Notes
Article history paragraph text
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 11/44/01. The protocol was agreed in September 2011. The assessment report began editorial review in February 2012 and was accepted for publication in July 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Notes
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence and/or academic-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence and academic-in-confidence data removed and replaced by the statement ‘commercial-in-confidence and/or academic in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk. The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all of the data considered in the original full NICE report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Wade et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK..
Chapter 1 Background and definition of the decision problem
Condition and aetiology
In 2007, 2828 women were diagnosed with cervical cancer in the UK, making it the 11th most common cancer in women, and accounting for around 2% of all cancers among women. Cervical cancer is the most common cancer in females aged < 35 years; 702 women aged < 35 years were diagnosed with cervical cancer in the UK in 2007. 1 Women will develop changes in the cervix many years before any progression to cancer. These precancerous changes are described as being high-grade cervical intraepithelial neoplasia (CIN); women may also develop low-grade CIN, which is not precancerous but can cause changes that can be detected at cervical screening.
Infection with certain genotypes of human papillomavirus (HPV), in particular HPV16 and HPV18, has been shown to be associated with the development of cervical cancer and CIN; almost all cervical cancers contain high-risk human papillomavirus (hrHPV) DNA. However, most HPV infections will not progress to CIN; the cell changes associated with HPV will regress to normal. Certain risk factors are associated with the progression of HPV infection to CIN, including the HPV genotype, early age at first intercourse, long duration of the most recent sexual relationship and cigarette smoking. 1
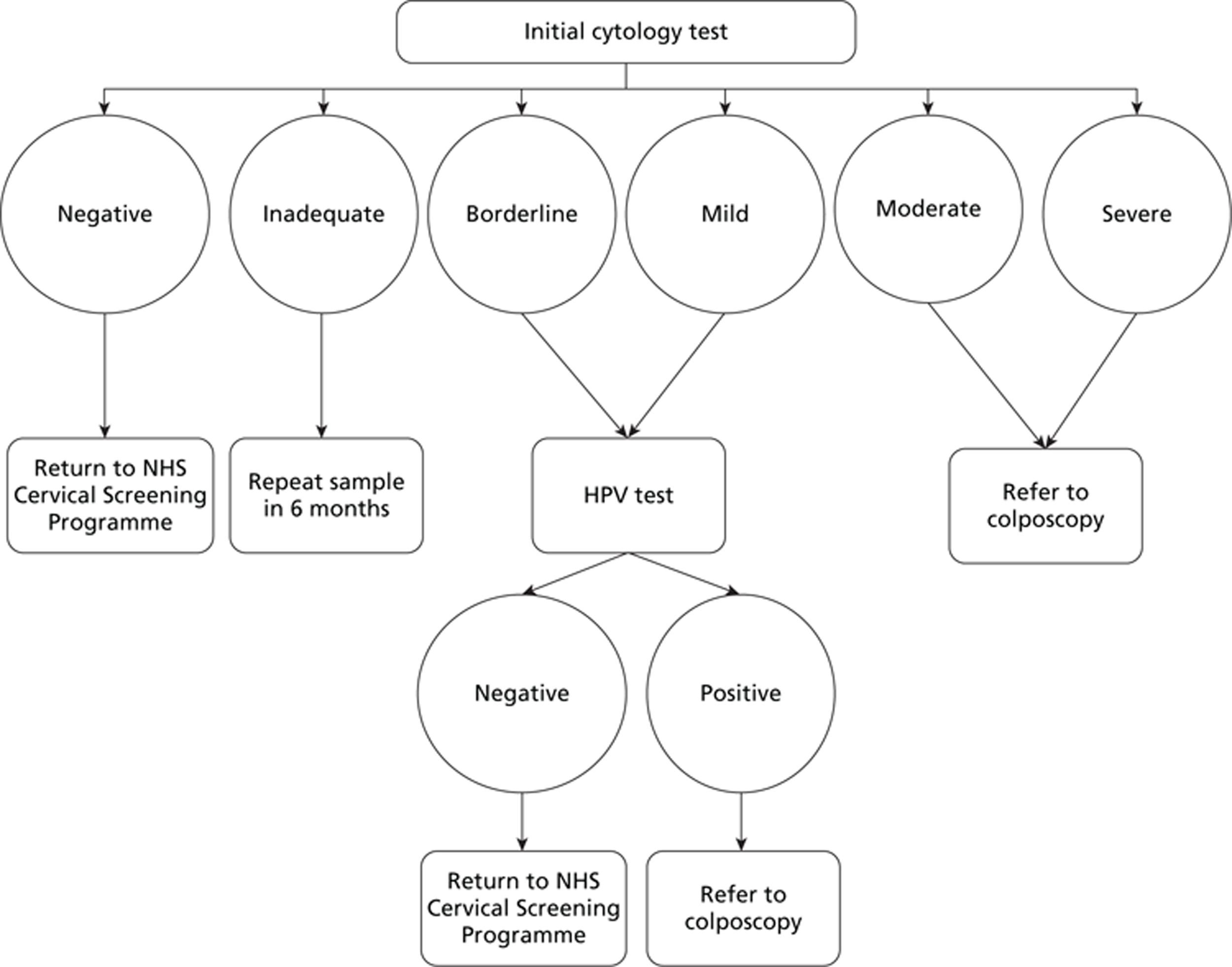
Women in England who are between the ages of 25 and 64 years are invited for regular cervical screening every 3 years (if aged between 25 and 49 years) or every 5 years (if aged between 50 and 64 years) under the NHS Cervical Screening Programme. 2 Most screening is conducted using liquid-based cytology (LBC); a sample of exfoliated cells is brushed from the transformation zone of the cervix for assessment in a pathology laboratory. Cytological assessment is performed to detect nuclear abnormalities, which are described as dyskaryotic. The degree of dyskaryosis can range from mild to severe, or borderline changes may be seen. There are three main terminology systems for reporting cervical cytology results. Table 1 shows a comparison of cytology classification systems. 3 At the scoping workshop, it was agreed that, where possible, the dyskaryosis terminology should be used in this assessment. 3
Just under 3.3 million women aged between 25 and 64 years attended for cervical screening in 2009–10; the percentage of eligible women who were recorded as screened at least once in the previous 5 years was 78.9%. Approximately 3.7 million samples were examined in 2009–10, of which 3.4 million (92.9%) were submitted by general practitioners (GPs) and NHS community clinics (suggesting that they were part of the NHS Cervical Screening Programme). 4
Just under 3.3 million women aged between 25 and 64 years attended for cervical screening in 2009–10; the percentage of eligible women who were recorded as screened at least once in the previous 5 years was 78.9%. Approximately 3.7 million samples were examined in 2009–10, of which 3.4 million (92.9%) were submitted by general practitioners (GPs) and NHS community clinics (suggesting that they were part of the NHS Cervical Screening Programme). 4
Overall, 2.9% of tests did not have a result, owing to an inadequate sample. This means that the sample did not contain sufficient cervical cells for analysis. This figure has dropped significantly (from approximately 9%) since the introduction of LBC, rather than the Papanicolaou test (known as the Pap test or smear test). Women with an inadequate sample should be recalled for a repeat test; if women have three consecutive inadequate results, they should be referred for colposcopy.
Table 2 presents a summary of cytology test results and management options for patients with an adequate test result, submitted by GPs and NHS community clinics. These recommendations are taken from the NHS Cervical Screening Programme guidelines published in 2010;2 however, the management of patients will change with the introduction of new guidelines for HPV triage, implemented in 2011–12. 5 These are discussed further below.
| Bethesda system | Dyskaryosis system | Papanicolaou system |
|---|---|---|
| Normal limits | Normal | I |
| Infection | Inflammatory atypia | II |
| Reactive and reparative changes | ||
| Atypical squamous cells of undetermined significance | Squamous atypia/HPV atypia | IIR |
| LSIL | Mild dyskaryosis | |
| HSIL | Moderate dyskaryosis | III |
| Severe dyskaryosis | IV | |
| Carcinoma in situ | ||
| Squamous cell carcinoma | Squamous cell carcinoma | V |
| Result | Definition | Actiona | Proportion (2009–10),b (%) |
|---|---|---|---|
| Negative | No nuclear abnormalities | Place on routine recall | 93.2 |
| Borderline changes | Nuclear changes that are not normal are present. Unsure whether the changes are dyskaryosis | Repeat the test in 6 months. Most will have reverted to normal. After 3 consecutive normal results, return to routine recall. If abnormality persists (three times) or worsens, refer for colposcopy. If in a 10-year period there are three borderline or more severe results, refer for colposcopy | 3.8 |
| Mild dyskaryosis | Nuclear abnormalities that are indicative of low-grade CIN | Refer for colposcopy (although it remains acceptable to repeat the test in 6 months instead – most will have reverted to normal after 6 months). Refer to colposcopy if changes persist on two occasions | 1.9 |
| Moderate dyskaryosis | Nuclear abnormalities reflecting probable CIN2 | Refer for colposcopy | 0.5 |
| Severe dyskaryosis | Nuclear abnormalities reflecting probable CIN3 | Refer for colposcopy | 0.6 |
There were 155,414 referrals for colposcopy in 2009–10; 78.6% of these were as a result of screening and 17.5% were clinically indicated, while 3.9% were for reasons not otherwise specified. Of women referred for colposcopy via the NHS Cervical Screening Programme, 48.8% were referred for borderline changes or mild dyskaryosis, 12.3% were referred for moderate dyskaryosis and 15.8% were referred for severe dyskaryosis or worse. There were a total of 453,947 appointments at colposcopy clinics in 2009–10, 41.9% of which were new appointments, 7.9% were return appointments for treatment and 50.2% were follow-up appointments. 4
In total, 27% of appointments were not attended: 2.6% were cancelled by the patient on the day, 10.2% were cancelled in advance, 10.5% were not attended with no advance warning and 3.7% were cancelled by the clinic. 4
Overall, 63.5% of women attending for colposcopy had some treatment or procedure at their first attendance, the most common being diagnostic biopsy, carried out at 45.5% of first attendances. For women referred for low-grade abnormalities, the most common procedure at first attendance was diagnostic biopsy and for women referred for high-grade abnormalities it was excision. The majority of those women presenting with high-grade abnormalities who had either no treatment or only diagnostic biopsy at first attendance, are likely to have received therapeutic treatment at a subsequent attendance. 4
New guidelines implemented in 2011/12 state that cytology samples from women with low-grade abnormalities (borderline changes or mild dyskaryosis) should be tested for hrHPV for triage for referral for colposcopy. 5 The test is performed on the LBC sample already obtained as part of the NHS Cervical Screening Programme. Women who test positive for hrHPV should be referred for colposcopy, whereas women who test negative for hrHPV should be returned to routine recall.
These new guidelines present the protocol for managing women in the NHS Cervical Screening Programme with the introduction of HPV triage. 5 The Guidelines for the NHS Cervical Screening Programme present additional treatment guidelines. 2
Treatment and screening options available include:
-
return to NHS Cervical Screening Programme (3- or 5-year recall, depending on age)
-
refer for rescreen at 6 months, with or without colposcopy
-
a diagnostic (punch) biopsy
-
a treatment biopsy
-
a treatment biopsy followed by cancer treatment.
If colposcopic findings are clear but cytology results are moderate or severe, then patients are reviewed at a ‘correlation meeting’ where the pathologists and colposcopists discuss the results and the management of patients. There is some variation in patient management among clinicians. Treatment and screening options are discussed further in Chapter 2 (see Model inputs).
The patient group of interest for this assessment is women referred for colposcopy through the NHS Cervical Screening Programme. Women referred because of symptoms indicative of cervical cancer (e.g. postcoital bleeding or appearance suggestive of cancer) are not of relevance to this assessment. Where possible, separate analyses will be performed according to cytology findings; these technologies may be more appropriate for patients with borderline changes, or mild or moderate dyskaryosis, as more severe abnormalities are easier to detect with standard colposcopy.
Description of the technologies under assessment
Three technologies have been included in this assessment: Dynamic Spectral Imaging System (DySIS), LuViva Advanced Cervical Scan and Niris Imaging System. All three are used as an adjunct to standard colposcopy, although LuViva also aims to reduce the number of patients requiring a colposcopy by screening out some patients referred for colposcopy. DySIS is a colposcope that incorporates a digital image analysis system [dynamic spectral imaging (DSI)], whereas LuViva and Niris are probes with image analysis systems designed to be used in conjunction with a standard colposcope.
DySIS (developed by DySIS Medical, Edinburgh, UK)
The Dynamic Spectral Imaging System (DySIS) is a digital video colposcope that incorporates a digital image analysing system (DSI) designed to detect cancerous and precancerous cervical tissue. DySIS can be used for full colposcopic evaluations of the vulva, vagina and cervix. DySIS maps the whitening effect following application of acetic acid (acetowhitening) on the epithelium of the cervix, to assist the clinician in selecting areas for biopsy and treatment. It does this by producing a quantitative measurement of the rate, extent and duration of acetowhitening, which is highly correlated with the altered structure and functionality of abnormal epithelial cells of the cervix. The dynamic map produced (known as DySISmap) can be overlaid on a colour image to assist in determining the presence and grade of any neoplastic lesion. DySIS is designed to work in conjunction with a bespoke DySIS speculum.
DySIS consists of an optical head with a white light-emitting diode for uniform illumination, magnification optics coupled to a digital colour charged, coupled device camera for image capture, and a computer and control electronics unit with a thin-film transistor monitor for image and data display. Linear polarisers are used in both the imaging and illumination pathways to reduce surface reflection (which might obscure the acetowhitening effect). The optical head does not come into contact with the tissue and magnifies images between 10 and 27 times. 6 It is mounted on a mechanical arm to position and stabilise it, and locked on to an extension shaft attached to the speculum, to ensure a stable field of view during image acquisition. For this reason, the speculum used with DySIS is different from the standard speculum used in colposcopy and gynaecology practice. The average length of use per examination is < 15 minutes.
New users can be trained in the use of DySIS, and in interpreting the DySISmap, in 2–4 hours. DySIS has a CE (Conformité Européenne) mark and the cost in the UK ranges from £18,000 to £22,000. Costs for specula are £3.50 per examination. 3
LuViva Advanced Cervical Scan (developed by Guided Therapeutics, Norcross, GA)
LuViva distinguishes between normal and diseased tissue by detecting biochemical and morphological changes at the cellular level. This is done using optical spectroscopy; light is directed at the cervix and the resulting fluorescence and reflectance spectra are collected and analysed. Areas with suspected disease are then identified and displayed. LuViva consists of a base unit with a results display, and a single-use guide, which is placed on the surface of the cervix. 7 LuViva is intended to be used before colposcopy to eliminate unnecessary colposcopies; a subset of patients would then go on to have colposcopy for additional assessment or to allow ‘see and treat’. The average length of use per examination (additional to colposcopy) is around 2 minutes.
New users can be trained in around 30 minutes. LuViva costs £11,500 and the single-use guide costs £17.25 per patient. 3 It was expected to receive a CE mark in 2012.
Niris Imaging System (developed by Imalux Corporation, Cleveland, OH)
The Niris Imaging System utilises optical coherence tomography (OCT) and is designed to work in conjunction with a standard speculum. Its imaging console produces near infrared light which is directed at the cervix. Optical light is backscattered from the tissue, collected by a detachable fibre optic probe, and combined with an internal reference signal to produce a high spatial resolution two-dimensional image of the superficial tissue microstructure. The intensity of light reflected back is a function of tissue structure and content, allowing differentiation of normal and abnormal tissue.
The system includes built-in protocols for image comparison with automated calculations for intensity and distance, with raw data also reported. Images can be monitored over time, allowing side-by-side comparisons of a patient's results from two time periods (images are exportable to an ancillary monitor). Niris is used following colposcopy in order to evaluate all abnormalities found during colposcopy.
Niris probes have a limited useful life of around 200 patient procedures but can be processed for re-use. The average length of use per examination (additional to colposcopy) is around 4 minutes. A probe sheath is used to provide physical stability and help prevent cross-contamination.
New users can be trained in around 2 hours. The Niris Imaging System costs US$49,500 (around £31,000) plus taxes and shipping. The probe costs US$2700 (around £1700) and a disposable sheath costs US$30 (around £19). 3 The device has received a CE mark and is now available in the UK. [Note: this is based on subsequent information from Imalux Corporation.]
Comparators
Standard colposcopy, with directed biopsy/treatment when necessary, is the current usual management for women referred with abnormal cytology results. A colposcope is a binocular field microscope used to examine the cervix following sequential application of saline, 3–5% acetic acid, and sometimes Lugol's iodine to identify any epithelial changes or capillary vessel patterns suggestive of disease. Histological examination of any biopsied tissue, which is the gold standard for diagnosis of CIN or invasive cervical cancer, is then undertaken. The initial outcome of colposcopy is classified as being adequate, where the whole of the transformation zone (and any lesions) can be viewed, or inadequate, where full visualisation is not possible, and where further investigation may be required. The skills of the colposcopist relate to training, experience, and the volume of patients seen. Colposcopy involves a significant amount of subjective assessment – results from the same patient may vary when assessed by different colposcopists. 8 Details of referral cytology results, other clinical information, the type of management available and the number of biopsies taken are also relevant when interpreting the results of colposcopy.
Typical durations of colposcopic procedures are 20 minutes for a new patient in whom large-loop excision of the transformation zone (LLETZ) is unnecessary, 30 minutes for a new patient who needs a LLETZ, and 15 minutes for a follow-up appointment (information supplied by clinical advisor). Colposcopes are also used for identifying other clinical conditions, such as vulval or vaginal intraepithelial neoplasia.
A meta-analysis of nine studies published in 1998 estimated the sensitivity and specificity of colposcopy as being 96% and 48%, respectively, for detecting normal tissue from any abnormal tissue, and 85% and 69%, respectively, for differentiating between normal/low-grade CIN and high-grade CIN/cancer,9 although most of the included studies appeared to be subject to bias. 10 More recently, better-quality studies have reported a sensitivity of around 57% for detecting CIN2+11 and around 56% for detecting CIN3+. 12
A standard colposcope costs around £17,500 (information provided by clinical advisors) and a disposable speculum costs £2.
Care pathways
Women with an abnormal cytology result, or repeated inadequate or borderline cytology results, are referred for colposcopy. According to the new HPV triage guidelines implemented in 2011–12, women with a borderline or mild dyskaryosis result should be referred for colposcopy only if they also test positive for hrHPV. 5 Colposcopy is used to visualise the cervix; if any abnormal area is identified then a biopsy is taken and sent for histopathological analysis. Colposcopy clinics are usually located within gynaecology or genitourinary medicine departments of general hospitals, although some colposcopy clinics may take place in primary care in the future.
Outcomes
The clinical outcomes of interest are diagnostic test accuracy outcomes (e.g. sensitivity and specificity), adverse effects and patient experience. Where other patient health outcomes are reported (e.g. morbidity and mortality from cancer or treatment) these will be included in the assessment.
Decision problem
The aim of this project is to determine the clinical effectiveness and cost-effectiveness of adjunctive colposcopy technologies for examination of the uterine cervix for patients referred for colposcopy through the NHS Cervical Screening Programme; the technologies under consideration are DySIS, LuViva Advanced Cervical Scan and Niris Imaging System.
Chapter 2 Assessment design and results by condition or aetiology
Systematic review of clinical effectiveness
Background
A systematic review was undertaken to assess the clinical effectiveness of adjunctive colposcopy technologies DySIS, LuViva Advanced Cervical Scan and Niris Imaging System for patients referred for colposcopy through the NHS Cervical Screening Programme.
The original scope for the assessment also included the APX 100 device (developed by Zilico Ltd, Manchester, UK). 3 However, this technology was removed from the assessment in December 2011, after the inclusion screening stage of the assessment.
Methods for reviewing clinical effectiveness
The systematic review was conducted following the general principles recommended in the Centre for Reviews and Dissemination (CRD) guidance13 and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 14
Search strategy
The literature search aimed to systematically identify research related to the clinical effectiveness and cost-effectiveness of adjunctive colposcopy technologies.
The base search strategy was constructed using MEDLINE and then adapted to the other resources searched. The search included the following components:
-
terms for cervix, and
-
terms for colposcopy (including both general colposcopy terms as well as specific technologies).
Searches of major bibliographic databases were limited by date (2000 onwards) reflecting the date of development of the new technologies. No language, study design or other limits were applied. Reference lists of all included studies were hand-searched to identify further relevant studies. Where necessary, authors of eligible studies were contacted for further information.
Search strategies were developed by an information specialist with input from the project team. The search strategy was checked by a second information specialist. Sources of information were identified by an information specialist with input from the project team.
As the technologies involved are relatively new, particular attention was given to identifying sources for ongoing trials and conference reports (by searching specialist sources such as Inside Conferences and ClinicalTrials.gov). Details of the search strategies are presented in Appendix 1.
The following resources were searched for relevant clinical effectiveness and cost-effectiveness research:
-
Allied and Complementary Medicine Database (AMED): via OvidSP, using the segment 1985 to September 2011, searched on 22 September 2011
-
BIOSIS Previews: via Dialog, using the segment 1993 to 2011 week 2 October, searched on 19 October 2011
-
Cochrane Database of Systematic Reviews (CDSR): via Wiley Cochrane Library website, Issue 9 of 12, September 2011, searched on 22 September 2011
-
Cochrane Central Register of Controlled Trials (CENTRAL): via Wiley Cochrane Library website, Issue 3 of 4, July 2011, searched on 22 September 2011
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL): via EBSCO, using the segment 1981 to 16 September 2011, searched on 22 September 2011
-
ClinicalTrials.gov: via website www.clinicaltrials.gov/, using the segment to September 2011, searched on 28 September 2011
-
Current Controlled Trials (CCT): via website www.controlled-trials.com/, using the segment to September 2011, searched on 28 September 2011
-
Database of Abstracts of Reviews of Effects (DARE): via Wiley Cochrane Library website Issue 3 of 4, July 2011, searched on 22 September 2011
-
EMBASE: via OvidSP, using the segment 1996 to week 37 2011, searched on 22 September 2011
-
Health Management Information Consortium (HMIC): via OvidSP, using the segment 1985 to September 2011, searched on 22 September 2011
-
Health Technology Assessment (HTA) database (via Wiley Cochrane Library website Issue 3 of 4, July 2011, searched on 22 September 2011
-
Inspec: via OvidSP, using the segment 1969 to week 36 2011, searched on 22 September 2011
-
Inside Conferences: via Dialog, using the segment 1993 to 18 October 2011, searched on 19 October 2011
-
MEDLINE: via OvidSP, using the segment 1948 to September week 2 2011, searched on 22 September 2011
-
NHS Economic Evaluation Database (NHS EED): via Wiley Cochrane Library website Issue 3 of 4, July 2011, searched on 22 September 2011
-
PASCAL: via Dialog, using the segment 1973 to 2011 week 2 October, searched on 19 October 2011
-
Science Citation Index Expanded (SCIE): via Web of Knowledge, using the segment 2000 to 22 September 2011, searched on 23 September 2011
-
Science Citation Index (SCI) – Conference Proceedings: via Web of Knowledge, using the segment 1990 to 22 September 2011, searched on 23 September 2011.
Additional searches were conducted to identify systematic reviews of colposcopy in an attempt to ascertain the diagnostic accuracy of colposcopy:
-
CDSR: via Wiley Cochrane Library website Issue 10 of 12, October 2011, searched on 25 October 2011
-
DARE: via CRD administration database, using the segment to 25 October 2011, searched on 25 October 2011
-
DARE: via Wiley Cochrane Library website Issue 4 of 4, October 2011, searched on 25 October 2011.
The following websites were searched for guidelines and care pathways:
-
Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/, searched on 16 June 2011)
-
National Institute for Health and Clinical Excellence (NICE) (www.nice.org.uk/, searched on 16 June 2011)
-
National Guideline Clearinghouse (www.guidelines.gov/, searched on 16 June 2011)
-
National Institute for Health Research (NIHR) Health Technology Assessment programme (www.hta.ac.uk/, searched on 16 June 2011)
-
NHS Evidence (www.evidence.nhs.uk/, searched on 16 June 2011)
-
TRIP database (www.tripdatabase.com/, searched on 16 June 2011).
Inclusion and exclusion criteria
Two reviewers independently screened all titles and abstracts. Full paper manuscripts of any titles/abstracts that appeared to be relevant were obtained, where possible, and the relevance of each study independently assessed by two reviewers according to the inclusion and exclusion criteria below. Studies that did not meet all of the criteria were excluded and their bibliographic details listed with reasons for exclusion. Any discrepancies were resolved through consensus, with involvement of a third reviewer when necessary.
As stated earlier, the original scope for the assessment also included the APX 100 device, developed by Zilico Ltd. 3 Since this technology was removed from the assessment in December 2011, after the inclusion screening stage of the assessment, inclusion criteria refer to the APX 100 device.
-
Study design Comparative studies, including diagnostic test accuracy studies and controlled trials, were included in the evaluation of clinical effectiveness, as this study design allows a comparison to be made between the new technology and current practice, which is essential for the economic model.
-
Intervention Studies assessing DySIS, LuViva Advanced Cervical Scan, Niris Imaging System or APX 100, alone or alongside colposcopy, were included in the evaluation of clinical effectiveness.
-
Comparators Studies that compared one of the adjunctive colposcopy technologies with standard colposcopy were included in the evaluation of clinical effectiveness.
-
Participants The population of interest is women referred for colposcopy through the NHS Cervical Screening Programme. Therefore, studies of women referred for colposcopy because of an abnormal cytology result were included in the evaluation of clinical effectiveness. Studies that also included women referred for colposcopy because of symptoms indicative of cervical cancer (e.g. postcoital bleeding) or women referred for colposcopy for follow-up of CIN were also eligible for inclusion; however, studies that included only women referred for symptoms or for follow-up were not eligible for inclusion.
-
Outcomes The clinical outcomes of interest were diagnostic test accuracy outcomes (e.g. sensitivity and specificity), adverse effects and patient experience. Where other patient health outcomes were reported (e.g. morbidity and mortality from cancer or treatment), these were also included in the assessment.
Data extraction strategy
Data on study and participant characteristics and outcomes were extracted by one reviewer using a standardised data extraction form and independently checked for accuracy by a second reviewer. Disagreements were resolved through consensus, with involvement of a third reviewer when necessary.
Where sufficient data were available, the following diagnostic accuracy statistics [with 95% confidence intervals (CIs)] were calculated, for each study, using the Canadian Institute of Health Research's Knowledge Translation statistics calculator:15 sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR), and negative LR. Subsequently, accuracy was also calculated (the proportion of true-positive and true-negative results).
To allow consistency when comparing studies, in our results section we have reported our calculated results, rather than those reported in the study reports (as our results sometimes differed slightly from those in the study reports). Where data were missing from publications or other study reports, the authors were contacted (via NICE in the case of the manufacturers of the technologies). Data from multiple publications of the same study were extracted as a single study. The data extraction tables are presented in Appendix 2.
Quality assessment strategy
The quality of the included studies was assessed using the QUADAS-2 quality assessment tool for diagnostic studies. 16 As well as adding review-specific questions to domains 2 and 3, three further quality-related questions were assessed (see Appendix 3 for details). The assessment was performed by one reviewer and independently checked by a second. Disagreements were resolved through consensus, with involvement of a third reviewer when necessary. Further details about QUADAS-2 and results of the quality assessment are presented in Chapter 2 (see Quality of research available) and Appendix 3.
Data analysis
In view of the heterogeneity between the included studies, in terms of participant characteristics and the different comparator technologies used, formal meta-analysis was not appropriate. Therefore, the studies were grouped according to the adjunctive technology used and a narrative synthesis was presented.
Results of the review of clinical effectiveness
Quantity of research available
A total of 7835 records were identified from the clinical effectiveness searches and an additional 69 records were identified via hand-searching or contact with the manufacturers (via NICE) (Figure 1).
FIGURE 1.
Flow diagram of the study selection process.
