Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/304/229. The contractual start date was in December 2007. The draft report began editorial review in September 2011 and was accepted for publication in May 2012. The authors identified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Woods et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Reminiscence interventions in dementia care
The development and evaluation of therapeutic interventions intended to benefit people with dementia and their family carers is the subject of much research interest at present. In view of the large and growing numbers of people with dementia, and the costs associated with meeting needs for care, there are clear advantages for health- and social-care services in supporting people with dementia in the community for longer but less intensively. However, there is consensus that this should not be at the cost of an additional burden on family carers. 1
Most attention has been given to pharmacological interventions, but there is increasing recognition that psychosocial interventions may have comparable value,2,3 and may be preferable in some contexts, for example where medication may be ineffective or have negative side-effects. 3,4 A number of systematic reviews of psychosocial interventions are now available,1,5,6 as well as a number of Cochrane reviews of specific approaches. 7,8
In the UK, reminiscence therapy appears to be the best-known therapeutic approach to working with people with dementia. For example, over half of care homes in Wales claimed to offer this approach to their residents according to a 2002 survey. 9 Reminiscence work with people with dementia has an extensive history,10,11 engendering enjoyable activities that promote communication and well-being. One factor in its popularity is that it works with early memories, which are often intact for people with dementia, thus drawing on the person’s preserved abilities rather than emphasising the person’s impairments. However, its popularity has not led to a corresponding body of evidence on its effects. The existing research literature was reviewed in our revised Cochrane review on reminiscence therapy for people with dementia. 12 Only four randomised controlled trials (RCTs) suitable for analysis were identified. Each examined different types of reminiscence work; all were small or of poor quality. The trials together identified significant improvements in cognition and mood 4–6 weeks after treatment and stress in caregivers who participated with the person with dementia in a reminiscence group. However, the review12 concluded that ‘in view of the limitations of the studies reviewed, there is an urgent need for more quality research in the field’. This dearth of evidence is reflected in the National Institute for Health and Clinical Excellence (NICE) and Social Care Institute for Excellence guideline on the management and treatment of dementia,3 which found insufficient evidence to recommend that reminiscence should be routinely offered to people with dementia, although its potential impact on mood of the person with dementia was highlighted.
Since the publication of the Cochrane systematic review,12 six papers have been identified as reporting research looking at various aspects of reminiscence therapy for people with dementia. Two papers13,14 appear to be based on the same community-living sample, with the later paper14 providing a reanalysis broken down by type of dementia (Alzheimer’s vs vascular dementia). A third paper15 reports an institutional sample. Positive findings on cognition and mood are reported from the institutional study, while the effects on people with vascular dementia emerge as longer lasting than those on people with Alzheimer’s in the community sample, in relation to withdrawal and cognition. None of the papers involved family caregivers in the groups. A further three papers16–18 report studies using technology to support reminiscence in dementia, all in a care home context, and none directly involving family caregivers. Gudex and colleagues16 note that the main effects of integrating reminiscence therapy into daily care were on staff, while Hsieh and colleagues17 found that reminiscence group therapy had significant efficacy in the treatment of depressed mood and apathy in nursing home residents with mild to moderate stage dementia. Haslam and colleagues18 found that group skittles appeared to perform better than reminiscence in relation to well-being, while group reminiscence was associated with better cognitive performance. However, a number of methodological flaws mean that these results should be viewed with caution.
To take research forward, there is a need to specify clearly the exact nature of the reminiscence work undertaken and its aims. Typically, a group approach has been implemented with ‘memory triggers’ (photographs, recordings, artefacts, etc.) used to promote personal and shared memories. A relatively recent development has been to include family carers in reminiscence groups alongside their relatives with dementia. Descriptive evaluations suggest that this joint approach [described as ‘Remembering Yesterday, Caring Today’ (RYCT)19] may improve the relationship between carer and person with dementia, benefiting both. 20 As it is the breakdown of this caregiving relationship that increases the likelihood of the person with dementia being placed in an alternative care setting, such as a care home, this effect could have far-reaching implications for families, society and public spending. Our group have reported a very small pilot study evaluating this joint reminiscence approach (seven patient–carer pairs in the treatment group; four in the waiting-list control group), which showed some trends in improved quality of life for patients and reduced stress for caregivers. 21 In a larger trial platform, funded by the Medical Research Council (MRC), improvements in autobiographical memory and carer depression were associated with reminiscence groups containing 50 patient–carer pairs.
The justification for evaluating the joint reminiscence approach specifically comes from these promising pilot data and the great interest in this approach in the field of reminiscence work. 10 More generally, a recent meta-analysis1 on interventions with family carers of people with dementia suggested that joint approaches may be more effective in improving carer outcomes than approaches targeted only at the carer. The previous tradition in dementia care of providing interventions for people with dementia and their carers separately from each other is being questioned. For example, in many areas of the UK, Alzheimer Café sessions have been established with an agenda including education as well as social contact, attended by both people with dementia and their carers. 22 The emphasis has shifted from ‘person-centred care’ to ‘relationship-centred care’, with recognition of the central importance of the patient–carer relationship to the benefit of both. 22 Although a joint focus on people with dementia and their caregivers is not possible for all people with dementia, only 6% of people with dementia have no identifiable caregiver,23 and these individuals have an increased risk of entering care homes.
Economics of dementia and the role of family carers
In the UK, the number of people with dementia is estimated to be > 800,000, a figure expected to rise owing to an ageing population. 24 Health-care services will face a significant challenge in meeting the needs of an ageing population, and in the case of people with dementia there will also be a sizeable burden on informal caregivers since two-thirds of people with dementia live in private residences. This informal care by friends and family contributes £12B (55%) of the estimated £23B annual cost of dementia to the UK economy. The direct cost to the health service is £1B, and £9B is accounted for by institutional care costs. 24 Worldwide, the estimated cost of informal care is estimated to be US$251B. 25 Considering that the informal care sector is a vast resource, it is essential that when reviewing dementia care, whether in terms of interventions delivered by the NHS or social services or as fiscal measures, the effects on the caregiver are taken into account. From an NHS perspective, this is line with the NICE ‘reference case’ which reports that ‘the perspective on outcomes should be all direct health effects, whether for patients or, when relevant, other people (principally carers)’. 26
Aim and objectives
This report presents data gathered from a pragmatic RCT to evaluate the effectiveness and cost-effectiveness of RYCT joint reminiscence groups, for people with mild to moderate dementia and their family caregivers, compared with ‘usual care’. The objectives of the trial were as follows:
-
To compare the effectiveness (in ameliorating the quality of life of people with dementia and the stress on their carers) of joint reminiscence groups with participants and carers followed by reminiscence-based maintenance with that of ‘usual treatment’.
-
To compare the incremental cost-effectiveness (in ameliorating the quality of life of people with dementia and the stress on their carers) of joint reminiscence groups with participants and carers followed by reminiscence-based maintenance with that of ‘usual treatment’.
Chapter 2 Methods
Intervention
Overview
The practice of using joint reminiscence groups attended by people with dementia and their carers19 emphasises active as well as passive forms of reminiscence by both carers and the people with dementia. This approach is known as RYCT. 19 People with dementia and their family caregivers attended 12 two-hour weekly sessions, in a social setting rather than a clinical setting where possible. Community centres and museums were among the venues employed.
Each session focused on a different theme, including childhood, schooldays, working life, marriage, and holidays and journeys (Box 1). Couples were encouraged to contribute with materials brought from home. Each session blended work in large and small groups, and a range of activities including art, cooking, physical re-enactment of memories, singing and oral reminiscence. The inclusion of the person with dementia was paramount. In the joint reminiscence groups, facilitators and volunteers guided carers to allow the person with dementia to respond and to value their contribution. In certain sessions a separate activity was arranged for the carer members of the group in a separate room for part of the session. This allowed the carers to share experiences and ask questions that they might have found difficult in front of the person with dementia.
-
Introductions – names and places
-
Childhood and family life
-
Schooldays
-
Starting work
-
Going out and having fun
-
Courting and marriage
-
Homes, gardens and animals
-
Food and cooking
-
The next generation – babies and children
-
Holidays and journeys
-
Festivals and special days
-
Rounding up and evaluation
After the 12 weekly sessions, maintenance sessions were held monthly for 7 months, following a more flexible programme aimed at responding to interests of group members, as well as revisiting popular topics, continuing to follow the same principles. A session might focus on a particular decade (e.g. the 1950s) with appropriate music and video clips.
Two facilitators led each session. These facilitators came from a variety of professional backgrounds, and included occupational therapists, mental health nurses, clinical psychologists, arts workers and community support workers.
A maximum of 12 dyads (participant with dementia and carer) were invited to attend each series of groups.
The manual
The manual19 was developed during the trial platform and incorporates the experience of running the groups in that context. It provides detailed session-by-session outlines for each of the 12 weekly sessions, as well as an account of the underlying principles (Box 2) and background. It includes a number of exercises and template forms for use at various points in the sessions.
-
Value each person’s contribution
-
Make people welcome and appreciated
-
Use a rich array of memory triggers – stimulate all the senses
-
Use non-verbal communication
-
Give people plenty of time to respond
-
Use creative ways to explore memories
-
Use failure-free activities
-
Divide time: large group/small group/feedback small-to-large group
-
Make connections between people
-
Celebrate differences, achievements, individual stories, shared experience
Volunteers
The two trained facilitators in each group were supported by several trained volunteers. Volunteers covered a range of ages and came from the voluntary sector (e.g. Alzheimer’s Society and Age Concern), health professional trainees and former carers with an understanding of working with older people. The presence of volunteers meant that if, for any reason, carers were not able to attend all the group sessions, the person with dementia could still contribute to the group sessions. A number of volunteers took part in the training sessions and the groups to contribute to their own professional development.
Training of facilitators
The training programme for facilitators and volunteers is also set out in the RYCT manual. Training engenders skills in listening, interpreting behaviours, group dynamics, and enthusing carers and people with dementia. Two half-day training sessions took place before each group commenced. After each session there was time for facilitators and volunteers to prepare session notes, complete attendance forms and collate evaluation forms on how the session had gone. Further evaluation forms were collected from carers and people with dementia at the end of the first session and at the end of the 12-week programme. The originator of this intervention approach, Pam Schweitzer, conducted training sessions in each centre and was available for consultation throughout the project. A number of meetings were held at a central location, where the group facilitators could discuss the treatment groups with Pam Schweitzer and with facilitators from other centres, offering peer supervision.
Treatment fidelity
It had been planned originally to videotape a sample of group sessions and to rate these videotapes for adherence to the intervention manual, but reviewers advised a lighter touch approach. Accordingly, group facilitators were asked to ensure that an adherence checklist was completed at the end of each session, often by a volunteer who had been in a position to observe the session. The checklist was based on the application of the essential principles of RYCT as well as relating to specific aspects of each session.
Trial platform
The current study is based on a pilot study comparing these joint reminiscence groups with usual treatment as part of a trial platform funded by the MRC (2004–6), which also refined outcome measures and prepared a detailed treatment manual. The trial platform also included an additional condition where people with dementia attended reminiscence groups without their carers.
Methods
Three university centres participated in the trial (Bangor University, Bradford University and University College London). Across the centres, three joint groups and two reminiscence alone groups were run. Participating dyads were randomised to either the joint reminiscence condition or to an active control condition (reminiscence alone) or a passive control condition (treatment as usual), depending on the centre. In the Bradford centre, the Zelen randomisation method27 was trialled; participants initially agreed to complete the assessment procedures at each time point; if randomised to an active intervention, further informed consent was then sought.
Participants were recruited from local NHS services, including Memory Clinics, and from voluntary agencies such as the Alzheimer’s Society. Inclusion criteria were a diagnosis of mild to moderate dementia and the absence of severe agitation and communication problems. All participants were required to have a family caregiver able and willing to attend reminiscence sessions with the person with dementia. Sixty-five participant dyads entered the trial and provided baseline data; 57 went on to receive the intervention to which they were randomised (seven of the eight lost at this point being stage 2 Zelen refusals). The post-treatment assessment was completed by 50 dyads; a 3-month follow-up assessment was completed by 45 dyads (10 treatment as usual, 24 joint reminiscence, 11 reminiscence alone). Most of the attrition at post-treatment and follow-up assessment was accounted for by death (six) and ill-health (four), with two withdrawals at the follow-up stage. A Consolidated Standards of Reporting Trials (CONSORT) diagram for the trial platform is provided in the trial protocol to be found in Appendix 10. The median age of the people with dementia was 78 years; that of the caregivers was 72 years. The average Mini-Mental State Examination28 score was 19.3 [standard deviation (SD) 5.0] (moderate dementia 12–20; mild dementia 21–26).
Primary outcome measures were quality of life in Alzheimer’s disease (QoL-AD),29 a quality-of-life measure completed with the person with dementia in a structured interview, which is also completed on a proxy basis by the caregiver; and Relatives’ Stress Scale (RSS),30 a self-report measure of the direct impact of caregiving. Secondary outcome measures included a measure of autobiographical memory (the type of personal memory over the lifespan that should be influenced by reminiscence work), adapted for the project to include more items and better coverage of the lifespan; measures of caregiver distress and depression [the General Health Questionnaire-28 item version (GHQ-28)31 and the Geriatric Depression Scale (GDS-15)];32 measures of the quality of relationship between the person with dementia and caregiver [quality of caregiver/patient relationship (QCPR)];33 and ratings of videotaped interactions between person with dementia and caregiver in two structured situations. 34
Results
All analyses reported were undertaken using analysis of covariance on post-treatment (or follow-up scores), with baseline scores as the covariate. For most of the measures in this small sample, differences between joint reminiscence and reminiscence alone were small. For the primary outcome measures, comparing either type of reminiscence with treatment as usual, the differences were not statistically significant; the effect sizes for QoL-AD, rated by the person with dementia, were small at post-treatment (0.17) and at 3 months’ follow-up (0.40); the initial rating for the caregiver rating of the quality of life for the person with dementia (a secondary outcome) was slightly higher (0.50), but the effect size at 3 months was similar (0.33). On the primary outcome for caregivers, the RSS, effect sizes were small to moderate (0.36 and 0.31).
On secondary outcome measures, people with dementia in the joint reminiscence group had significantly better autobiographical memory at post-treatment than those receiving treatment as usual (effect size 0.61; p = 0.007), but this was not maintained at follow-up. Caregivers involved in the joint reminiscence group reported less depression at post-treatment than those in the treatment as usual condition, a difference that was maintained at follow-up (effect size 0.57, p = 0.013, and effect size 0.42, p = 0.024, respectively). These findings were also clear when treatment as usual was compared with either type of reminiscence, with reminiscence work associated with better autobiographical memory at post-treatment, but not at follow-up, and the reminiscence conditions also associated with reduced caregiver depression and distress (on GHQ) at post-treatment and (on GDS and GHQ) at follow-up. Effect sizes for all these comparisons were in the range 0.48 to 0.6, except for autobiographical memory at follow-up, which was 0.13. The details of the comparisons between any form of reminiscence and treatment as usual are shown in Tables 1 and 2.
| Outcome measure | Baseline reminiscence | Baseline treatment as usual | Post-treatment reminiscence | Post-treatment treatment as usual | Effect size |
|---|---|---|---|---|---|
| QoL-AD (patient-rated) | 37.47 (5.46) | 35.50 (5.33) | 37.70 (5.22) | 34.83 (5.84) | 0.17 |
| RSS | 22.56 (13.77) | 20.50 (13.39) | 21.49 (12.77) | 24.33 (11.50) | 0.36 |
| GHQ-28 | 19.97 (9.94) | 21.82 (10.48) | 20.19 (10.66) | 27.64 (11.44) | 0.56 |
| GDS | 2.95 (3.45) | 3.09 (2.88) | 3.08 (3.22) | 5.09 (4.93) | 0.56 |
| AMI(E) | 69.01 (23.83) | 72.86 (27.96) | 67.58 (29.73) | 58.14 (30.54) | 0.54 |
| QoL-AD (carer-rated) | 30.82 (5.82) | 30.35 (4.71) | 30.99 (6.37) | 27.60 (4.97) | 0.50 |
| Outcome measure | Baseline reminiscence | Baseline treatment as usual | Follow-up reminiscence | Follow-up treatment as usual | Effect size |
|---|---|---|---|---|---|
| QoL-AD (patient-rated) | 37.08 (5.38) | 35.36 (5.57) | 35.49 (4.99) | 31.64 (11.79) | 0.40 |
| RSS | 20.11 (12.98) | 20.50 (13.39) | 22.78 (12.63) | 27.33 (13.85) | 0.31 |
| GHQ-28 | 18.97 (10.25) | 22.00 (10.01) | 21.14 (11.55) | 30.33 (13.24) | 0.62 |
| GDS | 2.46 (2.98) | 3.09 (2.88) | 3.41 (2.85) | 5.64 (4.70) | 0.48 |
| AMI(E) | 70.01 (23.30) | 72.86 (27.96) | 58.94 (28.96) | 58.59 (35.18) | 0.13 |
| QoL-AD (carer-rated) | 30.96 (5.56) | 29.59 (5.13) | 30.11 (6.50) | 26.82 (5.65) | 0.33 |
Implications of trial platform for the full trial
-
The Zelen method of randomisation led to several refusals to accept experimental interventions, thus weakening the effect of those interventions as Zelen analyses by ‘intention to treat’ (ITT); as there was no evidence that it otherwise assisted recruitment and retention in this field, it was not used in the current study.
-
Though the trial platform necessarily generated wide confidence intervals (CIs), the difference in effects between joint reminiscence and reminiscence alone appeared to be small, as one might have predicted a priori from the similar resources allocated to each. Indeed, reminiscence alone may have beneficial effects for caregivers also. This may be because of the brief respite afforded to the caregiver, or from the benefits they perceive the person with dementia is receiving.
-
Although it was considered that the further comparison of joint reminiscence and individual reminiscence would be of interest in providing a test of the additional effects of joint working and of relationship-centred care, the size and complexity of trial that would be required, given the probable small effect size for any difference between the two conditions, was judged not to be feasible. Accordingly, the current study focused on joint reminiscence groups.
-
Participants in the joint reminiscence groups requested monthly reunion meetings following the end of the 12 weekly sessions. They wished these to continue to have a reminiscence focus in addition to social contact. These maintenance sessions over the follow-up period have been incorporated into the current study.
Methods
Design
A pragmatic multicentre parallel group RCT of joint reminiscence and maintenance compared with usual treatment was carried out. Participants were randomised to the two groups using a restricted dynamic method of randomisation. The overall allocation ratio was 1 : 1, but this was restricted to ensure intervention groups were of a viable size. Data collection points were at baseline before randomisation, at 3 months immediately following completion of the weekly reminiscence sessions and at 10 months following completion of the seven monthly maintenance sessions of the therapy. The primary outcomes were assessed at all time points with the primary hypothesis examining these outcomes at the 10-month interval.
Ethics approval
A protocol was submitted for ethical scrutiny to the Multicentre Research Ethics Committee (MREC) for Wales (ref. no. 07/MRE09/58) in September 2007, with provisional approval being granted in October 2007. The issues identified by the committee as needing to be addressed were as follows:
-
Information sheets needed to be modified to make it clear that interviews and questionnaires could be completed over two sessions and that interviewees could take breaks if necessary.
-
Reference to section 32 of the Mental Capacity Act35 in the information sheets should be removed.
-
A protocol was required to deal with issues of neglect or ill treatment of people with dementia, especially in instances where the carer was the perpetrator.
After addressing these issues, final approval was granted in November 2007. Participating centres obtained approval from the appropriate Local Research Ethics Committee (LREC) and the relevant NHS Trust research and development (R&D) department.
Intervention and control conditions
Participants randomised to the intervention condition were invited to attend reminiscence group meetings as outlined above. Transport was arranged if required.
The control condition in this trial was designated as ‘treatment as usual’. The services and interventions available to people with dementia and family carers randomised to receive usual treatment varied between and within centres and over time. In principle, all the interventions offered to this group were also available to those in the active treatment groups as we were evaluating the additional effects of reminiscence work. The only exception to this was when reminiscence groups occurred at the same time as an alternative intervention. Our commitment to costing services and interventions received allowed us to monitor whether or not control groups were receiving alternative interventions in this way. Though changes and developments in the availability of medications for Alzheimer’s and other dementias should have affected both groups equally, this was also monitored through the service-use information collected.
Participants in the usual treatment group may have engaged in some form of reminiscence work during the 10 months of the study period. This is a popular approach in day-care centres, and reminiscence materials are widely available. However, it is unlikely that structured reminiscence work would have been offered in any of the centres, and even less likely that it would have been offered jointly to carers. It is this systematic group-based approach, rather than a general exhortation to reminisce to improve communication, that is the focus of this evaluation.
Study population
Eight centres in England and Wales were involved in the study: Bangor (covering north Wales), Bradford, Hull, London (north – covering the boroughs of Barking and Dagenham, Havering, Redbridge, Waltham Forest), London (south – covering the boroughs of Bexley, Bromley, Greenwich), Manchester (double centre – covering Bolton, Salford, Trafford) and Newport in south Wales (covering mainly Newport and Caerphilly). Researchers in six centres were based in the universities shown in Table 3, whereas those in Hull and Newport were based in NHS mental health services. Recruitment commenced in May 2008 and was completed in July 2010.
| REMCARE centre name | Organisations involved |
|---|---|
| Bangor | Bangor University |
| Conwy and Denbighshire NHS Trusta | |
| North East Wales NHS Trusta | |
| North West Wales NHS Trusta | |
| Bradford | University of Bradford |
| West Yorkshire Research and Development Consortiumb | |
| Hull | Humber Mental Health Teaching NHS Trustc |
| London (north) | University College London |
| North East London Mental Health NHS Trustd | |
| London (south) | University College London |
| Oxleas NHS Foundation Trust | |
| Manchester | University of Manchester |
| Greater Manchester West Mental Health NHS Foundation Trust | |
| Newport | Gwent Healthcare NHS Truste |
Eligibility criteria
Inclusion
All participants were people with dementia who at the time of the baseline assessment:
-
met the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) criteria for dementia of any type, including Alzheimer’s, vascular, Lewy body type and mixed
-
were in the mild to moderate stage of dementia (Clinical Dementia Rating)
-
could communicate and understand communication, shown by a score of 1 or 0 on the relevant items of the Clifton Assessment Procedures for the Elderly – Behaviour Rating Scale
-
could engage in group activity
-
were living in the community and had a relative or other caregiver who maintained regular contact, could act as informant, and was willing and able to participate in the intervention with the person with dementia.
Exclusion
Potential participants were excluded if they had any characteristic which could affect participation, for example:
-
major physical illness
-
uncorrected sensory impairment
-
disability or
-
high level of agitation.
Sample size
The original target sample size was 400 patients completing data collection for the trial after 10 months, comprising 200 in the intervention condition and 200 receiving treatment as usual. In the MRC trial platform intraclass correlation coefficients (ICCs) within randomised groups were negative (i.e. not significantly different from zero) for both the carer-specific GHQ and the carer-rated QoL-AD, but close to 0.1 for the QoL-AD rated by the person with dementia. Using a 5% significance level, comparison of 200 pairs completing reminiscence groups with 200 people with dementia receiving treatment as usual yields 80% power of detecting a standardised difference of 0.28 in the GHQ or the carer-rated QoL-AD. In contrast, because the patient-rated QoL-AD was estimated to suffer a ‘variance inflation factor’ of approximately 1.74 [namely 1 + 0.1 × (average completed group size of 8.4 – 1)], this yielded a power of 80% of detecting a standardised difference of 0.38. The trial platform, which had a sample size of 57 in three centres, suggested that these differences between 0.28 and 0.38 for the outcomes are plausible. In our judgement these differences also fall within the range of effects that are clinically important. Furthermore, because previous work had been exploratory, and therefore more heterogeneous than the current definitive trial, ICCs and SDs were expected to fall. To achieve a sample size of approximately 400, we allowed for 12% attrition between recruitment and the post-treatment assessment (estimated from our trial platform) and a further 18% over the following 7 months (estimated from a community study). 36 Hence, we set an initial sample size of 576, requiring 24 treatment groups initially comprising 12 dyads and another 288 randomised to usual treatment.
A review of the sample size calculation was completed in July 2009, as part of an extension application. This review revealed that as suspected the ICCs were lower than accommodated for within the original sample size calculation. The baseline data collected up to July 2009 showed that the ICCs for the patient-rated QoL-AD, using the difference method, was 0.0214. Given the smaller than anticipated group sizes in the study (estimated mean seven at 10-month follow-up) this led to a much reduced variance inflation factor (VIF) of VIF = 1 + 0.0214 × 6 = 1.1284. The revised recruitment target of 508 provided a potential sample size of 366 at 10-month follow-up, assuming 72% retention across the 10-month period. This provided 80% power to detect a standardised difference of 0.30 in the GHQ or carer-rated QoL-AD at the 5% significance level, and 80% power to detect a standardised difference of 0.31 in the patient-rated QoL-AD. The slight loss in power to detect a difference in the carer-rated measures is more than compensated for by the increased power to detect a difference on the patient-rated primary outcome measure.
Recruitment procedures
People with dementia and their family caregivers were recruited through mental health services for older people in each area [especially Memory Clinics, Community Mental Health Teams (CMHTs) for older people and associated professionals including psychiatrists, occupational therapists and Admiral Nurses®], associated day services and through relevant local voluntary sector agencies such as the Alzheimer’s Society. (Admiral Nurses are specialist mental health nurses, working primarily with carers of people with dementia. The service is available in a number of locations in England. ) The centres in Wales benefited from the support of Clinical Studies Officers accessed through the National Institute for Social Care and Health Research Clinical Research Centre (NISCHR-CRC). In Manchester and north London, support was given by the Dementias and Neurodegenerative Disease Research Network (DeNDRoN). In Hull, towards the end of the study, recruitment was extended to include certain general practitioner (GP) surgeries, as this was considered a potential additional source of participants.
Recruitment was in waves (3–5, depending on the centre), which offered the opportunity to focus on different geographical areas within the remit of each centre for each group. The project was briefly outlined to the potential participants by a member of the clinical team or Alzheimer’s Society worker, and permission for them to be contacted by a member of the research team was obtained. The research worker would then arrange to meet the potential participants and offer full details, respond to questions and, where the participants were willing to join the study, undertake the process of consent.
Informed consent
Participants were allowed to enter the study only after giving signed informed consent in accordance with the provisions of the Mental Capacity Act 2005. 35 For each couple participating in the trial, separate informed consent was sought from the person with dementia and their family caregiver. Participants with dementia were in the mild to moderate stages of dementia, and therefore could generally be expected to be competent to give informed consent for participation, provided that appropriate care was taken to explain the research and sufficient time allowed for them to reach a decision. In every case, participants with dementia were given at least 24 hours to consider the information provided. Wherever possible, the involvement of a family member, or other supporter, was sought.
It was made clear to both the person with dementia and the family caregiver that no disadvantage would accrue if they chose not to participate.
In seeking consent, current guidance from the British Psychological Society37 was followed on the evaluation of capacity. In this context, consent has to be regarded as a continuing process rather than a one-off decision, and willingness to continue participating was continually checked through discussion with the person with dementia during the assessments.
Where the participant’s level of impairment increased, so that he or she was no longer able to provide informed consent, the provisions of the Mental Capacity Act were followed, with the family caregiver as personal consultee. Where the person with dementia had him- or herself given informed consent initially, this provided a clear indication of the person’s likely perspective on continuing at later time points. The same procedure applied where the person with dementia appeared to lack capacity to consent initially but met the other criteria for the project. If at any point a person with dementia became distressed by the assessments, they were discontinued.
Ethical arrangements
There appear to be no documented harmful side effects from participating in reminiscence groups. Some past memories can be unhappy, and even traumatic, but with a skilled and trained facilitator participants will share only those aspects they feel comfortable with. Additional support on a one-to-one basis was given in the small number of cases where distressing memories surfaced.
Prospective participants were fully informed of the potential risks and benefits of the project. A reporting procedure was put in place to ensure that serious adverse events (SAEs) were reported to the chief investigator. On becoming aware of an adverse event involving a participant or carer, a member of the research team assessed whether or not it was ‘serious’. A SAE was defined in the trial as an untoward occurrence experienced by either a participant or carer which:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was otherwise considered medically significant by the investigator
-
came within the scope of the Protection of Vulnerable Adults (POVA) protocol, which was in place to ensure that suspected cases of abuse or neglect were followed-up in an appropriate manner.
A reporting form was submitted to the chief investigator who assessed whether or not the SAE was:
-
related to the conduct of the trial
-
unexpected.
Serious adverse events that were judged to be related and unexpected were to be reported to MREC and the trial Data Monitoring and Ethics Committee (DMEC) within 15 days.
Randomisation
Randomisation was completed using a dynamic allocation method38 stratifying for spousal or non-spousal relationship of the dyad. Complete list randomisation for each wave of recruitment within each centre was completed. All participants for a wave were intended to be recruited before being randomised, although provision was made within the system to allow additional randomisations within a group to be performed. Although the overall allocation ratio was 1 : 1, it was stipulated that the RYCT groups needed at least eight participants to prove viable; the randomisation system was restricted in order to accommodate this. This restriction does mean that overall allocation to the intervention was higher than to the control, although within each wave this was constrained to within an acceptable level. The final overall allocation ratio was not sufficiently different from 1 : 1 to cause any issue for the analysis.
Allocation concealment
By undertaking a complete list randomisation for each wave at each centre, allocation knowledge of the next assignment would be irrelevant as all participants for a centre would be randomised together. Unblinded researchers were the only staff informed at each of the centres of the participant’s allocation.
Implementation
Researchers completed a randomisation request form detailing all participants to be randomised. This form was then emailed to the responsible trials unit [North Wales Organisation for Randomised Trials in Health (& Social Care)], the centralised randomisation centre, where allocation was performed. The allocations were filled out on the request form and returned to the nominated unblinded researcher in each centre.
Blinding
Owing to the nature of the intervention, it was not possible to blind the participants to the allocation that they received. Within each centre there was a nominated blinded researcher and an unblinded researcher. Both researchers were able to complete the baseline assessments with the participants and request the randomisation of participants. Once randomised, the unblinded researcher received the allocations and took the role of informing participants of their allocation and organising the joint reminiscence groups. The unblinded researcher in the majority of the centres was also the facilitator for the joint reminiscence group. The blinded researcher carried out all follow-up assessments. As part of the follow-up assessments, the researcher completed a perception sheet that indicated their prediction of which treatment arm a participant was in.
The analysts remained blind to the allocation for the main analysis. Analyses including the joint reminiscence groups attendance records were scheduled to be completed after the main analysis to ensure that blinding was kept intact for as long as possible.
Data-collection procedures
Primary and secondary measures were completed at baseline, 3 months after baseline (first follow-up) and 10 months after baseline (second follow-up and primary end point). Centres were instructed to conduct baseline assessments within a 2-month window prior to the first joint reminiscence group being held. The interviews for the first follow-up were conducted within 2 months of the completion of the weekly joint reminiscence group sessions, while the interviews for the second follow-up were scheduled within 2 months of the final monthly maintenance session.
Interviews were usually conducted in the family home, and though provision was made in the protocol for alternative venues to be used if required, this seldom happened in practice. The questionnaire measures were arranged in a number of booklets for ease of administration. In particular, self-reported health questionnaires for the carer and proxy measures completed by the carer with respect to the person with dementia were incorporated into booklets designed for self-completion. Where local resources allowed, two assessors would visit a couple, one interviewing the person with dementia while the other interviewed the carer in a separate room if possible. Assessors operating on their own were encouraged to ask the carer to complete their booklets in a separate room while the interview with the person with dementia took place. A second visit was sometimes made to complete assessments where an interviewee became tired, or where it was otherwise requested by participants or deemed appropriate by the assessor.
Measures
Primary outcome measures
-
Quality of life of the person with dementia, using the QoL-AD scale,29 which covers 13 domains of quality of life. This is reliable and valid for people with mild and moderate degrees of dementia when they take part in structured interviews with trained interviewers. 39,40 A higher score on the scale indicates a better quality of life.
-
Caregiver’s mental health, assessed using the 28-item, self-completed GHQ,31 which has been widely used in caregiver research. 41,42 We used the scoring system with 4-point Likert scales ranging from 0 to 3. The questionnaire includes indicators of anxiety, depression, insomnia, social dysfunction and somatic symptoms. We chose the GHQ over the RSS as the primary outcome because it is more general in scope and more widely used. A higher score on the scale indicates more distress for the carer.
Secondary outcome measures
-
Autobiographical memory, assessed using an extended version of the autobiographical memory interview [AMI(E)]. 43 The AMI(E) assesses the person with dementia’s recall of personal memories relating to both factual (semantic) information (e.g. names of schools or teachers) and specific incidents. In the trial platform, we validated an additional section covering the period from middle-age to retirement, to cover the lifespan of our participants. A higher score on the scale indicates a better memory recall function.
-
Quality of relationship, assessed by both person with dementia and carer using the QCPR. 33 Originally developed in Belgium, this scale comprises 14 items with five-point Likert scales designed to assess the warmth of the relationship and the absence of conflict and criticism. In the trial platform, the QCPR had good internal consistency for carers (α = 0.85) and for people with dementia (α = 0.80), and concurrent validity with other measures of relationship quality and carer stress. A higher score on the QCPR scale indicates a better perceived relationship. Two subscales provide separate measures of warmth and absence of conflict/criticism.
-
Depression and anxiety, using the Cornell Scale for Depression in Dementia (CSDD)44 and the Rating Anxiety in Dementia (RAID)45 for the person with dementia; and the Hospital Anxiety and Depression Scale (HADS)46 for the carer. The CSDD is a 19-item scale, derived from interviews with the people with dementia and their carers in which the interviewer describes signs and symptoms to the interviewee. Where there is a discrepancy between carer’s and assessor’s ratings, the interviewer re-interviews the carer before making a final judgement. A higher score on the scale indicates more depressive symptoms. The RAID is an 18-item scale to rate anxiety in people with dementia based on structured interviews with them and their carers. A higher RAID score indicates more anxiety symptoms. The HADS is a well-validated 14-item, self-completed scale that measures both anxiety and depression, and is suitable for use with adults of all ages. Higher scores on the two HADS’ subscales denote the presence of more anxiety and depressive symptoms.
-
Stress specific to caregiving, using the RSS,30 which asks the caregiver to complete 15 five-point Likert items. A higher score overall on this scale indicates more stress specific to caregiving.
-
Quality of life of the person with dementia, rated by the caregiver using the proxy version of the QoL-AD,29 identical in structure and content to the version completed by the person with dementia. The proxy QoL-AD works on the same scale as the self-completed version, with a higher score indicating a better quality of life for the person with dementia, in this case as the carer perceives it.
-
General quality of life of both caregiver and person with dementia, using the European Quality of Life-5 Dimensions (EQ-5D). 47 The EQ-5D is a validated generic, health-related, preference-based measure comprising five domains: mobility, self-care, usual activities, pain and discomfort, and anxiety and depression. Each domain has three levels (no problems, some problems and many problems). The EQ-5D scoring system defines 243 (35) possible health states with two additional states (dead and unconscious), where death has a value of 0 and best imaginable health has a value of 1. The questions are complemented by a visual analogue scale (VAS), with 0 representing worst imaginable health and 100 representing best imaginable health, on which respondents are asked to indicate their current health. Caregivers completed the measure from their own perspective and for the person with dementia; the measure was also completed with the person with dementia whenever possible.
-
Functional ability of the person with dementia, using the Bristol Activities of Daily Living Scale,48 a 20-item scale completed by the carer. A higher score on this scale indicates less functional independence of the person with dementia.
