Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/126/02. The contractual start date was in February 2010. The draft report began editorial review in September 2011 and was accepted for publication in June 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Incidence and prevalence
Lung cancer is the most common cancer in the world and the second most common cancer diagnosed in the UK after breast cancer. In 2008, there were 40,806 new cases of lung cancer diagnosed in the UK, with 32,546 in England and 2403 in Wales. 1 Lung cancer is rarely diagnosed in people< 40 years of age, and 86% of cases occur in people > 60 years. 1Table 1 provides an overview of lung cancer statistics in the UK. The European age-standardised incidence rate of lung cancer in 2008 was 45.6 per 100,000 population in England and 52.2 per 100,000 population in Wales. 1 The UK incidence rate in men is similar to incidence rates in most of Western Europe and is lower than incidence rates in most of Eastern Europe. 1 The UK incidence rate in women is one of the highest rates in the European Union. 1 There is an increased incidence of lung cancer in individuals from the lowest socioeconomic strata. 2 In 2008, around 65,000 individuals were living with lung cancer in the UK;1 the majority of these individuals were men. 1
| Lung cancer | Men | Women | Total |
|---|---|---|---|
| Number of new cases (UK 2008) | 22,846 | 17,960 | 40,806 |
| Rate per 100,000 populationa | 59.4 | 38.8 | 47.8 |
| Number of deaths (UK 2008) | 19,868 | 15,393 | 35,261 |
| Rate per 100,000 populationa | 51.0 | 32.0 | 40.3 |
| One-year survival rate (for patients diagnosed 2004–6, England) | 27% | 30% | – |
| Five-year survival rate (for patients diagnosed 2004–6, England) | 7% | 9% | – |
Causation
Smoking causes around 90% of lung cancer deaths in men and > 80% of lung cancer deaths in women in the UK. 1 Other causes include radon exposure, air pollution, heredity and occupational exposure such as to asbestos and industrial chemicals. 3
Survival
There were 35,261 lung cancer-related deaths in the UK in 2008. 1 Prognosis is very poor; lung cancer is usually asymptomatic in the early stages, and two-thirds of patients are diagnosed at a late stage when curative treatment is not possible. In total, 27% of male and 30% of female lung cancer patients in England and Wales survive for 1 year; 7% and 9%, respectively, survive to 5 years. 1 According to the National Lung Cancer Data Audit (LUCADA) report4 for 2006–8, the median survival for individuals with lung cancer in England is 203 days (interquartile range 62 to 545 days).
There are many factors that affect lung cancer survival rates, including smoking status, general health, sex, race and cancer treatments. For example, Asian individuals with lung cancer have a significantly higher percentage survival at 1 and 3 years than white patients, regardless of age. 1
Diagnosis
As noted earlier, lung cancer at an early stage is usually asymptomatic and thus diagnosis is often at a late stage. Unfortunately, two-thirds of patients are diagnosed when the cancer has metastasised. Across England and Wales a significant proportion of each age group presents with late-stage metastatic disease. 4 According to recently updated National Institute for Health and Clinical Excellence (NICE) guidelines,5 urgent referral for chest radiography should be offered when a patient presents with haemoptysis or any of the following unexplained or persistent (i.e. lasting > 3 weeks) symptoms or signs:
-
cough
-
chest/shoulder pain
-
dyspnoea
-
weight loss
-
chest signs
-
hoarseness
-
finger clubbing
-
features suggestive of metastasis from a lung cancer (e.g. in brain, bone, liver or skin)
-
cervical/supraclavicular lymphadenopathy.
The updated NICE guidelines5 for the diagnosis and treatment of lung cancer recommend that, if a chest radiograph or chest computerised tomography (CT) scan suggests lung cancer, patients should be offered an urgent referral, usually to a chest physician, who should choose further investigations that give the most information about diagnosis and staging with the least risk to the patient. There are various diagnostic and staging techniques for non-small cell lung cancer (NSCLC) in the UK. Within this diagnostic process there are a number of key issues that need to be considered, including disease staging, performance status (PS), histology and the presence of comorbid disease.
Disease staging
The stage of lung cancer at diagnosis reflects the degree of spread of cancer and is crucially important to determine which patients have potentially curative disease and which do not; this helps to estimate a patient's prognosis. The TNM classification provides a system for staging the extent of cancer. T refers to the size and extent of the primary tumour, N refers to the involvement of the lymph nodes and M refers to the presence of metastases or distant spread of the disease. Recently, changes have been made to the predominantly used Union Internationale Contre le Cancer (UICC) TNM system for classification of NSCLC disease stage5 (previous UICC versions are available from the American Joint Committee on Cancer). There are several differences in staging between UICC versions 6 and 7 that are specifically relevant to patients with advanced lung cancer. For example, pleural effusion is classed as stage IIIB in version 6 and has been reclassed as stage IV in version 7. Table 2 shows how the stages from the sixth edition that have been modified compare with the new stages in the seventh edition. Table 3 shows the stage groupings in the seventh edition of the TNM classification.
| Sixth edition (2002) | Seventh edition (2009) | |
|---|---|---|
| TNM stage | TNM stage | Descriptor |
| T1 | T1a | Maximum dimension ≤ 2 cm |
| T1b | Maximum dimension 2–3 cm | |
| T2 | T2a | Maximum dimension 3–5 cm |
| T2b | Maximum dimension 5–7 cm | |
| T3 | Maximum dimension > 7 cm | |
| T4 | T3 | Additional nodule in same lobe |
| M1 | T4 | Additional nodule in ipsilateral different lobe |
| M1 | M1a | Additional nodules in contralateral lung |
| M1 | M1a | Ipsilateral pleural effusion |
| Stage | T | N | M |
|---|---|---|---|
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1a, b | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T1a, b | N1 | M0 |
| T2a | N1 | M0 | |
| T2b | N0 | M0 | |
| Stage IIB | T2b | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T1, T2 | N2 | M0 |
| T3 | N1, N2 | M0 | |
| T4 | N0, N1 | M0 | |
| Stage IIIB | T4 | N2 | M0 |
| Any T | N3 | M0 | |
| Stage IV | Any T | Any N | M1a, b |
Performance status
Performance status is a measure used to quantify cancer patients' general well-being and is used to determine whether or not a patient is fit enough to receive treatment and to assess how much supportive care a patient needs. There are three main scales used to measure PS: the World Health Organization (WHO) PS scale,6 the Karnofsky PS scale (KPS)6 and the Eastern Cooperative Oncology Group (ECOG) PS scale. 7 A summary of the WHO PS scale is shown in Table 4 as this is the most commonly used scale in clinical practice in the UK. 6 A score of 0 on the WHO scale indicates that a patient is completely able to look after him- or herself and a score of 4 shows that a patient requires a lot of support.
| Scale | WHO criteria |
|---|---|
| 0 | Patient is fully active and more or less the same as before illness |
| 1 | Patient is unable to carry out heavy physical work but can do anything else |
| 2 | Patient is up and about more than half the day; able to look after him/herself but not well enough to work |
| 3 | Patient is in bed or sitting in a chair for more than half the day; needs some help in looking after him/herself |
| 4 | Patient is in bed or a chair all the time and needs a lot of looking after |
Histology
Histological confirmation (i.e. a diagnosis made by taking a sample of tissue or cells) is an important element of diagnosis because it helps to determine a patient's treatment pathway. However, a proportion of diagnoses are based on clinical examination and radiological investigations alone, without histological evidence. The LUCADA data show that, for England and Wales, histological confirmation of the cancer diagnosis is made in 72% of cases, with wide (regional) variation from 25% to > 85%. 4 Recent NICE guidance for the first-line treatment of NSCLC recommends histological testing and therefore histological testing rates are expected to increase. 4
There are two main types of lung cancer: NSCLC accounts for approximately 84% of all lung cancers diagnosed and the remaining 15% are small cell lung cancer. The main subtypes of NSCLC are squamous cell carcinoma (33%) and non-squamous cell carcinoma (29%); the latter includes adenocarcinoma (25%) and large cell carcinoma (4%). Approximately 36% of patients are listed as being NSCLC ‘not otherwise specified’, 1% are carcinoma in situ and 1% are bronchioloalveolar. 9
Squamous cell carcinoma commonly begins in the bronchi, centrally in the lungs. Adenocarcinoma starts in the periphery of the lungs and can be present for a long time before detection. It is usually the type of lung cancer found in non-smokers and is the most common type seen in women. Large cell carcinomas often occur in the outer regions of the lungs and tend to grow rapidly and spread more quickly than some other forms of NSCLC. 10
Treatment options for non-small cell lung carcinoma
Figure 1 shows a treatment pathway for patients with NSCLC and estimates of the proportions and numbers of patients with NSCLC along the treatment pathway in England and Wales based on histology and staging data, NICE guidelines5 and NICE guidance. 10–14 A proportion of patients have small cell disease and recommendations for this group are not discussed in this report.
FIGURE 1.
Treatment pathway for patients with NSCLC. PLAT, platinum (cisplatin or carboplatin); S, stage. a, M Peak, data from the National Lung Cancer Audit, audit period 2009, personal communication, 2011; b, M Peak, data from the National Lung Cancer Audit, audit period 2008, personal communication, 2011.

In total, 30% of patients with NSCLC are diagnosed with stage I–IIIA disease. These patients may be suitable for potentially curative surgery or radical radiotherapy (RT). Surgery for NSCLC consists of lobectomy, pneumonectomy and wedge resection. Approximately 50% of patients undergoing these procedures will relapse and will then be eligible for further treatment. Patients with stage IIIA–IIIB disease who are not amenable to surgery can be treated with potentially curative chemoradiation (CTX-RT). CTX-RT can be delivered in different ways: as induction, sequential, concurrent and/or consolidation CTX-RT. For example, gemcitabine (GEM)-, vinorelbine (VNB)-, docetaxel (DOC)- and paclitaxel (PAX)-based CTX can be used alongside RT.
The majority (70%) of patients with NSCLC have stage IIIB or IV disease and a PS of 0 or 1 at the time of diagnosis. These patients are assessed for their suitability for first-line chemotherapy (CTX); less than half (48%) of patients who are assessed are considered suitable and actually receive treatment. Among those who receive CTX, almost half will respond to treatment and have either a complete or a partial response. Of these patients, a relatively small proportion can go on to have maintenance treatment and only 28% are suitable for second-line CTX.
The majority of patients with NSCLC are diagnosed late and have metastatic or locally advanced disease. Therefore, up to 50% of patients are treated with best supportive care (BSC) alone. During all stages of treatment, patients receive BSC or ‘active supportive care’ in addition to any anticancer treatment. In the recently published lung cancer guidelines,5 NICE defines ‘supportive care’ as ‘the multidisciplinary holistic care offered to all patients and their carers throughout the pathway to help them cope with cancer and treatment of it. BSC packages include options for information giving, symptom control and psychological, social and spiritual support. Palliative care provides a similar holistic approach, but is specific to those patients with advanced progressive illness’ (p. 98).
Combined chemotherapy and radiotherapy treatment
Radiotherapy is the treatment of cancer with high-energy radiation. RT can be either radical (potentially curative) or palliative. For patients of good PS in whom the disease can be encompassed within a radical RT treatment volume (mainly stage IIIA and selected stage IIIB), high doses of RT at conventional fractionation were the standard treatment with potential curative intent until the 1990s. Developments in RT regimens, including improved techniques and fractionation schedules,5 coupled with the addition of CTX have improved local control, systemic relapse and overall survival (OS). 19 Radical RT now commonly means potentially curative external beam RT and includes several fractionation schedules, including conventional fractionated RT, split-course RT, hyperfractionated RT, continuous hyperfractionated accelerated RT (CHART) and hyperfractionated accelerated RT (HART). In addition, there have been considerable recent technological advances in RT equipment [e.g. four-dimensional planning to account for tumour movement over the breathing cycle and On-Board Imager® treatment verification (Varian Medical Systems, Palo Alto, CA, USA)] that allow RT to be more accurately delivered to the tumour with less damage to normal tissues. 5 Also, recent innovation has enabled new approaches to be developed, for example stereotactic body RT. 5
The aim of adding CTX to RT is to improve the cure rate obtained with RT alone; CTX is used as a systemic treatment to control micrometastases and the risk of systemic relapse. In addition, many CTX agents have a radiation-sensitising effect and offer potential benefits in locoregional control. 5 RT is aimed at improving local control of the tumour. CTX-RT is described in different ways depending on the timing of the CTX relative to the RT (Table 5).
| Terms | Description |
|---|---|
| Radical RT | All RT that is not palliative in intent. Minimum dose of 50 Gy in 25 daily fractions or its radiobiological equivalent |
| Combined CTX-RT | Treatment given to patients eligible for potential curative RT at presentation; the treatments are given either sequentially or concurrently |
| Concurrent CTX-RT | CTX given on the same days as RT treatment |
| Sequential CTX-RT | CTX given before a course of RT but not during RT |
| Consolidation CTX-RT | CTX given subsequent to RT |
| Induction CTX | CTX given before CTX-RT |
Compared with RT alone, both sequential and concurrent CTX-RT have shown a 4% improvement in 2-year survival rates. 20,21 A Cochrane meta-analysis22 of 13 trials (2214 patients) confirmed a statistically significant reduction in risk of death at 2 years with concurrent CTX-RT compared with RT alone, although there was significant heterogeneity across the trials. An update of the Cochrane review19 compared sequential with concurrent CTX-RT. The authors of the review demonstrated a statistically significant benefit in median survival for concurrent CTX-RT (16–17 months) over sequential CTX-RT (13–15 months). However, the treatment-related mortality was almost twice as high in the concurrent arm and the incidence of acute oesophagitis was 19% in the concurrent arm compared with 3% in the sequential arm. The authors of the Cochrane review19 recognise that there was considerable heterogeneity related to the frequency of administration and doses of CTX.