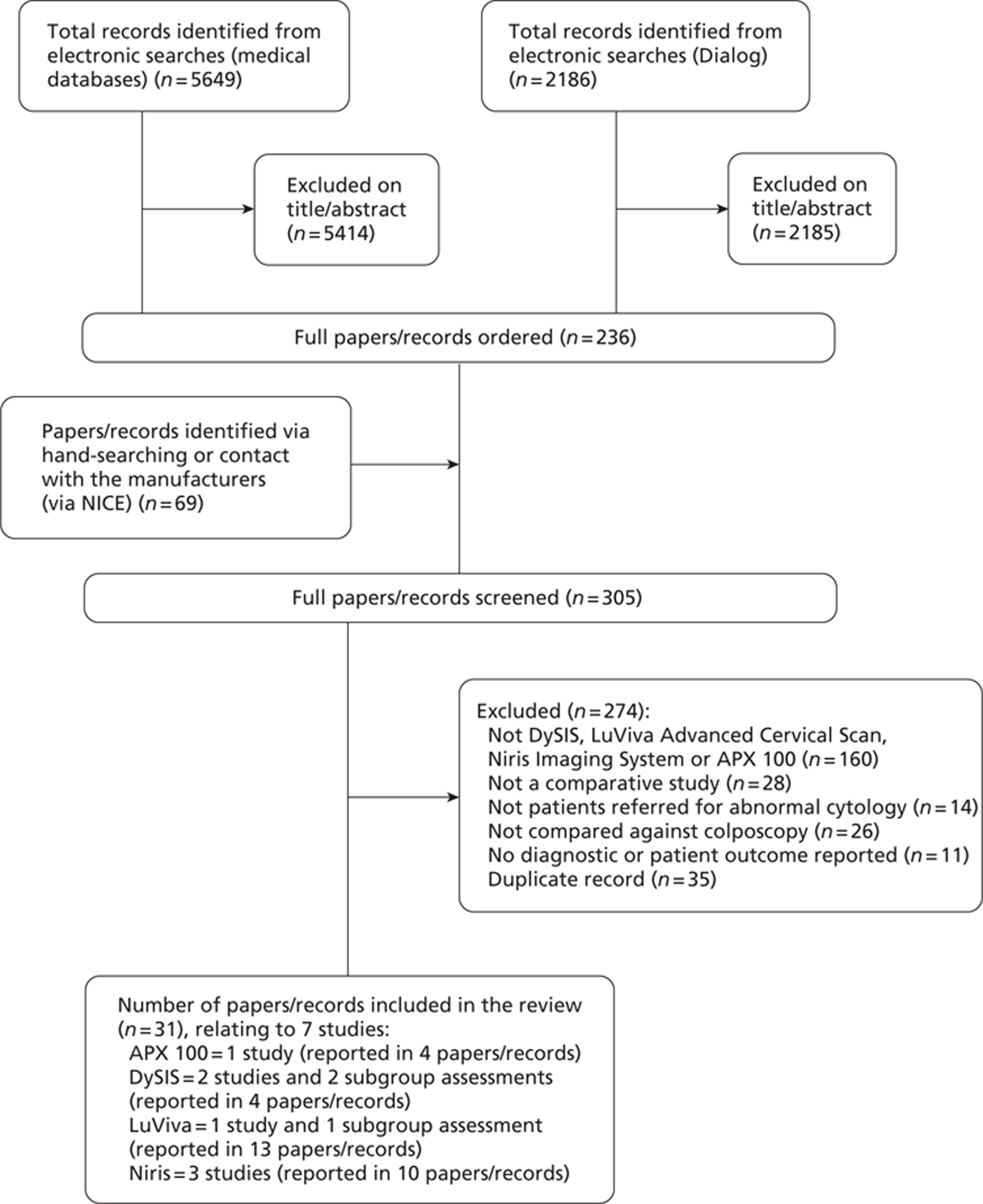
Seven studies (reported in 31 references) met the inclusion criteria. Details of studies excluded at the full publication stage are provided in Appendix 4.
On 21 December 2011, after we had finished screening studies for inclusion, we were informed by NICE that the APX 100 device, developed by Zilico Ltd, should be omitted from the assessment (one study, reported in four references). Therefore, six studies (reported in 27 references) were included in the review.
There were two main studies of the DySIS technology6,17 and two additional subgroup assessments; the two main studies6,17 were published in full, whereas one of the subgroup assessments was an unpublished draft manuscript (Zaal et al. , The VU University Medical Center, Amsterdam, the Netherlands, 2011) and the other subgroup assessment18 was reported in a conference abstract.
There was one study (Flowers et al. , University of Emory School of Medicine, Atlanta, GA, 2011) and one subgroup assessment19 of the LuViva Advanced Cervical Scan. The main study was an unpublished draft manuscript, whereas the subgroup assessment was reported in a conference poster. 19 he remaining 11 records were conference abstracts,20–23 presentations,24–26 a flyer,27 a ClinicalTrials.gov record,28 the manufacturer's presentation for NICE29 and the manufacturer's response to a question from the US Food and Drug Administration (FDA). 30 In addition, we received further clarification of methods and additional results via personal correspondence with the manufacturer on a number of occasions. However, there were some inconsistencies in the information we received; therefore, we are not entirely confident in the accuracy of these additional data. Results data received via personal correspondence have been highlighted as such in the summary of study characteristics and results (see Table 6) and the data extraction tables in Appendix 2.
There were three studies of the Niris Imaging System, all published in full. 31–33 The remaining seven records were conference abstracts,34–35 presentations36 and posters,37–38 the draft manuscript for one of the published papers (Liu et al. , Peking University Shenzhen Hospital, Shenzhen, China, 2009) and a draft book chapter that described one of the published studies. 39
Quality of research available
The QUADAS-2 tool, developed to improve, and to allow greater rating transparency than the original QUADAS tool, separates the evaluation of study quality into two main areas: risk of bias, and concerns regarding applicability. The tool consists of four domains: patient selection, index test, reference standard, and flow and timing. For individual studies each domain is assessed as being at a high, low, or unclear risk of bias, with the first three domains also assessed in terms of applicability concerns (also using high, low, or unclear ratings). The domains are supported by signalling questions, to help judge risk of bias. 16
Table 3 summarises the results of the QUADAS-2 assessments. Across almost all of the studies there were few applicability concerns in relation to appropriate patient recruitment and reference standard use. However, for the majority of studies, there were often difficulties in appraising risk of bias due to poor reporting, and there were also various applicability concerns about the conduct or interpretation of the adjunctive technologies. In general, study quality differed according to the type of adjunctive technology.
| Study | Concerns | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Applicability | ||||||||
| Index test | Index test | ||||||||
| Patient selection | Adjunct | Comparator | Reference standarda | Flow and timing | Patient selection | Adjunct | Comparator | Reference standard | |
| Soutter,17 DySIS | Low | Low | Low | Low | High | Low | High | High | Low |
| Louwers,6 DySIS | Low | Low | Low | Unclearb | Low | Low | Unclearb | High | Low |
| Flowers, unpublished, LuViva | Unclear | Unclear | Unclear | Low | High | Low | High | Low | Low |
| Escobar,31 Niris | Unclear | Unclear | Unclear | Low | Low | Low | High | Low | Low |
| Liu,32 Niris | Unclearb | Unclearb | Unclearb | Unclearb | Unclear | Low | High | Low | Low |
| Gallwas,33 Niris | Unclear | Unclear | Unclear | Low | High | Low | High | Unclear | Unclear |
DySIS
The two DySIS studies were judged to be at low risk of bias in terms of both patient selection and conduct and interpretation of the DySIS and colposcopy examinations. 6,17 However, there were applicability concerns in both studies relating to the conduct of colposcopy; video colposcopy using the DySIS colposcope was used, rather than the conventional colposcopy methods and equipment used in the NHS. The accuracy of colposcopy in these studies may therefore not be an accurate reflection of current NHS practice. Furthermore, in the earlier study a precommercial model was used, raising both applicability and bias concerns; around one-third of patients were excluded, largely due to equipment or software developmental problems. 17 These problems lessened during the later study, although 13% of patients were still excluded. 6 The earlier study clearly reported that histopathologists were unaware of DySIS results prior to assessing biopsies;17 details were unclear for the later study. 6 [Note: DySIS Medical have subsequently confirmed that histopathologists were unaware of DySIS results prior to assessing biopsies for this study also.]
LuViva Advanced Cervical Scan
The only study of LuViva (Flowers et al. , University of Emory School of Medicine, Atlanta, GA, 2011, unpublished) utilised two prototype systems that were referred to as LightTouch, rather than LuViva. The risk of bias assessment was hindered by poor reporting; it was unclear whether patients were enrolled consecutively, and there were uncertainties regarding possible bias arising from the conduct of the tests (most importantly, there was a lack of reporting on the level of training LightTouch assessors had been given). It was unclear whether the standard of care results could possibly have been influenced by knowledge of the biopsy results. The reference standard biopsy procedure was also poorly reported. After seeking further clarification from the manufacturers, it became apparent that only areas seen as being abnormal according to colposcopy were biopsied, with endocervical curettage and/or diagnostic excision biopsy being used for other patients. Applicability concerns regarding the conduct and interpretation of the tests were low for standard of care (where results were interpreted in the knowledge of both cytology and HPV test results) and high for LightTouch (where the cytology and HPV results were not used).
Niris Imaging System
For all three studies of the Niris Imaging System there was an unclear risk of bias in terms of patient selection (none of the studies indicated whether or not patients were recruited consecutively). 31–33 [Note: Imalux Corporation have subsequently confirmed that patients were enrolled consecutively in the study by Liu et al. 32] Similarly, all three studies were at an unclear risk of bias arising from the conduct of the tests (arising particularly from the absence of reporting on the level of training Niris assessors had been given). [Note: Imalux Corporation have subsequently confirmed that in the study by Liu et al. 32 expert colposcopists undertook the colposcopy examination and an OCT expert provided the OCT impression.] The risk of bias relating to the conduct and interpretation of biopsies was low in the two studies reporting that Niris images were anonymised,31,33 but was unclear in the remaining study. 32 [Note: Imalux Corporation have subsequently confirmed that histopathologists were unaware of Niris results prior to assessing biopsies for this study also.] The most recent study was at a high risk of bias for the flow and timing domain, since biopsies were taken only from suspicious areas (meaning false-negative results would not be identified). 33 For the earliest study the risk was low (random biopsies were performed). 31 For the remaining study the risk was unclear (it was unclear whether all recruited patients were included in the analyses). 32 Applicability concerns were high for all three studies regarding the conduct and interpretation of the Niris test. In both the earlier studies the Niris system could not provide cut-offs more specific than being ‘normal’, ‘abnormal’ or ‘indeterminate’ (see the data extraction table for the Escobar et al. study,31 in Appendix 2, for definitions),31–32 whereas for the latest study although results using CIN1+, CIN2+, and CIN3+ as cut-offs were provided, the images were not interpreted during the colposcopic examination. 33 Applicability concerns relating to colposcopy were low for the two earlier studies where the procedure was clearly described,31–32 and unclear for the later study where few details were provided. 33
Synthesis of the included studies
Table 4 displays the participant characteristics and comparator technologies used in the included studies. There was considerable heterogeneity between the included studies, in terms of participant characteristics and comparator technologies used, therefore no quantitative synthesis has been undertaken. The studies have been synthesised, narratively, for each adjunctive technology separately.
| Study | ||||
|---|---|---|---|---|
| Louwers et al., 20116 | Zaal et al., unpublished | Soutter et al., 200917 | Soutter et al., conference abstract18 | |
| Study | ||||
| Flowers et al., unpublished | Flowers and Tadros, conference poster19 | |||
| Study | ||||
| Gallwas et al., 201133 | Liu et al., 201032 | Escobar et al., 200631 | ||
| Participant characteristics | 239 women with abnormal cervical cytology or follow-up of a CIN1 or 2 lesion | Subgroup assessment of women in Louwers study6 who had an adequate HPV test result (n = 177) | 308 women with abnormal cervical cytology or symptoms suggesting the possibility of cervical neoplasia | Subgroup assessment of women in Soutter study17 in which the grade of the abnormal smear was known (n = 299) |
| Prevalence of CIN2+ = 45.2% | Prevalence of CIN2+ = 48% | Prevalence of CIN2+ = 23.4% |
|
|
| Analysis: per patient | Analysis: per patient | Analysis: per patient | Analysis: per patient | |
| Comparator technology | Colposcopy using DSI colposcope | Colposcopy using DSI colposcope | Colposcopy using DSI colposcope | Colposcopy using DSI colposcope |
| Diagnostic accuracy (CIN2+): | Diagnostic accuracy (CIN2+): | Diagnostic accuracy (CIN2+): | Diagnostic accuracy (CIN2+): | |
| Sensitivity = 51.9% | Sensitivity = 55% | Sensitivity = 48.6% | Women referred with a low-grade smear: | |
| Specificity = 81.7% | Specificity = 85% | Specificity = 89.4% | ||
| Sensitivity = 19.4% | ||||
| Specificity = 93.3% | ||||
| Women referred with a high-grade smear: | ||||
| Sensitivity = 72.5% | ||||
| Specificity = 68.6% | ||||
| Participant characteristics | AiC information removed | Subgroup assessment of women in Flowers et al., unpublished study; women aged 16–20 years (n = 245) | ||
| Prevalence of CIN2+ = 18.8% | ||||
| Analysis: per patient | ||||
| Comparator technology | AiC information removed | Current standard of care (consisting of Pap result, HPV and colposcopically directed biopsy) | ||
| Diagnostic accuracy (CIN2+): | ||||
| Sensitivity = 80% | ||||
| Participant characteristics | Women with abnormal cervical cytology (number unknown) | 299 women with abnormal cervical cytology or HPV positive for one of the hrHPV types (1237 paired images) | 212 women with abnormal cervical cytology or suspicious lesions (1215 images) | |
| Prevalence of CIN2+ = 52.9% | Prevalence of CIN2+ = 18% | Prevalence of CIN2+ = 15.3% | ||
| Analysis: per image | Analysis: per patient, per lesion and per ‘most severe biopsy per woman’ | Analysis: per patient and per lesion | ||
| Comparator technology | Conventional colposcopy | Conventional colposcopy | Conventional colposcopy | |
| Diagnostic accuracy (CIN2+): | Diagnostic accuracy (CIN2+): | Diagnostic accuracy (CIN2+): | ||
| Sensitivity = 99% | Low grade: | Sensitivity = 37.5% | ||
| Specificity = 61% | Sensitivity = 74% | Specificity = 70.6% | ||
| Specificity = 67% | ||||
| High grade: | ||||
| Sensitivity = 22.6% | ||||
| Specificity = 96.3% | ||||
DySIS
The main characteristics and results of the included DySIS studies are presented in Table 5; further details are presented in Appendix 2. There were two main studies of the DySIS technology6,17 and two additional subgroup assessments; one subgroup assessment of women according to their hrHPV type [HPV16 vs non-16 hrHPV (Zaal et al. , The VU University Medical Center, Amsterdam, the Netherlands, 2011, unpublished)] and one subgroup assessment18 of women according to the cytology test result (high grade vs low grade).
| Study | ||||||
|---|---|---|---|---|---|---|
| Louwers et al., 20116 | Zaal et al., unpublished | Soutter et al., 200917 | Soutter et al., conference abstract18 | |||
| Recruitment dates | 1 July 2008 to 1 September 2009 | August 2004 to July 2005 | ||||
| Number recruited | 275 | 447 | ||||
| Number analysed | 239 | 177 | 308 | 299 | ||
| Patient inclusion criteria | Women with abnormal cervical cytology or follow-up of a CIN1 or 2 lesion | Subgroup assessment of women in Louwers et al.6 study assessed as per protocol, who had an adequate HPV test result | Women with abnormal cervical cytology or symptoms suggesting the possibility of cervical neoplasia | Subgroup assessment of women in Soutter et al.17 for whom the grade of the abnormal smear was known | ||
| Patient age | Mean: 36.7 (range 18.7–62.6) years | Median: 33.6 (range 18.7–62.6) years | Median 37 years (upper and lower quartiles 29–46) | NR | ||
| Other relevant patient information | Result of last smear: | Result of last smear: | No women with clinically apparent cancer were included | 224 women were referred with a low-grade smear, 75 women were referred with a high-grade smear | ||
| normal = 5 (2.1%), borderline or mild dyskaryosis = 153 (64.0%), worse than borderline/mild dyskaryosis = 81 (33.9%) | normal = 4 (2.3%), borderline or mild dyskaryosis = 113 (63.8%), worse than borderline/mild dyskaryosis = 60 (33.9%) | Four women were referred with AGUS cervical cytology result | ||||
| hrHPV test: negative = 73 (30.5%), positive = 158 (66.1%), not performed = 8 (3.3%) | hrHPV test: positive = 133 women; 10 lrHPV+, 80 non-16 hrHPV+, 42 hrHPV16+, in one case the typing was inconclusive | |||||
| Adjunctive technology characteristics | DSI colposcope – DySIS v2.1, with colour-coded map | DSI colposcope – DySIS v2.1, with colour-coded map | Precommercial DySIS model (FPC-03), with PCM | Precommercial DySIS model (FPC-03), with PCM | ||
| Colposcopy characteristics | Colposcopic examination using DySIS as a regular video colposcope | Colposcopic examination using DySIS as a regular video colposcope | Colposcopic examination using DySIS as a regular video colposcope | Colposcopic examination using DySIS as a regular video colposcope | ||
| Reference standard | Histology result. Biopsies were taken from all suspicious areas identified by the DySIS colour-coded map or colposcopic impression. If both tests evaluated the cervix as normal, one biopsy was taken from the transformation zone at the 12 o'clock position | Histology result. Biopsies were taken from all suspicious areas identified by the DySIS colour-coded map or colposcopic impression. If both tests evaluated the cervix as normal, one biopsy was taken from the transformation zone at the 12 o'clock position | Histology result. Biopsies were taken from all suspicious areas identified by the DySIS colour-coded map or colposcopic impression, and also from sites that did not seem to contain CIN in order to reduce verification bias | Histology result. Biopsies were taken from all suspicious areas identified by the DySIS colour-coded map or colposcopic impression, and also from sites that did not seem to contain CIN, in order to reduce verification bias | ||
| Analysis presented | Per patient, ITT | Analysis: per patient, per protocol | Per patient | Per patient | ||
| Primary outcome | Histologically confirmed high-grade cervical disease (CIN2+) | Difference in colposcopic impression and histological outcome in women positive for HPV16 (HPV16+) vs women negative for HPV16 but positive for at least one hrHPV type (non-16 hrHPV+) | Incremental DySIS test characteristics over conventional colposcopy, using histology as a reference | The sensitivities and specifcities of DySIS and conventional colposcopy were calculated separately for patients referred with low-grade smears and patients referred with high-grade smears | ||
| Diagnostic accuracy results for DySIS adjunctive technology | CIN2+ | CIN2+ | High-grade disease | Patients referred for a low-grade smear | ||
| Sensitivity = 64.8% (95% CI 55.4 to 73.2) | Total population: Sensitivity = 80% (95% CI 70 to 88) | Sensitivity = 79.2% (95% CI 68.4 to 86.9) | Sensitivity = 77.4% | |||
| Specificity = 77.2% | Specificity = 70.2% (95% CI 61.9 to 77.4) | Specificity = 77% (95% CI 67 to 85) | Specificity = 75.8% (95% CI 70.0 to 80.9) | |||
| Patients referred for a high-grade smear: | ||||||
| PPV = 64.2% (95% CI 54.9 to 72.6) | Non-16 hrHPV+ population: Sensitivity = 74% (95% CI 57 to 87) | PPV = 50.0% (95% CI 41.0 to 59.0) | Sensitivity = 80.0% | |||
| NPV = 70.8% (95% CI 62.4 to 77.9) | Specificity = 67% (95% CI 50 to 80) | NPV = 92.3% (95% CI 87.6 to 95.3) Accuracy = 76.6% | Specificity = 74.3% | |||
| Accuracy = 67.8% LR+ = 2.18 (95% CI 1.62 to 2.93) | HPV16+ population: Sensitivity = 97% (95% CI 84 to 100) | LR+ = 3.28 (95% CI 2.54 to 4.23) | ||||
| LR− = 0.50 (95% CI 0.38 to 0.66) | Specificity = 100% (95% CI 69 to 100) | LR− = 0.28 (95% CI 0.17 to 0.43) | ||||
| Prevalence = 45.2% | Prevalence = 23.4% | |||||
| Diagnostic accuracy results for colposcopy | CIN2+ | CIN2+ | High-grade disease | Patients referred with a low-grade smear | ||
| Sensitivity = 51.9% (95% CI 42.5 to 61.0) | Total population: Sensitivity = 55% (95% CI 44 to 66) | Sensitivity = 48.6% (95% CI 37.4 to 59.9) | Sensitivity = 19.4% | |||
| Specificity = 81.7% (95% CI 74.2 to 87.4) | Specificity = 85% (95% CI 76 to 91) | Specificity = 89.4% (95% CI 84.8 to 92.7) | Specificity = 93.3% | |||
| PPV = 70.0% (95% CI 59.2 to 78.9) | Non-16 hrHPV+ population: Sensitivity = 61% (95% CI 43 to 76) | PPV = 58.3% (95% CI 45.7 to 69.9) | Patients referred with a high-grade smear: | |||
| NPV = 67.3% (95% CI 59.7 to 74.1) | Specificity = 83% (95% CI 69 to 93) | NPV = 85.1% (95% CI 80.1 to 89.0) | Sensitivity = 72.5% | |||
| Accuracy = 68.2% | HPV16+ population: Sensitivity = 53% (95% CI 35 to 71) | Accuracy = 79.9% | Specificity = 68.6% | |||
| LR+ = 2.83 (95% CI 1.89 to 4.24) | LR+ = 4.59 (95% CI 2.96 to 7.13) | |||||
| LR− = 0.59 (95% CI 0.48 to 0.73) | Specificity = 90% (95% CI 55 to 100) | LR− = 0.58 (95% CI 0.46 to 0.72) | ||||
| Prevalence = 45.2% | Prevalence = 23.4% | |||||
| Diagnostic accuracy results for DySIS adjunctive technology and colposcopy combined | Sensitivity = 79.6% (95% CI 71.1 to 86.1) | |||||
| Specificity = 62.6% (95% CI 54.1 to 70.4) | ||||||
| PPV = 63.7% (95% CI 55.3 to 71.3) | ||||||
| NPV = 78.8% (95% CI 70.0 to 85.6) | ||||||
| Accuracy = 70.3% | ||||||
| LR+ = 2.13 (95% CI 1.67 to 2.71) | ||||||
| LR− = 0.33 (95% CI 0.22 to 0.48) | ||||||
| Prevalence = 45.2% | ||||||
| Adverse effects | No adverse events were reported during the study period | NR | No adverse events were reported | NR | ||
| Patient satisfaction | DSI colposcopy, compared with conventional colposcopy, was no extra burden for the majority of women | NR | NR | NR | ||
The participants in the main studies were similar: women referred for colposcopy with an abnormal cervical cytology result or follow-up of a CIN1 or CIN2 lesion,6 or women referred with an abnormal cervical cytology result or symptoms suggesting the possibility of cervical neoplasia. 17 However, the prevalence of CIN2+ was considerably higher in the study by Louwers et al. ,6 at 45%, than in the study by Soutter et al. 17 (23%). The average age of participants was 37 years in both of the main studies. The Louwers et al. 6 results presented below are those for the ‘intention-to-treat’ (ITT) cohort of patients, rather than the ‘according-to-protocol’ (ATP) cohort, from which 56 women were excluded as their management did not strictly adhere to the protocol. 6 Results for the ATP cohort are reported in Appendix 2.
The DySIS technology used in the earlier study by Soutter et al. 17 was a precommercial model (FPC-03), which had some technical problems relating to the software, speculum and a batch of faulty disposable nozzles, leading to the exclusion of a large proportion of participants from the analyses. 17 DySIS v2.1 was used in the later study by Louwers et al. ; 6 therefore, this study is the most relevant for clinical practice. Both studies used the DySIS colposcope as a regular video colposcope as the comparator technology, and histology result was the reference standard. All patients underwent both DySIS colposcopy and the comparator colposcopic examination during the same appointment.
The sensitivity of DySIS was higher than that of conventional colposcopy (using the DySIS colposcope as a regular video colposcope) for distinguishing between normal or low-grade (CIN 0–1) and high-grade (CIN2+) disease: 64.8% compared with 51.9% in the study by Louwers et al. 6 and 79.2% compared with 48.6% in the study by Soutter et al. 17 However, the specificity was lower with DySIS; 70.2% compared with 81.7% in the study by Louwers et al. 6 and 75.8% compared with 89.4% in the study by Soutter et al. 17 The sensitivity and specificity of DySIS (the DSI colour-coded map) combined with conventional colposcopy were 79.6% and 62.6% respectively, compared with 51.9% and 81.7% for conventional colposcopy alone. 6 The differences in sensitivity and specificity between DySIS and conventional colposcopy and between DySIS combined with conventional colposcopy and conventional colposcopy alone were statistically significant (asymptotic McNemar test in the study by Louwers et al. , 6 Fisher's exact two-sided test in the study by Soutter et al. 17).
In the study by Louwers et al. 6 the overall diagnostic accuracy of DySIS was similar to that of conventional colposcopy: 67.8% compared with 68.2%. In the study by Soutter et al. 17 the overall diagnostic accuracy of DySIS was slightly lower than that of conventional colposcopy: 76.6% compared with 79.9%. The accuracy of DySIS combined with conventional colposcopy was also assessed using data from the study by Louwers et al. ,6 and was similar to that of conventional colposcopy alone, 70.3%.
In a subgroup assessment of women referred with a high-grade cytology test result, both sensitivity and specificity were higher with DySIS than conventional colposcopy; 80% compared with 72.5% for sensitivity and 74.3% compared with 68.6% for specificity, although this was based on a subgroup assessment of just 75 women. 18 In a subgroup of women referred with a low-grade cytology test result, sensitivity was higher with DySIS (77.4% compared with 19.4%), but specificity was lower (77.2% compared with 93.3%), based on a subgroup assessment of 224 women. 18
In a subgroup assessment of women with hrHPV16, both sensitivity and specificity were higher with DySIS than conventional colposcopy: 97% compared with 53% for sensitivity and 100% compared with 90% for specificity, although this was based on a subgroup assessment of just 42 women. In the subgroup of women with non-16 hrHPV, sensitivity was higher with DySIS (74% vs 61%), but specificity was lower (67% vs 83%), based on a subgroup assessment of 80 women (Zaal et al. , unpublished).
The two main studies stated that no adverse events were reported. 6,17
The study by Louwers et al. 6 assessed patient satisfaction using a questionnaire; the majority of women reported that DySIS was no extra burden compared with conventional colposcopy.
LuViva Advanced Cervical Scan
The main characteristics and results of the included LuViva study are presented in Table 6; further details are presented in Appendix 2. There was one main study of LuViva (Flowers et al. , unpublished) and one additional subgroup assessment of women aged 16–20 years. 19 However, women in England are not invited for cervical screening under the NHS Cervical Screening Programme until the age of 25 years;2 therefore, the subgroup population is not of direct relevance to this assessment.
| Study | ||
|---|---|---|
| Flowers et al., unpublished | Flowers and Tadros, conference poster19 | |
| Recruitment dates | AiC information has been removed | |
| Number recruited | ||
| Number analysed | 245 | |
| Patient inclusion criteria | Subgroup assessment of women in Flowers et al., unpublished study; women aged 16–20 years | |
| Patient age | 16–20 years | |
| Other relevant patient information | ||
| Adjunctive technology characteristics | MHS LightTouch | |
| Comparator technology characteristics | Current standard of care (consisting of Pap result, HPV and colposcopically directed biopsy) | |
| Reference standard | Histology result and clinical follow-up | |
| Analysis presented | Per patient | |
| Primary outcome | Prevalence of CIN2+ or worse disease in women of < 21 years and performance of MHS in this population | |
| Diagnostic accuracy results for LuViva adjunctive technology | CIN2+ | |
| Sensitivity = 91.3% (95% CI 79.7 to 96.6) | ||
| Specificity = 28.6% (95% CI 22.8 to 35.3) | ||
| PPV = 22.8% (95% CI 17.4 to 29.4) | ||
| NPV = 93.4% (95% CI 84.3 to 97.4) | ||
| Accuracy = 40.4% | ||
| LR+ = 1.28 (95% CI 1.13 to 1.45) | ||
| LR− = 0.30 (95% CI 0.12 to 0.79) | ||
| Prevalence = 18.8% | ||
| Diagnostic accuracy results for the current standard of care | CIN2+ | |
| Sensitivity = 80% | ||
| Adverse effects | NR | |
| Patient satisfaction | NR | |
The main study of LuViva was reported in an academic-in-confidence (AiC) unpublished report; therefore, the data cannot be presented in this report.
The name of the technology has been changed since the study was conducted; at the time of the study the LuViva Advanced Cervical Scan was called LightTouch. The comparator used in the study was the ‘current standard of care’, consisting of the cytology test result, HPV test result and colposcopically directed biopsy. Histology result was the reference standard; however, this was based on biopsy for abnormal-looking areas, and endocervical curettage when no lesion was seen on colposcopy [although if patients had been referred with low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells with possible high-grade squamous intraepithelial lesion (ASC-H) or high-grade squamous intraepithelial lesion (HSIL), diagnostic excision biopsy was performed]. In addition, around half of the patients had 2-year clinical follow-up. All patients underwent the LightTouch scan during the standard colposcopy appointment.
