Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/14/02. The contractual start date was in March 2011. The draft report began editorial review in July 2011 and was accepted for publication in February 2012. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Ramsay et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the underlying health problem
The decision about which treatment is best for a man diagnosed with cancer of the prostate, a sex gland located at the base of the bladder in the pelvis, presents an abundance of different but inter-related aspects that have been the focus of a number of previous Health Technology Assessments (HTAs) worldwide. 1–3 The present review was tasked with determining whether, for the UK NHS, complete removal of the prostate (radical prostatectomy) is best achieved using laparoscopic (keyhole) surgery or robotic surgery.
To understand the need for the review it is first necessary to consider changes in the characteristics of men diagnosed with prostate cancer over the last 30 years (see Evolution of prostate cancer diagnosis) and the resultant evolution of the technique of radical prostatectomy during that time period (see Development of radical prostatectomy). The technologies to be considered will then be described (see Description of the interventions) followed by an outline of the current demand for their use in the NHS (see Current use in the UK NHS).
Evolution of prostate cancer diagnosis
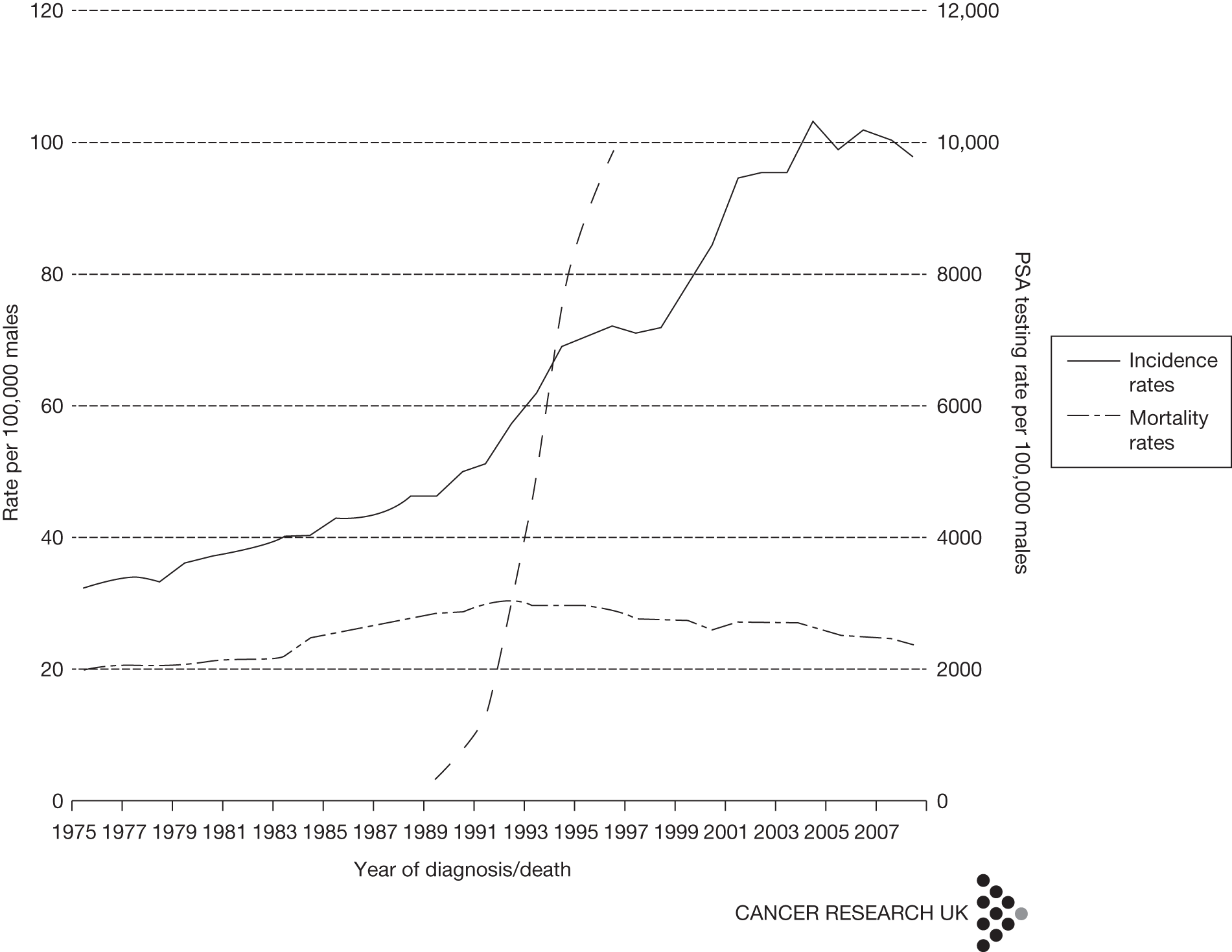
The discovery of prostate-specific antigen (PSA) in 1979 as an organ-specific serum marker of prostate cancer, followed by its introduction as a commercially available laboratory test in 1986, transformed the way that prostate cancer was diagnosed and managed worldwide. 4 Before PSA testing, men were generally diagnosed with prostate cancer following an abnormal digital rectal examination, with worsening urinary symptoms or with symptoms of metastatic disease such as bone pain. This meant that approximately 70% had locally advanced or metastatic disease on presentation. 5 Although complete removal of the prostate (radical prostatectomy) was a treatment option for locally advanced disease, most men progressed to metastasis when only palliative treatment such as androgen ablation (castration) could be offered, resulting in 5-year survival rates of < 50%. 6 The advent of PSA testing allied to systematic biopsy of the prostate gland changed this situation dramatically. It was realised that men with a serum PSA raised above a threshold value, originally set at 4 ng/ml7 and more recently in the UK at age-specific values of between 3 and 5 ng/ml,8 were more likely to have prostate cancer, which, if present, was usually at a preclinical stage without symptoms and was not detectable on digital rectal examination. Autopsy studies had previously showed that small foci of prostate cancer were common in men older than 45 years and that this prevalence increased with age. It was therefore not surprising that widespread adoption of PSA testing resulted in a substantial increase in the number of men diagnosed with prostate cancer during the 1980s and 1990s9 (Figure 1). Areas of the world that adopted PSA testing have subsequently experienced falling mortality rates for prostate cancer, but whether this is due to more successful radical treatment or a mixture of length and lead-time bias remains uncertain. 11
FIGURE 1.
Change in rates of PSA testing and prostate cancer diagnosis in the UK. 10 Adapted with the permission of Cancer Research UK.
Development of radical prostatectomy
This sudden rise in incidence of localised prostate cancer inevitably led to an increased demand for curative treatments. The initial focus was on open radical prostatectomy, a surgical operation to completely remove the prostate together with its surrounding thin layers of connective tissue through a lower abdominal incision. 12 This procedure was historically associated with excessive blood loss, complete loss of erectile function and a high rate of urinary incontinence together with an appreciable mortality. 13 Rapid expansion of the number of predominantly asymptomatic men requiring treatment for PSA-detected cancer stimulated development of surgical techniques to reduce the morbidity and mortality of open radical prostatectomy while achieving long-term cancer cure. It was realised that routine use of specific manoeuvres to prevent blood loss together with precise identification and preservation of the nerves and blood vessels that supply the erectile tissue of the penis and urinary sphincter allowed the operation to be performed within an acceptable margin of safety without compromising cancer cure. 14,15 These techniques were further refined by many surgeon innovators, establishing the three main principles of radical prostatectomy termed the ‘trifecta’: to cure the cancer, to preserve continence and to preserve erectile function. Despite these developments, the outcome of open radical prostatectomy remains less than ideal, with 20% of men requiring a blood transfusion, 7% having long-term urinary incontinence and 40% suffering erectile dysfunction after surgery, although surgeons who perform larger numbers of cases tend to have better results. 16–18 The risk of these longer-term adverse effects is an important part of counselling for men having to face treatment choices for PSA-detected localised prostate cancer given that most will have normal urinary and sexual function before intervention. Surgeons and technology researchers have therefore continued to seek ways to reduce the functional disturbance of the procedure but maintain its disease-curing potential, leading to the development during the last decade of first laparoscopic prostatectomy,19 and subsequently robotic prostatectomy, to enhance the accuracy of surgical dissection and further reduce blood loss. 20 Although not the prime focus of this review, it must be noted that the technique of open prostatectomy also continues to evolve with the same aim of minimising harms. Large high-volume single-institution series, particularly from the USA, suggest that open prostatectomy remains an option for men considering surgery for localised prostate cancer. 21
Description of the interventions
Technical description
Laparoscopic prostatectomy
Experience in gall bladder and kidney surgery highlighted the advantages of a laparoscopic approach to intra-abdominal organ removal. Insufflation of the abdominal cavity and use of endoscopic lens and digital camera systems for image magnification greatly enhanced surgical view, aiding accurate dissection, and reduced bleeding. Technological development in instrument design and the use of differing energy sources for haemostasis added further potential benefits over open surgery. Appreciation of these advantages led to the first series of men undergoing laparoscopic radical prostatectomy being reported in 1997. 22
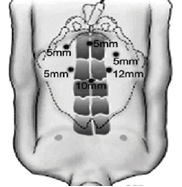
For standard laparoscopic radical prostatectomy the patient is anaesthetised and positioned supine on the operating table with legs abducted. Following skin cleansing and draping, the abdomen is punctured with a trocar at the umbilicus under vision using a Hassan technique and a pneumoperitoneum induced with CO2 gas, which is then maintained throughout the operation at a pressure of 10–12 mmHg. A telescopic camera is then inserted though the insufflation port (10 mm diameter) and a further three 5-mm ports and one 12-mm port are inserted in a specific configuration to allow ergonomic access to the pelvis without instrument clashes (Figure 2). The operating table is then adjusted with the patient in a 45° head-down position. The principal operating surgeon then proceeds with dissection of the prostate under televisual control using long narrow instruments such as a diathermy knife, scissors, graspers and needle holders passed through the ports while one or two assistant surgeons maintain the magnified view projected on two television screens by manipulating the telescopic camera and removing blood and fluid by suction. 23 Alternatively, the camera can be operated by a single active robotic manipulator arm that is controlled through voice commands from the operating surgeon. 24 Generally, blood loss is prevented by securing visible blood vessels with clips, diathermy and the use of other energy devices such as ultrasound. By considering preoperative findings and direct inspection of the prostate the surgeon will decide whether to preserve one or both neurovascular bundles attached to the posterolateral surface of the prostate that supply the urinary sphincter and penile erectile tissue. Once the prostate is dissected free it is placed in a retrieval bag within the abdomen and the continuity between the bladder and urethra restored by anastomosis using up to six interrupted sutures or by single continuous suture; a urinary catheter is then placed. One of the 12-mm ports is widened slightly to allow retrieval of the excised prostate, which is sent for pathological examination, haemostasis is then confirmed and the port sites closed with sutures. Anaesthesia is then reversed and the patient transferred to the recovery area for initial observation. The procedure typically takes 3.5–4 hours of operating theatre time. Increasing experience with the technique has demonstrated that it does result in reduced blood loss and earlier return to full activity compared with open prostatectomy, but any reduction in rates of erectile dysfunction and incontinence remains uncertain. 25,26
FIGURE 2.
Configuration of differently sized abdominal port sites through which instruments are introduced for laparoscopic prostatectomy. 23 Reproduced with permission from the International Brazilian Journal of Urology.
Robotic prostatectomy
A surgical robot can be defined as a powered device with artificial sensing that can be programmed or externally controlled by a surgeon to position and manipulate instruments to undertake surgical tasks. The key surgical benefits of robotic technology are to tirelessly make precise repetitive movements to move, locate and hold tools and to respond quickly to changes in commands. Robots are intended to assist rather than replace the surgeon, who retains control at all times. They can be broadly classified into three groups: passive, active and master–slave telemanipulators. 27,28 Early positive experience with passive devices, such as frames to accurately position instruments during brain surgery, and active devices programmed to respond to voice- or pedal-activated commands, such as extra ‘arms’ to position the endoscopic camera during standard laparoscopic surgery, led to the design of master–slave surgical manipulators. Here, the surgeon sits at a master console in the operating theatre separate from the patient and remotely controls arms that position and operate the camera and tools inserted into the patient through ports. The control mechanism can be through a joystick, pedals or, more appropriately for surgery, gloved handles that mimic the movements of the slave manipulator. The technology allows the scaling of motion whereby the relatively gross hand movements of the surgeon are translated to micromotions of the robotic arms. This is further enhanced by ‘wrists’ built into the instruments that allow six degrees of freedom of movement, which more closely approximates the range of movements possible by the human hand during open prostatectomy, rather than the more limited four degrees of freedom possible with standard laparoscopic instruments. An advanced camera lens system allows three-dimensional vision and 10–15 × magnification to be transmitted to the master console. Such master–slave telemanipulators were initially developed from previous US military designs by two commercial companies and used for coronary artery bypass surgery,29 but a subsequent commercial merger resulted in a single company, Intuitive Surgical Incorporated (Sunnyvale, CA, USA), which developed the da Vinci® system for wider clinical use. 30
The advantages of the multi-armed robotic telemanipulator system in terms of improved dexterity of operation of laparoscopic instruments by increasing articulation and scaling together with the three-dimensional magnified image all set in an ergonomic platform encouraged a number of centres, particularly in the USA, to apply this system to radical prostatectomy. It was also thought that the greater scope for telemedicine mentoring and the ability of the robot to scale surgeon movements and hence reduce unwanted movements such as tremor would widen the group of surgeons who could achieve competency at keyhole prostatectomy. 31,32
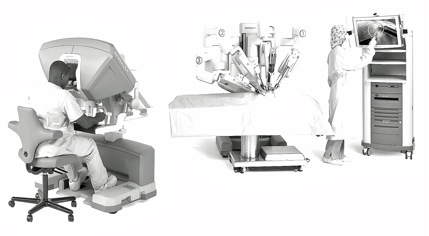
The initial preparation for robotic prostatectomy is identical to that for the standard laparoscopic procedure. The operating theatre is required to be of a minimum size to accommodate the extra equipment, although this is now standard for newer hospital facilities, including those within the UK NHS. Once the ports (generally six) are placed and the patient tilted in a 45° head-down position, the robot is then ‘docked’ to the patient, which generally takes 15–20 minutes. The docking requires the attachment of one robotic ‘slave’ arm to the telescopic camera while the other two (for the three-arm model) or three (for the four-arm model) are attached to the operating instruments that will be manipulated remotely by the lead surgeon. The arms are housed on a cart that is positioned adjacent to the patient. The assistant surgeon generally operates the suction device or retracting instruments through the remaining ports. The operating surgeon sits at a teleconsole within the operating theatre linked to the robot by cable, although more remote wireless locations are possible (Figure 3). 33 The console comprises a three-dimensional display monitor for the camera-fed operative view, ‘master’ arms linked to the ‘slave’ arms, which allow the surgeon to direct and operate the instruments, camera-positioning controls, foot pedals controlling diathermy for haemostasis and finally a central processing unit to regulate the system. Additional controls can adjust the display, the offset angle of the telescopic camera lens and the ratio of the scaling of surgeon’s movements to instrument movements. The procedure typically takes 3.5–4.5 hours of operating theatre time. Robotic prostatectomy also results in reduced blood loss and quicker return to full activity but again the hoped-for reduction in rates of incontinence and erectile dysfunction as a result of improved vision remains uncertain. 34 A deficiency of the robotic technique is the lack of transmission of the feel of the tissues from the remote instruments; reproduction of this haptic sense is a key aim of future development.
FIGURE 3.
da Vinci surgical robot system showing, from left to right, surgeon at remote console; three-armed (labelled 1–3) telemanipulator for docking to patient; and assistant adjusting room monitor. ©[2011] Intuitive Surgical, Inc. Reproduced with permission from ©2010 Intuitive Surgical, Inc.
It should be noted that the robotic technology within the da Vinci system continues to evolve and advancements tend to be added by Intuitive Surgical as options to the basic platform at extra cost. Currently, purchasers of the system can choose to have a fourth robotic arm, reducing the number of surgical assistants required, more advanced image transmission and an additional console to allow mentoring of surgeons under training (similar to dual controls for a motor car).
Current use in the UK NHS
Requirement for radical treatment of prostate cancer in the UK NHS
In the UK prostate cancer is generally detected by PSA testing of men complaining of lower urinary tract symptoms, although the numbers of asymptomatic men requesting a PSA test to assess their risk of having or developing prostate cancer is increasing, particularly among more affluent socioeconomic groups in the south of England. 35 For men with a serum PSA above a diagnostic threshold currently set in the UK at 3 ng/ml for men in their 50s, 4 ng/ml for those in their 60s and 5 ng/ml for those in their 70s, prostate biopsy is recommended. 8,36 Biopsy involves obtaining 10–12 cores of prostate tissue measuring 10 × 2 mm by transrectal ultrasound (TRUS)-guided needle biopsy as an outpatient procedure under local anaesthetic. This procedure is uncomfortable and is often associated with mild adverse effects such as bleeding and urinary tract infection (30–80%); more severe adverse effects such as systemic sepsis are uncommon (< 1%). 37
At present, approximately 25% of men with PSA levels above threshold will have cancer detected on biopsy,38 with 37,051 men being registered with the diagnosis in the UK during 2008. 11 Following diagnosis a treatment decision has to be made, which will involve consideration of the PSA level, the clinical stage of the cancer categorised on the tumour, node, metastasis (TNM) staging system,39 the aggressiveness of the cancer classified by grading the degree of disruption of the normal glandular architecture of the prostate seen on microscopic examination using the Gleason score40 and person factors such as life expectancy and treatment preference. 12,41,42 For men with apparent localised disease confined to the prostate gland (preoperative clinical classification of tumour stage cT1 and cT2, N0, M0), radical treatment by either surgery or radiation is an option, together with active surveillance programmes, with deferred treatment for men with a Gleason score ≤ 6. 43 Current evidence suggests that any benefit to the individual receiving radical treatment for prostate cancer takes at least 10 years to accrue and therefore these options are best used for men whose comorbidity and age suggests a life expectancy of > 10 years. 44 Finally, evidence is increasing that more aggressive cancers, categorised by a Gleason score of ≥ 8 out of 10 and a PSA of > 20 ng/ml, are likely to already have developed metastases and therefore such patients are considerably less likely to benefit from radical treatment alone. 45 The typical man who undergoes radical prostatectomy therefore is generally fit [American Society of Anesthesiologists (ASA) grade 0–2] and aged < 70 years and has tumour characteristics suggesting low or intermediate risk of disease progression according to the D’Amico risk classification system (Table 1). 46
| Group | PSA (ng/ml) | Gleason score (0–10)a | Clinical stagea | ||
|---|---|---|---|---|---|
| Low risk | < 10 | and | ≤ 6 | and | cT1–cT2a |
| Intermediate risk | 10–20 | or | 7 | or | cT2b–cT2c |
| High risk | > 20 | or | 8–10 | or | cT3–cT4 |
Estimated demand for radical prostatectomy
Assuming that 45% of men diagnosed with prostate cancer in the UK are aged < 70 years11 and that the disease is localised to the prostate in 86% of cases,47 approximately 14,000 men would have the option of radical treatment each year. Health episode statistics recorded for NHS England48 show that approximately 4000 (28% of the estimated total) men underwent radical prostatectomy in the year 2009–10, this being a similar proportion to that seen for men diagnosed with cancer in the control arm of the European Randomised Study of Screening for Prostate Cancer [946/3402 (28%)]. 49 [It is noted that there is a discrepancy between differing NHS datasets in the numbers of men coded as having a radical prostatectomy in NHS England in the financial year 2009–10: 4100 using the Office of Population Census and Surveys (OPCS) four-character procedure codes compared with 4703 using Healthcare Resource Group (HRG) codes.] The remaining men chose alterative treatment options such as implantation of radioactive seeds (brachytherapy, 15%), external beam radiotherapy (40%) or decided on an active surveillance protocol (17%). Demographic trends in terms of the increasing number of men at risk together with an anticipated continued rise in the use of PSA testing in the UK suggest that the demand for prostatectomy and other options to treat localised prostate cancer will increase over the next 10 years. Using the hypothetical scenario of increased ‘on demand’ use of PSA testing up to the rate currently practised in the USA would give an estimated figure of 7000 men per year,50 and this would rise further to an estimated 11,000 men per year with the hypothetical scenario of a national programme of PSA screening. 49,50
Current use of technologies in UK NHS
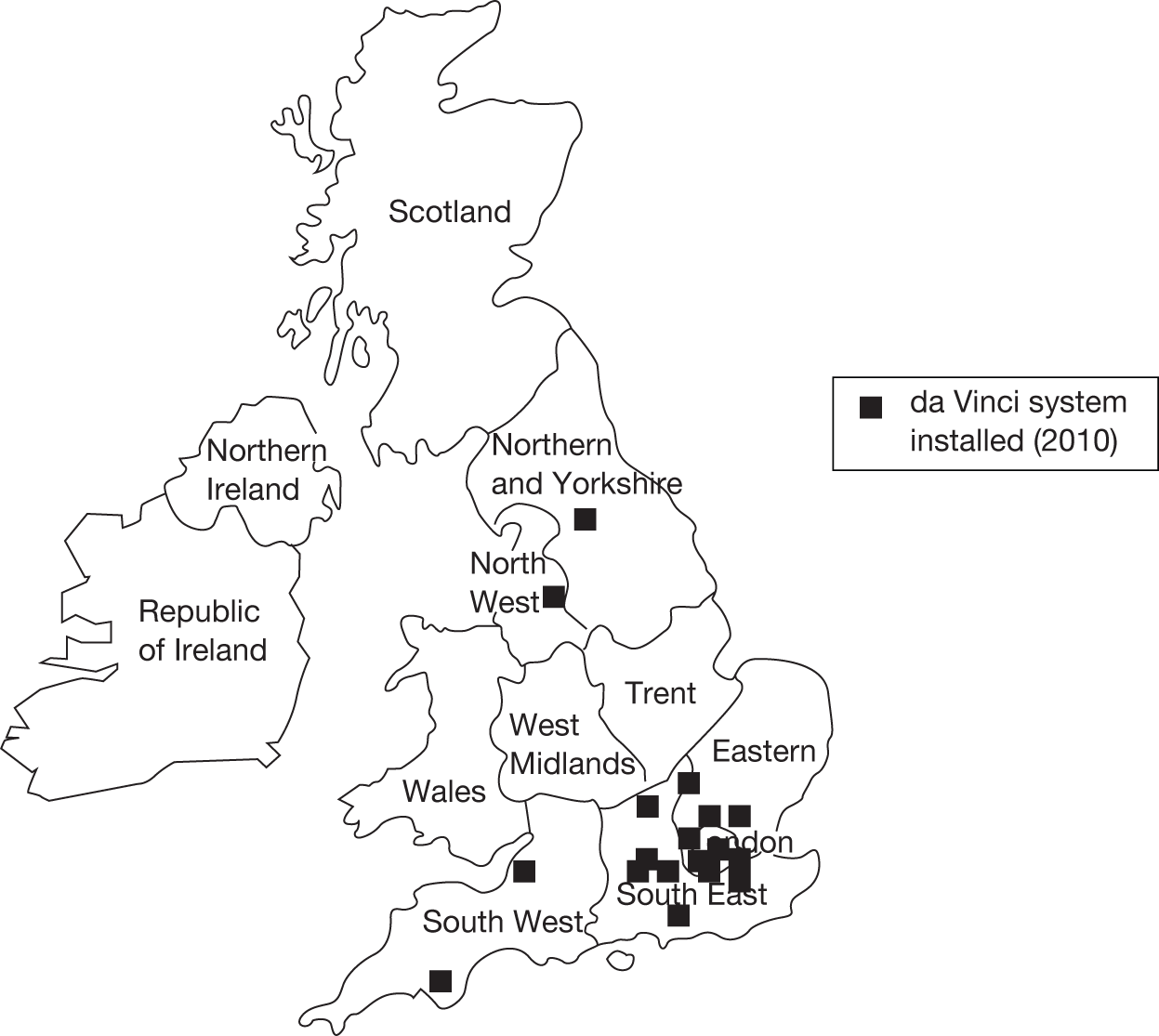
Under the NHS Cancer Plan pelvic cancer surgery, including radical prostatectomy, is concentrated within 60 UK cancer centres, of which approximately 20 perform at least some procedures laparoscopically [personal communication from expert panel members (D Neal, C Eden, R Kodelburg, N Soomro, A McNeil), 2010]. In 2010, 16 had access to a da Vinci robotic system, although most robotic systems in the UK were installed in 2009–10 and were not yet fully operational at the time of carrying out this review (Figure 4). 30,51 NHS England reference cost data recorded 1816 laparoscopic/robotic procedures in the year 2009–10, suggesting that these options were used for 46% of all radical prostatectomies. 52 Our own survey of cancer units known to be carrying out laparoscopic and robotic radical prostatectomies suggests a current 50 : 50 split between laparoscopic and robotic techniques, meaning that approximately 23% of radical prostatectomies carried out in the UK at present are performed using the robotic technique. Other areas of the world have experienced a greater uptake of robotic prostatectomy, for example in the USA it was estimated that 43% of all radical prostatectomies were performed using the robotic technique in the year 2006–7 and approximately 70% in 2008. 17,53,54
FIGURE 4.
UK sites with an installed da Vinci robotic surgical system in 2010.
Current costs for the UK NHS
NHS reference costs for England for the financial year 2009–10 published by the UK government’s Department of Health show an average tariff for open radical prostatectomy (HRG code LB21Z) of £4614 with 2897 procedures claimed by NHS hospitals giving a total annual cost of £1,336,758. For laparoscopic and robotic prostatectomy (HRG code LB22Z), the average tariff was £5257, with 1816 procedures claimed, giving a total annual cost of £9,546,712. (It is noted that there is a discrepancy between differing NHS datasets in the numbers of men coded as having a radical prostatectomy in NHS England in the financial year 2009–10: 4100 using OPCS four-character procedure codes compared with 4703 using HRG codes.) These data suggest a grand total tariff-based cost to the English NHS of £10,883,470 for the year 2009–10. Both an increase in the number of radical prostatectomies required and an increase in the proportion of procedures carried out using a laparoscopic or robotic technique would substantially increase the cost to the NHS. For example, a scenario of increased use of PSA testing leading to a demand for 7000 procedures per year that were all carried out laparoscopically or robotically would increase the tariff-based cost by 240% to £36,799,000.
Summary
Policy-makers within the UK NHS are therefore faced with the need to plan service provision for the increasing number of men diagnosed with localised prostate cancer who decide on radical prostatectomy as their preferred treatment option. A keyhole technique of radical prostatectomy either by standard laparoscopy or with the aid of robotic technology does appear to offer advantages in terms of reduced morbidity over the traditional open surgical approach. Advocates of the robotic system claim greater precision in dissection and more rapid gaining of surgeon competence for the procedure but this comes at a substantially greater equipment cost. This review has therefore been designed to help inform decisions regarding the commissioning and use of robotic surgery for men with localised prostate cancer in the NHS.
Aim of the review
This study aimed to determine the relative clinical effectiveness and cost-effectiveness of robotic prostatectomy compared with laparoscopic prostatectomy in the treatment of localised prostate cancer within the UK NHS (the full study protocol is available at www.hta.ac.uk/2169). The specific objectives of the study were to:
-
describe clinical care pathways for laparoscopic and robotic prostatectomy in a UK context
-
determine the relative clinical effectiveness and safety of each procedure
-
determine the influence of the learning curve on estimates of effectiveness and safety
-
perform a systematic review of existing economic evaluations of each procedure
-
determine which procedure is most likely to be cost-effective for implementation in the NHS
-
identify future research needs.
Chapter 2 Description of the care pathway
Introduction
The described care pathway (Figure 5) was constructed using available evidence and consensus building through two meetings of the expert panel convened for this review. Although it is primarily constructed to plan the systematic assembly of evidence and design the mathematical model that will estimate effectiveness and cost-effectiveness, the pathway is consistent with previously published clinical pathways of care. 43,45,51,55,56 This chapter will describe each component of the pathway.
FIGURE 5.
Summary flow chart showing complete care pathway used to frame the systematic review questions and the health economic model. BCR, biochemical (PSA) recurrence.
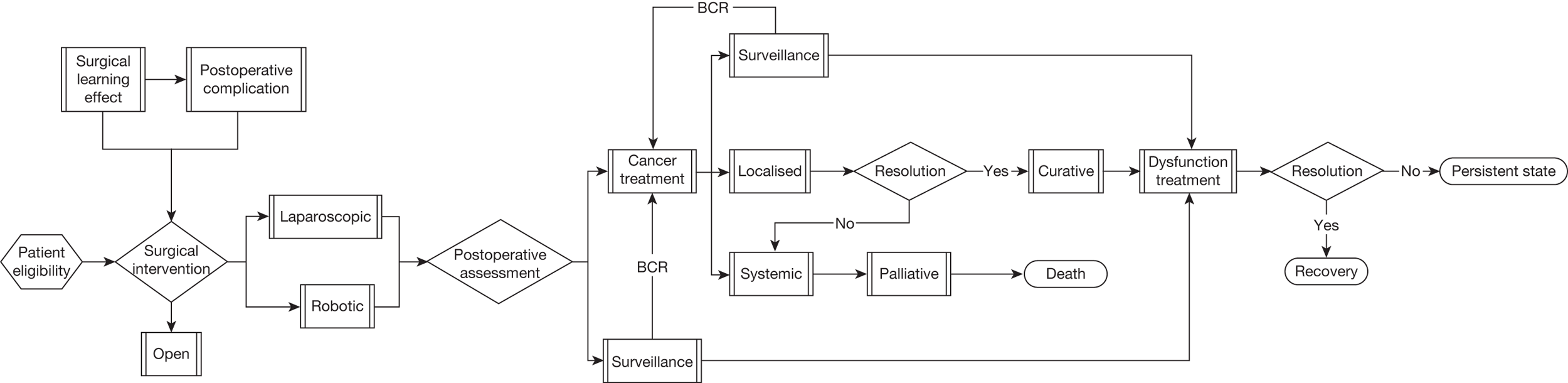
Preoperative characteristics of men undergoing radical prostatectomy
Patient characteristics
The population of patients considered for this review are men with localised prostate cancer undergoing radical prostatectomy at designated pelvic cancer surgical treatment centres within the UK NHS. The patient variables that define this population include age and comorbidity that together determine an estimated life expectancy of at least 10 years. The great majority of such men are able to undergo radical prostatectomy by either standard laparoscopic or robotic techniques; the few exceptions suited only to the open approach are those with poor respiratory reserve, morbid obesity or previous extensive pelvic surgery.
Disease factors are focused on the estimated risk of developing recurrent disease from metastases not identified at preoperative assessment or because of failure to completely remove localised disease. The approximate magnitude of this risk for an individual man diagnosed with prostate cancer can be calculated using a nomogram developed from linear regression models, the most commonly used version being hosted by the Memorial Sloan Kettering Cancer Institute in web-based form. 57 These models use the preoperative disease factors of age, PSA, clinical tumour stage, Gleason grade and number of needle biopsy cores positive for cancer.
Preoperative level of prostate-specific antigen
The preoperative serum PSA level is an independent statistically significant predictor of future recurrence but on its own is limited in reliability and predictive value. For prognostic purposes the value is defined in groupings corresponding to low (< 10 ng/ml), intermediate (10–20 ng/ml) and high (> 20 ng/ml) risk of disease progression.
Staging of prostate cancer
The stage of an individual’s cancer is categorised according to the Union for International Cancer Control (UICC) 2009 classification (Table 2). 39 Preoperatively this is determined by clinical assessment using digital rectal examination and imaging with the allocated tumour stage (T) given the prefix ‘c’, for example cT1. Following prostatectomy, pathological examination of the prostate and, in some cases, adjacent lymph nodes may result in a change in the staging as more accurate information concerning the size of the tumour and whether it has breached the external surface of the prostate will be available. To indicate this more accurate evaluation, the T stage assigned following pathological examination of the whole prostate is given the prefix ‘p’, for example pT2a. Rarely, no tumour will be found on pathological examination of the prostate following radical prostatectomy for biopsy-proven cancer; this is designated pT0.
| Stage | Substage | Description |
|---|---|---|
| T0 | No evidence of cancer found on complete pathological examination of the prostate | |
| T1 | Clinically unapparent tumour, not detected by digital rectal examination nor visible by imaging | |
| T1a | Incidental histological finding; ≤ 5% of tissue resected during TURP | |
| T1b | Incidental histological finding; > 5% of tissue resected during TURP | |
| T1c | Tumour identified by needle biopsy | |
| T2 | Confined within the prostate | |
| T2a | Tumour involves half of the lobe or less | |
| T2b | Tumour involves more than half of one lobe but not both lobes | |
| T2c | Tumour involves both lobes | |
| T3 | Tumour extends through the prostate capsule but has not spread to other organs | |
| T3a | Extracapsular extension (unilateral or bilateral) including bladder necka | |
| T3b | Tumour invades seminal vesicle(s) | |
| T4 | Tumour is fixed or invades adjacent structures other than seminal vesicles | |
| T4a | Tumour invades external sphincter and/or rectum | |
| T4b | Tumour invades levator muscles and/or is fixed to pelvic wall |
Gleason grading
The qualitative low-magnification microscopic histological description of prostate cancer first suggested by Gleason58 remains an essential aspect of prognostic categorisation although there have been substantial modifications over the subsequent years. 40 The classification grades individual areas of prostate cancer according to the degree of disruption of normal glandular architecture, with grade 1 indicating minimal disruption, grade 5 complete loss of normal glandular arrangement and grades 2, 3 and 4 intermediate between these two extremes. Standard practice consists of identifying the first and second most prevalent patterns within a set of biopsy cores, which give the primary and secondary Gleason grades (each rated 1–5). These are then added together to give the overall Gleason sum score (2–10). Recent consensus tends to limit the use of grades 1 and 2 and therefore scores generally range between 6 and 10. 59 Any tertiary higher disease areas are also reported irrespective of their extent. Higher individual grade and total sum score indicate more aggressive disease with the primary grade being more predictive. For example, an individual whose tumour is categorised as Gleason score 4 + 3 = 7 will tend to have a worse prognosis than an individual with a Gleason score of 3 + 4 = 7. 60 Recent consensus mandates that pathological reporting of prostate cancer using the Gleason grading system should include the most prevalent pattern (primary grade), the second most prevalent pattern (secondary grade) and the presence of any areas that are assigned a higher grade than that assigned to either the primary or secondary patterns (tertiary grade). For needle biopsies the Gleason score is obtained by summing the higher of the secondary or tertiary grades. For radical prostatectomy specimens the Gleason score is obtained by summing the primary and secondary grades, any higher-grade tertiary pattern being stated separately if it occupies < 5% of the tumour.
Cancer extent
There is some evidence that the tumour extent on needle core biopsy estimated by measuring the number of cores positive for cancer, the percentage of needle core tissue affected by cancer and the length in millimetres of the core segments with cancer present is also an independent prognostic factor predictive of future disease progression. 61 Similarly, the total volume of cancer identified by pathological examination of the whole prostate after radical prostatectomy has been assessed as a possible predictive factor for recurrence but was found not to be independently significant on multivariate analysis. 62 These pathological measures of cancer extent have not been included in our care pathway given the current uncertainty of the evidence base.
Summary
Variables collected preoperatively for men undergoing radical prostatectomy including age, tumour stage, Gleason score and tumour volume can predict the risk of disease progression at some time after surgery, with stage and Gleason sum score being most useful. It is therefore important that studies comparing treatments, such as this review, include an assessment of whether or not the patient groups undergoing each procedure are balanced for these variables.
Perioperative care
Introduction
For the purposes of this review it is assumed that the procedures being considered will be carried out in hospitals that have the necessary resources in terms of staff, facilities and NHS cancer plan approval to carry out either laparoscopic prostatectomy or robotic prostatectomy on a routine basis. This will comprise operating theatre and recovery facilities including critical care and standard urology wards, the required clinical and technical expertise including surgeons, anaesthetists, theatre nursing team, pathologists and technicians, and continued care including outpatient review, repeat imaging and facilities for further treatment for adverse events or cancer progression. The procedures have been described in Chapter 1. 30,63 For the safe conduct of both procedures it is important that all members of the operating theatre team have had specific training in the performance of the procedures, this being particularly crucial from a technical point of view for the robotic procedure.
Surgeon learning curve
Both laparoscopic and robotic prostatectomy are currently being implemented in the UK NHS, requiring the training of surgeons to perform the procedures. The performance of repeated tasks tends to improve with experience and this improvement is characteristically rapid at first and then slower as a steady state expert level is reached, leading to the use of the term ‘learning curve’ to describe the process. Learning of surgical procedures can be additionally influenced by the previous experience of the surgeon or surgical team, case-mix selection, use of multiple outcomes defining ‘success’ and continued development of the technology. 64 The learning curve effect is often crudely quantified by the number of procedures required to reach competence or the reducing time taken to perform the procedure; in open prostatectomy, for example, experience-related changes in performance may continue even after 250 procedures. 18 As use of laparoscopic prostatectomy increased it was realised that the procedure was difficult to master, requiring a high number of training procedures to achieve competence, and that the skills required did not translate directly from those used in open surgery. 65 This is a particular problem in countries such as the UK, where few centres undertake more than 50 cases per year, the suggested volume required for training and maintenance of competency. 66 Findings from individual case series suggest that robotic prostatectomy reduces the number of cases required for competence, enabling the surgeon to reach an expert level quicker, and that previous experience of laparoscopic prostatectomy is not essential. 67 In addition, it is possible that some surgeons who are unable to master the laparoscopic technique can take advantage of the greater movement control offered by the robotic system to become competent in robotic prostatectomy. Any evaluation of effectiveness and safety of the prostatectomy procedures must therefore balance the relative effects of the learning curves.
Pelvic lymphadenectomy
Men whose disease is characterised preoperatively as intermediate or high risk (see Table 1) may be advised to undergo pelvic lymphadenectomy as part of their laparoscopic or robotic radical prostatectomy in order to detect occult lymph node metastases. The lymphadenectomy is performed as the first part of the radical prostatectomy procedure using a standard dissection template and the package of lymph nodes is removed separately from the prostate for subsequent pathological examination. The prostatectomy would be aborted only if there was gross visible lymph node enlargement, which, given preoperative imaging, is a very rare circumstance. For the purposes of this evaluation we chose, in consultation with the expert panel, to assume that all men with intermediate- or high-risk disease undergoing laparoscopic or robotic prostatectomy would also have a pelvic lymphadenectomy. This is in line with current guidance but we do acknowledge the controversy in this area. 45
Hospital stay
Men are generally admitted to hospital either on the day of surgery or the evening before. A rectal enema is administered to clear the lower bowel. Just before surgery prophylactic antibiotics are given according to local policy and venous thrombosis/embolism prophylaxis also commenced. After surgery the patient is routinely nursed on a standard ward in the UK although specific comorbidities or intraoperative complications may require a period in a critical care area. In the UK, men are typically discharged home after 3 days with an indwelling catheter although this can be reduced by managed care programmes. They then return to the ward after a further 7–14 days according to local protocol as a day patient for urinary catheter removal and voiding check.
Perioperative adverse events
General
Although men undergoing this surgery generally do not have concurrent comorbidity that is a persistent threat to their health a proportion will be expected to suffer adverse events associated with major surgery and prolonged anaesthesia such as cardiac ischaemia, pulmonary embolism and prolonged loss of bowel function (ileus). In addition, specific complications include urinary and bloodstream infection, inadvertent injury to adjacent organs, particularly rectal perforation, excessive blood loss requiring transfusion and prolonged urinary or lymphatic leakage from abdominal drains. The adverse effect of these complications in terms of their severity and requirement for additional interventions and hospital stay can be summarised according to the Clavien–Dindo system (Table 3). 68,69
| Grade | Definition | Exclusions |
|---|---|---|
| Grade 0 | No deviation from planned postoperative course considering procedure and pre-existing comorbidity | |
| Grade I | Any deviation from the normal postoperative course without the need for specific pharmacological treatment or surgical, endoscopic and radiological interventions | |
| Grade II | Requiring pharmacological treatment with drugs other than those allowed for grade I complications. Includes blood transfusions and total parenteral nutrition | Treatments listed under grade I |
| Grade IIIa | Requiring surgical, endoscopic or radiological intervention not under general anaesthesia | |
| Grade IIIb | Requiring surgical, endoscopic or radiological intervention under general anaesthesia | |
| Grade IVa | Life-threatening complication affecting single organ system requiring IC/ICU management | TIA |
| Grade IVb | Life-threatening complication affecting more than one organ system requiring IC/ICU management | TIA |
| Grade V | Death of a patient |
Bladder neck contracture
An additional specific short-term complication is fibrosis and contracture of the sutured join between the top of the urethra and bladder outlet, the vesico-urethral anastomosis, termed bladder neck contracture or bladder neck stenosis. This will become noticeable after removal of the draining catheter with the narrowing of the urine channel, resulting in voiding problems reported by the patient over the next 3–6 weeks according to the severity of contracture. It is treated by endoscopic incision of the narrowed area, which requires an additional short hospital stay and a 7-day period of catheterisation. For most men the problem is cured by a single incision although for some this may need to be repeated once or twice. 70
Pathological examination of the prostate
Careful and thorough microscopic examination of the removed prostate by an experienced pathologist is required to determine the true extent of the disease and to identify whether or not the surgery may have been unable to remove all of the contained cancer (positive margin), whether or not the cancer has spread outside the prostate (extraprostatic extension) and, if lymphadenectomy has been performed, the presence of lymph node metastatic disease. In addition, a more comprehensive assessment of the Gleason patterns within the cancer is possible. 71 This examination will recategorise the disease according to stage and, if appropriate, lymph node status (pT and pN) and postoperative Gleason sum score, which will allow more accurate estimation of prognosis according to available post-radical prostatectomy prognostic nomograms57 and inform whether early additional (adjuvant) treatment should be advised. The crucial nature of this examination has led to regular international plenary meetings of expert pathologists who have made consensus recommendations guiding best practice for specimen collection, processing, examination and analysis in order to promote consistency in pathologist reporting of radical prostatectomy specimens. 59,72
Surveillance following radical prostatectomy
Follow-up schedule
Men who have undergone radical prostatectomy are generally seen by the operating team as outpatients 6 weeks after their surgery and then 3-monthly for the first year and 6-monthly for the next 4 years. At each follow-up consultation serum PSA is checked for evidence of tumour recurrence and a qualitative assessment made for continence and desired sexual function. If further assessment or treatment is required for any of these aspects then the pathway of care will be changed accordingly (see Figure 5).
Detection of persistent or recurrent disease
The risk of disease recurrence is higher if one or more of the following disease factors are present: preoperative PSA > 20 ng/ml, pathological Gleason score > 7, pathological extraprostatic disease (pT3/pT4), pathological positive margin or positive lymph nodes (pN1/pN2). If positive lymph nodes are found or the likelihood of disease persistence or recurrence is otherwise deemed to be very high then immediate adjunctive treatment may be offered. For the majority of men, however, PSA surveillance is started according to a standard schedule, for example that defined in the preceding paragraph. Following removal of the prostate, serum PSA (half-life 2.2 days) levels will rapidly fall to an undetectable level, defined as values less than the sensitivity of the assay. Generally, ultrasensitive PSA assays are used for men following radical prostatectomy giving postoperative values of < 0.01 ng/ml. Definitions of the threshold of PSA rise that signifies cancer recurrence vary but generally the finding of two successive PSA readings > 0.2 ng/ml is used, this being denoted biochemical recurrence. 73,74 Once biochemical recurrence occurs a decision will be made with the patient whether to continue surveillance or commence adjuvant treatment. This decision will be informed by tests such as magnetic resonance imaging and radionuclide bone scanning designed to demonstrate the site of recurrence as being in the prostatic bed (localised) or as lymph node or bony metastases (systemic).
Adjuvant treatment
For purely localised recurrence radical radiotherapy is recommended as defined in the RADICALS trial protocol. 75 The treatment consists of delivery of up to 66 Gy of radiation divided into daily doses over 4–6 weeks. It is uncertain whether or not the addition of short-term androgen deprivation is beneficial for presumed localised disease, a research question that RADICALS is designed to address. For men with likely systemic recurrence, long-term, typically life-long, androgen deprivation therapy (medical castration) most commonly achieved with a luteinising hormone-releasing hormone (LHRH) agonist is recommended. This consists of 3-monthly subdermal injections of a depot preparation of the chosen drug. Alternatively, some men may choose surgical castration, removing both testicles (bilateral orchiectomy). The use of long-term androgen deprivation therapy or bilateral orchiectomy for metastatic disease is thought to be palliative because at some point the disease will lose androgen dependency (castrate-resistant prostate cancer). The duration from start of therapy to escape from androgen control, signified by a further substantial rise in PSA values, varies according to the aggressiveness and extent of disease, with a median time of approximately 12 months. Side effects of androgen deprivation therapy include hormonal changes leading to hot flushes, gynaecomastia and altered fat distribution together with osteoporosis. Men with castrate-resistant prostate cancer have a median survival of approximately 18 months and further treatment is usually palliative with symptom control and use of corticosteroid drugs to improve well-being. The chemotherapeutic agent docetaxel does have some activity, extending survival by 3 months on average, but is suited only to men with good performance status. 76
Urinary incontinence
Recovery of continence following radical prostatectomy can take up to 12 months although most men will regain continence by 6 months. In general, therefore, men suffering urinary incontinence will be advised to use containment devices such as absorbent pads or penile sheath drainage for the initial 12 months. If bothersome leakage persists beyond this time then the main treatment options will be surgical implantation of an artificial urinary sphincter (AUS) or continued use of containment devices. For the purposes of this evaluation we used the individual definition of urinary incontinence given in each study without attempting to separate out differing definitions or categorisation of severity. A recently reported randomised controlled trial (RCT) of pelvic floor muscle therapy following radical prostatectomy demonstrated that the rate of urinary incontinence beyond 12 months using patient-reported measures and data collection independent of the clinical team was higher than that given by most of the studies used in our meta-analysis. 77
Erectile dysfunction
For men who were sexually active before surgery, approximately 40% will experience worsening of their sexual function and in particular difficulty initiating and sustaining penile erection sufficient for desired sexual activity. This is particularly dependent on preservation of one or both neurovascular bundles at the time of radical prostatectomy. Similar to urinary incontinence full recovery can take up to 12–18 months following surgery. For men with persistent and bothersome erectile dysfunction, treatment options will include drug treatment taken on an as-required basis, a vacuum constriction device or penile implant surgery. Most men will first trial an oral phosphodiesterase type V inhibitor, with a suggested prescribing frequency of one treatment per week according to NHS guidance. The next option will be alprostadil (Carerject®, Pfizer) given as an intraurethral pellet or an intracavernosal injection with suggested NHS prescribing frequency again of one treatment per week. For men who achieve satisfactory restoration of sexual activity with these drugs their use will continue long term. If drug treatments are unsuccessful men may trial a vacuum constriction device or consider surgical implantation of a penile prosthesis. The proportion of men pursuing these last two options is small as most will accept their loss of sexual function in the longer term. In addition, it should be noted that, although this outcome is an important aspect determining treatment selection for many men with localised prostate cancer, the definition of any deterioration is not standardised and collection of data concerning sexual function before and after surgery is generally poor. Most studies do not separately categorise those men who were sexually active before surgery and who underwent deliberate nerve-sparing surgery with the aim of preserving sexual function.
Chapter 3 Methods of the systematic review of clinical effectiveness
Methods
Comprehensive electronic searches were conducted to identify reports of published studies. Highly sensitive search strategies were designed including appropriate subject headings and text word terms, interventions under consideration and specific study designs. There was no language restriction but searches were restricted to years from 1995 onwards, reflecting the time of introduction of the techniques. MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, BIOSIS, Science Citation Index and Cochrane Central Register of Controlled Trials (CENTRAL) were searched for primary studies while the Cochrane Database of Systematic Reviews (CDSR), the Database of Abstracts of Reviews of Effects (DARE) and the HTA database were searched for reports of evidence syntheses. Reference lists of all included studies were scanned to identify additional potentially relevant reports. The expert panel provided details of any additional potentially relevant reports.
Conference abstracts from meetings of the European, American and British Urological Associations were searched. Ongoing studies were identified through searching Current Controlled Trials, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry and the National Institutes of Health (NIH) Research Portfolio Online Reporting Tools Expenditures and Results (RePORTER). Websites of manufacturers, professional organisations, regulatory bodies and the HTA were checked to identify unpublished reports. Full details of the search strategies used are detailed in Appendix 2.
Inclusion and exclusion criteria
Types of study
Evidence was considered from RCTs, non-randomised comparative studies and, for estimates of learning curve effects only, case series. For estimating learning curve effects robotic or laparoscopic arms of comparative studies were treated as separate case series. Conference abstracts and non-English-language reports were included only if they were of comparative studies.
Types of participants
The types of participants considered were men with clinically localised prostate cancer (cT1 or cT2), defined as cancer confined to the prostate gland and considered curable by radical removal of the prostate. Studies were included if ≥ 90% of the included men fulfilled this definition.
Types of interventions and comparators
Robotic radical prostatectomy was considered as the intervention and laparoscopic radical prostatectomy as the comparator. Open radical prostatectomy was also considered in studies comparing open radical prostatectomy with robotic radical prostatectomy and/or laparoscopic radical prostatectomy so that such studies could be included in a mixed-treatment comparison model (see Data analysis) assessing the relative effectiveness of robotic and laparoscopic radical prostatectomy.
Types of outcome measures
The following types of outcome measures were considered:
-
complications and adverse events including blood transfusion, anastomotic leak, bladder neck contracture, wound infection, organ injury, ileus, deep-vein thrombosis and pulmonary embolism
-
cancer related:
-
– rate of positive margin in resected specimen
-
– biochemical (PSA) recurrence
-
– need for further cancer treatment
-
– disease-free survival, defined as absence of clinically detectable disease
-
– survival
-
– mortality
-
-
functional:
-
– recovery of sexual (penile erection) function, quantified where possible by validated scores such as the International Index of Erectile Function-5 (IIEF-5)
-
– urinary continence, defined as use of one thin pad or less per day and/or as assessed on a validated symptom score
-
-
patient driven
-
– pain, quantified on a validated pain score, and analgesic requirements
-
– productivity (time to return to full activity)
-
– generic and disease-specific quality of life, measured through validated scores
-
-
descriptors of care
-
– equipment failure
-
– conversion to open procedure
-
– operative time
-
– duration of catheterisation
-
– hospital stay
-
–learning curve.
-
Exclusion criteria
The following types of report were excluded:
-
studies of men with metastatic disease
-
case series of open radical prostatectomy.
Data extraction strategy
Three reviewers independently screened titles and abstracts of all identified items. Full-text copies of all potentially relevant reports were obtained and independently assessed by two reviewers to determine whether or not they met the inclusion criteria. Three reviewers extracted details of study design, methods, participants, interventions and outcomes onto a data extraction form (see Appendix 3). Each reviewer’s data extraction was independently checked by a second reviewer for errors or inconsistencies. Any disagreements were resolved through consensus or arbitration by a third party. For studies reporting adverse events, two surgeons categorised each complication using the Clavien–Dindo classification of surgical complications68 (see Table 3) with a third surgeon acting as arbiter in cases of disagreement about classification.
Quality assessment strategy
Risk of bias
A modified version of the Cochrane risk of bias tool78 was adapted to include potential topic-specific confounders, which were identified through discussions with members of our project advisory group and our knowledge of existing literature. The topic-specific confounders related to specific outcomes are shown in the modified risk of bias tool (see Appendix 4). Three sets of two reviewers independently assessed the risk of bias of included full-text studies, with the exception of non-English publications and conference abstracts. Any differences in assessment or issues of uncertainty were resolved by discussion and consensus between the reviewers. The risk of bias assessment was summarised at the study level using judgements incorporating individual outcomes as well as study-level risk of bias domains. Individual outcomes were categorised as high risk of bias, low risk of bias or unclear risk of bias. The categories were weighted to reflect higher disagreement between the two clear categories of low and high risk with lower weighting for disagreement between either high- or low-risk and unclear judgements. Any disagreements were resolved by consensus or arbitration by a third party. The kappa statistic was used to assess inter-rater agreement between assessors of the risk of bias in each study, with 0–0.2 as slight agreement, 0.21–0.4 as fair agreement, 0.41–0.6 as moderate agreement, 0.61–0.8 as substantial agreement and 0.81–1 as perfect agreement. 79 If there was a sufficient number of low risk of bias studies, a meta-analysis would be performed restricted to only these studies (see Data analysis).
Determination of surgical margin status
Various protocols are described for the standardisation of processing and reporting of radical prostatectomy specimens, to identify pathological factors that could accurately predict patient outcome. 59,80–82 Variations in the protocols employed may potentially affect the determination of surgical margin status. Details of the methods described for the handling, processing and reporting of radical prostatectomy specimens were tabulated and summarised (see Table 7). The categories for the tabulations were derived from the findings of a recent international consensus conference on handling and staging of radical prostatectomy specimens, which convened following a web-based survey of members of the International Society of Urological Pathology (ISUP) with the intention to promote consistency in pathological reporting and the collection of appropriate prognostic information. 83,84 If there was a sufficient number of studies, a meta-analysis would be performed restricted to only the studies that reported all criteria (see Data analysis).
Data analysis
Data from each study were tabulated and summarised for each procedure in a form appropriate for the mixed-treatment comparison model. The lack of RCT evidence precluded undertaking a standard two-group meta-analysis; therefore, an indirect comparison (cross design) approach allowing inclusion of non-randomised comparative data was adopted85 within a mixed-treatment comparison framework. The models implemented were based on mixed-treatment comparison models developed by Lu and Ades. 86 The main parameters in the models for dichotomous outcomes are the logarithm of the odds ratios (log-ORs) of each procedure compared with the reference procedure open surgery. A random-effects model was adopted that incorporated an adjustment for the correlation between arms in studies that compared all three procedures. The model parameters were estimated within Bayesian methodology with the use of WinBUGS software version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK).
For continuous data for duration of operation, a similar model was constructed using means and standard errors instead of log-ORs and standard errors. This was carried out only in studies that compared robotic with laparoscopic procedures directly. Some assumptions were made because of the inconsistent reporting of duration of operation. If a median was reported but no mean the median was used as a substitute for the mean. Furthermore, if the standard deviation (SD) was not reported, imputation was conducted using the method proposed by Marinho and colleagues. 87 In this method, a linear regression of log (standard deviation) on log (mean) for all studies that reported a mean and standard deviation is first undertaken. The resultant predictive formula is then used to impute standard deviations for studies missing this value given the reported mean. This was conducted for each radical prostatectomy procedure separately.
Odds ratios (ORs) and associated 95% central credible intervals (CrIs) were estimated between laparoscopic surgery (the base case) and robotic surgery; if the OR is > 1 the calculated odds of a particular event are higher for robotic surgery than for laparoscopic radical prostatectomy, whereas if the OR is < 1 the calculated odds of a particular event are higher for laparoscopic radical prostatectomy. The CrI will show the degree of uncertainty around these calculated values. The statistical probability of the OR being different from 1, and hence the probability that robotic radical prostatectomy was better or worse than laparoscopic radical prostatectomy for specific outcomes, was calculated (this is sometimes called the ‘Bayesian p-value’ and is the proportion of the samples in the simulation in which the OR was < 1). In this report we have assumed that a probability equal to 0.95 is ‘statistically significant’. Finally, an individual estimate of the probability of the event occurring for each type of radical prostatectomy was calculated. These estimates were calculated from the model by using a prior distribution for the probability of an event when using the reference treatment (which was open radical prostatectomy) and combining that with the OR between each type of surgery and open surgery. The prior distribution for the event rate for open surgery was estimated using the data for open surgery in the included studies only and by applying a normal distribution to the log-OR of the probability of each outcome, with its mean and variance being estimated from a standard Bayesian random-effects model.
When there were a sufficient number of studies, the heterogeneity of effects was explored by repeating the analyses including only data from studies assessed at low risk of bias. In addition, for surgical margins, if there was a sufficient number of studies, the heterogeneity of effects was explored by repeating the analysis including only data from studies that reported all key pathological data (see Quality assessment strategy).
Vague prior distributions were used on the necessary parameters: the log-ORs of intervention procedures compared with open surgery, the individual study event rates and the random-effects standard deviation. For most outcomes a burn-in period of 20,000 iterations was adequate to achieve convergence and a further 100,000 samples were taken for each outcome.
Assessment of learning curves
The approach developed by members of our project team to estimate the learning effects on key outcomes was used. 88 In this approach, the expertise of the participating surgeons or centres described in each included study was first categorised according to previous experience (number of previous radical prostatectomies undertaken using open, laparoscopic or robotic techniques) and according to occurrence of the key outcomes of positive surgical margin rate. Positive margin rate was then plotted against previous experience to describe learning curve effects in the included studies. Data on the three key features of learning (starting level, rate of learning and expert level) were extracted where possible and a random-effects meta-analysis performed to estimate the pooled effect of the key features together with an appropriate measure of uncertainty [95% confidence interval (CI)].
The robustness of the above approach was assessed by extending the inclusion criteria to include case series of laparoscopic and robotic radical prostatectomy that included > 200 men. Positive surgical margin rates for the first and last cases were abstracted from each included case series (together with any other parameters used in the studies to assess learning). A test for a logarithmic shape of learning was undertaken using a linear least-squares regression (using the natural logarithm of procedure number as the independent variable and the natural logarithm of the positive surgical margin rate as the dependent variable). A dummy variable for robotic compared with laparoscopic case series was included in the analysis to test for any difference in rate of learning between the two radical procedures and the associated 95% CI was calculated.
Chapter 4 Clinical effectiveness of robotic compared with laparoscopic techniques
Quantity and quality of evidence
Number of studies identified
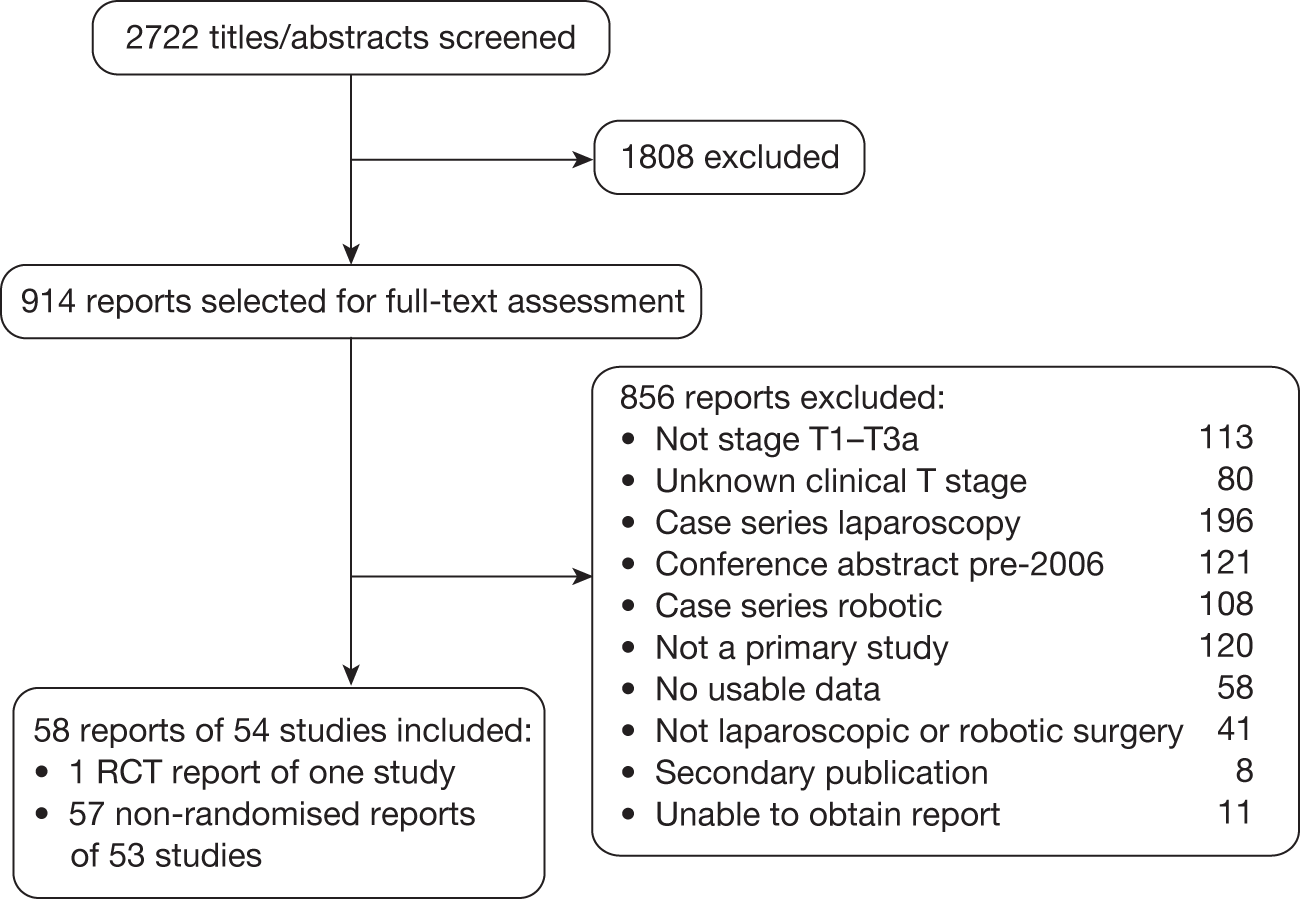
The searches identified 2722 potentially relevant titles and abstracts (Figure 6), from which 914 reports were selected for full-text eligibility screening. Of these, 58 reports (54 studies) were included and 856 reports were excluded with reasons for exclusion detailed in Figure 6. We attempted to obtain further details for 69 of the 80 (86%) reports that were excluded because of lack of clear information on the number of patients for each baseline clinical stage and which had contact details available. Nineteen replies were obtained. Only one of these 19 reports89 was subsequently deemed eligible for inclusion, but confirmation of this was received too late for it to be included in the review. Appendices 5 and 6 give the bibliographic details of the included and excluded studies respectively.
FIGURE 6.
Flow chart of the number of potentially relevant reports of identified studies and the numbers subsequently included and excluded from the clinical effectiveness review.
Number and type of included studies
The searches identified one RCT of laparoscopic versus open radical prostatectomy90 and 57 non-randomised comparative reports of 53 studies from 40 different clinical institutions: eight robotic versus laparoscopic prostatectomy;91–98 four robotic versus laparoscopic versus open prostatectomy [three primary,99–101 one secondary102 (earlier report of the same study but containing unique data)]; 18 robotic versus open prostatectomy (16 primary,103–118 two secondary119,120) and 27 laparoscopic versus open prostatectomy (26 primary,121–146 and one secondary147). There were three conference abstracts: two comparing robotic versus laparoscopic prostatectomy94,97 and one comparing robotic versus laparoscopic versus open prostatectomy. 102 Four studies were considered to include potential patient overlap: the study conducted by Menon and colleagues95 was a comparison of 40 laparoscopic and 40 robotic prostatectomies performed between 23 October 2000 and 22 October 2001; Tewari and colleagues116 report an extension of this work but compared 100 open and 200 robot operations between October 1999 and December 2002. As these studies included different comparators, they were treated as separate studies but the potential for overlap of robotic prostatectomy patients was noted. Similarly, Joseph and colleagues94 report a comparison including 800 laparoscopic cases from the Henri Mondor hospital, France, and 745 robotic cases from the University of Rochester, USA, between 2002 and 2006. An earlier publication93 analysed the last 50 cases from a series of 70 laparoscopic and 200 robotic cases from the University of Rochester (dates not given). The studies were treated as separate. Similar affiliated institution details of first authors were noted for seven studies: those by Anastasiadis122 and Salomon,140 Ficarra106 and Fracalanza,107 and Greco,129 Jurczok131 and Fornara. 127 These studies report overlapping treatment dates and similar procedures but it is unclear whether or not they include patient overlap as details of the institutions where the men were treated are not clearly given within the reported text. Similarly, we noted similar author institution details for another seven studies: those by Malcolm,110 Ball99 and Soderdahl,142 Trabulsi98 and Brown,125 and Loeb109 and Wagner146 although these involved different comparison groups and were treated as separate studies.
The 57 non-randomised comparative reports (of 53 studies) included 28 prospective and 17 retrospective reports. Three studies92,112,114 included a mixture of prospective and retrospective data and eight96,97,100,119,123,132,134,138 did not report the method of data collection. The method of data collection was uncertain in the study by Kim and colleagues132 because of a limited translation of the full-text version. Table 4 provides further details of the number and type of included studies.
| Comparison | Study report | Data collection | Number of reports |
|---|---|---|---|
| Robotic vs laparoscopic | RCT | 0 | |
| Non-randomised comparative | Prospective | 2 | |
| Retrospective | 3 | ||
| Both | 1 | ||
| Not reported | 2 | ||
| Total | 8 | ||
| Robotic vs laparoscopic vs open | RCT | 0 | |
| Non-randomised comparative | Prospective | 1 | |
| Retrospective | 1 | ||
| Not reported | 2 | ||
| Total | 4 | ||
| Robotic vs open | RCT | 0 | |
| Non-randomised comparative | Prospective | 8 | |
| Retrospective | 6 | ||
| Both | 2 | ||
| Not reported | 2 | ||
| Total | 18 | ||
| Laparoscopic vs open | RCT | Prospective | 1 |
| Non-randomised comparative | Prospective | 15 | |
| Retrospective | 7 | ||
| Unclear | 1 | ||
| Not reported | 4 | ||
| Total | 28 |
The RCT conducted by Guazzoni and colleagues90 comparing laparoscopic with open prostatectomy was set in Italy. Half of the included non-randomised studies were conducted in the USA (28/57, 49%). The remaining studies were conducted in France,91,94–96,101,122,140 Italy,106,107,114,123,129,134 Germany,127,131,137 Japan,135,136,144 Canada;121,130 there was one study from each of Australia,105 Austria,139 Brazil,141 Chile,133 Croatia,143 Republic of Korea,132 Spain,138 Sweden104 and Taiwan, Province of China. 113 Of the non-randomised comparative studies comparing robotic with laparoscopic radical prostatectomy, three primary full-text studies92,93,98 and one conference abstract97 were set in the USA, one conference abstract was set in both the USA and France94 and three studies were set in France. 91,95,96 Of the non-randomised comparative studies comparing robotic, laparoscopic and open radical prostatectomy, two primary studies99,100 and one secondary report102 were set in the USA and one study was set in France. 101 Of the non-randomised comparative studies comparing robotic and open radical prostatectomy, 10 primary studies103,108–112,115–118 and two secondary reports119,120 were set in the USA, one study was set in Australia,105 three primary studies106,107,114 were set in Italy, one study was set in Sweden104 and one was set in Taiwan, Province of China. 113 Of the non-randomised comparative studies comparing laparoscopic and open radical prostatectomy, seven primary studies124–126,128,142,145,146 and one secondary report147 were set in the USA, three primary studies127,131,137 were set in Germany, three primary studies135,136,144 were set in Japan, three primary studies123,129,134 were set in Italy, two primary studies122,140 were set in France and one study each was set in Austria,139 Brazil,141 Canada,121 Chile,133 Croatia,143 Republic of Korea132 and Spain. 138
The four full-text publications that required translation paired with their original language were Fornara and Zacharias127 (German), Kim132 (Korean), Soric143 (Croatian) and Raventos Busquets and colleagues138 (Spanish).
Characteristics of patients
The 58 reports included 21,126 men at enrolment. Excluding secondary reports and following exclusions because of ineligibility or participant dropout, the final study analyses included 19,064 men, of whom 6768 underwent robotic radical prostatectomy, 4952 underwent laparoscopic radical prostatectomy and 7344 underwent open radical prostatectomy. The demographic and disease characteristics of these included men are summarised in Table 5.
| Variable | Robotic | Laparoscopic | Open |
|---|---|---|---|
| n | 6768 | 4952 | 7344 |
| Age (years), median | 60.7 | 61.9 | 63 |
| Interquartile range (years) | 59.8–62 | 60.0–63.65 | 60.5–64.8 |
| Clinical stage, n (%) | |||
| cT1 | 4380 (64.7) | 3257 (65.8) | 3956 (53.9) |
| cT2 | 1743 (25.8) | 1312 (26.5) | 2194 (29.9) |
| cT3 | 58 (0.9) | 26 (0.5) | 148 (2.0) |
| cT4 | 1 (0.01) | 8 (0.2) | 0 (0) |
| Missing/unknowna | 586 (8.7) | 349 (7.0) | 1046 (14.2) |
| Preoperative Gleason score, n (%) | |||
| ≤ 6 | 2179 (32.2) | 989 (20.0) | 2389 (32.5) |
| 7 | 949 (14.0) | 429 (8.7) | 1574 (21.4) |
| 8–10 | 198 (2.9) | 54 (1.1) | 333 (4.5) |
| Missing/unknowna | 3442 (50.9) | 3480 (70.3) | 3048 (41.5) |
| Preoperative PSA (ng/ml), median | 6.3 | 7.2 | 7.9 |
| Interquartile range (ng/ml) | 5.4–7.1 | 6.3–8.6 | 6.0–9.3 |
| Postoperative whole prostate radical prostatectomy Gleason score, n (%) | |||
| ≤ 6 | 1200 (17.7) | 485 (9.8) | 1666 (22.7) |
| 7 | 1110 (16.4) | 415 (8.4) | 1634 (22.2) |
| 8–10 | 161 (2.4) | 49 (1.0) | 379 (5.2) |
| Missing/unknowna | 4297 (63.5) | 4003 (80.8) | 3665 (49.9) |
| Pathological tumour stage, n (%) | |||
| pT0 | 7 (0.1) | 6 (0.1) | 22 (0.3) |
| pT1 | 0 (0) | 29 (0.6) | 25 (0.3) |
| pT2 | 2060 (30.4) | 2373 (47.9) | 4246 (57.8) |
| pT3 | 571 (8.4) | 669 (13.5) | 1368 (18.6) |
| pT3/4b | 23 (0.3) | 45 (0.9) | 76 (1.0) |
| pT4 | 7 (0.1) | 17 (0.3) | 33 (0.4) |
| Missing/unknowna | 4203 (62.1) | 1710 (34.5) | 1574 (21.4) |
All studies reported age with a median (interquartile range) of 62 (60–64) years and a total range of 35–84 years.
Baseline clinical tumour staging data were reported for all studies except that conducted by Bolenz and colleagues;100 however, clinical staging data for this study were available from an earlier report in abstract form. 102 Eight reports107,111,120,126,139,141,143,147 did not report specific baseline clinical stage, simply reporting their inclusion criterion as ‘≤ cT1–T2’, and one109 did not report clinical stage by procedure. The baseline clinical tumour staging was similar between the laparoscopic and robotic radical prostatectomy patients with 68% and 69%, respectively, categorised as T1.
Less than half of the included reports (23/58, 40%)91,98,99,101,103,105–108,110,115,117–121,125,128,135,136,142,145,146 gave detailed biopsy Gleason scores for men undergoing prostatectomy in the format we required: numbers of men categorised as Gleason score ≤ 6, 7 or ≥ 8. Seven studies90,95,97,111,126,139,141 and one secondary report147 did not report biopsy Gleason grades or score. Over one-third of the included reports (21/58, 36%) reported either mean93–95,113,122–124,129,130,132,139,140,143,144 or median104,114,127,131,133,134,137 scores. The remaining reports presented details using different scoring formats90,92,102,138,141 or did not present separately by procedure. 100 Two-thirds of men undergoing both laparoscopic and robotic radical prostatectomy had a Gleason score ≤ 6.
Fifty reports90,91,93–101,103–109,112–119,122–125,127–146 gave preoperative PSA values, with the majority (38/50, 76%) reporting mean PSA for each group of men. Nine studies106–108,131,134,141,142,144,145 reported median group PSA values, whereas two studies135,136 reported mean and median PSA and one study119 reported PSA range only. Combining the median and mean PSA values across all of the studies demonstrated slightly lower levels of preoperative PSA in the robotic than in the laparoscopic procedures: 6.3 ng/ml and 7.2 ng/ml respectively. Three studies92,121,126 reported the number of men in each group falling into varying ranges of PSA values but as the ranges were inconsistent we were unable to include these data in the summary.
The postoperative Gleason sum score following pathological examination of the prostate was similar between the robotic and laparoscopic patients with 50% of the men in both groups with combinable Gleason information having a Gleason score ≤ 6. Pathological staging assigned following consideration of the operative finding during surgery and pathological examination of the removed prostate was similar between the robotic and laparoscopic patients with 78% of the men with combinable staging information in both groups categorised as pT2. There was a trend towards worse disease characteristics in men undergoing open prostatectomy with 55% having a post-prostatectomy Gleason score > 6 and 30% categorised as pT2 or higher.
Twenty-nine primary reports90–93,96,99,100,106,108,110–113,118,122,123,125,126,128,129,132,135–137,139,142,144–146 and two secondary reports102,119 reported the use of nerve-sparing techniques.
Overview of types of outcomes reported
The numbers and types of included studies reporting our main considered outcomes are summarised below.
Efficacy
Thirty-nine studies (67%)90,94–98,101,103,105–109,112–116,118,122,123,125–127,129–134,137–141,143–146 reported data on the rate of positive surgical margins in the excised prostate specimen.
Thirteen studies (22%)95,101,103,108,109,112,113,115,116,123,133,137,140 reported the rate of biochemical recurrence, but the time points at which this was censored, the definition of biochemical recurrence and the threshold values of PSA used varied between studies.
The need for and outcome of further treatment for prostate cancer recurrence was reported by one study. Dahl and colleagues126 reported information on the numbers of men requiring further cancer treatment consisting of salvage external beam radiation therapy, androgen deprivation therapy or both for cohorts of men undergoing laparoscopic or open prostatectomy.
Eight studies90,111,116,130,135–137,139 reported quality-of-life data using validated measures.
Safety
The majority of reports (45/58, 78%) included data on perioperative adverse events.
Thirteen primary reports93,94,99,103,109,110,130,135,136,141,142,144,145 and one secondary report147 did not report perioperative safety outcomes.
Four studies104,105,126,140 reported deaths within 30 days postoperatively because of surgical complications.
Postoperative incontinence and sexual dysfunction
Twenty-one studies (36%)91,93,97,99,106,108,110,113,114,116,123,126,128–130,133,135–137,142,146 provided data on urinary incontinence postoperatively. Three other studies112,122,139 reported continence data in a form that could not be converted to the numbers of incontinent men, which was our required format for meta-analysis. Two studies also reported data that we were unable to use because of presentation in graph format rather than numbers of incontinent men105 or because of presentation of immature data. 95 The study conducted by Carlsson and colleagues104 reported the number of patients requiring additional surgery for urinary incontinence between 30 days and 15 months after radical prostatectomy.
Nineteen studies (33%)93,99,106,108,110,112–114,116,122,123,126,128,129,133,135,136,142,146 provided data on sexual function following prostatectomy.
Risk of bias
Overall assessment of risk of bias
Forty-eight reports from 28 individual author-affiliated institutes were assessed for risk of bias. The secondary reports by Dahl and colleagues147 and Chan and colleagues119 contained unique outcomes not included in the associated primary studies103,126 and we therefore conducted risk of bias assessment for both reports. Twenty-four reports (50%)92,93,95,96,98,104–108,112,113,115,116,124,126,130,134,136,139,142,144,146,147 were judged to be at high overall risk of bias, 13 (27%)90,99–101,103,117,118,122,128,129,137,141,145 were low risk and 11 (23%)109,111,114,119,121,123,125,131,135,140 were judged unclear. Analysis of inter-rater agreement for overall assessment of risk of bias gave a kappa = 0.34 and a weighted kappa = 0.35, indicating moderate agreement.
Only the RCT conducted by Guazzoni and colleagues90 was judged to be at low risk of bias for sequence generation and the study by Touijer and colleagues145 was judged to be at low risk for allocation concealment. All other studies were high risk or unclear for these two key domains.
Risk of bias for reported outcomes
The risk of bias assessments for our chosen main outcomes of efficacy (predominantly surgical margins status), urinary incontinence and erectile dysfunction and perioperative adverse events are summarised in Figures 7–10 respectively.
FIGURE 7.
Summary of risk of bias assessment for reports of efficacy (n = 37).
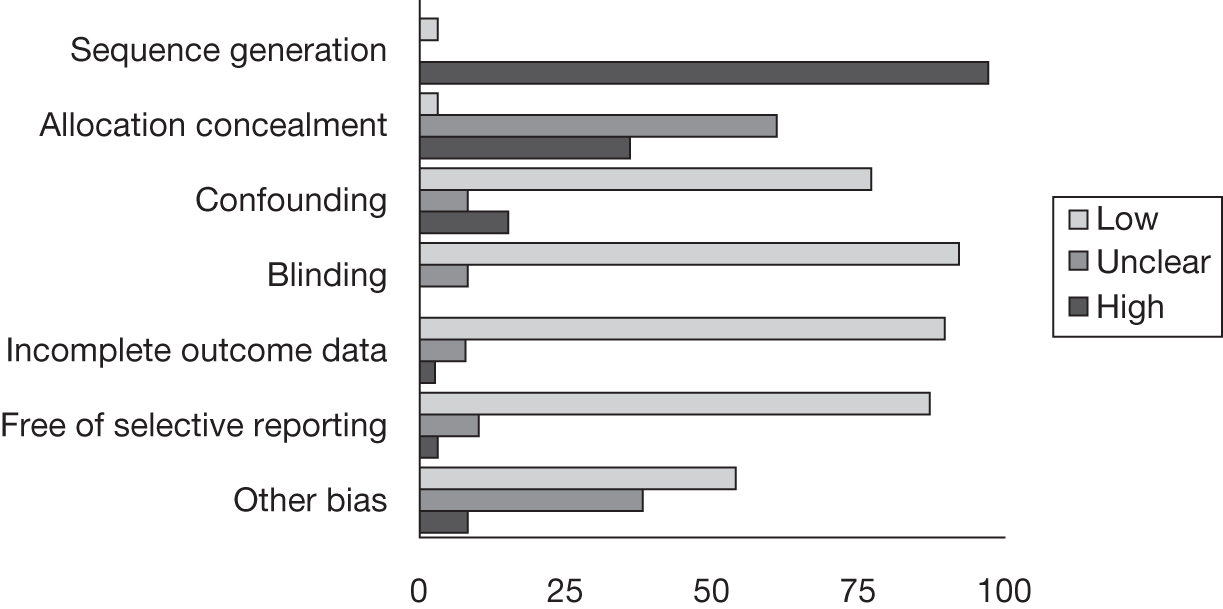
FIGURE 8.
Summary of risk of bias assessment for reports of urinary dysfunction (n = 23).
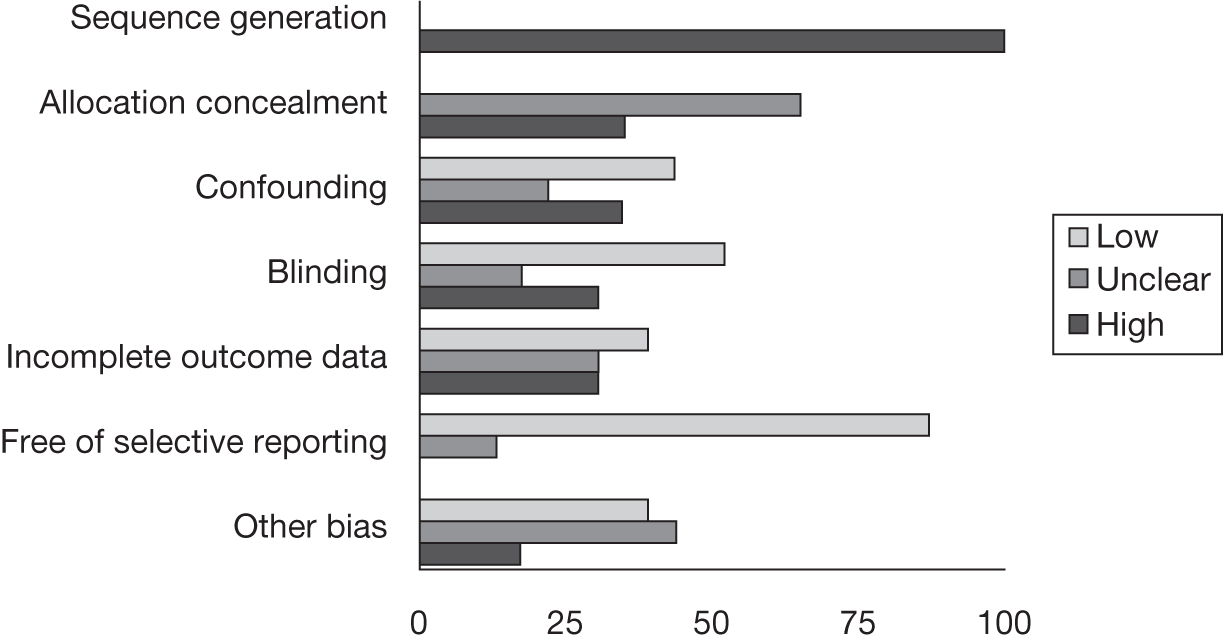
FIGURE 9.
Summary of risk of bias assessment for reports of erectile dysfunction (n = 20).
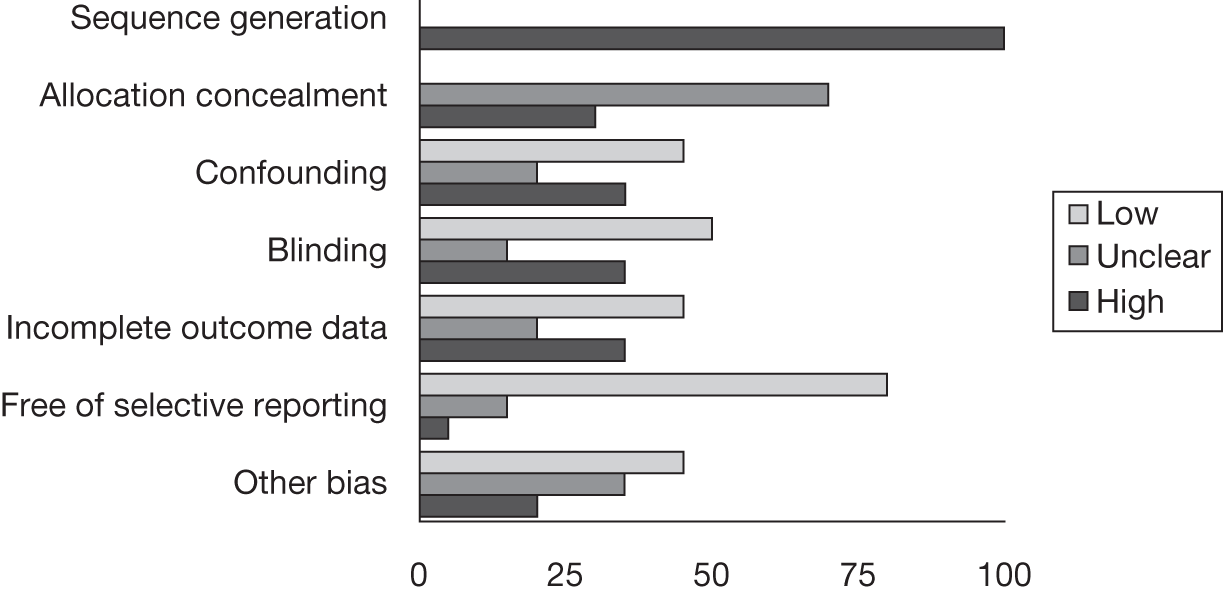
FIGURE 10.
Summary of risk of bias assessment for reports of perioperative safety (n = 35).
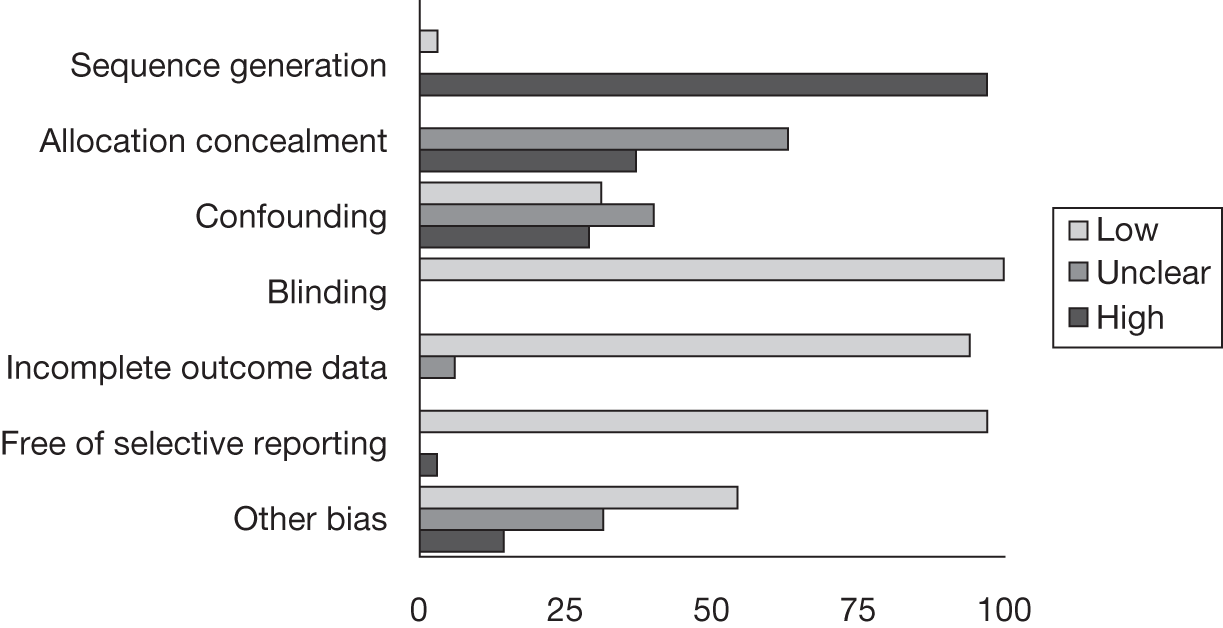
Efficacy
Thirty-seven reports90,93,95,96,98,101,103,105–109,112–118,122–126,128–131,134,137,139–141,144–147 were assessed for risk of bias for efficacy outcomes. Of these, 30 (81%)90,95,96,98,101,103,106,108,113–118,122–126,128,129,131,137,139–141,144–147 were considered to be at low risk of bias for confounding factors.
Urinary dysfunction
Twenty-three studies93,95,99,105,106,108,110,112–114,116,122,123,126,128–130,135–137,139,142,146 were assessed for risk of bias for reporting of urinary incontinence outcomes. Of these, 10 (43%)99,108,110,114,116,122,126,128,129,146 were considered to be at low risk of bias for confounding factors.
Erectile dysfunction
Twenty studies93,95,99,106,108,110,112–114,116,122,123,126,128,129,135–137,142,146 were assessed for risk of bias for reporting of erectile dysfunction. Of these, nine studies (39%)99,110,114,122,126,128,129,135,137 were considered to be at low risk of bias for confounding.
Perioperative safety
Thirty-five studies90,92,93,95,96,98,100,101,104–108,111–117,119,121–126,128,129,131,134,137,139,140,146 were assessed for risk of bias for reporting of perioperative adverse events. Of these, 11 (31%) were judged to be at low risk of bias for confounding factors. 90,96,100,106,114,116,122,124–126,131
Assessment of effectiveness
Data concerning outcomes included in the meta-analysis are detailed in Tables 6–16. A detailed description of all outcomes abstracted from the included studies is given in tables contained in Appendix 9.
Positive margins
Meta-analysis of data from the 37 included studies90,94–98,101,103,105–109,112–116,118,122,123,125,127,129–134,137, 139–141,143,144,146,147 that reported positive surgical margin rates (Table 6) showed a statistically significant improvement for robotic compared with laparoscopic prostatectomy (OR 0.69; 95% CrI 0.51 to 0.96; probability outcome favours robotic prostatectomy = 0.987). The probability of a positive margin predicted by the mixed-treatment comparison model was 17.0% following robotic prostatectomy compared with 23.6% following laparoscopic prostatectomy. Restriction of the meta-analysis to studies at low risk of bias did not change the direction of effect but did decrease the precision of the effect size (OR 0.73; 95% CrI 0.29 to 1.75), with the probability that the event rate was lower for robotic prostatectomy being no longer statistically significant (p = 0.782).
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| aAnastasiadis 2003122 | 61/230 (26.5) | 20/70 (28.6) | |
| Artibani 2003123 | 21/71 (29.6) | 12/50 (24.0) | |
| Barocas 2010103 | 281/1413 (19.9) | 148/491 (30.1) | |
| Brown 2004125 | 10/59 (16.9) | 12/60 (20.0) | |
| Dahl 2006147 | 43/286 (15.0) | 124/714 (17.4) | |
| Doumerc 2010105 | 45/212 (21.2) | 84/502 (16.7) | |
| aDrouin 2009101 | 12/71 (16.9) | 16/85 (18.8) | 15/83 (18.1) |
| Ficarra 2009106 | 35/103 (34.0) | 21/105 (20.0) | |
| Fornara 2004127 | 5/32 (15.6) | 7/32 (21.9) | |
| Fracalanza 2008107 | 10/35 (28.6) | 6/26 (23.1) | |
| aGreco 2010129 | 12/150 (8.0) | 17/150 (11.3) | |
| aGuazzoni 200690 | 16/60 (26.7) | 13/60 (21.7) | |
| Jacobsen 2007130 | 22/67 (32.8) | 60/148 (40.5) | |
| Joseph 200794 | 99/754 (13.1) | 246/800 (30.8) | |
| Jurczok 2007131 | 63/163 (38.7) | 104/240 (43.3) | |
| Kim 2007132 | 11/30 (36.7) | 11/45 (24.4) | |
| Krambeck 2009108 | 46/294 (15.6) | 100/588 (17.0) | |
| Lama 2009133 | 16/56 (28.6) | 21/59 (35.6) | |
| Loeb 2010109 | 22/152 (14.5) | 25/137 (18.2) | |
| Martorana 2004134 | 12/50 (24.0) | 13/50 (26.0) | |
| Menon 200295 | 7/40 (17.5) | 10/40 (25.0) | |
| Nadler 2010112 | 5/50 (10.0) | 12/50 (24.0) | |
| Ou 2009113 | 15/30 (50.0) | 6/30 (20.0) | |
| Poulakis 2007137 | 15/72 (20.8) | 16/70 (22.9) | |
| Remzi 2005139 | 10/39 (25.6) | 8/41 (19.5) | |
| Rocco 2009114 | 26/120 (21.7) | 60/240 (25.0) | |
| Rozet 200796 | 26/133 (19.5) | 21/133 (15.8) | |
| Salomon 2002140 | 32/155 (20.6) | 30/151 (19.9) | |
| Schroeck 2008115 | 106/362 (29.3) | 122/435 (28.0) | |
| Silva 2007141 | 22/90 (24.4) | 37/89 (41.6) | |
| Soric 2004143 | 6/26 (23.1) | 3/26 (11.5) | |
| Sundaram 200497 | 2/10 (20.0) | 2/10 (20.0) | |
| Terakawa 2008144 | 54/137 (39.4) | 52/220 (23.6) | |
| Tewari 2003116 | 18/200 (9.0) | 23/100 (23.0) | |
| Trabulsi 200898 | 3/50 (6.0) | 35/190 (18.4) | |
| Wagner 2007146 | 7/75 (9.3) | 14/75 (18.7) | |
| aWhite 2009118 | 11/50 (22.0) | 18/50 (36.0) | |
| Predicted probability of event | 0.176 | 0.236 | 0.238 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.69 (0.51 to 0.96); 0.987 | |
| Low-risk studies only | 0.73 (0.29 to 1.75); 0.782 |
Pathological examination of the prostate
Details of the methods described for the handling, processing and pathologist reporting of radical prostatectomy specimens were given in 24 included study reports90,94,96,98,101,103,105,106,109,112, 114,116,118,122,123,134,137–141,144 and are summarised in Table 7. In 10 (42%) of these studies reference was made to a published standardised protocol for examination of radical prostatectomy specimens: four studies gave one of three alternative references for the Stanford protocols148–150 and one122 specified the Stanford protocol without citing a relevant reference; the remaining studies referenced other protocols published from various centres. 82,151–153
| Study | Gland inked | Positive margin | Embedding | Blocks | Slice thickness | Apex slice sections | Base slice sections | Location of positive margin | Review | Other details | Method reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anastasiadis 2003122 | NS | NS | NS | NS | NS | NS | NS | NS | One pathologist | Stanford protocol unreferenceda | |
| Artibani 2003123 | Inked | Defined for RRPb | Complete | Whole mounts | RRP 2–3 mm, LRP 4–6 mm | Sagittal | Sagittal | NS | Two pathologists | Unreferenced protocol for LRP | McNeal 1990149 |
| Barocas 2010103 | Inked | Definedb | Systematic sample | NS | 3 mm | Sagittal | Sagittal | NS | NS | Srigley 200682 | |
| Dahl 2006147 | Inked | Definedb | NS | NS | NS | Radial | Radial | Classifiedc | Urological pathologists | ||
| Doumerc 2010105 | Inked | Definedb | Complete | Small blocks | NS | Sagittal | Sagittal | NS | One urological pathologist | ||
| Drouin 2009101 | Inked | Definedb | NS | NS | NS | NS | NS | NS | NS | ||
| Ficarra 2009106 | NS | NS | Complete | Whole mounts | 4 mm | NS | NS | NS | NS | ||
| Guazzoni 200690 | Inked | Definedb | NS | NS | NS | NS | NS | NS | NS | ||
| Joseph 200794 | Inked | Definedb | NS | NS | NS | NS | NS | NS | NS | ||
| Loeb 2010109 | Inked | Definedb,d | Complete | NS | 2–3 mm | Sagittal | 1-mm shave | NS | One urological pathologist | ||
| Martorana 2004134 | Inked | Definedb | Complete | NS | 2–3 mm | Sagittal | Sagittal | Classifiede | One pathologist | McNeal 1990149 | |
| Nadler 2010112 | Inked | Definedb | NS | NS | NS | NS | NS | NS | One pathologist | ||
| Poulakis 2007137 | Inked | Defineda | Complete | Small blocks | 3 mm | Shave | Shave | NS | One pathologist | Humphrey 1993148 | |
| Raventos Busquets 2007138 | NS | Definede | NS | NS | NS | NS | NS | NS | NS | ||
| Remzi 2005139 | NS | NSf | NS | NS | NS | NS | NS | NS | NS | ||
| Rocco 2009114 | Inked | Defineda | NS | NS | NS | NS | NS | NS | NS | ||
| Rozet 200796 | Inked | Defineda | NS | NS | NS | NS | NS | NSg | NS | Apex commonest +ve siteg | |
| Salomon 2002140 | Inked | NS | Complete | Small blocks | 3 mm | Shave | Shave | NS | One pathologist | Stamey 1988150 | |
| Silva 2007141 | NS | Definedh | NS | NS | NS | NS | NS | Classifiedb | Pathology services | ||
| Terakawa 2008144 | Inked | Defineda | NS | Whole mount | 3 mm | Parasagittal sections | Parasagittal sections | Classifiedi | One pathologist | ||
| Tewari 2003116 | Inked | Defineda,j | NS | NS | 5 mm | NS | NS | NS | NS | Ohori 1995152 | |
| Touijer 2007145 | Inked | Defineda | NS | NS | 3–4 mm | Parasagittal sections | NS | NS | One GU pathologist | ||
| Trabulsi 200898 | Inked | Defineda | Complete | Whole mount | NS | Parasagittal sections | Parasagittal sections | Classifiedk | Multidisciplinary conference review | Brown 2003151 | |
| White 2009118 | Inked | Defineda | Complete | Whole mount | 2–3 mm | NS | NS | Classifiedb | NS | True 1994153 |
Concerning established key features of quality-assured pathological examination, 19 (79%) studies described preliminary dyeing of the surface of the prostate to accurately identify the location of the surgical margin. The accepted definition of a positive margin in terms of tumour cells touching or in contact with the dyed prostate surface was specified by 18 (75%) studies; alternative descriptions used were ‘an extension of tumour at the surface of incision’141 and ‘a malignant margin is considered a positive margin’,138 but these studies did not comment on whether or not the specimen was dyed before sectioning. One study defined margin positivity following robotic prostatectomy as ‘cancer seen in the intra-operative distal biopsies’116 whereas a further study reported use of ‘frozen section to control for negative margins’. 139 Concerning the methods used to prepare microscope slides (sections) for examination of the prostate gland, the recommended technique of embedding the whole gland for sectioning was specified by nine (38%) studies98,105,106,109,118,123,134,137,140 whereas one (4%) specified systematic partial sampling103 and the sampling method was not specified or unclear in the remaining 14 (58%) studies. 90,94,96,101,112,114,116,122,138,141,144,145,147 Section thickness was specified within the recommended range of 2–6 mm in 11 (46%) studies.
The recommended technique of examining sagittal sections from both the apical and the basal slices of the prostate was specified by six (25%) studies. 98,103,105,123,134,144 Of the remainder, one study147 used radial sections, two studies137,140 used sagittal sections for the apex only and two studies137,140 used shave margins for both apex and base. No information was given or practice was unclear in the remaining 13 (54%) studies. 90,94,96,101,106,112,114,116,118,122,138,139,141
The site of positive margin was specified in six (24%) studies;98,118,134,141,144,147 in four studies118,134,141,147 locations were defined, with some variation in terminology, as apex, base or bladder neck, lateral or posterolateral and multiple and in two further studies98,144 as apex, base, anterior or posterior and apex, base or other. No study gave the extent in millimetres of positive margins in the results.
Given that no studies reported the same methodology for ascertainment of positive margin status it was not possible to undertake a meta-analysis restricted to studies using appropriate methodology.
In summary, these studies showed variation in the pathology protocols employed, which may have affected the determination of positive margin status and thereby increased the risk of bias in the results.
Biochemical recurrence
Biochemical recurrence rates up to 1 year following radical prostatectomy were reported in six studies (Table 8). 108,113,115,123,133,137 There was no evidence of a difference in the rates of biochemical recurrence calculated by the mixed-treatment comparison model between robotic and laparoscopic prostatectomy (OR 0.89; 95% CrI 0.24 to 3.34; probability outcome favours robotic prostatectomy = 0.588). Restriction of the meta-analysis to only the studies at low risk of bias was not possible because all studies were at high risk.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Artibani 2003123 | 12/63 (19.0) | 5/44 (11.4) | |
| Krambeck 2009108 | 14/248 (5.6) | 32/492 (6.5) | |
| Lama 2009133 | 6/56 (10.7%) | 7/59 (11.9) | |
| Ou 2009113 | 6/30 (20.0) | 5/30 (16.7) | |
| Poulakis 2007137 | 17/204 (8.3) | 11/70 (15.7) | |
| Schroeck 2008115 | 29/362 (8.0) | 54/435 (12.4) | |
| Predicted probability of event | 0.087 | 0.097 | 0.110 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.89 (0.24 to 3.34); 0.588 | |
| Low-risk studies only | Not estimable |
Urinary incontinence
The 22 studies that reported urinary incontinence used a variety of measures at different time points. Measures included observed urinary leakage,93 pad use,91,97,108,112–114,116,122,128,129,137,139,146 fluid volume voiding diary130 and validated questionnaire scores [University of California Los Angeles – Prostate Cancer Index (UCLA-PCI)99,110,135,136,142 and International Consultation of Incontinence Questionnaire (ICIQ-UI)106]. Artibani and colleagues123 measured both urinary leakage and pad use. The study conducted by Lama and colleagues133 did not give a definition of incontinence. The results from the 10 studies106,108,113,114,126,128–130,133,146 that reported urinary incontinence at a standard time point of 12 months following prostatectomy are given in Table 9. There was no evidence of a difference in the rates of urinary incontinence between robotic and laparoscopic prostatectomy (OR 0.55; 95% CrI 0.09 to 2.84; probability outcome favours robotic prostatectomy = 0.783). Restriction of the meta-analysis to only the studies at low risk of bias was not possible because all studies were at high risk.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Dahl 2009126 | 17/78 (21.8) | 9/72 (12.5) | |
| Ficarra 2009106 | 3/103 (3.0) | 12/105 (11.4) | |
| Ghavamian 2006128 | 7/70 (10.0) | 8/65 (12.3) | |
| Greco 2010129 | 4/150 (2.7) | 13/150 (8.7) | |
| Jacobsen 2007130 | 10/57 (17.5) | 19/148 (12.8) | |
| Krambeck 2009108 | 20/244 (8.2) | 30/476 (6.3) | |
| Lama 2009133 | 0/56 | 2/59 (3.4) | |
| Ou 2009113 | 0/30 | 1/30 (3.3) | |
| Rocco 2009114 | 2/79 (2.5) | 26/217 (12.0) | |
| Wagner 2007146 | 24/67 (35.8) | 35/66 (53.0) | |
| Predicted probability of event | 0.045 | 0.079 | 0.109 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.55 (0.09 to 2.84); 0.783 | |
| Low-risk studies only | Not estimable |
The study conducted by Carlsson and colleages104 reported 7/1253 (0.6%) patients requiring further postoperative surgery for incontinence between 30 days and 15 months after their initial robotic operation compared with 11/485 (2.2%) requiring further postoperative surgery for incontinence after undergoing an open radical prostatectomy.
Erectile dysfunction
As described in Overview of type of outcomes reported, a total of 19 studies provided data on sexual function. The time point following surgery when the outcome was assessed and the measure used to quantify the outcome varied between studies. Erectile dysfunction was variously defined as the inability to achieve and maintain a spontaneous or drug-assisted erection suitable for sexual intercourse93,108,113,114,116,122,123,126,129 or by validated symptom questionnaire scores [UCLA-PCI,99,110,135,136,142 IIEF-5106,128 Expanded Prostate Cancer Index Composite, sexual function subscale (EPIC-SFSS)146 and Sexual Health Inventory for Men (SHIM)112]. The study conducted by Lama and colleagues133 did not report a definition of erectile dysfunction. Given the diversity of definitions and types of data (continuous and dichotomous) it was not possible to collate data from individual studies into a form suited to meta-analysis. Of the two studies directly comparing robotic and laparoscopic prostatectomy that reported erectile dysfunction, one99 showed earlier recovery of sexual function following the robotic prostatectomy procedure, with 35% compared with 21% returning to baseline functioning at 3 months post surgery and 43% compared with 25% returning to baseline functioning at 6 months, and the other93 favoured laparoscopic prostatectomy (46% required drug aid vs 36% at 3 months in the robotic and laparoscopic groups, respectively).
Quality of life
Quality of life following prostatectomy as measured by validated patient-reported questionnaires was reported in 10 studies: European Quality of Life-5 Dimensions (EQ-5D) visual analogue scale (VAS); 90,116,139 Short Form questionnaire-36 items (SF-36);135,136 Short Form questionnaire-12 items (SF-12);111 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30);137 the quality-of-life item contained within the International Prostate Symptom Score (I-PSS);130 the International Continence Society (ICS)91 and the Expanded Prostate Cancer Index Composite urinary incontinence and sexual function subscales (EPIC-UISS-SFSS). 146 Full details are given in Appendix 9. Quality-of-life measurements following robotic prostatectomy were reported by two studies111,116 with a maximum observation period of 6 weeks. The data were insufficient to enable us to assess any difference in quality of life following robotic or laparoscopic prostatectomy. Three studies135–137 reported that preoperative physical functioning level was not achieved in all patients by 6 months postoperatively but the clinical significance of the differences was unclear.
Pain
There were no direct comparative studies of robotic and laparoscopic procedures reporting pain. It was therefore not possible to report any difference in pain between the procedures either postoperatively or in the long term.
Need for further cancer treatment
Dahl and colleagues126 was the only report that included information on the numbers of men requiring further treatment for cancer persistence or recurrence, with rates of 5/104 (5%) for laparoscopic prostatectomy and 2/102 (2%) for open prostatectomy.
Death
Four studies104,105,126,140 reported deaths resulting from complications in the 30-day postoperative period. These included two fatal cardiac arrests104,126 and one cerebrovascular accident105 following open prostatectomy. Salomon and colleagues140 also reported one death due to pulmonary embolism following laparoscopic prostatectomy. Five studies92,95,96,137,154 involving 1600 men specifically reported no postoperative deaths. Drouin and colleagues101 reported one death due to prostate cancer 5 years after open prostatectomy and four deaths due to cardiovascular complications without specifying which procedure these men had received. Krambeck and colleagues108 reported all-cause mortality rates of 4/248 (1.6%) for men undergoing robotic prostatectomy and 4/492 (0.8%) after open prostatectomy at a median follow-up time of 1.3 years.
Perioperative adverse events
Data on the perioperative adverse events of blood transfusion, anastomotic leak, bladder neck contracture, wound infection, organ injury, ileus, deep-vein thrombosis and pulmonary embolism are presented in Tables 10–17. Abstracted data concerning other specific adverse events not included in the meta-analysis are detailed in Appendix 10. All adverse events were additionally categorised according to the Clavien–Dindo system and the data meta-analysed according to Clavien–Dindo score (see Tables 59–70).
Blood transfusion
Meta-analysis of data from the 30 studies90–92,94–96,100,101,104–108,112,113,116,119–123,125,127–129,132–134,137,140 that reported blood transfusion rates (Table 10) showed a relative reduced need for blood transfusion with robotic prostatectomy compared with laparoscopic prostatectomy (OR 0.71; 95% CrI 0.31 to 1.62) but this was not statistically significant (probability outcome favours robotic prostatectomy = 0.780). The predicted rate of blood transfusion in the mixed-treatment comparison model was 3.5% for robotic prostatectomy and 5% for laparoscopic prostatectomy. Restriction of the meta-analysis to the studies at low risk of bias changed the direction of effect to favour the laparoscopic procedure but precision was reduced (OR 1.45; 95% CrI 0.38 to 6.21; probability that outcome favours laparoscopic prostatectomy = 0.257).
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Al-Shaiji 2010121 | 3/70 (4.3) | 42/70 (60.0) | |
| aAnastasiadis 2003122 | 6/230 (2.6) | 6/70 (8.6) | |
| Artibani 2003123 | 45/71 (63.4) | 17/50 (34.0) | |
| aBolenz 2010100 | 12/262 (4.6) | 4/211 (1.9) | 32/156 (20.5) |
| Brown 2004125 | 1/60 (1.7) | 31/60 (51.7) | |
| Carlsson 2010104 | 58/1253 (4.6) | 112/485 (23.1) | |
| Chan 2008119 | 5/660 (0.8) | 11/340 (3.2) | |
| Doumerc 2010105 | 2/212 (0.9) | 10/502 (2.0) | |
| Drouin 2009101 | 4/71 (5.6) | 5/85 (5.9) | 8/83 (9.6) |
| Ficarra 2009106 | 2/103 (1.9) | 15/105 (14.3) | |
| Fornara 2004127 | 2/32 (6.3) | 6/32 (18.8) | |
| Fracalanza 2008107 | 7/35 (20.0) | 12/26 (46.2) | |
| aGhavamian 2006128 | 5/70 (7.1) | 22/70 (31.4) | |
| Gosseine 200991 | 4/122 (3.3) | 8/125 (6.4) | |
| aGreco 2010129 | 3/150 (2.0) | 9/150 (6.0) | |
| aGuazzoni 200690 | 8/60 (13.3) | 32/60 (53.3) | |
| Hu 200692 | 5/322 (1.6) | 8/358 (2.2) | |
| Joseph 200794 | 10/754 (1.3) | 35/800 (4.4) | |
| Kim 2007132 | 7/30 (23.3) | 10/45 (22.2) | |
| Kordan 2010120 | 7/830 (0.8) | 14/414 (3.4) | |
| Krambeck 2009108 | 15/294 (5.1) | 77/588 (13.1) | |
| Lama 2009133 | 7/56 (12.5) | 23/59 (39.0) | |
| Martorana 2004134 | 1/50 (2.0) | 5/50 (10.0) | |
| Menon 200295 | 0/40 | 1/40 (2.5) | |
| Nadler 2010112 | 10/50 (20.0) | 45/50 (90.0) | |
| Ou 2009113 | 4/30 (13.3) | 18/30 (60.0) | |
| aPoulakis 2007137 | 2/72 (2.8) | 13/70 (18.6) | |
| Rozet 200796 | 13/133 (9.8) | 4/133 (3.0) | |
| Salomon 2002140 | 3/155 (1.9) | 31/151 (20.5) | |
| Tewari 2003116 | 0/200 | 67/100 (67.0) | |
| Predicted probability of event | 0.035 | 0.050 | 0.227 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.71 (0.31 to 1.62); 0.780 | |
| Low-risk studies only | 1.45 (0.38 to 6.21); 0.257 |
Bladder neck contracture
Meta-analysis of data from the 13 studies92,104,106,108,112,113,124–126,128,133,139,146 reporting bladder neck contracture (Table 11) showed a reduced rate for men undergoing robotic prostatectomy but this was not statistically significant (probability outcome favours robotic prostatectomy = 0.805). The predicted event probability in the mixed-treatment comparison model was 1% for robotic and 2.2% for laparoscopic prostatectomy. Restriction of the meta-analysis to only the studies at low risk of bias was not possible because all studies were categorised as high risk.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Bhayani 2003124 | 0/33 | 6/24 (25.0) | |
| Brown 2004125 | 0/60 | 2/60 (3.3) | |
| Carlsson 2010104 | 3/1253 (0.2) | 22/485 (4.5) | |
| Dahl 2009126 | 2/104 (2.0) | 0/102 | |
| Ficarra 2009106 | 3/103 (3.0) | 6/105 (5.7) | |
| Ghavamian 2006128 | 1/70 (1.4) | 3/70 (4.3) | |
| Hu 200692 | 2/322 (0.6) | 8/358 (2.2) | |
| Krambeck 2009108 | 3/248 (1.2) | 23/492 (4.7) | |
| Lama 2009133 | 5/56 (8.9) | 1/59 (1.7) | |
| Nadler 2010112 | 2/50 (4.0) | 7/50 (14.0) | |
| Ou 2009113 | 1/30 (3.3) | 0/30 | |
| Remzi 2005139 | 3/80 (3.8) | 4/41 (9.8) | |
| Wagner 2007146 | 2/75 (2.7) | 12/75 (16.0) | |
| Predicted probability of event | 0.010 | 0.021 | 0.049 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.48 (0.09 to 2.93); 0.805 | |
| Low-risk studies only | Not estimable |
Anastomotic leak
Meta-analysis of data from 14 studies90,94,96,97,101,104,112,113,125,126,128,134,139,140 that reported anastomotic leak (Table 12) showed a statistically significant reduced rate of anastomotic leaks in men following robotic prostatectomy (OR 0.21; 95% CrI 0.05 to 0.76; probability outcome favours robotic prostatectomy = 0.990). Predicted probability of this event in the model was 1.0% following robotic and 4.4% following laparoscopic prostatectomy. Restriction of the meta-analysis to only studies at low risk of bias was not possible because the zero event rate in the robotic studies produced unstable model convergence.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Brown 2004125 | 9/60 (15.0) | 2/60 (3.3) | |
| Carlsson 2010104 | 13/1253 (1.0) | 8/485 (1.6) | |
| Dahl 2009126 | 2/104 (1.9) | 0/102 | |
| aDrouin 2009101 | 0/71 | 2/85 (2.4) | 1/83 (1.2) |
| aGhavamian 2006128 | 2/70 (2.9) | 3/70 (4.3) | |
| aGuazzoni 200690 | 8/60 (13.3) | 20/60 (33.3) | |
| Joseph 200794 | 12/754 (1.6) | 112/800 (14.0) | |
| Martorana 2004134 | 1/50 (2.0) | 2/50 (4.0) | |
| Nadler 2010112 | 2/50 (4.0) | 2/50 (4.0) | |
| Ou 2009113 | 0/30 | 2/30 (6.7) | |
| Remzi 2005139 | 8/80 (10.0) | 6/41 (14.6) | |
| Rozet 200796 | 1/133 (0.8) | 1/133 (0.8) | |
| Salomon 2002140 | 4/155 (2.6) | 2/151 (1.3) | |
| Sundaram 200497 | 0/10 | 1/10 (10.0) | |
| Predicted probability of event | 0.010 | 0.044 | 0.033 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.21 (0.05 to 0.76); 0.990 | |
| Low-risk studies only | Not estimable |
Wound or urinary infection
Meta-analysis of data from 12 studies92,96,101,104,108,116,123,125–128,140 that reported infection rates (Table 13) showed a reduction in the rate of this event after robotic prostatectomy compared with laparoscopic prostatectomy but this was not statistically significant (probability outcome favours robotic prostatectomy = 0.662). The probability of an infection predicted by the model was 0.8% following robotic prostatectomy and 1.1% for laparoscopic prostatectomy. Restriction of the meta-analysis to only the studies at low risk of bias changed the direction of effect but precision was reduced.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Artibani 2003123 | 16/71 (22.5) | 8/50 (16.0) | |
| Brown 2004125 | 0/60 | 2/60 (3.3) | |
| Carlsson 2010104 | 25/1253 (2.0) | 8/50 (16.0) | |
| Dahl 2009126 | 1/104 (1.0) | 0/102 | |
| aDrouin 2009101 | 1/71 (1.4) | 0/85 | 6/83 (7.2) |
| Fornara 2004127 | 0/32 | 2/32 (6.3) | |
| aGhavamian 2006128 | 1/70 (1.4) | 1/70 (1.4) | |
| Hu 200692 | 7/322 (2.2) | 16/358 (4.5) | |
| Krambeck 2009108 | 3/248 (1.2) | 9/249 (3.6) | |
| Rozet 200796 | 12/133 (9.0) | 5/133 (3.8) | |
| Salomon 2002140 | 2/155 (1.3) | 14/151 (9.3) | |
| Tewari 2003116 | 0/200 | 4/100 (4.0) | |
| Predicted probability of event | 0.008 | 0.011 | 0.048 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.75 (0.18 to 3.35); 0.662 | |
| Low-risk studies only | 2.26 (0.02 to 295); 0.349 |
Organ injury
In descending order of frequency the reported injuries affected the rectum, ureter and bowel. Meta-analysis of data from the 17 studies93,101,104–106,113,116,123–125,127–129,133,134,139,140 that reported organ injuries (Table 14) showed a reduction in the event rate following the robotic procedure that was statistically significant (OR 0.16; 95% CrI 0.03 to 0.76; probability outcome favours robotic prostatectomy = 0.987). The event probability predicted by the model was 0.4% for robotic prostatectomy and 2.9% for laparoscopic prostatectomy. Restriction of the meta-analysis to only the studies at low risk of bias maintained the direction and magnitude of effect.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Artibani 2003123 | 4/71 (5.6) | 0/50 | |
| Bhayani 2003124 | 1/33 (3.0) | 0/24 | |
| Brown 2004125 | 2/60 (3.3) | 0/60 | |
| Carlsson 2010104 | 6/1253 (0.5) | 10/485 (2.0) | |
| Doumerc 2010105 | 1/212 (0.5) | 0/502 | |
| aDrouin 2009101 | 0/71 | 1/85 (1.2) | 1/83 (1.2) |
| Ficarra 2009106 | 2/103 (2.0) | 0/105 | |
| Fornara 2004127 | 1/32 (3.1) | 0/32 | |
| aGhavamian 2006128 | 2/70 (2.9) | 0/70 | |
| aGreco 2010129 | 2/150 (1.3) | 1/150 (0.7) | |
| Hu 200693 | 3/322 (0.9) | 23/358 (6.4) | |
| Lama 2009133 | 0/56 | 1/59 (1.7) | |
| Martorana 2004134 | 2/50 (4.0) | 0/50 | |
| Ou 2009113 | 2/30 (6.7) | 1/30 (3.3) | |
| Remzi 2005139 | 1/80 (1.3) | 1/41 (2.4) | |
| Salomon 2002140 | 4/155 (2.6) | 3/151 (2.0) | |
| Tewari 2003116 | 0/200 | 1/100 (1.0) | |
| Predicted probability of event | 0.004 | 0.029 | 0.008 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.16 (0.03 to 0.76); 0.987 | |
| Low-risk studies only | 0.00 (0.00 to 0.20); 0.992 |
Ileus
Meta-analysis of data from 12 studies92,95,106,108,112,116,123,125,128,134,139,140 that reported ileus (slowness of recovery of bowel function) rates (Table 15) showed a reduction in the event rate following the robotic procedure that was not statistically significant (OR 0.46; 95% CrI 0.12 to1.51; probability outcome favours robotic prostatectomy = 0.920). The predicted probability of ileus was 1.1% with the robotic procedure and 2.4% with the laparoscopic procedure. This difference should be treated with caution given that one study92 contributed one-third of all data. Restriction of the meta-analysis to only the studies at low risk of bias was not possible because all studies were categorised as high risk.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Artibani 2003123 | 1/71 (1.4) | 0/50 | |
| Brown 2004125 | 2/60 (3.3) | 3/60 (5.0) | |
| Ficarra 2009106 | 1/103 (1.0) | 1/105 (1.0) | |
| Ghavamian 2006128 | 2/70 (2.9) | 1/70 (1.4) | |
| Hu 200692 | 9/322 (2.8) | 19/358 (5.3) | |
| Krambeck 2009108 | 5/286 (1.7) | 10/564 (1.8) | |
| Martorana 2004134 | 1/50 (2.0) | 0/50 | |
| Menon 200295 | 1/40 (2.5) | 1/40 (2.5) | |
| Nadler 2010112 | 2/50 (4.0) | 0/50 | |
| Remzi 2005139 | 1/80 (1.3) | 0/41 | |
| Salomon 2002140 | 4/155 (2.6) | 0/151 | |
| Tewari 2003116 | 3/200 (1.5) | 3/100 (3.0) | |
| Predicted probability of event | 0.011 | 0.024 | 0.009 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.46 (0.12 to 1.51); 0.920 | |
| Low-risk studies only | Not estimable |
Deep-vein thrombosis
Meta-analysis of data from eight studies that reported deep-vein thrombosis rates (Table 16) showed an increased risk following the robotic procedure that was not statistically significant (OR 2.67; 95% CrI 0.26 to 50.3; probability outcome favours robotic prostatectomy = 0.193). The predicted probability of a deep-vein thrombosis was 0.6% with the robotic procedure and 0.2% with the laparoscopic procedure. Restriction of the meta-analysis to only the studies at low risk of bias was not possible because all studies were categorised as high risk.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Brown 2004125 | 0/60 | 2/60 (3.3) | |
| Ghavamian 2006128 | 1/70 (1.4) | 1/70 (1.4) | |
| Hu 200692 | 2/322 (0.6) | 0/358 | |
| Krambeck 2009108 | 1/248 (0.4) | 6/492 (1.2) | |
| Lama 2009133 | 0/56 | 1/59 (1.7) | |
| Nadler 2010112 | 0/50 | 1/50 (2.0) | |
| Salomon 2002140 | 1/155 (0.6) | 2/151 (1.3) | |
| Tewari 2003116 | 1/200 (0.5) | 1/100 (1.0) | |
| Predicted probability of event | 0.006 | 0.002 | 0.014 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 2.67 (0.26 to 50.3); 0.193 | |
| Low-risk studies only | Not estimable |
Pulmonary embolism
Because of the low event rate and the small number of studies reporting this outcome (Table 17) meta-analysis was not possible. Using crude combining of events across all studies, the percentage of men suffering pulmonary emboli was 2/1634 (0.1%) for robotic prostatectomy and 2/392 (0.5%) for laparoscopic prostatectomy.
