Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 07/37/32. The contractual start date was in May 2009. The draft report began editorial review in June 2011 and was accepted for publication in November 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
This project was funded by the NIHR Health Technology Assessment programme (project number 07/37/32) and the authors’ institutions received money through this. Only BH received salary from the grant (through employer, University of Edinburgh). PA holds a grant from the European Society of Intensive Care Medicine for a trial of therapeutic hypothermia in traumatic brain injury (Eurotherm3235 trial). Otherwise none declared.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Harris et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Background
The conditions and incidence: traumatic brain injury and stroke
Brain injuries resulting from stroke and trauma are common and costly in human and resource terms. In England, approximately 130,000 people have a stroke each year, of whom about one-quarter die and half of the survivors are left dependent on others. 1 The incidence of head injury is similar to that for stroke,2 although the incidence of death is lower, at 6–10 per 100,000 population per year. 3 However, head injury is more common in younger people, and it has been estimated that 4700 of those admitted to hospital each year would be unable to return to work at 6 weeks. 2 A Scottish study found that 78% of patients with a severe injury had moderate or severe disability 1 year later. 4
Aside from the often devastating consequences for patients and their families, these brain insults are expensive. Morbidity from head injury ‘far exceeds the capacity of UK neurorehabilitation services’3 and the costs of stroke to the NHS are estimated at £2.8B per year, with the cost to the wider economy about £1.8B more in disability and lost productivity. 1
Although the primary mechanisms of brain injury are different in trauma, haemorrhage and ischaemia [whether focal, as in ischaemic stroke, or global, as in cardiac arrest and neonatal hypoxic–ischaemic encephalopathy (HIE)], the result is a cascade of excitotoxity, apoptosis and inflammation. 5,6 Inflammation, cell death and infection, if present, mean that increased temperature is common after both stroke and brain injury. 7,8 There is no universally agreed definition of the threshold for pyrexia or where and how temperature should be measured in these patients but, in one study, nearly 68% of patients had a rectal temperature ≥ 37 °C within 48 hours after severe traumatic brain injury (TBI)9 and 54% had an axillary temperature of > 37.5 °C within 48 hours after stroke. 10
Increased temperature is associated with worse outcome after both stroke and TBI. 9,11 The exact nature of the relationship in humans is hard to determine, as the time of onset of raised temperature has an influence and temperature elevation can be a marker of more severe injury and of infection, both of which are also associated with worse outcome,12 although one systematic review11 suggests that infection may not play a significant part in the relationship in stroke. There is considerable evidence from animal research that reducing temperature, and, more especially, inducing hypothermia, reduces the extent of injury and that the sooner cooling is instigated the more effective it is. 6 However, there is insufficient high-quality prospective evidence to show that normothermic or hypothermic temperature interventions improve functional outcome in humans after TBI and stroke. 13–15 This may be because it is difficult to cool patients early and quickly enough and/or because the side effects of hypothermia, such as increased infection, may outweigh the benefits in some circumstances.
Nevertheless, the usual clinical goal in TBI and stroke is to reduce raised temperature to normothermia, although consistently achieving this can be difficult. 16,17 In stroke it is recommended that temperature is treated if > 37.5 °C. 18 In brain injury, body temperature control is recommended in the context of treating raised intracranial pressure (ICP). 19 There are no standard recommendations on the site of temperature measurement or methods of temperature reduction. In practice, choice of site of measurement is variable20,21 and cooling interventions are usually systemic. Pharmacological intervention, generally with paracetamol, is the most common first-line treatment, followed by a variety of physical systemic cooling interventions, which include cooling blankets, ice packs and fanning. 21,22
The intervention: non-invasive head cooling
Physical cooling methods can be classified into those targeted systemically and those targeted at the head to cool the brain directly, and include invasive and non-invasive methods. Non-invasive head cooling is the subject of this review and therefore invasive methods, such as antegrade and retrograde cerebral perfusion and devices applied to brain tissue, which are mainly used during surgery,23 are not included.
Methods of non-invasive head cooling are categorised into:
-
Heat loss from the upper airways This takes place by convection with gas or fluid flow or by conduction with nasal or pharyngeal balloons – whether or not these devices are truly non-invasive is a moot point, but they have been included in this review.
-
Heat loss through the skull This takes place by convection (fanning, hoods delivering cold air or water) or by conduction (passive, e.g. ice, gel caps or active, e.g. liquid cooling); some of the devices also have a neck band that theoretically may help cool the brain by reducing the temperature of the carotid blood supply. 24,25
Heat loss occurs as flow down temperature gradients from warm to cool. Convective cooling methods use air/gas flow to remove heat; molecules are removed in bulk and transfer heat in the process. Convective methods also allow heat loss by evaporation, a form of convection in which bulk movement of molecules is achieved by water loss (changing water into water vapour requires large amounts of heat). With conductive methods energy (heat) moves but the molecules do not. Heat from the head is conducted through the wall of the device and either actively removed by the circulating liquid coolant or passively absorbed by the frozen material (ice/gel). Devices containing frozen material will warm up in this process and must be replaced regularly to maintain cooling efficiency.
Non-invasive head-cooling methods are generally quick and easy to apply and may be suitable for pre-hospital use, which are important considerations in reducing time to cooling if neuroprotection is the aim. They also have potentially wide application because they can be used in patients with a range of severity of illness, not just the most severely ill.
How the intervention might work
Although cooling interventions are more commonly delivered systemically, the logic behind head cooling is that it targets cooling where it is needed because it is brain rather than trunk temperature that is important in cerebral protection. It is also thought that head cooling may reduce the complications of hypothermia because less body temperature reduction is required, although the evidence for this is not robust. 23
The great advantage of cooling, by comparison with most other neuroprotective interventions, is that it has many potentially beneficial effects with regard to secondary injury mechanisms and therefore cerebral protection. Hypothermia has even been described as ‘the ultimate neuroprotective cocktail’. 7 The effects of cooling are not fully understood but include reduction in metabolic rate, modulation of cerebral blood flow, and the inflammatory response and reduction of excitotoxic damage and cerebral oedema. 6,26 Because cooling can be very effective in reducing refractory ICP this is the most usual reason for instigating therapeutic hypothermia in severe traumatic and haemorrhagic brain injury. 27,28 In ischaemic stroke it is considered possible that therapeutic hypothermia could extend the time window within which restoration of blood supply, for example with thrombolysis, might be effective. 29
Measurement of temperature reduction
If cooling, however delivered, is to have a neuroprotective effect, brain temperature must be reduced. The primary measure of the effectiveness of head cooling with regard to temperature reduction is a decrease in intracranial temperature. For the purposes of this review, intracranial temperature is defined as temperature inside the skull and within the dura. In the absence of intracranial temperature data, the secondary measure for this review is reduction in core trunk temperature with head cooling, measured in an artery (usually pulmonary), the oesophagus, bladder or rectum, on the assumption that for core trunk temperature to be reduced there must have been some reduction in intracranial temperature. (For further explanation see Appendix 1.)
Cardiac arrest and neonatal hypoxic–ischaemic encephalopathy
The principal focus of this review is head cooling in TBI and stroke, in which the primary problem is in the brain. However, in global (whole body) ischaemia, following cardiac arrest, therapeutic hypothermia is considered to improve outcome, specifically with return of circulation after ventricular fibrillation,30–32 although doubts have been raised over the quality of the evidence. 33 Therefore, during the protocol review process, we were asked to include the cardiac arrest literature on head cooling in our searches because this could contribute information about how effective these interventions are in reducing temperature, and on their ease of use and side effects. Studies in cardiac arrest were not relevant for assessment of functional outcome in this review. However, in our opinion, it is not yet clear to what extent whole-body cooling, which includes myocardial cooling, contributes to improved outcome with hypothermia after cardiac arrest, and whether or not head cooling alone is as effective as systemic cooling in this systemic ischaemic injury. There is no comparative randomised controlled trial (RCT) but there is some evidence, for example, that myocardial reperfusion injury, which can be ameliorated by hypothermia, may contribute to post-arrest morbidity and mortality. 34,35
Neonatal HIE is the other global ischaemic condition in which therapeutic hypothermia has been shown to be of benefit. 36,37 Head cooling has been commonly used as the means of achieving hypothermia in neonatal HIE but whether or not it has advantages over systemic cooling has not yet been assessed in a comparative RCT. 36,38 However, a recent systematic review and meta-analysis in neonatal HIE includes a subgroup analysis of systemic hypothermia (seven studies) and head cooling (six studies) compared with normothermia, which shows that more adverse functional outcomes were reduced with systemic cooling than with head cooling. 38 It is relatively easy to cool infants with head cooling as they have a smaller body–head ratio than adults and therefore have less counterwarming from the trunk; also their skulls are not closed because their fontanelles have not fused. Intracranial temperature is not measured clinically in infants with neonatal HIE, but head cooling has a considerable ‘knock-on’ effect on body temperature and body warming is required to control systemic hypothermia. 39 The effect of head cooling on temperature in neonates does not extrapolate to adults, but neonatal head-cooling research could contribute information on adverse effects of methods and devices therefore it was included in the review for this purpose.
The reason for undertaking this review
Systematic reviews of cooling interventions after brain injury and stroke have not differentiated between cooling methods. The only Cochrane review of a specific cooling intervention, for example, is that of paracetamol for fever in children. 40 In the reviews of cooling for acute stroke14 and of hypothermia for head injury15 the effect of temperature reduction on outcome has been the focus rather than the method(s) of achieving this, although a distinction was made between pharmacological and physical methods in stroke. Yet physical cooling methods differ in their effectiveness and complications. The reason for using head cooling is that, theoretically, it may have advantages over systemic cooling. Cooling is targeted to the site of injury where it is most needed, therefore requiring less body temperature reduction relative to brain temperature, which means that it may have fewer side effects than systemic physical methods. In order to determine whether or not head cooling has an effect and whether or not there are advantages it was necessary to review head cooling as an intervention.
Chapter 2 Aim and objectives
The aim of this review was to assess the effectiveness and cost-effectiveness of non-invasive head cooling in adults after TBI and stroke and provide a comprehensive assessment of head-cooling research in these patients.
The objectives were:
-
Assessment of temperature change To assess what effect non-invasive head cooling has on intracranial temperature and/or core trunk temperature in patients after TBI and stroke. This objective was informed by studies in cardiac arrest as well as those in TBI and stroke.
-
Assessment of head cooling on outcome To assess what impact non-invasive head cooling has on disability, assessed with a validated outcome score, and mortality in adults after TBI and stroke.
-
Complications associated with head cooling To determine any adverse effects or complications associated with head cooling or the specific devices and methods used. Studies in TBI, stroke, cardiac arrest and neonatal HIE all provided information for this objective.
-
Health economic assessment To assess the cost-effectiveness of head cooling in TBI and stroke.
-
Public involvement To present the results of the review to members of the general public, in order to hear their views on the concept and possible use and effectiveness of head cooling, and provide information on their views for clinicians and researchers planning to use or trial head cooling.
Chapter 3 Review methods
Differences between protocol and review
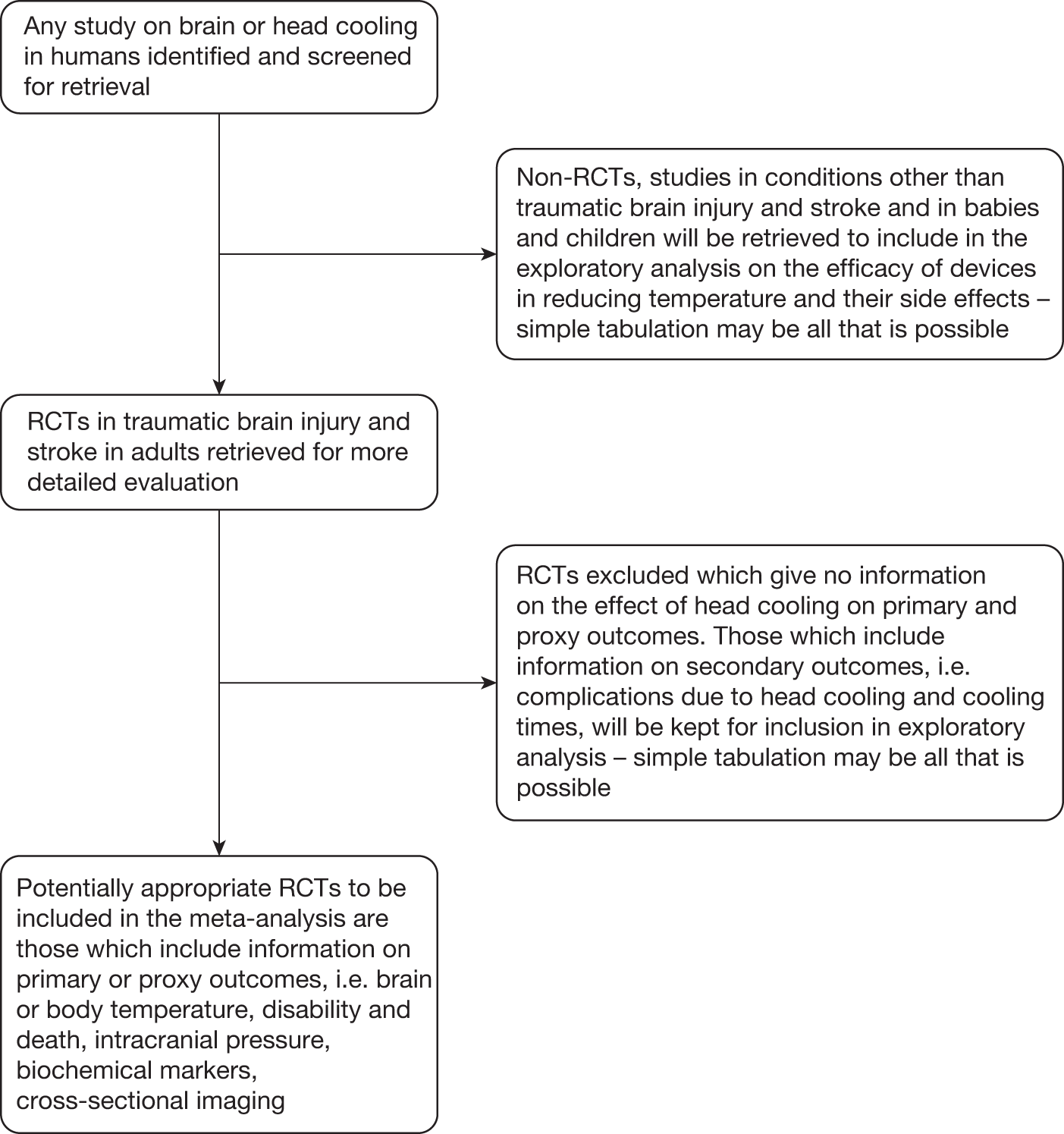
The review protocol can be found in Appendix 2. We had consultancy support from Brenda Thomas, Cochrane Stroke Group Trials Search Co-ordinator, and on her advice the outline search strategy in the protocol was considerably extended to include, for example, EMBASE classic, the British Library’s Electronic Table of Contents (Zetoc), British Nursing Index (BNI) and BNI Archive, and Web of Science conference proceedings. Had time allowed we would also have included additional country-specific databases in addition to those in the protocol (e.g. WanFang, Panteleimon, IndMED, KoreaMed), Web of Science cited reference search (forward search) and more hand-searching. The formal patent search was omitted owing to lack of time. Of the head-cooling reports in the review (see Figure 1, which corresponds to the results of stage 2, trial identification and selection in the protocol) only studies that could potentially have been RCTs were screened, assessed and had data extracted by two reviewers.
Criteria for considering studies for this review
Types of studies
Studies or case reports of any kind in adult humans after TBI and stroke, using any form of non-invasive head cooling were searched for. Studies of head cooling in cardiac arrest and neonatal HIE were also searched for to obtain information on temperature reduction (cardiac arrest) and adverse effects of cooling methods and devices (cardiac arrest and neonatal HIE).
Types of participants
All adults (aged ≥ 18 years) admitted to hospital with TBI, or ischaemic or haemorrhagic stroke, of any severity, and after resuscitation from cardiac arrest for the purposes of assessing efficacy of head cooling in reducing temperature. Studies of cooling in neonatal HIE were included only for information on adverse effects.
Types of intervention
Studies of any method of non-invasive head cooling of any duration given for the purposes of fever reduction, inducing normothermia or hypothermia, or reducing disability and mortality or reducing ICP were included. Studies in which head cooling was used solely during surgery or combined with another cooling intervention, excepting antipyretic drugs, such as paracetamol, were excluded.
Cooling intervention comparisons could include:
-
no cooling intervention or standard care
-
physical cooling interventions applied systemically or to parts of the body other than the head, for example tepid sponging, ice packs, cooling blankets, intravascular cooling catheters
-
pharmacological cooling interventions, for example paracetamol, non-steroidal anti-inflammatory drugs, cyclo-oxygenase inhibitors, ethymisole.
Outcome measures
Primary outcomes
-
Intracranial temperature (inside the skull and within the dura) or core trunk temperature (measured in an artery, the oesophagus, bladder or rectum). Comparisons could include temperature with and without head cooling, temperature at baseline compared with temperature at the end of cooling or the lowest temperature achieved.
-
All-cause mortality by end of follow-up.
-
Outcome assessed with a validated outcome score, i.e. Glasgow Outcome Scale (GOS),41 and acute, functional or outcome assessments listed on the Internet Stroke Center. 42
Other outcomes
-
Reduction in ICP.
-
Improvement in biochemical markers of injury, for example lactate–pyruvate ratio, glutamate, cytokines.
-
Improvement in cross-sectional imaging.
-
Time from brain injury or onset of stroke to start of cooling, cooling rate (hourly temperature reduction), and time from injury to target temperature and from device application to achieving target temperature. These are indicators of the effectiveness of head-cooling methods and devices and their ease of use, for example how quickly and easily they can be applied.
Adverse effects
Complications actually or possibly attributable to the head-cooling intervention or the specific device, for example infections, prolonged clotting time and bleeding complications, scalp damage.
Search methods for identification of studies
Appendix 3 (search strategies) contains details of the searches and search terms. The searches were not restricted by publication status, date or language.
Electronic searches
Dates given are for the most recent search.
Major international medical bibliographical databases
MEDLINE 1950 to 12 March 2011.
OLDMEDLINE 1948–65.
EMBASE 1980 to 2011 Week 10.
EMBASE Classic 1947–79.
Cumulative Index of Nursing and Allied Health Literature (CINAHL) 1937 to April 6 2010.
British Nursing Index (BNI) and BNI Archive 1985 to May 2010.
Web of Science Conference Proceedings Citation Index-Science (CPCI-S) 1990 to 19 July 2010.
Zetoc Conference Proceedings (8 August 2010).
ProQuest Dissertations & Theses (PQDT) database (25 March 2011).
The Cochrane Library
Cochrane Central Register of Controlled Trials (CENTRAL) (2011 Issue 1).
Cochrane Database of Systematic Reviews (CDSR) (2011 Issue 3).
Database of Abstracts of Reviews of Effects (DARE) (2011 Issue 1).
Health Technology Assessment (HTA) database (2011 Issue 1).
NHS Economic Evaluation Database (NHS EED) (2011 Issue 1).
Cochrane specialised trials registers
Cochrane Injuries Group (14 June 2010).
Cochrane Stroke Group (5 May 2010).
Other trial registers (last update all registers 6 March 2011)
World Health Organization International Clinical Trials Registry Platform (WHO ICTR).
Current Controlled Trials: the meta-register of controlled trials and International Standard Randomised Controlled Trial Number (ISRCTN) register.
ClinicalTrials.gov.
National Research Register archive.
Stroke Trials Registry.
Country-specific databases
Informit Health Collection (includes Australasian Medical Index) (6 February 2011).
China National Knowledge Database (CNKI): China Academic Journals (CAJ) Medicine and Public Health (hygiene) database (14 January 2011).
Japan Science and Technology Agency (JST): J-EAST (16 August 2010), J-STAGE (5 February 2011), journal@rchive (4 February 2011).
Latin American Caribbean Health Sciences Literature (LILACS) (5 February 2011).
Russian Academy of Sciences Bibliographies (25 March 2011).
Web search engines
Scirus (7 March 2011).
Google Scholar (26 March 2011).
Searching other resources
Reference lists of relevant studies and reviews and of books on therapeutic hypothermia and the proceedings of hypothermia conferences were checked. Investigators and manufacturers of head-cooling equipment were contacted in writing.
Data collection and analysis
Selection of studies
Bridget Harris conducted the searches with advice and help from Brenda Thomas, Cochrane Stroke Group Trials Search Co-ordinator. All retrieved results were imported into Reference Manager (version 11, Thomson Reuters, CA, USA), de-duplicated, and titles and abstracts were screened by BH to remove anything that did not meet the review criteria with regard to study type, participants, intervention and outcome (details above). Where full review or further information to determine relevance was required the complete paper was obtained and screened by BH. This resulted in a final data set of studies that met the review criteria, with full text, where this existed, for detailed assessment regarding inclusion and exclusion for analysis. If there was more than one report of a study all were included in order to facilitate complete data extraction. The method for screening and assessing papers in languages other than English is detailed below. The study assessment and data collection form was piloted by BH and PA (Appendix 4 contains the final version used for the review). It includes the quality checklist we used to assess RCTs, which was developed by the Cochrane Renal Group. 43 Trials were not included or excluded on the basis of an overall score on this checklist but according to whether they met the prespecified inclusion criteria for the review.
From the final data set any studies that purported to be RCTs were independently assessed for quality by BH and PA. Trials that had an adequate method of randomisation (see Appendix 4) were eligible for inclusion for formal analysis of the effect of head cooling on patient outcome. Trials in which the assessor of disability outcome was not blinded were excluded from the formal analysis as prespecified in the protocol. One of the reasons for this was because the intervention could not be blinded.
In addition to RCTs any studies, including proof of concept and case studies, that contained information on head-cooling devices and methods (presented in full in Appendix 7), their efficacy in reducing temperature, ease of use and adverse effects were included for descriptive reporting (as prespecified in the protocol). These studies were not formally assessed for quality and bias; they are described and the temperature data and adverse effects tabulated. It was considered that temperature, being a physical measure of a physiological variable, is less susceptible to interpretation and bias than, for example, functional outcome, and it was therefore reasonable to include information on the effect of head cooling on temperature, even if the studies were not randomised or controlled, because this provides some evidence of proof of concept (or otherwise).
Papers in languages other than English
A number of papers in foreign languages required full-text review: French (13), Italian (1), Slovakian (1), German (11), Japanese (3), Russian (8) and Chinese (26). Some of these had no, or an inadequate, English abstract so that it was not clear, for example, if the research was in humans or animals or whether head cooling or systemic cooling had been used without reading at least part of the paper. We had assistance from colleagues and friends with the requisite languages and used Google Translate (http://translate.google.com) to eliminate papers that were not relevant.
A Chinese-speaking intensive care doctor helped with the Chinese papers. She read them all, translated parts and went through them in detail with BH to assess quality and extract data. This did not highlight any that, on grounds of quality, warranted formal professional translation but we did have the study comparing head cooling with systemic cooling translated, as this was a particular comparison of interest with very few studies. 44 Because the other Chinese studies were not formally translated in full it has been possible only to report the main points and reasons for exclusion Appendix 6 (see Characteristics of Excluded Studies) compared with some of the studies in English where we have reported in more detail, although this is sometimes simply because there was more detail to report (the Chinese papers were mostly short).
Papers on head cooling in neonatal HIE in languages other than English were not assessed because this was not the primary condition of interest and there are recent systematic reviews (see Appendix 5, References to studies in neonatal hypoxic–ischaemic encephalopathy), which were also consulted for information on adverse effects of head-cooling methods and devices, i.e. the reason why papers on neonatal HIE were of interest.
Data extraction
BH and PA independently extracted data from RCTs using a standard form (see Appendix 4). They were not blinded to authors, journal or results. Disagreements were resolved by discussion. BH extracted data from all other studies. Where multiple reports of a trial were available, discrepancies between the reports were noted. Where there was missing information attempts were made to contact investigators.
Assessment of risk of bias
Randomised controlled trials were assessed for adequacy of the randomisation and allocation concealment process, potential for selection bias after allocation and level of masking (blinding of treatment provider, patient, outcome assessor, investigators and analysers of the data) (see Appendix 4).
Data synthesis
We were unable to carry out the full analysis plan specified in the protocol (see Appendix 2) because there were insufficient good-quality RCTs to undertake formal outcome analysis.
Briefly, had there been suitable RCT data, the following analysis was planned. For temperature data the difference in means would have been calculated with 95% confidence intervals (CIs). If sufficient good-quality trials for a meta-analysis had been found then a weighted mean difference would have been calculated. Pooled relative risk and 95% CIs for all-cause mortality and good neurological outcome would have been calculated using a random-effects model. Statistical heterogeneity would have been assessed using the chi-squared test.
However, it was recognised in the protocol that, depending on what was found, description of results might be all that was possible and the available temperature data are tabulated as a descriptive record of the effect of head cooling. No attempt has been made to draw any statistical inference. Data on adverse effects are reported descriptively.
Chapter 4 Results
The main results are presented first – the description of studies and effects of the interventions. The searches also provided examples of other conditions in which head cooling has been used as a therapy and some descriptions of head cooling that are of historical interest, and these are presented after the main results.
Description of studies
Refer to Appendix 6 for detailed information on studies included, excluded, awaiting assessment and ongoing. Studies that included mixed populations of TBI and stroke are classified as studies in brain injury.
Results of the search
Figure 1 shows the results of the search and selection process. In the box ‘Head-cooling reports in the review’ the number of studies is given of each type found, with the number of reports in parentheses, i.e. some studies had more than one report associated with them. There were 46 studies (with 52 associated reports) in TBI, stroke and brain injury and 12 studies (15 reports) in cardiac arrest.
FIGURE 1.
Search results. a, Some studies had more than one report and the number in parentheses refers to the total number of reports. b, Where it was possible to de-duplicate search results on import to Reference Manager duplicates were counted. Otherwise, a record was not kept of whether the citation was excluded because it was a duplicate (less common) or irrelevant.
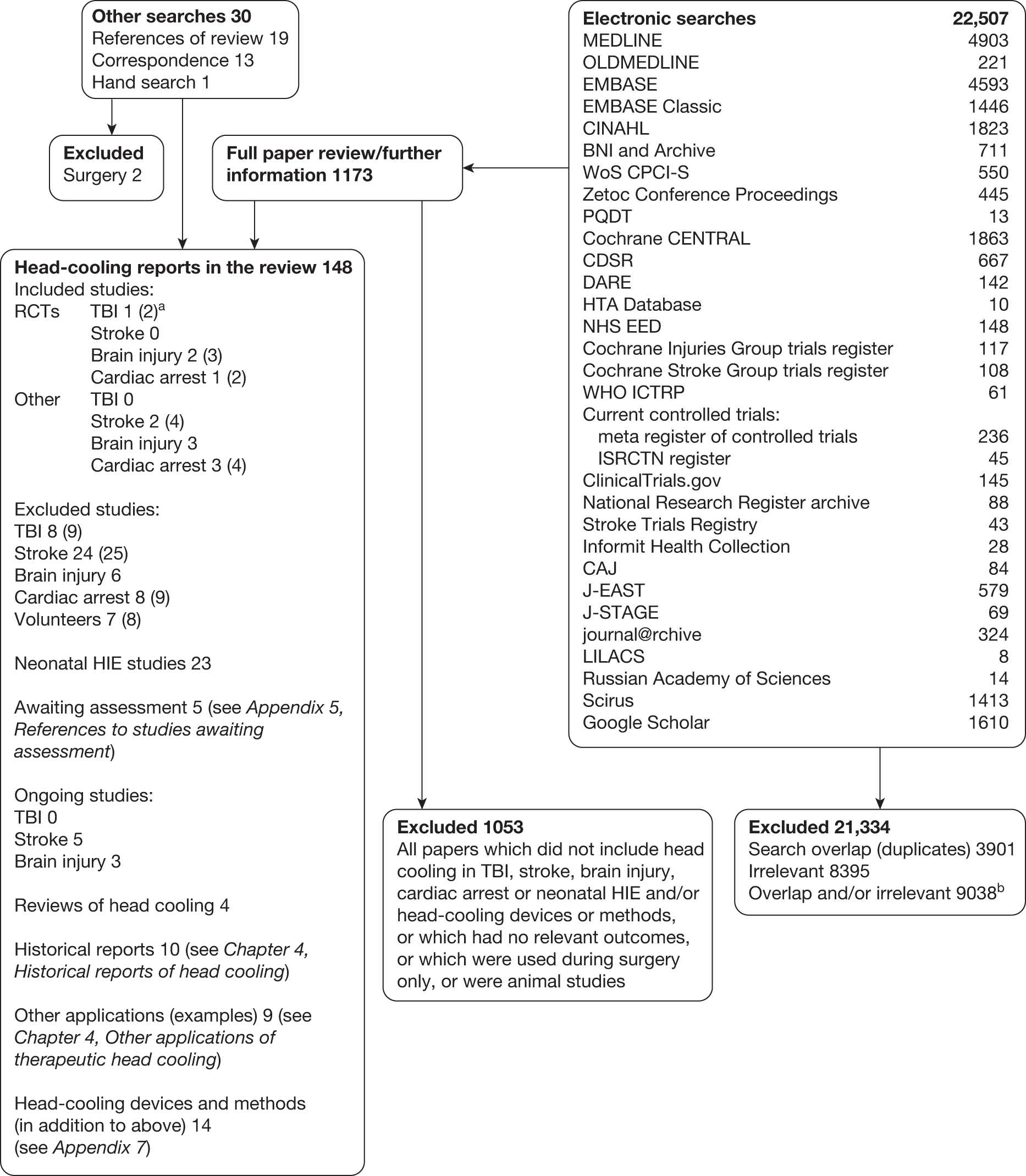
From the information available we were unable to reliably determine that there were any high-quality RCTs in TBI or stroke with blinded outcome assessment (see Appendix 6, Characteristics of included studies and Apendix 6, Characteristics of excluded studies).
Included studies
Most studies did not provide sufficient detail on temperatures for inclusion, for example the target temperature was reported rather than the actual temperature reduction or they used temperature measurement sites that did not meet the review criteria (see Appendix 6, Characteristics of excluded studies). Temperature measurement sites that were valid for inclusion were intracranial (inside the skull and within the dura) and/or core trunk (arterial, oesophageal, bladder or rectal).
Twelve studies did have useable data on the effect of head cooling on intracranial and/or core trunk temperature (see Table 2). Five were RCTs: one in TBI,45 two crossover trials in brain injury46,47 and two in cardiac arrest. 48,49 The other seven included studies were descriptive reports: two in stroke,50,51 three in brain injury52–54 and two in cardiac arrest. 55,56
All information on cooling method or device-related adverse effects that could be found in included or excluded studies, studies in neonatal HIE, reviews of head cooling or in other applications of head cooling was included. Studies in neonatal HIE are not described in Appendix 6 because they were only relevant for information on adverse effects and advantages of head-cooling devices and methods. References to studies in neonatal HIE lists all of the studies that were found on searches and read to extract these data.
Papers on head-cooling methods and devices were retained for information even if they contained limited or no clinical data and are included in Appendix 7, which describes the methods and devices that were found in this review.
Risk of bias in included studies
The four included RCTs had good allocation concealment, but none of the three in TBI and brain injury45–47 had blinded outcome assessment. The last two46,47 were also crossover trials with a primary physiological outcome. They were designed to assess proof of concept of intracranial temperature reduction in response to particular cooling methods, with short intermittent cooling periods rather than cooling as a sustained therapy that might influence outcome. Therefore, there were no outcome data on TBI or stroke suitable for inclusion in the review. However, these RCTs and the RCT in cardiac arrest49 did have data on temperature reduction with head cooling and are included for that reason. Detailed assessment of all studies can be found in Appendix 6.
Types of interventions
In brief, the interventions used in included studies (listed in Appendix 5, References to studies included in this review) were:
-
heat loss from the upper airways:
-
– nasal gas flow
-
– nebulised intranasal perfluorocarbon with oxygen (Rhinochill)
-
-
heat loss through the skull:
-
– convective head fanning
-
– conductive – passive ice and frozen gel caps
-
– conductive – active liquid head- and neck-cooling devices.
-
None of the devices had automatic (closed-loop) temperature feedback. In a comparative study of systemic cooling devices, those with automatic temperature control were shown to be more effective and less labour intensive than manually controlled devices. 17
Details of the applications of cooling are given in Table 1 and Appendix 6 (see Characteristics of included studies). Details of the cooling methods and devices can be found in Appendix 7.
| Authors | Type and purpose of study | Subjects | Head-cooling intervention | Effect of cooling on intracranial temperature | Effect of cooling on core trunk temperature |
|---|---|---|---|---|---|
| Andrews 2005,46 Harris 201057 | Randomised, controlled crossover trial of effect of restoration of nasal airflow on brain temperature in orally intubated patients | TBI and SAH (n = 15) | 30-minute baseline, randomised to 6-hour airflow or 6 hours of no airflow then crossed over for further 6 hours. Airflow: continuous through both nostrils at total rate of 115 ml/kg/minute (commensurate with normal minute volume), range 6–13 l |
Parenchymal Within-patient change in mean temperature with 6-hour airflow compared with 6 hours of no airflow –0.13 °C, SD 0.55 °C, 95% CI –0.43 °C to 0.17 °C. Range of temperature change: +0.55 °C to –0.9 °C |
Oesophageal Not reported |
| Sung 2009,54 Abou-Chebl 201158 and unpublished | Non-randomised single group safety and feasibility study of intranasal cooling induction with the Rhinochill device | Stroke and TBI with clinical indication for cooling (n = 15) | Intranasal cooling (Rhinochill) for 1 hour for fever control (n = 9) or neuroprotection/ICP reduction (n = 6) (followed by local standard cooling methods) |
Parenchymal n = 11: mean reduction after 1 hour of cooling 1.4 ± 0.4 °C |
Arterial, oesophageal, bladder or rectal n = 15: mean reduction after 1 hour of cooling 1.1 ± 0.6 °C |
| Andreas 200855 | Prospective observational study of feasibility and safety of Rhinochill device | Cardiac arrest after ROSC (n = 7) | Intranasal cooling (Rhinochill) for 1 hour (followed by cooling to 33 °C up to 24 hours with another device) | N/A |
Oesophageal Median (first to third quartile) baseline temperature : 35.4 °C (34.7 °C to 36 °C) After 1 hour: 34.1 °C (33.4 °C to 34.9 °C) Difference: 1.3 °C Cooling rate: 1.6 °C (1 °C to 1.7 °C)/hour |
| Busch 2008;56 201059 | Descriptive study of effectiveness, feasibility and safety of Rhinochill device | Cardiac arrest after ROSC (n = 84) | Intranasal cooling (Rhinochill) device for 1 hour (range 25–195 minutes) (followed by cooling to 33 °C up to 12–24 hours with a systemic device) | N/A |
Arterial, oesophageal, bladder or rectal Cooling rate median (first to third quartile): 1.1 °C (0.7 °C to 1.5 °C)/hour |
|
PRINCE trial |
RCT of safety, feasibility, cooling efficacy of Rhinochill device | Witnessed out-of-hospital cardiac arrest pre-ROSC (n = 194); 93 cooled (75 survived to hospital), 101 uncooled control patients (42 survived to hospital) | Intranasal cooling (Rhinochill) started during arrest and continued until after hospital arrival (median duration 32 minutes), target temperature 34 °C | N/A |
Rectal, bladder or intravascular Mean difference between cooled (n = 75) and control patients (n = 42) after hospital admission: –0.7 °C (p = 0.01) |
| Authors | Type and purpose of study | Subjects | Head-cooling intervention | Effect of cooling on intracranial temperature | Effect of cooling on core trunk temperature |
|---|---|---|---|---|---|
| Harris 2007;47 201057 | Randomised controlled crossover factorial trial of effect on temperature of enhanced nasal airflow and bilateral head fanning | TBI and SAH (n = 12) |
Thirty-minute baseline, each of four interventions in random order for 30 minutes with washout between (1) enhanced nasal airflow, (2) head fanning (no head bandages), (3) (1) + (2), and (4) no intervention 1 = continuous unhumidified airflow through both nostrils at twice the patient’s ventilated minute volume + 20 ppm nitric oxide 2 = bilateral head fanning with ambient air, total air speed approximately 8 m s-1 |
Parenchymal Difference in mean temperature over last 5 minutes of preceding washout minus mean over last 5 minutes of intervention = 0.15 °C with nasal airflow (p = 0.001, 95% CI 0.06 °C to 0.23 °C) and 0.26 °C with head fanning (p < 0.001, 95% CI 0.17 °C to 0.34 °C) Estimate of combined effect of airflow and fanning on temperature = 0.41 °C |
Oesophageal Difference in mean temperature over the last 5 minutes of preceding washout minus mean over the last 5 minutes of intervention = 0.13 °C with nasal airflow (p = 0.005, 95% CI 0.04 °C to 0.21 °C) and 0.19 °C with head fanning (p < 0.001, 95% CI 0.11 °C to 0.28 °C) Estimate of combined effect of airflow and fanning on temperature = 0.32 °C |
| Authors | Type and purpose of study | Subjects | Head-cooling intervention | Effect of cooling on intracranial temperature | Effect of cooling on core trunk temperature |
|---|---|---|---|---|---|
| Callaway 200248 | RCT with convenience sample of pre-hospital head cooling during cardiac arrest | Out-of-hospital cardiac arrest (n = 27); 14 cooled (5 excluded from analysis because of incomplete temperature data); 13 uncooled control patients | Head cooling with three 500-ml bags of ice applied to head + one across neck (duration 5–10 minutes) | N/A |
Oesophageal Cooled group mean baseline: 35.5 ± 1.0 °C; control patients 35.3 ± 1.7 °C; temperatures at end of cooling not reported Mean rate of temperature change in cooled group: 0.07 ± 0.06 °C/minute (95% CI –0.11 to –0.03) Mean change in control patients: 0.02 ± 0.06 °C/minute (95% CI –0.05 to 0.02) Difference: –0.05 °C/minute (95% CI –0.106 to 0.007) |
| Forte 200952 | Retrospective study of the effect of ice packs on ICP and brain temperature – not reported if prospective or retrospective | TBI, SAH, stroke, brain tumour, after decompressive craniectomy for refractory intracranial hypertension (n = 23) | Ice packs over decompressive craniectomy site, duration 61.7 hours (range 20–96 hours) depending on ICP and CT | Intracranial: Mean at baseline 37.1 °C (range 35.3–38.9 °C), mean over 48 hours of cooling 35.2 °C (range 33.6–37.6 °C); range of temperature change with cooling +0.3 °C to –4.5 °C |
Oesophageal Not reported |
| Authors | Type and purpose of study | Subjects | Head- and neck-cooling intervention | Effect of cooling on intracranial temperature | Effect of cooling on core trunk temperature |
|---|---|---|---|---|---|
| COOL BRAIN Stroke Trial Wang 2003;61 2004;50 200462 | Prospective, non-randomised pilot trial of the effectiveness of head cooling in reducing brain temperature | Stroke + ≥ 1 TBI (n = 14); 8 cooled, 6 ‘control patients’ (not reported here) |
Pressurised liquid cooling helmet, temperature of coolant not reported, heads shaved Cooling duration unclear – up to 72 hours. Active body warming to maintain bladder temperature > 33 °C, 35 °C if aged > 45 years |
Parenchymal Mean brain temperature reduction 1.84 °C (range 0.9–2.4 °C) within 1 hour |
Note: Active body warming Bladder: Not reported Mean brain minus bladder temperature difference during cooling = –1.6 °C |
| Harris 200945 | RCT to evaluate a head-cooling device in the management of TBI | TBI (n = 25); 11 cooled, 10 uncooled control patients (missing temperature data n = 4) | Pressurised liquid cooling helmet, coolant temperature not reported, heads not shaved, duration 24 hours, target intracranial temperature 33 °C, active body warming to maintain bladder temperature 36 °C |
Parenchymal or ventricular Cooled group: Mean baseline = 37.9 °C; at 12 hours, 36.8 °C; at 24 hours, 36.9°C Control patients: Mean baseline and 12 hours, 37.9°C; at 24 hours 38.1°C; difference from baseline in cooled group at 12 hours = 1.1°C, at 24 hours = 1°C 12-hour mean difference between cooled patients and control patients = –1.1 °C, at 24 hours –1.2 °C |
Note: Active body warming Bladder Not reported Mean intracranial minus bladder temperature over the 24-hour cooling period: –0.67 °C in the cooled group; +0.05 °C in the control patients (neither statistically significant) |
| Gaida 200851 | Observational study of head cooling for refractory fever management | SAH (n = 6) | Liquid cooling helmet (CSZ Blanketrol) for brain temperature > 37.8 °C after 2 hours of standard fever management, duration 6 hours |
Ventricular Mean baseline = 38.5 ± 0.6 °C Mean at 6 hours = 37.5 ± 0.4 °C Difference = 1 °C |
Arterial Mean baseline = 38.2 ± 0.6 °C Mean at 6 hours = 37.4 ± 0.5 °C Difference 0.8 °C |
|
TraumaTec Neuro-Wrap Neuro ICU Study Miller 200953 and unpublished |
Descriptive single group study to determine rate and degree of brain cooling with TraumaTec Neuro-Wrap |
Brain injury Interim data on n = 9, study aim n = 20 |
Liquid cooling helmet (TraumaTec Neuro-Wrap) for 8 hours Target temperature N/A |
Intracranial Mean baseline temperature 37.5 ± 1 °C Lowest temperature 35.5 ± 1.4 °C Difference 2.0 °C |
Body temperature remained between 36.7 °C and 37.8 °C |
Effects of interventions
Effect of non-invasive head cooling on temperature
Table 1 (12 studies) shows the effect of head cooling on intracranial and/or core trunk temperature and includes 99 patients who were cooled after TBI/stroke and 198 patients (data available for 175) who were cooled after cardiac arrest. In addition to different patient populations (TBI, stroke and cardiac arrest), there was considerable heterogeneity of cooling intervention (methods and duration), indications for cooling and reporting of temperature data (including some with no summary measure – for example, mean/median – and spread of temperature change with cooling), therefore the results have simply been tabulated. There is no straightforward way of presenting the data that addresses all of the sources of heterogeneity but because the purpose is to assess the effect of head cooling on temperature the data are presented by method of cooling. All of the TBI and stroke patients and none of the cardiac arrest patients had intracranial temperature monitoring. Cardiac arrest data are not presented separately from TBI and stroke but the aim of cooling after cardiac arrest was always hypothermia (target 33 °C or 34 °C). Baseline temperatures in the out-of-hospital cardiac arrest patients were low (around 35.5 °C), which makes a hypothermic target easier to achieve than in TBI and stroke patients who were not hypothermic at baseline. Hypothermia was the aim in only two of the eight TBI and stroke studies in Table 1. Two of the studies in Table 1 showed no effect of head cooling. Replication of normal, ambient temperature nasal airflow in intubated, brain-injured patients for 6 hours46 and ice packs to the head for 5–30 minutes in patients after cardiac arrest who were already cool (mean oesophageal temperature ≤ 35.5 °C). 48
Table 2 (nine studies) summarises the temperature reduction data in Table 1 and includes all those studies45,47,50–55,59 that had data on mean (or median) temperature reduction with head cooling. The studies that are omitted are the Pre-ROSC IntraNasal Cooling Effectiveness (PRINCE) trial,49 which did not report the temperature reduction in cooled patients, and the two studies which showed no effect46,48 (only one of these46 had data on average temperature reduction).
| Head-cooling method | Cooling duration | Intracranial temperature reduction (total no. of cooled patients) | Core trunk temperature reduction (total no. of cooled patients) |
|---|---|---|---|
|
Rhinochill |
1 hour | 1.4 °C (n = 11) | 1.1–1.3 °Ca (n = 106) |
|
Nasal airflow + head fanning Harris 200747 |
30 minutes | 0.41 °C (n = 12) | 0.32 °C (n = 12) |
|
Ice packs to craniectomy site Forte 200952 |
48 hours | 1.9 °C (n = 23) | Not reported |
|
Liquid cooling of head and neck Wang 2004,50 Harris 2009,45 Gaida 2008,51 TraumaTec Neuro-Wrap ICU Study Miller 200953 |
1–24 hours | 1–2 °C (n = 34) | 0.8 °C (n = 6) |
Functional outcome and mortality
We prespecified that only good-quality RCTs with blinded outcome assessment would be used to assess functional outcome and mortality, and we were unable to establish that any of the trials with control groups met these criteria. The RCTs in TBI and brain injury in Table 1 could not be included in this analysis because of crossover design46,47 (these were designed to assess proof of concept of intracranial temperature reduction with cooling applied for short periods only rather than as a sustained therapy) and because we were unable to verify if outcome assessment was blinded (no response from investigator). 45 The primary outcome of this latter study45 was determination of the effect of the head-cooling device on temperature in patients with TBI and specifically maintenance of a core body–brain temperature gradient using active body warming. Comparative assessment of outcome (mortality, GOS and functional independence measure) at hospital discharge or 28 days after injury (whichever was sooner) was a secondary objective. Six out of 12 patients in the cooled group and 4 out of 13 control patients died, but there was no statistically significant difference between the groups on any of the outcome measures. However, this study was too small (n = 25) to be powered to detect a difference in functional outcome (no sample size calculation was provided to show how study size was determined).
For other trials that had information on outcome (details in Appendix 6, Characteristics of excluded studies) the reasons for exclusion included insufficient information on methods, for example to assess whether they were RCTs or to complete the quality checklist, outcome assessments that did not meet the review criteria and either unblinded outcome assessment or insufficient information to determine if outcome assessment was blinded.
Adverse effects and complications associated with head-cooling devices and methods
All adverse effects that were reported in included or excluded studies, studies in neonatal HIE, reviews of head cooling or in other applications of head cooling are included here, although many studies reported no specific device or cooling method-related adverse effects. Adverse effects are reported and described under the broad headings of heat loss from the upper airways and heat loss through the skull, and were generally self-limiting and not serious (Table 3 provides a summary). Descriptions of the methods and devices can be found in Appendix 7. Unless stated to the contrary, all of the patients were unconscious and sedated.
| Adverse effects | Contraindications/precautions | ||
|---|---|---|---|
| Unconscious/sedated | Conscious/unsedated | ||
| Heat loss from upper airways | |||
| Nasal gas flow | Nasal erosion |
Base of skull fracture ?Facial fractures ?Sinusitis Requires sedation Keep mouth open for exit flow |
|
| Rhinochill intranasal coolant |
Cold-related nasal whitening (one severe) Nose/mouth bleeds (one severe) Periorbital oedema Nasal erythema/discharge |
Base of skull fracture ?Facial fractures Requires protected airway Requires sedation Keep face uncovered and mouth open for exit flow/reduce cold-related side effects |
|
| QuickCool nasal balloons | Headache, rhinorrhoea, redness, ulcers |
Base of skull fracture ?Facial fractures ?Sinusitis |
|
| Heat loss through skull | |||
| Face/head fanning | Face fanning uncomfortable | ||
| Ice/frozen gel caps |
Headache Cold can be hard to tolerate |
||
| Liquid head and neck cooling |
Skin erosion? Scalp oedema (neonates) |
Pressure on scalp/skull with pressurised devices | |
Heat loss from the upper airways: convection
Nasal gas flow
The method used by Dohi and colleagues63 (nasal airflow through a Foley catheter with an inflated balloon and the other nostril occluded with an epistaxis balloon) caused nasal erosion in ‘several’ patients, even although the intervention was used for a only ‘short period’. There was no sinusitis, tympanic membrane injury or olfactory dysfunction. Dohi and colleagues63 commented that ‘the procedure may cause an oppressive feeling due to the high volume of circulating air’ and was suitable only in sedated patients (p. 410). They stressed the importance of the air being able to exit through the mouth, as did Harris and colleagues. 47 The methods used by Andrews and colleagues46 and Harris and colleagues47 did not occlude the nostrils and no erosion was seen, although care and lubrication was required when inserting the nasal catheters to avoid causing a nosebleed. In personal testing, Harris57 found that it was difficult to swallow, as unless the air delivery tubing was able to blow back out of the nostrils the air had nowhere to go and could cause discomfort in the ears. With high flows of dry air stinging of the nasal mucosa could also occur initially. 57 Nasal gas flow is contraindicated with base of skull fracture, possibly with certain facial fractures, and with sinusitis if an occlusive balloon is used.
