Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 09/145/01. The contractual start date was in March 2011. The draft report began editorial review in November 2011 and was accepted for publication in June 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Bond et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
In 1988, the NHS introduced a national breast screening programme (NHSBSP) for women aged 50–64 years in response to recommendations by the Forrest Committee. 1 In 2001, the age range was extended to women aged 50–70 years, currently it is being expanded to women aged 47–73 years. In the UK, women are invited for routine screening by mammography every 3 years.
Rate of uptake
The most recent statistics from the Health and Social Care Information Centre show that in 2009–10 more than 2.24 million women in this age group in England were invited to take part in the programme, of whom 73.2% attended a screening clinic. 2 The rate of response varied according to the history of previous screening. Women who had previously attended routine screening were more likely to reattend (87.2%) than those who had received their first invitation (69.0%). 2 Of the 1,639,953 women (aged 50–70 years) who attended for routine breast screening in 2009–10 in England, 64,104 (3.9%) were recalled for further assessment. This included additional mammography, ultrasound, cytology, fine-needle aspiration (FNA), core biopsy and/or open biopsy of tissue. Another 1089 women (0.07%) were put on the early recall system and invited for further screening 6 or 12 months later. 2 Of the 64,104 women recalled, 12,525 (19.5%) were diagnosed with cancer through routine screening in England in 2009–10. Thus, 51,579 women of those recalled did not have breast cancer in 2009–10 (80.5% of those recalled and 3.1% of those screened). It is this group of women who are the subject of this systematic review.
Definition of false-positive mammogram
For the purposes of this study the definition of a false-positive mammogram is that given by the World Health Organization (WHO): ‘an abnormal mammogram (one requiring further assessment) in a woman ultimately found to have no evidence of cancer’. 3 This definition is rejected by some clinicians because having a positive screening mammogram is not a diagnosis of cancer in itself, but an indication that further assessment is needed. Nevertheless, for the purposes of this systematic review it is necessary to use the definition most commonly adopted in academic journals.
Incidence of breast cancer
Breast cancer is the most common cancer in the UK, with 48,034 new diagnoses in 2008. 4 It accounts for 31% of all cancers in women, with a one in nine lifetime risk. 4 The incidence of breast cancer in the separate countries within the UK can be seen in Table 1.
| England | Wales | Scotland | Northern Ireland | UK | |
|---|---|---|---|---|---|
| Cases (n) | 39,681 | 2624 | 4232 | 1156 | 47,693 |
| Crude rate per 100,000 population | 151.8 | 171.4 | 158.6 | 127.9 | 152.6 |
| Age-standardised rate (European) per 100,000 population (95% CI) | 123.8 (122.6 to 125.0) | 128.4 (123.5 to 133.4) | 123.6 (119.8 to 127.3) | 116.6 (109.9 to 123.3) | 123.9 (122.8 to 125.0) |
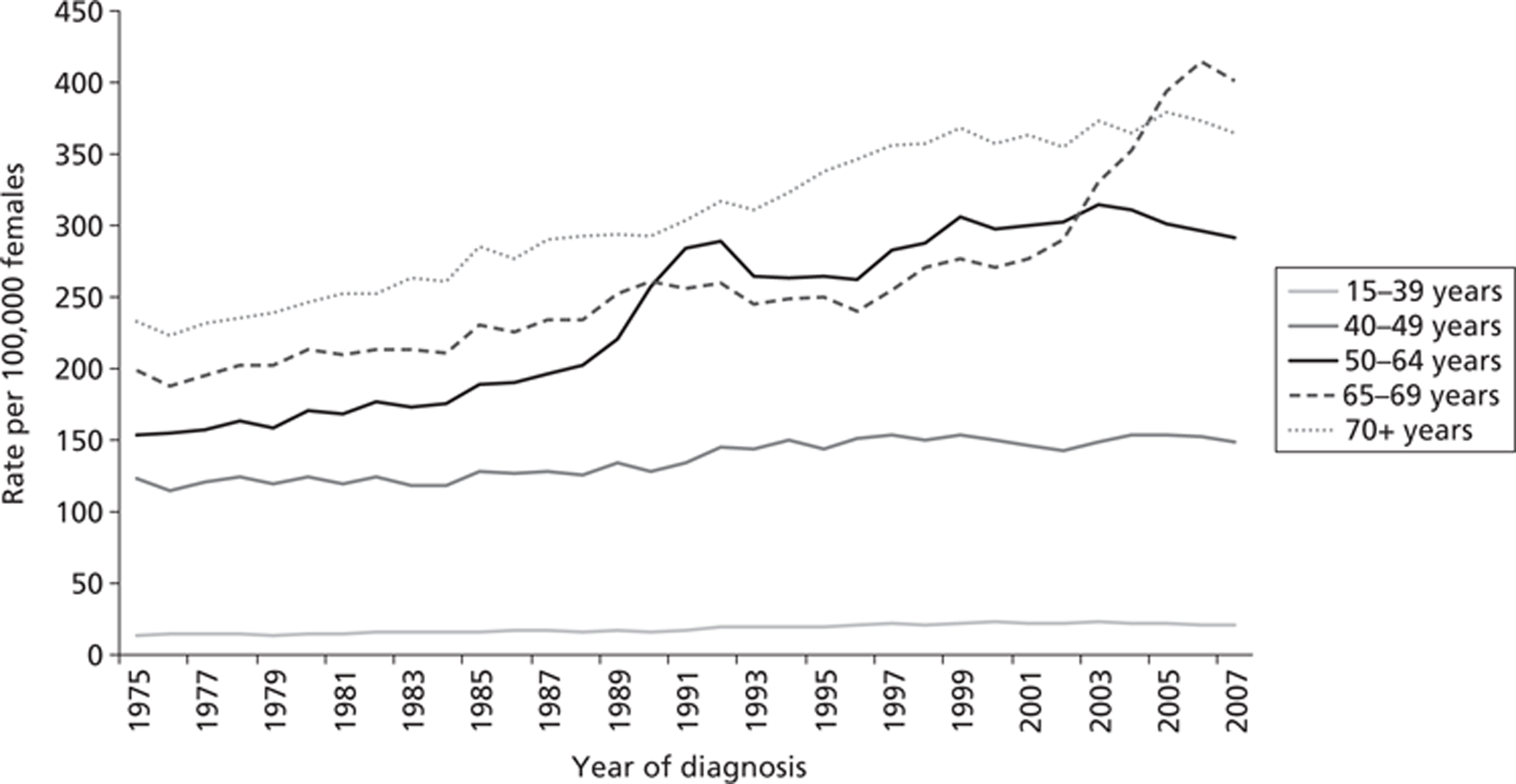
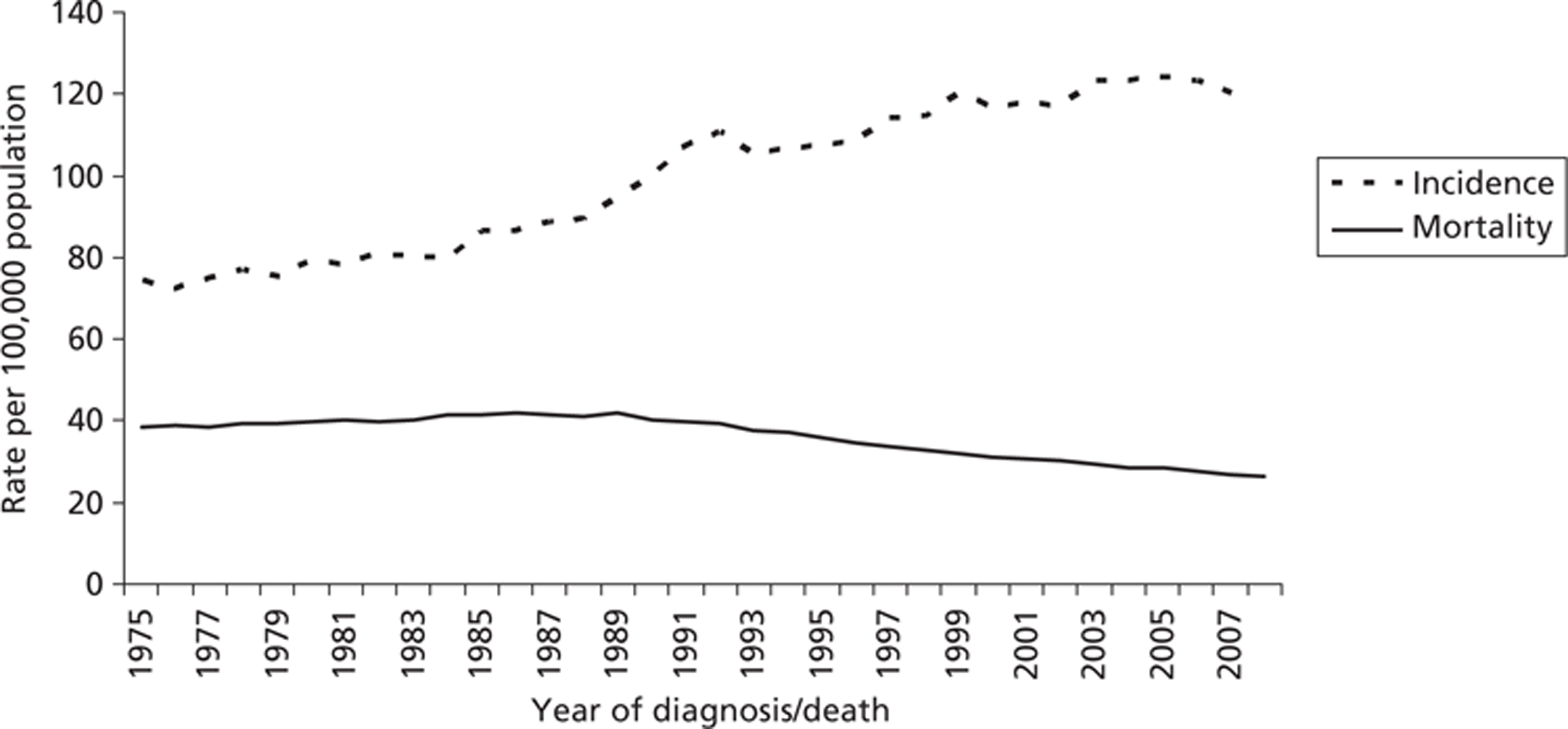
The number of cases of breast cancer in women has been steadily increasing in the UK over the last 30 years, with the annual incidence rising from 24,120 in 1978 to 47,693 in 2007. When the age of the women is standardised, the European age-standardised incidence rate increased by more than half (57%) over this time, from 77 per 100,000 in 1978 to 124 per 100,000 in 2008. 4 However, since the introduction of the national screening programme in 1988, the mortality rate has declined (Figure 1).
FIGURE 1.
Age-standardised European incidence and mortality rates for breast cancer in women, UK, 1975–2008. 4
The rise in incidence has been greatest in women in higher socioeconomic groups. 5 It is thought that this may be linked to their greater use of hormone replacement therapy for menopausal symptoms6 and the trend for having babies later in life. 7
When the figures are broken down by age, the effects of the screening programme can be seen by the sharp increase in incidence over this time among women aged 50–64 years. 8–10 The screening programme will detect cancers that would not have been noted in the patient's lifetime (overdiagnosis) and will bring forward the date of identification of cancer, finding it at an earlier stage, thus producing lead-time bias (Figure 2).
Mortality from breast cancer
The magnitude of the effect of mammography screening on breast cancer mortality is highly contested. Statistics for mortality from breast cancer in England are not available for 2009–10, but in 2008 a little over 10,000 women died from this disease, a rate of 26 per 100,000. 11 It can be shown that mortality from breast cancer began to decline in England at about the same time that the national breast screening programme was introduced, from about 40 per 100,000 to about 26 per 100,000 in 2008. 11 However, the exact effect that breast screening has had on breast cancer mortality is difficult to determine because it is hard to disaggregate the effects of improved treatments and other factors from the effects of screening.
However, a recent retrospective trend analysis by Autier et al. 12 has attempted to do this. Autier et al. 12 compared breast cancer mortality trends in three pairs of neighbouring European countries using WHO data from 1989 to 2006. The pairs of countries had similar demographics, quality and availability of health care. They differed in when breast cancer screening was introduced, with one country introducing it in about 1990 and the other about 10–15 years later. Autier et al. 12 calculated changes in breast cancer mortality using linear regressions of age-adjusted death rates. They found that although there was a wide difference in timing of the introduction of breast cancer screening in the pairs of countries there was a striking similarity in the rate of reduction of breast cancer mortality from 1990. They concluded that this reduction in breast cancer mortality was unlikely to have been the result of mammography screening. Similar findings have been replicated by the US Preventative Services Task Force,13 who have revised their endorsement of routine screening for women aged < 50 years. 13 In a letter to the British Medical Journal (BMJ), Bleyer14 presented a graph comparing US data with data from Autier et al. 12 Bleyer14 concluded that improved treatment, rather than screening, is the main reason for the reduction in mortality.
This research by Autier et al. 12 has been criticised by de Koning15 for being based on geographical comparisons which are unreliable and for the use of standardised all-age mortality. Further criticisms are that Autier et al. 12 have not accounted for the delay in time from the introduction of screening to realising its benefits.
Furthermore, in a recent systematic review of breast screening randomised controlled trials (RCTs), Gotzsche and Nielsen16 (n = 600,000) estimated that breast screening led to a 15% reduction in breast cancer mortality, but, conversely, that there was also a 30% increase in overtreatment of women whose cancer would never become apparent in their lifetime. 16 They estimated that, over 10 years, one woman would be saved from death by breast cancer for every 2000 women invited for screening. Additionally, 10 healthy women who would have remained undiagnosed would have been treated unnecessarily for breast cancer. On top of this, for the same cohort, at least another 200 women would go through the possible distress of a false-positive outcome. 16
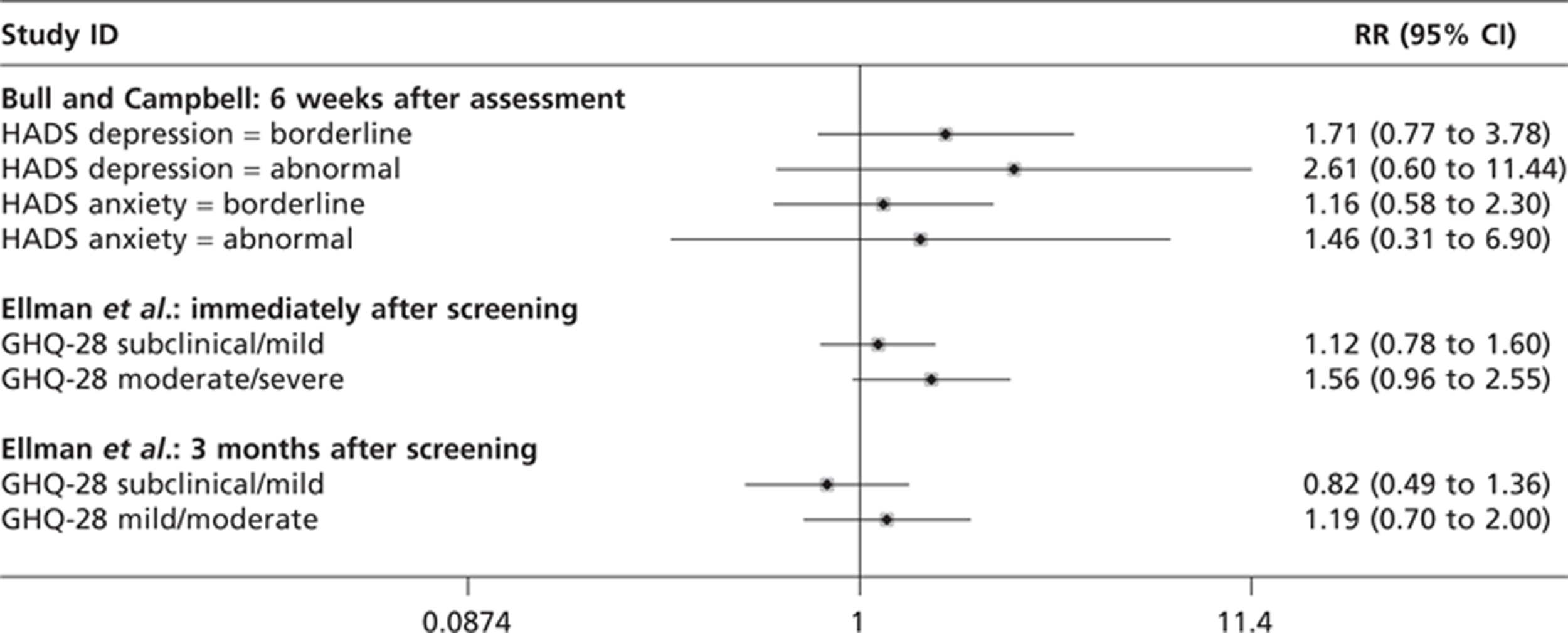
Of the eight eligible trials in the Gotzsche and Nielsen review16 (New York 1963,17–19 Malmo 197620–22 and Malmo II 1978,23 Two–County 1977,24–26 Edinburgh 1978,27–29 Canada 1980,30–33 Stockholm 1981,34–37 Goteborg 198238,39 and UK Age Trial 199140–44), one was excluded from meta-analysis because the randomisation was seriously flawed and the data held to be unreliable (Edinburgh 197828,29). Gotzsche and Nielsen16 found that only three of the remaining trials had adequate randomisation. Pooling the data from these trials revealed no statistically significant benefit from screening on breast cancer deaths after 7 years {relative risk (RR) 0.93 [95% confidence interval (CI) 0.79 to 1.09]} or after 10 years (RR 0.90; 95% CI 0.79 to 1.02). When the data from these trials were combined with data from the other four suboptimally randomised trials, a statistically significant reduction in death from breast cancer was found after both 7 and 13 years [RR 0.81 (95% CI 0.72 to 0.90) and RR 0.81 (95% CI 0.74 to 0.87), respectively]. The pooled data from the adequately randomised trials similarly showed that there was no significant effect from breast screening on all-cause mortality after 7 years (RR 0.98; 95% CI 0.94 to 1.03) and after 13 years (RR 0.99; 95% CI 0.93 to 1.06). 16 Gotzsche and Nielsen16 did not present data on all-cause mortality from all the included trials because the estimates were unreliable.
FIGURE 3.
(a) Percentages of women taking part in mammography screening in each country. (b) Change in national breast cancer mortality rate relative to country's mean rate during 1980–5. Reproduced from Bleyer A. Breast cancer mortality is consistent with European data. BMJ 2011;343:d5630,14 with permission from BMJ Publishing Group Ltd.
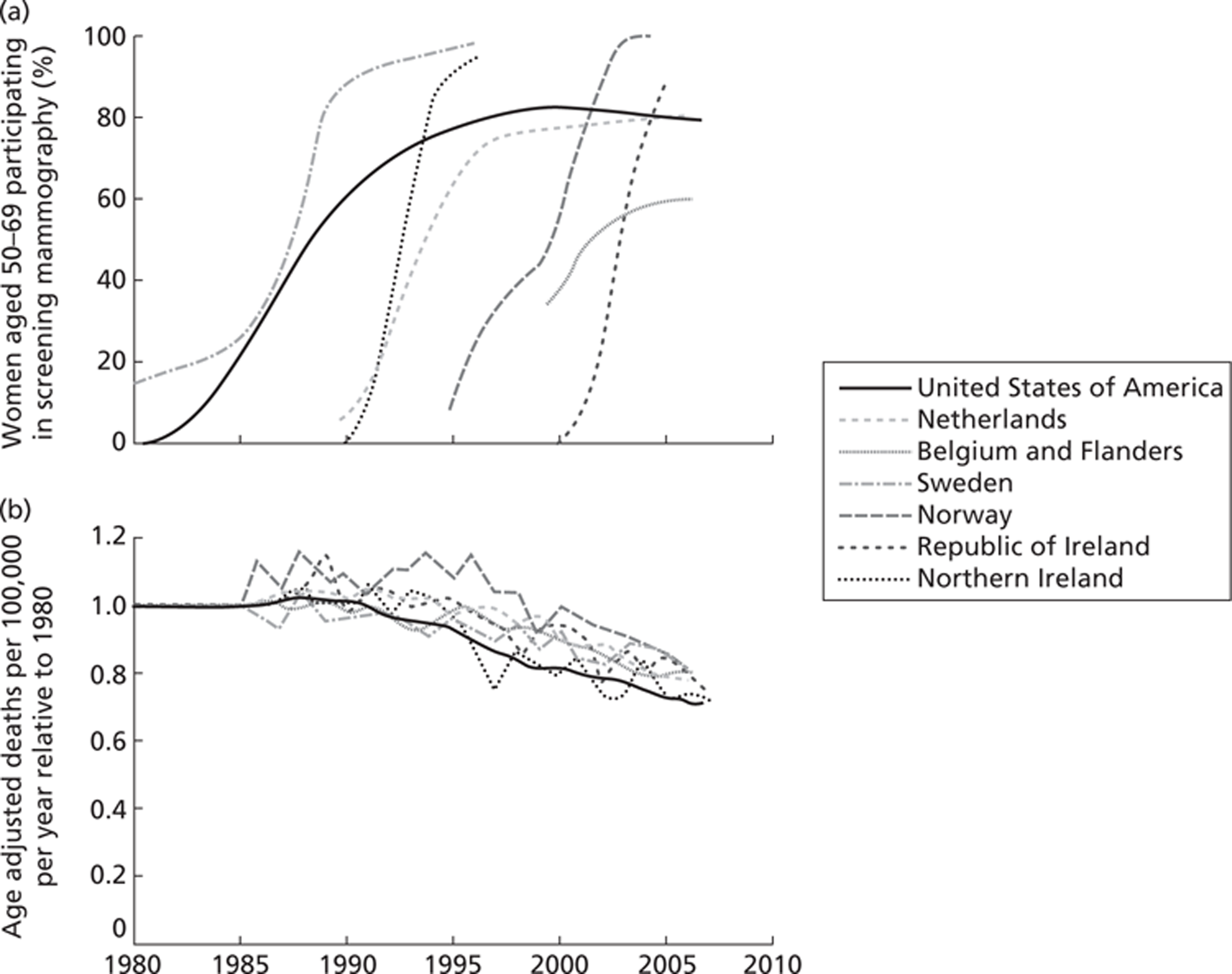
These results are controversial and a lively debate continues about the benefits and harms of breast cancer screening. Gotzsche and Nielsen's16 findings have been heavily criticised by Kopans et al. ,45,46 who claim that the reduction in breast cancer mortality due to screening is of the order of 20–25%. Others are also highly critical and have estimated the reduction in breast cancer due to screening to be as much as 30%. 47,48 Indeed, one modelling study estimated the reduction in mortality due to screening to be between 28% and 65%. 49 The best methods for arriving at an accurate estimate of mortality reduction are also contested. 50 It is beyond the scope of this systematic review to attempt to resolve these differences. However, currently (2011), Professor Sir Mike Richards is undertaking a review to evaluate the benefits and harms of the NHS breast cancer screening services.
Significance for patients
The negative psychological impact of false-positive screening results has been documented in the fields of prenatal and cervical cancer screening. 51,52 Their impact on the psychological well-being and behaviour of women who receive false-positive results from routine mammography has been less well researched and synthesised, particularly in the UK population.
A brief examination of observational studies, looking at the psychological consequences of false-positive mammograms, showed conflicting results. Some studies indicate that, while women show increased distress between receiving the information about the need for a follow-up appointment and receiving the all-clear, in the longer term their anxieties about breast cancer and mammography are not increased. 53–55 Other studies report that there are long-term adverse psychological consequences to receiving a false-positive mammogram. 56,57 The outcomes of studies looking at whether or not having false-positive results affects future attendance at breast screening appointments are similarly conflicting. 58–61
A quantitative systematic review in 2007 by Brewer et al. 62 found that the impact of a false-positive mammogram on subsequent screening attendance varied with nationality, although the reasons for this were unclear. They also reported a varying impact on long-term psychological distress, anxiety and depression, and on other behaviours such as frequency of breast self-examination. However, their review did not report the reasons for this variation in response. Furthermore, Brewer et al. 's review62 found no statistically sound studies that investigated if anxiety over a false-positive mammogram directly affects whether or not women return for routine screening or increase breast self-examination. There was little evidence about the effects on quality of life or trust of health-care services and no evidence about whether or not women who felt anxious after a false-positive screening result replaced routine screening attendance with breast self-examination.
However, the significance of receiving a false-positive mammogram result may go beyond distress and other effects on behaviour. McCann et al. 61 conducted a retrospective cohort study of 140,387 women, aged 49–63 years, attending NHSBSP routine screening clinics. They found that, among those women who were recalled for assessment which showed that they did not have cancer, the risk of interval cancer was increased more than threefold [rate per 1000 women screened, 9.6 (95% CI 6.8 to 12.4) compared with 3.0 (95% CI 2.7 to 3.4); odds ratio (OR) 3.19 (95% CI 2.34 to 4.35)] and these women were more than twice as likely to have cancer detected at their next routine screen in 3 years' time [rate per 1000, 8.4 (95% CI 5.8 to 10.9) vs 3.9 (95% CI 3.5 to 4.3); OR 2.15 (95% CI 1.55 to 2.98)]. This, of course, brings into question whether these women had false-positive or true-positive screening mammograms or whether or not something else explains these phenomena. It is beyond the scope of this systematic review to investigate this further.