-
Use of health care, social care and voluntary services. In a face-to-face interview, participants with dementia and their carers were each asked to recall, at baseline, 3 months and 10 months their contacts with health care, social care and voluntary services. This was done using an adapted Client Services Receipt Inventory (CSRI),49 used extensively in studies of mental health and dementia (e.g. Knapp and colleagues). 50
Data checking
Data for the project were collected in questionnaire packs completed by the researcher during an interview with the participants. All AMI(E) questionnaires were double scored at the centres before being sent for scanning to ensure consistency of scoring between individuals. This double scoring system was introduced and training was given at the first training day for researchers.
The completed questionnaire packs were then returned to the trials unit, the North Wales Organisation for Randomised Trials in Health (and Social Care). Each questionnaire book was scanned into Verity Teleform version 9.1 (Verity Inc., Sunnydale, CA), where the data underwent a verification and validation process before being exported to SPSS files. SPSS PASW version 18 (IBM Corporation, New York, NY) was used for all further data manipulations and analysis. A process of cleaning the SPSS files was undertaken. Variables were checked for out-of-range values and consistency. All corrections made to SPSS files were logged together with reasons for their change. A proportion of the questionnaire books were cross-checked with the SPSS data to allow identification of any issues with particular variables. Once issues had been identified then further in-depth cross-checking could take place. For example, the majority of the EQ-5D VASs required double checking, and all CSRIs required cross-checking with the hard copy owing to the amount of free-text contained in the form.
Data analysis
Missing data
There were two types of missing data within this data set: missing items within a measure and missing measures at a particular time point.
For items missing within a measure, the rules for completing missing data for the relevant measure were applied. The missing data rules implemented for each measure are considered part of the validated tool and were therefore used as designed in line with the original validation. This rule affected two measures used in this study: QoL-AD and the HADS. For QoL-AD, up to two missing items are replaced with the mean score of the remaining items. For the HADS, a single missing item from a subscale may be replaced by the mean of the remaining six items.
Once the measure rules had been implemented, missing time point data were considered. For baseline, a linear regression model was applied, taking into account age, gender, spousal care, centre, wave and other baseline measurement scores. This gave a complete baseline data set. For the follow-up time points, a linear regression was again used, this time within a measure. The linear regression model was fitted for each of the outcome measures separately, taking into account age, gender, spousal care, centre, wave, treatment group allocation and all previous time point scores. For follow-up 1 (FU1), baseline scores were used in the model, whereas for follow-up 2 (FU2), baseline and FU1 scores were used in the model. The imputations for the follow-up time points were carried out as a multiple imputation providing five replicate data sets for assessment.
Baseline characteristics
As recommended, no formal tests were carried out for significant differences of baseline characteristics between the treatment arms. 51 Data were tabulated for the whole sample, intervention and control groups for both demographic and clinical variables.
Interim analyses
No interim analyses had been scheduled for the data. During the course of the trial, no additional analyses were identified or requested by the DMEC.
Primary effectiveness analyses
A linear-mixed model analysis of covariance (ANCOVA) was used to analyse the quantitative repeated measures. For each quantitative response the baseline value was used as a covariate. The treatment group was a fixed factor. The different centres were random factors. The participants within a centre were also random factors.
The usual ‘wide data’ format is where each row represents a participant and each column represents a measurement made on that participant. The data file was transformed to the ‘long’ format where there are two rows for each participant, one for the measurements made at 3 months and one for the measurements made at 10 months. A column was created to indicate at which time point the measurements were made. Time was included in the model as a fixed factor, together with the interaction between treatment group and time. The advantage of this method of analysis is that all participants are included and all observations that are collected are included in the analysis.
The above analysis gives consistent unbiased estimates of the treatment effect provided the data are missing completely at random. This was assessed by seeing if any variables, for example treatment, centre, baseline values, gender, age, and so on, predicted whether or not an observation was missing using a logistic regression. Any predictors identified were included as covariates in the linear-mixed model ANCOVA, allowing the missing completely at random conclusion to be drawn.
-
Model 1: Is QoL-AD affected by treatment and time, taking into account covariates of age, gender, baseline score, centre and spousal care?
-
Model 2: Is the GHQ affected by treatment and time, taking into account covariates of age, gender, baseline score, centre and spousal care?
Secondary effectiveness analyses
The initial secondary models replace the 10-month outcome with the 3-month outcome.
-
Model 3: All models repeated for the secondary outcomes.
-
Model 4: Treatment adherence incorporated into the models.
Additional analyses
Additional analyses looked at the stipulated subscales of the GHQ, AMI(E) and the QCPR.
Economic analyses
Perspective
A public sector perspective was adopted spanning the NHS (dementia services, primary and secondary care) and local government (social services).
Micro-costing of reminiscence group therapy and maintenance
Micro-costing is a necessary part of economic evaluation. It allows a bottom-up construction of the costs of setting up and delivering a new programme by recording the types and quantities of resource input including, in the case of REMCARE, staff time, materials, room rental, recruitment and supervision of staff. Unit costs, tariffs or prices are then assigned for a particular currency and year. Within a country, results from micro-costing can be transferred between different settings and situations transparently. 52,53
Patterns of health care, social care and voluntary sector service use and associated costs by participants with dementia and their carers
In a face-to-face interview, participants with dementia and their carers were each asked to recall, at baseline, 3 months and 10 months, their contacts with health care, social care and voluntary services. This was done using an adapted CSRI. 49 We developed the CSRI by looking at instruments used in previous dementia studies and through consultation with the principal investigator on the REMCARE trial. As part of the CSRI asking about services use, interviewers asked participants with dementia and their carers about the drugs they had been prescribed and were taking. We were particularly interested in drugs prescribed for dementia, anxiety and to aid sleep.
Valuing resource use
The costs of resource use were estimated using national unit costs obtained from the Department of Health54 and Curtis. 55 Drug prices were obtained from the British National Formulary. 56 Unit cost data are listed in Appendix 1.
Imputation
The imputed values derived for the effectiveness analysis (as described in Missing data) were used, where appropriate, in the health economics analyses (i.e. for missing GHQ, QoL-AD, EQ-5D data). Missing costs were not imputed; only cases with full cost data were used.
Cost-effectiveness analysis
Effectiveness was evaluated in terms of the primary clinical outcomes: the disease-specific quality-of-life measure QoL-AD for participants with dementia and the GHQ for carers at the primary end point. Non-parametric bootstrapping (5000 replications) was used to address the uncertainty associated with point estimates of costs and outcomes.
Secondary cost–utility analysis
A cost–utility analysis (CUA) was conducted using EQ-5D47 completed by participants with mild to moderate dementia to calculate quality-adjusted life-years (QALYs) for these participants (1) assuming full compliance (i.e. all those allocated to the intervention group attended the joint reminiscence and maintenance sessions) and (2) using a compliance threshold (i.e. attending a minimum number of joint reminiscence and maintenance sessions). Subsequent analyses are planned that will use EQ-5D data collected from carers relating to their own health, and carer proxy measures that relate to the participant with dementia.
Triangulation substudy to compare self-report of service use by participants with dementia with their general practitioner records
A small substudy (n = 36) was undertaken to compare the self-report service use by participants with dementia with their GP records. Cases were selected randomly from those participants with dementia who had completed all three sets of assessments, with the aim of having an equal number from the intervention and control groups. Although three centres (Bangor, Hull and London) were initially selected from which to draw the sample, it subsequently proved not possible to collect data in London. This was mainly due to the difficulty of engaging the selected GP practices to assist with the study, as well as logistical issues in arranging visits to collect data. Consequently, it was decided that efforts to collect these data would be focused on the Bangor and Hull centres.
Data were collected for service utilisation relating to primary care, secondary care, as well as medication (dementia and other), for a period of 13 months (corresponding to baseline recall of 3 months plus the 10-month trial period). The substudy aimed to identify any systematic differences between contacts reported by study participants and GP records of frequency of GP visits, practice nurse visits, community psychiatric nurse (CPN) visits, psychiatrist appointments and hospital use. Weighted kappa was used to measure the level of agreement. 57
A comparison of European Quality of Life-5 Dimensions scores of trial participants with UK norms
The EQ-5D is a commonly used generic health-related quality-of-life measure supported by NICE (2008),26 used in economic evaluation of health-care interventions in the UK and internationally. The EQ-5D measure is described in detail in the Measures, (f) section above. A recent review of EQ-5D in dementia studies showed that it could be used in studies of people with mild to moderate dementia. 58 We wanted to compare the scores of participants with dementia and carers in the REMCARE trial with UK population norms,59 based on a survey of a representative sample of 3395 men and women aged ≥ 18 years, living in the UK.
Summary of changes to protocol
Approval was sought and obtained from MREC for 10 substantial amendments to the protocol during the trial. One of these was related to the production of a leaflet to assist with recruitment. Four were related to two bolt-on studies (not reported as part of the trial) undertaken by the centres in London and Bangor (one in each). The remaining five were connected to participant recruitment covering additional sites (Hull, London, Manchester), increased numbers (Bangor, London) and the inclusion of primary care trusts in Hull to facilitate recruitment through GP surgeries.
Chapter 3 Results
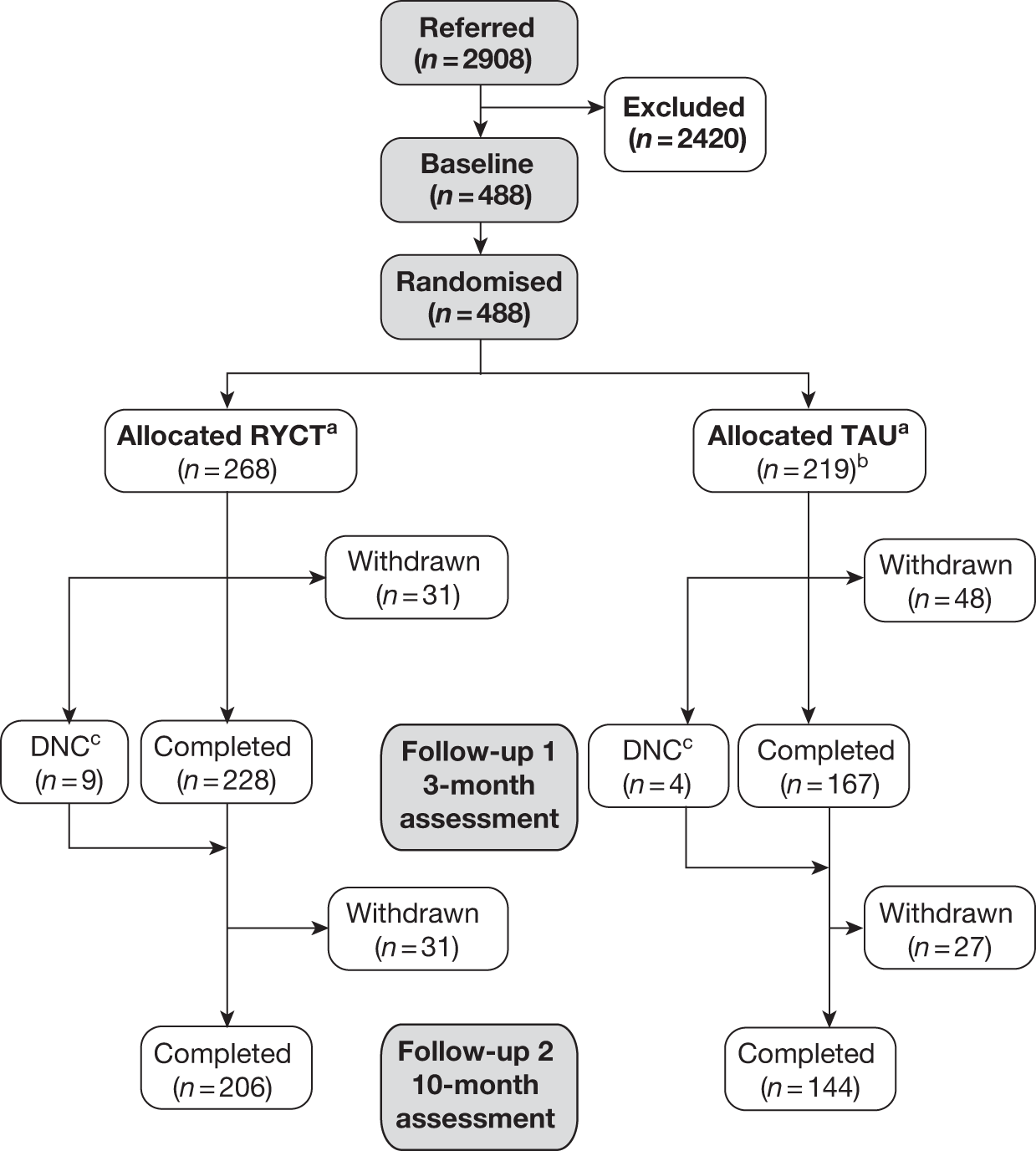
Figure 1a and b presents the details of the flow of participants through the trial. In total, 2908 people were considered for inclusion in the study. From these, 488 were ultimately randomised, although the final sample size was 487 (as one participant who was inadvertently recruited again to a later wave was excluded). The commonest reason for loss between referral or screening and randomisation was potential participants not wishing to participate in the research. The exclusion and clinical criteria accounted for around 15% of the losses and as such indicated no barrier to recruitment (Table 4).
FIGURE 1a.
Participant flow throughout the trial.
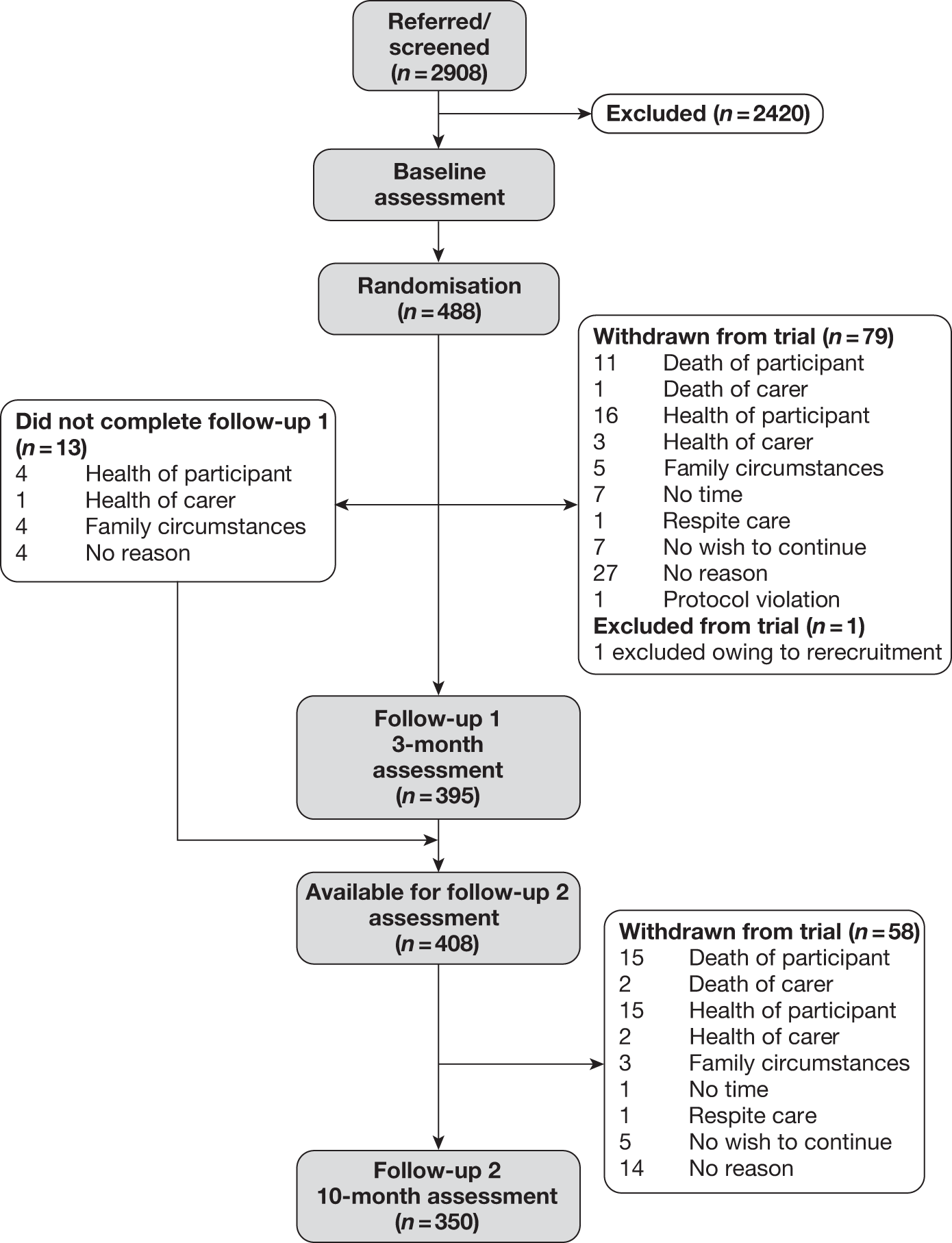
FIGURE 1b.
Participant flow through the trial indicating treatment allocation. a, Constrained randomisation to ensure viable RYCT groups against TAU (treatment as usual); b, one dyad removed owing to rerecruitment; c, the participants did not complete (DNC) FU1 assessment.
| Reason | Total (%) |
|---|---|
| Total referred or screened | 2908 |
| Unable to find Memory Clinic record | 115 (5) |
| Could not make contact by telephone | 393 (16) |
| Does not wish to take part | 863 (36) |
| Does not meet clinical criteria | 108 (4) |
| No suitable carer | 69 (3) |
| Now in residential care | 95 (4) |
| Already participating in similar study | 14 (< 1) |
| Exclusion criteria apply | 91 (4) |
| Unable to attend on day that joint reminiscence groups are being held | 113 (5) |
| Other | |
| Family situation at the time | 41 (2) |
| Carer or participant died | 96 (4) |
| Health issues for participant or carer | 168 (7) |
| Participant unaware of dementia diagnosis | 5 (< 1) |
| Not available | 209 (9) |
| Does not like groups – reference to dislike of intervention | 17 (< 1) |
| Unknown | 23 (1) |
| Total lost between referral/screening and randomisation | 2420 |
| Total number randomised | 488 |
| Conversion rate | 17% |
Table 5 indicates that the majority of referrals, 73%, to the project came from Memory Clinics in the various centres.
| Source | Total (%) |
|---|---|
| Memory Clinic | 2112 (73) |
| CMHT | 406 (14) |
| Alzheimer’s Society | 90 (3) |
| Psychology/psychiatry referral | 77 (3) |
| Day centres/well-being cafe | 60 (2) |
| Admiral Nurse/Memory Clinic nurse | 60 (2) |
| No information | 50 (2) |
| Other | 33 (1) |
| Age Concern | 21 (1) |
| Total | 2908 |
Randomised allocation
The 488 dyads gave informed consent and were randomised after baseline assessment between June 2008 and July 2010. A total of 268 dyads were randomised to the joint reminiscence groups and 220 were randomised to the control group. There was a differential rate of conversion between the centres [χ2 = 109.1, degrees of freedom (df) = 6; p < 0.001], presumably reflecting differences in referral and screening practices (Table 6). For example, the London centres relied more on screening of case records to identify potentially suitable participants. As mentioned above, one dyad was excluded at this point because of the same dyad being rerecruited a second time into the trial. This reduced the total sample size to 487 overall.
| Centre | Total referrals | Total randomisations (%) |
|---|---|---|
| Bangor | 375 | 71 (19) |
| Bradford | 116 | 50 (43) |
| Hull | 129 | 66 (51) |
| London (north) | 848 | 96 (11) |
| London (south) | 1000 | 91 (9) |
| Manchester | 195 | 88 (45) |
| Newport | 245 | 26 (11) |
| Total | 2908 | 488 |
Follow-up retention rates
Retention rates at 3-month time point
Between randomisation and FU1 there were 92 losses (Table 7). Seventy-nine of these were complete withdrawals from the trial, which includes 12 deaths. Thirteen of the dyads were not available to complete FU1 assessment but were available to complete the final follow-up assessment. There were differential retention rates between the centres at first follow-up (χ2 = 30.7; df = 6; p < 0.001).
| Centre | Baseline | Completed 3-month FU1 (retention rate) (%) | Completed 10-month FU2 (retention rate) (%) |
|---|---|---|---|
| Bangor | 71 | 64 (90) | 59 (83) |
| Bradford | 50 | 46 (92) | 42 (84) |
| Hull | 66 | 53 (80) | 53 (80) |
| London (north) | 96 | 73 (76) | 62 (65) |
| London (south) | 90 | 62 (69) | 50 (56) |
| Manchester | 88 | 78 (87) | 68 (77) |
| Newport | 26 | 19 (73) | 16 (62) |
| Total | 487 | 395 (81) | 350 (72) |
Retention rates at 10-month time point
At final follow-up (see Table 7) a further 58 dyads withdrew from the study, which includes a further 17 deaths. This gave a total of 137 complete withdrawals from the trial (including 29 deaths), which equates to a retention rate of approximately 72%, which was the predicted retention rate used in the updated sample size calculations. There were differential retention rates between the centres at FU2 (χ26 = 37.9; p < 0.001). In terms of withdrawals from the study (i.e. excluding deaths), the attrition rate was 22%.
Retention rates by allocated group
Table 7 indicates that during the course of the trial there were a total of 137 withdrawals. There were 62 (23%) withdrawals from the intervention group and 75 (34%) withdrawals from the control group. A comparison of baseline characteristics of those who dropped out did not indicate any significant differences between those in the intervention group and those in the control group. Baseline characteristics considered were age of participant with dementia [joint reminiscence 78.37 (7.41), control 78.36 (5.83); F1,135 = 0; p = 0.99], age of carer [joint reminiscence 70.47 (11.95), control 70.26 (11.68); F1,134 = 0.01; p = 0.92], gender of participant with dementia [joint reminiscence 30/32, control 39/36 (female/male); χ21 = 0.18; p = 0.67] and gender of carer [joint reminiscence 41/21, control 48/25 (female/male); χ21 = 0.002; p = 0.96). The baseline values for the two primary outcomes were also tested with no significant difference found, QoL-AD [joint reminiscence 36.42 (5.84), control 36.47 (5.40); F1,129 = 0.002; p = 0.96] and GHQ-28 [joint reminiscence 23.33 (10.43), control 24.65 (11.76); F1,123 = 0.43; p = 0.51]. Assessing the difference between the Clinical Dementia Rating (CDR)60 scores for the two groups did lead to an almost significant difference between the withdrawals from each group (joint reminiscence 3/33/26, control 5/53/17 for CDR scores 0.5/1/2, respectively; χ21 = 5.85; p = 0.054) with the dropouts from the control group having a lower level of severity.
Maintenance of ‘blind’ follow-up assessments
Perception sheets were completed for 389 (out of 395) FU1 interviews. Table 8 indicates that where researchers were able to make a judgement as to which condition the person had been allocated to, they were indeed more likely to be correct than incorrect in the direction of their prediction. However, they were certain of their judgement in only one-quarter of instances, and in the majority of instances were not able to correctly judge group allocation. It is interesting to note that in seven cases the researcher felt he or she definitely knew which allocated group the participant was in but this turned out to be incorrect.
| Researcher perception | Actual treatment group allocation | Total (%) | |
|---|---|---|---|
| Joint reminiscence (%) | Control (%) | ||
| ‘Definite’ judgement – incorrect | 4 (2) | 3 (2) | 7 (2) |
| ‘More likely’ judgement – incorrect | 35 (15) | 12 (7) | 47 (12) |
| Equally in control or joint reminiscence group | 97 (43) | 73 (45) | 170 (44) |
| ‘More likely’ judgement – correct | 25 (11) | 51 (31) | 76 (20) |
| ‘Definite’ judgement – correct | 65 (29) | 24 (15) | 89 (23) |
| Total | 226 | 163 | 389 |
Perception sheets were completed for 346 (out of 350) FU2 interviews. Table 9 shows that researchers were again more likely to be correct than incorrect when they felt able to make a judgement, but again they were only able to make a definite judgement in one-quarter of instances. At this time point, in five cases the researcher believed that he or she definitely knew which allocated group the participant was in but this turned out to be incorrect. Given the discrepancy between correct and incorrect judgements there is clearly likely to have been some degree of unblinding occurring at the two follow-up assessment points, but the proportion of correct definite judgements remains low, at around 25%, reflecting the considerable remaining uncertainty.
| Researcher perception | Actual treatment group allocation | Total (%) | |
|---|---|---|---|
| Joint reminiscence (%) | Control (%) | ||
| ‘Definite’ judgement – incorrect | 4 (2) | 1 (< 1) | 5 (1) |
| ‘More likely’ judgement – incorrect | 37 (18) | 4 (3) | 41 (12) |
| Equally in control or joint reminiscence group | 74 (36) | 51 (36) | 125 (36) |
| ‘More likely’ judgement – correct | 25 (12) | 63 (44) | 88 (25) |
| ‘Definite’ judgement – correct | 64 (32) | 23 (16) | 87 (25) |
| Total | 204 | 142 | 346 |
Analysis
Baseline characteristics of randomised dyads
Demographic information
Demographic information has been split into two main tables, one describing the demographics of the person with dementia (Table 10) and one for the carer data (Table 11). For the whole sample there is a high proportion of white married people who own their own homes.
| Characteristic | Total (%) | Joint reminiscence (%) | Control (%) |
|---|---|---|---|
| Female person with dementia | 242/487 (50) | 127/268 (47) | 115/219 (53) |
| Ethnicity: white | 447/469 (95) | 254/259 (98) | 193/210 (92) |
| Marital status: married | 337/468 (72) | 187/258 (72) | 151/210 (72) |
| Owner-occupied accommodation | 410/485 (85) | 218/268 (81) | 192/217 (88) |
| Characteristic | Total (%) | Joint reminiscence (%) | Control (%) |
|---|---|---|---|
| Female carer | 325/485 (67) | 188/268 (70) | 137/217 (63) |
| Ethnicity: white | 448/467 (96) | 254/259 (98) | 194/208 (93) |
| Marital status: married | 394/466 (85) | 222/256 (87) | 172/210 (82) |
| Carer accommodation owner-occupieda | 71/84 (85) | 43/51 (84) | 28/33 (85) |
Table 12 indicates the means, SDs and range of ages of the participants. The age of carers ranged from 23 to 91 years, and of people with dementia from 54 to 95 years. Within the sample there were 345 spousal dyads recruited and 142 non-spousal dyads. The 142 non-spousal dyads were made up of son/daughter (96), son/daughter-in-law (5), brother/sister (7), other relative (4), friend (15), partner (11), foster carer (1), carer (1), spouse–separated (1) and missing (1 randomised as other).
| Participant type | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Range | |||||||
| Person with dementia | 487 | 77.53 | 7.3 | 54–95 | ||||||
| Carer | 486a | 69.65 | 11.6 | 23–91 | ||||||
| Carer spousal | 345 | 73.95 | 7.8 | 44–91 | ||||||
| Carer non-spousal | 141 | 58.94 | 12.6 | 23–91 | ||||||
| Joint reminiscence | Control | |||||||||
| n | Mean | SD | Range | n | Mean | SD | Range | |||
| Person with dementia | 268 | 77.72 | 7.4 | 56–93 | 219 | 77.30 | 7.18 | 54–95 | ||
| Carer | 268 | 69.55 | 11.7 | 30–90 | 218 | 69.66 | 11.6 | 23–91 | ||
| Carer spousal | 189 | 74.26 | 7.6 | 45–89 | 156 | 73.58 | 8.1 | 44–91 | ||
| Carer non-spousal | 79 | 58.28 | 12.0 | 30–90 | 62 | 59.79 | 13.2 | 23–91 | ||
Of the 236 female–male dyads (Table 13), 218 were spousal relationships and of the 151 male–female dyads 127 were spousal relationships. Of the nine male–male dyads, eight of these were a child–parent relationship whereas the final dyad was noted as being a partner relationship. There was a majority of female-carer led dyads in both spousal and non-spousal stratifications.
| Gender of participant | Total | ||
|---|---|---|---|
| Female | Male | ||
| Gender of carer | |||
| Female | 89 | 236 | 325 |
| Male | 151 | 9 | 160 |
| Total | 240 | 245 | 485 |
Details of dementia diagnosis subtype were not collected initially. This information proved difficult to ascertain in a number of the centres, and was ultimately obtained for 38% of the sample. In a fifth of these cases the subtype of dementia was not known (Table 14). The great majority of the 147 participants where a subtype was reported were thought to have Alzheimer’s disease alone (72%), or in combination with vascular dementia (11%).
| Diagnosis | Total (%) | Joint reminiscence (%) | Control (%) |
|---|---|---|---|
| Alzheimer’s | 106/183 (58) | 58/105 (55) | 48/78 (62) |
| Vascular | 23/183 (13) | 15/105 (14) | 8/78 (10) |
| Lewy body | 1/183 (< 1) | 0/105 | 1/78 (1) |
| Mixed Alzheimer’s and vascular | 17/183 (9) | 11/105 (10) | 6/78 (8) |
| Not known | 36/183 (20) | 21/105 (20) | 15/78 (19) |
Table 15 indicates that the proxy ratings of depression for the participants with dementia reached the threshold for probable major depression in 24% of cases (CSDD score > 10), whereas the proxy ratings for anxiety reached clinically significant levels of anxiety in 31% of cases (RAID score ≥ 11).
| Mood measure | Thresholds | Total (%) | Joint reminiscence (%) | Control (%) |
|---|---|---|---|---|
| CSDD – person with dementia | < 6 Absence of depressive symptoms | 190/395 (48) | 101/215 (47) | 89/180 (49) |
| > 10 Probable major depression | 85/395 (22) | 48/215 (22) | 37/180 (21) | |
| > 18 Definite major depression | 11/395 (3) | 4/215 (2) | 7/180 (4) | |
| RAID – person with dementia | ≥ 11 Significant clinical anxiety | 130/425 (31) | 67/234 (29) | 63/191 (33) |
| HADS (depression) – carer | ≥ 8 Mild disturbance | 60/483 (12) | 28/266 (11) | 32/217 (15) |
| ≥ 11 Moderate disturbance | 24/483 (5) | 14/266 (5) | 10/217 (5) | |
| ≥ 15 Severe disturbance | 4/483 (1) | 4/266 (2) | 0/217 (0) | |
| HADS (anxiety) – carer | ≥ 8 Mild disturbance | 92/483 (19) | 53/266 (20) | 39/217 (18) |
| ≥ 11 Moderate disturbance | 72/483 (15) | 42/266 (16) | 30/217 (14) | |
| ≥ 15 Severe disturbance | 16/483 (3) | 10/266 (4) | 6/217 (3) |
For carers’ own symptoms, self-reporting of clinically relevant levels of depressive symptoms were at 18% (HADS depression subscale ≥ 8). Self-reported levels of anxiety were almost double this at 37% (HADS anxiety subscale ≥ 8). From the baseline data the RSS appears to be significantly correlated with many of the other carer measures. Pearson’s biserial correlation with: proxy QoL-AD –0.609; QCPR –0.618; HADS depression subscale 0.636; HADS anxiety subscale 0.632; and GHQ-28: 0.615. This indicates that the higher the reported stress levels of the carer the higher the depression, anxiety and GHQ-28 scores, whereas the carer’s perception of the person with dementia’s quality of life is lower as is their perception of their quality of relationship. Other strongly correlated measures are HADS subscales with GHQ-28: 0.741 (anxiety) and 0.664 (depression). RAID and CSDD is correlated at 0.721. The two subscales of the AMI are also strongly correlated at 0.608. Baseline correlations are given in Appendix 5.
Primary analysis of outcomes
The mean values for the two treatment groups at each of the three time points are given in Table 16. The primary ITT analysis did not demonstrate any evidence of a difference between the two treatment groups at any time point. The primary model fitted was an ANCOVA using 10-month outcome as the dependent variable, baseline score on the outcome measure and the age of the person with dementia as covariates, treatment allocation, gender of the person with dementia, spousal (spouse/other) as fixed factors and location and wave as random factors, with the interaction between location and allocation also being taken into account. Carer age and gender were also added for carer and proxy outcomes.
| Outcome measure | Baseline | 3 months | 10 months | |||
|---|---|---|---|---|---|---|
| Joint reminiscence | Control | Joint reminiscence | Control | Joint reminiscence | Control | |
| Participant with dementia | ||||||
| QoL-AD | 37.48 (5.32) | 36.96 (5.35) | 36.91 (5.61) | 36.97 (5.88) | 36.63 (5.63) | 35.96 (5.28) |
| AMIF | 56.07 (23.00) | 54.28 (24.20) | 54.31 (25.28) | 48.95 (24.76) | 50.53 (25.81) | 46.95 (25.55) |
| AMIM | 12.46 (6.93) | 12.94 (7.79) | 11.72 (7.61) | 11.20 (7.63) | 11.33 (8.21) | 10.61 (8.04) |
| EQ-5D VAS | 71.85 (20.33) | 70.72 (19.79) | 72.64 (18.40) | 71.82 (19.73) | 73.02 (18.32) | 72.42 (18.32) |
| EQ-5D utility | 0.75 (0.25) | 0.76 (0.26) | 0.77 (0.23) | 0.78 (0.24) | 0.77 (0.24) | 0.79 (0.23) |
| QCPR | 57.83 (6.42) | 57.45 (6.10) | 57.89 (6.52) | 57.37 (6.71) | 57.64 (6.25) | 57.08 (6.72) |
| QCPR warmth | 34.39 (3.58) | 34.46 (3.51) | 34.29 (4.10) | 34.29 (3.80) | 34.06 (3.65) | 33.57 (3.89) |
| QCPR negative | 23.37 (3.67) | 22.91 (3.76) | 23.63 (3.64) | 23.04 (3.78) | 23.47 (3.50) | 23.41 (3.58) |
| Carer | ||||||
| GHQ-28 | 22.75 (11.71) | 23.06 (12.00) | 22.67 (11.80) | 22.90 (10.37) | 24.34 (13.07) | 22.79 (12.50) |
| GHQ-28 somatic | 5.68 (3.81) | 6.03 (4.33) | 5.73 (3.96) | 6.13 (3.79) | 6.47 (4.45) | 6.27 (4.47) |
| GHQ-28 anxiety | 7.19 (4.74) | 7.26 (4.63) | 7.20 (4.53) | 6.80 (4.15) | 7.77 (4.61) | 6.70 (4.63) |
| GHQ-28 social | 7.98 (2.68) | 7.79 (2.52) | 7.80 (2.66) | 7.75 (2.14) | 8.05 (3.06) | 8.01 (2.41) |
| GHQ-28 depression | 2.01 (3.37) | 2.03 (3.22) | 2.08 (3.30) | 2.05 (2.98) | 2.32 (3.76) | 1.92 (3.14) |
| HADS anxiety | 6.43 (4.33) | 6.02 (4.17) | 6.61 (4.33) | 5.91 (4.18) | 6.98 (4.57) | 5.58 (4.21) |
| HADS depression | 4.34 (3.50) | 4.07 (3.37) | 4.40 (3.19) | 3.99 (3.09) | 5.06 (3.56) | 4.35 (3.21) |
| EQ-5D utility | 0.78 (0.23) | 0.77 (0.24) | 0.76 (0.23) | 0.75 (0.23) | 0.73 (0.28) | 0.77 (0.25) |
| EQ-5D VAS | 74.29 (17.77) | 72.90 (19.70) | 71.59 (20.17) | 70.99 (19.23) | 72.08 (18.91) | 71.59 (17.59) |
| RSS | 21.84 (10.89) | 21.29 (10.89) | 22.81 (10.48) | 21.14 (10.21) | 23.04 (10.92) | 21.10 (10.74) |
| QCPR | 53.45 (8.76) | 53.57 (8.64) | 52.45 (9.01) | 53.43 (8.74) | 53.06 (9.64) | 53.18 (9.15) |
| QCPR warmth | 32.40 (5.37) | 32.71 (5.33) | 31.67 (5.56) | 32.18 (5.30) | 31.90 (5.83) | 32.22 (5.42) |
| QCPR negative | 21.04 (4.45) | 20.82 (4.27) | 20.79 (4.32) | 21.27 (4.45) | 21.23 (4.71) | 20.96 (4.81) |
| Proxy | ||||||
| BADLS | 16.61 (9.43) | 15.06 (9.75) | 18.13 (10.16) | 16.53 (10.14) | 19.67 (10.81) | 19.03 (10.70) |
| RAID | 8.81 (7.45) | 8.15 (6.58) | 8.44 (6.92) | 7.87 (6.45) | 8.19 (7.44) | 7.50 (6.29) |
| CSDD | 7.04 (4.93) | 6.87 (5.13) | 6.80 (4.95) | 7.33 (5.50) | 6.70 (5.39) | 6.73 (5.09) |
| QoL-AD | 31.51 (6.25) | 31.47 (6.50) | 30.28 (6.10) | 31.13 (6.59) | 29.82 (5.84) | 30.74 (6.31) |
| EQ-5D utility | 0.57 (0.29) | 0.60 (0.27) | 0.56 (0.29) | 0.56 (0.29) | 0.54 (0.31) | 0.58 (0.28) |
| EQ-5D VAS | 62.64 (18.06) | 59.33 (19.87) | 59.34 (18.55) | 58.70 (19.09) | 59.20 (19.05) | 60.52 (19.90) |
As stipulated in the data collection procedures, centres could collect data 2 months prior to commencing the intervention, up to 2 months after completion of the intervention and up to 2 months after completion of the monthly sessions. This meant that the FU1 could potentially be from 3 months to 7 months from baseline. Similarly, FU2 could occur from 10 months up to 14 months after baseline. Given the extent of these windows, an assessment was made of any differences in them between the two treatment groups to see if this would be likely to have an impact on the outcomes. In the intervention group, the mean number of months between baseline and FU1 was 4.44 (SD 0.95; range 3–7) months. In the control group, the mean number of months between baseline and FU2 was 4.50 (SD 0.89; range 3–7). Between baseline and FU2, the mean number of months was 11.61 (SD 1.08; range 9–14) in the intervention group and 11.50 (SD 1.07; range 10–15) in the control group. As there were no systematic differences in the length of data collection window between the two groups then this would be unlikely to have an impact on any analysis model used. Therefore, this variation was not taken into account within any of the analysis models.
