Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/84/01. The contractual start date was in January 2008. The draft report began editorial review in February 2011 and was accepted for publication in May 2012. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Czoski-Murray et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The pre-operative preparation of patients undergoing any surgery involves a multidisciplinary approach. The surgical team assess the appropriateness of the surgery and the anaesthetists assess the patient’s fitness for surgery. The development of pre-operative assessment clinics in the last 20 years has seen nursing staff take a key role in the assessment preparation of patients for surgery. Protocols were developed and implemented locally to facilitate the patient care pathway in an environment where skill mix within teams was evolving.
In 2003 the National Institute for Health and Clinical Excellence (NICE) published Clinical Guideline 3 (CG3), which reviewed the use of routine pre-operative tests prior to routine surgery. 1 Prior to the guideline preparation, a systematic review was undertaken by Munro et al. 2 on behalf of the Health Technology Assessment (HTA) programme in 1997. The guideline development group undertook its own review of the literature. These two reviews defined and updated the purpose of pre-operative testing of apparently healthy patients.
Of the evidence base used to produce the guideline, > 50% was graded as amber (i.e. the benefit of the test was unknown). Therefore, despite the existence of some primary research, the evidence on which to base pre-operative testing protocols was inconclusive. Alongside this there has been an increasing awareness of the possibility of subjecting patients to unnecessary tests, and of the issues involved in dealing with the results of tests that may alarm patients but have little clinical significance.
The OPCECK study, undertaken in 19993 by members of this research team, suggested that it was somewhat difficult to attribute pre-operative examination and testing to perioperative and post-operative outcomes for patients. This study was designed to evaluate the performance of appropriately trained nurses and house officers in pre-operative assessment. Both the nurses and doctors were assessed by a clinical fellow in anaesthesia in the areas of clinical examination, history taking and ordering of appropriate tests for the patient. The nurses adhered to test ordering by following local protocols and thus performed far better at this task than junior doctors, who overinvestigated. The outcomes of interest in this study were the correct assessment of the patient, overassessment, underassessment not affecting management and underassessment possibly affecting management. The last was the primary outcome. Patients were followed up to establish if admission and surgery had proceeded as planned. Cancellation of surgery after the assessment clinic for any reason was noted as was the ordering of any additional tests by the surgeon or anaesthetist on admission.
It was difficult in this study to establish the link between patient outcomes and the quality of their pre-operative assessment. Test results were often outwith normal limits but the surgery went ahead anyway as the anaesthetist used his or her clinical judgement to assess the risks to the patient. Linking any change in clinical management resulting from a biochemistry test carried out pre-operatively was problematic.
The NICE review of the evidence that produced the guidelines identified change in clinical management as a result of a pre-operative biochemistry test as an outcome measure. 1 They found that, although some studies reported this as an outcome, they did not refine this further than delay, cancellation and alteration of treatment. Nor did this include any further explanation of what the change comprised or its impact on patients. 1
Answering these questions is central to understanding the need for and the impact of pre-operative biochemistry testing on patients who meet the criteria within the guidelines.
Being able to demonstrate that a test was carried out or not carried out as per protocol has little relevance unless the outcomes for patients are improved by these actions. This could be either by avoiding an unnecessary test or by avoiding complications by actions taken on the results of an abnormal test.
Of the 3 million or so surgical procedures carried out in the UK every year, a significant number of patients will be in the category of interest to this review. Although many of these tests are individually cheap, the NHS spends literally millions of pounds each year on tests.
Previous reviews4,5 were wide-ranging and included a wide range of patients, surgical procedures and tests. By contrast, this review is highly focused in terms of the tests, patients and surgeries under consideration. These are defined below.
The aims of this study
The aims of this study were to:
-
undertake a systematic review of the literature of the clinical effectiveness of routine testing of full blood count (FBC), electrolytes and renal function [urea and electrolytes test (U&E)] and pulmonary function [pulmonary function test (PFT)] as part of the pre-operative assessment procedures for patients classified as American Society of Anaesthesiologists (ASA) grades 1 and 2 who are undergoing minor or intermediate procedures
-
evaluate the cost-effectiveness of mandating or withdrawing each of these tests from routine pre-operative assessment for patients ASA grades 1 and 2 and minor and intermediate surgery
-
compare the evidence with the recommendations in the NICE CG3 and observed practice in NHS hospitals
-
identify using modelling techniques the expected value of information (EVI) whether or not there is value in the NHS in commissioning further primary research into the routine use of FBC, U&Es and PFTs in this patient population.
The patient group
The patient group to be considered in this review is those classified as ASA grades 1 and 2 undergoing minor or intermediate surgery. ASA produced guidelines for the classification from one to four according to their health status, comorbidities and, therefore, anaesthetic risk.
The patient group was limited to those undergoing minor or intermediate surgery as defined in the NICE guideline as, for example:
-
minor (grade 1): excision of lesion of skin, drainage of breast abscess, etc.
-
intermediate (grade 2): primary repair of inguinal hernia, excision of varicose veins of leg, tonsillectomy, knee arthroscopy.
Other minor and intermediate surgical procedures were included in the review and the detailed classification was obtained from Clinical Classification and Schedule Development Group (CCSD) Schedule of Procedures, 2005. 6
The tests
The tests defined in the review are FBC undertaken for:
-
known or suspected anaemia
-
symptomatic cardiovascular or pulmonary disease
-
condition-causing pre-operative blood loss
-
bleeding/bruising disease or history of bleeding/bruising disease
-
blood disorder (e.g. sickle cell disease, thalassaemia)
-
anticoagulant drugs
-
chronic disease (e.g. rheumatoid, renal disease). 1
Urea and electrolytes test (electrolyte, creatinine) for:
-
diabetes
-
renal disease
-
patients taking digoxin, diuretics, steroids, lithum. 1
Pulmonary function testing for:
-
spirometry
-
measurement of respiratory mechanics
-
measurement of transfer function
-
exercise testing
-
blood gas analysis. 1
Comorbidities
This review concentrates on the common comorbidities of cardiovascular, renal and respiratory disease. The scope of the review explicitly excludes diabetes.
Outcomes
The outcomes of interest from the literature were:
-
clinical benefit and costs of the tests (primary outcome)
-
the chances of finding an abnormal result
-
length of stay post-operatively
-
post-operative complication rates
-
number of operations cancelled due to abnormal test results on the day of operation.
The purpose of routine testing
The main purpose of pre-operative investigation is to provide additional diagnostic and prognostic information to supplement the clinical history of a patient with the aim of:
-
providing information that may confirm or question the correctness of the current course of clinical management
-
using this information to reduce the possible harm or increase the benefit to patients by altering their clinical management if necessary
-
using this information to assess the risk to the patient and opening up the possibility of discussing potential increases of risk with the patient
-
predicting post-operative complications
-
establishing a baseline measurement for later reference (to refer back to post-operatively); and
-
carrying out opportunistic screening that is unrelated to the surgery. 1
The routine testing of these patients would aim to identify, for example, unexpected anaemia or electrolytes and pulmonary function abnormalities that could impact on their planned anaesthetic or surgical management. By definition, ASA grade 1 and grade 2 patients will have a low incidence of unheralded abnormal tests, and then only a small fraction of these abnormal tests will lead to a measurable change in care. A proportion of the tests will not indicate any disease process, but will simply reflect the outliers in the normal population. The low incidence of abnormal tests makes identification of benefit using a conventional randomised controlled trial (RCT) very difficult.
National Institute for Health and Clinical Excellence guidance
The NICE guideline group set out the best available evidence for undertaking tests and for when these tests would not be necessary. The published evidence was supplemented by additional consensus work with clinical experts. The guideline concluded that there is no evidence to justify the practice of routinely testing patients aged < 50 years who do not present with comorbidities. Only investigations clinically indicated should be carried out.
Abacus survey
The National Institute for Health and Clinical Excellence commissioned Abacus to carry out a survey auditing the implementation of CG3 in 2005. 7 The focus of the survey commissioned by NICE was on the uptake of the guideline and the opinions of the respondents on the usefulness of the guideline, its impact on clinical practice and measure established to undertake internal audit.
We repeated this survey in 2008 with an emphasis on the tests of interest and ASA grade 1 and 2 patients.
How this study has changed from protocol
The paucity of published literature which could be linked to the specific tests and patient group made the building of a cost-effectiveness model problematic. We had proposed undertaking expert elicitation for some model parameters as we expected deficiencies in the evidence base. However, to populate the proposed model would have entailed undertaking expert elicitation for the majority of parameters, including those concerning clinical effectiveness and test performance. The degree of uncertainty that would result from such an undertaking would render such a model unworkable. After extensive discussion within the research team and consultation with external experts, we explored alternative avenues for estimating the clinical effectiveness and cost-effectiveness of routine pre-operative tests. We undertook econometric analyses of routine pre-operative test data held at the Leeds Teaching Hospitals Trust, linked to Hospital Episode Statistics data on outcomes, to estimate the impact of the use of these tests on outcomes.
The econometric work showed that EVI modelling to estimate the cost to the NHS of undertaking further primary research into the value of these tests was not relevant.
Chapter 2 Clinical effectiveness
Methods for reviewing clinical effectiveness
Identification of studies
A comprehensive literature search was performed in March to April 2008. Searches were designed to retrieve studies which evaluated the clinical effectiveness of routine pre-operative testing of FBC, electrolytes and renal function (U&E) and pulmonary function (PFT) in adult patients classified as ASA grades 1 and 2 undergoing elective minor (grade 1) or intermediate (grade 2) surgical procedures.
In addition, relevant citations from retrieved papers were followed up.
Sources searched
The following electronic bibliographic databases were searched:
-
BIOSIS
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
EMBASE
-
MEDLINE
-
MEDLINE In-Process & Other Non-Indexed Citations
-
NHS Database of Abstracts of Reviews of Effects (DARE)
-
NHS HTA Database
-
Science Citation Index (SCI).
To identify grey and unpublished literature, the Controlled Clinical Trials database, National Research Register Archive, National Institute for Health Research Clinical Research Network Portfolio database and the Copernic Meta-search Engine were searched.
In an attempt to identify the consequences of not undertaking routine testing, or of false-positive or false-negative test results, further searches were undertaken in June 2008 to retrieve papers which published data on intra- or post-operative adverse events occurring in relevant patients together with information on their test status and/or results.
As few relevant papers were identified by these searches, additional searches were undertaken in April and May 2009 to retrieve papers including information relating to adverse effects associated with commonly used anaesthetics in relation to the patients’ test status. In addition to the databases listed above, the following sources were also searched:
-
US Food and Drug Administration
-
British National Formulary
-
HTA agencies
-
drug companies manufacturing the anaesthetic.
Search strategies
The MEDLINE search strategies are presented in Appendix 1. The MEDLINE strategies were adapted for use in the other databases, and these search strategies are available on request.
Search restrictions
The searches were not restricted by date or language.
Inclusion and exclusion criteria
Inclusion criteria
Population
-
Adult patients classified as ASA grade 1 or 2 undergoing minor (grade 1) or intermediate (grade 2) surgery (including elective general surgery, day surgery and minor orthopaedic procedures) as classified by the CCSD Schedule of Procedures, 2005. 6 It was intended, where possible, to subdivide these into the following subgroups:
-
– apparently healthy patients with no clinical indication for testing FBC, electrolytes and renal function and pulmonary function
-
– patients with common comorbidities (e.g. respiratory disease, renal disease)
-
– patients receiving treatments likely to alter test results (e.g. diuretics).
-
It was originally planned to limit the population to adults aged 16–60 years. However, because of the paucity of relevant studies which met this inclusion criterion, the population was later extended to include all adult patients.
Intervention
-
Routine pre-operative testing of:
-
– FBC [including haemoglobin (Hb) concentration, haematocrit, platelet count and white blood cell count]
-
– electrolytes and renal function (U&E) (including sodium, potassium, urea and creatinine)
-
– pulmonary function test (PFT) (including some or all of spirometry, blood gas analysis, measurement of respiratory mechanics, measurement of transfer function and exercise testing of respiratory system).
-
Comparator
-
No routine pre-operative testing.
Outcomes
-
Abnormal test results.
-
Changes in management following abnormal test results in patients whose pre-operative clinical examinations were normal.
-
Adverse events possibly related to the test result.
-
Adverse events probably or possibly caused by the process of testing.
-
All-cause mortality.
Setting
-
Any country.
Date
-
1980 onwards.
Study type
-
RCTs.
-
Controlled non-randomised studies (e.g. cohort studies).
-
Case–control studies.
-
Case series.
-
Case reports.
-
Systematic reviews.
-
Economic evaluations.
Exclusion criteria
The following publication types were excluded from the review:
-
animal models
-
narrative reviews, editorials and opinions.
Systematic reviews of primary studies were also excluded from the review, but were read in case they led to the identification of additional relevant trials.
In addition, studies were excluded if they were considered methodologically unsound, did not report results in sufficient detail, or reported the use of a package of pre-operative tests from which it was not possible to distinguish the interventions studied in this review.
Sifting
The references identified by the electronic literature searches were sifted in three stages. They were screened for relevance first by title and then by abstract. Those papers which seemed from their abstracts to be relevant were then read in full, as were those for which abstracts were not available. At each step, studies which did not satisfy the inclusion criteria were excluded.
Data extraction strategy
A customised data extraction form based on that proposed by the NHS Centre for Reviews and Dissemination (CRD) was used. 8 Where possible, data were extracted by one reviewer and thoroughly checked by a second reviewer; any disagreements were resolved by discussion. However, with the exception of a study in Hebrew9 for which a translation was obtained, data from studies which were published in a language other than English were extracted by a single reviewer.
Where available, data relating to the following outcomes were extracted:
-
all-cause mortality
-
significant abnormal test findings
-
change of management
-
length of hospital stay
-
adverse effects probably or possibly related to the test result
-
adverse events probably or possibly caused by the process of testing.
Quality assessment strategy
It was proposed to use criteria based on those proposed by the NHS CRD8 (see Appendix 2) to assess the methodological quality of randomised trials which met the inclusion criteria.
It was proposed to assess the methodological and reporting quality of case series studies which met the inclusion criteria using a customised quality tool that combined generic criteria proposed by the NHS CRD8 and Chambers et al. 10 with review-specific criteria, as follows:
Generic criteria:
-
Were patients recruited prospectively?
-
Were patients recruited consecutively?
-
Were at least 90% of those included at baseline followed up (prospective studies only)?
-
Was loss to follow-up reported or explained (prospective studies only)?
-
Was follow-up long enough for important events to occur?
-
Were outcomes assessed using objective criteria or was blinding used?
-
Was an appropriate measure of variability reported?
Review-specific criteria:
-
Were the patients’ ages and ASA statuses adequately reported?
-
Was the operation type and/or risk classification adequately reported?
-
Were all operations elective?
-
Were all the tests conducted genuinely routine, or might some have been indicated?
-
Was a definition of normal or abnormal results provided?
Meta-analysis strategy
It was intended that, where appropriate, meta-analysis would be used to pool results, summary statistics would be derived for each study and a weighted average of the summary statistics would be computed across the studies. In the event, this was not possible because of the diversity of outcome measures used in the different studies.
The statistical calculations were performed using the following software packages:
-
Proportions and confidence intervals (CIs) – the confidence interval for proportion calculator produced by Dimension Research (Dimensions Research & Marketing Consultancy, Sharjah, United Arab Emirates).
-
The CIs around absolute risk changes – GraphPad software (GraphPad Software Inc., CA, USA).
Results
Quantity and quality of research available
Number of studies of clinical efficacy identified
The electronic literature searches identified 11,953 potentially relevant articles. Of these, four articles related to four studies9,11–13 which met the review’s inclusion criteria (Figure 1).
FIGURE 1.
Clinical effectiveness: summary of study selection and exclusion (electronic literature searches).
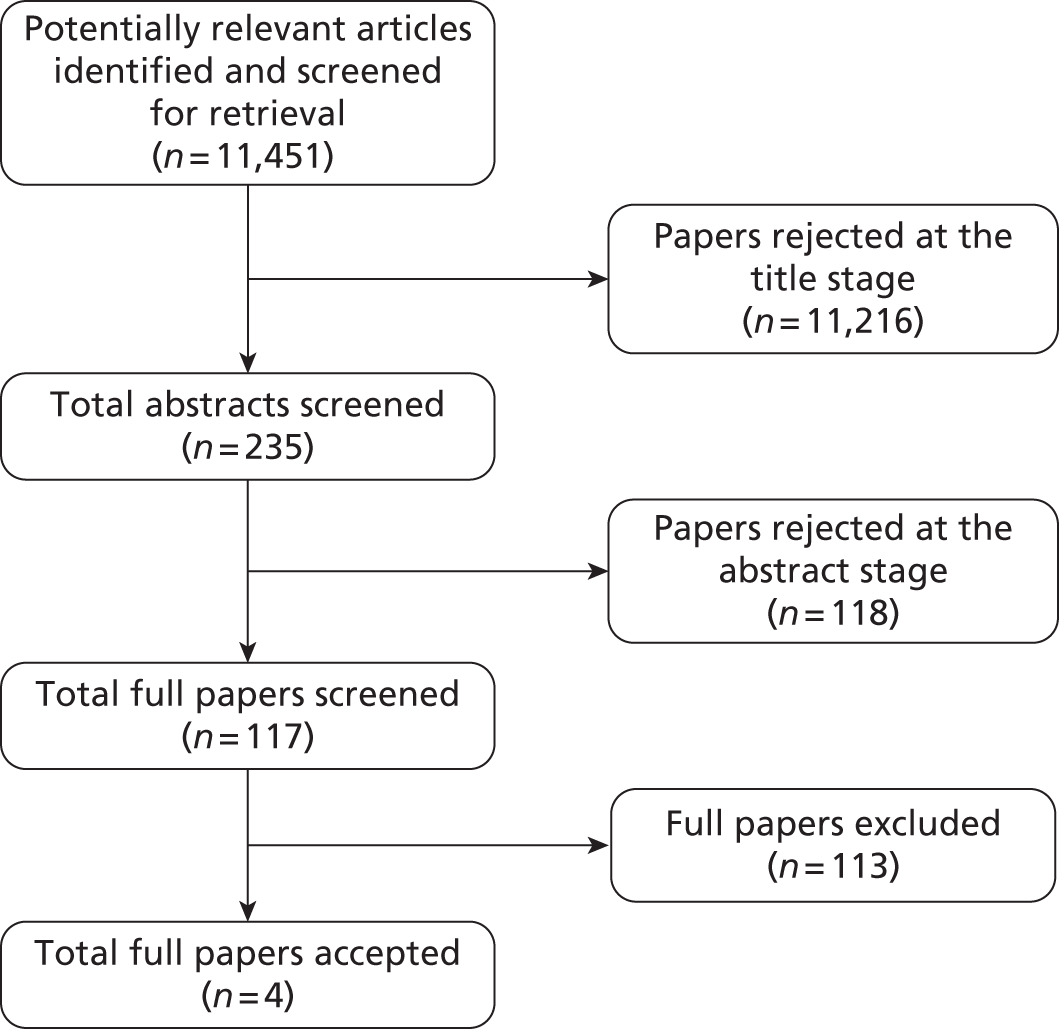
Two additional relevant studies, by Roukema et al. 14 and Turnbull and Buck,15 were identified only from citations.
Number and type of studies included
Six studies9,11–15 met the inclusion criteria for the review of clinical effectiveness; none was a RCT of pre-operative testing. A pseudo-randomised trial by Roukema et al. 14 used year of birth to allocate patients to treatment groups; it studied the effectiveness of pre- and post-operative breathing exercises in preventing pulmonary complications after upper abdominal surgery. However, because all participants underwent pre-operative PFTs, data from the control group could be utilised in the current review as a prospective case series examining the ability of such testing to predict pulmonary complications (see Quantity of research available). The remaining five studies were designed as prospective or retrospective case series. 9,11–13,15
Number and type of studies excluded, with reasons
As may be seen from Number of studies of clinical efficacy identified, a substantial number of the references identified by the electronic searches related to studies which did not meet the review’s inclusion criteria, and which were therefore excluded during the sifting process. Details are therefore given only of those references which:
-
appeared potentially relevant, but could not be obtained
-
were excluded after a full reading, if the reason for exclusion is potentially not readily apparent from the full text; or
-
might appear from their titles to be particularly pertinent to the subject of the review.
Such references are listed in Appendix 4, together with the reasons for their exclusion.
Quantity and quality of research available
Quantity of research available
As noted above, six studies were identified which reported results relating to one or more of the three tests in adult patients in ASA grades 1 and 2 (Table 1). 9,11–15 Although Gnocchi et al. 11 included in their study patients in ASA grades 1–3, routine testing was performed only in ASA grade 1 patients (tests for patients in ASA grades 2 and 3 were requested according to the conditions identified by, or suspected from, the clinical history and examination). Consequently, only the results relating to ASA grade 1 patients are relevant to, and included in, this review. Roukema et al. 14 and Turnbull and Buck15 did not specify the ASA status of their patients, but described them in terms which strongly suggest that they would appropriately have been categorised as ASA grade 1 or 2; these studies have therefore been included.
| Study | Country | Study design | Date of operation | All tests routine | Relevant tests | Number of patients | Age range (mean) (years) | ASA status | Operation type (risk classification) |
|---|---|---|---|---|---|---|---|---|---|
| Gnocchi et al. 200011 | Argentina | Prospective case series | 1 September 1995 to 30 April 1998 | Yes |
Aged 16–59 years: haemogram Aged ≥ 60 years: haemogram, plasma creatinine |
214 |
≥ 16 (ASA grade 1 47.8 ± 19.6; ASA grade 2 61.0 ± 16.5) |
1 |
Abdominal surgery including hernia repair, laparoscopic cholecystectomy and benign proctological surgery (2 or 3) |
| Haug and Reifeis 199913 | USA | Prospective case series | 1 February 1994 to 30 November 1994 | Yes | FBC, Hb, haematocrit, platelet count, mean corpuscular Hb, mean corpuscular volume, lymphocyte count, mean corpuscular Hb concentration |
458 enrolled (GA: 281; i.v. sedation: 177) 380 returned for scheduled procedure (GA: 235; i.v. sedation: 145) |
15–54 (23.4) |
1 or well-controlled 2 |
Dental surgery under general anaesthetic or i.v. sedation (Not specified) |
| Roukema et al. 198814 | Netherlands | Pseudo-RCT/prospective case series | No data | Yes | PFT (vital capacity and FEV1) | 84 in control group | ≤ 70; implicitly limited to adults | Not specified; patients described as ‘without pre-operative risk factors’ |
Upper abdominal surgery for benign biliary disease or duodenal ulcer (Not specified) |
| Szmuk et al. 20029 | Israel | Retrospective case series | No data | Yes | Determined by the surgeon; included blood count, urea nitrogen, electrolytes | 300 | 18–40 | 1 |
Unspecified (‘Minor’) |
| Tallo et al. 200712 | Brazil | Retrospective case series | January 2005 to December 2005 | Not clear | Hb, haematocrit, serum sodium, serum potassium, creatinine | 1254 |
Implicitly adult (ASA grades 1–3 68.1 ± 11.6) |
1–3; individual patient data provided for all patients suffering adverse events |
Cataract surgery (2) |
| Turnbull and Buck 198715 | Canada | Retrospective case series | 1973–84 | Not clear | Any screening tests received by the patient, including complete blood cell count, sodium, potassium, creatinine, urea | 1010 | Implicitly adult | Not specified; patients described as ‘otherwise healthy’ (i.e. apart from the need for cholecystectomy) and as having no other ‘active or ongoing disease on admission to hospital’ |
Cholecystectomy (Not specified) |
The studies related to three surgical specialties:
-
general surgery (including cholecystectomy,15 abdominal surgery,11 upper abdominal surgery14 and unspecified ‘minor’ surgery9)
-
ophthalmology (specifically cataract surgery12)
Five studies assessed the use of both FBCs and U&Es. 9,11–13,15 Only one study, that by Roukema et al. ,14 assessed the use of routine PFTs. The paucity of data relating to routine PFTs reflects the fact that this test is seldom routinely performed in asymptomatic patients; thus, Turnbull and Buck’s retrospective review15 of records relating to 1010 patients found that only three PFTs were performed, in each case in a patient whose history or physical examination had suggested some abnormality of pulmonary function.
Only one study, that by Szmuk et al.,9 specifically met the original criterion that all patients should fall within the 16–60 years age group (see Table 1). Of the remainder, the studies by Roukema et al. ,14 Tallo et al. 12 and Turnbull and Buck15 did not explicitly state that the study population was limited to adults; however, these studies have been retained as there is no indication that they included children.
As noted in Number and type of studies included in this review, data from the control group of Roukema et al. ’s pseudo-RCT14 of pre- and post-operative breathing exercises in preventing pulmonary complications after upper abdominal surgery are utilised as a prospective case series examining the ability of routine pre-operative PFTs to predict pulmonary complications.
The remaining studies were designed as case series. Two of these were prospective:
-
Gnocchi et al. 11 studied all ASA grade 1–3 patients, aged ≥ 16 years who were scheduled for elective abdominal surgery classified as grade 2 (low risk) or 3 (moderate risk) by the Johns Hopkins Risk Classification System in one hospital in Argentina between 1 September 1995 and 30 April 1998. As noted above, routine testing was undertaken only in patients in ASA grade 1.
-
Haug and Reifeis13 included all ASA grade 1 or 2 patients aged 15–54 years undergoing dental surgery under general anaesthesia or intravenous sedation in one American oral and maxillofacial surgery clinic between 1 February and 30 November 1994.
The remaining three case series were retrospective record reviews:
-
Szmuk et al. 9 reviewed the records of 300 ASA grade 1 patients aged 18–40 years who had undergone minor elective operations (most commonly hernia repair) in an Israeli public hospital at an unspecified point in time.
-
Tallo et al. 12 reviewed the records of 1254 patients who had undergone cataract surgery in a single hospital in Brazil between January and December 2005.
-
Turnbull and Buck15 reviewed the records of 1010 otherwise healthy individuals who had undergone cholecystectomy in two Canadian teaching hospitals between 1973 and 1984.
Quality of research available
Quality assessment using the customised tool described in Quality assessment strategy suggested that the prospective studies were of higher quality than the retrospective record reviews (for details see Appendix 5). However, in some instances this may reflect reporting quality rather than the quality of the study design. So, for example, Szmuk et al. 9 and Turnbull and Buck15 do not specify that the records that they reviewed were those of consecutive patients who met the study’s inclusion criteria, although this seems probable. Furthermore, Turnbull and Buck15 do not make it entirely clear whether or not all the operations were elective. Moreover, as they talk throughout about the number of tests performed, rather than the number of patients tested, it is not wholly clear that each test was performed only once in each patient, nor is it specified that the blood counts and multiphasic screening tests (which included tests for urea and electrolytes) were routinely performed, although this seems likely since, if each test was performed only once in each patient, > 98% of patients would have undergone these tests. Turnbull and Buck15 also failed to provide definitions of normal or abnormal results. Tallo et al. 12 also failed to specify whether or not the tests they reported were routine although, again, this seems probable; the very high proportion of patients with at least one abnormal test result suggests that most, if not all, underwent testing (see Full blood counts).
Several studies may be biased because of attrition. In the study by Gnocchi et al. ,11 777 patients in ASA grades 1–3 attended an Argentine hospital for pre-operative evaluation, but only 507 (62.3%) returned for surgery; the primary reason why the remaining 270 did not do so was lack of insurance cover for medical expenses. As noted above, Gnocchi et al. 11 undertook routine testing only in patients in ASA grade 1, but it is not clear how many of the original 777 patients were assessed as being in grade 1 because test results are presented only for the 214 grade 1 patients who returned to the hospital for a second interview, of whom 210 (98.1%) were deemed to be fit for surgery, but only 139 (66.2%) actually underwent the operation for which they were scheduled; again, the main reason why the remainder did not appeared to be lack of cover for medical expenses. No details were given of the health status of those patients who dropped out at either point in the study compared with those who underwent their scheduled operation, and therefore the study incorporates the potential for systematic bias at both points. Although attrition was lower in Haug and Reifeis’ study13 of patients undergoing dental surgery in the USA, 78 of 458 patients (17%) failed to return on the appointed day. The remaining four studies9,12,14,15 are less explicit about the pathway from assessment to operation, and the degree of attrition involved; they may also contain a potential for bias, related to financial or other, unknown, factors.
Assessment of clinical effectiveness
Because the included studies had different aims, they did not all report the same data. It has therefore been necessary to summarise each study on its own terms before attempting to compare their findings.
Full blood counts
The prospective case series by Gnocchi et al. 11 assessed:
-
the prevalence of asymptomatic disease in ASA grade 1 patients
-
the frequency of diagnoses which led to the cancellation or postponement of surgery in such patients
-
the incidence of perioperative complications in those patients who underwent surgery.
As noted in Quality of research available, it is not clear how many ASA grade 1 patients originally entered the study; results are presented only for the 214 who returned to the hospital for a second interview. In addition, it is not clear how many of this 214 were scheduled for grade 2 operations, and how many for grade 3. The number of ASA grade 1 patients with abnormal test results was not reported, although three patients initially classified as ASA grade 1 were reclassified as ASA grade 2 as a result of a diagnosis of hypertension (a reconstruction of the apparent patient flow is represented diagrammatically in Appendix 6). Moreover, as published, the results relating to the cancellation or postponement of surgery appear potentially contradictory: on the one hand, four ASA grade 1 patients (1.9%) were said to have had their operations postponed as a consequence of routine testing, but, on the other hand, the authors claimed that no operation was postponed or cancelled because of an unknown disease and stated that, in asymptomatic (i.e. ASA grade 1) patients, routinely requested laboratory tests showed no benefit in terms of either anaesthetic management or the detection of pathologies. All four ASA grade 1 patients whose operations were postponed had severe asymptomatic anaemia (Hb < 8 mg/dl).
As noted above, Gnocchi et al. 11 found that only 139 of the 210 ASA grade 1 patients deemed fit for surgery (66.2%) actually underwent the operation for which they were scheduled; the reason why the remaining 71 patients did not do so appeared to be lack of cover for medical expenses rather than any medical reason. One hundred and thirty of the 139 who underwent surgery had grade 2 operations; none suffered intraoperative complications and, although four had post-operative complications, a rate of 3.08% (95% CI 0.11% to 6.05%), these complications were considered to be unrelated to the pre-operative tests (two patients had wound infections, one had a haemorrhage from the site of the surgical drain which stopped spontaneously without requiring a blood transfusion and one had a clinical lower limb deep-vein thrombosis). There were no intraoperative or post-operative deaths in ASA grade 1 patients undergoing grade 2 operations.
The other prospective case series, that by Haug and Reifeis,13 sought to determine whether or not routine laboratory testing affected clinicians’ pre-operative evaluation and clinical decision-making. Seven of the 380 patients who returned for their dental procedure had an abnormal test result, a rate of 1.8% (95% CI 0.5% to 3.2%): three had borderline low red blood cell counts, one had borderline low haematocrit, one had a borderline low white blood count and two patients being treated for dentoalveolar abscesses had elevated white blood cell counts. No planned procedures in these patients were postponed, and the authors concluded that the routine laboratory tests had little or no effect on the clinicians’ decision-making process.
Szmuk et al. 9 evaluated the clinical benefit and cost of routine screening. Only nine patients were found to have abnormal test results, a rate of 3.0% (95% CI 1.1% to 4.9%). All nine had light anaemia (11–12 g), which in each case was attributed to increased menstrual flow and was consonant with the case history or physical examination. No operations were cancelled or delayed as a consequence of the test results. Szmuk et al. 9 therefore suggested that blood counts should not be routinely undertaken before minor operations in healthy patients, but should be performed only when indicated by the patient’s age, gender, case history and the findings of the physical examination.
Tallo et al. 12 sought to determine whether or not pre-operative testing prevented pre- and post-operative adverse events in patients in ASA grades 1–3 undergoing cataract surgery. Seventy-five per cent of these patients had at least one recorded abnormal result on a range of tests, which included fasting blood glucose, electrocardiography (ECG) and chest radiography, as well as Hb, haematocrit, serum sodium, potassium and creatinine. However, only 1.3% had an adverse clinical event which was considered to be related to the anaesthesia or surgery (Table 2), and no relationship was observed between abnormal test results and adverse events (chi-squared p = 0.334). One hundred and eighty-one patients (14%) were referred to a specialist for pre-operative assessment, of whom 104 were asymptomatic (57.5%; 95% CI 50.3% to 64.7%). Only 20% of these asymptomatic patients underwent any clinical intervention as a consequence of the specialist assessment, compared with 86% of symptomatic patients (Table 3). The blood count result was abnormal in only 1 of the 13 patients in ASA grades 1 or 2 who had an adverse clinical event that was considered to be related to the anaesthesia or surgery (Table 4).
| Patient outcomes | Number | Rate | 95% CI |
|---|---|---|---|
| Patients with at least one abnormal test result | 936/1254 | 74.6% | 72.2% to 77.1% |
| Patients referred for specialist assessment (includes symptomatic and asymptomatic patients) | 181/1254 | 14.4% | 12.5% to 16.4% |
| Patients who had an adverse clinical event considered to be related to the anaesthesia or surgery | 16/1254 | 1.3% | 0.7% to 1.9% |
| Patients referred for specialist assessment | Clinical intervention consequent on specialist pre-operative assessment | |
|---|---|---|
| No | Yes | |
| Asymptomatic | 83/104 (79.8%, 95% CI 72.1% to 87.5%) | 21/104 (20.2%, 95% CI 12.5% to 27.9%) |
| Symptomatic | 11/77 (14.3%, 95% CI 6.5% to 22.1%) | 66/77 (85.7%, 95% CI 77.9% to 93.5%) |
| ASA class | Age | Sex | Comorbidities | Abnormal results on relevant tests | Referred for specialist assessment | Change of management | Clinical adverse event |
|---|---|---|---|---|---|---|---|
| 1 | 52 | F | None | No | No | No | Bradycardia |
| 1 | 76 | M | None | No | No | No | Hypertension |
| 2 | 45 | M | None | No | No | No | Hypertension |
| 2 | 59 | M | Diabetes mellitus | No | Yes (endocrinology – fasting blood glucose = 186) | Yes | Hyperglycaemia |
| 2 | 61 | M | None | No | No | No | Bradycardia |
| 2 | 62 | M | None | No | No | No | Bradycardia |
| 2 | 68 | F | Systemic arterial hypertension, diabetes mellitus | No | No | No | Hypertension |
| 2 | 70 | M | Systemic arterial hypertension | No | Cardiology | No | Cerebrovascular accident |
| 2 | 78 | M | Systemic arterial hypertension, diabetes mellitus, chronic renal insufficiency | Creatinine = 2.2 | No | No | Hypertension |
| 2 | 81 | F | Diabetes mellitus, hypothyroidism | No | Yes (because of results of ECG and chest radiography; specialty not recorded) | Yes | Bronchospasm |
| 2 | 81 | M | Systemic arterial hypertension | No | No | No | Bronchospasm |
| 2 | 82 | F | None | No | No | No | Acute MI |
| 2 | 85 | F | Systemic arterial hypertension | No | No | No | Bradycardia |
Turnbull and Buck15 sought to assess the clinical value of routine pre-operative screening in otherwise healthy patients undergoing cholecystectomy. They reported the number of patients with abnormal results, the number of patients with abnormal results who received clinical interventions consequent on those results and the number of patients who developed a complication relevant to a test – in other words, a complication of which the test was intended to predict an increased risk. Thus, perioperative hypotension or a post-operative Hb concentration < 10.0 g/dl were deemed to be complications relevant to low Hb. The complications relevant to low white blood cell counts were not specified and high white cell counts were not reported as abnormal because of the possibility that they were caused by the patient’s cholecystitis. 15
A total of 1005 complete blood cell counts were undertaken in 1010 patients, but only eight tests were reported as having abnormal results; assuming that each test was undertaken in a different patient, this indicates a rate of 0.8% (95% CI 0.3% to 1.4%). Seven patients had low Hb concentrations (the lowest being 9.9 g/dl); one had a low white cell count (3200/mm3). Action was taken only in relation to two of the patients with low Hb concentrations [assuming that each test was undertaken in a different patient, this represents a rate of 0.2% (95% CI –0.1% to 0.5%)]. These two patients received pre-operative blood transfusions; despite this, one developed a relevant complication, as did one of the five patients with low Hb who did not receive a transfusion. Rates of relevant complications were therefore substantially higher in patients with abnormal Hb (2/7, 28.6%; 95% CI –4.9% to 62.0%) than in those with normal Hb, 14 of whom had relevant complications [assuming that each test was undertaken in a different patient, the denominator is 998, representing a rate of 1.4% (95% CI 0.7% to 2.1%)]. The one patient with a low white blood cell count did not suffer a relevant complication, although such complications were noted in 110 patients with a normal white blood cell count [assuming that each test was undertaken in a different patient, the denominator is 1004, representing a rate of 11.0% (95% CI 9.0% to 12.9%)] (see Appendix 7, Table 29). One patient died as a result of a post-operative pulmonary embolus.