Niris Imaging System
The main characteristics and results of the included Niris studies are presented in Table 7; further details are presented in Appendix 2. There were three studies of the Niris Imaging System. 31–33
| Study | |||
|---|---|---|---|
| Gallwas et al., 201133 | Liu et al., 201032 | Escobar et al., 200631 | |
| Recruitment dates | July 2008 to May 2010 | NR | NR |
| Number recruited | Unclear, although 1375 images were taken from 120 women (1165 images were from unsuspicious areas, and 210 were compared with histology) | Unclear | 220 |
| Number analysed | 210 images (number of women unknown) | 299 women (1237 paired diagnoses) | 212 (1215 images) |
| Patient inclusion criteria | Women with abnormal cervical cytology | Women with abnormal cervical cytology or a positive test for one of the high-risk types of HPV | Women with abnormal cervical cytology or suspicious lesions |
| Patient age | Mean: 31.1 (range 18–46) years | Median: 36.7 (range 19.2–67.9) years | Mean: 35.5 (range 18–80) years |
| Other relevant patient information | Result of last smear | 10% of women were menopausal | Result of last smear |
| PAP II, 19; PAP IIW, 14; PAP III, 5; PAP IIID, 44; PAP IVA, 32; PAP IVB, 5; PAP V, 1 | 48 (23%) had ASCUS, 142 (67%) had LSIL, 22 (10%) had HSIL | ||
| hrHPV test | 189 were premenopausal and 23 were postmenopausal | ||
| 93 women tested positive | |||
| Adjunctive technology characteristics | Colposcopy-guided OCT using the Niris Imaging system | Niris Imaging System | Imalux OCT device |
| Colposcopy characteristics | Conventional colposcopy | Conventional colposcopy | Conventional colposcopy |
| Reference standard | Histology result. Biopsies were taken from suspicious areas identified using OCT. (Biopsy procedure details were unclear for the colposcopy assessment.) | Histology result. Biopsies were taken at all positive areas and at the 2, 4, 8 and 10 o'clock positions at the squamocolumnar junction. Endocervical curettage was also performed on every patient | Histology result. Biopsies were taken at all positive areas and at the 2, 4, 8 and 10 o'clock positions at the squamocolumnar junction. Endocervical curettage was also performed on every patient |
| Analysis presented | Per image | Per patient, per lesion and per ‘most severe biopsy per woman’ | Per patient and per lesion |
| Primary outcome | CIN using cut-offs at CIN1+, CIN2+ and CIN3+ | CIN using cut-offs at indeterminate or abnormal | CIN using cut-offs at indeterminate or abnormal |
| Diagnostic accuracy results for Niris adjunctive technology | CIN1+ | Per-patient analysis | Per-patient analysis |
| Sensitivity = 97.9% (95% CI 94.1 to 99.3) | CIN2+ Indeterminate/abnormal | CIN2+ Indeterminate/abnormal | |
| Specificity = 39.1% (95% CI 28.1 to 51.3) | Sensitivity = 45.3% (95% CI 32.7 to 58.5) | Sensitivity = 93.8% (95% CI 79.9 to 98.3) | |
| PPV = 78.6% (95% CI 72.1 to 83.9) | Specificity = 86.1% (95% CI 81.2 to 89.9) | Specificity = 10.7% (95% CI 7.0 to 16.2) | |
| NPV = 89.3% (95% CI 72.8 to 96.3) | PPV = 41.4% (95% CI 29.6 to 54.2) | PPV = 16.0% (95% CI 11.4 to 21.9) | |
| Accuracy = 80.0% | NPV = 87.9% (95% CI 83.2 to 91.5) | NPV = 90.5% (95% CI 71.1 to 97.3) | |
| LR+ = 1.61(95% CI 1.32 to 1.96) | Accuracy = 78.9% | Accuracy = 23.4% | |
| LR− = 0.05(95% CI 0.02 to 0.17) | LR+ = 3.26(95% CI 2.12 to 5.02) | LR+ = 1.05(95% CI 0.95 to 1.16) | |
| Prevalence = 69.5% | LR− = 0.64(95% CI 0.50 to 0.82) | LR− = 0.58(95% CI 0.14 to 2.38) | |
| CIN2+ | |||
| Sensitivity = 86.5% (95% CI 78.9 to 91.6) | Prevalence = 17.8% | Prevalence = 15.3% | |
| Abnormal | Abnormal | ||
| Specificity = 63.6% (95% CI 53.8 to 72.4) | Sensitivity = 32.1% (95% CI 21.1 to 45.5) | Sensitivity = 56.3% (95% CI 39.3 to 71.8) | |
| PPV = 72.7% (95% CI 64.6 to 79.6) | Specificity = 93.1% (95% CI 89.2 to 95.6) | Specificity = 59.3% (95% CI 52.0 to 66.3) | |
| NPV = 80.8% (95% CI 70.7 to 88.0) | PPV = 50% (95% CI 34.1 to 65.9) | PPV = 20.0% (95% CI 13.0 to 29.4) | |
| Accuracy = 75.7% | NPV = 86.4% (95% CI 81.7 to 90.0) | NPV = 88.2% (95% CI 81.2 to 92.9) | |
| LR+ = 2.38(95% CI 1.81 to 3.12) | Accuracy = 82.2% | Accuracy = 58.9% | |
| LR− = 0.21(95% CI 0.13 to 0.35) | LR+ = 4.62(95% CI 2.53 to 8.45) | LR+ = 1.38(95% CI 0.97 to 1.97) | |
| Prevalence = 52.9% | LR− = 0.73(95% CI 0.61 to 0.88) | LR− = 0.74(95% CI 0.49 to 1.11) | |
| CIN3+ | |||
| Sensitivity = 87.2% (95% CI 78.0 to 92.9) | Prevalence = 17.8% | Prevalence = 15.3% | |
| Specificity = 81.1% (95% CI 73.5 to 86.8) | |||
| PPV = 73.1% (95% CI 63.3 to 81.1) | |||
| NPV = 91.5% (95% CI 85.0 to 95.3) | |||
| Accuracy = 83.3% | |||
| LR+ = 4.60(95% CI 3.20 to 6.62) | |||
| LR− = 0.16(95% CI 0.09 to 0.28) | |||
| Prevalence = 37.1% | |||
| Diagnostic accuracy results for colposcopy | CIN1+ | Per-patient analysis | CIN2+ |
| Sensitivity = 99% | CIN2+ | Sensitivity = 37.5% (95% CI 22.9 to 54.7) | |
| Specificity = 19% | Low grade | ||
| CIN2+ | Sensitivity = 74% (95% CI 60 to 84) | Specificity = 70.6% (95% CI 63.5 to 76.8) | |
| Sensitivity = 99% | |||
| Specificity = 61% | Specificity = 67% (95% CI 61 to 73) | PPV = 18.8% (95% CI 11.1 to 30.0) | |
| CIN3+ | High grade | NPV = 86.2% (95% CI 79.7 to 90.9) | |
| Sensitivity = 78% | |||
| Specificity = 74% | Sensitivity = 22.6% (95% CI 13.5 to 35.5) | Accuracy = 65.6% | |
| Specificity = 96.3% (95% CI 93.2 to 98.1) | LR+ = 1.28(95% CI 0.77 to 2.11) | ||
| PPV = 57.1% (95% CI 36.5 to 75.5) | LR− = 0.89(95% CI 0.67 to 1.18) | ||
| NPV = 85.3% (95% CI 80.6 to 88.9) | Prevalence = 15.3% | ||
| Accuracy = 83.3% | |||
| LR+ = 6.19(95% CI 2.75 to 13.94) | |||
| LR− = 0.80(95% CI 0.69 to 0.93) | |||
| Prevalence = 17.7% | |||
| Adverse effects | NR | NR | NR |
| Patient satisfaction | NR | NR | NR |
The participants were similar in all three studies: women referred for colposcopy with an abnormal cervical cytology result,33 women referred with an abnormal cervical cytology result or hrHPV,32 or women referred with an abnormal cervical cytology result or suspicious lesions. 31 However, the prevalence of CIN2+ was considerably higher in the study by Gallwas et al. ,33 at 53%, than in the study by Liu et al. 32 (18%) and the study by Escobar et al. (15%). 31 The average age of participants in the studies was between 31 and 37 years.
In the study by Gallwas et al. ,33 Niris images were evaluated as normal, inflammation, CIN1, CIN2, CIN3 or squamous carcinoma. 33 In the earlier study by Liu et al. ,32 Niris images were evaluated as normal, indeterminate or abnormal. 32 In the earliest study by Escobar et al. ,31 the system was referred to as the Imalux OCT device, it had different technical specifications to the other two studies,32,33 and this study31 also evaluated images as normal, indeterminate or abnormal. Images were evaluated as being normal if a well-organised, simple two-layer structure with a sharp interface between the surface epithelium and underlying stromal layer was seen. Images were evaluated as being abnormal if the tissue was unstructured with no apparent interface present. Images were evaluated as being indeterminate if irregularities on the images suggested artefacts or physiological alterations and did not meet criteria for normal or abnormal. The study by Gallwas et al. 33 is the most relevant for clinical practice because of the cut-offs used for categorising images.
All three studies31–33 used conventional colposcopy as the comparator technology, and histology result was the reference standard. However, biopsies were taken from only the suspicious areas in the study by Gallwas et al. ,33 therefore the results from this study are unreliable. All patients underwent OCT imaging using the Niris technology during the standard colposcopy appointment.
In the study by Gallwas et al. 33 the sensitivity of Niris was lower than that of conventional colposcopy for detecting CIN2+ disease: 86.5% and 99%, respectively. However, the sensitivity of 99% for conventional colposcopy is not representative of colposcopy in practice. In this study, biopsies for reference standard assessment were taken only from suspicious areas; thus, false-negative results would not have been detected, resulting in a falsely increased sensitivity result. 33 Therefore, the results from this study are unreliable. The specificity of Niris was similar to that of colposcopy: 63.6% and 61% respectively. The overall accuracy of Niris was 75.7%; it was not possible to calculate overall accuracy for conventional colposcopy. However, the lack of reference standard assessment of patients for whom no suspicious areas were identified also affects the specificity and overall accuracy results.
For detecting CIN1+ disease, the sensitivity of Niris was 97.9% compared with 99% for colposcopy, specificity was 39.1% for Niris and 19% for colposcopy, and accuracy was 80% for Niris. For detecting CIN3+, disease the sensitivity of Niris was 87.2% compared with 78% for colposcopy, specificity was 81.1% for Niris and 74% for colposcopy, and accuracy was 83.3% for Niris. 33 Again, these results are unreliable, owing to biopsies for reference standard assessment being taken only from suspicious areas.
In the study by Liu et al. ,6 the sensitivity of Niris was higher than that of conventional colposcopy for distinguishing between normal/indeterminate and abnormal lesions: 32.1% compared with 22.6%. 32 The specificity of Niris was slightly lower than that of conventional colposcopy: 93.1% compared with 96.3%. The overall diagnostic accuracy of Niris was similar to that of conventional colposcopy: 82.2% compared with 83.3% for conventional colposcopy (for determining ‘high-grade’ lesions with colposcopy).
For distinguishing between normal and indeterminate/abnormal lesions, the sensitivity of Niris was lower than that of colposcopy; 45.3% compared with 74% for conventional colposcopy (for determining ‘low-grade’ lesions with colposcopy). The specificity of Niris was higher than that of colposcopy: 86.1% compared with 67% for conventional colposcopy. The overall diagnostic accuracy of Niris was 78.9%; overall accuracy was not reported for conventional colposcopy.
In the study by Escobar et al. ,31 the sensitivity of Niris was higher than that of colposcopy for distinguishing between normal/indeterminate and abnormal lesions: 56.3% compared with 37.5%. However, the specificity of Niris was lower than that of colposcopy: 59.3% compared with 70.6%. The overall diagnostic accuracy of Niris was lower than that of conventional colposcopy: 58.9% compared with 65.6% for conventional colposcopy.
For distinguishing between normal and indeterminate/abnormal lesions, the sensitivity of Niris was 93.8% and the specificity was 10.7%. The overall diagnostic accuracy of Niris was 23.4%.
Summary of results for the most clinically relevant studies
Table 8 summarises the diagnostic accuracy results for the three most clinically relevant studies: the study of the most recent model of the DySIS technology by Louwers et al. ,6 the study of the LuViva Advanced Cervical Scan (under its former name of LightTouch) by Flowers et al. (unpublished) and the study of the most recent model of the Niris Imaging System by Gallwas et al. 33 (the only Niris study to report results using a CIN2 cut-off).
| Study | |||||||
|---|---|---|---|---|---|---|---|
| Louwers et al., 20116 | Flowers et al., unpublished | Gallwas et al., 201133 | |||||
| DySIS alone | DySIS + conventional colposcopy | Conventional colposcopy | AiC information has been removed | AiC information has been removed | AiC information has been removed | Niris Imaging System | Conventional colposcopy |
| Sensitivity = 64.8% (95% CI 55.4 to 73.2) | Sensitivity = 79.6% (95% CI 71.1 to 86.1) | Sensitivity = 51.9% (95% CI 42.5 to 61.0) | Sensitivity = 86.5% (95% CI 78.9 to 91.6) | Sensitivity = 99% | |||
| Specificity = 70.2% (95% CI 61.9 to 77.4) | Specificity = 62.6% (95% CI 54.1 to 70.4) | Specificity = 81.7% (95% CI 74.2 to 87.4) | Specificity = 63.6% (95% CI 53.8 to 72.4) | Specificity = 61% | |||
| Accuracy = 67.8% | Accuracy = 70.3% | Accuracy = 68.2% | Accuracy = 75.7% | NR | |||
| LR+ = 2.18 (95% CI 1.62 to 2.93) | LR+ = 2.13 (95% CI 1.67 to 2.71) | LR+ = 2.83 (95% CI 1.89 to 4.24) | LR+ = 2.38 (95% CI 1.81 to 3.12) | NR | |||
| LR− = 0.50 (95% CI 0.38 to 0.66) | LR− = 0.33 (95% CI 0.22 to 0.48) | LR− = 0.59 (95% CI 0.48 to 0.73) | LR− = 0.21 (95% CI 0.13 to 0.35) | NR | |||
| Prevalence = 45.2% | Prevalence = 45.2% | Prevalence = 45.2% | Prevalence = 52.9% | NR | |||
The results of the studies suggest that the sensitivity of the adjunctive technologies is higher for DySIS, DySIS plus conventional colposcopy, and LuViva than conventional colposcopy alone, and for LuViva sensitivity is also higher than the current standard of care (consisting of the cytology test result, HPV test result and colposcopically directed biopsy). For DySIS the specificity is lower for DySIS and DySIS plus conventional colposcopy than conventional colposcopy alone; resulting in an overall diagnostic accuracy similar to that of conventional colposcopy alone. The specificity of LuViva is lower than that of colposcopy alone, although the specificity of LuViva cannot be compared against the standard of care, as the relevant data were not reported. The sensitivity of Niris was found to be lower than that of conventional colposcopy and the specificity of Niris appears to be similar to that of conventional colposcopy. However, the results from this study33 are unreliable because biopsies for reference standard assessment were taken only from suspicious areas.
Discussion
Interpretation of study results and quality assessment
The systematic review identified a limited amount of evidence on the three adjunctive colposcopy technologies: two studies of the DySIS colposcope,6,17 one study of the LuViva Advanced Cervical Scan (Flowers et al. , unpublished) and three studies of the Niris Imaging System. 31–33
Both studies of the DySIS colposcope6,17 found that the sensitivity of DySIS was statistically significantly higher than that of conventional colposcopy for identifying CIN2+ disease, although specificity was significantly lower with DySIS. 6,17 Taking both sensitivity and specificity into account, the overall diagnostic accuracy was similar to that of conventional colposcopy. The LRs indicated that DySIS was only a fair predictor of how much a test result will change the (pre-test) odds of having CIN2+. The combination of the DSI colour-coded map and conventional colposcopy resulted in the highest result for sensitivity, although specificity was lowered further. 6 The authors did not define what was meant by ‘DSI colour-coded map and conventional colposcopy combined’, although it appears that patients were considered positive if either the DSI colour-coded map or conventional colposcopy were positive. [Note: DySIS Medical has subsequently confirmed that this assumption is correct.] It appears that this would be workable in clinical practice, with the colposcopist performing the examination using DySIS as a conventional colposcope, followed by assessment using the DSI colour-coded map.
The sensitivity of DySIS remained high in the subgroup of women referred for colposcopy with a low-grade cytology test result, whereas the sensitivity of conventional colposcopy was low in this subgroup of women. 18
In a subgroup analysis, the sensitivity of DySIS was higher in women with hrHPV16 than in women with non-16 hrHPV. Therefore, when the prevalence of hrHPV16 reduces in the screening population, as females who have been vaccinated against this strain of HPV reach the age for cervical cancer screening, DySIS sensitivity will reduce. However, the sensitivity of DySIS was still higher than that of conventional colposcopy in women with non-16 hrHPV, as well as women with hrHPV16 (Zaal et al. , unpublished).
The study of the LuViva Advanced Cervical Scan (Flowers et al., unpublished) reported higher sensitivity than the standard of care (consisting of the cytology test result, HPV test result and colposcopically directed biopsy) for identifying CIN2+ disease, although the specificity of LuViva was low. The authors of the study (Flowers et al. , unpublished) stated that the study evaluated the potential of the new technology to effectively triage women at risk for moderate and high-grade dysplasia rather than as an adjunct to colposcopy.
The most recent study33 of the Niris Imaging System was the most relevant for clinical practice because of the cut-offs used for categorising patients. This study reported a lower sensitivity for Niris for identifying CIN2+ disease than with conventional colposcopy, and a similar specificity.
From the results of our quality assessment, it appears that only the results of the DySIS study by Louwers et al. 6 can be interpreted as being both reliable and clinically applicable. The only concern with this study6 was whether conventional colposcopy was represented appropriately, although the authors pointed out that the results were similar to other studies evaluating conventional colposcopy. The authors also noted a limitation in that a second DySIS examination could not be performed after the first (the acetowhitening effect can last up to 45 minutes, which can interfere with DySIS measurements). This would restrict the use of DySIS when a repeat examination was required e.g. when only part of the transformation zone could be visualised in the first examination. For most of the other studies, the lack of clear reporting meant that the risk of bias was often ‘unclear’, although the reported issues that cast doubt on their reliability or relevance included a high dropout rate;17 use of different reference standard procedures across the population (Flowers et al. , unpublished); lack of a clinically relevant cut-off;31–32 reference standard not performed for all patients; and results not provided in real time. 33
Test accuracy may be overestimated in studies at risk of bias. 40 The STARD (STAndards for the Reporting of Diagnostic accuracy studies) statement was produced with the aim of improving the quality of reporting of diagnostic accuracy studies;41–42 although it appears so far to have had a minimal tangible effect on reporting quality, even in papers published in journals which explicitly endorse the STARD statement. 43–44 This has led to a call for authors, editors and peer reviewers to adhere to, and enforce, STARD statement guidelines. 44
Strengths and limitations
This systematic review addressed a clear research question using predefined inclusion criteria. Comprehensive literature searches were performed to locate all relevant published and unpublished studies without any language restrictions, thereby minimising the potential for publication bias and language bias. Hand-searching was also performed in order to identify additional relevant studies and the manufacturers were asked whether any other potentially relevant studies were available. Study selection was undertaken independently by two reviewers. Data extraction and quality assessment were checked by a second reviewer to minimise the potential for reviewer bias or error. The authors of studies were contacted, when necessary, for clarification of study details and for additional diagnostic accuracy data. Study quality assessment was undertaken using a validated checklist for diagnostic studies, with additional review-specific quality assessment items added. We are therefore confident that we have identified all relevant evidence and have appropriately critically appraised and synthesised the included studies.
However, the studies included in the review were clinically and methodologically heterogeneous, which meant that statistical pooling of results was not appropriate. The ways in which the studies varied, and the implications of the variation, are discussed below.
Participants
Women in England are not invited for cervical screening under the NHS Cervical Screening Programme until the age of 25 years. The studies included in the review included women aged 18 years, so the youngest women included in the studies would not be seen in practice. The mean or median age of participants was > 35 years for all studies except the study by Gallwas et al. 33 (mean age 31 years) and the study by Flowers et al. (unpublished), in which the mean age was not reported. From the data presented in the study by Flowers et al. (unpublished) it was apparent that around one-third of the participants were aged < 25 years. This limits the applicability of this study's results to an NHS setting.
The prevalence of CIN2+ varied considerably between studies, demonstrating heterogeneity between participants in the included studies. The reasons for this variation are not clear, although the inclusion criteria differed slightly between studies, and there was some variation in the setting of the included studies; the studies were conducted in the Netherlands, England and Greece, the USA, Germany, China, and the USA and the Dominican Republic. The two studies with the highest prevalence of CIN2+ were conducted in the Netherlands and Germany. 6,33
The implications of this variation in prevalence of CIN2+ on the results are that the sensitivity may be reduced in studies with a lower prevalence of CIN2+, as colposcopists who are less familiar with the characteristics of CIN2+ may be less able to recognise them on colposcopic examination.
Intervention
Some studies of both DySIS17 and the Niris Imaging System31,32 related to earlier versions of the technology, meaning that their results were of limited clinical value and/or more prone to bias; in addition, the earlier Niris studies31,32 did not use clinically appropriate cut-offs for categorising patients. In clinical practice, patient management decisions are made based on the colposcopist's impression of CIN grade and the reason for referral for colposcopy from the NHS Cervical Screening Programme.
The authors of the study of the LuViva Advanced Cervical Scan (Flowers et al. , unpublished) suggest that the intended use of the technology is to triage women for colposcopy, rather than as an adjunct to colposcopy. Therefore, this technology has a different place in the care pathway from the other technologies included in this assessment.
In clinical practice, colposcopists have access to cytology test results and are aware of other patient characteristics, such as age, etc. However, in most of the included studies it was unclear whether these data were available to colposcopists when interpreting the results of the new technology. Only two studies17,31 reported that cytology test results were available when interpreting the results of the new technology. [Note: Based on subsequent information from DySIS Medical and Imalux Corporation, four studies6,17,31,32 reported that cytology test results were available when interpreting the results of the new technology.]
Comparator
The comparators used varied across the technologies. In the studies of DySIS,6,17 the comparator was video colposcopy using the DySIS colposcope, rather than the conventional colposcopy methods and equipment used in the NHS. Therefore, the accuracy of conventional colposcopy in these studies may not be an accurate reflection of current NHS practice. In the study of the LuViva Advanced Cervical Scan (Flowers et al. , unpublished) the comparator was ‘standard of care’, which consisted of the cytology test result, HPV test result and colposcopically directed biopsy. The comparator used in the studies31–33 of the Niris Imaging System was conventional colposcopy.
The accuracy of colposcopy varied considerably between studies, which may reflect differences in colposcopic examination and biopsy procedures, the expertise of colposcopists and also the differences in prevalence of CIN2+ between studies.
Reference standard
The reference standard was histology for all of the included studies, although in the study of the LuViva Advanced Cervical Scan (Flowers et al. , unpublished) some patients also had 2-year clinical follow-up. Both of the studies of DySIS6,17 used random biopsies to assess negative colposcopy results. In the study of the LuViva Advanced Cervical Scan the histology result was based on biopsy for abnormal-looking areas and endocervical curettage when no lesion was seen on colposcopy (although some women may have had diagnostic excision biopsy), which has implications for the reliability of the reference standard. In the most clinically relevant study of the Niris Imaging System by Gallwas et al. ,33 biopsies were taken only from suspicious areas, so false-negative results would not be identified.
It is difficult to obtain a definite reference standard for patients with negative index test results; random biopsies are likely to be the most accurate, although they may miss diseased areas. Long-term follow-up may result in high dropout rates and the possibility that disease spotted at long-term follow-up began in the interim period, i.e. may not have been present at initial assessment. The LuViva study (Flowers et al. , unpublished) followed up around only half of the participants at 2 years, although the reasons for participants not receiving a 2-year follow-up were not explicit.
Outcomes
In order to re-calculate and confirm the reported results, full 2 × 2 data were required. However, these data were reported for only the adjunctive technology and the comparator in the two studies of DySIS. 6,17 Full 2 × 2 data were provided by the study authors for two further studies, on request. 31–32
All except one of the studies reported results ‘per patient’; the study of the Niris Imaging System by Gallwas et al. 33 reported results ‘per image’, meaning that not all of the data were independent observations as some women may have contributed multiple images. Furthermore, in this study it was unclear whether all participants contributed to the analysis.
As discussed earlier, in clinical practice patient management decisions are made based on the colposcopist's diagnosis and the reason for referral for colposcopy from the NHS Cervical Screening Programme. The majority of studies used the cut-off of CIN2+ for determining the sensitivity and specificity of the adjunctive technology. However, management guidelines are different for women with CIN1 from women with no CIN. 5 Therefore, the ability to distinguish between normal, CIN1, CIN2 and CIN3 is important in practice.
Conclusions
The DySIS colposcope is significantly more sensitive than conventional colposcopy for identifying CIN2+ disease, although specificity is significantly lower. The combination of the DSI colour-coded map and conventional colposcopy results in the highest sensitivity, although specificity is lowered further. Based on study quality assessment, these results are likely to be reliable.
The study of the LuViva Advanced Cervical Scan (Flowers et al. , unpublished) and the clinically relevant study of the Niris Imaging System33 contain significant biases and uncertainties; therefore, their results can not be relied on. In addition, the authors of the study of LuViva (Flowers et al. , unpublished) suggest that the intended use of the technology is to triage women for colposcopy, rather than as an adjunct to colposcopy.
Review of existing economic evaluations
Methods
Systematic searches of the literature were conducted to identify potentially relevant studies for inclusion in the assessment of cost-effectiveness (see Search strategy).
Results
The systematic literature search identified no economic evaluation studies of colposcopy or colposcopic adjuncts (DySIS, LuViva Advanced Cervical Scan and Niris Imaging System) that met the inclusion criteria for review. The searches did identify economic evaluations of HPV vaccines, screening strategies, referral strategies to colposcopy and options for managing abnormalities. None of the studies identified were found to be directly relevant to the decision problem addressed in this assessment. The main disadvantage of the studies identified was that each evaluation considered only a small part of the total treatment pathway of concern here. This was particularly evident with studies in which colposcopy was a part of the modelled treatment pathway. The accuracy of colposcopy was either assumed to be 100%45–46 or combined with the accuracy of biopsy. 47–50 However, those studies undertaking analysis from a UK perspective provided many useful inputs, described in more detail below (see Model inputs).
From the review, two UK-based evaluations were identified as potentially relevant. Each was a recent evaluation which used a Markov structure to model the costs and outcomes from a UK perspective. 46,51 The institutions were contacted to discuss the possibility of collaboration. As researchers from the University of Sheffield had recently updated their model and were undertaking updated analyses, an agreement was reached in which their most recent electronic model was provided to the External Assessment Group (EAG). This updated model has been most recently described in a graduate thesis. 52
Description of decision-analytic model
Overview
A decision-analytic model was developed to assess the cost-effectiveness of the three devices (DySIS, LuViva Advanced Cervical Scan and Niris Imaging System). It compared these with standard colposcopy for examination of the uterine cervix, for the detection of cancerous and precancerous cervical tissue in patients referred for colposcopy through the NHS Cervical Screening Programme. As a result of the weaknesses in the studies of Niris and LuViva (discussed in detail in Systematic review of clinical effectiveness), these devices were excluded in the base-case analysis. The analysis adopted the perspective of the UK NHS. The model provides a framework for the synthesis of data from the review of clinical effectiveness (see Systematic review of clinical effectiveness) and other relevant parameters.
Outcomes in the model are expressed in terms of quality-adjusted life-years (QALYs). Costs are evaluated from the perspective of the NHS and Personal Social Services, expressed in UK pounds sterling at 2011 prices. Both costs and outcomes are discounted using an annual discount rate of 3.5%, in line with current methods guidelines. 53 All stages of the work were informed by discussion with our clinical advisor and members of the specialist committee to provide feedback on specific aspects of the analysis, such as the modelling approach, data inputs and assumptions.
The following sections outline the structure of the model and provide details of the key assumptions and data sources used to populate the model.
Modelling approach
The decision-analytic model involved two stages. First, a decision tree to model the diagnostic and treatment pathways for patients referred to colposcopy from the NHS Cervical Screening Programme. Second, a Markov model, which simulates the natural history of patients and captures future cytological screening and referrals to colposcopy, to estimate the outcomes of the initial diagnosis and treatment choices. The decision tree has been developed for this appraisal, whereas the Markov model is based on a revised version of the model used by Hadwin et al. ,51 henceforth referred to as the Sheffield model. 52
Diagnostic and treatment decision tree
The diagnostic and treatment decision tree was developed to model the short-term diagnostic and treatment pathways and the outcomes of patients referred to colposcopy from the NHS Cervical Screening Programme. Patients are referred for colposcopy through the NHS Cervical Screening Programme for a variety of reasons (e.g. moderate or severe cytology). 2 For any given referral reason there is a distribution of the true underlying health states (this is discussed in further detail in Model inputs). The diagnostic treatment decision tree first allocates patients to their true underlying health state, with the distribution being dependent on their reason for referral, and then sends them down the diagnostic and treatment pathways dependent on probabilities for diagnostic accuracy, treatment and treatment effectiveness. Examples of parts of the decision trees are shown in Figure 2, showing the distribution of true underlying health state by reason for referral; Figure 3, showing the diagnostic and treatment pathways for a patient with a true underlying health state of CIN1; and Figure 4, showing the diagnostic and treatment pathways for a patient with cervical cancer.
FIGURE 2.
Distribution of true underlying health state by reason for referral.
FIGURE 3.
Diagnostic decision tree for patients with CIN1.
FIGURE 4.
Diagnostic decision tree for patients with cancer.
The decision tree captures the initial diagnosis of the patient by the colposcopist and any subsequent treatments or screening options based on their diagnosis at colposcopy and the reason for referral from the NHS Cervical Screening Programme. The effectiveness of any treatment is based on the true underlying condition of the patient. Treatment and screening options available include:
-
return to the NHS Cervical Screening Programme
-
refer for rescreen at 6 months: patients can be referred for rescreen with a cytological smear and HPV test, or can be referred for rescreen with colposcopy or adjunct
-
a diagnostic biopsy
-
a treatment biopsy
-
a treatment biopsy followed by cancer treatment.
Figure 3 shows the diagnostic and treatment decision tree for a patient whose true health state is CIN1. The patient receives an initial diagnosis by the colposcopist and can be incorrectly identified as clear, correctly identified as CIN1, incorrectly identified as CIN2/3 or incorrectly identified as having invasive cervical cancer (patients are not diagnosed as HPV+ by the colposcopist). The initial diagnosis is based on the diagnostic accuracy of the device (this is discussed in more detail in Model inputs). Following identification, the patient will be assigned to one of the five treatment and screening options discussed above (although, in Figure 3, option 5 is excluded as it is assumed that no patient with CIN1 can receive cancer therapy incorrectly, as histology resulting from the treatment biopsy is assumed to be 100% sensitive and specific). The patient's allocation to the treatment/screening option is based on the colposcopist's diagnosis and the reason for their referral for colposcopy from the NHS Cervical Screening Programme (this is discussed in more detail in Model inputs).