Rhinochill (Benechill Inc., San Diego, CA, USA)
The Rhinochill device delivers inert perfluorocarbon coolant mixed with oxygen through bilateral nasal prongs to the nasal cavity where the coolant is nebulised and evaporates removing heat in the process. There is an overpressure relief valve. A protected airway is required and it is contraindicated with base of skull fracture and some facial fractures. It is designed for induction of cooling rather than prolonged use. The Rhinochill device has had considerable safety and feasibility testing in animals and humans. Device-related adverse events have been generally mild and self-resolving provided the device is managed correctly. The trial49 (n = 93) and study59 (n = 84) in cardiac arrest reported a total of 23 cases of nasal whitening (cold induced), five of epistaxis (one in a patient with underlying coagulopathy), two of periorbital oedema, one of perioral bleed and one of coolant in sinus. These all resolved. Patients who were able to undergo olfactory function assessment were within normal limits. 59 Busch and colleagues59 reported that one patient with cardiogenic shock who was given high-flow oxygen (60–80 l/minute) sustained cold-induced tissue damage, which persisted until death owing to cardiac failure. They commented that ‘Essential safety measures that prevent tissue damage include uncovering the face and keeping the mouth open during cooling, so that coolant vapor can escape from mouth and nostrils’ (p. 947).
In the Rhinochill study in brain-injured patients (n = 15), transient minor nasal erythema and discharge was seen on rhinoscopy (Dr Barbut, Benechill Inc., San Diego, CA, 14 April 2011, personal communication) and there was one device-related serious adverse event – hypertension attributed to patient discomfort – which resolved by stopping the device and giving sedation [www.benechill.com/wp/clinical-program/clinical/neuro-icu-cooling-study/ (accessed 1 November 2010)]. There were no cold-related injuries in this study, but it seems logical that cardiac arrest patients with reduced cardiac output and subnormal body temperatures pre-hospital64 would be more at risk of cold-related tissue damage to the nose than brain-injured patients in hospital who are likely to have a more normal cardiac output and above-normal temperature.
One possible advantage of head cooling noted in the study in brain-injured patients, on the basis of cooling results in two morbidly obese patients, was that brain temperature reduction may be less affected by body mass than core trunk temperature reduction [www.benechill.com/wp/clinical-program/clinical/neuro-icu-cooling-study/ (accessed 1 November 2010)] and Dr Barbut, personal communication).
Heat loss from the upper airways: conduction
QuickCool (Lund, Sweden)
These bilateral nasal balloon catheters, perfused with cold saline, have been tested in unsedated healthy volunteers (n = 10). 65 Adverse effects were minor and resolved spontaneously. Ear nose and throat examination showed increased nasal secretions (n = 9), redness (n = 3) and small ulcers (n = 3). Subjects reported headache (n = 4), dizziness (n = 1) and rhinorrhoea (n = 7), and rated the balloons as pleasant (n = 1), neutral (n = 3) and unpleasant (n = 6).
Heat loss through the skull: convection
Fanning of face or head
Mariak66 used face fanning for fever reduction in six conscious neurosurgical patients and noted that ‘Generally all patients reported an unpleasant sensation when fanned’ (p. 281). Head fanning, avoiding blowing air into the eyes, on the other hand is not generally perceived as uncomfortable. It is sometimes assumed that the use of fans in the intensive care unit (ICU) is associated with infection risk,67 but a review found no published data that electric fans spread infection in clinical areas. 68
Heat loss through the skull: conduction
Passive: ice and gel caps
Callaway and colleagues48 used bags of ice round the head for cooling after cardiac arrest; there were no adverse effects (if ineffectiveness in this instance is discounted) but it was difficult to secure them for transport. 48 In two studies69,70 with gel caps, both also in cardiac arrest, no adverse effects were found but the investigators commented on the ease and speed (< 30 seconds) with which the caps could be applied.
The scalp cooling studies, found with other applications of therapeutic head cooling (see below), have provided some information on the effect of intense head cooling in conscious, unsedated patients. Headache was quite common and some patients could not tolerate the cold. With the lower-temperature gel devices in particular the dropout rate could be high – 9 out of 15 patients in one study using a gel cap at –26 °C. 71 Scalp cooling therapy seemed to be more tolerable with liquid cooling caps at less cold temperatures, although warm clothing, blankets and even hot water bottles and electric blankets are sometimes recommended to improve comfort (e.g. with the Penguin Cold Cap).
Active: liquid head and neck cooling devices
Two (of 17) stroke patients72 and two (of 12) patients with TBI45 undergoing liquid head and neck cooling had skin erosion/decubitus ulcers, but it is not clear if these were device related. Yamada and colleagues72 give no details of the device but in the Harris and colleagues trial45 the helmet was pressurised at 15 mmHg (coolant temperature is not reported). Despite it being pressurised, Harris and colleagues45 comment that the cap was not fully effective and give one reason as ‘insufficient cap contact with the scalp’ (p. 1263). 45 Wang and colleagues50 used a similar device (coolant temperature and pressure not reported) and mention no device-related problems. However, the necessity for close scalp contact may be problematic following brain injury, in the presence of wounds and skull fractures for example, and if the constriction causes an increase in ICP.
With an unpressurised liquid cooling helmet (TraumaTec Neuro-Wrap, TraumaTec Inc., San Antonio, TX, USA) there were no device-related systemic or local complications including ‘skin irritation of the scalp or neck, restriction of jugular venous drainage by the neck section resulting in ICP elevations, or compression of neck structures resulting in barostimulation and changes in blood pressure’ (Professor Robertson, Baylor College of Medicine, Houston, TX, 3 January 2011, personal communication).
The studies in neonatal HIE that were assessed to find information on complications and benefits related to head cooling are listed in Appendix 5 (see References to studies in neonatal hypoxic–ischaemic encephalopathy). These were not limited to RCTs because any studies that were specific to head cooling or head-cooling methods were of interest for this purpose. The only device-related complications noted were in studies using liquid cooling caps (unpressurised, coolant 8–12 °C, 72-hour cooling) and these were scalp oedema39,73,74 and two cases of sclerodema,44 which can be caused by cold stress in neonates. All resolved spontaneously.
Complications and possible benefits: head cooling compared with systemic cooling
We found no high-quality RCT evidence on the relative complications and benefits of head cooling compared with systemic cooling in TBI and stroke, or cardiac arrest. What has been found is presented descriptively here.
Three studies in TBI or brain injury directly compared head cooling and systemic cooling; all also had normothermic control groups. 44,75,76 One study was not randomised and had a somewhat unusual head-cooling protocol, which meant that some patients may not have received head cooling as it was applied intermittently ‘on each of three successive days for 0–6 hours (average 4.5 hours) according to the patient’s condition’ (p. 59). 75 Possibly this may account for why there seems to have been little difference in the temperatures in the head-cooled and systemically cooled groups, although the actual temperatures are not reported. Another may have been randomised but had insufficient detail to assess trial quality (e.g. method of randomisation, blinded follow-up) and no actual temperatures reported, plus 40 out of 96 patients were not followed up (GOS at 1 year). 76 The third, in brain injury, also may have been randomised but provided insufficient detail for assessment of study quality and no actual temperature data. 44 Therefore, none were able to be included in the review for formal analysis, but the main findings of the two studies that may have been randomised trials are briefly described here for information.
Qiu and colleagues76 (n = 96) assessed thrombocytopenia (platelet count < 100 × 109/l) and found similar rates in head-cooled (77%) and systemically cooled (75%) patients and lower rates in control patients (36%). The patients who had thrombocytopenia were followed up (GOS at 1 year, none lost to follow-up) and those in the control group had better outcomes (GOS score 4–5): control patients 80%, head cooled 39%, systemically cooled 35%. 76 This seems to imply that the effect of cooling impacted adversely on outcome independently of thrombocytopenia as long as 1 year later.
In the study by Zhao and colleagues44 (n = 69), complication rates (pneumonia, gastrointestinal bleed, arrhythmias, renal failure) were similar in systemically cooled patients (90.91%) and control patients (91.67%) and head-cooled patients (39.13%). But good outcome (GOS score 5) and mortality at hospital discharge were similar in head-cooled (56.5% and 21.7%, respectively) and systemically cooled (54.5% and 22.7%, respectively) patients and worse in control patients (25% and 45.8%, respectively). 44
In neonatal HIE there were no noteworthy differences in systemic complications with head cooling compared with standard care (for studies consulted, see Appendix 5, References to studies in neonatal hypoxic–ischaemic encephalopathy). No RCTs in neonatal HIE have directly compared systemic cooling with head cooling. However, Sarkar and colleagues77,78 carried out an observational study and assessed the difference in multiorgan and pulmonary function between head cooling and whole-body cooling and found no difference. They speculate that the reasons for this may be that the target temperatures are not low enough to produce significant adverse effects from hypothermia, and the differences in core temperatures between head-cooled and whole body-cooled infants are not large enough (around 1 °C) to produce differences in benefit or adverse effects.
A possible benefit of head cooling was observed in a small case series in neonatal HIE that compared head cooling (n = 14) with whole-body cooling (n = 20) and found that it reduced the incidence of severe cortical lesions. 79 Whether or not this would translate to adults with TBI and stroke is unknown, although logic suggests that cooling the more metabolically active cortices, as non-invasive head cooling may do, could be of benefit.
Other applications of therapeutic head cooling
There were a number of databases in which limiting the search terms beyond cooling and brain/head terms was not feasible (see Appendix 3). These searches provided examples of a range of other conditions in which head cooling has been used as a therapy. The papers were not retained as part of the formal search results but the conditions are listed here for information, with selected citations. Head cooling has been used for headache,80 epilepsy,81 multiple sclerosis,82 sudden deafness from ischaemia of the inner ear,83 and to alleviate environmental, occupational and exertional heat strain. 84,85 A more common use of head cooling is to cool the scalp to reduce hair loss with certain types of chemotherapy. 71,86,87 There are several commercially available scalp-cooling devices. The Paxman Cooler and the DigniCap circulate coolant at around –5 °C and +5 °C, respectively, and the Penguin Cold Cap and ChemoCap contain frozen gel at –25 °C. Sizing the caps to fit closely improves contact and therefore cooling; sometimes hair is wetted prior to application to increase cooling effectiveness (e.g. with the DigniCap).
Historical reports of head cooling
The use of cooling for injuries has a very long history with documentary evidence as far back as Ancient Egypt (see Swan88 for a review), but references to head cooling are less common. However, the searches did produce some descriptions of therapeutic head cooling that are of historical interest, although with insufficient detail to be of use for the review.
In 1897, Charles Phelps, a New York surgeon, wrote a book on TBI – pistol wounds in particular. He advocates shaving the head to aid diagnosis but also to facilitate heat loss and to ‘permit the effective application of the ice-cap, which next to trephination, under indicated conditions, is most nearly a directly curative resource’ (p. 223). 89 The perceived benefit was reduction of temperature and swelling, and a case study is reported of a patient who repeatedly became lucid when the ice cap was in situ and feverish and delirious without it.
Oliver Waugh, a Canadian surgeon, described treatment of skull fractures in 1926. In cases of ‘mild’ skull fracture, i.e. patients who had experienced only brief disturbance of consciousness, ‘treatment should be an initial saline purgation (one ounce of Epsom salts), an ice cap applied to the head and absolute rest for from ten days to two weeks’ (p. 1476). 90 Patients with more severe injury had a lumbar puncture with removal of cerebrospinal fluid if the pressure was raised, an ice cap and regular Epsom salts orally or rectally ‘for its dehydrating effect on the brain’ (p. 1478). 90 Rest and quiet, with morphine if necessary, are emphasised. The rationale for using ice caps is not explicitly stated, but the implication is that it was primarily for reduction of swelling rather than reduction of temperature.
A 1962 German paper recommends ‘selective cranial hypothermia’ as an effective method of reducing hypoxic damage and cerebral oedema after inadvertent perioperative cardiac arrest. 91 The brain was cooled to 32–33 °C, about 1–1.5 °C lower than body temperature, and cooling maintained for 4–6 days depending on the patient’s condition, followed by slow rewarming. Details of the brain cooling method were to be the subject of a separate paper, but this seems not to have been published.
There are a number of papers in Russian on head cooling from the 1960s and 1970s. These contain descriptions of devices (see Appendix 7) and examples of conditions treated, which included TBI,92,93 epilepsy and even psychiatric patients in whom head cooling apparently provided temporary healing but did not prevent death. 94 There were no RCTs and insufficient detail on temperatures for formal inclusion in the review, but as they are relatively early reports of head cooling it seemed logical to include them in this historical section. Brain temperatures in the range of 22–30 °C with body temperatures of 33–36 °C are described. 92,94 Ear canal temperature (external auditory meatus/auditory canal wall near the tympanic membrane) was used as a proxy for intracranial temperature,93,95,96 but Ioffe and Sumskii92 also measured intracranial temperature directly in some of their patients using specially made temperature sensors. These were sited in the silicone drains which were placed in the parenchyma or subdural space during surgery, thus simultaneously achieving drainage and temperature measurement. 92
This is an early report of the use of intracranial temperature measurement in humans outside the operating theatre. Brain temperature was also sometimes inferred from a nomogram, developed from experimental work and clinical experience, to predict intracranial temperatures at various depths from observed body temperature, taking into account the patient’s weight and the start time of cooling. 97,98
Chapter 5 Modelling of cost-effectiveness of head cooling
The review searches produced no suitable data for economic modelling and therefore this was unable to be undertaken. The purpose of the economic analysis presented here is to create an exploratory model of possible treatment effects and the cost-effectiveness of head cooling using local data for patients with TBI. Although the model will not formally assess the cost-effectiveness of head cooling, it will enable a discussion regarding whether or not the treatment is potentially cost-effective.
Literature review, Glasgow Coma Scale and Glasgow Outcome Scale
There are currently no economic evaluation studies published on the cost-effectiveness of head cooling in adult patients with TBI. This is because there has been insufficient research and the clinical effectiveness of the treatment is not established.
The Glasgow Coma Scale (GCS) is a standardised neurological scale for recording and communicating the conscious state following a brain injury. The GCS can be transformed to a coma score and is used to evaluate patients from ‘3’ (deep coma or death) to ‘15’ (fully awake). It is commonplace to use the scores to classify patients’ injury as severe (GCS ≤ 8), moderate (GCS 9–12) or minor (GCS 13–15),99 and this approach will be taken in the economic evaluation. It should be noted, however, that this is not a linear scale.
The GOS and the extended GOS assess the longer-term effects through measuring the health and functional status of a patient after a treatment or an intervention. The GOS classifies patient outcome into five categories (eight in the extended scale), which range from death to good recovery. 100
Sources of data and eligibility
Data used to inform the economic modelling process were taken from the Scottish Intensive Care Society (SICS) WardWatcher database, which contains a record of patients who received treatment in the critical care unit of the Western General Hospital, Edinburgh. There is no single diagnostic category for TBI and Appendix 8 explains how patients were identified from that database. From September 1994 to July 2010, 1039 patients with TBI were admitted, but pre-sedation GCS was available only for 695 of these patients and outcome data (five-point GOS at 12 months) for 168, those admitted in 2007–9.
The data set is relevant as it was planned that head cooling would take place in critical care and be available to all TBI patients in such a unit. Therefore, any patient in the data set would be eligible for head cooling. Usually, a patient will be admitted to a critical care ward if he or she has a GCS score of ≤ 12, which includes both moderately and severely injured patients.
Limitations of the data
A severe limitation with the available information is lack of head-cooling outcome data. There are outcome data, i.e. the GOS data described above, which are available for 168 patients who received treatment for TBI in the critical care unit. But what is missing is a measure of effectiveness of head cooling for a patient with TBI.
Ideally, outcome data that are specific to head cooling would be available, and would include the health impact of the intervention and patients’ characteristics (e.g. age, gender, time and severity of injury, etc.). The outcome information might then be generalisable to the local data set used in this model. For example, if, according to a published study, head cooling is most effective when administered quickly to younger patients, and a male aged 25 years old with a short time to arrival to hospital is present in the data set, it would then be possible to predict the effect of head cooling on that patient. These data could then be combined with the cost information to provide some indication of the cost-effectiveness of the intervention.
However, the effect of head cooling is not yet established. It is not possible to say that if a patient with a certain set of characteristics receives head cooling then there is evidence to suggest there will be an improvement in health outcome. A method of counteracting the gaps in the literature is to use expert opinion, for example a clinician could suggest what may happen to a patient after head cooling. However, this method was discarded, as it was considered to be stretching the definition of ‘expert opinion to support the data’ too far by asking a clinician to suggest a whole set of outcomes for the data set.
It should be stressed that every effort was made to create a robust economic model. This included multiple meetings with hospital consultants at a variety of hospital locations across Scotland, meetings and discussions with academics associated with the University of Edinburgh, and thorough literature reviews. However, despite the search for available evidence, it was concluded there are very few data.
Similar head-cooling studies that include economic modelling, such as that of Gray and colleagues,101 are not relevant, as these papers are focused on neonates, whereas our interest is in adults. In fact, the paper by Gray and colleagues101 highlights the main issue surrounding the economic modelling of head cooling in adults, i.e. the lack of head cooling outcome data for adults. The outcome of head cooling in infants is modelled by taking data from a RCT. 39 Therefore, in their economic model Gray and colleagues101 can base their outcome data on published evidence, and it is exactly this sort of information which is missing for adults. It is not appropriate to alter a variable, for example head cooling reduces the number of deaths by an arbitrary amount that is not based on reliable evidence.
A large RCT with outcome data would provide a solution to the above problems and enable a more informative economic model to be developed.
Model
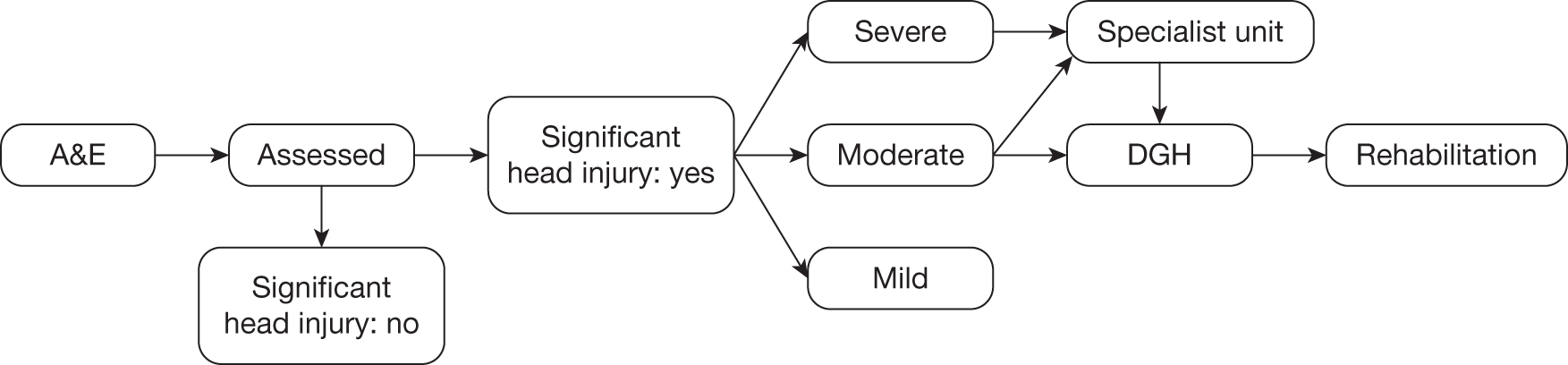
A simple diagrammatic model of the TBI pathway is provided in Figure 2. The pathway starts with assessment which includes GCS. From this point the patient would usually go to a specialised unit (critical care), a district general hospital (DGH) or home. The severe and some moderate patients tend to be admitted to critical care, which is where head-cooling treatment will be delivered. These are the patients for whom data are available, as explained above.
FIGURE 2.
The TBI pathway. A&E, accident and emergency department; DGH, district general hospital.
Methodology
Using the available data, the possible financial impact of head cooling on length of stay was modelled, i.e. if head cooling changes the length of stay of patients within the critical care unit would this have an economic impact? If the model assumes that the GCS can act as a rough proxy for how severely injured the patient is, then it is possible to model the financial impact of head cooling if it alters the length of stay associated with that level of injury.
Three scenarios are modelled:
-
First, the cost associated with the status quo, which takes into account the proportional split of patients between moderate and severe levels of injury and the cost of treating these patients, is modelled.
-
The second scenario investigates the financial cost if head-cooling increases by 1.5 days the length of stay of moderately and severely injured patients. This scenario was modelled in case applying head cooling lengthens patients’ stay in critical care as they undergo an additional treatment.
-
The third scenario examines the financial cost of head cooling decreasing a patient’s length of stay by 1 day, with respect to moderately and severely injured patients. This scenario was modelled in case the health benefits of head cooling enabled the patient to be moved on from critical care earlier than in current practice.
Descriptive statistics
Presented below are background information contained in the data.
| Variable | No. |
|---|---|
| Individuals in model | 695 |
| Mean age (years) | 42.31 |
| Median age (years) | 42.00 |
| Males | 532 |
| Females | 163 |
The mean lengths of stay of a moderately or severely injured patient, with the associated GCS scores, are tabulated below. The mean lengths of stay associated with scenarios 2 and 3 are also tabulated below.
| Severity of injury | GCS score | Length of stay (bed-days) |
|---|---|---|
| Severe | ≤ 8 | 6.38 |
| Moderate | 9–12 | 6.75 |
| Severity of injury | GCS score | Length of stay (bed-days) |
|---|---|---|
| Severe | ≤ 8 | 7.88 |
| Moderate | 9–12 | 8.25 |
| Severity of injury | GCS score | Length of stay (bed-days) |
|---|---|---|
| Severe | ≤ 8 | 5.38 |
| Moderate | 9–12 | 5.75 |
In addition, the cost per bed-day and equipment costs are outlined below. It is assumed that no additional staff time would be needed to provide the head-cooling intervention, as these patients have 1 : 1 nursing care, which would usually be sufficient to accommodate delivery of cooling interventions. The cost per hospital bed-day is calculated from 2008–9 data published by Information Services Division (ISD) Scotland for the ICU at the Western General Hospital, Edinburgh. Equipment costs are based on the Olympic CoolCap System, which includes reuseable cooling caps [costs were provided by the UK supplier Genesys Medical Solutions (UK) Ltd in December 2010].
| Variable | Cost (£) |
|---|---|
| Cost per hospital bed-day | 1472.13 |
| Cost of equipment | 13,500 |
| Costs of staff | 0 |
Results
It is expected that 69 patients per year would receive head cooling (because this was the average number of patients admitted to critical care each year in this data set). The proportional split between moderate and severe patients is 15% and 85%, respectively. The results of the three scenario models are presented below.
| Severity of injury | Cost (£) |
|---|---|
| Severe | 550,664.44 |
| Moderate | 102,915.78 |
| Total | 653,580.22 |
| Severity of injury | Cost (£) |
|---|---|
| Severe | 680,174.87 |
| Moderate | 125,770.56 |
| Total | 805,945.43 a |
| Severity of injury | Cost (£) |
|---|---|
| Severe | 464,324.15 |
| Moderate | 87,679.25 |
| Total | 552,003.40 a |
Discussion
The insight gained from the modelling above is limited. Essentially, the model is taking the GCS as a rough proxy for how severely injured the patient is and suggests that if head cooling could impact on length of stay then there may be a substantial change in costs owing to the expensive location of the treatment. 102 Head cooling is delivered in critical care, which generates a relatively expensive cost per bed-day. If the treatment alters the length of stay, and therefore the number of bed-days, then the change in cost between the current treatment and treatment with head cooling may be significant. However, it has been suggested by expert clinical opinion that head cooling may not impact on the length of stay of any section of the pathway outlined in Figure 2 (length of stay in critical care, DGH or rehabilitation).
The main benefit of head cooling for TBI is proposed to be improving the quality of life and reducing disability over the patient’s lifetime, i.e. what happens to patients after they go home and leave the mainly hospital-based pathway outlined in Figure 2. In addition, if there is an improvement in the long-term health of the patient then this will not only impact on the lifestyle of the patient, but will also require fewer health- and social-care support resources from the NHS and local authorities. This information will therefore significantly impact on whether or not the intervention is cost-effective, depending on the degree of health improvement and the size of the health and social costs.
Unfortunately, there are no UK lifetime TBI costs and no head-cooling cost data available in the literature, and therefore it is not possible to directly assess the long-term cost of TBI. We found the lack of lifetime costs for TBI surprising but requests for information to the Department of Health, the Scottish Office, the NHS Information Centre, the Trauma Audit and Research Network, and the Scottish Acquired Brain Injury Managed Clinical Network confirmed the lack of data. It stems, in part, from difficulty identifying patients who have had a TBI. The International Classification of Diseases, Tenth Edition, codes S00–S09, covers injuries to the head (S06 is specifically ‘intracranial injury’), but on initial admission to hospital it may not always be obvious whether or not brain injury is due to trauma, and patients may have other injuries and complex disease classification. In the USA there has been a concerted effort to collect data and consequently much more information is available,103,104 but, unfortunately, the health-care system is too different for this to translate to the UK. However, because this review has highlighted the lack of lifetime data on TBI, steps are now being taken in Scotland to address the situation and we are working with a group of people under the auspices of the Acquired Brain Injury Managed Clinical Network to improve data collection on TBI. We hope that, in the longer term, access to better data on the course of TBI will lead to benefits for these patients.
Although not related to TBI, Comas-Herrera and Wittenberg105 estimate that the lifetime health- and social-care costs of those aged ≥ 65 years in England are £31,500 per person at 2006–7 prices, averaged across males and females. The lifetime costs for a male reported in this study are £18,650 and £41,350 for a woman. These results compare favourably with those of Forder and Fernández,106 who estimate that the average lifetime expected cost of care for a male is £22,300, whereas for a female it is £40,400. The average for both genders is £31,700 per person in old age.
As seen in the descriptive statistics provided above, the average age of a patient with TBI is around 42 years, which is much lower than the 65 years used as the cut-off point for the estimates presented by Comas-Herrera and Wittenberg. 105 In addition, the individuals in their study are not reported to be disabled (although their health may deteriorate with age). The data in Comas-Herrera and Wittenberg105 highlight just how expensive health- and social-care costs can be and they may be much more if based on patients with TBI who have an average age of 42 years. It is reasonable to suggest that the long-term cost implications of TBI are substantial, which implies that if head cooling can improve the health of the patient and reduce the long-term costs of health and social care then it has a realistic chance of being cost-effective.
In addition to a lack of lifetime TBI cost data, there are also no available outcome data, i.e. health outcome data that evidence what happens to a patient after head cooling. There is no way to test the argument set out above – that head cooling may be cost-effective if it achieves positive health outcome and reduces the associated lifetime costs of health and social care. Thus, the model does not capture the main benefit of head cooling, which is the impact on quality of life and disability over the lifetime, and stops short of being a full economic evaluation, as there is no synthesis of outcome data with costs.
Conclusion: modelling of cost-effectiveness
The limited model presented here does display the sensitivity of the costs to changes in length of stay due to the expensive location of where head-cooling treatment is most likely to be delivered. Critical care has a relatively high cost per bed-day; thus, if length of stay changes, the impact on costs may be significant.
Unfortunately, good-quality data on outcome after head cooling are lacking and it is difficult to surmise from the model presented here whether or not the intervention is currently cost-effective. However, what the process has highlighted (and is the main conclusion of this model) is that if head cooling can positively impact on the quality of life for patients with TBI then the intervention may be cost-effective, owing to the high health- and social-care costs of severe brain injury and resulting disability.
Chapter 6 Public involvement
In the UK to date, head cooling in adults has been a research intervention and is not part of normal clinical care. As a result, there have been very few service users of head cooling. Those patients who have had head cooling were critically ill, sedated and unconscious, with consequently very limited or no awareness of the intervention. Even if we could have contacted them, it was difficult to know how they might contribute to this review from personal experience of head cooling. However, almost any member of the public might be a potential service user in the future, and thrust into that situation without prior warning because head cooling is an acute intervention for sudden and unexpected health emergencies – TBI, stroke and cardiac arrest. Therefore, during preparation of this report, the results of the review were presented to members of the general public in order to give them an opportunity to comment on and discuss the concept, possible use and effectiveness of head cooling, and also issues of consent to research in sudden and acute illness.
People at a city centre church in Edinburgh were asked if they would like to take part in a discussion, and 10 agreed. They ranged in age from 40 years to > 80 years: eight were women and two men. The discussions took place in two groups (n = 4 and n = 6), with one of the men in each group. Each person was given a one-page summary of the findings of the review, with a brief overview of consent for this type of research, i.e. research in critically ill patients who lack capacity to consent (see Appendix 9), together with photos of an intranasal cooling device and a liquid head-cooling device. After they had read the information, they were invited to comment, ask questions or discuss any aspect of the information. The researcher (BH) had no pre-set agenda and what was said and discussed was led entirely by the group members, with the researcher responding to questions and providing additional information as necessary. These were not focus groups: the group members were not research participants but partners in an opportunity for exchange of information, mutual learning and promotion of understanding. What follows is an overview of the aspects discussed to indicate what was of interest and of note to these members of the public.
With regard to head cooling, discussion ranged over the history of cooling, whether or not testing had been carried out in animals or human volunteers and to what extent this was relevant to people with brain injuries, hibernation in animals, whether or not cooling therapy was more natural than drugs, group members’ experiences of cooling therapy, and the nature of head cooling as an intervention compared with whole-body cooling.
Two people knew of babies with neonatal HIE: one had been cooled and was doing well. Another knew of a child who had fallen into icy water and been submerged for some time but recovered. Generally, it was felt to be important that cooling therapy was better known about by the public and health-care professionals in some instances, because one group member said she was surprised that a midwife friend did not know that cooling could be a therapy for babies with birth asphyxia.
With regard to research, matters discussed included being asked to consider research when in a state of shock after a relative’s sudden illness and how they might feel, how to decide what is best, what happens if something goes wrong, what randomisation means, and differences in the law between England and Scotland (something of which group members were not aware). One retired general practitioner reported being better disposed to agree to take part in a cooling trial than a drug trial because of concerns about pharmaceutical company-driven research. Most group members had not considered that the reason for a trial is because there is uncertainty over a treatment or that taking part in a trial does not necessarily mean getting an additional treatment over and above normal care, but may mean getting no additional treatment.
Although the group members were initially not sure what they would be able to contribute, they appreciated that this kind of research might be something that people could be confronted with ‘out of the blue’. They were interested to learn more about head cooling, and research in situations of critical illness. It was certainly helpful from the research and clinical point of view to get an idea of the range of questions and issues that may be relevant to people presented with this for the first time, as would be the case with patients, and their families, who might be eligible for head-cooling research in the future.
Chapter 7 Discussion
This was a complex review that included TBI and stroke, and also cardiac arrest for temperature reduction data, and cardiac arrest and neonatal HIE for adverse effects of methods and devices.
Impact of head cooling on functional outcome
The searches found 46 studies of non-invasive head cooling in TBI, stroke and brain injury, of which three were assessed as RCTs with good allocation concealment (see Figure 1). Good-quality RCTs with blinded outcome assessment were prespecified for inclusion in analysis of functional outcome, but none of the three met this criterion and was suitable for this purpose.
Temperature reduction with head cooling
For assessing the effect of head cooling on temperature, studies and trials in cardiac arrest were eligible in addition to those in TBI and stroke. Twelve studies had useable data, including four RCTs and four in cardiac arrest (see Table 1). There was considerable heterogeneity (of patients, reasons for cooling and interventions) in these studies, making it difficult to summarise the data.
Two studies showed no effect,46,48 but in both cases this seems likely to be because of the methods used. In one of these, ambient temperature nasal airflow delivered to intubated, ventilated patients to replicate normal nose breathing showed no effect on intracranial temperature at a distance from the nasopharynx. 46 With this method the temperature gradient between the patient and the airflow was relatively small. In the other, ice bags on the head and neck for 5–30 minutes did not further reduce temperature in patients who were already on average hypothermic. 48 This method does not actively remove heat by coolant flow.
However, in broad terms the data indicate that liquid head- and neck-cooling devices and the Rhinochill intranasal cooling device can reduce intracranial and/or core trunk temperature by around 1 °C or more, within 1 hour in some studies (see Table 1). (These methods create a relatively steep temperature gradient between the patient and the coolant, and actively remove heat by coolant flow.) This is promising and, in particular, suggests that there may be a role for liquid head-cooling devices for induction and maintenance of modest temperature reduction in TBI and stroke (the Rhinochill device is not designed for prolonged use). A small observational study51 showed that it was possible to successfully treat fever refractory to standard management (paracetamol, metamizole, alcohol washing and ice packs) in this way (see Table 1). It is noteworthy that, even in the presence of active body warming, intracranial temperature was reduced with a liquid head-cooling device and could be reduced below core trunk temperature. 45,50
This is in contrast with mathematical modelling studies of head cooling, which are sometimes cited to support the view that external head cooling with various devices, including liquid cooling helmets, has a very limited effect on intracranial temperature. The modelling data, even when incoming carotid temperature is varied (which is more realistic than models that treat it as fixed at 37 °C) suggest that these devices reduce brain temperature only superficially, up to about 18 mm below the parenchymal surface. 107,108 Even if the usual site of parenchymal temperature measurement – at 1 cm below the brain surface – is considered too superficial to provide valid information on whether or not deeper brain is cooled, the data in this review include examples of ventricular temperature and core body temperature reduction with head cooling (see Table 1). It is hard to conceive that core trunk temperature would be reduced with head cooling, for example with the Rhinochill intranasal cooling device, in the absence of some deeper brain temperature reduction (see Appendix 1).
Head cooling compared with systemic cooling
The reason commonly given for using head cooling is that there may be fewer side effects than with systemic hypothermia. 109 Some investigators simply assume that cooling the head and keeping the body warm will minimise systemic complications of hypothermia. 45,50 Some head-cooling device providers have also made that assumption. The TraumaTec website states: ‘Selective brain cooling with the Neuro-Wrap™ avoids the complications seen with full body cooling … Complications of systemic hypothermia do not occur as systemic normothermia is maintained’ (www.traumatec.com/traumatec-brain-injury.htm; accessed 28 April 2011). Also, on the Benechill website, regarding the Rhinochill device: ‘it is core temperature reduction that causes problems in cooling – not brain temperature reduction’ (www.benechill.com/wp/resource/; accessed 28 April 2011).
Harris and colleagues45 were unable to achieve an intracranial temperature of 33 °C with head cooling while maintaining bladder temperature at 36 °C with active warming (see Table 1), although whether or not such a large temperature gradient is necessary or desirable remains to be determined. Because a statistically significant intracranial body temperature was not achieved, they concluded that their head-cooling device was not useful in management of TBI. Nevertheless, mean intracranial temperature was reduced below body temperature by 0.67 °C, and Wang and colleagues,50 also in the presence of active body warming, achieved a mean 1.6 °C reduction of intracranial temperature below body temperature. This is a reversal of the norm, in which intracranial temperature is usually higher than body temperature,110 and could well be considered clinically relevant for that reason, although whether there is therapeutic benefit or otherwise is not known. It is difficult to measure intracranial temperature gradients in humans but, in animals, head cooling in the presence of body warming to normothermia has been shown to significantly increase intracranial temperature gradients compared with systemic normothermia and hypothermia, although, again, it is not known if this is harmful. 111,112
This review found no high-quality RCT evidence on the relative complications and benefits of head compared with systemic cooling in adults and there are no RCTs making that comparison in neonatal HIE. However, there is some circumstantial evidence from other sources that is relevant to the question of whether or not a hypothermic brain and relatively warmer body may produce fewer complications than systemic hypothermia.
The side effects of systemic hypothermia at temperatures of 33–35 °C include pulmonary oedema, rebound increases in ICP on rewarming, higher temperatures post hypothermia, coagulation abnormalities, metabolic effects and immune suppression. 113 However, the reporting and definition of complications in clinical trials of systemic hypothermia is variable, which makes assessing their impact difficult. Nevertheless, systematic reviews of trials of systemic hypothermia after brain injury have shown a non-significant increase in occurrence of infections with cooling therapies (i.e. not only with hypothermic therapy) in stroke14 and of pneumonia in hypothermia for TBI. 15 But whether or not head cooling results in fewer complications than systemic hypothermia is likely to depend on the mechanisms by which the complications are caused, how the cooling and warming of the blood as it circulates through a cooler brain and relatively warmer body affects these, and how extreme the temperature gradients are.
With regard to infection, because immune response is modulated by the brain114,115 it seems unwise to assume that brain cooling, with the body relatively warmer, will cause less immune depression and infection than systemic cooling. The primary hormonal pathway for brain–immune system interactions is the hypothalamic–pituitary–adrenal axis115 and, consequently, it is thought that brain cooling does suppress immune function because the pituitary is cooled. 116 Furthermore, if immune defence is accelerated and more efficient at increased temperatures,117 reducing brain temperature even to the normothermic range might increase morbidity and mortality from infection.
Another undesirable effect of cooling is shivering, with the requirement for sedation to prevent it. Shivering and stress response to cold may occur even if brain temperature alone is reduced below the ‘set-point’, as cooling of the preoptic area of the hypothalamus is sufficient to cause heat production and retention responses. 118,119 Lim120 controlled brain temperature and core trunk temperature independently in anaesthetised dogs using bilateral carotid antegrade cerebral perfusion with independent control of body temperature. Cool brain–warm body and warm brain–cool body conditions both produced shivering. Therefore, brain cooling, even if the body is warm, may not prevent shivering.
If the question of whether or not head cooling does produce fewer complications than systemic cooling is to be answered then a good-quality RCT is needed. There is a small safety and efficacy study ongoing in stroke. The Cerebral Hypothermia in Ischaemic Lesion (CHIL) trial has a head-cooling arm in China, a systemic hypothermia arm in Australia and a normothermic control group, with blinded outcome assessment of the National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS) and Barthel Index (BI) at 90 days (see Appendix 6, Characteristics of ongoing studies).
Head-cooling terminology and search terms
There is no agreed terminology to describe cooling that is directed specifically at the head and brain. In the absence of this we have previously suggested the term direct brain cooling and developed the classification of head-cooling methods used in this review. 23 This was in part an attempt to provide an alternative to the term selective brain cooling, which has been commonly and erroneously used in clinical papers to describe head-cooling interventions. Selective brain cooling is a natural thermoregulatory mechanism in which brain temperature is reduced below carotid blood, defined in the Glossary of terms for thermal physiology. 121 Although applying cooling to the head can reduce brain temperature below body temperature (see Table 1), this is not physiological selective brain cooling.
Because the purpose of therapeutic cooling is to reduce brain temperature, investigators may use head- and brain-related terms to describe cooling, even when they have used systemic methods. Hayashi’s group in Japan for example refer to ‘brain hypothermia therapy’ and ‘cerebral hypothermia’ but they use whole-body cooling. 116,122
The lack of standard terminology makes literature searching more difficult. Key words are variable, if used at all, in relation to cooling method. Medical subject headings (MeSH) do not specifically help in searches for cooling interventions or cooling targeted at particular organs. Gastric hypothermia is the only named method in the MeSH tree structure for therapeutic cooling and brain cooling crosses a number of subject areas. We used MeSH terms within our searches but it would refine indexing and aid searching if cooling interventions were incorporated, at least on the basic level of whether they are invasive or non-invasive, brain directed or systemic.
A helpful consensus has recently been reached on a number of factors related to targeted temperature management in critical care123 and this could usefully be extended to agreeing terminology for cooling methods.
Poor reporting of methods and temperature data
Poor reporting of study methodology and/or temperature data were the main reasons why studies were excluded (see Appendix 6, Characteristics of excluded studies). Poor reporting has ethical implications as well as being frustrating for readers and reviewers. It is a recognised problem that is being actively addressed by the CONSORT (Consolidated Standards of Reporting Trials) group (www.consort-statement.org) with some success. 124
If the studies found for this review, even if not randomised, had reported temperatures satisfactorily there would have been more information on proof of concept of head cooling with regard to temperature reduction. But many did not adequately report the cooling interventions, the temperature outcomes, where temperature was measured or temperature management in control groups (see Appendix 6).
A consensus report from a meeting of five international critical care societies has recently been published, which includes criteria for reporting studies of targeted temperature management in critical care. The therapeutic effect, safety and reproducibility of temperature management should be reported just as with a drug, including:
Accurate reporting of the indication for temperature management, the interval between disease onset and cooling, the management profile, including the rates of decrement and increment as well as the temperatures achieved, and a comprehensive description of the effects on each body system. (p. 1114). 123
Therefore, in addition to rigour in reporting study design,125 information reported in head-cooling studies should include sufficient detail on the cooling method(s) to allow replication, the temperature measurement sites, actual temperatures in intervention and control groups at baseline and with cooling and during rewarming, temperature management strategy (e.g. normothermia or no intervention) and temperature in control groups, and complications/adverse effects from cooling. Providing this information is particularly important because head-cooling research is still largely at the explanatory stage of whether and to what extent different methods reduce temperature.
Chinese studies of head cooling
Twenty-six of the studies on head cooling found for this review were Chinese. The reports were generally relatively short and, unfortunately, none gave sufficient detail on methods to allow trial quality to be adequately assessed or sufficient information on temperature. Poor reporting was also found in a review of leading Chinese medical journals by the Chinese Cochrane Centre. 126 It may be partly explained by lack of formal training in research methods and failure of journals to adopt reporting criteria such as CONSORT,126,127 but is not limited to Chinese trials124 (and see Appendix 6).
Information on the method of randomisation was missing or scanty (e.g. ‘computerised’, ‘number method’) and sample size calculation and whether or not the analysis was on an intention-to-treat basis were not reported. It was unclear if analysis was prespecified and sometimes the time scales of result reporting were ambiguous (days on which blood tests were carried out, for example), which suggested that positive results may have been selected for reporting (e.g. see Xu and colleagues128). None of the Chinese studies reported on blinding of treatment allocation, analysis or outcome assessment. In cooling studies it is not necessarily feasible to blind investigators to treatment allocation but we considered that blinded outcome assessment was important and therefore prespecified in the review protocol that studies in which this was not undertaken would be excluded from the formal analysis.
Recently the Chinese Cochrane Centre conducted a study to assess the adequacy of randomisation of peer-reviewed trials published in Chinese that purported to be RCTs. 129 Trial investigators were interviewed on the telephone and of 2235 studies only 207 (6.8%; 95% CI 5.9% to 7.7%) were found to be authentic RCTs. Most of those interviewed (85.6%) did not fully understand randomisation when they claimed that their trials were randomised. However, 5.1% did understand randomisation and still claimed that their trials were properly randomised when they were not. Although we had limited success in contacting Chinese authors for this review, the corresponding author of one study who did respond said that the trial was not randomised, although in the paper it was reported to be randomised using a randomisation table. 75
Selection bias owing to inadequate randomisation may be one reason for the relatively high proportion of positive results that has been noted in Chinese trials. 129 Those found in the searches for this review were all positive and, although the inadequate reporting of methods has made it impossible to assess their quality, it is plausible that selection bias and bias from unblinded outcome assessment and analysis were contributing factors.
Typically, if hypothermia was the aim, only the target temperature for head cooling was reported or, for reduction of fever, for example, the temperature at which the cooling device was set. The actual temperatures prior to cooling and during induction, maintenance and reversion in the intervention groups were not reported nor was the site of temperature measurement always specified [when it was, this is noted under characteristics of included/excluded studies (see Appendix 6)]. General information on the time to reach target temperature was sometimes provided, for example Yang and colleagues130 noted that it took 30–60 minutes to achieve a brain temperature of 35 °C and 3–4 hours to reach 32–35 °C. The implication is that large reductions in brain temperature can be achieved rapidly with non-invasive head cooling, which could be important and clinically relevant if more detailed information was available. There was also little information on temperature management in control groups, for example normothermia. Most papers simply stated that groups were treated the same with the exception of cooling.
A number of the Chinese stroke studies assessed outcome with the neurological deficiency score (NDS) (see Appendix 6). This is not an assessment of functional outcome and is not considered well validated by the Cochrane Stroke Group. It was not one of our prespecified assessment tools (www.strokecenter.org/trials/scales/scales-overview.htm; accessed 24 April 2011).
Cerebral oedema volume was used as an outcome measure in several Chinese stroke studies and a Japanese stroke study (see Appendix 6), but the methods used were not adequately explained. This was not one of our prespecified outcomes because the validity of cerebral oedema volume as a measure of improvement in brain injury is not established and there is no agreed method of measuring it (e.g. see Degos and colleagues131 and Lescot and colleagues132).
Ethical review and informed consent was not always reported in the Chinese studies. Medical ethics in China has been described as ‘anaemic’, a state of affairs attributed to there having been no equivalent to the Nuremberg Code because Second World War atrocities were not addressed, as they were in Europe by the Nuremberg Trials. 133 However, there have been considerable developments in medical research ethics in China since the 1990s, which include the requirement for ethical review. 134 But there are philosophical differences between the principle of individual autonomy on which ethics in Europe and North America are based, and the traditional Chinese focus on ‘social harmony over individual interests’ (p. 1867). 134 No single approach necessarily has the monopoly on ethical ‘correctness’ and sensitivity to cultural differences is important. 135
Medical treatment is not free in China. 136 Hospitals receive little government subsidy and therefore have to sell services. Drugs and medical consumables in particular are relatively expensive and may be subject to corrupt pricing,137 and corrupt purchasing and prescribing in hospitals. 138 The issue of cost was touched on in some of the Chinese study reports and has implications for trial validity. In one study, patients who were allocated to head cooling but could not afford the head-cooling device were cooled with ice packs. 139 Ou and colleagues140 investigated different durations of head cooling and noted that longer cooling increased the cost for patients. This also meant that the timing of patients’ discharge from hospital was not necessarily dictated by their condition but by their ability to pay for continued care, which is a potentially confounding factor in studies where functional or neurological outcome after head cooling was followed up on hospital discharge (e.g. see Dong and colleagues141).
This is not intended to single out Chinese studies for special criticism: it just happens that they formed a large proportion of the studies found by searches for this review. In summary, what we found with regard to reporting quality in the Chinese studies is consistent with a recent systematic review of the quality of Chinese RCTs142 and a review of reporting quality in leading Chinese medical journals,126 both undertaken by the Chinese Cochrane Centre. There are initiatives to improve trial conduct and reporting in China, and such initiatives are already having an effect elsewhere, although reporting is still not all that it should be. 143
Potential biases in the review process
This systematic review addresses clear research questions and used predefined inclusion criteria to select and appraise studies. We conducted extensive and sensitive searches but the possibility of publication bias remains. There may be, for example, more trials published in Chinese journals than we found. The majority of the trials found were small and/or, on the basis of the reports, of low methodological quality. If the trial reports did not reflect the true quality of the trials then it is possible that there are excluded trials that should have been included.
Agreements or disagreements with other reviews
Four previous reviews of head cooling were found: two, published in Chinese, in patients with cerebral haemorrhage,144,145 one that included human and animal studies in TBI146 and one that included human and animal studies in TBI, stroke, cardiac arrest and neonatal HIE. 23 None were systematic.
No head-cooling studies were included in the most recent Cochrane systematic reviews on hypothermia for traumatic head injury,15 modest cooling therapies (35 °C to 37.5 °C) for TBI,13 and cooling therapy for acute stroke (ischaemic or haemorrhagic). 14 Sydenham and colleagues15 excluded two head-cooling studies, one75 because the cooling intervention was of < 12 consecutive hours’ duration, the other147 was not a RCT, another study76 was awaiting assessment. Saxena and colleagues13 excluded five head-cooling studies46,47,50,76,147 for physiological end points46,47, methodological reasons76 and target temperature outside the review scope,50,147 respectively. den Hertog and colleagues14 excluded three head-cooling studies50,148,149 because the outcome measures were not relevant to the review (relevant outcome measures were functional outcome, mortality, mean temperature 24 hours after start of cooling, cerebral haemorrhage, complications).