Current related guidance
The following guidelines relate to this systematic review.
2011
NHS Breast Screening Programme 59: Quality Assurance Guidelines for Breast Cancer Screening Radiology. 63 These guidelines aim to raise radiology standards in breast cancer screening and relate to the transition to full-field digital mammography and the extension of the invitation to screening to women aged 47–73 years in England. They include minimising the numbers of women who are recalled and, therefore, the numbers of false-positives.
2010
NHS Breast Screening Programme 49: Clinical Guidelines for Breast Cancer Screening Assessment. 64 These guidelines set out minimum standards for breast cancer screening assessment. The guidelines state that this should be done using mammography or ultrasound, with clinical examination and image-guided biopsy if necessary. Women who are not diagnosed with cancer should receive written confirmation of the outcome.
2009
NHS Breast Screening Programme: Quality Assurance Guidelines for Surgeons in Breast Cancer Screening. 65 Among other guidelines for surgeons in breast cancer screening, these guidelines set out waiting-time targets for non-operative biopsy results to be given in < 1 week and for the time between the decision to refer for surgical assessment and the surgery taking place to be ≤ 1 week. They also aim to minimise the numbers of benign diagnostic open surgical biopsies to ≤ 15 per 10,000, prevalent screen and quantity of tissue taken to ≤ 20 g.
Current service provision
The UK NHSBSP is extending its service to invite women aged 47–73 years to attend for screening by mammography every 3 years. The purpose is to detect breast cancer in the general population. Contact details of eligible women are obtained through lists of registered patients by general practitioner (GP) surgery.
Mammography involves having an X-ray taken of the breasts. In the UK, two views are taken: craniocaudal (head-to-foot) and mediolateral oblique (angled side view). The mammogram is then read by two radiologists. Methods of resolving differences in opinion vary from unit to unit, but most commonly arbitration is used and a third radiologist will review the mammogram. If it is found to be normal, then the woman is put on routine recall and will receive another screening invitation in 3 years' time. There may be technical problems with the quality of the film, in which case the woman will be recalled to have the technically inadequate views repeated. Alternatively, the mammogram may show a suspicious area and the woman will be recalled for further assessment. It takes a maximum of 2 weeks from having a mammogram until a letter with the normal results is received. If further assessment is needed, the appointment for this must be within 3 weeks of the initial mammogram.
The further assessment may include another mammogram, ultrasound or core biopsy with or without FNA. FNA employs a 21-gauge needle to remove cells which are then cytologically assessed; biopsy requires a larger 14-gauge needle, which allows histologists to see the architectural context within which cells are placed and so allows more accurate diagnosis. In the UK, the lesions found are graded on a system of increasing severity: B1 is normal, B2 is benign, B3 is suspicious but probably benign, B4 is suspicious and probably cancer and B5 is cancer. B5 is further subdivided into (a) non-invasive disease, most commonly ductal carcinoma in situ (DCIS), a non-invasive, obligate precursor to invasive breast cancer, but a lesion which may not develop into invasive cancer in the lifetime of the woman, which is situated in the milk ducts; and (b) invasive cancer. It is not possible yet to assess which cases of DCIS will progress to invasive cancer and which will not do so in the lifetime of any given woman. It is likely that many such lesions would not affect the woman's lifespan. Furthermore, some lesions that are invasive may not progress to cause morbidity in the life of the individual woman. There is a debate over the amount of overdiagnosis, as this is described, that occurs.
If cancer is found, then the woman will be transferred from screening services to an oncology department. Treatment will normally be received within the NHS 62-day target from initial screening. It is usual to offer treatment for invasive cancer and non-invasive cancer as well as for many indeterminate lesions (B3 and B4).
Throughout the screening and assessment process women should have access to a clinical nurse specialist (CNS) or breast care nurse, whose role is to educate, inform and support her in a manner which is sensitive and timely and recognises her need for safety, comfort and dignity. 66 Women who are recalled are told in their letter that a breast care nurse will be available to talk to at the clinic or before the clinic by telephone if they have any concerns. Screening clinics, which may be some way from a hospital, do not routinely have breast care nurses on site. However, the invitation letter for screening will often mention the availability of a breast care nurse by telephone to allay any concerns.
Research questions
The aim of this research was to conduct a systematic review to identify the psychological impact on women of false-positive screening mammograms and any evidence for the effectiveness of interventions designed to reduce this impact. This is necessary because there is uncertainty about the nature and magnitude of their psychological impact on women, including what the predictors are of negative psychological outcomes that may affect attendance at future mammography screening. There is also a need to identify whether or not these effects differ in women from different backgrounds. This research is important because of the large number of false-positive results that come from routine mammography screening (see above).
The questions that this systematic review will address are:
-
What evidence is there for medium or long-term adverse psychological consequences from false-positive screening mammograms (≥ 1 month after assessment)?
-
Do the types of psychological consequences differ between different groups of women?
-
-
What evidence is there of interventions that reduce adverse psychological consequences?
Measurement of psychological consequences
A number of different measures of psychological morbidity are used in the primary research studies included in this systematic review. A brief summary of their characteristics is given below.
Disease specific
Psychological Consequences Questionnaire
The Psychological Consequences Questionnaire (PCQ)67 is a reliable and validated questionnaire that was developed specifically to measure the psychological consequences of mammography screening. It comprises two subscales, one consisting of 12 statements relating to possible negative consequences of mammography screening (i.e. how often in the past week the woman has experienced loss of sleep, change of appetite, feeling depressed, being scared, feeling tense, feeling under strain, being secretive, being irritable, withdrawing socially, having difficulty in doing ordinary activities at home and work or feeling worried about the future). The second subscale relates to potential benefits from screening and has 10 items covering feeling reassured, being able to cope better with everyday life, feeling less anxious about breast cancer, feeling more hopeful and a greater sense of well-being. The statements are scored on a four-point Likert scale ranging from not at all, rarely, some of the time to quite a lot of the time, with a range of 0–36 for the negative subscale and 0–30 for the positive subscale.
Cancer Worries Scale-Revised
The Cancer Worries Scale-Revised (CWS-R)68 is a six-item questionnaire that assesses the frequency of worries about developing cancer and how these worries affect daily mood and activities. It has been shown to be reliable and valid in at-risk populations. 68–70 The items are scored on a four-point Likert scale ranging from 1 (not at all or rarely) to 4 (almost all of the time).
Generic
Brief COPE
This is a shortened version of the COPE scale, which was developed to assess people's coping responses to stressful situations. The brief COPE71 has been used in breast cancer patients, although the work has not been published, and in hurricane survivors. The brief version has 28 items measuring a range of coping strategies (e.g. self-distraction, active coping, denial, substance use, emotional support, instrumental support, disengagement, venting, positive reframing, planning, humour, acceptance, religion and self-blame).
General Health Questionnaire
The General Health Questionnaire (GHQ) was designed as a screening tool for psychiatric illness in the context of general practice or general medical outpatients (i.e. non-psychiatric settings). 72 The GHQ covers the four domains of depression, anxiety, objectively observable behaviour and hypochondrias with 60 items. This instrument looks at recent experience and elicits responses on a four-point Likert scale using the statements less than usual, no more than usual, rather more than usual and much more than usual. A number of shorter versions have been developed: GHQ-12, GHQ-20, GHQ-28 and GHQ-30. 73 Items are scored 0–3 to give a total score or can be scored dichotomously with a particular threshold deemed to indicate that the respondent is ‘a case’.
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) was developed to screen for psychiatric disorders in general hospital settings, excluding psychiatric wards. 74 It has two subscales, anxiety and depression, which are measured with 14 items using a four-point Likert scale (0–3). The items are totalled to give an overall score for anxiety or depression. Respondents with a score of 8–10 are considered to be ‘doubtful cases’ and ≥ 11 are considered to be ‘cases’.
Life Orientation Test-Revised
The Life Orientation Test75 is a validated measure of dispositional optimism. It has 10 items that are responded to on a five-point Likert scale, ranging from 0 (I strongly disagree) to 4 (I strongly agree).
State–Trait Anxiety Inventory
The State–Trait Anxiety Inventory (STAI) measures anxiety in adults as both a current state and an enduring trait. 76 It consists of a 20-item scale of how the respondent feels in general and a 20-item scale of how they feel now. Each item is scored on a four-point scale that ranges from ‘not at all’ to ‘very much so’. Higher scores are related to higher anxiety.
Chapter 2 Methods
The systematic review was carried out following the principles published by the NHS Centre for Reviews and Dissemination (CRD). 77 The study protocol can be found in Appendix 1.
Methods for reviewing studies
Identification of studies and search strategy
The search strategy comprised the following main elements:
-
searching of electronic bibliographic databases
-
internet searches
-
scrutiny of references of included studies
-
contacting experts in the field.
The following electronic databases were searched in December 2010 for studies which met the inclusion criteria: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Health Management Information Consortium (HMIC), Cochrane Central Register for Controlled Trials, Cochrane Database of Systematic Reviews, CRD Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment (HTA), Cochrane Methodology, Web of Science, Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index-Science, Conference Proceeding Citation Index-Social Science and Humanities, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Sociological Abstracts, the International Bibliography of the Social Sciences (IBSS) and the British Library's Electronic Table of Contents. Ongoing trials were searched for at: UK Clinical Research Network (UKCRN), ControlledTrials.com, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), UK Database of Uncertainties about the Effects of Treatments (DUETs), a filter was applied to capture qualitative research as well as quantitative designs. Further searches for qualitative and grey literature were run in January 2011 on the following databases: MEDLINE In-Process & Other Non-Indexed Citations, EMBASE Classic and EMBASE, British Nursing Index and Archive, Social Policy and Practice, CINAHL plus, The Cochrane Library, HMIC, PsycINFO, Applied Social Sciences Index and Abstracts, Sociological Abstracts, Web of Science, CRD and IBSS. All searches were run from inception to 25 January 2011. Bibliographies of included studies were searched for further relevant studies. References were managed using Reference Manager version 11 (Thomson ResearchSoft, San Francisco, CA, USA) and EPPI-Reviewer 4 (Evidence for Policy and Practice Information and Co-ordinating Centre, University of London, London, UK).
Initial searches were carried out between 8 October 2010 and 25 January 2011. Update searches were carried out on 26 October 2011 and 23 March 2012.
Refer to Appendix 2 for the search strategy for MEDLINE.
Inclusion criteria
The inclusion criteria for the systematic review are summarised in Table 2.
| Question(s) | Criteria | Specification | Notes |
|---|---|---|---|
| 1 and 2 | Population | Women who had received a positive result from routine mammography screening in the UK and had been invited for further assessment which showed that they did not have breast cancer | Where data permitted we looked at subgroups (including socioeconomic status and ethnic group) |
| 2 | Intervention | Those interventions delivered to individuals to address the adverse psychological and behavioural consequences of a false-positive mammogram result | These were individual interventions not group ones |
| 1 | Comparator | Women who had received a negative (normal) result from routine mammography screening in the UK | |
| 2 | Comparator | An absence of an individual intervention in the same population | |
| 1 and 2 | Outcomes | Psychological and behavioural outcomes and those from qualitative studies | Including subsequent attendance at routine mammography screening and quality of life |
| 1 and 2 | Setting | UK | Secondary care |
| 1 and 2 | Study design | Systematic reviews, randomised, non-randomised, observational and qualitative studies | We did not consider individual case studies |
| 1 and 2 | Length of follow-up | At least 1 month from the ‘all-clear’ | Measured over the medium- to long-term (i.e. not the immediate response to receiving a false-positive result) |
| 1 and 2 | Language | English language only | Non-English-language papers were included in the searches and screened, so that the number of potentially includable foreign-language papers is known |
Exclusion criteria
The following types of studies were excluded: narrative reviews, editorials, opinion pieces, non-English-language papers, individual case studies and studies only reported as posters or by abstract where there is insufficient information to assess the quality of the study.
Study selection
Based on the above inclusion/exclusion criteria, papers were selected for review from the titles and abstracts generated by the search strategy. This was done independently by two reviewers (MB, TP); discrepancies were resolved by discussion, with the involvement of a third reviewer if necessary. Retrieved papers were again reviewed and selected against the inclusion criteria by the same independent process.
Data extraction
Data were extracted from included studies by one reviewer using standardised data extraction forms and checked by another reviewer. Authors of studies were contacted to provide missing information, as necessary. Data were gathered on the design, participants, methods, outcomes, baseline characteristics and results of the studies. The data extraction forms can be found in Appendix 3.
Critical appraisal – assessing risk of bias
Studies were assessed for internal and external validity according to criteria suggested by the updated NHS CRD Report No. 4, according to study type. 77,78 The quality of systematic reviews was evaluated using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 79 Individual RCTs were appraised with the Consolidated Standards of Reporting Trials (CONSORT) statement80 and individual observational studies with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 81 There were insufficient studies in each domain to produce a meaningful assessment of publication bias with a funnel plot.
Internal validity
Consideration of internal validity addresses how well potential sources of bias and confounding are acknowledged and accounted for. Bias can be characterised as potentially undermining an experimental study in four ways: through selection bias, so that the participants in each group are dissimilar; performance bias, where the treatment of the different groups varies apart from the intervention; detection bias, which can occur if the study assessors are aware of which groups participants are in; and attrition bias, where all participants are not fully accounted for or violations of the study protocol have occurred. In particular, checks of study internal validity should address the following: whether or not there is sufficient description of the inclusion criteria, outcomes, study design, setting and the intervention to ascertain that study groups were similar in all respects and were treated in similar ways except for the intervention; if a justification for the sample size is given; if appropriate data analysis techniques were used; if dropouts and withdrawals are accounted for; if the technique used to account for missing data is described and adequate; and if assessors were blind to the group status of participants.
Another threat to validity can come from confounding. This is where an unknown agent is acting independently on the outcome being measured and the matter under investigation, so that an association appears to be occurring between the outcome measure and the matter of interest, but which is an artefact of the independent relationships.
External validity
External validity was judged according to the ability of a reader to consider the applicability of findings to a patient group and service setting. Study findings can only be generalisable if they describe a cohort that is representative of the affected population at large. Studies that appeared representative of the UK breast cancer screening population with regard to these considerations were judged to be externally valid.
Methods for analysis and synthesis
Analysis
Analysis was carried out using StatSEv12 software (TX, USA). The principal summary measure was RR with 95% CIs.
Synthesis
All study designs had a narrative synthesis. Additionally:
Randomised controlled trials and controlled trials There was only one RCT and no controlled trials.
Observational studies Observational studies had possible sources of heterogeneity carefully considered before any meta-analysis was attempted to avoid potentially spurious relationships being found. Heterogeneity was explored through assessment of the studies' populations, methods and interventions. The heterogeneity of the data did not permit meta-analysis.
Chapter 3 Results
Quantity of research available
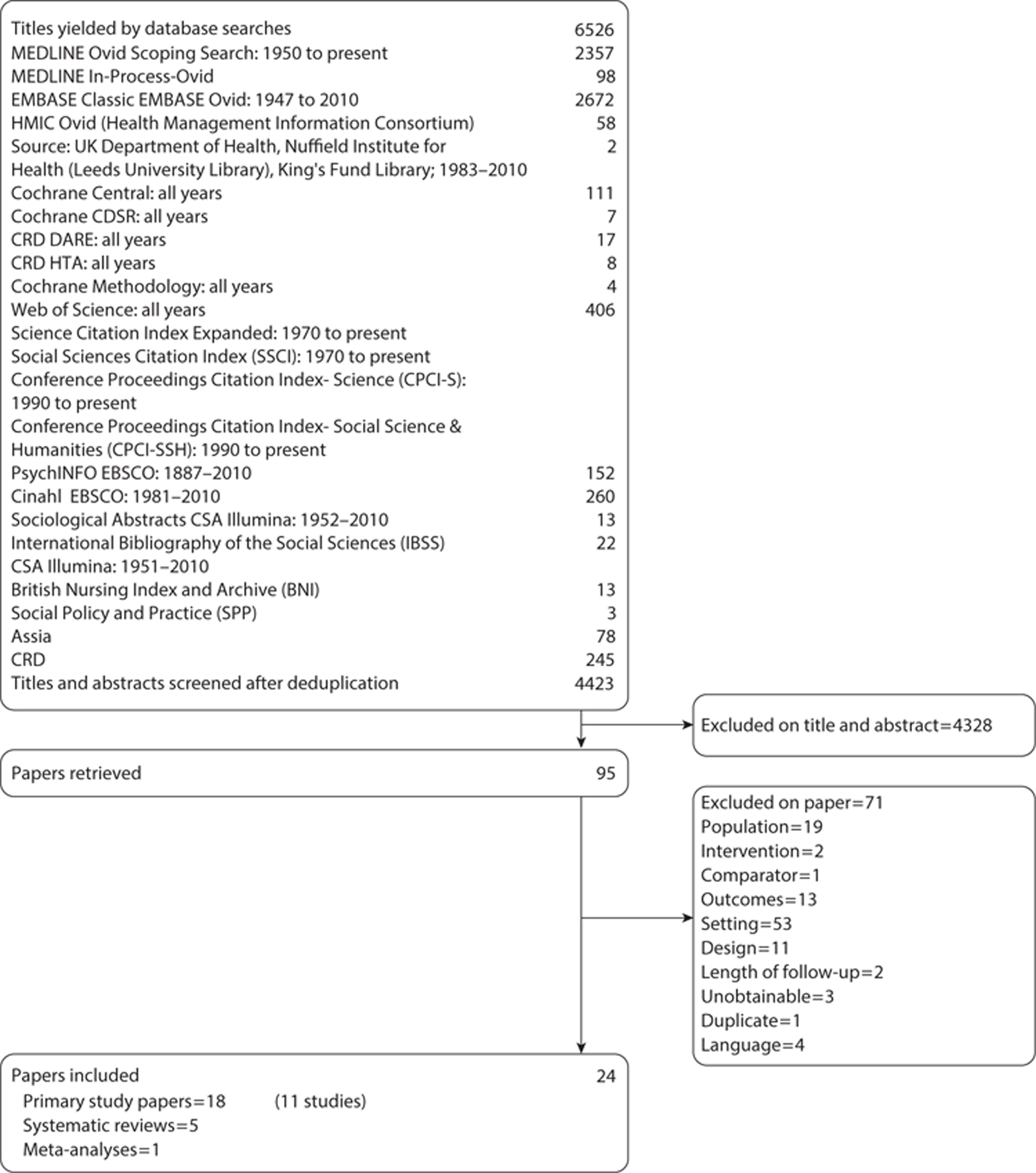
Number and type of studies included
Electronic database searches were conducted between 8 October 2010 and 25 January 2011. The initial searches found 883 titles and abstracts after deduplication. When these were screened, 67 papers were requested for further review and two PhD theses were unobtainable. Of the 65 papers that were available, 20 were found to meet the study inclusion criteria. Four of these were systematic reviews, one was a meta-analysis and 15 were research papers; although a qualitative search filter was used, none of the papers had qualitative designs.
Further, more sensitive, qualitative searches were then conducted to see if they led to admissible studies. These searches yielded 2350 titles and abstracts after deduplication; when these had been screened 14 papers were requested. The review of these papers led to the inclusion of three primary research papers and one more systematic review. One of these papers was a summary of a nested qualitative study from an included study; this had been published only as a conference poster. Contact with the author revealed that this study had not been published in full. No published qualitative studies were found.
In order to be certain that the search strategy was picking up all includable papers, a highly sensitive further scoping search of breast cancer (and breast cancer terms) and qualitative research [as a medical subject heading (MeSH)/EMTREE/controlled syntax term] or qualitative methods (free-text terms) and qualitative terms (free text) were used. This was followed by a further scoping search of the breast cancer terms with the qualitative cluster of terms. These searches produced an enormous number of titles and abstracts (n = 189,580). A sample of these was screened (n = 258) and no further includable studies were found.
A search for grey literature produced 13 titles after deduplication. One paper was retrieved but was subsequently excluded. Breast cancer charity websites were also searched; however, no includable papers were found.
After consultation with the information specialist (CC) it was decided to do a forwards chase of citations (n = 48) and a backwards chase of references (n = 50) from one of the included systematic reviews, by Brett et al. ,82 to see if this was a more productive strategy for finding includable papers. This led to the retrieval of another eight papers, none of which were included. However, it was concluded that screening bibliographies and chasing citations of retrieved papers, together with contacting experts and authors, was more likely to produce includable studies than pursuing highly specific but extremely insensitive searches. This strategy is supported by Greenhalgh and Peacock,83 who found only 30% of includable studies through searches from protocol inclusion criteria. This approach produced no further includable papers.
Update searches were carried out on 26 October 2011 and 23 March 2012; no new includable studies were found. The search strategy is available in Appendix 2 and the updated search strategy is available from the authors.
In total, 24 papers were included (18 primary studies, five systematic reviews and one meta-analysis). The list of included papers was sent to experts in the field to confirm that there were no more relevant published papers; it was accepted that saturation had been obtained. A list of ongoing studies can be found in Appendix 4.
No studies were found that were either about or that had subgroups of women from different ethnic, socioeconomic or other groups. One study was found of women who had a false-positive mammogram and had a family history of breast cancer (FHBC).
A flow chart of the selection process can be found in Figure 4. A list of papers excluded at the paper review stage with reasons for their exclusion is available in Appendix 5.
FIGURE 4.
Flow chart of published evidence included in this systematic review.
Quality of studies – study characteristics and risk of bias
Systematic reviews and meta-analyses
The searches identified five systematic reviews and one meta-analysis that partly or wholly addressed the research questions of this systematic review. It was decided to evaluate the quality of all these studies against PRISMA criteria which, although not designed as a quality assessment tool, can be used for the critical appraisal of systematic reviews and meta-analyses. 79Table 3 provides a summary of the inclusion criteria of these studies.
