphoma/progressive disease | 13 (8) | 22 (14) | 1 (0) | 9 (4) | 7 | 17 | – | – |
| Infection | – | – | 4 (2) | 4 (2) | – | – | – | – |
| Cardiac failure | – | – | 0 | 1 (0) | – | – | – | – |
| Apoplectic insult | – | – | 0 | 1 (0) | – | – | – | – |
| GVHD after ASCT | – | – | 0 | 1 (0) | – | – | – | – |
| Unknown | – | – | 1 (0) | 1 (0) | – | – | – | – |
Subgroup analyses
The MS61 reported data on the safety from the GLSG-2000 trial92 for the elderly population (≥ 60 years of age, n = 221). As for the whole trial population, the most common AEs were blood and bone marrow disorders, gastrointestinal disorders, skin toxicities, neurological disorders, cardiac disorders, infections and fever. Most of the AEs were mild to moderate in intensity, except for alopecia, leucopenia and neutropenia, which were mainly of grade 3/4 in intensity. The most common grade 3/4 AEs in the elderly population were blood and bone marrow disorders and alopecia. The remaining three trials did not provide AE data for subgroup populations.
Postmarketing data (taken from the manufacturer’s submission)
Over 1 million patients (length of exposure not known), predominantly NHL patients, have received rituximab since its first marketing authorisation. Worldwide safety data submitted to the Periodic Safety Update Reports (PSURs) (with a cut-off date of April 2007) have recorded 13,008 AEs. Of these reported AEs, 10,184 were classified as serious. For 7174 events, the report came from spontaneous sources (postmarketing experience). Other sources include clinical trials in oncology and rheumatoid arthritis (company- and investigator-sponsored trials). The MS62 presents a summary of AEs in the global safety database for rituximab (as of 30 April 2007) and this is presented in Table 26. The most frequently reported events were infection and infestation (15%), blood and lymphatic system disorders (14%), general disorders and administration site conditions (11%) and respiratory, thoracic and mediastinal disorders (10%).
| System organ class | SAEs | % SAEs | Total AEs | % Total AEs |
|---|---|---|---|---|
| Blood and lymphatic system disorders | 1586 | 16 | 1775 | 14 |
| Cardiac disorders | 566 | 6 | 604 | 5 |
| Congenital, familial and genetic disorders | 9 | 0 | 10 | 0 |
| Ear and labyrinth disorders | 31 | 0 | 44 | 0 |
| Endocrine disorders | 13 | 0 | 15 | 0 |
| Eye disorders | 61 | 1 | 106 | 1 |
| Gastrointestinal disorders | 601 | 6 | 767 | 6 |
| General disorders and administration site conditions | 770 | 8 | 1400 | 11 |
| Hepatobiliary disorders | 163 | 2 | 165 | 1 |
| Immune system disorders | 399 | 4 | 480 | 4 |
| Infections and infestations | 1852 | 18 | 1986 | 15 |
| Injury, poisoning and procedural complications | 177 | 2 | 281 | 2 |
| Investigations | 433 | 4 | 603 | 5 |
| Metabolism and nutrition disorders | 118 | 1 | 137 | 1 |
| Musculoskeletal and connective tissue disorders | 331 | 3 | 523 | 4 |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 495 | 5 | 513 | 4 |
| Nervous system disorders | 454 | 4 | 611 | 5 |
| Pregnancy, puerperium and perinatal conditions | 15 | 0 | 30 | 0 |
| Psychiatric disorders | 58 | 1 | 78 | 1 |
| Renal and urinary disorders | 174 | 2 | 188 | 1 |
| Reproductive system and breast disorders | 26 | 0 | 44 | 0 |
| Respiratory, thoracic and mediastinal disorders | 1136 | 11 | 1348 | 10 |
| Skin and subcutaneous tissue disorders | 271 | 3 | 711 | 5 |
| Social circumstances | 6 | 0 | 8 | 0 |
| Surgical and medical procedures | 47 | 0 | 51 | 0 |
| Vascular disorders | 392 | 4 | 530 | 4 |
| Total | 10,184 | 100 | 13,008 | 100 |
The updated summary of product characteristics from the EMA84 also discusses cases of progressive multifocal leucoencephalopathy (PML) being associated with the use of rituximab. All patients treated with MabThera for rheumatoid arthritis must be given a patient alert card with each infusion, which contains important safety information for patients including signs and symptoms to watch out for. However, cases of PML reported during postmarketing use of rituximab in NHL are very rare (numbers/percentages are not reported).
Discussion
The results from four randomised trials91–96 (of good quality) comparing the combination of R-chemotherapy with chemotherapy alone showed an improvement in a number of clinical effectiveness outcomes. This included trials evaluating R-CVP,95,96 R-CHOP,91,92 R-MCP93 and R-CHVPi94 in each case against their respective chemotherapy regimen.
Evidence from the four trials91–96 on the primary outcome of interest in this appraisal, OS, showed a benefit for rituximab and chemotherapy compared with chemotherapy alone, for all chemotherapy regimens. The difference in OS rates ranged from 6% to 14% when the R-chemotherapy arms were compared with the chemotherapy-alone arms. The difference in OS rates was statistically significant in three trials, the exception being the FL2000 trial94 (p = 0.1552). However, the follow-up period for the four trials is approximately 4–5 years and the median OS has yet to be reached for each arm (intervention and comparator) within each trial. The median survival of FL is reported as 8–10 years,29 although some have commented that this figure has increased in the last decade,14,15 and thus the evidence for the effect of R-chemotherapy on OS might be strengthened by a longer follow-up period. It is also noted that data in three trials are confounded by additional trial treatments (interferon-alpha maintenance/consolidation and SCT – for further details see Summary of trials), which needs to be considered when interpreting the OS and other time-to-event data. However, given the relapsing and remitting nature of FL, it is unlikely that a trial could be ethically undertaken to remove the effect of subsequent therapies, i.e. when a patient relapses they will receive subsequent treatment to induce remission.
Progression-free survival was measured only in the OSHO-39 trial93 and was significantly prolonged for the R-chemotherapy arm compared with the chemotherapy-alone arms (R-MCP) (median 28.8 months for MCP and not reached for R-MCP, p < 0.0001). Other time-to-event data such as EFS, TTP and TTNT showed similar benefits in effect, although these were inconsistently defined and not directly comparable between trials.
Overall response rates were significantly improved in all four trials,91–96 with a difference in 5–24% between the R-chemotherapy and chemotherapy arms. CR rates were also improved, with a difference between the R-chemotherapy and chemotherapy arms of 2–25%, which was reported as significant in three studies93–96 (the GLSG-2000 trial92 of R-CHOP vs CHOP did not report a p-value). Differences in PR rates were generally smaller (level of significance not reported); however, this might be explained by a potential way R-chemotherapy shifts patients from non-responders to PRs and PRs to CRs. There was some evidence that the response quality differed among the four R-chemotherapy combinations. For example, greater ORR was observed in the GSLG-2000 trial91,92 (R-CHOP vs CHOP) compared with the M39021 trial95,96 (R-CVP vs CVP), whereas CR rates were greater in the M39021 trial95,96 than the GLSG-2000 trial. 91,92 Others have noted these differences between R-chemotherapy regimens. 81 Clinical advice to the AG noted that R-CHOP/CHOP is reserved for more aggressive disease, and this would have implications on the quality of response. However, the baseline characteristics of the patients were generally similar in each of the four trials. 91–96
Considerable statistical heterogeneity was observed in exploratory meta-analyses undertaken to provide a summary of effect of response rates. Differences in treatment effects, study sample sizes, and chemotherapeutic agents and regimens are plausible reasons for this heterogeneity. Owing to the high level of heterogeneity, meta-analysis of response rates is not considered appropriate. Thus, response rate results from individual studies are considered more robust.
The safety data show that the addition of rituximab to chemotherapy does not result in clinically relevant adverse outcomes. Although an increased statistically significant incidence of leucocytopenia, neutropenia and granulocytopenia was observed in the trials in the R-chemotherapy arms, this was of limited clinical significance as the rate of infection did not increase in the R-chemotherapy arms (infection is associated with leucocytopenia, neutropenia and granulocytopenia). However, considerable numbers of patients were affected by grade 3 or 4 alopecia in both the R-CHOP and CHOP arms of the GSLG-2000 trial. 91,92 This side effect is as a result of the CHOP component of the treatment and is an important side effect to consider particularly in terms of patient acceptance, tolerance and choice.
It is noted that the median age of patients within the trials (52–61 years) is considerably younger than that seen in clinical practice, where over 70% are aged > 60 years at diagnosis and clinical advice suggests that the ECOG performance status is better than that seen in UK clinical practice. 10 This affects the generalisability of the findings to the clinical FL population; however, limited analyses undertaken within the trials did not show a differential affect for different clinical and demographic subgroups. Specifically, the GSLG-2000 trial91,92 showed that adding rituximab to chemotherapy was beneficial for both patients aged > 60 years and aged < 60 years.
Our own searches of the randomised evidence were exhaustive and we are confident that we have not missed any published reports of RCTs or other systematic reviews of R-chemotherapy in the treatment of FL.
In conclusion, the addition of rituximab to chemotherapy results in better clinical outcomes for patients when compared with chemotherapy alone, for all chemotherapeutic backbones examined in this review, i.e. CVP, CHOP, MCP and CHVPi. This is achieved with minimal additional AEs or toxicity, which are deemed to be clinically relevant.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
This chapter describes a review of the existing evidence on the cost-effectiveness of the addition of rituximab to chemotherapy in patients with untreated, symptomatic stage III/IV FL. This includes a systematic review of published evidence and evidence included in the MS. 62
Methods
A systematic search was performed to identify studies addressing the cost-effectiveness of the addition of rituximab to chemotherapy for the first-line treatment of FL. Only full economic evaluations published in English addressing the cost-effectiveness of the addition of rituximab to chemotherapy compared with chemotherapy alone in patients with FL were included in the review.
Eight databases were searched for relevant published literature including MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations (Ovid); CINAHL; EMBASE; NHS EED and HTA databases; SCI; and BIOSIS. In addition, literature searches were undertaken for the clinical effectiveness review and quality-of-life review (see Identification of studies) and relevant cost papers were identified from these searches. In addition, the reference lists of relevant articles and the MS62 were handsearched. Full details of the search strategies used in MEDLINE are presented in Appendix 5 (these have been adapted for use in other databases). Searches were not restricted by language or publication date.
Studies were selected for inclusion through a two-stage process. Titles and abstracts were examined for inclusion by one reviewer. Full manuscripts of selected citations were retrieved and assessed by one reviewer. The quality of the cost-effectiveness studies were assessed using a critical appraisal checklist adapted from the Drummond and Jefferson109 and Eddy110 checklists.
Results
Identified studies
The search retrieved 280 citations relating to cost-effectiveness (Figure 5). Two hundred and fifty-four articles were excluded at title stage, and 21 articles were excluded at abstract level. Four studies (corresponding to five references) were examined at full-text level111–115 and three studies (corresponding to four references) were identified as meeting the inclusion criteria of the systematic review of economic evaluations. 111–113,115 This included the Evidence Review Group (ERG) report submitted to NICE for TA110112 in which the addition of rituximab to CVP in first-line induction treatment was evaluated. Gomez et al. 114 was excluded from the review as this reference was unobtainable.
FIGURE 5.
Flow diagram of economic evaluation selection/exclusion.
An economic model was described in two studies: an HTA monograph113 and an ERG report. 112 Both studies are based on a critique undertaken by the ERG of the model submitted by the manufacturer (Roche) for TA110,83 a single technology appraisal (STA).
Overall, three different economic models were identified.
Summary of published cost-effectiveness studies
The three identified economic models111–113,115 were similar and used a Markov approach. There were differences in the comparators used between the studies. Dundar et al. 112,113 and Hornberger et al. 115 evaluated the cost-effectiveness of the addition of rituximab to CVP only. Ray et al. 111 reported the cost-effectiveness of the addition of rituximab to a CVP, CHOP, MCP and CHVPi regimen.
Ray et al. 111 and Dundar et al. 112,113 adopted the perspective of the UK NHS and Personal Social Services (PSS) with costs and benefits discounted at an annual rate of 3.5%. Hornberger et al. 115 conducted an economic evaluation in the USA, with costs and benefits discounted at 3.0%.
The impact of main model parameters was examined in univariate sensitivity analyses (SAs)in all economic evaluations identified by the AG. 111–113,115 Probabilistic sensitivity analyses (PSAs) were performed in the two UK models only. 111–113
The two UK economic evaluations produced broadly similar incremental cost-effectiveness ratios (ICERs) for the comparison of R-CVP with CVP. Dundar et al. 112,113 reported a cost per quality-adjusted life-year (QALY) gained of £8290 for the addition of rituximab to a CVP regimen in the MS62 model. Ray et al. 111 reported an ICER of £8613 per QALY gained for the same comparison and reported an ICER of £10,676, £7455 and £8498 per QALY gained for the addition of rituximab to a CHOP, MCP and CHVPi regimen, respectively. The two UK economic evaluations111–113 showed that the addition of rituximab to chemotherapy compared with chemotherapy alone has a cost per QALY gained under £20,000. In the US, Hornberger et al. 115 reported a cost per QALY gained of US$28,565 for the comparison between R-CVP and CVP.
A tabulated summary of key features and data sources for studies included in the review is presented in Table 27.
| Parameters | Ray et al.111 | Dundar et al.112,113 (including ERG report) | Hornberger et al.115 |
|---|---|---|---|
| Comparators |
R-CVP vs CVP R-CHOP vs CHOP R-MCP vs MCP R-CHVPi vs CHVPi |
R-CVP vs CVP | R-CVP vs CVP |
| Model structure | Markov model with three HSs: PFS; progressive disease; death | Markov model with three HSs: PFS; progressive disease; death | Markov model with three HSs: PFS; progressive disease; death |
| Age (years) | 53 | 53 | 50 |
| BSA at baseline | NR: M39021 trial95,96 | NR | 1.72 |
| Time horizon | Lifetime (not specified) | 10 years and 25 years | 30 years |
| Sources of effectiveness evidence (first-line induction) |
R-MCP vs MCP93 R-CHVPi vs CHVPi94 |
R-CVP vs CVP95,96 |
R-CVP vs CVP95,96 (only 40 months) Extrapolation based on observational studies |
| Sources of effectiveness evidence (second-line/progression) | Scotland and Newcastle Lymphoma Group116 | Scotland and Newcastle Lymphoma Group116 | Observational studies5,27,28,117 |
| Utilities |
PFS: 0.805 Disease progression: 0.618 |
NR |
PFS: 0.805 Disease progression: 0.618 |
| Base-case results (£/QALY gained) |
R-CVP vs CVP: £8613 R-CHOP vs CHOP: £10,676 R-MCP vs MCP: £7455 CHVPi vs CHVPi: £8498 |
R-CVP vs CVP: £8290 (MS: 25 years) ERG estimate: £9015 |
R-CVP vs CVP: US$28,565 |
A full description of each of the three cost-effectiveness studies along with a quality assessment checklist is presented below.
Critical appraisal of economic evaluation
The included cost-effectiveness studies;111–113,115 were assessed against a critical appraisal checklist adapted from the Drummond and Jefferson109 and Eddy110 checklists (Table 28).
| Critical appraisal items | Ray et al.111 | Dundar et al.112,113 (including ERG report) | Hornberger et al.115 | |
|---|---|---|---|---|
| Modelling assessments should include | ||||
| 1 | A statement of the problem | Yes | Yes | Yes |
| 2 | A discussion of the need for modelling vs alternative methodologies | Yes | Yes | Yes |
| 3 | A description of the relevant factors and outcomes | Yes | Yes | Yes |
| 4 | A description of the model including reasons for this type of model and a specification of the scope including; time frame, perspective, comparators and setting. (Note: n = no. of HSs within submodel) | Yes | Yes | Yes |
| 5 | A description of data sources (including subjective estimates), with a description of the strengths and weaknesses of each source, with reference to a specific classification or hierarchy of evidence |
Yes No reference to a hierarchy of evidence |
Yes No reference to a hierarchy of evidence |
Yes |
| 6 | A list of assumptions pertaining to: the structure of the model (e.g. factors included, relationships, and distributions) and the data | Yes | Yes | Yes |
| 7 | A list of parameter values that will be used for a base-case analysis, and a list of the ranges in those values that represent appropriate confidence limits and that will be used in a SA | Yes | Yes | Yes |
| 8 | The results derived from applying the model for the base case | Yes | Yes | Yes |
| 9 | The results of the SAs; unidimensional; best/worst case; multidimensional (Monte Carlo/parametric); threshold | Yes | Yes | Yes |
| 10 | A discussion of how the modelling assumptions might affect the results, indicating both the direction of the bias and the approximate magnitude of the effect | Yes | Yes | Yes |
| 11 | A description of the validation undertaken including:
|
Unclear |
Yes Model checked by the ERG |
Unclear |
| 12 | A description of the settings to which the results of the analysis can be applied and a list of factors that could limit the applicability of the results | Unclear | Yes | Yes |
| 13 | A description of research in progress that could yield new data that could alter the results of the analysis | Unclear | Unclear | Unclear |
Description and results of the published economic evaluations
Review of Ray et al. An evaluation of the cost-effectiveness of rituximab in combination with chemotherapy for the first-line treatment of FL in the UK
The aim of the study was to estimate the cost-effectiveness of the addition of rituximab to four chemotherapy regimens (CVP, CHOP, MCP, CHVPi) for patients with advanced FL in the UK. The model used a Markov approach and followed patients over a lifetime in three possible health states (HSs): PFS; progressive disease and death. The study adopted the perspective of the UK NHS and costs and QALYs were discounted at 3.5%. The mean age of patients entering the model was 53 years old. This study was commissioned by Roche and was available as a full paper.
The effectiveness in first-line induction was derived from four randomised Phase III clinical trials in patients with FL assessing the addition of rituximab to CVP,95,96 CHOP,91,92 MCP93 and CHVPi. 94 Publicly available data were used, i.e. from journal manuscripts, as the authors did not have access to individual patient-level data for those trials. Ray et al. 111 estimated the risk of progression by fitting a Weibull and exponential distributions to the data for the ‘chemotherapy’ arm only. The exponential distribution was selected for CVP, CHOP and MCP, whereas CHVPi was modelled using a Weibull distribution. The best fit was selected after analyses of the R2-value. Ray et al. 111 also calculated a HR for the addition of rituximab compared with chemotherapy alone derived from the PFS curves from the paper (through a calculation of the cumulative hazard by summing the negative log of the survival probabilities). These HRs were then applied to the estimated baseline curves to represent the risk of progression for patients receiving rituximab in addition to chemotherapy. The authors assumed that at the end of the PFS period, all patients progressed rather than dying. The rate of mortality while in PFS was assumed to be that reported in UK life tables. After relapse following first-line induction treatment, patients entered a ‘progressive’ HS (including subsequent relapses and lines of treatment) with patients remaining in this HS until death. The rate of progression from the ‘progressive’ HS to death was calculated using registry data from the Scotland and Newcastle Lymphoma Group (SNLG) assuming an exponential distribution. Deaths from other causes were included using the rates reported in UK life tables. Utility values were estimated using the European Quality of Life-5 Dimensions (EQ–5D) and were extracted from the Oxford Outcome Study,118,119 which was conducted in a cohort of 222 patients with FL in the UK. Patients in PFS were assumed to have a utility value of 0.805, whereas patients in the progressive HS had a utility value of 0.618. AEs were not included in the base-case analysis. However, a scenario analysis was conducted to estimate the impact of including additional costs associated with treating AEs and infusion site reactions on the cost-effectiveness of rituximab added to chemotherapy.
Drug costs were taken from the Monthly Index of Medical Specialities using the mean doses administered in the trials91–93,95,96 (except for CHVPi). Administration costs were taken from the NHS reference costs and transformed into a monthly cost (£309 per month for chemotherapy alone and £430 per month for R-chemotherapy). Drug costs for patients in the ‘progressive’ HS were derived from the published literature and assumptions (£195 per month). 83,120 The model also incorporated the cost of routine management for patients in PFS (one outpatient visit every 3 months) and in the progressive HS (one outpatient visit every month: £103). The cost of AEs was not included in the base case.
In the base-case analysis (Table 29), the addition of rituximab to CVP, CHOP, MCP and CHVPi led to a gain of 0.914, 0.831, 1.184 and 0.458 discounted QALYs, respectively, compared with chemotherapy alone. 111 The incremental discounted cost of the addition of rituximab to chemotherapy was estimated to be £7878, £8872, £8826 and £3892, respectively. The ICER associated with the addition of rituximab to CVP, CHOP, MCP and CHVPi compared with chemotherapy alone was estimated to be £8613, £10,676, £7455 and £8498 per QALY gained, respectively.
| Regimen | LY | QALY | Cost (£) | £/QALY gained |
|---|---|---|---|---|
| CVP vs R-CVP | ||||
| CVP | 6.710 | 4.748 | 20,708 | |
| R-CVP | 7.764 | 5.392 | 28,582 | 8613 |
| CHOP vs R-CHOP | ||||
| CHOP | 7.887 | 5.504 | 20,922 | |
| R-CHOP | 8.842 | 6.335 | 29,794 | 10,676 |
| MCP vs R-MCP | ||||
| MCP | 7.954 | 5.563 | 20,900 | |
| R-MCP | 9.312 | 6.747 | 29,725 | 7455 |
| CHVPi vs R-CHVPi | ||||
| CHVPi | 7.900 | 5.508 | 29,621 | |
| R-CHVPi | 8.428 | 5.966 | 33,513 | 8498 |
One-way SAs showed that the results were most sensitive to the time horizon and whether or not the treatment effect extended beyond the trial period. PSAs were also conducted. The uncertainty regarding the estimates of costs and QALYs were expressed using cost-effectiveness acceptability curves (CEACs) and cost-effectiveness frontiers. There was a high probability that the addition of rituximab to chemotherapy has a cost per QALY gained of < £20,000.
Ray et al. 111 also conducted incremental analysis comparing across chemotherapy regimens. The authors reported that MCP was cost-effective compared with CVP alone (£235 per QALY gained). CHOP, CHVPi and R-CVP were dominated by MCP, as those regimens provided lower QALYs at a higher cost. Similarly, R-CHOP and R-CHVPi were dominated by R-MCP. This analysis assumed that the treatment effect extended over a lifetime. Ray et al. 111 also presented an additional scenario by restricting the treatment effect of the addition of rituximab to 53 months. Overall, the authors found that MCP dominated R-CVP and CHOP. R-MCP dominated R-CHVPi and CHVPi. R-CHOP was extendedly dominated by R-MCP.
It was not possible for the AG to check the economic model as only the publication was available in the public domain. Based on the description of the model, this appears to be a reasonably well-conducted cost-effectiveness analysis. The generalisability of results from this study are, however, limited. The baseline age of the modelled cohort is not representative of patients with FL who are in first-line treatment in the UK (younger). Furthermore, the authors only explored the use of exponential or Weibull distributions to represent the rate of progression in patients treated in first-line induction. Alternative distributions might provide a better fit to the data. Similarly, the rate of progression in second line was modelled using an exponential distribution and no goodness-of-fit statistics were provided.
An important limitation is the source of effectiveness used for patients treated in first-line induction with CHOP, MCP and CHVPi, with or without rituximab. Responders to first-line induction with CHOP with or without rituximab were randomised to maintenance with interferon or SCT. 91,92 Responders to MCP with or without rituximab received maintenance interferon. 93 Similarly, the effectiveness for patients treated with CHVPi in first line with or without rituximab is confounded by the introduction of interferon during induction and the differences in treatment received post induction. 94 This is likely to overestimate the absolute gain in life-years (LYs)/QALYs associated with the addition of rituximab to chemotherapy. The model also did not consider that at the end of the PFS period, the outcome could be death rather than progression.
Some assumptions were also made by the authors and were not discussed. Patients were assumed to receive the same treatment post progression, irrespective of the choice of first-line treatment. Similarly, the source of effectiveness used to represent the rate of progression after relapse did not incorporate changes in the treatment pathways in the UK for relapsed patients (use of R-chemotherapy in combination with maintenance rituximab). It was also unclear from the study if patients were previously treated with rituximab or the type of chemotherapy received in first-line induction.
Finally, Ray et al. 111 conducted incremental analyses comparing across chemotherapy regimens. After discussion with clinical experts, the AG disagrees with this approach as the choice of chemotherapy is also based on patients’ characteristics and not solely the effectiveness of the chemotherapy (see Methods, for further discussion).
Review of Dundar et al. Rituximab for the first-line treatment of stage III–IV FL
Two studies were available: an HTA monograph113 and the ERG report. 112 Both studies are based on a critique undertaken by the ERG of the model submitted by the manufacturer (Roche) in TA110,83 a STA.
There is no published work with a first-hand description of the model. Our review is based on the ERG report112 for TA110,83 as this provided more detailed description on the economic evaluation submitted by the manufacturer. The submission made by the manufacturer was not publicly available.
The aim of the study was to evaluate the MS62 that estimated the cost-effectiveness of the addition of rituximab to CVP for first-line treatment of patients with advanced FL in the UK. The economic evaluation submitted by the manufacturer shared several features with the model published by Ray et al. 111 The model used a Markov approach and followed patients over 25 years in three possible HSs: PFS, progressive disease and death. The study also adopted the perspective of the UK NHS, with costs and QALYs discounted at 3.5%. The mean age of patients entering the model was 53 years.
The effectiveness in first-line induction was derived from a randomised Phase III clinical trial in patients with FL assessing the addition of rituximab to CVP. 95,96 Log-logistic distributions were fitted to individual patient-level data from the trial to represent the risk of progression after first-line induction treatment. AEs were omitted. After relapse following first-line induction, patients entered a ‘progressive’ HS (which included subsequent relapses and lines of treatment), with patients remaining in this HS until death. The rate of progression from the ‘progressive’ HS to death was calculated using registry data from the SNLG assuming an exponential distribution. Deaths from other causes were included using UK life tables. Utility values were estimated using the EQ-5D and were extracted from the Oxford Outcome Study. 117,118 The utility values used for the PFS and ‘progressive’ HS were marked as commercial in confidence.
Patients were assumed to receive eight cycles of treatments (assigned to the first cycle in the model). The surveillance costs in PFS were calculated to be £32.33 per month assuming four annual oncology visits. 112 Drug costs for patients in the progressive HS were derived from the published literature and assumptions and were assumed to be £193.33 per month. 120
In the base-case analysis, the addition of rituximab to CVP led to a gain of 1.251 discounted QALYs compared with chemotherapy alone. The incremental discounted cost of the addition of rituximab to chemotherapy alone was estimated to be £10,370. The ICER associated with the addition of rituximab to CVP compared with chemotherapy alone was estimated to be £8290 per QALY gained. One-way SAs showed that the results were most sensitive to the time horizon and treatment length and whether or not the treatment effect extended beyond the trial. PSAs were conducted and indicated that at a threshold of £30,000 per QALY gained, there was 100% probability that R-CVP was cost-effective compared with CVP. The ERG corrected errors identified in the MS62 and made some modifications to the economic model (translation of gain in PFS into OS and use of a Weibull distribution to represent the risk of progression in the ‘progressive’ HS. The ICER estimated by the ERG was £9015 per QALY gained (with 64% of PFS translating into OS). If no OS gain was assumed, the ICER increased to £20,593 per QALY gained.
As the report112 is based on a previous review of the economic model submitted by the manufacturer, the AG did not perform an independent assessment of this economic evaluation owing to resource constraints and the availability of a previous critic of the model (i.e. the ERG assessment). The ERG identified mistakes/inconsistencies after reviewing the economic model. More details are available in the ERG report. 111 In addition to the errors, the ERG highlighted some limitations in the manufacturer’s model:
-
The manufacturer assumed that most of the gain in PFS translated into a gain in OS (79% according to the ERG).
-
The baseline age was not representative of the patients in the UK receiving first-line therapy.
-
Utility values used – the manufacturer did not age adjust utility values, and utilities were calculated from a small sample size (especially for the ‘progressive’ HS).
-
The progression rate for patients in the ‘progressive’ HS. The ERG indicated that the exponential distribution selected by the manufacturer did not provide a good fit to the data and that a Weibull distribution would provide a more reasonable fit. Furthermore, the ERG questioned data from the SNLG in the absence of details about the characteristics of included patients.
-
The cost in the ‘progressive’ HS included the cost of first-line therapy, and therefore inflated the cost for patients remaining longer in the ‘progressive’ HS.
Review of Hornberger et al. Economic evaluation of rituximab and CVP for advanced FL
The aim of the study was to assess the cost-effectiveness of R-CVP compared with CVP in the USA. The economic evaluation shared several features with the model assessed by the ERG in TA11083,112,113 and Ray et al. 111 The model used a Markov approach and followed patients over 30 years in three possible HSs: PFS, progressive disease and death. The study adopted a societal perspective with costs and QALYs discounted at 3.0%. The mean age of patients entering the model was 50 years.
The effectiveness in first-line induction was derived from a randomised Phase III clinical trial in patients with FL assessing the addition of rituximab to CVP. 95,96 The PFS and OS Kaplan–Meier from the M39021 trial95,96 was used for the first 4 years and extrapolated beyond the trial based on published findings of a long-term observational study. 5,28,29,117 An annual mortality rate of 6.9% was applied.
The utility values for the time spent in each HS was extracted from the Oxford Outcome Study. 118,119 The utility values for patients in progression-free and progression HS were 0.805 and 0.618, respectively. The economic model also incorporated the disutility associated with chemotherapy (–0.15), SCT (–0.20) and end of life (–0.30). 121 There is no indication on how long the disutility was assumed to be.
Unit drug costs were derived from Medicare J-codes using the Mosby 2006 drug costs. The model assumed a BSA of 1.72 m2 and drug wastage was considered. Administration costs were derived from the number of hours of infusions and the cost per hour of administration from the current procedural terminology. 122 The models incorporated grade 3 and 4 AEs that had at least a 2% rate difference between the two arms. The cost of subsequent treatment regimens was derived from the cost of most common regimens recommended by the National Comprehensive Cancer Network. Maintenance after second-line induction for responders to chemotherapy was considered in the analysis.
Subsequent treatments had no impact on OS and were included only for costing purpose. Subsequent treatments were applied at the median TTP and 1 year thereafter. Salvage therapy was also included and it was assumed that 10% of patients undergo SCT as part of subsequent therapy. Finally, the economic evaluation included the cost of end of life. 123
In the base-case analysis, the addition of rituximab to CVP led to a gain of 0.93 discounted QALYs compared with chemotherapy alone. The incremental discounted cost of the addition of rituximab to chemotherapy alone was estimated to be US$26,439. The ICER associated with the addition of rituximab to CVP compared with chemotherapy alone was estimated to be US$28,565 per QALY gained.
One-way SAs showed that the results were most sensitive to utility values and the cost for a course of rituximab. Hornberger et al. 115 reported that none of the SAs generated a cost per QALY gained of > US$50,000 per QALY gained.
It was not possible for the AG to check the economic model as only the publication was available in the public domain. Based on the description of the model, this appears to be a reasonably well-conducted cost-effectiveness analysis. 115 The generalisability of results from this study, however, may be limited as the study was conducted in the USA. Furthermore, the baseline age of the modelled cohort (50 years) was not representative of patients with FL in who were in first-line treatment in the UK. Hornberger et al. 115 provided a very detailed description of the derivation of costs. However, the description of clinical effectiveness was poor. It is unclear how the PFS and OS Kaplan–Meier were extrapolated after 4 years.
Assessment of the manufacturer’s submission
There was one industry submission to NICE from Roche. 62 The MS62 included a full report and an electronic model submitted in Microsoft Excel© version 12 (Microsoft Corporation, Redmond, WA, USA). The economic model submitted by the manufacturer was reviewed to check that the parameters presented in the report corresponded to those used in the economic model. The economic model included in the MS62 was assessed using a critical appraisal checklist adapted from the Drummond and Jefferson109 and Eddy110 checklists (Table 30).
| Checklist | MS61 | |
|---|---|---|
| Modelling assessments should include | ||
| 1 | A statement of the problem | Yes |
| 2 | A discussion of the need for modelling vs alternative methodologies | Yes |
| 3 | A description of the relevant factors and outcomes | Yes |
| 4 | A description of the model including reasons for this type of model and a specification of the scope including; time frame, perspective, comparators and setting. Note: n = no. of HSs within submodel | Yes |
| 5 | A description of data sources (including subjective estimates), with a description of the strengths and weaknesses of each source, with reference to a specific classification or hierarchy of evidence | Yes |
| 6 | A list of assumptions pertaining to: the structure of the model (e.g. factors included, relationships and distributions) and the data | Yes |
| 7 | A list of parameter values that will be used for a base-case analysis, and a list of the ranges in those values that represent appropriate confidence limits and that will be used in a sensitivity analysis | Yes |
| 8 | The results derived from applying the model for the base case | Yes |
| 9 | The results of the SAs; unidimensional; best/worst case; multidimensional (Monte Carlo/parametric); threshold | Yes |
| 10 | A discussion of how the modelling assumptions might affect the results, indicating both the direction of the bias and the approximate magnitude of the effect | Yes |
| 11 | A description of the validation undertaken including:
|
Unclear |
| 12 | A description of the settings to which the results of the analysis can be applied and a list of factors that could limit the applicability of the results | Unclear |
| 13 | A description of research in progress that could yield new data that could alter the results of the analysis | Unclear |
Description of the manufacturer’s submission
Overview
The MS62 used a state-transition model with individuals moving between four possible HSs: PFS/first-line induction treatment (PFS1); PFS/second-line treatment (PFS2); progressive disease; and death (Figure 6). The model compared the cost-effectiveness of the addition of rituximab to CVP, CHOP, MCP and CHVPi for patients with advanced FL in the UK. The starting age in the model was 60 years and patients were followed up for 25 years. The study adopted the perspective of the UK NHS, with costs and QALYs discounted at 3.5%. A tabulated summary of key features and data sources of the economic model included in the MS62 is presented in Table 31.
| Parameters | MS61 |
|---|---|
| Comparators |
R-CVP vs CVP R-CHOP vs CHOP R-MCP vs MCP R-CHVPi vs CHVPi |
| Model structure | State transition approach with four HSs: PFS1, PFS2, progressive disease, death |
| Age, BSA at baseline | Age 60 years; BSA 1.8528 m2 |
| Time horizon | 25 years |
| Sources of effectiveness evidence (first-line induction) |
R-MCP vs MCP93 R-CHVPi vs CHVPi94 Parametric extrapolation (log-logistic, Weibull, exponential) |
| Sources of effectiveness evidence (second line/progression) |
EORTC 20981 trial;74,75 inclusion of second-line maintenance Parametric extrapolation (exponential) |
| Utilities |
PFS1: 0.88 PFS2: 0.79 Progressive disease: 0.62 |
| Base-case results (£/QALY gained) |
R-CVP vs CVP: £1529–5611 R-CHOP vs CHOP: £5758 R-MCP vs MCP: £4861 R-CHVPi vs CHVPi: £9251 |
Summary of effectiveness data
The effectiveness in first-line induction treatment was derived from four randomised Phase III clinical trials in patients with FL comparing the addition of rituximab to CVP,95,96 CHOP,91,92 MCP93 and CHVPi. 94 Individual patient-level data from the M39021 trial from journal manuscripts95,96 and the MS62 were used to estimate the rate of progression among patients treated with CVP or R-CVP in first-line induction assuming a log-logistic distribution. Individual patient-level data for the trials that compared CHOP with R-CHOP, MCP with R-MCP and CHVPi with R-CHVPi91–94 were not available to the manufacturer and therefore only publicly available data were used. A similar methodology to Ray et al. 111 was used by fitting a Weibull or exponential distribution (to the digitised data from the papers) to patients treated in first line with chemotherapy alone. The exponential distribution was selected for CHOP and MCP, whereas the Weibull distribution was chosen for CHVPi based on the R2-value. A HR was then applied to the estimated curves for the first 53 months to estimate the reduction in the risk of progression for patients receiving rituximab in addition to chemotherapy. Deaths in PFS1 were derived from the number of deaths and follow-up duration from the M39021 trial. 95,96
The effectiveness in PFS2/second-line treatment was based on data from the EORTC 20981 trial74,75 conducted among patients treated with CHOP or R-CHOP with or without maintenance rituximab in second line. Digitised data from the paper74,75 were used in the absence of individual patient-level data. The manufacturer used exponential distributions to estimate the risk of progression. The manufacturer stated that:
In order to avoid overcomplicating the model, the transition probabilities of progressing from PFS2 were not varied over time. Varying the probabilities over time would require tracking patients’ progression within the model and would result in an exponential increase of the size and complexity of the model with limited impact to the cost effectiveness of rituximab in first-line.
The most up-to-date data from the EORTC 20981 trial74 were used to estimate the progression rate from PFS2 to the progressive HS, and from the progressive HS to death [post-progression survival (PPS)]. The PPS have been calculated as a function of PFS and OS, assuming that the rate of progression in PPS equalled the sum of the rate of progression in OS and PFS. The manufacturer also attempted to apply a rule so that patients treated with rituximab in first-line induction and who relapse within 6–12 months would not receive rituximab in second-line induction.
Utilities were extracted from a study commissioned by the manufacturer (Oxford Outcomes Study). 118,119 The following utility values were used in the economic model; PFS1 = 0.88 (disease free); PFS2 = 0.79 (remission/full response); progressive disease = 0.62. AEs were not included in the MS. 62
Summary of resource utilisation and cost data
Drug costs were taken from the British National Formulary (BNF),86 using the planned dose from the trials. Administration costs were taken from NHS reference costs. The manufacturer also assumed that rituximab treatment was administered as a hospital day case.
The cost associated with monitoring/surveillance after induction treatment was derived from a study commissioned by the manufacturer. Supportive care costs for patients in the progressive HS (£500.53 per month) were derived from the cost used in the MS62 for an ongoing NICE appraisal124 from the post-protocol treatment from the EORTC 20891 trial74,75 and the cost of palliative care in the UK. 125
Summary of cost-effectiveness
Two analyses were presented for R-CVP compared with CVP. The first analysis fitted separate curves to each arm using individual patient-level data, whereas the second analysis assumed a HR (for R-CVP) for the first 53 months and fitted a parametric curve to CVP using the same approach as for CHOP, MCP and CHVPi.
In the base-case analysis, the addition of rituximab to CVP, CHOP, MCP and CHVPi led to a gain of 0.867/0.443, 1.096, 1.289 and 0.675 discounted QALYs compared with chemotherapy alone, respectively. The incremental discounted cost of the addition of rituximab to chemotherapy was estimated to be £1325/£2486, £6312, £6268 and £6247, respectively. Thus, the addition of rituximab to CVP, CHOP, MCP and CHVPi compared with chemotherapy alone resulted in an ICER of £1529/£5611, £5758, £4861 and £9251 per QALY gained, respectively (Table 32).
| Regimen | LYs | QALY | Cost (£) | £/QALY gained |
|---|---|---|---|---|
| CVP vs R-CVP | ||||
| CVP | 7.618 | 5.828 | 43,061 | |
| R-CVP | 8.386 | 6.695 | 44,386 | 1529 a |
| CVP | 7.342 | 5.544 | 44,570 | |
| R-CVP | 7.668 | 5.987 | 47,056 | 5611 b |
| CHOP vs R-CHOP | ||||
| CHOP | 8.279 | 6.479 | 42,717 | |
| R-CHOP | 9.407 | 7.575 | 49,029 | 5758 |
| MCP vs R-MCP | ||||
| MCP | 8.332 | 6.532 | 42,072 | |
| R-MCP | 9.671 | 7.821 | 48,340 | 4861 |
| CHVPi vs R-CHVPi | ||||
| CHVPi | 8.297 | 6.487 | 47,885 | |
| R-CHVPi | 9.039 | 7.162 | 54,132 | 9251 |
One-way SAs showed that the results were robust to parameter changes with none of the SAs increasing the ICER above £20,000 per QALY gained. PSAs were conducted. The PSA results indicated that the addition of rituximab to chemotherapy compared with chemotherapy alone was highly cost-effective assuming a willingness-to-pay (WTP) threshold of £20,000 per QALY gained. No incremental analysis was presented to compare across chemotherapy regimens.
Critique of the manufacturer’s submission
The AG reviewed the economic model and report included in the MS. 62 A detailed critique is presented below. In summary, there are concerns with the MS62 analyses. 62 Errors and inconsistencies were identified in the economic model. The model had also limitations relating to the source of effectiveness for patients treated in first or second line. For readability, we critique each section in turn.
Review of previous analyses and quality-of-life data
No economic review or quality-of-life reviews were included in the MS. 62
Sources of effectiveness for CHOP, MCP and CHVPi with or without rituximab
The trials used91–94 were likely to overestimate the effect of rituximab given that responders to first-line induction treatment received subsequent treatments with interferon maintenance or SCT (see Chapter 3, Summary of trials). This issue was not discussed in the MS. 62
Method used to estimate the rate of progression in the absence of patient-level data
Owing to the lack of individual patient-level data, the manufacturer assumed that the TTP was represented by either a Weibull or an exponential distribution, with these distributions estimated using ordinary least squares regression method. This approach is commonly used in health economic models when only data from manuscripts are available. However, it appears that there a number of errors and inconsistencies in the process used by the manufacturer to estimate the exponential distribution. By definition, the exponential distribution is composed of only one parameter (λ), as the rate is constant and does not vary with time. However, the manufacturer fitted a linear regression model (y = α × t + λ) to the transformed data (log scale) that contained two parameters: λ (constant) and α (variable time dependent). In some parts of the economic model, the rate of progression was calculated using λ only or the sum of λ and α. The inconsistency in the approach used limits the interpretation of the estimated coefficients used throughout the economic model. Furthermore, this approach is not correct, as this sometimes includes or excludes a time-dependent variable. The linear model has to be of the following form in order to estimate the parameter of the exponential distribution: y = α × t.
Inconsistencies and errors were identified between the risk of progression presented in the report and the risk of progression used in the economic model, notably in second line. In most cases, the fitted exponential distribution (using an ordinary least squares methodology) was not found to provide a reasonable fit to the data. Therefore, it appears that the manufacturer adjusted the parameters of the exponential distribution ‘manually’ by adding extra parameters in order to provide a reasonable visual fit to the data. This was not discussed by the manufacturer in the report and was identified by the AG only after review of the economic model. In some instances, the unadjusted coefficients were used (instead of the coefficient artificially adjusted to fit the data) in the economic model. For example, considering the PFS for patients treated with R-CHOP as induction in second line and maintenance with rituximab (Figure 7). The curve presented by the manufacturer in the report (grey line) was estimated after the addition of extra parameters (manual adjustment). However, in the economic model the dashed grey curve (before adjustment) was used (estimated by the AG), which provided a poorer fit.
FIGURE 7.
Kaplan–Meier plot and exponential distributions presented in the MS62 and used in the economic model for patients that respond to R-CHOP second-line induction treatment and receive maintenance rituximab. KM, Kaplan–Meier.
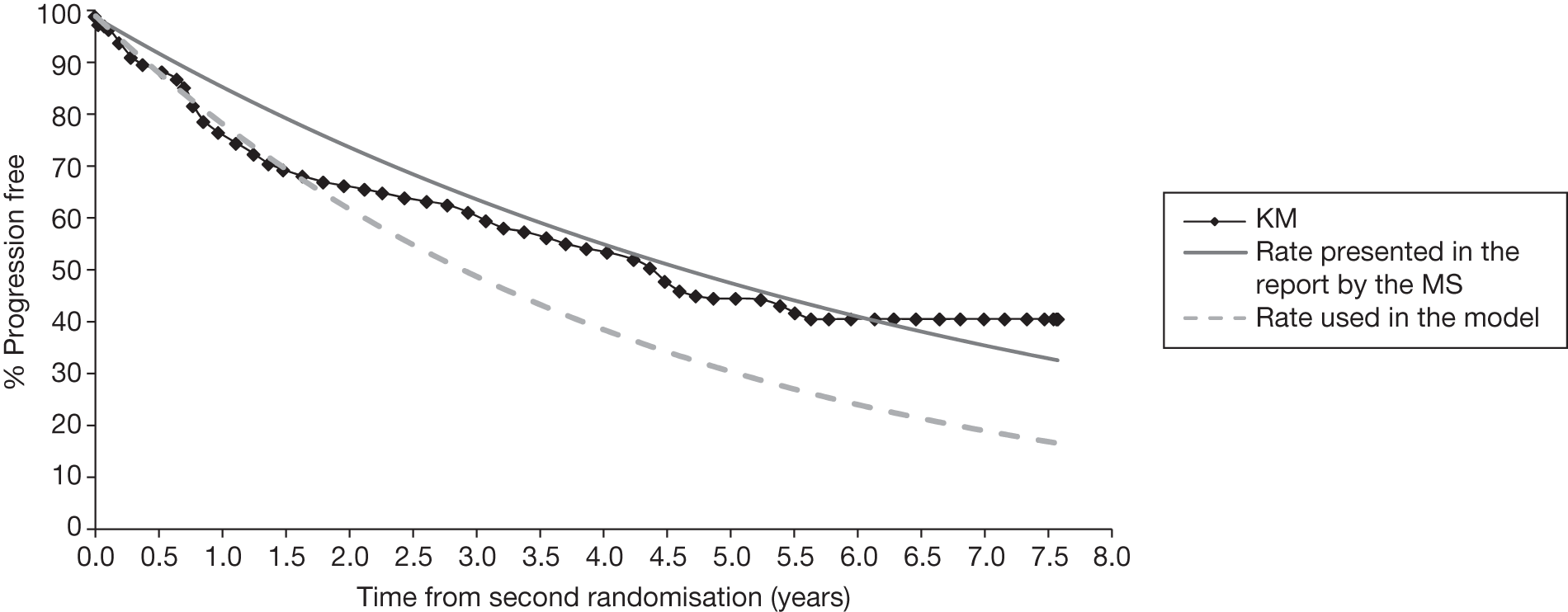
Approach used to estimate the hazard ratio
The HRs were calculated by taking the cumulative hazard (estimated by the sum of the negative log of the survival) from the PFS Kaplan–Meier curve estimated from the appropriate trials. The AG acknowledges that the approach was necessary in the absence of individual patient-level data. However, the AG note that such an approach might introduce bias, as the calculated cumulative hazard is dependent on the number of point estimates considered. A better approach would be to estimate the HR from the baseline parametric survival curve.
Duration of benefits
The manufacturer assumed that the treatment effect (HR) lasts 53 months based on the median follow-up duration in the M39021 trial. 95,96 However, the follow-up was different in other trials used. 91–94
Rule for patients previously treated with rituximab
The manufacturer wished to apply a rule whereby patients that relapsed within 6–12 months after first-line induction treatment with rituximab would not be eligible for rituximab in second-line treatment if previously exposed to rituximab. However, the decision of the manufacturer to simplify the economic model structure meant that several assumptions had to be made as the model was not able to track patients over time.
Treatment pathway
The manufacturer assumed that patients can receive only CHOP or R-CHOP in second-line treatment (followed, or not, by maintenance rituximab). Discussion with clinical experts indicated that CHOP-containing regimens are aggressive and therefore mainly used in younger patients. Older patients are likely to receive less aggressive chemotherapy regimens, such as FC with or without rituximab. Furthermore, clinical experts indicated that anthracycline-containing regimens (CHOP, MCP, CHVP) should only be used once in a lifetime and therefore patients previously treated with anthracycline regimens are likely to receive SCT in second line if they are fit enough or less aggressive chemotherapies (FC) if they are not considered to be sufficiently fit.
Source of effectiveness for patients treated in second line
The manufacturer used data from the EORTC 20981 trial74,75 to estimate the risk of progression for patients treated with CHOP or R-CHOP in second line with or without maintenance. However, patients in this study were rituximab naive, i.e. not previously treated with rituximab. The applicability of outcomes from this study to patients previously treated with rituximab is unclear. Furthermore, because data from second randomisation (i.e. after response induction) were used, the time spent in second-line induction (where the risk is zero for responders) was missing from calculations of PFS and OS. Furthermore, outcomes for non-responders were missed.
Estimation of post-progression survival
The manufacturer estimated PPS as a function of PFS and OS from the EORTC 20981 trial. 74,75 The AG, however, has some concerns about the approach used by the manufacturer. The manufacturer calculated PPS as the additive risk of OS and PFS (using the coefficients of the exponential distribution) so that PPS = OS + PFS. It is unclear why the addition of the coefficient of PFS and OS would be equal to the coefficient of PPS. Furthermore, the manufacturer used direct coefficients of the exponential distribution to estimate PPS before their ‘manual adjustment’ (curves are artificially modified to fit the data). This means that the curves for OS and PFS used to calculate PPS no longer fitted the data (Figures 8 and 9). Finally, the manufacturer used the combined data for patients randomised to observation or maintenance, therefore implying that the PPS would be the same following CHOP or R-CHOP induction.
FIGURE 8.
Kaplan–Meier plot and exponential distribution used to calculate PPS reported in the MS62 and actually used in the economic model for patients receiving observation after response to second-line induction treatment. Exp, exponential distribution; KM, Kaplan–Meier.
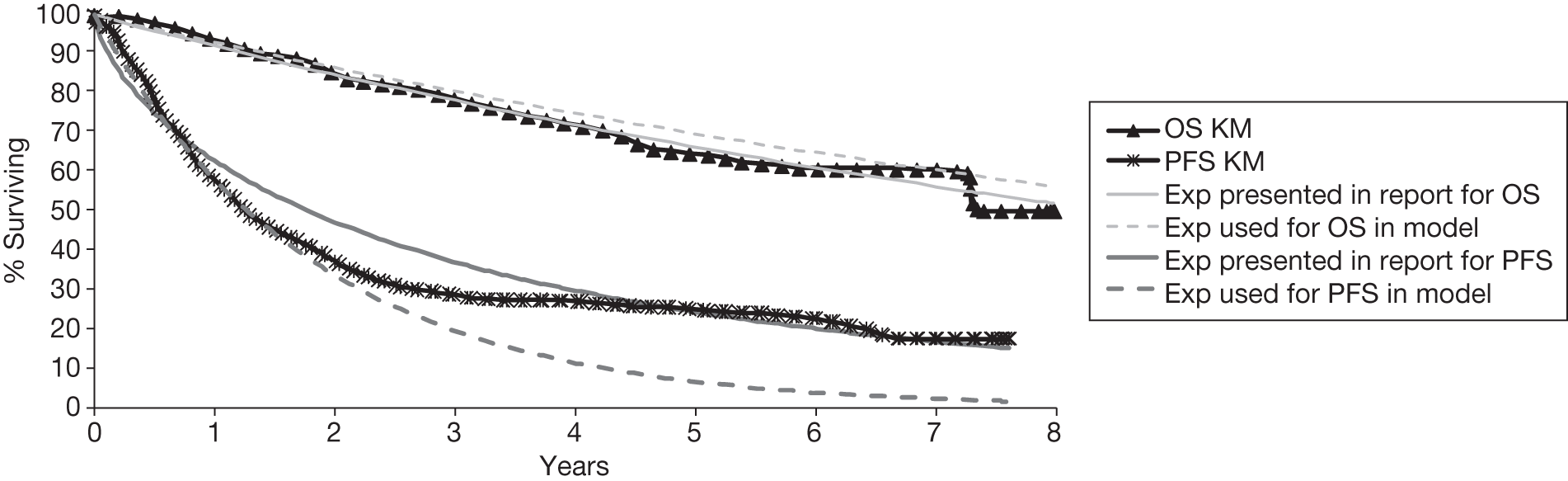
FIGURE 9.
Kaplan–Meier plot and exponential distribution used to calculate PPS reported in the MS62 and actually used in the economic model for patients receiving maintenance rituximab after response to second-line induction treatment. Exp, exponential distribution; KM, Kaplan–Meier. Note: The curve for OS used in the model is superimposed on the curve for OS presented in the report as this was derived correctly by the MS. 62
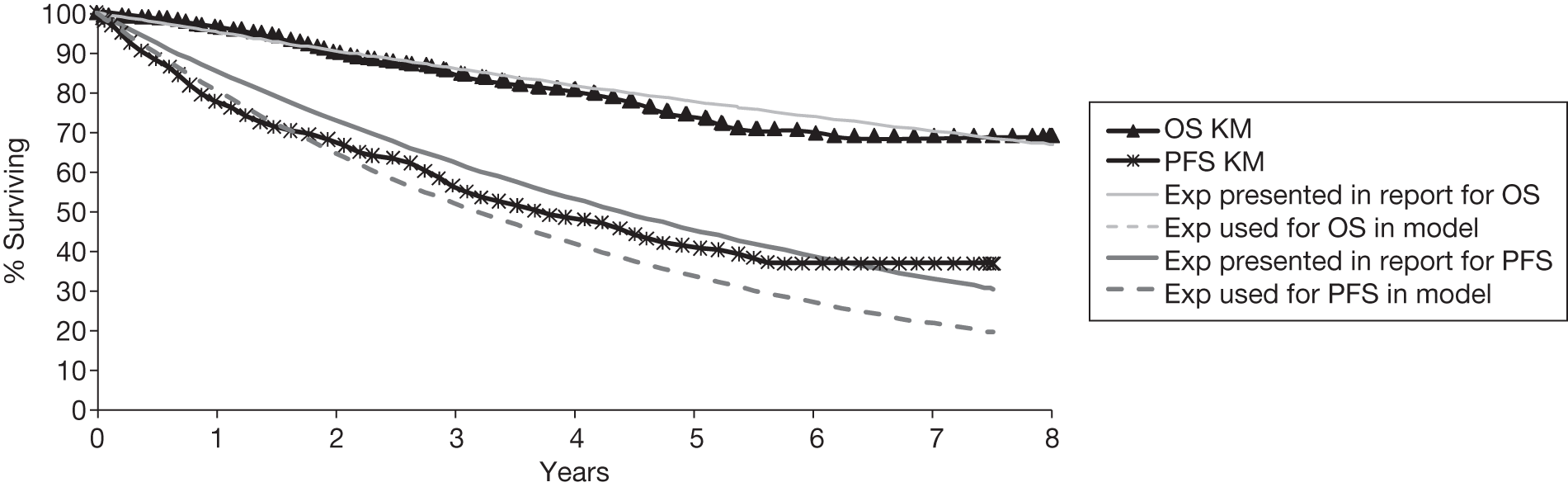
Model structure
Although Markov models are commonly used for oncology treatments, the Markov approach requires assumptions and can be inflexible. The manufacturer used exponential distributions to ‘avoid over-complicating’ the model. However, in most cases, the exponential distribution did not fit the data well.
Adverse events
The MS62 did not include the impact of AEs either in terms of costs or impact on quality of life, stating that there is no clinically significant difference between the rates and/or severity of AEs observed in the rituximab arms of each of the four first-line clinical trials91–96 when compared with the respective comparator arms. However, the clinical effectiveness review indicated that a greater number of blood and bone marrow AEs occurred in the R-chemotherapy arm than in the chemotherapy-alone arm, for example neutropenia, leucocytopenia. Despite these AEs not resulting in a difference in infection rates and thus being clinically significant, they would still incur costs to treat and their exclusion might bias the cost-effectiveness in favour of rituximab.
Treatment/management costs
Several errors/inconsistencies were identified by the AG after review of the economic model. First, the planned number of cycles in the EORTC 20981 trial74,75 (used to represent second-line treatment) is six cycles of CHOP or R-CHOP. Assuming a cost per cycle of £1462 for R-CHOP (estimated by the manufacturer), the maximum cost that a patient can incur is £8772 (£1462 × 6). In the economic model, the cost for patients treated with R-CHOP (accounting for the fact that some patients receive less than the planned dose owing to progression) was estimated to be £11,305 by the MS. 62 This is because of an error in the translation between month and cycle. The same error was found for the calculation of the cost of administration in second line.
The MS62 also used a complicated formula to estimate the cost associated with maintenance based on the area under the curve from the most up-to-date EORTC 20981 trial data. 74 The cost was then applied to the first cycle in the economic model. The AG had some difficulty in following the logic; however, we believe that costs were discounted twice.
Inconsistencies were also identified in the approach used to estimate the management costs in the ‘progressive’ HS. The manufacturer calculated a cost per month including the cost associated with the post-protocol treatment from the EORTC 20981 trial74,75 and the cost of palliative care. 125 This had the effect of inflating the cost for patients who spend a longer time in the ‘progressive’ HS and bias the cost-effectiveness in favour of rituximab.
The manufacturer also assumed no drug wastage. This might not be true if chemotherapies are not given in a large centre and vial sharing is not possible.
Utilities
The economic model included in the MS62 used utility values from the Oxford Outcomes Study. 118,119 The manufacturer assumed that the utility in PFS1 was similar to the utility of patients considered to be disease free (0.88, 95% CI 0.81 to 0.95). The utility for patients in remission/full response to therapy (0.79, 95% CI 0.72 to 0.86) was used to represent the utility for patients in PFS2. Finally, the utility for progressive disease was assumed to be 0.62 (95% CI 0.48 to 0.76). As suggested by the ERG in the ongoing appraisal for first-line maintenance,126,127 it seems inappropriate to assume that patients in PFS1 and PFS2 have different utility values given that these patients are in remission. This choice by the manufacturer to use the utility for patients considered to be ‘disease free’ to represent the utility in patients in PFS1 also appears to be inappropriate as these patients are in a ‘remission’ state and not ‘disease free’.
Other assumptions
The MS62 assumed that there were no resistance effects among patients previously treated with rituximab implying that the efficacy would be equal regardless of previous treatment. The MS62 referred to two studies to support the assumption of the absence of a resistance effect to rituximab. 128,129 However, the AG does not believe that the data from these two studies provide conclusive evidence that the resistance of rituximab is not a consideration. Further studies identified by the AG in other types of lymphoma130–132 suggest that there might be a resistance effect to rituximab.
Relevance of cost-effectiveness evidence for NICE decision-making
Three modelling studies (corresponding to four references)62,111–113 are potentially relevant for UK decision making. However, there are number of issues in the economic models identified that require further considerations (see Systematic review of existing cost-effectiveness evidence and Assessment of the manufacturer’s submission). These include:
-
The baseline age of the modelled cohort. The baseline age was not representative of the age of patients receiving first-line treatment in the UK.
-
The sources of effectiveness for patients treated with CHOP, MCP and CHVPi in first-line induction treatment with or without rituximab. The effectiveness values were derived from trials where patients have received subsequent treatment, such as interferon maintenance or SCT. Further details are available in Chapter 3 (see Summary of trials).
-
The source of effectiveness in patients receiving second-line treatment induction with or without maintenance rituximab. The effectiveness values were derived from patients not previously treated with rituximab. Additionally, in the MS,62 the time period when patients receive second-line induction treatment and outcomes for non-responders were not captured.
-
The choice of utility values. There was a mismatch between the utility values used and the HSs.
-
Costs for patients treated in second-line or in progressive disease; errors/inconsistencies were identified in the model in the MS. 62
-
Constraints imposed by the chosen model structure. The identified models used a Markov approach that required strong assumptions about timing and progression rate. For example, the manufacturer fitted exponential distributions in patients treated in second line and these did not fit the data.
-
Incorporation of death from non-FL causes.
Independent economic assessment
Methods
Introduction
The review of published economic evaluations111–113,115 were used. The main limitations identified were the description of the treatment pathway, the sources of effectiveness and assumptions that were made.
Previous guidance by NICE (TA110) was issued for the use of rituximab in combination with CVP for the first-line induction treatment of FL. 83 Since this guidance was produced, the licence of rituximab was extended for use in combination with any chemotherapy-containing regimen. 85 In 2008, NICE issued guidance recommending the use of rituximab in combination with chemotherapy in second-line induction treatment and for rituximab monotherapy as maintenance treatment in patients responding to second-line induction chemotherapy with or without rituximab. 72 At the time of writing, NICE is currently considering the use of rituximab monotherapy for first-line maintenance treatment of patients responding to first-line induction treatment with rituximab in addition to chemotherapy. 127 The final guidance is expected to be issued after delivery of this assessment report. A summary of previous guidance issued by NICE is presented in Chapter 1 (see Current service provision and Table 4).
This section describes the development of a de novo economic model addressing the main limitations identified in existing economic evaluations. 62,111–113,115 The key objective of the economic assessment is to address the cost-effectiveness of the addition of rituximab to chemotherapy in previously untreated, stage III/IV, patients with FL in England and Wales in line with changes in the licensing of rituximab85 and previous guidance issued by NICE. 73,82
Population appraised
The population under assessment is previously untreated, symptomatic, stage III–IV patients with FL in England and Wales.
Interventions/comparators
A probabilistic decision analytic model was developed to estimate the costs and QALYs of the addition of rituximab to three chemotherapy regimens: CVP, CHOP and MCP. The choice of chemotherapies was primarily based on available data91–96 and the robustness of the evidence in order to address the NICE scope defined for this appraisal. 133
No comparison was provided for the addition of rituximab to a CHVP regimen with interferon. This was because of issues in the design of the FL2000 trial,94 which compares the addition of rituximab with a CHVP regimen with interferon. There were differences in the interventions in the FL2000 trial. 94 The control/comparator group received 12 courses of a CHVP regimen administered every 28 days for six courses and then every 56 days for an additional six courses combined with 18 months of interferon, whereas the active treatment group received only six courses of a CHVP regimen administered every 28 days in addition to rituximab, with interferon delivered for 18 months. Clinical opinion suggests that CHVP regimens are very rarely used in the UK, and that interferon might not be used because of toxicity.
Description of the de novo economic model
The main source of effectiveness data were obtained from the three main trials conducted in first-line induction treatment which compared CVP against R-CVP,95,96 CHOP against R-CHOP91,92 and MCP against R-MCP. 93
The economic model was programmed using R software® (The R Foundation for Statistical Computing, Vienna, Austria), version 2.11.1, and uses a 25-year time horizon in the base case to capture costs and benefits as in the MS. 62 Shorter horizons (5 years, 10 years) and a lifetime horizon are presented in SAs. In accordance with the NICE guide for the methods of technology appraisal,97 the economic model adopts the perspective of the UK NHS and PSS with costs and benefits discounted at an annual rate of 3.5%.
Treatment pathway and clinical practice in the UK
The modelled treatment pathway incorporates guidance issued by NICE73,83 for the treatment of patients with FL in England and Wales and tries to replicate the treatment pathway observed in clinical practice. Owing to the possibility that first-line maintenance rituximab could be recommended by NICE, an alternative scenario including this option has been included.
Clinical opinion was sought and two clinicians completed a short questionnaire via a telephone interview. A summary of the answers is presented in Table 33. Overall, clinical opinion suggested that:
| Response status and time of relapse | Age (years) | First-line therapy | |||
|---|---|---|---|---|---|
| CVP | R-CVP | CHOP | R-CHOP | ||
| Relapse within 6 months after start of therapy (non-responders) | < 65 | R-CHOP | R-CHOP | R-HDT (± ASCT) | HDT (± ASCT) |
| ≥ 65 | R-FC | R-FC | R-FC | FC | |
| Responders at 6 months, but relapse within 6 months after end of therapy | < 65 | R-CHOP | R-CHOP | R-HDT (± ASCT) | HDT (± ASCT) |
| ≥ 65 | R-FC | R-FC | R-FC | FC | |
| Responders at 6 months, but relapse > 6 months after end of therapy | < 65 | R-CHOP | R-CHOP | R-HDT (± ASCT) | R-HDT (± ASCT) |
| ≥ 65 | R-FC | R-FC | R-FC | R-FC | |
-
In clinical practice, patients relapsing within 6–12 months after rituximab in combination with chemotherapy are not likely to be retreated with rituximab as recommended by the ESMO guideline. 19 An exception was for patients previously treated with R-CVP.
-
Anthracycline-containing regimens (CHOP, MCP) can be given only once in a lifetime. Thus, in second-line treatment, patients previously treated with an anthracycline-containing regimen will be considered for alternative treatments with salvage therapy [high-dose chemotherapy (HDT)] with or without rituximab in addition to ASCT, if aged < 65 years and are fit enough. Older or unfit patients are likely to receive less aggressive chemotherapies with or without rituximab, such as FC (note: this reflects the view of the clinical experts consulted by the AG). Rituximab may not to be given in second line as part of the salvage treatment for those patients previously treated with rituximab that relapse within 6–12 months after first-line induction treatment.