Clavien–Dindo scores
The predicted event rates based on the meta-analysis statistical models for each Clavien–Dindo category are shown in Table 18. The individual study data contributing to each meta-analysis are given in Appendix 9. The OR for each Clavien–Dindo score was in favour of the robotic procedure but only that for Clavien IIIb, adverse event requiring intervention under general anaesthesia, was statistically significant (Figure 11).
| Clavien–Dindo category (see Table 3) | Robotic (%) | Laparoscopic (%) | Open (%) |
|---|---|---|---|
| Clavien I | 2.1 | 4.1 | 4.2 |
| Clavien II | 3.9 | 7.2 | 17.5 |
| Clavien IIIa | 0.5 | 2.3 | 1.8 |
| Clavien IIIb | 0.9 | 3.6 | 2.5 |
| Clavien IVa | 0.6 | 0.8 | 2.1 |
| Clavien V | < 0.1 | 0.2 | 0.2 |
FIGURE 11.
Odds ratio and 95% CrI by Clavien–Dindo score.
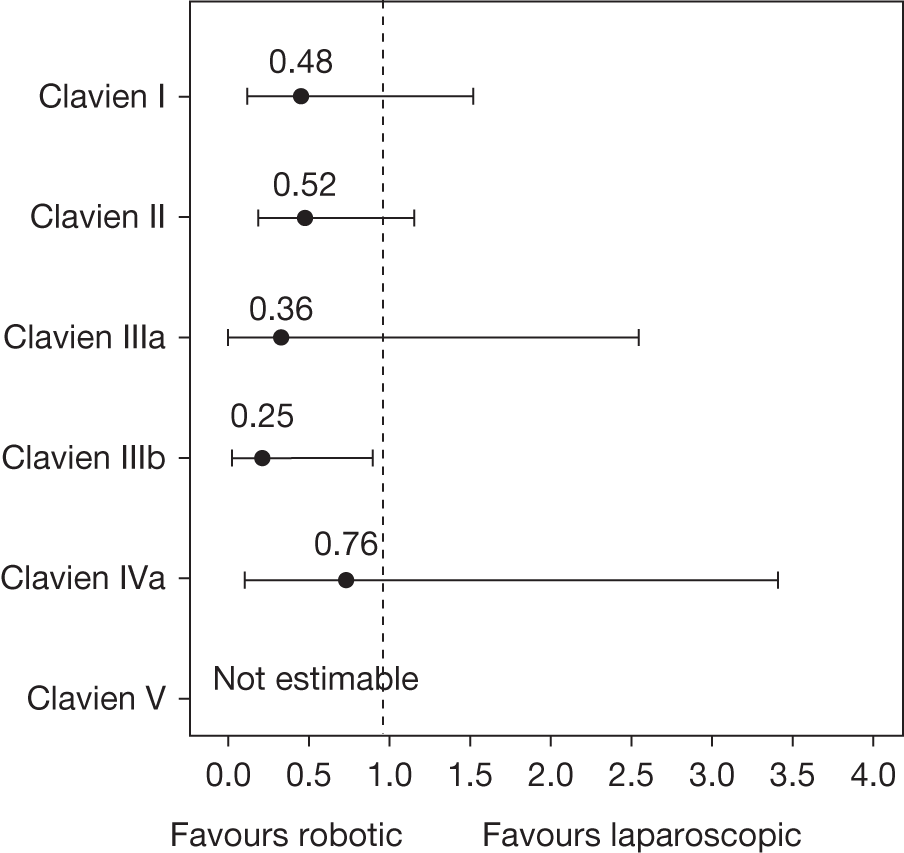
Descriptors of care
Equipment failure
Two studies reported equipment failure affecting the performance of the prostatectomy equipment. Menon and colleagues95 reported eight initial problems with the voice recognition system of the voice-controlled AESOP camera holder (Computer Motion, Goleta, CA, USA) during laparoscopic prostatectomy while Hu and colleagues92 reported two cases of equipment malfunction during robotic prostatectomy.
Conversion to open surgery
Meta-analysis of data from the 17 studies that reported rates of conversion from robotic or laparoscopic to open prostatectomy surgery (Table 19) showed lower rates for robotic prostatectomy but the difference was not statistically significant (OR 0.28; 95% CrI 0.03 to 2.00; probability outcome favours robotic prostatectomy = 0.893). The rate of conversion to open surgery predicted by the model was 0.3% with the robotic procedure and 0.9% with the laparoscopic procedure. Restriction of the meta-analysis to only the studies at low risk of bias was not possible because all studies were categorised as high risk.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) |
|---|---|---|
| Bhayani 2003124 | 3/36 (8.3) | |
| Chan 2008119 | 6/660 (0.9) | |
| Drouin 2009101 | 0/71 | 1/85 (1.2) |
| Ghavamian 2006128 | 0/70 | |
| Greco 2010129 | 0/150 | |
| Hu 200692 | 0/322 | 3/358 (0.8) |
| Jurczok 2007131 | 0/163 | |
| Martorana 2004134 | 0/50 | |
| Menon 200295 | 0/40 | 1/40 (2.5) |
| Namiki 2005135 | 0/45 | |
| Ou 2009113 | 2/30 (6.7) | |
| Remzi 2005139 | 1/80 (1.3) | |
| Rozet 200796 | 4/133 (3.0) | 0/133 |
| Soric 2004143 | 3/26 (11.5) | |
| Tewari 2003116 | 0/200 | |
| Trabulsi 200898 | 0/50 | 7/197 (3.6) |
| White 2009118 | 0/50 | |
| Predicted probability of event | 0.003 | 0.009 |
| OR (95% CrI); probability outcome favours robotic prostatectomy | All studies | 0.28 (0.03 to 2.00); 0.893 |
| Low-risk studies only | Not estimable |
Operation time
The criteria used to define and measure operation time varied considerably between studies and are detailed in Appendix 9. To attempt to minimise the effect of substantive variation between studies, meta-analysis was restricted to eight studies that directly compared robotic and laparoscopic operation times (Table 20). The pooled estimate demonstrated a statistically significant reduction in operation time of –12.4 minutes (95% CrI –16.5 minutes to –8.1 minutes) in favour of robotic prostatectomy. This difference should be treated with caution given uncertainty in whether robot docking time before commencing the surgery was included in the measured operation time in all studies.
| Study | Robotic, n, mean (SD) | Laparoscopic, n, mean (SD) |
|---|---|---|
| Bolenz 2009102 | 264, 198a (58.7) | 220, 235a (66.9) |
| Drouin 2009101 | 71, 199.6 (36.6)b | 85, 257.3 (94.3)b |
| Gosseine 200991 | 122, 237 (67.4) | 125, 241 (68.3) |
| Hu 200692 | 322, 186a (55.9) | 358, 246a (69.3) |
| Joseph 200794 | 754, 194 (57.8) | 800, 179 (54.3) |
| Menon 200295 | 40, 274 (94.3)b | 40, 258 (80.3)b |
| Rozet 200796 | 133, 166 (51.2) | 133, 160 (49.8) |
| Sundaram 200497 | 10, 290 (78.7) | 10, 394 (99.7) |
| Predicted mean time (minutes) | 225.1 | 237.5 |
| Mean difference (95% CrI) | All studies | –12.4 (–16.5 to –8.1) |
| Low-risk studies only | Not estimable |
Duration of catheterisation
Postoperative catheterisation policies varied considerably across the 23 studies90,91,94,96,101,105,106,113,114,116,122–124,127,129,131–134,137,139,140,143 that included relevant details and no meta-analysis was possible given the diversity of type of summary outcome measures reported. Of the four directly comparative studies of robotic and laparoscopic procedures, two94,96 reported a shorter duration of catheterisation in men undergoing laparoscopic prostatectomy and two91,101 reported a shorter duration of catheterisation for robotic prostatectomy. Only the report by Gosseine and colleagues92 showed that the difference in duration of catheterisation was statistically significant, being 1.5 days shorter for robotic prostatectomy (p = 0.01).
Length of hospital stay
Length of hospital stay varied considerably across the 28 studies91,96,97,102,105–108,112–114,116,119,121,123–128, 131–134,137–140,143 that gave this information and no meta-analysis was possible given the diversity of type of summary outcome measures reported. Of the four studies directly comparing robotic and laparoscopic prostatectomy,91,96,97,102 two reported a 1-day shorter length of stay for laparoscopic prostatectomy and two reported a 1-day shorter length of stay for robotic prostatectomy; none demonstrated any statistical significance.
Assessment of the learning curve
The variables of numbers of surgeons acting as lead operator, the number of procedures conducted by each surgeon prior to study commencement, the number of procedures carried out by each surgeon during the study and reported outcomes used to assess learning were abstracted from each included study (see Appendix 9). In general, the extent of reporting of relevant data on these variables was limited and data were often not given in a clear form suited to meta-analysis. The number of surgeons performing the surgery on men included in each study for both procedures was reported in 43/58 (74%) studies (see Appendix 9). Of these, nine90,91,97,105,109,112,113,128,134 were single-surgeon studies. Studies that provided information on surgeons’ previous experience did so in a number of different ways including using categories such as ‘experienced’, ‘fellowship trained’ or ‘performed radical retropubic prostatectomies for 15 years prior to study’.
We focused on the rate of positive surgical margins as the key outcome to assess the effect of increasing surgeon experience to maintain consistency with the findings of the systematic review and the importance of this outcome to the economic modelling (see Chapter 5). The proportion of positive surgical margins for robotic and laparoscopic radical prostatectomy was plotted against the number of procedures carried out by the participating surgeons in each included study (Figure 12). Regression modelling illustrated that there was no evidence of trends across increasing experience (the dashed line is the predicted linear relationship for laparoscopic studies and the solid line is the predicted linear relationship for robotic studies), with R2 < 0.02%, demonstrating no statistical significance.
FIGURE 12.
Proportion of positive surgical margins with increasing experience in included studies.
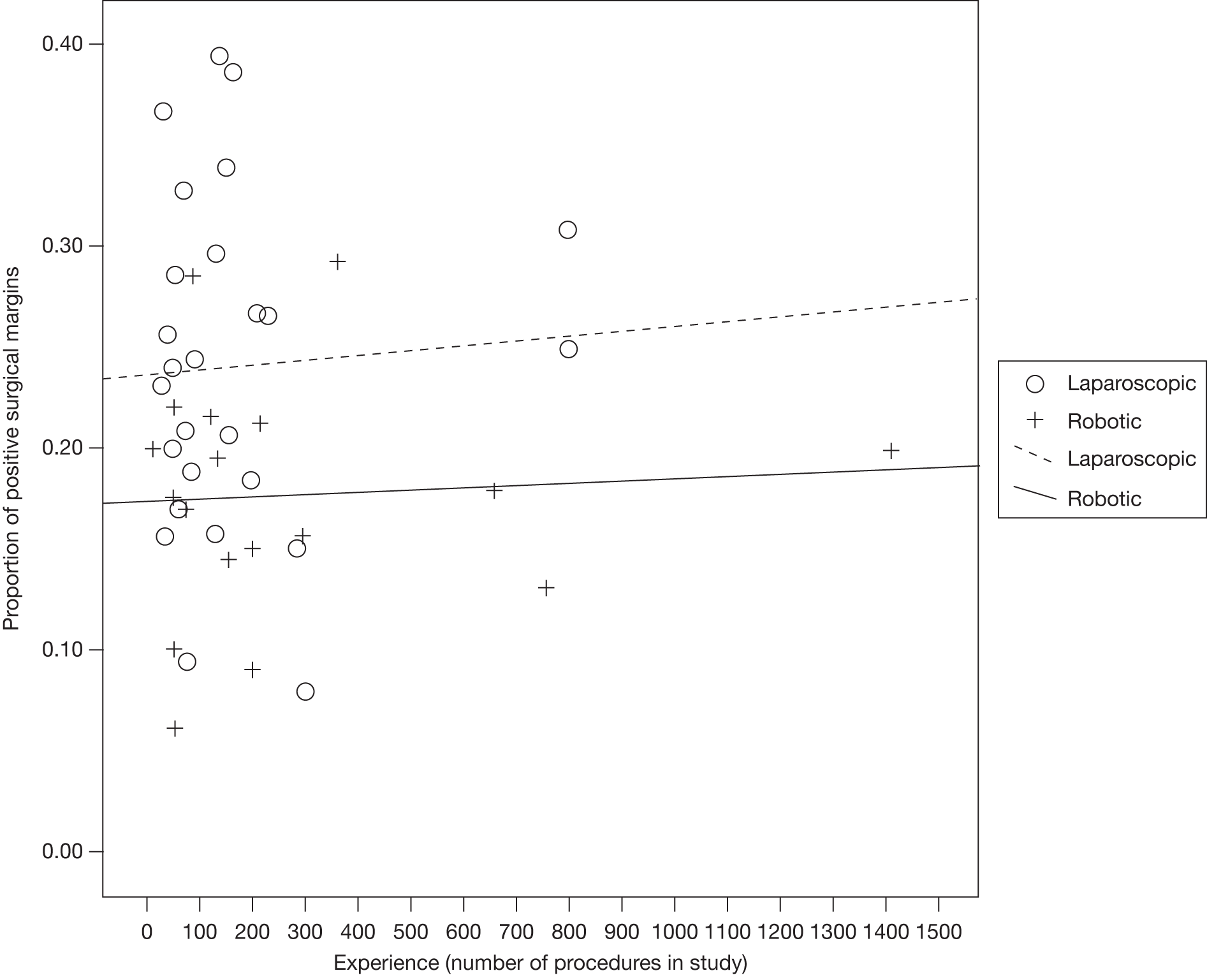
No data on parameters of the ‘shape’ of the learning curve, such as rates of positive margins for set number of cases performed, were identified in the included comparative studies. The inclusion criteria were therefore extended to include case series of laparoscopic and robotic radical prostatectomy that included more than 200 men. This specific extended search identified six robotic case series and four laparoscopic case series (Table 21). Two studies155,156 reported only a mathematical shape to the learning curve, thereby precluding any formal modelling of the learning curve parameters (starting point, rate of learning and asymptote). All studies reported a decrease in positive surgical margin rate with increasing surgeon experience except for that by Eden and colleagues157 who reported a consistently low rate throughout the series of men undergoing laparoscopic prostatectomy. The positive margin rate data plotted against the first and last reported level of experience for each case series are shown in Figure 13. There was some evidence that a non-linear (logarithmic) relationship with increasing experience fitted the data better than a linear relationship; however, this was not statistically significant (log-experience –0.02; 95% CI –0.043 to 0.003; p = 0.08). This equated to an average surgical margin rate of 25.6% at case one, reducing to 14.5% by 250 cases and 11.7% by 1000 cases. The data provided no evidence that learning contributed differently to positive margin rates between the two procedures (mean difference in level –0.02; 95% CI –0.16 to 0.12; p = 0.755).
| Study | Reported outcomes/measures | Number of cases | Robotic | Laparoscopic | Other information reported in study |
|---|---|---|---|---|---|
| Secin 2010158 | Margin rate | 6274 |
Case 1: 24% Case 250: 9% |
||
| Hong 2010155 | Margin rate | 469 |
Case 1: 27% Case 200: 25% Case 400: 21% |
Linear trend | |
| Tewari 2010154 | Margin rate | 1340 |
Case 1: 9% Case 100: 7% |
||
| McNeill 2010156 | Margin rate | 300 |
Case 1–50: 27% Case 251–300: 14.7% |
Log-linear trend | |
| Operation time |
Case 1: 200 minutes Case 200: 140 minutes |
||||
| Complications |
Case 1: 29% Case 250: < 1% |
||||
| Samadi 2010159 | Margin rate | 1181 |
Case 1: 8.5% Case 590: 4.3% |
||
| Rodriguez 2010160 | Margin rate | 400 |
Case 1: 32% Case 400: 13.3% |
||
| Jaffe 2009161 | Margin rate | 278 |
Case 1–12: 58% Case 12–189: 23% Case 278: 9% |
||
| Operation time |
Case 1–12: 250 minutes Case 12–189: 165 minutes Case 278: 134 minutes |
||||
| Eden 2009157 | Margin rate | 1000 | Series average: 13.3% | No trend noted | |
| Complications | No trend noted | ||||
| Blood loss | Series average: 200 ml | Stabilised after 200 cases | |||
| Potency |
Case 1: 23% Case 1000: 86% |
Stabilised after 700 cases | |||
| Operation time | Series average: 177 minutes | Stabilised after 200 cases | |||
| Vickers 2009162 | Biochemical recurrence | 4702 |
Case 10: 16% Case 250: 15.5% Case 750: 8.2% |
||
| Martinez-Pineiro 2006163 | Margin rate | 604 | Decreased significantly by 101 cases | ||
| Blood transfusion |
Case 1: 25% Case 600: 7% |
Stabilised by 200 cases | |||
| Operation time | Series average: 201 minutes |
FIGURE 13.
Proportion of positive surgical margins with increasing experience in case series.
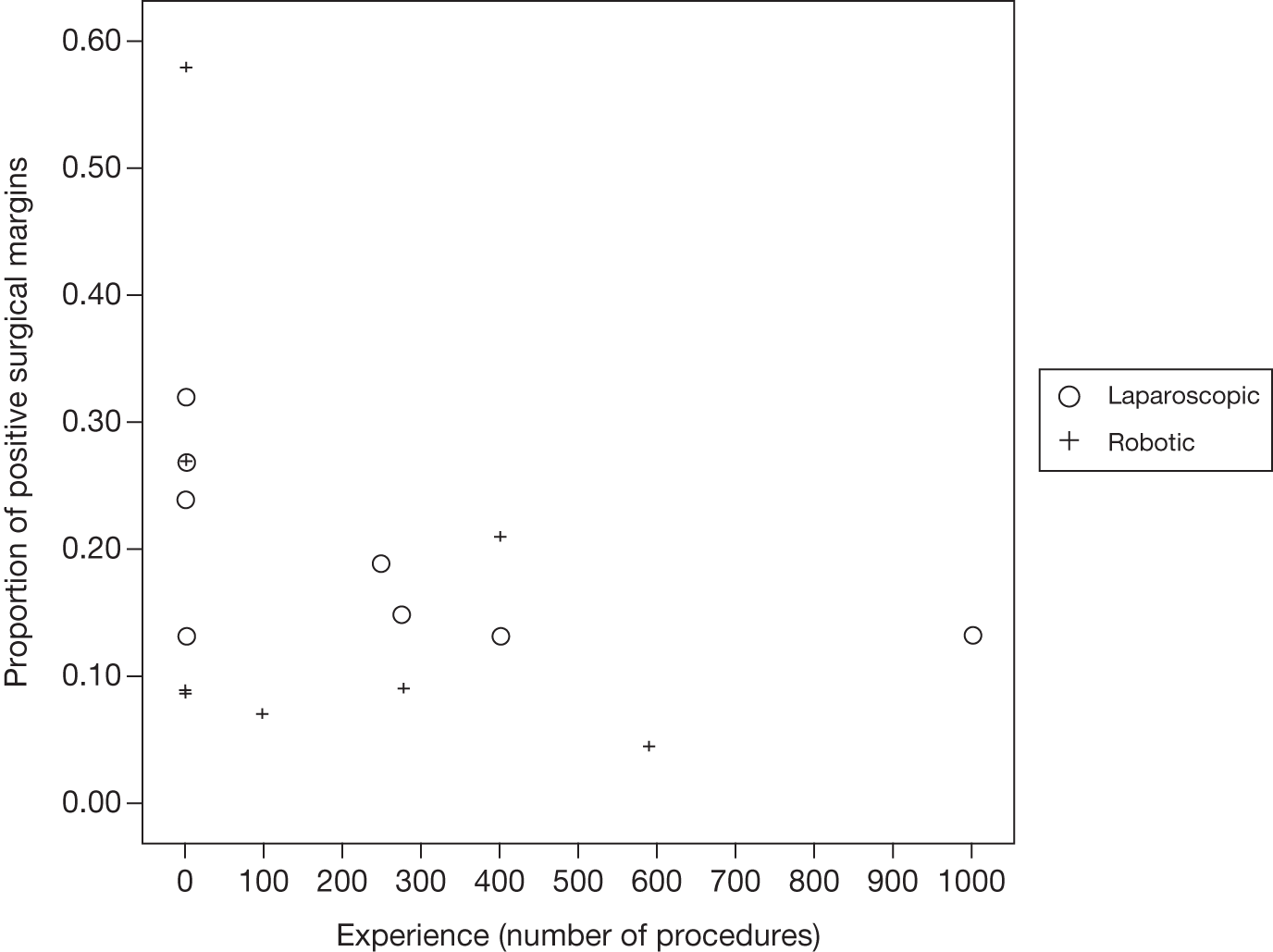
To summarise the results, the two approaches to assessing whether or not surgeon learning affected the rate of positive margins gave conflicting findings. Across the studies included in the meta-analyses of positive margin rates, there was no evidence that experience contributed as a significant confounder to the results, whereas the larger case series suggested a reduction over time in positive margin rates. There was no empirical evidence, however, that the rate of learning differed between the two surgical procedures. Caution is therefore required in the interpretation of these findings.
Summary and conclusions of the evidence of comparative effectiveness
This review considered data from 19,064 patients across one RCT and 53 non-randomised comparative studies with very few studies considered at low risk of bias. Results should be interpreted cautiously to reflect the poor quality of the evidence base and the variation in definitions of outcomes. It was noteworthy that, when meta-analyses were restricted to studies assessed to be at low risk of bias, the effect sizes tended to move from favouring robotic prostatectomy towards no difference. There were limited published data on long-term efficacy of robotic and laparoscopic radical prostatectomy in reducing morbidity and no data comparing mortality from prostate cancer. We found no evidence for any difference in patient-reported outcomes. There was strong statistical evidence that positive surgical margin rates, a proxy measure for cancer control, may be reduced by the use of robotic radical prostatectomy; however, it was unclear in the literature how these differences impact on cancer recurrence and long-term efficacy outcomes and restricting the analysis to low risk of bias studies showed no statistical evidence of a difference. This finding should therefore be interpreted with caution. In addition, the studies showed variation in the pathology protocols employed, which may have biased the determination of positive margin status and prevented accurate comparison between studies. Improvement in reporting pathology findings is necessary if evidence syntheses across studies are to be undertaken. The recent ISUP Consensus Conference72 aims to promote consistency in the handling and reporting of radical prostatectomy specimens and provide detailed guidelines that are feasible for most practising pathologists to implement and may be a major advance towards providing more comparable data in the published literature.
There was a general trend for robotic surgery to have fewer perioperative adverse events, apart from rarely reported deep-vein thrombosis, and the differences reached statistical significance for anastomotic leak and organ injury in particular, and those classified as Clavien IIIb in general. There were limited data on the important longer-term functional adverse effects of urinary incontinence and erectile dysfunction. The available data suggested no evidence of a difference in the proportion of men suffering urinary incontinence at 12 months. There were insufficient data to draw any conclusions on the likely size of any differential effect on rates of erectile dysfunction.
There was conflicting evidence on the impact of the learning curve for both procedures. There was no evidence that experience contributed as a significant confounder to the meta-analysis results, but case series data suggested a reduction over time in positive margin rates. There was, however, no empirical evidence that the rate of learning as expressed by changes in positive margin rates differed between the two surgical procedures and therefore little support for including the learning curve relationship in the base-case economic model.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Figure 14. This should be interpreted in light of the comments made earlier in the chapter.
FIGURE 14.
Summary of the clinical effect sizes (ORs and 95% CrIs) from meta-analyses. To improve visual display the upper CrI has been truncated to 5.0. Low RoB denotes estimate from low risk of bias studies only.
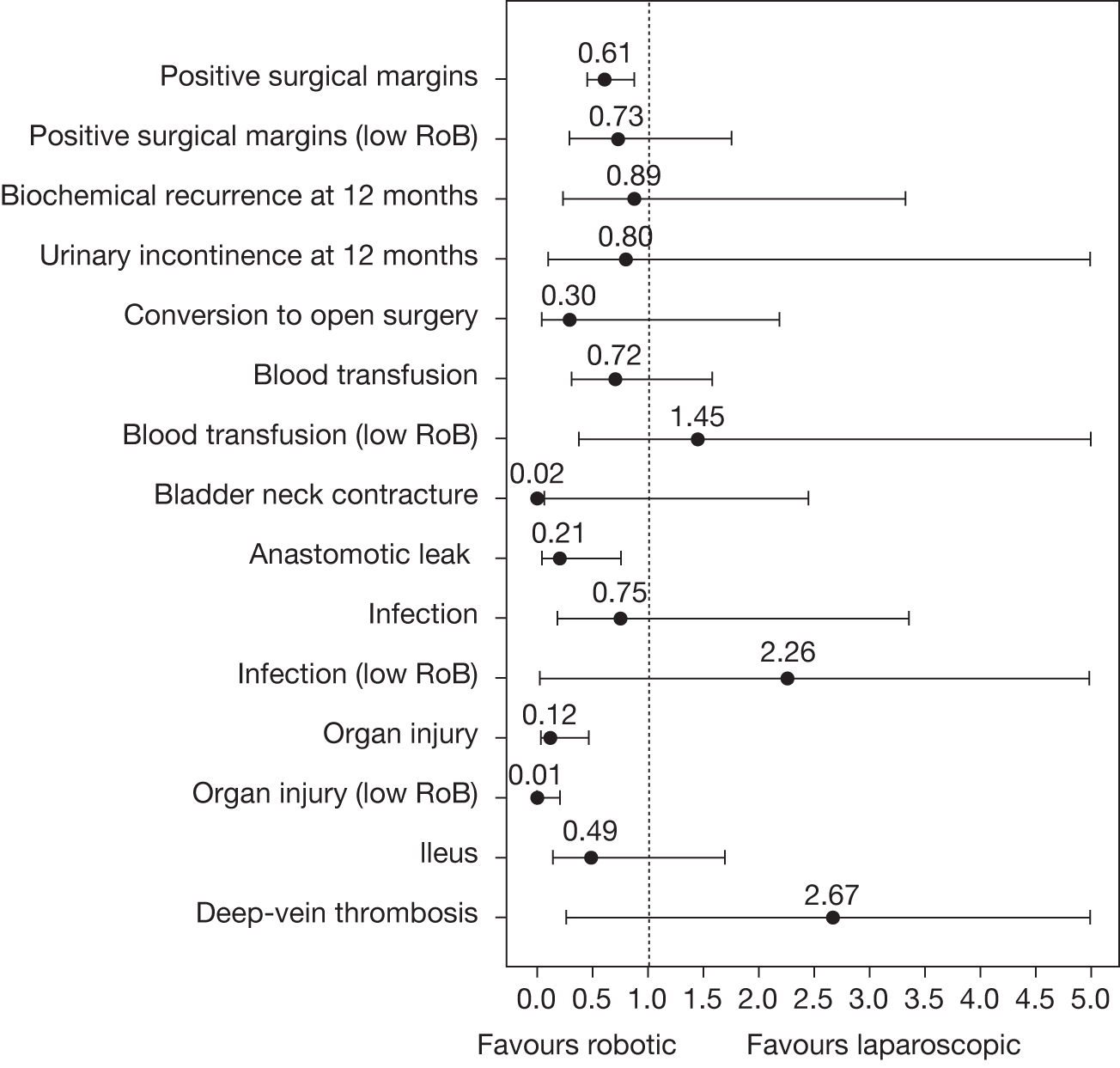
Chapter 5 Methods for health economic evaluation
Introduction
In this chapter we report the methodology and parameter value selection for a health economic evaluation comparing robotic radical prostatectomy with laparoscopic radical prostatectomy. This economic evaluation was conducted using a discrete-event simulation model described in detail in subsequent sections. This represents a change to the modelling specified in the original protocol. This change was required to account for the degree of complexity encountered while defining the treatment care pathways.
The original study protocol (see Appendix 1) specified the use of a Markov state transition model in order to explore aspects of heterogeneity within cohorts undergoing treatment for localised prostate cancer. Once the treatment care pathways were defined, however, it became clear that the use of a state transition model would be impracticable for several reasons:
-
The number of potential health states and their transitions was large.
-
The discrete-event model explicitly included multiple adverse events that may occur during progression along the care pathway trajectory while also accounting for potential feedback to one or more previous states within the care pathway. Inclusion of multiple event states would necessitate very large transition matrices.
-
The study required a modelling approach that would provide a high degree of flexibility in modelling interconnected care processes while also accounting for heterogeneity in the populations modelled. In addition, the discrete-event simulation adopted allows the incorporation of interdependent and simultaneously occurring health events and internal feedback loops, a characteristic found within the treatment care pathways. These would be difficult to achieve using a Markov-type approach; this is an important limitation of decision tree-based approaches. The approach adopted also provided more detailed reporting of each individual’s journey through the disease trajectory.
Before conducting the economic evaluation we attempted to identify and summarise any existing economic evaluations on this topic systematically (see the following section). The economic evaluation itself involved several stages, described later in this chapter.
Systematic review of previous economic evaluations
We searched for economic evaluations comparing both costs and outcomes of the two surgical procedures systematically. To be included studies had to include costs and effects, regardless of the way that each were estimated. We found no economic evaluations that fully met the inclusion criteria (see Appendix 11). Three publications were identified that reported cost comparisons between robotic and open radical prostatectomy,164–166 five publications reported cost comparisons between laparoscopic and open radical prostatectomy121,167–170 and three publications reported cost comparisons between robotic and laparoscopic surgery. 171–173 The publications by Bolenz and colleagues171 and Lotan and colleagues172 estimated the procedure costs of robotic and laparoscopic prostatectomy for a USA setting based on a retrospective patient cohort and a hypothetical costing exercise respectively. In both cases, excluding the capital cost of the robotic system, robotic prostatectomy was $500–700 more expensive per case than laparoscopic surgery. Bolenz and colleagues171 reported that the additional purchase and maintenance costs of a single robotic da Vinci system were $340,000 per year, while Lotan and colleagues172 reported that, assuming 300 cases per year, the cost of purchase plus maintenance costs were an additional $857 per case. Following a financial appraisal, again conducted in a USA setting, Steinberg and colleagues173 concluded that robotic prostatectomy was not financially viable in low-volume centres performing fewer than approximately 80 procedures per year under current tariffs. Although the method used to establish procedure costs in these three papers was clear, none considered costs beyond the hospital period and none attempted to compare procedures in terms of both costs and outcomes. Although the paucity of the evidence base was anticipated at the outset of the study, the results of this systematic attempt to identify relevant economic evaluations have highlighted the need for the economic evaluation that is reported in this monograph.
Methods
Model specification: purpose and design
The purpose of this model was to simulate the outcomes and costs during and following a radical prostatectomy procedure using either a robotic or laparoscopic technique performed in an appropriate UK NHS hospital on a man with clinically apparent localised prostate cancer. 43 The model was specified to follow the predefined care pathway for individual men for 10 years from the time of surgery, this being the anticipated duration of use of the current robotic technology under study (Intuitive Surgical, June 2010, personal communication). We also included as a sensitivity analysis the ability to specify the model over the lifetime of the individual, consistent with the epidemiological characteristics of localised prostate cancer, which typically has a long natural history with survival benefits for radical treatment needing at least 10–15 years to accrue. 44
We selected an individual-based event model in which surgical procedure, steps in the care pathway, the occurrence of longer-term adverse events and ultimately death are modelled as discrete events for individuals within the model. 174 The transition of individual men between events was driven by the previous health states, processes involved in their clinical treatment and subsequent care that arose as a consequence of the surgery, the underlying disease and natural lifespan. These included adverse events associated with the prostatectomy, events during clinical management of individuals who were cured of prostate cancer by the surgery and events driven by disease persistence or recurrence following prostatectomy. The clinical characteristics of individuals entering the simulation could be varied to represent the complete spectrum of patient and disease characteristics among the overall population of men with localised prostate cancer requiring radical prostatectomy. Each event and each subsequent patient management decision at all decision points in the pathway was modelled probabilistically based on available data relevant to patient care in the UK NHS. The hierarchy of data sources used was in the order of the associated systematic review, available relevant literature including web-based sources and consultation with relevant experts. The model was parameterised using data obtained from these sources describing disease progression, survival and the prevalence of adverse events. Data on costs to the UK NHS of laparoscopic and robotic prostatectomy were predominantly obtained directly from the manufacturer of the robotic system, Intuitive Surgical,30 and from national and local NHS sources (see Costs). To enable analysis of cost-effectiveness, utility values for the various health states within the care pathway were obtained from the literature (see Utilities). The model was constructed using the scripting language available for the R statistical package for computing. 175
State variables and timescales
State variables
Postoperatively each individual was assigned a combination of eight state variables. The first was age at the time of surgery. This was simulated by drawing a random deviate from a triangular distribution with minimum, peak and maximum shape parameters derived respectively from the 25th percentile, median and 75th percentile of the age distribution of men undergoing radical prostatectomy. The age range for each intervention was identical.
Four variables specified individual disease characteristics following pathological examination of the removed prostate:
-
surgical margin: negative or positive
-
tumour stage: pT0–T2 or pT3–pT4
-
Gleason sum score: ≤ 7 or 8–10
-
lymph node status: unknown, negative or positive.
Three variables indicated adverse events arising from prostatectomy that would not be resolved in the 3-month treatment phase:
-
bladder neck contracture (stenosis): absent or present
-
urinary incontinence (moderate or severe): absent or present
-
erectile dysfunction (bothersome to individual): absent or present.
Time step
The modelled time step (cycle length) was a quarter (3 months). For variables for which only annual data could be obtained the probabilities were converted to a standard time base of a quarter using Equation 1:
where P is the yearly probability of an event occurring and P ′ is the probability of an event occurring in a 3-month period.
Time horizons
The base-case time horizon for the model was 10 years, this being consistent with the anticipated duration of use of the current technology under test – the da Vinci surgical robotic system. A longer time horizon (40 years) that would cover the expected lifetime of the men included in the model was also used, consistent with the epidemiology of localised prostate cancer. 176
Assumptions within the model
Modelled events at each decision point within the pathway were discrete and independent. For example, surveillance for biochemical recurrence was simulated in the same way irrespective of events previously experienced by the individual. In the absence of suitable data the probability of further biochemical recurrence was independent of previous biochemical recurrences that had been successfully treated. In practice, care options inevitably are affected by previous disease characteristics and other related events, but the multitude of possibilities of care for particular individuals during the course of their cancer care subsequent to radical prostatectomy could not be fully parameterised in the model in the absence of sufficiently detailed individual-level data sets. Proportions of individuals undergoing different procedures within the care pathway were defined by the probability of being assigned to those procedures. This simplification was necessary because of the lack of data on the underlying causal factors leading to events; they were therefore modelled as random processes (see Modelling of discrete events).
The imprecision/uncertainty surrounding parameter estimates used within the model was characterised by assigning statistical distributions to parameters. For parameter estimates provided by the systematic review, the log-normal distribution was used to define the degree of surrounding uncertainty. Other parameters derived from the literature or other sources were considered for accuracy, credibility and plausibility at meetings of the expert panel. Identifying a suitable distribution for estimates and describing the uncertainty around these values was problematic. In such circumstances, uncertainty was calculated as a potential range of plausible values of ±25% of the estimate.
For parameters not defined by the systematic review we assumed that the point estimate was the most likely ‘real’ value and therefore did not consider that a uniform rectangular distribution was appropriate. Furthermore, by defining the extreme limits of the distribution using the triangular method (as described above) we ensured that the upper and lower bounds of variability did not exceed clinical plausibility. And finally, the way in which variability was calculated ensured that the degree of uncertainty applied to each intervention equally.
Modelling the care pathway
Following robotic or laparoscopic prostatectomy each individual was entered into the specific pathway dictated by his clinical and disease state after the operation (Figure 15). This state was characterised in terms of, first, cancer status and, second, the presence of one or more of the three adverse events that were deemed to persist beyond the treatment period: bladder neck contracture, urinary incontinence and erectile dysfunction. The individuals then proceeded through a series of events dependent on where they were in the care pathway and which would result in changes to, or resolutions of, differing health states. This would particularly include remission or relapse following additional treatment for recurrent prostate cancer or resolution of a longer-term adverse event by treatment.
FIGURE 15.
Schematic showing care pathway for the perioperative state during and immediately after radical prostatectomy. A0, perioperative health state; A1, postoperative health state; B2, surgeon learning effect; B3, perioperative complication classified using the Clavien–Dindo system; LRP, laparoscopic radical prostatectomy; ORP, open radical prostatectomy; RRP, robotic radical prostatectomy.
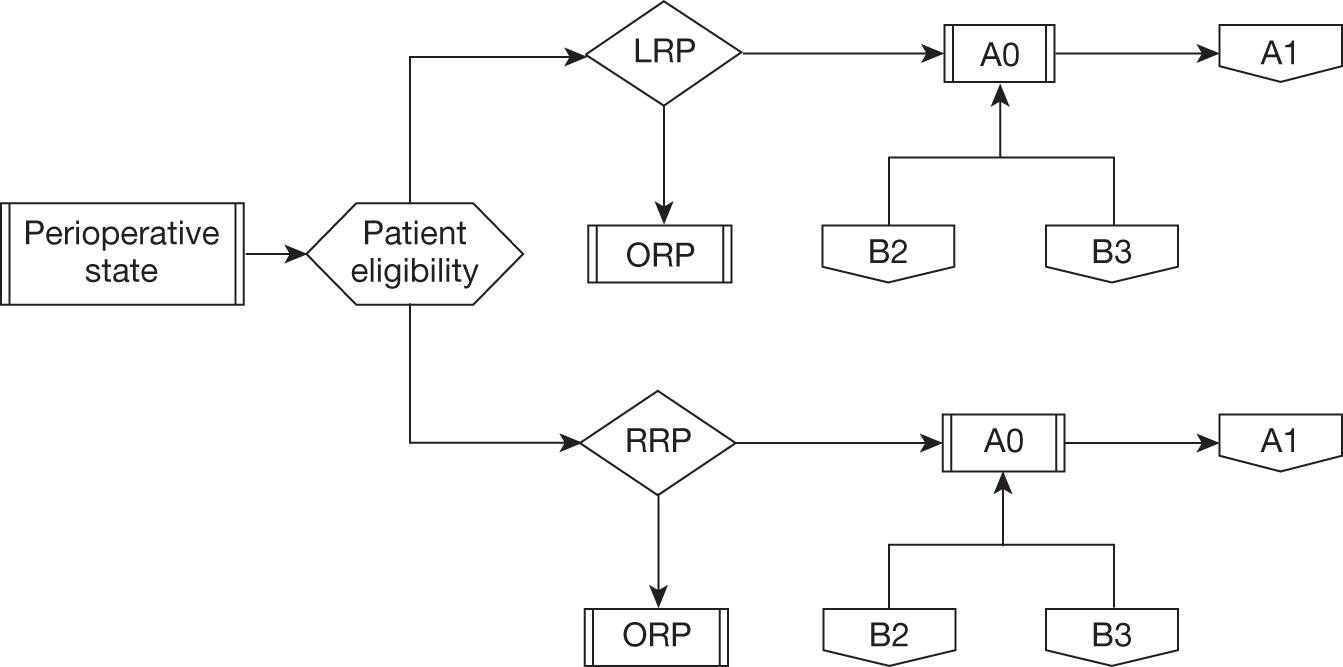
Events were modelled probabilistically using data derived from the hierarchy of sources defined previously in Model specification: purpose and design. Where possible the data used were relevant to both the clinical context of radical prostatectomy and current practice in the UK NHS. 43 Parameters, their values, their distributions and their sources are listed in abbreviated form in the relevant sections. Events experienced by individuals were scheduled in interacting ‘streams’. Surveillance, cancer treatment and mortality were first simulated either until the end of the time horizon if the individual survived or until a process within the care pathway led to death either from prostate cancer or from any other cause (see Figure 15). This provided the framework for each individual’s trajectory through the cancer care pathway. The second set of events simulated the management of the three postoperative dysfunctions: bladder neck contracture, urinary incontinence and erectile dysfunction. If a process led to an intervention event, such as surgery for urinary incontinence, this was scheduled only after at least 12 months of surveillance without a cancer-related event.
Modelling of discrete events
All events were assumed to be binomial in the sense that an event either occurred, 1, or did not occur, 0. Simulation of the occurrence of an event for an individual was undertaken by drawing random uniform deviates and comparing the observed deviate with the known probability of that event occurring given the relevant conditions. Thus, if x represents the proportion of men who experienced bothersome erectile dysfunction after laparoscopic prostatectomy, any random deviate drawn for an individual that was less than x would lead to that individual suffering the dysfunction and progressing down the appropriate pathway of care, whereas any deviate greater than x corresponded to no dysfunction. The proportion of men experiencing each event in each pathway was derived where possible from the systematic review reported in detail in Chapter 4. Other relevant literature or expert opinion were used where necessary.
Model health states and associated parameter values
Perioperative state
In line with the objective of this HTA all patients were assumed to have undergone radical prostatectomy by either laparoscopic or robotic means (see Figure 15). In addition, those individuals deemed to be at intermediate or high risk of early biochemical recurrence according to preoperative disease characteristics (Table 22) were allocated to undergo a concurrent pelvic lymph node dissection; the probability of this was defined from an appropriate additional literature source177 as the information was not available from the systematic review. Adverse events during surgery could initiate two further model events. First, the probability of suffering perioperative adverse events, categorised using systematic review data according to the Clavien–Dindo system into one of six levels, was defined as the proportion of patients who suffered that event68,69 (Figure 16). Second, and independently of adverse events categorised by the Clavien–Dindo system, a proportion of men undergoing laparoscopic or robotic prostatectomy were deemed to require conversion to an open procedure because of intraoperative difficulties. The rate for each of the procedures was determined from the systematic review and the consequence in terms of costs was defined as an extra 3-day hospital stay, decided by expert opinion (see Table 22).
| Perioperative state | Value | Probability | Interquartile range | Assigned distribution | Source |
|---|---|---|---|---|---|
| Robotic surgery | |||||
| Age (years) | 61.5 | 39–74 | Triangular | Systematic review | |
| Rate of pelvic lymphectomy (%) | 58.20 | 43.65–72.75 | Triangular | Sharma 2011177 | |
| Conversion to alternative surgical technique (%) | 0.3 | 0.03–2.16 | Triangular | Ollendorf 20102 | |
| Operative time (minutes) | 225 | NA | Systematic review | ||
| Clavien risk factor I | 1 | 0.021 | 0.006–0.064 | Log-normal | Systematic review |
| Clavien risk factor II | 2 | 0.039 | 0.016–0.064 | Log-normal | Systematic review |
| Clavien risk factor IIIa | 3 | 0.005 | 0.000–0.033 | Log-normal | Systematic review |
| Clavien risk factor IIIb | 3 | 0.009 | 0.002–0.033 | Log-normal | Systematic review |
| Clavien risk factor IVa | 4 | 0.006 | 0.001–0.027 | Log-normal | Systematic review |
| Clavien risk factor V (death) | 5 | 1.39 × 10–19 | 1.22 × 10–61–1.60 × 10–20 | Log-normal | Systematic review |
| Laparoscopic surgery | |||||
| Age (years) | 63 | 43–76 | Triangular | Systematic review | |
| Rate of pelvic lymphectomy | 58.94% | 43.7–72.8% (triangular) | Sharma 2011177 | ||
| Conversion to alternative surgical technique | 0.009% | 0.000–0.018 (triangular) | Ollendorf 20102 | ||
| Operative time (minutes) | 237.5 | N/A | Systematic review | ||
| Clavien risk factor I | 1 | 0.041 | 0.000–0.167 (log-normal) | Systematic review | |
| Clavien risk factor II | 2 | 0.072 | 0.019–0.143 (log-normal) | Systematic review | |
| Clavien risk factor IIIa | 3 | 0.013 | 0.000–0.077 (log-normal) | Systematic review | |
| Clavien risk factor IIIb | 3 | 0.036 | 0.010–0.160 (log-normal) | Systematic review | |
| Clavien risk factor IVa | 4 | 0.008 | 0.000–0.039 (log-normal) | Systematic review | |
| Clavien risk factor V (death) | 5 | 0.002 | 0.0004–0.0023 (log-normal) | Systematic review | |
FIGURE 16.
Flow chart illustrating the classification of various intraoperative adverse events using the Clavien–Dindo system for the grading of operative complications and their input into the perioperative care pathway. A0, perioperative health state; CRF, Clavien–Dindo risk factor; B3, perioperative complication categorised by the Clavien–Dindo system.
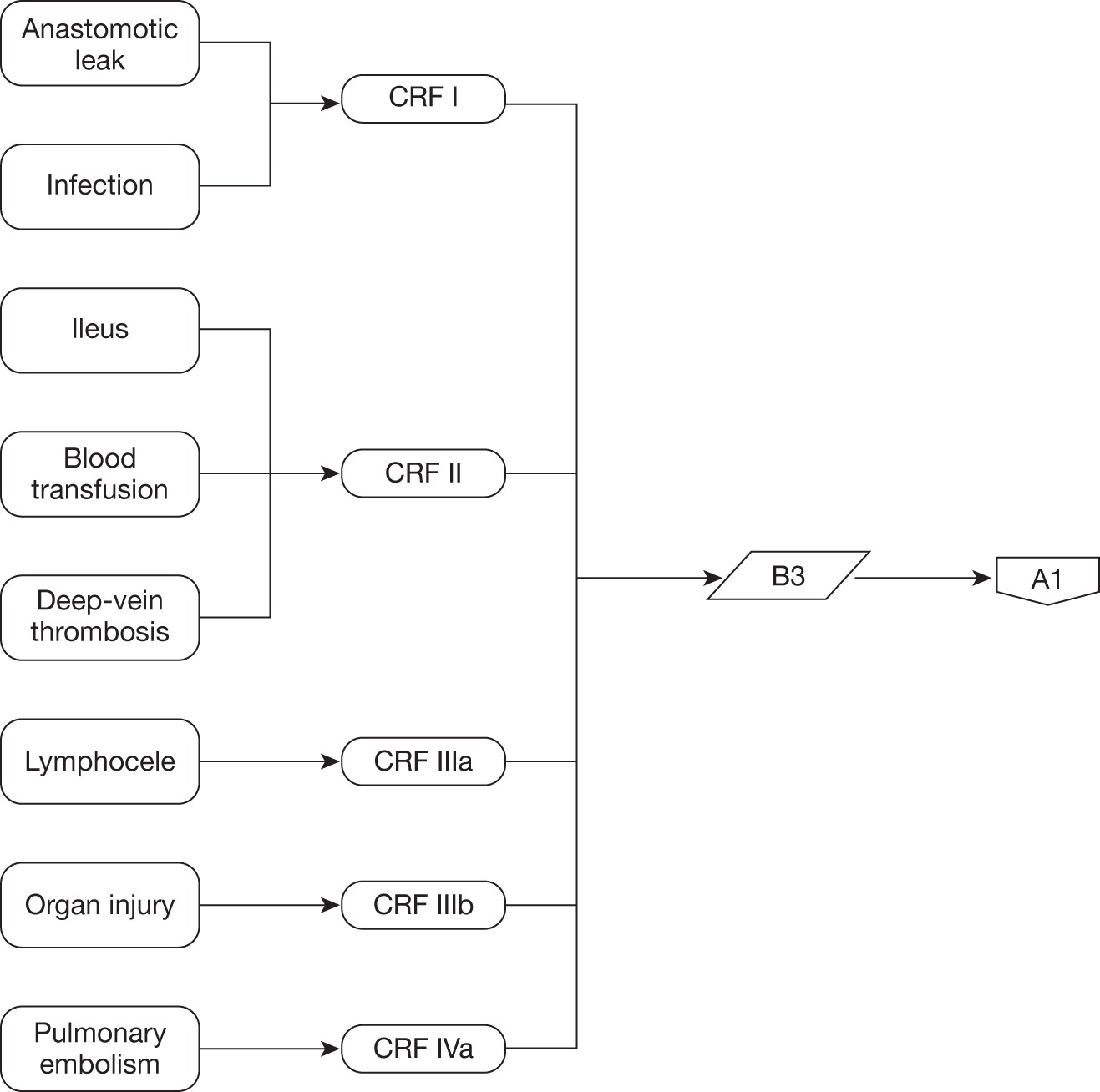
For each specific Clavien–Dindo level or adverse event the associated financial cost was modelled solely through the extra duration of hospitalisation measured in days that a patient would require according to expert opinion (Table 23). These events were assumed to have resolved during the 3-month perioperative state.
| Clavien–Dindo category | Number of additional bed-days |
|---|---|
| I | 1 |
| II | 2 |
| IIIa | 3 |
| IIIb | 3 |
| IVa | 4 |
| V | NA (results in death) |
| Conversion to open procedure | 3 |
Postoperative state
Immediate further cancer treatment
A proportion of men were assigned to require and undergo immediate further cancer treatment; the probability of this occurring was defined according to the findings of the systematic review, other literature sources and consensus of expert opinion (Table 24). First, men who had undergone pelvic lymphadenectomy as part of their radical prostatectomy and were found to have lymph node metastases on pathological examination of the removed lymph nodes were automatically selected for immediate further treatment. 178 The proportion of men who underwent lymphadenectomy and the proportion of those who were positive were assigned independently from other variables according to the observed rates following either type of surgery from literature sources validated by our expert panel. 177,179 Expert opinion deemed that all men with positive lymph nodes were assigned to further cancer treatment without the opportunity for a period of surveillance.
| Perioperative state | Probability | Lower limita | Upper limita | Assigned distribution | Source |
|---|---|---|---|---|---|
| Robotic surgery | |||||
| Positive margin | 0.163 | 0.119 | 0.225 | Log-normal | Systematic review |
| Lymph node metastases | 0.026 | 0.0195 | 0.0325 | Triangular | Kawakami 2006179 |
| Laparoscopic surgery | |||||
| Positive margin | 0.236 | 0.080 | 0.394 | Log-normal | Systematic review |
| Lymph node metastases | 0.026 | 0.0195 | 0.0325 | NA | Kawakami 2006179 |
Second, men who had two or more of the following features found on pathological examination of the removed prostate were considered for immediate further treatment:
-
positive surgical margin
-
Gleason score 8–10
-
tumour stage pT3–pT4.
If only one of these pathological disease characteristics was present the individual entered the surveillance pathway (Figure 17).
FIGURE 17.
Schematic representation of postoperative care pathway for men being considered for immediate further cancer treatment. A1, postoperative health state; A2, further cancer treatment state; A3, long-term adverse event dysfunction state; A4, surveillance state; BCR, biochemical recurrence.
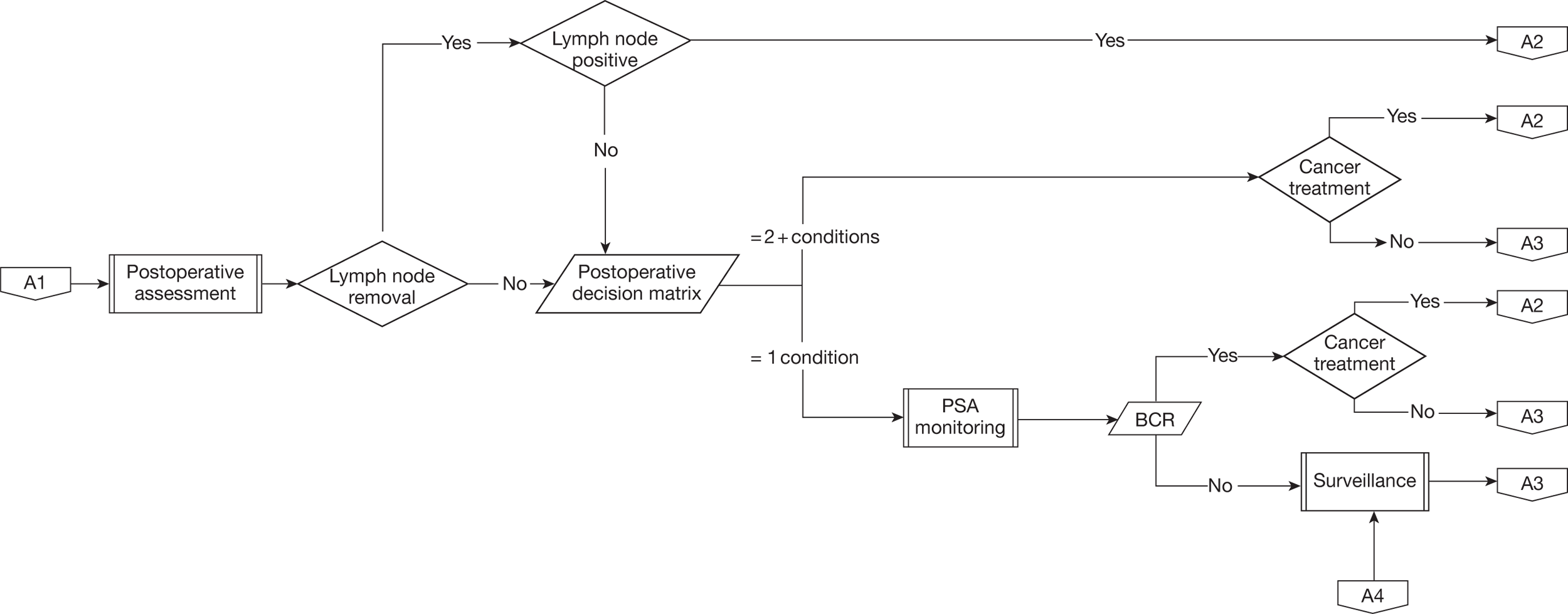
Parameterisation of this decision-based approach required linked data for individuals concerning the three features and this was not available from the systematic review. We therefore decided on the following approach. Linked values of postoperative Gleason sum score and postoperative tumour stage for 4669 individuals were kindly provided from a large single institutional database of men undergoing radical prostatectomy maintained at the Vanderbilt-Ingram Cancer Center, TN, USA (D Barocas, February 2011, personal communication). The numbers of men from this data set with each combination of Gleason sum score and tumour stage were then multiplied by the probability of men having a negative or positive surgical margin following robotic or laparoscopic prostatectomy defined by the systematic review and meta-analysis (see Table 24). The calculated patient numbers were then converted to percentages of the sample population, which defined the probability of each combination of the three variables (margin, Gleason sum score and tumour stage) for each procedure. These probabilities were then mapped to the decision matrix. The decision matrix, which directed the subsequent care pathway for individual men in the model, was formulated by rounds of consensus building with relevant members of the expert panel. The decision to be made was whether men would enter the surveillance state or proceed to further cancer treatment (Tables 25 and 26). The decision matrix gave total probabilities of 0.098 following robotic prostatectomy and 0.113 following laparoscopic prostatectomy for individual men requiring consideration for immediate further treatment.
| Margin status | Tumour stage | Gleason score | Number (%) of men in category | Probability of event in model | Management decision |
|---|---|---|---|---|---|
| Negative | Negative | Negative | 2900 (62.1) | 0.621 | Surveillance |
| Negative | Negative | Positive | 132 (2.8) | 0.028 | Surveillance |
| Negative | Positive | Negative | 612 (13.1) | 0.131 | Surveillance |
| Negative | Positive | Positive | 264 (5.6) | 0.056 | Treatment |
| Positive | Negative | Negative | 565 (12.1) | 0.121 | Surveillance |
| Positive | Negative | Positive | 26 (0.6) | 0.005 | Treatment |
| Positive | Positive | Negative | 119 (2.6) | 0.026 | Treatment |
| Positive | Positive | Positive | 51 (1.1) | 0.011 | Treatment |
| Margin status | Tumour stage | Gleason score | Number (%) of men in category | Probability of event in model | Management decision |
|---|---|---|---|---|---|
| Negative | Negative | Negative | 2647 (56.7) | 0.567 | Surveillance |
| Negative | Negative | Positive | 121 (2.6) | 0.026 | Surveillance |
| Negative | Positive | Negative | 558 (12.0) | 0.120 | Surveillance |
| Negative | Positive | Positive | 241 (5.2) | 0.052 | Treatment |
| Positive | Negative | Negative | 818 (17.5) | 0.175 | Surveillance |
| Positive | Negative | Positive | 37 (0.8) | 0.008 | Treatment |
| Positive | Positive | Negative | 173 (3.7) | 0.037 | Treatment |
| Positive | Positive | Positive | 74 (1.6) | 0.016 | Treatment |
Death due to causes other than prostate cancer
The age-related quarterly probability of non-prostate cancer-related mortality was obtained from actuarial tables published by the UK Office for National Statistics180 and was treated as a separate event from prostate cancer-related mortality.
Biochemical recurrence
The probability of biochemical recurrence was calculated for each 3-month time step according to the time since either prostatectomy or the most recent localised cancer event for men successfully treated for recurrent localised cancer by radical radiotherapy. The 12-month probability of biochemical recurrence was derived from the systematic review and then was assumed to decline exponentially according to published longer-term data (Table 27). 181 As described later, the use of selected alternative values for biochemical recurrence was explored in a sensitivity analysis.
| Variable | Value | Probability (quarterly) | Lower limita | Upper limita | Assigned distribution | Source |
|---|---|---|---|---|---|---|
| Biochemical recurrence rate | ||||||
| Biochemical recurrence event rate 1 year | 4.9% | 0.0125 | 0.0094 | 0.0156 | Triangular | Menon 2010181 |
| Biochemical recurrence event rate 3 years | 9.4% | 0.0109 | 0.0082 | 0.0136 | Triangular | Menon 2010181 |
| Biochemical recurrence event rate 5 years | 13.4% | 0.0095 | 0.0072 | 0.0119 | Triangular | Menon 2010181 |
| Biochemical recurrence event rate 7 years | 18.9% | 0.0099 | 0.0074 | 0.0124 | Triangular | Menon 2010181 |
| Further cancer treatment | ||||||
| Radiotherapy | 20.0% | NA | 0.150 | 0.250 | Triangular | Moreira 2010182 |
| Androgen deprivation therapy | 21.0% | NA | 0.158 | 0.263 | Triangular | Moreira 2010182 |
| Combined treatment | 10.0% | NA | 0.075 | 0.125 | Triangular | Moreira 2010182 |
| Surveillance | 49.0% | NA | 0.368 | 0.613 | Triangular | Moreira 2010182 |
| Prostate cancer mortality | ||||||
| Cancer-specific survival | NA | 0.76 | 0.69 | 0.83 | Triangular | Bria 2009183 |
| Overall survival | NA | 0.86 | 0.80 | 0.93 | Triangular | Bria 2009183 |
At each decision point the individual would continue surveillance without recurrence or experience a biochemical recurrence leading to further treatment or die from causes other than prostate cancer. In base-case simulations with a 10-year time horizon an individual could remain in the surveillance state or else be in a recurrence state at the end of the simulation and would be recorded as surviving without or with recurrent cancer respectively. If biochemical recurrence occurred, this was recorded before initiating the further cancer treatment process. Each time step that an individual spent under surveillance incurred a utility and a cost (described in Costs and Utilities).
Cancer treatment allocation
Men with pathologically involved lymph nodes or with two or more adverse pathological characteristics listed earlier were immediately assigned to the cancer treatment process following prostatectomy (Figure 18). The extent of the likely residual disease was defined as localised or systemic (metastatic) and this was randomly determined according to known probabilities using the same method described in Modelling of discrete events; this was independent of the precise cancer state variables (see Table 27). A similar process was used for men who underwent an initial period of surveillance and then suffered biochemical recurrence.
FIGURE 18.
Schematic diagram for the further cancer treatment care pathway. A2, cancer treatment health state; A3, long-term adverse event dysfunction health state; A4, surveillance health state.
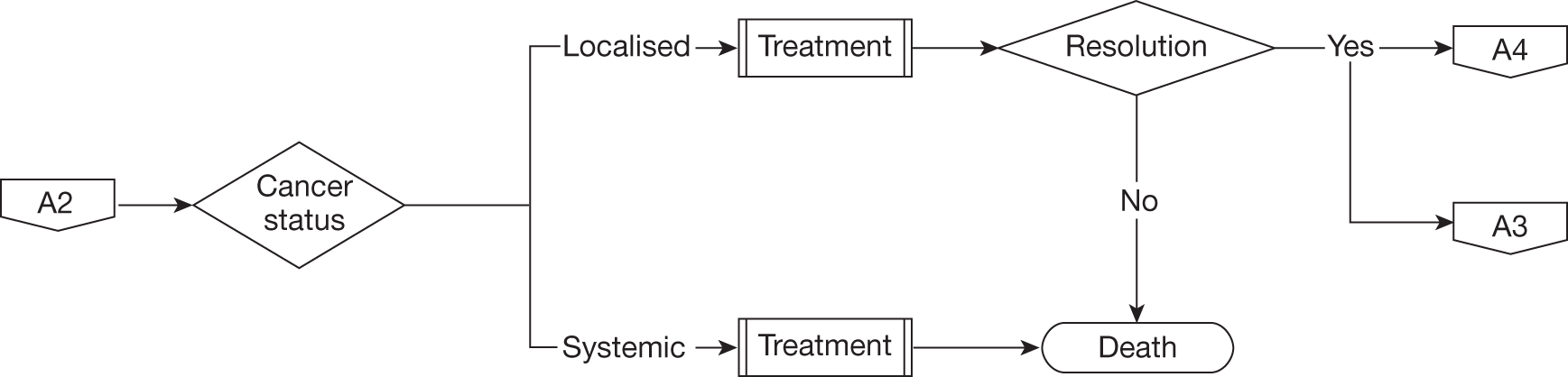
Localised cancer treatment
Diagnosis of persistent or recurrent cancer localised to the prostatic bed was an event with three outcomes. First, further cancer treatment in the form of radical radiotherapy with or without a 6-month course of androgen deprivation therapy could be successful, resulting in the remission event; these men then returned to the surveillance process. Second, further cancer treatment could be unsuccessful, leading to metastases, further treatment for systemic cancer by lifelong androgen deprivation therapy and cancer-related death. The probability of either of these two events was determined by survival rates from the literature concerning radical radiotherapy used to treat localised recurrence after prostatectomy (see Table 27). Finally, the individual could suffer non-prostate cancer-related mortality before completing treatment. For the base-case simulation individuals could be in the further cancer treatment state at the end of the 10-year period and were considered to be survivors with prostate cancer recurrence. The time from further cancer treatment and remission or cancer-related death was randomly determined according to rates of survival obtained from the literature.
Systemic (metastatic) cancer treatment
Diagnosis of systemic cancer was an event occurring because of unfavourable disease characteristics such as positive lymph nodes in the immediate postoperative period or because of failure of radical radiotherapy for localised recurrence or following the process of biochemical recurrence. Such men were treated with androgen deprivation therapy (medical castration) until cancer-related death, the only outcome possible. In the base-case simulation with a 10-year time horizon it was possible for men to survive if they remained in the systemic cancer treatment state at the end of the 10 years; the duration of survival while on treatment for systematic cancer was randomly determined according to known metastatic prostate cancer mortality rates (see Table 27).
Persistent adverse event states
Introduction
The incidence of the considered postoperative adverse events or dysfunctions – bladder neck contracture, urinary incontinence and erectile dysfunction – was defined according to the standard parameterisation hierarchy described above. Management of these postoperative dysfunctions was modelled by treating them as independent processes. If dysfunctions were found to be present, self-management and/or treatment began immediately according to current clinical practice (Figures 19 and 20). Each of the three dysfunction-related state variables was recorded as a categorical variable encoding the presence or absence of the pathological condition. These three variables were randomly determined to be present according to the observed rates following either type of surgery defined by the systematic review, other literature source or expert opinion (Table 28). We assumed that there was no systematic co-occurrence of dysfunctions, so they were assigned independently. In this way it was possible for an individual to experience each dysfunction simultaneously.
FIGURE 19.
Schematic showing care pathway for individuals in long-term adverse event state with bladder neck contracture, urinary incontinence and erectile dysfunction. A3, adverse event state; B1, treatment of adverse event.
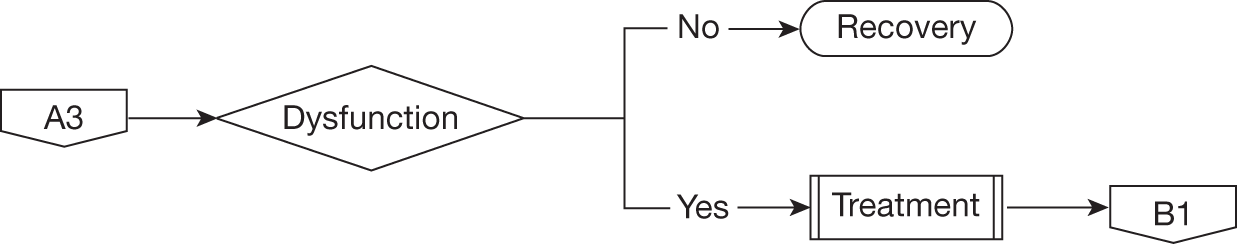
FIGURE 20.
Expanded care pathway for the management and treatment of individuals incurring long-term postoperative adverse events. A4, surveillance health state; B1, treatment of long-term adverse events.
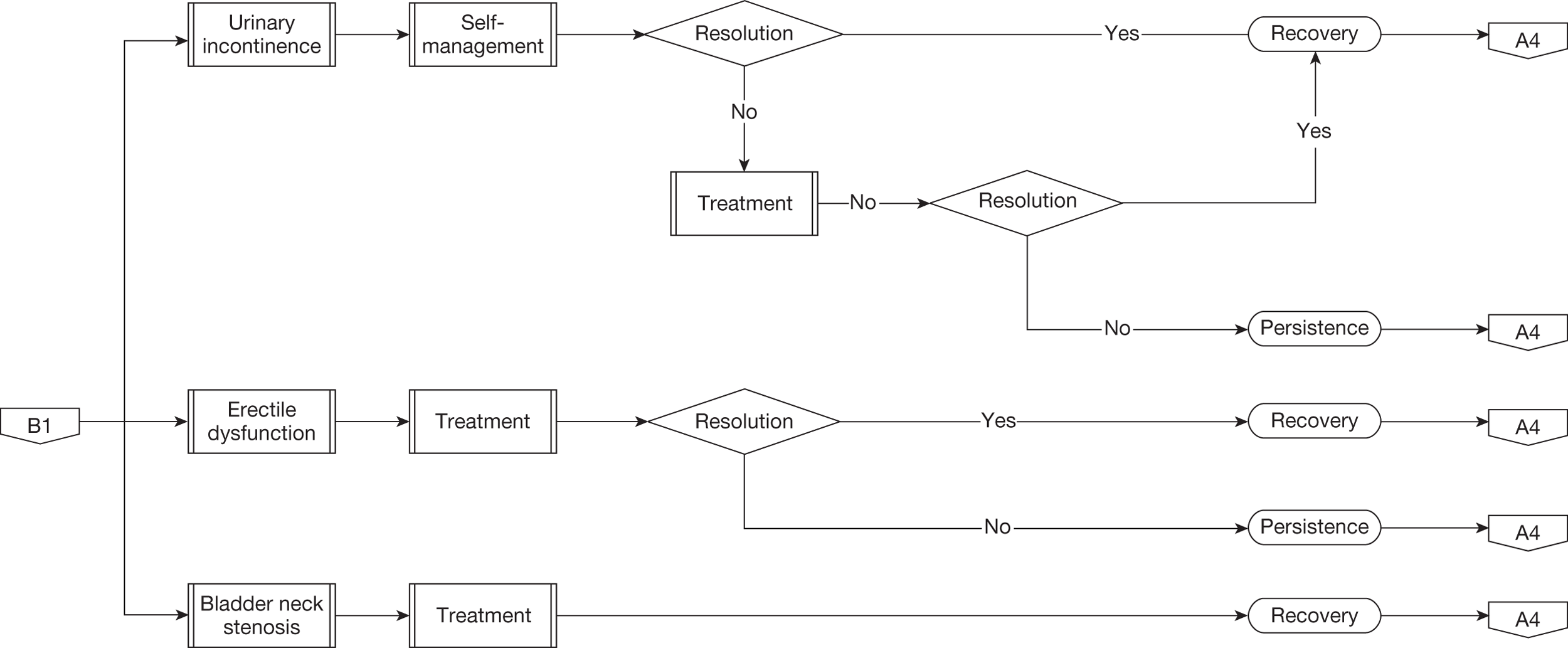
| Longer-term adverse event | Value | Probability | Lower limita | Upper limita | Assigned distribution | Source |
|---|---|---|---|---|---|---|
| Bladder neck contracture | ||||||
| Procedure rate robotic | 0.008 | 0.002 | 0.052 | Log-normal | Systematic review | |
| Procedure rate laparoscopic | 0.021 | 0.008 | 0.150 | Log-normal | Systematic review | |
| Urinary dysfunction management | ||||||
| Self-management < 1 year robotic | 0.043 | 0.007 | 0.224 | Log-normal | Systematic review | |
| Self-management success at 1 year | 0.957 | 0.720 | 1.000 | Log-normal | MAPS cohort77 | |
| Self-management < 1 year laparoscopic | 0.079 | 0.000 | 0.357 | Log-normal | Systematic review | |
| Surgical implantation of AUS | 5.20% | 3.90% | 6.50% | Triangular | Clinical expert panel | |
| AUS success rate | 90.00% | 67.50% | 100.00% | Triangular | Clinical expert panel | |
| Erectile dysfunction | ||||||
| Erectile dysfunction at 6 months | 80.20% | 60.00% | 100.00% | Triangular | Stanford 2000187 | |
| Erectile dysfunction at 1 year | 71.80% | 54.00% | 90.00% | Triangular | Stanford 2000187 | |
| Erectile dysfunction at 2 years | 59.90% | 45.00% | 75.00% | Triangular | Stanford 2000187 | |
| Erectile dysfunction management | ||||||
| Treatment for erectile dysfunction | 57.00% | 42.80% | 71.30% | Triangular | MAPS cohort (table 7.9)77 | |
| Reduction in erectile dysfunction treatment rate at 1 year | 50.00% | 37.50% | 62.50% | Triangular | Matthew 2005188 | |
| Sildenafil: 100 mg once weekly | 82.20% | 61.70% | 100.00% | Triangular | Schover 2002189 | |
| Sildenafil success rate overall | 31.00% | 0.690 | 23.30% | 38.80% | Triangular | Blander 2000190 |
| Alprostadil: 20 µg once weekly | 15.40% | 11.60% | 19.30% | Triangular | Schover 2002189 | |
| Alprostadil success rate overall | 57.10% | 0.429 | 42.80% | 71.40% | Triangular | Costabile 1998191 |
| Penile prosthesis implantation | 0.24% | 0.20% | 0.30% | Triangular | Schover 2002189 | |
| Penile prosthesis success rate | 92.00% | 69.00% | 100.00% | Triangular | Meuleman 2003192 | |
Bladder neck contracture
All men who suffered bladder neck contracture (stenosis) were assumed to require treatment during the first quarter time step following radical prostatectomy. The intervention required was taken to be endoscopic bladder neck incision. This event incurred a one-off cost that was included in the first-year costs for that individual, and an appropriate utility value was assigned to the quarter during which the individual suffered the condition (see Costs and Utilities). Discussion within our expert group suggested that recovery was likely to occur in most cases following a single treatment and this was supported by the available literature. 70 For the purposes of the model we therefore chose to assume that recovery occurred after a single incision in all cases with no continuing costs and utility returned to that of the surveillance state. We acknowledge, however, that this is likely to be a simplification of day-to-day patient care.
Urinary incontinence
In the second quarter immediately following their prostatectomy, men with moderate or severe urinary incontinence commenced self-management using containment pads, which incurred a cost and was associated with a specific utility value every quarter. There were three outcomes allowed for this self-management: spontaneous recovery, further surgery consisting of insertion of an AUS, or a persistent state that remained until the end of the studied time horizon or the man’s death and continued to accrue costs and associated disutility. The probability of the first two outcomes was assessed at each time step; if neither event occurred then the patient remained in a state of persistent incontinence. Men who recovered ceased to incur a cost and their utility was returned to that of the surveillance state. Men with persistent incontinence were eligible for insertion of an artificial sphincter as long as they had spent at least 12 months in the surveillance state since prostatectomy without biochemical recurrence, were not currently undergoing cancer treatment and had not previously undergone unsuccessful sphincter insertion. Surgical insertion of an artificial sphincter resulted in either recovery (success) or persistence (failure) of urinary incontinence according to published success rates of this surgery. The surgery incurred a one-off cost that was assigned to that year’s total cost for the individual. We chose to assume that implantation of an artificial sphincter would continue to successfully resolve symptoms throughout the studied time horizon without the need for any further treatment of incontinence. The proportion of men suffering recurrent incontinence after initial successful implantation is approximately 25% at 5 years but given the low overall probability of need for this device and the lack of difference in incontinence rates between the procedures under study we elected not to build this failure rate into our model. 184
Erectile dysfunction
Immediately following prostatectomy men who suffered bothersome erectile dysfunction were assigned to either self-management or drug therapy, incurring extra costs if relevant and associated with a defined utility value every quarter. Costs for drug treatments were obtained from the British National Formulary185 whereas cost information relating to surgical intervention was obtained from the Department of Health’s reference costs 2008–9. 186 Self-management was defined as no active treatment. Men undergoing drug therapy were assumed to be taking either oral medication, with sildenafil (Viagra®, Pfizer Inc., USA) being the index drug, or intrapenile medication, with intracavernosal injection of alprostadil (Caverject®, Pfizer Inc., USA) being the index treatment. The rates of use of these options were obtained from relevant literature. There were three outcomes of both self-management and drug therapy: the man could recover, undergo surgical implantation of a penile prosthesis to cure erectile dysfunction or enter a persistent state of continued self-management or drug use that remained until the end of the time horizon or the man’s death. The probability of the first two outcomes was assessed at each time step; if neither event occurred then the patient remained in a state of persistent erectile dysfunction. Men who recovered ceased to incur a cost and their utility returned to that of the cancer surveillance state. Individuals were eligible for penile prosthesis implantation if after at least 12 months of surveillance they did not have a biochemical recurrence, were not currently undergoing cancer treatment and had not already had a penile prosthesis implanted. Implantation of a penile prosthesis resulted in either recovery of erectile function or a persistent state, which was determined according to the success rates of this surgery published in the literature. The surgery incurred a one-off cost assigned to that year’s total cost for the specific individual.
Costs
Perioperative costs
General
A general cost for the standard length of hospital stay was derived from the relevant excess NHS bed-day cost tariff for the procedure (LB22Z) of £255186 multiplied by the average hospital stay for robotic/laparoscopic prostatectomy within the NHS of 3.48 days obtained from hospital episode statistics for 2008–9. 48 Hospital stay estimates from the systematic review were not used because they derived from a number of different heath-care systems. A cost per hour of NHS operating theatre time was derived from the baseline information calculated from General Hospital (Acute) obtained from ISD (Information Services Division) Scotland Theatre Services R140193 (Table 29). This was then multiplied by the duration of laparoscopic and robotic prostatectomy derived from the systematic review (see Table 22). The cost of pathological examination of the removed prostate and lymph nodes of £329.82 was obtained from the Newcastle upon Tyne Hospitals NHS Foundation Trust (D Evans, May 2010, personal communication).
| Variable | Mean (£) | Median (£) | Minimum (£) | Maximum (£) |
|---|---|---|---|---|
| Operating theatre cost per hour | 1155.79 | 1051.11 | 376.7 | 2574.06 |
Equipment costs
The cost of undertaking one procedure using either intervention was obtained by adding together the basic unit cost of each surgical system, the cost of any specialised surgical equipment and the cost of any consumables. These costs were then adjusted for the lifetime of the equipment and by the number of cases performed per year to obtain a cost for each procedure. This cost did vary with the number of procedures performed in each centre per year, principally because the contribution of capital equipment costs was different.
The specific costs to the NHS in terms of specialised equipment were obtained from individual NHS units carrying out the procedures, including hospitals in Aberdeen, Cambridge and Newcastle upon Tyne, UK. The list of reusable equipment and consumables used during a laparoscopic radical prostatectomy came from the Newcastle upon Tyne Hospitals NHS Foundation Trust (Maggie Birkbeck, Urology Theatre Manager, personal communication, June 2010). UK costs for the robotic system and ancillary devices or instruments were obtained from the manufacturer of the da Vinci system, Intuitive Surgical. 30 For the robotic system we chose to use for the base-case analysis the capital and maintenance costs of the most expensive system available (a four-arm manipulator and two consoles) but also performed sensitivity analyses using the least costly system available. For both procedures the process of calculating costs involved summing the following costs per procedure: unit cost + service contract cost (for robotic procedure only) + specialised equipment cost + consumables cost.
For the robotic system, as an alternative to outright purchase, various permutations of payment and leasing plans were considered, such as payments spread over differing number of years, paid either in advance or in arrears. The cost per procedure varied markedly between these payment options; it also varied by the anticipated throughput of patients per annum. The cost per procedure according to number of procedures performed per year using the equipment purchase plan defined for the base-case analysis is shown in Table 30. These costs are based on the use of the most expensive system option consisting of a four-arm manipulator and two consoles and are calculated on the basis of different throughputs, with 200 cases per year representing a maximum number and 50 cases per year representing the throughput of one of the smaller UK centres. These costs represent the higher range of expected costs of equipment and in sensitivity analysis we explore the impact of using less expensive system options. The costs of laparoscopic equipment were similarly estimated. For laparoscopic equipment we have assumed that reusable equipment was reused 200 times per year. The cost per procedure of laparoscopic equipment was £94.48. Appendices 12 and 13 describe the equipment costs in detail for both robotic and laparoscopic surgery.
| Total system cost (£) | Number of procedures | Service life | Cost per procedure (£) | Cost of surgical equipment (£) | Cost of consumables (£) | Total cost (£) |
|---|---|---|---|---|---|---|
| 3,090,000 | 200 | 7 | 2207.14 | 66.10 | 1194.11 | 3467.35 |
| 3,090,000 | 150 | 7 | 2942.86 | 88.14 | 1194.11 | 4225.11 |
| 3,090,000 | 100 | 7 | 4414.29 | 132.21 | 1194.11 | 5740.61 |
| 3,090,000 | 50 | 7 | 8828.57 | 264.42 | 1194.11 | 10,287.10 |
Costs associated with perioperative adverse events
As described in Model health states and associated parameter values, Perioperative state, perioperative adverse events were categorised using the Clavien–Dindo classification. For each Clavien level a judgement was made by the project team and expert panel about the implications for further care of a particular adverse event occurring (Table 31). This extra care was categorised in terms of the extra length of stay that an individual would undergo, which was combined with information on the cost of an additional day in hospital186 to obtain a cost of each adverse event. A similar process was followed for the cost of conversion to open surgery.
| Perioperative adverse event: | Unit cost (£)a | Equivalent cost of Clavien–Dindo risk factor/conversion (£) | Number of extra bed-days |
|---|---|---|---|
| Clavien level I | 255.00 | 255.00 | 1 |
| Clavien level II | 255.00 | 510.00 | 2 |
| Clavien level IIIa | 255.00 | 765.00 | 3 |
| Clavien level IIIb | 255.00 | 765.00 | 3 |
| Clavien level IVa | 255.00 | 1020.00 | 4 |
| Conversion to open surgery | 255.00 | 765.00 | 3 |
Costs associated with postoperative care
Surveillance
The cost of a single PSA test at £5.91 was obtained from the Newcastle upon Tyne Hospitals NHS Foundation Trust laboratory services directorate and applied throughout the period of surveillance according to the defined follow-up schedule (Table 32).
| PSA testing | Number of units per year | Unit cost (£)a | Cost per year (£) |
|---|---|---|---|
| During first year | 4 | 5.91 | 23.64 |
| Beyond year 1 | 1 | 5.91 | 5.91 |
The costs of further cancer treatment were derived from the tariff applied to the relevant HRG code186 in the case of radiotherapy and from the British National Formulary185 in the case of drug treatments. The one-off cost used for radiotherapy was calculated on the basis of 33 treatments at £135 = £4455. The cost of androgen deprivation therapy was based on an initial 14-day course of cyproterone acetate at £63.08 followed by a monthly cost for the LHRH agonist goserelin acetate (Zoladex®, Astra Zeneca) of £403.80, which was continued for the specified duration of treatment (6 months for localised recurrent cancer and lifelong for systemic recurrent cancer) (Table 33).
| Cancer treatment | Unit cost (£) |
|---|---|
| 33 sessions of radiotherapy | 4455.00a |
| Monthly cost of goserelin acetate | 403.80185 |
| 14-day course of cyproterone acetate | 63.08185 |
The costs of treatment of adverse events beyond the perioperative period were again derived from the relevant NHS tariff through the HRG code186 or from the British National Formulary185 or from a recent HTA-funded trial of conservative treatment for urinary incontinence after prostatectomy (men after prostate surgery trial, MAPS; C Glazener, Aberdeen University 2011, personal communication; Table 34). 77 We did not apply costs related to outpatient visits for follow-up or GP visits for associated care. Patient costs and societal costs were also not included.
| Long-term adverse event | Unit cost (£) |
|---|---|
| Bladder neck contracture | |
| Bladder neck incision (HRG LB27Z) | 1269.00a |
| Urinary incontinence | |
| Self-management per year | 263.5977 |
| Implantation of AUS (HRG LB50Z) | 3928.00a |
| AUS device | 4918.00a |
| Erectile dysfunction | |
| Sildenafil 100 mg once weekly | 5.88185 |
| Alprostadil 20 µg once weekly | 11.94185 |
| Penile prosthesis implantation (HRG LB47Z) | 2262.00a |
| Penile prosthesis device | 5023.00a |
Utilities
A utility value was assigned to each individual in each 3-month time step over the 10-year or lifetime horizon. The utility value encompassed the cancer management state (surveillance, biochemical recurrence, localised cancer, systemic cancer) and the longer-term adverse event state (bladder neck contracture, urinary incontinence and erectile dysfunction) (Table 35). Individuals present in more than one state during any 3-month step – localised recurrence and urinary incontinence, for example – were assigned a utility value equal to the product of the utility values applying to each of the states.
| Variable | Value | Lower limita | Upper limita | Assigned distribution | Source |
|---|---|---|---|---|---|
| General states – surveillance | |||||
| Postoperative state 1 year | 0.900 | 0.750 | 1.000 | Triangular | Korfage 2005194 |
| Death | 0 | Triangular | |||
| Further cancer treatment | |||||
| Biochemical recurrence | 0.730 | 0.548 | 0.913 | Triangular | Cowen 1998195 |
| Localised recurrence | 0.820 | 0.660 | 0.984 | Triangular | Korfage 2005194 |
| Systemic recurrence | 0.420 | 0.311 | 0.529 | Triangular | Cowen 1998195 |
| Longer-term adverse event | |||||
| Bladder neck contracture | 0.720 | 0.560 | 0.930 | Triangular | Volk 2004196 |
| Urinary incontinence | 0.830 | 0.750 | 1.000 | Triangular | Volk 2004196 |
| Erectile dysfunction | 0.840 | 0.770 | 1.000 | Triangular | Volk 2004196 |
Data analysis
The model compared effectiveness and cost-effectiveness [defined as incremental cost per quality-adjusted life-year (QALY)] for robotic compared with laparoscopic radical prostatectomy. The timing and nature of each event was recorded, allowing the construction of individual trajectories through the care pathways. When processes incurred costs, these were added to the total costs accrued for that patient in that year. When processes led to a change in utility then the value of that new utility was multiplied by the current QALYs for that patient in that year. Estimates of the mean costs, QALYs and incremental cost per QALY were obtained by simulating the outcomes for a group of 5000 men for each treatment. In the base-case analysis the time horizon has been taken to be 10 years. Both costs and QALYs are discounted at 3.5%. 197
Variations around the estimates of mean costs and QALYs were obtained by producing 1000 bootstrap estimates for mean costs and QALYs for each treatment. These data were then used to produce cost-effectiveness acceptability curves (CEACs). In the base-case analysis CEACs have been used to illustrate the imprecision surrounding the results caused by the variation in care and events experienced by the men modelled. These curves illustrate the likelihood that a strategy is cost-effective at various threshold values for society’s willingness to pay for an additional QALY. The CEACs are the product of a probabilistic analysis. In this analysis we have assumed that each of the parameters is associated with a degree of imprecision, as described in each of the data input tables, characterised by a triangular distribution. This distribution was chosen as the data available to inform an alternative distributional form were sparse.
Sensitivity analyses
Extension of the time horizon to 40 years
In this sensitivity analysis we explored the impact of extending the time horizon. Conceptually this should allow more time for any benefits of robotic surgery to offset the increased procedure costs.
Changes in the costs of robotic equipment
Robotic equipment comes in several different variants and can be obtained from the manufacturers using several different payment plans. The precise cost of each of these variants may vary between provider and Appendix 12 provides illustrative examples of the cost variants. These costs have been converted into an annual cost, assuming the manufacturers’ recommended lifespan of the equipment of 7 years, and a cost per procedure estimated. In this analysis we explore what the impact on the incremental cost per QALY is of using a lower cost for the robotic system. This analysis has been repeated for the different numbers of annual cases performed (from 50 per year to 200 per year). From these results it was possible to determine the effect on estimates of cost-effectiveness of varying the cost per procedure of robotic prostatectomy consequent to any particular payment plan or throughput.
Changes in the risk of having a positive margin
The estimates of positive margin rates following robotic and laparoscopic surgery were based on the point estimates derived from the systematic review. In this sensitivity analysis we explored the impact of using both the lower and the upper 95% CrI limits of the OR of the difference in positive margin rates between robotic and laparoscopic surgery (base-case OR 0.69; 95% CrI 0.506 to 0.955). The further cancer treatment matrices defined by using the lower and higher risks of having a positive margin following robotic surgery are shown in Tables 36 and 37, respectively. The probabilities for laparoscopic surgery remained the same as in the base case.
| Margin status | Tumour stage | Gleason score | Number (%) of men in category | Probability of event in model | Management decision |
|---|---|---|---|---|---|
| Negative | Negative | Negative | 3053 (65.4) | 0.654 | Surveillance |
| Negative | Negative | Positive | 139 (3.0) | 0.030 | Surveillance |
| Negative | Positive | Negative | 664 (13.8) | 0.138 | Surveillance |
| Negative | Positive | Positive | 278 (5.9) | 0.059 | Treatment |
| Positive | Negative | Negative | 412 (8.8) | 0.088 | Surveillance |
| Positive | Negative | Positive | 19 (0.4) | 0.004 | Treatment |
| Positive | Positive | Negative | 87 (1.9) | 0.019 | Treatment |
| Positive | Positive | Positive | 37 (0.8) | 0.008 | Treatment |
| Margin status | Tumour stage | Gleason score | Number (%) of men in category | Probability of event in model | Management decision |
|---|---|---|---|---|---|
| Negative | Negative | Negative | 2685 (59.59) | 0.575 | Surveillance |
| Negative | Negative | Positive | 122 (2.72) | 0.026 | Surveillance |
| Negative | Positive | Negative | 567 (12.57) | 0.121 | Surveillance |
| Negative | Positive | Positive | 244 (5.42) | 0.052 | Treatment |
| Positive | Negative | Negative | 780 (14.62) | 0.167 | Surveillance |
| Positive | Negative | Positive | 36 (0.67) | 0.008 | Treatment |
| Positive | Positive | Negative | 164 (3.08) | 0.035 | Treatment |
| Positive | Positive | Positive | 71 (1.33) | 0.015 | Treatment |
Combining change in costs per procedure and positive margin rates
In this analysis we explored the impact on the incremental cost per QALY of changes in both the cost per procedure and the risk of a positive margin. These data have been presented as plots of the incremental cost per QALY against the positive margin rate, defined in terms of an OR, for different numbers of procedures performed per year.
Changes in the risk of biochemical recurrence
In the base-case analysis it was assumed that the risk of biochemical recurrence was the same regardless of which procedure a man received. The rationale behind this assumption was that the meta-analysis reported in Chapter 4 provided no evidence of any difference; the CI surrounding the OR was wide and included 1. In the first sensitivity analysis concerning biochemical recurrence rates we assumed that on average robotic surgery was associated with a lower rate of biochemical recurrence. This lower rate was estimated by combining the long-term rates from Menon and colleagues181 with the point estimate of the OR for risk of biochemical recurrence at 12 months obtained from the systematic review (0.89). The CIs around the OR were not clinically plausible and therefore we assumed a triangular distribution with upper and lower limits for the 12-month risk of biochemical recurrence for robotic surgery set at ±2% (based on the finding of Menon and colleagues181; Table 38).
| Variable | Probability | Lower limita | Upper limita |
|---|---|---|---|
| Robotic surgery | |||
| Biochemical recurrence event rate 1 year | 0.0112 | 0.0084 | 0.0140 |
| Biochemical recurrence event rate 3 years | 0.0097 | 0.0073 | 0.0121 |
| Biochemical recurrence event rate 5 years | 0.0085 | 0.0064 | 0.0106 |
| Biochemical recurrence event rate 7 years | 0.0088 | 0.0066 | 0.0110 |
| Laparoscopic surgeryb | |||
| Biochemical recurrence event rate 1 year | 0.0125 | 0.0094 | 0.0156 |
| Biochemical recurrence event rate 3 years | 0.0109 | 0.0082 | 0.0136 |
| Biochemical recurrence event rate 5 years | 0.0095 | 0.0072 | 0.0119 |
| Biochemical recurrence event rate 7 years | 0.0099 | 0.0074 | 0.0124 |
In a second sensitivity analysis around the risk of biochemical recurrence we explored the impact of there being a higher rate of biochemical recurrence. The rationale behind this analysis was that the rates reported by Menon and colleagues181 were approximately 50% of those predicted in the meta-analysis. Therefore, in this sensitivity analysis we have simply doubled the rates observed by Menon and colleagues181 (Table 39).
| Variable | Probability | Lower limita | Upper limita |
|---|---|---|---|
| Robotic surgery | |||
| Biochemical recurrence event rate 1 year | 0.0222 | 0.0167 | 0.0278 |
| Biochemical recurrence event rate 3 years | 0.0164 | 0.0123 | 0.0205 |
| Biochemical recurrence event rate 5 years | 0.0170 | 0.0127 | 0.0212 |
| Biochemical recurrence event rate 7 years | 0.0177 | 0.0133 | 0.0221 |
| Laparoscopic surgery | |||
| Biochemical recurrence event rate 1 year | 0.0250 | 0.0187 | 0.0312 |
| Biochemical recurrence event rate 3 years | 0.0218 | 0.0164 | 0.0273 |
| Biochemical recurrence event rate 5 years | 0.0191 | 0.0143 | 0.0239 |
| Biochemical recurrence event rate 7 years | 0.0199 | 0.0149 | 0.0248 |
Chapter 6 Results of the health economic evaluation
Base-case analysis
In the base-case analysis robotic surgery was compared with laparoscopic surgery over a 10-year time horizon under the scenario that a centre with a single robot would perform 200 procedures per year and was using a da Vinci Si HD Dual Console that was purchased outright. Under this scenario, robotic surgery is more costly (primarily because of the cost of the equipment) but more effective (primarily because of the lower risk of having a positive margin). As a consequence, the incremental cost per QALY gained from robotic compared with laparoscopic surgery is £18,329, well below the threshold typically adopted by the National Institute for Health and Clinical Excellence (NICE) (Table 40). 197 These data do not suitably illustrate the uncertainty surrounding the costs and QALYs and the incremental cost per QALY. This is illustrated in the plot of cost and QALY pairs for each individual in the cohort for each treatment (Figure 21). Further details of the distribution of costs and QALYs are shown in Figure 22; here, density plots compare the distribution of costs and QALYs for each sample of 5000 men who received each intervention.
| Surgical capacity | Intervention | Mean cost (£) | Mean QALYs | ICER (£) | Probability cost-effective at different threshold values for WTP per QALY | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| 200 | Robotic | 9040 | 6.517 | 18,329 | 0.00 | 0.03 | 0.56 | 0.79 | 0.92 |
| Laparoscopic | 7628 | 6.440 | 1.00 | 0.97 | 0.44 | 0.21 | 0.08 | ||
| 150 | Robotic | 9799 | 6.517 | 28,172 | 0.00 | 0.00 | 0.20 | 0.53 | 0.82 |
| Laparoscopic | 7628 | 6.440 | 1.00 | 1.00 | 0.80 | 0.47 | 0.18 | ||
| 100 | Robotic | 11,312 | 6.517 | 47,822 | 0.00 | 0.00 | 0.00 | 0.11 | 0.52 |
| Laparoscopic | 7628 | 6.440 | 1.00 | 1.00 | 1.00 | 0.89 | 0.48 | ||
| 50 | Robotic | 15,859 | 6.517 | 106,839 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Laparoscopic | 7628 | 6.440 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 200a | Robotic | 8168 | 6.517 | 7009 | 0.00 | 0.72 | 0.93 | 0.96 | 0.97 |
| Laparoscopic | 7628 | 6.440 | 1.00 | 0.28 | 0.07 | 0.04 | 0.03 | ||
FIGURE 21.
Plot of costs and QALYs for each sample of 5000 men who received each intervention.
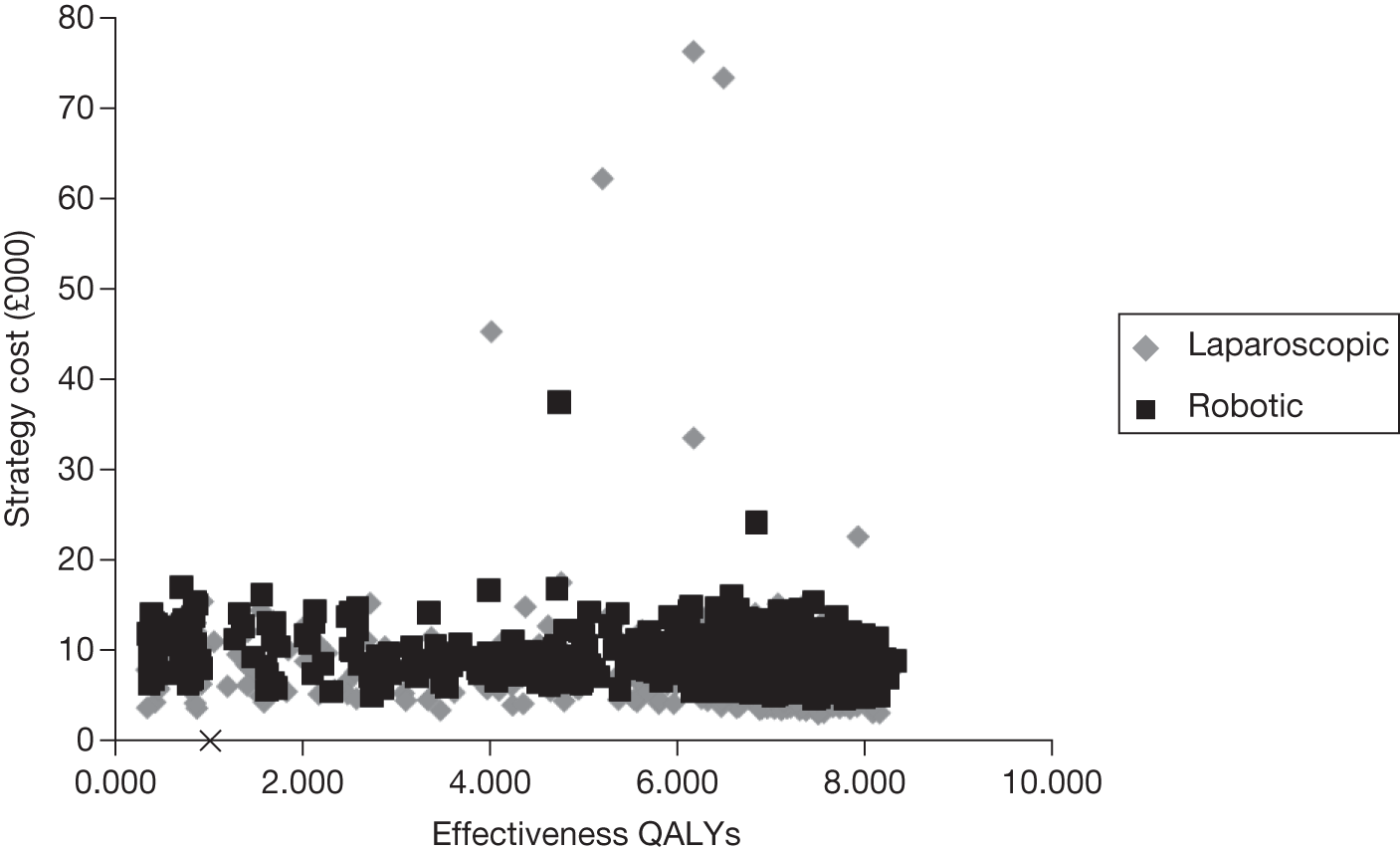
FIGURE 22.
Density charts describing the distribution of total costs (a) and QALYs (b) for the cohorts of modelled men.
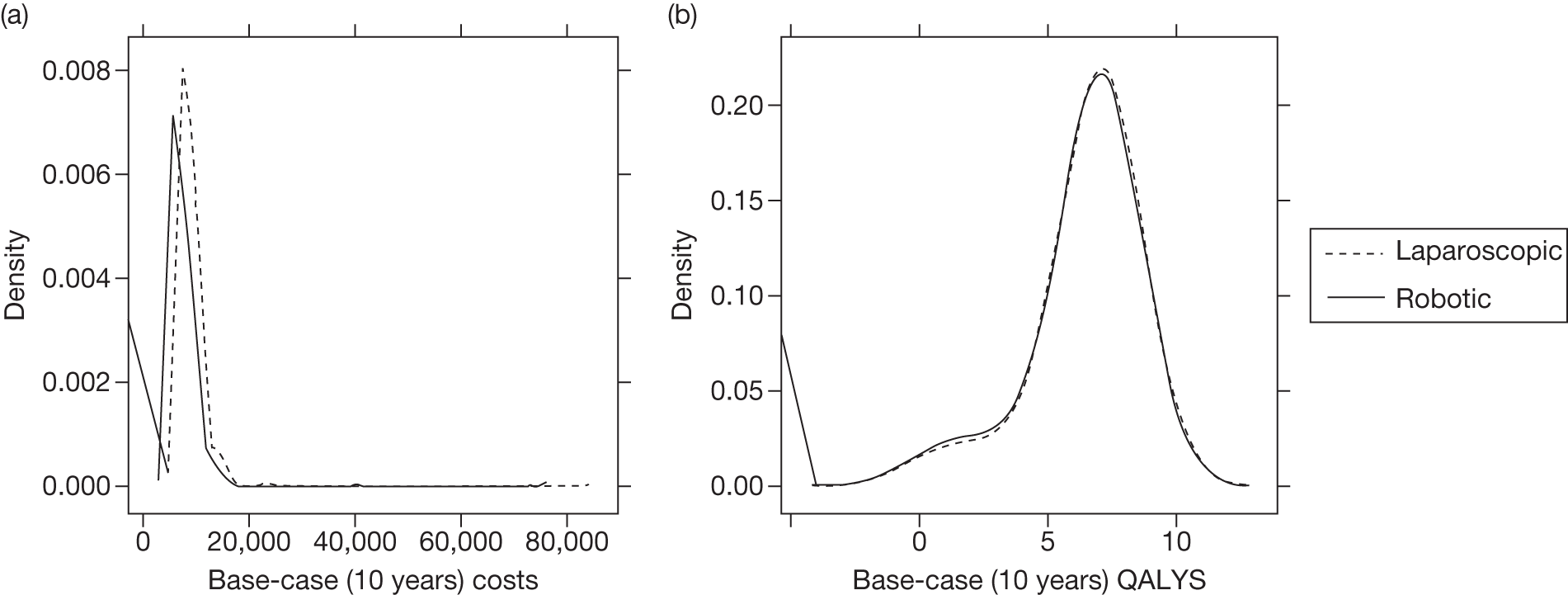
Figure 23 shows the plot of bootstrapped estimated mean costs and QALYs for each treatment; as this figure shows, it appears likely that the robotic surgery is both more costly and more effective than laparoscopic surgery. Thus, as Figure 24 illustrates, the robotic surgery has an approximately 95% chance of being considered cost-effective compared with laparoscopic surgery when society’s maximum willingness to pay for a QALY is £30,000.
FIGURE 23.
Plot of bootstrapped estimated mean costs and QALYs for each treatment for the base-case analysis.
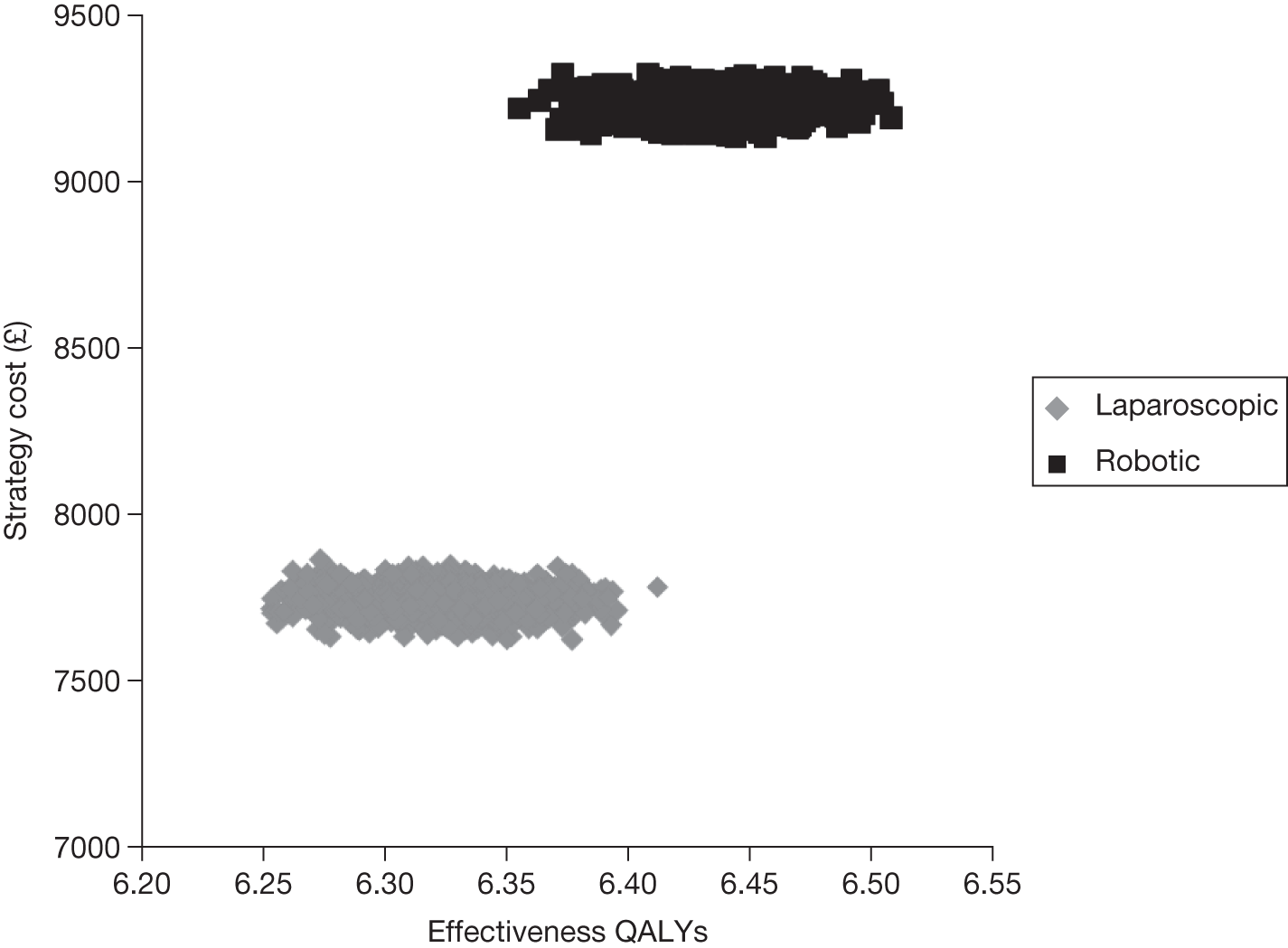
FIGURE 24.
Cost-effectiveness acceptability curves for the base-case analysis.
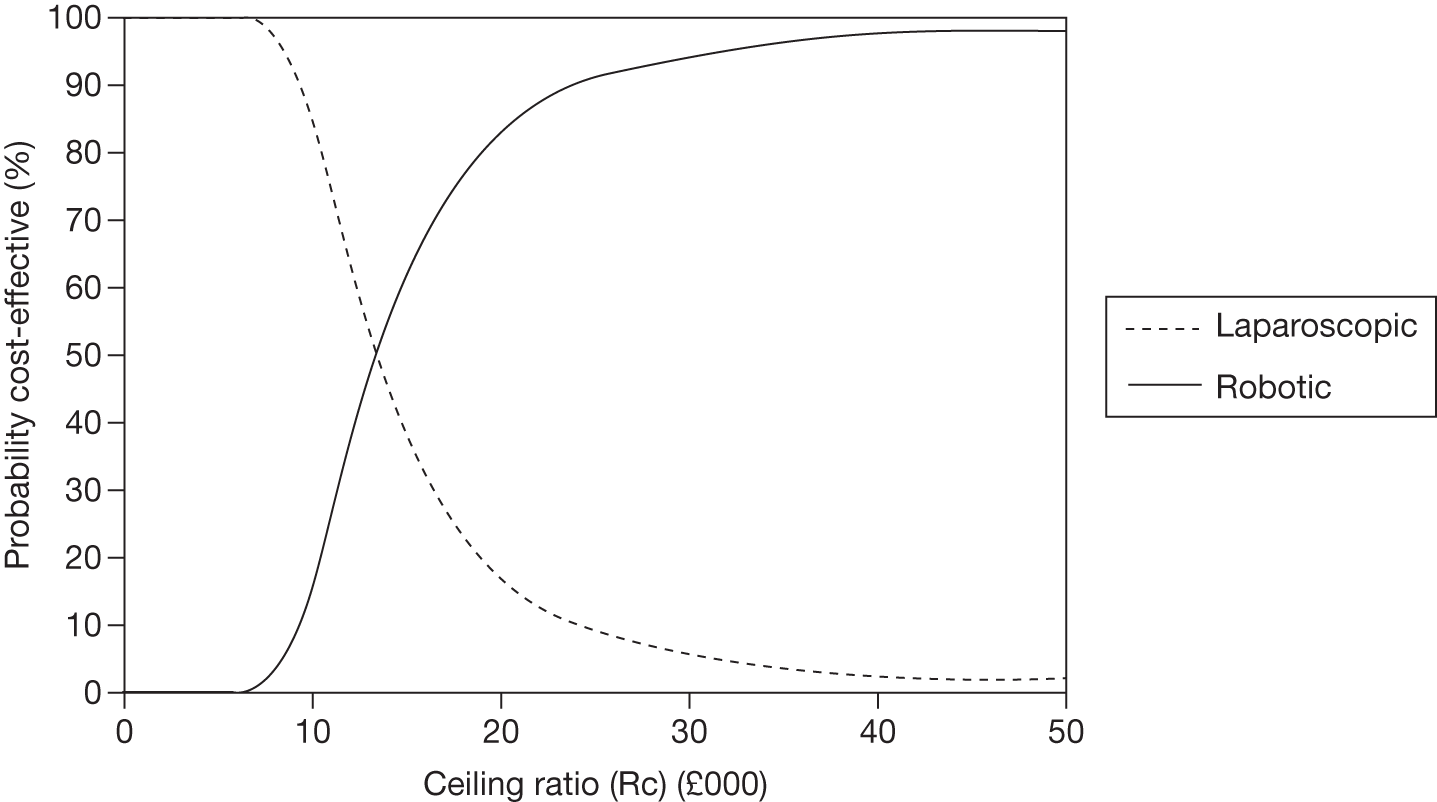
The results of the base-case analysis are sensitive to the costs of the robotic equipment. This is illustrated by exploring the impact of changing the number of surgeries performed per year (from 200 down to 50). As the number of procedures per year falls, the cost of the robotic equipment per procedure increases. As Table 40 illustrates, as the number of procedures per year falls from 200 to 50 and hence the cost of robotic equipment per procedure increases from £3467 to £10,287 (see Appendix 12 for details of how these costs were estimated), the mean incremental cost per QALY increases from £18,329 to £106,839. Consequently, the probability that robotic surgery would be considered cost-effective at a cost per QALY threshold typically used by NICE (£20,000) falls from 56% in the base-case analysis to virtually zero when the number of procedures per year is 50.
These data are based on the use of more expensive robotic equipment (da Vinci Si HD Dual Console). Should a less costly set-up be used instead, such as the da Vinci S EZ (three-arm) system, the equipment costs for the robotic procedure would be £2596 and in this situation the incremental cost per QALY gained for robotic compared with laparoscopic surgery would be £7009.
Sensitivity analysis
For each of the sensitivity analyses, mean costs and QALYs are shown for each treatment along with the incremental cost per QALY. Also shown is the likelihood that an intervention would be cost-effective at different threshold values for society’s willingness to pay for a QALY. Appendix 15 shows the plots of mean costs and QALYs and CEACs for each sensitivity analysis. Appendix 14 shows estimates of survival for each sensitivity analysis.
Increasing the time horizon
When the time horizon increases, the costs and QALYs for both types of surgery increase; however, for all of the scenarios that were modelled (Table 41), costs increase only slightly whereas there is a much larger proportionate increase in QALYs. As a consequence, the incremental cost per QALY for all scenarios modelled is lower than in the base case and the probability of robotic surgery being cost-effective at threshold values for a QALY that society might be willing to pay197 increases towards 1.
| Surgical capacity | Intervention | Mean cost (£) | Mean QALYs | ICER (£) | Probability cost-effective at different threshold values for WTP per QALY | ||||
|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| 200 | Robotic | 9179 | 12.12 | 1436 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Laparoscopic | 8075 | 11.36 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 150 | Robotic | 9937 | 12.12 | 2422 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Laparoscopic | 8075 | 11.36 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 100 | Robotic | 11,184 | 12.12 | 4045 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Laparoscopic | 8075 | 11.36 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 50 | Robotic | 15,998 | 12.12 | 10,306 | 0.00 | 0.41 | 1.00 | 1.00 | 1.00 |
| Laparoscopic | 8075 | 11.36 | 1.00 | 0.59 | 0.00 | 0.00 | 0.00 | ||
| 200a | Robotic | 8309 | 12.12 | 304 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Laparoscopic | 8075 | 11.36 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
Changes to the positive margin rate
In the base-case analysis we assumed that the OR for the difference in the positive margin rate between robotic and laparoscopic surgery was 0.69. In the first sensitivity analysis we took the difference in positive margin rates to be equal to the lower end of the CrI of the OR calculated in the meta-analysis reported in Chapter 4 (OR = 0.506). This resulted in robotic surgery having a lower rate of positive margins than in the base case and consequently a lower incremental cost per QALY (Table 42). Conversely, when the upper CrI limit of the OR for positive margins was used (OR = 0.955) the difference in positive margin rate between robotic and laparoscopic surgery was smaller than in the base case. As would be expected, the incremental cost per QALY increased as the number of procedures performed per year decreased. Indeed, only for the most optimistic scenario for robotic surgery modelled (the procurement cost of robotic equipment being equivalent to £2596) was the incremental cost per QALY < £30,000, and even in this scenario the likelihood that robotic surgery would be considered cost-effective was still only 60% at typical threshold values for society’s willingness to pay for a QALY (Table 43). 197 Overall, this sensitivity analysis illustrates the sensitivity of the results to changes in the effectiveness of robotic surgery because at the lower levels of throughput the mean incremental cost per QALY approaches or exceeds typical threshold values for society’s willingness to pay for a QALY (see Table 43). 197
| Surgical capacity | Intervention | Mean cost (£) | Mean QALYs | ICER (£) | Probability cost-effective at different threshold values for WTP per QALY | ||||
|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| 200 | Robotic | 9095 | 6.57 | 11,731 | 0.00 | 0.27 | 0.92 | 0.99 | 0.99 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 0.73 | 0.08 | 0.01 | 0.01 | ||
| 150 | Robotic | 9853 | 6.57 | 17,798 | 0.00 | 0.00 | 0.65 | 0.92 | 0.99 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 0.35 | 0.08 | 0.01 | ||
| 100 | Robotic | 11,097 | 6.57 | 27,743 | 0.00 | 0.00 | 0.09 | 0.60 | 0.94 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 0.91 | 0.40 | 0.06 | ||
| 50 | Robotic | 15,914 | 6.57 | 66,259 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 |
| Laparoscopic | 7628 | 6.44 | 1.000 | 1.00 | 1.00 | 1.00 | 0.88 | ||
| 200a | Robotic | 8223 | 6.57 | 4760 | 0.00 | 0.97 | 0.99 | 0.99 | 1.00 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 0.03 | 0.01 | 0.01 | 0.00 | ||
| Surgical capacity | Intervention | Mean cost (£) | Mean QALYs | ICER (£) | Probability cost-effective at different threshold values for WTP per QALY | ||||
|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| 200 | Robotic | 9099 | 6.47 | 50,502 | 0.00 | 0.00 | 0.13 | 0.30 | 0.49 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 0.87 | 0.70 | 0.51 | ||
| 150 | Robotic | 9859 | 6.47 | 76,564 | 0.00 | 0.00 | 0.02 | 0.12 | 0.34 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 0.98 | 0.88 | 0.66 | ||
| 100 | Robotic | 11,105 | 6.47 | 119,342 | 0.00 | 0.00 | 0.00 | 0.01 | 0.15 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 1.00 | 0.98 | 0.85 | ||
| 50 | Robotic | 15,923 | 6.47 | 284,694 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 200a | Robotic | 8230 | 6.47 | 20,675 | 0.000 | 0.214 | 0.48 | 0.60 | 0.67 |
| Laparoscopic | 7628 | 6.44 | 1.000 | 0.786 | 0.52 | 0.40 | 0.33 | ||
Changes in the costs and positive margin rates
To explore the relationship between positive margin rates, incremental cost per QALY and cost per procedure, we have plotted the incremental cost per QALY for the different ORs for positive margin against the changing cost of the procedure determined by varying the number of procedures performed per year and the purchase cost of the robotic system (Figure 25). The data have been presented in this way as the cost per procedure is likely to vary markedly between centres according to throughput. The costs per procedure for different throughputs and for five alternative scenarios of robotic system cost are summarised in Table 44 (see Appendix 12 for details of how these costs were estimated).
FIGURE 25.
Incremental cost per QALY for different costs per procedure and relative differences in positive margin rates for robotic versus laparoscopic surgery. OR, OR for positive margin rate for robotic versus laparoscopic surgery.