Outcome measures
Survival is considered to be the most reliable cancer end point within a randomised controlled trial (RCT) and, when trials can be conducted to adequately assess survival, it is usually the preferred end point. OS is a measure of the time from randomisation to death from any cause; median OS is the point in time at which 50% of people with a condition will have died and 50% are still alive. Year 1 and year 2 survival risk is defined as the probability of survival in intervals of time elapsed from randomisation to year 1 and year 2 respectively.
Many trials also report progression-free survival (PFS) as an intermediate surrogate measure of survival. PFS measures the amount of time between randomisation until tumour progression or death from any cause. In most trials tumour progression is defined using RECIST criteria (Response Evaluation Criteria in Solid Tumors) as at least a 20% growth in the size of the tumour or spread of the tumour since the beginning of treatment with a 5-mm absolute increase in size. 23 Time to progression (TTP) is defined as the time from randomisation until tumour progression (and does not include death). The majority of RCTs measure overall response rate (ORR), which is the proportion of patients who have a response (the tumour shrinks), which can be complete or partial. Stable disease is recorded when there is no response and the tumour does not change in size. Stable disease also means that no new tumours have developed and that the cancer has not spread to any new regions of the body. 24
Adverse event (AE) rates and health-related quality-of-life (HRQoL) data are also measures of important clinical benefit and provide information on how well patients are able to tolerate CTX treatments. AEs within trials are graded for severity (1–4), and usually the more severe events at grades 3 and 4 are of interest to clinicians. These are discussed in more detail in Chapter 3 (see Adverse events).
In advanced NSCLC, CTX-RT treatment is also given in an effort to improve HRQoL. Commonly used HRQoL tools within NSCLC trials include the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C3025 and the lung cancer-specific module QLQ-LC13,26 the Lung Cancer Symptom Scale (LCSS)27 and the Functional Assessment of Chronic Illness Therapy – Lung (FACT-L) questionnaire. 28 The European Quality of Life-5 Dimensions (EQ-5D)29 is a standardised generic instrument for measuring HRQoL that may be used in lung cancer trials. It provides a utility score for health and a self-rating of HRQoL. AEs, both from the disease itself and from CTX, have a considerable impact on HRQoL. 30
Despite HRQoL being both a vitally important measure of a patient's general emotional, physical and mental well-being and a very relevant measure of the ‘success’ of CTX treatment, because advanced-stage NSCLC is not curable, only a minority of trials address HRQoL issues.
Current UK guidelines and guidance
The National Institute for Health and Clinical Excellence produces clinical guidance and guidelines recommending appropriate treatments and care for people with NSCLC; NICE issues recommendations based on the best available clinical effectiveness and cost-effectiveness evidence. Comprehensive guidelines31 on the management of patients with lung cancer published by NICE in 2005 concluded that sequential CTX-RT is more beneficial than RT alone for patients with locally advanced NSCLC. The clinical evidence available appeared to support the use of concurrent CTX-RT, but the results of further clinical efficacy studies were required to ensure that informed decision-making could take place.
NICE guidelines5 on the diagnosis and treatment of lung cancer were updated in 2011. The updated guidelines state that CTX-RT is now an established approach to treatment, with curative intent of patients with NSCLC when surgery is not suitable, and the potential benefit in survival needs to be balanced with the risk of additional toxicities. Current NICE guidelines5 recommend that CTX-RT is the treatment of first choice for patients with stage II and stage III cancer who are not suitable for surgery; however, how currently available CTX and RT regimens should be optimally combined within concurrent CTX-RT remains unclear.
Rationale for this review
Given the advances in first-line treatment of NSCLC it was felt that an updated review was required. The Assessment Group conducted this review as part of a larger systematic review32 of all first-line CTX and CTX-RT treatments for patients with locally advanced or metastatic NSCLC. It was the opinion of the members of the clinical panel for the project that patients with potentially curable disease (e.g. stage IIIA) are different from those who are considered only for palliative treatment of more advanced disease and therefore that the results relating to these former patients should be reported separately.
Chapter 2 Definition of the decision problem
Decision problem
The population of interest is adult patients who are CTX naive, with locally advanced NSCLC.
Any first-line CTX-RT therapy (induction, sequential, concurrent or consolidation) was included. The Assessment Group did not place any restrictions on the type of CTX drug or RT included in the review.
The primary outcome was OS.
Secondary outcomes included the following:
-
PFS
-
survival risk at year 1 and year 2
-
ORR
-
AEs
-
HRQoL.
Overall aim and objective of the assessment
The objective of the assessment is to evaluate the clinical effectiveness of first-line CTX-RT for adult patients with locally advanced NSCLC. This review aims to identify the optimal combination of CTX and RT for this group of patients.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Identification of trials
A comprehensive search strategy was developed; search terms included a combination of index terms (e.g. non-small cell lung carcinoma) and free-text words (e.g. lung cancer or lung tumour or lung carcinoma). The search of MEDLINE and EMBASE was restricted to papers with abstracts published in the English language. MEDLINE was searched from January 1990 to March Week 3 2009 and EMBASE was searched from January 1990 to Week 13 2009. The Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials and Health Technology Assessments) was searched in Issue 3, July 2010. An updated search was performed of MEDLINE and EMBASE to identify trials published up until September 2010. All references were exported to the EndNote® reference database (version X4; Thomson Reuters, CA, USA). Details of the search strategies are available in Appendix 1. The Liverpool Reviews and Implementation Group (LRiG) team was expanded prior to searching to include clinicians with relevant experience in specialist CTX-RT treatment options.
The protocol was revised to exclude trials that had been published before 2000 because of the large numbers of references identified by the original searches and, more importantly, to reflect recent advances in CTX and RT treatments (e.g. third-generation CTX drugs and HART).
The Assessment Group carried out a number of targeted searches to ensure the completeness of the review, including in the database of the American Society for Clinical Oncology (ASCO) and on the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) websites.
A key review of CTX treatments for patients with NSCLC by Clegg et al. 33 was searched for relevant trials. An updated Cochrane review19 of concurrent CTX-RT has recently been published and communication with the lead author (Noelle O'Rourke) has ensured that all trials relevant to this review have been included. Reference lists of included trials were also searched to identify any further relevant trials.
Inclusion and exclusion criteria
The citations identified by the search strategy were assessed for inclusion; reviewers independently screened all the titles and abstracts identified by electronic searching of MEDLINE and EMBASE and The Cochrane Library (Issue 3, July 2010). The search of MEDLINE and EMBASE was updated to September 2010. Potentially relevant references were obtained as full-text copies and each reference was assessed independently by two reviewers using the inclusion criteria outlined in Table 6. The inclusion/exclusion assessment by each reviewer was recorded on a pretested, standardised form.
| Patient population | CTX-naive adult patients with locally advanced NSCLC |
| Intervention | Any first-line CTX-RT |
| Comparator | Any first-line CTX-RT |
| Outcomes | OS and/or PFS estimates |
| Study design | RCTs |
Data extraction strategy
Data extraction forms were developed and piloted on a sample of included trials. Data on trial design, population characteristics and outcomes were extracted by one reviewer and independently checked for accuracy by a second reviewer. Microsoft Access® software (Microsoft Corporation, Redmond, WA, USA) was used to store extracted data from the included trials. Appendix 2 contains details of the data extraction.
Critical appraisal strategy
All included trials were assessed for methodological quality using criteria based on the Centre for Reviews and Dissemination guidance for undertaking reviews in health care34 and adapted to reflect the characteristics of patients with NSCLC. Data relating to quality assessment were extracted by one reviewer and independently checked for accuracy by a second reviewer. Appendix 3 contains the trial quality assessment extraction details. Where necessary, any disagreements between reviewers were discussed in consultation with a third reviewer to achieve consensus.
Evidence synthesis
The trends in locally advanced NSCLC treatment in the UK indicate that concurrent CTX-RT has emerged as the standard of care in the NHS. 19,35 However, given the wide range of CTX-RT options, the optimum timing, dosing and choice of systemic agents to achieve the best therapy remain controversial and are subject to ongoing debate. For instance, two recent reviews/meta-analyses19,35 that evaluated concurrent and sequential CTX-RT schedules reported similar findings in terms of OS but their conclusions on whether or not concurrent CTX-RT should remain a standard of care in locally advanced NSCLC were different. The Aupérin et al. 35 review concluded that concurrent CTX-RT should remain a standard of care for patients with locally advanced disease but the authors of the Cochrane review19 concluded that the evidence base was weak.
In addition, most of the clinical trials19,35 that have led to concurrent CTX-RT becoming the standard of care have restricted enrolment to patients with good PS, limited weight loss and adequate lung function. Meanwhile, patients who do not meet these criteria are treated with low doses of CTX-RT, sequential CTX-RT, induction/concurrent CTX-RT or concurrent/consolidation CTX-RT. Despite evidence from these reviews,19,35 it is not immediately apparent what conclusions can be drawn from the clinical evidence regarding the relative efficacy of different CTX-RT treatments as neither of the reviews compared concurrent, sequential, induction/concurrent and concurrent/consolidation options.
Data from eligible studies were synthesised to estimate relative treatment effects. The aim of this evidence synthesis was to identify the best treatment options for the management of locally advanced NSCLC in terms of the optimal timing and sequencing of CTX-RT. Studies with at least two CTX-RT treatment arms comprising any CTX-RT including concurrent, sequential, induction/concurrent and concurrent/ consolidation treatments were eligible for inclusion in our evidence synthesis.
The Assessment Group planned analyses using standard meta-analyses and mixed-treatment comparison (MTC) where sufficient clinically and statistically homogeneous data were available from the included studies. The primary outcome for the evidence synthesis was OS, defined as time from randomisation to death of any cause. Planned secondary outcomes included PFS (defined as time from date of randomisation to the earliest sign of disease progression or death from any cause).
Direct evidence synthesis
All planned analyses were based on intention-to-treat (ITT) populations where possible. Where appropriate, standard meta-analyses were undertaken for each pair-wise treatment comparison using the ‘metan’ command within Stata version 9.2 (StataCorp LP, College Station, TX, USA). Where appropriate, for time-to-event outcomes (OS and PFS) the trial-level estimate of log hazard ratio (HR) and its variance were extracted directly from trial publications if available. In the absence of direct estimates from published papers, previously reported methods that used published data such as Kaplan–Meier survival curves or log-rank statistics were used to estimate the required trial-level log HR and its variance. 36,37 A random-effects (frequentist) inverse-variance weighted approach was used to pool estimates of log HR across trials. We assessed statistical heterogeneity by considering the chi-squared test for heterogeneity, with a 10% level of significance, and the I2 statistic, with a value of 50% representing moderate heterogeneity. 38,39
Mixed-treatment comparison: direct and indirect comparisons
As trials conducting head-to-head comparisons of all treatments under evaluation were not available or sparse, the possibility of conducting an indirect comparison was investigated by the Assessment Group. This approach fulfils the objective of providing simultaneous comparison of all of the relevant treatment alternatives and can provide information about the associated decision uncertainty or sufficient information for economic evaluation. Hence, for the purposes of decision-making, it was planned that a Bayesian MTC framework would be adopted to synthesise information on all technologies simultaneously using Markov chain Monte Carlo (MCMC) methods to estimate the posterior distributions for our outcomes of interest. The MCMC simulation begins with an approximate distribution and, if the model is a good fit to the data, the distribution converges to the true distribution. The MTC analysis allows for the synthesis of data from direct and indirect comparisons and allows for the ranking of different treatments in order of efficacy and estimation of the relative treatment effect of competing interventions. This approach assumes ‘exchangeability’ of treatment effect across all included trials, such that the observed treatment effect for any comparison could have been expected to arise if it had been measured in all other included trials. Exchangeability would be judged through examination of the trial populations and comparability of outcomes in the common treatment group facilitating the comparison. Inconsistency in the treatment effects between pair-wise comparisons was planned to be investigated by comparing the direct and indirect evidence together with the 95% confidence intervals (CIs).