All outcomes were assessed for normality. The GHQ-28 exhibited some non-normality trends and, thus, a natural log-transform was used. Tables 17–19 present the ANCOVA df, F-values and p-values for the original data and the ranges of these values seen for the multiple imputations at the 10-month primary end point. These tables also include the mean difference between the treatment groups seen in the original data and a pooled value for the multiple imputations. The pooled CIs have been calculated assuming a standard normal distribution. Tables 20–22 give the ANCOVA df, F-values and p-values for the original data and the ranges of these values seen for the multiple imputations at the 3-month end point. Full model specifications for the QoL-AD [person (people) with dementia (PwD)] and GHQ-28, the primary outcomes, are given in Appendix 7.
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range: (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| QoL-AD | Complete case | 1, 4.2 | 0.48 | 0.53 | None | 0.067 | 0.649 | –1.210 | 1.345 |
| Multiple imputations | (1, 5.2) (1, 2.5) | 0.01–7.80 | 0.084–0.93 | Age, location, wave | 0.286 | 0.710 | –1.11 | 1.68 | |
| AMI(E) memory | Complete case | 1, 2.6 | 0.65 | 0.49 | Wave, location | 0.159 | 0.790 | –1.395 | 1.714 |
| Multiple imputations | (1, 4.3) (1, 4.3) | 0.02–0.72 | 0.44–0.89 | Gender (2), spousal (2), wave (5), location (5) | –0.362 | 0.691 | –1.72 | 0.99 | |
| AMI(E) factual | Complete case | 1, 5.3 | 0.04 | 0.85 | Wave | 0.576 | 1.989 | –3.338 | 4.491 |
| Multiple imputations | (1, 5.1) (1, 4.3) | 0.07–4.59 | 0.094–0.80 | Age (1), spousal (2), wave (5) | –1.786 | 1.921 | –5.55 | 1.98 | |
| QCPR | Complete case | 1, 4.4 | 0.24 | 0.65 | Age | 0.670 | 0.845 | –0.993 | 2.333 |
| Multiple imputations | (1, 4.1) (1, 4.4) | 0.03–1.96 | 0.23–0.87 | Age (5), spousal (2) | 0.637 | 0.724 | –0.78 | 2.06 | |
| QCPR warmth | Complete case | 1, 4.9 | 1.26 | 0.31 | PwD age | 0.773 | 0.480 | –0.172 | 1.717 |
| Multiple imputations | (1, 5.2) (1, 4.7) | 0.40–2.19 | 0.20–0.55 | PwD age (5) | 0.555 | 0.377 | –0.18 | 1.29 | |
| QCPR negative | Complete case | 1, 3.45 | 0.64 | 0.47 | None | –0.158 | 0.464 | –1.071 | 0.754 |
| Multiple imputations | (1, 5.0) (1, 3.8) | 0.08–13.21 | 0.024–0.79 | None | –0.340 | 0.524 | –1.37 | 0.69 | |
| EQ-5D utility | Complete case | 1, 4.2 | 0.15 | 0.72 | Gender | –0.001 | 0.029 | –0.058 | 0.055 |
| Multiple imputations | (1, 4.8) (1, 5.3) | 0.06–0.15 | 0.71–0.82 | Gender (5), wave (1), allocation × location (1) | 0.004 | 0.028 | –0.05 | 0.06 | |
| EQ-5D VAS | Complete case | 1, 4.7 | 0.001 | 0.98 | None | –0.405 | 2.332 | –5.000 | 4.186 |
| Multiple imputations | (1, 2.8) (1, 4.0) | 0.05–1.40 | 0.33–0.84 | Location (1) | 0.271 | 1.977 | –3.6 | 4.15 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range: (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| GHQ-28 (log-transform) | Complete case | 1, 4.2 | 1.13 | 0.35 | Carer gender, carer age | 0.090 | 0.062 | –0.032 | 0.211 |
| Multiple imputations | (1, 4.7) (1, 4.3) | 0.13–3.22 | 0.14–0.74 | Carer gender, carer age, wave | 0.071 | 0.058 | –0.04 | 0.18 | |
| GHQ somatic | Complete case | 1, 4.1 | 0.69 | 0.45 | Carer gender | 0.442 | 0.520 | –0.582 | 1.465 |
| GHQ anxiety | Complete case | 1, 4.2 | 8.28 | 0.04 | Carer gender | 1.252 | 0.511 | 0.246 | 2.257 |
| Multiple imputations | (1, 4.9) (1, 2.7) | 4.7–14.7 | 0.04–0.08 | Carer gender (3), carer age (1), location (1) | 0.904 | 0.455 | 0.01 | 1.8 | |
| GHQ social | Complete case | 1, 3.0 | 2.12 | 0.24 | Carer age, carer gender | –0.225 | 0.354 | –0.923 | 0.472 |
| Multiple imputations | (1, 4.2) (1, 3.2) | 0.85–7.30 | 0.07–0.406 | Carer gender (5), carer age (4) | –0.253 | 0.349 | –0.94 | 0.43 | |
| GHQ depression | Complete case | 1, 4.3 | 1.14 | 0.34 | Carer age, spousal | 0.508 | 0.376 | –0.233 | 1.248 |
| Multiple imputations | (1, 4.3) (1, 3.6) | 0.06–1.74 | 0.27–0.82 | Carer age (4), carer gender (1), spousal (5) | 0.230 | 0.303 | –0.36 | 0.82 | |
| RSS | Complete case | 1, 4.8 | 0.004 | 0.95 | None | 0.483 | 1.078 | –1.638 | 2.603 |
| Multiple imputations | (1, 4.5) (1, 5.0) | 0.06–0.51 | 0.51–0.82 | Wave (1) | 0.057 | 0.977 | –1.86 | 1.97 | |
| HADS anxiety | Complete case | 1, 4.8 | 2.89 | 0.15 | None | 0.996 | 0.460 | 0.092 | 1.901 |
| Multiple imputations | (1, 4.8) (1, 4.7) | 1.18–3.13 | 0.14–0.33 | Carer age (1), PwD gender (1) | 0.592 | 0.396 | –0.18 | 1.37 | |
| HADS depression | Complete case | 1, 4.3 | 0.36 | 0.58 | PwD gender | 0.246 | 0.390 | –0.522 | 1.014 |
| Multiple imputations | (1, 4.2) (1, 3.9) | 0.01–1.30 | 0.31–0.94 | Carer age (3), PwD gender (3) | 0.089 | 0.367 | –0.63 | 0.81 | |
| QCPR | Complete case | 1, 4.3 | 0.70 | 0.45 | None | 0.069 | 1.002 | –1.903 | 2.042 |
| Multiple imputations | (1, 2.8) (1, 2.7) | 0.21–8.63 | 0.07–0.68 | Carer age (1), spousal (1) | 0.545 | 0.884 | –1.19 | 2.28 | |
| QCPR warmth | Complete case | 1, 5.0 | 0.16 | 0.70 | PwD age | –0.009 | 0.594 | –0.178 | 1.161 |
| Multiple imputations | (1, 4.5) (1, 5.2) | 0.02–2.63 | 0.17–0.89 | PwD age (5), spousal (3) | 0.309 | 0.604 | –0.87 | 1.49 | |
| QCPR negative | Complete case | 1, 4.0 | 0.57 | 0.49 | None | 0.011 | 0.556 | –1.083 | 1.104 |
| Multiple imputations | (1, 1.7) (1, 0.7) | 13.7–45.5 | 0.08–0.62 | Carer age (1), wave (2) | 0.278 | 0.477 | –0.66 | 1.21 | |
| EQ-5D utility | Complete case | 1, 4.4 | 4.03 | 0.11 | PwD age, PwD gender | –0.064 | 0.031 | –0.124 | –0.003 |
| Multiple imputations | (1, 4.7) (1, 4.8) | 2.05–3.06 | 0.14–0.22 | PwD age (3), PwD gender (5) | –0.044 | 0.026 | –0.09 | 0.01 | |
| EQ-5D VAS | Complete case | 1, 4.0 | 0.06 | 0.82 | PwD gender | 0.968 | 2.231 | –3.423 | 5.358 |
| Multiple imputations | (1, 4.7) (1, 4.0) | 0.04–0.60 | 0.48–0.85 | PwD gender (5), wave (1) | 1.025 | 1.899 | –0.04 | 0.18 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range: (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| Proxy QoL-AD | Complete case | 1, 2.2 | 3.25 | 0.20 | None | –0.504 | 0.617 | –1.717 | 0.709 |
| Multiple imputations | (1, 1.2) (1, 2.1) | 0.07–1.42 | 0.38–0.82 | Carer gender (3), wave (2), location (1) | –0.240 | 0.537 | –1.29 | 0.81 | |
| BADLS | Complete case | 1, 3.0 | 2.69 | 0.20 | None | –0.737 | 0.955 | –2.617 | 1.143 |
| Multiple imputations | (1, 4.2) (1, 3.6) | 2.24–6.51 | 0.07–0.21 | PwD age (2), spousal (4) | –1.129 | 0.755 | –2.61 | 0.35 | |
| EQ-5D utility | Complete case | 1, 3.5 | 1.05 | 0.37 | None | –0.038 | 0.034 | –0.104 | 0.029 |
| Multiple imputations | (1, 3.3) (1, 3.8) | 0.44–2.20 | 0.22–0.55 | Carer age (3), spousal (5) | –0.021 | 0.027 | –0.07 | 0.03 | |
| EQ-5D VAS | Complete case | 1, 4.9 | 0.34 | 0.59 | None | –1.105 | 2.211 | –5.455 | 3.244 |
| Multiple imputations | (1, 5.3) (1, 5.3) | 0.04–2.25 | 0.19–0.85 | Spousal (1), wave (4) | –1.878 | 1.932 | –5.66 | 1.91 | |
| CSDD | Complete case | 1, 3.7 | 0.15 | 0.72 | None | 0.120 | 0.665 | –1.190 | 1.430 |
| Multiple imputations | (1, 4.8) (1, 4.2) | 0.02–1.32 | 0.31–0.91 | Location (1) | 0.379 | 0.541 | –0.68 | 1.44 | |
| RAID | Complete case | 1, 4.7 | 0.35 | 0.58 | None | 0.317 | 0.816 | –1.290 | 1.924 |
| Multiple imputations | (1, 4.8) (1, 3.9) | < 0.001–4.12 | 0.11–0.99 | PwD age (2) | 0.442 | 0.727 | –0.98 | 1.87 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range: (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| QoL-AD | Complete case | 1, 4.6 | 0.04 | 0.86 | None | –0.756 | 0.592 | –1.921 | 0.409 |
| Multiple imputations | (1, 4.9) (1, 5.1) | 0.001–0.49 | 0.51–0.97 | Gender, allocation × location | –0.643 | 0.596 | –1.81 | 0.53 | |
| AMI(E) memory | Complete case | 1, 3.4 | 2.03 | 0.24 | PwD gender, location | 0.594 | 0.710 | –0.802 | 1.990 |
| Multiple imputations | (1, 4.9) (1, 4.3) | 0.89–2.81 | 0.17–0.39 | Gender (4), location (3) | 0.170 | 0.574 | –0.96 | 1.3 | |
| AMI(E) factual | Complete case | 1, 3.1 | 3.92 | 0.14 | None | 2.340 | 1.676 | –0.956 | 5.636 |
| Multiple imputations | (1, 4.0) (1, 3.8) | 1.48–6.51 | 0.067–0.29 | Wave (2) | 1.450 | 1.399 | –1.29 | 4.19 | |
| QCPR | Complete case | 1, 0.04 | 29.93 | 0.84 | Spousal | 0.482 | 0.987 | –1.460 | 2.424 |
| Multiple imputations | (1, 4.6) (1, 4.7) | 0.004–2.47 | 0.18–0.96 | Spousal (5), wave (3) | 0.307 | 0.733 | –1.13 | 1.74 | |
| QCPR warmth | Complete case | 1, 4.3 | 0.76 | 0.43 | None | 0.263 | 0.490 | –0.701 | 1.227 |
| Multiple imputations | (1, 5.0) (1, 4.9) | 0.40–1.44 | 0.29–0.56 | Spousal (4), wave (2) | 0.258 | 0.365 | –0.46 | 0.97 | |
| QCPR negative | Complete case | 1, 1.62 | 2.25 | 0.30 | None | 0.501 | 0.490 | –0.462 | 1.465 |
| Multiple imputations | (1, 4.1) (1, 2.3) | 1.78–6.79 | 0.11–0.25 | Spousal (3), wave (1) | 0.404 | 0.368 | –0.32 | 1.13 | |
| EQ-5D utility | Complete case | 1, 2.9 | 0.01 | 0.95 | None | 0.008 | 0.027 | –0.045 | 0.061 |
| Multiple imputations | (1, 3.2) (1, 4.8) | 0.007–0.04 | 0.85–0.94 | Age (1), location (3) | 0.014 | 0.023 | –0.03 | 0.06 | |
| EQ-5D VAS | Complete case | 1, 2.6 | 0.68 | 0.48 | None | 0.425 | 2.370 | –4.327 | 5.088 |
| Multiple imputations | (1, 5.1) (1, 4.2) | < 0.001–0.69 | 0.45–0.99 | Age (1), location (1) | 0.788 | 1.961 | –3.06 | 4.63 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range: (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| GHQ-28 (log-transform) | Complete case | 1, 4.6 | 0.04 | 0.86 | None | 0.021 | 0.051 | –0.080 | 0.122 |
| Multiple imputations | (1, 4.9) (1, 5.1) | 0.001–0.49 | 0.51–0.97 | Wave, spousal, gender (carer and PwD) | 0.017 | 0.059 | –0.10 | 0.13 | |
| GHQ somatic | Complete case | 1, 3.9 | 0.12 | 0.74 | None | –0.120 | 0.427 | –0.960 | 0.721 |
| Multiple imputations | (1, 4.8) (1, 4.6) | 0–1.88 | 0.24–0.99 | None | –0.007 | 0.428 | –0.85 | 0.83 | |
| GHQ anxiety | Complete case | 1, 4.9 | 1.99 | 0.22 | Carer gender | 0.959 | 0.435 | 0.103 | 1.815 |
| Multiple imputations | (1, 4.8) (1, 4.8) | 2.5–3.9 | 0.11–0.18 | Carer gender (5), spousal (2), allocation × location (1) | 0.824 | 0.376 | 0.09 | 1.56 | |
| GHQ social | Complete case | 1, 3.9 | 0.004 | 0.95 | None | –0.100 | 0.271 | –0.633 | 0.432 |
| Multiple imputations | (1, 4.8) (1, 3.7) | 0.26–2.43 | 0.20–0.63 | Spousal (2), PwD age (1) | 0.016 | 0.319 | –0.61 | 0.64 | |
| GHQ depression | Complete case | 1, 4.3 | 0.05 | 0.83 | Carer gender | –0.072 | 0.304 | –0.670 | 0.527 |
| Multiple imputations | (1, 4.5) (1, 4.7) | 0.03–0.20 | 0.68–0.88 | Carer gender (4), PwD gender (2), spousal (1), carer age (1) | –0.142 | 0.255 | –0.64 | 0.36 | |
| RSS | Complete case | 1, 0.8 | 14.89 | 0.21 | PwD age, PwD gender | 0.975 | 0.885 | –7.65 | 2.716 |
| Multiple imputations | (1, 4.0) (1, 0.5) | 3.27–4.93 | 0.09–0.48 | PwD age (5), PwD gender (4), wave (1) | 0.748 | 0.811 | –0.84 | 2.34 | |
| HADS anxiety | Complete case | 1, 4.1 | 0.30 | 0.61 | Carer gender | 0.346 | 0.375 | –0.391 | 1.083 |
| Multiple imputations | (1, 3.6) (1, 4.2) | 0.13–1.13 | 0.35–0.74 | Carer gender (5), carer age (10) PwD age (1), spousal (1), wave (2) | 0.182 | 0.313 | –0.43 | 0.8 | |
| HADS depression | Complete case | 1, 4.9 | 0.06 | 0.83 | PwD gender, wave | 0.107 | 0.305 | –0.494 | 0.708 |
| Multiple imputations | (1, 4.5) (1, 4.3) | 0.03–0.21 | 0.67–0.88 | PwD gender (4), wave (2) | –0.075 | 0.277 | –0.62 | 0.47 | |
| QCPR | Complete case | 1, 4.4 | 1.81 | 0.24 | Spousal | –1.493 | 0.824 | –3.114 | 0.128 |
| Multiple imputations | (1, 5.4) (1, 3.8) | 0.77–6.60 | 0.066–0.42 | Spousal (5), allocation × location (1) | –1.180 | 0.775 | –2.70 | 0.34 | |
| QCPR warmth | Complete case | 1, 4.8 | 0.63 | 0.46 | PwD age | –0.652 | 0.495 | –1.626 | 0.321 |
| Multiple imputations | (1, 5.2) (1, 4.3) | 1.03–0.11 | 0.36–0.75 | PwD age (5) | –0.199 | 0.496 | –1.17 | 0.77 | |
| QCPR negative | Complete case | 1, 4.1 | 3.84 | 0.12 | None | –0.840 | 0.462 | –1.749 | 0.069 |
| Multiple imputations | (1, 4.4) (1, 3.1) | 2.80–18.38 | 0.02–0.16 | None | –0.867 | 0.387 | –1.63 | –0.11 | |
| EQ-5D utility | Complete case | 1, 5.0 | 0.10 | 0.77 | PwD gender | 0.010 | 0.021 | –0.031 | 0.051 |
| Multiple imputations | (1, 5.3) (1, 5.0) | 0.04–0.18 | 0.16–0.95 | PwD gender (5), carer gender (2), wave (2), allocation × location (1), carer age (1) | 0.014 | 0.018 | –0.02 | 0.05 | |
| EQ-5D VAS | Complete case | 1, 2.81 | 0.26 | 0.64 | Carer age, carer gender, wave | –0.963 | 2.083 | –5.06 | 3.13 |
| Multiple imputations | (1, 0.79) (1, 3.39) | 0.00–4.89 | 0.32–0.99 | Carer age (5), carer gender (5), wave (3), location (1) | 0.066 | 1.789 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range: (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| Proxy QoL-AD | Complete case | 1, 5.4 | 0.64 | 0.46 | PwD age | –0.835 | 0.543 | –1.904 | 0.233 |
| Multiple imputations | (1, 5.6) (1, 5.3) | 0.02–1.99 | 0.22–0.89 | PwD age (2), allocation × location (3) | –0.703 | 0.534 | –1.75 | 0.34 | |
| BADLS | Complete case | 1, 4.1 | 0.80 | 0.42 | Carer age | 0.523 | 0.685 | –0.825 | 1.872 |
| Multiple imputations | (1, 5.0) (1, 4.0) | 0.01–4.13 | 0.11–0.92 | Carer age (5), PwD age (2) | 0.476 | 0.666 | –0.83 | 1.78 | |
| EQ-5D utility | Complete case | 1, 4.5 | 0.06 | 0.81 | 0.018 | 0.028 | –0.038 | 0.074 | |
| Multiple imputations | (1, 5.0) (1, 5.1) | 0.17–1.48 | 0.28–0.70 | 0.024 | 0.026 | –0.03 | 0.07 | ||
| EQ-5D VAS | Complete case | 1, 4.8 | 0.21 | 0.67 | –2.458 | 2.102 | –6.592 | 1.676 | |
| Multiple imputations | (1, 4.9) (1, 5.0) | 0.23–1.85 | 0.23–0.66 | PwD age (2) | –2.491 | 1.833 | –6.08 | 1.10 | |
| CSDD | Complete case | 1, 2.3 | 1.30 | 0.36 | 0.019 | 0.661 | –1.281 | 1.319 | |
| Multiple imputations | (1, 4.6) (1, 3.3) | 0.08–10.01 | 0.044–0.79 | Wave (2), location (1) | –0.292 | 0.604 | –1.48 | 0.89 | |
| RAID | Complete case | 1, 3.8 | 2.12 | 0.22 | 1.222 | 0.806 | –0.0364 | 2.807 | |
| Multiple imputations | (1, 3.7) (1, 4.9) | 0.11–1.11 | 0.24–0.76 | Carer age (3), PwD age (1), wave (2) | 0.664 | 0.615 | –0.54 | 1.87 |
Linear-mixed models were also fitted to allow change over the three time points to be taken into account. There was no evidence of differences between the two treatment groups for any of the outcomes for these models. Equivalent tables for these models are presented in Appendix 6.
Analyses of secondary outcome measures
There was no evidence of any difference between the two groups with respect to any of the secondary outcome measures at any time point for either of the ANCOVA models or the linear-mixed models fitted, with the exception of carer variables (HADS anxiety and depression, RSS) in the unadjusted analyses, but not with the multiple imputations. Details for the secondary outcomes at the 10-month end points are in Tables 17–19, which include the df, F-values and p-values for the treatment group allocation factor of the model and an indication of any other variables that were significant in the model. It is noted how many times a particular factor was significant for the multiple imputations. The ANCOVA models are similarly described for the 3-month time point in Tables 20–22. The tables for the corresponding linear-mixed models are presented in Appendix 6.
Subscale analysis
The GHQ-28 has four subscales that make up the total score: somatic, anxiety, social and depression. There was a significant difference between the two treatment groups for the anxiety subscale measure at the 10-month time point. There was no evidence of this difference at the 3-month time point. However, this finding was further reinforced by the linear-mixed model, which indicated that there were significant differences between the two treatment groups. The two groups have a mean difference of 1.25, standard error (SE) of 0.5, with the intervention group having higher scores on average (higher anxiety).
The QCPR has two subscales, one denoting warmth of the relationship and the other denoting negative aspects of the relationship. There was some evidence that there was a significant difference between the carer negative relationship scores at 3 months. However, this was only with the imputed data and was not backed up by the linear-mixed model. The two groups have a mean difference at 3 months of 0.84, SE 0.46, with the joint reminiscence group having the lower mean score (worse relationship).
Compliance analysis
To assess the impact of treatment compliance on the treatment effects, the influence of the number of sessions attended by the dyads randomised to the intervention group was evaluated. This was done in two ways:
-
The number of weekly sessions attended and number of monthly sessions attended was added to the primary models as two linear variables fitted to investigate whether or not there was any effect evident from the number of sessions attended. For the 3-month ANCOVA models only the weekly data were utilised in the model.
-
Attendance was also added to the model as two binary variables, one for weekly attendance and one for monthly attendances. The threshold for creating this binary variable was based on the clinical expectation of the number of sessions required to attend to achieve a perceived benefit. The criteria for compliance were discussed by the principal investigators and the reminiscence group consultant before finalising the analysis plan. The consensus of clinical experience of the intervention was that participants would have needed to attend six or more of the 12 weekly sessions and, in addition, three or more of the 7 monthly sessions before they could be said to have engaged with the weekly and maintenance interventions, respectively.
These variables were added in as an interaction between the control and the treatment group allocation. This allowed a comparison between the three groups : control; joint reminiscence group, compliers; and joint reminiscence group, non-compliers. Table 23 denotes the number of weekly sessions attended by the 254 dyads randomised to the joint reminiscence group condition available for analysis. The single dyad that had received the intervention despite being randomised to the control group attended 12 weekly sessions and six of the monthly sessions. For the compliance analysis this dyad was included as a ‘complier’ rather than a ‘control’. Seventy per cent of the intervention group complied with the weekly intervention, as defined by the compliance of attending six or more weekly sessions and three or more monthly sessions. The compliance rate dropped to 57% for attendance at three or more of the monthly sessions.
| Attendance | Number of sessions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Number attended | 29 | 22 | 7 | 7 | 4 | 5 | 6 | 9 | 17 | 21 | 34 | 41 | 52 |
| Percentage | 11 | 9 | 3 | 3 | 1 | 2 | 2 | 4 | 7 | 8 | 13 | 16 | 21 |
Results of the compliance analysis
For the majority of models there was no significant effect of the number of sessions attended for the outcome measures, for either the continuous applied models or the binary model.
However, there was some evidence that the number of weekly sessions attended is associated with the performance of the person with dementia on the AMI (memory) scale at the 3-month time point, for both the continuous model (F = 5.69; df = 1, 349.4; p = 0.018) and the binary model (F = 5.18; df = 1, 349.7;, p = 0.023). This significant result was replicated in three of the five multiple imputations. Table 63 (Appendix 8) provides the estimated group means for the person with dementia outcome measures. In general, those that regularly attended the joint reminiscence group treatment have a higher score in comparison with the other two groups.
For the person with dementia QCPR and the person with dementia EQ-5D utility score there was some evidence that the 10-month score is associated with the number of monthly sessions attended. For the QCPR (F = 4.09; df = 1, 278.3; p = 0.044), three of the five multiple imputations were significant for the continuous applied model and (F = 4.12; df = 1, 279.8; p = 0.043) one of the five multiple imputations was significant for the binary applied model. This effect appears to be evident on the warmth subscale, for both continuous and binary models, but not on the negative subscale.
For the EQ-5D utility at 10 months (F = 9.30; df = 1, 291.6; p = 0.002) all multiple imputations were significant for the continuous applied model (F = 6.40; df = 1, 293.0; p = 0.012), with three out of five multiple imputations significant for the binary applied model. These results are also detailed in Appendix 8, Table 63.
There was some evidence that the number of weekly sessions attended is associated with the 10-month outcome for the carer outcome measures on the RSS (continuous applied model: F = 7.03; df =299.1; p = 0.008; binary applied model with four out of five multiple imputations for each model significant: F = 8.01; df = 1, 309.07; p = 0.005). Those who continued to attend the reminiscence groups regularly scored 23.36 (SE 1.33), those in the control scored 21.57 (SE 1.19), whereas those who did not attend the groups or attended infrequently scored 18.70 (SE 1.77).
Health economics
Micro-costing of reminiscence group therapy and maintenance
Cost of Remembering Yesterday, Caring Today programme
Table 24 summarises the direct costs for 19 of the 28 RYCT programmes that ran over the course of the trial. Each programme comprised 12 weekly joint reminiscence groups followed by seven monthly maintenance sessions. Complete cost data were obtained for programmes running in Bangor (4), Bradford (2), Hull (4), north London (3), south London (1) and Manchester (5). For nine programmes cost data were either incomplete (e.g. only collected for joint reminiscence sessions) or unavailable (not collected). The Newport centre was the final centre to run groups, and it was not possible to collect the costs data within the project time frame.
| Expenditure type | n | Mean cost for the provision of each 19-session programme (£) | SD | Minimum (£) | Maximum (£) | Mean cost per session for 19-session programme (£) |
|---|---|---|---|---|---|---|
| Training relateda | 19 | 299 | 242 | 0 | 797 | 16 |
| Group facilitatorsb,c | 19 | 4931 | 1531 | 2795 | 7511 | 260 |
| Supporting staff (salary)c,d | 19 | 906 | 1028 | 0 | 3163 | 48 |
| Travel costs (facilitators and staff) | 19 | 266 | 461 | 0 | 1900 | 14 |
| Subtotal (staff-related) | 6402 | 338 | ||||
| Venue | 19 | 378 | 486 | 0 | 1846 | 20 |
| Participant and carer transport | 19 | 2258 | 1583 | 100 | 4750 | 119 |
| Reminiscence materials, resources, etc. | 19 | 158 | 114 | 0 | 330 | 8 |
| Refreshments | 19 | 185 | 38 | 95 | 237 | 10 |
| Administration | 19 | 52 | 35 | 0 | 102 | 3 |
| Total | 9433 e | 498 |
Approximately two-thirds of the mean total cost of running a programme was accounted for by staff-related costs, of which the largest subcategory was for group facilitators. The mean number of facilitators was 2 for weekly sessions and 1.76 for monthly sessions. The large variation in facilitator costs between programmes reflects the use of freelancers (who would typically be paid a fixed fee to run a session) compared with using NHS or university employees where additional employment costs were incurred.
In addition to group facilitators a number of assistants were recruited to help run each of the reminiscence sessions. Assistants comprised mainly unpaid volunteers and, in some centres, NHS and local authority staff who had been released from their usual duties to help with sessions to gain additional experience and skills. The salary cost of this latter group has not been included in calculating the cost of running an RYCT programme. Supporting staff comprised paid assistants and those providing administrative and clinical support. The mean number of assistants was 4.72 and 3.77 for weekly and monthly sessions, respectively. Typically a 2-hour joint reminiscence or maintenance session would require assistants to be present for between 3 and 4 hours. The effect on total costs should salary costs be incurred for this input would be considerable. Using a salary at the mid-point of NHS Band 2 salary scale, the hourly cost of employment for a health-care assistant would be around £11 per hour. Taking a mean of 3.5 hours per session and a mean of five and four assistants for each session of weekly and monthly sessions, respectively, an additional £3388 would be incurred per programme.
The second highest category of costs was related to providing transport for participants with dementia and their carers. There was also considerable variation between programmes both within and between different trial centres. This was because transport had to be tailored to the individual circumstances of those attending sessions, for while some carers were able to drive their own vehicles to venues, many couples had to rely on the provision of taxis (at considerable cost) to enable them to attend sessions. In estimating the costs of establishing a programme, where transport was to be provided for those attending, this category would have the greatest level of uncertainty.
Although venue costs were relatively modest compared with other categories, it should be noted the mean venue cost of £378 per programme presented here is a conservative estimate (see Table 24). Eight of the 19 programmes did not incur any costs for the venue and one wave only incurred a charge of £50 for the first session. The mean costs for venue increase to £652 (total) for the 19-session programme and to £34 per session if only waves incurring a charge are included (see Table 24).
The costing of reminiscence materials and resources was problematic. Although the cost of consumable items such as stationery and photographic printing can be accurately ascertained, props and memorabilia loaned by individuals or organisations are difficult to value and may be used by several programmes. Costs presented in this category reflect the direct costs incurred by programmes in purchasing materials and resources and do not include any attempt at costing items brought to sessions by individuals or organisations, or recycled from previous sessions.
Cost per dyad
The mean cost per dyad for the provision of a 19-session RYCT programme was £964 (based on a mean of 9.79 dyads per programme). A summary of data relating to the 19 RYCT programmes is provided in Table 25.
| Item | Number/cost |
|---|---|
| Number of RYCT programmes used in micro-costing | 19 |
| Maximum number of sessions possible | 3534 |
| Number of dyads randomised to these 19 RYCT programmes | 186 |
| Total number of sessions actually attended by dyads | 2035 |
| Mean number of dyads per RYCT programme | 9.79 |
| Mean cost per RYCT programme (£) (see Table 24) | 9433 |
| Mean cost per RYCT session (£) (see Table 24) | 498 |
| Mean cost per dyad (£) | 964 |
| Mean cost per session assuming full attendance (£) | 51 |
| Mean cost per session based on actual attendance (£) | 88 |
The mean number of dyads per programme for the trial was 9.57, which is close to the mean number of dyads per programme (9.79) used in the micro-costing analysis (Table 25).