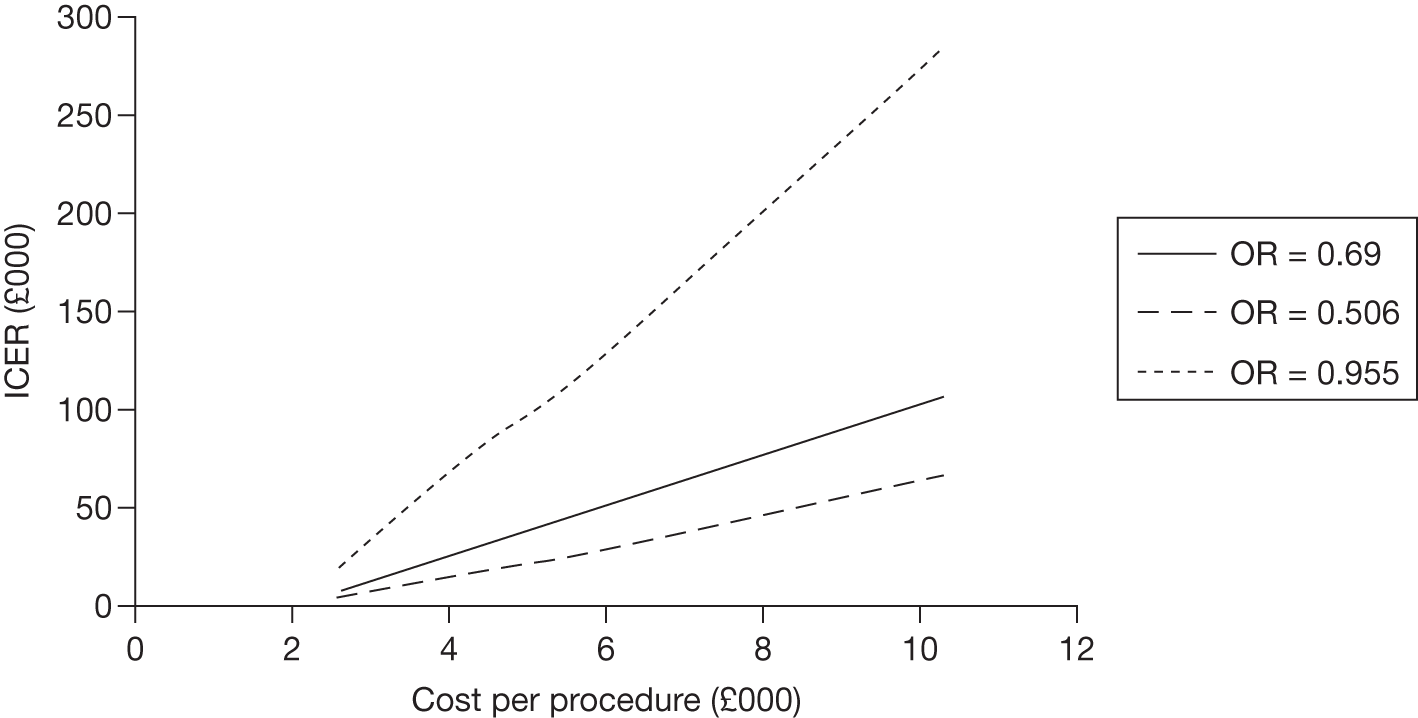
| Procedures per year | Type of equipment | Cost per procedure |
|---|---|---|
| 200 | da Vinci S EZ (three arm) | £2595.92 |
| 200 | da Vinci Si HD Dual Console | £3467.35 |
| 150 | da Vinci Si HD Dual Console | £4225.10 |
| 100 | da Vinci Si HD Dual Console | £5740.60 |
| 50 | da Vinci Si HD Dual Console | £10,287.09 |
As Figure 25 illustrates, as the cost per procedure increases with lower throughput and the OR for positive margin rate approaches 1 (no difference between procedures), the incremental cost per QALY increases beyond threshold values that society might be willing to pay. 197
For illustrative purposes these data have also been presented to show how the incremental cost per QALY changes as the relative difference in positive margin rate changes for different annual throughputs (Figure 26). As this figure illustrates, the incremental cost per QALY increases as the OR approaches 1.
FIGURE 26.
Incremental cost per QALY plotted against the OR for the relative difference in positive margin rate between robotic and laparoscopic surgery and for different numbers of procedures performed per year.
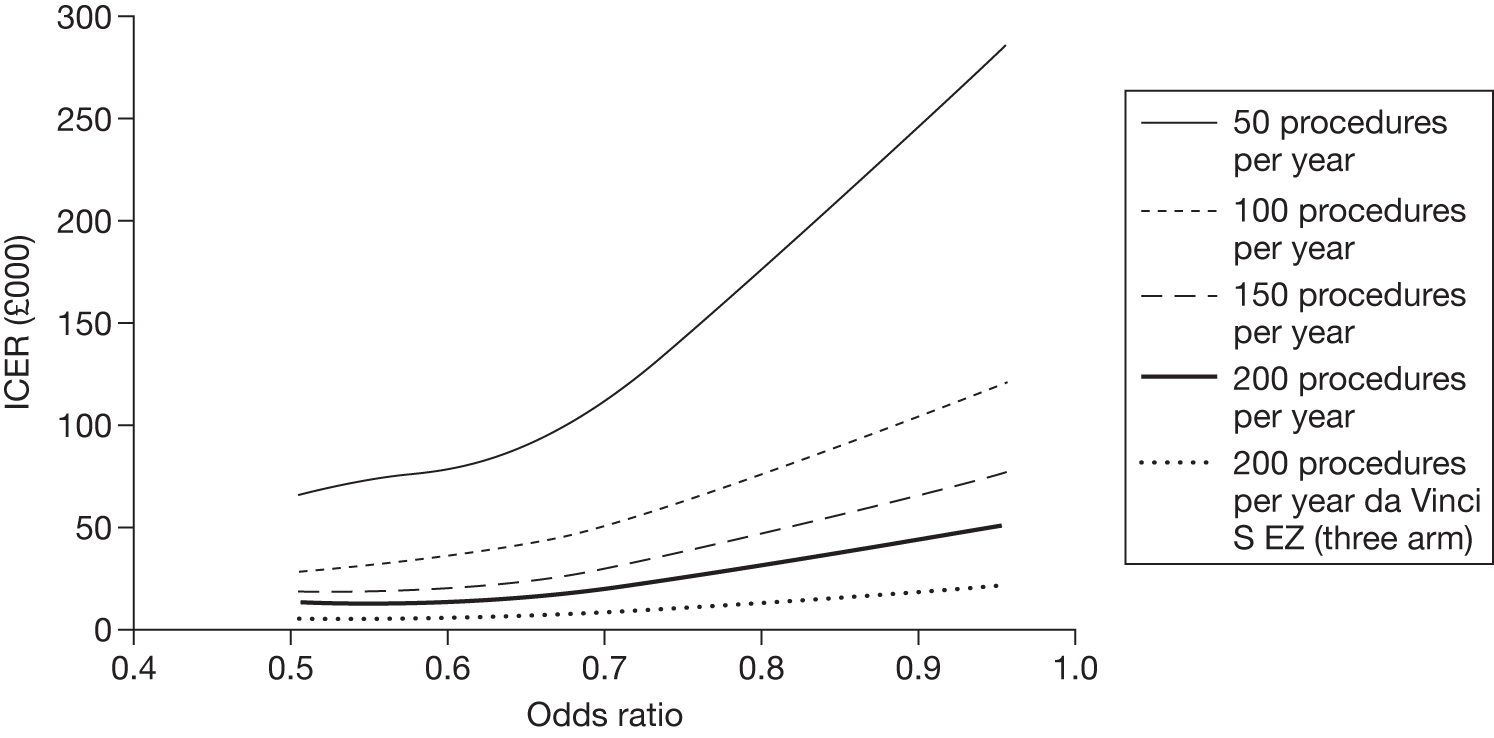
Changes to the risk of biochemical recurrence
In the base-case analysis it was assumed that the risk of biochemical recurrence was the same for both robotic and laparoscopic surgery. In the sensitivity analysis it has been assumed that on average robotic surgery is associated with a lower risk of biochemical recurrence (although the distribution attached to the value includes the possibility that there is no difference). A priori it would be expected that this would improve the relative efficiency of robotic surgery compared with laparoscopic surgery and, as Table 45 illustrates, on average this is what happened; however, the probability that robotic surgery would be considered cost-effective compared with the base case does not greatly alter over all threshold values considered.
| Surgical capacity | Intervention | Mean cost (£) | Mean QALYs | ICER (£) | Probability cost-effective at different threshold values for WTP per QALY | ||||
|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| 200 | Robotic | 9056 | 6.52 | 16,859 | 0.00 | 0.06 | 0.63 | 0.85 | 0.95 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 0.94 | 0.37 | 0.15 | 0.05 | ||
| 150 | Robotic | 9813 | 6.52 | 25,795 | 0.00 | 0.00 | 0.25 | 0.61 | 0.88 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 0.75 | 0.39 | 0.12 | ||
| 100 | Robotic | 11,059 | 6.52 | 40,506 | 0.00 | 0.00 | 0.01 | 0.21 | 0.65 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 0.99 | 0.79 | 0.35 | ||
| 50 | Robotic | 15,877 | 6.52 | 97,393 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 | ||
| 200a | Robotic | 8183 | 6.52 | 6546 | 0.00 | 0.789 | 0.949 | 0.97 | 0.98 |
| Laparoscopic | 7628 | 6.44 | 1.00 | 0.211 | 0.051 | 0.03 | 0.02 | ||
In a second sensitivity analysis on biochemical recurrence rate we explored the impact of a higher risk of biochemical recurrence for both robotic and laparoscopic surgery (Table 46). The impact of this was to increase the costs of and reduce the QALYs from robotic surgery. As a consequence the incremental costs per QALY increased and for situations in which the annual number of procedures was ≤ 100 the incremental cost per QALY would be above thresholds currently adopted by NICE. 197 Consequently, the probability that robotic surgery would be considered cost-effective increases compared with the base case although at the lowest throughputs considered robotic surgery is still highly unlikely to be considered cost-effective (see Table 40).
| Surgical capacity | Intervention | Mean cost (£) | Mean QALYs | ICER (£) | Probability cost-effective at different threshold values for WTP per QALY | ||||
|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | |||||
| 200 | Robotic | 9190 | 6.47 | 11,890 | 0.00 | 0.29 | 0.90 | 0.97 | 0.99 |
| Laparoscopic | 7842 | 6.35 | 1.00 | 0.71 | 0.10 | 0.03 | 0.01 | ||
| 150 | Robotic | 9949 | 6.47 | 18,582 | 0.00 | 0.00 | 0.58 | 0.89 | 0.97 |
| Laparoscopic | 7842 | 6.35 | 1.00 | 1.00 | 0.42 | 0.12 | 0.03 | ||
| 100 | Robotic | 11,194 | 6.47 | 29,567 | 0.00 | 0.00 | 0.07 | 0.52 | 0.90 |
| Laparoscopic | 7842 | 6.35 | 1.00 | 1.00 | 0.93 | 0.48 | 0.10 | ||
| 50 | Robotic | 16,008 | 6.47 | 72,029 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 |
| Laparoscopic | 7842 | 6.35 | 1.00 | 1.00 | 1.00 | 1.00 | 0.91 | ||
| 200a | Robotic | 8317 | 6.47 | 4191 | 0.00 | 0.96 | 0.99 | 1.00 | 1.00 |
| Laparoscopic | 7842 | 6.35 | 1.00 | 0.04 | 0.01 | 0.00 | 0.00 | ||
Summary of results of modelling cost-effectiveness of procedures
In the base-case analysis we have taken the best available evidence to inform the model, which in turn has been structured to reflect the current process of care. This analysis was based on the use of the most costly variant of the robotic equipment and explored the impact of variations in the number of procedures performed per year. As the number of procedures per year was reduced to < 150, the incremental cost per QALY became greater than threshold values that society might typically be willing to pay. 197
Given the available data, the main determinants of relative cost-effectiveness are the cost that centres would need to pay per procedure for the robotic equipment and the positive margin rate. The costs per procedure are influenced by the capital cost of the robotic system and the rate of use of each robotic system. The capital cost is determined by a number of different factors including the purchase plan taken for the robotic equipment, the type of equipment used and, not considered in this evaluation, the cost of any alterations to existing facilities. The rate of use of each system will also determine the cost per procedure, with higher throughput centres gaining significant economies of scale. The second key determinant of cost-effectiveness is the positive margin rate because of the effect of this parameter on determining subsequent cancer outcomes. The positive margin rate, along with other model parameters, is associated with considerable imprecision, but because of its role in determining management (see Tables 25 and 26) it was not possible to incorporate this uncertainty into the probabilistic sensitivity analysis. Nevertheless, when the uncertainty surrounding the OR for positive margins for robotic compared with laparoscopic surgery was incorporated into a deterministic sensitivity analysis the incremental cost per QALY was shown to increase as the OR approached 1. Indeed, when the OR was 0.955, higher than the point estimate based on data from studies at a low risk of bias, the incremental cost per QALY typically increased well beyond usual thresholds, especially when the number of procedures per year was low.
Overall, the results of the economic evaluation are suggestive that robotic radical prostatectomy could potentially be cost-effective but that this will depend on the long-term performance of robotic surgery in terms of cancer control and the number of procedures that can be performed per year in a centre where a robotic system is installed. This suggests that robotic surgery is more likely to be considered worthwhile in larger centres that manage ≥ 200 cases per year.
Chapter 7 Discussion
This review sought to answer the following question posed by the UK National Institute for Health Research HTA programme: ‘What is the clinical effectiveness of robotic surgery compared with laparoscopic surgery in the management of localised prostate cancer?’
Summary of findings
This HTA review, using the best available evidence and an appropriately complex health economic model, found that robotic prostatectomy was more effective but more costly than laparoscopic prostatectomy, and predicted that in the UK NHS it may be cost-effective provided that a minimum throughput is achieved for each robotic system and the cost of the system can be minimised. The implications of this review in terms of planning the best care in the NHS for men who require radical prostatectomy for treatment of their localised prostate cancer are therefore substantial, but the uncertainty surrounding our findings, associated with the inadequate evidence base, encourages a cautious approach. At present, of the 5000 men undergoing radical prostatectomy each year in the UK, approximately 50% are operated on using the open technique, 25% using the laparoscopic technique and 25% using the robotic technique. 52 With a further five robots being installed in UK NHS hospitals during 2011 to join the 16 already in service, it is likely that the proportion of men undergoing robotic surgery will increase. This review will help inform the setting of criteria, particularly related to monitoring of positive margin rate and minimum throughput, by which these robotic systems should be used to provide most benefit for men with localised prostate cancer and to the NHS. For the future there is an urgent need to standardise recording and reporting of relevant outcomes of treatments for localised prostate cancer within the NHS to allow better analysis of relative effectiveness and modelling of health economic benefits.
Clinical effectiveness
The methodology used in this report makes best use of the current evidence comparing the safety and outcome of radical prostatectomy performed for men with localised prostate cancer by open, laparoscopic or robotic techniques. In the mixed-treatment meta-analysis, only studies that involved a comparator arm were included when estimating differences between treatments. It is noteworthy that none of the studies eligible for inclusion in the meta-analysis comes from a UK centre. The prevalence of radical prostatectomy for localised prostate cancer within a particular community or health-care system is predominantly governed by the prevalence of PSA testing, which continues to be low in the UK relative to other countries with similarly developed health-care systems. 35 Although we used uncontrolled data derived from studies performed in many different countries, we did not find any large discrepancies in demographic and disease variables that may have resulted in differences in outcome between UK men undergoing radical prostatectomy and those from other countries. In terms of the surgical teams, most will have undergone mentored training in established laparoscopic and robotic centres elsewhere in Europe or in the USA, with updates from conference and ‘master class’ attendance. Generalisation of our results to the UK context does seem appropriate given this face validity, but a degree of caution needs to be exercised.
As is commonly the case with attempts to summarise outcomes from treatments for prostate cancer, we were unable to identify comparative estimates of cancer survival. Instead, we had to use proxy measures of disease outcome including positive surgical margins and rates of biochemical recurrence at 1 year. 74 Although both are considered to be predictive of cancer-specific survival, proof of this relationship is lacking. 199,200 Despite these caveats, the findings from the systematic review on differences in the process of care, safety and cancer outcome between robotic and laparoscopic prostatectomy appear to have face validity. The systematic review involved > 19,000 men with an average age of 61 years with preoperative cancer characteristics that were balanced between the groups and consistent with current recommendations for the use of this treatment. 43 Overall, 96% of men had cT1–cT2 disease and 94% a Gleason sum score on preoperative biopsy of ≤ 7. Latest data from the British Association of Urological Surgeons (BAUS)201 on 2225 men undergoing radical prostatectomy, submitted by participating institutions in the UK during 2010, suggest that disease characteristics are similar in the UK, with a median age of 60 years, 92% having cT1 or cT2 disease and 93% a preoperative Gleason sum score of ≤ 7. Following surgery, the meta-analysis showed an overall upstaging, with 21% of men in both the laparoscopic and robotic groups being pT3, but no overall worsening of Gleason sum score. The proportion of men having pT3 disease is a key variable because it is predictive of both positive surgical margin rates and ultimate survival. Data from the 60 UK centres contributing to the BAUS 2010 dataset showed that 36% of men undergoing radical prostatectomy had pT3 disease. Additional recent case series from UK centres performing purely laparoscopic or robotic prostatectomy reported pT3 rates of 26% and 46% respectively. 156,177 In summary, men included in our study were broadly typical of the population requiring this intervention in the UK NHS, but with a possible lower rate of pT3 disease, reflecting higher use of on-demand PSA testing in the USA and other Western European countries.
Patient-driven outcomes
Safety
Both laparoscopic and robotic radical prostatectomy had a good safety profile, with low rates of major morbidity and only one treatment-related death across all included studies. For most perioperative adverse events the direction of effect was in favour of robotic prostatectomy, suggesting potentially lower rates using the robotic system. The likelihood of this being a real difference was high only for the Clavien IIIb category concerning adverse events that required an additional operative intervention, particularly inadvertent rectal injury. The better vision and instrument dexterity afforded by the robotic system may have contributed to this although it should be noted that the absolute rates were low, increasing the chance that this was a random rather than a systematic difference between the procedures. There was no evidence of any difference in the rate of conversion to an open procedure, even though conversion could occur as an additional risk of machine failure in the case of robotic radical prostatectomy. Although we were unable to assess other relevant patient outcomes such as analgesic requirement, return to full activities or return to employment, given the similarity between these two minimally invasive approaches it is unlikely that there would be any differences. 33,202 Overall, our results do suggest that the improved vision and instrument manipulation afforded by the robotic system translates to improved operative patient safety.
Cancer control
All men with localised prostate cancer who embark on radical prostatectomy do so with the expectation that the operation will be curative and save them from the morbidity and early death associated with metastatic disease. 203,204 Information that our economic model of longer-term effectiveness could provide on this issue was dependent on estimates of positive margin rates (17.6% for robotic prostatectomy vs 23.6% for laparoscopic prostatectomy) and biochemical recurrence at 1 year (no evidence of a difference), which were the only relevant outcomes obtained from the meta-analysis. Although the evidence was that positive surgical margin rates, a proxy measure for cancer control, may be reduced by the use of robotic radical prostatectomy, the relevance of this in terms of cancer recurrence and long-term efficacy outcomes was unclear. This finding differed from that reported in a previous systematic review,205 which provided no evidence of a statistically significant difference in pooled estimates of surgical margin positivity. Restricting our analysis to low risk of bias studies continued to provide evidence of a lower rate of positive margin rates following robotic prostatectomy but with greater uncertainty and a lower probability that the difference was real. Our conclusion that robotic radical prostatectomy resulted in a lower rate of positive margins should therefore be interpreted with caution given this increased uncertainty around the estimates. In addition, a thorough review by our pathologist expert of the pathology protocols used in included studies showed that they provided limited detail and illustrated technical variation, which may have biased the categorisation of positive margin status and prevented accurate comparison between studies.
We used the best evidence from other literature and help from our expert panel to project, using a mathematical model, these short-term cancer outcome data from our systematic review to estimate long-term cancer-free survival over the subsequent 10 years or the individual’s lifetime. The findings suggest that overall survival was higher at 10 years for men undergoing robotic radical prostatectomy than for men undergoing laparoscopic radical prostatectomy, even if the upper CrI limit of the difference in positive margin rates (worse case) was used. In the base case the use of robotic prostatectomy resulted in an average gain of 0.045 life-years. Sensitivity analyses using lower differences in positive margin rates reduced the differences in 10-year overall survival as did increasing the overall biochemical recurrence rate. In all cases the estimates for 10-year survival rates were in the range of 70–80%, in line with those found in previous systematic reviews. 41
Long-term adverse events
Although the point estimate for the rate of bladder neck contracture was lower for robotic prostatectomy the degree of uncertainty meant that this was unlikely to represent a true difference. The lack of difference in rates of persistent urinary incontinence (∼6% after either procedure) or persistent erectile dysfunction (∼40% after either procedure) suggests that both techniques provide similar preservation of the key structures of urinary sphincter and neurovascular bundles. It is likely that erectile dysfunction in particular is highly dependent on preoperative sexual activity status and ability to preserve one or both neurovascular bundles at operation rather than on the type of surgery. 192,206 The reduced risks of rectal injury and anastomotic leak seen with robotic prostatectomy suggest that a greater accuracy of surgical dissection may be achieved. We do not, however, have sufficient comparative data at present on longer-term continence and sexual function rates to determine whether this translates to improved functional outcomes over the standard laparoscopic technique.
Surgeon outcomes
Uptake of robotic technology among surgeons who undertake radical prostatectomy has generally been enthusiastic, particularly in well-funded health-care systems where detection rates for localised prostate cancer are high. The experience from the USA, where 80,000 men underwent radical prostatectomy in 2007, suggests that if urologists have a choice between practising laparoscopic or robotic procedures most will concentrate on the robotic technique. 54 It is unclear how this experience will relate to surgeon preference in countries with lower rates of both use of radical prostatectomy and health-care expenditure. One suggested advantage of the robotic technique is that surgeons may need fewer cases to become fully competent in the procedure as mentoring and learning are facilitated by the console-based surgery. 207 Case series with > 200 men were reviewed together with the previously included comparative studies to ascertain possible learning effects and we found some evidence of improved positive margin rates with increasing experience; however, in contrast to previous studies we found no evidence of a differential learning effect for surgeons using laparoscopic or robotic techniques – the same learning curve was identified for both procedures. Part of the reason for this may have been our use of a patient-relevant outcome – positive margin rate – rather than operating time or blood transfusion rates, which are more often used for such comparisons. These data are consistent with the suggestion that it is the individual surgeon’s rate of learning that is the dominant factor rather than the technology used. 208 The volume of cases was not a confounding factor for the estimation of positive margin rates in the meta-analysis although, as stated above, there was a decrease in positive margin rates with increasing experience when the large case series were included.
Another stated advantage from the surgeon’s perspective is the ergonomic advantage of a seated position and scaling of hand movements available with the robotic system, causing less discomfort and a lower risk of chronic cervical pain. 209 To some extent this may relate to operating time. We did find that robotic prostatectomy was 15 minutes quicker on average to perform although the different ways of calculating this measure, in particular whether or not the docking time was included for the robotic procedure, give rise to some uncertainty. This saving of time is too small to allow increased productivity but may facilitate a greater rest period for the robotic surgical team. 210 Perhaps the most technically taxing part of the operation is achieving a watertight sutured join between the bladder neck and proximal urethral stump that remains patent in the longer term. We did find a significantly lower rate of urine leakage immediately postoperatively in the robotic prostatectomy group, suggesting a more reliable anastomosis, but this did not translate into higher rates of bladder neck contracture. Overall, the evidence that the robotic technology improved surgical operative performance for this particular step of the operation is weak.
Cost-effectiveness
No economic evaluations that compared the alternative forms of surgery from a UK perspective were identified and an economic evaluation based on a discrete-event simulation was planned. As described above, the findings of the systematic review were incorporated into the model and as a consequence the key determinants of cost-effectiveness were the time horizon, differences in positive margin rates and the relative costs of equipment. When a lifetime time horizon was adopted the costs and QALYs for both procedures increased but the increase in QALYs more than compensated for the increase in costs and hence the incremental cost per QALY was < £30,000 for all scenarios considered. This includes a scenario in which the number of procedures performed per year was 50 and in which the most costly robotic equipment was used. The principal reason for this is that adopting a longer time horizon allows more time for any benefits of robotic surgery to accrue and offset the initial higher equipment costs. Caution should, however, be exercised in interpreting the results as they rely on the extrapolation of relatively sparse short-term data within the model. There is uncertainty arising from both the quality of data and the mechanism for extrapolation.
The differences in positive margin rates translated into differences in QALYs and costs. For example, a higher positive margin rate resulted in lower QALYs, a greater need for further treatment and hence higher costs. With respect to costs, the cost per procedure was determined by the acquisition cost of the robotic system (which in turn depended on the specification of the equipment and the payment plan) and the number of procedures that might be performed annually using each robotic system. The costs of acquisition are to a certain degree under the control of a centre and depend on their own specific requirements and negotiations with the manufacturer. The number of procedures performed is a function of clinical need in the population that a centre serves and the population size. The results of the economic evaluation suggest that, when the difference in positive margins is equivalent to the point estimate estimated in the meta-analysis of all included studies, robotic radical prostatectomy was on average associated with an incremental cost per QALY that is less than threshold values typically adopted by the NHS when the cost of acquisition was low or the number of procedures was at the upper end of what could plausibly be achieved under current UK NHS provision (approaching 150 procedures per year). 197 This result holds except when the costs of acquisition were at the upper end of those estimated (see Appendix 12). Because the point estimate for difference in positive margin rate was uncertain, sensitivity analysis that progressively changed the difference in rates between robotic and laparoscopic prostatectomy was performed. At more optimistic values (OR = 0.506) the incremental cost per QALY would be less on average than threshold values typically adopted by the NHS when the number of procedures per year approached 100 or the procurement costs were at the lower end of those considered. Not unexpectedly, increasing the OR (OR = 0.955) resulted in a reduction in the QALY gain associated with the use of robotic prostatectomy and an increased cost. With the scenario of an OR for positive margin difference of 0.955 the incremental cost per QALY was only below the threshold if the number of procedures performed using each robotic system was increased to 200 and the lowest procurement cost for robotic equipment was assumed.
The mean estimates of incremental cost per QALY presented, although suggestive that robotic radical prostatectomy could potentially be cost-effective at conventional thresholds compared with laparoscopic prostatectomy, do not fully illustrate the degree of imprecision that exists. In the base-case robotic radical prostatectomy had an approximately 80% chance of being cost-effective when the threshold value for a QALY was £30,000. 197 However, caution should be exercised as this result does not incorporate the statistical imprecision surrounding variation in positive margin rates, a key predictor of longer-term outcomes in the model. This indicates the need for further data on the comparative long-term performance of the two forms of surgery. In addition, the sensitivity of estimates from cost-effectiveness for robotic prostatectomy to volume of surgery carried out in each centre argues for careful planning of NHS provision. As an illustration of the current service provision of the 60 UK centres that contributed to the BAUS radical prostatectomy database in 2010, 13 performed > 50 cases per year, of which three performed > 150 cases per year. 201 It should be noted, however, that less invasive management options for localised prostate cancer are emerging, including active surveillance, that may slow the growth in use of radical prostatectomy. 211
Strengths and weaknesses
Clinical effectiveness
The strength of the study is the systematic approach taken to review the literature. Exhaustive systematic searches were made of the major electronic databases. All potential studies were reviewed for eligibility, including non-English-language publications. The risk of bias for each included study was assessed using the best available tool. To prevent biases caused by selective data extraction all outcome parameters were predetermined by expert panel consensus and any data were extracted using standard forms. Despite these efforts it is possible that some relevant data remained hidden as a result of non-publication.
In total, 54 primary comparative studies were included. Although this haul of relevant studies is impressive, not every study contributed data to each outcome. Furthermore, differences in reporting between studies also limited the opportunities for comprehensive meta-analysis. As a consequence of the limited evidence base, the CIs around many estimates of differences were wide and included differences that would be clinically important but could favour either treatment. Another major limitation resulted from the fact that the majority of comparisons were made against open radical prostatectomy, with few head-to-head comparisons of robotic and laparoscopic technologies. Thus, the estimates generated by the meta-analysis make use of indirect comparisons. The mixed-treatment comparison models used to handle such data are an effective method of handling evidence from many trials on several interventions in one analysis. 85 Like all analyses they require assumptions to be made that may or may not be reasonable and accordingly the results should be interpreted with a degree of caution. There were 80 non-randomised comparative studies in which the clinical stage of cancer at baseline was unclear, thereby excluding the studies from the review. Although every effort was made to contact the authors of those papers, only 19 replied. The subsequent finding that exclusion of 18 was appropriate provides some reassurance that these studies do not represent a source of missed useable data but there remains a possibility that some were excluded because of their inadequate reporting.
The review attempted to include only unique data from included studies but we experienced difficulty determining secondary publications because of a lack of clarity in reporting details of treatment centres. There were four study sets (Anastasiadis and colleagues122 and Salomon and colleagues;140 Ficarra and colleagues106 and Fracalanza and colleagues;107 Barocas and colleagues103 and Chan and colleagues;119 Greco and colleagues,129 Jurczok and colleagues131 and Fornara and colleagues127) in which details of the affiliated institute of the first author, type of treatment and treatment dates were similar but it was unclear from the reported text whether or not these studies included an overlap of the same men. It is therefore possible that five studies107,119,127,131,140 have contributed to an overinclusion of men for some perioperative and efficacy outcomes.
The risk of bias assessment in the conduct of a systematic review is important. For this review a robust combined checklist, developed by the Cochrane Collaboration Non-Randomised Studies Methods Group [Barnaby C, Reeves, Jonathan J, Deeks, Julian PT, Higgins, et al. on behalf of the Cochrane Non-Randomised Studies Methods Group. Chapter 13: Including non-randomized studies. In Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. URL: www.cochrane-handbook.org (accessed March 2011)], assessing different sources of bias was produced. A scoring scale approach based on design features was avoided as this has been reported to be inaccurate concerning the direction of bias and can include items that are unrelated to the internal validity of a study. 212 For example, the terms ‘prospective study’ and ‘retrospective study’ are particularly ambiguous. ‘Prospective study’ should imply that all design aspects were planned, including hypothesis generation, recruitment of participants, baseline data collection and outcome data collection. In practice, how prospective a study is can often be unclear as some aspects of a study can be prospective, such as hypothesis generation and determination of outcomes, whereas others are retrospective, such as length of stay data collection from hospital records. The potential for bias in designs with different attributes can therefore vary considerably. This systematic review identified few studies at low risk of bias. The moderate inter-rater agreement between the two independent reviewers that was found in our review illustrates that risk of bias can be interpreted in different ways by different people. This is particularly likely in the newly developing methodological area of summarising non-randomised studies in which the level of reporting is often poor.
Many studies failed to report point estimates and measures of variability, hindering their use in estimating weighted mean differences, which require mean estimates for each intervention and standard deviations. It is possible that if means and standard deviations were reported more consistently, effect sizes would be different. However, in the systematic review, when an appropriate measure of variability was not reported for continuous outcomes, consistency across studies reporting the outcome was investigated and this would serve to eliminate biases when determining the direction of effect, even though the magnitude of effect remains uncertain.
A more specific methodological limitation that frustrated pooled analysis was the use of differing definitions and measures of functional outcomes for both urinary and erectile dysfunction. The variety of different ways of measuring dysfunction reduced the ability to compare data or to conduct a comprehensive meta-analysis. This was in part reflected by changing measurement methodologies for dysfunction across the time frame over which the studies were conducted, but it will remain a problem until consensus on important outcome measurements in this clinical area can be agreed. Initiatives such as the UK Medical Research Council-funded Core Outcome Measures in Effectiveness Trials (COMET) initiative213 may be useful in this context. Such initiatives aim to help researchers and clinicians across all specialities to develop a standardised set of outcomes (or core outcomes) that should be measured and reported as a minimum in all clinical trials of a specific condition, in order to make it easier to compare, contrast and synthesise the results of trials, to reduce the risk of inappropriate outcomes being measured and to reduce outcome reporting bias. 214
The examination of the influence of learning curves on the results was limited by poor reporting in the included studies. Given the general lack of data reported on the experiences of the centres included in the review, a proxy measure of ‘experience’ was used – namely the number of procedures performed. This measure may be inadequate to detect the differences between the interventions. In addition, when learning curve data were obtained from case series, the reported improvement with increasing experience may have limited applicability to current practice. This is partly because of the early reports of the effects of laparoscopic procedures focusing on refining the technique rather than on the acquisition of the technical skills required to perform the procedure in routine practice. If future studies conform to CONSORT reporting standards for non-pharmaceutical interventions215 this may help to alleviate some of the problems.
In summary, we believe that we have used the best available techniques to identify, review and meta-analyse the data that were available to us. This approach has enabled us to make robust broad conclusions concerning the relative beneficial and adverse effects of robotic prostatectomy compared with laparoscopic prostatectomy but which are associated with a defined degree of uncertainty.
Discrete-event model and economic evaluation
The economic evaluation was based on a discrete-event model. The purpose of this model was not just to estimate relative cost-effectiveness but also to investigate potential differences in clinical outcome between laparoscopic and robotic radical prostatectomy. As the model is a further level of evidence synthesis that builds on the systematic review and meta-analysis, many of the limitations applicable to the clinical data also apply to the economic data.
The decision context, like many of those faced in the evaluation of health-care interventions, was complex. Within a clinical context there is considerable variation between individuals in terms of demographic status and disease progression. In addition, the range, frequency and management of postoperative adverse events following surgery and the variations required in the care pathways necessitated the use of a more complex model than originally envisaged. The model form adopted was able to incorporate the degree of heterogeneity needed to simulate the life trajectory of individuals following surgery. In developing this model, we did not compromise realism in defining how care was implemented in the model. Elements of care that could occur in a given clinical setting were included insofar as they were recognised by the expert panel of practitioners. This inclusive approach effectively led to a complex suite of pathways that could not be modelled using ‘off-the shelf’ modelling packages often used in economic evaluations.
The complexity of the model permitted the simulation of a multitude of possible patient trajectories through the model. This can be illustrated by taking the example of a man who presents with a tumour of stage cT1 and undergoes surgery for presumed localised cancer. On pathological examination of the removed prostate it might be found that the tumour margin is positive but he is counselled to continue under surveillance with regular PSA checks. Happily there is no sign of biochemical recurrence and he remains in the surveillance state until the end of the 10-year time horizon of the study. In a more complex case, a man might remain under surveillance without cancer recurrence but require treatment for urinary dysfunction; he then subsequently requires further treatment for a localised recurrence, which is unfortunately unsuccessful, and he dies of prostate cancer following a period on androgen deprivation therapy. These complexities are required to model the costs and consequences of the differential outcomes of clinical effectiveness found in the systematic review but have the disadvantage of increasing the potential for error and misattribution. To guard against this the longer-term outputs of the model were checked for plausibility and credibility against existing literature sources and the opinions of our expert panel.
The major drivers of model design were heterogeneity in disease status and the requirement to describe realistic care pathways reflecting the range of postoperative adverse events and their treatment. Each health event and postoperative change in management was modelled probabilistically based on available data. As described in Chapter 5 this involved first defining the risk of an event occurring and then, for each man in a simulated cohort, generating a random number between 0 and 1. If the random number was less than the defined risk then the event was assumed to have occurred for that man. This process inevitably led to a large data requirement and a trade-off between model accuracy and data availability.
The data used within the model came from a number of, often independent, sources, which ranged from quantitative data derived from the systematic review through to qualitative data provided by clinical expert members of our advisory panel. Furthermore, parameter estimates for each event were assumed to be unbiased and representative of the population of men requiring radical prostatectomy for localised prostate cancer in the UK NHS. The use of different data sources, although unavoidable, may have introduced biases into the model estimates as the data came from different samples of the worldwide population of men undergoing radical prostatectomy. Furthermore, it was not always possible to assess the likelihood of non-independence in the parameter estimates. To overcome these limitations the parameters estimates were validated by the expert panel and model output discussed within the project team for clinical plausibility.
To address the imprecision we incorporated estimates of uncertainty for some parameters from the results of the meta-analysis. For other parameters we assumed triangular distributions when we had some information on mid-point and upper and lower limits for parameters and then used sensitivity analysis to investigate the behaviour of the model when we varied parameters for which we had only a point estimate and which were crucial to the model output. The sensitivity of health-related and economic outcomes was explored by determining the impact of varying the two parameters perceived to be of crucial importance to overall outcome: rates of pathological positive margin status and incidence of biochemical recurrence. In the case of positive margin rates the parameter was only one of the inputs used for deciding the need for further cancer treatment postoperatively. This precluded the exploration of imprecision in the probabilistic analysis and therefore this parameter was the focus of extensive deterministic sensitivity analysis.
When considering the impacts of each intervention strategy on health states, further treatment for cancer following radical prostatectomy was estimated as a less frequent event following robotic surgery than following laparoscopic surgery. This resulted in fewer cancer-specific deaths following robotic radical prostatectomy than following laparoscopic radical prostatectomy. The consequence of this was greater QALYs following robotic surgery and it also partly compensated for the increased costs of the robotic equipment.
Despite considerable efforts to elicit relevant information it was not possible to precisely quantify the extra cost of the robotic surgery equipment per procedure. This was because there are a plethora of different procurement strategies provided by the manufacturer, Intuitive Surgical, which varied by both method of payment and specification of equipment. Furthermore, the number of procedures performed each period using a given piece of equipment is variable. In the base case we chose to use the highest procurement cost and the highest plausible throughput of 200 cases per year. Repeating the analysis using lower procurement costs and a reduced number of procedures resulted in variation in the proportion of the cost of the robotic system attributed to each procedure, from £3500 to £10,200 (see Table 40). In the base-case analysis, only when the cost was at the higher level determined by a throughput of approximately 150 cases per year was the incremental cost per QALY around £30,000. It should be noted that more favourable assumptions around the positive margin rate tended to reduce the incremental cost per QALY but the incremental cost per QALY would still be > £30,000 for annual throughputs of approximately 100 cases (or a cost of robotic equipment per procedure of approximately £6000). It should also be noted that less favourable but still plausible assumptions concerning the difference in positive margin rates also increased the incremental cost per QALY to > £30,000, particularly when combined with lower throughput of cases. These results indicate that further research is required to more accurately determine positive margin rates and also how they predict long-term cancer outcomes.
In addition to clinical data and costs the model also attempted to incorporate information on the value of different events to the men under treatment – health-state utilities – so that QALYs could be estimated. Searches were conducted to identify data of most relevance to a UK decision-making context but few data were found and not all data were available from a single source. It is possible that we may have misvalued some events, which, if these events occurred at different rates between the two procedures, would have introduced a bias into the analysis. Ideally, health-state utilities data applicable to a UK population should be elicited to overcome this shortcoming.
One aspect of cost not included in the model was the use of unscheduled GP and outpatient visits. There was a lack of data on the frequency of these events with which to model. Previous experience from trials that include men after treatment of prostate cancer would suggest that these costs are relatively modest compared with the cost of surgery. Furthermore, given the apparent lack of difference in effects we did not expect there to be a substantial differential use of these services between groups.
In summary, the discrete-event model attempted to synthesise current clinical practice with the best available estimates of economic and health data to evaluate the potential benefits of robotic prostatectomy in comparison with standard laparoscopic prostatectomy. The model was conservative in that we did not model processes for which we had no evidence of a difference between the two surgical approaches. Furthermore, it did not assume dependence between processes when there was no information available to support a modelled relationship. The model demonstrated that there are circumstances when robotic prostatectomy could be cost-effective as judged against conventional thresholds for willingness to pay for a QALY, especially if lower costs of equipment can be secured and when the surgical capacity is high.
Chapter 8 Conclusions
Implications for health care
There are currently approximately 5000 men who require radical prostatectomy in the UK each year. This number is most likely to increase over the next 5 years as increased detection of localised prostate cancer occurs, associated with more widespread use of PSA testing in the target population. 35 Emergence of less invasive treatments may, however, slow any growth in the use of radical prostatectomy. 211
The results of this study, although associated with some uncertainty and lack of long-term direct measures of effectiveness, demonstrated that the outcomes were generally better for robotic than for laparoscopic surgery for major adverse events, and importantly for positive margin rates. This may lead to better cancer-related outcomes and fewer episodes of adjuvant radiotherapy for localised recurrence. At worst this review found no evidence to suggest that robotic prostatectomy is inferior to the standard laparoscopic technique.
Robotic prostatectomy will always be more costly to the NHS because of the fixed capital and maintenance charges for the robotic system. Our modelling does show, however, that this excess cost can be reduced by either or a combination of two mechanisms: minimisation of capital costs for purchase and maintenance of the robotic system by commercial negotiation, and maintenance of high usage by ensuring at least 100–150 procedures per year. Our study does provide some evidence that the cost-effectiveness of each procedure is dependent on the volume of cases but there was no evidence that this relationship differed between the procedures. It is self-evident that a higher throughput of cases facilitates training, mentoring and comparative auditing of surgeon performance in a sustainable team-based approach, which is required for effective use of complex equipment. 216
At present our information suggests that eight centres in the UK NHS achieve these levels of throughput using a varying combination of open, laparoscopic and robotic techniques. It should be noted that surgeon interest in using the robotic system is expanding into renal surgery, gynaecology and complex head and neck surgery, potentially allowing required throughput to be shared between specialties. Offsetting capital costs in this way would have consequences for case volume and may reduce the reliance on high prostatectomy throughput to improve the cost-effectiveness of the robotic technique compared with alternatives.
Implications for research
The main gaps in the evidence base are the lack of direct comparative studies of robotic and laparoscopic prostatectomy with low risk of bias and the lack of longer-term data with more certain measures of cancer control, such as cancer-specific mortality and overall mortality. Given the current increasing adoption of the robotic technology into the NHS, it may be difficult to undertake a randomised comparison against open or laparoscopic prostatectomy in the UK. A feasibility study for such a comparison has been initiated with the support of Cancer Research UK through the LOPERA trial (http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=6766). It is at present uncertain whether recruitment trends will be sufficient to encourage a definitive trial.
A brief updated search of abstracts related to robotic prostatectomy only was conducted in November 2011. We identified a further 15 comparative studies of robotic compared with laparoscopic prostatectomy (including one possible RCT), four studies comparing robotic, laparoscopic and open prostatectomy and nine studies comparing robotic and open prostatectomy. Therefore, internationally, there continues to be a number of studies published, suggesting that the trajectory of the evidence base is still upwards. However, the quality of the studies is uncertain and there continues to be a lack of evidence from RCTs. If a formal RCT is not possible then the following are areas in which further research would be important:
-
Well-designed prospective cohort studies directly comparing robotic and laparoscopic prostatectomies are required. Ideally such studies would be multicentre with long-term follow-up and would include predefined assessment of prostate cancer-specific survival as well as independent recording of learning curve, dysfunction and health-related quality-of-life measures.
-
Further evidence as to how positive margin rates impact on long-term cancer control outcomes.
-
Research to elicit the short- and long-term postoperative health-state valuations (e.g. utility values) associated with prostatectomy and the contribution of different dysfunctions as perceived by men.
-
Agreed definitions of outcomes in urology and measures for recording them. This would require consensus work in partnership with governing bodies such as BAUS and national initiatives such as COMET.
-
Research into strategies to improve planning of evaluation and potential dissemination of costly new technology in the UK NHS.
Acknowledgements
We thank Michael Trevor-Barnston (MBE JP DL), Peter Barton and Prostate UK for providing valuable consumer insight and advice through their participation as members of the study advisory group; Prokar Dasgupta (Professor of Robotic Surgery, Urological Surgeon and Urological Innovation/Consultant), Roger Kockelbergh (BAUS Chairman and Clinical Director of Urology) Alan McNeil (Consultant Urological Surgeon) and Anna O’Riordan (Consultant Urologist) for providing their clinical expertise as members of the project advisory group; Mr David Evans (Laboratory Manager) for providing pathological prostatectomy specimen sampling costing information; Surgical Intuitive Incorporated for providing procurement cost information for the da Vinci surgical robot system; Lara Kemp, Eleanor Lockhart and Winnie Yiu for providing secretarial support; and Graeme MacLennan for providing statistical advice and support.
This report was commissioned by the National Institute for Health Research (NIHR) HTA programme as project number 09/14/02. The Health Services Research Unit is core funded by the Chief Scientist Office of the Scottish Government Health Directorates. The views and opinions expressed are those of the authors and do not necessarily reflect those of the funders.
Contribution of authors
Craig Ramsay (co-principal investigator, Health Care Assessment Programme Director) oversaw and co-ordinated all aspects of the study and wrote the executive summary, the methods and results for the systematic review of clinical effectiveness and the discussion and conclusions chapters. Robert Pickard (co-principal investigator and Professor of Urology) jointly co-ordinated the study with Craig Ramsay, led and co-ordinated the economic evaluation and expert advisory group participation and wrote the background, the description of care pathways and the discussion and conclusions chapters. Clare Robertson (Research Fellow) led the day-to-day running of the study and reviewed the evidence for clinical effectiveness of the technologies with assistance from Tara Gurung (Research Fellow), Xueli Jia (Research Fellow), Graham Mowatt (Senior Research Fellow) and Pawana Sharma (Research Fellow). Andrew Close (Postdoctoral Research Associate) developed the care pathways with clinical advice from Robert Pickard and conducted the economic evaluation with supervision from Luke Vale (Professor of Health Economics), Mark Shirley (Research Associate) and Stephen Rushton (Professor of Biological Modelling). Andrew Close, Mark Shirley, Stephen Rushton, Luke Vale and Robert Pickard wrote the economic evaluation methods and results chapters. Nigel Armstrong (Health Economist) provided advice for conducting the economic evaluation at the start of the study. Daniel Barocas (MD Urologist) provided additional data for the economic evaluation. Cynthia Fraser (Information Specialist) developed and ran the search strategies and was responsible for obtaining full-text papers and for reference management. David Jenkinson (Research Fellow) provided statistical support. Thomas Lam (Senior Specialist Registrar and Honorary Clinical Lecturer) and Justine Royle (Consultant Urological Surgeon) classified reported adverse events into the Clavien–Dindo classification of surgical complications. Mary Robinson (Consultant Urological Pathologist) reviewed the quality of methods described for the handling, processing and pathologist reporting of radical prostatectomy specimens by papers included in the systematic review of clinical effectiveness. Christopher Eden (Consultant Urologist), David Neal (Professor of Surgical Oncology) and Naeem Soomro (Consultant Urologist) provided expert clinical advice on service and surgical aspects. All authors commented on drafts of the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Hummel S, Paisley S, Morgan A, Currie E, Brewer N. Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review. Health Technol Assess 2001;7.
- Ollendorf DA, Hayes J, McMahon P, Pearson SD. Active surveillance and radical prostatectomy for the management of low-risk, clinically localized prostate cancer. Boston, MA: Institute for Clinical and Economic Review; 2010.
- Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic Radical Prostatectomy – Accelerated Systematic Review 2005. www.surgeons.org/racs/research-and-audit/asernip-s/asernip-s-publications/accelerated-systematic-reviews/laparoscopic-radical-prostatectomy (accessed June 2011).
- Hernandez J, Thompson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer 2004;101:894-90.
- Elder JS, Scott WW, Nyberg LM. Overview of past and current philosophy of prostatic cancer. Prostate 1980;1:287-301.
- Alyea EP, Dees JE, Glenn JF. An aggressive approach to prostatic cancer. J Urol 1977;118:211-15.
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 1987;317:909-16.
- NHS Cancer Screening Programmes . Prostate Cancer Risk Management Programme 2010. www.cancerscreening.nhs.uk/prostate/publications.html (accessed May 2011).
- Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA 1995;273:548-52.
- Cancer Research UK . Prostate Cancer: Key Facts on Prostate Cancer 2009. http://info.cancerresearchuk.org/cancerstats/types/prostate/?a=5441 (accessed March 2011).
- Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, et al. Cancer surveillance series: interpreting trends in prostate cancer – Part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst 1999;91:1017-24.
- Bott SR, Birtle AJ, Taylor CJ, Kirby RS. Prostate cancer management: (1) an update on localised disease. Postgrad Med J 2003;79:575-80.
- Boxer RJ, Kaufman JJ, Goodwin WE. Radical prostatectomy for carcinoma of the prostate: 1951–1976. A review of 329 patients. J Urol 1977;117:208-13.
- Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol 1982;128:492-7.
- Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate 1983;4:473-85.
- Bhatnagar V, Kaplan RM. Treatment options for prostate cancer: evaluating the evidence. Am Fam Physician 2005;71:1915-22.
- Hu JC, Gu X, Lipsitz SR, Barry MJ, D’Amico AV, Weinberg AC, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302:1557-64.
- Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007;99:1171-7.
- Guillonneau B, Cathelineau X, Barret E, Rozet F, Vallancien G. Laparoscopic radical prostatectomy: technical and early oncological assessment of 40 operations. Eur Urol 1999;36:14-20.
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2002;20:557-66.
- Lepor H, Kaci L. Contemporary evaluation of operative parameters and complications related to open radical retropubic prostatectomy. Urology 2003;62:702-6.
- Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology 1997;50:854-7.
- Frota R, Turna B, Barros R, Gill IS. Comparison of radical prostatectomy techniques: open, laparoscopic and robotic assisted. Int Braz J Urol 2008;34:259-68.
- Unger SW, Unger HM, Bass RT. AESOP robotic arm. Surg Endosc 1994;8.
- Eden CG. Minimal access prostatectomy: how is it shaping up?. BJU Int 2008;101:791-2.
- Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic radical prostatectomy for localized prostate cancer: a systematic review of comparative studies. J Urol 2006;175:2011-17.
- Davies B. A review of robotics in surgery. J Eng Med 2000;214:129-40.
- Sutcliffe P, Czoski-Murray C, Chattle M, Ayiku L, Parry G. Emerging technology briefing paper on the use of robots in surgery. University of Sheffield: Review body for interventional procedures; 2006.
- Carpentier A, Loulmet D, Aupecle B, Berrebi A, Relland J. Computer-assisted cardiac surgery. Lancet 1999;353:379-80.
- Intuitive Surgical Incorporated . Intuitive Surgical – Products 2009. www.intuitivesurgical.com/products/index.aspx (accessed March 2011).
- Abbou CC, Hoznek A, Salomon L, Olsson LE, Lobontiu A, Saint F, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol 2001;165:1964-6.
- Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int 2001;87:408-10.
- Smith JA, Herrell SD. Robotic-assisted laparoscopic prostatectomy: do minimally invasive approaches offer significant advantages?. J Clin Oncol 2005;23:8170-5.
- Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol 2007;51:45-5.
- Williams N, Hughes LJ, Turner EL, Donovan JL, Hamdy FC, Neal DE, et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int 2011;108:1402-8.
- Oesterling JE, Cooner WH, Jacobsen SJ, Guess HA, Lieber MM. Influence of patient age on the serum PSA concentration. An important clinical observation. Urol Clin North Am 1993;20:671-80.
- Raja J, Ramachandran N, Munneke G, Patel U. Current status of transrectal ultrasound-guided prostate biopsy in the diagnosis of prostate cancer. Clin Radiol 2006;61:142-53.
- Roddam AW, Duffy MJ, Hamdy FC, Ward AM, Patnick J, Price CP, et al. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA level of 2–10 ng/ml: systematic review and meta-analysis. Eur Urol n.d.;48:386-99.
- Sobin DH, Gospodariwicz M, Witteking CH. TNM classification of malignant tumours. New York, NY: Wiley-Blackwell; 2009.
- Helpap B, Egevad L. Modified Gleason grading. An updated review. Histol Histopathol 2009;24:661-6.
- Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 2008;148:435-48.
- Zeliadt SB, Ramsey SD, Penson DF, Hall IJ, Ekwueme DU, Stroud L, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer 2006;106:1865-74.
- National Insititute for Health and Clinical Excellence . Prostate Cancer: Diagnosis and Treatment; Full Guidance 2008. www.nice.org.uk/nicemedia/pdf/cg58fullguideline.pdf (accessed March 2011).
- Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84.
- Heidenreich A, Bolla M, Joniau S, Mason MD, Matveev V, Mottet N, et al. Guidelines on prostate cancer. Arnhem, the Netherlands: European Association of Urology; 2011.
- D’Amico AV, Whittington R, Kaplan I, Beard C, Schultz D, Malkowicz SB, et al. Calculated prostate carcinoma volume: the optimal predictor of 3-year prostate specific antigen (PSA) failure free survival after surgery or radiation therapy of patients with pretreatment PSA levels of 4–20 nanograms per milliliter. Cancer 1998;82:334-41.
- South West Cancer Intelligence Service . Prostate Cancer Survival by Stage 2008. www.swpho.nhs.uk/resource/item.aspx?rid=41287 (accessed June 2011).
- HESonline . Main Procedures and Interventions: 4 Character 2009–10 2011. www.hesonline.nhs.uk/ (accessed May 2011).
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-38.
- Andriole GL, Grubb RL, Buys SS, Chia D, Church TR, Fouad MN, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-19.
- UK National Health Service . NHS Cancer Plan 2009. http://nhscancerplan.com/page/nhs-cancer-plan (accessed May 2011).
- Department of Health . NHS Reference Costs 2009–10 2011. http://data.gov.uk/dataset/nhs-reference-costs-2009-10 (accessed May 2011).
- Hu JC, Hevelone ND, Ferreira MD, Lipsitz SR, Choueiri TK, Sanda MG, et al. Patterns of care for radical prostatectomy in the United States from 2003 to 2005. J Urol 2008;180:1969-74.
- Barbash GI, Glied SA. New technology and health care costs – the case of robot-assisted surgery. N Engl J Med 2010;363:701-4.
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer 2011. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed May 2011).
- National Insititute for Health and Clinical Excellence . Improving Outcomes in Urological Cancers – Manual 2002. http://guidance.nice.org.uk/csguc/guidance/pdf/english (accessed May 2011).
- Memorial Sloan-Kettering Cancer Center . Prostate Cancer Nomograms 2009. www.mskcc.org/mskcc/html/10088.cfm (accessed March 2011).
- Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep 1966;50:125-8.
- Epstein JI, Amin M, Boccon-Gibod L, Egevad L, Humphrey PA, Mikuz G, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl 2005;216:34-63.
- Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3?. J Clin Oncol 2009;27:3459-64.
- Zhou M, Epstein JI. The reporting of prostate cancer on needle biopsy: prognostic and therapeutic implications and the utility of diagnostic markers. Pathology 2003;35:472-9.
- van der Kwast TH, Amin MB, Billis A, Epstein JI, Griffiths D, Humphrey PA, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol 2011;24:16-25.
- Fulmer BR, Schwartz BF. Laparoscopic and Robotic Radical Prostatectomy 2011. http://emedicine.medscape.com/article/458677-overview#showall (accessed May 2011).
- Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Statistical assessment of the learning curves of health technologies. Health Technol Assess 2001;5.
- Secin F. The learining curve for laparoscopic radical prostatectomy: an interbational multicenter study. J Endourol 2008;22.
- Hellawell GO, Stolzenburg JU. Ending the ‘learning curve’. BJU Int 2009;103:1454-5.
- Descazeaud A, Peyromaure M, Zerbib M. Will robotic surgery become the gold standard for radical prostatectomy?. Eur Urol 2007;51:9-11.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13.
- Rabbani F, Yunis LH, Pinochet R, Nogueira L, Vora KC, Eastham JA, et al. Comprehensive standardized report of complications of retropubic and laparoscopic radical prostatectomy. Eur Urol 2010;57:371-86.
- Breyer BN, Davis CB, Cowan JE, Kane CJ, Carroll PR. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int 2010;106:1734-8.
- Srigley JR, Amin MB, Epstein JI, Grignon DJ, Humphrey PA, Renshaw AA, et al. Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland. Arch Pathol Lab Med 2006;130:936-46.
- Samaratunga H, Montironi R, True L, Epstein JI, Griffiths DF, Humphrey PA, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 1: specimen handling. Mod Pathol 2011;24:6-15.
- Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007;177:540-5.
- Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Lilja H, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol 2006;24:3973-8.
- Medical Research Council . RADICALS: Radiotherapy and Androgen Deprivation in Combination After Local Surgery. A Randomised Controlled Trial in Prostate Cancer 2011. http://focus3trial.org/research_areas/study_details.aspx?s=28 (accessed May 2011).
- Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2011;59:572-83.
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): Two parallel randomised controlled trials. Lancet 2011;378:328-37.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of intervention. The Cochrane Collaboration; 2008.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74.
- Association of Clinical Pathologists . Guidelines for the macroscopic processing of radical prostatectomy and pelvic lymphadenectomy specimens. J Clin Pathol 2008;61:713-21.
- Montironi R, van der Kwast T, Boccon-Gibod L, Bono AV, Boccon-Gibod L. Handling and pathology reporting of radical prostatectomy specimens. Eur Urol 2003;44:626-36.
- Srigley JR. Key issues in handling and reporting radical prostatectomy specimens. Arch Pathol Lab Med 2006;130:303-17.
- Egevad L, Srigley JR, Delahunt B. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens: rationale and organization. Mod Pathol 2011;24:1-5.
- Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 5: surgical margins. Mod Pathol 2011;24:48-57.
- Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 2001;10:277-303.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24.
- Marinho VC, Higgins JP, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;1.
- Cook JA, Ramsay CR, Fayers P. Using the literature to quantify the learning curve: a case study. Int J Technol Assess Health Care 2007;23:255-60.
- Grossi FS, Di LS, Barnaba D, Larocca L, Raguso M, Sallustio G, et al. Laparoscopic versus open radical retropubic prostatectomy: a case–control study at a single institution. Arch Ital Urol Androl 2010;82:109-12.
- Guazzoni G, Cestari A, Naspro R, Riva M, Centemero A, Zanoni M, et al. Intra- and perioperative outcomes comparing radical retropubic and laparoscopic radical prostatectomy: results from a prospective, randomised, single-surgeon study. Eur Urol 2006;50:98-104.
- Gosseine PN, Mangin P, Leclers F, Cormier L. Pure laparoscopic versus robotic-assisted laparoscopic radical prostatectomy: comparative study to assess functional urinary outcomes. Prog Urol 2009;19:611-17.
- Hu JC, Nelson RA, Wilson TG, Kawachi MH, Ramin SA, Lau C, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol 2006;175:541-6.
- Joseph JV, Vicente I, Madeb R, Erturk E, Patel HR. Robot-assisted vs pure laparoscopic radical prostatectomy: are there any differences?. BJU Int 2005;96:39-42.
- Joseph JV, Salomon L, Capello SA, Patel HR, Abbou CC. Laparoscopic or robot-assisted extraperitoneal radical prostatectomy: 1554 cases from from two high volume institutions performed extraperitoneally. J Urol 2007;177:525-6.
- Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology 2002;60:864-8.
- Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol 2007;178:478-82.
- Sundaram C. Comparison of early experience with laparoscopic radical prostatectomy with and without robotic assistance. J Endourol 2004;18.
- Trabulsi EJ, Linden RA, Gomella LG, McGinnis DE, Strup SE, Lallas CD. The addition of robotic surgery to an established laparoscopic radical prostatectomy program: effect on positive surgical margins. Can J Urol 2008;15:3994-9.
- Ball AJ, Gambill B, Fabrizio MD, Davis JW, Given RW, Lynch DF, et al. Prospective longitudinal comparative study of early health-related quality-of-life outcomes in patients undergoing surgical treatment for localized prostate cancer: a short-term evaluation of five approaches from a single institution. J Endourol 2006;20:723-31.
- Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. The influence of body mass index on the cost of radical prostatectomy for prostate cancer. BJU Int 2010;106:1188-93.
- Drouin SJ, Vaessen C, Hupertan V, Comperat E, Misrai V, Haertig A, et al. Comparison of mid-term carcinologic control obtained after open, laparoscopic, and robot-assisted radical prostatectomy for localized prostate cancer. World J Urol 2009;27:599-605.
- Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. Cost comparison of robotic, laparoscopic and open radical prostatectomy. Eur Urol Suppl 2009;8.
- Barocas DA, Salem S, Kordan Y, Herrell SD, Chang SS, Clark PE, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. J Urol 2010;183:990-6.
- Carlsson S, Nilsson AE, Schumacher MC, Jonsson MN, Volz DS, Steineck G, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology 2010;75:1092-7.
- Doumerc N, Yuen C, Savdie R, Rahman MB, Rasiah KK, Pe BR, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 2010;106:378-84.
- Ficarra V, Novara G, Fracalanza S, D’Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int 2009;104:534-9.
- Fracalanza S, Ficarra V, Cavalleri S, Galfano A, Novara G, Mangano A, et al. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int 2008;101:1145-9.
- Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int 2009;103:448-53.
- Loeb S, Epstein JI, Ross AE, Schultz L, Humphreys EB, Jarow JP. Benign prostate glands at the bladder neck margin in robotic vs open radical prostatectomy. BJU Int 2010;105:1446-9.
- Malcolm JB, Fabrizio MD, Barone BB, Given RW, Lance RS, Lynch DF, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol 2010;183:1822-8.
- Miller J, Smith A, Kouba E, Wallen E, Pruthi RS. Prospective evaluation of short-term impact and recovery of health related quality of life in men undergoing robotic assisted laparoscopic radical prostatectomy versus open radical prostatectomy. J Urol 2007;178:854-8.
- Nadler RB, Casey JT, Zhao LC, Navai N, Smith ZL, Zhumkhawala A, et al. Is the transition from open to robotic prostatectomy fair to your patients? A single-surgeon comparison with 2-year follow-up. J Robot Surg 2010;3:201-7.
- Ou YC, Yang CR, Wang J, Cheng CL, Patel VR. Comparison of robotic-assisted versus retropubic radical prostatectomy performed by a single surgeon. Anticancer Res 2009;29:1637-42.
- Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int 2009;104:991-5.
- Schroeck FR, Sun L, Freedland SJ, Albala DM, Mouraviev V, Polascik TJ, et al. Comparison of prostate-specific antigen recurrence-free survival in a contemporary cohort of patients undergoing either radical retropubic or robot-assisted laparoscopic radical prostatectomy. BJU Int 2008;102:28-32.
- Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int 2003;92:205-10.
- Truesdale MD, Lee DJ, Cheetham PJ, Hruby GW, Turk AT, Badani KK. Assessment of lymph node yield after pelvic lymph node dissection in men with prostate cancer: a comparison between robot-assisted radical prostatectomy and open radical prostatectomy in the modern era. J Endourol 2010;24:1055-60.
- White MA, De Haan AP, Stephens DD, Maatman TK, Maatman TJ. Comparative analysis of surgical margins between radical retropubic prostatectomy and RALP: are patients sacrificed during initiation of robotics program?. Urology 2009;73:567-71.
- Chan RC, Barocas DA, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. Effect of a large prostate gland on open and robotically assisted laparoscopic radical prostatectomy. BJU Int 2008;101:1140-4.
- Kordan Y, Barocas DA, Altamar HO, Clark PE, Chang SS, Davis R, et al. Comparison of transfusion requirements between open and robotic-assisted laparoscopic radical prostatectomy. BJU Int 2010;106:1036-40.
- Al-Shaiji TF, Kanaroglou N, Thom A, Prowse C, Comondore V, Orovan W, et al. A cost-analysis comparison of laparoscopic radical prostatectomy versus open radical prostatectomy: the McMaster Institute of Urology experience. Can Urol Assoc J 2010;4:237-41.
- Anastasiadis AG, Salomon L, Katz R, Hoznek A, Chopin D, Abbou CC. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology 2003;62:292-7.
- Artibani W, Grosso G, Novara G, Pecoraro G, Sidoti O, Sarti A, et al. Is laparoscopic radical prostatectomy better than traditional retropubic radical prostatectomy? An analysis of perioperative morbidity in two contemporary series in Italy. Eur Urol 2003;44:401-6.
- Bhayani SB, Pavlovich CP, Hsu TS, Sullivan W, Su LM. Prospective comparison of short-term convalescence: laparoscopic radical prostatectomy versus open radical retropubic prostatectomy. Urology 2003;61:612-16.
- Brown JA, Garlitz C, Gomella LG, McGinnis DE, Diamond SM, Strup SE. Perioperative morbidity of laparoscopic radical prostatectomy compared with open radical retropubic prostatectomy. Urol Oncol 2004;22:102-6.
- Dahl DM, Barry MJ, McGovern FJ, Chang Y, Walker-Corkery E, McDougal WS. A prospective study of symptom distress and return to baseline function after open versus laparoscopic radical prostatectomy. J Urol 2009;182:956-65.
- Fornara P, Zacharias M. Minimal invasiveness of laparoscopic radical prostatectomy: reality or dream?. Aktuelle Urologie 2004;35:395-40.
- Ghavamian R, Knoll A, Boczko J, Melman A. Comparison of operative and functional outcomes of laparoscopic radical prostatectomy and radical retropubic prostatectomy: single surgeon experience. Urology 2006;67:1241-6.
- Greco F, Wagner S, Hoda M, Kawan F, Inferrera A, Lupo A, et al. Laparoscopic vs open retropubic intrafascial nerve-sparing radical prostatectomy: surgical and functional outcomes in 300 patients. BJU Int 2010;106:543-7.
- Jacobsen NE, Moore KN, Estey E, Voaklander D. Open versus laparoscopic radical prostatectomy: a prospective comparison of postoperative urinary incontinence rates. J Urol 2007;177:615-19.
- Jurczok A, Zacharias M, Wagner S, Hamza A, Fornara P. Prospective non-randomized evaluation of four mediators of the systemic response after extraperitoneal laparoscopic and open retropubic radical prostatectomy. BJU Int 2007;99:1461-6.
- Kim Y-J. Comparison of perioperative outcomes of extraperitoneal laparoscopic radical prostatectomy (ELRP) versus open radical retropubic prostatectomy (RRP): single surgeon’s initial experience. Korean J Urol 2007;48:131-7.
- Lama MK, Salinas NRO, Martinez JMF, Gribbell RAO, Cabrera OS, Sudy CAF. Prospective study and comparative of surgical and oncologic outcome between laparoscopic and retropubical radical prostatectomy. Actas Urol Esp 2009;33:167-71.
- Martorana G, Manferrari F, Bertaccini A, Malizia M, Palmieri F, Severini E, et al. Laparoscopic radical prostatectomy: oncological evaluation in the early phase of the learning curve comparing to retropubic approach. Arch Ital Urol Androl 2004;76:1-5.
- Namiki S, Egawa S, Baba S, Terachi T, Usui Y, Terai A, et al. Recovery of quality of life in year after laparoscopic or retropubic radical prostatectomy: a multi-institutional longitudinal study. Urology 2005;65:517-23.
- Namiki S, Egawa S, Terachi T, Matsubara A, Igawa M, Terai A, et al. Changes in quality of life in first year after radical prostatectomy by retropubic, laparoscopic, and perineal approach: multi-institutional longitudinal study in Japan. Urology 2006;67:321-7.
- Poulakis V, Witzsch U, de Vries R, Dillenburg W, Becht E. Laparoscopic radical prostatectomy in men older than 70 years of age with localized prostate cancer: comparison of morbidity, reconvalescence, and short-term clinical outcomes between younger and older men. Eur Urol 2007;51:1341-8.
- Raventos Busquets CX, Gomez Lanza E, Cecchini Rossell L, Trilla Herrera E, Orsola los de Santos A, Planas Morin J, et al. Comparison between open and laparoscopic approach in radical prostatectomy. Actas Urol Esp 2007;31:141-5.
- Remzi M, Klingler HC, Tinzl MV, Fong YK, Lodde M, Kiss B, et al. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy verus open retropubic radical prostatectomy. Eur Urol 2005;48:83-9.
- Salomon L, Levrel O, Anastasiadis AG, Saint F, de la Taille A, Cicco A, et al. Outcome and complications of radical prostatectomy in patients with PSA < 10 ng/ml: comparison between the retropubic, perineal and laparoscopic approach. Prostate Cancer Prostatic Dis 2002;5:285-90.
- Silva E, Ferreira U, Silva GD, Mariano MB, Netto NR, Billis A, et al. Surgical margins in radical prostatectomy: a comparison between retropubic and laparoscopic surgery. Int Urol Nephrol 2007;39:865-9.
- Soderdahl DW, Davis JW, Schellhammer PF, Given RW, Lynch DF, Shaves M, et al. Prospective longitudinal comparative study of health-related quality of life in patients undergoing invasive treatments for localized prostate cancer. J Endourol 2005;19:318-26.
- Soric T. Laparoscopic radical prostatectomy. Med Jadertina 2004;34:87-90.
- Terakawa T, Miyake H, Tanaka K, Takenaka A, Inoue TA, Fujisawa M. Surgical margin status of open versus laparoscopic radical prostatectomy specimens. Int J Urol 2008;15:704-7.
- Touijer K, Kuroiwa K, Eastham JA, Vickers A, Reuter VE, Scardino PT, et al. Risk-adjusted analysis of positive surgical margins following laparoscopic and retropubic radical prostatectomy. Eur Urol 2007;52:1090-6.
- Wagner AA, Link RE, Trock BJ, Sullivan W, Pavlovich CP. Comparison of open and laparoscopic radical prostatectomy outcomes from a surgeon’s early experience. Urology 2007;70:667-71.
- Dahl DM, He W, Lazarus R, McDougal WS, Wu CL. Pathologic outcome of laparoscopic and open radical prostatectomy. Urology 2006;68:1253-6.
- Humphrey PA. Complete histologic serial sectioning of a prostate gland with adenocarcinoma. Am J Surg Pathol 1993;17:468-72.
- McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am J Surg Pathol 1990;14:240-7.
- Stamey TA, McNeal JE, Freiha FS, Redwine E. Morphometric and clinical studies on 68 consecutive radical prostatectomies. J Urol 1988;139:1235-41.
- Brown JA, Garlitz C, Gomella LG, Hubosky SG, Diamond SM, McGinnis D, et al. Pathologic comparison of laparoscopic versus open radical retropubic prostatectomy specimens. Urology 2003;62:481-6.
- Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol 1995;154:1818-24.
- True LD. Surgical pathology examination of the prostate gland. Practice survey by American society of clinical pathologists. Am J Clin Pathol 1994;102:572-9.
- Tewari AK, Patel ND, Leung RA, Yadav R, Vaughan ED, El-Douaihy Y, et al. Visual cues as a surrogate for tactile feedback during robotic-assisted laparoscopic prostatectomy: posterolateral margin rates in 1340 consecutive patients. BJU Int 2010;106:528-36.
- Hong YM, Sutherland DE, Linder B, Engel JD. ‘Learning curve’ may not be enough: assessing the oncological experience curve for robotic radical prostatectomy. J Endourol 2010;24:473-7.
- McNeill AS, Nabi G, McLornan L, Cook J, Bollina P, Stolzenberg JU. Endoscopic extraperitoneal radical prostatectomy: critical analysis of outcomes and learning curve. BJU Int 2010;106:1537-43.
- Eden CG, Neill MG, Louie-Johnsun MW. The first 1000 cases of laparoscopic radical prostatectomy in the UK: evidence of multiple ‘learning curves’. BJU Int 2009;103:1224-30.
- Secin FP, Savage C, Abbou C, de la Taille A, Salomon L, Rassweiler J, et al. The learning curve for laparoscopic radical prostatectomy: an international multicenter study. J Urol 2010;184:2291-6.
- Samadi DB, Muntner P, Nabizada-Pace F, Brajtbord JS, Carlucci J, Lavery HJ. Improvements in robot-assisted prostatectomy: the effect of surgeon experience and technical changes on oncologic and functional outcomes. J Endourol 2010;24:1105-10.
- Rodriguez AR, Rachna K, Pow-Sang JM. Laparoscopic extraperitoneal radical prostatectomy: impact of the learning curve on perioperative outcomes and margin status. J Soc Laparoendosc Surg 2010;14:6-13.
- Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, et al. Robot-assisted laparoscopic prostatectomy: a single-institutions learning curve. Urology 2009;73:127-33.
- Vickers AJ, Savage CJ, Hruza M, Tuerk I, Koenig P, Martinez-Pineiro L, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol 2009;10:475-80.
- Martinez-Pineiro L. Learning curve of laparoscopic radical prostatectomy in a university teaching hospital: experience after the first 600 cases. Eur Urol Suppl 2006;5:914-24.
- Burgess SV. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol 2006;20:827-30.
- Hohwu L, Ehlers L, Borre M, Pedersen KV. Cost-effectiveness study of robot-assisted laparoscopic versus open retropubic radical prostatectomy. Eur Urol Suppl 2010;9.
- Scales CD, Jones PJ, Eisenstein EL, Preminger GM, Albala DM. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol 2005;174:2323-9.
- Anderson JK, Murdock A, Cadeddu JA, Lotan Y. Cost comparison of laparoscopic versus radical retropubic prostatectomy. Urology 2005;66:557-60.
- Link RE, Su LM, Bhayani SB, Pavlovich CP. Making ends meet: a cost comparison of laparoscopic and open radical retropubic prostatectomy. J Urol 2004;172:269-74.
- Mouraviev V, Nosnik I, Sun L, Robertson CN, Walther P, Albala D, et al. Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: a single-institution experience. Urology 2007;69:311-14.
- Satoh T. Cost comparison of curative therapies for localized prostate cancer in Japan: a single-institution experience. Jap J Radiol 2009;27:348-54.
- Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol 2010;57:453-8.
- Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy: cost comparison of open, laparoscopic and robot assisted techniques. J Urol 2004;172:1431-5.
- Steinberg PL, Merguerian PA, Bihrle W, Seigne JD. The cost of learning robotic-assisted prostatectomy. Urology 2008;72:1068-72.
- Karnon J. Alternative decision modelling techniques for the evaluation of health care technologies: Markov processes versus discrete event simulation. Health Econ 2003;12:837-48.
- R Development Core Team . The R Project for Statistical Computing 2011. www.r-project.org/ (accessed May 2011).
- Chamberlain J, Melia J, Moss S, Brown J. The diagnosis, management, treatment and costs of prostate cancer in England and Wales. Health Technol Assess 2001;1.
- Sharma NL, Papadopoulos A, Lee D, McLoughlin J, Vowler SL, Baumert H, et al. First 500 cases of robotic-assisted laparoscopic radical prostatectomy from a single UK centre: learning curves of two surgeons. BJU Int 2011;108:739-47.
- Palapattu GS, Singer EA, Messing EM. Controversies surrounding lymph node dissection for prostate cancer. Urol Clin North Am 2010;37:57-65.
- Kawakami J, Meng MV, Sadetsky N, Latini DM, Duchane J, Carroll PR, et al. Changing patterns of pelvic lymphadenectomy for prostate cancer: results from CaPSURE. J Urol 2006;176:1382-6.
- UK Office for National Statistics . Mortality Statistics: Deaths Registered in England and Wales 2011. www.statistics.gov.uk/statbase/product.asp?vlnk=15096%26pos=2%26colrank=1%26rank=160 (accessed May 2011).
- Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol 2010;58:838-46.
- Moreira DM, Banez LL, Presti JC, Aronson WJ, Terris MK, Kane CJ, et al. Predictors of secondary treatment following biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. BJU Int 2010;105:28-33.
- Bria E, Cuppone F, Giannarelli D, Milella M, Ruggeri EM, Sperduti I, et al. Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer?: meta-analysis of randomized trials. Cancer 2009;115:3446-56.
- Herschorn S, Bruschini H, Comiter C, Grise P, Hanus T, Kirschner-Hermanns R, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn 2010;29:179-90.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2011. http://bnf.org/bnf/index.htm (accessed June 2011).
- UK Department of Health . NHS Reference Costs 2008–2009 2010. www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_111591 (accessed May 2011).
- Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-60.
- Matthew AG, Goldman A, Trachtenberg J, Robinson J, Horsburgh S, Currie K, et al. Sexual dysfunction after radical prostatectomy: prevalence, treatments, restricted use of treatments and distress. J Urol 2005;174:2105-10.
- Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer 2002;95:2397-407.
- Blander DS, Sanchez-Ortiz RF, Wein AJ, Broderick GA. Efficacy of sildenafil in erectile dysfunction after radical prostatectomy. Int J Impot Res 2000;12:165-8.
- Costabile RA, Spevak M, Fishman IJ, Govier FE, Hellstrom WJ, Shabsigh R, et al. Efficacy and safety of transurethral alprostadil in patients with erectile dysfunction following radical prostatectomy. J Urol 1998;160:1325-8.
- Meuleman EJ, Mulders PF. Erectile function after radical prostatectomy: a review. Eur Urol 2003;43:95-101.
- Information Services Division (ISD) Scotland . NHS Costs 2009 2010. www.isdscotlandarchive.scot.nhs.uk/isd/6480.html (accessed June 2011).
- Korfage IJ, Essink-Bot ML, Borsboom GJ, Madalinska JB, Kirkels WJ, Habbema JD, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer 2005;116:291-6.
- Cowen ME, Miles BJ, Cahill DF, Giesler RB, Beck JR, Kattan MW. The danger of applying group-level utilities in decision analyses of the treatment of localized prostate cancer in individual patients. Med Decis Making 1998;18:376-80.
- Volk RJ, Cantor SB, Cass AR, Spann SJ, Weller SC, Krahn MD. Preferences of husbands and wives for outcomes of prostate cancer screening and treatment. J Gen Intern Med 2004;19:339-48.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/b52/a7/tamethodsguideupdatedjune2008.pdf (accessed May 2011).
- Kattan MW, Yu C, Salomon L, Vora K, Touijer K, Guillonneau B. Development and validation of preoperative nomogram for disease recurrence within 5 years after laparoscopic radical prostatectomy for prostate cancer. Urology 2011;77:396-401.
- Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer 2008;113:3075-99.
- Yossepowitch O, Bjartell A, Eastham JA, Graefen M, Guillonneau BD, Karakiewicz PI, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol 2009;55:87-99.
- Fowler S. Analyses of complex operations and the newly recognised registry for urological cancers January – December 2010. London: British Association of Urological Surgeons, Section of Oncology; 2011.
- Rocco B. Robotic prostatectomy: facts or fiction?. Lancet 2007;369:723-4.
- Anandadas CN, Clarke NW, Davidson SE, O’Reilly PH, Logue JP, Gilmore L, et al. Early prostate cancer – which treatment do men prefer and why?. BJU Int 2011;107:1762-8.
- Holmberg L. Prostate cancer screening: the need for problem-solving that puts men’s interests first. Eur Urol 2009;56:34-7.
- Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009;55:1037-63.
- Briganti A, Capitanio U, Chun FK, Karakiewicz PI, Salonia A, Bianchi M, et al. Prediction of sexual function after radical prostatectomy. Cancer 2009;115:3150-9.
- Kaul SA, Peabody JO, Shah N, Neal D, Menon M. Establishing a robotic prostatectomy programme: the impact of mentoring using a structured approach. BJU Int 2006;97:1143-4.
- Vickers AJ. Great meaningless questions in urology: which is better, open, laparoscopic, or robotic radical prostatectomy?. Urology 2011;77:1025-6.
- Bagrodia A, Raman JD. Ergonomics considerations of radical prostatectomy: physician perspective of open, laparoscopic, and robot-assisted techniques. J Endourol 2009;23:627-33.
- Mayer EK, Winkler MH, Aggarwal R, Karim O, Ogden C, Hrouda D, et al. Robotic prostatectomy: the first UK experience. Int J Med Robotic Comp Assis Surg 2006;2:321-8.
- Nguyen CT, Jones JS. Focal therapy in the management of localized prostate cancer. BJU Int 2011;107:1362-8.
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054-60.
- University of Liverpool . COMET Initiative (Core Outcome Measures in Effectiveness Trials) 2011. www.liv.ac.uk/nwhtmr/comet/core_outcomes.htm (accessed May 2011).
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309.
- Zorn KC, Gautam G, Shalhav AL, Clayman RV, Ahlering TE, Albala DM, et al. Training, credentialing, proctoring and medicolegal risks of robotic urological surgery: recommendations of the Society of Urologic Robotic Surgeons. J Urol 2009;182:1126-32.
- Shadish WR, Cook TD, Campbell DT. xperimental and quasi-experimental designs for generalized causal inference. Boston, MA: Houghton Mifflin; 2002.
- Walsh PC, Partin AW, Wien AJ, Kavoussi LR, Novick AC. Campbell-Walsh urology. Philadelphia, PA: WB Saunders; 2006.
- Patel VR, Palmer KJ, Coughlin G, Samavedi S. Robot-assisted laparoscopic radical prostatectomy: perioperative outcomes of 1500 cases. J Endourol 2008;22:2299-305.
- Latiff A. Preservation of bladder neck fibers in radical prostatectomy. Urology 1993;41:566-7.
- Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol 2007;51.
- McCarthy J, Catalona W, Marshall FF. Textbook of operative urology. Oxford: WB Saunders; 1996.
- Menon M, Tewari A, Peabody J. The VIP Team . Vattikuti Institute prostatectomy: technique. J Urol 2003;169:2289-92.
- Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the montsouris technique. J Urol 2001;163:1643-9.
- He W, Dahl DM, McDougal WS, Wu C-L. Laparoscopic versus open radical prostatectomy: analysis of 1000 cases at Massachusetts General Hospital. Mod Path 2006;19.
- Stolzenburg JU, Do M, Rabenalt R, Pfeiffer H, Horn L, Truss MC, et al. Endoscopic extraperitoneal radical prostatectomy: initial experience after 70 procedures. J Urol 2003;169:2066-71.
- Bollens R, Vanden Bossche M, Roumeguere T, Damoun A, Ekane S, Hoffmann P, et al. Extraperitoneal laparoscopic radical prostatectomy: results after 50 cases. Eur Urol 2001;40:65-9.
Appendix 1 Protocol
PROTOCOL FOR A SYSTEMATIC REVIEW AND ECONOMIC MODELLING OF THE RELATIVE CLINICAL BENEFIT AND COST-EFFECTIVENESS OF LAPAROSCOPIC SURGERY AND ROBOTIC SURGERY FOR REMOVAL OF THE PROSTATE IN MEN WITH LOCALISED PROSTATE CANCER
1. Background
Prostate cancer causes approximately 13% of cancer-related deaths and 4% of all deaths in the UK with an age-standardised mortality rate of 26/100,000, amounting to 10,000 men each year. 1 In the UK 35,000 new cases were reported in 2005. 1,2 In 1997 the annual cost to the NHS was estimated at £55 million3 whereas in 2007 the drug cost alone was approximately £130 million4 and with added costs for surgery, radiotherapy, and hospital and community care the current annual cost is likely to exceed £200 million.
The largest rise in incidence seen recently is among relatively younger men as a consequence of case-finding and screening for asymptomatic disease5,6 using the serum marker, prostate specific antigen (PSA) and multiple trans-rectal ultrasound (TRUS) guided needle biopsies of the prostate. 5,6 The majority of these asymptomatic cancers appear confined to the prostate on clinical staging and are therefore amenable to cure through radical treatment.
Radical prostatectomy, whereby the prostate is completely removed surgically, remains the favoured curative treatment option for localised prostate cancer and has been demonstrated to improve disease-specific survival compared with watchful waiting, although this benefit takes 10 years to accrue. 7
Open prostatectomy
Open radical prostatectomy involves the removal of the prostate gland together with the surrounding thin layers of connective tissue and is usually performed through a lower abdominal incision. 8 During the operation care is taken to minimise blood loss and to preserve the normal continence mechanism and, when tumour characteristics allow, the nerves and arteries supplying the penile erectile tissue. Despite this approximately 15% of men require blood transfusion, 7% have long-term urinary incontinence and 40% suffer erectile dysfunction after surgery although surgeons who perform larger numbers of cases tend to have better results. 9,10 These longer-term adverse effects reduce men’s general level of well-being and surgeons have therefore sought ways to reduce the functional disturbance of the procedure but maintain its disease-curing potential. 11
Laparascopic prostatectomy
Laparoscopic prostatectomy involves the insertion of five ports in the abdomen through which long, narrow instruments can be passed together with a camera. The ports are positioned ergonomically to enable the surgeon to dissect the prostate using the instruments with their handles located outside the body. Increasing experience with the technique has demonstrated that it does result in reduced blood loss compared with open prostatectomy but hoped for reduction in rates of erectile dysfunction and incontinence remains uncertain and is likely to depend on surgeon experience. 12–15
Robotic prostatectomy
The use of robotic technology allows the surgeon to control the surgical instruments from a console. Robotic prostatectomy involves the preliminary insertion of an umbilical camera port and three other ports for the instruments controlled by the four robotic arms. Additional ports are used for instruments operated by a human assistant and maintenance of pneumoperitoneum. The procedure is then carried out in an identical fashion to laparoscopic prostatectomy but with the surgeon remotely controlling the three or four slave manipulator arms whilst seated at a console which is usually, although not necessarily, sited adjacent to the patient in the operating room. 16 Over recent years there has been a rapid expansion in the availability of the ‘da Vinci®’ robot to the NHS for radical prostatectomy. 17–19
Rationale
The main advantage claimed for robotic prostatectomy is a reduction in the learning curve due to increased degrees of freedom of the robotic arms that hold the instruments. 20 However, the impact of this has only been considered in one comparison,21 in which the authors found that the direct costs associated with robotic procedures decreased substantially once their learning curve of 50 cases had been surpassed. Although the impact of more rapid gaining of competency on outcomes may be small, the impact on operating times, and hence on procedural costs might be significant and contribute to lower procedure costs in higher volume centres. 22,23 There is therefore a clear need to assess the relative clinical benefit and cost-effectiveness of laparoscopic and robotic prostatectomy in men with localised prostate cancer, including differential learning curve effects.
2. Aims and Objectives
The study aims to determine the clinical effectiveness and cost-effectiveness of robotic prostatectomy compared with laparoscopic prostatectomy in the treatment of patients with localised prostate cancer.
The specific objectives of the study are to:
-
Describe clinical care pathways for laparoscopic and robotic prostatectomy in a UK context;
-
Determine the clinical effectiveness and safety of each procedure;
-
Determine the influence of the learning curve on estimates of effectiveness and safety;
-
Perform a systematic review of existing economic evaluations of each procedure;
-
Determine which procedure is most likely to be cost-effective for implementation into the UK NHS; and
-
Identify future research needs.
3. Methods
3.1 Eligibility criteria
Types of study
We will consider evidence from randomised controlled trials (RCTs), non-randomised comparative studies and case series, the latter primarily for estimates of rare adverse events and longer-term effects. For estimating learning curve effects, information on the robotic or laparoscopic arms of comparative studies will be treated as case series. Systematic reviews of open prostatectomy will be considered in order to obtain evidence on the clinical effectiveness of open prostatectomy for the purposes of informing the economic model. We will include conference abstracts and non-English language reports of comparative studies only.
Types of participants
The types of participants considered will be men with localised prostate cancer, defined as cancer confined to the prostate gland and considered curable by radical removal of the prostate.
Types of interventions and comparators
The intervention considered will be robotic prostatectomy and the comparator laparoscopic prostatectomy. Open prostatectomy will also be considered as a comparator in studies comparing robotic prostatectomy with open prostatectomy, or laparoscopic prostatectomy with open prostatectomy, in order that such studies can be included in a mixed treatment comparison model assessing the relative effectiveness of robotic and laparoscopic prostatectomy.
Types of outcome measures
The following types of outcome measures will be considered:
-
Cancer related
-
Functional
-
– Recovery of sexual (penile erection) function, quantified by validated score (IIEF-5); and
-
– Urinary continence, defined as use of ≤ 1 thin pad per day and/or validated symptom score.
-
-
Adverse events
-
– Peri-operative:
-
– Blood loss – quantified as transfusion rate;
-
– Conversion to open procedure;
-
– Delayed discharge; and
-
– Death.
-
-
– Long term:
-
– Anastomotic stricture.
-
-
Two surgeons will categorise each complication using the Clavien–Dindo Classification of Surgical Complications (as detailed in Chapter 2, Table 3)26 with a third surgeon acting as arbitrar.
-
Procedural
-
– Learning curve;
-
– Equipment failure;
-
– Operative time;
-
– Hospital stay; and
-
– Duration of catheterisation.
-
-
Patient-driven
-
– Pain, quantified by validated pain score and analgesic requirements;
-
– Productivity (time to return to full activity); and
-
– Generic and disease-specific quality of life, measured through validated quality of life scores.
-
Exclusion criteria
The following types of report will be excluded:
-
Studies of men with metastatic disease;
-
Case series of open prostatectomy.
3.2 Search strategy
Comprehensive electronic searches will be conducted to identify reports of published studies. Highly sensitive search strategies will be designed, including appropriate subject headings and text word terms, interventions under consideration and included study designs. There will be no language restriction but searches will be restricted to years from 1995 onwards, reflecting the introduction of the techniques. Medline, Medline In Process, Embase, CINAHL, Biosis, Science Citation Index, Cochrane Controlled Trials Register (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Review of Effects (DARE) and the HTA databases will be searched. Reference lists of all included studies will be scanned in order to identify additional potentially relevant reports. We will also ask our expert panels to provide details of any additional potentially relevant reports.
Conference abstracts for the years 2006 onwards from meetings of the European, American and British Urological Associations will be searched. Ongoing studies will be identified through searching Current Controlled Trials, Clinical Trials, NIHR Portfolio and WHO International Clinical Trials Registry. Websites of manufacturers, professional organisations, regulatory bodies and the HTA will be checked to identify unpublished reports.
3.3 Quality assessment
We will use a modified version of the Cochrane risk of bias tool27 which we have adapted to include potential topic-specific confounders, which were identified through discussions with members of our project advisory group and our knowledge of existing literature. The topic-specific confounders related to specific outcomes as shown in the modified risk of bias tool (see Appendix 4). Three sets of two reviewers will independently assess the risk of bias of included full text studies, with the exception of non-English publications and conference abstracts. Any differences in assessment or issues of uncertainty will be resolved by discussion and consensus. For the risk of bias tool individual outcomes will be scored as High risk of bias, Low risk of bias or Unclear. Any disagreements will be resolved by consensus or by a third party.
3.4 Data extraction
Three reviewers will independently screen titles and abstracts of all identified items. Full text copies of all potentially relevant reports will be obtained and independently assessed by two reviewers to determine whether they meet inclusion criteria. Three reviewers will independently extract details of study design, methods, participants, interventions and outcomes onto a data extraction form (see Appendix 3). Each reviewer’s data extraction will be independently checked by a second reviewer for errors or inconsistencies. Any disagreements will be resolved through consensus or arbitration by a third party.
3.5 Data analysis
Data from each study will be tabulated and summarised for each procedure in a form appropriate for the mixed treatment comparison model. The lack of RCT evidence precludes undertaking a standard meta-analysis. Therefore we intend to adopt an indirect comparison (cross design) approach allowing inclusion of non-randomised comparative data and case series. 28 Reasons for heterogeneity of effects will be explored, including differences in populations, studies, outcome assessment and learning curve effects. We will examine heterogeneity between and within different study designs using a Bayesian hierarchical random effects model enabling use of all available evidence. 29
We will use a previously successful approach developed by members of our project team to estimate the learning effects on key outcomes. 30 The expertise of the participating surgeons or centres in each included study will first be categorised by previous experience. Data on the three key features of learning, starting level, rate of learning and expert level, will then be extracted. A random effects meta-analysis will be performed to estimate the pooled effect of the key features together with an appropriate measure of uncertainty. These estimates will be used to determine the likely ‘shape’ of the learning curve and will be validated by our experienced and novice clinical experts. The pooled data will be used firstly to investigate heterogeneity of effects on the key outcomes in the systematic review of effectiveness and secondly to inform the economic modelling on the likely change over time on the key outcomes and patient mix. This approach will account for possible differences in an individual surgeon’s learning curve for particular outcomes.
4. Cost-effectiveness
4.1 Systematic review of economic evaluations
Given that the results of any economic evaluation are particular to setting and time the main purpose of a review is to inform the modelling methodology and any parameter sources. This does not require a systematic review, but a review of key sources, i.e. those with a signal of high quality such as HTA reports. Therefore, there will be two reviews, a systematic one detailed below to identify the current status of the evidence on the technologies of interest and one of HTA reports, their citations and sources citing them looking at any technology for prostate cancer that uses modelling.
Search strategy
Highly sensitive search strategies will be designed to identify any economic evaluations where at least one of the technologies was laparoscopic or robotic surgery for prostate cancer. The following databases will be searched without language restriction for the years 1995 onwards: NHS EED, HTA Database, Medline, Medline In Process, Embase, Science Citation Index and Health Management Information Consortium (HMIC) database. Websites of HTA organisations will be consulted for additional reports. Reference lists of all included studies will be scanned and appropriate experts will be contacted for details of additional reports.
Quality assessment
Quality will be assessed according to the BMJ criteria, on which the NHS EED abstracts were largely based. 31
Data extraction
Two reviewers will independently screen the titles and abstracts of all items identified by the search strategy. Full text copies of all potentially relevant reports will be obtained and assessed by two reviewers independently against the inclusion criteria. Any disagreements will be resolved by consensus or arbitration by a third person. Two reviewers will independently extract details of study design such as economic perspective and type of analysis, methods such as model structure and costing, population, technologies, and outcomes such as QALYs onto specific data extraction forms in line with the NHS EED abstracts.
Reporting
Summaries of all studies will be tabulated. A brief critique according to model structure, paramaterisation and dealing with uncertainty will then be performed to identify methods that can be used together with limitations and recommendations for improvement that can be taken forward to the proposed model. Any sources of evidence of possible use in the proposed model will be recorded and reviewed by the research team.
4.2 Economic evaluation
Implications for the economic analysis
As no prior economic evaluation has been conducted from the perspective of the UK NHS we propose to construct a decision analytic model (DAM) comparing the cost-effectiveness of the two surgical techniques, which will make the best use of the evidence obtained from the systematic review32 A novel aspect of this work will be the emphasis on the learning curves for surgical procedures and economies of scale from changes in centre volumes which are likely to drive differences in costs for the considered technologies, something that in a typical CEA as recommended by NICE33 might be ignored. These particular facets are likely to be instrumental in driving differences in costs for the considered technologies and therefore need to be accorded greater weight in the analysis. In addition to this the impact of capital costs (approximately £1.5 million) and maintenance costs (approximately £150,000/year) for robotic prostatectomy are likely to be significant, particularly in lower volume centres. Changes from the recommended standard procedure would take time to implement, and require more intensive re-training involving use of mentors which, although associated with a briefer learning curve,34 may have additional resource implications and therefore require consideration in the model.
Model structure
In order to incorporate the effect of disease progression and possible need for subsequent treatments for each patient undergoing laparoscopic or robotic prostatectomy, a state transition model will be used which estimates consequences for a cohort beginning treatment at the same time. However, in order to estimate effects due to the learning curves for laparoscopic and robotic techniques a multiple cohort analysis will be used. 35,36 Such an approach, by allowing for changing numbers of patients eligible for surgery over time, also permits estimation of capital outlay as a function of demand, which was the approach used in a previous model. 37 However, even if demand remains constant, it also allows availability of technology, which is a function of surgeon competence, to be expressed as a function of patient numbers. This also enables consideration of the most efficient number of treatment centres. A multiple cohort approach additionally allows for population heterogeneity in age; those who are eligible for treatment will vary by age38 requiring the introduction of one cohort per age band per year. Although the technologies will be assumed to have a finite lifetime decided by manufacturer and clinical expert opinion and tested in a sensitivity analysis, each individual cohort will be followed up for various periods including the duration of patient lifetime in order to account for consequences for that cohort. 39
The design for the state transition model* used for each cohort was informed by expert opinion and published models of the progression of prostate cancer. 40–42 Patient eligibility is defined according to:
-
Male.
-
Cancer localised to prostate
[*Please note that during consultation with the advisory group the modelling approach was changed to a discrete-event simulation model. Full details and rationale in Chapter 5, Introduction.]
These criteria, including age will thus define an initial pre-operative state. A patient will then undergo one of the procedures whereby a set of short-term complications can occur according to corresponding probabilities each of which are assumed to be resolved within a the cycle time of 3 months. Micro-simulation43 will be used to analyse the model whereby an individual follows a random path over a lifetime using Monte Carlo Simulation (MCS). This reduces the need to define a separate health state of each of the set of criteria used to define a health state, e.g. presence or absence of each complication. Therefore, subsequent health states will be defined according to the following set of state variables:
-
Age
-
Margin (positive or negative)
-
Postoperative Gleason score (high or low)
-
Recurrence (none, local, systemic)
-
Erectile dysfunction (present or not)
-
Urinary incontinence (present or not)
Therefore transition probabilities (probability of moving to some health state in 3 months given current health state) will be defined according to the status of each of the state variables. For example, mortality rate increases with age and type of recurrence. Also, as can be seen in the care pathway, further treatments also depend on state variables so that, for example, the presence of urinary incontinence implies treatment for this condition. Postoperative evaluation of the surrounding tissue may lead to further treatment conditional on determining a positive or negative margin (Fig. 2). Where tissue margins are observed to be positive, then Gleason scores are used to identify an appropriate treatment within the pathway. Patients with high Gleason scores are immediately referred for further cancer treatment, whereas patients exhibiting low Gleason scores are monitored for Biochemical recurrence. Should biochemical recurrence be observed, patients may then devolve to additional treatment for cancer, otherwise surveillance will continue. Patients with a negative margin will be referred for surveillance with the possibility of further cancer treatment if necessary.
Pathways for treatments available to patients with prostate cancer are described in Figure 3. The treatment of localised cancers devolves into curative or palliative sub-pathways. Each sub-pathway may then lead to dysfunctions associated with the underlying condition and treatment. Ultimately, patients will reach a state of resolution or death. In the case of resolution of cancer, patients may then still be treated for the presence of one or more dysfunctions (Fig. 4–5). Patients may suffer from one or more dysfunctions simultaneously. In either case, interventions strategies may vary according to the severity of dysfunction. Ultimately, a patient may recover or reach a persistent state.
The economic perspective will be that of the United Kingdom National Health Service and discounting in the base case will be at 3.5%. 33 All modelling will pay attention to best practice44 and guidance from the project expert advisory group. The model will be constructed in two software packages according to best practice44 in C for speed and flexibility and TreeAge for presentation including any sensitivity analysis on demand.
[Please note that during consultation with the advisory group the modelling approach was changed to a discrete-event simulation model. Full details and rationale are given in Chapter 5, Introduction.]
Costing
Given the variation in costs due to learning and requirement for capital expenditure, it is essential to estimate the independent effect of staffing, equipment and overheads. As described above, some costs will be incurred as each patient progresses through the care pathway and thus would count as variable (with demand). However, a machine (and any additional building space) must be purchased regardless of numbers to be treated at least beyond the capacity of any existing machine. Therefore such a cost is fixed at least in the short term. The most appropriate sources will be used for each of these, such as expert opinion to determine appropriate staff mix, the systematic review to estimate operation times and length of stay as a function of technology, and purchase/maintenance costs from manufacturers and local users and their finance departments. Unit costs will be taken from appropriate routine sources for staffing,45 British National Formulary for drugs, and from equipment manufacturers. Variability in parameters will be tested by one-way sensitivity analyses.
Utilities
A cost utility analysis (CUA) will be performed with outcomes estimated in quality-adjusted life years (QALYs). 46 Each health state of the state transition model will require a utility estimated using the best available data, ideally derived using EQ-5D. 47–50 If necessary, plausible assumptions will be made in order to use utility values derived from different patient population (e.g. using an additive model to combine the effects of disease progression and adverse events in one age group to estimate the effect in a different age group).
Epidemiology
Two main items of epidemiological data are required for the economic model: one at the individual level to estimate the transition probabilities of the state transition model and another at the population level for the incidence of eligible patients. The former will be based on data from the systematic review and include any effect of surgeon experience/learning. The latter will be informed by incidence data and any likely trends informed by expert opinion. Each parameter will correspond to transitions between states in the model, such as from first treatment to remission.
Uncertainty
Deterministic sensitivity analyses will be carried out to test for the effect of assumptions and variability. 51 Costs and QALYs will be estimated as the expectation over the joint distribution of the parameters, informed from the systematic review, other sampling distributions or expert opinion according to best practice. Any correlations, informed where possible by the systematic review, will be incorporated. A probabilistic sensitivity analysis will also be undertaken allowing presentation of results in a series of cost-effectiveness acceptability curves (CEAC) and the construction of the cost-effectiveness acceptability frontier (CEAF) for various threshold values of the willingness to pay (WTP) for a QALY. 52
Identification of future research needs
A value of information analysis53 will be conducted to identify the expected value of perfect information (EVPI) over the expected lifetime of the considered procedures and the value of further research to identify more precise and reliable estimates of parameters used in the model.
5. Timescale
Start of project: 1st March 2010
Develop protocol and data extraction form: March – April 2010
Run search strategies: April 2010
Assess studies for inclusion: April – June 2010
First expert panel meeting: May 2010
Data extraction and quality assessment: July – September 2010
First progress report: 10 October 2010
Data analysis: October – December 2010
Second expert panel meeting: February 2011
Economic modelling: May 2010 – March 2011
Second progress report: February 2011
Report writing: January – April 2011
Report submission: 16th May 2011
6. References
- Westlake S. Cancer incidence and mortality in the United Kingdom and constituent countries, 2003–05. Health Statistics Quarterly 40. [document on the Internet]. London: Office for National Statistics; 2008.
- UK Prostate cancer incidence statistics [website on the Internet]. London: Cancer Research UK; 2008.
- Chamberlain J, Melia J, Moss S, Brown J. The diagnosis, management, treatment and costs of prostate cancer in England and Wales. Health Technol Assess 2001;1.
- Prescription Cost Analysis 2007 [webpage on the Internet]. NHS Information Centre; 2008.
- Prostate cancer: diagnosis and treatment; full guidance. CG58 [document on the Internet]. London: National Insititute for Health and Clinical Excellence; 2008.
- Bosanquet N, Sikora K. The economics of cancer care in the UK. Lancet Oncology 2004;5:568-74.
- Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84.
- Bott SR, Birtle AJ, Taylor CJ, Kirby RS. Prostate cancer management: (1) an update on localised disease. Postgrad Med J 2003;79:575-80.
- Bhatnagar V, Kaplan RM. Treatment options for prostate cancer: evaluating the evidence. Am Fam Physician 2005;71:1915-22.
- Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007;99:1171-7.
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2002;20:557-66.
- Eden CG. Minimal access prostatectomy: how is it shaping up?. BJU Int 2008;101:791-2.
- Herkommer K, Fuchs TA, Hautmann RE, Volkmer BG. Radical prostatectomy for men aged < 56 years with prostate cancer. Cost of illness analysis. Urologe (Ausg 1185;A) n.d.;44:1183-4.
- Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology 2002;60:864-8.
- Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic radical prostatectomy for localized prostate cancer: a systematic review of comparative studies. J Urol 2006;175:2011-7.
- Smith JA, Herrell SD. Robotic-assisted laparoscopic prostatectomy: do minimally invasive approaches offer significant advantages?. J Clin Oncol 2005;23:8170-5.
- Goldstraw MA, Patil K, Anderson C, Dasgupta P, Kirby RS. A selected review and personal experience with robotic prostatectomy: implications for adoption of this new technology in the United Kingdom. Prostate Cancer Prostat Dis 2007;10:242-9.
- Kaul SA, Peabody JO, Shah N, Neal D, Menon M. Establishing a robotic prostatectomy programme: The impact of mentoring using a structured approach. BJU Int 2006;97:1143-4.
- Mayer EK, Winkler MH, Aggarwal R, Karim O, Ogden C, Hrouda D, et al. Robotic prostatectomy: the first UK experience. MRCAS 2006;2:321-8.
- Rozet F, Harmon J, Cathelineau X, Barret E, Vallancien G. Robot-assisted versus pure laparoscopic radical prostatectomy. World J Urol 2006;24:171-9.
- Burgess SV, Atug F, Castle EP, Davis R, Thomas R. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol 2006;20:827-30.
- Ellison LM, Heaney JA, Birkmeyer JD. The effect of hospital volume on mortality and resource use after radical prostatectomy. J Urol 2000;163:867-9.
- Ramirez A, Benayoun S, Briganti A, Chun J, Perrotte P, Kattan MW, et al. High radical prostatectomy surgical volume is related to lower radical prostatectomy total hospital charges. Eur Urol 2006;50:58-62.
- Epstein JI, Amin M, Boccon-Gibod L, Egevad L, Humphrey PA, Mikuz G, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl 2005;216:34-63.
- Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Lilja H, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol 2006;24:3973-8.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [document on the Internet]. The Cochrane Collaboration; 2008.
- Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 2001;10:277-303.
- Prevost TC, Abrams KR, Jones DR. Hierarchical models in generalized synthesis of evidence: an example based on studies of breast cancer screening. Stat Med 2000;19:3359-76.
- Cook JA, Ramsay CR, Fayers P. Using the literature to quantify the learning curve: a case study. Int J Technol Assess Health Care 2007;23:255-60.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Stat Med 2003;22:3687-709.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal [document on the Internet] 2008. URL: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf.
- Fabrizio MD, Tuerk I, Schellhammer PF. Laparoscopic radical prostatectomy: decreasing the learning curve using a mentor initiated approach. J Urol 2003;169:2063-5.
- Goldman L, Gaspoz JM. Cost-effectiveness of clopidogrel: seeing through the smoke. Med Decis Making 2008;28:803-9.
- Lourenco T, Armstrong N, Nabi G, Deverill M, Pickard R, Vale L, et al. Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement (BPE). Health Technol Assess 2008;12.
- Armstrong N, Vale L, Deverill M, Nabi G, McClinton S, N’Dow J, et al. Surgical treatments for men with benign prostatic enlargement: cost effectiveness study. Br Med J 2009;338:1187-90.
- Cancer Statistics Registration. Registration of Cancer Diagnosis in 2006, England. [document on the Internet] 2008. URL: http://www.statistics.gov.uk/downloads/theme_health/MB1-37/MB1_37_2006.pdf.
- Karnon J, Brennan A, Akehurst R. A critique and impact analysis of decision modeling assumptions. Med Decis Making 2007;27:491-9.
- Alibhai SM, Naglie G, Nam R, Trachtenberg J, Krahn MD. Do older men benefit from curative therapy of localized prostate cancer?. J Clin Oncol 2003;21:3318-27.
- Calvert NW, Morgan AB, Catto JW, Hamdy FC, Akehurst RL, Mouncey P, et al. Effectiveness and cost-effectiveness of prognostic markers in prostate cancer. Br J Cancer 2003;88:31-5.
- Svatek RS, Lee JJ, Roehrborn CG, Lippman SM, Lotan Y. The cost of prostate cancer chemoprevention: a decision analysis model. Cancer Epidemiol Biomarkers Prevent 2006;15:1485-9.
- Karnon J, Brown J. Selecting a decision model for economic evaluation: a case study and review. Health Care Manag Sci 1998;1:133-40.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Curtis L. Unit costs of health and social care. University of Kent: Personal Social Services Research Unit; 2008.
- Drummond MF, Sculpher M, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford University Press; 1997.
- Albertsen PC, Nease RF, Potosky AL. Assessment of patient preferences among men with prostate cancer. J Urol 1998;159:158-63.
- Dale W, Basu A, Elstein A, Meltzer D. Predicting utility ratings for joint health states from single health states in prostate cancer: empirical testing of 3 alternative theories. Med Decis Making 2008;28:102-12.
- Krahn M, Ritvo P, Irvine J, Tomlinson G, Bremner KE, Bezjak A, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care 2003;41:153-64.
- Smith DS, Krygiel J, Nease RF, Sumner W, Catalona WJ. Patient preferences for outcomes associated with surgical management of prostate cancer. J Urol 2002;167:2117-22.
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000;17:479-500.
- Briggs AH, McGuire M, Drummond AM, McGuire A. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64.
Appendix 2 Search strategies
Clinical effectiveness of robotic compared with laparoscopic techniques
MEDLINE (1966–October week 3 2010), EMBASE (1980–2010 week 42) (MEDLINE In-Process & Other Non-Indexed Citations 25 October 2010)
Ovid Multifile Search URL: https://shibboleth.ovid.com/
-
exp prostatic neoplasms/su use mesz
-
exp prostate cancer/su use emez
-
prostatectomy/
-
(radical adj5 prostatectom$).tw.
-
or/1-4
-
prostatic neoplasms/ use mesz
-
exp prostate cancer/ use emez
-
(cancer adj3 (prostate or prostatic)).tw.
-
(carcinoma adj3 (prostate or prostatic)).tw.
-
(neoplas$ adj3 (prostate or prostatic)).tw.
-
(malignan$ adj3 (prostate or prostatic)).tw.
-
or/6-11
-
surgical procedures,operative/ use mesz
-
surgery/ use emez
-
su.fs.
-
(surgery or surgical or surgeon$).tw.
-
(resect$ or operation$ or operat$).tw.
-
or/13-17
-
12 and 18
-
5 or 19
-
laparoscopy/
-
laparoscopic surgery/ use emez
-
endoscopy/
-
video-assisted surgery/
-
surgical procedures, minimally invasive/ use mesz
-
minimally invasive surgery/ use emez
-
laparoscop$.tw.
-
endoscop$.tw.
-
(minimal$ adj3 (invasiv$ or access$)).tw.
-
(key hole or keyhole or robot$).tw.
-
video assist$.tw.
-
(trans peritoneal or transperitoneal or extra peritoneal).tw.
-
(montsouris or heilbronn).tw.
-
(da vinci or zeus).tw.
-
or/21-34
-
20 and 35
-
meta-analysis.pt.
-
review.pt.
-
meta-analysis/
-
systematic review/
-
randomized controlled trials/
-
(controlled or design or evidence or extraction).ab.
-
(sources or studies).ab.
-
or/37-43
-
exp clinical trial/
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomization/ use emez
-
randomi?ed.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/45-54
-
comparative study/ use mesz
-
follow-up studies/ use mesz
-
time factors/ use mesz
-
Treatment outcome/ use emez
-
major clinical study/ use emez
-
controlled study/ use emez
-
clinical trial/ use emez
-
(preoperat$ or pre operat$).mp. use mesz
-
(chang$ or evaluat$ or reviewed or baseline).tw.
-
(prospective$ or retrospective$).tw. use mesz
-
(cohort$ or case series).tw. use mesz
-
(compare$ or compara$).tw. use emez
-
or/56-67
-
36 and (44 or 55 or 68)
-
animals/ not (humans/ and animals/)
-
nonhuman/ not (human/ and nonhuman/)
-
69 not (70 or 71)
-
limit 72 to yr=”1995-2010”
-
remove duplicates from 73
Science Citation Index (1995–23 October 2010), BIOSIS (1995–19 October 2010)
ISI Web of Knowledge URL: http://wok.mimas.ac.uk/
#1 TS=prostatectomy
#2 TS= (cancer SAME (prostate or prostatic))
#3 TS= (carcinoma SAME (prostate or prostatic))
#4 TS= (neoplas* SAME (prostate or prostatic))
#5 TS= (malignan* SAME (prostate or prostatic))
#6 #2 or #3 or #4 or #5
#7 #6 and TS=surgery
#8 #6 and TS=surgical
#9 #6 and TS=resect*
#10 #6 and TS=operat*
#11 #1 OR #7 OR #8 OR #9 OR #10
#12 #11 and TS=laparoscop*
#13 #11 and TS=endoscop*
#14 #11 and TS=(key hole or keyhole or robot*)
#15 #11 and TS=(minimal* SAME (invasive* or access*))
#16 #11 and TS=video assist*
#17 #11 and TS=(trans peritoneal or transperitoneal or extra peritoneal)
#18 #11 and TS=(montsouris or heilbronn or da vinci or zeus)
#19 #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 #19 and TS=trial*
#21 #19 and TS=random*
#22 #19 and TS=(compare or comparative or comparison)
#23 #19 and TS=evaluat*
#24 #19 and TS=cohort
#25 #19 and TS=case series
#26 #19 and TS=meta analysis
#27 #19 and TS=review*
#28 #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
The Cochrane Library (CDSR Issue 10 2010, CENTRAL Issue 4 2010)
URL: http://www3.interscience.wiley.com/
#1 MeSH descriptor Prostatic Neoplasms explode all trees with qualifier: SU
#2 MeSH descriptor Prostatectomy, this term only
#3 (radical NEAR prostatectom*)
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Prostatic Neoplasms explode all trees
#6 (cancer NEAR/3 (prostate or prostatic))
#7 (carcinoma NEAR/3 (prostate or prostatic))
#8 (neoplas* NEAR/3 (prostate or prostatic))
#9 (malignan* NEAR/3 (prostate or prostatic))
#10 (#5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Surgical Procedures, Operative, this term only
#12 Any MeSH descriptor with qualifier: SU
#13 (surgery or surgical or surgeon*)
#14 (resect* or operation* or operat*)
#15 (#11 OR #12 OR #13 OR #14)
#16 (#10 AND #15)
#17 (#4 OR #16)
#18 MeSH descriptor Laparoscopy, this term only
#19 MeSH descriptor Endoscopy, this term only
#20 MeSH descriptor Video-Assisted Surgery, this term only
#21 MeSH descriptor Surgical Procedures, Minimally Invasive, this term only
#22 (laparoscop*) or (endoscop*) or (minimal* NEAR/3 (invasiv* OR access*)) or (key hole or keyhole) or (video assist*) or (robot*)
#23 (trans peritoneal OR transperitoneal) or (extra peritoneal) or (montsouris or heilbronn) or (da vinvi or zeus)
#24 (#18 OR #19 OR #20 OR #21 OR #22 OR #23)
#25 (#17 AND #24)
HTA/DARE (October 2010)
Centre for Reviews and Dissemination URL: http://nhscrd.york.ac.uk/welcome.htm
#1 MeSH prostatic neoplasms QUALIFIERS SU EXPLODE 1 2 3 4
#2 MeSH prostatectomy EXPLODE 1
#3 MeSH prostatic neoplasms EXPLODE
#4 surg* or laparoscop* or robot*
#5 (#2 or #3)
#6 #4 and #5
#7 #1 or #6
ClinicalTrials.gov (October 2010)
URL: http://clinicaltrials.gov/ct/gui/c/r
Condition=prostatic neoplasms AND (laparoscop* or robot*)
Current Controlled Trials (October 2010)
URL: www.controlled-trials.com/
Prostat% and (laparoscop% or robot%)
International Clinical Trials Registry Platform (ICTRP) (October 2010)
World Health Organization URL: www.who.int/ictrp/en/
Prostat* and (laparoscop* or robot*)
NIH RePORTER (October 2010)
URL: http://projectreporter.nih.gov/reporter.cfm
Prostat% and laparoscop%
Prostat% and robot%
Conference proceedings
American Society of Clinical Oncology (URL: www.asco.org)
Annual Meeting, Chicago, IL, 1–5 June 2007
Annual Meeting, Chicago, IL, 30 May–2 June 2008
Annual Meeting, Orlando, FL, 29 May–2 June 2009
Annual Meeting, Chicago, IL, 4–8 June 2010
American Urological Association (URL: www.auanet.org/)
Annual Meeting, Orlando, FL, 12–22 May 2008
Annual Meeting, Chicago, IL, 25–30 April 2009
Annual Meeting, San Francisco, CA, 29 May–3 June 2010
British Association of Urological Surgeons (URL: www.baus.org.uk/)
Annual Scientific Meeting, Manchester, UK, 23–27 June 2008.
Annual Scientific Meeting, Glasgow, UK, 22–25 June 2009
Annual Scientific Meeting, Manchester, UK, 21–24 June 2010
European Association of Urology (URL: www.uroweb.org/)
22nd Annual Congress, Berlin, Germany, 21–24 March 2007
23rd Annual Congress, Milan, Italy, 26–29 March 2008
24th Annual Congress, Stockholm, Sweden, 17–21 March 2009
25th Annual Congress, Barcelona, Spain, 16–20 April 2010
European Robotic Urology Symposium, Bordeaux, France, 29 September–1 October 2010
Websites consulted
American Society of Clinical Oncology (URL: www.asco.org)
American Urological Association (URL: www.auanet.org/)
British Association of Urological Surgeons (URL: www.baus.org.uk/)
Cancer Research UK (URL: http://info.cancerresearchuk.org/cancerstats/)
European Association of Urology (URL: www.uroweb.org/)
Intuitive Surgical – da Vinci prostatectomy (URL: www.davinciprostatectomy.com/)
Cost-effectiveness of robotic compared with laparoscopic techniques
MEDLINE (1966–October week 4 2010), EMBASE (1980–2010 week 43) (MEDLINE In-Process & Other Non-Indexed Citations 3 November 2010)
Ovid Multifile Search URL: https://shibboleth.ovid.com/
-
exp prostatic neoplasms/su use mesz
-
exp prostate cancer/su use emez
-
prostatectomy/
-
(radical adj5 prostatectom$).tw.
-
or/1-4
-
prostatic neoplasms/ use mesz
-
exp prostate cancer/ use emez
-
(cancer adj3 (prostate or prostatic)).tw.
-
(carcinoma adj3 (prostate or prostatic)).tw
-
(neoplas$ adj3 (prostate or prostatic)).tw.
-
(malignan$ adj3 (prostate or prostatic)).tw.
-
or/6-11
-
surgical procedures,operative/ use mesz
-
surgery/ use emez
-
su.fs.
-
(surgery or surgical or surgeon$).tw.