As with all meta-analyses, MTC may be conducted using either fixed- or random-effects models. Random-effects models allow for the possibility that the true treatment effect may differ between trials. In our analyses, random-effects models would be used throughout. Model fit would be assessed based on residual deviance and deviance information criteria. Adjustment for multiarm trials would be performed as estimates of relative treatment effects from trials with more than two treatment arms will be correlated because of their joint dependence on the reference treatment arm.
In each MCMC simulation we planned to rank the absolute log HR and then use it to calculate the probability that each treatment was best across all simulations. 40,41 If a treatment is statistically significantly better than all other treatments in the MTC, the probability of it being the most effective treatment will be at least 95%. A probability < 95% indicates that there is at least one other treatment that is not significantly different to the ‘best’ treatment (at the 5% level). Use of a non-informative (flat prior) normal distribution was planned for the log HR and log relative risk (RR) of each relative comparison; thus, the observed results are completely influenced by the data and not the choice of prior.
Where appropriate, WinBUGS version 1.4 statistical software42 (MRC Biostatistics Unit, Cambridge, UK) would be used for the MTC analysis by adapting code (presented in Appendix 4) from the Multi-parameter Evidence Synthesis Research Group. 43 It was planned to use two chains to ensure that model convergence was met after 90,000 iterations with a burn-in of 10,000. Formal convergence of the models would be assessed using trace plots and the Gelman–Rubin approach44 and through inspection of the history plots.
Results of the review of clinical effectiveness
Quantity of research available
As shown in Figure 2, the electronic searches identified 5378 citations (Table 7 describes in detail the results of the database searching). Initial screening identified 330 potentially relevant references; these were obtained as full-text copies and the 240 references that were published post 2000 were assessed for eligibility for inclusion. Overall, 19 trials were included that compared different regimens of CTX-RT. The initial search identified a relatively large number of ‘hits’ because this review was part of a more extensive systematic review32 that examined CTX compared with CTX as well as CTX-RT compared with CTX-RT.
FIGURE 2.
Flow diagram of inclusion of CTX-RT trials.
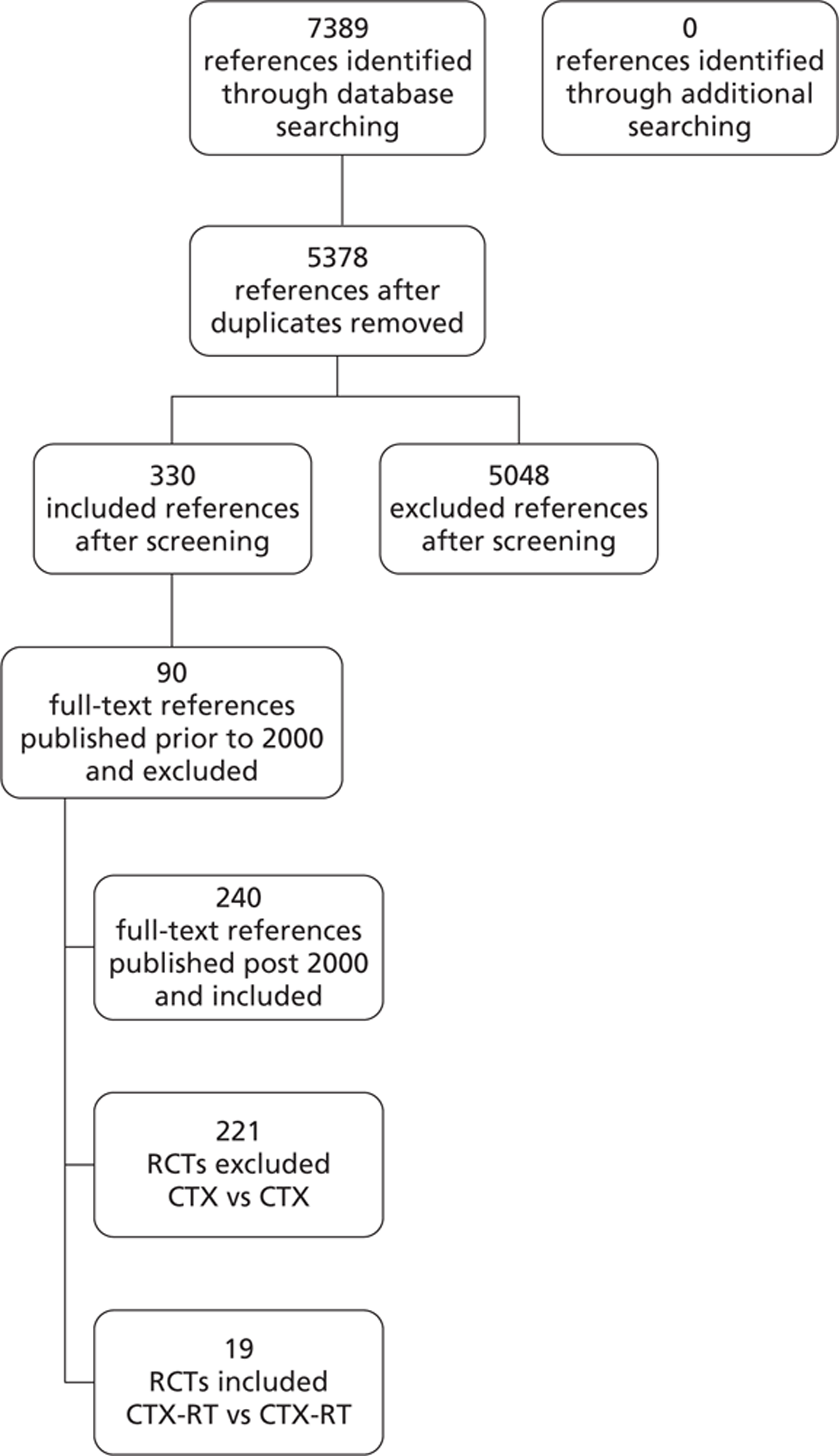
| Database | Dates | Number | Deduplicated | First screen | Second screen | 2000–10 | CTX-RT vs CTX-RT |
|---|---|---|---|---|---|---|---|
| MEDLINE | 1990–March Week 3 2009 | 2594 | 3848 | 329 | 265 | 175 | 11 |
| EMBASE | 1990–Week 13 2009 | 3034 | |||||
| MEDLINE | 2009–August Week 3 2010 | 316 | 455 | 35 | 34 | 34 | 5 |
| EMBASE | 2009–Week 34 2010 | 370 | |||||
| Cochrane Central Register of Controlled Trials | 2000–Issue 3 of 4, July 2010 | 1034 | 1034 | 174 | 31 | 31 | 3 |
| Cochrane Database of Systematic Reviews | 2000–Issue 3 of 4, July 2010 | 4 | 4 | 0 | 0 | 0 | 0 |
| Database of Abstracts of Reviews of Effects | 2000–Issue 3 of 4, July 2010 | 22 | 22 | 0 | 0 | 0 | 0 |
| Health Technology Assessment | 2000–Issue 3 of 4, July 2010 | 15 | 15 | 0 | 0 | 0 | 0 |
| Total number of references | 7389 | 5378 | 538 | 330 | 240 | 19 | |
| Total number of RCTs | 19 |
Assessment of effectiveness
In total, 19 RCTs met the inclusion criteria of the systematic review and compared CTX-RT with CTX-RT. 45–63
Quality
The results of the methodological quality assessment of trials are presented in Table 8. Overall methodological quality of included trials was poor, with nearly all trials failing to report relevant methodology; in particular, methods of randomisation, allocation concealment and blinding were inadequately described.
| Reference ID | Randomisation | Baseline comparability | Eligibility criteria specified | Co-interventions identifiedb | Blinding | Withdrawals | ITT | Other outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Truly random | Allocation concealment | Number stated | Presented | Achieveda | Assessors | Administration | Participants | Procedure assessed | > 80% in final analysis | Reasons stated | |||||
| Jeremic 200163 | NS | NS | ✓ | ✓✗ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Komaki 200250 | NS | ✓ | ✓ | ✓ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Schild 200262 | NS | NS | ✓ | ✓ | ✓ | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Vokes 200247 | NS | ✓ | ✓ | ✓ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Zatlouka 200451 | NS | ✗ | ✓ | ✓ | ✓ | ✓ | NS | ✗ | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
| Belan 200552 | NS | NS | ✓ | ✓ | ✓✗ | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Fournel 200549 | NS | ✓ | ✓ | ✓✗ | ✓✗ | ✓ | NS | ✗ | ✗ | ✗ | NA | ✓ | ✓ | ✗ | ✗ |
| Reinfuss 200556 | ✓ | NS | ✓ | ✓ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Dasgupta 200656 | NS | NS | ✓ | ✓ | NS | ✓ | ✓✗ | NS | NS | NS | NS | ✓ | NA | ✗c | ✗ |
| Gouda 200659 | NS | NS | ✓ | ✓ | ✓ | ✓ | NS | NS | NS | NS | NS | ✓ | NA | ✓ | ✗ |
| Belderbos 200754 | NS | NS | ✓ | ✓ | ✓✗ | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✓ | ✓ |
| Vokes 200748 | NS | ✓ | ✓ | ✓✗ | ✓✗ | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✓ | ✗ |
| Liu 200853 | NS | NS | ✓ | ✓ | ✓✗ | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Socinski 200855 | NS | NS | ✓ | ✓ | ✓✗ | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Berghmans 200945 | ✓ | ✓ | ✓ | ✓ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✗ | ✗ |
| Crvenkova 200957 | NS | NS | ✓ | ✓✗ | ✓ | ✓ | NS | NS | NS | NS | NS | ✓ | ✗ | NS | ✗ |
| Nyman 200958 | NS | NS | ✓ | ✓ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✗ | ✗ | ✓ |
| Zhu 200960 | NS | NS | NS | ✓✗ | NS | ✓ | NS | NS | NS | NS | NS | NS | NA | NS | ✗ |
| Movsas 201061 | NS | NS | ✓ | ✓ | NS | ✓ | NS | NS | NS | NS | NS | ✓ | ✓ | ✓ | ✗ |
Only two RCTs45,46 provided sufficient information regarding randomisation methods. Random assignment was performed centrally in five trials45,47–50 and so allocation concealment was assessed as adequate in these trials. Another trial used randomisation by envelope to conceal allocation. 51 Eighteen trials clearly reported the number of participants randomised. 45–59,61–63 All trials reported details of participant comparability at baseline.
Six trials48,49,52–55 reported imbalance between trial groups at baseline; these were assessed as achieving partial comparability. Two trials54,55 reported significant imbalance between treatment groups for baseline disease. In one trial55 62% of patients had stage IIIB disease in the concurrent GEM plus carboplatin (CARB)-RT arm compared with 32% of patients in the concurrent PAX plus CARB-RT arm. In the trial by Belderbos et al. ,54 47.4% of the patients in the sequential CTX-RT arm had stage IIIB disease compared with 63.8% in the concurrent CTX-RT arm.
All trials reported eligibility criteria. One trial56 reported details of co-interventions; however, it was unclear how many patients in each treatment arm had received any of the co-interventions. Two trials49,51 were reported as ‘open’ and it was assumed that assessors, administrators and participants were not blinded to treatment; blinding was not stated in the remaining 17 trials. Over 80% of patients were assessed in 18 trials. 45–59,61–63 Fourteen trials45–55,61–63 reported reasons for withdrawals, two trials57,58 failed to report this and in three trials56,59,60 there were no withdrawals. Six trials46,48,51,54,59,61 used an ITT approach and assessed all participants according to the groups to which they were randomised. The trial by Dasgupta et al. 56 intended to exclude non-completers from analyses; however, there were no non-completers and so all patients were assessed.
Two trials54,58 intended to measure HRQoL. In the trial by Belderbos et al. 54 it is not clear whether or not HRQoL was measured as it was not reported, and in the trial by Nyman et al. 58 HRQoL outcomes were measured and are to be reported in a future publication.
Five of the 19 trials were closed prematurely for the following reasons: confirmation of the benefit of concurrent CTX-RT,52 poor accrual,54 poor accrual due to administrative problems,45 high rate of serious AEs in the induction/concurrent CTX-RT arm55 and slow accrual coupled with interim analysis results that demonstrated a statistically significant difference in survival in favour of the concurrent CTX-RT arm. 51 One trial experienced slow accrual that led to a reduction in the target number of participants recruited. 62
Trial characteristics
Trial characteristics are presented in Appendix 6. The 19 trials were published between 2001 and 2010. Of the 13 multicentre trials, three have international centres. 45,54,61 There were seven Phase II47,50,52,54,55,58,61 and six Phase III trials45,48,49,56,62,63 and six trials in which the phase is unclear. 46,51,53,57,59,60 Eight trials47,48,50,51,54,55,62,63 were funded by research grants, four trials49,52,58,61 were funded by pharmaceutical companies and the funding source was not stated in seven trials. 45,46,53,56,57,59,60 Five trials47,48,58,62,63 were sufficiently powered to evaluate the primary outcome of each trial, which included TTP, median OS, response rate and 2-year survival rate. Five trials45,49,51,54,61 were inadequately powered and the power of nine trials46,50,52,53,55–57,59,60 was unclear. Median follow-up of patients ranged from 16.5 to 60 months.