The evidence relating to the value of routine pre-operative FBCs for ASA grade 1 or 2 patients undergoing elective minor to intermediate surgery is limited in both quantity and quality, as it is derived from five observational studies: data are available for a total of 1982 patients in ASA grades 1–2 (or equivalent) from the studies by Gnocchi et al. ,11 Haug and Reifeis,13 Szmuk et al. 9 and Turnbull and Buck,15 and a further unspecified number from the study by Tallo et al. 12
As may be seen from the summary in Table 5, this limited evidence suggests that the proportion of patients with an abnormal result in any component of the full blood test is low (range 0.8–3.0%), and the proportion with both an abnormal test result and a consequent change in clinical management is lower (range 0–1.9%). No deaths were specifically reported in patients with abnormal test results; Turnbull and Buck15 reported that one patient died as a consequence of a post-operative pulmonary embolus, but did not state whether or not this patient had an abnormal result on any test.
| Study | Number of patients tested | Definition of abnormal result | Number of patients with abnormal results (%; 95% CI) | Number of patients with operation postponed or cancelled because of test result (%; 95% CI) | Number of patients with other change in management because of test result (%) | Number of patients with abnormal test result and related adverse event (%) | Number of deaths in patients with abnormal test result (%) |
|---|---|---|---|---|---|---|---|
| Any component of FBC | |||||||
| Gnocchi et al. 200011 | 214 | N/A | Not reported | 4/214 (1.9%; 0.1% to 3.7%) | 0 | 0 | 0 |
| Haug and Reifeis 199913 | 380 | N/A | 7/380 (1.8%; 0.5% to 3.2%) | 0 | 0 | Not reported; implicitly none | Not reported; implicitly none |
| Szmuk et al. 20029 | 300 | N/A | 9 (3.0%; 1.1% to 4.9%)a | 0 | Not reported | Not reported; implicitly none | Not reported; implicitly none |
| Tallo et al. 200712 | Not clear | N/A | Not reported | Not reported | Not reported | 0 | 0 |
| Turnbull and Buck 198715 | 1005b | N/A | 8c (0.8%; 0.3% to 1.4%) | 0 | 2d (0.2%; –0.1% to 0.5%) | 0 | 0 |
| Individual components of FBC | |||||||
| Hb | |||||||
| Gnocchi et al. 200011 | 214 | < 8 g/dl | Not reported | 4/214 (1.9%; 0.1% to 3.7%) | 0 | 0 | 0 |
| Haug and Reifeis 199913 | 380 | Not specified | 3/380 (0.8%; –0.1 to 1.7%) | 0 | 0 | Not reported; implicitly none | Not reported; implicitly none |
| Szmuk et al. 20029 | Records of 300 patients examined. Not clear how many underwent each test | < 12 g/dl | 9 (3.0%; 1.1% to 4.9%)a | 0 | Not reported | Not reported; implicitly none | Not reported; implicitly none |
| Tallo et al. 200712 | Not clear | Men < 14 mg/dl; women < 12 mg/dl | Not reported | Not reported | Not reported | 0 | 0 |
| Turnbull and Buck 198715 | 1005b | Implicitly < 10.0 g/dl | 7c (0.7%; 0.2% to 1.2%) | 0 | 2d (0.2%; –0.1% to 0.5%) | 0 | 0 |
| Haematocrit | |||||||
| Haug and Reifeis 199913 | 380 | Not specified | 1/380 (0.3%; –0.3% to 0.8%) | 0 | 0 | Not reported; implicitly none | Not reported; implicitly none |
| Tallo et al. 200712 | Not clear | Not specified | Not reported | Not reported | Not reported | 0 | 0 |
| Platelet count | |||||||
| Turnbull and Buck 198715 | 1005b | Abnormal according to ‘generally accepted laboratory standards’ | 0 | N/A | N/A | N/A | N/A |
| White blood cell count | |||||||
| Haug and Reifeis 199913 | 380 | Not specified | 3/380e (0.8%; –0.1% to 1.7%) | 0 | 0 | Not reported; implicitly none | Not reported; implicitly none |
| Turnbull and Buck 198715 | 1005b | Abnormal according to ‘generally accepted laboratory standards’ | 1 (0.1%; –0.1% to 1.3%) | 0 | 1 (0.1%; –0.1% to 1.3%) | 0 | 0 |
Electrolytes and renal function (U&Es)
Four studies9,11,12,15 evaluated the use of tests for electrolytes and renal function. Three of these9,12,15 assessed such tests in all patients included in the study. However, Gnocchi et al. 11 limited routine testing for creatinine to the unspecified number of ASA grade 1 patients in their study who were aged ≥ 60 years; no abnormal results were found in this group.
In the study by Szmuk et al. ,9 no patients were said to have abnormal sodium, potassium or creatinine levels. Two patients were found to have slightly high urea nitrogen levels (45–48 mg); these were attributed to mild dehydration which, in both cases, was consonant with the case history or physical examination. As both patients had creatinine levels which were considered normal, with no evidence of any kidney damage, their operations were not cancelled or postponed as a consequence of the abnormal urea nitrogen results.
In the study by Tallo et al. ,12 only one patient in ASA grade 1 or 2 suffered a relevant adverse clinical event and had an abnormal U&E result. This was a 78-year-old male with hypertension, diabetes mellitus and chronic renal insufficiency; he had an abnormal creatinine result which had not triggered a specialist referral or a change of clinical management (Table 6).
| Study | Number of patients tested | Definition of abnormal result | Number of patients with abnormal results (%; 95% CI) | Number of patients with operation postponed or cancelled because of test result (%; 95% CI) | Number of patients with other change in management because of test result (%) | Number of patients with abnormal test result and related adverse event (%) | Number of deaths in patients with abnormal test result (%) |
|---|---|---|---|---|---|---|---|
| Any component of U&E | |||||||
| Gnocchi et al. 200011 | Not cleara | Test was for creatinine only; no definition of abnormal result given | 0 | N/A | N/A | N/A | 0 |
| Szmuk et al. 20029 | 300b | N/A | 2 (0.7%; –0.3% to 1.6%) | 0 | Not reported | Not reported; implicitly none | Not reported; implicitly none |
| Tallo et al. 200712 | Not clear | N/A | Not reported | Not reported | Not reported | 1c | 0 |
| Turnbull and Buck 198715 | 995d | N/A | Not reported | Not reported | Not reported | Not reported | 0 |
| Individual components of U&E | |||||||
| Sodium | |||||||
| Tallo et al. 200712 | Not clear | < 135 or > 145 mmol/l | Not reported | Not reported | Not reported | 0 | 0 |
| Turnbull and Buck 198715 | 995d | Abnormal according to ‘generally accepted laboratory standards’ | 5 (0.5%; 0.06% to 0.9%) | 0 | 0 | Not reported | 0 |
| Potassium | |||||||
| Tallo et al. 200712 | Not clear | < 3.2 or > 5.0 mmol/l | Not reported | Not reported | Not reported | 0 | 0 |
| Turnbull and Buck 198715 | 995d | Abnormal according to ‘generally accepted laboratory standards’ | 14 (1.4%; 0.7% to 2.1%) | Not reported | 4/995e (0.4%; 0.01% to 0.8%) | 1/995f (0.1%; –0.1% to 0.3%) | 0 |
| Urea nitrogen | |||||||
| Szmuk et al. 20029 | 300a | < 45 mg | 2 (0. 7%; –0.3% to 1.6%) | 0 | Not reported | Implicitly none | Implicitly none |
| Turnbull and Buck 198715 | 995d | Abnormal according to ‘generally accepted laboratory standards’ | 1 (0.1%; –0.1% to 0.3%) | 0 | 0 | 0 | 0 |
| Creatinine | |||||||
| Gnocchi et al. 200011 | Not clearb | None given | 0 | N/A | N/A | N/A | 0 |
| Tallo et al. 200712 | Not clear | > 1.0 mg/dl | Not reported | Not reported | Not reported | 1c | 0 |
| Turnbull and Buck 198715 | 995d | Abnormal according to ‘generally accepted laboratory standards’ | 2 (0.2%; –0.1% to 0.5%) | 0 | 0 | 0 | 0 |
Turnbull and Buck15 reported that 995 multiphasic screening tests (Sequential Multiple Analysis-12) were undertaken in 1010 patients (Table 7). Although 14 patients were said to have abnormally low potassium levels, the definition of ‘abnormal’ is not provided and only three were said to be outside ‘the traditionally accepted surgical and anaesthetic limits of 3.2 to 5.8 mEq/l (3.2 to 5.8 mmol/dl)’; the lowest value was 3.1 mmol/l. Four of the 14 patients received pre-operative supplementation with potassium; despite this, one of the four suffered post-operative hypokalaemia. None of the patients with low potassium suffered a cardiac complication. Two patients had clinically significantly elevated creatinine (1.8 and 3.2 mg/dl), but no consequent modification of surgical or anaesthetic management was recorded, and there were no relevant complications. Five tests showed abnormal sodium results and one patient had an abnormal urea level, but these abnormalities were said not to be clinically significant. For details, see Appendix 7, Table 30.
| Complication | Number (%; 95% CI) |
|---|---|
| Diaphoresis, near syncope | 105/4050 (2.6%; 2.1% to 3.1%) |
| Syncope | 24/4050 (0.6%; 0.4% to 0.8%) |
| Convulsive syncope | 6/4050 (0.1%; 0.03% to 0.3%) |
| Ventricular tachycardia | 1/4050 (0.02%; 0% to 0.1%) |
| Total | 136/4050 (3.4%; 2.8% to 3.9%) |
The evidence relating to the value of routine U&Es for ASA grade 1 or 2 patients undergoing elective minor to intermediate surgery is limited in both quantity and quality, being derived from only four observational studies: data are available for a total of 1310 patients in ASA grade 1–2 (or equivalent) from the studies by Szmuk et al. 9 and Turnbull and Buck,15 and a further unspecified number from the studies by Gnocchi et al. 11 and Tallo et al. 12
As may be seen from Table 6, only one study, that by Szmuk et al. ,9 reported the proportion of patients with an abnormal result in any component of the test; this figure was low, at 0.7%, and did not lead to any change in clinical management. No deaths were specifically reported in patients with abnormal test results although, as previously noted, Turnbull and Buck15 reported that one patient died as a consequence of a post-operative pulmonary embolus, but did not state whether or not this patient had an abnormal result on any test.
Venepuncture: adverse events
Blood samples for FBCs and U&Es are obtained by venepuncture. As none of the included studies reported adverse events relating to this process, additional systematic searches were carried out which were designed to identify studies of adverse events in adults who:
-
were comparable in terms of health status with the population included in the review of the clinical effectiveness of routine pre-operative testing [in other words, who either were stated to be ASA grade 1 or 2 or were said to be generally healthy, with no underlying medical conditions or medications (such as anticoagulants) which might influence the incidence of adverse events]; and
-
were undergoing simple venepuncture for diagnostic or screening purposes (see Appendix 8).
Studies which related to blood donors were excluded because:
-
the withdrawal of larger volumes of blood makes it difficult to differentiate between vasovagal reactions and transient relative hypotension due to blood loss16
-
the use of needles with a larger bore than the 20–22 gauge generally used in blood sampling may increase the risk of injury. 17
Studies were also excluded if they used more invasive methods of blood collection (cannulation or catheterisation), or collected arterial or capillary rather than venous blood samples.
Case series or case reports were included only if they related to adverse events for which data were not available from larger, higher-quality studies (observational or before-and-after studies).
The searches identified eight relevant articles:
-
Observational studies by Galena16 and Deacon and Abramowitz. 18
-
An uncontrolled before-and-after study by Godwin et al. 19
-
Case reports by Nouri et al. ,20 Pradhan and Gupta,21 Saeed and Gatens,4 Sander et al. 22 and Zubairy23 [for quality of reporting of meta-analyses (QUOROM) diagram, see Appendix 9].
-
Three additional relevant articles, by Berry and Wallis,24 Horowitz,17 and Yuan and Cohen,25 were identified from citations.
The adverse events identified by these articles fall into three categories:
-
vasovagal reactions
-
pain and bruising
-
more serious nerve injury.
These adverse events are discussed in turn below.
Vasovagal reactions result from an abnormal reflex stimulation of the vagus nerve. The trigger factors may be emotional or somatic. 26 In most patients, the signs and symptoms (which may include pallor, sweating, nausea, dizziness or light-headedness) are light or moderate and resolve spontaneously. However, some patients experience bradycardia with consequent hypotension, loss of consciousness and, in very severe cases, death. 26
Because data relating to vasovagal reactions are available from two large observational studies,16,18 lower-quality studies (case reports and small case series) relating to such adverse events have been excluded.
The larger observational study, that by Galena,16 recorded adverse effects associated with venepuncture carried out in outpatient settings between October 1988 and April 1991 on 4050 patients who were applying for life insurance. A 20- or 22-gauge needle was used to obtain a maximum of 30 μl of blood from each patient. Delayed reactions were identified using telephone calls made an unspecified length of time after the venepuncture. Potentially serious vasovagal reactions were experienced by 3.4% of patients (Table 7); these were significantly more common in men than in women (4.0% vs 1.3%; p < 0.001). None of those who experienced convulsive syncope had a previous history of seizure disorder.
Deacon and Abramowitz18 found lower rates of vasovagal reactions in 3315 adults undergoing venepuncture in three hospital outpatient phlebotomy clinics over a 3-week period, even though 80% had fasted prior to their venepuncture (Table 8). Although the rate indicated by the phlebotomists was higher, at 0.9%, than that reported by the patients, it was still substantially lower than the rate of 3.4% reported by Galena. 16
| Complication | Number (%; 95% CI) |
|---|---|
| Patient reported feeling very or extremely faint | 13/3315 (0.4%; 0.2% to 0.6%) |
| Patient reported losing consciousness | 7/3315 (0.2%; 0.1% to 0.4%) |
| Phlebotomist reported using strategies to manage fainting symptomsa with patient | 30/3315 (0.9%; 0.6% to 1.2%) |
Because data relating to pain and bruising are available from one large observational study16 and one uncontrolled before-and-after study,19 lower-quality studies (case reports and small case series) relating to such adverse events have been excluded.
In Galena’s large observational study,16 14.2% of patients reported adverse events related to pain and bruising (Table 9). Such adverse effects were significantly more common in women than in men (38.1% vs 7.9%; p < 0.001), a result which Galena16 suggested was probably related to narrower veins in women. No cases of local cellulitis or phlebitis were reported.
| Complication | Number (%; 95% CI) |
|---|---|
| Bruising | 416/4050 (10.3%; 9.3% to 11.2%) |
| Haematoma | 80/4050 (2.0%; 1.6% to 2.4%) |
| Pain | 80/4050 (2.0%; 1.6% to 2.4%) |
| Total | 576/4050 (14.2%;, 13.1% to 15.3%) |
Godwin et al. 19 reported higher overall rates of bruising. This small before-and-after study audited bruising in two groups of 100 consecutive medical and surgical inpatients aged ≥ 15 years who were not receiving anticoagulants and did not have extensive pre-existing bruises. Venepuncture was performed by phlebotomists using a pre-evacuated tube collection system to take blood from the antecubital fossa. A clean cotton wool ball was then taped to the venepuncture site; the phlebotomist instructed patients in the first group to apply pressure for a few minutes after the venepuncture, but remained with patients in the second group until the bleeding had stopped. The venepuncture site was then assessed 24 hours later. Bruising was less common in the second group (45% vs 25%; p < 0.01), and such bruises as occurred were also smaller in this group. The difference between the groups was more marked in older patients (Table 10) and the investigators suggested that this was perhaps because they were less able than younger patients to apply pressure to the venepuncture site. 19
| Patient age (years) | Number of patients with bruising (%; 95% CI) | |
|---|---|---|
| Patient pressure | Phlebotomist pressure | |
| < 60 | 11/37 (30%; 15% to 44%) | 7/42 (17%; 5% to 28%) |
| > 60 | 34/63 (54%; 42% to 66%) | 18/58 (31%; 19% to 43%) |
| Total | 45/100 (45%; 35% to 55%) | 25/100 (25%; 17% to 33%) |
The potentially most serious adverse events associated with venepuncture relate to nerve injury. Such adverse events can have disabling consequences. The only identified publications that report venepuncture-associated nerve injuries sufficiently severe to be brought to medical attention take the form of case reports and one small case series.
The case series presented data relating to 11 patients who were referred to a specialist with a particular interest in nerve injuries because of causalgia following routine venepuncture. 17 However, only four of these patients had undergone venepuncture for blood sampling; in the remainder, the venepuncture was for blood donation, insertion of intravenous lines or intravenous medication. A later paper by Horowitz5 combined data relating to these 11 patients with data from 13 patients who had subsequently been evaluated; this could not be utilised because it presented aggregated data from patients who had undergone venepuncture for blood sampling and patients who had undergone venepuncture for other reasons.
Data relating to the cases identified in the case reports, together with the four relevant patients from Horowitz’s case series,17 are summarised in Table 11. They demonstrate that nerve damage consequent on venepuncture can cause long-lasting pain, loss of muscle power and manual dexterity, and may also lead to clinical depression. Two studies specifically stated that a 20-gauge needle was used. In 4 of the 11 cases, venepuncture was specifically said to have been difficult.
| Study | Subject | Purpose and site of venepuncture | Diagnosis | Outcome | Comment |
|---|---|---|---|---|---|
| Berry and Wallis 197724 | 50-year-old woman | Blood grouping; left antecubital fossa | Injury to the medial cutaneous nerve | Pain and swelling in the forearm developed within 24 hours into hyperaesthesia in the whole forearm. A striking improvement was noted 24 hours after treatment with carbamazepine and 3 days later the only symptom was slight pain on moving the arm. Treatment was discontinued after 5 weeks, when the patient had no symptoms except slightly impaired touch sensation in the sensory distribution of the left medial cutaneous nerve | 20-gauge needle used |
| Horowitz 199417 | 61-year-old woman | Blood sampling; antecubital fossa | Causalgia affecting medial antebrachial cutaneous nerve | Increased symptoms and motor abnormalities of disuse, with joint contracture and psychiatric depression requiring antidepressant medication, observed at 7 years | |
| Horowitz 199417 | 61-year-old man | Blood sampling; antecubital fossa | Causalgia affecting lateral antebrachial cutaneous nerve | Increased symptoms and motor abnormalities of disuse, with joint contracture and psychiatric depression requiring antidepressant medication, observed at 4 years | |
| Horowitz 199417 | 56-year-old woman | Blood sampling; antecubital fossa | Causalgia affecting medial antebrachial cutaneous nerve | Increased symptoms, with joint contracture and motor abnormalities of disuse, observed at 18 months | |
| Horowitz 199417 | 35-year-old man | Blood sampling; wrist | Causalgia affecting superficial radial nerve | The burning pain resolved spontaneously over a 2-week period, but hyperpathia and allodynia in the injured nerve distribution persisted at 2.5 years | |
| Nouri et al. 200020 | 59-year-old woman | Routine phlebotomy for pre-operative assessment; radial vein | Causalgia affecting radial nerve | Immediate acute pain and numbness; dysaesthesia, hyperaesthesia, allodynia and loss of muscular power still persisted a year later. Following treatment with paroxetine, tramadol and capsaicin (Zacin®, Cephalon) and six nerve blocks, the pain in the arm and forearm was almost completely resolved, and that in the hand and wrist was somewhat reduced | 20-gauge needle used. Venepuncture said to be difficult, requiring three attempts |
| Pradhan and Gupta 199521 | 32-year-old woman with a minor pyrexial illness | Routine blood testing; cubital vein | Median nerve | Immediate intense pain in whole of left arm persisting on the palmar aspect of the forearm and hand, and accompanied by weakness and tingling. The paraesthesia subsided in 2 months; mild anaesthesia in radial side of palm persisted for 4 months; muscle power returned to normal with physiotherapy, but minimal wasting was still observed after 1 year | Venepuncture said to be very difficult because of non-visibility of veins |
| Saeed and Gatens 19834 | 47-year-old man | Pre-operative phlebotomy; cubital vein | Anterior interosseous syndrome | Pain in forearm and inability to flex thumb noted 4 days after surgery. Surgical tendon transfer required 14 months later to enable appropriate movement of the thumb | Venepuncture said to have been very difficult |
| Sander et al. 199822 | 64-year-old woman | Phlebotomy (purpose not stated); antecubital | Lateral antebrachial cutaneous neuropathy | Acute pain on insertion of needle followed by pain and numbness persisting, with some improvement, for 5 months | |
| Yuan and Cohen 198525 | 31-year-old man | Routine phlebotomy for pre-operative blood tests; cubital vein | Laceration of the lateral antebrachial cutaneous nerve with neuroma formation | Excruciating pain followed by numbness noted during venepuncture, followed by pain and numbness in the forearm persisting for 3 weeks, and resistant to treatment with butazolidin; lidocaine and steroid injection did not produce lasting relief. Surgery was performed on two occasions: the first was ineffective; the second relieved the pain but left permanent numbness. However, motor function was unimpaired | Repeated attempts at venepuncture were required |
| Zubairy 200223 | 44-year-old woman | Routine post-operative blood sampling; cubital fossa | Severe anterior interosseus nerve lesion | Loss of function in the thumb and index finger; weakness of pronation. Management was conservative. The first sign of spontaneous recovery was observed at 20 months and normal function at 34 months after the injury |
The case studies summarised above do not provide any indication of the rate of incidence of nerve injuries related to venepuncture, other than to imply that they were rare. A more specific impression of the incidence rate can be obtained only by considering two studies from blood transfusion centres. In a New Zealand blood transfusion unit performing approximately 80,000 venepunctures a year, Berry and Wallis24 found that, over a 2-year period, six people suffered injuries to the median nerve or medial and lateral cutaneous nerves which were severe enough for them to seek medical attention – an overall rate of approximately 1 in 25,000 (0.004%). Of those six, only one (noted above) was undergoing venepuncture for diagnostic purposes, using a 20-gauge needle; the remaining five were undergoing venepuncture for blood donation, using a larger 16-gauge needle. As this study gave no indication of the number or proportion of venepunctures undertaken for purposes of diagnosis rather than blood donation, it has not been possible to calculate a rate of nerve injury specific to diagnostic venepuncture; however, it seems likely that it would be lower than the overall rate.
A higher nerve injury rate was reported from a blood donation centre in the USA where nurses routinely reported all donor injuries. Over a 2-year period, 419,000 blood donations were collected using a 16-gauge needle and 66 cases of neurological nerve injury were identified from nursing records – a rate of 1 in 6300 (0.016%). 27 This figure is not directly comparable with the New Zealand figure because it includes cases which were not brought to medical attention, but the data for donors who requested a physician consultation (17 of the 56 individuals with nerve injury for whom follow-up data were available) also indicates a rate of approximately 1 in 25,000 (0.004%) (Table 12). This is a conservative estimate: 9 of the 66 donors with nerve injury could not be contacted for telephone follow-up and one was deliberately not contacted because of pending litigation. 27
| Recovery period | Number of donors with nerve injury and follow-up data (n = 56) (% of total; 95% CI) | Number requesting physician consultation(s) (% of category, 95% CI) | Number with residual neurological defecta (% of category, 95% CI) |
|---|---|---|---|
| < 3 days | 22 (39%; 27% to 52%) | 0 (0%) | 0 (0%) |
| 3–29 days | 17 (30%; 18% to 42%) | 5 (29%; 8% to 51%) | 0 (0%) |
| 1–3 months | 13 (4%; 0% to 8%) | 8 (62%; 35% to 88%) | 2 (15%; 0% to 35%) |
| 3–6 months | 2 (23%; 12% to 34%) | 2 (100%) | 1 (50%; 0% to 100%) |
| > 6 months | 2 (23%; 12% to 34%) | 2 (100%) | 1 (50%; 0% to 100%) |
In relation to the more common adverse effects associated with venepuncture undertaken for diagnostic or screening purposes in healthy patients, the evidence base is arguably more substantial than that relating to the value of routine pre-operative testing. Vasovagal reactions were reported by two large observational studies, by Galena16 and Deacon and Abramowitz;18 these included 7365 individuals. Data relating to pain and bruising from 4250 patients were available from Galena’s large observational study16 and a small before-and-after study by Godwin. 19 Unfortunately, data relating to nerve injuries in patients specifically undergoing venepuncture for diagnostic or screening purposes were available only from case series or case reports.
The adverse events which were most commonly reported were those related to pain and bruising: these affected between 14% and 45% of patients. Vasovagal reactions were rarer, affecting between 0.9% and 3.4%. No incidence data are available relating to nerve injuries; although these injuries are potentially disabling, they appear to be rare, and it seems likely that the incidence rate would be lower than the 0.004% reported in blood donors.
Pulmonary function testing
Only one study, the pseudo-RCT of pre- and post-operative breathing exercises by Roukema et al. ,14 provided evidence relating to the benefits of PFTs (Table 13). Four of the 84 patients in the control group (4.8%; 95% CI 0.2% to 9.3%) had an abnormal result, defined as a vital capacity < 75% of normal; these patients also had an abnormal forced expiratory volume in 1 second (FEV1). Only two of the four patients (50.0%; 95% CI 1.0% to 99.0%) subsequently experienced post-operative pulmonary complications, compared with 48 of the 80 patients with normal vital capacities (60.0%; 95% CI 49.3% to 70.7%), and the investigators therefore concluded that pre-operative PFT had no predictive value.
| Study | Number of patients tested | Definition of abnormal result | Number of patients with abnormal results (%; 95% CI) | Number of patients whose operation postponed or cancelled because of test result (%) | Number of patients with abnormal test result who had a related adverse event (%; 95% CI) | Number of deaths (%) |
|---|---|---|---|---|---|---|
| Roukema et al. 198814 | 84 | Vital capacity < 75% of normal | 4/84 (4.8%; 0.2% to 9.3%) | None reported | 2/4 (50%; 1% to 99%) | 0 |
The evidence relating to the value of routine PFTs for ASA grade 1 or 2 patients undergoing elective minor to intermediate surgery is extremely limited, being restricted to 84 patients in the control arm of a RCT conducted for another purpose. 14 The proportion of patients with an abnormal result was relatively low, at 4.8%, and did not lead to a change in management in any of the patients.
As the included study did not report adverse events relating to PFTs and the clinical effectiveness searches identified only one relevant case report,28 additional systematic searches were carried out; these were designed specifically to identify studies which reported adverse events associated with PFTs in patients without obvious predisposing health conditions (for the MEDLINE search strategy, see Appendix 8). These additional searches identified two relevant articles, by Krasnick29 and Oliphant et al. 30 (for QUOROM diagram, see Appendix 10); a further three relevant articles, by Manço et al. ,31 Nemet et al. 32 and Varkey and Cory,33 were identified from citations. A seventh paper, reporting a case of short-lived pneumoparotid apparently caused by PFTs, was excluded because the patient had a predisposition to this condition: he could sometimes produce facial swelling intentionally by coughing or blowing forcefully against his closed mouth, and had had bilateral facial swelling after an aeroplane flight. 34
Krasnick29 states that the adverse effects of PFTs include dizziness from hyperventilation and vasovagal reactions. However, such adverse events were not reported in the included studies, which reported only potentially more serious adverse events which appeared to be related to increased pressure in the mouth, throat or chest: pneumomediastinum, pneumothorax, subcutaneous emphysema and incarceration of existing inguinal hernia. One study30 reported an adverse event of a different nature, namely bilateral temporomandibular joint dislocation. The authors noted that, to the best of the their knowledge, this was unique as an adverse effect of PFT: most such dislocations result from wide opening of the mouth, which is not required for PFT (Table 14).
| Study | Subject | Specific test used | Adverse event | Treatment | Resolution |
|---|---|---|---|---|---|
| Krasnick 200129 | 32-year-old man with upper-chest tightness | Investigational spirometry before and after administration of nebulised levalbuterol hydrochloride | Pneumomediastinum (symptoms severe throat and neck pain and raised vocal pitch) | Apparently none | Symptoms resolved within 36 hours; a chest radiograph 5 days later was normal |
| Nemet et al. 200432 | Healthy 22-year-old, non-smoking female volunteer | Repeated FEV1s performed ‘with great vigour’ as part of research study | Pneumomediastinum and subcutaneous emphysema | Admitted to hospital overnight and treated with supplemental oxygen | Condition had improved sufficiently by the following day for hospital discharge; a full recovery was made within 2 weeks |
| Manço et al. 199031 | Healthy 25-year-old, non-smoking male physician | Standard spirometry, including repeated measurement of PEmax, undertaken as part of investigation to establish normal values | Pneumomediastinum, bilateral pneumothorax and subcutaneous emphysema (symptoms discomfort in the neck, mild dysphagia and dysphonia) | Apparently none | Symptoms resolved in about 3 days |
| Varkey and Kory 197333 | Healthy 23-year-old male medical student | FVC undertaken for familiarisation with PFT procedures | Mediastinal and subcutaneous emphysema (symptoms anterior chest pain, dizziness, swollen neck and mild discomfort on swallowing) | Hospital admission for observation, symptomatic treatment for pain | Symptoms subsided completely in the following 5 days |
| Patel et al. 199228 | Two male patients (one aged 63 and one aged 80 years) with inguinal hernia | Pre-operative PFT | Incarceration of existing inguinal hernia | Emergency surgery | Uncomplicated recovery; discharged from hospital on the fifth and fourth post-operative day |
| Oliphant et al. 200830 | 78-year-old man | PFT (purpose not specified) | Bilateral temporomandibular joint dislocation | Supplemental oxygen via nasal cannula; reduction of dislocation under conscious sedation | Discharged with follow-up arranged with a maxillofacial surgeon; PFT repeated uneventfully 6 weeks later |
The studies summarised above provide little indication of the rate of incidence of adverse events related to PFTs. Four29,30,32,33 of the six studies were individual case reports; as such, they provide no estimate of the incidence of the adverse events which they report other than to imply that, in the authors’ experience, they were unusual. Manço et al. 31 reported that pneumomediastinum, bilateral pneumothorax and subcutaneous emphysema occurred in 1 of 30 normal subjects in whom repeated measurement of maximum static expiratory (PEmax) mouth pressure had been undertaken for research purposes; the remaining 29 subjects suffered no ill effects. However, it seems highly unlikely that the incidence of this complication in normal clinical practice is as high as 1 in 30 as the authors stated that they had not previously observed any complications during extensive use of the technique in normal subjects and patients. 31 Moreover, it is perhaps noteworthy that the subjects of two other case reports were volunteers undertaking PFT for purposes of research32 or familiarisation:33 they are likely to have performed the manoeuvres more frequently or more vigorously than would be normal in pre-operative testing. Following their observation that two patients with inguinal hernia developed incarceration in that hernia following routine pre-operative PFT, apparently as a result of the prolonged increase in intra-abdominal pressure caused by forced expiratory spirometry, Patel et al. 28 undertook a retrospective review which identified that the remaining six patients with inguinal hernia who were referred for pre-operative spirometry in the same hospital during the same 12-month period did not suffer this adverse event, suggesting an incidence rate of one in four in this particular patient group. They identified no clinical or physiological criteria which differentiated the patients who developed incarceration from those who did not, and therefore concluded that, to prevent this complication, the use of a truss should be considered when undertaking PFT in all male patients with hernias.
Only one of the six studies, that by Patel et al. ,28 specifically stated that the adverse effects occurred after routine pre-operative PFT. In the case report by Oliphant et al. ,30 the purpose of testing was not clear, while in the case reported by Krasnick29 it was carried out for investigational purposes. In the remaining three cases, PFT was carried out either for research purposes31,32 or for familiarisation with the process;33 it is possible therefore that they were not representative of patients undergoing PFT for routine pre-operative testing. Thus, in the case reported by Manço et al. ,31 repeated measurement of PEmax mouth pressure was performed in an exercise designed to establish normal values for that laboratory, while Nemet et al. 32 reported that the subject performed FEV1 manoeuvres ‘with great vigour’, perhaps implicitly greater than usual in clinical practice, and to have continued despite feeling chest pain after the first FEV1 manoeuvre; she did not report this pain, but ran on a treadmill for 10 minutes before repeating the FEV1 manoeuvre twice more, by which time the symptoms had increased. Finally, Varkey and Kory33 reported the case of a healthy 23-year-old male medical student, described as ‘most eager to perform as well as possible on the pulmonary function tests’, who also continued with the tests despite noticing slight chest pain after the first manoeuvre, and increasing symptoms thereafter. It thus appears possible that, in these three cases, the symptoms may have been caused by particularly energetic performance of the manoeuvres, and exacerbated by continuation with testing despite the existence of those symptoms; such scenarios may not be typical of routine patient testing.
Discussion
The systematic review of the evidence for the clinical effectiveness of routine pre-operative testing in ASA grade 1 or 2 patients undergoing elective surgery has demonstrated the weakness of that evidence base. Despite thorough searching, no relevant RCTs and only six relevant observational studies9,11–15 were identified; only one14 of these related to PFTs. Moreover, not all of the observational studies reported the proportion of patients with abnormal test results, and fewer reported the more clinically useful measure, the number of patients whose management was changed as a result of an abnormal test result.
Furthermore, there are concerns that none of the included studies incorporated UK data, and those which were conducted in countries in which health care is funded by private health insurance, namely Argentina and the USA, incorporate a potential source of bias. As noted in Quality of research available, Gnocchi et al. 11 state that 270 of the 777 patients in ASA grades 1–3 who attended an Argentine hospital for pre-operative evaluation did not return for surgery (35%; 95% CI 31% to 38%); the primary reason was said to be lack of insurance cover for medical expenses. They do not state how many of the original 777 patients were in grade 1 and therefore scheduled for routine pre-operative testing, but present the results of such testing only for the 214 grade 1 patients who returned to the hospital for a second interview. Of the 210 patients (98%; 95% CI 96% to 100%) who were deemed to be fit for surgery, 71 (34%; 95% CI 27% to 40%) did not undergo the operation for which they were scheduled; again, the main reason appeared to be lack of cover for medical expenses. As no details are given of the health status of the patients who dropped out at either point in the study, compared with those who underwent the scheduled operation, the study incorporates the potential for systematic bias at both points. Although attrition was lower in Haug and Reifeis’s study13 of patients undergoing dental surgery in the USA, 78 of 458 patients (17%; 95% CI 14% to 20%) failed to return on the appointed day; no reasons were given for this. As the other studies are less explicit about the pathway from assessment to operation, they may also contain the potential for bias related to financial or other, unknown, factors.
Chapter 3 Cost-effectiveness
Aim of the cost-effectiveness review
A review of existing literature was undertaken to identify and quality assess all English-language economic evaluations of the routine pre-operative ordering of FBC tests, PFTs and U&Es. This was done in order to:
-
assess the quality of published evaluations of these tests that consider both costs and effects simultaneously
-
identify and explore the trade-offs involved in undertaking a test to identify a problem that would change the management of the patient, or not undertaking that test and incurring the potential risks to the patient
-
explore the uncertainty produced by limitations of empirical data
-
identify the areas where further primary research would be most valuable.
Review methods
Identification of studies
A systematic search of the literature to identify evidence on cost-effectiveness of routine pre-operative testing was performed between March and April 2008. Searches were designed to identify cost-effectiveness studied on pre-operative testing of apparently healthy individuals. Pre-operative tests included FBC, electrolytes and renal function (U&E), and pulmonary function (PFT) in the adult patient population, specifically in individuals classified as ASA grade 1 and 2 undergoing elective minor (grade 1) or intermediate (grade 2) surgical procedures.
Search strategy
The search strategy was developed by the Information Resources team at the School of Health and Related Research, University of Sheffield. Additionally, economics filters used by the NHS CRD were used to populate the NHS Economic Evaluation Database (NHS EED) and were adapted to other databases.
The core search strategy used for the review was designed for searching the MEDLINE electronic database, and was adapted as appropriate for all other databases searched, taking into account differences in indexing terms and search syntax for each database. Appendix 11 provides the search strategies employed.
Databases were searched from their date of inception to the most recent date available at that time. There was no restriction of study by country of origin, date of publication or language.
References were imported into Reference Manager (Thomson ResearchSoft, San Francisco, CA, USA) and then exported into an EndNote (version X2; Thomson Reuters, CA, USA) database, where they were managed.
Sources searched
A range of databases were searched to locate information on economic evaluations of routine pre-operative testing. The aim was to evaluate how relevant studies assessed the cost-effectiveness of the relevant pre-operative tests and the methodology that was adopted.
The following electronic bibliographic databases were searched:
-
MEDLINE
-
MEDLINE In-Process & Other Non-Indexed Citations
-
EMBASE
-
The Cochrane Library (include the CDSR, CENTRAL, NHS EED, NHS HTA and DARE)
-
BIOSIS
-
SCI.
Inclusion criteria
Studies that evaluated the cost-effectiveness, cost–utility or cost–benefit of routine pre-operative testing were eligible for further appraisal as long as they met our abstract selection criteria. More specifically, the analysis had to compare both costs and outcomes of alternative tests and report the results in an incremental basis (e.g. cost per life-year saved or cost per quality-adjusted life-year saved).
Abstract selection criteria:
-
language – English
-
study stetting – UK-based study population
-
patient age – adults (aged 16–60 years)
-
patients – ASA grade 1 classification (completely fit and healthy) or ASA grade 2 classification (some illness but no effect on normal daily activity)
-
surgical procedures – minor (grade 1, for example excision of lesion of skin or drainage of breast abscess) or intermediate (grade 2, for example primary repair of inguinal hernia, excision of varicose veins of leg, tonsillectomy or knee arthroscopy)
-
types of procedures – elective general surgery, day surgery or minor orthopaedic procedures
-
pre-operative tests – FBC, U&E and PFT (these include the following: some or all of spirometry, blood gas analysis, measurement of respiratory mechanics, measurements of transfer function, exercise testing of the respiratory system; generally, tests that identify unexpected anaemia, electrolyte abnormalities or abnormalities of respiratory function)
-
details of economic evaluation – resource use/cost and outcome comparison undertaken.
Exclusion criteria
Studies were also excluded if at least one of the three tests under investigation was not carried out (FBC, U&E or PFT). Finally, papers were excluded if the study was carried out on a paediatric or pregnant population.
Papers were initially excluded from further review if the study did not conduct a full economic evaluation, i.e. if there was not an incremental comparison of costs and effects.
However, in addition, economic evaluations that did not contain incremental analysis and partial economic evaluations (e.g. cost analysis) were identified if they satisfied all the other criteria (with the exception of being UK based) in order to extract data that might be used in an economic model.