Overall, the quality of methods and reporting in these systematic reviews is not high. This is despite them being conducted in the post-QUORUM (quality of reporting of meta-analyses) and, in some cases, post-PRISMA era. The exception to this is the review published by the UK HTA programme. 84 The features most commonly missing are information about access to the study protocol, presentation or access to the full electronic searches and, more worryingly, consideration of the risk of bias both within and across studies. It seems that most authors were happy to accept the results of their included studies de facto, despite being largely observational with many opportunities for bias and confounding to affect the results. A summary of the quality of the included systematic review can be found in Table 4.
| Author, year and reference | Title (no. of included studies) | Participants | Intervention | Comparator | Outcomes | Design | Exclusion criteria | Comment |
|---|---|---|---|---|---|---|---|---|
| Salz et al. 201085 | Meta-analysis of the effect of false-positive mammograms on generic and disease-specific psychosocial outcomes (17) | Women aged ≥ 40 years who had been invited to mammography screening | Screening with mammography | No comparator | Measures of well-being and behaviour | Observational studies | Mammography prompted by symptoms, hypothetical studies | This study is linked to the systematic review by Brewer et al. 200762 |
| False-positive vs normal results | BDI, CES-D, CWS-R, GHQ, HADS, HSCL, IAS, IES, K6, PCQ, RPCS, STAI, ad hoc | |||||||
| Hafslund and Nortvedt 200986 | Mammography screening from the perspective of quality of life: a review of the literature (17) | Women aged 40–74 years who had been invited to mammography screening | Screening with mammography | No comparator | Quality of life | Observational studies | Women with an increased risk of breast cancer, a diagnosis of cancer, aged ≥ 74 years or < 40 years, or the intervention was focused on anxiety. Non-English-language papers | Narrative synthesis |
| False-positive vs normal results | BCAI, BDI, GHQ, HADS, HQ, PCQ, SCL-90, STAI, TTO, | |||||||
| Brewer et al. 200762 | Systematic review: the long-term effects of false-positive mammograms (23) | Women aged ≥ 40 years who had been invited to mammography screening | Screening with mammography | No comparator | Return for routine screening | Observational studies | Mammography prompted by symptoms, hypothetical studies. Non-English-language papers | Includes a meta-analysis of the effects of false-positives on returning for routine screening |
| False-positive vs normal results | Measures of behaviour, well-being and beliefs | |||||||
| BDI, CES-D, GHQ, HADS, HSCL, IAS, IES, K6, PCQ, SCL-90, STAI, ad hoc | ||||||||
| Measured ≥ 1 month after assessment | ||||||||
| Armstrong et al. 200787 | Clinical guidelines. Screening mammography in women aged 40–49 years: a systematic review for the American College of Physicians (22) | Women aged 40–49 years who had been invited to mammography screening. The subgroup of false-positive women included those ≥ 50 years old | Screening with mammography | No comparator | Measures of behaviour and well-being | Observational studies | Case series | The false-positive studies were a subgroup of a more general review of screening mammography |
| False-positive vs normal results | FCS, GHQ, HADS, HSCL, IES, PCQ, STAI, ad hoc | Narrative synthesis | ||||||
| Brett et al. 200582 | The psychological impact of mammographic screening. A systematic review (52) | Women who had been invited to mammography screening | Screening with mammography | No comparator | Measures of behaviour and well-being | Observational studies | True-positive as a result of screening or symptomatic at the time of screening. Studies where the focus was on the impact of an intervention on anxiety. Non-English-language papers | Narrative synthesis |
| False-positive vs normal results | BDI, HADS, HSCL, GHQ, PCQ, POMS, STAI, ad hoc | |||||||
| Bankhead et al. 200384 | The impact of screening on future health-promoting behaviours and health beliefs. A systematic review (28) | Women who had been invited to mammography screening | Screening with mammography | No comparator | Health-promoting behaviours, attitudes and beliefs that result from breast screening | All study designs | Non-English-language papers and papers focusing on anxiety, pain or discomfort unless these affected health-promoting behaviour. Interventions to improve uptake of screening | The false-positive mammography studies were a subset of a more general review of screening that included breast, cervical and cholesterol screening |
| False-positive vs normal results | Ad hoc | Narrative synthesis |
| Section/topic | Item | Checklist item | Salz et al. 201085 | Hafslund and Nortvedt 200986 | Brewer et al. 200762 | Armstrong et al. 200787 | Brett et al. 200582 | Bankhead et al. 200384 |
|---|---|---|---|---|---|---|---|---|
| Title | ||||||||
| Title | 1 | identify the report as a systematic review, meta-analysis or both | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Abstract | ||||||||
| Structured summary | 2 | Provide a structured summary including, as applicable, background, objectives, data sources, study eligibility criteria, participants, interventions, study appraisal and synthesis methods, results, limitations, conclusions and implications of key findings, systematic review registration number | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Introduction | ||||||||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods | ||||||||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed and if available, provide registration information including registration number | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Eligibility criteria | 6 | Specify study characteristics and report characteristics used as criteria for eligibility, giving rationale | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Information sources | 7 | Describe all information sources in the search and date last searched | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | ∼a | ∼a | ∼a | ∼a | ∼a | ✓ |
| Study selection | 9 | State the process for selecting studies | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Data collection process | 10 | Describe method of data extraction from reports and any processes for obtaining and confirming data from investigators | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Data items | 11 | List and define all variables for which data are sort and any assumptions and simplifications made | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies and how this information is to be used in any data synthesis | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ |
| Summary measures | 13 | State the principal summary measures | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measure of consistency for each meta-analysis | ✓ | – | ✓ | ✗ | – | – |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ |
| Additional analyses | 16 | Describe methods of additional analyses, if done, indicating which were pre-specified | – | – | – | – | – | – |
| Results | ||||||||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally from a flow diagram | ✗ | ∼a | ✓ | ✗ | ∼c | ∼d |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted and provide the citations | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome-level assessments | ✗ | ✓ | ✗ | ✗ | ✗ | ∼b |
| Results of individual studies | 20 | For all outcomes considered, present for each study (a) simple summary data for each intervention group and (b) effect estimates and CIs, ideally with a forest plot | ✗ | ∼c | ∼d | ∼f | ✓ | ✓ |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measure of consistency | ✓ | – | ✓ | – | – | – |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ |
| Additional analysis | 23 | Give results of additional analyses, if done | – | – | – | – | – | – |
| Discussion | ||||||||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome: consider their relevance for key groups | ∼e | ∼hh | ∼h | ∼f | ✓ | ✓ |
| Limitations | 25 | Discuss limitation at study and outcome level and at review level | ∼g | ∼h | ✓ | ✗ | ✓ | ✓ |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implication for future research | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Funding | ||||||||
| Funding | 27 | Describe sources of funding for the systematic review and other support and role of funders for the systematic review | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
The most recent study is a meta-analysis of the detection of the psychological impact of false-positive screening mammograms by generic and disease-specific psychosocial measures by Salz et al. 85 This is a secondary analysis of their previous systematic review data, published in 2007. 62 The authors were interested in comparing the difference in sensitivity of generic and disease-specific outcome measures to detect the degree of well-being experienced by women who had received a false-positive screening mammogram. Their searches found 17 observational studies of women aged ≥ 40 years that compared psychological outcomes between women who had had a false-positive mammogram with those whose screening results had been normal.
This meta-analysis has some weaknesses. There is no indication that any consideration of the risk of bias or confounding within or across studies was made, although the inclusion criteria and methods for study selection are clearly stated.
Additionally, Salz et al. 85 do not account for the number of studies screened or provide a flow chart or details of the results of the individual studies they have included. In the results section, rather than present forest plots for each analysis, they present a table of pooled effect sizes for the different criteria of outcome (e.g. distress or depression). In the discussion, although they summarise their main findings, no comment was made on the strength of the findings or their limitations at study level.
This meta-analysis was preceded by Hafslund and Nortvedt,86 who conducted a systematic review, published in 2009, that looked at the impact on quality of life of mammography screening in women aged ≥ 40 years, comparing those who received a false-positive result with those whose result was normal. Psychological measures were taken in the short to medium term. The authors found 17 observational studies, which were given a narrative synthesis.
Overall, this is a poor-quality systematic review. Although methods for assessing the risk of bias in individual studies are described, insufficient information is given about the data collection process and the kind of data to be collected. The results section gives an estimation of the risk of bias within studies but the results of individual studies are inadequately presented, with little summary data. There is no assessment of the risk of bias across studies. Although the review's findings are summarised in the discussion, this is without relation to the strength of the evidence under review.
The systematic review by Brewer et al. 62 is of reasonable quality. Brewer et al. 62 were interested in the long-term effects of false-positive screening mammograms on women's attendance at their next routine breast screening clinic. They were also interested in the impact on psychological outcomes, which they measured at least 1 month after the assessment. They found 23 observational studies that met their inclusion criteria.
The rationale and methods for conducting the study are well reported, with the notable exception of those for evaluating the risk of bias in individual studies, although methods for countering the cumulative effects of bias and assessing publication bias are given. Not surprisingly, the results section does not report the risk of bias within studies and the reporting of individual studies' results for psychological outcomes is inadequate as only a vote-counting approach is taken. However, individual, summary and pooled data for reattendance at routine screening are presented. The discussion provides a good summary of the evidence and incorporates a consideration of the limitations of the review and its components, although the strength of the evidence is not reflected on.
In the same year (2007), Armstrong et al. 87 published a systematic review with the aim of assessing the evidence of risks and benefits of screening mammography for women aged 40–49 years, but included a subgroup of women aged up to 71 years who had received false-positive results. This older population was compared with those with a normal screening outcome. They found 22 observational studies about false-positive mammograms.
Unfortunately, this is a poor-quality systematic review. The methods used are inadequately described with no mention of the outcomes included, methods of data synthesis or any assessment of the risk of bias across studies. The authors used a crude measure of study quality that is solely based on design, which does not consider risk of bias or confounding within studies. Furthermore, there is no indication that they have thought how bias might affect the validity of their results. The study results are particularly poorly reported; there is no account of the results of the study screening process, no results of assessment of risk of bias, inadequately described results of individual studies and a narrative synthesis almost devoid of quantified outcomes. The very brief discussion does not consider the review's limitations at any level or offer an interpretation of the results with reference to other reviews.
A systematic review by Brett et al. 82 was of better quality, although still lacking many of the markers of a good-quality systematic review. The authors aimed to assess the negative psychological impact of mammography screening and how long this lasted; this included the impact on women given the all-clear after screening as well as those with false-positive results. They found 52 observational studies that met their inclusion criteria.
The abstract and introduction are clear. However, the methods section is confusing to read as it contains paragraphs that should be in the introduction and results sections. The process of study selection is described, but there is no flow chart showing the progress of screening. Furthermore, the risk of bias affecting the results of individual studies or across studies is not considered in the methods or results sections. The findings of individual studies are given a comprehensive narrative summary. Recognition is given in the discussion to the shortcomings of some of the instruments used and that bias and confounding could have influenced the results. However, how this might relate to individual studies or the overall conclusions is not discussed.
The final systematic review that has been included is a good-quality HTA programme publication by Bankhead et al. 84 This is a broad-ranging systematic review looking at the impact of several types of screening, including mammography, on health behaviours and beliefs. Bankhead et al. 84 found 28 observational studies that looked at women with false-positive mammography results.
The introduction and methods are clear and comprehensive and consideration is given to the risk of bias in individual studies. However, the risk of bias across studies is not mentioned. The results section, together with the tables in the appendices, gives a full account of the findings by outcome and study, although, again, the possible effects of risk of bias across studies are not reported. The discussion section gives a clear and thorough summary of the findings and the limitations of the study with suggestions for further research.
Primary research
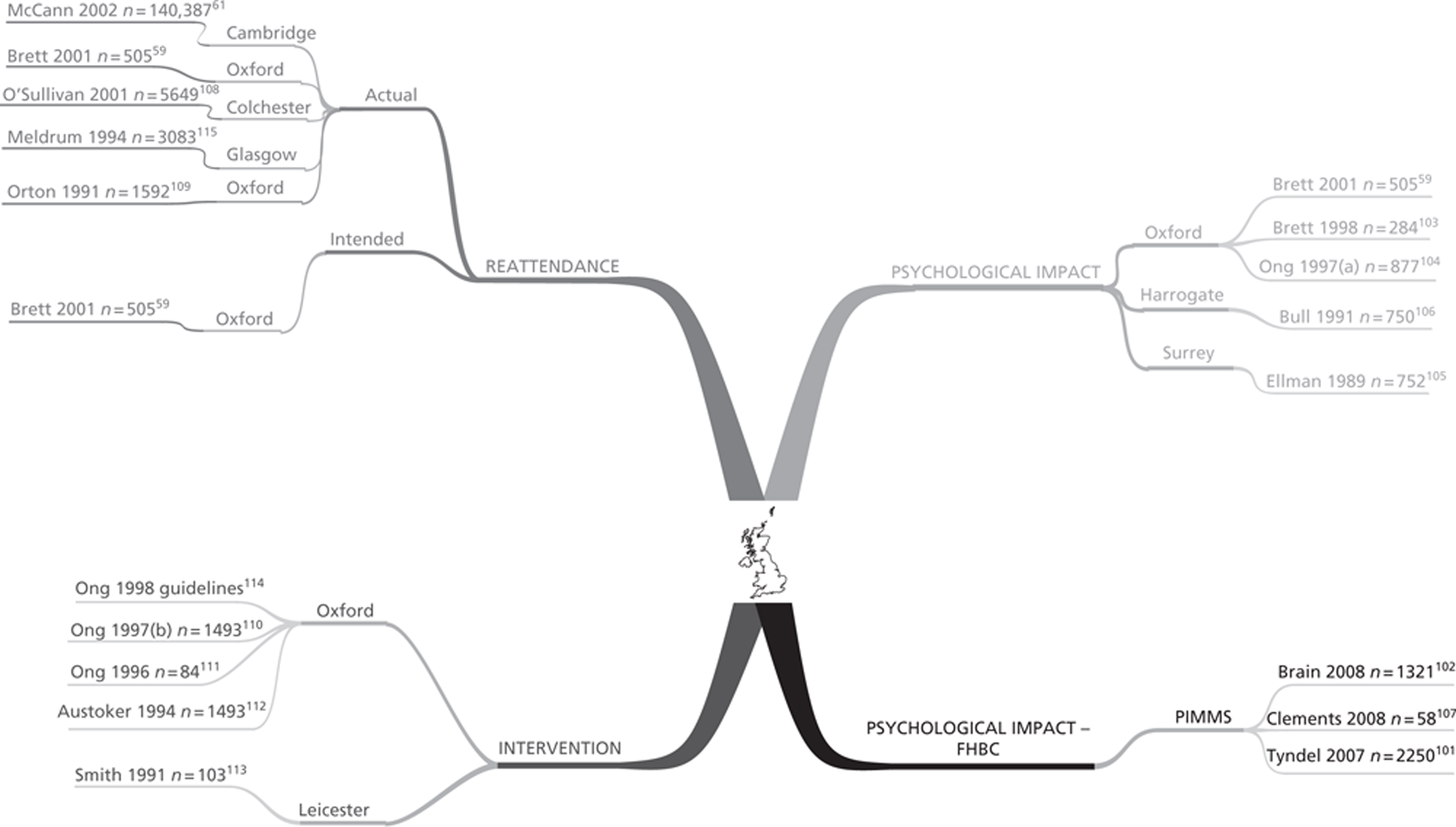
The searches returned 11 primary research studies (18 papers) that met the inclusion criteria (including being conducted in the UK). Four of the studies were prospective cohorts,59,101–106 one of these studies107 included a nested interview study but this was only published as a conference poster, four were retrospective cohorts,55,61,108,109 two had a cross-sectional design,110–113 one of these studies114 produced national guidelines that contained research findings and one was a RCT115 of an intervention to improve reattendance.
Four studies looked at the psychological impact of false-positive mammograms in the normal-risk population. 55,59,103–106 One study looked at the impact of having a false-positive screening mammogram among a population of women with a FHBC. 101,102,107 Five studies looked at the impact on returning for routine mammography screening59,61,103,108,109,115 and two studies investigated the impact of written information on distress or reattendance. 110–114
In some of the studies, groups of women were included with characteristics outside the scope of this systematic review; in these cases only data from the study population included in this review are extracted and reported.
Although worded slightly differently, the definition of woman with a false-positive mammogram is consistent in all of the included studies and agrees with the definition used in this systematic review (i.e. a woman who is recalled for assessment of any kind on the basis of a routine screening mammogram who is not then diagnosed with breast cancer).
This section first of all gives an overview of the relationship of the studies to each other according to their domain of interest (Figure 5). Some papers report outcomes in more than one domain and are therefore shown accordingly in Figure 5. After this, the characteristics and quality of the studies are discussed according to their domain. This is followed by a summary table of the characteristics of each paper (Table 5). Finally, a summary in Table 6 gives an overview of the quality of the observational studies. More detailed information about all the primary studies can be found in the data extraction forms in Appendix 3. A summary description of the measures used in the included primary research can be found in Chapter 1, Measurement of psychological consequences.
FIGURE 5.
Mind map showing the relationship of included studies to their domain of interest.
| Study, author, year (funding) | Design | n | Participants | Intervention group | Control group | Outcomes | Length of follow-up | Exclusion criteria | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Psychological impact | |||||||||
| Brett and Austoker 200159 (Cancer Research Campaign) | Prospective cohort, multicentre | 505 | Women invited for routine screening by mammography, already participating in the study at 5 months | Routine screening by mammography with a false-positive result (n = 375) | Routine screening by mammography with a normal result (n = 130) | PCQ, intention to reattend and actual reattendance satisfaction with service ad hoc questionnaire | 3 years (35 months) after assessment | Aged > 65 years, symptomatic referral, in another study, developed cancer | |
| Psychological impact | |||||||||
| Brett et al. 1998103 (Cancer Research Campaign) | Prospective cohort, multicentre | 284 | Women invited for routine screening by mammography, already participating in the study at 1 month | Routine screening by mammography with a false-positive result (n = 163) | Routine screening by mammography with a normal result (n = 52) | PCQ, intention to reattend, ad hoc questionnaire | 5 months after assessment | Aged > 65 years, symptomatic referral, in another study, developed cancer | 69 (24%) women chose not to return the questionnaire |
| Psychological impact | |||||||||
| Ong et al. 1997104 (Cancer Research Campaign, NHSBSP) | Prospective cohort, multicentre | 877 | Women invited for routine screening by mammography who were recalled for assessment | Women placed on early recall (< 3 years) (n = 182) | Women placed on routine recall after mammography (n = 173), further mammography assessment (n = 166), FNA (n = 109) or biopsy (n = 31) | PCQ | NA | Not reported | This study was primarily about the effects of early recall on women who had been called back for assessment after their mammogram, measures taken 1 month after assessment |
| Psychological impact | |||||||||
| Bull and Campbell 1991106 (Yorkshire Regional Health Authority) | Prospective cohort | 750 | Women invited for routine screening by mammography who were recalled for assessment | Routine screening by mammography with a false-positive result (n = 308) | Routine screening by mammography with a normal result (n = 420) | Ad hoc questionnaire including frequency of breast self-examination, HADS | 6 weeks after the ‘all-clear’ | Not reported | It is not known if the women had previously had cancer or were in a high-risk group |
| Psychological impact | |||||||||
| Ellman et al. 1989105 (DHSS Research Management Division) | Prospective cohort | 752 | Women invited for routine mammography screening and those recalled for further assessment and those with symptoms being further investigated | Routine screening by mammography with a false-positive result (n = 271) | Routine screening by mammography with a normal result (n = 295), symptomatic women who did not have cancer (n = 134), symptomatic or recalled screened women who did have cancer (n = 38), history of breast cancer with or without symptoms (n = 14) | GHQ-28, ad hoc questionnaire | 3 months after clinic attendance | Not reported | Participants also received clinical examination. Only those groups meeting the inclusion criteria will be considered in this systematic review |
| Psychological impact | |||||||||
| Psychological impact with a FHBC | |||||||||
| Brain et al. 2008102 (Cancer Research UK) | Prospective cohort | 1321 | Women aged 35–49 years invited for routine annual screening by mammography with a FHBC | Routine annual screening by mammography with a false-positive result (n = 112) | Routine annual screening by mammography with a normal result (n = 1174) | Questionnaire including: CWS-R, cognitive appraisal, COPE, perceived risk of breast cancer, dispositional optimism | 6 months, measures taken at 1 month before screening and 1 and 6 months after the ‘all-clear’ | Previous history of breast cancer or family history of ovarian cancer | This study aimed to find predictive variables of cancer-specific distress |
| Psychological impact | |||||||||
| Clements et al. 2008107 (Cancer Research UK) | In-depth interviews | 58 | Women aged 35–49 years invited for routine annual screening by mammography with a FHBC | Routine annual screening by mammography with a false-positive result (n = 22) | Routine annual screening by mammography with a normal result (n = 36) | Reactions of women to false-positive recall and value placed on the screening programme | NA | Previous history of breast cancer or family history of ovarian cancer | It is not known if the women had previously had cancer or were in a high risk group |
| Psychological impact | |||||||||
| Tyndel et al. 2007101 (Cancer Research UK) | Prospective cohort | 2321 | Women aged 35–49 years invited for routine annual screening by mammography with a FHBC | Routine annual screening by mammography with a false-positive result (n = 166) | Routine annual screening by mammography with a normal result (n = 2084) | CWS-R, PCQ | 6 months, measures taken at 1 month before screening and 1 and 6 months after the ‘all-clear’ | Previous history of breast cancer or family history of ovarian cancer | |
| Psychological impact | |||||||||
| Impact on reattendance | |||||||||
| McCann et al. 200261 (NHS Executive Eastern Region) | Retrospective cohort | 140,387 | Women aged 49–63 years invited for routine breast screening by mammography | Routine screening by mammography with a false-positive result (n = 4792) | Routine screening by mammography with a normal result (n = 108,617) | Subsequent attendance at routine screening after a false-positive result and rate of interval cancer – from records | 3 years | Women who were older than 63 years at follow-up | |
| Reattendance and interval cancer | |||||||||
| O'Sullivan et al. 2001108 (Cancer Research Campaign) | Retrospective cohort | 5649 | Women invited for mammography screening for the second or subsequent time | Routine screening by mammography with a false-positive result (n = 248) | Routine screening by mammography with a normal result (n = 5401) | Subsequent attendance at routine screening after a false-positive result – from records | Unclear, probably from 1989 to 1997 | Women invited for the first time and women who had been previously invited but had never attended | Effects of a false-positive result on reattendance for those on early recall and routine recall |
| Reattendance | |||||||||
| Brett and Austoker 200159 (Cancer Research Campaign) | As above in Psychological impact | ||||||||
| Brett et al. 1998103 (Cancer Research Campaign) | As above in Psychological impact | ||||||||
| Meldrum et al. 1994115 (Scottish Offce Home and Health Department) | RCT nested telephone interview study | 3083 | All women invited for second round routine mammography screening (aged 50–65 years) | Tailored invitation with a false-positive result (n = 115) and with a normal result (n = 800) | Standard invitation with a false-positive result (n = 112) and with a normal result (n = 791) | Subsequent attendance at routine screening and effect of a tailored invitation on subgroups | Not reported | Women with breast cancer and those whose screening history was not available | Trial comparing the effect of a tailored invitation on second-round screening attendance with a standard invitation |
| Orton etal. 1991109 (funding not reported) | Retrospective cohort | 1582 | Women, aged 45–64 years, invited to attend for second-round screening by mammography | Routine screening by mammography with a false-positive result (n = 50) | Routine screening by mammography with a normal result (n = 1532) | Reattendance, acceptability of screening | NA | Not reported | Data are not available for the acceptability of screening for false-positive participants |
| Reattendance | |||||||||
| Interventions to reduce the impact of false-positive mammograms | |||||||||
| Ong et al. 1998114 (Cancer Research Campaign, NHSBSP) | Guidelines and summary evidence from cross section, multicentre | NA | Women invited for routine screening by mammography who were recalled for assessment | NA | NA | Ad hoc questionnaire and criteria for evaluating breast screening information material developed by Austoker and Ong 1994112 | NA | Women recalled due to poor quality X-rays | National guidelines about information given prior to recall for further assessment based on the findings of Ong et al. 1997,104 1996111 and Austoker and Ong 1994112 |
| Information intervention | |||||||||
| Ong and Austoker 1997110 (Cancer Research Campaign, NHSBSP) | Cross section, multicentre | 1493 | Women invited for routine screening by mammography who were recalled for assessment | n = 1493 | NA | Ad hoc questionnaire | NA | Women recalled due to poor quality X-rays | Evaluation of women’s experiences at the assessment clinic and their information needs there and afterwards. Discourse analysis of open questions |
| Information intervention | |||||||||
| Ong et al. 1996111 (Cancer Research Campaign, NHSBSP) | Cross section, multicentre | 84 | UK breast screening assessment centres | Evaluation of information given in the initial letter/leaflet and prior to recall for further assessment | NA | Criteria for evaluating breast screening information material developed by Austoker and Ong 1994112 | NA | NA | |
| Information intervention | |||||||||
| Austoker and Ong 1994112 (Cancer Research Campaign, NHSBSP) | Cross section, multicentre | 1493 | Women invited for routine screening by mammography who were recalled for assessment | n = 1493 | NA | Ad hoc questionnaire | NA | Women recalled due to poor quality X-rays | Evaluation of information given prior to recall for further assessment from eight UK breast screening centres |
| Information intervention | |||||||||
| Smith et al. 1991113 (funding not reported) | Cross section | 103 | Women attending assessment clinic following recall after routine mammography screening | False-positive (N = 91) | NA | Ad hoc questionnaire | NA | Not reported | Survey of different versions of an invitation to return for further assessment. The responses from both groups of women are aggregated |
| Survey | Other outcome (N = 12) | ||||||||
The UK research in the field of false-positive mammography screening has been dominated by the University of Oxford Primary Care Education Research Group (OPCERG) who first published a series of papers from 1997 about the information needs of women recalled for further assessment following a screening mammogram. This work then developed to investigate the psychological impact of false-positive mammograms on the general population of screened women and how this affected their reattendance at their next routine screening, and latterly looked at the psychological impact of receiving a false-positive mammogram on women who have a FHBC. Other research groups in England and Scotland have also studied these four aspects and in many cases preceded the Oxford research.
Psychological impact
The papers from the OPCERG study in this domain span 5 years, with measures being taken at 1 month (T1), 5 months (T2) and 35 months (T3) from the last screening appointment. This study is a multicentre prospective cohort at 13 NHSBSP clinics in England and Scotland. The papers follow the same cohort of women over this time but the methods used appear to vary at different time points and it is not always clear on what basis the women included in the study have been selected or how the main outcome measure, the PCQ, has been used. The study includes women with normal mammograms as the control group and compares them with women with false-positive outcomes according to the process used in their further assessment (another mammogram, FNA or biopsy) or if they had been placed on early recall of 6 or 12 months following further assessment.
The first publication by OPCERG, by Ong et al. 104 (n = 877), reported primarily on the adverse psychological consequences of being placed on early recall (6 or 12 months recall rather than 3 years) at T1. These effects were measured using the PCQ negative scale which has 12 items on a four-point Likert scale (scored 0–3). 67 Participants were considered to be ‘cases’ if they responded positively to at least one item.
The study by Ong et al. 104 is a moderately good-quality study that used appropriate methods to address its aims. The authors reported almost all the key criteria specified by the STROBE statement. 81 However, they omitted to provide demographic data for all participants, not just those on early recall, which makes it difficult to interpret the results. They also failed to fully discuss the limitations of their study and its generalisability to other situations.
Following on from the study by Ong et al. ,104 Brett et al. 103 (n = 284) recruited from the same pool of women to find out what difference, if any, a further 4 months had on how these women were feeling after their false-positive mammogram. This was also 1 month before those on early recall were due for another screening mammogram. In this prospective cohort study, the previous studies' results (Ong et al. 104) were used as the baseline measures for comparison with the same subgroups. This study only included 12 centres, as one centre did not put any women on early recall.
Although this was a fairly good-quality study,103 it did not quite meet the same standards of reporting as the previous one. 104 Omissions include not considering potential sources of bias, giving an explanation of how missing data were handled and not providing demographic information.