-
(resect $ or operation$ or operat$).tw.
-
or/13-17
-
12 and 18
-
5 or 19
-
laparoscopy/
-
laparoscopic surgery/ use emez
-
endoscopy/
-
video-assisted surgery/
-
surgical procedures, minimally invasive/ use mesz
-
minimally invasive surgery/ use emez
-
laparoscop$.tw.
-
endoscop$.tw.
-
(minimal adj3 (invasiv$ or access$)).tw.
-
(key hole or keyhole or robot$).tw.
-
video assist$.tw
-
(trans peritoneal or transperitoneal or extra peritoneal).tw.
-
(montsouris or heilbronn).tw.
-
(da vinci or zeus).tw.
-
or/21-34
-
20 and 35
-
exp “costs and cost analysis”/
-
exp economic evaluation/ use emez
-
economics
-
exp economics,hospital/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp budgets/
-
exp models, economic/
-
exp decision theory/
-
ec.fs. use mesz
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economics$ or pharmacoeconomic$ or pharmo-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(value adj2 (money or monetary)).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
or/37-59
-
36 and 60
-
remove duplicates from 61
Science Citation Index (1995–30 October 2010)
ISI Web of Knowledge URL: http://wok.mimas.ac.uk/
#1 TS=prostatectomy
#2 TS=(cancer SAME (prostate or prostatic))
#3 TS=(cancinoma SAME (prostate or prostatic))
#4 TS=(neoplas* SAME (prostate or prostatic))
#5 TS=(malignan* SAME (prostate or prostatic))
#6 #2 or #3 or #4 or #5
#7 #6 and TS=(surgery or surgical)
#8 #6 and TS=(resect* or operat*)
#9 #1 or #7 or #8
#10 #9 AND TS=LAPAROSCOP*
#11 #9 AND TS=endoscop*
#12 #9 AND TS=(keyhole or key hole or robot*)
#13 #9 AND TS=(minimal* SAME (invasive* or access*))
#14 #9 AND TS=video assist*
#15 #9 AND TS=(montsouris or heilbronn or da vinci or zeus)
#16 #10 or #11 or #12 or #13 or #14 or #15
#17 TS=(cost* SAME effective*)
#18 TS=(cost* SAME benefit*)
#19 TS=(cost* SAME ( utility or utilities))
#20 TS=(cost* SAME (minimis* or minimiz*))
#21 TS=economic*
#22 TS=(price OR pricing)
#23 TS=(financial OR finance OR finances OR financed)
#24 TS=(value SAME (money OR monetary))
#25 TS=(markov OR monte carlo)
#26 TS=(decision SAME (tree* OR analy* OR model*))
#27 #16 AND (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #25 OR #26)
Health Management Information Consortium (1979–October 2010)
Ovid Multifile Search URL: http://gateway.ovid.com/athens
-
prostate cancer/
-
prostatectomy/
-
(radical adj5 prostatectom$).tw.
-
((prostate or prostatic) adj3 (cancer or carcinoma or neoplas$ or malignan$ or tumo?r$)).tw.
-
or/1-4
-
minimally invasive therapy/
-
laparoscop$.tw.
-
(key hole or keyhole or robot$).tw.
-
(minimal$ adj3 (invasiv$ or access$)).tw.
-
video assist$.tw.
-
(da vinci or zeus).tw.
-
(montsouris or heilbronn).tw.
-
or/6-12
-
5 and 13
NHS Economic Evaluation Database (October 2010)
Centre for Reviews and Dissemination URL: http://nhscrd.york.ac.uk/welcome.htm
#1 MeSH prostatic neoplasms QUALIFIERS SU EXPLODE
#2 MeSH prostatectomy EXPLODE
#3 MeSH prostatic neoplasms EXPLODE
#4 surg* or laparoscop* or robot*
#5 (#2 or #3)
#6 #4 and #5
#7 #1 or #6
Quality of life for robotic compared with laparoscopic techniques
MEDLINE (1966–October week 4 2010), EMBASE (1980–2010 week 43) (MEDLINE In-Process & Other Non-Indexed Citations 3 November 2010)
Ovid Multifile Search URL: https://shibboleth.ovid.com/
-
exp prostatic neoplasms/su use mesz
-
exp prostate cancer/su use emez
-
prostatectomy/
-
(radical adj5 prostatectom$).tw.
-
or/1-4
-
prostatic neoplasms/ use mesz
-
exp prostate cancer/ use emez
-
(cancer adj3 (prostate or prostatic)).tw.
-
(carcinoma adj3 (prostate or prostatic)).tw
-
(neoplas$ adj3 (prostate or prostatic)).tw.
-
(malignan$ adj3 (prostate or prostatic)).tw.
-
or/6-11
-
surgical procedures,operative/ use mesz
-
surgery/ use emez
-
su.fs.
-
(surgery or surgical or surgeon$).tw.
-
(resect $ or operation$ or operat$).tw.
-
or/13-17
-
12 and 18
-
5 or 19
-
laparoscopy/
-
laparoscopic surgery/ use emez
-
endoscopy/
-
video-assisted surgery/
-
surgical procedures, minimally invasive/ use mesz
-
minimally invasive surgery/ use emez
-
laparoscop$.tw.
-
endoscop$.tw.
-
(minimal adj3 (invasiv$ or access$)).tw.
-
(key hole or keyhole or robot$).tw.
-
video assist$.tw
-
(trans peritoneal or transperitoneal or extra peritoneal).tw.
-
(montsouris or heilbronn).tw.
-
(da vinci or zeus).tw.
-
or/21-34
-
20 and 35
-
quality of life/
-
quality adjusted life year/
-
“Value of Life”/ use mesz
-
health status indicators/ use mesz
-
health status/ use emez
-
sickness impact profile/ use mesz
-
disability evaluation/ use mesz
-
disability/ use emez
-
activities of daily living/ use mesz
-
exp daily life activity/ use emez
-
cost utility analysis/ use emez
-
rating scale/
-
questionnaires/
-
(quality adj1 life).tw.
-
quality adjusted life.tw.
-
disability adjusted life.tw.
-
(qaly? or qald? or qale? or qtime? or daly?).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw.
-
health$ year$ equivalent$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
(health adj3 (utilit$ or disutili$)).tw.
-
(health adj3 (state or status)).tw.
-
(sf36 or sf 36 or short form 36 or shortform 36).tw.
-
(sf6 or sf 6 or short form 6 or shortform 6).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20).tw.
-
willingness to pay.tw.
-
standard gamble.tw.
-
trade off.tw.
-
conjoint analys?s.tw.
-
discrete choice.tw.
-
or/37-70
-
(case report or editorial or letter).pt.
-
case report/
-
71 not (72 or 73))
-
36 and 74
-
remove duplicates from 75
Science Citation Index (1995–30 October 2010)
ISI Web of Knowledge URL: http://wok.mimas.ac.uk/
#1 TS=prostatectomy
#2 TS=(cancer SAME (prostate or prostatic))
#3 TS=(cancinoma SAME (prostate or prostatic))
#4 TS=(neoplas* SAME (prostate or prostatic))
#5 TS=(malignan* SAME (prostate or prostatic))
#6 #2 or #3 or #4 or #5
#7 #6 and TS=(surgery or surgical)
#8 #6 and TS=(resect* or operat*)
#9 #1 or #7 or #8
#10 #9 AND TS=LAPAROSCOP*
#11 #9 AND TS=endoscop*
#12 #9 AND TS=(keyhole or key hole or robot*)
#13 #9 AND TS=(minimal* SAME (invasive* or access*))
#14 #9 AND TS=video assist*
#15 #9 AND TS=(montsouris or heilbronn or da vinci or zeus)
#16 #10 or #11 or #12 or #13 or #14 or #15
#17 TS=quality of life
#18 TS=quality adjusted life
#19 TS=disability adjusted life
#20 TS= (qaly* OR qald* OR qale* OR qtime* OR daly)
#21 TS=(euroqol* OR euro qol* OR eq5d OR eq 5d)
#22 TS=(hql OR hqol OR h qol OR hrqol OR hr qol)
#23 TS=health* year* equivalent*
#24 TS=(hye OR hyes OR hui OR hui1 OR hui2 OR hui3)
#25 TS=(health utilit* OR disutilit*)
#26 TS=willingness to pay
#27 TS=standard gamble
#28 TS=discrete choice.
#29 TS=trade off
#30 TS= conjoint analys*
#31 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30
#32 #16 and #31
Appendix 3 Data extraction form
Appendix 4 Risk of bias form
Cochrane risk of bias table (non-randomised studies)
Laparoscopic versus robotic prostatectomy for localised prostate cancer
Assessor initial: Date evaluated:
Study ID:
| Item | Judgementa | Description (quote from paper or describe key information) | ||
|---|---|---|---|---|
| 1. Sequence generation | ||||
| 2. Allocation concealment | ||||
| 3a. Confoundingb | Outcome 1 (perioperative safety) | Confounders balancedb | ||
| Surgeon experience | ||||
| Comorbidity (ASA/Charlson score) | ||||
| Prostate size | ||||
| 3b. Confoundingb | Outcome 2 (urinary dysfunction) | Confounders balancedb | ||
| Surgeon experience | ||||
| Age | ||||
| Neurovascular bundle excision | ||||
| Anastomotic stricture | ||||
| 3c. Confoundingb | Outcome 3 (erectile dysfunction) | Confounders balancedb | ||
| Preoperative dysfunction/status | ||||
| Neurovascular bundle excision | ||||
| Surgeon experience | ||||
| Age/comorbidity | ||||
| 3d. Confoundingb | Outcome 4 (efficacy) | Confounders balancedb | ||
| Gleason score balanced at baseline | ||||
| Surgeon experience | ||||
| PSA score balanced at baseline | ||||
| Clinicalc tumour stage/nodal stage balanced at baseline | ||||
| 4a. Blinding? | Outcome 1 (perioperative safety) | |||
| 4b. Blinding? | Outcome 2 (urinary dysfunction) | |||
| 4c. Blinding? | Outcome 3 (erectile dysfunction) | |||
| 4d. Blinding? | Outcome 4 (efficacy) | |||
| 5a. Incomplete outcome data addressed? | Outcome 1 (perioperative safety) | |||
| 5b. Incomplete outcome data addressed? | Outcome 2 (urinary dysfunction) | |||
| 5c. Incomplete outcome data addressed? | Outcome 3 (erectile dysfunction) | |||
| 5d. Incomplete outcome data addressed? | Outcome 4 (efficacy) | |||
| 6a. Free of selective reporting? | Outcome 1 (perioperative safety) | |||
| 6b. Free of selective reporting? | Outcome 2 (urinary dysfunction) | |||
| 6c. Free of selective reporting? | Outcome 3 (erectile dysfunction) | |||
| 6d. Free of selective reporting? | Outcome 4 (efficacy) | |||
| 7. Free of other bias? | ||||
| 8. A priori protocol?d | ||||
| 9. A priori analysis plan?e | ||||
General decision rules
-
When a paper does not report details of confounders/other source of bias this should be judged as unclear.
-
When a paper does not report considered outcomes this should be judged as not applicable.
-
Allocation concealment should be judged as high risk of bias if groups are allocated by factors such as surgeon decision, patient preference. Allocation by hospital/institution = low risk. When no details are given, judge as unclear.
-
Surgeon experience: assume that surgeons performing open prostatectomy are experienced unless stated otherwise.
-
Absence of blinding is likely to have a low risk of bias for perioperative and efficacy outcomes.
-
Free of other bias: default is low risk unless there is a fundamental flaw with the study (e.g. inadequate follow-up time for dysfunction outcomes, data not presented for learning curve effects if these are likely to influence outcomes).
-
Judging overall direction of bias for individual outcomes: if confounding is judged unbalanced, outcome should be judged as high risk of bias.
Risk of bias tool (non-randomised studies)
Studies for which risk of bias tool is intended
Only suitable for ‘cohort-like’ studies, individually or cluster allocated. Include secondary analyses of clinical databases providing that the analysis is clearly structured as a comparison of control and intervention participants. Refer to Chapter 13, tables 13.2.a and b [Barnaby C, Reeves, Jonathan J, Deeks, Julian PT, Higgins, et al. on behalf of the Cochrane Non-Randomised Studies Methods Group. Chapter 13: Including non-randomized studies. In Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. URL: www.cochrane-handbook.org (accessed March 2011)]:
Table 13.2.a: individually allocated study designs:
-
RCT – randomised controlled trial
-
Q-RCT – quasi-randomised controlled trial
-
NRCT – non-randomised controlled trial
-
CBA – controlled before-and-after study (not common use of this label, see CChBA below)
-
PCS – prospective cohort study
-
RCS – retrospective cohort study.
Table 13.2.b: cluster-allocated study designs:
-
ClRCT – cluster randomised controlled trial
-
ClQ-RCT – cluster quasi-randomised controlled trial
-
ClNRCT – cluster non-randomised controlled trial
-
CITS – controlled interrupted time series
-
CChBA – controlled cohort before-and-after study. 217
Assessment of risk of bias
Issues when using modified risk of bias tool to assess cohort-like non-randomised studies:
-
use existing principle: score judgement and provide information (preferably direct quote) to support judgement
-
additional item on confounding
-
5-point scale for some items (distinguish ‘unclear’ from intermediate risk of bias
-
keep in mind the general philosophy – assessment is not about whether researchers could have done better but about the risk of bias; the assessment tool must be used in a standard way whatever the difficulty/circumstances of investigating the research question of interest and whatever the study design used
-
use of 5-point scale is uncharted territory; very interested to know whether this makes things easier or more difficult for reviewers
-
anchors?: ‘1/no/low risk’ of bias should correspond to a high-quality RCT; ‘5/high risk’ of bias should correspond to a risk of bias which means that the findings should not be considered (too risky, too much bias, more likely to mislead than inform).
-
1. Sequence generation
-
– low/high/unclear risk of bias item
-
– always high risk of bias (not random) for a non-randomised study
-
– might argue that this item redundant for non-randomised studies as it is always high – but important to include in risk of bias table (‘level playing field’ argument).
-
-
2. Allocation concealment
-
– low/high/unclear risk of bias item
-
– potentially low risk of bias for a non-randomised study, for example quasi-randomised (so high risk of bias to sequence generation) but concealed (reviewer judges that the people making decisions about including participants did not know how allocation was being carried out, e.g. odd/even date of birth/hospital number).
-
-
3. Risk of bias from confounding (additional item for non-randomised studies; assess for each outcome)
-
– assumes a prespecified list of potential confounders defined in the protocol
-
– low(1)/2/3/4/high(5)/unclear risk of bias item
-
– judgement needs to factor in:
-
– proportion of confounders (from prespecified list) that were considered
-
– whether most important confounders (from prespecified list) were considered
-
– resolution/precision with which confounders were measured
-
– extent of imbalance between groups at baseline
-
– care with which adjustment was carried out (typically a judgement about the statistical modelling carried out by authors)
-
-
– low risk of bias requires that all important confounders are balanced at baseline (not primarily/not only a statistical judgement or measured ‘well’ and ‘carefully’ controlled for in the analysis).
-
We have provided an optional ‘worksheet’ to help reviewers focus on the task (rows = confounders and columns = factors to consider).
-
4. Risk of bias from lack of blinding (assess for each outcome, as per existing risk of bias tool)
-
– low(1)/2/3/4/high(5)/unclear risk of bias item
-
– judgement needs to factor in:
-
– nature of outcome (subjective/objective; source of information)
-
– who was/was not blinded and the risk that those who were not blinded could introduce performance or detection bias
-
– see Chapter 8 [Higgins JP, Altman D, Sterne J, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. URL: www.cochrane-handbook.org (accessed March 2011)].
-
-
-
5. Risk of bias from incomplete outcome data (assess for each outcome, as per existing risk of bias tool)
-
– low(1)/2/3/4/high(5)/unclear risk of bias item
-
– judgement needs to factor in:
-
– reasons for missing data
-
– whether amount of missing data is balanced across groups, with similar reasons
-
– see Chapter 8 [Higgins JP, Altman D, Sterne J, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. URL: www.cochrane-handbook.org (accessed March 2011)].
-
-
-
6. Risk of bias from selective reporting (assess for each outcome; note: different to existing Chapter 8 recommendation) [Higgins JP, Altman D, Sterne J, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. URL: www.cochrane-handbook.org (accessed March 2011)]
-
– low(1)/2/3/4/high(5)/unclear risk of bias item
-
– judgement needs to factor in:
-
– existing risk of bias guidance on selective outcome reporting
-
– see Chapter 8 [Higgins JP, Altman D, Sterne J, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. URL: www.cochrane-handbook.org (accessed March 2011).]
-
– also, extent to which analyses (and potentially other choices) could have been manipulated to bias the findings reported, for example choice of method of model fitting, potential confounders considered/included
-
– look for evidence that there was a protocol in advance of carrying out any analysis/obtaining the data (difficult unless explicitly reported); non-randomised studies very different from RCTs – RCTs must have a protocol in advance of starting to recruit [for Research Ethics Committe (REC)/Institutional Review Board (IRB)/other regulatory approval] but non-randomised studies need not (especially older studies)
-
– hence, separate yes/no items asking reviewers whether they think that the researchers had a prespecified protocol and analysis plan?
-
-
Appendix 5 List of included studies
Al-Shaiji 2010
Al-Shaiji TF, Kanaroglou N, Thom A, Prowse C, Comondore V, Orovan W, et al. A cost-analysis comparison of laparoscopic radical prostatectomy versus open radical prostatectomy: the McMaster Institute of Urology experience. Can Urol Assoc J 2010;4:237–41.
Anastasiadis 2003
Anastasiadis AG, Salomon L, Katz R, Hoznek A, Chopin D, Abbou CC. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology 2003;62:292–7.
Artibani 2003
Artibani W, Grosso G, Novara G, Pecoraro G, Sidoti O, Sarti A, et al. Is laparoscopic radical prostatectomy better than traditional retropubic radical prostatectomy? An analysis of peri-operative morbidity in two contemporary series in Italy. Eur Urol 2003;44:401–6.
Ball 2006
Ball AJ, Gambill B, Fabrizio MD, Davis JW, Given RW, Lynch DF, et al. Prospective longitudinal comparative study of early health-related quality-of-life outcomes in patients undergoing surgical treatment for localized prostate cancer: a short-term evaluation of five approaches from a single institution. J Endourol 2006;20:723–31.
Barocas 2010
Barocas DA, Salem S, Kordan Y, Herrell SD, Chang SS, Clark PE, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. J Urol 2010;183:990–6.
Kordan Y, Barocas DA, Altamar HO, Clark PE, Chang SS, Davis R, et al. Comparison of transfusion requirements between open and robotic-assisted laparoscopic radical prostatectomy. BJU Int 2010;106:1036–40.
Chan RC, Barocas DA, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. Effect of a large prostate gland on open and robotically assisted laparoscopic radical prostatectomy. BJU Int 2008;101:1140–4.
Bhayani 2003
Bhayani SB, Pavlovich CP, Hsu TS, Sullivan W, Su LM. Prospective comparison of short-term convalescence: laparoscopic radical prostatectomy versus open radical retropubic prostatectomy. Urology 2003;61:612–16.
Bolenz 2010
Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. The influence of body mass index on the cost of radical prostatectomy for prostate cancer. BJU Int 2010;106:1188–93.
Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. Cost comparison of robotic, laparoscopic and open radical prostatectomy. Eur Urol Suppl 2009;8:364.
Brown 2004
Brown JA, Garlitz C, Gomella LG, McGinnis DE, Diamond SM, Strup SE. Perioperative morbidity of laparoscopic radical prostatectomy compared with open radical retropubic prostatectomy. Urol Oncol 2004;22:102–6.
Carlsson 2010
Carlsson S, Nilsson AE, Schumacher MC, Jonsson MN, Volz DS, Steineck G, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology 2010;75:1092–7.
Dahl 2009
Dahl DM, Barry MJ, McGovern FJ, Chang Y, Walker-Corkery E, McDougal WS. A prospective study of symptom distress and return to baseline function after open versus laparoscopic radical prostatectomy. J Urol 2009;182:956–65.
Dahl DM, He W, Lazarus R, McDougal WS, Wu CL. Pathologic outcome of laparoscopic and open radical prostatectomy. Urology 2006;68:1253–6.
Doumerc 2010
Doumerc N, Yuen C, Savdie R, Rahman MB, Rasiah KK, Pe BR, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 2010;106:378–84.
Drouin 2009
Drouin SJ, Vaessen C, Hupertan V, Comperat E, Misrai V, Haertig A, et al. Comparison of mid-term carcinologic control obtained after open, laparoscopic, and robot-assisted radical prostatectomy for localized prostate cancer. World J Urol 2009;27:599–605.
Ficarra 2009
Ficarra V, Novara G, Fracalanza S, D’Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int 2009;104:534–9.
Fornara 2004
Fornara P, Zacharias M. [Minimal invasiveness of laparoscopic radical prostatectomy: reality or dream?] Aktuel Urol 2004;35:395–405.
Fracalanza 2008
Fracalanza S, Ficarra V, Cavalleri S, Galfano A, Novara G, Mangano A, et al. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int 2008;101:1145–9.
Ghavamian 2006
Ghavamian R, Knoll A, Boczko J, Melman A. Comparison of operative and functional outcomes of laparoscopic radical prostatectomy and radical retropubic prostatectomy: single surgeon experience. Urology 2006;67:1241–6.
Gosseine 2009
Gosseine PN, Mangin P, Leclers F, Cormier L. [Pure laparoscopic versus robotic-assisted laparoscopic radical prostatectomy: comparative study to assess functional urinary outcomes.] Prog Urol 2009;19:611–17.
Greco 2010
Greco F, Wagner S, Hoda M, Kawan F, Inferrera A, Lupo A, et al. Laparoscopic vs open retropubic intrafascial nerve-sparing radical prostatectomy: surgical and functional outcomes in 300 patients. BJU Int 2010;106:543–7.
Guazzoni 2006
Guazzoni G, Cestari A, Naspro R, Riva M, Centemero A, Zanoni M, et al. Intra- and peri-operative outcomes comparing radical retropubic and laparoscopic radical prostatectomy: results from a prospective, randomised, single-surgeon study. Eur Urol 2006;50:98–104.
Hu 2006
Hu JC, Nelson RA, Wilson TG, Kawachi MH, Ramin SA, Lau C, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol 2006;175:541–6.
Jacobsen 2007
Jacobsen NE, Moore KN, Estey E, Voaklander D. Open versus laparoscopic radical prostatectomy: a prospective comparison of postoperative urinary incontinence rates. J Urol 2007;177:615–19.
Joseph 2005
Joseph JV, Vicente I, Madeb R, Erturk E, Patel HR. Robot-assisted vs pure laparoscopic radical prostatectomy: are there any differences? BJU Int 2005;96:39–42.
Joseph 2007
Joseph JV, Salomon L, Capello SA, Patel HR, Abbou CC. Laparoscopic or robot-assisted extraperitoneal radical prostatectomy: 1554 cases from two high volume institutions performed extraperitoneally. J Urol 2007;177:525–6.
Jurczok 2007
Jurczok A, Zacharias M, Wagner S, Hamza A, Fornara P. Prospective non-randomized evaluation of four mediators of the systemic response after extraperitoneal laparoscopic and open retropubic radical prostatectomy. BJU Int 2007;99:1461–6.
Kim 2007
Kim Y-J. Comparison of perioperative outcomes of extraperitoneal laparoscopic radical prostatectomy (ELRP) versus open radical retropubic prostatectomy (RRP): single surgeon’s initial experience. Kor J Urol 2007;48:131–7.
Krambeck 2009
Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int 2009;103:448–53.
Lama 2009
Lama MK, Salinas NRO, Martinez JMF, Gribbell RAO, Cabrera OS, Sudy CAF. Prospective study and comparative of surgical and oncologic outcome between laparoscopic and retropubical radical prostatectomy. Actas Urol Esp 2009;33:167–71.
Loeb 2010
Loeb S, Epstein JI, Ross AE, Schultz L, Humphreys EB, Jarow JP. Benign prostate glands at the bladder neck margin in robotic vs open radical prostatectomy. BJU Int 2010;105:1446–9.
Malcolm 2010
Malcolm JB, Fabrizio MD, Barone BB, Given RW, Lance RS, Lynch DF, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol 2010;183:1822–8.
Martorana 2004
Martorana G, Manferrari F, Bertaccini A, Malizia M, Palmieri F, Severini E, et al. Laparoscopic radical prostatectomy: oncological evaluation in the early phase of the learning curve comparing to retropubic approach. Arch Ital Urol Androl 2004;76:1–5.
Menon 2002
Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology 2002;60:864–8.
Miller 2007
Miller J, Smith A, Kouba E, Wallen E, Pruthi RS. Prospective evaluation of short-term impact and recovery of health related quality of life in men undergoing robotic assisted laparoscopic radical prostatectomy versus open radical prostatectomy. J Urol 2007;178:854–8.
Nadler 2010
Nadler RB, Casey JT, Zhao LC, Navai N, Smith ZL, Zhumkhawala A, et al. Is the transition from open to robotic prostatectomy fair to your patients? A single-surgeon comparison with 2-year follow-up. J Robotic Surg 2010;3:201–7.
Namiki 2005
Namiki S, Egawa S, Baba S, Terachi T, Usui Y, Terai A, et al. Recovery of quality of life in year after laparoscopic or retropubic radical prostatectomy: a multi-institutional longitudinal study. Urology 2005;65:517–23.
Namiki 2006
Namiki S, Egawa S, Terachi T, Matsubara A, Igawa M, Terai A, et al. Changes in quality of life in first year after radical prostatectomy by retropubic, laparoscopic, and perineal approach: multi-institutional longitudinal study in Japan. Urology 2006;67:321–7.
Ou 2009
Ou YC, Yang CR, Wang J, Cheng CL, Patel VR. Comparison of robotic-assisted versus retropubic radical prostatectomy performed by a single surgeon. Anticancer Res 2009;29:1637–42.
Poulakis 2007
Poulakis V, Witzsch U, de Vries R, Dillenburg W, Becht E. Laparoscopic radical prostatectomy in men older than 70 years of age with localized prostate cancer: comparison of morbidity, reconvalescence, and short-term clinical outcomes between younger and older men. Eur Urol 2007;51:1341–8.
Raventos Busquets 2007
Raventos Busquets CX, Gomez Lanza E, Cecchini Rossell L, Trilla Herrera E, Orsola los de Santos A, Planas Morin J, et al. [Comparison between open and laparoscopic approach in radical prostatectomy.] Actas Urol Esp 2007;31:141–5.
Remzi 2005
Remzi M, Klingler HC, Tinzl MV, Fong YK, Lodde M, Kiss B, et al. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy versus open retropubic radical prostatectomy. Eur Urol 2005;48:83–9.
Rocco 2009
Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int 2009;104:991–5.
Rozet 2007
Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol 2007;178:478–82.
Salomon 2002
Salomon L, Levrel O, Anastasiadis AG, Saint F, de la Taille A, Cicco A, et al. Outcome and complications of radical prostatectomy in patients with PSA < 10 ng/ml: comparison between the retropubic, perineal and laparoscopic approach. Prostate Cancer Prostatic Dis 2002;5:285–90.
Schroeck 2008
Schroeck FR, Sun L, Freedland SJ, Albala DM, Mouraviev V, Polascik TJ, et al. Comparison of prostate-specific antigen recurrence-free survival in a contemporary cohort of patients undergoing either radical retropubic or robot-assisted laparoscopic radical prostatectomy. BJU Int 2008;102:28–32.
Silva 2007
Silva E, Ferreira U, Silva GD, Mariano MB, Netto NR Jr, Billis A, et al. Surgical margins in radical prostatectomy: a comparison between retropubic and laparoscopic surgery. Int Urol Nephrol 2007;39:865–9.
Soderdahl 2005
Soderdahl DW, Davis JW, Schellhammer PF, Given RW, Lynch DF, Shaves M, et al. Prospective longitudinal comparative study of health-related quality of life in patients undergoing invasive treatments for localized prostate cancer. J Endourol 2005;19:318–26.
Soric 2004
Soric T. Laparoscopic radical prostatectomy. Medica Jadertina 2004;34:87–90.
Sundaram 2004
Sundaram C. Comparison of early experience with laparoscopic radical prostatectomy with and without robotic assistance. J Endourol 2004;18:A125.
Terakawa 2008
Terakawa T, Miyake H, Tanaka K, Takenaka A, Inoue TA, Fujisawa M. Surgical margin status of open versus laparoscopic radical prostatectomy specimens. Int J Urol 2008;15:704–7.
Tewari 2003
Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int 2003;92:205–10.
Touijer 2007
Touijer K, Kuroiwa K, Eastham JA, Vickers A, Reuter VE, Scardino PT, et al. Risk-adjusted analysis of positive surgical margins following laparoscopic and retropubic radical prostatectomy. Eur Urol 2007;52:1090–6.
Trabulsi 2008
Trabulsi EJ, Linden RA, Gomella LG, McGinnis DE, Strup SE, Lallas CD. The addition of robotic surgery to an established laparoscopic radical prostatectomy program: effect on positive surgical margins. Can J Urol 2008;15:3994–9.
Truesdale 2010
Truesdale MD, Lee DJ, Cheetham PJ, Hruby GW, Turk AT, Badani KK. Assessment of lymph node yield after pelvic lymph node dissection in men with prostate cancer: a comparison between robot-assisted radical prostatectomy and open radical prostatectomy in the modern era. J Endourol 2010;24:1055–60.
Wagner 2007
Wagner AA, Link RE, Trock BJ, Sullivan W, Pavlovich CP. Comparison of open and laparoscopic radical prostatectomy outcomes from a surgeon’s early experience. Urology 2007;70:667–71.
White 2009
White MA, De Haan AP, Stephens DD, Maatman TK, Maatman TJ. Comparative analysis of surgical margins between radical retropubic prostatectomy and RALP: are patients sacrificed during initiation of robotics program? Urology 2009;73:567–71.
Appendix 6 List of excluded studies: comparative studies in which number of patients for each baseline clinical stage was unclear
-
Abe T, Shinohara N, Harabayashi T, Sazawa A, Suzuki S, Kawarada Y, et al. Postoperative inguinal hernia after radical prostatectomy for prostate cancer. Urology 2007;69:326–9.
-
Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon’s outcomes. Urology 2004;63:819–22.
-
Albadine R, Jeong JY, Tavora F, Epstein JI, Gonzalgo M, Pavlovich C, et al. Characteristics of positive surgical margins in robotic assisted laparoscopic radical prostatectomy (RobRP), open retropubic radical prostatectomy (RRP) and laparoscopic radical prostatectomy (LapRP): a comparative study from a single academic center. Lab Invest 2009;89(Suppl. 1):699.
-
Atallah F, Khedis M, Seguin P, Fourcade O, Samii K. Postoperative analgesia and recovery after open and laparoscopic prostatectomy. Anesth Analg 2004;99:1878–9.
-
Baumert H. Laparoscopic simple prostatectomy vs. open simple prostatectomy: the first comparative study. Eur Urol Suppl 2006;5:310.
-
Baumert H, Ballaro A, Dugardin F, Kaisary AV. Laparoscopic versus open simple prostatectomy: a comparative study. J Urol 2006;175:1691–4.
-
Bianchi G, Annino F, Sighinolfi MC, Beato A, De Came C, Micali S, et al. Positive surgical margin rate in organ-confined prostate cancer. Comparative analysis between open and robotic surgery during and after robotic learning curve in a single surgeon experience. Anticancer Res 2010;30:177.
-
Binbay M, Tefekli AH, Yoruk E, Tepeler K, Sanlar O, Muslumanoglu AY, et al. Prospective comparison of quality of life in patients treated with either laparoscopic radical prostatectomy or open retro pubic radical prostatectomy. Eur Urol Suppl 2008;7:690.
-
Boris RS, Bhandari A, Krane LS, Eun D, Kaul S, Peabody JO. Salvage robotic-assisted radical prostatectomy: initial results and early report of outcomes. BJU Int 2009:103:952–6.
-
Burgess SV. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol 2006;20:827–30.
-
Caballero Romeu JP, Palacios RJ, Pereira Arias JG, Gamarra QM, Astobieta OA, Ibarluzea GG. [Radical prostatectomy: evaluation of learning curve outcomes laparoscopic and robotic-assisted laparoscopic techniques with radical retropubic prostatectomy.] Actas Urolog Espanol 2008;32:968–75.
-
Colombel M. Anatomical retrograde laparoscopic prostatectomy improves postoperative erections without increasing of surgical margins: a comparative study. Eur Urol Suppl 2006;5:51.
-
D’Alonzo RC, Gan TJ, Moul JW, Albala DM, Polascik TJ, Robertson CN, et al. A retrospective comparison of anesthetic management of robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy. J Clin Anesth 2009;21:322–8.
-
D’Elia C, Novara G, Galfano A, Boscolo-Berto R, Cavalleri S, Artibani W, et al. Prospective, non-randomized trial comparing robot-assisted laparoscopic and retro pubic radical prostatectomy in a single European institution: evaluation of positive surgical margin rates. Eur Urol Suppl 2009;8:281.
-
Desai P, Lipke M, Sundaram C, Gardner T, Koch M. Robotic assisted laparoscopic prostatectomy vs. open radical retropubic prostatectomy: characteristics of pathologic positive surgical margin in high risk patients. J Endourol 2006;20(Suppl. 1):A157.
-
Diaz JI, Corica A, McKenzie R, Schellhammer PF. [Comparative study of surgical efficacy in open versus laparoscopic prostatectomy: virtual prostate reconstruction and periprostatic tissue quantification.] Actas Urolog Espanol 2007;31:1045–55.
-
Durand X, Vaessen C, Bitker MO, Richard F. [Retropubic, laparoscopic and robot-assisted total prostatectomies: comparison of postoperative course and histological and functional results based on a series of 86 prostatectomies.] Prog Urol 2008;18:60–7.
-
Farnham SB, Webster TM, Herrell SD, Smith JA Jr. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology 2006;67:360–3.
-
Fehr J-L. From conventional laparoscopic prostatectomy to da Vinci prostatectomy. J Urol Urogynakolog 2006;13:11–13.
-
Fraga PC, Collins J, Mugnier C. Functional and histological comparative results of laparoscopic radical prostatectomy and robotic-assisted laparoscopic radical prostatectomy: prospective study by one surgeon. Eur Urol Suppl 2009;8:279.
-
Gainsburg DM, Wax D, Reich DL, Carlucci JR, Samadi DB. Intraoperative management of robotic-assisted versus open radical prostatectomy. J Soc Laparoendosc Surg 2010;14:1–5.
-
Gaitonde K, Frankl N, Bianchi GD, Zaki S, Donovan JF, Bracken RB. Time to continence after radical prostatectomy – comparison between open surgery and robot-assisted laparoscopic radical prostatectomy (RALRP). J Endourol 2006;20:A219.
-
Gettman M, Frank I. Radical retropubic prostatectomy versus robotic-assisted radical prostatectomy: an assessment of biochemical recurrence rates by d’Amico risk group and surgeon volume. J Urol 2010;183(4 Suppl. 1):e412.
-
Gonzalez-Berjon JM, Miles BJ, Shen S, Gardner JM, Zhai Q, Ayala AG, et al. A comparative histopathologic study of prostate cancer treated by conventional radical prostatectomy and robotic-assisted laparoscopic radical prostatectomy: a series of 1006 cases. Mod Pathol 2008;21(Suppl. 1):719.
-
Gonzalgo ML, Magheli A, Brotzman M, Su LM. Single surgeon comparison between conventional laparoscopic and robot-assisted radical prostatectomy: pathological and functional outcomes. J Urol 2008;179:344.
-
Grossi FS, Di LS, Barnaba D, Larocca L, Raguso M, Sallustio G, et al. Laparoscopic versus open radical retropubic prostatectomy: a case–control study at a single institution. Arch Ital Urol Androl 2010;82:109–12.
-
Hakimi AA, Blitstein J, Feder M, Shapiro E, Ghavamian R. Direct comparison of surgical and functional outcomes of robotic-assisted versus pure laparoscopic radical prostatectomy: single-surgeon experience. Urology 2009;73:119–23.
-
Hara I, Kawabata G, Miyake H, Nakamura I, Hara S, Okada H, et al. Comparison of quality of life following laparoscopic and open prostatectomy for prostate cancer. J Urol 2003;169:2045–8.
-
Herrell SD, Smith JA Jr. Robotic-assisted laparoscopic prostatectomy: what is the learning curve? Urology 2005;66(Suppl. 5):105–7.
-
Hicks JA, Manners J, Solomon LZ, Holmes SAV, Eden C. A comparison of post operative inguinal hernia rates after laparoscopic, retropubic and perineal radical prostatectomy. BJU Int 2007;99(Suppl. 4):35.
-
Hu JC, Wood DP, Andriole GL, Dunn RL, Dahl DM, Hollenbeck BK, et al. Perioperative quality care indicators of retropubic, laparoscopic, and robotic prostatectomy: results from a national, multi-center, prospective cohort. J Urol 2006;175(Suppl. 4):1151.
-
Hubosky SG, Fabrizio MD, Davis JW, Given RW, Lynch DF, Gambill BB, et al. Comparison of health-related quality of life (QOL) parameters in patients undergoing robotically assisted prostatectomy, open radical prostatectomy and laparoscopic radical prostatectomy. J Endourol 2006;20(Suppl. 1):A153.
-
Hyo K, Sung G, Cho W, Lee W. A comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single surgeon experience. J Endourol 2009;23(Suppl. 1):A89.
-
Hyo KT, Sung GT. A comparison of robotic assisted versus extraperitoneal laparoscopic radical prostatectomy: a single surgeon experience. J Robot Surg 2010;4:76:A89.
-
Jaffe J, Rozet F, Brand G, Harmon J, Cathelineau X, Barret E, et al. A direct comparison of robotic assisted vs pure laparoscopic radical prostatectomy: a single institution’s experience. J Endourol 2007;21(Suppl. 1):A68.
-
Jung H, Kaswik J, Wuerstle J, Williams S. The learning curve of laparoscopic compared to robotic surgeons during the implementation of a robotic prostatectomy program. J Urol 2010;183(Suppl. 1):e517.
-
Kang T, Park J, Song C, Hong J, Park H, Ahn H. Comparison of functional outcomes between robot-assisted radical prostatectomy and radical retropubic prostatectomy: a single surgeon experience. J Endourol 2009;23(Suppl. 1):A112.
-
Kaufman M, Baumgartner R, Anderson L, Smith J, Chang S, Herrell S, et al. Evidence based pathway for perioperative management of open and robotic assisted laparoscopic radical prostatectomy. J Endourol 2006;20(Suppl. 1):a278.
-
Keikha M, Ahmad N, Ooi J. Laparoscopic versus open radical prostatectomy: a review of outcomes at Western Hospital, Footscray, Victoria, Australia. BJU Int 2008;101:1–2.
-
Klingler HC, Remzi M, Kiss B, Katzenbeisser D, Marberger M. Endoscopic radical prostatectomy – da Vinci (TM) vs. laparoscopy in a single centre experience. J Endourol 2006;20(Suppl. 1):a219.
-
Koch MO, Smith W. Robotic vs. open radical prostatectomy: a single institution, single surgeon comparison of outcome. J Urol 2008;179(Suppl. 1):610.
-
Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Blute ML, Gettman MT. Radical prostatectomy for prostatic adenocarcinoma: matched comparison of retropubic and robot assisted techniques. J Urol 2008;179:555–6.
-
Menon M. Robotic radical retropubic prostatectomy. BJU Int 2003;91:175–6.
-
Menon M, Tewari A, Peabody JO, Shrivastava A, Kaul S, Bhandari A, et al. Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: experience of over 1100 cases. Urol Clin North Am 2004;31:701–17.
-
Menon M, Shrivastava A, Tewari A. Laparoscopic radical prostatectomy: conventional and robotic. Urology 2005;66:101–4.
-
Mikulasovich M, Noreen S, Samadi D, Idrees M, Liu Y, Nabizada-Pace F, et al. Comparison between robotic radical prostatectomy and open radical prostatectomy: surgical margin status against TNM stage, Gleason’s score, and tumor volume. Lab Invest 2009;89(Suppl. 1):183A.
-
Miller J, Smith A, Kouba E, Wallen EM, Pruthi RS. Prospective evaluation of short-term impact and recovery of health-related quality of life (HRQOL) in men undergoing robotic-assisted laparoscopic radical prostatectomy vs. open radical prostatectomy (ORP). J Urol 2007;177:189–90.
-
Mondejar RR, Moreno MJD, Navarro HP, Lopez PC, Ruiz JM, Guzman JMP, et al. Comparative study between radical retropubic prostatectomy and laparoscopic prostatectomy in our initial series (1988–1997 and 2005–2006). J Endourol 2007;21(Suppl. 1):A67.
-
Montorsi F, Gadda G, Gallina A, Buffi N, Briganti A, Suardi N, et al. Intrafascial bilateral nerve-sparing radical prostatectomy: a comparative functional analysis between open and robotic-assisted video-laparoscopic approaches. J Sexual Med 2009;6:411–12.
-
Nelson B, Kaufman M, Broughton G, Cookson MS, Chang SS, Herrell SD, et al. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol 2007;177:929–31.
-
Okabe T, Kim C, Yamanashi Y, Sakamoto A. [Anesthesia management for laparoscopic prostatectomy and open prostatectomy.] Masui – Jap J Anesthesiol 2007;56:1404–7.
-
Park S, Jaffer O, Lotan Y, Saboorian H, Roehrborn CG, Cadeddu JA. Contemporary laparoscopic and open radical retropubic prostatectomy: pathologic outcomes and Kattan postoperative nomograms are equivalent. Urology 2007;69:118–22.
-
Plainard X, Druet CM, Descazeaud A, Paulhac P, Lesaux N, Dumas JP, et al. [Study of urinary continence after radical prostatectomy. Comparison between laparoscopic and retropubic prostatectomy based on a series of 251 cases.] Prog Urol 2008;18:364–71.
-
Ploussard G, Xylinas E, Paul A, Gillion N, Salomon L, Allory Y, et al. Is robot assistance affecting operating room time compared with pure retroperitoneal laparoscopic radical prostatectomy? J Endourol 2009;23:939–43.
-
Porpiglia F, Fiori C, Chiarissi M, Manfredi M, Grande S, Scarpa R. Last 50 laparoscopic radical prostatectomy (of a series of more than 400 patients) vs first 50 robot-assisted laparoscopic radical prostatectomy: our results. J Endourol 2009;23(Suppl. 1):A266.
-
Rassweiler J, Seemann O, Schulze M, Teber D, Hatzinger M, Frede T. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol 2003;169:1689–93.
-
Rassweiler J, Hruza M, Teber D, Su LM. Laparoscopic and robotic assisted radical prostatectomy – critical analysis of the results. Eur Urol 2006;49:612–24.
-
Rigatti L, Guazzoni G, Naspro R, Cestari A, Centemero A, Riva M. Radical retropubic (RRP) and laparoscopic prostatectomy (LRP): a prospective urodynamic comparison of post-operative continence. Eur Urol Suppl 2007;6:210.
-
Roumeguere T, Bollens R, Vanden Bossche M, Rochet D, Bialek D, Hoffman P, et al. Radical prostatectomy: a prospective comparison of oncological and functional results between open and laparoscopic approaches. World J Urol 2003;20:360–6.
-
Schachter L, Herrell S, Baumgartner R, Dietrich M, Cookson M, Chang S, et al. Return of urinary continence after radical prostatectomy: results of a prospective comparative trial of an open retropubic vs. robotic approach. J Endourol 2006;20(Suppl. 1):A31.
-
Schachter LR, Herrell SD, Baumgartner R, Dietrich MS, Cookson MS, Chang SS, et al. Early and delayed return of urinary continence after radical prostatectomy: results of a prospective comparative trial of an open retropubic vs. robotic approach. J Urol 2007;177(Suppl. 1):532.
-
Schmeller N, Keller H, Janetschek G. Head-to-head comparison of retropubic, perineal and laparoscopic radical prostatectomy. Int J Urol 2007;14:402–5.
-
Schroeck FR, Krupski TL, Sun L, Albala DM, Price MM, Polascik TJ, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol 2008;54:785–93.
-
Secin FP, Salas RS, Karanikolas NT, Bianco FJ, Touijer K, Eastham J, et al. Comparative analysis of the impact of prostate volume on positive surgical margin incidence and location between open and laparoscopic radical prostatectomy. J Endourol 2006;20(Suppl. 1):A152.
-
Secin FP, Sanchez Salas R, Bianco F, Romero Otero J, Touijer K, Eastham JA, et al. Comparative analysis of the impact of prostate volume on positive surgical margin incidence and location between open and laparoscopic radical prostatectomy. Eur Urol Suppl 2007;6:210.
-
Secin FP, Touijer K, Romero Otero J, Bianco F, Eastham JA, Scardino PT, et al. Patient assessed erectile function recovery after open and laparoscopic radical prostatectomy: head-to-head prospective comparison. Eur Urol Suppl 2007;6:207.
-
Srinualnad S. Early experience of robotic assisted laparoscopic radical prostatectomy. J Med Assoc Thai 2008;91:377–82.
-
Srinualnad S, Nualyong C, Udompunturak S, Kongsuwan W. Endoscopic extraperitoneal radical prostatectomy (EERPE): a new approach for treatment of localized prostate cancer. J Med Assoc Thai 2006;89:1601–8.
-
Touijer K, Eastham JA, Secin FP, Romero OJ, Serio A, Stasi J, et al. Comprehensive prospective comparative analysis of outcomes between open and laparoscopic radical prostatectomy conducted in 2003 to 2005. J Urol 2008;179:1811–17.
-
Trabulsi E, Chandrasekar T, Lee F, Mccue P, Lallas C, Colon A. Lymph node yields with pelvic lymphadenectomy during robotic-assisted laparoscopic radical prostatectomy are higher than with open radical retropubic prostatectomy. J Endourol 2009;239(Suppl. 1):A89–90.
-
Trabulsi EJ, Zola JC, Gomella LG, Lallas CD. Transition from pure laparoscopic to robotic-assisted radical prostatectomy: a single surgeon institutional evolution. Urol Oncol 2010;28:81–5.
-
Uvin P, de Meyer JM, Van Holderbeke G. A comparison of the peri-operative data after open radical retropubic prostatectomy or robotic-assisted laparoscopic prostatectomy. Acta Chir Belg 2010;110:313–16.
-
Vodopija N, Zupancic M, Korsic L, Kramer F, Parac I. Laparoscopic radical prostatectomy – analysis of our first 100 consecutive cases. Coll Antropol 2004;28:429–37.
-
Vogeli T, Akbarov I, Lehnhardt M. Pain assessment after radical retro-pubic vs extraperitoneal laparoscopic radical prostatectomy – a prospective trial. J Endourol 2009;23(Suppl. 1):A97.
-
Webster TM, Herrell SD, Chang SS, Cookson MS, Baumgartner RG, Anderson LW, et al. Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol 2005;174:912–14.
-
Weizer AZ, Strope S, Wood DP Jr. Margin control in robotic and laparoscopic prostatectomy: what are the REAL outcomes? Urol Oncol 2010;28:210–14.
-
Williams SB. Radical retropubic prostatectomy and robotic-assisted laparoscopic prostatectomy: likelihood of positive surgical margin(s). J Urol 2010;184:1984–5.
-
Woellner J, Neisius A, Woellner G, Gillitzer R, Thueroff J, Hampel C. Robotic-assisted laparoscopic radical prostatectomy operative details and functional outcome. J Endourol 2009;23(Suppl. 1):A91.
-
Wood DP, Schulte R, Dunn RL, Hollenbeck BK, Saur R, Wolf JS, et al. Short-term health outcome differences between robotic and conventional radical prostatectomy. Urology 2007;70:945–9.
-
Yates J, Haleblian G, Stein B, Miller B, Renzulli J, Pareek G. The impact of robotic surgery on pelvic lymph node dissection during radical prostatectomy for localized prostate cancer: the Brown University early robotic experience. Can J Urol 2009;16:4842–6.
Appendix 7 Characteristics of the included studies
| Study details | Participant characteristics | Intervention characteristics | Outcomes | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author, year: Guazzoni 200690 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Italy Recruitment/treatment dates: not reported Prospective/retrospective data collection: prospective Randomisation method: consecutive and age-matched patients randomised using computer-generated randomisation table Length of follow-up: not reported Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: consecutive and age-matched patients who were diagnosed with clinically localised prostate cancer (cT1–cT2); patients who are aged < 70 years, with PSA < 20 ng/dl, Gleason score ≤ 7 Exclusion criteria: those with previous hormone blockade therapy or any previous prostatic bladder neck, urethral or pelvic surgery and total prostate volume ≥ 60 ml; patients without an indwelling catheter ABPatients enrolled, n120Patients randomised, n6060Patients analysed, n6060Age (years), mean (SD)62.29 (8.2)2.9 (7.4)PSA (ng/ml), mean (SD)6.9 (2.9)6.5 (3)Clinical stage, n (%)T145 (75)50 (83)T215 (25)10 (17) Digital rectal examination, TRUS, abdominal computed tomography scan and bone scan used for staging |
A | B | Patients enrolled, n | 120 | Patients randomised, n | 60 | 60 | Patients analysed, n | 60 | 60 | Age (years), mean (SD) | 62.29 (8.2) | 2.9 (7.4) | PSA (ng/ml), mean (SD) | 6.9 (2.9) | 6.5 (3) | Clinical stage, n (%) | T1 | 45 (75) | 50 (83) | T2 | 15 (25) | 10 (17) |
A. Laparoscopic prostatectomy: performed according to Montsouris technique; the urethra–vesicle anastomosis was performed with 8-10, 3-0 interrupted sutures performed intracorporeally after insertion of a metal bougie to expose the urethral stump; transperitoneal route was used Nerve sparing: Unilateral: 11/60 (18.3%) Bilateral: 25/60 (41.7%) Pelvic lymphadenectomy: 24/60 (40.0%) B. Open prostatectomy: performed by anatomic technique; a xenon head light and 2.5 magnification loops were used. The urethra–vesicle anastomosis was performed with 8-10, 3-0 interrupted sutures with a 5/8 needle Nerve sparing: Unilateral: 8/60 (13.3%) Bilateral: 31/60 (51.7%) Pelvic lymphadenectomy: 27/60 (45.0%) For both A and B: Lymph node dissection was performed when total serum PSA level was ≥ 10 ng/ml and/or Gleason score = 7 Nerve sparing was performed whenever possible according to preoperative parameters such as age, clinical stage and preoperative potency (recorded by the IIEF questionnaire and penile power Doppler ultrasound evaluation) (data not reported) |
Safety: open conversion, surgical complications, operating time, discharge time, catheterisation, blood loss, mobilisation, oral feeding Efficacy: margins, pT stage Quality of life: pain |
||||||
| A | B | |||||||||||||||||||||||||||||||
| Patients enrolled, n | 120 | |||||||||||||||||||||||||||||||
| Patients randomised, n | 60 | 60 | ||||||||||||||||||||||||||||||
| Patients analysed, n | 60 | 60 | ||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 62.29 (8.2) | 2.9 (7.4) | ||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 6.9 (2.9) | 6.5 (3) | ||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||
| T1 | 45 (75) | 50 (83) | ||||||||||||||||||||||||||||||
| T2 | 15 (25) | 10 (17) | ||||||||||||||||||||||||||||||
| Study details | Participant characteristics | Intervention characteristics | Outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author, year: Ball 200699 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: January 2000–April 2005 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: 6 months Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients with newly diagnosed clinically localised prostate cancer Exclusion criteria: not reported ABCPatients, nEnrolled821241351 month7693823 months561021226 months2211291Age (years), mean (SD)60 (7)61 (7)59 (6)PSA (ng/ml), mean (SD)6.0 (2.4)7.2 (7.1)7.8 (5.6)Clinical stage, nT166100116T2152419T3100Biopsy Gleason score, n≤ 659948571522378–108813 |
A | B | C | Patients, n | Enrolled | 82 | 124 | 135 | 1 month | 76 | 93 | 82 | 3 months | 56 | 102 | 122 | 6 months | 22 | 112 | 91 | Age (years), mean (SD) | 60 (7) | 61 (7) | 59 (6) | PSA (ng/ml), mean (SD) | 6.0 (2.4) | 7.2 (7.1) | 7.8 (5.6) | Clinical stage, n | T1 | 66 | 100 | 116 | T2 | 15 | 24 | 19 | T3 | 1 | 0 | 0 | Biopsy Gleason score, n | ≤ 6 | 59 | 94 | 85 | 7 | 15 | 22 | 37 | 8–10 | 8 | 8 | 13 |
A. Robotic prostatectomy: trade name of robot: da Vinci B. Laparoscopic prostatectomy: used awell-described technique, reference given C. Open prostatectomy: used astandard radical retropubic technique Nerve sparing for erectile function: ABCNon-nerve sparing, n (%)18 (22)67 (54)40 (30)Unilateral, n (%)9 (11)23 (19)30 (22)Bilateral, n (%)54 (66)34 (27)65 (48)Unknown, n (%)1 (1)00 |
A | B | C | Non-nerve sparing, n (%) | 18 (22) | 67 (54) | 40 (30) | Unilateral, n (%) | 9 (11) | 23 (19) | 30 (22) | Bilateral, n (%) | 54 (66) | 34 (27) | 65 (48) | Unknown, n (%) | 1 (1) | 0 | 0 |
Efficacy: pT stage Dysfunction: urinary incontinence, erectile dysfunction |
|||||||||||
| A | B | C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Enrolled | 82 | 124 | 135 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 76 | 93 | 82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 56 | 102 | 122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 22 | 112 | 91 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 60 (7) | 61 (7) | 59 (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 6.0 (2.4) | 7.2 (7.1) | 7.8 (5.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 66 | 100 | 116 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 15 | 24 | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 1 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 59 | 94 | 85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 15 | 22 | 37 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 8 | 8 | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 18 (22) | 67 (54) | 40 (30) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 9 (11) | 23 (19) | 30 (22) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 54 (66) | 34 (27) | 65 (48) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unknown, n (%) | 1 (1) | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Bolenz 2010100 Language: English Publication type: full text Number of study centres: 1 Setting: not reported Country: USA Recruitment/treatment dates: September 2003–April 2008 Prospective/retrospective data collection: not reported Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: TG |
Inclusion/exclusion criteria: not reported ABCPatients, n262211156Age (years), median625961BMI < 30 kg/m262 (56–66)59 (54–63)61.5 (57–66)BMI > 30 kg/m260 (57–65)56.5 (52–63)60.5 (54–64)PSA (ng/ml), median (range)BMI < 30 kg/m25.2 (4.1–7)5 (4.2–6.5)5.6 (4.4–7.2)BMI > 30 kg/m25.4 (4.3–7)5.1 (4–7.2)4.7 (4.1–5.9)BMI, body mass index. Biopsy Gleason scores for total sample: ≤ 6: 341 7: 236 8–10: 48 |
A | B | C | Patients, n | 262 | 211 | 156 | Age (years), median | 62 | 59 | 61 | BMI < 30 kg/m2 | 62 (56–66) | 59 (54–63) | 61.5 (57–66) | BMI > 30 kg/m2 | 60 (57–65) | 56.5 (52–63) | 60.5 (54–64) | PSA (ng/ml), median (range) | BMI < 30 kg/m2 | 5.2 (4.1–7) | 5 (4.2–6.5) | 5.6 (4.4–7.2) | BMI > 30 kg/m2 | 5.4 (4.3–7) | 5.1 (4–7.2) | 4.7 (4.1–5.9) | BMI, body mass index. |
A. Robotic prostatectomy: nerve sparing B. Laparoscopic prostatectomy: nerve sparing C. Open prostatectomy: nerve sparing |
Safety: blood transfusion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 262 | 211 | 156 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median | 62 | 59 | 61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI < 30 kg/m2 | 62 (56–66) | 59 (54–63) | 61.5 (57–66) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI > 30 kg/m2 | 60 (57–65) | 56.5 (52–63) | 60.5 (54–64) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI < 30 kg/m2 | 5.2 (4.1–7) | 5 (4.2–6.5) | 5.6 (4.4–7.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI > 30 kg/m2 | 5.4 (4.3–7) | 5.1 (4–7.2) | 4.7 (4.1–5.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Bolenz 2009;102 secondary to Bolenz 2010100 Language: English Publication type: conference abstract Number of study centres: 1 Setting: not reported Country: USA Recruitment/treatment dates: September 2003–April 2008 Prospective/retrospective data collection: not reported Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported |
Inclusion/exclusion criteria: not reported ABCPatients, n264220162Age (years), median615961BMI (kg/m2), median27.827.327.2PSA (ng/ml), median5.355.3Clinical stage, nT1c198193107T2a92017T2b7210T2c47022Not provided201Unknown155Biopsy Gleason score 8–10 (%)6.108.409.40Prostate size (ml)464645BMI, body mass index. Clinical stage data obtained via correspondence with lead author. |
A | B | C | Patients, n | 264 | 220 | 162 | Age (years), median | 61 | 59 | 61 | BMI (kg/m2), median | 27.8 | 27.3 | 27.2 | PSA (ng/ml), median | 5.3 | 5 | 5.3 | Clinical stage, n | T1c | 198 | 193 | 107 | T2a | 9 | 20 | 17 | T2b | 7 | 2 | 10 | T2c | 47 | 0 | 22 | Not provided | 2 | 0 | 1 | Unknown | 1 | 5 | 5 | Biopsy Gleason score 8–10 (%) | 6.10 | 8.40 | 9.40 | Prostate size (ml) | 46 | 46 | 45 | BMI, body mass index. Clinical stage data obtained via correspondence with lead author. |
A. Robotic prostatectomy: nerve sparing 85%, lymph node dissection 11% B. Laparoscopic prostatectomy: nerve sparing 96%, lymph node dissection 22% C. Open prostatectomy: nerve sparing 90%, lymph node dissection 100% |
Safety: operating time, hospital stay | |||||||||||||||||||||||||||||||
| A | B | C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 264 | 220 | 162 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median | 61 | 59 | 61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), median | 27.8 | 27.3 | 27.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median | 5.3 | 5 | 5.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 198 | 193 | 107 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 9 | 20 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 7 | 2 | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 47 | 0 | 22 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not provided | 2 | 0 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unknown | 1 | 5 | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score 8–10 (%) | 6.10 | 8.40 | 9.40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml) | 46 | 46 | 45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. Clinical stage data obtained via correspondence with lead author. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Drouin 2009101 Language: English Publication type: full text Number of study centres: not reported Setting: hospital Country: France Recruitment/treatment dates: January 2000–August 2004 Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: months, mean (range): total: 49.7 (18–103); A: 40.9 (18–60); B: 48.4 (18–84); C: 57.7 (18–103) Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients treated for prostate cancer with surgery Exclusion criteria: evidence of lymph node involvement during preoperative work-up or in case of clinical signs of non-localised disease ABCPatients, n718583Age (years), mean (range)60.4 (46–70)61.8 (39–73)60.5 (45–81)BMI (kg/m2), mean (range)22.6 (22–25)23 (22–25.2)23.3 (22.6–24.8)PSA (ng/ml), mean (range)7.8 (3–24)8.9 (3.4–37)9.2 (1.2–60)Clinical stage, nT1a–b002T1c505538T2a–b172228T2c4815Biopsy Gleason score, n≤ 660625971121248–10020BMI, body mass index. |
A | B | C | Patients, n | 71 | 85 | 83 | Age (years), mean (range) | 60.4 (46–70) | 61.8 (39–73) | 60.5 (45–81) | BMI (kg/m2), mean (range) | 22.6 (22–25) | 23 (22–25.2) | 23.3 (22.6–24.8) | PSA (ng/ml), mean (range) | 7.8 (3–24) | 8.9 (3.4–37) | 9.2 (1.2–60) | Clinical stage, n | T1a–b | 0 | 0 | 2 | T1c | 50 | 55 | 38 | T2a–b | 17 | 22 | 28 | T2c | 4 | 8 | 15 | Biopsy Gleason score, n | ≤ 6 | 60 | 62 | 59 | 7 | 11 | 21 | 24 | 8–10 | 0 | 2 | 0 | BMI, body mass index. |
A. Robotic prostatectomy: robot trade name: da Vinci system; approaches: transperitoneal; 34/71 had lymph node dissection B. Laparoscopic prostatectomy: approaches: transperitoneal; 42/85 had lymph node dissection C. Open prostatectomy: 58/83 had lymph node dissection |
Safety: surgical complications, open conversion, operating time, catheterisation, blood loss Efficacy: margins, pT stage, PSA recurrence Death |
||||||||||||||||||||||||||||||||||
| A | B | C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 71 | 85 | 83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 60.4 (46–70) | 61.8 (39–73) | 60.5 (45–81) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (range) | 22.6 (22–25) | 23 (22–25.2) | 23.3 (22.6–24.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 7.8 (3–24) | 8.9 (3.4–37) | 9.2 (1.2–60) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a–b | 0 | 0 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 50 | 55 | 38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a–b | 17 | 22 | 28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 4 | 8 | 15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 60 | 62 | 59 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 11 | 21 | 24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 0 | 2 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant characteristics | Intervention characteristics | Outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author, year: Gosseine 200991 Language: French Publication type: full text Number of study centres: 1 Setting: hospital Country: France Recruitment/treatment dates: March 2004–April 2007 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: 3 years Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: not reported Exclusion criteria: not reported ABPatients, n122125Age (years), mean (SD)60.6 (6.1)61.7 (6.8)BMI (kg/m2), mean (SD)26.7 (3.4)27.2 (3.5)Previous TURP, n24PSA (ng/ml), mean (SD)7.37 (4.3)7.87 (5.09)Clinical stage, n (%)T170 (57.4)78 (62.4)T252 (42.6)47 (37.6)Biopsy Gleason score, n (%)≤ 673 (59.8)86 (68.8)742 (34.4)36 (28.8)8–107 (5.8)3 (2.4)BMI, body mass index; TURP, transurethral resection of the prostate. |
A | B | Patients, n | 122 | 125 | Age (years), mean (SD) | 60.6 (6.1) | 61.7 (6.8) | BMI (kg/m2), mean (SD) | 26.7 (3.4) | 27.2 (3.5) | Previous TURP, n | 2 | 4 | PSA (ng/ml), mean (SD) | 7.37 (4.3) | 7.87 (5.09) | Clinical stage, n (%) | T1 | 70 (57.4) | 78 (62.4) | T2 | 52 (42.6) | 47 (37.6) | Biopsy Gleason score, n (%) | ≤ 6 | 73 (59.8) | 86 (68.8) | 7 | 42 (34.4) | 36 (28.8) | 8–10 | 7 (5.8) | 3 (2.4) | BMI, body mass index; TURP, transurethral resection of the prostate. |
A. Robotic prostatectomy: trade name of robot: da Vinci system B. Laparoscopic prostatectomy: Nerve sparing for erectile function: ABNon-nerve sparing, n (%)30 (25)45 (36)Unilateral, n (%)16 (13)13 (10.4)Bilateral, n (%)76 (62)64 (5.12)Bladder neck preservation, n (%)97 (79)53 (42)Not reported n (%)03 (2.4) |
A | B | Non-nerve sparing, n (%) | 30 (25) | 45 (36) | Unilateral, n (%) | 16 (13) | 13 (10.4) | Bilateral, n (%) | 76 (62) | 64 (5.12) | Bladder neck preservation, n (%) | 97 (79) | 53 (42) | Not reported n (%) | 0 | 3 (2.4) |
Safety: surgical complications, operating time, hospital stay, catheterisation, blood loss Dysfunction: urinary incontinence |
|||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 122 | 125 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 60.6 (6.1) | 61.7 (6.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 26.7 (3.4) | 27.2 (3.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous TURP, n | 2 | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 7.37 (4.3) | 7.87 (5.09) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 70 (57.4) | 78 (62.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 52 (42.6) | 47 (37.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 73 (59.8) | 86 (68.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 42 (34.4) | 36 (28.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 7 (5.8) | 3 (2.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index; TURP, transurethral resection of the prostate. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 30 (25) | 45 (36) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 16 (13) | 13 (10.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 76 (62) | 64 (5.12) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bladder neck preservation, n (%) | 97 (79) | 53 (42) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported n (%) | 0 | 3 (2.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Hu 200692 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: US Recruitment/treatment dates: A: June 2003–June 2004; B: October 2000–January 2003 Prospective/retrospective data collection: mixture Patient recruited consecutively, Y/N: no Length of follow-up: not reported Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients had radical prostatectomies with laparoscopic or robotic procedures Exclusion criteria: patients with neoadjuvant hormonal therapy ABPatient enrolled671517Patient analysed322358Age, mean (range)62.1 (41-84)63.7 (40-83)BMI, median (range)27.5 (17.8-51.5)27.4 (17.9-43.8)Previous abdominal surgery37/322 (11.5%)39/358 (10.9%)PSA, ng/ml0–466 (20.6%)55 (15.4%)4–10213 (66.4%)247 (69%)1042 (13.1%)56 (15.6%)Clinical stage, n (%)T1a1 (0.3)6 (1.7)T1b02 (0.6)T1c231 (74.5)261 (72.9)T2a59 (19.0)72 (20.%)T2b11 (3.5)4 (1.1)T2c7 (2.3)10 (2.8)T3a1 (0.3)1 (0.3)T3b02 (0.6)Biopsy Gleason score, n (%)1–55 (1.6)9 (2.5)6–7289 (93.5)322 (90.2)8–1015 (4.9)26 (7.3) |
A | B | Patient enrolled | 671 | 517 | Patient analysed | 322 | 358 | Age, mean (range) | 62.1 (41-84) | 63.7 (40-83) | BMI, median (range) | 27.5 (17.8-51.5) | 27.4 (17.9-43.8) | Previous abdominal surgery | 37/322 (11.5%) | 39/358 (10.9%) | PSA, ng/ml | 0–4 | 66 (20.6%) | 55 (15.4%) | 4–10 | 213 (66.4%) | 247 (69%) | 10 | 42 (13.1%) | 56 (15.6%) | Clinical stage, n (%) | T1a | 1 (0.3) | 6 (1.7) | T1b | 0 | 2 (0.6) | T1c | 231 (74.5) | 261 (72.9) | T2a | 59 (19.0) | 72 (20.%) | T2b | 11 (3.5) | 4 (1.1) | T2c | 7 (2.3) | 10 (2.8) | T3a | 1 (0.3) | 1 (0.3) | T3b | 0 | 2 (0.6) | Biopsy Gleason score, n (%) | 1–5 | 5 (1.6) | 9 (2.5) | 6–7 | 289 (93.5) | 322 (90.2) | 8–10 | 15 (4.9) | 26 (7.3) |
A. Robotic prostatectomy: trade name of robot: da Vinci system; approaches: trans-peritoneal B. Laparoscopic prostatectomy: approaches: trans-peritoneal (both Montsouris technique); nerve sparing ABUnilateral, n (%)27 (8.4)23 (6.4)Bilateral, n (%)259 (80.4)237 (66.2)Non-sparing, n (%)35 (0.9)87 (24.3) All patients (A and B) had bilateral pelvic lymph node dissection |
A | B | Unilateral, n (%) | 27 (8.4) | 23 (6.4) | Bilateral, n (%) | 259 (80.4) | 237 (66.2) | Non-sparing, n (%) | 35 (0.9) | 87 (24.3) |
Safety: surgical complications, operation time Death Learning curve: operating time |
||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient enrolled | 671 | 517 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient analysed | 322 | 358 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age, mean (range) | 62.1 (41-84) | 63.7 (40-83) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, median (range) | 27.5 (17.8-51.5) | 27.4 (17.9-43.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous abdominal surgery | 37/322 (11.5%) | 39/358 (10.9%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA, ng/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–4 | 66 (20.6%) | 55 (15.4%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4–10 | 213 (66.4%) | 247 (69%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | 42 (13.1%) | 56 (15.6%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 1 (0.3) | 6 (1.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 0 | 2 (0.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 231 (74.5) | 261 (72.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 59 (19.0) | 72 (20.%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 11 (3.5) | 4 (1.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 7 (2.3) | 10 (2.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3a | 1 (0.3) | 1 (0.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3b | 0 | 2 (0.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1–5 | 5 (1.6) | 9 (2.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6–7 | 289 (93.5) | 322 (90.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 15 (4.9) | 26 (7.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 27 (8.4) | 23 (6.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 259 (80.4) | 237 (66.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-sparing, n (%) | 35 (0.9) | 87 (24.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Joseph 200794 Language: English Publication type: conference abstract Number of study centres: 2 Setting: hospital Country: France/USA Recruitment/treatment dates: A: 2003–6 at the University of Rochester Medical Centre; B: 2002–6 at Henri Mondor Hospital of Creteil Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: none Systematic reviewer: XJ |
Inclusion criteria: patients underwentprostatectomy Exclusion criteria: not reported ABPatients enrolled, n754800Age (years), mean (range)60.0 (40–78)64.9 (43–77)BMI (kg/m2), mean (range)28.5 (17.7–56.2)27.2 (16.5–44.8)PSA (ng/ml), mean (range)6.6 (0.1–39.0)10.1 (1.5–99)Clinical stage, n (%)T1a–b014 (1.8)T1c452 (75.2)643 (80.4)T2148 (24.6)141 (17.8)T31 (0.2)0Not reported1532Biopsy Gleason score, mean (range)6.3 (4–9)6.2 (4–9)Prostate size (g), mean (range)55.4 (21–141)55.6 (22–192)BMI, body mass index. |
A | B | Patients enrolled, n | 754 | 800 | Age (years), mean (range) | 60.0 (40–78) | 64.9 (43–77) | BMI (kg/m2), mean (range) | 28.5 (17.7–56.2) | 27.2 (16.5–44.8) | PSA (ng/ml), mean (range) | 6.6 (0.1–39.0) | 10.1 (1.5–99) | Clinical stage, n (%) | T1a–b | 0 | 14 (1.8) | T1c | 452 (75.2) | 643 (80.4) | T2 | 148 (24.6) | 141 (17.8) | T3 | 1 (0.2) | 0 | Not reported | 153 | 2 | Biopsy Gleason score, mean (range) | 6.3 (4–9) | 6.2 (4–9) | Prostate size (g), mean (range) | 55.4 (21–141) | 55.6 (22–192) | BMI, body mass index. |
A. Robotic prostatectomy B. Laparoscopic prostatectomy: approaches: extraperitoneal Lymph node dissection:ABYes, n (%)281 (37.3)322 (40.3)No (%)(62.6)(59.7) |
A | B | Yes, n (%) | 281 (37.3) | 322 (40.3) | No (%) | (62.6) | (59.7) | Efficacy: margins, pathological Gleason score | ||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients enrolled, n | 754 | 800 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 60.0 (40–78) | 64.9 (43–77) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (range) | 28.5 (17.7–56.2) | 27.2 (16.5–44.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 6.6 (0.1–39.0) | 10.1 (1.5–99) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a–b | 0 | 14 (1.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 452 (75.2) | 643 (80.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 148 (24.6) | 141 (17.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 1 (0.2) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 153 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (range) | 6.3 (4–9) | 6.2 (4–9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (g), mean (range) | 55.4 (21–141) | 55.6 (22–192) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yes, n (%) | 281 (37.3) | 322 (40.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No (%) | (62.6) | (59.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Joseph 200593 (considered separate to Joseph 200794 but may include patient overlap for US patients) Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: not reported Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: last 50 patients in a series with localised prostate cancer who had laparoscopic radical prostatectomy or robot-assisted prostatectomy Exclusion criteria: first 50 cases in each laparoscopic and robot-assisted series ABPatients enrolled, n5050Age (years), mean (95% CI)59.6 (1.6)61.8 (1.6)PSA (ng/ml), mean (95% CI)7.3 (1.2)6.0 (0.83)Clinical stage, nT1c4334T2a614T2b12Biopsy Gleason score, mean6 (0.15)6 (0.14)Prostate size (g), mean53 (5.3)51 (4.1) |
A | B | Patients enrolled, n | 50 | 50 | Age (years), mean (95% CI) | 59.6 (1.6) | 61.8 (1.6) | PSA (ng/ml), mean (95% CI) | 7.3 (1.2) | 6.0 (0.83) | Clinical stage, n | T1c | 43 | 34 | T2a | 6 | 14 | T2b | 1 | 2 | Biopsy Gleason score, mean | 6 (0.15) | 6 (0.14) | Prostate size (g), mean | 53 (5.3) | 51 (4.1) |
A. Robotic prostatectomy B. Laparoscopic prostatectomy Nerve sparing: ABUnilateral, n (%)1 (2)10 (20)Bilateral, n (%)46 (92)24 (48)Non-sparing, n (%)3(6)16 (32) |
A | B | Unilateral, n (%) | 1 (2) | 10 (20) | Bilateral, n (%) | 46 (92) | 24 (48) | Non-sparing, n (%) | 3(6) | 16 (32) | Dysfunction: urinary incontinence, erectile dysfuntion, potency | |||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients enrolled, n | 50 | 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (95% CI) | 59.6 (1.6) | 61.8 (1.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (95% CI) | 7.3 (1.2) | 6.0 (0.83) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 43 | 34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 6 | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 1 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean | 6 (0.15) | 6 (0.14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (g), mean | 53 (5.3) | 51 (4.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 1 (2) | 10 (20) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 46 (92) | 24 (48) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-sparing, n (%) | 3(6) | 16 (32) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Menon 200295 Language: English Publication type: full text Number of study centres: one Setting: hospital Country: France Recruitment/treatment dates: October 2000–October 2001 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: mean (SD): A: 3 (1.3) months; B: 8.5 (3.2) months Length of follow-up for functional outcomes, mean: A: 1.5 months; B: 6.5 months Follow-up carried out with telephone survey by third party Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: patients with clinically localised prostate cancer undergoing prostatectomy; patients medically fit to undergo surgery, weighing < 250 lb (those weighing > 250 lb were recommended for open radical prostatectomy), waist size < 45 inches, body mass index < 35 kg/m2; patients with previous abdominal surgery were included ABPatients enrolled, n5048Patients analysed, n4040Age (years), mean (SD)60.7 (7.6)62.8 (7.0)BMI (kg/m2), mean (SD)27.7 (3.2)27.7 (2.5)PSA (ng/ml), mean (SD)5.7 (3.2)6.9 (4.4)Clinical stage, n (%)T1c28 (70)26 (65)T212 (30)14 (35)BMI, body mass index. |
A | B | Patients enrolled, n | 50 | 48 | Patients analysed, n | 40 | 40 | Age (years), mean (SD) | 60.7 (7.6) | 62.8 (7.0) | BMI (kg/m2), mean (SD) | 27.7 (3.2) | 27.7 (2.5) | PSA (ng/ml), mean (SD) | 5.7 (3.2) | 6.9 (4.4) | Clinical stage, n (%) | T1c | 28 (70) | 26 (65) | T2 | 12 (30) | 14 (35) | BMI, body mass index. |
A. Robotic prostatectomy: first 22 patients were operated using Montsouris technique; later 18 patients were operated using Vattikuti Institute technique B. Laparoscopic prostatectomy: performed using classical Montsouris technique |
Equipment failure Safety: surgical complications, operating time, discharge, blood loss Efficacy: margins, pT stage, pathological Gleason score, PSA recurrence Death (none) Learning curve: operating time |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients enrolled, n | 50 | 48 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients analysed, n | 40 | 40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 60.7 (7.6) | 62.8 (7.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 27.7 (3.2) | 27.7 (2.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 5.7 (3.2) | 6.9 (4.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 28 (70) | 26 (65) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 12 (30) | 14 (35) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Rozet 200796 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: France Recruitment/treatment dates: May 2003–May 2005 Prospective/retrospective data collection: not reported Patient recruited consecutively, Y/N: yes for group A Length of follow-up: not reported Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients underwent robotic or laparoscopic prostatectomy ABPatient enrolled, n133758 (operated at the same period)Patient analysed, n133133 (match-pair)Age, mean (range)62.0 (49–76)62.5 (47–74)BMI, mean (range)24.8 (18.8–35.5)25.3 (19.3–32.7)Previous abdominal/pelvic surgery5151PSA, ng/ml, mean (range)7.6 (0.9–38.0)7.8 (3.2–19.0)Clinical stage, n (%)T1b01 (0.8)T1c76 (57.1)90 (67.7)T2a51 (38.3)39 (29.3)T2b6 (4.5)2 (1.5)T3a01 (0.8)Biopsy Gleason score, mean (range)6.3 (4.0–9.0)6.3 (4.0–9.0)≤ 6101 (76%)93 (70%)729 (21.8%)37 (27.8%)8–103 (2.2%)3 (2.2%) |
A | B | Patient enrolled, n | 133 | 758 (operated at the same period) | Patient analysed, n | 133 | 133 (match-pair) | Age, mean (range) | 62.0 (49–76) | 62.5 (47–74) | BMI, mean (range) | 24.8 (18.8–35.5) | 25.3 (19.3–32.7) | Previous abdominal/pelvic surgery | 51 | 51 | PSA, ng/ml, mean (range) | 7.6 (0.9–38.0) | 7.8 (3.2–19.0) | Clinical stage, n (%) | T1b | 0 | 1 (0.8) | T1c | 76 (57.1) | 90 (67.7) | T2a | 51 (38.3) | 39 (29.3) | T2b | 6 (4.5) | 2 (1.5) | T3a | 0 | 1 (0.8) | Biopsy Gleason score, mean (range) | 6.3 (4.0–9.0) | 6.3 (4.0–9.0) | ≤ 6 | 101 (76%) | 93 (70%) | 7 | 29 (21.8%) | 37 (27.8%) | 8–10 | 3 (2.2%) | 3 (2.2%) |
A. Robotic prostatectomy: robot trade name: da Vinci system; approaches: extra-peritoneal B. Laparoscopic prostatectomy: approaches: extra-peritoneal nerve sparing ABUnilateral, n (%)35 (27.8)30 (23.8)Bilateral, n (%)91 (72.2)96 (76.2) Lymph node dissection: ABNo, n (%)131 (98.5)130 (97.7)Yes, n (%)2 (1.5)3 (2.3) |
A | B | Unilateral, n (%) | 35 (27.8) | 30 (23.8) | Bilateral, n (%) | 91 (72.2) | 96 (76.2) | A | B | No, n (%) | 131 (98.5) | 130 (97.7) | Yes, n (%) | 2 (1.5) | 3 (2.3) |
Safety: surgical complications, operating time, catheterisation, blood loss, blood transfusion Efficacy: margins, pT stage, pathological Gleason score Death Learning curve: operating time |
|||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient enrolled, n | 133 | 758 (operated at the same period) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient analysed, n | 133 | 133 (match-pair) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age, mean (range) | 62.0 (49–76) | 62.5 (47–74) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, mean (range) | 24.8 (18.8–35.5) | 25.3 (19.3–32.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous abdominal/pelvic surgery | 51 | 51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA, ng/ml, mean (range) | 7.6 (0.9–38.0) | 7.8 (3.2–19.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 0 | 1 (0.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 76 (57.1) | 90 (67.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 51 (38.3) | 39 (29.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 6 (4.5) | 2 (1.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3a | 0 | 1 (0.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (range) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.3 (4.0–9.0) | 6.3 (4.0–9.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 101 (76%) | 93 (70%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 29 (21.8%) | 37 (27.8%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 3 (2.2%) | 3 (2.2%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 35 (27.8) | 30 (23.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 91 (72.2) | 96 (76.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No, n (%) | 131 (98.5) | 130 (97.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yes, n (%) | 2 (1.5) | 3 (2.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Sundaram 200497 Language: English Publication type: conference abstract Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: not reported Prospective/retrospective data collection: not reported Patients recruited consecutively: yes in robotic group, not reported for laparoscopic group Length of follow-up: mean: 3 months Source of funding: not reported Systematic reviewer: XJ |
Inclusion and exclusion criteria: not reported ABPatients, n1010Age (years), mean (range)59.5 (53–69)58.7 (50–66)PSA (ng/ml), mean (range)5.2 (3–7.9)5.3 (4.7–6)Clinical stage, nT1c972a13 |
A | B | Patients, n | 10 | 10 | Age (years), mean (range) | 59.5 (53–69) | 58.7 (50–66) | PSA (ng/ml), mean (range) | 5.2 (3–7.9) | 5.3 (4.7–6) | Clinical stage, n | T1c | 9 | 7 | 2a | 1 | 3 |
A. Robotic prostatectomy B. Laparoscopic prostatectomy |
Safety: operating time, hospital stay, surgical complications, blood loss Efficacy: margins Dysfunction: urinary incontinence |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 10 | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 59.5 (53–69) | 58.7 (50–66) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 5.2 (3–7.9) | 5.3 (4.7–6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 9 | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2a | 1 | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Trabulsi 200898 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: A: October 2005–August 2006 B: March 2000–December 2005 Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: men with clinically localised prostate cancer treated with either robotic or laparoscopic prostatectomy ABPatients, n50190Age (years), mean (range)57.7 (37–60)58.6 (43–74)BMI (kg/m2), mean (range)28.4 (20.4–36.6)26.8 (18.8–51.8)PSA (ng/ml), mean (range)5.5 (1.1–21.1)6.5 (0.4–46)Clinical stage, n (%)T1c41 (82)145 (76)T2a9 (18)40 (21)Not reported05Biopsy Gleason score, n (%)≤ 636 (72)136 (72)3 + 48 (16)31 (16)4 + 34 (8)6 (3)≥ 82 (4)3 (2)Prostate size (g), mean (range)41 (16–102)43.3 (14–156)BMI, body mass index. |
A | B | Patients, n | 50 | 190 | Age (years), mean (range) | 57.7 (37–60) | 58.6 (43–74) | BMI (kg/m2), mean (range) | 28.4 (20.4–36.6) | 26.8 (18.8–51.8) | PSA (ng/ml), mean (range) | 5.5 (1.1–21.1) | 6.5 (0.4–46) | Clinical stage, n (%) | T1c | 41 (82) | 145 (76) | T2a | 9 (18) | 40 (21) | Not reported | 0 | 5 | Biopsy Gleason score, n (%) | ≤ 6 | 36 (72) | 136 (72) | 3 + 4 | 8 (16) | 31 (16) | 4 + 3 | 4 (8) | 6 (3) | ≥ 8 | 2 (4) | 3 (2) | Prostate size (g), mean (range) | 41 (16–102) | 43.3 (14–156) | BMI, body mass index. |
A. Robotic prostatectomy: used da Vinci system; surgical approaches intraperitoneal; lymph nodes dissected when indicated (in intermediate- and high-risk patients): 14 (28%) B. Laparoscopic prostatectomy: surgical approaches transperitoneal; lymph nodes dissection: same indication as above: 51 (27%) |
Safety: open conversion, blood loss Efficacy: margins, pT stage, pathological Gleason score |
||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 50 | 190 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 57.7 (37–60) | 58.6 (43–74) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (range) | 28.4 (20.4–36.6) | 26.8 (18.8–51.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 5.5 (1.1–21.1) | 6.5 (0.4–46) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 41 (82) | 145 (76) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 9 (18) | 40 (21) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 0 | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 36 (72) | 136 (72) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 + 4 | 8 (16) | 31 (16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 + 3 | 4 (8) | 6 (3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 8 | 2 (4) | 3 (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (g), mean (range) | 41 (16–102) | 43.3 (14–156) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant characteristics | Intervention characteristics | Outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author, year: Barocas 2010103 Language: English Publication type: full text Number of study centres: 1 Setting: medical centre Country: USA Recruitment/treatment dates: June 2003–January 2008 Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up, median [interquartile range (IQR)]: total: 10 (2–23) months; A: 8 (2–20) months; B: 17 (8–34) months Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients undergoing radical prostatectomy for clinically localised prostate cancer Exclusion criteria: patients with earlier treatment, missing data, lymph node involvement ABPatients, n1413491Age (years), mean (SD)61 (7.3)62 (7.3)PSA (ng/ml), median (IQR)5.4 (4.3–7.4)5.8 (4.6–8.4)Clinical stage, n (%)T1a3 (0.21)3 (0.61)T1b1 (0.07)0T1c1086 (77.3)342 (69.94)T2a267 (19)89 (18.2)T2b37 (2.63)42 (8.59)T2c4 (0.28)12 (2.45)T3a7 (0.5)0T3b01 (0.2)Missing8 patients were missing clinical stage; 2 patients were missing procedure typeBiopsy Gleason score, n (%)≤ 6986 (69.9)327 (66.6)7353 (25.0)116 (23.5)8–1072 (5.1)48 (9.8)Missing20 |
A | B | Patients, n | 1413 | 491 | Age (years), mean (SD) | 61 (7.3) | 62 (7.3) | PSA (ng/ml), median (IQR) | 5.4 (4.3–7.4) | 5.8 (4.6–8.4) | Clinical stage, n (%) | T1a | 3 (0.21) | 3 (0.61) | T1b | 1 (0.07) | 0 | T1c | 1086 (77.3) | 342 (69.94) | T2a | 267 (19) | 89 (18.2) | T2b | 37 (2.63) | 42 (8.59) | T2c | 4 (0.28) | 12 (2.45) | T3a | 7 (0.5) | 0 | T3b | 0 | 1 (0.2) | Missing | 8 patients were missing clinical stage; 2 patients were missing procedure type | Biopsy Gleason score, n (%) | ≤ 6 | 986 (69.9) | 327 (66.6) | 7 | 353 (25.0) | 116 (23.5) | 8–10 | 72 (5.1) | 48 (9.8) | Missing | 2 | 0 |
A. Robotic prostatectomy: trade name of robot: da Vinci system B. Open prostatectomy: performed by standard techniques with small modifications described by Walsh and Partin218 |
Efficacy: margins, pT stage, pathological Gleason score, PSA recurrence | ||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 1413 | 491 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 61 (7.3) | 62 (7.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (IQR) | 5.4 (4.3–7.4) | 5.8 (4.6–8.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 3 (0.21) | 3 (0.61) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 1 (0.07) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 1086 (77.3) | 342 (69.94) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 267 (19) | 89 (18.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 37 (2.63) | 42 (8.59) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 4 (0.28) | 12 (2.45) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3a | 7 (0.5) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3b | 0 | 1 (0.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | 8 patients were missing clinical stage; 2 patients were missing procedure type | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 986 (69.9) | 327 (66.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 353 (25.0) | 116 (23.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 72 (5.1) | 48 (9.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | 2 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Kordan 2010120 (secondary to Barocas 2010103) Language: English Publication type: full text Number of study centres: 1 Setting: university medical centre Country: USA Recruitment/treatment dates: June 2003–July 2006 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: not reported Source of funding: not reported Systematic reviewer: TG |
Inclusion criteria: clinically localised prostate cancer Exclusion criteria: not reported ABPatients, n830414Age (years), mean (SD)60.5 (7.2)61.5 (7.5)BMI (kg/m2), mean (SD)28.2 (4.2)28.0 (4.6)PSA (ng/ml), median (IQR)5.5 (4.4–7.3)6.0 (4.6–9.1)Clinical stage (clinically palpable > cT2), n (%)204 (24.8)128 (31.2)Biopsy Gleason score, n (%)≤ 6578 (69.8)261 (63.0)7211 (25.5)104 (15.1)8–1039 (47.1)49 (11.8)Not reported20Prostate size (ml) (range)46 (37–58)41 (31–52)BMI, body mass index. |
A | B | Patients, n | 830 | 414 | Age (years), mean (SD) | 60.5 (7.2) | 61.5 (7.5) | BMI (kg/m2), mean (SD) | 28.2 (4.2) | 28.0 (4.6) | PSA (ng/ml), median (IQR) | 5.5 (4.4–7.3) | 6.0 (4.6–9.1) | Clinical stage (clinically palpable > cT2), n (%) | 204 (24.8) | 128 (31.2) | Biopsy Gleason score, n (%) | ≤ 6 | 578 (69.8) | 261 (63.0) | 7 | 211 (25.5) | 104 (15.1) | 8–10 | 39 (47.1) | 49 (11.8) | Not reported | 2 | 0 | Prostate size (ml) (range) | 46 (37–58) | 41 (31–52) | BMI, body mass index. |
A. Robotic prostatectomy B. Open prostatectomy |
Safety: blood transfusion, blood loss | |||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 830 | 414 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 60.5 (7.2) | 61.5 (7.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 28.2 (4.2) | 28.0 (4.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (IQR) | 5.5 (4.4–7.3) | 6.0 (4.6–9.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage (clinically palpable > cT2), n (%) | 204 (24.8) | 128 (31.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 578 (69.8) | 261 (63.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 211 (25.5) | 104 (15.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 39 (47.1) | 49 (11.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 2 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml) (range) | 46 (37–58) | 41 (31–52) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Chan 2008119 (secondary to Barocas 2010103) Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: May 2003–August 2006 Prospective/retrospective data collection: not reported Patients recruited consecutively: yes Length of follow-up: none Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: patient with clinically localised carcinoma of the prostate Data reported based on prostate size (large vs small). Here we have extracted the combined data wherever possible. When mean (range) were reported, only ranges have been extracted ABPatients, n660340Age (years), range36–7840–81PSA (ng/ml), range0.18–760.5–51.7Clinical stage, n (%)T1497 (75)225 (66)T2160 (24)111 (33)T33 (1)4 (1)Biopsy Gleason score, n (%)≤ 6459 (70)212 (62)7173 (26)87 (26)8–1028 (4)41 (12)Prostate size (g), range15–1810.7–224 |
A | B | Patients, n | 660 | 340 | Age (years), range | 36–78 | 40–81 | PSA (ng/ml), range | 0.18–76 | 0.5–51.7 | Clinical stage, n (%) | T1 | 497 (75) | 225 (66) | T2 | 160 (24) | 111 (33) | T3 | 3 (1) | 4 (1) | Biopsy Gleason score, n (%) | ≤ 6 | 459 (70) | 212 (62) | 7 | 173 (26) | 87 (26) | 8–10 | 28 (4) | 41 (12) | Prostate size (g), range | 15–181 | 0.7–224 |
A. Robotic prostatectomy: performed using a five-port technique Nerve sparing: Unilateral: 8/28 Bilateral: 86/522 Non-nerve sparing: 25/110 B. Open prostatectomy: performed via an infra-umbilical midline incision Nerve sparing: Unilateral: 12/30 Bilateral: 52/183 Non-nerve sparing: 52/127 |
Safety: open conversion, operating time, hospital stay Learning curve: operating time |
|||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 660 | 340 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), range | 36–78 | 40–81 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), range | 0.18–76 | 0.5–51.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 497 (75) | 225 (66) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 160 (24) | 111 (33) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 3 (1) | 4 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 459 (70) | 212 (62) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 173 (26) | 87 (26) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 28 (4) | 41 (12) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (g), range | 15–181 | 0.7–224 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Carlsson 2010104 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Sweden Recruitment/treatment dates: January 2002–August 2007 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: median: A: 19 months; B: 30 months Source of funding: Swedish Cancer Society, Avtal om läkarutbildning och forskning (Agreement on Medical Education and Research; ALF) and the Johanna Hagstrand and Sigfrid Linnér Foundation Systematic reviewer: XJ |
Inclusion criteria: patients underwent robotic or retropubic prostatectomy for clinically localised prostate cancer ABPatients, n1253485Age (years), median (range)62 (35–78)63 (47–77)PSA (ng/ml), median (range)6.0 (4–9)6.0 (4–10)Clinical stage, n (%)cT1770 (61.5)251 (51.8)cT2435 (34.7)183 (37.8)cT348 (3.8)50 (10.4)Not reported01Biopsy Gleason score, median (range)6.3 (0.4–50)7.4 (0.1–135)Prostate size (ml), median (range)38.0 (16–206)38.0 (16–130) |
A | B | Patients, n | 1253 | 485 | Age (years), median (range) | 62 (35–78) | 63 (47–77) | PSA (ng/ml), median (range) | 6.0 (4–9) | 6.0 (4–10) | Clinical stage, n (%) | cT1 | 770 (61.5) | 251 (51.8) | cT2 | 435 (34.7) | 183 (37.8) | cT3 | 48 (3.8) | 50 (10.4) | Not reported | 0 | 1 | Biopsy Gleason score, median (range) | 6.3 (0.4–50) | 7.4 (0.1–135) | Prostate size (ml), median (range) | 38.0 (16–206) | 38.0 (16–130) |
A. Robotic prostatectomy B. Open prostatectomy: modification of Walsh ‘anatomical radical retropubic prostectomy218 Both A and B: a limited lymph node dissection performed if indicated (Gleason score 4 + 4 = 8 or PSA > 20 ng/ml) |
Safety: surgical complications Further treatment: urinary incontinence Death |
|||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 1253 | 485 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | 62 (35–78) | 63 (47–77) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | 6.0 (4–9) | 6.0 (4–10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| cT1 | 770 (61.5) | 251 (51.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| cT2 | 435 (34.7) | 183 (37.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| cT3 | 48 (3.8) | 50 (10.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 0 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, median (range) | 6.3 (0.4–50) | 7.4 (0.1–135) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), median (range) | 38.0 (16–206) | 38.0 (16–130) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Doumerc 2010105 Language: English Publication type: full text paper Number of study centres: not reported Setting: referral institution Country: Australia Recruitment/treatment dates: February 2006–December 2008 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up, mean (SD): A: 11.2 (9.4) months; B: 17.2 (9.7) months Source of funding: NIH Grant 5R01DK077116 Systematic reviewer: TG |
Inclusion criteria: clinically localised prostate cancer Exclusion criteria: patients with factors considered to increase surgical difficulty, e.g. morbid obesity, prostate size ≥ 100 ml, large middle lobe, previous TURP, a history of laparoscopic hernia mesh repair, multiple abdominal operations, high-volume tumour ABPatients, n212502Age (years), mean (range)61.3 (41–76)60.1 (40–78)PSA (ng/ml), mean (range)7.1 (0.7–41)8.3 (0.9–64)Clinical stage, n (%)T1a4 (2)5 (1)T1b2 (1)5 (1)T1c99 (47)201 (40)T2a59 (28)111 (22)T2b16 (7)70 (14)T2c32 (15)95 (19)T3015 (3)Gleason score n (%)≤ 673 (34)126 (25)7128 (61)321 (64)8–1012 (5.6)55 (11)Prostate size (ml), mean (range)50 (16–140)53.2 (20–145)Data for robotic Gleason scores as reported by study authors. |
A | B | Patients, n | 212 | 502 | Age (years), mean (range) | 61.3 (41–76) | 60.1 (40–78) | PSA (ng/ml), mean (range) | 7.1 (0.7–41) | 8.3 (0.9–64) | Clinical stage, n (%) | T1a | 4 (2) | 5 (1) | T1b | 2 (1) | 5 (1) | T1c | 99 (47) | 201 (40) | T2a | 59 (28) | 111 (22) | T2b | 16 (7) | 70 (14) | T2c | 32 (15) | 95 (19) | T3 | 0 | 15 (3) | Gleason score n (%) | ≤ 6 | 73 (34) | 126 (25) | 7 | 128 (61) | 321 (64) | 8–10 | 12 (5.6) | 55 (11) | Prostate size (ml), mean (range) | 50 (16–140) | 53.2 (20–145) | Data for robotic Gleason scores as reported by study authors. |
A. Robotic prostatectomy: described by Patel;219 transperitoneal surgical approach; trade name and manufacturer of robot not reported B. Open prostatectomy: performed via infra-umbilical incision Lymph node dissection: ABNo lymph node, n (%)158/212 (74.5)239/502 (47.6)Negative, n (%)54/54 (100)247/263 (94)1 positive, n (%)011/263 (4)> 1 positive, n (%)05/263 (2) |
A | B | No lymph node, n (%) | 158/212 (74.5) | 239/502 (47.6) | Negative, n (%) | 54/54 (100) | 247/263 (94) | 1 positive, n (%) | 0 | 11/263 (4) | > 1 positive, n (%) | 0 | 5/263 (2) |
Safety: surgical complications, operating time, hospital stay, catheterisation, blood loss Efficacy: margins, pT stage, pathological Gleason score Death |
||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 212 | 502 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 61.3 (41–76) | 60.1 (40–78) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 7.1 (0.7–41) | 8.3 (0.9–64) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 4 (2) | 5 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 2 (1) | 5 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 99 (47) | 201 (40) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 59 (28) | 111 (22) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 16 (7) | 70 (14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 32 (15) | 95 (19) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 0 | 15 (3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gleason score n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 73 (34) | 126 (25) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 128 (61) | 321 (64) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 12 (5.6) | 55 (11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (range) | 50 (16–140) | 53.2 (20–145) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data for robotic Gleason scores as reported by study authors. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No lymph node, n (%) | 158/212 (74.5) | 239/502 (47.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Negative, n (%) | 54/54 (100) | 247/263 (94) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 positive, n (%) | 0 | 11/263 (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 1 positive, n (%) | 0 | 5/263 (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Ficarra 2009106 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Italy Recruitment/treatment dates: February 2006–April 2007 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: 1 year Source of funding: partially funded by the Italian Ministry for University and Research Systematic reviewer: XJ |
Inclusion criteria: all patients undergoing robotic or open prostatectomy for clinically localised prostate cancer Exclusion criteria: not reported ABPatients, n103105Age (years), median (IQR)61 (57–67)65 (61–69)BMI (kg/m2), median (IQR)26 (24–28)26 (24–28)PSA (ng/ml), median (IQR)6.4 (4.6–9)6 (5–10)Clinical stage, n (%)T1c77 (75)66 (63)T2a-b22 (21)32 (30)T2c4 (4)7 (7)Biopsy Gleason score, n (%)n = 97n = 104≤ 671 (73)67 (64)718 (19)29 (28)8–108 (8)8 (8)Prostate size (ml), median (IQR)37.5 (30–48)40 (30–47)BMI, body mass index. |
A | B | Patients, n | 103 | 105 | Age (years), median (IQR) | 61 (57–67) | 65 (61–69) | BMI (kg/m2), median (IQR) | 26 (24–28) | 26 (24–28) | PSA (ng/ml), median (IQR) | 6.4 (4.6–9) | 6 (5–10) | Clinical stage, n (%) | T1c | 77 (75) | 66 (63) | T2a-b | 22 (21) | 32 (30) | T2c | 4 (4) | 7 (7) | Biopsy Gleason score, n (%) | n = 97 | n = 104 | ≤ 6 | 71 (73) | 67 (64) | 7 | 18 (19) | 29 (28) | 8–10 | 8 (8) | 8 (8) | Prostate size (ml), median (IQR) | 37.5 (30–48) | 40 (30–47) | BMI, body mass index. |
A. Robotic prostatectomy: trade name of robot: da Vinci system; approaches: extraperitoneal; 64 (62%) had bilateral nerve sparing; lymph node dissected in patients with high risk of lymph node involvement B. Open prostatectomy: approaches: extraperitoneal; 41 (39%) had bilateral nerve sparing; same indication as above for lymph node dissection |
Safety: surgical complications, operating time, hospital stay, catherisation, blood loss Efficacy: margins, pT stage Dysfunction: urinary incontinence, erectile dysfunction |
|||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 103 | 105 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (IQR) | 61 (57–67) | 65 (61–69) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), median (IQR) | 26 (24–28) | 26 (24–28) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (IQR) | 6.4 (4.6–9) | 6 (5–10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 77 (75) | 66 (63) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a-b | 22 (21) | 32 (30) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 4 (4) | 7 (7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | n = 97 | n = 104 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 71 (73) | 67 (64) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 18 (19) | 29 (28) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 8 (8) | 8 (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), median (IQR) | 37.5 (30–48) | 40 (30–47) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Fracalanza 2008107 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Italy Recruitment/treatment dates: May 2006–October 2006 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: none Source of funding: Italian ministry for University and Research Systematic reviewer: PS |
Inclusion criteria: patients with clinically localised prostate cancer (cT1–2) ABPatients, n3526Age (years), mean (range)62 (56–68)68.5 (59–71)BMI (kg/m2), mean (SD)25.5 (2.7)26.4 (3.7)PSA (ng/ml), median (range)6.2 (4.2–10.2)6.2 (4.5–9.1)Biopsy Gleason score, n (%)≤ 614 (40)6 (23)713 (37)16 (62)8–98 (23)4 (15)Prostate size (ml), median (range)40 (30–60)36 (30–40)Charlson score, mean (SD)4 (3–4)4.5 (3.7–5)BMI, body mass index. |
A | B | Patients, n | 35 | 26 | Age (years), mean (range) | 62 (56–68) | 68.5 (59–71) | BMI (kg/m2), mean (SD) | 25.5 (2.7) | 26.4 (3.7) | PSA (ng/ml), median (range) | 6.2 (4.2–10.2) | 6.2 (4.5–9.1) | Biopsy Gleason score, n (%) | ≤ 6 | 14 (40) | 6 (23) | 7 | 13 (37) | 16 (62) | 8–9 | 8 (23) | 4 (15) | Prostate size (ml), median (range) | 40 (30–60) | 36 (30–40) | Charlson score, mean (SD) | 4 (3–4) | 4.5 (3.7–5) | BMI, body mass index. |
A. Robotic prostatectomy: trade name: da Vinci system; performed with transperitoneal approach with an antegrade prostatic dissection; lymph node dissection carried out in men with a high risk of lymph node involvement B. Open prostatectomy: performed according to the Walsh technique;218 all patients had lymph node dissection, including external iliac and obturatory lymph nodes |
Safety: surgical complications, operating time, hospital stay, blood loss, surgical incision, time to mobilisation, oral feeding Efficacy: margins, pT stage Learning curve: operating time |
||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 35 | 26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 62 (56–68) | 68.5 (59–71) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 25.5 (2.7) | 26.4 (3.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | 6.2 (4.2–10.2) | 6.2 (4.5–9.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 14 (40) | 6 (23) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 13 (37) | 16 (62) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–9 | 8 (23) | 4 (15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), median (range) | 40 (30–60) | 36 (30–40) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charlson score, mean (SD) | 4 (3–4) | 4.5 (3.7–5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Krambeck 2009108 Language: English Publication type: full text Number of study centres: 1 Setting: clinic Country: USA Recruitment/treatment dates: August 2002–December 2005 Prospective/retrospective data collection: retrospective Patients recruited consecutively: yes in the robotic group, no in the open group. Length of follow-up: median 1.3 years Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients undergoing clinically localised prostate cancer Exclusion criteria: not reported ABPatients, n294588Age (years), median (range)61.0 (38.0–76.0)61.0 (41.0–77.0)PSA (ng/ml), median (range)4.9 (0.5–33.5)5.0 (0.6–39.7)Clinical stage, n (%)T1a/b04 (0.7)T1c214 (72.8)418 (71.1)T2a75 (25.5)130 (22.1)T2b4 (1.4)28 (4.8)T3/41 (0.3)8 (1.4)Biopsy Gleason score, n (%)≤ 6214 (72.8)441 (75.0)770 (23.8)133 (22.6)8–910 (3.4)14 (2.3) |
A | B | Patients, n | 294 | 588 | Age (years), median (range) | 61.0 (38.0–76.0) | 61.0 (41.0–77.0) | PSA (ng/ml), median (range) | 4.9 (0.5–33.5) | 5.0 (0.6–39.7) | Clinical stage, n (%) | T1a/b | 0 | 4 (0.7) | T1c | 214 (72.8) | 418 (71.1) | T2a | 75 (25.5) | 130 (22.1) | T2b | 4 (1.4) | 28 (4.8) | T3/4 | 1 (0.3) | 8 (1.4) | Biopsy Gleason score, n (%) | ≤ 6 | 214 (72.8) | 441 (75.0) | 7 | 70 (23.8) | 133 (22.6) | 8–9 | 10 (3.4) | 14 (2.3) |
A. Robotic prostatectomy: robot trade name: da Vinci system; all patients had pelvic lymphadenectomy B. Open prostatectomy: all patients had pelvic lymphadenectomy Nerve sparing: ABUnilateral, n (%)20 (6.8)26 (4.4)Bilateral, n (%)221 (75.1)509 (86.6) |
A | B | Unilateral, n (%) | 20 (6.8) | 26 (4.4) | Bilateral, n (%) | 221 (75.1) | 509 (86.6) |
Safety: surgical complications, operating time, hospital stay Efficacy: margins, pathological Gleason score, PSA recurrence, local recurrence, metastatic recurrence Dysfunction: urinary incontinence, erectile dysfunction Death Learning curve: operating time |
||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 294 | 588 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | 61.0 (38.0–76.0) | 61.0 (41.0–77.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | 4.9 (0.5–33.5) | 5.0 (0.6–39.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a/b | 0 | 4 (0.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 214 (72.8) | 418 (71.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 75 (25.5) | 130 (22.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 4 (1.4) | 28 (4.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3/4 | 1 (0.3) | 8 (1.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 214 (72.8) | 441 (75.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 70 (23.8) | 133 (22.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–9 | 10 (3.4) | 14 (2.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 20 (6.8) | 26 (4.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 221 (75.1) | 509 (86.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Loeb 2010109 Language: English Publication type: full text Number of study centres: not reported Setting: medical institution Country: USA Recruitment/treatment dates: 2005–8 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: TG |
Inclusion criteria: not reported Exclusion criteria: not reported ABTotalPatients, n152137289Age (years), mean (SD)––58.1 (5.6)PSA (ng/ml), mean (SD)––5.4 (2.9)Clinical stage, n (%)T1c––220 (76.1)T2––67 (23.1)T3––1 (0.4)Missing––1 (0.4)Gleason score, n (%)≤ 6––199 (68.9)7––73 (25.2)8–10––17 (5.9) |