Chapter 8 Conclusions
Head cooling after cerebral insults potentially encompasses a wide spectrum from simple fanning of the head after a mild stroke through to neuropreservation of the head by cryonics after legal death but before biological death (Alcor Foundation, www.alcor.org/; accessed 6 August 2010). In this review we have concentrated on non-invasive therapeutic head cooling after TBI and stroke, although studies in cardiac arrest were also included for data on temperature reduction, and studies in both cardiac arrest and neonatal HIE for adverse effects of methods and devices. We found a larger number of studies than expected, but few RCTs of confirmable quality and none that allowed us to determine if head cooling improves functional outcome in TBI or stroke. The review has shown that some methods of head cooling can reduce intracranial temperature, which is an important first step in determining effectiveness, but the evidence is not robust.
However, a fundamental issue in TBI and stroke, regardless of the method of cooling, is that the magnitude of temperature reduction (if any) necessary to improve functional outcome is still unknown. 13–15 Large RCTs are in progress to assess the effect of therapeutic hypothermia compared with normothermia. Two in TBI – one of systemic hypothermia as a neuroprotectant [the Prophylactic Hypothermia Trial to Lessen Traumatic Brain Injury (POLAR-RCT), http://clinicaltrials.gov/ct2/show/NCT00987688] and the other of raised ICP (Eurotherm3235Trial, www.controlled-trials.com/ISRCTN34555414/). In stroke, the EuroHYP trials (www.eurohyp.org), for example, are collectively planned to assess therapeutic hypothermia using various systemic cooling devices and also head cooling (see ‘i-Cool’ in Appendix 5, References to ongoing studies, Stroke). The CHIL trial (see Appendix 5, References to ongoing studies, Stroke) is comparing systemic hypothermia with head cooling and normothermia in stroke. There is still a need for RCTs of the effect of normothermia (or near normothermia) compared with no cooling in TBI and stroke. 13,150,151
Recommendations for research in TBI and stroke:
-
We suggest that active head-cooling devices are the most promising for further research, i.e. those that flow/circulate gas or liquid coolant.
-
More robust proof of concept of temperature reduction with head cooling is required. The effectiveness of head cooling in achieving and maintaining both normothermia and hypothermia should be assessed. Intracranial temperature should be measured (whenever feasible), as well as core trunk temperature in the oesophagus (or pulmonary artery), otherwise bladder, with rectal temperature being a last resort. It should be absolutely clear in study reports whether or not temperature has changed with cooling and by how much. Baseline temperatures, duration of cooling, temperatures achieved with cooling, and temperature change with cooling should be reported, with measures of central tendency and spread.
-
Head cooling, both with and without body warming, should be compared with systemic cooling to determine if complications – including shivering, infection and coagulation abnormalities – are fewer.
-
In volunteers, the effect on brain temperature gradients of different methods of head cooling with and without body warming might be assessed with magnetic resonance spectroscopy temperature measurement.
-
Head cooling as a method of treating raised ICP should be investigated.
-
The efficacy of head-cooling methods in maintaining cooling after induction of therapeutic hypothermia with cold intravenous fluids should be assessed.
-
The tolerability and effectiveness (infection, shivering, temperature reduction, functional outcome) of head cooling in achieving normothermia and hypothermia in awake patients should be assessed.
-
In stroke patients, the effect of head cooling prior to, and during, thrombolysis should be evaluated.
-
In stroke, the efficacy and tolerability of intranasal cooling combined with external head cooling should be investigated. (Intranasal cooling may not be suitable for trauma patients.)
Implications for practice in TBI and stroke:
-
Head cooling has potential as a method of reducing raised intracranial temperature when this is clinically indicated but there is insufficient evidence to recommend its use outside of research trials.
-
Improved methods of recording and tracking patients after TBI are required throughout the UK so that the impact and costs can be measured.
The overall aim of this review was to provide a comprehensive picture of head-cooling research in adults after TBI and stroke. We believe that we have done this and that the review shows that head cooling has promise as an intervention after TBI and stroke and therefore deserves further investigation.
Acknowledgements
Contribution of authors
Bridget Harris (Research Fellow, University of Edinburgh, and Clinical Research Specialist, Critical Care, NHS Lothian) managed the project, carried out the searches, wrote to investigators and manufacturers, organised obtaining individual patient data for the economic model and cleaned the data set, screened studies that were obtained on the searches for inclusion, extracted data, compiled the presentation of the data, undertook the presentation of the results to the public, and wrote the report.
Peter Andrews (Consultant, Critical Care Services and Honorary Professor, University of Edinburgh) assisted with project management, and provided expert opinion for the economic model, screening of studies for inclusion, extraction of data, and writing of the report.
Gordon Murray (Professor of Medical Statistics, University of Edinburgh) gave statistical and methodological advice and support, and assisted with writing the report.
John Forbes (Reader in Health Economics, University of Edinburgh) supervised the economic modelling and contributed to the report.
Owen Moseley (Health Economist, NHS Ayrshire and Arran) researched and wrote the exploratory model on cost-effectiveness of head cooling in patients with TBI.
Thanks to those who helped
We are most grateful to:
Brenda Thomas, Cochrane Stroke Group Trials Search Co-ordinator, for essential expert guidance and help with the searches and for searching the Cochrane Stroke Group trial register.
Karen Blackhall, Cochrane Injuries Group Trials Search Co-ordinator, for searching the Cochrane Injuries Group trial register.
Dr Cathie Sudlow, Mike McDowell, Dr Alasdair FitzGerald, Angela Kellacher and Lillian Ford for help sourcing individual patient outcome data in stroke and TBI.
Dr Wai Kwong for invaluable assistance with papers written in Chinese.
Dr Carlotta Bagna, Alison Bruce, Cristina Guarrera and Junko MacKenzie for assistance with papers written in Italian, German, Russian and Japanese, and Dora Wirth (Languages) Ltd for Russian and Chinese translations.
The members of the public who commented on, and discussed the findings of, the review.
We are also grateful to the following investigators and companies who did respond to our requests for information: Dr Jasmin Arrich; Dr Denise Barbut (Benechill Inc.); Dr Lucian Covaciu; Professor Wang Desheng; Dr Kenji Dohi; Dr Hinnerk Doll; Dr Inan Guler; Dr Rainer Kollmar; Dr Sven Poli; Dr Wusi Qiu; Professor Claudia Robertson; Dr Muzaffar Siddiqui; Professor Martin Smrcka; Professor Fritz Sterz; Dr Yoshimasa Takeda; Dr Frank Tortella; Dr William Walsh; Dr Huan (John) Wang; Ioannis Anastasakis, TechNiche International; Heidi Hughes, Cincinnati Sub-Zero Products Inc.; Becky Inderbitzen, Benechill Inc.; Richard Paxman, Paxman Coolers Ltd; Polar Products, Inc.; Susanne Richard, TraumaTec Inc.; Lindsay Shearer, Genesys Medical Solutions (UK) Ltd (Olympic Cool-Cap); Martin Waleij, Dignitana; and Todd Yelavich, Penguin Cold Caps NZ Ltd.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- National Audit Office (NAO) . Reducing Brain Damage: Faster Access to Better Stroke Care 2005.
- Tennant A. Admission to hospital following head injury in England: incidence and socio-economic associations. BMC Public Health 2005;5.
- National Institute for Clinical Excellence (NICE) . Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults 2007.
- Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000;320:1631-5.
- Mcilvoy LH. The effect of hypothermia and hyperthermia on acute brain injury. AACN Clin Issues 2005;16:488-500.
- Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res 2007;162:201-17.
- Corbett D, Thornhill J. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol 2000;10:145-52.
- Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis 2003;12:163-73.
- Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 2002;19:869-74.
- Castillo J, Davalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke 1998;29:2455-60.
- Hajat C, Hajat S, Sharma P. Effects of post stroke pyrexia on stroke outcome: a meta-analysis of studies in patients. Stroke 2000;31:410-4.
- Leira R, Blanco M, Rodriguez-Yanez M, Flores J, Garcia-Garcia J. Non-pharmacological neuroprotection: role of emergency stroke management. Cerebrovasc Dis 2006;21:89-98.
- Saxena M, Andrews PJ, Cheng A. Modest cooling therapies (35°C to 37.5°C) for traumatic brain injury. Cochrane Database Syst Rev 2008;3.
- den Hertog HM, van der Worp HB, Tseng MC, Dippel DW. Cooling therapy for acute stroke. Cochrane Database Syst Rev 2009;1.
- Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev 2009;2.
- Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery 2000;47:850-6.
- Hoedemaekers CC, Ezzahti MM, Gerritsen AA, van der Hoeven JG. Comparison of different cooling methods to induce and maintain normo- and hypothermia in ICU patients: a prospective intervention study. Crit Care 2007;24.
- The European Stroke Initiative Executive Committee and the EUSI Writing Committee . European Stroke Initiative Recommendations for Stroke Management: update 2003. Cerebrovasc Dis 2003;16:311-37.
- Brain Trauma Foundation . Management and prognosis of severe traumatic brain injury. J Neurotrauma 2000;17:453-627.
- Childs C, Hadcock J, Ray A, King AT, Protheroe R. Temperature measurement after severe head injury. Anaesthesia 2004;59:192-3.
- Johnston NJ, King AT, Protheroe R, Childs C. Body temperature management after severe traumatic brain injury: methods and protocols used in the United Kingdom and Ireland. Resuscitation 2006;70:254-62.
- Thompson HJ, Kirkness CJ, Mitchell PH, Webb DJ. Fever management practices of neuroscience nurses: national and regional perspectives. J Neurosci Nurs 2007;39:151-62.
- Harris BA, Andrews PJD, Mayer SA, Sessler DI. Therapeutic hypothermia. New York, NY: Marcel Dekker; 2005.
- Harris BA, Andrews PJ, Marshall I, Robinson TM, Murray GD. Forced convective head cooling device reduces human cross-sectional brain temperature measured by magnetic resonance: a non-randomized healthy volunteer pilot study. Br J Anaesth 2008;100:365-72.
- Diller KR, Zhu L. Hypothermia therapy for brain injury. Ann Rev Biomed Eng 2009;11:135-62.
- Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Design 2007;13:2310-23.
- Tokutomi T, Morimoto K, Miyagi T, Yamaguchi S, Ishikawa K, Shigemori M. Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery 2003;52:102-11.
- Clayton TJ, Nelson RJ, Manara AR. Reduction in mortality from severe head injury following introduction of a protocol for intensive care management. Br J Anaesth 2004;93:761-7.
- Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis 2005;14:107-14.
- Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009;4.
- Deakin CD, Morrison LJ, Morley PT, Callaway CW, Kerber RE, Kronick SL, et al. Part 8: Advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2010;81:e93-174.
- National Institute for Clinical Excellence (NICE) . Therapeutic Hypothermia Following Cardiac Arrest 2011.
- Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J. Hypothermia after cardiac arrest should be further evaluated – a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol 2011;151:334-41.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121-35.
- Zhao D, Abella BS, Beiser DG, Alvarado JP, Wang H, Hamann KJ, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008;77:242-9.
- Jacobs SE, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007;4.
- National Institute for Health and Clinical Excellence (NICE) . Therapeutic Hypothermia With Intracorporeal Temperature Monitoring for Hypoxic Perinatal Brain Injury 2010.
- Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010;15:238-46.
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663-70.
- Meremikwu M, Oyo-Ita A. Paracetamol for treating fever in children. Cochrane Database Syst Rev 2002;2.
- Teasdale GT, Pettigrew LEL, Wilson JTL, Murray G, Jennet B. Analysing outcome of treatment of severe head injury: a review and update on advancing use of the Glasgow outcome scale. J Neurotrauma 1998;15:587-97.
- Edwards D. Internet Stroke Trials Registry: Stroke Scales and Clinical Assessment Tools n.d. www.strokecenter.org/trials/scales/ (accessed 20 August 2007).
- Nigwekar SU, Navaneethan SD, Strippoli GF. Thyroxine for acute kidney injury. Cochrane Database Syst Rev 2007;3.
- Zhao B, Huang H, Zhang G. Treatment of severe brain injury with selective mild hypothermia of brain. J Trauma Surg 2003;5:420-2.
- Harris OA, Muh CR, Surles MC, Pan Y, Rozycki G, Macleod J, et al. Discrete cerebral hypothermia in the management of traumatic brain injury: a randomized controlled trial. J Neurosurg 2009;110:1256-64.
- Andrews PJ, Harris B, Murray GD. Randomized controlled trial of effects of the airflow through the upper respiratory tract of intubated brain-injured patients on brain temperature and selective brain cooling. Br J Anaesth 2005;94:330-5.
- Harris BA, Andrews PJ, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain-injured, mechanically ventilated patients: a randomized, crossover, factorial trial. Br J Anaesth 2007;98:93-9.
- Callaway CW, Tadler SC, Katz LM, Lipinski CL, Brader E. Feasibility of external cranial cooling during out-of-hospital cardiac arrest. Resuscitation 2002;52:159-65.
- Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010;122:729-36.
- Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg 2004;100:272-7.
- Gaida BJ, Yaldizli OO, Mnk S, Muroi C, Mudra R, Fröhlich J. Treatment of resistant fever with local cerebral cooling: P 029. Eur J Anaesthesiol 2008;25.
- Forte LV, Peluso CM, Prandini MN, Godoy R, Rojas SSO. Regional cooling for reducing brain temperature and intracranial pressure. Arq Neuropsiquiatr 2009;67:480-7.
- Miller E. Determination of the rate and degree of selective brain cooling in adults with the TraumaTec Neuro-Wrap. Abstract P94. J Neurotrauma 2009;26.
- Sung G, Torbey M, Abou-Chebl A. Rhinochill: a novel brain hypothermia delivery device. Neurology 2009;72.
- Andreas J, Losert H, Bayegan K, Haugk M, Arrich J, Krizanac D, et al. Nasal cooling with a new cooling device in patients after cardiac arrest and successful resuscitation. Resuscitation 2008;77.
- Busch HJ, Janata A, Eichwede F, Fodisch M, Wobker G, Stephan T, et al. Abstract P63: safety and feasibility of a new innovative cooling approach for immediate induction of therapeutic hypothermia in patients after successful resuscitation. Trans-nasal cooling after cardiac arrest. Circulation 2008;118.
- Harris BA. Heat Loss from the Upper Airways and through the Skull: Studies of Direct Brain Cooling in Humans 2010.
- Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction via intranasal cooling with the Rhinochill device: preliminary safety data in brain-injured patients. Stroke 2011;42:2164-69.
- Busch H-J, Eichwede F, Fodisch M, Taccone FS, Wobker G, Schwab T, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation 2010;81:943-9.
- Castrén M. and PRINCE collaborators . Intra-Arrest Trans-Nasal Evaporative Cooling: A Randomized Pre-Hospital Multicenter Study: PRINCE (Pre-ROSC Intra Nasal Cooling Effectiveness) n.d.
- Wang DZ, Wang H, Lanzino G, Rose JA, Honings DS, Rodde MI, et al. Cooling Helmet for Patients With Brain Ischemic and Hemorrhagic Infarctions: The COOL BRAIN-STROKE Trial n.d.
- Wang H, Wang D, Olivero W, Lanzino G, Honings D, Rodde M. Selective brain hypothermia can be achieved with a cooling helmet: preliminary findings of the COOL BRAIN-stroke trial. Stroke 2004;35.
- Dohi K, Jimbo H, Abe T, Aruga T. Positive selective brain cooling method: a novel, simple, and selective nasopharyngeal brain cooling method. Acta Neurochirurg Suppl 2006;96:409-12.
- Lyon RM, Richardson SE, Hay AW, Andrews PJ, Robertson CE, Clegg GR. Esophageal temperature after out-of-hospital cardiac arrest: an observational study. Resuscitation 2010;81:867-71.
- Covaciu L. Intranasal Cooling for Cerebral Hypothermia Treatment 2010.
- Mariak Z. Intracranial temperature recordings in human subjects. The contribution of the neurosurgeon to thermal physiology. J Therm Biol 2002;27:219-28.
- Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality. Part 2: practical aspects and side effects. Intensive Care Med 2004;30:757-69.
- Body R. BET 4 Do fans spread infection in clinical areas?. Emerg Med J 2008;25.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001;51:275-81.
- Storm C, Schefold JC, Kerner T, Schmidbauer W, Gloza J, Krueger A, et al. Prehospital cooling with hypothermia caps (PreCoCa): a feasibility study. Clin Res Cardiol 2008;97:768-72.
- Macduff C, Mackenzie T, Hutcheon A, Melville L, Archibald H. The effectiveness of scalp cooling in preventing alopecia for patients receiving epirubicin and docetaxel. Eur J Cancer Care 2003;12:154-61.
- Yamada K, Moriwaki H, Oe H, Yamawaki T, Nagatsuka K, Oomura M, et al. The feasibility and safety of mild brain hypothermia with local surface cooling in acute stroke. Stroke 2004;35.
- Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics 2003;111:244-51.
- Zhou W-H, Cheng G, Shao X, Liu X, Shan R, Zhuang D, et al. for the China Study Group. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010;157:367-72.
- Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res 2006;34:58-64.
- Qiu WS, Wang WM, Du HY, Liu WG, Shen H, Shen LF, et al. Thrombocytopenia after therapeutic hypothermia in severe traumatic brain injury. Chin J Traumatol 2006;9:238-41.
- Sarkar S, Barks JD, Bhagat I, Donn SM. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol 2009;29:558-63.
- Sarkar S, Barks JD, Bhagat I, Dechert R, Donn SM. Pulmonary dysfunction and therapeutic hypothermia in asphyxiated newborns: whole body versus selective head cooling. Am J Perinatol 2009;26:265-70.
- Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005;116:1001-6.
- Kramer BA, Kadar AG, Clark K. Use of the Neuro-Wrap system for severe post-electroconvulsive therapy headaches. J ECT 2008;24:152-5.
- Bagic A, Theodore WH, Bonwetsch R, Greenfield J, Sato S. Treating epilepsy with head-neck cooling without sedation. Epilepsia 2005;46.
- Reynolds L. The Effects of Head and Neck Cooling on Symptoms of Multiple Sclerosis 2007.
- Hato N, Hyodo J, Takeda S, Takagi D, Okada M, Hakuba N, et al. Local hypothermia in the treatment of idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx 2010;37:626-30.
- Simmons SE, Saxby BK, McGlone FP, Jones DA. The effect of passive heating and head cooling on perception, cardiovascular function and cognitive performance in the heat. Eur J Appl Physiol 2008;104:271-80.
- Wickwire PJ, Bishop PA, Green JM, Richardson MT, Lomax RG, Casaru C, et al. Physiological and comfort effects of a commercial “cooling cap” worn under protective helmets. J Occup Environ Hyg 2009;6:455-9.
- Massey CS. A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs 2004;8:121-30.
- Dougherty L. Comparing methods to prevent chemotherapy-induced alopecia. Cancer Nurs Pract 2006;5:25-31.
- Swan H. Clinical hypothermia: a lady with a past and some promise for the future. Surgery 1973;73:736-58.
- Phelps C. The classics of neurology and neurosurgery library. New York, NY: D Appleton and Company; 1897.
- Waugh OS. Acute cranial-cerebral injuries. Can Med Assoc J 1926;16:1475-9.
- Schlag G. Cardiac arrest and resuscitation. Zbl Chir 1962;87:1273-90.
- Ioffe I, Sumskii LI. Cranio-cerebral hypothermia in the treatment of patients with cranio-cerebral injuries. Zh Vopr Neirokhir Im NN Burdenko 1977;1:9-14.
- Zhmurko SF. Cranio-cerebral hypothermia in patients with acute cranio-cerebral injury. Khirurgiia 1971;47:40-3.
- Ivanov VV, Kolenko EA. Local and cerebral hypothermia using thermoelectric apparatus in severe craniocerebral trauma. Vestn Khirurg Im II Grek 1969;102:105-7.
- Bukov VA, Bobkov IG, Smirnov OA, Zol’nikov SM. The relationship between the temperature of various regions of the brain and the body in craniocerebral hypothermia in clinical practice. Khirurgiia 1967;43:14-21.
- Ugriumov VM, Zotov I, Bezukh MS. Cranio-cerebral hypothermia and neuro-vegetative blockade in the systematic treatment of injuries to the skull and brain. Vopr Neirokhirurgii 1975;6:11-7.
- Lojka J, Kravcova V, Kovacikova E, Gajdosova D, Nadvornik P. 1st experiences with craniocerebral hypothermia. Rozhl Chir 1973;52:812-16.
- Smirnov O, Meshcherinov I. Analytical method for determination of temperature distribution in the human head during craniocerebral hypothermia induced by ‘Kholod-2F’ apparatus. Biomed Eng 1970;4:192-7.
- Jennett B. Development of Glasgow Coma and Outcome Scales. Nepal J Neurosci 2005;2:24-8.
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998;15:573-85.
- Gray J, Geva A, Zheng Z, Zupancic JA. CoolSim: using industrial modeling techniques to examine the impact of selective head cooling in a model of perinatal regionalization. Pediatrics 2008;121:28-36.
- Morris S, Ridley S, Lecky FE, Munro V, Christensen MC. Determinants of hospital costs associated with traumatic brain injury in England and Wales. Anaesthesia 2008;63:499-508.
- Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost–benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma 2007;63:1271-8.
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
- Comas-Herrera A, Wittenberg R. Expected lifetime costs of social care for people aged 65 and over in England. Canterbury, UK: Personal Social Services Research Unit; 2010.
- Forder J, Fernández J-L. Analysing the Costs and Benefits of Social Care Funding Arrangements in England: Technical Report 2009.
- Zhu L, Diao C. Theoretical simulation of temperature distribution in the brain during mild hypothermia treatment for brain injury. Med Biol Eng Comput 2001;39:681-7.
- Diao C, Zhu L, Wang H. Cooling and rewarming for brain ischemia or injury: theoretical analysis. Ann Biomed Eng 2003;31:346-53.
- Gunn AJ. Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Curr Opin Pediatr 2000;12:111-15.
- Mcilvoy L. Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs 2004;36:23-31.
- Gelman B, Schleien CL, Lohe A, Kuluz JW. Selective brain cooling in infant piglets after cardiac arrest and resuscitation. Crit Care Med 1996;24:1009-17.
- Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics 2001;108:1103-10.
- Schubert A. Side effects of mild hypothermia. J Neurosurg Anesthesiol 1995;7:139-47.
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005;6:775-86.
- Wrona D. Neural–immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol 2006;172:38-5.
- Hayashi N, Hayashi N, Dietrich DW. Brain hypothermia treatment. Tokyo: Springer-Verlag; 2004.
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev 1991;71:93-127.
- Boulant J, Mackowiak PA. Fever: basic mechanisms and management. Philadelphia, PA: Lippincott-Raven; 1997.
- Bligh J. Mammalian homeothermy: an integrative thesis. J Therm Biol 1998;23:143-258.
- Lim TP. Central and peripheral control mechanisms of shivering and its effects on respiration. J Appl Physiol 1960;15:567-74.
- Commission for Thermal Physiology of the International Union of Physiological Sciences . Glossary of terms for thermal physiology. 3rd edn. Jpn J Physiol 2001;51:245-80.
- Hayashi N. The brain hypothermia therapy for prevention of vegetation after severe brain injury. Nippon Geka Gakkai Zasshi 1999;100:443-8.
- Nunnally MEM, Jaeschke RM, Bellingan GJM, Lacroix JM, Mourvillier BM, Rodriguez-Vega GMM, et al. Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Medicine 2011;39:1113-25.
- Hopewell S, Dutton S, Yu L-M, Chan A-W, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ 2010;340.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340.
- Xu L, Li J, Zhang M, Ai C, Wang L. Chinese authors do need CONSORT: reporting quality assessment for five leading Chinese medical journals. Contemp Clin Trials 2008;29:727-31.
- Wang J. Evidence-based medicine in China. Lancet 2010;375:532-3.
- Xu L, Han X, Li X, Yan C, Tang S. Effects of head hypothermia on levels of NO, SOD, Glut in serum of patients with hypertensive intracerebral hemorrhage encephalocele. Pract J Med Pharm 2004;21:868-9.
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10.
- Yang C, Liu H, Bo W, Wu H, Lang M, Xin X, et al. The effects of selective brain cooling on prevention and treatment of common complications of severe brain injury. Chin J Otorhinolaryngol Skull Base Surg 2006;12:410-13.
- Degos V, Pereira AR, Lescot T, Sanchez-Pena P, Daoudi M, Zouaoui A, et al. Does brain swelling increase estimated specific gravity?. Neurocrit Care 2008;9:338-43.
- Lescot T, Degos V, Puybasset L. Does the brain become heavier or lighter after trauma?. Eur J Anaesthesiol 2008;25:110-14.
- Kleinman A. Remaking the moral person in China: implications for health. Lancet 2010;375:1074-5.
- Wang R, Henderson GE. Medical research ethics in China. Lancet 2008;372:1867-8.
- Bosch X, Titus SL. Cultural challenges and international research integrity. Lancet 2009;373:610-12.
- Hu S, Tang S, Liu Y, Zhao Y, Escobar ML, de Ferranti D. Reform of how health care is paid for in China: challenges and opportunities. Lancet 2008;372:1846-53.
- Zhang H. China’s irrational medical pricing scheme. Lancet 2010;375.
- Yang Z, Fan D. How to solve the crisis behind Bribegate for Chinese doctors. Lancet 2011;379:e12-15.
- Liu RP, Li DX, Liang JZ, Liu CJ, Chen YF, Wang DX, et al. Nursing of hypothermia in treatment of patients with acute cerebrovascular diseases. Chin J Nurs 1999;34:724-5.
- Ou X, Hou S, Yang Y, Chen Q. Research of the treatment time of head hypothermia on hyperthermia after hypertensive intracerebal hemorrhage. Heilongjiang Nurs J 2005;11:342-3.
- Dong G, Ou X, Yang Y, Chen Q. The effect of head hypothermia on the hypertensive intracerebral hemorrhage associated with hyperthermia patients serumCORT, SOD and LPO levels. Pract J Med Pharm 2005;22:1057-9.
- Zhang D, Yin P, Freemantle N, Jordan R, Zhong N, Cheng KK. An assessment of the quality of randomised controlled trials conducted in China. Trials 2008;9.
- Mills EJ, Wu P, Gagnier J, Devereaux PJ. The quality of randomized trial reporting in leading medical journals since the revised CONSORT statement. Contemp Clin Trials 2005;26:480-7.
- Jiang Y-B, Zhao S-H, Ye K-H. Effects of local mild hypothermia on cerebral edema volume and the rehabilitation of neurological function in patients with cerebral hemorrhage: a meta analysis. Chin J Clin Rehabil 2005;9:6-7.
- Lan R, Jiang Y. Effect of local mild hypothermia on motor function in patients with cerebral hemorrhage and the Meta analysis of its mechanism. Chin J Clin Rehabil 2005;9:16-8.
- Christian E, Zada G, Sung G, Giannotta SL. A review of selective hypothermia in the management of traumatic brain injury. Neurosurgical Focus 2008;25.
- Qiu W, Shen H, Zhang Y, Wang W, Liu W, Jiang Q, et al. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci 2006;13:995-1000.
- Feng H, Shi D, Wang D, Xin X, Feng L, Zhang Y, et al. Effect of local mild hypothermia on treatment of acute intracerebral hemorrhage, a clinical study. Chung-Hua I Hsueh Tsa Chih (Chin Med J) 2002;82:1622-4.
- Su Z-Q, Wang Y, Zhao Q-J, Sun X-Y, Yang H-Y, Wang D-S. Recent effect of local mild hypothermia for improving neurological deficits in patients with cerebral hemorrhage. Chin J Clin Rehabil 2004;8:1816-17.
- Aiyagari V, Diringer MN. Fever control and its impact on outcomes: what is the evidence?. J Neurol Sci 2007;261:39-46.
- Childs C, Wieloch T, Lecky F, Machin G, Harris B, Stocchetti N. Report of a consensus meeting on human brain temperature after severe traumatic brain injury: its measurement and management during pyrexia. Front Neur 2010;1.
- Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol 2006;60:438-46.
- Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics 2001;108:1103-10.
- Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med 1998;26:562-7.
- Rossi S, Zanier ER, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry 2001;71:448-54.
- Moran JL, Peter JV, Solomon PJ, Grealy B, Smith T, Ashforth W, et al. Tympanic temperature measurements: are they reliable in the critically ill? A clinical study of measures of agreement. Crit Care Med 2007;35:155-64.
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 1948;27:476-83.
- Harris BA, Andrews PJD, Mayer SA, Sessler DI. Therapeutic hypothermia. New York, NY: Marcel Dekker; 2005.
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663-70.
- Harris BA, Andrews PJD, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain-injured, mechanically ventilated patients: a randomized, crossover, factorial trial. Br J Anaesth 2007;98:93-9.
- Harris B. Hypothermia. J Neurosurg 2009;111.
- Kang, Yang J, Lisheng L, Wei Y. Selective mild hypothermia therapy on immune function in patients with traumatic brain injury and prognosis. Shandong Med J 2004;44:35-6.
- Qiu W, Wang Y, Zhou Y, Ru J, Wang W. The clinical effects of selective hypothermia and decompressive craniectomy on severe traumatic brain injury. J Hangzhou Normal Univ 2007;27:10-2.
- Qiu W. A preliminary study on clinical effects of combinating noninvasive selective brain cooling and decompressive craniectomy in severe traumatic brain injury n.d.:45-7.
- Fang A. The clinical effects of selective hypothermia and decompressive craniectomy on severe traumatic brain injury. Zhejiang J Trauma Surg 2009;14:97-9.
- Xu E, Qi F, Wang J, Yan J, Pan L, Lu X. The clinical study of selective brain cooling in the treatment of acute cerebral infarction. J Apoplexy Nerv Dis 2003;20:434-6.
- Xia Q, Yan C. Investigation into therapeutic effect of cerebral cryotherapy on large-acreage cerebral infarction. Clin J Med Officer 2004;32:14-5.
- Li XL, Xia Q, Cheng ZX, Zhang YW, Liu QC. Influence of beginning time of hypothermia treatment on prognosis of extensive cerebral infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue Chin Crit Care Med 2005;17:180-2.
- Takenobu Y, Oe H, Imakita S, Naito H, Naritomi H. Neuroprotective effect of local surface cooling in acute ischemic stroke: P295. Stroke 2005;36.
- Chen Q, Ou X, Yang Y, Li X, Liu Q. The effect of head hypothermia on the large-acreage cerebral infarction (LCI) and hyperthermia patients serum CORT, SOD and LPO level. Chin J Prim Med 2006;13:20-1.
- Wang H, Wang D, Lanzino G, Elkins W, Olivero W. Differential interhemispheric cooling and ICP compartmentalization in a patient with left ICA occlusion. Acta Neurochir 2006;148:681-3.
- Inoue T, Kimura K, Iguchi Y, Shibazaki K, Matsumoto N, Iwanaga T. Local brain hypothermia with use of cooling hat may improve patients’ outcome in acute severe ischemic stroke. Stroke 2007;38.
- Hao Q, Zhang Z-B, Yang Y-F, Liu C-H. Assessment of batroxobin combined with local mild hypothermia in the treatment of cerebral infarction. Chin J Cerebrovasc Dis 2008;5:121-4.
- Shuaib A, Kanthan R, Goplen G, Griebel R, el-Azzouni H, Miyashita H, et al. In-vivo microdialysis study of extracellular glutamate response to temperature variance in subarachnoid hemorrhage. Acta Neurochir Suppl 1996;67:53-8.
- Dohi K, Jimbo H, Ikeda Y, Matsumoto K. Pharmacological brain cooling (PBC) by indomethacin; a non-selective cyclooxygenase (COX) inhibitor in acute hemorrhagic stroke. Nosotchu 2000;22:429-34.
- Dohi K, Jimbo H, Ikeda Y, Fujita S, Ohtaki H, Shioda S, et al. Pharmacological brain cooling with indomethacin in acute hemorrhagic stroke: anti-inflammatory cytokines and antioxidative effects. Acta Neurochir 2006;696:57-60.
- Xu LX, Li XL, Zhang XD, Wu XF, Zhang XM. Clinical efficacy of head mild hypothermia in treatment of hypertensive intracerebral hemorrhage. Chin J Geriatr Cardiovasc Cerebrovasc Dis 2002;4:327-9.
- Zhang X, Liu X, Li W, Chen R. Effects of local mild hypothermia treatment on plasma neuropeptide Y (NPY), neurotensin (NT), calcitonin gene-related peptide (CGRP) and endotheline (ET) in patients with cerebral hemorrhage. Chin J Emerg Med 2006;15:47-9.
- Xia Q, Qin S, Li X, . Efficacy of minimal injury aspiration in combination with head hypothermia in the treatment of hypertensive intracerebral hemorrhagic cerebral hernia. Chin J Geriatr Cardiovasc Cerebrovasc Dis 2003;5:184-5.
- Zhang XM, Li XL, Tang SH, Liu QC. Effect of head hypothermia on serum inflammatory cytokines levels in patients with hypertensive intracerebral hemorrhage. Chinese 2006;18:294-6.
- Tang Y, Zhang Y, Liu W, Wang D. Selective and mild hypothermia in the management of brain injury. J Neurochem 2008;106:65-6.
- Zhang Y, Wang D, Tang Y, Zhang Y. Effect of local mild hypothermia on glucose metabolism in human brain observed with positron emission tomography. Chin J Clin Rehabil 2005;9:110-12.
- Wu L, Wang D, Zhou Y, Liu N, Xu S, Hui K, et al. Effect of adhesive dressing local and mild hypothermia treatment for acute stroke. Chin J Immunol Neurol 2010;17:117-19.
- Mellergard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery 1992;31:671-7.
- Mariak Z, Lewko J, Jadeszko M, Dudek H. Cerebral and Related Temperatures in Resting, Normothermic Subjects 1993.
- Wang W, Jiang Q, Chen J, Lu F, Zhao Z, Wu J. The study on cases of severe brain injury treated with selective brain cooling. J Hangzhou Med Coll 2001;22:198-200.
- Hachimi-Idrissi S, Huyghens L, Van der Auwera M, Lauwaert I, Van Geffen G, Corne L. Doc, Keep the Head Cool! (abstract no. 301). Annual Meeting of the Society for Academic Emergency Medicine (SAEM). Acad Emerg Med 1999;6.
- Hachimi-Idrissi S, Zizi M, Nguyen DN, Schiettecate J, Ebinger G, Michotte Y, et al. The evolution of serum astroglial S-100 [beta] protein in patients with cardiac arrest treated with mild hypothermia. Resuscitation 2005;64:187-92.
- Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest:systematic review and individual patient data meta-analysis. Crit Care Med 2005;33:414-18.
- Ikeda K, Kuroki Y, Yosikawa K, Yokoyama T, Utino H. Changes in urinary 8-hydroxy-2-deoxyguanosine in patients with global brain ischemia undergoing brain hypothermia therapy: comparison of whole body and selective head cooling (P330). Crit Care 2007;11.
- Busch H-J, Brunner M, Schwab H, Inderbitzen B, Barbut D, Schwab T. Pre-treatment with transnasal cooling for the induction of therapeutic hypothermia in patients with cardiac arrest leads to a significant faster achievement of target temperature during systemic cooling (poster). Freiburg: Albert Ludwigs University Freiburg; 2008.
- Nordberg P, Castrén M, Svensson L, Barbut D. New Method of Intra-Arrest Trans-Nasal Cooling in Stockholm: The PRINCE II Study n.d.
- Takeda Y, Fumoto K, Naito H, Morimoto N. Development of a pharyngeal cooling system that enables brain temperature to be immediately reduced. Crit Care Med 2009;37:S250-7.
- Wandaller C, Holzer M, Sterz F, Wandaller A, Arrich J, Uray T, et al. Head and neck cooling after cardiac arrest results in lower jugular bulb than esophageal temperature. Am J Emerg Med 2009;27:460-5.
- Shiraki K, Sagawa S, Tajima F, Yokota A, Hashimoto M, Brengelmann GL. Independence of brain and tympanic temperatures in an unanesthetized human. J Appl Physiol 1988;65:482-6.
- Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. J Appl Physiol 1999;87:1609-13.
- Mariak Z, White MD, Lyson T, Lewko J. Tympanic temperature reflects intracranial temperature changes in humans. Pflugers Arch 2003;446:279-84.
- Kuhnen G, Einer-Jensen N, Tisherman SA, Tisherman SA, Sterz F. Therapeutic Hypothermia. Springer-Verlag: New York, NY; 2005.
- Corbett RJT, Laptook AR. Failure of localized head cooling to reduce brain temperature in adult humans. Neuroreport 1998;9:2721-5.
- Skulec R, Truhlar A, Ostadal P, Telekes P, Knor J, Tichacek M, et al. Current cooling methods for induction of mild hypothermia in cardiac arrest survivors. Vnitrni Lekarstvi 2009;55:1060-9.
- Skulec R, Truhlar A, Knor J, Seblova J, Cerny V. The practice of therapeutic mild hypothermia in cardiac arrest survivors in the Czech republic. Minerva Anestesiol 2010;76:617-23.
- Merrill TL, Mayer SA, Sessler DI. Therapeutic hypothermia. New York, NY: Marcel Dekker; 2005.
- Thoresen M. Cooling the newborn after asphyxia: physiological and experimental background and its clinical use. Semin Neonatol 2000;5:61-73.
- Robertson NJ, Kendall GS, Thayyil S. Techniques for therapeutic hypothermia during transport and in hospital for perinatal asphyxial encephalopathy. Semin Fetal Neonatal Med 2010;15:276-86.
- Covaciu L, Allers M, Enblad P, Lunderquist A, Wieloch T, Rubertsson S. Intranasal selective brain cooling in pigs. Resuscitation 2008;76:83-8.
- Grande PO, Reinstrup P, Romner B. Active cooling in traumatic brain-injured patients: a questionable therapy?. Acta Anaesthesiol Scand 2009;53:1233-8.
- Takeda Y, Fumoto K, Naito H, Morimoto N. Development of a pharyngeal cooling system that enables brain temperature to be immediately reduced. Crit Care Med 2009;37.
- Doll H, Truebel H, Kipfmueller F, Schaefer U, Neugebauer EAM, Wirth S, et al. Pharyngeal selective brain cooling improves neurofunctional and neurocognitive outcome after fluid percussion brain injury in rats. J Neurotrauma 2009;26:235-42.
- Doll H, Maegele M, Bohl J, Storkel S, Kipfmueller F, Schafer U, et al. Pharyngeal selective brain cooling is associated with reduced CNS cortical lesion after experimental traumatic brain injury in rats. J Neurotrauma 2010;27:2245-54.
- Smirnov O. New method for cooling (or heating) of the body and an apparatus for craniocerebral hypothermia. Biomed Eng 1968;2:343-7.
- Smirnov O. A method of increasing the efficiency of air hypotherms and an apparatus for craniocerebral cooling. Biomed Eng 1969;3:257-60.
- Schwartz AE, Stone JG, Pile-Spellman J, Finck AD, Sandhu AA, Mongero LB, et al. Selective cerebral hypothermia by means of transfemoral internal carotid artery catheterization. Radiology 1996;201:571-2.
- Natale JA, D’Alecy LG. Protection from cerebral ischemia by brain cooling without reduced lactate accumulation in dogs. Stroke 1989;20:770-7.
- Barone FC, Feuerstein GZ, White RF. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci Biobehav Rev 1997;21:31-44.
- Li X. Principle and clinical application of brain cooling treatment system. Chin Med Devices 2010;25:50-1.
- Kapidere M, Ahiska R, Guler I. A new microcontroller-based human brain hypothermia system. J Med Syst 2005;29:501-12.
- Ahmed S, Shimada T, Funakubo A, Fukui Y, Higami T. Development of a cooling unit using Peltier effect for injured head. J Life Support Eng 2005;17:44-5.
- Ahmed S, Shimada T, Funakubo A, Fukui Y, Higami T. Development of a cooling unit for the emergency treatment of head injury. World Congress on Medical Physics and Biomedical Engineering 2006. IFMBE Proc 2007;14:3243-6.
- Kolenko E, Bezukh M. Electronic device for hypothermia of the brain. Biomed Eng 1971;5:239-41.
- Reut NI. Technic of continuous therapeutic craniocerebral hypothermia in acute craniocerebral injury. Ortop Travmatol Protez 1970;31:47-8.
- Okhrimenko NN, Zaikin VS. Voen Med Zh 1974;1:68-9.
- Mink S, Schwarz U, Mudra R, Gugi C, Froehlich J, Keller E. Treatment of resistant fever: new method of local cerebral cooling. Neurocrit Care 2010;13.
- Kallmunzer B, Koehn J, Abrar D, Schwab S, Kollmar R. First Experiences With the New EMCOOLS Device for Selective Neck-Cooling for Patients With Acute Ischemic Stroke n.d.
- Minimizing neural damage . Patent granted for brain cooling and monitoring device. Can Fam Physician 2005;51.
- Kim DE, Labat K. Design process for developing a liquid cooling garment hood. Ergonomics 2010;53:818-28.
Appendix 1 Temperature measurement with head cooling
If cooling, however delivered, is to have a neuroprotective effect, brain temperature must be reduced. Intracranial temperature is recognised as the gold standard for measuring brain temperature, but invasive measurement is usually possible only in critically ill patients and at a single site. The intracranial sites most commonly used clinically are subdural, parenchymal (typically a centimetre or two into a frontal lobe) and ventricular. Invasive brain monitoring is uncommon in stroke patients. A drawback is that a single site of measurement may not reflect temperature across the brain, especially after injury and in the presence of head cooling when hotter and cooler areas and gradients may be more marked. 152,153
Intracranial temperature cannot be reliably predicted from body temperature. In neurosurgical patients, temperature differences have, for example, been shown to range within patients from –0.3 °C to 2.1 °C between frontal lobe and rectal temperatures,154 and between patients from –0.7 °C to 2.3 °C between ventricular and pulmonary artery temperatures. 155 Proxy measures of intracranial temperature used in clinical practice include jugular bulb temperature (the jugular bulbs are not within the cranium) and infrared thermometry in the ear canal. However, a number of studies have questioned the accuracy and precision of the latter (e.g. Moran and colleagues156) and both are potentially susceptible to contamination by scalp blood temperature,157 which is of relevance with external head-cooling interventions and significant changes in environmental temperature. True tympanic membrane temperature is rarely measured clinically, especially in unconscious patients, because of the risk of perforating the ear drum.
However, head cooling does not reduce brain temperature in isolation, and venous return from the cooled brain is likely to affect core trunk temperature. 158 Indeed, in neonates body warming is applied to prevent too great a core body temperature drop with head cooling. 159 Therefore, in the absence of intracranial temperature measurement, if core body temperature is reduced, this is an indication that there has been heat loss from the head. Furthermore, the temperature of the carotid supply to the brain will be reduced, which will, in turn, have a cooling effect on the brain. If core body temperature is not reduced at all it is probably not unreasonable to assume that intracranial temperature has not been significantly reduced. Thus, if core trunk temperature (in an artery, e.g. pulmonary artery, oesophagus, bladder or rectum) reduces with head cooling it can be inferred that the brain has been cooled. 24,160
Therefore, for the purposes of assessing the effect of head cooling on brain temperature for this review, intracranial temperature is defined as any temperature within the skull inside the dura. A primary measure of the effectiveness of head cooling is reduction in intracranial temperature. Core trunk temperature is used as proxy for brain temperature in the absence of intracranial temperature measurement and defined as temperature measured in an artery (usually pulmonary), the oesophagus, bladder or rectum. A secondary measure of the effectiveness of head cooling is reduction in core trunk temperature on the assumption that for this to be reduced there must have been some reduction in intracranial temperature.
Appendix 2 Review protocol (final agreed version December 2008)
Detailed project description
Systematic Review of Head Cooling in Adults after Traumatic Brain Injury and Stroke (Project Reference: 07/37/32)
Authors
Bridget Harris RGN, DipN, MSc, PhD
Peter JD Andrews MD, MBChB, FRCA
Gordon D Murray PhD, C Stat, FRCPE, FFPH, FRSE
John Forbes BA, MSc, PhD
NB the headings we have used are based on those for Cochrane Reviews. 1
Although we are using Cochrane review methodology, this review does not qualify as a Cochrane review because it straddles two Cochrane groups, stroke and injuries, and also because Cochrane reviews of cooling in stroke and head injury do not as yet differentiate between methods of achieving cooling. Nevertheless we have established links with the Cochrane Stroke Group, which is based locally, and have discussed the review with the co-ordinating editor of the Cochrane Stroke Group, the trials search co-ordinator and the statistical editor. They have agreed to give consultancy support and this is costed for in the budget. Although we cannot have formal access to the Cochrane Stroke Group, other than by paying for consultancy, informally it is possible to seek to discuss any aspect of the review as members of the Cochrane Stroke Group are colleagues. Ownership of the findings will remain with the review authors.
Background
The condition and incidence – traumatic brain injury and stroke
Brain injury due to stroke and trauma are common and costly in human and resource terms. Stroke affects 130,000 people a year with about 450,000 living with severe disability;2 it ‘is the third biggest cause of death in the United Kingdom and the largest single cause of severe disability’. 3 The incidence for head injury is similar to that for stroke,4 although the incidence of death is lower at 6–10 per 100,000 population per year. 5 However, head injury is more common in younger people and it has been estimated that 4,700 of those admitted to hospital each year would be unable to return to work at 6 weeks. 4 In Scotland, 78% of patients with a severe injury had moderate or severe disability one year later. 6
Although the primary mechanisms of brain injury are different in trauma, haemorrhage and ischaemia (whether focal as in ischaemic stroke or global as in cardiac arrest and neonatal hypoxic ischaemic encephalopathy), the result is a cascade of excitotoxity, apoptosis and inflammation. 7,8 Inflammation, cell death, and infection if present, means that increased temperature is common after both stroke and brain injury. 9,10 There is no universal definition of the threshold for pyrexia or where and how temperature should be measured but, for example, within 48 hours nearly 68% of patients had a rectal temperature ≥37 °C after severe traumatic brain injury11 and 54% had an axilla temperature > 37.5 °C after stroke. 12
Increased temperature is associated with worse outcome after both stroke and traumatic brain injury. 11,13 The exact nature of the relationship is hard to determine since the time of onset of raised temperature has an influence and temperature elevation can be a marker of more severe injury and of infection, both of which are also associated with worse outcome,14 although one systematic review suggests infection may not play a significant part in the relationship in stroke. 13 Nevertheless there is considerable evidence from animal research that reducing temperature, and more especially inducing hypothermia, reduces the extent of the injury and that the sooner cooling is instigated the more effective it is. 8 However, with the exception of cardiac arrest and neonatal hypoxic ischaemic encephalopathy, induction of hypothermia in humans has not yet translated to improved outcome. This may be because it is difficult to cool patients early and quickly enough and because the side effects of hypothermia, such as increased infection, may outweigh the benefits in some circumstances. 15–19
Although the focus of this review is head cooling in traumatic brain injury and stroke, in which the primary problem is neurological, it is in cardiac arrest that hypothermia is clinically advised20 and most commonly used. Therefore we will include the cardiac arrest literature on head cooling in our searches because this could contribute information about how effective these interventions are in reducing temperature, and on their ease of use and side effects. A head cooling study in cardiac arrest is included in systematic reviews of hypothermia for cardiac arrest21,22 but in this study systemic hypothermia (bladder temperature 34 °C) was achieved. 23 In our opinion it is not yet clear to what extent myocardial cooling contributes to improved outcome with hypothermia after cardiac arrest, and whether head cooling alone is as effective as systemic cooling in the absence of myocardial cooling. There is some evidence that myocardial reperfusion injury, which can be ameliorated by hypothermia, may contribute to post-arrest morbidity and mortality. 24,25
The intervention – non-invasive head cooling
Cooling methods can be classified into those targeted systemically and those targeted at the head to cool the brain. The brain is where cooling is needed after stroke and brain injury. Within these two classifications there are invasive and non-invasive methods. Systemic cooling is the most common intervention in standard practice.
Methods of non-invasive head cooling either induce heat loss from the upper airways by nasal gas flow and nasal lavage, or heat loss through the skull by convection (fanning, cooling hoods) and conduction (ice, cooling caps and helmets). 26 Some cooling caps and helmets also have a neck band which theoretically may cool the brain by decreasing the temperature of the carotid blood supply. 27 These non-invasive methods are generally quick and easy to apply and may be suitable for pre-hospital use, which are important considerations in reducing time to cooling. They also have potentially wide application because they can be used in patients with a range of severity of illness, not just the most severely ill. Head cooling, however, is not in common use in adults, the main application has been neonatal hypoxic-ischaemic injury,28 but around the world there is developing interest in its use in adults.