-
Patients who are not in complete or partial remission at the end of first-line induction treatment (i.e. stable disease) with chemotherapy with or without rituximab are likely to be offered second-line treatment despite the absence of progression.
Clinicians were only asked to define the treatment pathway in patients treated with CVP- or CHOP-containing regimens with or without rituximab. The pathway for MCP and R-MCP were assumed to be identical to CHOP and R-CHOP on the rationale that both were anthracycline regimens. The AG stresses that the treatment pathway defined in Table 33 is a simplification of treatment options given in second line and acknowledges that the treatment decisions taken includes other parameters, such as the presence of comorbidities and patient’s preferences.
The treatment pathways used in the economic model are presented in Figures 10–13, based on our discussions with clinical experts (see Table 33) and previous guidance issued by NICE. 72,82 Within the model, an age cut-off of 65 years was selected to classify eligibility for treatment in second line; however, the AG acknowledges that in clinical practice, patient age would not be the sole criteria as older patients who were fit enough may be eligible for SCT. Non-responders that did not progress at the end of treatment induction, were assumed to receive second-line treatment at the end of first-line induction treatment. Furthermore, we considered early relapse as relapse within 12 months after the start of treatment.
FIGURE 10.
Treatment pathways modelled in the economic model for CVP.
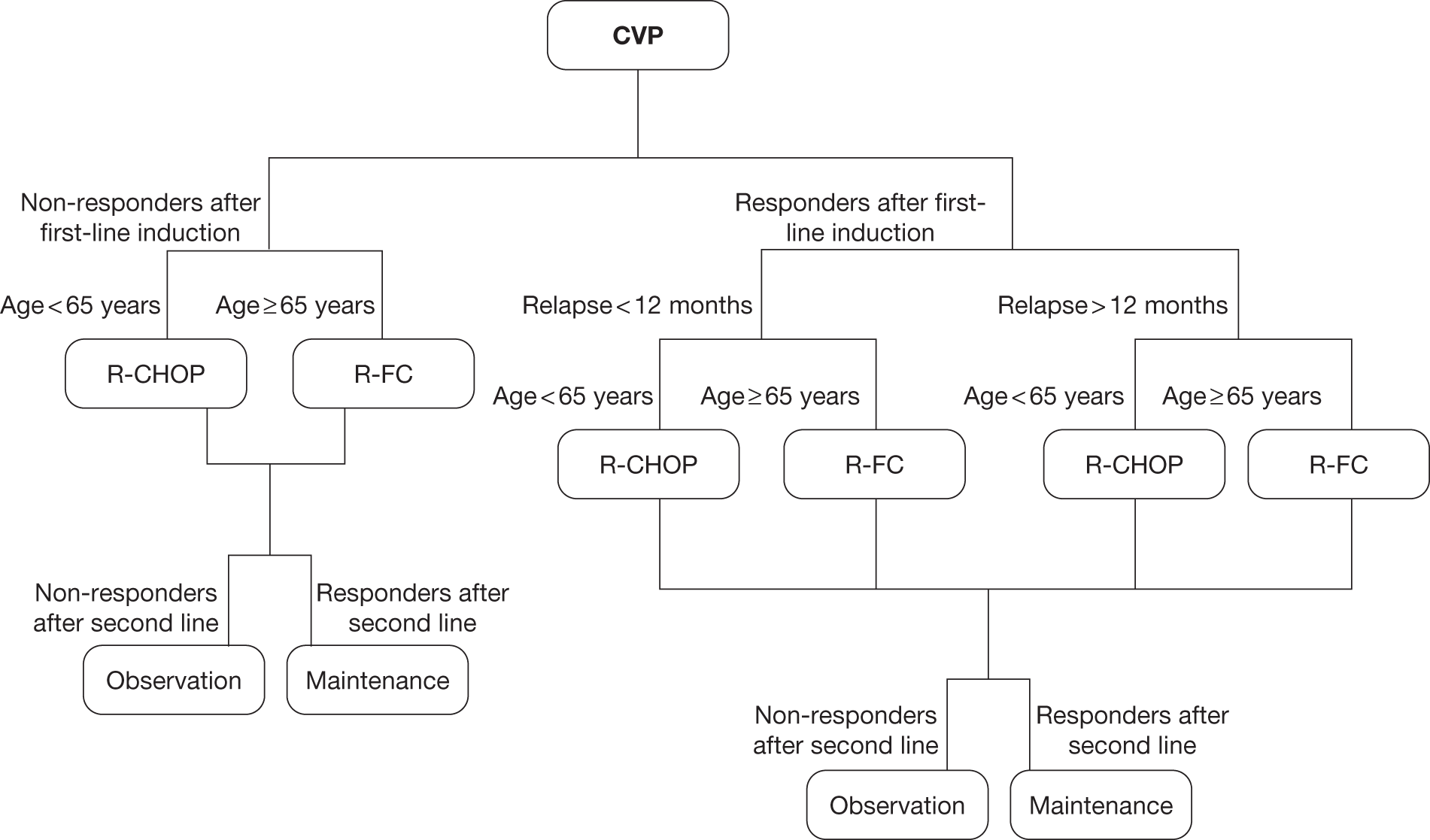
FIGURE 11.
Treatment pathways modelled in the economic model for R-CVP.
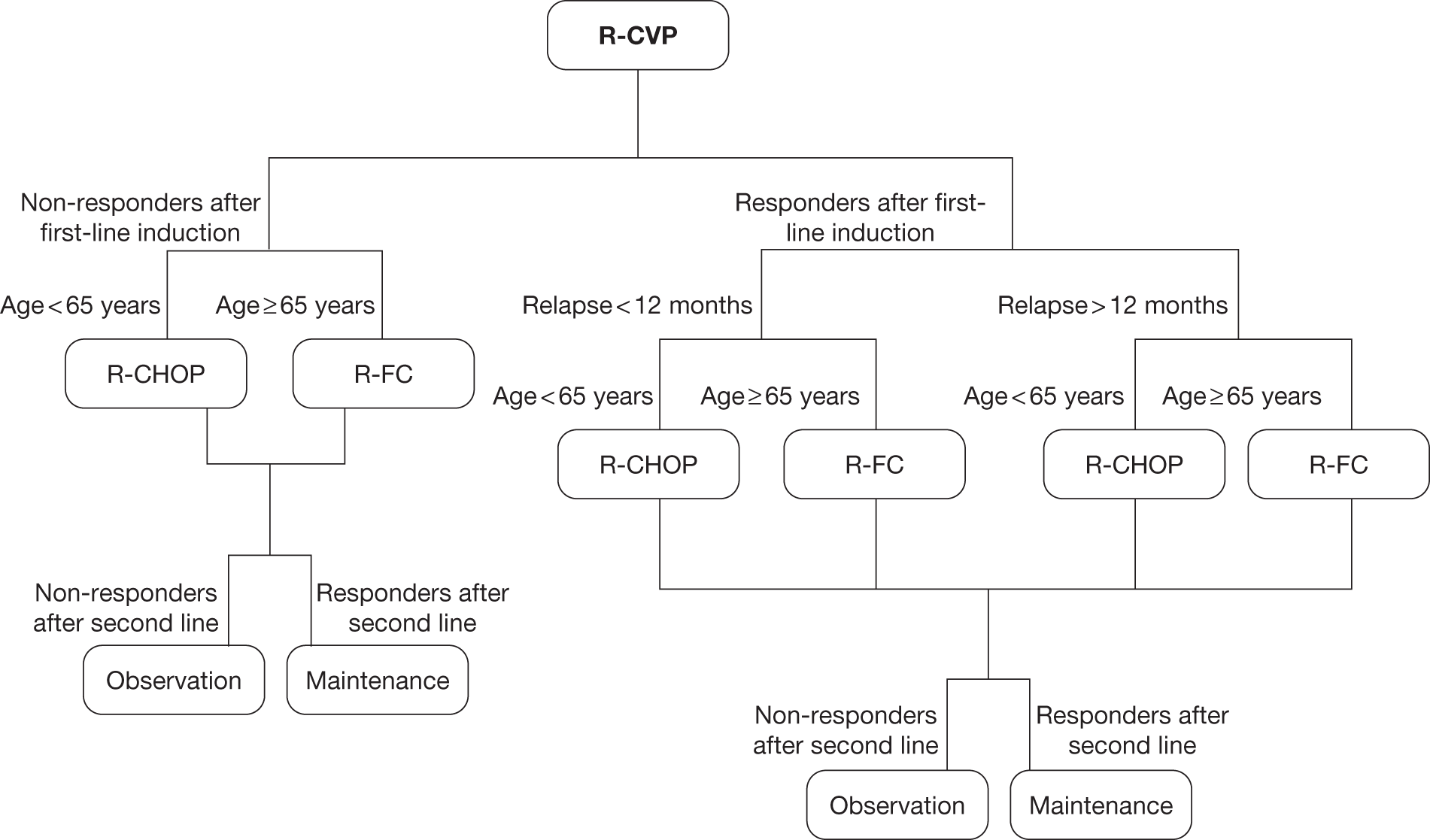
FIGURE 12.
Treatment pathways modelled in the economic model for CHOP/MCP.
FIGURE 13.
Treatment pathways modelled in the economic model for R-CHOP/R-MCP.
For the scenario analysis, clinical opinion was sought to determine the treatment pathway after first-line maintenance treatment in patients treated in first-line induction with rituximab. This is largely unknown, as first-line maintenance is not currently part of clinical practice. After discussion with clinical experts, the treatment pathway presented in Table 34 was used in the economic model for responders to first-line induction with rituximab in addition to chemotherapy.
| Response status and time of relapse | Age (years) | Second-line treatment | |
|---|---|---|---|
| R-CVP | R-CHOP/R-MCP | ||
| Relapse within 12 months after start of induction therapy (i.e. relapse after about < 6 months after start of maintenance) | < 65 | CHOP | R-HDT (± ASCT) |
| ≥ 65 | FC | FC | |
| Relapse after 12 months after start of induction therapy (i.e. relapse after > 6 months after start of maintenance) | < 65 | R-CHOP | R-HDT (± ASCT) |
| ≥ 65 | R-FC | R-HDT (± ASCT) | |
Note that the choice of second-line treatment for patients treated with chemotherapies only (i.e. without rituximab) in first-line induction was not amended (see Figures 10–13), as first-line maintenance is only considered an option by NICE in the ongoing appraisal for patients treated with rituximab in addition to chemotherapy in first-line induction therapy.
In addition to the base case, a range of SAs were conducted exploring the impact of the treatment pathway. As described later (see Effectiveness in patients treated with CHOP with or without rituximab in second line), there is a gap between evidence available and the treatment in clinical practice. No robust evidence were available for the effectiveness of FC-containing regimens with or without rituximab in patients aged ≥ 65 years at the time of relapse after first-line induction treatment. There were also no trials identified providing a direct comparison of ASCT in addition to salvage therapy with HDT with or without rituximab in patients with relapsed FL. Finally, the identified studies in patients with relapsed FL74,75,134 were conducted in cohorts of patients with FL that were not previously treated with rituximab (see Resistance to rituximab in patients previously exposed to rituximab treatment).
The following assumptions were explored in SAs:
-
Patients previously treated with R-CVP not being retreated with rituximab if relapsing < 12 months after the start of treatment (in the base case, those patients receive rituximab despite early relapse).
-
Patients previously treated with an anthracycline-containing regimen and aged < 65 years old receiving CHOP or R-CHOP in second line (in the base case, those patients receive salvage therapy with or without rituximab ± ASCT).
-
Patients aged > 65 years old receiving a CHOP-containing regimen (CHOP or R-CHOP) (in the base case, those patients receiving FC or R-FC).
-
Patients receiving second-line treatment after progression only (in the base case, patients with stable disease at the end of treatment induction are considered to be non-responders and undergo further line of treatment).
An additional scenario is also presented assuming that patients responding to first-line induction treatment with rituximab in combination with chemotherapy receive first-line maintenance rituximab for up to 2 years. This scenario is presented to explore the potential impact of the addition of first-line maintenance into the treatment pathway if NICE issue positive guidance. No final guidance was issued by NICE at the time of writing of this report. 127
Definition of progression
In the economic model, the need for further treatments is driven by the presence of progression, i.e. that patient receive second-line treatment only after relapse/progression. However, trials use different definitions for the TTP (see Chapter 3, Summary of trials and Appendix 12). A comparison of TTF (that includes next antilymphoma treatment and stable disease at cycle 4 as event), TTP and TTNT curves from the M39021 trial95,96 suggests that some patients might have received further/second-line treatments before progression.
In the economic model, we used TTP from the M39021 trial,95,96 as patient-level data were available (data provided by Roche, 15 February 2011, personal communication) for this outcome. PFS or EFS have been used in second line according to the data available. The AG acknowledges the potential differences between the outcomes, and refers to progression outcomes as PFS for simplicity and consistency.
Structure of the economic model
The structure of the economic model developed by the AG is similar to the model included in the MS62 in terms of HSs, with patients moving between four possible HSs: PFS1 (first-line induction treatment/progression-free), PFS2 (second line/progression free), progressive disease (including subsequent lines of chemotherapy), and death.
Health states were selected to represent the natural history in patients with patients with FL to incorporate previous NICE guidance. 72 The AG acknowledges that patients are likely to receive more than two lines of therapy in clinical practice; however, there is no robust evidence available that would allow the effectiveness after second-line treatment to be modelled with accuracy.
The economic model developed by the AG for this appraisal differs from the economic model included in the MS62 in the following manner:
-
use of a continuous time method over a traditional Markov process
-
treatment pathways reflecting more accurately clinical practice in England and Wales (see Figures 10–13)
-
responders and non-responders are modelled as two separate subgroups
-
use of a different source of evidence to model the effectiveness in patients treated with CHOP or MCP with or without rituximab (see Progression-free survival in patients treated with CHOP in first-line induction with or without rituximab).
The economic model treats responders and non-responders as two separate subgroups and therefore does not use the PFS curve calculated for the whole trial population. This choice has been made after reviewing the evidence available in first-line induction for patients treated with CHOP or MCP with or without rituximab (see Effectiveness in patients treated with MCP with or without rituximab in first line). This choice of model structure allows the implementation of maintenance after first- or second-line induction treatment. 73,127
A simplified schematic of the model structure is provided in Figure 14. A cohort of 100,000 individual patients were simulated, each with individual demographic characteristics (age, gender and BSA). The age at death owing to non-FL causes was sampled from a Gompertz distribution estimated from life tables in the UK,135 conditional on the patient being alive at the start of the simulation. In PFS1, patients received CVP, CHOP or MCP with or without rituximab. Patients remaining in PFS1 at the end of the induction treatment were assumed to be monitored but to not receive any further treatments. For each of the therapies examined, the response rates from the applicable trials91–93,95,96 were used to classify patients into responders and non-responders.
FIGURE 14.
Economic model structure.
The TTP is then sampled according to the PFS curves for responders and non-responders as appropriate, with non-responders having a faster disease progression (see Figure 27). If the estimated time of progression is later than the estimated time to non-FL death, patients are assumed to die before progression. For patients progressing before the age at death from all causes, the event (relapse or death) is determined based on the proportion of progression attributable to death (see Death in progression-free survival after first line). Patients do not continue in the simulation if progression is attributable to death. Patients dying incur no further costs and accrue no further QALYs. Patients relapsing move to second-line treatment.
Patients treated in second line are classified as either responders or non-responders. Responders to CHOP, R-CHOP, FC or R-FC receive maintenance rituximab for up to 2 years at the end of the induction phase as per NICE guidance. 73 Patients responding to HDT or R-HDT receive ASCT. Patients remaining in PFS2 at the end of treatment induction, maintenance or ASCT are assumed not to receive further treatment but would be monitored. The TTP is sampled and patients who progress before the age at death from all causes receive further lines of therapies (third/subsequent line). The time to death from the receipt of second-line treatment is also calculated to identify the cause of death (FL or all causes). Patients dying incur no further costs and accrue no further QALYs. Patients relapsing move to progressive disease. Those patients are assumed to incur additional costs associated with palliative and terminal care as appropriate.
Patient characteristics
A patient’s baseline characteristics were derived from registry data in England3,135 and Wales (data provided by the Welsh Cancer Intelligence & Surveillance Unit, 200816) and the demographics from the trials conducted in patients with FL.
Gender
The proportion of male patients (47%) is estimated from registry data in England3 and Wales (data provided by the Welsh Cancer Intelligence & Surveillance Unit, 200816).
Baseline age
The baseline age is derived from registry data in England3 and Wales (data provided by the Welsh Cancer Intelligence & Surveillance Unit, 200816) using a two-stage process. For consistency, a 5-year age band was assumed for patients aged ≥ 85 years (Figure 15). We then estimated the age within each age band, assuming a uniform distribution (i.e. equal probability). First, the age band of the patients was sampled. Then the precise age was estimated assuming a uniform distribution within the age band.
Body surface area
The BSA is estimated from the height (cm) and weight (kg) of patients from patient-level data from the PRIMA study,71,124 by gender, using the Mosteller formula: √[(cm × kg)/3600] (Table 35). Age-specific BSA values were considered but were not used as the use of an average greatly reduced the uncertainty associated with the BSA.
| Gender | Height (cm ± SD) | Weight (kg ± SD) | Estimated BSA (Mosteller formula) |
|---|---|---|---|
| Male | 175.01 ± 7.3 | 79.68 ± 13.34 | 1.97 |
| Female | 161.44 ± 6.75 | 67.83 ± 14.39 | 1.74 |
In the PSA, height and weight were sampled independently assuming no correlation. Although this is a limitation, we did not have access to patient-level data from the trial. 71
Age at death from all causes
The age at death from all causes is derived from UK life table data135 by fitting a Gompertz distribution to the data for males and for females.
The coefficients of the Gompertz distribution are presented below (see Analytic methods and Table 54). The AG acknowledges a limitation in the approach used, namely that deaths from FL were not excluded from the survival curve and therefore, double counting may occur. However, as it is possible that some of the deaths observed in the trials may be owing to non-FL causes this may be partly offset. The AG believes that the exclusion/inclusion of FL-related deaths from life tables data is likely to have a very minimal impact on the ICER.
Response rate after first-line induction treatment
In the economic model, patients are separated into responders and non-responders according to the response rates after first- or second-line induction treatment. The response rates in first-line induction treatment were extracted from the proportion of responders observed in the three main first-line remission induction trials (Table 36). 91–93,95,96
| First-line induction | CVP95,96 | R-CVP95,96 | CHOP91,92 | R-CHOP91,92 | MCP93 | R-MCP93 |
|---|---|---|---|---|---|---|
| Total no. of patients | 159 | 162 | 278 | 279 | 96 | 105 |
| No. of responders | 90 | 131 | 253 | 268 | 72 | 97 |
| Response rate (%) | 56.60 | 80.86 | 90.01 | 96.06 | 75.00 | 92.38 |
Owing to absence of relevant data for PFS by response category, no distinction was made between partial and CRs.
Progression-free survival in patients treated with CVP in first-line induction with or without rituximab
Progression-free survival in responders to CVP and R-CVP
Individual patient-level data from the M39021 trial95,96 have been provided by the manufacturer after a request from the AG (Roche, personal communication). The manufacturer provided the Kaplan–Meier plots from first randomisation (i.e. from start of treatment) and consequently, the Kaplan–Meier curve is flat for responders for the first 6 months corresponding to the initial period of induction treatment. Because of this, it was not appropriate to fit a distribution the entire Kaplan–Meier curve. Consequently, in the economic model, we assumed no progression for responders during treatment induction (196 days for eight cycles of 21 days + 28 days), with a distribution fitted from the end of this period.
To preserve the correlation between treatments in the PSA, the AG fitted a parametric distribution to all responders using treatment as a covariate. This was shown to provide an adequate fit to the data (Figures 16–18). The parametric distribution was selected through an iterative process after evaluating goodness-of-fit criteria, the visual plot of the curve to the observed data, the plausibility of the extrapolation at the end of clinical evidence and the plot of the hazard.
Different parametric models incorporate different hazard functions. Exponential models are only suitable if the observed hazard is approximately constant and positive. Weibull and Gompertz models incorporate monotonic hazards, whereas the logged models (log-logistic, log-normal) can incorporate non-monotonic hazards, but typically have long tails owing to a reducing hazard as time increases beyond a certain point. 136
The Akaike information criterion (AIC) and Bayesian information criterion (BIC) were calculated, and suggest that the log-normal model provides the best fit to the data (Table 37). Broadly similar AIC and BIC values were observed for the log-logistic and Gompertz distribution. However, goodness-of-fit criteria provide an indication of the goodness of fit to only the observed period and do not categorically indicate that one distribution should be preferred to the remaining distributions. The observed Kaplan–Meier data were plotted against the five fitted parametric distributions (exponential, Weibull, Gompertz, log-logistic and log-normal). The Gompertz, log-logistic and log-normal distributions provided a plausible fit to the observed data (see Figures 16 and 17). Similarly, visual inspection of the plot of the hazard (see Figure 18) suggests that the log-normal, log-logistic and Gompertz distributions were suitable, as the plot was broadly linear.
| Model | Observed | ll(null) | ll(model) | df | AIC | BIC |
|---|---|---|---|---|---|---|
| Exponential | 221 | –333.225 | –316.846 | 2 | 637.692 | 644.488 |
| Weibull | 221 | –330.528 | –315.636 | 3 | 637.271 | 647.466 |
| Gompertz | 221 | –322.177 | –309.133 | 3 | 624.266 | 634.460 |
| Log-logistic | 221 | –323.495 | –307.567 | 3 | 621.134 | 631.328 |
| Log-normal | 221 | –320.175 | –304.582 | 3 | 615.165 | 625.359 |
As single parametric distributions provided reasonable and plausible fit to the data, the AG did not considered other methodologies, such as the use of piecewise exponentials.
From the values of the AIC/BIC, the visual inspections of the fit to the observed period and hazards, the AG believes that the Gompertz, log-logistic and log-normal distributions provided a reasonable and plausible fit to the data. However, the AG believed that the log-normal distribution provided a more plausible long-term extrapolation compared with the Gompertz distribution (see Figure 44). The risk of progression using the Gompertz distribution flatten out after about 60 months, implying that about 40% of responders would never progress. FL is considered as an incurable disease and therefore the use of the Gompertz distribution may be implausible. In the base case, the log-normal distribution was selected by the AG as this was believed to be the most plausible parametric extrapolation. The Weibull and Gompertz distributions have been used in SA as these provided a different extrapolation. The AG did not test the log-logistic as the curve was very similar to the log-normal distribution. The log-normal regression model and variance–covariance matrix are presented in Table 38.
| No. of subjects = 221 | No. of observations = 221 | ||||
| No. of failures = 136 | |||||
| Time at risk = 5913.331 | |||||
| LR χ2(1) = 31.18 | |||||
| Log-likelihood = –304.582 | Prob > χ2 = 0 | ||||
| _t | Coef. | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| trt | 1.16341 | 0.204687 | 5.68 | 0 | 0.76223 to 1.56459 |
| _cons | 2.591318 | 0.152575 | 16.98 | 0 | 2.292276 to 2.89036 |
| /ln_sig | 0.335348 | 0.065793 | 5.1 | 0 | 0.206395 to 0.464301 |
| sigma | 1.398427 | 0.092007 | 1.229239 to 1.590901 | ||
| Variance–covariance matrix | |||||
| _t: | _t: | ln_sig: | |||
| trt | _cons | _cons | |||
| _t:trt | 0.041897 | ||||
| _t:_cons | –0.02258 | 0.023279 | |||
| ln_sig:_cons | 0.001846 | 0.001042 | 0.004329 | ||
Progression-free survival in non-responders to CVP and R-CVP
A similar process to that detailed for responders to CVP and R-CVP has been used to estimate the risk of progression among non-responders to CVP and R-CVP; however, Kaplan–Meier data from the start of treatment induction was used95,96 (data provided by Roche, personal communication). The goodness-of-fit criteria (Table 39), visual plot of the Kaplan–Meier to the observed period (Figures 19 and 20) and the plot of the hazard (Figure 21) indicate that the Gompertz, log-logistic and log-normal distributions again provide a plausible fit to the data. In the base-case analysis, the log-normal distribution was selected (Table 40), with other distributions tested in SA.
| Model | Observations | ll(null) | ll(model) | df | AIC | BIC |
|---|---|---|---|---|---|---|
| Exponential | 93 | –158.965 | –158.490 | 2 | 320.979 | 326.044 |
| Weibull | 93 | –156.174 | –155.819 | 3 | 317.637 | 325.235 |
| Gompertz | 93 | –149.751 | –149.509 | 3 | 305.019 | 312.616 |
| Log-logistic | 93 | –147.759 | –147.431 | 3 | 300.863 | 308.461 |
| Log-normal | 93 | –147.316 | –147.070 | 3 | 300.139 | 307.737 |
| No. of subjects = 93 | No. of observations = 93 | ||||
| No. of failures = 80 | |||||
| Time at risk = 1425.807 | |||||
| LR χ2(1) = 0.49 | |||||
| Log-likelihood = –147.07 | Prob > χ2 = 0.4823 | ||||
| _t | Coef. | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| trt | –0.20573 | 0.292553 | –0.7 | 0.482 | –0.77912 to 0.367666 |
| _cons | 2.273996 | 0.16551 | 13.74 | 0 | 1.949602 to 2.59839 |
| /ln_sig | 0.254605 | 0.081224 | 3.13 | 0.002 | 0.095409 to 0.413802 |
| sigma | 1.289952 | 0.104776 | 1.100108 to 1.512558 | ||
| Variance–covariance matrix | |||||
| _t: | _t: | ln_sig: | |||
| trt | _cons | _cons | |||
| _t:trt | 0.085587 | ||||
| _t:_cons | –0.02731 | 0.027394 | |||
| ln_sig:_cons | –0.00035 | 0.000945 | 0.006597 | ||
Progression-free survival in patients treated with CHOP in first-line induction with or without rituximab
Progression-free survival in responders to CHOP and R-CHOP
In the GLSG 2000 trial,91,92 patients of < 60 years of age achieving CR or PR following first-line induction treatment were randomised to either SCT or maintenance with interferon. Patients aged ≥ 60 years received maintenance with interferon. Consequently, the reported effectiveness in responders is confounded by the effect of maintenance interferon or SCT.
The AG believes that data from the GLSG 2000 trial91,92 would lead to an overestimate of the absolute gain of the addition of rituximab to CHOP because of the additional treatments provided to responders. Alternative sources of effectiveness have therefore been considered to model the risk of progression among responders to CHOP first-line induction with or without rituximab.
The PRIMA study71 provides data on the progression rate of patients responding to first-line induction with chemotherapy in combination with rituximab only (R-CVP, R-CHOP, R-FCM). Patients were randomised to maintenance rituximab or observation up to 2 years from the end of first-line treatment induction (R-CHOP, R-CVP or R-FCM). The majority of patients (90%) had stage IV FL and most of patients received R-CHOP as first-line induction treatment (74%).
Individual patient-level data from the PRIMA study71 were made available to the AG by the manufacturer (Roche, personal communication). The Kaplan–Meier curves for the responders randomised to observation for R-CHOP and R-CVP from the end of treatment induction have been compared (Figure 22). Although apparently visually different, the difference between the two curves was not statistically significant (p = 0.0970). However, the AG acknowledges that the absence of statistical differences might be attributable to small sample sizes (R-CVP, n = 113; R-CHOP, n = 386) and that this does not necessarily means that the two curves are similar. 71
FIGURE 22.
Comparison of the Kaplan–Meier data for responders to R-CHOP and R-CVP in the PRIMA study. 71 Analysis of individual patient-level data provided by the manufacturer (Roche, personal communication).
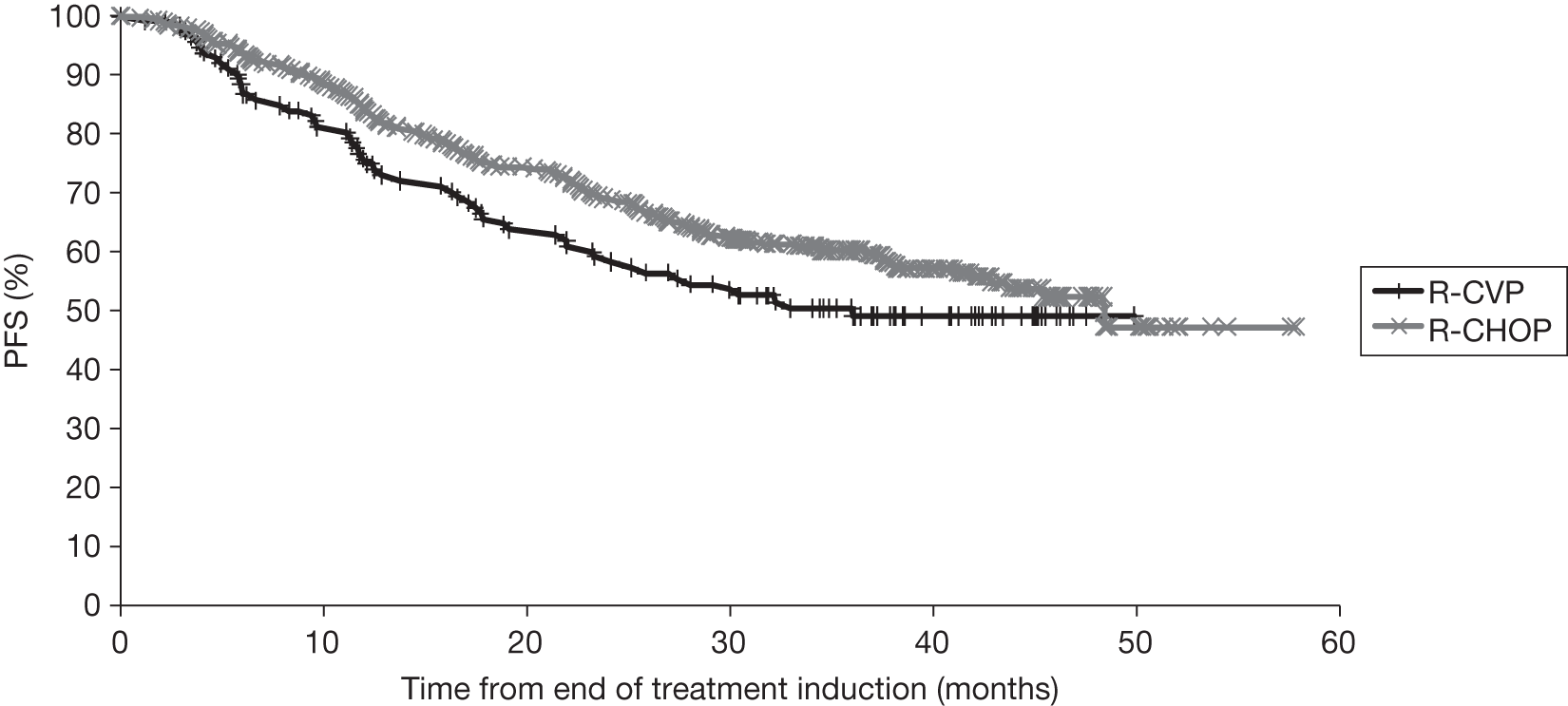
No robust sources of effectiveness were identified for the risk of progression for patients treated with CHOP first-line induction without rituximab. Most of the studies identified have been conducted in populations with other lymphomas, used a different study designs (retrospective) or were confounded by subsequent therapies for patients in remission. 137,138 Clinical opinion was sought about the mechanism of action of rituximab. This suggested that the addition of rituximab might provide the same relative benefit compared with chemotherapy alone, irrespective of the choice of chemotherapy.
Although patient-level data from the PRIMA study71 (data provided by Roche, personal communication) could have been used, the AG was not comfortable to use direct data from the trial owing to the high degree of censoring, which was noted by the ERG in the ongoing appraisal on rituximab for first-line maintenance treatment. 126 Furthermore, if a parametric function is fitted to patient-level data from the PRIMA study,71 the curve between R-CHOP and R-CVP curves would cross, as the curve for R-CVP becomes relatively flat after about 50 months. It is unclear if this is only an artefact of the limitation in the data used. 71,95,96
Given the limited evidence available on the progression for patients treated with CHOP and R-CHOP in first-line induction, the absence of a statistically significant difference for the risk of progression among responders to first-line induction with R-CVP and R-CHOP (p = 0.0970) and the suggestion by clinicians of a similar mechanism of action of rituximab for the different type of chemotherapies assessed, the AG used patient-level PFS data from the M39021 trial95,96 (data provided by Roche, personal communication) as a proxy of the PFS for patients responding to CHOP and R-CHOP, respectively.
The assumptions made were supported by additional analyses comparing the risk of progression among responders to R-CVP from the PRIMA study71 (data provided by Roche, personal communication) and responders to R-CVP from the M39021 trial95,96 (data provided by Roche, personal communication). Overall, the PFS from end of treatment induction was found to be broadly similar between the two trials (Figure 23).
Progression-free survival in non-responders to CHOP and R-CHOP
In the absence of evidence, the progression rates in patients that do not respond to first-line induction treatment with CHOP with or without rituximab were assumed to be equal to the rates of progression observed with CVP in combination with or without rituximab (see Progression-free survival in patients treated with CVP in first-line induction with or without rituximab). Although this is a limitation, it is consistent with the assumption that the rates of progression for responders to CHOP and R-CHOP equalled that of CVP and R-CVP.
Additionally, it is believed that this assumption would have little impact on the ICER, as only a small proportion of patients do not respond to first-line induction treatment with R-CHOP or CHOP (3.94% and 8.99%, respectively). 91,92 Clinical opinion was sought and suggested that this is a reasonable assumption.
Comparison of the modelled R-CHOP by the Assessment Group against data from an alternative randomised controlled trial77
Rummel et al. 78 report data from a Phase III trial comparing R-CHOP with R-bendamustine in patients treated for FL, Waldenström’s, marginal zone lymphoma, small lymphocytic lymphoma and MCL. Fifty-four per cent of patients had FL and patients treated with R-CHOP received a maximum of six cycles. The median age was 63 years and 77% of patients had stage IV disease. Thirty-three per cent and 48% of patients randomised had a FLIPI score of 2 or 3/> 3, respectively. The median observation time was 36 months. The response rate for all patients randomised to R-CHOP was 91.3% (all lymphoma types) and the median overall PFS (from randomisation) was 46.7 months in patients with FL who are treated with R-CHOP in first-line induction (which included all patients with FL). Although patients’ characteristics for patients with FL are not presented separately, patients’ characteristics for the whole trial population randomised to R-CHOP78 are broadly similar to the characteristics of the population included in other first-line induction trials for FL. 91–93,95,96
The PFS for patients with FL from Rummel et al. 78 was compared with our estimated combined PFS (responders and non-responders) for patients treated with R-CHOP assuming a response rate of 91.3% and that patients receive up to six cycles of treatment in the induction phase. Overall, the PFS predicted by the AG for R-CHOP is broadly similar to the PFS reported in Rummel et al. 78 (Figure 24).
Effectiveness in patients treated with MCP with or without rituximab in first line
As with CHOP-containing regimens, data from the first-line trial for R-MCP and MCP93 are confounded by responders receiving subsequent maintenance therapy with interferon-alpha. No robust alternative sources were identified by the AG.
To provide an estimation of the cost-effectiveness of rituximab in addition to MCP, a scenario analysis is presented, assuming that the PFS for responders and non-responders treated with MCP with or without rituximab are identical to the PFS in patients treated with CVP/CHOP with or without rituximab.
It is commented that although the PFS for responders and non-responders are assumed equal for R-CVP, R-CHOP and R-MCP, and are assumed equal for CVP, CHOP and MCP, the differences in response rates (see Table 36), number of cycles and time between cycles (see Table 44) result in different prognoses between interventions (see Figure 25).
FIGURE 25.
Progression-free survival for all patients treated with CVP, CHOP and MCP with or without rituximab.
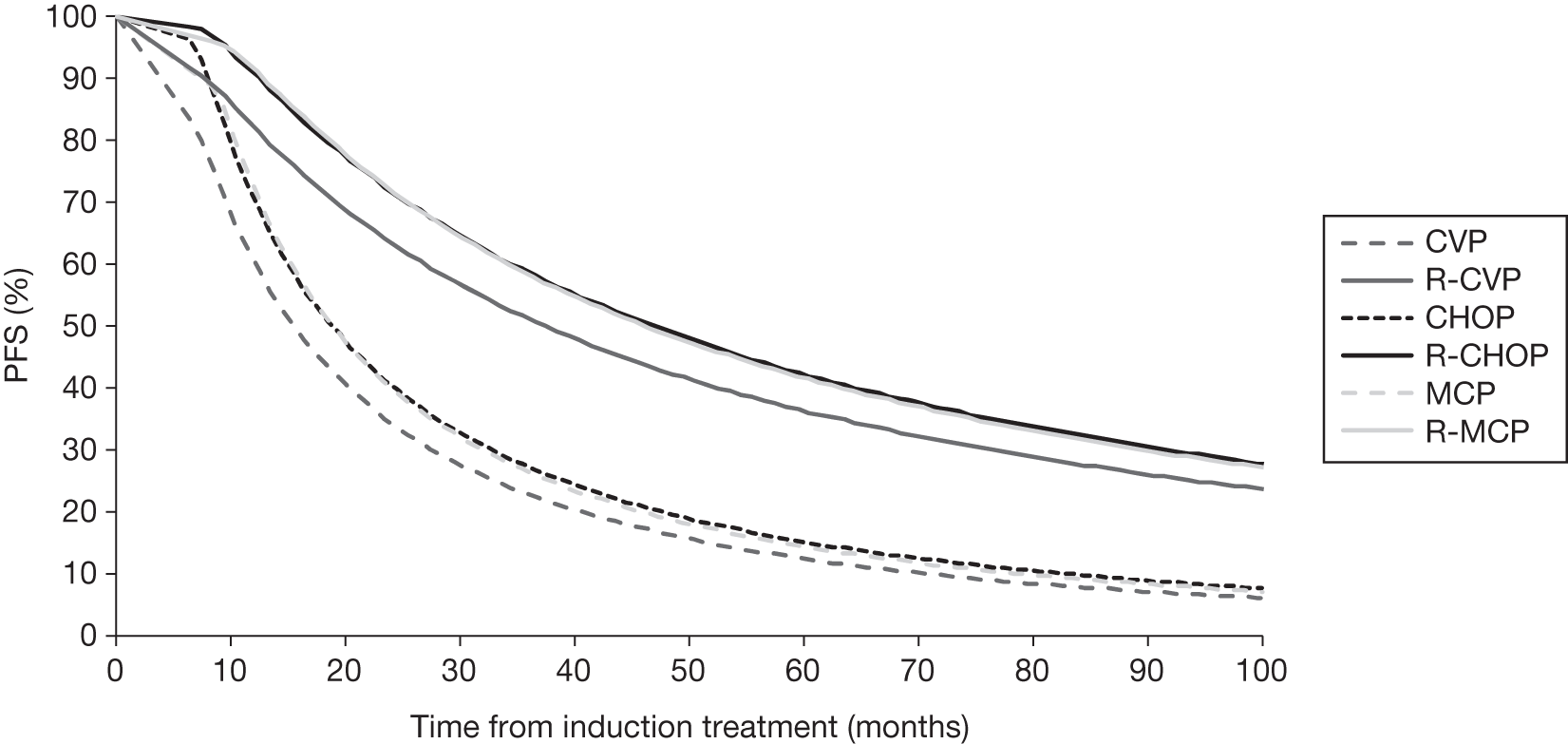
Summary of modelled progression-free survival in first-line induction
The modelled combined PFS (including both responders and non-responders) for patients treated with CVP, CHOP and MCP with or without rituximab is presented in Figure 25.
Effectiveness of rituximab first-line maintenance for patients that respond to first-line induction with chemotherapy in combination with rituximab (scenario analysis)
First-line maintenance was incorporated into the economic model by altering the risk of progression for patients responding to first-line induction with R-chemotherapy. The HR from the PRIMA study71 was used to alter the risk of progression (observation vs maintenance). Although there were differences in the HR for patients treated with R-CHOP (HR 0.51, 95% CI 0.39 to 0.65) and R-CVP (HR 0.68, 95% CI 0.45 to 1.02), we used data for the whole randomised population as differences might have been attributable to small sample sizes. Consequently, a HR of 0.55 (95% CI 0.44 to 0.68) was applied to the rate of progression for responders to R-chemotherapy for the first 42 months as clinical opinion suggests that the lasting effect ranges between 36 and 48 months. 127 SAs were conducted varying the lasting effect of first-line maintenance rituximab between 36 and 72 months.
Response rates in patients receiving second-line chemotherapy
The response rates for patients treated with CHOP and R-CHOP second-line induction treatment were extracted from the EORTC 20981 trial (Table 41). 74,75 The response rates were not available for FC-containing regimens used in older patients. As FC-containing regimens are less aggressive therapies, a lower effectiveness is expected. In the absence of evidence, we arbitrarily assumed that FC is 20% less effective than CHOP. SAs were conducted varying the response rates for patients treated with FC with or without rituximab.
Effectiveness in patients treated with CHOP with or without rituximab in second-line
Data from the EORTC 20981 trial74,75 were used to model the PFS in patients with FL who were treated with CHOP and R-CHOP in second-line induction, with or without rituximab maintenance. Patients were included in the EORTC 20981 trial74,75 if they had relapsed but had no more than two previous non-anthracycline-containing chemotherapy regimens. The study was conducted before the introduction of rituximab and therefore patients are rituximab naive, i.e. not previously exposed to rituximab. The initial results of the EORTC 20981trial75 were updated in a second publication74 that included 6 years of follow-up data. Patients were randomised to second-line induction treatment with either CHOP or R-CHOP; those patients who achieved a CR or PR had a second randomisation to either maintenance treatment with rituximab (once every 3 months) or observation for 2 years or until relapse.
Where possible, data from the latest follow-up duration74 were used in the economic model. The PFS and OS curves for responders to CHOP and R-CHOP second-line induction treatment were extracted from the latest follow-up of the EORTC 20981 trial. 74 However, the PFS and OS curves for non-responders to treatment induction were extracted from data presented by the manufacturer in a previous submission to NICE. 73
van Oers et al. 74 reported only OS data for all responders regardless of whether treatment induction was CHOP or R-CHOP randomised to either maintenance treatment with rituximab or observation. Data by treatment induction have been presented by the manufacturer in a previous NICE appraisal;73 however, this used a shorter follow-up duration (median = 39.4 months from first randomisation). These data indicated that the OS curves for patients randomised to observation or maintenance rituximab were broadly similar whether patients received CHOP or R-CHOP in second-line treatment induction (see figure 10 in MS62 for TA137). In the economic model, it was assumed that the OS for patients treated with CHOP or R-CHOP was the same, although patients receiving observation did less well than those who had maintenance with rituximab.
The PFS and OS for responders using the latest follow-up data from the EORTC 2098174 are presented from second randomisation, i.e. from the end of treatment induction. Consequently, the risk of PFS and OS are assumed to be zero during treatment induction in the economic model. A summary of data used in the economic model is presented in Table 42.
| Treatment | PFS | OS | ||
|---|---|---|---|---|
| First randomisation | Second randomisation | First randomisation | Second randomisation | |
| Non-responders | ||||
| CHOP |
✓ TA13773 |
✓ TA13773 |
||
| R-CHOP |
✓ TA13773 |
✓ TA13773 |
||
| Responders | ||||
| CHOP: observation |
✓ van Oers et al. 74 |
✓ Combined observation arm van Oers et al. 74 |
||
| R-CHOP: observation |
✓ van Oers et al. 74 |
|||
| CHOP: maintenance |
✓ van Oers et al. 74 |
✓ Combined maintenance arm van Oers et al. 74 |
||
| R-CHOP: maintenance |
✓ van Oers et al. 74 |
|||
It was not possible to have access to individual patient-level data from the EORTC 20981 trial,74,75 and therefore only data available in the public domain were used.
The digitised Kaplan–Meier curves included in the MS62 were used to fit several parametric distributions to represent the risk of progression or the risk to death. In the absence of individual patient-level data, the distributions have been fitted using the Solver function within Microsoft Excel in order to find the parameter values that minimise the root-mean-square error (RMSE) between the observed and predicted Kaplan–Meier. The best distribution was selected using an iterative approach after analysing the visual plot of the curve, the hazard plot and the RMSE. Overall, the Weibull and exponential distributions provided the poorest fit to the data. The Gompertz and log-logistic distribution provided a reasonable fit to only part of the data. The log-normal distribution fitted all the data reasonably well.
The plot of the PFS Kaplan–Meier and predicted log-normal distribution for patients responding to second-line treatment induction with CHOP and R-CHOP are presented in Figures 26 and 27.
The plot of the OS Kaplan–Meier and log-normal distribution for patients responding to second-line treatment induction is presented in Figure 28.
Finally, the plot of the OS and PFS Kaplan–Meier for non-responders to CHOP and R-CHOP in second-line treatment is presented in Figure 29.
FIGURE 29.
Plot of the OS and PFS Kaplan–Meier data and log-normal distribution for non-responders to second-line induction treatment (from start of induction treatment). 73
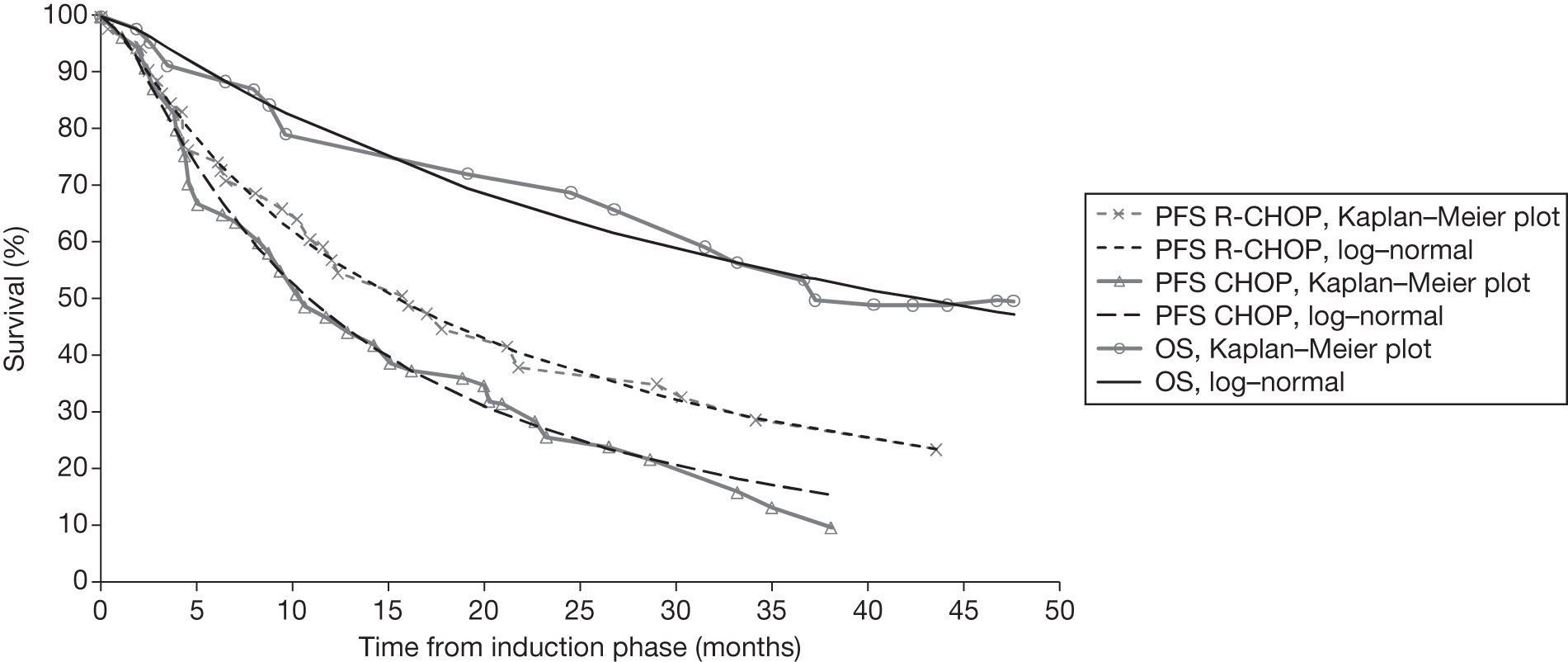
However, the distribution that provided the best fit to the data (the log-normal) hampered uncertainty analysis. In the PSA, we varied the mean PFS and OS by ± 5% by changing the mean parameter of the log-normal distribution but assuming the same standard deviation (SD). PFS and OS curves were sampled independently; however, the same random number was used to preserve the correlation between OS and PFS.
Effectiveness in patients treated with FC in combination or not with rituximab in second-line treatment
Clinical opinion sought by the AG suggested that FC or R-FC would be used for patients that cannot tolerate aggressive therapy (such as CHOP or HDT with or without ASCT), in particular older patients.
The published literature was searched for potential sources to estimate the effectiveness of FC-containing regimens with or without rituximab in patients with relapsed FL aged > 65 years; however, no data were identified. The AG was aware of a trial conducted in second-line treatment that compared fludarabine, cyclophosphamide, mitoxantrone (FCM) with R-FCM,139 with or without maintenance rituximab. The median age of patients randomised to FCM or R-FCM was approximately 60 years. This trial also had a different maintenance schedule compared with that of van Oers et al. ,74,75 which compared CHOP with R-CHOP in second-line induction. A previous NICE technology appraisal73 reported that the data were not mature in the R-FCM compared with FCM trial but, despite this limitation, the outcomes for R-FCM and R-CHOP are broadly similar.
The PFS and OS curves for responders and non-responders to CHOP and R-CHOP73–75 in second line (see Effectiveness in patients treated with CHOP with or without rituximab in second line) have been used a proxy for the risk of progression for patients treated with FC and R-FC. However, because we assumed a lower response rate for FC-containing regimen (20% lower) and the shorter induction period for FC/R-FC (four cycles instead of six), the overall modelled effectiveness for FC-containing regimens will be reduced compared with CHOP-containing regimens. SAs were conducted varying both the response rate and PFS curves (see Appendix 15).
Effectiveness in patients receiving salvage therapy with high-dose therapy with or without rituximab and autologous stem cell transplantation in relapsed patients with follicular lymphoma
Clinical advice sought by the AG indicated that patients previously treated with an anthracycline-containing regimen (CHOP, MCP) would not be retreated with an anthracycline regimen and would probably receive salvage therapy with HDT with or without rituximab before ASCT in cases for those that respond to chemotherapy.
Discussion with clinical experts suggested that the most commonly used HDT are up to four cycles of ESHAP (etoposide, methylprednisolone, cytarabine and cisplatin) or DHAP (dexamethasone, cytarabine and cisplatin) chemotherapy with or without rituximab. Stem cell harvest is then obtained for responders only, with patients for whom the harvest was successful eligible for BEAM (BCNU®/carmustine, cytarabine, etoposide and melphalan) conditioning plus ASCT (Figure 30).
FIGURE 30.
Treatment pathway for patients treated with HDT with or without rituximab.
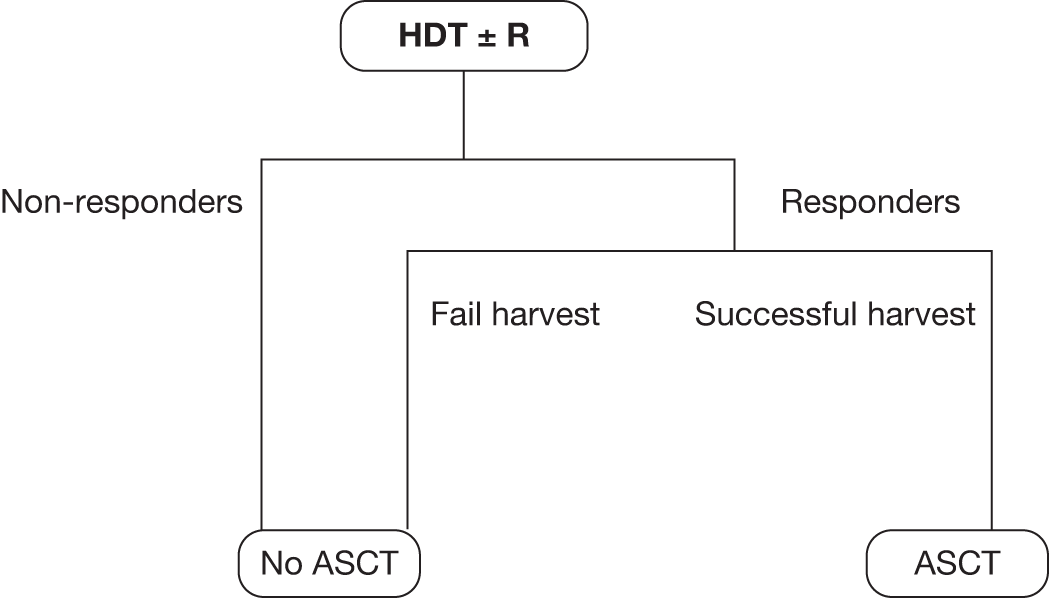
The literature was searched to identify studies that reported the impact of the addition of rituximab to salvage therapy before ASCT in patients with relapsed FL, although given the resource constraints it was not possible to perform a systematic search of the literature.
Sebban et al. 134 reported the impact of rituximab with or without HDT with transplant at the time of relapse in patients with FL. This retrospective study included patients that received CHVP alone or in addition to interferon in first-line induction. Relapsed patients receive salvage therapies, with the most used regimens being dexamethasone, high-dose cytarabine and cisplastin, ifosfamide, carboplatin and etoposide, mesna, mitoxantrone and etoposide, and fludarabine-based regimens. Rituximab was also offered to a proportion of patients with or without chemotherapy as part of the salvage treatment. Sebban et al. 134 reported that the 5-year EFS after first relapse (EFSR) was 52% in patients receiving rituximab as part of the salvage therapy (with or without chemotherapy) and 29% in patient receiving salvage therapy without rituximab. The 5-year survival after first relapse (SAR) rate was 81% and 44%, respectively.
Clinical opinion was sought regarding the validity of using evidence from this study134 to model the effectiveness of salvage therapy in addition to ASCT with or without rituximab. Overall, the clinical experts found the study appropriate, but cautioned that there were potential limitations in the study design. The addition of rituximab to salvage therapy is associated with considerable benefit, although it is unclear if the magnitude of the observed improvement is because of the retrospective nature of the study. 134 The study was also conducted in a pre-rituximab era, and therefore patients were not previously exposed to rituximab. Also, the proportion of patients that responded to HDT (for whom the harvest was successful) is unclear from the study, as well as the proportion of patients that received ASCT in both arms.
Despite these potential limitations, data from Sebban et al. 134 were used in the economic model to represent the effectiveness of salvage therapy with or without rituximab. Data for EFSR and SAR after salvage therapy with or without rituximab were taken from figure 3 in Sebban et al. 134 TechDig© software was used to estimate the data points and allow parametric distributions to be fitted. We examined different distributions using the Solver function within Microsoft Excel and overall, the log-normal was found to provide the best fit to the data. The plot of the Kaplan–Meier and estimated log-normal data is presented in Figure 31 for EFS and Figure 32 for OS.
FIGURE 31.
Event-free survival for patients treated with ASCT and salvage chemotherapy with or without rituximab. 134
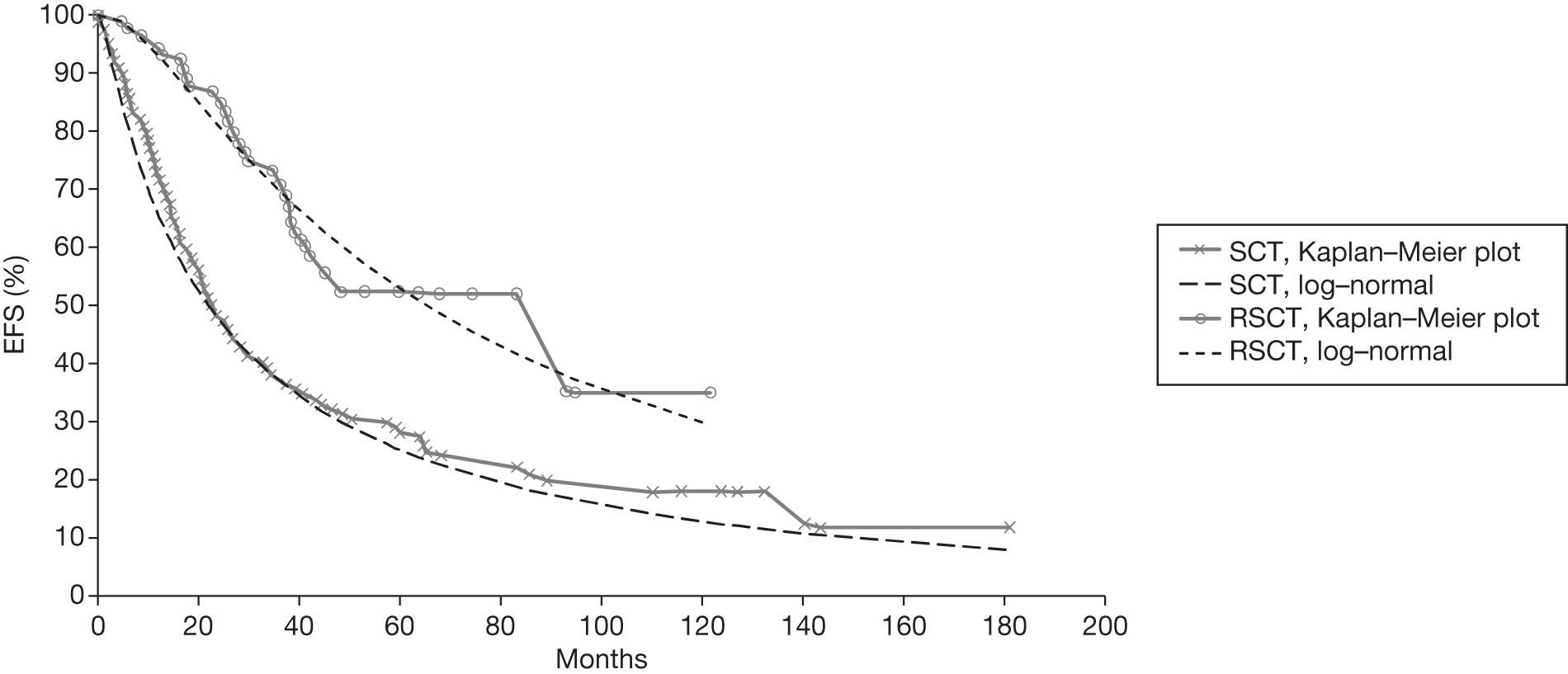
FIGURE 32.
Overall survival for patients treated with ASCT and salvage chemotherapy with or without rituximab. 134
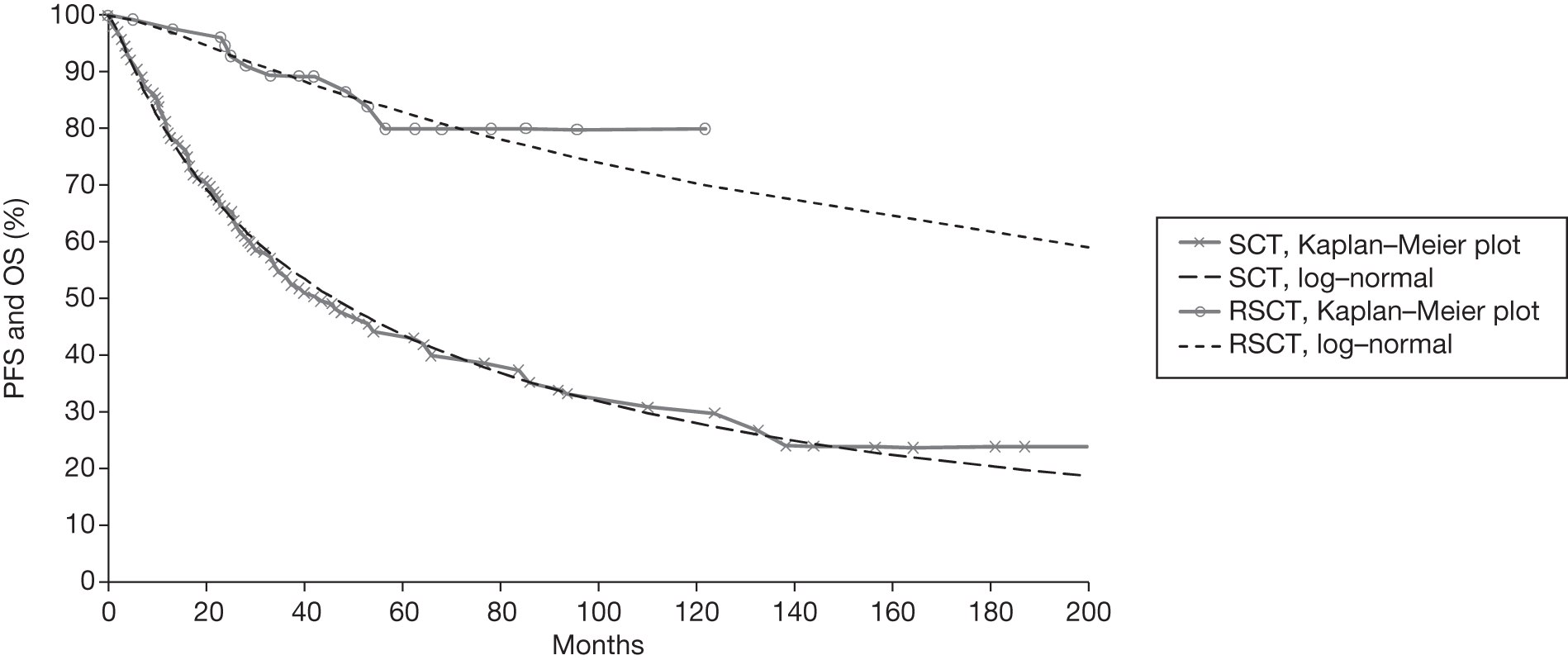
The mean effectiveness was varied by ± 5% in the PSA, with the SD of the log-normal distribution assumed constant.
Resistance to rituximab in patients previously exposed to rituximab treatment
A key assumption of the economic model submitted by the manufacturer is the absence of resistance in patients previously treated with rituximab.
Evidence of resistance in patients with relapsed FL have been estimated in cohorts of patients that have not been previously exposed to rituximab, although clinical opinion expressed in a previous NICE appraisal of rituximab73 suggested that there might be little or no loss of efficacy for retreatment with rituximab, given its mechanism of action. In the MS,62 two studies are referenced to support the assumption of the absence of a resistance effect to rituximab. 128,129 However, the AG does not believe that the data from these two studies128,129 provide conclusive evidence that resistance to rituximab can be discounted.
Johnston et al. 128 report that second-line response rates were only marginally reduced in patients with FL when compared with first-line response rates (ORR 88% to 76%, CR 52% to 44% and PR 36% to 32% in first line and second line, respectively). However, a comparison between patients who had received chemotherapy alone in first and second line and patients who had received R-chemotherapy in first and second line demonstrated that PFS following the second-line treatment was no different between the two patients groups, indicating that the second rituximab treatment had little benefit. There were several problems, however, with the study undertaken by Johnston et al. 128 in terms of its ability to prove or disprove resistance to rituximab. First, the number of patients with FL (n = 50) was small and the patients were not representative of UK patients with FL (median age at start of second treatment was young: 59 years). In addition, the comparisons being made were not ideal in determining the existence of rituximab resistance: R-chemotherapy (first line) and R-chemotherapy (second line) were compared with chemotherapy alone (first line) and chemotherapy alone (second line). The correct comparison would be R-chemotherapy (first line) + R-chemotherapy (second line) compared with chemotherapy alone (first line) and R-chemotherapy (second line). A substantial number of patients were also receiving R-monotherapy, which is not recommended in the UK unless all other options have been exhausted.