A | B | Total | Patients, n | 152 | 137 | 289 | Age (years), mean (SD) | – | – | 58.1 (5.6) | PSA (ng/ml), mean (SD) | – | – | 5.4 (2.9) | Clinical stage, n (%) | T1c | – | – | 220 (76.1) | T2 | – | – | 67 (23.1) | T3 | – | – | 1 (0.4) | Missing | – | – | 1 (0.4) | Gleason score, n (%) | ≤ 6 | – | – | 199 (68.9) | 7 | – | – | 73 (25.2) | 8–10 | – | – | 17 (5.9) |
A. Robotic prostatectomy: various techniques but the prostatic dissection was always antegrade with division of the bladder neck from anterior and posterior B. Open prostatectomy: performed in the standard anatomical fashion described by Latiff and Gomez220 |
Efficacy: margins, PSA recurrence | ||||||||||||||||||||||||
| A | B | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 152 | 137 | 289 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | – | – | 58.1 (5.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | – | – | 5.4 (2.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | – | – | 220 (76.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | – | – | 67 (23.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | – | – | 1 (0.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | – | – | 1 (0.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | – | – | 199 (68.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | – | – | 73 (25.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | – | – | 17 (5.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Malcolm 2010110 Language: English Publication type: full text Number of study centres: 1 Setting: prostate centre/institution Country: USA Recruitment/treatment dates: February 2000–December 2008 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: A: 20 months; B: 31.5 months Source of funding: not reported; three authors declared financial interest with In Touch Health Inc., Endocare Inc., Intuitive Surgical Inc., Dendreon Crop, southwest Oncology Group, ContraVac and Theralogix Systematic reviewer: CR |
Inclusion criteria: undergoing operative treatment for localised prostate cancer. Included in the analysis if a baseline and at least one follow-up questionnaire were completed (149 excluded) Exclusion criteria: patients were excluded from the analysis if multimodal treatment was administered. 195 patients with a UCLAPCI function/bother score ≤ 30 at baseline excluded from stat analysis ABPatients, n447135Age (years), mean (SD)59 (6)59 (7)Clinical stage, n (%)≤ T1c340 (76)112 (83)T2a68 (15)17 (13)T2b32 (7)6 (4)Unknown7 (2)0Biopsy Gleason score, n (%)≤ 6269 (60)93 (69)7154 (34)34 (25)≥ 824 (5)8 (6)PSA (ng/ml), median (IQR)5.2 (3.9–6.8)5.7 (4.7–7.3) |
A | B | Patients, n | 447 | 135 | Age (years), mean (SD) | 59 (6) | 59 (7) | Clinical stage, n (%) | ≤ T1c | 340 (76) | 112 (83) | T2a | 68 (15) | 17 (13) | T2b | 32 (7) | 6 (4) | Unknown | 7 (2) | 0 | Biopsy Gleason score, n (%) | ≤ 6 | 269 (60) | 93 (69) | 7 | 154 (34) | 34 (25) | ≥ 8 | 24 (5) | 8 (6) | PSA (ng/ml), median (IQR) | 5.2 (3.9–6.8) | 5.7 (4.7–7.3) |
A. Robotic prostatectomy: nerve-sparing techniques used where clinically appropriate as determined by the surgeon B. Open prostatectomy: nerve-sparing techniques used where clinically appropriate as determined by the surgeon; retropubic or perineal route Nerve sparing: ABSpared, n (%)366 (82)95 (70)Not spared, n (%)81 (18)40 (30) |
A | B | Spared, n (%) | 366 (82) | 95 (70) | Not spared, n (%) | 81 (18) | 40 (30) | Dysfunction: urinary function, sexual function | |||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 447 | 135 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 59 (6) | 59 (7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ T1c | 340 (76) | 112 (83) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 68 (15) | 17 (13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 32 (7) | 6 (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unknown | 7 (2) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 269 (60) | 93 (69) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 154 (34) | 34 (25) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 8 | 24 (5) | 8 (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (IQR) | 5.2 (3.9–6.8) | 5.7 (4.7–7.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spared, n (%) | 366 (82) | 95 (70) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not spared, n (%) | 81 (18) | 40 (30) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Miller 2007111 Language: English Publication type: full text Number of study centres: 1 Setting: hospital institution Country: USA Recruitment/treatment dates: July 2002–August 2006 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: 6 weeks Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients with clinically localised (cT1-2) prostate cancer Exclusion criteria: not reported ABPatients, n42120Age (years), mean61.160.6 |
A | B | Patients, n | 42 | 120 | Age (years), mean | 61.1 | 60.6 |
A. Robotic prostatectomy: robot trade name: da Vinci system (four robotic and two assistant ports in a manner similar to that of Menon et al. 221) B. Open prostatectomy: anatomical retropubic radical prostectomy via a 10–12 cm infra-umbilical midline incision For both A and B: nerve sparing was performed when oncologically appropriate and in patients who were potent preoperatively |
Safety: blood loss Quality of life |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 42 | 120 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean | 61.1 | 60.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Nadler 2010112 Language: English Publication type: full text paper Number of study centres: 1 Setting: not reported Country: USA Recruitment/treatment dates: A: October 2005–October 2006; B: July 2002–February 2006 Prospective/retrospective data collection: both Patients recruited consecutively: yes Length of follow-up: 2 years Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: not reported Exclusion criteria: not reported ABPatients, n5050Age (years), mean (range)59.7 (44–77)60 (40–75)BMI (kg/m2), mean (range)28.6 (23.3–42)28.2 (21–42.6)PSA (ng/ml), mean (range)6.5 (1.5–18.8)8.5 (1.9–95.6)Clinical stage, n (%)T141 (82)41 (82)T29 (18)9 (18)Biopsy Gleason score, mean (range)6.42 (6–9)6.66 (6–10)Prostate size (ml), mean (range)49.4 (27.2–109.1)62.8 (14.9–135.8)American Urological Association risk stratification, n (%)Low30 (60)28 (56)Moderate14 (28)12 (24)High6 (12)10 (20)BMI, body mass index. |
A | B | Patients, n | 50 | 50 | Age (years), mean (range) | 59.7 (44–77) | 60 (40–75) | BMI (kg/m2), mean (range) | 28.6 (23.3–42) | 28.2 (21–42.6) | PSA (ng/ml), mean (range) | 6.5 (1.5–18.8) | 8.5 (1.9–95.6) | Clinical stage, n (%) | T1 | 41 (82) | 41 (82) | T2 | 9 (18) | 9 (18) | Biopsy Gleason score, mean (range) | 6.42 (6–9) | 6.66 (6–10) | Prostate size (ml), mean (range) | 49.4 (27.2–109.1) | 62.8 (14.9–135.8) | American Urological Association risk stratification, n (%) | Low | 30 (60) | 28 (56) | Moderate | 14 (28) | 12 (24) | High | 6 (12) | 10 (20) | BMI, body mass index. |
A. Robotic prostatectomy: four-arm, fiveport technique B. Open prostatectomy: performed as described by McCarthy and Catalona222 Nerve sparing: ABBilateral, n (%)38 (76)43 (86)Unilateral, n (%)8 (16)0Non-nerve sparing47 Lymph node dissection: A: 29/50 (58%) B: 50/50 (100%) ABBilateral, n (%)16 (55)45 (90)Unilateral, n (%)13 (45)5 (10) |
A | B | Bilateral, n (%) | 38 (76) | 43 (86) | Unilateral, n (%) | 8 (16) | 0 | Non-nerve sparing | 4 | 7 | A | B | Bilateral, n (%) | 16 (55) | 45 (90) | Unilateral, n (%) | 13 (45) | 5 (10) |
Safety: surgical complications, operating time, hospital stay, blood loss Efficacy: margins, pT stage, PSA recurrence Dysfunction: urinary continence, potency |
||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 50 | 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 59.7 (44–77) | 60 (40–75) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (range) | 28.6 (23.3–42) | 28.2 (21–42.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 6.5 (1.5–18.8) | 8.5 (1.9–95.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 41 (82) | 41 (82) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 9 (18) | 9 (18) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (range) | 6.42 (6–9) | 6.66 (6–10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (range) | 49.4 (27.2–109.1) | 62.8 (14.9–135.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| American Urological Association risk stratification, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Low | 30 (60) | 28 (56) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 14 (28) | 12 (24) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| High | 6 (12) | 10 (20) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 38 (76) | 43 (86) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 8 (16) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing | 4 | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 16 (55) | 45 (90) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 13 (45) | 5 (10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Ou 2009113 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Taiwan, Province of China Recruitment/treatment dates: April 2004–April 2007 Prospective/retrospective data collection: retrospective Patients recruited consecutively: yes Length of follow-up: 15 months Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients undergoing prostatectomy ABPatients, n3030Age (years), mean (SD)67.3 (6.2)70.0 (6.1)BMI (kg/m2), mean (SD)24.2 (3.2)24.1 (3.3)PSA (ng/ml), mean (SD)16.5 (18.8)15.9 (14.1)Clinical stage, nT1159T21519T302Biopsy Gleason score, mean (SD)6.1 (0.9)6.2 (1.6)BMI, body mass index. |
A | B | Patients, n | 30 | 30 | Age (years), mean (SD) | 67.3 (6.2) | 70.0 (6.1) | BMI (kg/m2), mean (SD) | 24.2 (3.2) | 24.1 (3.3) | PSA (ng/ml), mean (SD) | 16.5 (18.8) | 15.9 (14.1) | Clinical stage, n | T1 | 15 | 9 | T2 | 15 | 19 | T3 | 0 | 2 | Biopsy Gleason score, mean (SD) | 6.1 (0.9) | 6.2 (1.6) | BMI, body mass index. |
A. Robotic prostatectomy: performed as described by Patel219 with minor modification; 22/30 (73.3%) patients had bilateral lymph node dissection B. Open prostatectomy: performed using Walsh’s technique;218 30/30 (100%) patients had bilateral lymph node dissection Nerve sparing: ABUnilateral, n (%)5 (16.7)1 (3.3)Bilateral, n (%)11 (36.7)1 (3.3)Non-nerve sparing, n (%)14 (46.7)28 (93.3) |
A | B | Unilateral, n (%) | 5 (16.7) | 1 (3.3) | Bilateral, n (%) | 11 (36.7) | 1 (3.3) | Non-nerve sparing, n (%) | 14 (46.7) | 28 (93.3) |
Safety: open conversion, surgical complications, operating time, hospital stay, catheterisation, blood loss Efficacy: margins, pathological Gleason score, PSA recurrence Dysfunction: urinary incontinence, erectile dysfunction Learning curve: operating time |
||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 30 | 30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 67.3 (6.2) | 70.0 (6.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 24.2 (3.2) | 24.1 (3.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 16.5 (18.8) | 15.9 (14.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 15 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 15 | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 0 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 6.1 (0.9) | 6.2 (1.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 5 (16.7) | 1 (3.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 11 (36.7) | 1 (3.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 14 (46.7) | 28 (93.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Rocco 2009114 Language: English Publication type: full text Number of study centres: 1 Setting: institution Country: Italy Recruitment/treatment dates: A: November 2006–December 2007; B: May 2004–February 2007 Prospective/retrospective data collection: A: prospective; B: retrospective Patients recruited consecutively: yes in laparoscopic group Length of follow-up: 1 year Source of funding: not reported Systematic reviewer: XJ |
ABPatients, n120240Age (years), median (range)63 (47–76)63 (46–77)PSA (ng/ml), median (range)6.9 (0.4–23.0)6.7 (0.7–22.0)Clinical stage, n (%)T1c82 (69%)145 (6%)T2a36 (31%)93 (39%)Missing22Biopsy Gleason score, median (range)6 (4–9)6 (4–10) | A | B | Patients, n | 120 | 240 | Age (years), median (range) | 63 (47–76) | 63 (46–77) | PSA (ng/ml), median (range) | 6.9 (0.4–23.0) | 6.7 (0.7–22.0) | Clinical stage, n (%) | T1c | 82 (69%) | 145 (6%) | T2a | 36 (31%) | 93 (39%) | Missing | 2 | 2 | Biopsy Gleason score, median (range) | 6 (4–9) | 6 (4–10) |
A. Robotic prostatectomy: Patel technique219 B. Open prostatectomy: Walsh technique218 |
Safety: operating time, hospital stay, catheterisation, blood loss Efficacy: margins, pT stage, pathological Gleason score Dysfunction: urinary incontinence, erectile dysfunction |
|||||||||||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 120 | 240 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | 63 (47–76) | 63 (46–77) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | 6.9 (0.4–23.0) | 6.7 (0.7–22.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 82 (69%) | 145 (6%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 36 (31%) | 93 (39%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | 2 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, median (range) | 6 (4–9) | 6 (4–10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Schroeck 2008115 Language: English Publication type: full text Number of study centres: 1 Setting: not reported Country: USA Recruitment/treatment dates: August 2003–January 2007 Prospective/retrospective data collection: retrospective Patients recruited consecutively: yes Length of follow-up, mean: A: 1.09 years; B: 1.37 years Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: not reported Exclusion criteria: conversion to open procedure ABPatients, n362435Age (years), median (range)59.2 (54.5–63.8)60.3 (55.3–64.7)BMI (kg/m2), median (range)27.8 (25.7–29.9)27.7 (25.5–30.4)PSA (ng/ml), median (range)5.4 (4.1–7.1)5.3 (4.1–7.2)Clinical stage, nT1281296T257101T3012Not reported22Biopsy Gleason score, n≤ 62542417891278–10942Not reported1025Prostate size (ml), median (range)42.9 (34.3–55)41.3 (24.4–52)BMI, body mass index. |
A | B | Patients, n | 362 | 435 | Age (years), median (range) | 59.2 (54.5–63.8) | 60.3 (55.3–64.7) | BMI (kg/m2), median (range) | 27.8 (25.7–29.9) | 27.7 (25.5–30.4) | PSA (ng/ml), median (range) | 5.4 (4.1–7.1) | 5.3 (4.1–7.2) | Clinical stage, n | T1 | 281 | 296 | T2 | 57 | 101 | T3 | 0 | 12 | Not reported | 2 | 2 | Biopsy Gleason score, n | ≤ 6 | 254 | 241 | 7 | 89 | 127 | 8–10 | 9 | 42 | Not reported | 10 | 25 | Prostate size (ml), median (range) | 42.9 (34.3–55) | 41.3 (24.4–52) | BMI, body mass index. |
A. Robotic prostatectomy: robot trade name: da Vinci system; performed using Vattikuti Institute technique; lymph node dissection 271/362 (74.9%) B. Open prostatectomy: lymph node dissection 313/435 (72%) |
Safety: blood loss Efficacy: margins, pathological Gleason score, PSA recurrence |
|||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 362 | 435 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | 59.2 (54.5–63.8) | 60.3 (55.3–64.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), median (range) | 27.8 (25.7–29.9) | 27.7 (25.5–30.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | 5.4 (4.1–7.1) | 5.3 (4.1–7.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 281 | 296 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 57 | 101 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 0 | 12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 2 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 254 | 241 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 89 | 127 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 9 | 42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 10 | 25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), median (range) | 42.9 (34.3–55) | 41.3 (24.4–52) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Tewari 2003116 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: October 1999–December 2002 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes for open group, not reported for robotic group Length of follow-up, mean: A: 236 days; B: 556 days Systematic reviewer: XJ |
ABPatients, n200100Age (years), mean (range)59.9 (40–72)63.1 (42.8–72)BMI (kg/m2), mean (range)27.7 (19–38)27.6 (17–41)Previous abdominal and hernia surgery20%19%PSA (ng/ml), mean (range)6.4 (0.6–41)7.3 (1.9–35)Clinical stage (%, as reported by study authors)T1a0.50T1c4959T2a1010T2b3935T3a1.54Biopsy Gleason score (%)≤ 66752728358–10613Mean score6.56.6Prostate size (ml), mean (range)58.8 (18–140)48.4 (24.2–70)BMI, body mass index. | A | B | Patients, n | 200 | 100 | Age (years), mean (range) | 59.9 (40–72) | 63.1 (42.8–72) | BMI (kg/m2), mean (range) | 27.7 (19–38) | 27.6 (17–41) | Previous abdominal and hernia surgery | 20% | 19% | PSA (ng/ml), mean (range) | 6.4 (0.6–41) | 7.3 (1.9–35) | Clinical stage (%, as reported by study authors) | T1a | 0.5 | 0 | T1c | 49 | 59 | T2a | 10 | 10 | T2b | 39 | 35 | T3a | 1.5 | 4 | Biopsy Gleason score (%) | ≤ 6 | 67 | 52 | 7 | 28 | 35 | 8–10 | 6 | 13 | Mean score | 6.5 | 6.6 | Prostate size (ml), mean (range) | 58.8 (18–140) | 48.4 (24.2–70) | BMI, body mass index. |
A. Robotic prostatectomy: robot trade name: da Vinci system (robotically assisted Vattikuti Institute prostatectomy) B. Open prostatectomy: conducted using the anatomical technique For A and B: some patients had lymph node dissection |
Safety: open conversion, surgical complications, hospital stay, catheterisation, blood loss Efficacy: margins, pT stage, pathological Gleason score, PSA recurrence Dysfunction: urinary incontinence, erectile dysfunction Quality of life Pain Death (none) |
|||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 200 | 100 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 59.9 (40–72) | 63.1 (42.8–72) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (range) | 27.7 (19–38) | 27.6 (17–41) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous abdominal and hernia surgery | 20% | 19% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 6.4 (0.6–41) | 7.3 (1.9–35) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage (%, as reported by study authors) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 0.5 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 49 | 59 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 10 | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 39 | 35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3a | 1.5 | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 67 | 52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 28 | 35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 6 | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean score | 6.5 | 6.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (range) | 58.8 (18–140) | 48.4 (24.2–70) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Truesdale 2010117 Language: English Publication type: full text Number of study centres: 1 Setting: academic institution Country: USA Recruitment/treatment dates: January 2005–November 2009 Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: patients who had undergone open or robot-assisted radical prostatectomy with concurrent pelvic lymph node dissection for histologically proven, clinically localised prostate cancer Exclusion criteria: not reported ABPatients, n99217Age (years), mean (SD)59.2 (7.1)61.7 (6.8)BMI (kg/m2), mean (SD)24.6 (8.3)23.1 (9.1)PSA (ng/ml), mean (SD)7.04 (7.5)8.35 (7.62)Clinical stage, n (%)T2a57 (57.6)155 (71.4)T2b4 (4)12 (5.5)T2c38 (38.4)50 (23)Biopsy Gleason score, n (%)≤ 628 (28.3)63 (29)734 (34.3)95 (43.8)8–1037 (3.4)59 (27.2)D’Amico risk, n (%)Low43 (43.4)64 (29.5)Intermediate36 (36.4)94 (43.3)High20 (20.2)59 (27.2)BMI, body mass index. |
A | B | Patients, n | 99 | 217 | Age (years), mean (SD) | 59.2 (7.1) | 61.7 (6.8) | BMI (kg/m2), mean (SD) | 24.6 (8.3) | 23.1 (9.1) | PSA (ng/ml), mean (SD) | 7.04 (7.5) | 8.35 (7.62) | Clinical stage, n (%) | T2a | 57 (57.6) | 155 (71.4) | T2b | 4 (4) | 12 (5.5) | T2c | 38 (38.4) | 50 (23) | Biopsy Gleason score, n (%) | ≤ 6 | 28 (28.3) | 63 (29) | 7 | 34 (34.3) | 95 (43.8) | 8–10 | 37 (3.4) | 59 (27.2) | D’Amico risk, n (%) | Low | 43 (43.4) | 64 (29.5) | Intermediate | 36 (36.4) | 94 (43.3) | High | 20 (20.2) | 59 (27.2) | BMI, body mass index. |
A. Robotic prostatectomy: pelvic lymph node dissection carried out; positive lymph node 1/99 (1%) B. Open prostatectomy: pelvic lymph node dissection carried out; positive lymph node 19/217 (8.8%) Overall lymph node positivity rate 6.3% |
Safety: operating time, blood loss Efficacy: pT stage, pathological Gleason score |
||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 99 | 217 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 59.2 (7.1) | 61.7 (6.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 24.6 (8.3) | 23.1 (9.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 7.04 (7.5) | 8.35 (7.62) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 57 (57.6) | 155 (71.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 4 (4) | 12 (5.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 38 (38.4) | 50 (23) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 28 (28.3) | 63 (29) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 34 (34.3) | 95 (43.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 37 (3.4) | 59 (27.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D’Amico risk, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Low | 43 (43.4) | 64 (29.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intermediate | 36 (36.4) | 94 (43.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| High | 20 (20.2) | 59 (27.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: White 2009118 Language: English Publication type: full text Number of study centres: 1 Setting: community urological practice Country: USA Recruitment/treatment dates: December 2005–March 2008 Prospective/retrospective data collection: retrospective; laparoscopic procedures were conducted before the initiation of the robotic programme Patients recruited consecutively: yes in robotic group, no in the laparoscopic group Length of follow-up: not reported Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients had clinically localised carcinoma of the prostate ABPatients, n5050aAge (years), mean6264.7PSA (ng/ml), mean4.635.04Clinical stage, n (%)T140 (80)38 (76)T210 (20)12 (24)T300Biopsy Gleason score, n (%)≤ 639 (78)40 (80)710 (20)9 (18)8–101 (2)1 (2)Matched to the robotic group according to clinical stage, baseline PSA level, age, Gleason score. |
A | B | Patients, n | 50 | 50a | Age (years), mean | 62 | 64.7 | PSA (ng/ml), mean | 4.63 | 5.04 | Clinical stage, n (%) | T1 | 40 (80) | 38 (76) | T2 | 10 (20) | 12 (24) | T3 | 0 | 0 | Biopsy Gleason score, n (%) | ≤ 6 | 39 (78) | 40 (80) | 7 | 10 (20) | 9 (18) | 8–10 | 1 (2) | 1 (2) | Matched to the robotic group according to clinical stage, baseline PSA level, age, Gleason score. |
A. Robotic prostatectomy: technique as described by Menon et al. 223 B. Open prostatectomy: performed in the traditional fashion For both A and B: nerve sparing was performed in all patients, but not reported whether unilateral or bilateral |
Safety: open conversion Efficacy: margins, pT stage, pathological Gleason score |
|||||||||||||||||||||||||||||||||||||
| A | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 50 | 50a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean | 62 | 64.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean | 4.63 | 5.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 40 (80) | 38 (76) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 10 (20) | 12 (24) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 39 (78) | 40 (80) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 10 (20) | 9 (18) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 1 (2) | 1 (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Matched to the robotic group according to clinical stage, baseline PSA level, age, Gleason score. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant characteristics | Intervention characteristics | Outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author, year: Al-Shaiji 2010121 Language: English Publication type: full text Number of study centres: 1 Setting: health centre Country: Canada Recruitment/treatment dates: November 2004–November 2005 Prospective/retrospective data collection: retrospective Patients recruited consecutively: yes Length of follow-up: not reported Source of funding: not reported Systematic reviewer: TG |
Inclusion criteria: those diagnosed with organ-confined prostate cancer Exclusion criteria: not reported ABPatients, n7070Age (years), mean (range) SD60 (48–73) 5.8462 (46–75) 6.33PSA level, n0–10 ng/ml6756> 10 ng/ml314Clinical stage, nT1c5541T2a1424T2b13T2c02Biopsy Gleason score, n< 7343373230> 747 |
A | B | Patients, n | 70 | 70 | Age (years), mean (range) SD | 60 (48–73) 5.84 | 62 (46–75) 6.33 | PSA level, n | 0–10 ng/ml | 67 | 56 | > 10 ng/ml | 3 | 14 | Clinical stage, n | T1c | 55 | 41 | T2a | 14 | 24 | T2b | 1 | 3 | T2c | 0 | 2 | Biopsy Gleason score, n | < 7 | 34 | 33 | 7 | 32 | 30 | > 7 | 4 | 7 |
A: Laparoscopic prostatectomy: not reported B. Open prostatectomy: not reported |
Safety: blood loss, operating time, hospital stay | ||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 70 | 70 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) SD | 60 (48–73) 5.84 | 62 (46–75) 6.33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA level, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–10 ng/ml | 67 | 56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 10 ng/ml | 3 | 14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 55 | 41 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 14 | 24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 1 | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 0 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 7 | 34 | 33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 32 | 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 7 | 4 | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Anastasiadis 2003122 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: France Recruitment/treatment dates: May 1998–December 2001 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up, median: A: 15.1 months; B: 15.5 months Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: men with localised prostate cancer Exclusion criteria: patients using vacuum erection devices, pharmacological injection therapy or transurethral alprostadil were not included in the questionnaire group ABPatients, n23070Age (years), mean (range) SD64.1 (46–77) 6.464.8 (50–75) 6.4PSA (ng/ml), mean (range) SD10.7 (1.2–80) 8.811.2 (1.2–70) 9.7Clinical stage, n (%)T1a–b10 (4.3)2 (2.8)T1c156 (67.8)50 (71.4)T2a58 (25.2)17 (24.3)T2b6 (2.6)1 (1.4)Biopsy Gleason score, mean (range) SD5.8 (2–9) 1.26.1 (3–10) 1.1 |
A | B | Patients, n | 230 | 70 | Age (years), mean (range) SD | 64.1 (46–77) 6.4 | 64.8 (50–75) 6.4 | PSA (ng/ml), mean (range) SD | 10.7 (1.2–80) 8.8 | 11.2 (1.2–70) 9.7 | Clinical stage, n (%) | T1a–b | 10 (4.3) | 2 (2.8) | T1c | 156 (67.8) | 50 (71.4) | T2a | 58 (25.2) | 17 (24.3) | T2b | 6 (2.6) | 1 (1.4) | Biopsy Gleason score, mean (range) SD | 5.8 (2–9) 1.2 | 6.1 (3–10) 1.1 |
A. Laparoscopic prostatectomy: performed with a descending technique B. Open prostatectomy: performed with an ascending technique For both interventions the indication for preserving one bundle [laparoscopic n = 33 (14.3%); open n = 4 (5.7%)] or both bundles [laparoscopic n = 77 (33.4%); open n = 28 (40.0%)] depended on pre- and intraoperative factors. If all biopsies from one lobe were positive that bundle was usually sacrificed, prioritising cancer control before sexual function |
Safety: catheterisation, surgical complications Efficacy: margins, pT stage, pathological Gleason score Dysfunction: urinary continence |
|||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 230 | 70 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) SD | 64.1 (46–77) 6.4 | 64.8 (50–75) 6.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) SD | 10.7 (1.2–80) 8.8 | 11.2 (1.2–70) 9.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a–b | 10 (4.3) | 2 (2.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 156 (67.8) | 50 (71.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 58 (25.2) | 17 (24.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 6 (2.6) | 1 (1.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (range) SD | 5.8 (2–9) 1.2 | 6.1 (3–10) 1.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Artibani 2003123 Language: English Publication type: full text Number of study centres: 2 Setting: hospital Country: Italy Recruitment/treatment dates: January 2001–December 2001 Prospective/retrospective data collection: not reported Patients recruited consecutively: yes Length of follow-up: median (range): A: 10 (4–16) months; B: 10 (4–18) months Source of funding: not reported Additional information: two groups of patients were from two different hospitals in the same city Systematic reviewer: XJ |
Inclusion criteria: patients undergoing prostatectomy ABPatients, n7150Age (years), mean (SD)63 (5.8)64 (6.6)PSA (ng/ml), mean (SD)15.7 (17)11 (9)Clinical stage, n (%)T1b1 (1.5)4 (8)T1c20 (28)26 (52)T2a34 (48)15 (30)T2b10 (14)4 (8)T36 (8.5)1 (2)Biopsy Gleason score, mean (SD)5.8 (1.3)5.7 (1.2) |
A | B | Patients, n | 71 | 50 | Age (years), mean (SD) | 63 (5.8) | 64 (6.6) | PSA (ng/ml), mean (SD) | 15.7 (17) | 11 (9) | Clinical stage, n (%) | T1b | 1 (1.5) | 4 (8) | T1c | 20 (28) | 26 (52) | T2a | 34 (48) | 15 (30) | T2b | 10 (14) | 4 (8) | T3 | 6 (8.5) | 1 (2) | Biopsy Gleason score, mean (SD) | 5.8 (1.3) | 5.7 (1.2) |
A. Laparoscopic prostatectomy: surgical approaches: extraperitoneal B. Open prostatectomy Nerve sparing: ABUnilateral, n (%)9 (12.7)0Bilateral, n (%)9 (12.7)0Non-nerve sparing, n (%)53 (74.6)50 (100) Lymph node dissection: A: not carried out if PSA < 10 ng/ml and biopsy Gleason score < 7 B: all had lymph node dissection |
A | B | Unilateral, n (%) | 9 (12.7) | 0 | Bilateral, n (%) | 9 (12.7) | 0 | Non-nerve sparing, n (%) | 53 (74.6) | 50 (100) |
Safety: hospital stay, catheterisation, surgical complications Efficacy: margins, pT stage, pathological Gleason score, PSA recurrence Dysfunction: urinary incontinence, erectile dysfunction |
|||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 71 | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 63 (5.8) | 64 (6.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 15.7 (17) | 11 (9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 1 (1.5) | 4 (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 20 (28) | 26 (52) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 34 (48) | 15 (30) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 10 (14) | 4 (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 6 (8.5) | 1 (2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 5.8 (1.3) | 5.7 (1.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 9 (12.7) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 9 (12.7) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 53 (74.6) | 50 (100) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Bhayani 2003124 Language: English Publication type: full text Number of study centres: 1 Setting: urological institute/medical centre Country: USA Recruitment/treatment dates: July 2001–June 2002 Prospective/retrospective data collection: retrospective Patients recruited consecutively: unclear Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: all patients undergoing laparoscopic and open radical prostatectomy for localised prostate cancer Exclusion criteria: not reported ABPatients, n3324Age (years), mean (SD)57.4 (6.3)60.5 (6.4)PSA (ng/ml), mean (SD)6.74 (3.8)8.6 (9.1)Clinical stage, nT1a01T1c2114T2a118T2b11Biopsy Gleason score, mean (SD)6.06 (0.25)6.13 (0.44) |
A | B | Patients, n | 33 | 24 | Age (years), mean (SD) | 57.4 (6.3) | 60.5 (6.4) | PSA (ng/ml), mean (SD) | 6.74 (3.8) | 8.6 (9.1) | Clinical stage, n | T1a | 0 | 1 | T1c | 21 | 14 | T2a | 11 | 8 | T2b | 1 | 1 | Biopsy Gleason score, mean (SD) | 6.06 (0.25) | 6.13 (0.44) |
A. Laparoscopic prostatectomy: performed using the Guillonneau and Vallancien technique222 B. Open prostatectomy: performed using the Walsh technique218 |
Safety: open conversion, operating time, hospital stay, surgical complications, catheterisation, blood loss Efficacy: pT stage |
|||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 33 | 24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 57.4 (6.3) | 60.5 (6.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 6.74 (3.8) | 8.6 (9.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 0 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 21 | 14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 11 | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 1 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 6.06 (0.25) | 6.13 (0.44) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Brown 2004125 Language: English Publication type: full text Number of study centres: 1 Setting: urological institution Country: USA Recruitment/treatment dates: March 2000–March 2002 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: not reported Exclusion criteria: patients requiring conversion to open procedure and patients receiving neoadjuvant hormonal therapy or with metastatic disease ABPatients, n6060Age (years), mean (median)58.8 (58.5)59 (59)PSA (ng/ml), mean (median)6.4 (6)5.6 (5.1)Clinical stage, nT1a–b01T1c4745T2a1311T2b03Biopsy Gleason score, n≤ 64741713188–1001 |
A | B | Patients, n | 60 | 60 | Age (years), mean (median) | 58.8 (58.5) | 59 (59) | PSA (ng/ml), mean (median) | 6.4 (6) | 5.6 (5.1) | Clinical stage, n | T1a–b | 0 | 1 | T1c | 47 | 45 | T2a | 13 | 11 | T2b | 0 | 3 | Biopsy Gleason score, n | ≤ 6 | 47 | 41 | 7 | 13 | 18 | 8–10 | 0 | 1 |
A. Laparoscopic prostatectomy: performed using the Guillonneau and Vallancien technique. 224 Simultaneous bilateral pelvic lymph node dissection performed in 11 patients B. Open prostatectomy: performed in the standard fashion with simultaneous modified bilateral pelvic lymph node dissection. Unilateral or bilateral nerve sparing was performed when indicated |
Safety: operating time, hospital stay, readmission, surgical complications Efficacy: margins, pT stage Learning curve: operating time |
||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 60 | 60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (median) | 58.8 (58.5) | 59 (59) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (median) | 6.4 (6) | 5.6 (5.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a–b | 0 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 47 | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 13 | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 0 | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 47 | 41 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 13 | 18 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 0 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Dahl 2009126 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: 16 June 2003–22 July 2004 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: 12 months Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients 40–70 years old scheduled to undergo open or laparoscopic radical prostatectomy for clinical stage T1–2 N0M0 prostate cancer by any one of three experienced surgeons Exclusion criteria: not reported ABnAt baseline1041026 months757812 months7873Age (years), mean59.559.9PSA (ng/ml), n (%)0–2.512 (12)11 (11)2.6–4.020 (19)26 (25)4.1–7.042 (40)40 (39)7.1–10017 (16)14 (14)> 10013 (13)11 (11) |
A | B | n | At baseline | 104 | 102 | 6 months | 75 | 78 | 12 months | 78 | 73 | Age (years), mean | 59.5 | 59.9 | PSA (ng/ml), n (%) | 0–2.5 | 12 (12) | 11 (11) | 2.6–4.0 | 20 (19) | 26 (25) | 4.1–7.0 | 42 (40) | 40 (39) | 7.1–100 | 17 (16) | 14 (14) | > 100 | 13 (13) | 11 (11) |
A. Laparoscopic prostatectomy B. Open prostatectomy Nerve sparing: ABUnilateral, n (%)5 (5)4 (4)Bilateral, n (%)98 (94)98 (96)Non-nerve sparing, n (%)1 (1)0 |
A | B | Unilateral, n (%) | 5 (5) | 4 (4) | Bilateral, n (%) | 98 (94) | 98 (96) | Non-nerve sparing, n (%) | 1 (1) | 0 |
Safety: surgical complications Dysfunction: urinary incontinence, erectile dysfunction Further treatment: cancer treatment |
||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| At baseline | 104 | 102 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 75 | 78 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 months | 78 | 73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean | 59.5 | 59.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–2.5 | 12 (12) | 11 (11) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.6–4.0 | 20 (19) | 26 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1–7.0 | 42 (40) | 40 (39) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1–100 | 17 (16) | 14 (14) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 100 | 13 (13) | 11 (11) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 5 (5) | 4 (4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 98 (94) | 98 (96) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 1 (1) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Dahl 2006147 (secondary to Dahl 2009126) Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: 2001–5 Prospective/retrospective data collection: not reported Patients recruited consecutively: yes Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: patients who underwent radical prostatectomy Exclusion criteria: not reported From He 2006225 (secondary to Dahl 2006): Baseline characteristics: PSA: 10 ng/ml in > 90% of patients; T1c: 89% Quote: ‘similar distributions of clinical stages, preoperative PSA levels and Gleason scores on biopsy were seen between two groups’ |
A. Laparoscopic prostatectomy: n = 286; performed using modified Guillonneau and Vallancien technique224 B. Open prostatectomy: n = 714 |
Efficacy: margins, pT stage, pathological Gleason score | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Fornara 2004127 Language: German Publication type: full text Number of study centres: 1 Setting: institution Country: Germany Recruitment/treatment dates: January 2003–April 2004 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported? Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: Clinically localised prostate cancer Exclusion criteria: unknown ABPatients, n3232Age (years), mean (range)62.9 (42–74)64.8 (57–74)PSA (ng/ml), mean (range)7.9 (3.6–20.8)7.25 (4.4–17.3)Clinical stage, nT1a21T1c1615T2a1212T2b24Biopsy Gleason score, median (range)5.7 (3–7)5.3 (3–7)Prostate weight (g), median (range)37 (18–72)62.3 (20–120) |
A | B | Patients, n | 32 | 32 | Age (years), mean (range) | 62.9 (42–74) | 64.8 (57–74) | PSA (ng/ml), mean (range) | 7.9 (3.6–20.8) | 7.25 (4.4–17.3) | Clinical stage, n | T1a | 2 | 1 | T1c | 16 | 15 | T2a | 12 | 12 | T2b | 2 | 4 | Biopsy Gleason score, median (range) | 5.7 (3–7) | 5.3 (3–7) | Prostate weight (g), median (range) | 37 (18–72) | 62.3 (20–120) |
A. Laparoscopic prostatectomy: pre-peritoneal B. Open prostatectomy: ascending technique Both A and B involved removal of the prostate gland and seminal vesicles All patients had lymph node dissection prior to prostatectomy |
Safety: surgical complications, operating time, hospital stay, catheterisation, blood loss Efficacy: margins, pT stage, pathological Gleason score |
||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 32 | 32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 62.9 (42–74) | 64.8 (57–74) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 7.9 (3.6–20.8) | 7.25 (4.4–17.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 2 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 16 | 15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 12 | 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 2 | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, median (range) | 5.7 (3–7) | 5.3 (3–7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate weight (g), median (range) | 37 (18–72) | 62.3 (20–120) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Ghavamian 2006128 Language: English Publication type: full text Number of study centres: 1 Setting: university hospital Country: USA Recruitment/treatment dates: A: 2001–2; B:1999–2001 Prospective/retrospective data collection: retrospective Patients recruited consecutively: unclear Length of follow-up: at least 18 months Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: clinically localised prostate cancer with low comorbidities and a greater than 10-year life expectancy Exclusion criteria: not reported ABPatients, n7070Age (years), mean (range) SD60.8 (43–72) 6.157.8 (44–72) 7.3PSA (ng/ml), mean (range) SD7.6 (3–16.5) 8.09.9 (2.3–33.7) 7.1Clinical stage, n (%)T1c54 (77.1)49 (70)T2a–b7 (10)9 (12.85)T2c9 (12.86)12 (17.1)Biopsy Gleason score, mean (SD)6.4 (0.8)6.7 (1.3)Biopsy Gleason score, n (%)5–64943719218–1026Prostate volume (ml), mean (range)40.8 (20–114)53.2 (19–135) |
A | B | Patients, n | 70 | 70 | Age (years), mean (range) SD | 60.8 (43–72) 6.1 | 57.8 (44–72) 7.3 | PSA (ng/ml), mean (range) SD | 7.6 (3–16.5) 8.0 | 9.9 (2.3–33.7) 7.1 | Clinical stage, n (%) | T1c | 54 (77.1) | 49 (70) | T2a–b | 7 (10) | 9 (12.85) | T2c | 9 (12.86) | 12 (17.1) | Biopsy Gleason score, mean (SD) | 6.4 (0.8) | 6.7 (1.3) | Biopsy Gleason score, n (%) | 5–6 | 49 | 43 | 7 | 19 | 21 | 8–10 | 2 | 6 | Prostate volume (ml), mean (range) | 40.8 (20–114) | 53.2 (19–135) |
A. Laparoscopic prostatectomy: performed using the Stolzenburg et al. 226 and Bollens et al. 227 technique. Extraperitoneal n = 40; transperitoneal n = 30. Nerve sparing performed when appropriate. Lymphadenectomy performed when PSA > 10 ng/nl or Gleason score ≥ 7 B. Open prostatectomy: performed using modified Walsh technique. 218 Nerve sparing performed when appropriate. Lymphadenectomy performed when PSA > 10 ng/nl or Gleason score ≥ 7 |
Safety: open conversion, surgical complications, operating time, hospital stay, blood loss Dysfunction: urinary incontinence, erectile dysfunction |
|||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 70 | 70 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) SD | 60.8 (43–72) 6.1 | 57.8 (44–72) 7.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) SD | 7.6 (3–16.5) 8.0 | 9.9 (2.3–33.7) 7.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 54 (77.1) | 49 (70) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a–b | 7 (10) | 9 (12.85) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 9 (12.86) | 12 (17.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 6.4 (0.8) | 6.7 (1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5–6 | 49 | 43 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 19 | 21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 2 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate volume (ml), mean (range) | 40.8 (20–114) | 53.2 (19–135) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Greco 2010129 Language: English Publication type: full text Number of study centres: 1 Setting: clinic Country: Italy Recruitment/treatment dates: January 2005–November 2007 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: 1 year Source of funding: not reported Systematic reviewer: TG |
Inclusion criteria: PSA < 10 ng/ml, Gleason score < 7 and only two positive of at least 12 biopsy cores Exclusion criteria: not reported ABPatients, n150150Age (years), mean (range)60.5 (45–76)61.5 (49–74)BMI (kg/m2), mean (range)32 (26–38)29 (25–53)PSA (ng/ml), mean (range)6.3 (2.4–10)6.95 (3.4–10)Clinical stage, nT1a1815T1b2320T1c106110T2a35Biopsy Gleason score, mean (range)5 (3–7)5 (3–7)Prostate size (ml), mean (range)45 (18–72)54 (20–88)BMI, body mass index. |
A | B | Patients, n | 150 | 150 | Age (years), mean (range) | 60.5 (45–76) | 61.5 (49–74) | BMI (kg/m2), mean (range) | 32 (26–38) | 29 (25–53) | PSA (ng/ml), mean (range) | 6.3 (2.4–10) | 6.95 (3.4–10) | Clinical stage, n | T1a | 18 | 15 | T1b | 23 | 20 | T1c | 106 | 110 | T2a | 3 | 5 | Biopsy Gleason score, mean (range) | 5 (3–7) | 5 (3–7) | Prostate size (ml), mean (range) | 45 (18–72) | 54 (20–88) | BMI, body mass index. |
A. Laparoscopic prostatectomy: nerve sparing B. Open prostatectomy: nerve sparing |
Safety: open conversion, surgical complications, operating time, catheterisation, blood loss Efficacy: margins, pT stage Dysfunction: urinary incontinence, erectile dysfunction |
||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 150 | 150 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 60.5 (45–76) | 61.5 (49–74) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (range) | 32 (26–38) | 29 (25–53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 6.3 (2.4–10) | 6.95 (3.4–10) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 18 | 15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 23 | 20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 106 | 110 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 3 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (range) | 5 (3–7) | 5 (3–7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (range) | 45 (18–72) | 54 (20–88) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Jacobsen 2007130 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Canada Recruitment/treatment dates: October 1999–July 2002 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: 12 months Source of funding: the Northern Alberta Urology Foundation and Alberta Heritage Foundation for Medical Research Systematic reviewer: XJ |
Inclusion criteria: all men with clinically localised prostate cancer scheduled for radical prostatectomy (open, retropubic or laparoscopic) at the University of Alberta between October 1999 and July 2002 Exclusion criteria: previous pelvic radiotherapy, a stated subjective complaint of incontinence at baseline or a neurological impairment known to affect bladder function A (first half)A (second half)BPatients, n67172Lost to follow-up at 1 year, n (%)10 (12)24 (13)Patients, n2928148Age (years), mean (SD)62.3 (6.4)60.9 (6.6)63.7 (5.7)BMI (kg/m2), mean (SD)26.87 (2.4)27.54 (2.8)28.1 (4.0)PSA, mean (SD)6.9 (2.0)7.2 (3.0)9.8 (8.2)Clinical stage, n (%)T1b002 (2)T1c15 (56)16 (57)61 (49)T2a8 (29)8 (29)41 (33)T2b3 (11)08 (6)T2c1 (4)4 (14)12 (10)T3a001 (0.8)Biopsy Gleason score, mean (SD)6.5 (0.51)6.4 (0.64)6.4 (0.77)BMI, body mass index. |
A (first half) | A (second half) | B | Patients, n | 67 | 172 | Lost to follow-up at 1 year, n (%) | 10 (12) | 24 (13) | Patients, n | 29 | 28 | 148 | Age (years), mean (SD) | 62.3 (6.4) | 60.9 (6.6) | 63.7 (5.7) | BMI (kg/m2), mean (SD) | 26.87 (2.4) | 27.54 (2.8) | 28.1 (4.0) | PSA, mean (SD) | 6.9 (2.0) | 7.2 (3.0) | 9.8 (8.2) | Clinical stage, n (%) | T1b | 0 | 0 | 2 (2) | T1c | 15 (56) | 16 (57) | 61 (49) | T2a | 8 (29) | 8 (29) | 41 (33) | T2b | 3 (11) | 0 | 8 (6) | T2c | 1 (4) | 4 (14) | 12 (10) | T3a | 0 | 0 | 1 (0.8) | Biopsy Gleason score, mean (SD) | 6.5 (0.51) | 6.4 (0.64) | 6.4 (0.77) | BMI, body mass index. |
A. Laparoscopic prostatectomy: approaches: transperitoneal. No lymph node dissection B. Open prostatectomy: approaches: transperitoneal. Lymph node dissection was conducted when indicated Additional information: patients with risk factors for lymphatic metastases (PSA ≥ 20 ng/ml, clinical stage ≥ T3, Gleason score 8–10) were offered an open procedure in lieu of a laparoscopic procedure |
Efficacy: margins, pT stage, pathological Gleason score Dysfunction: urinary incontinence Quality of life |
|||||||||||
| A (first half) | A (second half) | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 67 | 172 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lost to follow-up at 1 year, n (%) | 10 (12) | 24 (13) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 29 | 28 | 148 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 62.3 (6.4) | 60.9 (6.6) | 63.7 (5.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 26.87 (2.4) | 27.54 (2.8) | 28.1 (4.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA, mean (SD) | 6.9 (2.0) | 7.2 (3.0) | 9.8 (8.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1b | 0 | 0 | 2 (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 15 (56) | 16 (57) | 61 (49) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 8 (29) | 8 (29) | 41 (33) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 3 (11) | 0 | 8 (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 1 (4) | 4 (14) | 12 (10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3a | 0 | 0 | 1 (0.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 6.5 (0.51) | 6.4 (0.64) | 6.4 (0.77) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Jurczok 2007131 Language: English Publication type: full text Number of study centres: 1 Setting: university hospital Country: Germany Recruitment/treatment dates: January 2003–April 2006 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: clinical locally confined prostate carcinoma that had been confirmed histologically Exclusion criteria: not reported ABPatients, n163240Age (years), median (range)62.9 (42–74)64.8 (52–76)PSA (ng/ml), median (range)7.9 (2.4–10.2)7.25 (4.4–11.3)Clinical stage, nT1a06T1c7975T2a1412T2b77Not reported63140Biopsy Gleason score, median5.75.3Prostate size (ml), mean (range)37 (18–72)42.3 (20–120) |
A | B | Patients, n | 163 | 240 | Age (years), median (range) | 62.9 (42–74) | 64.8 (52–76) | PSA (ng/ml), median (range) | 7.9 (2.4–10.2) | 7.25 (4.4–11.3) | Clinical stage, n | T1a | 0 | 6 | T1c | 79 | 75 | T2a | 14 | 12 | T2b | 7 | 7 | Not reported | 63 | 140 | Biopsy Gleason score, median | 5.7 | 5.3 | Prostate size (ml), mean (range) | 37 (18–72) | 42.3 (20–120) |
A. Laparoscopic prostatectomy: pre-peritoneal technique with pelvic lymphadenectomy B. Open prostatectomy: ascending retropubic technique as described by Walsh218 with pelvic lymphadenectomy |
Safety: open conversion, surgical complications, operating time, hospital stay, catheterisation, blood loss Efficacy: margins, pT stage, pathological Gleason score |
|||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 163 | 240 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | 62.9 (42–74) | 64.8 (52–76) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (range) | 7.9 (2.4–10.2) | 7.25 (4.4–11.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a | 0 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 79 | 75 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 14 | 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 7 | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | 63 | 140 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, median | 5.7 | 5.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (range) | 37 (18–72) | 42.3 (20–120) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Kim 2007132 Language: Korean Publication type: full text Number of study centres: 1 Setting: hospital Country: Republic of Korea Recruitment/treatment dates: A: 2005–6, B: 2003–6 Prospective/retrospective data collection: uncertain Patients recruited consecutively: uncertain Length of follow-up: uncertain Source of funding: uncertain Systematic reviewer: PS |
Inclusion criteria: uncertain Exclusion criteria: uncertain ABPatients, n3045Age (years), mean (SD)66.7 (4.4)63.2 (9.2)BMI (kg/m2), mean (SD)24.4 (2.3)24.5 (2.7)PSA (ng/ml), mean (SD)11.1 (12.5)9.3 (10.4)Clinical stage, n (%)T1c21 (70)30 (66.7)T29 (30)15 (33.3)Biopsy Gleason score, mean (SD)6.5 (0.9)6.5 (0.8)BMI, body mass index. |
A | B | Patients, n | 30 | 45 | Age (years), mean (SD) | 66.7 (4.4) | 63.2 (9.2) | BMI (kg/m2), mean (SD) | 24.4 (2.3) | 24.5 (2.7) | PSA (ng/ml), mean (SD) | 11.1 (12.5) | 9.3 (10.4) | Clinical stage, n (%) | T1c | 21 (70) | 30 (66.7) | T2 | 9 (30) | 15 (33.3) | Biopsy Gleason score, mean (SD) | 6.5 (0.9) | 6.5 (0.8) | BMI, body mass index. |
A. Laparoscopic prostatectomy: extraperitoneal: all B. Open prostatectomy Nerve sparing: A: unilateral = 3/30; bilateral = 7/30; non-nerve sparing = 20/30 B: unilateral = 7/45; bilateral = 25/45; non-nerve sparing = 13/45 |
Safety: surgical complications, operating time, hospital stay, catheterisation Efficacy: margins, pT stage, pathological Gleason score |
|||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 30 | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 66.7 (4.4) | 63.2 (9.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 24.4 (2.3) | 24.5 (2.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 11.1 (12.5) | 9.3 (10.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 21 (70) | 30 (66.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 9 (30) | 15 (33.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 6.5 (0.9) | 6.5 (0.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Lama 2009133 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Chile Recruitment/treatment dates: January 2003–March 2007 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: 3 years Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients having localised prostate cancer, no previous prostate surgery, prostate < 100 g, a Gleason score < 8 and complete data to obtain an adequate follow-up of at least 1 year were recruited ABPatients, n5659Age (years), mean64.463.5PSA (ng/ml), mean (range)7.94 (1.8–35)8.85 (2.5–34)Clinical stage, nT1c3940T2a1514T2b15T2c10Biopsy Gleason score, mode (range)5 (3–7)5 (3–7) |
A | B | Patients, n | 56 | 59 | Age (years), mean | 64.4 | 63.5 | PSA (ng/ml), mean (range) | 7.94 (1.8–35) | 8.85 (2.5–34) | Clinical stage, n | T1c | 39 | 40 | T2a | 15 | 14 | T2b | 1 | 5 | T2c | 1 | 0 | Biopsy Gleason score, mode (range) | 5 (3–7) | 5 (3–7) |
A. Laparoscopic prostatectomy B. Open prostatectomy |
Safety: surgical complications, operating time, hospital stay, catheterisation Efficacy: margins, PSA recurrence Dysfunction: urinary incontinence, erectile dysfunction Learning curve: operating time |
|||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 56 | 59 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean | 64.4 | 63.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 7.94 (1.8–35) | 8.85 (2.5–34) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 39 | 40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 15 | 14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 1 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 1 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mode (range) | 5 (3–7) | 5 (3–7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Martorana 2004134 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Italy Recruitment/treatment dates: March 2002–November 2003 Prospective/retrospective data collection: not reported Patients recruited consecutively: yes Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: not reported Exclusion criteria: not reported ABPatients, n5050Age (years), median (SD)64.6 (7.54)66.9 (5.46)PSA (ng/ml), median (SD)10.85 (9.02)13.62 (10.53)Clinical stage, nT12720T22227T313Biopsy Gleason score, median (SD)5.56 (1.28)5.68 (1.35) |
A | B | Patients, n | 50 | 50 | Age (years), median (SD) | 64.6 (7.54) | 66.9 (5.46) | PSA (ng/ml), median (SD) | 10.85 (9.02) | 13.62 (10.53) | Clinical stage, n | T1 | 27 | 20 | T2 | 22 | 27 | T3 | 1 | 3 | Biopsy Gleason score, median (SD) | 5.56 (1.28) | 5.68 (1.35) |
A: Laparoscopic prostatectomy: performed according to the Montsouris technique222 B. Open prostatectomy |
Safety: open conversion, surgical complications, operating time, hospital stay, catheterisation Efficacy: margins, pT stage, pathological Gleason score Learning curve: operating time |
||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 50 | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (SD) | 64.6 (7.54) | 66.9 (5.46) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (SD) | 10.85 (9.02) | 13.62 (10.53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 27 | 20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 22 | 27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 1 | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, median (SD) | 5.56 (1.28) | 5.68 (1.35) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Namiki 2005135 Language: English Publication type: full text Number of study centres: 4 Setting: hospital Country: Japan Recruitment/treatment dates: January 2002–April 2003 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: not reported Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: newly diagnosed prostate cancer T1–T3N0M0 Exclusion criteria: PSA failure > 0.1 ng/ml within 12 months following surgery ABPatients, n45121Age (years), mean, median, SD (range)64.7, 64, 5.8 (54–75)66.5, 67, 5.8 (49–78)Comorbidities, nDiabetes57Cardiovascular39Other cancer410Hypertension933Gastrointestinal523PSA (ng/ml), mean, median, SD (range)8.3, 7.3, 4.5 (2.3–26)8.9, 7.3, 5.8 (2–54)Clinical stage, nT12761T21855T305Biopsy Gleason score, n≤ 6194872673 |
A | B | Patients, n | 45 | 121 | Age (years), mean, median, SD (range) | 64.7, 64, 5.8 (54–75) | 66.5, 67, 5.8 (49–78) | Comorbidities, n | Diabetes | 5 | 7 | Cardiovascular | 3 | 9 | Other cancer | 4 | 10 | Hypertension | 9 | 33 | Gastrointestinal | 5 | 23 | PSA (ng/ml), mean, median, SD (range) | 8.3, 7.3, 4.5 (2.3–26) | 8.9, 7.3, 5.8 (2–54) | Clinical stage, n | T1 | 27 | 61 | T2 | 18 | 55 | T3 | 0 | 5 | Biopsy Gleason score, n | ≤ 6 | 19 | 48 | 7 | 26 | 73 |
A. Laparoscopic prostatectomy: performed using the Guillonneau and Vallancien technique224 with minor modifications B. Open prostatectomy: performed using the Walsh technique218 ABUnilateral, n (%)21 (47)71 (59)Bilateral, n (%)3 (6)20 (16)Non-nerve sparing, n (%)21 (47)30 (25) Indications for nerve sparing depended on preoperative and intraoperative factors, prioritising cancer control |
A | B | Unilateral, n (%) | 21 (47) | 71 (59) | Bilateral, n (%) | 3 (6) | 20 (16) | Non-nerve sparing, n (%) | 21 (47) | 30 (25) |
Efficacy: pT stage, pathological Gleason score Dysfunction: urinary function, sexual function Quality of life |
|||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 45 | 121 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean, median, SD (range) | 64.7, 64, 5.8 (54–75) | 66.5, 67, 5.8 (49–78) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comorbidities, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diabetes | 5 | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cardiovascular | 3 | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other cancer | 4 | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypertension | 9 | 33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gastrointestinal | 5 | 23 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean, median, SD (range) | 8.3, 7.3, 4.5 (2.3–26) | 8.9, 7.3, 5.8 (2–54) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 27 | 61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 18 | 55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 0 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 19 | 48 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 26 | 73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 21 (47) | 71 (59) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 3 (6) | 20 (16) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 21 (47) | 30 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Namiki 2006136 Language: English Publication type: full text Number of study centres: 4 Setting: hospital Country: Japan Recruitment/treatment dates: April 2003–March 2004 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: 1 year Source of funding: study supported by a grant from the Suzuki Urological Foundation and the Japanese Ministry of Health and Welfare Systematic reviewer: CR |
Inclusion criteria: patients with localised prostate cancer Exclusion criteria: only patients with preoperative health-related quality-of-life data and data from at least two later time points were included in the analysis AB1B2Patients, n6421865Age (years), mean, median, SD (range)64.7, 64, 5.8 (54–77)67.1, 67, 5.6 (49–78)68.6, 70, 5.5 (56–78)PSA (ng/ml), mean, median, SD (range)10.1, 8.9, 6.3 (2.3–32)11.8, 8.4, 10.6 (2.8–67)7.9, 6.8 4.4 (2.5–25.4)Clinical stage, nT1339746T2289118T33301Biopsy Gleason score, n≤ 620471874417147 |
A | B1 | B2 | Patients, n | 64 | 218 | 65 | Age (years), mean, median, SD (range) | 64.7, 64, 5.8 (54–77) | 67.1, 67, 5.6 (49–78) | 68.6, 70, 5.5 (56–78) | PSA (ng/ml), mean, median, SD (range) | 10.1, 8.9, 6.3 (2.3–32) | 11.8, 8.4, 10.6 (2.8–67) | 7.9, 6.8 4.4 (2.5–25.4) | Clinical stage, n | T1 | 33 | 97 | 46 | T2 | 28 | 91 | 18 | T3 | 3 | 30 | 1 | Biopsy Gleason score, n | ≤ 6 | 20 | 47 | 18 | 7 | 44 | 171 | 47 |
A. Laparoscopic prostatectomy B. Open prostatectomy: B1: retropubic B2: perineal AUnilateral, n (%)28 (44)105 (37)Bilateral, n (%)3 (5)39 (1)Non-nerve sparing, n (%)33 (51)139 (49) Indications for nerve sparing depended on preoperative and intraoperative factors, prioritising cancer control |
A | Unilateral, n (%) | 28 (44) | 105 (37) | Bilateral, n (%) | 3 (5) | 39 (1) | Non-nerve sparing, n (%) | 33 (51) | 139 (49) |
Efficacy: pathological Gleason score Dysfunction: urinary function, sexual function Quality of life |
|||||||||||||||||||
| A | B1 | B2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 64 | 218 | 65 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean, median, SD (range) | 64.7, 64, 5.8 (54–77) | 67.1, 67, 5.6 (49–78) | 68.6, 70, 5.5 (56–78) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean, median, SD (range) | 10.1, 8.9, 6.3 (2.3–32) | 11.8, 8.4, 10.6 (2.8–67) | 7.9, 6.8 4.4 (2.5–25.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 33 | 97 | 46 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 28 | 91 | 18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 3 | 30 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 20 | 47 | 18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 44 | 171 | 47 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 28 (44) | 105 (37) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 3 (5) | 39 (1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 33 (51) | 139 (49) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Poulakis 2007137 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Germany Recruitment/treatment dates: A: January 2004 – not reported; B: July 2000 – not reported Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: not < 6 months Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: patients who underwent extra peritoneal laparoscopy and pelvic lymphadenectomy since January 2004 for clinically localised prostate cancer Exclusion criteria: patients with follow-up of < 6 months ABGroup IGroup IIPatients, n7213270Age (years), mean (SD)74.1 (2.3)57.3 (2.2)74 (1.9)BMI (kg/m2), mean (SD)29 (4)27 (5)30 (5)Previous abdominal or pelvic surgery, n (%)18 (25)41 (31)17 (24.3)PSA (ng/ml), mean (SD)13.5 (6.4)9.1 (7.1)13.7 (6.8)Clinical stage, naTotal5113353T1c6336T2a/b276430T2c183617Biopsy Gleason score, median (range)7 (5–9)6 (5–9)7 (5–9)Prostate size (ml), mean (SD)51 (14)47 (16)53 (15)Comorbidity, mean (range)2 (1–2)1 (1–3)2 (1–2)BMI, body mass index. a Data as reported by study authors. |
A | B | Group I | Group II | Patients, n | 72 | 132 | 70 | Age (years), mean (SD) | 74.1 (2.3) | 57.3 (2.2) | 74 (1.9) | BMI (kg/m2), mean (SD) | 29 (4) | 27 (5) | 30 (5) | Previous abdominal or pelvic surgery, n (%) | 18 (25) | 41 (31) | 17 (24.3) | PSA (ng/ml), mean (SD) | 13.5 (6.4) | 9.1 (7.1) | 13.7 (6.8) | Clinical stage, na | Total | 51 | 133 | 53 | T1c | 6 | 33 | 6 | T2a/b | 27 | 64 | 30 | T2c | 18 | 36 | 17 | Biopsy Gleason score, median (range) | 7 (5–9) | 6 (5–9) | 7 (5–9) | Prostate size (ml), mean (SD) | 51 (14) | 47 (16) | 53 (15) | Comorbidity, mean (range) | 2 (1–2) | 1 (1–3) | 2 (1–2) | BMI, body mass index. | a Data as reported by study authors. |
A. Laparoscopic prostatectomy: group 1: ≥ 71 years; group 2: ≤ 59 years Nerve sparing: Unilateral: group 1: 13 (18%); group 2: 41 (31%) Bilateral: group 1: 2 (2.8%); group 2: 30 (22.7%) B. Open prostatectomy: historical cohort from July 2000 Nerve sparing: Unilateral: 11 (5.7%) Bilateral: 3 (4.3%) Only group 1 was compared with the cohort who underwent open prostatectomy |
Safety: surgical complications, operating time, hospital stay, catherisation, blood loss, mobilisation, oral feeding Efficacy: margins, pT stage, pathological Gleason score, PSA recurrence Dysfunction: urinary incontinence Death (none) |
|||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group I | Group II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 72 | 132 | 70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 74.1 (2.3) | 57.3 (2.2) | 74 (1.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 29 (4) | 27 (5) | 30 (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous abdominal or pelvic surgery, n (%) | 18 (25) | 41 (31) | 17 (24.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 13.5 (6.4) | 9.1 (7.1) | 13.7 (6.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, na | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 51 | 133 | 53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 6 | 33 | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a/b | 27 | 64 | 30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2c | 18 | 36 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, median (range) | 7 (5–9) | 6 (5–9) | 7 (5–9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (SD) | 51 (14) | 47 (16) | 53 (15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comorbidity, mean (range) | 2 (1–2) | 1 (1–3) | 2 (1–2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Data as reported by study authors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Raventos Busquets 2007138 Language: Spanish Publication type: full text Number of study centres: not reported Setting: hospital Country: Spain Recruitment/treatment dates: January 2004–January 2006 Prospective/retrospective data collection: not reported Patients recruited consecutively: yes Length of follow-up: none Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: not reported Exclusion criteria: not reported ABPatients, n10575Age (years), mean, (SD)65 (5.9)65.6 (6.7)PSA (ng/ml), mean, (SD)7.1 (2.2)9.28 (NR)Clinical stage, n (%)T178 (74)58 (76.9)T227 (26)17 (23.1)Biopsy Gleason score, n (%)≤ 655 (52.6)40 (53)> 650 (47.4)35 (47)NR, not reported. |
A | B | Patients, n | 105 | 75 | Age (years), mean, (SD) | 65 (5.9) | 65.6 (6.7) | PSA (ng/ml), mean, (SD) | 7.1 (2.2) | 9.28 (NR) | Clinical stage, n (%) | T1 | 78 (74) | 58 (76.9) | T2 | 27 (26) | 17 (23.1) | Biopsy Gleason score, n (%) | ≤ 6 | 55 (52.6) | 40 (53) | > 6 | 50 (47.4) | 35 (47) | NR, not reported. |
A. Laparoscopic prostatectomy: extraperitoneal procedure: 20/105 B. Open prostatectomy: 41% did not undergo lymph node dissection |
Safety: operating time, hospital stay Efficacy: margins, pT stage Learning curve: Operating time |
||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 105 | 75 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean, (SD) | 65 (5.9) | 65.6 (6.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean, (SD) | 7.1 (2.2) | 9.28 (NR) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1 | 78 (74) | 58 (76.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 27 (26) | 17 (23.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 55 (52.6) | 40 (53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 6 | 50 (47.4) | 35 (47) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NR, not reported. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Remzi 2005139 Language: English Publication type: full text Number of study centres: 1 Setting: not reported Country: Austria Recruitment/treatment dates: January 2002–October 2003 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: at least 12 months, mean 14.9 months Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: histologically confirmed adenocarcinoma of the prostate and clinically ≤ T2 Exclusion criteria: A1A2BPatients, n394141Age (years), mean (SD)61 (11)59 (12)60 (14)PSA (ng/ml), mean (SD)5.5 (3.7)8.1 (6.1)6.9 (4.4)Gleason score, mean (SD)5.1 (1.2)5.5 (1.3)4.7 (1.5)Prostate size (ml), mean (SD)37 (16)32 (14)44 (18) |
A1 | A2 | B | Patients, n | 39 | 41 | 41 | Age (years), mean (SD) | 61 (11) | 59 (12) | 60 (14) | PSA (ng/ml), mean (SD) | 5.5 (3.7) | 8.1 (6.1) | 6.9 (4.4) | Gleason score, mean (SD) | 5.1 (1.2) | 5.5 (1.3) | 4.7 (1.5) | Prostate size (ml), mean (SD) | 37 (16) | 32 (14) | 44 (18) |
A. Laparoscopic prostatectomy: cutting and dissection performed using a harmonic scalpel and bipolar forceps. A voice-controlled robotic arm (AESOP) was used for camera guidance A1: transperitoneal approach performed using Guillonneau and Vallancien technique;224 37/39 (95%) had staging lymphadenectomy A2: extraperitoneal approach performed using Bollens et al. technique;227 41/41(100%) had staging lymphadenectomy B. Open prostatectomy: 29/41 (71%) had staging lymphadenectomy Nerve sparing: ABNerve sparing, n (%)46 (57.5)29 (71)Non-nerve sparing, n (%)34 (42.5)12 (29) |
A | B | Nerve sparing, n (%) | 46 (57.5) | 29 (71) | Non-nerve sparing, n (%) | 34 (42.5) | 12 (29) |
Safety: open conversion, operating time, hospital stay, surgical complications, catheterisation, blood loss Efficacy: margins, pT stage, pathological Gleason score Dysfunction: urinary continence Quality of life: postoperative pain |
|||||||||||||||||||||||||||||||||||
| A1 | A2 | B | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 39 | 41 | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 61 (11) | 59 (12) | 60 (14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 5.5 (3.7) | 8.1 (6.1) | 6.9 (4.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gleason score, mean (SD) | 5.1 (1.2) | 5.5 (1.3) | 4.7 (1.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate size (ml), mean (SD) | 37 (16) | 32 (14) | 44 (18) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nerve sparing, n (%) | 46 (57.5) | 29 (71) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 34 (42.5) | 12 (29) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Salomon 2002140 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: France Recruitment/treatment dates: 1988–2001 Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up, mean (range): B1: 4.7 (0.27–13.9) years; B2: 5.4 (1.7–8.6) years; A: 1.3 (0.1–3.5) years Source of funding: not reported Systematic reviewer: CR |
Inclusion criteria: PSA < 10 ng/ml Exclusion criteria: not reported ABPatients, n155151Age (years), mean63.5B1: 63.8; B2: 65.9PSA (ng/ml), mean6.6B1: 5.5; B2: 6.5Clinical stage, nT1a–b715T1c10671T2a4057T2b28Biopsy Gleason score, mean5.7B1: 5.6; B2: 5.7 |
A | B | Patients, n | 155 | 151 | Age (years), mean | 63.5 | B1: 63.8; B2: 65.9 | PSA (ng/ml), mean | 6.6 | B1: 5.5; B2: 6.5 | Clinical stage, n | T1a–b | 7 | 15 | T1c | 106 | 71 | T2a | 40 | 57 | T2b | 2 | 8 | Biopsy Gleason score, mean | 5.7 | B1: 5.6; B2: 5.7 |
A. Laparoscopic prostatectomy B. Open prostatectomy B1: retropubic n = 86 B2: perineal n = 65 Lymphadenectomy: B1: all B2: preoperative Gleason score ≥ 7 A: preoperative Gleason score ≥ 7 |
Safety: blood transfusion, operating time, hospital stay, catheterisation, surgical complications Efficacy: margins, pT stage, pathological Gleason score, PSA recurrence |
|||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 155 | 151 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean | 63.5 | B1: 63.8; B2: 65.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean | 6.6 | B1: 5.5; B2: 6.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1a–b | 7 | 15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 106 | 71 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 40 | 57 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b | 2 | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean | 5.7 | B1: 5.6; B2: 5.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Silva 2007141 Language: English Publication type: full text Number of study centres: 2 Setting: hospital/private practice Country: Brazil Recruitment/treatment dates: A: May 2000–August 2004; B: June 1999–October 2003 Prospective/retrospective data collection: retrospective Patients recruited consecutively: yes Length of follow-up: none Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: patients with PSA ≤ 15 ng/ml,Gleason score ≤ 7 in the prostate biopsy, patients with maximum clinical stage of T2 Exclusion criteria: ABPatients, n9089Age (years), median (range)63 (46–78)63 (46–76)PSA (ng/ml), median7.367.99Variance for values not specified. |
A | B | Patients, n | 90 | 89 | Age (years), median (range) | 63 (46–78) | 63 (46–76) | PSA (ng/ml), median | 7.36 | 7.99 | Variance for values not specified. |
A. Laparoscopic prostatectomy B. Open prostatectomy Detail of interventions not reported |
Efficacy: margins, pT stage, pathological Gleason score | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 90 | 89 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | 63 (46–78) | 63 (46–76) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median | 7.36 | 7.99 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Variance for values not specified. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Soderdahl 2005142 Language: English Publication type: full text Number of study centres: 1 Setting: medical centre Country: USA Recruitment/treatment dates: 2001–3 Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: 12 months Source of funding: US Army and the Department of Defence Systematic reviewer: XJ |
Inclusion criteria: patients with newly diagnosed clinically localised prostate cancer Exclusion criteria: not reported ABPatients, n116186Complete survey data, n9386Age (years), median6159PSA (ng/ml), median5.716Clinical stage (%)T1c81.7084.90T218.3015.10Gleason score, n (%)≤ 674 (79.6)58 (67.4)716 (17.2)22 (25.6)8–103 (3.2)6 (7.0) |
A | B | Patients, n | 116 | 186 | Complete survey data, n | 93 | 86 | Age (years), median | 61 | 59 | PSA (ng/ml), median | 5.71 | 6 | Clinical stage (%) | T1c | 81.70 | 84.90 | T2 | 18.30 | 15.10 | Gleason score, n (%) | ≤ 6 | 74 (79.6) | 58 (67.4) | 7 | 16 (17.2) | 22 (25.6) | 8–10 | 3 (3.2) | 6 (7.0) |
A. Laparoscopic prostatectomy B. Open prostatectomy Nerve sparing: ABUnilateral, n (%)16 (17)23 (27)Bilateral, n (%)20 (22)38 (44)Non-nerve sparing, n (%)57 (61)25 (29) |
A | B | Unilateral, n (%) | 16 (17) | 23 (27) | Bilateral, n (%) | 20 (22) | 38 (44) | Non-nerve sparing, n (%) | 57 (61) | 25 (29) |
Efficacy: pT stage Dysfunction: urinary function, sexual function |
||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 116 | 186 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complete survey data, n | 93 | 86 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median | 61 | 59 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median | 5.71 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 81.70 | 84.90 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 18.30 | 15.10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gleason score, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 74 (79.6) | 58 (67.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 16 (17.2) | 22 (25.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 3 (3.2) | 6 (7.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 16 (17) | 23 (27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 20 (22) | 38 (44) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-nerve sparing, n (%) | 57 (61) | 25 (29) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Soric 2004143 Language: Croatian Publication type: full text Number of study centres: 1 Setting: medical centre Country: Croatia Recruitment/treatment dates January 2004–January 2005 Prospective/retrospective data collection: prospective Patients recruited consecutively: unclear Length of follow-up: Source of funding: not reported Systematic reviewer: CR |
ABPatients, n2626Age (years), mean (range)62 (52–70)64 (50–70)PSA (ng/ml), mean (range)10.54 (1.25–27)14.65 (4.9–60)Clinical stage T1–T2, n2626Gleason score, mean (range)5.5 (3–7)5.5 (4–7)Comorbidity,a n026a Abdominal surgery, abdominal or pelvic radiotherapy, adipose patients and patients with anaesthetic contraindications. | A | B | Patients, n | 26 | 26 | Age (years), mean (range) | 62 (52–70) | 64 (50–70) | PSA (ng/ml), mean (range) | 10.54 (1.25–27) | 14.65 (4.9–60) | Clinical stage T1–T2, n | 26 | 26 | Gleason score, mean (range) | 5.5 (3–7) | 5.5 (4–7) | Comorbidity,a n | 0 | 26 | a Abdominal surgery, abdominal or pelvic radiotherapy, adipose patients and patients with anaesthetic contraindications. |
A. Laparoscopic prostatectomy B. Open prostatectomy |
Safety: open conversion, surgical complications, operating time, hospital stay, catheterisation Efficacy: margins, pT stage, pathological Gleason score |
|||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 26 | 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 62 (52–70) | 64 (50–70) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (range) | 10.54 (1.25–27) | 14.65 (4.9–60) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage T1–T2, n | 26 | 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gleason score, mean (range) | 5.5 (3–7) | 5.5 (4–7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comorbidity,a n | 0 | 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Abdominal surgery, abdominal or pelvic radiotherapy, adipose patients and patients with anaesthetic contraindications. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Terakawa 2008144 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: Japan Recruitment/treatment dates: January 2000–April 2007 Prospective/retrospective data collection: retrospective Patients recruited consecutively: not reported Length of follow-up: none Source of funding: not reported Systematic reviewer: PS |
Inclusion criteria: patients who underwent both systematic TRUS-guided needle biopsy of the prostate and radical prostatectomy without any neoadjuvant therapies Exclusion criteria: ABPatients, n137220Age (years), mean (SD)67.3 (5.8)69.1 (5.9)PSA (ng/ml), mean (SD)10.9 (8.5)12.9 (15.1)Clinical stage, n (%)T1c51 (37)74 (34)T286 (63)146 (66)Biopsy Gleason score, mean (SD)6.5 (0.9)6.4 (1.3) Digital rectal examination, transrectal ultrasonography, PSA assay, TRUS-guided needle biopsy, pelvic computerised tomography and bone scan were used for staging. |
A | B | Patients, n | 137 | 220 | Age (years), mean (SD) | 67.3 (5.8) | 69.1 (5.9) | PSA (ng/ml), mean (SD) | 10.9 (8.5) | 12.9 (15.1) | Clinical stage, n (%) | T1c | 51 (37) | 74 (34) | T2 | 86 (63) | 146 (66) | Biopsy Gleason score, mean (SD) | 6.5 (0.9) | 6.4 (1.3) |
A. Laparoscopic prostatectomy Nerve sparing: Unilateral: 13 (9.5%) Bilateral: 17 (12.4%) Surgical procedure described elsewhere B. Open prostatectomy Nerve sparing: Unilateral: 19 (8.6%) Bilateral: 17 (7.7%) Surgical procedure described elsewhere |
Efficacy: margins, pT stage | |||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 137 | 220 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 67.3 (5.8) | 69.1 (5.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 10.9 (8.5) | 12.9 (15.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 51 (37) | 74 (34) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 86 (63) | 146 (66) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, mean (SD) | 6.5 (0.9) | 6.4 (1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Touijer 2007145 Language: English Publication type: full text Number of study centres: 1 Setting: hospital Country: USA Recruitment/treatment dates: January 2003–June 2005 Prospective/retrospective data collection: prospective Patients recruited consecutively: yes Length of follow-up: none Source of funding: National Cancer Institute Systematic reviewer: PS |
Inclusion criteria: men with clinically localised (cT1–cT3a) adenocarcinoma of the prostate Exclusion criteria: those receiving hormone therapy before surgery (n = 36/1213 excluded) ABPatients enrolled, n1213Patients analysed, n485692Age (years), median (IQR)60 (55–65)59 (54–64)PSA (ng/ml), median (IQR)5.3 (4.0–7.5)5.3 (4.1–7.1)Clinical stage, n (%)T1c348 (71.7)451 (65)T2125 (25.8)213 (31)T312 (2.5)28 (4)Biopsy Gleason score, n (%)6307 (63)405 (59)7151 (31)228 (33)8–927 (6)57 (8)Partin probability of non-organ-confined disease, median (IQR)0.37 (0.33–0.510.45 (0.33–0.62) Magnetic resonance imaging (MRI) was used for clinical staging. |
A | B | Patients enrolled, n | 1213 | Patients analysed, n | 485 | 692 | Age (years), median (IQR) | 60 (55–65) | 59 (54–64) | PSA (ng/ml), median (IQR) | 5.3 (4.0–7.5) | 5.3 (4.1–7.1) | Clinical stage, n (%) | T1c | 348 (71.7) | 451 (65) | T2 | 125 (25.8) | 213 (31) | T3 | 12 (2.5) | 28 (4) | Biopsy Gleason score, n (%) | 6 | 307 (63) | 405 (59) | 7 | 151 (31) | 228 (33) | 8–9 | 27 (6) | 57 (8) | Partin probability of non-organ-confined disease, median (IQR) | 0.37 (0.33–0.51 | 0.45 (0.33–0.62) |
A. Laparoscopic prostatectomy: n = 485. Performed using modified Montsouris technique222 Nerve sparing: Unilateral preservation: 6% Bilateral preservation: 89% Bilateral resection: 5% B. Open prostatectomy: n = 692. Standard technique Nerve sparing: Unilateral preservation: 6% Bilateral preservation: 91% Bilateral resection: 3% |
Efficacy: margins, pT stage, pathological Gleason score | ||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients enrolled, n | 1213 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients analysed, n | 485 | 692 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (IQR) | 60 (55–65) | 59 (54–64) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), median (IQR) | 5.3 (4.0–7.5) | 5.3 (4.1–7.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 348 (71.7) | 451 (65) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2 | 125 (25.8) | 213 (31) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 12 (2.5) | 28 (4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 307 (63) | 405 (59) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 151 (31) | 228 (33) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–9 | 27 (6) | 57 (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Partin probability of non-organ-confined disease, median (IQR) | 0.37 (0.33–0.51 | 0.45 (0.33–0.62) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Author, year: Wagner 2007146 Language: English Publication type: full text Number of study centres: 1 Setting: institution Country: USA Recruitment/treatment dates: not reported Prospective/retrospective data collection: prospective Patients recruited consecutively: not reported Length of follow-up: mean: total: > 2 years; A: 26 months; B: 27 months Source of funding: not reported Systematic reviewer: XJ |
Inclusion criteria: patients undergoing prostatectomy Exclusion criteria: not reported ABPatients, n7575Age (years), mean (SD)58 (6.9)59 (6.9)BMI (kg/m2), mean (SD)27 (3.0)29 (4.5)PSA (ng/ml), mean (SD)6.2 (4.22)8.1 (6.27)Clinical stage, n (%)T1c47 (63)45 (60)T2a21 (28)24 (32)T2b–2c7 (9)6 (8)T300Biopsy Gleason score, n (%)≤ 661 (81)48 (64)712 (16)23 (31)8–102 (3)4 (5)BMI, body mass index. Author admitted there was a selection bias. |
A | B | Patients, n | 75 | 75 | Age (years), mean (SD) | 58 (6.9) | 59 (6.9) | BMI (kg/m2), mean (SD) | 27 (3.0) | 29 (4.5) | PSA (ng/ml), mean (SD) | 6.2 (4.22) | 8.1 (6.27) | Clinical stage, n (%) | T1c | 47 (63) | 45 (60) | T2a | 21 (28) | 24 (32) | T2b–2c | 7 (9) | 6 (8) | T3 | 0 | 0 | Biopsy Gleason score, n (%) | ≤ 6 | 61 (81) | 48 (64) | 7 | 12 (16) | 23 (31) | 8–10 | 2 (3) | 4 (5) | BMI, body mass index. | Author admitted there was a selection bias. |
A. Laparoscopic prostatectomy: Montsouris technique222 was used B. Open prostatectomy: anatomical approach of Walsh218 was used Nerve sparing: ABUnilateral, n (%)22 (29)9 (12)Bilateral, n (%)47 (63)62 (83) |
A | B | Unilateral, n (%) | 22 (29) | 9 (12) | Bilateral, n (%) | 47 (63) | 62 (83) |
Safety: operating time, surgical complications, blood loss Efficacy: margins, pT stage Dysfunction: urinary incontinence, erectile dysfunction |
|||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients, n | 75 | 75 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 58 (6.9) | 59 (6.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2), mean (SD) | 27 (3.0) | 29 (4.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSA (ng/ml), mean (SD) | 6.2 (4.22) | 8.1 (6.27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical stage, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T1c | 47 (63) | 45 (60) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2a | 21 (28) | 24 (32) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T2b–2c | 7 (9) | 6 (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T3 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biopsy Gleason score, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 6 | 61 (81) | 48 (64) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 12 (16) | 23 (31) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8–10 | 2 (3) | 4 (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI, body mass index. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author admitted there was a selection bias. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unilateral, n (%) | 22 (29) | 9 (12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bilateral, n (%) | 47 (63) | 62 (83) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Appendix 8 Detailed risk of bias assessment for the included studies
| Study | Sequence generation | Allocation concealment | Confounding | Blinding | Incomplete outcome data | Free of selective reporting | Other bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perioperative safety | Urinary dysfunction | Erectile dysfunction | Efficacy | Perioperative safety | Urinary dysfunction | Erectile dysfunction | Efficacy | Perioperative safety | Urinary dysfunction | Erectile dysfunction | Efficacy | Perioperative safety | Urinary dysfunction | Erectile dysfunction | Efficacy | ||||
| Al-Shaiji 2010121 | ✗ | ? | ? | ? | ? | ? | ? | ||||||||||||
| Anastasiadis 2003122 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | × |
| Artibani 2003123 | ✗ | ? | ? | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ball 200699 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ? | ? | ? | ||||||||
| Barocas 2010103 | ✗ | ? | ✓ | ✓ | ? | ✓ | ? | ||||||||||||
| Bhayani 2003124 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Bolenz 2010100 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ? | ||||||||||||
| Brown 2004125 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ||||||||
| Carlsson 2010104 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ||||||||||||
| Chan 2008119 | ✗ | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Dahl 2006147 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Dahl 2009126 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Doumerc 2010105 | ✗ | ✗ | ✗ | ? | ✗ | ? | ? | ? | ✓ | ✗ | ✓ | ✓ | ? | ✓ | ? | ||||
| Drouin 2009101 | ✗ | ? | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Ficarra 2009106 | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Fracalanza 2008107 | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Ghavamian 2006128 | ✗ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Greco 2010129 | ✗ | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Guazzoni 200690 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Hu 200692 | ✗ | ? | ? | ✓ | ✓ | ✓ | ✗ | ||||||||||||
| Jacobsen 2007130 | ✗ | ? | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ? | ||||||||
| Joseph 200593 | ✗ | ? | ? | ✗ | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Jurczok 2007131 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Malcolm 2010110 | ✗ | ? | ✓ | ✓ | ✗ | ✗ | ? | ? | ✓ | ✓ | ✗ | ||||||||
| Martorana 2004134 | ✗ | ? | ✗ | ✗ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ? | ||||||||
| Menon 200295 | ✗ | ? | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | × | ✓ | ✗ |
| Miller 2007111 | ✗ | ? | ? | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Nadler 2010112 | ✗ | ? | ✗ | ✗ | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ? | ? | ? |
| Namiki 2005135 | ✗ | ? | ? | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ? | ||||||||
| Namiki 2006136 | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ||||||||
| Ou 2009113 | ✗ | ✗ | ? | ? | ? | ✓ | ✓ | ? | ? | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Poulakis 2007137 | ✗ | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Remzi 2005139 | ✗ | ✗ | ✗ | ? | ✓ | ✓ | ? | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ? | ||||
| Rocco 2009114 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Rozet 200796 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Salomon 2002140 | ✗ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Schroeck 2008115 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Silva 2007141 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ? | ||||||||||||
| Soderdahl 2005142 | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ||||||||
| Terakawa 2008144 | ✗ | ✗ | ✓ | ✓ | ✓ | ? | ? | ||||||||||||
| Tewari 2003116 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ? | ✓ | ? | ? | ✗ | ? |
| Touijer 2007145 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ||||||||||||
| Trabulsi 200898 | ✗ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ? | ✓ | ||||||||
| Truesdale 2010117 | ✗ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Wagner 2007146 | ✗ | ? | ✗ | ✓ | ? | ✓ | ✓ | ? | ? | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ? |
| White 2009118 | ✗ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
Appendix 9 Data tables
| Study | Outcome reported as | Robotic, n/N (%)a | Laparoscopic, n/N (%)a | Open, n/N (%)a | Notes | |
|---|---|---|---|---|---|---|
| Equipment failure | ||||||
| Hu 200692 | Robot malfunction (unresponsive and refractory to troubleshooting measures) | 2/333 (0.6) | 0 | First case converted to laparoscopic radical prostatectomy and second case occurred after second robot replacement | ||
| Menon 200295 | Reported as excluded from analysis and not as equipment failure | Not reported | 8; initial problems with the voice recognition system of the AESOP camera holder | ‘The problem was corrected after the first 4 cases. Inclusion of these 8 patients in analysis would have increased the average operative times for laparoscopic prostatectomy by 10 mins’ | ||
| Converted to other intervention | ||||||
| Bhayani 2003124 | Converted to other intervention | 3/36 (8.3) | 0/24 | |||
| Chan 2008119 | Converted to other intervention | 6/660 (0.9), to open | Secondary report of primary study Barocas 2010104 | |||
| Drouin 2009101 | Converted to other intervention | 0/71 | 1/85 (1.2) | 0/83 | ||
| Ghavamian 200678 | Converted to other intervention | 0/70 | 0/70 | |||
| Greco 2010129 | Converted to other intervention | 0/150 | 0/150 | |||
| Guazzoni 200690 | Converted to other intervention | 0/60 | RCT | |||
| Hu 200692 | Converted to other intervention | 0/322 | 3/358 (0.8), first 3, to open | |||
| Jurczok 2007131 | Converted to other intervention | 0/163 | 0/240 | |||
| Martorana 2004134 | Converted to other intervention | 0/50 | 0/50 | |||
| Menon 200295 | Converted to other intervention | 0/40, to open | 1/40 (2.5), to open | |||
| Namiki 2005135 | Converted to other intervention | 0/45 | 0/121 | |||
| Ou 2009113 | Converted to other intervention | 2/30 (6.7) | 0/30 | |||
| Remzi 2005139 | Converted to other intervention | 1/80 (1.3) | 0/41 | |||
| Rozet 200796 | Converted to other intervention | 4/133 (3.0) | 0/133 | |||
| Soric 2004143 | Converted to other intervention | 3/26 (11.5) | 0/26 | |||
| Tewari 2003116 | Converted to other intervention | 0/200 | 0/100 | |||
| Trabulsi 200898 | Converted to other intervention | 0/50 | 7/197 (3.6) | |||
| White 2009118 | Converted to other intervention | 0/50 | Not reported | |||
| Blood transfusion | ||||||
| Al-Shaiji 2010121 | Blood transfusion | 3/70 (4.3) | 42/70 (60.0) | |||
| Anastasiadis 2003122 | Blood transfusion during surgery | 6/230 (2.6) | 6/70 (8.6) | |||
| Artibani 2003123 | Blood transfusion | 45/71 (63) | 17/50 (34.0) | |||
| Bolenz 2010100 | Blood transfusion | 12/262 (4.6) | 4/211 (1.9) | 32/156 (20.5) | ||
| Brown 2004125 | Blood transfusion | 1/60 (1.7) | 31/60 (51.7) | |||
| Carlsson 2010104 | Blood transfusion | 58/1253 (4.6) | 112/485 (23.1) | |||
| Chan 2008119 | Blood transfusion | 5/660 (0.8) | 11/340 (3.2) | |||
| Doumerc 2010105 | Blood transfusion | 2/212 (0.9) | 10/502 (2.0) | |||
| Drouin 2009101 | Blood transfusion | 4/71 (5.6) | 5/85 (5.9) | 8/83 (9.6) | ||
| Ficarra 2009106 | Blood transfusion | 2/103 (1.9) | 15/105 (14.3) | |||
| Fornara 2004127 | Blood transfusion | 2/32 (6.3) | 6/32 (18.8) | |||
| Fracalanza 2008107 | Blood transfusion | |||||
| During surgery | 6/35 (17.1) | 9/26 (34.6) | ||||
| After surgery | 1/35 (2.9) | 3/26 (11.5) | ||||
| Ghavamian 2006128 | Blood transfusion | 5/70 (7.1) | 22/70 (31.4) | |||
| Gosseine 200991 | Blood transfusion | 4/122 (3.3) | 8/125 (6.4) | |||
| Greco 2010129 | Blood transfusion | 3/150 (2.0) | 9/150 (6.0) | |||
| Guazzoni 200690 | Blood transfusion | RCT | ||||
| Homologous | 0/60 | 5/60 (8.3) | ||||
| Autologous | 8/60 (13.3) | 27/60 (45.0) | ||||
| Hu 200692 | Blood transfusion | 5/322 (1.6) | 8/358 (2.2) | |||
| Joseph 200794 | Blood transfusion | 10/754 (1.3) | 35/800 (4.4) | Abstract | ||
| Jurczok 2007131 | Blood transfusion | 5/163 (3) | 22/240 (9) | n/N calculated from reported percentages | ||
| Kim 2007132 | Blood transfusion | 7/30 (23.3) | 10/45 (22.2) | |||
| Kordan 2010120 | Blood transfusion | 7/830 (0.8) | 14/414 (3.4) | Secondary to Barocas 2010104 | ||
| Krambeck 2008108 | Blood transfusion | 15/294 (5.1) | 77/588 (13.1) | |||
| Lama 2009133 | Blood transfusion | 7/56 (12.5) | 23/59 (39.0) | |||
| Martorana 2004134 | Blood transfusion | 1/50 (2.0) | 5/50 (10.0) | |||
| Menon 200295 | Blood transfusion | 0/40 | 1/40 (2.5) | |||
| Nadler 2010112 | Blood transfusion | 10/50 (20.0) | 45/50 (90.0) | |||
| Ou 2009113 | Blood transfusion | 4/30 (13.3) | 18/30 (60.0) | |||
| Poulakis 2007137 | Blood transfusion (unit) |
Group I: 2/72 (2.7) Group II: 3/132 (2.3) |
13/70 (18.6) | Groups I and II split by age (data not combined) | ||
| Rozet 200796 | Blood transfusion | 13/133 (9.8) | 4/133 (3.0) | |||
| Salomon 2002140 | Blood transfusion | 3/155 (1.9) | 31/151 (20.5) | |||
| Soric 2004143 | Blood transfusion (ml), mean | 130 | 240 | |||
| Tewari 2003116 | Blood transfusion | 0/200 | 67/100 (67.0) | |||
| Operating time, minutes (convert hours to minutes: hours x 60 = minutes) | ||||||
| Al-Shaiji 2010121 | Operating time, mean (range) | 232 (132–348) | 170 (108–330) | |||
| Bhayani 2003124 | Operating time, mean (SD) | 348 (72) | 168 (33) | |||
| Bolenz 2009102 (secondary to Bolenz 2010100) | Operating time, median | 198 | 235 | 225 | ||
| Brown 2004125 | Operating time, mean (median) | 348 (330) | Not reported | From time of skin incision to time of completion of wound closure | ||
| Chan 2008119 | Operating time, range | 63–483 | 82–245 | Range reported from two groups of different prostate size | ||
| Doumerc 2010105 | Operating time, mean (range) | 192 (119–525) | 148 (75–330) | |||
| Drouin 2009101 | Operating time, mean (SD) | 199.6 (36.6) | 257.3 (94.3) | 208.5 (76) | ||
| Ficarra 2009106 | Operating time, median | 185 | 135 | |||
| Fornara 2004127 | Operating time, median (range) | 220 (180–360) | 140 (120–190) | |||
| Fracalanza 2008107 | Operating time, mean (SD) | 195.6 (45) | 127.2 (31.7) | Robotics: insertion of the Veress needle to the suture of the last laparoscopic port; open: from skin incision to suture | ||
| Ghavamian 2006128 | Operating time, mean (SD) | 246.4 (46.1) | 181.8 (18.7) | Skin incision to closure | ||
| Gosseine 20091 | Operating time, mean | 237 | 241 | |||
| Greco 2010129 | Operating time, mean (range) | 165 (90–240) | 120 (60–180) | |||
| Guazzoni 200690 | Operating time, mean (SD) | N235 (49.9) | 170 (34.2) |
RCT Total time in the operating room from entry to exit |
||
| Hu 200692 | Operating time, median (range) | 186 (114–528) | 246 (150–768) | |||
| Joseph 200794 | Operating time, mean (range) | 194 (91–486) | 179 (75–450) |
Abstract Skin incision to closure |
||
| Jurczok 2007131 | Operating time, median (range) | 180 (120–240) | 120 (80–190) | |||
| Kim 2007132 | Operating time, mean (SD) | 335.9 (93.7) | 201.9 (62.8) | |||
| Krambeck 2008108 | Operating time, median (25th–75th percentile) | 236 (204–285) | 204 (162–268) | |||
| Lama 2009133 | Operating time, mean (SD) | 203 (52) | 151 (30) | |||
| Martorana 2004134 | Operating time, mean (range) | 358 (180–565) | 159 (115–225) | |||
| Menon 200295 | Operating time, mean (SD) | 274 (94.3) | 258 (80.3) | Start of dissection to closure | ||
| Nadler 2010112 | Operating time, mean (range) | 341 (175–591) | 235 (152–352) | |||
| Ou 2009113 | Operating time, mean (SD) | 205 (103) | 213 (37) | |||
| Poulakis 2007137 | Operating time, mean (SD) |
Group I: 144 (36) Group II: 144 (30) |
150 (30) | Two age groups | ||
| Raventos Busquets 2007138 | Operating time, mean (SD) | 172.3 (43.7) | 145.1 (32.9) | |||
| Remzi 2005139 | Operating time, mean (SD) |
Transperitoneal: 279 (70) Extraperitoneal: 217 (51) |
195 (72) | |||
| Rocco 2009114 | Operating time, median (range) | 215 (165–450) | 160 (90–240) | Skin incision to closure | ||
| Rozet 200796 | Operating time, mean (range) | 166 (90–300) | 160 (90–270) | |||
| Salomon 2002140 | Operating time, mean, SD, (range) | 266, 73 (120–510) |
Retropubic: 181, 46 (120–360) Perineal: 163, 58 (80–325) |
Total operative time included pelvic lymphadenectomy | ||
| Soric 2004143 | Operating time, mean (range) | 302 (183–513) | 272 (197–304) | |||
| Sundaram 200497 | Operating time, mean (range) | 290 (210–340) | 394 (240–480) | Abstract | ||
| Truesdale 2010117 | Operating time, mean (SD) | 153.4 (51.3) | 204 (32.9) | |||
| Wagner 2007146 | Operating time, mean (SD) | 282 (53.4) | 162 (39.0) | |||
| Hospital stay, days | ||||||
| Al-Shaiji 2010121 | Hospital stay, mean, SD, (range) | 3.4, 1.84 (2–12) | 5.6, 1.49 (2–10) | |||
| Artibani 2003123 | Hospital stay, mean, SD, (range) | 7.2, 3.4 (2–19) | 10.2, 2 (7–15) | |||
| Bhayani 2003124 | Hospital stay, mean (SD) | 2.97 (0.55) | 3.04 (0.21) | |||
| Bolenz 2009102 | Hospital stay, median | 2 | 1 | 2 | ||
| Brown 2004125 | Hospital stay, mean, median (range) | 2.8, 2 (6–15) | 3, 3 (2–5) | |||
| Chan 2008119 | Hospital stay, range | 0.6–8.8 | 0.7–3.6 | Range reported from two groups of different prostate size | ||
| Doumerc 2010105 | Hospital stay, mean (range) | 2.8 (2–7) | 505 (3–10) | |||
| Ficarra 2009106 | Hospital stay, median (range) | 6 (5–8) | 7 (6–9) | |||
| Fornara 2004127 | Hospital stay, mean | 12.4 | 11.2 | |||
| Fracalanza 2008107 | Hospital stay, median (range) | 5 (9–6) | 8 (5–9) | |||
| Ghavamian 2006128 | Hospital stay, mean | 2 | 3 | |||
| Gosseine 200991 | Hospital stay, mean (SD) | 9 (2.1) | 10.2 (3.2) | |||
| Jurczok 2007131 | Hospital stay, median | 9.4 | 11.2 | |||
| Kim 2007132 | Hospital stay, mean (SD) | 6.7 (3.7) | 6.9 (2.6) | |||
| Krambeck 2008108 | Hospital stay (days), n/N (%) | |||||
| 1 | 86/294 (29.3) | 114/588 (19.4) | ||||
| 2 | 176/294 (59.9) | 400/588 (68.0) | ||||
| 3–6 | 31/294 (10.5) | 65/588 (11.1) | ||||
| ≥7 | 1/294 (0.3) | 9/588 (1.5) | ||||
| Lama 2009133 | Hospital stay, mean (SD) | 7.3 (4.7) | 10.7 (9.2) | |||
| Martorana 2004134 | Hospital stay, mean | 5 (3–39) | 6.9 (4–17) | |||
| Nadler 2010112 | Hospital stay, mean (range) | 2.5 (1.12) | 2.8 (2–6) | |||
| Ou 2009113 | Hospital stay, mean (SD) | 7.3 (2.3) | 8.37 (2.2) | |||
| Poulakis 2007137 | Hospital stay, mean (SD) |
Group I: 9 (2) Group II: 9 (3) |
11 (3) | Groups I and II are two age groups (data not combined) | ||
| Raventos Busquets 2007138 | Hospital stay, mean (SD) | 4.8 (1.3) | 5.79 (1.67) | |||
| Remzi 2005139 | Hospital stay, mean (SD) |
Transperitoneal: 7 (2) Extraperitoneal: 7 (2) |
10 (4) | |||
| Rocco 2009114 | Hospital stay, mean (range) | 3 (2–12) | 6 (3–16) | |||
| Rozet 200796 | Hospital stay, mean (range) | 5.4 (3–26) | 4.9 (3–20) | |||
| Salomon 2002140 | Hospital stay, mean, SD (range) | 6.8, 3 (4–21) |
Retropubic: 12.1, 7.6 (5–55) Perineal: 7.9, 4.1 (2–22) |
|||
| Soric 2004143 | Hospital stay, mean | 12 | 12 | |||
| Sundaram 200497 | Hospital stay, mean (range) | 1.3 (1–3) | 2.2 (1–3) | Abstract | ||
| Tewari 2003116 | Hospital stay, mean (range) | 1.2 (< 1–5) | 3.5 (3–6) | |||
| Proportion of included men discharged from hospital within the stated interval | ||||||
| Guazzoni 200690 | Discharged on day 6 with or without catheter | 54/60 (90.0) | 52/60 (86.7) |
RCT Delayed discharge was due to fever, persistent lymphorrhea and rectal damage |
||
| Menon 200295 | Discharge home < 1day | 32/40 (80.0) | 26/40 (65.0) | |||
| Readmission | ||||||
| Brown 2004125 | Readmission due to surgical complications | 0/60 | 1/60 (1.7) | Because of deep-vein thrombosis | ||
| Need critical care | ||||||
| No studies | ||||||
| Bladder neck stenosis/anastomotic stricture | ||||||
| Bhayani 2003124 | Bladder neck contracture | 0/33 | 6/24 (25.0) | |||
| Brown 2004125 | Bladder neck contracture | 0/60 | 2/60 (3.3) | |||
| Carlsson 2010104 | Bladder neck contracture (30 days–15 months) | 3/1253 (0.2) | 22/485 (4.5) | |||
| Dahl 2009126 | Bladder neck contracture | 2/104 (2.0) | 0/102 | |||
| Ficarra 2009106 | Stenosis of the urethrovesical anastomosis | 3/103 (3.0) | 6/105 (5.7) | |||
| Ghavamian 2006128 | Bladder neck contracture | 1/70 (1.4) | 3/70 (4.3) | |||
| Hu 200693 | Bladder neck contracture | 2/322 (0.6) | 8/358 (2.2) | |||
| Krambeck 2008108 | Bladder neck contracture, 1 year | 3/248 (1.2) | 23/492 (4.7) | |||
| Stricture, 1 year | 8/286 (2.8) | 6/492 (1.2) | ||||
| Lama 2009133 | Bladder neck stenosis | 5/56 (8.9) | 1/59 (1.7) | |||
| Nadler 2010112 | Bladder neck contracture | 2/50 (4.0) | 7/50 (14.0) | |||
| Ou 2009113 | Mild vesicourethral anastomosis stricture | 1/30 (3.3) | 0/30 | |||
| Remzi 2005139 | Anastomotic stricture | 3/80 (3.8) | 4/41 (9.8) | |||
| Wagner 2007146 | Bladder neck contracture | 2/75 (2.7) | 12/75 (16.0) | |||
| Catheterisation, days | ||||||
| Anastasiadis 2003122 | Catheterisation, mean | 5.8 | 7.8 | |||
| Artibani 2003123 | Catheterisation, mean, SD (range) | 8, 2.8 (4–18) | 8.4, 0.9 (7–12) | |||
| Bhayani 2003124 | Catheterisation, mean (SD) | 14 (6.9) | 19 (1.22) | |||
| Doumerc 2010105 | Catherisation, mean (range) | 6.3 (6–21) | 7.9 (6–20) | |||
| Drouin 2009101 | Catheterisation, mean (range) | 8.1 (3–31) | 8.9 (3–91) | 14.7 (6–28) | ||
| Ficarra 2009106 | Catheterisation, median (range) | 5 (4–7) | 6 (5–12) | |||
| Fornara 2004127 | Catheterisation, mean | 17.9 | 13.2 | |||
| Gosseine 200991 | Catheterisation, mean | 5.5 | 6.5 | |||
| Greco 2010129 | Catheterisation, mean | 7 | 9 | |||
| Guazzoni 200690 | 5-day catheterisation, n/N (%) | 52/60 (86.7) | 40/60 (66.7) |
RCT Patients requiring 5 days of catherisation |
||
| Joseph 200794 | Catheterisation, mean (range) | 10.2 (7–21) | 6.1 (1–48) | Abstract | ||
| Jurczok 2007131 | Catheterisation, median or mean | 8.9 | 10.2 | |||
| Kim 2007132 | Catheterisation, mean (SD) | 10.7 (7.8) | 12.1 (6.7) | |||
| Lama 2009133 | Catheterisation, mean (SD) | 8.8 (3.9) | 14.9 (6.2) | |||
| Martorana 2004134 | Catheterisation, mean (range) | 13 (6–36) | 15 (11–21) | |||
| Ou 2009113 | Catheterisation, mean (SD) | 7.7 (2.1) | 9.2 (2.9) | |||
| Poulakis 2007137 | Catheterisation, mean (SD) |
Group I: 7 (3) Group II: 7 (2) |
22 (6) | Groups I and II are two age groups (data not combined) | ||