Identification of relevant data to inform the economic model
Sifting
The sifting of the references identified by the literature searches for relevant papers to the present study was shared between two reviewers (CMc and YO). Both reviewers screened references by title and abstract. Once potentially relevant studies were identified the full manuscripts of those that were not excluded at this stage were obtained for a more detailed appraisal. The screening of full manuscripts was split between the two reviewers. The full papers that were identified as being potentially relevant were shared between the two reviewers for further screening (half of the full manuscripts were assessed by each of the reviewers). Each reviewer selected papers if they met the abstract selection criteria. Abstract selection tables were filled out by the reviewers to identify studies of relevance (see Appendix 12). Once references were selected data extraction was undertaken by one of the reviewers (YO) using customised data extraction forms.
Data extraction
Data extraction of the identified references was undertaken by collecting details on specific aspects of the studies that could inform the design and parameterisation of a cost-effectiveness model. The following details were identified in the data extraction form:
-
characteristics of studies, type of evaluation and synthesis
-
– type of test (FBC, U&E or PFT)
-
– interventions (surgery type)
-
– study population
-
– country
-
– duration of study
-
– type of model used
-
– perspective
-
– model assumptions (with regard to outcomes and model construction)
-
-
cost and resource-use data sources
-
– unit costs
-
– unit cost data sources
-
– resource use
-
– resource data source
-
– – currency and currency year
-
– discount rate
-
– efficacy data and health outcomes/utility efficacy data
-
– efficacy data sources
-
– health outcomes/utility
-
– health outcome data sources
-
– discount rate
-
– cost-effectiveness ratios
-
– total costs
-
– total incremental costs
-
– total outcome
-
– total incremental outcomes
-
-
sensitivity analyses
-
– sensitivity analysis methods
-
– sensitivity analysis results
-
-
author conclusions.
Further in-depth assessment of studies included
A thorough assessment of the identified references that were selected for data extraction was undertaken. This involved a detailed appraisal of the data that were provided in the studies. Information on the patient population was further assessed to see if clear details regarding the patients’ ASA grades were reported and, if so, whether or not it was possible to unpick these data relating to just ASA grade 1 and 2 patients. Additionally, a detailed assessment of the surgical interventions carried out in the studies was undertaken. An evaluation of the data provided in relation to minor or major surgery (as per the definition above) was undertaken. Finally, data regarding the pre-operative tests undertaken in the studies were reviewed to see if they aligned with the tests in question.
Quality assessment of full evaluations included
In order to assess the evidence provided in the studies included in the review, quality assessment of the full economic evaluation included in the review was undertaken. Quality assessment criteria were based on a widely used quality assessment checklist specifically for economic evaluations. The Drummond Checklist35 was used in order to assess the methodological quality of economic evaluations which met the inclusion criteria. This is a standard checklist for the critical appraisal of economic evaluations and contains a list of questions used to interrogate published studies. An assessment of the evidence provided in the study relating to the cost-effectiveness, cost and utility data reported was undertaken, as well as an assessment of the suitability of this evidence for use in an economic evaluation within the scope of the present study.
No quality assessment was carried out for the partial economic evaluations.
Results
Literature search results
Figure 2 shows the results of the literature search which identified 5151 references in total. Of these, there were 252 duplicated references. Thirty-two references were identified as not being in the English language. Non-English-language references were identified electronically by scanning through the database entries. Two hundred and eighty-two references were identified as relevant from the title and abstract sifting using the abstract selection criteria. Of the 282 full manuscripts that were obtained for further assessment, eight papers36–43 (one full economic evaluation36 and seven partial economic evaluations37–43) were identified for data extraction.
FIGURE 2.
Results of cost-effectiveness literature search.
Full papers excluded
All 282 full papers that were identified as relevant were assessed based on the abstract selection criteria. An abstract selection table was used to log the key characteristic of each paper. Papers were excluded from further detailed assessment and data extraction if they did not fit the inclusion criteria. For example, papers were not included if they were not in the English language or were of studies not carried out in the UK or that did not assess the relevant pre-operative tests. The result of the full paper screening based on the 282 references identified is given in Table 15. Manuscripts were excluded if they did not fit one or more of the inclusion criteria. (Characteristics of all the 282 full papers in relation the abstract selection criteria are presented in Appendix 12. )
| Stages | No. of references identified | |
|---|---|---|
| Manuscripts obtained for more detailed appraisal after title and abstract screening | 282 | |
| Manuscripts excluded from data extraction | ||
| Language – not English | 39 | |
| Patients 1 – not aged 16–60 years | 17 | |
| Surgical procedures – not minor or intermediate | 1 | |
| Tests – not FBC, U&E or PFT | 12 | |
| Economic evaluation: not incremental analysis of costs and outcomes and not | ||
| Patients 2 – not ASA grade 1 or 2 classification, or | ||
| Surgical procedures – not minor or intermediate, or | ||
| Tests – not FBC, U&E or PFT | 205 | |
| Total no. manuscripts excluded from data extraction | 274 | |
| Manuscripts identified for data extraction | 8 | |
| Full economic evaluations (i.e. report incremental cost-effectiveness ratio) | 1 | |
| Partial economic evaluations | 7 | |
Two hundred and five papers were excluded because they did not provide a full economic evaluation. This was in addition to not assessing the patient population, surgical procedures and pre-operative tests relevant to the current study. A further 39 papers were found not to be in the English language. Seventeen papers were excluded because they did not assess the age range of patients relevant to this study. A further 13 papers did not meet the criteria for the pre-operative tests and the surgical procedures under study.
Studies identified for inclusion
A final set of eight papers were identified for inclusion in the cost-effectiveness review and data extraction (see Appendix 13 for data extraction tables). On closer inspection of the data relating to the patient ASA grade, surgical interventions and pre-operative tests performed, it became clear that the studies identified did not provide enough relevant data to inform the model structure or parameterisation of the economic model for any of the three tests currently under study (Table 16).
| Study | Type of evaluation and synthesis | Type of test | Interventions | Study population | Country | Duration of study | Comments |
|---|---|---|---|---|---|---|---|
| Full economic evaluations | |||||||
| Lawrence et al. 198936 | Full cost-effectiveness and cost–benefit analyses | Routine UA | Use of routine UA in the prevention of wound infection for elective clean-wound, non-prosthetic knee procedures. A comparison of routine, or screening, pre-operative UA vs no screening UA for UTI remote from the operative site | Surgical subset of elective, clean wound, intra-articular, non-prosthetic knee procedures not involving osteotomy | USA | Not stated | Insufficient detail given to assume data was limited to ASA grade 1, or adults |
| Partial economic evaluations | |||||||
| Capdenat Saint-Martin et al. 199837 | Not a full economic evaluation – assessed the number and type of pre-operative tests ordered within the study period, the estimated savings and mean cost of pre-operative testing | T/SA, Hb, PT/PTT, Plt/BT, Elec/Glu and BUN/creat | Local adaptation of national guidelines combined with active feedback and organisational analysis | Anaesthetists in 15 surgical wards of Bordeaux University Hospital, Region Aquitain, France | France | 1 month in 1993 and 1 month in 1994 | ASA grade 1 patients, but includes children and emergency surgery |
| Fischer 199638 | Not a full economic evaluation – total pre- and post-clinic implementation costs were evaluated to assess the cost saving of the introduction of the clinic | CBC, platelets, UA, PT/PTT, general survey panel, electrolytes, renal panel, CXRs and ECGs | APEC | 8972 adult patients attending the APEC who were evaluated for surgery and consultation | USA | 6-month period pre-APEC and 1-year post APEC | Includes ASA grade 3 and 4 patients. Not enough detail given to grade of surgery |
| Imasogie et al. 200339 | Not a full economic evaluation – provides an assessment of cost savings when routine pre-operative testing is discontinued | Includes CBC, electrolytes, creatinine, urea, glucose and ECG | The discontinuation of pre-operative tests in ambulatory cataract surgery – two groups compared: (a) testing group with (b) non-testing group | Consecutive cataract patients’ charts were reviewed, during the period of June to September 2000 representing the patients who had routine pre-operative tests (testing group) and June to September 2001 representing the patients who had no routine pre-operative tests (non-testing group) | Canada | 4 months | Appears to include any ASA grade |
| Johnson and Mortimer 200240 | Not a full economic evaluation – a cost-saving analysis was presented in terms of the selective ordering of tests |
The medical notes of 100 surgical patients were scrutinised pre-operatively prior to induction of anaesthesia The results of FBC, U&Es⁄creatinine and random glucose were recorded |
Prospective audit of medical notes of patients undergoing elective surgery | 100 patients undergoing elective surgical procedures under general anaesthesia | UK | 1995–6 | ASA status and surgical intervention not reported |
| Kitz et al. 198841 | Cost analysis – not full economic evaluation, results present include a cost analysis of operating and recovery room time, staff costs and total costs of pre-operative tests in the two study settings | CBC, UA, ECG, Panel 6 and chest radiogram | Inpatient and ambulatory logs were examined to identify hospital resources used for comparable groups of INPTs and DSU patients undergoing surgical arthroscopy and laparoscopy: level I – visual examination of pelvic viscera; level II –also includes fallopian tube with methylene blue or radio-opaque dye | INPTs and DSU patients undergoing surgical arthroscopy of the knee or diagnostic laparoscopy (ASA grade 1 and 2 patients) | USA | January to June 1984 | Limited data, only providing a cost summary |
| Larocque and Maykut 199442 | Not a full economic evaluation – provides unit costs of each of the pre-operative tests employed and a count of each time a test was performed which was compared for the pre- and post-protocol period | CBC, liver profile, chest radiography, electrocardiography and UA | Implementation of a guideline for pre-operative laboratory investigation using retrospective chart audit | Patients operated on including minor surgery and major surgery | Canada | December 1991 to July 1992 | Included patients ASA grades 3–5 and major surgery |
| MacPherson et al. 200543 | Not a full economic evaluation – the average number of tests per patient and cost of tests per patient were calculated | Coags; CPM; EUC; FBC; G&H; LFT and TFT | Assessment of a protocol-based test ordering system or guideline in the ordering of pathology tests in surgical patients attending PAC | All elective adult surgical patients attending the hospital PAC | Australia | Pre-guideline implementation – Group I (700 individuals attending the PAC between April and June 2002); immediate post-guideline introduction (720 individuals between April and June 2003) | Insufficient detail given to assume data was limited to ASA grade 1, or adults, or grade 1–2 surgery |
The Lawrence et al. study36 is the only full economic evaluation identified by the literature search. However, the focus of the study is urinalysis (UA), and it does not report results for any of the tests in the scope of the review.
Capdenat Saint-Martin et al. 37 assessed the use of a local adaptation of national guidelines combined with active feedback and organisational analysis on the ordering of pre-operative investigations for fit ASA grade 1 patients undergoing surgery in 15 wards in a university hospital in France. Pre-operative tests ordered were assessed over 1 month, before and after the local guideline was employed. The sample population included low-risk patients. The patient population included in the study comprised both children aged < 18 years and adults: pre-intervention, n = 536 (47% of the sample were aged < 15 years); post-intervention, n = 516 (50% were aged < 15 years). Given that the data were not split by age group, it is not possible to report the findings for the adult population aged 16–60 years independently.
Pre-operative tests assessed included blood typing and screening for unexpected antibodies, Hb, prothrombin time and partial thromboplastin time, platelet count and bleeding time, electrolytes and blood glucose, blood urea nitrogen and creatinine.
Outcome measures reported in the study included the number and type of pre-operative tests ordered within the study period (1 month in 1993 and 1 month in 1994 representing the pre- and post-guideline time periods) and the estimated savings. Mean costs of pre-operative testing were calculated for the two measurement periods, costs were reported in francs, dollars and the European currency unit (ECU – an artificial ‘basket’ currency that was used by the member states of the European Union as their internal accounting unit at that time).
The study population includes a significant number of patients aged < 15 years. As the current study is concerned with the adult population, this study is not relevant for informing the cost-effectiveness analysis for these tests, as per the scope of the review. The data are presented for the whole sample of patients. There was no subsample analysis that would have enabled the teasing out of data specifically relating to the patient population aged 16–60 years. Additionally, some of the patients underwent emergency surgical procedures. The data for each type of surgery was not presented separately. It was not possible to identify only those patients who had minor or intermediate surgery.
The article by Fischer38 addresses the development and implementation of an Anaesthesia Preoperative Evaluation Clinic (APEC) at a university hospital in the USA. The clinic aimed to provide a service to support physicians in deciding which pre-operative tests their patients might need. All consultations, physical evaluations, educational resources, laboratory and electrocardiographic services, and hospital admissions and registrations were made available in one centralised location. Fischer38 compared pre-operative tests ordered by surgeons and primary care physicians for a 6-month period before the clinic was introduced in the hospital and the 6-month period that occurred 1 year after the introduction of the clinic when ordering of pre-operative assessments was carried out by the anaesthesiologist. Over a 1-year period in 1995, the APEC evaluated 8972 adult patients (age range was not reported) for surgery and consultation. Patient assessments included ASA grade 1 (12%), grade 2 (29%) and grade 3 (54%). Some patients (< 5%) of ASA grade 4 status were also included in the study. Patients evaluated in the clinic were either undergoing surgery the following day (the authors state approximately 70% of the sample) or undergoing procedures 2–7 days after evaluation (28%). No further details are given about the types of surgery that patients underwent.
The pre-operative tests assessed in the study were as follows: complete blood count (CBC), platelets, UA, general survey panel [renal panel, liver function test (LFT), glucose, calcium, albumin, magnesium and uric acid], electrolytes, renal panel and prothrombin time/partial thromboplastin time. These were recorded as the number of each of the tests carried out between the two time periods.
Outcomes assessed in the study included the number of tests ordered, the number of operating room cancellations and number of delays or adverse patient events. The cost of each test was determined using an in-house system. The total pre- and post-clinic implementation costs were evaluated to assess the cost saving resulting from the introduction of the clinic.
The applicability of this evidence is limited for the purposes of this study. A significant number of patients were ASA grade 3 or grade 4 (just under 60%) and thus outside the scope of the review. Additionally, detailed information regarding surgical procedures was not available; thus, we are unable to identify whether procedures undertaken were minor or intermediate. As a result, the relevance of the results of Fischer38 to the patients/procedures specified in the scope of the review is unclear.
The study by Imasogie et al. 39 aimed to evaluate the potential cost savings accruing when routine pre-operative testing is discontinued in ambulatory cataract surgery patients. The hospital-based study was set in Canada and assessed the introduction of a new policy of discontinuing routine laboratory testing prior to cataract surgery.
The charts of cataract patients were reviewed over a 4-month period prior to (testing group) and after the introduction of the new policy a year later. This provided data on 636 patients in the testing group and 595 patients in the non-testing group.
The pre-operative tests assessed included LFT, ECG, echocardiogram, chest radiography, CBC, INR, partial thromboplastin time, Hb, sickle screen, electrolytes, urea and creatinine (EUC); glucose and cardiac stress test.
Clinical data were collected on ASA grade, past medical history and medications, perioperative events (cancellations, intraoperative hypertension, arrhythmia, hypotension), and post-operative events including unanticipated admission and readmissions.
The costs of individual tests were identified from the hospital finance department. Based on the tests ordered and the cost of each, the total costs of laboratory tests of individual patients were calculated. The outcomes evaluated were perioperative hypertension, hypotension, bradycardia arrhythmias, myocardial ischaemia, myocardial infarction, congestive heart failure, syncope, hypoglycaemia, oxygen saturation of < 90% and airway obstruction.
The authors found that there was no difference in the incidence of pre-operative, intraoperative or post-operative events between the two groups of patients. They found a significant reduction in the number of tests per patient ordered in the non-testing group: 0.4 tests per patient compared with 5.8 tests in the testing group. A 90% reduction in laboratory costs per patient was achieved.
Details of patients’ ASA status were not reported in the paper; the authors reported only that these data were collected and that the two groups were not significantly different in terms of ASA status. Although the study provided detailed information on the three tests that are the focus of this study, the lack of information on ASA status, test indication and subsequent treatment and outcomes of treatment, means that generalising from this study to the tightly specified patients in the scope of this review is unlikely to be appropriate.
Johnson and Mortimer40 carried out a prospective audit of the medical notes of 100 patients (between 1995 and 1996) undergoing elective surgical procedures under general anaesthesia in a teaching hospital in the UK (Manchester Teaching Hospital) in order to determine the value of routine pre-operative screening investigations. These investigations included FBC, U&Es and random glucose. The investigations were performed on all patients presenting for elective surgery.
A total of 773 pre-operative screening investigations were analysed in terms of frequency of abnormalities and whether or not the perioperative management was changed when the result was abnormal. Notes were taken from different specialties (39 vascular, 35 breast and 26 urology), but no further details were given about the surgical operations that were undertaken. The costs of the tests were also examined.
The authors found that 9.1% of test results were abnormal. Perioperative management was altered as a result of only two abnormal results (0.2%). Eight complications occurred perioperatively, none of which could have been predicted by the pre-operative screening tests. A cost analysis was presented using selective ordering of tests.
The study does not give sufficient detail of the patient population or the surgical interventions that were undertaken to assume that the evidence presented is in line with the requirements of the present study. Also, given that the data from the study were derived from one hospital in the UK, generalisability of the results is limited.
The comparison of the use of pre-operative tests, operating and recovery room time for comparable groups of patients receiving inpatient or ambulatory care was undertaken by Kitz et al. 41 Hospital costs for the pre-operative tests and for nursing labour costs, based on operating and recovery room times, were also assessed.
Patients undergoing surgical arthroscopy of the knee and diagnostic laparoscopy were included in the study. Diagnostic laparoscopies were divided into two groups: (1) level 1 – visual examination of the pelvic viscera only; and (2) laparoscopy with fallopian tube lavage with methylene blue of radio-opaque dye.
The study utilised inpatient and ambulatory logs to identify patients who underwent inpatient or ambulatory surgical arthroscopy from January to June 1984. Pre-operative tests assessed included CBC, UA, ECG, Panel 6 and chest radiography.
The study provided a cost analysis including the costs of individual laboratory and radiology services. Total hospital costs for the tests were calculated for the inpatients and for the day surgery unit. The study did not aim to assess the cost-effectiveness of the tests or provide a full economic evaluation; for example, no utility data are presented in the study. The evidence is limited for informing the cost-effectiveness model as it provides only a summary of the costs for each of the tests. The costs are based on data from one institution in in the USA. Given that the study was carried out over 20 years ago, the applicability of these costs to the current study setting is extremely limited.
Larocque and Maykut42 assessed the implementation of guidelines for pre-operative laboratory investigations using a retrospective chart audit. The charts of patients were taken from a Canadian university teaching hospital (between 1991 and 1992).
Patients who had undergone both minor (e.g. cataract extraction, transurethral resection of the prostate) and major (e.g. laparoscopic cholecystectomy, hip arthroplasty, abdominal hysterectomy, breast reduction and radial neck dissection) surgery were included in the study. The study also collected data on the age of patients, any pre-existing conditions, medications, ASA status, type of surgery and type of anaesthesia. Patients in ASA grades 1–5 as well as patients undergoing both minor and major surgery were included. The results were not reported separately for each of the ASA subgroups or combinations thereof.
The outcome measures used in the study included reduction in the number of tests performed and the impact of a reduction in tests on morbidity and mortality.
The study reports the unit cost of each of the pre-operative tests and the number of tests performed. This count was compared for the pre- and post-protocol period. These data are specific to the Canadian teaching hospital in which the study was conducted and thus of limited relevance to a UK analysis. In addition, the study data were reported in an aggregate form (e.g. the total number of investigations), meaning that insufficient detail is available for use in parameterising a UK cost-effectiveness analysis.
MacPherson et al. 43 assessed whether or not the introduction of a protocol-based test ordering system (or a guideline) would reduce ordering of inappropriate pathology tests in surgical patients attending a pre-admission clinic (PAC) in a hospital based in Australia. The guideline provided information in two parts: the first contained information about tests to be ordered on the basis of the proposed surgical procedure and the second provided a list of test to be ordered according to a pre-existing medical condition.
The data were obtained from three cohorts of patients attending the PAC over three different time periods: before guideline implementation – group 1 (700 individuals attending the PAC between April and June 2002); immediate post guideline introduction – group 2 (720 individuals between April and June 2003); and the final group (group 3) included individuals attending the PAC clinic the subsequent 3-month period after the introduction of the guideline (763 individuals attending PAC from July to August 2003). The following tests were included in the study: tests of coagulation (Coags), calcium, phosphate and magnesium (CPM), EUC, FBC, group and hold tests (G&Hs), LFTs and thyroid function tests (TFTs).
The study examined the numbers of patients in each group for whom any of eight standard pathology tests had been ordered. The average number of tests per patient (group 1, 2.48; group 2, 1.88; and group 3, 1.91), and cost of tests per patient (group 1, A$42.22; group 2, A$31.89; and group 3, A$33.05) were presented. Further details of the assessment of outcome measures were not given. As with many of the other studies, the usefulness of this study as an information source for a UK cost-effectiveness analysis is limited by the lack of detailed information about the ASA status of patients and surgical interventions undertaken.
Discussion
The systematic review shows the lack of available data involving full economic evaluation of the routine pre-operative ordering of FBC, PFTs and U&Es at present. Only one full economic evaluation was identified. 36 Although we additionally reported seven further partial economic evaluations37–43 with a view to extracting data that might be used in an economic model, these too provided few data that could be utilised.
Overall, the studies identified were either non-UK based, did not involve ASA grade 1 or 2 patients or did not assess electrolytes and renal function and pulmonary function pre-operative tests. The one cost-effectiveness analysis identified explored the implications of carrying out and not carrying out pre-operative testing; however, it did not include utility-based outcomes, was more than 20 years old and was carried out in the USA. Insufficient evidence was available to construct, or aid construction of, a decision probability model for the three tests.
The seven further partial economic evaluations lacked detailed information about the study population and the surgical interventions. Three studies presented findings of guideline or protocol implementation. 37,42,43 These studies focused on deriving total costs and costs per patient to show the benefits of carrying out a reduced number of routine testing. They did not provide enough detailed cost data to inform the building of an economic model.
The demographics of the patients included in the studies were also problematic. Once again, few details were given; one study37 included a large proportion of patients aged < 16 years in the analysis and without details of any subanalysis was not applicable to our study setting. Similarly, the ASA grades included in the studies did not fit our criteria, in as much as none of the papers separated the results by ASA grade 1 and ASA grade 2 classes, which are the focus of our study.
There are some limitations to the review that should be noted. The search strategy identified a large number of studies that were not relevant. This may perhaps be the result of utilising broad search terms. However, this was necessary to ensure that relevant studies were not excluded. Additionally, papers that were not in the English language were excluded from the cost-effectiveness review. Some of these papers may have been relevant to the study setting. However, the applicability of non-UK-based studies in informing the model is likely to be limited.
Future studies assessing the cost-effectiveness of pre-operative tests would benefit from providing disaggregate information about the patients’ ASA status and the type of surgery proposed, as well as detailed data on any amendments to perioperative management in response to test results, cancellations and delays of operations and perioperative outcomes. The data would allow a better comparison between studies as well helping to characterise the clinical pathway for a cost-effectiveness model.
In terms of data required to reflect the real-world application of the tests, evidence regarding the delivery of tests would be of value in informing an economic model (i.e. delivered in a bundle or in sequence could be a valuable distinction in building an economic model).
Chapter 4 Survey of current practice on pre-operative testing in ASA grade 1 and ASA grade 2
The purpose of the survey was to capture current practice of ordering tests for patients classed as ASA grades 1 and 2 undergoing elective minor or intermediate surgery. To do this we chose to approach hospital-based pre-operative assessment clinics directly. We wanted to obtain as wide a picture as possible from those working in a wide variety of settings. Previously, the Abacus International Survey7 comprised a paper and online survey. The investigators contacted members of the Royal College of Anaesthetists (RCoA) Pre-operative Assessment Association and the British Anaesthetic & Recovery Nurses Association (BARNA) and requested that they complete their survey which covered all of the recommendations of the clinical guidelines. This audit was commissioned by NICE to gauge the impact of CG3 on clinical practice.
The questionnaire development
We used some of the questions developed by the Abacus survey7 but excluded those which asked about major surgery and ASA grades above 1 and 2. The questions specifically asked if the indicated tests were carried out routinely. This was to distinguish between those tests that could be considered for the patient in accordance with CG3. We included questions on the testing of patients with common comorbidities of cardiovascular disease, renal disease and respiratory disease. We restricted this to minor and intermediate surgery and for patients aged < 60 years as indicated by the briefing document. We also undertook a very brief snapshot of the level of compliance with CG3 in the range of tests presented in CG3 for ASA grades 1 and 2 and minor and intermediate surgery. We did not include any of the questions relating to the respondents’ opinion regarding the NICE guidance. We included questions about electronic patient administration services (PAS) including how data from patients results were recorded and whether or not the system differentiated between which pre-operative clinic ordered the test. The original survey7 included a number of questions specifically about neurosurgery and cardiovascular surgery that we did not include as these questions were poorly answered in the Abacus survey7 owing to the smaller numbers of centres undertaking this type of surgery. We asked those completing the questionnaire to include a copy of their protocol, if it was locally developed, for use in ASA grade 1 and 2 patients. (See Appendix 14 for questionnaire.)
Once we had the basic structure we consulted with anaesthetic colleagues locally in Sheffield who had an interest in pre-operative assessment. The short questionnaire was ready to be tested once we had checked the status of the project with the National Research Ethics Service (NRES).
We sent details of the project along with the questionnaire to NRES and it was confirmed that this work was classed as service evaluation and did not require ethics approval. The questionnaire requested details about the professional responsibilities of the person completing it. The respondents were assured of the confidentiality of the responses. We had a code for the hospital trust for monitoring purposes so that reminders were not sent to those who had already returned the questionnaire.
As part of our consultation process on the questionnaire we also asked if our strategy of sending directly to the pre-operative assessment clinics would be appropriate. We were advised that this would be likely to obtain a response from those directly involved on a daily basis in assessing patients and ordering tests according to protocols. In the covering letter we asked if the questionnaire could be passed to other clinics run by different specialties in their hospital if they thought that they were using different protocols. We included additional copies of the questionnaire with pre-paid return envelopes.
In the summer of 2008 we sent out 20 questionnaires to hospitals selected to represent teaching hospitals and district general hospitals. Initially we did not receive any back and sent out reminders. We then received four questionnaires. We reviewed the questionnaires and found that only two had sent a copy of their protocol, which was a copy of the NICE guidance in both cases.
We decided to keep with this strategy and the full survey was sent out to pre-operative assessment clinics in 486 hospitals in England and Wales in the autumn of 2008. These hospitals were identified through internet searches for hospitals that appeared to have a surgical unit. Children’s hospitals were excluded. The previous Abacus7 survey did not report on whether or not their respondents (anaesthetists and pre-operative nurses) worked at the same hospital.
To comply with Welsh-language requirements we asked if the respondents would prefer to have a Welsh-language version available.
The survey results
We did not undertake any statistical analysis and these results presented are descriptive.
From the first mailing of questionnaires, 30 questionnaires were returned. We sent out reminders and a further 53 questionnaires were returned, of which five were blank. This gave a total of 83 questionnaires returned (a response rate of 17%). Twenty-four of these had a protocol attached. All of these protocols were copies of the NICE guidance. It was not possible to compare our low response rate with that of the Abacus study7 as they were not clear how many potential respondents they contacted. In addition, a number of the questions they asked were skipped by a large number of respondents, which does not allow for comparisons. However, obtaining a high response rate from busy professionals in a clinical setting is always a challenge.
As expected, all those completing the questionnaire were nurses involved in pre-operative assessment, and all were involved in ordering tests. No one completed a questionnaire passed to them by another pre-operative clinic in the same hospital, i.e. no questionnaires named the same hospital more than once.
We included questions on the number of surgical patients and the proportion of minor, intermediate and major surgeries. In addition, we asked for a breakdown of the numbers of patients in ASA grades 1–4. These were so poorly answered that it was obvious that this information was not readily available to the nurses completing the questionnaire. We asked for this information as it could have potentially been of use in the economic model. We have not reported on these results.
The results tables
The tables below are the results from the survey showing the individual responses to the questions on test ordering.
Table 17 shows that there is 100% compliance with the NICE guidance for those aged < 40 years. The older age groups show more variation, particularly with ECG. Where NICE recommends considering undertaking ECG, FBC, U&E, random glucose and UA in patients aged > 40 years,1 we could perhaps assume that these tests are carried out so frequently in this group as to be classed as routine. However, we did not include a section for tests under consideration which may have limited respondents’ choices.
| Age (years) | CXR | ECG | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|
| 16–40 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 41–60 | 0 (0%) | 15 (18%) | 7 (8%) | 7 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
Tables 18 and 19 show the results for patients ASA grade 2 with cardiovascular comorbidity undergoing minor and intermediate surgery. The results are very similar for minor and intermediate surgery. NICE recommends considering FBC and U&E in this group of patients.
| Age (years) | Chest radiography | ECG | Haemostasis | Blood gas analysis | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|---|---|
| 16–40 | 0 (0%) | 83 (100%) | 0 (0%) | 0 (0%) | 7 (8%) | 7 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 41–60 | 0 (0%) | 83 (100%) | 3 (4%) | 0 (0%) | 12 (14%) | 16 (19%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Age (years) | Chest radiography | ECG | Haemostasis | Blood gas analysis | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|---|---|
| 16–40 | 0 (0%) | 83 (100%) | 0 (0%) | 0 (0%) | 7 (8%) | 16 (19%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 41–60 | 0 (0%) | 83 (100%) | 3 (4%) | 0 (0%) | 12 (14%) | 19 (23%) | 0 (0%) | 0 (0%) | 0 (0%) |
Table 20 shows the results for ASA grade 2 patients with respiratory comorbidity.
| Age (years) | Chest radiography | ECG | Haemostasis | Blood gas analysis | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|---|---|
| 16–40 | 3 (4%) | 15 (18%) | 0 (0%) | 0 (0%) | 7 (8%) | 7 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 41–60 | 14 (16%) | 21 (25%) | 3 (4%) | 0 (0%) | 10 (12%) | 16 (19%) | 0 (0%) | 0 (0%) | 10 (12%) |
Table 21 shows the results for ASA grade 2 patients with respiratory comorbidity. Those with respiratory comorbidities are slightly less likely to be considered for U&Es. NICE guidance recommends considering testing U&Es in this patient group.
| Age (years) | Chest radiography | ECG | Haemostasis | Blood gas analysis | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|---|---|
| 16–40 | 1 (1%) | 15 (18%) | 0 (0%) | 0 (0%) | 7 (8%) | 7 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 41–60 | 14 (16%) | 32 (38%) | 3 (4%) | 0 (0%) | 12 (14%) | 16 (19%) | 0 (0%) | 0 (0%) | 10 (12%) |
Tables 22 and 23 show the results for patients with renal comorbidity. NICE recommends U&Es for these patients and to consider FBC.
| Age (years) | Chest radiography | ECG | Haemostasis | Blood gas analysis | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|---|---|
| 16–40 | 0 (0%) | 15 (18%) | 0 (0%) | 0 (0%) | 15 (18%) | 83 (100%) | 13 (15%) | 0 (0%) | 0 (0%) |
| 41–60 | 0 (0%) | 21 (25%) | 3 (4%) | 0 (0%) | 21 (25%) | 83 (100%) | 17 (20%) | 0 (0%) | 0 (0%) |
| Age (years) | Chest radiography | ECG | Haemostasis | Blood gas analysis | FBC | U&E | Random glucose analysis | UA | PFT |
|---|---|---|---|---|---|---|---|---|---|
| 16–40 | 0 (0%) | 15 (18%) | 0 (0%) | 0 (0%) | 15 (18%) | 83 (100%) | 13 (15%) | 0 (0%) | 0 (0%) |
| 41–60 | 0 (0%) | 32 (38%) | 6 (7%) | 0 (0%) | 21 (25%) | 83 (100%) | 17 (20%) | 0 (0%) | 0 (0%) |
The types of hospital responding were teaching hospitals (n = 32) and district general hospitals (n = 51). Slightly more district general hospitals than teaching hospitals responded.
Discussion
In this section of the study we concentrated on finding out if there was still a culture of routine tests for FBC, electrolytes and renal function and pulmonary function in ASA grade 1 and 2 patients undergoing minor and intermediate surgery. Our results show a substantial level of compliance in the reduction of the routine testing of FBC, electrolytes and renal function and pulmonary function in ASA grade 1 and 2 patients. No one reported carrying out PFTs in this patient group.
There was more variation in reporting of tests in patients with comorbidities. NICE guidance recommends that FBC and U&Es be considered for most of these patients with common comorbidities. Our results suggest that in some places these tests may be part of the routine pre-operative work-up. However, the numbers are small and it is equally likely that a clinical judgement is being made whether or not individual patients actually require these tests.
However, we recognise that the ASA grading of patients is likely to be variable and may be subject to grade inflation to enable testing to be carried out within the NICE guidelines. It is possible that there is a degree of familiarity with the guidance in the 7 years since publication and the time of this survey.
There are other considerations including the increasing standardisation of care throughout the NHS and the work of pre-operative assessment clinics. However, we recognise that these do not follow the same structure in each hospital, and indeed some may not have a formal ‘clinic’ setting.
We attempted to spread our net fairly widely so that we could reach a wider group. However, we recognise that in places where there was no formal pre-operative clinic we could still have failed to reach our intended respondents. We targeted those units with a formal set clinic by addressing the questionnaires to them. We are likely not to have any responses from hospitals relying on trainee medical staff to undertake this role. Our demographics showed that only nurses completed this survey. Other categories of staff may not have seen the questionnaire. As we have shown our response rate was relatively poor and our own local very large trust did not respond as part of the survey. By consulting with our anaesthetic colleagues and with our nursing contacts involved in pre-operative assessment we decided that the responses from nurses would reflect local practice. There was some discussion that nurses would be more aware of any deviations from protocol across the board owing to preferences in testing by senior medical staff.
Undertaking surveys of this kind may be an inefficient method of collecting this kind of information. As part of its guidance, NICE recommends the use of internal audit and the use of routine collected data available through electronic systems. This, of course, disadvantages hospitals with less sophisticated methods of accessing test results.
We did not ask about audit arrangements; in contrast, the Abacus survey7 in 2005 found that there was relatively poor preparation to undertake audit of the compliance with the guidance.
Chapter 5 Cost-effectiveness of pre-operative testing of full blood count, electrolytes and renal function and pulmonary function in the management of ASA grade 1 and grade 2 surgical patients undergoing minor and intermediate surgery
Introduction
Routine pre-operative testing is a high-volume, low-cost activity within the NHS. The high volume of the tests drives a substantial total budget impact, which means that it is important to establish whether or not the tests are a high-value use of limited NHS resources. The potential savings to the NHS by eliminating these tests if they do not represent good value is significant.
Data on health status on admission, from the National Enquiry into Perioperative Deaths, indicate that, even among the elderly (patients aged ≥ 80 years), patients in categories ASA grades 1 and 2 account for > 50% of all patients. 44
The aim of this part of the study was to work with clinical experts in the team to construct a decision-analytic modelling framework capable of establishing the value of each of the routinely used pre-operative tests, either individually or in combination, in terms of the incremental costs and outcomes associated with their use for patients in ASA grades 1 and 2, undergoing intermediate or minor surgery. Routine use means use when the test is not clinically indicated on the basis of the patient history or factor identified during the physical examination.
The evaluation considers three tests that historically have been used routinely in all surgeries: FBC, which is used to check for anaemia; U&Es, which checks renal function and sodium levels; and PFT, which assesses lung capacity.
Methods
The first stage in developing a decision-analytic model is to identify the clinical pathway of patients in the scope of the evaluation, the place of the intervention or interventions being evaluated in that clinical pathway and the potential impact of the interventions on the patient pathway.
To do this, we interviewed the consultant anaesthetists within the study team (Charles Reilly and Duncan Young) to map out a representative patient pathway for otherwise healthy (ASA grades 1 and 2) elective minor/intermediate surgery patients and the impact of each of the tests on the clinical pathway. We also asked them to identify appropriate measures of effect for capturing the impact of the tests, as well as the appropriate time horizon and cycle length for the cost-effectiveness model.
The information obtained was then used to construct a decision tree model with accompanying narrative. This was shared with the consultant anaesthetists for confirmation that it accurately reflected the information they had provided and their expert opinion on the conceptual role of pre-operative tests in this particular indication. The decision tree was then finalised in the light of any further comments.
The finalised decision tree which represented the clinical pathway then provided a framework for identifying the evidence on costs, effectiveness and outcomes required from the systematic literature reviews.
Results
Figure 3 shows a truncated version of the decision tree.
FIGURE 3.
Cost-effectiveness of pre-operative test – model structure. Example using FBC. –ve, negative; +ve, positive. Notes: Assess: the patient is further assessed given the unclear test result; Augmented post-operative care: the post-operative management of the patient is not according to standard care; Delay operation: the patient’s operation is delayed; Investigate: the patient undergoes further investigation to confirm his or her health status; Marginal: patient’s test result is unclear; Not marginal: the patient’s test result is not marginal (a clear test result); Standard post-operative care: the patient has standard post-operative management; Successful/unsuccessful: successful or unsuccessful treatment or operation; Treat: the patient is given a treatment if his or her test result is not clearly negative.
The underlying principle for the use of these routine pre-operative tests is the identification of an asymptomatic condition that could impact on the perioperative and/or post-operative care, prior to surgery in order to allow either an amendment to the care plan, deferring of the procedure to allow the condition to be treated so that the individual is fit for surgery or the cancellation of the surgery if the test results indicate that the balance of risks and benefits of the surgery is no longer positive and treatment of the identified condition is not likely to change the balance of risk and benefits in a relevant time scale.
For each test there is an underlying probability that a patient has the unrecognised condition and a set of test performance characteristics that indicate whether the administration of the test will correctly or incorrectly provide positive or negative results.
Associated with each test result is a clinical strategy based on the measured test result (positive or negative) and each treatment strategy has costs and outcomes associated with it. These differ according to whether the individual is a true-positive, true-negative, false-positive or false-negative. Each pathway has a health state value (utility) associated with it.