Details of trial interventions are presented in Appendix 7. Concurrent CTX is defined as CTX given on RT treatment days (whether before or after each fraction of RT). Sequential CTX-RT is defined as CTX given before or after a course of RT but not during. Consolidation CTX-RT is defined as CTX given subsequent to RT, and induction CTX-RT is defined as CTX given prior to RT.
Four trials46,51,54,60 compared sequential with concurrent CTX-RT, four trials49,52,56,57 compared sequential with concurrent/consolidation CTX-RT, three trials48,50,59 compared induction/concurrent CTX-RT with concurrent CTX-RT and two trials45,52 compared induction/consolidation CTX-RT with concurrent/ consolidation CTX-RT. The remaining six trials47,55,57,58,61,62 could not be grouped for comparison as they compared a variety of different CTX-RT regimens.
Different CTX agents were used both across and within trials. It is worth noting that GEM, PAX and VNB were used by a similar number of trials, whereas DOC was used by relatively few trials. Etoposide (ETOP) plus platinum (cisplatin or carboplatin) (PLAT) was used in seven trials. 49,50,56,57,61–63
Radiation doses, number of fractions, schedule, relative dose intensity (RDI) and overall treatment time also varied between and within trials. Details of RDI are presented in Appendix 8. Twelve trials45,46,49–56,60,61 reported details of actual treatment received including median time to complete treatment, percentage of patients who completed treatment as per protocol and details of reductions and delays in CTX and RT. A sample of the within-trial differences that are demonstrated to be statistically significantly different are discussed here.
In the trial by Fournel et al. ,49 88% of patients in the concurrent CTX-RT arm received at least 60 Gy RT, compared with 59.4% in the sequential CTX-RT arm (p < 0.001); 54% received two planned cycles of consolidation CTX, 7% received only one course and 39% received no consolidation CTX. In the Zatloukal et al. trial,51 only 58% of patients in the sequential CTX-RT group completed four courses of CTX, compared with 83% in the CTX-RT concurrent group (p < 0.0007), and only 64% of the sequential CTX-RT group received RT, compared with 94% of concurrent CTX-RT group (p = 0.0002). The required time for completing treatments was statistically significantly different between the two groups in the trial by Zhuan and Wu;60 the concurrent CTX-RT group took, on average, 31 days less than the sequential CTX-RT group to complete treatment (p = 0.05).
In the trial by Reinfuss et al.,46 treatment was administered to 75% of patients in the concurrent CTX-RT arm on average, compared with 96.7% of participants in the sequential CTX-RT arm; the difference is statistically significant (log-rank test, p < 0.01). The only difference in treatment between the trial groups was the sequence of CTX and RT. Reported toxicity was significantly higher in the concurrent CTX-RT group than in the sequential CTX-RT group. Because of this toxicity, treatment was not completed in 21.4% of participants in the concurrent CTX-RT arm compared with 2.2% in the sequential CTX-RT arm.
It appears that the percentage of patients who completed treatment, and a higher dose intensity, was higher for all three CTX drugs regimens in the concurrent/consolidation CTX-RT arm than in the induction/concurrent CTX-RT arm of the trial by Berghmans et al. 45 Significantly fewer patients completed 7 weeks of CTX in the induction/concurrent CTX-RT arm than in the concurrent/consolidation CTX-RT arms of the trial by Belani et al. 52
In the trial by Movsas et al. ,61 in which patients received consolidation with either GEM or GEM/DOC after identical CTX-RT, 90.6% received all three planned cycles of GEM, compared with 68.8% who received all three cycles of GEM/DOC.
In the trial by Socinski et al. ,55 87.2% completed therapy to at least 74 Gy in the PAX plus CARB-RT arm, compared with 78.3% in the GEM plus CARB-RT arm. Rates of compliance with induction CTX, initiation and completion of concurrent CTX-RT and average dose and completion of thoracic RT were all higher in the PAX plus CARB-RT arm than in the GEM plus CARB-RT arm (this arm was closed prematurely because of the high rate of grade 4/5 pulmonary toxicity).
Patient characteristics
Details of patient characteristics are given in Appendix 9. The inclusion/exclusion criteria adopted by each of the trials are presented in Appendix 10. The number of patients randomised to trial arms ranged from 2059 to 184. 48 More than 50% of patients within the trials were male, with a median age of 40–64 years. With the exception of three trials,45,50,54 all trials included patients with disease stage IIIA or IIIB only. All but seven trials49,51,53,54,56,60,61 specifically excluded pleural effusions (see Appendix 10). The majority of patients within each trial had either adenocarcinoma or squamous cell carcinoma, and three trials46,48,63 failed to report details describing patient histology. In the majority of trials PS ranged from 0 to 1 [using a variety of PS criteria: ECOG, the Cancer and Leukemia Group B (CALGB)48, WHO] or from 60 to 100 (KPS). One trial51 included a small percentage of patients with ECOG PS 2 and one trial63 included a small percentage of patients with KPS 50.
Outcomes
Trial outcomes are presented in Table 9. Across the trials, median OS ranged from 12 to 29.5 months (16 trials), survival rate ranged from 37% to 80% at 1 year (14 trials) and from 14.3% to 66.6% at 2 years (16 trials). Across the trials, median PFS ranged from 5.4 to 14.9 months (11 trials) and median TTP ranged from 7.3 to 13.3 months (two trials). Across the trials, tumour ORR ranged from 33% to 88% (14 trials).
| Reference ID | Treatment | Median OS | Median PFS | Survival 1 year | Survival 2 years | Tumour ORR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | 95% CI | HR (95% CI) | Months | 95% CI | HR (95% CI) | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Jeremic 200163 | CTX + (HFX)RT days 1–5 | 20 | NR | NR | NR | NR | NR | 80 | NR | 47 | NR | 85 | NR |
| CTX + (HFX)RT days 1–7 | 22 | NR | NR | NR | NR | NR | 78 | NR | 49 | NR | 88 | NR | |
| Komaki 200250 | CTX → CTX + RT | 16.4 | NR | NR | 9.4 | NR | NR | 63 | NR | 39 | NR | 73 | NR |
| CTX + (HFX)RT | 15.5 | NR | NR | 8.2 | NR | NR | 58 | NR | 32 | NR | 71 | NR | |
| Schild 200262 | CTX + RT (2× daily) | 14 | NR | NR | 9.4 | NR | NR | 37 | 29 to 47 | 47 | NR | NR | NR |
| CTX + RT (4× daily) | 15 | NR | NR | 9.6 | NR | NR | 40 | 32 to 50 | 50 | NR | NR | NR | |
| Vokes 200247 | CTX (GEM + CIS) → CTX + RT | 18.3 | 13.8 to 23.6 | NR | 8.4 | NR | NR | 68 | 56 to 79 | 37 | NR | 74 | 60 to 86 |
| CTX (VNB + CIS) → CTX + RT | 14.8 | 12 to 19.5 | NR | 9.1 | NR | NR | 62 | 50 to 75 | 29 | NR | 67 | 52 to 80 | |
| CTX (PAX + CARB) → CTX + RT | 17.7 | 12.4 to 24.7 | NR | 11.5 | NR | NR | 65 | 53 to 78 | 40 | NR | 73 | 57 to 85 | |
| Zatlouka 200451 | CTX + RT | 16.6. | NR | 0.61 (0.39 to 0.93) | 11.9a | NR | NR | 69.2 | NR | 34.2 | NR | 80 | 62 to 98 |
| CTX → RT | 12.9 | NR | NR | 8.5a | NR | NR | 53 | NR | 14.3 | NR | 47 | 33 to 61 | |
| Belani 200552 | CTX → RT | 13 | NR | NR | 9.0 | NR | NR | 57 | NR | 30 | NR | NR | NR |
| CTX → CTX + RT | 12.7 | NR | NR | 6.7 | NR | NR | 53 | NR | 25 | NR | NR | NR | |
| CTX + RT → CTX | 16.3 | NR | NR | 8.7 | NR | NR | 63 | NR | 31 | NR | NR | NR | |
| Fournel 200549 | CTX → RT | 14.5 | 8.3 to 27.4 | NR | NR | NR | NR | 59.8 | 50 to 69 | 26.5 | 17.9 to 35.0 | 54 | NR |
| CTX + RT → CTX | 16.3 | 5.8 to 34.8 | NR | NR | NR | NR | 60.4 | 50.8 to 69.9 | 39.3 | 29.7 to 48.9 | 49 | NR | |
| Reinfuss 200546 | CTX → CT | NR | NR | NR | NR | NR | NR | NR | NR | 26.7 | NR | NR | NR |
| CTX + RT | NR | NR | NR | NR | NR | NR | NR | NR | 33.3 | NR | NR | NR | |
| Dasgupta 200656 | CTX → RT | NR | NR | NR | NR | NR | NR | 88.4 | NR | 57 | NR | 65.7 | NR |
| CTX + R | NR | NR | NR | NR | NR | NR | 86.1 | NR | 66.6 | NR | 66.7 | NR | |
| Gouda 200659 | CTX → CTX + RT | NR | NR | NR | NR | NR | NR | 55 | NR | 40 | NR | 75 | NR |
| CTX + RT | NR | NR | NR | NR | NR | NR | 65 | NR | 45 | NR | 79 | NR | |
| Belderbos 200754 | CTX → RT | 16.2 | 12.8 to 22.6 | 1.06 (074 to 1.52 | 10.8 | 9.0 to 15.0 | 0.79 (0.56 to 1.10) | 69 | 58.7 to 79.3 | 33.6 | 23 to 44.2 | 69.7 | 58.1 to 79.8 |
| CTX + RT | 16.5 | 11.3 to 24.3 | 8.5 | 6.4 to 10.9 | 55.9 | 45.0 to 66.9 | 38.5 | 27.6 to 49.4 | 60.8 | 47.8 to 72.4 | |||
| Vokes 200748 | CTX + RT | 12 | 10 to 16 | NR | 7.0 | NR | NR | NR | NR | 29 | NR | 67 | 60 to 74 |
| CTX → CTX + RT | 14 | 11 to 16 | NR | 8.0 | NR | NR | NR | NR | 31 | NR | 61 | 59 to 69 | |
| Liu 200853 | Low-dose weekly CTX (DOC) + RT (3D conformal) → CTX (DOC + CIS) | 20 | NR | NR | NR | NR | NR | 69.8 | NR | 48.1 | NR | 81.8 | NR |
| Systemic CTX + RT (3D conformal) → CTX (DOC + CIS) | 16 | NR | NR | NR | NR | NR | 66.5 | NR | 40.2 | NR | 86.4 | NR | |
| Socinski 2008a55 | CTX → CTX (PAX + CARB) + RT | 24.3 | 12.3 to 36.4 | NR | 14.9 | 7.9 to 24.3 | NR | 66.7 | 50.3 to 78.7 | NR | NR | 66.6 | 50.5 to 80.4 |
| CTX → CTX (GEM + CARB) + RT | 12.5 | 9.4 to 27.6 | NR | 7.7 | 5.1 to 11.0 | NR | 50 | 29.9 to 67.2 | NR | NR | 69.2 | 48.2 to 85.7 | |
| Berghmans 200945 | CTX + RT → CTX | 17.0 | 9.3 to 24.6 | NR | 7 3s | 5.0 to 9.6 | NR | NR | NR | NR | NR | 57 | 36 to 78 |
| CTX → CTX + RT | 23.9 | 13.3 to 34.5 | NR | 13.3a | 8.7 to 17.6 | NR | NR | NR | NR | NR | 79 | 64 to 94 | |
| Crvenkova 200957 | CTX → RT | 13 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CTX + RT → CTX | 22 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Nyman 200958 | CTX → CTX + accelerated RT | 17.69 | 11.15 to 36.25 | NR | 8.75 | 7.4 to 15.16 | NR | 64 | NR | 41 | NR | 33 | NR |
| CTX → CTX daily + conventional RT | 17.68 | 11.97 to NR | NR | 10.27 | 8.31 to 14.3 | NR | 62 | NR | 39 | NR | 70 | NR | |
| CTX → CTX weekly + conventional RT | 20.63 | 12.72 to 25.09 | NR | 9.31 | 7.33 to 13.78 | NR | 62 | NR | 38 | NR | 45 | NR | |
| Zhu 200960 | CTX + RT (3D conformal) | 19.7 | NR | NR | NR | NR | NR | 70.5 | NR | 47.7 | NR | 68 | NR |
| CTX → RT (3D conformal) | 18.2 | NR | NR | NR | NR | NR | 66.7 | NR | 42.9 | NR | 62 | NR | |
| Movsas 201061 | CTX + RT → CTX (GEM) | 16.1 | 9.8 to 34.0 | NR | 5.4 | 2.7 to 7.9 | NR | 65.6 | 46.9 to 79.3 | 40.6 | 23.8 to 56.8 | NR | NR |
| CTX + RT → CTX (GEM + DOC) | 29.5 | 16.4 to 52.0 | NR | 13.4 | 4.6 to 23.3 | NR | 71.9 | 52.9 to 84.3 | 55.7 | 36.8 to 70.9 | NR | NR | |
Results of evidence synthesis
Overall, population data describing just over 2000 patients in the 19 trials were eligible for consideration as part of the Assessment Group's approach to evidence synthesis. Detailed characteristics of all included trials are described in Appendix 6 and are also presented in the appendices. The Assessment Group investigated the possibility of conducting both meta-analysis and MTC analysis using the large quantity of trial data available. The Assessment Group concluded that the data available were heterogeneous: there were variations in CTX agents and different RT doses both across and within trials; number of fractions, schedules, intensity and overall treatment time also varied between trials. In summary, the 19 trials were disparate in terms of the interventions and comparators being compared (Table 10) and comprised eight distinct comparisons. As such, the conduct of a MTC was considered to be inappropriate. Direct meta-analysis using OS data was undertaken where possible: sequential CTX-RT compared with concurrent CTX-RT; sequential CTX-RT compared with concurrent/consolidation CTX-RT and sequential CTX-RT compared with concurrent CTX-RT with or without consolidation. The Assessment Group was unable to undertake any meta-analysis on PFS because of limited data.