Coiffier et al. 129 presented results from a small sample of patients (n = 59) who received one of the following combinations: R-monotherapy/R-monotherapy; R-chemotherapy/R-chemotherapy, R-monotherapy/R-chemotherapy; and R-chemotherapy/R-monotherapy. The findings showed that the second-line response rate and TTP did not appear to be affected by rituximab in patients who had received rituximab in first line. However, the number of patients who received R-monotherapy is unknown and the participants in the study were patients diagnosed with a B-cell lymphoma, thus the numbers of patients with FL within the study is unknown.
From a non-systematic review of the literature via web searching, the AG identified further studies conducted in other types of lymphoma suggesting that retreatment with rituximab might be associated with a loss of efficacy. 130–132
Borgerding et al. 130 reported a very low response rate in a cohort of 28 patients with DLBCL after prior exposure to rituximab. The authors reported an ORR of 32% (9 of 28 patients). Furthermore, Weide et al. 132 examined the use of bendamustine in combination with mitoxantrone and rituximab (BMR) in patients with stage III/IV relapsed or refractory indolent lymphomas and MCL with or without prior rituximab-containing chemoimmunotherapy treatment. Fifty-seven patients were recruited, 39% of whom had received prior R-chemotherapy. The median age was 66 years (range 40–83 years). Approximately 50% of patients had FL. The ORR was 89% (35% CR and 54% PR). ORR in R-chemotherapy pre-treated patients was lower at 76% (38% CR and 38% PR).
Similarly, Martin et al. 131 report a Phase III trial comparing the response rates to R-ICE (rituximab, ifosfamide, carboplatin and etoposide) and R-DHAP salvage therapy followed by HDT with ASCT (CORAL trial) for patients with relapsed or refractory DLBCL. Martin et al. 131 report that prior exposure to rituximab was associated with a significant loss of efficacy. Patients in the rituximab group had a significantly worse PFS (17% vs 57% at 3 years) and OS (38% vs 67% at 3 years) compared with patients who were not previously treated with rituximab. Prior exposure to rituximab was an independent adverse prognostic factor for both PFS (RR 2.0, 95% CI 1.2 to 3.3, p = 0.008) and OS (RR 2.2, 95% CI 1.3 to 3.9, p = 0.004). The AG acknowledges that the effectiveness between patients previously treated with rituximab and those naive to rituximab might be confounded by the level of disease aggressiveness; those relapsing early on rituximab might have a more aggressive disease.
Overall, the two studies128,129 reported by the manufacturer do not provide conclusive evidence to prove or disprove rituximab resistance. Further studies identified by the AG130–132 suggest that there might be a resistance effect to rituximab. The AG sought clinical advice on this issue, which indicated that resistance of rituximab is unknown; however, the clinicians believed that there is little or no loss of effectiveness considering its mechanism of action.
In the base case, no resistance is assumed. SAs are conducted, exploring the potential development of resistance after rituximab retreatment. SAs are conducted by increasing the rate of progression in patients receiving rituximab in second line when they had previously been treated with rituximab.
Incorporation of adverse events in the economic assessment
The economic model includes the impact of AEs in terms of management costs and impairment in quality of life. Only grade 3 and 4 AEs were included, as these are deemed of clinical and economic importance by the AG. Furthermore, only those that occurred in the first-line induction setting were included because of the lack of robust data in patients treated in second-line and subsequent lines of treatment.
After reviewing the relative frequency of AEs within patients treated with chemotherapy with and without rituximab and the likely management cost and impact on HRQoL, the AG included the following AEs in first-line induction: leucopenia, granulocytopenia, neutropenia, anaemia, alopecia, infection, cardiac arrhythmia and cardiac dysfunction.
Only grade 3/4 neutropenia and leucopenia have been included in first-line maintenance as these were the most commonly reported grade 3 or 4 AEs in the PRIMA study. 71
The management costs associated with the treatment of AEs were extracted from the costs used in a submission by the manufacturer for an ongoing NICE appraisal. 124 It is also assumed that grade 3 and 4 AEs would incur the same costs. We further assumed that each AE led to a reduction in HRQol by 15% for 45 days. It was not possible to independently estimate the management costs of AEs and the effect on HRQoL owing to resource constraints. The management costs were varied by ± 20% in SAs. The disutility was also varied in SAs.
The AG acknowledges the limitations of the inclusion of AE in the economic model, in that it is very simplistic. However, SAs presented later indicated that AE had a limited impact on the ICER (see Appendix 15). Table 43 provides a summary of AEs included in the economic model.
| AE | Rates (%) | Cost used in the economic model (£) | Source for costs | |||||
|---|---|---|---|---|---|---|---|---|
| CVP95,96 | R-CVP95,96 | CHOP92 | R-CHOP92 | MCP93 | R-MCP93 | |||
| Leucopenia | 8.81 | 11.73 | 60.98 | 69.06 | 58.33 | 71.43 | 0 | MS for ongoing NICE appraisal124 |
| Granulocytopenia | – | – | 53.17 | 63.06 | – | – | 1514 | MS for ongoing NICE appraisal124 |
| Neutropenia | 13.84 | 24.07 | – | – | – | – | 3272 | MS for ongoing NICE appraisal124 |
| Anaemia | – | – | 10.24 | 8.97 | 4.17 | 2.86 | 445 | SA09F: Other red blood cell disorders without CC140 |
| Alopecia | – | – | 60.98 | 66.82 | – | – | 44 | MS for ongoing NICE appraisal; assumed to be the same as depression124 |
| Infection | – | – | 6.83 | 4.95 | 8.33 | 6.67 | 1077 | MS for ongoing NICE appraisal124 |
| Cardiac dysfunction | – | – | 0.98 | 3.14 | – | – | 606 | MS for ongoing NICE appraisal; assumed to be the same as arrythmia124 |
| Cardiac arrhythmia | – | – | 0.00 | 1.79 | – | – | 606 | MS for ongoing NICE appraisal124 |
Drug acquisition and administration costs
The planned dose from the three main trials92,93,95,96 were used to calculate the drug acquisition cost in the absence of detailed information about dose reduction/increase for each separate arms in the trials.
The planned number of cycles were also used in the economic model. Patients treated with CHOP or R-CHOP were assumed to receive a maximum of eight cycles in first-line induction and six cycles in second-line induction. A SA was conducted assuming that patients received a maximum of six cycles of CHOP and R-CHOP in first-line induction. Patients treated with FC or R-FC were assumed to receive a maximum of four cycles in second-line induction. A SA was conducted assuming that patients treated with FC-containing regimens would receive a maximum of six cycles. The planned dose and maximum number of cycles used in the economic model are summarised in Table 44.
| Treatment | CVP95,96 | R-CVP95,96 | CHOP92 | R-CHOP92 | MCP93 | R-MCP93 |
|---|---|---|---|---|---|---|
| Cyclophosphamide | 750 mg/m2 i.v. day 1 | 750 mg/m2 i.v. day 1 | 750 mg/m2 i.v. day 1 | 750 mg/m2 i.v. day 1 | ||
| Vincristine | 1.4 mg/m2 i.v. day 1 | 1.4 mg/m2 i.v. day 1 | 1.4 mg/m2 i.v. day 1 | 1.4 mg/m2 i.v. day 1 | ||
| Prednisone/prednisolonea | 40 mg/m2 days 1–5 | 40 mg/m2 days 1–5 | 100 mg/m2 days 1–5 | 100 mg/m2 days 1–5 | 25 mg/m2 days 1–5 | 25 mg/m2 days 1–5 |
| Mitoxantrone | 8 mg/m2 i.v. days 1 and 2 | 8 mg/m2 i.v. days 1 and 2 | ||||
| Chlorambucil | 3 × 3 mg/m2, orally, days 1–5 | 3 × 3 mg/m2, orally, days 1–5 | ||||
| Doxorubicin | 50 mg/m2 i.v. day 1 | 50 mg/m2 i.v. day 1 | ||||
| Rituximab | 375 mg/m2 i.v. day 1 | 375 mg/m2 i.v. day 1 | 375 mg/m2 i.v. day 1 | |||
| Maximum no. of cycles | 8 | 8 | 6–8b | 6–8b | 8 | 8 |
| Interval between cycles | 21 | 21 | 21 | 21 | 28 | 28 |
In the economic model, the number of cycles a patient receives is calculated from the PFS curve to account for patients that withdraw before the end of planned treatment owing to progression. Withdrawal from toxicity was not modelled; however, this was shown to be uncommon in the first-line trials. 91–93,95,96
The acquisition costs of the intervention are calculated from the protocol defined/planned dose, the BSA (see Table 35) and unit costs extracted from the BNF. 86 No vial sharing is assumed.
The costs associated with the administration of each cycle of treatment are derived from NHS Reference Costs 2009/10140 and assumptions included in the MS. 62 Chemotherapies are assumed to be administered on a day-case basis. The unit costs and Healthcare Resource Groups used are presented in Table 45. In addition to the administration costs from the NHS reference costs, patients who receive rituximab are assumed to incur additional pharmacy costs based on the costs included in the MS62 (£15.54). A SA is conducted assuming a cost of £32 as used by the manufacturer in an ongoing NICE appraisal for maintenance rituximab. 124 Pharmacy costs were included separately because the manufacturer stated that other treatment costs (i.e. chemotherapy drugs, including any pharmacy dispensing costs and associated drugs to manage the side effects of the chemotherapy) are excluded from NHS reference costs. Finally, the cost associated with transport is also included assuming that 30% of patients require NHS transportation. 62
| Regimen | Administration cost (£) | Source |
|---|---|---|
| R-chemotherapy | 309.17 | SB14Z: Deliver complex chemotherapy, including prolonged infusional treatment at first attendance140 |
| Maintenance | 284.45 | SB15Z: Deliver subsequent elements of a chemotherapy cycle140 |
| Chemotherapy alone | 270.62 | SB13Z: Deliver more complex parenteral chemotherapy at first attendance140 |
| Pharmacy cost | 15.54 | MS61 |
| Transport | 39.24 | PTS: Patient Transport Services140 |
A summary of drug acquisition and administration costs by chemotherapy cycle in first-line induction per patient is presented in Table 46, assuming a BSA of 1.80.
| Costs (£) | ||||||
|---|---|---|---|---|---|---|
| CVP | R-CVP | CHOP | R-CHOP | MCP | R-MCP | |
| Drug acquisition cost/cycle | 60.48 | 1282.89 | 233.08 | 1455.49 | 218.78 | 1441.19 |
| Administration cost/cyclea | 297.93 | 336.49 | 297.93 | 336.49 | 568.55b | 607.10b |
| Total treatment cost/cycle | 358.41 | 1619.38 | 531.01 | 1791.98 | 787.33 | 2048.29 |
| Total treatment cost/patient according to the protocol defined dose | 2867 | 12,955 | 4248 | 14,336 | 6299 | 16,386 |
It is not clear from Sebban et al. 134 which salvage therapies or which rituximab regimens was used. It is also unclear what were the proportion of patients that responded to salvage therapy, the proportion that had a successful harvest and the proportion of patients that receive ASCT.
In the economic model, we assumed that patients receive two cycles of ESHAP with or without rituximab before ASCT with BEAM. The planned dose has been extracted from the clinical policies and protocol document from Surrey, West Sussex and Hampshire Cancer Network,141 presented in Table 47. We assumed that rituximab is administered at 375 mg/m2. The cost of salvage therapy with or without rituximab in patients with relapsed FL is estimated from the BNF. 85
| Day | Drug | Dose |
|---|---|---|
| 1–4 (four doses) | Cisplatin | 25 mg/m2/day |
| 1–5 (five doses) | Methylprednisolone | 500 mg/day |
| 1 only | Cytarabine | 2000 mg/m2 |
| 1–4 (four doses) | Etoposide | 40 mg/m2/day |
| 1–6 (six doses) | Corticosteroid eye drops, e.g. prednisolone 0.5% | One drop |
In the base case, we assumed the response rates for HDT with or without rituximab to be 10% higher than the response rates for CHOP and R-CHOP in second-line treatment. 74,75 We further assumed that 80% of patients have a successful harvest after response to HDT. The AG stresses that these assumptions have been made with extremely limited supportive data. SAs were conducted varying both the response rate for HDT and proportion of patients with successful harvest.
For patients responding to HDT with or without rituximab and for whom the harvest was successful, the cost of ASCT + BEAM was assumed to be £30,400, based on a costing exercise commissioned by the London Specialised Commissioning Group. 142 The cost includes pre-transplant mobilisation, stem cell harvest and storage, pre-transplant assessment, patient work-up, transplant admission and cost up to 1 year after discharge.
Management at the end of treatment induction/maintenance: monitoring and surveillance cost
The management of the disease at the end of treatment induction and/or maintenance is adapted from the monitoring reported in the MS62 after discussion with our clinical experts. Compared with the monitoring reported in the MS,62 the monitoring defined by our clinical experts (Table 48) was less intensive, particularly with regard to scanning and imaging.
| Items | Frequency | |
|---|---|---|
| Treatment induction: first 6 months after end of treatment induction | Maintenance: first 24 months after end of maintenance | |
| Period 1 | ||
| Haematologist led | One every month | One every 3 months |
| CT scans | One CT scan at end of treatment | One CT scan at end of treatment |
| FBC, patient history, physical examination, LFT, U&E | One every month | One every 3 months |
| Period 2 | ||
| Remaining months | Remaining months | |
| Haematologist led | One every 4 months | One every 4 months |
| CT scans | No CT scan | No CT scan |
| FBC, patient history, physical examination | One every 4 months | One every 4 months |
| Immunoglobulin tests, LFT, U&E, LDH | One every 4 months | One every 4 months |
The AG comments that the monitoring used in the economic model is simplistic, but that SAs indicated that the results were not markedly influenced by this parameter (see Appendix 15).
After first- and second-line induction treatment the monitoring was separated into two phases:
-
first 6 months after the end of treatment induction
-
remaining months.
The monitoring after maintenance treatment with rituximab has also been separated into two phases:
-
first 24 months after the end of maintenance
-
remaining months.
Unit costs have been extracted from the NHS Reference Costs 2009/10 and costs used in the Sheffield Teaching Hospital Trust (2005–6, personal communication). Costs are summarised in Table 49.
| Resource | Unit cost (£) | Definition/source |
|---|---|---|
| Hospital clinic visit with haematologist | 128.67 | Code: 303 – Clinical haematology consultant led: follow-up attendance non-admitted face to face140 |
| CT scan | 146.16 | Code: RA14Z – CT scan, more than three areas140 |
| FBC | 5.50 | Sheffield hospital (Sheffield Teaching Hospital Trust, 2005–6, personal communication) |
| Patient history/physical examination | 5.44 | Code: DAP842–Other pathology service140 |
| Full profile (U&E, LFT, calcium) | 14.98 | Sheffield hospital (Sheffield Teaching Hospital Trust, personal communication) |
| Serum IgG, IgA, IgM and electrophoresis | 21.99 | Sheffield hospital (Sheffield Teaching Hospital Trust, personal communication) |
| LDH test | 11.12 | Sheffield hospital (Sheffield Teaching Hospital Trust, personal communication) |
Health service costs associated with management in third/subsequent lines
Patients that progress after second-line treatment with CHOP, R-CHOP, FC or R-FC (induction or maintenance) and who are still alive are assumed to undergo third/subsequent lines of therapy. A one-off cost was applied in the economic model according to the choice of treatment received in second-line (induction and maintenance).
The management costs were estimated from the post-protocol treatments observed in the EORTC 20981 trial. 74,75 The frequency of resources used for patients treated with CHOP only, R-CHOP only, CHOP in addition to maintenance rituximab, and R-CHOP in addition to maintenance rituximab74 were multiplied by the unit costs used by the manufacturer in a previous NICE appraisal (Table 50). 73 Unit costs were not inflated as main costs were drug and procedure costs.
| Treatment | Unit cost (£)72 | Treatment received in second line, %74 | |||
|---|---|---|---|---|---|
| CHOP | R-CHOP | CHOP-Rx | R-CHOP-Rx | ||
| Chemotherapy | 3232 | 49.28 | 33.67 | 34.21 | 38.46 |
| Radiotherapy | 1620 | 23.19 | 18.37 | 17.11 | 17.58 |
| ASCT | 18,998 | 4.35 | 8.16 | 7.89 | 5.49 |
| Allogeneic SCT | 41,721 | 7.25 | 7.14 | 10.53 | 4.40 |
| Rx, single | 8490 | 37.68 | 13.27 | 10.53 | 5.49 |
| Rx, combination | 11,206 | 28.99 | 14.29 | 17.11 | 8.79 |
| Other | 0 | 11.59 | 12.24 | 7.89 | 18.68 |
| Total cost (£) | 12,265 | 8644 | 10,085 | 5857 | |
Patients treated with HDT with or without rituximab are assumed to go directly on to palliative care and no costs were applied for the further lines of treatments. This assumption was made in the absence of data about the post-progression treatment after HDT with or without ASCT and the assumption that fewer treatments are available after relapse to ASCT or HDT. A SA was conducted assuming no costs for third-line treatment for all patients.
Health service costs associated with palliative and/or terminal care
The costs associated with palliative care were estimated from the cost of palliative care for different type of advanced cancers (breast, colon, lung, uterus, ovary, prostate, stomach/oesophagus) from the start of strong opioid treatment until death. 143 The average cost per month was calculated excluding the cost of hospitalisation, as it is likely that hospitalisation costs represent terminal care. The costs per month have been inflated to 2010 prices and are estimated to be £180.68 per month.
In addition to the cost of palliative care, the cost associated with terminal care, i.e. the management before death, was included. This cost was applied only to patients whose cause of death is attributable to FL. The cost of terminal care is sourced from the NICE clinical guidance on cancer palliative/supportive care125 and includes the cost of support provided by specialist hospital/community palliative care teams, including hospice type care, day care, hospital inpatient/outpatient support, bereavement services and continuous support for dying patients. The cost per cancer death is assumed to be £4077 (£3236 inflated to 2010 prices). 125
The AG acknowledges that it is possible that there might be double-counting, as two separate sources have been used. SAs were conducted assuming no cost for terminal care.
Death in progression-free survival after first line
We used PFS as a proxy for progression; however, PFS includes both relapse and death as an event. The MS62 reported that seven deaths occurred in the CVP arm and three deaths in the R-CVP arm. At the end of the trial follow-up period, it was estimated that the number of events (death and/or progression) were 136 and 98, respectively, based on the Kaplan–Meier curves and number of patients randomised. Consequently, we estimated that 5.15% (CVP) and 3.06% (R-CVP) of progression events were attributable to death. The rate of death in CVP was applied to CHOP and MCP. The rate of death in R-CVP was applied to R-CHOP and R-MCP. The rate is then varied using a beta distribution in the PSA.
Health-state utilities
This section of the report presents a systematic review of HS utilities in patients with FL and describes the assignment of utilities in the economic model.
Systematic review of health-state utilities in patients with follicular lymphoma
A systematic search was performed to identify studies addressing the impairment in quality of life in patients with FL. Full papers and abstracts were included in the review. Only studies conducted in patients with FL or studies conducted in a mix of similar patients when the majority of patients had FL have been included. As the AG was aware of data using the EQ-5D in patients with FL and, given resource constraints, only studies assessing the quality of life using the EQ-5D have been considered for the review, as this is the preferred valuation method of HRQol by NICE. 97 The AG acknowledges that this may be a limitation.
The following databases were searched for relevant published literature: MEDLINE including MEDLINE In-Process & Other Non-Indexed Citations (Ovid); CINAHL; EMBASE; The Cochrane Library including the CDSR, CENTRAL, DARE, NHS EED and HTA databases; SCI; and BIOSIS. Ongoing research have been searched using clinical trials databases and registers, including NIHR Clinical Research Network Portfolio; National Research Register (NRR) archive 2000–7; Current Controlled Trials and ClinicalTrials.gov. Finally, relevant conference proceedings were searched, including the ASCO, ESCO, ASH, BSH and the EHA. Full details of the main search strategy for this review are presented in Appendix 5. In addition, the MS62 was handsearched62 to identify relevant references.
Studies were selected for inclusion through a two-stage process. Titles and abstracts were examined for inclusion by one reviewer. Full manuscripts of selected citations have been retrieved and assessed by one reviewer.
The search retrieved 712 citations relating to quality of life (Figure 33). Six hundred and sixty-nine articles were excluded at title stage, and 28 articles were excluded at abstract level. Fifteen studies have been examined at full-text level and two studies (corresponding to three references) were identified meeting the criteria for the systematic review of quality-of-life data. The study conducted by Wild et al. 118,119 is unpublished and was commissioned by the manufacturer. The full report was made available to the AG and is referred as the ‘Oxford Outcome Study’. The second study, by Friedlich et al. 144 was available in only the abstract form and was conducted in a mix of patients with follicular and other indolent lymphomas. A summary of included studies is below. Reasons for exclusion were the absence of EQ-5D data (use of other instruments or EQ-5D data not presented), Q-TWiST analysis or utilities estimated in a different population.
FIGURE 33.
Flow diagram of quality-of-life review selection/exclusion.
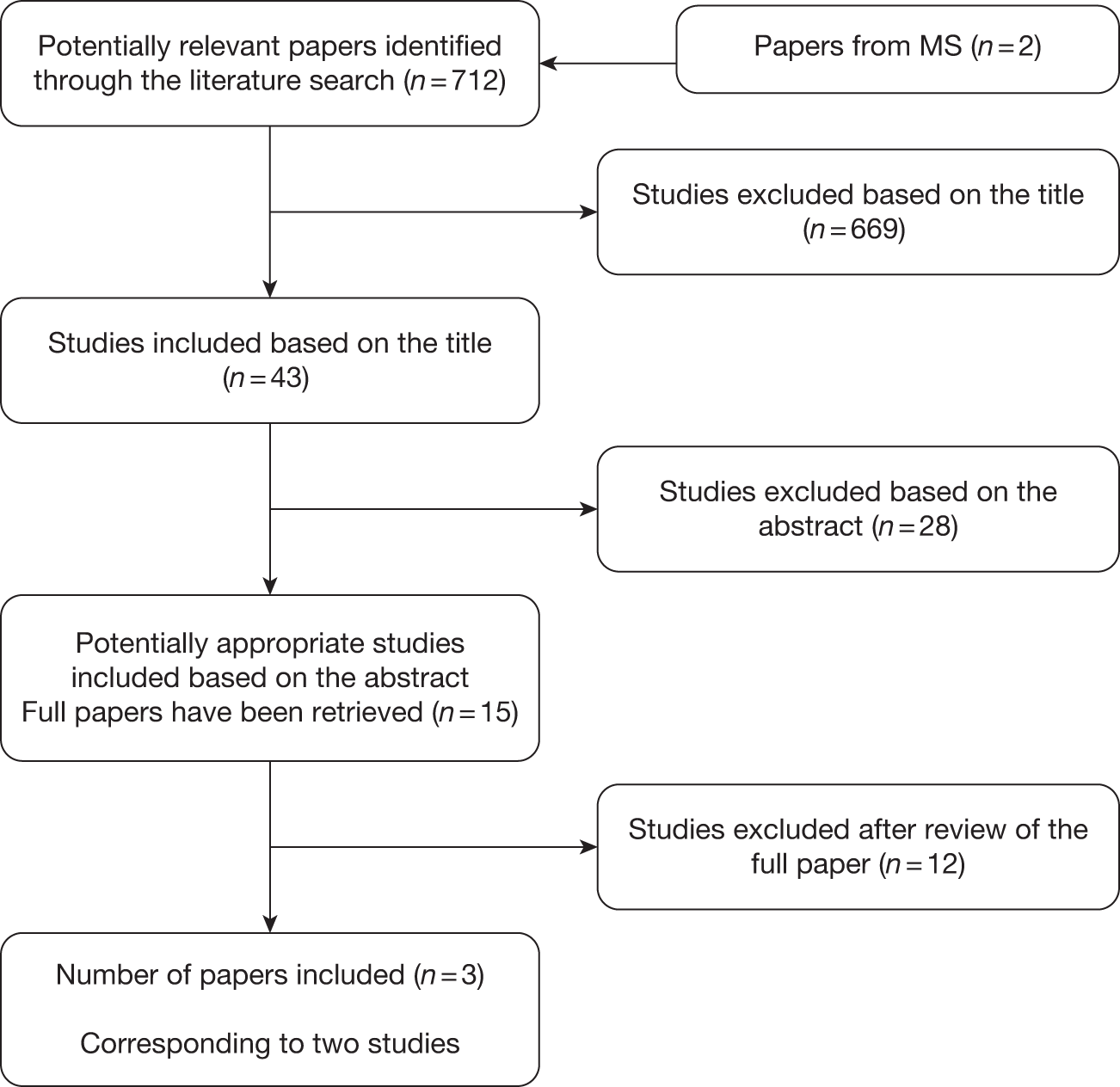
Review of the Oxford Outcomes Study
The review is based on the unpublished report of the study119 made available to the AG by the manufacturer. This study was commissioned by the manufacturer and was used in their economic model.
The study included 222 patients, aged ≥ 18 years with histologically confirmed FL and an ECOG performance status of 0–2. Patients were recruited from eight UK sites. Utilities were elicited from patients using the ED-5D questionnaire. The visual analogue scale (VAS) score is also presented. Patients also completed other outcome measures such as the Functional Assessment of Cancer Therapy-G [FACT (general)] and FACT-LYM (lymphoma).
Of the 222 returned case report forms, 215 participants returned completed EQ-5D questionnaires and 218 returned completed VAS data. The main analysis separated patients into five possible health states (HSs):
-
active disease: newly diagnosed (HS1)
-
active disease relapsed (HS2)
-
PR to therapy (HS3)
-
CR to therapy/remission (HS4)
-
disease free (no detectable diseases) (HS5).
The authors state:
Four of the five categories relate to the known stage of the disease and in particular to patients response to treatment. Patients who are disease free have essentially had the best response to treatment, those in remission the next, followed by PR and, finally, those without response (or whose response has relapsed). The newly diagnosed stage represents patients who have active disease and have started (or may be about to start) treatment, but for whom their response to treatment and therefore the relevant response categorisation is unknown.
Additional analyses are also presented aggregating the following HSs:
-
‘partial response to therapy’ (HS3), ‘complete response to therapy/remission’ (HS4), ‘disease free’ (no detectable diseases) (HS5)
-
‘active disease: newly diagnosed’ (HS1), ‘active disease relapsed’ (HS2).
Differences in the HSs utilities between groups have been examined using the Kruskal–Wallis H-test or Mann–Whitney U-test. Analyses are also presented estimating HS utility using ordinary-least-square regression analysis. The study also examined the impact of current and previous treatment with chemotherapy, but was not powered to examine this issue.
HSs utilities for the five HSs defined in the main analysis are presented in Table 51.
| Disease state | n | Mean (SD) [SE] | Range | |
|---|---|---|---|---|
| Minimum | Maximum | |||
| Active disease: newly diagnosed (HS1) | 50 | 0.83 (0.22) [0.03] | –0.24 | 1.00 |
| Active disease: relapsed (HS2) | 33 | 0.62 (0.32) [0.06] | –0.08 | 1.00 |
| PR to therapy (HS3) | 39 | 0.77 (0.21) [0.03] | 0.02 | 1.00 |
| Remission/full response to therapy (HS4) | 66 | 0.79 (0.23) [0.03] | –0.08 | 1.00 |
| Disease free (HS5) | 27 | 0.88 (0.15) [0.03] | 0.49 | 1.00 |
Additional analyses aggregating HSs are presented in Table 52.
The definition of selected HSs is poorly described. Following the short description provided by the authors, it appears that the HSs relate to the degree of response to chemotherapy but not the number of previous lines of chemotherapy (Table 53). Forty-two per cent of patients achieving PR to therapy received two or more chemotherapies; the proportion of patients in remission/full response to therapy that received two or more previous chemotherapy is about 28%.
| No. of previous chemotherapies | Disease state (%) | ||||
|---|---|---|---|---|---|
| Active disease: newly diagnosed (n = 51) | Active disease: relapsed (n = 34) | PR to therapy (n = 40) | Remission/full response to therapy (n = 67) | Disease free (n = 26) | |
| 0 | 94.1 | 20.6 | 10.0 | 22.4 | 11.5 |
| 1 | 2.0 | 17.6 | 47.5 | 49.3 | 30.8 |
| 2 | 2.0 | 20.6 | 20.0 | 13.4 | 23.1 |
| 3 | 2.0 | 26.5 | 5.0 | 6.0 | 23.1 |
| 4 | 0.0 | 5.9 | 7.5 | 6.0 | 3.8 |
| 5 | 0.0 | 8.8 | 7.5 | 3.0 | 7.7 |
| 6 | 0.0 | 0.0 | 2.5 | 0.0 | 0.0 |
In the main analysis, in which patients were separated into five possible HSs, there are some concerns about the small sample size of patients included within each HSs (range 27–50). Inaccuracy could be easily introduced when working with such small sample sizes. The description of included patients is also poorly detailed within the report, but is available in a related publication. 145 Thirty-three per cent of patients had stage I/II FL. Utility values are expected to be lower when only patients with FL with stage III/IV are included. Finally, there are some inconsistencies between the subgroup analyses (see Table 52) when HSs were aggregated.
Review of Friedlich et al.
Only the abstract form of the study144 was available. The study was conducted in patients with indolent lymphoma or FL attending an outpatient malignant haematology clinic in Toronto (Canada). Patients were asked to complete a questionnaire including utility measures (EQ-5D, FACT).
Eighty-four patients completed the questionnaire. The mean age was 58.7 years (SD 13.8) and 55% were male. The majority of patients had FL (55%). Similarly, the majority of patients had stage III/IV FL (65%).
The mean utility score for the population was 0.84 ± 0.24 SD. The authors reported that utilities were higher (p = 0.049) in patients being observed (0.91 ± 0.16 SD) compared with those in first remission (0.84 ± 0.25 SD), subsequent remissions (0.81 ± 0.20 SD) or those who were receiving active chemotherapy (0.75 ± 0.27 SD). The authors also reported that patients who were being followed in ongoing remission also trended to higher health status values (mean 0.88 ± 0.21) compared with those who were not in remission (0.80 ± 0.22 SD, p = 0.15).
Health-state utilities used in the economic model
The economic model included in the MS61 uses utility values from the Oxford Outcomes Study. 118,119 The manufacturer assumed that the utility in PFS1 was similar to the utility of patients considered to be disease free (0.88, 95% CI 0.81 to 0.95). The utility for patients in remission/full response to therapy (0.79, 95% CI 0.72 to 0.86) was used to represent the utility for patients in PFS2. Finally, the utility for progressive disease was assumed to be 0.62 (95% CI 0.48 to 0.76).
The ERG in the ongoing appraisal for first-line maintenance suggested that it is inappropriate to assume that patients in PFS1 and PFS2 have different utility values given that these patients are in remission. 126 The ERG also noted that the utility for patients in the progressive state was estimated from a small sample size (n = 33) and did not account for patients that would be in ‘remission’ in the third/subsequent lines of treatment. In addition to these limitations, the AG noted that using the utility for patients considered to be ‘disease free’ to represent the utility in patients in PFS1 also appears to be inappropriate as these patients are in a ‘remission’ state and not ‘disease free’. 118,119
The Oxford Outcomes Study118,119 reported additional analyses aggregating health states into ‘disease progression’ and ‘progression free’ (see Table 52). This was considered more appropriate by the AG as the health-state utilities in the main analysis were calculated from the degree of response to therapy and not the number of lines of treatment. Furthermore, aggregating utility values provided larger sample sizes and was expected to decrease the uncertainty and potential inaccuracy in the mean estimate. There also appears to be some errors in some of the subgroup analysis (see Table 50).
In the base case, the utility value in PFS1 and PFS2 was assumed to be 0.805, against 0.7363 for patients in the progressive health state (see Table 52). SAs were conducted to examine the impact of HRQoL in the ICER. HS utilities were varied by ± 20%. The values included in the MS62 were also examined in SAs. HS utilities from a separate source144 were also tested.
Utilities were varied in the PSA assuming a beta distribution. We assumed that the SE for the utility in progressive state was 5% around the mean in the absence of information in the study. Utility values were not age adjusted.
Analytic methods
Results are presented in terms of mean undiscounted LYs, discounted lifetime costs and discounted QALYs.
The following strategies were compared and the ICER was calculated for:
-
CVP against R-CVP
-
CHOP against R-CHOP
-
MCP against R-MCP.
Incremental analyses to determine the most cost-effective combination of chemotherapy with or without rituximab were not conducted by the AG as this was not considered relevant. Discussions with our clinical experts suggested that the choice of chemotherapy was based on additional factors such as patient’s disease characteristics and/or the presence of comorbidities as well as the efficacy of the regimen.
A range of scenarios were presented varying the main model assumptions to identify parameters that had the greatest impact on the ICER.
Probabilistic sensitivity analysis were also carried out using Monte Carlo simulation. The uncertainty in each parameter was represented using a probability distribution. The distribution with the key model parameters are presented in Table 54. The decision uncertainty was shown as the probability that each intervention is the most cost-effective at a given cost-effectiveness threshold. The probability of being the most cost-effective intervention was provided for WTP thresholds of £20,000 and £30,000 per QALY gained.
| Description | Deterministic | PSA – distribution | Source |
|---|---|---|---|
| Gender distribution | |||
| No. of males | 879 |
✓ (Beta distribution) |
Registry data in England3 and Walesa |
| No. of females | 990 | ||
| Age distribution | See Figure 15 | ✗ | Registry data in England3 and Walesa |
| All-cause mortality (Gompertz distribution) | |||
| Scale (male) | 0.0000312171 | ✗ | Derived from UK life table135 |
| Shape (male) | 0.0965411930 | ||
| Scale (female) | 0.0000115556 | ||
| Shape (female) | 0.1042325152 | ||
| BSA | See Table 35 | ✓ (Normal distribution) | Derived from the height and weight from the PRIMA study71,124 |
| Response rate | See Tables 36 and 41 | ✓ (Beta distribution) | First-line induction trials91–93,95,96 and second-line induction trial74,75 |
| PFS in responders and non-responders to first-line induction treatment | See Tables 39 and 42 | ✓ (Multivariate normal distribution) | Analysis of patient-level data from the M39021 trial,95,96 provided by the manufacturer (Roche, personal communication) |
| PFS for responders in second-line treatment with CHOP or R-CHOP with or without maintenance (log-normal distribution – see Figures 26 and 27) | |||
| Scale (CHOP) | 2.394999 | ✓ (Normal distribution, the scale parameter was varied assuming a SE of 5% around the scale) | Derived from van Oers et al.74 |
| Shape (CHOP) | 0.167823 | ||
| Scale (CHOP–R) | 3.623044 | ||
| Shape (CHOP–R) | 0.381342 | ||
| Scale (R-CHOP) | 3.277728 | ||
| Shape (R-CHOP) | 0.633029 | ||
| Scale (R-CHOP–R) | 3.984251 | ||
| Shape (R-CHOP–R) | 0.643069 | ||
| PFS for non-responders in second-line treatment with CHOP or R-CHOP with or without maintenance (log-normal distribution – see Figure 29) | |||
| Scale (CHOP) | 2.389454 | ✓ (Normal distribution, the scale parameter was varied assuming a SE of 5% around the scale) | Derived from van Oers et al.74 |
| Shape (CHOP) | 0.210479 | ||
| Scale (CHOP–R) | 2.741266 | ||
| Shape (CHOP–R) | 0.359914 | ||
| OS for responders in second line (log-normal distribution – see Figure 28) | |||
| Scale (observation) | 4.623707 | ✓ (Normal distribution, the scale parameter was varied assuming a SE of 5% around the scale) | Derived from van Oers et al.74 |
| Shape (observation) | 0.288565 | ||
| Scale (maintenance) | 5.104284 | ||
| Shape (maintenance) | 0.385508 | ||
| OS for non-responders in second line (log-normal distribution – see Figure 29) | |||
| Scale | 3.759047 |
✓ (Normal distribution, the scale parameter was varied assuming a SE of 5% around the scale) |
Derived from Van Oers et al.73 |
| Shape | 0.453447 | ||
| PFS for patients receiving salvage treatment in second line (log-normal distribution – see Figure 31) | |||
| Scale (HDT) | 3.092036 |
✓ (Normal distribution, the scale parameter was varied assuming a SE of 5% around the scale) |
Derived from Sebban et al.134 |
| Shape (HDT) | 0.406642 | ||
| Scale (HDT + R) | 4.179713 | ||
| Shape (HDT + R) | 0.137204 | ||
| OS for patients receiving salvage treatment in second line (log-normal distribution – see Figure 32) | |||
| Scale (HDT) | 3.835276 | ✓ (Normal distribution, the scale parameter was varied assuming a SE of 5% around the scale) | Derived from Sebban et al.134 |
| Shape (HDT) | 0.498643 | ||
| Scale (HDT + R) | 5.675053 | ||
| Shape (HDT + R) | 0.506431 | ||
| Proportion of AE | See Table 43 | ✓ (Beta distribution) | First-line induction trials91–93,95,96 |
| Cost of AE | See Table 43 | ✓ (Normal distribution, assuming a SE of 5% around the mean costs) | MS for ongoing maintenance appraisal124 |
| Health-state utility | |||
| PFS1, PFS2 | 0.805 (0.018 SE) |
✓ (Beta distribution) |
Wild et al.118,119 |
| Progressive disease | 0.7633 (SE assumed to be 5% around the mean) | Wild et al.118,119 | |
| Monitoring cost, administration cost | See Tables 45 and 49 | ✓ (Log-normal distribution or normal distribution assuming a SE of 5% around the mean costs) | See Tables 45 and 49 |
| Cost: third line | See Table 50 | ✓ (Normal distribution, assuming a SE of 5% around the mean costs) | Derived from van Oers et al.74 and units used in TA137 by the MS73 |
| Cost: palliative care | £4077 | ✓ (Normal distribution, assuming a SE of 5% around the mean costs) | Guidance on Cancer Services125 |
Results of the School of Health and Related Research economic assessment
Results are presented for two scenarios:
-
base-case analysis assuming no first-line maintenance in patients responding to R-chemotherapy first-line induction
-
scenario analysis incorporating first-line maintenance in patients responding to R-chemotherapy first-line induction.
Base-case analysis assuming no first-line maintenance in patients responding to R-chemotherapy first-line induction
Deterministic results
The results of the deterministic base-case cost-effectiveness analysis are presented in Tables 55–57. Analyses indicate that the addition of rituximab to CVP leads to a gain of 0.96 discounted QALYs for an additional cost of about £7389. The cost per QALY gained of CVP in combination with rituximab compared with CVP alone is £7720 (see Table 55).
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY |
|---|---|---|---|
| CVP | 9.86 | 30,793 | 5.99 |
| R-CVP | 11.50 | 38,183 | 6.95 |
| Cost per QALY (£) | 7720 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY |
|---|---|---|---|
| CHOP | 11.55 | 34,983 | 6.84 |
| R-CHOP | 12.40 | 40,708 | 7.37 |
| Cost per QALY (£) | 10,834 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY |
|---|---|---|---|
| MCP | 11.45 | 36,103 | 6.79 |
| R-MCP | 12.35 | 41,370 | 7.36 |
| Cost per QALY (£) | 9316 |
The addition of rituximab to CHOP leads to a gain of 0.53 QALYs for an additional cost of £5725. The cost per QALY gained of CHOP in combination with rituximab compared with CHOP alone is £10,834 (see Table 56).
Finally, the addition of rituximab to MCP leads to a gain of 0.57 QALYs for an additional cost of about £5267. The cost per QALY gained of MCP in combination with rituximab compared with MCP alone is £9316 (see Table 57).
Patients treated without rituximab in first-line induction spend less time in PFS1, but generally more time in PFS2 and in the progressive disease health state compared with patients receiving chemotherapies in addition to rituximab (Figure 34). A similar pattern is observed for the accrued QALYs (Figure 35). The fact that more patients in the R-chemotherapy group do not progress before death than in the chemotherapy group means that the average time in PFS1 is longer for the R-chemotherapy group, but the average duration in PFS2 and disease progression are shorter, as the patients who remain in PFS1 have zero times within these states.
FIGURE 34.
Base-case analysis: undiscounted LYs. PD, progression.
FIGURE 35.
Base-case analysis: discounted QALYs. PD, progression.
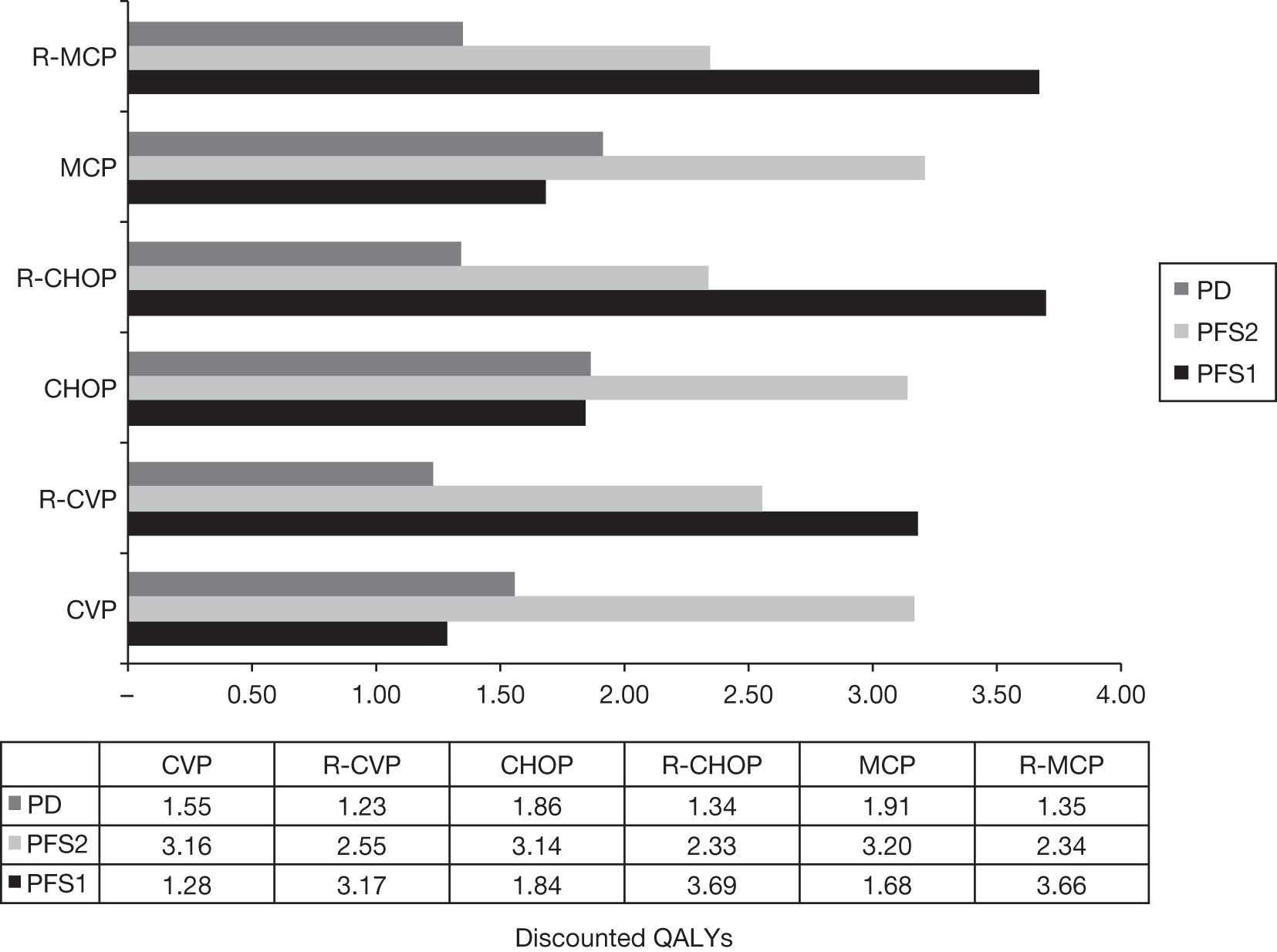
The addition of rituximab is associated with an increase in treatment costs, the management of AEs and monitoring/surveillance in first-line induction treatment compared with patients treated with chemotherapy alone (Figures 36–38). However, patients treated with chemotherapy alone incur more costs in second line and subsequent lines of treatment.
FIGURE 36.
Base-case analysis: management and treatment costs for patients treated with CVP in first-line induction with or without rituximab.
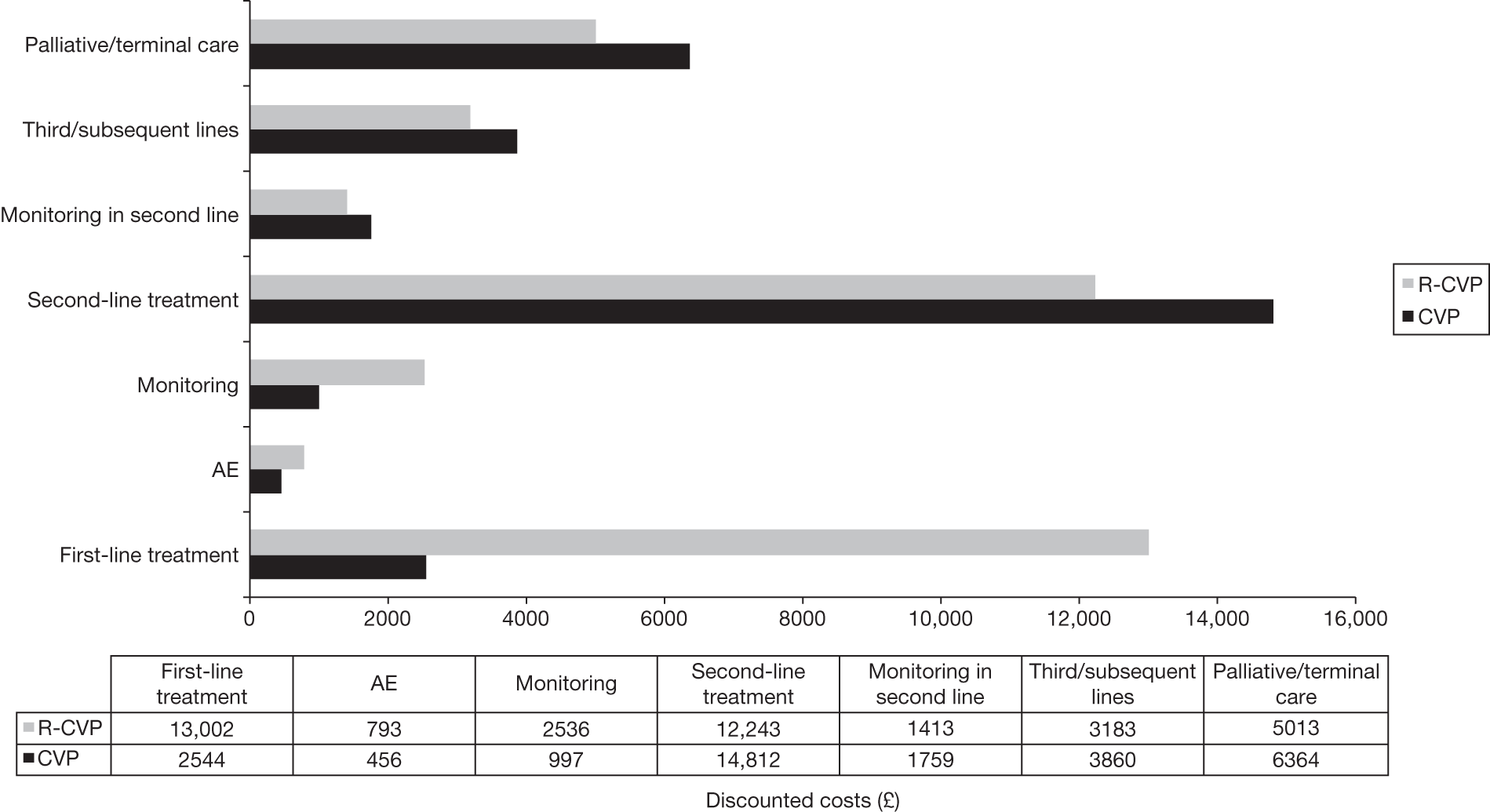
FIGURE 37.
Base-case analysis: management and treatment costs for patients treated with CHOP in first-line induction with or without rituximab.
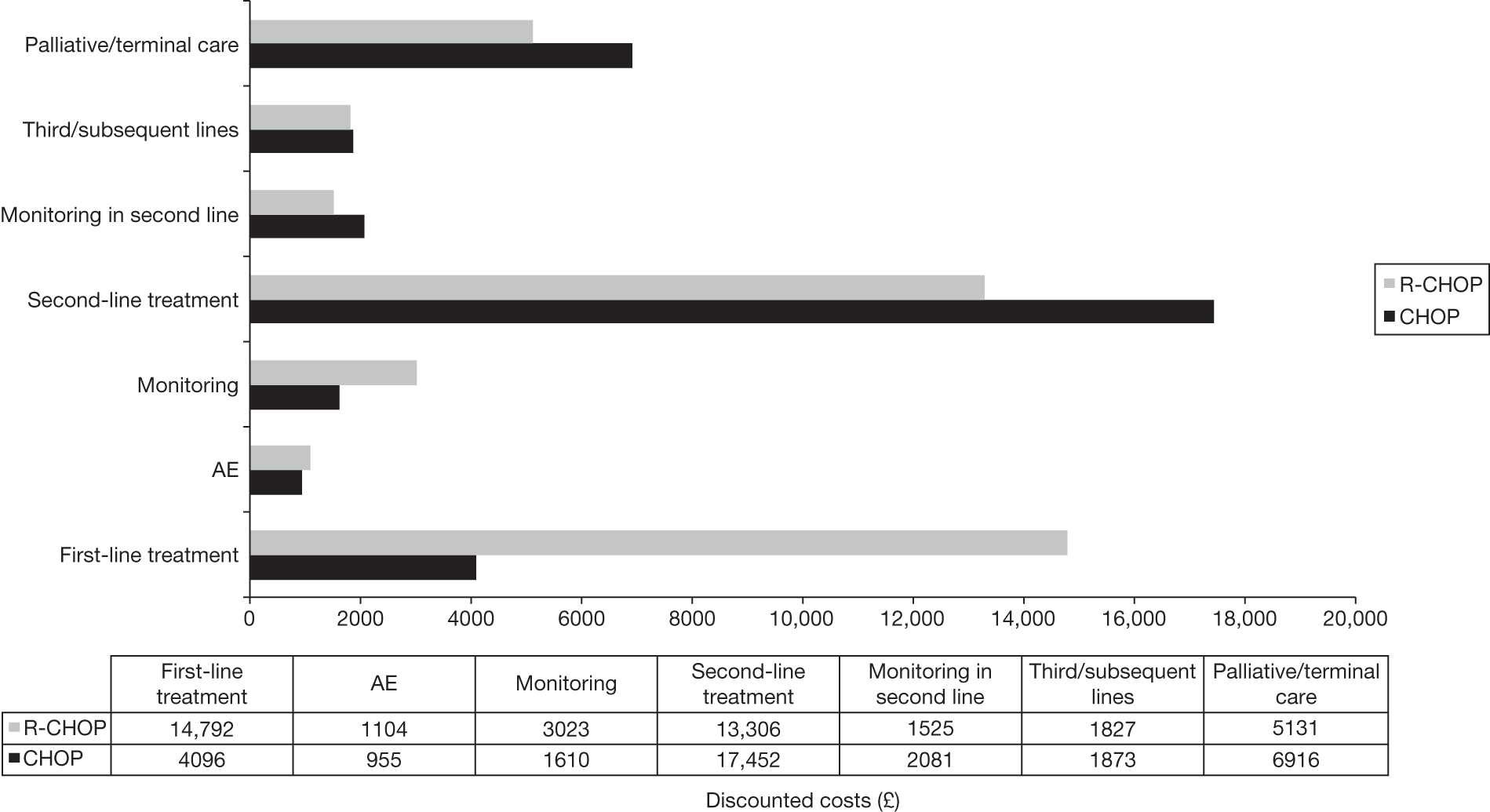
FIGURE 38.
Base-case analysis: management and treatment costs for patients treated with MCP in first-line induction with or without rituximab.
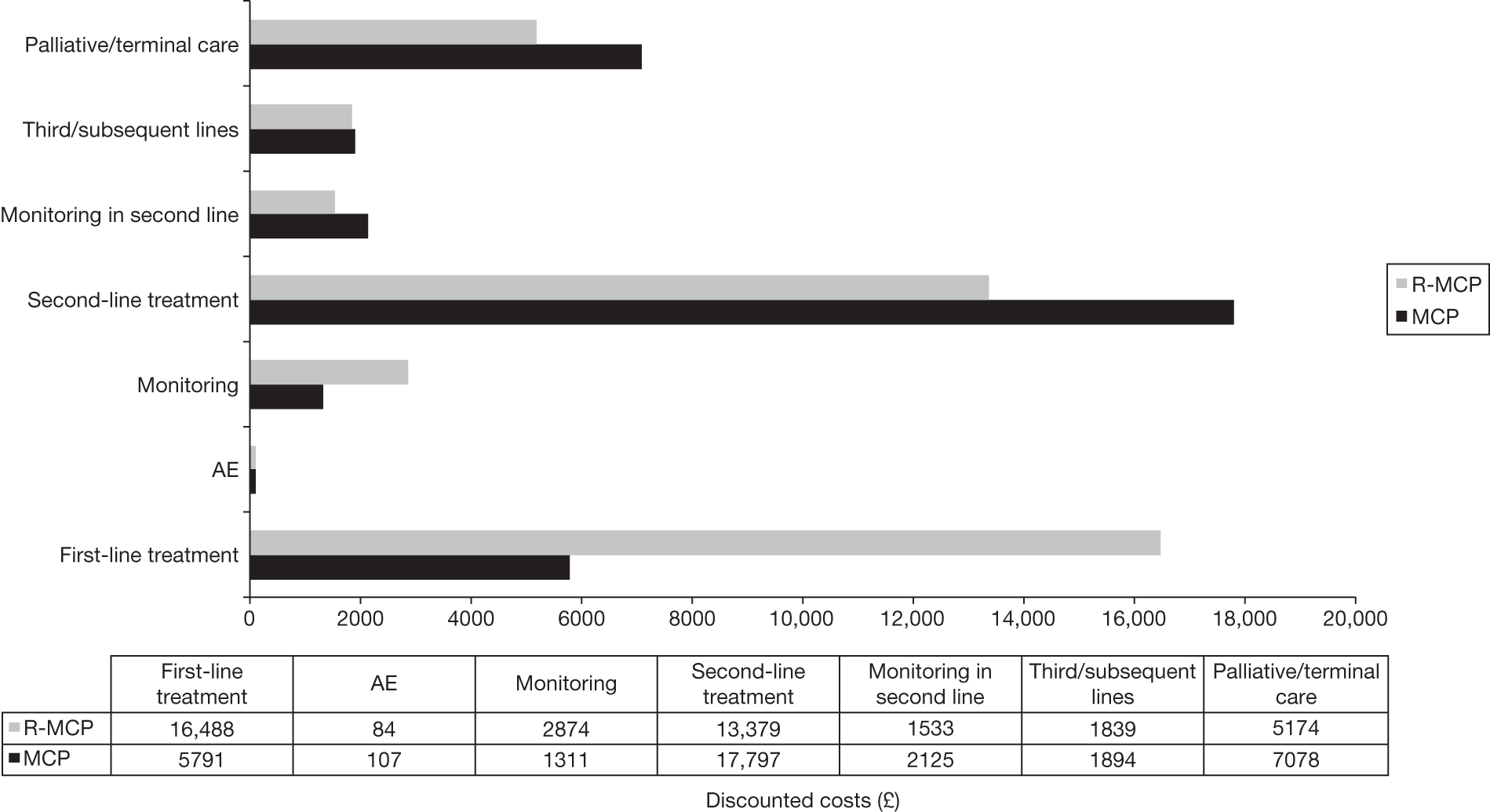
Probabilistic results
Results from the PSA differ slightly compared with the deterministic results owing to non-linearities within the model. The ICER in the PSA for the addition of rituximab to CVP, CHOP and MCP is estimated to be £7735, £10,855 and £9313 per QALY gained, respectively (Tables 58–60). The probabilities of being cost-effective at different WTP thresholds are presented in Figures 39–41 for R-CVP compared with CVP, R-CHOP compared with CHOP and R-MCP compared with MCP, respectively. The CEACs show that the addition of rituximab to chemotherapy (CVP, CHOP and MCP) in first-line induction have a high probability of being cost-effective at a cost-effectiveness threshold of £20,000 per QALY gained.
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY | Probability CE (%) at £20,000 | Probability CE (%) at £30,000 |
|---|---|---|---|---|---|
| CVP | 9.91 | 30,651 | 6.02 | ||
| R-CVP | 11.56 | 38,050 | 6.97 | ||
| Cost per QALY (£) | 7735 | 100.00 | 100.00 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY | Probability CE (%) at 20,000 | Probability CE (%) at 30,000 |
|---|---|---|---|---|---|
| CHOP | 11.60 | 34,881 | 6.85 | ||
| R-CHOP | 12.39 | 40,608 | 7.38 | ||
| Cost per QALY (£) | 10,855 | 88.50 | 95.70 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY | Probability CE (%) at £20,000 | Probability CE (%) at £30,000 |
|---|---|---|---|---|---|
| MCP | 11.50 | 35,970 | 6.80 | ||
| R-MCP | 12.21 | 41,248 | 7.37 | ||
| Cost per QALY (£) | 9313 | 92.10 | 96.70 |
FIGURE 39.
Base-case analysis: CEAC for R-CVP vs CVP alone.
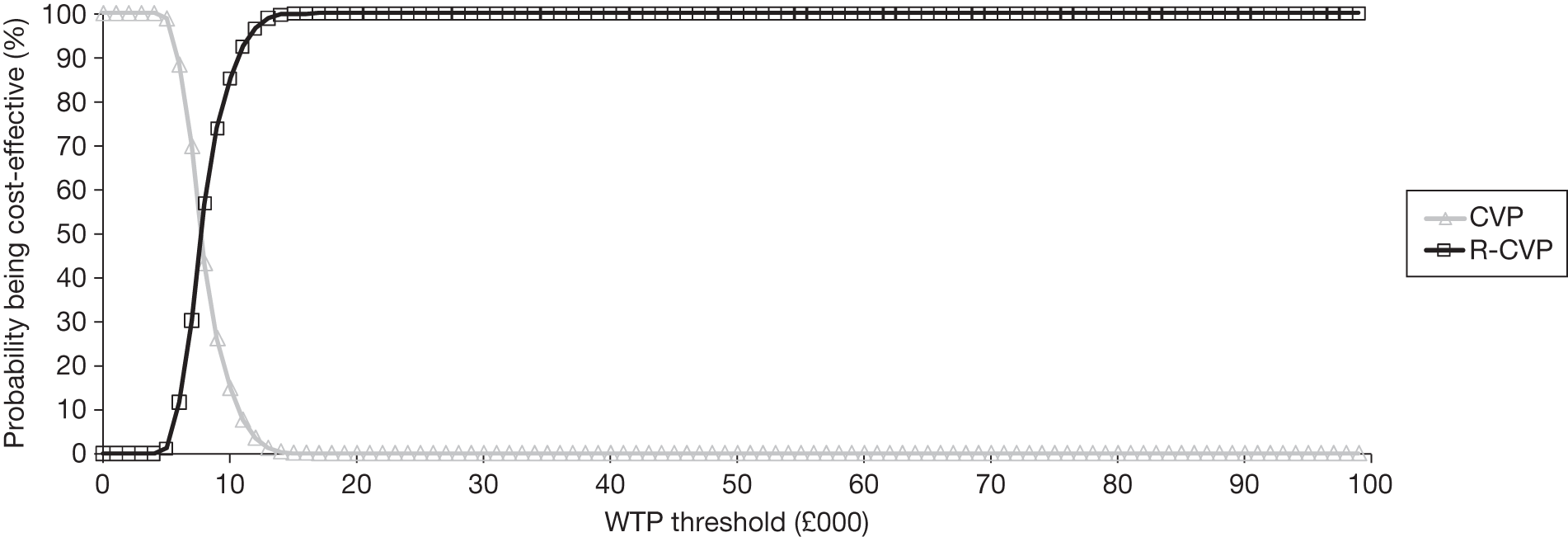
FIGURE 40.
Base-case analysis: CEAC for R-CHOP vs CHOP alone.
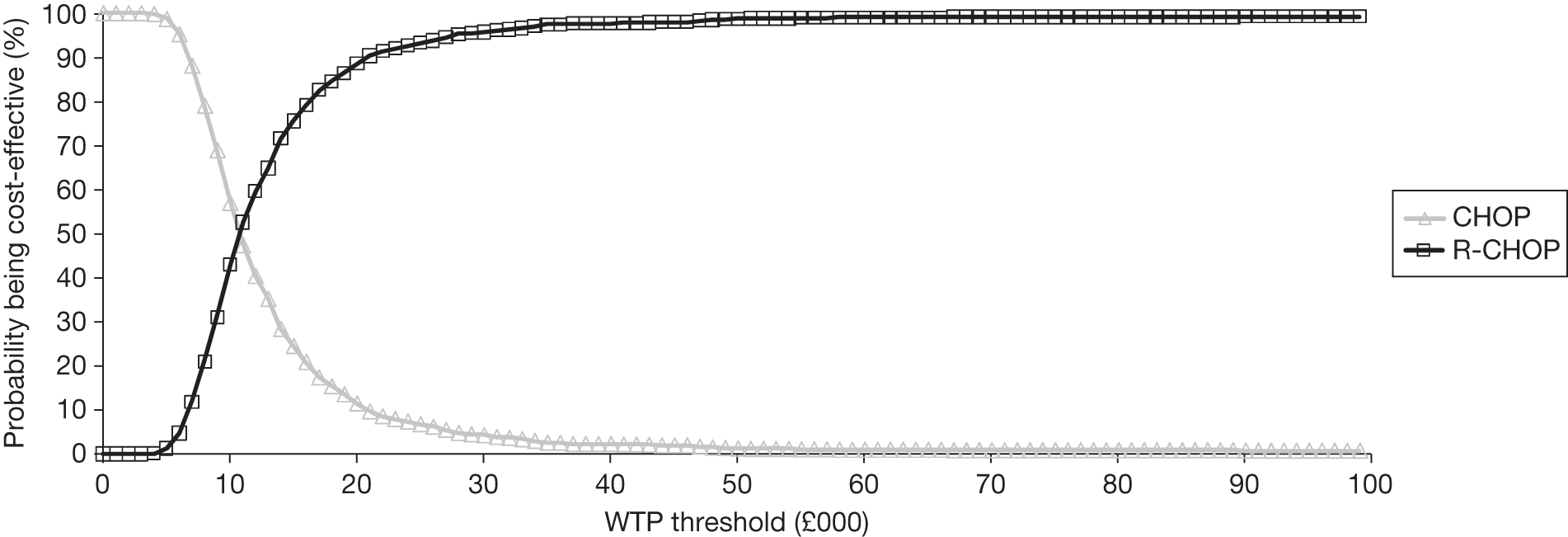
FIGURE 41.
Base-case analysis: CEAC for R-MCP vs MCP alone.