Patterns of health care, social care and voluntary sector service use and associated costs by participants with dementia and their carers
Frequency of service use
The mean frequency of community-based service for participants with dementia appears to be relatively low for all categories overall, with little difference between the intervention and control groups over the 10-month study period. Even the largest difference, for home-care workers, was not statistically significant (Table 26).
| Service | Reminiscence (n = 196) | Control (n = 140) | Difference in mean | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| District nurse | 2.36 | 8.794 | 1.20 | 3.713 | 1.16 | 0.443 |
| GP | 3.77 | 4.161 | 3.63 | 4.334 | 0.14 | 0.524 |
| Practice nurse (GP clinic) | 1.51 | 1.968 | 1.62 | 3.062 | –0.11 | 0.258 |
| Health visitor | 0.01 | 0.143 | 0.01 | 0.119 | 0.00 | 0.382 |
| Community psychiatrist | 0.54 | 1.130 | 0.58 | 1.404 | –0.04 | 0.632 |
| Psychologist | 0.36 | 1.238 | 0.16 | 0.566 | 0.20 | 0.169 |
| Counsellor | 0.02 | 0.174 | 0.01 | 0.845 | 0.01 | 0.495 |
| CPN/CMHT | 1.09 | 2.704 | 1.83 | 5.327 | –0.74 | 0.425 |
| Physiotherapist | 0.28 | 2.284 | 0.26 | 1.295 | 0.02 | 0.225 |
| Occupational health therapist | 0.63 | 2.781 | 0.44 | 1.384 | 0.19 | 0.596 |
| Care manager | 0.10 | 0.459 | 0.04 | 0.252 | 0.06 | 0.118 |
| Social worker | 0.71 | 1.746 | 0.54 | 1.456 | 0.17 | 0.349 |
| Home-care worker | 6.77 | 26.71 | 15.61 | 75.302 | –8.84 | 0.968 |
| Care attendant | 8.18 | 54.002 | 9.01 | 47.859 | –0.83 | 0.835 |
| Sitting scheme | 1.94 | 8.803 | 1.14 | 4.559 | 0.80 | 0.766 |
| Family support worker | 0.66 | 3.580 | 1.67 | 14.323 | –1.01 | 0.898 |
| Chiropodist | 0.68 | 1.433 | 0.65 | 1.308 | 0.03 | 0.641 |
| Dietician | 0.01 | 0.101 | 0.07 | 0.607 | –0.06 | 0.209 |
Given that day-care services are likely to be very similar between service providers, it is appropriate to consider the total day-care services received by participants with dementia (Table 27) and their carers (see Table 30). Day-care service use for participants with dementia is higher for the intervention group than the control group with no statistically significant difference overall (though significant differences are indicated for both local authority and NHS day care) (see Table 27). A comparison of day-care service use for participants with dementia for the 3-month period prior to baseline did not show any statistically significant differences between the control and intervention groups.
| Service provider | Reminiscence (n = 196) | Control (n = 140) | Difference in mean | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Local authority | 12.72 | 30.075 | 6.96 | 22.220 | 5.76 | 0.032 |
| Voluntary organisation | 6.48 | 60.546 | 3.61 | 12.579 | 2.87 | 0.573 |
| NHS (not hospital) | 4.14 | 50.814 | 2.36 | 9.208 | 1.78 | 0.027 |
| Mean total day care received | 23.34 | 84.514 | 12.93 | 30.237 | 10.41 | 0.201 |
A comparison between the intervention and control groups for frequency of hospital service use shows that for many categories (continuing care/respite, medical ward, outpatient services, accident and emergency) service use was higher for the control group although these differences were not statistically significant (Table 28). Exceptions to this trend were days in assessment/rehabilitation wards and day hospital service use, which were recorded as being significantly higher for the intervention group.
| Inpatient services | Reminiscence (n = 196) | Control (n = 140) | Difference in mean | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Assessment/rehabilitationb | 1.41 | 9.237 | 0.20 | 1.420 | 1.21 | 0.032 |
| Continuing care/respiteb | 0.70 | 4.490 | 0.78 | 5.484 | –0.08 | 0.821 |
| Medical wardb | 1.19 | 7.535 | 4.09 | 36.241 | –2.90 | 0.438 |
| Other inpatient wardb | 0.94 | 5.413 | 0.29 | 1.796 | 0.65 | 0.340 |
| Other services | ||||||
| Outpatient servicesc | 2.64 | 8.904 | 3.31 | 11.993 | –0.67 | 0.073 |
| Accident and emergencyd | 0.23 | 0.635 | 0.80 | 5.262 | –0.57 | 0.635 |
| Day hospitald | 0.48 | 3.008 | 0.10 | 0.834 | 0.38 | 0.028 |
For carers, differences between the intervention and control groups for the frequency of community care, day care and hospital service use were small and not statistically significant (Tables 29–31).
| Service | Reminiscence (n = 196) | Control (n = 140) | Difference in mean | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| District nurse | 0.07 | 0.527 | 0.09 | 0.424 | –0.02 | 0.443 |
| GP | 1.11 | 1.522 | 1.16 | 1.619 | –0.05 | 0.524 |
| Practice nurse (GP clinic) | 0.56 | 1.643 | 0.34 | 0.774 | 0.22 | 0.258 |
| Health visitor | 0.01 | 0.101 | 0.06 | 0.676 | –0.05 | 0.382 |
| Community psychiatrist | 0.00 | 0.000 | 0.00 | 0.000 | 0.00 | 0.632 |
| Psychologist | 0.05 | 0.309 | 0.05 | 0.346 | 0.00 | 0.169 |
| Counsellor | 0.10 | 0.716 | 0.09 | 1.014 | 0.01 | 0.495 |
| CPN/CMHT | 0.08 | 0.539 | 0.06 | 0.436 | 0.02 | 0.425 |
| Physiotherapist | 0.11 | 0.631 | 0.21 | 1.141 | –0.10 | 0.225 |
| Occupational health therapist | 0.02 | 0.159 | 0.01 | 0.119 | 0.01 | 0.596 |
| Care manager | 0.01 | 0.101 | 0.00 | 0.000 | 0.01 | 0.118 |
| Social worker | 0.06 | 0.307 | 0.03 | 0.206 | 0.03 | 0.349 |
| Home-care worker | 0.00 | 0.000 | 0.21 | 2.371 | –0.21 | 0.968 |
| Care attendant | 0.43 | 6.000 | 0.00 | 0.000 | 0.43 | 0.835 |
| Sitting scheme | 0.01 | 0.071 | 0.00 | 0.000 | 0.01 | 0.766 |
| Family support worker | 0.05 | 0.362 | 0.79 | 0.466 | –0.74 | 0.898 |
| Chiropodist | 0.71 | 0.277 | 0.08 | 0.295 | –0.09 | 0.641 |
| Dietician | 0.02 | 0.225 | 0.01 | 0.085 | 0.01 | 0.209 |
| Service provider | Reminiscence (n = 196) | Control (n = 140) | Difference in mean | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Local authority | 0.12 | 1.714 | 0.00 | 0.000 | 0.12 | 0.398 |
| Voluntary organisation | 0.04 | 0.500 | 0.79 | 7.148 | –0.75 | 0.375 |
| NHS (not hospital) | 0.00 | 0.000 | 0.00 | 0.000 | 0.00 | 0.237 |
| Mean total day care received | 0.16 | 1.783 | 0.79 | 7.148 | –0.63 | 0.400 |
| Inpatient services | Reminiscence (n = 196) | Control (n = 140) | Difference in mean | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Assessment/rehabilitationb | 0.29 | 1.919 | 0.04 | 0.348 | 0.25 | 0.074 |
| Continuing care/respiteb | 0.08 | 1.004 | 0.17 | 1.246 | –0.09 | 0.065 |
| Medical wardb | 0.45 | 2.108 | 0.40 | 2.031 | 0.05 | 0.665 |
| Other inpatient wardb | 0.09 | 0.763 | 0.17 | 1.003 | –0.08 | 0.134 |
| Other services | ||||||
| Outpatient servicesc | 3.56 | 16.865 | 2.99 | 9.236 | 0.57 | 0.783 |
| Accident and emergencyd | 0.10 | 0.329 | 0.21 | 1.354 | –0.11 | 0.642 |
| Day hospitald | 0.10 | 0.541 | 2.01 | 20.317 | –1.91 | 0.591 |
Alternative accommodation
In addition to collecting data for community care, day care and hospital service use, carers were also asked to indicate whether or not the participants with dementia in their care had spent time in accommodation away from their usual place of residence. Table 32 summarises the number of days spent away from home in different types of accommodation.
| Type of accommodation | Reminiscence (n = 196) | Control (n = 140) | ||
|---|---|---|---|---|
| Mean number of nights | SD | Mean number of nights | SD | |
| Sheltered accommodation | 0 | 0 | 0 | 0 |
| Residential home | 1.71 | 14.522 | 0 | 0 |
| Nursing home | 0 | 0 | 0 | 0 |
| Dual registered home | 0 | 0 | 0.39 | 4.564 |
| General medical ward | 0.01 | 0.071 | 0.04 | 0.423 |
| Rehabilitation ward | 0 | 0 | 0 | 0 |
| Acute psychiatric ward | 0.61 | 8.500 | 0 | 0 |
Cost of service use
The costs of service use were derived from using national unit costs. 54,55 Unit costs for each health-care and social-care service (shown in Appendix 1) were multiplied by the frequencies recorded in the CSRI completed by participants with dementia and carers. Unit costs are shown in Appendix 1 and the mean costs for community care, day care and hospital service use are shown in Appendix 2. The analysis has been restricted to cases where full cost data could be calculated (i.e. where health and social-care service use data had been obtained at both 3- and 10-month follow-ups). The price year used was 2010. Given that follow-up was for < 12 months, discounting was not applied to either costs or outcomes.
Table 33 summarises the mean total costs of health-care and social-care service use for the intervention and control groups for participants with dementia and carers over 10 months. A more detailed breakdown of costs is presented in Appendix 3. Although the mean total costs for participants with dementia in the intervention group were 13.5% (£580) higher overall than for the control group, this difference was not statistically significant (see Table 33). Most of the difference is accounted for by the higher mean cost of day care for the intervention group, which is 80% higher than the mean cost for the control group. This higher mean cost reflects the increased frequency of day service use (although not statistically significant overall) for participants with dementia in the intervention group (see Table 27). It is not clear why this should be the case, although it is possible that increased social contact through attending joint reminiscence groups may increase service uptake through improved knowledge of local service provision and availability. For carers, mean costs for the intervention group are 12.7% higher overall than the control group over the 10-month period (£172), although again this was not statistically significant.
| Service | Reminiscence (n = 196) | Control (n = 140) | Difference in mean total costs (£) | Asymptotic significancesa | ||
|---|---|---|---|---|---|---|
| Mean total costs (£) | SD | Mean total costs (£) | SD | |||
| Participants with dementia | ||||||
| Community care | 1072 | 1809 | 1170 | 1983 | –98 | 0.674 |
| Day care | 1098 | 4451 | 610 | 1415 | 488 | 0.230 |
| Hospital use | 2719 | 7106 | 2529 | 8087 | 190 | 0.801 |
| Total (participant with dementia) | 4889 | 8806 | 4309 | 8729 | 580 | 0.471 |
| Carers | ||||||
| Community care | 258 | 339 | 283 | 449 | –25 | 0.505 |
| Day care | 7 | 77 | 34 | 307 | –27 | 0.400 |
| Hospital use | 1266 | 3752 | 1043 | 3622 | 223 | 0.694 |
| Total (carer) | 1531 | 4647 | 1360 | 1459 | 171 | 0.800 |
| Grand total | 6419 | 5667 | 751 | |||
Cost-effectiveness analysis
Cost-effectiveness was evaluated in terms of the primary clinical outcomes: the disease-specific quality-of-life measure QoL-AD for participants with dementia and the GHQ-28 for carers. Non-parametric bootstrapping (5000 replications) was used to address the uncertainty associated with point estimates of costs and outcomes. The incremental cost-effectiveness for the QoL-AD was £2586 (i.e. the mean cost of a one point change on the scale reflecting an improvement in quality of life) (Table 34). It should be noted that the 95% CIs for this estimate were extremely broad. The analysis for the GHQ for carers showed that the small mean difference in scores between the intervention and control groups was positive, indicating poorer mental health for carers in the intervention group as compared with the control group over the 10 months of the study (see Table 45, Appendix 3).
| Group | Mean total cost (SD) | Mean QoL-AD score (SD) | Difference in cost (£) | Difference in effect (QoL-AD score) | ICER (£) (95% CI) |
|---|---|---|---|---|---|
| Intervention (n = 196) | 5853 (8806) | 37.013 (4.768) | 1544 | 0.597 | 2586 (–20,280 to 24,340) |
| Control (n = 140) | 4309 (8729) | 36.416 (4.692) |
Cost–utility analysis
Cost–utility analysis was undertaken separately for participants with dementia and their carers using the total cost of health-care and social-care services and QALYs generated from the self-completed EQ-5D. Cases included all those for whom complete cost data were available (n = 336) and the analysis was conducted on an ITT basis. Total QALYs were calculated using the area under the curve method. For cases with missing EQ-5D values, imputed values were generated in accordance with the procedure outlined in Chapter 2.
While a full CUA had been planned as part of the economic evaluation of the REMCARE trial, the results showed that generating cost-effectiveness acceptability curves (CEACs) would not be meaningful. The mean costs of health-care and social-care service use were higher for the intervention group (see Table 33) and the costs of the joint reminiscence and maintenance sessions only applied to the intervention arm, giving a higher overall mean cost for the intervention group compared with the control group. Coupled with the lack of any statistically significant effect from the self-reported EQ-5D, this indicated that the intervention could not be cost-effective. Results shown in Table 35 confirm that the mean difference in QALYs between intervention and control arms for both participants with dementia and carers were negligible. Given that these would generate meaninglessly high incremental cost per QALY figures, they have not been calculated. Cost-effectiveness planes generated from 5000 non-parametric bootstrapped replications are presented in Appendix 3 for reference.
| Participant | Group | Mean total cost (£) | SD of mean total cost (£) | Mean QALYs | SD of mean QALYs | Difference in cost (£) | Difference in effect (QALYs) |
|---|---|---|---|---|---|---|---|
| Person with dementia | Reminiscence (n = 196) | 5853 | 8806 | 0.644 | 0.141 | 1544 | 0.001 |
| Control (n = 140) | 4309 | 8729 | 0.643 | 0.150 | |||
| Carer | Reminiscence (n = 196) | 2495 | 3866 | 0.632 | 0.175 | 1136 | –0.000 |
| Control (n = 140) | 1359 | 3743 | 0.633 | 0.179 |
Patterns of drug use at baseline in the REMCARE trial
Table 36 indicates that at baseline, 61.9% (63.1% reminiscence group; 60.6% control group) of participants across the whole sample were taking one medication relating specifically to dementia (i.e. an acetylcholinesterase inhibitor). At baseline, 26.1% (22.4% reminiscence group; 30.7% control group) of participants across the whole sample were taking one antidepressant drug. At baseline, 9.9% (10.1% reminiscence group; 9.6% control group) of carers across the whole sample were taking one antidepressant drug. Fewer than 5% of the sample as a whole were receiving one or more antipsychotic medications.
| Participant drug use | Reminiscence group (%) | Control group (%) | Whole sample (%) |
|---|---|---|---|
| Participants on one dementia drug | 169 (63.1) | 132 (60.6) | 301 (61.9) |
| Participants on two dementia drugs | 3 (1.1) | 6 (2.8) | 9 (1.9) |
| Participants on one antidepressant drug | 60 (22.4) | 67 (30.7) | 127 (26.1) |
| Participants on two antidepressant drugs | 2 (0.07) | 1 (0.5) | 3 (0.06) |
| Participants on one sleeping drug | 7 (2.6) | 9 (4.1) | 16 (3.3) |
| Participants on two sleeping drugs | 1 (0.04) | 1 (0.2) | |
| Participants on one antipsychotic drug | 11 (4.1) | 13 (6.0) | 24 (4.9) |
| Participants on two antipsychotic drugs | 2 (1.0) | 2 (0.04) | |
| Carer on one antidepressant drug | 27 (10.1) | 21 (9.6) | 48 (9.9) |
| Carer on two antidepressant drugs | 1 (0.04) | 1 (0.5) | 2 (0.04) |
| Carer on one sleeping drug | 9 (3.3) | 10 (4.6) | 19 (3.9) |
| Carer on one antipsychotic drug | 3 (1.1) | 2 (1.0) | 5 (1.0) |
A comparison of European Quality of Life-5 Dimensions scores of trial participants with UK norms
Tables 46–51 (Appendix 4) show that, overall, mean EQ-5D and EQ-5D VAS scores for both participants and carers were lower than population norms. Mean population norms59 tend to decrease with age, and this was also observed in the REMCARE trial.
Triangulation substudy to compare self-report of service use by participants with dementia with their general practitioner records
Health economists have two options for collecting information about the frequency and type of contacts that participants have in RCTs with health-care and social-care services. These are to ask participants to recall contacts over a specified period of time or to interrogate GP or hospital notes directly. There have been previous studies looking at the level of agreement or otherwise between these two methods. 57 We opted to ask REMCARE study participants to recall their type and frequency of contacts as a less intensive research method than interrogation of patient records. This triangulation substudy was designed to check the level of agreement or otherwise of participant recall with their GP records. Weighted kappa, κ, was used to assess the degree of agreement between self-reported health-care service use for a sample of participants with dementia (n = 36; 18 from intervention group and 18 from control group, randomly selected from the Hull and Bangor groups) and records maintained by their GPs. The results are presented in Table 37. A higher kappa coefficient (maximum value 1) indicates a higher level of agreement. Coefficients may be grouped to form a scale, such as those devised by Landis and Koch63 and Altman. 64 The scale of kappa coefficients presented by Altman64 gives five levels of agreement: < 0.20 = poor; 0.20–0.40 = fair; 0.41–0.60 = moderate; 0.61–0.80 = good; and > 0.80 = very good. Levels of agreement at baseline, 3 months and 10 months between self-reported contacts and GP records were consistently good or very good, with the exception of psychiatrist contacts at baseline and 3 months, GP visits at 3 months and practice nurse at 10 months. Overall, there was a high level of agreement between data collected from participants with dementia and data collected from their GPs. Although based on a small subsample of REMCARE participants, the results of this triangulation study suggest that we can be relatively confident about the recall of participants with mild to moderate dementia and their carers about their contacts with services in the REMCARE trial.
| Service type | Baselinea | 3 months | 10 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPb | CSRIc | κd | z-score | p-value | GPb | CSRIc | κd | z-score | p-value | GPb | CSRIc | κd | z-score | p-value | |
| GP visit | 1.42 (1.89) | 1.58 (2.03) | 0.82 | 4.91 | < 0.001 | 1.33 (1.31) | 1.39 (1.29) | 0.58 | 3.48 | < 0.001 | 2.22 (3.02) | 2.78 (3.53) | 0.75 | 4.52 | < 0.001 |
| Practice nurse | 0.61 (0.87) | 0.50 (0.85) | 0.80 | 4.86 | < 0.001 | 0.47 (0.91) | 0.36 (0.87) | 0.89 | 5.4 | < 0.001 | 0.89 (1.01) | 0.47 (0.65) | 0.31 | 2.31 | 0.02 |
| CPN | 0.50 (1.03) | 0.56 (1.13) | 0.88 | 5.28 | < 0.001 | 0.33 (1.37) | 0.58 (2.03) | 0.74 | 4.77 | < 0.005 | 0.39 (1.20) | 0.50 (1.83) | 0.83 | 5.04 | < 0.001 |
| Psychiatrist | 0.56 (0.23) | 0.25 (0.55) | 0.07 | 0.67 | 0.51 | 0.56 (0.23) | 0.28 (0.57) | 0.19 | 1.88 | 0.06 | 0.58 (1.84) | 0.78 (1.84) | 0.60 | 3.77 | < 0.001 |
| Hospital use | 0.97 (2.43) | 1.03 (2.43) | 0.82 | 4.92 | < 0.001 | 0.97 (3.12) | 0.97 (3.12) | 0.93 | 5.57 | < 0.001 | 1.47 (2.35) | 1.42 (2.38) | 0.99 | 5.94 | < 0.001 |
Chapter 4 Discussion
Main findings
The REMCARE trial evaluated the impact of being offered the opportunity to attend joint reminiscence groups on people with dementia and their caregivers.
With 487 people with dementia/family caregiver pairs, this is the largest trial of any reminiscence-based intervention for people with dementia in the world literature. The primary planned analyses do not show any benefit from being allocated to receive the reminiscence intervention for either people with dementia or their caregivers, in terms of quality of life, for the person with dementia, or psychological distress, for the family caregiver. No effect was found on the autobiographical memory of people with dementia, or on their mood or functional level when examining the secondary outcome variables. Similarly, no benefits for the family caregiver, in terms of mood and stress, were identified. Neither people with dementia nor their caregivers reported any improvement in quality of relationship. A difference does emerge on one of the four subscales of the primary outcome measure for caregivers, the anxiety subscale of the GHQ. On this subscale, those caregivers randomised to receive reminiscence report higher levels of anxiety at the 10-month end point, whereas those allocated to receive treatment as usual show a reduced level of anxiety at this point.
This was the first economic evaluation alongside a trial of joint reminiscence groups, and we examined, from a public sector, multiagency perspective, the relationship between costs and outcomes for both participants with dementia and their family carers. We undertook a thorough micro-costing of the RYCT programme and found that approximately two-thirds of the mean total cost of running a RYCT programme was accounted for by group leader and assistant costs, with transport being the next most significant cost. The costs of running a RYCT programme depend on whether group leaders are NHS salaried or freelance and whether assistants are paid or voluntary. Variation in RYCT programme costs between research centres in the REMCARE trial was accounted for by payment method and grade of group leaders and assistants.
There were very few statistically significant differences in the frequency and total cost of NHS, social care and voluntary sector service use between groups. On a subsample comparing carers’ self-report of service use with primary care records, there was evidence overall of good agreement on most aspects of service usage. The intervention group reported using more local authority and NHS day-care services and day-care hospital services than the control group over the 10-month study period. It may be that one of the additional effects of attending a reminiscence group with other carers is the opportunity to find out about other available services and how to access them.
Participants with dementia were able to complete the EQ-5D in a face-to-face interview, in line with evidence on suitability of this health-related quality-of-life instrument in this patient group. 58 However, at baseline, our sample (both in the intervention group and control group), both participants and carers, reported lower health-related quality of life than UK norms by age and gender. 59
The primary end point effectiveness results of this trial mean that RYCT could not be cost-effective. The differences in QALYs between intervention and control groups over the 10-month study period were negligible. Service costs and the cost of the intervention meant that there were greater overall costs for no apparent benefits on the measures used. Although calculation of an estimated cost per point improvement on the QoL-AD was possible, the CIs were so broad as to make this impossible to interpret. A more precise estimate would have allowed comparison with the best-established psychosocial intervention for people with dementia: cognitive stimulation. This has been shown to have a QoL-AD benefit of between 1 and 2 points. 2
These findings stand in contrast to those of our trial platform, where, in a much smaller sample, significant differences on autobiographical memory and caregiver depression were identified after 12 weekly group reminiscence sessions, in favour of the reminiscence condition. Notably, the treatment as usual control group showed a decline in these and other outcomes over the 3 months of the study, whereas those receiving the reminiscence intervention maintained their baseline levels. In the current study, the marked decline in carers’ stress and strain over the treatment and follow-up period is not evident in the treatment as usual group as it was in the trial platform. Failure to replicate the trial platform may be due to a range of factors, some resulting from the rigorous application of the learning from the trial platform in relation to an appropriate control group and randomisation method.
The extension of the study from three to eight centres meant that there were a number of new group facilitators. Although the trial platform demonstrated that the approach could be delivered by new facilitators, with training and supervision, and a detailed treatment manual had been produced, it was necessary in the current study to ensure that the RYCT approach was delivered as planned. This was achieved by each centre receiving training from the originator of the approach, having regular opportunities to meet with other facilitators for peer supervision, and completion after each session of a checklist indicating adherence to the approach as set out in the treatment manual. A number of group sessions in several centres were videotaped for the purposes of providing a record for group members, and these reinforce the extent to which the approach was implemented as planned.
Overall, 81% of participants completed the 3-month assessment and 72% of participants completed the 10-month end point assessment. The latter figure was consistent with our projections for a sample of people with dementia at this mild to moderate degree of severity initially living in the community. Attrition through death and illness was the most common reason for not being available for follow-up assessments, with the rates of attrition being less for the intervention than the control group (23% and 34%, respectively). There is no evidence that the differential attrition rate influenced the findings, with little difference in the characteristics of those dropping out between the groups, with the exception that those dropping out of the treatment as usual condition did so at a slightly lower level of dementia severity.
Although attrition from the study was less marked in the intervention arm of the study, a considerable minority of those allocated to receive the reminiscence intervention did not in fact participate as planned. Over 1 in 10 (11%) of participants randomised did not attend any group sessions at all, and one-quarter in total attended three or fewer sessions. In some cases this was due to illness or death, in others it was for logistical reasons and in other cases either the caregiver or the person with dementia decided not to continue. A threshold of attending six or more of the 12 weekly sessions was set, based on a consensus of clinical experience of the approach. Similarly, a threshold of three or more of the potential seven monthly sessions was set to indicate compliance with the maintenance intervention.
Analysing the data in relation to whether or not the intervention was actually received, rather than by allocation, indicated some differences in outcomes between those receiving the intervention and those who did not. Autobiographical memory, in relation to being able to recount memories related to particular events in the person’s life, showed a significant benefit at the 3-month assessment for those who attended more weekly sessions. The quality of relationship between the person with dementia and caregiver, as rated by the person with dementia, was higher at the 10-month end point for those who attended more monthly sessions. Quality of life of the person with dementia, rated on the EQ-5D by the person with dementia, showed a similar positive effect for those who had attended more monthly sessions. (Those who attended more monthly sessions were a subgroup of those who attended more weekly sessions.) Finally, those caregivers attending more weekly sessions reported a higher level of stress on the RSS at the 10-month follow-up. Thus, in relation to the person with dementia, these results are more consistent with the trial platform, but continue to show a contrasting, negative effect on the caregiver. It should be borne in mind that these compliance findings may reflect a ‘survivor’ effect, with those able to continue having higher scores on certain measures, irrespective of the intervention. They are also the result of a number of statistical comparisons and may be chance findings. The attribution to the intervention cannot then be clear. However, the analyses do control for baseline levels of each measure, as well as key demographic factors.
Implications for health care
Should joint reminiscence group interventions be withdrawn?
Given the largely negative findings, and a strong suggestion that any effects on family caregivers are to increase stress and anxiety, the question must be raised as to whether or not such joint reminiscence groups should be withdrawn at this stage. The findings are unexpected and not consistent with systematic reviews,1 which suggest that involving people with dementia and caregivers together in an intervention leads to better outcomes for family caregivers.
Feedback from caregivers who have participated in the programme will be of great value in interpreting these results. Feedback was collected regularly as part of the group programme, and is now being analysed thematically to assist in the interpretation of the trial findings. Given the finding that it is those who remain with the groups who show the worst outcomes, on the RSS at least, their comments are especially important to consider.
The triangulation of feedback from participants with the trial findings was not part of the original trial protocol, and is outside the scope of the current report. However, it is a necessary piece of work in order to enable a fuller understanding and interpretation of the trial findings, and will be reported in due course.
The trial does indicate clearly that this is not an intervention that would be taken up by all people with dementia and caregivers, with one-quarter not taking up the opportunity when offered to engage with the groups. Some expressed a discomfort with groups, whereas some caregivers found it logistically difficult to attend on a regular basis. Some caregivers were doubtful the group would be helpful, and there were certainly examples of people with dementia who had shown some enjoyment of the groups being withdrawn by their relative who was less enthusiastic about attending. Outside the research context, joining such a group would clearly be a matter of personal preference, and potentially more choice regarding the person with dementia attending alone might be offered.
Further work also needs to be undertaken to identify whether or not there are particular factors that lead to particular people with dementia and caregivers benefiting from attending the groups, looking at factors that predict outcomes. This will be linked with consideration of clinical significance. At baseline, over one-third (37%) of the family caregivers showed levels of anxiety of clinical concern on the HADS; if the negative findings from caregivers had an influence on these rates, this would be of particular relevance. At the 10-month time point, the rate for carers who attended six or more RYCT sessions was 39%, compared with 37% for those who had attended fewer group sessions, with no clear difference between groups emerging. The difficulty from a clinical perspective may well be how to balance positive benefits for people with dementia with negative effects on family caregivers. Given the crucial role of family caregivers in maintaining people with dementia in the community, should the impact on the caregiver be given precedence?
Factors to consider in research on psychosocial interventions
One of the limitations of the REMCARE trial that has emerged is a lack of emphasis on process evaluation alongside the intervention. A more systematic approach to recording the experiences of those participating in the interventions (and those who decided not to continue), would have had the potential to illuminate the unexpected findings of the trial. As with many other studies in the field, the evaluation frame has focused on changes outside the group context, and it would have been helpful to have further evaluations of changes within the treatment context, for example in participation, communication and interaction, and in enjoyment – for both people with dementia and their caregivers.
Originally, it had been planned to examine in more detail the delivery of the treatment, through videotaping and rating a sample of sessions in each centre; however, a lighter touch to monitoring adherence was recommended during the review process, and so the checklist approach was utilised. It would have been helpful to have had more details regarding how caregivers who were expressing stress and difficulty were supported in the groups. The treatment manual schedules separate time for caregivers to meet together in a separate room, away from the people with dementia, during certain (not all) group sessions. There was some discussion during the project regarding these sessions, which appeared necessary in some groups and less so in others. Potentially, having an opportunity to ventilate in such a context might be helpful for one caregiver, but may spark increased concerns in another, perhaps regarding what lies ahead in the caregiving journey. One possibility is that the sessions primed expressions of stress and anxiety, but because this was not the primary focus did not adequately bring these towards resolution. However, this is difficult to ascertain at this juncture.
The study also lacks a means of balancing and reconciling discrepant outcomes for the person with dementia and his or her caregiver. A rating of global outcome for each dyad would have been one approach to this difficult area. Perhaps because caregiver and person with dementia outcomes are typically interlinked, this area of outcome assessment has not been developed in this field. The outcome measures used in the study tend to focus on negative outcomes for the carer, rather than positive well-being. Depression, anxiety and stress arising from caregiving are well covered, but positive aspects much less so – perhaps only through the ‘warmth’ aspect of the relationship measure.
After the trial had been running for some months, some evidence from the literature emerged that there could be a differential response to reminiscence work, according to the type of dementia, Alzheimer’s or vascular. This variable was added to the data set, but proved difficult to obtain in many settings. The diagnosis of subtype of dementia still appears to be challenging in many NHS services in England and Wales, and makes this type of comparison much more difficult.
A number of instrumental variables were considered, in attempting to understand the findings of the study. These are variables that could potentially have a role in mediating the relationships identified in the statistical analyses, by influencing attendance at the groups which would affect the outcome variables indirectly only through attendance. In this trial, insufficient information was collected to include any of these variables as instrumental variables in the immediate analysis. However, the variables are worth considering for future studies of this type.
The variables suggested included the timing of the group in relation to the current relationship status. Some caregivers were reaching the end of their time caring at home, and their stress was increasing rapidly. The timing of the intervention, as with many psychosocial interventions, may be crucial. Although data are available on severity of dementia, these do not necessarily indicate the difficulties being experienced by the caregiver. Specifically, no measure of behavioural difficulties was included (as this was not the focus of the study). However, such a measure may have been a useful indicator of how the caregiver viewed the person with dementia’s difficulties in day-to-day life.
In a clinical context, attempts might be made to assemble a group with complementary characteristics. In the research context, facilitators were required to develop group cohesion even, as in one group, where there were group members with an antipathy arising from contacts with each other well before the group started. Logistic issues, such as meeting times, ease of access with travel and transport were also inflexible in the research context. Even though transport could be provided, this was potentially stressful for some, in view of the distance between the person’s home and the meeting place. As in some cases some time passed between participants agreeing to take part and the group commencing, the person’s initial interest may have waned, or other circumstances may have changed.
The facilitator and volunteer characteristics may also be important to consider further. Despite adherence to the intervention protocol, there are variations in style that could potentially have been captured by measures of therapeutic alliance, cognitive style and interpersonal style. Variations even in the use of telephone reminders for the dyads prior to the sessions could presumably influence attendance rates. Some of these factors, if they had been influential, might perhaps have been picked up in the inclusion of location and wave in the analysis models, which effectively allows effects specific to a particular group to emerge, if very different from the others.
Recommendations for future research
Two major areas for further research arise from this trial and its findings. First, given the findings on caregiver anxiety and stress, a direct comparison with other caregiver interventions would be of particular interest. This is currently under way, through the National Institute of Health Research (NIHR)-funded Support at Home – Interventions to Enhance Life in Dementia Programme ‘SHIELD’ (principal investigator: Professor Martin Orrell, University College London ), in which joint reminiscence groups are being compared with a caregiver support programme, based on the Befriending and Costs of Caring (BECCA) intervention, evaluated in a previous HTA trial. 65–67
The second relates to the effects of reminiscence groups for people with dementia alone. This was considered in the trial platform for REMCARE, in which outcomes appeared similar whether or not caregivers were included. If the putative negative effects on caregivers could be avoided if the caregiver is not required to attend with the person with dementia, then it may be that this still constitutes a useful intervention for people with dementia. However, an evaluation of its effects should focus on within-session and proximal benefits, rather than longer-term benefits, as have been evaluated in the current trial, with little apparent effect. The research would then address the potential benefits for people with dementia of reminiscence groups, in terms of affect and well-being and autobiographical memory within and soon after group participation.
Conclusions
The REMCARE trial indicates that offering participation in a programme of joint reminiscence groups for people with dementia and caregivers is neither an effective nor a cost-effective intervention in relation to quality of life for people with dementia or psychological distress for family caregivers. Indeed, it may be associated with an increase in anxiety amongst caregivers. ITT analyses do not indicate any benefits to participants with dementia on secondary outcomes such as autobiographical memory, mood or quality of relationship with the caregiver. One-quarter of those randomised to attend reminiscence groups attended three or fewer of the planned 19 sessions. There is some evidence from a planned compliance analysis suggesting that those who do attend as planned show improvements in autobiographical memory at the 3-month follow-up, and in quality of life and quality of relationship after 10 months of the intervention. However, caregivers who attend report greater stress. These compliance analysis results should be viewed with some caution – they are exploratory and changes may be attributed to other factors rather than the intervention received.
Further work is needed to understand these findings in the context of reports from those participating in the groups. A comparison of the joint reminiscence approach with a caregiver support intervention is already underway, and together these projects should be able to indicate whether the joint approach should be reconsidered, despite the evident enjoyment and satisfaction of those involved.
Acknowledgements
This study was supported by the NIHR Health Technology Assessment (HTA) programme.
We wish to thank all the people with dementia and their family caregivers who took part in the study for their time and their contribution to the research.
Special thanks to Pam Schweitzer MBE, who developed the RYCT approach and inspired the project. Pam served as the reminiscence consultant for the trial, providing training and supervision for the group facilitators.
The Trial Steering Committee (TSC) was chaired by Jane Fossey, and included Penny Davies and Professor Alistair Burns. The DMEC comprised Amanda Farrin (chairperson), Professor Gail Mountain and Dr Fiona Goudie. We are grateful to both committees for their diligent oversight of the trial.
We wish to thank all those who facilitated groups and took part in data collection:
Bangor: Joan Woods, Rhiannon Williams, Eileen Bulkeley, Jill Coleman, Martin Jones, Julia Roberts, Eryl Martin, Nathan Bray, Melissa van der Bilj and Teresa Gill.
Bradford: Rachel Muir, Tricia Gallagher, Karen Flowers, Jean Harland, Rachael Lilley, Paul Martin, Louise Richardson, Jenny Shackleton, Dominic Slingsby, Jessica Tasker, Anna Tatton and Sarah Hodgson.
Hull: Hannah Wilkinson, Kerry Dyer, Marcus Tredinnick, Len Stevens, Katie Swift, Cathryn Hart, Sue Whiteing, Karen Hawcroft and Dr Emma Wolverson.
London: Dr Jennifer Wenborn, Nyla Bhatti, Fara Hamidi, Louise Owen, Dr Kay Rawnsley, Dr Henriëtte van der Roest, Cherya Sequeira, Maria Stavrinaki and Adrian Dassrath.
Manchester: Ricca Felixson, Elizabeth Johnson, Naomi Lewis, Lynne Owen and Octavia Smart.
Newport: Bethan Jones, Anna Jenkins, Sarah Hunt, Matthew Williams and Anne Carpenter.
Many colleagues in the Institute of Medical and Social Care Research, Bangor University contributed greatly, including: Ceri Bray, Michelle Williams (project secretary), Brenda Ellis, Rhiannon Whitaker, Huw Roberts, Shubha Sreenivas, Carys Jones and Natalia Hounsome.
Great help was received in recruiting participants and in other aspects of the project from the NISCHR-CRC (formerly Clinical Research Collaboration Cymru), working with NEURODEM Cymru in Wales, and from DeNDRoN in England. The Alzheimer’s Society in all areas and several local Age Concern organisations (including Age Concern, Salford) gave valuable support, as did a number of galleries and museums, including Salford Museum and Gallery, Hull City Council (museums) and the Streetlife Museum, Hull.