Invasive methods of head cooling, such as antegrade and retrograde cerebral perfusion and open irrigation of the brain surface, are used during surgery26 and will not be included in this review.
Standard temperature management in stroke and brain injury is generally aimed at reducing hyperthermia, since there is insufficient evidence that inducing hypothermia improves outcome. In stroke it is recommended that temperature is treated if above 37.5 °C. 29 In brain injury maintaining normothermia is recommended in the context of treating raised intracranial pressure. 30 Some neurological units have specific protocols,31 for example treating temperature if above 38 °C and inducing hypothermia to 35 °C as part of the treatment for treating raised intracranial pressure. 32 There are no standard recommendations on the site of temperature measurement or methods of temperature reduction. In practice choice of site of measurement is variable31,33 and cooling interventions are systemic. Pharmacological intervention with paracetamol is the most common first line treatment, followed by a variety of physical systemic cooling interventions which include cooling blankets, ice packs and fanning. 31,34
How the intervention might work – mechanisms and temperature reduction
Mechanisms
Although cooling interventions are more commonly delivered systemically, the logic behind brain cooling is that it targets cooling where it is needed because it is brain rather than trunk temperature which is important in cerebral protection. It is also thought that brain cooling may reduce the complications of hypothermia because less body temperature reduction is required, although the evidence for this is not robust. 26
The great advantage of cooling, by comparison with most other neuroprotective interventions, is that it has multifactorial effects with regard to cerebral protection and prevention and reduction of secondary insults. Hypothermia has even been described as ‘the ultimate neuroprotective cocktail’. 9 The effects of cooling are not fully understood but include reduction of metabolic rate, modulation of cerebral blood flow and the inflammatory response and reduction of excitotoxic damage and cerebral oedema. 8,35 Because cooling can be very effective in reducing refractory intracranial pressure this is the most usual reason for instigating physical cooling interventions in severe traumatic and haemorrhagic brain injury. 32,36 One of the attractions of cooling therapies is that it is possible cooling could extend the time window within which restoration of blood supply, e.g. with thrombolysis or resuscitation, might be effective.
Even if head cooling has a relatively modest effect on reduction of disability after brain injury and stroke it may be cost effective since morbidity from head injury ‘far exceeds the capacity of UK neurorehabilitation services’5 and the costs of stroke to the NHS are estimated at £2.8 billion per year. 3
Measurement of temperature reduction
If cooling, however delivered, is to have a neuroprotective effect brain temperature must be reduced. Intracranial temperature is generally recognised as the gold standard for measuring brain temperature, but it is usually only possible to measure it in critically ill patients and at a single site, which may not reflect temperature across the brain especially after injury. Invasive brain monitoring is uncommon in stroke patients. Proxy measures of brain temperature include jugular bulb temperature (the jugular bulb is not within the cranium and is therefore not a true intracranial temperature ) and infrared thermometry in the ear canal. But a number of studies have questioned the accuracy and precision of the latter (e.g. 37) and both are susceptible to contamination by head skin temperature, which is a relevant factor with most head cooling interventions.
However, head cooling does not reduce brain temperature in isolation and the venous return from the cooled brain is likely to affect core trunk temperature. 26 In the absence of intracranial temperature measurement therefore, if core body temperature is reduced this indicates there has been heat loss from the head and, furthermore, the temperature of the carotid supply to the brain will be reduced which will in turn have a cooling effect on the brain. If core body temperature is not reduced at all it is not unreasonable to assume that intracranial temperature has not been significantly reduced either. Thus if core trunk temperature (e.g. in the pulmonary artery, oesophagus, bladder or rectum) reduces with head cooling it can be inferred that the brain has been cooled. 27,38 Indeed in neonates body warming is applied to prevent too great a core body temperature drop with head cooling. 28
Therefore, for the purposes of this head cooling review, brain temperature constitutes any intracranial temperature and body temperature is core trunk temperature measured in the pulmonary artery, oesophagus, bladder or rectum. Core trunk temperature will be used as proxy for brain temperature in the absence of intracranial temperature measurement. However, we will look at all temperature measurement data in studies of head cooling for the purposes of descriptive reporting.
Why it is important to do this review
Reviews of cooling interventions after brain injury and stroke have not differentiated between cooling methods. Among Cochrane reviews, for example, the only review of the effectiveness of a specific cooling intervention is that of paracetamol for fever in children. 39 In the reviews of cooling for acute stroke and of hypothermia for head injury the effect of temperature reduction on outcome is the focus and not the method(s) of achieving this. 15,40 Yet cooling methods differ in their effectiveness and complications and as further research is undertaken it may become more obvious that not all cooling methods are equal, and that discrimination between methods is necessary in reviews of the effect of cooling on outcome. By confining this review to head cooling we are correcting for inconsistencies in methodology, equipment and temperature measurement to a greater extent than existing systematic reviews of cooling after stroke, brain injury and cardiac arrest. Theoretically head cooling has advantages over systemic cooling because it targets cooling where it is needed, requires less body temperature reduction relative to brain temperature reduction and therefore may have fewer side effects. We believe a review of non-invasive head cooling as an intervention is now warranted. If we find sufficient good quality head cooling research on either stroke or brain injury we will review the effects separately.
Service user involvement
To date, in the UK at least, head cooling has been a research intervention and not part of normal clinical care. As a result there have been very few adult service users of head cooling. Those patients who have had head cooling were severely ill and heavily sedated or unconscious and consequently unaware of the intervention. Even if we could contact them, it is therefore difficult to know how they might contribute to this review from personal experience of head cooling. This is why the service users engaged with so far have been those who have had a stroke or brain injury who are known to or have been cared for by the Lead Applicant.
However, during preparation of the report, the results of the review will be presented to members of the general public, which might include people who have had a stroke or brain injury. It will be of value and interest to hear their views on the concept and possible use and effectiveness of head cooling and could provide useful information for planning future trials of head cooling. Presenting research to the community is a strategy which has been used in the US to address emergency research without consent, so called ‘waiver of consent’, for example in the National Acute Brain Injury Study Hypothermia II (NABIS-H II). Since head cooling is an acute intervention for unexpected and sudden health emergencies – stroke, brain injury and cardiac arrest – we consider this would be an appropriate way to engage with people who might be candidates for head cooling (service users) in the future.
Aims and objectives
Our overall aim is to assess the effectiveness of non-invasive head cooling in adults after traumatic brain injury and stroke.
In order to achieve this we intend:
Firstly, to address the explanatory, mechanistic question of what the cooling interventions achieve in terms of reducing brain temperature. If there is a clear effect, then we will explore what characteristics of the intervention and/or patients are associated with the extent of brain cooling. This objective will be informed by studies in cardiac arrest as well as those in stroke and traumatic brain injury.
Secondly, to address the pragmatic, clinical question of what impact brain cooling has on patient outcomes. Only the studies in stroke and traumatic brain injury will be relevant here, although studies in cardiac arrest may highlight some potential adverse effects of cooling.
Thirdly, we aim to assess the cost effectiveness of head cooling in traumatic brain injury and stroke.
Fourthly, we intend to present the results of the review to members of the general public in order to hear their views on the concept and possible use and effectiveness of head cooling.
Ideally this will establish whether and to what extent head cooling is effective, in both brain injury and stroke, and which methods are most suitable in which circumstances. However, the degree to which we can establish this does depend on the nature and quality of the research available. Nevertheless, we intend to provide a comprehensive picture of head cooling research which will inform clinicians and guide researchers and which we can update as further studies become available.
Specific objectives:
-
To assess the effects of non-invasive head cooling on:
-
intracranial temperature and/or core trunk temperature (pulmonary artery, oesophageal, bladder, rectal)
-
disability assessed with a validated outcome score
-
mortality
-
in adults after traumatic brain injury and stroke.
-
-
To determine any adverse effects or complications associated with head cooling or the specific devices used.
-
To model the economic implications of managing traumatic brain injury and stroke using head cooling.
-
To present the results of the review to members of the general public, in order to hear their views on the concept and possible use and effectiveness of head cooling and provide information on their views for clinicians and researchers planning to use or trial head cooling.
-
To provide a comprehensive picture of head cooling research which will inform clinicians and guide researchers.
Criteria for considering studies for this review
Types of studies
A record will be made of all studies or case reports in adult humans using any form of non-invasive head cooling.
Of these only randomised controlled trials will be included in the formal analysis, but proof of concept studies which give information on temperature reduction will be tabulated and the temperature reduction assessed. Full blinding may not be feasible given the nature of the intervention but any trials in which the assessor of disability outcome was not blinded will be excluded from the analysis. Temperature, being a concrete measure of a physiological variable, is less susceptible to interpretation.
Types of participants
All adults (≥ 18 years) admitted to hospital with traumatic brain injury, or ischaemic or haemorrhagic stroke, of any severity (and cardiac arrest for the purposes of assessing efficacy of devices).
Types of intervention
Any method of non-invasive head cooling of any duration given for the purposes of fever reduction, inducing normothermia or hypothermia, or reducing disability and mortality or reducing intracranial pressure will be included. Studies in which direct brain cooling is combined with another cooling intervention, excepting antipyretic drugs such as paracetamol, will be excluded.
Types of outcome
Primary outcomes
Proxy outcomes
-
Reduction in intracranial pressure.
-
Improvement in biochemical markers of injury e.g. lactate/pyruvate ratio, glutamate, cytokines.
-
Improvement in cross-sectional imaging.
Secondary outcomes
Complications actually or possibly attributable to the head cooling intervention or the specific device, e.g. infections, prolonged clotting time and bleeding complications, scalp damage; time from brain injury or onset of stroke to start of cooling, cooling rate (hourly temperature reduction), and time from injury to target temperature and from device application to achieving target temperature. These are indicators of the effectiveness of the devices and their ease of use, e.g. how quickly and easily they can be applied.
Note on outcome scales
Typically, outcome scales are dichotomised to reflect people’s level of dependence, although independence does not necessarily mean that a person has no residual deficit. With the five point GOS, for example, 1 is death, scores of 2 or 3 indicate dependence and scores of 4 or 5 independence of others in daily life. The American Heart Association Stroke Outcome Classification is similar with levels I and II indicating independence, and III-V partial or complete dependence. The Barthel Index on the other hand scores patients from 0 (total dependence) to 100 (total independence) with a score of > 70 being classed as good outcome. On the Scandinavian Stroke Scale a score of <30 points (range 0–58) has been defined as poor outcome. 43
Comparisons
Possible cooling intervention comparisons include:
-
no cooling intervention or standard care
-
physical cooling interventions applied systemically or to parts of the body other than the head e.g. tepid sponging, ice packs, cooling blankets, intravascular cooling catheters
-
pharmacological cooling interventions e.g. paracetamol, non-steroidal anti-inflammatory drugs, cyclo-oxygenase inhibitors, ethymisole.
For evidence of temperature reduction, possible comparisons include temperature with and without the device, temperature at baseline compared with target temperature or the lowest temperature achieved.
Search methods for identification of studies
Support with searches has been budgeted for from the Cochrane Stroke Group Trials Search Co-ordinator.
The planned search strategy is extensive because we suspect there is publication bias with head cooling research. Dealing with publication bias is acknowledged to be problematic in the Cochrane Handbook. The primary strategy is comprehensive searching, which is why our search strategy goes beyond databases of published material. Furthermore head cooling is a less mainstream intervention than other forms of cooling and trials may be found outside the more usual databases. It is known, for example, that brain cooling has been used in China and Japan and possibly South America and Russia, therefore the complete search strategy specifically includes databases relevant to these countries. No language, publication date or publication state restrictions will be imposed in search terms.
Databases to be searched:
-
The Cochrane Library including: Cochrane Database of Systematic Reviews; Database of Abstracts of Reviews of Effects; Cochrane Central Register of Controlled Trials (The Cochrane Library 2005, Issue 1); Health Technology Assessment Database; NHS Economic Evaluation Database.
-
The Cochrane Injuries Group and the Cochrane Stroke Group will be asked for access to their subject specific trials register and existing hand searches of journals and conference proceedings.
-
Other trials databases:
-
Clinical Trials
-
Current Controlled Trials
-
Find a Clinical Trial
-
National Research Register archive
-
MEDLINE (January 1966 to most recent)
-
Old MEDLINE (1950 to 1965)
-
Ovid MEDLINE in process and other non-indexed citations
-
EMBASE (1980 to most recent)
-
Australasian Medical Index
-
Chinese Biomedical Literature Database
-
Japan Information Centre of Science and Technology
-
Latin American Caribbean Health Sciences Literature
-
Cumulative Index of Nursing and Allied Healthcare
-
Scirus.
The search terms require refining but the principle employed will be to search in the first instance for everything on the subject of head or brain cooling, aiming for sensitivity rather than specificity. The initial search may be run without age limits in order to identify the full range of brain cooling studies (including those in neonates), which will allow investigators and manufacturers involved with this technology to be identified and contacted. The titles and abstracts of the studies found will then be searched to retrieve those which include adults with traumatic brain injury or stroke or cardiac arrest.
Possible MEDLINE search terms:
-
((brain or head or crani$or skull or cerebral or cortex or cortical or scalp or face or nasal or nose or nasopharyngeal or airways) adj2 (cool$or cold or hypothermia)).mp.
-
limit 1 to humans
-
limit 2 to (“adult (19 to 44 years)” or “middle age (45 to 64 years)” or “middle aged (45 plus years)” or “all aged (65 and over)” or “aged (80 and over)”)
Because the devices used for head cooling are varied and described in a number of different ways it is likely to be unhelpful to search more specifically e.g. for helmets, caps, fans, etc.
‘Grey’ literature:
-
Google Scholar: search with the exact phrases i) ‘head cooling’ and ii) ‘brain cooling’. Include subject areas: biology, life sciences, and environmental science; engineering, computer science and mathematics; medicine, pharmacology and veterinary science.
-
Russian Academy of Science.
-
Patent offices: UK Intellectual Property Office, European Patent Office, US Patent and Trademark Office.
Reference lists:
-
Reference lists of textbooks on hypothermia, traumatic brain injury and stroke, of reviews and of relevant studies will be searched.
Correspondence:
-
Investigators involved in previous studies, manufacturers of cooling equipment and persons who have lodged patents for head cooling devices will be contacted.
Methods of the review
Some consultancy support has been budgeted for from the Cochrane Stroke Group.
Trial identification and selection
-
BH will conduct the searches and screen out from the results anything unrelated to non-invasive head cooling, resolving uncertainty by discussion with PJDA. Any studies which include head cooling as an intervention, regardless of reason for use, will be kept. This will identify manufacturers of equipment, and possible complications and difficulties with head cooling as a technology. The references of these studies will be searched. From the information obtained in this initial screen BH will undertake the correspondence as outlined above.
-
BH will screen the results from stage 1 for any reports on head cooling in traumatic brain injury or stroke, resolving uncertainty by discussion with PJDA. Studies in other disease states, e.g. cardiac arrest, will be kept if they can add to information about the efficacy of devices in reducing temperature, their ease of use and side effects and contribute to the exploratory analysis.
-
BH and PJDA will independently screen titles and abstracts of the reports resulting from stage 2. Final identification for inclusion for formal analysis will be randomised controlled trials of non-invasive head cooling in traumatic brain injury or stroke. Disagreements at any stage will be resolved by discussion and consulting with GDM if necessary. Non-English studies selected for inclusion will be translated. If more than one report of a trial is found all will be included in order to facilitate complete data extraction.
-
Studies not suitable for inclusion for the formal primary and secondary analysis will be reviewed for information suitable for exploratory analysis.
Data extraction
BH and PJDA will independently extract data using a standard form with disagreements resolved by consultation with GDM. They will not be blinded to authors, journal or results. Where multiple reports of a trial are included any discrepancies between the reports will be noted. If there is missing information investigators of included trials will be written to.
The data to be collected will include: study name/ID; methods; participants – mechanism of injury, age, gender, total number randomised, total randomised to each group, baseline temperature ; interventions being tested – cooling methods and devices, target temperature, duration of cooling; outcomes – intracranial and/or core trunk temperature attained, mortality, dependency. Also information on the proxy and secondary outcomes listed above.
Quality assessment (assessment of validity of included studies)
The quality of the included studies will be assessed as part of the data collection procedure (above) with regard to:
-
adequacy of the randomization process
-
adequacy of the allocation concealment process
-
potential for selection bias after allocation
-
level of masking (treatment provider, patient, outcome assessor, investigators and analysers of the data).
The following quality checklist, developed by the Cochrane Renal Group,44 will be used (the Cochrane Stroke Group does not have such a checklist):
-
Allocation concealment:
-
– A. Adequate – randomisation method described that would not allow investigator or participant to know or influence intervention group before eligible participant entered in the study.
-
– B. Unclear – Randomisation stated but no information on method used is available.
-
– C. Inadequate – Method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence the intervention group.
-
-
Blinding:
-
– Blinding of investigators: Yes/No/not stated
-
– Blinding of participants: Yes/No/not stated
-
– Blinding of outcome assessor: Yes/No/not stated
-
– Blinding of data analysis: Yes/No/not stated
-
– The above are not considered blinded if the treatment group can be identified in > 20% of participants because of the side effects of treatment.
-
-
Intention to treat:
-
– Yes – specifically reported by the authors that intention-to-treat analysis was undertaken and this was confirmed on study assessment.
-
– Yes – not stated, but confirmed on study assessment.
-
– No – not reported and lack of intention-to-treat analysis confirmed on study assessment. (Patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study, or were not included because of protocol violation).
-
– No – stated but not confirmed upon study assessment.
-
– Not stated.
-
-
Completeness of follow-up:
-
– Per cent of patients excluded or lost to follow-up.
-
Only randomised controlled trials which meet the study quality criteria, having obtained missing data from the investigators if necessary, will be included in the formal analysis, taking into account that blinding of investigators and participants to head cooling may not be feasible. But any trials in which the assessor of disability outcome was not blinded will be excluded from the analysis.
We plan to pace our work in order to spend sufficient time on the review in the early stages so that the searches can be conducted and researchers written to as soon as possible to allow maximum time for follow up. If there is ultimately still reasonable doubt over the quality of studies we will not include them in the formal analysis, although we will log them in the interests of a better description of this field of research. We intend that this should be an ongoing review and will continue to pursue delayed information for inclusion in updates.
Data analysis
Primary analysis
For temperature data the difference in means will be calculated with 95% confidence intervals. If there are sufficient good quality trials for a meta-analysis a weighted mean difference will be calculated. Pooled relative risk and 95% confidence intervals for all-cause mortality and good neurological outcome will be calculated using a random-effects model. Statistical heterogeneity will be assessed using the chi-squared test.
It is likely to be appropriate to conduct sensitivity analyses of some aspects of head cooling, in relation to all-cause mortality for example, but it is difficult to prespecify these precisely. Factors which may be relevant include target temperature, cooling rate/time to target temperature, duration of cooling and rate of rewarming.
All analyses and forest plots will be stratified for stroke versus traumatic brain injury, and versus cardiac arrest for studies looking at temperature as the outcome. Observational data will be tabulated as a descriptive record of available information but no attempt will be made to draw any statistical inference.
Secondary analysis
The secondary analysis will assess the effect of cooling on proxy outcomes (intracranial pressure, biochemical markers of injury, cross-sectional imaging).
Subgroup analyses
It is desirable to prespecify a limited number of relevant subgroup analyses but we are totally dependent upon the data the trial authors have collected and reported. Based on the existing literature all our specified sub-group analyses are of potential interest but in practice we are only likely to have data to investigate one or two in any detail. Which ones we are ultimately able to investigate will depend on the trials which are suitable for inclusion, as will the precise definitions of the subgroup criteria which will of necessity be determined by the available data.
Almost all of the analyses will be descriptive, with forest plots stratified where possible by relevant subgroups. If sufficient high quality studies can be identified then it will be possible to explore subgroup effects more formally using meta-regression techniques, i.e. modelling how study characteristics (relating to the patients or the interventions) impact upon the results.
Pre-specified subgroup analyses:
-
Target temperature :
-
– normothermia versus hypothermia,
-
– mild versus moderate hypothermia.
-
(But definitions of these temperature ranges are not always consistent and therefore studies may have non-equivalent target temperature ranges. 45)
-
Cooling duration, e.g. 24 hrs or less, 24 to 48 hrs, > 48 hrs. Time from injury to achieving target temperature, e.g. within 6 hours, 12 hours, more than 12 hours.
-
Rewarming strategy:
-
– passive versus active,
-
– rate of rewarming – if this data is available this would be the preferable analysis.
-
-
Pharmacological adjuncts to physical cooling:
-
– paracetamol versus no paracetamol,
-
– any pharmacological cooling versus no pharmacological cooling.
-
-
Head cooling method:
-
– head versus head and neck,
-
– temperature controlled devices versus methods without temperature control,
-
– heat loss from the upper airways versus heat loss through the skull.
-
-
ICP management strategy:
-
– barbiturates versus no barbiturates.
-
In addition to the above there are other factors which would be relevant for subgroup analysis if there is data, for example complications and adverse events, cooling rate (°C per hour), age > 60 versus 60 or younger in traumatic brain injury (there is a significant increase in poor outcome above 60 years30).
Exploratory analysis
Studies not suitable for inclusion for formal analysis will be reviewed for information relating to the primary, proxy and secondary outcomes, temperature reduction in particular, and this will be tabulated and assessed.
Publication bias
We will attempt to assess publication bias using funnel plots as suggested in the Cochrane Handbook.
Costs and economic analysis
The aim of the economic analysis is to assess the cost effectiveness of head cooling. The perspective is the NHS and the relevant comparisons will include no use of head cooling versus head cooling protocols for selected patient groups. The economic implications of managing traumatic brain injury and stroke using direct brain cooling will be modelled using a discrete event simulation model, rather than a simple cohort simulation model based on a Markov process. The primary justification for the discrete event simulation model is the very different patient sub-groups who undergo direct brain cooling and the variability in baseline characteristics, history, prognosis and expected health outcomes and resource costs. This will allow more realistic modelling of virtual patient histories which are summarised to permit estimates of resource costs and other treatment effects measured in terms of survival and health related quality of life. Discrete event simulation has been used in a wide range of studies examining new health technologies,46 including an investigation of the impact of selective health cooling in a perinatal population. 47
The model will be developed to compare different policies and guidelines for patient selection, choice of technique and clinical setting for head cooling. Comparisons will include: no use of head cooling and scenarios that add a cooling protocol to patient management. Particular attention will be placed on how a cooling protocol could be introduced and scaled up to accommodate a wider range of patients treated in critical care and other clinical units where head cooling is feasible (e.g. acute stroke units).
The short run input parameters into the simulation will be obtained from the systematic overview of the literature on treatment effects following head cooling. These will be combined with generic estimates of key parameters for medium and longer run events for patients following traumatic brain injury and stroke. Trauma and stroke care are both areas of medicine where existing predictive models and the factors influencing survival and health related quality of life are relatively well understood. Our plan is to calibrate the discrete event simulation with information drawn from population based studies of functional outcomes following traumatic brain injury,48 comparisons of outcomes observed for brain injury and stroke patients49 and longitudinal studies of patients with hypoxia of cardiac aetiology.
The introduction of head cooling will have direct and indirect effects on the organisation and use of resources in critical care and other acute hospital settings. The human resources and the equipment needed to monitor, induce and maintain hypothermic patients will be quantified and valued using estimates based on a synthesis of reports derived from published literature and unpublished material related to the costs of equipment acquisition and maintenance. Building on our experience gained in an earlier HTA project where we modelled the costs and effects of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke,50,51 this information will be complemented by expert opinion on the expected resource consequences arising from the effects of head cooling on acute hospital pathways measured in terms of the intensity and duration of care. We will populate the model initially with parameter values based on our knowledge of resource requirements and then validate the base case and distributions using expert concurrence from specialists with a particular interest in and experience of head cooling who we will survey using our network of research contacts. Sensitivity analysis will be conducted using both scenario analysis, allowing sub-sets of model parameters to vary according to key clinical and economic decisions involving the numbers and case-mix of patients who are head cooled, and probabilistic sensitivity analysis for patient level simulation models using the approach of O’Hagen, Stevenson and Madan. 52
With the same caveats as those outlined under subgroup analysis above, our prespecified economic subgroup analyses are:
-
Time from injury to achieving target temperature, e.g. within 6 hours, 12 hours, more than 12 hours.
-
Treatment in specialist unit versus not. Specialist units would include those specialising in neuro or critical care; there is some evidence that they have better outcomes and are more cost effective. 53,54
Research governance
The sponsor for this systematic review is the University of Edinburgh.
Time-chart for major activities (in months)
-
1–14 Searches for published and unpublished studies
-
2–4 Pilot test of inclusion criteria
-
4–14 Inclusion assessments
-
3–4 Pilot test of validity criteria
-
4–14 Validity assessments
-
3–4 Pilot test of data collection
-
4–14 Data collection
-
4–14 Data entry
-
5–16 Missing information
-
14–17 Analysis
-
1–18 Preparation of report
Expertise
Bridget Harris will manage the project, carry out the searches, write to investigators and manufacturers, screen studies for inclusion, extract data, carry out the analyses and write the report. She will be supervised and assisted by Professor Andrews and Professor Murray with whom she has worked and published before in a similar balance of roles.
Professor Andrews will give support with project management, screening of studies for inclusion, extraction of data and assist with writing the report.
Professor Murray will give statistical and methodological advice and support, assist in the event of uncertainty and disagreements over screening of studies and data extraction and with writing the report.
Dr Forbes will supervise the economics analysis by an economics/operations research graduate with experience of decision modelling, and contribute to the report.
We have links with the Cochrane Stroke Group who are based locally and have negotiated access to support from them. We also have links with the Cochrane Injuries Group as Professor Andrews is a reviewer.
Justification of support required
We are asking for salary for Bridget Harris (6 months WTE MH50, 70) and a contribution to time for Professor Andrews (6 weeks WTE), Professor Murray and Dr Forbes (4 weeks WTE each) over the 18 months we expect it will take us to complete the review. We believe this represents good value because we are deploying our time and expertise cost-effectively, with the least expensive member of the team (the lead applicant) having the largest workload but well supervised by the more experienced members with their excellent specialist skills.
The only NHS costs are Professor Andrews’ time which is costed at current Consultant National Rates.
Seven days consultancy costs for members of the Cochrane Stroke Group at £300/day are required for support with searches and conduct of the review (total £2100).
Dr Forbes will supervise the economic analysis with ten days assistance from an economics/operations research graduate with experience of decision modelling at £300/day (total £3000). For this analysis a software license (TreeAge Pro Suite) is required at a cost of £350.
We have estimated £3000 for translation costs. This is based on possibly requiring 6 papers of 3–3500 words each to be translated at a cost of £150/1000 words. It is hard to obtain translation costs without having the papers, but the cost/1000 words is based on discussion with the Cochrane Stroke Group, the School of Literature, Languages and Culture at the University of Edinburgh and commercial translating services.
We have estimated £300 for interlibrary loans and photocopying, although we aim to work electronically whenever possible in order to reduce paper use and avoid adding to greenhouse gas emissions.
Reference list
- Higgins JPT, Green SE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] 2006. URL: http://www.cochrane%20org/resources/handbook/hbook.htm (accessed 2008 Feb. 7).
- The Stroke Association . Resource Sheet 11: Stroke Statistics 2006.
- Department of Health 2007. URL: http://www.dh.gov.uk/en/Policyandguidance/Healthandsocialcaretopics/Stroke/index%20htm (accessed 2007 Aug. 12).
- Tennant A. Admission to hospital following head injury in England: Incidence and socioeconomic associations. BMC Publ 2005;5.
- National Institute for Clinical Excellence . Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults 2007.
- Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000;320:1631-5.
- Mcilvoy LH. The effect of hypothermia and hyperthermia on acute brain injury. AACN Clin Issues 2005;16:488-500.
- Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res 2007;162:201-17.
- Corbett D, Thornhill J. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol 2000;10:145-52.
- Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis 2003;12:163-73.
- Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 2002;19:869-74.
- Castillo J, Davalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke 1998;29:2455-60.
- Hajat C, Hajat S, Sharma P. Effects of post stroke pyrexia on stroke outcome: a meta-analysis of studies in patients. Stroke 2000;31:410-14.
- Leira R, Blanco M, Rodriguez-Yanez M, Flores J, Garcia-Garcia J. Non-pharmacological neuroprotection: role of emergency stroke management. Cerebrovasc Dis 2006;21:89-98.
- Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database Syst Rev 2004. URL: 10%201002/14651858%20CD001048%20pub2.
- Cheung KW, Green RS, Magee KD, Cheung KW, Green RS, Magee KD. Systematic review of randomized controlled trials of therapeutic hypothermia as a neuroprotectant in post cardiac arrest patients. CJEM Can J Emerg Med Care 2006;8:329-37.
- Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007;4.
- den Hertog H, van der Worp B, van Gemert M, Dippel D. Therapeutic hypothermia in acute ischemic stroke. Expert Rev Neurother 2007;7:155-64.
- Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma 2008;25:62-71.
- Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003;108:118-21.
- Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med 2005;33:414-18.
- Cheung KW, Green RS, Magee KD, Cheung KW, Green RS, Magee KD. Systematic review of randomized controlled trials of therapeutic hypothermia as a neuroprotectant in post cardiac arrest patients. CJEM Can J Emerg Med Care 2006;8:329-37.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001;51:275-81.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury (review). N Engl Med 2007;357:1121-35.
- Zhao D, Abella B.S, Beiser DG, Alvarado JP, Wang H, Hamann KJ, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2007.
- Harris BA, Andrews PJD, Mayer SA, Sessler DI. Therapeutic hypothermia. New York: Marcel Dekker; 2005.
- Harris BA, Andrews PJD, Marshall I, Robinson TM, Murray GD. Forced convective head cooling device reduces human cross-sectional brain temperature measured by magnetic resonance: a non-randomized healthy volunteer pilot study. Br J Anaesth 2008;100:365-72.
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663-70.
- The European Stroke Initiative Executive Committee and the EUSI Writing Committee . European Stroke Initiative Recommendations for Stroke Management – Update 2003. Cerebrovasc Dis 2003;16:311-37.
- Brain Trauma Foundation . Management and prognosis of severe traumatic brain injury. J Neurotrauma 2000;17:453-627.
- Johnston NJ, King AT, Protheroe R, Childs C. Body temperature management after severe traumatic brain injury: Methods and protocols used in the United Kingdom and Ireland. Resuscitation 2006;70:254-62.
- Clayton TJ, Nelson RJ, Manara AR. Reduction in mortality from severe head injury following introduction of a protocol for intensive care management. Br J Anaesth 2004;93:761-7.
- Childs C, Hadcock J, Ray A, King AT, Protheroe R. Temperature measurement after severe head injury (letter). Anaesthesia 2004;59:192-3.
- Thompson HJ, Kirkness CJ, Mitchell PH, Webb DJ. Fever management practices of neuroscience nurses: national and regional perspectives. J Neurosci Nurs 2007;39:151-62.
- Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des 2007;13:2310-23.
- Tokutomi T, Morimoto K, Miyagi T, Yamaguchi S, Ishikawa K, Shigemori M. Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery 2003;52:102-11.
- Moran JL, Peter JV, Solomon PJ, Grealy B, Smith T, Ashforth W, et al. Tympanic temperature measurements: are they reliable in the critically ill? A clinical study of measures of agreement. Crit Care Med 2007;35:155-64.
- Harris BA, Andrews PJD, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain-injured, mechanically ventilated patients: a randomized, crossover, factorial trial. Br J Anaesth 2007;98:93-9.
- Meremikwu M, Oyo-Ita A. Cochrane Database Syst Rev. John Wiley & Sons, Ltd Chichester, UK; 2002.
- Correia M, Silva M, Veloso M. Cochrane Database Syst Rev. John Wiley & Sons, Ltd, Chichester, UK; 1999.
- Teasdale GT, Pettigrew LEL, Wilson JTL, Murray G, Jennet B. Analysing outcome of treatment of severe head injury: a review and update on advancing use of the Glasgow outcome scale. J Neurotrauma 1998;15:587-97.
- Edwards D. Stroke Scales and Clinical Assessment Tools. Internet Stroke Center Stroke Trials Registry 2007. URL: http://www.strokecenter.org/trials/scales/.
- Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996;347:422-5.
- Nigwekar SU, Navaneethan SD, Strippoli GF. Cochrane Database Syst Rev Protocols. John Wiley & Sons, Ltd Chichester, UK; 2007.
- Fink ELM, Callaway CW, Tisherman SA, Kochanek PM. Hypothermia in traumatic brain injury (letter). Crit Care Med 2007;35:1999-2000.
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8:1-91.
- Gray J, Geva A, Zheng Z, Zupancic JA. CoolSim: using industrial modeling techniques to examine the impact of selective head cooling in a model of perinatal regionalization. Pediatrics 2008;121:28-36.
- Corrigan JD, Selassie AW, Lineberry LA, Millis SR, Wood KD, Pickelsimer EE, et al. Comparison of the Traumatic Brain Injury (TBI) Model Systems national dataset to a population-based cohort of TBI hospitalizations. Arch Phys Med Rehabil 2007;88.
- Eng JJ, Rowe SJ, McLaren LM. Mobility status during inpatient rehabilitation: a comparison of patients with stroke and traumatic brain injury. Arch Phys Med Rehabil 2002;83.
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS. Health Technol Assess 2002;6:1-112.
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke 2004;35:1490-7.
- O’Hagan A, Stevenson M, Madan J. Monte Carlo probabilistic sensitivity analysis for patient level simulation models: efficient estimation of mean and variance using ANOVA. Health Economics 2007;16:1009-23.
- Mirski MA, Chang CW, Cowan R. Impact of a neuroscience intensive care unit on neurosurgical patient outcomes and cost of care: evidence-based support for an intensivist-directed specialty ICU model of care. J Neurosurg Anesthesiol 2001;13:83-92.
- Stroke Unit Trialists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2007.
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900.
Appendix 3 Search strategies
Major international medical bibliographic databases
MEDLINE 1950 to 12 March 2011 (last update)
Summary of search terms
-
1-8 = cooling/cooling therapies
-
10-15 = head/brain
-
18 = cooling/cooling therapies AND head/brain limited to human
-
19-23 = stroke
-
24-29 = traumatic brain injury
-
30-32 = cardiac arrest
-
33-42 = neonatal hypoxic ischaemic encephalopathy
-
45-47 = citations indexed as neither human nor animal
Running the search to line 18, i.e. cooling/cooling therapies AND head/brain limited to human in MEDLINE 1950 to March Week 3 2010 produced 17,088 results. This was judged too many to manage, so the search was made more specific by including selection terms for stroke, TBI, cardiac arrest and neonatal hypoxic ischaemic encephalopathy.
During the process of developing and refining the MEDLINE search terms, it became apparent that there were some studies that were not indexed as either human or animal, although some were in fact human. Therefore, the final version of the search terms included a strategy to capture these ‘not human not animal’ studies in order that human studies that were not indexed as ‘human’ did not get missed.
Search terms
-
hypothermia/ or hypothermia, induced/ or cryotherapy/
-
cold temperature / or ice/ or refrigeration/ or extreme cold/ or fever/th
-
(hypotherm$ or normotherm$).tw.
-
((low or lower or reduc$) adj5 temperature $).tw.
-
(cool$ or cold or chill$ or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems).tw.
-
(fan or fans or fanned or fanning).tw.
-
(cryother$ or cryogen$ or cryotreat$).tw.
-
(ice or icy or iced or ice-pack or icepack or refrigerat$ or froz$ or freez$).tw.
-
or/1-8
-
exp brain/ or exp head/ or exp skull/
-
(head or crani$ or skull or scalp or face).tw.
-
(brain or intracranial or cerebral or cerebrocranial or cortex or cortical or forebrain or hemispher$).tw.
-
(neck or pharyn$ or nasopharyn$ or naso-pharyn$ or airway$).tw.
-
(intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril$ or naso-oral or nasooral or oro-nasal or oronasal).tw.
-
(hat or helmet or cap or hood or collar).tw.
-
or/10-15
-
9 and 16
-
limit 17 to humans
-
cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp “intracranial embolism and thrombosis”/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/
-
(stroke or poststroke or post-stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
-
((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm)).tw.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
-
or/19-22
-
craniocerebral trauma/ or brain injuries/ or exp brain concussion/ or exp brain hemorrhage, traumatic/ or diffuse axonal injury/ or epilepsy, post-traumatic/
-
coma, post-head injury/ or exp head injuries, closed/ or head injuries, penetrating/ or exp intracranial hemorrhage, traumatic/ or exp skull fractures/ or brain edema/
-
((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).tw.
-
((brain or cerebral or intracranial) adj5 (edema or oedema or swell$)).tw.
-
(TBI or diffuse axonal injur$).tw.
-
or/24-28
-
heart arrest/ or exp heart failure/ or exp cardiopulmonary resuscitation/ or resuscitation/ or heart massage/
-
((cardiac or heart or cardiopulmonary or cardio pulmonary or cardio-pulmonary or circulat$) adj5 (arrest or resuscita$ or massage or life support or reanimat$)).tw.
-
30 or 31
-
Hypoxia-Ischemia, Brain/
-
((brain or cerebral or global) adj (hypox$ or anox$) adj (ischaemi$ or ischemi$)).tw.
-
((hypox$ or anox$) adj (ischaemi$ or ischemi$) adj encephalopath$).tw.
-
hypoxia, brain/ or asphyxia neonatorum/
-
((birth or newborn or encephalopath$) adj5 (asphyxia$ or respiratory failure)).tw.
-
or/33-37
-
Exp infant, newborn/
-
(birth or infant$ or neonat$ or newborn$ or new born$ or perinatal or peri-natal or baby or babies).tw.
-
39 or 40
-
38 and 41
-
23 or 29 or 32 or 42
-
18 and 43
-
17 and (23 or 29 or 32 or 42) [ = everything including human]
-
45 NOT 44
-
46 NOT (humans/ or animals/)
OLDMEDLINE 1948–65
Search date: 4 April 2010.
Search terms
-
hypothermia/ or hypothermia, induced/ or cryotherapy/
-
cold temperature / or ice/ or refrigeration/ or extreme cold/ or fever/th
-
(hypotherm$ or normotherm$).tw.
-
((low or lower or reduc$) adj5 temperature $).tw.
-
(cool$ or cold or chill$ or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems).tw.
-
(fan or fans or fanned or fanning).tw.
-
(cryother$ or cryogen$ or cryotreat$).tw.
-
(ice or icy or iced or ice-pack or icepack or refrigerat$ or froz$ or freez$).tw.
-
or/1-8
-
exp brain/ or exp head/ or exp skull/
-
(head or crani$ or skull or scalp or face).tw.
-
(brain or intracranial or cerebral or cerebrocranial or cortex or cortical or forebrain or hemispher$).tw.
-
(neck or pharyn$ or nasopharyn$ or naso-pharyn$ or airway$).tw.
-
(intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril$ or naso-oral or nasooral or oro-nasal or oronasal).tw.
-
(hat or helmet or cap or hood or collar).tw.
-
or/10-15
-
9 and 16
EMBASE 1980 to 2011 week 10
Last update: 12 March 2011.
The same issue with studies not indexed as either human or animal occurred in EMBASE as in MEDLINE and lines 48–53 in the search were added to capture these.
Search terms
-
hypothermia/ or induced hypothermia/ or profound induced hypothermia/ or chill/ or shivering/ or cryotherapy/ or low temperature / or low temperature procedures/
-
cold/ or cold air/ or cold exposure/ or cold treatment/ or cooling/ or cooling water/ or ice/ or freezing/ or fever/th
-
(hypotherm$ or normotherm$).tw.
-
((low or lower or reduc$) adj5 temperature $).tw.
-
(cool$ or cold or chill$ or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems).tw.
-
(fan or fans or fanned or fanning).tw.
-
(cryother$ or cryogen$ or cryotreat$).tw.
-
(ice or icy or iced or ice-pack or icepack or refrigerat$ or froz$ or freez$).tw.
-
or/1-8
-
exp brain/ or exp head/ or exp skull/ or exp neck/ or exp pharynx/
-
(head or cranium or crani$ or skull or scalp or face).tw.
-
(brain or intracranial or cerebral or cerebrocranial or cortex or cortical or forebrain or hemispher$).tw.
-
(neck or pharyn$ or nasopharyn$ or naso-pharyn$ or airway$).tw.
-
(intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril$ or naso-oral or nasooral or oro-nasal or oronasal).tw.
-
helmet/ or (hat or helmet or cap or hood or collar).tw.
-
or/10-15
-
9 and 16
-
limit 17 to human
-
cerebrovascular disease/ or basal ganglion hemorrhage/ or brain hematoma/ or brain hemorrhage/ or brain infarction/ or brain ischemia/ or carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or intracranial aneurysm/ or occlusive cerebrovascular disease/ or stroke/ or stroke patient/ or stroke unit/
-
(stroke or poststroke or post-stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
-
((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm)).tw.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
-
or/19-22
-
head injury/ or brain injury/ or traumatic brain injury/ or skull injury/ or brain concussion/ or brain contusion/ or brain damage/ or brain stem injury/ or cerebellum injury/ or diffuse axonal injury/
-
exp skull fracture/ or postconcussion syndrome/ or traumatic epilepsy/ or coma/ or exp skull fracture/ or brain edema/
-
((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).tw.
-
((brain or cerebral or intracranial) adj5 (edema or oedema or swell$)).tw.
-
(TBI or diffuse axonal injur$).tw.
-
or/24-28
-
exp heart failure/ or heart arrest/ or resuscitation/ or heart massage/
-
((cardiac or heart or cardiopulmonary or cardio pulmonary or cardio-pulmonary or circulat$) adj5 (arrest or resuscita$ or massage or life support or reanimat$)).tw.
-
30 or 31
-
brain ischemia/ or brain hypoxia/
-
((brain or cerebral or global) adj (hypox$ or anox$) adj (ischaemi$ or ischemi$)).tw.
-
((hypox$ or anox$) adj (ischaemi$ or ischemi$) adj encephalopath$).tw.
-
(encephalopath$ adj5 (asphyxia$ or respiratory failure)).tw.
-
33 or 34 or 35 or 36
-
exp newborn/
-
(birth or infant$ or neonat$ or newborn$ or new born$ or perinatal or peri-natal or baby or babies).tw.
-
38 or 39
-
37 and 40
-
newborn hypoxia/
-
((birth or newborn or new born or neonat$) adj5 (asphyxia$ or hypoxia or respiratory failure)).tw.
-
42 or 43
-
41 or 44
-
23 or 29 or 32 or 45
-
18 and 46
-
17 and 46 [main search not restricted to human]
-
limit 48 to human
-
limit 48 to animals
-
limit 48 to animal studies
-
49 or 50 or 51
-
48 not 52
EMBASE Classic 1947–79
Search date: 13 May 2010.
During development of these search terms it was found necessary to add a strategy to increase removal of animal studies (line 49).
Search terms
-
hypothermia/ or induced hypothermia/ or profound induced hypothermia/ or chill/ or shivering/ or cryotherapy/ or low temperature / or low temperature procedure/
-
cold/ or cold air/ or cold exposure/ or cold treatment/ or cooling/ or cooling water/ or ice/ or freezing/ or fever/th
-
(hypotherm$ or normotherm$).tw.
-
((low or lower or reduc$) adj5 temperature $).tw.
-
(cool$ or cold or chill$ or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems).tw.
-
(fan or fans or fanned or fanning).tw.
-
(cryother$ or cryogen$ or cryotreat$).tw.
-
(ice or icy or iced or ice-pack or icepack or refrigerat$ or froz$ or freez$).tw.
-
or/1-8
-
exp brain/ or exp head/ or exp skull/ or exp neck/ or exp pharynx/
-
(head or cranium or cranial or skull or scalp or face).tw.
-
(brain or intracranial or cerebral or cortex or cortical or forebrain or hemispher$).tw.
-
(neck or pharyn$ or nasopharyn$ or naso-pharyn$ or airway$).tw.
-
(intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril$ or naso-oral or nasooral or oro-nasal or oronasal).tw.
-
helmet/ or (hat or helmet or cap or hood or collar).tw.
-
or/10-15
-
9 and 16
-
limit 17 to human
-
cerebrovascular disease/ or basal ganglion hemorrhage/ or brain hematoma/ or brain hemorrhage/ or brain infarction/ or brain ischemia/ or carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or intracranial aneurysm/ or occlusive cerebrovascular disease/ or stroke/ or stroke patient/ or stroke unit/
-
(stroke or poststroke or post-stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
-
((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm)).tw.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
-
or/19-22
-
head injury/ or brain injury/ or traumatic brain injury/ or skull injury/ or brain concussion/ or brain contusion/ or brain damage/ or brain stem injury/ or cerebellum injury/ or diffuse axonal injury/
-
exp skull fracture/ or postconcussion syndrome/ or traumatic epilepsy/ or coma/ or exp skull fracture/ or brain edema/
-
((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).tw.
-
((brain or cerebral or intracranial) adj5 (edema or oedema or swell$)).tw.
-
(TBI or diffuse axonal injur$).tw.
-
or/24-28
-
exp heart failure/ or heart arrest/ or resuscitation/ or heart massage/
-
((cardiac or heart or cardiopulmonary or cardio pulmonary or cardio-pulmonary or circulat$) adj5 (arrest or resuscita$ or massage or life support or reanimat$)).tw.
-
30 or 31
-
brain ischemia/ or brain hypoxia/
-
((brain or cerebral or global) adj (hypox$ or anox$) adj (ischaemi$ or ischemi$)).tw.
-
((hypox$ or anox$) adj (ischaemi$ or ischemi$) adj encephalopath$).tw.
-
(encephalopath$ adj5 (asphyxia$ or respiratory failure)).tw.
-
33 or 34 or 35 or 36
-
exp newborn/
-
(birth or infant$ or neonat$ or newborn$ or new born$ or perinatal or peri-natal or baby or babies).tw.
-
38 or 39
-
37 and 40
-
newborn hypoxia/
-
((birth or newborn or new born or neonat$) adj5 (asphyxia$ or hypoxia or respiratory failure)).tw.
-
42 or 43
-
41 or 44
-
23 or 29 or 32 or 45
-
18 and 46
-
17 and 46
-
(rat or rats or cat or cats or dog or dogs or gerbil or gerbils or rabbit or rabbits or baboon or baboons).ti.
-
48 not 49
Cumulative Index of Nursing and Allied Healthcare (CINAHL) 1937 to 6 April 2010
This search was conducted through EBSCO therefore no disease terms were included because these make the search too complex for EBSCO.
Search terms
-
S18 S10 and S17
-
S17 S11 or S12 or S13 or S14 or S15 or S16
-
S16 TI ((hat or helmet or cap or hood or collar)) or AB ((hat or helmet or cap or hood or collar))
-
S15 TI ((intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril* or naso-oral or nasooral or oro-nasal or oronasal) ) or AB ( (intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril* or naso-oral or nasooral or oro-nasal or oronasal))
-
S14 TI ((neck or pharyn* or nasopharyn* or naso-pharyn* or airway*)) or AB ((neck or pharyn* or nasopharyn* or naso-pharyn* or airway*))
-
S13 TI ((brain or intracranial or cerebral or cortex or cortical or forebrain or hemispher*)) or AB ((brain or intracranial or cerebral or cortex or cortical or forebrain or hemispher*))
-
S12 TI ((head or cranium or cranial or skull or scalp or face)) or AB ((head or cranium or cranial or skull or scalp or face))
-
S11 (MH “Brain+”) or (MH “Head+”) or (MH “Skull+”)
-
S10 (S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9)
-
S9 TI ((hypotherm* or normotherm*)) or AB ( (hypotherm* or normotherm*))
-
S8 TI ((ice or icy or iced or ice-pack or icepack or refrigerat* or froz* or freez*)) or AB ((ice or icy or iced or ice-pack or icepack or refrigerat* or froz* or freez*))
-
S7 TI ((cryother* or cryogen* or cryotreat*)) or AB ((cryother* or cryogen* or cryotreat*))
-
S6 TI ((fan or fans or fanned or fanning)) or AB ((fan or fans or fanned or fanning))
-
S5 TI ((cool* or cold or chill* or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems)) or AB ((cool* or cold or chill* or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems))
-
S4 TI ((low* N5 temperature *) or (reduc* N5 temperature *)) or AB ((low* N5 temperature *) or (reduc* N5 temperature *))
-
S3 (MH “Shivering”)
-
S2 (MH “Cryotherapy”) or (MH “Cold+”) or (MH “Ice”) or (MH “Refrigeration”) or (MH “Fever/TH”)
-
S1 (MH “Hypothermia”) or (MH “Hypothermia (NANDA)”) or (MH “Hypothermia (Saba CCC)”) or (MH “Hypothermia, Induced”)
British Nursing Index (BNI) and BNI Archive 1985 to May 2010 (last update)
Search terms
-
hypothermia/
-
(hypotherm$ or normotherm$).tw.