Patients referred for rescreen at 6 months or returned to the NHS Cervical Screening Programme without receiving treatment enter the Markov model (described in detail in Description of decision-analytic model) in the same underlying health state in which they entered the diagnostic and treatment decision tree (in the case of Figure 3, CIN1). Patients receiving diagnostic biopsy will then receive a subsequent treatment or screening option (treatment biopsy, referred for rescreen at 6 months or returned to the NHS Cervical Screening Programme) based on their true underlying histology (as diagnostic biopsy and subsequent histology is assumed to be 100% sensitive and specific). Patients receiving treatment biopsy, at the initial colposcopy, or at a subsequent colposcopy as the result of a treatment decision based on a diagnostic biopsy, will be either ‘cured’ or ‘not cured’, i.e. the treatment biopsy has a failure rate that is described in more detail later in this chapter. Those patients who are cured will be referred for rescreen at 6 months or returned to the NHS Cervical Screening Programme and will enter the Markov model in the ‘clear’ health state. Those patients who are not cured will be referred for rescreen at 6 months or returned to the NHS Cervical Screening Programme and will enter the Markov model in their original health state, in the case of Figure 3, CIN1.
In our model we have split the types of biopsies into treatment and diagnostic biopsies. Different types of biopsies may be used for either reason, but the important distinction in the model is that a diagnostic biopsy is not curative and provides further information on the patient (in the model it is assumed to be perfect information). A treatment biopsy is undertaken with curative intent. Treatment biopsy may be a LLETZ, but in some cases less invasive treatment may be used.
Figure 4 shows the diagnostic decision tree for a patient with invasive cervical cancer. Similarly to Figure 3 for patients with CIN1, the patient receives an initial diagnosis by the colposcopist and can be incorrectly identified as clear, incorrectly identified as CIN1, incorrectly identified as CIN2/3 or correctly identified as having invasive cervical cancer. In contrast with patients with CIN1, patients with invasive cervical cancer who receive a treatment biopsy will also receive appropriate cancer treatment. As a result of the cancer therapy, they can be cured and returned to the NHS Cervical Screening Programme, or referred for rescreen at 6 months, entering the Markov model as ‘clear’ but receiving a QALY decrement and cost associated with survival of cervical cancer (this is discussed in detail in Model inputs) or they will die of cancer. Those patients who die of cancer do not enter the Markov model but instead receive an expected QALY ‘pay-off’ and costs associated with dying from cancer (this is discussed in detail in Model inputs).
Natural history and screening model
The natural history and screening model is based on the Sheffield model, a revised version of the model used by Hadwin et al. 51
The natural history model consists of nine states: clear, HPV, CIN1, CIN2/3, invasive cancer stages 1, 2, 3 and 4, and death (Figure 5). Patients enter the natural history model in the state based on their outcome from the diagnostic and treatment decision tree described above. Patients were allowed to progress and regress between these states every 6 months, based on age-related transition probabilities. 47 Possible transitions during any period in the model are represented by the arrows in the figure. Although the transition probabilities in the Hadwin et al. paper51 were largely based on an earlier study,54 the revised version of the model used the probabilities from Myers et al. 47 This model allowed for the regression of CIN2/3 to the less severe states of CIN1 and clear, transitions which were not allowed for in the earlier model.
At different time points during the model, the patients will also enter a screening pathway model (shown in Figure 6). For those returned to the NHS Cervical Screening Programme, screening will take place every 3 years between the ages of 25 and 49 years, and every 5 years between the ages of 50 and 64 years. For those referred for rescreen by cytology at 6 months, this will occur 6 months after the initial colposcopy. Following cytological screening, and HPV screening where required, a patient may be re-referred for colposcopy, based on the reasons for referral for colposcopy from the NHS Cervical Screening Programme. 5 When they are re-referred for colposcopy, they re-enter the diagnostic and treatment decision tree described previously.
It should be noted that not all patients with invasive cervical cancer will be identified as a result of cytological screening or colposcopy. These patients will be missed by screening but may subsequently be identified as having cancer, as a result of their cancer becoming symptomatic. These patients would then be treated appropriately, and some will be cured, and will transition to the ‘clear’ health state in the natural history model but receive an appropriate QALY decrement and cost associated with cancer treatment, whereas some will not be cured and will die of cancer, and will exit the model immediately but receive a QALY pay-off and cost associated with cancer treatment. As previously stated, these pay-offs and decrements associated with cancer are described in detail in Model inputs.
Model inputs
Diagnostic accuracy
From the systematic review of clinical effectiveness, sensitivities and specificities for various cut-offs are provided for colposcopy and the various adjuncts (see Synthesis of the included studies). However, as discussed in the clinical effectiveness section, the studies relating to the LuViva Advanced Cervical Scan and the Niris Imaging System contain significant biases and uncertainties so their results are not reliable to use in the model. Therefore, for our primary analyses, we compare only DySIS, DySIS plus colposcopy and colposcopy alone. Table 9 details the sensitivities and specificities used in the cost-effectiveness analyses (based on the CIN2+ cut-off as described in Synthesis of the included studies). It should be noted that data here are presented in terms of probabilities rather than in percentages, as in the clinical section.
| Diagnostic device | Sensitivity | Specificity | Reference |
|---|---|---|---|
| Colposcopy alone | 0.519 | 0.817 | Louwers et al.6 |
| DySIS | 0.648 | 0.702 | Louwers et al.6 |
| DySIS +colposcopy | 0.796 | 0.626 | Louwers et al.6 |
Although sensitivities and specificities are available, the dichotomous nature of their derivation, based on the use of a CIN2+ cut-off, means these are not sufficient for the model. The model requires the probability of the diagnoses of the different stages of disease made by the new technologies or colposcopy, whether correct or otherwise, conditional on the true underlying disease status. For example, for a patient with CIN1, we need to estimate the probability that they are correctly diagnosed as CIN1, as well as the probabilities that they are incorrectly diagnosed as clear, or found to be ‘CIN2/3’ or ‘cancer’. Therefore, further assumptions are required to convert the sensitivities and specificities into the probabilities required for the model.
It should be apparent that just because a true ‘clear’ patient was found to be below CIN2+, and is therefore defined as a true-negative for the device, it does not mean that they were correctly diagnosed as ‘clear’, as they could also have been diagnosed as ‘CIN1’. Similarly, a ‘clear’ patient found to be CIN2+, so that they fall into a false-positive for the device, does not necessarily have to be found to be ‘CIN2/3’, as they could also be found to be ‘cancer’. The same issues exist for those who are true ‘CIN2/3’ or worse. Therefore, to move from sensitivities and specificities based on a CIN2+ cut-off to the probabilities required for the model some information about the distribution of diagnoses conditional on disease status and whether, based on a CIN2+ cut-off, they are true-negative, false-positive, true-positive or false-negative, is required.
Gallwas et al. 33 provide information on the probability of a particular diagnosis, based on the device conditional on the true health state as measured by histology (e.g. the probability of being diagnosed CIN1 at colposcopy conditional on being CIN1 or the probability of being diagnosed with cancer conditional on being CIN1). This information can also be used to calculate the probability of being found to be in a particular health state conditional on the true disease state and the result of a diagnostic test. For example, the probability of being found to be CIN1 conditional on the true disease state being CIN1 and the (colposcopic) diagnostic test finding them to be negative at a CIN2+ cut-off. By combining this evidence with the sensitivities and specificities (i.e. the evidence on whether they are true-negative, false-positive, true-positive or false-negative at a cut-off of CIN2+), we can calculate the required probabilities for the model. The probabilities used to convert sensitivities and specificities into model parameters are shown in Table 10. Note the assumption here is that, although the data from Gallwas et al. 33 are based on the Niris Imaging System, it is assumed that these also apply to colposcopy and the other new technologies being assessed. There are concerns with the Gallwas et al. 33 study as histology was undertaken only in patients with a suspicious lesion and thus the table below does not represent the full population. This will have the effect of underestimating the probability of a patient being diagnosed as clear for all negative (clear or CIN1) test results. This has been explored in sensitivity analyses in which we assume that all patients that are found to be negative are diagnosed as clear. Patients considered as inflamed in the study were excluded from the table as it was unclear whether they would be considered clear or CIN1.
| True health state | Result based on CIN2+ cut-off | Diagnosis based on colposcopy or new technology | Probability |
|---|---|---|---|
| Clear | True-negative | Clear | 0.935 |
| CIN1 | 0.065 | ||
| False-positive | CIN2/3 | 1.000 | |
| Cancer | 0.000 | ||
| CIN1 | True-negative | Clear | 0.255 |
| CIN1 | 0.745 | ||
| False-positive | CIN2/3 | 1.000 | |
| Cancer | 0.000 | ||
| CIN2/3 | False-negative | Clear | 0.432 |
| CIN1 | 0.568 | ||
| True-positive | CIN2/3 | 0.966 | |
| Cancer | 0.034 | ||
| Cancer | False-negative | Clear | 0.333 |
| CIN1 | 0.667 | ||
| True-positive | CIN2/3 | 0.077 | |
| Cancer | 0.923 |
For example, a patient with true underlying health state CIN1 has a probability of being found to be CIN1 by colposcopy/new technologies of 0.61. This is calculated by multiplying the probability of her being found to be below the CIN2+ threshold by the diagnostic test, the specificity (0.817), by the probability of her being identified at CIN1, given that she is CIN1 and the diagnostic test found her to be below CIN2+ (0.745).
As stated previously, and in the clinical review section (see Systematic review of clinical effectiveness), the evidence on the LuViva Advanced Cervical Scan and the Niris Imaging System contains significant biases and has, therefore, been excluded from the main analyses. Even if the evidence were considered reliable, the heterogeneity between the studies raises issues about their comparability. In the light of the unreliability of the evidence, the heterogeneity and the dearth of formal methods for mixed-treatment comparisons of diagnostic devices, no formal attempt at synthesising the studies has been made.
True health states
The initial model population consists of patients who are referred to colposcopy from the NHS Cervical Screening Programme. To model the underlying progression of the disease and the likelihood of correct diagnoses it is necessary to estimate the true underlying health states of patients entering the model. As the model population is first identified by the reason for referral, we have estimated the true health state by the reason for referral.
Data for this analysis were provided by the Northern Gynaecological Oncology Centre, Queen Elizabeth Hospital, Gateshead (hereafter referred to as the Gateshead data). All patients who visited the colposcopy clinic from 1 January 2006 to 29 November 2011 were included – 4533 patients in total. The percentage of patients in each health state was calculated by the reason for referral as described below. Patients' true health states were estimated, based either on (1) biopsy alone or (2) biopsy if available, otherwise colposcopy. For the case in which only biopsies were used to determine the true health states the population was limited to those who underwent biopsies (Table 11).
| True health state (biopsy result) | Reasons for referral | ||||||
|---|---|---|---|---|---|---|---|
| Borderline changes (%) (n = 1168) | Mild dyskaryosis (%) (n = 639) | Moderate dyskaryosis, % (n = 576) | Severe dyskaryosis (%) (n = 847) | Possible invasion (%) (n = 10) | Possible glandular neoplasia (%) (n = 122) | Inadequate (%) (n = 22) | |
| Normal | 22 | 15 | 4 | 2 | 0 | 18 | 64 |
| HPV | 40 | 30 | 7 | 2 | 10 | 27 | 32 |
| CIN1 | 22 | 32 | 11 | 2 | 0 | 9 | 5 |
| CIN2/3 | 16 | 22 | 77 | 87 | 50 | 29 | 0 |
| Cancer | 0 | 0 | 1 | 6 | 40 | 17 | 0 |
In the case of using ‘biopsy, otherwise colposcopy’ to estimate the true health state, biopsy results were used for those that underwent a biopsy and the colposcopy result was used for those who did not have a biopsy (Table 12). This provides a larger sample size but the true health state of those added to the sample is determined by the colposcopic finding, which is considered less accurate.
| True health state (biopsy, otherwise colposcopy result) | Reasons for referral | ||||||
|---|---|---|---|---|---|---|---|
| Borderline changes (%) (n = 1360) | Mild dyskaryosis (%) (n = 715) | Moderate dyskaryosis (%) (n = 633) | Severe dyskaryosis (%) (n = 917) | Possible invasion (%) (n = 11) | Possible glandular neoplasia (%) (n = 151) | Inadequate (%) (n = 141) | |
| Normal | 28 | 20 | 5 | 3 | 0 | 28 | 88 |
| HPV | 37 | 28 | 7 | 2 | 9 | 22 | 11 |
| CIN1 | 20 | 31 | 12 | 3 | 0 | 11 | 1 |
| CIN2/3 | 14 | 20 | 76 | 86 | 45 | 25 | 0 |
| Cancer | 0 | 0 | 0 | 6 | 45 | 14 | 0 |
In the data set, some patients had multiple biopsy results from a single visit. In such circumstances, the most severe result was considered the ‘true’ health state. Furthermore, in the data set the patients were separately identified as having adenocarcinoma or invasive cancer, so these diagnoses were combined to make up the cancer population within the model. The data do not indicate the stage of cancer being diagnosed; in the base case those diagnosed as adenocarcinoma or invasive cancer as a result of the screening were assumed to have stage 1 cancer, based on clinical advice that nearly all cancers identified at screening are stage 1. One limitation of the data is that they do not capture patients who underwent a biopsy under a general anaesthetic, so it is not clear whether these patients would be different from those in the current data set.
Treatment probabilities
Based on clinical advice, treatment decisions are assumed to be based on cytological and colposcopic results (Table 13). Two sets of treatment probabilities were tested in the model, the first based on clinical guidelines and clinical advice and the second based on treatment patterns from the Gateshead data. From the Gateshead data we estimated the probabilities of different treatment options for each combination of cytological and colposcopic results. In some cases, multiple cytological and colposcopic results were reported, in these cases we considered the most severe result to be that which was used for decisions.
| Reason for referral | Colposcopy or new technology results | Treatment possibilities | Guidelines and clinical advice (%) | Gateshead data (%) |
|---|---|---|---|---|
| Borderline cytology + HPV positivea | Normal | Discharge and return to normal screening | 100 | 10.7 |
| Follow-up | 0 | 15.1 | ||
| Immediate treatment – excision biopsy | 0 | 0.8 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 73.5 | ||
| Low grade | Discharge and return to normal screening | 0 | 0.2 | |
| Follow-up | 0 | 2.7 | ||
| Immediate treatment – excision biopsy | 0 | 0.9 | ||
| Biopsy, no curative intent (punch or small excision) | 100 | 96.2 | ||
| High grade | Discharge and return to normal screening | 0 | 0.6 | |
| Follow-up | 0 | 1.2 | ||
| Immediate treatment – excision biopsy | 0 | 4.9 | ||
| Biopsy, no curative intent (punch or small excision) | 100 | 93.3 | ||
| Cancer (I–IV) | Discharge and return to normal screening | 0 | DNO | |
| Follow-up | 0 | DNO | ||
| Immediate treatment – excision biopsy | 90 | DNO | ||
| Biopsy, no curative intent (punch or small excision) | 10 | DNO | ||
| Mild dyskaryosis + HPV positivea | Normal | Discharge and return to normal screening | 100 | 9.4 |
| Follow-up | 0 | 16.4 | ||
| Immediate treatment – excision biopsy | 0 | 0.5 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 73.7 | ||
| Low grade | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 4.1 | ||
| Immediate treatment – excision biopsy | 0 | 1.8 | ||
| Biopsy, no curative intent (punch or small excision) | 100 | 94.2 | ||
| High grade | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 2.4 | ||
| Immediate treatment – excision biopsy | 0 | 13.0 | ||
| Biopsy, no curative intent (punch or small excision) | 100 | 84.6 | ||
| Cancer (I–IV) | Discharge and return to normal screening | 0 | DNO | |
| Follow-up | 0 | DNO | ||
| Immediate treatment – excision biopsy | 90 | DNO | ||
| Biopsy, no curative intent (punch or small excision) | 10 | DNO | ||
| Moderate dyskaryosis | Normal | Discharge and return to normal screening | 0 | 8.6 |
| Follow-up | 100 | 28.6 | ||
| Immediate treatment – excision biopsy | 0 | 8.6 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 54.3 | ||
| Low grade | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 7.1 | ||
| Immediate treatment – excision biopsy | 0 | 11.1 | ||
| Biopsy, no curative intent (punch or small excision) | 100 | 81.7 | ||
| High grade | Discharge and return to normal screening | 0 | 1.3 | |
| Follow-up | 0 | 5.4 | ||
| Immediate treatment – excision biopsy | 80 | 84.9 | ||
| Biopsy, no curative intent (punch or small excision) | 20 | 8.4 | ||
| Cancer (I–IV) | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 0.0 | ||
| Immediate treatment – excision biopsy | 90 | 100.0 | ||
| Biopsy, no curative intent (punch or small excision) | 10 | 0.0 | ||
| Severe dyskaryosis | Normal | Discharge and return to normal screening | 0 | 0.0 |
| Follow-up | 100 | 28.6 | ||
| Immediate treatment – excision biopsy | 0 | 33.3 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 38.1 | ||
| Low grade | Discharge and return to normal screening | 0 | 1.4 | |
| Follow-up | 0 | 8.1 | ||
| Immediate treatment – excision biopsy | 70 | 33.8 | ||
| Biopsy, no curative intent (punch or small excision) | 30 | 56.8 | ||
| High grade | Discharge and return to normal screening | 0 | 1.4 | |
| Follow-up | 0 | 5.6 | ||
| Immediate treatment – excision biopsy | 100 | 88.7 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 4.3 | ||
| Cancer (I–IV) | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 0.0 | ||
| Immediate treatment – excision biopsy | 90 | 66.7 | ||
| Biopsy, no curative intent (punch or small excision) | 10 | 33.3 | ||
| Possible glandular neoplasia | Normal | Discharge and return to normal screening | 0 | 5.3 |
| Follow-up | 100 | 26.3 | ||
| Immediate treatment – excision biopsy | 0 | 31.6 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 36.8 | ||
| Low grade | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 12.9 | ||
| Immediate treatment – excision biopsy | 100 | 38.7 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 48.4 | ||
| High grade | Discharge and return to normal screening | 0 | 3.7 | |
| Follow-up | 0 | 0 | ||
| Immediate treatment – excision biopsy | 100 | 88.9 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 7.4 | ||
| Cancer (I–IV) | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 0.0 | ||
| Immediate treatment – excision biopsy | 90 | 50.0 | ||
| Biopsy, no curative intent (punch or small excision) | 10 | 50.0 | ||
| Possible invasion | Normal | Discharge and return to normal screening | 0 | DNO |
| Follow-up | 50 | DNO | ||
| Immediate treatment – excision biopsy | 50 | DNO | ||
| Biopsy, no curative intent (punch or small excision) | 0 | DNO | ||
| Low grade | Discharge and return to normal screening | 0 | DNO | |
| Follow-up | 0 | DNO | ||
| Immediate treatment – excision biopsy | 100 | DNO | ||
| Biopsy, no curative intent (punch or small excision) | 0 | DNO | ||
| High grade | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 0.0 | ||
| Immediate treatment – excision biopsy | 100 | 100.0 | ||
| Biopsy, no curative intent (punch or small excision) | 0 | 0.0 | ||
| Cancer (I–IV) | Discharge and return to normal screening | 0 | 0.0 | |
| Follow-up | 0 | 0.0 | ||
| Immediate treatment – excision biopsy | 90 | 100.0 | ||
| Biopsy, no curative intent (punch or small excision) | 10 | 0.0 |
In the Gateshead data, some of the possible combinations of cytological and colposcopic results did not occur. For instance, in patients with a cytological result of possible invasive cancer there were no cases of colposcopic results of normal or mild in the Gateshead data. Given this lack of data, we were not able to estimate treatment probabilities for some combinations of cytological and colposcopic results. In these cases, we assumed that patients in the Gateshead data would receive the treatment according to guidelines and clinical opinion.
For each combination of cytological and colposcopic results we calculated the percentage of patients receiving a treatment biopsy, diagnostic biopsy, follow-up or 3- to 5-year screening. We assumed follow-up would occur within 6 months and, in the case of cytological results of moderate, severe, possible invasion or possible glandular neoplasia and a normal colposcopic finding, we assumed this follow-up would occur after having a correlation meeting. A correlation meeting is a meeting of colposcopists and pathologists to review the cytological and colposcopic findings and determine the most appropriate next steps of treatment. Following clinical advice we assumed that a correlation meeting for a patient with moderate cytology and a normal colposcopy or severe cytology and normal colposcopy would result in 10%–30% of patients being followed up in 6 months and 70–90% returning for a diagnostic biopsy. We were also advised that a cytological result of possible glandular neoplasia followed by a normal colposcopy would result in the correlation meeting finding the need for additional diagnostic biopsies or possibly a treatment biopsy. Clinical advice suggested that 50% of patients with cytological findings of invasive cancer and normal colposcopy would have an immediate treatment biopsy, and the other 50% would be reviewed during a correlation meeting of which all were likely to result in further diagnostic testing.
Treatment effectiveness
Probability of cure from treatment biopsy
In a 2011 study by Ghaem-Maghami et al. ,55 retrospective data on 2455 consecutive women treated for CIN for the first time between 1989 and 2004 using excision were used to examine the failure rates. Failure was measured by the detection of high-grade cervical disease after treatment, defined as cytological findings of moderate dyskaryosis or more severe or histological findings of CIN2+. The median length of follow-up was 238 weeks. The authors reported that the cumulative failure rate at 10 years was 4.9% for CIN1 (n = 570), 9.8% for CIN2 (n = 886) and 10.3% for CIN3 (n = 999). From this we calculated a weighted excision failure rate of CIN2/3 as 10.1% and a total excision failure rate of 8.9% (Table 14). This estimate was higher than estimates from a 2007 meta-analysis on failure rates with excision,56 from which we calculated the total excision failure rate to be 5.78% (915/15,828).
All-cause mortality excluding cervical cancer
Mortality rates from causes other than cervical cancer were calculated using data from the Office for National Statistics (ONS)57 by subtracting the deaths due to cervical cancer (ICD-10:C53) from the total number of deaths for each age group and dividing by the UK population for each age group also from the ONS data (Table 15). 58
| Cause of mortality | Annual probability of dying from all other causes (%) | ||||
|---|---|---|---|---|---|
| Age groups (years) | All causes | Cervical cancer | Not cervical cancer | Population | |
| All ages | 255,326 | 816 | 254,510 | 28,011,900 | 0.91 |
| < 1 | 1420 | − | 1420 | 346,200 | 0.41 |
| 1–4 | 229 | − | 229 | 1,330,700 | 0.02 |
| 5–9 | 135 | − | 135 | 1,497,900 | 0.01 |
| 10–14 | 147 | − | 147 | 1,542,800 | 0.000 |
| 15–19 | 342 | − | 342 | 1,680,400 | 0.02 |
| 20–24 | 394 | 6 | 388 | 1,855,700 | 0.02 |
| 25–29 | 577 | 25 | 552 | 1,844,700 | 0.03 |
| 30–34 | 772 | 31 | 741 | 1,718,100 | 0.04 |
| 35–39 | 1363 | 50 | 1313 | 1,882,100 | 0.07 |
| 40–44 | 2259 | 56 | 2203 | 2,069,800 | 0.11 |
| 45–49 | 3351 | 61 | 3290 | 2,041,700 | 0.16 |
| 50–54 | 4807 | 74 | 4733 | 1,770,000 | 0.27 |
| 55–59 | 6744 | 58 | 6686 | 1,605,100 | 0.42 |
| 60–64 | 10,786 | 65 | 10,721 | 1,707,800 | 0.63 |
| 65–69 | 13,347 | 71 | 13,276 | 1,343,700 | 0.99 |
| 70–74 | 19,352 | 78 | 19,274 | 1,153,800 | 1.67 |
| 75–79 | 29,015 | 72 | 28,943 | 977,800 | 2.96 |
| 80–84 | 43,008 | 73 | 42,935 | 786,900 | 5.46 |
| 85–89 | 54,862 | 68 | 54,794 | 547,300 | 10.01 |
| 90+ | 62,416 | 28 | 62,388 | 309,200 | 20.18 |
Cancer mortality
In the previous versions of the model,51,52 detected cancer was assumed to have a 100% cure rate. In this version we have relaxed this assumption adding cancer mortality for detected cancer patients as described below, based on data from Cancer Research UK. 59
Stage 1 cancer is considered to be curable with the prognosis dependent on the depth and width of the cancer. Stage 1a1 is estimated to have a cure rate of 98–99%, stage 1a2 a cure rate of 95–98%, stage 1b1 a cure rate of 90–95% and stage 1b2 a cure rate of 80%. Where stage 1 is generally limited to the cervix, stage 2 cancers have spread outside the neck of the womb into the surrounding tissues, but have not spread into the muscles or ligaments that line the pelvis or to the lower part of the vagina. Stage 2a cancer has spread into the top part of the vagina and the 5-year survival rate is 70–90%; in stage 2b cancer there is further spreading and the 5-year survival rate is 60–70%. In stage 3 cancer it has spread away from the cervix into surrounding structures in the pelvic area and the 5-year survival rate is 30–50%. Stage 4 cancer has spread to other body organs outside the cervix and womb and the 5-year survival rate is 20%.
This analysis assumes that the 1-year cure rate of stage 1 cancer is 95% and that the 5% of patients who progress have the same 5-year outcomes as stage 2 patients. We also assume that patients who live beyond 5 years with higher stages of cancer are cured. The 5-year cure rates of stages 2, 3 and 4 were estimated to be 75%, 50% and 20%, respectively. For stage 3, the high end of the 5-year survival rate was chosen.
Modelling cancer outcomes
As discussed in the diagnostic and treatment decision tree section previously, patients identified with cancer are assumed to either be cured, and re-enter the model as ‘clear’, or to die as a result of cancer and exit the model immediately. For those patients who are cured, they receive a QALY decrement and cost associated with cancer treatment by cancer stage. This QALY decrement represents the QALYs as a result of cancer symptoms and treatment when compared with full health. This QALY decrement occurs immediately, although the effects that are used to calculate the decrement are assumed to have occurred over 5 years. Those patients who die receive an expected QALY pay-off and cost associated with cancer mortality. The QALY pay-off represents the QALYs that a patient who dies of cancer is expected to receive before their death although they will exit the model at the point it is determined they will die. The methods used to calculate these are described in detail below; first, for patients with cancer stages 2–4, and, second, for patients with cancer stage 1.
Cancer stages 2–4
Five-year mortality rates were identified for cancer stages 2–4. 59 Based on the assumption that mortality is distributed exponentially, survival curves were drawn for patients by stage of cancer. These curves were then separated into those patients who survived until 5 years and those who died within 5 years. For those patients who died as a result of cancer, their survival curve was converted into quality-adjusted survival, by multiplying by the associated health-related quality of life (HRQoL) given cancer stage and treatment. The quality-adjusted survival over 5 years was discounted at a rate of 3.5% to calculate the QALY pay-off for a patient who dies as a result of cancer at the point when they exit the model.
For those patients who survived, the difference between HRQoL based on being in the ‘clear’ state and HRQoL as a result of treatment of cancer by stage was calculated over 5 years. This was discounted at a rate of 3.5%, to calculate the QALY decrement as a result of cancer and cancer treatment. In the scenario analyses, the length of time a patient experiences a reduction in HRQoL as a result of cancer and cancer treatment was varied.
Cancer stage 1
For cancer stage 1, 5-year survival probabilities were not available as a result of the low mortality associated with the disease if caught at an early stage. Instead, the probability of being cured was identified. For those patients who are cured, the QALY decrement associated with cancer treatment was calculated in a similar way as for patients with cancer stages 2–4, although the difference was only calculated over 1 year rather than 5 years (i.e. the difference between HRQoL based on being in the ‘clear’ state and HRQoL as a result of treatment of cancer by stage was calculated over 1 year). Those patients who were not cured were assumed to progress immediately to cancer stage 2 with its associated mortality and HRQoL decrements. Therefore, a proportion who were not cured were assumed to survive cancer stage 2 and receive the HRQoL decrement described above, and the rest were assumed to die as a result of cancer stage 2 and receive the QALY pay-off described above.
Full details of the cancer outcomes are provided in Table 16.
| Outcomes | Cancer stage | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| QALY decrement for those who survivea | 0.0495737 | 0.3707 | 0.3707 | 1.2973 |
| QALY pay-off for those who dieb | N/A | 2.079227 | 1.953032 | 1.276931 |
| Cost of cancer treatment (£) | 13,920.37 | 22,930.51 | 22,779.62 | 24,244.24 |
Health-related quality of life and quality-adjusted life-year decrements
The QALYs in the current model are those published previously in the Sheffield model,52 which had been previously used in other models. 60–62 Changes have been made to the HRQoL and QALY inputs as described below. HRQoL refers to the patient's health measured on an interval scale, where ‘0’ represents death and ‘1’ represents perfect health. QALY estimates combine both HRQoL of health states and the time spent in those health states, with 1 QALY representing a year in perfect health. A QALY decrement is the decrease in the HRQoL over a set time period converted into lost QALYs.
Quality-adjusted life-year decrement of colposcopy
Previously the authors used an estimate of 0.03 for the QALY decrement of undergoing a colposcopy and the associated treatment, which may or may not include a biopsy. In the current model it is important to consider the possible health improvements of avoiding biopsies with more accurate colposcopies. We use the following data to separate the QALY decrement associated with a colposcopy and that associated with a biopsy. In a 2003 time trade-off analysis, the authors report the HRQoL of ‘three repeat Pap smears’ to be 0.958 and the HRQoL of an ‘immediate colposcopy with no pathology’ to be 0.927, and they estimate the difference to be 0.031 (95% CI 0.007 to 0.055). 63 The difference of 0.031 is, therefore, used in the model as the QALY decrement associated with a colposcopy.