In Hull, North and East Yorkshire and Northern Lincolnshire Comprehensive Local Research Network and local GPs supported recruitment and support for excess treatment costs was provided by the Hull York Medical School, with support from Professor Ian Greer (then Dean), Humber NHS Foundation Trust, Hull Primary Care Trust and East Riding of Yorkshire Primary Care Trust (now NHS East Riding of Yorkshire). In Wales, excess treatment costs were provided by the Wales Office for R&D in health and social care (later NISCHR), and in Bradford through the West Yorkshire R&D Consortium.
We wish to thank all the NHS Trusts and Health Boards who supported the research in a number of ways, and their staff who assisted in identifying potential participants. These include Aneurin Bevan Health Board, Conwy and Denbighshire NHS Trust, North East Wales NHS Trust, North West Wales NHS Trust, Betsi Calwaladr University Health Board, Humber NHS Foundation Trust, Greater Manchester West Mental Health NHS Foundation Trust, Salford Primary Care Trust, North East London Foundation Trust and the Oxleas Foundation Trust.
Many staff in the NHS and voluntary sector organisations were of great assistance, including:
North Wales site: Nia Williams, Rowena Spencer, John Hughes Roberts, Jean Thompson, Lorraine Davies, Dorothy Roberts-Jones, Dr Bobby Kurian, Sian Bowyer, Ann Jones, Lisa Foulkes, Andrea Ganley, Mandy Rowlands, Sandra Dowling, Amanda Hewitt, Jacky Baldini, Joan Davies, Tracy Stockin, Denise Roberts, Valerie Smith and The Old Brewery Centre for Younger People with Dementia, Shotton.
Newport site: Dr Pauline Ruth, Dr Bapuji Rao, Dr Caroline Linton, Dr Patrick Chance and CMHT colleagues.
Manchester site: Alexandra House Day Centre (now known as the Humphrey Booth Resource Centre), Brierley House Day Centre and Walkden Gateway.
Contributions of authors
Robert T Woods (Professor, Clinical Psychology of Older People) conceived, planned and designed the study and supervised its conduct. He was principal investigator for the north Wales site and drafted sections of the final report.
Errollyn Bruce (Lecturer, Dementia Studies) conceived, planned and designed the study and was principal investigator for the Bradford site.
Rhiannon T Edwards (Professor, Health Economics) oversaw the health economics aspects of the study.
Ruth Elvish (Clinical Psychologist) contributed to data collection and group facilitation at the Manchester site and contributed to analysis of qualitative data arising from the trial.
Zoe Hoare (Research Officer, Statistics) was trial statistician, developed the statistical analysis plan, undertook the statistical analyses and drafted sections of the final report.
Barry Hounsome (Research Fellow, Health Economics) was trial manager for the project, undertook the health economics evaluation and drafted sections of the final report.
John Keady (Professor, Mental Health Nursing of Older People) was principal investigator for the Manchester site and oversaw analysis of qualitative data arising from the trial.
Esme D Moniz-Cook (Professor, Clinical Psychology and Ageing) was principal investigator for the Hull site.
Vasiliki Orgeta (Research Officer, Ageing and Mental Health) contributed to and co-ordinated data collection in the London sites.
Martin Orrell (Professor, Ageing and Mental Health) conceived, designed and planned the study and was principal investigator for the south and north London sites.
Janice Rees (Consultant Clinical Psychologist) was principal investigator for the Newport site.
Ian T Russell (Professor, Clinical Trials) contributed to the planning and design of the study and oversaw the methodological aspects of the trial.
All study authors contributed to the preparation and review of this report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc 2003;51:657-64.
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry 2003;183:248-54.
- National Institute for Health and Clinical Excellence and the Social Care Institute of Excellence (NICE-SCIE) . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care. Clinical Guideline 42 2006.
- McShane R, Keene J, Gedling K, Fairburn C, Jacoby R, Hope T. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up. BMJ 1997;314:266-70.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos GG. Old Age Task Force of the World Federation of Biological Psychiatry. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn 2010;30:161-78.
- Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2003;4.
- Woods B, Spector AE, Prendergast L, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2005;4.
- Dementia Services Development Centre (DSDC) . Services for People With Dementia in Wales. Report No. 1: Residential and Nursing Home Care in Wales 2002. www.bangor.ac.uk/imscar/dsdc/documents/DSDCreport1Homes_000.pdf (accessed 9 September 2012).
- Gibson F. The past in the present: using reminiscence in health and social care. Baltimore, MD: Health Professions Press; 2004.
- Woods RT, McKiernan F, Haight BK, Webster J. The art and science of reminiscing: theory, research, methods and applications. Washington, DC: Taylor & Francis; 1995.
- Woods RT, Bruce E, Edwards RT, Hounsome B, Keady J, Moniz-Cook ED, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev 2005;2.
- Tadaka E, Kanagawa K. A randomized controlled trial of a group care program for community-dwelling elderly people with dementia. Jpn J Nurs Sci 2004;1:19-25.
- Tadaka E, Kanagawa K. Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatr Gerontol Int 2007;7:167-73.
- Wang J-J. Group reminiscence therapy for cognitive and affective function of demented elderly in Taiwan. Int J Geriatr Psychiatry 2007;22:1235-40.
- Gudex C, Horsted C, Jensen A, Kjer M, Sørensen J. Consequences from use of reminiscence – a randomised intervention study in ten Danish nursing homes. BMC Geriatr 2010;10.
- Hsieh C-J, Chang C, Su S-F, Hsiao Y-L, Shih Y-W, Han W-H, et al. Reminiscence group therapy on depression and apathy in nursing home residents with mild to moderate dementia. JECM 2010;2:72-8.
- Haslam C, Haslam SA, Jetten J, Bevins A, Ravenscroft S, Tonks J. The social treatment: the benefits of group interventions in residential care settings. Psychol Aging 2010;25:157-67.
- Schweitzer P, Bruce E. Remembering Yesterday, Caring Today: reminiscence in dementia care – a guide to good practice. London: Jessica Kingsley Publishers; 2008.
- Bruce E, Gibson F. Remembering Yesterday, Caring Today: Evaluatiors’ Report 1998.
- Thorgrimsen L, Schweitzer P, Orrell M. Evaluating reminiscence for people with dementia: a pilot study. Art Psychother 2002;29:93-7.
- Nolan M, Davies S, Ryan T, Keady J. Relationship-centred care and the ‘Senses’ framework. J Dementia Care 2008;16:26-8.
- Tuokko H, MacCourt P, Heath Y. Home alone with dementia. Aging Ment Health 1999;3:21-7.
- Luengo-Fernandez R, Leal J, Gray A. The economic burden of dementia and associated research funding in the United Kingdom. Cambridge: Alzheimer’s Research Trust; 2010.
- Wimo A, Prince M. Alzheimer’s Disease International World Alzheimer Report 2010: The Global Economic Impact of Dementia 2010.
- National Institue for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Homer C. Using the Zelen design in randomized controlled trials: debates and controversies. J Adv Nurs 2002;38:200-7.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975;12:189-98.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19.
- Agar S, Moniz-Cook E, Orbell S, Elston C, Wang M. Measuring the outcome of psychosocial intervention for family caregivers of dementia sufferers: a factor analytic study. Aging Ment Health 1997;1:166-75.
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med 1979;9:139-45.
- Yesavage JA, Brink TL, Rose TL. Development and validation of a geriatric depression scale: a preliminary report. J Psychiatry Res 1983;17:37-49.
- Spruytte N, Van Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychol Psychother 2002;75:295-311.
- Gallagher-Thompson D, Dal-Canto PG, Darnley S, Basilio LA, Whelan L, Jacob T. A feasibility study of videotaping to assess the relationship between distress in Alzheimer’s disease caregivers and their interaction style. Aging Ment Health 1997;1:346-55.
- The National Archives . Mental Capacity Act 2005. www.legislation.gov.uk/ukpga/2005/9/contents (accessed 13 September 2011).
- Woods RT, Wills W, Higginson IJ, Hobbins J, Whitby M. Support in the community for people with dementia and their carers: a comparative outcome study of specialist mental health service interventions. Int J Geriatr Psych 2003;18:298-307.
- Dobson C. Conducting research with people not having the capacity to consent to their participation. Leicester: British Psychological Society; 2008.
- Russell D, Hoare ZSJ, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34.
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods RT, et al. Whose quality of life is it anyway?: the validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis. Assoc 2003;17:201-8.
- Orrell M, Hoe J, Hancock G, Livingston G. Quality of life of people with dementia in residential care homes. Br J Psychiatry 2006;188:460-4.
- Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry 1998;13:248-56.
- Marriott A, Donaldson C, Tarrier N, Burns A. Effectiveness of cognitive—behavioural family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psychiatry 2000;176:557-62.
- Kopelman MD, Wilson BA, Baddeley A. The autobiographical memory interview. Bury St Edmunds: Thames Valley Test Company; 1990.
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry 1988;23:271-84.
- Shankar KK, Walker M, Frost D, Orrell MW. The development of a valid and reliable scale for rating anxiety in dementia (RAID). Aging Ment Health 1999;3:39-4.
- Flint AJ, Rifat SL. Validation of the hospital anxiety and depression scale as a measure of severity of geriatric depression. Int J Geriatr Psychiatry 1996;11:991-4.
- EuroQol EQ-5D n.d. www.euroqol.org (accessed 13 September 2011).
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living scale. Age Ageing 1996;25:113-20.
- Beecham J, Knapp M. Costing psychiatric interventions. London: Gaskell; 1992.
- Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods RT, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry 2006;188:574-80.
- Matthews J. An introduction to clinical trials. London: Arnold/Hodder Headline; 2000.
- Kinsella S. Ten Lesson for Micro-Costing in Health Economics 2004. www.stephenkinsella.net/2008/07/04/985 (accessed 24 August 2010).
- Morris S, Devlin N, Parkin D. Economic analysis in health care. Chichester: Wiley and Sons; 2007.
- Department of Health . NHS Reference Costs 2009–10 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123459 (accessed 9 September 2012).
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit (PSRRU), University of Kent; 2010.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2011.
- Mistry H, Buxton M, Longworth L, Chatwin J, Peveler R. Comparison of general practitioner records and patient self-report questionnaires for estimation of costs. Eur J Health Econ 2005;6:261-6.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Centre for Health Economics Discussion Paper 172. York: University of York; 1999.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72.
- Royal College of Nursing . NHS Agenda for Change Pay Scales – 2010 2011 n.d. www.rcn.org.uk/__data/assets/pdf_file/0018/233901/003303.pdf (accessed 9 September 2012).
- Bangor University . Bangor University Pay Scales 2010 n.d. www.bangor.ac.uk/finance/py/documents/pcalc11b_000.pdf (accessed 9 September 2012).
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74.
- Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991.
- Charlesworth GM, Shepstone L, Wilson E, Reynolds S, Mugford M, Price D, et al. Befriending carers of people with dementia: randomised controlled trial. BMJ 2008;336.
- Charlesworth G, Burnell K, Beecham J, Hoare Z, Hoe J, Wenborn J, et al. Peer support for family carers of people with dementia, alone or in combination with group reminiscence in a factorial design: study protocol for a randomised controlled trial. Trials 2011;12.
- Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F. Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial. Health Technol Assess 2008;12.
Appendix 1 Unit costs and sources
| Item | Unit | Cost (£) | Source/notes |
|---|---|---|---|
| NHS district nurse, home visit | Per visit | 27 | District nurse home visit, including qualification costs (PSSRU, 2010 , p. 159)55 |
| NHS district nurse, clinic visit | Per visit | 73 | District nurse per hour spent with patient, including qualification costs (PSSRU, 2010, p. 159)55 |
| GP surgery visit | Per visit | 3.10 | GP per surgery/clinic minute including qualification costs and direct care staff costs (PSSRU, 2010, p. 167)55 |
| GP home visit | Per visit | 120 | Per home visit lasting 23.4 minutes, including travel time, qualification costs and direct care staff costs (PSSRU, 2010, p. 167)55 |
| Nurse surgery visit | Per visit | 12 | Per consultation, including qualification costs (PSSRU, 2010, p. 164)55 |
| Nurse home visit | Per visit | 20 | Per home visit, including qualification costs (PSSRU, 2010, p. 164)55 |
| NHS health visitor, home visit | Per visit | 42 | Health visitor home visit, including qualification costs (PSSRU, 2010, p. 161)55 |
| NHS health visitor, clinic visit | Per visit | 84 | Health visitor per hour of clinic contact, including qualification costs (PSSRU, 2010, p. 161)55 |
| NHS psychiatrist, home visit | Per hour | 328 | Assumed same as clinic visit. Consultant: psychiatric cost per hour patient contact, including qualification costs (PSSRU, 2010, p. 220)55 |
| NHS psychiatrist, clinic visit | Per hour | 328 | Consultant: psychiatric cost per hour patient contact, including qualification costs (PSSRU, 2010, p. 220)55 |
| NHS psychologist, home visit | Per hour | 81 | Hourly rate assumed same as for clinic visit. Per hour of client contact, clinical psychologist. £1.50 per visit for travel (PSSRU, 2010, p. 155)55 |
| NHS psychologist, clinic visit | Per hour | 81 | Per hour of client contact, clinical psychologist (PSSRU, 2010, p. 155)55 |
| NHS counsellor, home visit | Per hour | 44 | Assumed same as clinic visit. Per hour of client contact for counselling service in primary care (PSSRU, 2010, p. 78)55 |
| NHS counsellor, clinic visit | Per hour | 44 | Per hour of client contact for counselling service in primary care (PSSRU, 2010, p. 78)55 |
| NHS CPN, home visit | Per hour | 56 | Hourly rate assumed same as for clinic visit. Per hour of face-to-face contact, including qualification costs. £1.50 per visit for travel (PSSRU, 2010, p. 160)55 |
| NHS CPN, clinic visit | Per hour | 56 | Per hour of face-to-face contact, including qualification costs (PSSRU, 2010, p. 160)55 |
| NHS physiotherapist, home visit | Per visit | 47 | Per physiotherapist home visit, including qualification costs (PSSRU, 2010, p. 151)55 |
| NHS physiotherapist, clinic visit | Per visit | 17 | Per physiotherapist clinic visit, including qualification costs (PSSRU, 2010, p. 151)55 |
| NHS occupational therapist, home visit | Per visit | 46 | Per home visit, NHS community occupational therapist, including qualification costs (PSSRU, 2010, p. 152)55 |
| NHS occupational therapist, clinic visit | Per visit | 17 | Per clinic visit, NHS community occupational therapist, including qualification costs (PSSRU, 2010, p. 152)55 |
| Care manager, home visit | Per visit | 36 | Based on registered manager for the intensive management of older people, cost per home visit (PSSRU, 2010, p. 178)55 |
| Care manager, clinic visit | Per hour | 112 | Based on registered manager for the intensive management of older people, cost per hour of face-to-face contact (PSSRU, 2010, p. 178)55 |
| Social worker, home visit | Per hour | 213 | Assumed to be same as clinic visit. Per hour of face-to-face contact, including qualification costs (PSSRU, 2010, p. 172)55 |
| Social worker, clinic visit | Per hour | 213 | Per hour of face-to-face contact, including qualification costs (PSSRU, 2010, p. 172)55 |
| Local authority home-care worker | Per hour | 25 | Per hour of weekday face-to-face contact, local authority home-care worker (PSSRU, 2010, p. 176)55 |
| Care attendant | Per hour | 25 | Assumed same as for local authority home-care worker |
| Sitting scheme | Per session | 12.99 | Based on five agencies in Torbay 2005 (Charlesworth, et al. 2008 , p. 52),65 adjusted to 2010 prices |
| Carer’s support worker, home visit | Per hour | 49 | Assumed same as clinic visit, per hour of client related work for family support worker, including training costs (PSSRU, 2010, p. 179)55 |
| Carer’s support worker, clinic visit | Per hour | 49 | Per hour of client related work for family support worker, including training costs (PSSRU, 2010, p. 179)55 |
| NHS chiropodist home visit | Per visit | 20 | Per home visit, community chiropodist (PSSRU, 2010, p. 154)55 |
| NHS chiropodist clinic visit | Per visit | 11 | Per clinic visit, community chiropodist (PSSRU, 2010, p. 154)55 |
| NHS dietician, home visit | Per hour | 57 | Per hour of home visiting, hospital-based dietician including qualification costs £2.80 per visit for travel (PSSRU, 2010, p. 198)55 |
| NHS dietician, clinic visit | Per hour | 32 | Per hour in clinic, hospital-based dietician including qualification costs (PSSRU, 2010, p. 198)55 |
| Local authority day care | Per day | 43 | Per day, local authority social services day care for people with mental health problems (PSSRU, 2010, p. 74)55 |
| Voluntary-sector day care | Per day | 42 | Per day, voluntary/not-for-profit organisation providing day care for people with mental health problems (PSSRU, 2010, p. 75)55 |
| NHS day care | Per day | 66 | Per day, NHS trust day care for people with mental health problems (PSSRU, 2010, p. 73)55 |
Appendix 2 Cost of health-care and social-care service use
| Service | Intervention (n = 196) | Control (n = 140) | Difference in mean (£) | ||
|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | ||
| District nurse | 59.57 | 220.863 | 30.03 | 98.398 | 29.24 |
| GP | 211.88 | 292.578 | 259.03 | 910.627 | –47.15 |
| Practice nurse (GP clinic) | 11.43 | 16.958 | 14.77 | 36.236 | –3.34 |
| Health visitor | 0.43 | 6.000 | 0.60 | 5.002 | –0.17 |
| Community psychiatrist | 92.32 | 213.669 | 123.59 | 372.755 | –31.27 |
| Psychologist | 36.78 | 150.584 | 10.47 | 44.795 | 26.31 |
| Counsellor | 1.01 | 10.037 | 0.31 | 3.719 | 0.70 |
| CPN/CMHT | 57.19 | 202.857 | 100.24 | 356.289 | –43.05 |
| Physiotherapist | 5.14 | 39.397 | 9.42 | 56.255 | –4.28 |
| Occupational health therapist | 20.58 | 76.994 | 19.01 | 62.671 | 1.57 |
| Care manager | 3.73 | 17.861 | 1.29 | 9.069 | 2.44 |
| Social worker | 152.96 | 597.054 | 99.98 | 258.967 | 52.98 |
| Home-care worker | 127.53 | 507.398 | 233.10 | 1054.595 | –105.57 |
| Care attendant | 199.51 | 1126.772 | 165.13 | 770.121 | 34.38 |
| Sitting scheme | 25.25 | 114.356 | 14.75 | 59.215 | 10.50 |
| Family support worker | 57.50 | 313.970 | 78.87 | 550.407 | –21.37 |
| Chiropodist | 8.81 | 19.713 | 8.11 | 17.931 | 0.70 |
| Dietician | 0.32 | 3.189 | 1.63 | 13.842 | –1.31 |
| Total | 1071.94 | 1809.059 | 1170.33 | 1982.962 | –98.42 |
| Service provider | Intervention (n = 196) | Control (n = 140) | Difference in mean (£) | ||
|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | ||
| Local authority | 547.15 | 1293.234 | 299.46 | 955.450 | 247.69 |
| Voluntary organisation | 278.62 | 2603.460 | 155.11 | 540.912 | 123.51 |
| NHS (not hospital) | 273.09 | 3353.707 | 155.57 | 607.746 | 117.52 |
| Total mean cost | 1098.86 | 4451.440 | 610.14 | 1414.61 | 488.72 |
| Inpatient services | Intervention (n = 196) | Control (n = 140) | Difference in mean (£) | ||
|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | ||
| Assessment/rehabilitation | 555 | 3618 | 76 | 556 | 479 |
| Continuing care/respite | 301 | 1882 | 345 | 2429 | –44 |
| Medical ward | 579 | 2826 | 859 | 4694 | –280 |
| Other inpatient ward | 513 | 2827 | 548 | 3928 | –35 |
| Other services | |||||
| Outpatient services | 431 | 1609 | 620 | 2927 | –189 |
| Accident and emergency | 21 | 56 | 49 | 245 | –28 |
| Day hospital | 319 | 2508 | 32 | 286 | 287 |
| Total mean cost | 2719 | 7106 | 2529 | 8087 | 190 |
| Service | Intervention (n = 196) | Control (n = 140) | Difference in mean (£) | ||
|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | ||
| District nurse | 8.07 | 48.881 | 3.33 | 11.764 | 4.74 |
| GP | 153.01 | 219.865 | 155.30 | 218.392 | –2.29 |
| Practice nurse (GP clinic) | 20.51 | 135.639 | 10.27 | 33.872 | 10.24 |
| Health visitor | 4.07 | 34.794 | 2.70 | 28.593 | 1.37 |
| Community psychiatrist | 0.00 | 0.000 | 5.85 | 69.303 | –5.85 |
| Psychologist | 10.50 | 72.076 | 16.81 | 126.615 | –6.31 |
| Counsellor | 8.64 | 45.135 | 12.15 | 120.918 | –3.51 |
| CPN/CMHT | 3.25 | 17.105 | 4.82 | 30.123 | –1.57 |
| Physiotherapist | 5.20 | 19.616 | 11.02 | 53.929 | –5.82 |
| Occupational health therapist | 3.45 | 27.382 | 0.78 | 5.649 | 2.67 |
| Care manager | 1.31 | 11.36 | 0.00 | 0.000 | 1.31 |
| Social worker | 14.13 | 88.830 | 12.58 | 65.679 | 1.55 |
| Home-care worker | 7.11 | 68.437 | 35.39 | 317.641 | –28.28 |
| Care attendant | 7.60 | 105.002 | 0.00 | 0.000 | 7.60 |
| Sitting scheme | 0.13 | 1.856 | 0.37 | 4.391 | –0.24 |
| Family support worker | 7.90 | 42.181 | 8.95 | 52.939 | –1.05 |
| Chiropodist | 2.33 | 7.709 | 1.89 | 6.590 | 0.44 |
| Dietician | 0.98 | 7.826 | 0.68 | 5.924 | 0.30 |
| Total | 258.19 | 339.341 | 282.89 | 448.779 | –24.70 |
| Service provider | Intervention (n = 196) | Control (n = 140) | Difference in mean (£) | ||
|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | ||
| Local authority | 5.27 | 73.714 | 0.00 | 0.000 | 5.27 |
| Voluntary organisation | 1.54 | 21.500 | 33.17 | 307.334 | –31.63 |
| NHS (not hospital) | 0.00 | 0.000 | 0.94 | 11.156 | –0.94 |
| Total mean cost | 6.81 | 76.680 | 34.11 | 307.434 | –27.30 |
| Inpatient services | Intervention (n = 196) | Control (n = 140) | Difference in mean (£) | ||
|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | ||
| Assessment/rehabilitation | 135 | 815 | 36 | 352 | 99 |
| Continuing care/respite | 33 | 443 | 58 | 374 | –25 |
| Medical ward | 501 | 2554 | 461 | 3103 | 40 |
| Other inpatient ward | 58 | 551 | 89 | 600 | –31 |
| Other services | |||||
| Outpatient services | 458 | 2,046 | 276 | 933 | 182 |
| Accident and Emergency | 9 | 32 | 19 | 122 | –10 |
| Day hospital | 72 | 478 | 104 | 658 | –32 |
| Total mean cost | 1266 | 3752 | 1043 | 3622 | 223 |
Appendix 3 Cost-effectiveness analysis
| Group | Mean total cost (£) (SD) | Mean GHQ-28 score (SD) | Difference in cost (£) | Difference in effect (GHQ-28 score) |
|---|---|---|---|---|
| Intervention (n = 196) | 2495 (3866) | 23.162 (10.807) | 1136 | 0.922 |
| Control (n = 140) | 1359 (3743) | 22.240 (10.043) |
FIGURE 2.
Cost-effectiveness plane for participants with dementia (quality of life, derived from self-completed EQ-5D).
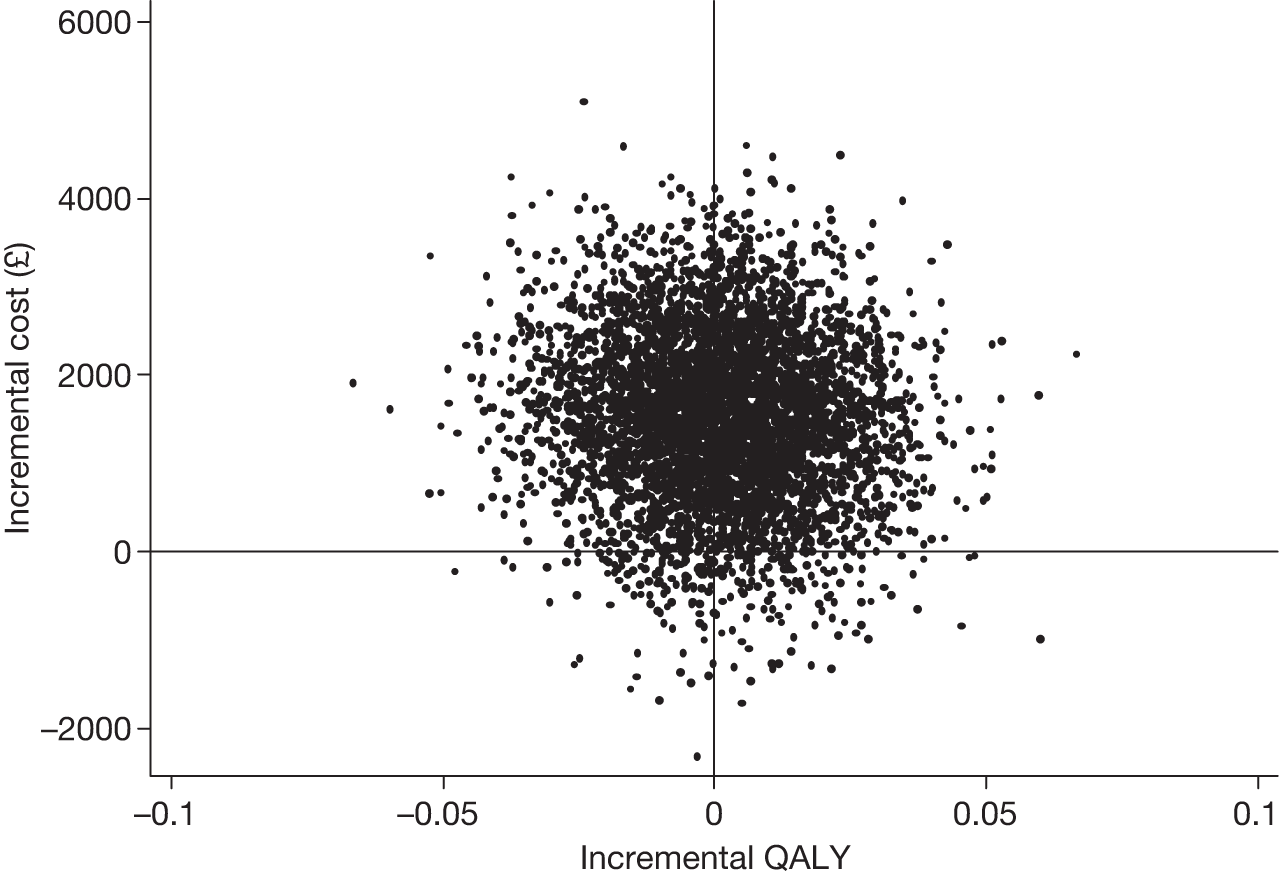
FIGURE 3.
Cost-effectiveness plane for carers’ quality of life, derived from self-completed EQ-5D.
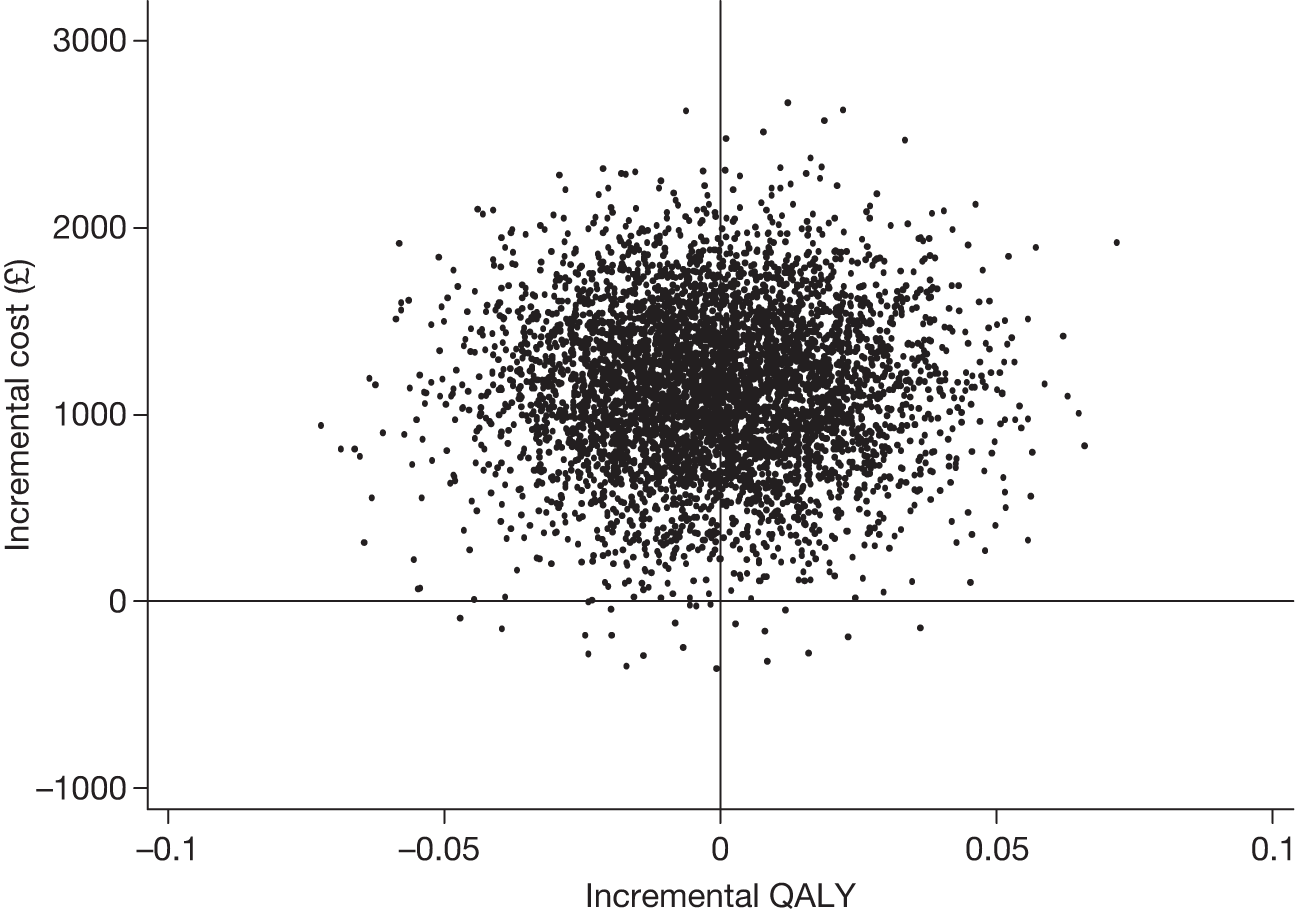
Appendix 4 European Quality of Life-5 Dimensions scores of trial participants compared with UK norms
| PwD age group (years) | Gender of participant | RYCT group | Control group | All participants | Population norms |
|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD) | ||
| 45–54 | Female | 0.80 (n/a ), 1 | 0.80 (n/a), 1 | 0.85 (0.23) | |
| Male | 0.81 (n/a), 1 | 0.81 (n/a), 1 | 0.84 (0.27) | ||
| Total | 0.80 (0.01), 2 | 0.80 (0.01), 2 | 0.85 (0.25) | ||
| 55–64 | Female | 0.80 (0.15), 5 | 0.82 (0.16), 3 | 0.81 (0.14), 8 | 0.81 (0.26) |
| Male | 0.66 (0.17), 6 | 0.76 (0.05), 2 | 0.69 (0.14), 8 | 0.78 (0.28) | |
| Total | 0.72 (0.17), 11 | 0.80 (0.12), 5 | 0.75 (0.15), 16 | 0.80 (0.26) | |
| 65–74 | Female | 0.70 (0.30), 30 | 0.83 (0.15), 22 | 0.75 (0.26), 52 | 0.78 (0.25) |
| Male | 0.71 (0.29), 44 | 0.73 (0.36), 35 | 0.72 (0.32), 79 | 0.78 (0.28) | |
| Total | 0.71 (0.29), 74 | 0.77 (0.30), 57 | 0.73 (0.30), 131 | 0.78 (0.26) | |
| 75 + | Female | 0.76 (0.24), 89 | 0.75 (0.25), 81 | 0.75 (0.24), 170 | 0.71 (0.27) |
| Male | 0.77 (0.23), 87 | 0.75 (0.24), 64 | 0.76 (0.23), 151 | 0.75 (0.28) | |
| Total | 0.76 (0.23), 176 | 0.75 (0.24), 145 | 0.76 (0.24), 321 | 0.73 (0.27) |
| PwD age group (years) | Gender of participant | RYCT group | Control group | All participants | Population norms |
|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD) | ||
| 45–54 | Female | 55.00 (n/a ), 1 | 55.00 (n/a), 1 | 82.42 (17.23) | |
| Male | 80.00 (n/a), 1 | 80.00 (n/a), 1 | 81.56 (19.23) | ||
| Total | 67.50 (17.68), 2 | 67.50 (17.68), 2 | 82.03 (18.15) | ||
| 55–64 | Female | 50.60 (34.88), 5 | 87.50 (17.68), 2 | 61.14 (34.46), 7 | 80.26 (17.67) |
| Male | 65.50 (30.67), 6 | 50.00 (43.59), 3 | 60.33 (33.51), 9 | 78.99 (19.04) | |
| Total | 58.73 (31.90), 11 | 65.00 (38.08), 5 | 60.69 (32.77), 16 | 79.74 (18.23) | |
| 65–74 | Female | 75.74 (22.40), 31 | 69.80 (27.69), 25 | 72.55 (24.73), 55 | 76.55 (18.61) |
| Male | 69.14 (20.98), 42 | 75.11 (17.92), 35 | 71.86 (19.75), 77 | 78.19 (27.40) | |
| Total | 71.95 (21.69), 73 | 72.90 (22.45), 60 | 72.14 (21.87), 132 | 77.32 (18.05) | |
| 75 + | Female | 71.86 (19.36), 91 | 69.73 (16.78), 85 | 70.65 (18.03), 175 | 74.07 (18.47) |
| Male | 73.73 (18.23), 89 | 71.03 (19.84), 65 | 72.59 (18.91), 154 | 72.90 (18.99) | |
| Total | 72.78 (18.78), 180 | 70.29 (18.12), 150 | 71.56 (18.44), 329 | 73.66 (18.63) |
| Carer age group (years) | Gender of carer | RYCT group | Control group | All carers | Population norms |
|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD) | ||
| < 25 | Female | 0.71 (n/a ), 1 | 0.71 (n/a), 1 | 0.94 (0.12) | |
| Total | 0.71 (n/a), 1 | 0.71 (n/a), 1 | 0.94 (0.12) | ||
| 25–34 | Male | 0.80 (n/a), 1 | 0.80 (n/a), 1 | 0.93 (0.16) | |
| Total | 0.80 (n/a), 1 | 0.80 (n/a), 1 | 0.93 (0.15) | ||
| 35–44 | Female | 0.88 (0.16), 5 | 0.69 (0.52), 4 | 0.80 (0.35), 9 | 0.91 (0.15) |
| Male | 0.87 (0.09), 4 | 0.85 (0.05), 2 | 0.87 (0.07), 6 | 0.91 (0.17) | |
| Total | 0.88 (0.13), 9 | 0.74 (0.41), 6 | 0.83 (0.27), 15 | 0.91 (0.16) | |
| 45–54 | Female | 0.85 (0.19), 15 | 0.90 (0.11), 10 | 0.87 (0.16), 25 | 0.85 (0.23) |
| Male | 0.90 (0.09), 3 | 0.94 (0.09), 6 | 0.93 (0.09), 9 | 0.84 (0.27) | |
| Total | 0.85 (0.18), 18 | 0.92 (0.10), 16 | 0.88 (0.15), 34 | 0.85 (0.25) | |
| 55–64 | Female | 0.81 (0.19), 38 | 0.79 (0.21), 30 | 0.80 (0.20), 68 | 0.81 (0.26) |
| Male | 0.79 (0.31), 9 | 0.86 (0.11), 6 | 0.82 (0.25), 15 | 0.78 (0.28) | |
| Total | 0.81 (0.21), 47 | 0.80 (0.20), 36 | 0.81 (0.21), 83 | 0.80 (0.26) | |
| 65–74 | Female | 0.74 (0.25), 70 | 0.73 (0.28), 57 | 0.73 (0.26), 127 | 0.78 (0.25) |
| Male | 0.82 (0.30), 20 | 0.80 (0.21), 20 | 0.81 (0.26), 40 | 0.78 (0.28) | |
| Total | 0.76 (0.26), 90 | 0.75 (0.26), 77 | 0.75 (0.26), 167 | 0.78 (0.26) | |
| 75 + | Female | 0.74 (0.22), 58 | 0.68 (0.25), 31 | 0.72 (0.23), 89 | 0.71 (0.27) |
| Male | 0.77 (0.23), 43 | 0.80 (0.20), 43 | 0.78 (0.21), 86 | 0.75 (0.28) | |
| Total | 0.76 (0.22), 101 | 0.75 (0.23), 74 | 0.75 (0.22), 175 | 0.73 (0.27) |
| Carer age group (years) | Gender of carer | RYCT group | Control group | All carers | Population norms |
|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD) | ||
| < 25 | Female | 100.00 (n/a), 1 | 100.00 (n/a), 1 | 86.00 (13.43) | |
| Total | 100.00 (n/a), 1 | 100.00 (n/a), 1 | 86.49 (13.60) | ||
| 25–34 | Male | 76.00 (n/a), 1 | 76.00 (n/a), 1 | 86.87 (14.41) | |
| Total | 76.00 (n/a), 1 | 76.00 (n/a), 1 | 86.84 (14.41) | ||
| 35–44 | Female | 76.80 (22.66), 5 | 67.50 (33.04), 4 | 72.67 (26.27), 9 | 86.35 (14.88) |
| Male | 87.50 (9.57), 4 | 82.50 (17.68), 2 | 85.83 (11.14), 6 | 86.81 (12.39) | |
| Total | 81.56 (17.97), 9 | 72.50 (27.88), 6 | 77.93 (21.99), 15 | 86.56 (13.79) | |
| 45–54 | Female | 73.67 (13.16), 15 | 75.50 (17.73), 10 | 74.40 (14.82), 25 | 82.42 (17.23) |
| Male | 85.00 (5.00), 3 | 86.33 (11.45), 6 | 85.89 (9.41), 9 | 81.56 (19.23) | |
| Total | 75.56 (12.82), 18 | 79.56 (16.18), 16 | 77.44 (14.41), 34 | 82.03 (18.15) | |
| 65–74 | Female | 74.56 (18.16), 71 | 69.76 (20.09), 58 | 72.40 (19.13), 129 | 76.55 (18.61) |
| Male | 77.15 (16.61), 20 | 76.09 (21.92), 22 | 76.60 (19.35), 42 | 78.19 (27.40) | |
| Total | 75.13 (17.77), 91 | 71.50 (20.66), 80 | 73.43 (19.21), 171 | 77.32 (18.05) | |
| 75 + | Female | 70.08 (18.62), 59 | 71.12 (16.28), 34 | 70.46 (17.71), 93 | 74.07 (18.47) |
| Male | 74.65 (17.05), 43 | 73.43 (20.40), 44 | 74.03 (18.72), 87 | 72.90 (18.99) | |
| Total | 72.01 (18.03), 102 | 72.42 (18.64), 78 | 72.19 (18.24), 180 | 73.66 (18.63) |
| PwD age group (years) | Gender of carer | RYCT group | Control group | All | Population norms |
|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD) | ||
| 45–54 | Female | 0.81 (n/a ), 1 | 0.81 (n/a), 1 | 0.85 (0.23) | |
| Male | 0.62 (n/a), 1 | 0.62 (n/a), 1 | 0.84 (0.27) | ||
| Total | 0.72 (0.14), 2 | 0.72 (0.14), 2 | 0.85 (0.25) | ||
| 55–64 | Female | 0.53 (0.29), 7 | 0.63 (0.31), 4 | 0.56 (0.29), 11 | 0.81 (0.26) |
| Male | 0.62 (0.41), 5 | 0.71 (0.00), 2 | 0.65 (0.34), 7 | 0.78 (0.28) | |
| Total | 0.57 (0.33), 12 | 0.65 (0.24), 6 | 0.60 (0.30), 18 | 0.80 (0.26) | |
| 65–74 | Female | 0.58 (0.29), 48 | 0.67 (0.26), 35 | 0.62 (0.28), 83 | 0.78 (0.25) |
| Male | 0.59 (0.29), 27 | 0.60 (0.29), 22 | 0.59 (0.29), 49 | 0.78 (0.28) | |
| Total | 0.58 (0.29), 75 | 0.64 (0.27), 57 | 0.61 (0.28), 132 | 0.78 (0.26) | |
| 75 + | Female | 0.55 (0.29), 131 | 0.57 (0.28), 95 | 0.56 (0.29), 226 | 0.71 (0.27) |
| Male | 0.62 (0.26), 48 | 0.61 (0.26), 54 | 0.61 (0.26), 102 | 0.75 (0.28) | |
| Total | 0.57 (0.28), 179 | 0.58 (0.28), 149 | 0.58 (0.28), 328 | 0.73 (0.27) |
| PwD age group (years) | Gender of carer | RYCT group | Control group | All | Population norms |
|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD) | ||
| 45–54 | Female | 82.00 (n/a ), 1 | 82.00 (n/a), 1 | 82.42 (17.23) | |
| Male | 25.00 (n/a), 1 | 25.00 (n/a), 1 | 81.56 (19.23) | ||
| Total | 53.50 (n/a), 2 | 53.50 (40.31), 2 | 82.03 (18.15) | ||
| 55–64 | Female | 62.14 (19.12), 7 | 72.50 (18.48), 4 | 65.91 (18.68), 11 | 80.26 (17.67) |
| Male | 62.60 (22.70), 5 | 85.00 (7.07), 2 | 69.00 (21.71), 7 | 78.99 (19.04) | |
| Total | 62.33 (19.67), 12 | 76.67 (16.02), 6 | 67.11 (19.34), 18 | 79.74 (18.23) | |
| 65–74 | Female | 65.43 (19.13), 47 | 61.86 (18.94), 37 | 63.86 (19.01), 84 | 76.55 (18.61) |
| Male | 65.33 (15.07), 27 | 62.77 (18.70), 22 | 64.18 (16.66), 49 | 78.19 (27.40) | |
| Total | 65.39 (17.64), 74 | 62.20 (18.70), 59 | 63.98 (18.12), 133 | 77.32 (18.05) | |
| 75 + | Female | 61.31 (18.64), 133 | 55.29 (20.68), 94 | 58.81 (19.69), 227 | 74.07 (18.47) |
| Male | 62.15 (16.69), 48 | 61.81 (18.37), 52 | 61.97 (17.49), 100 | 72.90 (18.99) | |
| Total | 61.53 (18.10), 181 | 57.61 (20.07), 146 | 59.78 (19.08), 327 | 73.66 (18.63) |
Appendix 5 Baseline correlational matrices
This appendix contains two correlational matrices, the first depicting the relationships between the participant with dementia measures and the second the relationships between the carer and proxy outcome measures. These have been calculated on the baseline unimputed values for each of the measures.