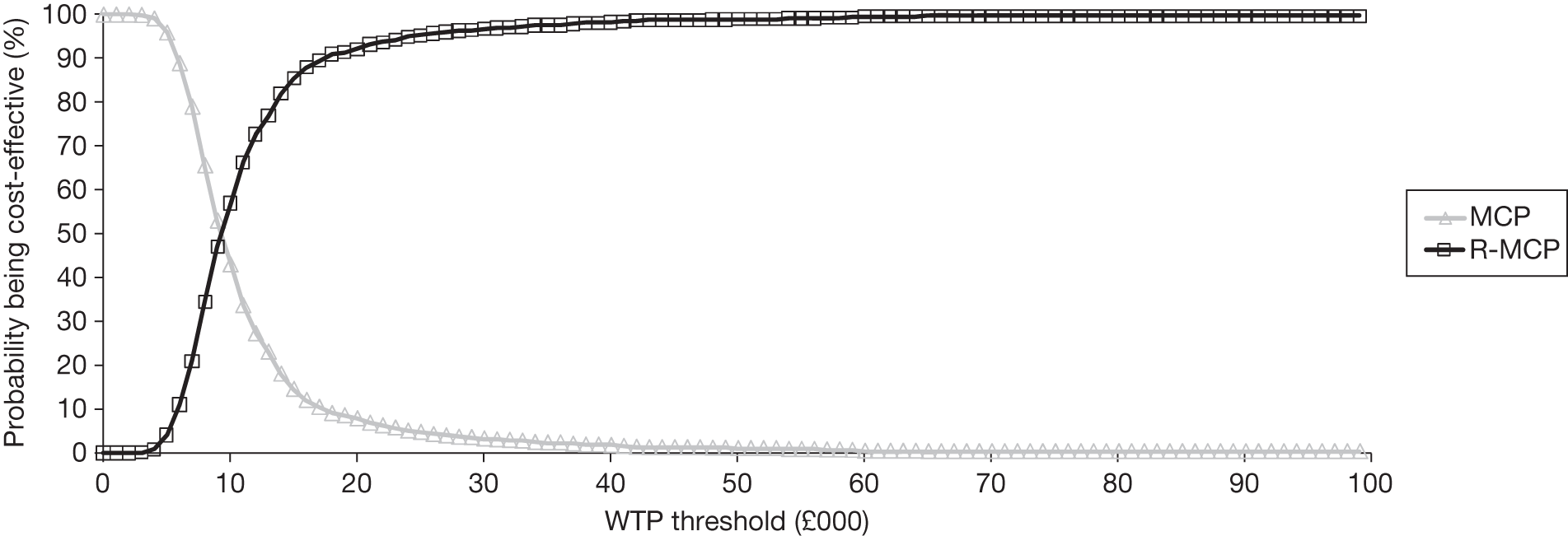
The probabilities of the addition of rituximab to CVP being cost-effective compared with CVP alone are 100% when assuming a WTP of £20,000 and £30,000 per QALY gained, respectively (see Table 58 and Figure 39).
The probabilities of the addition of rituximab to CHOP being cost-effective compared with CHOP alone are 88.50% and 95.70%, assuming a WTP of £20,000 and £30,000 per QALY gained, respectively (see Table 59 and Figure 40).
The probabilities of the addition of rituximab to MCP being cost-effective compared with MCP alone are 92.10% and 96.70% assuming a WTP of £20,000 and £30,000 per QALY gained, respectively (Table 60 and Figure 41).
Univariate sensitivity analyses: impact of main model parameters
A range of univariate SAs were undertaken to assess the impact of main model parameters and assumption on the cost per QALY gained. Full results of SAs performed are presented in Appendix 15 for the comparison between R-CVP and CVP, R-CHOP and CHOP, and R-MCP and MCP. The main findings from the SAs are described below.
We explored different time horizon (5 years, 10 years and lifetime). The ICER was sensitive to the assumption about the time horizon and becomes more favourable to rituximab for all comparisons as the time horizon increases (Table 61).
| Time horizon | Cost (£) | ||
|---|---|---|---|
| R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP | |
| Base case (25 years) | 7720 | 10,834 | 9316 |
| 5 years | 20,998 | 33,975 | 24,366 |
| 10 years | 11,287 | 16,650 | 13,598 |
| Lifetime | 7360 | 10,362 | 8963 |
We explored different assumptions about the discount rates, assuming either no discounting and either costs or benefits discounted. Results were not sensitive to the assumption about discounting (see Appendix 15). As an illustration, the ICER for R-CHOP compared with CHOP ranged from £11,788 (assuming no discounting for costs but QALY discounted at 3.5%) to £7634 (assuming no discounting for QALYs but costs discounted at 3.5%) per QALY gained.
In the base case, the effectiveness was modelled fitting a log-normal to the Kaplan–Meier curve from the M39021 trial. 95,96 In SAs, we explored the use of two alternative distributions (Gompertz and Weibull distributions). These two distributions were selected as they provided a plausible but different extrapolation compared with the log-normal distribution. The ICER was broadly similar (Table 62) assuming a Weibull distribution compared with our base-case assumption (log-normal extrapolation). However, the ICER was particularly sensitive if a Gompertz distribution was selected (see Table 60). For example, the ICER of R-CHOP against CHOP was £3941 per QALY gained when assuming a Gompertz distribution compared with £10,834 using a log-normal distribution (base-case assumption).
| Distribution | Cost (£) | ||
|---|---|---|---|
| R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP | |
| Base case | 7720 | 10,834 | 9316 |
| Weibull | 8054 | 12,030 | 10,594 |
| Gompertz | 4174 | 3941 | 3146 |
The Differences between the log-normal and Gompertz estimates are probably caused by differences in the extrapolation at the end of clinical evidence, with the risk of progression using the Gompertz distribution flattening out after about 60 months (Figure 42).
FIGURE 42.
Comparison of the extrapolation using the log-normal and Gompertz distribution for responders to R-CVP. KM, Kaplan–Meier.
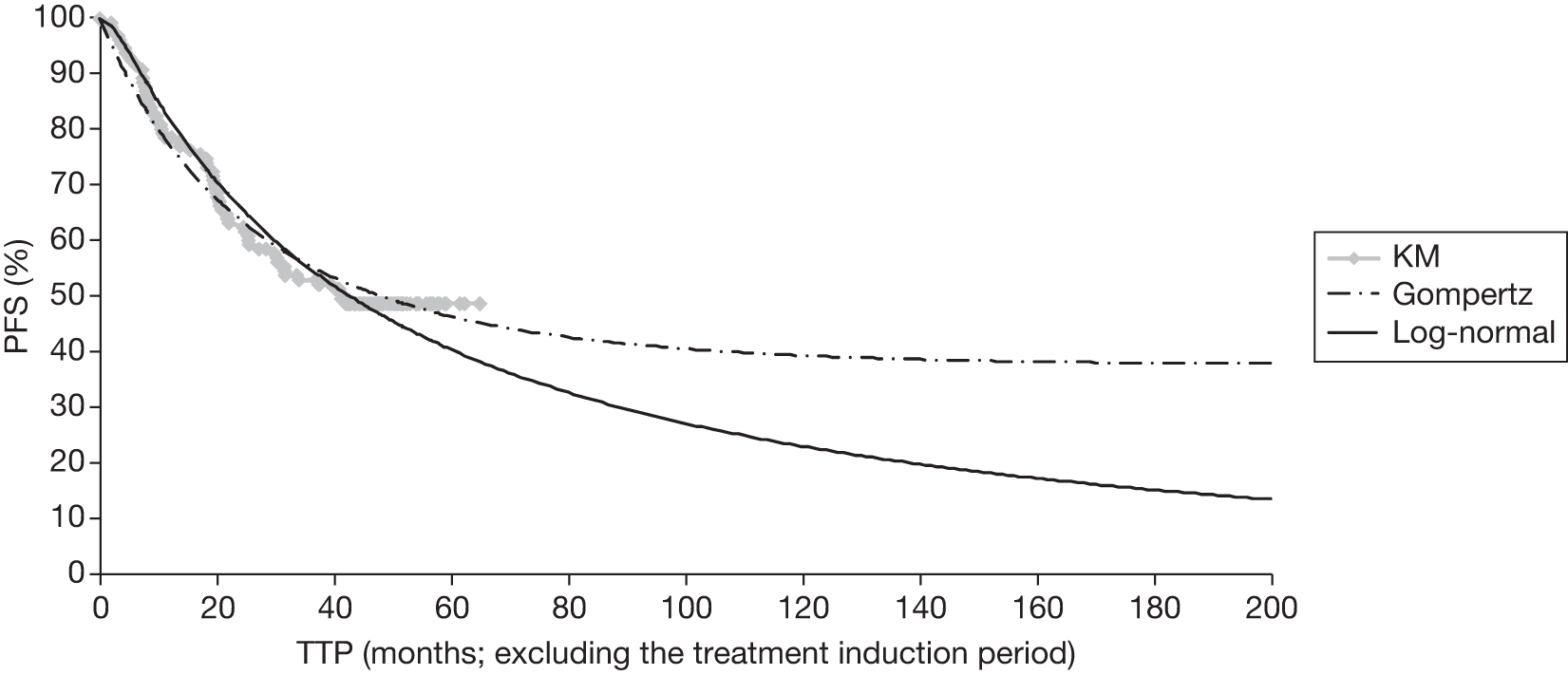
As both curves provided a plausible fit to the observed data, the ICERs may be overestimated. However, as FL is usually considered as incurable, the Gompertz extrapolation might not be plausible.
The proportion of progression attributable to death in first-line induction was derived from the M39021 trial. 62,95,96 SAs were conducted assuming that no progressions are attributable to death or that the same proportion of progression is attributable to death in the two arms (Table 63). The impact on the ICER was minimal.
| Rate of progression | Cost (£) | ||
|---|---|---|---|
| R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP | |
| Base case (5% for CVP, 3% for R-CVP) | 7720 | 10,834 | 9316 |
| None | 8224 | 13,463 | 11,192 |
| Using the rate from the CVP arm in both arms | 7984 | 11,872 | 10,023 |
| Using the rate from the R-CVP arm in both arms | 8080 | 12,470 | 10,457 |
As previously mentioned, the effect of rituximab resistance after retreatment with rituximab is unknown. In the base case, we assumed the same rate of progression after rituximab in combination with chemotherapy or salvage therapy in rituximab naive or rituximab pre-treated patients.
A SA was conducted exploring the potential impact of resistance among previously treated patients with rituximab. The resistance was modelled by reducing the rate of progression or death of rituximab in second line for patients previously treated with rituximab. A reduction up to 30% was examined in SAs to avoid the rate of progression/death in second line being higher for patients not receiving rituximab as part of the second-line treatment.
The ICER was very sensitive when a lower effectiveness was assumed in patients previously treated with rituximab (Table 64). For example, the ICER for R-CHOP against CHOP was > £20,000 per QALY gained if a reduction in effectiveness of > 20% was assumed (see Table 64).
| Reduced effectiveness in previously treated rituximab patients | Cost (£) | ||
|---|---|---|---|
| R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP | |
| Base case | 7720 | 10,834 | 9316 |
| –10% | 9379 | 13,843 | 11,718 |
| –15% | 10,616 | 16,328 | 13,632 |
| –20% | 12,328 | 20,163 | 16,494 |
| –25% | 14,870 | 26,939 | 21,253 |
| –30% | 19,102 | 42,361 | 30,902 |
Results of this SA have to be considered with caution, as the existence of a resistance effect is unknown and, if it does exist, how this would translate.
In the base case, a proportion of patients might not progress and remain in PFS1 during the entire simulation because of the parametric extrapolation. We examined a scenario in which we truncated the survival curves, assuming that patient can remain in PFS1 only for a maximum duration.
As expected, the ICER was very sensitive to this assumption. The ICER for the addition of rituximab to CHOP and MCP rose to > £20,000 per QALY gained if patients were assumed to be progression free in first line for a maximum duration of approximately 9 years (Table 65).
| Maximum time that a patient can stay in PFS1 | Cost (£) | ||
|---|---|---|---|
| R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP | |
| Base case | 7720 | 10,834 | 9316 |
| 5 years | 16,656 | 43,733 | 36,602 |
| 6 years | 14,527 | 32,857 | 27,820 |
| 7 years | 13,044 | 26,749 | 22,799 |
| 8 years | 11,964 | 22,835 | 19,527 |
| 9 years | 11,143 | 20,149 | 17,277 |
| 10 years | 10,513 | 18,210 | 15,642 |
| 11 years | 10,016 | 16,745 | 14,403 |
| 12 years | 9613 | 15,607 | 13,437 |
| 13 years | 9287 | 14,718 | 12,685 |
| 14 years | 9018 | 13,999 | 12,074 |
| 15 years | 8797 | 13,427 | 11,584 |
| 16 years | 8616 | 12,963 | 11,188 |
| 17 years | 8461 | 12,576 | 10,855 |
| 18 years | 8331 | 12,256 | 10,579 |
| 19 years | 8223 | 11,995 | 10,352 |
In the base-case analysis, we assumed the same OS for patients treated with CHOP (FC) and R-CHOP (R-FC) in second-line induction after maintenance or observation. A SA was presented assuming an increase in the mean OS for patients receiving R-CHOP or R-FC in second-line induction treatment compared with CHOP or FC. As shown in Table 66, the impact on the cost per QALY was modest. This SA mainly effects the comparison between CVP against R-CVP as patients treated with CHOP or MCP regimens do not receive CHOP or R-CHOP in second-line induction treatment but only FC and R-FC if aged > 65 years.
| Increase in mean OS | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 7720 | 10,834 | 9316 |
| 5% | 8067 | 11,213 | 9620 |
| 10% | 8441 | 11,588 | 9918 |
| 15% | 8837 | 11,950 | 10,208 |
| 20% | 9232 | 12,283 | 10,468 |
| 25% | 9613 | 12,565 | 10,691 |
The ICER increases as more patients treated with chemotherapy alone are expected to receive rituximab as part of their second line.
There were uncertainties in the health-state utility values used in the economic model. In the base case, we assumed that the utility values in PFS1, PFS2 and progressive health state were 0.805, 0.805 and 0.7366, respectively.
A SA was conducted assuming the same utility values as in the MS62 (0.880, 0.790 and 0.620) and resulted in an improvement in the ICER (Table 67). A SA was also performed using utility values estimated in Canada in a cohort of patients with different types of lymphoma (0.84, 0.81 and 0.74)144 and showed a modest impact on the ICER (see Table 67).
| HS utility values | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 7720 | 10,834 | 9316 |
| Utility values used in the MS61 | 6180 | 7167 | 6165 |
| Utility values estimated in a mixed cohort of patients with lymphoma143 | 7147 | 9518 | 8186 |
| Reduction in utility values by 10% | 8578 | 12,038 | 10,352 |
| Reduction in utility values by 20% | 9650 | 13,543 | 11,646 |
| Reduction in utility values by 30% | 11,029 | 15,478 | 13,309 |
| Assuming a 10% higher utility values in PFS1 compared with PFS2 | 6447 | 8019 | 6898 |
| Assuming no disutility | 7704 | 10,760 | 9291 |
| Disutility of 10% | 7715 | 10,809 | 9308 |
| Disutility of 20% | 7725 | 10,860 | 9325 |
| Disutility of 30% | 7736 | 10,910 | 9342 |
We examined a reduction in utility values ranging from 10% to 30%. Assuming a reduction in utility values of 30% had a modest impact on the ICER. A scenario is presented assuming that the utility in PFS1 is 10% higher compared with the utility values in PFS 2. The impact on the ICER was modest.
Finally, a range of SAs were conducted examining different assumptions about disutility owing to AEs. These had a minimal impact on the ICER.
Changes in the treatment pathway were examined given the shortcoming in evidence available. Overall, using different evidence to model the effect of second-line treatment had a modest impact on the cost per QALY. Assuming that patients treated with CHOP or MCP regimens in first-line induction regimens received CHOP or R-CHOP in second line instead of HDT ± ASCT had a modest impact on the cost per QALY gained (Table 68). Similarly, we examined a scenario in which older patients received CHOP and R-CHOP in second-line induction instead of FC and R-FC. The impact on the cost per QALY was minimal (see Table 68).
| Modelled treatment pathway | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 7720 | 10,834 | 9316 |
| Patients receive second-line after progression only | 9230 | 10,945 | 10,125 |
| Patients on R-CVP are not retreated with rituximab in second line if early relapse | 8123 | 10,834 | 9316 |
| Patients treated with an anthracycline regimen receive CHOP with or without rituximab in second line | 7720 | 8058 | 7155 |
| Older patients receive with or without rituximab in second line | 7742 | 10,833 | 9232 |
| Combination of the three previous scenarios | 7841 | 7967 | 7035 |
| All patients receive R-HDT | 8506 | 8745 | 7574 |
| All patients receive HDT | 6159 | 6245 | 5604 |
| All patients receive CHOP | 7553 | 7714 | 6907 |
| All patients receive R-CHOP | 7742 | 7933 | 7041 |
The ICER was mainly sensitive whether the same treatment was given post-progression for patients previously treated with R-chemotherapy or chemotherapy alone.
We also examined different assumptions about the effectiveness of FC-containing regimens in older patients assuming a reduced effectiveness compared with CHOP-containing regimens. The impact on the cost per QALY was minimal, with the ICER for R-CHOP against CHOP ranging from £10,019 (reduction in the rate of progression by 30%) to £11,268 (response rate reduced by 10% compared with CHOP/R-CHOP).
There were considerable uncertainties about the response rate for HDT, the proportion of patients with successful harvest and number of cycles of HDT.
In SAs we varied the response rate of HDT, assuming different success rates for harvest and assuming up to four cycles of HDT. The impact on the ICER was minimal with the ICER ranging from £9430 (assuming four cycles) to £11,221 (assuming the same response rate as CHOP/R-CHOP) per QALY gained for the comparison between R-CHOP and CHOP (see Appendix 15).
Assumptions of the occurrence (assuming no AE) and management costs of AEs (± 20%) had a minimal impact on the cost per QALY for all regimens (see Appendix 15).
The ICER between R-CHOP and CHOP improved assuming that patients only receive six cycles (£5951 per QALY gained compared with £10,834 in the base case).
The ICER was not very sensitive to assumptions about management costs (Table 69).
| Management costs | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 7720 | 10,834 | 9316 |
| Administration cost +20% | 7724 | 10,859 | 9370 |
| Administration cost –20% | 7716 | 10,810 | 9263 |
| Rx pharm (£35) | 7847 | 11,089 | 9549 |
| No monitoring | 6475 | 9214 | 7600 |
| Monitoring +20% | 7969 | 11,159 | 9660 |
| Monitoring –20% | 7471 | 10,510 | 8973 |
| No third-line cost | 8427 | 10,921 | 9413 |
| No palliative care | 8715 | 13,744 | 12,228 |
| No terminal care | 8138 | 11,303 | 9773 |
| No palliative or terminal care | 9132 | 14,213 | 12,684 |
Varying the maximum age at which patients can receive aggressive therapies (60–80 years) had a small impact on the cost per QALY gained (Table 70).
| Age to receive aggressive therapies | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 7720 | 10,834 | 9316 |
| 60 years | 7690 | 9832 | 8528 |
| 70 years | 7735 | 11,758 | 9973 |
| 75 years | 7748 | 12,763 | 10,659 |
| 80 years | 7747 | 13,377 | 11,099 |
Finally, the impact in model results of varying the BSA was minimal (Table 71).
| BSA | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 7720 | 10,834 | 9316 |
| 1.6 | 6095 | 7384 | 6164 |
| 1.7 | 7192 | 9712 | 8289 |
| 1.8 | 7192 | 9712 | 8289 |
| 1.9 | 8318 | 12,094 | 10,469 |
Scenario analysis: including first-line maintenance with rituximab in responders to R-chemotherapy
The AG explored a scenario in which first-line maintenance was incorporated into the treatment pathway. At the time of writing of the report, no guidance has been issued by NICE and, therefore, results are presented to help the Appraisal Committee in case a positive recommendation is made by NICE for the use of rituximab monotherapy as a first-line maintenance treatment in patients responding to R-chemotherapy first-line induction.
Deterministic results incorporating first-line maintenance into the treatment pathway
The cost-effectiveness results for the scenario analysis incorporating first-line maintenance for responders to R-chemotherapy in first-line induction treatment are presented in Tables 72–74. Analyses indicate that the addition of rituximab to CVP leads to a gain of 1.25 discounted QALYs for an additional cost of about £18,727. The cost per QALY gained of CVP in combination with rituximab compared with CVP alone is £14,959 (see Table 72).
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY |
|---|---|---|---|
| CVP | 9.86 | 30,793 | 5.99 |
| R-CVP | 12.03 | 49,520 | 7.25 |
| Cost per QALY (£) | 14,959 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY |
|---|---|---|---|
| CHOP | 11.55 | 34,983 | 6.84 |
| R-CHOP | 13.02 | 54,134 | 7.72 |
| Cost per QALY (£) | 21,687 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY |
|---|---|---|---|
| MCP | 11.45 | 36,103 | 6.79 |
| R-MCP | 12.89 | 54,079 | 7.67 |
| Cost per QALY (£) | 20,493 |
The addition of rituximab to CHOP leads to a gain of 0.88 QALYs for an additional cost of £19,150. The cost per QALY gained of CHOP in combination with rituximab compared with CHOP alone is £21,687 (see Table 73).
Finally, the addition of rituximab to MCP leads to a gain of 0.88 QALYs for an additional cost of about £17,976. The cost per QALY gained of MCP in combination with rituximab compared with MCP alone is £20,493 (see Table 74).
Details about the number of LYs, discounted QALY and costs by health states are presented in Appendix 16.
Probabilistic results for the scenario analysis incorporating first-line maintenance rituximab in responders to R-chemotherapy
The ICER in the PSA for the addition of rituximab to CVP, CHOP and MCP are estimated to be £15,017, £21,625 and £20,418, respectively (Tables 75–77). The probabilities of being cost-effective at different WTP thresholds are presented in Figures 43–45 for R-CVP vs CVP, R-CHOP vs CHOP, R-MCP vs MCP, respectively.
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY | Probability CE (%) at 20,000 | Probability CE (%) at 30,000 |
|---|---|---|---|---|---|
| CVP | 9.91 | 30,651 | 6.02 | ||
| R-CVP | 12.09 | 49,477 | 7.27 | ||
| Cost per QALY (£) | 15,017 | 95.60 | 100.00 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY | Probability CE (%) at £20,000 | Probability CE (%) at £30,000 |
|---|---|---|---|---|---|
| CHOP | 11.60 | 34,881 | 6.85 | ||
| R-CHOP | 12.94 | 54,063 | 7.74 | ||
| Cost per QALY (£) | 21,625 | 36.00 | 91.50 |
| Regimen | Undiscounted LY | Discounted cost (£) | Discounted QALY | Probability CE (%) at £20,000 | Probability CE (%) at £30,000 |
|---|---|---|---|---|---|
| MCP | 11.50 | 35,970 | 6.80 | ||
| R-MCP | 12.90 | 54,004 | 7.69 | ||
| Cost per QALY (£) | 20,418 | 44.90 | 91.90 |
FIGURE 43.
Scenario analysis: CEAC for R-CVP vs CVP alone.
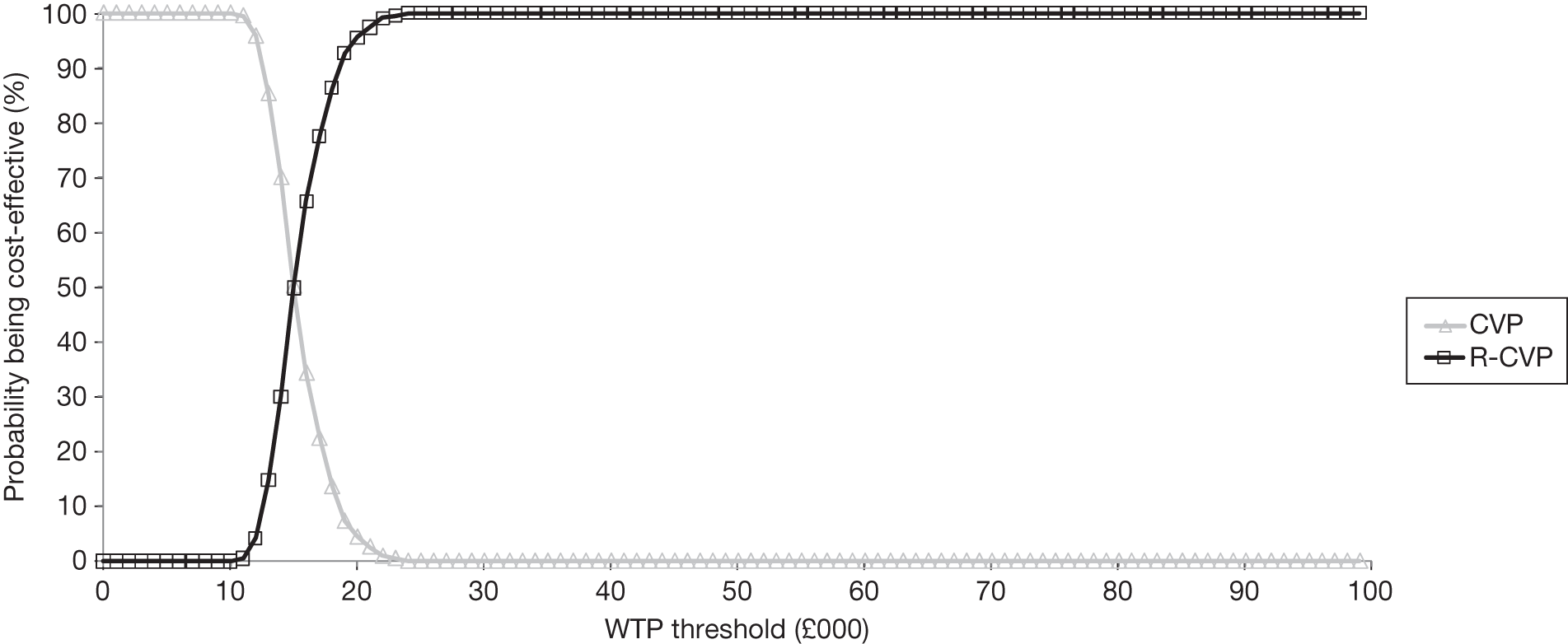
FIGURE 44.
Scenario analysis: CEAC for R-CHOP vs CHOP alone.
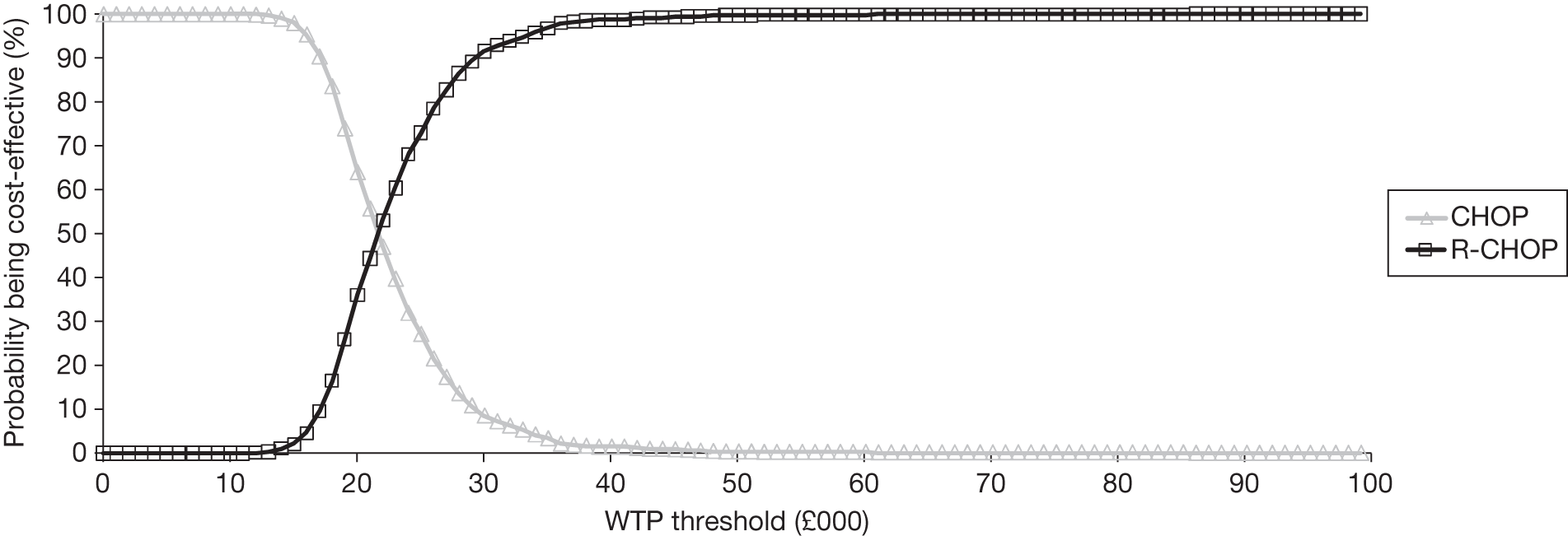
FIGURE 45.
Scenario analysis: CEAC for R-MCP vs MCP alone.
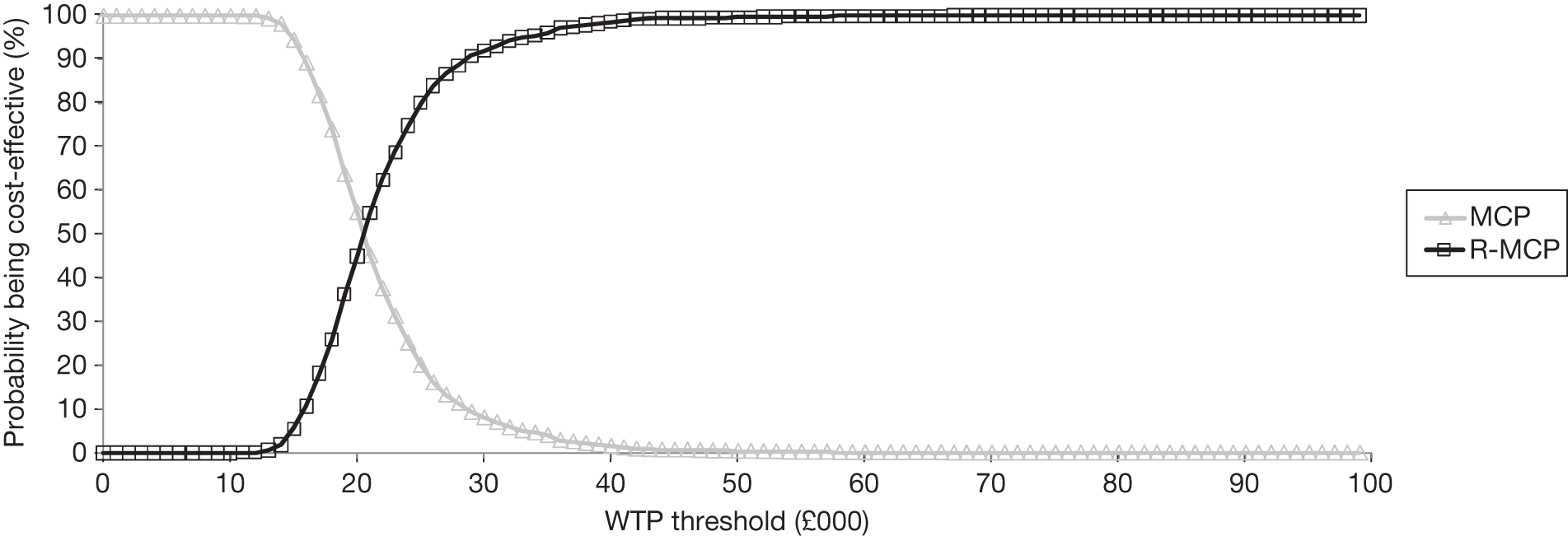
The probabilities of the addition of rituximab to CVP being cost-effective compared with CVP alone are 95.60% and 100.00% assuming a WTP of £20,000 and £30,000 per QALY gained respectively (see Table 75 and Figure 43).
The probabilities of the addition of rituximab to CHOP being cost-effective compared with CHOP alone are 36.00% and 91.50% assuming a WTP of £20,000 and £30,000 per QALY gained, respectively (see Table 76 and Figure 44).
The probabilities of the addition of rituximab to MCP being cost-effective compared with MCP alone are 44.90% and 91.90% assuming a WTP of £20,000 and £30,000 per QALY gained, respectively (see Table 77 and Figure 45).
Univariate sensitivity analyses: impact of main model parameters in the scenario analysis incorporating first-line maintenance in responders to R-chemotherapy
A range of univariate SAs were undertaken to assess the impact of main model parameters and assumption on the cost per QALY gained. A limited number of SAs are presented in the main section of the report for readability. Full results of SAs performed are presented in Appendix 15 for the comparison between R-CVP and CVP, R-CHOP and CHOP and R-MCP and MCP for the scenario analysis.
We explored different time horizons (5 years, 10 years and lifetime). The ICER was sensitive to the assumption about the time horizon with an improvement in the ICER for all comparisons as the time horizon increases (Table 78).
| Time horizon | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case (25 years) | 14,959 | 21,687 | 20,493 |
| 5 years | 54,094 | 91,356 | 80,497 |
| 10 years | 24,126 | 36,367 | 33,482 |
| Lifetime | 14,125 | 20,533 | 19,510 |
Again, the ICER was very sensitive when a Gompertz distribution was used instead of a log-normal distribution (Table 79).
| Distribution | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 14,959 | 21,687 | 20,493 |
| Weibull | 15,958 | 23,824 | 22,833 |
| Gompertz | 9419 | 12,490 | 11,653 |
We also explored different assumptions about the effect of first-line maintenance, varying the HRs using the CIs (0.48 to 0.66) or varying the assumption of the treatment duration effect (36–72 months). Results are presented in Table 80 and showed a modest impact on the cost per QALY gained.
| Length of first-line ;maintenance effect | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 14,959 | 21,687 | 20,493 |
| 36 months | 15,469 | 22,703 | 21,436 |
| 48 months | 14,524 | 20,827 | 19,712 |
| 60 months | 13,828 | 19,478 | 18,470 |
| 72 months | 13,305 | 18,495 | 17,547 |
| HR: 0.48 | 14,205 | 20,051 | 19,063 |
| HR: 0.66 | 16,210 | 24,628 | 23,044 |
As previously mentioned, the effect of rituximab resistance after retreatment with rituximab is unknown. In the base case, we assumed the same rate of progression after rituximab in combination with chemotherapy or salvage therapy in rituximab-naive or rituximab pre-treated patients.
Again, the ICER was very sensitive when a lower effectiveness was assumed in patients previously treated with rituximab (Table 81).
| Reduced effectiveness in previously treated rituximab patients | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 14,959 | 21,687 | 20,493 |
| –10% | 16,851 | 24,447 | 23,067 |
| –15% | 18,100 | 26,301 | 24,788 |
| –20% | 19,650 | 28,629 | 26,946 |
| –25% | 21,624 | 31,646 | 29,731 |
| –30% | 24,234 | 35,734 | 33,489 |
In the base case, a proportion of patients might not progress and remain in PFS1 during the entire simulation because of the parametric extrapolation (Table 82). We examined a scenario in which we truncated the survival curves, assuming that patient can remain in PFS1 only for a maximum duration.
| Maximum time that a patient can stay in PFS1 | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 14,959 | 21,687 | 20,493 |
| 5 years | 31,354 | 61,115 | 60,170 |
| 6 years | 27,043 | 49,043 | 47,647 |
| 7 years | 24,178 | 41,756 | 40,277 |
| 8 years | 22,151 | 36,904 | 35,414 |
| 9 years | 20,651 | 33,528 | 32,065 |
| 10 years | 19,516 | 31,050 | 29,618 |
| 11 years | 18,645 | 29,166 | 27,766 |
| 12 years | 17,951 | 27,698 | 26,330 |
| 13 years | 17,394 | 26,544 | 25,206 |
| 14 years | 16,944 | 25,615 | 24,305 |
| 15 years | 16,577 | 24,869 | 23,580 |
| 16 years | 16,274 | 24,252 | 22,984 |
| 17 years | 16,023 | 23,746 | 22,496 |
| 18 years | 15,815 | 23,326 | 22,089 |
| 19 years | 15,642 | 22,985 | 21,758 |
Again, the ICER was very sensitive to this assumption.
Changes in the treatment pathway were examined given the shortcomings in evidence available. The ICER was sensitive when it was assumed that the same treatment post-progression was used in both arms (Table 83). In clinical practice, it is expected that patients not previously treated with rituximab are more likely to receive rituximab as part of the second-line treatment, and therefore would have a greater benefit in second line.
| Modelled treatment pathway | R-CVP vs CVP | R-CHOP vs CHOP | R-MCP vs MCP |
|---|---|---|---|
| Base case | 14,959 | 21,687 | 20,493 |
| Patients receive second-line after progression only | 16,828 | 21,576 | 20,944 |
| Patients on R-CVP are not retreated with rituximab in second line if early relapse | 15,816 | 21,687 | 20,493 |
| Patients treated with an anthracycline regimen receive CHOP with or without rituximab in second line | 14,959 | 16,517 | 15,261 |
| Older patients receive with or without rituximab in second line | 15,145 | 22,251 | 21,026 |
| Combination of the three previous scenarios | 15,919 | 16,750 | 15,452 |
| All patients receive R-HDT | 18,325 | 20,293 | 18,491 |
| All patients receive HDT | 11,273 | 12,153 | 11,227 |
| All patients receive CHOP | 14,127 | 15,337 | 14,146 |
| All patients receive R-CHOP | 15,034 | 16,436 | 15,111 |
Comparison of the base-case cost-effectiveness for the addition of rituximab to chemotherapy estimated by Assessment Group and estimated by manufacturer
Only results for the base-case analysis are compared as the manufacturer62 did not present a scenario analysis allowing responders to R-chemotherapy in first-line induction to receive first-line maintenance. Greater LYs were estimated by the AG compared with the manufacturer’s estimate (Figure 46).
Similarly, the mean discounted QALYs were usually higher in the AG model compared with the manufacturer’s estimate (Figure 47).
On the other hand, the manufacturer’s estimate of mean discounted management and treatment costs were greater compared with the costs estimated by the AG (Figure 48).
Those differences translated into differences in the ICER estimated by the AG and included in the MS62 (Table 84).
| R therapies vs non-R therapies | AG model | MS61 model |
|---|---|---|
| R-CVP vs CVP | 7720 | 1529 |
| R-CHOP vs CHOP | 10,834 | 5758 |
| R-MCP vs MCP | 9316 | 4861 |
The AG believes that differences in results are explained by the following differences in the modelling approach and assumptions used:
-
The MS62 used time-to-event data from the GLSG-200091,92 and OSHO39 trials93 to model the effectiveness of CHOP/R-CHOP and MCP/R-MCP in first-line induction. However, responders received subsequent therapies (maintenance interferon and SCT) in those trials and therefore the effectiveness is likely to be confounded. The AG used a more conservative approach combining data from the M39021 trial95,96 but response rates from the trials. 91–93 A separate source indicated that median PFS was about 46.7 months in patients with FL treated with R-CHOP in first-line induction. 78 The modelled median PFS using the AG approach was close at about 43 months. The modelled median PFS using the MS62 approach was about 64 months.
-
There were differences in the modelled treatment pathway. The AG model provides a more detailed description of the treatment pathway in patients with FL owing to the flexibility in the model structure. The AG considered the use of salvage therapy (HDT) with or without rituximab in addition to ASCT in patients previously treated with an anthracycline regimen. The AG also considered the use of FC in second-line treatment for patients aged > 65 years. The economic model included in the MS62 assumed that patients can only receive CHOP or R-CHOP in second-line induction. The source of effectiveness in second-line is different between the two economic evaluations.
-
As previously mentioned, there were some errors in the approach used by the manufacturer to model second-line treatment. This included:
-
the derivation of the transition probability
-
the calculation of PPS
-
errors in the estimation of costs in second line.
-
More details are available in Assessment of the manufacturer’s submission.
-
The manufacturer fitted exponential distributions to data in second line from the EORTC 20981 trial. 74,75 However, the distributions did not provide a reasonable fit to the data. The AG used log-normal distribution that provided a better fit to the data.
-
The economic model submitted by the manufacturer missed the time spend in second-line induction treatment. PFS and OS are calculated after induction treatment in second line. The AG model included the time spent at induction treatment. This was possible as the AG modelled the impact of maintenance more accurately by separating responders from non-responders.
-
The AG used a different approach to model the OS in second-line using direct Kaplan–Meier curves for OS. The manufacturer estimated OS derived from PFS and an estimated PPS. However, there were some concerns on the approach used to derived PPS.
-
The AG used different utility values (PFS1 0.805, PFS2 0.805; disease progression 0.7363) compared with the utility values included in the MS61 (PFS1 0.88, PFS2 0.79; disease progression 0.62).
-
The model developed by the AG was also more flexible allowing to track patients over time, requiring less assumptions and therefore providing a more accurate description of outcomes over time.
Summary and conclusions to the cost-effectiveness section
The review of existing economic evaluations,111–113,115 the manufacturer’s model and the economic evaluation carried out by the AG suggests that the addition of rituximab to chemotherapy compared with chemotherapy alone has a cost per QALY gained < £20,000 assuming that responders to R-chemotherapy do not receive first-line maintenance. The ICERs estimated by the AG for the addition of rituximab to CVP, CHOP and MCP is £7720, £10,834 and £9316 per QALY gained, respectively, assuming no first-line maintenance for responders to R-chemotherapy.
The AG presented a scenario analysis incorporating first-line maintenance in responders to R-chemotherapy in first-line induction. The ICER estimated by the AG for the addition of rituximab to CVP, CHOP and MCP is £14,959, £21,687 and £20,493 per QALY gained, respectively, assuming that responders to R-chemotherapy receive first-line maintenance rituximab.
Results are not directly comparable across chemotherapies, as they are selected in clinical practice with regard to factors including age, performance status and disease aggressiveness.
A range of SAs were conducted and suggested that the ICER was sensitive to the assumptions about the time horizon (Table 61 and 78), the parametric extrapolation of evidence in first-line induction (Tables 62 and 79), resistance to rituximab in previously exposed patients (Tables 64 and 81), maximum time a patient can remain progression free after first-line induction (Tables 65 and 82) and the assumed treatment pathway (Table 68 and 83).
There were large uncertainties in the source of effectiveness in the absence of robust evidence. Therefore, the results presented should be interpreted with consideration of the assumption used.
Generalisability
There is no evidence to suggest that the results of the analysis cannot be generalised across all patients who have stage III/IV FL. However, it is noted that patients included in the trials were generally younger than those seen in clinical practice in the UK. Furthermore, despite the AG attempting to provide an accurate description of the treatment pathway in patients with FL, there were considerable uncertainties in the source of effectiveness of treatments used in second line, notably for the effect of salvage therapy in patients previously treated with an anthracycline regimen or the effectiveness in patients previously treated with rituximab in first-line induction. This assessment is based on data involving the following chemotherapeutic agents: CVP, CHOP and MCP. It is not certain that the results can be generalised to other R-chemotherapy regimens and other second/subsequent lines of treatment.There are limitation in the pathway assumed within the model. Although SAs have been undertaken to provide an indication of the effect on the ICER when these assumptions are altered, not all possible second- and third-line therapies have been evaluated.
Strengths and limitations of analysis
The economic evaluation has several strengths compared with previous studies. The modelled treatment pathways in our model incorporates guidance issued by NICE73 for the treatment of patients with FL and tried to provide an accurate description of the treatment pathway observed in clinical practice, whereas other models have not undertaken this in as great a detail. Notably, the economic model takes into account the fact that in clinical practice, patients previously treated with an anthracycline regimen (CHOP, MCP) would be offered alternative treatment with salvage therapy with or without rituximab in addition to ASCT if evidence of response and aged < 65 years and are sufficiently fit. Furthermore, the model evaluates the option that patients who are not in remission (complete or partial) at the end of first-line remission induction treatment with R-chemotherapy or chemotherapy alone are likely to be offered further treatment (second-line treatment) despite the absence of progression as observed in clinical practice.
The model also uses a continuous time method over a traditional Markov process. The continuous time approach confers numerous advantages over the Markov process used in previous cost-effectiveness models, notably in terms of flexibility. The rate of progression can be easily represented by distributions that are time dependent.
There was uncertainty regarding the effectiveness of CHOP and MCP with or without rituximab as first-line induction treatment owing to the confounding effect of maintenance therapy with interferon or SCT for responders in the main trials. 91–93 The AG used data from the M39021 trial95,96 and the response rate from the appropriate trial91–93 and showed that the median predicted PFS for R-CHOP was similar to the median PFS from a separate study. 78
A range of SAs were also conducted. The model considered different assumptions regarding the risk of resistance and maximum time a patient can remain progression free in first-line induction. The model also incorporated the impact of AEs in terms of costs and impairment in quality of life. Although the implementation is simplistic, the conclusion was that these had a limited impact on the results.
Finally, a scenario analysis is also presented incorporating the impact of first-line maintenance among patients responding to first-line induction with rituximab in combination with chemotherapy.
There are several limitations of the study. There were considerable uncertainties in the effectiveness in first-line induction with CHOP, R-CHOP, MCP and R-MCP. The approach used by the AG provided a reasonable fit to R-CHOP when compared with a separate source,78 although this was considered the best approach by the AG there is still uncertainty regarding the applicability of this assumption.
Another limitation relates to the data used to model the risk of progression after second-line treatment. We used data from the EORTC 20981 trial74,75 to model the progression rate for patients treated in second line with CHOP and R-CHOP with or without maintenance rituximab. However, patients were rituximab naive (i.e. not previously treated with rituximab) and therefore results from this study might not be applicable to patients previously treated with rituximab. SAs have been conducted assuming a lower effectiveness for patients previously treated with rituximab and showed that the results were highly sensitive to the assumption about the development of resistance.
Furthermore, we assumed that patients previously treated with an anthracycline regimen (CHOP, MCP) with or without rituximab would be eligible for salvage therapy with or with rituximab in addition to ASCT if there was evidence of response to chemotherapy. However, the effectiveness for patients treated with salvage therapy was extracted from a single study. Biases might have been introduced. The addition of rituximab to salvage therapy was associated with considerable benefit although it was unclear if the magnitude of the observed improvement was owing to the retrospective nature of the study. 134 The study was also conducted in a pre-rituximab era, and therefore patients were not previously exposed to rituximab. It is also unclear from the study the proportion of patients that responded to HDT, the proportion for whom the harvest was successful and the proportion of patients that received ASCT in both arms.
There were also uncertainties regarding the utility values used to describe health states in the economic model. Utility values have been extracted from a single unpublished study. 117,118 The study included 33% patients with stage I/II FL and utility values were presented according to the degree of response to therapy. The applicability of data to populate the economic model was limited because the health states in the economic model did not match health-state categories from the study. However, a range of SAs were conducted and showed a modest impact on the ICER.
Further potential limitation is the use of log-normal distribution to represent the risk of progression in first and second-line treatment. The log-normal distribution is non-monotonic and can have a long tail. In first-line treatment, the log-normal provided a plausible and reasonable fit to the data and was therefore used. The ICER was very sensitive, and became more favourable to rituximab if the Gompertz distribution was used. The AG believed that the log-normal distribution provided a more plausible long-term extrapolation (see Figure 42). The use of log-normal distribution in second-line treatment also hampered the uncertainty analysis, but this disadvantage was outweighed by the better fit of the log-normal distribution to the data compared with other distributions.
The inclusion of first-line maintenance in responders to R-chemotherapy in first-line induction was also modelled in a simplistic manner. The treatment pathway is unknown as not part yet of clinical practice.
Finally, our results are in line with findings from previous cost-effectiveness analyses; that the addition of rituximab to chemotherapy compared with chemotherapy alone (CVP, CHOP and MCP) is likely to have a cost per QALY gained of < £25,000.
Chapter 5 Assessment of factors relevant to the NHS and other parties
The Department of Health’s updated cancer plan, issued in January 2011, has outlined the government’s commitment to providing and expanding patient choice of treatment by 2013/14. This includes:
-
when to have treatment
-
where to have treatment (some treatments can be given in hospital or in the community)
-
which organisation delivers treatment and care
-
which team delivers the treatment, and
-
what form of clinically appropriate treatment to have.
The paper also states that one of the NHS outcomes is to prevent people from dying prematurely and cancer is identified as a specific improvement area. One- and five-year cancer survival rates will be key indicators with regards to meeting this outcome.
No budget impact analysis was undertaken in this assessment report, as clinical experts and the evidence suggests that rituximab is already routinely used alongside CVP in the UK. The addition of rituximab to further chemotherapies is not expected to incur significant costs. There would be minimal additional staff or infrastructure costs.
Chapter 6 Discussion
Statement of principal findings
Four RCTs91–96 comparing rituximab and chemotherapy with chemotherapy alone in untreated, symptomatic stage III–IV patients with FL were identified. Rituximab and chemotherapy compared with chemotherapy alone increased the likelihood of a response to treatment in all four trials, with additional toxicity of limited clinical relevance. In three trials, numbers of CRs were significantly greater in the R-chemotherapy arm when compared with the chemotherapy-alone arm. Over a follow-up period of 4–5 years, R-chemotherapy increased the OS rate compared with chemotherapy alone. Median OS values have not yet been reached in either the intervention or comparator arms in the trials; however, this is not unexpected given the median survival for patients with FL is 8–10 years. 29 The four trials91–96 presented evidence that R-chemotherapy prolonged other clinical outcomes such as response duration, TTF, TTP, TTNT, EFS and DFS.
The ICERs for the addition of rituximab to CVP, CHOP and MCP are £7720, £10,834 and £9316 per QALY gained, respectively, when it was assumed that first-line rituximab maintenance was not used. When it was assumed that patients responding to first-line induction with R-chemotherapy receive first-line maintenance rituximab for up to 2 years, the ICERs increase to £14,959, £21,687 and £20,493 per QALY gained, respectively.
Sensitivity analyses indicated that the ICER was mostly sensitive to the assumptions about the time horizon, the choice of parametric distribution to model the effectiveness in first-line induction, the maximum time a patient can remain progression free, assumptions regarding resistance to rituximab and the modelled treatment pathway.
There were large uncertainties in the source of effectiveness in the absence of robust evidence. Therefore, the results presented should be interpreted with consideration of the assumption used. We have made assumptions, and the appraisal is based on a small set of trials with a great degree of heterogeneity in design and effectiveness. This may limit the generalisability of the findings.
Finally, results are not directly comparable across chemotherapies since they are selected in clinical practice with regard to factors including age, performance status and disease aggressiveness. This assessment is based on data involving the following chemotherapeutic agents: CVP, CHOP, MCP and CHVPi. It is not certain that the results can be generalised to other R-chemotherapy regimens.
Strengths and limitations of the assessment
This assessment provides a systematic review of RCTs comparing rituximab and chemotherapy with chemotherapy alone in the first-line treatment of untreated, symptomatic stage III–IV FL, using the most up-to-date data (more mature data from the GLSG-2000 trial using data from the Buske and Hoster91 presentation at the ASH 2008 conference). We undertook comprehensive searches for trials and are confident that we have not missed any reports of RCTs or other systematic reviews of R-chemotherapy compared with chemotherapy alone.
Previous reviews have been carried out investigating the use of rituximab in FL but have included trials evaluating the use of R-chemotherapy compared with chemotherapy alone in both untreated and patients with relapsed FL. 146–148 These previous reviews present meta-analysed results for ORR, with findings in agreement with our own results, i.e. R-chemotherapy improves response rates when compared with chemotherapy alone. However, the AG believes the response rates from the individual trials to be a more robust estimator of the efficacy of the specific R-chemotherapy regimens than meta-analysed response rates. This is owing to problems with the validity of the meta-analyses, namely the high level of statistical heterogeneity. Ideally, this high level of heterogeneity would be explored further and explained by estimating the predictive distribution of a new study. This was not undertaken in this assessment because of resource constraints.
Data for other outcomes such as OS are compromised in three studies owing to other trial treatments. Longer OS data follow-up would strengthen findings as median OS has not yet been reached in any of the trials.
This assessment provides an indication of the cost-effectiveness of the addition of rituximab to CVP, CHOP and MCP alone in the UK. The results of our model are consistent with the findings from previous cost-effectiveness analyses. The model developed by the AG extends the analysis undertaken in previous economic models in terms of a greater level of detail in the modelled treatment pathway. A wide range of assumptions have also been examined given the high uncertainty in model parameters. However, there are some limitations relating to the sources of data used for the effectiveness in first- and second-line and utility values. Assumptions have been made owing to the confounding effects of other trial treatments within two of the three trials in first-line induction. Data from a single trial have been used to represent the effectiveness for patients treated with salvage therapy with or without rituximab and studies reporting the effectiveness of treatment in second line were conducted in rituximab-naive patients. There were large uncertainties in the source of effectiveness in the absence of robust evidence. Therefore, the results presented should be interpreted with consideration of the assumption used.
Uncertainties
There was uncertainty regarding the effectiveness of CHOP and MCP with or without rituximab as first-line induction treatment owing to the confounding effect of maintenance therapy with interferon or SCT for responders in the main trials. There were also uncertainties about the inclusion of first-line maintenance in responders to R-chemotherapy in first-line induction as no guidance was issued by NICE at the time of writing of the report. Another uncertainty relates to the data used to model the risk of progression after second-line treatment. Furthermore, we also assumed that patients previously treated with an anthracycline regimen (CHOP, MCP) with or without rituximab would be eligible for salvage therapy with or with rituximab in addition to ASCT if there was evidence of response to chemotherapy. However, the effectiveness for patients treated with salvage therapy was extracted from a single study. Biases might have been introduced. Studies reporting the effectiveness of CHOP, R-CHOP and salvage therapy in second-line treatment were conducted in a pre-rituximab era and, therefore, patients were not previously exposed to rituximab. Therefore, results from these studies might not be applicable to patients previously treated with rituximab.
Other relevant factors
Other relevant factors to this assessment report include:
-
The outcome of the NICE appraisal assessing the use of rituximab monotherapy as a first-line maintenance treatment in FL.
-
Whether or not bendamustine becomes licensed for use as a first-line chemotherapy in FL and, if so, whether or not it is subsequently approved by NICE.
Chapter 7 Conclusions
Implications for service provision
The addition of rituximab to CVP, CHOP and MCP is likely to be clinically effective in the first-line treatment of stage III–IV FL. The cost per QALY gained is estimated to be < £25,000 for all scenarios and is considerably lower if first-line rituximab maintenance is not assumed. The main uncertainties in terms of influencing the ICER relate to the effectiveness of rituximab retreatment (i.e. resistance) and the effect of salvage treatment in patients previously treated with anthracycline regimens. The context for care and the mode of delivery are very similar to the comparator therapy, thus there are no major implications that do not also apply to chemotherapy alone.
Suggested research priorities
Future research priorities include:
-
effectiveness of rituximab retreatment (determination of resistance)
-
trials comparing an R-chemotherapy with another R-chemotherapy in populations that are eligible to receive both therapies
-
more studies are required assessing HRQoL in FL using the EQ-5D
-
effectiveness of salvage treatment for patients previously treated with an anthracycline regimen
-
non-confounded data for assessment of first-line treatment
-
effectiveness of therapies in older patients (R-FC/FC)
-
standardisation of time-to-event outcome measures.
Acknowledgements
The following team of clinical advisors gave substantial advice throughout the project and provided peer review of the report:
-
Professor Patrick Chu, Consultant in Haematology, Royal Liverpool University Hospital.
-
Dr Andrew McMillan, Consultant in Haematology, Nottingham University Hospitals NHS Trust.
The authors wish to thank Gill Rooney for her help in preparing and formatting the report, and Professor Adrian Bagust, Dr Matthew Lyttelton, Dr Josh Wright and Dr Eva Kaltenthaler for peer reviewing the report.
Declared competing interests of the clinical advisors/peer reviewers
Dr Andrew McMillan, Consultant in Haematology (Clinical Advisor):
-
Personal pecuniary interest:
-
– financial support from Roche to attend conferences of the American Society of Haematology (ASH), the European Hematology Association (EHA) and the International Conference on Malignant Lymphoma (ICML) over the last years (5+)
-
– honoraria from Roche Advisory Boards (5+)
-
– honoraria from Roche for lectures, for example Satellite Symposia (3+)
-
– honoraria from Napp pharmaceuticals for advisory board for bendamustine hydrochloride.
-
Dr Matthew Lyttelton, Consultant Haematologist, Kettering General Hospital NHS Foundation Trust (commented on a draft version of this report):
-
Personal non-pecuniary interest:
-
– Supported by educational grants from Roche for travel and accommodation at international meetings.
-
Contributions of authors
Diana Papaioannou was the AG lead and undertook the clinical effectiveness review.
Rachid Rafia undertook the cost-effectiveness review and developed the cost-effectiveness model.
Matt Stevenson advised on the cost-effectiveness review and development of the cost-effectiveness model.
John Rathbone helped undertake the clinical effectiveness review.
Helen Buckley-Woods performed the literature searches.
John Stevens provided statistical advice.
About the School of Health and Related Research
The School of Health and Related Research (ScHARR) is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of the public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR Health Technology Assessment programme on behalf of a range of policy-makers, including NICE. ScHARR-TAG is part of a wider collaboration of five units from other regions. The other units are Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsula Technology Assessment Group (PenTAG), University of Exeter; and NHS CRD, University of York.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Cancer Research UK . Cancer in the UK: July 2010 n.d. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@sta/documents/generalcontent/018070.pdf.
- Cancer Research UK . Non-Hodgkin Lymphoma (NHL) Statistics – Key Facts 2010. http://info.cancerresearchuk.org/cancerstats/types/nhl/.
- Office for National Statistics (ONS) . Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2008 2010.
- Cancer Research UK . Non-Hodgkin Lymphoma – UK Mortality Statistics 2010. http://info.cancerresearchuk.org/cancerstats/types/nhl/mortality/.
- Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Pract Res Clin Haematol 2005;18:1-10.
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1998;16:2780-95.
- The Non-Hodgkin’s Lymphoma Classification Project . A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood 1997;89:3909-18.
- Macmillan Cancer Support . Follicular Lymphoma 2009.
- Cancer Research UK . Outlook for Low Grade Lymphomas 2010. www.cancerhelp.org.uk/type/non-hodgkins-lymphoma/treatment/statistics-and-outlook-for-non-hodgkins-lymphoma#low.
- Cancer Research UK . Non-Hodgkin Lymphoma (NHL) Statistics. Cancer Statistics 2010. http://info.cancerresearchuk.org/cancerstats/types/nhl/incidence/.
- Freytes CO, Burzynski JA. Lymphoma Follicular 2009. http://emedicine.medscape.com/article/203268-overview.
- Tan D, Rosenberg SA, Levy R, Lavori R, Tibshiranio R, Hoppe RT, et al. Survival in follicular lymphoma. The Stanford Experience 1960–2003. Blood 2007;110.
- Tan D, Rosenberg SA, Slava B, Levy R, Lavori R, Hoppe RT, et al. Closing the gap: a comparison of observed versus expected survival in follicular lymphoma at Stanford University from 1960–2003. J Clin Oncol 2008;26.
- Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol 2005;23:8447-52.
- Liu Q, Fayad L, Cabanillas F, Hagemeister FB, Ayers GD, Hess M, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas MDAnderson Cancer Center. J Clin Oncol 2006;24:1582-9.
- Welsh Cancer Intelligence &Amp; Surveillance Unit 2008.
- National Cancer Intelligence Network (NCIN) . One, Five and Ten Year Cancer Prevalence 2010.
- Cancer Research UK . Non-Hodgkin Lymphoma – UK Incidence Statistics: Trends over Time 2010.
- Dreyling M. Newly diagnosed and relapsed follicular lymphoma: ESMOClinical Practice Guidelines for diagnosis treatment and follow-up. Ann Oncol. 2010;21:181-3.
- Fisher SG, Fisher RI. The epidemiology of non-Hodgkin’s lymphoma. Oncogene 2004;23:6524-34.
- Serraino D, Salamina G, Franceschi S, Dubois D, La Vecchia C, Brunet JB, et al. The epidemiology of AIDS-associated non-Hodgkin’s lymphoma in the World Health Organization European Region. Br J Cancer 1992;66:912-16.
- Ioachim HL. Neoplasms associated with immune deficiencies. Pathol Annu 1987;22:177-222.
- Kersey JH, Shapiro RS, Filipovich AH. Relationship of immunodeficiency to lymphoid malignancy. Pediatr Infect Dis J 1988;7:S10-12.
- Cartwright RA, McNally RJQ. Epidemiology of non-Hodgkin’s lymphoma. Hematol Oncol 1994;3:1-34.
- Filipovich AH, Mathur A, Kamat D, Shapiro RS. Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer Res 1992;52:S5465-7.
- Skarin AT, Dorfman DM. Non-Hodgkin’s lymphomas: current classification and management. CA Cancer J Clin 1997;47:351-72.
- British Committee for Standards in Haematology . Guidelines on Diagnosis and Therapy: Nodal Non-Hodgkin’s Lymphoma 2003.
- Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood 2004;104:1258-65.
- Horning SJ. Natural history of and therapy for the indolent non-Hodgkin’s lymphomas. Semin Oncol 1993;20:75-88.
- Gallagher CJ, Gregory WM, Jones AE, Stansfeld AG, Richards MA, Dhaliwal, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol 1986;4:1470-80.
- Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:5165-9.
- Olin RL, Kanetsky PA, Ten Have TR, Nasta SD, Schuster SJ, Andreadis C. Determinants of the optimal first-line therapy for follicular lymphoma: a decision analysis. Am J Hematol 2010;85:255-60.
- Coiffier B. First-line treatment of follicular lymphoma in the era of monoclonal antibodies. Clin Adv Hematol Oncol 2005;3:484-91.
- Carbone PP, Meier HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 1971;31.
- Hiddemann W, Buske C, Dreyling M, Weigert O, Lenz G, Unterhalt M. Current management of follicular lymphomas. Br J Haematol 2007;136:191-202.
- Knight C, Hind D, Brewer N, Abbott V. Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation. Health Technol Assess 2004;8.
- Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;110:695-708.
- National Cancer Institute SEER Training 2010. http://training.seer.cancer.gov/lymphoma/abstract-code-stage/morphology/formulation.html.
- Lennert K, Feller AC, Diebold J, Paulli A, Le Torneau A. Histopathology of non-Hodgkin’s lymphomas (based on the updated Kiel Classification). New York, NY: Springer-Verlag; 1992.
- Longo DL. What’s the deal with follicular lymphomas?. J Clin Oncol. 1993;11:202-8.
- Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Leger-Falandry C, Cogliatti S, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol 2005;16:1675-82.
- Luminari S, Federico M. Prognosis of follicular lymphomas. Hematol Oncol 2006;24:64-72.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55.
- Decaudin D, Lepage E, Brousse N, Brice P, Harousseau JL, Belhadj K, et al. Low-grade stage III-IV follicular lymphoma: multivariate analysis of prognostic factors in 484 patients: a study of the groupe d’Etude des lymphomes de l’Adulte. J Clin Oncol 1999;17:2499-505.
- Soubeyran P, Eghbali H, Bonchion F, Trojanic M, Richaudd P, Hoernia B. Low-grade follicular lymphomas: analysis of prognosis in a series of 281 patients. Eur J Cancer 1991;27:1606-13.
- Morrison VA. Non-Hodgkin’s lymphoma in the elderly. Part 1: overview and treatment of follicular lymphoma. Oncology 2007;21:1104-10.
- Warnke RA, Kim H, Fuks Z, Dorfman RF. The coexistence of nodular and diffuse patterns in nodular non-Hodgkin’s lymphomas: significance and clinicopathologic correlation. Cancer 1977;40:1229-33.
- Hicks EE, Rappaport H, Winter WJ. Follicular lymphoma: a re-evaluation of its position in the scheme of malignant lymphoma based on a survey of 253 cases. Cancer 1956;9:792-821.
- Ezdinli EZ, Costello WG, Kucuk O, Berard CW. Effect of the degree of nodularity on the survival of patients with nodular lymphomas. J Clin Oncol 1987;5:413-18.
- Hu E, Weiss LM, Hoppe RT, Horning SJ. Follicular and diffuse mixed small-cleaved and large-cell lymphoma: a clinicopathologic study. J Clin Oncol 1985;3:1183-7.
- Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 2005;106:2169-74.
- Medeiros LJ, Picker LJ, Gelb AB, Strickler JG, Brain SW, Weiss LM, et al. Numbers of host ‘helper’ T cells and proliferating cells predict survival in diffuse small-cell lymphomas. J Clin Oncol 1989;7:1009-17.
- Strickler JG, Copenhaver CM, Rojas VA, Horning SJ, Warnke RA. Comparison of ‘host cell infiltrates’ in patients with follicular lymphoma with and without spontaneous regression. Am J Clin Pathol 1988;90:257-61.