The latest publication from OPCERG on the population at normal risk of breast cancer is by Brett and Austoker59 (n = 505). This study follows the same cohort of women as Brett et al. 103 in 13 NHSBSP clinics in England and Scotland. They took measures of adverse psychological consequences with the PCQ at 35 months after participants' last assessment (i.e. 1 month before their next routine screening mammogram was due). Brett and Austoker59 deemed that a total score ≥ 12 (range 0–36) on the PCQ showed negative psychological consequences and so it is only the percentage of scores ≥ 12 that are reported. It is not clear why they have chosen this total score as the cut-off point for showing psychological harm. The original validation paper by Cockburn et al. 67 does not have this cut-off but indicates that the bottom quartile of scores represent no dysfunction, the second quartile mild psychological disturbance, the third quartile moderate disturbance and the top quartile marked disturbance.
Brett and Austoker59 also used an ad hoc questionnaire to measure satisfaction with the breast screening service and assess factors that may influence women's level of anxiety. They also asked women about their intention to attend their next routine mammography screening. This last outcome was compared with their actual attendance.
Again, this is a generally well-described study but with similar omissions as before: no explanation of how missing data were dealt with or consideration of the role bias may have played in the results. However, they do provide some demographic information about the participants (marital status, home ownership and educational level).
Prior to the work by the Oxford team, Sutton et al. 55 (n = 1021) conducted a retrospective cohort study that was primarily interested in the levels of anxiety experienced by women attending routine mammography screening who had normal outcomes. However, they also looked at anxiety levels in women who had false-positive results. Nine months after the pre-screening baseline, they asked these women and others with normal results to retrospectively reflect on how anxious they had felt at six stages of the screening process: (1) receiving the invitation; (2) waiting at the clinic for the mammogram; (3) at the clinic after the mammogram; (4) waiting for the results; (5) after reading the results letter; and (6) now. These measures were taken on a three-point ad hoc scale, ranging from not anxious (1), a bit anxious (2) to very anxious (3). The retrospective results are only reported numerically at stages 3–5, therefore these data have been extracted. The data at other time points are reported in graphical form only and so could not be reliably extracted.
| STROBE statement – checklist of items that should be included in reports of observational studies | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item no. | Recommendation | Brett and Austoker 200159 | Brett et al. 1998103 | Ong et al. 1997104 | Sutton et al. 199555 | Bull and Campbell 1991106 | Ellman et al. 1989105 | Brain et al. 2008107 | Tyndel et al. 2007101 | McCann et al. 200261 | O'Sullivan 2001108 | Orton et al. 1991109 | Ong and Austoker 1997110 | Ong et al. 1996111 | Austoker and Ong 1994112 | Smith et al. 1991113 | |
| Title and abstract | |||||||||||||||||
| 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ||
| Introduction | |||||||||||||||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objectives | 3 | State specific objectives, including any pre-specified hypotheses | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods | |||||||||||||||||
| Study design | 4 | Present key elements of study design early in the paper | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up and data collection | ✓ | ✓ | ✓ | ✓ | P | ✓ | ✓ | ✓ | ✓ | P | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants | 6 | (a) Cohort study – give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NA | NA | NA | NA |
| Case–control study – give the eligibility criteria and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Cross-sectional study – give the eligibility criteria and the sources and methods of selection of participants | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ✓ | ✓ | ✓ | ✓ | ||
| (b) Cohort study – for matched studies, give matching criteria and number of exposed and unexposed | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | P | ||
| Case–control study – for matched studies, give matching criteria and the number of controls per case | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ✓ | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders and effect modifiers. Give diagnostic criteria, if applicable | ✓ | ✓ | ✓ | ✓ | P | P | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | P |
| Data sources/measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | ✓ | ✓ | ✓ | P | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Bias | 9 | Describe any efforts to address potential sources of bias | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Study size | 10 | Explain how the study size was arrived at | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✓ | NA | NA | ✗ | ✗ | ✗ | ✗ | ✗ |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ |
| (b) Describe any methods used to examine subgroups and interactions | NA | NA | ✓ | NA | NA | NA | NA | NA | ✗ | NA | NA | NA | NA | NA | NA | ||
| (c) Explain how missing data were addressed | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | NA | NA | ✗ | ✗ | NA | ✗ | ✗ | ||
| (d) Cohort study – if applicable, explain how loss to follow-up was addressed | ✗ | ✗ | NA | ✗ | ✗ | ✗ | ✗ | ✓ | NA | NA | NA | NA | NA | NA | NA | ||
| Case–control study – if applicable, explain how matching of cases and controls was addressed | NA | NA | NA | NA | NA | NA | ✓ | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Cross-sectional study – if applicable, describe analytical methods taking account of sampling strategy | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ✗ | NA | NA | NA | ✗ | ||
| (e) Describe any sensitivity analyses | NA | NA | NA | NA | NA | NA | ✓ | ✓ | NA | NA | NA | NA | NA | NA | NA | ||
| Results | |||||||||||||||||
| Participants | 13 | (a) Report numbers of individuals at each stage of study, e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up and analysed | ✓ | ✓ | ✓ | ✓ | P | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| (b) Give reasons for non-participation at each stage | ✓ | ✗ | NA | ✗ | ✗ | ✗ | ✗ | ✗ | NA | NA | ✗ | ✗ | NA | NA | ✗ | ||
| (c) Consider use of a flow diagram | ✗ | ✗ | NA | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Descriptive data | 14 | (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders | ✓ | ✗ | P | P | P | P | ✓ | ✓ | P | ✗ | ✗ | ✗ | NA | ✗ | P |
| (b) Indicate number of participants with missing data for each variable of interest | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | NA | NA | ✗ | ✗ | NA | ✓ | ✗ | ||
| (c) Cohort study – summarise follow-up time (e.g. average and total amount) | ✓ | ✓ | NA | ✗ | ✗ | ✗ | ✗ | ✗ | NA | NA | NA | NA | NA | NA | NA | ||
| Outcome data | 15 | Cohort study – report numbers of outcome events or summary measures over time | ✓ | ✓ | NA | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NA | NA | NA | NA | NA |
| Case–control study – report numbers in each exposure category, or summary measures of exposure | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Cross-sectional study – report numbers of outcome events or summary measures | NA | NA | ✓ | NA | NA | NA | NA | NA | NA | NA | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g. 95% CI). Make clear which confounders were adjusted for and why they were included | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | P | ✓ | ✓ | ✓ | ✓ |
| (b) Report category boundaries when continuous variables were categorised | NA | NA | NA | NA | ✓ | ✓ | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| (c) If relevant, consider translating estimates of RR into absolute risk for a meaningful time period | ✗ | ✗ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Other analyses | 17 | Report other analyses done (e.g. analyses of subgroups and interactions, and sensitivity analyses) | NA | ✓ | ✓ | ✓ | NA | NA | ✓ | ✓ | NA | NA | NA | NA | NA | NA | NA |
| Discussion | |||||||||||||||||
| Key results | 18 | Summarise key results with reference to study objectives | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | ✗ | ✓ | ✗ | P | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | P | ✗ |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies and other relevant evidence | ✓ | ✓ | P | P | ✓ | P | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | P | ✓ | P |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Other information | |||||||||||||||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
The baseline measures were taken using different measures (STAI76 and the GHQ72); however, the results of the women with false-positive mammograms are not disaggregated from the rest of the participants and so are not reportable.
This is a poorly designed and executed study. Initially, a cohort were recruited and followed up through the screening process. Additionally, another sample group was recruited which overlapped with the original sample group. A subset that contained participants from both groups was used for the main analysis (which compared women with normal mammograms with non-attenders and is therefore outside the scope of this review), while the whole of the first sample was used in the retrospective analysis of anxiety. There was no consideration of bias, confounding or the weakness of the design that relied on memory going back 9 months using an unvalidated measure. The results of the false-positive substudy are only partially numerically reported, the whole being shown in a figure that does not allow accurate disaggregation. Furthermore, there is no accounting of attrition or information about how missing data were dealt with. There is, however, acknowledgement that if they had used a disease-specific measure, such as the PCQ, they might have found different results.
Another similarly poor-quality study was conducted by Bull and Campbell106 in 1991 (n = 750). They followed a cohort of women who had attended mammography screening from their invitation to screening until 6 weeks after they had received the results of their follow-up assessment. The women were divided into four groups: (A) those invited for screening; (B) those with a normal mammogram; (C) those who went for further assessment with a mammogram, ultrasound or FNA; and (D) those who had a surgical biopsy for further assessment. The sample in groups A and B overlapped. However, no rationale is given for the design of the study. The outcomes measured were anxiety and depression using HADS,74 frequency of breast self-examination and impressions of the screening programme using an open question in an ad hoc questionnaire.
This study had a number of reporting flaws; in particular, there was no comment on the possibility of bias from the design and conduct of the study, nor was there any explanation of how missing data were accounted for. The only description of the participants was their age and no information was given about attrition or reasons for this. The discussion speculates at length about possible reasons for the findings, but neglects to mention any limitations of the study. Bull and Campbell106 also fail to comment on the generalisability of their findings.
The prospective cohort study by Ellman et al. 105 (n = 752) is of similarly poor quality. Ellman et al. 's105 aim was to compare the psychiatric morbidity experienced by women who had attended breast screening or a review clinic and had one of five results (group A, normal; group B, false-positive; group C, symptomatic with benign result; group D, symptomatic or recalled women who did have cancer; or group E, women with a history of breast cancer with or without symptoms). Only women in groups A and B were within the inclusion criteria of this systematic review and had their results reported. Morbidity was measured with GHQ-2872 either at the breast screening clinic (groups A and B) or the review clinic (groups C–E) before participants saw the clinician and was measured again 3 months later. An ad hoc questionnaire seeking opinions about the clinic and their experience of treatment was given to participants at follow-up.
Ellman et al. 's study105 failed to show an understanding of the limitations of their design. There was no consideration of potential sources of bias, no explanation of how missing data were accounted for or how this might affect the results. Although the tables of results were clearly presented and the results summarised in the discussion, there was no consideration of how generalisable they might be.
Psychological impact with family history of breast cancer
Although the population in this section is not part of the general breast cancer screening population or programme, it is interesting to look at this research and compare the results with that of the general population. The OPCERG and others collaborated in the Psychological Impact of Mammography Screening (PIMMS) research programme to look at the effects of having a false-positive screening mammogram in a population of women with a FHBC. The three papers reported in this section are from the PIMMS group.
Tyndel et al. 101 published the first paper in the series in 2007 (n = 2321). This was a prospective cohort study of women aged 35–49 years who had a moderate-to-high risk of breast cancer due to their family history and were on an annual screening programme. They were interested in comparing the psychological impact of having a false-positive mammogram with that of having a normal one within this group. Women who had previously had cancer and those with a family history of ovarian cancer were excluded. Disease-specific outcomes (PCQ and CWS-R68) were measured 1 month before participants' screening mammogram, 1 month after the ‘all-clear’ and 6 months after assessment.
This is a reasonably good-quality observational study. It is well-designed and well-reported; almost all the quality indicators in the STROBE checklist are met. The only substantial omission by Tyndel et al. 101 is a failure to report the extent of missing data and how these items were accounted for in the analysis.
The follow-on study is by Brain et al. 102 (n = 1286) who conducted a multiple regression analysis of the participants of the Tyndel et al. study101 to find out which pre-screening variables were predictive of cancer-specific distress. Initially, they conducted partial correlations of pre-screening variables on a number of scales (CWS-R,68 brief COPE,71 perceived risk of cancer and dispositional optimism) to see how they correlated with cancer worry at 1 and 6 months after assessment. This was followed by a hierarchical multiple regression to show predictive associations between these baseline variables and cancer worry at 1 and 6 months.
This second study from the PIMMS group was also of reasonable quality. The main criticism is the same: a failure to report how many data were missing and how missing data were dealt with in the analysis.
A qualitative interview study was nested within the PIMMS research. However, this was published only as the summary of a conference poster. The summary is reasonably detailed and, although it does not give the level of detail that would be expected in a qualitative research paper, it has been included because it is part of an included study and it gives some insight into the results found by the quantitative research. This interview study was conducted by Clements et al. 107 on 58 women who were part of the PIMMS cohort study (false-positive result = 22, normal result = 36).
As this study was published only as the summary of a poster, many of the expected quality criteria are unreported. However, the design is suitable for the research question and the thematic method of analysis appropriate. There is a lack of detail about the results and their interpretation as well as a lack of a theoretical framework and justification for the methods used. Therefore, it is not possible to comment thoroughly on the robustness of this study.
Impact of false-positive mammograms on returning for routine screening
The studies in this section have been subdivided into those that report on participants' actual returning for future breast screening and those that report on the intention to reattend.
Actual reattendance
Five studies measured the reattendance at routine mammography screening of women who had a false-positive screening mammogram.
The most recent study to report the actual reattendance of women who had a false-positive screening mammogram was by McCann et al. 61 (n = 140,387). This was a large, retrospective study that looked at records going back 3.5 years from NHS screening units in East Anglia and Cancer Registry databases. As well as looking at reattendance, this study aimed to quantify the increased risk of interval cancer (cancer detected between screenings) found among women in this group and establish if there was also an increased risk of cancer detection at second-round screening if women had been false-positive in the first round. The risk of interval cancer is outside the scope of this systematic review, therefore these results are not reported.
This large, convincing, retrospective cohort study gives clearly presented results that are well discussed. However, the statistical methods used are not described and the description of demographic characteristics is limited to age.
Prior to this, O'Sullivan et al. 108 (n = 5649) conducted a similar retrospective cohort study of women who had taken part in the NHS screening programme in east and central London. Their focus was only on the effects of having a false-positive mammogram on reattendance at the next screening round.
This data registry study clearly presents its descriptive results. However, O'Sullivan et al. 108 fail to provide any information about the characteristics of the participants, the number of screening centres involved or the possible role of bias and no comment is made about the study's generalisability to other screening centres.
The 2001 study by Brett and Austoker59 (n = 505) looked at actual reattendance as well as the psychological impact of false-positive mammograms. A summary of this paper can be found above in Primary research.
Previously, Meldrum et al. 115 (n = 3083) conducted a RCT on reattendance that compared a standard invitation letter for second-round screening (3 years later) with a letter tailored to the outcome of the previous screening round (e.g. false-positive or normal). Additionally, a telephone interview study was conducted of 66 women who had tailored intervention letters to gauge their views of the letters.
This is a poor-quality RCT. It is not clear whether or not the participants were aware that they were taking part in a study. Very little detail is given about the intervention or the control letters, certainly not enough to replicate the study. Although the method of randomisation is reported, it is unclear whether or not the assessors were blinded to the groups. Most significantly, the analysis uses some inappropriate methods. In particular, an adjustment is made for multiple comparisons when only a single comparison is made. There is no explanation for this. This leads to an inaccurate interpretation of the results in the discussion, which fails to acknowledge any limitations of the study. The interview study is inadequately reported with no description of methods of analysis and what probably is very selective reporting of results. A CONSORT assessment of quality can be found in Appendix 3.
The final study in this group is by Orton et al. 109 (n = 1582) using data from three GP practices. They looked at whether or not the acceptability of the first round of screening or having a false-positive mammogram could help predict attendance at second-round screening 3 years later. Unfortunately, the authors did not disaggregate their data to show the effect of screening satisfaction for women with false-positive mammograms on attendance. The only usable data is a comparison of second-round attendance of women who had false-positive mammograms in the first screening round with those who had a normal result.
This retrospective cohort study reports most of the quality criteria for observational studies. The rationale and methods are clearly described. However, there is no description of the demographic characteristics of the participants, so it is not possible to judge whether or not the differences in outcomes may have been because of bias or confounding factors. The discussion fails to comment on the limitations of the study, although it does remark on the generalisability of the study.
Intended reattendance
The most recent research looking at the intention to reattend the next screening round following a false-positive mammogram is the 2001 study by Brett and Austoker. 59 This was not the main focus of the paper: the data consist of women's responses to a questionnaire about which external influences have affected their attitude to returning for screening in 1 months' time. It is not possible to compare this intentional data with the actual reattendance. A summary of this paper can be found above in Psychological impact.
In addition to measuring adverse psychological consequences at 5 months post assessment, Brett et al. 103 (n = 284) also measured participants' intention to attend their next breast screening appointment. For most of the cohort, this was 2.5 years away, but for those placed on early recall it was only 1 month away. A summary of this paper can be found above in Psychological impact.
Interventions to reduce the impact of being recalled for further assessment
No studies were found that directly addressed the issue of testing an intervention to relieve the negative psychological consequences of false-positive mammograms. However, two studies were found that looked at the information needs of women prior to further assessment, one from OPCERG (1994–7)110–112 and one from Smith et al. 113 These studies do not disaggregate the data according to the outcome of the recall (i.e. distinguish between those who had false-positive and true-positive outcomes). This breaches the inclusion criteria for this systematic review. However, these studies are included because at the time of recall the final outcome is unknown to the women and there is no reason to suspect that, prior to the event, women with false-positive outcomes would have different information needs from those with other outcomes.
From 1994 to 1998 Austoker, Ong and others from OPCERG published a series of three papers about their research into the information needs of women who were recalled for further assessment following routine mammography screening, plus national guidelines on behalf of NHSBSP. This research will be presented first followed by a study by Smith et al. 113 of recall invitation letters.
The original work that OPCERG studies came from is by Austoker and Ong112 (n = 1493). Their aim was to assess the need for written information among women who had been recalled for assessment following routine screening mammography. They did this by conducting a multicentre study of eight breast screening centres in the UK.
First, 484 women were invited for a questionnaire interview after recall and before assessment. Two weeks after assessment these and other women (total n = 1493) were given an ad hoc questionnaire to assess their response to being recalled and how worrying or reassuring the messages contained in recall literature were. Second, Austoker and Ong112 also collected samples of the literature sent to women from the different centres and found a wide variation in the amount of information given and the language used. They assessed the breadth of information given in the recall letters and leaflets and whether or not the women thought they had been given sufficient information on matters such as the reason for recall, the location of the centre, who could come with them, how long the appointment would take, what tests they would undergo, who they would see, when they would know the outcome and how to get more information. They also assessed the language used in the information literature and categorised it according to whether the participants found it to be particularly worrying or reassuring. Simple descriptive statistics are reported together with a few examples of comments made in the open questions.
This is a well-presented and clearly reported study. However, Austoker and Ong112 failed to gather information about the demographic profile of their participants; given that this was a large study, it would have been very interesting to see what light this information might have shone on the results.
Following on from this, and based on their original development of criteria for evaluating breast screening information material, Ong et al. 111 (n = 84) evaluated the health education literature in 84 breast screening units in the UK. They considered both the initial screening letter given to women and the recall letter and leaflet sent to those with suspicious mammograms. The evaluation criteria were divided into words and phrases that were particularly worrying and those that were particularly stress relieving. The number of centres that mentioned items in their literature in these criteria was recorded.
As this is an evaluation of written material with simple descriptive statistics, many of the STROBE quality criteria do not apply. The methods and results were clearly described and comprehensively discussed. The main criticism is that there is a lack of discussion of the limitations of the study.
This study led into the most recent of the primary research papers, which is by Ong and Austoker110 (n = 1493). They used an ad hoc questionnaire to elicit views of women about their experience of being recalled, in particular the quality of communication at the clinic (who was available to talk to at the clinic and related information needs). The questionnaire also had open questions that allowed a free response that underwent a discourse analysis.
Overall, this is a well-conducted and clearly reported survey. However, there are some omissions. The authors have failed to report the demographic characteristics of the participants or discussed what effect these may have had on the results, given no indication of how bias or confounding factors may have played a role in the results and have not considered other limitations of their study. Furthermore, the methods and results of the discourse analysis are not described, except to say that the most frequently occurring items or those deemed to be most important by the analysts are tabulated.
The most recent publication in this series is by Ong et al. 114 and is the national guidelines for improving the quality of written information sent to women who are recalled for assessment. The guidelines are based on the findings of the previous three papers and give advice on the content and wording of assessment invitation letters. The research evidence that is included in the report is a conflation of the findings of the studies and does not contain new evidence. Therefore, these data have not been extracted as they are already in the primary research paper data extraction forms. The guidelines are mentioned here for completeness.
The final study in this section is a poorly described survey of women's satisfaction with the Leicestershire Breast Screening Service in 1989–90 by Smith et al. 113 (n = 103). This includes assessing the adequacy of information given to women recalled for further assessment. Their aim was to compare three different versions of a recall letter giving different amounts of information, including telephone access to a breast care nurse, to see which was most acceptable to recalled women.
This study is described as an audit, but it goes further than that as it includes experimental testing of the different forms of the recall letter. The reporting of the study is very inadequate. Not enough information about the methods used is provided; it is often difficult to reconcile the text with the tables and there is no acknowledgement of the limitations of their methods.
Results and comment
Systematic reviews and meta-analyses
The scope of the included systematic reviews was broader than that of this review; they included non-UK studies and outcomes measured for < 1 month from women receiving the ‘all-clear’ after their follow-up assessment. Therefore their results may not reflect the medium- to long-term outcomes in the UK that are the subject of this review. Nevertheless, they are of interest as a comparison to this review's findings.
All the systematic reviews and meta-analyses summary results showed a negative impact from receiving a false-positive mammogram on measures of well-being, depression and anxiety compared with women with normal screening results. The primary studies included used both disease-specific and generic measures, which were usually analysed together in the reviews. The exception to this was the meta-analysis of generic psychological measures by Salz et al. 85 that showed no difference in psychological outcomes between women with false-positive results and those with normal ones except for anxiety, which was positively correlated with having a false-positive mammogram [0.03 (95% CI 0.00 to 0.07)]. The evidence varied concerning whether psychological distress had a short-term (< 1 month after assessment) or long-term impact. There was some evidence that the degree of impact varied with the severity of the reassessment test, with women undergoing biopsy showing greater psychological distress than those with a repeat mammogram. 82
The results for the impact of receiving a false-positive mammogram on self-care behaviour and returning for the next routine screening mammogram give a more complex picture. Two reviews, Salz et al. 85 and Armstrong et al. ,87 looked at effects of having a false-positive mammogram on frequency of breast self-examination. In both evidence syntheses, women with false-positive mammograms reported increased breast self-examination, which may indicate increased anxiety about the risk of developing breast cancer. However, Armstrong et al. 87 found there was no statistically significant difference between groups in the likelihood of returning for routine breast screening, although it is not clear whether the studies were reporting actual attendance or intention to attend. This may be important as Bankhead et al. 84 found that women were more likely to say that they had an intention to attend their next routine mammogram than actually do so. Other studies showed a variation in the effect of a false-positive mammogram on returning for screening according to location, with European women unaffected in this domain, Canadian women less likely to return and women from the USA more likely to return for routine mammography. 62Table 7 gives a summary of the results of the included systematic reviews and meta-analyses.
| Author | No. of included false-positive studies | Total false-positive study population | Results summary: effects of false-positive mammograms | Direction of change | |||
|---|---|---|---|---|---|---|---|
| Well-being impact | Behavioural impact | ||||||
| Salz et al. 201085 | 17 | 20,781 | Disease-specific measures detected associations with increased psychological distress and increased breast care-related activity on all eight outcomes. However, generic measures only detected an effect on one outcome (generalised anxiety) | ↓a | ↓ | ||
| –b | |||||||
| Outcome | Breast cancer-specific | Generic | |||||
| Correlation coefficient, r (95% CI) | Correlation coefficient, r (95% CI) | ||||||
| Distress | 0.16 (0.10 to 0.22) | −0.04 (−0.08 to 0.01) | |||||
| Somatisation | 0.12 (0.05 to 0.19) | 0.01 (−0.09 to 0.11) | |||||
| Depression | 0.00 (−0.04 to 0.04) | ||||||
| Fear | 0.08 (0.03 to 0.14) | 0.11 (−0.18 to 0.39) | |||||
| Anxiety | 0.22 (0.18 to 0.27) | 0.03 (0.00 to 0.07) | |||||
| Worry | 0.12 (0.08 to 0.16) | 0.02 (−0.04 to 0.08) | |||||
| Perceived likelihood of cancer | 0.09 (0.04 to 0.14) | ||||||
| Perceived benefits of mammography | 0.11 (0.06 to 0.17) | ||||||
| Frequency of breast self-examination | 0.11 (0.04 to 0.19) | ||||||
| Hafslund and Nortvedt 200986 | 13 | 14,530 | Having a false-positive result had a negative impact on women's well-being and quality of life that continued for weeks or months after assessment | ↓ | Not measured | ||
| Brewer et al. 200762 | 23 | 313,967 | Women who had a false-positive mammogram were more distressed and anxious than those who did not. A meta-analysis of the effect on likelihood of returning for routine screening varied with location: women from Europe showed a non-significant trend towards not returning, risk ratio 0.97 (95% CI 0.93 to 1.01), Canadian women were less likely to return, risk ratio 0.63 (95% CI 0.50 to 0.80) and women from the USA were more likely to return, risk ratio 1.07 (95% CI 1.02 to 1.12), following a false-positive mammogram | ↓ | ∼c | ||
| ↓d | |||||||
| ↑e | |||||||
| Armstrong et al. 200787 | 22 | 167,969 | Overall, there was a short-term association between anxiety and depression and having a false-positive mammogram. However, some studies found this to be prolonged and recurring prior to the next routine screening. There was no increased or decreased likelihood of returning for screening among women who had a false-positive mammogram compared with those with a normal one. Although there was an increase in breast self-examination and breast- and non-breast-related health-care visits | ↓ | –f | ||
| ↓g | |||||||
| Brett et al. 200582 | 29 | 17,394 | In general, there was a negative psychological impact on women who had a false-positive mammogram compared with those with normal mammograms. The greatest effect was found with those who had undergone biopsy. There was conflicting evidence about the duration of the impact and what the effects were on the likelihood of women returning for routine mammography | ↓ | ↓ | ||
| ↑ | |||||||
| Bankhead et al. 200384 | 13 | 40,495 | There was a difference between women's stated intention to reattend for routine mammography following a false-positive mammogram and their actual attendance, which was lower than had been said | Not reported | ↓ | ||
Primary research
As in the previous section, the results of the primary research studies have been organised according to their domain of interest. Figure 5 (see Primary research) shows the relationship of the included primary studies to the outcome categories.