-
((low or lower or reduc$) adj5 temperature $).tw.
-
(cool$ or cold or chill$ or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems).tw.
-
(fan or fans or fanned or fanning).tw.
-
(cryother$ or cryogen$ or cryotreat$).tw.
-
(ice or icy or iced or ice-pack or icepack or refrigerat$ or froz$ or freez$).tw.
-
or/1-7
During the development process this search was run with the addition of head terms but in the end the search with hypothermia/cooling terms alone (as above) was used for the review because there was some concern that relevant papers were being missed when head terms were added. However, when the two sets of results were compared it turned out that no trials were missed by including head terms, i.e. the search was sufficiently sensitive, therefore in future both sets of terms could be used, as follows, which will increase specificity:
-
hypothermia/
-
(hypotherm$ or normotherm$).tw.
-
((low or lower or reduc$) adj5 temperature $).tw.
-
(cool$ or cold or chill$ or RapidCool or QuickCool or Rhinochill or Benechill or CoolSystems).tw.
-
(fan or fans or fanned or fanning).tw.
-
(cryother$ or cryogen$ or cryotreat$).tw.
-
(ice or icy or iced or ice-pack or icepack or refrigerat$ or froz$ or freez$).tw.
-
or/1-7
-
(head or cranium or cranial or skull or scalp or face).tw.
-
(brain or intracranial or cerebral or cortex or cortical or forebrain or hemispher$).tw.
-
(neck or pharyn$ or nasopharyn$ or naso-pharyn$ or airway$).tw.
-
(intra-nasal or intranasal or nasal or transnasal or trans-nasal or nose or nostril$ or naso-oral or nasooral or oro-nasal or oronasal).tw.
-
(hat or helmet or cap or hood or collar).tw.
-
or/9-13
-
8 and 14 (set downloaded)
-
8 not 15 (set downloaded)
Web of Science Conference Proceedings Citation Index-Science (CPCI-S) 1990 to 19 July 2010
After some initial investigation the search was confined to conference proceedings and did not include science citations, as these were likely to be found on other databases.
Search terms
Topic (i.e. title, abstract, keywords, authors’ keywords), selected for each search line
Timespan all years
brain same hypotherm* or brain same cool*
Or
head same hypotherm* or head same cool*
Refine by subject area – the following subject areas were included (the number of results in each area are shown, which facilitates the decision on what to include):
-
Clinical neurology
-
Surgery
-
Critical care medicine
-
Neurosciences
-
Cardiac & cardiovascular systems
-
Respiratory system
-
Thermodynamics
-
Engineering, electrical & electronic
-
Emergency Medicine
-
Multidisciplinary sciences
-
Anesthesiology
-
Engineering, biomedical
-
Physiology
-
Medicine, research & experimental
-
Medicine, general & internal
-
Neuroimaging
-
Engineering, multidisciplinary
Zetoc Conference Proceedings
Last update: 8 August 2010.
Search limited to conference proceedings because Journals are covered by MEDLINE and other databases searched.
Searched separately for the following terms in ‘all fields’: brain hypotherm*, head hypotherm*, brain cool*, head cool*
ProQuest Dissertations & Theses (PQDT)
Last update: 25 March 2011.
Search terms: brain OR head AND cooling OR hypothermia in title
The Cochrane Library
Last update: CENTRAL, DARE, HTA, NHS EED 2011 Issue 1.
Last update: CDSR 2011 Issue 3.
Cochrane Central Register of Controlled Trials (CENTRAL)
Cochrane Database of Systematic Reviews (CDSR)
Database of Abstracts of Reviews of Effects (DARE)
Health Technology Assessment (HTA) database
NHS Economic Evaluation Database (EED)
Search terms for CENTRAL (search terms for CDSR, DARE, HTA, EED based on CENTRAL search terms):
-
#1 MeSH descriptor hypothermia this term only
-
#2 MeSH descriptor hypothermia, induced this term only
-
#3 MeSH descriptor cryotherapy this term only
-
#4 MeSH descriptor Cold Temperature this term only
-
#5 MeSH descriptor Ice this term only
-
#6 MeSH descriptor Refrigeration this term only
-
#7 MeSH descriptor Extreme Cold this term only
-
#8 MeSH descriptor Fever this term only with qualifiers: TH
-
#9 MeSH descriptor Shivering this term only
-
#10 (low* in Title, Abstract or Keywords near/6 temperature * in Title, Abstract or Keywords)
-
#11 (reduc* in Title, Abstract or Keywords near/6 temperature * in Title, Abstract or Keywords)
-
#12 (hypotherm* in Title, Abstract or Keywords or normotherm* in Title, Abstract or Keywords)
-
#13 (cool* in Title, Abstract or Keywords or cold in Title, Abstract or Keywords or chill* in Title, Abstract or Keywords or RapidCool in Title, Abstract or Keywords or QuickCool in Title, Abstract or Keywords or Rhinochill in Title, Abstract or Keywords or Benechill in Title, Abstract or Keywords or CoolSystems in Title, Abstract or Keywords)
-
#14 (fan in Title, Abstract or Keywords or fans in Title, Abstract or Keywords or fanned in Title, Abstract or Keywords or fanning in Title, Abstract or Keywords)
-
#15 (cryother* in Title, Abstract or Keywords or cryogen* in Title, Abstract or Keywords or cryotreat* in Title, Abstract or Keywords)
-
#16 (ice in Title, Abstract or Keywords or icy in Title, Abstract or Keywords or iced in Title, Abstract or Keywords or ice-pack in Title, Abstract or Keywords or icepack in Title, Abstract or Keywords or refrigerat* in Title, Abstract or Keywords or froz* in Title, Abstract or Keywords or freez* in Title, Abstract or Keywords)
-
#17 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16)
-
#18 MeSH descriptor Brain explode all trees
-
#19 MeSH descriptor Head explode all trees
-
#20 MeSH descriptor Skull explode all trees
-
#21 (head in Title, Abstract or Keywords or cranium in Title, Abstract or Keywords or cranial in Title, Abstract or Keywords or skull in Title, Abstract or Keywords or scalp in Title, Abstract or Keywords or face in Title, Abstract or Keywords)
-
#22 (brain in Title, Abstract or Keywords or intracranial in Title, Abstract or Keywords or cerebral in Title, Abstract or Keywords or cortex in Title, Abstract or Keywords or cortical in Title, Abstract or Keywords or forebrain in Title, Abstract or Keywords or hemispher* in Title, Abstract or Keywords)
-
#23 (neck in Title, Abstract or Keywords or pharyn* in Title, Abstract or Keywords or nasopharyn* in Title, Abstract or Keywords or naso-pharyn* in Title, Abstract or Keywords or airway* in Title, Abstract or Keywords)
-
#24 (intra-nasal in Title, Abstract or Keywords or intranasal in Title, Abstract or Keywords or nasal in Title, Abstract or Keywords or transnasal in Title, Abstract or Keywords or trans-nasal in Title, Abstract or Keywords or nose in Title, Abstract or Keywords or nostril* in Title, Abstract or Keywords or naso-oral in Title, Abstract or Keywords or nasooral in Title, Abstract or Keywords or oro-nasal in Title, Abstract or Keywords or oronasal in Title, Abstract or Keywords)
-
#25 (hat in Title, Abstract or Keywords or helmet in Title, Abstract or Keywords or cap in Title, Abstract or Keywords or hood in Title, Abstract or Keywords or collar in Title, Abstract or Keywords)
-
#26 (#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25)
-
#27 (#17 and #26)
-
#28 MeSH descriptor cerebrovascular disorders explode all trees
-
#29 (stroke in Title, Abstract or Keywords or poststroke in Title, Abstract or Keywords or post-stroke in Title, Abstract or Keywords or cerebrovasc* in Title, Abstract or Keywords or “brain vasc*” in Title, Abstract or Keywords or “cerebral vasc*” in Title, Abstract or Keywords or cva* in Title, Abstract or Keywords or apoplex* in Title, Abstract or Keywords or SAH in Title, Abstract or Keywords)
-
#30 (brain* in Title, Abstract or Keywords or cerebr* in Title, Abstract or Keywords or cerebell* in Title, Abstract or Keywords or cortical in Title, Abstract or Keywords or vertebrobasilar in Title, Abstract or Keywords or hemispher* in Title, Abstract or Keywords or intracran* in Title, Abstract or Keywords or intracerebral in Title, Abstract or Keywords or infratentorial in Title, Abstract or Keywords or supratentorial in Title, Abstract or Keywords or MCA in Title, Abstract or Keywords or “anterior circulation” in Title, Abstract or Keywords or “posterior circulation” in Title, Abstract or Keywords or “basal ganglia” in Title, Abstract or Keywords)
-
#31 (isch* in Title, Abstract or Keywords or infarct* in Title, Abstract or Keywords or thrombo* in Title, Abstract or Keywords or emboli* in Title, Abstract or Keywords or occlus* in Title, Abstract or Keywords or hypox* in Title, Abstract or Keywords or vasospasm in Title, Abstract or Keywords)
-
#32 (#31 and #31)
-
#33 (brain* in Title, Abstract or Keywords or cerebr* in Title, Abstract or Keywords or cerebell* in Title, Abstract or Keywords or intracerebral in Title, Abstract or Keywords or intracran* in Title, Abstract or Keywords or parenchymal in Title, Abstract or Keywords or intraventricular in Title, Abstract or Keywords or infratentorial in Title, Abstract or Keywords or supratentorial in Title, Abstract or Keywords or “basal gangli*” in Title, Abstract or Keywords or subarachnoid in Title, Abstract or Keywords)
-
#34 (haemorrhage* in Title, Abstract or Keywords or hemorrhage* in Title, Abstract or Keywords or haematoma* in Title, Abstract or Keywords or hematoma* in Title, Abstract or Keywords or bleed* in Title, Abstract or Keywords)
-
#35 (#33 and #34)
-
#36 (#28 or #29 or #32 or #35)
-
#37 MeSH descriptor Craniocerebral Trauma this term only
-
#38 MeSH descriptor Brain Injuries this term only
-
#39 MeSH descriptor brain concussion explode all trees
-
#40 MeSH descriptor Brain Hemorrhage, Traumatic explode all trees
-
#41 MeSH descriptor Diffuse Axonal Injury this term only
-
#42 MeSH descriptor Epilepsy, Post-Traumatic this term only
-
#43 MeSH descriptor Coma, Post-Head Injury this term only
-
#44 MeSH descriptor Head Injuries, Closed explode all trees
-
#45 MeSH descriptor Head Injuries, Penetrating this term only
-
#46 MeSH descriptor Intracranial Hemorrhage, Traumatic explode all trees
-
#47 MeSH descriptor skull fractures explode all trees
-
#48 MeSH descriptor brain edema this term only
-
#49 (head in Title, Abstract or Keywords or crani* in Title, Abstract or Keywords or cerebr* in Title, Abstract or Keywords or capitis in Title, Abstract or Keywords or brain* in Title, Abstract or Keywords or forebrain* in Title, Abstract or Keywords or skull* in Title, Abstract or Keywords or hemispher* in Title, Abstract or Keywords or intra-cran* in Title, Abstract or Keywords or inter-cran* in Title, Abstract or Keywords)
-
#50 (injur* in Title, Abstract or Keywords or trauma* in Title, Abstract or Keywords or damag* in Title, Abstract or Keywords or wound* in Title, Abstract or Keywords or fracture* in Title, Abstract or Keywords or contusion* in Title, Abstract or Keywords)
-
#51 (#49 and #50)
-
#52 (brain in Title, Abstract or Keywords or cerebral in Title, Abstract or Keywords or intracranial in Title, Abstract or Keywords)
-
#53 (edema in Title, Abstract or Keywords or oedema in Title, Abstract or Keywords or swell* in Title, Abstract or Keywords)
-
#54 (#52 and #53)
-
#55 (TBI in Title, Abstract or Keywords or “diffuse axonal injur*” in Title, Abstract or Keywords)
-
#56 (#37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #51 or #54 or #55)
-
#57 MeSH descriptor Heart Arrest this term only
-
#58 MeSH descriptor Heart Failure explode all trees
-
#59 MeSH descriptor Cardiopulmonary Resuscitation explode all trees
-
#60 MeSH descriptor Resuscitation this term only
-
#61 MeSH descriptor Heart Massage this term only
-
#62 (cardiac in Title, Abstract or Keywords or heart in Title, Abstract or Keywords or cardiopulmonary in Title, Abstract or Keywords or “cardio pulmonary” in Title, Abstract or Keywords or cardio-pulmonary in Title, Abstract or Keywords or circulat* in Title, Abstract or Keywords)
-
#63 (arrest in Title, Abstract or Keywords or resuscita* in Title, Abstract or Keywords or massage in Title, Abstract or Keywords or “life support” in Title, Abstract or Keywords or reanimat* in Title, Abstract or Keywords)
-
#64 (#62 and #63)
-
#65 (#57 or #58 or #59 or #60 or #61 or #64)
-
#66 MeSH descriptor Hypoxia-Ischemia, Brain this term only
-
#67 MeSH descriptor Hypoxia, Brain this term only
-
#68 MeSH descriptor Asphyxia Neonatorum this term only
-
#69 (brain in Title, Abstract or Keywords or cerebral in Title, Abstract or Keywords or global in Title, Abstract or Keywords)
-
#70 (hypox* in Title, Abstract or Keywords or anox* in Title, Abstract or Keywords)
-
#71 (ischaemi* in Title, Abstract or Keywords or ischemi* in Title, Abstract or Keywords)
-
#72 (#69 and #70 and #71)
-
#73 (hypox* in Title, Abstract or Keywords or anox* in Title, Abstract or Keywords)
-
#74 (ischaemi* in Title, Abstract or Keywords or ischemi* in Title, Abstract or Keywords)
-
#75 encephalopath* in Title, Abstract or Keywords
-
#76 (#73 and #74 and #75)
-
#77 (birth in Title, Abstract or Keywords or newborn in Title, Abstract or Keywords or encephalopath* in Title, Abstract or Keywords)
-
#78 (asphyxia* in Title, Abstract or Keywords or “respiratory failure” in Title, Abstract or Keywords)
-
#79 (#77 and #78)
-
#80 (#66 or #67 or #68 or #72 or #76 or #79)
-
#81 MeSH descriptor Infant, Newborn explode all trees
-
#82 (birth in Title, Abstract or Keywords or infant* in Title, Abstract or Keywords or neonat* in Title, Abstract or Keywords or newborn* in Title, Abstract or Keywords or “new born*” in Title, Abstract or Keywords or perinatal in Title, Abstract or Keywords or peri-natal in Title, Abstract or Keywords or baby in Title, Abstract or Keywords or babies in Title, Abstract or Keywords)
-
#83 (#81 or #82)
-
#84 (#80 and #83)
-
#85 (#36 or #56 or #65 or #84)
-
#86 (#27 and #85)
Cochrane specialised trials registers
Cochrane Injuries Group
Search date: 14 June 2010.
Search terms: (head or brain or intracranial or cerebral or cerebrocranial or cortex or cortical or forebrain or hemisphere*) and ((Hypotherm* or normotherm* or cryother* or cryogen* or cryotreat*) or ((low or lower* or reduc*) and temperature *))
Cochrane Stroke Group
Search date: 5 May 2010.
Search codes: Search method:1; Stage: Not specified; Condition: Not specified; Intervention type: OTHER; Intervention code: hypothermia
Other trial registers
Last update: 6 March 2011 all registers.
Search terms: hypothermia and cooling (cool* where truncation was allowed); both terms were searched for separately if OR was not an option.
Ongoing trials were only included as relevant if in stroke or TBI. Trials in cardiac arrest and neonatal HIE which had not completed were excluded.
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTR) – this includes:
-
– Australian New Zealand Clinical Trials Registry
-
– Chinese Clinical Trial Registry
-
– Clinical Trials Registry – India
-
– Clinical Research Information Service - Republic of Korea
-
– German Clinical Trials Register
-
– Iranian Registry of Clinical Trials
-
– Japan Primary Registries Network
-
– Pan African Clinical Trial Registry
-
– Sri Lanka Clinical Trials Registry
-
– The Netherlands National Trial Register.
-
– [Note: Each of the databases in WHO ICTR was searched individually because Brenda Thomas (Trials Search Co-ordinator Cochrane Stroke Group) had found that when the whole WHO ICTR was searched there were fewer results than when each element was searched individually.]
-
-
Current Controlled Trials: the meta-register of controlled trials and International Standard Randomised Controlled Trial Number (ISRCTN) register
-
ClinicalTrials.gov
-
National Research Register archive
-
Stroke Trials Registry.
Country-specific databases
Informit Health Collection
As of 1 January 2010 this replaced and includes the Australasian Medical Index
Last update: 6 February 2011.
Search terms: hypothermia OR cool* in title with no limits.
China Academic Journals (CAJ) Medicine and public health (hygiene) database in China Academic Journals
This forms part of the China National Knowledge Database (CNKI). CNKI is considered the Chinese equivalent of PubMed and China Academic Journals (CAJ) is the most comprehensive, full-text database of Chinese journals in the world, starting from 1915.
Last update: 14 January 2011.
Search terms – these were devised to work with head-cooling terms which are compatible with translation from Chinese (including Google Translate):
In title (matching = precise) for:
-
brain cooling or head cooling
-
brain hypothermia or head hypothermia
-
local hypothermia or local cooling
-
focal hypothermia or focal cooling
-
selective hypothermia or selective cooling
-
focal moderate hypothermia or focal moderate hypothermia
-
cerebral hypothermia or cerebral cooling
-
cerebral cryotherapy or brain cryotherapy or head cryotherapy
-
local cryotherapy or focal cryotherapy or selective cryotherapy.
Japan Science and Technology Agency
J-EAST (updating of this database ceased in 2007) through Science Links Japan
Last update: 16 August 2010.
Search terms – four separate searches using respectively: brain cooling, head cooling, brain hypothermia, head hypothermia in title or keywords, no language or date limits.
J-STAGE (Japan Science and Technology Information Aggregator, Electronic)
Last update: 5 February 2011.
Search included journals, proceedings, reports and JST reports. Subject areas: ‘clinical medicine’ and ‘general medicine, social medicine and nursing sciences’. Search terms: head OR brain AND cooling OR hypothermia, no language or date limits.
journal@rchive
Last update: 4 February 2011.
Search terms: brain or head AND cooling or hypothermia in title, no language or date limits.
Latin-American and Caribbean System on Health Sciences Information (LILACS)
Last update: 5 February 2011.
Search terms: hypothermia OR cooling AND brain OR head in title.
Russian Academy of Sciences Bibliographies (coverage 1992 to present)
Accessed through University of Edinburgh portal.
Last update: 25 March 2011.
Search terms: hypothermia or cooling in keywords.
Web search engines
Scirus
Last update: 7 March 2011.
Search terms: hypothermia or cooling in title; subject area: medicine; limited to humans; limited to therapeutic hypothermia (the most relevant of the available options). (Note: human filter let through a number of animal studies.)
Google Scholar
Last update: 26 March 2011.
Search terms: at least one of the words ‘Head cooling’ ‘brain cooling’ anywhere in the article.
Date limit: 2006–11.
Subject areas: biology, life sciences, and environmental science; medicine, pharmacology and veterinary science.
The date limit was set to 2006 onwards because a previous Google Scholar search had been carried out in February 2006. The four papers found on this previous search were already in the search results database, as they had been identified by other searches for this review.
Reference lists
Reference lists of books on hypothermia and of reviews and relevant studies were searched.
Books searched
-
Hayashi N. (editor) Brain hypothermia: pathology, pharmacology and treatment of severe brain injury. Tokyo: Springer-Verlag; 2000.
-
Hayashi N, Bullock R, Dietrich DW, Maekawa T, Tamura A. Hypothermia for acute brain damage: pathomechanism and practical aspects. Conference proceedings of 1st International Brain Hypothermia Symposium. Tokyo: Springer-Verlag; 2004.
-
Hayashi N, Dietrich DW (editors). Brain hypothermia treatment. Tokyo: Springer-Verlag; 2004.
-
Maier CM, Steinberg GK (editors). Hypothermia and cerebral ischemia. New York, NY: Humana Press; 2004.
-
Mayer SA, Sessler DI (editors). Therapeutic hypothermia. New York, NY: Marcel Dekker; 2005.
-
Tisherman SA, Stertz F (editors). Therapeutic hypothermia. New York, NY: Springer; 2005.
Conference proceedings
We searched the proceedings of all three International Hypothermia Symposia (Tokyo 2004, Miami 2007, Lund 2009) and of the Therapeutic Temperature Management Conference (Barcelona 2008).
Writing to investigators and device manufacturers
Investigators and manufacturers of head-cooling devices were written to with varying success. A read receipt was asked for on e-mails but, unfortunately, even when investigators indicated that they had read the e-mail a reply was not necessarily forthcoming. Manufacturers of devices are perhaps understandably reluctant to release details of ongoing human research, although there were notable exceptions, including Benechill, TraumaTec and Paxman.
The language barrier was a considerable problem in communicating with Chinese investigators, mostly rendering it impossible to make contact. Given the amount of research being undertaken in China and the ongoing work on quality improvement with regard to conduct and reporting, it would seem sensible to include someone who can read and write Chinese and understands the subject area as a member of the review team if at all possible. We have plans to do this for the next iteration of the review.
Patent search
A formal patent search was included in the search strategy in the protocol but was not undertaken owing to lack of time. Nevertheless, we had a number of patents on file as a result of ongoing alerts for head cooling information through Google, and this helped with identifying manufacturers of devices to contact.
Appendix 4 Study assessment and data collection form: systematic review of head cooling (version 3)
Appendix 5 References to head-cooling studies
References to studies included in this review
Included traumatic brain injury studies
Harris B. Hypothermia (letter and authors’ response). J Neurosurg 2009;111:1296–7.
Harris OA, Muh CR, Surles MC, Pan Y, Rozycki G, Macleod J, et al. Discrete cerebral hypothermia in the management of traumatic brain injury: a randomized controlled trial. [Erratum appears in J Neurosurg 2009 Jun;110:1322.] J Neurosurg 2009;110:1256–64.
Included stroke studies (ischaemic and haemorrhagic)
Gaida BJ, Yaldizli OO, Mnk S, Muroi C, Mudra R, Fröhlich J. Treatment of resistant fever with local cerebral cooling: P 029. Eur J Anaesthesiol 2008;25:11.
Wang DZ, Wang H, Lanzino G, Rose JA, Honings DS, Rodde MI, et al. Cooling helmet for patients with brain ischemic and hemorrhagic infarctions: the COOL BRAIN-STROKE Trial. The American Stroke Association 28th International Stroke Conference, 13 February 2003, Phoenix, AZ.
Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg 2004;100:272–7.
Wang H, Wang D, Olivero W, Lanzino G, Honings D, Rodde M. Selective brain hypothermia can be achieved with a cooling helmet: preliminary findings of the COOL BRAIN-stroke trial (conference abstract). Stroke 2004;35:293.
Included brain injury studies (with traumatic brain injury and stroke)
Andrews PJ, Harris B, Murray GD. Randomized controlled trial of effects of the airflow through the upper respiratory tract of intubated brain-injured patients on brain temperature and selective brain cooling. Br J Anaesth 2005;94:330–5.
Forte LV, Peluso CM, Prandini MN, Godoy R, Rojas SSO. Regional cooling for reducing brain temperature and intracranial pressure. Arq Neuropsiquiatr 2009;67:480–7.
Harris BA. Heat loss from the upper airways and through the skull: studies of direct brain cooling in humans. PhD Thesis. Edinburgh: University of Edinburgh; 2010.
Harris BA, Andrews PJ, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain-injured, mechanically ventilated patients: a randomized, crossover, factorial trial. Br J Anaesth 2007;98:93–9.
Miller E. Determination of the rate and degree of selective brain cooling in adults with the TraumaTec Neuro-Wrap (abstract P94). J Neurotrauma 2009;26:A25.
Sung G, Torbey M, Abou-Chebl A. Rhinochill: a novel brain hypothermia delivery device. Neurology 2009;72:A75.
Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction via intranasal cooling with the Rhinochill device: preliminary safety data in brain-injured patients. Stroke 2011;42:2164–69.
Included cardiac arrest studies
Andreas J, Losert H, Bayegan K, Haugk M, Arrich J, Krizanac D, et al. Nasal cooling with a new cooling device in patients after cardiac arrest and successful resuscitation. Resuscitation 2008;77:S29.
Busch H-J, Eichwede F, Fodisch M, Taccone FS, Wobker G, Schwab T, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation 2010;81:943–9.
Busch HJ, Janata A, Eichwede F, Fodisch M, Wobker G, Stephan T, et al. Safety and feasibility of a new innovative cooling approach for immediate induction of therapeutic hypothermia in patients after successful resuscitation. trans-nasal cooling after cardiac arrest (abstract P63). Circulation 2008;118:S1459.
Callaway CW, Tadler SC, Katz LM, Lipinski CL, Brader E. Feasibility of external cranial cooling during out-of-hospital cardiac arrest. Resuscitation 2002;52:159–65.
Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010;122:729–36.
Castrén M and PRINCE collaborators. Intra-arrest trans-nasal evaporative cooling:a randomized pre-hospital multicenter study: PRINCE (Pre-ROSC Intra Nasal Cooling Effectiveness). American Heart Association Resuscitation Science Symposium, 15 November, 2009, Orlando, FL.
References to studies excluded from this review
Excluded traumatic brain injury studies
Fang A. The clinical effects of selective hypothermia and decompressive craniectomy on severe traumatic brain injury. Zhejiang J Trauma Surg 2009;14:97–9.
Ioffe I, Sumskii LI. Cranio-cerebral hypothermia in the treatment of patients with cranio-cerebral injuries.] [Russian.] Zh Vopr Neirokhir Im NN Burdenko 1977;1:9–14.
Kang, Yang J, Lisheng L, Wei Y. Selective mild hypothermia therapy on immune function in patients with traumatic brain injury and prognosis. Shandong Med J 2004;44:35–6.
Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res 2006;34:58–64.
Qiu W. A preliminary study on clinical effects of combinating noninvasive selective brain cooling and decompressive craniectomy in severe traumatic brain injury. Proceedings of the International Conference on Recent Advances in Neurotraumatology, Tianjin, China, 19–22 September 2007;45–47.
Qiu W, Shen H, Zhang Y, Wang W, Liu W, Jiang Q, et al. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci 2006;13:995–1000.
Qiu WS, Wang WM, Du HY, Liu WG, Shen H, Shen LF, et al. Thrombocytopenia after therapeutic hypothermia in severe traumatic brain injury. Chin J Traumatol 2006;9:238–41.
Qiu W, Wang Y, Zhou Y, Ru J, Wang W. The clinical effects of selective hypothermia and decompressive craniectomy on severe traumatic brain injury. J Hangzhou Normal Univ 2007;27:10–12.
Zhmurko SF. Cranio-cerebral hypothermia in patients with acute cranio-cerebral injury. [Russian.] Khirurgiia 1971;47:40–3.
Excluded stroke studies (ischaemic and haemorrhagic)
Chen Q, Ou X, Yang Y, Li X, Liu Q. The effect of head hypothermia on the large-acreage cerebral infarction (LCI) and hyperthermia patients serum CORT, SOD and LPO level. Chin J Prim Med 2006;13:20–1.
Dohi K, Jimbo H, Ikeda Y, Matsumoto K. Pharmacological brain cooling (PBC) by indomethacin; a non-selective cyclooxygenase (COX) inhibitor in acute hemorrhagic stroke. Nosotchu 2000;22:429–34.
Dohi K, Jimbo H, Ikeda Y, Fujita S, Ohtaki H, Shioda S, et al. Pharmacological brain cooling with indomethacin in acute hemorrhagic stroke: antiinflammatory cytokines and antioxidative effects. Acta Neurochir 2006;96(Suppl.):57–60.
Dong G, Ou X, Yang Y, Chen Q. The effect of head hypothermia on the hypertensive intracerebral hemorrhage associated with hyperthermia patients serum CORT, SOD and LPO levels. Pract J Med Pharm 2005;22:1057–9.
Feng H, Shi D, Wang D, Xin X, Feng L, Zhang Y, et al. [Effect of local mild hypothermia on treatment of acute intracerebral hemorrhage, a clinical study.] [Chinese.] Chung-Hua i Hsueh Tsa Chih [Chin Med J] 2002;82:1622–4.
Hao Q, Zhang Z-B, Yang Y-F, Liu C-H. Assessment of batroxobin combined with local mild hypothermia in the treatment of cerebral infarction. Chin J Cerebrovasc Dis 2008;5:121–4.
Inoue T, Kimura K, Iguchi Y, Shibazaki K, Matsumoto N, Iwanaga T. Local brain hypothermia with use of cooling hat may improve patients’ outcome in acute severe ischemic stroke. Stroke 2007;38:499.
Li XL, Xia Q, Cheng ZX, Zhang YW, Liu QC [Influence of beginning time of hypothermia treatment on prognosis of extensive cerebral infarction.] [Chinese.] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue/Chinese Critical Care Medicine 2005;17:180–2.
Liu RP, Li DX, Liang JZ, Liu CJ, Chen YF, Wang DX, et al. Nursing of hypothermia in treatment of patients with acute cerebrovascular diseases. Chin J Nurs 1999;34:724–5.
Ou X, Hou S, Yang Y, Chen Q. Research of the treatment time of head hypothermia on hyperthermia after hypertensive intracerebal hemorrhage. Heilongjiang Nurs J 2005;11:342–3.
Shuaib A, Kanthan R, Goplen G, Griebel R, el-Azzouni H, Miyashita H, et al. In-vivo microdialysis study of extracellular glutamate response to temperature variance in subarachnoid hemorrhage. Acta Neurochirurgica Suppl 1996;67:53–8.
Su Z-Q, Wang Y, Zhao Q-J, Sun X-Y, Yang H-Y, Wang D-S. Recent effect of local mild hypothermia for improving neurological deficits in patients with cerebral hemorrhage. Chin Clin Rehabil 2004;8:1816–17.
Takenobu Y, Oe H, Imakita S, Naito H, Naritomi H. Neuroprotective effect of local surface cooling in acute ischemic stroke: P295. [Abstract 30th International Stroke Conference.] Stroke 2005;36:495A.
Tang Y, Zhang Y, Liu W, Wang D. Selective and mild hypothermia in the management of brain injury. J Neurochem 2008;106:65–6.
Wang H, Wang D, Lanzino G, Elkins W, Olivero W. Differential interhemispheric cooling and ICP compartmentalization in a patient with left ICA occlusion. Acta Neurochir 2006;148:681–3.
Wu L, Wang D, Zhou Y, Liu N, Xu S, Hui K, et al. Effect of adhesive dressing local and mild hypothermia treatment for acute stroke. Chin J Immunol Neurol 2010;17:117–19.
Xia Q, Qin S, Li X, et al. Efficacy of minimal injury aspiration in combination with head hypothermia in the treatment of hypertensive intracerebral hemorrhagic cerebral hernia. Chin J Geriatr Cardiovasc Cerebrovasc Dis 2003;5:184–5.
Xia Q, Yan C. Investigation into therapeutic effect of cerebral cryotherapy on large-acreage cerebral infarction. Clin J Med Officer 2004;32:14–15.
Xu LX, Li XL, Zhang XD, Wu XF, Zhang XM. Clinical efficacy of head mild hypothermia in treatment of hypertensive intracerebral hemorrhage. Chin J Geriatr Cardiovasc Cerebrovasc Dis 2002;4:327–9.
Xu E, Qi F, Wang J, Yan J, Pan L, Lu X. The clinical study of selective brain cooling in the treatment of acute cerebral infarction. J Apoplexy Nerv Dis 2003;20:434–6.
Xu L, Han X, Li X, Yan C, Tang S. Effects of head hypothermia on levels of NO, SOD, Glut in serum of patients with hypertensive intracerebral hemorrhage encephalocele. Pract J Med Pharm 2004;21:868–9, 872.
Yamada K, Moriwaki H, Oe H, Yamawaki T, Nagatsuka K, Oomura M, et al. The feasibility and safety of mild brain hypothermia with local surface cooling in acute stroke. Stroke 2004;35:298.
Yang Y, Ou X, Chen Q. A study on time of head hypothermy for large acreage cerebral infarction patients with central high fever. Chin Nurs Res 2006;20:45–6.
Zhang X, Liu X, Li W, Chen R. Effects of local mild hypothermia treatment on plasma neuropeptide Y (NPY), neurotensin (NT), calcitonin gene-related peptide (CGRP) and endotheline (ET) in patients with cerebral hemorrhage. Chin J Emerg Med 2006;15:47–9.
Zhang XM, Li XL, Tang SH, Liu QC. [Effect of head hypothermia on serum inflammatory cytokines levels in patients with hypertensive intracerebral hemorrhage.] [Chinese.] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue/Chin Crit Care Med 2006;18:294–6.
Excluded brain injury studies (with traumatic brain injury and stroke)
Dohi K, Jimbo H, Abe T, Aruga T. Positive selective brain cooling method:a novel, simple, and selective nasopharyngeal brain cooling method. Acta Neurochir Suppl 2006;96:409–12.
Mariak Z. Intracranial temperature recordings in human subjects. The contribution of the neurosurgeon to thermal physiology. J Therm Biol 2002;27:219–28.
Mellergard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery 1992;31:671–7.
Wang W, Jiang Q, Chen J, Lu F, Zhao Z, Wu J. The study on cases of severe brain injury treated with selective brain cooling. J Hangzhou Med Coll 2001;22:198–200.
Yang C, Liu H, Bo W, Wu H, Lang M, Xin X, et al. The effects of selective brain cooling on prevention and treatment of common complications of severe brain injury. Chin J Otorhinolaryngol Skull Base Surg 2006;12:410–13, 417.
Zhao B, Huang H, Zhang G. Treatment of severe brain injury with selective mild hypothermia of brain. J Traum Surg 2003;5:420–2.
Excluded cardiac arrest studies
Busch H-J, Brunner M, Schwab H, Inderbitzen B, Barbut D, Schwab T. Pre-treatment with trans-nasal cooling for the induction of therapeutic hypothermia in patients with cardiac arrest leads to a significant faster achievement of target temperature during systemic cooling (poster). Albert Ludwigs University Freiburg, Department of Cardiology and Angiology, Freiburg, Germany, 2008.
Hachimi-Idrissi S, Huyghens L, Van der Auwera M, Lauwaert I, Van Geffen G, Corne L. Doc, Keep the Head Cool! (abstract no. 301). Annual Meeting of the Society for Academic Emergency Medicine (SAEM). Acad Emerg Med 1999;6:472.
Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001;51:275–81.
Hachimi-Idrissi S, Zizi M, Nguyen DN, Schiettecate J, Ebinger G, Michotte Y, et al. The evolution of serum astroglial S-100 [beta] protein in patients with cardiac arrest treated with mild hypothermia. Resuscitation 2005;64:187–92.
Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest:systematic review and individual patient data meta-analysis. Crit Care Med 2005;33:414–18.
Ikeda K, Kuroki Y, Yosikawa K, Yokoyama T, Utino H. Changes in urinary 8-hydroxy-2-deoxyguanosine in patients with global brain ischemia undergoing brain hypothermia therapy: comparison of whole body and selective head cooling (P330). Crit Care 2007;11:P330.
Nordberg P, Castrén M, Svensson L, Barbut D. New method of intra-arrest trans-nasal cooling in Stockholm: The PRINCE II study. Third International Hypothermia Symposium, 2 September 2009, Lund, Sweden.
Storm C, Schefold JC, Kerner T, Schmidbauer W, Gloza J, Krueger A, et al. Prehospital cooling with hypothermia caps (PreCoCa): a feasibility study. Clin Res Cardiol 2008;97:768–72.
Takeda Y, Fumoto K, Naito H, Morimoto N. Development of a pharyngeal cooling system that enables brain temperature to be immediately reduced. Crit Care Med 2009;37:S250–7.
Wandaller C, Holzer M, Sterz F, Wandaller A, Arrich J, Uray T, et al. Head and neck cooling after cardiac arrest results in lower jugular bulb than esophageal temperature. Am J Emerg Med 2009;27:460–5.
Excluded studies in volunteers
Corbett RJT, Laptook AR. Failure of localized head cooling to reduce brain temperature in adult humans. Neuroreport 1998;9:2721–5.
Covaciu L. Intranasal cooling for cerebral hypothermia treatment. PhD thesis. Sweden: Uppsala University; 2010.
Harris BA, Andrews PJ, Marshall I, Robinson TM, Murray GD. Forced convective head cooling device reduces human cross-sectional brain temperature measured by magnetic resonance: a non-randomized healthy volunteer pilot study. Br J Anaesth 2008;100:365–72.
Kuhnen G, Einer-Jensen N, Tisherman SA. Cooling methods. In Tisherman SA, Sterz F, editors. Therapeutic hypothermia. Springer-Verlag: New York, NY; 2005. pp. 211–33.
Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. J Appl Physiol 1999;87:1609–13.
Mariak Z, White MD, Lyson T, Lewko J. Tympanic temperature reflects intracranial temperature changes in humans. Pflugers Arch 2003;446:279–84.
Shiraki K, Sagawa S, Tajima F, Yokota A, Hashimoto M, Brengelmann GL. Independence of brain and tympanic temperatures in an unanesthetized human. J Appl Physiol 1988;65:482–6.
References to studies in neonatal hypoxic–ischaemic encephalopathy
Akisu M, Huseyinov A, Yalaz M, Cetin H, Kultursay N. Selective head cooling with hypothermia suppresses the generation of platelet-activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostag Leukotr Ess 2003;69:45–50.
Alkharfy TM. A simplified method for head cooling: feasibility and safety. J Neonatal Perinat Med 2010;3:127–34.
Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics 2001;107:480–4.
Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics 2003;111:244–51.
Battin MR, Thoresen M, Robinson E, Polin RA, Edwards AD, Gunn AJ; Cool Cap Trial Group. Does head cooling with mild systemic hypothermia affect requirement for blood pressure support? Pediatrics 2009;123:1031–6.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics 1998;102:885–92.
Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R, et al. ; CoolCap Study Group. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr 2008;152:55–8.
Horn AR, Woods DL, Thompson C, Eis I, Kroon M. Selective cerebral hypothermia for post-hypoxic neuroprotection in neonates using a solid ice cap. S Afr Med J 2006;96:976–81.
Kilani RA. The safety and practicality of selective head cooling in asphyxiated human newborn infants, a retrospective study. J Med Liban 2002;50:17–22.
Kumazawa K, Ibara S, Kobayashi K, Tokuhisa T, Maruyama H, Maede Y, et al. Changes of blood glutamate levels in hypoxic ischemic encephalopathy patients undergoing brain hypothermia. In Hayashi N, Bullock R, Dietrich DW, Maekawa T, Tamura A, editors. Hypothermia for Acute Brain Damage: Pathomechanism and Practical Aspects. Tokyo: Springer Verlag; 2004. pp. 320–4.
Lin ZL, Yu HM, Lin J, Chen SQ, Liang ZQ, Zhang ZY. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia:an experience from a single neonatal intensive care unit. J Perinatol 2006;26:180–4.
Liu C-Q, Xia Y-F, Yuan Y-X, Li L, Qiu X-L. Effects of selective head cooling with mild hypothermia on serum levels of caspase-3 and IL-18 in neonates with hypoxic-ischemic encephalopathy. Chin J Contemp Pediatr 2010;12:690–2.
Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005;116:1001–6.
Sarkar S, Barks JD, Bhagat I, Dechert R, Donn SM. Pulmonary dysfunction and therapeutic hypothermia in asphyxiated newborns:whole body versus selective head cooling. Am J Perinatol 2009;26:265–70.
Sarkar S, Barks JD, Bhagat I, Donn SM. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol 2009;29:558–63.
Shao XM, Zhou WH. Efficacy and safety of selective head cooling with mild systemic hypothermia after neonatal hypoxic ischemic encephalopathy. Hot Topics in Neonatology, 3 December 2005, Washington, DC.
Simbruner G, Haberl C, Harrison V, Linley L, Willeitner AE. Induced brain hypothermia in asphyxiated human newborn infants:a retrospective chart analysis of physiological and adverse effects. Intensive Care Med 1999;25:1111–17.
Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 2000;106:92–9.
Whitelaw A, Thoresen M. Clinical experience with therapeutic hypothermia in asphyxiated infants. Dev Med Child Neurol Suppl 2001;86:30–1.
Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, et al. ; CoolCap Study Group. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119:912–21.
Zhou W-H. Safety study of hypothermia for treatment of hypoxic-ischemic brain damage in term neonates. Acta Pharmacol Sin 2002;23:64–8.
Zhou W-H, Cheng G, Shao X, Liu X, Shan R, Zhuang D, et al. ; China Study Group. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010;157:367–72.
Reviews in neonatal hypoxic–ischaemic encephalopathy
Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363.
Jacobs SE, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007;4:CD003311.
Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy: are we there yet? BMC Pediatr 2007;7:30.
Shah PS. Hypothermia:a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010;15:238–46.
References to studies awaiting assessment
We have been unable to obtain the papers for the first three of these and have had no response to requests for further information for the other two (see Appendix 6 for further details).
Bunatyan AA, Zolnikov SM, Smirnov OA. [Present day problems of anesthesiology and recovery of consciousness.] [Russian.] L’vov: 1969. p.294.
Bunatyan AA, Zolnikov SM, Smirnov OA. Fourth International Symposium on Anesthesiology, 1969, Varna, Bulgaria, p. 503.
Ioffe YS, Smirnov OA. In Comatose states following cranio-cerebral trauma. Moscow; 1969. p. 126.
Skulec R, Truhlar A, Knor J, Seblova J, Cerny V. The practice of therapeutic mild hypothermia in cardiac arrest survivors in the Czech republic. Minerva Anestesiol 2010;76:617–23.
Skulec R, Truhlar A, Ostadal P, Telekes P, Knor J, Tichacek M, et al. [Current cooling methods for induction of mild hypothermia in cardiac arrest survivors.] Vnitrni Lekarstvi 2009;55:1060–9.
References to ongoing studies
Stroke
i-Cool (Induction of Cooling) Pilot: a randomised trial comparing three methods for rapid induction of therapeutic hypothermia in stroke patients:
-
start date: 2010
-
Dr Sven Poli.
The Cerebral Hypothermia in Ischaemic Lesion (CHIL) Trial: a randomised trial evaluating systemic and local mild hypothermia on infarct expansion and salvage of the ischaemic penumbra in acute ischaemic stroke (ACTRN12609000690257):
-
start date: 2009
-
Professor Christopher Levi.
Multiple Interventions for Neuroprotection Utilizing Thermal Regulation in the Emergent Treatment of Stroke (MINUTES) study:
-
start date: 2006
-
Dr Muzaffar Siddiqui
-
Siddiqui MM, Ludwig Y, Hussain MS, Manawadu D, Mateer A, Beaulieu C, Saqqur M, Shuaib A. Multiple interventions for neuroprotection utilizing thermal regulation in the emergent treatment of stroke:the MINUTES study. Int J Stroke 2008;3:140. (abstract PO01–195).
Emergency room trial of brain cooling in stroke with the Rhinochill device:
-
Dr Denise Barbut
-
not started yet.
Study of brain cooling in stroke with the DigniCap:
-
Martin Waleij, CEO Dignitana www.dignitana.com/
-
start date: ?
-
awaiting further details.
Brain injury
Determination of the rate and degree of selective brain cooling in adults with the TraumaTec Neuro-Wrap®:
-
started: 2009–10
-
Miller E. Determination of the rate and degree of selective brain cooling in adults with the TraumaTec Neuro-Wrap (abstract P94). J Neurotrauma 2009;26:A25.
Brain Cooling Study, Carle Foundation Hospital, Urbana, IL:
-
Dr Huan Wang
-
start date: June 2011
-
awaiting further details
Trials of QuickCool nasal cooling balloons:
-
‘QuickCool is currently enrolling patients in clinical trials in Sweden and Denmark. These studies investigate the safety and efficacy of the novel QuickCool Intranasal Brain Cooling System in the following clinical areas: cardiac arrest, traumatic brain injury and subarachnoid hemorrhage.’ www.quickcool.se//Contents.asp?id%20=%20358 (accessed 8 May 2011).
References to other applications of head cooling
Bagic A, Theodore WH, Bonwetsch R, Greenfield J, Sato S. Treating epilepsy with head-neck cooling without sedation. Epilepsia 2005;46:233.
Dougherty L. Comparing methods to prevent chemotherapy-induced alopecia. Cancer Nurs Pract 2006;5:31.
Hato N, Hyodo J, Takeda S, Takagi D, Okada M, Hakuba N, et al. Local hypothermia in the treatment of idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx 2010;37:626–30.
Kramer BA, Kadar AG, Clark K. Use of the Neuro-Wrap system for severe post-electroconvulsive therapy headaches. J ECT 2008;24:152–5.
Macduff C, Mackenzie T, Hutcheon A, Melville L, Archibald H. The effectiveness of scalp cooling in preventing alopecia for patients receiving epirubicin and docetaxel. Eur J Cancer Care 2003;12:154–61.
Massey CS. A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs 2004;8:121–30.
Reynolds, L. The effects of head and neck cooling on symptoms of multiple sclerosis. Thesis. Halifax, NS: Dalhousie University; 2007.
Simmons SE, Saxby BK, McGlone FP, Jones DA. The effect of passive heating and head cooling on perception, cardiovascular function and cognitive performance in the heat. Eur J Appl Physiol 2008;104:271–80.
Wickwire PJ, Bishop PA, Green JM, Richardson MT, Lomax RG, Casaru C, et al. Physiological and comfort effects of a commercial ‘cooling cap’ worn under protective helmets. J Occup Environ Hyg 2009;6:455–9.
References to historical reports of head cooling
Bukov VA, Bobkov IG, Smirnov OA, Zol’nikov SM. [The relationship between the temperature of various regions of the brain and the body in craniocerebral hypothermia in clinical practice.] Khirurgiia (Mosk) 1967;43:14–21.
Ioffe I, Sumskii LI. [Cranio-cerebral hypothermia in the treatment of patients with cranio-cerebral injuries.] [Russian.] Zh Vopr Neirokhir Im NN Burdenko 1977;1:9–14.
Ivanov VV, Kolenko EA. [Local and cerebral hypothermia using thermoelectric apparatus in severe craniocerebral trauma.] [Russian.] Vestn Khir Im II Grek 1969;102:105–7.
Lojka J, Kravcova V, Kovacikova E, Gajdosova D, Nadvornik P. [1st experiences with craniocerebral hypothermia.] [Slovak.] Rozhl Chir 1973;52:812–16.
Phelps C. Traumatic injuries of the brain and its membranes. In The classics of neurology and neurosurgery library. New York, NY: Appleton and Company; 1897. pp. 223–4.
Schlag G. Cardiac arrest and resuscitation. Zbl Chir 1962;87:1273–90.
Smirnov O, Meshcherinov I. Analytical method for determination of temperature distribution in the human head during craniocerebral hypothermia induced by ‘Kholod-2F’ apparatus. Biomed Eng 1970;4:192–7.
Ugriumov VM, Zotov I, Bezukh MS. [Cranio-cerebral hypothermia and neuro-vegetative blockade in the systematic treatment of injuries to the skull and brain.] [Russian.] ZhVopr Neirokhir 1975;6:11–17.
Waugh OS. Acute cranial-cerebral injuries. Can Med Assoc J 1926;16:1475–9.
Zhmurko SF. [Cranio-cerebral hypothermia in patients with acute cranio-cerebral injury.] [Russian.] Khirurgiia 1971;47:40–3.