- Federico M, Vitolo U, Zinzani PL, Chisesi T, Clo V, Bellesi G, et al. Prognosis of follicular lymphoma: a predictive model based on a retrospective analysis of 987 cases. Intergruppo Italiano Linfomi. Blood 2000;95:783-9.
- Bastion Y, Berger F, Bryon PA, Felman PF, French M, Coiffier B. Follicular lymphomas: assessment of prognostic factors in 127 patients followed for 10 years. Ann Oncol 1991;2:123-9.
- Romaguera JE, McLaughlin P, North L, Dixon D, Silvermintz KB, Garnsey LA, et al. Multivariate analysis of prognostic factors in stage IV follicular low-grade lymphoma: a risk model. J Clin Oncol 1991;9:762-9.
- Lopez-Guillermo A, Montserrat E, Bosch F, Terol MJ, Campo E, Rozman C. Applicability of the International Index for Aggressive Lymphomas to patients with low-grade lymphoma. J Clin Oncol 1994;12:1343-8.
- Solal-Celigny P, Cahu X, Cartron G. Follicular lymphoma prognostic factors in the modern era: what is clinically meaningful?. Int J Hematol 2010;92:246-54.
- Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009;27:4555-62.
- Morschhauser F, Dreyling M, Rohatiner A, Hagemeister F, Bischof Delaloye A. Rationale for consolidation to improve progression-free survival in patients with non-Hodgkin’s lymphoma: a review of the evidence. Oncologist 2009;14:17-29.
- Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A. Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation. Health Technol Assess 2002;6.
- Roche . MabThera® (rituximab) for the First-Line Treatment of Follicular Non-Hodgkin’s Lymphoma: Submission to the National Institute for Health and Clinical Excellence (NICE) 2010.
- Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin’s lymphomas. N Engl J Med 1984;311:1471-5.
- Martinsson U, Glimelius B, Hagberg H, Sundstrom C. Primarily asymptomatic low-grade non-Hodgkin lymphomas: prediction of symptom-free survival and total survival. Eur J Haematol 1989;43:332-8.
- Brice P, Bastion Y, Lepage E, Brousse N, Haioun C, Moreau P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy prednimustine or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 1997;15:1110-17.
- Ardeshna KM, Smith P, Norton A, Hancock B, WHoskin PJ, MacLennan KA, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet 2003;362:516-22.
- Young RC, Longo DL, Glatstein E, Ihde DC, Jaffe ES, DeVita VT. The treatment of indolent lymphomas: watchful waiting v aggressive combined modality treatment. Semin Hematol 1988;25:11-6.
- National Institute for Health and Clinical Excellence (NICE) . Rituximab for the Treatment of Follicular Lymphoma (technology Appraisal Guidance 110) 2006.
- Heinzelmann F, Ottinger H, Engelhard M, Soekler M, Bamberg M, Weinmann M. Advanced-stage III/IV follicular lymphoma: treatment strategies for individual patients. Strahlenther Onkol 2010;186:247-54.
- Hiddemann W. Rituximab maintenance therapy in follicular lymphoma comes of age. Leukemia Res 2006;30:S1-2.
- Salles G, Seymour JF, Feugier P, Offner F, Lopez-Guillermo A, Bouabdallah R. Rituximab Maintenance for 2 Years in Patients With Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy (Abstract 8004) n.d.
- Rohatiner A, Gregory WM, Peterson E, Borden P, Solal-Celigny P, Hagenbeek A. Meta-Analysis to evaluate the role of interferon in follicular lymphoma. J Clin Oncol 2005;23:2215-23.
- National Institute for Health and Clinical Excellence (NICE) . Rituximab for the Treatment of Relapsed or Refractory Stage III or IV Follicular Non-Hodgkin’s Lymphoma (review of Technology Appraisal Guidance 137) 2008.
- van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 2010;28:2853-8.
- van Oers MH, Klasa R, Marcus R, Wolf H, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood 2006;108:3295-304.
- British Committee for Standards in Haematology (BCSH) . Best Practice in Lymphoma Diagnosis and Reporting 2010.
- British Committee for Standards in Haematology (BCSH) . Best Practice in Lymphoma Diagnosis and Reporting: Specific Disease Appendix 2010.
- Rummel M, Niederle N, Maschmeyer G, Banat A, von Gruenhagen U, Losem C, et al. Bendamustine Plus Rituximab Is Superior in Respect of Progression Free Survival and CR Rate When Compared to CHOP Plus Rituximab As First-Line Treatment of Patients With Advanced Follicular Indolent Mantle Cell Lymphomas: Final Results of a Randomized Phase III Study of the StiL (Study Group Indolent Lymphomas, Germany 2009.
- Multicentric Study . Three Randomized Arms (R-CVP Vs R-CHOP Vs R-FM) for Patients With Stage II-IV Follicular Lymphoma n.d. http://clinicaltrials.gov/ct2/show/NCT00774826.
- First-Line R-CVP vs R-CHOP . Immunochemotherapy for Indolent Lymphoma and R Maintenance. (PLRG4) n.d. http://clinicaltrials.gov/ct2/show/NCT00801281.
- Alkylator Combination In Follicular Lymphoma Immuno-Chemotherapy for Older Patients: A Phase III Comparison of First-Line R-CVP Versus R-FC (previous Acronym: RiCH FLO) n.d. http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=6968.
- Siddhartha G, Vijay P. R-CHOP versus R-CVP in the treatment of follicular lymphoma: a meta-analysis and critical appraisal of current literature. J Hematol Oncol 2009;2.
- National Institute for Health and Clinical Excellence (NICE) . Rituximab for the Treatment of Follicular Lymphoma: NICE Technology Appraisal Guidance 110 2006.
- Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood 1984;63:1424-33.
- European Medicines Agency (EMA) . MabThera: European Public Assessment Report (EPAR)-Product Information: Annex I – Summary of Product Characteristics 2011.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2011.
- British Committee for Standards in Haematology (BCSH) . Follicular Lymphoma 2011.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses The PRISMA Statement. J Clin Epidemiol 2009;62:1006-12.
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCISponsored International Working Group. J Clin Oncol 1999;17:1244-53.
- Centre for Reviews and Dissemination (CRD) . Centre for Reviews and Dissemination Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Buske C, Hoster E, Dreyling M, Forstpointner R, Kneba M, Schmitz N, et al. Rituximab in combination with CHOP in patients with follicular lymphoma: analysis of treatment outcome of 552 patients treated in a randomized trial of the German Low Grade Lymphoma Study Group (GLSG) after a follow up of 58 months. Blood 2008;112.
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide doxorubicin vincristine prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725-32.
- Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, et al. East German Study Group Hematology and Oncology Study . Rituximab added to first-line mitoxantrone chlorambucil prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol 2007;25:1986-92.
- Salles G, Mounier N, de Guibert S, Morschhauser F, Doyen C, Rossi JF, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMSFL2000 study. Blood 2008;112:4824-31.
- Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide vincristine prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008;26:4579-86.
- Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005;105:1417-23.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- A Study of MabThera (Rituximab) in Elderly Patients With Untreated Follicular Non-Hodgkin’s Lymphoma (NHL) n.d. http://clinicaltrials.gov/ct2/show/NCT01144364.
- BNLIRCT of MCD Vs FMD in Follicular NHL n.d. http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=908.
- National Cancer Institute (NCI) . Common Toxicity Criteria (CTC) 2011.
- World Health Organization (WHO) Handbook for reporting results of cancer treatment. WHO offset publication No. 48. Neoplasm 1980;20:37-46.
- Solal-Celigny P, Imrie K, Belch A, Robinson K-S, Cunningham D, Rueda A, et al. (Rituximab) plus CVP chemotherapy for first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma (NHL): confirmed efficacy with longer follow-up. Blood 2005;106.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34.
- European Medicines Agency (EMA) . Assessment Report for MabThera: International Non-Proprietary Name Common Name: Rituximab Procedure No EMEA H C 000165 II 0053 2008.
- Buske C, Hoster E. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood 2006;108:1504-8.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2009.
- Brundage MD, Pater JL, Zee B. Assessing the reliability of two toxicity scales: implication for interpreting toxicity data. J Nat Cancer Inst 1993;85.
- Moher D, Schulz KF, Altman DG. for the Consort Group . The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. Ann Intern Med 2001;134:657-62.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83.
- Eddy DM. Assessing Medical Technologies. Washington, DC: National Academy Press; 1985.
- Ray JA, Carr E, Lewis G, Marcus R. An evaluation of the cost-effectiveness of rituximab in combination with chemotherapy for the first-line treatment of follicular non-Hodgkin’s lymphoma in the UK. Value Health 2010;13:346-57.
- Dundar Y, Hounsome J, McLeod C, Bagust A, Boland A, Davis H, et al. Rituximab for the first line treatment of stage III-IV follicular non-Hodgkin’s lymphoma. London: NICE; 2006.
- Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al. Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma. Health Technol Assess 2009;13:23-8.
- Gomez J, Rios E, Rubio C, Castro Gomez A, Varela C. Pharmacoeconomic analysis of adding rituximab to chemotherapy as first line treatment for patients with advanced follicular lymphoma. Pharmacoeconomics 2010;7:55-67.
- Hornberger J, Reyes C, Lubeck D, Valente N, Hornberger J, Reyes C, et al. Economic evaluation of rituximab plus cyclophosphamide vincristine and prednisolone for advanced follicular lymphoma. Leuk Lymphoma 2008;49:227-36.
- Scottish and Newcastle Lymphoma Group (SNLG) . Database Analysis (Roche Data on File) 2004.
- Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol 2005;23:5019-26.
- Wild D, Pettengell R, Lewis G. Utility Elicitation in Patients With Follicular Lymphoma 2006.
- Wild D, Pettengell R, Lewis G. Utility Elicitation in Patients With Follicular Lymphoma 2005.
- Tolley K, Morgan G, Cartwright R, Williams R. Economic aspects of non-Hodgkin’s lymphoma. London: Office of Health Economics; 1998.
- Guadagnolo BA, Punglia RS, Kuntz KM, Mauch PM, Ng AK. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol 2006;24:4116-22.
- ASP . Drug Pricing Files 2006.
- Hoover D, Crystal S, Kumar R, Sambamoorthi U, Cantor J. Medical expenditures during the last year of life: findings from the 1992–1996 Medicare current beneficiary survey. Health Serv Res 2002;37:1625-42.
- Roche . Rituximab for the First Line Maintenance Treatment of Follicular Non-Hodgkin’s Lymphoma: Roche Submission to the National Institute for Health and Clinical Excellence 2010.
- Ward S, Salzano S, Sampson F, Cowan J. Guidance on Cancer Services: Improving Supportive and Palliative Care for Adults with CANCER. London: NICE; 2004.
- Greenhalgh J, Bagust A, Boland A, Blundell M, Oyee J, Dundar Y, et al. Rituximab for the first-line maintenance treatment of follicular non-Hodgkin’s lymphoma: a single technology appraisal 2012.
- National Institute for Health and Clinical Excellence (NICE) . Rituximab for the Maintenance Treatment of Follicular Non-Hodgkin’s Lymphoma Following Response to First-Line Chemotherapy 2011.
- Johnston A, Bouafia-Sauvy F, Broussais-Guillaumot F, Michallet AS, Traulle C, Salles G, et al. Re-treatment with rituximab in 178 patients with relapsed and refractory B-cell lymphomas: a single instiution case control study. Leuk Lymphoma 2010;51:399-405.
- Coiffier B, Bouafia-Sauvy F, Thieblemont C, Hequet O, Arnaud P, Dumontet C, et al. Rituximab re-treatment in B-cell lymphoma patients: efficacy and toxicity in 59 patients terated in one center. Blood 2002;100.
- Borgerding A, Hasenkamp J, Glass B, Wulf G, Trumper L. Rituximab retherapy in patients with relapsed aggressive B cell and mantle cell lymphoma. Ann Hematol 2010;89:283-9.
- Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, et al. DR-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. AGEL/TAMO study. Haematologica 2008;93:1829-36.
- Weide R, Hess G, Koppler H, Heymanns J, Thomalla J, Aldaoud A, et al. High anti-lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A multicenter phase II study of the German Low Grade Lymphoma Study Group (GLSG). Leuk Lymphoma 2007;48:1299-306.
- National Institute for Health and Clinical Excellence (NICE) . Rituximab for the First-Line Treatment of Stage III-IV Follicular Lymphoma (Review of TA 110): Final Scope. 2010.
- Sebban C, Brice P, Delarue R, Haioun C, Souleau B, Mounier N, et al. Impact of rituximab and/or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA study. J Clin Oncol 2008;26:3614-20.
- Office for National Statistics (ONS) . Interim Life Tables England &Amp; Wales 1980–82 to 2007–09 2010.
- Collet D. Modelling survival data in medical research. London: Chapman & Hall/CRC; 2003.
- Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Trumper L, Reiser M, et al. Combined cyclophosphamide vincristine doxorubicin prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer 2006;107:1014-22.
- Montserrat E, Garcia-Conde J, Vinolas N, Lopez-Guillermo A, Hernandez-Nieto L, Zubizarreta A, et al. CHOP vs. ProMACE-CytaBOM in the treatment of aggressive non-Hodgkin’s lymphomas: long-term results of a multicenter randomized trial. (PETHEMA: Spanish Cooperative Group for the Study of Hematological Malignancies Treatment, Spanish Society of Hematology). Eur J Haematol 1996;57:377-83.
- Forstpointer R, Unterhalt M, Dreyling M, Bock HP, Repp RW, Pott C. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab fludarabine cyclophosphamide and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006;108:4003-8.
- UK Department of Health (DoH) . National Health Service Reference Costs (2009 10) 2011.
- Surrey West Sussex and Hampshire Cancer Network . NHS Clinical Policies & Protocols: 2nd Line Salvage Regimens for Lymphoma. ESHAP 2010. www.swshcn.nhs.uk/healthcare-professionals/clinical-policies-and-protocols/haematology_protocols/2nd-line-salvage-regimens-for-lymphoma/ESHAP.pdf.
- London Specialised Commissioning Group . Adult BMT Costing and Pricing Model Workshop 2009.
- Guest JF, Ruiz FJ, Greener MJ, Trotman IF. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. Eur J Cancer Care 2006;15:65-73.
- Friedlich JD, Cheung MC, Imrie KR, Hales B, Mittmann N, Buckstein R. Discrimination of health states in follicular lymphoma with utilities derived from the EuroQOL EQ5D instrument. Blood 2006;108.
- Pettengell R, Donatti C, Hoskin PJ, Poynton C, Kettle PJ, Hancock BW, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol 2008;19:570-6.
- Gao G, Liang X, Jiang J, Zhou X, Huang R, Chu Z, et al. A systematic review and meta-analysis of immunochemotherapy with rituximab for B-cell non-Hodgkin’s lymphoma. Acta Oncol 2010;49:3-12.
- Schulz H, Bohlius J, Skoetz N, Trelle S, Kober T, Reiser M, et al. Chemotherapy plus rituximab versus chemotherapy alone for B-cell non-Hodgkin’s lymphoma. Cochrane Database Syst Rev 2007;4. 10.1002/14651858.CD003805.pub2.
- Schulz H, Bohlius J, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma. J Nat Cancer Inst 2007;99:706-14.
- Office for National Statistics (ONS) . Mid Year Population Estimates 2008 2010.
- Cancer Research UK . Performance Status 2011. http://cancerhelp.cancerresearchuk.org/about-cancer/cancer-questions/performance-status (accessed 28 June 2012).
Appendix 1 Incidence calculations and data sources for non-Hodgkin’s lymphoma and follicular lymphoma
| Incidence | All | Male | Female |
|---|---|---|---|
| Total populationa | 54,454,800 | 26,782,800 | 27,672,000 |
| NHL casesb | 10,319 | 5534 | 4785 |
| FL casesb | 1869 | 879 | 990 |
| Crude incidence rate NHL per 100,000 ((NHL cases/population) × 100,000) | 18.9 | 20.7 | 17.3 |
| Crude incidence rate FL per 100,000 ((FL cases/population) × 100,000) | 3.4 | 3.3 | 3.6 |
| Incidence | All | Male | Female |
|---|---|---|---|
| Total populationa | 51,464,700 | 25,323,500 | 26,141,200 |
| NHL casesb | 9676 | 5186 | 4490 |
| FL casesb | 1757 | 827 | 930 |
| Crude incidence rate NHL per 100,000 ((NHL cases/population) × 100,000) | 18.8 | 20.5 | 17.2 |
| Crude incidence rate FL per 100,000 ((FL cases/population) × 100,000) | 3.4 | 3.3 | 3.6 |
| Incidence | All | Male | Female |
|---|---|---|---|
| Total populationa | 2,990,100 | 1,459,300 | 1,530,800 |
| NHL casesb | 643 | 348 | 295 |
| FL casesb | 112 | 52 | 60 |
| Crude incidence rate NHL per 100,000 ((NHL cases/population) × 100,000) | 21.5 | 23.8 | 19.3 |
| Crude incidence rate FL per 100,000 ((FL cases/population) × 100,000) | 3.7 | 3.6 | 3.9 |
Appendix 2 Ann Arbor staging system
The standard staging system used for FL is the same as that proposed for Hodgkin’s disease at the Ann Arbor Conference in 1971. It classifies four stages of disease (Table 88).
Each stage of disease is divided into two subsets of patients according to the presence (A) or absence (B) of systemic symptoms. Fever of not evident cause, night sweats and weight loss of > 10% of body weight are considered to be systemic symptoms.
| Stage I | One lymph node region (I), or localised involvement of a single extralymphatic organ or site (IE) |
| Stage II | Two or more lymph node regions on the same side of the diaphragm (II), or localised involvement of a single associated extralymphatic organ or site and its regional nodes with or without other lymph node regions on the same side of the diaphragm (IIE) |
| Stage III | Lymph node regions on both sides of the diaphragm (III), which may also be accompanied by localised involvement of an extralymphatic organ or site (IIIE), by involvement of the spleen (IIIS), or both (IIIE + S) |
| Stage IV | Disseminated (multifocal) involvement of one or more extralymphatic organs with or without associated lymph node involvement, or isolated extralymphatic organ involvement with distant (non-regional) nodal involvement. Involved organs should be designated by subscript letters (P, lung; H, liver; M, bone marrow) |
Appendix 3 Eastern Cooperative Oncology Group performance status
| Grade | ECOG |
|---|---|
| 0 | You are fully active and more or less as you were before your illness |
| 1 | You cannot carry out heavy physical work but can do anything else |
| 2 | You are up and about more than half the day; you can look after yourself but are not well enough to work |
| 3 | You are in bed or sitting in a chair for more than half the day; you need some help in looking after yourself |
| 4 | You are in bed or a chair all the time and need a lot of looking after |
Appendix 4 Deaths in England and Wales (including cancer and non-Hodgkin’s lymphoma deaths)
| Deaths in England and Wales | No. of deaths |
|---|---|
| Cancer deaths in England and Wales in 2008 | 137,831 |
| No. of deaths in England and Wales in 2008 | 509,090 |
| No. of NHL deaths in England and Wales in 2008 | 3978 |
Appendix 5 Literature search strategies
Sample search for clinical effectiveness evidence using a RCT filter in MEDLINE including MEDLINE In-Process & Other Non-Indexed Citations (Ovid):
-
Cyclophosphamide.af.
-
Cyclophosphamide/
-
1 or 2
-
vincristine.af.
-
Vincristine/
-
4 or 5
-
vindesine.af.
-
Vindesine/
-
7 or 8
-
(prednisolone or prednisone).af.
-
Prednisolone/or Prednisone/
-
10 or 11
-
doxorubicin.af.
-
Doxorubicin
-
13 or 14
-
(mitoxantrone or mitozantrone).af.
-
Mitoxantrone/
-
16 or 17
-
(cholorambucil or chlorambucil).af.
-
Chlorambucil/
-
19 or 20
-
fludarabine.af.
-
Bendamustine.af.
-
3 and 6 and 12
-
3 and 15 and 6 and 12
-
3 and 18 and 6 and 12
-
3 and 15 and 9 and 12
-
18 and 21 and 12
-
22 and 3 and 18
-
18 and 22
-
24 or 25 or 26 or 27 or 28 or 29 or 30 or 23
-
(CVP or CHOP or CNOP or CHVP or MCP or FCM or FM).af.
-
31 or 32
-
(rituximab or mabthera or mab thera or rituxan or IDEC-102 or IDEC-C2B8 or Rituksimabi or Rituximabum or anti-CD20 or immunotherapy or 131I-rituximab or rituximab–alliinase conjugate or monoclonal antibod$).af.
-
Antibodies, Monoclonal/
-
33 or 34 or 35
-
(follicular lymphoma or indolent lymphoma or low grade lymphoma or lymphoma or NHL).ti,ab.
-
(Lymphoma$adj5 non-hodgkin$).ti,ab.
-
(follic$adj5 (lymphocyte$or lymphoma$)).ti,ab.
-
Lymphoma, Follicular/
-
Lymphoma, Non-Hodgkin/
-
37 or 38 or 39 or 40 or 41
-
36 and 42
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
44 or 45 or 46 or 47 or 48 or 49 or 50
-
(clinic$adj trial$1).tw.
-
((singl$or doubl$or treb$or tripl$) adj (blind$3 or mask$3)).tw.
-
Placebos/
-
Placebo$.tw.
-
Randomly allocated.tw.
-
(allocated adj2 random).tw.
-
52 or 53 or 54 or 55 or 56 or 57
-
51 or 58
-
Case report.tw.
-
Letter/
-
Historical article/
-
Review of reported cases.pt.
-
Review, multicase.pt.
-
60 or 61 or 62 or 63 or 64
-
59 not 65
-
43 and 66
In addition, searching was undertaken in October to November 2010 to identify literature on chlorambucil and fludarabine using the terms (cholorambucil or chlorambucil).af. or (Chlorambucil/) or (fludarabine).af.) combined with population terms (steps 37–42) and RCT terms (steps 44–66) (using Boolean AND).
Example of economics/cost-effectiveness filter
-
Economics/
-
exp “Costs and Cost Analysis”/
-
economic value of life/
-
exp economics hospital/
-
exp economics medical/
-
economics nursing/
-
exp models economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp budgets/
-
ec.fs.
-
(cost or costs or costed or costly or costing$).tw.
-
(economic$or pharmacoeconomic$or price$or pricing$).tw.
-
quality adjusted life years/
-
(qaly or qaly$).af.
-
or/1–15
Example of quality-of-life filter (combined with population terms only)
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.tw
-
(qaly$or qald$or qale$or qtime$).tw
-
disability adjusted life.tw
-
daly$.tw
-
health status indicators/
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw
-
(euroqol or euro qol or eq5d or eq 5d).tw
-
(hql or hqol or h qol or hrqol or hr qol).tw
-
(hye or hyes).tw
-
health$year$equivalent$.tw
-
health utilit$.tw
-
(hui or hui1 or hui2 or hui3).tw
-
disutili$.tw
-
rosser.tw
-
quality of wellbeing.tw
-
quality of wellbeing.tw
-
qwb.tw
-
willingness to pay.tw
-
standard gamble$.tw
-
time trade off.tw
-
time tradeoff.tw
-
tto.tw
-
or/1–28
Appendix 6 Response criteria for non-Hodgkin’s lymphoma89
Complete response requires the following:
-
Complete disappearance of all detectable clinical and radiographic evidence of disease and disappearance of all disease-related symptoms if present before therapy, and normalisation of those biochemical abnormalities (e.g. LDH) definitely assignable to NHL.
-
All lymph nodes and nodal masses must have regressed to normal size (≤ 1.5 cm in their greatest transverse diameter for nodes of > 1.5 cm before therapy). Previously involved nodes that were 1.1–1.5 cm in their greatest transverse diameter before treatment must have decreased to ≤ 1 cm in their greatest transverse diameter after treatment, or by > 75% in the sum of the products of the greatest diameters (SPD).
-
The spleen, if considered to be enlarged before therapy on the basis of a CT scan, must have regressed in size and must not be palpable on physical examination. Any macroscopic nodules in any organs detectable on imaging techniques should no longer be present. Similarly, other organs considered to be enlarged before therapy owing to involvement by lymphoma, such as liver and kidneys, must have decreased in size.
-
If the bone marrow was involved by lymphoma before treatment, the infiltrate must be cleared on repeat bone marrow aspirate and biopsy of the same site. The sample on which this determination is made must be adequate (≥ 20-mm biopsy core).
Complete response/unconfirmed complete response includes those patients who fulfil criteria 1 and 3 above, but with one or more of the following features:
-
A residual lymph node mass of > 1.5 cm in greatest transverse diameter that has regressed by > 75% in the SPD. Individual nodes that were previously confluent must have regressed by > 75% in their SPD compared with the size of the original mass.
-
Indeterminate bone marrow (increased number or size of aggregates without cytological or architectural atypical).
Partial response requires the following:
-
A decrease of ≥ 50% in the SPD of the six largest dominant nodes or nodal masses.
-
No increase in the size of the other nodes, liver or spleen.
-
Splenic and hepatic nodules must regress by at least 50% in the SPD.
-
With the exception of splenic and hepatic nodules, involvement of other organs is considered assessable and not measurable disease.
-
Bone marrow assessment is irrelevant for determination of a PR because it is assessable and not measurable disease; however, if positive, the cell type should be specified in the report, for example large-cell lymphoma or low-grade lymphoma (i.e. small, lymphocytic small cleaved, or mixed small and large cells).
-
No new sites of disease.
Stable disease is defined as less than a PR but is not progressive disease.
Progressive disease requires the following:
-
An increase of ≥ 50% from nadir in the SPD of any previously identified abnormal node for PRs or non-responders.
-
Appearance of any new lesion during or at the end of therapy.
Appendix 7 List of unobtainable references
-
Agarwal, PK. Monoclonal antibody therapy for low grade follicular lymphoma: rituximab. Ind J Hematol and Blood Transfus 2000;18:71–4.
-
Bilgir O, Atesalan F, Cengiz E, Gediz F. The comparison of fludarabine and CHOP therapies for first line treatment in untreated low and intermediate grade non-Hodgkin lymphomas (NHL). Blood 2000;96:234b.
-
De Acquatella GS. Comparative study of the CHOP protocol versus COP-BLAM I. Revista Inst Nac Cancerol 1993;39:1867–79.
-
Herold M. Phase III randomised study of therapy for low grade non-Hodgkin’s lymphoma in patients with advanced stage: chemotherapy versus chemotherapy plus rituximab. Onkologie 1999;22:4–6.
-
Herold M, Fiedler F, Pasold R, Knauf W, Freund M, Naumann R, et al. Efficacy and toxicity of rituximab plus mitoxantrone, chlorambucil, prednisolone (MCP) versus MCP alone in advanced indolent NHL: interim results of a clinical phase III study of the East German Study Group Hematology/Oncology (OSHO). Blood 2001;98: (Suppl 1): 601.
-
Li TS. Clinical observation of CHOP regimen and CHOEP regimen in the treatment of Non-Hodgkin’s lymphoma. Chin J Cancer Prev Treat 2007;14:1019–20.
-
Lopez-Guillermo A, Garcia-Conde J, Alvarez-Carmona AM, Leon P, Maldonado J, Alcala A, et al. Comparison of chemotherapy CHOP vs CHOP/VIA in the treatment of aggressive non-Hodgkin’s lymphoma: a randomized multicenter study of 132 patients. The PETHEMA group. Program for Study and Therapeutics of malignant hemopathies. Spanish Association of Hematology and Hemotherapy. Med Clin-Barcelona 1998;110:601–4.
-
Lopez-Guillermo A, Garcia Conde J, Alvarez-Carmona AM, Leon P, Maldonado J, Alcala A, et al. Comparison between chemotherapy regimens CHOP vs CHOP/VIA for the treatment of aggressive non-Hodgkin’s lymphomas. Randomized multicenter study on 132 patients. Med Clin-Barcelona 1998;110:601–4.
-
Meusers P, Engelhard M, Bartels H, Binder T, Fülle HH, Görg K, et al. On the unfavourable prognosis of advanced centrocytic lymphoma: Results of a multicentric, randomized, therapeutic study (COP- vs CHOP- protocol). Klin Wochenschr 1989;67:98–9.
-
Moreno-Nogueira JA, Bezwoda WR, MacDonald MM, Moules IK, Akitt LM, Posner LE, et al. Phase III randomized multicenter trial comparing Cyclophosphamide, Adriamycin, Vincristin and Prednisone (CHOP) vs Cyclophosphamide, Mitoxantrone, Vincristin and Prednisone (CNOP) in the treatment of stage II-IV diffused non-Hodgkin and acute lymphomas. Oncología 1988;34.
-
Paul S. Technology evaluation: CpG-7909, Coley. Curr Opin Mol Ther 2003;5:553–9.
-
Prentice AG, Johnson SAN, Harper P, Hewins M, Hewins P, Tyrrell CJ, et al. A randomised, prospective trial of chlorambucil and prednisolone (CP) v mitozantrone, chlorambucil and prednisolone (MCP) in de novo, stage II or more advanced, low-grade non-Hodgkins lymphoma (LG-NHL). B J Haematol 1996;93:74.
-
Remy F, Ribrag V, Mounier N, Haioun C, Salles G, Golfier JB, et al. Preliminary evaluation of efficacy and toxicity of two doses schedules of bortezomib plus R-CHOP regimen in front-line B lymphoma patients. Haematologica 2006;91:68.
-
Solal-Celigny P, Brice P, Brousse N, Caspard H, Bastion Y, Haioun C, et al. Phase II trial of fludarabine monophosphate as first-line treatment in patients with advanced follicular lymphoma: a multicenter study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 1996;14:514–19.
-
Wendling, P. Bendamustine outshines CHOP in advanced lymphomas. Oncol Rep 2010;30.
Appendix 8 List of reports of four included studies
M39021 trial
-
Imrie K, Belch A, Pettengell R, Rueda A, McKendrick J, Solal Celigny P, et al. CVP plus rituximab compared to CVP alone in previously untreated patients with follicular lymphoma: impact of baseline prognostic factors. Ann Oncol 2005;16(Suppl. 5):109–10.
-
Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. An international multi-centre, randomized, open-label, phase III trial comparing rituximab added to CVP chemotherapy or CVP chemotherapy alone in untreated stage III/IV follicular non-Hodgkin’s lymphoma. Blood 2003;102:28a.
-
Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005;105:1417–23.
-
Marcus R, Imrie K, Solal Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008;26:4579–86.
-
Solal-Celigny P, Imrie K, Belch A, Robinson KS, Cunningham D, Rueda A, et al. Mabthera (Rituximab) plus CVP chemotherapy for first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma (NHL): confirmed efficacy with longer follow-up. Blood 2005;106:106A.
GLSG-2000 trial
-
Buske C, Kneba M, Forstpointner R, Schmitz N, Lengfelder E, Schmits R. et al. Combined immuno-chemotherapy (R-CHOP) results in significantly superior response rates and time to treatment failure in first line treatment of patients with advanced stage follicular lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Onkologie 2005;28:37–8.
-
Buske C, Kneba M, Lengfelder E, Pfreundschuh M, Ludwig WD, Graeven U, et al. Front-line combined immuno-chemotherapy (R-CHOP) significantly improves the time to treatment failure and overall survival in elderly patients with advanced stage follicular lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006;108:146–7.
-
Buske C, Hoster E, Dreyling M, Forstpointner R, Kneba M, Schmitz N, et al. Rituximab in Combination with CHOP in patients with follicular lymphoma: analysis of treatment outcome of 552 patients treated in a randomized trial of the German Low Grade Lymphoma Study Group (GLSG) after a follow up of 58 months. Blood 2008;112:Abstract no. 2599.
-
Buske C, Kneba M, Lengfelder E, Pfreundschuh M, Ludwig WD, Graeven U, et al. The first line combined immunochemotherapy R-CHOP prolongs overall survival in elderly patients with advanced stage follicular lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Onkologie 2007;30:70.
-
Buske C, Hoster E. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood 2006;108:1504–8.
-
Buske C, Hoster E, Dreyling M, Hasford J, Unterhalt M, Hiddemann W. The Follicular Lymphoma International Prognostic Index (FLIPI) predicts treatment outcome in patients with advanced stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). Blood 2005;106:272A.
-
Hiddemann W, Dreyling MH, Forstpointer R, Kneba M, Woermann B, Lengfelder E, et al. Combined immuno-chemotherapy (R-CHOP) significantly improves time to treatment failure in first line therapy of follicular lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Blood 2003;102:104a.
-
Hiddemann W, Forstpointner R, Kneba M, Schmitz N, Schmits R, Metzner B, et al. The addition of rituximab to combination chemotherapy with CHOP has a long lasting impact on subsequent treatment in remission in follicular lymphoma but not in mantle cell lymphoma: results of two prospective randomized studies of the German Low Grade Lymphoma Study Group (GLSG). Blood 2004;104:50a.
-
Hiddemann W, Dreyling M, Forstpointner R, Kneba M, Schmitz N, Schmits R, et al. Combined immuno-chemotherapy (R-CHOP) has a long lasting impact on subsequent consolidation in remission in follicular lymphoma but not in mantle cell lymphoma. Ann Oncol 2005;16(Suppl. 5):111.
-
Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725–32.
OSHO-39 trial
-
Herold M, Dolken G, Fiedler F, Franke A, Freund M, Helbig W, et al. Randomized phase III study for the treatment of advanced indolent non-Hodgkin’s lymphomas (NHL) and mantle cell lymphoma: chemotherapy versus chemotherapy plus rituximab. Ann Hematol 2003;82:77–9.
-
Herold M, Pasold R, Srock S, Neser S, Niederwieser D, Neubauer A, et al. Results of a Prospective Randomised Open Label Phase III Study Comparing Rituximab Plus Mitoxantrone, Chlorambucile, Prednisolone Chemotherapy (R-MCP) Versus MCP Alone in Untreated Advanced Indolent Non-Hodgkin’s Lymphoma (NHL) and Mantle-Cell-Lymphoma (MCL). Blood 2004;104:169.
-
Herold M, Haas A, Srock S, Neser S, Al-Ali K, Neubauer A, et al. Addition of Rituximab to First-Line MCP (Mitoxantrone, Chlorambucil, Prednisolone) Chemotherapy Prolongs Survival in Advanced Follicular Lymphoma 4 Year Follow-Up Results of a Phase III Trial of the East German Study Group Hematology and Oncology (OSHO39). Blood 2006;108:147.
-
Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol 2007;25:1986–92.
FL2000 trial
-
Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled on to the GELA-GOELAMS FL-2000 trial. J Clin Oncol 2008;26:440–6.
-
Foussard C, Mounier N, Van Hoof A, Delwail V, Casasnovas O, Deconinck E, et al. Update of the FL2000 randomized trial combining rituximab to CHVP-Interferon in follicular lymphoma (FL) patients (pts). J Clin Oncol: ASCO Annual Meeting Proceedings 2006;24:424.
-
Salles GA, Foussard C, Nicolas M, Franck M, Chantal, D, Thierry L, et al. Rituximab added to aIFN+CHVP improves the outcome of follicular lymphoma patients with a high tumor burden: first analysis of the GELA-GOELAMS FL-2000 randomized trial in 359 patients. Blood 2004;104:a49–50.
-
Salles GA, Mounier N, de Guibert S, Morschhauser F, Doyen C, Rossi J, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: final analysis of the GELA-GOELAMS FL2000 study with a 5-year follow-up. Blood 2007;110:4617–26.
-
Salles G, Mounier N, de Guibert S, Morschhauser F, Doyen C, Rossi JF, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood 2008;112:4824–31.
Appendix 9 List of excluded studies
Excluded studies for head-to-head evidence review (n = 108)
-
No author. A phase III trial of CHOP vs CHOP + rituximab vs CHOP + 1–131 tositumomab for newly diagnosed follicular non-Hodgkin lymphoma. Clin Adv Hematol Oncol 2006;4:536–8.
-
No author. BiovaxlD could prolong remission duration in follicular lymphoma patients in Phase III clinical trial. Immunotherapy 2009;1:522.
-
No author. Clinical studies for patients with indolent non-Hodgkin’s lymphoma. Lymphoma Today 2000;6:10.
-
No author. Improved survival in aggressive NHL. Eur J Cancer 2001;37:1326.
-
No author. Low-grade malignant non-Hodgkin’s lymphoma: initial treatment with the monoclonal antibody rituximab. Deutsche Apotheker Zeitung 2004;144:57–8.
-
No author. Positive results announced from pivotal study of bendamustine in non-Hodgkin’s lymphoma. Oncology 2007;21:1496.
-
No author. Rituxan delays disease progression in indolent non-Hodgkin’s lymphoma. Oncology (Williston Park) 2002;16:1472.
-
No author. Rituximab for initial treatment of indolent non-Hodgkin’s lymphoma. Deutsche Apotheker Zeitung 2004;144:50–1.
-
Ahmad K. Conventional treatment for non-Hodgkin lymphoma for the CHOP. Lancet Oncol 2004;5:266.
-
Alberta Heritage Foundation for Medical Research. Rituxan® (rituximab) (structured abstract). Edmonton, AB: Heritage Foundation for Medical Research; 2000.
-
Al-Salman JS. Successful treatment of gastrointestinal follicular lymphoma with rituxan and combination chemotherapy. Medical Oncology 2001;18:277–83.
-
Anderson T, Bender RA, Fisher RI, DeVita VT, Chabner BA, Berard CW, et al. Combination chemotherapy in non-Hodgkin’s lymphoma: results of long-term followup. Cancer Treat Rep 1977;61:1057–66.
-
Anlan AY. Different chemotherapy protocols for intermediate and high grade malignant non-Hodgkin’s lymphoma. Chin J Oncol 1997;19:215–17.
-
Aviles AD. Combined therapy in advanced stages (III and IV) of follicular lymphoma increases the possibility of cure: results of a large controlled clinical trial. Eur J Haematol 2002;68:144–9.
-
Azim HA, Santoro L, Bociek RG, Gandini S, Malek RA, Azim HA, Jr, et al. High dose intensity doxorubicin in aggressive non-Hodgkin’s lymphoma: a literature-based meta-analysis. Ann Oncol 2010;21:1064–71.
-
Bachy EB. Long-term follow-up of patients with newly diagnosed follicular lymphoma in the prerituximab era: effect of response quality on survival – a study from the groupe d’etude des lymphomes de l’adulte. J Clin Oncol 2010;28:822–9.
-
Badin FH. Rituximab in the treatment of B-cell non-Hodgkin lymphoma, focus on outcomes and comparative effectiveness. Clinicoeconom Outcomes Res 2010;2:37–45.
-
Baltazar S, Tripp G, Baez E, Rivas S, Solis L, Ignacio G, et al. CNOP vs CNOP-rituximab vs rituximab alone as first line therapy for indolent non-Hodgkin lymphoma (INHL): preliminary disease-free/overall survival analysis. The Ninth Congress of the European Hematology Association, Geneva, Switzerland, 10–13 June 2004.
-
Baltazar S, Tripp G, Baez E, Rivas S, Solis L, Ignacio G, et al. CNOP vs CNOP-rituximab vs rituximab alone as first line therapy for indolent non-Hodgkin lymphoma (INHL): preliminary disease-free/overall survival analysis. Hematology J 2004;5:10.
-
Bassi S, Mancuso P, Gigli F, Antoniotti P, Bertazzoni P, Quarna J, et al. Lymphoid subpopulations and safety profile in untreated-follicular lymphoma receiving rituximab monotherapy as induction and maintenance therapy. Blood 2006;108:259.
-
Bjorkholm M, Andersson T, Ahlbom A, Osby E. CNOP (mitoxantrone) chemotherapy is inferior to CHOP (doxorubicin) in the treatment of patients with aggressive non-Hodgkin lymphoma (meta-analysis) (structured abstract). Eur J Haematol 2008;80:477–82.
-
Boettcher S, Pott C, Ritgen M, Hiddemann W, Unterhalt M, Kneba M. Evidence for FcY receptor IIIA-independent rituximab effector mechanisms in patients with follicular lymphoma treated with combined immuno-chemotherapy. Blood 2004;104:a170–1a.
-
Braga P, Carvalho S, Gomes M, Guerra L, Lucio P, Marques H, et al. Health outcomes and costs of rituximab in combination with cyclophosphamide, vincristine and prednisolone in the treatment of patients with advanced follicular lymphoma in Portugal. Blood 2009;114:978–9.
-
Brandt L, Kimby E, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in indolent non-Hodgkin’s lymphoma. Acta Oncol 2001;40:213–23.
-
Brice P, Salles G, Haioun C, Tilly H, Le Gouill S, Foussard C, et al. Long term follow up of 566 patient with follicular lymphoma included from 1986 to 1995 in the GELF86 study before rituximab era. Haematologica 2006;91:383.
-
Buske C, Hoster E, Dreyling MH, Forstpointner R, Kneba M, Schmitz N, et al. Rituximab overcomes sex as a strong adverse prognostic factor for treatment outcome in patients with follicular lymphoma: analysis of patients treated with Rituximab/CHOP or CHOP in randomized trials of the German Low Grade Lymphoma Study Group (GLSG). Blood 2009;114:1427.
-
Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchauser F, et al. The poor prognosis value of high intra-tumoral macrophages counts in follicular lymphoma patients requires selection of appropriate cut-off and can be circumvented by rituximab therapy. Blood 2006;108:247.
-
Chauvergne J, Durand M, Hoerni B, Hoerni-Simon G, Brunet R, Lagarde C, et al. Induction chemotherapy of non-Hodgkin’s malignant lymphomas. Preliminary results of a controlled trial. Eur J Cancer 1977;13:399–400.
-
Cheung MC, Meyer R, Hux J, Evans W, Nefsky M, Imrie KR. Rituximab in lymphoma: a review of a province-wide initiative after one year. Blood 2001;98:a432.
-
Cheung MC, Meyer R, Evans W, Zanke B, Imrie KR. Rituximab for indolent lymphoma: an analysis of outcomes in a large population-wide Study. Blood 2003;102:b505–6.
-
Cheung MC, Haynes AE, Meyer RM, Stevens A, Imrie KR, Members of the Hematology, Disease Site Group of the Cancer Care Ontario Program in Evidence-Based Care. Rituximab in lymphoma: a systematic review and consensus practice guideline from Cancer Care Ontario. Cancer Treat Rev 2007;33:161–76.
-
Chisesi T. Chlorambucil vs chlorambucil + interferon in the therapy of low grade non-Hodgkin’s lymphoma. Ann Hematol 1998;77(Suppl. 1):A23.
-
Coiffier B. First-line treatment of follicular lymphoma in the era of monoclonal antibodies. Clin Adv Hematol Oncol 2005;3:484–91.
-
Dana BW, Dahlber S, Nathwani BN, Chase E, Coltman C, Miller TP, et al. Long-term follow-up of patients with low-grade malignant lymphomas treated with doxorubicin-based chemotherapy or chemoimmunotherapy. J Clin Oncol 1993;11:644–51.
-
DeGrendele HG. Fludarabine/mitoxantrone versus CHOP followed by rituximab consolidation in chemotherapy-naive follicular lymphoma. Clin Lymphoma 2003;4:10–15.
-
Doelken G, Schueler F. Rapid clearance of circulating lymphoma cells from peripheral blood of follicular lymphoma patients treated with chemotherapy and rituximab as compared to patients treated with chemotherapy alone. European Hematology Association. URL: www.ehaweb.org/. 2002.
-
Doorduijn JK, van der Holt B, van der Hem KG, van-Imhoff G, Kramer MHH. Standard CHOP-chemotherapy in elderly patients with intermediate-/high-grade non-Hodgkin’s lymphoma (NHL) has acceptable toxicity. Ann Oncol 1999;10:109.
-
Dreyling M, Forstpointner R, Ludwig W, Gramatzki M, Boeck H, Haenel M, et al. The addition of rituximab to a fludarabine combination (R-FCM) significantly improves remission rates and overall survival in recurrent follicular as well as mantle cell lymphoma – follow up a prospective randomized trial of the German low grade lymphoma study group (GLSG). Ann Oncol 2005;16(Suppl. 5):110–11.
-
Dreyling M, Forstpointner R, Gramatzki M, Bock I, Hanel M, Seymour J, et al. Rituximab maintenance improves progression-free and overall survival rates after combined immuno-chemotherapy (R-FCM) in patients with relapsed follicular and mantle cell lymphoma: final results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol: ASCO Annual Meeting Proceedings 2006;24:422.
-
Fehrenbacher L, Polikoff JA, Hermann R, Jhangiani H, Bjerke J, Loeck D, et al. Rituximab therapy after response to R-CHOP induction therapy for patients with previously untreated indolent Non-Hodgkin’s Lymphoma (NHL): clinical outcomes and pharmacokinetics (PK). Blood (ASH Annual Meeting Abstracts) 2007;110:1295.
-
Feng JJY. Meta-analysis on the efficacy and safety of R-CHOP chemotherapy for the treatment of low and moderate malignant B cell non-Hodgkin lymphoma. J Leuk Lymphoma 2009;18:216–19.
-
Fisher RI. Overview of Southwest Oncology Group Clinical Trials in non-Hodgkin Lymphoma. S0016. A phase III trial of CHOP vs CHOP + rituximab vs CHOP + iodine131-labeled monoclonal anti-B1 antibody (tositumomab) for treatment of newly diagnosed follicular NHL. Clin Adv Hematol Oncol 2005;3:544–6.
-
Freedman AS, Hamlin PA, Neelapu S, Nichols C, Robertson M, Bergier G, et al. Phase III trial of active immunotherapy (FavId, Id/KLH) following rituximab induction therapy: clinical responses in patients (pts) with follicular non-Hodgkin’s lymphoma (fNHL). Blood 2006;108:780.
-
Gao G, Liang X, Jiang J, Zhou X, Huang R, Chu Z, et al. A systematic review and meta-analysis of immunochemotherapy with rituximab for B-cell non-Hodgkin’s lymphoma. Acta Oncol 2010;49:3–12.
-
Gaynor ER, Fisher RI, Gaynor ER, Fisher RI. CHOP therapy: has shorter-course therapy had an impact on lymphomas? Cancer Invest 1998;16:26–32.
-
Genadieva Stavrik S, Siljanovski N, Stojanovik A, Georgievski B, Karanfilski O, Panovska I, et al. Rituximab plus CHOP is superior to standard CHOP as initial treatment for patients with previously untreated aggressive non-Hodgkin’s lymphoma: a single centre experience. 10th Congress of the European Hematology Association, Stockholm, Sweden, 2–5 June 2005.
-
Ghielmini M. Adding rituximab to cyclophosphamide, vincristine and prednisone increases time to treatment failure or progression in people with untreated stage III/IV follicular lymphoma. Cancer Treat Rev 2005;31:644–7.
-
Ghielmini M. Follicular lymphoma: chemotherapy and/or antibodies? Blood 2006;108:3235.
-
Gomez HL, Samanez C, Campana F, Vera L, Leon J, Flores C, et al. A phase II-b trial of amifostine plus CHOP vs CHOP in elderly patients with aggressive non-Hodgkin’s lymphoma. Proc Am Soc Clin Oncol 2003;22:743.
-
Hainsworth JD, Litchy S, Morrissey LH, Andrews MB, Grimaldi M, McCarty M, et al. Rituximab plus short-duration chemotherapy as first-line treatment for follicular non-Hodgkin’s lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2005;23:1500–6.
-
Hamlin, PA. Meta-analysis: making sense of maintenance rituximab? Comments. Curr Oncol Rep 2009;11:329–30.
-
Hensel M, Scheuer L, Salwender H, Finke J, Buerkle D, Duerk HA, et al. How much rituximab do we need: a multicenter, randomized trial comparing 1, 3 or 6 infusions of Rituximab added to 6 cycles of CHOP chemotherapy in untreated patients with advanced follicular non-Hodgkins lymphoma (HD2000-Trial). Blood 2004;104:b230.
-
Herold M, Pasold R, Srock S, Neser S, Niederwieser D, Neubauer A, et al. Rituximab plus mitoxantrone, chlorambucil, prednisolone (R-MCP) is superior to MCP alone in advanced indolent and follicular lymphoma: results of a phase III study (OSHO39). Ann Oncol 2005;16(Suppl. 5):51–2.
-
Hiddemann W, Forstpointner R, Fiedler F, Gramatzski M, Dorken B, Illiger H, et al. Combined immunochemotherapy (R-FCM) is superior to chemotherapy (FCM) alone in recurrent indolent lymphoma: results of a prospective randomized comparison. Eur J Cancer 2001;37:S46.
-
Hiddemann W, Hoster E, Buske C, Dreyling M, Kneba M, Hallek M, et al. Rituximab is the essential treatment modality that underlies the significant improvement in short and long term outcome of patients with advanced stage follicular lymphoma: a 10 year analysis of GLSG trials. Blood 2006;108:147.
-
Hieke K, Herold M. Adding rituximab to standard chemotherapy appears dominant vs chemotherapy alone in advanced stage NHL: interim results from a randomized clinical trial (RCT). Value Health 2005;8:A38.
-
Hieke K, Pasold R, Neser S, Neiderwieser D, Neubauer A, Doelken G, et al. Cost evaluation of Rituximab plus MCP vs MCP alone in advanced stage indolent non-Hodgkin’s-lymphoma based on a randomized controlled multicenter trial. Blood 2004;104:28–29a.
-
Hirt C, Schueler F, Kiefer T, Schwenke C, Herold M, Doelken G. High molecular remission rate in follicular lymphoma patients after combination therapy with rituximab and chemotherapy: clinical significance of quantitative monitoring of minimal residual disease. Onkologie 2005;28:144.
-
Hirt C, Schuler F, Kiefer T, Schwenke C, Haas A, Niederwieser D, et al. Rapid and sustained clearance of circulating lymphoma cells after chemotherapy plus rituximab: clinical significance of quantitative t(14;18) PCR monitoring in advanced stage follicular lymphoma patients. Br J Haematol 2008;141:631–40.
-
Hochster HS, Weller E, Ryan T, Habermann TM, Gascoyne R, Frankel SR, et al. Results of E1496: A phase III trial of CVP with or without maintenance rituximab in advanced indolent lymphoma (NHL). J Clin Oncol: ASCO Annual Meeting Proceedings 2004;22:556.
-
Hunault-Berger MI. Intensive therapies in follicular non-Hodgkin lymphomas. Blood 2002;100:1141–52.
-
Itoh K, Igarashi T, Ogura M, Morishima Y, Hotta T, Kinoshita A, et al. Randomized phase II study of concurrent and sequential combinations of rituximab (R) plus CHOP (R-CHOP) in untreated indolent B-NHL: 7-year follow-up results. J Clin Oncol: ASCO Annual Meeting Proceedings 2008;26:482.
-
Jaeger G, Brezinschek R, Neumeister P, Linkesch W. CHOP-induction and anti-CD20-(mAb-TheraTM) consolidation as first-line treatment of follicular lymphoma. Preliminary data of a phase II study. Onkologie 2000;23:151.
-
Kouroukis CT, Browman GP, Esmail R, Meyer RM. Chemotherapy for older patients with newly diagnosed, advanced-stage, aggressive-histology non-Hodgkin lymphoma: a systematic review (Structured abstract). Ann Intern Med 2002;136:144–52.
-
Ladetto M, Ricca I, Benedetti F, Vitolo U, Patti C, Rambaldi A, et al. Rituximab-supplemented high-dose sequential chemotherapy (HDS) has superior response rate and event-free survival (EFS) compared to R-CHOP in poor risk follicular lymphoma (FL) at diagnosis: results from a multicenter randomized GITMO trial. Proceedings of the 47th Annual Meeting of the American Society of Hematology, Atlanta, GA. 10–13 December 2005. Abstract no. 675.
-
Ladetto M, Ricca I, Benedetti F, Vitolo U, Patti C, Rambaldi A, et al. Rituximab-supplemented high-dose sequential chemotherapy (HDS) has superior response rate and event-free survival (EFS) compared to R-CHOP in poor risk follicular lymphoma (FL) at diagnosis: results from a multicenter randomized GITMO trial. Blood 2005;106.
-
Ladetto M, De Marco F, Benedetti F, Vitolo U, Patti C, Rambaldi A, et al. Clinical and molecular results of the multicenter randomized GITMO-IIL trial in poor risk follicular lymphoma (FL) at diagnosis: rituximab-supplemented high-dose sequential chemotherapy (R-HDS) is superior to CHOP-R in molecular remissions rate, EFS and PFS. Blood 2006;108:101.
-
Lenz G. Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J Clin Oncol 2004;22:4926–33.
-
Leonard, JPR. New Agents in development for Non-Hodgkin’s lymphoma. Semin Hematol 2007;44:S18–21.
-
Maloney DG, Press OW, Braziel RM, Unger JM, LeBlanc ML, Grogan T, et al. A phase II trial of CHOP followed by rituximab chimeric monoclonal anti-CD20 antibody for treatment of newly diagnosed follicular non-Hodgkin’s lymphoma: SWOG 9800. Blood 2001;98:843a.
-
Maloney DG, Pender-Smith B, Unger JM, Braziel RM, Radich J, Press OW, et al. Fc(gamma) receptor polymorphisms do not influence progression free survival (PFS) of follicular NHL patients treated with chop followed by rituximab (SWOG 9800). Blood 2004;104:170A.
-
Marcus R. Use of rituximab in patients with follicular lymphoma. Clin Oncol 2007;19:38–49.
-
Martinelli G, Schmitz S, Hess U, Okkinga E, Stupp R, Taverna C, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol 2010;28:4480–4.
-
Matsumoto K, Maeda Y, Ono Y, Tamiya T, Furuta T, Ohmoto T. Primary malignant lymphoma in the central nervous system treated with high dose methotrexate (MTX)-CHOP (M-CHOP). Noshinkeigeka 1998;26:889–95.
-
McClanahan F, Hielscher T, Rieger M, Hensel M, Bentz M, Schmidt-Wolf I, et al. How much rituximab do we need: a multicenter, randomized trial comparing 1, 3 or 6 infusions of rituximab combined with 6 cycles of CHOP chemotherapy in untreated patients with advanced follicular lymphoma (HD2000-Trial). Blood 2009;114:1051–2.
-
Meckenstock G, Martin S, Kobbe G, Moller P, Bentz M, Rhagavachar A, et al. Fludarabine/mitoxantrone versus high-dose chemotherapy, each combined with CD20-antibody rituximab, as first-line treatment of advanced follicular lymphoma. Onkologie 2002;25:60.
-
Meckenstock G, Martin S, Kobbe G, Moller P, Bentz M, Aul C, et al. Firstline treatment of advanced follicular lymphoma comparing fludarabine/Mitoxantrone and high-dose chemotherapy, each combined with CD20-antibody Rituximab. Onkologie 2003;26:174.
-
Meckenstock G, Kobbe G, Kronenwett R, Martin S, Viardot A, Aul C, et al. First line treatment of advanced follicular lymphoma comparing fludarabine/mitoxantrone and high-dose chemotherapy, each combined with CD20-antibody rituximab. Onkologie 2005;28:153.
-
Mori M, Murai Y, Suzuki K, Nonaka Y, Sato H, Tomiyama J, et al. Study of optimal CHOP dose ranges for elderly patients with non-Hodgkin’s lymphoma. Rinsho Ketsueki 1999;40:199–204.
-
Nagai H, Yano T, Watanabe T, Uike N, Okamura S, Hanada S, et al. Remission induction therapy containing rituximab markedly improved the outcome of untreated mature B cell lymphoma. Br J Haematol 2008;143:672–80.
-
National Horizon Scanning Centre. Rituximab for 1st line low-grade non-Hodgkin’s lymphoma: horizon scanning review (Brief record). Birmingham: National Horizon Scanning Centre; 2004.
-
National Horizon Scanning Centre. Rituximab (Mabthera) for non Hodgkin’s lymphoma: first line maintenance therapy: horizon scanning technology briefing (Project record). Birmingham: National Horizon Scanning Centre; 2009.
-
National Institute for Clinical Excellence (NICE). Rituximab for aggressive non-Hodgkin’s lymphoma (structured abstract). London: NICE; 2003; p. 22.
-
National Institute for Health and Clinical Excellence (NICE). Rituximab for the treatment of follicular lymphoma. London: NICE; 2006. p. 20.
-
Oborilova AS. Fludarabine in treatment of follicular lymphoma. Klinická Onkologie 2003;16:203–10.
-
Psyrri A, Dimopoulos MA, Fountzilas G, Tsatalas C, Anagnostopoulos A, Papageorgiou S, et al. Treatment of low-grade non-Hodgkin lymphomas with fludarabine and mitoxantrone followed by rituximab maintenance: results of a phase II study. Blood 2006;108:B261–2.
-
Rambaldi A, Lazzari M, Manzoni C, Carlotti E, Arcaini L, Baccarani M, et al. Monitoring of minimal residual disease after CHOP and rituximab in previously untreated patients with follicular lymphoma. Blood 2002;99:856–62.
-
Rivas Vera S, Baez E, Sobrevilla CP, Baltazar S, Tripp F, Vela J, et al. Is first line single agent rituximab the best treatment for indolent non-Hodgkin’s lymphoma? Update of a multicentric study comparing rituximab vs CNOP vs rituximab plus CNOP. Blood 2005;106:684.
-
Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Nat Cancer Inst 200;99:706–14.
-
Shen T, Guan ZZ, Shen Z, She Y, Zhu J. Results of multicentre phase IV study of rituximab in combination with CHOP chemotherapy in patients with previously untreated non-Hodgkin’s lymphoma. Blood 2004;104:B239–40.
-
Shen Z, Li J, Wang A, Chen Y. Survival benefits of patients with non-Hodgkin’s lymphoma treated with rituximab combined with chemotherapy. Blood 2004;104:240B.
-
Siddhartha G, Vijay P. R-CHOP versus R-CVP in the treatment of follicular lymphoma: a meta-analysis and critical appraisal of current literature. J Hematol Oncol 2009;2:14.
-
Sonneveld P, van Putten W, Biesma D, Holte H, van-Marwijk KM, Kramer MHH, et al. Phase III trial of 2-Weekly CHOP with rituximab for aggressive B-Cell non-Hodgkin’s lymphoma in elderly patients. Blood 2006;108:66.
-
Stone JM, Page FJ, Laidlaw CR, Cooper I, Stone JM, Page FJ, et al. Selection of patients for randomised trials: a study based on the MACOP-B vs CHOP in NHL study. Aust NZ J Med 1994;24:536–40.
-
Taskinen M, Karjalainen-Lindsberg ML, Leppa S, Taskinen M, Karjalainen-Lindsberg ML, Leppa S. Prognostic influence of tumor-infiltrating mast cells in patients with follicular lymphoma treated with rituximab and CHOP. Blood 2008;111:4664–7.
-
Tripp F, Baez E, Avila E, Baltazar S, Garces O, Reyes G, et al. Efficacy and Safety of Rituximab + CHOP in IR and maintenance therapy with rituximab vs observation in de novo aggressive non-Hodgkin Lymphoma. Blood 2007;110:b193–4.
-
Vose JML. Long-term update of a phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma 2005;46:1569–73.
-
Vose JM, Link BK, Grossbard ML, Czuczman M, Grillo-Lopez A, Benyunes M, et al. Long term follow-up of a Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated aggressive non-Hodgkin’s lymphoma (NHL). Proceedings of the 44th Annual Meeting of the American Society of Hematology. Philadelphia, PA, 2002. Abstract no. 1396.
-
Walewski J, Kraszewska E, Mioduszewska O, Romejko-Jarosinska J, Hellmann A, Czyz J, et al. Rituximab (Mabthera, Rituxan) in patients with recurrent indolent lymphoma: evaluation of safety and efficacy in a multicenter study. Med Oncol 2001;18:141–8.
-
Wilson WH. R-CHOP strikes again with survival benefit in follicular lymphoma. Blood 2005;106:3678–9.
-
Winter JNW. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood 2006;107:4207–13.
-
Winter JN, Weller E, Horning SJ, Krajewska M, Variakojis D, Habermann TM, et al. Rituximab (R) alters the prognostic indicator profile in diffuse aggressive non-Hodgkin lymphomas. Blood 2003;102:a101–2.
-
Wunderlich A, Kloess M, Reiser M, Rudolph C, Truemper L, Bittner S, et al. Practicability and acute haematological toxicity of 2- and 3-weekly CHOP and CHOEP chemotherapy for aggressive non-Hodgkin’s lymphoma: results from the NHL-B trial of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 2003;14:881–93.
-
Xie CL. Efficacy of Rituximab combined with CHOP for B cell non-Hodgkin’s lymphoma. Chin J Clin Oncol 2009;36:1398–400.
-
Xu ZQ. Clinical study of Rituximab combined with CHOP in treatment of B cell non-Hodgkin’s lymphoma. Chin J Cancer Prev Treat 2007;14:1589–90.
-
Zinzani PL. A randomized trial of fludarabine and mitoxantrone plus rituximab versus CHOP plus rituximab as first-line treatment in patients with follicular lymphoma. Blood 2001;98:842a.
-
Zinzani PL, Biosis A. Multicenter randomized trial of fludarabine and mitoxantrone (FM) plus rituximab versus CHOP plus rituximab as first-line treatment in patients with follicular lymphoma (FL). Blood 2002;100:305b.
-
Zinzani PL, Pulsoni A, Perrotti A, Soverini S, Zaja F, De Renzo A, et al. Fludarabine plus mitoxantrone with and without rituximab versus CHOP with and without rituximab as front-line treatment for patients with follicular lymphoma. J Clin Oncol 2004;22:2654–61.
Excluded studies that could potentially inform a network meta-analysis (n = 104)
-
Al-Ismail SA, Whittaker JA, Gough J, Al-Ismail SA, Whittaker JA, Gough J. Combination chemotherapy including epirubicin for the management of non-Hodgkin’s lymphoma. Eur J Cancer Clin Oncol 1987;23:1379–84.
-
Alliot C. Fludarabine versus cyclophosphamide, vincristine, and prednisone in recurrent low-grade lymphomas. J Clin Oncol 2003;21:2626–7.
-
Alliot C. CHOP versus CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2005;23:4797–9.
-
Andersen J, Thorling K, Bentzen SM, Brincker H, Christensen BE, Pedersen M, et al. Phase III trial of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) versus cisplatin, etoposide, bleomycin and prednisone (CisEBP) for the treatment of advanced non-Hodgkin’s lymphoma of high grade malignancy. The Danish Lymphoma Study Group. Acta Oncol 1990;29:995–9.
-
Aurer I, Eghbali H, Raemackers JM, Khaled HM, Van Glabbeke M, Baila L, et al. (R)CHOP versus (R)CHOP: a randomized Phase II study of gemcitabine combined with (R)CHOP in untreated aggressive Non-Hodgkin’s lymphoma – EORTC Lymphoma Group Protocol 20021. Blood 2008;112:1234–5.
-
Ben Shahar M, Gepstein R, Haim N, Polliack A. Phase III trial of a combination of chlorambucil (L) and prednisone (P) with or without mitoxantrone (M) in advanced low grade non-Hodgkin’s lymphoma (NHL) and CLL. Proc Am Soc Clin Oncol 1998;17:20a.
-
Bessell EM, Burton A, Haynes AP, Glaholm J, Child JA, Cullen MH, et al. A randomised multicentre trial of modified CHOP versus MCOP in patients aged 65 years and over with aggressive non-Hodgkin’s lymphoma. Ann Oncol 2003;14:258–67.
-
Bezwoda WR, Rastogi RB. A randomized comparative study of cyclophosphamide, vincristine, doxorubicin, and prednisolone and cyclophosphamide, vincristine, mitoxantrone, and prednisolone regimens in the treatment of intermediate- and high-grade lymphoma with 8 years’ follow-up. Semin Hematol 1994;31:3.
-
Bezwoda W, Rastogi R, Erazo Valla A, Diaz-Maqueo J, Pavlovsky S, Morioka H, et al. Long-term results of a multicentre randomised, comparative phase III trial of CHOP versus CNOP regimens in patients with intermediate- and high-grade non-Hodgkin’s lymphomas. Eur J Cancer 1995;31A:903–11.