Quality-adjusted life-year decrement of biopsy
The authors also report the HRQoL of a ‘cone biopsy after immediate colposcopy’ to be 0.922. 63 Therefore, we use the difference between the colposcopy with no pathology and colposcopy with a cone biopsy to estimate the HRQoL decrement of a biopsy, which is 0.005. We assume the HRQoL decrement lasts for 1 year and thus a QALY decrement of 0.005 or 1.8 healthy days associated with biopsy. We use 0.005 in the model as the QALY decrement for both diagnostic and treatment biopsies, as we do not have differential HRQoL estimates. This assumption may be important as it may underestimate the negative health effects of a treatment biopsy. The TOMBOLA (Trial of Management of Borderline and Other Low-Grade Abnormal Smears) study64 suggests that LLETZ compared with biopsy resulted in more pain (67% vs 53%), more discharge (63% vs 46%) and more bleeding (87% vs 79%). Not only did these events occur more often, but also bleeding and discharge were reported to have a significantly longer duration. 64
In a 2008 meta-analysis of adverse pregnancy outcomes associated with treatment of CIN the authors report that LLETZ was not associated with a significant increase in adverse pregnancy outcomes. 64 Although six out of seven studies suggested a positive but non-significant association with perinatal mortality, five of these compared patients with LLETZ with healthy control subjects, patients without CIN. These studies were also very small and had up to three events. In the one study that compared LLETZ with a population of patients with CIN, the relative risk of adverse pregnancy outcomes was 1.08 (95% CI 0.65 to 1.80), in a study with 2273 events. 65 This result suggests that there is no additional risk of adverse pregnancy outcomes associated with LLETZ. This is an important comparison to help clarify whether there is an additional risk of LLETZ, as the authors report that patients with CIN are known to have an increased risk of adverse obstetric characteristics. 65 As described previously treatment biopsy includes all types of biopsies used for treatment including LLETZ and small excision biopsies.
The QALY decrement associated with biopsy was explored in a sensitivity analysis owing to the uncertainty around this parameter.
Quality-adjusted life-year decrement of cytology
In the previous version of the model the QALY decrement associated with cytology was 0.02. This represents 1 week of life and was much higher than the QALY decrement for biopsy. This seems implausible so we searched other sources for the disutility of cytology. We decided on a QALY decrement of 0.0016 or a disutility of 0.02 over 1 month as was used in other models. 49
Health-related quality of life of underlying true health states
The previous version of the model also used different HRQoL values for the clear, HPV, CIN1 and CIN2/3 health states, with HRQoL scores of 1 (i.e. perfect health) for those who were clear or had HPV, whereas CIN1 had a score of 0.91 and CIN2/3 a score of 0.87. The model assumes that clear and patients with HPV are in perfect health, whereas the other HRQoL scores are based on the study data of Insinga et al. 62 and Chuck. 45 However, given CIN1 and CIN2/3 health states are considered to be asymptomatic, in the base case it was assumed that all patients who were clear, HPV, CIN1 or CIN2/3 would have the same HRQoL (Table 17).
| Health state | HRQoL score | Source |
|---|---|---|
| Clear | 0.91 | Insinga et al.62 and assumptions |
| HPV | 0.91 | Insinga et al.62 and assumptions |
| CIN1 | 0.91 | Insinga et al.62 |
| CIN2/3 | 0.91 | Insinga et al.62 and assumptions |
| Cancer stage 1 | 0.65 | Chuck45 |
| Cancer stage 1 with treatment | 0.86 | Chuck45 |
| Cancer stage 2 | 0.67 | Chuck45 |
| Cancer stage 2 with treatment | 0.83 | Chuck45 |
| Cancer stage 3 | 0.56 | Chuck45 |
| Cancer stage 3 with treatment | 0.83 | Chuck45 |
| Cancer stage 4 | 0.48 | Chuck45 |
| Cancer stage 4 with treatment | 0.63 | Chuck45 |
Costs
An estimate of the average cost per procedure of each of the technologies being assessed is required for a cost-effectiveness analysis. The average cost of a procedure is determined by the set-up cost, annual recurring costs and per patient costs. The set-up costs consist of the capital cost of the machine. The recurring costs consist of the annual maintenance costs and the costs involved in replacing equipment and overheads. Per patient costs consist of the consumables utilised for each procedure and of the cost of staff required.
Information provided by the manufacturers has been used to estimate the costs of each of the technologies being assessed. The purchase price and maintenance costs of colposcopy were provided by clinical advisors (Table 18).
| Cost component | Colposcopy | DySIS | DySIS + colposcopy | LuViva | Niris |
|---|---|---|---|---|---|
| Assumed useful life of the equipment (years) | 15 | 5 | 5 | 5 | 10 |
| Purchase price (£) | 10,000 | 20,000 | 20,000 | 11,500 | 37,769 |
| Equivalent annual cost (£)a | 839 | 4280 | 4280 | 2461 | 4388 |
| Annual maintenance costs (£) | 1000 | 1600 | 1600 | 160 | 0 |
| Other cost (per patient) (£) | 0 | 0 | 0 | 3.50 | 3.50 |
| Disposables (per patient) (£) | 2.00 | 3.50 | 3.50 | 17.25 | 33.19 |
| Total cost per patient (£)b | 3.50 | 8.29 | 8.29 | 22.88 | 40.26 |
The purchase price of each technology was annuitised over the expected lifetime of the technology. Clinical advisors estimated the lifetime of a colposcope to be 15–20 years. The lifetime of the LuViva and DySIS devices were estimated to be 5 years and the Niris device to be 7–10 years by their manufacturers. In the base case we assumed the useful life of the colposcope to be 15 years, DySIS and LuViva to be 5 years, and that of Niris to be 10 years. The equivalent annual cost was calculated from the purchase price of the technology and the useful life of the equipment using the discount rate of costs of 3.5%.
The annual maintenance cost of the colposcope is 10% of the purchase price as suggested by the clinical advisors. The per-patient cost of a speculum was estimated to be £2. The annual maintenance costs and disposable costs of the adjunct technologies were provided by the manufacturers (see Table 18).
As the LuViva and Niris trials both used colposcopy to guide the probe or to confirm diagnosis, the cost of the colposcope was also added to their total costs.
To estimate the total cost per patient, it was necessary to know the number of patients expected to be treated each year in order to distribute the fixed costs across the patients. We requested the number of patients managed on a single colposcope from our clinical advisors. The average across available responses from the clinical advisors was 1229 patients per device per year.
To capture the additional costs of a colposcopy visit, treatment costs from the TOMBOLA study were used as provided by a personal communication with Professor Dave Whynes (lead economist in that study) (Table 19). 66 These costs were inflated to 2011 prices. The additional cost of a diagnostic biopsy was estimated to be £20.28 and the additional cost of treatment biopsy to be £97.16. As the TOMBOLA cost of a colposcopy examination includes the cost of the colposcope, the colposcopy costs as calculated in Table 18 were subtracted from the inflated estimates from the TOMBOLA trial (see Table 19) to estimate the cost of an examination excluding normal colposcope costs.
| Treatment | Unit costs (£) | Costs inflated to 2011 prices (£) |
|---|---|---|
| Colposcopy examination only | 111.44 | 132.40 |
| Colposcopy with biopsya | 130.19 | 152.68 |
| Colposcopy with LLETZb | 193.22 | 229.57 |
To calculate the total cost of each examination by device, the per-patient cost of each device, as calculated in Table 18, was added to the cost of an examination excluding normal colposcope costs, £128.90, which is the cost of the colposcopy examination, £132.40, less the cost of the colposcope and disposables, £3.50 (Table 20).
| Device | Cost used in model (£) |
|---|---|
| Colposcopy | 132.40 (128.90 + 3.50) |
| DySIS alone | 137.19 |
| DySIS plus colposcopy | 137.19 |
| LuViva | 151.78 |
| Niris | 169.16 |
The model does not consider the additional cost of a correlation meeting.
Cancer costs by stage were taken from published UK sources and inflated to 2011 prices (Table 21). 67
| Cancer stage | Cost used in model (£) |
|---|---|
| 1 | 14,304 |
| 2 | 23,562 |
| 3 | 23,407 |
| 4 | 24,912 |
Analyses
Below, we summarise the analyses undertaken for the report. All analyses are conducted separately for each reason for referral and then a weighted average of cost-effectiveness is reported across all reasons for referral.
The characteristics of the base case are as follows:
-
Patients entered the model at the age of 36 years (the average age of those referred for colposcopy from the NHS Cervical Screening Programme).
-
Treatment probabilities were based on guidelines and clinical advice.
-
The distribution of underlying health states was based on Kelly et al. 68 for those referred for borderline plus HPV+ and mild plus HPV+, and on biopsy data from Gateshead for the other reasons for referral.
-
HRQoL scores were based on the Eggington study69 and the assumption that there was no differential HRQoL between clear, HPV, CIN1 and CIN2/3 (see Health-related quality of life and quality-adjusted life-year decrements).
-
Duration of the HRQoL decrement as a result of cancer was assumed to be 1 year for stage 1 and 5 years for stages 2, 3 and 4.
-
No patients were lost to follow-up.
All of the other scenarios considered used the same assumptions and parameter values as the base case unless stated. For the scenario analyses we considered the following variations to our assumptions:
-
The patient's age (25 and 45 years old).
-
The duration of the HRQoL decrement as a result of cancer for stages 2, 3 and 4 (1 year's and 3 years' duration).
-
Cancer treatment costs (50% lower and higher).
-
The HRQoL estimates from the Sheffield model were used (i.e. clear and HPV states were assumed to be in perfect health).
-
The QALY decrement associated with treatment biopsy was varied (increased by 200%, 500% and 2000%).
-
The QALY decrement associated with cytological screening was varied (increased and decreased by 50%).
-
Alternative costs of a colposcope were used (£5000 and £20,000).
-
Alternative treatment probabilities were used (based on the Gateshead data).
-
Patients testing negative by colposcopy or adjuncts would be diagnosed as clear.
Key assumptions for modelling and inputs
A number of key assumptions have been made in the decision-analytic model and these are listed below.
-
Treatment and screening decisions are based on the reason for referral for colposcopy and the colposcopist's findings at that examination only (i.e. for those patients re-entering the diagnostic and treatment decision tree, prior history plays no part in the diagnosis and treatment).
-
Patients referred to 6-month cytological rescreen after colposcopy require only one inadequate test to be referred again to colposcopy, unlike those on the NHS Cervical Screening Programme who require three inadequate tests.
-
Cancer patients with stage 2, 3 or 4 who survive are assumed to receive treatment for 5 years and therefore incur the associated decrements in HRQoL.
-
All cancer patients who die as a result of cancer exit the model immediately and receive a QALY pay-off and cost associated with cancer mortality.
-
All patients who survive 5 years after cancer treatment are assumed to be cured.
-
All patients attend cytology and colposcopy; there is no loss to follow-up.
-
Patients who are cured of CIN have the same risk of future CIN as the general population.
-
The Gallwas et al. study33 used to convert sensitivities and specificities into the required probabilities of the model is reasonable for all the technologies.
Cost-effectiveness results
The base-case analysis compares only DySIS or DySIS plus colposcopy to colposcopy alone for each reason for referral and for the whole population, because of the lack of reliable evidence for the LuViva and Niris devices. In the sensitivity analyses undertaken on the base case, it was determined that the consequences of treatment biopsy required further exploration.
A secondary analysis was also undertaken assuming a higher QALY decrement and cost for treatment biopsy, as this was shown to be of importance in the model. This secondary analysis was also combined with each of the sensitivity analyses previously described (see Analyses).
The whole population was a weighted average of the results of each reason for referral. These estimates were based on data from the NHS Cervical Screening Programme,2 together with Kelly et al. ,68 to account for the reduced numbers of borderline and mild patients as a result of the introduction of HPV triage. The weighted population analyses are 51.3% borderline + HPV, 30.1% mild dyskaryosis + HPV, 8.2% moderate dyskaryosis, 9.3% severe dyskaryosis, 0.4% possible invasion and 0.7% possible glandular neoplasia.
As a result of the unreliable data for Niris and LuViva, an indicative analysis was undertaken to test the needed sensitivity to be considered cost-effective given their reported costs and an assumed specificity.
Results of the base-case analysis
In most instances colposcopy alone was dominated by DySIS or DySIS plus colposcopy (Table 22). In other words, colposcopy alone had worse expected outcomes in terms of QALYs and was more costly than either of the DySIS arms. In the case of patients referred for possible invasion, possible neoplasia or inadequate screens, DySIS was more cost-effective than colposcopy alone, as long as the cost-effectiveness threshold was at least £2000 per additional QALY. However, in these cases, colposcopy was still dominated by DySIS plus colposcopy. For all reasons for referral, DySIS alone was more costly and less effective than (dominated by) DySIS plus colposcopy. Therefore, the base case indicates that DySIS plus colposcopy was the cost-effective form of management conditional on the assumptions and evidence used.
| Reasons for referral | Borderline + HPV | Mild + HPV | Moderate | Severe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER | Costs (£) | QALYs | ICER | Costs (£) | QALYs | ICER | Costs (£) | QALYs | ICER | ||
| Reasons for Referral | Possible invasion | Possible neoplasia | 3 × inadequate | Whole populationa | |||||||||
| Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | ||
| Colposcopy vs DySIS alone | |||||||||||||
| Colposcopy | 1188.55 | 20.40278 | 1223.09 | 20.39607 | 1106.85 | 20.45252 | 1754.00 | 20.45968 | |||||
| DySIS alone | 1163.45 | 20.41037 | Dominant | 1192.53 | 20.40468 | Dominant | 1071.80 | 20.46103 | Dominant | 1739.28 | 20.46442 | Dominant | |
| Colposcopy vs DySIS + colposcopy | |||||||||||||
| Colposcopy | 1188.55 | 20.40278 | 1223.09 | 20.39607 | 1106.85 | 20.45252 | 1754.00 | 20.45968 | |||||
| DySIS + colposcopy | 1131.10 | 20.41738 | Dominant | 1155.54 | 20.41249 | Dominant | 1031.45 | 20.46903 | Dominant | 1716.98 | 20.46942 | Dominant | |
| Colposcopy vs DySIS alone | |||||||||||||
| Colposcopy | 6500.85 | 20.34731 | 3313.68 | 20.41361 | 753.02 | 20.47523 | 1313.59 | 20.41339 | |||||
| DySIS alone | 6501.71 | 20.34877 | 592.59 | 3316.53 | 20.41546 | 1545.34 | 755.20 | 20.47653 | 1687.09 | 1287.18 | 20.42098 | Dominant | |
| Colposcopy vs DySIS + colposcopy | |||||||||||||
| Colposcopy | 6500.85 | 20.34731 | 3313.68 | 20.41361 | 753.02 | 20.47523 | 1313.59 | 20.41339 | |||||
| DySIS + colposcopy | 6496.13 | 20.35026 | Dominant | 3312.33 | 20.41711 | Dominant | 751.27 | 20.47768 | Dominant | 1254.00 | 20.42805 | Dominant | |
The scenario analyses described above were undertaken (see Appendix 6). Overall, colposcopy alone had higher costs and lower health outcomes than DySIS or DySIS plus colposcopy for all sensitivity analyses undertaken. The least cost-effective result occurred when colposcopy alone was compared with DySIS alone for patients who were referred because of inadequate cytology. For a population of 25-year-old patients, DySIS alone cost £13,614 per additional QALY compared with colposcopy alone. For all reasons for referral and for all sensitivity analyses, DySIS or DySIS plus colposcopy was cost-effective compared with colposcopy alone as long as the cost-effectiveness threshold was at least £15,000 per QALY.
The base-case results (see Table 22) also demonstrated that patients referred with possible invasion had the highest expected costs and worst expected outcomes. Patients referred with inadequate screens had the lowest costs and the best outcomes. Patients referred with borderline/mild cytology and HPV+ had slightly higher costs and worse outcomes than patients referred with moderate/severe cytology. The model suggests this was a result of the difference in treatment patterns between the two groups. More severe patients underwent treatment biopsy which in the model was very effective and had low additional costs (£97) and low QALY decrement (0.005). Less-severe patients returned for multiple treatments, which increased the costs by £132.40 per visit, while they remained at risk of cancers that went undetected. Also, each cytological test and colposcopy visit was associated with a QALY decrement of 0.0016 and 0.03, respectively. This effect will be further magnified by patients being lost to follow-up, which has not been considered in the model.
Further sensitivity analysis demonstrated that, using the base-case inputs, an increase in a diagnostic device's specificities resulted in worse outcomes. This suggests that it is better to falsely identify patients as CIN2/3 than to find that they are truly CIN1. This occurs because of the difference in treatments for each diagnosis. This result suggests that, as above, given the low additional costs and low QALY decrement of treatment biopsy, it may be a better treatment option for patient with CIN1s than watchful waiting. Further sensitivity analysis was, therefore, undertaken to determine which inputs could be changed in the model to ensure that an increase in specificity resulted in improved outcomes. Three model inputs were identified as important.
-
QALY decrement of treatment biopsy
-
cost of treatment biopsy
-
treatment patterns.
These three inputs were tested to determine the threshold of each input at which an increase in specificity for a given management option would improve outcomes. It was found that the QALY decrement of treatment biopsy would have to be increased from 0.005 (see Health-related quality of life and quality-adjusted life-year decrements) to 0.13 (or 47.5 days of healthy life), the cost of treatment biopsy would have to be increased from £97 (see Costs) to £2758 or treatment patterns would have to include treatment biopsy of CIN1.
Results of the secondary analyses
Separate secondary analyses were undertaken for the scenarios in which the QALY decrement of treatment biopsy is 0.13 (from 0.005 in the base case) or the cost of treatment biopsy is £2758 (from £97 in the base case). At these values the model generates improved outcomes as the specificity of a given management option is increased.
In the case of increasing the QALY decrement associated with treatment biopsy, the results of the overall analysis suggested that colposcopy alone is more costly and less effective than (i.e. is dominated by) both DySIS alone and DySIS plus colposcopy (Table 23) for the overall (weighted) population.
| Reasons for referral | Borderline + HPV | Mild + HPV | Moderate | Severe | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER | Costs (£) | QALYs | ICER | Costs (£) | QALYs | ICER | Costs (£) | QALYs | ICER | |
| Reasons for Referral | Possible invasion | Possible neoplasia | 3 × inadequate | Whole populationa | ||||||||
| Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | |
| Colposcopy vs DySIS alone | ||||||||||||
| Colposcopy | 1188.55 | 20.35130 | 1223.09 | 20.33886 | 1106.85 | 20.32422 | 1754.00 | 20.31718 | ||||
| DySIS alone | 1163.45 | 20.35538 | Dominant | 1192.53 | 20.34380 | Dominant | 1071.80 | 20.32932 | Dominant | 1739.28 | 20.32049 | Dominant |
| Colposcopy vs DySIS + colposcopy | ||||||||||||
| Colposcopy | 1188.55 | 20.35130 | 1223.09 | 20.33886 | 1106.85 | 20.32422 | 1754.00 | 20.31718 | ||||
| DySIS + colposcopy | 1131.10 | 20.35991 | Dominant | 1155.54 | 20.34901 | Dominant | 1031.45 | 20.33499 | Dominant | 1716.98 | 20.32451 | Dominant |
| Colposcopy vs DySIS alone | ||||||||||||
| Colposcopy | 6500.85 | 20.20776 | 3313.68 | 20.29134 | 753.02 | 20.46303 | 1313.59 | 20.33799 | ||||
| DySIS alone | 6501.71 | 20.20771 | Dominated | 3316.53 | 20.28840 | Dominated | 755.20 | 20.46282 | Dominated | 1287.18 | 20.34230 | Dominant |
| Colposcopy vs DySIS + colposcopy | ||||||||||||
| Colposcopy | 6500.85 | 20.20776 | 3313.68 | 20.29134 | 753.02 | 20.46303 | 1313.59 | 20.33799 | ||||
| DySIS + Colposcopy | 6496.13 | 20.20819 | Dominant | 3312.33 | 20.28688 | 303.35 | 751.27 | 20.46297 | 32,008.73 | 1254.00 | 20.34705 | Dominant |
This was also the case for most of the individual referral groups. The only exceptions were that, in the case of colposcopy compared with DySIS alone, DySIS alone was found to be dominated in patients referred with possible neoplasia, possible invasion and three inadequate cytology tests. In the case of colposcopy compared with DySIS plus colposcopy, DySIS plus colposcopy was found to be less costly and less effective than colposcopy in patients referred with possible neoplasia and three inadequate cytology tests. In the former referral group, the incremental cost-effectiveness ratio (ICER) for colposcopy alone was £303, suggesting that colposcopy alone is cost-effective. In the latter referral group, the ICER for colposcopy alone compared with DySIS plus colposcopy was £32,009, which is above NICE's conventional cost-effectiveness threshold (£20,000–£30,000 per QALY gained) and suggests that DySIS plus colposcopy is the cost-effective option.
The sensitivity analyses of this secondary analysis show that although both DySIS comparators are not always cost-effective in the possible invasion and possible neoplasia referral groups and, in some scenarios are dominated, in the overall weighted population both DySIS comparators were found to be less costly and more effective than colposcopy alone (see Appendix 6). The intuition for this is discussed further below (see Discussion).
A further sensitivity analysis was conducted to establish the QALY decrement with treatment biopsy, which would result in DySIS plus colposcopy having an ICER compared with colposcopy alone of £20,000 and £30,000, respectively (i.e. the QALY decrements at which the combined form of management would potentially no longer be cost-effective). It was established that the QALY decrement would have to be 0.38 or 139 healthy days (rather than 0.005 or 1.8 healthy days in the base case, and 0.13 or 47 healthy days in the secondary analysis) for DySIS alone not to be cost-effective compared with colposcopy alone at a £20,000 per QALY threshold. If the QALY decrement is 0.42 or 153 healthy days then DySIS plus colposcopy is not cost-effective compared with colposcopy alone at the £20,000 per QALY threshold.
In the case of increasing the cost of treatment biopsy to £2758 (from £97 in the base case) the results of the overall (weighted) analysis suggested that colposcopy alone was less costly and less effective than both DySIS alone and DySIS plus colposcopy (Table 24). DySIS alone resulted in £13,808 per additional QALY compared with colposcopy alone and DySIS plus colposcopy resulted in £12,761 per additional QALY, suggesting that both DySIS-based strategies were cost-effective compared with colposcopy at standard cost-effectiveness thresholds. The cost-effectiveness between referral groups varied widely with DySIS alone costing £74,876 per additional QALY compared with colposcopy alone in those patients referred for possible neoplasia. All comparisons with colposcopy alone in referral groups of moderate, severe, possible invasion, possible neoplasia and inadequate cytology produced cost-effectiveness results greater than £25,000 per additional QALY. However, both DySIS-based strategies were cost-effective in the referral groups borderline + HPV and mild + HPV, which comprise 51.3% and 30.1% of the modelled population, respectively.
| Reasons for referral | Borderline + HPV | Mild + HPV | Moderate | Severe | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | |
| Reasons for referral | Possible invasion | Possible neoplasia | 3 × inadequate | Whole populationa | ||||||||
| Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | Costs | QALYs | ICER | |
| Colposcopy vs DySIS alone | ||||||||||||
| Colposcopy | 1686.37 | 20.40278 | 1781.46 | 20.39607 | 2432.19 | 20.45252 | 3829.41 | 20.45968 | ||||
| DySIS alone | 1747.22 | 20.41037 | 8023.00 | 1841.51 | 20.40468 | 6977.35 | 2692.37 | 20.46103 | 30,568.11 | 4046.35 | 20.46442 | 45,852.74 |
| Colposcopy vs DySIS + colposcopy | ||||||||||||
| Colposcopy | 1686.37 | 20.40278 | 1781.46 | 20.39607 | 2432.19 | 20.45252 | 3829.41 | 20.45968 | ||||
| DySIS + colposcopy | 1776.64 | 20.41738 | 6181.54 | 1869.32 | 20.41249 | 5351.36 | 2951.27 | 20.46903 | 31,437.41 | 4274.52 | 20.46942 | 45,714.04 |
| Colposcopy vs DySIS alone | ||||||||||||
| Colposcopy | 8957.49 | 20.34731 | 5553.71 | 20.41361 | 933.31 | 20.47523 | 2161.56 | 20.41339 | ||||
| DySIS alone | 9049.45 | 20.34877 | 63,055.50 | 5691.59 | 20.41546 | 74,876.31 | 975.73 | 20.47653 | 32,725.11 | 2266.39 | 20.42098 | 13,807.91 |
| Colposcopy vs DySIS + colposcopy | ||||||||||||
| Colposcopy | 8957.49 | 20.34731 | 5553.71 | 20.41361 | 933.31 | 20.47523 | 2161.56 | 20.41339 | ||||
| DySIS + colposcopy | 9133.52 | 20.35026 | 59,664.66 | 5794.90 | 20.41711 | 68,952.75 | 1002.11 | 20.47768 | 28,090.58 | 2348.62 | 20.42805 | 12,760.81 |
The sensitivity analyses of this secondary analysis shows that both DySIS comparators are cost-effective in the overall weighted population in all sensitivity analyses undertaken at a threshold of £10,000 per QALY. Although the DySIS comparators were not always cost-effective in each of the individual referral groups, they were always more effective than colposcopy alone (see Appendix 7).
A further sensitivity analysis was conducted to establish the cost of treatment biopsy which would result in DySIS plus colposcopy having an ICER compared with colposcopy alone of £20,000 and £30,000, respectively (i.e. the cost at which the combined form of management would potentially no longer be cost-effective). It was established that the cost would have to be £7698 (rather than £97 in the base case and £2758 in the secondary analysis) for DySIS alone not to be cost-effective compared with colposcopy alone at a £20,000 per QALY threshold, and £8912 for DySIS plus colposcopy not to be cost-effective at a £20,000 per QALY threshold. The cost of treatment biopsy would have to be £11,068 to find DySIS alone compared with colposcopy alone not cost-effective at a £30,000 per QALY threshold and £12,695 to find DySIS plus colposcopy not cost-effective compared with colposcopy alone at a £30,000 per QALY threshold.
Indicative analysis of LuViva and Niris
Two further analyses were undertaken based only on the costs of the LuViva Advanced Cervical Scan and the Niris Imaging System. Owing to the unreliability of the evidence on these devices, these analyses are indicative only and should be interpreted with caution. Given the costs of LuViva and assuming the same specificity as DySIS plus colposcopy, the sensitivity of LuViva would have to be 83% to be considered cost-effective at £20,000 per QALY compared with DySIS plus colposcopy. Given the costs of Niris and assuming the same specificity as DySIS plus colposcopy, the sensitivity of Niris would have to be 86% to be considered cost-effective at £20,000 per QALY compared with DySIS plus colposcopy. DySIS plus colposcopy was chosen as the comparator, as it was found to be the cost-effective option at a £20,000 per QALY threshold in the base case.
It should be emphasised that this evaluation is not comparable to the sensitivities reported above (see Synthesis of the included studies). The evaluation provides the sensitivities of Niris and LuViva needed to be cost-effective assuming that they are being used in a population similar to that used for the DySIS studies. The previous quality assessments reported above (see Quality of research available) make it clear that these studies are not comparable. Similarly, issues exist for the specificities thus in this analysis we have assumed that it will be the same as DySIS plus colposcopy. It is unclear how reasonable this assumption is.
Discussion
The literature review did not identify any cost-effectiveness analyses of colposcopy or of any of the adjunctive technologies. However, economic evaluations of other parts of the management pathway did inform the current evaluation. In particular, an economic model developed at the University of Sheffield, which evaluated the cost-effectiveness of HPV testing triage of women with low-grade abnormal cervical smears, was available for adaptation and was further developed by the EAG. The new model allowed for the comparison of colposcopy with other similar diagnostics and was based on the sensitivity and specificity of each device, as well as the costs of the device and its consumables. Given current practice, treatment was determined by the reason for referral and the results of the colposcopy or adjunct. In the diagnosis of CIN1, watchful waiting was practiced but in the case of CIN2/3 the patients were more likely to receive a treatment biopsy. The results of treatment and the future chances of detection, whether from colposcopic follow-up or from routine screening, determined the future risk of cervical cancer.
The underlying progression in the model along with many of the model inputs were used in the previous economic analyses. However, the EAG updated the model to incorporate treatment decisions based on cytological and colposcopic findings, the effectiveness of cancer treatment, the QALY decrement of biopsy, and the fixed and variable costs of colposcopy and the new technologies. Data were lacking for the long-term costs and consequences of a treatment biopsy, and the model did not incorporate the long-term costs of treatment biopsy; this was considered an important variable, as it influenced the direction of effect of the specificities of the diagnostic technologies.
Sufficient data were available to compare DySIS alone and DySIS plus colposcopy with colposcopy alone. The base-case analysis suggests that both DySIS management options dominate (i.e. are less costly and more effective than) colposcopy alone in the overall weighted population. In the few instances where DySIS alone did not dominate colposcopy alone, the ICERs were £593, £1545 or £1687 per QALY for the referral groups ‘possible invasion’, ‘possible glandular neoplasia’ or ‘inadequate cytology’, respectively. For all reasons for referral, DySIS plus colposcopy is less costly and more effective than DySIS alone. The results of the overall weighted population were robust to the ranges tested in the sensitivity analysis; the highest ICER was £13,614 per QALY in the inadequate cytology referral group in a 25-year-old population comparing DySIS alone with colposcopy alone.
In the base-case analysis, increasing the specificity of a given technology had the effect of lowering its predicted health outcomes and worsening its cost-effectiveness. Three important variables were identified as influencing the direction of effect of specificity:
-
QALY decrement of treatment biopsy
-
costs of treatment biopsy
-
treatment patterns of CIN1.