Appendix 6 Characteristics of studies
Contents
-
Characteristics of included studies:
-
– traumatic brain injury
-
– stroke – ischaemic, haemorrhagic, mixed
-
– brain injury – heterogeneous cerebral problems including TBI and stroke
-
– cardiac arrest
-
-
Characteristics of excluded studies:
-
– traumatic brain injury
-
– stroke – ischaemic, haemorrhagic, mixed
-
– brain injury – heterogeneous
-
– cardiac arrest
-
– studies in volunteers
-
-
Studies awaiting assessment:
-
– randomised controlled trials
-
– other studies
-
-
Characteristics of ongoing studies:
-
– stroke
-
– brain injury.
-
Within these headings, papers are listed in date (oldest first) and then alphabetical order. The full reference details for all the papers in this appendix can be found in Appendix 5.
Studies in neonatal HIE were included in the report only if they provided information on complications/adverse effects/advantages of head cooling and are not included in Characteristics of studies. The studies are listed in Appendix 5 (see References to studies in neonatal hypoxic–ischaemic encephalopathy).
Characteristics of included studies
Randomised controlled trials: traumatic brain injury
Harris and colleagues 2009,45 Harris 2009161
| Methods | RCT to evaluate the Discrete Cerebral Hypothermia head-cooling system in the management of TBI |
| Participants |
Total n = 25, age mean ± SD cooled 38.1 (± 15) (one missing data), control patients 33.2 (± 20), age range 18–90 years, 22 male TBI GCS ≤ 8 Participating sites: Level 1 trauma centre Multicentre: No – single US site Language: English Allocation concealment: ‘Blindly randomized’ – computer-generated random numbers determined by Department of Biostatistics ‘assigned to each patient based on their order in the study and GCS score on initial assessment [severe (5–8) (n = 18) vs critical (3–4) (n = 7)], to allow for block randomization and to provide an initial balance in severity between the 2 groups’ p. 1258 Outcome assessor blind: Not reported Data analysis blinded: Not reported Intention to treat: Yes, for as long as they contributed data (see Follow-up, below) Groups comparable: The cooled group spent less time in the Emergency Department before enrolment. Four cooled had craniotomy vs one control patient Follow-up complete: No – complete data available for 21/25 patients, 11 cooled, 10 control patients. Two patients withdrawn by families, one ICP monitor dislodged, one incomplete data acquisition owing to unreliable systemic temperature measurement Temperature measurement sites: Intracranial: cooled group – two parenchymal, 10 ventricular; control patients – one parenchymal, 12 ventricular; bladder |
| Interventions |
Head and neck cooling, head not shaved, water circulating device (Discrete Cerebral Hypothermia System) set to maximum cooling (?temp), pressurised to 15 mmHg, with active body warming to bladder temperature 36 °C (‘to avoid systemic hypothermia’) (n = 12) vs no head cooling – temperature management if any in this group (e.g. aim of normothermia) is not reported (n = 13). Treatment of both groups ‘in accordance with the Brain Trauma Foundation’s (BTFs) Guidelines for the Management of Severe Traumatic Brain Injury’ p. 1258 (BTF guidelines. J Neurotrauma 2007;24:S37–44). (Note these contain nothing on temperature management apart from induced hypothermia so do not explain control group temperature management) Time from injury to start of cooling: Within 48 hours of hospital admission Time from start of cooling to target: Within 24 hours of cooling period Target temperature: 33 °C intracranial, 2 of 11 patients with complete temperature data achieved target Duration of cooling: 24 hours Rewarming: Controlled rewarming 0.5 °C every 3 hours over 24 hours, monitoring continued to 72 hours from start of cooling |
| Outcomes |
1. Effectiveness of the cooling cap in reducing intracranial temperature and establishing a core body/brain temperature gradient (36 °C body/33 °C brain) 2. Mortality, GOS and FIM at days 1, 2, 3, 7, 14, 21 and 28, and at hospital discharge if this was prior to 1 month Result 1 Baseline (estimated) intracranial temperature in the treatment group: 37.9 °C (95% CI 37.4 °C to 38.5 °C). After 12 hours cooling mean intracranial temperature 36.8 °C (95% CI 36.1 °C to 37.5 °C). At 24 hours, 36.9 °C Baseline mean intracranial temperature in the control group: 37.9 °C (95% CI 37.6 °C to 38.2 °C), after 12 hours 37.9 °C (95% CI 37.5 °C to 38.3 °C), at 24 hours 38.1 °C Mean difference between intracranial and bladder temperature for 12-hour intervention period was –0.67 °C (p = 0.07) for the treatment group and 0.05 °C (p = 0.67) for the control patients. ‘This showed a trend toward a greater temperature gradient in the treatment group than in the control patients However, the cooling cap neither established nor maintained a significant cranial-bladder temperature gradient’ Result 2 ‘Six (50.0%) of 12 patients in the treatment group and 4 (30.8%) of 13 in the control group died (p = 0.43). The medians of the maximum change in GOS and FIM scores during the study period (28 days) for both groups were 0. There was no significant difference in complications between the groups (p-value range 0.20–1.0)’ Complications respiratory failure, shock, septicaemia, decubitus ulcer, cardiac arrest but no significant difference between groups. Patients were checked every 12 hours for cold damage to skin while cooling cap was in situ Two patients had decubitus ulcers, both in cooled group – ulcer location not reported |
| Notes |
Unclear whether or not the analysis plan was prespecified and no power calculation. There was no significant difference in any of the outcome measures but lack of information regarding blinded follow-up excluded the outcome data from formal analysis. However, the primary purpose of this study was feasibility not assessment of outcome and the temperature data are suitable for inclusion in the review, with the caveat regarding why baseline intracranial temperature in cooled group was ‘estimated’ and how? Awaiting response from investigator regarding blinded outcome analysis, estimated baseline brain temperature, baseline and 12-hour bladder temperatures for both groups and whether or not decubitus ulcers were device related |
Randomised controlled trials: stroke (ischaemic, haemorrhagic, mixed)
None.
Randomised controlled trials: brain Injury
Andrews and colleagues 2005,46 Harris 201057
| Methods | Crossover RCT of the effect of nasal airflow on intracranial and oesophageal temperature |
| Participants |
Total n = 15, mean age 43 (range 17–70) years, 9 female TBI n = 9; SAH n = 6 Participating sites: Neurological ICU Multicentre: No – single UK site Language: English Allocation concealment: Sealed, opaque envelopes provided by the trial statistician, opened during baseline period Outcome assessor blind: No Data analysis blinded: No, primary outcome analysis was prespecified Intention to treat: Yes Groups comparable: Within patient comparison, i.e. crossover trial Follow-up complete: Yes Temperature measurement sites: Intracranial (parenchyma), oesophageal |
| Interventions |
Thirty-minute baseline, randomised to 6 hours of airflow or 6 hours of no airflow then crossed over for further 6 hours. Airflow continuous through both nostrils, at total rate of 115 ml/kg/minute (commensurate with normal minute volume), range 6–13 l Time from injury to start of cooling: 0–5 days but proof of concept of temperature reduction, not for neuroprotection Time from start of cooling to target: N/A, proof of concept Target temperature : N/A, proof of concept Duration of cooling: 6 hours Rewarming: Passive (airflow stopped) |
| Outcomes |
Primary (prespecified): Within-patient change in mean intracranial temperature over 6-hour nasal airflow compared with 6 hours with no airflow Result: Mean –0.13 °C, SD 0.55 °C, 95% CI –0.43 °C to 0.17 °C. Range of temperature change: +0.55 °C to –0.9 °C Secondary (exploratory): Difference between mean brain temperatures over last 5 minutes before airflow started and last 5 minutes of the first half hour with airflow Result: Mean –0.04 °C, SD 0.16 °C, 95% CI –0.13 to 0.04 °C. Range of temperature change: +0.18 °C to –0.52 °C |
| Notes |
The published paper (Andrews and colleagues 2005) contains an error in the temperature data (minus signs were omitted) and therefore the results reported in Harris 2010 are used in the review. Harris 2010 also supplied detailed information on methods. The patients were orally intubated and ventilated and the purpose of the study was to see if flowing air through their noses would reduce intracranial temperature (proof of concept) |
Harris and colleagues 2007,47 Harris 201057
| Methods | Randomised controlled crossover factorial trial of the effect of enhanced nasal airflow and bilateral head fanning on intracranial and oesophageal temperature |
| Participants |
Total n = 12, mean age 43 (range 20–67) years, 6 female TBI n = 8, SAH n = 4 Participating sites: Neurological ICU Multicentre: No – single UK site Language: English Allocation concealment: Sealed, opaque envelopes provided by the trial statistician, opened during baseline period Outcome assessor blind: No Data analysis blinded: No, primary outcome analysis was prespecified Intention to treat: Yes Groups comparable: Within-patient comparison, i.e. crossover trial Follow-up complete: 1 of 12 lost to follow-up at 6 months Temperature measurement sites: Intracranial (parenchyma); oesophageal |
| Interventions |
30-minute baseline, each of four interventions in random order: (1) enhanced nasal airflow; (2) head fanning (no head bandages); (3) 1 + 2; (4) no intervention. [(1) = continuous unhumidified airflow through both nostrils at twice the patient’s ventilated minute volume + 20 ppm. nitric oxide; (2) = bilateral head fanning with ambient air, total air speed approximately 8 m/second-1] Time from injury to start of cooling: 0–4 days but proof of concept of temperature reduction, not for neuroprotection Time from start of cooling to target: N/A, proof of concept Target temperature: N/A, proof of concept Duration of cooling: Thirty minutes per intervention Rewarming: Passive (airflow stopped) |
| Outcomes |
Primary (prespecified): Within-patient comparison of each patient’s mean brain temperature for the last 5 minutes of each intervention with the last 5 minutes of the preceding washout Result: Difference in mean brain temperature over the last 5 minutes of preceding washout minus mean over the last 5 minutes of intervention = 0.15 °C with nasal airflow (p = 0.001, 95% CI 0.06 °C to 0.23 °C) and 0.26 °C with head fanning (p < 0.001, 95% CI 0.17 °C to 0.34 °C). Estimate of combined effect of airflow and fanning on brain temperature was 0.41 °C Difference in mean oesophageal temperature over last 5 minutes of preceding washout minus the mean over the last 5 minutes of intervention = 0.13 °C with nasal airflow (p = 0.005, 95% CI 0.04 °C to 0.21 °C) and 0.19 °C with head fanning (p < 0.001, 95% CI 0.11 °C to 0.28 °C). Estimate of combined effect of airflow and fanning on temperature was 0.32 °C |
| Notes | The patients were orally intubated and ventilated, and the purpose of the study was to see if the cooling interventions would reduce intracranial temperature (proof of concept) |
Randomised controlled trials: cardiac arrest
Pre-ROSC Intra-Nasal Cooling Effectiveness (PRINCE) trial
Castrén and colleagues 201049 (main study report); Castrén and colleagues 200960 (conference abstract); http://clinicaltrials.gov/ct2/show/NCT00808236 (trials registration entry)
| Methods | RCT of pre-hospital intra-arrest cooling with the Rhinochill device |
| Participants |
Total n = 194; mean age 66.1 years, 67 male cooled group; mean age 64.2 years, 79 male control patients Out-of-hospital witnessed cardiac arrest, with CPR initiated by Emergency Medical Service within 20 minutes of collapse, no organised rhythm or palpable pulse (i.e. no ROSC) by the time the airway was secured Participating sites: Emergency Medical Service in 15 sites in five European countries, all sites had a pre-hospital physician service Multicentre: Yes Language: English Allocation concealment: Numbered, sealed envelopes Outcome assessor blind: Intended but not necessarily achieved Data analysis blinded: Not reported Intention to treat: Yes Groups comparable: Yes Follow-up complete: No, three in each group had no outcome data Temperature measurement sites: Infrared tympanic temperature pre-hospital and on admission then core temperature rectal (60%), bladder (35%), intravascular (5%) |
| Interventions | Intra-arrest intranasal cooling with Rhinochill device until transition to systemic cooling in hospital, median cooling duration 32 minutes (IQR 21–60 minutes) (n = 93) vs no cooling (n = 101). Target temperature 34 °C |
| Outcomes |
Primary: ROSC rate Secondary: Survival to hospital discharge (but not powered to detect outcome differences), 24-hour SAE rate Temperature results: Median time to target temperature (core) of 34 °C in the treatment group was 155 minutes (IQR 124–315 minutes) vs 284 minutes (IQR 172–471 minutes) in control patients. The mean core temperature was significantly lower in treated patients when measured after hospital arrival: 35.1 °C (SD ± 1.3 °C) vs 35.8 °C (SD ± 0.9 °C), p = 0.01 Rates of survival: 31% control patients, 43.8% cooled group (p = 0.04, RR = 1.9) Adverse events: Nasal whitening 13 (14%) of 93 patients during nasal cooling, resolved spontaneously in all five resuscitated patients. No relationship between longer duration of treatment and nasal discoloration. Epistaxis: three patients (3.2%), serious in one patient with an underlying coagulopathy secondary to hepatic failure. This was the only device-related SAE. Periorbital emphysema occurred 75 minutes into treatment in one patient and resolved spontaneously within 24 hours. The total number of SAEs that occurred within 7 days was seven in the treatment group and 14 in the control group |
Callaway and colleagues 200248
| Methods | RCT of pre-hospital intra-arrest head cooling |
| Participants |
Total n = 27, but five cooled patients were excluded because temperature measurements could not be completed, mean 68 ± 15 years hypothermia group; mean 80 ± 10 years control group; 18 male Out-of-hospital cardiac arrest, convenience sample Participating sites: City of Pittsburgh Emergency Medical Services Multicentre: No Language: English Allocation concealment: Not reported Outcome assessor blind: No Intention to treat: No, five subjects with incomplete temperature measurements were excluded from analysis Groups comparable: Unclear Follow-up complete: Yes (all patients died in hospital) Temperature measurement sites: Nasopharyngeal, tympanic, oesophageal |
| Interventions | Pre-hospital head cooling with three 500-ml bags of ice round the head plus one bag over the neck during CPR after cardiac arrest (n = 9) vs normothermia subjects receiving usual care but without cooling (n = 13). Temperature measurements every 5 minutes for 15 minutes until ROSC or discontinuation of resuscitation. Cooling was discontinued if core temperature reduced below 34 °C or if spontaneous circulation was restored for at least 5 minutes |
| Outcomes |
Reduction in temperature, in-hospital mortality, adverse events/complications Temperature results: Baseline oesophageal temperatures: 35.5 ± 1.0 °C cooled group, 35.3 ± 1.7 °C normothermia [sic] group, i.e. patients were already cool. Temperatures were actually measured for 5–30 minutes in each group The mean rate of change (± SD) of oesophageal temperature did not differ between hypothermia (–0.07 ± 0.06 °C/minute; 95% CI –0.11 to –0.03) and normothermia (–0.02 ± 0.06 °C/minute; 95% CI –0.05 to 0.02) groups Outcome at hospital discharge: No patient survived to hospital discharge Adverse events/complications: No problems from use of ice bags for cooling except difficulty of securing them round the head for transport |
| Notes |
Convenience sample who were randomised to cooling and control group, method of randomisation not reported, not blinded, five subjects were excluded from analysis because serial temperature measurements could not be completed. Control group is described as ‘normothermia’ group, although mean baseline temperature in both groups was below normal and did not change Probably not reasonable to expect ice bags to reduce temperature within 5–30 minutes |
Other studies (not randomised controlled trials): traumatic brain injury
None.
Other studies (not randomised controlled trials): stroke (ischaemic, haemorrhagic, mixed)
COOL BRAIN-stroke trial
Wang and colleagues 200361 (conference abstract), Wang and colleagues 200462 (conference abstract); Wang and colleagues 200450 (main study report); www.strokecenter.org/trials/TrialDetail.aspx?tid%20=%20473 (Stroke Trials Registry entry)
| Methods | Prospective, non-randomised pilot study (according to Stroke Trials Registry entry) of the effectiveness of a head-cooling device in reducing brain temperature |
| Participants |
Total n = 14 of whom eight were cooled; age and gender not reported Acute ischaemic or haemorrhagic stroke (+ ≥ 1 TBI in main report) Participating sites: single Neuro ICU, USA Language: English Follow-up complete: Follow-up not reported Temperature measurement sites: Intracranial (parenchymal), bladder |
| Interventions |
Head and neck cooling with water-circulating cooling helmet, head shaved, body warming to maintain bladder temperature > 33 °C, > 35 °C if age > 45 years + ‘standard’ stroke care (n = 8); ‘control patients’ had ‘standard’ stroke care, no information about temperature management (n = 6) Time from injury to start of cooling: Within 24 hours of admission Time from start of cooling to target: ‘Mean of 3.4 hours (range 2–6 hours) to achieve a brain temperature < 34 °C’ Target temperature: Brain not stated but probably ≤ 34 °C; bladder 33–35 °C Duration of cooling: Unclear but helmet in situ for up to 72 hours Rewarming: ‘Mean 0.63°C/hour (range 0.15–1.45°C/hour) passive rewarming rate was observed’ |
| Outcomes |
Change in intracranial temperature (0.8 cm below cortical surface) minus bladder temperature, cooling rate, complications (The trials registry entry included NIHSS, mRS, BI and mortality as outcomes but these were not reported) Temperature results in cooled patients: Mean overall brain–bladder temperature change –1.6 °C Mean brain temperature reduction of 1.84 °C (range 0.9–2.4 °C) within 1 hour. Brain temperature < 34 °C in mean 3.4 hours, bladder temperature < 36 °C in mean 6.67 hours |
| Notes |
There are discrepancies and omissions in the reports of this trial According to the Stroke Trials Registry entry, the paper in the Journal of Neurosurgery is the main published report on the COOL BRAIN-stroke trial, although the trial is not referred to by name in the paper (Wang and colleagues 2004). That report of the trial includes at least one patient with TBI in addition to stroke patients, says the trial was randomised and includes a ‘control’ group but no information on the comparability of the groups at baseline (Wang and colleagues 2004). However, a published abstract (Wang and colleagues 2003), the preliminary report (Wang and colleagues 2004) and the completed entry in the Stroke Trials Registry (which cites the published abstract and the Journal of Neurosurgery paper as the publications) state that the study included only stroke patients and was not randomised. The results in the completed entry in the Stroke Trials Registry are the same as those given in the Journal of Neurosurgery paper (Wang and colleagues 2004), with the addition of the following: ‘There were no serious complications or adverse events. Efficacy data (NIHSS, Rankin) was not published’. The source of the results is given as the two cited publications and ‘correspondence with the trial co-ordinator’ It is not reported how long patients were cooled for. According to the Stroke Trials Registry the helmet was to remain in situ for 72 hours. Brain temperature was monitored for ‘a mean of 48–72 hours’ (Wang and colleagues 2004a). Data are shown for an ‘illustrative case’ with TBI and this patient appears to have been cooled for 24 hours and the helmet removed at 48 hours Apart from the illustrative case there is no information on age, gender, number with stroke vs TBI in cooled group or ‘control patients’, i.e. the comparability of the groups is unknown The preliminary report (Wang and colleagues 2004) includes six cooled stroke patients and the temperature results are based on 300 data hours. The main report (Wang and colleagues 2004) includes eight cooled patients and the temperature results are based on 277 data hours. There are no data on baseline temperature and no explanation of how the brain–bladder temperature was calculated. There is no information on how many patients actually had body warming, but as it took up to 12 hours to reach a bladder temperature of < 36 °C some patients may not have received it With caveats, the temperature data in the cooled patients contribute to proof of concept of temperature reduction with head cooling and is included in the review |
Gaida and colleagues 200851 (abstract only)
| Methods | Observational study of head and neck cooling for fever management |
| Participants |
n = 6, age and gender not reported Aneurysmal SAH Participating sites: Single Swiss Neuro ICU Language: English Temperature measurement sites: Intracranial (ventricular), arterial blood |
| Interventions |
Standard management (paracetamol, metamizole, alcohol washing, ice packs) plus head and neck cooling (CSZ Blanketrol head and neck wrap) if brain temperature was still > 37.8 °C after 2 hours Time from injury to start of cooling: N/A – fever management Time from start of cooling to target: Achieved by 6 hours Target temperature: Not reported but probably 37.5 °C Duration of cooling: 6 hours Rewarming: N/A |
| Outcomes |
Intracranial (ventricular) and arterial blood temperature after 6 hours of standard care plus head and neck cooling Results: ‘Tbrain and Tblood after 6h of wrap cooling decreased significantly from Tbrain 38.5 ± 0.6 °C and Tblood 38.2 ± 0.6 °C to 37.5 ± 0.4 °C and 37.4 ± 0.5 °C (p < 0.0001 for both)’ |
| Notes | The temperature setting used is not stated but the circulating water temperature could be set between 4 °C and 42 °C |
Other studies (not randomised controlled trials): brain injury
Forte and colleagues 200952
| Methods | Retrospective study of the effect of ice over decompressive craniectomy site on ICP and temperature reduction |
| Participants |
n = 23, mean age 48.9 (range 16–83) years, 13 female Severe TBI n = 6: SAH 10; ischaemic stroke, four; brain tumour, two; ICH, one; plus refractory intracranial hypertension and decompressive craniectomy Participating sites: Neurosurgical ICUs in two Portuguese hospitals Language: English Follow-up complete: Yes Temperature measurement sites: Intracranial (site not specified); oesophageal |
| Interventions |
Cooling by ice packs over decompressive craniectomy site Time from injury to start of cooling: Not reported but cooling was for ICP reduction not neuroprotection Time from start of cooling to target: Target was ICP reduction and cooling was continued to achieve this Target temperature: Target variable was ICP ≤ 20 mmHg Duration of cooling: Mean 61.7 (range 20–96) hours; time depended on stable ICP during rewarming and improvement on CT Rewarming: ‘Gradual and passive rewarming of the brain, with the intermittent application of ice packs to the area of the craniectomy’ keeping ICP stable and avoiding ‘abrupt’ rise in brain temperature |
| Outcomes |
ICP, temperature, mortality in ICU and GOS on discharge from ICU Temperature results: Mean intracranial temperature reduced from 37.1 °C (range 35.3–38.9 °C), prior to cooling, to mean 35.2 °C (range 33.6–37.6 °C) over 48 hours following start of cooling (p < 0.0001) Range of temperature change with cooling +0.3 °C to –4.5 °C ICP results: Mean ICP reduced from 28 mmHg (18–64 mmHg), in the pre-cooling period, to 13 mmHg (2–51 mmHg) in the post-cooling period (p = 0.0001). ‘During the pre-cooling period, 19 of the 23 (82.60%) patients presented ICP higher than or equal to 20 mmHg and only two patients (8.69%) maintained an ICP over 20 mmHg after cooling’ |
TraumaTec Neuro-Wrap Neuro ICU Study
Miller 200953 (abstract of the protocol); interim data on nine patients provided by principal investigator, Professor Claudia Robertson (3 January 2011)
| Methods | Descriptive, non-randomised single group study to determine rate and degree of brain cooling with TraumaTec Neuro-Wrap |
| Participants |
Total n = 20, with the data provided are for the first nine; age and gender not reported Brain injury Participating sites: Single neuro ICU in USA Language: English Follow-up complete: No – interim data Temperature measurement sites: Intracranial and core body (sites not specified) |
| Interventions | Head and neck cooling for 8 hours with circulating water device (TraumaTec Neuro-Wrap) |
| Outcomes |
Rate and degree of intracranial cooling, complications Temperature results: Mean start brain temperature 37.5 ± 1 °C; lowest brain temperature 35.5 ± 1.4 °C, difference 2.0 °C, body temperature remained constant between 37.8 °C and 36.7 °C. Lowest brain temperature achieved was 33.1 °C, with corresponding core temperature of 37.1 °C. Lowest core body temperature seen in any subject was 36.2 °C Complications: No systemic complications or local complications attributable to the device, e.g. skin irritation of the scalp or neck, restriction of jugular venous drainage by the neck section resulting in ICP elevations, or compression of neck structures resulting in barostimulation and changes in blood pressure |
| Notes | Graph supplied with unpublished data shows cooling for 6.5 hours |
Rhinochill Neuro ICU Study
Sung and colleagues 200954 (abstract of protocol and interim data); abstract of completed study www.benechill.com/wp/clinical-program/clinical/neuro-icu-cooling-study/ (accessed 1 November 2010); full report (submitted for publication) provided by Dr Denise Barbut (14 April 2011), now published as Abou-Chebl and colleagues 201158
| Methods | Non-randomised single group safety and feasibility study of intranasal cooling induction with the Rhinochill device |
| Participants |
Total n = 15, mean age 50.3 (range 21–88) years, 9 female Five ICH, 5 ischaemic stroke, 5 TBI with a clinical indication for cooling (e.g. raised ICP or fever), baseline NIHSS 26.7 ± 6.7 Participating sites: Three neuro ICUs in USA Language: English Follow-up complete: Yes, to planned 24 hours but one patient only received 30-minute cooling Temperature measurement sites: Intracranial (parenchyma) n = 11; tympanic n = 10; core trunk rectal n = 10, bladder n = 2, pulmonary artery n = 2, oesophageal n = 1 |
| Interventions | Intranasal cooling (Rhinochill) for 1 hour for fever control (n = 9) or neuroprotection/ICP reduction (n = 6), followed by local standard cooling methods |
| Outcomes |
Temperature reduction and ICP reduction at 1 hour, adverse events, 24-hour follow-up (temperatures, vital signs, neurological examination, rhinoscopy, chest radiograph, brief smell identification test if conscious), repeat rhinoscopy and smell test at 2 weeks after cooling or discharge if sooner Temperature results after 1 hour of cooling: Intracranial temperature reduction mean 1.4 ± 0.4 °C (n = 11 of 15), core trunk temperature reduction 1.1 ± 0.6 °C (n = 15) ICP results: Mean initial ICP = 16 mmHg, mean reduction after 1 hour of cooling = 5.2 mmHg (32.5%) Complications: Transient minor nasal erythema and discharge was seen on rhinoscopy One device-related serious adverse event: Mean arterial pressure rise (75–94 mmHg) within 15 minutes of start of cooling, resolved by stopping the device and administering sedation |
| Notes |
Mean ICP was not raised, presumably because ≤ 6 of 15 patients were being cooled for raised ICP Smell tests were possible in no patients at 2 weeks because dead (n = 6) or not conscious Funded by Benechill Inc. |
Other studies (not randomised controlled trials): cardiac arrest
Andreas and colleagues 200855 (conference abstract)
| Methods | Prospective observational case series to assess preliminary safety and effectiveness of Rhinochill device |
| Participants |
Total n = 7, median (first to third quartile) age 68 (range 66–74) years, 6 male Successfully resuscitated after cardiac arrest Participating sites: The emergency department of a tertiary care university hospital in Austria Language: English Follow-up complete: Unclear – neurological outcome was assessed but when and whether in all patients is not reported Temperature measurement sites: Oesophageal |
| Interventions | Intranasal cooling with Rhinochill device for 60 minutes (followed by cooling to 33 °C up to 24 hours with another device) |
| Outcomes |
Temperature reduction at 60 minutes, safety Temperature results: Median (first to third quartile) oesophageal temperature at baseline 35.4 °C (range 34.7–36 °C). After 60 minutes, 34.1 °C (range 33.4–34.9 °C). Cooling rate 1.6 °C (range 1–1.7 °C)/hour Safety results: No adverse events related to cooling device Two patients favourable neurological outcome (CPC 1 or 2), assessment point not reported but possibly hospital discharge |
| Notes |
Busch and colleagues 200856 (conference abstract interim report); Busch and colleagues 201059
| Methods | Multicentre single arm descriptive study of effectiveness, safety and feasibility of Rhinochill device |
| Participants |
Total n = 84, median (first to third quartile) age 71 (range 63 to 79) years, 64 male Successfully resuscitated after cardiac arrest Participating sites: 11 European emergency departments and ICUs (Austria, Belgium, Germany) Language: English Follow-up complete: Yes Temperature measurement sites: Tympanic (n = 82); core: arterial (n = 17), oesophageal (n = 19), rectal (n = 22), bladder (n = 26) = 84 (the four sites of core temperature measurement were analysed together as a composite core temperature) |
| Interventions | Intranasal cooling with Rhinochill device for 60 minutes (followed by cooling to 33 °C up to 12–24 hours with a systemic device) |
| Outcomes |
Primary end points: cooling rate, time needed to achieve mild hypothermia (34 °C) and target temperature (33 °C), evaluation of possible side effects of evaporative cooling in the nasopharynx and elsewhere Secondary end points: survival rate and neurologic outcome (CPC) at hospital discharge Adverse events: from time of enrolment to hospital discharge and olfactory function Temperature results: actual cooling duration with Rhinochill median 60 (range 25–195) minutes. Composite core cooling rate (n = 84) median (first to third quartile) 1.1 (0.7; 1.5) °C/hour (n = 84) Cooling rate with arterial and oesophageal temperature – faster reacting sites – (n = 36) 1.4 (0.9; 2.0) °C/hour Cooling rate with bladder and rectal temperature – slower reacting sites – (n = 48) 0.9 (0.5; 1.2) °C/hour No patient reached 33 °C core temperature within 1 hour Outcome results: 34 of 84 patients survived, 26 of 34 with favourable neurological outcome (CPC 1–2). Device-related adverse events: In one patient with cardiogenic shock given high oxygen flow rate 60–80 l/minute, cold-induced tissue damage (persisted until death due to cardiac failure), reversible cold-related nasal discolouration n = 10 (these patients had lower oxygen flow rate 40–50 l/minute), epistaxis n = 2 (resolved), coolant in sinus (n = 1) (resolved), periorbital gas emphysema n = 1 (resolved). Nine patients showed aspiration on chest radiograph but this did not have characteristic appearance of liquid coolant aspiration Eleven patients had olfactory function assessed and all were within normal limits The authors’ note: ‘Essential safety measures that prevent tissue damage include uncovering the face and keeping the mouth open during cooling, so that coolant vapor can escape from mouth and nostrils’ (p. 947) |
| Notes |
Benechill Inc. provided per patient payment in support of this study If these patients are cool at baseline [mean core temperature was approximately 36 °C at baseline (figure 2)] and also have low flow/cardiogenic shock nasal tissue discolouration/freezing is presumably more likely than in, for example, brain-injured patients, who are warmer and possibly better perfused peripherally |
Characteristics of excluded studies
Traumatic brain injury
|
Zhmurko 197193 (USSR study, language Russian) TBI, n = 149 cooled Interventions: Head cooling with passive (ice and salt) cooling helmet, target temperature not reported. Cooling duration: mild TBI 8 hours (if ICP normal), 12 hours (raised ICP); moderate TBI (usually raised ICP) 2 × 8-hour cooling, 16-hour interval; severe TBI 2 × 12 hours’ cooling, 12-hour interval; skull fracture 12 hours or 24 hours cooling × 2 or more Outcomes: ‘Disappearance of pathological symptoms’, hospital length of stay |
Not a randomised trial although there was a control group (n = 128) but no information is given about their care. ICP values not reported Insufficient information on temperature to assess temperature reduction. Temperature measurement sites: axilla and ?ear canal (i.e. method described by Bukov VA, Vinogradov VI. Khirurgiya, 1968;10:50). It seems likely this was ear canal temperature because a slightly later paper by Bukov and Vinogradov reports that ‘temperature of the auditory canal wall near the tympanic membrane reflects temperature of the basal brain portion with a precision up to ± 0.4 °C’ (Bukov et al. Determination of brain temperature during cooling of the head. Vestn Khir Im II Grek 1970;104:113–14) |
|
Ioffe and Sumskii 197792 [USSR study (Moscow), language Russian] TBI, n = 56 (33 comatose, 9 sedated, 18 with tracheostomy for assisted breathing) Interventions: Convective head cooling with ‘fluidocraniotherm’ device, lowest air temperature –5 °C, target temperature of cerebral cortex 28–30 °C, maintaining rectal temperature at 33–34 °C. Duration: 6–29 hours with repeat cooling if patients’ condition worsened (e.g. ICP rise). Two patients required body warming (45 °C air) because of excessive rectal temperature reduction Outcomes: Mortality (? in hospital) |
Not a randomised trial Insufficient information on temperature to assess temperature reduction. In hyperthermic patients the target brain temperature of 28–30 °C was not always achieved but normothermia was achievable (decrease from 39.5–40 °C to 36.5–37 °C). Temperature measurement sites: intracranial (n = 15), external auditory meatus, oesophagus, rectum. Note: This is an early report of the clinical use of intracranial temperature measurement in humans |
|
Kang and colleagues 2004162 (Language Chinese) Total n = 81, of whom severe TBI (GCS 3–8) n = 40 Interventions: In severe TBI group: head and neck cooling with cooling pads and ‘drugs that reduce temperature ’ (n = 22) vs ‘normal care’ (n = 18) Outcomes: Immune function, mortality and disability |
Insufficient information on methods to assess quality. Patients ‘divided’ into groups Insufficient information on temperature to assess temperature reduction |
|
Liu and colleagues 200675 (Chinese study, published in English) TBI (GCS ≤ 8), total n = 66 Interventions: Cooling started on admission or after craniotomy. Head cooling with water circulating hood and neck cooling with frozen gel pads – brain surface temperature reduced to 33–35 °C ‘within 2 hours in most patients’, rectal temperature 36.5–37.5 °C (n = 22) vs systemic hypothermia – aim rectal temperature 33–35 °C but ‘about 37 °C’ in results (n = 21) vs normothermia – brain and rectal temperatures ‘about 37 °C throughout’ (n = 23) Outcomes: ICP, SOD, complications of hypothermia, GOS at 2 years |
Not a randomised trial. Although the paper reports that ‘each patient was assigned to one of three groups according to a randomization table’ [p. 59, according to the corresponding author it was not a randomised trial (personal communication, 12 January 2007)]. Described as ‘double-blind’ but unclear how investigators were blinded to the intervention, no information on blinding of analysis or outcome assessment or intention to treat (but intention to treat presumed for GOS because follow-up numbers reported and no mention of crossover) Some patients in the head-cooled group may not have received head cooling. as it was applied intermittently ‘on each of three successive days for 0–6 hours (average 4.5 hours) according to the patient’s condition’ (p. 59) Insufficient information on temperature to assess temperature reduction. In the results rectal temperature in the systemic hypothermia group is reported as ‘about 37 °C’ but the aim was 33–35 °C, communication with the corresponding author failed to clarify this. Temperature measurement sites: ‘brain surface temperature’, rectal Mean (SD) ICP data are reported at 24, 48 and 72 hours after injury and was not significantly different between head-cooled and systemically cooled patients, and was significantly lower in these groups than in the uncooled patients. But mean ICP was high in all groups at all time points. The lowest reported values were in systemically cooled patients at 72 hours: mean 26.48 ± 3.75 mmHg |
|
Qiu and colleagues 2006a147 (Chinese study published in English) TBI (GCS ≤ 8), total n = 90 Interventions: Mean 4.1 hours after admission or after craniotomy. Head cooling with water circulating hood and neck cooling with frozen gel pads – brain temperature 33–35 °C ‘within 2 hr’, rectal temperature 37.5 °C (n = 45) vs normothermia – brain temperature ‘about’ 37 °C, rectal temperature ‘about’ 38 °C (n = 45) Outcomes: ICP at 24, 48 and 72 hours after injury; complications of hypothermia; GOS 6 months |
Not a randomised trial Temperature measurement sites: intracranial (10 mm below cortex or where ICH was evacuated), rectal Figure 1 shows brain temperature every 12 hours for 96 hours and figure 2 the rectal temperature. But the scale was judged too small to reliably extract the data and it is not clear how the data were calculated (e.g. at a single time point or over each 12-hour period). Cooling duration was 72 hours, followed by ‘natural rewarming’. By 90 hours, mean brain temperature still appears to be in the cooled range below 35°C, i.e. 20 hours after start of cooling (figure 1). The text reports that: ‘After cessation of hypothermia, the brain temperature returned to baseline in 8.4–20.6 h’ (p. 997). Baseline was approximately 37 °C (figure 1) Brain temperature appears to be consistently approximately 1 °C lower than rectal temperature in the non-cooled patients (figures 1 and 2), which seems unusual |
|
Qiu and colleagues 2006b76 (Chinese study published in English) TBI (GCS ≤ 8), total n = 96 Interventions: Head cooling with water circulating hood and neck cooling with frozen gel pads to nasopharyngeal temperature 33–35 °C (n = 24) vs systemic cooling – rectal temperature 34.5–36 °C ‘or at the similar nasopharyngeal temperature’ to the head cooled group (n = 30) vs ‘normothermia’ (n = 42) Outcomes: Thrombocytopenia days 1, 3, 5 and 7; GOS at 1 year |
Insufficient information on methods to assess quality. Patients ‘randomised’/’categorised’ but no details. No intention-to-treat analysis. Loss to follow-up: 40 of 96 patients enrolled did not have GOS at 1 year reported because it seems that only the patients who developed thrombocytopenia were followed up Insufficient information on temperature to assess temperature reduction. Temperature measurement sites: nasopharyngeal, intracranial (10 mm below cortex or where ICH was evacuated), rectal |
|
Qiu and colleagues 2007163 (language Chinese) and Qiu and colleagues 2007164 (conference abstract in English) TBI (GCS 4–8), total n = 37 Interventions: Head and neck cooling + decompressive craniectomy (n = 16) vs normothermic control patients (n = 21) Outcomes: ICP, complications, GOS 6 months |
Not a randomised trial Insufficient information on temperature to assess temperature reduction |
|
Fang 2009165 (language Chinese) TBI (GCS 3–8), total n = 91 Interventions: Hemicraniectomy + head and neck cooling (n = 49) vs hemicraniectomy (n = 42) Outcomes: ICP and prognosis |
Retrospective study – not a randomised trial Insufficient information on temperature to assess temperature reduction |
Stroke: ischaemic
|
Xu and colleagues 2003166 (language Chinese) Ischaemic stroke, total n = 44 Interventions: Head cooling (cooling hat) + routine care (n = 24) vs routine care (n = 20) Outcomes: ESS days 7, 21, 90; BI days 21, 90 |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation is reported Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: axilla |
|
Xia and Yan 2004167 (language Chinese) Ischaemic stroke, total n = 61 Interventions: Head cooling + routine care (n = 31) vs routine care (n = 30) Outcomes: Infarct size at day 7, in-hospital mortality, NDS and ADL at hospital discharge |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation is reported Insufficient information on temperature to assess temperature reduction |
|
Yamada and colleagues 200472 (Japanese study, conference abstract in English) Ischaemic stroke NIHSS > 1, n = 17 Interventions: Head and neck cooling for 3–7 days, unsedated patients (‘no anaesthesia’) Outcomes: adverse events, mortality, BI 3–10 months after stroke |
Not a randomised trial – feasibility and safety study No intracranial temperature measurements. Bladder temperature reported as unchanged with cooling but no actual bladder temperatures given Sites of temperature measurement: jugular bulb, tympanic membrane, bladder, axilla Two patients had skin erosion but whether that was due to the head-cooling device is not reported Contact with the author has not produced more information |
|
Li and colleagues 2005168 (language Chinese) Ischaemic stroke, total n = 92 Interventions: Head cooling within 6 hours of stroke (n = 31) vs within 7–10 hours (n = 31) vs within 11–14 hours (n = 30) Outcomes: Infarct volume (CT) 2–3 days after cooling; in-hospital mortality, NDS at hospital discharge |
Not a randomised trial Insufficient information on temperature to assess temperature reduction |
|
Takenobu and colleagues 2005169 (Japanese study, conference abstract in English) Ischaemic stroke, total n = 38 Interventions: Head and neck cooling with circulating coolant at 5 °C (MC-3000, Mac-Eight, Japan) (n = 24) vs no cooling (n = 14) Outcomes: Oedema volume day 6 (median), ischaemic volume day 33 (median) on CT |
Not a randomised trial, control patients matched for age and ischaemic area on CT No temperature data |
|
Chen and colleagues 2006170 (language Chinese) Ischaemic stroke with body temperature 39–40 °C, total n = 122 Interventions: Head cooling (n = 49) vs tepid sponging and ice cold saline bowel irrigation (n = 43) vs healthy control patients (n = 30) Outcomes: serum cortisol, lipid peroxide, SOD at days 2, 3, 4 after raised temperature; NDS and ADL at hospital discharge |
Insufficient information on methods to assess quality, though mentions ‘computerised’ randomisation. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: body (?where) |
|
Wang and colleagues 2006171 (US study, language English) Severe ischaemic stroke, 1.7-cm midline shift, n = 1 Intervention: Head and neck cooling, with body warming to maintain body temperature at 35 °C Outcomes: Intracranial temperature and ICP in each hemisphere |
Case study of head cooling in a patient with terminal ischaemic stroke in the COOL BRAIN-stroke trial Sites of temperature measurement: bilateral parenchymal (0.8 cm below cortical surface), body (?where) Although there was temperature data with head cooling this case study was judged not relevant for inclusion in the review The patient was hypothermic at baseline with body temperature of 35 °C, the non-infarcted hemisphere temperature 35.1 °C and the infracted hemisphere approximately 33.3 °C. When treatment was withdrawn at the family’s request after 10 hours of cooling the intracranial temperatures were approximately 27.5 °C and 19 °C. The increase in temperature difference between the hemispheres was interpreted as poorly perfused brain tissue being more susceptible to head cooling, but in a moribund patient this is perhaps of more academic interest than clinical relevance. The body warming and head cooling may simply have accentuated the reduction in brain temperature below body temperature which has been shown to occur with brain death (Lyson, et al. Neurol Neurochir Pol 2006;40:269–75) |
|
Yang and colleagues 2006130 (language Chinese) Ischaemic stroke with temperature of ≥ 39 °C, total n = 136 Interventions: Head cooling until body temperature ≤ 37.5 °C (n = 30) vs head cooling for 1–2 days (n = 34) vs head cooling for 3–4 days (n = 41) vs head cooling for 5–6 days (n = 31) Outcomes: recurrence of pyrexia; NDS and ADL at hospital discharge |
Not a randomised trial. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction |
|
Inoue and colleagues 2007172 (Japanese study, conference abstract in English) Ischaemic stroke, total n = 53 Interventions: Active head and neck cooling at 5 °C for 3 days (n = 37); control patients with no head cooling (n = 16) Outcomes: Mortality, complications, frequency of haemorrhagic infarct and brain herniation within 7 days of stroke |
Not a randomised trial Insufficient information on temperature to assess temperature reduction. ‘Ear drum’ temperature 35.2 ± 0.3 °C during cooling. Sites of temperature measurement: ear drum, core body temperature (?where) |
|
Hao and colleagues 2008173 (language Chinese) Ischaemic stroke, total n = 45 Interventions: Head cooling + batroxobin (n = 22) vs normal temperature + batroxobin (n = 23) Outcomes: modified Edinburgh-Scandavian Stroke Scale at days 7, 14 and 21 |
Insufficient information on methods to assess quality (‘number method’ randomisation). No relevant functional outcome measures Site of temperature measurement: tympanic |
Stroke: haemorrhagic
|
Shuaib and colleagues 1996174 (US study, language English) SAH – three awake and alert, one GCS ≤ 8, total n = 4 Intervention: ‘Mild’ head cooling for 1 hour/day for 3–4 days (‘cooling hat’, Manson and Manson Engineering, Longview, WA) (n = 4) Outcomes: Extracellular glutamate (microdialysis) 1 hour before, during and 1 hour after head cooling |
Not a randomised trial No temperature data: ‘Uncertain about the exact degree of cooling produced by the cooling hats.’ (p. 57). Site of temperature measurement: body (?where) |
|
Dohi and colleagues 2000175 (Japanese study, language Japanese); Dohi and colleagues 2006176 (Japanese study, language English) Haemorrhagic stroke (ICH/SAH), total n = 89 Intervention: Cooling induction immediately post operative (SAH n = 11) or post admission (ICH n = 35) with rectal indomethacin 100 mg plus 8–12 l/minute chilled air (24 °C) via a ‘balloon catheter’ in one nostril with airflow exiting through the mouth; cooling maintenance – brain temperature 36.5–37.5 °C – with rectal indomethacin 6 mg/kg/day plus room temperature regulation plus additional indomethacin to maximum of 600 mg/day if needed (n = 46). Convenience control group (ICH 13, SAH 30, n = 43). Outcomes: CSF interleukin-1β and serum bilirubin at 1, 2, 4 and 7 days; vasospasm (in patients with SAH); GOS 3 months |
Not a randomised trial Insufficient information on temperature to assess temperature reduction with nasal airflow. In the Japanese paper the mean temperatures for cases (37.8 ± 0.29 °C) and control patients (38.0 ± 0.18 °C) over the 4-day cooling period are reported, which is an indication of the effect of indomethacin, but not for the period of induction of cooling with nasal airflow. How long nasal airflow was administered for induction is not reported in these two papers but Dohi and colleagues (200663) says ‘in general … for a short period’. Site of temperature measurement: cerebral ventricle |
|
Feng colleagues 2002148 (language Chinese) ICH, total n = 40 Intervention: Active head cooling ‘controllable semiconductor brain-protecting freezer’ set at 6 °C + routine care (n = 20) vs routine care (n = 20) Outcomes: Cerebral oedema volume on CT (Tada formula) and ESS at 1 and 2 weeks |
Insufficient information on methods to assess quality. 1 : 1 matched groups – not strictly randomised. No relevant outcome measures Insufficient information on temperature to assess temperature reduction |
|
Xu and colleagues 2002177 (language Chinese) Haemorrhagic stroke, total n = 58 Interventions: Head cooling with ‘head temperature control instrument’ (n = 28) vs control (n = 30) Outcomes: Cerebral oedema volume on CT, flow velocity in middle cerebral artery, pulsatility index, NDS at 21 days |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation reported, not intention to treat. No relevant outcome measures Insufficient information on temperature to assess temperature reduction |
|
Su and colleagues 2004149 (language Chinese) ICH, total n = 42 Interventions: Active head cooling with cooling pads (Harbin Institute of Technology) on area of haemorrhage shown on CT (n = 21) + routine care vs routine care (n = 21) Outcomes: Cerebral oedema volume on CT (Duotian formula) and ESS at 1 and 2 weeks |
Insufficient information on methods to assess quality. Patients ‘divided’ 1 : 1 but no details on how. No relevant outcome measures Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: tympanic |
|
Zhang and colleagues 2006178 (language Chinese) ICH, total n = 70 Interventions: Head and neck cooling + routine care (n = 35) vs routine care (n = 35) Outcomes: Neuropeptide Y, neurotensin, calcitonin gene-related peptide, endotheline at day 1, 7 and 14 post stroke |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation is reported. No relevant outcome measures Insufficient information on temperature to assess temperature reduction |
|
Xia and colleagues 2003179 (language Chinese) Haemorrhagic stroke, total n = 263 Interventions: Direct microscopic haematoma evacuation + head cooling (n = 132) vs direct microscopic haematoma evacuation alone (n = 131) Outcomes: Re-bleeding rate, mortality, NDS and ADL on hospital discharge |
Probably case control. Insufficient information on methods to assess quality Insufficient information on temperature to assess temperature reduction |
|
Xu and colleagues 2004128 (language Chinese) Haemorrhagic stroke, total n = 91 Interventions: Head cooling (n = 31) vs observation group (n = 30) vs healthy control patients (n = 30) Outcomes: Serum nitric oxide, SOD, glutamate, NDS and ADL at day 2 and day 7 or 10 (unclear) |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation is reported. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: scalp skin |
|
Dong and colleagues 2005141 (language Chinese) Haemorrhagic stroke with body temperature 38–40°C (high temperature), total n = 91 Interventions: Head cooling (n = 54) vs tepid sponging and ice cold saline bowel irrigation (n = 37) Outcomes: Serum cortisol, lipid peroxide, SOD at days 2, 3, 4 after high temperature; NDS and ADL at high temperature and on hospital discharge |
Insufficient information on methods to assess quality. Patients ‘divided’ into groups, no details of what that means. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: body (?where) |
|
Ou and colleagues 2005140 (language Chinese) Haemorrhagic stroke, total n = 170 Interventions: Head cooling until body temperature ≤ 37.5 °C (n = 43) vs head cooling for 1–2 days (n = 51) vs head cooling for 3–4 days (n = 38) vs head cooling for 5–6 days (n = 38) Outcomes: Rate of pyrexia recurrence, NDS and ADL at hospital discharge |
Insufficient information on methods to assess quality. Mentions ‘computer’ randomisation but no details. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: body (?where) |
|
Zhang and colleagues 2006180 (language Chinese) Haemorrhagic stroke, total n = 124 Interventions: Head cooling (n = 63) vs control (n = 61) Outcomes: Interleukin-6, tumour necrosis factor alpha prior to treatment and day 8; NDS and ADL ?at hospital discharge |
Insufficient information on methods to assess quality. Mentions ‘computer’ randomisation but no details. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction |
Stroke: mixed
|
Liu and colleagues 1999139 (language Chinese) Mixed stroke, total n = 62 Interventions: Cooling (n = 31): either cooling mattress + ‘skull temperature reducing instruments’ (in young patients) or head cooling + ice packs to abdomen and axillae (old and frail patients) or head cooling alone with hat or ice packs (patients sensitive to cooling) vs control patients (n = 31) Outcomes: In-hospital mortality and length of hospital stay |
Insufficient information on methods to assess quality. ‘Computerised’ randomisation is reported but those who could not afford the head-cooling device were cooled with ice packs Insufficient information on temperature to assess temperature reduction |
|
Tang and colleagues 2008181 (Chinese study, English abstract) Mixed stroke, n = not reported |
Conference abstract relating to development of a head-cooling device, which says ‘Data will also be presented from human positron emission tomography studies showing a decrease in glucose metabolism in the selectively cooled brain areas, and from clinical trials in haemorrhagic and ischemic stroke patients’. One of the authors provided a full paper on the PET study (Zhang and colleagues 2005182 in volunteers – see Appendix 7) but had no copy of the full paper with the clinical data |
|
Wu and colleagues 2010183 (language Chinese) Mixed stroke, total n = 32 Interventions: Head cooling over area of infarct or haemorrhage (controllable semiconductor refrigeration apparatus, Harbin Institute of Technology) within 6 hours of stroke for 48 hours or within > 6 hours for 96 hours + conventional care (n = 16) vs conventional care (n = 16) Outcomes: infarct and oedema volume 14 days; ESS at 14 and 30 days |
Insufficient information on methods to assess quality. Abstract says ‘randomised’ but paper suggests this was probably a case control study – matched 1 : 1 – but unclear. No relevant functional outcome measures Insufficient information on temperature to assess temperature reduction. Sites of temperature measurement: nasal and tympanic |
Brain injury
|
Mellergard 1992184 (Swedish study, language English) TBI and SAH (n = 5), all with shaved and bandaged headsOutcomes: Temperature change |
Case studies of head cooling and nasopharyngeal cooling – judged to have insufficient detail for inclusion Some patients had more than one head-cooling method but not at the same time. The cooling was of an exploratory ad hoc nature, meaning that coincidental temperature change or lack of it for other reasons cannot be ruled out. There is a graph for each cooling method; each graph shows a single patient’s response. Otherwise the actual temperatures are not reported Sites of temperature measurement: intracranial (ventricular), epidural, rectal Ventricular temperature reduction with: |
|
Mariak and colleagues 200266 (Polish study, language English) Brain injury with fever (n = 6) Face fanning, airflow 3.5 m/second, duration not reported, patients had dressings on their heads |
Case studies of face fanning for fever reduction – judged to have insufficient detail for inclusion This refers to earlier research presented in a conference abstract (Mariak and colleagues 1993185), which does not report actual temperatures. The temperature reduction is reported in Mariak and colleagues 200266 Sites of temperature measurement: intracranial (subdural), tympanic In three of six patients subdural temperature decreased. Mean reduction = 0.15 ± 0.18 °C. The authors comment that the low air speed and head dressings may help to explain why there was not a greater temperature reduction |
|
Dohi and colleagues 200663 (Japanese study, language English) Severe TBI, (n = 2) 8–12 l/minute chilled air (24 °C) via a 16 g Foley ‘balloon’ catheter in one nostril, the other nostril occluded with an epistaxis balloon, the air exited through the mouth plus head fanning. Duration of cooling: ‘in general … for a short period’ |
Technical note on nasopharyngeal cooling including two case studies – judged to have insufficient detail for inclusion Sites of temperature measurement: intracranial (ventricular) In patients with SAH, ventricular temperature reduced from 37.8 °C to 34 °C (3.8 °C) in 45 minutes, in patients with TBI from 39 °C to 37 °C (2 °C) in 120 minutes |
|
Wang and colleagues 2001186 (language Chinese) Severe brain injury (GCS < 8), total n = 45 Interventions: Passive head and neck cooling (blue ice strips) (n = 22) vs routine care (n = 23) Outcomes: Mortality and GOS at hospital discharge |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation is reported Insufficient information on temperature to assess temperature reduction. Sites of temperature measurement: parenchymal or brain surface and rectal |
|
Zhao and colleagues 200344 (language Chinese) Severe brain injury (GCS ≤ 8) admitted within 12 hours of injury and having had either evacuation of haematoma or decompressive craniectomy, total n = 69 Interventions: Head cooling (n = 23) vs systemic hypothermia (n = 22) vs normothermia (n = 24). When cooling was started is not reported Outcomes: In-hospital complications (pneumonia, gastrointestinal bleed, arrhythmias, renal failure), mortality and GOS at hospital discharge |
Insufficient information on methods to assess quality; ?’computer’ randomisation Insufficient information on temperature to assess temperature reduction |
|
Yang and colleagues 2006130 (language Chinese) Severe brain injury (GCS ≤ 8), total n = 87 Interventions: Head and neck cooling (hat closely bandaged on) (n = 44) vs control patients (n = 43) Outcomes: ICP; ‘early complications’ – hyperglycaemia, epilepsy, vasospasm, stress ulcer at 1 week after cooling; GOS 3 months |
Insufficient information on methods to assess quality, although ‘computerised’ randomisation is reported Insufficient information on temperature to assess temperature reduction. Site of temperature measurement: brain (?where) |
Cardiac arrest
|
Hachimi-Idrissi and colleagues 1999187 (conference abstract, Belgian study, language English) Prehospital cardiac arrest, total n = 21, the first 11 were cooled, the subsequent 10 were control patients Interventions: Head cooling with a helmet device (?passive) during resuscitation for up to 4 hour (11) vs no cooling (n = 10) Outcomes: Speed and effectiveness of helmet device to cool to target temperature of 34 °C ‘No complication’ from the cooling helmet |
Non-randomised precursor to Hachimi-Idrissi and colleagues 2001, little information on cooling device (‘new helmet device’), probably Frigicap Insufficient information on temperature to assess temperature reduction Site of temperature measurement: tympanic and bladder |
|
Hachimi-Idrissi and colleagues 200169; Hachimi-Idrissi and colleagues 2005188 (study of S100β with cooling which includes the patients in Hachimi-Idrissi and colleagues 2001); additional information in Holzer and colleagues 2005189 (Belgian study, language English) Cardiac arrest – asystole or pulseless electrical activity, total n = 30 (plus three reported in Hachimi-Idrissi and colleagues 2005 and Holzer and colleagues 2005) Interventions: Passive head cooling after ROSC and stabilisation in emergency room with an aqueous glycerol helmet (Frigicap) –4°C, applied over paper cap and changed every hour, duration of cooling 4 hours or until bladder temperature 34 °C (n = 16) vs no cooling – passive rewarming to 37 °C if hypothermic, paracetamol if temperature >38 °C (n = 14) Outcomes: Feasibility and speed of helmet device to cool to target temperature of 34 °C. CPC at hospital discharge ‘No complication’ from the cooling helmet |
RCT. Hachimi-Idriss and colleagues 2001 has inadequate information on randomisation method (‘prospectively blindly randomised’) or blinding but Holzer and colleagues 2005 reports the method (random number tables, opaque envelopes) and that outcome assessors were blinded and includes data on an additional three patients Insufficient information on temperature to assess temperature reduction. Hachimi-Idrissi and colleagues 2001 reports baseline tympanic temperature but not baseline bladder, and time to target but not actual end temperatures Hachimi-Idrissi and colleagues 2005 (reports target was 33 °C) and Holzer and colleagues 2005 include no temperature data Site of temperature measurement: tympanic (infrared thermometer) and bladder |
|
Ikeda and colleagues 2007190 (conference abstract, Japanese study, language English) Cardiac arrest, total n = 12 Interventions: Selective head cooling (n = 7) vs whole body cooling (n = 5), duration not reported, target temperature 34±1°C Outcomes: Urinary 8-hydroxy-2-deoxyguanosine, outcome at 28 days after admission |
Not a RCT No information on cooling methods except ‘selective head’ and ‘whole body’ Insufficient information on temperature to assess temperature reduction. No information on temperature measurement sites No response from authors to request for further information |
|
Busch and colleagues 2008191 (conference abstract, German study, language English) Cardiac arrest after ROSC, total n = 70 Interventions: transnasal head-cooling cooling (Rhinochill) followed by intravascular cooling (n = 19) vs intravenous 4 °C saline followed by intravascular cooling (n = 41) vs intravascular cooling alone (n = 10) Outcomes: Time from hospital admission to target temperature; CPC and mortality at 7 days and hospital discharge |
Non-randomised feasibility study of induction of hypothermia by transnasal cooling with historic control patients who had had standard care Insufficient information on temperature to assess body temperature reduction with head cooling. Temperature measurement sites: tympanic and bladder or rectal These patients may also have been included in the paper by Busch and colleagues 2010 (under included studies above) |
|
Storm and colleagues 200870 (German study, language English) Cardiac arrest, total n = 49 Interventions: Pre-hospital passive head cooling with gel cap after return of ROSC (n = 24) vs standard care control patients (n = 25) Outcomes: Change in tympanic temperature from pre-cooling to hospital admission, adverse events until hospital admission (none related to the device, e.g. freezing, tissue necrosis), outcome at hospital discharge |
Non-randomised feasibility study: prehospital cooling with hypothermia caps (PreCoCa) Temperature measurement site (tympanic) did not meet inclusion criteria for this review |
|
Nordberg and colleagues 2009192 (conference abstract, Swedish study, language English) Cardiac arrest, total planned n = 100, at time of report n = 15 Interventions: Prehospital, intra-arrest transnasal cooling with Rhinochill device (n = 7) vs standard care (n = 8), cooling duration not reported Outcomes: Outcome at hospital discharge, adverse effects |
RCT – early report of Pre-ROSC Intra-Nasal Cooling Effectiveness II (PRINCE II) No details on methods No temperature data reported |
|
Takeda and colleagues 2009193 (preliminary data); www.controlled-trials.com/ISRCTN98089900 (Japanese study, conference abstract in English) Cardiac arrest, n = 300, n = 3, reported in abstract Interventions: Active pharyngeal cooling during or immediately after resuscitation Outcomes: Tympanic temperature, neurological recovery, mortality |
RCT. This trial has completed, report is in preparation, and a follow-on trial is planned to look at outcome (with Dr Yoshimasa Takeda, 18 April 2011, personal communication) Temperature measurement site (tympanic) did not meet inclusion criteria for this review |
|
Wandaller and colleagues 2009194 (Austrian study, language English) Cardiac arrest, total n = 11: n = 5 series 1, n = 6 series 2 Interventions: Series 1 active head cooling for 1 hour after ROSC with MedCool Rapid Cooling System (n = 5); series 2, active head cooling + neck cooling (n = 6). Rescue therapy: endovascular cooling if temperature not reduced by 1 °C after 1 hour, required by 4/5 in series 1 and 2/6 in series 2, total cooling time 12 hours Outcome: Difference between jugular bulb temperature and oesophageal temperature Device-related adverse events: None |
Non-randomised feasibility study of head cooling and head and neck cooling Data not available for temperature change with head/head and neck cooling alone: ‘We regret that we were not able to distinguish between the effects of head or head and neck cooling vs endovascular cooling on the different temperature sites’ (p. 464) Site of temperature measurement: tympanic, jugular bulb, oesophageal |
Studies in volunteers
The following studies were excluded because they were proof-of-concept assessments of the effect of a head-cooling method or device on intracranial temperature in volunteers, i.e. studies that were not for therapeutic purposes in TBI, stroke or cardiac arrest. In four of the studies (listed first), participants had brain injuries but most were conscious and eligible for these studies because they had intracranial temperature monitoring as part of their normal care rather than because of their injuries. The healthy volunteers (three studies) had non-invasive magnetic resonance spectroscopy brain temperature measurement. Some of these studies provided information on adverse effects.