-
Bishop JF, Wiernik PH, Kaplan RS. High dose cyclophosphamide, vincristine and prednisone plus or minus adriamycin (CAVP vs CVP) in advanced poorly differentiated lymphocytic and lymphocytic-histiocytic non-Hodgkin’s lymphoma (NHL): a randomized trial. Proc Am Assoc Cancer Res 1981;C-727.
-
Bishop JF, Wiernik PH, Wesley MN, Kaplan RS, Diggs CH, Barcos MP, et al. A randomized trial of high dose cyclophosphamide vincristine and prednisone plus or minus doxorubicin CVP versus CAVP with long-term follow-up in advanced non-Hodgkin’s Lymphoma. Leukemia (Basingstoke) 1987;1:508–13.
-
Bruno S, Corrado C, Santarelli MT, Garay G, Somoza N, Tezanos Pinto M, et al. Randomized trial of cyclophosphamide versus cyclophosphamide, vincristine and prednisone in low grade lymphoma. Proc Am Soc Clin Oncol 1988;7:243, Abstract.
-
Burchardt CA, Brugger W, Maschmeyer G, Kofahl-Krause D, Fischer L, Roller F, et al. Peripheral blood stem cell mobilization after bendamustine containing chemotherapy in indolent lymphomas is possible. Results From the Phase III Study of B-R Vs. CHOP-R (NHL 1–2003 trial) of the StiL (Study group indolent Lymphomas, Germany). Blood 2009;114:1048–9.
-
Burton C, Cunningham D, Qian W, Maclennan K, Hancock B. A phase III trial comparing CHOP to PMITCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin’s lymphoma. Ann Oncol 2005;16(Suppl. 5):103.
-
Burton C, Smith P, Vaughan-Hudson G, Qian W, Hoskin P, Cunningham D. Comparison of CHOP versus CIOP in good prognosis younger patients with histologically aggressive non-Hodgkin lymphoma. Br J Haematol 2005;130:536–41.
-
Burton C, Linch D, Hoskin P, Milligan D, Dyer MJ, Hancock B, et al. A phase III trial comparing CHOP to PMitCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin’s lymphoma. Br J Cancer 2006;94:806–13.
-
Carrato A, Koziner B, Wong G, Lowenthal DA, Chapman D, Little C, et al. Randomized comparison of CHOP vs BLEO-CHOP for the treatment of non-Hodgkins lymphoma: a five year update. Proc Am Soc Clin Oncol 1987;6:198.
-
Chen SF, Chen GQ, Lao Y, Zhang JZ, Li ZQ, Wang WR, et al. Clinical comparison of ProMACE-CytaBOM and CHOP regimen for the treatment of Non-Hodgkin’s lymphoma. Chin J Cancer 1997;16:438–40.
-
Cooper IA, Wolf MM, Robertson TI, Fox RM, Matthews JP, Stone JM, et al. Randomized comparison of MACOP-B with CHOP in patients with intermediate-grade non-Hodgkin’s lymphoma. The Australian and New Zealand Lymphoma Group. J Clin Oncol 1994;12:769–78.
-
Dell’Olio M, Bodenizza C, Carella AM, Falcone A, Greco MM, La Sala A, et al. R-COMP vs R-CHOP in the treatment in newly diagnosed elderly patients with aggressive non-Hodgkin’s lymphoma. Haematologica 2007;92:471.
-
Diggs CH, Wiernik PH. Cyclophosphamide, vinristine, and prednisone (CVP) vs cyclophosphamide, adriamycin, vincristine and prednisone (CAVP) for disseminated non-Hodgkin’s lymphomas. Proc Am Assoc Cancer Res 1977;16:288.
-
Ding Y, Huabang D, Yuhua F, Xiaoyu F, Zhijiu L, Yuhua K. A randomised study of comparison of regimen ABVD vs CHOP in patients with intermediate and high grade lymphoma. Ann Oncol 1999;10:105.
-
Economopoulos T, Dimopoulos MA, Mellou S, Pavlidis N, Samantas E, Nicolaides C, et al. Treatment of intermediate- and high-grade non-Hodgkin’s lymphoma using CEOP versus CNOP. Eur J Haematol 2002;68:135–43.
-
Ezzat A, Ibrahim E, Stuart R, Ajarim D, Bazarbashi S, El-Foudeh M, et al. Adding high-dose tamoxifen to CHOP does not influence response or survival in aggressive non-Hodgkin’s lymphoma: an interim analysis of a randomized phase III trial. Med Oncol 2000;17:39–46.
-
Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993;328:1002–6.
-
Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. A phase III comparison of CHOP vs m-BACOD vs ProMACE-CytaBOM vs MACOP-B in patients with intermediate- or high-grade non-Hodgkin’s lymphoma: results of SWOG-8516 (Intergroup 0067), the National High-Priority Lymphoma Study. Ann Oncol 1994;5(Suppl. 2):91–5.
-
Fisher RI. Cyclophosphamide, doxorubicin, vincristine, and prednisone versus intensive chemotherapy in non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol 1997;40:S42–6.
-
Foussard C, Deconinck E, Desablens B, Ghandouri C, Milpied N, Vilque JP, et al. A randomized trial of fludarabine, mitoxantrone (FM) versus doxorubicin, cyclophosphamide, vindesine, prednisone (CHEP) as first line treatment in patients with advanced low-grade non- Hodgkin lymphoma (LG-NHL): a multicenter study by Goelams Group. Blood 1998;92:316.
-
Foussard C, Deconinck E, Desablens B, Ghandour C, Lucas V, Pignon B. Fludarabine – mitoxantrone combination: preliminary results of a randomized trial versus m-chef as first line treatment in patients with advanced low-grade non-Hodgkin lymphoma by Goelams group. Ann Oncol 1999;10:127.
-
Foussard C, Deconninck E, Desablens B, Maisonneuve H, Gressin R, Pignon B, et al. Improved response after fludarabine, mitoxantrone (FM) in first line treatment of patients with advanced low-grade non-Hodgkin’s lymphoma (LG-LNH) as compared to prednisone, doxorubicin, vindesine, cyclophosphamide (CHEP): a randomized trial by Goelams Group. Proc Am Soc Clin Oncol 2001;20(Pt 1):280a.
-
Foussard C, Colombat P, Maisonneuve H, Berthou C, Gressin R, Rousselet MC, et al. Long-term follow-up of a randomized trial of fludarabine-mitoxantrone, compared with cyclophosphamide, doxorubicin, vindesine, prednisone (CHVP), as first-line treatment of elderly patients with advanced, low-grade non-Hodgkin’s lymphoma before the era of monoclonal antibodies. Ann Oncol 2005;16:466–72.
-
Fridrik MA, Hausmaninger H, Greil R, Lang A, Drach J, Fortelny A, et al. Cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) versus cyclophosphamide, epirubicin, vincristine, prednisolone, ifosfamide, VP-16, methotrexate, dexamethasone (CEOP/IMVP-Dexa) for aggressive non-Hodgkin’s lymphoma. A multicenter randomized trial of the Austrian working party medical oncology. Blood 1999;94:261b.
-
Fridrik MA, Hausmaninger H, Greil R, Lang A, Drach J, Fortelny A, et al. Cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) versus cyclophosphamide, epirubicin, vincristine, prednisolone, ifosfamide, VP-16, methotrexate, dexamethasone (CEOP/IMVP-Dexa) for aggressive non-Hodgkin’s lymphoma. A multicenter randomized trial of the Austrian working party medical oncology. Proceedings of the 44th Annual Meeting of the American Society of Hematology, Philadelphia, PA, 7–10 December 2002. Abstract no. 3065.
-
Gams RA, Rainey M, Dandy M, Bartolucci AA, Silberman H, Omura G, et al. Phase III study of BCOP v CHOP in unfavorable categories of malignant lymphoma: a Southeastern Cancer Study Group trial. J Clin Oncol 1985;3:1188–95.
-
Hagberg H, Bjorkholm M, Glimelius B, Lindemalm C, Mellstedt H, Killander A, et al. CHOP vs MEV for the treatment of non-Hodgkin’s lymphoma of unfavourable histopathology: a randomized clinical trial. Eur J Cancer Clin Oncol 1985;21:175–9.
-
Hagenbeek A, Eghbali H, Monfardini S, Resegotti E, Hoskin J, deWolf Peeters C, et al. Fludarabine versus conventional CVP chemotherapy in newly diagnosed patients with stages III and IV low grade malignant non-Hodgkin’s lymphoma. Preliminary results from a prospective, randomized phase III clinical trial in 381 patients. Blood 1998;92:315a.
-
Hagenbeek A, Eghbali H, Monfardini S, Resegotti E, Hoskin J, deWolf Peeters C, et al. Fludarabine compared with CVP chemotherapy in newly diagnosed patients with stages III and IV low grade malignant non- Hodgkin’s lymphoma. Final analysis of a prospective randomized phase III intergroup study in 381 patients. Blood 2001;98:843a.
-
Hagenbeek A, Eghbali H, Monfardini S, Vitolo U, Hoskin PJ, de Wolf Peeters C, et al. Phase III intergroup study of fludarabine phosphate compared with cyclophosphamide, vincristine, and prednisone chemotherapy in newly diagnosed patients with stage III and IV low-grade malignant non-Hodgkin’s lymphoma. J Clin Oncol 2006;24:1590–6.
-
Hou MY. Comparison of short-term effect of ProMACE-CytaBOM regimen with CHOP regimen for non-Hodgkin’s lymphoma. Chin J Clin Oncol 1995;22:857–9.
-
Hou M, Li L, Qiu M, Yan X, Zhu J, Gou HF, et al. Clinical randomized comparative trial of ProMACE-CytaBOM regimen and CHOP regimen in treating non-Hodgkin’s lymphoma. Aizheng 2005;24:461–4.
-
Jerkeman M, Anderson H, Cavallin-Stahl E, Dictor M, Hagberg H, Johnson A, et al. CHOP versus MACOP-B in aggressive lymphoma: a Nordic Lymphoma Group randomised trial. Ann Oncol 1999;10:1079–86.
-
Kimby E, Bjorkholm M, Gahrton G, Glimelius B, Hagberg H, Johansson B, et al. Chlorambucil/prednisone vs CHOP in symptomatic low-grade non-Hodgkin’s lymphomas: a randomized trial from the Lymphoma Group of Central Sweden. Ann Oncol 1994;5(Suppl. 2):67–71.
-
Kitamura K, Mori M, Masuda M, Hotta T, Miyazaki T, Miura R, et al. Improved survival with THP-COP compared to CHOP for non-Hodgkin’s lymphoma in the elderly: a Japanese cooperative study. Blood 1994;84:164.
-
Klasa R, Meyer R, Shustik C, Sawka C, Smith A, Grenier JF, et al. Fludarabine versus CVP in previously treated patients with progressive low grade non-Hodgkin’s lymphomas (lg-NHL). Proc Am Soc Clin Oncol 1999;18:9a.
-
Koenig HJ, Mueller G. A randomized trial of chop versus Ceop e Equals Epirubicin in intermediate and high grade non-Hodgkin’s lymphoma Nhl. J Cancer Res Clin Oncol 1990;116:613.
-
Koppler H, Pfluger KH. CHOEP versus alternating hCHOP/IVEP for high grade non-Hodgkin’s lymphomas: a randomized multicentre trial. Onkologie 1991;14:181–4.
-
Koppler H, Pfluger KH, Eschenbach I, Pfab R, Bruntsch U, Zeller W, et al. Sequential versus alternating chemotherapy for high-grade non-Hodgkin’s lymphomas: preliminary results of a phase III multicentre trial. Onkologie 1990;13:28–32.
-
Koppler H, Pfluger KH, Eschenbach I, Pfab R, Birkmann J, Zeller W, et al. Sequential versus alternating chemotherapy for high grade non-Hodgkin’s lymphomas: a randomized multicentre trial. Hematol Oncol 1991;9:217–23.
-
Kruglova GV, Finogenova IA. COP vs CHOP in aggressive non-Hodgkin’s lymphomas (NHL). Ann Hematol 1998;77(Suppl. 1):A30.
-
Liang R, Todd D, Chan TK, Chiu E, Lie A, Ho F, et al. COPP chemotherapy for elderly patients with intermediate and high grade non-Hodgkin’s lymphoma. Hematol Oncol 1993;11:43–50.
-
Lorusso V, Palmieri G, Bianco AR, Abate G, Catalano G, De Vita F, et al. CEOP-B/VIMB vs promace-CytaBOM in the treatment of intermediate or high grade non-Hodgkin’s lymphoma: a randomised multicenter study of Southern Italy Cooperative Group. Int J Oncol 2000;16:149–54.
-
Luminari S. The GISL HD2000 trial comparing ABVD with BEACOPP and with CEC as initial treatment of patients with advanced Hodgkin lymphoma. Am J Hematol Oncol 2009;8.
-
Meusers P, Engelhard M, Bartels H, Binder T, Fulle HH, Gorg K, et al. Multicenter randomized therapeutic study for advanced centrocytic lymphoma: COP vs CHOP regimen. Blut 1988;57:222.
-
Meusers P, Engelhard M, Bartels H, Binder T, Fulle HH, Gorg K, et al. Multicentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosis. Hematol Oncol 1989;7:365–80.
-
Meyer RM, Benger A, Browman G, Bryant D, Frank G, Leber B, et al. Randomized phase II comparison of standard CHOP with weekly ‘CHOP’ for elderly patients with intermediate grade lymphoma (IGL). Proc Am Soc Clin Oncol 1994;13:369.
-
Meyer RM, Benger A, Findlay B, Friedman E, Haines P, Leber B, et al. A randomized phase II comparison of CHOP vs CHOP + etoposide (CHOPE) in patients with intermediate grade lymphoma. Proc Am Soc Clin Oncol 1998;17:32a, Abstract.
-
Meyer RM, Browman GP, Samosh ML, Benger AM, Bryant-Lukosius D, Wilson WE, et al. Randomized phase II comparison of standard CHOP with weekly CHOP in elderly patients with non-Hodgkin’s lymphoma. J Clin Oncol 1995;13:2386–93.
-
Miller TP, Dalberg S, Cassady JR, Spier C, Grogan TM, Carlin S, et al. Superiority of a brief course of chop plus radiotherapy (RT) over chop alone in localized unfavorable non-Hodgkin’s lymphoma (NHL) is seen in lowest risk subgroups: a southwest oncology group study. Ann Oncol 1996;7:23.
-
Mishima Y, Sugimura N, Matsumoto-Mishima Y, Terui Y, Takeuchi K, Asai S, et al. An imaging-based rapid evaluation method for complement-dependent cytotoxicity discriminated clinical response to rituximab-containing chemotherapy. Clinical Cancer Research 15–5–2009;15:3624–32.
-
Monfardini S, Tancini G, DeLena M. Cyclophosphamide, vincristine, and prednisone (CVP) versus adriamycin, bleomycin, and prednisone (ABP) in stage IV non Hodgkin’s lymphomas. Med Pediatr Oncol 1977;3:67–74.
-
Montoto S. High clinical and molecular response rates with fludarabine, cyclophosphamide and mitoxantrone in previously untreated patients with advanced stage follicular lymphoma. Haematologica 2008;93:207–14.
-
Montoto S, Lopez-Guillermo A, Domingo-Domenech E, Ribera J, Altes A, Estany C, et al. High clinical and molecular response rates with fludarabine, cyclophosphamide and mitoxantrone (FCM) in previously untreated patients with advanced stage follicular lymphoma (FL). Blood 2003;102:401a.
-
Mori M, Miura AB. Study of optimal CHOP dose ranges for elderly patients with non-Hodgkin’s lymphoma. Rinsho Ketsueki 1999;40:199–204.
-
Mori M, Kitamura K, Masuda M, Hotta T, Miyazaki T, Miura AB, et al. Long-term results of a multicenter randomized, comparative trial of modified CHOP versus THP-COP versus THP-COPE regimens in elderly patients with non-Hodgkin’s lymphoma. Int J Hematol 2005;81:246–54.
-
Morishima Y, Ogura M, Itoh K, Ohtsu T, Sasaki Y, Kasai M, et al. A Randomized Comparison of PBSC Mobilization By Lenograstim During Biweekly CHOP Vs High-CHOP in Pts with Non-Hodgkin’s Lymphoma. Proc Am Soc Clin Oncol 1999;18:63a-
-
Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Truemper L, Reiser M, et al. Anthracyclin-containing regimens (CHOP) achieve higher response rates but not superior overall survival in follicular and mantle cell lymphoma. Ann Oncol 2005;16(Suppl. 5):151–2.
-
Nickenig C, Buske C, Dreyling M, Kneba M, Lengfelder E, Pfreundschuh M, et al. Immune chemotherapy (R-CHOP) significantly improve the time until therapy failure as well as total survival in older patients with follicular lymphoma – GLSG study results. Med Klinik 2007;102:35.
-
Niitsu N, Umeda M. Prognostic factors of follicular lymphoma treated with combination chemotherapy. Rinsho Ketsueki 1997;38:496–504.
-
Ogura M, Morishima Y, Kagami Y, Watanabe T, Itoh K, Igarashi T, et al. Randomized phase II study of concurrent and sequential rituximab and CHOP chemotherapy in untreated indolent B-cell lymphoma. Cancer Sci 2006;97:305–12.
-
Ohnoshi T, Hayashi K, Ueno K, Murashima M, Tada A, Mizuta J, et al. Chemotherapy of aggressive non-Hodgkin’s lymphomas: a retrospective analysis of a single institution. Rinsho Ketsueki 1989;30:1201–4.
-
Oriuchi N, Higuchi T, Endo K, Tsukamoto N, Matsuda H, Kuji I, et al. Application of 18F-FDG PET for the assessment of early response to the treatment and prognosis of patients. Kaku Igaku 2009;46:96–9.
-
Pan D, Qin J, Farber C, Zelenetz AD, O’Brien JP, Portlock CS. Intensification with high dose cyclophosphamide following CHOP offers no significant benefit compared to CHOP alone as initial therapy for indolent non-Hodgkin’s lymphomas. Proc Am Soc Clin Oncol 2001;20(Pt 2):226b.
-
Pan D, Qin J, Farber C, O’Brien J, Filippa D, Portlock CS, et al. CHOP with high dose cyclophosphamide consolidation versus CHOP alone as initial therapy for advanced stage, indolent non-Hodgkin’s lymphomas. Leuk Lymphoma 2003;44:967–71.
-
Pan D, Chen P, Cai XJ. The studies on clinical efficacy of biweekly regimen of CHOP chemotherapy on non-Hodgkin’s malignant lymphoma. Chin J Clin Oncol 2006;33:455–7.
-
Pavlovsky S, Erazo A, Diaz-Maqueo JC, Somoza N, Cervantes G, Garay G, et al. A randomized trial of CHOP versus CNOP (n = Mitoxantrone) in intermediate and high grade non-Hodgkin’s lymphoma. Proc Am Soc Clin Oncol 1989;8:258.
-
Pavlovsky S, Santarelli MT, Erazo A, Diaz Maqueo JC, Somoza N, Lluesma Gonalons M, et al. Results of a randomized study of previously-untreated intermediate and high grade lymphoma using CHOP versus CNOP. Ann Oncol 1992;3:205–9.
-
Pavlovsky S, Milone G, Somoza N, Corrado C, Magnasco H, Cerutti I, et al. [Comparative study of 1st generation versus 2d generation (CAVPE) combinations in intermediate and high-grade lymphomas.] Sangre 1993;38:17–23.
-
Portlock CS, Rosenberg SA, Glatstein E, Kaplan HS. Stage IV non-Hodgkin’s lymphoma: a prospective trial comparing CVP (Cyclophosphamide, Vincristine, Prednisone) alone vs split course CVP with total lymphoid irradiation (CVP-RAD) vs continuous single alkylating agent (SA). Proc Am Assoc Cancer Res 1975;16:266.
-
Prentice AG, Johnson SA, Harper P, Ewings M, Ewings P, Tyrell CJ, et al. A trial of chlorambucil and prednisolone vs mitoxantrone, chlorambucil and prednisolone in non-Hodgkin’s lymphoma (NHL) of working formulation (WF) classes A to F at stage II or greater. Blood 1997;90:249a.
-
Rolski J, Koralewski P, Pawlicki M, Rolski J, Koralewski P, Pawlicki, M. Comparison of effectiveness of adriamycin and epirubicin administered in the CHOP protocol to patients with malignant non-Hodgkin’s lymphoma. Wiad Lek 1993;46:356–9.
-
Sonneveld P, van der Lelie H, Nieuwenhuis K, Schouten H, de Rider M, Hop W, et al. Phase III study of doxorubicin (CHOP) compared with mitoxantrone (CNOP) in elderly patients with intermediate/high risk non-Hodgkin’s lymphoma. Blood 1994;84:384a.
-
Sonneveld P, de Ridder M, van der Lelie H, Nieuwenhuis K, Schouten H, Mulder A, et al. Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin’s lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol 1995;13:2530–9.
-
Stuart R, Ezzat A, Ibrahim E, Ranginwala M, Ajarim D, El Foudeh M, et al. CHOP versus CHOP+high-dose tamoxifen in the management of aggressive non-Hodgkin’s lymphoma: Interim analysis of a prospective randomized trial. Proc Am Soc Clin Oncol 1999;18:38a.
-
Takagi T, Sampi K, Sawada U, Sakai C, Oguro M, Takagi T, et al. A comparative study of CHOP versus MEVP (mitoxantrone, etoposide, vindesine, prednisolone) therapy for intermediate-grade and high-grade non-Hodgkin’s lymphoma: a prospective randomized study. Int J Hematol 1993;57:67–71.
-
Thyss A, Sotto JJ, Mari V, Rossi JF, Fabbro M, Maisonneuve H. Aggressive non-Hodgkin’s lymphoma in the elderly: Early results of a phase III study comparing CHOP-like regimen and MEMID. Ann Oncol 1999;10:59.
-
Thyss A, Gressin R, Foussard C, Lepeu G, Fabbro M, Delwail V, et al. Phase III study of CHOP-like regimen compared with MEMID in elderly patients with aggressive Hodgkin’s lymphoma: preliminary results. Blood 1998;92:622a.
-
Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. A randomized comparison of ACVBP and CHOP in the treatment of advanced aggressive non-Hodgkin’s lymphoma: the LNH93–5 study. Blood 2000;96:832a.
-
Tilly H, Mounier N, Coiffier B, Bouabdallah R, Casasnovas O, Reman O, et al. ACVBP regimen vs CHOP in the treatment of advanced aggressive non-Hodgkin’s lymphoma (NHL). Results of the LNH93–5 study with a median follow-up of 5 years. Proc Am Soc Clin Oncol 2003;22:574.
-
Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood 2003;102:4284–9.
-
Tilly H, Mounier N, Coiffier B, Casasnovas O, Boubdallah R, Reman O, et al. Superiority of intensive conventional chemotherapy ACVBP over CHOP in advanced aggressive non-Hodgkin’s lymphoma (NHL). Results of the CELA study LNH93–5 with a median follow-up of 5 years. Eighth Congress of the European Hematology Association, Lyon, France. 13–15 June, 2003.
-
Tirelli U, Carbone A. 20-year experience on malignant lymphomas in patients aged 70 and older at a single institute. Crit Rev Oncol Hematol 2001;37:153–8.
-
Tirelli U, Van Glabbeke M, Teodorovic I, Thomas J, Bron D, Kluin-Nelemans JC, et al. A randomized comparison of CHOP vs a new combination of VP-16, mitoxantrone and prednimustine (VMP) in patients (pts) more than 70 years of age with intermediate-high grade NHL: an EORTC lymphoma group study. Proc Am Soc Clin Oncol 1995;14:398, Abstract.
-
Tirelli U, Van Glabbeke M, Teodorovic I, Rosti G, Zagonel V, Monfardini S. A randomized comparison of CHOP vs a combination of VP16, mitoxantrone and prednimustine (VMP) in patients (PTS)> 70 years of age with intermediate-high grade NHL. Tumori 1996;82:59.
-
Tsurumi H, Yamada T, Sawada M, Kasahara S, Kanemura N, Kojima Y, et al. Biweekly CHOP or THP-COP regimens in the treatment of newly diagnosed aggressive non-Hodgkin’s lymphoma. A comparison of doxorubicin and pirarubicin: a randomized phase II study. J Cancer Res Clin Oncol 2004;130:107–13.
-
Urasinski I, Zdziarska B, Wichert K, Zuk E, Urasinski I, Zdziarska B, et al. Results of treating patients with highly malignant non-Hodgkin’s lymphomas; comparison of treatment efficacy with CHOP and CBVPM-AVBP. Pol Merkur Lekarski 1998;4:137–9.
-
Uyl-de-Groot CA, Hagenbeek A, Verdonck LF, Löwenberg B, Rutten FF. Cost-effectiveness of ABMT in comparison with CHOP chemotherapy in patients with intermediate- and high-grade malignant non-Hodgkin’s lymphoma (NHL). Bone Marrow Transplant 1995;16:463–70.
-
Vaughan HG. BNLI trial of CHOP vs chlorambucil in aggressive low grade stage III/IV non Hodgkin’s lymphoma: a preliminary report. Br J Haematol 1990;74:13.
-
Vose JM, Link BK, Grossbard ML, Czuczman M, Grillo-Lopez A, Gilman P, et al. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2001;19:389–97.
-
Wolf M, Cooper I, Robertson T, Fox R, Matthews J, Stone, J. Randomized comparison of macop-b with chop for intermediate-grade non-Hodgkin’s lymphoma: long-term follow-up. Ann Oncol 1996;7:24.
-
Wolf M, Matthews JP, Stone J, Cooper IA, Robertson TI, Fox RM, et al. Long-term survival advantage of MACOP-B over CHOP in intermediate-grade non-Hodgkin’s lymphoma. The Australian and New Zealand Lymphoma Group. Ann Oncol 1997;8(Suppl.)1:71–5.
-
Woof M, Matthews JP, Stone J, Grigg A, Benson W. Confirmation of a long-term survival advantage of MACOP-B over CHOP in intermediate-grade non-Hodgkin’s lymphoma (NHL). Ann Oncol 1999;10:59.
-
Zinzani PL, Tura S, Cajozzo A, Leone G, Papa G, Gentilini P, et al. Phase III comparative trial adriamycin vs idarubicin (CHOP vs CIOP) in the treatment of intermediate grade non-Hodgkin’s lymphoma. Blood 1992;80:476a.
-
Zinzani PL, Martelli M, Storti S, Musso M, Cantonetti M, Leone G, et al. Phase III comparative trial using CHOP vs CIOP in the treatment of advanced intermediate-grade non-Hodgkin’s lymphoma. Leuk Lymphoma 1995;19:329–35.
Appendix 10 List of excluded studies that met criteria for a network meta-analysis
-
Coiffier B, Stamatoullas A, Belanger C, Bouabdallah R, Haioun C, Neidhardt EM, et al. CHVP + interferon alpha 2b treatment is associated with a longer survival than fludarabine alone in elderly patients with high risk follicular lymphoma: a randomized study from the GELA. Blood 1998;92:486a.
-
Coiffier B, Neidhardt-Berard EM, Tilly H, Belanger C, Bouabdallah R, Haioun C, et al. Fludarabine alone compared to CHVP plus interferon in elderly patients with follicular lymphoma and adverse prognostic parameters: a GELA study. Ann Oncol 1999;10:1191–7.
-
Nickenig C, Dreyling MH, Schiegnitz E, Pfreundschuh M, Truemper LH, Reiser M, et al. CHOP Improves response rates but not overall survival in follicular and mantle cell lymphoma (MCL): results of a randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Blood 2004;104:176.
-
Nickenig C, Dreyling M, Schiegnitz E, Wandt H, Huber C, Trumper L, et al. CHOP significantly improves overall response and overall survival in patients with advanced follicular lymphoma: results of a randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Onkologie 2004;27:15.
-
Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Trumper L, Reiser M, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer 2006;107:1014–22.
-
Rummel MJ, von Gruenhagen U, Niederle N, Ballo H, Weidmann E, Welslau M, et al. Bendamustine plus rituximab versus CHOP plus rituximab in the first-line-treatment of patients with follicular, indolent and mantle cell lymphomas: results of a randomized Phase III study of the Study Group Indolent Lymphomas (StiL). Abstract no. 2596. Blood 2008;112:900.
-
Rummel MJ, Niederle N, Maschmeyer G, Banat A, von Gruenhagen U, Losem C, et al. Bendamustine plus rituximab is superior in respect of progression free survival and CR rate when compared to CHOP plus rituximab as first-line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: final results of a randomized Phase III study of the StiL (Study Group Indolent Lymphomas, Germany). Blood 2009;114:168–9.
Appendix 11 Data extraction tables
M39021 trial (Marcus et al.)95,96
Methods
Allocation: Randomised (1 : 1 ratio using stratification according to IPI scores).
Blinding: Open label.
Setting: Multicentre, 47 centres in Australia, Belgium, Brazil, Canada, France, Israel, Poland, Portugal, Spain, Switzerland and the UK.
Treatment duration: Treated every 21 days for a maximum of eight cycles.
Follow-up: Median 53 months (no range reported).
Design: Parallel group, ITT.
Power calculation: Yes.
Participants
Diagnosis: Follicular lymphoma, n = 322 (one CVP-enrolled patient withdrew consent).
Age: Median age – R-CVP 52 years, CVP = 53 years.
Gender: Males 174, females 148.
Inclusion criteria: Patients 18 years or older with untreated CD20-positive follicular lymphoma (NCI Working Formulation Groups B, C, D; WHO follicular lymphoma grades 1–3) confirmed by lymph node biopsy. All patients had to have stage III or IV disease, a performance status of 0–2 according to ECOG criteria, a life expectancy of > 3 months, and a need for therapy in the opinion of the participating clinician.
Exclusion criteria: Patients were ineligible if there was evidence of histological transformation to high-grade lymphoma or DLBCL, central nervous system (CNS) involvement, or a history of severe cardiac disease or previous malignancy other than in situ carcinoma of the cervix and basal cell carcinoma of the skin. Patients were also excluded if they had impaired renal or hepatic function.
Enrolment details and diagnosis
A total of 322 patients enrolled between 2000 and 2002 from 47 sites in Australia, Belgium, Brazil, Canada, France, Israel, Poland, Portugal, Spain, Switzerland and the UK. Patients diagnosed with CD20-positive follicular lymphoma (NCI) and were previously untreated.
Interventions
-
CVP Dose 750 mg/m2 cyclophosphamide intravenously (i.v.) on day 1; 1.4 mg/m2 vincristine, up to a maximal dose of 2 mg i.v., on day 1; and 40 mg/m2 prednisone per day, orally, on days 1–5; n = 159.
-
Rituximab + CVP Dose 375 mg/m2 rituximab i.v. on day 1 of eight therapy cycles; n = 162.
Patients in both groups were treated every 21 days for a maximum of eight cycles.
Maintenance therapy
None.
Tumour response and progression was determined using the guidelines by Cheson et al. Stable disease after cycle 4 was considered a ‘treatment failure’ event by the independent DSMC, who believed that patients with stable disease would be more likely to continue the same therapy in the R-CVP arm but would be more likely to start a new treatment in the CVP arm; these patients were withdrawn from treatment.
Time to progression was defined as the interval between randomisation and progression, relapse after response or death from any cause. Time to treatment failure (TTF) was defined as the time between randomisation and any one of the following events: progressive disease (PD), relapse after response, institution of new antilymphoma treatment (NLT), stable disease after cycle 4 (SD4) or death by any cause.
Disease-free survival was defined as the time between complete response and relapse or death (not specified).
Time to next antilymphoma treatment was defined as the time between randomisation and the date of next/new treatment or death (not specified).
Response duration was defined as the time between response and relapse or death (not specified).
Overall survival was defined as the time from randomisation to the date of death by any cause.
| Baseline characteristics | R-CVP, no. (%) (n = 162) | CVP, no. (%) (n = 159) |
|---|---|---|
| Age/gender | ||
| Median age (years) | 52 | 53 |
| < 40 years | 24 (15) | 16 (10) |
| 40–50 years | 48 (30) | 45 (28) |
| 51–60 years | 49 (30) | 54 (34) |
| ≥ 60 years | 41 (25) | 44 (28) |
| Male sex | 88 (54) | 85 (54) |
| Performance status (ECOG score)a | ||
| 0 | 93 (57) | 90 (57) |
| 1 | 65 (40) | 60 (38) |
| > 1 | 4 (3) | 8 (5) |
| Not evaluable/missing | 0 | 1 (1) |
| Histology class (IWF classification): local review | ||
| A (CLL) | 0 | 2 (1) |
| B (FL grade 1) | 59 (36) | 53 (33) |
| C (FL grade 2) | 87(54) | 89 (56) |
| D (FL grade 3) | 14 (9) | 13 (8) |
| Other | 1 (1) | 1 (1) |
| Not evaluable/missing | 1 (1) | 1 (1) |
| Histology class (IWF classification): central review | ||
| A (CLL) | 0 | 2 (1) |
| B (FL grade 1) | 38 (23) | 46 (29) |
| C (FL grade 2) | 82 (51) | 69 (43) |
| D (FL grade 3) | 19 (12) | 19 (12) |
| Other | 7 (4) | 6 (2) |
| Not evaluable/missing | 16 (10) | 17 (11) |
| Stage (Ann Arbor) | ||
| II | 2 (1) | 2 (1) |
| III-1b | 5 (3) | 4 (3) |
| III-2c | 40 (25) | 41 (26) |
| IV | 114 (70) | 112 (70) |
| Not evaluable/missing | 1 (1) | 0 |
| IPI scored | ||
| 0 | 1 (1) | 1 (1) |
| 1 | 72 (44) | 69 (43) |
| 2 | 57 (35) | 57 (36) |
| 3 | 19 (12) | 21 (13) |
| 4 | 2 (1) | 3 (2) |
| Not evaluable/missing | 11 (7) | 8 (5) |
| FLIPI scored | ||
| 0–2 | 80 (49) | 75 (47) |
| 3–5 | 71 (44) | 75 (47) |
| Not evaluable/missing | 11 (7) | 9 (6) |
| One or more B symptomse | 65 (40) | 51 (32) |
| Bulky diseasef | 63 (39) | 73 (46) |
| Bone marrow involvement | 103 (64) | 102 (64) |
| One or more extranodal sites | 28 (17) | 27 (17) |
| Elevated LDHg | 39 (26) | 39 (26) |
| Parameter | R-CVP, no. (%) (n = 162)a | CVP, no. (%) (n = 159)a |
|---|---|---|
| Method of selecting patients | ||
| BNLI criteria | 45 (27.8) | 46 (28.9) |
| Not BNLI criteria | 117 (72.2) | 113 (71.1) |
| B symptomsb | ||
| At least one present | 65 (40.1) | 51 (32.1) |
| All absent | 97 (59.9) | 108 (67.9) |
| Bulky diseasec | ||
| Yes | 63 (38.9) | 73 (45.9) |
| No | 99 (61.1) | 86 (54.1) |
| More than three nodal sites with diameters > 3 cm | ||
| Yes | 44 (27.2) | 32 (20.1) |
| No | 118 (72.8) | 127 (79.9) |
| Baseline haemoglobin (R-CVP = 161, CVP = 158) | ||
| < 100 g/l (%) | 7 (4.3) | 7 (4.4) |
| ≥ 100 g/l (%) | 154 (95.7) | 151 (95.6) |
| Baseline WBC (R-CVP = 161, CVP = 158) | ||
| < 3.0 × 109/l | 1 (0.6) | 1 (0.6) |
| 3.0 × 109/l | 160 (99.4) | 157 (99.4) |
| Baseline neutrophils (R-CVP = 160, CVP = 155) | ||
| < 1.5 × 109/l | 1 (0.6) | 3 (1.9) |
| ≥ 1.5 × 109/l | 159 (99.4) | 152 (98.1) |
| Baseline platelets (R-CVP = 161, CVP = 158) | ||
| < 100 × 109/l | 5 (3.1) | 6 (3.8) |
| ≥ 100 × 109/l | 156 (96.9) | 152 (96.2) |
| Baseline β2-microglobulin (R-CVP = 147, CVP = 141) | ||
| < 3 mg/dl | 1 (0.7) | 0 |
| ≥ 3 mg/dl | 146 (99.3) | 141 (100) |
| Baseline LDH (R-CVP = 152, CVP = 152) | ||
| < 2 ULN | 39 (25.7) | 39 (25.7) |
| ≥ 2 ULN | 113 (74.3) | 113 (74.3) |
| Baseline performance status ECOG (R-CVP = 162, CVP = 158) | ||
| < 1 | 4 (2.5) | 8 (5.1) |
| ≥ 1 | 158 (97.5) | 150 (94.9) |
| Macroscopic liver involvement (R-CVP = 162, CVP = 159) | ||
| Yes | 10 (6.2) | 9 (5.7) |
| No | 152 (93.8) | 150 (94.3) |
| Macroscopic renal involvement (R-CVP = 162, CVP = 159) | ||
| Yes | 4 (2.5) | 2 (1.3) |
| No | 158 (97.5) | 157 (98.7) |
| At least one symptom | 132 (81.5) | 125 (78.6) |
| Parameter | M39021,95,96 median follow-up = 53 months | |
|---|---|---|
| R-CVP (n = 162) | CVP (n = 159) | |
| Overall response: no. (%) | 131 (81) (95% CI 74% to 87%) | 90 (57) (95% CI 49% to 64%) |
| p-value | < 0.0001 | |
| CR (includes CRu): no. (%) | 66 (41) (95% CI 33% to 49%) | 16 (10) (95% CI 6% to 16%) |
| p-value | < 0.0001 | |
| PR: no. (%) | 65 (40) | 74 (47) |
| No p-value reported | ||
| Stable disease | 12 (7) | 33 (21) |
| p-value | No p-value reported | |
| Progressive disease | 17 (11) | 31 (20) |
| p-value | No p-value reported | |
| OS rate (% alive using Kaplan–Meier estimate at 4 years) | 83 (95% CI 77 to 89) | 77 (95% CI 70 to 83) |
| p-value | < 0.0290 | |
| Median OS | Not reached | Not reached |
| No. of deaths (42-month follow-up) (%)101 | 23 (14) | 35 (22) |
| p-value | No p-value reported | |
| Deaths owing to lymphoma: no. (%) | 13 (8) | 22 (14) |
| Median TTF | 27 months (95% CI 25 to 37) | 7 months (95% CI 6 to 9) |
| p-value | < 0.0001 | |
| Median response duration | 38 months (95% CI 28 to NE) | 14 months (95% CI 9 to 18) |
| p-value | < 0.0001 | |
| Median time to next treatment, months | 49 (32 to NE) | 12 (10–18) |
| p-value | < 0.0001 | |
| Median DFS, months | Not reached (35 to NE) | 21 (14–38) |
| p-value | 0.0001 | |
| Median TTP, months | 34 (27–48) | 15 (12–18) |
| p-value | < 0.0001 | |
| AEs (grade 3/4) | M3902195,96 | |
|---|---|---|
| R-CVP, n = 162 | CVP, n = 159 | |
| Neutropenia | 39 (24) | 22 (14) |
| Leucopenia taken from MS61 (could not be confirmed in manuscripts) | 19 (12) | 14 (9) |
| Experiencing at least one AE | 157 (97) | 153 (96) |
| Experiencing an AE with 24 hours of infusion | 115 (71) | 81 (51) |
| Experiencing a total of six life-threatening AEs | 5(3) | 0 |
| Grade 3 or 4 rituximab infusion-related reaction | 14 (9) | Not applicable |
| Leaving study before completing four cycles | 6 (4) | 13 (8%) |
| Leaving study early before completing eight cycles | 25 (15) | 51 (32) |
| Treatment-related deaths | 0 (0) | 0 (0) |
| R-CVP (n = 162) | CVP (n = 159) |
|---|---|
| Eight cycles administered to n = 144 (89%) | Eight cycles administered to n = 103 (65%) |
| Ninety per cent of patients received the planned dose of prednisolone and vincristine at each administered cycle and this was similar between the R-CVP and CVP arms. The proportion of patients who received > 90% of cyclophosphamide was higher in the CVP group (> 94%) than the R-CVP group (> 85%). Ninety-six per cent of patients received > 90% of the planned dose of rituximab at each administered cycle | |
|
Subgroup analyses Multivariate analysis assessed the prognostic value of various parameters (BNLI criteria, age, extranodal sites, LDH, FLIPI, IPI, bone marrow involvement, elevated B2-microglobulin, B symptoms, bulky disease, nodal areas, haemoglobin level) on outcome in terms of TTP in the presence of the trial treatment effect. Only the FLIPI (categorised as 0–2 vs 3–5 in the analysis) was a significant prognostic parameter for TTP in addition to the trial treatment. Patients with a FLIPI score of 0–2, who received R-CVP, had the longest TTP. No other prognostic factor improved the predictive power. In two further multivariate analyses (one utilising IPI instead of FLIPI, the other considering neither of the composite factors FLIPI and IPI), only haemoglobin level and number of nodal areas were found to be statistically significant predictors of TTP in addition to trial treatment |
|
| Prognostic factor | R-CVP (n = 162) | CVP (n = 159) | |||||
|---|---|---|---|---|---|---|---|
| No. | Median TTP (months) | 95% CI | No. | Median TTP (months) | 95% CI | p-value | |
| FLIPI score | |||||||
| 0–1 | 28 | Not reached | 38 to NE | 23 | 22 | 16 to 40 | 0.0085 |
| 2 | 62 | 37 | 28 to NE | 56 | 17 | 13 to 25 | 0.0003 |
| 3–5 | 61 | 26 | 16 to 34 | 71 | 11 | 10 to 15 | 0.0004 |
| IPI score | |||||||
| 0–1 | 73 | 44 | 30 to NE | 70 | 20 | 13 to 26 | < 0.0001 |
| 2 | 57 | 27 | 20 to 39 | 57 | 14 | 10 to 17 | 0.0003 |
| 3–4 | 21 | 40 | 11 to NE | 24 | 12 | 8 to 25 | 0.0333 |
| Histology at central review (IWF) | |||||||
| Class B | 38 | 34 | 27 to NE | 46 | 17 | 11 to 24 | 0.0037 |
| Class C | 82 | 35 | 26 to NE | 69 | 15 | 10 to 21 | < 0.0001 |
| Class D | 19 | Not reached | 30 to NE | 19 | 14 | 7 to 24 | < 0.0046 |
| B symptoms | |||||||
| ≥ 1 | 65 | 32 | 22 to NE | 51 | 17 | 12 to 23 | 0.0014 |
| All absent | 97 | 37 | 26 to 48 | 108 | 14 | 11 to 20 | < 0.0001 |
| Bulky disease | |||||||
| Yes | 63 | 38 | 25 to 48 | 73 | 13 | 11 to 21 | < 0.0001 |
| No | 99 | 32 | 26 to NE | 86 | 16 | 13 to 21 | < 0.0001 |
| Haemoglobin (g/dl) | |||||||
| ≥ 12 | 132 | 39 | 31 to NE | 121 | 17 | 13 to 22 | < 0.0001 |
| < 12 | 29 | 11 | 9 to 28 | 35 | 12 | 10 to 16 | 0.3941 |
| FLIPI | R-CVP (n = 162) | CVP (n = 159) |
|---|---|---|
| FLIPI 0–1 (good prognosis) | Not reached | 22 |
| FLIPI 2 (intermediate prognosis) | 37 | 17 |
| FLIPI 3–5 (poor prognosis) | 26 | 11 |
GLSG-2000 trial91,92
Methods
Allocation: Randomised (computer generated, in blocks stratified).
Blinding: Open label.
Setting: Germany, multicentre.
Treatment duration: Six to eight cycles (up to 24 weeks).
Follow-up: Median 58 months.
Design: Parallel group, ITT analysis.
Power calculation: Yes.
Participants
Diagnosis: Follicular lymphoma (advance stage III–IV), untreated, grades I and II (WHO) classification.
Number: 630 enrolled (not reported how many randomised).
Age: Median age 57 years (range 21–90 years).
Gender: 266 males and 291 females.
Inclusion criteria: Patients 18 years of age and older previously untreated, advanced-stage FL grades 1 and 2 according to the WHO classification, Stage III or IV disease and a requirement for therapeutic intervention as defined by the presence of B symptoms (night sweats, fever or weight loss), bulky disease (mediastinal lymphomas of > 7.5 cm or other lymphomas of > 5 cm in maximal diameter), impairment of normal hematopoiesis with haemoglobin levels of < 100 g/l, granulocyte count of < 1.5 × 109/l, thrombocyte count of < 100 × 109/l, or rapidly progressive disease.
Exclusion criteria: Patients were ineligible if they had FL grade III, were pregnant or lactating, or were women of childbearing potential and not using a reliable method of contraception.
Interventions
-
CHOP Dose: 750 mg/m2 cyclophosphamide; 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine: all given i.v. on day 1. Prednisolone given 100 mg/m2 daily on days 1–5 orally; n = 278.
-
Rituximab + CHOP Dose rituximab: 375 mg/m2 the day before the respective R-CHOP course; n = 279.
Patients achieving CR after four cycles were treated with a total of six cycles only, whereas all other patients received eight courses of CHOP or R-CHOP.
Treatment cycles Every 3 weeks for a total of six to eight cycles; number of cycles, patients achieving CR after four cycles were treated with a total of six cycles; all other patients received eight cycles. Patients with progressive disease at anytime during R-CHOP or CHOP therapy were withdrawn from treatment.
Maintenance therapy
Patients aged < 60 years achieving CR or PR after CHOP or R-CHOP were offered a second randomisation for treatment in remission to either intensification by the Dexa-BEAM regimen consisting of dexamethasone 3 × 8 mg/day orally on days 1–10, bischloroethylnitrosourea (BCNU) 60 mg/m2 daily on day 2, melphalan 20 mg/m2 daily i.v. on day 3, etoposide 75 mg/m2 daily i.v. on days 4–7, and cytosine arabinoside 2 × 100 mg/m2 every 12 hours i.v. on days 4–7 with subsequent stem cell harvest followed by myeloablative radiochemotherapy with total body irradiation (12 Gy) and cyclophosphamide 60 mg/kg daily for 2 days and stem cell retransfusion or long-term interferon-alpha maintenance initiated at a dose of 3 × 5 million international units (MIU)/week and reduced according to observed adverse effects. Interferon maintenance therapy was given until lymphoma progression or the development of intolerable adverse effects. Second randomisation stratified for type of initial therapy (R-CHOP or CHOP) and the response (CR or PR). Only 25 patients did not receive either of these options.
Enrolment details and diagnosis
A total of 630 patients enrolled from 200 institutions between May 2000 and August 2003. In June 2003, significantly longer TTF was recorded for the R-CHOP arm (p = 0.001) and randomisation stopped according to the protocol in August 2003. Grade 1 or 2 histological diagnosis for 390 patients confirmed by a central pathology review, 38 patients’ results still pending.
Evaluation response and definitions
Tumour response and progression was determined using the guidelines by Cheson et al. 89 Response to therapy assessed every two cycles and 4 weeks after completion of last course, and consisted of:
-
physical examination – every 3 months
-
blood count and LDH level – every 3 months
-
ultrasound of abdomen – every 3 months
-
CT scan of previously involved areas – every 6 months
-
patients fulfilling CR criteria had bone marrow biopsy – every 3 months.
Time to treatment failure was defined as the interval between the start of treatment and the documentation of resistance to initial therapy, disease progression or death. Response duration was defined as the interval from the end of successful induction therapy to the documentation of disease progression or death. Overall survival was defined as the interval between start of treatment and death. Time to next antilymphoma treatment was not defined.
| Characteristic | R-CHOP (n = 279) | CHOP (n = 278) | p-value | ||
|---|---|---|---|---|---|
| Median age (years), min.–max. | 57 | 27–90 | 57 | 21–81 | 0.79 |
| Male | 120 | 43% | 146 | 53% | 0.027 |
| Ann Arbor stage IV | 194 | 70% | 191 | 69% | 0.85 |
| Bone marrow involved | 180 | 65% | 179 | 64% | 1.00 |
| B symptoms | 108 | 39% | 113 | 41% | 0.60 |
| Elevated LDH | 73 | 26% | 66 | 24% | 0.56 |
| Hb < 120 g/l | 54 | 20% | 56 | 20% | 0.83 |
| ECOG performance status 0 | 97 | 36% | 88 | 32% | 0.82 |
| ECOG performance status 1 | 155 | 57% | 167 | 61% | NR |
| ECOG performance status > 2 | 18 | 7% | 19 | 7% | NR |
| FLIPI low risk | 39 | 14% | 31 | 11% | 0.61 |
| FLIPI intermediate risk | 114 | 41% | 119 | 44% | NR |
| FLIPI high risk | 123 | 45% | 123 | 45% | NR |
| Outcome | GLSG-2000 trial91,92 (median follow-up = 56 months) | |
|---|---|---|
| R-CHOP (n = 279) | CHOP (n = 278) | |
| OR: no. (%) | 271 (97) (no CI reported) | 253 (91) (no CI reported) |
| p-value reported in study | 0.0046 | |
| CR: no. (%) | 53 (20) | 47 (17) |
| p-value reported in study | No p-value reported | |
| PR (includes CRus): no. (%) | 215 (77) | 187 (74) |
| No p-value reported | ||
| Stable disease including minor response | 6 (2) | 17 (6) |
| p-value reported in study | No p-value reported | |
| Progressive disease | 3 (1) | 6 (2) |
| p-value reported in study | No p-value reported | |
| OS 5-year rate % | 90 (no CI reported) | 84 (no CI reported) |
| p-value reported in study | 0.0493 | |
| Median OS | Not reached | Not reached |
| No. of deaths reported at 3 years92 | 6 | 17 |
| p-value reported in study | = 0.016 | |
| Median TTF | Not reached | 35 months |
| p-value | < 0.0001 | |
| Duration of response at 5 years | 66% | 35% |
| p-value reported in study | p < 0.0001 | |
| Median time to next antilymphoma treatment (reported at 18-month follow-up)92 | NR | NR |
| p-value reported in study | 0.001 | |
Adverse events
| Cause of death/time of death | R-CHOP (n = 223) | CHOP (n = 205) |
|---|---|---|
| Death owing to lymphoma | 1 (0) | 9 (4) |
| Death owing to infection | 4 (2) | 4 (2) |
| Death owing to cardiac failure | 0 | 1 (0) |
| Apoplectic insult | 0 | 1 (0) |
| Death owing to GVHD after ASCT | 0 | 1 (0) |
| Death cause unknown | 1 (0) | 1 (0) |
| Death by 18 months | 2 (1) | 2 (1) |
| Death by 36 months | 6 (3) | 17 (8) |
| (p = 0.016) |
| AE | Grade 1 and 2 | Grade 3 | Grade 4 | |||
|---|---|---|---|---|---|---|
| R-CHOP (n = 223) | CHO (n = 205) | R-CHOP (n = 223) | CHOP (n = 205) | R-CHOP (n = 223) | CHOP (n = 205) | |
| Haemoglobin level | 112 (50) | 100 (49) | 18 (8) | 18 (9) | 2 (1) | 2 (1) |
| Leucocyte | 54 (24) | 57 (28) | 96 (43) | 78 (38) | 58 (26) | 47 (23) |
| Granulocyte | 42 (19) | 41 (20) | 49 (22) | 47 (23) | 91 (41) | 62 (30) |
| Platelets count | 38 (17) | 33 (16) | 9 (4) | 10 (5) | 4 (2) | 6 (3) |
| Infection | 74 (33) | 59 (29) | 11 (5) | 12(6) | 0 (0) | 2 (1) |
| Bleeding | 9 (4) | 6 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea/vomiting | 100 (45) | 90 (44) | 9 (4) | 12(6) | 0 (0) | 0 (0) |
| Stomatitis | 58 (26) | 59 (29) | 2 (1) | 4 (2) | 0 (0) | 0 (0) |
| Obstipation | 33 (15) | 27 (13) | 4 (2) | 2 (1) | 0 (0) | 0 (0) |
| Diarrhoea | 25 (11) | 23 (11) | 4 (2) | 6 (3) | 0 (0) | 0 (0) |
| Fever | 65 (29) | 45 (22) | 0 (0) | 2 (1) | 0 (0) | 0 (0) |
| Cardiac dysfunction | 7 (3) | 8 (4) | 4 (2) | 2 (1) | 2 (1) | 0 (0) |
| Alopecia | 42 (19) | 51 (25) | 140 (63) | 115 (56) | 9 (4) | 10 (5) |
| Cardiac arrhythmia | 13 (6) | 8 (4) | 2 (1) | 0 (0) | 2 (1) | 0 (0) |
| Neurotoxicity | 76 (34) | 86 (42) | 2 (1) | 4 (2) | 0 (0) | 0 (0) |
| CNS toxicity | 4 (2) | 4 (2) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| Allergy | 13 (6) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| R-CHOP (n = 223) | CHOP (n = 205) | |||||
| Infections including fevers of unknown origin | 11 (5) | 14 (7) | ||||
| Stopped treatment due to AEs | 2 (1) | 0 (0) | ||||
| Early cessation of rituximab AEs (%) | 2 (1) | 0 (0) | ||||
Number of treatment cycles and dose administered: not reported
| Subgroup | Result |
|---|---|
| Age, years | |
| < 60 | Median TTF not reached for CHOP (p-value for Cox regression = 0.003). Estimated RR for TTF for R-CHOP: 0.417 (95% CI 0.233 to 0.747) |
| ≥ 60 | Median TTF 29 months for CHOP (p-value for Cox regression = 0.004). Estimated RR for TTF for R-CHOP: 0.354 (95% CI 0.175 to 0.715) |
| IPI score | |
| 1–2 | Median not reached (p-value for Cox regression = 0.001). Estimated RR for TTF for R-CHOP 0.412 (95% CI 0.242 to 0.701) |
| 3–5 | 29 months (p-value for Cox regression = 0.009). Estimated RR for TTF for R-CHOP 0.33 (95% CI 0.144 to 0.761) |
| Elderly patients | |
| Estimated 4 years’ PFS was 62.2% for R-CHOP (n = 109) vs 27.9 % after CHOP (n = 112) (log-rank test: p < 0.0001). R-CHOP (n = 109) prolonged OS in elderly patients with an estimated 4 years’ OS of 90% after immunochemotherapy vs 81% after CHOP (n = 112) alone (log-rank test: p = 0.039) | |
| FLIPI score | |
| Low-risk group | R-CHOP prolonged 5 years’ TTF: R-CHOP 84% vs 46% CHOP (p = 0.0021) |
| Intermediate-risk group | TTF prolonged 5 years’ R-CHOP 73% vs 37% CHOP (p < 0.0001) |
| High-risk group | TTF prolonged 5 years’ R-CHOP 49% vs CHOP 23% (p < 0.0001) |
OSHO-39 trial (Herold et al.)93
Methods
Allocation: Randomised (random number list).
Blinding: Open label.
Setting: Germany, 34 centres.
Treatment duration: Total of 32 weeks consisting of eight treatment cycles of rituximab.
Follow up: Median 49 months for R-MCP, 42 months MCP (no range reported).
Design: Parallel group, ITT analysis.
Power calculation: Yes (using primary popaulton of follicular lymphoma).
Participants
Diagnosis: Follicular lymphoma.
Number: 358 total (201 with FL).
Age: Median age, MCP arm = 57 years (range 31–75 years), R-MCP arm = 60 years (33–78 years).
Gender: 89 males and 112 females.
Inclusion criteria: Age 18–75 years, untreated, histologically confirmed, CD20 indolent NHL (FL, grade 1 and 2 only; lymphoplasmacytic lymphoma) and MCL. Stage III or IV disease according to the Ann Arbor classification General performance status of ≤ 2 according to the ECOG scale. Needing treatment for either, B symptoms or extranodal manifestation, haematopoietic insufficiency, rapid tumour growth, bulky disease [lymphoma of > 7.5 cm in diameter, mediastinal tumor one-third of thorax diameter at thoracic vertebra 5/6, or immunohaematological phenomena (e.g. haemolytic anemia or immune thrombocytopenia)].
Exclusion criteria: Patients with concomitant diseases and/or restricted organ function not caused by lymphoma or patients with HIV infection were excluded from the study.
Interventions
-
MCP Dose mitoxantrone 8 mg/m2 i.v. on days 3 and 4, chlorambucil (3 × 3mg/m2 orally) on days 3–7, and prednisolone (25 mg/m2 orally) on days 3–7; n = 96.
-
Rituximab-MCP Dose rituximab 375 mg/m2 i.v. on day 1 of each therapy cycle, followed by mitoxantrone (8 mg/m2 i.v.) on days 3 and 4, chlorambucil (3 × 3mg/m2 orally) on days 3–7, and prednisolone (25 mg/m2 orally) on days 3–7; n = 105.
Maintenance
Maintenance therapy with interferon alpha-2a (4.5 MIU three times per week until relapse) was planned in all study patients with FL who had achieved PR or CR and was initiated within 4–8 weeks after treatment completion; thus 3 × 4.5 MIU per week until disease progression was initiated in 97% (n = 102) and 92% (n = 88) of planned patients in the R-MCP group and MCP group, respectively.
Enrolment details and diagnosis
Enrolment occurred between October 1998 and September 2003 at 34 centres in Germany. Follicular lymphoma was confirmed histologically by a designated reference pathologist.
Evaluation response and definitions
After completion of induction treatment, patients were observed every 8 weeks during the first year, at 3-month intervals during the second year, and then every 6 months from the third year onward. Tumour responses were assessed after two treatment cycles, after six treatment cycles, and 4 weeks after completion of study treatment. Response assessment included all diagnostic measures used in the pre-therapeutic staging (including CT scans of neck, chest, abdomen and pelvis, and bone marrow biopsy).
Patients with disease progression after two cycles of therapy were prematurely withdrawn from study treatment and were considered as having treatment failure in the analysis of EFS. Patients who had not reached a PR or CR after six cycles of therapy were also classified as experiencing treatment failure in the EFS analysis. Patients with a CR or a PR after six cycles of chemotherapy or immunochemotherapy, respectively, received a further two consolidation cycles of MCP or R-MCP for a total of eight treatment cycles.
Progression-free survival was defined as randomisation to disease progression or death from NHL. Overall survival was defined as the time from randomisation to the date of death by any cause. Response duration was defined as the time between response to treatment and disease progression or death by any cause. EFS was defined as the time between randomisation and relapse, disease progression or disease progression after two cycles or PR after six cycles. Time to next antilymphoma treatment was defined as time between randomisation and date of next/new treatment.
| Characteristic | R-MCP (n = 105), no. (%) | MCP (n = 96), no. (%) |
|---|---|---|
| Age, median (range) | 57 (31–75) | 60 (33–79) |
| Males | 36 (37) | 53 (50) |
| Ann Arbor stage III | 22 (23) | 30 (29) |
| Ann Arbor stage IV | 74 (77) | 75 (71) |
| ECOG performance status 0 | 54 (56) | 69 (65) |
| ECOG performance status 1 | 36 (39) | 29 (29) |
| ECOG performance status 2 | 6 (6) | 7 (7) |
| LDH > normal | 30 (31) | 31 (30) |
| Bone marrow infiltrate | 71 (74) | 73 (70) |
| B symptoms: nightly sweating | 34 (35) | 46 (44) |
| B symptoms: fever > 38°C | 2 (2) | 4 (4) |
| B symptoms: weight loss > 10% within 6 months | 20 (21) | 16 (15) |
| FLIPI low (0–1) | 6 (6) | 9 (9) |
| FLIPI intermediate (2) | 37 (39) | 39 (36) |
| FLIPI high (3–5) | 53 (55) | 59 (56) |
| Outcome | OSHO-3993 (median follow-up) | |
|---|---|---|
| R-MCP (n = 105) | MCP (n = 96) | |
| OR: no. (%) | 97 (92) (no CI reported) | 72 (75) (no CI reported) |
| p-value reported in study | 0.0009 | |
| CR: no. (%) | 52 (50) | 24 (25) |
| p-value reported in study | 0.0004 | |
| PR (includes CRus): no. (%) | 45 (43) | 48 (50) |
| No p-value reported | ||
| Stable disease | NRa | NRa |
| p-value reported in study | No p-value reported | |
| Progressive disease |
3 (3) (after two cycles] |
10 (10) (after two cycles) |
| p-value reported in study | No p-value reported | |
| OS rate at 4 years (%) | 87 (CI NR) | 74 (CI NR) |
| p-value reported in study | 0.0096 | |
| Median OS | Not reached | Not reached |
| No. of deaths at 4 years | 15 | 25 |
| p-value reported in study | No p-value reported | |
| Median PFS, months | Not reached | 28.8 |
| p-value reported in study | < 0.0001 | |
| No. of events, n (%) | 30 (29) | 50 (52) |
| % PFS at 4 years | 71 | 40 |
| Median EFS, months | Not reached | 26 |
| p-value | < 0.0001 | |
| Median response duration, months | Not reached | 35 |
| p-value | < 0.0001 | |
| Median TTNT, months | Not reached | 29.4 |
| p-value | 0.0002 | |
| Cause of death | R-MCP (n = 105) | MCP (n = 96) |
|---|---|---|
| Death cause unknown | 15 (14) | 25 (26) 0 |
| Cause-specific deaths (p = 0.0159) | 7 (7) | 17 (18) |
| AE | Grade 1 or 2 | Grade 3 | Grade 4 | |||
|---|---|---|---|---|---|---|
| R-MCP (n = 105) | MCP (n = 96) | R-MCP (n = 105) | MCP (n = 96) | R-MCP (n = 105) | MCP (n = 96) | |
| Haemoglobin level | 18 (17) | 18 (19) | 2 (2) | 3 (3) | 1 (1) | 1 (1) |
| Leucocyte/WBC | 3 (3) | 8 (8) | 25 (24) | 21 (22) | 50 (48) | 35 (36) |
| Platelets count | 31 (30) | 32 (33) | 4 (4) | 6 (6) | 0 (0) | 1 (1) |
| Infection | 44 (42) | 34 (35) | 6 (6) | 7 (7) | 1 (1) | 1 (1) |
| Nausea/vomiting | 25 (24) | 14 (14) | 1 (1) | 6 (6) | 0 (0) | 0 (0) |
| Stomatitis | 11 (10) | 7 (7) | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Diarrhoea | 11 (10) | 4 (4) | 2 (2) | 0 (0) | 0 (0) | 2 (2) |
| Rash | 16 (15) | 1 (1) | 0 (0) | 2 (2) | 0 (0) | 0 (0) |
| Heartburn | 15 (14) | 3 (3) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Insomnia | 15 (14) | 7 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bone pain | 10 (10) | 10 (10) | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal | 9 (9) | 5 (5) | 2 (2) | 1 (1) | 0 (0) | 1 (1) |
| Other (not specified) | 11 (10) | 8 (8) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| R-MCP (n = 105) | MCP (n = 96) |
|---|---|
| Eight cycles administered to n = 92 (88%) | Eight cycles administered to n = 64 (67%) |
|
The mean dose of study drugs administered in the OHSO–39 trial93 were rituximab, 660−680 mg/cycle; mitoxantrone, 24−28 mg/cycle; chlorambucil, 68−81 mg/cycle and prednisolone, 226−231 mg/cycle. The authors stated that the dose intensity of the chemotherapy did not differ between treatment arms93 Interferon-alpha maintenance treatment (3 x 4.5 MIU per week until disease progression) was initiated in 97% and 92% of responding patients in the R-MCP and MCP arms, respectively |
|
Subgroup analyses
None.
FL2000 trial (Salles et al.)94
Methods
Allocation: Randomised (methods not specified).
Blinding: Open label.
Setting: France and Belgium, 54 centres.
Treatment duration: 72 weeks.
Follow up: Median 5 years (range: 0.2–6.4 years).
Design: Parallel, ITT anlayses.
Power calculation: Yes.
Participants
Diagnosis: Follicular lymphoma.
Number: = 60 (358 analysed; one patient withdrew consent after registration; one patient had a major inclusion violation, which was registered at relapse).
Age: Median 61 years (range 25–75 years).
Gender: 178 males and 180 females.