With the exception of the intervention study by Meldrum et al. ,115 all the included studies had observational designs. These designs are necessarily used when looking at the effects of a past event. However, results from observational studies should be interpreted with caution as bias and confounding may have influenced them. There may be systematic differences between the groups that do and the groups that do not have false-positive mammograms: those in the false-positive group will have had greater exposure to NHS care which could have affected their response to the questionnaires, none of the studies were blinded, therefore the way that measures were taken may have varied between the groups, and attrition bias may have skewed the results either way, as those who withdrew from the study may have been more or less affected by their screening outcome than those who remained in the studies.
As reported above, some of the studies included participants who are outside the inclusion criteria for this systematic review. Therefore, only data from the study population included in this review have been extracted and reported.
Psychological impact
In the first paper from OPCERG, by Ong et al. 104 (n = 877), adverse psychological consequences were measured at T1 (1 month after the last screening appointment) using the negative PCQ subscale. The proportion of participants within each of the screening outcomes was recorded. The results are presented simply as proportions; therefore, the review authors (MB) have calculated RRs to provide greater insight into the relationship between false-positive and normal mammograms with adverse psychological consequences. Table 8 shows the outcomes at T1. These results show an increased risk of psychological distress at 1 month after the last screening appointment for women who had a false-positive result compared with women who had a normal mammogram. The risk of distress increases in line with the intrusiveness of the assessment process, so that women who had another mammogram had a RR of 1.71 (95% CI 1.24 to 2.35), whereas women who had a biopsy were at the greatest risk, RR 2.96 (95% CI 2.19 to 4.01). Those put on early recall or who had a FNA also showed increased distress, RR 2.13 (95% CI 1.58 to 2.87) and RR 1.97 (95% CI 1.44 to 2.69), respectively.
| Outcome of screening | Women reporting adverse PCs at T1 n/N (%) | RRa(95% CI) |
|---|---|---|
| Normal mammogram | 38/130 (29) | Baseline |
| False-positive after | ||
| Further mammography | 64/128 (50) | 1.71 (1.24 to 2.35)**** |
| FNA | 61/106 (58) | 1.97 (1.44 to 2.69)**** |
| Biopsy | 26/30 (87) | 2.96 (2.19 to 4.01)**** |
| ER after assessment | 81/130 (62) | 2.13 (1.58 to 2.87)**** |
The next paper in this series is by Brett et al. 103 (n = 284) and reports outcomes 5 months after the last screening appointment (T2). Although all the women included at T2 were respondents at T1, it appears that they are a selected subset of Ong et al. 's104 participants, who were matched with T1 respondents placed on 6 months early recall, using undisclosed criteria. A comparison between T1 results in Brett et al. 103 (the selected subgroup) and T1 in Ong et al. 104 indicates that the Brett et al. 's103 subset has a higher proportion of adverse PCQ scores than the original study found (compare Table 8 with column 2 of Table 9), thus leading to higher RR estimates in the study by Brett et al. 103 than in that by Ong et al. 104
| Outcome of screening | Women reporting adverse PCs at T1 n/N (%) | RRa (95% CI) | Women reporting adverse PCs at T2 n/N (%) | RRa (95% CI) |
|---|---|---|---|---|
| Normal mammogram | 9/52 (17) | Baseline | 5/52 (10) | Baseline |
| False-positive after | ||||
| Further mammography | 29/51 (57) | 3.29 (1.73 to 6.23)*** | 23/51 (45) | 4.69 (1.93 to 11.38)*** |
| FNA | 26/41 (63) | 3.66 (1.93 to 6.93)**** | 18/41 (44) | 4.57 (1.85 to 11.26)*** |
| Biopsy | 21/23 (91) | 5.28 (2.87 to 9.68)**** | 14/23 (61) | 6.33 (2.59 to 15.50)**** |
| ER after assessment | 32/46 (70) | 4.02 (2.15 to 7.50)**** | 27/46 (59) | 6.10 (2.56 to 14.54)**** |
There is another source of uncertainty about the comparability of Brett et al. 's T1 results103 with Ong et al. 's T1 results:104 it is unclear what the proportion of women experiencing adverse psychological events represents. In the earlier paper (Ong et al. 104) the proportions represent those experiencing at least one psychological event on the PCQ and in the last paper in the series (Brett and Austoker59) it is the proportion of women scoring ≥ 12 on the PCQ. However, Brett et al. 103 do not disclose the method used for calculating the proportion. One clue is that they report that the PCQ is scored 1–4 rather than the conventional 0–3. If they have transposed the scores in this way, then a score ≥ 12 would be equivalent to a score of at least 1 on the original paper (Ong et al. 104) where the PCQ is scored 0–3. Assuming that the scores are directly comparable in this way it can be seen that, for all screening outcome categories, the level of psychological adverse effects has reduced over time (T1–T2) for the matched subset (see Table 9). This suggests that the matched subset in Brett et al. 103 is not representative of the population in Ong et al. 104 which would introduce a potential bias leading to overestimates of adverse psychological consequences at T1 for women having false-positive mammograms.
Furthermore, there is disagreement between tables within the paper by Brett et al. 103 about the benign biopsy and early recall results. In their table 1, 61% of women placed on early recall and 59% of women who had a biopsy show adverse psychological consequences, but in their table 2 these figures are reversed, although we are told that the difference between the scores at T1 and T2 was lower for women placed on early recall than for those who had a biopsy. Therefore, it has been assumed that table 2 is correct. The authors were contacted about this but no reply was received.
At T2 the results show that for all groups of women the level of distress had reduced. The greatest decrease in distress was for those women who had a biopsy at assessment (−30%). However, the RR of distress compared with those with normal mammograms had increased for all false-positive subgroups. Those women who had a biopsy continued to be at a greater risk of distress than those with a normal mammogram (RR 6.33; 95% CI 2.59 to 15.50). Those women who were put on 6 months early recall were also at a high RR (6.10; 95% CI 2.56 to 14.54). This is probably because they were only 1 month away from their next screening appointment (see Table 9).
Brett et al. 103 also conducted a logistic regression to find out which personal characteristics might be influencing the results. This showed that only having psychological consequences at 1 month (OR 5.82; 95% CI 2.70 to 12.56) and the kind of further investigation that women had (OR 4.40; 95% CI 1.35 to 14.35) were related to having psychological consequences at 5 months (Table 10).
| Variable | OR (95% CI) |
|---|---|
| Psychological consequences at 1 month | 5.82 (2.70 to 12.56)*** |
| Result group (type of investigation) | 4.40 (1.35 to 14.35)** |
| Age of women | 1.00 (0.98 to 1.03) NS |
| Apprehensiveness about attending | 0.92 (0.80 to 1.07) NS |
| Greater perceived likelihood of ever getting breast cancer compared with the average woman | 0.91 (0.35 to 2.34) NS |
| Likelihood of attending future breast screening | 0.61 (0.03 to 11.93) NS |
| Need to discuss breast screening with someone | 0.50 (0.24 to 1.02) NS |
In the final paper in the series, Brett and Austoker59 (n = 505) report outcomes at 35 months (T3) from the last screening appointment and compare them with the same participants at T1. It appears that this cohort has not been matched, as in the previous paper by Brett et al. ,103 but is the same cohort as in the first paper by Ong et al. 104 However, in this last paper, cases are those with a PCQ score ≥ 12. If the assumption of comparability is made between methods used to calculate T1 and T3 scores, then the proportion of women with normal mammograms experiencing psychological adverse events is similar at T1 (26%) and T3 (25%), although these results are higher than at T2 (10%). For those women with false-positive mammograms (other screening outcomes) the proportion experiencing adverse outcomes has reduced over time, although for those who were assessed with FNA the proportion who were distressed at T3 (45%) is similar that at T2 (44%) (Table 11).
| Last breast screening results group (1995) | Women reporting adverse PCs at T1 n/N (%) | RRa (95% CI) | Women reporting adverse PCs at T3 n/N (%) | RRa (95% CI) |
|---|---|---|---|---|
| Normal mammogram | 26/99 (26) | Baseline | 25/99 (25) | Baseline |
| False-positive after | ||||
| Further mammography | 47/93 (51) | 1.92 (1.31 to 2.83)*** | 30/93 (32) | 1.28 (0.82 to 2.00) NS |
| FNA | 36/66 (55) | 2.08 (1.40 to 3.09)*** | 30/66 (45) | 1.80 (1.17 to 2.77)*** |
| Biopsy | 15/21 (71) | 2.72 (1.78 to 4.17)**** | 11/21 (52) | 2.07 (1.22 to 3.52)** |
| ER after assessment | 62/100 (62) | 2.36 (1.64 to 3.40)**** | 46/100 (46) | 1.82 (1.22 to 2.72)** |
An examination of the RR of experiencing psychological consequences at T3 and T1 show that this risk has diminished substantially for those assessed with a further mammogram, RR 1.28 (95% CI 0.82 to 2.00), as they were not statistically significantly more distressed than women who had a normal mammogram. Also, women who were placed on 6-months early recall showed a 23% reduction in their RR of distress at T3, RR 1.82 (95% CI 1.22 to 2.72). However, women who were assessed by FNA had the least reduction in RR (0.28) with a 35-month RR of 1.80 (95% CI 1.17 to 2.77). Those who had a biopsy maintained their status of having the highest RR of distress at T3, RR 2.07 (95% CI 1.22 to 3.52). Table 11 shows the results comparing T1 and T3.
The results from the OPCERG study indicate that there is an enduring relationship, lasting at least 3 years, between having a false-positive mammogram and exhibiting negative psychological consequences. This effect is shown to be in proportion to the degree of invasiveness of the assessment procedure, with 52% of women who were assessed by biopsy, 45% assessed by FNA, 32% assessed by further mammogram and 46% of those placed on 6 months early recall, compared with 25% of those with a normal mammogram, still experiencing distress 3 years after their last screening appointment. The numbers of distressed women may partly be explained by the impending date for their next screening appointment, although the proportions are still likely to reflect their assessment procedure.
The forest plot in Figure 6 gives an overview of the RR of psychological distress from a false-positive mammogram in relation to time and the method of assessment when compared with a normal mammogram.
FIGURE 6.
Forest plot of the RRs of negative psychological consequences from having a false-positive mammogram compared with a normal one by type of false-positive assessment at T1 (1 month after assessment), T2 (5 months after assessment) and T3 (35 months after assessment), measured with the PCQ.
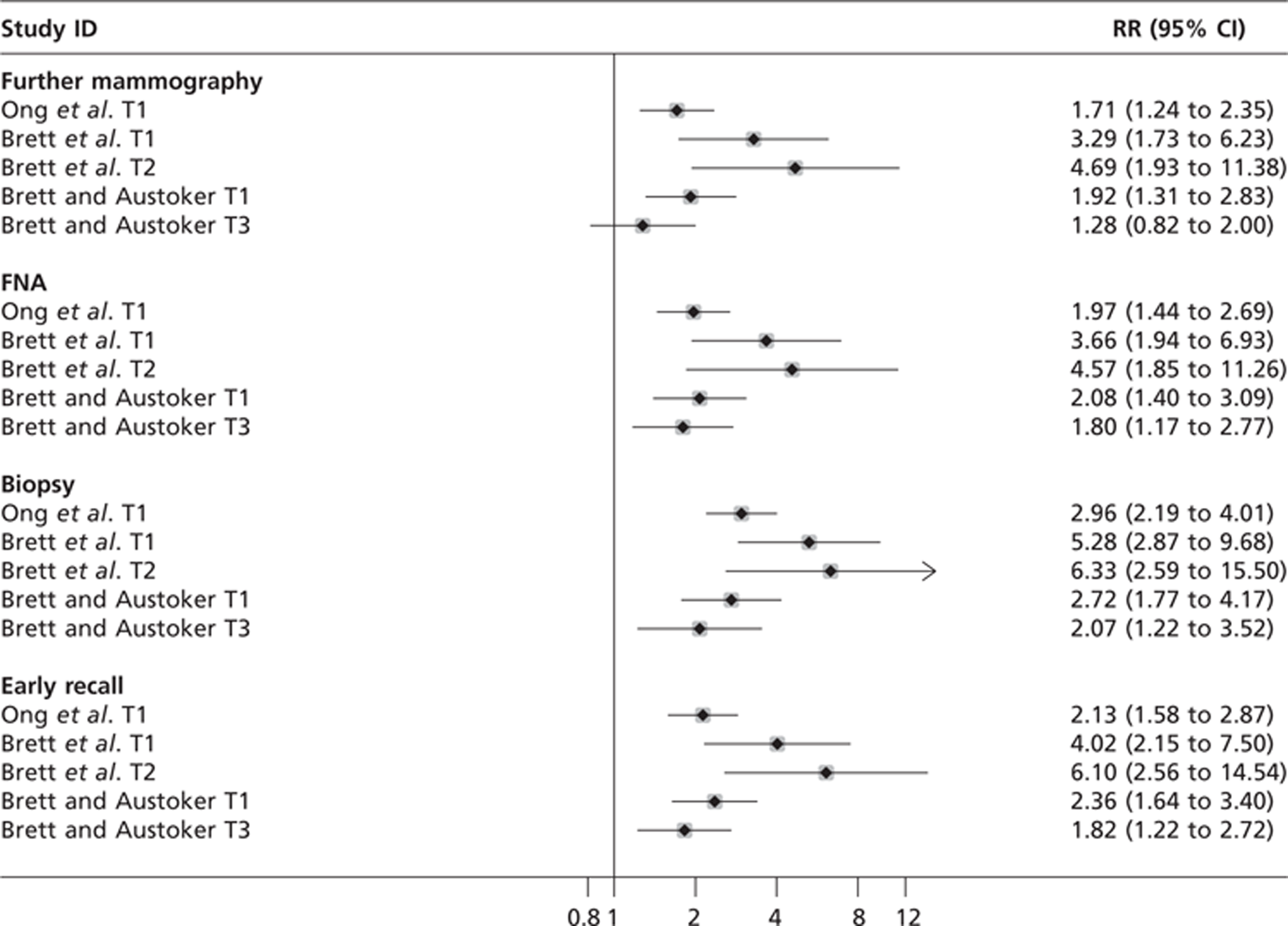
The results from the ad hoc questionnaire, given at 35 months (T3), reported by Brett and Austoker59 shed some light on factors that may have influenced the level of distress experienced. Table 12 shows the items that were statistically significantly correlated with psychological distress at 1 month before the next routine screening (T3). The highest correlation with distress is with the lack of opportunity to talk to someone after the screening appointment (r = 0.35). The other most highly correlated factors are the waiting time between screening and assessment (r = 0.30), communication problems at the screening appointment due to anxiety (r = 0.29), fear of radiation (r = 0.28), amount of written information (r = 0.28), the performance of health workers (r = 0.27) and the unnecessary worry caused by the last breast screen (r = 0.26).
| Statements about last screening appointment | False-positive coefficient |
|---|---|
| Opportunity to talk to somebody after the breast screening appointment | 0.35*** |
| Waiting between appointment letter and appointment(s) | 0.30*** |
| Difficulties with taking in verbal information at breast screening appointment because of anxiety | 0.29*** |
| Fear of radiation | 0.28*** |
| Amount of written information | 0.28*** |
| Perceived performance of health workers | 0.27*** |
| Unnecessary worry experienced as a result of the last breast screening | 0.26*** |
| The amount of time spent on verbal communication at assessment | 0.24*** |
| Verbal communication: chance to say what is on one's mind | 0.23*** |
| Waiting for test results | 0.22*** |
| Quality of verbal communication | 0.21*** |
| Women's understanding of test result | 0.21*** |
| Amount of information provided in advance | 0.18** |
| Postal notification of mammographic results | 0.14* |
Preceding the work of OPCERG, Sutton et al. 55 (n = 1021) published results from their retrospective cohort study. These results show that, on their ad hoc questionnaire, when looking back over the previous 9 months, women who had false-positive mammograms were more likely to report that they were more anxious than women with normal mammograms at three time points: at the clinic after the mammogram, mean difference 0.24 (95% CI 0.03 to 0.45); while waiting for the results letter, 0.25 (95% CI 0.02 to 0.48); and after reading the results letter, 1.69 (95% CI 1.54 to 1.84). Unfortunately, Sutton et al. 55 do not numerically report how anxious the two groups were at the time they completed the questionnaire; these results are only presented as a graph which it is not possible to accurately read. The findings from this study are questionable as, apart from other methodological considerations of bias, the experience of having a false-positive mammogram may have coloured the women's view of how they felt at the time of screening and confounded their responses. It would seem unlikely that there should be any genuine difference between the two groups prior to receiving the results letter when it might be expected to find a difference in anxiety levels. A summary of the results from this study can be found in Table 13.
| Outcome | Stage 1: receive screening invitation, mean (SD) | Stage 2: while waiting for the mammogram, mean (SD) | Stage 3: at the clinic after the mammogram, mean (SD) | Stage 4: after screening and before receiving the results, mean (SD) | Stage 5: after reading the results letter, mean (SD) | Stage 6: now, mean (SD) |
|---|---|---|---|---|---|---|
| False-positive (n = 24) | Not reported | Not reported | 1.60 (0.68) | 1.95 (0.09) | 2.85 (0.37) | Not reported |
| Normal mammogram (n = 671) | Not reported | Not reported | 1.36 (0.52) | 1.70 (0.57) | 1.16 (0.36) | Not reported |
| Difference in means (95% CI) | – | – | 0.24 (0.03 to 0.45) | 0.25 (0.02 to 0.48) | 1.69 (1.54 to 1.84) | – |
The results of Bull and Campbell's (n = 750) cohort study106 are ambiguous. The authors' ad hoc questionnaire of the frequency of breast self-examination found that 6 weeks after receiving the ‘all-clear’ women who had a false-positive mammogram examined their breasts more frequently than women who had a normal mammogram. Women who had a biopsy at assessment were more likely to self-examine once a week or more than those who had less invasive assessments. This may indicate that the women with false-positive results were more anxious about having breast cancer than those with normal results, although there is uncertainty about the meaning of very frequent breast self-examination. These results can be seen in Table 14.
| Frequency of breast self-examination by group | Normal mammogram (N = 102), n (%) | False-positive (not biopsy) (N = 204), n (%) | False-positive (biopsy) (N = 49), n (%) |
|---|---|---|---|
| Never | 22 (22) | 24 (12) | 7 (14) |
| Less than once a month | 23 (23) | 34 (17) | 7 (14) |
| Once a month | 47 (46) | 97 (48) | 18 (37) |
| Once a week | 10 (10) | 41 (20) | 12 (24) |
| More than once a week | 0 | 8 (4) | 5 (10) |
| No response | 0 | 0 | 0 |
When the results of both false-positive groups are combined there is a clear link between frequency of breast self-examination and having a false-positive mammogram, with the RR increasing with the frequency of self-examination (Table 15).
| Frequency of breast self-examination | Normal mammogram (N = 102), n (%) | All false-positive (N = 253), n (%) | RRacomparing normal mammogram with all false-positive (95% CI) |
|---|---|---|---|
| Never | 22 (22) | 31 (12) | 0.57 (0.35 to 0.93)* |
| Less than once a month | 23 (23) | 41 (16) | 0.71 (0.46 to 1.13) NS |
| Once a month | 47 (46) | 115 (45) | 0.99 (0.77 to 1.27) NS |
| Once a week | 10 (10) | 63 (25) | 2.54 (1.36 to 4.75)** |
| More than once a week | 0 | 13 (5) | (0.66 to 182.48) NS |
| No response | 0 | 0 |
However, Bull and Campbell's106 results with the HADS tell a different story. They show no statistically significant difference in anxiety or depression between the groups at 6 weeks after assessment, with the majority in all groups being within the normal range on both subscales. Borderline anxiety and depression were higher in the false-positive group that did not have a biopsy, depression (12%) or anxiety (12%) than in the false-positive group that had a biopsy, depression (6%) or anxiety (8%). This may have been because a biopsy was seen as a more conclusive declaration of health than other methods of assessment (Table 16).
| HADS subscales | Normal mammogram n/N (%) | False-positive (not biopsy) n/N (%) | False-positive (biopsy) n/N (%) |
|---|---|---|---|
| Depression | |||
| Normal (0–7) | 95/104 (91) | 168/202 (83) | 43/49 (88) NS |
| Borderline (8–10) | 7/104 (7) | 25/202 (12) | 3/49 (6) NS |
| Abnormal (≥ 10) | 2/104 (2) | 9/202 (5) | 3/49 (6) NS |
| Anxiety | |||
| Normal (0–7) | 91/103 (88) | 174/202 (86) | 42/49 (86) NS |
| Borderline (8–10) | 10/103 (10) | 24/202 (12) | 4/49 (8) NS |
| Abnormal (≥ 10) | 2/103 (2) | 4/202 (2) | 3/49 (6) NS |
When the false-positive groups are combined, the RR of experiencing anxiety or depression is not statistically significantly greater in the false-positive group (Table 17).
| HADS subscales | Normal mammogram n/N (%) | False-positive n/N (%) | RRa (95% CI) |
|---|---|---|---|
| Depression | |||
| Normal (0–7) | 95/104 (91) | 211/251(84) | Baseline |
| Borderline (8–10) | 7/104 (7) | 28/251 (11) | 1.71 (0.77 to 3.78) NS |
| Abnormal (≥ 10) | 2/104 (2) | 12/251 (5) | 2.61 (0.60 to 11.44) NS |
| Anxiety | |||
| Normal (0–7) | 91/103 (88) | 216/251 (86) | Baseline |
| Borderline (8–10) | 10/103 (10) | 28/251 (11) | 1.16 (0.59 to 2.30) NS |
| Abnormal (≥ 10) | 2/103 (1) | 7/251 (3) | 1.46 (0.31 to 6.90) NS |
The prospective cohort study by Ellman et al. 105 (n = 752) used the GHQ-28,72 another generic instrument, to measure psychological morbidity at the screening visit and 3 months later. They found that there were no statistically significant differences between women who had normal mammograms and those with false-positive ones. Scores ≥ 4 on the GHQ-28 are deemed to be ‘cases’ (Table 18).
| Time | Normal mammogram n/N (%) | False-positive n/N (%) |
|---|---|---|
| Screening visit | 71/295 (24) | 216/721 (30) NS |
| 3 months later | 56/295 (19) | 137/721 (19) NS |
The distribution of scores on the four subscales of somatic, anxiety, social dysfunction and depression can be seen in Table 19. These show that the proportion of scores above ‘case’ level declined in both groups between baseline and follow-up, with the greatest decline in the false-positive group for the somatic and anxiety subscales (40% to 26% and 44% to 29%, respectively).
When the RRs are calculated for psychological morbidity these again show that there is no statistically significant difference between those women with and those without false-positive mammograms at baseline and 3 months later (Table 20).
| Symptom subscale | Normal mammogram | False-positive | ||
|---|---|---|---|---|
| Screening visit n/N (%) | 3 months later n/N (%) | Screening visit n/N (%) | 3 months later n/N (%) | |
| Somatic | 113/295 (38) | 98/287 (34) | 108/271 (40) | 69/266 (26) |
| Anxiety | 104/295 (35) | 75/287 (26) | 119/271 (44) | 77/266 (29) |
| Social dysfunction | 104/295 (35) | 86/287 (30) | 89/271 (33) | 77/266 (29) |
| Depression | 42/295 (14) | 29/287 (10) | 38/271 (14) | 27/266 (10) |
| Time | Normal mammogram n/N (%) | False-positive n/N (%) | RR20(95% CI) |
|---|---|---|---|
| Screening visit score | |||
| Normal (0–4) | 222/295 (75) | 189/271 (70) | Baseline |
| Subclinical/mild (5–9) | 49/295 (17) | 48/271 (18) | 1.12 (0.78 to 1.60) NS |
| Clinical mild/moderate (10–28) | 24/295 (8) | 34/271 (13) | 1.56 (0.96 to 2.55) NS |
| 3 months later score | |||
| Normal (0–4) | 232/287 (81) | 216/266 (81) | Baseline |
| Subclinical/mild (5–9) | 31/287 (11) | 23/266 (9) | 0.82 (0.49 to 1.36) NS |
| Clinical mild/moderate (10–28) | 24/287 (8) | 27/266 (10) | 1.19 (0.70 to 2.00) NS |
The forest plot in Figure 7 shows the results from Bull and Campbell106 and Ellman et al. 105 Here it can be seen that, although none of the results show clinical levels of general anxiety and depression, there is a trend in that direction.