The tests operate independently, i.e. the clinical response, costs and outcomes from each test are not dependent on the results of either of the other two tests. However, a positive test result on any one of the tests is a sufficient condition to lead to an operation being delayed.
The proposed cycle length for the model was 1 week and the time horizon for the model was 6 weeks. The cycle length was chosen on the basis of the time it takes for treatments to be initiated and treatment strategies changed. The time horizon was based on the time a clinician would allow for resolution of the types of asymptomatic problems identified by these tests before choosing to cancel the operation. Inevitably there is a substantial element of judgement determining these parameters.
Table 24 reports the parameters required for the construction of the cost-effectiveness model. The evidence for each of the parameters based on the systematic literature reviews reported earlier in this report and national cost databases such as the NHS Reference Costs 2007/8 is also described in the following chapter (see third column, Table 27). 46 There is no evidence in the reported literature for the majority of the parameters required to populate the cost-effectiveness model structure developed.
| Parameter | Baseline value | Published evidence |
|---|---|---|
| FBC | ||
| Probability of positive/negative test result | N/A | No |
| Probability of marginal positive/negative test result | N/A | No |
| Probability of successful/unsuccessful treatment after positive test result | N/A | No |
| Probability of successful/unsuccessful operation | N/A | No |
| PFT | ||
| Probability of positive/negative test result | N/A | No |
| Probability of marginal positive/negative test result | N/A | No |
| Probability of successful/unsuccessful treatment after positive test result | N/A | No |
| Probability of successful/unsuccessful operation | N/A | No |
| Costs45,46 | ||
| Cost of FBC Test | £6 | Yes |
| Cost of U&E | £4 | Yes |
| Cost of PFT | £66 | Yes |
| Cost of successful operationa | £781–1204 | Yes |
| Cost of post-test investigation | £225 | Yes |
| Cost of treatment for conditions identified by pre-operative testb | £3.28–71 per month | Yes |
| Additional cost of post-operative managementc | £814–5226 | Yes |
| U&E | ||
| Probability of positive/negative test | N/A | No |
| Probability of marginal positive/negative test | N/A | No |
| Probability of successful/unsuccessful treatment after positive test result | N/A | No |
| Probability of successful/unsuccessful operation | N/A | No |
| Utilities | ||
| Pre-operative utility | N/A | No |
| Post-operative utilities – successful operation | N/A | No |
| Post-operative utility – unsuccessful operation | N/A | No |
Discussion
Conceptually, the role of routine pre-operative tests is easy to describe. However, the evidence base to support the clinical effectiveness of these three tests in any area of surgery is extremely limited. In the context of minor and intermediate surgery for otherwise healthy patients, we could find no published research on their clinical effectiveness. In addition we found no evidence on the test performance characteristics any of the three tests.
Although we had envisaged having to undertake expert elicitation for some parameters owing to a lack of published evidence, to populate the proposed model structure would entail undertaking expert elicitation for the majority of parameters, including those concerning effectiveness and test performance. After extensive discussion within the research team and consultation with external experts we concluded that the results of analyses based on such extensive expert elicitation would lack credibility with the policy and clinical practitioner audiences that the work was designed to inform.
Therefore, we decided to examine alternative avenues for estimating the clinical effectiveness and cost-effectiveness of routine pre-operative tests. Specifically, we would undertake de novo econometric analyses of routine pre-operative test data held at the Leeds Teaching Hospitals Trust, linked to Hospital Episode Statistics data on outcomes, to estimate the impact of the use of these tests on outcomes. These econometric models could then be linked to cost data to estimate the incremental cost-effectiveness of the tests.
Assuming a robust relationship between the use of these tests and outcomes of surgery could be established, the uncertainty in the parameters in the estimated model would allow simulation modelling to examine the value of further research to reduce that uncertainty, via a simple attribution of a value of health to the modelled outcome.
The econometric modelling is reported in Chapter 6.
Chapter 6 Routine pre-operative testing regression analysis report
Introduction
The objective of the study reported in this chapter was to estimate the relationship between the administration of FBC, U&E and PFT and outcomes on otherwise healthy patients undergoing minor or intermediate surgery. The study was undertaken in response to the lack of published evidence on the clinical effectiveness and/or cost-effectiveness of the use of these tests.
We identified a large routine patient-level data set on tests, surgical procedure and outcomes at Leeds Teaching Hospitals Trust and proposed to utilise econometric methods to questions concerning the value of routine testing. Unfortunately, the data set does not report the use of PFTs; therefore, our analysis was constrained to the role of FBC and U&Es.
Methods
An extract was taken from the PAS system of the Leeds Teaching Hospitals NHS Trust for patients admitted for elective surgery in 2008. There were 114,209 records in the full extract, of which 104,021 were for patients aged ≥ 16 years. Procedure codes were reviewed to identify all minor and intermediate procedures; this left 21,905 unique records. To further simplify the analysis, we eliminated records with more than one hospital episode. This left a sample of 21,792 observations.
By linking the patient’s episode to any previous or subsequent episodes recorded in PAS, the following variables were constructed:
-
Readmission30Day – the patient was readmitted to Leeds Teaching Hospitals NHS Trust within 30 days.
-
Readmission3Month – the patient was readmitted to Leeds Teaching Hospitals NHS Trust within 3 months.
-
Readmission12Month – the patient was readmitted to Leeds Teaching Hospitals NHS Trust within 12 months.
-
DiedInHospital – the patient died during this episode.
-
DiedInHospital30Day – the patient died in Leeds Teaching Hospitals NHS Trust within 30 days.
-
DiedInHospital3Month – the patient died in Leeds Teaching Hospitals NHS Trust within 3 months.
-
DiedInHospital12Month – the patient died in Leeds Teaching Hospitals NHS Trust within 12 months.
-
LengthOfStay – number of days in the episode.
The ASA grade was not recorded in the PAS system. As a proxy for the ASA grade of the patient we calculated both the Charlson score of comorbidity47 and the total bed-days using any episodes in the 12 months preceding the operative admission. These variables were labelled as CharlsonScore and PreviousBedDays, respectively.
Using the patient’s postcode of residence, the IMD2004 decile was looked up and added to the data set.
A probabilistic record linkage was performed between the PAS extract and an extract from the pathology laboratory results database of the tests performed between 2007 and 2009 (1.4 million records), based on the patient’s NHS number, forename, surname, date of birth and gender. This resulted in 997 episodes not being linked to any record in the test result database. This may indicate that either no test had ever been performed for that patient or the degree of agreement between the matching variables was lower than the threshold.
For those episodes where test results were linked, the earliest FBC and U&E preceding the operative episode were returned. Tests more than 60 days prior to the admission were excluded. The following variables were then constructed:
-
FBC – the patient had a FBC or not.
-
FBCOrderDate – the date the FBC was ordered.
-
FBC_DaysBefore – the number of days the FBC was before the episode start.
-
FBC_OutsideNormalRange – the test result was outside the normal range.
-
U&E – the patient had a U&E or not.
-
U&EOrderDate – the date the U&E was ordered.
-
U&E_DaysBefore – the number of days the U&E was before the episode start.
-
U&E_OutsideNormalRange – the test result was outside the normal range.
Three alternative outcome measures were identified:
-
length of stay (continuous in days)
-
readmission within 30 days
-
hospital mortality.
The key explanatory variables for the analysis were the two dichotomous variables representing whether or not patients had FBCs and U&Es.
In addition, we included a number of pre-specified conditioning variables, specifically:
-
age (in years)
-
sex (1 = female, 0 = male)
-
ethnicity
-
socioeconomic status (IMD04 deciles)
-
primary diagnosis [Office of Population Census and Surveys: Classification of Interventions and Procedures (OPCS) codes]
-
surgical procedure [International Classification of Diseases, Tenth Edition (ICD10) codes].
Probit regression models are used to predict the probability of an individual being discharged within 30 days of the procedure, conditioned on a set of individual characteristics. The probit model is
where P is probability, Y = 1 is the observation that an individual is discharged within 30 days of the procedure, X is a vector of explanatory variables which include age, sex, Charlson comorbidity index,47 ethnicity, socioeconomic status (IMD04), primary diagnosis (OPCS) and surgical procedure (ICD10) and ɸ is the cumulative distribution function of the standard normal distribution. The parameters are estimated by maximum likelihood in Stata version 11 (StataCorp LP, College Station, TX, USA). Models are estimated separately for FBC test and U&E test.
Probit regression models were also used to estimate the likelihood that an individual would receive (a) a FBC or (b) a U&E.
Results
Descriptive statistics for variables included in the analysis are reported in Table 25. The first thing to note is that the frequency of test use is not consistent with the hypothesis of their routine use. FBCs were performed in only 58% of patients in the data set and U&Es in only 57%.
| Variable | Mean | SD | Min. | Max. |
|---|---|---|---|---|
| fbc | 0.583 | 0.493 | 0 | 1 |
| ue | 0.567 | 0.495 | 0 | 1 |
| sex | 1.584 | 0.493 | 1 | 2 |
| age | 57.031 | 20.154 | 15 | 101 |
| Charlson Score | 0.066 | 0.334 | 0 | 6 |
| imd1 | 0.212 | 0.409 | 0 | 1 |
| imd2 | 0.108 | 0.310 | 0 | 1 |
| imd3 | 0.116 | 0.320 | 0 | 1 |
| imd4 | 0.078 | 0.268 | 0 | 1 |
| imd5 | 0.092 | 0.289 | 0 | 1 |
| imd6 | 0.117 | 0.322 | 0 | 1 |
| imd7 | 0.094 | 0.291 | 0 | 1 |
| imd8 | 0.064 | 0.245 | 0 | 1 |
| imd9 | 0.086 | 0.280 | 0 | 1 |
| imd10 | 0.033 | 0.178 | 0 | 1 |
| race1 | 0.699 | 0.459 | 0 | 1 |
| race2 | 0.004 | 0.060 | 0 | 1 |
| race3 | 0.006 | 0.080 | 0 | 1 |
| race4 | 0.002 | 0.048 | 0 | 1 |
| race5 | 0.001 | 0.026 | 0 | 1 |
| race6 | 0.002 | 0.039 | 0 | 1 |
| race7 | 0.001 | 0.033 | 0 | 1 |
| race8 | 0.012 | 0.109 | 0 | 1 |
| race9 | 0.013 | 0.113 | 0 | 1 |
| race10 | 0.002 | 0.045 | 0 | 1 |
| race11 | 0.005 | 0.071 | 0 | 1 |
| race12 | 0.007 | 0.083 | 0 | 1 |
| race13 | 0.006 | 0.076 | 0 | 1 |
| race14 | 0.002 | 0.047 | 0 | 1 |
| race15 | 0.003 | 0.052 | 0 | 1 |
| race16 | 0.006 | 0.080 | 0 | 1 |
| race17 | 0.229 | 0.420 | 0 | 1 |
| opcs1 | 0.018 | 0.133 | 0 | 1 |
| opcs2 | 0.036 | 0.187 | 0 | 1 |
| opcs3 | 0.264 | 0.441 | 0 | 1 |
| opcs4 | 0.018 | 0.135 | 0 | 1 |
| opcs5 | 0.033 | 0.179 | 0 | 1 |
| opcs6 | 0.027 | 0.163 | 0 | 1 |
| opcs7 | 0.033 | 0.179 | 0 | 1 |
| opcs8 | 0.019 | 0.137 | 0 | 1 |
| opcs9 | 0.010 | 0.099 | 0 | 1 |
| opcs10 | 0.205 | 0.157 | 0 | 1 |
| opcs11 | 0.017 | 0.130 | 0 | 1 |
| opcs12 | 0.025 | 0.404 | 0 | 1 |
| opcs13 | 0.137 | 0.344 | 0 | 1 |
| opcs14 | 0.039 | 0.194 | 0 | 1 |
| opcs15 | 0.066 | 0.248 | 0 | 1 |
| opcs16 | 0.050 | 0.218 | 0 | 1 |
| opcs17 | 0.001 | 0.032 | 0 | 1 |
| icd1 | 0.001 | 0.029 | 0 | 1 |
| icd2 | 0.130 | 0.336 | 0 | 1 |
| icd3 | 0.008 | 0.086 | 0 | 1 |
| icd4 | 0.017 | 0.130 | 0 | 1 |
| icd5 | 0.249 | 0.433 | 0 | 1 |
| icd6 | 0.013 | 0.114 | 0 | 1 |
| icd7 | 0.016 | 0.125 | 0 | 1 |
| icd8 | 0.029 | 0.169 | 0 | 1 |
| icd9 | 0.087 | 0.281 | 0 | 1 |
| icd10 | 0.021 | 0.144 | 0 | 1 |
| icd11 | 0.035 | 0.185 | 0 | 1 |
| icd12 | 0.159 | 0.365 | 0 | 1 |
| icd13 | 0.040 | 0.196 | 0 | 1 |
| icd14 | 0.006 | 0.076 | 0 | 1 |
| icd15 | 0.071 | 0.257 | 0 | 1 |
| icd16 | 0.020 | 0.141 | 0 | 1 |
| icd17 | 0.098 | 0.297 | 0 | 1 |
Using one outcome, readmission within 30 days, to illustrate the problem, just over 10% of the full sample is readmitted within 30 days. This is 13% if they had FBCs or U&Es but only 8–9% if they did not, which is contrary to our expectations. A probit model for the outcome confirms this. Significant positive coefficients are obtained on the dichotomous variables FBC and U&E suggesting that those who had the tests are more likely to be readmitted within 30 days (Table 26).
| Independent variable | Coefficient | Standard error | z-value | p > |z| | |
|---|---|---|---|---|---|
| ue | 0.254708 | 0.074060 | 3.44 | 0.001 | |
| fbc | 0.063463 | 0.072282 | 0.88 | 0.380 | |
| age | 0.002118 | 0.001038 | 2.04 | 0.041 | |
| sex | 0.002802 | 0.038476 | 0.07 | 0.942 | |
| race2 | –0.103160 | 0.265286 | –0.39 | 0.697 | |
| race3 | 0.156642 | 0.194972 | 0.80 | 0.422 | |
| race4 | 0.132060 | 0.314043 | 0.42 | 0.674 | |
| race8 | 0.010705 | 0.148053 | 0.07 | 0.942 | |
| race9 | –0.196660 | 0.162084 | –1.21 | 0.225 | |
| race10 | 0.129890 | 0.380266 | 0.34 | 0.733 | |
| race11 | –0.273210 | 0.284263 | –0.96 | 0.336 | |
| race12 | –0.482300 | 0.263230 | –1.83 | 0.067 | |
| race13 | –0.242640 | 0.256709 | –0.95 | 0.345 | |
| race14 | 0.327621 | 0.272929 | 1.20 | 0.230 | |
| race15 | –0.375850 | 0.325198 | –1.16 | 0.248 | |
| race16 | –0.128960 | 0.226173 | –0.57 | 0.569 | |
| race17 | –0.310970 | 0.040869 | –7.61 | 0.000 | |
| opcs41dum2 | 0.195883 | 0.261810 | 0.75 | 0.454 | |
| opcs41dum3 | 0.465534 | 0.272863 | 1.71 | 0.088 | |
| opcs41dum4 | –0.524520 | 0.396648 | –1.32 | 0.186 | |
| opcs41dum5 | 0.373183 | 0.269113 | 1.39 | 0.166 | |
| opcs41dum6 | 0.171577 | 0.270358 | 0.63 | 0.526 | |
| opcs41dum7 | 0.719664 | 0.275405 | 2.61 | 0.009 | |
| opcs41dum8 | 0.436592 | 0.294112 | 1.48 | 0.138 | |
| opcs41dum9 | 0.412275 | 0.402044 | 1.03 | 0.305 | |
| opcs41dum10 | 0.848982 | 0.256433 | 3.31 | 0.001 | |
| opcs41dum11 | 0.217559 | 0.284765 | 0.76 | 0.445 | |
| opcs41dum12 | 0.531826 | 0.273651 | 1.94 | 0.052 | |
| opcs41dum13 | 0.273787 | 0.257670 | 1.06 | 0.288 | |
| opcs41dum14 | –0.126560 | 0.268081 | –0.47 | 0.637 | |
| opcs41dum15 | 0.823398 | 0.260753 | 3.16 | 0.002 | |
| opcs41dum16 | –0.024050 | 0.275910 | –0.09 | 0.931 | |
| opcs41dum17 | 0.434054 | 0.443554 | 0.98 | 0.328 | |
| icd101dum2 | 0.580444 | 0.559719 | 1.04 | 0.300 | |
| icd101dum3 | 0.104997 | 0.596036 | 0.18 | 0.860 | |
| icd101dum4 | –0.018470 | 0.617425 | –0.03 | 0.976 | |
| icd101dum5 | –0.206260 | 0.565385 | –0.36 | 0.976 | |
| icd101dum6 | 0.779125 | 0.652997 | 1.19 | 0.233 | |
| icd101dum7 | –0.522250 | 0.606628 | –0.86 | 0.389 | |
| icd101dum8 | –0.048780 | 0.571828 | –0.09 | 0.932 | |
| icd101dum9 | –0.172280 | 0.564082 | –0.31 | 0.760 | |
| icd101dum10 | 0.256588 | 0.572196 | 0.45 | 0.654 | |
| icd101dum11 | –0.281590 | 0.573682 | –0.49 | 0.624 | |
| icd101dum12 | –0.110940 | 0.560829 | –0.20 | 0.843 | |
| icd101dum13 | –0.210520 | 0.568083 | –0.37 | 0.711 | |
| icd101dum14 | 0.067331 | 0.593207 | 0.11 | 0.910 | |
| icd101dum15 | –0.184130 | 0.562386 | –0.33 | 0.743 | |
| icd101dum16 | –0.135020 | 0.562148 | –0.24 | 0.810 | |
| cons | –1.842350 | 0.616409 | –2.99 | 0.003 | |
| Probit regression | Number of obs = 11,561 | ||||
| LR χ2 (49) = 840.99 | |||||
| Prob > χ2 = 0.0000 | |||||
| Log-likelihood = –3669.0966 | Pseudo-R 2 = 0.1028 | ||||
The main models were estimated for patients with a Charlson score47 of 0 or 1 and for patients in whom the tests (if carried out) were carried out no more than 30 days prior to admission. However, results are similar if we use tests carried out up to 60 days prior to admission. The results are also similar for an alternative outcome measure (length of stay), with mean length of stay being longer for those in whom tests were performed, again contrary to expectations. It was not possible to investigate the third outcome measure, as hospital 30-day mortality is only 0.1% and 3-month mortality is only 0.4%.
To examine the hypothesis that these tests were not being used routinely, we estimated probit models to predict which patients would undergo FBCs and U&Es. The main explanatory variables are age, sex, Charlson score,47 OPCS and ICD codes for first procedure and primary diagnosis, race and IMD codes, which proxy for socioeconomic status via the patient’s postcode (Tables 27 and 28).
| FBC | Coefficient | Standard error | z-value | p > |z| |
|---|---|---|---|---|
| age | 0.013450 | 0.000577 | 23.33 | 0.000 |
| sex | 0.106167 | 0.020416 | 5.20 | 0.000 |
| Charlson Score | 0.263823 | 0.029817 | 8.85 | 0.000 |
| opcs41dum2 | 0.670388 | 0.154974 | 4.33 | 0.000 |
| opcs41dum3 | –0.143850 | 0.160748 | –0.89 | 0.371 |
| opcs41dum4 | –0.254620 | 0.188711 | –1.35 | 0.177 |
| opcs41dum5 | 0.020992 | 0.158024 | 0.13 | 0.894 |
| opcs41dum6 | –0.066390 | 0.156711 | –0.42 | 0.672 |
| opcs41dum7 | 0.629987 | 0.158717 | 3.97 | 0.000 |
| opcs41dum8 | 0.657884 | 0.167864 | 3.92 | 0.000 |
| opcs41dum9 | 0.871072 | 0.204211 | 4.27 | 0.000 |
| opcs41dum10 | 0.285404 | 0.148939 | 1.92 | 0.055 |
| opcs41dum11 | 0.056132 | 0.162890 | 0.34 | 0.730 |
| opcs41dum12 | 0.504439 | 0.160024 | 3.15 | 0.002 |
| opcs41dum13 | 0.544150 | 0.150607 | 3.61 | 0.000 |
| opcs41dum14 | –0.066820 | 0.153476 | –0.44 | 0.663 |
| opcs41dum15 | 0.461348 | 0.151350 | 3.05 | 0.002 |
| opcs41dum16 | 0.109387 | 0.151734 | 0.72 | 0.471 |
| opcs41dum17 | 0.409347 | 0.320709 | 1.28 | 0.202 |
| icd101dum2 | 0.419199 | 0.323975 | 1.29 | 0.196 |
| icd101dum3 | 0.571920 | 0.341416 | 1.68 | 0.094 |
| icd101dum4 | 0.424852 | 0.355662 | 1.19 | 0.232 |
| icd101dum5 | 0.378974 | 0.329364 | 1.15 | 0.250 |
| icd101dum6 | 0.835885 | 0.352417 | 2.37 | 0.018 |
| icd101dum7 | 0.422676 | 0.342337 | 1.23 | 0.217 |
| icd101dum8 | 0.718548 | 0.329996 | 2.18 | 0.029 |
| icd101dum9 | 0.651206 | 0.326306 | 2.00 | 0.046 |
| icd101dum10 | 0.430405 | 0.329973 | 1.30 | 0.192 |
| icd101dum11 | 0.190715 | 0.329757 | 0.58 | 0.563 |
| icd101dum12 | 0.537712 | 0.324084 | 1.66 | 0.097 |
| icd101dum13 | 0.625161 | 0.327138 | 1.91 | 0.056 |
| icd101dum14 | 0.566180 | 0.343715 | 1.65 | 0.100 |
| icd101dum15 | 0.384594 | 0.325289 | 1.18 | 0.237 |
| icd101dum16 | 0.230977 | 0.331380 | 0.70 | 0.486 |
| icd101dum17 | 0.356863 | 0.324831 | 1.10 | 0.272 |
| race2 | –0.057350 | 0.146366 | –0.39 | 0.695 |
| race3 | 0.020869 | 0.110840 | 0.19 | 0.851 |
| race4 | –0.015280 | 0.181693 | –0.08 | 0.933 |
| race5 | 0.270222 | 0.359077 | 0.75 | 0.452 |
| race6 | –0.056870 | 0.219913 | –0.26 | 0.796 |
| race7 | 0.031166 | 0.263853 | 0.12 | 0.906 |
| race8 | 0.069126 | 0.079941 | 0.86 | 0.387 |
| race9 | 0.278117 | 0.080876 | 3.44 | 0.001 |
| race10 | 0.036855 | 0.199437 | 0.18 | 0.853 |
| race11 | 0.450421 | 0.130483 | 3.45 | 0.001 |
| race12 | –0.027840 | 0.107624 | –0.26 | 0.796 |
| race13 | 0.025517 | 0.117997 | 0.22 | 0.829 |
| race14 | –0.154780 | 0.185048 | –0.84 | 0.403 |
| race15 | –0.394880 | 0.171781 | –2.30 | 0.022 |
| race16 | 0.128506 | 0.112562 | 1.14 | 0.254 |
| race17 | –0.308750 | 0.021965 | –14.06 | 0.000 |
| imd2 | –0.099260 | 0.033301 | –2.98 | 0.003 |
| imd3 | –0.142500 | 0.032641 | –4.37 | 0.000 |
| imd4 | –0.133270 | 0.037335 | –3.57 | 0.000 |
| imd5 | –0.18264 | 0.035058 | –5.19 | 0.000 |
| imd6 | –0.17627 | 0.032634 | –5.40 | 0.000 |
| imd7 | –0.20314 | 0.035058 | –5.79 | 0.000 |
| imd8 | –0.23122 | 0.039905 | –5.79 | 0.000 |
| imd9 | –0.17274 | 0.036184 | –4.77 | 0.000 |
| imd10 | –0.28820 | 0.052508 | –5.49 | 0.000 |
| _cons | –1.18645 | 0.356942 | –3.32 | 0.001 |
| Number of obs = 21,742 | ||||
| LR χ2 (60) = 2074.02 | ||||
| Prob > χ2 = 0.0000 | ||||
| Log-likelihood = –13,732.975 | Pseudo-R 2 = 0.0702 | |||
| U&E | Coefficient | Standard error | z-value | p > |z| | |
|---|---|---|---|---|---|
| age | 0.022855 | 0.000594 | 38.47 | 0.000 | |
| sex | –0.003950 | 0.020949 | –0.19 | 0.851 | |
| Charlson Score | 0.284033 | 0.032064 | 8.86 | 0.000 | |
| opcs41dum2 | 0.797629 | 0.157573 | 5.06 | 0.000 | |
| opcs41dum3 | –0.022990 | 0.163867 | –0.14 | 0.888 | |
| opcs41dum4 | –0.170800 | 0.193337 | –0.88 | 0.377 | |
| opcs41dum5 | 0.114608 | 0.161157 | 0.71 | 0.477 | |
| opcs41dum6 | –0.068140 | 0.159945 | –0.43 | 0.670 | |
| opcs41dum7 | 0.831243 | 0.161683 | 5.14 | 0.000 | |
| opcs41dum8 | 0.841626 | 0.170565 | 4.93 | 0.000 | |
| opcs41dum9 | 1.136425 | 0.209070 | 5.44 | 0.000 | |
| opcs41dum10 | 0.466354 | 0.151633 | 3.08 | 0.002 | |
| opcs41dum11 | 0.256710 | 0.166039 | 1.55 | 0.122 | |
| opcs41dum12 | 0.385461 | 0.161810 | 2.38 | 0.017 | |
| opcs41dum13 | 0.171809 | 0.153065 | 1.12 | 0.262 | |
| opcs41dum14 | 0.065127 | 0.156098 | 0.42 | 0.677 | |
| opcs41dum15 | 0.293185 | 0.154021 | 3.20 | 0.001 | |
| opcs41dum16 | 0.221133 | 0.154649 | 1.43 | 0.153 | |
| opcs41dum17 | 0.520082 | 0.322032 | 1.62 | 0.106 | |
| icd101dum2 | 0.045329 | 0,317082 | 0.14 | 0.886 | |
| icd101dum3 | 0.293213 | 0.335272 | 0.87 | 0.382 | |
| icd101dum4 | 0.190669 | 0.350457 | 0.54 | 0.586 | |
| icd101dum5 | 0.109618 | 0.323055 | 0.34 | 0.734 | |
| icd101dum6 | 0.276315 | 0.348033 | 0.79 | 0.427 | |
| icd101dum7 | –0.149160 | 0.336500 | 0.27 | 0.790 | |
| icd101dum8 | 0.086230 | 0.323538 | 0.27 | 0.790 | |
| icd101dum9 | 0.223007 | 0.319466 | 0.70 | 0.485 | |
| icd101dum10 | 0.006708 | 0.323477 | 0.02 | 0.983 | |
| icd101dum11 | –0.142200 | 0.323248 | –0.44 | 0.660 | |
| icd101dum12 | 0.006975 | 0.317239 | 0.02 | 0.982 | |
| icd101dum13 | –0.504150 | 0.322016 | –1.57 | 0.117 | |
| icd101dum14 | 0.188721 | 0.338003 | 0.56 | 0.577 | |
| icd101dum15 | –0.015310 | 0.318523 | –0.05 | 0.962 | |
| icd101dum16 | –0.142580 | 0.325056 | –0.44 | 0.661 | |
| icd101dum17 | –0.043780 | 0.318076 | –0.14 | 0.891 | |
| race2 | –0.143600 | 0.146939 | –0.98 | 0.328 | |
| race3 | 0.147874 | 0.114834 | 1.29 | 0.198 | |
| race4 | –0.179890 | 0.199040 | –0.90 | 0.366 | |
| race5 | 0.275862 | 0.369010 | 0.75 | 0.455 | |
| race6 | 0.343529 | 0.231213 | 1.49 | 0.137 | |
| race7 | 0.187172 | 0.274663 | 0.68 | 0.496 | |
| race8 | –0.052790 | 0.081704 | –0.65 | 0.518 | |
| race9 | 0.141052 | 0.079758 | 1.77 | 0.077 | |
| race10 | –0.160120 | 0.200413 | –0.80 | 0.424 | |
| race11 | 0.118722 | 0.124858 | 0.95 | 0.342 | |
| race12 | –0.041280 | 0.110269 | –0.37 | 0.708 | |
| race13 | 0.048866 | 0.121306 | 0.40 | 0.687 | |
| race14 | –0.281780 | 0.192301 | –1.47 | 0.143 | |
| race15 | –0.609830 | 0.187086 | –3.26 | 0.001 | |
| race16 | 0.046919 | 0.115946 | 0.40 | 0.687 | |
| race17 | –0.281570 | 0.022645 | –12.43 | 0.000 | |
| imd2 | –0.031230 | 0.034310 | –0.91 | 0.363 | |
| imd3 | –0.053370 | 0.033667 | –1.59 | 0.113 | |
| imd4 | –0.060660 | 0.038563 | –1.57 | 0.116 | |
| imd5 | –0.165130 | 0.036124 | –4.57 | 0.000 | |
| imd6 | –0.078110 | 0.033622 | –2.32 | 0.020 | |
| imd7 | –0.228320 | 0.035911 | –6.36 | 0.000 | |
| imd8 | –0.177080 | 0.041008 | –4.32 | 0.000 | |
| imd9 | –0.158880 | 0.037101 | –4.28 | 0.000 | |
| imd10 | –0.231320 | 0.053657 | –4.31 | 0.000 | |
| _cons | –1.263400 | 0.351905 | –3.59 | 0.000 | |
| Number of obs = 21,742 | |||||
| LR χ2 (60) = 4056.30 | |||||
| Prob > χ2 = 0.0000 | |||||
| Log-likelihood = –12,841.233 | Pseudo-R 2 = 0.1364 | ||||
There are a large number of statistically significant variables, and, in the case of both FBCs and U&Es, the models correctly predict which patients will be tested in > 70% of cases.
Conclusion
The frequencies of the use of the two tests indicate that they are not used routinely for otherwise healthy patients in minor or intermediate surgery. Two sets of probit models confirm this: the first links the use of the tests to a lower probability of being discharged within 30 days of the procedure and the second demonstrates that it is possible to predict quite accurately which patients will receive these tests. Therefore, it appears that clinical practice has changed such that the research question the study was designed to address is no longer relevant.
It must be noted that these data, although for a large number of observations, are from one trust only (even though that trust consists of a number of hospitals). Although the finding is consistent with the survey findings reported elsewhere in this report, it is quite possible that tests are being use routinely in other NHS hospitals.
It must also be noted that we have been unable to undertake an equivalent analysis for PFTs. Thus, we cannot comment on the use of this test or its impact on the outcome from surgery.
The absence of ASA grade in the data set and deriving this from the Charlson score, although useful, is not the same as having the actual grade recorded by the anaesthetist. Therefore, it is possible that the case mix of the sample of patients included in the data set for the analysis reported in this chapter is more or less diffuse than that specified in the scope of the original study proposal.
A final caveat is that the data set on which the analysis was undertaken is constructed on the basis of a probabilistic linkage of two separate data sets. Although the linkage results were strong, there is a possibility that the test and outcome data do not relate to the same individuals and our findings are spurious.
Chapter 7 Discussion
The original objective of the study reported here was to review the literature on the clinical effectiveness and cost-effectiveness of the routine pre-operative use of three diagnostic tests – FBC, U&E and PFT – in the context of minor and intermediate surgery for otherwise healthy patients, and to synthesise the evidence identified in the context of a de novo cost-effectiveness model.
A comprehensive and systematic search of both the effectiveness and cost-effectiveness literature identified a large number of potentially relevant studies. However, when these studies were subjected to detailed review and quality assessment it became clear that the literature provides no evidence on the effectiveness and cost-effectiveness of these specific tests in the specific patient groups in the context of the UK NHS.
The limitations of the published clinical effectiveness literature – from the perspective of this study – included but were not limited to:
-
inadequate reporting of the surgery that patients were being prepared for
-
inadequate reporting of the specific tests undertaken and the results of the individual tests
-
inadequate reporting of the clinical response to test results; and
-
inadequate reporting of the outcomes of the surgery.
These limitations were by and large shared by the published cost-effectiveness literature. In addition, there were almost no studies from the UK NHS, which meant that the estimates of the resource use and cost reported in the identified papers were unlikely to be relevant to the NHS. The studies also failed to report disaggregated information on resource utilisation and cost and focussed on short-term clinical outcomes rather than health outcomes.
The cost-effectiveness literature that was identified did not look at the longer-term outcomes attributable to the use or non-use of these pre-operative tests (i.e. it focused on the difference in the incidence of perioperative complications and the costs associated with these). This is perhaps attributable to the fact that the studies were generally small and investigators quite possibly did not have the resources necessary to undertake longer-term follow-up. It might also be because the relationships between perioperative complications and longer-term health outcomes are insufficiently understood to allow the construction of models to predict these longer-term consequences in the absence of data.
Whatever the reason for the lack of longer-term health outcome data for these pre-operative tests, the literature does not support any robust conclusions about the value of the routine use of these pre-operative tests compared with alternative uses of the limited health-care resources.
In addition to the literature reviews, we repeated a survey of current practice commissioned by NICE as part of their guideline review process in 2005. The results indicate that the degree of uptake of the NICE guidance on pre-operative testing has increased substantially since the original study. The responses suggest that routine pre-operative testing in minor surgery in patients aged < 40 years has all but disappeared from the NHS. 7
The results of the survey of practice could not be directly verified by this study. However, owing to the lack of published evidence we undertook an additional piece of work, analysing routine testing data from one large teaching hospital trust. The results of this analysis are discussed in more detail below, but they are consistent with the results of the survey and thus may represent a weak validation of these survey results.
The analysis of routine testing and surgical outcome data was not part of the original proposal. However, given the lack of published evidence on the clinical effectiveness and/or cost-effectiveness of these tests, and the importance of the question given the high volumes of pre-operative testing across the NHS as a whole, we deemed it important to exhaust all reasonable avenues of enquiry in pursuit of relevant evidence.
We were fortunate that Leeds Teaching Hospitals Trust had maintained a database of all tests ordered that could be linked, at the individual patient level, to a number of measures of outcome of surgery. This provided a substantial number of observations on which we could estimate regression models. Although the details of the analysis are reported elsewhere, it is worth reiterating that the essence of the work was to estimate the relationship between utilisation of any of the three tests in the pre-operative assessment and the outcomes of surgery in a cohort of otherwise healthy patients undergoing minor or intermediate surgical procedures. It should be noted that we had to approximate the ASA grade 1 and ASA grade 2 score retrospectively. This is not the same as having an original anaesthetist’s score, which further increases the uncertainty in the interpretation of the results, as the case mix of the patients in the sample may be more or less diffuse than in the study scope.
If the tests were being used routinely and they were having a positive impact on outcomes, we would expect to see that patients who received the tests were likely to have shorter lengths of stay and more likely to be discharged from hospital by 30 days. The modelled relationships were exactly the opposite of what was expected. Many patients did not undergo any of these tests and those who did were more likely to have longer lengths of stay and less likely to be discharged by 30 days post operation.
In constructing a decision-analytic model for the cost-effectiveness of these tests it became clear that a number of key determinants of the value of these tests were dependent on the specific cause of the abnormal test result. There are multiple potential causes for abnormal tests results for all three tests. The appropriate clinical response, its resource implication and the expected outcomes of the treatment and hence the potential cost-effectiveness of the test are all dependent on the underlying cause. Constructing models for each possible abnormal test/cause combination was outside the scope of this project. However, any future work examining the cost-effectiveness of these tests in pre-operative assessment will have to frame the decision problem in this context if each parameter in the decision problem is going to be clearly specified.
The most defensible conclusion to be drawn from this study is that there is insufficient evidence to support the utilisation of these three tests as part of the routine pre-operative assessment in otherwise healthy patients undergoing minor and intermediate surgery. The survey and analysis of routine data from the Leeds Teaching Hospitals NHS Trust indicate that the time of universal utilisation of these tests in pre-operative assessment may indeed have passed. However, concerns over response rates and the risks of generalising from data on a single trust make this conclusion tentative.
This study raises the question of how to proceed in an evidence-based decision-making context when there is effectively no evidence related to the decision problem. We had originally proposed to address weaknesses in the evidence by using expert elicitation. However, when it became clear that virtually all of the decision parameters in the decision problem would require expert elicitation, the appropriateness of this strategy became questionable. Challenges associated with establishing who would be the appropriate experts for different parameters in the decision model, how to ensure the representativeness of the sample, and synthesising the evidence provided by different experts on different parameters meant that wholesale elicitation was methodologically questionable and pragmatically beyond the resources of this project.
We considered that establishing a representative sample of experts for the elicitation would be essential if the results of the analysis were to be credible to the medical and decision-making community. However, it would be equally problematic as we are not aware of methods for establishing that the relatively small samples of experts that would be feasible within project resources are representative of such a large community of practitioners. For these reasons, formal elicitation of expert opinion does not appear to offer an analytical solution to the health-care decision-maker’s dilemma of how to make an evidence-based decision in the absence of evidence.
Recommendations for further research
The total expenditure on pre-operative tests across the NHS remains significant. Given the almost complete absence of published evidence on the clinical effectiveness, safety and cost-effectiveness of routine use of these tests in uncomplicated patients undergoing ASA grade 1 and 2 procedures, any well-designed research would add to the current state of knowledge. However, to recommend specific research questions it would be necessary for us to have a view as to the value of additional information to decision-makers in the NHS. To assess the likely value of such research it would be necessary to have a robust assessment of the current scale of the routine use of these tests in the patient/procedure combinations of interest.
The low response rate to our survey, despite significant efforts at follow-up, suggests that this type of survey will not be a satisfactory strategy for scoping the scale of the research opportunity. A systematic identification of routine test databases held by NHS trusts is necessary to establish the feasibility of undertaking a multicentre version of the routine data analysis that we report for Leeds Teaching Hospitals Trust.