Inclusion criteria: Untreated patients 18–75 years of age; histological diagnosis of FL grade 1, 2, 3a performed in last 3 months on lymph node biopsy (pathologic review by panel of three expert pathologists) stage II–IV (Ann Arbor); fulfil any one of following criteria for high tumour burden: (1) presence of a bulk tumour defined by either one of the following: tumour lesion with a largest diameter of ≥ 7 cm, spleen enlargement with a craniocaudal diameter of >20 cm, existence of three lymph nodes in three distinct nodal areas with a diameter of > 3 cm, pleural effusion, ascites, or symptomatic compressive syndrome; (2) presence of B symptoms (fever, night sweats, or weight loss); (3) a performance status on the ECOG scale > 1; (4) elevated serum levels of LDH (above normal values) or β2-microglobulin (≥ 30 mg/dl).
Exclusion criteria: Patients with contraindications to anthracyclines, interferon, or rituximab, with known positivity for HIV or active viral hepatitis, or with a previous malignancy were not eligible for the study.
Interventions
-
CHVPi Twelve courses: six courses every 28 days, six courses every 56 days: 600 mg/m2 cyclophosphamide i.v. on day 1, 25 mg/m2 adriamycin/doxorubicin intravenously on day 1, 100 mg/m2 etoposide i.v. on day 1, 40 mg/m2 predisolone orally from days 1–5. Interferon-alpha2a s.c. during 18 months, three times a week at an initial dose of 4.5 MIU per injection for patients of < 70 years or 3 MU per injection for patients aged > 70 years; n = 183.
-
Rituximab + CHVPi Doses as per comparator arm on same days of cycle. Rituximab = 375 mg/m2, six cycles every 28 days; however, cycles 1 and 2 CHVPi only; cycles 3 and 4 R-CHVP-I (plus extra rituximab on day 8 of cycle); cycles 5 and 6 RCHVP-I: and cycles 7–12 interferon only every 56 days; n = 175.
Maintenance therapy
None.
Evaluation and response and outcomes definitions
-
Evaluation of response performed after six chemotherapy courses (6 months) and at the end of the whole treatment (18 months).
-
Disease evaluation for response assessments was recommended in the International Workshop criteria: CR, disappearance of all lesions and of radiological or biological abnormalities observed at diagnosis and the absence of new lesions; CRu, CR with persistence of some radiologic abnormalities, which had to have regressed in size by at least 75%; PR, regression of all measurable lesions by > 50%, the disappearance of non-measurable lesions, and the absence of new lesions and ‘stable disease’, regression of any measurable lesion by ≤ 50% or no change in the non-measurable lesions, but without growth of existing lesions or the appearance of new lesions.
-
Progressive disease – appearance of a new lesion, any growth of the initial lesion by > 25%, or growth of any measurable lesion that had regressed during treatment by > 50% from its smallest dimensions.
-
Responding patients with previous bone marrow involvement for which bone marrow evaluation was missing at evaluation were considered has having a PR even if they met the criteria of CRu or CR. Any residual marrow infiltrate that could not be demonstrated to be a reactive infiltrate using immunostaining was considered as a positive bone marrow biopsy, and the response, if other criteria were met, as a PR.
-
Patients who completed their treatment had a complete clinical examination every 3 months for the first year and then every 6 months for 5 years. A CT scan was performed yearly, and a new bone marrow biopsy was performed 18 months after treatment completion or when clinically indicated.
Overall survival was defined as the time from randomisation to the date of death by any cause. EFS was defined as time from randomisation to disease progression, death any cause, relapse or new antilymphoma treatment. Response duration was defined as the time from response to disease progression, death from any cause, or relapse.
Enrolment details and diagnosis
Patients were enrolled between May 2000 and May 2002. Histological diagnosis of FL grades 1, 2 and 3a performed in last 3 months on lymph node biopsy (pathological review by panel of three expert pathologists for 344 patients, four diagnoses of FL could not be formally confirmed because of technical problems, 12 cases were classified as non-FL subtypes), according to WHO criteria.
| Patient characteristics | R-CHVPi (n, %) | CHVPi (n, %) | Missing values |
|---|---|---|---|
| ECOG performance status > 1 | 11 (6) | 16 (9) | 0 |
| B symptoms presence | 38 (22) | 52 (29) | 1 |
| Ann Arbor stage III or IV | 152 (87) | 165 (91) | 2 |
| No. of nodal sites involved > 4 | 86 (49) | 78 (43) | 0 |
| Bone marrow involvement: | 108 (62) | 121 (67) | 4 |
| Extranodal sites > 1 | 60 (35) | 73 (40) | 3 |
| LDH more than upper normal value | 64 (37) | 66 (36) | 5 |
| Haemoglobin < 12 g/dl | 37 (21) | 30 (17) | 2 |
| β2-microglobulin > 3 mg/l | 62 (38) | 56 (33) | 28 |
| IPI score > 2 | 60 (36) | 71 (39) | 10 |
| FLIPI 0–1 factors | 28 (16) | 37 (21) | 9 |
| FLIPI 2 factors | 63 (37) | 59 (33) | 9 |
| FLIPI 3 factors or more | 79 (46) | 83 (46) | 9 |
| Outcome | 6-month follow-up data | 18-month follow-up data (response rate only) | ||
|---|---|---|---|---|
| R-CHVPi (n = 175) | CHVPi (n = 183) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| OR: no. (%) (no CI reported) | 164 (94) | 156 (85) | 142 (81) | 131 (72) |
| p-value reported in study | NR | NR | NR | NR |
| CR: no. (%) | 63(36) | 29(16) | 90 (51) | 71 (39) |
| p-value reported in study | < 0.001a | 0.035a | ||
| PRd: no. (%) | 101(58) | 127 (69) | 52 (30) | 60 (33) |
| < 0.001a | 0.035a | |||
| Stable disease | 2 (1) | 9 (5) | 1 (1) | 3 (2) |
| p-value reported in study | < 0.001a | 0.035a | ||
| Progressive disease | 8 (5) | 18 (10) | 31 (18) | 47 (26) |
| p-value reported in study | < 0.001a | 0.035a | ||
| OS rate at 5 years, % | 84, (95% CI 78 to 84) | 79, (95% CI 72 to 84) | ||
| p-value reported in study | 0.1552 | |||
| Median OS | NR | |||
| No. of deaths at 18 months | 1 (1) | 2 (1) | ||
| p-value reported in study | No p-value reported | |||
| Median EFS, months | Not reached | 35 | ||
| p-value reported in study | 0.0004 | |||
| 5-year EFS | 53% (95% CI 45% to 60%) | 37% (95% CI 29% to 44%) | ||
| p-value reported in study | 0.001 | |||
| Duration of response at 4 years | 64%b (95% CI 55% to 72%) | 37% (95% CI 29% to 44%) | ||
| p-value reported in study | 0.012 | |||
| AE | Induction (6 months of treatment) | Consolidation additional (12 months of treatment) | ||
|---|---|---|---|---|
| R-CHVPi (n = 175) | CHVPi (n = 183) | R-CHVPi (n = 175) | CHVPi (n = 183) | |
| Haemoglobin level | 6 (3) | 9 (5) | 1 (1) | 4 (2) |
| Neutrophil | 103 (59) | 114 (62) | 11 (6)a | 69 (38) |
| Platelet count | 5 (3) | 6 (3) | 2 (2) | 4 (2) |
| Fever | 2 (1) | 2 (1) | 0 (0) | 1 (1) |
| Infection | 4 (2) | 0 (0) | 2 (1) | 2 (1) |
| Cardiac dysfunction | 2 (1) | 3 (2) | 0 (0) | 1 (1) |
Numbers of cycles administered
-
In total, 95% of patients in the R-CHVPi arm and 94% of patients in the CHVPi arm received the initial six cycles of treatment.
-
Among patients who did not progress during therapy, 161 (98%) and 153 (98%) of the patients received the planned chemotherapy courses during the first 6 months in the R-CHVPi and CHVPi arms, respectively.
-
In the CHVPi arm, 116 (87%) of 134 patients without death or progression received the six planned cycles of chemotherapy consolidation.
-
A total of 237 (66%) patients followed the interferon treatment according to the protocol, with dose adaptation (45 patients) or short (< 4 weeks) interruptions (55 patients), without significant differences in adaptation between the two study arms.
-
Interferon treatment was stopped in 50 patients resulting from disease progression (R-CHVPi arm, 19 cases; CHVPi arm, 31 cases, respectively) and was interrupted either for > 1 month (16 cases) or definitively (72 cases) resulting from toxicity. These major interruptions were observed in 41 patients in the RCHVPi arm and 47 patients in the CHVPi arm.
Subgroup analyses
Because the FL2000 trial94 was not stratified by the FLIPI, checked for effects of prognostic factors on outcome resulting from sampling fluctuation in the treatment groups using multivariate analysis of survival. The Cox regression model included FLIPI and treatment as explanatory variables. The interactions between risk factors and treatment were also included in the model.
Results
Significantly different outcomes for each group both for 5-year EFS and OS (p < 0.001 for each). When the low- and intermediate-risk groups were considered together and compared with the high-risk group, this index was also able to discriminate risk groups for patients in each treatment arm. When considering together the 187 patients who presented either a low or an intermediate FLIPI score, no significant difference in outcome was observed according to each treatment arm. However, the outcome of the 162 patients with the highest FLIPI score (three to five adverse prognostic factors) was found to be significantly different both for 5-year EFS (p = 0.001) and OS (p = 0.025) between the CHVPi- and R-CHVPi-treated patients. Five-year OS probability for patients in the FL200094 in the different FLIPI prognostic subgroups (low, intermediate, and high) was found to be 95%, 89% and 70% as opposed to 91%, 78% and 53%, respectively.
Appendix 12 Outcomes definitions for time-to-event data
Note that the definitions do not include OS or PFS.
| Trial | Death not specified | Relapse | Disease progression | Death any cause |
|---|---|---|---|---|
| M3902195,96 | ✓ | ✓ | ||
| GLSG-200091,92 | ✓ | ✓ | ||
| OSHO-3993 | ✓ | |||
| FL200094 | ✓ | ✓ | ✓ |
| Trial | Resistance to initial therapy | Disease progression | Death any cause | Death not specified | Relapse after response | New antilymphoma treatment | Stable disease after cycle 4 |
|---|---|---|---|---|---|---|---|
|
From randomisation |
✓ | ✓ | ✓ | ✓ | |||
|
From start of treatment |
✓ | ✓ | ✓ |
| Trial | Date of next/new treatment | Death not specified |
|---|---|---|
| M3902195,96 | ✓ | ✓ |
| GLSG200091,92 | Not defined | |
| OSHO-3993 | ✓ |
Appendix 13 Chi-squared test analysis for response rate data
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CVP | CVP | R-CVP | CVP | |
| CR | 49 | 12 | 30.8 | 30.2 |
| PR (includes CRu) | 82 | 78 | 80.7 | 79.3 |
| Stable disease | 12 | 33 | 22.7 | 22.3 |
| Disease progression | 17 | 31 | 24.2 | 23.8 |
| Death | 2 | 5 | 3.5 | 3.5 |
| Treatment arm totals | 162 | 159 | 162.0 | 159.0 |
| p-value | < 0.001 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CVP | CVP | R-CVP | CVP | |
| CR | 49 | 12 | 30.8 | 30.2 |
| PR (includes CRu) | 82 | 78 | 80.7 | 79.3 |
| Stable disease | 12 | 33 | 22.7 | 22.3 |
| Disease progression + dead | 19 | 36 | 27.8 | 27.2 |
| Treatment arm totals | 162 | 159 | 162.0 | 159.0 |
| p-value | < 0.001 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CHOP | CHOP | R-CHOP | CHOP | |
| CR | 53 | 47 | 50.1 | 49.9 |
| PR (includes CRu) | 215 | 206 | 210.9 | 210.1 |
| Stable disease (includes ‘minor response’ as well) | 6 | 17 | 11.5 | 11.5 |
| Disease progression | 3 | 6 | 4.5 | 4.5 |
| Dead | 2 | 2 | 2.0 | 2.0 |
| Treatment arm totals | 279 | 278 | 279 | 278 |
| p-value | 0.15 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CHOP | CHOP | R-CHOP | CHOP | |
| CR | 53 | 47 | 50.1 | 49.9 |
| PR (includes CRu) | 215 | 206 | 210.9 | 210.1 |
| Stable disease (includes ‘minor response’ as well) | 6 | 17 | 11.5 | 11.5 |
| Disease progression + dead | 5 | 8 | 6.5 | 6.5 |
| Treatment arm totals | 279 | 278 | 279.0 | 278.0 |
| p-value | 0.09 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-MCP | MCP | R-MCP | MCP | |
| CR | 52 | 24 | 39.7 | 36.3 |
| PR | 45 | 48 | 48.6 | 44.4 |
| < PR + disease progression | 8 | 24 | 16.7 | 15.3 |
| Treatment arm totals | 105 | 96 | 105 | 96 |
| p-value | < 0.001 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CHVPi | CHVPi | R-CHVPi | CHVPi | |
| CR | 63 | 29 | 45.0 | 47.0 |
| PR (includes CRu) | 101 | 127 | 111.5 | 116.5 |
| Stable disease | 2 | 9 | 5.4 | 5.6 |
| Disease progression | 8 | 18 | 12.7 | 13.3 |
| Dead | 1 | 0 | 0.5 | 0.5 |
| Treatment arm totals | 175 | 183 | 175.0 | 183.0 |
| p-value | < 0.001 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CHVPi | CHVPi | R-CHVPi | CHVPi | |
| CR | 63 | 29 | 45.0 | 47.0 |
| PR (includes CRu) | 101 | 127 | 111.5 | 116.5 |
| Stable disease + disease progression + dead | 11 | 27 | 18.6 | 19.4 |
| Treatment arm totals | 175 | 183 | 175.0 | 183.0 |
| p-value | < 0.001 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CHVPi | CHVPi | R-CHVPi | CHVPi | |
| CR | 90 | 71 | 78.70112 | 82.29888268 |
| PR (includes CRu) | 52 | 60 | 54.7486 | 57.25139665 |
| Stable disease | 1 | 3 | 1.955307 | 2.044692737 |
| Disease progression | 31 | 47 | 38.12849 | 39.87150838 |
| Dead | 1 | 2 | 1.46648 | 1.533519553 |
| Treatment arm totals | 175 | 183 | 175 | 183 |
| p-value | 0.123063805 | |||
| Outcome | Observed | Expected | ||
|---|---|---|---|---|
| R-CHVPi | CHVPi | R-CHVPi | CHVPi | |
| CR | 90 | 71 | 78.70112 | 82.29888268 |
| PR (includes CRu) | 52 | 60 | 54.7486 | 57.25139665 |
| Stable disease + disease progression + dead | 33 | 52 | 41.55028 | 43.44972067 |
| Treatment arm totals | 175 | 183 | 175 | 183 |
| p-value | 0.031978375 | |||
Appendix 14 Exploratory meta-analyses
Three exploratory meta-analyses were conducted to explore the results of synthesising the ORR, CR and PR from the four trials.
There were several problems with the validity of these analyses. First, the level of statistical heterogeneity calculated in RevMan using the I2-statistic was very high (range I2 = 56–88%). The I2-value describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance),106 and an I2-value of > 50% is considered to be a high enough level of heterogeneity to suggest that meta-analysis is not appropriate. Ideally, this high level of heterogeneity would be explored further and explained by estimating the predictive distribution of a new study. This was not undertaken owing to resource constraints.
Reasons for the high level of heterogeneity could be because of differences in treatment effects in the four trials. Examination of the CIs for the results from the individual trials showed that there was little overlap in the meta-analyses for CR, and to a lesser extent for PR, indicating evidence for heterogeneity of intervention effects. Indeed, the GLSG-200091,92 trial observed much higher ORR (a combination of CR and PR) for both the R-chemotherapy and chemotherapy-alone arms in comparison with the other studies. This was mostly accounted for by an increase in the numbers of PR (20% CR and 77% PR in the R-CHOP arm), whereas in the OSHO-39 trial93 there was a more even split between the CR/PR categories (50% CR and 43% PR in the R-MCP arm). As well as evidence for different intervention effects in the four trials, there are other possible explanations for the high level of heterogeneity. First, each study administered a different therapeutic intervention with respect to the chemotherapy regimen used: this included different chemotherapeutic agents (CVP, CHOP, MCP and CHVPi) and different regimens of treatment (3-weekly vs 4-weekly cycles; six cycles of treatment vs eight cycles of treatment). Second, there was a difference in the sample sizes of the studies, for example the GLSG-2000 trial91,92 was the largest trial with an ITT population of n = 557 patients, whereas the OSHO-39 trial93 was substantially smaller (n = 201).
The AG also notes that the choice of chemotherapeutic regimen is not solely determined by clinical efficacy. For example, R-CHOP is less likely to be given to patients who are elderly or unfit, but more likely to be given to treat aggressive or bulky disease, which may impact on the perceived efficacy. Additionally, the analyses assume that rituximab has no synergistic interaction with the chemotherapeutic component of a regimen for the treatment effect. The AG also comment that the analyses of ORR, CR and PR are not independent analyses given that the same patients are counted in more than one analysis.
The AG therefore believes the response rates from the individual trials to be a more robust estimator of the efficacy of the specific R-chemotherapy regimens. These are subsequently used in the decision model (see Chapter 4) rather than meta-analysed response rates. The findings from the meta-analyses are presented below for completeness, but the use of these is strongly cautioned against.
Overall response rate
The addition of rituximab to chemotherapy showed a significant improvement in ORR compared with chemotherapy alone when the four trials were combined, with a RR of 1.18 (95% CI 1.04 to 1.33, p = 0.01) (Figure 49). This translated as an 18% increased likelihood of being a responder (complete or partial) to treatment if receiving R-chemotherapy compared with chemotherapy alone.
Complete response rate
The addition of rituximab to chemotherapy showed a significant improvement in CR compared with chemotherapy alone when the four trials were combined, with a RR of 2.05 (95% CI 1.27 to 3.30, p = 0.003) (Figure 50). This translated as a 105% (i.e. over double) increased likelihood of being a CR to treatment if receiving R-chemotherapy compared with chemotherapy alone.
Partial response rate
The meta-analysis of PR incorporated the results from three trials (M39021 trial95,96 not being directly comparable: see Chapter 3, Summary of trials, for further details). For PR, the addition of rituximab to chemotherapy did not show a significant improvement in PR compared with chemotherapy; the RR calculated as 0.95 (95% CI 0.83 to 1.08, p = 0.44); this translated as a 5% decreased likelihood of being a PR if receiving R-chemotherapy compared with chemotherapy alone (Figure 51).
The meta-analysed PR appears counterintuitive when compared with the meta-analysed results for ORR and CR. However, this might be explained by the way in which the rituximab–chemotherapy combination affects the movement of the number of patients within each response category (‘non-responder’, ‘partial responder’ and ‘complete responder’). It is plausible that the rituximab–chemotherapy combination might ‘shift’ more non-responders to PRs relative to the chemotherapy alone group, thus increasing the numbers within the PR group. However, at the same time the rituximab–chemotherapy combination appears to have an effect in patients who would otherwise be PRs and ‘shift’ such patients to ‘complete responders’. This effect of shifting PRs to CRs would thus reduce the numbers within the PR group, negating the increase in numbers with the PR group as a result of the ‘non-responder’ to ‘PR’ conversion. These two effects may result in the number of PRs in the R-chemotherapy arm being similar to the number of PRs in the chemotherapy alone group.
Using the FL2000 18-month response rate data
The 6-month response rate data from the FL2000 trial94 were considered most appropriate for the meta-analysis of response rates, as the intervention and comparator treatment arms up until that time point were comparable with the other three trials. The trial participants went on to receive a further 12 months of treatment, which consisted of interferon only for both treatment arms and bimonthly CHVP for the comparator arm. The results are presented in Figures 52–54. The use of the 18-month response rate data did not materially affect the results, with the exception of reducing the median RR by 0.4 for CR and reducing statistically heterogeneity considerably in the analysis of PR.
FIGURE 49.
Forest plot for meta-analysis of ORR of the four trials. Chemo, chemotherapy.
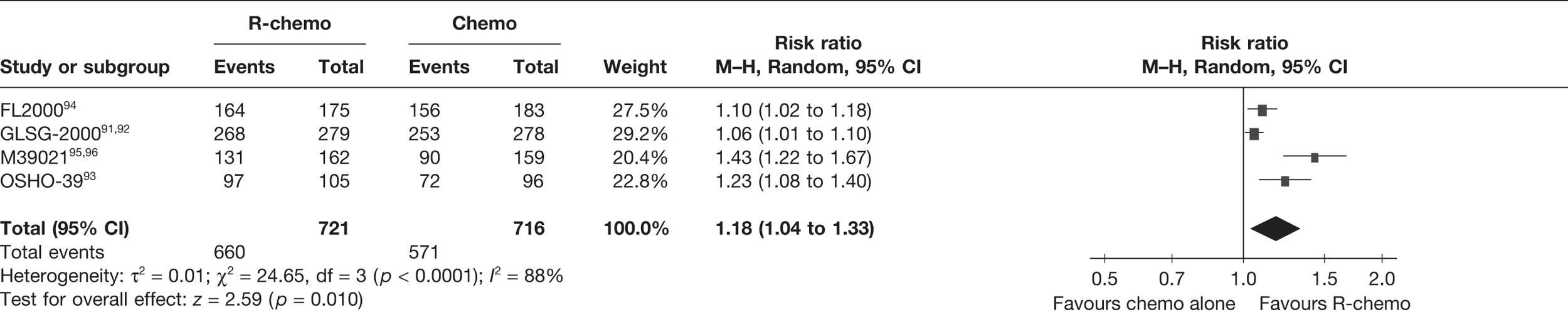
FIGURE 50.
Forest plot for meta-analysis of CR rate of the four trials. Chemo, chemotherapy.
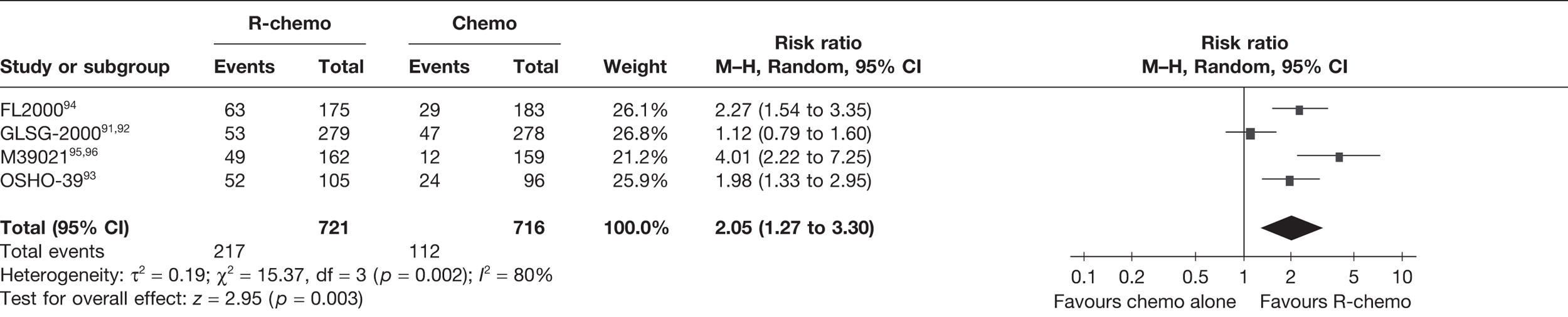
FIGURE 51.
Forest plot for meta-analysis of PR rate of the four trials. Chemo, chemotherapy.
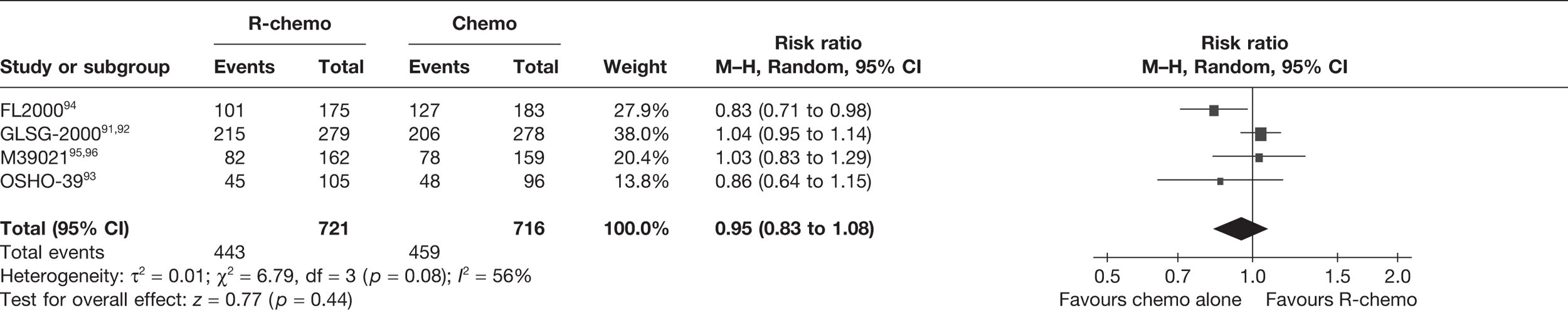
FIGURE 52.
Forest plot for meta-analysis of ORR using the FL200094 18-month response rates. Chemo, chemotherapy.
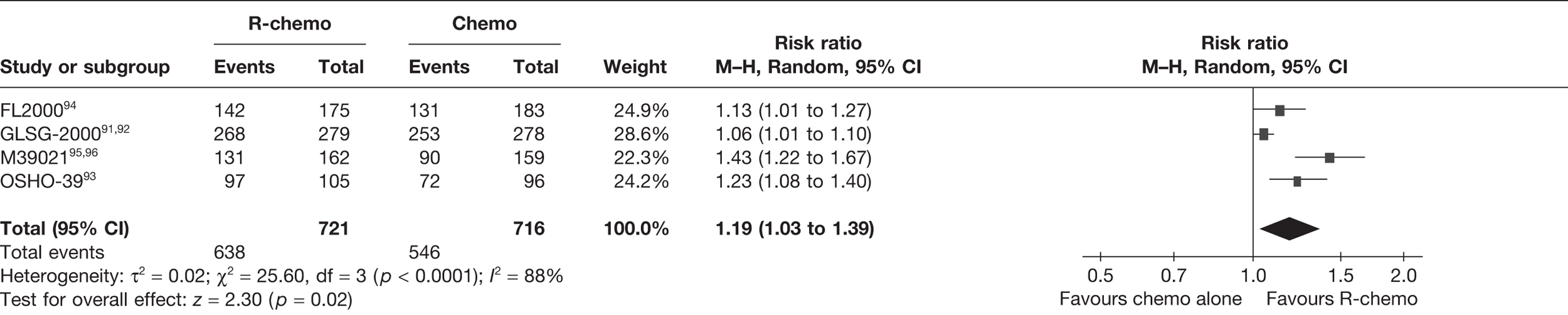
FIGURE 53.
Forest plot for meta-analysis of CR rate using the FL200094 18-month response rates. Chemo, chemotherapy.
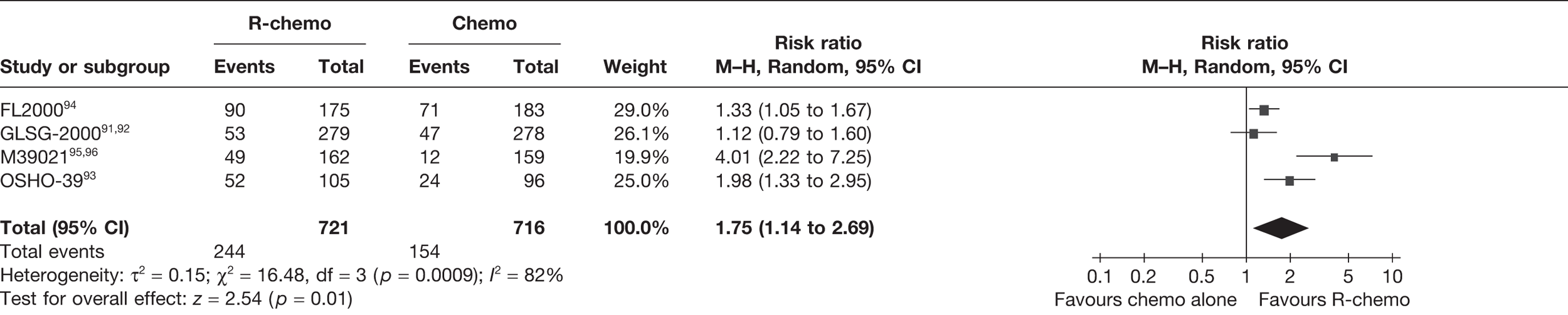
FIGURE 54.
Forest plot for meta-analysis of PR using the FL200094 18-month response rates. Chemo, chemotherapy.
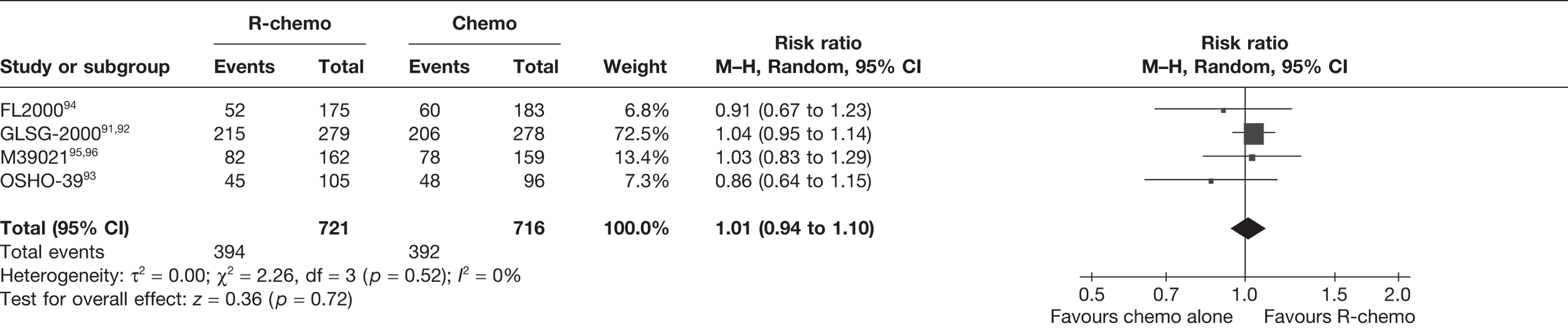
Appendix 15 Full results of sensitivity analyses
| Parameters | CVP | R-CVP (base case) | R-CVP (scenario) | ICER – cost per QALY gained | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LY | QALY | Cost | LY | QALY | Cost | LY | QALY | Cost | Base case | Scenario | |
| Base case | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Time horizon | |||||||||||
| 5 years | 4.06 | 2.98 | 23,278 | 4.37 | 3.22 | 28,360 | 4.43 | 3.27 | 38,683 | 20,998 | 54,094 |
| 10 years | 6.57 | 4.51 | 27,472 | 7.38 | 5.07 | 33,813 | 7.60 | 5.22 | 44,673 | 11,287 | 24,126 |
| Lifetime | 10.80 | 6.24 | 31,278 | 12.69 | 7.26 | 38,795 | 13.30 | 7.57 | 50,186 | 7360 | 14,125 |
| Discounting | |||||||||||
| 0% costs, 0% benefits | 9.86 | 7.73 | 35,632 | 11.50 | 9.09 | 44,002 | 12.03 | 9.52 | 56,241 | 6147 | 11,469 |
| 0% costs, 3.5% benefits | 9.86 | 5.99 | 35,632 | 11.50 | 6.95 | 44,002 | 12.03 | 7.25 | 56,241 | 8745 | 16,463 |
| 3.5% costs, 0% benefits | 9.86 | 7.73 | 30,793 | 11.50 | 9.09 | 38,183 | 12.03 | 9.52 | 49,520 | 5426 | 10,421 |
| Parametric distribution | |||||||||||
| Weibull | 9.76 | 5.94 | 31,041 | 11.37 | 6.89 | 38,669 | 11.81 | 7.15 | 50,199 | 8054 | 15,958 |
| Gompertz | 9.97 | 6.05 | 30,279 | 12.18 | 7.26 | 35,349 | 12.91 | 7.66 | 45,421 | 4174 | 9419 |
| Death event in PFS | |||||||||||
| None | 10.30 | 6.25 | 32,058 | 11.72 | 7.07 | 38,766 | 12.22 | 7.35 | 50,046 | 8224 | 16,386 |
| CVP arm | 9.86 | 5.99 | 30,793 | 11.35 | 6.87 | 37,759 | 11.89 | 7.17 | 49,139 | 7984 | 15,599 |
| R-CVP arm | 10.04 | 6.10 | 31,327 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 8080 | 15,914 |
| Resistance to rituximab (%) | |||||||||||
| –10 | 9.86 | 5.99 | 30,793 | 11.19 | 6.79 | 38,229 | 11.76 | 7.11 | 49,565 | 9379 | 16,851 |
| –15 | 9.86 | 5.99 | 30,793 | 11.01 | 6.70 | 38,246 | 11.61 | 7.03 | 49,579 | 10,616 | 18,100 |
| –20 | 9.86 | 5.99 | 30,793 | 10.82 | 6.60 | 38,249 | 11.45 | 6.95 | 49,586 | 12,328 | 19,650 |
| –25 | 9.86 | 5.99 | 30,793 | 10.62 | 6.50 | 38,235 | 11.28 | 6.86 | 49,580 | 14,870 | 21,624 |
| –30 | 9.86 | 5.99 | 30,793 | 10.41 | 6.38 | 38,210 | 11.09 | 6.77 | 49,563 | 19,102 | 24,234 |
| Utility values | |||||||||||
| PFS1 = 0.805; PFS2 = 0.805; disease progression = 0.7363 | 9.86 | 5.81 | 30,793 | 11.50 | 7.01 | 38,183 | 12.03 | 7.39 | 49,520 | 6180 | 11,862 |
| PFS1 = 0.805; PFS2 = 0.805; disease progression = 0.7363 | 9.86 | 6.08 | 30,793 | 11.50 | 7.11 | 38,183 | 12.03 | 7.43 | 49,520 | 7147 | 13,804 |
| –10% | 9.86 | 5.40 | 30,793 | 11.50 | 6.26 | 38,183 | 12.03 | 6.52 | 49,520 | 8578 | 16,621 |
| –20% | 9.86 | 4.80 | 30,793 | 11.50 | 5.56 | 38,183 | 12.03 | 5.80 | 49,520 | 9650 | 18,699 |
| –30% | 9.86 | 4.20 | 30,793 | 11.50 | 4.87 | 38,183 | 12.03 | 5.07 | 49,520 | 11,029 | 21,370 |
| Higher in PFS1 (+10%) | 9.86 | 6.12 | 30,793 | 11.50 | 7.27 | 38,183 | 12.03 | 7.63 | 49,520 | 6447 | 12,395 |
| No disutility | 9.86 | 6.00 | 30,793 | 11.50 | 6.96 | 38,183 | 12.03 | 7.25 | 49,520 | 7704 | 14,928 |
| Disutility = –10% | 9.86 | 6.00 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7715 | 14,949 |
| Disutility = –20% | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.24 | 49,520 | 7725 | 14,969 |
| Disutility = –30% | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.24 | 49,520 | 7736 | 14,990 |
| Treatment pathway | |||||||||||
| Second line after progression | 10.14 | 6.17 | 30,228 | 11.60 | 7.01 | 37,977 | 12.13 | 7.31 | 49,315 | 9230 | 16,828 |
| R-CVP no retreatment if early relapse | 9.86 | 5.99 | 30,793 | 11.31 | 6.83 | 37,550 | 11.87 | 7.15 | 49,026 | 8123 | 15,816 |
| Patients receive CHOP and R-CHOP instead of salvage HDT and R-HDT | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Patients receive CHOP and R-CHOP instead of FC and R-FC | 10.11 | 6.14 | 31,913 | 11.73 | 7.08 | 39,172 | 12.24 | 7.36 | 50,394 | 7742 | 15,145 |
| Last three scenarios | 10.11 | 6.14 | 31,913 | 11.52 | 6.94 | 38,193 | 12.07 | 7.25 | 49,636 | 7841 | 15,919 |
| All patients receive salvage with rituximab | 12.14 | 7.14 | 38,358 | 13.36 | 7.86 | 44,421 | 13.72 | 8.07 | 55,262 | 8506 | 18,325 |
| All patients receive salvage without rituximab | 6.99 | 4.45 | 28,838 | 9.18 | 5.74 | 36,776 | 9.96 | 6.18 | 48,402 | 6159 | 11,273 |
| All patients receive CHOP | 9.36 | 5.67 | 28,377 | 11.11 | 6.71 | 36,174 | 11.71 | 7.05 | 47,764 | 7553 | 14,127 |
| All patients receive R-CHOP | 10.11 | 6.14 | 31,913 | 11.73 | 7.08 | 39,172 | 12.27 | 7.38 | 50,519 | 7742 | 15,034 |
| Effectiveness of FC | |||||||||||
| No loss of response | 10.10 | 6.14 | 31,188 | 11.73 | 7.08 | 38,530 | 12.24 | 7.36 | 49,834 | 7827 | 15,271 |
| Response 10% lower than CHOP regimens | 9.98 | 6.07 | 30,990 | 11.62 | 7.01 | 38,358 | 12.13 | 7.30 | 49,679 | 7776 | 15,114 |
| Response 30% lower than CHOP regimens | 9.74 | 5.93 | 30,598 | 11.39 | 6.89 | 38,010 | 11.93 | 7.19 | 49,362 | 7676 | 14,832 |
| Response 40% lower than CHOP regimens | 9.61 | 5.85 | 30,400 | 11.28 | 6.83 | 37,840 | 11.82 | 7.14 | 49,204 | 7615 | 14,673 |
| Response 50% lower than CHOP regimens | 9.49 | 5.78 | 30,212 | 11.17 | 6.77 | 37,676 | 11.72 | 7.08 | 49,056 | 7565 | 14,523 |
| PFS reduction –10% | 9.72 | 5.92 | 30,835 | 11.39 | 6.89 | 38,223 | 11.92 | 7.19 | 49,559 | 7625 | 14,754 |
| PFS reduction –20% | 9.57 | 5.83 | 30,870 | 11.25 | 6.81 | 38,259 | 11.79 | 7.12 | 49,593 | 7520 | 14,525 |
| PFS reduction –30% | 9.39 | 5.73 | 30,875 | 11.09 | 6.73 | 38,270 | 11.64 | 7.04 | 49,608 | 7409 | 14,282 |
| Costing of salvage therapy | |||||||||||
| Response rate same as CHOP regimens | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Response rate 20% greater than CHOP regimens | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Response rate 30% greater than CHOP regimens | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| No. of cycles of ESHAP = 3 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| No. of cycles of ESHAP = 4 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Harvest success rate: 1 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Harvest success rate: 0.95 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Harvest success rate: 0.90 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Harvest success rate: 0.85 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Harvest success rate: 0.75 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Only one administration | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| One additional administration | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| AEs | |||||||||||
| No AEs | 9.86 | 6.00 | 30,337 | 11.50 | 6.96 | 37,390 | 12.03 | 7.25 | 48,637 | 7353 | 14,588 |
| Costs +20% | 9.86 | 5.99 | 30,884 | 11.50 | 6.95 | 38,341 | 12.03 | 7.25 | 49,697 | 7791 | 15,027 |
| Costs –20% | 9.86 | 5.99 | 30,702 | 11.50 | 6.95 | 38,024 | 12.03 | 7.25 | 49,344 | 7650 | 14,891 |
| No. of cycles | |||||||||||
| Six cycles for CHOP | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.03 | 7.25 | 49,520 | 7720 | 14,959 |
| Six cycles for FC | 9.87 | 6.01 | 32,540 | 11.52 | 6.96 | 39,728 | 12.04 | 7.26 | 50,926 | 7521 | 14,714 |
| Management costs | |||||||||||
| Cost +20% | 9.86 | 5.99 | 31,730 | 11.50 | 6.95 | 39,123 | 12.03 | 7.25 | 50,962 | 7724 | 15,362 |
| Cost –20% | 9.86 | 5.99 | 29,856 | 11.50 | 6.95 | 37,242 | 12.03 | 7.25 | 48,078 | 7716 | 14,556 |
| Cost pharmacy = 35 | 9.86 | 5.99 | 30,948 | 11.50 | 6.95 | 38,459 | 12.03 | 7.25 | 49,951 | 7847 | 15,179 |
| No monitoring costs | 9.86 | 5.99 | 28,037 | 11.50 | 6.95 | 34,234 | 12.03 | 7.25 | 46,450 | 6475 | 14,708 |
| Monitoring cost +20% | 9.86 | 5.99 | 31,344 | 11.50 | 6.95 | 38,972 | 12.03 | 7.25 | 50,134 | 7969 | 15,009 |
| Monitoring cost –20% | 9.86 | 5.99 | 30,242 | 11.50 | 6.95 | 37,393 | 12.03 | 7.25 | 48,906 | 7471 | 14,909 |
| No third-line treatment costs | 9.86 | 5.99 | 26,933 | 11.50 | 6.95 | 34,999 | 12.03 | 7.25 | 46,495 | 8427 | 15,626 |
| No cost palliative care | 9.86 | 5.99 | 26,223 | 11.50 | 6.95 | 34,564 | 12.03 | 7.25 | 46,232 | 8715 | 15,984 |
| No terminal care costs | 9.86 | 5.99 | 29,000 | 11.50 | 6.95 | 36,789 | 12.03 | 7.25 | 48,253 | 8138 | 15,379 |
| No terminal or palliative care costs | 9.86 | 5.99 | 24,429 | 11.50 | 6.95 | 33,170 | 12.03 | 7.25 | 44,965 | 9132 | 16,404 |
| Maximum age (years) at which aggressive therapy is given | |||||||||||
| 60 | 9.75 | 5.93 | 30,441 | 11.41 | 6.90 | 37,903 | 11.95 | 7.21 | 49,281 | 7690 | 14,821 |
| 70 | 9.95 | 6.05 | 31,118 | 11.59 | 7.00 | 38,465 | 12.10 | 7.29 | 49,764 | 7735 | 15,040 |
| 75 | 10.02 | 6.09 | 31,419 | 11.65 | 7.03 | 38,724 | 12.17 | 7.32 | 49,993 | 7748 | 15,117 |
| 80 | 10.07 | 6.12 | 31,653 | 11.70 | 7.06 | 38,929 | 12.21 | 7.34 | 50,172 | 7747 | 15,149 |
| BSA (m2) | |||||||||||
| 1.6 | 9.86 | 5.99 | 28,432 | 11.50 | 6.95 | 34,266 | 12.03 | 7.25 | 43,586 | 6095 | 12,105 |
| 1.7 | 9.86 | 5.99 | 30,110 | 11.50 | 6.95 | 36,994 | 12.03 | 7.25 | 47,684 | 7192 | 14,038 |
| 1.8 | 9.86 | 5.99 | 30,110 | 11.50 | 6.95 | 36,994 | 12.03 | 7.25 | 47,684 | 7192 | 14,038 |
| 1.9 | 9.86 | 5.99 | 31,550 | 11.50 | 6.95 | 39,512 | 12.03 | 7.25 | 51,584 | 8318 | 16,003 |
| Maximum time (years) in PFS1 | |||||||||||
| 5 | 9.68 | 5.90 | 31,256 | 10.60 | 6.48 | 40,882 | 10.79 | 6.60 | 53,183 | 16,656 | 31,354 |
| 6 | 9.72 | 5.92 | 31,170 | 10.74 | 6.56 | 40,500 | 10.99 | 6.72 | 52,669 | 14,527 | 27,043 |
| 7 | 9.74 | 5.94 | 31,103 | 10.86 | 6.63 | 40,182 | 11.16 | 6.81 | 52,240 | 13,044 | 24,178 |
| 8 | 9.77 | 5.95 | 31,051 | 10.97 | 6.69 | 39,911 | 11.30 | 6.89 | 51,873 | 11,964 | 22,151 |
| 9 | 9.78 | 5.96 | 31,009 | 11.05 | 6.74 | 39,682 | 11.41 | 6.95 | 51,564 | 11,143 | 20,651 |
| 10 | 9.80 | 5.97 | 30,975 | 11.13 | 6.78 | 39,490 | 11.52 | 7.01 | 51,301 | 10,513 | 19,516 |
| 11 | 9.81 | 5.97 | 30,948 | 11.19 | 6.81 | 39,326 | 11.60 | 7.05 | 51,080 | 10,016 | 18,645 |
| 12 | 9.82 | 5.98 | 30,926 | 11.25 | 6.84 | 39,183 | 11.68 | 7.09 | 50,885 | 9613 | 17,951 |
| 13 | 9.82 | 5.98 | 30,906 | 11.29 | 6.86 | 39,058 | 11.74 | 7.12 | 50,717 | 9287 | 17,394 |
| 14 | 9.83 | 5.98 | 30,890 | 11.33 | 6.88 | 38,948 | 11.79 | 7.15 | 50,568 | 9018 | 16,944 |
| 15 | 9.84 | 5.99 | 30,876 | 11.37 | 6.89 | 38,854 | 11.84 | 7.17 | 50,439 | 8797 | 16,577 |
| 16 | 9.84 | 5.99 | 30,864 | 11.40 | 6.91 | 38,774 | 11.88 | 7.18 | 50,329 | 8616 | 16,274 |
| 17 | 9.84 | 5.99 | 30,855 | 11.42 | 6.92 | 38,701 | 11.91 | 7.20 | 50,230 | 8461 | 16,023 |
| 18 | 9.85 | 5.99 | 30,846 | 11.44 | 6.93 | 38,635 | 11.94 | 7.21 | 50,141 | 8331 | 15,815 |
| 19 | 9.85 | 5.99 | 30,838 | 11.46 | 6.93 | 38,576 | 11.97 | 7.22 | 50,063 | 8223 | 15,642 |
| Greater OS (%) for R-CHOP compared with CHOP | |||||||||||
| 5 | 10.41 | 6.26 | 31,444 | 11.95 | 7.16 | 38,677 | 12.41 | 7.42 | 49,930 | 8067 | 15,969 |
| 10 | 10.94 | 6.51 | 32,043 | 12.37 | 7.35 | 39,130 | 12.76 | 7.58 | 50,307 | 8441 | 17,080 |
| 15 | 11.42 | 6.73 | 32,572 | 12.74 | 7.52 | 39,531 | 13.08 | 7.72 | 50,638 | 8837 | 18,263 |
| 20 | 11.85 | 6.92 | 33,033 | 13.07 | 7.66 | 39,878 | 13.36 | 7.84 | 50,924 | 9232 | 19,489 |
| 25 | 12.21 | 7.09 | 33,424 | 13.36 | 7.79 | 40,170 | 13.60 | 7.94 | 51,163 | 9613 | 20,696 |
| Maintenance duration effect (months) | |||||||||||
| 36 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 11.97 | 7.22 | 49,684 | 7720 | 15,469 |
| 48 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.08 | 7.27 | 49,373 | 7720 | 14,524 |
| 60 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.17 | 7.32 | 49,115 | 7720 | 13,828 |
| 72 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.24 | 7.36 | 48,896 | 7720 | 13,305 |
| HR maintenance | |||||||||||
| 0.48 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 12.13 | 7.31 | 49,411 | 7720 | 14,205 |
| 0.66 | 9.86 | 5.99 | 30,793 | 11.50 | 6.95 | 38,183 | 11.88 | 7.16 | 49,676 | 7720 | 16,210 |
| Parameters | CHOP | R-CHOP (base case) | R-CHOP (scenario) | ICER – cost per QALY gained | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LY | QALY | Cost | LY | QALY | Cost | LY | QALY | Cost | Base case | Scenario | |
| Base case | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 13.02 | 7.72 | 54,134 | 10,834 | 21,687 |
| Time horizon | |||||||||||
| 5 years | 4.28 | 3.13 | 25,929 | 4.44 | 3.25 | 30,003 | 4.52 | 3.31 | 42,241 | 33,975 | 91,356 |
| 10 years | 7.19 | 4.90 | 30,458 | 7.63 | 5.21 | 35,660 | 7.90 | 5.40 | 48,618 | 16,650 | 36,367 |
| Lifetime | 13.15 | 7.23 | 35,994 | 14.07 | 7.78 | 41,705 | 14.79 | 8.16 | 55,183 | 10,362 | 20,533 |
| Discounting | |||||||||||
| 0% costs, 0% benefits | 11.55 | 9.01 | 40,994 | 12.40 | 9.76 | 47,222 | 13.02 | 10.28 | 61,687 | 8306 | 16,295 |
| 0% costs, 3.5% benefits | 11.55 | 6.84 | 40,994 | 12.40 | 7.37 | 47,222 | 13.02 | 7.72 | 61,687 | 11,788 | 23,434 |
| 3.5% costs, 0% benefits | 11.55 | 9.01 | 34,983 | 12.40 | 9.76 | 40,708 | 13.02 | 10.28 | 54,134 | 7634 | 15,081 |
| Parametric distribution | |||||||||||
| Weibull | 11.43 | 6.77 | 35,483 | 12.16 | 7.25 | 41,186 | 12.75 | 7.59 | 55,009 | 12,030 | 23,824 |
| Gompertz | 11.72 | 6.92 | 34,115 | 12.87 | 7.58 | 36,733 | 13.80 | 8.09 | 48,766 | 3941 | 12,490 |
| Death event in PFS | |||||||||||
| None | 12.04 | 7.11 | 36,344 | 12.61 | 7.48 | 41,296 | 13.21 | 7.82 | 54,651 | 13,463 | 25,867 |
| CVP arm | 11.55 | 6.84 | 34,983 | 12.25 | 7.28 | 40,281 | 12.88 | 7.65 | 53,759 | 11,872 | 23,141 |
| R-CVP arm | 11.76 | 6.95 | 35,559 | 12.40 | 7.37 | 40,708 | 13.02 | 7.72 | 54,134 | 12,470 | 24,200 |
| Resistance to rituximab | |||||||||||
| –10% | 11.55 | 6.84 | 34,983 | 12.18 | 7.25 | 40,769 | 12.82 | 7.62 | 54,194 | 13,843 | 24,447 |
| –15% | 11.55 | 6.84 | 34,983 | 12.06 | 7.19 | 40,796 | 12.71 | 7.57 | 54,220 | 16,328 | 26,301 |
| –20% | 11.55 | 6.84 | 34,983 | 11.93 | 7.13 | 40,814 | 12.59 | 7.51 | 54,239 | 20,163 | 28,629 |
| –25% | 11.55 | 6.84 | 34,983 | 11.78 | 7.05 | 40,822 | 12.46 | 7.45 | 54,252 | 26,939 | 31,646 |
| –30% | 11.55 | 6.84 | 34,983 | 11.62 | 6.97 | 40,826 | 12.32 | 7.38 | 54,260 | 42,361 | 35,734 |
| Utility values | |||||||||||
| PFS1 = 0.805; PFS2 = 0.805; disease progression = 0.7363 | 11.55 | 6.66 | 34,983 | 12.40 | 7.45 | 40,708 | 13.02 | 7.92 | 54,134 | 7167 | 15,113 |
| PFS1 = 0.805; PFS2 = 0.805; disease progression = 0.7363 | 11.55 | 6.95 | 34,983 | 12.40 | 7.55 | 40,708 | 13.02 | 7.94 | 54,134 | 9518 | 19,354 |
| –10% | 11.55 | 6.15 | 34,983 | 12.40 | 6.63 | 40,708 | 13.02 | 6.95 | 54,134 | 12,038 | 24,097 |
| –20% | 11.55 | 5.47 | 34,983 | 12.40 | 5.89 | 40,708 | 13.02 | 6.18 | 54,134 | 13,543 | 27,109 |
| –30% | 11.55 | 4.79 | 34,983 | 12.40 | 5.16 | 40,708 | 13.02 | 5.40 | 54,134 | 15,478 | 30,982 |
| Higher in PFS1 (+10%) | 11.55 | 7.02 | 34,983 | 12.40 | 7.73 | 40,708 | 13.02 | 8.17 | 54,134 | 8019 | 16,628 |
| No disutility | 11.55 | 6.87 | 34,983 | 12.40 | 7.40 | 40,708 | 13.02 | 7.75 | 54,134 | 10,760 | 21,580 |
| Disutility = –10% | 11.55 | 6.85 | 34,983 | 12.40 | 7.38 | 40,708 | 13.02 | 7.73 | 54,134 | 10,809 | 21,651 |
| Disutility = –20% | 11.55 | 6.83 | 34,983 | 12.40 | 7.35 | 40,708 | 13.02 | 7.71 | 54,134 | 10,860 | 21,724 |
| Disutility = –30% | 11.55 | 6.81 | 34,983 | 12.40 | 7.33 | 40,708 | 13.02 | 7.69 | 54,134 | 10,910 | 21,796 |
| Treatment pathway | |||||||||||
| Second line after progression | 11.60 | 6.87 | 34,821 | 12.48 | 7.41 | 40,765 | 13.10 | 7.76 | 54,190 | 10,945 | 21,576 |
| R-CVP no retreatment if early relapse | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 13.02 | 7.72 | 54,134 | 10,834 | 21,687 |
| Patients receive CHOP and R-CHOP instead of salvage HDT and R-HDT | 10.30 | 6.25 | 31,905 | 11.83 | 7.12 | 38,928 | 12.51 | 7.50 | 52,598 | 8058 | 16,517 |
| Patients receive CHOP and R-CHOP instead of FC and R-FC | 11.80 | 6.98 | 36,067 | 12.61 | 7.48 | 41,493 | 13.22 | 7.82 | 54,882 | 10,833 | 22,251 |
| Last three scenarios | 10.54 | 6.39 | 32,989 | 12.04 | 7.23 | 39,713 | 12.70 | 7.60 | 53,346 | 7967 | 16,750 |
| All patients receive salvage with rituximab | 12.45 | 7.32 | 39,045 | 13.63 | 8.00 | 45,002 | 14.06 | 8.25 | 57,917 | 8745 | 20,293 |
| All patients receive salvage without rituximab | 7.60 | 4.81 | 30,140 | 9.76 | 6.06 | 37,961 | 10.68 | 6.59 | 51,809 | 6245 | 12,153 |
| All patients receive CHOP | 9.83 | 5.95 | 29,624 | 11.56 | 6.95 | 37,321 | 12.27 | 7.35 | 51,130 | 7714 | 15,337 |
| All patients receive R-CHOP | 10.54 | 6.39 | 32,989 | 12.13 | 7.29 | 40,137 | 12.77 | 7.65 | 53,656 | 7933 | 16,436 |
| Effectiveness of FC | |||||||||||
| No loss of response | 11.80 | 6.97 | 35,363 | 12.61 | 7.48 | 41,013 | 13.21 | 7.82 | 54,415 | 11,268 | 22,509 |
| Response 10% lower than CHOP regimens | 11.67 | 6.91 | 35,174 | 12.50 | 7.42 | 40,861 | 13.11 | 7.77 | 54,274 | 11,045 | 22,098 |
| Response 30% lower than CHOP regimens | 11.44 | 6.77 | 34,790 | 12.30 | 7.31 | 40,548 | 12.92 | 7.67 | 53,986 | 10,669 | 21,348 |
| Response 40% lower than CHOP regimens | 11.31 | 6.70 | 34,599 | 12.19 | 7.25 | 40,390 | 12.82 | 7.62 | 53,840 | 10,489 | 20,980 |
| Response 50% lower than CHOP regimens | 11.20 | 6.63 | 34,420 | 12.09 | 7.20 | 40,237 | 12.73 | 7.57 | 53,706 | 10,331 | 20,622 |
| PFS reduction –10% | 11.42 | 6.76 | 35,026 | 12.29 | 7.30 | 40,744 | 12.91 | 7.66 | 54,170 | 10,582 | 21,233 |
| PFS reduction –20% | 11.27 | 6.68 | 35,061 | 12.15 | 7.23 | 40,774 | 12.79 | 7.60 | 54,200 | 10,310 | 20,733 |
| PFS reduction –30% | 11.10 | 6.58 | 35,071 | 12.00 | 7.15 | 40,784 | 12.65 | 7.53 | 54,216 | 10,019 | 20,199 |
| Costing of salvage therapy | |||||||||||
| Response rate same as CHOP regimens | 11.55 | 6.84 | 34,216 | 12.40 | 7.37 | 40,145 | 13.02 | 7.72 | 53,652 | 11,221 | 22,011 |
| Response rate 20% greater than CHOP regimens | 11.55 | 6.84 | 35,750 | 12.40 | 7.37 | 41,271 | 13.02 | 7.72 | 54,615 | 10,448 | 21,364 |
| Response rate 30% greater than CHOP regimens | 11.55 | 6.84 | 36,043 | 12.40 | 7.37 | 41,534 | 13.02 | 7.72 | 54,834 | 10,393 | 21,281 |
| No. of cycles of ESHAP = 3 | 11.55 | 6.84 | 36,271 | 12.40 | 7.37 | 41,624 | 13.02 | 7.72 | 54,921 | 10,132 | 21,121 |
| No. of cycles of ESHAP = 4 | 11.55 | 6.84 | 37,558 | 12.40 | 7.37 | 42,540 | 13.02 | 7.72 | 55,708 | 9430 | 20,555 |
| Harvest success rate: 1 | 11.55 | 6.84 | 37,093 | 12.40 | 7.37 | 42,255 | 13.02 | 7.72 | 55,457 | 9771 | 20,798 |
| Harvest success rate: 0.95 | 11.55 | 6.84 | 36,565 | 12.40 | 7.37 | 41,869 | 13.02 | 7.72 | 55,126 | 10,037 | 21,020 |
| Harvest success rate: 0.90 | 11.55 | 6.84 | 36,038 | 12.40 | 7.37 | 41,482 | 13.02 | 7.72 | 54,796 | 10,303 | 21,243 |
| Harvest success rate: 0.85 | 11.55 | 6.84 | 35,511 | 12.40 | 7.37 | 41,095 | 13.02 | 7.72 | 54,465 | 10,569 | 21,465 |
| Harvest success rate: 0.75 | 11.55 | 6.84 | 34,456 | 12.40 | 7.37 | 40,321 | 13.02 | 7.72 | 53,803 | 11,100 | 21,910 |
| Only one administration | 11.55 | 6.84 | 34,349 | 12.40 | 7.37 | 40,224 | 13.02 | 7.72 | 53,722 | 11,119 | 21,940 |
| One additional administration | 11.55 | 6.84 | 34,561 | 12.40 | 7.37 | 40,385 | 13.02 | 7.72 | 53,859 | 11,024 | 21,856 |
| AEs | |||||||||||
| No AEs | 11.55 | 6.87 | 34,028 | 12.40 | 7.40 | 39,604 | 13.02 | 7.75 | 52,920 | 10,479 | 21,288 |
| Costs +20% | 11.55 | 6.84 | 35,174 | 12.40 | 7.37 | 40,929 | 13.02 | 7.72 | 54,376 | 10,891 | 21,746 |
| Costs –20% | 11.55 | 6.84 | 34,792 | 12.40 | 7.37 | 40,487 | 13.02 | 7.72 | 53,891 | 10,778 | 21,629 |
| No. of cycles | |||||||||||
| Six cycles for CHOP | 11.51 | 6.81 | 34,234 | 12.27 | 7.29 | 37,122 | 12.93 | 7.67 | 50,718 | 5951 | 19,092 |
| Six cycles for FC | 11.57 | 6.85 | 36,680 | 12.42 | 7.37 | 42,054 | 13.03 | 7.73 | 55,398 | 10,206 | 21,261 |
| Management costs | |||||||||||
| Cost +20% | 11.55 | 6.84 | 35,813 | 12.40 | 7.37 | 41,550 | 13.02 | 7.72 | 55,591 | 10,859 | 22,398 |
| Cost –20% | 11.55 | 6.84 | 34,154 | 12.40 | 7.37 | 39,865 | 13.02 | 7.72 | 52,677 | 10,810 | 20,977 |
| Cost pharmacy = 35 | 11.55 | 6.84 | 35,062 | 12.40 | 7.37 | 40,921 | 13.02 | 7.72 | 54,545 | 11,089 | 22,064 |
| No monitoring costs | 11.55 | 6.84 | 31,292 | 12.40 | 7.37 | 36,160 | 13.02 | 7.72 | 50,627 | 9214 | 21,897 |
| Monitoring cost +20% | 11.55 | 6.84 | 35,722 | 12.40 | 7.37 | 41,617 | 13.02 | 7.72 | 54,835 | 11,159 | 21,646 |
| Monitoring cost –20% | 11.55 | 6.84 | 34,245 | 12.40 | 7.37 | 39,798 | 13.02 | 7.72 | 53,432 | 10,510 | 21,729 |
| No third-line treatment costs | 11.55 | 6.84 | 33,111 | 12.40 | 7.37 | 38,881 | 13.02 | 7.72 | 52,501 | 10,921 | 21,960 |
| No cost palliative care | 11.55 | 6.84 | 29,502 | 12.40 | 7.37 | 36,764 | 13.02 | 7.72 | 50,769 | 13,744 | 24,085 |
| No terminal care costs | 11.55 | 6.84 | 33,549 | 12.40 | 7.37 | 39,521 | 13.02 | 7.72 | 53,100 | 11,303 | 22,141 |
| No terminal or palliative care costs | 11.55 | 6.84 | 28,067 | 12.40 | 7.37 | 35,577 | 13.02 | 7.72 | 49,735 | 14,213 | 24,538 |
| Maximum age (years) at which aggressive therapy is given | |||||||||||
| 60 | 11.13 | 6.63 | 33,780 | 12.16 | 7.25 | 39,946 | 12.81 | 7.62 | 53,470 | 9832 | 19,745 |
| 70 | 11.90 | 7.02 | 36,129 | 12.63 | 7.47 | 41,530 | 13.24 | 7.82 | 54,886 | 11,758 | 23,230 |
| 75 | 12.17 | 7.16 | 37,152 | 12.80 | 7.56 | 42,270 | 13.40 | 7.90 | 55,592 | 12,763 | 24,704 |
| 80 | 12.33 | 7.25 | 37,973 | 12.91 | 7.61 | 42,906 | 13.52 | 7.96 | 56,187 | 13,377 | 25,559 |
| BSA (m2) | |||||||||||
| 1.6 | 11.55 | 6.84 | 33,716 | 12.40 | 7.37 | 37,617 | 13.02 | 7.72 | 48,435 | 7384 | 16,669 |
| 1.7 | 11.55 | 6.84 | 34,665 | 12.40 | 7.37 | 39,796 | 13.02 | 7.72 | 52,385 | 9712 | 20,067 |
| 1.8 | 11.55 | 6.84 | 34,665 | 12.40 | 7.37 | 39,796 | 13.02 | 7.72 | 52,385 | 9712 | 20,067 |
| 1.9 | 11.55 | 6.84 | 35,378 | 12.40 | 7.37 | 41,768 | 13.02 | 7.72 | 56,143 | 12,094 | 23,517 |
| Maximum time (years) in PFS1 | |||||||||||
| 5 | 11.33 | 6.71 | 35,877 | 11.56 | 6.91 | 44,464 | 11.86 | 7.09 | 59,233 | 43,733 | 61,115 |
| 6 | 11.37 | 6.74 | 35,698 | 11.69 | 6.99 | 43,896 | 12.03 | 7.20 | 58,465 | 32,857 | 49,043 |
| 7 | 11.41 | 6.76 | 35,567 | 11.79 | 7.05 | 43,429 | 12.18 | 7.29 | 57,834 | 26,749 | 41,756 |
| 8 | 11.43 | 6.78 | 35,462 | 11.88 | 7.11 | 43,038 | 12.31 | 7.37 | 57,300 | 22,835 | 36,904 |
| 9 | 11.45 | 6.79 | 35,379 | 11.96 | 7.15 | 42,708 | 12.42 | 7.43 | 56,854 | 20,149 | 33,528 |
| 10 | 11.47 | 6.80 | 35,312 | 12.03 | 7.19 | 42,428 | 12.51 | 7.48 | 56,474 | 18,210 | 31,050 |
| 11 | 11.49 | 6.81 | 35,262 | 12.09 | 7.22 | 42,202 | 12.59 | 7.52 | 56,167 | 16,745 | 29,166 |
| 12 | 11.50 | 6.81 | 35,220 | 12.14 | 7.25 | 42,006 | 12.66 | 7.56 | 55,900 | 15,607 | 27,698 |
| 13 | 11.51 | 6.82 | 35,184 | 12.19 | 7.27 | 41,837 | 12.72 | 7.59 | 55,673 | 14,718 | 26,544 |
| 14 | 11.52 | 6.82 | 35,153 | 12.23 | 7.29 | 41,686 | 12.78 | 7.61 | 55,470 | 13,999 | 25,615 |
| 15 | 11.53 | 6.82 | 35,128 | 12.26 | 7.30 | 41,559 | 12.82 | 7.64 | 55,298 | 13,427 | 24,869 |
| 16 | 11.53 | 6.83 | 35,106 | 12.29 | 7.32 | 41,454 | 12.86 | 7.65 | 55,152 | 12,963 | 24,252 |
| 17 | 11.54 | 6.83 | 35,089 | 12.31 | 7.33 | 41,359 | 12.90 | 7.67 | 55,024 | 12,576 | 23,746 |
| 18 | 11.54 | 6.83 | 35,074 | 12.34 | 7.34 | 41,275 | 12.93 | 7.68 | 54,910 | 12,256 | 23,326 |
| 19 | 11.55 | 6.83 | 35,060 | 12.35 | 7.35 | 41,201 | 12.95 | 7.69 | 54,811 | 11,995 | 22,985 |
| Greater OS (%) for R-CHOP compared with CHOP | |||||||||||
| 5 | 11.71 | 6.91 | 35,155 | 12.52 | 7.42 | 40,825 | 13.13 | 7.77 | 54,241 | 11,213 | 22,292 |
| 10 | 11.85 | 6.98 | 35,308 | 12.62 | 7.47 | 40,930 | 13.23 | 7.81 | 54,337 | 11,588 | 22,876 |
| 15 | 11.97 | 7.04 | 35,439 | 12.71 | 7.51 | 41,022 | 13.31 | 7.85 | 54,420 | 11,950 | 23,415 |
| 20 | 12.08 | 7.09 | 35,553 | 12.79 | 7.54 | 41,099 | 13.39 | 7.89 | 54,490 | 12,283 | 23,910 |
| 25 | 12.17 | 7.14 | 35,645 | 12.86 | 7.57 | 41,164 | 13.45 | 7.91 | 54,549 | 12,565 | 24,323 |
| Maintenance duration effect (months) | |||||||||||
| 36 | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 12.97 | 7.69 | 54,364 | 10,834 | 22,703 |
| 48 | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 13.07 | 7.75 | 53,931 | 10,834 | 20,827 |
| 60 | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 13.15 | 7.79 | 53,572 | 10,834 | 19,478 |
| 72 | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 13.22 | 7.83 | 53,276 | 10,834 | 18,495 |
| HR maintenance | |||||||||||
| 0.48 | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 13.13 | 7.78 | 53,961 | 10,834 | 20,051 |
| 0.66 | 11.55 | 6.84 | 34,983 | 12.40 | 7.37 | 40,708 | 12.85 | 7.62 | 54,390 | 10,834 | 24,628 |
| Parameters | MCP | R-MCP (base case) | R-MCP (scenario) | ICER– cost per QALY gained | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LY | QALY | Cost | LY | QALY | Cost | LY | QALY | Cost | Base case | Scenario | |
| Base case | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.89 | 7.67 | 54,079 | 9316 | 20,493 |
| Time horizon | |||||||||||
| 5 years | 4.25 | 3.12 | 27,233 | 4.43 | 3.26 | 30,660 | 4.49 | 3.31 | 42,324 | 24,366 | 80,497 |
| 10 years | 7.12 | 4.87 | 31,621 | 7.61 | 5.22 | 36,341 | 7.84 | 5.38 | 48,633 | 13,598 | 33,482 |
| Lifetime | 13.04 | 7.18 | 37,112 | 13.99 | 7.76 | 42,361 | 14.63 | 8.10 | 55,109 | 8963 | 19,510 |
| Discounting | |||||||||||
| 0% costs, 0% benefits | 11.45 | 8.94 | 42,032 | 12.35 | 9.73 | 47,913 | 12.89 | 10.19 | 61,663 | 7416 | 15,677 |
| 0% costs, 3.5% benefits | 11.45 | 6.79 | 42,032 | 12.35 | 7.36 | 47,913 | 12.89 | 7.67 | 61,663 | 10,401 | 22,379 |
| 3.5% costs, 0% benefits | 11.45 | 8.94 | 36,103 | 12.35 | 9.73 | 41,370 | 12.89 | 10.19 | 54,079 | 6643 | 14,356 |
| Parametric distribution | |||||||||||
| Weibull | 11.35 | 6.74 | 36,499 | 12.11 | 7.24 | 41,822 | 12.63 | 7.54 | 54,903 | 10,594 | 22,833 |
| Gompertz | 11.59 | 6.85 | 35,367 | 12.82 | 7.57 | 37,623 | 13.64 | 8.02 | 48,991 | 3146 | 11,653 |
| Death event in PFS | |||||||||||
| None | 11.95 | 7.07 | 37,490 | 12.56 | 7.47 | 41,961 | 13.08 | 7.77 | 54,602 | 11,192 | 24,562 |
| CVP arm | 11.45 | 6.79 | 36,103 | 12.19 | 7.27 | 40,942 | 12.75 | 7.60 | 53,702 | 10,023 | 21,849 |
| R-CVP arm | 11.67 | 6.91 | 36,690 | 12.35 | 7.36 | 41,370 | 12.89 | 7.67 | 54,079 | 10,457 | 22,899 |
| Resistance to rituximab (%) | |||||||||||
| –10 | 11.45 | 6.79 | 36,103 | 12.13 | 7.24 | 41,432 | 12.70 | 7.57 | 54,140 | 11,718 | 23,067 |
| –15 | 11.45 | 6.79 | 36,103 | 12.00 | 7.18 | 41,457 | 12.59 | 7.52 | 54,165 | 13,632 | 24,788 |
| –20 | 11.45 | 6.79 | 36,103 | 11.87 | 7.12 | 41,476 | 12.47 | 7.46 | 54,184 | 16,494 | 26,946 |
| –25 | 11.45 | 6.79 | 36,103 | 11.73 | 7.04 | 41,483 | 12.34 | 7.40 | 54,195 | 21,253 | 29,731 |
| –30 | 11.45 | 6.79 | 36,103 | 11.57 | 6.96 | 41,485 | 12.20 | 7.33 | 54,203 | 30,902 | 33,489 |
| Utility values | |||||||||||
| PFS1 = 0.805; PFS2 = 0.805; disease progression = 0.7363 | 11.45 | 6.59 | 36,103 | 12.35 | 7.44 | 41,370 | 12.89 | 7.86 | 54,079 | 6165 | 14,092 |
| PFS1 = 0.805; PFS2 = 0.805; disease progression = 0.7363 | 11.45 | 6.89 | 36,103 | 12.35 | 7.54 | 41,370 | 12.89 | 7.88 | 54,079 | 8186 | 18,216 |
| –10% | 11.45 | 6.11 | 36,103 | 12.35 | 6.62 | 41,370 | 12.89 | 6.90 | 54,079 | 10,352 | 22,770 |
| –20% | 11.45 | 5.43 | 36,103 | 12.35 | 5.88 | 41,370 | 12.89 | 6.13 | 54,079 | 11,646 | 25,616 |
| –30% | 11.45 | 4.75 | 36,103 | 12.35 | 5.15 | 41,370 | 12.89 | 5.37 | 54,079 | 13,309 | 29,275 |
| Higher in PFS1 (+10%) | 11.45 | 6.96 | 36,103 | 12.35 | 7.72 | 41,370 | 12.89 | 8.11 | 54,079 | 6898 | 15,572 |
| No disutility | 11.45 | 6.80 | 36,103 | 12.35 | 7.37 | 41,370 | 12.89 | 7.68 | 54,079 | 9291 | 20,440 |