| Remzi 2005139 | Catheterisation, mean (range) |
Transperitoneal: 7.2 (6–23) Extraperitoneal: 6.1 (4–24) |
10.9 (8–35) | |||
| Rocco 2009114 | Catheterisation, mean (range) | 6 (4–30) | 7 (4–35) | |||
| Rozet 200796 | Catheterisation, mean (range) | 9.2 (6–29) | 9.0 (7–31) | |||
| Salomon 2002140 | Catheterisation, mean, SD (range) | 5.7, 4.8 (2–30) |
Retropubic: 12.1, 8.1 (4–45) Perineal: 11.3, 4.6 (3–30) |
|||
| Soric 2004143 | Catheterisation, mean | 10 | 8 | |||
| Tewari 2003116 | Catheterisation, mean (range) | 7 (1–18) | 15.8 (7–28) | |||
| Anastomotic leak | ||||||
| Brown 2004125 | Anastomotic leak | 9/60 (15.0) | 2/60 (3.3) | |||
| Carlsson 2010104 | Anastomotic leak | 13/1253 (1.0) | 8/485 (1.6) | < 30 days postoperatively | ||
| Dahl 2009126 | Anastomotic leak | 2/104 (1.9) | 0/102 | > 200 ml/day | ||
| Drouin 2009101 | Anastomotic leak | 0/71 | 2/85 (2.4) | 1/83 (1.2) | ||
| Ghavamian 2006128 | Anastomotic leak | 2/70 (2.9) | 3/70 (4.3) | |||
| Guazzoni 200690 | Anastomotic leak | 8/60 (13.3) | 20/60 (33.3) | RCT | ||
| Joseph 200794 | Urine leak at cystogram | 12/754 (1.6) | 112/800 (14.0) | Abstract | ||
| Kim 2007132 | Anastomotic leak | 5/30 (16.7) | Not reported | > 14 days; managed by prolonged catheterisation | ||
| Martorana 2004134 | Anastomotic leak | 1/50 (2.0) | 2/50 (4.0) | |||
| Nadler 2010112 | Anastomotic leak | 2/50 (4.0) | 2/50 (4.0) | |||
| Ou 2009113 | Mild vesicourethral anastomosis leaking | 0/30 | 2/30 (6.7) | |||
| Remzi 2005139 | Anastomotic leak | 8/80 (10.0) | 6/41 (14.6) | |||
| Rozet 200796 | Anastomotic leak | 1/133 (0.8) | 1/133 (0.8) | |||
| Salomon 2002140 | Anastomotic leak | 4/155 (2.6) | 2/151 (1.3) | |||
| Sundaram 200497 | Anastomotic leak | 0/10 | 1/10 (10.0) | Abstract | ||
| Hernia (port/incision sites) | ||||||
| Menon 200295 | Hernia port/incision site | Not reported | 1/40 (2.5) | |||
| Nadler 2010112 | Inguinal hernia | 0/50 | 1/50 (2.0) | |||
| Tewari 2003116 | Wound dehiscence/hernia | 2/200 (1.0) | 1/100 (1.0) | |||
| Infection | ||||||
| Artibani 2003123 | Fever | 15 | 7 | |||
| Wound infection | 0 | 1 | ||||
| Port site infection | 1 | 0 | ||||
| Subtotal | 16/71 (22.5) | 8/50 (16.0) | ||||
| Brown 2004125 | Superficial wound infection | 0/60 | 2/60 (3.3) | |||
| Carlsson 2010104 | Infection | 18 | 44 | All occurred < 30 days postoperatively | ||
| Pneumonia | 0 | 4 | ||||
| Infected lymphocele | 1 | 3 | ||||
| Wound infection | 6 | 29 | ||||
| Subtotal | 25/1253 (2.0) | 80/485 (16.0) | ||||
| Dahl 2009126 | Wound infection | 1/104 (1.0) | 0/102 | |||
| Drouin 2009101 | Urinary infection | 1/71 (1.4) | 0/85 | 6/83 (7.2) | ||
| Fornara 2004127 | Wound infection | 0/32 | 2/32 (6.3) | |||
| Ghavamian 2006128 | Urinary tract infection | 1/70 (1.4) | 1/70 (1.4) | |||
| Hu 200692 | Cellulitis | 6 | 12 | |||
| Orchitis | 1 | 1 | ||||
| Clostridium difficile enterocolitis | 0 | 1 | ||||
| Pneumonia | 0 | 1 | ||||
| Bacterial peritonitis | 0 | 1 | ||||
| Subtotal | 7/322 (2.2) | 16/358 (4.5) | ||||
| Jurczok 2007131 | Wound infection | 5/163 (3.1) | 8/240 (3.4) | n/N calculated from reported percentages | ||
| Krambeck 2008108 | Sepsis, 1 month | 0 | 1 | |||
| Urinary tract infection, 1 month | 3 | 6 | ||||
| Abdominal abscess, 1 year | 0 | 2 | ||||
| Subtotal | 3/248 (1.2) | 9/249 (3.6) | ||||
| Rozet 200796 | Wound abscess | 1 | 0 | |||
| Infected pelvic haematoma | 3 | 2 | ||||
| Urinary infection | 6 | 1 | ||||
| Urinary sepsis | 2 | 2 | ||||
| Subtotal | 12/133 (9.0) | 5/133 (3.8) | ||||
| Salomon 2002140 | Wound infection | 2/155 (1.3) | 12/151 (7.9) | |||
| Sepsis | 0/155 | 2/151 (1.3) | ||||
| Subtotal | 2/155 (1.3) | 14/151 (9.3) | ||||
| Tewari 2003116 | Postoperative fever/pneumonia | 0/200 | 4/100 (4.0) | |||
| Organ injury | ||||||
| Artibani 2003123 | Rectal injury | 2 | 0 | |||
| Transient peripheral nerve injury | 2 | 0 | ||||
| Subtotal | 4/71 (5.6) | 0/50 | ||||
| Bhayani 2003124 | Epigastric artery injury | 1/33 (3.0) | 0/24 | |||
| Brown 2004125 | Ureteral injury | 2/60 (3.3) | 0/60 | One required reoperation | ||
| Carlsson 2010104 | Rectal injury | 2 | 8 | |||
| Small bowel injury | 1 | 0 | ||||
| Ureteral injury | 1 | 0 | ||||
| Femoral nerve injury | 2 | 0 | ||||
| Obturator nerve injury | 0 | 2 | ||||
| Subtotal | 6/1253 (0.5) | 10/485 (2.1) | ||||
| Doumerc 2010105 | Bowel injury | 1/212 (0.5) | 0/502 | |||
| Drouin 2009101 | Rectal injury | 0/71 | 1/85 (1.2) | 1/83 (1.2) | ||
| Ficarra 2009106 | Colon lesion | 1 | 0 | |||
| Rectal lesion | 1 | 0 | ||||
| Subtotal | 2/103 (1.9) | 0/105 | ||||
| Fornara 2004127 | Rectal lesion | 1/32 (3.1) | 0/32 (0) | |||
| Ghavamian 2006128 | Bladder injury | 1/70 (1.4) | 0/70 | |||
| Inferior epigastric injury | 1/70 (1.4) | 0/70 | ||||
| Subtotal | 2/70 (2.9) | 0/70 | ||||
| Greco 2010129 | Rectal injury | 2/150 (1.3) | 1/150 (0.7) | |||
| Guazzoni 200690 | Rectal injury | 1/60 (1.7) | Not reported |
RCT Rectal injury repaired with interrupted sutures intraoperatively |
||
| Hu 200692 | Artery injury | 0 | 3 | |||
| Nerve injury | 0 | 4 | ||||
| Intraoperative heocolonic injury | 2 | 1 | ||||
| Intraoperative urethral injury | 1 | 1 | ||||
| Intraoperative rectal injury | 0 | 7 | ||||
| Rectourethral fistulas | 0 | 7 | ||||
| Subtotal | 3/322 (0.9) | 23/358 (6.4) | ||||
| Kim 2007132 | Rectal injury | 1/30 (3.3) | Not reported | Managed by laparoscopic repair | ||
| Epigastric vessel injury | 1/30 (3.3) | Managed by simple closure | ||||
| Lama 2009133 | Rectal perforation | 0/56 | 1/59 (1.7) | |||
| Martorana 2004134 | Epigastric vessel injury | 1/50 (2.0) | 0/50 | |||
| Bladder wall lesion | 1/50 (2.0) | 0/50 | ||||
| Subtotal | 2/50 (4.0) | 0/50 | ||||
| Ou 2009113 | Bladder injury and vesicourethral anastomosis tear | 1 | 0 | |||
| Urinary bladder injury | 1 | 0 | ||||
| Rectal injury | 0 | 1 | ||||
| Subtotal | 2/30 (6.7) | 1/30 (3.3) | ||||
| Remzi 2005139 | Rectal injury | 1/80 (1.3) | 1/41 (2.4) | Repaired intraoperatively | ||
| Salomon 2002140 | Ureteral injury | 1/155 (0.6) | 0/151 | |||
| Rectal injury | 3/155 (1.9) | 3/151 (2.0) | ||||
| Subtotal | 4/155 (2.6) | 3/151 (2.0) | ||||
| Soric 2004143 | Ureter wound | 2/26 (7.7) | Not reported | |||
| Tewari 2003116 | Rectal injuries | 0/200 | 1/100 (1.0) | |||
| Ileus | ||||||
| Artibani 2003123 | Ileus | 1/71 (1.4) | 0/50 | |||
| Brown 2004125 | Prolonged ileus | 2/60 (3.3) | 3/60 (5.0) | |||
| Ficarra 2009106 | Ileus | 1/103 (1.0) | 1/105 (1.0) | |||
| Ghavamian 2006128 | Ileus | 2/70 (2.9) | 1/70 (1.4) | |||
| Hu 200692 | Ileus | 9/322 (2.8) | 19/358 (5.3) | |||
| Krambeck 2008108 | Ileus, 1 month | 5/286 (1.7) | 10/564 (1.8) | |||
| Martorana 2004134 | Ileus | 1/50 (2.0) | 0/50 | |||
| Menon 200295 | Ileus | 1/40 (2.5), transient | 1/40 (2.5), paralytic | |||
| Nadler 2010112 | Ileus | 2/50 (4.0) | 0/50 | |||
| Remzi 2005139 | Ileus | 1/80 (1.3) | 0/41 | |||
| Salomon 2002140 | Ileus | 4/155 (2.6) | 0/151 | |||
| Tewari 2003116 | Ileus | 3/200 (1.5) | 3/100 (3.0) | |||
| Deep-vein thrombosis | ||||||
| Brown 2004125 | Deep-vein thrombosis | 0/60 | 2/60 (3.3) | |||
| Ghavamian 2006128 | Deep-vein thrombosis | 1/70 (1.4) | 1/70 (1.4) | |||
| Hu 200692 | Deep-vein thrombosis | 2/322 (0.6) | 0/358 | |||
| Krambeck 2008108 | Deep-vein thrombosis | 1/248 (0.4) | 6/492 (1.2) | |||
| Lama 2009133 | Deep-vein thrombosis | 0/56 | 1/59 (1.7) | |||
| Nadler 2010112 | Deep-vein thrombosis | 0/50 | 1/50 (2.0) | |||
| Salomon 2002140 | Deep-vein thrombosis | 1/155 (0.6) | 2/151 (1.3) | |||
| Tewari 2003116 | Deep-vein thrombosis | 1/200 (0.5) | 1/100 (1.0) | |||
| Pulmonary embolism | ||||||
| Carlsson 2010104 | Pulmonary embolism | 2/1253 (0.2) | 5/485 (1.0) | |||
| Dahl 2009126 | Pulmonary embolism | 1/104 (1.0) | 0/102 | |||
| Krambeck 2008108 | Pulmonary embolism | 0/248 | 5/492 (1.0) | |||
| Rozet 200796 | Pulmonary embolism | 0/133 | 1/133 (0.8) | |||
| Salomon 2002140 | Pulmonary embolism | 1/155 (0.6) | 1/151 (0.7) | |||
| Blood loss (ml) | ||||||
| Al-Shaiji 2010121 | Blood loss, mean, SD (range) | 241.4, 167.0 (50–1200) | 849.6, 646.7 (100–3500) | |||
| Bhayani 2003124 | Blood loss (estimated), mean (SD) | 533 (212) | 1473 (768) | |||
| Doumerc 2010105 | Blood loss estimated | Numbers of patients with mean estimated blood loss | ||||
| < 499 | 208/212 (98.1) | 349/502 (69.5) | ||||
| 500–999 | 4/212 (1.9) | 147/502 (29.3) | ||||
| > 1000 | 0/212 | 6/502 (1.2) | ||||
| Drouin 2009101 | Blood loss, mean, SD (range) | 310.7, 205.5 (80–1800) | 558, 574 (110–1100) | 821.2, 582.3 (210–2200) | ||
| Ficarra 2009106 | Blood loss (intraoperative), median | 300 | 500 | |||
| Fornara 2004127 | Blood loss, median | 200 | 550 | |||
| Fracalanza 2008107 | Blood loss, median (range) | 300 (200–400) | 500 (250–650) | |||
| Ghavamian 2006128 | Blood loss (estimated), mean (SD) | 275.8 (43.1) | 563.2 (54.5) | |||
| Gosseine 200991 | Blood loss, mean | 551 | 538 | |||
| Greco 2010129 | Blood loss, mean (range) | 450 (150–750) | 650 (400–900) | |||
| Guazzoni 200690 | Blood loss, mean (SD) | 257.3 (177) | 853.3 (485) | RCT | ||
| Hu 200692 | Blood loss (estimated), median (range) | 250 (50–1600) | 200 (0–1500) | |||
| Joseph 200794 | Blood loss (estimated), mean (range) | 190.0 (20–1400) | 768 (100–2000) | Abstract | ||
| Jurczok 2007131 | Blood loss (estimated), median (range) | 200 (100–700) | 550 (200–1900) | |||
| Kordan 2010120 | Blood loss (estimated), median (range) | 100 (50–200) | 450 (300–600) | Secondary to Barocas 2010104 | ||
| Menon 200295 | Blood loss, mean (SD) | 256 (164.4) | 391 (278.9) | |||
| Miller 2007111 | Blood loss (estimated operative), mean | 232.1 | 490.4 | |||
| Nadler 2010112 | Blood loss, mean (range) | 533 (200–1500) | 1540 (500–5000) | |||
| Ou 2009113 | Blood loss, mean (SD) | 314 (284) | 912 (370) | |||
| Poulakis 2007137 | Blood loss (estimated intraoperative), mean (SD) |
Group I: 205 (81) Group II: 190 (84) |
486 (185) | Groups I and II two age groups (data not combined) | ||
| Remzi 2005139 | Blood loss, mean (SD) |
Transperitoneal: 290 (254) Extraperitoneal: 189 (140) |
385 (410) | |||
| Rocco 2009114 | Blood loss, median (range) | 200 (50–2000) | 800 (150–5000) | |||
| Rozet 200796 | Blood loss (operative), mean (range) | 609 (100–3000) | 512 (70–1800) | |||
| Schroeck 2008115 | Blood loss (estimated), median (range) | 150 (100–173) | 800 (500–1200) | |||
| Sundaram 200497 | Blood loss (estimated), mean (range) | 295 (50–500) | 620 (250–2000) | Abstract | ||
| Tewari 2003116 | Blood loss (estimated), mean (range) | 153 (25–750) | 910 (200–5000) | |||
| Trabulsi 200898 | Blood loss (estimated), median (range) | 287 (50–1500) | 370 (50–3200) | |||
| Truesdale 2010117 | Blood loss (estimated), mean (SD) | 157.7 (105.1) | 940.5 (615.0) | |||
| Wagner 2007146 | Blood loss (estimated), mean (SD) | 305 (164.2) | 1331 (709.8) | |||
| Surgical incision | ||||||
| Fracalanza 2008107 | Length of surgical incision (cm), median (range) | 3.5 (3–4) | 15 (12–17) | |||
| Other perioperative complications | ||||||
| Anastasiadis 2003122 | Surgical complications | 22/230 (9.6) | 9/70 (12.9) |
Including anastomotic leak, wound infection, rectal injury, temporary ileus, haematoma % complications for open reported as 13.1% in paper (9.17 patients) |
||
| Artibani 2003123 | Acute urinary retention | 1 | 2 | |||
| Pelvic haematoma | 1 | 0 | ||||
| Cardiovascular complications | 3 | 0 | ||||
| Subtotal | 5/71 (7.0) | 2/50 (4.0) | ||||
| Bhayani 2003124 | Major complications | |||||
| Hydroureteronephrosis | 1 | 0 | ||||
| Dislodged catheter requiring replacement | 1 | 0 | ||||
| Bladder neck contracture requiring operative bladder neck incision | 0 | 3 | ||||
| Subtotal | 2/33 (6.0) | 3/24 (12.5) | ||||
| Minor complications: | ||||||
| Calf myositis | 1 | 0 | ||||
| Obturator nerve palsy | 1 | 0 | ||||
| Postoperative hydrocele | 1 | 0 | ||||
| Epigastric artery injury | 1 | 0 | ||||
| Inadvertent cystotomy | 1 | 0 | ||||
| Subtotal | 5/33 (15.2) | 0/24 | ||||
| Overall subtotal | 7/33 (21.2) | 3/24 (12.5) | ||||
| Brown 2004125 | Ulnar neuropathy | 1/60 | 0/60 | |||
| Rectus haematoma | 1/60 | 0/60 | ||||
| Subtotal | 2/60 (1.7) | 0/60 | ||||
| Carlsson 2010104 | Myocardial infarction, < 30 days postoperatively | 1/1253 (0.1) | 2/485 (0.4) | |||
| Surgical reintervention, < 30 days postoperatively | 24/1253 (1.9) | 14/485 (2.9) | ||||
| Dahl 2009126 | Lymphocele | 4 | 0 | |||
| Hematuria | 5 | 1 | ||||
| Hematoma leading to contracture | 1 | 0 | ||||
| Fatal cardiac arrest | 0 | 1 | ||||
| Genital femoral nerve irritation | 3 | 0 | ||||
| Meatal stricture | 1 | 0 | ||||
| Urinary retention | 1 | 1 | ||||
| Seroma | 1 | 0 | ||||
| Vasovagal syncope | 1 | 0 | ||||
| Chronic pain in abdomen | 0 | 1 | ||||
| Subtotal | 17/104 (16.3) | 4/102 (3.9) | ||||
| Doumerc 2010105 | Bleeding | 2/212 (0.9) | 0/502 | |||
| Severe pain | 1/212 (0.5) | 0/502 | ||||
| Pelvic haematoma | 0/212 | 1/502 (0.2) | ||||
| Subtotal | 3/212 (1.4) | 1/502 (0.2) | ||||
| Drouin 2009101 | Retention | 1 | 3 | 3 | ||
| Postoperative bleeding | 4 | 0 | 0 | |||
| Lymphocele | 0/ | 0 | 1 | |||
| Subtotal | 5/71 (7.0) | 3/85 (3.5) | 4/83 (4.8) | |||
| Ficarra 2009106 | Postoperative bleeding | 7 | 7 | |||
| Cardiovascular complications | 0 | 2 | ||||
| Wound dehiscence | 0 | 1 | ||||
| Surgical re-exploration | 4 (due to bleeding) | 0 | ||||
| Subtotal | 11/103 (10.7) | 10/105 (9.5) | ||||
| Fornara 2004127 | Lymphocele | 0/32 | 1/32 (3.1) | |||
| Fracalanza 2008107 | Fever | 2/35 (5.7) | 4/26 (15.4) | ‘no other complications’ | ||
| Ghavamian 2006128 | Clot retention | 1 | 1 | |||
| Lymphocele | 2 | 2 | ||||
| Neuropraxia | 1 | 0 | ||||
| Subtotal | 4/70 (5.7) | 3/70 (4.3) | ||||
| Gosseine 200991 | Surgical complications | 5/122 (4.1) | 8/125 (6.4) | |||
| Guazzoni 200690 | Fever | 1 | 3 | RCT | ||
| Persistent lymphorrhea | 4 | 5 | ||||
| Acute urinary retention after removal of catheter | 1 | 1 | ||||
| Subtotal | 6/60 (10.0) | 9/60 (15.0) | ||||
| Hu 200692 | Myocardial infarction | 0 | 0 | |||
| Cerebrovascular accidents | 0 | 0 | ||||
| Lymphocele | 3 | 3 | ||||
| Urine retention | 13 | 20 | ||||
| Urine leak | 24 | 48 | ||||
| Clot retention | 1 | 1 | ||||
| Intra-abdominal drain retraction | 1 | 0 | ||||
| Acute tubular necrosis | 0 | 1 | ||||
| Subtotal | 42/322 (13.0) | 73/358 (20.4) | ||||
| Joseph 200794 | Urinary retention | 12/754 (1.6) | 48/800 (6.0) | Abstract | ||
| Jurczok 2007131 | Rectal lesion | 3/163 (1.8) | 4/240 (1.6) | n/N calculated from reported percentages | ||
| Lymphocele | 5/163 (3.2) | 7/240 (2.9) | ||||
| Revision | 2/163 (1.2) | 6/240 (2.5) | ||||
| Kim 2007132 | Subcutaneous emphysema | 4/30 (13.3) | Not reported | Conservative management | ||
| Krambeck 2008108 | Urinary retention, 1 month | 8/286 | 7/564 | |||
| Ureteric obstruction, 1 month | 0/286 | 1/564 | ||||
| Haemorrhage/haematoma, 1 month | 10/286 | 10/564 | ||||
| Renal failure, 1 month | 0/286 | 1/564 | ||||
| Drug reaction, 1 month | 2/286 | 7/564 | ||||
| Lymphocele, 1 year | 1/248 | 5/492 | ||||
| Lymphoedema, 1 year | 0/248 | 0/492 | ||||
| Myocardial infarction, 1 month | 0/286 | 0/564 | ||||
| Respiratory failure, 1 month | 2/286 | 3/564 | ||||
| Stroke, 1 month | 3/286 | 3/564 | ||||
| Subtotal | 26/248 (10.5) | 37/492 (7.5) | ||||
| Lama 2009133 | Urinary retention | 1 | 5 | |||
| Urinary leakage | 0 | 2 | ||||
| Bleeding | 1 | 3 | ||||
| Seroma | 1 | 0 | ||||
| Perioperative hypercapnia | 0 | 1 | ||||
| Embolic stroke | 0 | 1 | ||||
| Subtotal | 3/56 (5.4) | 12/59 (20.3) | ||||
| Martorana 2004134 | Uteral stretching | 1 | 0 | |||
| Lymphoceles | 0 | 2 | ||||
| Subtotal | 1/50 (2.0) | 2/50 (4.0) | ||||
| Menon 200295 | Entrapment of ureter in vesicourethral anastomotic stitch | 0/40 | 1/40 (2.5) | |||
| Nadler 2010112 | Pneumonia | 1 | 0 | |||
| Gastric ulcer | 1 | 0 | ||||
| Subtotal | 2/50 (4.0) | 0/50 | ||||
| Ou 2009113 | Intraoperative bleeding | 1 | 0 | |||
| Lymph leakage for 3 weeks | 1 | 0 | ||||
| Subtotal | 2/30 (6.7) | 0/30 | ||||
| Poulakis 2007137 | Group I | Group II | ||||
| Early complications (first 30 days after surgery): |
Data not combined Major, moderate and minor complications defined Medical comorbidity assessed with a scoring algorithm placing patients into four groups (but not defined) |
|||||
| Minor/moderate complications | ||||||
| Dehiscence/rupture of wound | 0 | 1 | 7 | |||
| Haematoma/haemorrhage | 2 | 2 | 7 | |||
| Urinary retention | 0 | 2 | 1 | |||
| Prolonged urinary leakage (> 2 weeks) | 1 | 0 | 3 | |||
| Lymphocele | 2 | 2 | 2 | |||
| Gastrointestinal symptoms including peritonitis and ileus | 0 | 0 | 7 | |||
| Delirium | 6 | 0 | 4 | |||
| Fever > 39°C (urosepsis) | 1 | 1 | 1 | |||
| Subtotal | 12/72 (16.7) | 8/132 (7) | 32/70 (43) | |||
| Major complications | ||||||
| Respiratory insufficiency | 2 | 0 | 2 | |||
| Cardiovascular including arrhythmias and myocardial infarction | 1 | 1 | 3 | |||
| Thrombophlebitis/pulmonary emboli/stroke | 1 | 1 | 2 | |||
| Subtotal | 4/72 (5.6) | 2/132 (1.5) | 7/70 (10.0) | |||
| Late complications (30 days after surgery) | ||||||
| Bladder neck contraction | 0 | 0 | 3 | |||
| Wound hernia | 0 | 1 | 3 | |||
| Subtotal | 0/72 | 1/132 (0.8) | 6/70 (8.6) | |||
| Remzi 2005139 | Haemorrhage | 1/80 (1.3) | 3/41 (7.3) | |||
| Rozet 200796 | Cardiac complications | 0 | 0 | |||
| Postoperative bleeding | 6 | 1 | ||||
| Retention | 1 | 3 | ||||
| Renal insufficiency | 2 | 0 | ||||
| Subtotal | 9/133 (6.8) | 4/133 (3.0) | ||||
| Salomon 2002140 | Lymphorrhea | 2 | 6 | |||
| Pelvic haematoma | 2 | 2 | ||||
| Postoperative neuropathy | 0 | 2 | ||||
| Subtotal | 4/155 (2.6) | 10/151 (6.7) | ||||
| Soric 2004143 | Blood vessel damage | 1/26 (3.8) | Not reported | |||
| Nerve damage | 1/26 (3.8) | Not reported | ||||
| Bladder neck sclerosis | 2/26 (7.7) | Not reported | ||||
| Sundaram 200497 | Transient urinary retention for 3 weeks after the catheter was removed | 1/10 (10.0) | 0/10 | Abstract | ||
| Tewari 2003116 | Lymphocele | 0 | 2 | |||
| Obturator neuropathy | 0 | 2 | ||||
| Myocardial infarction | 0 | 1 | ||||
| Postoperative bleeding/re-exploration | 1 | 4 | ||||
| Subtotal | 1/200 (0.5) | 9/100 (9.0) | ||||
| Early postoperative results | ||||||
| Mobilisation | ||||||
| Fracalanza 2008107 | Mobilisation (days), mean (SD) | 1 (0) | 1.2 (0.4) | |||
| Guazzoni 200691 | First flatus | RCT | ||||
| Day 1 | 21/60 (35.0) | 11/60 (18.3) | ||||
| Day 2 | 37/60 (61.7) | 45/60 (75.0) | ||||
| Day 3 | 2/60 (3.3) | 4/60 (6.7) | ||||
| Mobilisation | ||||||
| Day 1 | 55/60 (91.7) | 49/60 (81.7) | ||||
| Day 2 | 5/60 (8.3) | 11/60 (18.3) | ||||
| Day 3 | – | – | ||||
| Free ambulation | ||||||
| Day 1 | 14/60 (23.3) | 6/60 (10.0) | ||||
| Day 2 | 46/60 (76.7) | 54/60 (90.0) | ||||
| Day 3 | – | – | ||||
| Poulakis 2007137 | Time to full mobilisation (days), mean (SD) |
Group I: 3.7 (1.2) Group II: 3.2 (1.0) |
5.1 (1.7) | Groups I and II two age groups (data not combined) | ||
| Oral feeding | ||||||
| Fracalanza 2008107 | Resumption of oral feeding (days), mean (SD) | 1 (0.3) | 1.8 (0.7) | |||
| Guazzoni 200690 | Oral solid intake | RCT | ||||
| Day 1 | – | – | ||||
| Day 2 | 55/60 (91.7) | 58/60 (96.7) | ||||
| Day 3 | 5/60 (8.3) | 2/60 (3.3) | ||||
| Poulakis 2007137 | Time to first oral intake (days), mean (SD) |
Group I: 1.1 (0.5) Group II: 0.9 (0.6) |
2.3 (0.9) | Groups I and II two age groups (data not combined) | ||
| Poulakis 2007137 | Duration of parenteral fluid administration (days), mean (SD) |
Group I: 2.2 (0.9) Group II: 1.9 (0.8) |
3.1 (1.2) | Groups I and II two age groups (data not combined) | ||
| Study | Measures | Timing | Robotic, n/N (%)a | Laparoscopic, n/N (%)a | Open, n/N (%)a | Notes | |
|---|---|---|---|---|---|---|---|
| Urinary incontinence | |||||||
| Artibani 2003123 | Incontinence (any amount of urinary leakage) | > 12 months | 12/20 (60.0) | 5/14 (35.7) | |||
| Incontinence (need protection system) | > 12 months | 8/20 (40.0) | 3/14 (21.4) | ||||
| Ball 200699 | Urinary function (UCLA-PCI), mean (SD) | 6 months | Both validated measures | ||||
| Baseline | 88 (18) | 86 (24) | 88 (20) | ||||
| % baseline score | 69 (31) | 69 (40) | 75 (40) | ||||
| Urinary bother (UCLA-PCI), mean (SD) | 6 months | ||||||
| Baseline | 85 (24) | 81 (30) | 85 (26) | ||||
| % baseline score | 78 (45) | 75 (40) | 74 (40) | ||||
| AUA SI (American Urological Association Symptom Index), mean (SD) | 6 months | ||||||
| Baseline | 72 (22) | 70 (23) | 74 (21) | ||||
| % baseline score | 123 (52) | 106 (34) | 104 (42) | ||||
| Dahl 2009126 | Not returned to baseline continence | 12 months | 37/78 (47) | 37/72 (51) | 12-month data collected by mail survey | ||
| During last 4 weeks how often leaked urine? | 12 months | ||||||
| Every day | 14/78 (17.9) | 11/73 (15.1) | |||||
| About once/week | 8/78 (10.3) | 14/73 (19.2) | |||||
| Less than once/week | 24/78 (30.8) | 18/73 (24.7) | |||||
| Not at all | 32/78 (41.0) | 29/73 (39.7) | |||||
| Best description of urinary control during last 4 weeks | 12 months | ||||||
| No control whatsoever | 0/78 | 0/73 | |||||
| Frequent dribbling | 2/78 (2.6) | 1/73 (1.4) | |||||
| Occasional dribbling | 30/78 (38.5) | 37/73 (50.7) | |||||
| Total control | 46/78 (59.0) | 35/73 (47.9) | |||||
| How many pads/adult nappies daily during last 4 weeks? | 12 months | ||||||
| 3 or more | 0/78 | 0/73 | |||||
| 2 | 3/78 (3.8) | 1/73 (1.4) | |||||
| 1 | 10/78 (12.8) | 8/73 (11.0) | |||||
| 0 | 65/78 (83.3) | 63/73 (86.3) | |||||
| Ficarra 2009106 | Urinary incontinence (ICIQ-UI) | 12 months | 3/103 (2.9) | 12/105 (11.4) | |||
| Time to urinary continence, mean | – | 25 days (n = 103) | 75 days (n = 105) | ||||
| Ghavamian 2006128 | Continence, defined as no leakage and no pad use | Continence data converted to incontinence | |||||
| Diurnal | 3 months | 30/70 (42.9) | 31/70 (44.3) | ||||
| 6 months | 21/70 (30.0) | 20/70 (28.6) | |||||
| 12 months | 7/70 (10.0) | 8/65 (12.3) | |||||
| 18 months | 7/70 (10.0) | 5/63 (7.9) | |||||
| Nocturnal | 3 months | 27/70 (38.6) | 26/70 (37.1) | ||||
| 6 months | 19/70 (27.1) | 20/70 (28.6) | |||||
| 12 months | 5/70 (7.1) | 6/65 (9.2) | |||||
| 18 months | 4/70 (5.7) | 3/63 (4.8) | |||||
| Gosseine 200991 | I-PSS and ICS questionnaire scores | 1 year |
Study reports more than 92% questionnaire response rate 75% A and 70% B respondents reported continent at 6 months |
||||
| Using at least one pad for protection | 87% of those incontinent at 6 months (= 25% of respondents) | 71% of those incontinent at 6 months (= 30% of respondents) | |||||
| Using one or more pads for protection | 19% of those incontinent at 6 months (= 25% of respondents) | 17% of those incontinent at 6 months (= 30% of respondents) | |||||
| Greco 2010129 | Minimal stress incontinence (one or two pads per day) | 3 months | 13/150 (8.7) | 29/150 (19.3) | Data for absence of complete urinary continence converted from complete urinary continence data | ||
| Moderate stress incontinence (two or four pads per day) | 3 months | 3/150 (2.0) | 7/150 (4.7) | ||||
| Absence of complete urinary continence | 4 weeks | 86/150 (57.3) | 104/150 (69.3) | ||||
| 3 months | 16/150 (10.7) | 36/150 (24.0) | |||||
| 12 months | 4/150 (2.7) | 13/150 (8.7) | |||||
| Jacobsen 2007130 | Incontinence (24-hour pad testing, total pad weight gain > 8 mg) | 12 months | 10/57 (17.5) | 19/148 (12.8) | |||
| I-PSS [7-item (0, mildly to 35, severely symptomatic), subjectively administered urinary symptom questionnaire] | Baseline, mean (SD) | First half (n not reported): 7.9 (5.4); Second half (n not reported): 9.2 (6.7) | (n = 172) 7.3 (6.6) | ||||
| 12 months, mean (SD) | First half (n = 29): 5.9 (2.9); second half (n = 28): 5.7 (1.4) | (n = 148) 5.8 (5.0) | |||||
| Joseph 200593 | Continence verified by absence of leakage on Valsalva manoeuvre or coughing after catheter removal | Immediately | 27/50 (54.0) | 40/50 (80.0) | Converted to incontinence | ||
| 1 month | 37/50 (74.0) | 12/50 (24.0) | |||||
| 2 months | 46/50 (92.0) | 36/50 (72.0) | |||||
| 3 months | 45/50 (90.0) | 40/50 (80.0) | |||||
| Krambeck 2008108 | One to two pads/day | 12 months | 17/244 (7.0) | 23/476 (4.8) | |||
| Three pads/day | 3/244 (1.2) | 7/476 (1.5) | |||||
| Lama 2009133 | Incontinence (no definition) | 6 months | 1/56 (1.8) | 2/59 (3.4) | |||
| 12 months | 0/56 | 2/59 (3.4) | |||||
| Malcolm 2010110 | Urinary function (UCLA-PCI), mean (SD) | Baseline | 92 (13) | 89 (18) | 195 patients with function/bother score < 30 at baseline excluded from analysis | ||
| 3 months | 71 | 73 | |||||
| 6 months | 69 | 80 | |||||
| 12 months | 74 | 79 | |||||
| 18 months | 74 | 82 | |||||
| 24 months | 76 | 84 | |||||
| 30 months | 75 | 82 | |||||
| 36 months | 78 | 83 | |||||
| Urinary bother (UCLA-PCI), mean (SD) | Baseline | 93 (14) | 92 (15) | ||||
| 3 months | 65 | 68 | |||||
| 6 months | 77 | 77 | |||||
| 12 months | 81 | 84 | |||||
| 18 months | 81 | 85 | |||||
| 24 months | 83 | 87 | |||||
| 30 months | 85 | 88 | |||||
| 36 months | 86 | 88 | |||||
| Namiki 2005135 | Urinary function (UCLA-PCI), mean (SD) | Baseline | 94.3 (14.6) | 91.4 (18.1) | |||
| 1 month | 35.0 (18.8) | 63.2 (26.7) | |||||
| 3 months | 55.5 (29.5) | 68.9 (25.3) | |||||
| 6 months | 69.0 (27.5) | 80.2 (21.8) | |||||
| 12 months | 75.8 (19.2) | 83.3 (20.4) | |||||
| Urinary bother (UCLA-PCI), mean (SD) | Baseline | 82.4 (25.6) | 83.3 (27.1) | ||||
| 1 month | 53.8 (29.6) | 73.4 (26.6) | |||||
| 3 months | 63.8 (33.5) | 76.1 (28.0) | |||||
| 6 months | 75.0 (28.9) | 85.1 (24.4) | |||||
| 12 months | 75.6 (24.2) | 89.7 (20.5) | |||||
| Namiki 2006136 | Retropubic | Perineal | |||||
| Urinary function (UCLA-PCI), mean (SD) | Baseline | 95.1 (14.6) | 92.9 (18.1) | 91.0 (14.6) | |||
| 1 month | 43.2 (18.8) | 58.5 (26.7) | 51.7 (18.8) | ||||
| 3 months | 63.1 (29.5) | 62.1 (25.3) | 59.4 (29.5) | ||||
| 6 months | 75.1 (27.5) | 74.4 (21.8) | 71.6 (27.5) | ||||
| 12 months | 75.2 (19.2) | 77.9 (20.4) | 74.9 (19.2) | ||||
| Urinary bother (UCLA-PCI), mean (SD) | Baseline | 86.0 (25.6) | 88.8 (27.1) | 83.0 (25.6) | |||
| 1 month | 48.5 (29.6) | 67.0 (26.6) | 60.0 (29.6) | ||||
| 3 months | 74.1 (33.5) | 72.0 (28.0) | 65.6 (33.5) | ||||
| 6 months | 78.8 (28.9) | 81.3 (24.4) | 75.0 (28.9) | ||||
| 12 months | 77.8 (24.2) | 84.4 (20.5) | 80.9 (24.2) | ||||
| Ou 2009113 | Incontinence (need to wear a pad) | 1 week | 24/30 (80.0) | 29/30 (96.7) | Converted from continence data | ||
| 12 months | 0/30 | 1/30 (3.3) | |||||
| Poulakis 2007137 | Incontinence (use of any number of pads) | 6 months |
Group I: 38/72 (52.8) Group II: 12/132 (9.1) |
33/70 (47.1) | In paper reported as urinary continence (use of no pads) | ||
| Rocco 2009114 | Incontinence [use pads (except safety pad)] | 3 months | 34/115 (29.6) | 87/233 (37.3) | |||
| 6 months | 8/110 (7.3) | 40/229 (17.5) | |||||
| 12 months | 2/79 (2.5) | 26/217 (12.0) | |||||
| Soderdahl 2005142 | UCLA-PCI (score 0–100, with higher score indicating better function or less bother) |
% baseline score (defined as a score of at least 80% of the pretreatment score) Validated measure |
|||||
| Urinary function, % baseline score | 12 months | 70.7 (n = 93) | 71.0 (n = 86) | ||||
| Urinary bother, % baseline score | 12 months | 83.8 (n = 93) | 86.4 (n = 86) | ||||
| Sundaram 200497 | Use pads (any number) | Mean: 3 months | 3/10 (30.0) | 2/10 (20.0) |
Abstract Converted from continence data |
||
| Tewari 2003116 | Not achieved continence (continence defined as using no pads or a liner for security reasons only) | Not reported | 40/200 (20.0) | 56/100 (56.0) | A third party telephone interview asked patients about pad use to manage urinary incontinence | ||
| Wagner 2007146 | EPIC-UISS (score 1–100), mean (SD) | Baseline | 95.6 (9.56) | 88.2 (20.41) | |||
| % baseline score at 12 months, mean | 12 months | 64 (n = 55) | 73 (n = 39) | Mean postoperative UISS score as a percentage of baseline preoperative function | |||
| Pad use/day | Median: 12 months | ||||||
| 0 | 43/67 (64.2) | 31/66 (47.0) | |||||
| 1 | 12/67 (17.9) | 14/66 (21.2) | |||||
| 2 | 8/67 (11.9) | 10/66 (15.2) | |||||
| ≥3 | 4/67 (6) | 11/66 (16.7) | |||||
| Erectile dysfunction | |||||||
| Artibani 2003123 | Sexual function not recovered | > 6 months | 52/57 (91.2) | 36/40 (90.0) |
Erectile function recovery defined as the ability to have intercourse spontaneously or sildenafil assisted 5/57 (8.8) laparoscopic and 4/40 (10) open patients recovered sildenafil-assisted sexual function |
||
| Ball 200699 | Sexual function (UCLA-PCI), mean (SD) | 6 months | Validated measure | ||||
| Baseline | 65 (27) | 56 (29) | 59 (30) | ||||
| % baseline score | 43 (43) | 25 (21) | 33 (33) | ||||
| Sexual bother (UCLA-PCI), mean (SD) | 6 months | ||||||
| Baseline | 69 (33) | 60 (36) | 64 (38) | ||||
| % baseline score | 32 (41) | 38 (45) | 27 (41) | ||||
| Dahl 2009126 | Not returned to baseline state of erectile function | 12 months | 44/77 (57.1) (this group was encouraged earlier phosphodiesterase-5 inhibitor use) | 50/73 (68.5) | Returning of baseline erectile function converted to non-recovery of baseline function | ||
| During last 4 weeks usual quality of erections | 12 months | ||||||
| None at all | 21/77 (27.3) | 18/73 (24.7) | |||||
| Not firm enough for any activity | 15/77 (19.5) | 12/73 (16.4) | |||||
| Firm enough for masturbation | 16/77 (20.8) | 26/73 (35.6) | |||||
| Firm enough for intercourse | 25/77 (32.5) | 17/73 (23.3) | |||||
| Ficarra 2009106 | Erectile function not recovered (in those having bilateral nerve sparing) (potency defined as a score of > 17 on the IIEF-5) | 12 months | 12/64 (18.8) | 21/41 (51.2) | Converted from recovery data | ||
| Ghavamian 2006128 | Erectile function (potency defined as a score of ≥ 3 on the IIEF-5, questions 2 and 3 – able to achieve and maintain erection satisfactory for intercourse more than half the time) | Converted from potency data | |||||
| Bilateral nerve sparing | 3 months | 32/40 (80.0) | 25/30 (83.3) | ||||
| 6 months | 18/40 (45.0) | 17/30 (56.7) | |||||
| 12 months | 11/40 (27.5) | 12/29 (41.4) | |||||
| 18 months | 8/39 (20.5) | 8/29 (27.6) | |||||
| Unilateral nerve sparing | 3 months | 8/10 (80.0) | 11/12 (91.7) | ||||
| 6 months | 8/10 (80.0) | 9/12 (75.0) | |||||
| 12 months | 7/10 (70.0) | 7/11 (63.6) | |||||
| 18 months | 4/9 (44.4) | 6/11 (54.5) | |||||
| All | 3 months | 40/50 (80.0) | 36/42 (85.7) | ||||
| 6 months | 26/50 (52.0) | 26/42 (61.9) | |||||
| 12 months | 18/50 (36.0) | 19/40 (47.5) | |||||
| 18 months | 12/48 (25.0) | 14/40 (35.0) | |||||
| Greco 2010129 | Potency, defined as patient’s reported ability to achieve sexual intercourse with or without the use of phosphodiesterase-5 inhibitors | 1 year | 51/150 (34.0) | 73/150 (48.7) | Converted from potency data | ||
| Joseph 200593 | Requires drug aid (sildenafil or tadalafil) (%) | 3 months | 46 | 36 | Unclear if IIEF means are for those requiring drug aid only or also include those with spontaneous erections | % of patients interviewed at 3 months | |
| IIEF-5 score, mean (SD) | 34 (11) | 37 (15) | |||||
| Krambeck 2008108 | Impotent – erections satisfactory for intercourse with or without the use of phosphodiesterase-5 inhibitors | 12 months | 61/203 (30.0) | 155/417 (37.3) | |||
| Lama 2009133 | Erectile function not preserved (no definition) | 12 months | 41/56 (73.2) | 33/59 (60.0) | Converted from erectile function preserved data | ||
| Malcolm 2010110 | Sexual function (UCLA-PCI), mean (SD) | Baseline | 73 (17) | 74 (18) | |||
| 3 months | 28 | 24 | |||||
| 6 months | 33 | 37 | |||||
| 12 months | 40 | 43 | |||||
| 18 months | 42 | 48 | |||||
| 24 months | 45 | 46 | |||||
| 30 months | 41 | 50 | |||||
| 36 months | 46 | 48 | |||||
| Sexual bother (UCLA-PCI), mean (SD) | Baseline | 84 (20) | 86 (20) | ||||
| 3 months | 41 | 27 | |||||
| 6 months | 42 | 28 | |||||
| 12 months | 47 | 40 | |||||
| 18 months | 51 | 46 | |||||
| 24 months | 48 | 52 | |||||
| 30 months | 52 | 54 | |||||
| 36 months | 45 | 58 | |||||
| Namiki 2005135 | Sexual function (UCLA-PCI), mean (SD) | Baseline | 36.2 (23.3) | 39.3 (24.7) | |||
| 1 month | 5.4 (8.0) | 9.5 (15.6) | |||||
| 3 months | 9.1 (9.5) | 10.0 (11.6) | |||||
| 6 months | 7.5 (8.5) | 13.0 (13.9) | |||||
| 12 months | 8.4 (12.6) | 11.7 (15.2) | |||||
| Sexual bother (UCLA-PCI), mean (SD) | Baseline | 72.7 (21.4) | 71.5 (27.4) | ||||
| 1 month | 51.3 (34.9) | 48.4 (34.1) | |||||
| 3 months | 53.8 (32.3) | 54.0 (34.9) | |||||
| 6 months | 48.8 (33.6) | 51.5 (36.4) | |||||
| 12 months | 60.6 (34.8) | 59.0 (33.2) | |||||
| Namiki 2006136 | Retropubic | Perineal | |||||
| Sexual function (UCLA-PCI), mean (SD) | Baseline | 32.4 (23.3) | 33.4 (24.7) | 38.0 (23.3) | |||
| 1 month | 4.0 (8.0) | 7.5 (15.6) | 6.8 (8.0) | ||||
| 3 months | 7.8 (9.5) | 6.3 (11.6) | 7.1 (9.5) | ||||
| 6 months | 9.7 (8.5) | 7.2 (13.9) | 7.5 (8.5) | ||||
| 12 months | 10.2 (12.6) | 10.4 (15.2) | 8.8 (12.6) | ||||
| Sexual bother (UCLA-PCI), mean (SD) | Baseline | 68.5 (21.4) | 68.9 (27.4) | 67.9 (21.4) | |||
| 1 month | 56.8 (34.9) | 55.3 (34.1) | 49.0 (34.9) | ||||
| 3 months | 63.7 (32.3) | 56.2 (34.9) | 51.2 (32.3) | ||||
| 6 months | 54.4 (33.6) | 59.3 (36.4) | 55.1 (33.6) | ||||
| 12 months | 62.2 (34.8) | 58.2 (33.2) | 53.0 (34.8) | ||||
| Ou 2009113 | Impotent | 12 months | Converted from potency data | ||||
| Patients had bilateral nerve sparing | 0/11 | 0/1 | |||||
| Patients had unilateral nerve sparing | 2/5 (40.0) | 1/1 (100.0) | |||||
| Unable to have sexual intercourse | 12 months | ||||||
| Patients had bilateral nerve sparing | 2/11 (18.2) | 0/1 | |||||
| Patients had unilateral nerve sparing | 4/5 (80.0) | 1/1 (100.0) | |||||
| Rocco 2009114 | Potency not recovered (unable to have complete sexual intercourse) | 3 months | 80/116 (69.0) | 191/233 (82.0) | |||
| 6 months | 61/107 (57.0) | 158/229 (69.0) | |||||
| 12 months | 31/79 (39.2) | 127/215 (59.1) | |||||
| Soderdahl 2005142 | UCLA-PCI (score 0–100, with higher score indicating better function or less bother) |
% baseline score (defined as a score of at least 75% of the pretreatment score) Validated measures |
|||||
| Sexual function, % baseline score | 12 months | 35.9 (n = 93) | 46.0 (n = 86) | ||||
| Sexual bother, % baseline score | 12 months | 42.9 (n = 93) | 39.0 (n = 86) | ||||
| Tewari 2003116 | Time to return to erections (definition not reported) (days), mean | – | 180 | 440 | A third party telephone interviewer asked patients about preoperative sexual function, ability to obtain erection and use of sildenafil | ||
| Wagner 2007146 | EPIC-SFSS (score 1–100), mean (SD) | Baseline | 70.7 (14.75) | 71.2 (16.36) | |||
| % baseline score at 12 months, mean | 12 months | 45 (n = 37) | 37 (n = 25) | Mean postoperative UISS score as a % of baseline preoperative function | |||
| Impotent (not had sexual intercourse during the last 4 weeks) in those with nerve sparing | 12 months | 22/37 (59.5) | 14/25 (56.0) | Converted from potency data | |||
| Faecal incontinence | |||||||
| Ball 200699 | Bowel function (UCLA-PCI), mean (SD) | 6 months | |||||
| Baseline | 86 (14) | 84 (18) | 87 (15) | ||||
| % baseline score | 98 (24) | 102 (25) | 102 (26) | ||||
| Bowel bother (UCLA-PCI), mean (SD) | 6 months | ||||||
| Baseline | 90 (19) | 87 (25) | 90 (20) | ||||
| % baseline score | 99 (30) | 94 (27) | 99 (26) | ||||
| Malcolm 2010110 | Bowel function (UCLA-PCI), baseline: mean (SD), 3–36 months: mean % of baseline score | Baseline | 88 (14) | 87 (14) | |||
| 3 months | 101 | 98 | |||||
| 6 months | 102 | 102 | |||||
| 12 months | 103 | 102 | |||||
| 18 months | 103 | 103 | |||||
| 24 months | 101 | 104 | |||||
| 30 months | 102 | 102 | |||||
| 36 months | 102 | 101 | |||||
| Bowel bother (UCLA-PCI), baseline: mean (SD), 3–36 months: mean % of baseline score (PBS) | Baseline | 94 (13) | 92 (15) | ||||
| 3 months | 98 | 93 | |||||
| 6 months | 100 | 102 | |||||
| 12 months | 100 | 99 | |||||
| 18 months | 100 | 100 | |||||
| 24 months | 97 | 102 | |||||
| 30 months | 99 | 96 | |||||
| 36 months | 94 | 99 | |||||
| Namiki 2005135 | Bowel function (UCLA-PCI), mean (SD) | Baseline | 89.5 (13.9) | 88.3 (15.1) | |||
| 1 month | 81.6 (18.1) | 82.0 (20.1) | |||||
| 3 months | 86.8 (20.1) | 86.0 (18.3) | |||||
| 6 months | 89.2(13.8) | 91.0 (13.4) | |||||
| 12 months | 89.0 (10.6) | 90.2 (13.7) | |||||
| Bowel bother (UCLA-PCI), mean (SD) | Baseline | 91.5 (17.8) | 91.0 (20.9) | ||||
| 1 month | 86.0 (25.1) | 86.1 (24.5) | |||||
| 3 months | 87.5 (25.3) | 91.5 (17.7) | |||||
| 6 months | 93.5 (14.7) | 94.3 (13.3) | |||||
| 12 months | 86.5 (21.5) | 93.0 (15.9) | |||||
| Namiki 2006136 | Retropubic | Perineal | |||||
| Bowel function (UCLA-PCI), mean (SD) | Baseline | 89.1 (13.9) | 89.2 (15.1) | 85.9 (13.9) | |||
| 1 month | 83.0 (18.1) | 82.0 (20.1) | 81.0 (18.1) | ||||
| 3 months | 88.4 (20.1) | 85.1 (18.3) | 83.0 (20.1) | ||||
| 6 months | 87.6 (13.8) | 87.9 (13.4) | 88.3 (13.8) | ||||
| 12 months | 91.8 (10.6) | 85.3 (13.7) | 86.6 (10.6) | ||||
| Bowel bother (UCLA-PCI), mean (SD) | Baseline | 87.5 (17.8) | 90.5 (20.9) | 86.3 (17.8) | |||
| 1 month | 83.0 (25.1) | 88.0 (24.5) | 82.0 (25.1) | ||||
| 3 months | 91.7 (25.3) | 87.9 (17.7) | 84.0 (25.3) | ||||
| 6 months | 88.9 (14.7) | 89.9 (13.3) | 88.4 (14.7) | ||||
| 12 months | 91.7 (21.5) | 88.8 (15.9) | 87.7 (21.5) | ||||
| Urinary continence | |||||||
| Anastasiadis 2003122 | Diurnal continence | % reported as continent | |||||
| No pad use (%) | 6 months | 59.2 | 43.3 | ||||
| No pad use (%) | 1 year | 76.1 | 66.7 | ||||
| Including pad use without leakage (%) | 1 year | 89 | 77.7 | ||||
| Nocturnal continence | |||||||
| No pad use (%) | 1 year | 87.1 | 66.7 | ||||
| Including pad use without leakage (%) | 1 year | 96 | 90 | ||||
| Nadler 2010112 | Continence defined as one or less precautionary pads/day | 12 months | 39/44 (88.6) | 41/46 (89.1) | |||
| Remzi 2005139 | Early full continence (no pad) | 1 month |
Transperitoneal: 10/39 (25.6) Extraperitoneal: 11/41 (26.8) |
8/41 (19.5) | |||
| 12 months |
Transperitoneal: 33/39 (84.6) Extraperitoneal: 36/41 (87.8) |
33/41 (80.5) | |||||
| Potency | |||||||
| Anastasiadis 2003122 | Potency rate (%) | 1 year | 41 | 30 |
% reported potent Potency defined as the ability to achieve and maintain an erection suitable for sexual intercourse |
||
| Potency rate after preservation of one neurovascular bundle (%) | 1 year | 46 | 27 | ||||
| Potency rate after preservation of both neurovascular bundles (%) | 1 year | 53 | 44 | ||||
| Potency rate patients < 60 years with bilateral neurovascular preservation (%) | 1 year | 81 | 72 | ||||
| Joseph 200593 | % reporting spontaneous erections as assessed by interview | 3 months | 40 | 22 | |||
| Nadler 2010112 | Potency | 12 months | 8/22 (36.4) | 0/4 |
Analysis includes only patients potent at baseline, with bilateral nerve sparing and at least 12 months’ follow-up (27/50 robot, 34/50 open) Potency defined as score > 17 on SHIM |
||
| 18 months | 10/21 (47.6) | 3/6 (50.0) | |||||
| 24 months | 10/22 (45.5) | 11/17 (64.7) | |||||
| Satisfied with the outcome of surgery | |||||||
| Menon 200295 | Measure not reported |
Robotics: mean 1.5 months Laparoscopic: mean 6.5 months |
27/30 (90.0) | 38/40 (95.0) | |||
| Study | Subgroup | Timing | Robotic, n/N (%)a | Laparoscopic, n/N (%)a | Open, n/N (%)a | Notes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive margin | ||||||||||||||||||||||
| Anastasiadis 2003122 | 61/230 (26.5) | 20/70 (28.6) | ||||||||||||||||||||
| Artibani 2003123 | 21/71 (29.6) | 12/50 (24.0) | ||||||||||||||||||||
| Barocas 2010103 | 281/1413 (19.9) | 148/491 (30.1) | ||||||||||||||||||||
| Brown 2004125 | 10/59 (16.9) | 12/60 (20.0) | ||||||||||||||||||||
| Dahl 2006147 | 43/286 (15.0) | 124/714 (17.4) | ||||||||||||||||||||
| Doumerc 2010105 | Total | 45/212 (21.2) | 84/502 (16.7) | |||||||||||||||||||
| PT2 | 17/212 (8.0) | 33/502 (6.6) | ||||||||||||||||||||
| PT3 | 28/212 (13.2) | 51/502 (10.2) | ||||||||||||||||||||
| Drouin 2009101 | 12/71 (16.9) | 16/85 (18.8) | 15/83 (18.1) | |||||||||||||||||||
| Ficarra 2009106 | 35/103 (34.0) | 21/105 (20.0) | ||||||||||||||||||||
| Fornara 2004127 | 5/32 (15.6) | 7/32 (21.9) | ||||||||||||||||||||
| Fracalanza 2008107 | 10/35 (28.6) | 6/26 (23.1) | ||||||||||||||||||||
| Greco 2010129 | 12/150 (8.0) | 17/150 (11.3) | PT2a/b/c | |||||||||||||||||||
| Guazzoni 200690 | 16/60 (26.7) | 13/60 (21.7) |
RCT Positive surgical margin was considered as any ink on the specimen section regardless of pathological stage |
|||||||||||||||||||
| Jacobsen 2007130 | 22/67 (32.8) | 60/148 (40.5) | ||||||||||||||||||||
| Joseph 200794 | 99/754 (13.1) | 246/800 (30.8) | Abstract | |||||||||||||||||||
| Jurczok 2007131 | Total | 63/163 (38.7) | 104/240 (43.3) | % for pathological stage only reported in paper | ||||||||||||||||||
| T2 a/b/c | 16/163 (9.8) | 30/240 (12.5) | ||||||||||||||||||||
| T3 a/b | 47/163 (28.8) | 74/240 (30.8) | ||||||||||||||||||||
| Kim 2007132 | 11/30 (36.7) | 11/45 (24.4 | ||||||||||||||||||||
| Krambeck 2008108 | 46/294 (15.6) | 100/588 (17.0) | ||||||||||||||||||||
| Lama 2009133 | 16/56 (28.6) | 21/59 (35.6) | ||||||||||||||||||||
| Loeb 2010109 | 22/152 (14.5) | 25/137 (18.2) | ||||||||||||||||||||
| Martorana 2004134 | Total | 12/50 (24.0) | 13/50 (26.0) | |||||||||||||||||||
| T2 | 6/50 (12.0) | 5/50 (10.0) | ||||||||||||||||||||
| T3 | 6/50 (12.0) | 8/50 (16.0) | ||||||||||||||||||||
| Menon 200295 | 7/40 (17.5) | 10/40 (25.0) | ||||||||||||||||||||
| Nadler 2010112 | Total | 5/50 (10.0) | 12/50 (24.0) | |||||||||||||||||||
| PT2 | 2/43 (4.7) | 3/33 (9.1) | ||||||||||||||||||||
| PT3 | 3/7 (42.9) | 9/17 (52.9) | ||||||||||||||||||||
| Ou 2009113 | 15/30 (50.0) | 6/30 (20.0) | ||||||||||||||||||||
| Poulakis 2007137 |
Group I: 15/72 (20.8) Group II: 14/132 (10.6) |
16/70 (22.9) | Presence of tumour cells at the ink site of surgical specimen | |||||||||||||||||||
| Raventos Busquets 2007138 | 5.7% | 16.5% | The sum of the malignant . . . and malignant margin (unclear in translated version; Spanish paper) | |||||||||||||||||||
| Remzi 2005139 |
Transperitoneal: 10/39 (25.6) Extraperitoneal: 8/41 (19.5) |
8/41 (19.5) | ||||||||||||||||||||
| Rocco 2009114 | 26/120 (21.7) | 60/240 (25.0) | ||||||||||||||||||||
| Rozet 200796 | 26/133 (19.5) | 21/133 (15.8) | ||||||||||||||||||||
| Salomon 2002140 | 32/155 (20.6) | 30/151 (19.9) | ||||||||||||||||||||
| Schroeck 2008115 | 106/362 (29.3) | 122/435 (28.0) | ||||||||||||||||||||
| Silva 2007141 | 22/90 (24.4) | 37/89 (41.6) | ||||||||||||||||||||
| Soric 2004143 | 6/26 (23.1) | 3/26 (11.5) | ||||||||||||||||||||
| Sundaram 200497 | 2/10 (20.0) | 2/10 (20.0) | Abstract | |||||||||||||||||||
| Terakawa 2008144 | 54/137 (39.4) | 52/220 (23.6) | Presence of cancer at the inked margin of resection in the radical prostatectomy specimen | |||||||||||||||||||
| Tewari 2003116 | 18/200 (9.0) | 23/100 (23.0) | ||||||||||||||||||||
| Touijer 2007145 | Overall rate: 11.3% | Overall rate: 11% | Presence of cancer at the inked margin of resection in the radical prostatectomy specimen regardless of whether or not additional tissue was resected | |||||||||||||||||||
| Incidence of positive surgical margins over time, OR per 100 patients (95% CI) | Overall rate: 0.72 (0.56 to 0.89), p=0.003 | Overall rate: 1.06 (0.94 to 1.21), p = 0.3 | ||||||||||||||||||||
| Organ-confined disease: 0.60 (0.40 to 0.90), p = 0.01 | Organ-confined disease: 1.08 (0.80 to 1.46), p = 0.6 | |||||||||||||||||||||
| Non-organ-confined disease: 0.26 (0.06 to 1.05), p = 0.061 | Non-organ-confined disease: 1.39 (0.75 to 2.44), p = 0.3 | |||||||||||||||||||||
| Risk of positive surgical margins, OR (95% CI) | 1.156 (0.792 to 1.686) | Laparoscopic compared with open, adjusted for organ-confined probability (p = 0.5) | ||||||||||||||||||||
| Trabulsi 200898 | 3/50 (6.0) | 35/190 (18.4) | Used a whole-mount step section technique. Positive if tumour appeared at the inked margin | |||||||||||||||||||
| Wagner 2007146 | 7/75 (9.3) | 14/75 (18.7) | Extension of tumour to the inked surface of the resected specimen | |||||||||||||||||||
| White 2009118 | 11/50 (22.0) | 18/50 (36.0) | Presence of tumour tissue on the inked surface of the specimen | |||||||||||||||||||
| Pathology stage | ||||||||||||||||||||||
| Anastasiadis 2003122 | T2a | 165/230 (71.7) | 46/70 (65.7) | |||||||||||||||||||
| T3a | 38/230 (16.5) | 12/70 (17.1) | ||||||||||||||||||||
| T3b | 27/230 (11.7) | 12/70 (17.1) | ||||||||||||||||||||
| Artibani 2003123 | T2 | 42/71 (59.2) | 33/50 (66.0) | |||||||||||||||||||
| T3a | 18/71 (25.4) | 8/50 (16.0) | ||||||||||||||||||||
| T3b | 5/71 (7.0) | 5/50 (10.0) | ||||||||||||||||||||
| T4 | 4/71 (5.6) | 2/50 (4.0) | ||||||||||||||||||||
| N4 | 1/71 (1.4) | 2/50 (4.0) | ||||||||||||||||||||
| Ball 200699 | T2 | 58/82 (70.7) | 96/124 (77.4) | 86/135 (63.7) | ||||||||||||||||||
| T3/4 | 23/82 (28.0) | 26/124 (21.0) | 46/135 (34.1) | |||||||||||||||||||
| Unknown | 1/82 (1.2) | 2/124 (1.6) | 3/135 (2.2) | |||||||||||||||||||
| Barocas 2010103 | T0 | 7/1413 (0.5) | 3/491 (0.6) | |||||||||||||||||||
| T2 | 1136/1413 (80.4) | 342/491 (69.7) | ||||||||||||||||||||
| T3 | 268/1413 (19.0) | 144/491 (29.3) | ||||||||||||||||||||
| T4 | 0/1413 | 2/491 (0.4) | ||||||||||||||||||||
| Bhayani 2003124 | T0 | 0/33 | 1/24 (4.2) | |||||||||||||||||||
| T2 | 26/33 (78.8) | 14/24 (58.3) | ||||||||||||||||||||
| T3a | 6/33 (18.2) | 6/24 (25.0) | ||||||||||||||||||||
| T3b | 1/33 (3.0) | 3/24 (12.5) | ||||||||||||||||||||
| Brown 2004125 | T2a | 14/59 (23.7) | 13/60 (1.7) | |||||||||||||||||||
| T2b | 34/59 (57.6) | 39/60 (65.0) | ||||||||||||||||||||
| T3a | 8/59 (13.6) | 4/60 (6.7) | ||||||||||||||||||||
| T3b | 2/59 (3.4) | 3/60 (5.0) | ||||||||||||||||||||
| T4 | 1/59 (1.7) | 1/60 (1.7) | ||||||||||||||||||||
| Dahl 2006147 | Pathological stage for positive margins | |||||||||||||||||||||
| T0 | 0/0 | 8/714 (1.1) | T00/00/8T232/246 (13.0)77/583 (13.2)T311/40 (27.5)47/123 (38.2) | T0 | 0/0 | 0/8 | T2 | 32/246 (13.0) | 77/583 (13.2) | T3 | 11/40 (27.5) | 47/123 (38.2) | ||||||||||
| T0 | 0/0 | 0/8 | ||||||||||||||||||||
| T2 | 32/246 (13.0) | 77/583 (13.2) | ||||||||||||||||||||
| T3 | 11/40 (27.5) | 47/123 (38.2) | ||||||||||||||||||||
| T2 | 246/286 (86.0) | 583/714 (81.7) | ||||||||||||||||||||
| T3 | 40/286 (14.0) | 123/714 (17.2) | ||||||||||||||||||||
| Doumerc 2010105 | T2a | 18/212 (8.5) | 37/502 (7.4) | |||||||||||||||||||
| T2b | 12/212 (5.7) | 20/502 (4.0) | ||||||||||||||||||||
| T2c | 116/212 (54.7) | 268/502 (53.4) | ||||||||||||||||||||
| T3a | 55/212 (25.9) | 129/502 (25.7) | ||||||||||||||||||||
| T3b | 11/212 (5.2) | 48/502 (9.6) | ||||||||||||||||||||
| Drouin 2009101 | T2a | 3/71 (4.2) | 6/85 (7.1) | 5/83 (6.0) | ||||||||||||||||||
| T2b | 10/71 (14.1) | 6/85(7.1) | 5/83 (6.0) | |||||||||||||||||||
| T2c | 48/71 (67.6) | 58/85 (68.2) | 58/83 (69.9) | |||||||||||||||||||
| T3a | 9/71 (12.7) | 11/85 (12.9) | 13/83 (15.7) | |||||||||||||||||||
| T3b | 1/71 (1.4) | 4/85 (4.7) | 2/83 (2.4) | |||||||||||||||||||
| Ficarra 2009106 | T2 | 60/103 (58.3) | 49/105 (46.7) | |||||||||||||||||||
| T3a | 39/103 (37.9) | 42/105 (40.0) | ||||||||||||||||||||
| T3b | 4/103 (3.9) | 14/105 (13.3) | ||||||||||||||||||||
| Fornara 2004127 | T2a | 4/32 (12.5) | 4/32 (12.5) | |||||||||||||||||||
| T2b | 4/32 (12.5) | 2/32 (6.3) | ||||||||||||||||||||
| T2c | 23/32 (71.9) | 25/32 (78.1) | ||||||||||||||||||||
| T3a | 1/32 (3.1) | 1/32 (3.1) | ||||||||||||||||||||
| Fracalanza 2008107 | T2a | 4/35 (11.4) | 3/26 (11.5) | |||||||||||||||||||
| T2c | 19/35 (54.3) | 8/26 (30.8) | ||||||||||||||||||||
| T3a | 11/35 (31.4) | 11/26 (42.3) | ||||||||||||||||||||
| T3b | 1/35 (2.9) | 4/26 (15.4) | ||||||||||||||||||||
| Greco 2010129 | T2a | 120/150 (80.0) | 118/150 (78.7) | Laparoscopic T2a reported as 129/150. Contacted author to clarify if this is a typo and should be 120 (n = 159 otherwise) | ||||||||||||||||||
| T2b | 15/150 (10.0) | 17/150 (11.3) | ||||||||||||||||||||
| T2c | 12/150 (8.0) | 10/150 (6.7) | ||||||||||||||||||||
| T3a/3b | 3/150 (2.0) | 5/150 (3.3) | ||||||||||||||||||||
| Guazzoni 200690 | T2 | 45/60 (75.0) | 44/60 (73.3) | RCT | ||||||||||||||||||
| T3a | 12/60 (20.0) | 14/60 (23.3) | ||||||||||||||||||||
| T3b | 3/60 (5.0) | 2/60 (3.33) | ||||||||||||||||||||
| Jacobsen 2007130 | T0 | 1/67 (1.5) | 1/148 (0.7) | Numbers for open add to 144 but n = 148 – 4 not reported | ||||||||||||||||||
| T2a | 7/67 (10.4) | 16/148 (11.0) | ||||||||||||||||||||
| T2b | 1/67 (1.5) | 4/148 (2.7) | ||||||||||||||||||||
| T2c | 39/67 (58.2) | 78/148 (52.7) | ||||||||||||||||||||
| T3a | 6/67 (9.0) | 30/148 (20.3) | ||||||||||||||||||||
| T3b | 3/67 (4.5) | 15/148 (10.1) | ||||||||||||||||||||
| T4 | 0/67 | 0/148 | ||||||||||||||||||||
| Jurczok 2007131 | T2a | 26/162 (16.0) | 45/240 (18.8) | Percentages only reported in paper. Laparoscopic percentages add up to 99%. No mention of withdrawals. Figures total 162 instead of total 163 patients in group | ||||||||||||||||||
| T2b | 44/162 (27.2) | 53/240 (22.1) | ||||||||||||||||||||
| T2c | 38/162 (23.4) | 60/240 (25.0) | ||||||||||||||||||||
| T3a/b | 54/162 (33.3) | 82/240 (34.2) | ||||||||||||||||||||
| Kim 2007132 | T2 | 26/30 (86.7) | 36/45 (80.0) | Laparoscopic T2 reported as 16/30 (86.7%). Presumed 16 is an error and actual figure is 26/30 | ||||||||||||||||||
| T3 | 4/30 (13.3) | 5/45 (11.1) | ||||||||||||||||||||
| T4 | 0/30 | 4/45 (8.9) | ||||||||||||||||||||
| Martorana 2004134 | T2 | 31/50 (62.0) | 28/50 (56.0) | |||||||||||||||||||
| T3 | 19/50 (38.0) | 22/50 (44.0) | ||||||||||||||||||||
| Menon 200295 | T2a | 9/40 (22.5) | 7/40 (17.5) | |||||||||||||||||||
| T2b | 24/40 (60.0) | 30/40 (75.0) | ||||||||||||||||||||
| T3a | 4/40 (10.0) | 2/40 (5.0) | ||||||||||||||||||||
| T3b | 3/40 (7.5) | 0/40 | ||||||||||||||||||||
| T4a | 0/40 | 1/40 (2.5) | ||||||||||||||||||||
| Nadler 2010112 | T2 | 43/50 (86.0) | 33/50 (66.0) | |||||||||||||||||||
| T3 | 7/50 (14.0) | 17/50 (34.0) | ||||||||||||||||||||
| Namiki 2006136 | T2 | 53/64 (82.8) | 200/283 (70.7) | |||||||||||||||||||
| T3 | 11/64 (17.2) | 83/283 (29.0) | ||||||||||||||||||||
| Namiki 2005135 | T2 | 30/45 (66.7) | 103/121 (85.1) | |||||||||||||||||||
| T3 | 15/45 (33.3) | 17/121 (14.0) | ||||||||||||||||||||
| T4 | 0/45 | 1/121 (0.8) | ||||||||||||||||||||
| Poulakis 2007137 | Group I: | Group II: | Groups I and II two age groups (data not combined) | |||||||||||||||||||
| T2a | 3/72 (4.2) | 24/132 (18.2) | 4/70 (5.7) | |||||||||||||||||||
| T2b | 10/72 (13.9) | 28/132 (21.2) | 12/70 (17.1) | |||||||||||||||||||
| T2c | 27/72 (37.5) | 38/132 (28.8) | 24/70 (34.3) | |||||||||||||||||||
| T3a | 19/72 (26.4) | 26/132 (19.7) | 17/70 (24.3) | |||||||||||||||||||
| T3b | 13/72 (18.1) | 16/132 (12.1) | 13/70 (18.6) | |||||||||||||||||||
| Raventos Busquets 2007138 | T2 | 80% | 70.90% | Laparoscopic: n = 105; open: n = 75 | ||||||||||||||||||
| T3 | 20% | 29.10% | ||||||||||||||||||||
| Remzi 2005139 | Trans-peritoneal | Extra-peritoneal | ||||||||||||||||||||
| T2 | 24/39 (61.5) | 27/41 (65.9) | 26/41 (63.4) | |||||||||||||||||||
| T3 | 14/39 (35.9) | 14/41 (34.1) | 14/41 (34.1) | |||||||||||||||||||
| T4 | 1/39 (2.6) | 0 | 1/41 (2.4) | |||||||||||||||||||
| Rocco 2009114 | T2 | 88/120 (73.3) | 150/240 (62.5) | |||||||||||||||||||
| T3 | 29/120 (24.2) | 85/240 (35.4) | ||||||||||||||||||||
| T4 | 3/120 (2.5) | 5/240 (2.1) | ||||||||||||||||||||
| Rozet 200796 | T2a | 16/133 (12.0) | 11/133 (8.3) | |||||||||||||||||||
| T2b | 2/133 (1.5) | 6/133 (4.5) | ||||||||||||||||||||
| T2c | 92/133 (69.2) | 86/133 (64.7) | ||||||||||||||||||||
| T3a | 16/133 (12.0) | 22/133 (16.5) | ||||||||||||||||||||
| T3b | 7/133 (5.3) | 8/133 (6.0) | ||||||||||||||||||||
| Salomon 2002140 | Retropubic: | Figures presented in table 3 for perineal approach add to 100 instead of the 65 who received the procedure | ||||||||||||||||||||
| T2 | 126/155 (81.3) | 66/86 (76.7) | ||||||||||||||||||||
| T3a | 20/155 (12.9) | 13/86 (15.1) | ||||||||||||||||||||
| T3b | 9/155 (5.8) | 7/86 (8.2) | ||||||||||||||||||||
| Silva 2007141 | T2a | 9/90 (10.0) | 13/89 (14.6) | |||||||||||||||||||
| T2b | 11/90 (12.2) | 2/89 (2.2) | ||||||||||||||||||||
| T2c | 61/90 (67.8) | 61/89 (68.5) | ||||||||||||||||||||
| T3a | 1/90 (1.1) | 9/89 (10.1) | ||||||||||||||||||||
| T3b | 8/90 (8.9) | 4/89 (4.5) | ||||||||||||||||||||
| Soderdahl 2005142 | T0 | 1/93 (1.1) | 1/86 (1.2) | |||||||||||||||||||
| T2 | 73/93 (78.5) | 55/86 (64.0) | ||||||||||||||||||||
| T3/4 | 19/93 (20.4) | 30/86 (34.9) | ||||||||||||||||||||
| Soric 2004143 | T1 | 9/26 (34.6) | 6/26 (23.1) | |||||||||||||||||||
| T2 | 9/26 (34.6) | 14/26 (53.8) | ||||||||||||||||||||
| T3 | 6/26 (23.1) | 5/26 (19.2) | ||||||||||||||||||||
| Terakawa 2008144 | T2 | 106/137 (77.4) | 139/220 (63) | |||||||||||||||||||
| T3 | 31/137 (22.6) | 81/220 (36.8) | ||||||||||||||||||||
| Tewari 2003116 | T2a | 30/200 (15.0) | 18/100 (18.0) | |||||||||||||||||||
| T2b | 144/200 (72.0) | 75/100 (75.0) | ||||||||||||||||||||
| T3a | 14/200 (7.0) | 4/100 (4.0) | ||||||||||||||||||||
| T3b | 12/200 (6.0) | 3/100 (3.0) | ||||||||||||||||||||
| Touijer 2007145 | T0 | 3/485 (0.6) | 8/692 (1.2) | |||||||||||||||||||
| T1 | 29/485 (6.0) | 25/692 (3.6) | ||||||||||||||||||||
| T2a | 65/485 (13.4) | 89/692 (12.9) | ||||||||||||||||||||
| T2b | 261/485 (53.8) | 355/692 (51.3) | ||||||||||||||||||||
| T3a | 105/485 (21.6) | 170/692 (24.6) | ||||||||||||||||||||
| T3b | 17/485 (3.5) | 35/692 (5.1) | ||||||||||||||||||||
| T4 | 5/485 (1.0) | 10/692 (1.4) | ||||||||||||||||||||
| Trabulsi 200898 | T0 | 0/50 | 1/190 (0.5) | |||||||||||||||||||
| T2a | 12/50 (24.0) | 40/190 (21.1) | ||||||||||||||||||||
| T2b | 0/50 | 2/190 (1.1) | ||||||||||||||||||||
| T2c | 31/50 (62.0) | 119/190 (62.6) | ||||||||||||||||||||
| T3a | 5/50 (10.0) | 12/190 (6.3) | ||||||||||||||||||||
| T3b | 2/50 (4.0) | 6/190 (3.2) | ||||||||||||||||||||
| T4 | 0/50 | 10/190 (5.3) | ||||||||||||||||||||
| Truesdale 2010117 | T2 | 71/99 (71.7) | 136/217 (62.7) | % do not match those reported in paper | ||||||||||||||||||
| T3 | 23/99 (23.2) | 70/217 (32.3) | ||||||||||||||||||||
| T4 | 4/99 (4.0) | 7/217 (3.2) | ||||||||||||||||||||
| Wagner 2007146 | T0 | 1/75 (1.3) | 1/75 (1.3) | |||||||||||||||||||
| T2 | 67/75 (89.3) | 52/75 (69.5) | ||||||||||||||||||||
| T3 | 7/75 (9.3) | 21/75 (28.0) | ||||||||||||||||||||
| T4 | 0/75 | 1/75 (1.3) | ||||||||||||||||||||
| White 2009118 | T2a | 12/50 (24.0) | 12/50 (24.0) | |||||||||||||||||||
| T2c | 35/50 (70.0) | 35/50 (70.0) | ||||||||||||||||||||
| T3a | 3/50 (6.0) | 3/50 (6.0) | ||||||||||||||||||||
| Pathological Gleason score | ||||||||||||||||||||||
| Anastasiadis 2003122 | 6.7, 1.1 (4–10) | 6.9, 0.9 (5–10) | Mean, SD (range) | |||||||||||||||||||
| Artibani 2003123 | 6.4 (1.3) | 6.3 (0.9) | Mean (SD) | |||||||||||||||||||
| Barocas 2010103 | ≤ 6 | 723/1413 (51.2) | 221/491 (45.0) | |||||||||||||||||||
| 7 | 588/1413 (41.6) | 213/491 (43.4) | ||||||||||||||||||||
| 8–10 | 94/1413 (6.7) | 54/491 (11.0) | ||||||||||||||||||||
| Dahl 2006147 | ≤ 6 | 45/212 (21.2) | 76/502 (15.2) | Biopsy Gleason score for positive margins00/00/85–620/192 (10.4)60/452 (13.3)717/78 (21.8)48/199 (24.1)8–96/16 (7.5)16/55 (29.1) | Biopsy Gleason score for positive margins | 0 | 0/0 | 0/8 | 5–6 | 20/192 (10.4) | 60/452 (13.3) | 7 | 17/78 (21.8) | 48/199 (24.1) | 8–9 | 6/16 (7.5) | 16/55 (29.1) | |||||
| Biopsy Gleason score for positive margins | ||||||||||||||||||||||
| 0 | 0/0 | 0/8 | ||||||||||||||||||||
| 5–6 | 20/192 (10.4) | 60/452 (13.3) | ||||||||||||||||||||
| 7 | 17/78 (21.8) | 48/199 (24.1) | ||||||||||||||||||||
| 8–9 | 6/16 (7.5) | 16/55 (29.1) | ||||||||||||||||||||
| 7 | 149/212 (70.3) | 357/502 (71) | ||||||||||||||||||||
| 8–10 | 18/212 (8.5) | 69/502 (13.7) | ||||||||||||||||||||
| Doumerc 2010105 | ≤ 6 | 45/212 (21.2) | 76/502 (15.2) | |||||||||||||||||||
| 7 | 149/212 (70.3) | 357/502 (71) | ||||||||||||||||||||
| 8–10 | 18/212 (8.5) | 69/502 (13.7) | ||||||||||||||||||||
| Fornara 2004127 | 6.4 | 5.7 | Median | |||||||||||||||||||
| Jacobsen 2007130 | First half = 6.7 (0.61), Second half = 6.6 (0.74) | 6.6 (0.9) | Mean (SD) | |||||||||||||||||||
| Joseph 200794 | 6.5 (4–10) | 6.9 (6–10) |
Abstract Mean (range) |
|||||||||||||||||||
| Jurczok 2007131 | 6.4 | 5.7 | Median | |||||||||||||||||||
| Kim 2007132 | 6.6 (0.8) | 6.6 (0.7) | Mean (SD) | |||||||||||||||||||
| Krambeck 2008108 | ≤ 6 | 192/294 (65.3) | 391/588 (66.5) | |||||||||||||||||||
| 7 | 87/294 (29.6) | 167/588 (28.4) | ||||||||||||||||||||
| 8–10 | 14/294 (4.8) | 30/588 (5.1) | ||||||||||||||||||||
| Martorana 2004134 | 6.10 (0.91) | 6.16 (0.71) | Median (SD) | |||||||||||||||||||
| Menon 200295 | 6.8 (0.82) | 6.8 (0.82) | Mean (SD) | |||||||||||||||||||
| Namiki 2005135 | 6 | 19/45 (42) | 48/121 (39.7) | |||||||||||||||||||
| 7 | 26/45 (58) | 73/121 (60.3) | ||||||||||||||||||||
| Namiki 2006136 | ≤ 6 | 20/64 (31.3) | 65/283 (23.0) | |||||||||||||||||||
| ≥ 7 | 44/64 (68.8) | 218/283 (77.0) | ||||||||||||||||||||
| Ou 2009113 | 7.2 (1.1) | 6.7 (1.6) | Mean (SD) | |||||||||||||||||||
| Poulakis 2007137 |
Group I: 7 (5–9) Group II: 6 (5–9) |
7 (5–9) | Median (range). Groups I and II two age groups (data not combined) | |||||||||||||||||||
| Remzi 2005139 |
Transperitoneal: 5.1 (2.0) Extraperitoneal: 5.5 (1.9) |
4.7 (2.2) | Mean (SD) | |||||||||||||||||||
| Rocco 2009114 | 7 (4–9) | 7 (3–9) | Median (range) | |||||||||||||||||||
| Rozet 200796 | 6.5 (5–9) | 6.5 (5–9) | Mean (range) | |||||||||||||||||||
| Salomon 2002140 | 6.6 (4–10) |
Retropubic: 6.2 (3–10) Perineal: 6.1 (4–9) |
Median (range) | |||||||||||||||||||
| Schroeck 2008115 | ≤ 6 | 168/362 (46.4) | 177/435 (40.7) | |||||||||||||||||||
| 7 | 176/362 (48.6) | 199/435 (45.7) | ||||||||||||||||||||
| 8–10 | 18/362 (4.9) | 59/435 (13.6) | ||||||||||||||||||||
| Silva 2007141 | 7 | 7 | Median | |||||||||||||||||||
| Soric 2004143 | 6.25 (4–9) | 5.7 (4–7) | Median (range) | |||||||||||||||||||
| Tewari 2003116 | ≤ 6 | 87/200 (43.5) | 42/100 (42.0) | |||||||||||||||||||
| 7 | 80/200 (40.0) | 38/100 (38.0) | ||||||||||||||||||||
| 8–10 | 21/200 (10.5) | 20/100 (20.0) | ||||||||||||||||||||
| Touijer 2007145 | ≤ 6 | 184/485 (38.0) | 280/692 (40.5) | |||||||||||||||||||
| 7 | 270/485 (55.7) | 349/692 (50.4) | ||||||||||||||||||||
| 8–10 | 25/485 (5.2) | 56/692 (8.1) | ||||||||||||||||||||
| Missing | 6/485 (1.2) | 7/692 (1.0) | ||||||||||||||||||||
| Trabulsi 200898 | ≤ 6 | 33/50 (66.0) | 109/190 (57.4) | |||||||||||||||||||
| 7 | 15/50 (30.0) | 67/190 (35.3) | ||||||||||||||||||||
| ≥ 8 | 2/50 (4.0) | 8/190 (4.2) | ||||||||||||||||||||
| Truesdale117 | ≤ 6 | 14/99 14.1) | 26/217 (12.0) | |||||||||||||||||||
| 7 | 71/99 (71.7) | 135/217 (62.2) | ||||||||||||||||||||
| 8–10 | 14/99 (14.1) | 56/217 (25.8) | ||||||||||||||||||||
| White 2009118 | ≤ 6 | 25/50 (50.0) | 35/50 (70.0) | |||||||||||||||||||
| 7 | 24/50 (48.0) | 15/50 (30.0) | ||||||||||||||||||||
| 8–10 | 1/50 (2.0) | 0/50 | ||||||||||||||||||||
| PSA recurrence | ||||||||||||||||||||||
| Definition | ||||||||||||||||||||||
| Artibani 2003123 |
A: mean 10 (range 4–16) months B: mean 10 (range 4–18) months |
12/63 (19.0) | 5/44 (11.4) | PSA > 0.3 ng/ml | ||||||||||||||||||
| Barocas 2010103 | 3 years postoperatively | 181/425 (42.6) | 155/257 (60.3) | PSA > 0.2 ng/ml on one or more assays, or when a patient received postoperative hormone therapy, radiation or chemotherapy in the face of an increasing PSA | ||||||||||||||||||
| Drouin 2009101 | Mean 49.7 (range 18–103) months | 7/71 (9.9) | 10/85 (11.8) | 12/83 (14.5) | A single measure of PSA > 0.2 ng/ml | |||||||||||||||||
| Krambeck 2008108 | Median 1.3 years | 14/248 (5.6) | 32/492 (6.5) | PSA progression (no definition) | ||||||||||||||||||
| Lama 2009133 | 6 months | 6/56 (10.7) | 6/59 (10.2) | Biochemical relapse (no definition) | ||||||||||||||||||
| 1 year | 6/56 (10.7) | 7/59 (11.9) | ||||||||||||||||||||
| 2 years | 6/56 (10.7) | 9/59 (15.2) | ||||||||||||||||||||
| 3 years | 11/56 (19.6) | 12/59 (20.3) | ||||||||||||||||||||
| Loeb 2010109 | Not reported | 14/266 men with follow-up data had PSA > 0.2 ng/ml | ||||||||||||||||||||
| Menon 200295 | 38/40 (95.0) | 39/40 (97.5) | Undetectable postoperative PSA | |||||||||||||||||||
| Nadler 2010112 | During 27.1 months of follow-up | 4/50 (8.0) | 3/50 (6.0) | During 27.1 months of follow-up 92% and 94% reported undetectable PSA defined as PSA ≤ 0.1 ng/ml | ||||||||||||||||||
| Ou 2009113 | 15 months | 6/30 (20.0) | 5/30 (16.7) | Two consecutive postoperative PSA > 0.2 ng/ml | ||||||||||||||||||
| Poulakis 2007137 | 6 months |
Group I: 10/72 (13.9) Group II: 7/132 (5.3) |
11/70 (15.7) | PSA ≥ 0.1 ng/ml. Groups I and II two age groups (data not combined) | ||||||||||||||||||
| Salomon 2002140 | 3-year actuarial PSA recurrence-free rate | 86.2% |
Retropubic: 89.3% Perineal: 89.2% |
|||||||||||||||||||
| Schroeck 2008115 |
A: mean 1.09 years B: mean 1.37 years |
29/362 (8.0) | 54/435 (12.4) | Adjusted hazard ratio for risk of PSA recurrence and p-values reported in paper | ||||||||||||||||||
| Tewari 2003116 |
A: mean 236 days B: mean 556 days |
16/200 (8.0) | 15/100 (15.0) | > 0.2 ng/ml (converted from undetectable PSA% data) | ||||||||||||||||||
| Local recurrence | ||||||||||||||||||||||
| Krambeck 2009108 | Median 1.3 years | 3/248 (1.2) | 5/492 (1.0) | |||||||||||||||||||
| Metastatic recurrence | ||||||||||||||||||||||
| Krambeck 2009108 | Median 1.3 years | 1/248 (0.4) | 0/492 | Reported as ‘systematic progression’ | ||||||||||||||||||
| Study | Treatment/outcome | Timing/duration of follow-up | Robotic, n/N (%)a | Laparoscopic, n/N (%)a | Open, n/N (%)a |
|---|---|---|---|---|---|
| Further cancer treatment | |||||
| Dahl 2009126 | Radiation | 12 months | 3/104 | 0/102 | |
| Androgen deprivation | 1/104 | 2/102 | |||
| Both radiation and androgen deprivation | 1/104 | 0/102 | |||
| Subtotal: 5/104 (4.8) | Subtotal: 2/102 (2.0) | ||||
| Treatment of urinary incontinence | |||||
| Carlsson 2010104 | 30 days–15 months | 7/1253 (0.6) | 11/485 (2.3) | ||
| Treatment of erectile dysfunction | |||||
| No studies reported data on this outcome | |||||
| Treatment of faecal incontinence | |||||
| No studies reported data on this outcome | |||||
| Death, specify reasons | |||||
| Carlsson 2010104 | < 30 days postoperatively | 0/1253 | 1/485 (0.2) | ||
| Doumerc 2010105 | Death from cerebral vascular accident | 0/212 | 1/502 (0.2) | ||
| Drouin 2009101 | Pulmonary embolism | 5 years | 0/71 | 0/85 | 1/83 (1.2) |
| Hu 200692 | Not reported | 0/322 | 0/358 | ||
| Krambeck 2008108 | Death from prostate cancer | Median 1.3 years | 0/248 | 0/492 | |
| Death from any cause | 4/248 (1.6) | 4/492 (0.8) | |||
| Menon 200295 |
Robotic: mean 3 (SD 1.3) months Laparoscopic: mean 8.5 (SD 3.2) months |
0/40 | 0/40 | ||
| Poulakis 2007137 |
Group I: 0/72 Group II: 0/132 |
0/70 | |||
| Rozet 200796 | Not reported | 0/133 | 0/133 | ||
| Salomon 2002140 | Pulmonary embolism | First day post operation | 1/155 (0.6) | 0/151 | |
| Tewari 2003116 |
A: mean 236 days B: mean 556 days |
0/200 0/200 |
0/100 0/100 |
||
| Study | Measures | Timing | Robotic | Laparoscopic | Open | Notes, e.g. validated measure or not | ||
|---|---|---|---|---|---|---|---|---|
| Guazzoni 2006 90 | Postoperative pain, mean (SD) | Recovery room | 1.88 (1.31) | 1.92 (1.08) |
RCT Pain assessed with the use of a validated 10-point VAS for pain (0 = no pain, 10 = worst possible pain) |
|||
| 3 hours | 1.92 (1.46) | 2.75 (1.99) | ||||||
| Day 1 | 1.7 (1.45) | 2.65 (1.44) | ||||||
| Day 2 | 1.61 (0.9) | 1.96 (1.2) | ||||||
| Day 3 | 1.03 (0.82) | 1.53 (1.13) | ||||||
| Pain at discharge | Not reported | 2/60 (3.3) | ||||||
| Jacobsen 2007130 | I-PSS quality-of-life question (patient asked how he feels about tolerating his current level of urinary symptoms for the rest of his life: 0, mildly to 6, terrible), mean (SD) | Baseline | First half: 1.9 (1.8) (n not reported); Second half: 1.4 (1.2) (n not reported) | 1.6 (1.6) (n = 172) | ||||
| 1 year | First half: 1.9 (1.4) (n = 29); Second half: 1.9 (1.2) (n = 28) | 1.5 (1.4) (n = 148) | ||||||
| Miller 2007111 | SF-12 v.2 Physical and Mental Health Survey Acute Form | Validated tool, scale not reported | ||||||
| Mental component score, mean (SD) | Preoperatively | 49.8 (6.2) | 45.7 (9.8) | |||||
| 6 weeks | 57.4 (4.3) | 58.0 (4.7) | ||||||
| Physical component score, mean (SD) | Preoperatively | 57.6 (2.4) | 56.9 (6.0) | |||||
| 6 weeks | 56.4 (1.7) | 52.8 (4.7) | ||||||
| Namiki 2005135 | SF-36 | |||||||
| Physical function, mean (SD) | Baseline | 88.9 (11.8) | 88.9 (11.4) | |||||
| 1 month | 84.0 (15.8) | 85.5 (13.4) | ||||||
| 3 months | 88.7 (11.5) | 88.7 (9.2) | ||||||
| 6 months | 89.2 (11.1) | 87.4 (12.8) | ||||||
| 12 months | 87.8 (12.9) | 89.5 (11.0) | ||||||
| Role limitation, physical, mean (SD) | Baseline | 77.1 (27.2) | 83.3 (23.3) | |||||
| 1 month | 67.1 (29.9) | 73.2 (29.7) | ||||||
| 3 months | 75.2 (25.3) | 79.1 (23.6) | ||||||
| 6 months | 85.0 (18.7) | 83.2 (23.4) | ||||||
| 12 months | 82.4 (25.0) | 86.2 (22.0) | ||||||
| Bodily pain, mean (SD) | Baseline | 82.0 (21.2) | 84.6 (18.7) | |||||
| 1 month | 74.5 (22.6) | 71.2 (20.9) | ||||||
| 3 months | 82.3 (19.5) | 80.9 (19.8) | ||||||
| 6 months | 82.7 (21.9) | 86.0 (16.8) | ||||||
| 12 months | 84.2 (17.9) | 85.9 (17.1) | ||||||
| General health perception, mean (SD) | Baseline | 60.3 (17.3) | 60.9 (14.4) | |||||
| 1 month | 54.9 (16.6) | 57.3 (12.2) | ||||||
| 3 months | 61.3 (14.9) | 61.6 (16.1) | ||||||
| 6 months | 59.8 (13.3) | 64.0 (15.2) | ||||||
| 12 months | 61.0 (19.0) | 64.5 (16.4) | ||||||
| Mental health, mean (SD) | Baseline | 71.5 (16.4) | 69.1 (20.9) | |||||
| 1 month | 63.5 (13.2) | 68.7 (17.8) | ||||||
| 3 months | 70.9 (18.7) | 73.8 (20.4) | ||||||
| 6 months | 74.6 (16.1) | 75.9 (21.8) | ||||||
| 12 months | 75.1 (18.6) | 77.8 (18.6) | ||||||
| Role limitation, emotional, mean (SD) | Baseline | 78.2 (26.4) | 80.5 (22.9) | |||||
| 1 month | 66.7 (27.9) | 72.2 (26.9) | ||||||
| 3 months | 76.1 (27.0) | 77.9 (24.0) | ||||||
| 6 months | 82.3 (21.6) | 84.3 (20.4) | ||||||
| 12 months | 83.1 (22.3) | 86.6 (22.3) | ||||||
| Social function, emotional, mean (SD) | Baseline | 77.3 (22.3) | 80.9 (23.1) | |||||
| 1 month | 60.6 (28.1) | 76.6 (25.2) | ||||||
| 3 months | 74.7 (22.7) | 81.5 (22.3) | ||||||
| 6 months | 79.2 (25.2) | 85.6 (19.6) | ||||||
| 12 months | 84.3 (19.6) | 88.3 (19.9) | ||||||
| Vitality, mean (SD) | Baseline | 68.0 (17.0) | 68.7 (19.3) | |||||
| 1 month | 61.5 (17.6) | 63.3 (16.2) | ||||||
| 3 months | 67.0 (18.3) | 71.3 (22.4) | ||||||
| 6 months | 72.3 (13.8) | 71.5 (17.4) | ||||||
| 12 months | 70.7 (14.6) | 72.4 (19.0) | ||||||
| Namiki 2006136 | SF-36 | Retropubic | Perineal | |||||
| Physical function, mean (SD) | Baseline | 90.5 (10.6) | 86.9 (11.8) | 86.6 (14.0) | ||||
| 1 month | 89.6 (8.3) | 83.8 (16.8) | 84.3 (12.6) | |||||
| 3 months | 91.2 (8.5) | 85.7 (15.6) | 84.2 (13.7) | |||||
| 6 months | 90.5 (9.3) | 88.2 (16.7) | 82.6 (12.9) | |||||
| 12 months | 89.1 (9.0) | 87.0 (13.4) | 86.0 (14.0) | |||||
| Role limitation, physical, mean (SD) | Baseline | 83.4 (16.1) | 83.1 (22.7) | 80.8 (24.3) | ||||
| 1 month | 67.7 (25.3) | 61.8 (25.0) | 66.1 (23.2) | |||||
| 3 months | 77.4 (22.6) | 74.9 (23.6) | 72.7 (31.4) | |||||
| 6 months | 83.9(19.6) | 80.6 (21.8) | 80.1 (26.2) | |||||
| 12 months | 82.3 (24.4) | 83.2 (20.3) | 75.4 (27.1) | |||||
| Bodily pain, mean (SD) | Baseline | 87.9 (16.5) | 85.2 (20.1) | 80.7 (22.5) | ||||
| 1 month | 66.1 (22.3) | 66.1 (23.0) | 74.5 (23.2) | |||||
| 3 months | 87.4 (15.2) | 77.2 (20.7) | 77.0 (25.9) | |||||
| 6 months | 88.8 (16.6) | 84.1 (19.1) | 82.3 (24.9) | |||||
| 12 months | 88.9 (21.8) | 86.6 (18.1) | 75.8 (25.2) | |||||
| General health perception, mean (SD) | Baseline | 64.9 (14.7) | 57.4 (16.3) | 62.3 (16.3) | ||||
| 1 month | 50.4 (14.5) | 58.9 (16.5) | 61.3 (15.9) | |||||
| 3 months | 63.8 (16.4) | 58.9 (16.2) | 56.6 (17.1) | |||||
| 6 months | 63.6 (14.6) | 61.4 (16.3) | 60.4 (18.2) | |||||
| 12 months | 56.3 (14.5) | 61.1 (17.0) | 57.3 (20.2) | |||||
| Mental health, mean (SD) | Baseline | 68.9 (16.7) | 68.9 (16.7) | 72.3 (20.9) | ||||
| 1 month | 58.6 (20.3) | 58.6 (20.3) | 71.5 (25.4) | |||||
| 3 months | 75.7 (15.4) | 75.7 (15.4) | 66.1 (20.0) | |||||
| 6 months | 75.7 (15.2) | 75.7 (15.2) | 74.8 (18.1) | |||||
| 12 months | 71.7 (17.2) | 71.7 (17.2) | 72.5 (20.0) | |||||
| Role limitation, emotional, mean (SD) | Baseline | 86.7 (16.9) | 81.9 (22.6) | 78.4 (25.5) | ||||
| 1 month | 70.6 (20.8) | 65.4 (28.9) | 66.7 (26.3) | |||||
| Study | Robotic | Laparoscopic | Open | Reported outcomes/measures | Other information | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. surgeons | No. procedures conducted before this study | No. procedures conducted during this study | Learning curve outcome | Other information | No. surgeons | No. procedures conducted before this study | No. procedures conducted during this study | Learning curve outcome | Other information | No. surgeons | No. procedures conducted before this study | No. procedures conducted during this study | Learning curve outcome | Other information | |||
| Al-Shaiji 2010121 | 2/5 attending urologists | 70 | 3/5 attending urologists | 70 | Safety (blood loss, operating time, hospital stay) | ||||||||||||
| Anastasiadis 2003122 | 230 | 70 |
Safety (catheterisation, surgical complications) Efficacy (margins, pT stage, pathological Gleason score) Dysfunction (urinary continence) |
Laparoscopic and robotic radical prostatectomy performed by different surgeons with a high level of experience in their preferred technique | |||||||||||||
| Artibani 2003123 | 1 | > 60 | 71 | 1 | Experienced | 50 |
Safety (hospital stay, catheterisation, surgical complications) Efficacy (margin, pT stage, pathological Gleason score, PSA recurrence) Dysfunction (urinary incontinence, erectile) |
||||||||||
| Ball 200699 | 2 | 82 in total | Completed robotic training and proctoring | 2 | 124 in total | 3 | 135 in total | All fellowship-trained oncological surgeons |
Efficacy (pT stage) Dysfunction (urinary incontinence, erectile) |
||||||||
| Barocas 2010103 | 4 | 1413 | 4 | 491 | Efficacy (margins, pT stage, pathological Gleason score, PSA recurrence | ||||||||||||
| Bhayani 2003124 | 2 | 36 | 2 | 24 |
Safety (open conversion, operating time, hospital stay, surgical complications, catheterisation, blood loss) Efficacy (pT stage) |
Same two fellowship-trained surgeons in their first year of practice with comparable experience and training | |||||||||||
| Bolenz 2009102 (secondary to Bolenz 2010100) | NR | NR | 264 | NR | NR | 220 | NR | NR | 162 | Safety (operating time, hospital stay) | |||||||
| Bolenz 2010100 | 2 | 262 | A learning curve was included in robort-assisted laparoscopic prostatectomy patients, but between the 50 patients initially operated and the most recently treated 50 patients there was no significant difference in median operative time and median length of hospital stay | 1 | 211 | 3 | 156 | Performed by experienced surgeons after their learning curve in robotic and laporascopic radical prostataectomy procedures | Safety (blood transfusion) | ||||||||
| Brown 2004125 | NR | 0 | 60 | Operating time (minutes), mean: 1–10: 456; 11–20: 402; 21–30: 384; 31–60: 306 | NR | NR | 60 |
Safety (operating time, hospital stay, readmission, surgical complications) Efficacy (margins, pT stage) Learning curve (operating time) |
Procedures performed by or under the direction of two staff surgeons (different surgeons for each procedure) | ||||||||
| Carlsson 2010104 | 6 | I: 451; II: 444; III: 181; IV: 112; V: 35; VI: 30 | 9 (6 also performed robot) | I: > 250; II: > 250; III: < 7; IV: < 7; V: > 100; VI: > 250 | 485 in total | Safety (surgical complications) | |||||||||||
| Chan 2008119 | 2 | 660 in total | Operating time (minutes): 63–483 |
I: performed both; II: robotics only ‘experienced’ |
3 | 340 in total | Operating time (minutes): 82–245 |
III and IV: open only ‘experienced’ |
Safety (open conversion, operating time, hospital stay) Learning curve |
||||||||
| Dahl 2009126 | 1/3 | 104 | 1/3 | 102 |
Safety (surgical complications) Dysfunction (urinary incontinence, erectile) Further treatment |
1/3 experienced surgeons | |||||||||||
| Dahl 2006147 (secondary to Dahl 2009126) | 1 | 1 | 286 | 1/5 | 714 | Open surgery performed by five experienced urologists in the same department | Efficacy (margins, pT stage, pathological Gleason score) | ||||||||||
| Doumerc 2010105 | 1 | 212 | 1 | > 2000 | 502 |
Safety (surgical complications, operating time, hospital stay, catheterisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score) Dysfunction and learning curve data in graph form only |
Surgeries were performed by one experienced surgeon. Surgeon had performed > 2000 RRPs cases Learning curve was based on the number of cases needed to achieve competency in each of the following areas: console time, pathological outcome (over all pT2 and pT3 positive surgical margin rates) and early continence, i.e. 6 weeks Learning curve analysed by positive surgical margin rates and the EPIC score (%) at 6 weeks |
||||||||||
| Drouin 2009101 | 1 | 3 | 3 |
Safety (surgical complications, open conversion, operating time, catheterisation, blood loss) Efficacy (margins, pT stage, PSA recurrence) Death |
|||||||||||||
| Ficarra 2009106 | 2 | > 50/surgeon | 103 in total | 4 | > 400/surgeon | 105 in total |
Safety (surgical complications, operating time, hospital stay, catherisation, blood loss) Efficacy (margins, pT stage) Dysfunction (urinary incontinence, erectile) |
||||||||||
| Fornara 2004127 | 32 | 32 |
Safety (surgical complications, operating time, hospital stay, catheterisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score) |
German | |||||||||||||
| Fracalanza 2008107 | 1 | > 50 | 35 in total | Time (minutes), mean (SD): 195.6 (45) | 3 | > 200 | 26 in total | Time (minutes), mean (SD): 127.2 (31.7) |
Safety (surgical complications, operating time, hospital stay, blood loss, surgical incision, time to mobilisation, oral feeding) Efficacy (margins, pT stage) Learning curve |
‘experienced’ | |||||||
| Ghavamian 2006128 | 1 | 60 | 70 | First 60 cases not included in the comparison | 1 | > 300 |
Safety (open conversion, surgical complications, operating time, hospital stay, blood loss) Dysfunction (urinary incontinence, erectile) |
Same surgeon for both procedures with > 7 years practice at a major metropolitan academic university hospital | |||||||||
| Gosseine 200991 | 1 | 122 | 1 | 125 |
Safety (surgical complications, operating time, hospital stay, catheterisation, blood loss) Dysfunction (urinary incontinence) |
Performed by the same surgeon at the beginning of his experience (French) | |||||||||||
| Greco 2010129 | 2 | At least 60 nerve-sparing and 150 laparo-scopic radical prostatec-tomies | 150 | 2 | At least 60 nerve-sparing and 150 open prostatec-tomies |
Safety (open conversion, surgical complications, operating time, catheterisation, blood loss) Efficacy (margins, pT stage) Dysfunction (urinary incontinence, erectile) |
All surgical procedures performed by two surgeons | ||||||||||
| Guazzoni 200690 (RCT) | 1 | > 150 | 60 | 1 | Performed radical retropubic prostatec-tomies for 15 years prior to study | 60 |
Safety (open conversion, surgical complications, operating time, discharge time, catheterisation, blood loss, mobilisation, oral feeding) Efficacy (margins, pT stage) Quality of life (pain) |
Single surgeon (‘senior urologist’) not under learning curve, started general laparoscopic experience 12 years before the study and in particular laparoscopic radical prostatectomies in 1990 | |||||||||
| Hu 200692 | 3 | I: 126; II: 144; III: 52 | Time (minutes), median (range): 186 (114–528) | Same 3 | I: 167; II: 124; III: 65 | Time (minutes), median (range): 246 (150–768) |
Equipment failure (presume this is not learning curve dependent) Safety (surgical complications, operating time, blood loss) Learning curve?? (operating time) Death (none) |
||||||||||
| Jacobsen 2007130 | 10 | 0 | 67 in total | Same 10 | 172 in total |
Efficacy (margins, pT stage, pathological Gleason score) Dysfunction (urinary incontinence) Quality of life |
|||||||||||
| Joseph 200593 (linked to Joseph 200794) | NR | 150 | 50 (cases 151–200) | NR | 28 | 50 (cases 29–78) | Laparoscopic radical prostatectomy-experienced surgeons with assistants generally untrained in laparoscopic radical prostatectomy. Laparoscopic series completed first. University of Rochester Medical Centre | Dysfunction (urinary incontinence, erectile, potency) | |||||||||
| Joseph 200794 | NR | NR | 754 | University of Rochester Medical Centre | NR | NR | 800 | Henry Mondor Hospital | Efficacy (margins, pathological Gleason score) | Abstract | |||||||
| Jurczok 2007131 | 3 | 163 | 3 | 240 |
Safety (open conversion, surgical complications, operating time, hospital stay, catheterisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score) |
Performed by three experienced surgeons with no difference between the operative results of each | |||||||||||
| Kim 2007132 | 30 | 45 |
Safety (surgical complications, operating time, hospital stay, catheterisation) Efficacy (margins, pT stage, pathological Gleason score) |
Korean | |||||||||||||
| Kordan 2010120 (secondary to Barocas 2010103) | 2/4 | NR | 830 | 3/4 | NR | 414 | Safety (blood transfusion, blood loss) | One surgeon performed both robotic radical prostatectomy and robot-assisted laparoscopic prostatectomy | |||||||||
| Krambeck 2009108 | 3 | 294 | Time (minutes), median (25th–75th percentile): early: n = 94, 295 (248–357); middle: n = 100, 235 (201–268); late: n = 100, 211 (186–236) | 17 | 588 | Time (minutes), median (25th–75th percentile): early: n = 188, 190 (158–245); middle: n = 200, 206 (162–268); late: n = 200, 228 (169–288) |
Safety (surgical complications, operating time, hospital stay) Efficacy (margins, pathological Gleason score, PSA recurrence, local recurrence, metastatic recurrence) Dysfunction (urinary incontinence, erectile) Death Learning curve (operating time) |
||||||||||
| Lama 2009133 | 1 | 0 | 56 | Time (minutes), mean (SD): 202.5 (52.1) | Laparoscopic radical prostatectomy performed by a urologist trained in laparoscopy whose learning curve was completed for open prostatectomy | NR | NR | 59 |
Safety (surgical complications, operating time, hospital stay, catheterisation) Efficacy (margins, PSA recurrence) Dysfunction (urinary incontinence, erectile) Learning curve (operating time) |
RRP completed learning curve | |||||||
| Loeb 2010109 | 1 | 152 | 1 | > 1000 open | 137 | Efficacy (margins, PSA recurrence) | Single surgeon | ||||||||||
| Malcolm 2010110 | 1 | 447 | Robotic: performed by one of three fellowship-trained endourology or oncology surgeons | 1 | 135 | Open: performed by one of four fellowship-trained urological oncologists | Dysfunction (urinary function, sexual function) | ||||||||||
| Martorana 2004134 | 1 | 0 | 50 | Operating time (minutes), mean: patients 1–25: 399; patients 26–50: 316; patients 35–50: 265 | 1 | 50 | Operating time (minutes), mean: patients 1–50: 159 |
Safety (open conversion, surgical complications, operating time, hospital stay, catheterisation) Efficacy (margins, pT stage, pathological Gleason score) Learning curve (operating time) |
For both procedures, surgery was performed by the same first surgeon with experience in open but not laparoscopic surgery | ||||||||
| Menon 200295 (linked to Tewari 2003116) | 3 | 0 |
I and III: 4; II and III: 10; III: 36 Total: 50 |
Time (minutes), mean (SD): 274 (94.3) Time first year (minutes): 490.89 |
4 | I and II: 600; III: 0 (1000 open cases) |
I: 27; II: 19; IV: 2 Total: 48 |
Time (minutes), mean, (SD); 258 (80.3) Time first year (minutes): 228.08 |
III: assisted; I and II: experience in laparoscopic prostatectomy |
Equipment failure Safety (surgical complications, operating time, discharge, blood loss) Patient satisfaction Efficacy (margins, pT stage, pathological Gleason score, PSA recurrence) Death (none) Learning curve (operating time) |
|||||||
| Miller 2007111 | NR | NR | 42 | NR | NR | 120 |
Safety (blood loss) Quality of life |
||||||||||
| Nadler 2010112 | 1 | 50 | 1 | > 460 open and 24 laparo-scopic |
Safety (surgical complications, operating time, hospital stay, blood loss) Efficacy (margins, pT stage, PSA recurrence) Dysfunction (urinary continence, potency) |
Single-experience laparoscopic urologist. Before performing robotic surgery the surgeon attended a 2-day training course | |||||||||||
| Namiki 2005135 | 2 | > 50 | 45 | 5 | > 50 | 121 |
Efficacy (pT stage, pathological Gleason score) Dysfunction (urinary function, sexual function) Quality of life (SF-36) |
Staff urologist level UCLA-PCI figures available in graph form for baseline, 1 month, 3 months, 6 months and 12 months for urinary function, urinary bother, sexual function, sexual bother |
|||||||||
| Namiki 2006136 | 2 | > 100 | 65 in total | Retro-pubic: 5; perineal: 2 | Perineal: > 50 | Retro-pubic: 218; perineal: 66 | Considerable experience with retropubic surgery |
Efficacy (pathological Gleason score) Dysfunction (urinary function, sexual function) Quality of life (SF-36) |
|||||||||
| Ou 2009113 | 1 | 0 | 30 | Time (minutes), mean (SD): 205 (103) | Same one | 30 | Time (minutes), mean (SD): 213 (37) |
Safety (open conversion, surgical complications, operating time, hospital stay, catherisation, blood loss) Efficacy (margins, pathological Gleason score, PSA recurrence) Dysfunction (incontinence, erectile) Learning curve (operating time) |
|||||||||
| Poulakis 2007137 | NR | NR | 72 | NR | NR | 132 | NR | NR | 70 |
Safety (surgical complications, operating time, hospital stay, catherisation, blood loss, mobilisation, oral feeding) Efficacy (margins, pT stage, pathological Gleason score, PSA recurrence) Dysfunction (urinary incontinence) Death (none) |
|||||||
| Raventos Busquets 2007138 | 105 in total | Time (minutes), mean (SD): 172.3 (43.7) | 56% were conducted by surgeons experienced in laparoscopic surgery | Time (minutes), mean (SD): 145.1 (32.9) | 51% of cases were conducted by surgeons experienced in open surgery |
Safety (operating time, hospital stay) Efficacy (margins, pT stage) Learning curve (operating time) |
Spanish | ||||||||||
| Remzi 2005139 | 1 | > 300 major laparo-scopic surgeries | 80 in total | Experienced. Initial learning curve overcome | NR | 41 in total |
Safety (open conversion, operating time, hospital stay, surgical complications, catheterisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score) Dysfunction (urinary continence) Quality of life (postoperative pain) |
||||||||||
| Rocco 2009114 | 3 | Same three |
Safety (operating time, hospital stay, catherisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score) Dysfunction (urinary incontinence, potency) |
||||||||||||||
| Rozet 200796 | 4 | 133 | Time (minutes), mean (range): 166 (90–300) | 4 | 133 | Time (minutes), mean (range): 160 (90–270) |
Safety (open conversion, surgical complications, operating time, hospital stay, catherisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score) Death (none) |
||||||||||
| Salomon 2002140 | NR | NR | 155 | NR | NR | 151 |
Safety (blood transfusion, operating time, hospital stay, catheterisation, surgical complications) Efficacy (margins, pT stage, pathological Gleason score, PSA recurrence) |
||||||||||
| Schroeck 2008115 | 1/4 | NR | 362 | 1/6 | NR | 435 |
Safety (blood loss) Efficacy (margins, pathological Gleason score, PSA recurrence) |
Two surgeons performed both robotic radical prostatectomy and robot-assisted laparoscopic prostatectomy | |||||||||
| Silva 2007141 | 1 | 90 | ‘experienced single surgeon under a learning curve’ | 1 | 89 | ‘Resident physicians under a teacher’s supervision at University’ | Efficacy (margins, pT stage, pathological Gleason score) | ||||||||||
| Soderdahl 2005142 | 2 | 116 in total | Both fellowship trained | 3 | 186 in total | All fellowship trained |
Efficacy (pT stage) Dysfunction (urinary function, sexual function) |
||||||||||
| Soric 2004143 | NR | NR | 26 | NR | NR | 26 |
Safety (open conversion, surgical complications, operating time, hospital stay, catheterisation) Efficacy (margins, pT stage, pathological Gleason score) |
Croatian | |||||||||
| Sundaram 200497 | 1 | 0 | 10 | Time (minutes), mean (range): 290 (210–340) | Same one | > 40 | 10 | Time (minutes), mean (range): 394 (240–280) |
Safety (operating time, hospital stay, surgical complications, blood loss) Efficacy (margins) Dysfunction (urinary incontinence) |
Abstract | |||||||
| Terakawa 2008144 | 5 | I: 54; II: 42; III: 31; IV: 7; V: 3 | Paper stated that surgeons were well experienced in ‘laparaoscopy surgery’ | NR | 220 in total | Less experienced, residents in training | Efficacy (margins, pT stage) | ||||||||||
| Tewari 2003116 | 1 | 200 | 8 | Combined experience of > 1400 | 100 |
Safety (open conversion, surgical complications, hospital stay, catheterisation, blood loss) Efficacy (margins, pT stage, pathological Gleason score, PSA recurrence) Dysfunction (urinary incontinence, erectile) Quality of life (pain) Death (none) |
|||||||||||
| Touijer 2007145 | 2 | I: 398; II: 87 | 2 | III: 422; IV: 270 | Efficacy (margins, pT stage, pathological Gleason score) | ||||||||||||
| Trabulsi 200898 | 0 | 50 | Positive margins: 3/50 (6%) | 147 | 50 | Positive margins: 10/50 (20%) |
Safety (open conversion, blood loss) Efficacy (margins, pT stage, pathological Gleason score) |
||||||||||
| Truesdale 2010117 | 1 | 99 | Cases limited to a single high-volume surgeon | 4 | 217 | Cases limited to those performed at a single institution by four high-volume surgeons |
Safety (operating time, blood loss) Efficacy (pT stage, pathological Gleason score) |
||||||||||
| Wagner 2007146 | 1 | 0 | 75 | Time (minutes), mean (SD): 282 (53) | Same one | 0 | 75 | Time (minutes), mean (SD): 162 (39) |
Safety (operating time, surgical complications, blood loss) Efficacy (margins, pT stage) Dysfunction (urinary incontinence, erectile) |
Just out of training | |||||||
| White 2009118 | 1 | 2 | 50 | Same one | 50 |
Safety (open conversion) Efficacy (margins, pT stage, pathological Gleason score) |
|||||||||||
Appendix 10 Classification of reported adverse effects using the Clavien–Dindo classification of surgical complications68
| Study | Reported adverse effect(s) |
|---|---|
| Artibani 2003123 | Acute urinary retention, fever, wound infection |
| Bhayani 2003124 | Dislodged catheter requiring replacement, inadvertent cystotomy |
| Brown 2004125 | Anastomotic leak, rectus haematoma, ulnar neuropathy, wound infection |
| Carlsson 2010104 | Wound infection, infection, anastomotic leak |
| Dahl 2009126 | Anastomotic leak, chronic abdomen pain, genital femoral nerve irritation, seroma, urinary retention, vasovagal syncope, wound infection |
| Doumerc 2010105 | Anastomotic leak |
| Drouin 2009101 | Anastomotic leak, urinary retention, urinary infection |
| Fornara 2004127 | Wound infection |
| Fracalanza 2008107 | Fever |
| Ghavamian 2006128 | Anastomotic leak, clot retention, urinary infection |
| Guazzoni 200690 | Urinary retention, anastomotic leak, fever |
| Hu 200692 | Urinary retention, urinary leak, clot retention |
| Joseph 200794 | Urinary leakage, urinary retention |
| Jurczok 2007131 | Wound infection |
| Kim 2007132 | Subcutaneous emphysema, anastomotic leak |
| Krambeck 2009108 | Urinary retention, urinary infection, drug reaction |
| Lama 2009133 | Urinary leakage, urinary retention, seroma |
| Martorana 2004134 | Anastomotic leak |
| Nadler 2010112 | Anastomotic leak |
| Ou 2009113 | Anastomotic leak |
| Poulakis 2007137 | Urinary infection |
| Remzi 2005139 | Anastomotic leak |
| Rozet 200796 | Anastomotic leak, wound abscess?, urinary infection, retention, infected pelvic haematoma |
| Salomon 2002140 | Anastomotic leak, wound infection |
| Sundaram 200497 | Anastomotic leak, urinary retention |
| Tewari 2003116 | Obturator neuropathy |
| Study ID | Reported adverse effect(s) |
|---|---|
| Al-Shaji 2010121 | Blood transfusion |
| Anastasiadis 2003122 | Blood transfusion |
| Artibani 2003123 | Blood transfusion, cardiovascular complications, ileus, pelvic haematoma |
| Bhayani 2003124 | Calf myositis, obturator nerve palsy |
| Bolenz 2010100 | Blood transfusion |
| Brown 2004125 | Blood transfusion, deep-vein thrombosis, ileus |
| Carlsson 2010104 | Blood transfusion |
| Dahl 2009126 | Bladder neck contracture |
| Doumerc 2010105 | Pelvic haematoma, blood transfusion, blood loss |
| Drouin 2009101 | Blood transfusion, postoperative bleeding |
| Ficarra 2009106 | Postoperative bleeding, ileus, cardiovascular complications, blood loss, blood transfusion |
| Fornara 2004127 | Blood transfusion |
| Fracalanza 2008107 | Blood transfusion |
| Ghavamian 2006128 | Blood transfusion, deep-vein thrombosis, ileus, neuropraxia |
| Gosseine 200991 | Blood transfusion |
| Greco 2010129 | Blood transfusion |
| Guazzoni 200690 | Blood transfusion, lymphorrhea |
| Hu 200692 | Nerve damage/injury, intra-abdominal drain retraction, ileus, blood loss, blood transfusion |
| Joseph 200794 | Blood transfusion |
| Jurczok 2007131 | Blood transfusion |
| Kim 2007132 | Blood transfusion |
| Kordan 2010120 | Blood transfusion |
| Krambeck 2009108 | Blood transfusion, deep-vein thrombosis, haemorrhage/haematoma, ileus, lymphoedema |
| Lama 2009133 | Perioperative hypercapnia, deep-vein thrombosis, blood loss, blood transfusion |
| Martorana 2004134 | Blood transfusion, ileus |
| Menon 200295 | Ileus, blood transfusion |
| Nadler 2010112 | Ileus, deep-vein thrombosis, blood transfusion |
| Ou 2009113 | Blood transfusion, lymph leakage |
| Poulakis 2007137 | Haemorrhage/haematoma, gastrointestinal symptoms, fever > 39°C, delirium, blood loss, blood transfusion |
| Remzi 2005139 | Ileus, haemorrhage/haematoma |
| Rozet 200796 | Postoperative bleeding, cardiovascular complications |
| Salomon 2002140 | Blood transfusion, deep-vein thrombosis, ileus, lymphorrhea, pelvic haematoma, postoperative neuropathy |
| Soric 2004143 | Blood transfusion, nerve damage/injury |
| Tewari 2003116 | Blood transfusion, deep-vein thrombosis, ileus |
| Study | Reported adverse effect(s) |
|---|---|
| Dahl 2009126 | Lymphocele |
| Drouin 2009101 | Lymphocele |
| Fornara 2004127 | Lymphocele |
| Ghavamian 2006128 | Lymphocele |
| Hu 200692 | Lymphocele |
| Jurczok 2007131 | Lymphocele |
| Krambeck 2009108 | Abdominal abscess, lymphocele |
| Martorana 2004134 | Lymphocele |
| Poulakis 2007137 | Lymphocele, prolonged urinary leakage |
| Soric 2004143 | Ureter wound |
| Tewari 2003116 | Lymphocele |
| Study | Reported adverse effect(s) |
|---|---|
| Artibani 2003123 | Rectal injury/lesion |
| Bhayani 2003124 | Bladder neck contracture, epigastric artery/vessel injury, hydroureteronephrosis, postoperative hydrocele |
| Brown 2004125 | Bladder neck contracture, ureteral injury |
| Carlsson 2010104 | Ureteral injury, surgical reintervention, small bowel injury, rectal lesion/injury, bladder neck contracture |
| Dahl 2009126 | Hematoma leading to contracture, hematuria, meatal stricture |
| Doumerc 2010105 | Bowel injury |
| Drouin 2009101 | Rectal injury/lesion |
| Ficarra 2009106 | Wound dehiscence, surgical re-exploration, rectal lesion/injury, colon lesion |
| Fornara 2004127 | Rectal injury/lesion |
| Ghavamian 2006128 | Bladder injury, bladder neck contracture, inferior epigastric injury |
| Greco 2010129 | Rectal injury/lesion |
| Guazzoni 200690 | Rectal injury/lesion |
| Hu 200692 | Rectal injury/lesion, bladder neck contracture |
| Jurczok 2007131 | Rectal injury/lesion, revision |
| Kim 2007132 | Rectal injury/lesion, epigastric artery/vessel injury |
| Krambeck 2009108 | Bladder neck contracture, ureteric obstruction |
| Lama 2009133 | Rectal injury/lesion, bladder neck stenosis |
| Martorana 2004134 | Bladder injury, epigastric artery/vessel injury |
| Menon 200295 | Hernia, ureter entrapment |
| Nadler 2010112 | Hernia, bladder neck contracture |
| Ou 2009113 | Bladder injury, rectal injury, anastomotic stricture |
| Poulakis 2007137 | Dehiscence/rupture of wound, bladder neck contracture |
| Remzi 2005139 | Rectal injury/lesion, anastomotic stricture |
| Salomon 2002140 | Rectal injury/lesion, ureteral injury |
| Soric 2004143 | Bladder neck sclerosis, blood vessel damage, ureteral injury |
| Tewari 2003116 | Rectal injury/lesion, surgical re-exploration, wound dehiscence, wound hernia |
| Wagner 2007146 | Bladder neck contracture |
| Study | Reported adverse effect(s) |
|---|---|
| Carlsson 2010104 | Pulmonary embolism, myocardial infarction |
| Dahl 2009126 | Pulmonary embolism |
| Ficarra 2009106 | Re-exploration due to bleeding |
| Hu 200692 | Pulmonary embolism, myocardial infarction, cerebral vascular accident, acute tubular necrosis |
| Krambeck 2009108 | Pulmonary embolism, renal failure, myocardial infarction, stroke |
| Lama 2009133 | Embolic stroke |
| Poulakis 2007137 | Cardiovascular including arrhythmias and myocardial infarction, respiratory insufficiency |
| Rozet 200796 | Pulmonary embolism, renal insufficiency |
| Salomon 2002140 | Pulmonary embolism |
| Tewari 2003116 | Myocardial infarction |
| Study | Reported adverse effect(s) |
|---|---|
| Carlsson 2010104 | Fatal cardiac arrest |
| Dahl 2009126 | Fatal cardiac arrest |
| Doumerc 2010105 | Death due to cerebral vascular accident |
| Salomon 2002140 | Death due to pulmonary embolism |
No studies reported adverse effects classed as Clavien IVb or d.
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Artibani 2003123 | 16/71 (22.5) | 3/50 (6.0) | |
| Bhayani 2003124 | 2/33 (6.1) | 0/24 (0) | |
| Brown 2004125 | 11/60 (18.3) | 4/60 (6.7) | |
| Carlsson 2010104 | 37/1253 (3.0) | 83/485 (17.1) | |
| Dahl 2009126 | 9/104 (8.7) | 0/102 (0) | |
| Doumerc 2010105 | 1/212 (0.5) | 0/502 (0) | |
| Drouin 2009101 | 2/71 (2.8) | 0/85 (0) | |
| Fornara 2004127 | 0/32 (0) | 2/32 (6.3) | |
| Fracalanza 2008107 | 2/35 (5.7) | 4/26 (15.4) | |
| Ghavamian 2006128 | 3/70 (4.3) | 5/70 (7.1) | |
| Guazzoni 200690 | 10/60 (16.7) | 24/60 (40.0) | |
| Hu 200692 | 38/322 (11.8) | 69/358 (19.3) | |
| Joseph 200794 | 24/754 (3.2) | 160/800 (20.0) | |
| Jurczok 2007131 | 5/163 (3.1) | 8/240 (3.3) | |
| Kim 2007132 | 9/30 (30.0) | 0/45 (0) | |
| Krambeck 2009108 | 13/294 (4.4) | 20/588 (3.4) | |
| Lama 2009133 | 2/56 (3.6) | 7/59 (11.9) | |
| Martorana 2004134 | 1/50 (2%) | 2/50 (4%) | |
| Nadler 2010112 | 2/50 (4.0) | 2/50 (4.0) | |
| Ou 2009113 | 0/30 (0) | 2/30 (6.7) | |
| Poulakis 2007137 | 1/204 (0.5) | 1/70 (1.4) | |
| Remzi 2005139 | 8/80 (10.0) | 6/41 (14.6) | |
| Rozet 200796 | 12/133 (9.0) | 7/133 (5.3) | |
| Salomon 2002140 | 6/155 (3.9) | 14/151 (9.3) | |
| Sundaram 200497 | 1/10 (10.0) | 1/10 (10.0) | |
| Tewari 2003116 | 0/200 (0) | 2/200 (1.0) |
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Al-Shaji 2010121 | 3/70 (4.3) | 42/70 (60.0) | |
| Anastasiadis 2003122 | 6/230 (2.6) | 6/70 (8.6) | |
| Artibani 2003123 | 5/71 (7.0) | 0/50 (0) | |
| Bhayani 2003124 | 2/33 (6.1) | 0/24 (0) | |
| Bolenz 2010100 | 12/262 (4.6) | 4/211 (1.9) | 32/156 (20.5) |
| Brown 2004125 | 3/60 (5.0) | 36/60 (60.0) | |
| Carlsson 2010104 | 58/1253 (4.6) | 116/485 (23.9) | |
| Dahl 2009126 | 2/104 (1.9) | 0/104 (0) | |
| Doumerc 2010105 | 4/212 (1.9) | 11/502 (2.2) | |
| Drouin 2009101 | 8/71 (11.3) | 5/85 (5.9) | |
| Ficarra 2009106 | 10/103 (9.7) | 25/105 (23.8) | |
| Fornara 2004127 | 2/32 (6.3) | 6/32 (18.8) | |
| Fracalanza 2008107 | 7/35 (20.0) | 12/26 (46.2) | |
| Ghavamian 2006128 | 9/70 (12.9) | 24/70 (34.3) | |
| Gosseine 200991 | 4/122 (3.3) | 8/125 (6.4) | |
| Greco 2010129 | 3/150 (2.0) | 9/150 (6.0) | |
| Guazzoni 200690 | 12/60 (20.0) | 37/60 (61.7) | |
| Hu 200692 | 24/322 (7.5) | 33/358 (9.2) | |
| Joseph 200794 | 10/754 (1.3) | 35/800 (4.4) | |
| Jurczok 2007131 | 5/163 (3.1) | 22/240 (9.2) | |
| Kim 2007132 | 7/30 (23.3) | 10/45 (22.2) | |
| Kordan 2010120 | 7/830 (0.8) | 14/414 (3.4) | |
| Krambeck 2009108 | 31/294 (10.5) | 104/588 (17.7) | |
| Lama 2009133 | 8/56 (14.3) | 28/59 (47.5) | |
| Martorana 2004134 | 3/50 (6.0) | 4/50 (8.0) | |
| Menon 200295 | 1/40 (2.5) | 2/40 (5.0) | |
| Nadler 2010112 | 12/50 (24.0) | 46/50 (92.0) | |
| Ou 2009113 | 6/30 (20.0) | 18/30 (60.0) | |
| Poulakis 2007137 | 17/204 (8.3) | 32/70 (45.7) | |
| Remzi 2005139 | 2/80 (2.5) | 3/41 (7.3) | |
| Rozet 200796 | 21/133 (15.8) | 7/133 (5.3) | |
| Salomon 2002140 | 45/151 (29.8) | 12/155 (7.7) | |
| Soric 2004143 | 1/26 (3.9) | 0/26 (0) | |
| Tewari 2003116 | 4/200 (2.0) | 75/100 (75.0) |
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Dahl 2009126 | 4/104 (3.8) | 0/102 (0) | |
| Drouin 2009101 | 0/71 (0) | 0/85 (0) | 1/83 (1.2) |
| Fornara 2004127 | 0/32 (0) | 1/32 (3.1) | |
| Ghavamian 2006128 | 2/70 (2.9) | 2/70 (2.9) | |
| Hu 200692 | 3/322 (0.9) | 3/358 (0.8) | |
| Jurczok 2007131 | 5/163 (3.1) | 7/240 (2.9) | |
| Krambeck 2009108 | 1/294 (0.3) | 5/588 (0.9) | |
| Martorana 2004134 | 0/50 (0) | 2/50 (4.0) | |
| Poulakis 2007137 | 5/204 (2.5) | 5/70 (7.1) | |
| Soric 2004143 | 2/26 (7.7) | 0/26 (0) | |
| Tewari 2003116 | 0/200 (0) | 2/100 (2.0) |
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Artibani 2003123 | 2/71 (2.8) | 0/50 (0) | |
| Bhayani 2003124 | 3/33 (9.1) | 6/24 (25.0) | |
| Brown 2004125 | 2/60 (3.3) | 2/60 (3.3) | |
| Carlsson 2010104 | 31/1253 (2.5) | 44/485 (9.1) | |
| Dahl 2009126 | 7/104 (6.7) | 1/102 (1.0) | |
| Doumerc 2010105 | 1/212 (0.5) | 0/502 (0) | |
| Drouin 2009101 | 0/71 (0) | 1/85 (1.2) | 1/83 (1.2) |
| Ficarra 2009106 | 5/103 (4.9) | 7/105 (6.7) | |
| Fornara 2004127 | 1/32 (3.1) | 0/32 (0) | |
| Ghavamian 2006128 | 3/70 (4.3) | 3/70 (4.3) | |
| Greco 2010129 | 2/150 (1.3) | 1/150 (0.7) | |
| Guazzoni 200690 | 1/60 (1.7) | 0/60 (0) | |
| Hu 200692 | 3/322 (0.9) | 26/358 (7.3) | |
| Jurczok 2007131 | 5/163 (3.1) | 10/240 (4.2) | |
| Kim 2007132 | 2/30 (6.7) | 0/45 (0) | |
| Krambeck 2009108 | 3/294 (1.0) | 24/588 (4.1) | |
| Lama 2009133 | 5/56 (8.9) | 2/59 (3.4) | |
| Martorana 2004134 | 2/50 (4.0) | 0/50 (0) | |
| Menon 200295 | 0/40 (0) | 2/40 (5.0) | |
| Nadler 2010112 | 2/50 (4.0) | 8/50 (16.0) | |
| Ou 2009113 | 3/30 (10.0) | 1/30 (3.3) | |
| Poulakis 2007137 | 2/204 (1.0) | 13/70 (18.6) | |
| Remzi 2005139 | 4/80 (5.0) | 5/41 (12.2) | |
| Salomon 2002140 | 4/155 (2.6) | 3/151 (2.0) | |
| Soric 2004143 | 3/26 (11.5) | 0/26 (0) | |
| Tewari 2003116 | 2/200 (1.0) | 1/100 (1.0) | |
| Wagner 2007146 | 2/75 (2.7) | 12/75 (16.0) |
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Carlsson 2010104 | 3/1253 (0.2) | 7/485 (1.4) | |
| Dahl 2009126 | 1/104 (1) | 0/102 (0) | |
| Ficarra 2009106 | 4/103 (3.9) | 7/105 (6.7) | |
| Hu 200692 | 0/322 (0) | 1/358 (0.3) | |
| Krambeck 2009108 | 5/294 (1.7) | 12/588 (2.0) | |
| Lama 2009133 | 0/56 (0) | 1/59 (1.7) | |
| Poulakis 2007137 | 4/204 (2.0) | 5/70 (7.1) | |
| Rozet 200796 | 2/133 (1.5) | 1/133 (0.8) | |
| Salomon 2002140 | 0/155 (0) | 1/151 (0.7) | |
| Tewari 2003116 | 1/200 (0.5) | 5/100 (5.0) |
| Study | Robotic, n/N (%) | Laparoscopic, n/N (%) | Open, n/N (%) |
|---|---|---|---|
| Carlsson 2010104 | 0/1253 (0) | 1/485 (0.2) | |
| Dahl 2009126 | 0/140 (0) | 1/102 (1.0) | |
| Doumerc 2010105 | 0/212 (0) | 1/502 (0.2) | |
| Salomon 2002140 | 1/155 (0.7) | 0/151 (0) |
Not possible to meta-analyse Clavien V adverse events.
Appendix 11 Results of the systematic review of economic evaluations
-
802 titles and abstracts screened
-
23 selected for full-text assessment.
Reasons for exclusion
Not a primary study (n = 1)
-
Patel HRH. Robotic and laparoscopic surgery: cost and training. Surg Oncol 2009;18:242–6.
Clinical stage unclear (unsure if a relevant patient group is being considered) (n = 5)
-
Burgess SV. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol 2006;20:827–30.
-
Hohw L, Ehlers L, Borre M, Pedersen KV. Cost-effectiveness study of robot-assisted laparoscopic versus open retropubic radical prostatectomy. Eur Urol Suppl 2010;9:505.
-
O’Malley SP. Review of a decision by the Medical Services Advisory Committee based on health technology assessment of an emerging technology: the case for remotely assisted radical prostatectomy. Int J Technol Assess Health Care 2007;23:286–91.
-
Scales J, Jones PJ. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol 2005;174:2323–9.
-
Taylor J. Individualized predictions of disease progression following radiation therapy for prostate cancer. University of Michigan Department of Biostatistics Working Paper Series no. 1024. Berkeley, CA: Berkeley Electronic Press;2004.
Not laparoscopic or robot surgery (n = 8)
-
Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst 2000;92:1731–9.
-
Konski A, Sherman E, Krahn M, Bremner K, Beck JR, Watkins-Bruner D, et al. Economic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86-10). Int J Radiat Oncol Biol Physics 2005;63:788–94.
-
Konski A, Watkins-Bruner D, Brereton H, Feigenberg S, Hanks G. Long-term hormone therapy and radiation is cost-effective for patients with locally advanced prostate carcinoma. Cancer 2006;106:51–7.
-
Lazzaro C, Bartoletti R, Guazzoni G, Orestano F, Pappagallo GL, Prezioso D, et al. Economic evaluation of different hormonal therapies for prostate cancer: final results from the Quality of Life Antiandrogen Blockade Italian Observational Study (QuABIOS). Arch Ital Urol Androl 2007;79:104–7.
-
Neymark N, Adriaenssen I, Gorlia T, Caleo S, Bolla M. Estimating survival gain for economic evaluations with survival time as principal endpoint: a cost-effectiveness analysis of adding early hormonal therapy to radiotherapy in patients with locally advanced prostate cancer. Health Econ 2002;11:233–48.
-
Perez CA, Michalski J, Ballard S, Drzymala R, Kobeissi BJ, Lockett MA, et al. Cost benefit of emerging technology in localized carcinoma of the prostate. Int J Radiat Oncol Biol Physics 1997;39:875–83.
-
Ramsey S, Veenstra D, Clarke L, Gandhi S, Hirsch M, Penson D. Is combined androgen blockade with bicalutamide cost-effective compared with combined androgen blockade with flutamide. Urology 2005;66:835–9.
-
Samant RS, Dunscombe PB, Roberts GH. A cost-outcome analysis of long-term adjuvant goserelin in addition to radiotherapy for locally advanced prostate cancer. Semin Urol Oncol 2003;21:171–7.
Not cost-effectiveness analysis (form of cost comparison only) (n = 9)
-
Al-Shaiji TF, Kanaroglou N, Thom A, Prowse C, Comondore V, Orovan W, et al. A cost-analysis comparison of laparoscopic radical prostatectomy versus open radical prostatectomy: the McMaster Institute of Urology experience. Can Urol Assoc J 2010;4:237–41 (included in effectiveness review).
-
Anderson JK. Cost comparison of laparoscopic versus radical retropubic prostatectomy. Urology 2005;66:557–60.
-
Bolenz C. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol 2010;57:453–8.
-
Gregori A, Galli S, Goumas I, Scieri F, Stener S, Gaboardi F. A cost comparison of laparoscopic versus open radical cystoprostatectomy and orthotopic ileal neobladder at a single institution. Arch Ital Urol Androl 2007;79:127–9.
-
Link RE, Su LM, Bhayani SB, Pavlovich CP. Making ends meet: a cost comparison of laparoscopic and open radical retropubic prostatectomy. J Urol 2004;172:269–74.
-
Lotan Y. The new economics of radical prostatectomy: cost comparison of open, laparoscopic and robot assisted techniques. J Urol 2004;172:1431–5.
-
Mouraviev V. Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: a single-institution experience. Urology 2007;69:311–14.
-
Satoh T. Cost comparison of curative therapies for localized prostate cancer in Japan: a single-institution experience. Japn J Radiol 2009;27:348–54.
-
Steinberg PL, Merguerian PA, Bihrle W III, Heaney JA, Seigne JD. A da Vinci robot system can make sense for a mature laparoscopic prostatectomy program. J Soc Laparoendosc Surg 2008;12:9–12.