|
Shiraki and colleagues 1988195 (Japanese study, language English) n = 1, 12 years, male Conscious, 8 days after pineal tumour removal, undergoing radiotherapy, ventricular drain/temperature monitoring in situ Intervention: Face fanning with 25 °C (ambient) air, body covered. One convective session and one convective plus evaporative (body heating with electric blanket to produce facial sweating); 20 minutes with cooling followed by 20 minutes without Outcome: No convincing effect on intracranial or oesophageal temperature |
Observational study Site of temperature measurement: intracranial (lateral ventricle and parenchyma 1 cm above ventricle) and oesophageal |
|
Mariak and colleagues 1999196 (Polish study, language English) n = 4, age 38–55 years, 2 female Conscious, post surgery for minor SAH occurring 7–10 days earlier, mildly hyperthermic (active warming inducing sweating) Intervention/outcome: On extubation intracranial temperature above the cribriform plate reduced by 0.4–0.85 °C (mean 0.55 ± 0.21 °C). Intensive breathing induced a further reduction 0.20–0.30 °C (mean 0.26 ± 0.04 °C) |
Observational study of the effect of extubation and restoration of airflow through the upper respiratory tract on temperature Site of temperature measurement: intracranial (subdural and on midline between frontal lobes and cribriform plate), oesophageal |
|
Mariak and colleagues 2003197 (Polish study, language English) n = 14, age 28–70 years, 9 male Unanaesthetised patients after surgery for subdural haematoma (n = 8), ICH (n = 2), brain tumour (n = 4); eight conscious with no neurological deficit, four GCS 9–14, 2 GCS 8; all had head dressings of various kinds; mildly hyperthermic as a result of active post-op warming (n = 6), feverish > 38 °C (n = 4), normothermic (n = 4) Intervention: Face fanning 32.5 m/second for 20–30 minutes depending on individual tolerance Outcome: Mean decrease in subdural temperature 0.15 ± 0.18 °C, mean decrease in oesophageal temperature 0.05 ± 0.09 °C, mean decrease in rectal temperature 0.03 ± 0.07 °C Adverse event: ‘Generally all patients reported an unpleasant sensation when fanned’ (p. 281) |
Non-randomised observational study to identify extracranial temperature sites that reliably and repeatably reflect intracranial temperature Sites of temperature measurement: intracranial (subdural), tympanic membrane, oesophageal and rectal |
|
Kuhnen and colleagues 2005198 (language English) n = 1 Intubated patient before removal of a deep brain tumour Intervention/outcome: Nasal airflow increasing from 5–10–15 l over 20 minutes appeared to attenuate rise in brain temperature but the patient was hypothermic throughout (all brain temperature points < 36 °C) |
Observation of nasal airflow in an intubated patient with intracranial temperature measurement at four points (35-, 28-, 21- and 14-mm depth) |
|
Corbett and Laptook 1998199 (language English) Ten healthy volunteers, aged 22–47 years Intervention: Passive head-cooling session – two Elasto-gel head caps, inner cap precooled to 4 °C and outer to –20 °C. Control session – same caps but prewarmed to 34 °C (n = 9) or room temperature (n = 1). Duration 50 minutes with each set of caps Outcome: Mean superficial cortex temperature with cooling 36.8 °C, without 37 °C. Mean thalamic temperature with cooling 36.6 °C, without 36.6 °C. Oral and axillary temperatures unchanged between sessions |
Non-randomised crossover study – cap sequence and magnetic resonance spectroscopy measurement sequence alternated between subjects Site of temperature measurement: intracranial measured by magnetic resonance spectroscopy, oral, axilla |
|
Harris and colleagues 200824; Harris 201057 (PhD thesis) (language English) Five unsedated healthy volunteers, aged 31–48 years, 3 male Interventions: 30-minute head cooling followed by 30-minute head and neck cooling with prototype convective cooling helmet delivering air 42.5 l/second at 14.5 °C Outcome: Net brain temperature reduction with head cooling 0.45 °C (SD 0.23 °C, p = 0.01, 95% CI 0.17–0.74°C); with head and neck cooling 0.378 °C (SD 0.30 °C, p = 0.049, 95% CI 0.00 °C to 0.74 °C). Equivalent net reductions in oesophageal temperature 0.16 °C (SD 0.04 °C) and 0.36 °C (SD 0.12 °C) Adverse events: None |
Non-randomised observational study Site of temperature measurement: intracranial measured by magnetic resonance spectroscopy, oesophageal |
|
Covaciu 201065 (PhD thesis, Swedish study, language English) Ten unsedated healthy volunteers; mean age 22 years, range 21–62 years, 9 male Intervention: Active cooling with bilateral intranasal balloons (QuickCool) at 20 °C, unilateral in one subject, for 60 minutes Outcome: Brain temperature reduction measured by magnetic resonance spectroscopy –1.7 ± 0.8°C (n = 9), brain temperature reduction measured by phase mapping method –1.8 ± 0.8°C (n = 9), rectal temperature reduction –0.5 ± 0.3°C (n = 5) Adverse events: Ear, nose and throat examination showed increased nasal secretions (n = 9), redness (n = 3), small ulcers (n = 3). Headache (n = 4), dizziness (n = 1). Subsequent rhinorrhoea (n = 7). Balloons rated as pleasant (n = 1), neutral (n = 3), unpleasant (n = 6). All fully recovered by day 7 follow-up |
Non-randomised observational study Site of temperature measurement: intracranial measured by magnetic resonance spectroscopy, axilla (n = 4), rectal (n = 5) |
Studies awaiting assessment
Randomised controlled trials
No RCTs awaiting assessment
Other studies
Smirnov and Mescherinov98 referenced human studies with the Kholod 2-F device in the USSR: ‘Similarly we treated results obtained by use of apparatus, Kholod 2-F, in clinical practice on patients during operations on the heart, during neurosurgical operations, and during recovery of consciousness.’ The references were:
-
Bunatyan AA, Zolnikov SM, Smirnov OA. In Present Day Problems of Anesthesiology and Recovery of Consciousness. [Russian.] L’vov; 1969. p. 294.
-
Bunatyan AA, Zolnikov SM, Smirnov OA. Fourth International Symposium on Anesthesiology. [Russian.] Varna; 1969. p. 503.
-
Ioffe YS, Smirnov OA. In Comatose States following Cranio-Cerebral Trauma. [Russian.] Moscow; 1969. p. 126.
The data on head cooling ‘during recovery of consciousness’ could be relevant but we have been unable to obtain the papers, even through the National Library of Russia with whom our Russian-speaking librarian has established good links and which supplied some of the other papers in Russian.
Two papers200,201 suggested that head cooling was being used in cardiac arrest in Czechoslovakia. The second of these was a survey of ICUs about therapeutic hypothermia in cardiac arrest, in which 10% of the 90 units who responded reported using a helmet for cooling (Skulec and colleagues 2010201). A request for further information has produced no response.
Characteristics of ongoing studies
Ongoing studies of head cooling in cardiac arrest are not included.
Stroke
| Trial name or title | Induction of Cooling (i-Cool) Pilot: A randomised trial comparing three methods for rapid induction of therapeutic hypothermia in stroke patients |
| Methods | Prospective, open, randomised, single-centre study |
| Participants | Intubated, ventilated stroke patients with combined ICP-temperature probe (n = 30) |
| Interventions | Hypothermia to a target core temperature 34 °C is induced with one of the following:
|
| Outcomes |
Primary outcome: Speed of brain cooling during the first hour Secondary outcomes: Safety aspects – intracranial bleeding or pulmonary complications, co-medication; examination of effects on ICP and cerebral autoregulation |
| Starting date | 2010 |
| Contact information |
Dr Sven Poli Center of Clinical Neurosciences, Heidelberg University, INF 400, 69120 Heidelberg, Germany www.strokecenter.org/trials/TrialDetail.aspx?tid%20=%201098 (accessed 22 February 2011) |
| Notes |
| Trial name or title | The Cerebral Hypothermia in Ischaemic Lesion (CHIL) Trial: A randomised trial evaluating systemic and local mild hypothermia on infarct expansion and salvage of the ischaemic penumbra in acute ischaemic stroke (ACTRN12609000690257) |
| Methods | RCT (block randomisation) |
| Participants | Patients aged ≥ 18 years with acute hemispheric ischaemic stroke, NIHSS ≥ 8, presenting within 6 hours of onset of symptoms or within 6 hours of when last seen unaffected, with evidence of hypoperfused but viable hemispheric brain tissue on perfusion CT. n = 80 |
| Interventions |
Systemic hypothermia (Australian centre): Target temperature 33 °C – induction with 30 ml/kg ice-cold Hartmann’s solution, maintenance with intravascular cooling device Local head cooling (Chinese centre – Harbin, China): No information Control patients: Normothermia (< 38°C) as per standard care |
| Outcomes |
|
| Starting date | 2009 |
| Contact information |
Professor Christopher Levi (christopher.levi@hnehealth.nsw.gov.au) Hunter Stroke Research Group, John Hunter Hospital, Locked Bag 1, Hunter Region mail Centre NSW 2310, Australia www.anzctr.org.au/trial_view.aspx?ID%20=%20308341 (accessed 16 April 2011) |
| Notes |
Safety/efficacy study No response to request for details of local head-cooling intervention |
| Trial name or title | Multiple Interventions for Neuroprotection Utilizing Thermal Regulation in the Emergent Treatment of Stroke (MINUTES) study |
| Methods | Open label randomised study |
| Participants | Patients with cortical stroke within 12 hours of onset or 6 hours from awakening from sleep (n = 70) |
| Interventions | Combination therapy:
|
| Outcomes | NIHSS at 48 hours, 1 week and 90 days (blinded assessor) |
| Starting date | 2006 |
| Contact information |
Dr Muzaffar Siddiqui Division of Neurology University of Alberta/Grey Nuns Community Hospital, Edmonton, Canada |
| Notes |
Interim report: Siddiqui MM, Ludwig Y, Hussain MS, Manawadu D, Mateer A, Beaulieu C, et al. Multiple interventions for neuroprotection utilizing thermal regulation in the emergent treatment of stroke: the MINUTES study. Int J Stroke 2008;3(Suppl. 1):140 Contains no data on the effect of head cooling A methods paper is currently being prepared for publication (Dr Siddiqui,18 April 2011, personal communication) Alberta Health Services information: www.capitalhealth.ca/NewsAndEvents/Features/2006/MINUTESstudy.htm (accessed 16 April 2011) |
| Trial name or title | Emergency room trial of brain cooling in stroke with the Rhinochill device |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information |
Dr Denise Barbutt Becky Inderbitzen |
| Notes | This trial is in the planning stage |
| Trial name or title | Study of brain cooling in stroke with the DigniCap |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information |
Martin Waleij, CEO Dignitana |
| Notes | Further information not available yet |
Brain injury
| Trial name or title | Determination of the rate and degree of selective brain cooling in adults with the TraumaTec Neuro-Wrap |
| Methods |
Descriptive, non-randomised single group study to determine the rate and degree of brain cooling that can be achieved using a new device, the Neuro-Wrap (TraumaTec Inc.) Published protocol abstract: Miller (2009) Determination of the rate and degree of selective brain cooling in adults with the TraumaTec Neuro-Wrap. J Neurotrauma 26:A25 |
| Participants | Adult neurointensive care patients with intracranial temperature monitoring as part of standard care (n = 20) |
| Interventions | Application of Neuro-Wrap for 8 hours |
| Outcomes |
Rate and degree of change in brain and core body temperatures Occurrence of hypotension and ICP change |
| Starting date | 2010 |
| Contact information |
Professor Claudia Robertson Baylor College of Medicine, Houston, TX, USA Susanne Richard |
| Notes | Interim data has kindly been provided for nine subjects and is included in this review |
| Trial name or title | Delivery of selective hypothermia in brain injury patients using a cooling helmet, the COOL BRAIN Trial II: a feasibility and safety study |
| Methods | Prospective feasibility and safety study |
| Participants | Patients with TBI, stroke or pre-hospital cardiac arrest, aged 18–80 years (n = 80–100) |
| Interventions | Application of head- and neck-cooling device (WElkins, LLC, Roseville, CA, USA) pre-hospital, patients determined to have severe brain injuries on arrival in the emergency department will continue to wear the device for 72 hours. Temperature measurement sites intracranial and body core (rectal or bladder) |
| Outcomes |
Feasibility and safety Primary hypothesis: Initiation of selective cerebral hypothermia prior to hospital arrival, inpatient setting or ambulatory care units using a new head-and-neck cooling head cover is feasible and safe in patients with brain injury Secondary hypothesis: Effective selective cerebral hypothermia using a new head-and-neck cooling head cover can be achieved in patients with brain injury with their heads unshaved |
| Starting date | June 2012 |
| Contact information |
Huan (John) Wang, MD Assistant Professor of Neurosurgery Carle Foundation Hospital University of Illinois College of Medicine at Urbana-Champaign Director, Thermal Neuroscience Laboratory (TNL) The Beckman Institute of Advanced Technology and Sciences University of Illinois at Urbana-Champaign |
| Notes |
www.carle.org/notices/braincoolingstudy/brain-cooling-study.aspx (accessed 25 April 2011) Funder: Joint Improvised Explosive Devices Defense Office (JIEDDO), Department of Defense, USA, Contract No: HQ00342–10-C-0031 $700,000 |
| Trial name or title | Trials of QuickCool nasal cooling balloons |
| Methods | |
| Participants | TBI, cardiac arrest and SAH |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information |
QuickCool AB Ideon Science Park Visiting address: Beta 6, Scheelevägen 17 SE-223 70 Lund |
| Notes |
‘QuickCool is currently enrolling patients in clinical trials in Sweden and Denmark. These studies investigate the safety and efficacy of the novel QuickCool Intranasal Brain Cooling System in the following clinical areas: cardiac arrest, TBI and subarachnoid hemorrhage’ www.quickcool.se//Contents.asp?id%20=%20358 (accessed 8 May 2011) We are awaiting further information from the company Grande and colleagues give a very brief report of two patients who seem to have been cooled with QuickCool in their review of hypothermia after TBI: ‘Our preliminary results from two patients exposed to selective brain cooling via the nasal–oral cavity showed a relatively effective reduction of whole body temperature, but the difference between body temperature and brain temperature was only 0.1 °C’ (Grande, et al. Acta Anaesthesiol 2009;53:1233–8: 1237) |
Appendix 7 Non-invasive head-cooling methods and devices
Contents
-
Introduction.
-
Heat loss from the upper airways.
-
– Convective head-cooling methods and devices: nasal gas/nebulised coolant.
-
– Active conductive (liquid) head-cooling methods and devices: nasal and pharyngeal balloons.
-
-
Heat loss through the skull.
-
– Convective head-cooling methods and devices (air or water directed on the head).
-
– Active conductive (liquid) head-cooling devices (circulating cold fluid).
-
– Passive (non-circulating)conductive head-cooling methods and devices.
-
-
Scalp-cooling devices.
-
– Liquid (active) scalp-cooling caps.
-
– Frozen gel (passive) scalp-cooling caps.
-
-
Non-invasive neck-cooling devices.
-
Personal cooling garments.
Introduction
Devices included in this appendix are those that have been developed for brain injury or cardiac arrest or have been used in patients with these conditions. Where there is a current web address for companies this has been included to provide some indication of which devices are ‘active’.
Methods of non-invasive head cooling are categorised into:
-
Heat loss from the upper airways By convection with gas or fluid flow or by conduction with nasal or pharyngeal balloons. Whether or not the devices used are truly non-invasive is a moot point.
-
Heat loss through the skull By convection (fanning, hoods/caps delivering cold air or water) or by conduction (active, e.g. liquid cooling, or passive, e.g. ice, gel caps). Some of the devices also have a neck band, which, theoretically, may help cool the brain by reducing the temperature of the carotid blood supply. 24,25
Liquid (active) cooling helmets contain circulating water with and without antifreeze. Heat from the head is transferred by conduction through the helmet wall and then removed by the circulating coolant. 202 They have the benefit of being able to be maintained at a constant temperature and some have the facility for temperature adjustment. This is potentially important because there is a possibility of tissue freezing and necrosis if scalp temperature is reduced too much. Passive (non-circulating) cooling caps, containing frozen gel, for example, will thaw and have to be refrozen periodically but are simple and relatively inexpensive. The cheapest method is ice packs round the head. However, whether active or passive, the helmet/cap needs to be in close contact with the scalp for optimum heat removal and may be pressurised to achieve this.
With the possible exception of the liquid cooling device developed at Harbin Medical University in China, no head-cooling devices described here have automatic (closed-loop) temperature feedback. In a comparative study of systemic cooling devices, those with automatic temperature control were shown to be more effective and less labour intensive than manually controlled devices. 17
At the end of this appendix there are brief sections on scalp-cooling devices, which have sometimes been used in brain injury, non-invasive neck-cooling devices and personal cooling garments.
Neonatal head-cooling devices are not included here because they are unsuitable for adults, for example the Olympic Cool-Cap (Natus Medical Inc., San Carlos, CA, USA) is specifically designed and sized for neonates. For reviews that include examples of head-cooling techniques used in neonatal HIE see Thoreson,203 which includes an early method used in the USSR comprising induction by cold water spray over the head and maintenance with a liquid cooling cap, and also Robertson and colleagues,204 which includes the Olympic Cool-Cap and some low-technology methods including fans and water bottles.
Heat loss from the upper airways
Convective head-cooling methods and devices: nasal gas/nebulised coolant
Nasopharyngeal cooling through a Foley catheter (Figure 3)
Mellergard184 cut off the tip of a Foley catheter at an angle and passed the catheter through the patient’s nostril with the opening facing upwards. The balloon was inflated with sodium chloride 0.9% and the catheter pulled back until the balloon stopped behind the choane nasi. Oxygen at 5–10 l/minute was flowed through the catheter via a copper coil in a bucket of iced water.
FIGURE 3.
‘A schematic figure of how nasopharyngeal cooling was attempted through a Foley catheter positioned behind the choane nasi. As seen in the inserted rectal and intraventricular temperature curves, nasopharyngeal cooling had very limited effect on brain temperature’184 (figure 2). Reproduced with permission from Mellergard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery 1992;31:671–7.
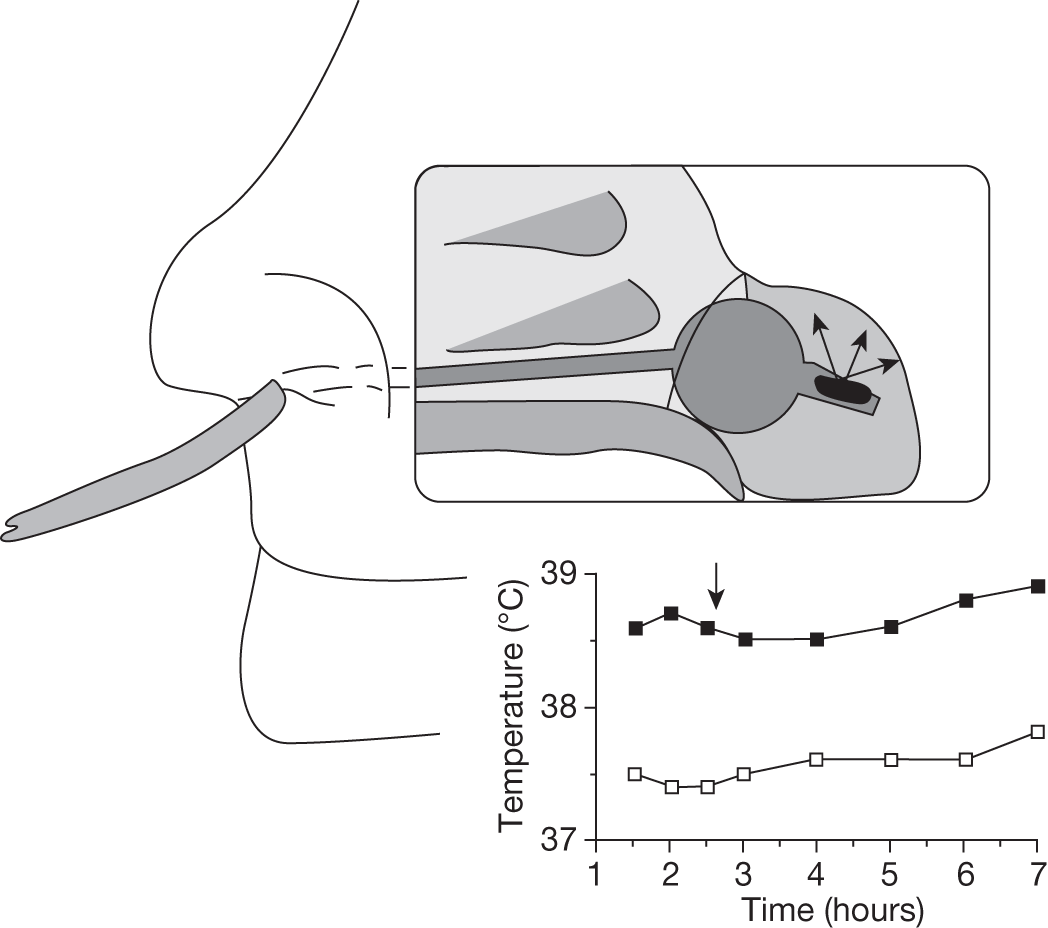
Placing the catheter this for back completely bypassed the nose and therefore did not utilise its capacity for heat loss which may partly explain the lack of cooling effect. However, in patients with a base of skull fracture this might mean less risk of pneumocephalus but the method would still be contraindicated because passing the catheter through the nose could risk worsening the fracture or the catheter entering the brain.
Thermo-radiating brain cooling (Figure 4)
A cooling induction method used by Dohi and colleagues;63,175,176 8–12 l/minute chilled air (24 °C) via a 16 fg Foley ‘balloon’ catheter in one nostril, the other nostril was occluded with an epistaxis balloon and the air exited through the mouth. It is not completely clear but it seems that the Foley catheter balloon was inflated to prevent air leaking back out of the nostril, which may have reduced the heat loss from the nose and contributed to nasal erosion. 63 The importance of enabling the air to exhaust from the mouth is emphasised. 175 This method is contraindicated with base of skull fracture and sinusitis. 63
FIGURE 4.
(a) Thermo-radiating Brain Cooling (TRBC) was performed by nasopharyngeal cooling. (b) Scheme of TRBC: artificial nasopharyngeal circulation with chilled air (24 °C, 9–12 l/minute)175 (figure 2, p. 431). Reproduced with permission from Dohi K, Jimbo H, Ikeda Y, Matsumoto K. Pharmacological brain cooling (PBC) by indomethacin; a non-selective cyclooxygenase (COX) inhibitor in acute hemorrhagic stroke. Nosotchu 2000;22:429–34.
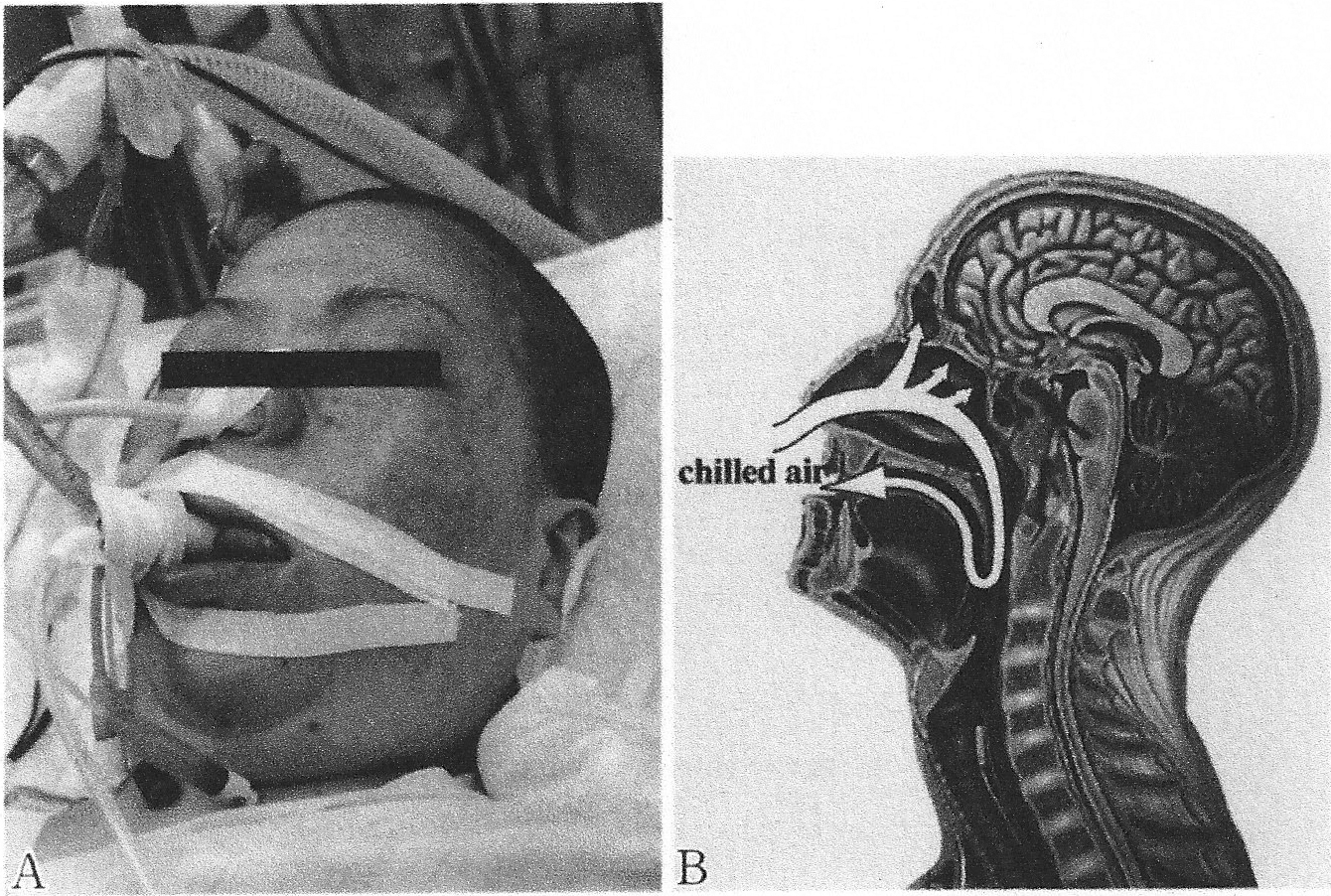
Bilateral nasal airflow
Continuous bilateral nasal airflow was used in two crossover trials in brain-injured patients. 46,47 In the first trial the air was delivered through a sponge-tipped oxygen catheter in each nostril, at room temperature and humidity and a rate of 115 ml/kg/minute (approximating normal, resting minute volume). 46 In the second, unhumidified air from the compressed air supply at twice the patients’ minute ventilation volumes, plus 20 parts per million (ppm) nitric oxide gas (mucosal vasodilatation to facilitate heat loss), and an 85-g lead weight over the facial vein on each side of the nose, to facilitate intracranial venous drainage, considered important in heat loss from the upper airway. Two methods of delivery were used. First, a Whispaflow valve with a paediatric (uncuffed) tracheal tube in each nostril and, second (and more successfully from the ease of delivery point of view), a double-airflow meter with oxygen tubing in each nostril. 47 Nasal airflow is contraindicated with base of skull fracture and possibly with facial fractures.
Rhinochill Intranasal Cooling System (Benechill Inc., San Diego, CA, USA): www.benechill.com/ (Figures 5 and 6)
This is a portable, battery-powered nasal cooling device that is primarily intended for induction of cooling, particularly pre-hospital in patients experiencing cardiac arrest. Bilateral nasal prongs (with rounded tips and spray ports on the dorsal surface) are inserted and inert perfluorocarbon coolant mixed with oxygen is nebulised in the nasal cavity where it evaporates, removing heat in the process. Gas exits through the nostrils or mouth along with any perfluorocarbon, which does not evaporate. There is an overpressure relief valve. There is no closed-loop temperature feedback. It is not designed for prolonged use (about 1 hour) and perfluorocarbon is expensive. Rhinochill has been trialled in humans after cardiac arrest49,59 and used in brain-injured patients. 54 Contraindications to use include base of skull, and possibly nasal and orbital, fractures and an unprotected airway.
FIGURE 5.
The Rhinochill device. Photo reproduced with permission from Benechill Inc., www.benechill.com.

FIGURE 6.
Rhinochill device. (a) Tubing set. (b) Control unit59 (figure 1, p. 944). Reprinted from Resuscitation81. Busch H-J, Eichwede F, Fodisch M, Taccone FS, Wobker G, Schwab T, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest, 943–9, 2010, with permission from Elsevier.
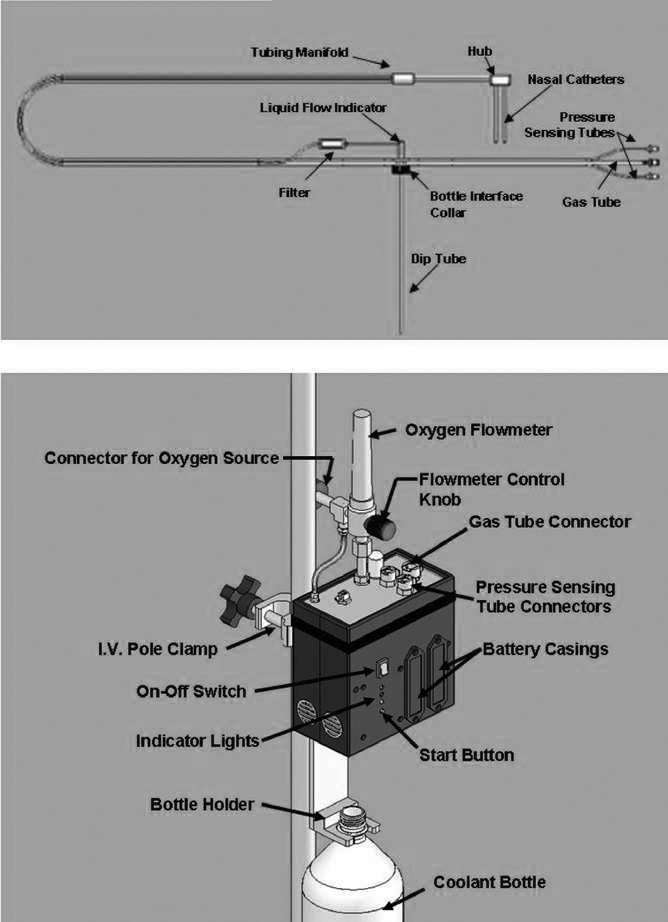
Rapid hypothermia induction device (Figure 7)
This device has been developed for use pre-hospital by biomedical engineering students and Professor Harikrishna Tandri at Johns Hopkins University. It consists of an air tank, a pressure regulator and control mechanism, and two nasal prongs that are inserted into the nostrils. Cold, dry air is flushed through the nostrils to increase evaporative heat loss from the nose and cool the brain. Animal tests have been carried out but we have had no response to a request for information on whether human testing has been conducted.
FIGURE 7.
The Rapid Hypothermia Induction Device, in development at Johns Hopkins University, is used by emergency or ambulance personnel to rapidly administer therapeutic hypothermia treatment to victims of cardiac arrest. Reproduced with permission from http://nciia.org/bmeidea2010 (accessed 4 March 2011).

Active conductive (liquid) head-cooling methods and devices: nasal and pharyngeal balloons
QuickCool Intranasal System (QuickCool AB, Lund, Sweden): www.quickcool.se/ (Figures 8 and 9)
This device comprises a portable pump and single patient use thin-walled balloon catheters. The catheters are inserted bilaterally into the nostrils and perfused with cold saline to cool the brain and to a lesser extent the body. There is no closed-loop temperature feedback. The device has been tested in healthy volunteers,65 used in patients206 and is currently being trialled in cardiac arrest, TBI and subarachnoid haemorrhage (SAH) (see Appendix 5, References to ongoing studies). The healthy volunteer testing (n = 9) used magnetic resonance spectroscopy to measure brain temperature: ‘After 60 minute of intranasal cooling brain temperature reduction was –1.7 ± 0.8 °C as measured by MRSI and –1.8 ± 0.9 °C as measured by phase mapping method’ (p. 36). 65
FIGURE 8.
Photo: QuickCool AB, Lund.
FIGURE 9.
‘Schematic representation of the cooling circuit used. Cold saline fills the nasal balloons by gravity and is actively aspirated by pumps in order to be directed through the heat-exchanger machine. The height of the bag related to nasopharynx is proportional to the pressure inside the balloons’205 (figure 1, p. 85). Reproduced from Resuscitation 76. Covaciu L, Allers M, Enblad P, Lunderquist A, Wieloch T, Rubertsson S. Intranasal selective brain cooling in pigs, 83–8, 2008, with permission from Elsevier.
Pharyngeal cooling cuff (Daiken Medical Company Ltd, Japan): www.daiken-iki.co.jp/ (Figures 10 and 11)
This is designed for use in cardiac arrest. 207 The pharyngeal cooling cuff is inserted into the pharynx after tracheal intubation and saline at 5 °C is circulated through it at 500 ml/minute at a pressure of 50 cmH2O. There is no closed-loop temperature feedback. The close proximity of the carotids to the pharynx is said to facilitate cooling of carotid blood and thence the brain.
FIGURE 10.
Pharyngeal cooling. Reproduced with permission from www.cc.okayama-u.ac.jp/∼cool/e-intou.html (accessed 17 April 2011).
FIGURE 11.
Pharyngeal cooling cuff: equipped with pressure and temperature sensors that transmit perfusion data to the circulator. Reproduced with permission from www.cc.okayama-u.ac.jp/∼cool/e-cuff.html (accessed 17 April 2011).
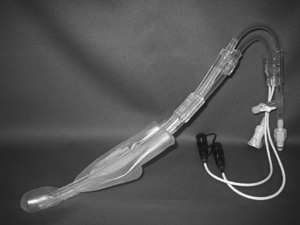
This cooling device was used during resuscitation after cardiac arrest in the Japanese i-Cool trial, which has completed but not reported yet [www.controlled-trials.com/ISRCTN98089900 (accessed 4 March 2011)]. A significant decrease in tympanic temperature was shown and another trial is planned to look at neurological outcome (Dr Yoshimasa Takeda, Principal Investigator, Okayama University Medical School, Okayama, Japan, 18 April 2011, personal communication).
Heat loss through the skull
Convective head-cooling methods and devices (air or water directed on the head)
Head fanning
Fanning the head or face with ambient air using electric fans is a simple and relatively cheap method of cooling the head. It does not produce large brain temperature reductions47 and will not on its own induce hypothermia but may help to reduce fever. 66 Face fanning can be uncomfortable. 197 Bilateral head fanning doubles the airflow and increases turbulence around the head, which will increase heat loss. It is sometimes assumed that the use of fans in ICU is associated with infection risk,67 but a review found no published data showing that electric fans spread infection in clinical areas. 68
RapidCool Hypothermia System (MedCool Inc., Wellesley, MA, USA) (Figure 12)
This device (United States Patent 7507250) was tested in patients after cardiac arrest but is not commercially available. It ‘… directs jets of cold water (1 °C to 4 °C) through the hair directly to the scalp. Water is removed from the cooling cap by an aspiration system located about the inner rim of the cap, fixed just above the ears of the patient’ (p. 461). 194
FIGURE 12.
MedCool rapid head-cooling device194 (figure 1, p. 461). Reproduced from Am J Emerg Med 27. Wandaller C, Holzer M, Sterz F, Wandaller A, Arrich J, Uray T, et al. Head and neck cooling after cardiac arrest results in lower jugular bulb than esophageal temperature, 460–5, 2009, with permission from Elsevier.