| Disutility = –10% | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.89 | 7.67 | 54,079 | 9308 | 20,475 |
| Disutility = –20% | 11.45 | 6.79 | 36,103 | 12.35 | 7.35 | 41,370 | 12.89 | 7.66 | 54,079 | 9325 | 20,510 |
| Disutility = –30% | 11.45 | 6.78 | 36,103 | 12.35 | 7.34 | 41,370 | 12.89 | 7.65 | 54,079 | 9342 | 20,546 |
| Treatment pathway | |||||||||||
| Second line after progression | 11.57 | 6.86 | 35,693 | 12.49 | 7.44 | 41,475 | 13.03 | 7.75 | 54,184 | 10,125 | 20,944 |
| R-CVP no retreatment if early relapse | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.89 | 7.67 | 54,079 | 9316 | 20,493 |
| Patients receive CHOP and R-CHOP instead of salvage HDT and R-HDT | 10.16 | 6.18 | 32,938 | 11.79 | 7.11 | 39,588 | 12.42 | 7.47 | 52,589 | 7155 | 15,261 |
| Patients receive CHOP and R-CHOP instead of FC and R-FC | 11.70 | 6.93 | 37,204 | 12.56 | 7.47 | 42,157 | 13.08 | 7.77 | 54,811 | 9232 | 21,026 |
| Last three scenarios | 10.41 | 6.33 | 34,038 | 12.00 | 7.23 | 40,374 | 12.62 | 7.57 | 53,321 | 7035 | 15,452 |
| All patients receive salvage with rituximab | 12.36 | 7.28 | 40,209 | 13.61 | 8.00 | 45,717 | 14.02 | 8.24 | 58,020 | 7574 | 18,491 |
| All patients receive salvage without rituximab | 7.41 | 4.71 | 31,113 | 9.70 | 6.05 | 38,621 | 10.59 | 6.55 | 51,811 | 5604 | 11,227 |
| All patients receive CHOP | 9.69 | 5.88 | 30,613 | 11.52 | 6.95 | 37,990 | 12.19 | 7.33 | 51,144 | 6907 | 14,146 |
| All patients receive R-CHOP | 10.41 | 6.33 | 34,038 | 12.10 | 7.29 | 40,820 | 12.70 | 7.63 | 53,697 | 7041 | 15,111 |
| Effectiveness of FC | |||||||||||
| No loss of response | 11.70 | 6.93 | 36,492 | 12.56 | 7.47 | 41,678 | 13.08 | 7.77 | 54,360 | 9655 | 21,314 |
| Response 10% lower than CHOP regimens | 11.58 | 6.86 | 36,299 | 12.45 | 7.41 | 41,525 | 12.98 | 7.72 | 54,218 | 9487 | 20,916 |
| Response 30% lower than CHOP regimens | 11.34 | 6.72 | 35,911 | 12.24 | 7.30 | 41,213 | 12.79 | 7.62 | 53,935 | 9188 | 20,156 |
| Response 40% lower than CHOP regimens | 11.21 | 6.65 | 35,717 | 12.14 | 7.24 | 41,055 | 12.69 | 7.56 | 53,789 | 9027 | 19,783 |
| Response 50% lower than CHOP regimens | 11.09 | 6.58 | 35,535 | 12.03 | 7.19 | 40,903 | 12.60 | 7.52 | 53,653 | 8896 | 19,442 |
| PFS reduction –10% | 11.32 | 6.72 | 36,146 | 12.23 | 7.29 | 41,409 | 12.78 | 7.61 | 54,117 | 9101 | 20,050 |
| PFS reduction –20% | 11.17 | 6.63 | 36,182 | 12.10 | 7.22 | 41,438 | 12.66 | 7.55 | 54,145 | 8865 | 19,558 |
| PFS reduction –30% | 10.99 | 6.53 | 36,192 | 11.95 | 7.14 | 41,447 | 12.52 | 7.47 | 54,159 | 8608 | 19,031 |
| Costing of salvage therapy | |||||||||||
| Response rate same as CHOP regimens | 11.45 | 6.79 | 35,317 | 12.35 | 7.36 | 40,803 | 12.89 | 7.67 | 53,591 | 9704 | 20,833 |
| Response rate 20% greater than CHOP regimens | 11.45 | 6.79 | 36,890 | 12.35 | 7.36 | 41,938 | 12.89 | 7.67 | 54,567 | 8929 | 20,152 |
| Response rate 30% greater than CHOP regimens | 11.45 | 6.79 | 37,189 | 12.35 | 7.36 | 42,206 | 12.89 | 7.67 | 54,798 | 8874 | 20,074 |
| No. of cycles of ESHAP = 3 | 11.45 | 6.79 | 37,423 | 12.35 | 7.36 | 42,293 | 12.89 | 7.67 | 54,872 | 8613 | 19,892 |
| No. of cycles of ESHAP = 4 | 11.45 | 6.79 | 38,743 | 12.35 | 7.36 | 43,215 | 12.89 | 7.67 | 55,665 | 7910 | 19,292 |
| Harvest success rate: 1 | 11.45 | 6.79 | 38,266 | 12.35 | 7.36 | 42,931 | 12.89 | 7.67 | 55,421 | 8251 | 19,557 |
| Harvest success rate: 0.95 | 11.45 | 6.79 | 37,725 | 12.35 | 7.36 | 42,541 | 12.89 | 7.67 | 55,086 | 8517 | 19,791 |
| Harvest success rate: 0.90 | 11.45 | 6.79 | 37,184 | 12.35 | 7.36 | 42,151 | 12.89 | 7.67 | 54,750 | 8784 | 20,025 |
| Harvest success rate: 0.85 | 11.45 | 6.79 | 36,644 | 12.35 | 7.36 | 41,761 | 12.89 | 7.67 | 54,415 | 9050 | 20,259 |
| Harvest success rate: 0.75 | 11.45 | 6.79 | 35,562 | 12.35 | 7.36 | 40,980 | 12.89 | 7.67 | 53,744 | 9583 | 20,727 |
| Only one administration | 11.45 | 6.79 | 35,453 | 12.35 | 7.36 | 40,882 | 12.89 | 7.67 | 53,659 | 9601 | 20,755 |
| One additional administration | 11.45 | 6.79 | 35,670 | 12.35 | 7.36 | 41,045 | 12.89 | 7.67 | 53,799 | 9506 | 20,667 |
| AEs | |||||||||||
| No AEs | 11.45 | 6.80 | 35,996 | 12.35 | 7.37 | 41,287 | 12.89 | 7.68 | 53,892 | 9331 | 20,348 |
| Costs +20% | 11.45 | 6.79 | 36,124 | 12.35 | 7.36 | 41,387 | 12.89 | 7.67 | 54,117 | 9308 | 20,511 |
| Costs –20% | 11.45 | 6.79 | 36,082 | 12.35 | 7.36 | 41,354 | 12.89 | 7.67 | 54,042 | 9324 | 20,474 |
| No. of cycles | |||||||||||
| Six cycles for CHOP | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.89 | 7.67 | 54,079 | 9316 | 20,493 |
| Six cycles for FC | 11.47 | 6.80 | 37,814 | 12.36 | 7.36 | 42,718 | 12.90 | 7.68 | 55,334 | 8704 | 20,036 |
| Management costs | |||||||||||
| Cost +20% | 11.45 | 6.79 | 37,328 | 12.35 | 7.36 | 42,626 | 12.89 | 7.67 | 55,919 | 9370 | 21,194 |
| Cost –20% | 11.45 | 6.79 | 34,878 | 12.35 | 7.36 | 40,115 | 12.89 | 7.67 | 52,239 | 9263 | 19,792 |
| Cost pharmacy = 35 | 11.45 | 6.79 | 36,182 | 12.35 | 7.36 | 41,581 | 12.89 | 7.67 | 54,478 | 9549 | 20,856 |
| No monitoring costs | 11.45 | 6.79 | 32,666 | 12.35 | 7.36 | 36,963 | 12.89 | 7.67 | 50,675 | 7600 | 20,529 |
| Monitoring cost +20% | 11.45 | 6.79 | 36,790 | 12.35 | 7.36 | 42,252 | 12.89 | 7.67 | 54,760 | 9660 | 20,485 |
| Monitoring cost –20% | 11.45 | 6.79 | 35,416 | 12.35 | 7.36 | 40,489 | 12.89 | 7.67 | 53,398 | 8973 | 20,500 |
| No third-line treatment costs | 11.45 | 6.79 | 34,209 | 12.35 | 7.36 | 39,531 | 12.89 | 7.67 | 52,404 | 9413 | 20,742 |
| No cost palliative care | 11.45 | 6.79 | 30,484 | 12.35 | 7.36 | 37,397 | 12.89 | 7.67 | 50,677 | 12,228 | 23,020 |
| No terminal care costs | 11.45 | 6.79 | 34645 | 12.35 | 7.36 | 40,170 | 12.89 | 7.67 | 53,016 | 9773 | 20,944 |
| No terminal or palliative care costs | 11.45 | 6.79 | 29,025 | 12.35 | 7.36 | 36,197 | 12.89 | 7.67 | 49,614 | 12,684 | 23,471 |
| Maximum age (years) at which aggressive therapy is given | |||||||||||
| 60 | 11.02 | 6.57 | 34,868 | 12.12 | 7.25 | 40,617 | 12.69 | 7.58 | 53,433 | 8528 | 18,492 |
| 70 | 11.81 | 6.97 | 37,258 | 12.58 | 7.47 | 42,200 | 13.10 | 7.77 | 54,815 | 9973 | 22,116 |
| 75 | 12.08 | 7.11 | 38,306 | 12.75 | 7.55 | 42,941 | 13.26 | 7.84 | 55,512 | 10,659 | 23,667 |
| 80 | 12.24 | 7.21 | 39,134 | 12.86 | 7.61 | 43,574 | 13.37 | 7.89 | 56,099 | 11,099 | 24,650 |
| BSA (m2) | |||||||||||
| 1.6 | 11.45 | 6.79 | 35,051 | 12.35 | 7.36 | 38,536 | 12.89 | 7.67 | 48,769 | 6164 | 15,638 |
| 1.7 | 11.45 | 6.79 | 35,855 | 12.35 | 7.36 | 40,541 | 12.89 | 7.67 | 52,455 | 8289 | 18,925 |
| 1.8 | 11.45 | 6.79 | 35,855 | 12.35 | 7.36 | 40,541 | 12.89 | 7.67 | 52,455 | 8289 | 18,925 |
| 1.9 | 11.45 | 6.79 | 36,427 | 12.35 | 7.36 | 42,346 | 12.89 | 7.67 | 55,957 | 10,469 | 22,264 |
| Maximum time (years) in PFS1 | |||||||||||
| 5 | 11.26 | 6.68 | 36,843 | 11.52 | 6.91 | 45,008 | 11.75 | 7.05 | 59,088 | 36,602 | 60,170 |
| 6 | 11.30 | 6.71 | 36,694 | 11.64 | 6.99 | 44,454 | 11.92 | 7.16 | 58,329 | 27,820 | 47,647 |
| 7 | 11.33 | 6.72 | 36,585 | 11.75 | 7.05 | 44,001 | 12.07 | 7.25 | 57,706 | 22,799 | 40,277 |
| 8 | 11.35 | 6.74 | 36,497 | 11.84 | 7.10 | 43,621 | 12.20 | 7.32 | 57,181 | 19,527 | 35,414 |
| 9 | 11.37 | 6.75 | 36,429 | 11.92 | 7.15 | 43,302 | 12.30 | 7.38 | 56,742 | 17,277 | 32,065 |
| 10 | 11.39 | 6.76 | 36,375 | 11.98 | 7.18 | 43,032 | 12.39 | 7.43 | 56,369 | 15,642 | 29,618 |
| 11 | 11.40 | 6.76 | 36,333 | 12.04 | 7.21 | 42,813 | 12.47 | 7.47 | 56,066 | 14,403 | 27,766 |
| 12 | 11.41 | 6.77 | 36,299 | 12.09 | 7.24 | 42,623 | 12.54 | 7.51 | 55,804 | 13,437 | 26,330 |
| 13 | 11.42 | 6.77 | 36,268 | 12.14 | 7.26 | 42,461 | 12.60 | 7.54 | 55,582 | 12,685 | 25,206 |
| 14 | 11.43 | 6.78 | 36,243 | 12.18 | 7.28 | 42,316 | 12.65 | 7.56 | 55,383 | 12,074 | 24,305 |
| 15 | 11.43 | 6.78 | 36,221 | 12.21 | 7.30 | 42,194 | 12.70 | 7.59 | 55,216 | 11,584 | 23,580 |
| 16 | 11.44 | 6.78 | 36,204 | 12.24 | 7.31 | 42,092 | 12.74 | 7.60 | 55,072 | 11,188 | 22,984 |
| 17 | 11.44 | 6.78 | 36,190 | 12.26 | 7.32 | 42,001 | 12.77 | 7.62 | 54,947 | 10,855 | 22,496 |
| 18 | 11.44 | 6.79 | 36,177 | 12.28 | 7.33 | 41,919 | 12.80 | 7.63 | 54,836 | 10,579 | 22,089 |
| 19 | 11.45 | 6.79 | 36,167 | 12.30 | 7.34 | 41,849 | 12.82 | 7.64 | 54,740 | 10,352 | 21,758 |
| Greater OS (%) for R-CHOP compared with CHOP | |||||||||||
| 5 | 11.61 | 6.87 | 36,277 | 12.46 | 7.41 | 41,489 | 12.99 | 7.72 | 54,185 | 9620 | 21,106 |
| 10 | 11.75 | 6.94 | 36,433 | 12.57 | 7.46 | 41,594 | 13.09 | 7.76 | 54,280 | 9918 | 21,704 |
| 15 | 11.88 | 7.00 | 36,565 | 12.66 | 7.50 | 41,686 | 13.18 | 7.80 | 54,361 | 10,208 | 22,261 |
| 20 | 11.99 | 7.05 | 36,680 | 12.74 | 7.54 | 41,764 | 13.25 | 7.83 | 54,431 | 10,468 | 22,766 |
| 25 | 12.08 | 7.09 | 36,773 | 12.81 | 7.57 | 41,830 | 13.31 | 7.86 | 54,488 | 10,691 | 23,191 |
| Maintenance duration effect (months) | |||||||||||
| 36 | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.84 | 7.64 | 54,299 | 9316 | 21,436 |
| 48 | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.93 | 7.69 | 53,884 | 9316 | 19,712 |
| 60 | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 13.01 | 7.73 | 53,546 | 9316 | 18,470 |
| 72 | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 13.08 | 7.77 | 53,263 | 9316 | 17,547 |
| HR maintenance | |||||||||||
| 0.48 | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.99 | 7.72 | 53,898 | 9316 | 19,063 |
| 0.66 | 11.45 | 6.79 | 36,103 | 12.35 | 7.36 | 41,370 | 12.74 | 7.58 | 54,338 | 9316 | 23,044 |
Appendix 16 Additional results for the scenario analysis incorporating first-line maintenance
FIGURE 55.
Scenario analysis: undiscounted LYs. PD, progression.
FIGURE 56.
Scenario analysis: discounted QALYs. PD, progression.
FIGURE 57.
Scenario analysis: management and treatment costs for patients treated with CVP in first-line induction with or without rituximab.
FIGURE 58.
Scenario analysis: management and treatment costs for patients treated with CHOP in first-line induction with or without rituximab.
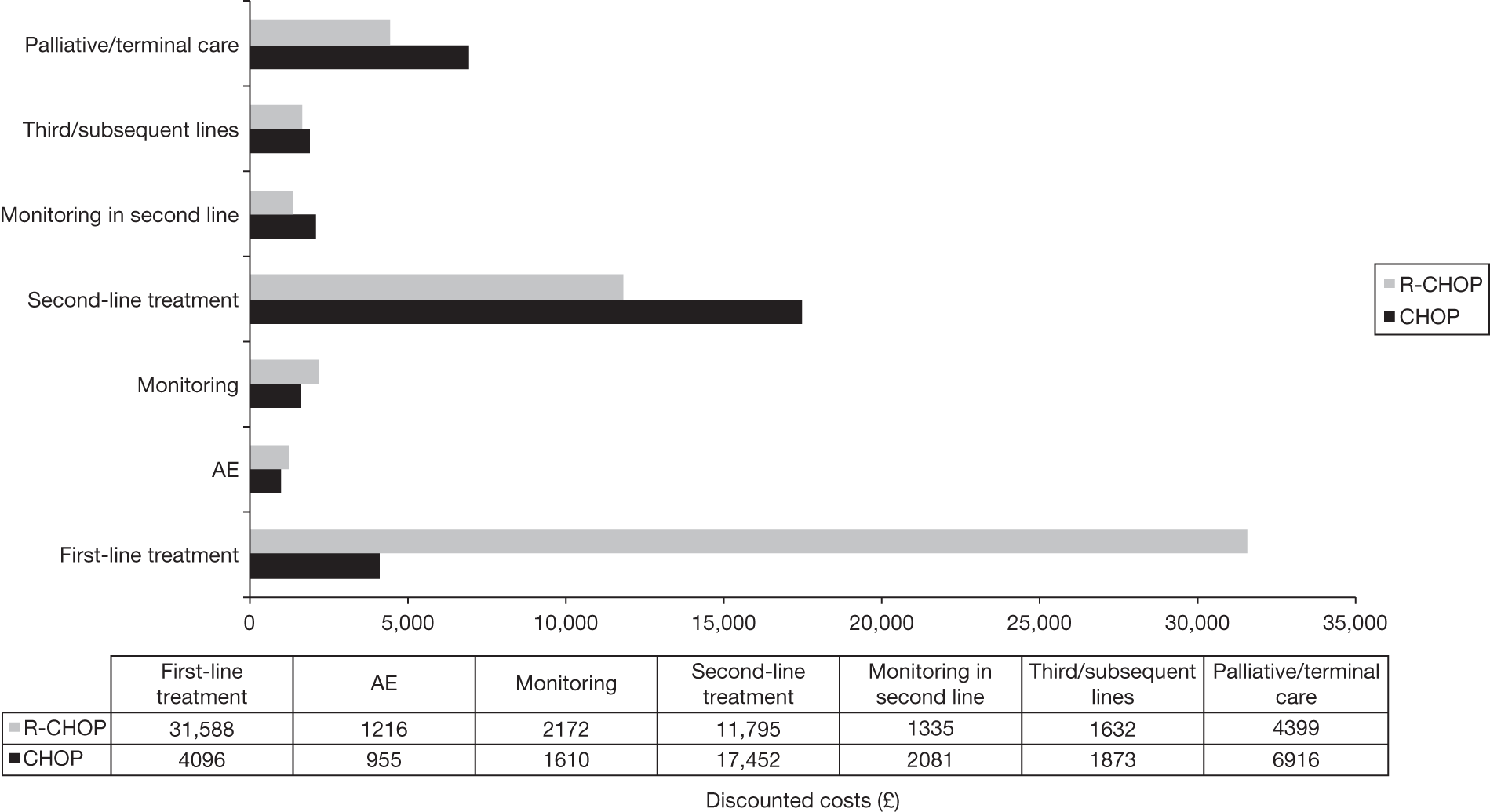
FIGURE 59.
Scenario analysis: management and treatment costs for patients treated with MCP in first-line induction with or without rituximab.
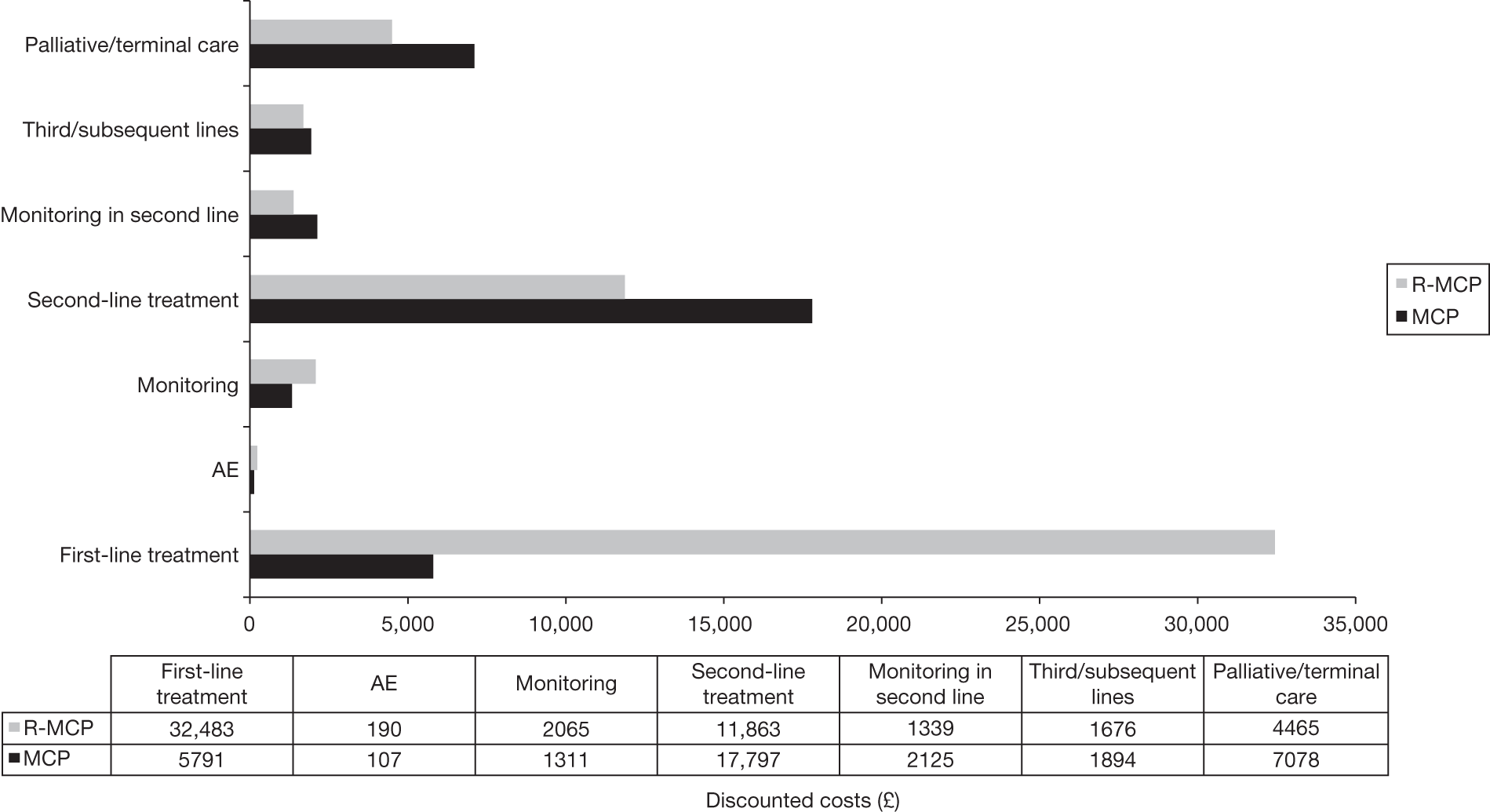
Appendix 17 Protocol
Technology Assessment Report commissioned by the NIHR HTA programme on behalf of the National Institute for Health and Clinical Excellence
Reference no: 09/141/01 (Batch 10).
Title of the project:
Rituximab for the first-line treatment of stage III–IV follicular lymphoma (review of TA110).
TAR team
School of Health and Related Research (ScHARR) Technology Assessment Group, The University of Sheffield.
Lead: Diana Papaioannou, Research Associate.
ScHARR, University of Sheffield, 30 Regent Court, Sheffield S1 4DATel: 0114 2220766
Fax: 0114 272 4095
E-mail: d.papaioannou@sheffield.ac.uk
Address for correspondence
All correspondences should be sent to the project lead (Diana Papaioannou, d.papaioannou@sheffield.ac.uk), the cost-effectiveness modeller (Matt Stevenson, M.D.Stevenson@sheffield.ac.uk), the managing director of ScHARR-TAG (Eva Kaltenthaler, e.kaltenthaler@sheffield.ac.uk) and the project administrator (Gill Rooney, g.rooney@sheffield.ac.uk).
Plain English summary
Lymphomas are cancers of the lymphatic system, which is a system of tubes and glands in the body which filters body fluid and fights infection. 1 There are two main types of lymphoma: Hodgkin’s and Non-Hodgkin’s Lymphoma (NHL). NHL can be divided into low-grade and high-grade lymphomas, depending on how quickly they grow and spread. Follicular lymphoma (FL) is a type of NHL low-grade lymphoma of cells called B-lymphocytes.
Grading and staging of the disease informs treatment pathways. Staging of NHL refers to how many lymph nodes are affected by the disease and informs the treatment and prognosis of the disease. There are four stages of NHL. Stage I disease involves only one group of lymph nodes or lymphoma in one organ of the body is affected. Stage II refers to disease that has spread to two groups of lymph nodes or an organ and one or more group of lymph nodes, with a criteria being that these are on the same side of the diaphragm. Stages III and IV are more advanced disease. Stage III includes lymph nodes affected on both sides of the diaphragm, and stage IV disease indicates that the NHL has spread from the lymph nodes, for example to the liver, bone marrow, or blood. 1
Histological grading of the disease is determined by the WHO classification grades I, II, IIIa or IIIb,2 which categorise disease into low-grade/indolent disease or high-grade/aggressive disease. There is consensus that grade IIIb disease should be classified as aggressive and treated as such. 2
NHL accounts for approximately 4% of all cancers diagnosed in the UK, with 9703 new cases registered in England and Wales in 2007, and 3978 registered deaths in 2008. 3 FL accounts for 30% of all low grade lymphomas1 and has a UK incidence
of approximately 4 per 100,000. 2 The median age of patients with FL is around 60 years and approximately 50% of patients will present with bone marrow involvement ( i.e. stage IV disease). 2 Over 70% of people with follicular lymphoma are still alive five years after the diagnosis,4 with median survival of nine to ten years. 5
Treatment of advanced (stage III or IV) FL is palliative; the aim of treatment being to prolong survival, achieve the longest possible remission and improve quality of life. Treatments are usually administered intermittently over a period of several years, with the expectation that the disease will relapse and remit during that time. 2
Currently, rituximab (Mabthera®, Roche Products) in combination with cyclophosphamide, vincristine and prednisolone (CVP regimen) is recommended by NICE guidance (TA110) as a first-line treatment option for symptomatic stage III or IV follicular lymphoma. 6 However, the market authorisation has changed for rituximab, and it is now licensed for use for the treatment of previously untreated patients with symptomatic stage III-IV follicular lymphoma in combination with other chemotherapies in addition to CVP. 7
The aim of this review is to systematically evaluate and appraise the clinical and cost-effectiveness of rituximab (in its licensed indication) in combination with chemotherapy compared with non-rituximab-containing chemotherapy, for the first-line treatment of symptomatic stage III-IV follicular lymphoma.
Decision problem
Purpose of the decision to be made
This assessment will address the question: ‘What is the clinical and cost-effectiveness of rituximab (in its licensed indication) with chemotherapy for the first-line treatment of symptomatic stage III-IV follicular lymphoma’.
Clear definition of the intervention
Rituximab (Mabthera®) is indicated for the treatment of previously untreated patients with stage III-IV follicular lymphoma in combination with chemotherapy at a recommended dose of 375 mg/m2 BSA per cycle, for up to eight cycles. 7 This assessment will include interventions where rituximab is given in combination with the following chemotherapy regimens:
-
CVP: cyclophosphamide, vincristine and prednisolone
-
CHOP: cyclophosphamide, doxorubicin, vincristine and prednisolone
-
CNOP: cyclophosphamide, mitoxantrone, vincristine and prednisolone
-
CHVP: cyclophosphamide, doxorubicin, vindesine, prednisolone
-
MCP: mitoxantrone, cholorambucil, and prednisolone
-
FCM: fludarabine, cyclophosphamide and mitoxantrone
-
FM: fludarabine and mitoxantrone
-
Bendamustine.
If the TAR team become aware of another widely used chemotherapy regimen used in combination with rituximab, this will be searched for separately at that time. Note that due to the scope specifying the intervention as rituximab given in combination with chemotherapy, interventions including rituximab and radio-immunotherapy or bone marrow/stem cell transplant are not considered as an intervention for this appraisal.
Bendamustine is not currently licensed as a first-line treatment with rituximab within this population but is included as a combination chemotherapy agent (with rituximab) as the anticipated date of licensing is not known and could occur within the time scales of the appraisal.
Rituximab (Mabthera®) is also licensed for treatment of follicular lymphoma at other stages within the treatment pathway, other types of NHL (and has indications for treatment of CLL and rheumatoid arthritis). These indications for use are not included within the final scope but are included below for completeness:
-
Rituximab maintenance therapy is indicated for patients with relapsed/refractory follicular lymphoma responding to induction therapy with chemotherapy with or without Mabthera.
-
Rituximab monotherapy is indicated for treatment of patients with stage III-IV follicular lymphoma who are chemoresistant or are in their second or subsequent relapse after chemotherapy.
-
Rituximab is indicated for the treatment of patients with CD20-positive diffuse large B cell non-Hodgkin’s lymphoma in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) chemotherapy. 7
Place of the intervention in the treatment pathway
The review will focus on the use of rituximab (in its licensed indication) in combination with chemotherapy as first-line treatment of symptomatic stage III-IV follicular lymphoma.
Relevant comparators
Non-rituximab-containing chemotherapies are the relevant comparators, and for this assessment the following comparators are considered:
-
CVP: cyclophosphamide, vincristine and prednisolone
-
CHOP: cyclophosphamide, doxorubicin, vincristine and prednisolone
-
CNOP: cyclophosphamide, mitoxantrone, vincristine and prednisolone
-
CHVP: cyclophosphamide, doxorubicin, vindesine, prednisolone
-
MCP: mitoxantrone, cholorambucil, and prednisolone
-
FCM: fludarabine, cyclophosphamide and mitoxantrone
-
FM: fludarabine and mitoxantrone
-
Bendamustine.
In addition, each intervention will be compared against each other.
Population and relevant subgroups
The population will comprise adults with symptomatic stage III-IV follicular lymphoma (a non-Hodgkin’s lymphoma) who have not received any previous treatment. If the evidence allows, subgroup analyses by type of chemotherapy regimen received will be considered, although initial clinical advice indicates that there are no relevant subgroups within the population that need to be addressed.
Key factors to be addressed
This review will aim to evaluate the following objectives:
-
Evaluate the clinical effectiveness of rituximab in combination with chemotherapy as first-line treatment in terms of overall survival, progression-free survival, response rates, duration of disease remission, and health-related quality of life.
-
Evaluate the adverse effect profile and toxicity.
-
Evaluate the cost-effectiveness of rituximab in combination with other.
-
chemotherapy in terms of incremental cost per quality-adjusted life year.
-
Estimate the possible overall cost in England and Wales.
Areas of agreement at the scoping workshop that are outside the scope of the appraisal and therefore do not require any detailed assessment
There was no scoping workshop for this appraisal.
Report methods for synthesis of evidence of clinical effectiveness
A systematic review of the evidence for clinical effectiveness will be undertaken following the general principles outlined in ‘Systematic Reviews: CRD’s guidance for undertaking reviews in health care8 and the principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (http://www.prisma-statement.org/). 9
Search strategy
A comprehensive search will be undertaken to systematically identify clinical and cost-effectiveness literature pertaining to rituximab for the treatment of follicular lymphoma.
The search strategy will comprise the following main elements:
-
Searching of electronic databases
-
Contact with experts in the field
-
Scrutiny of bibliographies of retrieved papers.
Electronic searches
Search strategies will be used to identify relevant trials (as specified under the inclusion criteria, below) and systematic reviews/meta-analyses (for identification of additional trials). Searches will not be restricted by language or publication date. An example of the Medline search strategy is shown in Appendix 1. This will be adapted for other databases. A comprehensive database of relevant published and unpublished articles will be constructed using Reference Manager© software.
Databases
The following electronic databases will be searched from inception: MEDLINE including Medline in process (Ovid); CINAHL; EMBASE; The Cochrane Library including the Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CENTRAL), DARE, NHS EED and HTA databases; Science Citation Index (SCI); NIHR Clinical Research Network Portfolio; National Research Register (NRR) archive 2000-2007; Current Controlled Trials; Clinical Trials.gov.; BIOSIS. Relevant conference proceedings will be searched, for example the American Society of Clinical Oncology (ASCO), European Society of Clinical Oncology (ESMO), American Society of Hematology (ASH) and the British Society for Haematology (BSH) will be searched.
Inclusion/exclusion criteria
Population
The population will comprise adults with symptomatic stage III-IV follicular lymphoma (a non-Hodgkin’s lymphoma) who have not received any previous treatment.
Interventions
Rituximab in combination with any of the following chemotherapy regimens: CVP, CHOP, CNOP, CHVP, MCP, FCM, FM and bendamustine.
Comparators
The comparator will be chemotherapy without Rituximab, which for this review are considered to be one of the following: CVP, CHOP, CNOP, CHVP, MCP, FCM, FM or bendamustine. In addition, the interventions will be compared against each other.
Outcomes
-
overall survival
-
progression free survival
-
response rates
-
duration of disease remission
-
adverse effects of treatment
-
health related quality of life.
Subgroups to be examined
If the evidence allows, subgroup analyses by type of chemotherapy regimen received will be considered.
Inclusion criteria
According to the accepted hierarchy of evidence, randomised controlled trials (RCTs) will be included for clinical effectiveness, as they provide the most authoritative form of evidence. If insufficient data are not available from RCTs, observational studies or clinical trials may be considered. Studies published as abstracts or conference presentations will only be included if sufficient details represented to allow an appraisal of the methodology and the assessment of the results to be undertaken. Systematic reviews and clinical guidelines will be used as sources of references.
Exclusion criteria
Reviews of primary studies will not be included in the analysis, but will be retained for discussion and identification of additional trials. Studies which are considered methodologically unsound will be excluded from the review as well as the following publication types: non-randomised studies (except for adverse events); animal models; preclinical and biological studies; narrative reviews, editorials, opinions; non-English-language papers and reports where insufficient methodological details are reported to allow critical appraisal of study quality.
Data extraction strategy
Studies will be selected for inclusion through a two-stage process according to the above inclusion/exclusion criteria. Titles and abstracts will be examined for inclusion by one reviewer. Screening will be checked by a second reviewer on ten per cent of citations and a kappa coefficient will be calculated to measure inter-rater reliability. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary. Full manuscripts of selected citations will be retrieved and assessed by one reviewer against the inclusion/exclusion criteria. Data will be extracted by one reviewer using a standardised data extraction form and checked by a second reviewer. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary. Where multiple publications of the same study are identified, data will be extracted and reported as a single study
Quality assessment strategy
The methodological quality of each included study will be assessed by one reviewer and checked by a second reviewer, according to (adapted) criteria based on those proposed by the NHS CRD for randomised controlled trials (RCTs). 8 (See Appendix 2.)
Consideration of study quality to assess RCTs will include the following factors: method of randomisation, allocation concealment, blinding of patients, outcome assessors and data-analysts, numbers of participants randomised, baseline comparability between groups, specification of eligibility criteria, whether intent to treat analysis is performed, completeness of follow up and whether study power calculations are performed and reported.
Methods of analysis/synthesis
Data will be tabulated and discussed in a narrative review. Where appropriate (i.e. populations, interventions and outcomes are comparable), meta-analysis will be employed to estimate a summary measure of effect on relevant outcomes based on ITT analyses.
Meta-analysis will be carried out using fixed or random effects models, using the Cochrane Collaboration Review Manager© Software (version 5.0). 10 Heterogeneity will be explored through consideration of the study populations, methods and interventions, by visualisation of results and, in statistical terms, by the χ2 test for homogeneity and the I2 statistic.
It is anticipated that the work will require a network meta-analysis to be undertaken to determine efficacy. This will be populated with all identified trials involving an intervention or a comparator. It is noted that the network meta-analysis could potentially be strengthened by the inclusion of RCTs involving two pharmaceuticals that were neither interventions nor comparators, provided there were RCTs comparing these pharmaceuticals with an intervention or a comparator. However, literature searches for all RCTs from these pharmaceuticals will not be conducted as they are likely to have little impact on the results of interest and would have significant resource implications.
Report methods for synthesising evidence of cost-effectiveness
Identifying and systematically reviewing published cost-effectiveness studies
Studies relating to the cost-effectiveness associated with rituximab in combination with chemotherapy will be identified using an economic search filter which will be integrated into the search strategy detailed in Section 5.1; this economic search filter is presented in Appendix 1. Relevant studies identified and included in the manufacturer’s submission will also be included. The quality of economic literature will be assessed using a combination of key components of the British Medical Journal11 checklist for economic evaluations together with the Eddy checklist on mathematical model12
Systematic literature search for other data related to cost-effectiveness
A search of the broader literature on follicular lymphoma will be undertaken to identify the evidence base on HRQoL (i.e. health state values). The literature search will identify relevant values for appropriate health states. Primary data collection will not be undertaken.
Searches for additional information regarding model parameters, patient preferences and other topics not covered within the clinical effectiveness and cost-effectiveness reviews will be based on the methodological discussion paper produced by InterTASC (January 2005).
Methods for estimating costs and cost-effectiveness
Where appropriate a mathematical model will be constructed by adapting an existing model or developing a new model using available evidence. The model developed will estimate the cost per QALY gained for rituximab and chemotherapy. It is hoped that suitable quality of life data will be identified from the literature, in the absence of quality of life data; the model may use indirect evidence on quality of life from alternative sources. The model will use efficacy data from the key RCTs identified through the systematic searches. Cost data for the economic model will be extracted from a variety of published sources.
A sensitivity analysis will be undertaken to identify the key parameters that determine the cost-effectiveness of the intervention with the objective of identifying how secure the results of the economic analyses are, given the available evidence. Uncertainty with respect to model parameters will be explored with a probabilistic sensitivity analysis (PSA), where uncertainty of all input variables is modelled with probability distribution of their value. The information derived from PSA will be summarised graphically using cost effectiveness acceptability curves.
The time horizon of the analysis will be a patient’s lifetime in order to reflect the chronic nature of the disease. The perspective will be that of the National Health Services and PSS. Both cost and QALY will be discounted at 3.5%.
Handling the company submission(s)
All data submitted by the manufacturers/sponsors will be considered if received by the TAR team no later than 20 December 2010. Data arriving after this date may not be considered. If the data meet the inclusion criteria for the review they will be extracted and quality assessed in accordance with the procedures outlined in this protocol. Any economic evaluations included in the company submission, provided it complies with NICE’s advice on presentation, will be assessed for clinical validity, reasonableness of assumptions and appropriateness of the data used in the economic model. If the TAR team judge that the existing economic evidence is not robust, then further work will be undertaken, either by adapting what already exists or developing de-novo modelling.
Any ‘commercial in confidence’ data taken from a company submission will be underlined and highlighted in blue in the assessment report (followed by an indication of the relevant company name e.g. in brackets).Any academic in confidence data will be underlined and highlighted in yellow.
Competing interests of authors
Dr Andrew McMillan has attended Roche Advisory Boards (and received Honoraria) and received sponsorship from Roche to attend International meetings.
Appendix 1: Draft clinical effectiveness search strategy
-
Cyclophosphamide.af.
-
Cyclophosphamide/
-
1 or 2
-
vincristine.af.
-
Vincristine/
-
4 or 5
-
vindesine.af.
-
Vindesine/
-
7 or 8
-
(prednisolone or prednisone).af.
-
Prednisolone/ or Prednisone/
-
10 or 11
-
doxorubicin.af.
-
Doxorubicin/
-
13 or 14
-
(mitoxantrone or mitozantrone).af.
-
Mitoxantrone/
-
16 or 17
-
(cholorambucil or chlorambucil).af.
-
Chlorambucil/
-
19 or 20
-
fludarabine.af.
-
Bendamustine.af.
-
3 and 6 and 12
-
3 and 15 and 6 and 12
-
3 and 18 and 6 and 12
-
3 and 15 and 9 and 12
-
18 and 21 and 12
-
22 and 3 and 18
-
18 and 22
-
24 or 25 or 26 or 27 or 28 or 29 or 23
-
(CVP or CHOP or CNOP or CHVP or MCP or FCM or FM).af.
-
30 or 31
-
(rituximab or mabthera or mab thera or rituxan or IDEC-102 or IDEC-C2B8 or Rituksimabi or Rituximabum or anti-CD20 or immunotherapy or 131I-rituximab or rituximab-alliinase conjugate or monoclonal antibod$).af.
-
Antibodies, Monoclonal/
-
32 or 33 or 34
-
(follicular lymphoma or indolent lymphoma or low grade lymphoma or lymphoma or NHL).ti,ab.
-
(Lymphoma$ adj5 non-hodgkin$).ti,ab.
-
(follic$ adj5 (lymphocyte$ or lymphoma$)).ti,ab.
-
Lymphoma, Follicular/
-
Lymphoma, Non-Hodgkin/
-
36 or 37 or 38 or 39 or 40
-
35 and 41
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
43 or 44 or 45 or 46 or 47 or 48 or 49
-
(clinic$ adj trial$1).tw.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw.
-
Placebos/
-
Placebo$.tw.
-
Randomly allocated.tw.
-
(allocated adj2 random).tw.
-
51 or 52 or 53 or 54 or 55 or 56
-
50 or 57
-
Case report.tw.
-
Letter/
-
Historical article/
-
Review of reported cases.pt.
-
Review, multicase.pt.
-
59 or 60 or 61 or 62 or 63
-
58 not 64
-
42 and 65
Economics filter
-
Economics/
-
exp “Costs and Cost Analysis”/
-
economic value of life/
-
exp economics hospital/
-
exp economics medical/
-
economics nursing/
-
exp models economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp budgets/
-
ec.fs.
-
(cost or costs or costed or costly or costing$).tw.
-
(economic$ or pharmacoeconomic$ or price$ or pricing$).tw.
-
quality adjusted life years/
-
(qaly or qaly$).af.
-
or/1-15
Appendix 2: Draft quality assessment scale
| Was the method used to assign participants to the treatment groups really random? |
| What method of assignment was used? |
| Was the allocation of treatment concealed? |
| What method was used to conceal treatment allocation? |
| Was the number of participants who were randomised stated? |
| Were details of baseline comparability presented? |
| Was baseline comparability achieved? |
| Were the eligibility criteria for study entry specified? |
| Were any co-interventions identified that may influence the outcomes for each group? |
| Were the outcome assessors blinded to the treatment allocations? |
| Were the individuals who administered the intervention blinded to the treatment allocation? |
| Were the participants who received the intervention blinded to the treatment allocation? |
| Was the success of the blinding procedure assessed? |
| Were at least 80% of the participants originally included in the randomised process followed up in the final analysis? |
| Were the reasons for withdrawal stated? |
| Was an ITT analysis included? |
Appendix 3: Critical appraisal checklist for economic evaluations using key components of the British Medical Journal11 checklist for economic evaluation together with the Eddy checklist12 on mathematical models employed in technology assessments
| Reference ID | ||
| Title | ||
| Authors | ||
| Year | ||
| Modelling assessments should include: | Yes/No | |
| 1 | A statement of the problem; | |
| 2 | A discussion of the need for modelling vs. alternative methodologies; | |
| 3 | A description of the relevant factors and outcomes; | |
| 4 | A description of the model including reasons for this type of model and a specification of the scope including; time frame, perspective, comparators and setting. Note: n = number of health states within sub-model | |
| 5 | A description of data sources (including subjective estimates), with a description of the strengths and weaknesses of each source, with reference to a specific classification or hierarchy of evidence; | |
| 6 | A list of assumptions pertaining to: the structure of the model (e.g. factors included, relationships, and distributions) and the data; | |
| 7 | A list of parameter values that will be used for a base-case analysis, and a list of the ranges in those values that represent appropriate confidence limits and that will be used in a sensitivity analysis; | |
| 8 | The results derived from applying the model for the base case; | |
| 9 |
The results of the sensitivity analyses: unidimensional; best/worst case; multidimensional (Monte Carlo/parametric); threshold |
|
| 10 | A discussion of how the modelling assumptions might affect the results, indicating both the direction of the bias and the approximate magnitude of the effect; | |
| 11 |
A description of the validation undertaken including: concurrence of experts; internal consistency; external consistency; predictive validity. |
|
| 12 | A description of the settings to which the results of the analysis can be applied and a list of factors that could limit the applicability of the results; | |
| 13 | A description of research in progress that could yield new data that could alter the results of the analysis. | |
References
- Cancer Research UK Non-Hodgkin’s Lymphoma . Cancer Help UK 2010. URL: http://www.cancerhelp.org.uk/type/non-hodgkins-lymphoma/index.htm.
- British Committee for Standards in Haematology (BCSH) Guidelines on Nodal Non-Hodgkin’s Lymphoma 2002. URL: http://www.bcshguidelines.com/pdf/NHL_100903.pdf.
- Cancer Research UK UK Non-Hodgkin lymphoma (NHL) statistics . Cancer Statistics 2010. URL: http://info.cancerresearchuk.org/cancerstats/types/nhl/index.htm?script = true.
- Freytes CO, Burzynski JA. Lymphoma, Follicular 2009. URL: http://emedicine.medscape.com/article/203268-overview.
- Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Practice and Research Clinical Haematology 2005;18:1-10.
- National Institute for Health and Clinical Excellence . Rituximab for the Treatment of Follicular Lymphoma: NICE Technology Appraisal Guidance 110 2006.
- European Medicines Agency (EMEA) . Summary of Product Characteristics 2009. URL: http://www.ema.europa.eu/humandocs/PDFs/EPAR/Mabthera/emea-combined-h165en.pdf.
- Centre for Reviews and Dissemination Systematic Reviews . CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. and The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Journal of Clinical Epidemiology 2009;62:1006-12.
- Review Manager (RevMan) [Computer Program] 2008.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. British Medical Journal 1996;313:275-83.
- Eddy DM. The role of mathematical modeling. in Assessing medical technology. Technology Assessment 1985:144-5.
Glossary
- Antibody
- An immunoglobulin molecule that has a specific amino acid sequence by virtue of which it interacts only with the antigen that induced its synthesis in cells of the lymphoid series (especially plasma cells) or with an antigen closely related to it. Antibodies are classified, according to their mode of action, as agglutinins, bacteriolysins, haemolysins, opsonins, precipitins, etc.
- Antigen
- A substance that is capable, under appropriate conditions, of inducing a specific immune response and of reacting with the products of that response, i.e. with specific antibodies or specifically sensitised T lymphocytes, or both. Antigens may be soluble substances (such as toxins and foreign proteins) or particulates (such as bacteria and tissue cells); however, only the portion of the protein or polysaccharide molecule known as the antigenic determinant (epitopes) combines with antibody or a specific receptor on a lymphocyte.
- B cell
- A type of lymphocyte normally involved in the production of antibodies to combat infection. It is a precursor to a plasma cell. During infections, individual B-cell clones multiply and are transformed into plasma cells, which produce large amounts of antibodies against a particular antigen on a foreign microbe. This transformation occurs through interaction with the appropriate CD4 T-helper cells.
- CD20
- Unglycosylated phosphoproteins expressed only on B cells. They are regulators of transmembrane calcium conductance and are thought to play a role in B-cell activation and proliferation.
- Disease-free survival*
- The time from complete response to relapse or death (not specified) [as defined in the R-CVP vs CVP (M39021) trial].
- Event-free survival*
- The time period from randomisation to disease progression/relapse, death by any cause or new antilymphoma treatment (FL2000 trial). The time period from randomisation to disease progression after two cycles or partial response at six cycles or disease progression/relapse (OSHO-39 trial).
- FL2000 trial (follicular lymphoma-2000 trial)
- An open-label randomised controlled trial (RCT) comparing R-CHVPi (rituximab, cyclophosphamide, doxorubicin, etoposide, prednisolone and interferon-alpha) with CHVPi for the first-line treatment of stage III–IV follicular lymphoma (FL).
- Follicular lymphoma
- A type of non-Hodgkin’s lymphoma (NHL), named as such because of the location (lymphoid follicles) and behaviour (growth in a follicular fashion) of the cancerous cells.
- GLSG-2000 trial (German Low Grade Lymphoma Study-2000 trial)
- An open-label RCT comparing R-CHOP (rituximab, cyclophosphamide, doxorubicin/adriamycin, vincristine and prednisolone) with CHOP for the first-line treatment of stage III–IV FL.
- Granulocytopenia
- A decrease in the numbers of granulocytes, which are a type of white blood cell that helps to fight infection.
- Indolent disease
- Disease that develops slowly.
- Leucocytopenia
- A marked decrease in the numbers of white blood cells, which can increase the risk of infection.
- Lymph
- The almost colourless fluid that bathes body tissues and is found in the lymphatic vessels that drain the tissues of the fluid that filters across the blood vessel walls from blood. Lymph carries lymphocytes that have entered the lymph nodes from the blood.
- Lymphocyte
- White cells of the blood that are derived from stem cells of the lymphoid series. Two main classes are recognised, T and B lymphocytes, the latter responsible (when activated) for production of antibody, the former subdivided into subsets (helper, suppressor, cytotoxic T cells) and responsible both for cell-mediated immunity and for stimulating B cells.
- Lymphoma
- Malignant tumour of lymphoid cells. Lymphomas are of either Hodgkin’s or non-Hodgkin’s type.
- M39021 trial
- An open-label RCT comparing R-CVP (rituximab, cyclophosphamide, vincristine and prednisolone) with CVP for the first-line treatment of stage III–IV FL.
- Monoclonal antibodies
- An antibody made by a single clone of cells.
- Neutropenia
- A marked decrease in the numbers of neutrophils (a type of granulocyte), which can increase the risk of infection.
- Non-Hodgkin’s lymphoma
- A group of lymphomas that differ in important ways from Hodgkin’s disease and are classified according to the microscopic appearance of the cancer cells. There are many different subtypes of NHL; some of these are fast growing and life-threatening, whereas others are slow growing and may not require immediate treatment.
- OSHO-39 trial
- An open-label RCT comparing R-MCP (rituximab, mitoxantrone, chlorambucil and prednisolone) with MCP for the first-line treatment of stage III–IV FL.
- Overall survival
- The time from randomisation to the date of death by any cause.
- Progression-free survival
- The time from randomisation to disease progression or death.
- Response duration*
- The time from response achieved (complete or partial) to disease progression/relapse or death.
- T cell
- A class of lymphocytes, so called because they are derived from the thymus and have been through thymic processing. Involved primarily in controlling cell-mediated immune reactions and in the control of B-cell development. The T cells co-ordinate the immune system by secreting lymphokine hormones.
- Time to next antilymphoma treatment*
- The time from randomisation to date of next/new treatment (OSHO-39 and M39021 trials) or death (M39021 trial).
- Time to progression*
- The time from randomisation to disease progression, relapse after response, death by any cause (M39021 trial).
- Time to treatment failure*
- The time period from randomisation to death, relapse after response, new antilymphoma treatment or stable disease after cycle 4 (M39021 trial). The time period from start of treatment to resistance to initial therapy, disease progression or death (GLSG-2000 trial).
*No standard definitions exist. Definitions taken from four trials are included in this appraisal.
List of abbreviations
- AE
- adverse event
- AG
- Assessment Group
- AIC
- Akaike information criterion
- ASCT
- autologous stem cell transplant
- BCSH
- British Committee for Standards in Haematology
- BEAM
- BCNU®/carmustine, cytarabine, etoposide and melphalan
- BIC
- Bayesian information criterion
- BNF
- British National Formulary
- BSA
- body surface area
- CEAC
- cost-effectiveness acceptability curve
- CHOP
- cyclophosphamide, doxorubicin/adriamycin, vincristine and prednisolone
- CHVP
- cyclophosphamide, doxorubicin, etoposide and prednisolone
- CHVPi
- cyclophosphamide, doxorubicin, etoposide, prednisolone and interferon-alpha
- CI
- confidence interval
- CLL
- chronic lymphocytic leukaemia
- CNOP
- cyclophosphamide, mitoxantrone, vincristine and prednisolone
- CR
- complete response/responder
- CRD
- Centre for Reviews and Dissemination
- CRu
- unconfirmed complete response/responder
- CT
- computerised tomography
- CVP
- cyclophosphamide, vincristine and prednisolone
- DFS
- disease-free survival
- DHAP
- dexamethasone, cytarabine and cisplatin
- DLBCL
- diffuse large B-cell lymphoma
- ECOG
- Eastern Cooperative Oncology Group
- EFS
- event-free survival
- EFSR
- event-free survival after first relapse
- EMA
- European Medicines Agency
- EQ-5D
- European Quality of Life-5 Dimensions
- ERG
- Evidence Review Group
- ESHAP
- etoposide, methylprednisolone, cytarabine and cisplatin
- ESMO
- European Society for Medical Oncology
- FACT
- Functional Assessment of Cancer Therapy
- FACT-LYM
- Functional Assessment of Cancer Therapy (lymphoma)
- FC
- fludarabine and cyclophosphamide
- FCM
- fludarabine, cyclophosphamide and mitoxantrone
- FL
- follicular lymphoma
- FL2000
- follicular lymphoma-2000 trial (R-CHVPi vs CHVPi)
- FLIPI
- Follicular Lymphoma International Prognostic Index
- FM
- fludarabine and mitoxantrone
- GLSG-2000
- German Low Grade Lymphoma Study-2000 (R-CHOP vs CHOP)
- HDT
- high-dose chemotherapy
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HS
- health state
- ICER
- incremental cost-effectiveness ratio
- IPI
- International Prognostic Index
- ITT
- intention to treat
- i.v.
- intravenously
- IWF
- International Working Formulation
- LDH
- lactate dehydrogenase
- LFT
- liver function test
- LY
- life-year
- M39021
- R-CVP vs CVP trial
- MCL
- mantle cell lymphoma
- MCP
- mitoxantrone, chlorambucil and prednisolone
- MIU
- million international units
- MS
- manufacturer’s submission
- NCI-CTC
- National Cancer Institute Common Toxicity Criteria
- NE
- not estimable
- NHL
- non-Hodgkin’s lymphoma
- NICE
- National Institute for Health and Clinical Excellence
- NR
- not reported
- ORR
- overall response rate
- OS
- overall survival
- OSHO
- East German Society of Haematology and Oncology
- OSHO-39
- East German Society of Haematology and Oncology R-MCP vs MCP trial
- PET
- positron emission tomography
- PFS
- progression-free survival
- PFS1
- progression-free survival after first line
- PFS2
- progression-free survival after second line
- PML
- progressive multifocal leucoencephalopathy
- PPS
- post-progression survival
- PR
- partial response/responder
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- R
- rituximab
- R-C
- rituximab and chlorambucil
- R-chemotherapy
- rituximab and chemotherapy
- R-CHOP
- rituximab, cyclophosphamide, doxorubicin/adriamycin, vincristine and prednisolone
- R-CHVP
- rituximab, cyclophosphamide, doxorubicin, etoposide and prednisolone
- R-CHVPi
- rituximab, cyclophosphamide, doxorubicin, etoposide, prednisolone and interferon-alpha
- R-CVP
- rituximab, cyclophosphamide, vincristine and prednisolone
- R-DHAP
- rituximab, dexamethasone, cytarabine, cisplatin
- R-ESHAP
- rituximab, etoposide, methylprednisolone, cytarabine, cisplatin
- R-F
- rituximab and fludarabine
- R-FC
- rituximab, fludarabine and cyclophosphamide
- R-FCM
- rituximab, fludarabine, cyclophosphamide and mitoxantrone
- R-ICE
- rituximab, ifosfamide, carboplatin, etoposide
- R-MCP
- rituximab, mitoxantrone, chlorambucil and prednisolone
- RCT
- randomised controlled trial
- REAL
- Revised European–American Lymphoma
- RMSE
- root-mean-square error
- RR
- relative risk
- Rx
- maintenance rituximab
- SA
- sensitivity analysis
- SAR
- survival after first relapse
- ScHARR
- School of Health and Related Research
- SCT
- stem cell transplant
- SD
- standard deviation
- SE
- standard error
- SNLG
- Scotland and Newcastle Lymphoma Group
- SPD
- sum of the products of the greatest diameters
- STiL
- Study Group Indolent Lymphomas
- TTF
- time to treatment failure
- TTNT
- time to next antilymphoma treatment
- TTP
- time to progression
- U&E
- urea and electrolytes
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health