Appendix 12 Costs of robotic equipment
| Surgical system procurement | List price (£) | 4 years, arrears (£) | 5 years, advance (£) | 5 years, arrears (£) | 6 years, advance (£) | 6 years, arrears (£) | 7 years, advance (£) | Annual service contract (£) |
|---|---|---|---|---|---|---|---|---|
| Plan 1: da Vinci Si HD Dual Console | 2,100,000.00 | 487,200.00 | 386,400.00 | 417,900.00 | 338,100.00 | 365,400.00 | 310,800.00 | 165,000.00 |
| Plan 2: da Vinci Si HD Single Console | 1,600,000.00 | 371,000.00 | 294,400.00 | 318,400.00 | 259,200.00 | 278,400.00 | 236,800.00 | 140,000.00 |
| Plan 3: da Vinci S HD | 1,375,000.00 | 348,000.00 | 276,000.00 | 298,500.00 | 243,000.00 | 261,000.00 | 222,000.00 | 140,000.00 |
| Plan 4: da Vinci S HD reconditioned (four arm) | 1,250,000.00 | 324,800.00 | 257,600.00 | 278,600.00 | 226,800.00 | 243,600.00 | 207,200.00 | 140,000.00 |
| Plan 5: da Vinci S EZ (three arm) | 1,150,000.00 | 273,760.00 | NS | 234,820.00 | 191,160.00 | 205,320.00 | 174,640.00 | 120,000.00 |
| Total system cost (including service contract) (£) | Number of procedures | Service life | Cost per procedure (£) | Cost of surgical equipment (£) | Cost of consumables (£) | Total cost per procedure (£) |
|---|---|---|---|---|---|---|
| Procurement cost based on purchase plan 1 | ||||||
| 3,090,000.00 | 200 | 7 | 2207.14 | 66.10 | 1194.11 | 3467.35 |
| 3,090,000.00 | 150 | 7 | 2942.86 | 88.14 | 1194.11 | 4225.11 |
| 3,090,000.00 | 100 | 7 | 4414.29 | 132.21 | 1194.11 | 5740.61 |
| 3,090,000.00 | 50 | 7 | 8828.57 | 264.42 | 1194.11 | 10,287.10 |
| Procurement cost based on purchase plan 2 | ||||||
| 2,440,000.00 | 200 | 7 | 1742.86 | 66.10 | 1194.11 | 3003.07 |
| 2,440,000.00 | 150 | 7 | 2323.81 | 88.14 | 1194.11 | 3606.06 |
| 2,440,000.00 | 100 | 7 | 3485.71 | 132.21 | 1194.11 | 4812.03 |
| 2,440,000.00 | 50 | 7 | 6971.43 | 264.42 | 1194.11 | 8429.96 |
| Procurement cost based on purchase plan 3 | ||||||
| 2,215,000.00 | 200 | 7 | 1582.14 | 66.10 | 1194.11 | 2842.35 |
| 2,215,000.00 | 150 | 7 | 2109.52 | 88.14 | 1194.11 | 3391.77 |
| 2,215,000.00 | 100 | 7 | 3164.29 | 132.21 | 1194.11 | 4490.61 |
| 2,215,000.00 | 50 | 7 | 6328.57 | 264.42 | 1194.11 | 7787.10 |
| Procurement cost based on purchase plan 4 | ||||||
| 2,090,000.00 | 200 | 7 | 1492.86 | 66.10 | 1194.11 | 2753.07 |
| 2,090,000.00 | 150 | 7 | 1990.48 | 88.14 | 1194.11 | 3272.73 |
| 2,090,000.00 | 100 | 7 | 2985.71 | 132.21 | 1194.11 | 4312.03 |
| 2,090,000.00 | 50 | 7 | 5971.43 | 264.42 | 1194.11 | 7429.96 |
| Procurement cost based on purchase plan 5 | ||||||
| 1,870,000.00 | 200 | 7 | 1335.71 | 66.10 | 1194.11 | 2595.92 |
| 1,870,000.00 | 150 | 7 | 1780.95 | 88.14 | 1194.11 | 3063.20 |
| 1,870,000.00 | 100 | 7 | 2671.43 | 132.21 | 1194.11 | 3997.45 |
| 1,870,000.00 | 50 | 7 | 5342.86 | 264.41 | 1194.11 | 6801.38 |
| Surgical system upgrade | List price (£) | 4 years, arrears (£) | 5 years, advance (£) | 5 years, arrears (£) | 6 years, advance (£) | 6 years, arrears (£) | 7 years, advance (£) |
|---|---|---|---|---|---|---|---|
| da Vinci S HD to da Vinci Si HD | 600,000.00 | 139,020.00 | 110,400.00 | 119,400.00 | 97,200.00 | 104,400.00 | 88,800.00 |
| da Vinci Si HD Single Console to da Vinci Si HD Dual Console | 500,000.00 | 116,000.00 | 92,000.00 | 99,500.00 | 81,000.00 | 87,000.00 | 74,000.00 |
| da Vinci S EZ 3 Arm to 4 Arm | 220,000.00 | 51,040.00 | 40,480.00 | 43,780.00 | 35,640.00 | 38,280.00 | 32,560.00 |
| Surgical equipment | Number of units | Unit cost (capital) (£) | Operative service life | Number of procedures | Cost per procedure (£) | Total cost per procedure (£) |
|---|---|---|---|---|---|---|
| 200 cases per annum | ||||||
| Olympus EndoEYE® O DEG Telescope (Olympus Ltd, Japan) | 1 | 13,961.00 | 5 | 200 | 13.96 | 66.10 |
| Valleylab® Diathermy Generator (Tyco Healthcare Inc., USA) | 1 | 13,000.00 | 7 | 200 | 9.29 | |
| Olympus® Stack Unit (Insufflator) (Olympus Ltd, Japan) | 1 | 60,000.00 | 7 | 200 | 42.86 | |
| 150 cases per annum | ||||||
| Olympus EndoEYE O DEG Telescope | 1 | 13,961.00 | 5 | 150 | 18.61 | 88.14 |
| Valleylab Diathermy Generator | 1 | 13,000.00 | 7 | 150 | 12.38 | |
| Olympus Stack Unit (Insufflator) | 1 | 60,000.00 | 7 | 150 | 57.14 | |
| 100 cases per annum | ||||||
| Olympus EndoEYE O DEG Telescope | 1 | 13,961.00 | 5 | 100 | 27.92 | 132.21 |
| Valleylab Diathermy Generator | 1 | 13,000.00 | 7 | 100 | 18.57 | |
| Olympus Stack Unit (Insufflator) | 1 | 60,000.00 | 7 | 100 | 85.71 | |
| 50 cases per annum | ||||||
| Olympus EndoEYE O DEG Telescope | 1 | 13,961.00 | 5 | 50 | 55.84 | 264.42 |
| Valleylab Diathermy Generator | 1 | 13,000.00 | 7 | 50 | 37.14 | |
| Olympus Stack Unit (Insufflator) | 1 | 60,000.00 | 7 | 50 | 171.43 | |
| Consumables description (reusable) | Number of units | Unit cost (£) | Number of procedures | Total cost per procedure (£) |
|---|---|---|---|---|
| Hot Shears | 1 | 248.35 | 10 | 24.84 |
| Large Needle Driver | 2 | 195.80 | 10 | 39.16 |
| Maryland Bipolar Forceps | 1 | 240.90 | 10 | 24.09 |
| Pro-grasp® Forceps (Intuitive Surgical, CA, USA) | 1 | 195.80 | 10 | 19.58 |
| Total | 107.67 |
| Consumables description (disposable) | Number of units | Unit cost (£) | Number used per procedure | Total cost per procedure (£) |
|---|---|---|---|---|
| Anti-fog | 1 | 3.00 | 1 | 3.00 |
| Camera arm drape | 1 | 26.40 | 1 | 26.40 |
| Camera drape | 1 | 22.28 | 1 | 22.28 |
| Catheter tip syringe | 1 | 0.27 | 1 | 0.27 |
| Drain | 1 | 8.30 | 1 | 8.30 |
| Drape set | 1 | 8.20 | 1 | 8.20 |
| Hourly Uri-metre | 1 | 3.60 | 1 | 3.60 |
| Insufflation tubing | 1 | 2.70 | 1 | 2.70 |
| Major swab pack | 1 | 9.63 | 1 | 9.63 |
| Ports blunt | 1 | 40.00 | 1 | 40.00 |
| Ports sharp | 1 | 62.00 | 1 | 62.00 |
| Silastic catheter | 1 | 9.75 | 1 | 9.75 |
| Spigot | 1 | 0.08 | 1 | 0.08 |
| Stryker suction | 1 | 34.50 | 1 | 34.50 |
| Suction irrigation | 1 | 22.00 | 1 | 22.00 |
| Surgical blades × 2 | 2 | 0.11 | 2 | 0.22 |
| Tip cover accessory | 1 | 18.15 | 1 | 18.15 |
| Urinary catheter bag | 1 | 0.45 | 1 | 0.45 |
| Hypodermic needles × 2 | 2 | 0.05 | 2 | 0.10 |
| S-shaped retractors × 2a | 2 | 1.96 | 2 | 3.92 |
| Instrument arm drape | 3 | 40.15 | 3 | 120.45 |
| Ligamax® Endoclips 5 mm (Ethicon Inc., USA) (1–6 used, price each) | 3 | 108.66 | 3 | 325.98 |
| Memopouch bags | 3 | 31.60 | 3 | 94.80 |
| Seals | 3 | 13.42 | 3 | 40.26 |
| Velcro fastening strips × 3 | 3 | 1.20 | 3 | 3.60 |
| Syringes × 4 | 4 | 0.20 | 4 | 0.80 |
| Sutures × 9 | 9 | 25.00 | 9 | 225.00 |
| 1086.44 | ||||
| Total | 1194.11 |
Appendix 13 Costs of laparoscopic equipment
| Surgical equipment | Number of units | Unit cost (capital) (£) | Operative service life (years) | Numbers of procedures | Cost per procedure (£) |
|---|---|---|---|---|---|
| Olympus EndoEYE O DEG Telescope | 1 | 13,961.00 | 5 | 200 | 13.96 |
| Ethicon® Needle Holders ´ 2 (Ethicon Inc., USA) | 2 | 689.33 | 2 | 200 | 3.45 |
| Laparoscopic instruments and storage case | 1 | 8400.00 | 2 | 200 | 21.00 |
| Valleylab Diathermy Generator | 1 | 13,000.00 | 7 | 200 | 9.29 |
| Harmonic® Scalpel generator and Handpiece (Ethicon Inc., USA) | 1 | 5499.00 | 7 | 200 | 3.93 |
| Olympus Stack Unit | 1 | 60,000.00 | 7 | 200 | 42.86 |
| Total | 94.49 |
| Consumables description | Number of units | Unit cost (£) | Number used per procedure | Cost per procedure (£) |
|---|---|---|---|---|
| Anti-fog | 1 | 3.00 | 1 | 3.00 |
| Catheter tip syringe | 1 | 0.27 | 1 | 0.27 |
| Drain | 1 | 8.30 | 1 | 8.30 |
| Drape set | 1 | 8.20 | 1 | 8.20 |
| Harmonic shears | 1 | 405.00 | 1 | 405.00 |
| Hourly Uri-metre | 1 | 3.60 | 1 | 3.60 |
| Hypodermic needles × 2 | 2 | 0.05 | 2 | 0.10 |
| Insufflation tubing | 1 | 2.70 | 1 | 2.70 |
| Laparoscopic instrument pouch | 2 | 6.50 | 2 | 13.00 |
| Ligamax Endoclips 5 mm (1–6 used, price each) | 3 | 108.66 | 3 | 325.98 |
| Major swab pack | 1 | 9.63 | 1 | 9.63 |
| Memopouch bags | 3 | 31.60 | 3 | 94.80 |
| Ports blunt | 1 | 40.00 | 1 | 40.00 |
| Ports sharp | 1 | 62.00 | 1 | 62.00 |
| S-shaped retractors × 2a | 2 | 1.96 | 2 | 3.92 |
| Seals | 3 | 11.00 | 3 | 33.00 |
| Shears | 1 | 61.50 | 1 | 61.50 |
| Silastic catheter | 1 | 9.75 | 1 | 9.75 |
| Spigot | 1 | 0.08 | 1 | 0.08 |
| Stryker Suction | 1 | 34.50 | 1 | 34.50 |
| Suction irrigation | 1 | 22.00 | 1 | 22.00 |
| Surgical blades × 2 | 2 | 0.11 | 1 | 0.11 |
| Sutures × 9 | 9 | 25.00 | 9 | 225.00 |
| Syringes × 4 | 4 | 0.20 | 4 | 0.80 |
| Urinary catheter bag | 1 | 0.45 | 1 | 0.45 |
| Velcro fastening strips × 3 | 3 | 1.20 | 3 | 3.60 |
| Total | 1371.29 |
Appendix 14 Estimates of numbers of survivors and mean duration of survival
| Analysis | Outcome | Robotic | Laparoscopic |
|---|---|---|---|
| Base case (10 years) | Survivors | 3950/5000 | 3922/5000 |
| Life-years | 9.033 | 8.98 | |
| Base case (lifetime) | Survivors | 0/5000 | 0/5000 |
| Life-years | 21.810 | 20.26 | |
| Relative difference in positive margin rate was 0.61 | Survivors | 3932/5000 | 3922/5000 |
| Life-years | 9.108 | 8.975 | |
| Relative difference in positive margin rate was 0.88 | Survivors | 3874/5000 | 3922/5000 |
| Life-years | 8.978 | 8.975 | |
| Difference in biochemical recurrence was 0.89 | Survivors | 3976/5000 | 3922/5000 |
| Life-years | 9.05 | 8.98 | |
| Biochemical recurrence rates twice those of base case and difference was 0.89 | Survivors | 3913/5000 | 3822/5000 |
| Life-years | 9.001 | 8.600 |
Appendix 15 Density charts describing the distribution of total costs and quality-adjusted life-years for the cohort of modelled men for each analysis presented
FIGURE 27.
Distribution of costs and QALYs for each intervention in the base case, 10-year time horizon (200 procedures).
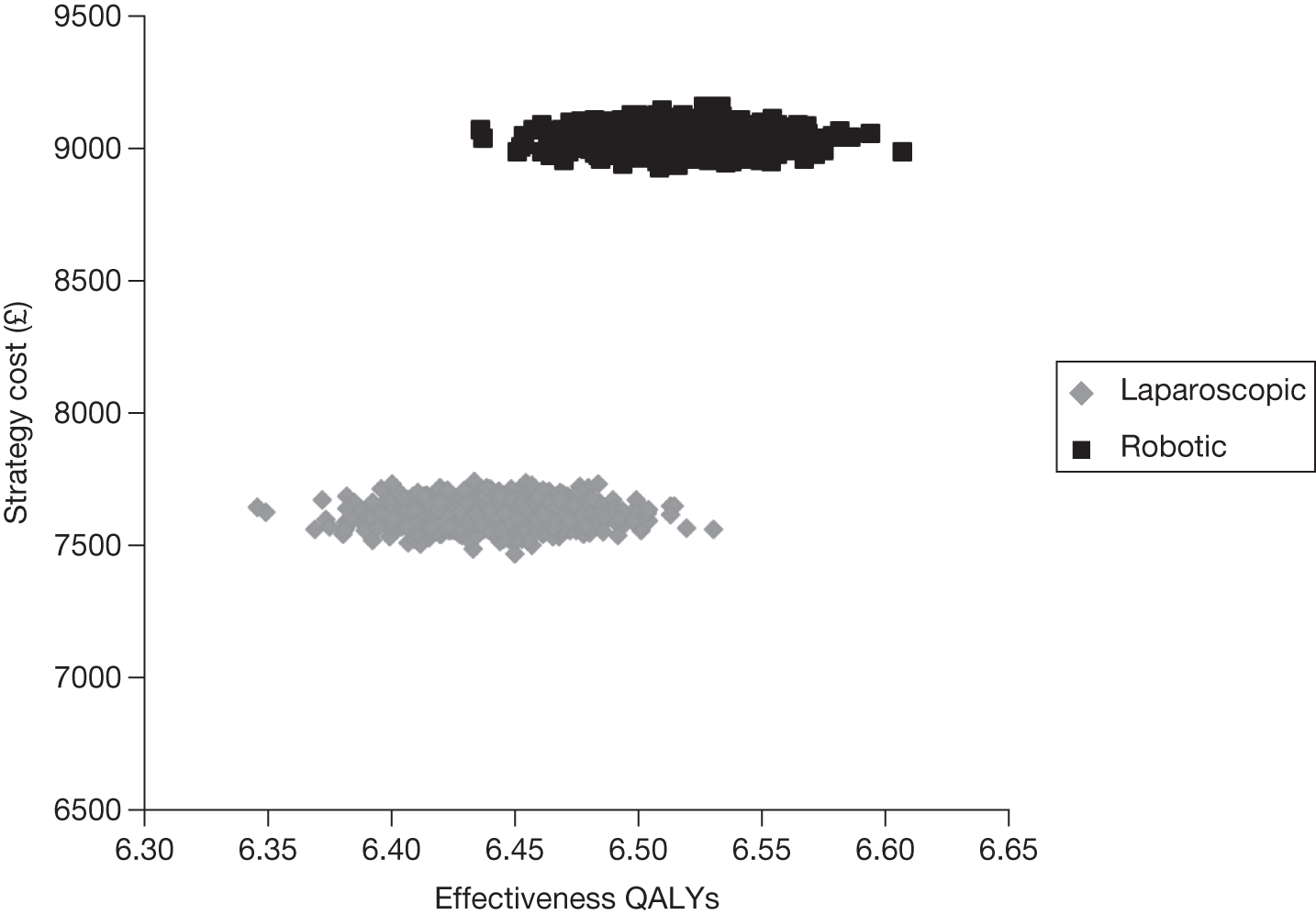
FIGURE 28.
Cost-effectiveness acceptability curve for each intervention in the base case, 10-year time horizon (200 procedures).
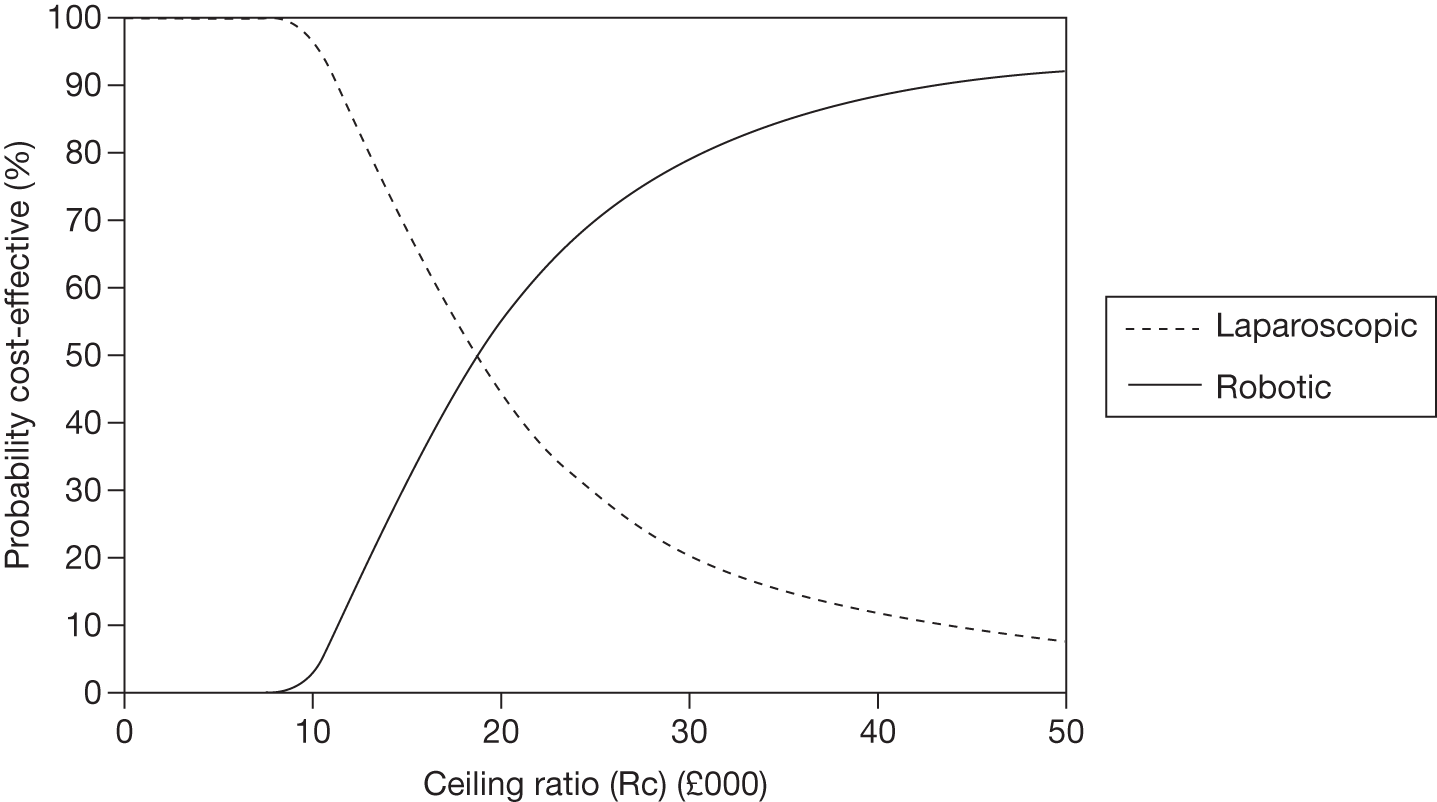
FIGURE 29.
Distribution of costs and QALYs for each intervention in the base case, 10-year time horizon (150 procedures).
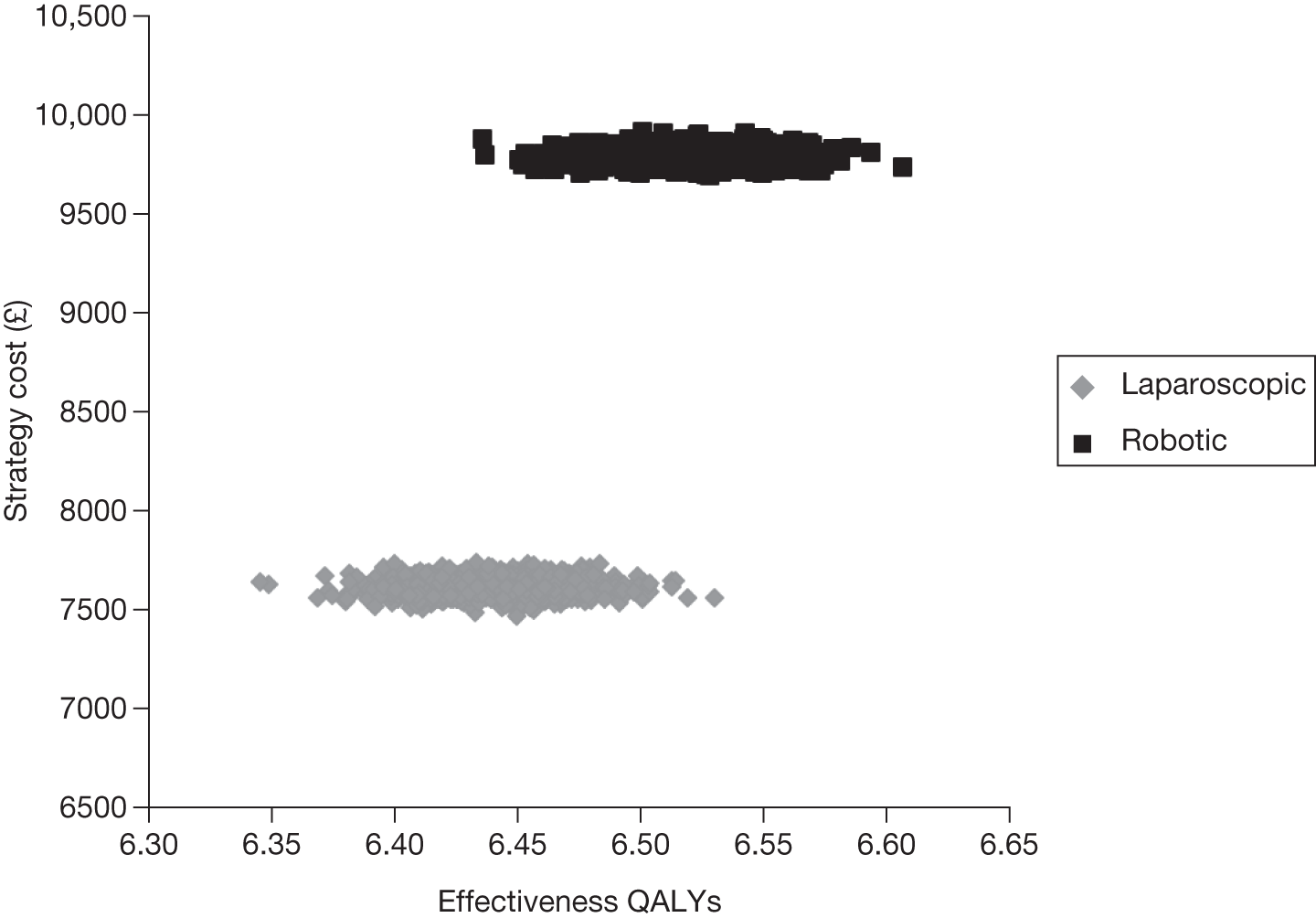
FIGURE 30.
Cost-effectiveness acceptability curve for each intervention in the base case, 10-year time horizon (150 procedures).
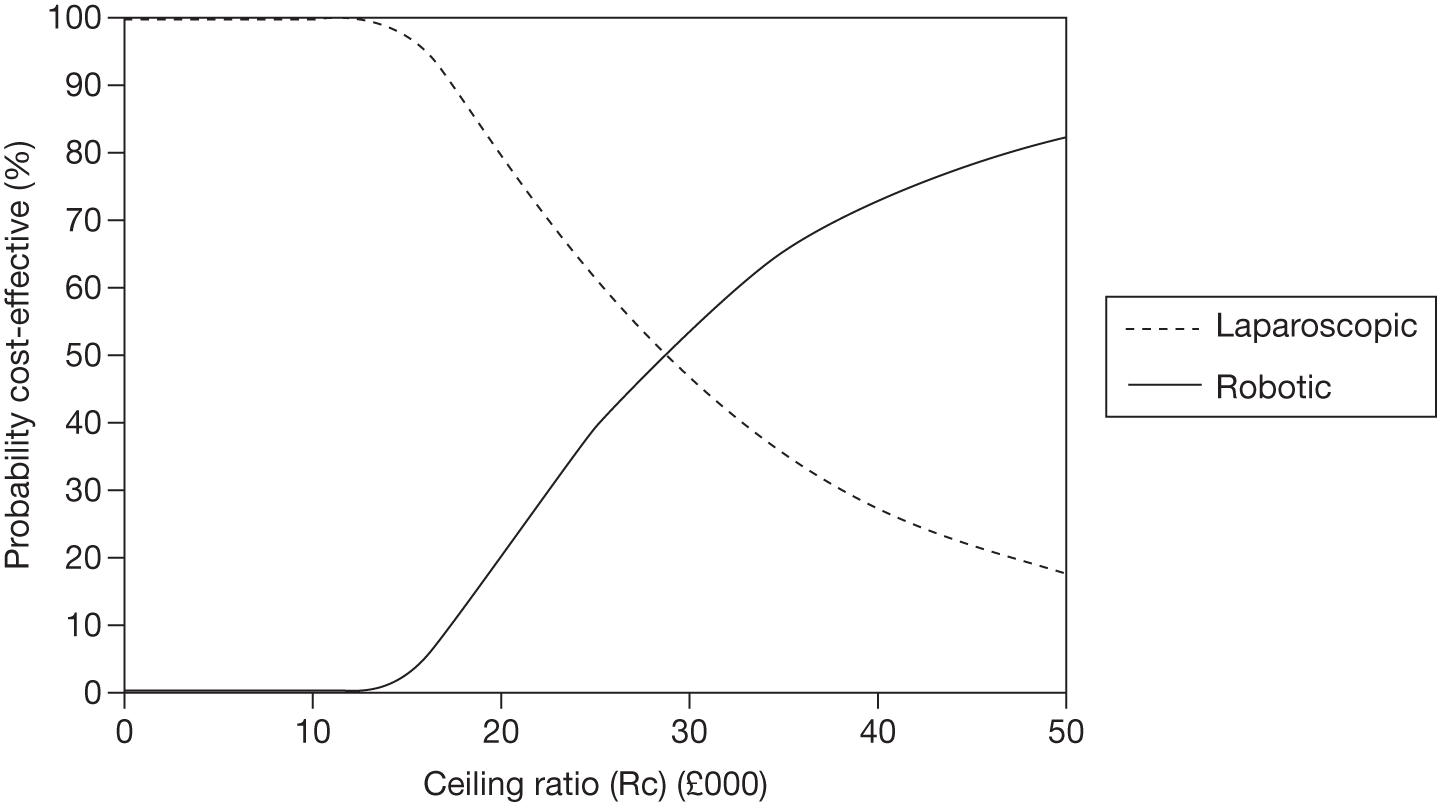
FIGURE 31.
Distribution of costs and QALYs for each intervention in the base case, 10-year time horizon (100 procedures).
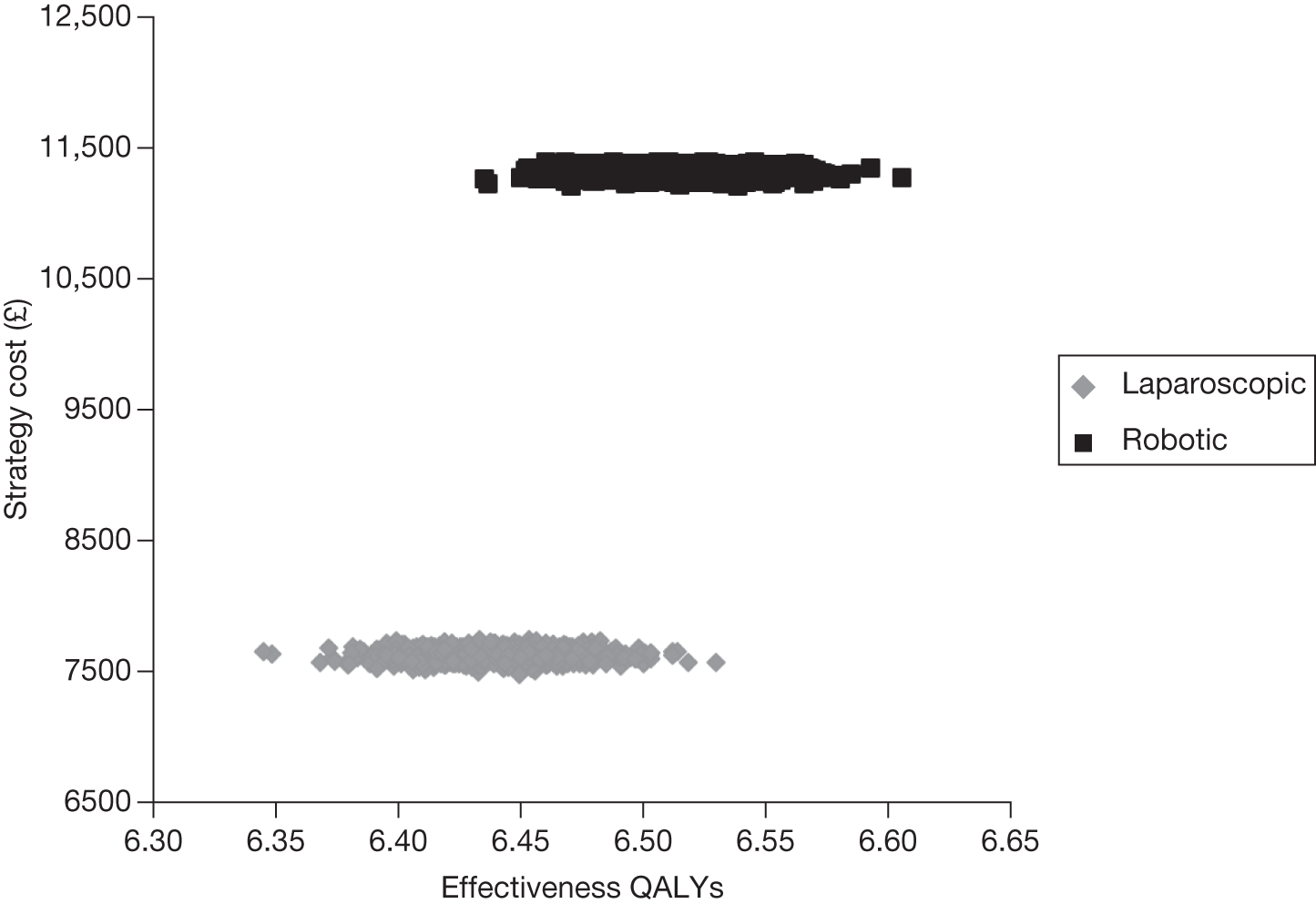
FIGURE 32.
Cost-effectiveness acceptability curve for each intervention in the base case, 10-year time horizon (100 procedures).
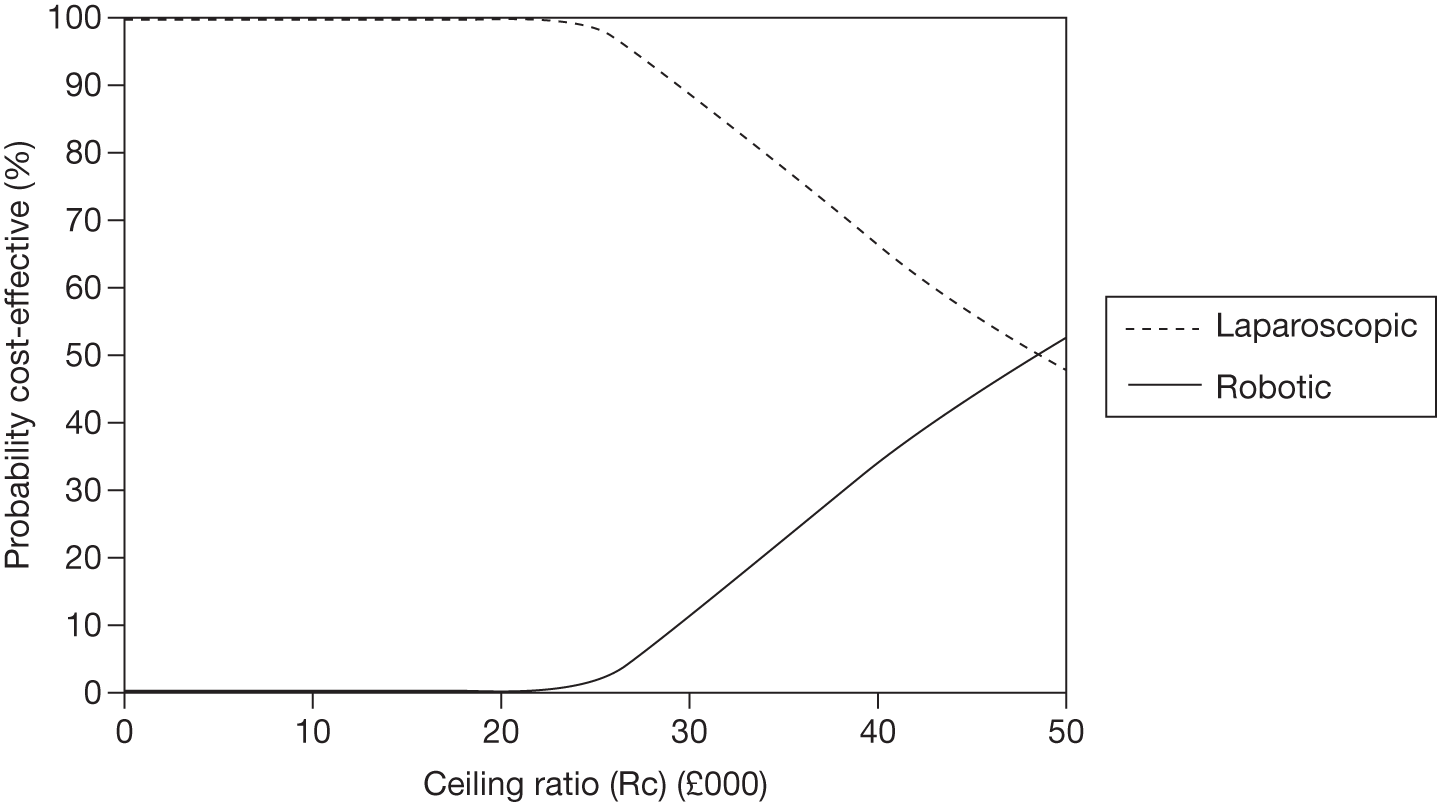
FIGURE 33.
Distribution of costs and QALYs for each intervention in the base case, 10-year time horizon (50 procedures).
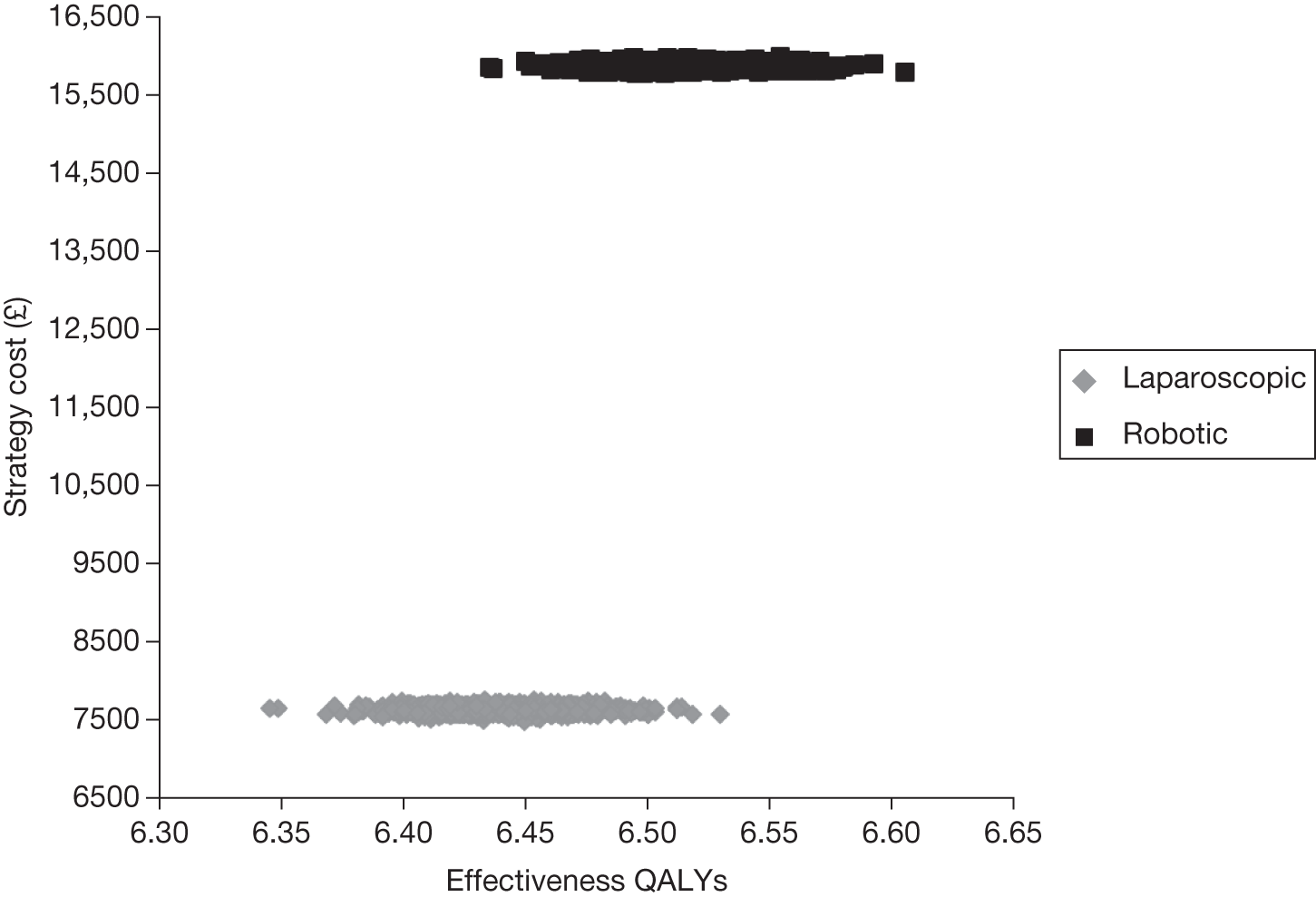
FIGURE 34.
Cost-effectiveness acceptability curve for each intervention in the base case, 10-year time horizon (50 procedures).
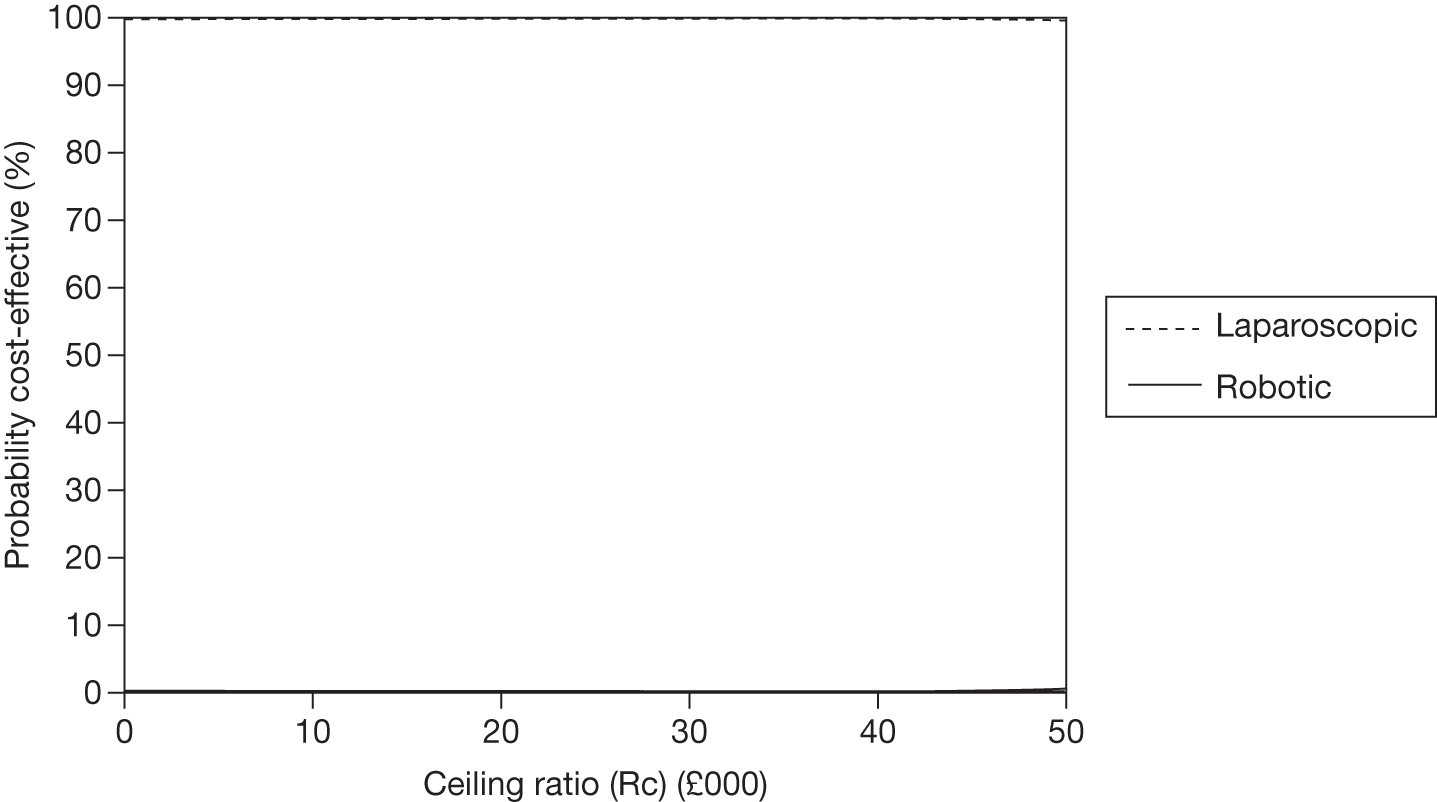
FIGURE 35.
Distribution of costs and QALYs for each intervention in the base case, 10-year time horizon (200 procedures using the least expensive procurement plan for the robotic system).
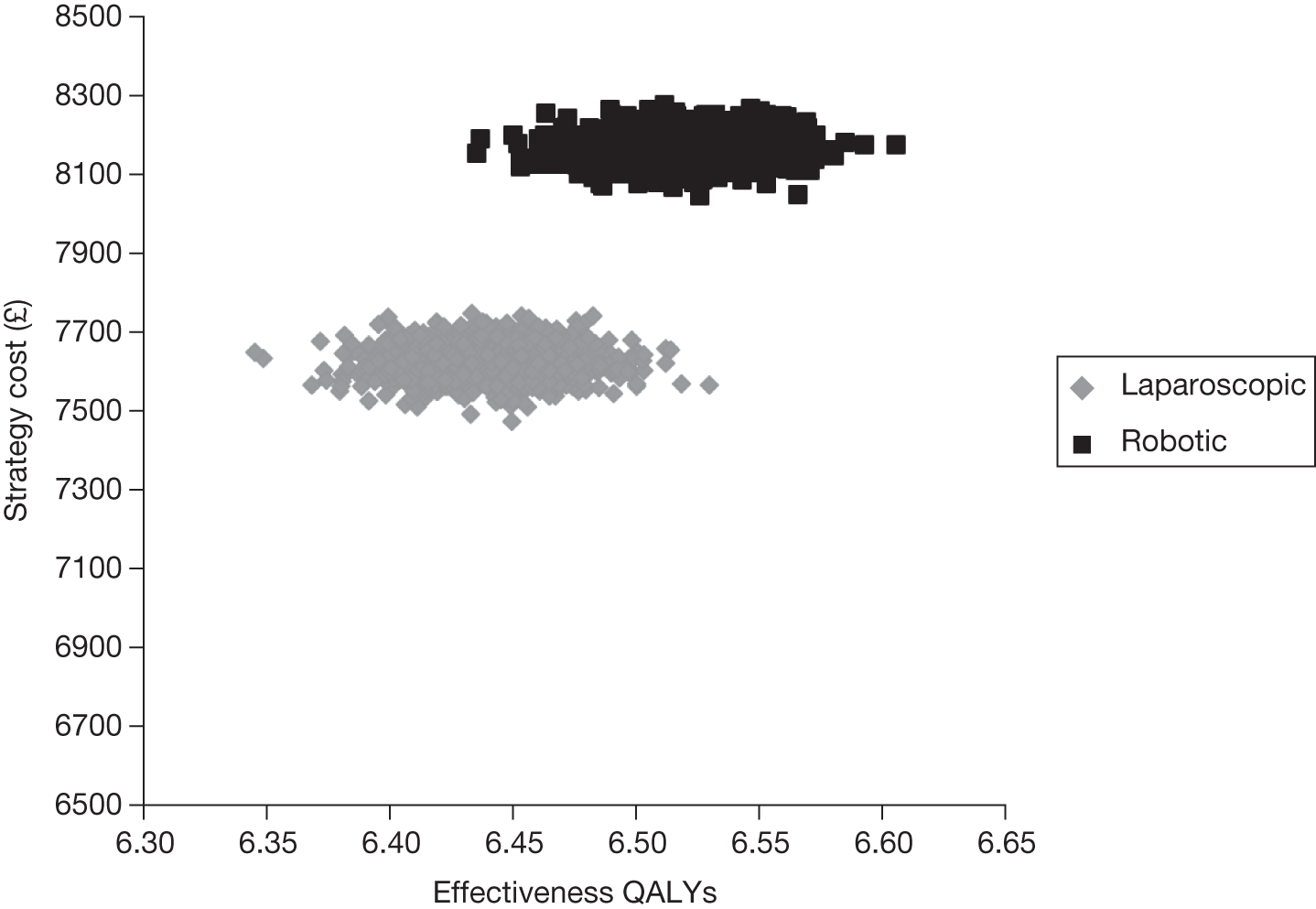
FIGURE 36.
Cost-effectiveness acceptability curve for each intervention in the base case, 10-year time horizon (200 procedures using the least expensive procurement plan for the robotic system).
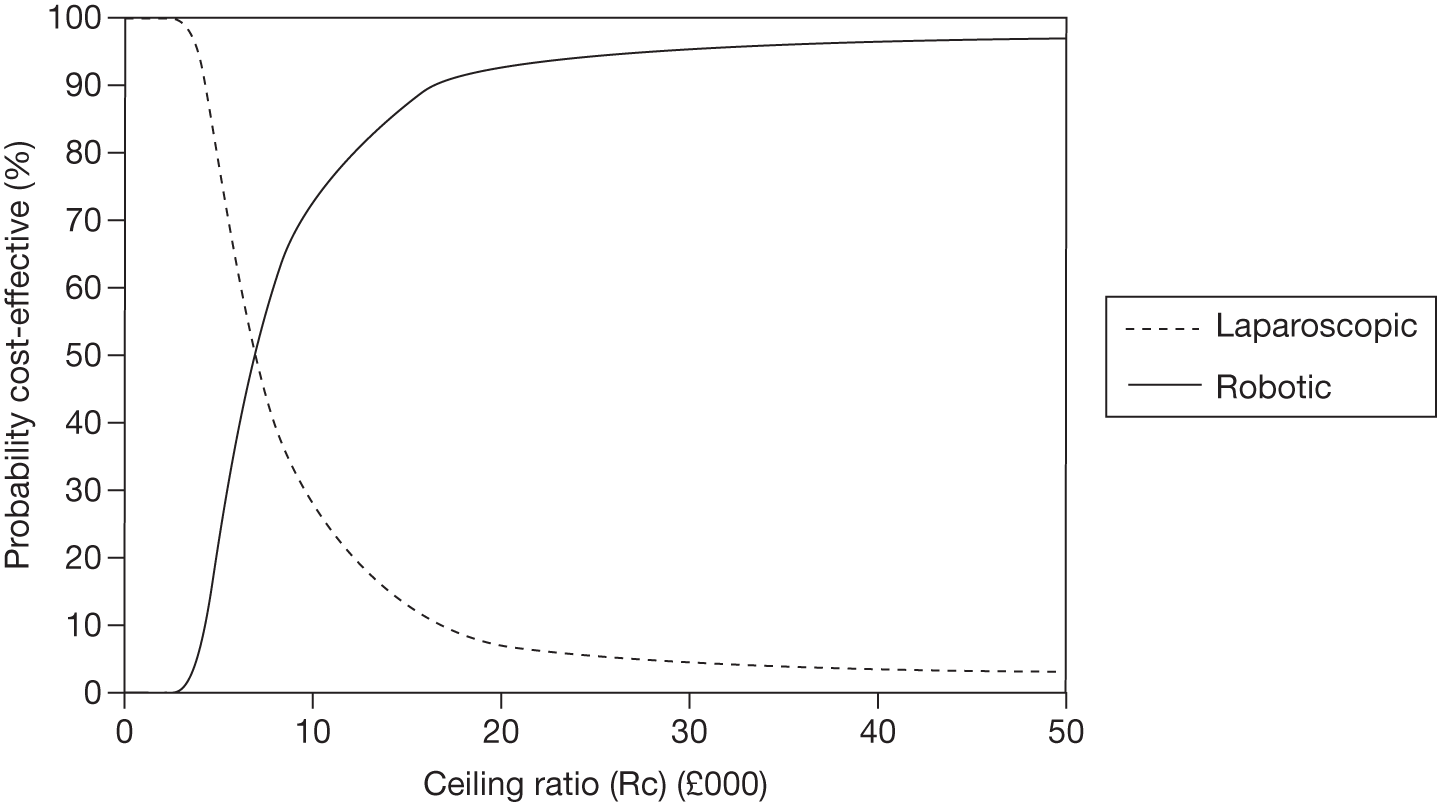
FIGURE 37.
Distribution of costs and QALYs for each intervention in the base case, 70-year time horizon (200 procedures).
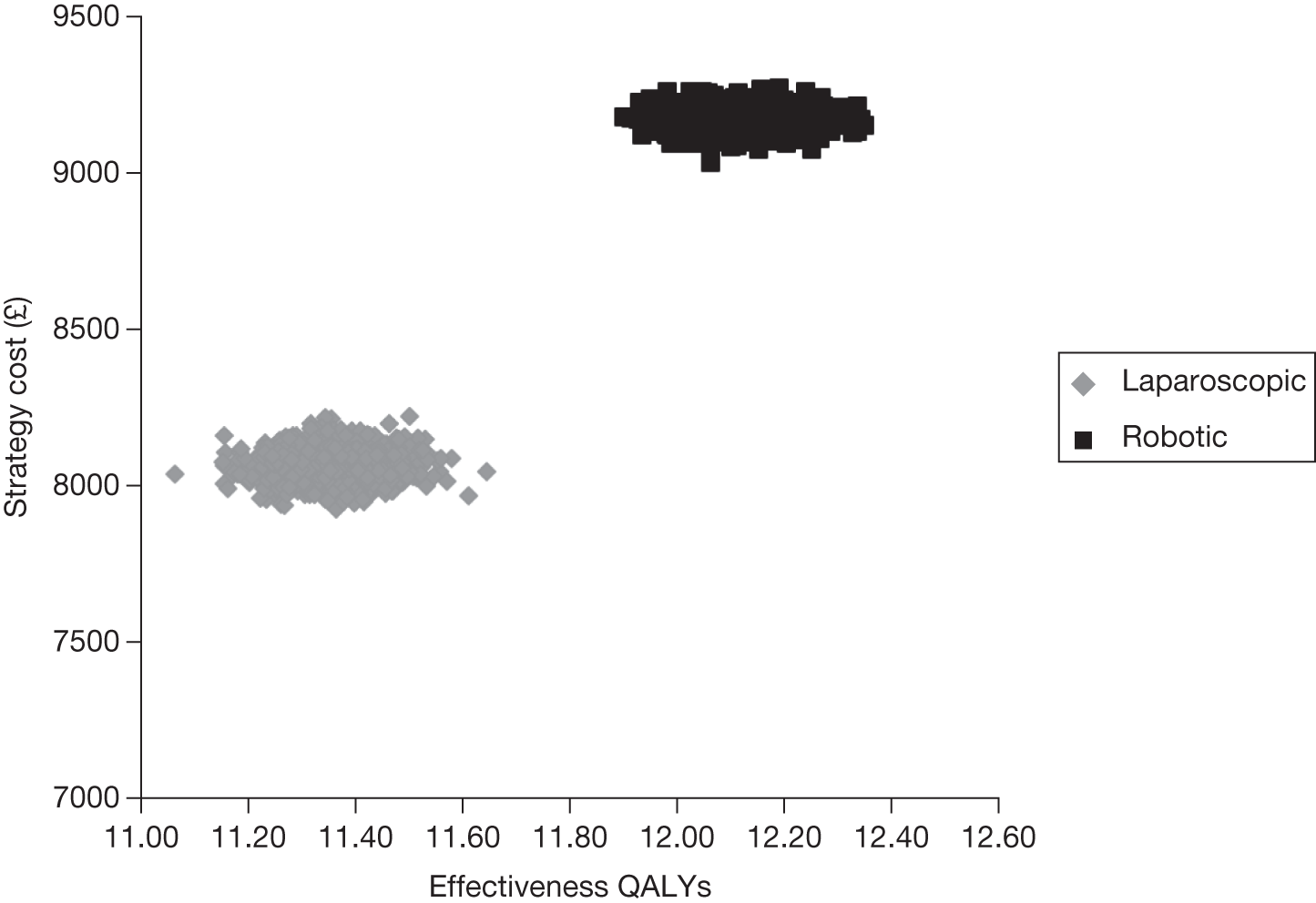
FIGURE 38.
Cost-effectiveness acceptability curve for each intervention in the base case, 70-year time horizon (200 procedures).
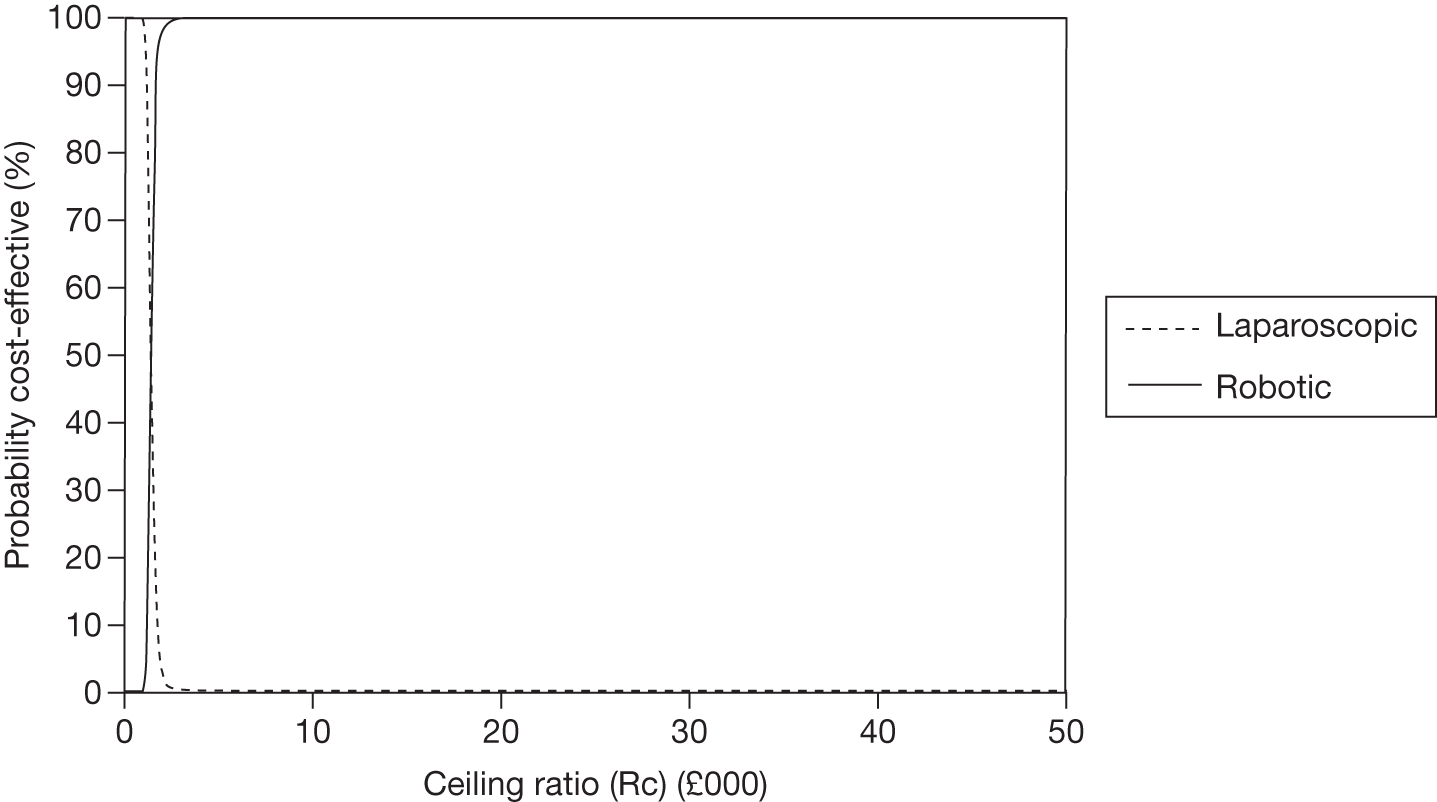
FIGURE 39.
Distribution of costs and QALYs for each intervention in the base case, 70-year time horizon (150 procedures).
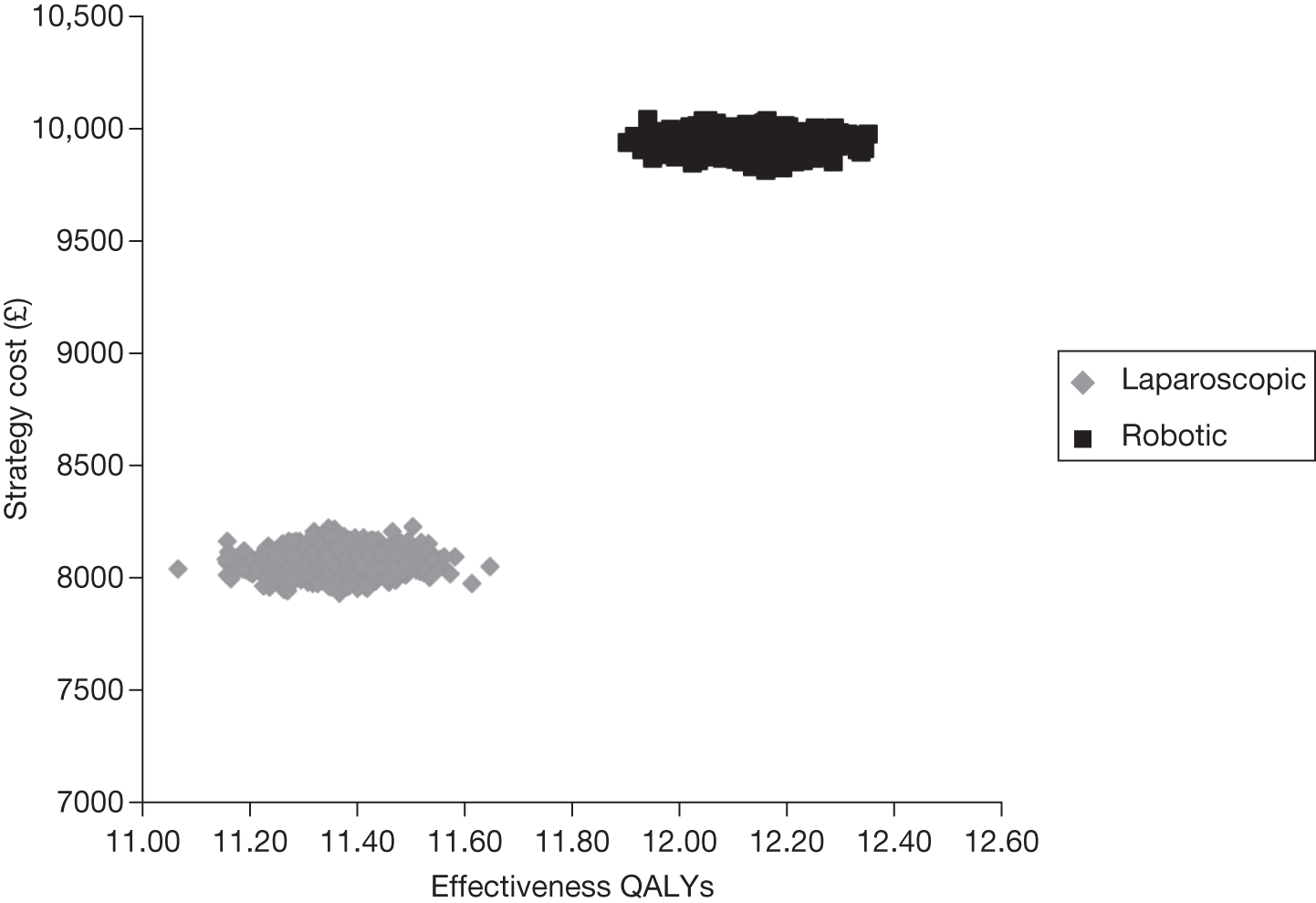
FIGURE 40.
Cost-effectiveness acceptability curve for each intervention in the base case, 70-year time horizon (150 procedures).
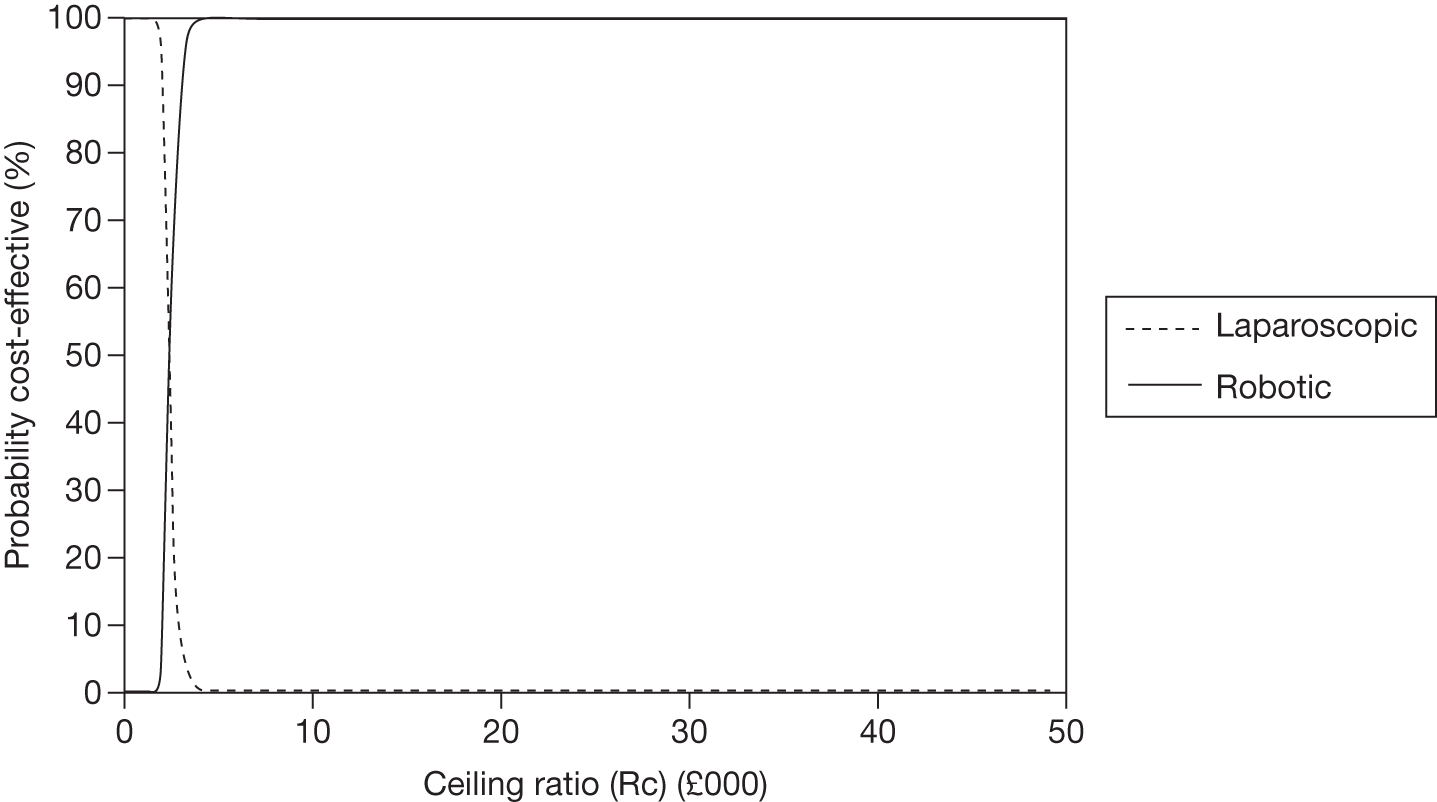
FIGURE 41.
Distribution of costs and QALYs for each intervention in the base case, 70-year time horizon (100 procedures).
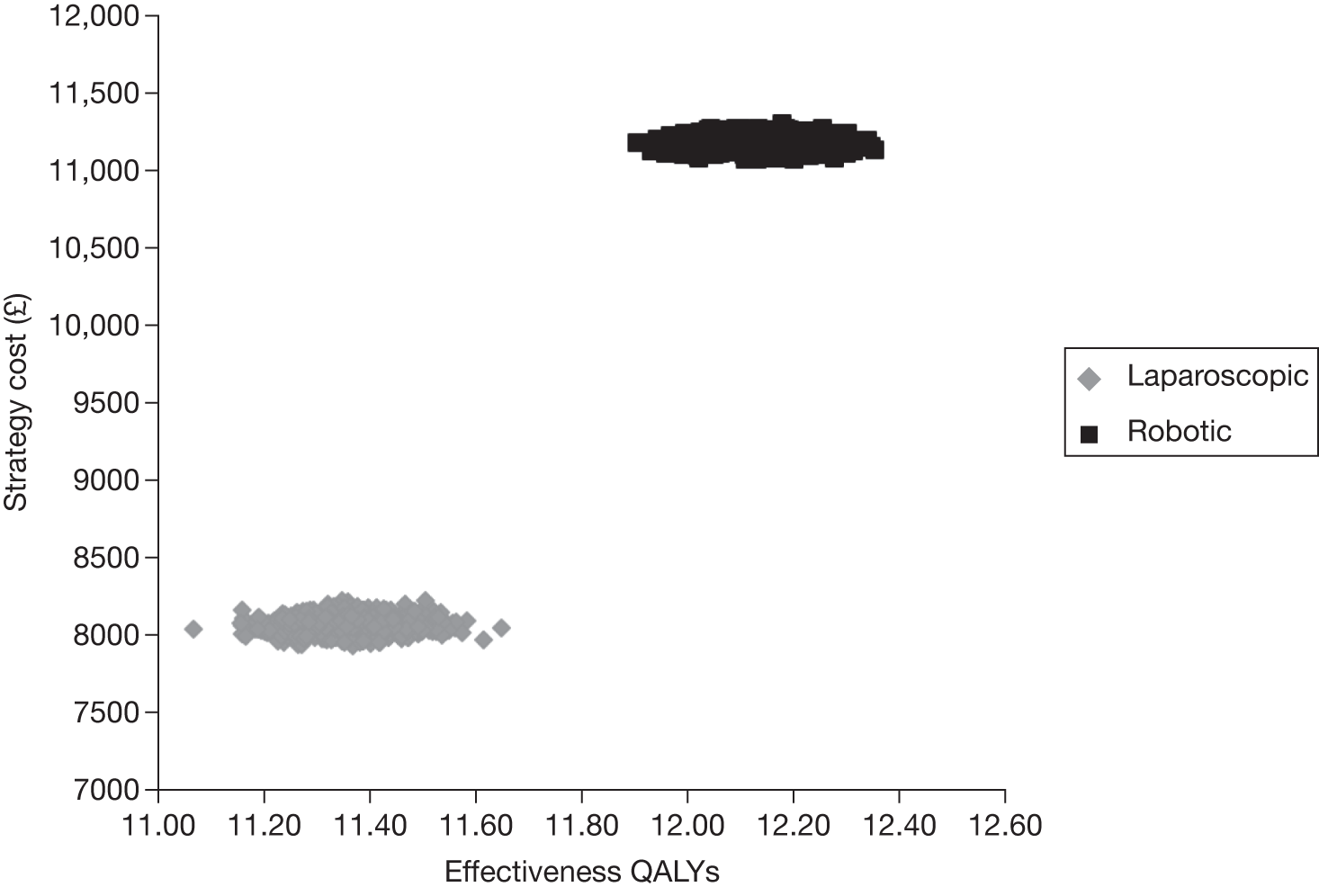
FIGURE 42.
Cost-effectiveness acceptability curve for each intervention in the base case, 70-year time horizon (100 procedures).
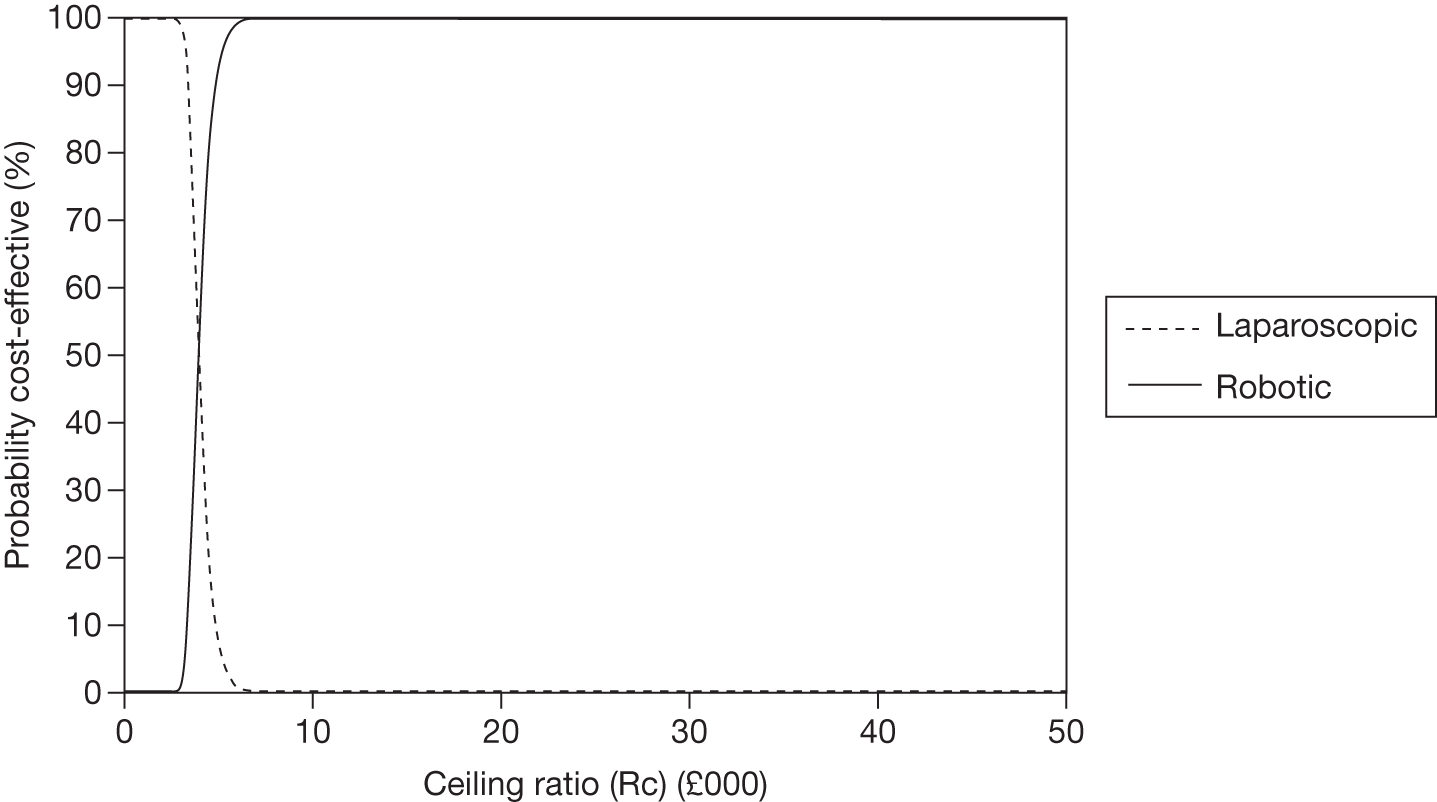
FIGURE 43.
Distribution of costs and QALYs for each intervention in the base case, 70-year time horizon (50 procedures).
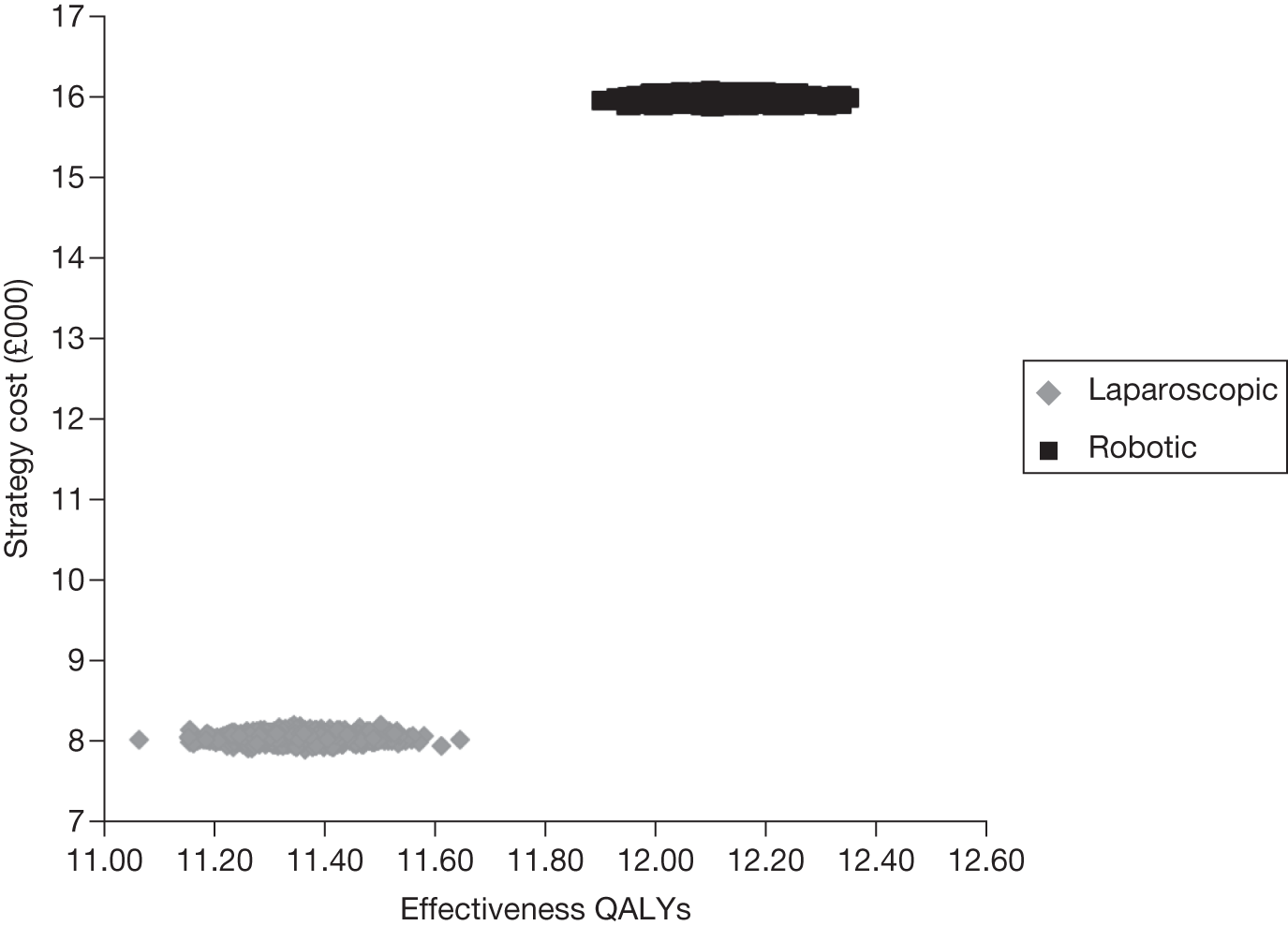
FIGURE 44.
Cost-effectiveness acceptability curve for each intervention in the base case, 70-year time horizon (50 procedures).
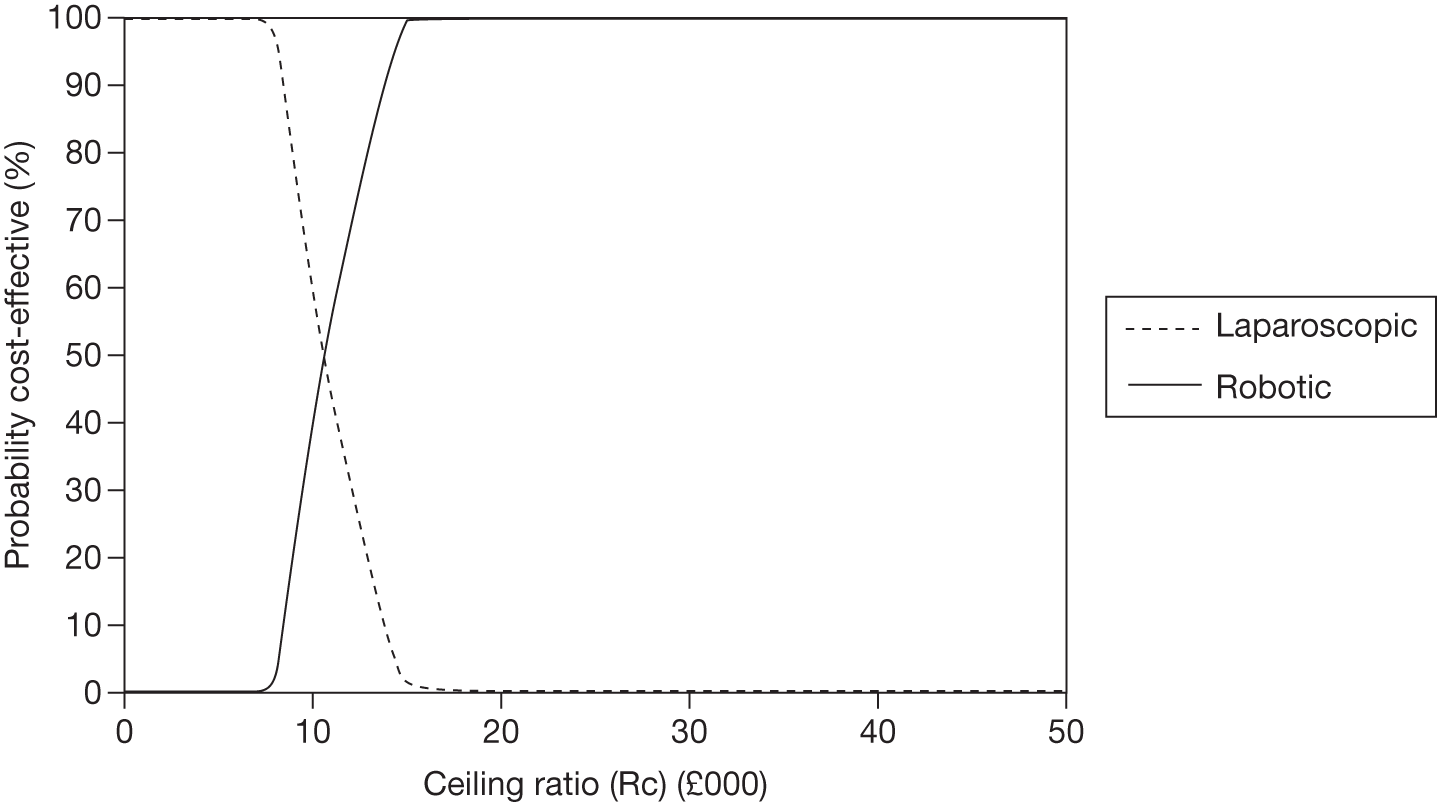
FIGURE 45.
Distribution of costs and QALYs for each intervention in the base case, 70-year time horizon (200 procedures using the least expensive procurement plan for the robotic system).
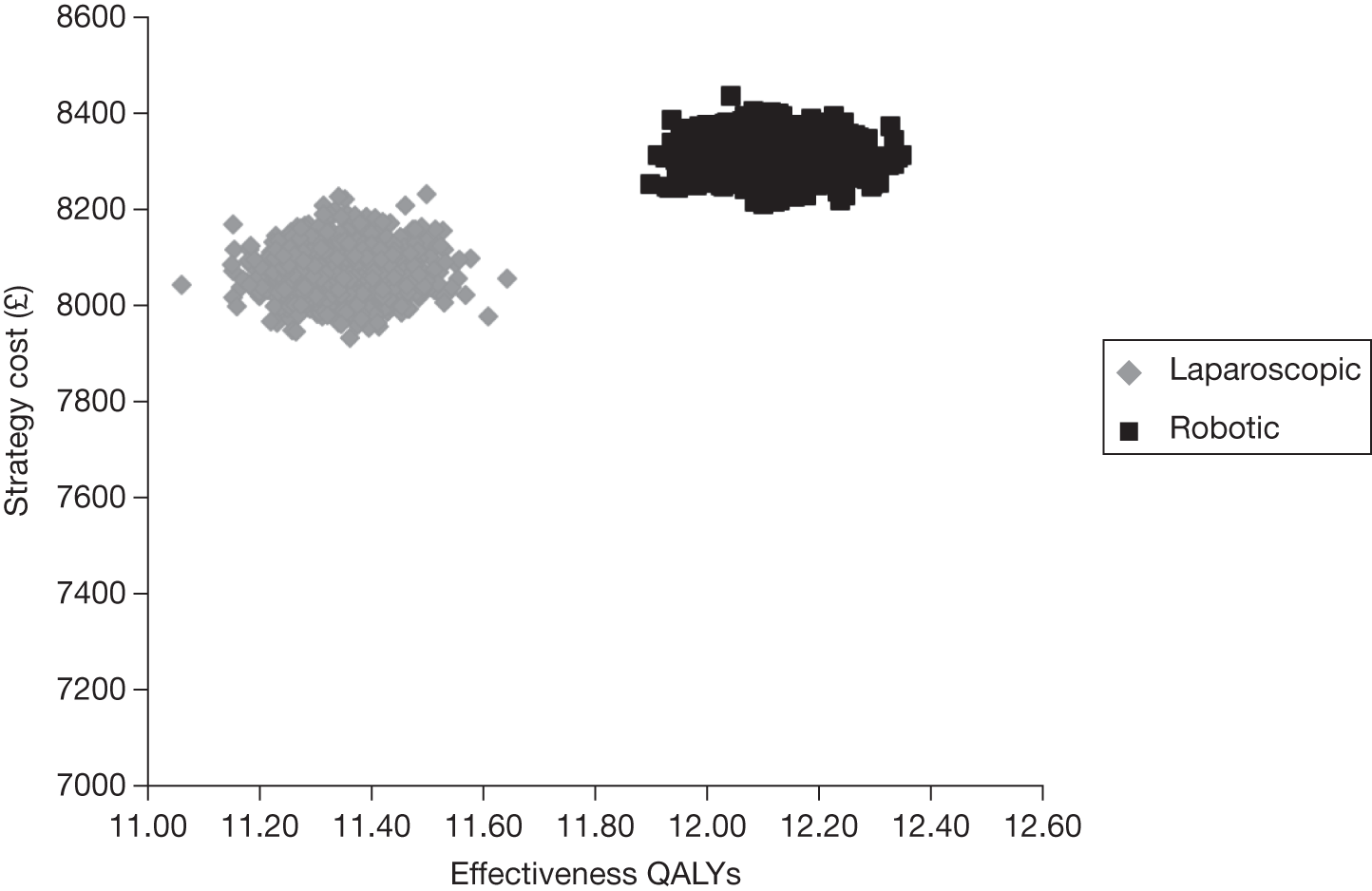
FIGURE 46.
Cost-effectiveness acceptability curve for each intervention in the base case, 70-year time horizon (200 procedures using the least expensive procurement plan for the robotic system).
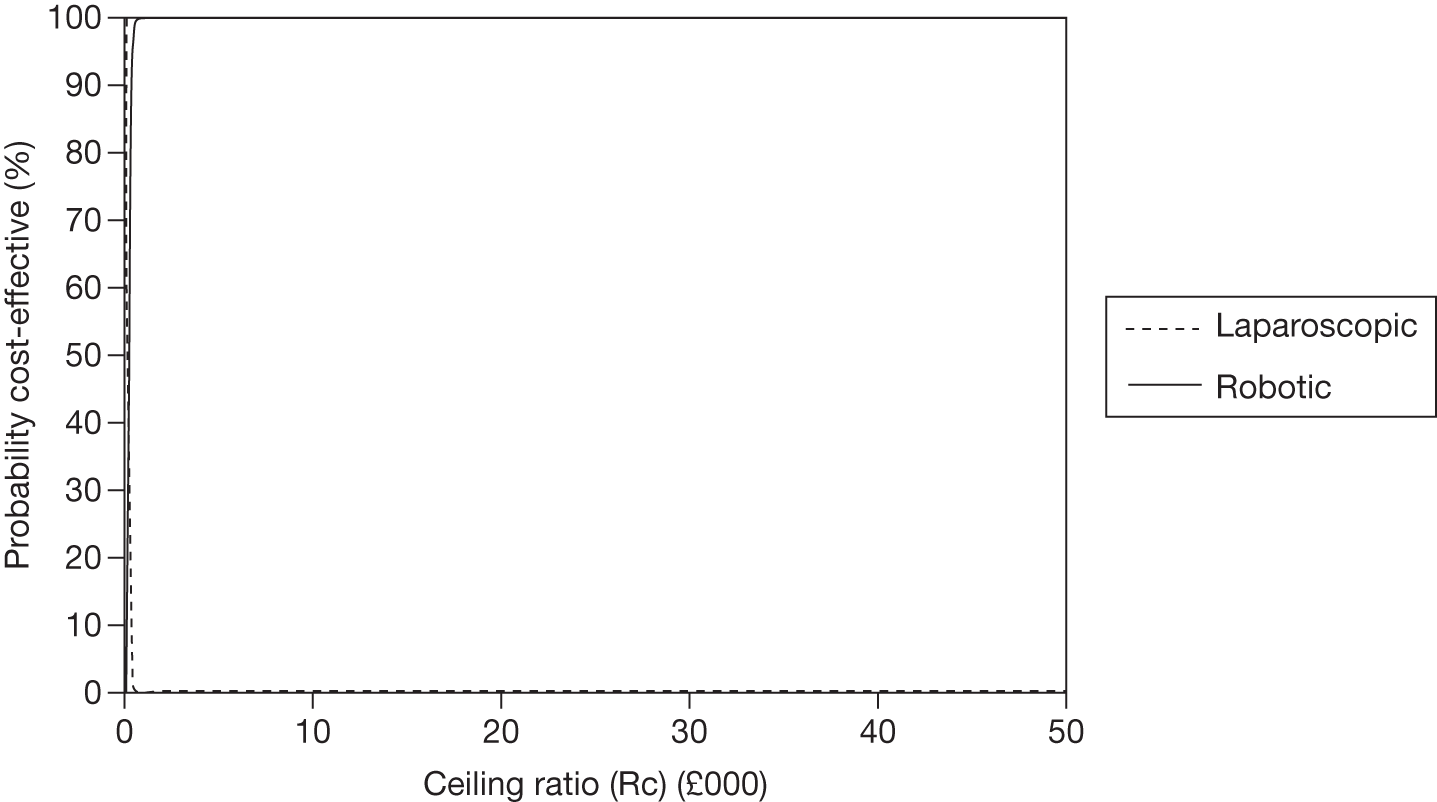
FIGURE 47.
Distribution of costs and QALYs for each intervention following sensitivity analysis at the higher rate of biochemical recurrence (200 procedures).
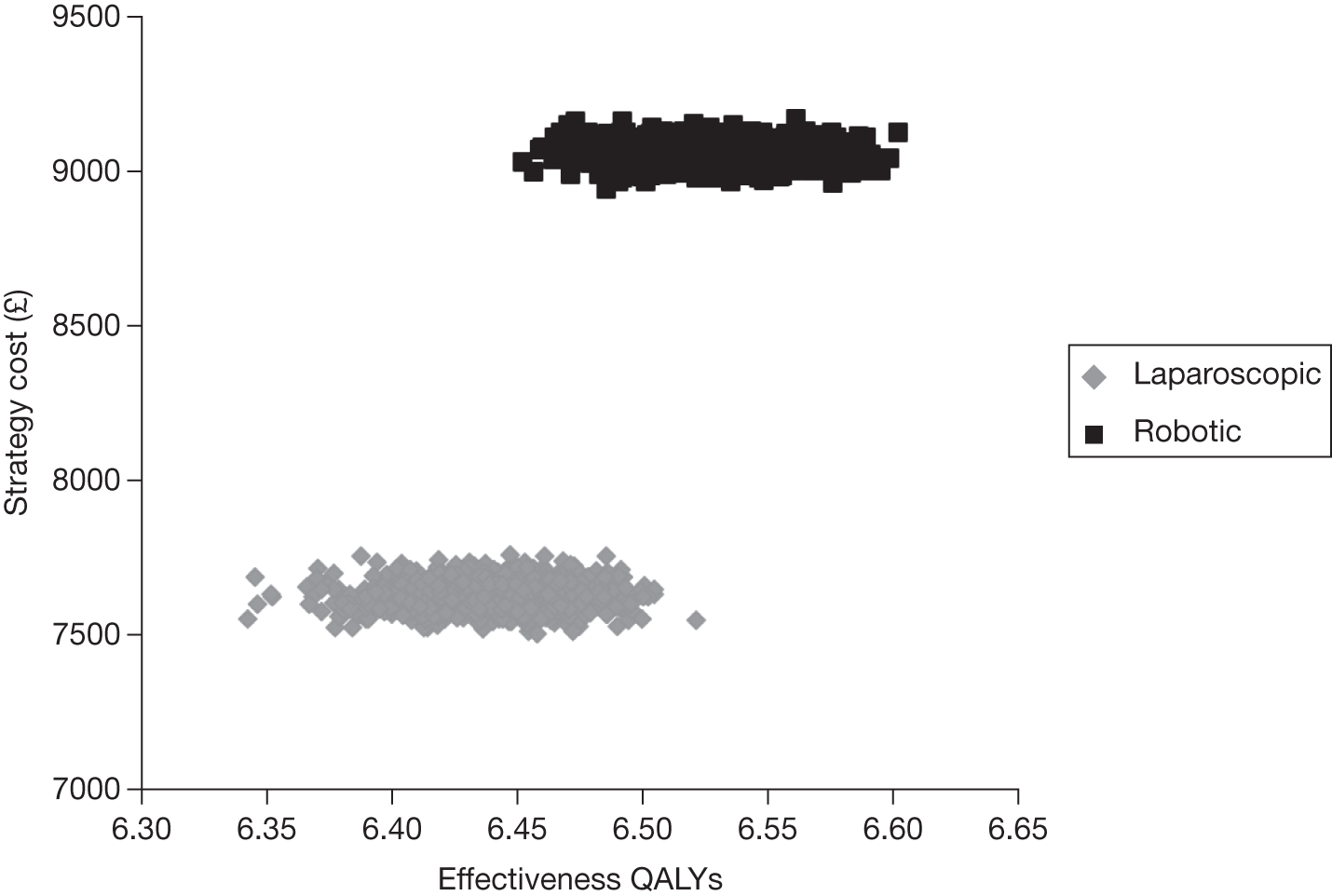
FIGURE 48.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis at the higher rate of biochemical recurrence (200 procedures).
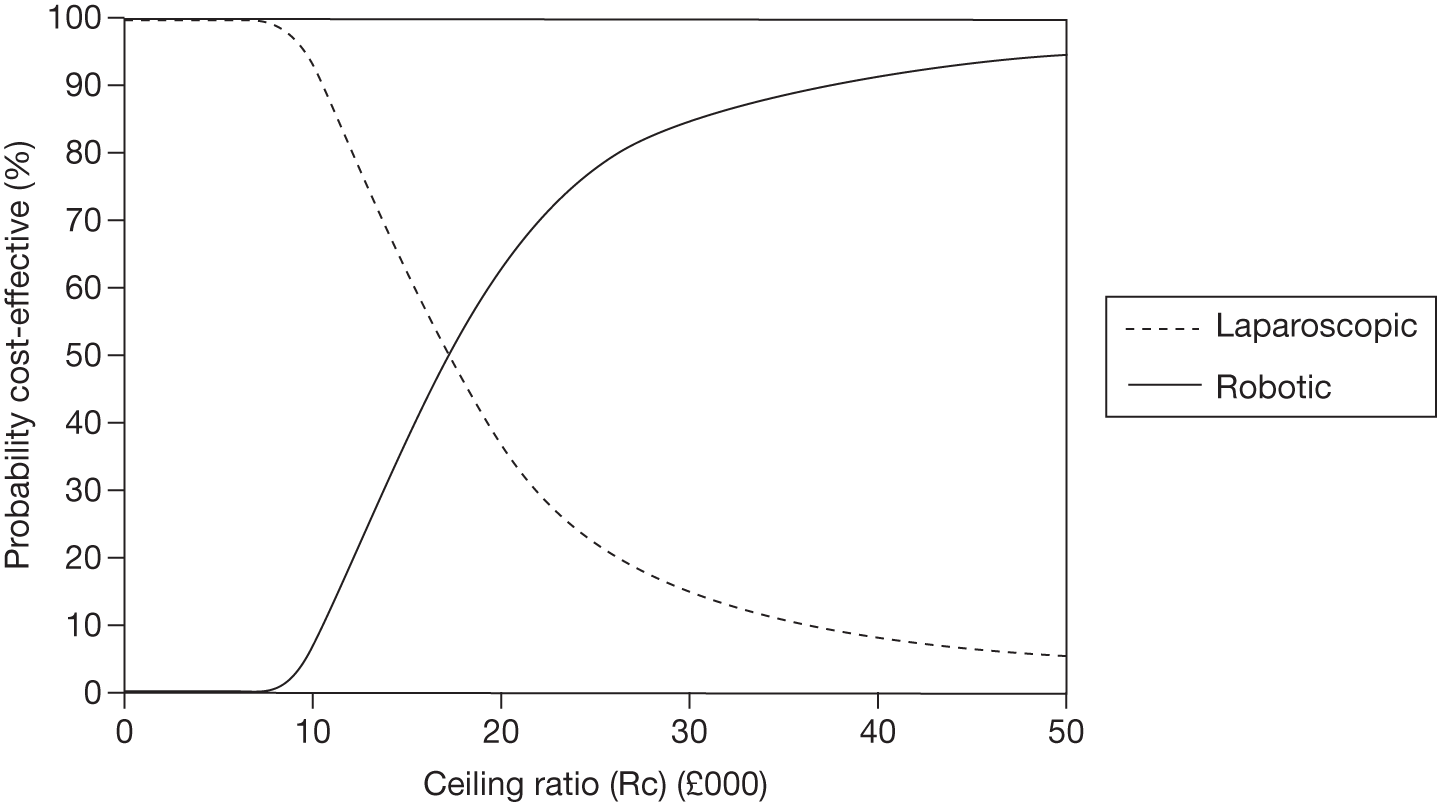
FIGURE 49.
Distribution of costs and QALYs for each intervention following sensitivity analysis at the higher rate of biochemical recurrence (150 procedures).
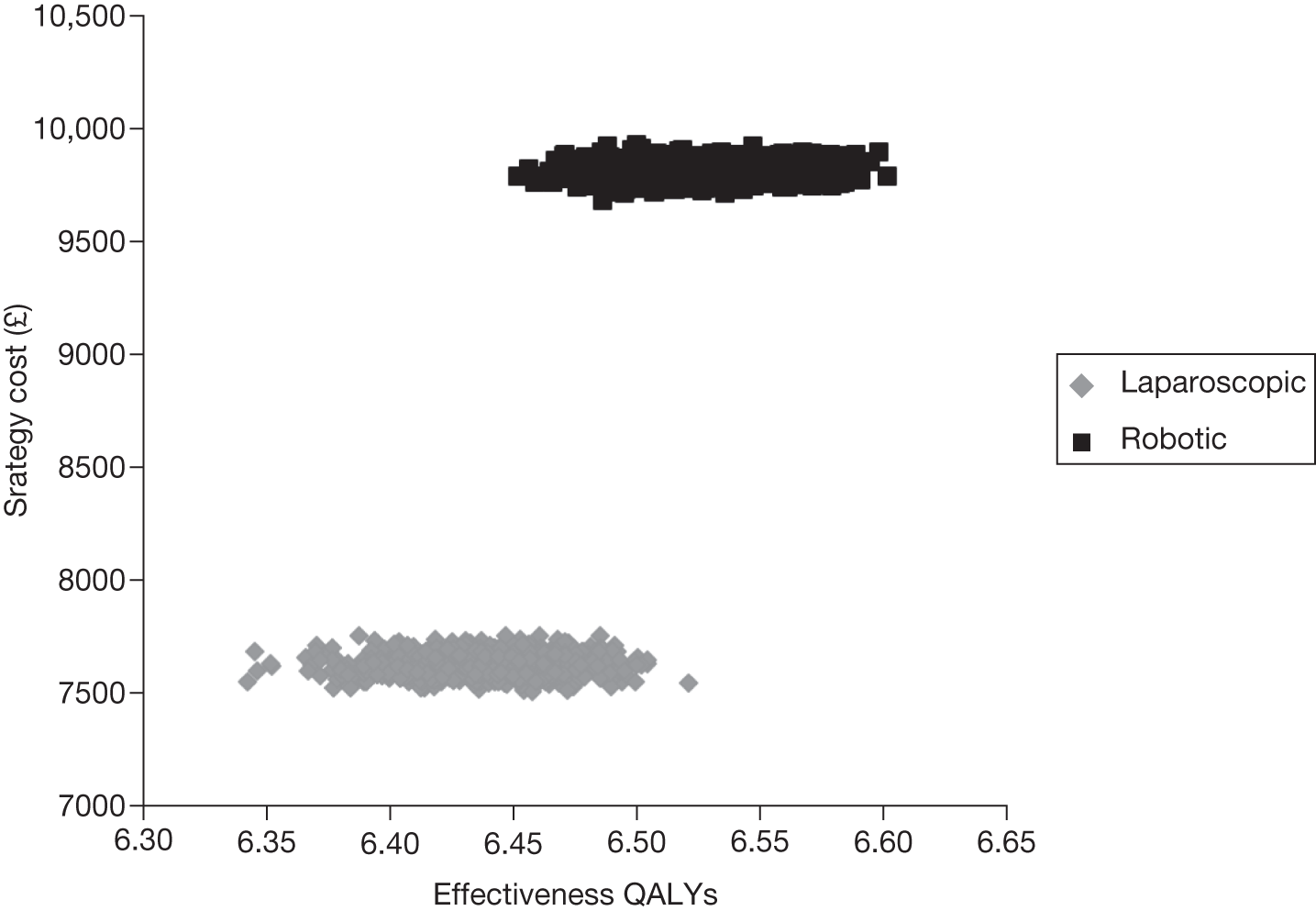
FIGURE 50.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis at the higher rate of biochemical recurrence (150 procedures).
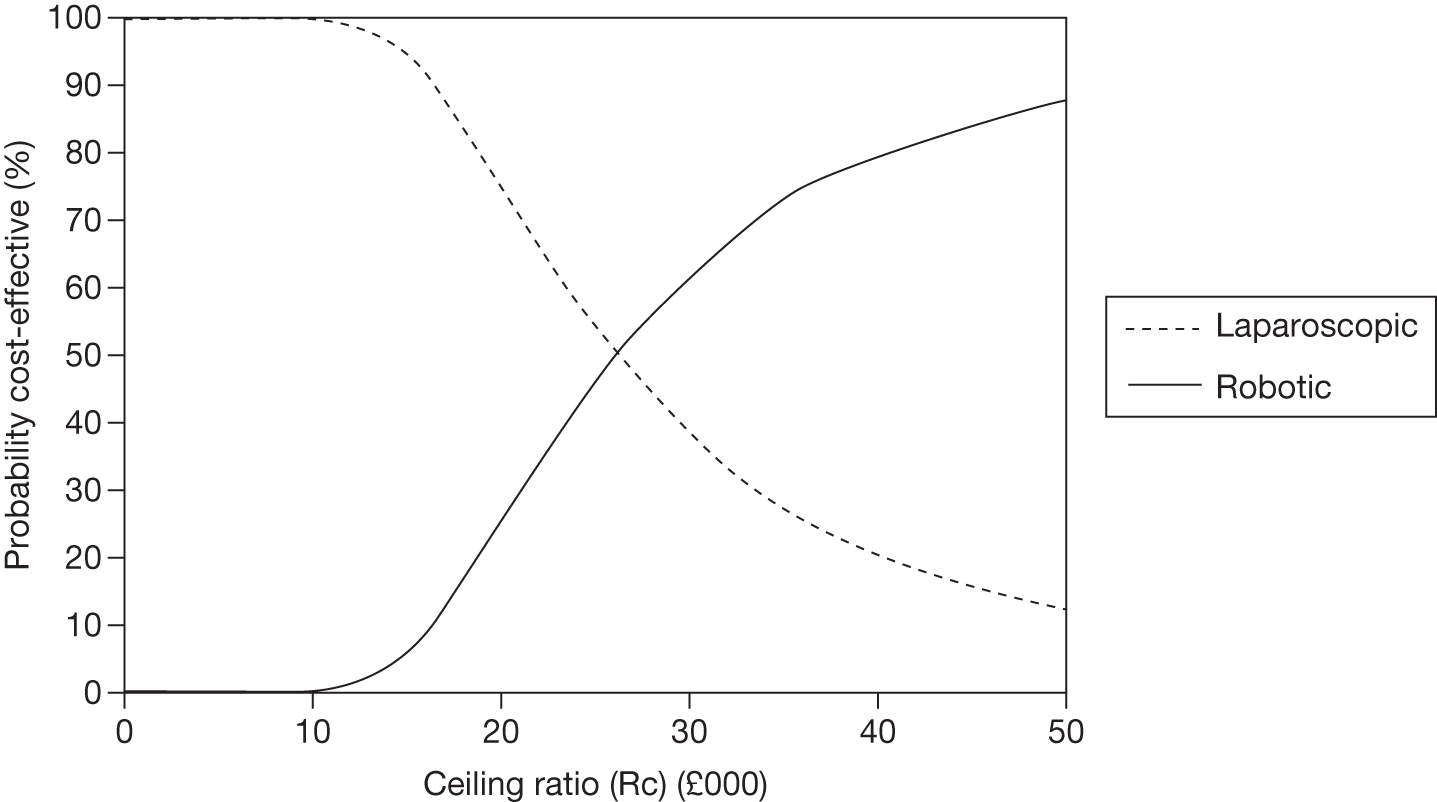
FIGURE 51.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the higher rate of biochemical recurrence (100 procedures).

FIGURE 52.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years at the higher rate of biochemical recurrence (100 procedures).
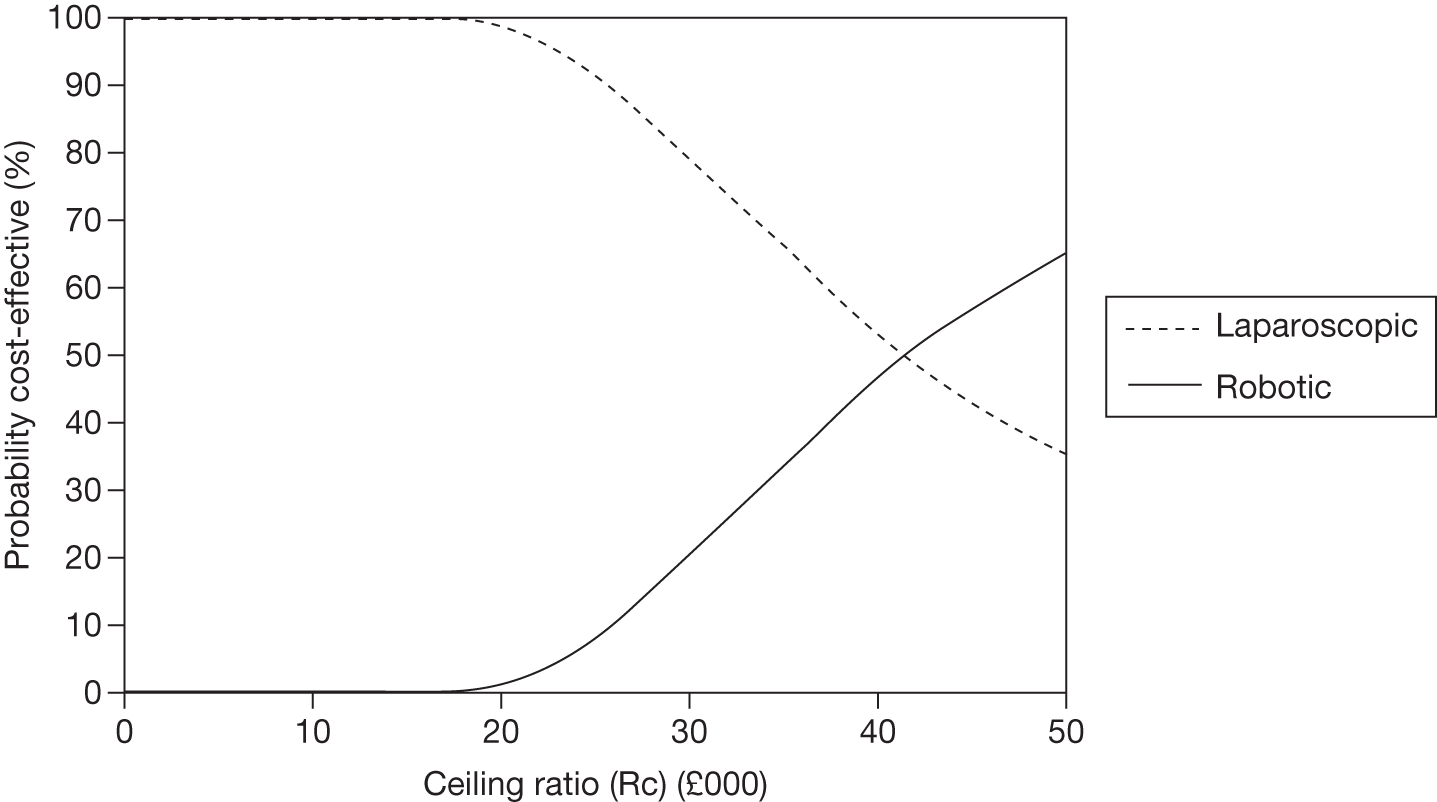
FIGURE 53.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the higher rate of biochemical recurrence (50 procedures).
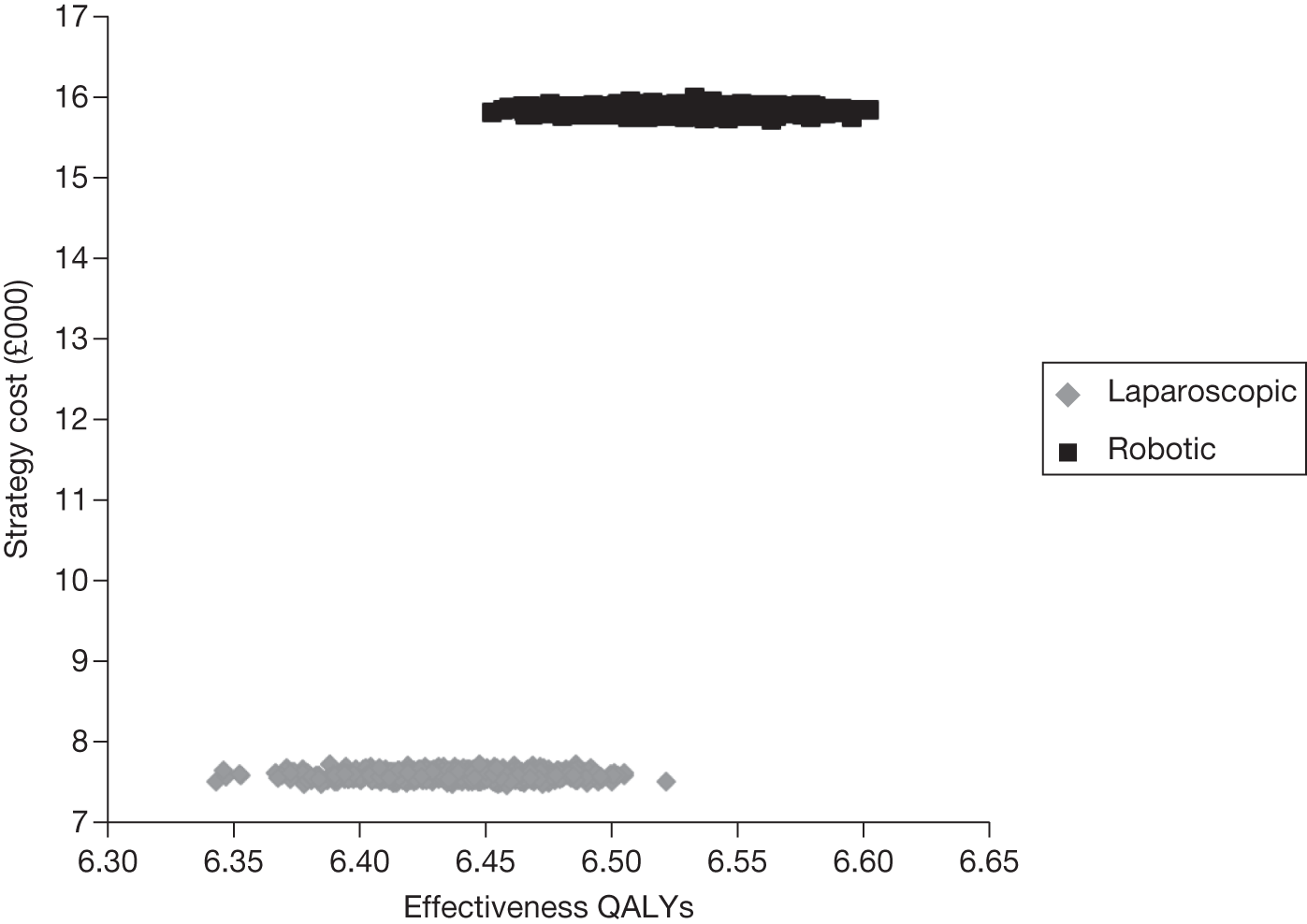
FIGURE 54.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years at the higher rate of biochemical recurrence (50 procedures).
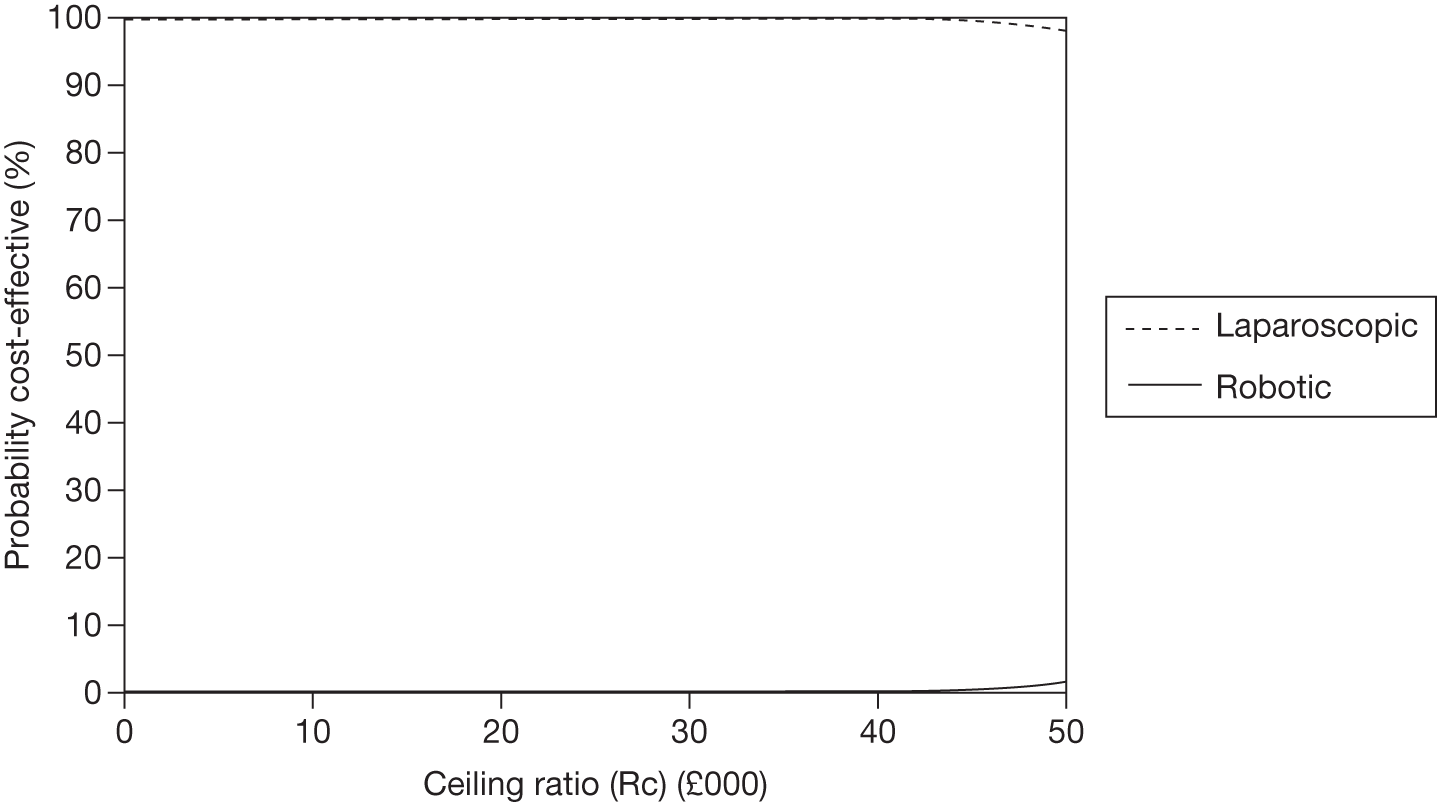
FIGURE 55.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the higher rate of biochemical recurrence (200 procedures using the least expensive procurement plan for the robotic system).
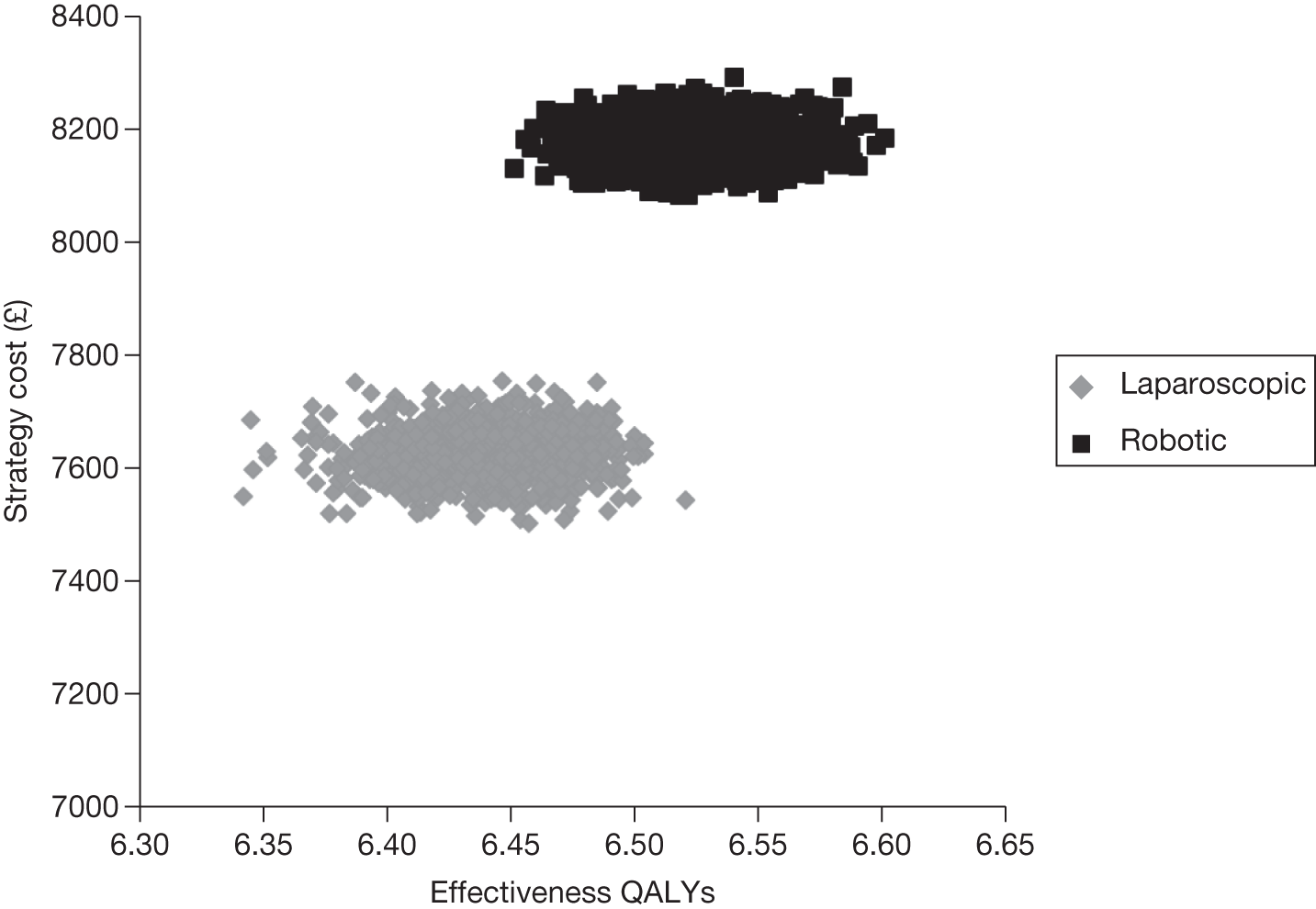
FIGURE 56.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years at the higher rate of biochemical recurrence (200 procedures using the least expensive procurement plan for the robotic system).