| Reference ID | Concurrent CTX-RT | Sequential CTX-RT | Induction/concurrent CTX-RT | Concurrent/consolidation CTX-RT |
|---|---|---|---|---|
| Jeremic 200163 | ETOP + CARB + HFXRT vs ETOP + CARB + HFXRT | |||
| Komaki 200250 | ETOP + CIS + HFXRT | VBL + CIS → CIS + RT | ||
| Schild 200262 | ETOP + CIS + RT vs ETOP + CIS + HFXRT | |||
| Vokes 200247 | GEM + CIS → GEM + CIS + RT vs PAX + CIS → PAX + CIS + RT vs VNB + CIS → VNB + CIS + RT | |||
| Zatloukal 200451 | VNB + CIS + RT | VNB + CIS → RT | ||
| Belani 200552 | PAX + CARB → RT | PAX + CARB → PAX + CARB + RT | PAX + RT → CARB | |
| Fournel 200549 | VNB + CIS → RT | ETOP + CIS + RT → VNB + CIS | ||
| Reinfuss 200546 | VNB + CIS + RT | VNB + CIS → RT | ||
| Dasgupta 200656 | ETOP + CIS → RT + ETOP + CIS | ETOP + CIS + RT → ETOP + CIS | ||
| Gouda 200659 | PAX + CARB + RT | PAX + CARB → PAX + CARB + RT | ||
| Belderbos 200754 | CIS + RT | GEM + CIS → RT | ||
| Vokes 200748 | PAX + CARB + RT | PAX + CARB → PAX + CARB + RT | ||
| Liu 200853 | DOC + CIS + RT → DOC + CIS vs DOC + RT → DOC + CIS | |||
| Socinski 2008a55 | GEM + CARB → GEM + RT vs PAX + CARB → PAX + CARB + RT | |||
| Berghmans 200945 | GEM + VNB + CIS → GEM + VNB + CIS + RT | GEM + VNB + CIS + RT → GEM + VNB + CIS | ||
| Crvenkova 200957 | ETOP + CARB—>RT | ETOP + CIS + RT → ETOP + CARB | ||
| Nyman 200958 | PAX + CARB → PAX + RT vs PAX + CARB → PAX + RT vs PAX + CARB → PAX + CARB + RT | |||
| Zhu 200960 | VNB + CIS + RT | VNB + CIS → RT | ||
| Movsas 201061 | ETOP + CIS + RT → GEM vs ETOP + CIS + RT → GEM + DOC |
Overall survival data available for inclusion in meta-analyses
Sequential chemoradiation compared with concurrent chemoradiation (n = 4)
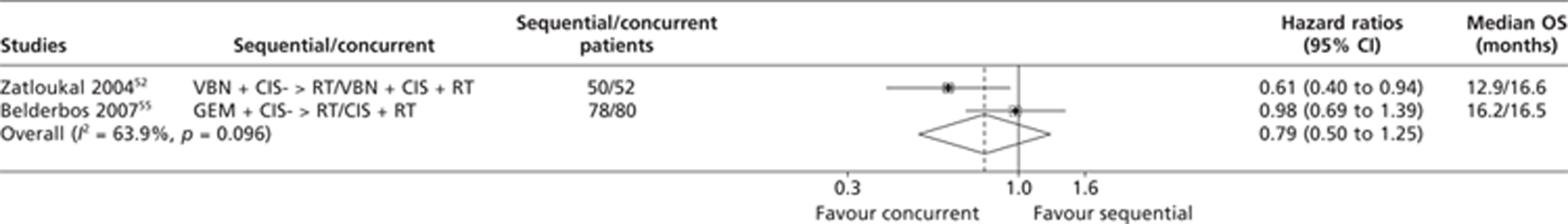
Four trials46,51,54,60 compared sequential CTX-RT with concurrent CTX-RT. The HRs for OS for two trials51,54 were extracted directly from the published trial papers. Two studies46,60 were excluded from the meta-analysis on OS because data were unavailable and it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. The trial by Zatloukal et al. 51 demonstrated significantly longer OS with concurrent CTX-RT (median survival 16.6 months) than with sequential CTX-RT (median survival 12.9 months) (p = 0.023, log-rank test; HR 0.61; 95% CI 0.39 to 0.93). The results from the trial described by Belderbos et al. 54 were not statistically significant. The pooled results from the OS meta-analysis comparing concurrent CTX-RT with sequential CTX-RT were therefore based on two trials. The results of this analysis are presented in Figure 3 and appear to show an OS advantage for concurrent CTX-RT arms over sequential arms; this result is not statistically significant (HR 0.79; 95% CI 0.50 to 1.25). Visual examination of the forest plot indicates a non-statistically significant chi-squared test for heterogeneity (p = 0.096) and an I2 statistic of 63.9%; the results suggest inconsistency in the direct evidence from the two trials. 51,54
FIGURE 3.
Summary of the direct evidence results for OS for trials comparing sequential CTX-RT with concurrent CTX-RT.
Sequential chemoradiation compared with concurrent/consolidation chemoradiation (n = 4)
Four trials49,52,56,57 compared sequential CTX-RT with concurrent/consolidation CTX-RT. Concurrent/consolidation CTX-RT significantly increased median OS in a trial by Crvenkova et al. 57 Three trials49,52,56 showed non-significant trends in favour of concurrent/consolidation CTX-RT compared with sequential CTX-RT.
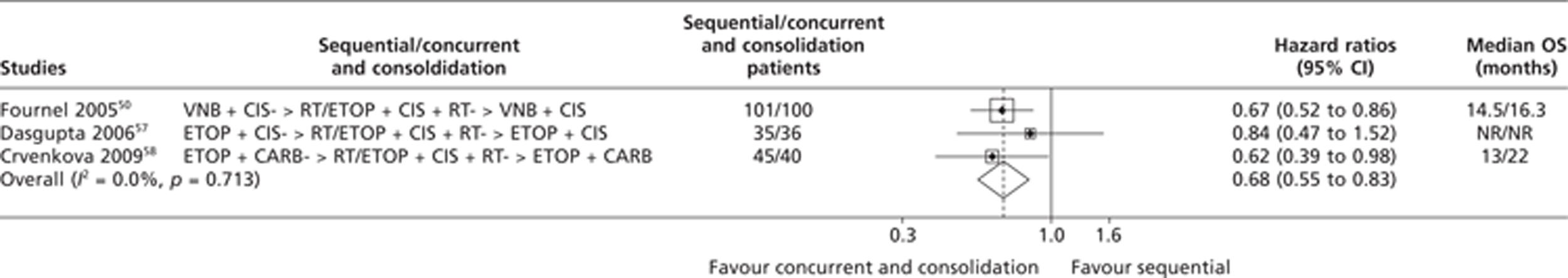
One52 of the four studies was excluded from the meta-analysis on OS because data were unavailable and it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. The OS HRs for one trial49 were extracted directly from the published trial paper, while HRs for two trials56,57 were estimated using summary statistics based on the methods described in the methods section of this report. The pooled results from the meta-analysis on OS comparing sequential CTX-RT with concurrent/consolidation CTX-RT were therefore based on data from three trials. The results of this analysis are presented in Figure 4 and appear to show a statistically significant OS advantage for concurrent/consolidation CTX-RT treatment over sequential treatment; this result is statistically significant (HR 0.68; 95% CI 0.55 to 0.83). Visual examinations of the forest plot, the chi-squared test for heterogeneity (p = 0.713) and the I2 statistic (0%) all suggest very good consistency.
FIGURE 4.
Summary of the direct evidence results for OS for trials comparing sequential CTX-RT with concurrent/consolidation CTX-RT. NR, not reported.
Sequential chemoradiation compared with concurrent chemoradiation with or without consolidation (n = 8)
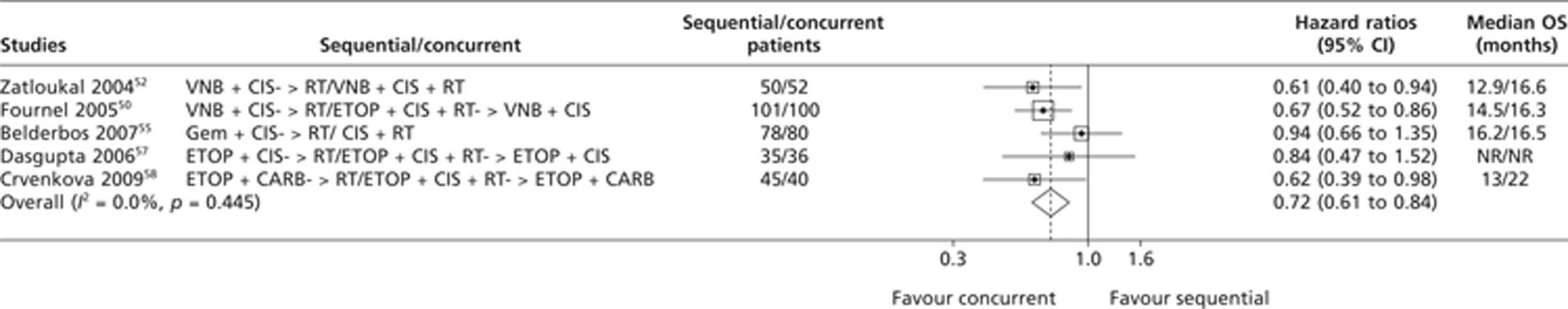
Eight trials46,49,51,52,54,56,57,60 compared sequential CTX-RT with concurrent CTX-RT with or without consolidation and were considered for inclusion in the meta-analysis on OS. The HRs for OS for three trials49,51,54 were extracted directly from the published trial papers, while HRs for two trials56,57 were estimated using summary statistics based on the methods described in the methods section of this report. Three studies47,52,61 were excluded from the meta-analysis on OS because data were unavailable and it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. Three trials49,51,57 demonstrated significantly longer OS with concurrent CTX-RT with or without consolidation (median survival ranged from 16.3 to 22 months) than with sequential CTX-RT (median survival ranged from 12.9 to 14.5 months). The pooled results from the meta-analysis on OS comparing sequential CTX-RT with concurrent CTX-RT with or without consolidation were based on data from five trials. The results of this analysis are presented in Figure 5 and appear to show a statistically significant OS advantage for concurrent CTX-RT with or without consolidation over sequential treatment; this result is statistically significant (HR 0.72; 95% CI 0.61 to 0.84). Visual examinations of the forest plot, the chi-squared test for heterogeneity (p = 0.445) and the I2 statistic (0%) all suggest very good consistency.
FIGURE 5.
Summary of the direct evidence results for OS for trials comparing sequential CTX-RT with concurrent CTX-RT with or without consolidation. NR, not reported
Overall survival data unavailable for inclusion in direct meta-analysis
Induction/concurrent chemoradiation compared with concurrent chemoradiation (n = 3)
Three trials48,50,59 compared induction/concurrent CTX-RT with concurrent CTX-RT. None of the studies presented OS HRs and therefore no meta-analysis was undertaken because it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. As shown in Table 10 the induction CTX used in two of the three trials was the same (PAX plus CARB). Direct results from each of the three studies indicated that the addition of induction CTX increased toxicity and provided no survival benefit over concurrent CTX-RT alone.