| Outcome measure | QoL AD | AMI(E) (factual) | AMI(E) (memory) | QCPR PwD | EQ-5D QALY | EQ-5D VAS |
|---|---|---|---|---|---|---|
| QoL-AD | ||||||
| Pearson correlation | 1 | 0.08 | 0.01 | 0.24 | 0.39 | 0.38 |
| Significance (two-tailed) | 0.1 | 0.83 | < 0.001 | < 0.001 | < 0.001 | |
| n | 468 | 468 | 468 | 441 | 456 | 461 |
| AMI(E) (factual) | ||||||
| Pearson correlation | 0.08 | 1 | 0.6 | 0.05 | 0.03 | 0.09 |
| Significance (two-tailed) | 0.1 | < 0.001 | 0.27 | 0.57 | 0.05 | |
| n | 468 | 485 | 485 | 451 | 471 | 477 |
| AMI(E) (memory) | ||||||
| Pearson correlation | 0.01 | 0.6 | 1 | 0.03 | –0.15 | 0.02 |
| Significance (two-tailed) | 0.83 | < 0.001 | 0.48 | < 0.001 | 0.74 | |
| n | 468 | 485 | 487 | 451 | 471 | 479 |
| QCPR PwD | ||||||
| Pearson correlation | 0.24 | 0.05 | 0.03 | 1 | 0.14 | 0.2 |
| Significance (two-tailed) | < 0.001 | 0.27 | 0.48 | < 0.001 | < 0.001 | |
| n | 441 | 451 | 451 | 451 | 445 | 447 |
| EQ-5D QALY | ||||||
| Pearson correlation | 0.39 | 0.03 | –0.15 | 0.14 | 1 | 0.41 |
| Significance (two-tailed) | < 0.001 | 0.57 | < 0.001 | < 0.001 | < 0.001 | |
| n | 456 | 471 | 471 | 445 | 471 | 464 |
| EQ-5D VAS | ||||||
| Pearson correlation | 0.38 | 0.09 | 0.02 | 0.2 | 0.41 | 1 |
| Significance (two-tailed) | < 0.001 | 0.05 | 0.74 | < 0.001 | < 0.001 | |
| n | 461 | 477 | 479 | 447 | 464 | 480 |
| Outcome measure | GHQ-28 | HADS depression | HADS anxiety | RSS | QCPR carer | Carer EQ-5D QALY | Carer EQ-5D VAS | Proxy QoL-AD | BADLS | Proxy EQ-5D QALY | Proxy EQ-5D VAS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GHQ-28 | |||||||||||
| Pearson correlation | 1 | 0.66 | 0.74 | 0.61 | –0.39 | –0.46 | –0.53 | –0.33 | 0.17 | –0.2 | –0.22 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 460 | 457 | 457 | 448 | 450 | 453 | 460 | 456 | 441 | 456 | 458 |
| HADS depression | |||||||||||
| Pearson correlation | 0.66 | 1 | 0.62 | 0.64 | –0.43 | –0.45 | –0.48 | –0.4 | 0.28 | –0.31 | –0.27 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 457 | 484 | 484 | 469 | 468 | 477 | 484 | 478 | 463 | 480 | 480 |
| HADS anxiety | |||||||||||
| Pearson correlation | 0.74 | 0.62 | 1 | 0.63 | –0.45 | –0.35 | –0.32 | –0.38 | 0.23 | –0.25 | –0.25 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 457 | 484 | 484 | 469 | 468 | 477 | 484 | 478 | 463 | 480 | 480 |
| RSS | |||||||||||
| Pearson correlation | 0.61 | 0.64 | 0.63 | 1 | –0.6 | –0.31 | –0.37 | –0.61 | 0.45 | –0.41 | –0.31 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 448 | 469 | 469 | 472 | 459 | 466 | 472 | 466 | 451 | 469 | 468 |
| QCPR carer | |||||||||||
| Pearson correlation | –0.39 | –0.43 | –0.45 | –0.6 | 1 | 0.15 | 0.25 | 0.52 | –0.29 | 0.21 | 0.16 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 450 | 468 | 468 | 459 | 471 | 463 | 471 | 467 | 451 | 467 | 468 |
| Carer EQ-5D QALY | |||||||||||
| Pearson correlation | –0.46 | –0.45 | –0.35 | –0.31 | 0.15 | 1 | 0.53 | 0.14 | –0.12 | 0.13 | 0.08 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.01 | < 0.001 | 0.08 | |
| n | 453 | 477 | 477 | 466 | 463 | 479 | 479 | 473 | 460 | 475 | 476 |
| Carer EQ-5D VAS | |||||||||||
| Pearson correlation | –0.53 | –0.48 | –0.32 | –0.37 | 0.25 | 0.53 | 1 | 0.3 | –0.2 | 0.16 | 0.36 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 460 | 484 | 484 | 472 | 471 | 479 | 488 | 481 | 465 | 483 | 483 |
| Proxy QoL-AD | |||||||||||
| Pearson correlation | –0.33 | –0.4 | –0.38 | –0.61 | 0.52 | 0.14 | 0.3 | 1 | –0.38 | 0.48 | 0.49 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 456 | 478 | 478 | 466 | 467 | 473 | 481 | 481 | 461 | 477 | 478 |
| BADLS | |||||||||||
| Pearson correlation | 0.17 | 0.28 | 0.23 | 0.45 | –0.29 | –0.12 | –0.2 | –0.38 | 1 | –0.49 | –0.33 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.01 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 441 | 463 | 463 | 451 | 451 | 460 | 465 | 461 | 465 | 461 | 462 |
| Proxy EQ-5D QALY | |||||||||||
| Pearson correlation | –0.2 | –0.31 | –0.25 | –0.41 | 0.21 | 0.13 | 0.16 | 0.48 | –0.49 | 1 | 0.42 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 456 | 480 | 480 | 469 | 467 | 475 | 483 | 477 | 461 | 483 | 479 |
| Proxy EQ-5D VAS | |||||||||||
| Pearson correlation | –0.22 | –0.27 | –0.25 | –0.31 | 0.16 | 0.08 | 0.36 | 0.49 | –0.33 | 0.42 | 1 |
| Significance (two-tailed) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.08 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| n | 458 | 480 | 480 | 468 | 468 | 476 | 483 | 478 | 462 | 479 | 483 |
Appendix 6 Linear-mixed model results for the models fitted to all outcomes
This appendix contains the results of the models for the linear-mixed models applied to the entire data set allowing consideration of a change over time to be taken into account.
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| QoL-AD | Complete case | 1, 655.6 | 0.17 | 0.68 | Gender | 0.210 | 0.438 | –0.649 | 1.070 |
| Multiple imputations | (1, 919.2) (1, 919.0) | 0.05–0.99 | 0.32–0.82 | Gender (5), time point (4) | 0.194 | ||||
| AMI(E) memory | Complete case | 1, 692.3 | 1.06 | 0.30 | Gender | 0.580 | 0.561 | –0.520 | 1.681 |
| Multiple imputations | (1, 916.3) (1, 912.8) | 0.004–0.12 | 0.73–0.95 | Spousal (1), gender (5) | –0.042 | ||||
| AMI(E) factual | Complete case | 1, 698.1 | 1.92 | 0.17 | Gender | 2.389 | 1.766 | –1.077 | 5.855 |
| Multiple imputations | (1, 917.9) (1, 916.9) | 0.06–0.34 | 0.56–0.85 | Gender (5), time point (5) | –0.155 | ||||
| QCPR | Complete case | 1, 637.5 | 1.12 | 0.29 | None | 0.554 | 0.524 | –0.474 | 1.583 |
| Multiple imputations | (1, 924.0) (1, 925.0) | 0.34–1.95 | 0.16–0.56 | Spousal (1) | 0.497 | ||||
| QCPR warmth | Complete case | 1, 659.9 | 0.86 | 0.35 | Spousal | 0.304 | 0.306 | –0.296 | 0.904 |
| Multiple imputations | (1, 924.7) (1, 923.4) | 0.67–1.28 | 0.26–0.41 | Spousal (3), time point (2) | 0.247 | ||||
| QCPR negative | Complete case | 1, 654.0 | 1.38 | 0.24 | None | 0.317 | 0.288 | –0.248 | 0.883 |
| Multiple imputations | (1, 922.9) (1, 925.5) | 0.00–1.26 | 0.26–0.98 | None | 0.134 | ||||
| EQ-5D utility | Complete case | 1, 672.5 | 0.34 | 0.56 | Gender | –0.011 | 0.018 | –0.046 | 0.025 |
| Multiple imputations | (1, 922.9) (1, 922.9) | 0.02–0.09 | 0.76–0.88 | Gender (2) | 0.001 | ||||
| EQ-5D VAS | Complete case | 1, 662.4 | 0.06 | 0.81 | None | 0.344 | 1.465 | –2.532 | 3.221 |
| Multiple imputations | (1, 920.9) (1, 923.5) | 0.12–0.38 | 0.54–0.73 | None | 0.560 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| GHQ-28 (log-transform) | Complete case | 1, 682.0 | 0.001 | 0.98 | Carer gender, PwD gender | 0.005 | 0.040 | –0.073 | 0.083 |
| Multiple imputations | (1, 910.6) (1, 910.5) | 0.005–0.52 | 0.47–0.94 | Carer gender (5), PwD gender (5) | 0.003 | ||||
| GHQ somatic | Complete case | 1, 718.5 | 0.61 | 0.43 | Carer gender, PwD gender | –0.222 | 0.307 | –0.826 | 0.382 |
| Multiple imputations | (1, 962.1) (1, 961.9) | 0.1–4.33 | 0.04–0.76 | Carer gender (5), PwD gender (5), time point (4) | –0.265 | ||||
| GHQ anxiety | Complete case | 1, 712.1 | 3.43 | 0.06 | Carer gender, PwD gender | 0.614 | 0.323 | –0.020 | 1.248 |
| Multiple imputations | (1, 959.2) (1, 959.1) | 1.21–4.23 | 0.04–0.27 | Carer gender (5), PwD gender (5), spousal (1) | 0.486 | ||||
| GHQ social | Complete case | 1, 718 | 0.0 | 0.99 | PwD gender, spousal | –0.005 | 0.196 | –0.391 | 0.381 |
| Multiple imputations | (1, 963) (1, 963) | 0.06–1.68 | 0.20–0.81 | Spousal (5), PwD gender (5), time point (2) | –0.046 | ||||
| GHQ depression | Complete case | 1, 720.9 | 0.45 | 0.50 | PwD gender | 0.176 | 0.245 | –0.306 | 0.658 |
| Multiple imputations | (1, 960.9) (1, 960.0) | 0–0.22 | 0.64–0.99 | Spousal (4), PwD gender (5) | 0.015 | ||||
| RSS | Complete case | 1, 709.9 | 4.34 | 0.038 | PwD gender, carer gender | 1.623 | 0.780 | 0.91 | 3.155 |
| Multiple imputations | (1, 907.9) (1, 908.0) | 0.80–3.67 | 0.056–0.37 | PwD gender (5), carer gender (2) | 1.026 | ||||
| HADS anxiety | Complete case | 1, 720.0 | 7.20 | 0.007 | PwD gender, carer gender | 0.848 | 0.309 | 0.242 | 1.454 |
| Multiple imputations | (1, 905.7) (1, 908.4) | 2.03–3.98 | 0.046–0.15 | PwD gender (5), carer gender (5) | 0.481 | ||||
| HADS depression | Complete case | 1, 720.6 | 3.91 | 0.048 | Spousal, PwD gender, carer gender, time point | 0.477 | 0.238 | 0.010 | 0.945 |
| Multiple imputations | (1, 909.0) (1, 909.8) | 0.31–2.71 | 0.10–0.58 | Spousal (5), PwD gender (5), carer gender (2), time point (5) | 0.235 | ||||
| QCPR | Complete case | 1, 694.4 | 0.22 | 0.64 | PwD gender | –0.296 | 0.687 | –1.645 | 1.053 |
| Multiple imputations | (1, 906.5) (1, 906.5) | 0–0.64 | 0.42–0.99 | PwD gender (5) | 0.176 | ||||
| QCPR warmth | Complete case | 1, 711.3 | 0.57 | 0.45 | Spousal, PwD gender | –0.306 | 0.413 | –1.116 | 0.505 |
| Multiple imputations | (1, 960.1) (1, 961.5) | 0.03–2.28 | 0.13–0.87 | Spousal (5), PwD gender (5), carer gender (5) | 0.081 | ||||
| QCPR negative | Complete case | 1, 708.0 | 0.03 | 0.85 | PwD gender | –0.038 | 0.339 | –0.703 | 0.628 |
| Multiple imputations | (1, 959.0) (1, 959.5) | 0.02–2.30 | 0.13–0.89 | Spousal (3), PwD gender (5), carer gender (2), allocation × time point (1) | 0.281 | ||||
| EQ-5D utility | Complete case | 1, 713.1 | 0.14 | 0.71 | Spousal, PwD gender, carer gender | –0.008 | 0.018 | –0.045 | 0.028 |
| Multiple imputations | (1, 605.8) (1, 906.3) | 0.001–0.41 | 0.52–0.98 | PwD gender (5), carer gender (5), spousal (5) | –0.007 | ||||
| EQ-5D VAS | Complete case | 1, 907.6 | 0.93 | 0.34 | PwD gender | 1.081 | 1.417 | –1.702 | 3.864 |
| Multiple imputations | (1, 909.8) (1, 909.9) | 0.63–2.59 | 0.11–0.43 | Spousal (3), PwD gender (5), carer gender (3) | 1.422 |
| Outcome measure | Complete case non-imputed but adjusted data | df hypothesis, error | F-value | p-value | Other significant variables | Mean difference | SE | LCI | UCI |
|---|---|---|---|---|---|---|---|---|---|
| Multiple imputations (five repetitions) | df range (low df) (high df) | F-value range | p-value range | No. of times significant in multiple imputations | Pooled mean difference | Pooled SE | Pooled LCI | Pooled UCI | |
| Proxy QoL-AD | Complete case | 1, 713.9 | 2.14 | 0.14 | Spousal, PwD gender, carer gender | –0.677 | 0.461 | –1.582 | 0.228 |
| Multiple imputations | (1, 908.9) (1, 905.8) | 0.02–1.25 | 0.26–0.88 | Spousal (5), PwD gender (5), carer gender (5) | –0.223 | ||||
| BADLS | Complete case | 1, 718.0 | 1.18 | 0.28 | Spousal, PwD gender, carer gender, time point | 0.816 | 0.779 | –0.712 | 2.345 |
| Multiple imputations | (1, 909.0) (1, 909.4) | 1.93–4.66 | 0.03–0.17 | Carer gender (5), time point (5) | 1.231 | ||||
| EQ-5D utility | Complete case | 1, 707.1 | 1.01 | 0.31 | Spousal | –0.024 | 0.022 | –0.067 | 0.019 |
| Multiple imputations | (1, 905.3) (1, 904.0) | 0.04–0.80 | 0.37–0.84 | Spousal (3) | –0.013 | ||||
| EQ-5D VAS | Complete case | 1, 716.0 | 0.01 | 0.94 | Spousal | –0.195 | 1.439 | –3.020 | 2.631 |
| Multiple imputations | (1, 907.7) (1, 907.3) | 0.01–1.63 | 0.20–0.94 | Spousal (5), PwD gender (1) | –0.329 | ||||
| CSDD | Complete case | 1, 595 | 0.66 | 0.42 | PwD gender | –0.333 | 0.435 | –1.187 | 0.520 |
| Multiple imputations | (1, 913) (1, 913) | 0.01–1.06 | 0.30–0.94 | PwD gender (3) | –0.095 | ||||
| RAID | Complete case | 1, 631.4 | 1.74 | 0.19 | None | 0.720 | 0.546 | –0.353 | 1.793 |
| Multiple imputations | (1, 906.4) (1, 903.4) | 0.11–2.59 | 0.11–0.51 | 0.533 |
Appendix 7 Full model descriptions for primary outcomes Quality of life in Alzheimer’s disease (QoL-AD) and General Health Questionnaire-28 item version
The tables contained in this appendix cover the full model descriptions for the primary outcomes. These include the 10-month ANCOVA, 3-month ANCOVA and the linear-mixed model results.
| Factor | Non-imputed but adjusted data | Multiple imputations (five repetitions) | ||||
|---|---|---|---|---|---|---|
| df hypothesis, error | F-value | p-value | df (low df) (high df) | F-value range | p-value range | |
| Baseline value | 1, 215.2 | 106.76 | < 0.001 | (1, 349.8) (1, 323.7) | 127.08–190.55 | All < 0.001 |
| Age | 1, 178.9 | 0.02 | 0.89 | (1, 159.3) (1, 210.8) | 0.01–4.19 | < 0.001–0.65 |
| Gender | 1, 278.0 | 0.67 | 0.42 | (1, 328.4) (1, 373.9) | 0.10–5.77 | 0.017–0.75 |
| Spousal | 1, 242.3 | 0.77 | 0.38 | (1, 318.5) (1, 353.0) | 0.15–1.57 | 0.21–0.70 |
| Location | 6, 4.6 | 3.5 | 0.10 | (6, 5.8) (6, 5.8) | 2.13–10.30 | 0.007–0.19 |
| Wave | 4, 286.5 | 0.90 | 0.46 | (4, 441.0) (4, 436.8) | 1.07–2.45 | 0.045–0.37 |
| Allocation | 1, 4.2 | 0.48 | 0.53 | (1, 5.2) (1, 2.5) | 0.01–7.80 | 0.084–0.93 |
| Allocation × location | 6, 282 | 0.88 | 0.51 | (6,435) (6, 435) | 0.31–1.77 | 0.10–0.93 |
| Factor | Non-imputed but adjusted data | Multiple imputations (five repetitions) | ||||
|---|---|---|---|---|---|---|
| df hypothesis, error | F-value | p-value | df (low df) (high df) | F-value range | p-value range | |
| Baseline value | 1, 279.8 | 186.29 | < 0.001 | (1, 429.7) (1, 433.9) | 148.16–237.87 | < 0.001 |
| PwD age | 1, 286.4 | 0.68 | 0.41 | (1, 333,2) (1, 391.3) | 0.05–2.72 | 0.10–0.82 |
| Carer gender | 1, 284.0 | 9.72 | 0.002 | (1, 429.4) (1, 416.3) | 0.91–12.3 | 0.001–0.34 |
| Carer age | 1, 296.1 | 3.22 | 0.07 | (1, 436.0) (1, 433.2) | 1.13–4.07 | 0.044–0.29 |
| PwD gender | 1, 298.3 | 0.66 | 0.42 | (1, 427.4) (1, 436.8) | 0.18–2.55 | 0.11–0.67 |
| Spousal | 1, 301.1 | 0.09 | 0.77 | (1, 433.9) (1, 429.4) | 0.04–0.96 | 0.34–0.85 |
| Location | 6, 5.00 | 1.76 | 0.28 | (6, 5.7) (6, 5.423) | 0.62–1.99 | 0.22–0.71 |
| Wave | 4, 299.1 | 0.53 | 0.71 | (4, 429.9) (4, 430.0) | 0.31–2.80 | 0.026–0.87 |
| Allocation | 1, 4.2 | 1.13 | 0.35 | (1, 4.7) (1, 4.3) | 0.13–3.22 | 0.14–0.74 |
| Allocation × location | 6, 295 | 0.87 | 0.52 | (6,425) (6, 425) | 0.69–1.39 | 0.22–0.66 |
| Factor | Original data | Multiple imputations (five repetitions) | ||||
|---|---|---|---|---|---|---|
| df hypothesis, error | F-value | p-value | df (low df) (high df) | F-value range | p-value range | |
| Baseline value | 1, 337.2 | 234.15 | < 0.001 | (1, 353.3) (1, 436.9) | 26.71–317.34 | All < 0.001 |
| Age | 1, 270.5 | 0.02 | 0.90 | (1, 323.1) (1, 347.9) | 0.01–0.02 | 0.67–0.92 |
| Gender | 1, 318.9 | 0.83 | 0.36 | (1, 440.3) (1, 433.0) | 0.07–2.83 | 0.093–0.80 |
| Spousal | 1, 316.8 | 0.86 | 0.35 | (1, 383.9) (1, 431.6) | 0.01–1.41 | 0.24–0.91 |
| Location | 6, 5.4 | 0.61 | 0.72 | (6, 5.7) (6, 5.4) | 0.75–1.63 | 0.30–0.63 |
| Wave | 4, 324.9 | 0.21 | 0.94 | (4, 439.8) (4, 440.9) | 0.06–0.22 | 0.93–0.93 |
| Allocation | 1, 5.2 | 0.26 | 0.63 | (1, 5.4) (1, 4.6) | 0.01–2.63 | 0.17–0.93 |
| Allocation × location | 6, 322 | 2.30 | 0.035 | (6, 435) (6, 435) | 1.02–2.25 | 0.038–0.41 |
| Factor | Original data | Multiple imputations (five repetitions) | ||||
|---|---|---|---|---|---|---|
| df hypothesis, error | F-value | p-value | df (low df) (high df) | F-value range | p-value range | |
| Baseline value | 1, 336.3 | 265.62 | < 0.001 | (1, 421.9) (1,429.6) | 235.63–322.48 | All < 0.001 |
| PwD age | 1, 338.5 | 0.89 | 0.35 | (1, 421.3) (1, 431.1) | 0.57–2.78 | 0.096–0.45 |
| Carer gender | 1, 323.9 | 3.82 | 0.051 | (1, 394.4) (1, 429.9) | 1.81–5.18 | 0.023–0.18 |
| Carer age | 1, 338.9 | 0.19 | 0.67 | (1, 436.6) (1, 435.7) | 0.08–3.49 | 0.062–0.77 |
| PwD gender | 1, 336.6 | 1.12 | 0.29 | (1, 436.8) (1, 436.3) | 0.04–5.16 | 0.024–0.84 |
| Spousal | 1, 337.2 | 0.24 | 0.62 | (1, 435.1) (1, 436.0) | 0.00–4.13 | 0.043–0.99 |
| Location | 6, 5.2 | 0.48 | 0.80 | (6, 5.8) (6, 5.6) | 0.29–0.64 | 0.70–0.92 |
| Wave | 4, 332.8 | 1.26 | 0.29 | (4, 429.3) (1, 430.9) | 1.30–2.94 | 0.020–0.27 |
| Allocation | 1, 4.6 | 0.04 | 0.86 | (1, 4.9) (1, 5.1) | 0.001–0.49 | 0.51–0.97 |
| Allocation × location | 6, 327 | 1.26 | 0.28 | (6, 425) (6, 425) | 0.95–1.81 | 0.097–0.46 |
| Factor | Original data | Multiple imputations (five repetitions) | ||||
|---|---|---|---|---|---|---|
| df hypothesis, error | F-value | p-value | df (low df) (high df) | F-value range | p-value range | |
| Spousal | 1, 652.0 | 6.57 | 0.011 | (1,919.0) (1, 924.7) | 4.80–9.53 | 0.002–0.029 |
| Gender | 1, 653.3 | 12.18 | 0.001 | (1, 923.7) (1, 924.4) | 5.55–14.53 | < 0.001–0.019 |
| Time point | 1, 649.6 | 1.64 | 0.20 | (1, 916.2) (1, 916.6) | 0.80–10.77 | 0.001–0.37 |
| Allocation | 1, 655.6 | 0.17 | 0.68 | (1, 919.2) (1, 919.0) | 0.05–0.99 | 0.32–0.82 |
| Time point × allocation | 1, 649.5 | 0.567 | 0.45 | (1, 916.3) (1, 916.5) | 0.14–5.60 | 0.018–0.71 |
| Factor | Original data | Multiple imputations (five repetitions) | ||||
|---|---|---|---|---|---|---|
| df hypothesis, error | F-value | p-value | df (low df) (high df) | F-value range | p-value range | |
| Spousal | 1, 682.0 | 4.51 | 0.034 | (1, 910.8) (1, 907.0) | 3.35–6.40 | 0.012–0.061 |
| PwD gender | 1, 681.4 | 47.81 | < 0.001 | (1, 902.6) (1, 900.5) | 21.88–56.03 | All < 0.001 |
| Carer gender | 1, 681.9 | 12.86 | < 0.001 | (1, 906.3) (1, 910.8) | 7.82–20.28 | < 0.001–0.005 |
| Time point | 1, 677.1 | 0.20 | 0.66 | (1, 905.8) (1, 904.3) | 0.39–1.11 | 0.29–0.53 |
| Allocation | 1, 682.0 | 0.001 | 0.98 | (1, 910.6) (1, 910.5) | 0.005–0.52 | 0.47–0.94 |
| Allocation × time point | 1, 677.1 | 1.43 | 0.23 | (1, 905.8) (1, 903.9) | 0.25–3.50 | 0.062–0.62 |
Appendix 8 Compliance analysis
| Outcome measure | Data set used | Group | 3-month weekly compliance | 10-month monthly compliance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimated mean | SE | LCI | UCI | Estimated mean | SE | LCI | UCI | |||
| QoL-AD | Complete case | Control | 37.68 | 0.61 | 36.48 | 38.89 | 36.78 | 0.69 | 35.42 | 38.15 |
| RYCT non-compliers | 36.23 | 0.73 | 34.89 | 37.76 | 36.46 | 0.85 | 34.78 | 38.13 | ||
| RYCT compliers | 37.08 | 0.53 | 36.04 | 38.11 | 37.44 | 0.92 | 35.62 | 39.25 | ||
| Pooled imputation | Control | 37.45 | 0.65 | 36.13 | 38.76 | 36.47 | 0.78 | 34.83 | 38.12 | |
| RYCT non-compliers | 36.10 | 0.66 | 34.80 | 37.40 | 36.86 | 0.66 | 35.56 | 38.15 | ||
| RYCT compliers | 37.04 | 0.55 | 35.96 | 38.12 | 36.48 | 0.93 | 34.58 | 38.39 | ||
| AMI(E) memory | Complete case | Control | 10.81 | 0.74 | 9.35 | 12.27 | 12.37 | 0.85 | 10.70 | 14.04 |
| RYCT non-compliers | 9.90 | 0.88 | 8.17 | 11.63 | 11.81 | 1.01 | 9.83 | 13.79 | ||
| RYCT compliers | 11.79 | 0.62 | 10.56 | 13.02 | 12.63 | 1.11 | 10.45 | 14.81 | ||
| Pooled imputation | Control | 11.11 | 0.62 | 9.90 | 12.37 | 12.60 | 0.85 | 10.88 | 14.33 | |
| RYCT non-compliers | 10.18 | 0.82 | 8.56 | 11.80 | 11.97 | 0.93 | 10.07 | 13.86 | ||
| RYCT compliers | 11.67 | 0.61 | 10.48 | 12.86 | 12.19 | 0.97 | 10.28 | 14.09 | ||
| AMI(E) factual | Complete case | Control | 50.02 | 1.76 | 46.56 | 53.47 | 49.24 | 2.14 | 45.02 | 53.46 |
| RYCT non-compliers | 49.86 | 2.09 | 45.76 | 53.47 | 48.74 | 2.54 | 43.75 | 53.73 | ||
| RYCT compliers | 52.91 | 1.48 | 50.00 | 55.82 | 48.58 | 2.81 | 43.05 | 54.11 | ||
| Pooled imputation | Control | 50.27 | 1.52 | 47.29 | 53.25 | 48.08 | 2.02 | 44.03 | 52.14 | |
| RYCT non-compliers | 49.40 | 2.25 | 44.82 | 53.98 | 44.49 | 2.38 | 41.41 | 49.34 | ||
| RYCT compliers | 52.49 | 1.49 | 49.55 | 55.43 | 47.19 | 2.37 | 42.61 | 51.83 | ||
| QCPR | Complete case | Control | 56.95 | 1.01 | 54.97 | 58.92 | 56.93 | 0.89 | 55.17 | 58.69 |
| RYCT non-compliers | 56.52 | 0.95 | 54.66 | 58.39 | 56.25 | 1.14 | 54.00 | 58.50 | ||
| RYCT compliers | 57.53 | 0.66 | 56.23 | 58.83 | 59.47 | 1.22 | 57.06 | 61.87 | ||
| Pooled imputation | Control | 56.58 | 0.75 | 55.08 | 58.08 | 56.76 | 0.71 | 55.36 | 58.15 | |
| RYCT non-compliers | 56.06 | 0.92 | 54.21 | 57.92 | 56.77 | 0.83 | 55.14 | 58.41 | ||
| RYCT compliers | 57.09 | 0.64 | 55.82 | 58.35 | 58.53 | 1.00 | 56.55 | 60.50 | ||
| EQ-5D utility | Complete case | Control | 0.76 | 0.028 | 0.70 | 0.81 | 0.81 | 0.032 | 0.75 | 0.87 |
| RYCT non-compliers | 0.79 | 0.033 | 0.72 | 0.85 | 0.74 | 0.037 | 0.66 | 0.81 | ||
| RYCT compliers | 0.76 | 0.024 | 0.71 | 0.80 | 0.87 | 0.041 | 0.79 | 0.95 | ||
| Pooled imputation | Control | 0.74 | 0.027 | 0.68 | 0.79 | 0.77 | 0.034 | 0.70 | 0.84 | |
| RYCT non-compliers | 0.75 | 0.030 | 0.69 | 0.81 | 0.73 | 0.033 | 0.67 | 0.80 | ||
| RYCT compliers | 0.75 | 0.022 | 0.71 | 0.80 | 0.82 | 0.038 | 0.74 | 0.89 | ||
| EQ-5D VAS | Complete case | Control | 71.10 | 2.51 | 66.17 | 76.03 | 71.05 | 2.51 | 66.11 | 75.99 |
| RYCT non-compliers | 68.13 | 3.13 | 61.98 | 74.29 | 68.59 | 3.12 | 62.48 | 74.71 | ||
| RYCT compliers | 71.26 | 2.23 | 66.88 | 75.64 | 70.97 | 3.36 | 64.37 | 77.58 | ||
| Pooled imputation | Control | 70.46 | 2.26 | 65.92 | 75.00 | 70.49 | 2.26 | 65.96 | 75.02 | |
| RYCT non-compliers | 68.41 | 3.77 | 60.12 | 76.71 | 68.83 | 3.38 | 61.39 | 76.27 | ||
| RYCT compliers | 71.51 | 2.35 | 66.69 | 76.34 | 71.75 | 3.37 | 64.79 | 78.71 | ||
Appendix 9 Serious adverse events
A SAE is an untoward occurrence experienced by either a participant or carer which:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
is otherwise considered medically significant by the investigator.