Summary: psychological impact in the general population
The OPCERG found that there was a statistically significant negative psychological impact from having a false-positive mammogram, whereas the main outcome measures of Bull and Campbell106 and those of Ellman et al. 105 did not. The difference in findings between those of OPCERG and others may be explained in a variety of ways by differences in their design, methods and populations or they may be an artefact of bias or confounding. However, one key difference stands out: OPCERG measured outcomes with disease-specific instruments, whereas Bull and Campbell106 and Ellman et al. 105 used generic measures designed to detect general anxiety and depression at clinically recognisable levels.
Further possible evidence of some distress comes from the Bull and Campbell study's106 frequency of breast self-examination results. In this study there is a clear relationship between the RR of increasingly frequent breast self-examination and having a false-positive mammogram, with those women assessing themselves once a week or more than this having RRs of 2.54 (95% CI 1.36 to 4.75) and 10.95 (95% CI 0.66 to 182.48), respectively. It may be reasonable to suggest that this level of frequency of self-examination is a proxy for anxiety.
The disagreement about the psychological impact of false-positive screening results may be explained by the type of outcome measures used, whether disease-specific or generic. Brodersen et al. ,116–119 from the University of Copenhagen, have written a number of papers on the subject of measuring psychological distress in women who have received false-positive mammograms. In particular, they conducted a literature review to find out how suitable the outcome measures used in studies of false-positive mammograms were for detecting psychological distress. 116 The review found 23 includable studies; the most commonly used outcome measures were the HADS, GHQ, STAI and PCQ. By judging the instruments' psychometric properties in the context of false-positive mammography, they found that HADS, GHQ and STAI were unsuitable for use in measuring psychological distress. This was because the content of HADS and the GHQ were not applicable to the screening context as they were designed to screen for general anxiety at clinical levels rather than the specific breast cancer anxiety that may be expected. The STAI had been validated using students before their exams and may not replicate the anxiety felt by women possibly facing cancer. None of these scales have had their content validity demonstrated in the breast cancer screening context and all contain items that are irrelevant to women being screened, which may lead to the items' omission by respondents. Brodersen et al. 116 also reported that the PCQ was the most suitable measure to use to test psychological morbidity resulting from breast cancer screening as it had been validated in this context and was specifically designed for this purpose. However, its ability to reliably measure the psychological consequences of mammography screening in the long term has yet to be determined. Table 21 shows the relationship between the types of measure used (generic or disease-specific) and the results of studies that compared women with false-positive mammograms with those with normal mammograms.
| Study | Year | Disease-specific | Generic | Negative psychological consequences: false-positive vs normal result | ||
|---|---|---|---|---|---|---|
| PCQ | HADS | GHQ-28 | Found | Not found | ||
| Brett and Austoker59 | 2001 | ✓ | ||||
| Brett et al.103 | 1998 | ✓ | ||||
| Ong et al.104 | 1997 | ✓ | ||||
| Bull and Campbell106 | 1991 | ✓ | ||||
| Ellman et al.105 | 1989 | ✓ | ||||
Therefore, it may be reasonable to speculate that, for those in the general population, having a false-positive screening mammogram can cause breast cancer-specific psychological distress that may endure for up to 3 years. However, it is less likely that there will be general anxiety detectable at clinically recognisable levels. Further research is needed in well-designed observational studies that use both disease-specific and generic outcomes to determine whether or not this is the case.
Psychological impact with family history of breast cancer
The PIMMS Management group's first results are from Tyndel et al. 's101 (n = 2321) study in the FHBC population. When their within-study group results are considered, the women who had a normal mammogram showed a statistically significant decrease in their distress levels between T1 (1 month before screening) and T2 (1 month after screening) on both measures (CWS-R and PCQ), whereas those in the recall group did not. However, when the between T2-and-T3 (6 months after screening) scores are compared, both groups show statistically significant reductions in distress over this 5-month period on both measures (Table 22).
| Questionnaire | False-positive result | Within false-positive result | Normal result | Within normal result | Between groups | |
|---|---|---|---|---|---|---|
| Mean (SD) | Paired t-test | Mean (SD) | Paired t-test | Difference in means | 95% CI | |
| CWS-R | ||||||
| T1 (n = 111, 1171) | 11.61 (2.90) | – | 10.99 (2.91) | – | 0.62 | 1.19 to 0.05, NS |
| T2 (n = 111, 1171) | 11.68 (2.89) | – | 10.56 (2.60) | – | 1.12 | 1.63 to 0.61, NS |
| T3 (n = 111, 1159) | 10.35 (2.65) | – | 10.12 (2.49) | – | 0.21 | 0.30 to 0.72, NS |
| Difference T1–T2 | – | −0.298, NS | – | 7.537** | ||
| Difference T2–T3 | – | 6.372** | – | 8.633** | ||
| PCQ | ||||||
| T1 (n = 110, 1167) | 7.32 (7.66) | – | 5.06 (6.71) | – | 2.26 | 3.59 to 0.93, NS |
| T2 (n = 110, 1167) | 7.1 (7.44) | – | 4.18 (6.19) | – | 2.92 | 4.05 to 1.69* |
| T3 (n = 110, 1169) | 4.61 (6.42) | – | 3.84 (6.00) | – | 0.77 | 1.95 to 0.41, NS |
| Difference T1–T2 | – | −0.051, NS | – | 6.935** | ||
| Difference T2–T3 | – | 5.752** | – | 3.183** | ||
The between-group scores are harder to interpret owing to the potential for bias to be introduced by lack of randomisation and demographic data that indicates that there are differences between the groups. The false-positive group have statistically significantly greater proportions of participants with biological children (p = < 0.05), a high-risk family history (p = < 0.05) and post-mammography hospital attendance for symptoms (p = < 0.05). The only statistically significant difference in the between-group scores is with the PCQ at T2, when those in the recall group {mean [standard deviation (SD)] 7.1 (7.44)} were more distressed than those in the normal result group [mean (SD) 4.08 (6.19)], this difference was no longer statistically significant 5 months later at T3 (see Table 22). However, these results, like those from the other studies, have not been adjusted for potential confounders (items on the PCQ and CWS-R). Tyndel et al. 101 present adjusted results (for the potential confounders listed above) in graphical form only (which could not be accurately transposed) that indicate that if these are considered then the recall group showed the greatest decrease in distress between T2 and T3 and the normal result group between T1 and T2 on both measures. The adjusted results were statistically significant; the level of significance is not reported.
Unusually, Tyndel et al. 101 also took measures on the positive subscale of the PCQ to see if there were any benefits from having a false-positive mammogram. They found that women who were recalled scored statistically significantly more highly at T2 [mean (SD) 13.02 (7.6)] than those with normal mammograms [mean (SD) 10.81 (6.9)]. However, this effect had diminished and was not statistically significant by T3. Additionally, when they had received their results at T2 and T3, women were asked whether their opinion of the benefits of breast screening had changed since their last visit. Women who were recalled were statistically significantly more likely to feel positive about the benefits of screening at both time points than those with normal mammograms, T2 = OR 3.16 (95% CI 2.14 to 4.70) and T3 = OR 2.35 (95% CI 1.53 to 3.61) (Table 23).
| Outcome | False-positive | Normal mammogram | Mann–Whitney U-test | |
|---|---|---|---|---|
| Positive PCQ at T2 | 51,561 | ** | ||
| Mean (SD) | 13.02 (7.6) | 10.81 (6.9) | ||
| Positive PCQ at T3 | 59,169 | NS | ||
| Mean (SD) | 12.65 (8.9) | 11.16 (7.0) | ||
| Benefits of screening more positive at T2 | OR | 95% CI | ||
| No. (%) | 112 (55) | 1164 (27) | 3.17 | 2.14 to 4.70*** |
| Benefits of screening more positive at T3 | ||||
| No. (%) | 105 (35) | 1085 (19) | 2.35 | 1.53 to 3.61*** |
Overall, the levels of distress were similar in both groups although at T2, on the PCQ, the false-positive group showed greater distress. Additionally, 1 month after the all-clear (T2) those women with false-positive mammograms saw statistically significantly more benefits from screening and were more positive about the benefits of screening at T2 and 6 months later (T3).
Brain et al. 102 (n = 1286) then used the results of Tyndel et al. 101 to investigate which factors, evident at pre-screening, predicted cancer distress at 1 month (T2) and 6 months (T3) after assessment. The results of hierarchical multiple regression showed that, among others, cancer worry at 1 month after screening was predicted by having a false-positive mammogram (p < 0.05). This was no longer the case at 6 months after the all-clear. The model accounted for 61% of the variance at T2 and 57% at T3. Table 24 gives all the variables predictive of cancer distress in the FHBC population.
| T1 (1 month before screening) variable | T2 (1 month after screening) CWS-R | T3 (6 months after screening) CWS-R |
|---|---|---|
| T1 cancer worry | 0.54*** | 0.58*** |
| High perceived lifetime risk of breast cancer | 0.09*** | 0.08** |
| Relative died of breast cancer in the last year | – | 0.05* |
| Belief in increased risk due to family history | 0.09*** | 0.08*** |
| First attendance at the screening programme | −0.07*** | −0.04* |
| Being recalled for further tests – false-positive | 0.06* | – |
| Low emotion focused coping potential | −0.06* | −0.05* |
| Use of religion as a coping strategy | 0.05** | – |
| Dispositional optimism | −0.05* | 0.00, NS |
| Low challenge appraisal | −0.04* | −0.02, NS |
| Substance use for coping | 0.04* | – |
In order to gain greater understanding of their results and how women valued being in the annual FHBC screening programme, the PIMMS Management Group conducted an interview study of their participants who did (n = 22) and did not (n = 36) have a false-positive mammogram. 107 As this work has been published only as a poster summary, it is only possible to report an overview of their findings.
The thematic analysis of the interviews by Clements et al. 107 (n = 58) showed that being part of the FHBC screening programme helped to relieve fear of breast cancer and resulted in women feeling more in control of their family history. Women believed that taking part in screening would enable the earlier detection of cancer and that this would lead to a positive outcome. They also believed that a mammogram was more likely to detect early-stage cancer than actually is the case. They thought that an all-clear result meant that they did not have cancer. Although women who had a false-positive result were initially distressed, when they received the all-clear they had increased feelings of reassurance and security and a greater faith in the screening process than those with an initial all-clear result. They felt that being recalled was positive proof that screening worked.
Summary: psychological impact in the family history of breast cancer population
Like the women in the normal-risk population, those in the false-positive FHBC group were significantly more distressed at 1 month after screening than were those with normal mammograms. However, this similarity had disappeared 5 months later, when, although the level of distress was higher than in the normal mammogram group, it was no longer significantly higher in the FHBC false-positive population. The results from the positive PCQ may shed some light on this, as women with a false-positive result scored statistically significantly higher at 1 month after assessment than those with normal mammograms (27%) and reported that they felt more positive about the benefits of screening than at their previous visit (55%). It may be that the women with a FHBC were anticipating a malignant result and felt reassured by having further tests that they remained free of cancer, whereas those in the general population believed they were well and were shocked when they were recalled. A few months later the negative effects of the false-positive experience had diminished for both populations. However, women with a FHBC still reported a statistically significantly more positive attitude to screening at 6 months after their last screening appointment than those who had a normal result in the FHBC population, although multiple regression indicated that a false-positive outcome was predictive of distress 1 month after screening.
Unfortunately, there are currently (2011) no published qualitative interview studies among members of the normal-risk UK population who have experienced a false-positive mammogram to help us better understand these differences and Clements et al. 's results. 107 It would seem likely that women in the general population approach breast screening with a different belief; they have no reason to anticipate breast cancer and believe themselves to be well. As Wardle and Pope120 surmise, their view of themselves as healthy people is challenged by being recalled and the spectre of cancer looms large. This is a very different scenario to those women with a family history of the disease, who may carry the belief that they will one day develop breast cancer. 121 Therefore, rather than seeing two assessments (screening and follow-up tests) as giving increased reassurance of good health, women in the general population may view being recalled as undermining their belief in their good health. In the absence of qualitative interview evidence in the general population, this is, of course, speculative.
Impact of a false-positive mammogram on returning for routine screening
Actual reattendance
The results from the large data registry review of mammography screening attendance in East Anglia by McCann et al. 61 (n = 140,387) show that women who had a false-positive mammogram were less likely than those with normal mammograms to reattend 3 years later for their next routine breast screen (RR 0.97; 95% CI 0.96 to 0.98). Additionally, women who had a biopsy in order to rule out cancer were less likely to reattend than those who were assessed by other methods (RR 0.93; 95% CI 0.89 to 0.97) (Table 25).
| Study group | Reattending (n) | Not reattending (n) | Reattendance, % (95% CI) | RRa (95% CI) |
|---|---|---|---|---|
| Normal mammogram | 93,081 | 15,536 | 85.7 (85.5 to 85.9) | Baseline |
| False-positive – all | 3981 | 811 | 83.1 (82.0 to 84.4) | 0.97 (0.96 to 0.98)**** |
| False-positive – no biopsy | 3572 | 706 | 83.5 (82.4 to 84.6) | 0.97 (0.96 to 0.99)**** |
| False-positive – biopsy | 409 | 105 | 79.6 (76.1 to 83.1) | 0.93 (0.89 to 0.97)*** |
These results do not replicate those of the earlier registry study, based in central and east London, by O'Sullivan et al. 108 (n = 5649), which found that, although reattendance rates were lower, there was no statistically significant difference in reattendance levels between women with normal mammograms and those who had false-positive ones. These findings included those who had been put on early recall following further assessment (Table 26).
| Result at initial screening | Reattending, n (%) | Not reattending, n (%) | Reattendance, % (95% CI) | RRa (95% CI) |
|---|---|---|---|---|
| Normal | 3841 (71) | 1560 (29) | 71 (69.56 to 72.44) | Baseline |
| False-positive – all | 175 (71) | 73 (29) | 71 (64.28 to 77.72) | 0.99 (0.91 to 1.08) NS |
| False-positive – routine recall | 119 (74) | 43 (26.5) | 74 (66.12 to 81.88) | 1.03 (0.94 to 1.13) NS |
| False-positive – early recall | 56 (65) | 30 (35) | 56 (52.51 to 77.49) | 0.92 (0.78 to 1.07) NS |
The difference in findings may be due to differences in study design. In McCann et al. 's study,61 participants were invited for their second round of screening, whereas O'Sullivan et al. 's participants108 were being invited for up to their fifth screening round. Therefore, the difference in findings may be partly caused by a general decrease in screening attendance over time. 122 However, this does not explain why those who attended more screenings and therefore had an increased risk of a false-positive mammogram should have similar levels of reattendance to those with normal mammograms in O'Sullivan et al. 's study. 108
The study by Brett and Austoker,59 published in 2001 (n = 505), into the psychological consequences of false-positive mammograms also included data on reattendance at the next screening clinic. Their results, which agree with those of McCann et al. ,61 showed that women who had a false-positive mammogram were less likely to reattend screening in 3 years' time. It is not clear how many mammography-screening invitations these women had received (Table 27).
| Result at initial screening | Reattending, n (%) | Not reattending, n (%) | Reattendance, % (95% CI) | RRa (95% CI) |
|---|---|---|---|---|
| Normal | 120 (92) | 10 (08) | 92 (87.15 to 96.85) | Baseline |
| False-positive | 319 (85) | 56 (15) | 85 (81.08 to 88.92) | 0.92 (0.86 to 0.98)* |
This study was preceded by the RCT by Meldrum et al. ,115 in 1994 (n = 3083), which aimed to find out the impact of invitation letters tailored to the outcome of the previous screening round (normal or false-positive) on reattendance. Their results show that, for the standard invitation letter, those with a normal mammogram were more likely to reattend (74%) 3 years later, than those with a false-positive one (70%), although there was no statistically significant difference in the RR of attendance between the two groups (Table 28).
| Result at initial screening | Reattending, n (%) | Not reattending, n (%) | Reattendance, % (95% CI) | RRa (95% CI) |
|---|---|---|---|---|
| Normal | 583 (74) | 208 (26) | 74 (71 to 77) | Baseline |
| False-positive | 78 (70) | 34 (30) | 70 (61 to 78) | 0.94 (0.83 to 1.08) NS |
However, those with a previous false-positive mammogram who received a tailored invitation were more likely to reattend (82%) than those with a previous normal mammogram (74%). There was a small statistically significant RR for reattendance, RR 1.10 (95% CI 1.00 to 1.21) (Table 29).
| Result at initial screening | Reattending, n (%) | Not reattending, n (%) | Reattendance, % (95% CI) | RRa (95% CI) |
|---|---|---|---|---|
| Normal | 594 (74) | 206 (26) | 74 (70.47 to 77.53) | Baseline |
| False-positive | 94 (82) | 21 (18) | 84 (74.23 to 89.77) | 1.10 (1.00 to 1.21)* |
Very little information is given about the subsequent interviews. Sixty-six women were interviewed about the acceptability and understandability of the tailored letter. No negative comments were made about the letter, but only one person spontaneously mentioned that the letter was tailored to her screening history. Most women appeared not to have paid much attention to the content as they had previously been through the screening process. None of the non-attenders responded that the tailored letter had discouraged them from being screened again.
The final study that reported actual reattendance data is by Orton et al. 109 (n = 1582). Their findings showed that more women with previous false-positive mammograms reattended mammography screening (92%) than those who had a normal outcome (89%). However, the RR of reattendance was not statistically significant (Table 30).
| Result at initial screening | Reattending, n (%) | Not reattending, n (%) | Reattendance, % (95% CI) | RRa (95% CI) |
|---|---|---|---|---|
| Normal | 1362 (89) | 170 (11) | 89 (87.43 to 90.57) | Baseline |
| False-positive | 46 (92) | 4 (08) | 92 (84.48 to 99.52) | 1.03 (0.95 to 1.13) NS |
Intended reattendance
The evidence about women's intention to reattend mammography screening when they have had a false-positive mammogram is very limited. It comes only from the final paper from the OPCERG study by Brett and Austoker (n = 505). 59 They reported the results from a questionnaire that asked participants which external factors had influenced their decision to attend their next routine screening in 1 months' time. These factors can be seen in Table 31, the only items that women reported as worrying influences being magazine or newspaper articles.
| Item | n/N | % (95% CI) | Cause worry % |
|---|---|---|---|
| Magazine or newspaper article | 83/288 | 29 (24 to 34) | 11 |
| Television programme | 72/288 | 25 (20 to 30) | 9 |
| GP attitude to screening | 69/288 | 24 (19 to 29) | – |
| Friend | 60/288 | 21 (16 to 26) | – |
| Poster or leaflet | 50/288 | 17 (13 to 22) | – |
| Family | 47/288 | 16 (12 to 20) | – |
| Radio programme | 37/288 | 13 (9 to 17) | – |
The logistic regression conducted by Brett et al. 103 of women with false-positive mammograms 5 months after assessment showed that intention to attend the next screening round was not related to experiencing psychological distress at that time (see Table 10).
Summary: impact of false-positive mammograms on reattendance
Figure 8 compares the RRs of the actual reattendance studies. A meta-analysis was not undertaken because of the lack of information about the demographic profiles of participants, making it impossible to assess the studies' homogeneity, together with underlying reservations about the validity of meta-analysis of observational data which can magnify underlying bias and confounding. Two studies showed a statistically significant effect on reattendance [RR 0.97 (95% CI 0.96 to 0.98)]61 and [RR 0.92 (95% CI 0.86 to 0.98)],59 whereas two did not [RR 0.99 (95% CI 0.91 to 1.08)]108 and [RR 1.03 (95% CI 0.95 to 1.13)]. 109 It could be argued that most notice should be taken of the study by McCann et al. 61 on the basis of size (n = 140,387) and quality. However, O'Sullivan et al. 108 was also a large study (n = 5549) and showed no such effect, although their participants differed from those in the other studies as they were not necessarily on their second screening round and there may be an effect of repeated screening reducing attendance. 122 The smallest study was by Brett and Austoker (n = 505)59 who found that significantly more women with false-positive mammograms failed to reattend the second round of screening, whereas Orton et al. (n = 1582)109 found a non-statistically significant trend in the opposite direction.
FIGURE 8.
Forest plot of the likelihood of failing to reattend the next round of mammography screening following a false-positive mammogram compared with a normal one.
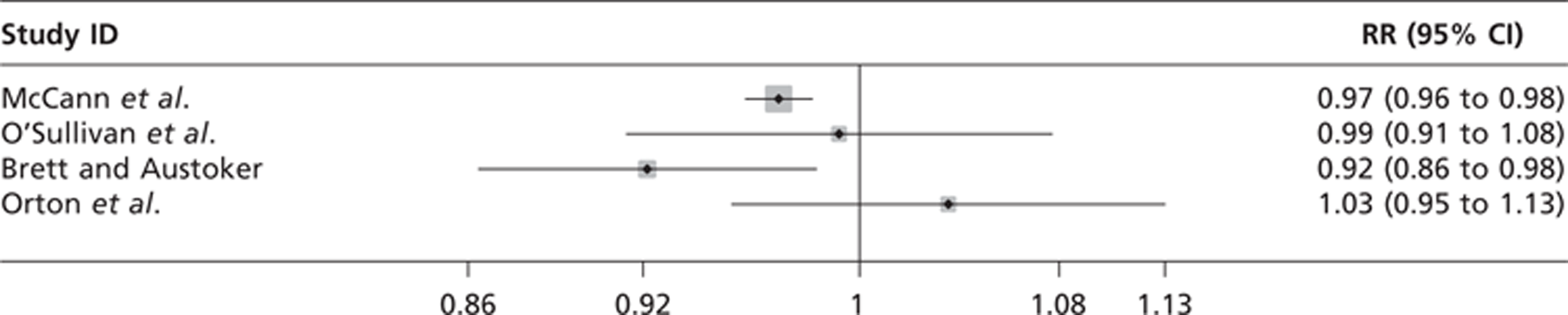
There is some evidence, from the only RCT in this systematic review, that these reattendance figures can be improved for the false-positive group by sending women tailored invitation letters for the next screening round that refer back to their previous outcome. The data from Brett and Austoker59 on external influences on reattendance following a false-positive mammogram indicate that the media may have a greater influence than GP, family or friends. The availability of a CNS at screening clinics, as well as assessment clinics, may help to answer questions and concerns resulting from the media.
Overall, it seems likely that the experience of having a false-positive mammogram has a detrimental effect on next-round screening attendance. Future studies should collect demographic information so that their data can be reasonably compared in meta-analysis and this hypothesis can be tested.
Interventions to reduce the impact of false-positive mammograms
As mentioned in Interventions to reduce the impact of being recalled for further assessment we found no studies that directly addressed this issue. However, two studies were found that looked at the information needs of women who are recalled after screening. The initial results from the multicentre OPCERG study by Austoker and Ong112 (n = 1493) found that 92% of women were distressed or very distressed when they received their recall letter for assessment following a screening mammogram. Although no standardised measures of anxiety were taken, the quotes from the answers to the open questions in Table 32 give an indication of the range of responses.
| Reaction | n (%) women | Sample comments |
|---|---|---|
| Pleased | 30 (2) | Very pleased to think I was having a proper check |
| Neutral/not distressed | 87 (6) | I just felt normal |
| Somewhat distressed | 497 (34) | Concerned though not unduly |
| I felt rather apprehensive | ||
| Nervous, but I think it is a good thing | ||
| Unpleasantly apprehensive | ||
| Distressed | 415 (28) | Nervous and very apprehensive |
| Anxious and worried | ||
| Frightened and worried | ||
| Worried, afraid | ||
| Very distressed | 439 (30) | I felt the whole bottom had fallen out of my world |
| I felt sick then faint, then I cried then I kept thinking what I have to do if I have cancer | ||
| Worried to death | ||
| Panic stricken, depressed. Convinced I was going to die | ||
| Completely devastated. Reason abandoned me | ||
| All women | 1468 (100) |
Further results from the questionnaire compared the responses of women who had been given particular items of information in recall letters or leaflets with those who had not. Austoker and Ong112 found that for all items women wanted more information; this was especially the case when the item was not mentioned in the recall literature. The item that the greatest proportion of women wanted more information about was the reason for their recall (item mentioned in recall literature 36%, item not mentioned in recall literature 46%) (Table 33).
| Item | Item mentioned in letter/leaflet | Item not mentioned in letter/leaflet | ||
|---|---|---|---|---|
| Women wanting more information | Women wanting more information | |||
| % | n/N | % | n/N | |
| Why they were recalled | 36 | 383/1070 | 46 | 179/388** |
| What tests would be done | 11 | 65/606 | 35 | 298/847**** |
| Who could come with them | 5 | 44/888 | 35 | 148/419**** |
| How to get more information | 18 | 143/783 | 33 | 212/633**** |
| Who they would see | 13 | 168/1266 | 33 | 62/186**** |
| How long the appointment would take | 8 | 17/222 | 28 | 248/900**** |
| How to get to the centre | 8 | 71/854 | 26 | 75/290**** |
| How to change the appointment | 2 | 33/1436 | – | |
Austoker and Ong112 then compared the level of distress at being recalled with the reported need for more information using an ad hoc questionnaire. They found that women who were distressed or very distressed at being recalled reported a greater need for more information than women who were somewhat or not at all distressed at the prospect of further assessment. The items with the strongest relationship between distress and information need were ‘wanting to have more information about why they were recalled’, RR 1.84 (95% CI 1.58 to 2.14), and ‘more information about how to get more information’, RR 1.55 (95% CI 1.27 to 1.89) (Table 34).
| Item | Distressed/very distressed women | Somewhat/not distressed women | RR (95% CI) | ||
|---|---|---|---|---|---|
| Women wanting more information | Women wanting more information | ||||
| % | n/N | % | n/N | ||
| Why they were recalled | 48 | 403/834 | 26 | 157/598 | 1.84 (1.58 to 2.14)**** |
| How to get more information | 29 | 237/811 | 19 | 116/616 | 1.55 (1.28 to 1.89)**** |
| What tests would be done | 27 | 224/828 | 22 | 130/599 | 1.25 (1.03 to 1.51)* |
| How long the appointment would take | 27 | 173/640 | 20 | 93/466 | 1.35 (1.08 to1.69)** |
| Who they would see | 18 | 146/826 | 13 | 80/598 | 1.32 (1.03 to 1.70)* |
| Who could come with them | 13 | 102/762 | 17 | 94/557 | 0.79 (0.61 to 1.03) NS |
| How to get to the centre | 13 | 83/659 | 13 | 64/497 | 0.98 (0.72 to 1.33) NS |
| How to change the appointment | 2 | 18/824 | 3 | 15/523 | 0.76 (0.39 to 1.50) NS |
Austoker and Ong112 also examined the information in the initial invitation to screening and compared levels of distress, using an ad hoc questionnaire, with whether or not the initial invitation referred to the possibility of recall. They found that when this was not mentioned in the letter or leaflet that women were more likely to be distressed or very distressed if they were recalled, RR 0.76 (95% CI 0.58 to 0.99). They also found an additional benefit from having an information leaflet as well as a recall letter. The RR of finding an aspect of the information about recall reassuring increased statistically significantly when participants were also given an information leaflet, RR 4.04 (95% CI 3.10 to 5.26). Having an additional leaflet also increased women's belief that they understood the assessment procedure, RR 1.27 (95% CI 1.22 to 1.33).