All of these inputs worked on the same premise that watchful waiting of CIN1 is only appropriate if the costs and health outcomes of a treatment biopsy outweigh the additional costs of follow-up and the risk of developing cancer from being misdiagnosed in the future. In the base case, the QALY decrement and the costs associated with treatment biopsy suggested that it was better to treat CIN1 with a treatment biopsy. This may be a genuine insight of the model but it may also reflect that the cost and QALY decrement in the model were too low. Scenario analyses were undertaken to determine the QALY decrement or cost of treatment biopsy necessary for specificity to have a positive effect on health outcomes and cost-effectiveness. In both cases these values were much higher than those used in the model. The QALY decrement of the treatment biopsy would have to increase to 0.13 from 0.005 and the cost of the treatment biopsy would have to increase to £2758 from £97. These parameter values are much larger than those used in the base case and may be implausible (a 2500% increase in the QALY decrement of the treatment biopsy or a 2700% increase in the cost of treatment biopsy). These parameters suggest that treatment biopsy would result in a loss of 45 days of life. However, it is possible that both the QALY decrement and the cost of treatment biopsy are simultaneously higher in which case they would both work in the same direction, and there are multiple combinations that would change the effect of specificity. More accurate estimates of both of these inputs would allow us to make more precise estimates of the cost-effectiveness of the alternative technologies, but only at extreme values would either of these inputs in isolation change the conclusion of the modelling that DySIS is cost-effective compared with colposcopy.
In the secondary analyses, colposcopy was more costly and less effective than either DySIS option in the overall weighted population. However, under some assumptions, neither DySIS option was cost-effective for some of the referral groups. This was the case when the cost of treatment biopsy was increased to £2758. Under this assumption, only borderline-plus-HPV and mild-plus-HPV groups were considered cost-effective. DySIS alone compared with colposcopy alone was £8023 and £6977 per QALY in the borderline-plus-HPV and mild-plus-HPV groups, respectively. DySIS plus colposcopy compared with colposcopy alone was £6182 and £5351 per QALY in the borderline-plus-HPV and mild-plus-HPV groups, respectively. As these groups constituted > 80% of the population overall, both DySIS comparators can still be considered cost-effective even under the assumptions of the secondary analysis. Although not cost-effective in the more severe reasons for referral, the DySIS comparators were still more effective in these groups.
For this secondary analysis, DySIS and DySIS plus colposcopy appear less favourable in patients with more serious reasons for referral, whereas they remain favourable in the other groups. This is true when either the QALY decrement or cost associated with treatment biopsy is increased. There is a combination of factors that contribute to this. First, the lower specificity of the devices and the more intensive treatment patterns as a result of the more severe cytology will result in more patients who are truly ‘clear’, ‘HPV’ or ‘CIN1’ receiving invasive treatment in the more serious referral groups. Second, as the treatment biopsies become more costly, either in terms of increased costs or lost health as a result of increased QALY decrement, then it is possible that capturing more patients with CIN2/3 as a result of the higher sensitivity of the devices will not prove beneficial, i.e. it might be better to miss such patients as the costs associated with treating them exceed the health benefits as a result of assuming a very high cost and QALY decrement for treatment biopsy. These issues are likely to affect the more serious referral groups more as a result of the split between the underlying true health states in these groups.
The differential cost-effectiveness between referral groups in the secondary analysis suggests that it may be more cost-effective to use different diagnostic devices in different groups. However, if the device is funded for one or more referral groups then the additional cost of using it in other referral groups is zero, with the exception of any differential in the cost of disposables. This suggests that although it may not be cost-effective to fund a device for each referral group separately, it still may be cost-effective to use it in all groups if it is cost-effective to fund it for a single group. To determine if it is cost-effective in a single group would require changing the expected throughput to that of the referral group being considered and will change the per-patient costs of each device. This is not expected to make an important difference in the case of DySIS, as it was found that DySIS was cost-effective in the borderline-plus-HPV and mild-plus-HPV groups that account for > 80% of those referred.
Only indicative sensitivity analyses based on the costs of the LuViva Advanced Cervical Scan and the Niris Imaging System were undertaken, and these do not allow us to draw any conclusions on their potential cost-effectiveness. This sensitivity analysis does allow us to say that given their costs, and assuming that they could obtain a specificity equal to DySIS plus colposcopy, their sensitivity would have to be 83% for LuViva and 86% for Niris to be considered cost-effective at £20,000 per QALY threshold compared with the most cost-effective option in our model – DySIS plus colposcopy. These results are not comparable to the sensitivities reported above (see Synthesis of the included studies) because of the differences in patient populations and the quality issues of the studies as described above.
Conclusions
From the economic analysis the EAG concludes that the effectiveness evidence on LuViva and Niris is too unreliable to be included in the analysis. The results of the analysis suggest that DySIS plus colposcopy is less costly and more effective than both DySIS alone or colposcopy alone, and that these results are robust to the numerous sensitivity analyses that were undertaken.
Chapter 3 Discussion
Statement of principal findings
The systematic review of the clinical effectiveness of adjunctive colposcopy technologies found a limited amount of data on three adjunctive technologies: two studies of the DySIS colposcope, one study of the LuViva Advanced Cervical Scan, and three studies of the Niris Imaging System.
The two studies of the DySIS colposcope were well reported and had a low risk of bias; they found statistically significantly higher sensitivity with DySIS (both alone and in combination with colposcopy) than conventional colposcopy alone for identifying CIN2+ disease, although specificity was significantly lower with DySIS.
The study of LuViva and those of Niris were all poorly reported and so the risk of bias in these studies was often unclear; where study methodology was reported there were a number of limitations that led to a high risk of bias. Consequently, the results of these studies cannot be considered reliable.
The base-case cost-effectiveness analysis suggests that both DySIS treatment options (DySIS alone and DySIS plus colposcopy) are less costly and more effective than (dominate) colposcopy alone in the overall weighted population. In the few instances where DySIS alone was more costly and more effective than colposcopy alone, the ICERs were £593, £1545 or £1687 per QALY for the referral groups ‘possible invasion’, ‘possible neoplasia’ or ‘inadequate cytology’, respectively. For all reasons for referral DySIS plus colposcopy is less costly and more effective than DySIS alone. The results of the overall weighted population were robust to the ranges tested in the sensitivity analysis; the highest ICER was £13,614 per QALY in the inadequate cytology referral group in a 25-year-old population comparing DySIS alone with colposcopy alone.
A finding of the base-case analysis was that immediate treatment of women with CIN1 was more effective and cost-effective than watchful waiting. This finding was sensitive to the parameter values for the QALY decrement and cost of treatment biopsy and assumed treatment patterns. In the secondary analyses the DySIS comparators were less costly and more effective in the scenario when the QALY decrement of treatment biopsy was increased to 0.13 (from 0.005) and cost-effective when the cost of treatment biopsy was increased to £2758 (from £97) with ICERs of £13,808 and £12,761 per QALY for DySIS alone and DySIS plus colposcopy, respectively, compared with colposcopy alone.
Only indicative sensitivity analyses based on the costs of the LuViva Advanced Cervical Scan and the Niris Imaging System were undertaken, which do not allow us to draw any conclusions on their potential cost-effectiveness.
When comparing the clinical effectiveness results with the cost-effectiveness results, a noticeable contrast is evident: although DySIS plus colposcopy had a very similar overall accuracy to colposcopy alone, it appears cost-effective. This disparity is as a result of several reasons. First, measures of diagnostic accuracy do not necessarily capture what is of importance when it comes to determining patient outcomes. When treating patients with suspected cancerous or precancerous lesions, simply knowing whether a patient is above or below a CIN2+ cut-off is not sufficient for determining treatment, which will in turn affect a patient's outcomes. Secondly, accuracy combines both the effects of sensitivity and specificity in a defined way that does not reflect the same values from the model that are determined by treatment patterns.
In the model the effects of sensitivity and specificity depend on the consequences of treatment. In the case where the treatment consequences (costs and adverse effects) are lower than the risk and consequences of missing treatment (cancer progression), but current practice does not treat, then it is better for a diagnostic technology to have lower specificity. If, however, the treatment consequences are more severe than the consequences of missing treatment, but current practice undertakes treatment, then it is better for a diagnostic technology to have lower sensitivity. This balance becomes particularly difficult using a binary cut-off (sensitivity and specificity at CIN2+) when actual diagnosis will decide treatment across more than two diagnoses (clear, CIN1, CIN2, CIN3, cancer). For increased sensitivity and specificity to be cost-effective, the treatment patterns for all diagnosed patients must also be cost-effective.
Strengths and limitations of the assessment
Strengths
We conducted a rigorous systematic review of the clinical effectiveness of adjunctive colposcopy technologies, which addressed a clear research question using predefined inclusion criteria. Comprehensive literature searches were performed to locate all relevant published and unpublished studies without any language restrictions. Hand-searching and contact with the manufacturers further reduced the potential for missing relevant studies. Therefore, we are confident that all relevant studies were included in the review. Study selection was undertaken independently by two reviewers and data extraction and quality assessment were checked by a second reviewer to minimise the potential for reviewer bias or error. Validity assessment was undertaken using a validated checklist for diagnostic studies, with additional review-specific quality assessment items added.
To model the decision problem, the adjunct technologies need to be located in the diagnostic and treatment pathway. The model captures the full complexity of this pathway and is also driven by an underlying natural history component that captures the progression of the disease. A previously validated economic model was made available from the University of Sheffield and updated to fit the current decision problem. The clinical experts were very involved in the model development and helped verify treatment patterns and other model inputs. The model facilitates a careful assessment of the uncertainties in the evidence available and assumptions underlying its structure. The cost-effectiveness results for DySIS are robust to most uncertainties in the model.
Limitations
The main limitation of the systematic review of the clinical effectiveness of adjunctive colposcopy technologies was the limited amount and quality of the evidence available. Some of the earlier studies assessed precommercial versions of the technologies, so are not comparable to the later studies, after technologies had been developed further.
Owing to potential biases in the studies of the LuViva Advanced Cervical Scan and the Niris Imaging System, only the results of the studies of the DySIS colposcope are likely to be reliable. Only one of the studies of the Niris Imaging System used clinically relevant cut-offs for classifying images; however, the lack of reference standard assessment for patients with no suspicious areas in this study means that the results are unreliable. The study of the LuViva Advanced Cervical Scan appeared to use a different reference standard for patients with no suspicious areas, thus reducing the reliability of the results of the study. In addition, the authors suggest that the intended use of LuViva is to triage women for colposcopy, rather than as an adjunct to colposcopy.
The findings of the economic analysis are limited by the effectiveness data available. These data were reported as sensitivity and specificity at a CIN2+ threshold. In practice, decisions are not made on whether a patient is CIN2+ or not, and more detailed information about how accurately patients were identified would be more appropriate. To compensate for this lack of data we assumed that DySIS would diagnose across the possible health states clear, CIN1, CIN2, CIN3 or cancer, similarly to Niris in the study by Gallwas et al. 32 The QALY decrement and costs associated with treatment biopsy may not fully take into account the long-term consequences of the procedure. When modelling the outcomes of cancer patients simplifying assumptions have been made.
The Sheffield model on which this model was based was not probabilistic and we were unable to make the complete model probabilistic within the time frame of the assessment. We did consider the inclusion of probabilistic analysis in the diagnostic model but were unaware of methods for capturing the bivariate distribution of sensitivity and specificity from single trial estimates, which is needed for the probabilistic analysis.
Uncertainties
The studies included in this assessment were based on populations of women primarily referred with abnormal cervical cytology. There is uncertainty about how generalisable the results of these studies are to the population of women referred for colposcopy in the future.
The recent introduction of the HPV triage test will alter the population of women referred for colposcopy through the NHS Cervical Screening Programme (women with low-grade abnormalities on screening will be referred for colposcopy only if they are positive for hrHPV). 5 In addition, the screening population is likely to change as females who have received the HPV vaccine reach screening age.
There is uncertainty associated with the method and data used to convert sensitivities and specificities to the required probabilities for the model. This is true, in particular, because of the use of data from a single technology to inform this parameter for all technologies. It is possible that this will be different across technologies. There is a lack of data available on the costs and QALY decrement of treatment biopsy and it is unclear whether the estimates used are robust. It is unclear how ‘see and treat’ and loss to follow-up might influence the cost-effectiveness of the adjunct devices.
These analyses consider the cost-effectiveness of purchasing a DySIS device rather than purchasing a new colposcope. A separate analysis might consider the cost-effectiveness of replacing a colposcope that has already been purchased. In this case, the per-patient costs of colposcope would exclude the annuitised cost of the colposcope (£1.50). It is expected that this difference will not change the decision being made, particularly if the replaced colposcope has value and can be sold to contribute to the purchase of the new device.
These analyses assume the average use of a colposcope or adjunct as indicated by our clinical advisors. In clinics where colposcopes would be used much less frequently, such as GP clinics, it is unclear whether DySIS would be cost-effective.
It is possible that the introduction of a new device will change treatment patterns. The cost-effectiveness results provided in this report are based on treatment patterns from current clinical opinion or from the Gateshead data, which are both based on the use of standard colposcopy.
Other relevant factors
Currently the economic model does not take account of patients with previous cancerous or precancerous lesions being at higher risk of recurrence than the general population.
The cost-effectiveness of each device may be affected by the level of ‘see and treat’ used and the amount of loss to follow-up; however, neither of these factors have been evaluated in the economic model.
These results depend on the use of current guidelines and clinical advice to determine treatment probabilities. Any changes to the guidelines will result in different cost-effectiveness.
Chapter 4 Conclusions
DySIS, particularly when combined with colposcopy, has higher sensitivity than conventional colposcopy alone. There is no reliable evidence on the clinical effectiveness of the other adjunctive colposcopy technologies: the LuViva Advanced Cervical Scan and the Niris Imaging System.
From the economic analysis, the EAG concludes that the results of the analysis suggest that DySIS plus colposcopy is less costly and more effective than both DySIS alone and colposcopy alone, and that these results are robust to the numerous sensitivity analyses that were undertaken. The effectiveness evidence on LuViva and Niris is not considered sufficiently reliable to be included in the economic analysis.
Implications for service provision
The introduction of any of these new devices may require additional staff training, which may result in additional upfront costs that were not considered in the analysis. These costs may be actual training costs paid to the manufacturer but might also be costs associated with the additional time or initial accuracy of staff as they learn to use the new device.
Suggested research priorities
In light of the risk of bias affecting the results of the studies of the LuViva Advanced Cervical Scan and the Niris Imaging System, further studies are necessary to reliably evaluate their diagnostic accuracy. The bias risk was a result of the reference standard methodologies used, with further uncertainty about study reliability stemming from the unclear reporting in relation to other possible sources of bias.
The findings of the current model suggest that treatment of CIN1 is cost-effective. However, current treatment guidelines suggest that watchful waiting is preferred for these patients. Further research is needed to assess the robustness of the current model findings to inform the appropriate management of CIN1.
Future studies on the diagnostic accuracy of such technologies should provide results for each diagnostic category (clear, CIN1, CIN2, CIN3, possible invasion and possible neoplasia) rather than sensitivity and specificity at a single cut-off. This could be done by completing Table 25.
| Histological result | Findings of new device | |||||
|---|---|---|---|---|---|---|
| Clear | CIN1 | CIN2 | CIN3 | Possible invasion | Possible neoplasia | |
| Clear | ||||||
| CIN1 | ||||||
| CIN2 | ||||||
| CIN3 | ||||||
| Possible invasion | ||||||
| Possible neoplasia | ||||||
With the information from this table we would not be required to use additional data or assumptions to convert the sensitivities and specificities into the required probabilities for the model.
Future studies should consider assessing interobserver agreement between colposcopists.
Given that a new device may change treatment patterns, further research could also consider collecting data on ‘see and treat’ rates and the number of biopsies performed.
Acknowledgements
We would like to thank the following for providing information and advice: Dr Maggie Cruickshank, Senior Lecturer in Gynaecology, University of Aberdeen; and Dr Hazel Squires and Dr Jim Chilcott, University of Sheffield.
We would also like to thank the following members of the Diagnostics Advisory Committee for additional information and advice: Dr Karin Denton, Consultant Cytopathologist, North Bristol NHS Trust; Mrs Phyllis Dunn, Clinical Lead Nurse, University Hospital of North Staffordshire; Mr Andrew Fish, Consultant Gynaecologist, Brighton and Sussex University Hospitals NHS Trust; Dr Sadaf Ghaem-Maghami, Senior Lecturer and Honorary Consultant in Gynaecological Oncology, Imperial College London; Mr Pierre Martin-Hirsch, Consultant Gynaecological Oncologist, Lancashire Teaching Hospitals NHS Foundation Trust; Mr Charles Redman, Consultant in Obstetrics and Gynaecology, University Hospital of North Staffordshire NHS Trust; and Dr Miren Turner, GP/Colposcopist.
Contribution of authors
Ros Wade (Research Fellow) was responsible for the clinical effectiveness section, writing the protocol, study selection, data extraction, validity assessment and writing the final report.
Eldon Spackman (Research Fellow) was responsible for the cost-effectiveness section, writing the protocol, study selection, data extraction, development of the economic model and writing the final report.
Mark Corbett (Research Fellow) was involved in the clinical effectiveness section, writing the protocol, study selection, data extraction, validity assessment and writing the final report.
Simon Walker (Research Fellow) was involved in the cost-effectiveness section, study selection, data extraction, development of the economic model and writing the final report.
Kate Light (Information Specialist) devised the search strategy, carried out the literature searches and wrote the search methodology sections of the final report.
Raj Naik (Consultant Gynaecological Oncologist) provided clinical advice and commented on drafts of the final report.
Mark Sculpher (Professor of Health Economics) provided input at all stages, was involved in the development of the economic model, commented on drafts of the report and had overall responsibility for the cost-effectiveness section of the report.
Alison Eastwood (Senior Research Fellow) provided input at all stages, commented on drafts of the report and had overall responsibility for the clinical effectiveness section of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Research UK . Cervical Cancer Statistics-UK 2012. http://info.cancerresearchuk.org/cancerstats/types/cervix/ (accessed 3 August 2011).
- Colposcopy and programme management: guidelines for the NHS Cervical Screening Programme. NHSCSP; 2010.
- Adjunctive colposcopy technologies for examination of the uterine cervix – DySIS, LuViva Advanced Cervical Scan, Niris Imaging System and Zilico APX-100: final scope. September 2011. London: NICE; 2011.
- The NHS Information Centre – Public Health Indicators and Population Statistics team . Cervical Screening Programme England 2009–10 2010. www.ic.nhs.uk/webfiles/publications/008_Screening/cervscreen0910/2009_10_Cervical_Bulletin_Final_Report_AI_v1F.pdf (accessed 13 January 2012).
- HPV triage and test of cure implementation guidance. NHSCSP good practice guide number 3. Sheffield: NHSCSP; 2011.
- Louwers JA, Zaal A, Kocken M, ter Harmsel WA, Graziosi GCM, Spruijt JWM, et al. Dynamic spectral imaging colposcopy: higher sensitivity for detection of premalignant cervical lesions. BJOG 2011;118:309-18. http://dx.doi.org/10.1111/j.1471-0528.2010.02806.x.
- LuViva™ Advanced Cervical Scan – adjunct to screening and colposcopy for cervical dyskaryosis. Birmingham: National Horizon Scanning Centre, University of Birmingham; 2010.
- Etherington IJ, Luesley DM, Shafi MI, Dunn J, Hiller L, Jordan JA. Observer variability among colposcopists from the West Midlands region. Br J Obstet Gynaecol 1997;104:1380-4. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11007.x.
- Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31. http://dx.doi.org/10.1016/S0029-7844(98)00006-4.
- Jordan J, Arbyn M, Martin-Hirsch P, Schenck U, Baldauf JJ, Da Silva D, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 2008;19:342-54. http://dx.doi.org/10.1111/j.1365-2303.2008.00623.x.
- Pretorius RG, Zhang W-H, Belinson JL, Huang M-N, Wu L-Y, Zhang X, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol 2004;191:430-4. http://dx.doi.org/10.1016/j.ajog.2004.02.065.
- ASCUS-LSIL Triage Study (ALTS) Group . A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol 2003;188:1393-400.
- Systematic reviews: CRD's guidance for undertaking reviews in health care. York: University of York; 2009.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2700.
- Centre for Evidence-Based Medicine, Toronto . Stats Calculator n.d. http://ktclearinghouse.ca/cebm/practise/ca/calculators/statscalc (accessed 18 January 2012).
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36.
- Soutter WP, Diakomanolis E, Lyons D, Ghaem-Maghami SA, Tosin A, Haidopoulos D, et al. Dynamic spectral imaging: improving colposcopy. Clin Cancer Res 2009;15:1814-20. http://dx.doi.org/10.1158/1078-0432.CCR-08-1636.
- Soutter WP, Diakomanolis E, Lyons D, Haidopoulos D, Balas C. Comparison of the diagnostic performance characteristics of dynamic spectral imaging and colposcopy in low and high grade referral smear populations, separately [abstract]. J Low Genit Tract Dis 2010;14.
- Flowers LC, Tadros TS. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results for adolescent patients. Norcross, GA: Guided Therapeutics; 2010.
- Chakhtoura NA, Twiggs LB, Wilkinson EJ, Raab SS. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results of follow up data [abstract]. J Low Genit Tract Dis 2010;14.
- Twiggs LB, Chakhtoura NA, Werner CL, Griffith WF, Flowers LC, Lashgari M, et al. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results of a 1,607 subject pivotal trial. J Low Genit Tract Dis 2010;14:248-9.
- Flowers LC, Tadros TS. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results for adolescent subjects [abstract]. J Low Genit Tract Dis 2010;14.
- Wilkinson EJ, Raab SS. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: histopathology review procedures and results. J Low Genit Tract Dis 2010;14:265-6.
- Chakhtoura N, Twiggs L, Wilkinson E, Raab S. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results of follow up data. Norcross, GA: Guided Therapeutics; 2010.
- Twiggs LB. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results of a 1,607 subject pivotal trial. Norcross, GA: Guided Therapeutics; 2010.
- Chakhtoura N, Twiggs LB. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: results of a 1,607 subject pivotal trial. Norcross, GA: Guided Therapeutics; 2010.
- Point-of-care, non-invasive cervical disease detection. Norcross, GA: Guided Therapeutics; 2010.
- Spectroscopic evaluation of cervical neoplasia. Bethesda, MD: National Institutes of Health; n.d.
- Distributor presentation United Kingdom and Ireland. Norcross, GA: Guided Therapeutics; 2010.
- Lerner HP. PMA P100037 Clinical Module Amendment 2. Norcross, GA: Guided Therapeutics; 2011.
- Escobar PF, Rojas-Espaillat L, Tisci S, Enerson C, Brainard J, Smith J, et al. Optical coherence tomography as a diagnostic aid to visual inspection and colposcopy for preinvasive and invasive cancer of the uterine cervix. Int J Gynecol Cancer 2006;16:1815-22. http://dx.doi.org/10.1111/j.1525-1438.2006.00665.x.
- Liu Z, Belinson SE, Li J, Yang B, Wulan N, Tresser NJ, et al. Diagnostic efficacy of real-time optical coherence tomography in the management of preinvasive and invasive neoplasia of the uterine cervix. Int J Gynecol Cancer 2010;20:283-7. http://dx.doi.org/10.1111/IGC.0b013e3181cd1810.
- Gallwas JKS, Turk L, Stepp H, Mueller S, Ochsenkuehn R, Friese K, et al. Optical coherence tomography for the diagnosis of cervical intraepithelial neoplasia. Lasers Surg Med 2011;43:206-12. http://dx.doi.org/10.1002/lsm.21030.
- Gallwas J, Turk L, Stepp H, Muller S, Friese K, Dannecker C. Visualization of cervical intraepithelial neoplasia (CIN) by optical coherence tomography (OCT) [abstract]. Arch Gynecol Obstet 2010;282.
- Wulan N, Belinson JL, Belinson SE, Rong X, Zhang W, Zhu YS, et al. Study of the diagnostic efficacy of ‘real time’ optical coherence tomography as an adjunct to colposcopy for cervical neoplasia. Cleveland, OH: Imalux Corporation; 2010.
- Rasool N, Wulan N, Belinson SE, Belinson JL, Wang C, Rong X, et al. Study of the diagnostic efficacy of ‘real time' optical coherence tomography as an adjunct to VIA in managing cervical neoplasia. Cleveland, OH: Imalux Corporation; 2008.
- Apisdorf J, Sperling J, Rojas-Espaillat L, Escobar P, Feldchtein FI, Tresser N, et al. Niris optical coherence tomography as an adjunct to cervical cancer diagnosis and treatment: model for a single episode of care. Cleveland, OH: Imalux Corporation; 2007.
- Rojas-Espaillat L, Escobar PF, Belinson JL, Tisc S, Brainard J, De la Rosa V, et al. Optical coherence tomography as a diagnostic adjunct to visual inspection and colposcopy for pre-invasive and invasive cancer of the uterine cervix. Cleveland, OH: Imalux Corporation; n.d.
- Kuznetsova IA, Gladkova ND, Gelikonov VM, Belinson JL, Shakhova NM, Feldchtein FI, et al. Optical coherence tomography: technology and applications. Berlin: Springer; 2008.
- Rutjes AWS, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PMM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006;174:469-76. http://dx.doi.org/10.1503/cmaj.050090.
- Bossuyt PM, Reitsma JB. The STARD initiative. Lancet 2003;361. http://dx.doi.org/10.1016/S0140-6736(03)12122-8.
- Bossuyt PMM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem 2003;49:7-8. http://dx.doi.org/10.1373/49.1.7.
- Bossuyt PMM. STARD statement: still room for improvement in the reporting of diagnostic accuracy studies. Radiology 2008;248:713-14. http://dx.doi.org/10.1148/radiol.2483080868.
- Wilczynski NL. Quality of reporting of diagnostic accuracy studies: no change since STARD statement publication: before-and-after study. Radiology 2008;248:817-23. http://dx.doi.org/10.1148/radiol.2483072067.
- Chuck A. Cost-effectiveness of 21 alternative cervical cancer screening strategies. Value Health 2010;13:169-79. http://dx.doi.org/10.1111/j.1524-4733.2009.00611.x.
- Legood R, Gray A, Wolstenholme J, Moss S. Lifetime effects, costs, and cost effectiveness of testing for human papillomavirus to manage low grade cytological abnormalities: results of the NHS pilot studies. BMJ 2006;332:79-85. http://dx.doi.org/10.1136/bmj.38698.458866.7C.
- Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol 2001;151:1158-71. http://dx.doi.org/10.1093/oxfordjournals.aje.a010166.
- Bergeron C, Largeron N, McAllister R, Mathevet P, Remy V. Cost-effectiveness analysis of the introduction of a quadrivalent human papillomavirus vaccine in France. Int J Technol Assess Health Care [serial Online] 2008:10-9.
- Kulasingam SL, Benard S, Barnabas RV, Largeron N, Myers ER. Adding a quadrivalent human papillomavirus vaccine to the UK cervical cancer screening programme: a cost-effectiveness analysis. Cost Effectiveness and Resource Allocation 2008;6. http://dx.doi.org/10.1186/1478-7547-6-4.
- Mandelblatt JS, Lawrence WF, Womack SM, Jacobsen D, Yo B, Hwang Y, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA 2002;287:2372-81. http://dx.doi.org/10.1001/jama.287.18.2372/NHSEED-22002008122/frame.html.
- Hadwin R, Eggington S, Brennan A, Walker P, Patnick J, Pilgrim H. Modelling the cost-effectiveness and capacity impact of changes to colposcopy referral guidelines for women with mild dyskaryosis in the UK Cervical Screening Programme. BJOG 2008;115:749-57. http://dx.doi.org/10.1111/j.1471-0528.2008.01683.x.
- Kim E-J. Modelling the Cost-Effectiveness of Human Papillomavirus (HPV) Testing for Triage of Women With Low-Grade Abnormal Cervical Smears: A Study Within the TOMBOLA Trial 2010.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Sherlaw-Johnson C, Gallivan S, Jenkins D, Jones MH. Cytological screening and management of abnormalities in prevention of cervical cancer: an overview with stochastic modelling. J Clin Pathol 1994;47:430-5. http://dx.doi.org/10.1136/jcp.47.5.430.
- Ghaem-Maghami S, De-Silva D, Tipples M, Lam S, Perryman K, Soutter W. Determinants of success in treating cervical intraepithelial neoplasia. BJOG 2011;118:679-84. http://dx.doi.org/10.1111/j.1471-0528.2010.02770.x.
- Ghaem-Maghami S, Sagi S, Majeed G, Soutter WP. Incomplete excision of cervical intraepithelial neoplasia and risk of treatment failure: a meta-analysis. Lancet Oncol 2007;8:985-93.
- Health and life events guidance and metadata. ONS; n.d.
- Subtopic: population estimates by age and sex. ONS; n.d.
- Cervical cancer statistics and outlook. UK: Cancer Research; 2010.
- Chuck A. Cost-effectiveness of 21 alternative cervical cancer screening strategies. Value Health 2010:169-79. http://dx.doi.org/10.1111/j.1524-4733.2009.00611.x.
- Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N. Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis. Health Technol Assess 2004;8.
- Insinga RP, Glass AG, Myers ER, Rush BB. Abnormal outcomes following cervical cancer screening: event duration and health utility loss. Med Decis Making 2007;27:414-22. http://dx.doi.org/10.1177/0272989X07302128.
- Birch S, Melnikow J, Kuppermann M. Conservative versus aggressive follow up of mildly abnormal Pap smears: testing for process utility. Health Econ 2003;12:879-84.