If feasible, this would allow the identification of the scale of the use of these tests in practice and the degree to which they are being used in otherwise healthy patients, rather than in response to a specific clinical indication. Only once this information is available will it be possible to establish whether or not any further research in this area is required and, if so, which research questions have the greatest potential value to the NHS.
Acknowledgements
Contribution of authors
Carolyn Czoski-Murray undertook sections of the reviewing, the survey of current practice and preparation of the report.
Myfanwy Lloyd Jones undertook the systematic review of the clinical effectiveness and the preparation of the report.
Chris McCabe constructed the exemplar cost-effectiveness model and contributed to health economics sections of the draught review.
Karl Claxton provided expert advice in health economics methodology and contributed to the review.
Yemi Oluboyede undertook the cost-effectiveness review and the preparation of the review.
Jenny Roberts undertook the econometric analyses and contributed to the review.
Jon Nicholl provided expert advice and contributed to the report.
Angie Rees undertook the searches and contributed to the report.
Charles Reilly and Duncan Young provided expert advice and both contributed to the report.
Tom Fleming managed the database from Leeds Teaching Hospitals NHS Trust and contributed to the main report.
Helen Light, Leeds Institute of Health Sciences, University of Leeds, provided administrative and clerical support in the formatting of the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- National Collaborating Centre for Acute Care Preoperative tests . The Use of Routine Preoperative Tests for Elective Surgery. Evidence, Methods & Guidance 2003. www.nice.org.uk/nicemedia/pdf/CG3NICEguideline.pdf (accessed January 2008).
- Munro J, Booth A, Nicholl J. Routine preoperative testing: a systematic review of the evidence. Health Technol Assess 1997;1.
- Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al. Effectiveness of appropriately trained nurses in preoperative assessment: randomised controlled equivalence/non inferiority trial. BMJ 2002;325.
- Saeed MA, Gatens PF. Anterior interosseous nerve syndrome: unusual etiologies. Arch Phys Med Rehabil 1983;64.
- Horowitz SH. Venipuncture-induced causalgia: anatomic relations of upper extremity superficial veins and nerves, and clinical considerations. Transfusion 2000;40:1036-40.
- Clinical Classification and Schedule Development Group Schedule of Procedures 2005. www.ccsd.org.uk/ (accessed August 2009–10).
- Abacus International Survey . A Survey Measuring the Impact of NICE Clinical Guideline 3: The Use of Routine Preoperative Tests for Elective Surgery 2005. www.nice.org.uk/nicemedia/live/10920/29099/29099.pdf (accessed January 2008).
- NHS Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness: CRD’s Guidance for Those Carrying Out or Commissioning Reviews. Report 4 2001;4.
- Szmuk P, Gurevich B, Dotan Z, Zabeeda D, Geva D, Ezri T. The significance and cost of preoperative laboratory screening in young healthy patients in a public hospital in Israel. Harefuah 2002;141:344-6.
- Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol 2009;6:1253-60.
- Gnocchi C, Risso J, Khoury M, Torn A, Noel M, Baredes N, et al. Application of a preoperative evaluation model in patients undergoing elective abdominal surgery. Medicina 2000;60:125-34.
- Tallo FS, Soriano ES, Alvarenga LS. Preoperative evaluation and cataract surgery. Arq Bras Oftalmol 2007;70:633-7.
- Haug RH, Reifeis RL. A prospective evaluation of the value of preoperative laboratory testing for office anesthesia and sedation. J Oral Maxillofac Surg 1999;57:16-20.
- Roukema JA, Carol EJ, Prins JG. The prevention of pulmonary complications after upper abdominal surgery in patients with noncompromised pulmonary status. Arch Surg 1988;123:30-4.
- Turnbull JM, Buck C. The value of preoperative screening investigations in otherwise healthy individuals. Arch Intern Med 1987;14:1101-5.
- Galena HJ. Complications occurring from diagnostic venipuncture. J Fam Pract 1992;34:582-4.
- Horowitz SH. Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology 1994;44.
- Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiet Disord 2006;20:946-60.
- Godwin PG, Cuthbert AC, Choyce A. Reducing bruising after venepuncture. J Qual Health Care 1992;1:245-6.
- Nouri M, Rozema C, Nouri M, Rouchet M, Bailly M. Radial neuropathy after peripheral venous puncture. Ann Fr Anesth Reanim 2000;19:39-41.
- Pradhan S, Gupta A. Iatrogenic median and femoral neuropathy. J Assoc Physicians India 1995;43.
- Sander HW, Conigliari MF, Masdeu JC. Antecubital phlebotomy complicated by lateral antebrachial cutaneous neuropathy. N Engl J Med 1998;339.
- Zubairy AI. How safe is blood sampling? Anterior interosseus nerve injury by venepuncture. Postgrad Med J 2002;78.
- Berry PR, Wallis WE. Venipuncture nerve injuries. Lancet 1977;1:1236-7.
- Yuan RT, Cohen MJ. Lateral antebrachial cutaneous nerve injury as a complication of phlebotomy. Plast Reconstr Surg 1985;76:299-300.
- Wakita R, Ohno Y, Yamazaki S, Kohase H, Umino M. Vasovagal syncope with asystole associated with intravenous access. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:e28-e32.
- Newman BH, Waxman DA. Blood donation-related neurologic needle injury: evaluation of 2 years’ worth of data from a large blood center. Transfusion 1996;36:213-15.
- Patel V, Raju L, Wollschlager C. Incarceration of existing inguinal hernia as a complication of pulmonary function testing. Chest 1992;101:876-7.
- Krasnick J. Pneumomediastinum following spirometry. Chest 2001;120.
- Oliphant R, Key B, Dawson C, Chung D. Bilateral temporomandibular joint dislocation following pulmonary function testing: a case report and review of closed reduction techniques. Emerg Med J 2008;25:435-6.
- Manço JC, Terra-Filho J, Silva GA. Pneumomediastinum, pneumothorax and subcutaneous emphysema following the measurement of maximal expiratory pressure in a normal subject. Chest 1990;98:1530-2.
- Nemet D, Suchard JR, DiBernardo LM, Mukai DS, Cooper DM. Pneumomediastinum and subcutaneous emphysema after pulmonary function tests in a young healthy woman. Eur J Emerg Med 2004;11:105-7.
- Varkey B, Kory RC. Mediastinal and subcutaneous emphysema following pulmonary function tests. Am Rev Respir Dis 1973;108.
- Kirsch CM, Shinn J, Porzio R, Trefelner E, Kagawa FT, Wehner JH, et al. Pneumoparotid due to spirometry. Chest 1999;116:1475-8.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- Lawrence VA, Gafni A, Gross M. The unproven utility of the preoperative urinalysis: economic evaluation. J Clia Epidemiol 1989;42:1185-92.
- Capdenat Saint-Martin E, Michel P, Raymond JM, Iskandar H, Chevalier C, Petitpierre MN, et al. Description of local adaptation of national guidelines and of active feedback for rationalising preoperative screening in patients at low risk from anaesthetics in a French university hospital. Qual Health Care 1998;7:5-11.
- Fischer S. Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital. Anesthesiology 1996;85:196-20.
- Imasogie N, Wong DT, Luk K, Chung F. Elimination of routine testing in patients undergoing cataract surgery allows substantial savings in laboratory costs. A brief report. Can J Anaesth 2003;50:243-8.
- Johnson RK, Mortimer AJ. Routine pre-operative blood testing: is it necessary?. Anaesthesia 2002;57:914-17.
- Kitz DS, Slusarz-Ladden C, Lecky JH. Hospital resources used for inpatients and ambulatory surgery. Anesthetiology 1988;69:383-6.
- Larocque BJ, Maykut RJ. Implementation of guidelines for preoperative laboratory investigations in patients scheduled to undergo elective surgery. Can J Surg 1994;37:397-401.
- MacPherson RD, Reeve SA, Stewart TV, Cunningham AES, Craven ML, Fox G, et al. Effective strategy to guide pathology test ordering in surgical patients. ANZ J Surg 2055;75:138-43.
- National Confidential Enquiry into Perioperative Deaths n.d. www.ncepod.org.uk/ (accessed February 2012).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2007.
- Department of Health . NHS Reference Costs 2007 8 2009.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83.
- Adams JG, Weigelt JA, Poulos E. Usefulness of preoperative laboratory assessment of patients undergoing elective herniorrhaphy. Arch Surg 1992;127:801-4.
- Ajimura FY, Maia AS, Hachiya A, Watanabe AS, Nunes MP, Martins MA, et al. Preoperative laboratory evaluation of patients aged over 40 years undergoing elective non-cardiac surgery. São Paulo Med J 2005;123:50-3.
- Alam M, Saqib M, ul Haq I. Pre-operative hematological profile: current practice. J Coll Physicians Surg Pak 2003;13:542-3.
- Medical memo . Preoperative tests. Harv Mens Health Watch 1999;4.
- Arieta CE, Nascimento MA, Lira RP, Kara-Jose N. Waste of medical tests in preoperative evaluation for cataract surgery. Cad Saude Publica 2004;20:303-10.
- Barazzoni F, Grilli R, Baggi M, Biegger P, Renella R. Evaluation of the impact of guidelines for rationalizing the prescription of preop tests for patients ASA 1 and 2 undergoing elective surgery. Epidemiol Prev 1999;23:37-46.
- Billings PJ, Davies JP, Richards R, Aubrey DA. An audit of the preoperative investigation of surgical patients. Ann R Coll Surg Engl 1993;75:205-10.
- Bryson GL, Wyand A, Bragg PR. Preoperative testing is inconsistent with published guidelines and rarely changes management. Can J Anaesth 2006;53:236-41.
- Cartana J, Amengual E, Yarnoz MC, Cortes J. Are routine preoperative studies justified?. Med Clin 1989;92.
- Desmonts JM. What to expect from complementary preoperative studies in asymptomatic subjects (ASA 1). Rev Med Suisse 1993;113:111-13.
- Diouf E, Kane O, Beye M, Diop M, Ndiaye F, Sall-Ka B. Evaluation of preoperative complementary examination ordering. Dakar Med 1998;43:1-4.
- Dunne JRM. Perioperative anemia: An independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002;102:237-44.
- Ebert TJ, Kharasch ED, Rooke GA, Shroff A, Muzi M. Myocardial ischemia and adverse cardiac outcomes in cardiac patients undergoing noncardiac surgery with sevoflurane and isoflurane. Sevoflurane Ischemia Study Group. Anesth Analg 1997;85:993-9.
- Finegan BA, Rashiq S, McAlister FA, O’Connor P. Selective ordering of preoperative investigations by anesthesiologists reduces the number and cost of tests. Can J Anaesth 2005;52:575-80.
- Fischer SP. Cost-effective preoperative evaluation and testing. Chest 1999;115:96S-100S.
- Fourcade RO. Le bilan pré-opératiore en urologie. [Preoperative evaluation in urology. A reassessment. Ann Urol 1989;23:422-5.
- Gallus AS, Hirsh J, Gent M. Relevance of preoperative and postoperative blood tests to postoperative leg-vein thrombosis. Lancet 1973;2:805-9.
- Golub R, Cantu R, Sorrento JJ, Stein HD. Efficacy of preadmission testing in ambulatory surgical patients. Am J Surg 1992;163:565-70.
- Halabe-Cherem J, Palomo-Pinon S, Flores-Padilla G, Romero E, Chong-Martinez BA, Nellen-Hummel H, et al. Preoperative assessment in adults. Gac Med Mex 1995;131:267-75.
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth 2006;97:164-70.
- Johnson H, Jr, Knee-Ioli S, Butler TA, Munoz E, Wise L. Are routine preoperative laboratory screening tests necessary to evaluate ambulatory surgical patients?. Surgery 1988;104:639-45.
- Johnson RK, Mortimer AJ. Routine pre-operative blood testing: is it necessary?. Anaesthesia 2002;57:914-17.
- Kamimura T, Koga T, Oshita Y, Hanada M, Nagafuchi Y, Takagi A, et al. Prevalence of previously undiagnosed airflow limitation in patients who underwent preoperative pulmonary function test. Kurume Med J 2006;53:53-7.
- Kaplan EB, Sheiner LB, Boeckmann AJ, Roizen MF, Beal SL, Cohen SN, et al. The usefulness of preoperative laboratory screening. JAMA 1985;253:3576-81.
- Keenan J, Henderson MH, Riches G. Orthopaedic pre-operative assessment: a two-year experience in 5,000 patients. Ann R Coll Surg Engl 1998;80:174-6.
- Kocabas A, Kara K, Ozgur G, Sonmez H, Burgut R. Value of preoperative spirometry to predict postoperative pulmonary complications. Respir Med 1996;90:25-33.
- Lira RP, Nascimento MA, Moreira-Filho DC, Kara-Jose N, Arieta CE. Are routine preoperative medical tests needed with cataract surgery?. Rev Panam Salud Publica 2001;10:13-7.
- Lira RPC, Nascimento M, Kara-Jose N, Arieta C. Predictive value of preoperative tests in facectomy. Rev Saude Publica 2003;37:197-202.
- MacPherson DS, Snow R, Lofgren RP. Preoperative screening: value of previous tests. Ann Intern Med 1990;113:969-73.
- Mantha S, Roizen MF, Madduri J, Rajender Y, Shanti NK, Gayatri K. Usefulness of routine preoperative testing: a prospective single-observer study. J Clin Anesth 2005;17:51-7.
- McAlister FAK. Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am J Respir Crit Care Med 2003;167:741-4.
- McCleane GJ. Preoperative measurement of haemoglobin concentration. Ulster Med J 1990;59:145-8.
- McKee RF, Scott EM. The value of routine preoperative investigations. Ann R Coll Surg Engl 1987;69:160-2.
- McKibbin M. The pre-operative assessment and investigation of ophthalmic patients. Eye 1996;10:138-40.
- Meguro K, Nagayama T, Ashidate Y. Preoperative evaluation of aged patients. Masui 1996;45:S143-S146.
- Mignonsin D, Degui S, Kane M, Bondurand A. Value of selective prescription of preanesthetic laboratory tests. Cah Anesthesiol 1996;44:13-7.
- Morales-Orozco C, Mata-Miranda MP, Cardenas-Lailson LE. Cost/benefit of preoperative examinations for routine elective surgery. Cir Cir 2005;73:25-30.
- Narr BJ, Hansen TR, Warner MA. Preoperative laboratory screening in healthy Mayo patients: cost-effective elimination of tests and unchanged outcomes. Mayo Clin Proc 1991;66:155-9.
- Nascimento MA, Lira RP, Soares PH, Spessatto N, Kara-Jose N, Arieta CE. Are routine preoperative medical tests needed with cataract surgery? Study of visual acuity outcome. Curr Eye Res 2004;28:285-90.
- Pfaff A, van der Linden CJ. Laboratory findings for the preoperative evaluation of patients with otherwise no organic disorders. Ned Tijdschr Geneeskd 1989;133:2291-3.
- Philip B, Lombard L, Roaf E, Drager L, Calalang I, Philip J. Sevoflurane vital capacity induction compared with propofol intravenous induction for adult ambulatory anesthesia. Anesthesiology 1997;87.
- Philip BK, Lombard LL, Roaf ER, Drager LR, Calalang I, Philip JH. Comparison of vital capacity induction with sevoflurane to intravenous induction with propofol for adult ambulatory anesthesia. Anesth Analg 1999;89:623-7.
- Roseano M, Calligaris L, Pozzetto B, Cibi N, Bortul M. Evaluation of surgical risk in elderly patients: a review of 207 cases. Chir Ital 2002;54:437-45.
- Schein OD, Katz J, Bass EB, Tielsch JM, Lubomski LH, Feldman MA, et al. The value of routine preoperative medical testing before cataract surgery. Study of medical testing for cataract surgery. N Engl J Med 2000;342:168-75.
- Stephens MB. Routine preoperative testing before cataract surgery. J Fam Pract 2000;49:299-300.
- Suh KD, Jeong YS, Kam BK, Lee JM, Huh D, Kim JD, et al. The prediction of postoperative pulmonary complications in the elderly patients. Tuberc Respir Dis 1997;44:321-8.
- Velanovich V. The value of routine preoperative laboratory testing in predicting postoperative complications: a multivariate analysis. Surgery 1991;109:236-43.
- Velanovich V. The effects of age, gender, race and concomitant disease on postoperative complications. J R Coll Surg Edinb 1993;38:225-30.
- Walters G, McKibbin M. The value of pre-operative investigations in local anaesthetic ophthalmic surgery. Eye 1997;11:847-9.
- Wattsman T-A, Davies RS. The utility of preoperative laboratory testing in general surgery patients for outpatient procedures. Am Surg 1997;63:81-90.
- Wittgen CM, Naunheim KS, Andrus CH, Kaminski DL. Preoperative pulmonary function evaluation for laparoscopic cholecystectomy. Arch Surg 1993;128:880-6.
- Wyatt WJ, Reed DN, Apelgren KN. Pitfalls in the role of standardized preadmission laboratory screening for ambulatory surgery. Am Surg 1989;55:343-6.
- Arieta CE, Nascimento MA, Lira RP, Kara-Jose N. Waste of medical tests in preoperative evaluation for cataract surgery. Cad Saude Publica 2004;20:303-10.
- Based NU. Cost control in preoperative anesthesiological screening. Anasthesiol Intensivmed 1998;39:392-6.
- Binder M, Schwarz S. Preanaesthetic evaluation: Strained relations of interests. Is there a benefit, is there a disadvantage?. Gesundheitsokon Qualitatsmanage 2002;7:173-6.
- Christian KW, Gervais H, Dick W. Value of preoperative screening studies. Anaesthesist 1988;37:694-703.
- Daher M. Usefulness of pre-operative investigations. Rev Med Liban 1996;8:96-100.
- De FG. Are preoperative routine tests always useful?. Recenti Prog Med 1984;75:418-19.
- Dempfle CE. Perioperative coagulation diagnostics. Anaesthesist 2005;54:167-78.
- Diouf E, Kane O, Beye M, Diop M, Ndiaye F, Sall-Ka B. Evaluation of preoperative complementary examination ordering. Dakar Med 1998;43:1-4.
- Dralle H, Lorenz K, Nguyen-Thanh P. Minimally invasive video-assisted parathyroidectomy – selective approach to localized single gland adenoma. Langenbecks Arch Surg 1999;384:556-62.
- Eisold H. Cost savings with preoperative ambulatory diagnosis in elective gastrointestinal operations. Leber Magen Darm 1996;26:326-37.
- Hoogbergen AJM. The value of preoperative examination (I: Reply I). Ned Tijdschr Geneeskd 1990;134:1234-5.
- Hunting AA. Are preoperative routine examinations necessary?. Tidsskr Nor Laegeforen 1989;109:2640-1.
- Irace L, Scialdone A, Aiello C, Villani C, Coppolino P, Di SM, et al. The diagnosis of the cardiologic risk in patients destined for lung removal surgery. Monaldi Arch Chest Dis 1990;45:175-86.
- Ise Y, Hagiwara K, Saitoh S, Honjo K, Soh S, Kato A, et al. Comparison of the effects of prophylactic antibiotic therapy and cost-effectiveness between Cefazolin (CEZ) and Sulbactam/Ampicillin (SBT/ABPC) in gastric cancer surgery employing clinical pathway. Yakugaku Zasshi 2004;124:815-24.
- Junger A, Engel J, Quinzio L, Banzhaf A, Jost A, Hempelmann G. Risk predictors, scoring systems and prognostic models in anesthesia and intensive care. Part I: anesthesia. Anasthesiol Intensivmed Notfallmed Schmerzther 2002;37:520-7.
- Langemeijer JJM. Effective preoperative examination in the anaesthesiological outpatient clinic. Ned Tijdschr Geneeske 1996;140:1723-6.
- Passamonti EP. Preoperative cardiac evaluation in non cardiac surgery. Monaldi Arch Chest Dis 2004;62:40-6.
- Persson S, Bake B. Routine preoperative ECG of younger patients is not justified. Lakartidningen 1992;89:1991-2.
- Pfaff A, Van Der Linden CJ. Laboratory findings for the preoperative evaluation of patients with otherwise no organic disorders. Ned Tijdschr Geneeskd 1989;133:2291-3.
- Prause G. The preoperative outpatient clinic in Graz: the first 15 years. Anaesthesist 1994;43:223-8.
- Raeder JC. Tidsskr Nor Laegeforen 1996;116:497-9.
- Ramschak H. Perioperative coagulation laboratory. Infusionsther Transfusionsmed 1997;24:43-5.
- Rassler B, Waurick S, Meinecke CD. The prognostic relevance of preoperative pulmonary function tests. Anaesthesist 1994;43:73-81.
- Reingruber B, Klein P, Schneider I, Hohenberger W. Verifying routine preoperative diagnosis between private practice and the surgical university clinic. Langenbecks Arch Chir Suppl Kongressbd 1997;114:782-4.
- Ritz JP, Germer CT, Buhr HJ. Preoperative routine chest x-ray: expensive and of little value. Langenbecks Arch Chir Suppl Kongressbd 1997;114:1051-3.
- Roewer N, Kehl F. An important step in the right direction. Improved preoperative diagnostics--a medical and economic necessity. Anaesthesist 2005;54:425-6.
- Rutten CL, Post D, Smelt WL. Outpatient preoperative examination by the anesthesiologist. I. Fewer procedures and preoperative hospital days. Ned Tijdschr Geneeskd 1995;139:1028-32.
- Sanchez-Alvarez J, Rivero M, Valencia M, Solozabal C. Usefulness of preoperative location tests in primary hyperparathyroidism. An Med Interna 1997;14:360-2.
- Scheidegger D. German . Preoperative assessment from the viewpoint of the anesthetist. Swiss Surg 1995:128-9.
- Schmitt KP, Boehm T, Fleck M, Kaiser WA. Cost-effectiveness of MR-imaging in the preoperative work-up of suspicious breast lesions. Rofo 2001;173:898-901.
- Schwilk B, Friess L, Friesdorf W, Ahnefeld FW, Georgieff M. Preoperative risk factors and intraoperative and postoperative risk management in 11,890 anesthesias. Initial results of a prospective study. Anasthesiol Intensivmed Notfallmed Schmerzther 1993;28:484-92.
- Stohr G, Weyland W, Post S, Becker H. Ambulatory co-disciplinary risk-adjusted preoperative care. Langenbecks Arch Chir Suppl Kongressbd 1998;115:861-3.
- Strom C, Kilger E, Von Scheidt W, Peter K. The role of echocardiography in preoperative of cardiac risk patients before non-cardiac-surgery interventions. Anaesthesist 1998;47:903-11.
- Van Aken H, Rolf N. Preoperative evaluation and preparation. The anesthetist’s view. Anaesthesist 1997;46:S80-S84.
- Van Der MJ. Drastic measures in preoperative laboratory diagnosis. Ned Tijdschr Geneeskd 1984;28:2357-8.
- Van Klei WA, Rutten CL, Moons KG, Lo B, Knape JT, Grobbee DE. Limited effect of Health Council guideline on outpatient preoperative evaluation clinics in the Netherlands: an inventory. Ned Tijdschr Geneeskd 2001;145:25-9.
- Van Melkebeke CD. Preoperative risk stratification for patients undergoing non-cardiac surgery. Ned Tijdschr Geneeskd 2002;58:260-7.
- Vesconi S, Riedo R, Ciceri G, Rusconi MG. Protocol for preoperative chest X-rays in elective surgery. Minerva Anestesiol 2000;66:11-6.
- Ansermino JM, Than M, Swallow PD. Pre-operative blood tests in children undergoing plastic surgery. Ann R Coll Surg Engl 1999;81:175-8.
- Atwell JD, Burn JM, Dewar AK, Freeman NV. Paediatric day-case surgery. Lancet 1973;302:895-6.
- Detsky AS, Baker JP, O’Rourke K, Goel V. Perioperative parenteral nutrition: a meta-analysis. Ann Intern Med 1987;107:195-203.
- Derkay CS. A cost-effective approach for preoperative hemostatic assessment in children undergoing adenotonsillectomy. Arch Otolaryngol Head Neck Surg 2000;126.
- Dzankic S, Pastor D, Gonzalez C, Leung JM. The prevalence and predictive value of abnormal preoperative laboratory tests in elderly surgical patients. Anesth Analg 2001;93:301-8.
- Ferrari LR. Preoperative evaluation of pediatric surgical patients with multisystem considerations. Anesth Analg 2004;99:1058-69.
- Ferrer FA, McKenna PH, Donnal JF. Noninvasive angiography in preoperative evaluation of complicated pediatric renal masses using phase-contrast magnetic resonance angiography. Urology 1994;44:254-9.
- Hoare TJ. Pre-operative haemoglobin estimation in paediatric ENT surgery. J Laryngol Otol 1993;107:1146-8.
- Hsia DC, Fleishman JA, East JA, Hellinger FJ. Pediatric human immunodeficiency virus infection: Recent evidence on the utilization and costs of health services. Arch Pediatr Adolesc Med 1995;149:489-96.
- Juliana H, Lim TA, Inbasegaran K. Pre-operative investigations: yield and conformity to national guidelines. Med J Malaysia 2003;58:5-16.
- Mallick MS. Is routine pre-operative blood testing in children necessary?. Saudi Med J 2006;27:1831-4.
- Meneghini L, Zadra N, Zanette G, Baiocchi M, Giusti F. The usefulness of routine preoperative laboratory tests for one-day surgery in healthy children. Paediatr Anaesth 1998;8:11-5.
- Parry DA, Booth T, Roland PS. Advantages of magnetic resonance imaging over computed tomography in preoperative evaluation of pediatric cochlear implant candidates. Otol Neurotol 2005;26:976-82.
- Rossello PJ, Ramos CA, Mayol PM. Routine laboratory tests for elective surgery in pediatric patients: are they necessary?. Bol Asoc Med P R 1980;72:614-23.
- Shah SA, Sajid T, Asif M, Khan F, Ghani R. Significance and cost effectiveness of pre-operative routine laboratory investigations in young healthy patients undergoing elective ear, nose & throat surgery. J Ayub Med College Abbottabad 2007;19:3-6.
- Tornebrandt K, Fletcher R. Pre-operative chest X-rays in elderly patients. Anaesthesia 1982;37:901-2.
- Wittkugel EPV. Pediatric preoperative evaluation – a new paradigm. Int Anesthesiol Clin 2006;44:141-58.
- Abayomi O, Dritschilo A, Emami B, Watring WG, Piro AJ. The value of ‘routine tests’ in the staging evaluation of gynecologic malignancies: a cost effectiveness analysis. Int J Radiat Oncol Biol Phys 1982;8:241-4.
- Amberg JA, Schneiderman LJ, Berry CC, Zettner A. The abnormal outpatient chemistry panel serum alkaline phosphatase: analysis of physician response, outcome, cost and health effectiveness. J Chronic Dis 1982;35:81-8.
- Costamagna G, Bianco MA, Rotondano G. Cost-effectiveness of endoscopic sphincterotomy. Endoscopy 1998;30:A212-A215.
- Edis AJ, Sheedy PF, Beahrs OH, Van Heerden JA. Results of reoperation for hyperparathyroidism, with evaluation of preoperative localization studies. Surgery 1978;84:384-93.
- Erickson RA, Carlson B. The role of endoscopic retrograde cholangiopancreatography in patients with laparoscopic cholecystectomies. Gastroenterology 1995;109:252-63.
- Hrung JM, Langlotz CP, Orel SG, Fox KR, Schnall MD, Schwartz JS. Cost-effectiveness of MR imaging and core-needle biopsy in the preoperative work-up of suspicious breast lesions. Radiology 1999;213:39-4.
- Ransom SB, Mcneeley SG, Malone JM. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol 1996;175:1201-3.
- Roberts JA. Allergic reactions to anaesthetics: economic aspects of pre-operative screening. Monogr Allergy 1992;30:207-21.
- Ruda JM, Stack BC, Hollenbeak CS. The cost-effectiveness of additional preoperative ultrasonography or sestamibi-SPECT in patients with primary hyperparathyroidism and negative findings on sestamibi scans. Arch Otolaryngol Head Neck Surg 2006;132:46-53.
- Shaw LJ, Hachamovitch R, Cohen M, Berman DS, Borges-Neto S, Udelson JE, et al. Cost implications of selective preoperative risk screening in the care of candidates for peripheral vascular operations. Am J Manag Care 1997;3:1817-27.
- Sonnenberg A, Townsend WF. Cost-benefit analysis of preoperative testing for fecal occult blood. Gastroenterology 1991;100.
- Sonnenberg A, Townsend WF. Preoperative testing for fecal occult blood: a questionable practice. Am J Gastroenterol 1992;87:1410-17.
- Weber AM, Taylor RJ, Wei JT, Lemack G, Piedmonte MR, Walters MD. The cost-effectiveness of preoperative testing (basic office assessment vs. urodynamics) for stress urinary incontinence in women. BJU Int 2002;89:356-63.
- Anonymous . Routine preoperative investigations are expensive and unnecessary. Lancet 1983;322:1466-7.
- Anonymous . Preoperative routines. The Swedish Council on technology assessment in health care. Int J Technol Assess Health Care 1991;7:95-100.
- Anonymous . Individualized testing protocol decreases costs and frustration. Minim Invasive Surg Nurs 1996;10.
- Abbott NK, Biala G, Pollock W. The impact of preoperative assessment on intraoperative nurse performance. AORN J 1983;37:43-58.
- Ainley-Walker JC. Routine preoperative chest X-rays. Anaesthesia 1979;34.
- Allison JG, Bromley HR. Unnecessary preoperative investigations: evaluation and cost analysis. Am Surg 1996;62:686-9.
- Alsumait BM, Alhumood SA, Ivanova T, Mores M, Edeia M. A prospective evaluation of preoperative screening laboratory tests in general surgery patients. Med Princ Pract 2002;11:42-5.
- Archer C, Levy AR, McGregor M. Value of routine preoperative chest x-rays: a meta-analysis. Can J Anaesth 1993;40:1022-7.
- Armstrong EP, Patrick KL, Erstad BL. Comparison of preoperative skin preparation products. Pharmacotherapy 2001;21:345-50.
- Asua J, Lopez-Argumedo M. Preoperative evaluation in elective surgery. INAHTA synthesis report. Int J Technol Assess Health Care 2000;16:673-83.
- Atkins RF. Predicting the utility of the preoperative electrocardiogram: ageism and its economic impact. J Clin Monit 1994;10:70-2.
- Bachman JW, Heise RH, Naessens JM, Timmerman MG. A study of various tests to detect asymptomatic urinary tract infections in an obstetric population. JAMA 1993;270:1971-4.
- Bader A. The preoperative assessment clinic: organization and goals. Ambulatory Surg 1999;7:133-8.
- Bahhady IJ, Unterborn J. Pulmonary function tests: An update. Consultant 2003;43:813-20.
- Barak M, Ben-Abraham R, Katz Y. ACC/AHA guidelines for preoperative cardiovascular evaluation for noncardiac surgery: a critical point of view. Clin Cardiol 2006;29:195-8.
- Barazzoni F, Grilli R, Amicosante AM, Brescianini S, Marca MA, Baggi M, et al. Impact of end user involvement in implementing guidelines on routine pre-operative tests. Int J Qual Health Care 2002;14:321-7.
- Bass EB, Steinberg EP, Luthra R, Schein OD, Tielsch JM, Javitt JC, et al. Do ophthalmologists, anesthesiologists, and internists agree about preoperative testing in healthy patients undergoing cataract surgery?. Arch Ophthalmol 1995;113:1248-56.
- Bellan L. Preoperative testing for cataract surgery. Can J Ophthalmol 1994;29:111-14.
- Berger GL, Sadlowski RW, Sharpe JR, Finney RP. Lack of value of routine preoperative bone and liver scans in cystectomy candidates. J Urol 1981;125:637-9.
- Best WR, Khuri SF, Phelan M, Hur K, Henderson WG, Demakis JG, et al. Identifying patient preoperative risk factors and postoperative adverse events in administrative databases: results from the Department of Veterans Affairs National Surgical Quality Improvement Program. J Am Coll Surg 2002;194:257-66.
- Bird BJ, Chrisp DB, Scrimgeour G. Extensive pre-operative shaving: a costly exercise. N Z Med J 1984;97:727-9.
- Blery C, Chastang C, Gaudy JH. Critical assessment of routine preoperative investigations. Eff Health Care 1983;1:111-14.
- Blitz SG, Cram P, Chernew ME, Monto AS, Fendrick AM. Diagnostic testing or empirical neuraminidase inhibitor therapy for patients with influenza-like illness: what a difference a day makes. Am J Manag Care 2002;8:221-7.
- Blomgren L, Zethraeus N, Johansson G, Jonsson B, Bergqvist D. Cost consequences of preoperative duplex examination before varicose vein surgery: a randomized clinical trial. Phlebology 2006;21:90-5.
- Boothe P, Finegan BA. Changing the admission process for elective surgery: an economic analysis. Can J Anaesth 1995;42:391-4.
- Bushick JB, Eisenberg JM, Kinman J, Cebul RD, Schwartz JS. Pursuit of abnormal coagulation screening tests generates modest hidden preoperative costs. J Gen Intern Med 1989;4:493-7.
- Callaghan LC, Edwards ND, Reilly CS. Utilisation of the pre-operative ECG. Anaesthesia 1995;50:488-90.
- Campbell HE. Health economics and surgical care. Surgery 2006;24:268-71.
- Campbell SC, Klein EA, Levin HS, Piedmonte MR. Open pelvic lymph node dissection for prostate cancer: a reassessment. Urology 1995;46:352-5.
- Caprini JA, Goldshteyn S, Glase CJ, Hathaway K. Thrombophilia testing in patients with venous thrombosis. Eur J Vasc Endovasc Surg 2005;30:550-5.
- Carty SE, Worsey MJ, Virji MA, Brown ML, Watson CG. Concise parathyroidectomy: the impact of preoperative SPECT 99mTc sestamibi scanning and intraoperative quick parathyroid hormone assay. Surgery 1997;122:1107-14.
- Cassidy J, Marley RA. Preoperative assessment of the ambulatory patient. J Perianesth Nurs 1996;11:334-43.
- Catchlove BR, Wilson RM, Spring S, Hall J. Routine investigations in elective surgical patients: their use and cost effectiveness in a teaching hospital. Med J Aust 1979;2:107-10.
- Chang L, Lo S, Stabile BE, Lewis RJ, Toosie K, De VC. Preoperative versus postoperative endoscopic retrograde cholangiopancreatography in mild to moderate gallstone pancreatitis: a prospective randomized trial. Ann Surg 2000;231:82-7.
- Chu UB, Clevenger FW, Imami ER, Lampard SD, Frykberg ER, Tepas JJ. The impact of selective laboratory evaluation on utilization of laboratory resources and patient care in a level-I trauma center. Am J Surg 1996;172:558-63.
- Cirasino L, Barosi G, Torre M, Crespi S, Colombo P, Belloni PA. Preoperative predictors of the need for allogeneic blood transfusion in lung cancer surgery. Transfusion 2000;40:1228-34.
- Clark E. Preoperative assessment. Primary care work-up to identify surgical risks. Geriatrics 2001;56:36-40.
- Clevenger FW, Tepas JJ. Preoperative management of patients with major trauma injuries. AORN J 1997;65:583-4.
- Clinton JE, Yaron M, Tsai SH. Chest radiography in the emergency department. Ann Emerg Med 1986;15:254-6.
- Cloutier MA. Informed consent for PSA testing. Clin Ethics Rep 1995;9:6-8.
- Clyne ME, Forlenza M. Consumer-focused preadmission testing: a paradigm shift. J Nurs Care Qual 1997;11:9-15.
- Collier PE. Changing trends in the use of preoperative carotid arteriography: the community experience. Cardiovasc Surg 1998;6:485-9.
- Collins RC. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol Assess 2007;11.
- Collins SL, Chendrasekhar A. Coordination of routine preoperative testing effects change in practice pattern. Anesthesiology (Hagerstown) 1995;83.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol 1998;3:698-703.
- Correa AJ, Reinisch L, Paty VA, Sanders DL, Duncavage JA. Analysis of a critical pathway in osteoplastic flap for frontal sinus obliteration. Laryngoscope 1999;109:1212-16.
- Crowther MA, Bates S, Schiff D, Dobranowski J, Meade M, McDonald E, et al. Comparison of compression ultrasound vs ascending contrast venography for proximal DVT in medical-surgical ICU patients. Blood 2004;104:712A-713A.
- Cutler BS, Leppo JA. Dipyridamole thallium 201 scintigraphy to detect coronary artery disease before abdominal aortic surgery. J Vasc Surg 1987;5:91-100.
- Daniell JF, Kurtz BR, Ke RW. Hysteroscopic endometrial ablation using the rollerball electrode. Obstet Gynecol 1992;80:329-32.
- Davenport DL, Henderson WG, Khuri SF, Mentzer RM, Richardson JD, Shemin RJ, et al. Preoperative risk factors and surgical complexity are more predictive of costs than postoperative complications: a case study using the National Surgical Quality Improvement Program (NSQIP) database. Ann Surg 2005;242:463-71.
- Davies JM, Pagenkopf D, Todd K, Werry B, Finegan BA. Comparison of selection of preoperative laboratory tests: the computer vs the anaesthetist. Can J Anaesth 1994;41:1156-60.
- De Nino LA, Lawrence VA, Averyt EC, Hilsenbeck SG, Dhanda R, Page CP. Preoperative spirometry and laparotomy – blowing away dollars. Chest 1997;111:1536-41.
- De VC, Pak S, Arnell T, Donayre C, Lewis RJ, Stabile BE, et al. Cardiac assessment prior to vascular surgery: is dipyridamole-sestamibi necessary?. Ann Vasc Surg 1996;10:325-9.