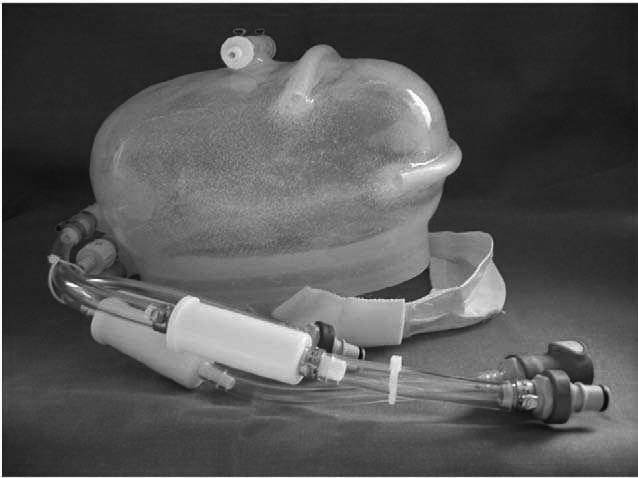
Kholod 2, Kholod 2-F and Thermokholod FV devices, USSR (Figures 13–17)
These are variations of a convective head-cooling device using water or water and alcohol [Kholod 2 (Figure 13) and 2-F (Figures 14 and 15)] and air [Thermokholod FV (Figure 16)], respectively. They were developed in the USSR in the mid-1960s and used in TBI, cerebral hypoxia (e.g. after cardiac arrest), epilepsy and surgery and do not seem to have had closed-loop temperature feedback. 96,97,210,211 Very limited patient data are reported, although ‘many clinical trials’ are mentioned. 210 Smirnov says of the Thermokholod FV that ‘As clinical tests have shown, the brain can be cooled at various controlled rates (up to 0.3 °C/minute) and the reduced temperature continuously maintained with an accuracy of ± 0.5 °C’ (p. 259). 211 The Kholod 2 and 2-F could also be used for warming. Because body temperature reduced as a result of head cooling, Smirnov and colleagues210 developed two body-warming devices – one electric and one using air delivered through a transparent ‘tent’ weighted at the sides (Figure 17) – to maintain body temperature (31–36 °C) during head cooling or for warming during surgery. 210 The electric body-warming device had closed-loop temperature control to the patient’s rectal temperature.
FIGURE 13.
Diagram of Kholod 2 helmet. Apparatus for cooling (heating) of the brain (helmet). 1, Main pipe; 2, openings for escape of heat carrier; 3, hollow elements (tubes); 4, collector; 5, stops restricting length of flow210 (figure 2, p. 344). Smirnov O. New method for cooling (or heating) of the body and an apparatus for craniocerebral hypothermia. Biomed Eng 1968;2:343–7. With kind permission from Springer Science and Business Media.
FIGURE 14.
The ‘Kholod 2-F’ cold unit. 1, Control panel; 2, access door; 3, casing; 4, front wheel locking lever; 5, carrying handles; 6, collecting chamber for heat carrier; 7, helmet210 (figure 3, p. 345). Smirnov O. New method for cooling (or heating) of the body and an apparatus for craniocerebral hypothermia. Biomed Eng 1968;2:343–7. With kind permission from Springer Science and Business Media.
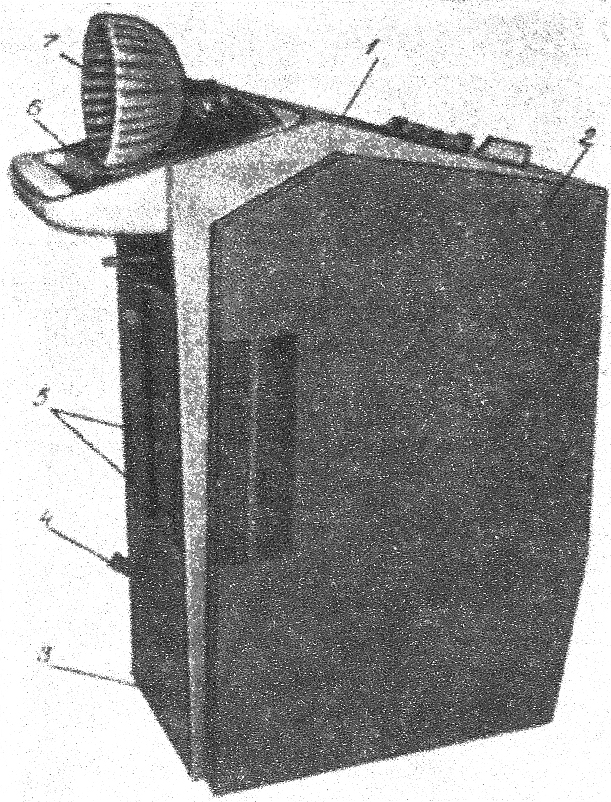
FIGURE 15.
Kholod 2-F device Photo: RIA Novosti 01.11.1970. Reproduced with permission from http://visualrian.com/images/item/31265 (accessed 8 March 2011).
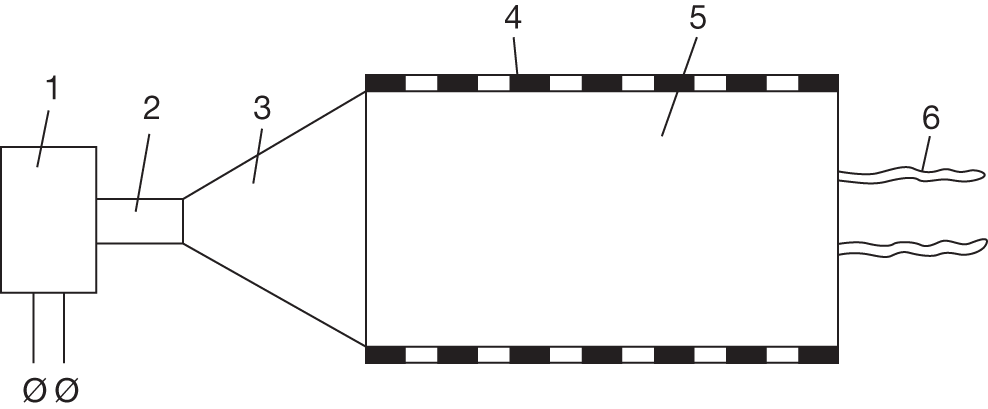
FIGURE 16.
The air and Freon system of the ‘Thermokholod FV’ apparatus. 1, VS-0.7–3 refrigerating plant; 2, heat exchanger; 3, TRV-2M heat-regulating valve; 4, centrifugal ventilator; 5, lower air pressure conduit; 6, upper air pressure conduit; 7, intake air conduit; 8, apparatus for jet cooling; 9, air collector211 (figure 3, p. 258). Smirnov O. A method of increasing the efficiency of air hypotherms and an apparatus for craniocerebral cooling. Biomed Eng 1969;3:257–60. With kind permission from Springer Science and Business Media.
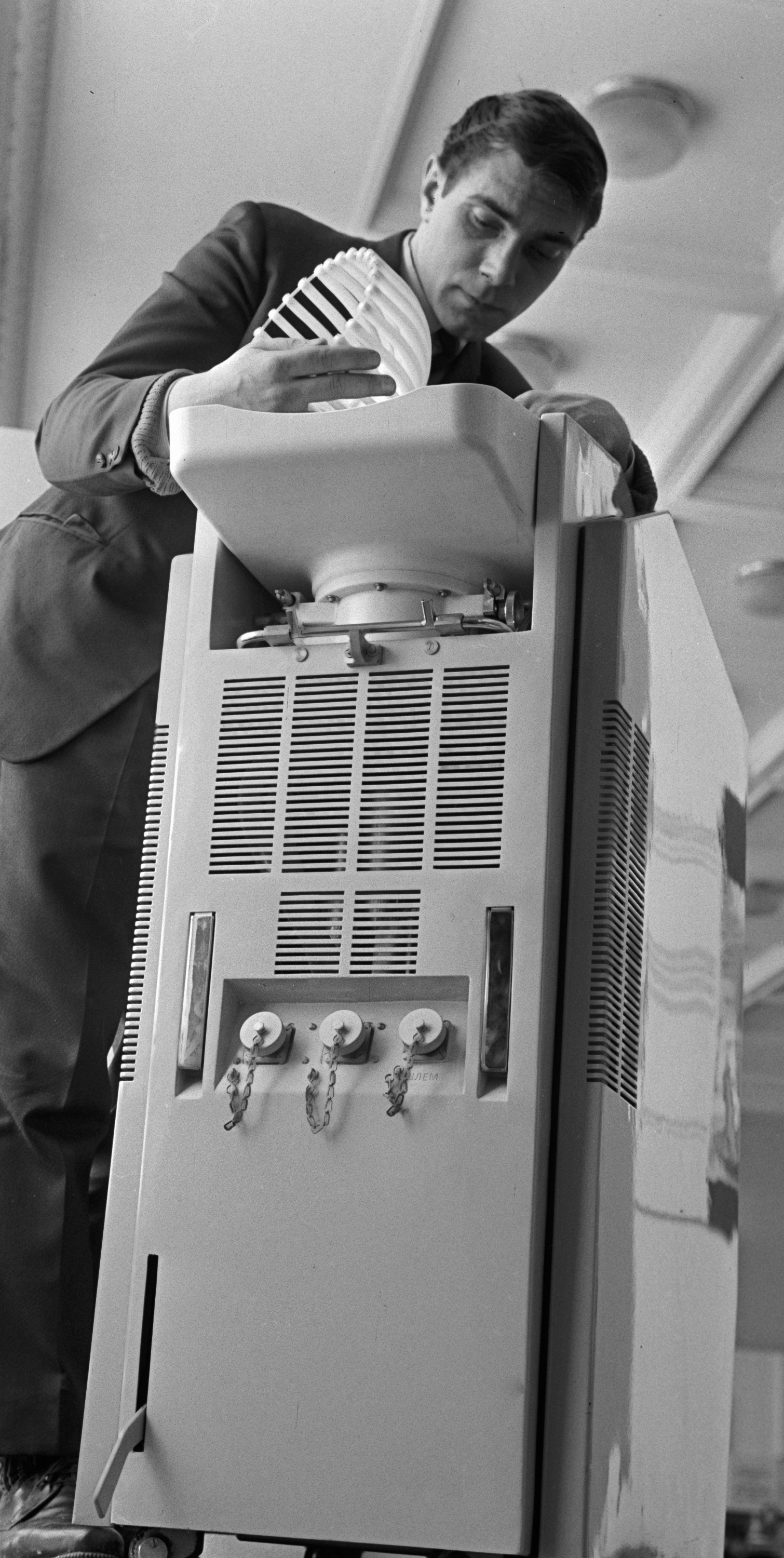
FIGURE 17.
Apparatus for air heating (cooling) of the body. 1, Hot-air machine; 2, flexible hose; 3, funnel; 4, weights; 5, pneumatic casing; 6, ties210 (figure 5, p. 345). Smirnov O. New method for cooling (or heating) of the body and an apparatus for craniocerebral hypothermia. Biomed Eng 1968;2:343–7. With kind permission from Springer Science and Business Media.
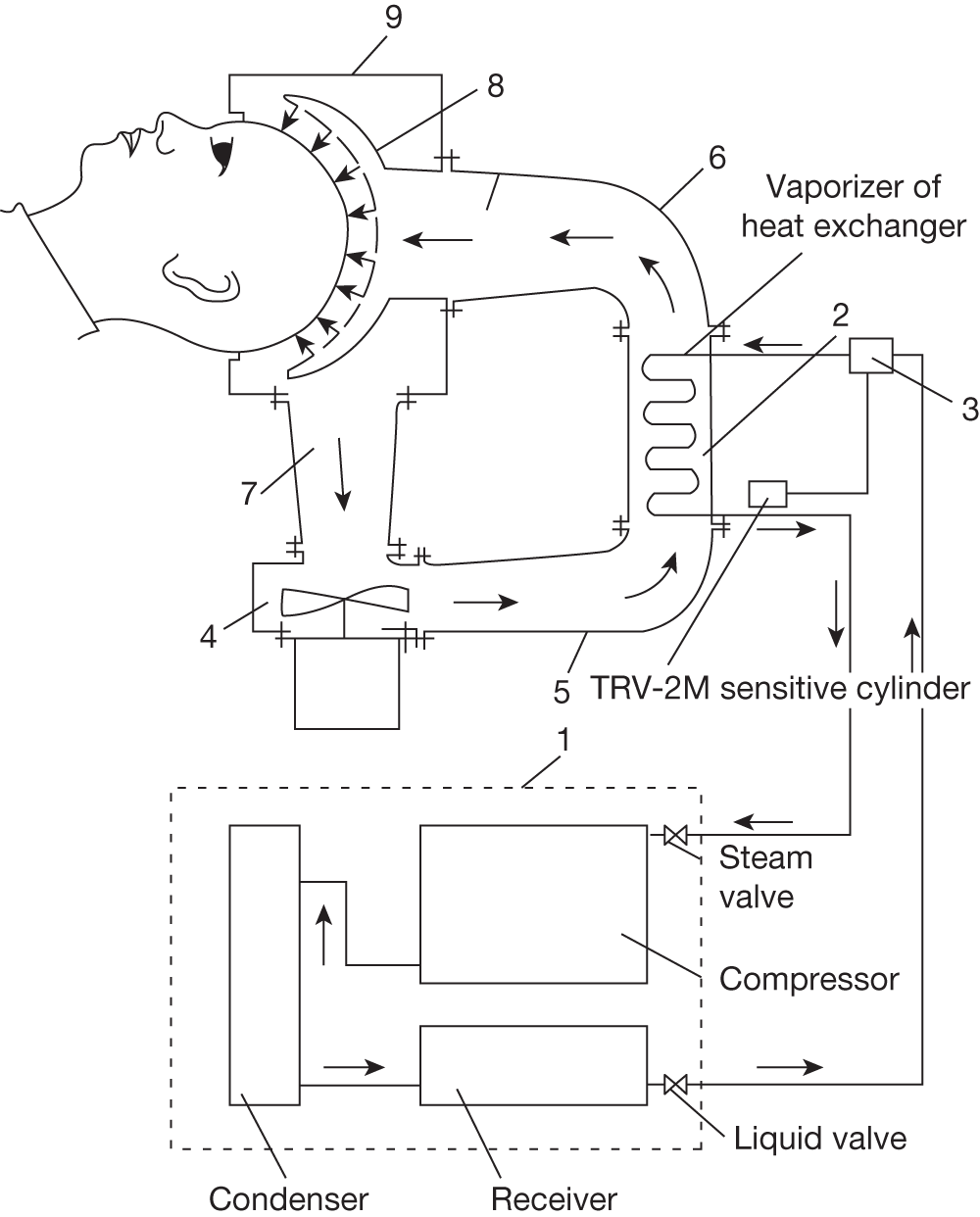
The complete ‘craniocerebral hypothermy’ system therefore consisted of three separate units: the head cooling (or warming) unit (Kholod 2, Kholod 2-F or Thermokholod FV), the body warming (or cooling) unit and a temperature measurement unit which could measure four temperatures (tympanic membrane, nasopharynx, oesophagus and rectum). It seems that intracranial temperature was inferred from tympanic temperature on the basis of experimental data in dogs.
The Kholod 2 and 2-F units are described by Smirnov. 210 They used water, or a 10–20% mixture of water and alcohol, as a coolant and had a helmet with a collecting chamber, an electric pump, a heat exchanger and a temperature controller, housed in a wheeled cabinet. The helmet was specially moulded to the head (anthropologists were consulted over the shape so that it would fit a range of head sizes) and had evenly distributed tubes, with openings on the head side and the coolant collecting chamber, which also acted as a head rest, under the head. The helmet was adjusted to the patient’s head by a hinge and with the collecting chamber was height adjustable; in the Kholod 2-F these were detachable. Coolant was pumped through the heat exchanger into the top of the helmet, through the tubes and sprayed out perpendicularly onto the head. ‘Stops’ restricted the reach of the coolant jets – quite how is not clear but presumably this was to avoid liquid spraying beyond the helmet. The coolant drained into the collecting chamber, which had openings for this purpose, and was recirculated. Coolant temperature could be automatically controlled between –3°C and +14°C during cooling and between 33°C and 43°C during warming. This method of head cooling was said to be more effective than other methods, such as rubber helmets, because it was convective rather than conductive and overcame ‘adverse conditions produced by the hair’.
The Thermokholod FV (Figure 16) blew cold air over the head and could be used over head dressings. 211 It seems to have been developed to avoid the problems associated with spraying water on the head in the presence of wounds. Air was delivered to the surface of the head in a way that broke up the current: ‘… into separate streams and distributed them evenly over the treated surface in such a way as to ensure run-off of “exhausted” air. In this way breakdown of the boundary layer of air at the surface of the head was achieved and a compulsory convective heat exchange assured between the surface of the head and the air current’ (p. 259). 211
‘Fluidocraniotherm’
This device is similar to the Thermokholod FV but was developed and used in-house at the NV Sklifosovskiy Scientific Research Institute of Emergency Medicine, Moscow, for craniocerebral hypothermia, i.e. ‘predominant cooling of the brain through the external layers of the head’. 92 It cooled by forced convection of air. The reason given for using air was that it was suitable directly after surgery; wounds were covered with ‘cerigelum’ or a collodion dressing. Fifty-six patients were cooled with the ‘Fluidocraniotherm’. Some received repeat cooling if their intracranial pressure and cerebral blood flow measurements showed their condition was worsening (how these were measured is not described). The device had an inbuilt fan which blew air into a helmet covering the whole head down to the eyebrows. The air temperature was controllable between –5°C and 40°C and the patient’s temperature was measured either in the ear canal, oesophagus, rectum or (n = 15) brain (see Chapter 4, Historical reports of head cooling for method). Target brain temperature (cortex) was 28–30°C with rectal temperature of 33–43°C. In patients without brain temperature monitoring (n = 41), brain temperature was inferred from nomograms (see Chapter 4, Historical reports of head cooling). Temperature control seems to have been manual rather than closed-loop. Cooling was delivered for 6–29 hours, during which time patients were anaesthetised. Once temperature was lowered (after 2–18 hours), cooling was maintained by ice packs on the head and over major blood vessels, and 1% aminopyrine given two or three times daily.
Prototype convective cooling hood (KCI, Ferndown, Dorset, UK)
This device was tested in volunteers (n = 5) with magnetic resonance spectroscopy brain temperature measurement; it is not commercially available. 24 A hood and collar made of a double layer of nylon, with holes for air flow in the inner layer, delivered air at approximately 14.5 °C and 15 m s–1 through neoprene insulated tubing from the cooling machine in the scanner control room. The hood and collar could be independently clamped off to allow head and/or neck cooling to be delivered.
Active conductive (liquid) head-cooling devices (circulating cold fluid)
Swedish Air Force head-cooling helmet
Mellergard borrowed an Air Force cooling helmet for clinical use. It was:
… made of fabric enclosing the whole head and part of the neck [with] a system of thin plastic channels on the inside, through which water circulated. The water was supplied from a thermostat bath, with an optional temperature range between 5 and 40 °C, and was continuously circulated through an electromechanical pump. 184
Cincinnati Sub-Zero (CSZ) head wrap (Cincinnati Sub-Zero Products Ltd, Cincinnati, OH, USA): www.cszmedical.com/ (Figures 18 and 19)
This is part of the whole-body hypothermia single-patient use Kool-Kit system, which is an example of a combined head and body device, though either part can be used on their own. Cold water is circulated through the head wrap by the Blanketrol III unit. The head wrap is not pressurised. The company thought that the head wrap had been used on its own for head cooling but had no details of any studies. Our searches showed it had been used by Gaida and colleagues. 51
FIGURE 18.
Cincinnati Sub-Zero (CSZ) head wrap. Photo reproduced with permission from CSZ Products Ltd, http://www.cszmedical.com/Products/Hyper-Hypothermia/wholebodyhypothermia.htm (accessed 25 April 2011).

FIGURE 19.
Blanketrol III. Photo reproduced with permission from CSZ Products Ltd, http://www.cszmedical.com/Products/Hyper-Hypothermia/Blanketrol-III.aspx (accessed 25 April 2011).

TraumaTec Neuro-Wrap (TraumaTec Inc., San Antonio, TX, USA) www.traumatec.com/ (Figure 20)
This is very similar to the CSZ head wrap but portable and is currently being trialled in Neurosciences ICU patients53 (see Appendix 5, References to ongoing studies). The following information was kindly provided by Professor Claudia Robertson the principal investigator:
The TraumaTec Neuro-Wrap is a helmet-shaped water blanket of soft plastic, with flaps that fit snugly over the head and circumferentially around the neck. The cranial flaps are designed to accommodate ICP monitors, head dressings, or other clinical paraphernalia, and can be adjusted to ensure maximal surface contact. The Wrap has high-flow fluid channels to create conductive heat transfer from the scalp and carotid arteries, thus achieving cooling of the brain. Fluid circulation is provided by a small portable refrigeration/pumping unit, designed specifically for this device.
(Professor Robertson, 3 January 2011, personal communication)
FIGURE 20.
TraumaTec Neuro-Wrap. Photo courtesy of Susanne Richard, TraumaTec, Inc., San Antonio, TX.
Cool Brain Cooling Helmet (Figure 21)
This device originated as a spin-off from the National Aeronautics and Space Administration (NASA) space suit technology and was used in the COOL BRAIN stroke study50. It consists of two components, the head/neck liner (Figure 21) and the conditioning unit. The inner liner is made of thin, urethane laminated nylon fabric with a circulating liquid cooling heat exchanger. Over that is a pneumatic liner which is pressurised to improve contact with the head and neck. The whole is adjustable to improve the fit and has access openings on each side over the Kocher points and anteriorly at the midline of the neck. The conditioning unit is portable (mains or battery power). It contains an insulated ice reservoir with heat exchanger and the control system for temperature and coolant circulation. This device is now commercially available as the WElkins EMT system (WElkins, LLC, Roseville, CA, USA; URL: welkinsmed.com).
FIGURE 21.
‘Helmet worn by William Elkins, a NASA scientist, who invented this technology. The cooling helmet has an outer pneumatic liner pressurized to allow close contact with the cranium and neck. The device also is adjustable to fit a significant range of head sizes’50 (figure 1, p. 273). Reproduced with permission from Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg 2004;100:272–7.

Discrete Cerebral Hypothermia System, CoolSystems Inc. (Alameda, CA, USA) (Figure 22)
Harris and colleagues45 described their experience with this device. Pros were ease and speed of application, facilitated by the portability of the device, and ease of use. Cons were problems with regulation of water temperature, inadequate contact of the cap with the head (this was despite the cap being pressurised) and the desired intracranial temperature and intracranial/bladder temperature gradient not being achieved. The researchers suggested that a coolant other than water might be better.
FIGURE 22.
‘The Discrete Cerebral Hypothermia System cooling cap. (a) Photograph of the cooling cap. (b) Photograph of a patient from our study wearing the cooling cap. The ICP and temperature monitor can also be seen’45 (figure 1, p. 1257). Reproduced with permission from Harris OA, Muh CR, Surles MC, Pan Y, Rozycki G, Macleod J, et al. Discrete cerebral hypothermia in the management of traumatic brain injury: a randomized controlled trial. J Neurosurg 2009;110:1256–64 [Erratum appears in J Neurosurg 2009;110:1322.]
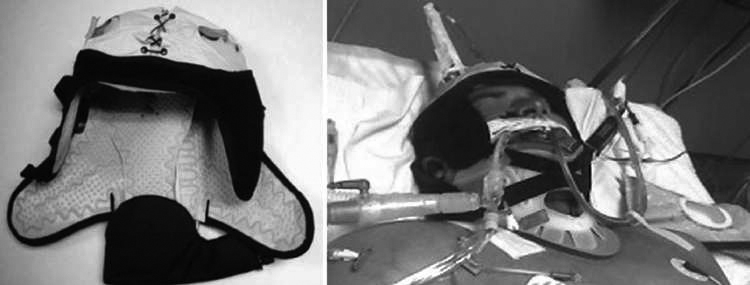
However, Harris and colleagues45 were aiming to achieve an intracranial temperature of 33 °C, while bladder temperature was maintained at 36 °C with body warming. This is a challenging target. The lack of success is attributed to deficiencies in the head-cooling device but it is questionable whether such a large brain/body temperature difference is achievable in humans without isolating the cerebral and corporal circulation from each other (e.g. Schwartz and colleagues212), although even steeper gradients have been achieved in animals (e.g. Natale and D’Alecy213 and Barone and colleagues214).
Device used at The Affiliated Hospital of Hangzhou Teachers College, Hangzhou, Zhejiang, China (Figures 23 and 24).
This was a cooling cap with circulating water (4 °C) (Figure 23) and an adjustable neck band containing frozen ‘blue ice’ packs (Figure 24). 75,147 The water was circulated by a ‘hypothermia machine’ (KN 01, EBM, Beijing, China) and the neck packs were replaced every 3–4 hours as they thawed.
Device developed at The First Clinical Hospital, Harbin Medical University, Harbin, Heilongjiang Province, China
Zhang and colleagues182 and Tang and colleagues181 contain information on the development of a head-cooling device. Zhang and colleagues182 is a study in two healthy volunteers with the head-cooling device showing reduction of cerebral glucose metabolism with positron emission tomography. Tang and colleagues181 refers to using the device in stroke patients but provides no details and we have been unable to find more information. The other Chinese studies conducted in Harbin found for the review appear to use the same device. 148,149,183 It is a circulating water device and described as ‘experimental apparatus for medical brain hypothermia controllable semiconductor protection type refrigeration apparatus TER-40A from the Harbin Institute of Technology Education thermal Research Development Office’. 183 The Chinese centre for the CHIL trial (see Appendix 5, References to ongoing studies) is Harbin, and we have contacted the Chief Investigator for CHIL (in Australia) for more information. Our request has been received but we have had no response yet.
Device developed by the Equipment Department, Jilin Provincial Brain Hospital, Siping, Jilin, China
Description of a computerised cooling device that circulates water through a hat and pads at temperatures of between 3 °C and 25 °C. 215
Human brain hypothermia system developed by the Electronics and Computer Education Department, Faculty of Technical Education Gazi University, Ankara, Turkey
The system comprises ‘a microcontroller-based control card, four different temperature measurement circuits, a electronic control card module, a water circulation system, a switching mode power supply, and a helmet’ (p. 502). 216 The temperature range is –5 °C to 46.15 °C because it is intended that the device could also be used for hyperthermic tumour therapy. This device is undergoing animal testing and has not yet been used in humans (Dr Guler, 28 February 2011, personal communication).
Helmet for emergency cooling in head injury, Japan
A device is described which was developed by a team from Niigata Sangyo University, Tokyo Denki University and Sapporo Medical University, School of Medicine. It uses a Peltier chip to cool water, which circulates through the helmet. 217,218 We have had no response to a request for information on whether or not this has been used in humans.
Device developed at the Institute of Semiconductors, Academy of Sciences of the USSR, A.P. Polenov Leningrad Scientific-Research Neurosurgical Institute, USSR (Figures 25 and 26)
This device (Figure 25) had been used clinically for 2 years, although details were not reported, and was said to have fewer complications than other methods of head cooling such as ‘spray helmets’ (possibly the Kholod 2) and to be easier to use, provide better temperature control and more reliable than devices that required the coolant to be replaced or Freon refrigerator devices. It had two separate parts: a helmet containing a thermopile and a control unit. The helmet had three layers (Figure 26). The outer part was reinforced glass fibre with 17 serially connected thermocouples [‘intermetallic compounds on a base of bismuth telluride with branches having p- and n-type conductivity’ (p. 240)]219 which generated cold. The middle layer, in which the thermocouple cold-junction collectors sat, contained a heat-transfer liquid with a low freezing point. This middle layer was filled and drained through a tube on the outside of the helmet. The inner layer was stretchy rubber which was conformed to the head by adjusting the quantity of heat-transfer liquid. A rubber hood covered with insulating material was placed over the whole helmet to hold it tight to the head. The control unit had a temperature measurement circuit, supplied the current (high current rectifier) and circulated the water which removed the heat from the thermopile.
FIGURE 25.
General view of electronic device for hypothermia of the brain219 (figure 1, p. 241). Kolenko E, Bezukh M. Electronic device for hypothermia of the brain. Biomed Eng 1971;5:239–41. With kind permission from Springer Science and Business Media.
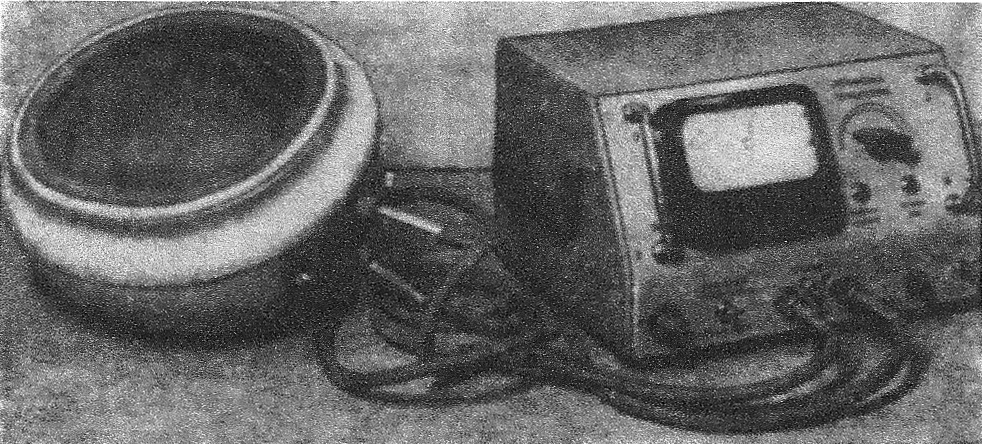
FIGURE 26.
Schematic cross-section of helmet. 1, Thermocouples; 2, collectors; 3, rubber membrane219 (figure 2, p. 241). Kolenko E, Bezukh M. Electronic device for hypothermia of the brain. Biomed Eng 1971;5:239–41. With kind permission from Springer Science and Business Media.
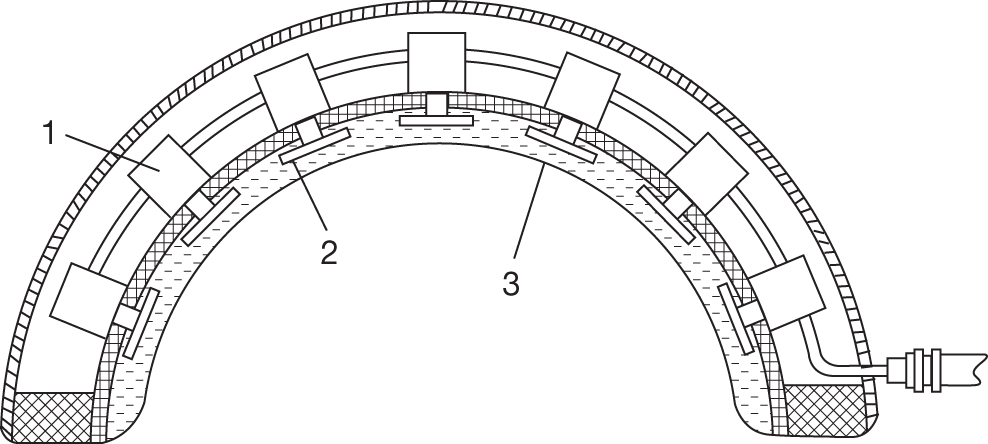
Rubber, water circulating head-cooling device, USSR (Figure 27)
This is described as an easy-to-use helmet device, made of readily obtainable parts, which circulates water at 8–10 °C. 220 Use of the device in nine patients with TBI is mentioned but few details are given.
FIGURE 27.
1, Rubber helmet; 2, rubber tubing; 3, tap to run water into serpentine coil (4) in a case (5) with insulation (6). The waste runs out into a washbasin220 (p. 47, translated from Russian). Reproduced with permission from Reut NI. [Technic of continuous therapeutic craniocerebral hypothermia in acute craniocerebral injury.] [Russian.] Ortop Travmatol Protez 1970;31:47–8.
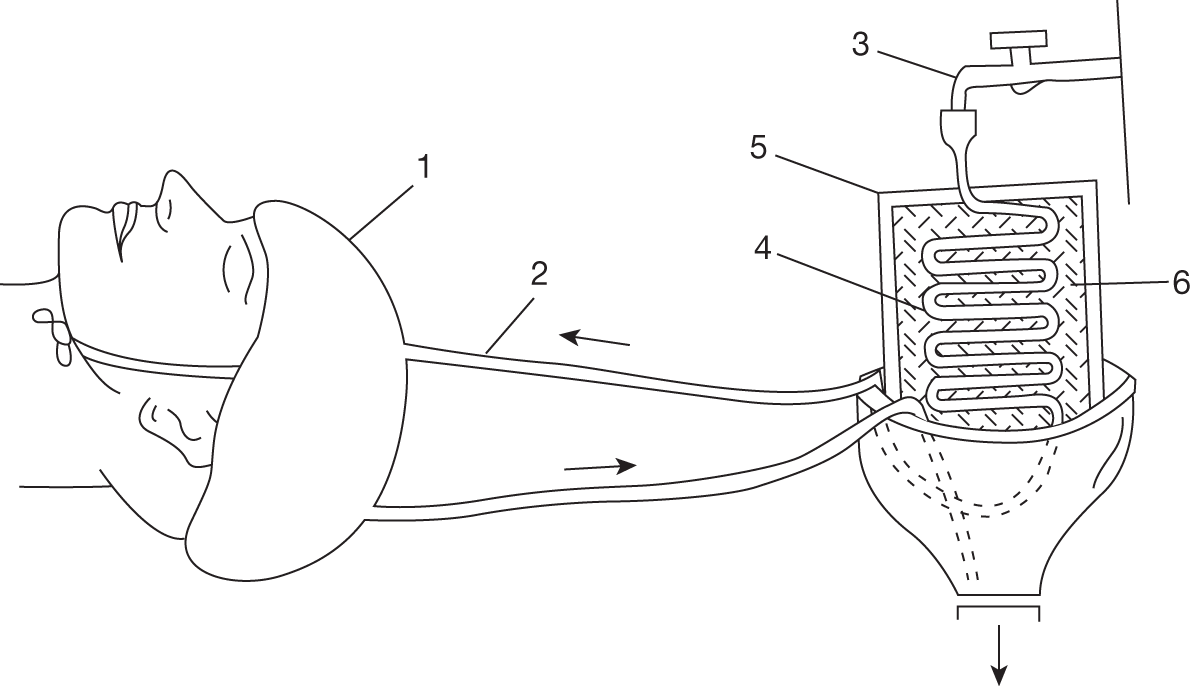
Helmet for focal or global head cooling, USSR (Figure 28)
This device is described as being suitable for severe TBI with haemorrhage. No patient data are reported. 221 It seems to have been designed for either focal cooling, if one pocket has coolant flow, or more global head cooling if all pockets are used.
FIGURE 28.
Helmet, which reduces temperature for focal and global hypothermia: a, front; b, side; c, back221 (p. 68, translated from Russian). Reproduced with permission from Okhrimenko NN, Zaikin VS. [Use of regional hypothermia of the head for treating acute disorders of the cerebral circulation.] [Russian.] Voen Med Zh 1974;1:68–9.
Passive (non-circulating) conductive head-cooling methods and devices
Ice packs
Ice packs to the head have been used for cooling in brain injury52 and cardiac arrest. 48 In patients during out-of-hospital resuscitation, Callaway and colleagues48 put three bags each containing 500 ml of shaved ice around the head and draped a fourth bag across the neck. Forte and colleagues’ patients52 had had decompressive craniectomy and the ice packs were applied to the site of bone removal; unsurprisingly, this may be more effective in reducing temperature than in patients with an intact cranium.
Rubber cooling helmet filled with ice and salt, Chita Medical Institute, Chita, USSR (Figures 29 and 30)
Zhmurko93 reports on the cooling helmet designed and used at the Chita Medical Institute (now the Chita State Academy of Medicine). This was adapted from a locally made rubber anti-gas helmet and was made in different sizes so that it could be fitted tightly to the head to help ensure uniform cooling. It had two layers, between which was a mixture of ground up ice or snow with 33% salt. This mixture produced a temperature of –21°C and could maintain hypothermia for up to 3 hours; if longer cooling was required the helmet was refilled.
FIGURE 29.
Rubber cooling helmet filled with ice and salt (figure 1 from Zhmurko93). Reproduced with permission from Zhmurko SF. [Cranio-cerebral hypothermia in patients with acute cranio-cerebral injury.] [Russian.] Khirurgiia 1971;47:40–3.

FIGURE 30.
Rubber cooling helmet shown on head (figure 2 from Zhmurko93). Reproduced with permission from Zhmurko SF. [Cranio-cerebral hypothermia in patients with acute cranio-cerebral injury.] [Russian.] Khirurgiia 1971;47:40–3.
Sovika head and neck cooling device (Sovika GmbH, Jesewitz, Germany) www.sovika.de/ (Figures 31 and 32)
This is a portable reusable device with gel-filled pads. It is stored flat at 4 °C and wrapped round the head and neck conforming to shape. This device is currently being trialled in the i-Cool pilot study in stroke patients (see Appendix 5, References to ongoing studies).
FIGURE 31.
Sovika head and neck cooling device. Photo courtesy of Sovika GmbH, Jesewitz, Germany.

FIGURE 32.
Sovika head and neck cooling device in situ. Photo courtesy of Sovika GmbH, Jesewitz, Germany.
Other makes of gel cap
Other gel caps that have been used for head cooling are the Hypotherm Gel Kap (Flexoversal, Hilden, Germany),184 the Frigicap (a scalp-cooling cap for chemotherapy) (Figure 33) refrigerated to –4 °C, applied over a paper cap and replaced hourly to keep the cap temperature low,69 and an elastogel cap (Figure 34) by Southwest Technologies (Kansas, MO) (www.elastogel.com/product-catalog/hot-a-cold-therapy/head-facial-therapy), which was carried in a mobile fridge at –5 °C to use in out-of-hospital cardiac arrest. 70
FIGURE 33.
Frigicap containing aqueous glycerol69 (figure 1, p. 277). Reproduced from Resuscitation 51. Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study, 275–81, 2001, with permission from Elsevier.

FIGURE 34.
Elastogel cap on a patient after cardiac arrest70 (figure 1, p. 98). Reproduced with permission from Storm C, Schefold JC, Kerner T, Schmidbauer W, Gloza J, Krueger A, et al. Prehospital cooling with hypothermia caps (PreCoCa): a feasibility study. Clin Res Cardiol 2008;97:768–72.
Scalp-cooling devices
Scalp-cooling devices are listed separately here because they are not marketed for use in brain injury. However, some of them may be suitable for brain cooling.
Liquid (active) scalp-cooling caps
-
Paxman Scalp Cooler (Paxman Coolers Ltd, Huddersfield, UK; www.paxman-coolers.co.uk). The company believes the device has been used in accident and emergency but has no information on this and does not support uses other than scalp cooling.
-
Dignicap (Dignitana, Lund, Sweden; www.dignitana.com/) is being used in a study in stroke patients but details are not yet available.
-
Scalp cooling system II (Amit Technology Science & Medicine Ltd, Doar Na Shimshon, Israel; www.scsii.co.il/ ). We do not know if this has been used in brain injury or whether it definitely uses liquid cooling; it is possibly a convective (air) cooling device. Requests for information from the company have met with no response.
Frozen gel (passive) scalp-cooling caps
-
Penguin Cold Cap (Penguin Cold Caps NZ Ltd, Greenhithe, New Zealand; http://penguincoldcaps.co.nz). This has not been used in brain injury.
-
ChemoCap (ChemoCap Products, Windsor, ON, Canada; www.chemocap.com/).
-
There has been no response to our query about whether this has been used in brain injury.
Non-invasive neck-cooling devices
There are some devices designed to be used only on the neck with the intention that cooling the carotids will cool the brain but there are limited patient data available.
Water-circulating Arctic Sun pads (Medivance Inc., Louisville, KY, USA; www.medivance.com/) that are specially shaped for placement over the carotid triangles were used in nine patients with SAH and intractable fever. 222 Mean brain temperature reduced by about 0.5 °C within a few hours but the reduction was not sustained. Emcools have developed their cooling pads for neck cooling (Stroke.Pad, Emcools, Vienna, Austria) but there are few data as yet. 223
There is a patent for a non-invasive neck cooling device that holds removable cold inserts over the carotids (US Patent 6682552 – Brain cooling device and monitoring system) intended for pre-hospital use in stroke patients. This was developed by Ramsden and colleagues224 at the University of Saskatchewan, Saskatoon, SK, Canada, but requests for information about whether it has actually been used in patients have met with no response.
The Sandhu Cerebral Cooling Collar (LifeCore Technologies Inc., Cleveland, OH, www.lifecoretech.com) is somewhat similar, with a removable cool pack that fits round the front of the neck. Ethical approval has been obtained to compare this device with a systemic surface cooling device (Gaymar Industries Inc., Orchard Park, NY) for fever control in stroke patients in neuro ICUs (investigator Dr Michael DeGeorgia, University Hospitals of Cleveland, Case Medical Center, Cleveland, OH). (Scott Raybuck, Life Core Technologies LLC, Cleveland, OH, personal communication).
Whether these latter two devices are much different from commercially available personal cooling neck collars, for example Black Ice (Lakeland, TS, USA), is not yet clear.
Personal cooling garments
There is a somewhat grey area between medical devices for therapeutic clinical head cooling and the myriad personal cooling systems for preventing heat strain, for example in multiple sclerosis and for military and firefighting personnel. Some of these personal cooling systems include head- and neck-cooling components, which are either soaked in water to activate and cool by evaporation or contain phase-change packs (e.g. Polar Products Inc., Arkon, OH, USA; http://store.polarsoftice.com). There are some liquid cooling garments but these are usually vests and not for head cooling. Polar Products active ice head cap is an exception and is designed for migraine sufferers. It has not been used in brain injury, although the company think it could be suitable for that purpose.
Kim and Labat225 describe the design process for a liquid cooling hood which formed part of the Minnesota Advanced Cooling Suit (MACS)-Delphi developed for use by astronauts (https://taskbook.nasaprs.com/Publication/index.cfm?action%20=%20public_query_taskbook_content%26TASKID%20=%207267; accessed 2 May 2011). The new design was made of mesh with tubing threaded through it (Figure 35).
FIGURE 35.
Minnesota Advanced Cooling Suit (MACS)-Delphi hood new design225 (figure 5, p. 825). Kim DE, Labat K. Design process for developing a liquid cooling garment hood. Ergonomics 2010;53:818–28, reprinted by permission of Taylor & Francis Ltd, http://www.tandf.co.uk/journals.

Appendix 8 Identifying patients with traumatic brain injury in the WardWatcher database
We are very grateful to Angela Kellacher, Clinical Co-ordinator for WardWatcher, for her help in setting up and running the initial search of WardWatcher.
The initial search used the Acute Physiology and Chronic Health Evaluation (APACHE) diagnosis and the SICS diagnoses. In WardWatcher, the APACHE classification categorises patients according to the primary diagnosis which has warranted their admission to intensive care. The SICS diagnoses group patients into categories and allow reason for hospital admission and multiple reasons for admission to intensive care to be captured. Neither would capture all patients with TBI, hence both were used.
The APACHE codes were:
Medical
-
21. head trauma
-
23. intracranial haemorrhage (ICH)/subdural haemorrhage (SDH)/SAH
Surgical
-
43. head trauma
-
44. craniotomy for ICH/SDH/SAH
SICS diagnoses were:
-
109. Diffuse brain injury
-
110. Intracerebral contusions/haematoma
-
111. Extradural haematoma
-
112. Subdural haematoma
-
113. Other TBI
-
137. Intracerebral haemorrhage
-
301. Diffuse head injury
-
302. Intracerebral contusions/haematoma
-
303. Extradural haematoma
-
304. Subdural haematoma
-
305. Skull fracture
-
306. Other head trauma
-
311. Multiple trauma (including diffuse brain injury)
-
312. Multiple trauma (including intracerebral contusions/haematoma)
-
313. Multiple trauma (including extradural haematoma)
-
314. Multiple trauma (including subdural haematoma)
Plus the following system failing code was included:
3. Neurological
The initial search produced a data set of patients with TBI but this also included patients who had not had TBI, therefore the data set had to be cleaned by hand. This was undertaken as follows.
Patients were removed if:
-
APACHE, primary diagnosis (hospital), primary diagnosis (unit) were all SAH/SAH (other) and/or coiling was mentioned
-
brain tumour as primary diagnosis/surgery for brain tumour when no mention of trauma
-
seizures/cerebral abscess/cerebral infarction as primary diagnoses with no mention of trauma/contusions
-
ICH with thoraco/abdominal aortic aneurysm as primary diagnosis
-
haemorrhage with hypertension as primary diagnoses and no mention of trauma/contusions
-
ICH with pregnancy as primary diagnosis
-
ICH associated with thrombotic disorders or leukaemia
-
other vascular/neurological disorder as primary diagnosis.
Patients were kept if:
-
trauma related to head was in any diagnosis (including relevant fractures, e.g. skull in ‘other’ diagnoses)
-
extradural, epidural, SDH (note: although SDH can on rare occasions be aneurysmal, expert consensus was that it was better to include these patients)
-
diffuse brain injury
-
intracerebral contusions.
Appendix 9 Information for members of the public
Head-cooling therapy after brain injury
You are being invited to read and comment on the following information because our research team is interested in the views of members of the public.
Brain injury and cooling
Patients who have suffered a brain injury, for example from an accident or a stroke, often have a raised temperature. This may increase damage from the injury and contribute to swelling in the brain, which can increase damage further still. It is usual to give these patients cooling therapy to try and restore their temperature to normal. If brain swelling is a particular problem, patients may be cooled to below normal temperature (hypothermia therapy).
The usual cooling therapies include drugs, such as paracetamol, washing with cool water and machines that circulate cold water through pads or blankets applied to the body. These therapies reduce the temperature of the whole body. But for some time it has been thought that it could be helpful to apply cooling to the head alone. This targets the site of the injury and may reduce the side effects of cooling the entire body, particularly when temperature is reduced to below normal.
Head cooling is already being successfully used in babies who suffer brain damage from lack of oxygen at birth. The problem with applying cooling to the head in adults is that it may not have enough effect on brain temperature to be helpful. Head cooling is therefore not yet part of normal care in adults and is still being researched.
Head-cooling research to date
We have recently reviewed the research evidence on head cooling in brain injury to assess the current state of knowledge. The patients who received head cooling in these research studies were very ill – unconscious and sedated – and had their brain temperature measured as part of their normal care. Two main methods of head cooling were used:
-
delivery of cooling gas through the nose
-
helmets with cold water circulating through the lining.
On the next page there are photos of these methods.
Side effects from the cooling methods were generally minor and got better after cooling stopped. They included whitening of the nose from cold and small areas of skin damage.
Head cooling reduced brain temperature by at least 1 °C within about 1 hour, which may be potentially helpful. However, the research so far does not provide information on whether patients recover better from their injuries as a result of head cooling. That is the real measure of effectiveness of any treatment.
Further research
In summary, we know that head cooling can reduce brain temperature but we do not yet know enough about its effects on recovery to use it as part of normal care. Further research is needed to assess this. Because this kind of research is done when people are very sick, they are not able to give their own consent to take part in the research. Some people have a welfare guardian who can give permission on their behalf, but otherwise a close family member is asked. The delay in finding someone to give permission can mean that the person cannot take part in the research at all. In England a doctor who is involved in the patient’s care, but who is not involved in the research, can give permission. It is possible that the law will be changed in Scotland to allow this.
Any comments you may have about head-cooling therapy and research, including the issue of permission, will be very much appreciated.
List of abbreviations
- ADL
- activities of daily living
- APACHE
- Acute Physiology and Chronic Health Evaluation
- BI
- Barthel Index
- CCH
- craniocerebral hypothermia
- CHIL
- Cerebral Hypothermia in Ischaemic Lesion trial
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPC
- cerebral performance category
- CSF
- cerebrospinal fluid
- CT
- computerised tomography
- DGH
- district general hospital
- ESS
- European Stroke Scale
- FIM
- Functional Independence Measure
- GCS
- Glasgow Coma Scale
- GOS
- Glasgow Outcome Scale
- HIE
- hypoxic–ischaemic encephalopathy
- ICH
- intracranial haemorrhage
- ICP
- intracranial pressure
- ICU
- intensive care unit
- MeSH
- medical subject heading
- mRS
- modified Rankin Scale
- N/A
- not applicable
- NASA
- National Aeronautics and Space Administration
- NDS
- neurological deficiency score
- NIHSS
- National Institutes of Health Stroke Scale
- ppm
- parts per million
- RCT
- randomised controlled trial
- ROSC
- return of spontaneous circulation
- SAH
- subarachnoid haemorrhage
- SD
- standard deviation
- SDH
- subdural haemorrhage
- SICS
- Scottish Intensive Care Society
- SOD
- superoxide dismutase
- TBI
- traumatic brain injury
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health