- Group T, Sharp L, Cotton S, Cochran C, Gray N, Little J, et al. After-effects reported by women following colposcopy, cervical biopsies and LLETZ: results from the TOMBOLA trial. BJOG 2009;116:1506-14. http://dx.doi.org/10.1111/j.1471-0528.2009.02263.x.
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1284.
- Group T. Options for managing low grade cervical abnormalities detected at screening: cost effectiveness study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2549.
- Wolstenholme JL, Whynes DK. Stage-specific treatment costs for cervical cancer in the United Kingdom. Eur J Cancer 1998;34:1889-93. http://dx.doi.org/10.1016/S0959-8049(98)00232-9.
- Kelly RS, Patnick J, Kitchener HC, Moss SM. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the Sentinel Sites study. Br J Canc 2011;105:983-8. http://dx.doi.org/10.1038/bjc.2011.326.
- Eggington S, Hadwin R, Brennan A, Walker P. Modelling the impact of referral guideline changes for mild dyskaryosis on colposcopy services in England. Sheffield: NHS Cancer Screening Programmes; 2006.
- Ferris DG, Macfee MS, Miller JA, Litaker MS, Crawley D, Watson D. The efficacy of telecolposcopy compared with traditional colposcopy. Obstet Gynecol 2002;99:248-54. http://dx.doi.org/10.1016/S0029-7844(01)01671-4.
- 2000.
- 2002.
- SpectRx cervical cancer test moves toward approval. Photomed Laser Surg 2004;22:259-60. http://dx.doi.org/10.1089/1549541041438650.
- American Society for Colposcopy and Cervical Pathology (ASCCP), Hagerstown, MD. Society news. J Low Genit Tract Dis 2006;10:69-71.
- New imaging system helps detect cervical precancer. AWHONN Lifelines 2006;10.
- American Society for Colposcopy and Cervical Pathology (ASCCP) . Biennial Meeting, Las Vegas, NV 24–28 March 2010, abstracts. J Low Genit Tract Dis 2010;14:241-74.
- Clinical positioning of APX 100. Zilico; 2011.
- Abdul S, Brown BH, Milnes P, Tidy JA. A clinical study of the use of impedance spectroscopy in the detection of cervical intraepithelial neoplasia (CIN). Gynecol Oncol 2005;99:S64-6. http://dx.doi.org/10.1016/j.ygyno.2005.07.046.
- Abdul S, Brown BH, Milnes P, Tidy JA. The use of electrical impedance spectroscopy in the detection of cervical intraepithelial neoplasia. Int J Gynecol Cancer 2006;16:1823-32. http://dx.doi.org/10.1111/j.1525-1438.2006.00651.x.
- Acosta-Mesa H-G, Cruz-Ramirez N, Hernandez-Jimenez R. Aceto-white temporal pattern classification using k-NN to identify precancerous cervical lesion in colposcopic images. Comput Biol Med 2009;39:778-84. http://dx.doi.org/10.1016/j.compbiomed.2009.06.006.
- Agrawal A, Harrell T, Bambot S, Faupel M, Ferris D. Multimodal multispectral imaging of the cervix in vivo for the detection of neoplasia. Proc SPIE 2001;4259:68-74. http://dx.doi.org/10.1117/12.432482.
- Alush A, Greenspan H, Goldberger J. Automated and interactive lesion detection and segmentation in uterine cervix images. IEEE Trans Med Imaging 2010;29:488-501. http://dx.doi.org/10.1109/TMI.2009.2037201.
- Alvarez RD, Wright TC. Optical Detection Group . Effective cervical neoplasia detection with a novel optical detection system: a randomized trial. Gynecol Oncol 2007;104:281-9. http://dx.doi.org/10.1016/j.ygyno.2006.08.056.
- Anastasiadou M, De Martino A, Clement D, Liege F, Laude-Boulesteix B, Quang N, et al. Polarimetric imaging for the diagnosis of cervical cancer. Physica Status Solidi C 2008;5:1423-6. http://dx.doi.org/10.1002/pssc.200777805.
- Atkinson EN. Age and FSH effects in fluorescence spectra from the cervix: an exploratory analysis. Gynecol Oncol 2005;99:S95-7. http://dx.doi.org/10.1016/j.ygyno.2005.07.051.
- Azar EG, Intes X. Bellingham, WA: SPIE; 2009.
- Badizadegan K, Backman V, Boone CW, Crum CP, Dasari RR, Georgakoudi I, et al. Spectroscopic diagnosis and imaging of invisible pre-cancer. Faraday Discuss 2004;126:265-79. http://dx.doi.org/10.1039/b305410a.
- Balas C. A novel optical imaging method for the early detection, quantitative grading, and mapping of cancerous and precancerous lesions of cervix. IEEE Trans Biomed Eng 2001;48:96-104. http://dx.doi.org/10.1109/10.900259.
- Balas C. The potential of spectral imaging in the non-invasive diagnostics of tissue cancers and pre-cancers. Proc SPIE 2002;5123:287-92.
- Balas C, Simos C, Sciadas Y. Dynamic spectral imaging for in vivo diagnostics, screening and guided therapeutics of cervical neoplasia. Indian J Med Res 2005;121.
- Balas CJ, Papoutsoglou G, Potirakis A. In vivo molecular imaging of cervical neoplasia using acetic acid as biomarker. IEEE J Sel Top Quantum Electron 2008;14:29-42. http://dx.doi.org/10.1109/JSTQE.2007.913396.
- Balas C, Papoutsoglou G, Diakomanolis E, Haidopoulos D, Soutter WP. Quantitative assessment of the AW phenomenon and determination of its biophysical origins as a means for in vivo assessing CIN-related micro-structural and functional changes. J Low Genit Tract Dis 2010;14:268-9.
- Balasubramani L, Brown BH, Healey J, Tidy JA. The detection of cervical intraepithelial neoplasia by electrical impedance spectroscopy: the effects of acetic acid and tissue homogeneity. Gynecol Oncol 2009;115:267-71. http://dx.doi.org/10.1016/j.ygyno.2009.08.010.
- Bazant-Hegemark F, Stone N, Read MD, McCarthy K, Wang RK. Optical coherence tomography (OCT) imaging and computer aided diagnosis of human cervical tissue specimens. Proc SPIE 2007;6627.
- Belinson J, Qiao YL, Pretorius R, Zhang WH, Elson P, Li L, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001;83:439-44. http://dx.doi.org/10.1006/gyno.2001.6370.
- Belinson JL. n.d.
- Belinson S, Ledford K, Wulan N, Rasool N, Wang C, Rollins A, et al. Epithelial brightness by optical coherence tomography distinguishes grades of dysplasia. Cleveland, OH: Imalux Corporation; 2009.
- Benavides JM, Chang SC, Park SY, Richards-Kortum R, Mackinnon N, MacAulay C, et al. Multispectral digital colposcopy for in vivo detection of cervical cancer. Opt Express 2003;11:1223-36. http://dx.doi.org/10.1364/OE.11.001223.
- Bogaards A, Aalders MC, Zeyl CC, de Blok S, Dannecker C, Hillemanns P, et al. Localization and staging of cervical intraepithelial neoplasia using double ratio fluorescence imaging. J Biomed Opt 2002;7:215-20. http://dx.doi.org/10.1117/1.1463045.
- Brookner C, Utzinger U, Follen M, Richards-Kortum R, Cox D, Atkinson EN. Effects of biographical variables on cervical fluorescence emission spectra. J Biomed Opt 2003;8:479-83. http://dx.doi.org/10.1117/1.1578642.
- Brown BH, Milnes P, Abdul S, Tidy JA. n.d.
- Brown BH, Tidy JA, Boston K, Blackett AD, Smallwood RH, Sharp F. Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet 2000;355:892-5. http://dx.doi.org/10.1016/S0140-6736(99)09095-9.
- Brown BH, Milnes P, Abdul S, Tidy JA. Detection of cervical intraepithelial neoplasia using impedance spectroscopy: a prospective study. BJOG 2005;112:802-6. http://dx.doi.org/10.1111/j.1471-0528.2004.00530.x.
- Bush J, Davis PG, Marcus MA. All-fiber optic coherence domain interferometric techniques. Boston, MA: Optiphase, Inc.; 2001.
- Cantor SB, Levy LB, Cardenas-Turanzas M, Basen-Engquist K, Le T, Beck JR, et al. Collecting direct non-health care and time cost data: application to screening and diagnosis of cervical cancer. Med Decis Making 2006;26:265-72. http://dx.doi.org/10.1177/027298906288679.
- Cantor SB, Yamal J-M, Guillaud M, Cox DD, Atkinson EN, Benedet JL, et al. Accuracy of optical spectroscopy for the detection of cervical intraepithelial neoplasia: testing a device as an adjunct to colposcopy. Int J Cancer 2011;128:1151-68. http://dx.doi.org/10.1002/ijc.25667.
- Chance B, Alfano RR, Tromberg BJ, Tamura M, Sevick-Muraca EM. Bellingham, WA: SPIE; 2005.
- Chang SK, Follen M, Malpica A, Utzinger U, Staerkel G, Cox D, et al. Optimal excitation wavelengths for discrimination of cervical neoplasia. IEEE Trans Biomed Eng 2002;49:1102-10. http://dx.doi.org/10.1109/TBME.2002.803597.
- Chang SK, Mirabal YN, Atkinson EN, Malpica A, Follen M, Richards-Kortum R. n.d.
- Chang SK, Pavlova I, Marin NM, Follen M, Richards-Kortum R. Fluorescence spectroscopy as a diagnostic tool for detecting cervical pre-cancer. Gynecol Oncol 2005;99:S61-3. http://dx.doi.org/10.1016/j.ygyno.2005.07.045.
- Chang SK, Mirabal YN, Atkinson EN, Cox D, Malpica A, Follen M, et al. Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt 2005;10. http://dx.doi.org/10.1117/1.1899686.
- Chang VT-C, Cartwright PS, Bean SM, Palmer GM, Bentley RC, Ramanujam N. Quantitative physiology of the precancerous cervix in vivo through optical spectroscopy. Neoplasia 2009;11:325-32.
- Chang VT-C, Bean SM, Cartwright PS, Ramanujam N. Visible light optical spectroscopy is sensitive to neovascularization in the dysplastic cervix. J Biomed Opt 2010;15. http://dx.doi.org/10.1117/1.3495730.
- Chang VT-C, Merisier D, Yu B, Walmer DK, Ramanujam N. Calibration schemes of a field-compatible optical spectroscopic system to quantify neovascular changes in the dysplastic cervix. Proc SPIE 2011;7891.
- Cheung TH, Wu T, Lo WK, Yu MY, Qu J, Monsonego J. 5th International Multidisciplinary Congress (EUROGIN 2003), Paris, France, 13–16 April, 2003. Bologna: Medimond Inc.; n.d.
- Claude I, Pouletaut P, Huault S, Boulanger JC. n.d.
- Measurement of digital colposcopy for fluorescence spectroscopy of cervical intraepithelial neoplasia. Bethesda, MD: National Institutes of Health; n.d.
- Spectroscopy in diagnosing dysplasia and neoplasia in the cervix in women undergoing colposcopy. Bethesda, MD: National Institutes of Health; n.d.
- Measurement of digital colposcopy for fluorescence spectroscopy of TNC using a second device. Bethesda, MD: National Institutes of Health; n.d.
- Digital colposcopy in finding cervical intraepithelial neoplasia in patients with an abnormal pap smear. Bethesda, MD: National Institutes of Health; n.d.
- Digital colposcopy in finding cervical intraepithelial neoplasia. Bethesda, MD: National Institutes of Health; n.d.
- Spectroscopy versus standard care in cervical cancer patients. Bethesda, MD: National Institutes of Health; n.d.
- Combined digital colposcopy analysis to improve cervical precancer and cancer detection. Bethesda, MD: National Institutes of Health; n.d.
- Cervical cancer detection using optical spectroscopy. Bethesda, MD: National Institutes of Health; n.d.
- Non-invasive, real-time technology for diagnosis of cervical tissue. Bethesda, MD: National Institutes of Health; n.d.
- Patient satisfaction & quality of life in patients with cervical cancer. Bethesda, MD: National Institutes of Health; n.d.
- Digital imaging aid for assessment of cervical dysplasia. Bethesda, MD: National Institutes of Health; n.d.
- Investigation of the uterine cervix using CervicalMD. Bethesda, MD: National Institutes of Health; n.d.
- High resolution digital imaging of the uterine cervix. Bethesda, MD: National Institutes of Health; n.d.
- Reflectance confocal imaging in cervical cancer patients. Bethesda, MD: National Institutes of Health; n.d.
- Collier T, Guillaud M, Follen M, Malpica A, Richards-Kortum R. Real-time reflectance confocal microscopy: comparison of two-dimensional images and three-dimensional image stacks for detection of cervical precancer. J Biomed Opt 2007;12. http://dx.doi.org/10.1117/1.2717899.
- Coppolillo EF, Eliseth MC, Malamud de Ruda Vega H, Perazzi BE. Three-dimensional digital colposcopy. Acta Obstet Gynecol Scand 2009;88:120-1. http://dx.doi.org/10.1080/00016340802534802.
- Dattamajumdar AK, Wells D, Parnell J, Lewis JT, Ganguly D, Wright TC. n.d.
- Dattamajumdar AK, Wells D, Parriell J, Ganguly D, Wright TC. Preliminary results from multi-center clinical trials for detection of cervical squamous intraepithelial lesions using a novel full-field evoked tissue fluorescence based imaging instrument. Proc SPIE 2001;4432:312-13. http://dx.doi.org/10.1117/12.447157.
- Dattamajumdar AK, Ferenczy A, Tuggy M, Rosing M, Waldman J, Hunter V, et al. 5th International Multidisciplinary Congress (EUROGIN 2003), Paris, France, 13–16 April 2003. Bologna: Medimond Inc.; n.d.
- DeSantis T, Chakhtoura N, Twiggs L, Ferris DG, Lashgari M, Flowers L, et al. Spectroscopic imaging as a triage test for cervical disease: a prospective multicenter clinical trial. J Low Genit Tract Dis 2007;11:18-24. http://dx.doi.org/10.1097/01.lgt.0000230207.50495.05.
- DeWeert MJ, Oyama J, McLaughlin E, Jacobson E, Hakansson J, Bignami GS, et al. Analysis of spatial variability in hyperspectral imagery of the uterine cervix in vivo. Proc SPIE 2003;4959:67-76. http://dx.doi.org/10.1117/12.479509.
- Dominik P, Witold K, Marcin P, Agata J, Maciej P, Marek S. Assessment of optoelectronic method and molecular test usefulness for cervical intraepithelial neoplasia and cervical cancer detection. Ginekol Pol 2010;81:426-30.
- Drezek R, Follen M, Richards-Kortum R. 2002.
- Escobar PF, Belinson JL, White A, Shakhova NM, Feldchtein FI, Kareta MV, et al. Diagnostic efficacy of optical coherence tomography in the management of preinvasive and invasive cancer of uterine cervix and vulva. Int J Gynecol Cancer 2004;14:470-4. http://dx.doi.org/10.1111/j.1048-891x.2004.14307.x.
- Escobar PF, Rojas-Espaillat L, Belinson JL. Optical diagnosis of cervical dysplasia. Int J Gynaecol Obstet 2005;89:63-4. http://dx.doi.org/10.1016/j.ijgo.2004.12.029.
- Feldchtein FI, Gladkova ND, Snopova L, Zagaynova E, Streltzova O, Shakhov A, et al. Blinded recognition of optical coherence tomography images of human mucosa precancer. Proc SPIE 2003;4956:89-94. http://dx.doi.org/10.1117/12.478926.
- Feldchtein F, Bush J, Gelikonov G, Gelikonov V, Piyevsky S. Cost effective, all-fiber autocorrelator based 1300 nm OCT system. Proc SPIE 2005;5690:349-55. http://dx.doi.org/10.1117/12.589502.
- Ferris DG, Lawhead RA, Dickman ED, Holtzapple N, Miller JA, Grogan S, et al. Multimodal hyperspectral imaging for the noninvasive diagnosis of cervical neoplasia. J Low Genit Tract Dis 2001;5:65-72.
- Ferris DG, Litaker MS, Dickman ED, Allmond LM, Smith KM, Arrington TL. Women's responses to cervical interrogation by fluorescent and reflective spectroscopy. J Low Genit Tract Dis 2003;7:299-303. http://dx.doi.org/10.1097/00128360-200310000-00013.
- Ferris DG, Li W, Gustafsson U, Lieberman RW, Galdos O, Santos C. Enhancing colposcopy with polarized light. J Low Genit Tract Dis 2010;14:149-54. http://dx.doi.org/10.1097/LGT.0b013e3181d52f59.
- Freeberg JA, Serachitopol DM, McKinnon N, Price R, Atkinson EN, Cox DD, et al. Fluorescence and reflectance device variability throughout the progression of a phase II clinical trial to detect and screen for cervical neoplasia using a fiber optic probe. J Biomed Opt 2007;12. http://dx.doi.org/10.1117/1.2750332.
- Fujii T, Nakamura M, Kameyama K, Saito M, Nishio H, Ohno A, et al. Digital colposcopy for the diagnosis of cervical adenocarcinoma using a narrow band imaging system. Int J Gynecol Cancer 2010;20:605-10. http://dx.doi.org/10.1111/IGC.0b013e3181d98da9.
- Gage JC, Safaeian M, Jeronimo J, Schiffman M. Spectroscopic imaging as triage test for cervical disease. J Low Genit Tract Dis 2008;12:52-3. http://dx.doi.org/10.1097/lgt.0b013e318149e232.
- Gallwas J, Turk L, Stepp H, Friese K, Dannecker C. Visualization of cervical intraepithelial neoplasias by optical coherence tomography: early results. Geburtshilfe Frauenheilkd 2009;69:931-4. http://dx.doi.org/10.1055/s-0029-1186174.
- Gallwas J, Chiapponi C, Turk L, Ochsenkuehn R, Friese K, Dannecker C. Optical coherence tomography: preliminary results with a new noninvasive technique for evaluating uterine cervical tissue and vulvar epithelium. Minerva Ginecol 2010;62:395-401. http://dx.doi.org/10.1002/uog.7656.
- Gallwas J, Turk L, Friese K, Dannecker C. Optical coherence tomography as a non-invasive imaging technique for preinvasive and invasive neoplasia of the uterine cervix. Ultrasound Obstet Gynecol 2010;36:624-9.
- Gandhi SV, Walker D, Milnes P, Mukherjee S, Brown BH, Anumba DOC. Electrical impedance spectroscopy of the cervix in non-pregnant and pregnant women. Eur J Obstet Gynecol Reprod Biol 2006;129:145-9. http://dx.doi.org/10.1016/j.ejogrb.2005.12.029.
- Georgakoudi I, Sheets EE, Crum CP, Mueller MG, Backman V, Feld MS. Tri-modal spectroscopy as a tool for detecting cervical squamous intraeptihelial lesions in vivo. Proc SPIE 2001;2:1-9. http://dx.doi.org/10.1117/12.447118.
- Georgakoudi I, Sheets EE, Muller MG, Backman V, Crum CP, Badizadegan K, et al. Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo. Am J Obstet Gynecol 2002;186:374-82. http://dx.doi.org/10.1067/mob.2002.121075.
- Ghanate AD, Kothiwale S, Singh SP, Bertrand D, Murali Krishna C. Comparative evaluation of spectroscopic models using different multivariate statistical tools in a multicancer scenario. J Biomed Opt 2011;16. http://dx.doi.org/10.1117/1.3548303.
- Gladkova ND, Shakhova NM, Shakhov BY, Snopova LB. Optic coherent tomography: a new high-resolution technology of visualization of tissue structures. Communication II. Optical images of benign and malignant entities. Vestn Roentgenol Radiol 2004;2:44-5.
- Gudibande R, Mozumder M, Singh R, Panigrahi PK, Gupta S, Pradhan A. Differentiating human cervical dysplastic and normal tissue through wavelet domain characterization of intrinsic fluorescence. Proc SPIE 2011;7902.
- What is LuViva?. Norcross: GA: Guided Therapeutics; n.d.
- Gustafsson U, McLaughlin E, Jacobson E, Hakansson J, Troy P, DeWeert MJ, et al. Fluorescence and reflectance monitoring of human cervical tissue in vivo: a case study. Proc SPIE 2003;4959:100-10. http://dx.doi.org/10.1117/12.479495.
- Gustafsson U, McLaughlin E, Jacobson E, Hakansson J, Troy P, DeWeert MJ, et al. In vivo fluorescence and reflectance imaging of human cervical tissue. Proc SPIE 2003;5031:521-30. http://dx.doi.org/10.1117/12.480413.
- Harper DM, Schmid-Saugeon P, Zelenchuk A, Pitts JD, Kaufman HB. Quantitative measurements of acetowhitening may provide an adjunct to colposcopy to identify cervical intraepithelial neoplasia 2 and 3. Obstet Gynecol 2004;103:S18-19.
- Hsiung P, Chudoba C, Pitris C, Li XD, Ko T, Fujimoto JG, et al. In vivo colposcopic imaging of neoplastic tissues using Optical Coherence Tomography. Proc SPIE 2001;4254:58-60. http://dx.doi.org/10.1117/12.427947.
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science 1991;254:1178-81. http://dx.doi.org/10.1126/science.1957169.
- Huang Z, Mo J, Zheng W, Low J, Ng J, Ilancheran A. Combining near-infrared autofluorescence and Raman spectroscopy improves the in vivo detection of cervical precancer. 2008 Quantum Electronics and Laser Science Conference (QELS). Piscataway, NJ: IEEE; 2008.
- Huh WK, Cestero RM, Garcia FA, Gold MA, Guido RS, McIntyre-Seltman K, et al. Optical detection of high-grade cervical intraepithelial neoplasia in vivo: results of a 604-patient study. Am J Obstet Gynecol 2004;190:1249-57. http://dx.doi.org/10.1016/j.ajog.2003.12.006.
- Huh WK. Optical detection of cervical neoplasia: results from an internally-controlled multicenter study. Gynecol Oncol 2005;99. http://dx.doi.org/10.1016/j.ygyno.2005.07.041.
- Report no. 0400–000018 Rev A. Cleveland, OH: Imalux Corporation; 2010.
- Report no. 0400–000019 Rev A. Cleveland, OH: Imalux Corporation; 2010.
- Cervical cancer [economic effects]. Cleveland, OH: Imalux Corporation; n.d.
- OCT technology: Imalux. Cleveland, OH: Imalux Corporation; n.d.
- Report No.: 0400–000025–0. Cleveland, OH: Imalux Corporation; n.d.
- Report No.: 0400–000024–0. Cleveland, OH: Imalux Corporation; n.d.
- Imalux . Niris Imaging System [Powerpoint Presentation Prepared for the National Institute for Health and Clinical Excellence] 2011.
- Jeronimo J, Schiffman M. Colposcopy at a crossroads. Am J Obstet Gynecol 2006;195:349-53. http://dx.doi.org/10.1016/j.ajog.2006.01.091.
- Jeronimo J, Massad LS, Schiffman M. Visual appearance of the uterine cervix: correlation with human papillomavirus detection and type. Am J Obstet Gynecol 2007;197.
- Johansson A, Kromer K, Sroka R, Stepp H. Clinical optical diagnostics – status and perspectives. Medical Laser Application 2008;23:155-74. http://dx.doi.org/10.1016/j.mla.2008.08.002.
- Kang W. Diagnostic efficacy of computer-extracted image features using optical coherence tomography in pre- cancerous cervix. Cleveland, OH: Imalux Corporation; 2008.
- Kang W, Qi X, Kareta M, Belinson JL, Rollins AM. Automated processing and classification of cervical images using optical coherence tomography. Washington, DC: Optical Society of America; 2008.
- Kang W, Qi X, Tresser NJ, Kareta M, Belinson JL, Rollins AM. Computer-aided diagnosis in OCT of the precancerous cervix. Cleveland, OH: Imalux; 2010.
- Kang W, Qi X, Tresser NJ, Kareta M, Belinson JL, Rollins AM. Diagnostic efficacy of computer extracted image features in optical coherence tomography of the precancerous cervix. Med Phys 2011;38:107-13. http://dx.doi.org/10.1118/1.3523098.
- Kanter EM, Majumder S, Kanter GJ, Woeste EM, Mahadevan-Jansen A. Effect of hormonal variation on Raman spectra for cervical disease detection. Am J Obstet Gynecol 2009;200:512-3. http://dx.doi.org/10.1016/j.ajog.2008.11.024.
- Knapp P, Zbroch T, Kobylec M, Knapp P. Computerized fluorocolposcopy: the significance in evaluation and therapy of early cervical lesions. Przegl Lek 2004;61:1393-9.
- Kuznetzova IA, Shakhova NM, Kachalina TS, Gladkova ND, Myakov AV, Iksanov RR, et al. Optical coherence tomography in diagnosing of cervical cancer. Proc SPIE 2000;3097:440-7. http://dx.doi.org/10.1117/12.386285.
- Lange H, Baker R, Hakansson J, Gustafsson UP. Reflectance and fluorescence hyperspectral elastic image registration. Proc SPIE 2004;5370:335-45. http://dx.doi.org/10.1117/12.535720.
- Lange H. Automatic detection of multi-level acetowhite regions in RGB color images of the uterine cervix. Proc SPIE 2005;5747:1004-17. http://dx.doi.org/10.1117/12.596064.
- Lange H. Automatic glare removal in reflectance imagery of the uterine cervix. Proc SPIE 2005;5747:2183-92. http://dx.doi.org/10.1117/12.596012.
- Lange H, Ferris DG. Computer-aided-diagnosis (CAD) for colposcopy. Proc SPIE 2005;5747:71-84. http://dx.doi.org/10.1117/12.594658.
- Ledford K, Tresser N. Epithelium brightness by optical coherence tomography (OCT) may distinguish low-grade from high-grade cervical dysplasia in real-time n.d.
- Lee JS, Follen M, MacAulay C, Pikkula B, Serachitopol D, Price R, et al. Sources of variability in fluorescence spectroscopic measurements in a phase II clinical trial of 850 patients. Gynecol Oncol 2007;107:S260-9. http://dx.doi.org/10.1016/j.ygyno.2007.07.012.
- Lee S-W, Kang J-H, Yoo J-Y, Kim B-M. n.d.
- Lee S-W, Yoo J-Y, Kang J-H, Kang M-S, Jung S-H, Chong Y, et al. n.d.
- Lee S-W, Yoo J-Y, Kang J-H, Kang M-S, Jung S-H, Chong Y, et al. Optical diagnosis of cervical intraepithelial neoplasm (CIN) using polarization-sensitive optical coherence tomography. Opt Express 2008;16:2709-19. http://dx.doi.org/10.1364/OE.16.002709.
- Li W, Van Raad V, Gu J, Hansson U, Hakansson J, Lange H, et al. Computer Vision for Biomedical Image Applications. First International Workshop, CVBIA 2005. Proceedings. Berlin: Springer-Verlag Berlin; 2005.
- Li W, Poirson A. Advances in visual computing. Second International Symposium, ISVC 2006, Lake Tahoe, NV, USA, November 6–8, 2006, Proceedings, Part II. Berlin: Springer-Verlag; 2006.
- Li W, Soto-Thompson M, Gustafsson U. A new image calibration system in digital colposcopy. Opt Express 2006;14:12887-901. http://dx.doi.org/10.1364/OE.14.012887.
- Li W, Soto-Thompson M, Yizhi X, Lange H. A new image calibration technique for colposcopic images. Proc SPIE 2006;6144.
- Li W, Gu J, Ferris D, Poirson A. Automated image analysis of uterine cervical images. Proc SPIE 2007;6514.
- Li W, Ferris DG, Lieberman RW. Computerized image analysis for acetic acid induced intraepithelial lesions. Proc SPIE 2008;6914.
- Li W, Lieberman RW, Nie S, Xie Y, Eldred M, Oyama J. Histopathology reconstruction on digital imagery. Proc SPIE 2009;7263.
- Li W, Venkataraman S, Gustafsson U, Oyama JC, Ferris DG, Lieberman RW. Using acetowhite opacity index for detecting cervical intraepithelial neoplasia. J Biomed Opt 2009;14. http://dx.doi.org/10.1117/1.3079810.
- Loning M, Lankenau E, Strunck C, Krokowski M, Hillbricht S, Diedrich K, et al. Optical coherence tomography: a noninvasive high resolution cross-sectional tissue imaging in addition to colposcopy. Geburtshilfe Frauenheilkd 2003;63:1158-61. http://dx.doi.org/10.1055/s-2003-815196.
- Luck B, Carlson K, Collier T, Sung K-B. Confocal microscopy. IEEE Potentials 2004;23:14-7. http://dx.doi.org/10.1109/MP.2004.1266933.
- Lukic A, Iannaccio S, Di Properzio M, Carico E, Camboni A, Vecchione A, et al. Microcolposcopy in the diagnostic evaluation of abnormal cervical cytology: when and why to do it. Clin Exp Obstet Gynecol 2009;36:26-30.
- MacKinnon NB, Cardeno M, Au S, MacAulay CE, Pikkula BM, Serachitopol D, et al. Design of a multispectral digital colposcope. Proc SPIE 2007;6430.
- Malpica A, Zuluaga A, Boiko I, Richards-Kortum R, Follen M. Assessment of cervical squamous intraepithelial lesions by optical coherence tomography and colposcopy [abstract]. Mod Pathol 2001;14.
- Malpica A, Zuluaga A, Boiko I, Richards-Kortum R, Follen M. Assessment of cervical squamous intraepithelial lesion by optical coherence tomography and colposcopy. Lab Invest 2001;81:1331-8.
- Margariti V, Zervakis M, Balas C. n.d.