- Degnore LT, Wilson FC. Surgical management of hemophilic arthropathy. Instr Course Lect 1989;38:383-8.
- Delahunt B, Turnbull PR. How cost effective are routine preoperative investigations?. N Z Med J 1980;92:431-2.
- Denham DW, Norman J. Cost-effectiveness of preoperative sestamibi scan for primary hyperparathyroidism is dependent solely upon the surgeon’s choice of operative procedure. J Am Coll Surg 1998;186:293-305.
- Devalia KL, Wright D, Sathyamurthy P, Prasad P, Bruce C. Role of preoperative arthrography in early Perthes disease as a decision-making tool. Is it really necessary?. J Pediatr Orthop B 2007;16:196-200.
- Dillon MF, Collins D, Rice J, Murphy PG, Nicholson P, Mac EJ. Preoperative characteristics identify patients with hip fractures at risk of transfusion. Clin Orthop Relat Res 2005;439:201-6.
- Dimakakos P, Vlahos L, Papadimitriou J. Surgery of varicose veins on ambulatory basis. Early and late results. Int Surg 1995;80:267-70.
- Diokno AC, Dimaculangan RR, Lim EU, Steinert BW. Office based criteria for predicting type II stress incontinence without further evaluation studies. J Urol 1999;161:1263-7.
- Dix FPB. A selective approach to histopathology of the gallbladder is justifiable. Surgeon 2003;1:233-5.
- Doering LV, Esmailian F, Laks H. Perioperative predictors of ICU and hospital costs in coronary artery bypass graft surgery. Chest 2000;118:736-43.
- Dorenbusch MJ, Maglinte DDT, Micon LT, Graffis RA, Turner WW. Intravenous cholangiography and the management of choledocholithiasis prior to laparoscopic cholecystectomy. Surg Laparosc Endosc 1995;5:188-92.
- Dublin BA, Karp NS, Kasabian AK, Kolker AR, Shah MH. Selective use of preoperative lower extremity arteriography in free flap reconstruction. Ann Plast Surg 1997;38:404-7.
- Dubois RW, Lim D, Hebert P, Sherwood M, Growe GH, Hardy JF, et al. The development of indications for the preoperative use of recombinant erythropoietin. Can J Surg 1998;41:351-65.
- D’ugo DM, Persiani R, Caracciolo F, Ronconi P, Coco C, Picciocchi A. Selection of locally advanced gastric carcinoma by preoperative staging laparoscopy. Surg Endosc 1997;11:1159-62.
- Dyson E, Will E, Davison A, O’Malley A, Shepherd H, Jones R. Use of the urinary protein creatinine index to assess proteinuria in renal transplant patients. Nephrol Dial Transplant 1992;7:450-2.
- Eagle KAB. Evaluating cardiac risk for surgery. J Am Coll Cardiol 2007;16:21-3.
- Eckman MH, Erban JK, Singh SK, Kao GS. Screening for the risk for bleeding or thrombosis. Ann Intern Med 2003;138:W15-W24.
- Einhorn N, Zurawski VR, Knapp RC, Bast RC. Preoperative Elevation of Ca 125 Ca 72 and Ca 15–3 in Patients With Nonmucinous Epithelial Ovarian Cancer n.d.;28.
- Eiseman B, Jones R, Mcclatchey M, Borlase B. Cost-effective diagnostic test sequencing. World J Surg 1989;13:272-6.
- Espallargues M, Alonso J, Castilla M. Preoperative testing practice in healthy cataract surgery patients. Results of a survey of ophthalmologists in Barcelona, Spain. Barcelona I-PORT Investigators. Int Ophthalmol 1996;20:315-22.
- Everett LL. Can the risk of postoperative nausea and vomiting be identified and lowered during the preoperative assessment?. Int Anesthesiol Clin 2002;40:47-62.
- Farrell SA, Epp A, Flood C, Lajoie F, Macmillan B, Mainprize T, et al. The evaluation of stress incontinence prior to primary surgery. J Obstet Gynaecol Can 2003;25:313-24.
- Fattahi T. Perioperative laboratory and diagnostic testing – what is needed and when?. Oral Maxillofac Surg Clin North Am 2006;18:1-6.
- Ferrando A, Ivaldi C, Buttiglieri A, Pagano E, Bonetto C, Arione R, et al. Guidelines for preoperative assessment: impact on clinical practice and costs. Int J Qual Health Care 2005;17:323-9.
- Finegan BA, Rashiq S, McAlister FA, O’Connor P. Selective ordering of preoperative investigations by anesthesiologists reduces the number and cost of tests. Can J Anesth 2005;52:575-80.
- Fischer SP. Do preoperative clinic improve operating room efficiency?. Semin Anesth Perioperat Med Pain 1999;18:273-80.
- Fischer SP. Preoperative assessment and preparation: new innovations. Curr Opin Anaesthesiol 1997;10:410-13.
- Fischer SP. Cost-effective preoperative evaluation and testing. Chest 1999;115:96S-100S.
- Harik-Khan RI, Fleg JL, Muller DC, Wise RA. The effect of anthropometric and socioeconomic factors on the racial difference lung function. Am J Respir Crit Care Med 2001;164:1647-54.
- Hilibrand AS, Dina TS. The use of diagnostic imaging to assess spinal arthrodesis. Orthop Clin North Am 1998;29:591-60.
- Hnatiuk MOW, Dillard LTA, Torrington CKG. Adherence to established guidelines for preoperative pulmonary-function testing. Chest 1995;107:1294-7.
- Hoeks SE, Schouten O, van der Vlugt MJ, Poldermans D. Preoperative cardiac testing before major vascular surgery. J Nucl Cardiol 2007;14:885-91.
- Hollenbeak CS, Lendel I, Beus KS, Ruda JM, Stack BC. The cost of screening for synchronous thyroid disease in patients presenting with primary hyperparathyroidism. Arch Otolaryngol Head Neck Surg 2007;133:1013-21.
- Hollenberg SM. Preoperative cardiac risk assessment. Chest 1999;115:51S-57S.
- Horton JB, Reece EM, Broughton G, Janis JE, Thornton JF, Rohrich RJ. Patient safety in the office-based setting. Plast Reconstr Surg 2006;117:61e-80e.
- Howard PA. Dalteparin: a low-molecular-weight heparin. Ann Pharmacother 1997;31:192-203.
- Howie JGR, Heaney DJ, Maxwell M, Walker JJ. A comparison of a Patient Enablement instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract 1998;15:165-71.
- Hux J. Preoperative testing prior to elective surgery. Hosp Q 2003;6:26-7.
- Imasogie NW. Elimination of routine testing in patients undergoing cataract surgery allows substantial savings in laboratory costs. A brief report. Can J Anesth 2003;50:246-8.
- Ishaq M, Kamal RS, Aqil M. Value of routine pre-operative chest X-ray in patients over the age of 40 years. J Pak Med Assoc 1997;47:279-81.
- Jaffer AK, Brotman DJ, Sridharan ST, Litaker DG, Michota FA, Frost SD, et al. Postoperative pulmonary complications: experience with an outpatient preoperative assessment program. J Clin Outcome Manag 2005;12:505-10.
- Jang HJ, Lim JH, Lee SJ, Park CK, Park HS, Do YS. Hepatocellular carcinoma: are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation?. Radiology 2000;215:373-80.
- Johnson H, Knee-Ioli S, Butler TA, Munoz E, Wise L. Are routine preoperative laboratory screening tests necessary to evaluate ambulatory surgical patients?. Surgery 1988;104:639-45.
- Jones T, Isaacson J. Preoperative screening: what tests are necessary?. Cleve Clin J Med 1995;62:374-8.
- Justice AC, King JT. The case for a full cost–benefit analysis of preoperative HIV screening. J Clin Epidemiol 1993;46:1229-31.
- Khandekar JD, Winchester DP, Scott Jones R, Murphy GP. Cancer surgery for the general surgeon. Philadelphia, PA: Lippincott Raven; 1999.
- Kitchens CS. Preoperative PTs, PTTs, cost-effectiveness, and health care reform. Radical changes that make good sense. Chest 1994;106:661-2.
- Lee H, Doig CJ, Ghali WA, Donaldson C, Johnson D, Manns B. Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med 2004;32:981-5.
- Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2007;25:625-33.
- Macario A, Roizen MF, Thisted RA, Kim S, Orkin FK, Phelps C. Reassessment of preoperative laboratory testing has changed the test-ordering patterns of physicians. Surg Gynecol Obstet 1992;175:539-47.
- MacPherson DS. Preoperative laboratory testing – should any tests be routine before surgery. Med Clin North Am 1993;77:289-308.
- Mancuso C. Effects of changes in routine pre-operative testing. J Gen Intern Med 1996;11.
- Mancuso CA. Impact of new guidelines on physicians’ ordering of preoperative tests. J Gen Intern Med 1999;14:166-72.
- Mantha S, Roizen MF, Madduri J, Rajender Y, Shanti NK, Gayatri K. Usefulness of routine preoperative testing: a prospective single-observer study. J Clin Anesth 2005;17:51-7.
- Marcello PW, Roberts PL. ‘Routine’ preoperative studies. Which studies in which patients?. Surg Clin North Am 1996;76:11-23.
- Marton KI, Tul V, Sox HC. Modifying test-ordering behavior in the outpatient medical clinic. A controlled trial of two educational interventions. Arch Intern Med 1985;145:816-21.
- Maurer WG, Borkowski RG, Parker BM. Quality and resource utilization in managing preoperative evaluation. Anesthesiol Clin 2004;22:155-75.
- McCleane G. Preoperative biochemical screening. BMJ 1988;297:1129-30.
- Morrison JE, Jacobs VR. Outpatient laparoscopic hysterectomy in a rural ambulatory surgery center. J Am Assoc Gynecol Laparosc 2004;11:359-64.
- Munro J, Booth A, Nicholl J. Routine preoperative testing: a systematic review of the evidence. Health Technol Assess 1997;1.
- Muskett AD, McGreevy JM. Rational preoperative evaluation. Postgrad Med J 1986;62:925-8.
- Nahas ZSF. A safe and cost-effective short hospital stay protocol to identify patients at low risk for the development of significant hypocalcemia after total thyroidectomy. Laryngoscope 2006;116:906-10.
- Nanthakrishnan N, Rao KM, Narasimhan R, Sethuraman KR, Reddy KSN, Veliath AJ. Preoperative differentiation of benign and malignant solitary thyroid nodules. Natl Med J India 1989;2:111-14.
- Naraghi R, O’Donnell WF, Bahnson RR. Routine frozen section of pelvic lymph node specimens prior to radical retropubic prostatectomy is unnecessary in patients with prostate specific antigen levels less than 20 ng/ml. J Urol 1995;153.
- Nardella A, Pechet L, Snyder LM. Continuous improvement, quality control, and cost containment in clinical laboratory testing. Effects of establishing and implementing guidelines for preoperative tests. Arch Pathol Lab Med 1995;119:518-22.
- Narr BJ, Hansen TR, Warner MA. Preoperative laboratory screening in healthy Mayo patients: cost-effective elimination of tests and unchanged outcomes. Mayo Clin Proc 1991;66:155-9.
- Nelson B, Carey WD, Vogt D, Beck G. Preoperative signs and laboratory values predict both survival after and hospital costs Hc of liver transplantation Lt. Gastroenterology 1987;92.
- Northup PGB. Preoperative delta-MELD score does not independently predict mortality after liver transplantation. Am J Transplant 2004;4:1643-9.
- Ntia IO, Okikiolu OA. Excretory urography before prostatectomy. Af J Med Med Sci 1996;25:75-9.
- Okelberry CR. Preadmission testing shortens preoperative length of stay. Hospitals 1974;49:71-2.
- Onder G, D’Arco C, Fusco D, Bernabei R. Preoperative assessment and risk factors in the surgical treatment of lung cancer: the role of age. Rays 2004;29:407-11.
- Oyama Y, Ali Y, Fishman M, Welles C, O’Connor N, Falco A, et al. Routine sinus CT scanning is unnecessary prior to hematopoietic stem cell transplantation. Blood 2001;98.
- Parker BM, Tetzlaff JE, Litaker DL, Maurer WG. Redefining the preoperative evaluation process and the role of the anesthesiologist. J Clin Anesth 2000;12:350-6.
- Parrish DOG. Exercise testing in special situations: ER, preoperative and disability evaluation. Prim Care 2001;28:199-208.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol 2005;19:663-79.
- Patel RIH. Laboratory tests in children undergoing ambulatory surgery: a review of clinical practice and scientific studies. Ambul Surg 2000;8:165-9.
- Pellikka PA, Roger VL, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. 2. Dobutamine stress echocardiography – techniques, implementation, clinical-applications, and correlations. Mayo Clin Proc 1995;70:16-27.
- Peredy TR, Powers RD. Bedside diagnostic testing of body fluids. Am J Emerg Med 1997;15:400-7.
- Phipps CG. Effectiveness of the clinical nurse specialist in preadmission testing. Health Matrix 1987;5:23-7.
- Poe RH, Kallay MC, Dass T, Celebic A. Can postoperative pulmonary complications after elective cholecystectomy be predicted?. Am J Med Sci 1988;295:29-34.
- Pokorny RM, Heniford T, Allen JW, Tuckson WB, Galandiuk S. Limited utility of preoperative studies in preparation for colostomy closure. Am Surg 1999;65:338-40.
- Pollard JB, Olson L. Early outpatient preoperative anesthesia assessment: does it help to reduce operating room cancellations?. Anesth Analg 1999;89:502-5.
- Pollard JB, Garnerin P, Dalman RL. Use of outpatient preoperative evaluation to decrease length of stay for vascular surgery. Anesth Analg 1997;85:1307-11.
- Pollard JB, Zboray AL, Mazze RI. Economic benefits attributed to opening a preoperative evaluation clinic for outpatients. Anesth Analg 1996;83:407-10.
- Popovic AD, Thomas JD, Neskovic AN, Cosgrove DM, Stewart WJ, Lauer MS. Time-related trends in the preoperative evaluation of patients with valvular stenosis. Am J Cardiol 1997;80:1464-8.
- Power LM, Thackray NM. Reduction of preoperative investigations with the introduction of an anaesthetist-led preoperative assessment clinic. Anaesth Intensive Care 1999;27:481-8.
- Putnis S, Nanuck J, Heath D. An audit of preoperative blood tests. J Perioper Pract 2008;18:56-9.
- Qiu CM. Anesthesia preoperative medicine clinic: beyond surgery cancellations. Anesthesiology 2006;105:224-5.
- Rabkin SW, Horne JM. Preoperative electrocardiography: its cost-effectiveness in detecting abnormalities when a previous tracing exists. CMAJ 1979;121:301-6.
- Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol 1995;86:346-8.
- Raw DN. Inadequate pre-operative evaluation and preparation 2. Anaesthesia 2001;56.
- Rennie M. Can we keep patients out of the ICU by pre-operative training?. Br J Intensive Care 2004.
- Reynolds TM. National Institute for Health and Clinical Excellence, Clinical Scince Reviews Committee of the Association for Clinical Biochemistry . National Institute for Health and Clinical Excellence guidelines on preoperative tests: the use of routine preoperative tests for elective surgery. Ann Clin Biochem 2006;43:13-6.
- Ricciardi G, Angelillo IF, Del PU, D’errico MM, Grasso GM, Gregorio P, et al. Routine preoperative investigation. Results of a multicenter survey in Italy. Collaborator Group. Int J Technol Assess Health Care 1998;14:526-34.
- Rich MW. The preoperative electrocardiogram: have we reached the end of an era?. J Am Geriatr Soc 2002;50:1301-3.
- Richie JP. The unproven utility of the preoperative urinalysis: economic evaluation. J Urol 1990;144:806-7.
- Ridgway EJ, Wilson AP, Kelsey MC. Preoperative screening cultures in the identification of staphylococci causing wound and valvular infections in cardiac surgery. J Hosp Infect 1990;15:55-63.
- Rink EH. Impact of introducing near patient testing for standard investigations in general practice. BMJ 1993;307:775-8.
- Roberts CJ, Fowkes FG, Ennis WP, Mitchell M. Possible impact of audit on chest X-ray requests from surgical wards. Lancet 1983;322:446-8.
- Robinson TN, Biffl WL, Moore EE, Heimbach JK, Calkins CM, Burch J. Routine preoperative laboratory analyses are unnecessary before elective laparoscopic cholecystectomy. Surg Endosc 2003;17:438-41.
- Roehrborn CG, Chinn HK, Fulgham PF, Simpkins KL, Peters PC. The role of transabdominal ultrasound in the preoperative evaluation of patients with benign prostatic hypertrophy. J Urol 1986;135:1190-3.
- Roizen M. Preoperative patient evaluation. Can J Anaesth 1989;36:S13-S19.
- Roizen MF. Preoperative evaluation of vascular patients: are the benefits worth the cost?. J Cardiothorac Vasc Anesth 1993;7:645-6.
- Roizen MF. Cost-effective preoperative laboratory testing. JAMA 1994;271:319-20.
- Roizen MF. Preoperative evaluation of patients: a review. Ann Acad Med Singapore 1994;23:49-55.
- Romfh RF. The appropriateness of routine diagnostic studies. Mil Med 1989;154:140-4.
- Russo V, Gostoli V, Lovato L, Montalti M, Marzocchi A, Gavelli G, et al. Clinical value of multidetector CT coronary angiography as a preoperative screening test before non-coronary cardiac surgery. Heart 2007;93:1591-8.
- Ryan P. The benefits of a nurse-led preoperative assessment clinic. Nurs Times 2000;96:42-3.
- Sanders DP, McKinney FW, Harris WH. Clinical evaluation and cost effectiveness of preoperative laboratory assessment on patients undergoing total hip arthroplasty. Orthopedics 1989;12:1449-53.
- Sanjay OP. Pre-operative serum potassium levels and peri-operative outcomes in patients undergoing cardiac surgery. Indian J Clin Biochem 2004;19:40-4.
- Schein OD. Assessing what we do. The example of preoperative medical testing. Arch Ophthalmol 1996;114:1129-31.
- Schroeder D. The preoperative period summary. Chest 1999;115:44S-46S.
- Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: A systematic review of the literature. Am J Med 2004;116:58-69.
- Sharaf RN, Weinshel EH, Bini EJ, Rosenberg J, Sherman A, Ren CJ. Endoscopy plays an important preoperative role in bariatric surgery. Obes Surg 2004;14:1367-72.
- Sheehan JJ, Ridge CA, Ward EV, Duffy GJ, Collins CD, Skehan SJ, et al. FDG PET in preoperative assessment of colorectal liver metastases combining ‘evidence-based practice’ and ‘technology assessment’ methods to develop departmental imaging protocols: should FDG PET be routinely used in the preoperative assessment of patients with colorectal liver metastases?. Acad Radiol 2007;14:389-97.
- Sihoe ADL, Lee TW, Ahuja AT, Yim APC. Should cervical ultrasonography be a routine staging investigation for lung cancer patients with impalpable cervical lymph nodes?. Eur J Cardiothoracic Surg 2004;25:486-91.
- Silecchia GP. Role of routine preoperative chest X-ray in patients candidate to laparoscopic surgery. Results of a prospective study. Chirurgia 2000;13:23-7.
- Silverstein MD, Boland BJ. Conceptual framework for evaluating laboratory tests: case-finding in ambulatory patients. Clin Chem 1994;40:1621-7.
- Singh B, Gupta R, Yadav SP. Pre-operative haemoglobin estimation is essential. J Laryngol Otol 1994;108:920-1.
- Sinha CK, Hamaker R, Hamaker RC, Freeman SB, Borrowdale RW, Huntley TC. Utility of preoperative radionuclide scanning for primary hyperparathyroidism. Laryngoscope 1997;107:753-8.
- Smetana GW, MacPherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003;87:7-40.
- Sommerville TE, Murray WB. Information yield from routine pre-operative chest radiography and electrocardiography. S Afr Med J 1992;81:190-6.
- Straube S, Derry S, Mcquay HJ, Moore RA. Effect of preoperative Cox-II-selective NSAIDs (coxibs) on postoperative outcomes: a systematic review of randomized studies. Acta Anaesthesiol Scand 2005;49:601-13.
- Swanson HL, Scheb DM. The role of the anesthesia care coordinator in preadmission testing. AORN J 1996;64:776-80.
- Tabas GH, Vanek MS. Is ‘routine’ laboratory testing a thing of the past? Current recommendations regarding screening. Postgrad Med 1999;105:213-20.
- Tait AR, Parr HG, Tremper KK. Evaluation of the efficacy of routine preoperative electrocardiograms. J Cardiothorac Vasc Anesth 1997;11:752-5.
- Takemura Y, Ishida H, Inoue Y, Beck JR. Yield and cost of individual common diagnostic tests in new primary care outpatients in Japan. Clin Chem 2002;48:42-54.
- Takemura Y, Ishida H, Inoue Y, Kobayashi H, Beck JR. Opportunistic discovery of occult disease by use of test panels in new, symptomatic primary care outpatients: yield and cost of case finding. Clin Chem 2000;46:1091-8.
- Tarazi EM, Ramirez R, Davoodian K. A survey of preoperative testing requirements. Anesthesiology Abstracts of Scientific Papers Annual Meeting 2000.
- Tawam MN, Talano JV, Chaudhry FA. Use of dobutamine stress echocardiography for risk assessment before noncardiac surgery. Am J Card Imaging 1996;10:128-32.
- Thompson RE. Determining the necessity for routine preoperative laboratory tests and x-rays: a cooperative PSRO/hospital study. QRB Qual Rev Bull 1979;5:15-7.
- Thompson RS, Kirz HL, Gold RA. Changes in physician behavior and cost savings associated with organizational recommendations on the use of ‘routine’ chest X-rays and multichannel blood tests. Prev Med 1983;12:385-96.
- Thue G, Sandberg S. Survey of office laboratory tests in general practice. Scand J Prim Health Care 1994;12:77-83.
- Tierney WM, Miller ME, McDonald CJ. The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests. N Engl J Med 1990;322:1499-504.
- Tigges S, Roberts DL, Vydareny KH, Schulman DA. Routine chest radiography in a primary care setting. Radiology 2004;233:575-8.
- Usal H, Nabagiez J, Sayad P, Ferzli GS. Cost effectiveness of routine type and screen testing before laparoscopic cholecystectomy. Surg Endosc 1999;13:146-7.
- Van Der Merwe WL, Coetzee AR. Pre-operative assessment and management of the patient with ischaemic coronary artery disease in non-cardiac surgery. S Afr J Surg 1992;30:99-103.
- Van Klei WA, Grobbee DE, Rutten CL, Hennis PJ, Knape JT, Kalkman CJ, et al. Role of history and physical examination in preoperative evaluation. Eur J Anaesthesiol 2003;20:612-18.
- Van Klei WA, Moons KG, Van Rheineck Leyssius AT, Kalkman CJ, Rutten CL. Validation of a preoperative prediction rule to predict RBC transfusions. Anesthesiology Abstracts of Scientific Papers Annual Meeting 2001:abstract-1083.
- Van Klei WA, Moons KG, Van Rheineck Leyssius AT, Knape JT, Rutten CL. Preoperative prediction of RBC transfusions: a reduction of type and screen investigations. Anesthesiology Abstracts of Scientific Papers Annual Meeting 2000.
- Van Klei W, Moons K, Leyssius A, Knape J, Rutten C, Grobbee D. A reduction in type and screen: preoperative prediction of RBC transfusions in surgery procedures with intermediate transfusion risks. Br J Anaesth 2001;87:250-7.
- Vanzuidewijn DBWD, Songun I, Hamming J, Kievit J, Vandevelde CJH, Veselic M. Preoperative diagnostic-tests for operable thyroid-disease. World J Surg 1994;18:506-11.
- Velanovich V. How much routine preoperative laboratory testing is enough?. Am J Med Qual 1993;8:145-51.
- Velanovich V. Preoperative laboratory screening based on age, gender, and concomitant medical diseases. Surgery 1994;115:56-61.
- Vogt AW, Henson LC. Unindicated preoperative testing: ASA physical status and financial implications. J Clin Anesth 1997;9:437-41.
- Wagner JD, Moore DL. Preoperative laboratory testing for the oral and maxillofacial surgery patient. J Oral Maxillofac Surg 1991;49:177-82.
- Walton GM. The cost benefit of routine pre-operative Hb investigations for oral surgery. Br Dent J 1988;165:406-7.
- Wattsman TAD. The utility of preoperative laboratory testing in general surgery patients for outpatient procedures. Am Surg 1997;63:81-90.
- West JC, Napoliello DA, Costello JM, Nassef LA, Butcher RJ, Hartle JE, et al. Preoperative dobutamine stress echocardiography versus cardiac arteriography for risk assessment prior to renal transplantation. Transpl Int 2000;13:S27-S30.
- Wetchler BV. Preoperative laboratory and diagnostic testing: cost vs. value. Ambul Surg 1999;7:1-2.
- Wiencek RG, Weaver DW, Bouwman DL, Sachs RJ. Usefulness of selective preoperative chest x-ray films. A prospective study. Am Surg 1987;53:396-8.
- Zwack GC, Derkay CS. The utility of preoperative hemostatic assessment in adenotonsillectomy. Int J Pediatr Otorhinolaryngol 1997;39:67-76.
Appendix 1 Systematic review of clinical effectiveness: MEDLINE search strategies
Terms for surgery/pre-operative care
-
Surgery/
-
surgery-elective.tw.
-
surgical procedures, elective/or surgical procedures, minor/
-
elective surgery.tw.
-
minor surgery.tw.
-
intermediate surgery.tw.
-
Ambulatory Surgical Procedures/
-
day surgery.tw.
-
asymptomatic.tw.
-
preoperative.tw.
-
pre-operative.tw.
-
pre operative.tw.
-
Ambulatory Care/
-
or/1-13
Terms for routine test
-
Diagnostic Tests, Routine/
-
Preoperative Care/
-
routine test$.tw.
-
routine assessment$.tw.
-
routine investigation$.tw.
-
Clinical Chemistry Tests/
-
Risk Assessment/
-
Blood Cell Count/
-
full blood count.tw.
-
fbc.tw.
-
Hematologic Tests/
-
Urea/
-
Urinalysis/
-
Electrolytes/
-
urine test$.tw.
-
blood test$.tw.
-
u&e.tw.
-
(electrolytes and renal function).tw.
-
Respiratory Function Tests/
-
pulmonary function test$.tw.
-
Spirometry/
-
spirometry.tw.
-
Blood Gas Analysis/
-
blood gas analysis.tw.
-
pft.tw.
-
measurement of respiratory mechanics.tw.
-
measurement of transfer function.tw.
-
Exercise Test/
-
exercise test$.tw.
-
Respiratory System/
-
(42 or 43) and 44
-
h?ematolog$test$.tw.
-
vitalograph.tw.
-
FEV1.tw.
-
Vital Capacity/
-
vital capacit$.tw.
-
transfer function.tw.
-
Pulmonary Diffusing Capacity/
-
diffusing capacit$.tw.
-
dlco.tw.
-
exp Lung Volume Measurements/
-
lung capacit$.tw.
-
cardiopulmonary exercise test$.tw.
-
cpx.tw.
-
maxim$oxygen uptake.tw.
-
V02max.tw.
-
Oxygen Consumption/
-
or/15-41,45,46-61
Adult terms
-
adult/or aged/or middle aged/
-
adult$.tw.
Diagnosis filter from McMaster University
-
sensitiv:.mp.
-
diagnos:.mp.
-
di.fs.
-
or/64-66
For the clinical effectiveness searches, the terms for surgery and pre-operative care were combined with the terms describing the routine test and what they measured/assessed (15–61). These terms were then combined with terms for adults, our target population. Because the tests are used for diagnostic purposes, the search was combined with the McMaster University filter for finding diagnosis studies.
Adverse events search
Adverse events terms
-
Diagnostic Errors/
-
False Negative Reactions/
-
False Positive Reactions/
-
Observer Variation/
-
diagnostic error$.tw.
-
false negative$.tw.
-
false positive$.tw.
-
OR/1-7
The above terms for adverse effects were combined with the surgery and pre-operative care terms, the routine test terms, and the adult terms.
Anaesthetic drug search
Anaesthetic drug terms
-
sevoflurane.af.
-
ultane.af.
-
28523-86-6.af.
-
desflurane.af.
-
57041-67-5.af.
-
suprane.af.
-
Propofol/
-
diprivan.af.
-
2078-54-8.af.
-
rocuronium.af.
-
esmeron.af.
-
143558-00-3.af.
-
sugammadex.af.
-
bridion.af.
-
organnon25969.af.
The common anaesthetic drug terms were combined with the surgery and pre-operative care terms, the routine test terms and the adult terms.
Appendix 2 Systematic review of clinical effectiveness: quality assessment of randomised controlled trial
Assessment tool based on NHS CRD Report No. 48
| Study | |
|---|---|
| Was the method used to assign participants to the treatment groups really random? | |
| What method of assignment was used? | |
| Was the allocation of treatment concealed? | |
| What method was used to conceal treatment allocation? | |
| Was the number of participants who were randomised stated? | |
| Were details of baseline comparability presented? | |
| Was baseline comparability achieved? | |
| Were the eligibility criteria for study entry specified? | |
| Were any co-interventions identified that may influence the outcomes for each group? | |
| Were the outcome assessors blinded to the treatment allocations? | |
| Were the individuals who administered the intervention blinded to the treatment allocation? | |
| Were the participants who received the intervention blinded to the treatment allocation? | |
| Was the success of the blinding procedure assessed? | |
| Were at least 80% of the participants originally included in the randomised process followed up in the final analysis? | |
| Were the reasons for withdrawal stated? | |
| Was an intention-to-treat analysis included? | |
Appendix 3 Final protocol
Background to the Study
In 2003 NICE published Clinical Guideline 3 which reviewed the use of routine pre-operative tests prior to routine surgery. Prior to the guideline preparation a systematic review by Munro, Booth and Nicholl was undertaken on behalf of the HTA programme in 1997. The guideline development group undertook their own review of the literature. These two reviews defined and updated the purpose of pre-operative testing of apparently healthy patients.
Of the evidence base used to produce the guideline over 50% was graded as amber i.e. the benefit of the test was unknown. Therefore, despite the existence of some primary research, the evidence on which to base pre-operative testing protocols was inconclusive. Alongside this there has been an increasing awareness of the possibility of subjecting patients to unnecessary tests, and of the issues involved in dealing with the results of tests that may alarm patients but have little clinical significance.
The aims of this study
The aims:
-
undertake a systematic review of the literature of the clinical effectiveness of routine testing of full blood count (FBC), electrolytes and renal function (U&E), and pulmonary function (PFT) as part of the pre-operative assessment procedures for patients classified as American Association of Anaesthesiologist (ASA) grade 1 and 2 who are undergoing minor or intermediate procedures.
-
Evaluate the cost effectiveness of mandating or withdrawing each of these tests from routine pre-operative assessment for patients ASA grade 1 and 2 and minor and intermediate surgery
-
Compare the evidence with the recommendations in the NICE Guidance (2003) and observed practice in NHS hospitals
-
Identify using modelling techniques the expected value of information (EVI) whether there is value in the NHS in commissioning further primary research into the routine use of FBC, U&Es and PFTs in this patient population.
Search restrictions
The searches will not be restricted by date of language.
Inclusion and exclusion criteria
Inclusion criteria
Population
-
Adult patients classified as ASA grade 1 or 2 undergoing minor (grade 1) or intermediate (grade 2) surgery (including elective general surgery, day surgery, and minor orthopaedic procedures) as classified by the CCSD Schedule of Procedures 2005. 1 Where possible, to subdivide these into the following subgroups:
-
– Apparently healthy patients with no clinical indication for testing FBC, U&Es and PFTs
-
– Patients with common comorbidities (e.g. respiratory disease, renal disease)
-
– Patients receiving treatments likely to alter test results (e.g. diuretics).
-
It was originally planned to limit the population to adults aged 16 to 60. However, because of the paucity of relevant studies which met this inclusion criterion, the population was later extended to include all adult patients.
Intervention
-
Routine preoperative testing of:
-
– Full blood count (FBC) (including haemoglobin concentration, haematocrit, platelet count, and white blood cell count)
-
– Electrolytes and renal function (U&E) (including sodium, potassium, urea, and creatinine)
-
– Pulmonary function (PFT) (including some or all of spirometry, blood gas analysis, measurement of respiratory mechanics, measurement of transfer function, and exercise testing of respiratory system)
-
Comparator
-
No routine preoperative testing
Outcomes
-
Abnormal test results
-
Changes in management following abnormal test results in patients whose preoperative clinical examinations were normal
-
Adverse events possibly related to the test result
-
Adverse events probably or possibly caused by the process of testing
-
All-cause mortality
Setting
-
Any country
Date
-
1980 onwards
Study type
-
RCTs
-
Controlled non-randomised studies (eg cohort studies)
-
Case–control studies
-
Case series
-
Case reports
-
Systematic reviews
-
Economic evaluations
Exclusion criteria
The following publication types will be excluded from the review:
-
Animal models
-
Narrative reviews, editorials, opinions.
Sifting
The references identified by the electronic literature searches will be sifted in three stages. Screening for relevance first by title and then by abstract. Those papers which seem from their abstract (or if there is no abstract available) to be relevant will be retrieved and read in full. At each step, studies which do not satisfy the inclusion criteria will be excluded.
Data extraction strategy
A customised data extraction form will be based on those proposed by the NHS Centre for Reviews and Dissemination. 8 Where possible, data will be extracted by one reviewer, and thoroughly checked by a second reviewer; any disagreements will be resolved by discussion.
Where available, data relating to the following outcomes will be extracted:
-
all-cause mortality
-
significant abnormal test findings
-
change of management
-
length of hospital stay
-
adverse effects probably or possibly related to the test result
-
adverse events probably or possibly caused by the process of testing.
Quality assessment strategy
We propose to assess to use criteria based on those proposed by the NHS Centre for Reviews and Dissemination8 (see Appendix 2) to assess the methodological quality of randomised trials which meet the inclusion criteria.
We will assess the methodological and reporting quality of case series studies which meet the inclusion criteria using a customised quality tool combining generic criteria proposed by the NHS Centre for Reviews and Dissemination8 and Chambers et al. 10 with review-specific criteria, as follows:
-
Generic criteria:
-
– Were patients recruited prospectively?
-
– Were patients recruited consecutively?
-
– Were at least 90% of those included at baseline followed up (prospective studies only)?
-
– Was loss to follow-up reported or explained (prospective studies only)?
-
– Was follow-up long enough for important events to occur?
-
– Were outcomes assessed using objective criteria or was blinding used?
-
– Was an appropriate measure of variability reported?
-
-
Review-specific criteria:
-
– Were the patients’ age and ASA status adequately reported?
-
– Was the operation type and/or risk classification adequately reported?
-
– Were all operations elective?
-
– Were all the tests conducted genuinely routine, or might some have been indicated?
-
– Was a definition of normal or abnormal results provided?
-
Meta-analysis strategy
Where appropriate, meta-analysis will be used to pool results, and summary statistics will be derived for each study and a weighted average of the summary statistics be computed across the studies.
The survey
We propose to survey the current protocol use in NHS Trusts in England and Wales to establish if the NICE Guidance is being adhered to. This will be carried out by sending paper questionnaires based on the Abacus study in 2005 to nurses involved in pre operative assessment care.
Economic evaluation
Analysis plan: final protocol
Background
The objective of the study is the value of routinely testing FBC, U&E and PFT in patients with (1) no apparent clinical indication and (2) subgroups with common comorbidities.
The originally proposed approach to construct an economic model based on published literature has not proved possible due to the lack of published evidence on the effectiveness of these tests.
We have identified a large routine patient level data set on tests, surgical procedure and outcomes at Leeds Teaching Hospital Trust with an excess of 1m records. We propose to utilise econometric methods to address the following questions.
-
Having adjusted for patient level characteristics and surgical intervention does not having the tests result in worse outcomes for patients without comorbidities?
-
Having adjusted for patient level characteristics and surgical intervention does not having the tests result in worse outcomes for patients with comorbidities?
Proposed methods
The subset of records for minor and intermediate risk surgical procedures have been identified on the basis of BUPA schedule of procedures. This is consistent with the methods used in our previous work on pre-operative assessment.
The outcomes to be used for these analyses are:
-
Length of stay – continuous in days
-
Readmission within 30 days
-
Hospital mortality
For categorical variables (Readmission within 30 days and Hospital mortality) we will logistic regression models; whilst for the continuous variable (length of stay) we will estimate linear regression models.
The pre-specified conditioning variables in each analysis will be:
-
Age –in years
-
Sex (1 = female, 0 = male)
-
Ethnicity
-
socio-economic status – IMD04_decile
-
Primary Diagnosis
-
Secondary Diagnosis
-
Surgical Procedure
The key explanatory variables will be:
-
Full Blood Count;
-
U&E; and
-
Pulmonary function test
These will be entered as dummy variables. We will also enter joint dummy variables for each possible combination of these tests; e.g. FBC and U&E.
The models will be estimated separately for patients with and without comorbidities. Comorbidities will be modelled in two ways. First we will use the presence or absence of a secondary diagnosis as evidence of a comorbidity. Secondly, we will use whether patients had additional tests as a proxy for the presence of a comorbidity. We believe the second measure may be a more sensitive measure for the presence of a comorbidity, although obviously less specific than the recorded secondary diagnosis.
The outputs of these analyses will be six separate models assessing whether the absence of any combination of the three tests is associated with a difference in any of the three measures of outcome. The models will be assessed using standard statistical measures for goodness of fit, specification, and collinearity.
We will report the models in full and whether there is a statistically significant relationship between the absence of any combination of the three tests and length of stay, 30 day re-admission and hospital mortality.
Where the models report a significant relationship we will examine the costs incurred for the tests and the costs associated with the different outcomes in order to assess the likely value of the tests to the NHS. Given the extremely large number of observations available for these analyses, we judge that the absence of statistically significant relationship is sufficient to treat absence of evidence as evidence of absence.