Induction/concurrent chemoradiation compared with concurrent/consolidation chemoradiation (n = 2)
Two trials45,52 comparing induction/concurrent CTX-RT with concurrent/consolidation CTX-RT were considered for inclusion in the meta-analysis on OS. The studies did not present OS HRs and therefore no meta-analysis was undertaken because it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. It is noted that the induction and consolidation chemotherapies used in the trials were different (see Table 10). Results from both trials demonstrate a longer median survival time with concurrent/consolidation CTX-RT than with induction/concurrent CTX-RT (16.3 months vs 12.7 months; 23.9 months vs 17 months); these results were not statistically significantly different.
Induction/concurrent chemoradiation compared with induction/concurrent chemoradiation (n = 3)
Three trials47,55,58 that compared induction/concurrent CTX-RT with induction/concurrent CTX-RT were considered for inclusion in the meta-analysis on OS. None of the studies presented OS HRs data and therefore no meta-analysis was undertaken because it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. The three trials were very different to each other as is shown in Table 10. In the trial by Nyman et al. ,58 all patients had the same induction CTX following randomisation into three arms. Concurrent weekly CTX with conventional RT was associated with a longer median survival (20.63 months) than concurrent CTX-RT with accelerated RT (17.69 months) and concurrent daily CTX with conventional RT (17.68 months).
The trial by Vokes et al. 47 compared induction CTX using GEM, VNB or PAX in combination with cisplatin (CIS) in addition to concurrent CTX-RT; median survival for all patients was 17 months (range 14.8–18.3 months). The trial by Socinski et al. 55 evaluated induction CTX with either PAX plus CARB or GEM plus CARB. On day 43, the PAX plus CARB arm received weekly CARB plus PAX whereas the GEM plus CARB arm received biweekly GEM. Both arms received CTX concurrently with 74 Gy of thoracic RT utilising three-dimensional treatment planning. The median survival time was 24.3 months in the PAX plus CARB arm compared with 12.5 months in the GEM plus CARB arm. The GEM plus CARB arm was closed prematurely because of a high rate of grade 4/5 pulmonary toxicity.
Concurrent chemoradiation compared with concurrent chemoradiation (n = 2)
Two trials62,63 comparing concurrent CTX-RT with concurrent CRX-RT were considered for inclusion in the meta-analysis on OS. None of the studies presented OS HRs data and therefore no meta-analysis was undertaken because it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. The trial by Jeremic et al. 63 aimed to investigate whether or not it is advantageous to add weekend CTX consisting of ETOP plus CARB to hyperfractionated RT and concurrent daily ETOP plus CARB. No difference was found regarding median survival time or 5-year survival rates (20 vs 22 months; 20% vs 23%; p = 0.57). The trial by Schild et al. 62 demonstrated no statistically significant difference in OS between ETOP plus CIS plus RT once daily and ETOP plus CIS plus RT twice daily (14 vs 15 months).
Concurrent/consolidation chemoradiation compared with concurrent/ consolidation chemoradiation (n = 2)
Two trials53,61 comparing concurrent/consolidation CTX-RT with concurrent/consolidation CTX-RT were considered for inclusion in the meta-analysis on OS. None of the studies presented OS HRs and therefore no meta-analysis was undertaken because it was impossible for the Assessment Group to estimate the OS HRs based on the published summary statistics. The two trials were very different to each other as shown in Table 10. The trial by Movsas et al. 61 compared two different consolidation CTX interventions (GEM compared with GEM plus DOC). Patients were randomised after they had all received the same concurrent CTX-RT. Consolidation therapy with GEM was associated with a median OS of 16.1 months compared with 29.5 months for GEM plus DOC. The trial by Liu et al. 53 showed no significant difference in 1- and 2-year survival rates between low-dose weekly DOC and standard DOC plus CIS – both groups received concurrent RT and the same consolidation CTX-RT. Median survival time was 20 months for the low-dose weekly DOC group and 16 months for the standard DOC plus CIS group.
Adverse events
Adverse events are presented in Appendix 11. Concurrent CTX-RT is associated with higher oesophageal toxicity than sequential CTX-RT. In the trial by Belani et al. 52 the most common locoregional grade 3/4 toxicity during and after thoracic RT was oesophagitis, which was more pronounced with concurrent CTX-RT than sequential CTX-RT. In the trial by Belderbos et al. 54 oesophagitis grade 3/4 was more frequent in the induction/concurrent CTX-RT arm than in the sequential CTX-RT arm (14% vs 5%); however, late oesophagitis grade 3 was 4% in both arms. Pneumonitis grade 3/4 was 14% in the sequential CTX-RT and 18% in the concurrent CTX-RT arm. In the trial by Crvenkova and Krstevska,57 acute oesophagitis and incidence of neutropenia were higher in the concurrent/consolidation CTX-RT arm than in the sequential CTX-RT arm; grade 3 oesophagitis was characteristic only of concurrent CTX-RT and was a reason for RT interruption (no longer than 7 days). In the trial by Fournel et al. ,49 oesophageal toxicity was significantly more frequent in the concurrent/consolidation CTX-RT arm than in the sequential CTX-RT arm (32% vs 3%). Treatment had to be stopped because of acute severe toxicity in 18% of patients in the sequential CTX-RT arm and 23% of patients in the concurrent/consolidation CTX-RT arm.
In the Komaki et al. 50 trial ,the incidence of acute oesophagitis was significantly higher among patients in the concurrent hyperfractionated RT group than among those in the induction/concurrent standard RT group (p < 0.0001). Analysis of late toxicity showed that chronic oesophageal toxicity was significantly more frequent in the concurrent hyperfractionated RT group than in the induction/concurrent standard RT group (p = 0.003). In addition, the incidence of acute haematological toxicity was significantly higher among patients treated with induction/concurrent standard RT (p = 0.01 for anaemia and p = 0.03 for other haematological toxicities) than among those treated with concurrent hyperfractionated RT.
In the Reinfuss et al. trial,46 the rate of toxicity in the concurrent CTX-RT arm was statistically significantly higher than the rate in the sequential arm. Full treatment according to the plan was given to 96.7% of patients treated sequentially and to 75% in the concurrently treated group. In 6.8% of patients undergoing sequential treatment and 14.3% of patients undergoing concurrent treatment, toxicity enforced breaks in irradiation, lasting 8–10 days, after which treatment was resumed and completed.
In the trial by Belderbos et al. ,54 acute haematological toxicity grade 3/4 was more pronounced in the sequential arm than in the concurrent arm (30% vs 6%). In the trial reported by Berghman et al. ,45 there was no difference in toxicity, except for more leucopenia and infection in the concurrent/consolidation CTX-RT arm than in the induction/concurrent CTX-RT arm. Secondary anaemia was more frequent in the sequential treatment group than in the concurrent arm in Crvenkova et al. 57 In the trial by Fournel et al. ,49 the incidence of neutropenia, including grade 4 neutropenia, was higher with sequential CTX-RT than with concurrent CTX-RT (p = 0.008). In addition, peripheral neuropathies were also more frequent in the sequential CTX-RT arm than in the concurrent arm.
In the trial by Jeremic et al. ,63 patients treated with the addition of weekend CTX had significantly more high-grade (> grade 3) haematological toxicity, including leucopenia, thrombocytopenia and anaemia (p = 0.0046). Late high-grade toxicity was not different between the two treatment groups.
Movsas et al. 61 reported that grade 3 or 4 events, including neutropenia (9/32 or 28.1% vs 18/32 or 56.3%; p = 0.03), anaemia (1/32 or 3.1% vs 6/32 or 18.8%; p = 0.05) and fatigue (2/32 or 6.3% vs 5/32 or 15.6%; not significant), were more frequent with consolidation GEM plus DOC than with consolidation GEM alone.
In the trial by Liu et al. 53 patients with grade 3/4 toxicity accounted for 14.3% of patients in the low-dose weekly DOC alone group and 28.6% of patients in the standard DOC plus CIS group (χ2 = 0.765, p = 0.382; χ2 = 1.108, p = 0.292, respectively). There were no statistically significant differences in toxicity except for nausea/vomiting, which was significantly higher in the standard DOC plus CIS group for grades 3/4.
Quality of life
Only one trial58 reported on HRQoL and the authors plan to report the results in full in a separate publication. Preliminary analyses showed no statistically significant differences between the trial arms for expected toxicity, dyspnoea, dysphagia and global HRQoL.
Summary of results
Nineteen RCTs met the inclusion criteria and compared CTX-RT with CTX-RT. The majority of patients were male and middle-aged and had disease stage III with adenocarcinoma or squamous cell carcinoma and a PS of 0–1.
Overall, the methodological quality of included trials was poor with nearly all trials failing to report relevant methodology; in particular, methods of randomisation and allocation concealment were reported inadequately. Six trials reported some imbalance between trial groups at baseline, of which two trials reported a statistically significant imbalance between treatment groups for baseline disease stage. None of the trials was reported as being blinded. Seven trials assessed all participants according to the groups to which they were randomised. Only one trial reported any HRQoL data and preliminary analysis showed no statistically significant differences between the arms according to expected toxicity, dyspnoea, dysphagia and global HRQoL.
Five trials were closed prematurely mainly because of poor accrual, and in one trial the GEM plus CARB arm was closed prematurely because of a high rate of grade 4/5 pulmonary toxicity. Only three trials had international centres and only six were clearly Phase III trials. Sources of funding were a mixture of pharmaceutical and research grants; seven trials failed to report the source of funding. Only five trials were powered sufficiently to evaluate the primary outcome of the trial, of which two trials were powered to detect differences between treatment groups in 2-year survival rate.
Twelve trials compared various regimens of sequential, concurrent and consolidation CTX-RT. Five trials compared different types of RT or CTX, one trial compared RT once daily with RT twice daily and another trial assessed the addition of weekend CTX. In addition, there were different CTX agents and different radiation doses both across and within trials, and number of fractions, schedule, intensity and overall treatment time also varied between trials.
Across the trials, median OS ranged from 12 to 29.5 months and survival rate ranged from 37% to 80% at 1 year and from 14.3% to 66.6% at 2 years. Median PFS ranged from 5.4 to 14.9 months and median TTP ranged from 7.3 to 13.3 months. Tumour ORR ranged from 33% to 88%.
Results of individual studies showed that concurrent CTX-RT is associated with significantly longer survival than sequential CTX-RT51,57 and that concurrent/consolidation CTX-RT is associated with significantly longer survival than induction/concurrent CTX-RT. 45,52
The Assessment Group performed several direct evidence comparisons (meta-analysis) using data combining induction, sequential, concurrent and consolidation CTX-RT. The results appear to show no statistically significant evidence to support OS advantage for concurrent CTX-RT arms over sequential CTX-RT arms. However, when concurrent/consolidation treatments were compared with sequential treatments, the difference in OS was shown to be statistically significant. When sequential CTX-RT was compared with concurrent CTX-RT with or without consolidation, the latter yielded a statistically significant improvement in OS.
Only 12 trials reported information about CTX and RT treatment received in terms of RDI. In the trials comparing sequential CTX-RT with concurrent CTX-RT, more patients in the concurrent arms tolerated higher doses of RT. Concurrent/consolidation CTX-RT may be easier to tolerate than induction/concurrent CTX-RT. However, concurrent CTX-RT is associated with greater oesophagus toxicity than sequential CTX-RT.
The Assessment Group concluded that the 19 trials included in the systematic review were too disparate to form any conclusion as to the effectiveness of individual CTX agents or types of RT.
Chapter 4 Discussion
Chemoradiation treatment is common practice for stage III cancers for those patients whom clinicians believe may be curable. This review was carried out as part of a larger project32 looking at first-line treatments for patients with locally advanced and/or metastatic lung cancer. It was the opinion of the members of the clinical panel for the project that patients with stage IIIA or IIIB potentially curable disease are different from those who are considered for only palliative treatment of more advanced disease. The clinical panel was of the opinion that the review of CTX-RT treatments for patients with potentially curable disease should be reported separately.
There have been previous reviews that looked at this question. 22,35,64 However, given the advances in treatment, in the areas of both CTX and RT, it was felt worthwhile to examine the question again and to limit the dates of included trials to represent current, rather than historical, clinical practice.