In addition, any cases where action has been taken under the REMCARE protocol for the protection of vulnerable adults (dealing with suspected abuse or neglect of participants) would be reported using this procedure.
Sites were asked to categorise the reported event into one of the following categories:
-
death
-
life-threatening
-
hospitalisation or prolongation of existing hospitalisation
-
persistent or significant disability or incapacity
-
otherwise considered medically significant by the investigator
-
alleged/suspected abuse/neglect, as detailed in protection of vulnerable adults protocol.
There were 102 SAEs reported to the chief investigator during the course of the trial. The categorisation of these events is given in Table 64. This table totals 103 events; this is because one SAE was reported both as a persistent or significant disability and under the protection of vulnerable adults categories. There were 71 events reported in the RYCT intervention group and 31 events reported in the control group. The discrepancy in number of reported events in each group can be attributed to the greater contact that the intervention group had with the trial team. If a dyad of an RYCT group did not attend then the facilitator was likely to follow that dyad up. This resulted in an imbalance of the number of reported incidents as the control group were only contacted at the follow-up time points and events may not have been retrospectively reported.
| Category | Total | Reminiscence | Control | Linked to trial |
|---|---|---|---|---|
| Death | 34 | 17 | 17 | 0 |
| Life-threatening | 1 | 1 | 0 | 0 |
| Hospitalisation | 37 | 23 | 14 | 0 |
| Disability | 9 | 9 | 0 | 0 |
| Medically significant | 19 | 19 | 0 | 1 |
| Protection of vulnerable adults | 3 | 3 | 0 | 0 |
| Total | 103 | 72 | 31 | 1 |
The 34 deaths reported break down into 30 deaths of participants with dementia and four carer deaths. There are only 29 deaths noted on the CONSORT flowchart as in five cases the remaining participant of the dyad wished to continue in the trial. These participants have not been included in the analysis.
There was one reported life-threatening event. This event related to a participant with dementia staying in a respite placement leaving the care home for a period of 3–4 hours unaccompanied. The person was found unharmed and returned to the care home.
There was one event recorded as linked to participation in the trial on the SAE form returned to the central team. This was when a participant became upset by participation in one of the intervention sessions related to marriage. The protocol for dealing with distressing events during the intervention was implemented and time was spent with the participant and carer after the session to ensure that the participant was all right. Subsequently the participant with dementia developed shingles. The carer believed that the upset felt by the participant during the session was linked to the development of the shingles. The carer had a discussion with the participant’s GP, who reassured the carer that attributing reasons for the onset of shingles was difficult. The chief investigator and trial management team felt that there was no reason to attribute a link between the onset of shingles and the event at the intervention group. This event was reported to the independent DMEC during the regular meeting. There was no concern raised from the committee that this incident should have been handled any differently. The DMEC was also not concerned at the raised concern of the link between the distressing event of the RYCT group and the onset of shingles owing to the singular occurrence during the course of the entire trial.
The distribution of types of events that led to reporting SAEs have been further broken down in Table 65. There are no particular events that occur with a much greater frequency than any other event with all events being those expected in this demography of participants.
| Category | Participant with dementia | Carer | Dyad |
|---|---|---|---|
| Back pain | 0 | 1 | 0 |
| Cataract | 2 | 0 | 0 |
| Chest infection/respiratory/flu | 8 | 0 | 0 |
| Death | 29 | 4 | 0 |
| Distress/stress | 1 | 1 | 0 |
| Fall | 12 | 1 | 0 |
| Other health issues not requiring hospitalisation | 8 | 2 | 0 |
| Heart condition | 4 | 0 | 0 |
| Hospitalised | 3 | 1 | 0 |
| Mistaken drug | 0 | 1 | 0 |
| Respite care | 4 | 0 | 0 |
| Safeguarding | 2 | 0 | 1 |
| Stroke | 5 | 1 | 0 |
| Surgery | 0 | 3 | 0 |
| UTI | 8 | 0 | 0 |
| Wandered away | 1 | 0 | 0 |
| Total | 87 | 15 | 1 |
Protocol violations
There were four protocol violations reported to DMEC throughout the trial. The majority of these were because of administrative errors.
A clerical error in north London resulted in an incorrect randomisation result being relayed to a participant. This participant had been randomised to the control group but had been informed that they had been randomised to the intervention group. This participant duly attended the intervention sessions but in line with the ITT analysis principle has been analysed as part of the control group. Procedures were put in place to prevent incorrect results being relayed to participants.
A group of participants from north London were incorrectly randomised as being from south London. The mistake was noticed before the participants were informed of the results. The participants were then rerandomised within the correct location. Clearer centre labelling on the forms was instigated with notes to centres about making sure their email requests are clearly labelled. With the introduction of clearer labelling this problem did not recur.
There was an issue regarding a local site approval for recruitment. MREC approval was sought and approved to allow recruitment from a Memory Clinic in south Manchester. The principal investigator in Manchester was sent the approval in order to apply for local approval of this centre. It was discovered that this local approval had not been sought before recruitment at this centre started. Recruitment at this centre was suspended immediately once this was realised. Local approval for this centre was sought and given. The problem appeared to be a miscommunication between the centre and trial manager. No further site approvals were needed during the duration of the trial.
In the Newport centre not all baseline assessments for one dyad were completed before randomisation. This was despite the trial management team stipulating on more than one occasion that baseline assessments needed to be completed before the randomisation. Primary outcome measures of QoL-AD and GHQ were completed before randomisation, questionnaire booklets for health economic data and some demographic information were not completed before randomisation. The dyad continued with the study and completed further assessments. The baseline questionnaires not completed before randomisation were treated as missing – as the dyad did not complete them. These data could be imputed as stipulated in the statistical analysis plan. There was no wider impact on the study as this was one of the last dyads to be recruited into the study.
Appendix 10 Study protocol
REMCARE
Reminiscence groups for people with dementia and their family caregivers: pragmatic 8-centre trial of joint reminiscence and maintenance v usual treatment
REMCARE Protocol
Version 3: 3 September 2009
ISRCTN042430123
Project title
Reminiscence groups for people with dementia and their family care-givers: pragmatic 8-centre trial of joint reminiscence and maintenance v usual treatment (REMCARE)
Planned investigation
Research objectives
-
To compare the effectiveness (in ameliorating the quality of life of people with dementia & the stress on their carers) of joint reminiscence groups with participants & carers followed by reminiscence-based maintenance with that of ‘usual treatment’.
-
To compare the incremental cost-effectiveness (in ameliorating the quality of life of people with dementia & the stress on their carers) of joint reminiscence groups with participants & carers followed by reminiscence-based maintenance with that of ‘usual treatment’.
Existing research
The development and evaluation of therapeutic interventions intended to benefit people with dementia and their family care-givers is the subject of much research interest at present. In view of the large and growing numbers of people with dementia, and the costs associated with meeting needs for care, there are clear advantages for health and social care services if people with dementia can be supported in the community for an extended period, with less intensive support. However, there is consensus that this must not be at the cost of additional burden to family care-givers (1).
Most attention has been given to pharmacological interventions, but there is increasing recognition that psychosocial interventions may have comparable value (2, 42), and may be preferable in some contexts, e.g. where medication may have negative side-effects (3, 42). A number of systematic reviews of psychosocial interventions are now available (e.g. 1, 4, 5), as well as a number of Cochrane reviews of specific approaches (e.g. 6, 7).
In practice, in the UK, Reminiscence Therapy appears to be the most well-known therapeutic approach to working with people with dementia. For example, over half of care homes in Wales claim to offer this approach to their residents (8). Reminiscence work with people with dementia has an extensive history (9, 10), involving enjoyable activities that promote communication and well-being. One factor in its popularity is that it works with early memories, which are often relatively intact for people with dementia, thus drawing on the person’s preserved abilities, rather than emphasising the person’s impairments. However, its popularity has not led to a corresponding body of evidence on its effects. The existing research literature has been brought together in our revised Cochrane review on reminiscence therapy for people with dementia (11). Only four randomised controlled trials (RCTs) suitable for analysis were identified. Each examined different types of reminiscence work; all were small or of poor quality. Taking the results from the studies together, some significant results were obtained in relation to cognition and mood 4-6 weeks after the treatment, and reduced care-giver stress where the care-giver participated with the person with dementia in a reminiscence group. However, the review concludes that ‘in view of the limitations of the studies reviewed, there is an urgent need for more quality research in the field’. This dearth of evidence is reflected in the NICE-SCIE Guideline on the management and treatment of dementia (42), which found insufficient evidence to recommend that reminiscence should be routinely offered to people with dementia, although its potential impact on mood of the person with dementia was highlighted.
In order to take research forward, there is a need to specify clearly the exact nature of the reminiscence work to be undertaken and its aims. Typically, a group approach has been used, with ‘memory triggers’ (photographs, recordings, artefacts etc.) used to promote personal and shared memories. A recent development has been to involve family care-givers in the groups alongside their relatives with dementia. Descriptive evaluations suggest that this joint approach (described as ‘Remembering Yesterday, Caring Today’ - RYCT) may improve the relationship between care-giver and person with dementia, benefiting both (12). As it is the breakdown of this care-giving relationship that increases the likelihood of the person with dementia being placed in institutional care, this effect could have far-reaching implications for families, society and public spending. Our group have reported a very small pilot study evaluating this joint reminiscence approach (7 patient-carer dyads in the treatment group; 4 in the waiting-list control group), which showed some trends in improved quality of life for patients and reduced stress for care-givers (13). In the next section, we shall present results from a larger trial platform, funded by the MRC, that has recently been completed, which developed this pilot work further.
The justification for evaluating the joint reminiscence approach specifically comes from this promising pilot data and the great interest in this approach in the field of reminiscence work (9). More generally, a recent meta-analysis (1) on interventions with family care-givers of people with dementia suggested that joint approaches may be more effective in improving care-giver outcomes than approaches targeted only at the care-giver. The previous tradition in dementia care of interventions for people with dementia and their care-givers separate from each other is being questioned. For example, in many areas of the UK, Alzheimer Café sessions have been established, with an agenda including education as well as social contact, attended by both people with dementia and their care-givers. The emphasis has shifted from ‘person-centred care’ to ‘relationship-centred care’, with recognition of the central importance of the relationship between person with dementia and care-giver to the well-being of both. Although a joint focus on people with dementia and their care-givers is not applicable to all people with dementia, the proportion of people with dementia without an identifiable care-giver has been reported to be as low as 6% (14), with such people being much more likely to enter care homes.
Reference methods
It is proposed to carry out a pragmatic randomised controlled trial of joint reminiscence groups v usual treatment.
Trial platform
The applicants have recently completed (31 May 2006) a pilot study comparing these joint reminiscence groups with usual treatment as part of a trial platform funded by the MRC, which also refined outcome measures and prepared a detailed treatment manual. The trial platform also included an additional condition where people with dementia attended reminiscence groups without their carers.
Methods
Three centres participated in the trial (Bangor, Bradford and UCL). Across the centres, three joint groups and two reminiscence alone groups were run. Participating dyads were randomised to either the joint reminiscence condition or to an active control condition (reminiscence alone) or a passive control condition (treatment as usual), depending on the centre. In the Bradford centre, the Zelen randomisation method (15) was trialled; participants initially agreed to complete the assessment procedures at each time-point; if randomised to an active intervention, further informed consent was then sought.
Participants were recruited from local NHS services, including memory clinics, and from voluntary agencies, such as the Alzheimer’s Society. Inclusion criteria were a diagnosis of mild to moderate dementia and the absence of severe agitation and communication problems. All participants were required to have a family care-giver able and willing to attend reminiscence sessions with the person with dementia. 65 participant dyads entered the trial and provided baseline data (see Appendix 1: CONSORT diagram for trial platform). 57 went on to receive the intervention they were randomised to (7 being lost through stage 2 Zelen refusals). The post-treatment assessment was completed by 50 dyads; a three-month follow-up assessment was completed by 45 dyads (10 treatment as usual, 24 joint reminiscence, 11 reminiscence alone).The median age of the people with dementia was 78 years; that of the care-givers was 72 years. The average Mini-Mental State Examination (16) score was 19.3 (sd 5.0) (moderate dementia: 12-20; mild dementia 21-26).
Primary outcome measures were: QoL-AD (17), a quality-of-life measure completed with the person with dementia in a structured interview, which is also completed on a proxy basis by the care-giver; RSS (18), a self-report measure of the direct impact of care-giving. Secondary outcome measures included: a measure of autobiographical memory (the type of personal memory over the lifespan that should be influenced by reminiscence work), adapted for the project to include more items and better coverage of the lifespan; measures of care-giver distress and depression (the General Health Questionnaire (GHQ-28) (19) and the Geriatric Depression Scale (GDS-15) (20); measures of the quality of relationship between the person with dementia and care-giver (Quality of Care-giver Patient Relationship – QCPR) (21), ratings of video-taped interactions between person with dementia and care-giver in two structured situations (22).
Results
All analyses reported were undertaken using analysis of covariance on post-treatment (or follow-up scores), with baseline scores as the covariate. For most of the measures in this small sample, differences between joint reminiscence and reminiscence alone were small. For the primary outcome measures, comparing either type of reminiscence with treatment as usual, the differences were not statistically significant; the effect sizes for QoL-AD, rated by the person with dementia were small at post-treatment (0.17) and at 3 month follow-up (0.40); the initial rating for the care-giver rating of the quality of life for the person with dementia (a secondary outcome) was slightly higher (0.50), but the effect size at three-months was similar (0.33). On the primary outcome for care-givers, the RSS, effect sizes were small to moderate (0.36 and 0.31).
On secondary outcome measures, people with dementia in the joint reminiscence group had significantly better autobiographical memory at post-treatment than those receiving treatment as usual (effect size 0.61; p=0.007), but this was not maintained at follow-up. Care-givers involved in the joint reminiscence group reported less depression at post-treatment than those in the treatment as usual condition, a difference that was maintained at follow-up (effect size 0.57; p=0.013 and effect size 0.42; p=0.024 respectively). These findings were also clear when treatment as usual was compared with either type of reminiscence, with reminiscence work associated with better autobiographical memory at post-treatment, but not follow-up, and the reminiscence conditions also associated with reduced care-giver depression and distress (on GHQ) at post-treatment and (on GDS and GHQ) at follow-up. Effect sizes for all these comparisons were in the range 0.48 to 0.6, except for autobiographical memory at follow-up, which was 0.13. The details of the comparisons between any form of reminiscence and treatment as usual are shown in Appendix 1.
Implications of trial platform for full trial proposal
-
The Zelen method of randomisation led to several refusals to accept experimental interventions, thus weakening the effect of those interventions as Zelen analyses by ‘intention to treat’; as there was no evidence that it otherwise assisted recruitment and retention in this field, we shall not use it in the proposed trial.
-
Though the trial platform necessarily generated wide confidence intervals, the difference in effects between joint reminiscence and reminiscence alone appear to be small, as one might have predicted a priori from the similar resources allocated to each. Indeed, reminiscence alone may have beneficial effects for care-givers also. This may be because of the brief respite afforded to the care-giver, or from the benefits they perceive the person with dementia receiving.
-
Although the further comparison of joint reminiscence and individual reminiscence would be of interest in providing a test of the additional effects of joint working, and of relationship-centred care, we are persuaded that the size and complexity of trial that would be required, given the probable small effect size for any difference between them, would not be feasible. Accordingly, we are now proposing to focus on joint reminiscence groups.
-
Participants in the joint reminiscence groups requested monthly reunion meetings following the end of the 12 weekly sessions. They wished these to continue to have a reminiscence focus in addition to social contact. These maintenance sessions over the follow-up period have been built into the current proposal.
-
It proved entirely feasible in the trial platform to recruit to two arms over a 6 month period in each centre.
-
The trial platform thoroughly tested the outcome measures for this population, which appear valid, reliable, responsive, relevant and acceptable.
-
The treatment manual for the joint intervention has been refined and tested. The training procedure for new group facilitators has been developed and adjusted. A simple treatment adherence schedule has been developed, which can be completed directly by a trained observer.
Recruitment and randomisation
As in the trial platform, recruitment will be through mental health services for older people in each area (especially memory clinics, Community Mental Health Teams for Older People and associated professionals including psychiatrists, occupational therapists and Admiral Nurses®), associated day services and through relevant local voluntary sector agencies such as the Alzheimer’s Society. Recruitment will be in three waves, offering the opportunity to focus on different geographical areas within the remit of each centre for each group. In each centre, there will be a six month period between one group commencing and the next; recruitment in each centre to the Trial Platform was achieved well within this period.
As in the trial platform, which had approval from the relevant LRECs, the project would be briefly outlined to the potential participants by a member of the clinical team or Alzheimer’s Society worker, and permission to contact with a member of the research team obtained. The research worker would then arrange to meet the potential participants and offer full details, respond to questions etc. and, where the participants were willing to join the study, undertake the process of consent. The NHS service costs associated with this proposal include an amount for the initial explanation of the project by the clinical team member and obtaining of the potential participants’ permission to introduce them to the research team. In the current application, this important process will be facilitated by the joint appointments of several of the research team with local NHS memory clinics and other services (Woods, Orrell, Moniz-Cook, Keady). In addition, in the Hull centre, there is an existing protocol where all memory clinic attendees are given the opportunity to give consent at the outset to be approached regarding future research projects in which the service is participating. NEURODEM Cymru has funding to introduce a similar ‘opt-in to research’ system to memory clinics and other services in Wales by the commencement of the project groups, which will similarly ensure that only those with some interest are approached. In each case, those who have opted in are under no obligation to participate in any particular project.
The local researcher who will not take part in any follow-up assessments will contact the remote randomisation service of the North Wales Organisation for Randomised Trials in Health (NWORTH) when they have 24 dyads ready for randomisation. NWORTH is a trials unit recognised & funded by the Clinical Research Collaboration Cymru specifically for HTA trials. The same researcher will make arrangements for the 12 dyads randomised to the intervention group to attend group sessions, and will liaise with the group facilitators.
Other biases
Trials of psychosocial interventions cannot be blind to therapists or participants because they are aware of which, if any, treatment they are delivering or receiving. In contrast, researchers who assess participants after randomisation should not know to which arm they belong. In particular post-treatment and follow-up assessors will not attend any of the group or maintenance sessions, and will not have access to attendance lists etc.
However, our experience in the trial platform (shared by similar projects) is that participants may occasionally and inadvertently inform researchers of the treatment they are receiving. We aim to reduce this effect by explicit reminders to participants before the assessment visit, and by the use of self-report measures wherever feasible. We shall also ask all assessors to record their impression of the arm to which each participant belongs, and their confidence in that prediction. This will enable us to test whether inadvertent loss of blinding leads to bias, and to adjust for any bias detected.
Centres
The proposal is based on the involvement of 8 centres. These are as follows:
1) Bangor; this centre will recruit from the 3 NHS Trusts in North Wales, running groups in each area across the 3 waves. Support in recruitment will be secured from NEURODEM Cymru, the Wales Dementias & Neurodegenerative Diseases Research Network, for which Professor Woods is the academic lead. The 4 Memory Clinics in North Wales already collaborate on research projects.
2) South Wales (Newport), again with support from NEURODEM Cymru. This service has a number of sectors covering distinct geographical areas.
3) London – Essex; this centre will recruit primarily from the North East London Mental Health Trust, covering 4 London boroughs with a population of 120,000 older people, and 3 Memory Clinics.
4) London – South; this centre will recruit from the Memorial Hospital, Woolwich and associated services, which participated in the trial platform, and where RYCT groups have been running since 1998.
5) Hull; this centre will recruit from Humber Mental Health Teaching NHS Trust and adjoining areas. Their Memory Clinics work closely with the Alzheimer’s Society in Hull, and cover a population of 80,000 older people. Participants will also be recruited through approaching GP surgeries within two local Primary Care Trusts: NHS Hull and NHS East Riding of Yorkshire.
6) Bradford: this centre will recruit from Memory Clinics and Alzheimer’s Society groups in Bradford.
7 & 8) Manchester; this double-centre will recruit from the large populations covered by the Bolton, Salford and Trafford Mental Health Trust, including specialist Admiral Nurses in Bolton and other well-developed services. Participants will also be recruited through the Manchester Mental Health and Social Care Trust (MMHSCT), including the Memory Clinic within Wythenshawe Hospital.
Adoption of the project by DeNDRoN UK has been discussed with the relevant Clinical Studies Groups and the project appears to meet the various criteria specified. In Wales, the project would be adopted by CRC Cymru through NEURODEM Cymru. Support for recruitment will be sought from the respective Research Professional Networks, following adoption. The Manchester and North London centres fall within the areas of DeNDRoN Local Research Networks, and the two Wales centres will receive support from NEURODEM Cymru. Bradford, Hull and South London are located outside areas covered by a thematic local research network, but if required, could access support from the Comprehensive Research Networks which are planned to be in place by the time the project commences.
Planned interventions
Joint reminiscence groups (JRGs)
This approach is known as ‘Remembering Yesterday, Caring Today’ (RYCT). It places emphasis on active, as well as passive forms of reminiscence, involving both care-givers and the person with dementia. Couples will attend 12 two-hour sessions, held, where possible, in a social as opposed to a clinic-based setting. Each session is structured around a different theme for example; childhood, schooldays, working life, marriage, and holidays and journeys. Couples are encouraged to contribute with materials brought from home. Each session involves a blend of large and small group work. Typical activities include art, cooking, physical re-enactment of memories, singing and verbal reminiscence. The emphasis is firmly placed on the inclusion of the person with dementia. In the joint reminiscence groups care-givers are guided by facilitators and volunteers into allowing time for the person with dementia to respond and to value the contributions of the person with dementia.
There is a maximum limit of 12 couples to two trained facilitators in each group, together with a number of trained volunteers. Our previous experience suggested that ideally volunteers should be a mixture of ages drawn from voluntary sector (Alzheimer’s Society, Age Concern), psychology graduates and former carers with an understanding of working with older people. The training programme for facilitators and volunteers is set out in the RYCT manual (developed during the MRC trial platform). Training involves acquiring skills in listening, group dynamics, interpretation of behaviours and learning methods to maximise inclusion of carers and people with dementia. Two half day training sessions take place before the group commences. After each session time is set aside for the facilitators and volunteers to prepare session notes and to complete attendance and evaluation forms. Evaluation forms from care-givers and people with dementia are collected at the end of the first session and at the end of the 12 week programme. The RYCT manual provides facilitators and volunteers with a recommended blend of activities for each session, based around the core principles of RYCT.
The availability of volunteers means that if, for any reason, carers are not able to attend all the group sessions, the person with dementia can still be involved and engaged in the group sessions. Maintenance sessions are held monthly, and follow a similar pattern – re-visiting some topics and introducing some new ones such as considering a particular decade, e.g. the 1950s, with the aid of relevant music and video clips.
Treatment as usual
The services and interventions available to people with dementia and family care-givers randomised to receive usual treatment will naturally vary between and within centres and may change over time. In general, the interventions offered to this group will also be available to those in the active treatment groups, so that we will be examining the additional effects of reminiscence work. The only exception to this would be where the active treatment is scheduled at the same time as an alternative intervention. Our approach to costing the services and interventions received should allow us to monitor whether the usual treatment group is receiving alternative interventions in this way. Changes and developments in the availability of medications for Alzheimer’s and other dementias will affect both groups equally, and will be recorded as part of the costing information collected.
It is entirely feasible that participants in the usual treatment group may be involved in some form of reminiscence work during the 10 months of the study period. It is a popular approach in day care centres; reminiscence materials are widely available. However, it is very unlikely that, in our experience, such a structured approach to reminiscence work will be offered in any of the centres, or that it will be offered jointly to carers. It is this systematic group-based approach, rather than a general exhortation to reminisce to improve communication, that is the concern of this evaluation.
Drop-out rates
It is anticipated that some participants will not complete the full number of treatment sessions; in the trial platform, ill health was usually cited as the reason; 12% of participating dyads were lost between beginning the active treatment and the post-treatment evaluation. Our sample size calculations allow for this attrition. Wherever possible, the dyad will be encouraged to continue with the assessment measures, allowing them to be included fully in an intention to treat analysis, irrespective of number of group sessions attended.
Adherence to treatment protocol
In order to check on the parity of treatment across different centres, in the Trial Platform we have developed a simple adherence to treatment schedule; sections of a small sample (around a quarter) of group sessions will be observed by a trained rater and rated on specific aspects of RYCT; for example aspects of communication, session structure and thematic content. These observations will be fed back to facilitators and will support the supervision process.
Recruitment and training of facilitators
The reminiscence groups require skilled facilitators to lead them. The presence of family care-givers, and the requirement to ensure that their concerns do not dominate the group, adds a further dimension. One of the aims of our trial platform was to ensure that new facilitators could be recruited and trained to carry out the approach in line with the principles established by its originators, Pam Schweitzer and Age Exchange. Accordingly, whilst Pam Schweitzer led one of the reminiscence groups in the trial platform, the remaining four were led by facilitators who received initial training from Pam Schweitzer, together with the opportunity to discuss issues as they arose once the groups were underway. These facilitators included an experienced community mental health nurse, an occupational therapist and a health-care assistant, with several years experience in a very active dementia day-care service, and community arts workers.
In the proposed trial, facilitators will be identified in collaboration with each centre, and training and supervision provided. We anticipate that the majority of facilitators will have a mental health nursing or occupational therapy or clinical psychology background, but large group facilitation skills, warmth, energy and enthusiasm are as important as any particular professional qualification.. The use of two facilitators for each group, and the inclusion of volunteers, enables effective de-briefing and learning to occur at the end of each session. Group facilitators will participate in monthly supervision sessions, with a supervision team including the project consultant Pam Schweitzer, and arrangements for more immediate access to supervision will also be made. The training programme for volunteers is set out in the RYCT manual (developed during the MRC trial platform). Training covers skills in listening, group dynamics, interpretation of behaviours and learning methods to maximise inclusion of carers and people with dementia. Two half day training sessions take place before the group commences. After each session time is set aside for the facilitators and volunteers to prepare session notes and to complete attendance and evaluation forms. The RYCT manual provides facilitators and volunteers with a recommended blend of activities for each session, based around the core principles of RYCT.
Planned inclusion criteria
-
Participants with dementia will meet the DSM-IV (24) criteria for dementia. All types of dementia will be included, including Alzheimer’s, vascular dementia, Dementia of Lewy Body type and mixed dementias.
-
Participants with dementia will be in the mild to moderate stage of dementia (Clinical Dementia Rating: (25)).
-
Participants with dementia will have some ability to communicate and understand communication: a score of 1 or 0 on the relevant items of the Clifton Assessment Procedures for the Elderly – Behaviour Rating Scale (26).
-
Participants with dementia will be living in the community at the time of the baseline assessment, and will have a relative or other care-giver who maintains regular contact, can act as an informant, and would be willing and able to participate in the intervention with the person with dementia.
Planned exclusion criteria
-
Participants will not have a major physical illness or sensory impairment or disability or high level of agitation which could affect participation.
Ethical arrangements
Risks and anticipated benefits for trial participants:
There appear to be no documented harmful side-effects from participating in reminiscence groups and no adverse reactions were apparent in the MRC trial platform. Some past memories can be unhappy, and even traumatic, but with a skilled and trained facilitator participants will share only those aspects they feel comfortable with, and if distressing memories were to surface, the person would be given additional support on a one-to-one basis.
Benefits are consistently reported by participants in the groups, including enjoyment, feelings of validation and self-worth. The desire of participants to continue meeting following the sessions provides an indication of the value placed on the benefits. Prospective participants will be fully informed of the potential risks and benefits of the project.
Consent
Participants will be in the mild to moderate stages of dementia, and therefore would generally be expected to be competent to give informed consent for participation, provided that appropriate care is taken in explaining the research and sufficient time is allowed for them to reach a decision. In every case, the participant will have had at least 24 hours to consider the information provided. It is helpful for a family member or other supporter to be involved, and we would aim to ensure that this is done wherever possible. Informed consent will be sought separately from the family care-giver, in relation to their own participation. It will be made clear to both participants and family care-givers that no disadvantage will accrue if they choose not to participate.
In seeking consent, we will follow current guidance from the British Psychological Society on evaluation of capacity. In this context, consent has to be regarded as a continuing process rather than a one-off decision, and willingness to continue participating will be continually checked through discussion with participants during the assessments.
Where the participant’s level of impairment increases, so that they are no longer able to provide informed consent, the provisions of the Mental Capacity Act will be followed, with the family care-giver as consultee. Where the person has themselves given informed consent initially, this provides a clear indication of the person’s likely perspective on continuing at later time-points. The same procedure will apply where the person with dementia appears to lack capacity to consent initially, but meets the other criteria for the project. At any point where a participant with dementia becomes distressed by the assessments they will be discontinued.
Retention of trial documentation
It is planned that anonymised data will be kept securely for a period of seven years following the completion of the trial, subject to discussion with relevant Ethics Committees.
Confidentiality
Only members of the research team will have access to the original data. Participants’ personal details will be stored separately from the data, and will be kept in a separate file on a password protected computer at the University of Wales Bangor. Each participant will be assigned an identification code, which will be used in all data storage files; these will not contain names or any other means of personal identification. All personal details will be deleted on completion of the study.
Proposed sample size
Our target sample size is 400 patients completing data collection for the trial after ten months, comprising 200 in JRGs and 200 receiving treatment as usual. In the trial platform intra-class correlation coefficients (ICCs) within randomised groups were negative (i.e. not significantly different from zero) for both the carer-specific GHQ-28 and the carer-rated QoL-AD, but close to 0.1 for the QoL-AD rated by the person with dementia. Using a 5% significance level, comparison of the 200 pairs completing JRGs with the 200 people with dementia receiving treatment as usual will yield 80% power of detecting a standardised difference of 0.28 in the GHQ or the carer-rated QoL-AD. In contrast the patient-rated QoL-AD is likely to suffer a ‘variance inflation factor’ of approximately 1.74 [viz. 1 + 0.1 x (average completed group size of 8.4 minus 1)], thus yielding a power of 80% of detecting a standardised difference of 0.38. Our trial platform, which had a sample size of 57 in 3 centres, suggests that these differences between 0.28 and 0.38 are plausible. In our judgement they also fall within the range of effects that are clinically important. Furthermore, because our trial platform was exploratory, and therefore more heterogeneous than the proposed definitive trial, ICCs and standard deviations are likely to fall. To achieve a sample size of approximately 400, we need to allow for 12% attrition between recruitment and the post-treatment assessment (estimated from our trial platform) and a further 18% over the following 7 months (estimated from a community study (27)). Hence, we shall seek an initial sample size of 576, requiring 24 treatment groups initially comprising 12 dyads and another 288 randomised to usual treatment.
Statistical analysis
We shall analyse by intention to treat, in that all available data will be included, however methods of imputation such as LOCF are of limited utility in dementia, where the expectation is decline for the usual treatment group, and participants will be lost through death and illness. Hence, our sample size calculations are based on the numbers estimated to be available at the study end point, 10 months after randomisation. Multilevel modelling will be used to address the issue of clustering within randomised groups. We shall also use analysis of covariance to adjust for baseline differences in outcome variables. Analyses will consider the evaluation ten months after randomisation as the primary end point in evaluating whether the intervention has had a substantive effect on the person with dementia and/or care-giver. Secondary analyses will consider the effects immediately following the intensive phase of 12 weekly group sessions.
Proposed outcome measures
Primary outcome measures
-
quality of life for the person with dementia, self-assessed by the QoL-AD (17), which has been shown to be reliable and valid for people with mild and moderate degrees of dementia (28), (29). The scale is completed in a structured interview with the person with dementia and covers 13 domains of life quality.
-
care-giver’s mental health, evaluated using the 28 item, self-report General Health Questionnaire GHQ-28 (19) which has been widely used in care-giver research (30, 31); the Likert scoring system 0-1-2-3 will be used. The scale includes indicators of anxiety, depression, insomnia, social dysfunction and somatic symptoms. This is preferred as the primary care-giver outcome to the Relatives’ Stress Scale in this study, in view of its more general focus and wide usage.
Secondary outcome measures
-
Autobiographical memory, assessed using an extended version of the Autobiographical Memory Interview (32). The extended AMI assesses recall of the person with dementia’s personal memories relating to both factual (semantic) information for example, names of schools or teachers and specific incidents. In the trial platform, we validated an additional section on middle-age to retirement, to give systematic coverage to the life-span of our participants.
-
Measure of relationship quality, self-completed by both person with dementia and carer: Quality of the Care-giving Relationship: QCPR (21). Originally developed in the Netherlands this scale comprises 14 items (with 5 point Likert scales) designed to assess the warmth of the relationship and the absence of conflict and criticism. In the trial platform, the QCPR had good internal consistency for carers α.85 and for people with dementia α.80 and concurrent validity with other measures of relationship quality and carer stress.