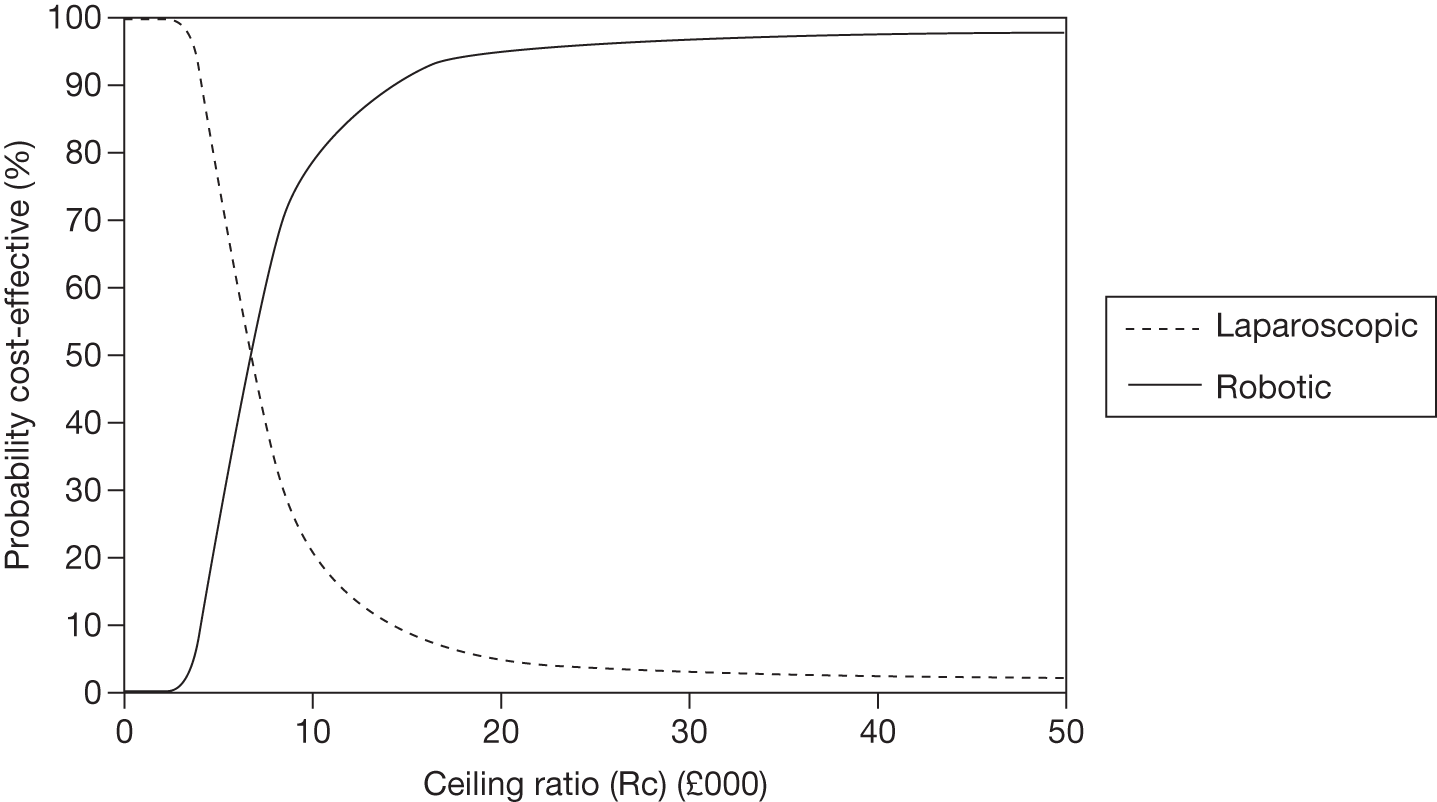
FIGURE 57.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the lower rate of biochemical recurrence (200 procedures).
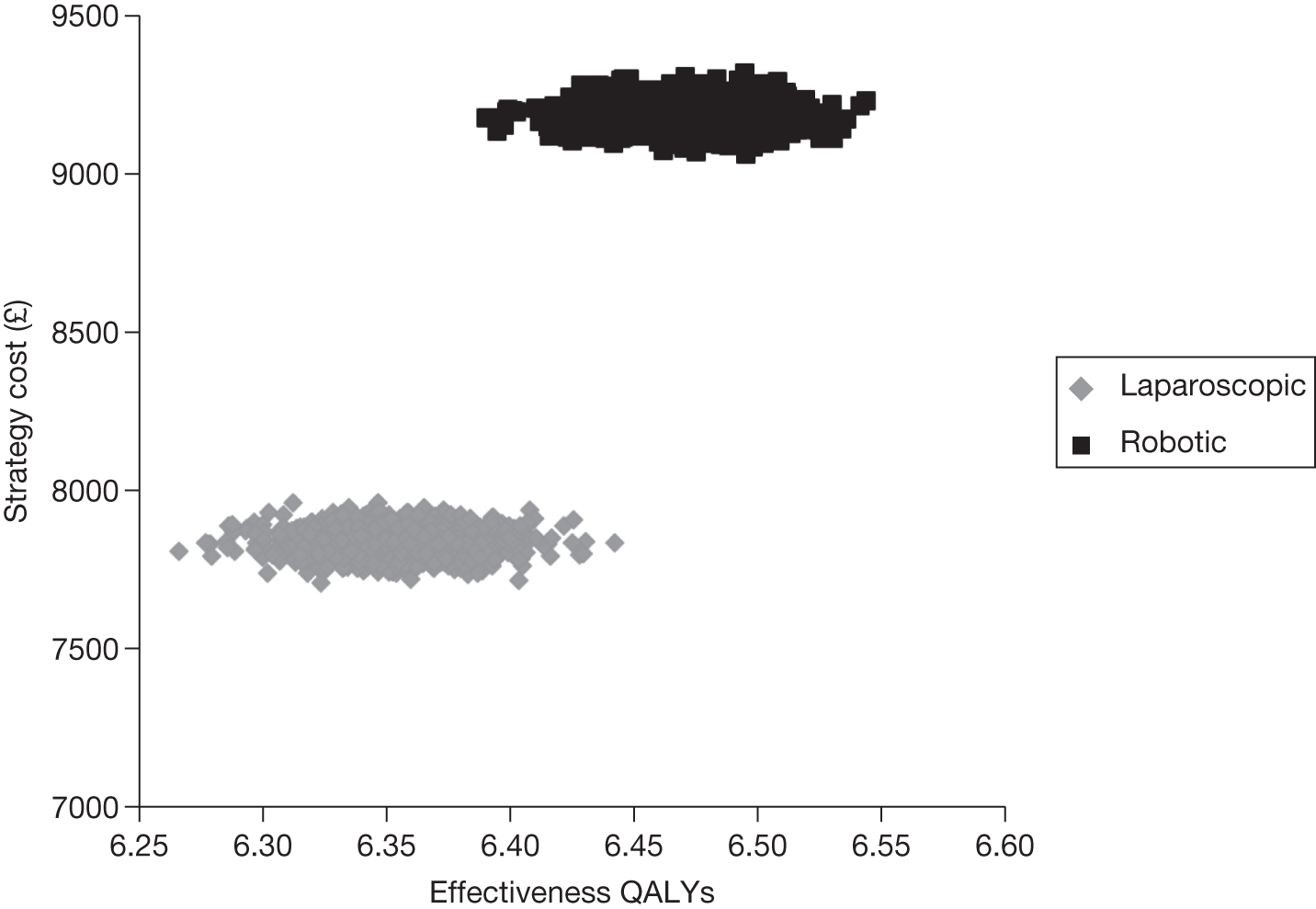
FIGURE 58.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years with rates of biochemical recurrence doubled (200 procedures).
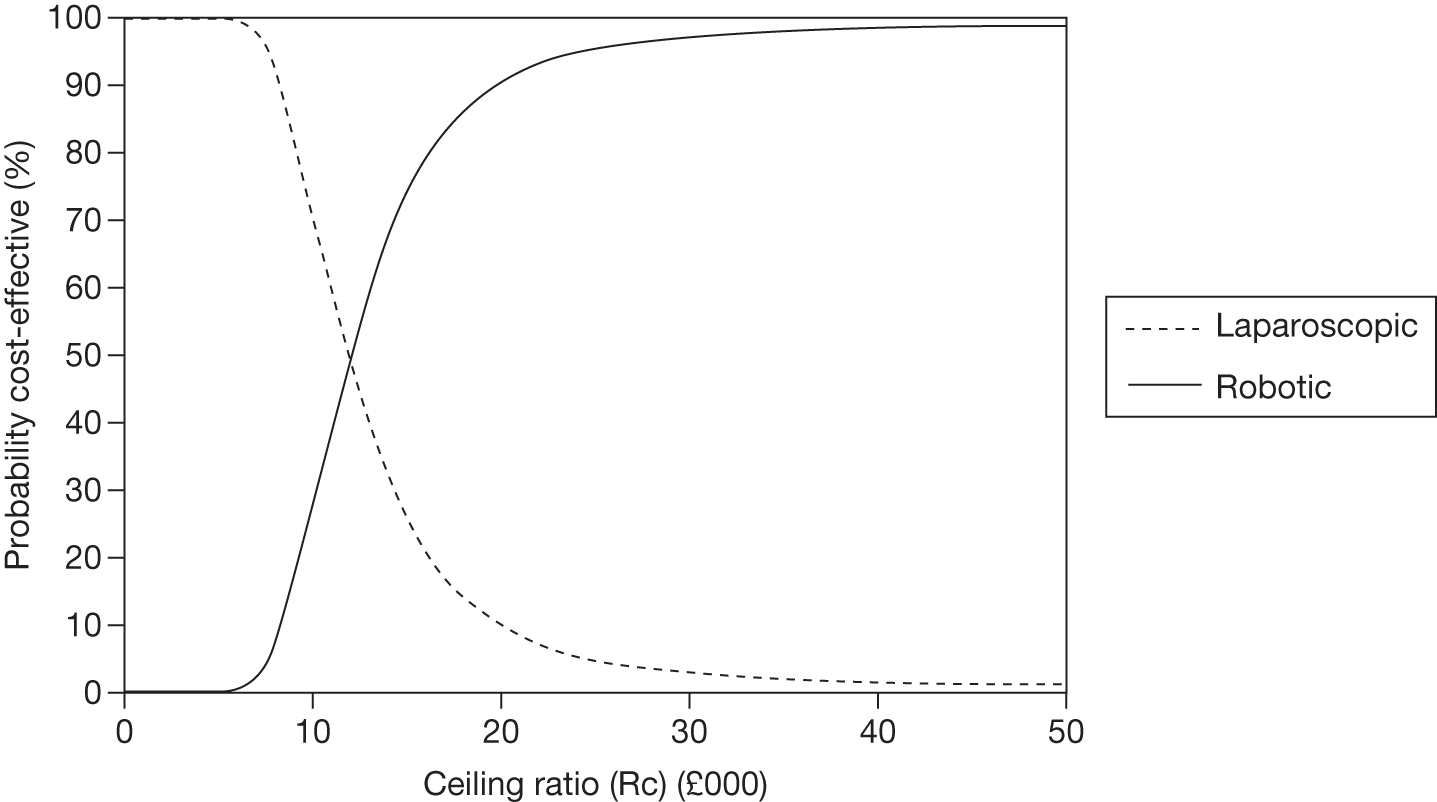
FIGURE 59.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the lower rate of biochemical recurrence (150 procedures).
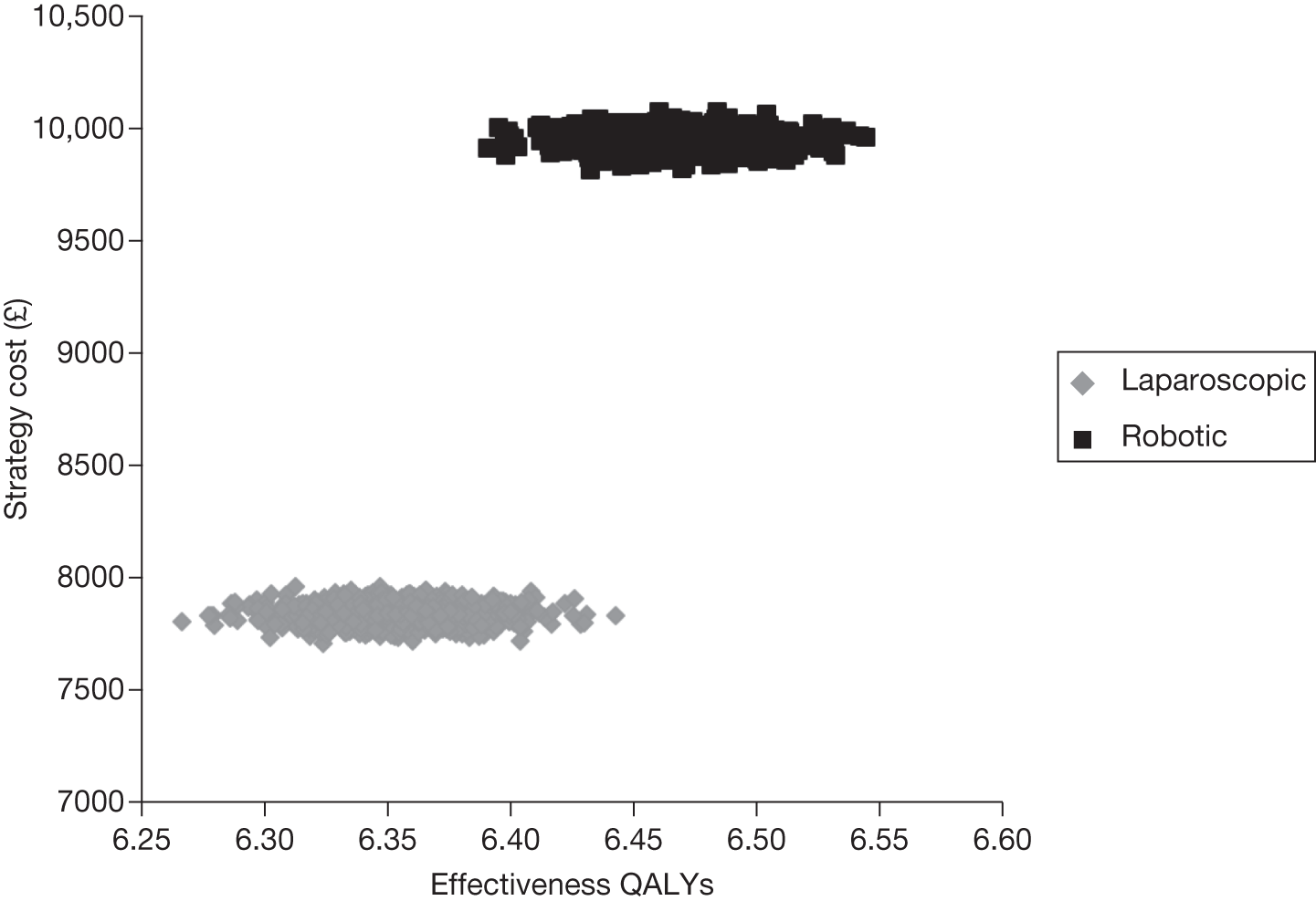
FIGURE 60.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years with rates of biochemical recurrence doubled (150 procedures).
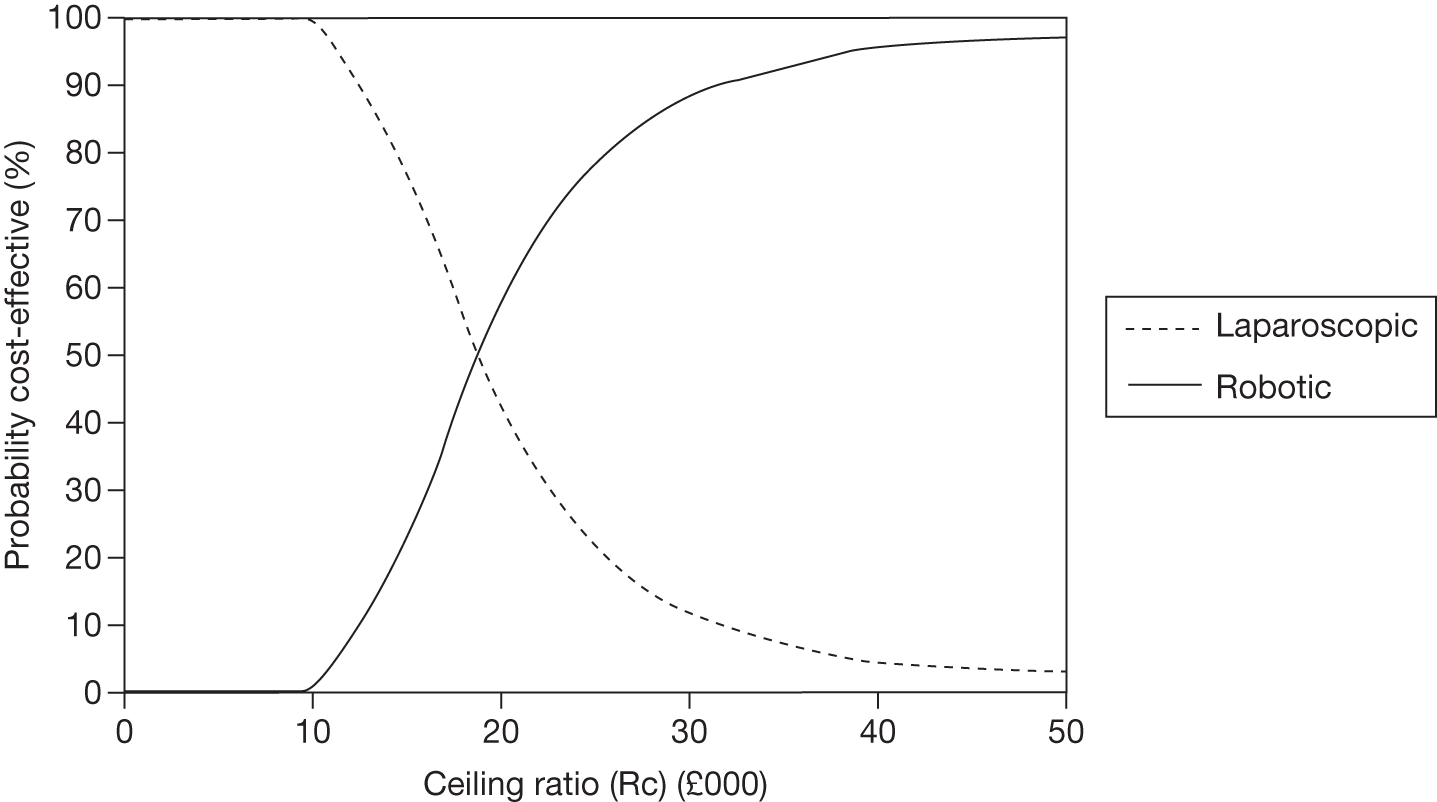
FIGURE 61.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the lower rate of biochemical recurrence (100 procedures).
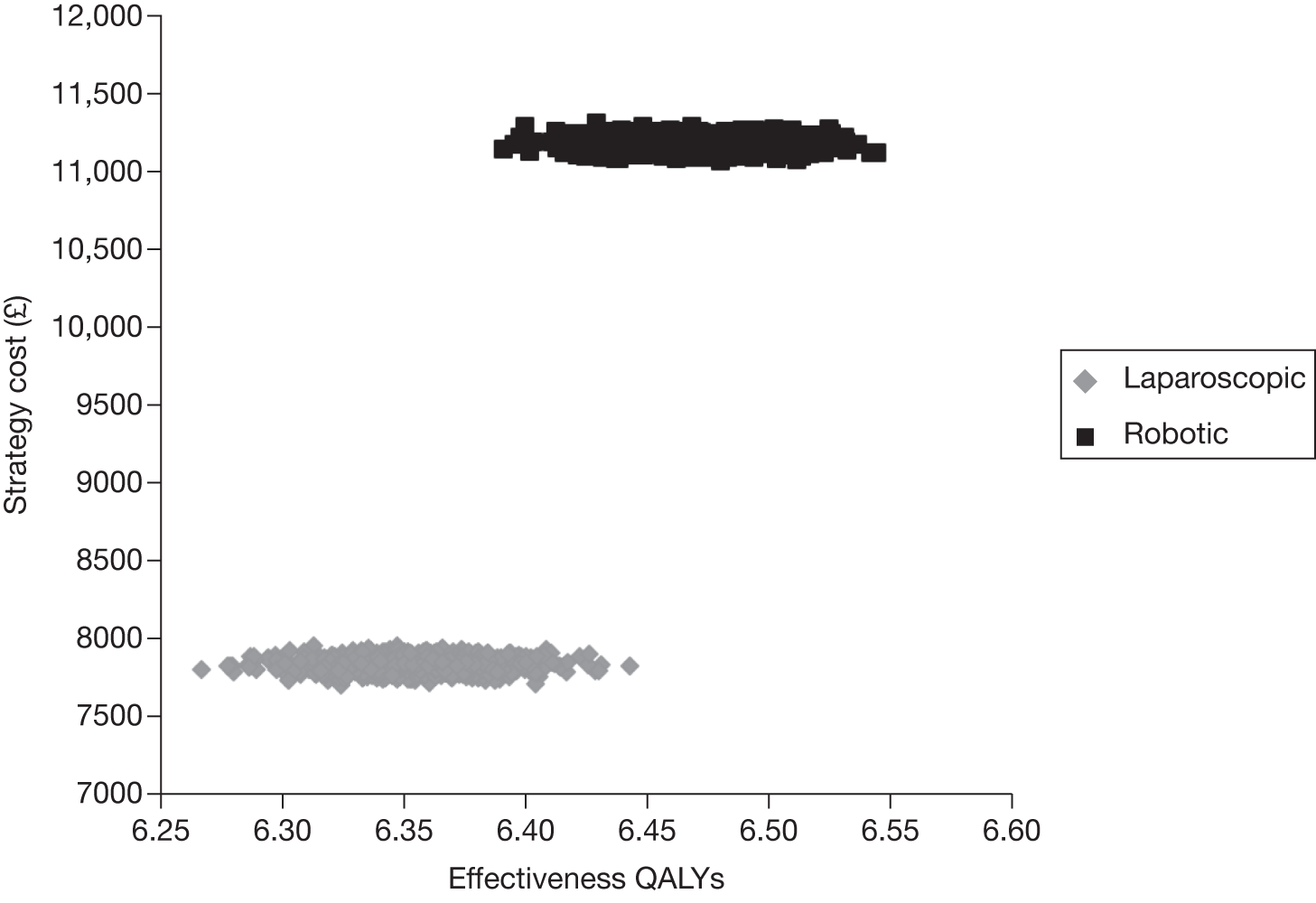
FIGURE 62.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years with rates of biochemical recurrence doubled (100 procedures).
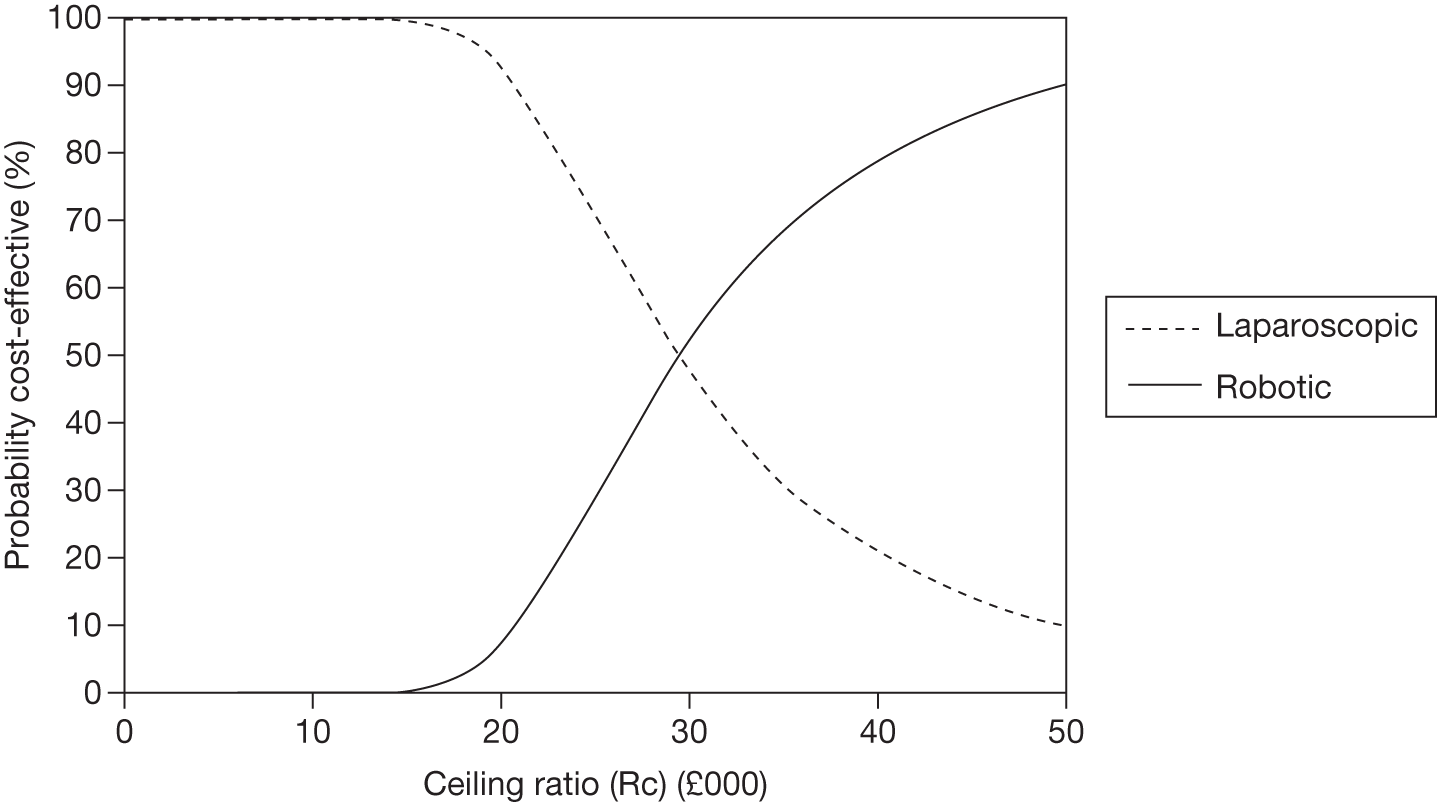
FIGURE 63.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the lower rate of biochemical recurrence (50 procedures).
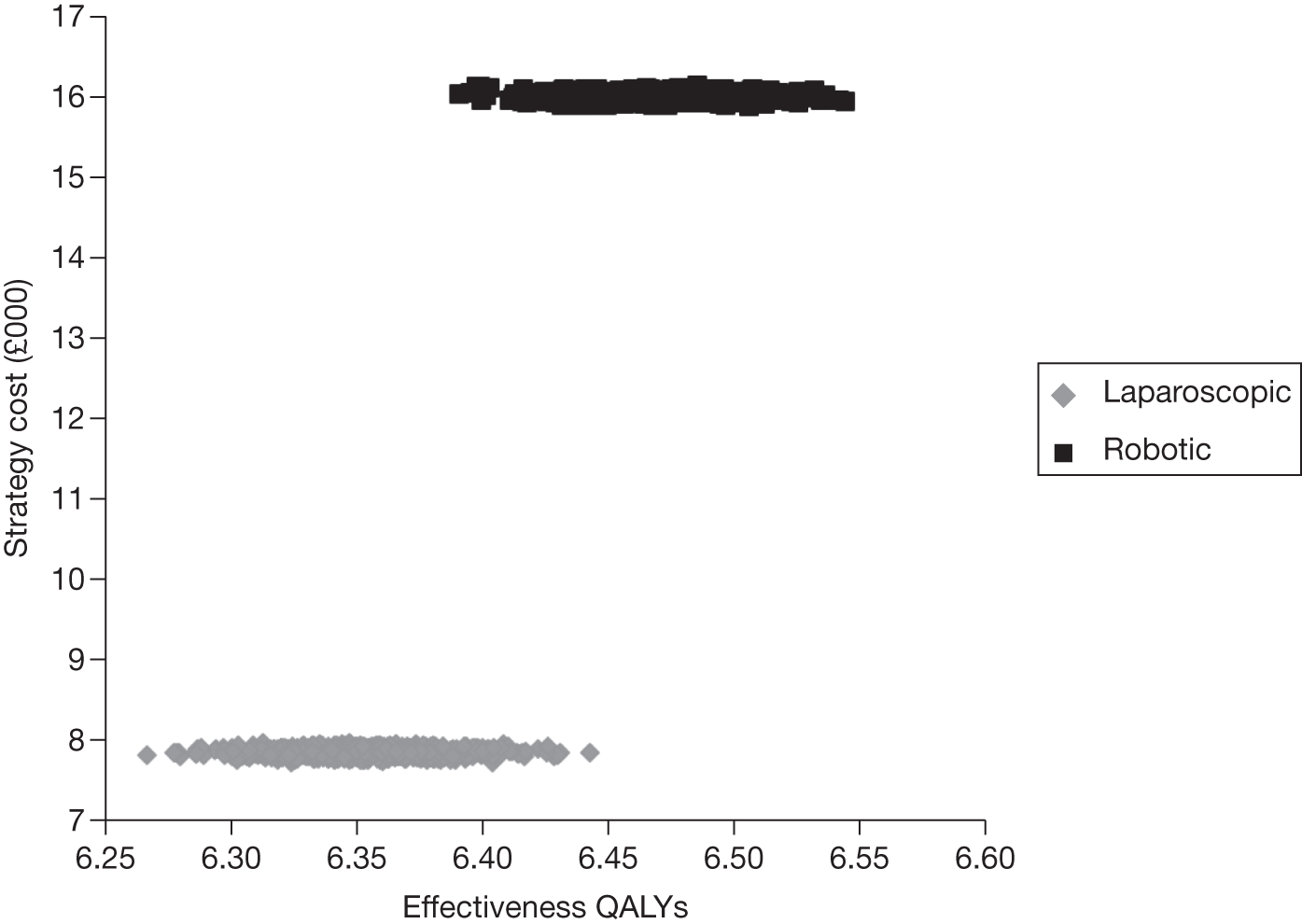
FIGURE 64.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years with rates of biochemical recurrence doubled (50 procedures).
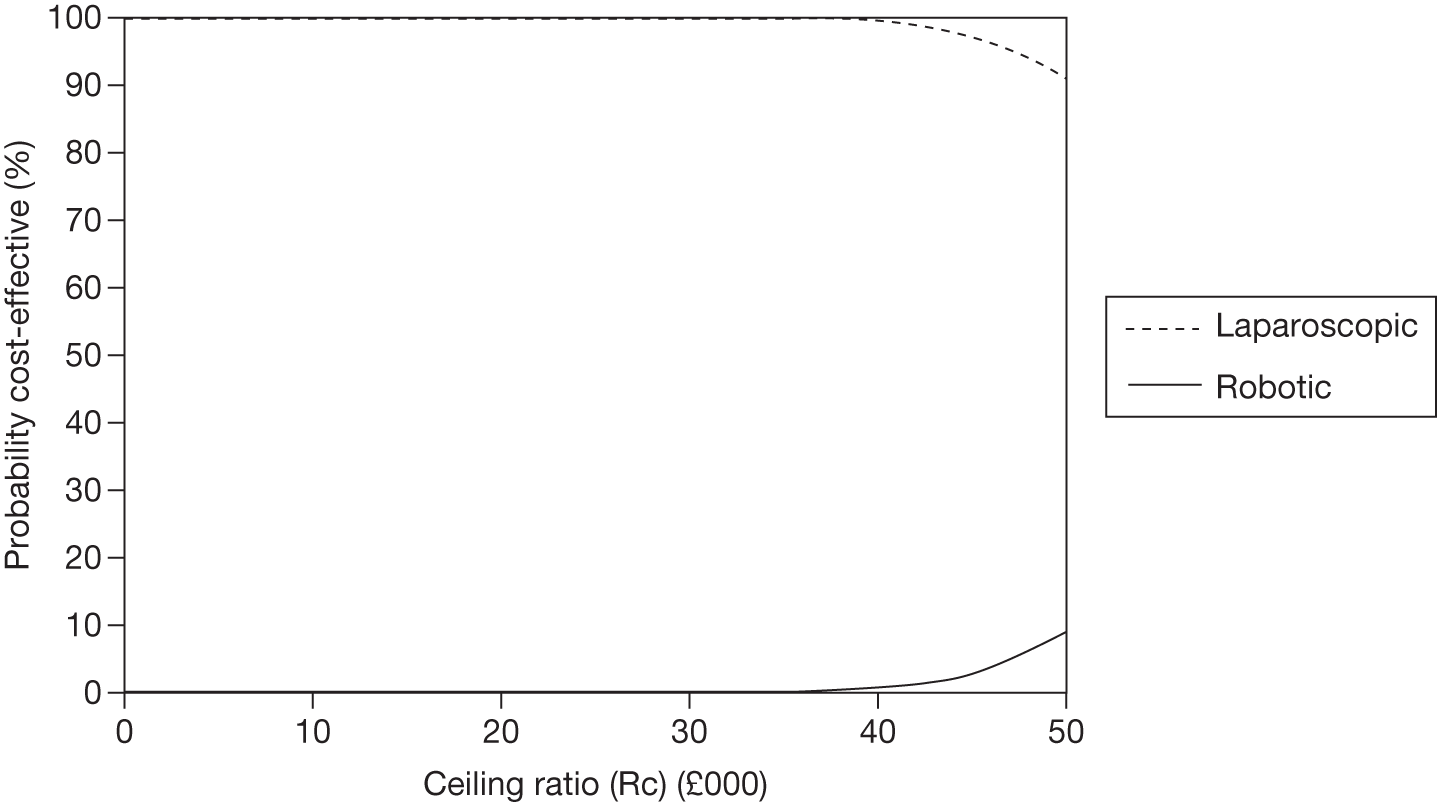
FIGURE 65.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years at the lower rate of biochemical recurrence (200 procedures using the least expensive procurement plan for the robotic system).
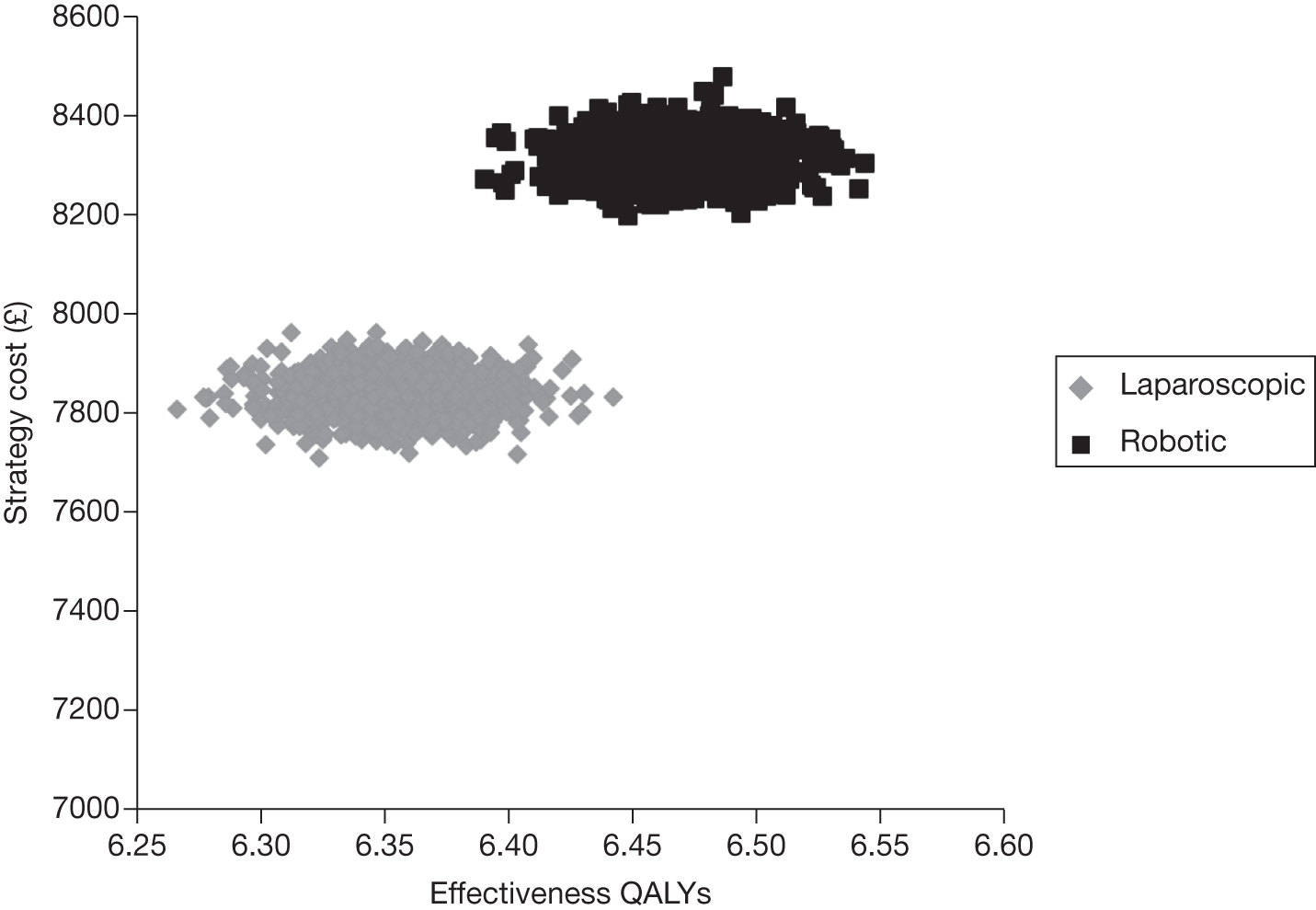
FIGURE 66.
Cost-effectiveness acceptability curve of each intervention following sensitivity analysis over 10 years with rates of biochemical recurrence doubled (200 procedures using the least expensive procurement plan for the robotic system).
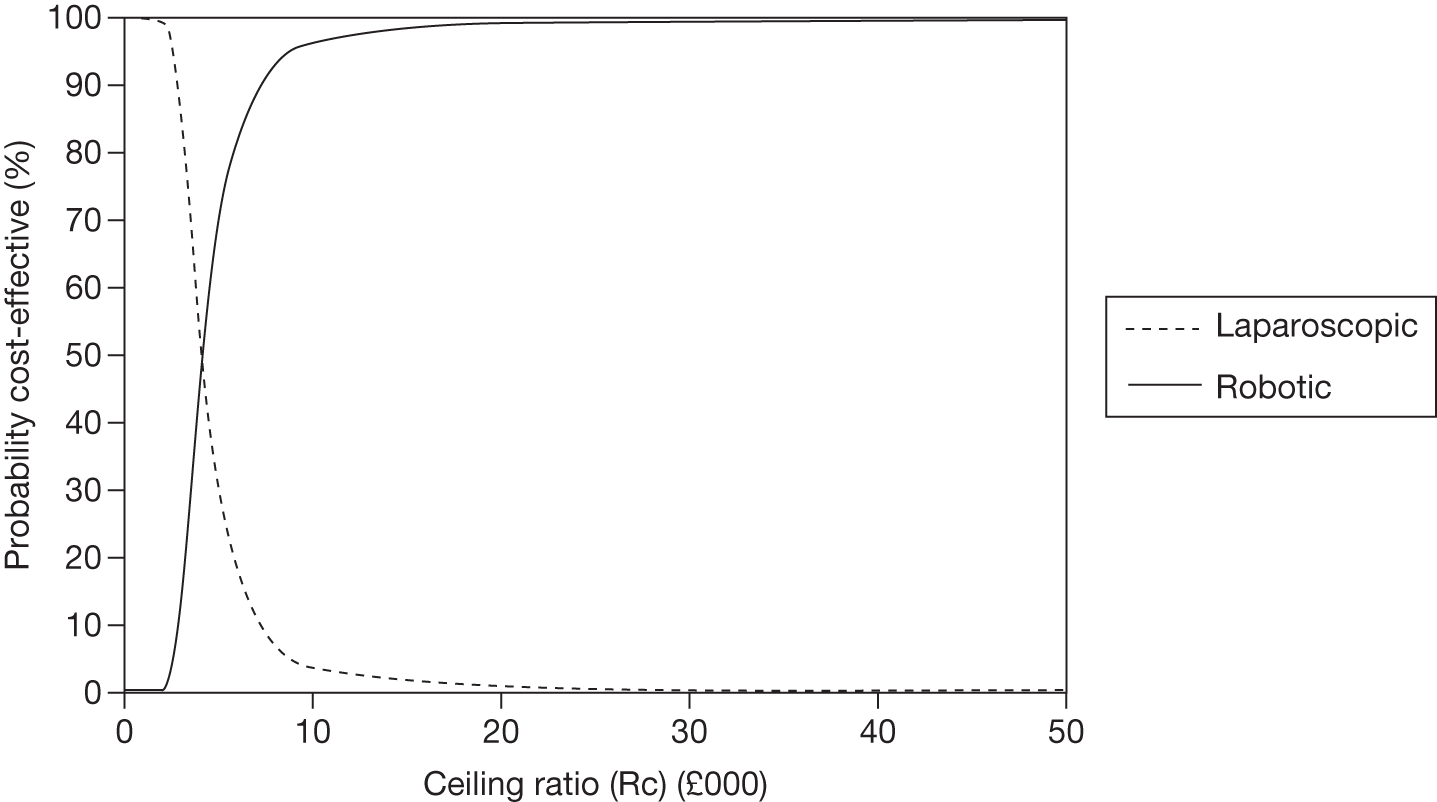
FIGURE 67.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (200 procedures).
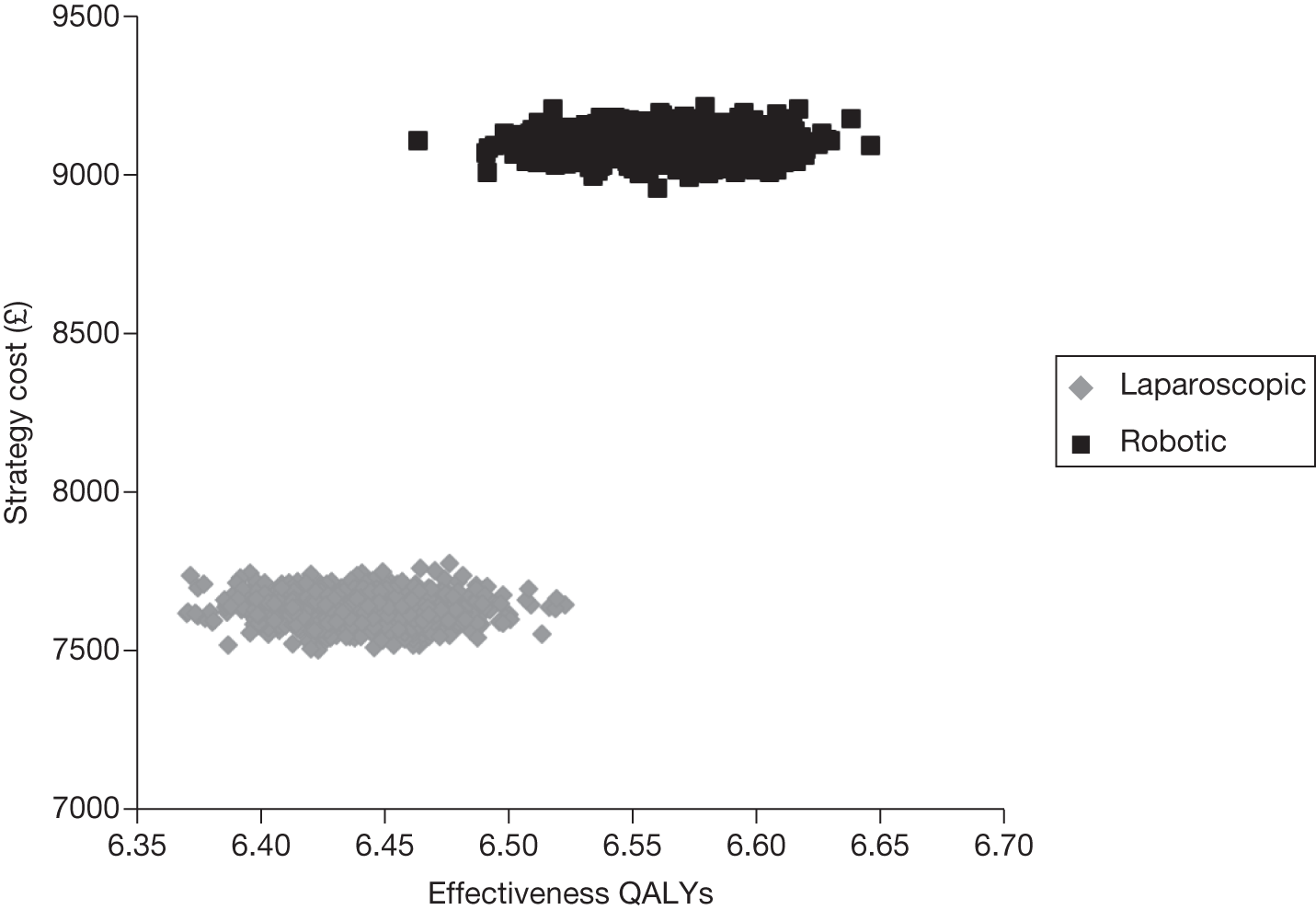
FIGURE 68.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (200 procedures).
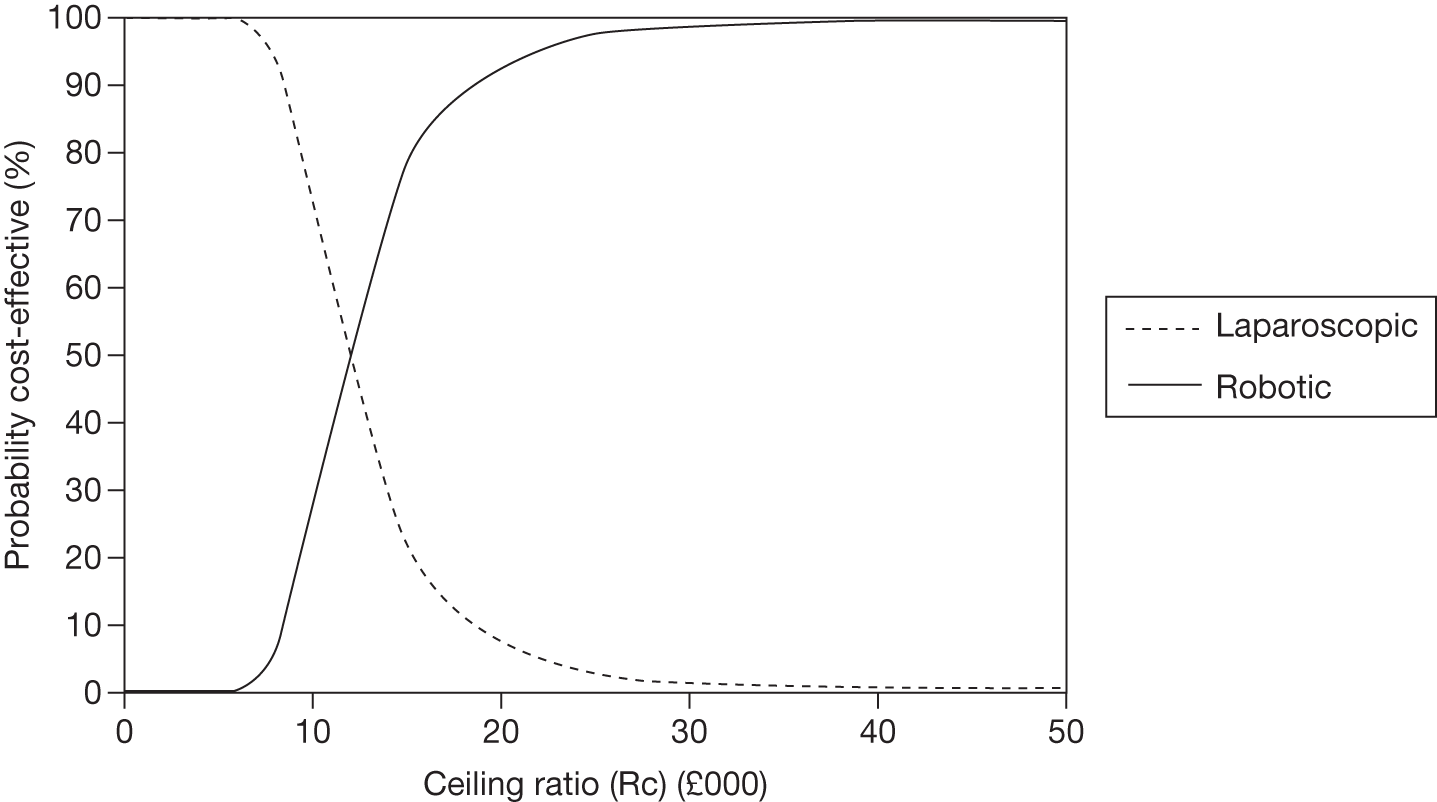
FIGURE 69.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (150 procedures).
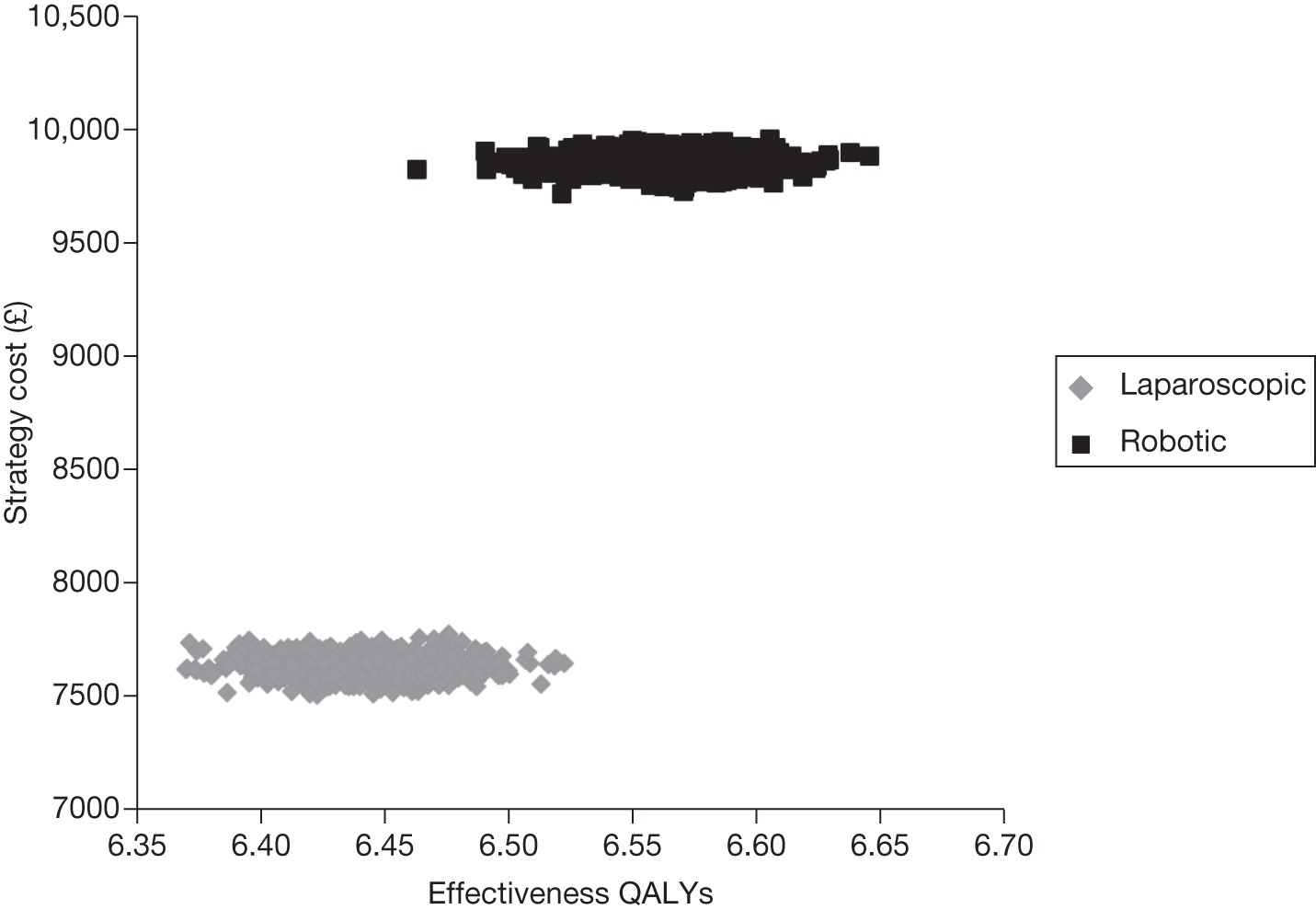
FIGURE 70.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (150 procedures).
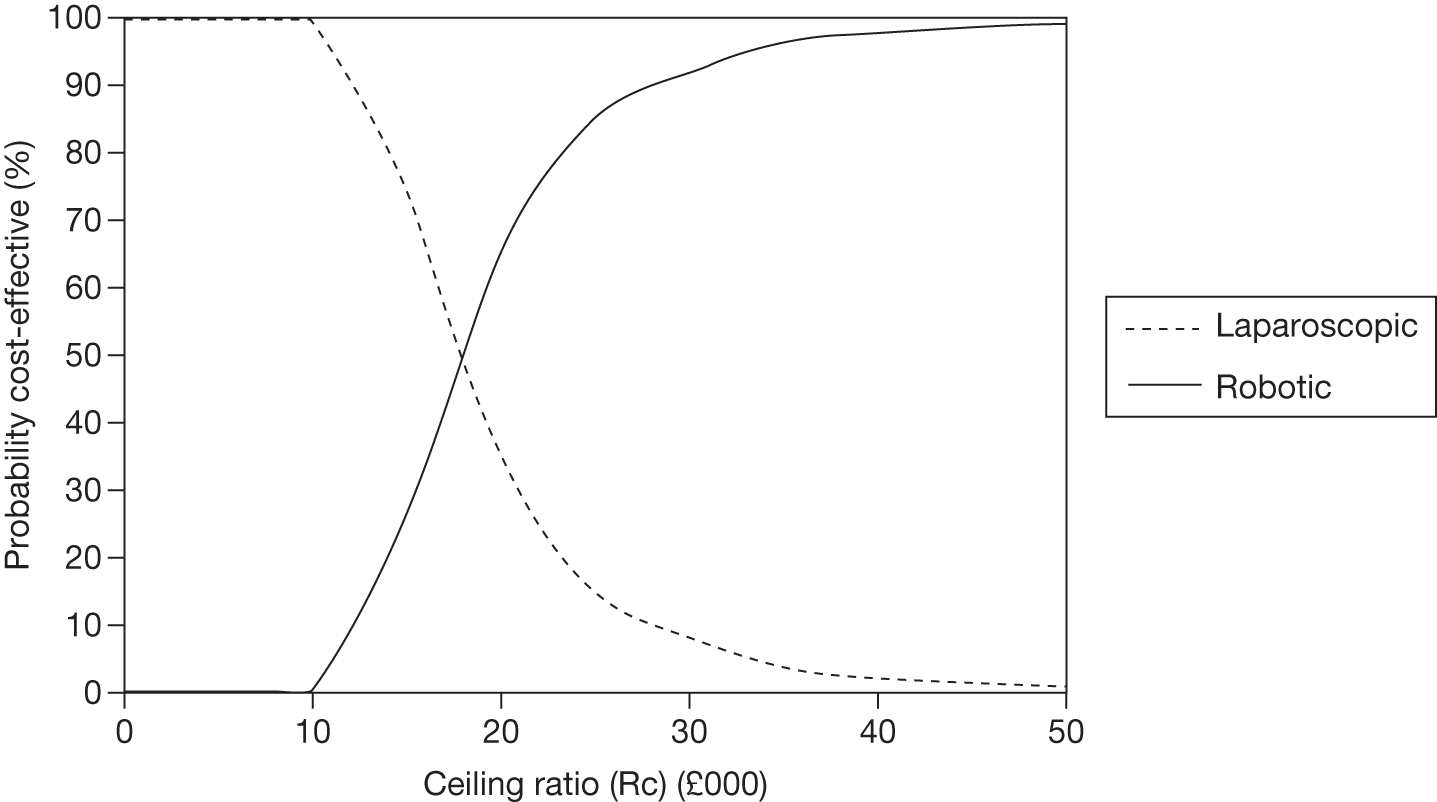
FIGURE 71.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (100 procedures).
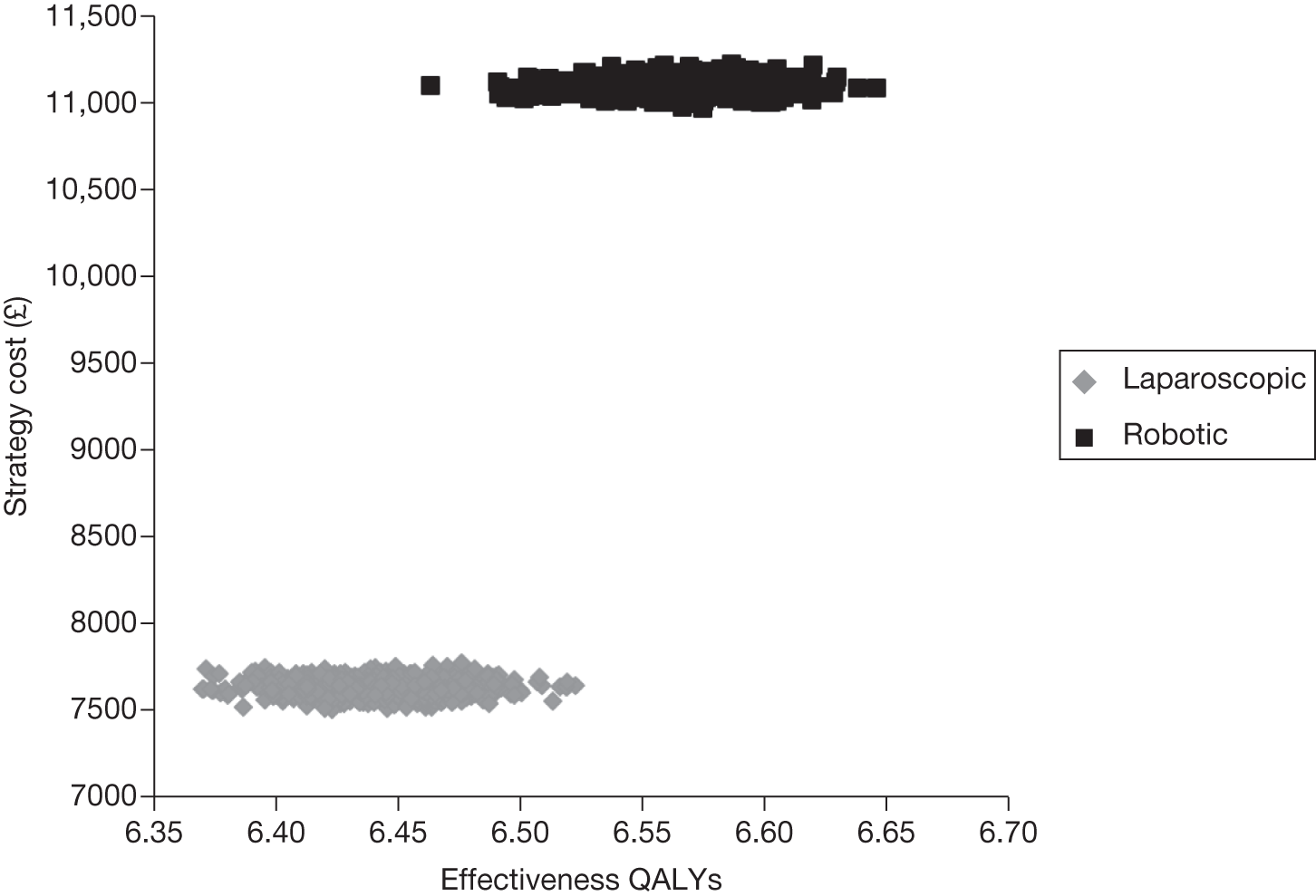
FIGURE 72.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (100 procedures).
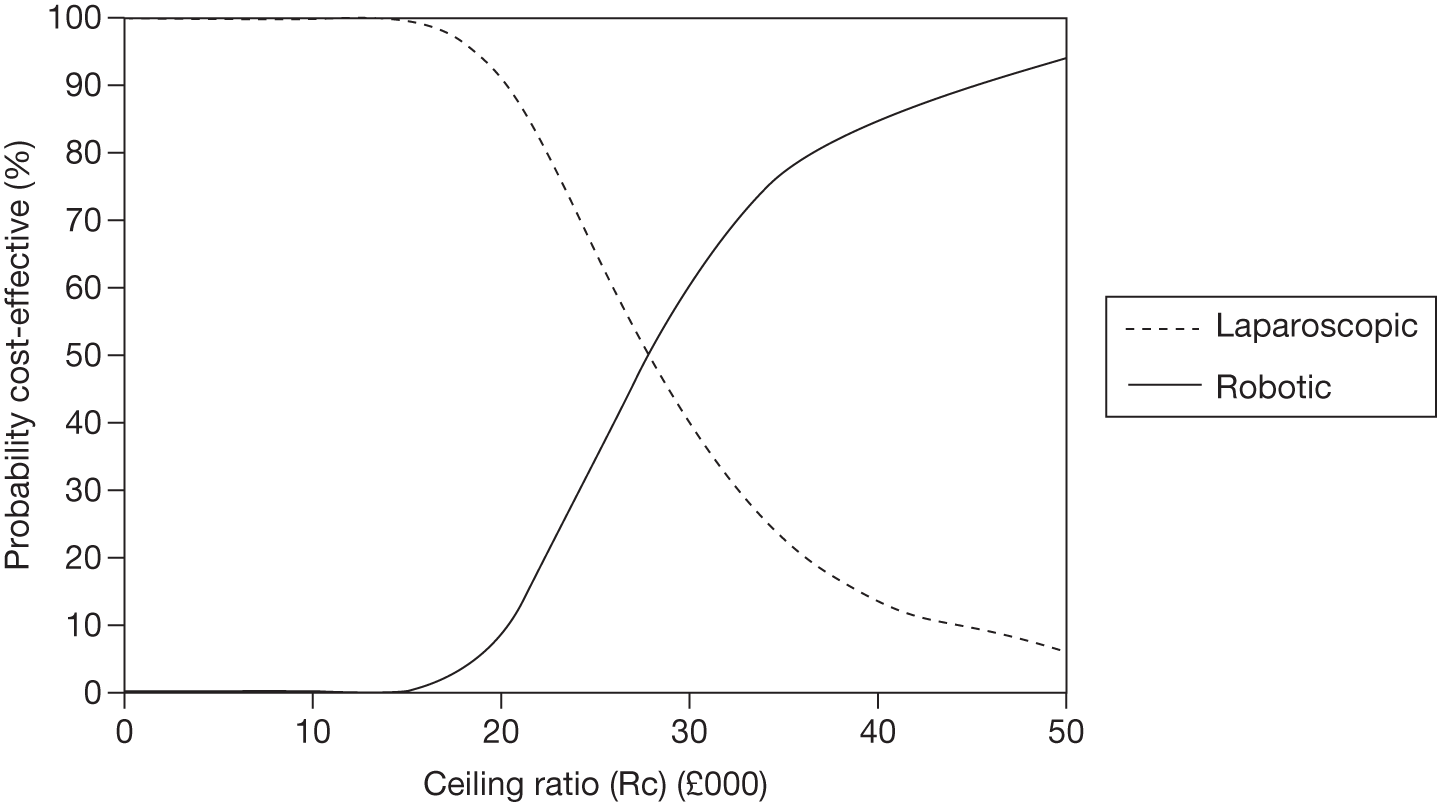
FIGURE 73.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (50 procedures).
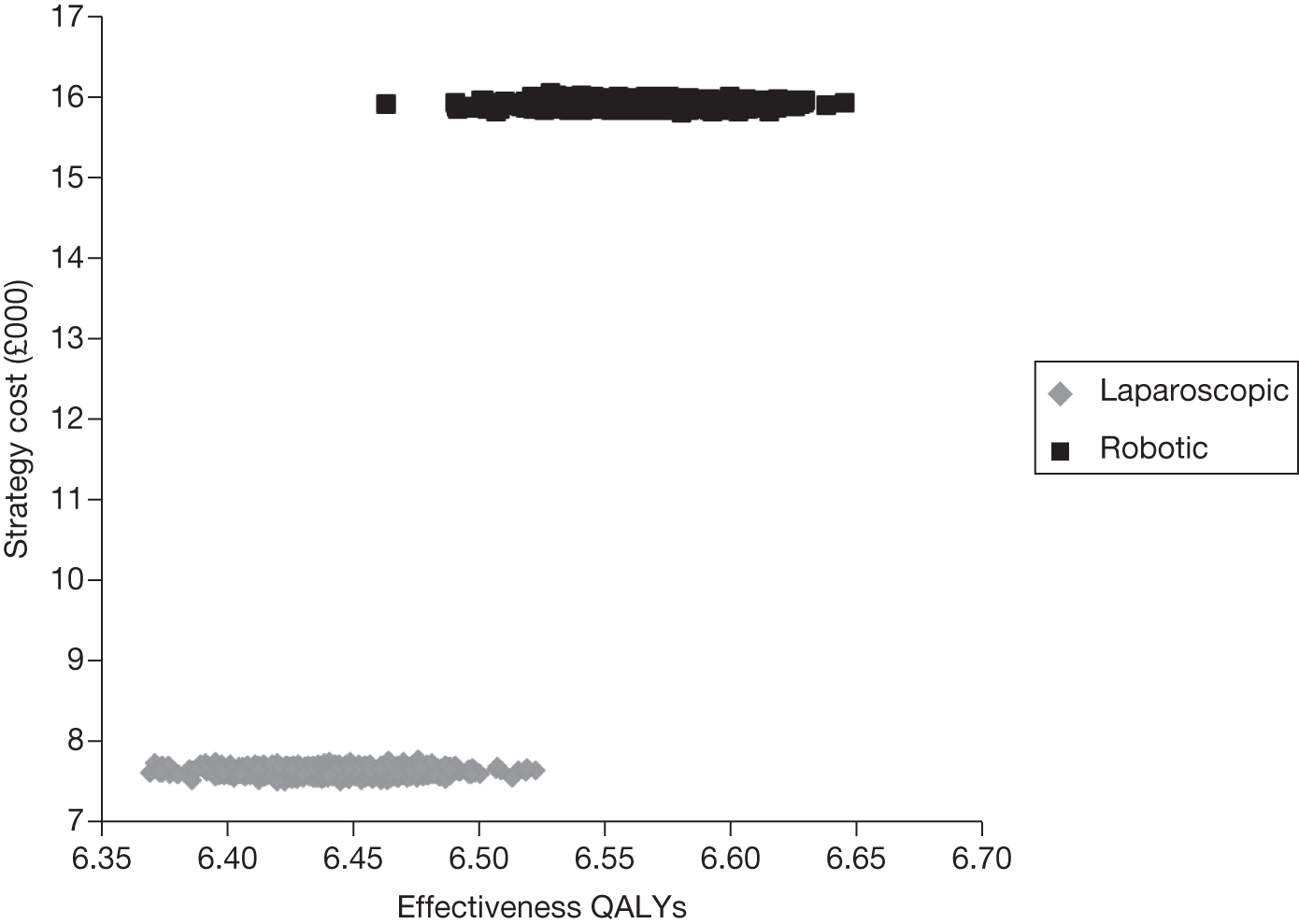
FIGURE 74.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (50 procedures).
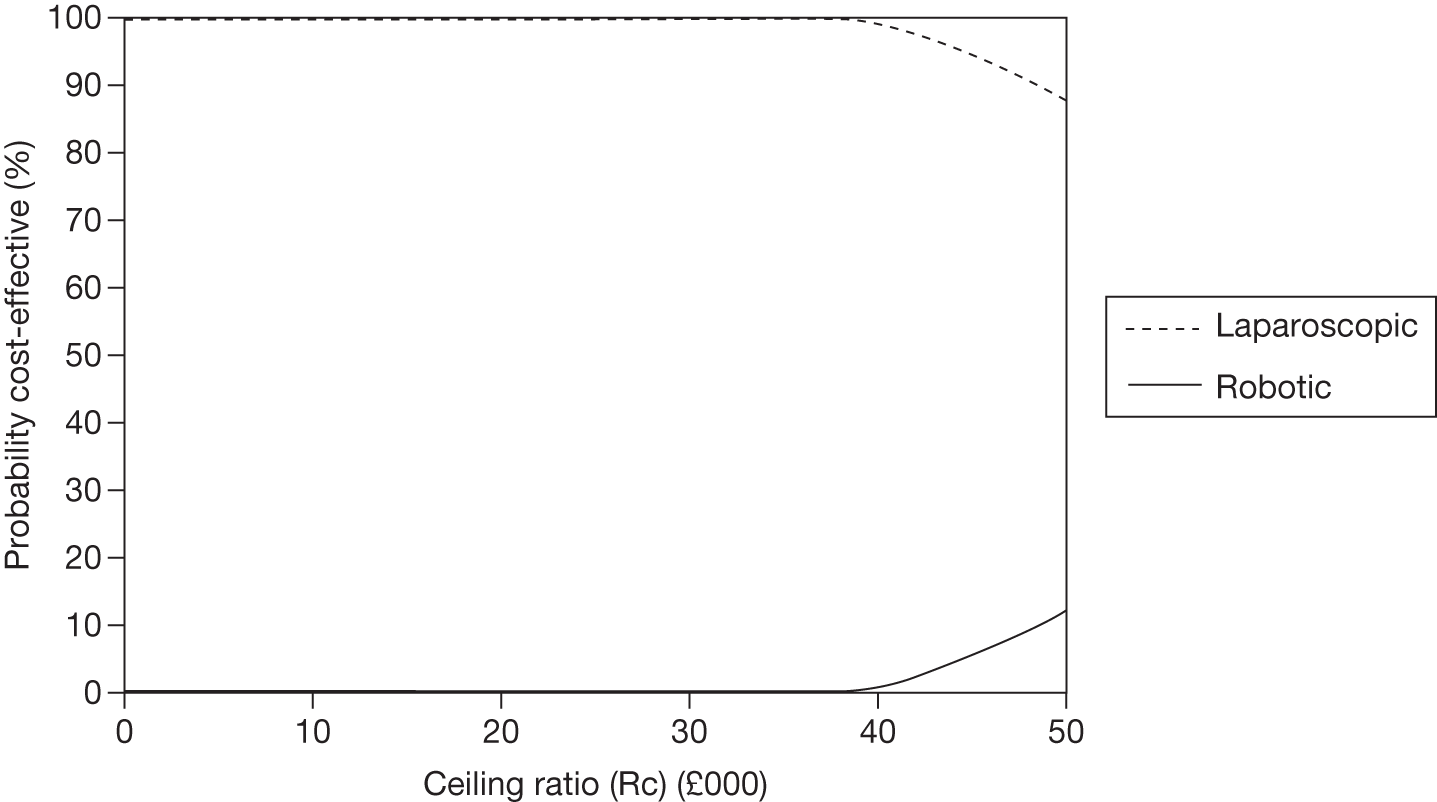
FIGURE 75.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (200 procedures using the least expensive procurement plan for the robotic system).
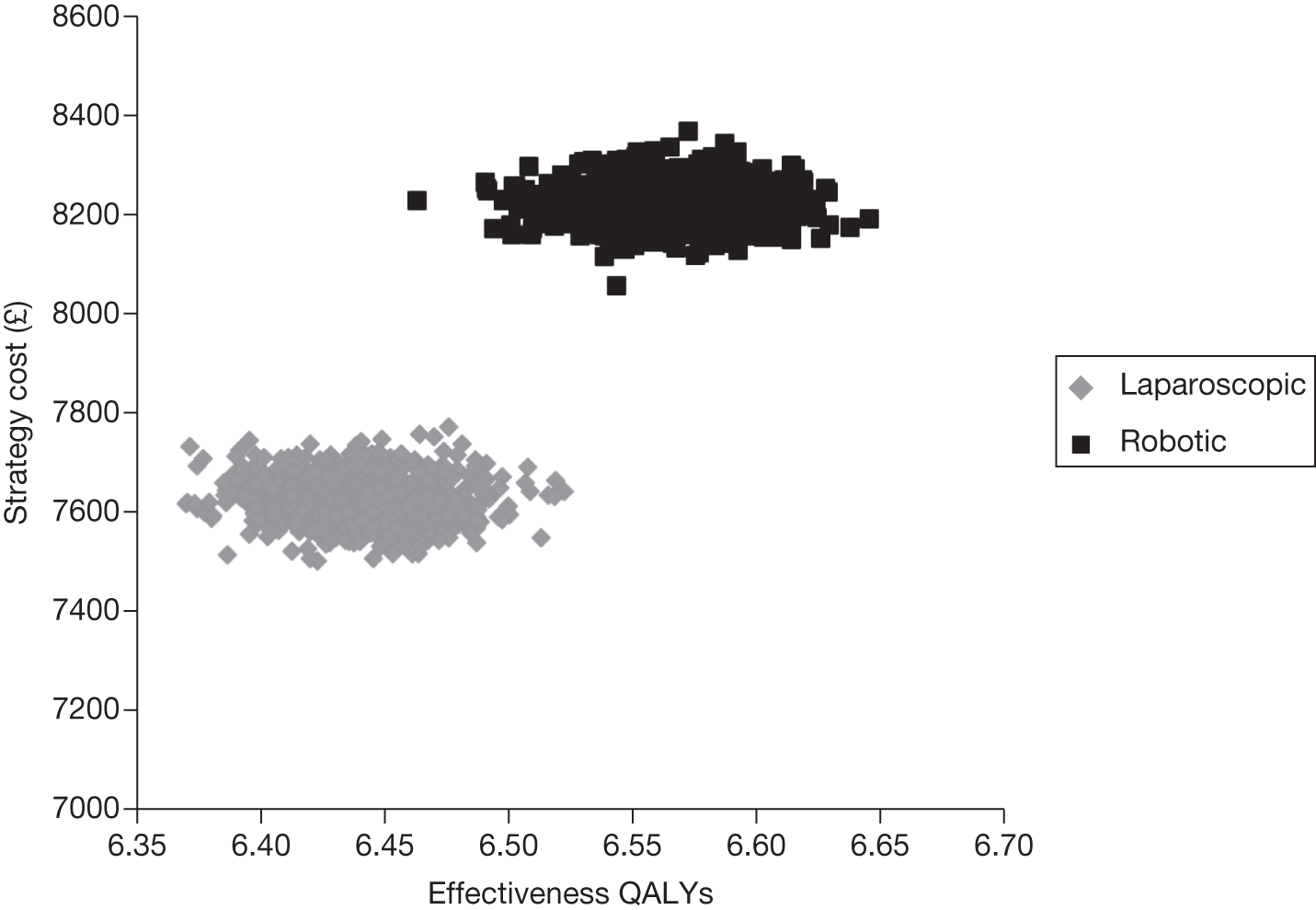
FIGURE 76.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.506 (200 procedures using the least expensive procurement plan for the robotic system).
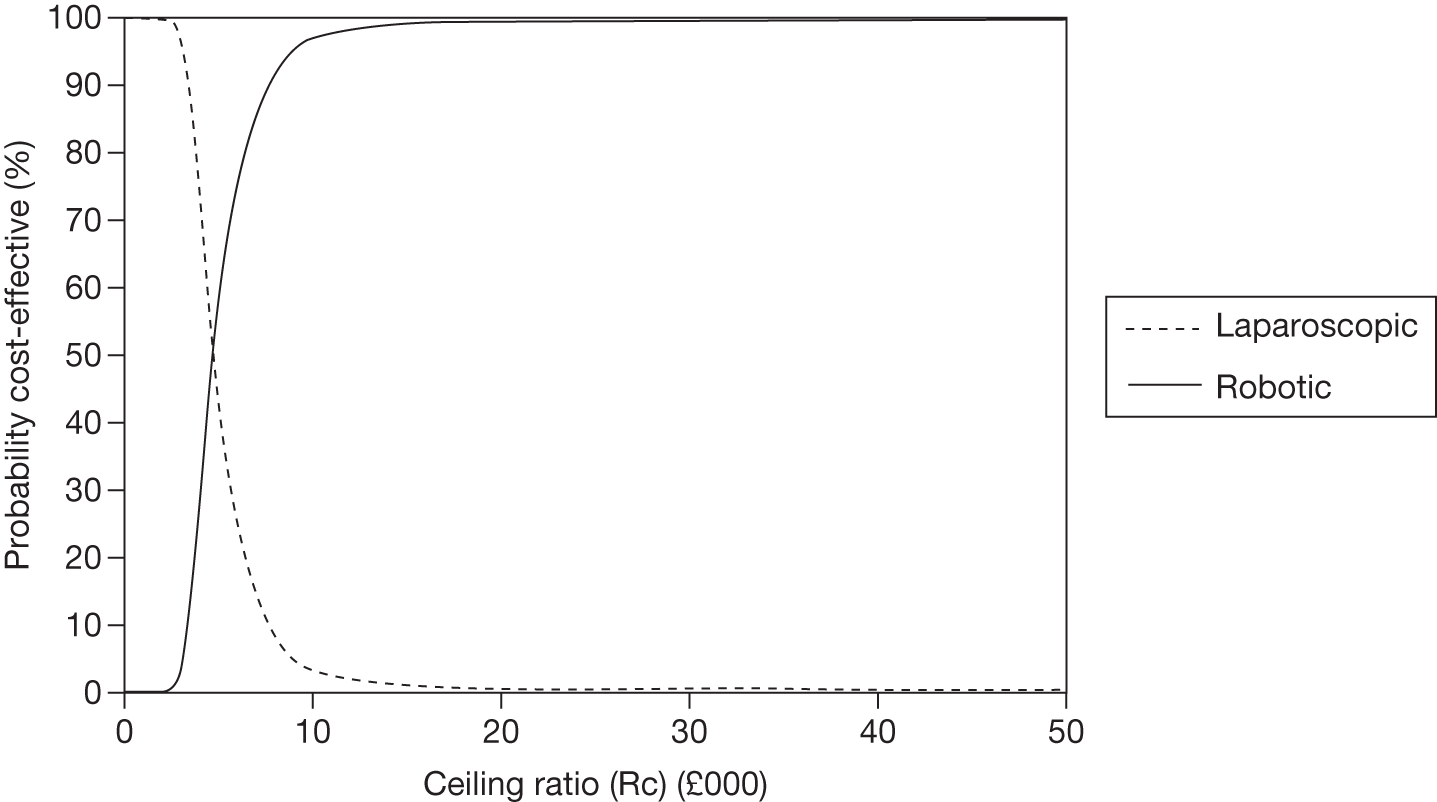
FIGURE 77.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (200 procedures).
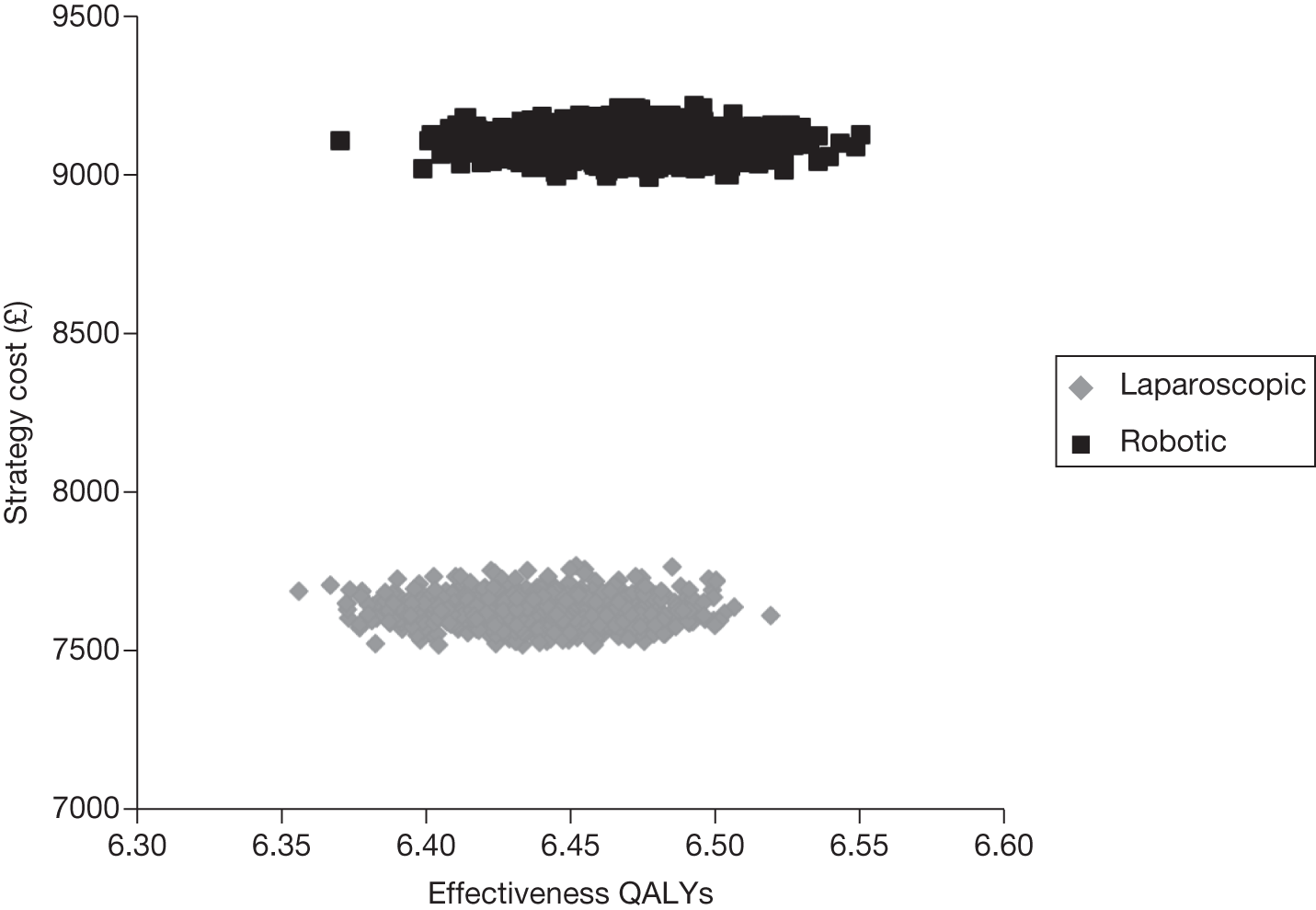
FIGURE 78.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (200 procedures).
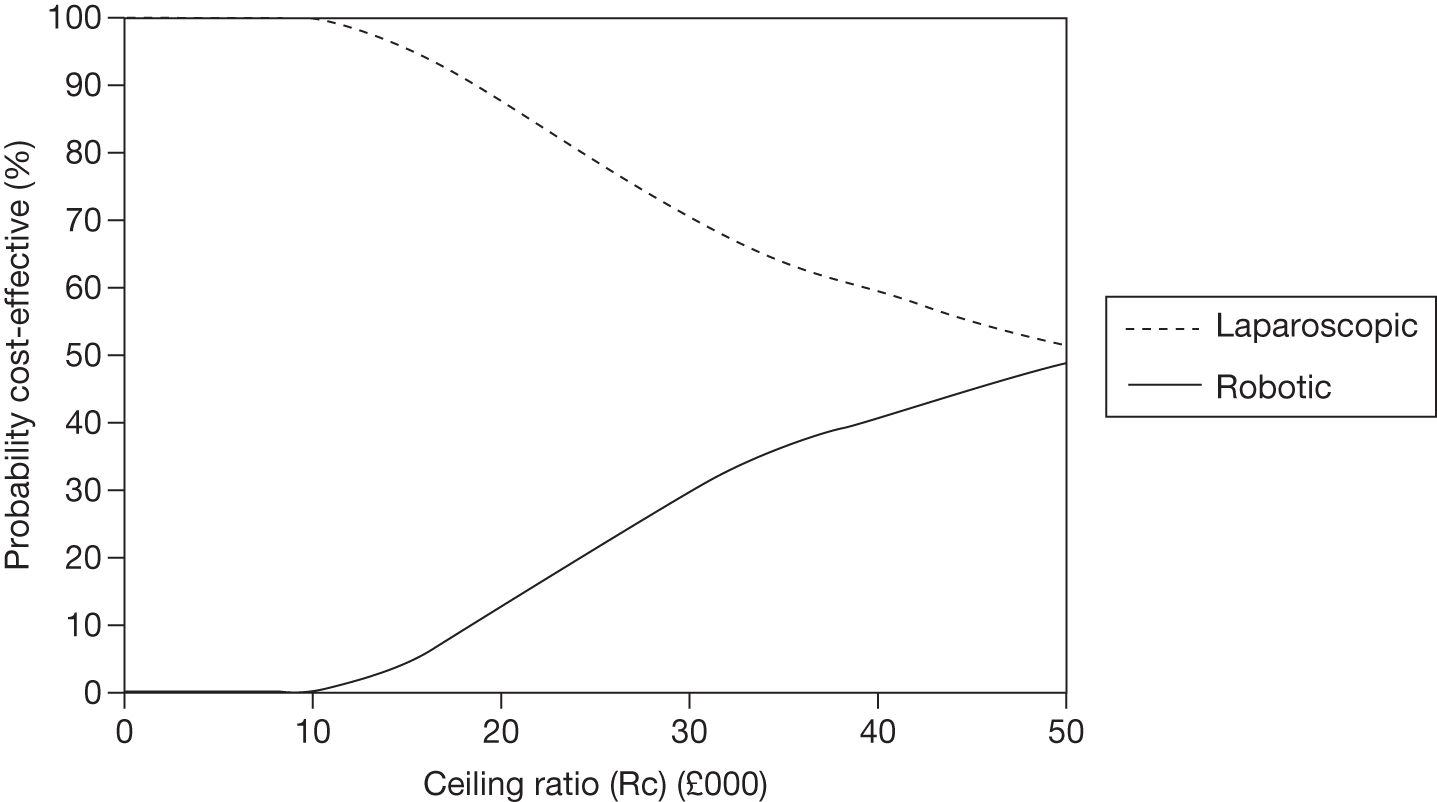
FIGURE 79.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (150 procedures).
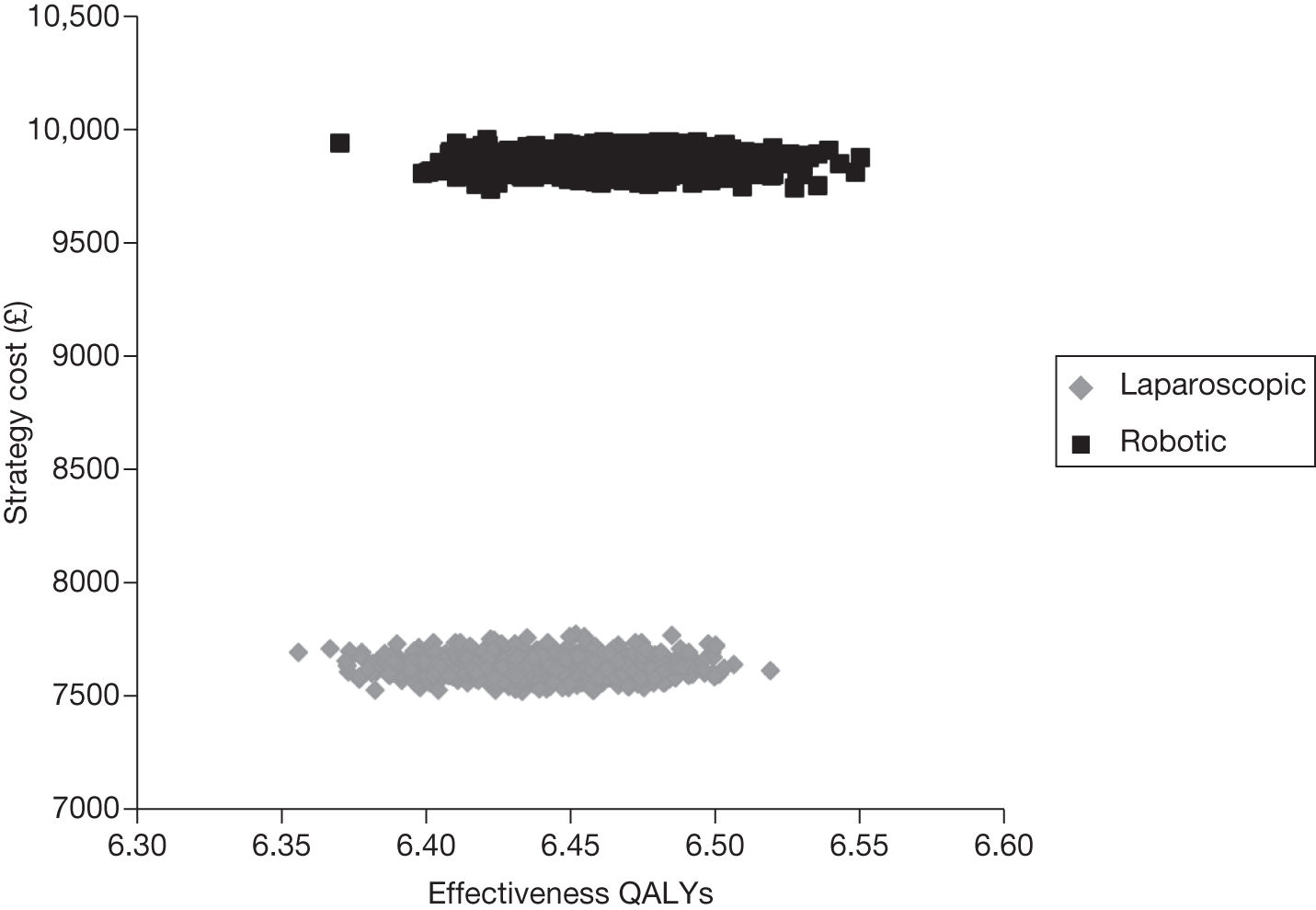
FIGURE 80.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (150 procedures).
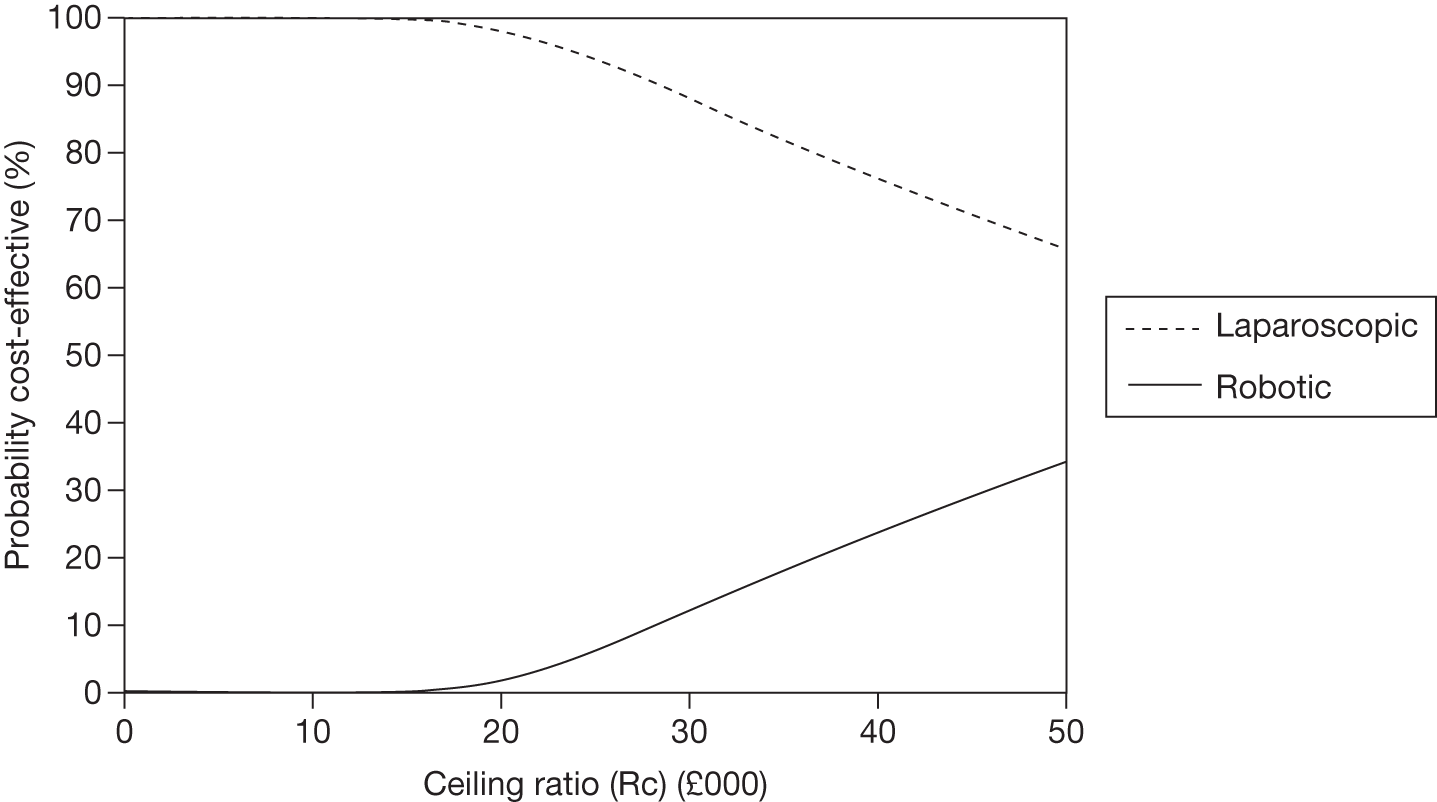
FIGURE 81.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (100 procedures).
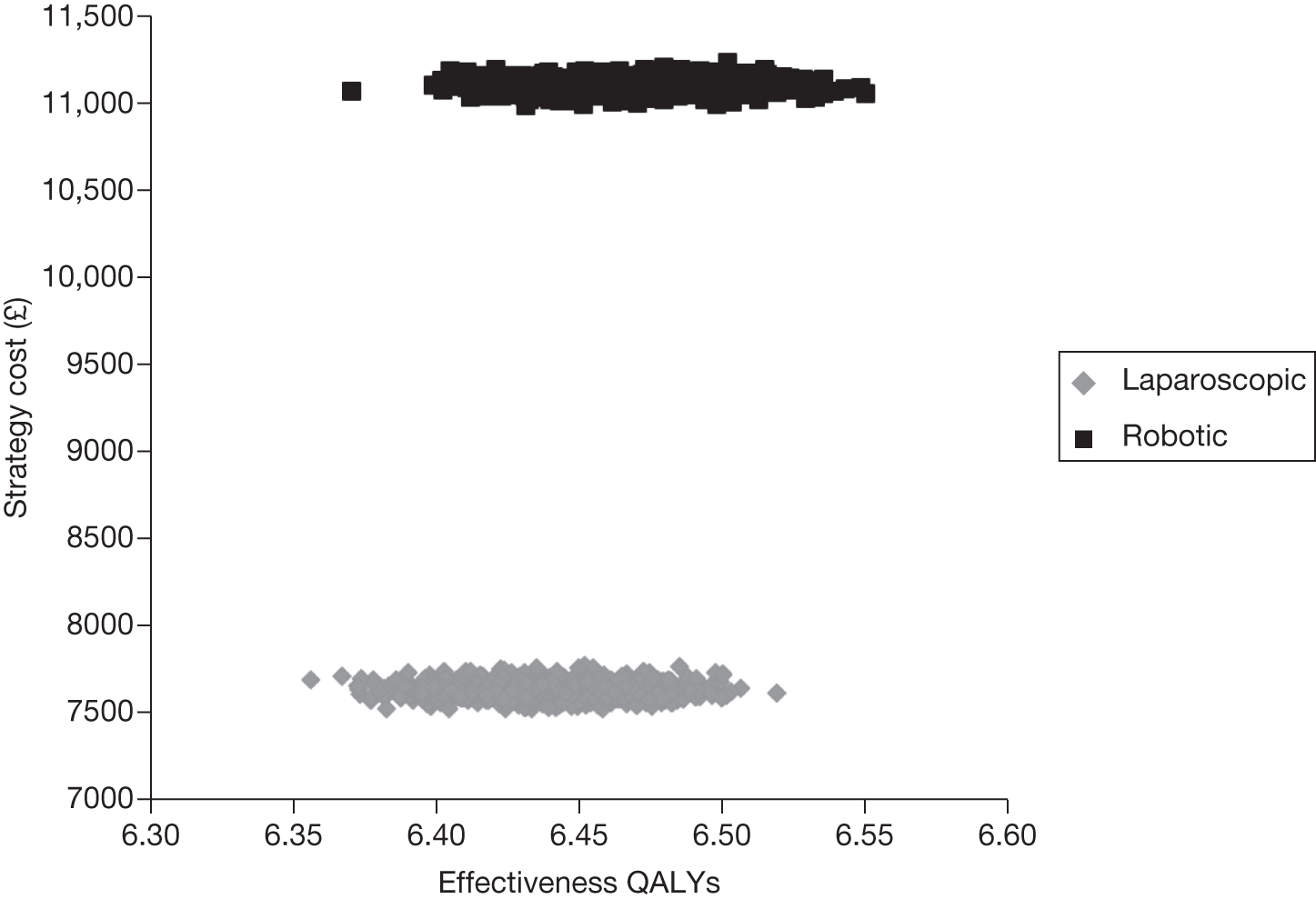
FIGURE 82.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (100 procedures).
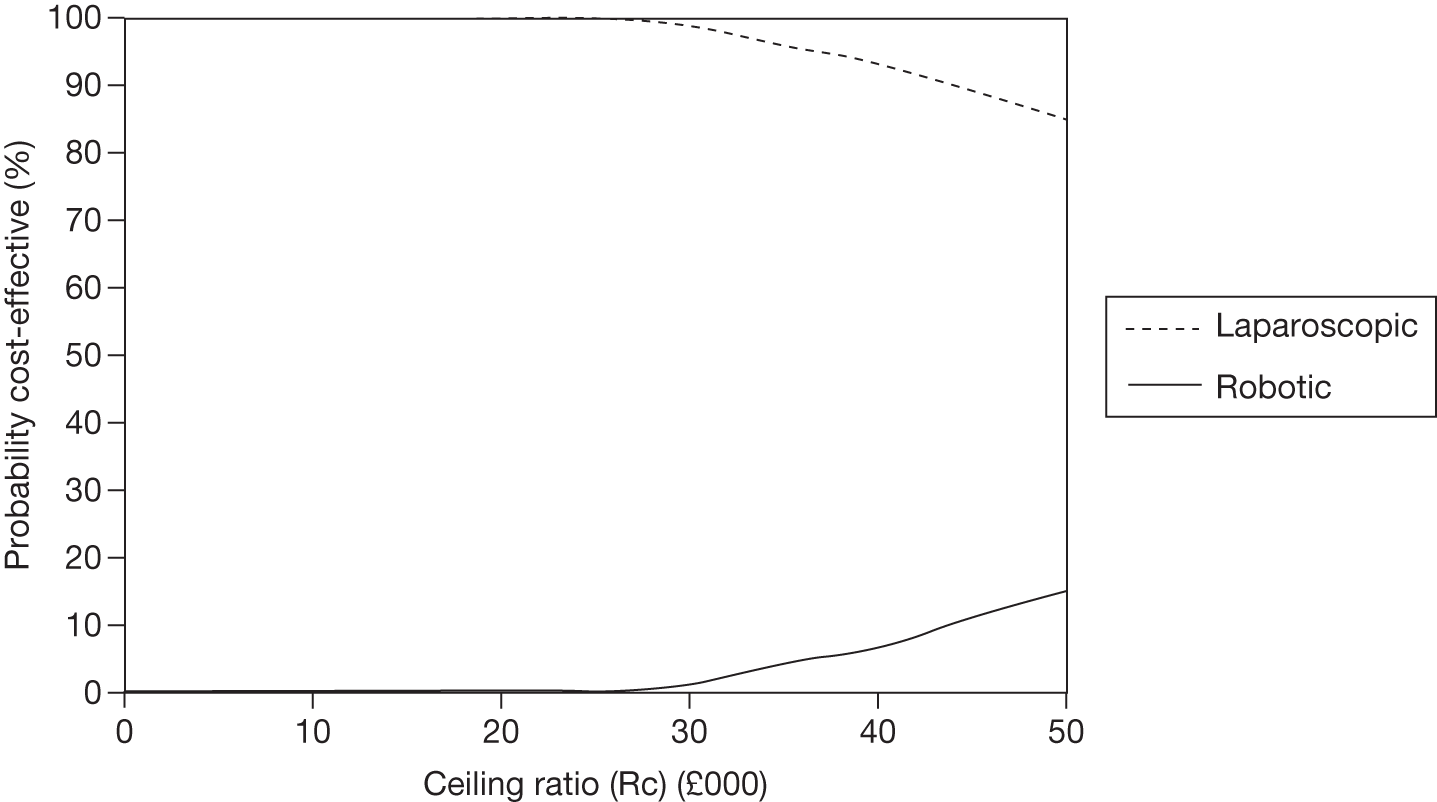
FIGURE 83.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (50 procedures).
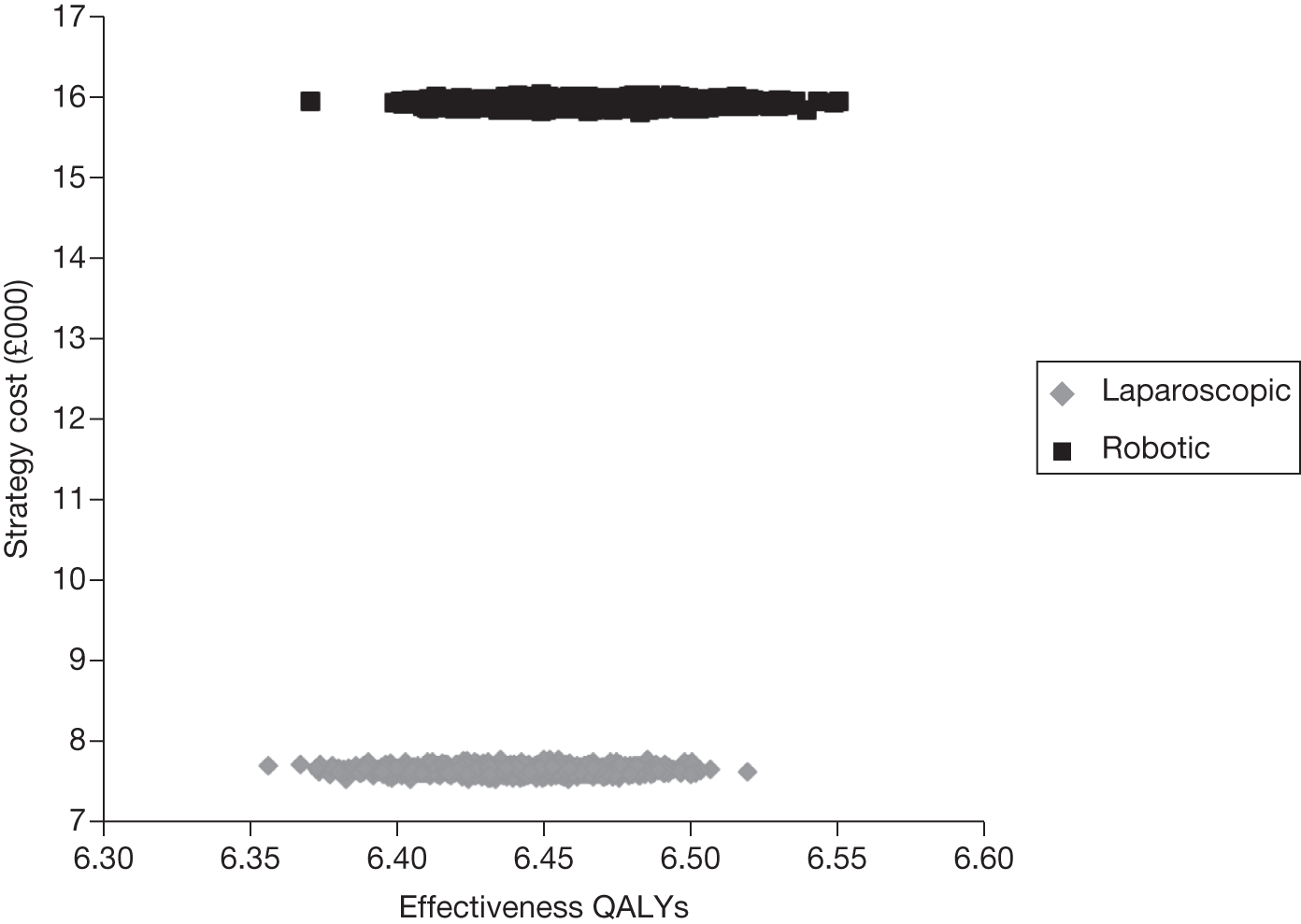
FIGURE 84.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (50 procedures).
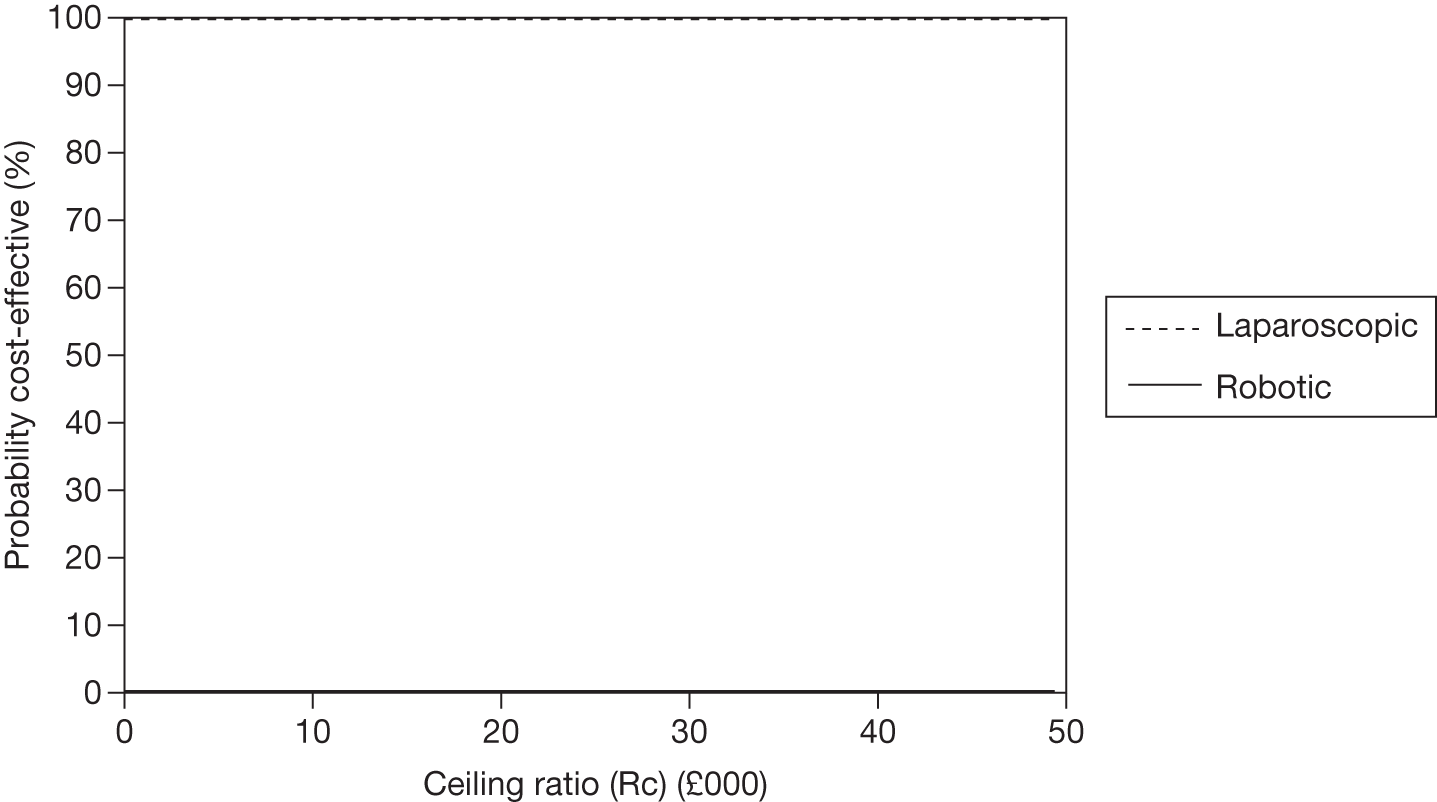
FIGURE 85.
Distribution of costs and QALYs for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (200 procedures using the least expensive procurement plan for the robotic system).
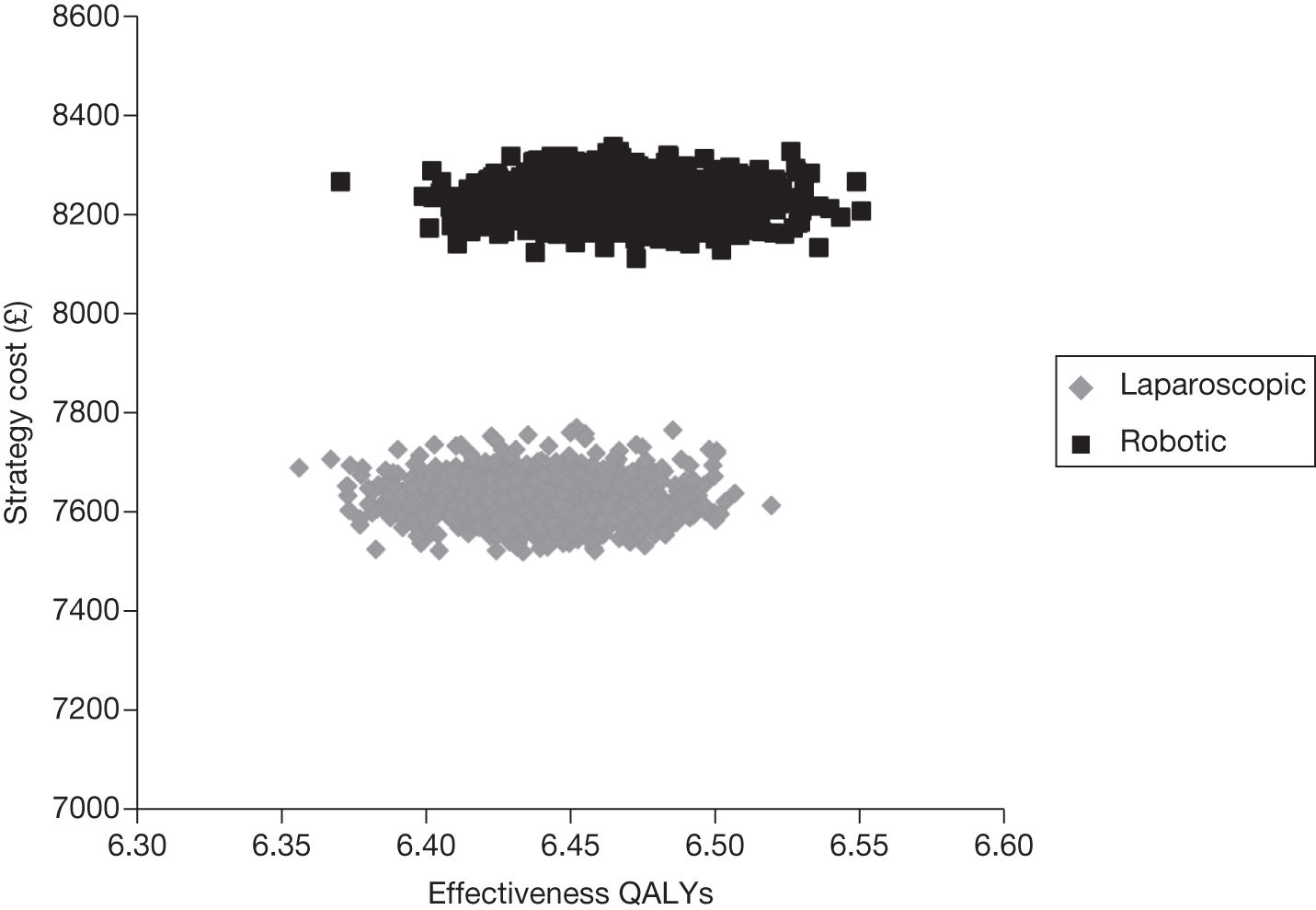
FIGURE 86.
Cost-effectiveness acceptability curve for each intervention following sensitivity analysis over 10 years with rates for positive margin status set at OR 0.955 (200 procedures using the least expensive procurement plan for the robotic system).
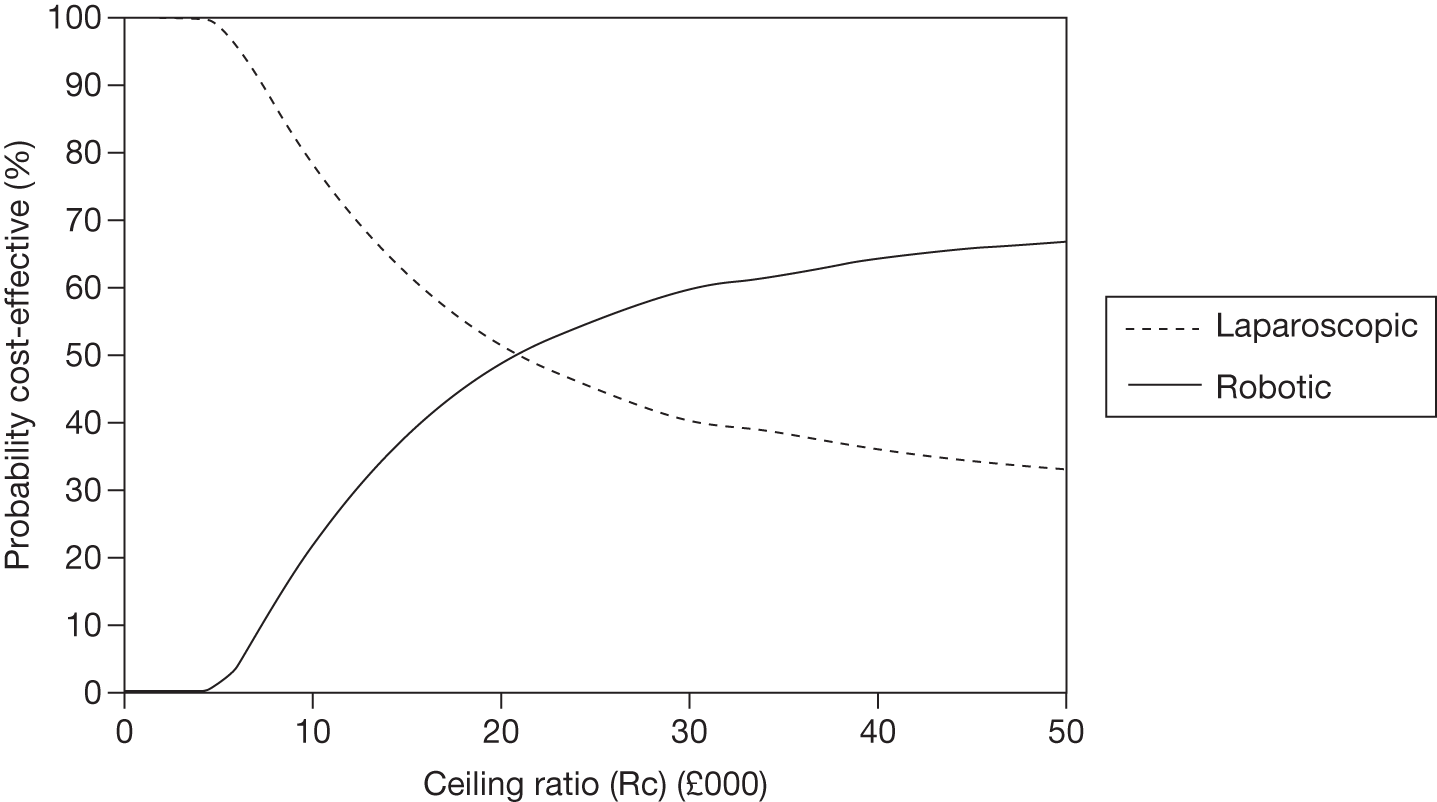
List of abbreviations
- ASA
- American Society of Anesthesiologists
- AUS
- artificial urinary sphincter
- BAUS
- British Association of Urological Surgeons
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- COMET
- Core Outcome Measures in Effectiveness Trials
- CrI
- central credible interval (for Bayesian analysis)
- cT
- preoperative clinical classification of tumour stage
- DARE
- Database of Abstracts of Reviews of Effects
- EQ-5D
- European Quality of Life-5 Dimensions
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30
- EPIC-UISS-SFSS
- Expanded Prostate Cancer Index Composite urinary incontinence and sexual function subscales
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICIQ-UI
- International Consultation of Incontinence Questionnaire
- ICS
- International Continence Society
- IIEF-5
- International Index of Erectile Function-5
- I-PSS
- International Prostate Symptom Score
- ISD
- Information Services Division (Scotland)
- ISUP
- International Society of Urological Pathology
- LHRH
- luteinising hormone-releasing hormone
- log-OR
- logarithm of the odds ratio
- MAPS
- men after prostate surgery trial
- NICE
- National Institute for Health and Clinical Excellence
- NIH
- National Institutes of Health
- NIHR
- National Institute for Health Research
- OPCS
- Office of Population Census and Surveys
- OR
- odds ratio
- PSA
- prostate-specific antigen
- pT
- postoperative pathological classification of tumour stage
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SHIM
- Sexual Health Inventory for Men
- TRUS
- transrectal ultrasound
- UCLA-PCI
- University of California Los Angeles – Prostate Cancer Index
- UICC
- Union for International Cancer Control
- VAS
- visual analogue scale
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known, such as NHS, or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health