- Marin NM, Milbourne A, Rhodes H, Ehlen T, Miller D, Benedet L, et al. Diffuse reflectance patterns in cervical spectroscopy. Gynecol Oncol 2005;99:S116-20. http://dx.doi.org/10.1016/j.ygyno.2005.07.054.
- Marsa L. Optical wand used to detect cervical cancer. Los Angeles Times 2004.
- Martinho HD. Optical biopsy of pre-malignant or degenerative lesions: the role of the inflammatory process. Proc SPIE 2011;7890.
- Massad LS, Collins YC. Strength of correlations between colposcopic impression and biopsy histology. Gynecol Oncol 2003;89:424-8. http://dx.doi.org/10.1016/S0090-8258(03)00082-9.
- McLaren W, Tan J, Quinn M, Monsonego J. 5th International Multidisciplinary Congress (EUROGIN 2003), Paris, France, 13–16 April 2003. Bologna: Medimond Inc.; n.d.
- Mehlhorn G, Munzenmayer C, Kage A, Benz M, Koch MC, Winter C, et al. Computer-assisted diagnosis (CAD) for colposcopy: evaluation of a pilot study. Endoskopie Heute 2010;23:290-6. http://dx.doi.org/10.1055/s-0030-1262651.
- Mikhail MS, Palan PR, Basu J, Romney SL. Computerized measurement of intercapillary distance using image analysis in women with cervical intraepithelial neoplasia: correlation with severity. Acta Obstet Gynecol Scand 2004;83:308-10.
- Milbourne A, Park SY, Benedet JL, Miller D, Ehlen T, Rhodes H, et al. Results of a pilot study of multispectral digital colposcopy for the in vivo detection of cervical intraepithelial neoplasia. Gynecol Oncol 2005;99:S67-75. http://dx.doi.org/10.1016/j.ygyno.2005.07.047.
- Mirabal YN, Chang SK, Atkinson EN, Malpica A, Follen M, Richards-Kortum R. Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt 2002;7:587-94. http://dx.doi.org/10.1117/1.1502675.
- Mirkovic J, Lau C, McGee S, Yu C-C, Nazemi J, Galindo L, et al. Effect of anatomy on spectroscopic detection of cervical dysplasia. J Biomed Opt 2009;14. http://dx.doi.org/10.1117/1.3194142.
- Mo J, Zheng W, Low J, Ng J, Ilancheran A, Huang Z. Near-infrared autofluorescence spectroscopy for in vivo diagnosis of cervical precancer. Proc SPIE 2008;6853. http://dx.doi.org/10.1117/12.762181.
- Mo J, Zheng W, Low JJH, Ng J, Ilancheran A, Huang Z. High wavenumber Raman spectroscopy for in vivo detection of cervical dysplasia. Anal Chem 2009;81:8908-15. http://dx.doi.org/10.1021/ac9015159.
- Mourant J, Kunapareddy P, Carpenter S, Freyer JP. Vibrational spectroscopy for identification of biochemical changes accompanying carcinogenesis and the formation of necrosis. Gynecol Oncol 2005;99:S58-60. http://dx.doi.org/10.1016/j.ygyno.2005.07.044.
- Mourant JR, Bocklage TJ, Powers TM, Greene HM, Bullock KL, Marr-Lyon LR, et al. In vivo light scattering measurements for detection of precancerous conditions of the cervix. Gynecol Oncol 2007;105:439-45. http://dx.doi.org/10.1016/j.ygyno.2007.01.001.
- Mourant JR, Bocklage TJ, Powers TM, Greene HM, Dorin MH, Waxman AG, et al. Detection of cervical intraepithelial neoplasias and cancers in cervical tissue by in vivo light scattering. J Low Genit Tract Dis 2009;13:216-23. http://dx.doi.org/10.1097/LGT.0b013e318195d91b.
- Mourant JR, Powers TM, Bocklage TJ, Greene HM, Dorin MH, Waxman AG, et al. In vivo light scattering for the detection of cancerous and precancerous lesions of the cervix. Appl Opt 2009;48:D26-35. http://dx.doi.org/10.1364/AO.48.000D26.
- Muller MG, Georgakoudi I, Feld MS, Chu S, Vuletic V, Kerman AJ, et al. Proceedings of the 15th International Conference on LASER Spectroscopy, Snowbird, UT, 10–15 June 2001. Singapore: World Scientific; 2002.
- Murali Krishna C, Prathima NB, Malini R, Vadhiraja BM, Bhatt RA, Fernandes DJ, et al. Raman spectroscopy studies for diagnosis of cancers in human uterine cervix. Vib Spectrosc 2006;41:136-41. http://dx.doi.org/10.1016/j.vibspec.2006.01.011.
- Muthuvelu K, Shanmugam S, Koteeswaran D, Srinivasan S, Venkatesan P, Aruna P, et al. Synchronous luminescence spectroscopic characterization of blood elements of normal and patients with cervical cancer. Proc SPIE 2011;7895.
- Nath A, Rivoire K, Chang S, West L, Cantor SB, Basen-Engquist K, et al. A pilot study for a screening trial of cervical fluorescence spectroscopy. Int J Gynecol Cancer 2004;14:1097-107. http://dx.doi.org/10.1111/j.1048-891X.2004.14607.x.
- Nordstrom RJ, Burke L, Niloff JM, Myrtle JF. Identification of cervical intraepithelial neoplasia (CIN) using UV-excited fluorescence and diffuse-reflectance tissue spectroscopy. Lasers Surg Med 2001;29:118-27. http://dx.doi.org/10.1002/lsm.1097.
- Nour El-Din N, Mohii El-Din AR, Sanad AS, Hegazy AR. The use of colposcopy versus colposcopy and optical detection system in diagnosis of squamous intraepithelial lesions of the cervix: a clinical comparative study. Int J Gynaecol Obstet 2009;107. http://dx.doi.org/10.1016/S0020-7292(09)61059-6.
- O’Connell MP, Tidy J, Wisher SJ, Avis NJ, Brown BH, Lindow SW. An in vivo comparative study of the pregnant and nonpregnant cervix using electrical impedance measurements. Br J Obstet Gynaecol 2000;107:1040-1. http://dx.doi.org/10.1111/j.1471-0528.2000.tb10410.x.
- Okimoto GS, Parker MF, Mooradian GC, Saggese SJ, Grisanti A, O’Connor DM, et al. New features for detecting cervical pre-cancer using hyperspectral diagnostic imaging. Proc SPIE 2001;4255:67-79. http://dx.doi.org/10.1117/12.426747.
- Orfanoudaki IM, Themelis GC, Sifakis SK, Fragouli DH, Panayiotides JG, Vazgiouraki EM, et al. A clinical study of optical biopsy of the uterine cervix using a multispectral imaging system. Gynecol Oncol 2005;96:119-31. http://dx.doi.org/10.1016/j.ygyno.2004.09.013.
- Papoutsoglou G, Potirakis A, Balas C. Modeling of epithelia transport phenomena related with the acetowhitening optical changes: potential for the in-vivo diagnosis of cervical neoplasia. Proc SPIE 2008;6991.
- Park SY, Follen M, Milbourne A, Rhodes H, Malpica A, MacKinnon N, et al. Automated image analysis of digital colposcopy for the detection of cervical neoplasia. J Biomed Opt 2008;13. http://dx.doi.org/10.1117/1.2830654.
- Park SY, Sargent D, Lieberman R, Gustafsson U. Domain-specific image analysis for cervical neoplasia detection based on conditional random fields. IEEE Trans Med Imaging 2011;30:867-78. http://dx.doi.org/10.1109/TMI.2011.2106796.
- Park SY, Sargent D, Wolters R, Lieberman RW. n.d. http://dx.doi.org/10.1109/ICSC.2010.85.
- Parker MF, Mooradian GC, Karins JP, O’Connor DM, Speer BA, Owensby PD, et al. Hyperspectral diagnostic imaging of the cervix: report on a new investigational device. J Low Genit Tract Dis 2000;4:119-24.
- Parker MF, Mooradian GC, Okimoto GS, O’Connor DM, Miyazawa K, Saggese SJ. Initial neural net construction for the detection of cervical intraepithelial neoplasia by fluorescence imaging. Am J Obstet Gynecol 2002;187:398-402. http://dx.doi.org/10.1067/mob.2002.123940.
- Parker MF. Emerging technology in cervical cancer screening: spectroscopy. Clin Obstet Gynecol 2005;48:209-17. http://dx.doi.org/10.1097/01.grf.0000151586.23981.74.
- Pfefer TJ, Sharma D, Agrawal A, Matchette LS. Evaluation of a fiberoptic-based system for optical property measurement in highly attenuating turbid media. Proc SPIE 2005;5691:163-71. http://dx.doi.org/10.1117/12.591101.
- Pitris C, Drexler W, Ghanta RK, Kartner FX, Morgner U, Fujimoto JG, et al. n.d. http://dx.doi.org/10.1109/CLEO.2000.907315.
- Pitris C, Hsiung P-L, Li X, Drexler W, Fujimoto JG, Goodman A, et al. n.d.
- Pogue BW, Kaufman HB, Zelenchuk A, Harper W, Burke GC, Burke EE, et al. Analysis of acetic acid-induced whitening of high-grade squamous intraepithelial lesions. J Biomed Opt 2001;6:397-403. http://dx.doi.org/10.1117/1.1412850.
- Porras C, Wentzensen N, Rodríguez AC, Morales J, Burk RD, Alfaro M, et al. Switch from cytology-based to human papillomavirus test-based cervical screening: implications for colposcopy. Int J Cancer 2012;130:1879-87. http://dx.doi.org/10.1002/ijc.26194.
- Pretorius RG, Bao Y-P, Belinson JL, Burchette RJ, Smith JS, Qiao Y-L. Inappropriate gold standard bias in cervical cancer screening studies. Int J Cancer 2007;121:2218-24. http://dx.doi.org/10.1002/ijc.22991.
- Qiang J, Engel J, Craine E. Classifying cervix tissue patterns with texture analysis. Pattern Recognition 2000;33:1561-73. http://dx.doi.org/10.1016/S0031-3203(99)00123-5.
- Qu JY, Cheung TH, Hua J, Lo WK. Calibrated autofluorescence imaging for classification of tissue and detection of early cervical cancer. Proc SPIE 2001;4707:97-102.
- Redden Weber C, Schwarz RA, Atkinson EN, Cox DD, MacAulay C, Follen M, et al. Model-based analysis of reflectance and fluorescence spectra for in vivo detection of cervical dysplasia and cancer. J Biomed Opt 2008;13. http://dx.doi.org/10.1117/1.3013307.
- Robichaux A, Lieber CA, Shappell H, Huff B, Jones H, Mahadevan-Jansen A. In vivo detection of cervical dysplasia with near-infrared Raman spectroscopy. Proc SPIE 2002;4614:145-51. http://dx.doi.org/10.1117/12.460793.
- Robichaux-Viehoever A, Kanter E, Shappell H, Billheimer D, Jones H. Characterization of Raman spectra measured in vivo for the detection of cervical dysplasia. Appl Spectrosc 2007;61:986-93. http://dx.doi.org/10.1366/000370207781746053.
- Sanad AS, Nour El-Din N, Mohii El-Din AR, Hegazy AR. The use of colposcopy versus colposcopy and optical detection system in diagnosis of squamous intraepithelial lesions of the cervix: a clinical comparative study. Middle East Fertil Soc J 2011;16:137-42. http://dx.doi.org/10.1016/j.mefs.2010.12.005.
- Sapozhnikova VV, Shakhova NM, Kamensky VA, Kuranov RV, Loshenov VB, Petrova SA. Complementary use of optical coherence tomography and 5-aminolevulinic acid induced fluorescent spectroscopy for diagnosis of neoplastic processes in cervix and vulva. Proc SPIE 2003;4956:81-8. http://dx.doi.org/10.1117/12.477738.
- Sapozhnikova VV, Shakhova NM, Kamensky VA, Petrova SA, Snopova LB, Kuranov RV. Capabilities of fluorescence spectroscopy using 5-ALA and optical coherence tomography for diagnosis of neoplastic processes in the uterine cervix and vulva. Laser Physics 2005;15:1664-73.
- Schomacker KT, Meese TM, Jiang C, Abele CC, Dickson K, Sum ST, et al. Novel optical detection system for in vivo identification and localization of cervical intraepithelial neoplasia. J Biomed Opt 2006;11. http://dx.doi.org/10.1117/1.2208987.
- Sergeev AM, Gelikonov VM, Gelikonov GV, Feldchtein FI, Kuranov RV, Gladkova ND, et al. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt Express 1997;1:432-40. http://dx.doi.org/10.1364/OE.1.000432.
- Shakhova NM, Kuznetsova IA, Kachalina TS, Yunusova EE. New approach to cervical cancer diagnosing using OCT-colposcopy [poster]. Cleveland, OH: Imalux Corporation; n.d.
- Shakhova N, Kuznetzova I, Kachalina T, Gladkova N, Gelikonov V, Kamensky V, et al. 5th International Multidisciplinary Congress (EUROGIN 2003), Paris, France, 13–16 April 2003. Bologna: Medimond Inc.; n.d.
- Shakhova NM, Gelikonov GV, Kamensky VA, Kuranov RV, Sapozhnikova VV, Turchin IV, et al. New advances in optical coherence tomography for diagnostics of cervical neoplasia. Proc SPIE 2003;5140:138-43. http://dx.doi.org/10.1117/12.500095.
- Shakhova NM, Sapozhnikova VV, Kamensky VA, Kuranov RV, Losheno VB, Petrova SA, et al. Optical methods for diagnosis of neoplastic processes in the uterine cervix and vulva. J Appl Res 2003;3:144-55.
- Shinn E, Le T, Gallegos J, Basen-Engquist K. A pilot analysis of multispectral digital colposcopy for women with high-grade squamous intraepithelial lesion (HGSIL) Pap smear results. Gynecol Oncol 2007;107:S83-5. http://dx.doi.org/10.1016/j.ygyno.2007.07.046.
- Sokolov K, Nieman LT, Myakov A, Gillenwater A. Polarized reflectance spectroscopy for pre-cancer detection. Technol Cancer Res Treat 2004;3:1-14.
- Srinivasan Y, Corona E, Nutter B, Mitra S, Bhattacharya S. A unified model-based image analysis framework for automated detection of precancerous lesions in digitized uterine cervix images. IEEE J Sel Top Signal Process 2009;3. http://dx.doi.org/10.1109/JSTSP.2008.2011102.
- Sung K-B, Richards-Kortum R, Follen M, Malpica A, Liang C, Descour MR. Fiber optic confocal reflectance microscopy: a new real-time technique to view nuclear morphology in cervical squamous epithelium in vivo. Opt Express 2003;11:3171-81. http://dx.doi.org/10.1364/OE.11.003171.
- Tan J, Quinn MA, Pyman JM, Delaney PM, McLaren WJ. Detection of cervical intraepithelial neoplasia in vivo using confocal endomicroscopy. BJOG 2009;116:1663-70. http://dx.doi.org/10.1111/j.1471-0528.2009.02261.x.
- Trokhanova OV, Chijova YA, Okhapkin MB, Korjenevsky AV, Tuykin TS. Using of electrical impedance tomography for diagnostics of the cervix uteri diseases. JPCS 2010;224.
- Tromberg BJ, Yodh AG, Tamura M, Sevick-Muraca EM. n.d.
- Utzinger U, Heintzelman DL, Mahadevan-Jansen A, Malpica A, Follen M, Richards-Kortum R. Near-infrared Raman spectroscopy for in vivo detection of cervical precancers. Appl Spectrosc 2001;55:955-9. http://dx.doi.org/10.1366/0003702011953018.
- Van Raad V. n.d.
- Van Raad V, Hamza MH. IASTED International Conference on Biomedical Engineering; Salzburg, Austria, 25–27 June 2003. Anaheim, CA: ACTA Press; n.d.
- Van Raad V, Liu Y, Jiang T, Zhang C. Computer vision for biomedical image applications. First international workshop, CVBIA 2005, Beijing, China, 21 October 2005. Proceedings. Berlin: Springer-Verlag; 2005.
- Van Raad V, Xue Z, Lange H. Lesion margin analysis for automated classification of cervical cancer lesions. Proc SPIE 2006;6144.
- Vargas G, Shilagard T, Johnston R, Bell B, Stegall RL, Vincent K, et al. Use of high-resolution confocal imaging of the vaginal epithelial microstructure to detect microbicide toxicity. J Infect Dis 2009;199:1546-52. http://dx.doi.org/10.1086/598221.
- Vargis E, Byrd T, Khabele D, Mahadevan-Jansen A. Using Raman spectroscopy to detect cervical dysplasia in minority populations. Gynecol Oncol 2010;116.
- Vargis E, Byrd T, Reese J, Khabele D, Al-Hendy A, Mahadevan-Jansen A. Detecting changes in the cervix with Raman spectroscopy. AIP Conf Proc 2010;1267:441-2. http://dx.doi.org/10.1063/1.3482605.
- Vargis E, Kanter E, Kanter G, Rao G, Mahadevan-Jansen A. Proximity to disease has a significant impact on automated disease classification of normal tissues. Gynecol Oncol 2010;116:S33-4.
- Vargis E, Kanter EM, Majumder SK, Keller MD, Beaven RB, Rao GG, et al. Effect of normal variations on disease classification of Raman spectra from cervical tissue. Analyst 2011;136:2981-7. http://dx.doi.org/10.1039/c0an01020k.
- Vengadesan N, Muthuvelu K, Anbupalam T, Madhuri S, Parmeswaran D, Aruna P, et al. Optical biopsy of normal and cancerous cervical tissues by native fluorescence spectroscopy. Proc SPIE 2001;4261:145-9. http://dx.doi.org/10.1117/12.424530.
- Vengadesan N, Anbupalam T, Hemamalini S, Ebenezar J, Muthvelu K, Koteeswaran D, et al. Characterization of cervical normal and abnormal tissues by synchronous luminescence spectroscopy. Proc SPIE 2002;4613:13-7. http://dx.doi.org/10.1117/12.465247.
- Vincent KL, Bell BA, Rosenthal SL, Stanberry LR, Bourne N, Sweeney YTC, et al. Application of optical coherence tomography for monitoring changes in cervicovaginal epithelial morphology in macaques: potential for assessment of microbicide safety. Sex Transm Dis 2008;35:269-75. http://dx.doi.org/10.1097/OLQ.0b013e31815abad8.
- Vincent KL, Bourne N, Bell BA, Vargas G, Tan A, Cowan D, et al. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: a promising model for testing safety of candidate microbicides. Sex Transm Dis 2009;36:312-18. http://dx.doi.org/10.1097/OLQ.0b013e31819496e4.
- Weingandt H, Stepp H, Baumgartner R, Diebold J, Xiang W, Hillemanns P. Autofluorescence spectroscopy for the diagnosis of cervical intraepithelial neoplasia. BJOG 2002;109:947-51. http://dx.doi.org/10.1111/j.1471-0528.2002.01311.x.
- Werner CL, Griffith WF, Ashfaq R, Gossett D, Wilkinson E, Raab S, et al. Comparison of human papilloma virus testing and spectroscopy combined with cervical cytology for the detection of high-grade cervical neoplasia. J Low Genit Tract Dis 2007;11:73-9. http://dx.doi.org/10.1097/01.lgt.0000230208.58118.58.
- Wilkinson EJ, Raab SS. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: histopathology review procedures and results. Norcross, GA: Guided Therapeutics; 2010.
- Winter ML. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: experience with a low cost commercial prototype. Norcross, GA: Guided Therapeutics; 2010.
- Winter ML, Sternfeld DR. Multimodal spectroscopy as a triage test for women at risk for cervical neoplasia: experience with a low cost commercial prototype. J Low Genit Tract Dis 2010;14.
- Wu T, Cheung TH, Lo KW, Qu JY. Detecting neoplastic growths in vivo with autofluorescence imaging. Proc SPIE 2003;4958:71-7. http://dx.doi.org/10.1117/12.479804.
- Wu T, Qu JY, Cheung T-H, Lo KW-K, Yu M-Y. Preliminary study of detecting neoplastic growths in vivo with real time calibrated autofluorescence imaging. Opt Express 2003;11:291-8. http://dx.doi.org/10.1364/OE.11.000291.
- Wu TT, Cheung TH, Lo KW, Wen Y, Qu JY. Fluorescence detection of neoplastic growths in vivo. Diagnostic Optical Spectroscopy in Biomedicine II 2003;5141:58-63.
- Wu TT, Cheung TH, Wen Y, Qu JY. n.d. http://dx.doi.org/10.1109/COS.2003.1278205.
- Wu TT, Cheung T-H, Yim S-F, Qu JY. Optical imaging of cervical precancerous lesions based on active stereo vision and motion tracking. Opt Express 2008;16:11224-30. http://dx.doi.org/10.1364/OE.16.011224.
- Wu TT, Cheung T-H, Yim S-F, Qu JY. Quantitative optical imaging of early cervical cancer: mechanisms, methods, and clinical study. Proc SPIE 2009;7187.
- Wu T, Cheung T-H, Yim S-F, Qu JY. Clinical study of quantitative diagnosis of early cervical cancer based on the classification of acetowhitening kinetics. J Biomed Opt 2010;15. http://dx.doi.org/10.1117/1.3365940.
- Wulan N, Rasool N, Belinson SE, Belinson JL, Wang C, Rong X, et al. Clevaland, OH: Imalux Corporation; 2008.
- Wulan N, Rasool N, Belinson SE, Wang C, Rong X, Zhang W, et al. Study of the diagnostic efficacy of real-time optical coherence tomography as an adjunct to unaided visual inspection with acetic acid for the diagnosis of preinvasive and invasive neoplasia of the uterine cervix. Int J Gynecol Cancer 2010;20:422-7. http://dx.doi.org/10.1111/IGC.0b013e3181d09fbb.
- Yang B, Pretorius RG, Belinson JL, Zhang X, Burchette R, Qiao Y-L. False negative colposcopy is associated with thinner cervical intraepithelial neoplasia 2 and 3. Gynecol Oncol 2008;110:32-6. http://dx.doi.org/10.1016/j.ygyno.2008.03.003.
- Yu Y, Huang J, Zhang S, Restif C, Huang X, Metaxas D. 2011.
- Zagaynova E, Gladkova N, Shakhov A, Terentyeva A, Snopova L, Shakhov N, et al. Optical coherence tomography: potentialities in clinical practice. Proc SPIE 2004;5474:103-14. http://dx.doi.org/10.1117/12.580771.
- Zagaynova E, Gladkova N, Shakhova HM, Gelikonov G, Gelikonov V. Endoscopic OCT with forward-looking-probe: clinical studies in urology and gastroenterology. J. Biophotonics 2008;1:114-28. http://dx.doi.org/10.1002/jbio.200710017.
- Zara JM, Lingley-Papadopoulos CA. Endoscopic OCT approaches toward cancer diagnosis. IEEE J Sel Top Quantum Electron 2008;14:70-81. http://dx.doi.org/10.1109/JSTQE.2007.912020.
- Zertuche J, Pena A. A new colposcopic technique in uterine cervical cancer. Int J Gynaecol Obstet 2009;107:S605-6. http://dx.doi.org/10.1016/S0020-7292(09)62163-9.
- Zhang S, Huang J, Metaxas D, Wang W, Huang X. Discriminative sparse representations for cervigram image segmentation n.d.
- Zhao H, Liang J, Ma J, Yang Z. n.d.
- Zhao F-H, Zhang W-H, Pan Q-J, Zhang X, Chen W, Liu B, et al. A study of cervical cancer screening algorithms. Chung Hua Chung Liu Tsa Chih 2010;32:420-4.
Appendix 1 Literature search strategies
Appendix 2 Data extraction tables
Appendix 3 Quality assessment
Appendix 4 Table of excluded studies with rationale
Appendix 5 Sensitivity analysis of the base case
Appendix 6 Sensitivity analyses of the secondary analysis quality-adjusted life-year decrement of treatment biopsy 0.13 (from 0.005)
Sensitivity analyses of the secondary analysis quality-adjusted life-year decrement of treatment biopsy 0.13 (from 0.005) (PDF download)
Appendix 7 Secondary analysis cost of treatment biopsy £2758 sensitivity analyses
Secondary analysis cost of treatment biopsy £2758 sensitivity analyses (PDF download)
Appendix 8 Protocol (submitted 22 September 2011)
Glossary
Technical terms and abbreviations are used throughout this report. The meaning is usually clear from the context but a glossary is provided for the non-specialist reader.
- Acetowhitening
- Whitening effect following application of acetic acid to epithelial tissue, which is a sign of increased nuclear protein.
- Adverse effect
- An abnormal or harmful effect caused by, and attributable to, exposure to a medication or other intervention, which is indicated by some result such as death, a physical symptom or visible illness. An effect may be classed as adverse if it causes functional or anatomical damage, causes irreversible change in the homeostasis of the organism, or increases the susceptibility of the organism to other chemical or biological stress.
- APX 100
- A digital image analysing system for detecting cancerous and precancerous cervical tissue. It works by measuring the resistivity (via electrical impedance spectroscopy) of cervical epithelial cells.
- Cervical intraepithelial neoplasia
- A term describing abnormal changes in the squamous epithelial cells of the cervix. The disorder is graded according to its pathological progress, from CIN1 to CIN3.
- Colposcope
- A magnifying instrument designed to facilitate visual inspection of the cervix.
- Correlation meeting
- A meeting where the pathologists and colposcopists discuss the results and the management of patients who have clear colposcopic findings, but moderate or severe cytology results.
- DySIS
- A digital video colposcope using dynamic spectral imaging for detecting cancerous and precancerous cervical tissue. It works, following application of acetic acid, by mapping the acetowhitening of the epithelium of the cervix (the DySISmap). [Note: Subsequent to the production of this report, DySIS Medical informed the assessment group that the current terminology for the DySIS technology is ‘DySIS colposcopy’when referring to the DySISmap and colposcopy combined, and ‘DySISmap’when referring to the DySISmap alone (this was previously known as ‘DSI map’or ‘DSI colour-coded map’).]
- Dyskaryosis
- A term describing abnormality of the cell nucleus (but not the cytoplasm).
- Electrical impedance spectroscopy
- A form of spectroscopy that works by utilising electric current patterns.
- Histology
- An abbreviation of histopathology.
- Histopathology
- The microscopic study of tissue samples to enable diagnosis.
- Human papillomavirus
- A type of virus that can affect the skin and the moist membranes lining parts of the body. Some types of human papillomavirus (known as high-risk human papillomaviruses) can cause dyskaryosis in the cells of the cervix.
- Liquid-based cytology
- A method of preparing cervical samples for laboratory examination.
- LuViva Advanced Cervical Scan
- A digital image analysing system for detecting cancerous and precancerous cervical tissue. It works by detecting biochemical and morphological changes at the cellular level (using optical spectroscopy).
- NHS Cervical Screening Programme
- The programme set up in the UK aimed at detecting and treating early abnormalities which, if left untreated, could lead to cervical cancer.
- Niris Imaging System
- A digital image analysing system for detecting cancerous and precancerous cervical tissue. It works using optical coherence tomography to produce a two-dimensional image of the tissue.
- Optical coherence tomography
- A technique for creating two- or three-dimensional cross-sectional images of tissue using infrared light.
- Pathologist
- The individual responsible for examining and interpreting cell and/or tissue samples.
- Quality of life
- A concept incorporating all the factors that might impact on an individual's life, including factors such as the absence of disease or infirmity, as well as other factors that might affect the individual's physical, mental and social well-being.
- Quality-adjusted life-year
- An index of health gain by which survival duration is weighted or adjusted by the patient's quality of life during the survival period. Quality-adjusted life-years have the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- See and treat
- The removal of an abnormal area during colposcopy.
- Spectroscopy
- An analytical method for studying the structural and biochemical features of tissue, most commonly by utilising electromagnetic spectra readings.
- Speculum
- An instrument for opening a body cavity in order to allow visual inspection.
- Statistical significance
- An estimate of the probability of an association (effect) as large or larger than what is observed in a study occurring by chance, usually expressed as a p-value.
- Threshold analysis
- Amount of variation needed in the parameter values of a model to achieve a specified outcome. In the context of cost-effectiveness analysis in the UK NHS, this specified outcome is usually the cost-effectiveness threshold of £20,000–30,000 per additional QALY gained.
- Transformation zone
- An area of the cervix where nearly all precancerous and cancerous changes occur.
List of abbreviations
- AGUS
- atypical glandular cells of undetermined significance
- AiC
- academic in confidence
- ASC-H
- atypical squamous cells with possible high-grade squamous intraepithelial lesion
- ATP
- according to protocol
- CE
- Conformité Européenne
- CI
- confidence interval
- CIN
- cervical intraepithelial neoplasia
- CRD
- Centre for Reviews and Dissemination
- DSI
- dynamic spectral imaging
- DySIS
- dynamic spectral imaging system
- EAG
- External Assessment Group
- GP
- general practitioner
- HPV
- human papillomavirus
- hrHPV
- high-risk human papillomavirus
- HRQoL
- health-related quality of life
- HSIL
- high-grade squamous intraepithelial lesion
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LBC
- liquid-based cytology
- LLETZ
- large-loop excision of the transformation zone
- LR
- likelihood ratio
- lrHPV
- low-risk human papillomavirus
- LSIL
- low-grade squamous intraepithelial lesion
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- NR
- not reported
- OCT
- optical coherence tomography
- ONS
- Office for National Statistics
- PCM
- pseudocolour map
- PPV
- positive predictive value
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- SIGN
- Scottish Intercollegiate Guidelines Network
- STARD
- STAndards for the Reporting of Diagnostic accuracy studies
- TOMBOLA
- Trial of Management of Borderline and Other Low-Grade Abnormal Smears
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.