-
Depression and anxiety for both people with dementia and carer (Cornell Scale & RAID for person with dementia; Hospital Anxiety & Depression Scale for carer); Cornell Scale for Depression in Dementia (CSDD) (33): A 19-item interviewer administered measure, using information from interview with the person with dementia and their carer. Signs and symptoms are described to the carer as they appear on the scale. Where there is a discrepancy between the carer and clinician’s ratings the carer is re-interviewed before the interviewer makes the final judgment. RAID (34): An 18 item rating scale to measure anxiety in a person with dementia based on a structured interview with the carer and the person with dementia. The Hospital Anxiety & Depression Scale (35) is a 14-item, self-report well-validated scale, which provides an index of both anxiety and depression, and is suitable for use with adults of all ages.
-
Stress specific to the care-giving situation - the Relatives’ Stress Scale (18): self-report scale for the care-giver, contains 15 items rated on a five-point Likert scale.
-
Quality of life of person with dementia, rated by the care-giver, using the proxy version of the QoL-AD (17), identical in structure and content to the self-report version above.
-
Costs, using the validated Client Services Receipt Inventory (CSRI) (36). The CSRI has been used extensively in studies of mental health and dementia care (e.g. (37)) and comprehensively gathers data on accommodation, medication and services accepted. In this case, the data collected will reflect the previous 3 months (at baseline and post-treatment) and 7 months (at follow-up).
-
Quality of life of care giver and person with dementia will also be measured using EQ-5D. EQ-5D (49) is a standardised instrument for use as a measure of health outcome, applicable to a wide range of health conditions and treatments. It provides a simple descriptive profile and a single index value for health status. EQ-5D was originally designed to complement other instruments but is now increasingly used as a ‘stand alone’ measure. EQ-5D is designed for self-completion by respondents and can be used in face-to-face interviews. It is cognitively simple, taking only a few minutes to complete. Instructions to respondents are included in the questionnaire. We did not include the EQ-5D originally, in view of concerns that use of a generic quality-of-life measure such as EQ-5D might not be sufficiently sensitive for use as the primary outcome measure with people with dementia. Our team has previously used the EQ-5D to evaluate the concurrent validity of the QoL-AD (28), and the two scales showed moderate correlation (0.54), but rather less of the sample of people with mild to moderate dementia were able to complete it, even though it was administered in an interview. Care-givers will be asked to complete the measure from their own perspective and for the person with dementia. The self-report of the person with dementia will also be obtained wherever possible.
-
The Bristol Activities of Daily Living Scale (50), a 20 item scale, completed by the carer, rating the functional ability of the person with dementia
Health economics analysis
Our approach
In this study, our principal chosen method of economic analysis is cost-effectiveness analysis. The study population offers an opportunity for us to conduct a secondary cost-utility analysis, and for transparency, we plan to set out all costs and effects for people with dementia and their carers in a cost-consequence analysis.
Cost data
This analysis takes a multisectoral public sector perspective spanning the NHS (dementia Services, primary and secondary care) and local government. The interventions received will be fully costed from the perspective of local dementia services to generate a total programme cost and cost per participant or per participant-carer pair.
We shall estimate the costs of dementia care through the validated Client Service Receipt Inventory (CSRI), completed with the family care-giver. The measurement of health service utilization is a routine part of the estimation of costs in economic evaluation. There is a growing literature on the reliability of patient recall as an alternative to accessing GP records, (e.g. 47) and our economic protocol is consistent with that used by health economists who have conducted trials in this field previously (36, 37). GP and other provider records are not necessarily an entirely accurate source of service utilization and hence costing information. These formal records, though mainly computer based, are sometimes incomplete or not sufficiently linked between provider agencies e.g. primary and secondary NHS care, NHS and social services. We consider that the costs of collecting data from GPs and other care providers for the whole sample would not be justified in terms of adding accuracy or reliability to the utilization and costing information used in the planned evaluation. We propose to triangulate with GP notes for a subsample to enable the estimation of any systematic differences in reports. What is important is that control and intervention groups are treated identically in terms of costing, as it is the difference in costs and effects between groups that is of interest. The triangulation exercise will be conducted with 40 participants (20 in the intervention group and 20 in the control group) to compare self-reported visits to primary and secondary care with recorded visits on GP notes for the 10-month study period, to validate this approach. We will use National costs (38, 44).
Costs will include:
-
Costs of running the joint reminiscence groups.
-
Costs of reminiscence-based maintenance groups following the initial intervention.
-
Direct costs of all primary and secondary health-care services used by participants in the intervention and control arms of the study (home/surgery telephone contacts with GP and practice nurse, outpatient and inpatient attendances at secondary care, prescribing).
-
Indirect costs associated with lost productivity and care-giver costs of attending group sessions.
-
(No intangible costs to be included).
Effectiveness data
Effectiveness will be evaluated in terms of the primary clinical outcomes: the specific quality-of-life measure QoL-AD and the GHQ-28 at the primary end-point.
Incremental cost-effectiveness analysis
The incremental cost-effectiveness ratio will indicate the change in costs and effectiveness of moving to joint reminiscence group therapy followed by reminiscence-based maintenance for the improvement of quality of life of people with dementia and amelioration of care-giver stress, as compared with no intervention. We will use bootstrap calculations for examining the uncertainty in the cost-effectiveness analysis, to provide an estimate of the probability distribution of the cost-effectiveness ratio, its confidence interval, or variance in the ratio. We will plot cost-effectiveness acceptability curves (CEACs), which have been widely adopted as a method to quantify and graphically represent uncertainty in economic evaluation studies of health-care technologies (39). They can equally be used in the evaluation of public health interventions.
Sensitivity analysis
Sensitivity analysis will be undertaken to test whether plausible changes in the values of the main variables affect the results of the analysis e.g. the age of the care-giver – there may be differences between spouse care-givers and those adult offspring care-givers who are in employment, for example.
Secondary cost-utility analysis
We will conduct a cost–utility analysis using EQ-5D to calculate QALYs (1) for carers and (2) for carers and people with dementia, on an experimental additive basis, where EQ-5D may have to be completed by proxy for people with dementia (45, 46). The addition of EQ-5D to the interview schedules for both care-givers and people with dementia allows us to undertake a secondary, more methodologically experimental, analysis which could measure and potentially combine the health utility gains to both people with dementia and their carers. This is in accord with the recommendation from the National Institute for Health and Clinical Excellence (NICE) that utility measures be included in trials of new drugs and interventions to facilitate cost per QALY calculation and that analysts consider the health effects of an intervention regardless of by whom they are accrued “For the reference case, the perspective on outcomes should be all direct health effects whether for patients or, where relevant, other individuals (principally carers).” (48, p.22). The potential impact on cost per QALY ratios in future, if health utility gains of carers were to be added to those of people with conditions such as dementia, has been recently highlighted (46). Given the findings of the trial platform, we consider that the costs of reminiscence therapy are not likely to be substantial, and the effects may well be modest, which could result in a cost per QALY ratio with a large standard error. This, taken together with our concerns about the use of a generic measure of quality of life with people with dementia, leads us to propose the utility analysis as secondary to the analysis of cost effectiveness.
Cost consequence analysis
The cost-consequence analysis is a variant of cost-effectiveness analysis in which the components of incremental costs and consequences (health outcomes) of alternative programmes are listed without aggregation. This will be used for a comparison of secondary outcome measures of participants in the intervention and control arms of the study at baseline, 3 months, and 10 months. The inclusion of a cost–consequence analysis in addition to the standard cost effectiveness and cost–utility analysis will set out clearly in a transparent manner the range of costs and consequences resulting from reminiscence therapy. This will provide the range of evidence required by commissioners and policy makers responsible for funding and co-ordinating services.
| Costs | Consequences |
|---|---|
| Costs of reminiscence therapy programmes | General and dementia-specific health-related quality of life of participants |
| Costs of primary and secondary sector health service utilisation | Use of dementia medication |
| Reminiscence based maintenance | Quality of life of carers |
| Carer stress | |
| Independent living in the community | |
| Residential care |
Research governance
The trial is sponsored by the University of Wales Bangor.
A Trial Steering Committee will be established with an independent chairperson and at least three other independent members, recruited from the UKCRC Dementias & Neurodegenerative Research Network (DeNDRoN) and the corresponding network in Wales, NEURODEM Cymru. By analogy with two trials currently funded by the NHS HTA Programme – COGNATE and FolATED – we shall create the Data Monitoring & Ethics Committee (DMEC) as a subcommittee of the TSC, so as to enhance continuity and make efficient use of expert scientific resources. The TSC will include user/carer representatives from the NEURODEM Cymru panel. The first TSC/DMEC meeting will be held in January 2008, followed by meetings in December 2008 and December 2009.
Project timetable and milestones
| December 1 2007 | Project commences: |
| Trial Manager & Co-ordinator in post | |
| Research Officers recruited. | |
| MREC approval and initial R&D approvals obtained | |
| Facilitator training begins. | |
| February 1 2008 | LREC and R&D approvals in place |
| February/March 2008 | Baseline assessments for first wave |
| April 2008 | First wave of treatment groups |
| Recruitment = 192 | |
| July 2008 | Post-treatment assessments for first wave |
| July/August 2008 | Baseline assessments for second wave |
| September 2008 | Second wave of treatment groups |
| Recruitment = 384 | |
| December 2008 | Post-treatment assessments for second wave |
| January/February 2009 | Baseline assessments for third wave |
| February 2009 | 10 month follow-up first wave |
| March 2009 | Third wave of treatment groups |
| Recruitment = 576 | |
| June 2009 | Post-treatment assessments for third wave |
| July 2009 | 10 month follow-up for second wave |
| January 2010 | 10 month follow-up for third wave |
| March 31 2010 | Database closed |
| April/May 2010 | Data analysis |
| November 30 2010 | Write-up of draft final report and draft paper complete |
Expertise
Our team offers a multidisciplinary approach, including expertise in clinical psychology, psychiatry, social work, mental health nursing, health economics and randomised trial methodology.
Bob Woods is a clinical psychologist, who has been developing and evaluating psychological approaches in dementia care, including reminiscence therapy, since 1977; he is amongst the pioneers of an evidence-based approach in this field, and is a co-author of three Cochrane systematic reviews. He led the trial platform from which this proposal has arisen, and will be responsible for the overall leadership and management of the project. He will manage the Trial Co-ordinator and the research staff at Bangor.
Ian Russell is a public health researcher who specialises in designing and conducting pragmatic RCTs, and developing patient-assessed measures of health outcomes for RCTs. He has recently brought these perspectives back to Wales, notably as founding director of the North Wales Organisation for Randomised Trials in Health (N-WORTH – a trials unit recognised and funded by CRCC, specifically for HTA trials), and as Chairperson of the Methodological Network of CRCC. N-WORTH will support the proposed trial, both methodologically and technically. In particular N-WORTH will adapt its trial software and Standard Operating Procedures (SOPs) to the trial, and contribute to the technical training and supervision of all researchers. He will also oversee the statistical, design, randomisation and data management aspects of the project.
Martin Orrell is an old age psychiatrist, who in a joint paper with BW (40) set out a manifesto for developing a rigorous evidence-based approach to the evaluation of psychological approaches in dementia care, which has resulted in a number of Cochrane reviews and a recently published RCT of a cognitive stimulation approach in dementia (2), including a health economics evaluation (37). He will manage the researchers based in London, covering a population base in Essex, through the North East London Mental Health Trust, and South London, where the Memorial Hospital, Woolwich will be a second centre, having participated n the trial platform and previous RYCT projects.
Errollyn Bruce is a key member of the Bradford Dementia Group, who has been involved in a number of innovative dementia care projects, including the development and descriptive evaluation of RYCT (12), working closely with Age Exchange. She will manage the researchers based in Bradford, and will also lead on the treatment adherence aspects of the trial.
Rhiannon Tudor Edwards is the Founding Director of the UWB Centre for Economics & Policy in Health, the largest group of health economists in Wales. She specialises in the economic evaluation of public health and complex interventions (43). She will manage and work with the dedicated trial health economics research officer, analyse results and write the health economics article describing trial findings.
John Keady has been at the forefront of developments in nursing research in dementia care, and has contributed greatly to the understanding of the perspectives of both people with dementia and their family care-givers, and has been instrumental in the development of relationship-centred care. In his new post at the University of Manchester, he is linked closely with clinical services in Bolton, Salford and Trafford Mental Health NHS Teaching Trust, and will be able to guide the implementation of the project in these large centres of population. He will manage the researchers based in Manchester.
Esme Moniz-Cook is a clinical psychologist who has been a pioneer of psychosocial interventions, in a variety of settings including primary care and care homes. She brings access to the Yorkshire and Humberside area through her position in the Humber Mental Health Teaching NHS Trust. She will manage the researchers based in Hull and the East Riding of Yorkshire.
Pam Schweitzer OBE is a key collaborator with and consultant to the project. She has been for many years Director of Age Exchange, a reminiscence-based charity, which has developed great expertise in reminiscence work with people with dementia and initiated the Remembering Yesterday, Caring Today project, which led to the joint reminiscence groups being evaluated here. She has published extensively on this topic (41), and been a key-note speaker at many national and international conferences. She established the European Reminiscence Network, and since her retirement from Age Exchange, works in developing the field further through this network. She will oversee the training of facilitators and contribute to the quality assurance of the treatment groups.
Service users
Service users have already been involved in discussions of this proposal. Following the completion of the joint reminiscence groups and the reminiscence alone groups in Bangor as part of the MRC trial platform, the participants (people with dementia and care-givers) met with the PI (Bob Woods) and their recommendations for future work were sought. They were generally very positive about the groups, and were keen to know the results. From their perspective the benefits were very clear, and they were keen for the NHS locally to fund similar projects. They recommended that meetings should continue monthly after the 12 weekly sessions, to maintain the momentum. They appreciated and enjoyed being able to re-watch, on video, clips from the sessions. In addition, the user-carer research steering group at the Centre for Mental Health & Ageing, Humber Mental Health Teaching NHS Trust have perused the proposal and expressed their support for it.
We would intend to involve service users in the course of the project through NEURODEM Cymru (the Wales Dementias and Neurodegenerative Diseases Research Network). This would involve the appointment of several service users from the NEURODEM panel to monitor the project and advise the project team. This has been most useful in relation to other recent projects e.g. with the Alzheimer’s Society providing monitors for a trial of cognitive rehabilitation at University of Wales Bangor.
References
- Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. Journal of American Geriatrics Society 2003;51:657-64.
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry 2003;183:248-54.
- McShane R, Keene J, Gedling K, Fairburn C, Jacoby R, Hope T. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow-up. BMJ 1997;314:266-70.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021.
- Woods RT, Qizilbash N. Evidence-based dementia practice. Oxford: Blackwell; 2002.
- Clare L, Moniz-Cook E, Orrell M, Spector A, Woods B. In: The Cochrane Library. Chichester: Wiley; 2004.
- Spector A, Orrell M, Davies S, Woods B. In: The Cochrane Library. Oxford: Update Software; 1998.
- DSDC-Wales . Services for People With Dementia in Wales 2002.
- Gibson F. The past in the present: using reminiscence in health and social care. Baltimore: Health Professions Press; 2004.
- Woods RT, McKiernan F, Haight BK, Webster J. The art and science of reminiscing: theory, research, methods and applications. Washington DC: Taylor & Francis; 1995.
- Woods B, Spector A, Jones C, Orrell M, Davies S. In: The Cochrane Database of Systematic Reviews. Chichester: Wiley; 2005.
- Bruce E, Gibson F. Remembering Yesterday, Caring Today: Evaluators’ Report 1998.
- Thorgrimsen L, Schweitzer P, Orrell M. Evaluating reminiscence for people with dementia: a pilot study. The Arts in Psychotherapy 2002;29:93-7.
- Tuokko H, MacCourt P, Heath Y. Home alone with dementia. Aging Ment Health 1999;3:21-7.
- Homer C. Using the Zelen design in randomized controlled trials: debates and controversies. J Adv Nurs 2002;38:200-7.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975;12:189-98.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-9.
- Agar S, Moniz-Cook E, Orbell S, Elston C, Wang M. Measuring the outcome of psychosocial intervention for family caregivers of dementia sufferers: a factor analytic study. Aging Ment Health 1997;1:166-75.
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med 1979;9:139-45.
- Yesavage JA, Brink TL, Rose TL. Development and validation of a geriatric depression scale: a preliminary report. J Psychiatry Res 1983;17:37-49.
- Spruytte N, Van-Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychology & Psychotherapy: Theory, Research &Amp; Practice 2002;75:295-311.
- Gallagher-Thompson D, Dal-Canto PG, Darnley S, Basilio LA, Whelan L, Jacob T. A feasibility study of videotaping to assess the relationship between distress in Alzheimer’s disease caregivers and their interaction style. Aging Ment Health 1997;1:346-55.
- Wenger GC, Woods B, Keady J, Scott A, Devakumar M. Dementia Action Research & Education: Older People With Dementia and Their Carers: Professional Intervention in Primary Care: Final Report to Department of Health 2000.
- American-Psychiatric-Association . Diagnostic and Statistical Manual of Mental Disorders (4th Edition) 1994.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72.
- Pattie AH, Gilleard CJ. Manual for the Clifton Assessment Procedures for the Elderly (CAPE). Sevenoaks: Hodder & Stoughton Educational; 1979.
- Woods RT, Wills W, Higginson I, Hobbins J, Whitby M. Support in the community for people with dementia and their carers: a comparative outcome study of specialist mental health service interventions. Int J Geriatr Psychiatry 2003;18:298-307.
- Thorgrimsen L, Selwood A, Spector A, Royan L, de-Madariaga-Lopez M, Woods RT, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life - Alzheimer’s Disease (QoL-AD) Scale. Alzheimer Dis Assoc Disord 2003;17:201-8.
- Hoe J, Hancock GA, Livingston G, Orrell M. Quality of life of people with dementia in residential care homes. Br J Psychiatry 2006;188:460-4.
- Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry 1998;13:248-56.
- Marriott A, Donaldson C, Tarrier N, Burns A. Effectiveness of cognitive-behavioural family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psychiatry 2000;176:557-62.
- Kopelman MD, Wilson BA, Baddeley A. The Autobiographical Memory Interview. Bury St Edmunds: Thames Valley Test Company; 1990.
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23:271-84.
- Shankar KK, Walker M, Frost D, Orrell MW. The development of a valid and reliable scale for rating anxiety in dementia (RAID). Aging Ment Health 1999;3:39-4.
- Flint AJ, Rifat SL. Validation of the Hospital Anxiety and Depression Scale as a measure of severity of geriatric depression. Int J Geriatr Psychiatry 1996;11:991-4.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring mental health needs. London: Gaskell; 1992.
- Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods B, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry 2006;188:574-80.
- Netten A, Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit, University of Kent; 2004.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves - facts fallacies and frequently asked questions. Health Econ 2004;13:405-1.
- Orrell M, Woods RT. Tacrine and psychological therapies in dementia - no contest?. Int J Geriatr Psychiatry 1996;11:189-92.
- Schweitzer P. Reminiscence in dementia care. London: Age Exchange; 1998.
- NICE-SCIE . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care 2006.
- Edwards RT, Ó Céilleachair AJ, Bywater T, Hughes D, Hutchings J. A Parenting Programme for Children at risk of developing Conduct Disorder: A Cost-Effectiveness Analysis. BMJ 2007.
- Department of Health (2005) n.d. URL: www.dh.gov.uk/PublicationsAndStatistics/Publications/PublicationsPolicyAndGuidance/PublicationsPolicyAndGuidanceArticle/fs/en?CONTENT_ID=4105545%26chk=znAfqu.
- Bell CM, Araki SS, Neumann PJ. The Association between care-giver burden and care-giver Health related Quality of Life, in Alzheimer Disease. Alzheimer Dis Assoc Disord 2001;15:129-36.
- Dixon S, Walker M, Salek S. Incorporating carer effects into economic evaluation. Pharmaeconomics n.d.:24-41.
- Mistry H, Buxton M, Longworth L, Chatwin J, Peveler R. on behalf of the AHEAD research team . Comparison of general practioner records and patient self-report questionnaires for the estimation of costs. Eur J Health Econom 2005;50.
- NICE . Guide to the Methods of Technology Appraisal 2004.
- n.d. URL: www.euroqol.org/.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age &Amp; Ageing 1996;25:113-20.
Appendices
-
Results from trial platform
-
Letter of support from Chairperson of Dementias Clinical Study Group, DeNDRoN UK
-
CONSORT diagram for the proposed trial
-
MRC trial platform CONSORT diagram
Appendix 1: Results from trial platform
| Baseline Reminiscence | Baseline Treatment as Usual | Post-treatment Reminiscence | Post-treatment Treatment as usual | Effect size | |
|---|---|---|---|---|---|
| QoL-AD (patient-rated) | 37.47 | 35.50 (5.33) | 37.70 | 34.83 (5.84) | 0.17 |
| (5.46) | (5.22) | ||||
| Relatives’ Stress Scale | 22.56 | 20.50 (13.39) | 21.49 | 24.33 | 0.36 |
| (13.77) | (12.77) | (11.50) | |||
| GHQ - 28 | 19.97 | 21.82 | 20.19 | 27.64 | 0.56 |
| (9.94) | (10.48) | (10.66) | (11.44) | ||
| GDS | 2.95 | 3.09 | 3.08 | 5.09 | 0.56 |
| (3.45) | (2.88) | (3.22) | (4.93) | ||
| AMI | 69.01 | 72.86 | 67.58 | 58.14 | 0.54 |
| (23.83) | (27.96) | (29.73) | (30.54) | ||
| QoL-AD (carer-rated) | 30.82 | 30.35 | 30.99 | 27.60 | 0.50 |
| (5.82) | (4.71) | (6.37) | (4.97) |
| Baseline Reminiscence | Baseline Treatment as Usual | Follow-up Reminiscence | Follow-up Treatment as usual | Effect size | |
|---|---|---|---|---|---|
| QoL-AD (patient-rated) | 37.08 | 35.36 | 35.49 | 31.64 | 0.40 |
| (5.38) | (5.57) | (4.99) | (11.79) | ||
| Relatives’ Stress Scale | 20.11 | 20.50 | 22.78 | 27.33 | 0.31 |
| (12.98) | (13.39) | (12.63) | (13.85) | ||
| GHQ - 28 | 18.97 | 22.00 | 21.14 | 30.33 | 0.62 |
| (10.25) | (10.01) | (11.55) | (13.24) | ||
| GDS | 2.46 | 3.09 | 3.41 | 5.64 | 0.48 |
| (2.98) | (2.88) | (2.85) | (4.70) | ||
| AMI | 70.01 | 72.86 | 58.94 | 58.59 | 0.13 |
| (23.30) | (27.96) | (28.96) | (35.18) | ||
| QoL-AD (carer-rated) | 30.96 | 29.59 | 30.11 | 26.82 | 0.33 |
| (5.56) | (5.13) | (6.50) | (5.65) |
Appendix 2: Letter of support from Chairperson of Dementias Clinical Study Group, DeNDRoN UK
Appendix 3: CONSORT diagram
Flow diagram for proposed trial
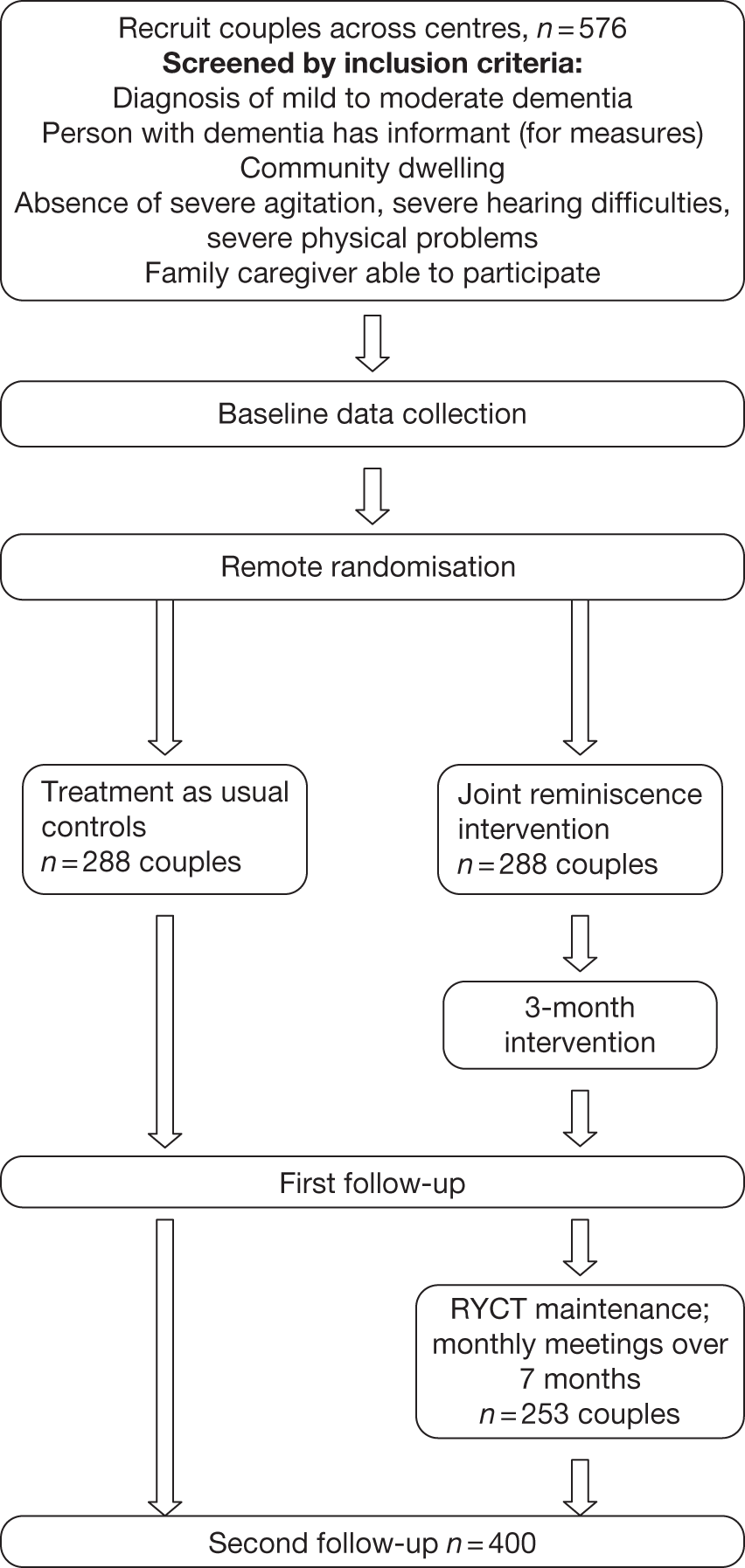
Appendix 4: MRC trial platform CONSORT diagram
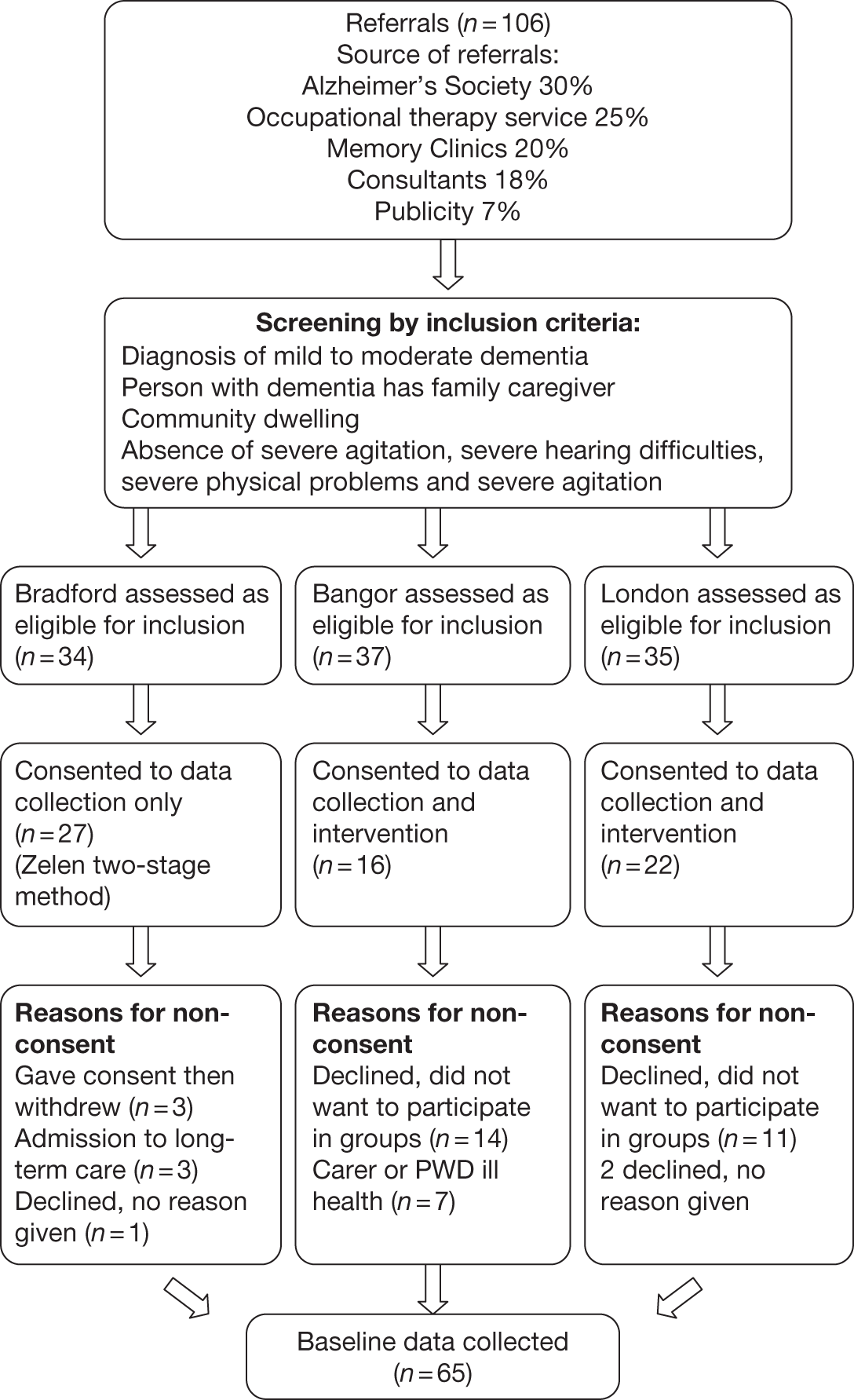
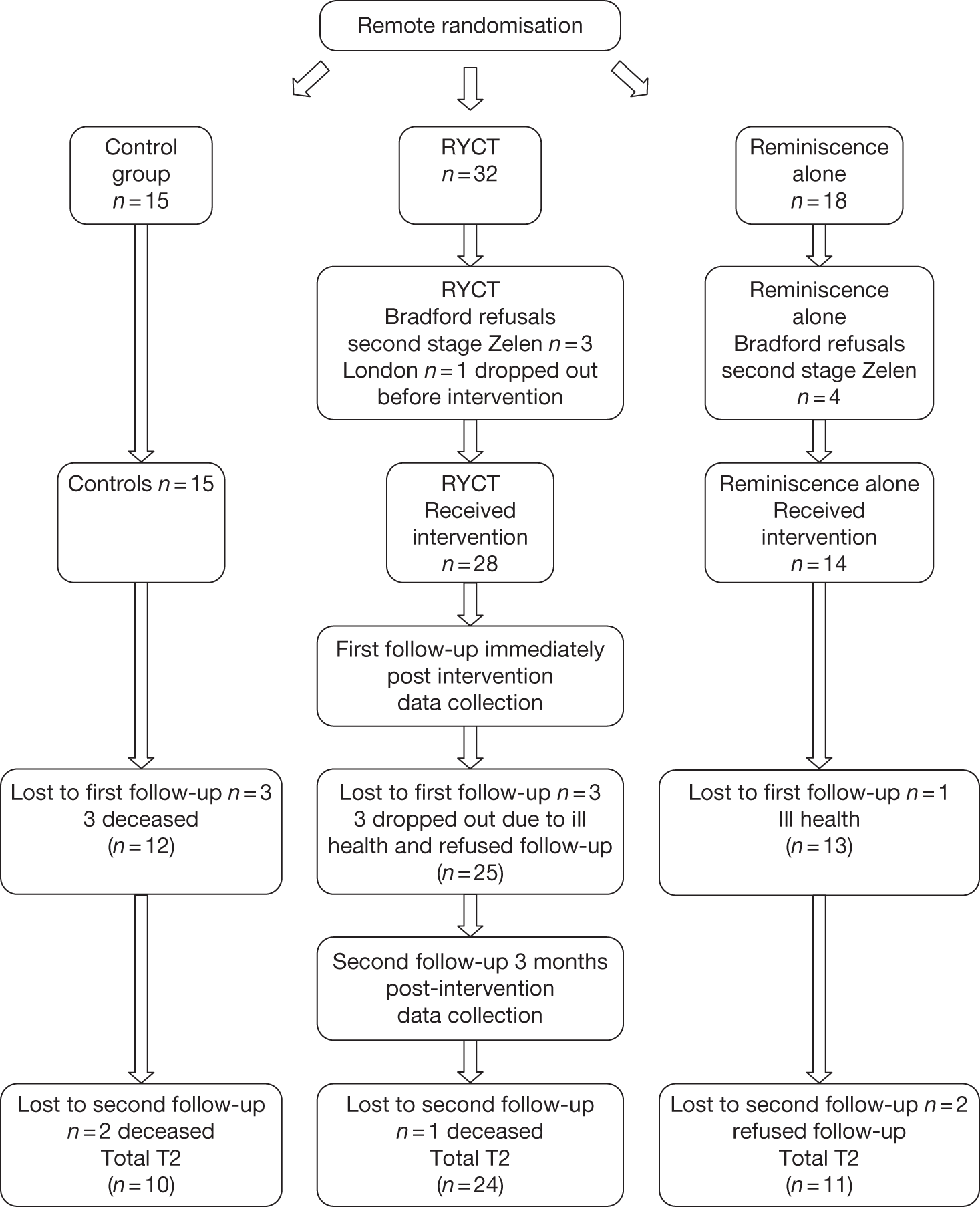
List of abbreviations
- AMI(E)
- autobiographical memory interview (extended version)
- ANCOVA
- analysis of covariance
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CONSORT
- Consolidated Standards of Reporting Trials
- CPN
- community psychiatric nurse
- CSDD
- Cornell Scale for Depression in Dementia
- CSRI
- Client Service Receipt Inventory
- CUA
- cost–utility analysis
- DeNDRoN
- Dementias and Neurodegenerative Disease Research Network
- DMEC
- Data Monitoring and Ethics Committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D
- European Quality of Life-5 Dimensions
- FU1
- first follow-up
- FU2
- second follow-up
- GDS
- Geriatric Depression Scale
- GHQ-28
- General Health Questionnaire-28 item version
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- ICC
- intraclass correlation coefficient
- ITT
- intention to treat
- LREC
- Local Research Ethics Committee
- MRC
- Medical Research Council
- MREC
- Multicentre Research Ethics Committee
- NICE
- National Institute for Health and Clinical Excellence
- NISCHR-CRC
- National Institute for Social Care and Health Research – Clinical Research Centre
- POVA
- Protection of Vulnerable Adults
- PwD
- person (people) with dementia
- QALY
- quality-adjusted life-year
- QCPR
- Quality of Caregiver/Patient Relationship
- QoL-AD
- quality of life in Alzheimer’s disease
- R&D
- research and development
- RAID
- Rating Anxiety in Dementia
- RCT
- randomised controlled trial
- RSS
- Relatives’ Stress Scale
- RYCT
- Remembering Yesterday, Caring Today
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health