Examining the value of more research
The estimated models can be used to explore the value of information associated with further research into these tests. The standard errors on the model parameters will provide a measure of the uncertainty associated with the relationship between the presence or absence of the test results and patient outcomes. Should we find evidence of a relationship between the routine tests and any of these outcomes, it will be appropriate to examine the value of undertaking further research, such as a prospective randomised controlled trial of these test, to inform future policy.
Using monte carlo simulation it is possible to simulate a distribution for the expected outcomes. The simulated distributions will describe the probability that the use of the routine tests are associated with difference in each of the outcomes and by extension the risk that using the central estimate to guide practice will lead to making the wrong decision. By attaching a value to each of the outcomes, e.g. the cost of a readmission or the value of a statistical life, it will be possible to attribute a value to reducing the risk of making the wrong decision through further research.
Appendix 4 Systematic review of clinical effectiveness: tabulation of excluded studies
| Study | Reason for exclusion |
|---|---|
| Adams et al. 199248 | Age range 13–80 years; no relevant subgroup analyses |
| Ajimura et al. 200549 | No data regarding ASA grade |
| Alam et al. 200350 | Age range 4–59 years; no relevant subgroup analyses |
| Anonymous 199951 | Brief summary of an unreferenced Mayo Clinic study |
| Arieta et al. 200452 | ASA grades 1–3; no relevant subgroup analyses |
| Barazzoni et al. 199953 | Includes children; no relevant subgroup analyses |
| Billings et al. 199354 | No data regarding age or ASA grade |
| Bryson et al. 200655 | No data regarding age or ASA grade |
| Cartana et al. 198956 | No data regarding ASA grade |
| Desmonts 199357 | Nature of surgery not recorded (very poorly reported study) |
| Diouf et al. 199858 | Article not available |
| Dunne et al. 200259 | ASA grades 1–5; no relevant subgroup analyses |
| Ebert et al. 199760 | ASA grades 2–4; no relevant subgroup analyses |
| Finegan et al. 200561 | ASA grades 1–4; no relevant subgroup analyses |
| Fischer 199962 | Not research study |
| Fourcade 198963 | ASA grades 1–4; no relevant subgroup analyses |
| Gallus et al. 197364 | Major surgery |
| Golub et al. 199265 | ASA grades 1–3; no relevant subgroup analyses |
| Halabe-Cherem et al. 199566 | Includes major and emergency surgery |
| Hans et al. 200667 | Includes major surgery |
| Johnson et al. 198868 | No data regarding ASA grade |
| Johnson et al. 200269 | No data regarding ASA grade |
| Kamimura et al. 200670 | No data regarding ASA grade |
| Kaplan et al. 198571 | No data regarding age or ASA grade |
| Keenan et al. 199872 | Focus not on specific tests |
| Kocabas et al. 199673 | Includes major surgery |
| Lira et al. 200174 | ASA grades 1–3; no relevant subgroup analyses |
| Lira et al. 200375 | ASA grades 1–3; no relevant subgroup analyses |
| MacPherson et al. 199076 | No data regarding ASA grade |
| Mantha et al. 200577 | No data regarding ASA grade |
| McAlister et al. 200378 | No data regarding ASA grade |
| McCleane 199079 | All ages and ASA grades 1–4; subgroup analyses by ASA grade but not by age |
| McKee and Scott 198780 | No data regarding ASA grade |
| McKibbin 199681 | No data regarding ASA grade |
| Meguro et al. 199682 | Article not available |
| Mignonsin et al. 199683 | All ages and ASA grades 1–3; subgroup analyses by ASA grade but not by age |
| Morales-Orozco et al. 200584 | Article not available |
| Narr et al. 199185 | Includes children; no relevant subgroup analyses |
| Nascimento et al. 200486 | ASA grades 1–3; no relevant subgroup analyses |
| Pfaff and van der Linden 198987 | No data regarding ASA grade |
| Philip et al. 199788 | Article not available; appears to be same study as Philip et al. 1999,89 which did not meet the study inclusion criteria |
| Roseano et al. 200290 | ASA grades 2–5; no relevant subgroup analyses; may include emergency surgery |
| Schein et al. 200091 | Intervention takes the form of a ‘standard battery of medical tests’ including electrocardiography and glucose as well as CBC and serum electrolytes, urea nitrogen and creatinine; results reported for the total package, not by individual test |
| Stephens 200092 | Summary of study by Schein et al. 200091 |
| Suh et al. 199793 | Article not available |
| Velanovich 199194 | No data regarding ASA grade |
| Velanovich 199395 | No data regarding ASA grade |
| Walters and McKibbin 199796 | ASA grades 1–3; no relevant subgroup analyses |
| Wattsman and Davies 199797 | ASA grades 1–3; no relevant subgroup analyses |
| Wittgen et al. 199398 | Mean ASA grade > 2; no relevant subgroup analyses |
| Wyatt et al. 198999 | No data regarding age or ASA grade |
Appendix 5 Systematic review of clinical effectiveness: quality assessment of non-randomised studies
| Criterion | Study | |||||
|---|---|---|---|---|---|---|
| Gnocchi et al.11 | Haug and Reifeis13 | Roukema et al.14 | Szmuk et al.9 | Tallo et al.12 | Turnbull and Buck15 | |
| Prospective recruitment | Yes | Yes | Yes | No | No | No |
| Consecutive recruitment | Yes | Yes | Yes | Not clear | Yes | Not clear |
| ≥ 90% followed up | No | No | Yes | Not applicable | No | Not applicable |
| Loss to follow-up reported or explained | Yes | Yes | Not applicable | Not applicable | Yes | Not applicable |
| Follow-up long enough | Yes | Yes | Not clear | Yes | Not clear | Not clear |
| Outcome assessment objective or blinded | Not clear | Yes | Not clear | Yes | Yes | Yes |
| Measure of variability | Yes | No | No | No | No | No |
| Age and ASA status reported | Yes | Yes | No | Yes | Yes | Not clear |
| Operation type and/or risk category adequately reported | Yes | Yes | Yes | Yes | Yes | Yes |
| All operations elective | Yes | Yes | Yes | Yes | Yes | Not clear |
| All tests routine | Yes | Yes | Yes | Yes | Not clear | Not clear |
| Normal/abnormal results defined | No | No | Yes | Yes | Yes | Not clear |
Appendix 6 Apparent patient flow in the study by Gnocchi et al.11
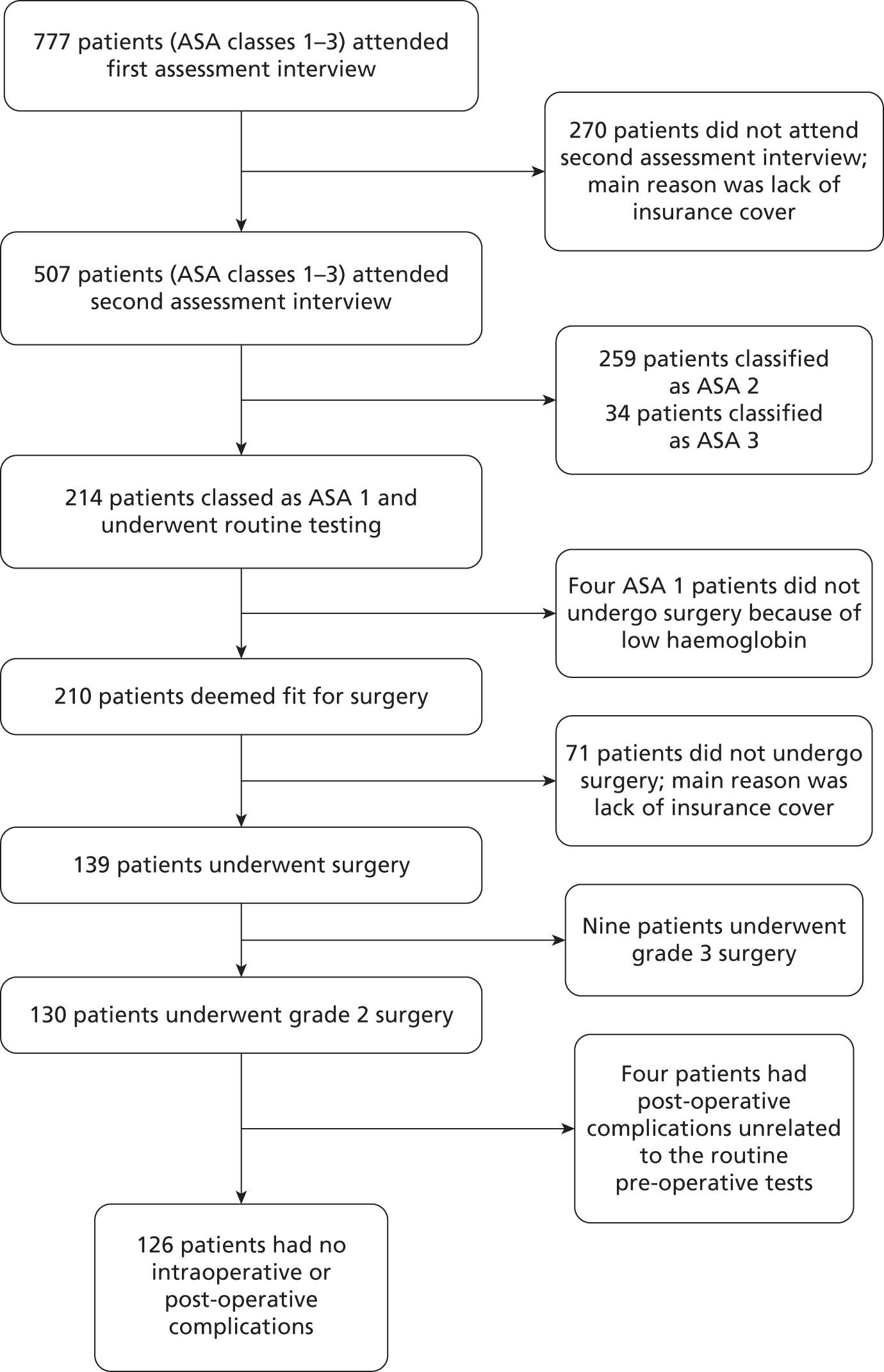
Appendix 7 Turnbull and Buck:15 summary of relevant pre-operative test results and relevant complications
| Test | Hb | Platelets | White blood cells |
|---|---|---|---|
| No. of abnormal test results | 7/1005 | 0/1005 | 1/1005 |
| Rate | 0.7% | 0% | 0.1% |
| 95% CI | 0.2% to 1.2% | Not applicable | –1.0% to 0.3% |
| Patients with relevant complications: total | 16/1010 | 37/1010 | 110/1010 |
| Rate | 1.6% | 3.7% | 10. 9% |
| 95% CI | 0.8% to 2.4% | 2.5% to 4.8% | 9.0% to 12.8% |
| Patients with relevant complications: normal test result | 14/998 | 37/1005 | 110/1004 |
| Rate | 1.4% | 3.7% | 11.0% |
| 95% CI | 0. 7% to 2.1% | 2.5% to 4.8% | 9.0% to 12.9% |
| Patients with relevant complications: abnormal test result | 2/7 | 0 | 0 |
| Rate | 28.6% | 0% | 0% |
| 95% CI | –4.9% to 62.0% | Not applicable | Not applicable |
| Patients with abnormal test result: action | 2/7 | Not applicable | 0 |
| Rate | 28.6% | Not applicable | 0% |
| 95% CI | –4.9% to 62.0% | Not applicable | Not applicable |
| Patients with abnormal test result: no action | 5/7 | Not applicable | 1/1 |
| Rate | 71.4% | Not applicable | Not applicable |
| 95% CI | 38.0% to 105.0% | Not applicable | Not applicable |
| Patients with abnormal test result and relevant complication: action | 1/2 | Not applicable | Not applicable |
| Rate | 50% | Not applicable | Not applicable |
| 95% CI | –19.3% to 119.3% | Not applicable | Not applicable |
| Patients with abnormal test result and relevant complication: no action | 1/2 | Not applicable | Not applicable |
| Rate | 50% | Not applicable | Not applicable |
| 95% CI | –19.3% to 119.3% | Not applicable | Not applicable |
| Test | Sodium | Potassium | Creatinine | Urea |
|---|---|---|---|---|
| No. of abnormal test results | 5/995 | 14/995 | 2/995 | 1/995 |
| Rate | 0.5% | 1.41% | 0.2% | 0.1% |
| 95% CI | 0.1% to 0.9% | 0.7% to 2.1% | –0.1% to 0.5% | –0.1% to 0.3% |
| Patients with relevant complications: total | 1/1010 | 21/1010 | 0/1010 | 0/1010 |
| Rate | 0.1% | 2.08% | 0% | 0% |
| 95% CI | –0.1% to 0.3% | 1.2% to 3.0% | Not applicable | Not applicable |
| Patients with relevant complications: normal test result | 1/990 | 20/981 | 0/993 | 0/994 |
| Rate | 0.1% | 2.0% | 0% | 0% |
| 95% CI | –0.1% to 0.3% | 1.2% to 2.9% | Not applicable | Not applicable |
| Patients with relevant complications: abnormal test result | 0/5 | 1/14 | 0/2 | 0/1 |
| Rate | 0% | 7.1% | 0% | 0% |
| 95% CI | Not applicable | –6.4% to 20.6% | Not applicable | Not applicable |
| Patients with abnormal test result: action | 0/5 | 4/14 | 0/2 | 0/1 |
| Rate | 0% | 28.6% | 0% | 0% |
| 95% CI | Not applicable | 4.9% to 52.2% | Not applicable | Not applicable |
| Patients with abnormal test result: no action | 5/5 | 10/14 | 2/2 | 1/1 |
| Rate | 100% | 71.4% | 100% | 100% |
| 95% CI | Not applicable | 47.8% to 95.1% | Not applicable | Not applicable |
| Patients with abnormal test result and relevant complication: action | Not applicable | 1/4 | Not applicable | Not applicable |
| Rate | Not applicable | 25.0% | Not applicable | Not applicable |
| 95% CI | Not applicable | –17.4% to 67.4% | Not applicable | Not applicable |
| Patients with abnormal test result and relevant complication: no action | Not applicable | Not applicable | Not applicable | |
| Rate | Not applicable | Not applicable | Not applicable | |
| 95% CI | Not applicable | Not applicable | Not applicable |
Appendix 8 Review of the adverse effects of venepuncture and pulmonary function testing: methods
Identification of studies
Literature searches were performed in August 2009 to retrieve papers on any adverse events associated with the performance of either venepuncture used to obtain samples for blood testing or PFTs.
Sources searched
The following electronic bibliographic databases were searched:
-
BIOSIS
-
CINAHL
-
CDSR
-
CENTRAL
-
EMBASE
-
MEDLINE
-
MEDLINE In-Process & Other Non-Indexed Citations
-
NHS DARE
-
NHS HTA Database
-
SCI.
Search strategies
The MEDLINE search strategies are as follows.
Blood test adverse events search
-
exp Hematologic Tests/ae [Adverse Effects]
-
Blood Specimen Collection/ae [Adverse Effects]
-
Phlebotomy/ae [Adverse Effects] (335)
-
1 or 2 or 3
-
adult/or aged/or middle aged/
-
adult$.tw.
-
5 or 6
-
4 and 7
Pulmonary function test adverse events search
-
exp Respiratory Function Tests/ae [Adverse Effects]
-
adult/or aged/or middle aged/
-
adult$.tw.
-
2 or 3
-
1 and 4
-
limit 6 to yr=“1999 -Current”
The MEDLINE strategies were adapted for use in the other databases; these search strategies are available on request.
Search restrictions
The searches were not restricted by language. Because of the large number of results retrieved, the PFT adverse effects search was limited to the last 10 years.
Inclusion and exclusion criteria
Inclusion criteria
Population
-
Adult patients classified as ASA grade 1 or 2, or otherwise stated to be healthy.
Intervention
-
Simple venepuncture for diagnostic or screening purposes.
-
Pulmonary function testing.
Outcomes
-
Adverse events probably or possibly caused by the process of testing.
Setting
-
Any country.
Study type
-
RCTs.
-
Controlled non-randomised studies (e.g. cohort studies).
-
Case–control studies.
-
Case series.
-
Case reports.
-
Systematic reviews.
-
Economic evaluations.
Exclusion criteria
As in the systematic review of clinical effectiveness, the following publication types were excluded:
-
animal models
-
narrative reviews, editorials and opinions.
In addition, studies were excluded if:
-
venepuncture was used only to obtain blood donations, or was used both to obtain blood donations and to obtain smaller samples for diagnostic or screening purposes, but no distinction was made between the two uses
-
cannulation or catheterisation were used to obtain blood samples
-
they related to the collection of arterial or capillary rather than venous blood samples.
Sifting
The same three-stage sifting process was used as was used in the systematic review of clinical effectiveness.
Data extraction strategy
Data were extracted directly to the tables included in the report.
Quality assessment strategy
Because many of the relevant studies took the form of case reports, a formal quality assessment was not undertaken. Larger studies (observational or before-and-after studies) were deemed to be of higher quality than case series or case reports, and the latter were included only if they related to adverse events for which data were not available from the larger studies.
Appendix 9 Review of the adverse effects of venepuncture: quantity of research available
The electronic literature searches identified 466 potentially relevant articles. Of these, eight articles4,16,18–23 met the review’s inclusion criteria (see Figure 4).
FIGURE 4.
Adverse effects of venepuncture: summary of study selection and exclusion (electronic literature searches).
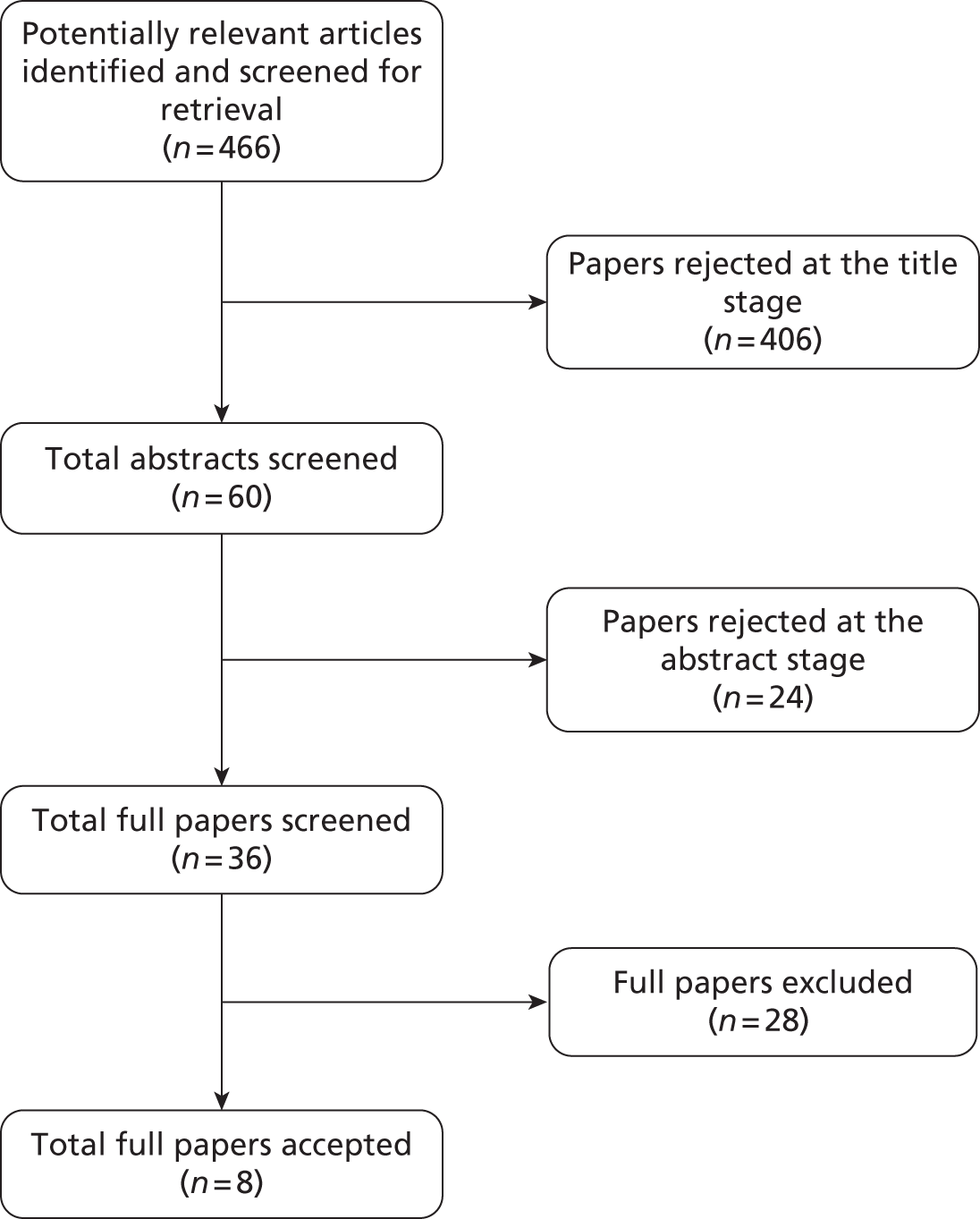
Three additional relevant articles, by Berry and Wallis,24 Horowitz17 and Yuan and Cohen,25 were identified only from citations.
Appendix 10 Review of the adverse effects of pulmonary function testing: quantity of research available
The electronic literature searches identified 396 potentially relevant articles. Of these, two articles14,28 met the review’s inclusion criteria (see Figure 5).
FIGURE 5.
Adverse effects of pulmonary function testing: summary of study selection and exclusion (electronic literature searches).
Three additional relevant articles, by Manço et al. ,31 Nemet et al. 32 and Varkey and Cory,33 were identified only from citations, and a fourth, by Patel et al. ,28 was identified by the clinical effectiveness searches.
Appendix 11 Search strategies for cost-effectiveness review
MEDLINE/MEDLINE In-Process & Other Non-Indexed Citations – Ovid MEDLINE(R) <1950 to January week 1 2009>
| # | Search history | Results |
|---|---|---|
| 1 | Surgery/ | 27,739 |
| 2 | surgery-elective.tw. | 64 |
| 3 | Surgical procedures, elective/or surgical procedures, minor/ | 5914 |
| 4 | Elective surgery.tw. | 4729 |
| 5 | Minor surgery.tw. | 1104 |
| 6 | Intermediate surgery.tw. | 23 |
| 7 | Ambulatory Surgical Procedures/ | 8227 |
| 8 | Day surgery.tw. | 1484 |
| 9 | asymptomatic.tw. | 74,729 |
| 10 | preoperative.tw. | 107,611 |
| 11 | pre-operative.tw. | 10,624 |
| 12 | Pre operative.tw. | 10,624 |
| 13 | Ambulatory Care/ | 29,659 |
| 14 | or/1-13 | 262,897 |
| 15 | Diagnostic Tests, Routine/ | 4708 |
| 16 | Preoperative Care/ | 40,716 |
| 17 | Routine test$.tw. | 1985 |
| 18 | Routine assessment$.tw. | 749 |
| 19 | Routine investigation$.tw. | 738 |
| 20 | Clinical Chemistry Tests/ | 677 |
| 21 | Risk Assessment/ | 96,059 |
| 22 | Blood Cell Count/ | 17,175 |
| 23 | Full blood count.tw. | 391 |
| 24 | fbc.tw. | 227 |
| 25 | Hematologic Tests/ | 3910 |
| 26 | Urea/ | 32,935 |
| 27 | Urinalysis/ | 2848 |
| 28 | Electrolytes/ | 17,466 |
| 29 | Urine test$.tw. | 1538 |
| 30 | Blood test$.tw. | 7113 |
| 31 | u&e.tw. | 451 |
| 32 | (electrolytes and renal function).tw. | 596 |
| 33 | Respiratory Function Tests/ | 32,143 |
| 34 | Pulmonary function test$.tw. | 6153 |
| 35 | Spirometry/ | 14,322 |
| 36 | spirometry.tw. | 6808 |
| 37 | Blood Gas Analysis/ | 17,075 |
| 38 | Blood gas analysis.tw. | 2756 |
| 39 | pft.tw. | 806 |
| 40 | Measurement of respiratory mechanics.tw. | 23 |
| 41 | Measurement of transfer function.tw. | 0 |
| 42 | Exercise Test/ | 39,143 |
| 43 | Exercise test$.tw. | 15,186 |
| 44 | Respiratory System/ | 10,648 |
| 45 | (42 or 43) and 44 | 64 |
| 46 | h?ematolog$test$.tw. | 526 |
| 47 | vitalograph.tw. | 138 |
| 48 | FEV1.tw. | 12,930 |
| 49 | Vital Capacity/ | 10,184 |
| 50 | Vital capacit$.tw. | 8648 |
| 51 | Transfer function.tw. | 2623 |
| 52 | Pulmonary Diffusing Capacity/ | 2884 |
| 53 | Diffusing capacit$.tw. | 2770 |
| 54 | dlco.tw. | 1275 |
| 55 | Exp Lung Volume Measurements/ | 25,821 |
| 56 | Lung capacit$.tw. | 2477 |
| 57 | Cardiopulmonary exercise test$.tw. | 1082 |
| 58 | cpx.tw. | 495 |
| 59 | maxim$ oxygen uptake.tw. | 3356 |
| 60 | V02max.tw. | 21 |
| 61 | Oxygen Consumption/ | 80,267 |
| 62 | or/15-41,45-61 | 395,740 |
| 63 | economics/ | 25,191 |
| 64 | Exp “costs and cost analysis”/ | 138,805 |
| 65 | Economic value of life/ | 4966 |
| 66 | Exp economics, hospital/ | 15,604 |
| 67 | Exp economics, medical/ | 11,574 |
| 68 | economics, nursing/ | 3775 |
| 69 | economics, pharmaceutical/ | 1965 |
| 70 | Exp models, economic/ | 6185 |
| 71 | Exp “fees and charges”/ | 23,781 |
| 72 | Exp budgets/ | 9949 |
| 73 | ec.fs. | 246,405 |
| 74 | (cost or costs or costed or costly or costing$).tw. | 198,747 |
| 75 | (economic$ or pharmacoeconomic$ or price$ or pricing).tw. | 106,075 |
| 76 | or/63-75 | 495,837 |
| 77 | 14 and 62 and 76 | 1480 |
Bioscience Information Service/Science Citation Index – Web of Knowledge
# 5
#4 AND #3
Databases=SCI-EXPANDED Timespan=All Years
# 4
TS=(cost* OR economic* OR “fees and charges” OR budget* OR price OR pricing OR pharmacoeconomic* OR pharmaco-economic* OR finance OR finances OR financing OR financial OR fee OR fees OR fiscal OR funding)
Databases=SCI-EXPANDED Timespan=All Years
# 3
#2 AND #1
Databases=SCI-EXPANDED Timespan=All Years
# 2
TS=(routine test* OR routine assessment* OR routine investigation* OR clinical chemistry test* OR blood cell count OR full blood count OR fbc OR hematologic test* OR haematologic test* OR urea OR urinalysis OR electrolytes OR urine test* OR blood test* OR u&e OR respiratory function test* OR pulmonary function test* OR spirometry OR blood gas analysis OR pft OR vitalograph OR FEV1 OR vital capacit* OR transfer function OR pulmonary diffusing capacit* OR dlco OR lung capacit* OR lung volume measurement OR cpx OR oxygen uptake OR V02max OR oxygen consumption OR cardiopulmonary exercise test*)
Databases=SCI-EXPANDED Timespan=All Years
# 1
TS=(surgery OR ambulatory surgical procedures OR asymptomatic OR preoperative OR pre-operative OR pre operative OR ambulatory care)
Databases=SCI-EXPANDED Timespan=All Years
EMBASE – Ovid <1980 to week 4 2009>
-
Surgery/
-
Elective Surgery/
-
elective surgery.tw.
-
minor surgery/
-
minor surgery.tw.
-
intermediate surgery.tw.
-
ambulatory surgery/
-
ambulatory care/
-
day surgery.tw.
-
asymptomatic.tw.
-
preoperative.tw.
-
pre-operative.tw.
-
pre operative.tw.
-
or/1-13
-
diagnostic test/
-
Preoperative Care/
-
routine test$.tw.
-
routine assessment$.tw.
-
routine investigation$.tw.
-
clinical chemistry/
-
risk assessment/
-
blood cell count/
-
full blood count.tw.
-
fbc.tw.
-
blood examination/
-
h?ematolog$ test$.tw.
-
Urea/
-
URINALYSIS/
-
Electrolyte/
-
urine test$.tw.
-
blood test$.tw.
-
u&e.tw.
-
(electrolytes and renal function).tw.
-
lung function test/
-
pulmonary function test$.tw.
-
respiratory function test$.tw.
-
spirometry/
-
spirometry.tw.
-
blood gas analysis/
-
blood gas analysis.tw.
-
pft.tw.
-
measurement of respiratory mechanics.tw.
-
measurement of transfer function.tw.
-
exercise test/
-
exercise test$.tw.
-
respiratory system/
-
44 or 45
-
46 and 47
-
vitalograph.tw.
-
FEV1.tw.
-
vital capacity/
-
vital capacit$.tw.
-
transfer function.tw.
-
lung diffusion capacity/
-
diffusing capacit$.tw.
-
dlco.tw.
-
lung volume/
-
lung capacit$.tw.
-
cardiopulmonary exercise test$.tw.
-
cpx.tw.
-
maxim$ oxygen uptake.tw.
-
V02max.tw.
-
oxygen consumption/
-
or/15-43,48-63
-
exp SOCIOECONOMICS/
-
exp “Cost Benefit Analysis”/
-
exp “Cost Effectiveness Analysis”/
-
exp “Cost of Illness”/
-
exp “Cost Control”/
-
exp Economic Aspect/
-
exp Financial Management/
-
exp “Health Care Cost”/
-
exp Health Care Financing/
-
exp Health Economics/
-
exp “Hospital Cost”/
-
(financial or fiscal or finance or funding).tw.
-
exp “Cost Minimization Analysis”/
-
(cost adj estimate$).mp.
-
(cost adj variable$).mp.
-
(unit adj cost$).mp.