Austoker and Ong112 categorised words and phrases in the recall letter and information sheet, according to responses in the questionnaire and women's additional comments, to produce lists of information that were either reassuring or worrying (Table 35).
| Reassuring aspects of the information | Worrying aspects of the information |
|---|---|
| To receive a leafet describing assessment as well as the recall letter | Receiving the recall letter on a Saturday |
| To be told that ‘being recalled is part of routine (or second stage) screening’ or that ‘the great majority are found to have normal breasts’ | The waiting time between receiving the recall letter and the appointment |
| To be told in the recall letter that more information can be obtained by phoning the centre | The vagueness of reasons given for recall – for example, ‘for a variety of reasons the tests have to be repeated’ |
| To be told that the woman could contact the breast care nurse at the centre | Being told ‘not to worry’ or ‘not to be alarmed’ |
| To be told when women will receive the results | Being told that the mammogram had been ‘unclear’ |
| Being told that the reason for being recalled would be given to the women when they were seen at the centre, the implication being that the centres knew something (assumed to be bad news) that was being withheld from the women | |
| Using the word ‘cancer’ specifically in the context of recall in the initial letter of invitation for mammography | |
| Using the words ‘cancer’, ‘something wrong’, ‘treatment’ or ‘abnormality’ in the recall letter | |
| Recall letters stating that ‘in the majority of women the results of the second visit show that they do not have cancer’ rather than ‘most of these (recalled) women are found to have normal breasts’ | |
| Being told in the recall letter/leafet that assessment was at a hospital rather than a centre/unit/clinic | |
| Being told that women would be seen ‘by a team of specialists’ | |
| Being told that the women could contact a nurse ‘counsellor’ (rather than a ‘breast care nurse’, which was reassuring), or that a ‘counsellor’ would be available at the centre | |
| Being given detailed descriptions in the recall leafet about FNA |
The study that followed on from this initial research into the information needs of recalled women, by Ong et al. ,111 used the categories developed above to evaluate the recall information letters and leaflets sent out by all the assessment centres in the UK. Of the total 87 centres, 84 (97%) sent their materials for evaluation.
The authors found that 99% of the information sent out with the initial screening invitation referred to the possibility of recall (Table 36).
| Topic | Mentioned in any of the written information: | Mentioned in neither leafet nor letter nor GP letter, % (no.) of centres | |||
|---|---|---|---|---|---|
| In the letter, % (no.) of centres | In GP letter, % (no.) of centres | In the leafet, % (no.) of centres | In both letter and leafet, % (no.) of centres | ||
| Possibility of recall | 46 (39/84) | 5 (4/84) | 99 (83/84) | 45 (38/84) | 1 (1/84) |
When Ong et al. 111 compared the list of ‘worrying information’ with that contained in the recall letters and leaflets they found that 54% of the literature included one of these items (Table 37).
| Topics mentioned | Mentioned in any of the written information: | Mentioned in neither recall leafet nor recall letter, % (no.) of centres | ||
|---|---|---|---|---|
| In recall letter, % (no.) of centres | In recall leafet, % (no.) of centres | In both recall leafet and letter, % (no.) of centres | ||
| One or more worrying items | 43 (35/82) | 18 (15/82) | 7 (6/82) | 46 (38/82) |
| Word ‘cancer’ | 9 (7/82) | 10 (8/82) | 1 (1/82) | 83 (68/82) |
| Words ‘treatment’, ‘something wrong’, ‘abnormality’, or ‘abnormal area of the breast’ | 20 (16/82) | 4 (3/82) | 1 (1/82) | 78 (64/82) |
| Word ‘hospital’a | 10 (8/82) | 1 (1/82) | 0 | 89 (73/82) |
| Words ‘not to worry’b | 22 (18/82) | 1 (1/82) | 0 | 77 (63/82) |
| Phrase ‘nurse counsellor’ | 5 (4/82) | 9 (7/82) | 0 | 87 (71/82) |
An examination of stress-relieving information found that 83% of recall literature contained at least one item (Table 38).
| Topics mentioned | Mentioned in any of the written information: | Mentioned in neither recall leafet nor recall letter, % (no.) of centres | ||
|---|---|---|---|---|
| In recall letter, % (no.) of centres | In recall leafet, % (no.) of centres | In both recall leafet and letter, % (no.) of centres | ||
| One or more stress-relieving messages | 68 (56/82) | 33 (27/82) | 20 (16/82) | 17 (14/82) |
| Recall is part of second stage/routine screening | 46 (77/82) | 26 (3/82) | 11 (9/82) | 38 (31/82) |
| Most recalled women are found to have normal breasts | 28 (23/82) | 6 (5/82) | 4 (3/82) | 30 (25/82) |
| A substantial number of women are recalled | 32 (26/82) | 11 (9/82) | 1 (1/82) | 60 (49/82) |
The data from the Austoker and Ong112 study was further analysed by Ong and Austoker110 (n = 1493) to find out women's views of the quality of communication at the assessment clinic, how this related to their level of distress and what role a breast care nurse might play in mitigating this distress. Table 39 shows that there was a strong link between whether or not participants would have liked to have talked with someone at the assessment centre [RR 1.42 (95% CI 1.15 to 1.74)], whether or not they thought they were given enough information about their physical examination [RR 1.71 (95% CI 1.02 to 2.86)] or X-rays [RR 2.34 (95% CI 1.47 to 3.73)] and whether they were distressed/very distressed or somewhat/not distressed about being recalled.
| Communication | Distressed/very distressed | Somewhat/not distressed | RR (95% CI) | ||
|---|---|---|---|---|---|
| % (n/N) | 95% CI | % (n/N) | 95% CI | ||
| Women who had not had the opportunity to talk with a health worker at the centre about the reason for recall | 33 (275/835) | 0.30 to 0.36 | 32 (191/597) | 0.28 to 0.36 | 1.03 (0.88 to 1.20) NS |
| Women who would have liked to talk about the reason for recall | 26 (214/835) | 0.23 to 0.29 | 18 (108/597) | 0.15 to 0.21 | 1.42 (1.15 to 1.74)**** |
| Women who thought they were not given enough information about their physical examination | 6 (46/757) | 0.04 to 0.08 | 4 (20/563) | 0.02 to 0.05 | 1.71 (1.02 to 2.86)**** |
| Women who thought they were not given enough information about their X-rays | 9 (72/773) | 0.07 to 0.12 | 4 (22/553) | 0.03 to 0.06 | 2.34 (1.47 to 3.73)**** |
The results in Table 40 clearly show that the availability of a breast care nurse at an assessment centre greatly increased the probability that participants would have talked to somebody at the centre about the reason for their recall, RR 0.62 (95% CI 0.59 to 0.66). Where the opportunity to talk to someone was not available women were far more likely to have liked to talk to someone, RR 7.46 (95% CI 4.57 to 12.16). Women were also more likely to have had their assessment tests explained to them and not to need more information about their tests if a breast care nurse was available at the assessment centre (see Table 40).
| Communication | Centres where women were not systematically provided with the opportunity to talk immediately before tests | Centres where the breast care nurse provided women with the opportunity to talk in private immediately before tests | RR (95% CI) | ||
|---|---|---|---|---|---|
| % (n/N) | 95% CI | % (n/N) | 95% CI | ||
| Women who had talked at the centre about the reason for recall | |||||
| With ‘somebody at the centre’ | 58 (611/1055) | 0.55 to 0.61 | 93 (374/401) | 0.90 to 0.96 | 0.62 (0.59 to 0.66)**** |
| With a doctor or radiologist | 31 (323/1035) | 0.28 to 0.34 | 7 (26/391) | 0.04 to 0.10 | 4.69 (3.20 to 6.88)**** |
| With a nurse | 9 (97/1035) | 0.08 to 0.11 | 60 (234/391) | 0.55 to 0.65 | 0.16 (0.13 to 0.19)**** |
| Women who would have liked to talk about reason for recall | 30 (310/1039) | 0.27 to 0.33 | 4 (16/400) | 0.02 to 0.06 | 7.46 (4.57 to 12.16)**** |
| Women who stated that the tests they had were not explained to them | |||||
| Physical examination by a doctor | 8 (82/981) | 0.07 to 0.10 | 2 (7/381) | 0.01 to 0.04 | 4.55 (2.12 to 9.75)**** |
| X-rays | 9 (88/996) | 0.05 to 0.11 | 1 (5/379) | 0.00 to 0.03 | 6.70 (2.74 to 16.36)**** |
| Ultrasound | 9 (39/413) | 0.07 to 0.13 | 2 (5/212) | 0.01 to 0.05 | 4.00 (1.60 to 10.01)*** |
| Women who wanted more information about their tests | |||||
| Physical examination by a doctor | 6 (59/964) | 0.05 to 0.08 | 2 (7/378) | 0.01 to 0.04 | 3.31 (1.52 to 7.17)*** |
| X-rays | 7 (68/971) | 0.05 to 0.09 | 2 (8/376) | 0.01 to 0.04 | 3.29 (1.60 to 6.78)*** |
| Ultrasound | 10 (39/401) | 0.07 to 0.13 | 3 (6/209) | 0.01 to 0.06 | 3.39 (1.46 to 7.87)*** |
The final study in this section is the survey of user satisfaction with the Leicestershire breast screening service by Smith et al. 113 (n = 103). Table 41 shows that they found that 75% of participants were upset or very upset when they received their recall letter.
| Reaction | n/% |
|---|---|
| Positive (e.g. ‘glad to be in such capable hands’) | 4 |
| Neutral (e.g. ‘I wasn't bothered’) | 10 |
| Surprised | 11 |
| Upset (e.g. ‘anxious’, ‘worried’, ‘upset’) | 44 |
| Very upset (e.g. ‘terrified’, ‘extremely anxious’) | 31 |
| Total | 100 |
The results of the survey about how satisfied the women were with information given in different versions of the recall letter about the reason for their recall and what would happen at the clinic showed that satisfaction varied considerably between the versions of the letter. Women were most satisfied with letter three that gave the most information about what would happen at the clinic and least satisfied with letter one that gave minimal information (Table 42).
| Letter version | Reasons for recall n (%) | Events at the clinic n (%) |
|---|---|---|
| 1. Minimal information about possible tests | 15(50) | 17 (63) |
| 2. Offer of telephoning a breast care nurse for more information | 25 (71) | 24 (74) |
| 3. Similar to letter one but with more detail about what would happen at the clinic | 26 (81) | 27 (90) |
| All versions | 66 (68) | 68 (76) |
| Chi-squared | 7.243 | 5.817 |
| p-value | 0.027 | 0.055 |
When participants were asked if they would have telephoned a breast care nurse if one was available, 98% said they would and 100% of the women who received letter two with the breast care nurse's telephone number used this facility (Table 43).
| Letter version | Answer | n |
|---|---|---|
| 1 – would telephone the BCN | Yes | 25 |
| No | 0 | |
| 2 – did telephone the BCN | Yes | 13 |
| No | 0 | |
| 3 – would telephone the BCN | Yes | 17 |
| No | 1 |
Summary: interventions to reduce the impact of false-positive mammograms
The results from these studies evidently show that most women want clear information about the reason for their recall and what will happen to them at their assessment and that having this information can reduce stress levels; however, too much information about the process of FNA was considered stressful. There was a benefit from having a recall leaflet as well as a letter, as this increased the likelihood that women believed they understood what would happen to them. The level of stress experienced by women when they were recalled was increased if the possibility of recall was not mentioned in the original screening invitation. The value of having a breast care nurse to talk to is very apparent by increasing satisfaction with the process of assessment, reducing distress and satisfying the need to have someone at the clinic to talk to. It is also interesting to note the effect of language in the recall literature, with 54% of the recall literature containing at least one worrying item and 83% containing at least one stress-relieving message.
Chapter 4 Discussion
Statement of principal findings
The aim of this systematic review was to identify the psychological impact on women of false-positive screening mammograms and any evidence for the effectiveness of interventions designed to reduce this impact. We were also looking for evidence of effects in subgroups of women.
Our searches retrieved 4423 titles and abstracts after deduplication. When screening was complete, we found five systematic reviews,62,82,85–87 one meta-analysis and 11 primary studies that met our inclusion criteria. No studies were found that were either about or reported subgroups of women from different ethnic, socioeconomic or other groups within the general screening population. One study101,102,107 was found that included women who had a false-positive mammogram and a FHBC. The quality of the primary research was variable: we found one poor-quality RCT115 and 10 observational studies. 55,59,61,101–114 The best-quality observational research was conducted by OPCERG and PIMMS groups and the observational studies reporting reattendance rates. However, even here there were shortcomings in reporting key information. Overall, the main weaknesses in reporting were a failure to consider the possible effects of bias and confounding on the results and a failure to report participants' demographic and other characteristics, making the interpretation of the results very difficult. These quality indicators appear to have been overlooked, as, in most cases, there was no consideration of the limitations of the methods or conduct of the study. Therefore, the results of this systematic review must be treated with caution, not just because they come from a limited number of observational studies, but also because many of these studies lack methodological robustness.
General population
The studies of the psychological impact of false-positive mammograms in the general population gave conflicting results. When disease-specific measures were used (PCQ), an enduring negative impact was found that lasted until 35 months from the last assessment. The degree of distress found was related to the level of invasiveness of the method of assessment used so that, at 35 months, women who had a biopsy were more distressed (RR 2.07; 95% CI 1.22 to 3.52) than women who had FNA (RR 1.80; 95% CI 1.17 to 2.77) and, non-significantly, further mammography (RR 1.28; 95% CI 0.82 to 2.00). Additionally, women placed on early recall were also at a greater RR of distress (RR 1.82; 95% CI 1.22 to 2.72). The greatest RR of distress was felt at 5 months after assessment and was significant for all assessment procedures.
Further evidence of psychological distress came from results of a comparison of the frequency of breast self-examination of women who had false-positive or normal mammograms. This found that significantly more women who were recalled examined themselves once a week or more often, which may be taken as a proxy for anxiety although the meaning of this behaviour is unclear.
Conversely, when generic measures of clinical levels of general anxiety and depression were used (HADS and GHQ-28) no significant differences were found between the two groups at 6 weeks after assessment and 3 months after screening.
Therefore, it may be reasonable to speculate that, for those in the general population, having a false-positive screening mammogram can cause breast cancer-specific psychological distress that may endure for up to 3 years. However, it is less likely that there will be general anxiety detectable at clinically recognisable levels.
Family history of breast cancer population
These results draw a slightly different picture to those in the general-risk population. Here the psychological distress of the false-positive group was statistically significantly greater than the normal group only at 1 month after screening [negative PCQ, difference in means 2.92 (95% CI 4.05 to 1.69)]. At the same time the false-positive group also scored significantly higher on the positive PCQ than those with normal mammograms (Mann–Whitney U-test 51,561; p< 0.05). They also rated the benefits of screening more highly than those with normal mammograms at 1 (T2) and 6 months (T3) after screening on an ad hoc questionnaire [OR: T2, 3.17 (95% CI 2.14 to 4.70); T3, 2.35 (95% CI 1.53 to 3.61)]. These results may appear to be conflicting at first glance. However, the summary results from the unpublished interview study suggest that the women in the false-positive group may have been rationalising their anxiety at being recalled by reassuring themselves that this meant that the programme was thorough and would detect early cancer that could be treated.
Impact of a false-positive mammogram on returning for routine screening
The evidence for the impact of having a false-positive mammogram on returning for the next screening round is conflicting. It comes mainly from four retrospective observational studies55,61,108,109 that collected data from registries and other NHS databases. The weight of evidence, in terms of the numbers of participants, is that women with false-positive mammograms are less likely to return for their next round of screening than women with normal mammograms. The largest study61 with this finding (n = 140,387) had a RR of not returning of 0.97 (95% CI 0.96 to 0.98). Two studies108,109 with a combined population of 7231 found that there was no such association. Evidence from a poor-quality RCT115 suggests that this finding can be reversed if women are given screening invitation letters that are tailored to the outcome of their last screening (RR 1.10; 95% CI 1.00 to 1.21).
Interventions to reduce the impact of false-positive mammograms
The above evidence suggests that in the general population there is a negative psychological impact from having a false-positive mammogram that may endure for 3 years and may deter women from attending their next round of screening. Unfortunately, we were unable to find any studies that directly addressed these problems. Nevertheless, we identified two studies110–113 that looked at the information needs of women who were recalled and the importance of communication. These studies showed that women wanted clear information about the reason they had been recalled. However, the ability of clinicians to address this is limited by the need to stage the information to ensure direct and face-to-face support in cases where radiologists are reasonably sure that the screening mammogram indicates cancer. Women also indicated that they wanted clear information about what would happen at their assessment, as well as access to a breast care nurse or CNS to talk through their concerns. Satisfaction with the service increased if women were sent a recall leaflet as well as a letter as participants believed that they had better understanding of what would happen to them at the assessment clinic. The importance of the language used in the recall literature was also evident, with particular words and phrases reducing or increasing stress. The research by the OPCERG was used to produce national guidelines on improving the quality of written information sent to women who were recalled for assessment in 1998. 114
These intervention studies are more than 10 years old, and it is unknown whether or not the recommendations in the national guidelines have been implemented. There is currently no national recall information leaflet similar to the NHS breast-screening leaflet.
Comparison with other systematic reviews
With the exception of the systematic review by Bankhead et al. ,84 the quality of the included secondary research was not very high. Of particular concern was the lack of consideration of the effects of bias and confounding in their included observational studies.
Our results agree with those of previous systematic reviews and meta-analyses. In particular, we agree with all these evidence syntheses that there can be negative psychological consequences from having a false-positive mammogram. We also supported their finding that having a false-positive mammogram increased the frequency of breast self-examination, which may be a proxy for anxiety. The meta-analysis by Salz et al. 85 that compared outcomes measured by disease-specific or generic measures of psychological distress agrees with our finding that the type of outcome measure used can affect whether or not an outcome is found to be statistically significant, although, unlike Salz et al. ,85 we were unable to find evidence of anxiety at clinical levels. We also agree with the finding that the evidence about reattendance is conflicted. However, the weight of evidence suggests that women with this result are less likely to reattend.
Strengths and limitations
The strengths of this systematic review are that it was conducted by an independent research team using robust methods. Our searches were comprehensive and we believe that we have retrieved all includable studies.
The systematic review may have been influenced by publication bias. However, there were insufficient studies in each domain to produce a funnel plot that would give meaningful information.
The robustness of the findings of this systematic review is limited by the reliability of the included studies. With the exception of one weak RCT all the studies were observational and so subject to the risks of bias and confounding that are associated with these designs, compounded by lack of reporting key information such as the baseline characteristics of the groups. However, the nature of the subject under study necessitates observational designs; therefore, great care should be taken in matching groups and reporting their characteristics.
Chapter 5 Conclusions
We conclude that the experience of having a false-positive screening mammogram, in the general risk of breast cancer population, can cause breast cancer-specific psychological distress that may endure for up to 3 years. However, it is less likely that there will be general anxiety detectable at clinically recognisable levels. The likelihood of women experiencing distress may be determined by the degree of invasiveness of the assessment procedure, with more invasive techniques increasing the probability of psychological distress.
The strongest evidence suggests that the distress caused by a false-positive mammogram may be sufficient to deter an additional 3% of women from attending their next breast cancer screening appointment.
It is important to provide women who are recalled with clear, carefully worded information about the reason for their recall and the process of the assessment (but not in such detail that they become distressed without the support of the screening staff being present), and to have a breast care nurse or CNS available to answer any concerns they may have.
There is some evidence that having a subsequent round of screening invitation letter that refers to the outcome of the previous screening round may encourage women with false-positive mammograms to reattend.
For women with a FHBC, having a false-positive mammogram, while increasing levels of distress, may also reassure them that they are being well looked after and that early cancer will be detected that could be treated.
Additionally, the evidence suggests that women with false-positive mammograms are at three times greater risk of interval cancer than those with normal mammograms and are more than twice as likely to have cancer detected at the next screening round. The reasons for this finding need further explanation.
Implications for health care
The evidence suggests that the availability of a CNS at screening clinics as well as assessment clinics may help to allay concerns raised by information sources other than official literature. There is also some evidence that including a reference to the previous recall in the next round invitation letter may facilitate attendance.
Research recommendations
The evidence found by this systematic review in the general population is at least 10 years old. Up-to-date studies are needed that reflect current screening practice.
-
A qualitative interview study of the general population of women who have had false-positive screening mammograms, in order to understand what this experience means to them.
-
Well-designed observational studies, in the general screening population, that use disease-specific and generic outcome measures in order to determine the level of severity of negative psychological outcomes. Including studies of women from different ethnic and socioeconomic groups.
-
The routine collection of demographic information in observational studies so that future systematic reviews may be able to judge whether or not the pooling of data is possible.
-
Currently there is no standard national recall letter following a suspicious screening mammogram. There should be a national survey of the recall literature sent out from NHSBSP services to see if the national guidelines produced in 1998 are being adhered to, followed by the development of such a letter.
-
There is some evidence to suggest that there may be a relationship between tailored invitation letters for next screening round for women who have had false-positive mammograms and reattendance. A well-designed RCT would be able to help us understand whether or not this relationship exists and a nested qualitative study would give insight into the important features of such a letter.
-
Developmental and pilot work of interventions both to relieve the distress of false-positive mammograms and to encourage women with this outcome to reattend routine screening. Promising interventions should then be tested in well-designed RCTs sufficiently powered to allow for subgroup analysis.
A list of ongoing studies can be found in Appendix 4. We found no ongoing UK studies about false-positive mammograms.
Acknowledgements
We would like to acknowledge Jenny Lowe and Sue Whiffin for their administrative support and especially Jaime Peters for her statistical advice.
Expert Advisory Group
We would particularly like to thank our expert advisors:
| Ms Kate Blackmore | Patient representative. |
| Dr Russell Davies | Consultant radiologist, Royal Devon & Exeter NHS Foundation Trust. |
| Professor Jenny Hewison | Professor of the Psychology of Healthcare, University of Leeds. |
| Ms Sue Milward | Patient representative. |
| Professor Carl Roobottom | Consultant radiologist, Plymouth Hospitals NHS Trust. |
| Dr Jim Steel | Director of Screening, Consultant breast radiologist, Plymouth Hospitals NHS Trust. |
Contributions of authors
| Mary Bond (Senior Research Fellow, HTA) | Provided project management, wrote the protocol, codesigned the searches, led the systematic review, wrote and edited the report. This systematic review is part of a continuing research project and PhD supported by the Collaboration for Leadership in Applied Health Research and Care (PenCLAHRC). |
| Toby Pavey (Research Fellow, HTA) | Acted as second reviewer and commented on the draught report. |
| Karen Welch (Information Specialist) | Designed and undertook literature searches. |
| Chris Cooper (Information Specialist) | Designed and undertook literature searches and commented on the draught report. |
| Ruth Garside (Senior Research Fellow, HTA) | Qualitative research advisor and commented on the draught report. |
| Sarah Dean (Senior Lecturer, HSR) | Health psychology advisor and commented on the draught report. |
| Chris Hyde (Professor Public Health and Clinical Epidemiology) | Commented on the draught report and was overall director and guarantor of the report. |
About the Peninsula Technology Assessment Group
The Peninsula Technology Assessment Group (PenTAG) is part of the Institute of Health Service Research at the Peninsula Medical School. PenTAG was established in 2000 and currently has three major work streams; independent Health Technology Assessments for NICE and the NIHR HTA Programme, systematic reviews as part of the Cochrane Collaboration Heart Group and evidence synthesis work in relation to the needs of PenCLAHRC, as well as for other local and national decision-makers.