Principal findings
The quality of the studies included in this review is generally poor and there are significant differences in the patient populations and treatments (both RT and CTX) within and across the studies, limiting the conclusions that can be drawn. The results of a series of meta-analyses conducted by the Assessment Group appear to demonstrate a statistically significantly improved OS with the use of concurrent/consolidation CTX-RT over sequential CTX-RT and statistically significantly improved OS with the use of concurrent CTX-RT (with or without consolidation) over sequential treatment. The Assessment Group did not find a statistically significant difference in OS between concurrent CTX-RT and sequential CTX-RT although there appears to be a trend towards an OS advantage for patients receiving concurrent CTX-RT. It is noted that concurrent CTX-RT is associated with significant oesophageal toxicity.
It should be acknowledged, however, that both the RT and CTX aspects of care for patients with NSCLC have changed significantly over the past 10 years and can be expected to continue to evolve, thus limiting the value of any comparison of treatments over time. This includes changes in methods of diagnosis and categorisation of the disease. In addition, ETOP appears to be commonly used in the UK but is not licensed for use in lung cancer.
Strengths and limitations
This report provides a summary and critical appraisal of a comprehensive evidence base of CTX-RT trials. It may be that additional trials have been reported since our last literature search but it is unlikely that their results, unless from very large, well-designed trials, would change the conclusions of the review.
Although CTX-RT is an established treatment regimen for eligible patients, how best to combine CTX and RT remains unclear. The optimal type and dose of CTX and RT also remain unclear. The updated NICE guidelines5 on the diagnosis and treatment of lung cancer recommend further research into the incorporation of accelerated RT fractionations within CTX treatment regimens and research into combinations of new targeted agents [e.g. epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors] and RT regimens. This highlights the uncertainty regarding best first-line treatment options for patients with NSCLC. Unfortunately the quality and the heterogeneity of the available data mean that this review has not been able to provide clear direction for clinicians regarding these important issues.
It is also disappointing that the quality of the research in this area does not meet the accepted quality standards regarding trial design and reporting. Quality assessment of included trials by the Assessment Group demonstrated that the included studies were generally of poor quality except for the criterion of patient follow-up. It could be argued that it is not possible to blind patients and care providers or that it is difficult to recruit enough patients to allow for trials with a sufficient size for conclusions to be drawn. However, there is no reason why concealment of allocation and appropriate randomisation cannot be achieved. The lack of balance across the arms in a number of trials indicates that randomisation procedures did not in fact provide equivalent groups in a number of instances.
In addition, there is no reason why outcome measures with appropriate statistical analysis cannot be presented (e.g. CIs around data points such as OS). This selective and incomplete reporting of outcomes in the included trials severely limited data synthesis. It is difficult to understand how authors of research reports can state that they have measured outcomes such as OS and then fail to report their data comprehensibly.
Despite its importance and relevance to patients and clinicians, there are few reports of HRQoL in the NSCLC trials. It is acknowledged that collecting and reporting HRQoL data in RCTs may be difficult. However, such data are critically important if the appropriate analyses are to be carried out to inform health policy decisions. Measuring HRQoL outcomes in patients with advanced NSCLC is reported to be particularly difficult because of the severity of symptoms, the side effects of CTX-RT treatment and early deaths associated with the disease. However, it is estimated that about half of all patients with advanced NSCLC could reasonably be expected to complete HRQoL instruments within a clinical trial setting (Paul Beckett, Consultant Physician for Burton Hospital NHS Trust, 2011, personal communication).
It is acknowledged that, even when HRQoL data are available, comparison across trials is complex. This is due to an array of factors including the number and timing of HRQoL measurements available and administered to patients; the intercountry cultural differences in how HRQoL is interpreted; and the resource requirements for its collection (patient explanations and assistance in completing questionnaires).
Uncertainties
As noted above, there have been recent changes in the diagnostic criteria used for lung cancer. There are differences between UICC version 6 and version 7 that are relevant to advanced lung disease; for example, pleural effusion is classed as stage IIIB in version 6 and has been moved to stage IV in version 7. Most of the trials included in the Assessment Group's systematic review will have used version 6, and so it should be noted that many of the patients classified as stage IIIB within the included trials would now be classified as stage IV and it is unclear what their treatment options would have been. This stage migration needs to be accounted for in future reviews when comparing outcomes across trials using different TNM classifications.
It is unknown what effect variance in exposure to treatment may have on clinical efficacy and safety outcomes. For example, it may be that the survival benefit associated with concurrent/consolidation CTX-RT could be accounted for by the significantly fewer patients in the sequential CTX-RT arms receiving a full course of RT rather than by concurrent CTX-RT increasing radiosensitisation (and therefore the effect of RT). More research in this area is warranted.
Other relevant factors
As noted earlier, there have been significant changes to the delivery of care for NSCLC patients. Over the past 10 years there has been a plethora of new CTX treatments approved for use including those that have been shown to be more effective in different disease categories [e.g. pemetrexed (Alimta®, Eli Lilly) for patients with non-squamous disease and gefitinib (Iressa®, AstraZeneca) for patients with EGFR mutation-positive tumours]. This requires changes in the diagnostic and treatment paths taken by patients as it is expected that in the near future all patients will have appropriate histological and genetic testing carried out. This will provide more information for clinicians to aid in planning patient care but will obviously make treatment choices more complex (Brown T, Pilkington G, Bagust A, Boland A, Oyee J, Tudur-Smith C, et al. Clinical and cost effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer: a systematic review and economic evaluation. Health Technol Assess, in preparation).
As noted above, these changes in diagnosis and treatment have been compounded by changes in the staging of NSCLC and these changes mean that comparison of results from past and future studies will be difficult and perhaps not possible.
Chapter 5 Conclusion
This review identified that the research conducted in the area of CTX-RT was generally of poor quality and suffered from a lack of reporting of all important clinical findings, including OS. In addition, there are within- and between-trial variations in treatment protocols including treatment duration, sequencing and length, RT exposure and type of CTX. These wide variations severely limited the combination of trial results.
Meta-analyses conducted as part of this review demonstrated a small but statistically significant improvement in OS in patients receiving concurrent/consolidation CTX-RT compared with sequential CTX-RT and statistically significantly improved OS with the use of concurrent CTX-RT (with or without consolidation) over sequential treatment. However, as noted, the variation in treatment protocols and the changes in the diagnostic criteria and staging used in NSCLC mean that the results of comparisons across these trials and with future trials need to be viewed with caution.
Acknowledgements
Expert Advisory Panel: Emer McKenna and Noelle O'Rourke. Thanks to Juliet Hockenhull for database expertise and Janet Atkinson for administrative support.
Contributions of authors
| Angela Boland | Input into all aspects of the clinical component of the review. |
| Tamara Brown | Project management, data management, systematic review of clinical effectiveness data and preparation of report. |
| Rumona Dickson | Input into all aspects of the clinical component of the review. |
| Yenal Dundar | Development of search strategies. |
| Gerlinde Pilkington | Data extraction and quality assessment of the clinical trials. |
| James Oyee | Assessment of statistics and data analysis including meta-analyses. |
| Elizabeth Richards | Data extraction of clinical trials. |
| Catrin Tudur Smith | Statistical expertise. |
| Rongrong Yang | Data extraction of clinical trials. |
All contributors took part in the editing and production of this report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Research UK . CancerStats. Lung Cancer and Smoking Statistics – Key Facts 2011. http://info.cancerresearchuk.org/cancerstats/types/lung/ (accessed 25 January 2011).
- Mackenbach J, Huisman M, Andersen O, Bopp M, Borgan J, Borrell C, et al. Inequalities in lung cancer mortality by the educational level in 10 European populations. Eur J Cancer 2004;40.
- Cancer Research UK . CancerHelp UK. Lung Cancer Risks and Causes n.d. www.cancerhelp.org.uk/type/lung-cancer/about/lung-cancer-risks-and-causes (accessed 2 February 2011).
- NHS, The Information Centre . National Lung Cancer Audit Report 2009. www.ic.nhs.uk/canceraudits/lung (accessed January 2011).
- The diagnosis and treatment of lung cancer. London: NICE; 2011.
- Cancer Research UK . CancerHelp UK. Performance Status n.d. www.cancerhelp.org.uk/about-cancer/cancer-questions/performance-status (accessed 1 February 2011).
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55.
- Zubrod C, Schneiderman M, Frei E III, Brindley C, Gold G, Shnider B, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis 1960;11:7-13.
- Information Centre for Health and Social Care . Report for the Audit Period 2006 2007.
- National Institute for Health and Clinical Excellence . Final Appraisal Determination: Pemetrexed for First-Line Treatment of Non-Small Cell Lung Cancer 2009. www.nice.org.uk/nicemedia/pdf/FADPemetrexedLungCancer.pdf.
- National Institute for Health and Clincial Excellence . Final Appraisal Determination: Gefitinib for the First-Line Treatment of Locally Advanced or Metastatic Non-Small-Cell Lung Cancer 2010. http://guidance.nice.org.uk/TA/Wave10/21/FAD/FinalAppraisalDetermination/pdf/English (accessed February 2011).
- National Institute for Health and Clinical Excellence . Lung Cancer (non-Small Cell) – Pemetrexed (maintenance) – Final Appraisal Determination 2010. www.nice.org.uk/guidance/index.jsp?action=download&o=48303 (accessed February 2011).
- National Institute for Health and Clinical Excellence . Lung Cancer (non-Small Cell) – Erlotinib: Guidance 2008. http://guidance.nice.org.uk/TA162 (accessed February 2011).
- National Institute for Health and Clincial Excellence . Lung Cancer (non-Small-Cell, Advanced or Metastatic Maintenance Treatment) – Erlotinib (monotherapy): Appraisal Consultation Document 2011. www.nice.org.uk/guidance/index.jsp?action=article&o=51743 (accessed February 2011).
- Cancer Research UK . Lung Cancer – UK Incidence Statistics n.d. http://info.cancerresearchuk.org/cancerstats/types/lung/incidence/ (accessed January 2011).
- Lung cancer: full guidelines. London: NICE; 2005.
- Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9.
- Greenhalgh J, McLeod C, Bagust A, Boland A, Fleeman N, Dundar Y, et al. Liverpool: LRiG: University of Liverpool; 2009.
- O’Rourke N, Roqué i Figuls M, Farré Bernadó N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2010;6.
- Auperin A, Le Pechoux C, Pignon JP, Koning C, Jeremic B, Clamon G, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473-83.
- Non-small Cell Lung Cancer Collaborative Group . Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899-90. www.ncbi.nlm.nih.gov/pmc/articles/PMC2550915/pdf/bmj00613–0017.pdf (accessed 2011).
- Rowell NP, O’Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004;4.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
- Wang J, Wu N, Cham M, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. Am J Roentgenol 2009;193:1090-6.
- Aaronson N, Ahmedzai S, Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76.
- Bergman B, Aaronson N, Ahmedzai S. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994.
- Hollen PJ, Gralla RJ. Comparison of instruments for measuring quality of life in patients with lung cancer. Semin Oncol 1996;23:31-40.
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy – Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220.
- EuroQoL Group . What Is EQ-5D? n.d. www.euroqol.org/ (accessed July 2011).
- Grutters J, Joore M, Wiegman E, Langendijk J, de Ruysscher D, Hochstenbag M. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax 2010;65:903-7.
- London: NICE; 2005.
- Brown T, Massey G, Bagust A, Boland A, Oyee J, Tudur-Smith C, et al. Clinical and cost effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer: a systematic review and economic evaluation. Health Technol Assess 2012.
- Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer. Health Technol Assess 2001;5.
- York: University of York; 2009.
- Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34.
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Multi-parameter Evidence Synthesis Research Group . Formal Methods for Multi-Parameter Evidence Synthesis and Their Role in Epidemiology and Economic Evaluation n.d. www.bristol.ac.uk/cobm/research/mpes.
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55.
- Berghmans T, Van Houtte P, Paesmans M, Giner V, Lecomte J, Koumakis G, et al. A phase III randomised study comparing concomitant radiochemotherapy as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2009;64:187-93.
- Reinfuss M, Skolyszewski J, Kowalska T, Glinski B, Dymek P, Walasek T, et al. Evaluation of efficacy of combined chemoradiotherapy in locoregional advanced, inoperable non-small cell lung cancer (clinical randomized trial). Nowotwory 2005;55:200-6.
- Vokes EE, Herndon IJE, Crawford J, Leopold KA, Perry MC, Miller AA, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol 2002;20:4191-8.
- Vokes EE, Herndon IJE, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer. J Clin Oncol 2007;25:1698-704.
- Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95–01 Study. J Clin Oncol 2005;23:5910-17.
- Komaki R, Seiferheld W, Ettinger D, Lee JS, Movsas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non-small-cell lung cancer: long-term follow-up of RTOG 92–04. Int J Radiat Oncol Biol Phys 2002;53:548-57.
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 2004;46:87-98.
- Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005;23:5883-91.
- Liu X, Xiao T, Jin Y, Chen H. Concurrent chemoradiotherapy with low-dose weekly docetaxel followed by consolidation chemotherapy with docetaxel and cisplatin in the treatment of stage III non-small cell lung cancer. Chin J Lung Cancer 2008;1:79-84.
- Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P, et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972–22973). Eur J Cancer 2007;43:114-21.
- Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer. J Clin Oncol 2008;26:2457-63.
- Dasgupta A, Dasgupta C, Basu S, Majumdar A. A prospective and randomized study of radiotherapy, sequential chemotherapy radiotherapy and concomitant chemotherapy-radiotherapy in unresectable non small cell carcinoma of the lung. J Cancer Res Ther 2006;2:47-51.
- Crvenkova S, Krstevska V. Sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small cell lung cancer: our experience. Prilozi 2009;30:197-20.
- Nyman J, Friesland S, Hallqvist A, Seke M, Bergstrom S, Thaning L, et al. How to improve loco-regional control in stages IIIa-b NSCLC?. Results of a three-armed randomized trial from the Swedish Lung Cancer Study Group. Lung Cancer 2009;65:62-7.
- Gouda YS, Kohail HM, Eldeeb NA, Omar AM, El-Geneidy MM, Elkerm YM. Randomized study of concurrent carboplatin, paclitaxel, and radiotherapy with or without prior induction chemotherapy in patients with locally advanced non-small cell lung cancer. J Egypt Natl Canc Inst 2006;1:73-81.
- Zhu L, Wu R. Sequential and concurrent radiochemotherapy for stage III non-small cell lung cancer. Chin J Cancer Prev Treat 2009;16:1105-7.
- Movsas B, Langer CJ, Ross HJ, Wang L, Jotte RM, Feigenberg S, et al. Randomized phase II trial of cisplatin, etoposide, and radiation followed by gemcitabine alone or by combined gemcitabine and docetaxel in stage III A/B unresectable non-small cell lung cancer. J Thorac Oncol 2010;5:673-9.
- Schild S, Stella P, Geyer S, Bonner J, Marks R, MCGinnis W, et al. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2002;54:370-8.
- Jeremic B, Shibamoto Y, Acimovic L, Milicic B, Milisavljevic S, Nikolic N, et al. Hyperfractionated radiation therapy and concurrent low-dose, daily carboplatin/etoposide with or without weekend carboplatin/etoposide chemotherapy in stage III non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys 2001;50:19-25.
- O’Rourke N, MacBeth F. Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer? A review of guidelines and evidence. Clin Oncol 2010;22:347-55.
- Fisher MD. Irinotecan/cisplatin versus vindesine/cisplatin versus irinotecan alone in advanced non-small-cell lung cancer. Clin Lung Cancer 2000;2:180-1.
- Negoro S, Masuda N, Takada Y, Sugiura T, Kudoh S, Katakami N, et al. Randomised phase III trial of irinotecan combined with cisplatin for advanced non-small-cell lung cancer. Br J Cancer 2003;88:335-41.
- Grigorescu AC, Draghici IN, Nitipir C, Gutulescu N, Corlan E. Gemcitabine (GEM) and carboplatin (CBDCA) versus cisplatin (CDDP) and vinblastine (VLB) in advanced non-small-cell lung cancer (NSCLC) stages III and IV: a phase III randomised trial. Lung Cancer 2002;37:9-14.
- Lin YC, Lin Y, Lin W, Du CW, Wu MY, Li DR. Comparison of effects of three chemotherapy regimens for advanced non-small cell lung cancer. Ai Zheng 2002;21:1359-61. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/105/CN-00558105/frame.html (accessed January 2011).
- Georgoulias V, Pallis AG, Kourousis C, Alexopoulos A, Ardavanis A, Agelidou A, et al. Docetaxel versus docetaxel/cisplatin in patients with advanced non-small-cell lung cancer: preliminary analysis of a multicenter, randomized phase III study. Clin Lung Cancer 2003;4:288-93.
- Georgoulias V, Ardavanis A, Agelidou A, Agelidou M, Chandrinos V, Tsaroucha E, et al. Docetaxel versus docetaxel plus cisplatin as front-line treatment of patients with advanced non-small-cell lung cancer: a randomized, multicenter phase III trial. J Clin Oncol 2004;22:2602-9.
- Leong SS, Tan EH, Fong KW, Wilder-Smith E, Ong YK, Tai BC, et al. Randomized double-blind trial of combined modality treatment with or without amifostine in unresectable stage III non-small-cell lung cancer. J Clin Oncol 2003;21:1767-74.
- Miller VA, Johnson DH, Krug LM, Pizzo B, Tyson L, Perez W, et al. Pilot trial of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib plus carboplatin and paclitaxel in patients with stage IIIB or IV non-small-cell lung cancer. J Clin Oncol 2003;21:2094-100.
- Semrau S, Fietkau R. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. Strahlenther Onkol 2003;179:502-3. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/045/CN-00474045/frame.html (accessed January 2011).
- Teng XY, Zhou NN, Su YS, Zhou ZM, Liu DG, He YJ. Comparison of cisplatin combined with gemcitabine versus cisplatin combined with vinorelbine regimen for treatment of patients with advanced non-small cell lung cancer. Ai Zheng 2003;22:404-6. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/787/CN-00436787/frame.html (accessed January 2011).
- Vansteenkiste J, Vandebroek J, Nackaerts K, Dooms C, Galdermans D, Bosquee L, et al. Influence of cisplatin-use, age, performance status and duration of chemotherapy on symptom control in advanced non-small cell lung cancer: detailed symptom analysis of a randomised study comparing cisplatin-vindesine to gemcitabine. Lung Cancer 2003;40:191-9.
- Vansteenkiste JF, Vandebroek JE, Nackaerts KL, Weynants P, Valcke YJ, Verresen DA, et al. Clinical-benefit response in advanced non-small-cell lung cancer: a multicentre prospective randomised phase III study of single agent gemcitabine versus cisplatin-vindesine. Ann Oncol 2001;12:1221-30.
- O’Brien ME, Anderson H, Kaukel E, O’Byrne K, Pawlicki M, Von Pawel J, et al. SRL 172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: Phase III results. Ann Oncol 2004;15:906-14.
- Gao Y, Shi ZQ, Cao CW, Zhu CL, Guo J. A randomized trial of docetaxol plus cisplatin versus gemzar plus cisplatin in treating advanced non-small cell lung cancer. Ai Zheng 2005;24:985-9. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/966/CN-00569966/frame.html (accessed January 2011).
- Liu L, Wang XW, Li L, Zhang X, Zhang WD, Yu XJ. A randomized comparative trial of three combined regimens containing cisplatin for treatment of advanced non-small cell lung cancer. Ai Zheng 2006;25:990-4. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/906/CN-00704906/frame.html (accessed January 2011).
- Ramalingam S, Barstis J, Perry MC, La Rocca RV, Nattam SR, Rinaldi D, et al. Treatment of elderly non-small cell lung cancer patients with three different schedules of weekly paclitaxel in combination with carboplatin: subanalysis of a randomized trial. J Thorac Oncol 2006;1:240-4.
- Belani CP, Barstis J, Perry MC, La Rocca RV, Nattam SR, Rinaldi D, et al. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol 2003;21:2933-9.
- Xu S, Wang W, Liu X. Clinical studies on the therapy of advanced non-small cell lung cancer (NSCLC) in the elderly using domestic gemcitabine in combination with carboplatin. Chin J Clin Oncol 2006;33:35-7. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/439/CN-00641439/frame.html (accessed January 2011).
- Gridelli C, Gallo C, Ceribelli A, Gebbia V, Gamucci T, Ciardiello F, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol 2007;8:500-12.
- Gridelli C, Butts C, Ciardiello F, Feld R, Gallo C, Perrone F. An international, multicenter, randomized phase III study of first-line erlotinib followed by second-line cisplatin/gemcitabine versus first-line cisplatin/gemcitabine followed by second-line erlotinib in advanced non-small-cell lung cancer: treatment rationale and protocol dynamics of the TORCH trial. Clin Lung Cancer 2008;9:235-8.
- Zhang S, Wang J, Wang Q, Yang X, Hu F, Li X, et al. A randomized study of docetaxel plus cisplatin versus paclitaxel plus cisplatin in previously untreated advanced non-small cell lung cancer. Chin J Lung Cancer 2008;11:110-4. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/044/CN-00708044/frame.html (accessed January 2011).
Appendix 1 Details of clinical search strategies
Appendix 2 Details of clinical data abstraction
Appendix 3 Details of clinical trial quality assessment
Appendix 4 Code from the Multi-parameter Evidence Synthesis Research Group
Code from the Multi-parameter Evidence Synthesis Research Group (PDF download)
Appendix 5 Excluded studies with reasons for exclusion
Appendix 6 Trial characteristics
Appendix 7 Intervention details
Appendix 8 Relative dose intensity
Appendix 9 Patient characteristics
Appendix 10 Inclusion/exclusion criteria of included studies
Inclusion/exclusion criteria of included studies (PDF download)
Appendix 11 Adverse events
Appendix 12 Protocol
Glossary
- Adenocarcinoma
- Cancer that begins in cells that line certain internal organs and which have glandular (secretory) properties.
- Chemo-naive
- Chemotherapy naive – having received no previous chemotherapy treatment.
- Chemoradiation
- Combined chemotherapy and radiation therapy.
- Chemotherapy
- Treatment with anticancer drugs.
- Heterogeneity
- Between-trial variation.
- Histological diagnosis
- A diagnosis made by examining a sample of tissue or cells.
- Intention to treat
- A method of data analysis in which all patients are analysed in the group that they were assigned to at randomisation regardless of treatment adherence.
- Large cell carcinoma
- A group of lung cancers in which the abnormal cells are large.
- Locally advanced disease
- Stages IIIA/IIIB non-small cell lung carcinoma.
- Meta-analysis
- A quantitative method for combining the results of many trials into one set of conclusions.
- Metastasis
- The spread of cancer from one part of the body to another. Tumours formed from cells that have spread are called ‘secondary tumours’ and contain cells that are like those in the original (primary) tumour. The plural is metastases.
- Non-small cell lung cancer
- A group of lung cancers that includes squamous cell carcinoma, adenocarcinoma and large cell carcinoma.
- Non-squamous cell carcinoma
- A classification of cancer that includes adenocarcinoma, which begins in the cells that line the alveoli, and large cell carcinoma that begin in types of large cells.
- Radiation therapy
- The use of high-energy radiation from X-rays, neutrons and other sources to kill cancer cells and shrink tumours. Radiation may come from a machine outside the body (external beam radiation therapy) or from material called radioisotopes. Radioisotopes produce radiation and are placed in or near a tumour or near cancer cells. This type of radiation treatment is called internal radiation therapy, implant radiation or brachytherapy. Systemic radiation therapy uses a radioactive substance, such as a radiolabelled monoclonal antibody, that circulates throughout the body. Also called radiotherapy.
- Relative risk
- The proportion of diseased people among those exposed to the relevant risk factor divided by the proportion of diseased people among those not exposed to the risk factor.
- Relative risk reduction
- Alternative way of expressing relative risk. It is calculated as RRR = (1 – RR) × 100%. The RRR can be interpreted as the proportion of the baseline ‘risk’ that was eliminated by a given treatment or by avoidance of exposure to a risk factor.
- Squamous cell carcinoma
- Cancer that begins in squamous cells, which are found in the tissue that forms the surface of the skin, the lining of the hollow organs of the body and the passages of the respiratory and digestive tracts. Also called epidermoid carcinoma.
List of abbreviations
- AE
- adverse event
- BSC
- best supportive care
- CALGB
- Cancer and Leukemia Group B
- CARB
- carboplatin
- CIS
- cisplatin
- CI
- confidence interval
- CT
- computerised tomography
- CTX
- chemotherapy
- CTX-RT
- chemoradiation
- DOC
- docetaxel
- ECOG
- Eastern Cooperative Oncology Group
- EGFR
- epidermal growth factor receptor
- ETOP
- etoposide
- GEM
- gemcitabine
- Gy
- Gray (unit of absorbed radiation dose)
- HART
- hyperfractionated accelerated radiotheraphy
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ITT
- intention to treat
- KPS
- Karnofsky performance status
- LUCADA
- National Lung Cancer Data Audit
- M +
- mutation-positive (EGFR)
- MCMC
- Markov chain Monte Carlo
- MTC
- mixed-treatment comparison
- NICE
- National Institute for Health and Clinical Excellence
- NSCLC
- non-small cell lung cancer
- ORR
- overall response rate
- OS
- overall survival
- PAX
- paclitaxel
- PFS
- progression-free survival
- PLAT
- platinum (cisplatin or carboplatin)
- PS
- performance status
- RCT
- randomised controlled trial
- RDI
- relative dose intensity
- RR
- relative risk
- RT
- radiotherapy
- TTP
- time to progression
- UICC
- Union Internationale Contre le Cancer
- VBL
- vinblastine
- VNB
- vinorelbine
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.