-
or/65-80
-
14 and 64 and 81
-
from 82 keep 1
The Cochrane Library
-
#1 MeSH descriptor Surgery explode all trees
-
#2 MeSH descriptor Surgical Procedures, Elective explode all trees
-
#3 MeSH descriptor Surgical Procedures, Minor explode all trees
-
#4 (elective surgery):ab or (elective surgery):ti
-
#5 (minor surgery):ab or (minor surgery):ti
-
#6 (intermediate surgery):ti,ab
-
#7 MeSH descriptor Ambulatory Surgical Procedures explode all trees
-
#8 (day surgery):ti,ab
-
#9 (asymptomatic):ti,ab
-
#10 preoperative:ti,ab
-
#11 pre operative:ti,ab
-
#12 pre operative:ti,ab
-
#13 MeSH descriptor Ambulatory Care, this term only
-
#14 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13)
-
#15 MeSH descriptor Diagnostic Tests, Routine explode all trees
-
#16 MeSH descriptor Preoperative Care, this term only
-
#17 (routine AND (test* OR assessment* OR investigation*)):ti,ab
-
#18 MeSH descriptor Clinical Chemistry Tests, this term only
-
#19 MeSH descriptor Risk Assessment, this term only
-
#20 MeSH descriptor Blood Cell Count, this term only
-
#21 (full blood count):ti,ab
-
#22 fbc:ti,ab
-
#23 MeSH descriptor Hematologic Tests, this term only
-
#24 (haematology test*):ti,ab
-
#25 (hematology test*):ti,ab
-
#26 MeSH descriptor Urea, this term only
-
#27 MeSH descriptor Urinalysis, this term only
-
#28 MeSH descriptor Electrolytes, this term only
-
#29 (urine test*):ti,ab
-
#30 (blood test*):ti,ab
-
#31 (u&e):ti,ab
-
#32 (electrolytes and renal function):ti,ab
-
#33 MeSH descriptor Respiratory Function Tests, this term only
-
#34 (pulmonary function test*):ti,ab
-
#35 MeSH descriptor Spirometry, this term only
-
#36 (spirometry):ti,ab
-
#37 MeSH descriptor Blood Gas Analysis, this term only
-
#38 (blood gas analysis):ti,ab
-
#39 pft:ti,ab
-
#40 (measurement of respiratory mechanics):ti,ab
-
#41 (measurement of transfer function):ti,ab
-
#42 MeSH descriptor Exercise Test explode all trees
-
#43 (exercise test*):ti,ab
-
#44 MeSH descriptor Respiratory System explode all trees
-
#45 (#42 OR #43)
-
#46 (#44 AND #45)
-
#47 (vitalograph):ti,ab
-
#48 FEV1:ti,ab
-
#49 MeSH descriptor Vital Capacity, this term only
-
#50 (vital capacit*):ti,ab
-
#51 (transfer function):ti,ab
-
#52 MeSH descriptor Pulmonary Diffusing Capacity explode all trees
-
#53 (diffusing capacit*):ti,ab
-
#54 dlco:ti,ab
-
#55 MeSH descriptor Lung Volume Measurements explode all trees
-
#56 (lung capacit*):ti,ab
-
#57 (cardiopulmonary exercise test*):ti,ab
-
#58 cpx:ti,ab
-
#59 (maxim* oxygen uptake):ti,ab
-
#60 V02max:ti,ab
-
#61 MeSH descriptor Oxygen Consumption, this term only
-
#62 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61)
-
#63 (#14 AND #62)
Appendix 12 Excluded studies: cost-effectiveness review
| Reference | Population: UK based |
|---|---|
| Arieta et al. 2004100 | No |
| Based 1998101 | No |
| Binder and Schwarz 2002102 | No |
| Christian et al. 1988103 | No |
| Daher 1996104 | No |
| De 1984105 | |
| Dempfle 2005106 | No |
| Diouf et al. 1998107 | No |
| Dralle et al. 1999108 | |
| Eisold 1996109 | |
| Hoogbergen 1990110 | |
| Hunting 1989111 | |
| Irace et al. 1990112 | No |
| Ise et al. 2004113 | |
| Junger et al. 2002114 | |
| Langemeijer 1996115 | No |
| Passamonti 2004116 | No |
| Persson and Bake 1992117 | No |
| Pfaff and Van Der Linden 1989118 | |
| Prause 1994119 | |
| Raeder 1996120 | No |
| Ramschack 1997121 | |
| Rassler et al. 1994122 | No |
| Reingruber et al. 1997123 | No |
| Ritz et al. 1997124 | No |
| Roewer and Kehl 2005125 | No |
| Rutten et al. 1995126 | No |
| Sanchez-Alvarez et al. 1997127 | No |
| Scheidegger 1995128 | |
| Schmitt et al. 2011129 | No |
| Schwilk et al. 1993130 | No |
| Stohr et al. 1998131 | |
| Strom et al. 1998132 | |
| Szmuk et al. 20029 | No |
| Van Aken and Rolf 1997133 | |
| Van Der 1984134 | |
| Van Klei et al. 2001135 | No |
| Van Melkebeke 2002136 | No |
| Vesconi et al. 2000137 | No |
| Reference | Patients 1: aged > 16 years |
|---|---|
| Ansermino et al. 1999138 | No |
| Atwell et al. 1973139 | No |
| Detsky et al. 1987140 | No |
| Derkay 2000141 | No |
| Dzankic et al. 2001142 | No |
| Ferrrari 2004143 | No |
| Ferrer et al. 1994144 | No |
| Hoare 1993145 | 2–15 years |
| Hsia et al. 1995146 | No |
| Juliana et al. 2003147 | > 12 years |
| Mallick 2006148 | No |
| Meneghini et al. 1998149 | No |
| Parry et al. 2005150 | No |
| Rossello et al. 1980151 | Patients aged < 14 years included |
| Shah et al. 2007152 | No – includes children (patients aged 5–40 years) |
| Tornebrandt and Fletcher 1982153 | No |
| Wittkugel 2006154 | No |
| Reference | Language: English? | Patients 1: aged > 16 years? | Patients 2: ASA grade 1 or 2 classification? | Surgical procedures: minor or intermediate? | Tests: FBC, U&E or PFT? | Economic evaluation: incremental analysis of costs and outcomes? |
|---|---|---|---|---|---|---|
| Abayomi et al. 1982155 | Yes | Yes | Patients with carcinoma of the cervix and endometrium stage 1–4 of the disease | No | FBC, U&E | Yes – cost per positive diagnostic test |
| Reference | Population: UK based? | Language: English? | Patients 1: aged > 16 years? | Patients 2: ASA grade 1 or 2 classification? | Surgical procedures: minor or intermediate? | Tests: FBC, U&E or PFT? | Economic evaluation: incremental analysis of costs and outcomes? |
|---|---|---|---|---|---|---|---|
| Amberg et al. 1982156 | No | Yes | Not clear if patient population are all adults | Not enough detail to identify ASA status of patients | Not enough detail regarding surgical grade of patients | No – alkaline phosphatase test | Yes |
| Costamaga et al. 1998157 | No | Yes | Yes | Not clearly stated | Patients who have an endoscopic intervention for the treatment of choledochlithiasis | No | Yes |
| Edis et al. 1978158 | No | Yes | Yes | No | Surgery for overactive parathyroid glands | No – venous sampling, arteriography | Yes |
| Erickson and Carlson 1995159 | No | Yes | Yes | Patients with cholelithiasis | No – laparoscopic cholecystectomy | No – endoscopic retrograde cholangiopancreatography/endoscopic sphincterotomy | Yes |
| Hrung et al. 1999160 | No | Yes | Yes | No – patients with suspected breast cancer | No | No – core-needle biopsy or excisional biopsy without pre-operative testing, and magnetic resonance imaging | Yes |
| Ransom et al. 1996161 | No | Yes | Yes | Unclear about ASA status of patients | No – elective and emergency laparoscopic procedures | No – pre-operative blood type and screen testing | Yes |
| Roberts 1992162 | No | Yes | No – allergy blood testing | Yes – based on simulated data | |||
| Ruda et al. 2006163 | No | Yes | no | No – patients with negative findings on scans with technetium (99mTc) sestamibi for sporadic primary hyperparathyroidism | No | No – comparison of pre-operative imaging using ultrasonography; single-photon emission computed tomography with technetium (99mTc) sestamibi; and control bilateral neck exploration | Yes |
| Shaw et al. 1997164 | No | Yes | Yes | No – patients with a history of coronary disease or angina | No – patients about to undergo vascular operations | No – dipyridamole myocardial perfusion imaging, dobutamine echocardiography and cardiac catheterisation before vascular operations | Yes |
| Sonnenberg and Townsend 1991165 | No | Yes | Yes | No | Patients with gastrointestinal symptoms who are hospitalised for major elective surgery | No – faecal occult blood testing | Yes |
| Sonnenberg and Townsend 1992166 | No | Yes | Yes | Screening for cancer | No | No – faecal occult blood testing | Yes |
| Weber et al. 2002167 | No | Yes | Yes | No – based on a hypothetical sample of women used in the model. ASA grade of women not provided – included women with stress incontinence symptoms | No – some patients had surgical intervention while others only had a medical intervention |
No – two methods of pre-operative assessment in the diagnosis of uncomplicated stress urinary incontinence: basic office assessment Urodynamic testing |
Yes |
| Total | 12 | ||||||
| Reference | Population: UK based? | Language: English? | Patients 1: aged > 16 years? | Patients 2: ASA grade 1 or 2 classification? | Surgical procedures: minor or intermediate? | Tests: FBC, U&E or PFT? | Economic evaluation: incremental analysis of costs and outcomes? |
|---|---|---|---|---|---|---|---|
| Anonymous 1983168 | Yes | Yes | No | No | |||
| Anonymous 1991169 | No | Yes | Yes | No | General surgery, chest, urology, orthopaedics and gynaecology | FBC, U&E | No – questionnaire survey to attending physicians, total cost of tests in Sweden estimated |
| Anonymous 1996170 | Yes | Yes | No | No | |||
| Abbott et al. 1983171 | Yes | Yes | Focus is on nurse performance | No | No | ||
| Ainley-Walker 1979172 | Yes | Yes | No | No | |||
| Allison and Bromley 1996173 | No | Yes | Yes | No | No – cost analysis | ||
| Alsumait et al. 2002174 | No | Yes | Yes | Not enough detail on ASA grade | No | ||
| Archer et al. 1993175 | No | Yes | Yes | No | No | ||
| Armstrong et al. 2001176 | Yes | Yes | No | No | |||
| Asua and Lopez-Argumedo 2000177 | Yes | Yes | No | No | |||
| Atkins 1994178 | Yes | Yes | No | No | |||
| Bachman et al. 1993179 | No | Yes | Yes | No – included pregnant women | No – screening techniques for detecting asymptotic UTIs | No | |
| Bader 1999180 | No | Yes | Yes | No | No | No | |
| Bahhady and Underborn 2003181 | No | Yes | Yes | PFT | No | ||
| Barak et al. 2006182 | No | Yes | Yes | No | No | No | |
| Barazzoni et al. 2002183 | Yes | Yes | No | No | No | ||
| Bass et al. 1995184 | Yes | Yes | Patients with cataracts | Patients undergoing cataracts surgery | FBC, U&E and PFT | No – frequency of tests reported | |
| Bellan 1994185 | No | Yes | Yes | No | No | ||
| Berger et al. 1981186 | No | Yes | Yes | Patients treated for bladder cancer | No | No | |
| Best et al. 2002187 | Yes | Yes | No | No | |||
| Bird et al. 1984188 | No | Yes | Yes | No | No – evaluated shaving procedure for different types of surgery | ||
| Blery et al. 1983189 | No | Yes | Yes | No | FBC | No | |
| Blitz et al. 2002190 | Yes | Yes | No | No | No | ||
| Blomgren et al. 2006191 | No | Yes | Yes | No | No | No | |
| Boothe and Finegan 1995192 | No | Yes | Yes | No | No | No | |
| Bushick et al. 1989193 | Yes | Yes | No | No | No | ||
| Callaghan et al. 1995194 | Yes | Yes | No | No | |||
| Campbell 2006195 | Yes | Yes | No | No | No | ||
| Campbell et al. 1995196 | Yes | Yes | No | No | No | ||
| Caprini 2005197 | Yes | Yes | No | No | |||
| Carty et al. 1997198 | No | Yes | Yes | Patient ASA status not given | Operation for sporadic primary hyperparathyroidism | No – comparison of single radionuclide imaging technique of 99mTc-sestamibi and single-photon emission computed tomography imaging | No |
| Cassidy and Marley 1996199 | Yes | Yes | No | No | No | ||
| Catchlove et al. 1979200 | No | Yes | Yes | No | FBC | No – cost analysis | |
| Chang et al. 2000201 | Yes | Yes | No | No | No | ||
| Chu et al. 1996202 | Yes | Yes | No | No | No | ||
| Cirasino et al. 2000203 | Yes | Yes | No | No | No | ||
| Clark 2001204 | Yes | Yes | No | No | No | ||
| Clevenger and Tepas 1997205 | Yes | Yes | No | No | |||
| Clinton et al. 1986206 | Yes | Yes | No | No | |||
| Cloutier 1995207 | No | Yes | Yes | No | No | No | No |
| Clyne and Forlenza 1997208 | Yes | Yes | No | No | |||
| Collier 1998209 | No | Yes | Yes | No | No | No | No |
| Collins 2007210 | Yes | Yes | No | No | No | ||
| Collins and Chendrasekhar 1995211 | Yes | Yes | No | No | |||
| Cook and Zitelli 1998212 | Yes | Yes | No | No | |||
| Correa et al. 1999213 | Yes | Yes | No | No | No | No | |
| Crowther et al. 2004214 | Yes | Yes | No | No | |||
| Cutler and Leppo 1987215 | Yes | Yes | No | Coronary artery surgery | No | ||
| Daniell et al. 1992216 | No | Yes | Yes | No | Endometrial ablation procedures | No | No |
| Davenport et al. 2005217 | Yes | Yes | No | No | |||
| Davies et al. 1994218 | Yes | Yes | No | No | No | ||
| De Nino et al. 1997219 | No | Yes | Yes | No | No | ||
| De et al. 1996220 | Yes | Yes | No | No | No | ||
| Degnore and Wilson 1989221 | Yes | Yes | No | No | |||
| Delahunt and Turnbull 1980222 | No | Yes | Yes | No | FBC, U&E | No – estimate cost saving | |
| Denham and Norman 1998223 | No | Yes | Yes | Patients with sporadic primary hyperparathyroidism | Surgery under monitored anaesthesia care compared with undirected bilateral exploration | No – (a) standard bilateral exploration and (b) sestamibi-guided limited exploration | No |
| Devalia et al. 2007224 | Yes | Yes | No | No | No | ||
| Dillon et al. 2005225 | Yes | Yes | No | No | |||
| Dimakakos et al. 1995226 | Yes | Yes | No | No | |||
| Diokno et al. 1999227 | Yes | Yes | No | No | |||
| Dix 2003228 | Yes | Yes | No | No | |||
| Doering et al. 2000229 | Yes | Yes | No | No | No | ||
| Dorenbusch et al. 1995230 | Yes | Yes | No | No | No – cost analysis | ||
| Dublin et al. 1997231 | Yes | Yes | No | No | |||
| Dubois et al. 1998232 | Yes | Yes | No | No | |||
| D’ugo et al. 1997233 | Yes | Yes | No | No | No | ||
| Dyson et al. 1992234 | Yes | Yes | No | No | |||
| Eagle 2007235 | No | Yes | Yes | No | No | No | No |
| Eckman et al. 2003236 | Yes | Yes | No | No | |||
| Einhorn et al. 1987237 | No | Yes | Yes | Patients who have undergone laparotomy for diagnosis of a pelvic mass | Detecting ovarian cancer in an apparently healthy population | Serum markers | No – abstract |
| Eiseman et al. 1989238 | Yes | Yes | No | No | |||
| Espallargues et al. 1996239 | No | Yes | Yes | Not enough detail on tests | No | ||
| Everett 2002240 | Yes | Yes | No – looking at post-operative nausea and vomiting | Yes | |||
| Farrell et al. 2003241 | No | Yes | Yes | No | Pelvic examinations | No – guideline recommendation document | |
| Fattahi et al. 2006242 | No | Yes | Yes | No | Yes | No | |
| Ferrando et al. 2005243 | Yes | Yes | No | No | |||
| Finegan et al. 2005244 | No | Yes | Yes | Includes ASA grades 3 and 4 patients | Not clearly defined | Yes | No – reported the total cost of tests ordered, mean number of tests ordered and mean cost of pre-operative testing |
| Fischer 1999245 | No | Yes | Yes | No | No | ||
| Fischer 1997246 | No | Yes | Yes | No | No | No | |
| Fischer 1999247 | No | Yes | Yes | No | No | Yes | No |
| Harik-Kahn et al. 2001248 | No | No | Lung function | No | |||
| Hilibrand and Dina 1998249 | Yes | Yes | No | No | No | No | |
| Hnatiuk et al. 1995250 | Yes | Yes | Not enough detail on ASA status | No | |||
| Hollenbeak et al. 2007251 | No | Yes | Yes | No | No | No | No |
| Hoeks et al. 2007252 | Yes | Yes | No | No | No | ||
| Hollenberg 1999253 | Yes | Yes | No | No | |||
| Horton et al. 2006254 | Yes | Yes | No | No | No | ||
| Howard 1997255 | Yes | Yes | No | No | No | No | |
| Howie et al. 1998256 | Yes | Yes | No | No | |||
| Hux 2003257 | No | Yes | Yes | No | Blood chemistry | Cost analysis | |
| Imasogie 2003258 | No | Yes | Yes | No | No | ||
| Ishaq et al. 1997259 | No | Yes | Yes | Patients undergoing elective non-acute and non-cardiopulmonary surgery | Routine chest radiography | No | |
| Jaffer et al. 2005260 | No | Yes | Yes | Patients undergoing elective non-cardiac surgery | Evaluation of post-operative pulmonary complications after the following surgical procedures: orthopaedic, urologic, neurosurgery, gynaecologic, colorectal or general | FBC, serum chemistries | No – total cost estimates for each patient group |
| Jang et al. 2000261 | Yes | Yes | No | No | No | No | |
| Johnson et al. 1988262 | No | Yes | Yes | No | Patients undergoing ambulatory surgical procedures | FBC, U&E | No – cost description |
| Jones and Isaacson 1995263 | Yes | Yes | No | No | U&E | No | |
| Justice and King 1993264 | Yes | Yes | No | No | |||
| Khandekar 1999265 | No | Yes | Yes | No | No | No | No |
| Kitchens 1994266 | No | Yes | Yes | Not enough detail | Not enough detail | No | Unclear |
| Lee et al. 2004267 | Yes | Yes | No | No | No | ||
| Liberato et al. 2007268 | Yes | Yes | No | No | No | No | |
| Macario et al. 1992269 | No | Yes | Yes | No | FBC, U&E | No – cost analysis | |
| MacPherson 1993270 | No | Yes | Yes | No | FBC, U&E | No | |
| Mancuso 1996271 | Yes | Yes | Ambulatory surgery patients | FBC, U&E | No – cost analysis | ||
| Mancuso 1999272 | Yes | Yes | Not enough detail | Not enough detail | No | ||
| Mantha et al. 2005273 | Yes | Yes | No | No | |||
| Marcello and Roberts 1996274 | No | Yes | Yes | FBC, U&E | No | ||
| Marton et al. 1985275 | No | Yes | Unclear | Not enough detail given to establish ASA status | No – included in the patients with chronic obstructive pulmonary disease, hypertension, cardiac disease and diabetes | Tests included aminophylline, serum electrolytes, serum digoxin and serum glucose | No |
| Maurer et al. 2004276 | No | Yes | Yes | No | No | No | |
| McCleane 1988277 | Yes | Yes | No | No | |||
| Morrison and Jacobs 2004278 | Yes | Yes | Patients undergoing laparoscopic hysterectomy | No – no pre-operative tests discussed | No – cost description but not relevant as study was not concerned with pre-operative tests | ||
| Munro et al. 1997279 | Yes | Yes | No | No | |||
| Muskett and McGreevy 1986280 | No | Yes | Yes | No | Patients undergoing a range of surgical procedures | FBC, U&E | No |
| Nahas 2006281 | No | Yes | Yes | Serum calcium | No | ||
| Nanthakrishnan et al. 1989282 | Yes | Yes | No | No | |||
| Naraghi et al. 1995283 | Yes | Yes | No | No | |||
| Nardella et al. 1995284 | No | Yes | Yes | No | FBC, U&E | No – cost description | |
| Narr et al. 1991285 | No | Yes | Yes | No | Hb concentration | No – cost analysis | |
| Nelson et al. 1987286 | Yes | Yes | Not enough detail on ASA status | Not enough detail | No – cost analysis | ||
| Northup 2004287 | Yes | Yes | No | No | No | No | |
| Ntia and Okikiolu 1996288 | No | Yes | Yes | No | No | No | |
| Okelberry 1974289 | Yes | Yes | No | No | |||
| Onder et al. 2004290 | No | Yes | Yes | No | No | No | No |
| Oyama et al. 2001291 | Yes | Yes | No | No | PFT | No | |
| Parker et al. 2000292 | Yes | Yes | No | No | No | ||
| Parrish 2001293 | No | Yes | Yes | No | No | No | No |
| Pasternak and Johns 2005294 | Yes | Yes | No | No | No | ||
| Patel 2000295 | No | Yes | Yes | No | No | No | |
| Pellikka et al. 1995296 | Yes | Yes | No | No | |||
| Peredy and Powers 1997297 | No | Yes | Yes | No | No | ||
| Phipps 1987298 | Yes | Yes | No | Looks at who delivers tests | No | ||
| Poe et al. 1988299 | No | Yes | Yes | No | PFT | No – cost analysis | |
| Pokorny et al. 1999300 | No | Yes | Yes | No | No | No | |
| Pollard and Olson 1999301 | Yes | Yes | No | No | |||
| Pollard et al. 1997302 | Yes | Yes | No | No | No | No | |
| Pollard et al. 1996303 | No | Yes | Yes | Unclear | Unclear | No | No |
| Popovic et al. 1997304 | No | Yes | Yes | No | No | No | No |
| Power and Thackray 1999305 | No | Yes | Yes | No | No | FBC, U&E | No |
| Putnis et al. 2008306 | No | Yes | Yes | ASA grades 1–3 | FBC | No | |
| Qiu 2006307 | Yes | Yes | No | No | |||
| Rabkin and Horne 1979308 | No | Yes | Yes | ASA status not given | No – elective or emergency operations | No | No |
| Ransom et al. 1995309 | No | Yes | Yes | No details on ASA grade given | Vaginal hysterectomy | No – pre-operative type-and-screen testing (cross match not full screen) | No |
| Raw 2001310 | Yes | Yes | Not enough detail on ASA status | No | No | ||
| Rennie 2004311 | Yes | Yes | No | No | |||
| Reynolds et al. 2006312 | Yes | Yes | Not just ASA grades 1 and 2 | Not just minor and intermediate surgery | no | ||
| Ricciardi et al. 1998313 | No | Yes | Yes | No | Laboratory tests, Hb, electrolytes, etc. | No – questionnaire used by Swedish study adapted to Italy | |
| Rich 2002314 | Yes | Yes | No | No | |||
| Richie 1990315 | Yes | Yes | No | No | No | ||
| Ridgway et al. 1990316 | Yes | Yes | No | No | No | No | |
| Rink 1993317 | Yes | Yes | No | No | |||
| Roberts et al. 1983318 | Yes | Yes | Yes | Unclear | Unclear | No | No |
| Robinson et al. 2003319 | Yes | Yes | No | No | No | ||
| Roehrborn et al. 1986320 | Yes | Yes | No | No | |||
| Roizen 1989321 | No | Yes | Yes | No | No | No | |
| Roizen 1993322 | No | Yes | Yes | Not enough detail on ASA status | Vascular surgery | Unclear | |
| Roizen 1994323 | No | Yes | Yes | No | No – just contains information on when tests are applicable | ||
| Roizen 1994324 | No | Yes | Yes | No | No | ||
| Romfh 1989325 | No | Yes | Yes | No | Blood glucose | No – cost analysis | |
| Russo et al. 2007326 | Yes | Yes | No | No | No | ||
| Ryan 2000327 | Yes | Yes | Orthopaedic surgery | Peak flow, blood pressure, UA | No | ||
| Sanders et al. 1989328 | No | Yes | Yes | No | No – total hip replacement surgery | FBC, U&E | Unclear |
| Sanjay 2004329 | Yes | Yes | No | No | No | No | |
| Schein 1996330 | Yes | Yes | No | Cataract surgery | No – description of a forthcoming study | ||
| Schroeder 1999331 | Yes | Yes | No | No | No | ||
| Shander et al. 2004332 | Yes | Yes | No | No | |||
| Sharaf et al. 2004333 | Yes | Yes | No | No | No | ||
| Sheehan et al. 2007334 | Yes | Yes | No | No | No | ||
| Sihoe et al. 2004335 | Yes | Yes | No | No | No | ||
| Silecchia 2000336 | No | Yes | Yes | No | No | No | No |
| Silverstein and Boland 1994337 | Yes | Yes | No | No | No | ||
| Singh et al. 1994338 | Yes | Yes | No | No – letter to editor, comment on a paper | |||
| Sinha et al. 1997339 | Yes | Yes | No | No | |||
| Smetana and MacPherson 2003340 | No | Yes | Yes | Not enough detail on ASA status | no | No | |
| Sommerville and Murray 1992341 | No | Yes | Yes | No | No | ||
| Straube et al. 2005342 | Yes | Yes | No | No | |||
| Swanson and Scheb 1996343 | Yes | Yes | No | No | No | ||
| Tabas and Vanek 1999344 | Yes | Yes | No | No | |||
| Tait et al. 1997345 | Yes | Yes | No | No | |||
| Takemura et al. 2002346 | No | Yes | Yes | No | |||
| Takemura et al. 2000347 | Yes | Yes | No | No | |||
| Tarazi et al. 2000348 | Yes | Yes | No | No | No | ||
| Tawam et al. 1996349 | No | Yes | Yes | No | No | No | |
| Thompson 1979350 | Yes | Yes | No | No | No | ||
| Thompson et al. 1983351 | No | Yes | Yes | No detail given on patient ASA status | No detail given on the surgical procedures undertaken | No – chest radiography and multichannel blood tests as a screening instrument for chronic obstructive pulmonary disease, tuberculosis, heart disease, and lung cancer in asymptomatic adults – not enough detail given on the set of tests | No |
| Thue and Sandberg 1994352 | No | Yes | Yes | No | Blood count | No | |
| Tierney et al. 1990353 | No | Yes | Yes | Not clear | Not clear | Testing physician’s knowledge about the costs of screening tests | No |
| Tigges et al. 2004354 | Yes | Yes | No | No | |||
| Turnbull and Buck 198715 | No | Yes | Yes | Patients admitted for cholecystectomy | No | FBC, PFT, U&E | No – cost description |
| Usal et al. 1999355 | No | Yes | Yes | No | No | No | No |
| Van Der Merwe and Coetzee 1992356 | No | Yes | Yes | No | No | No | |
| Van Klei et al. 2003357 | Yes | Yes | No | No | |||
| Van Klei et al. 2001258 | Yes | Yes | No | No | |||
| Van Klei et al. 2000359 | Yes | Yes | No | No | |||
| Van Klei et al. 2001360 | Yes | Yes | No | No | |||
| Vanzuidewijn et al. 1994361 | Yes | Yes | No | No | No | ||
| Velanovich 1993362 | No | Yes | Yes | No | No | ||
| Velanovich 1994363 | No | Yes | Yes | Not enough detail on ASA status | Elective operations in general, vascular, thoracic, and head and neck surgical services | FBC, U&E | No |
| Vogt and Henson 1997364 | No | Yes | Yes | No – includes ASA grade 3 patients | No – not enough detail given to the grade of surgery | Yes | No |
| Wagner and Moore 1991365 | No | Yes | Yes | Not enough detail on ASA status | FBC, U&E | No | |
| Walton 1988366 | Manchester UK | Yes | Yes | Not enough detail on ASA status | Dental surgery patients | FBC | No – cost description |
| Wattsman 1997367 | No | Yes | Yes | No | No – cost description | ||
| West et al. 2000368 | Yes | Yes | No | No | No | No | |
| Wetchler 1999369 | Yes | Yes | No | No | |||
| Wiencek et al. 1987370 | No | Yes | Yes | No | No | No | |
| Zwack and Derkay 1997371 | Yes | Yes | No | No | No | No | |
| Total | 205 | ||||||
Appendix 13 Data extraction tables for cost-effectiveness review
| Study | Type of model | Perspective | Model assumptions |
|---|---|---|---|
| Capdenat Saint-Martin et al. 199837 | N/A | N/A | N/A |
| Fischer 199638 | N/A | N/A | N/A |
| Imasogie et al. 200339 | N/A | N/A | N/A |
| Johnson and Mortimer 200240 | N/A | N/A | N/A |
| Kitz et al. 198841 | N/A | N/A | N/A |
| Larocque and Maykut 199442 | N/A | N/A | N/A |
| Lawrence et al. 198936 | Decision tree | The perspective of the analysis was that of third-party payers | The economic analysis was modified from the definition of a comprehensive evaluation that (1) clinical value or usefulness of the UA is not previously established; (2) clinical outcomes owing to an abnormal UA, other than the possibility of increased risk of wound infection, were not included (e.g. costs and consequences of further evaluation such as intravenous pyelography, cystoscopy, prostatic resection); and (3) the study estimated minimum direct benefits only and did not consider indirect costs or benefits |
| MacPherson et al. 200543 | N/A | N/A | N/A |
| Study | Cost items | Cost data sources | Resource use | Resource data source | Currency and currency year | Discount rate |
|---|---|---|---|---|---|---|
| Capdenat Saint-Martin et al. 199837 |
Blood typing Hb + platelet count Prothrombin time and partial thromboplastin time Bleeding time Fibrinogen Electrolyte + glucose + urea Hepatic enzymes + chest radiograph+ ECG |
Not stated | Not stated | Not stated | 1993–4 Franks, ECU and US $ | Not stated |
| Fischer 199638 | Pre-operative tests assessed in the study: CBC, platelets, US, general survey panel (renal panel, lung function test, glucose, calcium, albumin, magnesium and uric acid), electrolytes, renal panel and prothrombin time/partial thromboplastin time | Not stated | Not stated | Not stated | 1994 US $ | Not stated |
| Imasogie et al. 200339 | Costs of the individual | Ascertained from the hospital finance department | Based on the tests ordered and the cost of each test, total costs of laboratory tests of individual patients were calculated | Ascertained from the hospital finance department | CAN $ | Not given |
| Johnson and Mortimer 200240 |
Costings for tests Charges being based on a non-emergency basis during the hours of 9.00 a.m. to 4.00 p.m. for NHS patients. These were £3.67 for FBC, £1.62 for U&Es and £1.07 for glucose |
Obtained from the hospital pathology department | Not given | Not given | UK £ | Not given |
| Kitz et al. 198841 | Pre-operative tests | Within study institution | Nursing labour costs | Within study | Date not stated – assume US $ | Not stated |
| Larocque and Maykut 199442 | Cost per test: EUC CAN$4.02; glucose CAN$1.24; liver profile CAN$4.24; chest radiography CAN$30.43 | Biochemistry department | N/A | N/A | N/A | N/A |
| Lawrence et al. 198936 |
(a) Charges for laboratory tests, antibiotics, and professional fees (b) The DRG estimates reimbursement fees for procedures |
(a) Teaching hospital of the University of Texas Health Science Centre at San Antonio for 1986 (b) Based on a national urban average of $2967.43, which was not corrected for teaching hospital, area wage index, or disproportionate share of indigent patients. The relative weight of a diagnostic category is applied to this average to obtain the DRG fee |
Cost of the hospital day incurred when a planned procedure is postponed due to presence of remote UTI | Estimated with the per diem cost of an average US community hospital bed in 1983 adjusted to 1986 ($461/day) using the consumer price index | All costs are in 1986 US $ | Not stated |
| MacPherson et al. 200543 | FBC | Not stated | None stated | None stated | Australian $ | None stated |
| EUC | ||||||
| Lung function test | ||||||
| Thyroid function test | ||||||
| CPM | ||||||
| Coags | ||||||
| Glucose | ||||||
| G&H | ||||||
| Unit costs for each test not given |
| Study | Efficacy data | Efficacy data sources | Health outcomes/utility | Health outcome data sources | Discount rate |
|---|---|---|---|---|---|
| Capdenat Saint-Martin et al. 199837 | Not stated | Not stated | Perioperative morbidity; patient death | Within study | Not stated |
| Fischer 199638 | Not stated | Not stated | Operating room cancellations and delays or adverse patient events | Within study | Not stated |
| Imasogie et al. 200339 | Not stated | Not stated | Perioperative; hypertension, hypotension; bradycardia arrhythmias; myocardial ischaemia; myocardial infarction; congestive heart failure; syncope; hypoglycaemia; oxygen saturation of < 90%; and airway obstruction | Not stated | Not stated |
| Johnson and Mortimer 200240 | The numerical value of each result was classed as abnormal when its value fell outside the normal range | Determined by the stated reference range on the hospital blood form (mean ± 2 standard deviations) | Action taken pre-, intra- or post-operatively in consequence of the abnormal result. Complications occurring perioperatively were recorded in detail stating whether or not the pre-operative blood tests were normal | Patient’s notes | Not stated |
| Kitz et al. 198841 | Not stated | Not stated | Not stated | Not stated | Not stated |
| Larocque and Maykut 199442 | Not stated | Not stated | Mortality or morbidity | Within the study | Not stated |
| Lawrence et al. 198936 |
(a) Decision tree in figure 1 diagrams the relevant clinical outcomes, with respective probabilities, for elective clean wound knee procedures vis-a-vis the complication of wound infection (b) The base figure of 458,000 procedures annually and the baseline probability estimates for wound infection and its outcomes (shown in figure 1) yield the study results (table 1) (c) Authors estimated that 10% of patients undergoing these procedures would be aged ≥ 60 years. The 1% increase in risk from UTI, if not prevented, would result in 4.58 additional wound infections in the initial cohort of 458,000 procedures |
(a) These probabilities were estimated by the authors (who include an orthopaedic surgeon) after a literature review and after interviews with two other orthopaedic surgeons (b) REF: National Centre for Health Statistics, R. Pokras: Detailed diagnoses and procedures for patients discharged from short-stay hospitals, United States, 1985. Vital and Health Statistica. Series 13, No. 90. DHHS Pub. No. (PHlS) 87–1751. Public Health Service. Washington: U.S. Government Printing Office; April 1987 (c) REF b and Lawrence VA, Kroenke K. 1988 |
The health outcomes considered were final, one-time only, surgical outcomes. Benefits were prevention of wound infection and its sequelae | Literature and authors knowledge in the area | Not stated |
| MacPherson et al. 200543 | None stated | None stated | None stated | None stated | None stated |
| Study | Total costs | Total incremental costs | Total outcome | Total incremental outcomes | Cost-effectiveness ratios |
|---|---|---|---|---|---|
| Capdenat Saint-Martin et al. 199837 | Annual saving Fr 3.04M | None stated | Perioperative morbidity: n = 11; patient death: n = 0 | None stated | None stated |
| Fischer 199638 | Total cost per patient – pre intervention US$188.90, post intervention US$76.82 | None stated | Operating room cancellations: n = 148 unexpected cancellations | 55% decrease in tests ordered | None stated |
| Imasogie et al. 200339 | A potential cost saving of CAN$21,217.70 was possible for the management of 595 patients [(39.67 – 4.01) × 595]; 595 being a hypothetical number of patients in a 4-month period |
The cost per patient was CAN$4.01 ± 18.92 in the non-testing group compared with CAN$39.67 ± 19.04, in the testing group A 90% reduction in laboratory costs The cost of tests per patient was reduced from CAN$39.67 to CAN$4.01 |
Reduction in the proportion of patients referred to the anaesthesia consult clinic to 33% compared with 47% before the guidelines were introduced, but this was not statistically significant | Reduction in the number of tests ordered after the new policy was introduced, from 5.8 tests per patient to 0.4 tests per patient | None stated |
| Johnson and Mortimer 200240 | It is estimated that > £114,000 per year is spent on requests for FBC, U&E⁄creatinine and random glucose | None stated | 64 of 706 (9.1%) results were abnormal. Perioperative management was altered in only two patients (0.3%) | None stated | None stated |
| Kitz et al. 198841 | Total hospital costs for the tests – US$1261 (day surgery unit) and US$5893 (inpatients) | None stated | None stated | None stated | None stated |
| Larocque and Maykut 199442 | CAN$93,137.20 total annual saving | Saving CAN$5.20 per patient investigation |
Morbidity: 12.6% (pre protocol) and 9.2% (post protocol) Mortality: 0.4% pre protocol and 0% post protocol |
None stated | None stated |
| Lawrence et al. 198936 |
Estimated US$7M on pre-operative UA and associated costs Screening costs: for the cohort of 458,000 procedures, the added cost to the health-care system for routine pre-operative UA is US$6,870,000 or US$28,258,600 if one includes the cost of a hospital day to admit the patient, discover a possible UTI and then postpone the planned procedure |
Without routine screening, the cost of treating the additional cases of wound infection is US$13,226 for the baseline risk of 0.01 for wound infection after these procedures Dividing the added costs owing to introduction of a screening programme by the cost of treating the additional infections if UA were not done indicates that the cost of preventing an additional 4.58 infections is 500 times greater than the cost of treating these infections. The net ‘benefit’ is an approximate deficit of $6,857,000 annually (table 4) |
If UTI causes a 1% risk increase wound infection, and if physicians respond appropriately to all abnormal UAs (i.e. treat UTIs pre operatively), then an additional 4.58 wound infections would be prevented | Literature | US$1,500,000 per wound infection prevented. Cost of treating additional cases of wound infection, given no pre-operative urinalysis, is approximately 500-fold less than the cost of screening with routine UA |
| MacPherson et al. 200543 | Group 1: AUS$29,549; Group 2: AUS$22,941; Group 3: AUS$25,219 | N/A |
Test per patient: Group 1: AUS$1742; Group 2: AUS$1356; Group 3: AUS$1483 |
None stated | None stated |
| Study | Sensitivity analysis methods | Sensitivity analysis results |
|---|---|---|
| Capdenat Saint-Martin et al. 199837 | N/A | N/A |
| Fischer 199638 | N/A | N/A |
| Imasogie et al. 200339 | N/A | N/A |
| Johnson and Mortimer 200240 | N/A | N/A |
| Kitz et al. 198841 | N/A | N/A |
| Larocque and Maykut 199442 | N/A | N/A |
| Lawrence et al. 198936 |
(a) The authors tested the robustness of their results with sensitivity analysis using threshold calculations. In other words, they asked: At what charge for a UA would we break even, i.e. costs would equal benefits? At what charge would it be a toss-up between screening costs and expense of treating additional infections if screening were not done? (b) With a worst-case scenario approach and asking how much would UTI have to increase the risk of wound infection to make it ‘worthwhile’ to do screening UAs? |
(a) With a risk increase of 1%, the break-even point occurs at a charge of US$0.03 per UA (b) The highest estimate of risk increase found in the literature is fivefold, from data seriously flawed by lack of accounting for confounding variables. Using fivefold for the incremental risk imposed by UTI, the threshold cost of US$11.70 at best approaches current charges for a UA |
| MacPherson et al. 200543 | N/A | N/A |
| Study | Author conclusions |
|---|---|
| Capdenat Saint-Martin et al. 199837 | The authors found a sharp drop in the number of pre-operative tests ordered by anaesthetists after local adaptation of national guidelines combined with active feedback about their practice and implementation of practice and discussion about organisational changes. Clinical audit is not an appropriate design to establish a causal relation between intervention and effect and caution must be exercised in drawing such conclusions from studies of this type. Nevertheless, they conclude that the changes were profound and coincided not only with feedback of practice but also a radical appraisal of the organisational basis for pre-operative assessment |
| Fischer 199638 | A successful APEC can demonstrate significant clinical advantages, improve quality and value for customers, and provide visible leadership in responding to rapidly changing health-care demands. In 1995, the APEC evaluated 8972 outpatients and to-be-admitted patients. A US$112.09 per patient decrease in pre-operative testing during this year at Stanford has a potential cost-reduction to the hospital of US$1.01M |
| Imasogie et al. 200339 | In ambulatory cataract surgery, > 90% savings in laboratory costs is possible after elimination of routine tests |
| Johnson and Mortimer 200240 | Over 19,000 operations are performed annually (1995–6) in Wythenshawe Hospital. Using this figure, it is estimated that > £114,000 per year is spent on routine pre-operative blood tests. Our audit did not examine other investigations such as clotting studies, ECGs and chest radiography which are more expensive. We estimate that, in our hospital, elimination of unnecessary screening tests would save approximately £50,000 per annum. Extrapolating this to all acute hospitals in the NHS (approximately 280) could result in cost savings of several million pounds |
| Kitz et al. 198841 | Hospital costs for these tests were four times greater for inpatients than for day surgery unit patients. Operating room time was from 20 to 45 minutes longer for INPTs than for DSU patients (p < 0.05). Recovery room time was from 25 to 52 minutes longer for DSU patients (p < 0.05). Per patient nursing labour costs paralleled operating and recovery room times. These kinds of analyses are important in identifying opportunities to improve resource use, in assessing institutional costs for surgical care, and in designing strategies that allow institutions and physicians to respond to cost containment pressures |
| Larocque and Maykut 199442 | The observed reduction in the frequency of pre-operative laboratory investigations was attributed to the introduction of the guidelines |
| Lawrence et al. 198936 | We estimated that (1) nearly US$7M is spent annually on pre-operative urinalyses and associated costs; (2) given the best estimate of the increase in risk of wound infection attributable to UTI, 4.58 wound infections may be prevented annually, at a cost of US$91,500,000 per wound infection prevented; (3) the cost of treating additional cases of wound infection, given no pre-operative UA, is approximately 500-fold less than the cost of screening with routine UA. We conclude that the routine pre-operative UA is clinically and economically unsound before clean-wound, non-prosthetic knee surgery and probably before other types of clean-wound surgery. For this relatively inexpensive test, aggregate costs are disproportionately high and appear to outweigh clinical benefits. Routine pre-operative UA is clinically and economically unsound before clean wound, non-prosthetic knee surgery and probably before other types of clean-wound surgery. For this relatively inexpensive test, aggregate costs are disproportionately high and appear to outweigh clinical benefits |
| MacPherson et al. 200543 | The results of the introduction of the PAC have been significant and sustained since the full implementation of the scheme. The literature is replete with reports from studies that show pathology test ordering is excessive and wasteful |
Appendix 14 Questionnaire
Glossary
- Allodynia
- Excessive sensitivity to stimuli which does not usually cause pain.
- Anterior interosseous syndrome
- Flexion weakness of the thumb, index finger and sometimes the middle finger, with pronation weakness with flexed elbow; often associated with acute local trauma. (Saeed MA, Gatens PF. Anterior interosseous nerve syndrome: unusual etiologies. Arch Phys Med Rehabil 1983;64:182.)
- Bradycardia
- Slow heart rate.
- Causalgia
- A persistent burning, shooting pain in a specific peripheral nerve distribution. (Horowitz SH, Horowitz SH. Venipuncture-induced causalgia: anatomic relations of upper extremity superficial veins and nerves, and clinical considerations. Transfusion 2000;40:1036–40.)
- Convulsive syncope
- Loss of consciousness with tonic–clonic movements.
- Diaphoresis
- Excessive sweating.
- Haematocrit
- The proportion of blood volume occupied by red blood cells.
- Hyperpathia
- Excessive sensitivity to painful stimuli.
- Hypotension
- Abnormally low blood pressure.
- Pneumomediastinum
- Leakage of air into the mediastinum.
- Pneumoparotid
- Enlargement of the parotid gland because of a reflux of pressurised air from the mouth.
- Pronation
- Rotational movement of the forearm at the radioulnar joint without an associated movement at the shoulder. [Wikipedia Pronation. Wikipedia. 2009. URL: http://en.wikipedia.org/wiki/Mediastinum (accessed May 2009).]
- Syncope
- Fainting, loss of consciousness.
List of abbreviations
- APEC
- Anaesthesia Preoperative Evaluation Clinic
- ASA
- American Society of Anaesthesiologists
- CBC
- complete blood count
- CCSD
- Clinical Coding and Schedule Development
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CG3
- Clinical Guideline 3
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- Coags
- tests of coagulation
- CPM
- calcium, phosphate and magnesium
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- ECG
- electrocardiography
- ECU
- European currency unit
- EUC
- electrolytes, urea and creatinine
- EVI
- expected value of information
- FEV1
- forced expiratory volume in 1 second
- FBC
- full blood count
- G&H
- group and hold test
- Hb
- haemoglobin
- HTA
- Health Technology Assessment
- LFT
- liver function test
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Clinical Excellence
- PAC
- pre-admission clinic
- PAS
- patient administration services
- PEmax
- maximum static expiratory
- PFT
- pulmonary function test
- QUOROM
- quality of reporting of meta-analyses
- RCT
- randomised controlled trial
- SCI
- Science Citation Index
- TFT
- thyroid function test
- UA
- urine analysis
- U&E
- urea and electrolytes test
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has only been used once, or it is a non-standard abbreviation used only in figures/tables/appendices in which case the abbreviation is defined in the figure or table legend.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health