The group is multidisciplinary and draws on individuals' backgrounds in public health, health services research, computing and decision analysis, systematic reviewing, statistics and health economics. The Peninsula Medical School is a school within the Universities of Plymouth and Exeter. The Institute of Health Research is made up of discrete but methodologically related research groups, among which Health Technology Assessment is a strong and recurring theme. Recent PenTAG HTA projects include:
Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of TA111): a systematic review and economic model. Health Technol Assess 2012;16(21).
Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess 2010;14(2).
Hoyle M, Crathorne L, Garside R, Hyde C. Ofatumumab (Arzerra®) for the treatment of chronic lymphocytic leukaemia in patients who are refractory to fludarabine and alemtuzumab: a critique of the submission from GSK. Health Technol Assess 2011;15(Suppl. 1).
Rogers G, Hoyle M, Thompson-Coon J, Pitt M, Moxham T, Liu Z, et al. Dasatinib and nilotinib for imatinib-resistant or intolerant chronic myeloid leukaemia: a systematic review and economic evaluation. Health Technol Assess 2012;16(22).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Forrest P. Breast cancer screening. Report to the Health Ministers of England, Wales, Scotland and Northern Ireland. London: HMSO; 1986.
- The NHS Information Centre PHIT . Breast Screening Results from the NHSBSP 2009–10 2011. www.cancerscreening.nhs.uk/breastscreen/statistics.html (accessed 28 March 2011).
- World Health Organisation (WHO) International Agency for Research on Cancer . Breast Cancer Screening. International Agency for Research on Cancer 2002.
- Cancer Research UK . Breast Cancer-UK Incidence Statistics 2010. http://info.cancerresearchuk.org (accessed 4 May 2010).
- Beral V, Banks E, Reeves G, Wallis M. Hormone replacement therapy and high incidence of breast cancer between mammographic screens. Lancet 1997;349:1103-4. http://dx.doi.org/10.1016/S0140-6736(05)62328-8.
- Quinn MJ, Cooper N, Rachet B, Mitry E, Coleman MP. Survival from cancer of the breast in women in England and Wales up to 2001. Br J Cancer 2008;99:S53-S55. http://dx.doi.org/10.1038/sj.bjc.6604587.
- Parsa P, Parsa B. Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pac J Cancer Prev 2009;10:545-50.
- Cancer statistics registrations: registrations of cancer diagnosed in 2007, England. London: National Statistics; 2010.
- ISD Scotland . ISD Cancer Information Programme 2009. www.isdscotland.org (accessed 4 November 2012).
- Welsh Cancer Intelligence and Surveillance Unit . Cancer Incidence in Wales 2010. www.wales.nhs.uk (accessed 4 November 2010).
- Office for National Statistics . Breast Cancer: Incidence Rates Rise, Mortality Rates Fall 2011. www.statistics.gov.uk/CCI/nugget.asp?ID=575 (accessed 29 March 2011).
- Autier P, Boniol M, Gavin A, Vatten LJ. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ 2011;343.
- U.S. Preventative Services Task Force . Addendum and correction: screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2010;152.
- Bleyer A. US breast cancer mortality is consistent with European data. BMJ 2011;343.
- de Koning H. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d4411.
- Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2011;1.
- Shapiro S, Venet W, Strax P. Selection, follow-up, and analysis in the health insurance plan study: a randomized trial with breast cancer screening. J Natl Cancer Inst Monogr 1985;67:65-74.
- Strax P. Mass screening for breast cancer. Rev Fr Gynecol Obstet 1973;68:457-66.
- Fink R, Shapiro S, Roester R. Impact of efforts to increase participation in repetitive screenings for early breast cancer detection. Am J Public Health 1972;62:328-36. http://dx.doi.org/10.2105/AJPH.62.3.328.
- Andersson I, Aspegren K, Janzon L, Landberg T, Lindholm K, Linell F, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ 1988;297:943-8. http://dx.doi.org/10.1136/bmj.297.6654.943.
- Andersson I. Radiographic screening for breast carcinoma. II. Prognostic considerations on the basis of a short-term follow-up. Acta Radiol Diagn 1981;22:227-33. http://dx.doi.org/10.1111/j.1748-1716.1981.tb06887.x.
- Andersson I. Radiographic screening for breast carcinoma. I. Program and primary findings in 45–69 year old women. Acta Radiol Diagn 1981;22:185-94.
- Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Scand J Caring Sci 2002;359:909-19.
- Tabar L, Fagerberg CJ, Gad A, Baldetorp L, Holmberg LH, Grontoft O, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health & Welfare. Lancet 1985;i:829-32.
- Tabar L, Gad A. Screening for breast cancer: the Swedish trial. Radiology 1981;138:219-22.
- Tabar L, Fagerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radio Clin North Am 1992;30:187-210.
- Alexander F, Roberts MM, Lutz W, Hepburn W. Randomisation by cluster and the problem of social class bias. J Epidemiol Community Health 1989;43:29-36. http://dx.doi.org/10.1136/jech.43.1.29.
- Alexander FE, Anderson TJ, Brown HK, Forrest AP, Hepburn W, Kirkpatrick AE, et al. The Edinburgh randomised trial of breast cancer screening: results after 10 years of follow-up. Br J Cancer 1994;70:542-8. http://dx.doi.org/10.1038/bjc.1994.342.
- Alexander FE, Anderson TJ, Brown HK, Forrest APM, Hepburn W, Kirkpatrick AE, et al. 14 years of follow-up from the Edinburgh randomised trial of breast-cancer screening. Lancet 1999;353:1903-8. http://dx.doi.org/10.1016/S0140-6736(98)07413-3.
- Baines CJ. The Canadian National Breast Screening Study: a perspective on criticisms. Ann Intern Med 1994;120:326-34.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study – 2: 13–year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst 2000;92:1490-9. http://dx.doi.org/10.1093/jnci/92.18.1490.
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study – 1: breast cancer mortality after 11 to 16 years of follow-up. Ann Intern Med 2002;137.
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study: update on breast cancer mortality. J Natl Cancer Inst Monogr 1997;22:37-41.
- Frisell J, Lidbrink E, Hellstrom L, Rutqvist LE. Follow up after 11 years – update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res Treat 1997;45:263-70. http://dx.doi.org/10.1023/A:1005872617944.
- Frisell J, Glas U, Hellstrom L, Somell A. Randomized mammographic screening for breast cancer in Stockholm. Design, first round results and comparisons. Breast Cancer Res Treat 1986;8:45-54. http://dx.doi.org/10.1007/BF01805924.
- Frisell J, Eklund G, Hellstrom L, Lidbrink E, Rutqvist LE, Somell A. Randomized study of mammography screening – preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat 1991;18:49-56. http://dx.doi.org/10.1007/BF01975443.
- Frisell J, Eklund G, Hellstrom L, Glas U, Somell A. The Stockholm breast cancer screening trial – 5-year results and stage at discovery. Breast Cancer Res Treat 1989;13:79-87. http://dx.doi.org/10.1007/BF01806553.
- Bjurstam N, Bjorneld L, Warwick J, Sala E, Duffy SW, Nystrom L, et al. The Gothenburg Breast Screening Trial. Cancer 2003;97:2387-96. http://dx.doi.org/10.1002/cncr.11361.
- Bjurstam N, Bjorneld L, Duffy SW, Smith TC, Cahlin E, Eriksson O, et al. The Gothenburg breast screening trial. Cancer 1997;80:2091-9.
- UK Trial . First results on mortality reduction in the UK Trial of Early Detection of Breast Cancer. UK Trial of Early Detection of Breast Cancer Group. Lancet 1988.
- Moss S. A trial to study the effect on breast cancer mortality of annual mammographic screening in women starting at age 40. Trial Steering Group. J Med Screen 1999;6:144-8.
- Moss S, Waller M, Anderson TJ, Cuckle H. Randomised controlled trial of mammographic screening in women from age 40: predicted mortality based on surrogate outcome measures. Br J Cancer 2005;92:955-60. http://dx.doi.org/10.1038/sj.bjc.6602395.
- Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial 2006;368:2053-60.
- Moss S, Thomas I, Evans A, Thomas B, Johns L. Randomised controlled trial of mammographic screening in women from age 40: results of screening in the first 10 years. Br J Cancer 2005;92:949-54. http://dx.doi.org/10.1038/sj.bjc.6602396.
- Kopans DB, Smith RA, Duffy SW. Mammographic Screening and Overdiagnosis. Radiology 2011;260:616-20. http://dx.doi.org/10.1148/radiol.11110716.
- Otto SJ, Fracheboud J, Looman C, Broeders M, Boer R, Hendriks J, et al. Initiation of population-based mammography screening in Dutch municipalities and effect on breast-cancer mortality: a systematic review. Lancet 2003;361:1411-17. http://dx.doi.org/10.1016/S0140-6736(03)13132-7.
- Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen THH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen 2010;17:25-30. http://dx.doi.org/10.1258/jms.2009.009094.
- Wald NJ, Law MR, Duffy SW. Breast screening saves lives. BMJ 2009;339.
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer 2005;353:1784-92.
- Duffy S. Re: Estimate of Breast Screening Benefit Was 6 Times Too Large 2009. www.bmj.com/cgi/eletters/338/jan27_2/b86 (accessed 11 October 2011).
- Marteau TM, Cook R, Kidd J, Michie S, Johnston M, Slack J, et al. The psychological effects of false-positive results in prenatal screening for fetal abnormality: a prospective study. Prenat Diagn 1992;12:205-14. http://dx.doi.org/10.1002/pd.1970120309.
- French DP, Maissi E, Marteau TM. The psychological costs of inadequate cervical smear test results: three-month follow-up. Psychooncology 2006;15:498-50. http://dx.doi.org/10.1002/pon.980.
- Lampic C, Thurfjell E, Bergh J, Sjoden P-O. Short- and long-term anxiety and depression in women recalled after breast cancer screening. Eur J Cancer 2001;37:463-9. http://dx.doi.org/10.1016/S0959-8049(00)00426-3.
- Scaf-Klomp W, Sanderman R, van de Wiel HB, Otter R, van den Heuvel WJ. Distressed or relieved? Psychological side effects of breast cancer screening in The Netherlands. J Epidemiol Community Health 1997;51:705-10. http://dx.doi.org/10.1136/jech.51.6.705.
- Sutton S, Saidi G, Bickler G, Hunter J. Does routine screening for breast cancer raise anxiety? Results from a three wave prospective study in England. J Epidemiol Community Health 1995;49:413-18. http://dx.doi.org/10.1136/jech.49.4.413.
- Aro AR, Pilvikki Absetz S, van Elderen TM, van der Ploeg E, van der Kamp LJT. False-positive findings in mammography screening induces short-term distress – breast cancer-specific concern prevails longer. Eur J Cancer 2000;36:1089-97. http://dx.doi.org/10.1016/S0959-8049(00)00065-4.
- Gram IT, Lund E, Slenker SE. Quality of life following a false positive mammogram. Br J Cancer 1990;62:1018-22. http://dx.doi.org/10.1038/bjc.1990.430.
- Burman ML, Taplin SH, Herta DF, Elmore JG. Effect of false-positive mammograms on interval breast cancer screening in a health maintenance organization. Ann Intern Med 1999;131:1-6.
- Brett J, Austoker J. Women who are recalled for further investigation for breast screening: psychological consequences 3 years after recall and factors affecting re-attendance. J Public Health 2001;23:292-300. http://dx.doi.org/10.1093/pubmed/23.4.292.
- Lampic C, Thurfjell E, Sjoden P-O. The influence of a false-positive mammogram on a woman's subsequent behaviour for detecting breast cancer. Eur J Cancer 2003;39:1730-7. http://dx.doi.org/10.1016/S0959-8049(02)00451-3.
- McCann J, Stockton D, Godward S. Impact of false-positive mammography on subsequent screening attendance and risk of cancer. Breast Cancer Res 2002;4. http://dx.doi.org/10.1186/bcr455.
- Brewer NT, Salz T, Lillie SE. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med 2007;146:502-10.
- Wilson R, Liston J. Quality assurance guidelines for breast cancer screening radiology. Sheffield: NHS Cancer Screening Programmes; 2011.
- Liston J, Wilson R. Clinical guidelines for breast cancer screening assessment. Sheffield: NHS Cancer Screening Programmes; 2010.
- Sibbering M, Watkins R, Winstanely J, Patnick J. Quality assurance guidelines for surgeons in breast cancer screening. Sheffield: NHS Cancer Screening Programme; 2009.
- Quality assurance guidelines for clinical nurse specialists in breast cancer screening. Sheffield: NHS Cancer Screening Programmes; 2008.
- Cockburn J, De Luise T, Hurley S, Clover K. Development and validation of the PCQ: a questionnaire to measure the psychological consequences of screening mammography. So Sci Med 1992;34:1129-34. http://dx.doi.org/10.1016/0277-9536(92)90286-Y.
- Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991;114:657-61.
- Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychol 1991;10:259-67. http://dx.doi.org/10.1037/0278-6133.10.4.259.
- Watson M, Lloyd S, Davidson J, Meyer L, Eeles R, Ebbs S, et al. The impact of genetic counselling on risk perception and mental health in women with a family history of breast cancer. Br J Cancer 1999;79:868-74. http://dx.doi.org/10.1038/sj.bjc.6690139.
- Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997;4:92-100. http://dx.doi.org/10.1207/s15327558ijbm0401_6.
- Goldberg D. The detection of psychiatric illness by questionnaire. Oxford: Oxford University Press; 1972.
- Goldberg D, Williams P. London: NFER-Nelson; 1988.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67:1063-78. http://dx.doi.org/10.1037/0022-3514.67.6.1063.
- Speilberger C. Manual for the State–Trait Anxiety Inventory: STAI (form Y). Palo Alto, CA: Consulting Psychologists Press; 1983.
- Systematic reviews: CRD's guidance for undertaking reviews in health care. York: NHS Centre for Reviews and Dissemination; 2009.
- Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ; 2001.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. http://dx.doi.org/10.1016/S0140-6736(00)04337-3.
- Elm Ev, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. http://dx.doi.org/10.1136/bmj.39335.541782.AD.
- Brett J, Bankhead C, Henderson B, Watson E, Austoker J. The psychological impact of mammographic screening. A systematic review. Psycho Oncol 2005;14:917-38. http://dx.doi.org/10.1002/pon.904.
- Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 2005;33:1064-5. http://dx.doi.org/10.1136/bmj.38636.593461.68.
- Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al. The impact of screening on future health-promoting behaviours and health beliefs: a systematic review. Health Technol Assess 2003;7.
- Salz T, Richman AR, Brewer NT. Meta-analyses of the effect of false-positive mammograms on generic and specific psychosocial outcomes. Psycho Oncol 2010;19:1026-34. http://dx.doi.org/10.1002/pon.1676.
- Hafslund B, Nortvedt MW. Mammography screening from the perspective of quality of life: a review of the literature. Scand J Caring Sci 2009;23:539-48. http://dx.doi.org/10.1111/j.1471-6712.2008.00634.x.
- Armstrong K, Moye E, Williams S, Berlin JA, Reynolds EE. Clinical guidelines. Screening mammography in women 40 to 49 years of age: a systematic review for the American College of Physicians. Ann Intern Med 2007;146.
- Meystre-Agustoni G, Paccaud F, Jeannin A, Dubois-Arber F. Anxiety in a cohort of Swiss women participating in a mammographic screening programme. J Med Screen 2001;8:213-19. http://dx.doi.org/10.1136/jms.8.4.213.
- Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. http://dx.doi.org/10.1001/archpsyc.1961.01710120031004.
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults – evaluation of a short-form of the CES-D. Am J Prev Med 1994;10:77-84.
- Boer H. Psychological effects of breast cancer screening. Twente: University of Twente; 1993.
- Goldberg DP. Manual of the General Health Questionnaire. Windsor: NFER-Nelson Publishing; 1978.
- Walker LG, Cordiner CM, Gilbert FJ, Needham G, Deans HE, Affleck IR, et al. How distressing is attendance for routine breast screening?. Psycho Oncol 1994;3:299-304. http://dx.doi.org/10.1002/pon.2960030406.
- Derogatis L, Lipman RS, Rickels K, Uhlenhut E, Covi L. Hopkins Symptom Checklist (HSCL) – a self-report symptom inventory. Behav Sci 1974;19:1-15. http://dx.doi.org/10.1002/bs.3830190102.
- Kellner R. Abridged manual of the illness attitude scales. Albuquerque, NM: University of New Mexico; 1987.
- Horowitz M, Wilner N, Alvarez W. Impact of event scale – measure of subjective stress. Psychosom Med 1979;4:209-18.
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002;32:959-76. http://dx.doi.org/10.1017/S0033291702006074.
- McNaire D, Lorr M, Droppleman LF. Manual for the profiles of moods scale. San Diego, CA: Educational and Industrial Testing Services; 1971.
- Rakowski W, Andersen M, Stoddard AM, Urban N, Rimer BK, Lane DS, et al. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychology 1997;16:433-42. http://dx.doi.org/10.1037/0278-6133.16.5.433.
- Derogatis L. Administration, scoring and procedures manual 1 to the (revised) version. Baltimore, MD: Johns Hopkins University School of Medicine; 1977.
- Tyndel S, Austoker J, Henderson BJ, Brain K, Bankhead C, Clements A, et al. What is the psychological impact of mammographic screening on younger women with a family history of breast cancer? Findings from a prospective cohort study by the PIMMS Management Group. J Clin Oncol 2007;25:3823-30. http://dx.doi.org/10.1200/JCO.2007.11.0437.
- Brain K, Henderson BJ, Tyndel S, Bankhead C, Watson E, Clements A, et al. Predictors of breast cancer-related distress following mammography screening in younger women on a family history breast screening programme. Psychooncology 2008;17:1180-8. http://dx.doi.org/10.1002/pon.1355.
- Brett J, Austoker J, Ong G. Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi-centre follow-up study comparing different breast screening result groups five months after their last breast screening appointment. J Public Health 1998;20:396-403. http://dx.doi.org/10.1093/oxfordjournals.pubmed.a024793.
- Ong G, Austoker J, Brett J. Breast screening: adverse psychological consequences one month after placing women on early recall because of a diagnostic uncertainty. A multicentre study. J Med Screen 1997;4:158-68.
- Ellman R, Angeli N, Christians A, Moss S, Chamberlain J, Maguire P. Psychiatric morbidity associated with screening for breast cancer. Br J Cancer 1989;60:781-4. http://dx.doi.org/10.1038/bjc.1989.359.
- Bull AR, Campbell MJ. Assessment of the psychological impact of a breast screening programme. Br J Radiol 1991;64:510-15. http://dx.doi.org/10.1259/0007-1285-64-762-510.
- Clements A, Tyndel S, Henderson B, Brain K, Watson E, Austoker J, et al. ‘More positive about mammography’ – reactions of women to a false positive recall: a qualitative study of women at risk of familial breast cancer. Breast Cancer Res 2008;10. http://dx.doi.org/10.1186/bcr1974.
- O’Sullivan I, Sutton S, Dixon S, Perry N. False positive results do not have a negative effect on reattendance for subsequent breast screening. J Med Screen 2001;8:145-8. http://dx.doi.org/10.1136/jms.8.3.145.
- Orton M, Fitzpatrick R, Fuller A, Mant D, Mlynek C, Thorogood M. Factors affecting women's response to an invitation to attend for a second breast cancer screening examination. Br J Gen Pract 1991;41:320-2.
- Ong G, Austoker J. Recalling women for further investigation of breast screening: women's experiences at the clinic and afterwards. J Public Health Med 1997;19:29-36. http://dx.doi.org/10.1093/oxfordjournals.pubmed.a024582.
- Ong G, Austoker J, Brouwer A. Evaluation of the written information sent to women who are called back for further investigation of breast screening in the UK. Health Education Journal 1996;55:413-29. http://dx.doi.org/10.1177/001789699605500407.
- Austoker J, Ong G. Written information needs of women who are recalled for further investigation of breast screening: results of a multicentre study. J Med Screen 1994;1:238-44.
- Smith S, Botha JL, Goosey R, Daintith H. Audit of user satisfaction with the Leicestershire Breast Screening Service – women attending for assessment of abnormal mammograms. J Public Health Med 1991;13:166-71.
- Ong G, Austoker J, Brouwer A. Guidelines on improving the quality of the written information sent to women who are recalled for assessment. NHSBSP Publication no. 38. NHSBSP; 1998.
- Meldrum P, Turnbull D, Dobson HM, Colquhoun C, Gilmour WH, McIlwaine GM. Tailored written invitations for second round breast cancer screening: a randomised controlled trial. J Med Screen 1994;1:245-8.
- Brodersen J, Thorsen H, Cockburn J. The adequacy of measurement of short and long-term consequences of false-positive screening mammography. J Med Screen 2004;11:39-44. http://dx.doi.org/10.1258/096914104772950745.
- Brodersen J, Thorsen H, Kreiner S. Validation of a condition-specific measure for women having an abnormal screening mammography. Value Health 2007;10:294-30. http://dx.doi.org/10.1111/j.1524-4733.2007.00184.x.
- Brodersen J, McKenna SP, Doward LC, Thorsen H. Measuring the psychosocial consequences of screening. Health Qual Life Outcomes 2007;5. http://dx.doi.org/10.1186/1477-7525-5-3.
- Brodersen J, Thorsen H, Anderson JS. Short-term and long-term psychosocial consequences of false positive screening mammography – development of two new questionnaire based on the psychological consequences questionnaire (the PCQ). Value Health 2004;7:786-7. http://dx.doi.org/10.1016/S1098-3015(10)66105-0.
- Wardle J, Pope R. The psychological costs of screening for cancer. J Psychosom Res 1992;36:609-24. http://dx.doi.org/10.1016/0022-3999(92)90051-3.
- Chalmers K, Thomson K. Coming to terms with the risk of breast cancer: perceptions of women with primary relatives with breast cancer 1996;6:256-82.
- Scaf-Klomp W, van Sonderen FL, Stewart R, van Dijck JA, van den Heuvel WJ. Compliance after 17 years of breast cancer screening. J Med Screen 1995;2:195-9.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ 1995;311:109-12. http://dx.doi.org/10.1136/bmj.311.6997.109.
- Popay J, Rogers A, Williams G. Rationale and standards for the systematic review of qualitative literature in health services research. Qual Health Res 1998;8:341-51. http://dx.doi.org/10.1177/104973239800800305.
- Noblit GW, Hare RD. Meta-ethnography: synthesizing qualitative studies. Newbury Park, CA: Sage; 1988.
- Britten N, Campbell R, Pope C, Donovan J, Morgan M, Pill R. Using meta ethnography to synthesise qualitative research: a worked example. J Health Serv Res Policy 2002;7:209-15. http://dx.doi.org/10.1258/135581902320432732.
- Garside R, Britten N, Stein K. The experience of heavy menstrual bleeding: a systematic review and meta-ethnography of qualitative studies. J Adv Nurs 2008;63:550-62. http://dx.doi.org/10.1111/j.1365-2648.2008.04750.x.
- Rodgers M, Sowden A, Pettigrew M, Arai L, Roberts H, Britten N, et al. Testing Methodological Guidance on the Conduct of Narrative Synthesis in Systematic Reviews 2009. http://evi.sagepub.com (accessed 12 June 2009).
- Oliver S, Harden A, Rees R, Shepherd J, Brunton G, Oakley A. Young people and mental health: novel methods for systematic review of research on barriers and facilitators. Health Educ Res 2008;23:770-90. http://dx.doi.org/10.1093/her/cym038.
Appendix 1 Protocol
Appendix 2 Search strategy
Appendix 3 Data extraction forms
Appendix 4 Ongoing studies
Appendix 5 Papers excluded at full review
List of abbreviations
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CNS
- clinical nurse specialist
- CONSORT
- Consolidated Standards of Reporting Trials
- CRD
- Centre for Reviews and Dissemination
- CWS-R
- Cancer Worries Scale-Revised
- FHBC
- family history of breast cancer
- FNA
- fine-needle aspiration
- GHQ
- General Health Questionnaire
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HTA
- Health Technology Assessment
- IBSS
- International Bibliography of the Social Sciences
- MeSH
- medical subject heading
- NHSBSP
- NHS Breast Screening Programme
- OPCERG
- Oxford Primary Care Education Research Group
- OR
- odds ratio
- PCQ
- Psychological Consequences Questionnaire
- PIMMS
- Psychological Impact of Mammography Screening
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- STAI
- State–Trait Anxiety Inventory
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well-known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.